- Open access
- Published: 04 February 2022

Analysis of the COVID-19 pandemic: lessons towards a more effective response to public health emergencies
- Yibeltal Assefa ORCID: orcid.org/0000-0003-2393-1492 1 ,
- Charles F. Gilks 1 ,
- Simon Reid 1 ,
- Remco van de Pas 2 ,
- Dereje Gedle Gete 1 &
- Wim Van Damme 2
Globalization and Health volume 18 , Article number: 10 ( 2022 ) Cite this article
43k Accesses
10 Altmetric
Metrics details
The pandemic of Coronavirus Disease 2019 (COVID-19) is a timely reminder of the nature and impact of Public Health Emergencies of International Concern. As of 12 January 2022, there were over 314 million cases and over 5.5 million deaths notified since the start of the pandemic. The COVID-19 pandemic takes variable shapes and forms, in terms of cases and deaths, in different regions and countries of the world. The objective of this study is to analyse the variable expression of COVID-19 pandemic so that lessons can be learned towards an effective public health emergency response.
We conducted a mixed-methods study to understand the heterogeneity of cases and deaths due to the COVID-19 pandemic. Correlation analysis and scatter plot were employed for the quantitative data. We used Spearman’s correlation analysis to determine relationship strength between cases and deaths and socio-economic and health systems. We organized qualitative information from the literature and conducted a thematic analysis to recognize patterns of cases and deaths and explain the findings from the quantitative data.
We have found that regions and countries with high human development index have higher cases and deaths per million population due to COVID-19. This is due to international connectedness and mobility of their population related to trade and tourism, and their vulnerability related to older populations and higher rates of non-communicable diseases. We have also identified that the burden of the pandemic is also variable among high- and middle-income countries due to differences in the governance of the pandemic, fragmentation of health systems, and socio-economic inequities.
The COVID-19 pandemic demonstrates that every country remains vulnerable to public health emergencies. The aspiration towards a healthier and safer society requires that countries develop and implement a coherent and context-specific national strategy, improve governance of public health emergencies, build the capacity of their (public) health systems, minimize fragmentation, and tackle upstream structural issues, including socio-economic inequities. This is possible through a primary health care approach, which ensures provision of universal and equitable promotive, preventive and curative services, through whole-of-government and whole-of-society approaches.
The pandemic of Coronavirus Disease 2019 (COVID-19) is a timely reminder of the nature and impact of emerging infectious diseases that become Public Health Emergency of International Concern (PHEIC) [ 1 ]. The COVID-19 pandemic takes variable shapes and forms in how it affects communities in different regions and countries [ 2 , 3 ]. As of 12 January, 2022, there were over 314 million cases and over 5.5 million deaths notified around the globe since the start of the pandemic. The number of cases per million population ranged from 7410 in Africa to 131,730 in Europe while the number of deaths per million population ranged from 110 in Oceania to 2740 in South America. Case-fatality rates (CFRs) ranged from 0.3% in Oceania to 2.9% in South America [ 4 , 5 ]. Regions and countries with high human development index (HDI), which is a composite index of life expectancy, education, and per capita income indicators [ 6 ], are affected by COVID-19 more than regions with low HDI. North America and Europe together account for 55 and 51% of cases and deaths, respectively. Regions with high HDI are affected by COVID-19 despite their high universal health coverage index (UHCI) and Global Health Security index (GHSI) [ 7 ].
This seems to be a paradox (against the established knowledge that countries with weak (public) health systems capacity will have worse health outcomes) in that the countries with higher UHCI and GHSI have experienced higher burdens of COVID-19 [ 7 ]. The paradox can partially be explained by variations in testing algorithms, capacity for testing, and reporting across different countries. Countries with high HDI have health systems with a high testing capacity; the average testing rate per million population is less than 32, 000 in Africa and 160,000 in Asia while it is more than 800, 000 in HICs (Europe and North America). This enables HICs to identify more confirmed cases that will ostensibly increase the number of reported cases [ 3 ]. Nevertheless, these are insufficient to explain the stark differences between countries with high HDI and those with low HDI. Many countries with high HDI have a high testing rate and a higher proportion of symptomatic and severe cases, which are also associated with higher deaths and CFRs [ 7 ]. On the other hand, there are countries with high HDI that sustain a lower level of the epidemic than others with a similar high HDI. It is, therefore, vital to analyse the heterogeneity of the COVID-19 pandemic and explain why some countries with high HDI, UHCI and GHSI have the highest burden of COVID-19 while others are able to suppress their epidemics and mitigate its impacts.
The objective of this study was to analyse the COVID-19 pandemic and understand its variable expression with the intention to learn lessons for an effective and sustainable response to public health emergencies. We hypothesised that high levels of HDI, UHCI and GHSI are essential but not sufficient to prevent and control COVID-19.
We conducted an explanatory mixed-methods study to understand and explain the heterogeneity of the pandemic around the world. The study integrated quantitative and qualitative secondary data. The following steps were included in the research process: (i) collecting and analysing quantitative epidemiological data, (ii) conducting literature review of qualitative secondary data and (iii) evaluating countries’ pandemic responses to explain the variability in the COVID-19 epidemiological outcomes. The study then illuminated specific factors that were vital towards an effective and sustainable epidemic response.
We used the publicly available secondary data sources from Johns Hopkins University ( https://coronavirus.jhu.edu/data/new-cases ) for COVID-19 and UNDP 2020 HDI report ( http://hdr.undp.org/en/2019-report ) for HDI, demographic and epidemiologic variables. These are open data sources which are regularly updated and utilized by researchers, policy makers and funders. We performed a correlation analysis of the COVID-19 pandemic. We determined the association between COVID-19 cases, severity, deaths and CFRs at the 0.01 and 0.05 levels (2-tailed). We used Spearman’s correlation analysis, as there is no normal distribution of the variables [ 8 ].
The UHCI is calculated as the geometric mean of the coverage of essential services based on 17 tracer indicators from: (1) reproductive, maternal, newborn and child health; (2) infectious diseases; (3) non-communicable diseases; and, (4) service capacity and access and health security [ 9 ]. The GHSI is a composite measure to assess a country’s capability to prevent, detect, and respond to epidemics and pandemics [ 10 ].
We then conducted a document review to explain the epidemic patterns in different countries. Secondary data was obtained from peer-reviewed journals, reputable online news outlets, government reports and publications by public health-related associations, such as the WHO. To explain the variability of COVID-19 across countries, a list of 14 indicators was established to systematically assess country’s preparedness, actual pandemic response, and overall socioeconomic and demographic profile in the context of COVID-19. The indicators used in this study include: 1) Universal Health Coverage Index, 2) public health capacity, 3) Global Health Security Index, 4) International Health Regulation, 5) leadership, governance and coordination of response, 6) community mobilization and engagement, 7) communication, 8) testing, quarantines and social distancing, 9) medical services at primary health care facilities and hospitals, 10) multisectoral actions, 11) social protection services, 12) absolute and relative poverty status, 13) demography, and 14) burden of communicable and non-communicable diseases. These indicators are based on our previous studies and recommendation from the World Health Organization [ 3 , 4 ]. We conducted thematic analysis and synthesis to identify the factors that may explain the heterogeneity of the pandemic.
Heterogeneity of COVID-19 cases and deaths around the world: what can explain it?
Table 1 indicates that the pandemic of COVID-19 is heterogeneous around regions of the world. Figure 1 also shows that there is a strong and significant correlation between HDI and globalisation (with an increase in trade and tourism as proxy indicators) and a corresponding strong and significant correlation with COVID-19 burden.
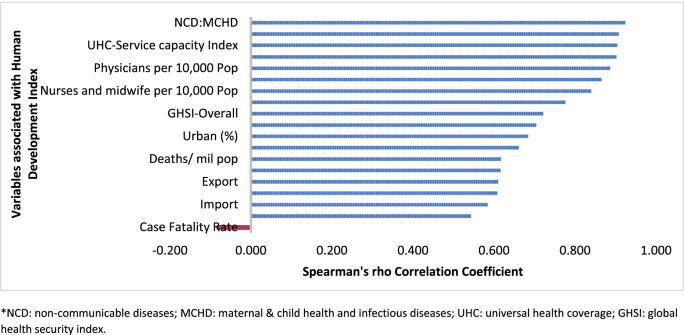
Human development index and its correlates associated with COVID-19 in 189 countries*
Globalisation and pandemics interact in various ways, including through international trade and mobility, which can lead to multiple waves of infections [ 11 ]. In at least the first waves of the pandemic, countries with high import and export of consumer goods, food products and tourism have high number of cases, severe cases, deaths and CFRs. Countries with high HDI are at a higher risk of importing (and exporting) COVID-19 due to high mobility linked to trade and tourism, which are drivers of the economy. These may have led to multiple introductions of COVID-19 into these countries before border closures.
The COVID-19 pandemic was first identified in China, which is central to the global network of trade, from where it spread to all parts of the world, especially those countries with strong links with China [ 12 ]. The epidemic then spread to Europe. There is very strong regional dimension to manufacturing and trading, which could be facilitate the spread of the virus. China is the heart of ‘Factory Asia’; Italy is in the heart of ‘Factory Europe’; the United States is the heart of ‘Factory North America’; and Brazil is the heart of ‘Factory Latin America’ [ 13 ]. These are the countries most affected by COVID-19 during the first wave of the pandemic [ 2 , 3 , 14 ].
It is also important to note that two-third of the countries currently reporting more than a million cases are middle-income countries (MICs), which are not only major emerging market economies but also regional political powers, including the BRICS countries (Brazil, Russia, India and South Africa) [ 3 , 15 ]. These countries participate in the global economy, with business travellers and tourists. They also have good domestic transportation networks that facilitate the internal spread of the virus. The strategies that helped these countries to become emerging markets also put them at greater risk for importing and spreading COVID-19 due to their connectivity to the rest of the world.
In addition, countries with high HDI may be more significantly impacted by COVID-19 due to the higher proportion of the elderly and higher rates of non-communicable diseases. Figure 1 shows that there is a strong and significant correlation between HDI and demographic transition (high proportion of old-age population) and epidemiologic transition (high proportion of the population with non-communicable diseases). Countries with a higher proportion of people older than 65 years and NCDs (compared to communicable diseases) have higher burden of COVID-19 [ 16 , 17 , 18 , 19 , 20 ]. Evidence has consistently shown a higher risk of severe COVID-19 in older individuals and those with underlying health conditions [ 21 , 22 , 23 , 24 , 25 ]. CFR is age-dependent; it is highest in persons aged ≥85 years (10 to 27%), followed by those among persons aged 65–84 years (3 to 11%), and those among persons aged 55-64 years (1 to 3%) [ 26 ].
On the other hand, regions and countries with low HDI have, to date, experienced less severe epidemics. For instance, as of January 12, 2022, the African region has recorded about 10.3 million cases and 233,000 deaths– far lower than other regions of the world (Table 1 ) [ 27 ]. These might be due to lower testing rates in Africa, where only 6.5% of the population has been tested for the virus [ 14 , 28 ], and a greater proportion of infections may remain asymptomatic [ 29 ]. Indeed, the results from sero-surveys in Africa show that more than 80% of people infected with the virus were asymptomatic compared to an estimated 40-50% asymptomatic infections in HICs [ 30 , 31 ]. Moreover, there is a weak vital registration system in the region indicating that reports might be underestimating and underreporting the disease burden [ 32 ]. However, does this fully explain the differences observed between Africa and Europe or the Americas?
Other possible factors that may explain the lower rates of cases and deaths in Africa include: (1) Africa is less internationally connected than other regions; (2) the imposition of early strict lockdowns in many African countries, at a time when case numbers were relatively small, limited the number of imported cases further [ 2 , 33 , 34 ]; (3) relatively poor road network has also limited the transmission of the virus to and in rural areas [ 35 ]; (4) a significant proportion of the population resides in rural areas while those in urban areas spend a lot of their time mostly outdoors; (5) only about 3% of Africans are over the age of 65 (so only a small proportion are at risk of severe COVID-19) [ 36 ]; (6) lower prevalence of NCDs, as disease burden in Africa comes from infectious causes, including coronaviruses, which may also have cross-immunity that may reduce the risk of developing symptomatic cases [ 37 ]; and (7) relative high temperature (a major source of vitamin D which influences COVID-19 infection and mortality) in the region may limit the spread of the virus [ 38 , 39 ]. We argue that a combination of all these factors might explain the lower COVID-19 burden in Africa.
The early and timely efforts by African leaders should not be underestimated. The African Union, African CDC, and WHO convened an emergency meeting of all African ministers of health to establish an African taskforce to develop and implement a coordinated continent-wide strategy focusing on: laboratory; surveillance; infection prevention and control; clinical treatment of people with severe COVID-19; risk communication; and supply chain management [ 40 ]. In April 2021, African Union and Africa CDC launched the Partnerships for African Vaccine Manufacturing (PAVM), framework to expanding Africa’s vaccine manufacturing capacity for health security [ 41 ].
Heterogeneity of the pandemic among countries with high HDI: what can explain it?
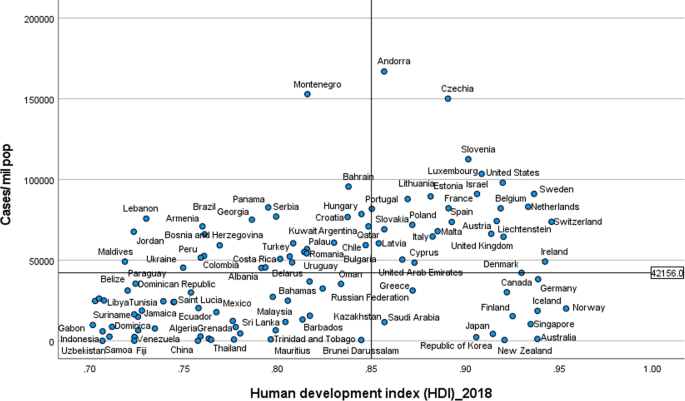
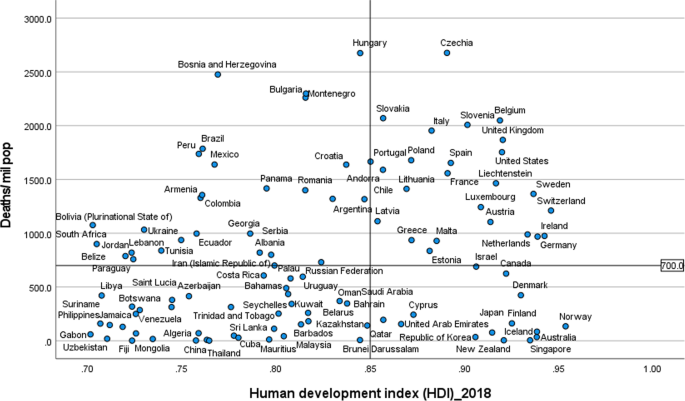
Figures 2 and 3 illustrate the variability of cases and deaths due to the COVID-19 pandemic across high-income countries (HICs). Contrary to the overall positive correlation between high HDI and cases, deaths and fatality rates due to COVID-19, there are outlier HICs, which have been able to control the epidemic. Several HICs, such as New Zealand, Australia, South Korea, Japan, Denmark, Iceland, and Norway, managed to contain their epidemics (Figs. 2 and 3 ) [ 15 , 42 , 43 ]. It is important to note that most of these countries (especially the island states) have far less cross-border mobility than other HICs.
Scatter plot of COVID-19 cases per million population in countries with high human development index (> 0.70)
Scatter plot of COVID-19 deaths per million population in countries with high human development index (> 0.70)
HICs that have been successful at controlling their epidemics have similar characteristics, which are related to governance of the response [ 44 ], synergy between UHC and GHS, and existing relative socio-economic equity in the country. Governance and leadership is a crucial factor to explain the heterogeneity of the epidemic among countries with high HDI [ 45 ]. There has been substantial variation in the nature and timing of the public health responses implemented [ 46 ]. Adaptable and agile governments seem better able to respond to their epidemics [ 47 , 48 ]. Countries that have fared the best are the ones with good governance and public support [ 49 ]. Countries with an absence of coherent leadership and social trust have worse outcomes than countries with collective action, whether in a democracy or autocracy, and rapid mobilisation of resources [ 50 ]. The erosion of trust in the United States government has hurt the country’s ability to respond to the COVID-19 crisis [ 51 , 52 ]. The editors of the New England Journal of Medicine argued that the COVID-19 crisis has produced a test of leadership; but, the leaders in the United States had failed that test [ 47 ].
COVID-19 has exposed the fragility of health systems, not only in the public health and primary care, but also in acute and long-term care systems [ 49 ]. Fragmentation of health systems, defined here to mean inadequate synergy and/ or integration between GHS and UHC, is typical of countries most affected by the COVID-19 pandemic. Even though GHS and UHC agendas are convergent and interdependent, they tend to have different policies and practices [ 53 ]. The United States has the highest index for GHS preparedness; however, it has reported the world’s highest number of COVID-19 cases and deaths due to its greatly fragmented health system [ 54 , 55 ]. Countries with health systems and policies that are able to integrate International Health Regulations (IHR) core capacities with primary health care (PHC) services have been effective at mitigating the effects of COVID-19 [ 50 , 53 ]. Australia has been able to control its COVID-19 epidemic through a comprehensive primary care response, including protection of vulnerable people, provision of treatment and support services to affected people, continuity of regular healthcare services, protection and support of PHC workers and primary care services, and provision of mental health services to the community and the primary healthcare workforce [ 56 ]. Strict implementation of public health and social intervention together with UHC systems have ensured swift control of the epidemics in Singapore, South Korea, and Thailand [ 57 ].
The heterogeneity of cases and deaths, due to COVID-19, is also explained by differences in levels of socio-economic inequalities, which increase susceptibility to acquiring the infection and disease progression as well as worsening of health outcomes [ 58 ]. COVID-19 has been a stress test for public services and social protection systems. There is a higher burden of COVID-19 in Black, Asian and Minority Ethnic individuals due to socio-economic inequities in HICs [ 59 , 60 ]. Poor people are more likely to live in overcrowded accommodation, are more likely to have unstable work conditions and incomes, have comorbidities associated with poverty and precarious living conditions, and reduced access to health care [ 59 ].
The epidemiology of COVID-19 is also variable across MICs, with HDI between 0.70 and 0.85, around the world. Overall, the epidemic in MICs is exacerbated by the rapid demographic and epidemiologic transitions as well as high prevalence of obesity. While India and Brazil witnessed rapidly increasing rates of cases and deaths, China, Thailand, Vietnam have experienced a relatively lower disease burden [ 15 ]. This heterogeneity may be attributed to a number of factors, including governance, communication and service delivery. Thailand, China and Vietnam have implemented a national harmonized strategic response with decentralized implementation through provincial and district authorities [ 61 ]. Thailand increased its testing capacity from two to over 200 certified facilities that could process between 10,000 to 100,000 tests per day; moreover, over a million village health volunteers in Thailand supported primary health services [ 62 , 63 ]. China’s swift and decisive actions enabled the country to contain its epidemic though there was an initial delay in detecting the disease. China has been able to contain its epidemic through community-based measures, very high public cooperation and social mobilization, strategic lockdown and isolation, multi-sector action [ 64 ]. Overall, multi-level governance (effective and decisive leadership and accountability) of the response, together with coordination of public health and socio-economic services, and high levels of citizen adherence to personal protection, have enabled these countries to successfully contain their epidemics [ 61 , 65 , 66 ].
On the other hand, the Brazilian leadership was denounced for its failure to establish a national surveillance network early in the pandemic. In March 2020, the health minister was reported to have stated that mass testing was a waste of public funding, and to have advised against it [ 67 ]. This was considered as a sign of a collapse of public health leadership, characterized by ignorance, neoliberal authoritarianism [ 68 ]. There were also gaps in the public health capacity in different municipalities, which varied greatly, with a considerable number of Brazilian regions receiving less funding from the federal government due to political tension [ 69 ]. The epidemic has a disproportionate adverse burden on states and municipalities with high socio-economic vulnerability, exacerbated by the deep social and economic inequalities in Brazil [ 70 ].
India is another middle-income country with a high burden of COVID-19. It was one of the countries to institute strict measures in the early phase of the pandemic [ 71 , 72 ]. However, the government eased restrictions after the claim that India had beaten the pandemic, which lead to a rapid increase in disease incidence. Indeed, on 12 January 2022, India reported 36 million cumulative cases and almost 485,000 total deaths [ 15 ]. The second wave of the epidemic in India exposed weaknesses in governance and inadequacies in the country’s health and other social systems [ 73 ]. The nature of the Indian federation, which is highly centripetal, has prevented state and local governments from tailoring a policy response to suit local needs. A centralized one-size-fits-all strategy has been imposed despite high variations in resources, health systems capacity, and COVID-19 epidemics across states [ 74 ]. There were also loose social distancing and mask wearing, mass political rallies and religious events [ 75 ]. Rapid community transmission driven by high population density and multigenerational households has been a feature of the current wave in India [ 76 ]. In addition, several new variants of the virus, including the UK (B.1.1.7), the South Africa (20H/501Y or B.1.351), and Brazil (P.1), alongside a newly identified Indian variant (B.1.617), are circulating in India and have been implicated as factors in the second wave of the pandemic [ 75 , 76 ].
Heterogeneity of case-fatality rates around the world: what can explain it?
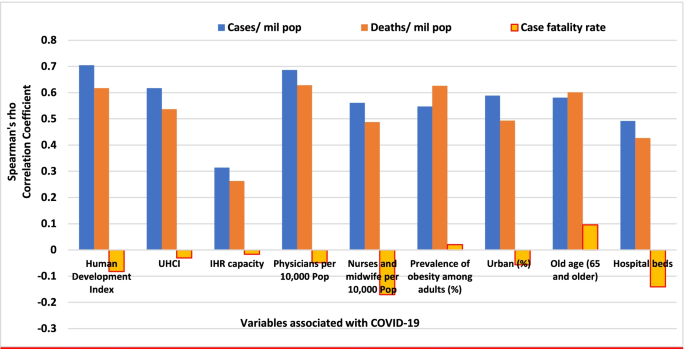
The pandemic is characterized by variable CFRs across regions and countries that are negatively associated with HDI (Fig. 1 ). The results presented in Fig. 4 show that the proportion of elderly population and rate of obesity are important factors which are positively associated with CFR. On the other hand, UHC, IHR capacity and other indicators of health systems capacity (health workforce density and hospital beds) are negatively associated with the CFR (Figs. 1 and 4 ).
Correlates of COVID-19 cases, deaths and case-fatality rates in 189 countries
The evidence from several research indicates that heterogeneity can be explained by several factors, including differences in age-pyramid, socio-economic status, access to health services, or rates of undiagnosed infections. Differences in age-pyramid may explain some of the observed variation in epidemic severity and CFR between countries [ 77 ]. CFRs across countries look similar when taking age into account [ 78 ]. The elderly and other vulnerable populations in Africa and Asia are at a similar risk as populations in Europe and Americas [ 79 ]. Data from European countries suggest that as high as 57% of all deaths have happened in care homes and many deaths in the US have also occurred in nursing homes. On the other hand, in countries such as Mexico and India, individuals < 65 years contributed the majority of deaths [ 80 ].
Nevertheless, CFR also depends on the quality of hospital care, which can be used to judge the health system capacity, including the availability of healthcare workers, resources, and facilities, which affects outcomes [ 81 ]. The CFR can increase if there is a surge of infected patients, which adds to the strain on the health system [ 82 ]. COVID-19 fatality rates are affected by numerous health systems factors, including bed capacity, existence and capacity of intensive care unit (ICU), and critical care resources (such as oxygen and dexamethasone) in a hospital. Regions and countries with high HDI have a greater number of acute care facilities, ICU, and hospital bed capacities compared to lower HDI regions and countries [ 83 ]. Differences in health systems capacity could explain why North America and Europe, which have experienced much greater number of cases and deaths per million population, reported lower CFRs than the Southern American and the African regions, partly also due to limited testing capacity in these regions (Table 1 ) [ 84 , 85 , 86 ]. The higher CFR in Southern America can be explained by the relatively lower health systems surge capacity that could not adequately respond to the huge demand for health services [ 69 , 86 ]. The COVID-19 pandemic has highlighted existing health systems’ weaknesses, which are not able to effectively prepare for and respond to PHEs [ 87 ]. The high CFRs in the region are also exacerbated by the high social inequalities [ 69 ].
On the other hand, countries in Asia recorded lower CFRs (~ 1.4%) despite sharing many common risk factors (including overcrowding and poverty, weak health system capacity etc) with Africa. The Asian region shares many similar protective factors to the African region. They have been able to minimize their CFR by suppressing the transmission of the virus and flattening the epidemic curve of COVID-19 cases and deaths. Nevertheless, the epidemic in India is likely to be different because it has exceeded the health system capacity to respond and provide basic medical care and medical supplies such as oxygen [ 88 ]. Overall, many Asian countries were able to withstand the transmission of the virus and its effect due to swift action by governments in the early days of the pandemic despite the frequency of travel between China and neighbouring countries such as Hong Kong, Taiwan and Singapore [ 89 ]. This has helped them to contain the pandemic to ensure case numbers remain within their health systems capacity. These countries have benefited from their experience in the past in the prevention and control of epidemics [ 90 ].
There are a number of issues with the use of the CFR to compare the management of the pandemic between countries and regions [ 91 ], as it does not depict the true picture of the mortality burden of the pandemic. A major challenge with accurate calculation of the CFR is the denominator on number of identified cases, as asymptomatic infections and patients with mild symptoms are frequently left untested, and therefore omitted from CFR calculations. Testing might not be widely available, and proactive contact tracing and containment might not be employed, resulting in a smaller denominator, and skewing to a higher CFR [ 82 ]. It is, therefore, far more relevant to estimate infection fatality rate (IFR), the proportion of all infected individuals who have died due to the infection [ 91 ], which is central to understanding the public health impact of the pandemic and the required policies for its prevention and control [ 92 ].
Estimates of prevalence based on sero-surveys, which includes asymptomatic and mildly symptomatic infections, can be used to estimate IFR [ 93 ]. In a systematic review of 17 studies, seroprevalence rates ranged from 0.22% in Brazil to 53% in Argentina [ 94 ]. The review also identified that the seroprevalence estimate was higher than the cumulative reported case incidence, by a factor between 1.5 times in Germany to 717 times in Iran, in all but two studies (0.56 times in Brazil and 0.88 times in Denmark) [ 94 , 95 ]. The difference between seroprevalence and cumulative reported cases might be due to asymptomatic cases, atypical or pauci-symptomatic cases, or the lack of access to and uptake of testing [ 94 ]. There is only a modest gap between the estimated number of infections from seroprevalence surveys and the cumulative reported cases in regions with relatively thorough symptom-based testing. Much of the gap between reported cases and seroprevalence is likely to be due to undiagnosed symptomatic or asymptomatic infections [ 94 ].
Collateral effects of the COVID-19 pandemic
It is important to note that the pandemic has significant collateral effects on the provision of essential health services, in addition to the direct health effects [ 96 ]. Disruptions in the provision of essential health services, due to COVID-19, were reported by nearly all countries, though it is more so in lower-income than higher-income countries [ 97 , 98 ]. The biggest impact reported is on provision of day-to-day primary care to prevent and manage some of the most common health problems [ 99 ].
The causes of disruptions in service delivery were a mix of demand and supply factors [ 100 ]. Countries reported that just over one-third of services were disrupted due to health workforce-related reasons (the most common causes of service disruptions), supply chains, community mistrust and fears of becoming infected, and financial challenge s[ 101 ]. Cognizant of the disruptive effects of the pandemic, countries have reorganized their health system.
Countries with better response to COVID-19 have mobilized, trained and reallocated their health workforce in addition to hiring new staff, using volunteers and medical trainees and mobilizing retirees [ 102 ]. Several strategies have also been implemented to mitigate disruptions in service delivery and utilization, including: triaging to identify the most urgent patient needs, and postponing elective medical procedures; switching to alternative models of care, such as providing more home-based care and telemedicine [ 101 ].
This study identifies that the COVID-19 pandemic, in terms f cases and deaths, is heterogeneous around the world. This variability is explained by differences in vulnerability, preparedness, and response. It confirms that a high level of HDI, UHCI and GHSI are essential but not sufficient to control epidemics [ 103 ]. An effective response to public health emergencies requires a joint and reinforcing implementation of UHC, health emergency and disease control priorities [ 104 , 105 ], as well as good governance and social protection systems [ 106 ]. Important lessons have been learned to cope better with the COVID-19 pandemic and future emerging or re-emerging pandemics. Countries should strengthen health systems, minimize fragmentation of public health, primary care and secondary care, and improve coordination with other sectors. The pandemic has exposed the health effects of longstanding social inequities, which should be addressed through policies and actions to tackle vulnerability in living and working conditions [ 106 ].
The shift in the pandemic epicentre from high-income to MICs was observed in the second global wave of the pandemic. This is due to in part to the large-scale provision of vaccines in HICs [ 15 ] as well as the limitations in the response in LMICs, including inadequate testing, quarantine and isolation, contact tracing, and social distancing. The second wave of the pandemic in low- and middle-income countries spread more rapidly than the first wave and affected younger and healthier populations due to factors, including poor government decision making, citizen behaviour, and the emergence of highly transmissible SARS-CoV-2 variants [ 107 ]. It has become catastrophic in some MICs to prematurely relax key public health measures, such as mask wearing, physical distancing, and hand hygiene [ 108 ].
There is consensus that global vaccination is essential to ending the pandemic. Universal and equitable vaccine delivery, implemented with high volume, speed and quality, is vital for an effective and sustainable response to the current pandemic and future public health emergencies. There is, however, ongoing concern regarding access to COVID-19 vaccines in low-income countries [ 109 ]. Moreover, there is shortage of essential supplies, including oxygen, which has had a major impact on the prevention and control of the pandemic. It is, therefore, vital to transform (through good governance and financing mechanisms) the ACT-A platform to deliver vaccines, therapeutics, diagnostics, and other essential supplies [ 109 , 110 ]. The global health community has the responsibility to address these inequalities so that we can collectively end the pandemic [ 107 ].
The Omicron variant has a huge role in the current wave around the world despite high vaccine coverage [ 111 ]. Omicron appears to spread rapidly around the world ever since it was identified in November 2021 [ 112 ]. It becomes obvious that vaccination alone is inadequate for controlling the infection. This has changed our understanding of the COVID-19 pandemic endgame. The emergence of new variants of concern and their spread around the world has highlighted the importance of combination prevention, including high vaccination coverage in combination with other public health prevention measures [ 112 ].
Overall, the COVID-19 pandemic and the response to it emphasise valuable lessons towards an effective and sustainable response to public health emergencies. We argue that the PHC approach captures the different preparedness and response strategies required towards ensuring health security and UHC [ 113 ]. The PHC approach enables countries to progressively realize universal access to good-quality health services (including essential public health functions) and equity, empower people and communities, strengthen multi-sectoral policy and action for health, and enhance good governance [ 114 ]. These are essential in the prevention and control of public health emergencies, to suppress transmission, and reduce morbidity and mortality [ 115 ]. Access to high-quality primary care is at the foundation of any strong health system [ 116 ], which will, in turn, have effect on containing the epidemic, and reducing mortality and CFR [ 117 ]. Australia is a good example in this regard because it has implemented a comprehensive PHC approach in combination with border restrictions to ensure health system capacity is not exceeded [ 56 ]. The PHC approach will enable countries to develop and implement a context-specific health strategy, enhance governance, strengthen their (public) health systems, minimize segmentation and fragmentation, and tackle upstream structural issues, including discrimination and socio-economic inequities [ 118 ]. This is the type of public health approach (comprehensive, equity-focused and participatory) that will be effective and sustainable to tackle public health emergencies in the twenty-first century [ 119 , 120 ]. In addition, it is vital to transform the global and regional health systems, with a strong IHR and an empowered WHO at the apex [ 121 ]. We contend that this is the way towards a healthier and safer country, region and world.
The COVID-19 pandemic demonstrates that the world remains vulnerable to public health emergencies with significant health and other socio-economic impacts. The pandemic takes variable shapes and forms across regions and countries around the world. The pandemic has impacted countries with inadequate governance of the epidemic, fragmentation of their health systems and higher socio-economic inequities more than others. We argue that adequate response to public health emergencies requires that countries develop and implement a context-specific national strategy, enhance governance of public health emergency, build the capacity of their health systems, minimize fragmentation, and tackle socio-economic inequities. This is possible through a PHC approach that provides universal access to good-quality health services through empowered communities and multi-sectoral policy and action for health development. The pandemic has affected every corner of the world; it has demonstrated that “no country is safe unless other countries are safe”. This should be a call for a strong global health system based on the values of justice and capabilities for health.
Availability of data and materials
Data are available in a public, open access repository: Johns Hopkins University: https://coronavirus.jhu.edu/data/new-cases , and UNDP: http://hdr.undp.org/en/2019-report ; WHO: https://www.who.int/publications/m/item/weekly-epidemiological-update%2D%2D-22-december-2020
Abbreviations
Coronavirus Disease 2019
Case-fatality rates
Human development index
Universal health coverage index
Global Health Security index
High-income countries
Middle-income countries
El Zowalaty ME, Järhult JD. From SARS to COVID-19: A previously unknown SARS-CoV-2 virus of pandemic potential infecting humans–Call for a One Health approach. One Health. 2020;9:100124. https://doi.org/10.1016/j.onehlt.2020.100124 .
Van Damme W, Dahake R, Delamou A, Ingelbeen B, Wouters E, Vanham G, et al. The COVID-19 pandemic: diverse contexts; different epidemics—how and why? BMJ Glob Health. 2020;5(7):e003098.
Article PubMed Google Scholar
World Health Organization (WHO): Coronavirus disease ( COVID-19): situation report, 150. 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200618-covid-19-sitrep-150.pdf .
Weekly epidemiological update - 22 December 2020 [ https://www.who.int/publications/m/item/weekly-epidemiological-update%2D%2D-22-december-2020 ].
Worldometer: COVID-19 coronavirus pandemic. 2020. https://www.worldometers.info/coronavirus/ .
Anand S, Sen A. Human Development Index: Methodology and Measurement. 1994. http://hdr.undp.org/en/content/human-development-index-methodology-and-measurement .
De Larochelambert Q, Marc A, Antero J, Le Bourg E, Toussaint J-F. Covid-19 mortality: a matter of vulnerability among nations facing limited margins of adaptation. Public Health. 2020;8. https://doi.org/10.3389/fpubh.2020.604339 .
de Winter JC, Gosling SD, Potter J. Comparing the Pearson and Spearman correlation coefficients across distributions and sample sizes: a tutorial using simulations and empirical data. Psychol Methods. 2016;21(3):273.
World Health Organization. Universal health coverage [ http://www.who.int/universal_health_coverage/en/ ].
Johns Hopkins Center for Health Security. Global Health Security Index [ https://www.ghsindex.org/ ].
Pol Antràs SJR. Esteban Rossi-Hansberg: how do globalisation and pandemics interact? Surprising insights from a new model. In: #LSEThinks | CEP | global development vol. 2020. London: London School of Economics; 2020.
Google Scholar
Cai P. Understanding China’s belt and road initiative; 2017.
Baldwin R, Tomiura E. Thinking ahead about the trade impact of COVID-19. Economics in the Time of COVID-19. 2020;59. https://repository.graduateinstitute.ch/record/298220?ln=en .
Organization WH: Coronavirus disease ( COVID-19): situation report, 182. 2020.
Johns Hopkins University: COVID-19 Map - Johns Hopkins Coronavirus Resource Center. In. Edited by Security JHUCfH; 2021. https://coronavirus.jhu.edu/map.html .
Wang T, Du Z, Zhu F, Cao Z, An Y, Gao Y, et al. Comorbidities and multi-organ injuries in the treatment of COVID-19. Lancet. 2020;395(10228):e52.
Article CAS PubMed PubMed Central Google Scholar
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. Jama. 2020;323(11):1061–9.
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506.
Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–13.
Hussain A, Bhowmik B, Do Vale Moreira NC. COVID-19 and diabetes: knowledge in progress. Diabetes Res Clin Pract. 2020;162:108142. https://doi.org/10.1016/j.diabres.2020.108142 . Epub 2020 Apr 9.
Covid C, COVID C, COVID C, Chow N, Fleming-Dutra K, Gierke R, Hall A, et al. Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019—United States, February 12–march 28, 2020. Morb Mortal Wkly Rep. 2020;69(13):382.
Group C-S. Characteristics of COVID-19 patients dying in Italy: report based on available data on March 20th, 2020. Rome: Instituto Superiore Di Sanita; 2020. https://www.epicentro.iss.it/coronavirus/bollettino/Report-COVID-2019_20_marzo_eng.pdf .
Guan W-j, Ni Z-y, Hu Y, Liang W-H, Ou C-Q, He J-X, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–20.
Article CAS PubMed Google Scholar
Arentz M, Yim E, Klaff L, Lokhandwala S, Riedo FX, Chong M, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington state. Jama. 2020;323(16):1612–4.
Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the new York City area. JAMA. 2020;323(20):2052–9. https://doi.org/10.1001/jama.2020.6775 .
Covid C, Team R. Severe outcomes among patients with coronavirus disease 2019 (COVID-19)—United States, February 12–march 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343–6.
Article Google Scholar
Mbow M, Lell B, Jochems SP, Cisse B, Mboup S, Dewals BG, et al. COVID-19 in Africa: dampening the storm? Science. 2020;369(6504):624–6.
Kavanagh MM, Erondu NA, Tomori O, Dzau VJ, Okiro EA, Maleche A, et al. Access to lifesaving medical resources for African countries: COVID-19 testing and response, ethics, and politics. Lancet. 2020;395(10238):1735–8.
Nordling L. Africa's pandemic puzzle: why so few cases and deaths? In: American Association for the Advancement of Science; 2020.
Oran DP, Topol EJ. Prevalence of asymptomatic SARS-CoV-2 infection: a narrative review. Ann Intern Med. 2020;173(5):362–7. https://doi.org/10.7326/M20-3012 . Epub 2020 Jun 3.
Nikolai LA, Meyer CG, Kremsner PG, Velavan TP. Asymptomatic SARS coronavirus 2 infection: invisible yet invincible. Int J Infect Dis. 2020;100:112–6. https://doi.org/10.1016/j.ijid.2020.08.076 . Epub 2020 Sep 3.
Rao C, Bradshaw D, Mathers CD. Improving death registration and statistics in developing countries: lessons from sub-Saharan Africa. Southern Afr J Demography. 2004:81–99.
Mehtar S, Preiser W, Lakhe NA, Bousso A, TamFum J-JM, Kallay O, et al. Limiting the spread of COVID-19 in Africa: one size mitigation strategies do not fit all countries. Lancet Glob Health. 2020;8(7):e881–3. Published online 2020 Apr 28. https://doi.org/10.1016/S2214-109X(20)30212-6 .
Nachega J, Seydi M, Zumla A. The late arrival of coronavirus disease 2019 (COVID-19) in Africa: mitigating pan-continental spread. Clin Infect Dis. 2020;71(15):875–8.
Gwilliam K, Foster V, Archondo-Callao R, Briceno-Garmendia C, Nogales A, Sethi K. Africa infrastructure country diagnostic: roads in sub-Saharan Africa: The World Bank; 2008.
Guengant J-P. Africa’s population: history, current status, and projections. In: Africa's Population: In Search of a Demographic Dividend: Springer; 2017. p. 11–31.
Collaborators GOD, Bernabe E, Marcenes W, Hernandez C, Bailey J, Abreu L, et al. Global, regional, and national levels and trends in burden of oral conditions from 1990 to 2017: a systematic analysis for the global burden of disease 2017 study. J Dent Res. 2020;99(4):362–73.
Cambaza EM, Viegas GC, Cambaza C. Manuel a: potential impact of temperature and atmospheric pressure on the number of cases of COVID-19 in Mozambique, southern Africa. J Public Health Epidemiol. 2020;12(3):246–60.
Lawal Y. Africa’s low COVID-19 mortality rate: a paradox? Int J Infect Dis. 2021;102:118–22.
Nkengasong JN, Mankoula W. Looming threat of COVID-19 infection in Africa: act collectively, and fast. Lancet. 2020;395(10227):841–2.
African Union and Africa CDC. African union and Africa CDC launches partnerships for African vaccine manufacturing (PAVM), framework to achieve it and signs 2 MoUs: African Union and Africa CDC; 2021.
Forbes. What Do Countries With The Best Coronavirus Responses Have In Common? Women Leaders [ https://www.forbes.com/sites/avivahwittenbergcox/2020/04/13/what-do-countries-with-the-best-coronavirus-reponses-have-in-common-women-leaders/#603bd9433dec ].
University of Notre Dame. What can we learn from Austria’s response to COVID-19? [ https://keough.nd.edu/what-can-we-learn-from-austrias-response-to-covid-19/ ].
Stoller JK. Reflections on leadership in the time of COVID-19. BMJ Leader. 2020. https://doi.org/10.1136/leader-2020-000244 .
Houston Public Media. What 6 Of The 7 Countries With The Most COVID-19 Cases Have In Common [ https://www.npr.org/sections/goatsandsoda/2020/07/31/896879448/the-nations-with-the-most-to-lose-from-covid-19 ].
Hale T, Petherick A, Phillips T, Webster S. Variation in government responses to COVID-19. Blavatnik school of government working paper. 2020;31. https://en.unesco.org/inclusivepolicylab/sites/default/files/learning/document/2020/4/BSG-WP-2020-031-v3.0.pdf .
The Editors. Dying in a leadership vacuum. N Engl J Med. 2020;383(15):1479–80.
The Cable News Network. US and UK are bottom of the pile in rankings of governments' handling of coronavirus pandemic [ https://edition.cnn.com/2020/08/27/world/global-coronavirus-attitudes-pew-intl/index.html ].
Huston P, Campbell J, Russell G, Goodyear-Smith F, Phillips RL, van Weel C, et al. COVID-19 and primary care in six countries. BJGP Open. 2020;4(4):bjgpopen20X101128. https://doi.org/10.3399/bjgpopen20X101128 .
Goodyear-Smith F, Kinder K, Eden AR, Strydom S, Bazemore A, Phillips R, et al. Primary care perspectives on pandemic politics. Glob Public Health. 2021;16(8-9):1304–19. https://doi.org/10.1080/17441692.2021.1876751 . Epub 2021 Jan 24.
Rutledge PE. Trump, COVID-19, and the war on expertise. Am Rev Public Adm. 2020;50(6-7):505–11.
Ugarte DA, Cumberland WG, Flores L, Young SD. Public attitudes about COVID-19 in response to president trump's social media posts. JAMA Netw Open. 2021;4(2):e210101.
Article PubMed PubMed Central Google Scholar
Lal A, Erondu NA, Heymann DL, Gitahi G, Yates R. Fragmented health systems in COVID-19: rectifying the misalignment between global health security and universal health coverage. Lancet. 2021;397(10268):61–7. https://doi.org/10.1016/S0140-6736(20)32228-5 . Epub 2020 Dec 1.
Tromberg BJ, Schwetz TA, Pérez-Stable EJ, Hodes RJ, Woychik RP, Bright RA, et al. Rapid scaling up of Covid-19 diagnostic testing in the United States—the NIH RADx initiative. N Engl J Med. 2020;383(11):1071–7.
Marwaha J, Halamka J, Brat G. Lifesaving ventilators will sit unused without a national data-sharing effort; 2020.
Kidd MR. Five principles for pandemic preparedness: lessons from the Australian COVID-19 primary care response. Br J Gen Pract. 2020;70(696):316–7. https://doi.org/10.3399/bjgp20X710765 . Print 2020 Jul.
Hsu LY, Tan M-H. What Singapore can teach the US about responding to COVID-19. STAT News. 2020. https://www.statnews.com/2020/03/23/singapore-teach-united-states-about-covid-19-response/ .
Horton R. Offline: COVID-19 is not a pandemic. Lancet (London, England). 2020;396(10255):874.
Article CAS Google Scholar
Raisi-Estabragh Z, McCracken C, Bethell MS, Cooper J, Cooper C, Caulfield MJ, et al. Greater risk of severe COVID-19 in black, Asian and minority ethnic populations is not explained by cardiometabolic, socioeconomic or behavioural factors, or by 25 (OH)-vitamin D status: study of 1326 cases from the UK biobank. J Public Health. 2020;42(3):451–60.
Hamidianjahromi A. Why African Americans are a potential target for COVID-19 infection in the United States. J Med Internet Res. 2020;22(6):e19934.
Tangcharoensathien V, Bassett MT, Meng Q, Mills A. Are overwhelmed health systems an inevitable consequence of covid-19? Experiences from China, Thailand, and New York State. BMJ. 2021;372. https://doi.org/10.1136/bmj.n83 .
Organization WH: COVID-19 health system response monitor, Thailand. 2020.
Narkvichien M. Thailand’s 1 million village health volunteers-“unsung heroes”-are helping guard communities nationwide from COVID-19. Nonthaburi: World Health Organization; 2020.
Kupferschmidt K, Cohen J. Can China's COVID-19 strategy work elsewhere? In: American Association for the Advancement of Science; 2020.
Al Saidi AMO, Nur FA, Al-Mandhari AS, El Rabbat M, Hafeez A, Abubakar A. Decisive leadership is a necessity in the COVID-19 response. Lancet. 2020;396(10247):295–8.
Forman R, Atun R, McKee M, Mossialos E. 12 lessons learned from the management of the coronavirus pandemic. Health Policy. 2020;124(6):577–80. https://doi.org/10.1016/j.healthpol.2020.05.008 .
Barberia LG, Gómez EJ. Political and institutional perils of Brazil's COVID-19 crisis. Lancet. 2020;396(10248):367–8.
Ortega F, Orsini M. Governing COVID-19 without government in Brazil: ignorance, neoliberal authoritarianism, and the collapse of public health leadership. Global Public Health. 2020;15(9):1257–77.
Ezequiel GE, Jafet A, Hugo A, Pedro D, Ana Maria M, Carola OV, et al. The COVID-19 pandemic: a call to action for health systems in Latin America to strengthen quality of care. Int J Qual Health Care. 2021;33(1):mzaa062.
Rocha R, Atun R, Massuda A, Rache B, Spinola P, Nunes L, et al. Effect of socioeconomic inequalities and vulnerabilities on health-system preparedness and response to COVID-19 in Brazil: a comprehensive analysis. Lancet Glob Health. 2021;9(6):e782–92. https://doi.org/10.1016/S2214-109X(21)00081-4 . Epub 2021 Apr 12.
Lancet T. India under COVID-19 lockdown. Lancet (London, England). 2020;395(10233):1315.
Siddiqui AF, Wiederkehr M, Rozanova L, Flahault A. Situation of India in the COVID-19 pandemic: India’s initial pandemic experience. Int J Environ Res Public Health. 2020;17(23):8994.
Article CAS PubMed Central Google Scholar
Taneja P, Bali AS. India’s domestic and foreign policy responses to COVID-19. Round Table. 2021;110(1):46–61.
Choutagunta A, Manish G, Rajagopalan S. Battling COVID-19 with dysfunctional federalism: lessons from India. South Econ J. 2021;87(4):1267–99.
Mallapaty S. India's massive COVID surge puzzles scientists. Nature. 2021;592(7856):667–8.
Thiagarajan K. Why is India having a covid-19 surge? In: British Medical Journal Publishing Group; 2021.
Fisman DN, Greer AL, Tuite AR. Age is just a number: a critically important number for COVID-19 case fatality. Ann Intern Med. 2020;173(9):762–3.
Sudharsanan N, Didzun O, Bärnighausen T, Geldsetzer P. The contribution of the age distribution of cases to COVID-19 case fatality across countries: a 9-country demographic study. Ann Intern Med. 2020;173(9):714–20. https://doi.org/10.7326/M20-2973 . Epub 2020 Jul 22.
Think Global Health. The Myth of South Asian Exceptionalism. In: He Myth of South Asian Exceptionalism: South Asia's young population conceals the effects that COVID-19 has on its older and more vulnerable people. vol. 2020; 2020.
Ioannidis JP, Axfors C, Contopoulos-Ioannidis DG. Population-level COVID-19 mortality risk for non-elderly individuals overall and for non-elderly individuals without underlying diseases in pandemic epicenters. Environ Res. 2020;188:109890.
Kim D-H, Choe YJ, Jeong J-Y. Understanding and interpretation of case fatality rate of coronavirus disease 2019. J Korean Med Sci. 2020;35(12):e137. https://doi.org/10.3346/jkms.2020.35.e137 .
Rajgor DD, Lee MH, Archuleta S, Bagdasarian N, Quek SC. The many estimates of the COVID-19 case fatality rate. Lancet Infect Dis. 2020;20(7):776–7.
Sorci G, Faivre B, Morand S. Explaining among-country variation in COVID-19 case fatality rate. Sci Rep. 2020;10(1):1–11.
Sen-Crowe B, Sutherland M, McKenney M, Elkbuli A. A closer look into global hospital beds capacity and resource shortages during the COVID-19 pandemic. J Surg Res. 2021;260:56–63.
Li M, Zhang Z, Cao W, Liu Y, Du B, Chen C, et al. Identifying novel factors associated with COVID-19 transmission and fatality using the machine learning approach. Sci Total Environ. 2021;764:142810.
Undurraga EA, Chowell G, Mizumoto K. COVID-19 case fatality risk by age and gender in a high testing setting in Latin America: Chile, march–august 2020. Infect Dis Poverty. 2021;10(1):1–11.
Taylor L. How Latin America is fighting covid-19, for better and worse. BMJ. 2020;370:m3319. https://doi.org/10.1136/bmj.m3319 .
Bhuyan A. Covid-19: India looks to import oxygen as cases surge, overwhelming hospitals. In: British Medical Journal Publishing Group; 2021.
An BY, Tang S-Y. Lessons from COVID-19 responses in East Asia: institutional infrastructure and enduring policy instruments. Am Rev Public Adm. 2020;50(6-7):790–800.
Chen H, Shi L, Zhang Y, Wang X, Sun G. A cross-country core strategy comparison in China, Japan, Singapore and South Korea during the early COVID-19 pandemic. Glob Health. 2021;17(1):1–10.
Kahathuduwa CN, Dhanasekara CS, Chin S-H. Case fatality rate in COVID-19: a systematic review and meta-analysis. J Prev Med Hyg. 2021;62(2):E311–20. https://doi.org/10.15167/2421-4248/jpmh2021.62.2.1627 . eCollection 2021 Jun.
Meyerowitz-Katz G, Merone L. A systematic review and meta-analysis of published research data on COVID-19 infection-fatality rates. Int J Infect Dis. 2020.
Seoane B. A scaling approach to estimate the COVID-19 infection fatality ratio from incomplete data. PLoS One. 2021;16(2):e0246831. https://doi.org/10.1371/journal.pone.0246831 . eCollection 2021.
Byambasuren O, Dobler CC, Bell K, Rojas DP, Clark J, McLaws M-L, et al. Comparison of seroprevalence of SARS-CoV-2 infections with cumulative and imputed COVID-19 cases: systematic review. PLoS One. 2021;16(4):e0248946.
Shakiba M, Nazemipour M, Salari A, Mehrabian F, Nazari SSH, Rezvani SM, et al. Seroprevalence of SARS-CoV-2 in Guilan Province, Iran, April 2020. Emerg Infect Dis. 2021;27(2):636.
Blanchet K, Alwan A, Antoine C, Cros MJ, Feroz F, Guracha TA, et al. Protecting essential health services in low-income and middle-income countries and humanitarian settings while responding to the COVID-19 pandemic. BMJ Glob Health. 2020;5(10):e003675.
Thome J, Coogan AN, Fischer M, Tucha O, Faltraco F. Challenges for mental health services during the 2020 COVID-19 outbreak in Germany. Psychiatry Clin Neurosci. 2020;74(7):407. https://doi.org/10.1111/pcn.13019 . Epub 2020 May 26.
Riley T, Sully E, Ahmed Z, Biddlecom A. Estimates of the potential impact of the COVID-19 pandemic on sexual and reproductive health in low-and middle-income countries. Int Perspect Sex Reprod Health. 2020;46:73–6.
World Health Organization (WHO): Maintaining essential health services: operational guidance for the COVID-19 context: interim guidance, 1 June 2020. In.: World Health Organization; 2020. https://www.who.int/publications/i/item/WHO-2019-nCoV-essential_health_services-2020.2 .
World Health Organization (WHO): Pulse survey on continuity of essential health services during the COVID-19 pandemic: interim report, 27 August 2020. In.: World Health Organization; 2020. https://www.who.int/publications-detail-redirect/WHO-2019-nCoV-EHS_continuity-survey-2020.1 .
World Health Organization (WHO): Second round of the national pulse survey on continuity of essential health services during the COVID-19 pandemic: interim report, 22 April 2021. In.: World Health Organization; 2021. https://www.who.int/publications/i/item/WHO-2019-nCoV-EHS-continuity-survey-2021.1 .
Organization WH. COVID-19: operational guidance for maintaining essential health services during an outbreak: interim guidance, 25 march 2020: World Health Organization; 2020.
Chen Y-Y, Assefa Y. The heterogeneity of the COVID-19 pandemic and national responses: an explanatory mixed-methods study. BMC Public Health. 2021;21(1):1–15.
World Health Organization (WHO). Thirteenth general programme of work 2019–2023. The seventy-first world health assembly. Geneva (Switzerland): World Health Organization; 2018.
Mahjour J, Mirza Z, Rashidian A, Atta H, Hajjeh R, Thieren M, et al. " promote health, keep the world safe, serve the vulnerable" in the eastern Mediterranean region. East Mediterr Health J. 2018;24(4):323–4.
Rollston R, Galea S. COVID-19 and the social determinants of health. Am J Health Promot. 2020;34(6):687–9. https://doi.org/10.1177/0890117120930536b .
Nachega JB, Sam-Agudu NA, Masekela R, van der Zalm MM, Nsanzimana S, Condo J, et al. Addressing challenges to rolling out COVID-19 vaccines in African countries. Lancet Glob Health. 2021;9(6):e746–8. https://doi.org/10.1016/S2214-109X(21)00097-8 . Epub 2021 Mar 10.
Skegg D, Gluckman P, Boulton G, Hackmann H, Karim SSA, Piot P, et al. Future scenarios for the COVID-19 pandemic. Lancet. 2021;397(10276):777–8.
Nhamo G, Chikodzi D, Kunene HP, Mashula N. COVID-19 vaccines and treatments nationalism: challenges for low-income countries and the attainment of the SDGs. Global Public Health. 2021;16(3):319–39.
Figueroa JP, Bottazzi ME, Hotez P, Batista C, Ergonul O, Gilbert S, et al. Urgent needs of low-income and middle-income countries for COVID-19 vaccines and therapeutics. Lancet. 2021;397(10274):562–4.
He X, Hong W, Pan X, Lu G, Wei X. SARS-CoV-2 omicron variant: characteristics and prevention. Med Comm (2020). 2021;2(4):838–45. https://doi.org/10.1002/mco2.110 .
Karim SSA, Karim QA. Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. Lancet. 2021;398(10317):2126–8.
Sanders D, Nandi S, Labonté R, Vance C, Van Damme W. From primary health care to universal health coverage—one step forward and two steps back. Lancet. 2019;394(10199):619–21.
Hone T, Macinko J, Millett C. Revisiting Alma-Ata: what is the role of primary health care in achieving the sustainable development goals? Lancet. 2018;392(10156):1461–72.
Redwood-Campbell L, Abrahams J. Primary health care and disasters—the current state of the literature: what we know, gaps and next steps. Prehospital Disaster Med. 2011;26(3):184–91.
Bitton A, Ratcliffe HL, Veillard JH, Kress DH, Barkley S, Kimball M, et al. Primary health care as a foundation for strengthening health systems in low-and middle-income countries. J Gen Intern Med. 2017;32(5):566–71.
Dunlop C, Howe A, Li D, Allen LN. The coronavirus outbreak: the central role of primary care in emergency preparedness and response. BJGP Open. 2020;4(1):bjgpopen20X101041. https://doi.org/10.3399/bjgpopen20X101041 .
Assefa Y, Gilks CF, van de Pas R, Reid S, Gete DG, Van Damme W. Reimagining global health systems for the 21st century: lessons from the COVID-19 pandemic. BMJ Glob Health. 2021;6(4):e004882.
Loewenson R, Accoe K, Bajpai N, Buse K, Abi Deivanayagam T, London L, et al. Reclaiming comprehensive public health. BMJ Glob Health. 2020;5(9):e003886.
Rawaf S, Allen LN, Stigler FL, Kringos D, Quezada Yamamoto H, van Weel C, et al. Lessons on the COVID-19 pandemic, for and by primary care professionals worldwide. Eur J Gen Pract. 2020;26(1):129–33.
Gostin LO, Friedman EA. A retrospective and prospective analysis of the west African Ebola virus disease epidemic: robust national health systems at the foundation and an empowered WHO at the apex. Lancet. 2015;385(9980):1902–9.
Download references
Acknowledgements
Not applicable.
No funding was obtained for this study.
Author information
Authors and affiliations.
School of Public Health, the University of Queensland, Brisbane, Australia
Yibeltal Assefa, Charles F. Gilks, Simon Reid & Dereje Gedle Gete
Institute of Tropical Medicine, Antwerp, Belgium
Remco van de Pas & Wim Van Damme
You can also search for this author in PubMed Google Scholar
Contributions
YA conceived the idea of the research, collected and analysed the data, and prepared the first and subsequent drafts. DG participated in data collection and analysis. CFG, SR, RVDP, DG and WVD provided comments during subsequent drafts. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Yibeltal Assefa .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Assefa, Y., Gilks, C.F., Reid, S. et al. Analysis of the COVID-19 pandemic: lessons towards a more effective response to public health emergencies. Global Health 18 , 10 (2022). https://doi.org/10.1186/s12992-022-00805-9
Download citation
Received : 04 August 2021
Accepted : 14 January 2022
Published : 04 February 2022
DOI : https://doi.org/10.1186/s12992-022-00805-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Heterogeneity
Globalization and Health
ISSN: 1744-8603
- General enquiries: [email protected]
- Research article
- Open access
- Published: 04 June 2021
Coronavirus disease (COVID-19) pandemic: an overview of systematic reviews
- Israel Júnior Borges do Nascimento 1 , 2 ,
- Dónal P. O’Mathúna 3 , 4 ,
- Thilo Caspar von Groote 5 ,
- Hebatullah Mohamed Abdulazeem 6 ,
- Ishanka Weerasekara 7 , 8 ,
- Ana Marusic 9 ,
- Livia Puljak ORCID: orcid.org/0000-0002-8467-6061 10 ,
- Vinicius Tassoni Civile 11 ,
- Irena Zakarija-Grkovic 9 ,
- Tina Poklepovic Pericic 9 ,
- Alvaro Nagib Atallah 11 ,
- Santino Filoso 12 ,
- Nicola Luigi Bragazzi 13 &
- Milena Soriano Marcolino 1
On behalf of the International Network of Coronavirus Disease 2019 (InterNetCOVID-19)
BMC Infectious Diseases volume 21 , Article number: 525 ( 2021 ) Cite this article
19k Accesses
36 Citations
14 Altmetric
Metrics details
Navigating the rapidly growing body of scientific literature on the SARS-CoV-2 pandemic is challenging, and ongoing critical appraisal of this output is essential. We aimed to summarize and critically appraise systematic reviews of coronavirus disease (COVID-19) in humans that were available at the beginning of the pandemic.
Nine databases (Medline, EMBASE, Cochrane Library, CINAHL, Web of Sciences, PDQ-Evidence, WHO’s Global Research, LILACS, and Epistemonikos) were searched from December 1, 2019, to March 24, 2020. Systematic reviews analyzing primary studies of COVID-19 were included. Two authors independently undertook screening, selection, extraction (data on clinical symptoms, prevalence, pharmacological and non-pharmacological interventions, diagnostic test assessment, laboratory, and radiological findings), and quality assessment (AMSTAR 2). A meta-analysis was performed of the prevalence of clinical outcomes.
Eighteen systematic reviews were included; one was empty (did not identify any relevant study). Using AMSTAR 2, confidence in the results of all 18 reviews was rated as “critically low”. Identified symptoms of COVID-19 were (range values of point estimates): fever (82–95%), cough with or without sputum (58–72%), dyspnea (26–59%), myalgia or muscle fatigue (29–51%), sore throat (10–13%), headache (8–12%) and gastrointestinal complaints (5–9%). Severe symptoms were more common in men. Elevated C-reactive protein and lactate dehydrogenase, and slightly elevated aspartate and alanine aminotransferase, were commonly described. Thrombocytopenia and elevated levels of procalcitonin and cardiac troponin I were associated with severe disease. A frequent finding on chest imaging was uni- or bilateral multilobar ground-glass opacity. A single review investigated the impact of medication (chloroquine) but found no verifiable clinical data. All-cause mortality ranged from 0.3 to 13.9%.
Conclusions
In this overview of systematic reviews, we analyzed evidence from the first 18 systematic reviews that were published after the emergence of COVID-19. However, confidence in the results of all reviews was “critically low”. Thus, systematic reviews that were published early on in the pandemic were of questionable usefulness. Even during public health emergencies, studies and systematic reviews should adhere to established methodological standards.
Peer Review reports
The spread of the “Severe Acute Respiratory Coronavirus 2” (SARS-CoV-2), the causal agent of COVID-19, was characterized as a pandemic by the World Health Organization (WHO) in March 2020 and has triggered an international public health emergency [ 1 ]. The numbers of confirmed cases and deaths due to COVID-19 are rapidly escalating, counting in millions [ 2 ], causing massive economic strain, and escalating healthcare and public health expenses [ 3 , 4 ].
The research community has responded by publishing an impressive number of scientific reports related to COVID-19. The world was alerted to the new disease at the beginning of 2020 [ 1 ], and by mid-March 2020, more than 2000 articles had been published on COVID-19 in scholarly journals, with 25% of them containing original data [ 5 ]. The living map of COVID-19 evidence, curated by the Evidence for Policy and Practice Information and Co-ordinating Centre (EPPI-Centre), contained more than 40,000 records by February 2021 [ 6 ]. More than 100,000 records on PubMed were labeled as “SARS-CoV-2 literature, sequence, and clinical content” by February 2021 [ 7 ].
Due to publication speed, the research community has voiced concerns regarding the quality and reproducibility of evidence produced during the COVID-19 pandemic, warning of the potential damaging approach of “publish first, retract later” [ 8 ]. It appears that these concerns are not unfounded, as it has been reported that COVID-19 articles were overrepresented in the pool of retracted articles in 2020 [ 9 ]. These concerns about inadequate evidence are of major importance because they can lead to poor clinical practice and inappropriate policies [ 10 ].
Systematic reviews are a cornerstone of today’s evidence-informed decision-making. By synthesizing all relevant evidence regarding a particular topic, systematic reviews reflect the current scientific knowledge. Systematic reviews are considered to be at the highest level in the hierarchy of evidence and should be used to make informed decisions. However, with high numbers of systematic reviews of different scope and methodological quality being published, overviews of multiple systematic reviews that assess their methodological quality are essential [ 11 , 12 , 13 ]. An overview of systematic reviews helps identify and organize the literature and highlights areas of priority in decision-making.
In this overview of systematic reviews, we aimed to summarize and critically appraise systematic reviews of coronavirus disease (COVID-19) in humans that were available at the beginning of the pandemic.
Methodology
Research question.
This overview’s primary objective was to summarize and critically appraise systematic reviews that assessed any type of primary clinical data from patients infected with SARS-CoV-2. Our research question was purposefully broad because we wanted to analyze as many systematic reviews as possible that were available early following the COVID-19 outbreak.
Study design
We conducted an overview of systematic reviews. The idea for this overview originated in a protocol for a systematic review submitted to PROSPERO (CRD42020170623), which indicated a plan to conduct an overview.
Overviews of systematic reviews use explicit and systematic methods for searching and identifying multiple systematic reviews addressing related research questions in the same field to extract and analyze evidence across important outcomes. Overviews of systematic reviews are in principle similar to systematic reviews of interventions, but the unit of analysis is a systematic review [ 14 , 15 , 16 ].
We used the overview methodology instead of other evidence synthesis methods to allow us to collate and appraise multiple systematic reviews on this topic, and to extract and analyze their results across relevant topics [ 17 ]. The overview and meta-analysis of systematic reviews allowed us to investigate the methodological quality of included studies, summarize results, and identify specific areas of available or limited evidence, thereby strengthening the current understanding of this novel disease and guiding future research [ 13 ].
A reporting guideline for overviews of reviews is currently under development, i.e., Preferred Reporting Items for Overviews of Reviews (PRIOR) [ 18 ]. As the PRIOR checklist is still not published, this study was reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2009 statement [ 19 ]. The methodology used in this review was adapted from the Cochrane Handbook for Systematic Reviews of Interventions and also followed established methodological considerations for analyzing existing systematic reviews [ 14 ].
Approval of a research ethics committee was not necessary as the study analyzed only publicly available articles.
Eligibility criteria
Systematic reviews were included if they analyzed primary data from patients infected with SARS-CoV-2 as confirmed by RT-PCR or another pre-specified diagnostic technique. Eligible reviews covered all topics related to COVID-19 including, but not limited to, those that reported clinical symptoms, diagnostic methods, therapeutic interventions, laboratory findings, or radiological results. Both full manuscripts and abbreviated versions, such as letters, were eligible.
No restrictions were imposed on the design of the primary studies included within the systematic reviews, the last search date, whether the review included meta-analyses or language. Reviews related to SARS-CoV-2 and other coronaviruses were eligible, but from those reviews, we analyzed only data related to SARS-CoV-2.
No consensus definition exists for a systematic review [ 20 ], and debates continue about the defining characteristics of a systematic review [ 21 ]. Cochrane’s guidance for overviews of reviews recommends setting pre-established criteria for making decisions around inclusion [ 14 ]. That is supported by a recent scoping review about guidance for overviews of systematic reviews [ 22 ].
Thus, for this study, we defined a systematic review as a research report which searched for primary research studies on a specific topic using an explicit search strategy, had a detailed description of the methods with explicit inclusion criteria provided, and provided a summary of the included studies either in narrative or quantitative format (such as a meta-analysis). Cochrane and non-Cochrane systematic reviews were considered eligible for inclusion, with or without meta-analysis, and regardless of the study design, language restriction and methodology of the included primary studies. To be eligible for inclusion, reviews had to be clearly analyzing data related to SARS-CoV-2 (associated or not with other viruses). We excluded narrative reviews without those characteristics as these are less likely to be replicable and are more prone to bias.
Scoping reviews and rapid reviews were eligible for inclusion in this overview if they met our pre-defined inclusion criteria noted above. We included reviews that addressed SARS-CoV-2 and other coronaviruses if they reported separate data regarding SARS-CoV-2.
Information sources
Nine databases were searched for eligible records published between December 1, 2019, and March 24, 2020: Cochrane Database of Systematic Reviews via Cochrane Library, PubMed, EMBASE, CINAHL (Cumulative Index to Nursing and Allied Health Literature), Web of Sciences, LILACS (Latin American and Caribbean Health Sciences Literature), PDQ-Evidence, WHO’s Global Research on Coronavirus Disease (COVID-19), and Epistemonikos.
The comprehensive search strategy for each database is provided in Additional file 1 and was designed and conducted in collaboration with an information specialist. All retrieved records were primarily processed in EndNote, where duplicates were removed, and records were then imported into the Covidence platform [ 23 ]. In addition to database searches, we screened reference lists of reviews included after screening records retrieved via databases.
Study selection
All searches, screening of titles and abstracts, and record selection, were performed independently by two investigators using the Covidence platform [ 23 ]. Articles deemed potentially eligible were retrieved for full-text screening carried out independently by two investigators. Discrepancies at all stages were resolved by consensus. During the screening, records published in languages other than English were translated by a native/fluent speaker.
Data collection process
We custom designed a data extraction table for this study, which was piloted by two authors independently. Data extraction was performed independently by two authors. Conflicts were resolved by consensus or by consulting a third researcher.
We extracted the following data: article identification data (authors’ name and journal of publication), search period, number of databases searched, population or settings considered, main results and outcomes observed, and number of participants. From Web of Science (Clarivate Analytics, Philadelphia, PA, USA), we extracted journal rank (quartile) and Journal Impact Factor (JIF).
We categorized the following as primary outcomes: all-cause mortality, need for and length of mechanical ventilation, length of hospitalization (in days), admission to intensive care unit (yes/no), and length of stay in the intensive care unit.
The following outcomes were categorized as exploratory: diagnostic methods used for detection of the virus, male to female ratio, clinical symptoms, pharmacological and non-pharmacological interventions, laboratory findings (full blood count, liver enzymes, C-reactive protein, d-dimer, albumin, lipid profile, serum electrolytes, blood vitamin levels, glucose levels, and any other important biomarkers), and radiological findings (using radiography, computed tomography, magnetic resonance imaging or ultrasound).
We also collected data on reporting guidelines and requirements for the publication of systematic reviews and meta-analyses from journal websites where included reviews were published.
Quality assessment in individual reviews
Two researchers independently assessed the reviews’ quality using the “A MeaSurement Tool to Assess Systematic Reviews 2 (AMSTAR 2)”. We acknowledge that the AMSTAR 2 was created as “a critical appraisal tool for systematic reviews that include randomized or non-randomized studies of healthcare interventions, or both” [ 24 ]. However, since AMSTAR 2 was designed for systematic reviews of intervention trials, and we included additional types of systematic reviews, we adjusted some AMSTAR 2 ratings and reported these in Additional file 2 .
Adherence to each item was rated as follows: yes, partial yes, no, or not applicable (such as when a meta-analysis was not conducted). The overall confidence in the results of the review is rated as “critically low”, “low”, “moderate” or “high”, according to the AMSTAR 2 guidance based on seven critical domains, which are items 2, 4, 7, 9, 11, 13, 15 as defined by AMSTAR 2 authors [ 24 ]. We reported our adherence ratings for transparency of our decision with accompanying explanations, for each item, in each included review.
One of the included systematic reviews was conducted by some members of this author team [ 25 ]. This review was initially assessed independently by two authors who were not co-authors of that review to prevent the risk of bias in assessing this study.
Synthesis of results
For data synthesis, we prepared a table summarizing each systematic review. Graphs illustrating the mortality rate and clinical symptoms were created. We then prepared a narrative summary of the methods, findings, study strengths, and limitations.
For analysis of the prevalence of clinical outcomes, we extracted data on the number of events and the total number of patients to perform proportional meta-analysis using RStudio© software, with the “meta” package (version 4.9–6), using the “metaprop” function for reviews that did not perform a meta-analysis, excluding case studies because of the absence of variance. For reviews that did not perform a meta-analysis, we presented pooled results of proportions with their respective confidence intervals (95%) by the inverse variance method with a random-effects model, using the DerSimonian-Laird estimator for τ 2 . We adjusted data using Freeman-Tukey double arcosen transformation. Confidence intervals were calculated using the Clopper-Pearson method for individual studies. We created forest plots using the RStudio© software, with the “metafor” package (version 2.1–0) and “forest” function.
Managing overlapping systematic reviews
Some of the included systematic reviews that address the same or similar research questions may include the same primary studies in overviews. Including such overlapping reviews may introduce bias when outcome data from the same primary study are included in the analyses of an overview multiple times. Thus, in summaries of evidence, multiple-counting of the same outcome data will give data from some primary studies too much influence [ 14 ]. In this overview, we did not exclude overlapping systematic reviews because, according to Cochrane’s guidance, it may be appropriate to include all relevant reviews’ results if the purpose of the overview is to present and describe the current body of evidence on a topic [ 14 ]. To avoid any bias in summary estimates associated with overlapping reviews, we generated forest plots showing data from individual systematic reviews, but the results were not pooled because some primary studies were included in multiple reviews.
Our search retrieved 1063 publications, of which 175 were duplicates. Most publications were excluded after the title and abstract analysis ( n = 860). Among the 28 studies selected for full-text screening, 10 were excluded for the reasons described in Additional file 3 , and 18 were included in the final analysis (Fig. 1 ) [ 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 ]. Reference list screening did not retrieve any additional systematic reviews.
PRISMA flow diagram
Characteristics of included reviews
Summary features of 18 systematic reviews are presented in Table 1 . They were published in 14 different journals. Only four of these journals had specific requirements for systematic reviews (with or without meta-analysis): European Journal of Internal Medicine, Journal of Clinical Medicine, Ultrasound in Obstetrics and Gynecology, and Clinical Research in Cardiology . Two journals reported that they published only invited reviews ( Journal of Medical Virology and Clinica Chimica Acta ). Three systematic reviews in our study were published as letters; one was labeled as a scoping review and another as a rapid review (Table 2 ).
All reviews were published in English, in first quartile (Q1) journals, with JIF ranging from 1.692 to 6.062. One review was empty, meaning that its search did not identify any relevant studies; i.e., no primary studies were included [ 36 ]. The remaining 17 reviews included 269 unique studies; the majority ( N = 211; 78%) were included in only a single review included in our study (range: 1 to 12). Primary studies included in the reviews were published between December 2019 and March 18, 2020, and comprised case reports, case series, cohorts, and other observational studies. We found only one review that included randomized clinical trials [ 38 ]. In the included reviews, systematic literature searches were performed from 2019 (entire year) up to March 9, 2020. Ten systematic reviews included meta-analyses. The list of primary studies found in the included systematic reviews is shown in Additional file 4 , as well as the number of reviews in which each primary study was included.
Population and study designs
Most of the reviews analyzed data from patients with COVID-19 who developed pneumonia, acute respiratory distress syndrome (ARDS), or any other correlated complication. One review aimed to evaluate the effectiveness of using surgical masks on preventing transmission of the virus [ 36 ], one review was focused on pediatric patients [ 34 ], and one review investigated COVID-19 in pregnant women [ 37 ]. Most reviews assessed clinical symptoms, laboratory findings, or radiological results.
Systematic review findings
The summary of findings from individual reviews is shown in Table 2 . Overall, all-cause mortality ranged from 0.3 to 13.9% (Fig. 2 ).
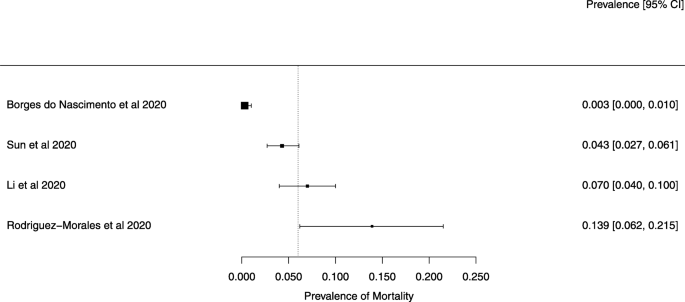
A meta-analysis of the prevalence of mortality
Clinical symptoms
Seven reviews described the main clinical manifestations of COVID-19 [ 26 , 28 , 29 , 34 , 35 , 39 , 41 ]. Three of them provided only a narrative discussion of symptoms [ 26 , 34 , 35 ]. In the reviews that performed a statistical analysis of the incidence of different clinical symptoms, symptoms in patients with COVID-19 were (range values of point estimates): fever (82–95%), cough with or without sputum (58–72%), dyspnea (26–59%), myalgia or muscle fatigue (29–51%), sore throat (10–13%), headache (8–12%), gastrointestinal disorders, such as diarrhea, nausea or vomiting (5.0–9.0%), and others (including, in one study only: dizziness 12.1%) (Figs. 3 , 4 , 5 , 6 , 7 , 8 and 9 ). Three reviews assessed cough with and without sputum together; only one review assessed sputum production itself (28.5%).
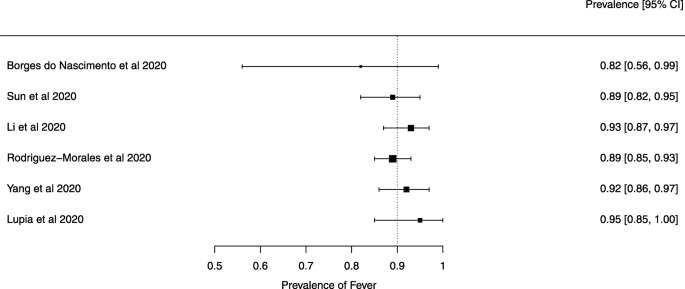
A meta-analysis of the prevalence of fever
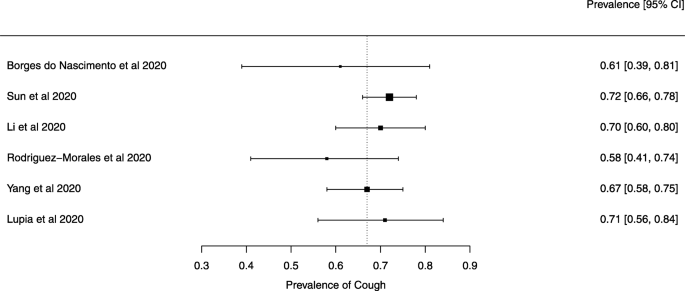
A meta-analysis of the prevalence of cough
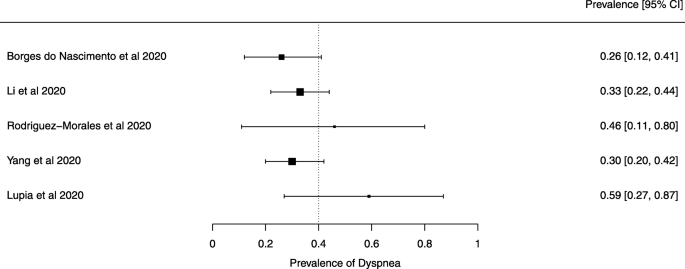
A meta-analysis of the prevalence of dyspnea
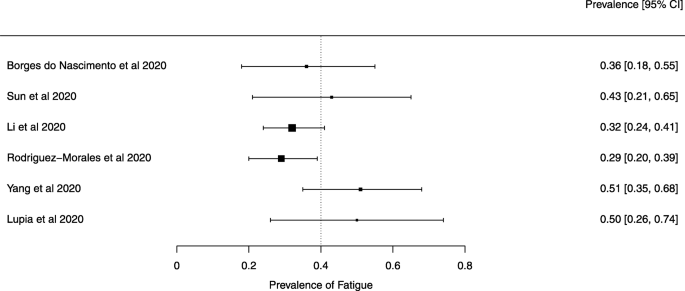
A meta-analysis of the prevalence of fatigue or myalgia
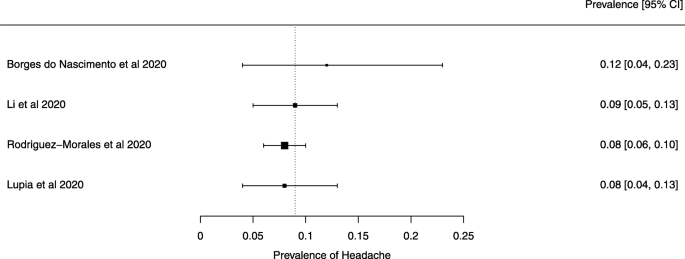
A meta-analysis of the prevalence of headache
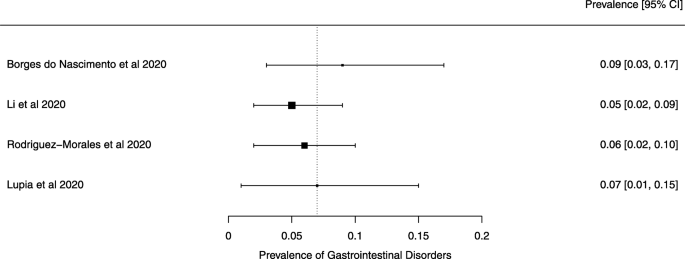
A meta-analysis of the prevalence of gastrointestinal disorders
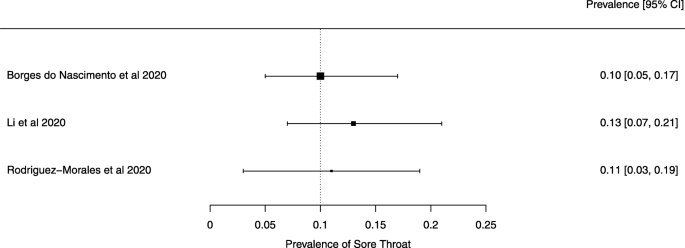
A meta-analysis of the prevalence of sore throat
Diagnostic aspects
Three reviews described methodologies, protocols, and tools used for establishing the diagnosis of COVID-19 [ 26 , 34 , 38 ]. The use of respiratory swabs (nasal or pharyngeal) or blood specimens to assess the presence of SARS-CoV-2 nucleic acid using RT-PCR assays was the most commonly used diagnostic method mentioned in the included studies. These diagnostic tests have been widely used, but their precise sensitivity and specificity remain unknown. One review included a Chinese study with clinical diagnosis with no confirmation of SARS-CoV-2 infection (patients were diagnosed with COVID-19 if they presented with at least two symptoms suggestive of COVID-19, together with laboratory and chest radiography abnormalities) [ 34 ].
Therapeutic possibilities
Pharmacological and non-pharmacological interventions (supportive therapies) used in treating patients with COVID-19 were reported in five reviews [ 25 , 27 , 34 , 35 , 38 ]. Antivirals used empirically for COVID-19 treatment were reported in seven reviews [ 25 , 27 , 34 , 35 , 37 , 38 , 41 ]; most commonly used were protease inhibitors (lopinavir, ritonavir, darunavir), nucleoside reverse transcriptase inhibitor (tenofovir), nucleotide analogs (remdesivir, galidesivir, ganciclovir), and neuraminidase inhibitors (oseltamivir). Umifenovir, a membrane fusion inhibitor, was investigated in two studies [ 25 , 35 ]. Possible supportive interventions analyzed were different types of oxygen supplementation and breathing support (invasive or non-invasive ventilation) [ 25 ]. The use of antibiotics, both empirically and to treat secondary pneumonia, was reported in six studies [ 25 , 26 , 27 , 34 , 35 , 38 ]. One review specifically assessed evidence on the efficacy and safety of the anti-malaria drug chloroquine [ 27 ]. It identified 23 ongoing trials investigating the potential of chloroquine as a therapeutic option for COVID-19, but no verifiable clinical outcomes data. The use of mesenchymal stem cells, antifungals, and glucocorticoids were described in four reviews [ 25 , 34 , 35 , 38 ].
Laboratory and radiological findings
Of the 18 reviews included in this overview, eight analyzed laboratory parameters in patients with COVID-19 [ 25 , 29 , 30 , 32 , 33 , 34 , 35 , 39 ]; elevated C-reactive protein levels, associated with lymphocytopenia, elevated lactate dehydrogenase, as well as slightly elevated aspartate and alanine aminotransferase (AST, ALT) were commonly described in those eight reviews. Lippi et al. assessed cardiac troponin I (cTnI) [ 25 ], procalcitonin [ 32 ], and platelet count [ 33 ] in COVID-19 patients. Elevated levels of procalcitonin [ 32 ] and cTnI [ 30 ] were more likely to be associated with a severe disease course (requiring intensive care unit admission and intubation). Furthermore, thrombocytopenia was frequently observed in patients with complicated COVID-19 infections [ 33 ].
Chest imaging (chest radiography and/or computed tomography) features were assessed in six reviews, all of which described a frequent pattern of local or bilateral multilobar ground-glass opacity [ 25 , 34 , 35 , 39 , 40 , 41 ]. Those six reviews showed that septal thickening, bronchiectasis, pleural and cardiac effusions, halo signs, and pneumothorax were observed in patients suffering from COVID-19.
Quality of evidence in individual systematic reviews
Table 3 shows the detailed results of the quality assessment of 18 systematic reviews, including the assessment of individual items and summary assessment. A detailed explanation for each decision in each review is available in Additional file 5 .
Using AMSTAR 2 criteria, confidence in the results of all 18 reviews was rated as “critically low” (Table 3 ). Common methodological drawbacks were: omission of prospective protocol submission or publication; use of inappropriate search strategy: lack of independent and dual literature screening and data-extraction (or methodology unclear); absence of an explanation for heterogeneity among the studies included; lack of reasons for study exclusion (or rationale unclear).
Risk of bias assessment, based on a reported methodological tool, and quality of evidence appraisal, in line with the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) method, were reported only in one review [ 25 ]. Five reviews presented a table summarizing bias, using various risk of bias tools [ 25 , 29 , 39 , 40 , 41 ]. One review analyzed “study quality” [ 37 ]. One review mentioned the risk of bias assessment in the methodology but did not provide any related analysis [ 28 ].
This overview of systematic reviews analyzed the first 18 systematic reviews published after the onset of the COVID-19 pandemic, up to March 24, 2020, with primary studies involving more than 60,000 patients. Using AMSTAR-2, we judged that our confidence in all those reviews was “critically low”. Ten reviews included meta-analyses. The reviews presented data on clinical manifestations, laboratory and radiological findings, and interventions. We found no systematic reviews on the utility of diagnostic tests.
Symptoms were reported in seven reviews; most of the patients had a fever, cough, dyspnea, myalgia or muscle fatigue, and gastrointestinal disorders such as diarrhea, nausea, or vomiting. Olfactory dysfunction (anosmia or dysosmia) has been described in patients infected with COVID-19 [ 43 ]; however, this was not reported in any of the reviews included in this overview. During the SARS outbreak in 2002, there were reports of impairment of the sense of smell associated with the disease [ 44 , 45 ].
The reported mortality rates ranged from 0.3 to 14% in the included reviews. Mortality estimates are influenced by the transmissibility rate (basic reproduction number), availability of diagnostic tools, notification policies, asymptomatic presentations of the disease, resources for disease prevention and control, and treatment facilities; variability in the mortality rate fits the pattern of emerging infectious diseases [ 46 ]. Furthermore, the reported cases did not consider asymptomatic cases, mild cases where individuals have not sought medical treatment, and the fact that many countries had limited access to diagnostic tests or have implemented testing policies later than the others. Considering the lack of reviews assessing diagnostic testing (sensitivity, specificity, and predictive values of RT-PCT or immunoglobulin tests), and the preponderance of studies that assessed only symptomatic individuals, considerable imprecision around the calculated mortality rates existed in the early stage of the COVID-19 pandemic.
Few reviews included treatment data. Those reviews described studies considered to be at a very low level of evidence: usually small, retrospective studies with very heterogeneous populations. Seven reviews analyzed laboratory parameters; those reviews could have been useful for clinicians who attend patients suspected of COVID-19 in emergency services worldwide, such as assessing which patients need to be reassessed more frequently.
All systematic reviews scored poorly on the AMSTAR 2 critical appraisal tool for systematic reviews. Most of the original studies included in the reviews were case series and case reports, impacting the quality of evidence. Such evidence has major implications for clinical practice and the use of these reviews in evidence-based practice and policy. Clinicians, patients, and policymakers can only have the highest confidence in systematic review findings if high-quality systematic review methodologies are employed. The urgent need for information during a pandemic does not justify poor quality reporting.
We acknowledge that there are numerous challenges associated with analyzing COVID-19 data during a pandemic [ 47 ]. High-quality evidence syntheses are needed for decision-making, but each type of evidence syntheses is associated with its inherent challenges.
The creation of classic systematic reviews requires considerable time and effort; with massive research output, they quickly become outdated, and preparing updated versions also requires considerable time. A recent study showed that updates of non-Cochrane systematic reviews are published a median of 5 years after the publication of the previous version [ 48 ].
Authors may register a review and then abandon it [ 49 ], but the existence of a public record that is not updated may lead other authors to believe that the review is still ongoing. A quarter of Cochrane review protocols remains unpublished as completed systematic reviews 8 years after protocol publication [ 50 ].
Rapid reviews can be used to summarize the evidence, but they involve methodological sacrifices and simplifications to produce information promptly, with inconsistent methodological approaches [ 51 ]. However, rapid reviews are justified in times of public health emergencies, and even Cochrane has resorted to publishing rapid reviews in response to the COVID-19 crisis [ 52 ]. Rapid reviews were eligible for inclusion in this overview, but only one of the 18 reviews included in this study was labeled as a rapid review.
Ideally, COVID-19 evidence would be continually summarized in a series of high-quality living systematic reviews, types of evidence synthesis defined as “ a systematic review which is continually updated, incorporating relevant new evidence as it becomes available ” [ 53 ]. However, conducting living systematic reviews requires considerable resources, calling into question the sustainability of such evidence synthesis over long periods [ 54 ].
Research reports about COVID-19 will contribute to research waste if they are poorly designed, poorly reported, or simply not necessary. In principle, systematic reviews should help reduce research waste as they usually provide recommendations for further research that is needed or may advise that sufficient evidence exists on a particular topic [ 55 ]. However, systematic reviews can also contribute to growing research waste when they are not needed, or poorly conducted and reported. Our present study clearly shows that most of the systematic reviews that were published early on in the COVID-19 pandemic could be categorized as research waste, as our confidence in their results is critically low.
Our study has some limitations. One is that for AMSTAR 2 assessment we relied on information available in publications; we did not attempt to contact study authors for clarifications or additional data. In three reviews, the methodological quality appraisal was challenging because they were published as letters, or labeled as rapid communications. As a result, various details about their review process were not included, leading to AMSTAR 2 questions being answered as “not reported”, resulting in low confidence scores. Full manuscripts might have provided additional information that could have led to higher confidence in the results. In other words, low scores could reflect incomplete reporting, not necessarily low-quality review methods. To make their review available more rapidly and more concisely, the authors may have omitted methodological details. A general issue during a crisis is that speed and completeness must be balanced. However, maintaining high standards requires proper resourcing and commitment to ensure that the users of systematic reviews can have high confidence in the results.
Furthermore, we used adjusted AMSTAR 2 scoring, as the tool was designed for critical appraisal of reviews of interventions. Some reviews may have received lower scores than actually warranted in spite of these adjustments.
Another limitation of our study may be the inclusion of multiple overlapping reviews, as some included reviews included the same primary studies. According to the Cochrane Handbook, including overlapping reviews may be appropriate when the review’s aim is “ to present and describe the current body of systematic review evidence on a topic ” [ 12 ], which was our aim. To avoid bias with summarizing evidence from overlapping reviews, we presented the forest plots without summary estimates. The forest plots serve to inform readers about the effect sizes for outcomes that were reported in each review.
Several authors from this study have contributed to one of the reviews identified [ 25 ]. To reduce the risk of any bias, two authors who did not co-author the review in question initially assessed its quality and limitations.
Finally, we note that the systematic reviews included in our overview may have had issues that our analysis did not identify because we did not analyze their primary studies to verify the accuracy of the data and information they presented. We give two examples to substantiate this possibility. Lovato et al. wrote a commentary on the review of Sun et al. [ 41 ], in which they criticized the authors’ conclusion that sore throat is rare in COVID-19 patients [ 56 ]. Lovato et al. highlighted that multiple studies included in Sun et al. did not accurately describe participants’ clinical presentations, warning that only three studies clearly reported data on sore throat [ 56 ].
In another example, Leung [ 57 ] warned about the review of Li, L.Q. et al. [ 29 ]: “ it is possible that this statistic was computed using overlapped samples, therefore some patients were double counted ”. Li et al. responded to Leung that it is uncertain whether the data overlapped, as they used data from published articles and did not have access to the original data; they also reported that they requested original data and that they plan to re-do their analyses once they receive them; they also urged readers to treat the data with caution [ 58 ]. This points to the evolving nature of evidence during a crisis.
Our study’s strength is that this overview adds to the current knowledge by providing a comprehensive summary of all the evidence synthesis about COVID-19 available early after the onset of the pandemic. This overview followed strict methodological criteria, including a comprehensive and sensitive search strategy and a standard tool for methodological appraisal of systematic reviews.
In conclusion, in this overview of systematic reviews, we analyzed evidence from the first 18 systematic reviews that were published after the emergence of COVID-19. However, confidence in the results of all the reviews was “critically low”. Thus, systematic reviews that were published early on in the pandemic could be categorized as research waste. Even during public health emergencies, studies and systematic reviews should adhere to established methodological standards to provide patients, clinicians, and decision-makers trustworthy evidence.
Availability of data and materials
All data collected and analyzed within this study are available from the corresponding author on reasonable request.
World Health Organization. Timeline - COVID-19: Available at: https://www.who.int/news/item/29-06-2020-covidtimeline . Accessed 1 June 2021.
COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). Available at: https://coronavirus.jhu.edu/map.html . Accessed 1 June 2021.
Anzai A, Kobayashi T, Linton NM, Kinoshita R, Hayashi K, Suzuki A, et al. Assessing the Impact of Reduced Travel on Exportation Dynamics of Novel Coronavirus Infection (COVID-19). J Clin Med. 2020;9(2):601.
Chinazzi M, Davis JT, Ajelli M, Gioannini C, Litvinova M, Merler S, et al. The effect of travel restrictions on the spread of the 2019 novel coronavirus (COVID-19) outbreak. Science. 2020;368(6489):395–400. https://doi.org/10.1126/science.aba9757 .
Article CAS PubMed PubMed Central Google Scholar
Fidahic M, Nujic D, Runjic R, Civljak M, Markotic F, Lovric Makaric Z, et al. Research methodology and characteristics of journal articles with original data, preprint articles and registered clinical trial protocols about COVID-19. BMC Med Res Methodol. 2020;20(1):161. https://doi.org/10.1186/s12874-020-01047-2 .
EPPI Centre . COVID-19: a living systematic map of the evidence. Available at: http://eppi.ioe.ac.uk/cms/Projects/DepartmentofHealthandSocialCare/Publishedreviews/COVID-19Livingsystematicmapoftheevidence/tabid/3765/Default.aspx . Accessed 1 June 2021.
NCBI SARS-CoV-2 Resources. Available at: https://www.ncbi.nlm.nih.gov/sars-cov-2/ . Accessed 1 June 2021.
Gustot T. Quality and reproducibility during the COVID-19 pandemic. JHEP Rep. 2020;2(4):100141. https://doi.org/10.1016/j.jhepr.2020.100141 .
Article PubMed PubMed Central Google Scholar
Kodvanj, I., et al., Publishing of COVID-19 Preprints in Peer-reviewed Journals, Preprinting Trends, Public Discussion and Quality Issues. Preprint article. bioRxiv 2020.11.23.394577; doi: https://doi.org/10.1101/2020.11.23.394577 .
Dobler CC. Poor quality research and clinical practice during COVID-19. Breathe (Sheff). 2020;16(2):200112. https://doi.org/10.1183/20734735.0112-2020 .
Article Google Scholar
Bastian H, Glasziou P, Chalmers I. Seventy-five trials and eleven systematic reviews a day: how will we ever keep up? PLoS Med. 2010;7(9):e1000326. https://doi.org/10.1371/journal.pmed.1000326 .
Lunny C, Brennan SE, McDonald S, McKenzie JE. Toward a comprehensive evidence map of overview of systematic review methods: paper 1-purpose, eligibility, search and data extraction. Syst Rev. 2017;6(1):231. https://doi.org/10.1186/s13643-017-0617-1 .
Pollock M, Fernandes RM, Becker LA, Pieper D, Hartling L. Chapter V: Overviews of Reviews. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane. 2020. Available from www.training.cochrane.org/handbook .
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions version 6.1 (updated September 2020). Cochrane. 2020; Available from www.training.cochrane.org/handbook .
Pollock M, Fernandes RM, Newton AS, Scott SD, Hartling L. The impact of different inclusion decisions on the comprehensiveness and complexity of overviews of reviews of healthcare interventions. Syst Rev. 2019;8(1):18. https://doi.org/10.1186/s13643-018-0914-3 .
Pollock M, Fernandes RM, Newton AS, Scott SD, Hartling L. A decision tool to help researchers make decisions about including systematic reviews in overviews of reviews of healthcare interventions. Syst Rev. 2019;8(1):29. https://doi.org/10.1186/s13643-018-0768-8 .
Hunt H, Pollock A, Campbell P, Estcourt L, Brunton G. An introduction to overviews of reviews: planning a relevant research question and objective for an overview. Syst Rev. 2018;7(1):39. https://doi.org/10.1186/s13643-018-0695-8 .
Pollock M, Fernandes RM, Pieper D, Tricco AC, Gates M, Gates A, et al. Preferred reporting items for overviews of reviews (PRIOR): a protocol for development of a reporting guideline for overviews of reviews of healthcare interventions. Syst Rev. 2019;8(1):335. https://doi.org/10.1186/s13643-019-1252-9 .
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Open Med. 2009;3(3):e123–30.
Krnic Martinic M, Pieper D, Glatt A, Puljak L. Definition of a systematic review used in overviews of systematic reviews, meta-epidemiological studies and textbooks. BMC Med Res Methodol. 2019;19(1):203. https://doi.org/10.1186/s12874-019-0855-0 .
Puljak L. If there is only one author or only one database was searched, a study should not be called a systematic review. J Clin Epidemiol. 2017;91:4–5. https://doi.org/10.1016/j.jclinepi.2017.08.002 .
Article PubMed Google Scholar
Gates M, Gates A, Guitard S, Pollock M, Hartling L. Guidance for overviews of reviews continues to accumulate, but important challenges remain: a scoping review. Syst Rev. 2020;9(1):254. https://doi.org/10.1186/s13643-020-01509-0 .
Covidence - systematic review software. Available at: https://www.covidence.org/ . Accessed 1 June 2021.
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008.
Borges do Nascimento IJ, et al. Novel Coronavirus Infection (COVID-19) in Humans: A Scoping Review and Meta-Analysis. J Clin Med. 2020;9(4):941.
Article PubMed Central Google Scholar
Adhikari SP, Meng S, Wu YJ, Mao YP, Ye RX, Wang QZ, et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect Dis Poverty. 2020;9(1):29. https://doi.org/10.1186/s40249-020-00646-x .
Cortegiani A, Ingoglia G, Ippolito M, Giarratano A, Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 2020;57:279–83. https://doi.org/10.1016/j.jcrc.2020.03.005 .
Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109(5):531–8. https://doi.org/10.1007/s00392-020-01626-9 .
Article CAS PubMed Google Scholar
Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, et al. COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(6):577–83. https://doi.org/10.1002/jmv.25757 .
Lippi G, Lavie CJ, Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): evidence from a meta-analysis. Prog Cardiovasc Dis. 2020;63(3):390–1. https://doi.org/10.1016/j.pcad.2020.03.001 .
Lippi G, Henry BM. Active smoking is not associated with severity of coronavirus disease 2019 (COVID-19). Eur J Intern Med. 2020;75:107–8. https://doi.org/10.1016/j.ejim.2020.03.014 .
Lippi G, Plebani M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chim Acta. 2020;505:190–1. https://doi.org/10.1016/j.cca.2020.03.004 .
Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta. 2020;506:145–8. https://doi.org/10.1016/j.cca.2020.03.022 .
Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109(6):1088–95. https://doi.org/10.1111/apa.15270 .
Lupia T, Scabini S, Mornese Pinna S, di Perri G, de Rosa FG, Corcione S. 2019 novel coronavirus (2019-nCoV) outbreak: a new challenge. J Glob Antimicrob Resist. 2020;21:22–7. https://doi.org/10.1016/j.jgar.2020.02.021 .
Marasinghe, K.M., A systematic review investigating the effectiveness of face mask use in limiting the spread of COVID-19 among medically not diagnosed individuals: shedding light on current recommendations provided to individuals not medically diagnosed with COVID-19. Research Square. Preprint article. doi : https://doi.org/10.21203/rs.3.rs-16701/v1 . 2020 .
Mullins E, Evans D, Viner RM, O’Brien P, Morris E. Coronavirus in pregnancy and delivery: rapid review. Ultrasound Obstet Gynecol. 2020;55(5):586–92. https://doi.org/10.1002/uog.22014 .
Pang J, Wang MX, Ang IYH, Tan SHX, Lewis RF, Chen JIP, et al. Potential Rapid Diagnostics, Vaccine and Therapeutics for 2019 Novel coronavirus (2019-nCoV): a systematic review. J Clin Med. 2020;9(3):623.
Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, Villamizar-Peña R, Holguin-Rivera Y, Escalera-Antezana JP, et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020;34:101623. https://doi.org/10.1016/j.tmaid.2020.101623 .
Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients. AJR Am J Roentgenol. 2020;215(1):87–93. https://doi.org/10.2214/AJR.20.23034 .
Sun P, Qie S, Liu Z, Ren J, Li K, Xi J. Clinical characteristics of hospitalized patients with SARS-CoV-2 infection: a single arm meta-analysis. J Med Virol. 2020;92(6):612–7. https://doi.org/10.1002/jmv.25735 .
Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–5. https://doi.org/10.1016/j.ijid.2020.03.017 .
Bassetti M, Vena A, Giacobbe DR. The novel Chinese coronavirus (2019-nCoV) infections: challenges for fighting the storm. Eur J Clin Investig. 2020;50(3):e13209. https://doi.org/10.1111/eci.13209 .
Article CAS Google Scholar
Hwang CS. Olfactory neuropathy in severe acute respiratory syndrome: report of a case. Acta Neurol Taiwanica. 2006;15(1):26–8.
Google Scholar
Suzuki M, Saito K, Min WP, Vladau C, Toida K, Itoh H, et al. Identification of viruses in patients with postviral olfactory dysfunction. Laryngoscope. 2007;117(2):272–7. https://doi.org/10.1097/01.mlg.0000249922.37381.1e .
Rajgor DD, Lee MH, Archuleta S, Bagdasarian N, Quek SC. The many estimates of the COVID-19 case fatality rate. Lancet Infect Dis. 2020;20(7):776–7. https://doi.org/10.1016/S1473-3099(20)30244-9 .
Wolkewitz M, Puljak L. Methodological challenges of analysing COVID-19 data during the pandemic. BMC Med Res Methodol. 2020;20(1):81. https://doi.org/10.1186/s12874-020-00972-6 .
Rombey T, Lochner V, Puljak L, Könsgen N, Mathes T, Pieper D. Epidemiology and reporting characteristics of non-Cochrane updates of systematic reviews: a cross-sectional study. Res Synth Methods. 2020;11(3):471–83. https://doi.org/10.1002/jrsm.1409 .
Runjic E, Rombey T, Pieper D, Puljak L. Half of systematic reviews about pain registered in PROSPERO were not published and the majority had inaccurate status. J Clin Epidemiol. 2019;116:114–21. https://doi.org/10.1016/j.jclinepi.2019.08.010 .
Runjic E, Behmen D, Pieper D, Mathes T, Tricco AC, Moher D, et al. Following Cochrane review protocols to completion 10 years later: a retrospective cohort study and author survey. J Clin Epidemiol. 2019;111:41–8. https://doi.org/10.1016/j.jclinepi.2019.03.006 .
Tricco AC, Antony J, Zarin W, Strifler L, Ghassemi M, Ivory J, et al. A scoping review of rapid review methods. BMC Med. 2015;13(1):224. https://doi.org/10.1186/s12916-015-0465-6 .
COVID-19 Rapid Reviews: Cochrane’s response so far. Available at: https://training.cochrane.org/resource/covid-19-rapid-reviews-cochrane-response-so-far . Accessed 1 June 2021.
Cochrane. Living systematic reviews. Available at: https://community.cochrane.org/review-production/production-resources/living-systematic-reviews . Accessed 1 June 2021.
Millard T, Synnot A, Elliott J, Green S, McDonald S, Turner T. Feasibility and acceptability of living systematic reviews: results from a mixed-methods evaluation. Syst Rev. 2019;8(1):325. https://doi.org/10.1186/s13643-019-1248-5 .
Babic A, Poklepovic Pericic T, Pieper D, Puljak L. How to decide whether a systematic review is stable and not in need of updating: analysis of Cochrane reviews. Res Synth Methods. 2020;11(6):884–90. https://doi.org/10.1002/jrsm.1451 .
Lovato A, Rossettini G, de Filippis C. Sore throat in COVID-19: comment on “clinical characteristics of hospitalized patients with SARS-CoV-2 infection: a single arm meta-analysis”. J Med Virol. 2020;92(7):714–5. https://doi.org/10.1002/jmv.25815 .
Leung C. Comment on Li et al: COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(9):1431–2. https://doi.org/10.1002/jmv.25912 .
Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, et al. Response to Char’s comment: comment on Li et al: COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(9):1433. https://doi.org/10.1002/jmv.25924 .
Download references
Acknowledgments
We thank Catherine Henderson DPhil from Swanscoe Communications for pro bono medical writing and editing support. We acknowledge support from the Covidence Team, specifically Anneliese Arno. We thank the whole International Network of Coronavirus Disease 2019 (InterNetCOVID-19) for their commitment and involvement. Members of the InterNetCOVID-19 are listed in Additional file 6 . We thank Pavel Cerny and Roger Crosthwaite for guiding the team supervisor (IJBN) on human resources management.
This research received no external funding.
Author information
Authors and affiliations.
University Hospital and School of Medicine, Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil
Israel Júnior Borges do Nascimento & Milena Soriano Marcolino
Medical College of Wisconsin, Milwaukee, WI, USA
Israel Júnior Borges do Nascimento
Helene Fuld Health Trust National Institute for Evidence-based Practice in Nursing and Healthcare, College of Nursing, The Ohio State University, Columbus, OH, USA
Dónal P. O’Mathúna
School of Nursing, Psychotherapy and Community Health, Dublin City University, Dublin, Ireland
Department of Anesthesiology, Intensive Care and Pain Medicine, University of Münster, Münster, Germany
Thilo Caspar von Groote
Department of Sport and Health Science, Technische Universität München, Munich, Germany
Hebatullah Mohamed Abdulazeem
School of Health Sciences, Faculty of Health and Medicine, The University of Newcastle, Callaghan, Australia
Ishanka Weerasekara
Department of Physiotherapy, Faculty of Allied Health Sciences, University of Peradeniya, Peradeniya, Sri Lanka
Cochrane Croatia, University of Split, School of Medicine, Split, Croatia
Ana Marusic, Irena Zakarija-Grkovic & Tina Poklepovic Pericic
Center for Evidence-Based Medicine and Health Care, Catholic University of Croatia, Ilica 242, 10000, Zagreb, Croatia
Livia Puljak
Cochrane Brazil, Evidence-Based Health Program, Universidade Federal de São Paulo, São Paulo, Brazil
Vinicius Tassoni Civile & Alvaro Nagib Atallah
Yorkville University, Fredericton, New Brunswick, Canada
Santino Filoso
Laboratory for Industrial and Applied Mathematics (LIAM), Department of Mathematics and Statistics, York University, Toronto, Ontario, Canada
Nicola Luigi Bragazzi
You can also search for this author in PubMed Google Scholar
Contributions
IJBN conceived the research idea and worked as a project coordinator. DPOM, TCVG, HMA, IW, AM, LP, VTC, IZG, TPP, ANA, SF, NLB and MSM were involved in data curation, formal analysis, investigation, methodology, and initial draft writing. All authors revised the manuscript critically for the content. The author(s) read and approved the final manuscript.
Corresponding author
Correspondence to Livia Puljak .
Ethics declarations
Ethics approval and consent to participate.
Not required as data was based on published studies.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: appendix 1..
Search strategies used in the study.
Additional file 2: Appendix 2.
Adjusted scoring of AMSTAR 2 used in this study for systematic reviews of studies that did not analyze interventions.
Additional file 3: Appendix 3.
List of excluded studies, with reasons.
Additional file 4: Appendix 4.
Table of overlapping studies, containing the list of primary studies included, their visual overlap in individual systematic reviews, and the number in how many reviews each primary study was included.
Additional file 5: Appendix 5.
A detailed explanation of AMSTAR scoring for each item in each review.
Additional file 6: Appendix 6.
List of members and affiliates of International Network of Coronavirus Disease 2019 (InterNetCOVID-19).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Borges do Nascimento, I.J., O’Mathúna, D.P., von Groote, T.C. et al. Coronavirus disease (COVID-19) pandemic: an overview of systematic reviews. BMC Infect Dis 21 , 525 (2021). https://doi.org/10.1186/s12879-021-06214-4
Download citation
Received : 12 April 2020
Accepted : 19 May 2021
Published : 04 June 2021
DOI : https://doi.org/10.1186/s12879-021-06214-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Coronavirus
- Evidence-based medicine
- Infectious diseases
BMC Infectious Diseases
ISSN: 1471-2334
- General enquiries: [email protected]
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

COVID-19 Pandemic: Knowledge and Perceptions of the Public and Healthcare Professionals
Priyanka a parikh, binoy v shah, ajay g phatak, amruta c vadnerkar, shraddha uttekar, naveen thacker, somashekhar m nimbalkar.
- Author information
- Article notes
- Copyright and License information
Somashekhar M. Nimbalkar [email protected]
Corresponding author.
Received 2020 Apr 15; Accepted 2020 May 15; Collection date 2020 May.
This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Background and objective
The recent pandemic due to the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has become a major concern for the people and governments across the world due to its impact on individuals as well as on public health. The infectiousness and the quick spread across the world make it an important event in everyone’s life, often evoking fear. Our study aims at assessing the overall knowledge and perceptions, and identifying the trusted sources of information for both the general public and healthcare personnel.
Materials and methods
This is a questionnaire-based survey taken by a total of 1,246 respondents, out of which 744 belonged to the healthcare personnel and 502 were laypersons/general public. There were two different questionnaires for both groups. The questions were framed using information from the World Health Organization (WHO), UpToDate, Indian Council of Medical Research (ICMR), Center for Disease Control (CDC), National Institute of Health (NIH), and New England Journal of Medicine (NEJM) website resources. The questions assessed awareness, attitude, and possible practices towards ensuring safety for themselves as well as breaking the chain of transmission. A convenient sampling method was used for data collection. Descriptive statistics [mean(SD), frequency(%)] were used to portray the characteristics of the participants as well as their awareness, sources of information, attitudes, and practices related to SARS-CoV-2.
The majority (94.3%) of the respondents were Indians. About 80% of the healthcare professionals and 82% of the general public were worried about being infected. Various websites such as ICMR, WHO, CDC, etc., were a major source of information for the healthcare professional while the general public relied on television. Almost 98% of healthcare professionals and 97% of the general public, respectively, identified ‘Difficulty in breathing” as the main symptom. More than 90% of the respondents in both groups knew and practiced different precautionary measures. A minority of the respondents (28.9% of healthcare professionals and 26.5% of the general public) knew that there was no known cure yet. Almost all respondents from both the groups agreed on seeking medical help if breathing difficulty is involved and self-quarantine if required.
Most healthcare professionals and the general public that we surveyed were well informed about SARS-CoV-2 and have been taking adequate measures in preventing the spread of the same. There is a high trust of the public in the government. There are common trusted sources of information and these need to be optimally utilized to spread accurate information.
Keywords: sars-cov-2 (severe acute respiratory syndrome coronavirus -2), covid 19, healthcare provider, public health and safety, knowledge mapping, infection control measures, covid-19 india, lockdown
Introduction
In December 2019, the 2019 novel coronavirus disease (COVID-19) caused by novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in China, followed by a rapid spread all over the world. On March 11, 2020, the World Health Organization (WHO) raised its pandemic alert. As of April 11, 2020, COVID-19 had caused over 95,269 deaths in 189 countries and overseas territories or communities [ 1 ].
In a connected world, fake news and rumor-mongering are common due to a surge in the use of the internet and social media. A confused comprehension in an emerging communicable disease of which even the experts have inadequate knowledge can lead to fear and chaos, even excessive panic, which has the probability to aggravate the disease epidemic [ 2 ]. During the SARS epidemic from 2002 to 2004, there were misconceptions and hence excessive panic in the general public concerning SARS. This led them to be resistant to comply with suggested preventive measures such as avoiding public transportation, going to a hospital when sick, etc. This contributed to the rapid spread of SARS and resulted in a more serious epidemic situation [ 3 ]. A similar experience occurred during the Ebola outbreak in 2009 in Africa. These experiences underscore the vital role of engaging with the general public and healthcare professionals and the importance of monitoring their perception of disease epidemic control, which may affect the compliance of community to the precautionary strategies. Understanding related factors affecting and influencing people to undertake precautionary behavior may also help decision-makers take appropriate measures to promote individual or community health. Hence, it is crucial to understand people’s risk perception and identify their trusted sources of information to effectively communicate and frame key messages in response to the emerging disease [ 4 ].
Since it is the novel coronavirus, its epidemiological features are not well known and new studies and publications will take anywhere from a month to a year making it important to know and understand the level of knowledge and preparedness of the healthcare personnel in terms of the managing the virus affected patients. Today healthcare professionals managing COVID-19 across the world are in an unprecedented situation, having to make tough decisions and working under extreme pressures. Decisions include equitable distribution of scant resources among the needy patients, balancing their own physical and mental healthcare needs along with those of the patients, aligning their desire and duty to patients with those to family and friends, and providing care for all unwell patients with constrained or inadequate resources. This may cause some to experience moral distress or mental health problems [ 5 ].
Effective communication is a priority in WHO’s COVID-19 roadmap; accurate and salient messages will enhance trust and enable the public to make informed choices based on recommendations [ 6 ].
As the outbreak intensified, social media has taken on new and increased importance with the large-scale implementation of social distancing, quarantine measures, and lockdown of complete cities. Social media platforms have become a way to enable homebound people to survive isolation and seek help, co-ordinate donations, entertain, and socialize with each other.
Social media platforms arguably support the conditions necessary for attitude change by exposing individuals to correct, accurate, health-promoting messages from healthcare professionals
In order to investigate community responses to SARS-CoV-2, we conducted this online survey among the general public and healthcare professionals to identify awareness of SARS-CoV-2 (perceived burden and risk), trusted sources of information, awareness of preventative measures and support for governmental policies and trust in authority to handle SARS-CoV-2 outbreak and put forward policy recommendations in case of similar future conditions.
We performed a cross-sectional survey of a convenient sample of respondents. The ethical approval for the study was taken from the Institutional Ethics Committee - 2, HM Patel Centre for Medical Care and Education, Karamsad via letter IEC/ HMPCMCE/ 2019 / Ex. 07/ dated March 23, 2020. All participants were above 18 years of age conveniently selected from the public at large by reaching out to the general public and healthcare professionals by the authors. The participants were largely from India. The consent of the participants was taken at the beginning of the survey. Two different self-administered questionnaires were used. The one for non-medical personnel (general public) is shown in Table 1 , while the one for medical and paramedical personnel is shown in Table 2 .
Table 1. COVID-19 (for non-medical personnel) question list.
Table 2. covid-19 (for medical and paramedical personnel) question list..
WHO, World Health Organization
The questions were framed using information from the WHO, UpToDate, Indian Council of Medical Research (ICMR), Center for Disease Control (CDC), National Institute of Health (NIH), and New England Journal of Medicine (NEJM) website resources as updated till March 19, 2020. They were validated consensually by experts from the Department of Pediatrics, Pulmonary Medicine, Public Health, and General Internal Medicine. The COVID-19 questions for healthcare professionals, i.e., medical and paramedical personnel were applicable to consultants, residents, interns, medical students, physiotherapists, physiotherapy students, nurses, nursing students, dentists, etc. The questionnaire was administered in English with the help of Google forms, which is a cloud-based data management tool used for designing and developing web-based questionnaires and available free. A link to the online surveys was sent out to them via e-mails and different social media platforms, namely WhatsApp, Facebook, LinkedIn, and Instagram messages, hence without any geographical barrier. The data collection was started on the March 23, 2020 and was continued up till March 27, 2020 midnight. The dates are important as on 22 March there was a self-imposed Janata Curfew in response to Prime Minister of India’s call while from the midnight of March 24, 2020, there was a nationwide lockdown across India. The data was automatically collected in the form of a google sheet and the collected data was being exported automatically to google sheets (similar to Microsoft Excel).
Descriptive statistics [mean (SD), frequency (%)] were used to portray the characteristics of the participants as well as their awareness, sources of information, attitudes, and practices related to SARS-CoV-2. Due to large sample sizes in the healthcare professional group as well as the general public group, exploratory visual comparisons were presented without typical statistical tests of significance.
A total of 744 health and allied professionals and 502 persons from people at large consented and completed the survey. A majority (94.3%) of the participants were Indian residents with insignificant responses from outside India. It is presumed that the majority of the respondents are of Indian residents but the possibility of a handful of them being non-Indians cannot be ruled out because we did not collect demographic data. A comparison of awareness about SARS-CoV-2 between the general public and healthcare professionals is shown in Table 3 .
Table 3. Awareness about SARS-CoV-2.
The gender distribution was equal in the healthcare professionals group, whereas it was more male-dominated in the general public group (49.7% vs 56.4% males). The respondents were younger in the healthcare professionals group as compared to the general public group [mean (SD) age: 29.55 (12.53) vs 32.16 (13.32) years].
The majority of the participants from the healthcare professionals group [594 (80%)] and the general public group [410 (82%)] were worried about getting SARS-CoV-2 infection. Those who were not worried expressed justified reasons (mainly precautions) for their attitude. Online resources, television, peer group discussions, and scientific literature constituted the main sources of information in the healthcare professionals group, whereas television, social networking sites, and newspapers/magazines constituted the main sources of information in the general population group. Participants in both groups reported WHO and official Indian Government websites (ICMR, Ministry of Health and Family Welfare (MOHFW)) as the most trusted online resources.
Most of the healthcare professionals reported that they had accessed videos by WHO/other sources [514 (69%)], read scientific articles [407 (54.7%)], and attended online lectures [242 (32.5%)] related to SARS-CoV-2.
Most healthcare professionals [727(98%)] as well as the general public [486(97%)] identified “difficulty in breathing” as the main symptom of SARS-CoV-2 infection along with cough and fever. Respondents from both the groups were aware of precautionary measures such as hand washing/sanitizer, wearing masks, social distancing, covering mouth while sneezing, and self-quarantine. Majority of the participants (62.7% in the general public and 71.8% in healthcare professionals) were aware of the infection period and the asymptomatic period (91% in the general public and 94.3% in healthcare professionals), but there appeared to be some confusion regarding curative treatment and vaccine availability in both the groups. Most participants rightly endorsed medical masks for healthcare workers, symptomatic patients, and persons who are coughing/sneezing. However, an appreciable proportion of healthcare professionals [303(40.7%)], as well as respondents from the general public [253(50.4%)], wrongly endorsed medical masks for healthy persons to protect themselves.
Most healthcare professionals [648(87.1%)] expressed their trust in the ICMR task force on SARS-CoV-2. Similar feelings were echoed by the general public [426(85%)] in trusting the current government.
Half of the general public respondents showed eagerness for the SARS-CoV-2 test without difficulty in breathing. A similar trend was observed among health professionals. Almost all respondents from the general public (98%) and the healthcare professionals (100%) endorsed seeking medical help if the breathing difficulty was involved.
Slightly more healthcare professionals reported regular influenza vaccination as compared to the general public [175(23.5%) vs 76(15.1%)]. Almost all the respondents agreed for self-isolation if needed. The majority of the respondents reported that they were washing the hands more frequently and knew the correct way of handwashing.
We present here a study of the awareness of SARS-CoV-2 among healthcare professionals and the general public with a comparison of many features among them. It is heartening to note that the knowledge with respect to SARS-CoV-2 is relatively high among the respondents.
There are, however, various limitations of the study and these are inherent due to the circumstances in which this survey was done. The study was begun on March 23, 2020, one day after Janata Curfew in India as requested by the Prime Minister and one day before the lockdown on March 24, 2020 [ 7 ]. The survey was filled during the days of the lockdown when the respondents had a lot of time on their hands and were probably active on social media as well as watching the television news. Hence, it is quite relevant that many individuals have their information from these two sources, making it important to ensure that accurate information through verified channels and healthcare professionals are presented and broadcasted to the people. This also points towards the importance of the right people being active on social media so that they can communicate the scientifically validated information to the masses.
The curfew and the lockdown ensured that the seriousness of the disease was impressed upon by the highest offices in the country, which is reflected in people taking good precautionary measures to protect themselves from the disease as well as break the chain of transmission. The cases in India have hence not risen to a very high number as rapidly as expected/projected, which also probably indicates that the message was well conveyed and well perceived. As this is a survey that was filled remotely, we need to be cautious in drawing strong conclusions.
Another limitation of the study is that the questionnaire was in the form of google forms and the language of conduct was English. This implies that the people who did not have access to the internet and were not literate were unable to be a part of this survey. But as the source of information for all the general public remains similar (television is ubiquitous in India), we can infer that they would have a similar response. We base this inference as the main sources of information of the public at large were newspapers, television, and WhatsApp despite having access to websites and other online sources. In villages, often the literate readout regional newspapers and news received on mobiles to the rest of the family/friends to ensure dissemination of information.
It is now known that the basic reproductive number (R0) of coronavirus is more in healthcare professionals as compared to the lay public and hence the relative indifference or "no worries" approach of healthcare professionals towards getting infected by SARS-CoV-2 is a concern. In the scenario where adequate personal protective equipment (PPE) may not be available to the healthcare facilities in India due to increased global demand, it is important that healthcare workers know their risk for being infected. In a recent study in Mumbai, 79% of the healthcare professionals were aware of the various PPE required with only 54.5% of them being aware of isolation procedures needed for SARS-CoV-2 infected patients [ 8 ]. The numbers for paramedical staff were also lower. India imports raw materials for PPE production from China and South Korea. Due to the shortage of materials and low rate of supply, the availability has taken a massive hit resulting in an acute shortage in the market. It is highly likely that many healthcare professionals will not use appropriate PPE, will get infected, and further spread infections to patients [ 9 - 11 ]. The Bhilwara cohort in Rajasthan is an example of how a healthcare professional needs to protect against infection since he/she is likely to transmit it to others [ 12 ]. Another example in Mumbai is Saifee hospital, which was shut down due to an infected healthcare professional who continued to work and passed on the infection to many during the asymptomatic phase. The SARS-CoV-2 disease presents a unique organism that can be spread for at least five days before developing symptoms and up to 37 days after presentation [ 13 , 14 ]. Given its high infectivity, it is a recipe for disaster if healthcare personnel gets it. We have not collected demographic information from the participants and hence it is possible that many of them work in situations where they may not anticipate getting infected. The previous few months have shown how surgeons, orthopedicians, dentists, etc., who typically do not deal with infectious diseases are getting infected by coronavirus [ 15 , 16 ]. In this scenario, it is worrying that only 80% of healthcare professionals were worried while the public was slightly more worried (82%).
The difference in the source of information for healthcare professionals and the general public is stark when we compare information garnered through social media. Social media at 78.3% is the second-highest source for the general public, while the healthcare professionals give it a measly 1%. Since social media is prone to fake news, it is heartening that healthcare professionals are not learning from it. However, the reliance of the general public on social media indicates that healthcare professionals, professional organizations, and government officers need to invest a significant proportion of their time and resources to be active on social media to disseminate correct news. The shots heard round the world rapid-response network is an example that needs to be followed [ 17 ]. In another example, we have Dr. Roberto Burioni who has successfully given accurate data on social media. If more healthcare professionals were to enrich social media, it would be a useful platform for the public [ 18 , 19 ]. While many government officials are active on Twitter in India, the platform that is commonly used in India is WhatsApp, Telegram, Instagram, and TikTok and these are dynamic and keep changing. WhatsApp in the middle of this pandemic reduced the forwarding to just one person for a message that had been forwarded five times from the previous number of forwarding to five people (which was unlimited initially) [ 20 ]. It indicates the importance of this platform across the world for the spreading of messages. The healthcare professionals rated scientific journals at just about 40.9%. It may be due to the low availability of high-quality evidence or poor access that many healthcare professionals in India have to scientific journals, which are mostly published out of developed countries [ 21 ]. In a pandemic situation, this disparity in access can be catastrophic and hence most journals have provided open access to all coronavirus-related publications. Healthcare professionals accessed websites such as WHO, Medscape, MOHFW, CDC, Worldometers, covid19.com , ICMR, UpToDate, and PubMed, for reliable information, which is an indicator of their faith in health organizations across the world. Interestingly though at a low 29.3%, much of the general public accessed similar websites such as WHO, MOHFW, CDC, and ICMR. At the time that the survey was administered, online webinars via zoom or other applications were just beginning in India to educate clinicians searching for answers. This is not reflected in our current study due to many of the responses being filled before the same or the respondents not being part of these audiences. The study authors have attended many of these meetings conducted by the Indian Academy of Pediatrics, etc., and this information is made available via email or WhatsApp messages. In a changing world, both healthcare professionals and the general public need to have reliable and accurate sources of information.
The severity of illness was well identified by all who were surveyed as being difficulty in breathing. Another heartening aspect was that precautionary measures were well known to both the groups of participants with appropriate hand washing techniques, avoidance of public gatherings, and covering of the mouth while coughing and sneezing as the top three precautionary measures. During the first week of March in India, all the telephone and cellular caller tunes were changed to advisories of how to prevent coronavirus disease and when to seek medical help, which included the above messages apart from appeals on television, etc [ 22 ].
There was less knowledge related to treatment and vaccine among both healthcare professionals and the general public, which was a disappointing finding for healthcare professionals as they were expected to be aware of this. The same could be said of the knowledge of the infectivity period and duration of being asymptomatic after infection. There was a good knowledge of the usage of masks among the general public and healthcare professionals except for the usage of medical masks for healthy people to protect themselves. The ICMR and other bodies have issued guidelines on the usage of masks and this seems to have been disseminated widely [ 23 ]. There was also a low insistence on the need for testing those without respiratory difficulty. In a scenario where testing resources are limited, this is an appropriate response but since it is possible to have the infection without respiratory difficulty, especially early on, this disinterest in getting tested, especially in healthcare personnel is worrisome when there is enough evidence of spread from asymptomatic and mildly symptomatic persons. It is also likely that this response may be due to the fact during the time that this questionnaire was administered, the total cases rose from 400+ to about 800+ and the testing strategy of ICMR was limited to those with contact or travel to SARS-CoV-2-affected areas [ 24 ].
Since writing this manuscript, except for a single source event of a religious gathering in Delhi, which caused the doubling of cases to increase from about seven days to 4.1 days, it is reasonable to conclude that adequate knowledge exists among the general public. We can only hope that this would be enough to ensure that lockdown to reduce transmission and flatten the curve will be successful [ 25 - 28 ].
Conclusions
The COVID-19 pandemic has affected the world in various ways. The deficiency of information, the need for accurate information, and the rapidity of its dissemination are important, as this pandemic requires the cooperation of entire populations. The rapid survey that we conducted had a good response and we show that healthcare professionals and the general public were quite well informed about the coronavirus. They are aware of the measures needed to be taken to reduce the spread of the disease. The knowledge present allows the authors to speculate that the lockdown in India would be effective. The public receives a large amount of information from social media such as WhatsApp and the medical fraternity and government need to develop strategies to ensure that accurate information needs to spread in these fora. The public awareness is quite high and it is important that the knowledge of communication channels be known and be kept at the topmost priority throughout the pandemic.
Acknowledgments
We are thankful to Dr. Mili Shah for language check of our manuscript.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained by all participants in this study. INSTITUTIONAL ETHICS COMMITTEE ‐ 2 H M PATEL CENTRE FOR MEDICAL CARE AND EDUCATION, KARAMSAD [ECR/1123/Inst/GJ/2018] issued approval IEC/ HMPCMCE/ 2019 / Ex. 07/. The following is part of the text of the approval letter indicating approval for the study. "Your research proposal ‘Response of the public and health care providers to a pandemic of a new virus’ was submitted for review and approval by committee members under Exempt Review. As it involves collection of data using anonymous online questionnaire with maintenance of privacy and confidentiality, it qualified for an Exempt from Full Committee Review. The matter was reviewed by Committee Members and decided to review it under ‘Exempt from full committee’ review. After review and subsequent clarification by you, the project is approved by IEC in its present form. As the online form has information and consent section, which needs to be read and accepted by the respondents before answering the study questions, committee waivers the need for any other consent for data collection."
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
- 1. WHO COVID-19 Dashboard. [Apr;2020 ];WHO COVID-19 Dashboard. (2020. https://who.sprinklr.com/ 2020
- 2. The public’s response to severe acute respiratory syndrome in Toronto and the United States. Blendon RJ, Benson JM, DesRoches CM, Raleigh E, Taylor-Clark K. Clin Infect Dis. 2004;38:925–931. doi: 10.1086/382355. [ DOI ] [ PubMed ] [ Google Scholar ]
- 3. Monitoring community responses to the SARS epidemic in Hong Kong from day 10 to day 62. Lau JTF, Yang X, Tsui H, Kim JH. J Epidemiol Commun Health. 2003;57:864–870. doi: 10.1136/jech.57.11.864. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 4. Epidemiology of severe acute respiratory syndrome (SARS): adults and children. Zhong NS, Wong GW. Paediatric Resp Rev. 2004;5:270–274. doi: 10.1016/j.prrv.2004.07.011. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 5. Managing mental health challenges faced by healthcare workers during covid-19 pandemic. Greenberg N, Docherty M, Gnanapragasam S, Wessely S. BMJ. 2020;368:0. doi: 10.1136/bmj.m1211. [ DOI ] [ PubMed ] [ Google Scholar ]
- 6. WHO - Communicating Risk in Public Health Emergencies. [Apr;2020 ]; http://www.who.int/risk-communication/guidance/download/en/ 2020
- 7. India will be under complete lockdown for 21 days: Narendra Modi - Economic Times. [Apr;2020 ]; https://economictimes.indiatimes.com/news/politics-and-nation/india-will-be-under-complete-lockdown-starting-midnight-narendra-modi/articleshow/74796908.cms 2020
- 8. COVID-19 awareness among healthcare students and professionals in Mumbai metropolitan region: a questionnaire-based survey. Modi PD, Nair G, Uppe A, Modi J, Tuppekar B, Gharpure AS, Langade D. Cureus. 2020;12:0. doi: 10.7759/cureus.7514. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 9. Coronavirus testing kits: India to buy PPEs, but no testing kits, from China - The Economic Times. [Apr;2020 ]; https://economictimes.indiatimes.com/news/politics-and-nation/india-to-buy-ppes-but-no-testing-kits-from-china/articleshow/74881474.cms 2020
- 10. Lack of PPE, poor infection control put medical staff at risk of Covid-19. [Apr;2020 ]; https://www.hindustantimes.com/india-news/lack-of-ppe-poor-infection-control-put-medical-staff-at-risk-of-covid-19/story-5jmeJgwUAaFuu4wfiCu8XN.html Covid-19. 2020
- 11. Covid-19 outbreak: protective health gear in short supply - The Economic Times. [Apr;2020 ]; https://economictimes.indiatimes.com/news/politics-and-nation/covid-19-outbreak-protective-health-gear-in-short-supply/articleshow/74765953.cms 2020
- 12. Bhilwara’s Tale of Negligence: Infected Docs, Latest COVID-19 Case Hint at Possible Community Spread News18. [Apr;2020 ]; https://www.news18.com/news/india/bhilwaras-tale-of-negligence-infected-doctors-latest-covid-19-case-hint-at-possibility-of-community-spread-2552595.html 2020
- 13. Guidance for discharge and ending isolation in the context of widespread community transmission of COVID-19. [Apr;2020 ]; https://www.ecdc.europa.eu/sites/default/files/documents/covid-19-guidance-discharge-and-ending-isolation-first%20update.pdf of COVID-19, 8 April. 2020
- 14. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Zhou F, Yu T, Du R, et al. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 15. Covid- 19: Around 50 doctors, medical staff test positive in India - Livemint. [Apr;2020 ]; https://www.livemint.com/news/india/covid-19-around-50-doctors-medical-staff-test-positive-in-india-11585912669844.html 2020
- 16. Coronavirus: AIIMS’ doctor tests positive for COVID-19 infection, says Sources - Deccan Herald. [Apr;2020 ]; https://www.deccanherald.com/national/north-and-central/coronavirus-aiims-doctor-tests-positive-for-covid-19-infection-says-sources-820422.html 2020
- 17. Shots Heard Round the World. [Apr;2020 ];Shots Heard round the world. (2020. https://www.shotsheard.com/blog/the-first-shot-1 2020
- 18. Countering anti-vaccination trends and changing online opinion. Signorelli C, Odone A. Eur J Public Health. 2019;29 [ Google Scholar ]
- 19. Pietrucci P. Routledge: Taylor & Francis; 2019. Blasting for science. In: The Routledge Handbook of Language and Science. [ Google Scholar ]
- 20. Covid fallout: WhatsApp changes limit on forwarded messages, users can send only 1 chat at a time - Economic Times. [Apr;2020 ]; https://economictimes.indiatimes.com/magazines/panache/whatsapp-changes-limit-on-forwarded-messages-users-can-send-only-1-chat-at-a-time/articleshow/75023912.cms 2020
- 21. A scientometric analysis of Indian research output in medicine during 1999-2008. Gupta BM, Bala A. J Nat Sci Biol Med. 2011;2:87–100. doi: 10.4103/0976-9668.82313. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 22. Coronavirus in India: On every call you make, you will hear a person coughing and that is annoying - IndiaToday. [Apr;2020 ]; https://www.indiatoday.in/technology/talking-points/story/coronavirus-in-india-on-every-call-you-make-you-will-hear-a-person-coughing-and-that-is-annoying-1653962-2020-03-09 2020
- 23. Ministry of Health and Family Welfare - Guidelines on use of mask by public. [Apr;2020 ]; https://www.mohfw.gov.in/pdf/Useofmaskbypublic.pdf 2020
- 24. ICMR - Revised Strategy of COVID19 testing in India (Version 3, dated 20/03/2020) [Apr;2020 ]; https://icmr.nic.in/sites/default/files/upload_documents/2020-03-20_covid19_test_v3.pdf 2020
- 25. Cases doubling in 4.1 days; without Jamaat, it would’ve been in 7.4 days - Economic Times. [Apr;2020 ]; https://m.economictimes.com/news/politics-and-nation/rate-of-doubling-of-covid-19-cases-4-1-days-without-tablighi-jamaat-incident-it-would-have-been-7-4-government/articleshow/74994181.cms 2020
- 26. How Tablighi Jamaat event became India’s worst coronavirus vector. [Apr;2020 ]; https://www.aljazeera.com/news/2020/04/tablighi-jamaat-event-india-worst-coronavirus-vector-200407052957511.html 2020
- 27. Flattening the much-talked COVID-19 curve—How close are we in India? - Research Matters. [Apr;2020 ]; https://researchmatters.in/news/flattening-much-talked-covid-19-curve%E2%80%94how-close-are-we-india 2020
- 28. Lockdown may help flatten coronavirus curve in India, says study - Business Today. [Apr;2020 ]; https://www.businesstoday.in/current/economy-politics/lockdown-may-help-flatten-coronavirus-curve-in-india-says-study/story/399955.html 2020
- View on publisher site
- PDF (150.6 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Current issue
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 76, Issue 2
- COVID-19 pandemic and its impact on social relationships and health
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- http://orcid.org/0000-0003-1512-4471 Emily Long 1 ,
- Susan Patterson 1 ,
- Karen Maxwell 1 ,
- Carolyn Blake 1 ,
- http://orcid.org/0000-0001-7342-4566 Raquel Bosó Pérez 1 ,
- Ruth Lewis 1 ,
- Mark McCann 1 ,
- Julie Riddell 1 ,
- Kathryn Skivington 1 ,
- Rachel Wilson-Lowe 1 ,
- http://orcid.org/0000-0002-4409-6601 Kirstin R Mitchell 2
- 1 MRC/CSO Social and Public Health Sciences Unit , University of Glasgow , Glasgow , UK
- 2 MRC/CSO Social and Public Health Sciences Unit, Institute of Health & Wellbeing , University of Glasgow , Glasgow , UK
- Correspondence to Dr Emily Long, MRC/CSO Social and Public Health Sciences Unit, University of Glasgow, Glasgow G3 7HR, UK; emily.long{at}glasgow.ac.uk
This essay examines key aspects of social relationships that were disrupted by the COVID-19 pandemic. It focuses explicitly on relational mechanisms of health and brings together theory and emerging evidence on the effects of the COVID-19 pandemic to make recommendations for future public health policy and recovery. We first provide an overview of the pandemic in the UK context, outlining the nature of the public health response. We then introduce four distinct domains of social relationships: social networks, social support, social interaction and intimacy, highlighting the mechanisms through which the pandemic and associated public health response drastically altered social interactions in each domain. Throughout the essay, the lens of health inequalities, and perspective of relationships as interconnecting elements in a broader system, is used to explore the varying impact of these disruptions. The essay concludes by providing recommendations for longer term recovery ensuring that the social relational cost of COVID-19 is adequately considered in efforts to rebuild.
- inequalities
Data availability statement
Data sharing not applicable as no data sets generated and/or analysed for this study. Data sharing not applicable as no data sets generated or analysed for this essay.
This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/ .
https://doi.org/10.1136/jech-2021-216690
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Introduction
Infectious disease pandemics, including SARS and COVID-19, demand intrapersonal behaviour change and present highly complex challenges for public health. 1 A pandemic of an airborne infection, spread easily through social contact, assails human relationships by drastically altering the ways through which humans interact. In this essay, we draw on theories of social relationships to examine specific ways in which relational mechanisms key to health and well-being were disrupted by the COVID-19 pandemic. Relational mechanisms refer to the processes between people that lead to change in health outcomes.
At the time of writing, the future surrounding COVID-19 was uncertain. Vaccine programmes were being rolled out in countries that could afford them, but new and more contagious variants of the virus were also being discovered. The recovery journey looked long, with continued disruption to social relationships. The social cost of COVID-19 was only just beginning to emerge, but the mental health impact was already considerable, 2 3 and the inequality of the health burden stark. 4 Knowledge of the epidemiology of COVID-19 accrued rapidly, but evidence of the most effective policy responses remained uncertain.
The initial response to COVID-19 in the UK was reactive and aimed at reducing mortality, with little time to consider the social implications, including for interpersonal and community relationships. The terminology of ‘social distancing’ quickly became entrenched both in public and policy discourse. This equation of physical distance with social distance was regrettable, since only physical proximity causes viral transmission, whereas many forms of social proximity (eg, conversations while walking outdoors) are minimal risk, and are crucial to maintaining relationships supportive of health and well-being.
The aim of this essay is to explore four key relational mechanisms that were impacted by the pandemic and associated restrictions: social networks, social support, social interaction and intimacy. We use relational theories and emerging research on the effects of the COVID-19 pandemic response to make three key recommendations: one regarding public health responses; and two regarding social recovery. Our understanding of these mechanisms stems from a ‘systems’ perspective which casts social relationships as interdependent elements within a connected whole. 5
Social networks
Social networks characterise the individuals and social connections that compose a system (such as a workplace, community or society). Social relationships range from spouses and partners, to coworkers, friends and acquaintances. They vary across many dimensions, including, for example, frequency of contact and emotional closeness. Social networks can be understood both in terms of the individuals and relationships that compose the network, as well as the overall network structure (eg, how many of your friends know each other).
Social networks show a tendency towards homophily, or a phenomenon of associating with individuals who are similar to self. 6 This is particularly true for ‘core’ network ties (eg, close friends), while more distant, sometimes called ‘weak’ ties tend to show more diversity. During the height of COVID-19 restrictions, face-to-face interactions were often reduced to core network members, such as partners, family members or, potentially, live-in roommates; some ‘weak’ ties were lost, and interactions became more limited to those closest. Given that peripheral, weaker social ties provide a diversity of resources, opinions and support, 7 COVID-19 likely resulted in networks that were smaller and more homogenous.
Such changes were not inevitable nor necessarily enduring, since social networks are also adaptive and responsive to change, in that a disruption to usual ways of interacting can be replaced by new ways of engaging (eg, Zoom). Yet, important inequalities exist, wherein networks and individual relationships within networks are not equally able to adapt to such changes. For example, individuals with a large number of newly established relationships (eg, university students) may have struggled to transfer these relationships online, resulting in lost contacts and a heightened risk of social isolation. This is consistent with research suggesting that young adults were the most likely to report a worsening of relationships during COVID-19, whereas older adults were the least likely to report a change. 8
Lastly, social connections give rise to emergent properties of social systems, 9 where a community-level phenomenon develops that cannot be attributed to any one member or portion of the network. For example, local area-based networks emerged due to geographic restrictions (eg, stay-at-home orders), resulting in increases in neighbourly support and local volunteering. 10 In fact, research suggests that relationships with neighbours displayed the largest net gain in ratings of relationship quality compared with a range of relationship types (eg, partner, colleague, friend). 8 Much of this was built from spontaneous individual interactions within local communities, which together contributed to the ‘community spirit’ that many experienced. 11 COVID-19 restrictions thus impacted the personal social networks and the structure of the larger networks within the society.

Social support
Social support, referring to the psychological and material resources provided through social interaction, is a critical mechanism through which social relationships benefit health. In fact, social support has been shown to be one of the most important resilience factors in the aftermath of stressful events. 12 In the context of COVID-19, the usual ways in which individuals interact and obtain social support have been severely disrupted.
One such disruption has been to opportunities for spontaneous social interactions. For example, conversations with colleagues in a break room offer an opportunity for socialising beyond one’s core social network, and these peripheral conversations can provide a form of social support. 13 14 A chance conversation may lead to advice helpful to coping with situations or seeking formal help. Thus, the absence of these spontaneous interactions may mean the reduction of indirect support-seeking opportunities. While direct support-seeking behaviour is more effective at eliciting support, it also requires significantly more effort and may be perceived as forceful and burdensome. 15 The shift to homeworking and closure of community venues reduced the number of opportunities for these spontaneous interactions to occur, and has, second, focused them locally. Consequently, individuals whose core networks are located elsewhere, or who live in communities where spontaneous interaction is less likely, have less opportunity to benefit from spontaneous in-person supportive interactions.
However, alongside this disruption, new opportunities to interact and obtain social support have arisen. The surge in community social support during the initial lockdown mirrored that often seen in response to adverse events (eg, natural disasters 16 ). COVID-19 restrictions that confined individuals to their local area also compelled them to focus their in-person efforts locally. Commentators on the initial lockdown in the UK remarked on extraordinary acts of generosity between individuals who belonged to the same community but were unknown to each other. However, research on adverse events also tells us that such community support is not necessarily maintained in the longer term. 16
Meanwhile, online forms of social support are not bound by geography, thus enabling interactions and social support to be received from a wider network of people. Formal online social support spaces (eg, support groups) existed well before COVID-19, but have vastly increased since. While online interactions can increase perceived social support, it is unclear whether remote communication technologies provide an effective substitute from in-person interaction during periods of social distancing. 17 18 It makes intuitive sense that the usefulness of online social support will vary by the type of support offered, degree of social interaction and ‘online communication skills’ of those taking part. Youth workers, for instance, have struggled to keep vulnerable youth engaged in online youth clubs, 19 despite others finding a positive association between amount of digital technology used by individuals during lockdown and perceived social support. 20 Other research has found that more frequent face-to-face contact and phone/video contact both related to lower levels of depression during the time period of March to August 2020, but the negative effect of a lack of contact was greater for those with higher levels of usual sociability. 21 Relatedly, important inequalities in social support exist, such that individuals who occupy more socially disadvantaged positions in society (eg, low socioeconomic status, older people) tend to have less access to social support, 22 potentially exacerbated by COVID-19.
Social and interactional norms
Interactional norms are key relational mechanisms which build trust, belonging and identity within and across groups in a system. Individuals in groups and societies apply meaning by ‘approving, arranging and redefining’ symbols of interaction. 23 A handshake, for instance, is a powerful symbol of trust and equality. Depending on context, not shaking hands may symbolise a failure to extend friendship, or a failure to reach agreement. The norms governing these symbols represent shared values and identity; and mutual understanding of these symbols enables individuals to achieve orderly interactions, establish supportive relationship accountability and connect socially. 24 25
Physical distancing measures to contain the spread of COVID-19 radically altered these norms of interaction, particularly those used to convey trust, affinity, empathy and respect (eg, hugging, physical comforting). 26 As epidemic waves rose and fell, the work to negotiate these norms required intense cognitive effort; previously taken-for-granted interactions were re-examined, factoring in current restriction levels, own and (assumed) others’ vulnerability and tolerance of risk. This created awkwardness, and uncertainty, for example, around how to bring closure to an in-person interaction or convey warmth. The instability in scripted ways of interacting created particular strain for individuals who already struggled to encode and decode interactions with others (eg, those who are deaf or have autism spectrum disorder); difficulties often intensified by mask wearing. 27
Large social gatherings—for example, weddings, school assemblies, sporting events—also present key opportunities for affirming and assimilating interactional norms, building cohesion and shared identity and facilitating cooperation across social groups. 28 Online ‘equivalents’ do not easily support ‘social-bonding’ activities such as singing and dancing, and rarely enable chance/spontaneous one-on-one conversations with peripheral/weaker network ties (see the Social networks section) which can help strengthen bonds across a larger network. The loss of large gatherings to celebrate rites of passage (eg, bar mitzvah, weddings) has additional relational costs since these events are performed by and for communities to reinforce belonging, and to assist in transitioning to new phases of life. 29 The loss of interaction with diverse others via community and large group gatherings also reduces intergroup contact, which may then tend towards more prejudiced outgroup attitudes. While online interaction can go some way to mimicking these interaction norms, there are key differences. A sense of anonymity, and lack of in-person emotional cues, tends to support norms of polarisation and aggression in expressing differences of opinion online. And while online platforms have potential to provide intergroup contact, the tendency of much social media to form homogeneous ‘echo chambers’ can serve to further reduce intergroup contact. 30 31
Intimacy relates to the feeling of emotional connection and closeness with other human beings. Emotional connection, through romantic, friendship or familial relationships, fulfils a basic human need 32 and strongly benefits health, including reduced stress levels, improved mental health, lowered blood pressure and reduced risk of heart disease. 32 33 Intimacy can be fostered through familiarity, feeling understood and feeling accepted by close others. 34
Intimacy via companionship and closeness is fundamental to mental well-being. Positively, the COVID-19 pandemic has offered opportunities for individuals to (re)connect and (re)strengthen close relationships within their household via quality time together, following closure of many usual external social activities. Research suggests that the first full UK lockdown period led to a net gain in the quality of steady relationships at a population level, 35 but amplified existing inequalities in relationship quality. 35 36 For some in single-person households, the absence of a companion became more conspicuous, leading to feelings of loneliness and lower mental well-being. 37 38 Additional pandemic-related relational strain 39 40 resulted, for some, in the initiation or intensification of domestic abuse. 41 42
Physical touch is another key aspect of intimacy, a fundamental human need crucial in maintaining and developing intimacy within close relationships. 34 Restrictions on social interactions severely restricted the number and range of people with whom physical affection was possible. The reduction in opportunity to give and receive affectionate physical touch was not experienced equally. Many of those living alone found themselves completely without physical contact for extended periods. The deprivation of physical touch is evidenced to take a heavy emotional toll. 43 Even in future, once physical expressions of affection can resume, new levels of anxiety over germs may introduce hesitancy into previously fluent blending of physical and verbal intimate social connections. 44
The pandemic also led to shifts in practices and norms around sexual relationship building and maintenance, as individuals adapted and sought alternative ways of enacting sexual intimacy. This too is important, given that intimate sexual activity has known benefits for health. 45 46 Given that social restrictions hinged on reducing household mixing, possibilities for partnered sexual activity were primarily guided by living arrangements. While those in cohabiting relationships could potentially continue as before, those who were single or in non-cohabiting relationships generally had restricted opportunities to maintain their sexual relationships. Pornography consumption and digital partners were reported to increase since lockdown. 47 However, online interactions are qualitatively different from in-person interactions and do not provide the same opportunities for physical intimacy.
Recommendations and conclusions
In the sections above we have outlined the ways in which COVID-19 has impacted social relationships, showing how relational mechanisms key to health have been undermined. While some of the damage might well self-repair after the pandemic, there are opportunities inherent in deliberative efforts to build back in ways that facilitate greater resilience in social and community relationships. We conclude by making three recommendations: one regarding public health responses to the pandemic; and two regarding social recovery.
Recommendation 1: explicitly count the relational cost of public health policies to control the pandemic
Effective handling of a pandemic recognises that social, economic and health concerns are intricately interwoven. It is clear that future research and policy attention must focus on the social consequences. As described above, policies which restrict physical mixing across households carry heavy and unequal relational costs. These include for individuals (eg, loss of intimate touch), dyads (eg, loss of warmth, comfort), networks (eg, restricted access to support) and communities (eg, loss of cohesion and identity). Such costs—and their unequal impact—should not be ignored in short-term efforts to control an epidemic. Some public health responses—restrictions on international holiday travel and highly efficient test and trace systems—have relatively small relational costs and should be prioritised. At a national level, an earlier move to proportionate restrictions, and investment in effective test and trace systems, may help prevent escalation of spread to the point where a national lockdown or tight restrictions became an inevitability. Where policies with relational costs are unavoidable, close attention should be paid to the unequal relational impact for those whose personal circumstances differ from normative assumptions of two adult families. This includes consideration of whether expectations are fair (eg, for those who live alone), whether restrictions on social events are equitable across age group, religious/ethnic groupings and social class, and also to ensure that the language promoted by such policies (eg, households; families) is not exclusionary. 48 49 Forethought to unequal impacts on social relationships should thus be integral to the work of epidemic preparedness teams.
Recommendation 2: intelligently balance online and offline ways of relating
A key ingredient for well-being is ‘getting together’ in a physical sense. This is fundamental to a human need for intimate touch, physical comfort, reinforcing interactional norms and providing practical support. Emerging evidence suggests that online ways of relating cannot simply replace physical interactions. But online interaction has many benefits and for some it offers connections that did not exist previously. In particular, online platforms provide new forms of support for those unable to access offline services because of mobility issues (eg, older people) or because they are geographically isolated from their support community (eg, lesbian, gay, bisexual, transgender and queer (LGBTQ) youth). Ultimately, multiple forms of online and offline social interactions are required to meet the needs of varying groups of people (eg, LGBTQ, older people). Future research and practice should aim to establish ways of using offline and online support in complementary and even synergistic ways, rather than veering between them as social restrictions expand and contract. Intelligent balancing of online and offline ways of relating also pertains to future policies on home and flexible working. A decision to switch to wholesale or obligatory homeworking should consider the risk to relational ‘group properties’ of the workplace community and their impact on employees’ well-being, focusing in particular on unequal impacts (eg, new vs established employees). Intelligent blending of online and in-person working is required to achieve flexibility while also nurturing supportive networks at work. Intelligent balance also implies strategies to build digital literacy and minimise digital exclusion, as well as coproducing solutions with intended beneficiaries.
Recommendation 3: build stronger and sustainable localised communities
In balancing offline and online ways of interacting, there is opportunity to capitalise on the potential for more localised, coherent communities due to scaled-down travel, homeworking and local focus that will ideally continue after restrictions end. There are potential economic benefits after the pandemic, such as increased trade as home workers use local resources (eg, coffee shops), but also relational benefits from stronger relationships around the orbit of the home and neighbourhood. Experience from previous crises shows that community volunteer efforts generated early on will wane over time in the absence of deliberate work to maintain them. Adequately funded partnerships between local government, third sector and community groups are required to sustain community assets that began as a direct response to the pandemic. Such partnerships could work to secure green spaces and indoor (non-commercial) meeting spaces that promote community interaction. Green spaces in particular provide a triple benefit in encouraging physical activity and mental health, as well as facilitating social bonding. 50 In building local communities, small community networks—that allow for diversity and break down ingroup/outgroup views—may be more helpful than the concept of ‘support bubbles’, which are exclusionary and less sustainable in the longer term. Rigorously designed intervention and evaluation—taking a systems approach—will be crucial in ensuring scale-up and sustainability.
The dramatic change to social interaction necessitated by efforts to control the spread of COVID-19 created stark challenges but also opportunities. Our essay highlights opportunities for learning, both to ensure the equity and humanity of physical restrictions, and to sustain the salutogenic effects of social relationships going forward. The starting point for capitalising on this learning is recognition of the disruption to relational mechanisms as a key part of the socioeconomic and health impact of the pandemic. In recovery planning, a general rule is that what is good for decreasing health inequalities (such as expanding social protection and public services and pursuing green inclusive growth strategies) 4 will also benefit relationships and safeguard relational mechanisms for future generations. Putting this into action will require political will.
Ethics statements
Patient consent for publication.
Not required.
- Office for National Statistics (ONS)
- Ford T , et al
- Riordan R ,
- Ford J , et al
- Glonti K , et al
- McPherson JM ,
- Smith-Lovin L
- Granovetter MS
- Fancourt D et al
- Stadtfeld C
- Office for Civil Society
- Cook J et al
- Rodriguez-Llanes JM ,
- Guha-Sapir D
- Patulny R et al
- Granovetter M
- Winkeler M ,
- Filipp S-H ,
- Kaniasty K ,
- de Terte I ,
- Guilaran J , et al
- Wright KB ,
- Martin J et al
- Gabbiadini A ,
- Baldissarri C ,
- Durante F , et al
- Sommerlad A ,
- Marston L ,
- Huntley J , et al
- Turner RJ ,
- Bicchieri C
- Brennan G et al
- Watson-Jones RE ,
- Amichai-Hamburger Y ,
- McKenna KYA
- Page-Gould E ,
- Aron A , et al
- Pietromonaco PR ,
- Timmerman GM
- Bradbury-Jones C ,
- Mikocka-Walus A ,
- Klas A , et al
- Marshall L ,
- Steptoe A ,
- Stanley SM ,
- Campbell AM
- ↵ (ONS), O.f.N.S., Domestic abuse during the coronavirus (COVID-19) pandemic, England and Wales . Available: https://www.ons.gov.uk/peoplepopulationandcommunity/crimeandjustice/articles/domesticabuseduringthecoronaviruscovid19pandemicenglandandwales/november2020
- Rosenberg M ,
- Hensel D , et al
- Banerjee D ,
- Bruner DW , et al
- Bavel JJV ,
- Baicker K ,
- Boggio PS , et al
- van Barneveld K ,
- Quinlan M ,
- Kriesler P , et al
- Mitchell R ,
- de Vries S , et al
Twitter @karenmaxSPHSU, @Mark_McCann, @Rwilsonlowe, @KMitchinGlasgow
Contributors EL and KM led on the manuscript conceptualisation, review and editing. SP, KM, CB, RBP, RL, MM, JR, KS and RW-L contributed to drafting and revising the article. All authors assisted in revising the final draft.
Funding The research reported in this publication was supported by the Medical Research Council (MC_UU_00022/1, MC_UU_00022/3) and the Chief Scientist Office (SPHSU11, SPHSU14). EL is also supported by MRC Skills Development Fellowship Award (MR/S015078/1). KS and MM are also supported by a Medical Research Council Strategic Award (MC_PC_13027).
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Read the full text or download the PDF:
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Open access
- Published: 16 May 2022
A comprehensive SARS-CoV-2 and COVID-19 review, Part 1: Intracellular overdrive for SARS-CoV-2 infection
- David A. Jamison Jr. 1 na1 ,
- S. Anand Narayanan ORCID: orcid.org/0000-0002-9783-5711 1 , 2 na1 ,
- Nídia S. Trovão ORCID: orcid.org/0000-0002-2106-1166 1 , 3 ,
- Joseph W. Guarnieri 1 , 4 ,
- Michael J. Topper 1 , 5 ,
- Pedro M. Moraes-Vieira ORCID: orcid.org/0000-0002-8263-786X 1 , 6 , 7 , 8 ,
- Viktorija Zaksas ORCID: orcid.org/0000-0001-7916-4902 1 , 9 ,
- Keshav K. Singh ORCID: orcid.org/0000-0002-0047-8616 1 , 10 ,
- Eve Syrkin Wurtele 1 , 11 &
- Afshin Beheshti ORCID: orcid.org/0000-0003-4643-531X 1 , 12 , 13 na2
European Journal of Human Genetics volume 30 , pages 889–898 ( 2022 ) Cite this article
25k Accesses
47 Citations
59 Altmetric
Metrics details
- Viral infection
COVID-19, the disease caused by SARS-CoV-2, has claimed approximately 5 million lives and 257 million cases reported globally. This virus and disease have significantly affected people worldwide, whether directly and/or indirectly, with a virulent pathogen that continues to evolve as we race to learn how to prevent, control, or cure COVID-19. The focus of this review is on the SARS-CoV-2 virus’ mechanism of infection and its proclivity at adapting and restructuring the intracellular environment to support viral replication. We highlight current knowledge and how scientific communities with expertize in viral, cellular, and clinical biology have contributed to increase our understanding of SARS-CoV-2, and how these findings may help explain the widely varied clinical observations of COVID-19 patients.
Similar content being viewed by others

A comprehensive SARS-CoV-2 and COVID-19 review, Part 2: host extracellular to systemic effects of SARS-CoV-2 infection

Molecular characteristics, immune evasion, and impact of SARS-CoV-2 variants
Small molecules in the treatment of COVID-19
Introduction.
As of November 21st, 2021, over 257 million cases of coronavirus disease 2019 (COVID-19) have been reported, and more than 5 million lives claimed globally [ 1 ]. The disease is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Development of vaccines against SARS-CoV-2 provide a major step forward in reducing COVID-19’s impact. However, the pandemic is ongoing, and the continued viral transmission allows for accumulation of mutations in the viral genome, which can provide advantages in replication, immune escape, increased transmissibility, or diagnostic detection failure [ 2 ]. With the quickly evolving SARS-CoV-2 variants and the slow rate of vaccination globally, it is critical to fully understand this novel virus and disease.
Coronaviruses are named as such because the S proteins resemble a halo or corona on scanning electron microscope imagery [ 3 ]. SARS-CoV-2 belongs to the genus Betacoronavirus. Of the human Betacoronavirus, including OC43, HKU1, SARS-CoV-1, and Middle East Respiratory Syndrome-Coronavirus (MERS-CoV) [ 4 ]. SARS-CoV-2 bears the highest genetic sequence similarity to SARS-CoV-1 [ 5 ]. Accordingly, COVID-19, caused by SARS-CoV-2, resembles SARS, caused by SARS-CoV-1, in many ways, but with some important differences [ 6 ]. Key characteristics of SARS-CoV-1 and 2 include: 1) a positive-sense RNA virus with a large genome of ~30 kilobases; 2) a large, enveloped virus containing a helical nucleocapsid with the virus’s genetic code, with an exterior studded in several spike proteins that facilitate the infection of host cells), and 3) similar genomic structures. The first 2/3 of both genomes encodes for two macro polypeptides pp1a/pp1b (see Fig. 1 ). Pp1a/pp1b are auto-proteolytically processed to generate 16 non-structural proteins (NSP).
Structural elements of the virus, including the spike protein, envelope, membrane, and internal components such as the viral single-stranded RNA and nucleocapsid proteins (above). SARS-CoV-2 genome components (below).
The main virus-specific functions of the SARS-CoV-2 NSPs are: NSP1 - cellular mRNA degradation, global translation inhibition; NSP2 - cell cycle progression disruption; NSP3 - formation of double-membrane vesicles (DMVs; SARS-CoV-2 protease); NSP4 - formation of DMVs; NSP5 - main SARS-CoV-2 protease; NSP6 - formation of DMVs, NSP7 - replication complex; NSP8 – primase; NSP9 - RNA binding protein; NSP10 - cofactor of NSP14 & NSP16; NSP11 - unknown, NSP12 - RNA-dependent RNA polymerase; NSP13 - RNA helicase, 5ʹ phosphatase, NSP14 - N7-MTase, 3ʹ-5ʹ exonuclease; NSP15 – endonuclease; and NSP16–2ʹ-O-MTase, mRNA capping.
The remaining 1/3 of the SARS-CoV-2 genome encodes for the structural proteins S (spike), E (envelope), M (membrane), and N (nucleocapsid), and several open reading frames (ORFs; (3a, 6, 7a, 7b, 8, 9b, and 10) [ 7 ]. The S protein binds the host cell receptor, which for SARS-CoV-1/2 is the human angiotensin-converting enzyme 2 (hACE2) (see Fig. 2 and Supplementary Table 1 ). These proteins share homology and function with SARS-CoV-1.
Structural interactions between the virus and target cell, including the viral spike protein, ACE2-receptor, TMPRSS2 reaction to cleave and begin the viral intracellular internalization (above, A ), and consequent signal transduction pathways stimulated by the virus as it hijacks pathways to turn the infected cell into a SARS-CoV-2 producing factory (below, B ).
There are two notable differences between SARS-CoV-1 and SARS-CoV-2. First is the presence of the ORF8 polypeptide found in SARS-CoV-2 but not in SARS-CoV-1. SARS-CoV-1 has a 29 nucleotide (nt) deletion (del) which splits it into ORF8a and ORF8b. Second, SARS-CoV-2 contains a gene encoding a novel orphan protein, ORF10, which is not present in SARS-CoV-1 [ 7 ].
SARS-CoV-2’s evolutionary rate has been estimated to be around 9’×’10 −4 substitutions per site per year [ 8 ], while also having a high transmissibility, large portion of asymptomatic cases [ 9 ], large pool of susceptible hosts to replicate in [ 10 , 11 ], and on-going environmental pressures (e.g., low vaccination rates and changes in policies allowing human carriers to continue to transmit the virus), which have allowed SARS-CoV-2 to accumulate mutation in its genome.
Mutations have been detected in all parts of the viral genome, including in the leader 5ʹ untranslated region (UTR), orf1ab (NSP1, NSP2, NSP3, NSP6, NSP12, NSP13, and NSP14), spike, ORF3a, ORF8, nucleocapsid, and ORF10 [ 8 ]. These genomic changes have been shown to influence viral immune evasion, inflammasome interaction, helicase, exonuclease proofreading mechanism, the activity of the RNA-dependent RNA polymerase (RdRp) and thereby viral replication, infectivity, and cell release [ 12 ].
Mutations associated with the spike are of particular interest, as they influence human-to-human transmission, as well as human-to-animal passage. Within the spike, mutations tend to fall into four general classes, those that affect the receptor-binding domain (RBD), which are of importance because some may provide both immune escape or a fitness advantage, as well as facilitate reverse zoonotic events. There are some mutations that occur in the N-terminal domain (NTD), which is the portion most exposed on the virus surface. There is evidence for immune selection in this region, and preliminary evidence that at least one of these changes (delH69/delV70) could improve fitness [ 13 ]. Mutations in or near the furin cleavage site, and several groupings close to the D614G mutation, possibly affect infection efficiency and can also be important for neutralizing antibodies.
This large SARS-CoV-2 genome diversity has been categorized by different nomenclature systems, describing variants of varied public health interest or concern. Pango lineages B.1.1.7 (Alpha), B.1.351 (Beta), P.1 (Gamma) and B.1.617.2 (Delta) have been classified as “variants of concern” (VOC) because they present mutations that have been shown to impact diagnostics, treatments, or vaccines, conferring increased transmissibility and increased disease severity. The impact of these mutations highlights the need for further research not only on the mechanisms of SARS-CoV-2 intracellular processes, but also how the extracellular environment may lead to further spread of the virus and subsequent public health burden.
The SARS-CoV-2 virus can exert physiological effects by directly infecting cells and via intercellular signaling by the infected cells. In this review, we provide insight into SARS-CoV-2 infection and intracellular host responses (targets, pathways, networks, biological processes, and functional adaptations) to viral invasion. We emphasize a canonical set of reactions induced by SARS-CoV-2, which we have organized for the reader’s consideration. However, there is tremendous variation in cellular responses to SARS-CoV-2, depending on factors including the cell type, organ type, metabolic and physiological context, patient genetics, individual clinical characteristics (e.g., age, sex, comorbidities), and stage/severity in the COVID-19 disease.
This is the first of a three-part comprehensive series of linked reviews on SARS-CoV-2 covering: intracellular effects (present study); extracellular consequences (review 2); and current and potential therapeutics (review 3). This review and the two that will follow aim to provide a foundational understanding of the current knowledge on SARS-CoV-2, from basic biology to clinical outcome and therapy avenues, that highlight future areas of research and could help inform public health interventions across the world.
Infected tissue and cell types
SARS-CoV-2 targets the nasal cavity and lungs; however, the detailed cellular tropism remains unclear, and likely varies among individuals. Furthermore, there is increased variability of viral cellular tropism with the emergent SARS-CoV-2 variants, which include Alpha, Beta, Gamma, Epsilon, Eta, Iota, Kappa, 1.617.3, Mu, Zeta, and in particular, Delta and Omicron, as well as the various lineages of each variant [ 14 ]. This is related with SARS-CoV-2’s mutation ability affecting its antigenic phenotype to circumvent immunity. The spike protein mediates attachment of the virus to host cell-surface receptors and fusion between virus and cell membranes; it is also the principal target for neutralizing antibodies generated following infection, and is the component for both mRNA and adenovirus-based vaccines [ 15 ]. Several studies have contributed to the current understanding of how mutations in the SARS-CoV-2 spike protein affect neutralization and emergence of new strains, which include studies of traditional escape mutation, targeted characterization of particular mutations, and wider investigations of large numbers of circulating variants [ 16 ]. These are active areas of research, in particular given the continued emergence of new lineages of new variants. A study of human, bat, non-human primate, and mouse cell lines showed various cell types were susceptible to the virus. These included pulmonary, intestinal, hepatic, renal, and neuronal, with cell lines expressing the hACE-2 receptor (hACE-2) having a generally greater viral load [ 17 ]. Although cell lines do not reflect physiological conditions, this research indicates that SARS-CoV-2 can infect many cell types, and that hACE-2 provides a critical entry mechanism [ 18 ]. Epithelial, vascular endothelial, pancreas, and mucosal cell types can all be infected by the virus [ 19 , 20 , 21 ].
Several investigations have employed 3D organoid cultures to simulate more physiological conditions than cell cultures [ 22 ]. In one such study, lung and colonic organoid models showed SARS-CoV-2 infection was reduced when various SARS-CoV-2 entry inhibitors were applied [ 22 ]. Another study illustrated the flexibility of different organoid models, such as pancreatic endocrine cells, liver organoids, cardiomyocytes, and dopaminergic neurons from human pluripotent stem cells, and adult primary cells (human islets, hepatocyte, and cholangiocytes) to test viral effects such as cytokine production, gene expression, and other physiological responses. The resultant data correlated well with some patient autopsy samples [ 22 ] indicating organoids provide a valuable disease modeling tool [ 18 ].
In one study of post-mortem patients, immunohistochemistry and immunofluorescence revealed viral antigen (spike protein) in pneumocytes and hyperplastic cells around the bronchioles, mucosal epithelia, submucosal glands, gland ducts of the trachea, glands of the small intestine, distal tubules and collecting ducts of the kidneys, islets of Langerhans, glands and intra-islet ducts of the pancreas, and vascular tissues of the brain and heart [ 23 ]. Few viral antigens were present in the large intestine and renal proximal tubules, and none in the liver. A follow-up colocalization analysis showed ACE2 and viral antigen in the lung, trachea, small intestine, kidney, pancreas, and heart. In the brain, ACE2-expressing cells were detected, but they were negative for the viral antigen [ 23 ]. Endothelial cells of multiple organs were infected, supporting the clinical observations of endotheliitis in some COVID-19 patients.
Single-cell RNA sequencing (scRNA-seq) demonstrated ACE2 receptor expression was primarily restricted to lung pneumocytes, gut absorptive enterocytes, and nasal mucosa goblet secretory cells [ 24 ]. In general, the distribution of ACE2 receptors may in part explain the systemic diversity and range of SARS-CoV-2’s effects. Further research into infection of these cell types versus others in mucosal barrier organs will be important to determine cell-types that serve as initial entry ways for the virus into the body.
Human autopsy studies [ 21 ] have shown that SARS-CoV-2 infects multiple organs including lungs, pharynx, liver, nasal mucosa, trachea, intestines, skin, pancreas, kidney, brain, and heart. A study of 27 patients showed multi-organ tropisms (lung, pharynx, heart, liver, brain, and kidneys), with the highest levels of SARS-CoV-2 copies per cell, as detected by in situ hybridization and indirect immunofluorescence, in the respiratory tract, and lower levels in the kidneys, liver, heart, and brain [ 21 ]. Transcriptional profiling of nasopharyngeal swabs, patient autopsy, and body-wide tissues (e.g. heart, liver, lung, kidney, and lymph nodes), provided further evidence of the physiologically systemic effects of SARS-CoV-2 [ 24 ].
These studies suggest that the virus has a varying range of expression within each organ, which may be influenced by levels of the ACE2-receptor and related entry factors (Transmembrane protease, serine-2 [TMPRSS2], transferrin receptor protein 1 [TRFC1], cluster of differentiation 4 [CD4], and neuropilin-1 [NRP1]) within each organ-type [ 24 ]. This further highlights the varied organ and tissues that are capable of being infected by the virus, and the resultant wide-range of patient symptoms.
The physiological status of the individual significantly affects COVID-19 morbidity and mortality [ 25 , 26 ]. Notably, patients with pre-existing conditions of obesity, hypertension, and diabetes have a less favorable disease outcome, likely in part due to the elevated levels of inflammation and metabolic disturbances associated with those conditions [ 25 ]. Conversely, SARS-CoV-2 infection may exacerbate pre-existing conditions, leading to more severe COVID-19 outcome [ 27 ].
SARS-CoV-2 Receptors – Angiotensin Converting Enzyme-2 (ACE2)
The cellular surface receptor ACE2, a key regulator of the Renin-Angiotensin Aldosterone System (RAAS). It is speculated to be the primary SARS-CoV-2 viral target for entry. SARS-CoV-2 is thought to infect multiple organs in part due to the widespread distribution, expression, and polymorphisms of ACE2 [ 28 , 29 ].
ACE2’s molecular function in the human RAAS pathway is to cleave Angiotensin I to produce Angiotensin 1–9, and break down Angiotensin II into Angiotensin 1–7. RAAS moderates blood pressure and osmolarity by means of hormonal feedback control. In response to binding of ACE2 to the ACE2 receptor (ACE2R), blood vessels vasoconstrict. This process is mediated by G-protein-signaling, activating phospholipase C and increasing cytosolic Ca 2+ concentrations. ACE2 also plays an important role in inactivating Des-Arg9-Bradykinin (DABK), a bradykinin involved in inflammation. This inactivation promotes C-X-C motif chemokine 5 (CXCL5), macrophage inflammatory protein-2 (MIPS2), keratinocytes-derived chemokine (KC), and tumor necrosis factor-〈 (TNF-α) activity, drawing leukocytes into the affected tissues [ 30 ].
Decreased ACE2 receptor expression can have detrimental effects. Computational models of COVID-19 suggest the role of a bradykinin storm in the pathophysiology of the disease. In this model, the Kallikrein-Kinin and Renin-Angiotensin-Aldosterone Systems are integrated, with cross-talk mediated by the degradation of bradykinin by ACE and prolylcarboxypeptidase [ 31 ]. This behavior makes the SARS-CoV-2 spike protein behave akin to an ACE-inhibiting drug [ 32 ]. Thus, disruption of ACE2 expression from SARS-CoV-2 binding can lead to altered tissue function and exacerbate disease.
The ~600-kDa trimeric S proteins can bind to ACE2 through the RBD required for membrane fusion (see Fig. 2 ). The binding initiates viral internalization, with the cleavage of S1/S2 inducing a conformational change from prefusion into post-fusion. S1 consists of the NTD, the RBD, and subdomain 1 and 2 (SD1 and SD2). S2 contains the hydrophobic fusion peptide and is responsible for the viral and cell membrane fusion [ 33 ]. SARS-CoV-2 S-protein shows varying states of conformational shifts of the RBD site progressing towards proteolytic processing, making the viral RBD more accessible to ACE2, with the cleavage at the S1/S2 leading towards RBD open confirmation and viral internalization [ 33 , 34 ]. The S- and RBD-viral sites are notable for affecting transmission and disease severity, and variants have been shown to accumulate mutations at these sites leading to increased S- and RBD affinity with ACE2 [ 35 ]. Understanding the biology of the SARS-CoV-2 surface interactions will help elucidate how the virus can invade multiple organ systems and cell types.
Calcium Ion (Ca 2+ ) Signaling
The calcium ion (Ca 2+ ) is essential for many aspects of cellular physiology and viral replication. Experimental data on the relation between Ca 2+ signaling and SARS-CoV-2 infection and replication is sparse. However, studies of other coronaviruses (e.g., SARS-CoV-1, MERS-CoV) have reported that these viruses utilize Ca 2+ for host fusion [ 36 ]. The fusion protein (FP) of MERS-CoV binds to one Ca 2+ ion, while the SARS-CoV-2 spike (S) protein has two FP domains, FP1 and FP2, and binds to two Ca 2+ ions for host cell entry [ 37 ]. SARS-CoV-2 appears to affect cellular function by altering the host Ca 2+ homeostasis in ways that promote viral infection and reproduction (see Fig. 3 ). One mechanism is through disruption of calcium channels and pumps (e.g., voltage-gated calcium channels (VGCCs), receptor-operated calcium channels, store-operated calcium channels, transient receptor-potential ion channels, and Ca 2+ -ATPase) [ 28 , 37 ]. This leads to increased intracellular Ca 2+ concentrations, resulting in virus-induced cell lysis [ 28 , 37 ]. The interaction between the virus and VGCCs may also promote virus-host cell fusion for entry [ 28 ].
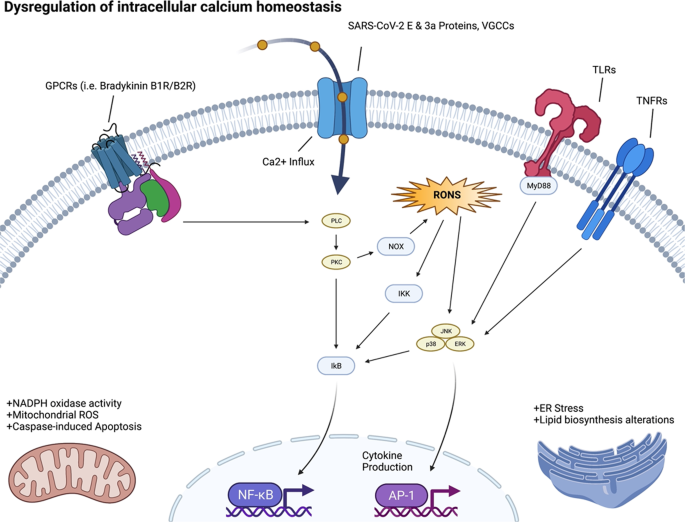
Structural elements of the virus, including the spike protein, envelope, membrane, and internal components such as the viral single-stranded RNA and nucleocapsid proteins (above).
Viroporins, transmembrane pore-forming proteins that alter membrane permeability to ions including Ca 2+ by forming membrane channels, are a characteristic of a diversity of virus. SARS-CoV-1/2 each encode viroporins. SARS-CoV-1 encodes for three viroporin proteins ORF3a, E and ORF8b, which alter ion homeostasis within the cell, and have important roles in pathogenesis and promoting viral fitness. SARS-CoV-2 encodes two of these viroporin proteins, E and ORF3a; however, the ORF8 protein of SARS-CoV-2 is highly divergent from SARS-CoV-1 ORF8b and lacks the viroporin sequence of SARS-CoV-1 ORF8b.
The E and ORF3a proteins of coronaviruses impact Ca 2+ homeostasis in the host, by acting as calcium ion channels, enhancing the virion’s entry and replication potential [ 38 ]. The SARS-CoV-2-E protein is a 76 amino acid (aa) integral membrane protein with one transmembrane domain (TMD) that allows the E protein to form protein-lipid channels in membranes that promote permeability to Ca 2+ ions. The SARS-CoV-2-ORF3a protein is 274 aa in length, harbors three helical TMD, and is a Na + or Ca 2+ ion channel protein. The alteration of Ca 2+ homeostasis by SARS-COV-1-E and SARS-COV-2-E proteins promotes SARS-CoV-1/2 fitness and elicits the production of chemokines and cytokines, contributing to pathogenesis. Ion channel activity modulation by the SARS-CoV-1-ORF3a protein also modulates viral release [ 39 ]. Therefore, when SARS-CoV-2 infects the human body, the resultant dysregulation of Ca 2+ homeostasis may contribute to morbidity and mortality. COVID-19 patients have been noted to have low serum calcium levels overall [ 40 ].
We speculate that Ca 2+ dysregulation could lead to increased cellular oxidative stress and shifts in metabolic activity. Low Ca 2+ may also be coupled with viral infection and internalization through the ACE2R, which synergizes with Ca 2+ signaling pathways. Understanding these reverberations will increase our insight into the basic biology of the effects of SARS-CoV-2 infection on the various organ systems.
Intracellular signaling
Viral infection and hijacking of cell-surface receptors begin to trigger activation of multiple intracellular pathways in addition to Ca 2+ signaling. As infection proceeds, SARS-CoV-2 manipulates, or totally reprograms, the normal metabolism and signaling of the host cell, optimizing the molecular environment to enable the viral replication cycle. This involves interfering with signaling pathways that regulate processes of DNA repair and replication, immune response, transcription, metabolism, cell cycle, and apoptosis [ 39 ].
SARS-CoV-2 infection alters phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT), Type I and III interferon, transforming growth factor-β (TGF-β), Toll-like receptors (TLR), and nuclear factor kappa-light chain enhancer (NF-κB) pathways. These pathways are dysregulated in the setting of SARS-CoV-2 to antagonize host antiviral responses and are vital for viral replication, entry, propagation, and/or apoptosis/viral release. For instance, severe COVID-19 is characterized by an inflammatory profile dominated by NF-κB activity [ 41 ]. The SARS-CoV-2-encoded NSP13 and Open Reading Frame 9c (ORF9c) proteins can interact directly with elements of the transducin-like enhancer (TLE) family of proteins and thus regulate the NF-κB inflammatory response [ 42 ]. While broad activation of NF-κB is induced by a variety SARS-CoV-2-encoded products, Open Reading Frame 7a (ORF7a) specifically is a potent stimulator of NF-κB associated proinflammatory chemo- and cytokines, which are elevated in the presence of severe COVID-19. NF-κB plays a similar role in other coronavirus infections.
Host antiviral immunity requires an optimal and coordinated response to control viral infections; this immunity is mediated by several host sensors, notably pattern recognition receptors (PRR). PRRs identify damage- and pathogen-associated molecular patterns (DAMPs and PAMPs, respectively). SARS-CoV-2 infects the cell via the endosomal compartment, and may activate TLRs, such as TLR4, resulting in increased NF-κB activity and expression [ 42 , 43 ]. The MyD88-mediated TRIF activation of TLR downstream pathways triggers the nuclear translocation of NF-κB, IFN regulatory factor 3 (IRF-3), and IFN regulatory factor 7 (IRF-7), resulting in the expression of innate immunity proinflammatory cytokines (interleukin-1 [IL-1], interleukin-6 [IL- 6], TNF-α) and interferons (IFNs). Continuous activation of TLR can increase MyD88 and Interleukin-1β (IL-1β), which then can further activate NF-κB [ 43 ]. RNA viruses are detected by several sensors, such as TLRs 3, 7 and 8. TLR3 recognizes double stranded RNA, while TLR7 and TLR8 single stranded RNA. In addition to ssRNA and dsRNA, viral proteins can act as PAMPS and potentiate inflammatory signaling through the stimulation of surface TLRs. Interestingly, in SARS-CoV-2, TLR2 is a critical mediator of envelope protein detection and driver of pathogenesis through inflammatory process augmentation [ 44 ]. Some individuals with severe COVID-19 have mutations in genes associated with type I and III IFN pathways [ 45 ]. Ten percent of individuals that progress to severe COVID-19 pneumonia display elevated amounts of neutralizing antibodies against type I IFN-α2 and IFN-ω [ 46 ]; these antibodies are not present in healthy or asymptomatic individuals. Of note, albeit TLR3 activation is critical for viral clearance, TLR3 hyperactivation can lead to a cytokine storm and the subsequent severe COVID-19. Other receptors are also involved in SARS-CoV-2 recognition, such as the proteins of retinoic acid-inducible gene I (RIG-I) and melanoma differentiation-associated gene 5 (MDA-5). Once inside the cell, double strand viral RNA can be recognized by RIG-I/MDA5, thus initiating an antiviral response through mitochondrial antiviral signaling (MAVS). MAVS activated the downstream pathways, IκB kinase α/β (IKK) and TBK1/IKKε, leading to translocation of NF-κB and/or IRF3 into the nucleus and induction of genes involved with innate antiviral immunity and the subsequent induction of IFN-stimulated genes (ISGs).
Inhibition of IFNs and ISGs is a tactic used by several viruses to evade host antiviral responses [ 47 ], and SARS-CoV-2-mediated IFNs and ISGs dysregulation appears to be an important strategy used by this virus to replicate and disrupt immune homeostasis. Furthermore, therapies with type I and III IFNs alone or combined with other drugs suppressed SARS-CoV-2, ameliorating COVID-19 disease [ 48 ].
The cytokine TGF-β triggers the Janus kinases (JAKs) / signal transducer and activator of transcription (STAT) proteins (JAK/STAT) pathway in certain contexts, while suppressing it in others [ 49 ]. It has been proposed that SARS-CoV-2 proteins, particularly NSP1 and ORF6, may dysregulate STAT1 and STAT3, leading to a positive feedback loop where coagulopathy triggers TLR4 via PAI-1 binding, circularly activating STAT; for this reason, therapeutic targeting of the Janus kinase pathway has been proposed [ 50 ].
The innate immune response is a first step to protecting against pathogens, which stimulate the interferon signaling pathway and expression of IFN-I, leading to an antiviral cellular response [ 51 ]. Coronaviruses have developed mechanisms to hinder IFN-expression and reduce the production of IFN. This suppression has been shown to correlate with disease severity and mortality [ 52 ]. This holds true for SARS-CoV-2, with recent studies showing that viral proteins ORF6, ORF8, and nucleocapsid being potent inhibitors of the IFN-I signaling pathway [ 53 ].
Metabolic adaptations
Viruses rely on host cell machinery to propagate, promoting anabolism for generation of macromolecules needed for virion replication and assembly (see Fig. 2 ). Consequently, viral proteins (see Supplementary Table 1 ) affect intracellular pathways, leading to subsequent adaptations by the cellular metabolism where the mitochondria plays a central role.
We reported recently through study of COVID-19 patient samples (i.e., nasopharyngeal swab samples, various organs from autopsy COVID-19 samples, murine lung tissues, and various organs from hamsters being infected with SARS-CoV-2) that heavy suppression occurs of mitochondrial functions in various organs [ 54 ]. Specifically, in the course of SARS-CoV-2 infection, the virus blocks the transcription of discrete groups of mitochondrial genes from major bioenergetic organs, while upregulation occurs in others as a compensatory mechanism to rescue the damage occurring in the major bioenergetic organs. This demonstrated a dynamic evolution of mitochondrial gene expression and cellular energetics as the virus progresses from one organ to the next. Transcriptomic changes in the nasopharyngeal infected samples revealed that during initial SARS-CoV-2 infection, nDNA coded mitochondrial genes are blocked and the co-inhibited genes were found to group together as components of preassembly modules of the mitochondrial oxidative phosphorylation (OXPHOS) complexes I, II, III, IV and V. At the time of death for COVID-19 patients, we showed virtually all mitochondrial function were inhibited in the heart, suggesting cardiac mitochondrial dysfunction in longer term COVID-19 pathology. In addition, mTOR signaling and the integrated stress response were highly dysregulated throughout all organs. Lastly, mitochondrial inhibition was shown to activate HIF-1α and its target genes shifting cellular metabolism away from catabolism and towards viral synthesis. Our results indicate that manipulation of mitochondrial function may be an important approach for mitigating the severity of COIVID-19.
SARS-CoV-2 infection of human monocytes [ 55 ] and human pulmonary alveolar epithelial (HPAEpiC) cells [ 56 ] induced mitochondrial reactive oxygen species (mROS) production, increased HIF-1α protein levels and upregulated expression of HIF-1α target genes [ 57 ]. The stability of hypoxia inducible factor-1α (HIF-1α) during a SARS-CoV-2 infection was shown to increase the production of pro-inflammatory cytokines and SARS-CoV-2 replication [ 55 , 56 , 57 ]. The expression of ORF3a in human embryonic kidney 293 T-antigen cells (HEK293T) cells increased the stability of HIF-1α and induced mROS production, which is an activator of HIF-1α. Together these results suggest that ORF3a induces mROS production to activate HIF-1α, which in turn triggers a shift in cellular metabolism to favor glycolysis, resulting in increased viral replication and transcription of pro-inflammatory cytokines.
Following host cell infection, the SARS-CoV-2 replication/transcription complex synthesizes ~30 kb viral genomes as well as the subgenomic RNAs required to encode for viral structural and mechanistic proteins. Between 1–5 h post-infection, the percentage of coronavirus-encoded protein per total cellular protein translation may increase by as much as 20,000 times, with the fraction of viral to cellular RNA ultimately reaching as high as 90% intracellularly [ 58 ]. To accommodate this huge shift towards viral replication, there is certainly a requirement of a shift in cellular metabolism to accommodate for viral synthesis. An investigation of SARS-CoV-2 metabolism during the initial 48-hours post viral infection showed that amino acid availability and synthesis are altered, de novo purine synthesis intermediates are accumulated, intracellular glucose and folate are depleted, and lactate levels are elevated [ 59 ]. This suggests a viral strategy of upregulating purine metabolism at the post-translational level to coincide with the shutting off of the majority of host proteins at translation levels.
Virus-infected cells also commonly exhibit the Warburg effect - increased glycolytic metabolism in the presence of inadequate oxygen for oxidative phosphorylation - to supply reducing equivalents and precursors for macromolecule biosynthesis, and to support generation of ATP needed for also increasing nucleotide and lipid biosynthesis. Metabolic shifts include dysregulated Ca 2+ signaling and increased mitochondrial generation of ROS. How SARS-CoV-2 induces host cell nucleotide metabolism remains unanswered.
Mitochondrial metabolism and function are highly impacted in multiple ways. With the shift towards glycolysis, there is a reduction in oxidative phosphorylation affecting the mitochondria and its function. SARS-CoV-2 may interact with the mitochondria to destabilize its oxidative phosphorylation capacity. Coronavirus replication requires the formation of double-membrane vesicles (DMVs) derived from endoplasmic reticulum (ER). These DMVs serve as a site for viral replication and help conceal the virus from host cellular defenses. Interestingly, mitochondrial stress is known to induce mitochondria-derived vesicles (MDVs) that communicate with the ER. It is conceivable that SARS-CoV-2 disruption of mitochondrial function results in the induction of (double-membrane) MDVs. SARS-CoV-2 RNA present in the mitochondria induces mitochondrial dysfunction. Increased DMVs can provide opportunity for viruses to hide and replicate [ 60 ].
SARS-CoV-2 and all subgenomic RNAs are enriched in the host mitochondria, and viral genome’s 5ʹ - and 3ʹ -UTRs contain distinct mitochondrial localization signals [ 61 ], indicating that the viral RNA may hijack the mitochondria, an interesting hypothesis for experimental validation [ 61 ]. Other recent studies have mapped physical interactions of SARS-CoV-2 encoded proteins with mitochondrial localized proteins. These interactions include: NSP8 interaction with mitochondrial ribosomal protein s2 (MRPS2), mitochondrial ribosomal protein s5 (MRPS5), mitochondrial ribosomal protein s25 (MRPS25), and mitochondrial ribosomal protein s27 (MRPS27) ribosomal proteins; ORF9c interaction with mitochondrial NADH:Ubiquinone Oxidoreductase Complex Assembly Factor 1 (NDUFAF1) and NADH:Ubiquinone Oxidoreductase Complex Assembly Factor 9 (NDUFB9); ORF10 interaction with TIMM8; and NSP7 interaction with mitochondrial NADH:Ubiquinone Oxidoreductase Complex Assembly Factor 2 (NDUFAF2). NDUFAF1, NDUFAF2, NDUFB9, and NADH:Ubiquinone Oxidoreductase Complex Assembly Factor 10 (NDUFA10) are all key players in the assembly of complex I, and NDUFA10 is suggested as being one of the master regulators of the SARS-CoV-2 pathology [ 7 ]. Interactions were also observed between viral M protein and ATPase Na + /K + Transporting Subunit Beta 1 (ATP1B1), ATPase H + Transporting V1 Subunit A (ATP6V1A), acyl-coenzyme A dehydrogenase (ACADM), Alpha-aminoadipic semialdehyde synthase (AASS), Peptidase, Mitochondrial Processing Subunit Beta (PMPCB), Pitrilysin Metallopeptidase 1 (PITRM1), Coenzyme Q8B (COQ8B), and Peptidase, Mitochondrial Processing Subunit Alpha (PMPCA); these proteins are each components of critical mitochondrial metabolic pathways. SARS-CoV-2-encoded ORF9b protein interacts and localizes with Translocase Of Outer Mitochondrial Membrane 70 (TOMM70) [ 7 ], a mitochondrial import receptor important for transporting proteins into mitochondria and, more importantly, in modulating anti-viral cellular defense pathways [ 62 ]. These mitochondrial interactions offer glimpses of the viral effect on glycolytic and oxidative phosphorylation pathways and the potential side effects.
Another example of COVID-19’s mitochondrial-related impacts is the over-production of cellular ROS [ 63 ]. ROS and reactive nitrogen species have diverse functions in biological systems; oxidatively attacking pathogens, regulating cell proliferation, and key signaling functions [ 64 ]. However, dysregulation of ROS is implicated in many diseases, including the hyper-inflammatory late phase of COVID-19 [ 65 ]. As a part of normal redox metabolism, superoxide radicals are converted into hydrogen peroxide by the action of superoxide dismutase. The hydrogen peroxide is subsequently broken down into water by glutathione peroxidase. During COVID-19, isoforms of enzymes, including glutathione peroxidase and thioredoxin reductase, may be directly targeted and proteolyzed by the SARS-CoV-2 protease, Mpro.
SARS-CoV-2 is thought to suppress the ROS-associated Nuclear factor erythroid 2-related factor 2 (Nrf2) pathway. Nrf2 is a transcription factor that regulates the expression of antioxidant proteins that protect against oxidative damage. Dysregulation of the Nrf2 pathway will exacerbate the pro-oxidative stress caused by the virus [ 66 ]. SARS-CoV-2 may suppress the accumulation of the selenoprotein transcripts, which are crucial for the correct functioning of Phospholipid hydroperoxide glutathione peroxidase (GPX4) and mitochondria function [ 67 ]. This redox impairment would lead to a buildup of hydrogen peroxide, which could trigger inflammation by promoting the activity of the mitogen-activated protein kinase (MAPK), NF-κB, and the nuclear NOD-like receptor (NLR) family pyrin domain containing 3 (NLRP3) inflammasome [ 68 ].
Due to the multiple effects of SARS-CoV-2 that alter cellular metabolic and oxidative states, there are multiple directions to deplete NAD+, loss of cellular ATP and reduced Poly-ADP-ribose polymerase (PARP) activity, each of which have cytotoxic effects of their own [ 69 ]. In general, NADPH, synthesized from NAD+, is necessary for many key redox reactions; a reduced level of NADPH could play a mechanistic role in cellular metabolic changes from SARS-CoV-2 infection.
SARS-CoV-2 mediated reduction in ATP and nitric oxide signaling induces cell stress
Cellular metabolism adapts to the alterations induced by SARS-CoV-2 infection of the cell (see Fig. 4 ). These adaptations depend on the cell and tissue type. Here, we focus on ATP signaling, which is relevant to epithelial cells, and nitric oxide (NO) signaling, which tends to be perturbed in endothelial cells [ 70 ]. Activation of each pathway at low levels provides protection to the host.
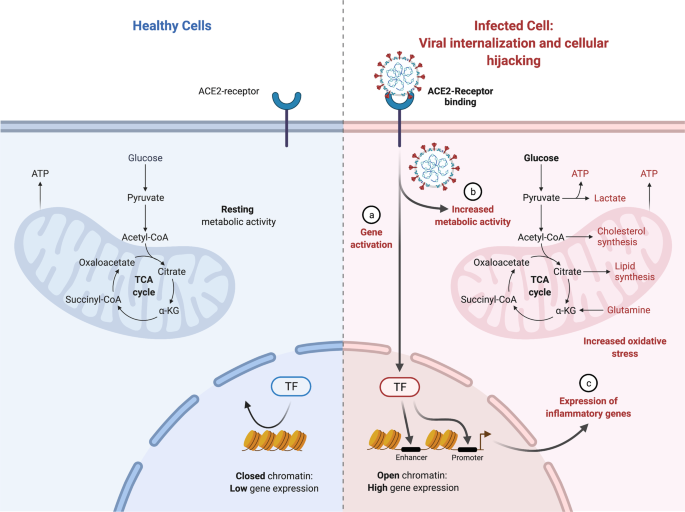
Metabolic pathways and shifts that lead to cellular dysregulation and viral activation to lead towards viral replication (above).
ATP production from oxidative phosphorylation, glycolysis, and other pathways is critical to support cellular physiology, but this molecule also has signaling properties, which can be particularly beneficial in epithelial cells [ 70 ]. Perturbations in ATP generation induced by the virus in epithelial cells [ 71 ] can lead to ATP release from the apical or basolateral spaces, and subsequent extracellular ATP signaling [ 72 ]. It can stimulate P2 receptors on neighboring epithelial cells to activate signal transduction pathways and alter cellular function in adjacent cells even if they are not infected, thus priming naive host cells for confrontation with the virus [ 71 , 72 ].
In the endothelium, nitric oxide directly affects mitochondrial metabolism through interaction with cytochrome C, providing cytoprotection against free radicals. However, reduction of NO bioavailability, due to the increased oxidative stress state caused by SARS-CoV-2-elevated superoxides, results in the formation of peroxynitrites (ONOO-). The reduced NO diffusion to neighboring vascular smooth muscle may impair vascular function [ 73 ]. Peroxynitrite also causes injury to the mitochondria and reduces ATP synthesis, with all of the concomitant negative effects. Therefore, loss of NO bioavailability has major cellular consequences, inducing shifts in multiple enzymatic pathways, cell injury, and death.
Like ATP, NO acts as a biological signaling molecule. This dissolved gas rapidly diffuses across cell membranes and regulates various functions across the body [ 73 ]. The vascular endothelium is the predominant cellular source of NO production, and it plays a critical role in maintaining cardiovascular function. Factors that reduce endothelial NO production (increased oxidative stress, changes in NO synthase synthesis) negatively affect endothelial function [ 73 ]. The cascade of inflammation and oxidative stress triggered by COVID-19 leads to the formation of superoxide free radicals, impairing biological processes and increasing cytotoxicity in the host cells [ 74 ]. The instantaneous reaction of superoxide and NO yields ONOO-, a powerful, cytotoxic nitrating agent. This reaction effectively destroys the NO, rendering it unavailable for its normal regulatory purposes. Thus, the downregulation of NO bioavailability is thought to be a central factor in the severity of COVID-19-associated endotheliitis and the onset of endothelial dysfunction [ 75 ].
The causative agent of the COVID-19 the pandemic, SARS-CoV-2, has caused loss of incomes, economic crises, morbidities, and loss of life worldwide. Here, we describe the virus and review state-of-the-art information about the processes it utilizes to enter and reprogram the human host machinery. We detail research on early infection using evidence from patient samples, organoids and cells, and non-human animal studies. Each of these has limitations but taken together provide unique observational and mechanistic insight on SARS-CoV-2 infection.
COVID-19 is a pleiotropic condition. Viral insults and subsequent cellular metabolic adaptations differ in the context of cell-type, genotype and environmental influences. Thus, much of what we have presented applies to specific cell types and contexts, and we have attempted to cover these contexts.
Key avenues of future research on SARS-CoV-2 infection and propagation include: 1) defining the mechanisms of how the virus enters cells, and the protein and receptor molecules that are critical to this process; 2) elucidating the dynamics of how protein machinery is captured and retrofitted for viral purposes in a cell-specific manner; 3) understanding how the host genetics and environment can affect the ability of the virus to infect; 4) understanding the impact of SARS-CoV-2 on glycolysis and oxidative phosphorylation; and 5) revealing how the mitochondria adapts to ultimately shift its physiology from steady-state.
In the best-case scenario for the SARS-CoV-2 virus, infection leads to a cascade of intracellular adaptations in which multiple networks are remodeled, from transcription to metabolism to signal transduction, shifting the invaded host cell from its original physiology into a SARS-CoV-2 replication system, and causing the emission of new viral particles and signaling molecules. The subsequent disease events will reverberate across the body’s cells and organs. This will be the subject of our Part 2 review (in preparation).
Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20:533–4.
Article CAS PubMed PubMed Central Google Scholar
Van Egeren D, Novokhodko A, Stoddard M, Tran U, Zetter B, Rogers M, et al. Risk of rapid evolutionary escape from biomedical interventions targeting SARS-CoV-2 spike protein. PloS One. 2021;16:e0250780.
Article PubMed PubMed Central CAS Google Scholar
Yao H, Song Y, Chen Y, Wu N, Xu J, Sun C, et al. Molecular architecture of the SARS-CoV-2 virus. Cell. 2020;183:730–8.
Liu J, Xie W, Wang Y, Xiong Y, Chen S, Han J, et al. A comparative overview of COVID-19, MERS and SARS. Int J Surg. 2020;81:1–8.
Article PubMed PubMed Central Google Scholar
Grifoni A, Sidney J, Zhang Y, Scheuermann RH, Peters B, Sette A. A sequence homology and bioinformatic approach can predict candidate targets for immune responses to SARS-CoV-2. Cell Host Microbe. 2020;27:671–80.
Caldaria A, Conforti C, Di Meo N, Dianzani C, Jafferany M, Lotti T, et al. COVID-19 and SARS: Differences and similarities. Dermatol Ther. 2020;33:e13395.
Gordon DE, Jang GM, Bouhaddou M, Xu J, Obernier K, White KM, et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583:459–68.
Benvenuto D, Giovanetti M, Salemi M, Prosperi M, De Flora C, Junior Alcantara LC, Angeletti S, Ciccozzi M. The global spread of 2019-nCoV: a molecular evolutionary analysis. Pathog Glob Health. 2020;114:64–7.
Alene M, Yismaw L, Assemie MA, Ketema DB, Mengist B, Kassie B, et al. Magnitude of asymptomatic COVID-19 cases throughout the course of infection: A systematic review and meta-analysis. PloS One. 2021;16:e0249090.
Mahdy MA, Younis W, Ewaida Z. An overview of SARS-CoV-2 and animal infection. Front Vet Sci. 2020;7:1084.
Article Google Scholar
Fang S, Li K, Shen J, Liu S, Liu J, Yang L, et al. GESS: a database of global evaluation of SARS-CoV-2/hCoV-19 sequences. Nucleic Acids Res. 2021;49:D706–14.
Article CAS PubMed Google Scholar
Banoun H. Evolution of SARS-CoV-2: Review of Mutations, Role of the Host Immune System. Nephron. 2021;145:392–403.
Meng B, Kemp SA, Papa G, Datir R, Ferreira IATM, Marelli S, et al. Recurrent emergence of SARS-CoV-2 spike deletion H69/V70 and its role in the Alpha variant B.1.1.7. Cell Rep. 2021;29:109292.
Centers for Disease Control and Prevention. “SARS-CoV-2 variant classifications and definitions.” (2021).
Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol. 2020;5:562–9.
Dai L, Gao GF. Viral targets for vaccines against COVID-19. Nat Rev Immunol. 2021;21:73–82.
Ali A, Vijayan R. Dynamics of the ACE2–SARS-CoV-2/SARS-CoV spike protein interface reveal unique mechanisms. Sci Rep. 2020;10:1–2.
Article CAS Google Scholar
Song W, Gui M, Wang X, Xiang Y. Cryo-EM structure of the SARS coronavirus spike glycoprotein in complex with its host cell receptor ACE2. PLoS Pathog. 2018;14:e1007236.
Chu H, Chan JF, Yuen TT, Shuai H, Yuan S, Wang Y, et al. Comparative tropism, replication kinetics, and cell damage profiling of SARS-CoV-2 and SARS-CoV with implications for clinical manifestations, transmissibility, and laboratory studies of COVID-19: an observational study. Lancet Microbe. 2020;1:e14–23.
Yan R, Zhang Y, Li Y, Ye F, Guo Y, Xia L, et al. Structural basis for the different states of the spike protein of SARS-CoV-2 in complex with ACE2. Cell Res. 2021;31:717–9.
Puelles VG, Lütgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L, et al. Multiorgan and renal tropism of SARS-CoV-2. N. Engl J Med. 2020;383:590–2.
Article PubMed Google Scholar
Menter T, Haslbauer JD, Nienhold R, Savic S, Hopfer H, Deigendesch N, et al. Postmortem examination of COVID‐19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology. 2020;77:198–209.
Ziegler CG, Allon SJ, Nyquist SK, Mbano IM, Miao VN, Tzouanas CN, et al. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020;181:1016–35.
Park J, Foox J, Hether T, Danko DC, Warren S, Kim Y, et al. System-wide transcriptome damage and tissue identity loss in COVID-19 patients. Cell Rep Med. 2022;3:100522.
Han Y, Duan X, Yang L, Nilsson-Payant BE, Wang P, Duan F, et al. Identification of SARS-CoV-2 inhibitors using lung and colonic organoids. Nature. 2021;589:270–5.
Ashraf UM, Abokor AA, Edwards JM, Waigi EW, Royfman RS, Hasan SA, et al. SARS-CoV-2, ACE2 expression, and systemic organ invasion. Physiological Genomics. 2021;53:51–60.
Suryamohan K, Diwanji D, Stawiski EW, Gupta R, Miersch S, Liu J, et al. Human ACE2 receptor polymorphisms and altered susceptibility to SARS-CoV-2. Commun Biol. 2021;4:1–1.
Ozono S, Zhang Y, Ode H, Sano K, Tan TS, Imai K, et al. SARS-CoV-2 D614G spike mutation increases entry efficiency with enhanced ACE2-binding affinity. Nat Commun. 2021;12:1–9.
Sodhi CP, Wohlford-Lenane C, Yamaguchi Y, Prindle T, Fulton WB, Wang S, et al. Attenuation of pulmonary ACE2 activity impairs inactivation of des-Arg9 bradykinin/BKB1R axis and facilitates LPS-induced neutrophil infiltration. Am J Physiol-Lung Cell Mol Physiol. 2018;314:L17–31.
Article PubMed CAS Google Scholar
Calixto JB, Medeiros R, Fernandes ES, Ferreira J, Cabrini DA, Campos MM. Kinin B1 receptors: key G‐protein‐coupled receptors and their role in inflammatory and painful processes. Br J Pharmacol. 2004;143:803–18.
Huang Y, Yang C, Xu XF, Xu W, Liu SW. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacologica Sin. 2020;41:1141–9.
Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S, et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215–20.
Davidson AM, Wysocki J, Batlle D. Interaction of SARS-CoV-2 and other coronavirus with ACE (angiotensin-converting enzyme)-2 as their main receptor: therapeutic implications. Hypertension 2020;76:1339–49.
Ko PJ, Woodrow C, Dubreuil MM, Martin BR, Skouta R, Bassik MC, et al. A ZDHHC5-GOLGA7 protein acyltransferase complex promotes nonapoptotic cell death. Cell Chem Biol. 2019;26:1716–24.
Van De Veerdonk F, Netea MG, Van Deuren M, Van Der Meer JW, De Mast Q, Bruggemann RJ, et al. Kinins and cytokines in COVID-19: a comprehensive pathophysiological approach.
Mohammad S, Bouchama A, Mohammad Alharbi B, Rashid M, Saleem Khatlani T, Gaber NS, et al. SARS-CoV-2 ORF8 and SARS-CoV ORF8ab: genomic divergence and functional convergence. Pathogens. 2020;9:677.
Article CAS PubMed Central Google Scholar
Castaño-Rodriguez C, Honrubia JM, Gutiérrez-Álvarez J, DeDiego ML, Nieto-Torres JL, Jimenez-Guardeño JM, et al. Role of severe acute respiratory syndrome coronavirus viroporins E, 3a, and 8a in replication and pathogenesis. MBio. 2018;9:e02325–17.
McClenaghan C, Hanson A, Lee SJ, Nichols CG. Coronavirus proteins as ion channels: Current and potential research. Front Immunol. 2020;11:2651.
Di Filippo L, Formenti AM, Rovere-Querini P, Carlucci M, Conte C, Ciceri F, et al. Hypocalcemia is highly prevalent and predicts hospitalization in patients with COVID-19. Endocrine. 2020;68:475–8.
Schett G, Manger B, Simon D, Caporali R COVID-19 revisiting inflammatory pathways of arthritis. Nat Rev Rheumatol. 2020;16:465–70.
Huang J, Hume AJ, Abo KM, Werder RB, Villacorta-Martin C, Alysandratos KD, et al. SARS-CoV-2 infection of pluripotent stem cell-derived human lung alveolar type 2 cells elicits a rapid epithelial-intrinsic inflammatory response. Cell Stem Cell. 2020;27:962–73.
Zheng M, Karki R, Williams EP, Yang D, Fitzpatrick E, Vogel P, et al. TLR2 senses the SARS-CoV-2 envelope protein to produce inflammatory cytokines. Nat Immunology. 2021;1-0.
Mukherjee R, Bhattacharya A, Bojkova D, Mehdipour AR, Shin D, Khan KS, et al. Famotidine inhibits toll-like receptor 3-mediated inflammatory signaling in SARS-CoV-2 infection. J Biolog Chem. 2021;297:100925.
Zhang Q, Bastard P, Liu Z, Le Pen J, Moncada-Velez M, Chen J, et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370:eabd4570.
Bastard P, Rosen LB, Zhang Q, Michailidis E, Hoffmann HH, Zhang Y, et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370:eabd4585.
Hung IF, Lung KC, Tso EY, Liu R, Chung TW, Chu MY, et al. Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395:1695–704.
Irvani SS, Golmohammadi M, Pourhoseingholi MA, Shokouhi S, Darazam IA. Effectiveness of Interferon Beta 1a, compared to Interferon Beta 1b and the usual therapeutic regimen to treat adults with moderate to severe COVID-19: structured summary of a study protocol for a randomized controlled trial. Trials. 2020;21:1–3.
Thevarajan I, Buising KL, Cowie BC. Clinical presentation and management of COVID-19. Med J Aust. 2020;213:134–9.
Tan L, Wang Q, Zhang D, Ding J, Huang Q, Tang YQ, et al. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5:1–3.
Google Scholar
Yang J, Wise L, Fukuchi KI. TLR4 Cross-talk with NLRP3 inflammasome and complement signaling pathways in Alzheimer’s disease. Front Immunol. 2020;11:724.
Li JY, Liao CH, Wang Q, Tan YJ, Luo R, Qiu Y, et al. The ORF6, ORF8 and nucleocapsid proteins of SARS-CoV-2 inhibit type I interferon signaling pathway. Virus Res. 2020;286:198074.
Lei X, Dong X, Ma R, Wang W, Xiao X, Tian Z, et al. Activation and evasion of type I interferon responses by SARS-CoV-2. Nat Commun. 2020;11:1–2.
Codo AC, Davanzo GG, de Brito Monteiro L, de Souza GF, Muraro SP, Virgilio-da-Silva JV, et al. Elevated glucose levels favor SARS-CoV-2 infection and monocyte response through a HIF-1α/glycolysis-dependent axis. Cell Metab. 2020;32:437–46.
Guarnieri JW, Dybas JM, Fazelinia H, Kim MS, Frere J, Zhang Y, et al. Targeted down regulation of core mitochondrial genes during SARS-CoV-2 infection. bioRxiv. 2022 Feb 22.
Wang P, Luo R, Zhang M, Wang Y, Song T, Tao T, et al. A cross-talk between epithelium and endothelium mediates human alveolar–capillary injury during SARS-CoV-2 infection. Cell death Dis. 2020;11:1–7.
Tian M, Liu W, Li X, Zhao P, Shereen MA, Zhu C, et al. HIF-1α promotes SARS-CoV-2 infection and aggravates inflammatory responses to COVID-19. Signal Transduct Target Ther. 2021;6:1–3.
Irigoyen N, Firth AE, Jones JD, Chung BY, Siddell SG, Brierley I. High-resolution analysis of coronavirus gene expression by RNA sequencing and ribosome profiling. PLoS Pathog. 2016;12:e1005473.
Singh KK, Chaubey G, Chen JY, Suravajhala P. Decoding SARS-CoV-2 hijacking of host mitochondria in COVID-19 pathogenesis. Am J Physiol-Cell Physiol. 2020;319:C258–67.
Ajaz S, McPhail MJ, Singh KK, Mujib S, Trovato FM, Napoli S, et al. Mitochondrial metabolic manipulation by SARS-CoV-2 in peripheral blood mononuclear cells of patients with COVID-19. Am J Physiol-Cell Physiol. 2021;320:C57–65.
Stukalov A, Girault V, Grass V, Karayel O, Bergant V, Urban C, et al. Multilevel proteomics reveals host perturbations by SARS-CoV-2 and SARS-CoV. Nature 2021;594:246–52.
Thaker SK, Ch’ng J, Christofk HR. Viral hijacking of cellular metabolism. BMC Biol. 2019;17:1–5.
Cecchini R, Cecchini AL. SARS-CoV-2 infection pathogenesis is related to oxidative stress as a response to aggression. Med Hypotheses. 2020;143:110102.
Suhail S, Zajac J, Fossum C, Lowater H, McCracken C, Severson N, et al. Role of oxidative stress on SARS-CoV (SARS) and SARS-CoV-2 (COVID-19) infection: a review. The protein journal. 2020;39:644–56.
Taylor EW, Radding W. Understanding selenium and glutathione as antiviral factors in COVID-19: Does the viral Mpro protease target host selenoproteins and glutathione synthesis? Front Nutr. 2020;7:143.
Wang Y, Huang J, Sun Y, Stubbs D, He J, Li W, et al. SARS-CoV-2 suppresses mRNA expression of selenoproteins associated with ferroptosis, endoplasmic reticulum stress and DNA synthesis. Food Chem Toxicol. 2021;153:112286.
Abais JM, Xia M, Zhang Y, Boini KM, Li PL. Redox regulation of NLRP3 inflammasomes: ROS as trigger or effector? Antioxid Redox Signal. 2015;22:1111–29.
Olagnier D, Farahani E, Thyrsted J, Blay-Cadanet J, Herengt A, Idorn M, et al. SARS-CoV2-mediated suppression of NRF2-signaling reveals potent antiviral and anti-inflammatory activity of 4-octyl-itaconate and dimethyl fumarate. Nat Commun. 2020;11:1–2.
CAS Google Scholar
Heer CD, Sanderson DJ, Voth LS, Alhammad YM, Schmidt MS, Trammell SA, et al. Coronavirus infection and PARP expression dysregulate the NAD metabolome: An actionable component of innate immunity. J Biol Chem. 2020;295:17986–96.
Agledal L, Niere M, Ziegler M. The phosphate makes a difference: cellular functions of NADP. Redox Rep. 2010;15:2–10.
Schwiebert EM, Zsembery A. Extracellular ATP as a signaling molecule for epithelial cells. Biochimica et Biophysica Acta (BBA)-Biomembranes. 2003;1615:7–32.
Novak I. ATP as a signaling molecule: the exocrine focus. Physiology. 2003;18:12–7.
Green SJ. Covid-19 accelerates endothelial dysfunction and nitric oxide deficiency. Microbes Infect. 2020;22:149.
Yamasaki H. Blood nitrate and nitrite modulating nitric oxide bioavailability: potential therapeutic functions in COVID-19. Nitric Oxide. 2020;103:29–30.
Lingappan K. NF-κB in oxidative stress. Curr Opin Toxicol. 2018;7:81–6.
Ozdemir B, Yazici A. Could the decrease in the endothelial nitric oxide (NO) production and NO bioavailability be the crucial cause of COVID-19 related deaths? Med Hypotheses. 2020;144:109970.
Download references
Acknowledgements
The opinions expressed in this article are those of the authors and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the United States government.
This work was supported by supplemental funds for COVID-19 research from Translational Research Institute of Space Health through NASA Cooperative Agreement NNX16AO69A (T-0404) to AB, and by a NASA Space Biology Postdoctoral Fellowship (80NSSC19K0426) to SAN. MJT is a recipient of The Evelyn Grollman Glick Scholar Award and supported by research funding from The Dr. Miriam and Sheldon G. Adelson Medical Research Foundation, Van Andel Research Institute through the Van Andel Research Institute – Stand Up To Cancer Epigenetics Dream Team. Stand Up To Cancer is a program of the Entertainment Industry Foundation, administered by AACR, and Specialized Program of Research Excellence (SPORE) program, through the National Cancer Institute (NCI), grant P50CA254897.
Author information
These authors contributed equally: David A. Jamison and S. Anand Narayanan.
This author jointly supervised this work: Afshin Beheshti.
Authors and Affiliations
COVID-19 International Research Team, Medford, MA, USA
David A. Jamison Jr., S. Anand Narayanan, Nídia S. Trovão, Joseph W. Guarnieri, Michael J. Topper, Pedro M. Moraes-Vieira, Viktorija Zaksas, Keshav K. Singh, Eve Syrkin Wurtele & Afshin Beheshti
Department of Nutrition & Integrative Physiology, Florida State University, Tallahassee, FL, USA
S. Anand Narayanan
Fogarty International Center, National Institutes of Health, Bethesda, MD, USA
Nídia S. Trovão
Center for Mitochondrial and Epigenomic Medicine, Children’s Hospital of Philadelphia, Philadelphia, PA, USA
Joseph W. Guarnieri
Department of Oncology, The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, MD, USA
Michael J. Topper
Department of Genetics, Evolution, Microbiology and Immunology, Institute of Biology, University of Campinas, Campinas, SP, Brazil
Pedro M. Moraes-Vieira
Obesity and Comorbidities research Center (OCRC), University of Campinas, Campinas, SP, Brazil
Experimental Medicine Research Cluster, University of Campinas, Campinas, Brazil
Center for Translational Data Science, University of Chicago, Chicago, IL, USA
Viktorija Zaksas
Department of Genetics, Heersink School of Medicine, University of Alabama at Birmingham, Birmingham, AL, USA
Keshav K. Singh
Center for Metabolic Biology, Bioinformatics and Computational Biology, and Genetics Development, and Cell Biology, Iowa State University, Ames, IA, USA
Eve Syrkin Wurtele
Stanley Center for Psychiatric Research, Broad Institute of MIT and Harvard, Cambridge, MA, USA
Afshin Beheshti
KBR, Space Biosciences Division, NASA Ames Research Center, Moffett Field, CA, USA
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization: DAJ; Writing – Original Draft: DAJ, SN; Writing – Review and Editing: AB, ESW, KKS, JWG, MJT, VZ, PMMV, DAJ, SN, NST. Visualization: DAJ, SN, JWG; Supervision: AB; Funding Acquisition: AB.
Corresponding authors
Correspondence to S. Anand Narayanan or Afshin Beheshti .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplemental table 1, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Jamison, D.A., Anand Narayanan, S., Trovão, N.S. et al. A comprehensive SARS-CoV-2 and COVID-19 review, Part 1: Intracellular overdrive for SARS-CoV-2 infection. Eur J Hum Genet 30 , 889–898 (2022). https://doi.org/10.1038/s41431-022-01108-8
Download citation
Received : 24 November 2021
Revised : 20 March 2022
Accepted : 12 April 2022
Published : 16 May 2022
Issue Date : August 2022
DOI : https://doi.org/10.1038/s41431-022-01108-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Liver injury in covid-19: an insight into pathobiology and roles of risk factors.
- Abbas Tazarghi
- Sahar Bazoq
- Hadi Razavi Nikoo
Virology Journal (2024)
Oxoammonium salts exert antiviral effects against coronavirus via denaturation of their spike proteins
- Ryosuke Segawa
- Yusuke Sasano
- Noriyasu Hirasawa
Scientific Reports (2024)
Possible temporal relationship between SARS-CoV-2 infection and anti-NMDA receptor encephalitis: a meta-analysis
- Veronika Vasilevska
- Paul C. Guest
- Johann Steiner
Translational Psychiatry (2024)
Dual Role of Extracellular Vesicles as Orchestrators of Emerging and Reemerging Virus Infections
- A. P. Athira
- Smrithi Sreekanth
- Anismrita Lahon
Cell Biochemistry and Biophysics (2024)
Toll-like receptors polymorphisms and COVID-19: a systematic review
- Barbara Rayssa Correia dos Santos
- Luana Karen Correia dos Santos
- Elaine Virginia Martins de Souza Figueiredo
Molecular and Cellular Biochemistry (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
REVIEW article
Coronavirus disease (covid-19): comprehensive review of clinical presentation.

- 1 Department of Medicine, King Edward Medical University/ Mayo Hospital, Lahore, Pakistan
- 2 Department of Anesthesia and Intensive Care, Post-Graduate Medical Institute/LGH, Lahore, Pakistan
- 3 Rajarshee Chhatrapati Shahu Maharaj Government Medical College, Kolhapur, India
- 4 Department of Medicine, Faculty of Medicine, University of Tlemcen, Tlemcen, Algeria
- 5 School of Tropical Medicine and Global Health, Nagasaki University, Nagasaki, Japan
- 6 Institute of Research and Development, Duy Tan University, Da Nang, Vietnam
COVID-19 is a rapidly growing pandemic with its first case identified during December 2019 in Wuhan, Hubei Province, China. Due to the rampant rise in the number of cases in China and globally, WHO declared COVID-19 as a pandemic on 11th March 2020. The disease is transmitted via respiratory droplets of infected patients during coughing or sneezing and affects primarily the lung parenchyma. The spectrum of clinical manifestations can be seen in COVID-19 patients ranging from asymptomatic infections to severe disease resulting in mortality. Although respiratory involvement is most common in COVID-19 patients, the virus can affect other organ systems as well. The systemic inflammation induced by the disease along with multisystem expression of Angiotensin Converting Enzyme 2 (ACE2), a receptor which allows viral entry into cells, explains the manifestation of extra-pulmonary symptoms affecting the gastrointestinal, cardiovascular, hematological, renal, musculoskeletal, and endocrine system. Here, we have reviewed the extensive literature available on COVID-19 about various clinical presentations based on the organ system involved as well as clinical presentation in specific population including children, pregnant women, and immunocompromised patients. We have also briefly discussed about the Multisystemic Inflammatory Syndrome occurring in children and adults with COVID-19. Understanding the various clinical presentations can help clinicians diagnose COVID-19 in an early stage and ensure appropriate measures to be undertaken in order to prevent further spread of the disease.
Introduction
COVID-19 is a growing pandemic with initial cases identified in Wuhan, Hubei province, China toward the end of December 2019. Labeled as Novel Coronavirus 2019 (2019-nCoV) initially by the Chinese Center for Disease Control and Prevention (CDC) which was subsequently renamed as Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2) due to its homology with SARS-CoV by the International Committee on Taxonomy of Viruses (ICTV) ( 1 , 2 ). The World Health Organization (WHO) later renamed the disease caused by SARS-CoV-2 as Coronavirus Disease-2019 (COVID-19) ( 3 ). COVID-19 is primarily a disease of the respiratory system affecting lung parenchyma with fever, cough, and shortness of breath as the predominant symptoms. Recent studies have shown that it can affect multiple organ systems and cause development of extra-pulmonary symptoms. Presence of extra-pulmonary symptoms can often lead to late diagnosis and sometimes even mis-diagnosis of COVID-19 which can be detrimental to patients. As researchers globally continue to understand COVID-19 and its implications on the human body, knowledge about the various clinical presentations of COVID-19 is paramount in early diagnosing and treatment in order to decrease the morbidity and mortality caused by the disease.
Epidemiology and Pathophysiology
While studying the early transmission dynamics of COVID-19 outbreak in Wuhan, many cases were found to be linked to the Huanan wholesale seafood market. Further investigation revealed <10% of the total cases could be linked to the market which led to the conclusion of human-to-human transmission of the virus occurring through respiratory droplets and contact transmission contributing to the rise in the number of affected individuals ( 4 ). The exponential rise in the number of cases in China and reporting of cases outside China in multiple countries led WHO to declare COVID-19 as a pandemic on 11th March 2020 ( 5 ).
SARS-CoV-2 tends to infect all age groups and is transmitted via direct contact or respiratory droplets generated during coughing or sneezing by the infected patient during both symptomatic or pre-symptomatic phase of infection. Other routes of transmission include fecal-oral route and fomites along with small risk of vertical transmission from mother to child if infection occurs during third trimester of pregnancy ( 6 , 7 ). There has also been evidence of asymptomatic transmission of COVID-19 ( 8 ). The concept of super spreaders in relation to COVID-19 is emerging where a single individual either symptomatic or asymptomatic can infect a disproportionately large number of individuals in an appropriate super spreading conditions such as mass gathering due to production of large number of infectious agent for prolonged duration of time ( 9 ). As per the literature, the incubation period of COVID-19 ranges from 2 to 14 days with a mean incubation period of 3 days ( 10 ). The basic Reproduction number (Ro) of SARS-CoV-2 is 2–2.5. Each individual infected with COVID-19 can infect 2–2.5 other individuals in a naïve population which also explains the exponential growth in the number of cases ( 10 ). The disease tends to be of mild to moderate severity in roughly 80% of patients, and severe disease is associated with infants, elderly patients above 65 years, and patients with other comorbidities such as diabetes mellitus, hypertension, coronary artery disease, and other chronic conditions ( 1 , 2 ). COVID-19 has also been found to be more severe in males than in females with a case fatality rate of 2.8% in males and 1.7% in females ( 11 ). The major organ system affected by the virus is the respiratory system, but it can affect other organ systems either directly or by the effect of host immune response. SARS-CoV-2, the causative agent of COVID-19, after entering the human host initially replicates in the epithelial mucosa of the upper respiratory tract (nose and pharynx) followed by migration to the lungs where further replication of virus occurs causing transient viraemia. The virus uses Angiotensin Converting Enzyme 2 (ACE2) receptor as a primary entry to cells. ACE2 is found abundantly in the mucosal lining of the respiratory tract, vascular endothelial cells, heart, intestine, and kidney. Thus, the virus has potential for replication in all these organs. After entry into cells, the virus undergoes further rapid replication within the target cells and induces extensive epithelial and endothelial dysfunction leading to exponential inflammatory response with the production of a large amount of proinflammatory cytokines and chemokines. Activation of proinflammatory cytokines and chemokines leads to neutrophil activation and migrations and results in the characteristic cytokine storm. The immunological downregulation of ACE2 by the virus contributes to acute lung injury in COVID-19. ACE2 also regulates the renin angiotensin system (RAS); thus, downregulation of ACE2 also causes dysfunction of RAS which contributes to enhanced inflammation ( 2 , 11 – 15 ). These entire factors contribute to symptoms of COVID-19 with sepsis, multi-organ dysfunction, acute respiratory distress syndrome (ARDS), and prothrombotic state leading to an exacerbation of organ dysfunction.
Clinical Manifestation
We review here the system based clinical features of COVID-19.
Respiratory
According to report from WHO-China-Joint Mission on COVID-19, 55,924 laboratories confirmed cases of COVID-19 had fever (87.9%), dry cough (67.7%), fatigue (38.1%), sputum production (33.4%), difficulty breathing (18.6%), sore throat (13.9%), chills (11.4%), nasal congestion (4.8%), and hemoptysis (0.9%) ( 1 ).
Some patients may rapidly progress to acute lung injury and ARDS with septic shock. The median interval between the onset of initial symptoms to development of dyspnea, hospital admission, and ARDS was 5, 7, and 8 days respectively ( 10 ). Some patients with COVID-19 may have reduced oxygen saturation in blood (≤ 93%) with oxygen saturation down to 50 or 60% but remained stable without significant distress, and as such, were termed as salient hypoxia or happy hypoxia ( 16 , 17 ). Trial of oxygen therapy, prone positioning, high flow continuous positive airway pressure, non-re-breathable mask alongside trial of anticoagulation are often used to manage these patients ( 16 , 17 ). However, further study is required to define the role of these strategies in management.
The most frequent radiological abnormality among 975 patients with COVID-19 in computed tomography (CT) scan of chest was ground glass opacity (56.4%) and bilateral patchy shadowing (51.8%) ( 18 ). A scientific review of 2,814 patients have shown that the most common chest CT finding in COVID-19 patients was ground glass opacity followed by consolidation. However, the findings can vary in different patients and at various stages of diseases. Other CT findings include interlobular septal thickening, reticular pattern, crazy paving, etc. Atypical findings like air bronchogram, bronchial wall thickening, nodule, pleural effusion, and lymphadenopathy have also been noted in some studies ( 19 ). A study showed that among 877 patients with non-severe diseases and 173 patients with severe diseases, 17.9 and 2.9% of the patients did not have any detectable radiological abnormalities, respectively ( 18 ).
ENT (Ear, Nose, and Throat)
ENT manifestations are one of the most frequent symptoms encountered by physicians in COVID-19. A peculiar clinical presentation in some COVID-19 patients includes the deterioration of sense, taste (dysgeusia), and loss of smell (anosmia). A systematic review and meta-analysis of 10 studies with 1,627 participants surveyed for olfactory deterioration and 9 studies with 1,390 participants examined for gustatory symptoms demonstrated prevalence of 52.73 and 43.93% of these symptoms among COVID-19 patients, respectively. These clinical features may often present at earlier stages of the disease ( 20 ). Additionally, sore throat, rhinorrhea, nasal congestion, tonsil edema, and enlarged cervical lymph nodes are commonly seen among otolaryngological dysfunctions in patients ( 21 ). A large observational study of 1,099 COVID-19 patients reported tonsils swelling in 23 patients (2.1%), throat congestion in 19 patients (1.7%) and enlarged lymph nodes in 2 patients (0.2%) ( 18 ). This can be explained by the fact that there is a high expression of ACE2 receptors on the epithelial cells of the oral and nasal mucosa including the tongue. It has been known that the novel coronavirus has a strong binding affinity to ACE2 receptors through which it invades host cells ( 22 ). This theory may explain the exhibition of extra-respiratory symptoms including ENT manifestations as part of COVID-19 symptoms.
Cardiovascular
Cardiac manifestation in patient with COVID-19 can occur due to cardiac strain secondary to hypoxia and respiratory failure, direct effect of SARS-CoV-2 on heart or secondary to inflammation and cytokine storm, metabolic derangements, rupture of plaque and coronary occlusion by thrombus, and consequences of drugs used for treatment ( 23 – 25 ). The need for intensive care admission, non-invasive ventilation (46.3 vs. 3.9%), and invasive mechanical ventilation (22 vs. 4.2%) were higher among patients with cardiac ailments as compared to those without cardiac involvement as well as higher hospital mortality than those without myocardial involvement (51.2 vs. 4.5%) ( 26 ). These patients tend to have electrocardiographic (ECG) changes as well as elevations in high sensitivity cardiac troponin (hsCTn) and N- terminal pro-B-type natriuretic peptide (NT proBNP) which corresponded to raised inflammatory markers. Hypertension, acute and fulminant myocarditis, ventricular arrhythmias, atrial fibrillation, stress cardiomyopathy, hypotension and heart failure, acute coronary syndrome (ACS) with ST elevation or depression MI with normal coronaries have been reported ( 23 , 27 ). In a Chinese cohort of 138 patients, 16.7% had arrhythmias with risk higher among those needing ICU care with no mention of the type of arrhythmia that was present ( 28 ). Less frequently, cardiac symptoms like chest pain or tightness and palpitation can be the initial presenting features without fever producing a diagnostic dilemma. Some of these patients eventually go on to develop respiratory symptoms as diseases progress ( 29 ). Patients who have recovered from acute illness may develop arrhythmias as a result of myocardial scar and need future monitoring ( 27 ). One important point to note is use of Renin Angiotensin Aldosterone System (RAAS) modulators in patients with COVID-19. Guidelines from ACC/AHA/HFSA recommends continuing them in high risk patient based on goal directed therapy approach supported by a recent systematic review and meta-analysis conducted by Hasan et. Al. which demonstrated use of ACEI/ARB in COVID-19 patients is associated with lower odds/ hazards of mortality and development of severe/critical diseases as compared to no use of ACEI/ARB ( 30 , 31 ).
Gastrointestinal
In the initial cohort of patients from China, nausea or vomiting and diarrhea were present in 5 and 3.7% of patients ( 1 ). Review of data from 2,023 patients showed anorexia to be the most frequently occurring gastrointestinal symptom in adults. Diarrhea was the most common presenting gastrointestinal symptom in both adults and children while vomiting was found to be more common in children ( 32 ). Other rare symptoms included nausea, abdominal pain, and gastrointestinal bleeding. There have been few instances where COVID-19 patients presented with only gastrointestinal symptoms without the development of fever or respiratory symptoms at the onset and during disease progression ( 33 ). In a smaller cohort of 204 patients, 50.5% had some form of intestinal symptoms and of those, 5.8% had only intestinal symptoms while the remaining patients developed respiratory symptoms subsequently. The most common symptoms reported among them was anorexia (78.64%), non-dehydrating diarrhea (34%), vomiting (3.9%), and abdominal pain (1.94%) ( 34 ). In addition, those with GI symptoms tend to have a longer interval between symptom onset and hospital admission (9 vs. 7.3 days) possibly due to lack of clinical suspicion and delay in diagnosis. Patients with gastrointestinal symptoms tend to have higher elevation in AST and ALT indicating coexistent liver injury ( 34 ). The mechanism behind GI illness is not clearly known but could be due to direct invasion of virus via ACE2 receptor in the intestinal mucosa. This can be supported by the fact that viral RNA can be detected in stool samples of COVID-19 patients which may also hint toward possible fecal-oral transmission ( 35 ). Liver dysfunction is likely secondary to the use of hepatotoxic drugs, hypoxia induced liver injury, systemic inflammation, and multi organ failure ( 36 ).
Renal manifestation in patients with COVID-19 can occur due to direct invasion of podocytes and proximal tubular cells by SARS-CoV-2 virus, secondary endothelial dysfunction causing effacement of foot process with vacuolation and detachment of podocytes, and acute proximal tubular dysfunction ( 37 ). Furthermore, hypoxia, cytokine storm, rhabdomyolysis, nephrotoxic drugs, and overlying infections can all exacerbate renal injury ( 38 ). Based on initial reports, prevalence of Acute Kidney Injury (AKI) among COVID-19 hospitalized patients range from 0.5 to 29%. In a cohort of 701 patients, proteinuria (43.9%), hematuria (26.7%), elevated creatinine (14.4%), elevated blood urea nitrogen (13.1%), and low glomerular filtration rate (≤ 60 ml/min/1.73 m 2 ) (13.1%) were present at the time of hospital admission with 5.1% developing AKI during the illness. AKI was more prevalent among those with baseline renal impairment ( 39 ). In another large cohort of 5,449 patients, 36.6% had AKI with prevalence higher among mechanically ventilated patients compared to non-ventilated patients (89.7 vs. 21.7%) ( 40 ). Patients developing renal impairment are prone to have higher mortality within the hospital. Another point to highlight is the presentation of COVID-19 in renal transplant recipients. Due to immunosuppression, these patients are likely to have low fever at presentation with swift clinical decline and requirement for mechanical ventilation with high mortality as compared to the general population ( 41 ).
Neurological
Most patients with COVID-19 develop neurological symptoms along with respiratory symptoms during the course of illness; however, several case reports in review of literature document patient presentation of neurological dysfunction without typical symptoms of fever, cough, and difficulty breathing ( 42 ). There is a 2.5-fold enhanced risk of severe illness and increased death in patients with a history of previous stroke with similar findings among those with Parkinson's diseases. The prevalence of neurological features ranges from 6 to 36% along with hypoxic ischemic encephalopathy up to 20% in some series of patients ( 43 ). Neurological symptoms tend to occur early in the course of illness (median 1–2 days) with most common neurological features being headache, confusion, delirium, anosmia or hyposmia, dysgeusia or ageusia, altered mental status, ataxia, and seizures ( 44 ). Among patients admitted with COVID-19, the prevalence of ischemic stroke ranges from 2.5 to 5% despite receiving prophylaxis for venous thromboembolism. Patients prone to have established cardiovascular risk factors are likely to have a more severe diseases ( 43 ). Other presentations include viral encephalitis, acute necrotizing encephalopathy (ANE), infectious toxic encephalopathy, meningitis, Guillain Barre Syndrome (GBS), Miller Fisher syndrome, and polyneuritis cranialis with GBS being the first feature of COVID-19 in few cases ( 42 , 43 , 45 ). In COVID-19 patients, CNS features are possibly due to direct invasion of neurons and glial cells by SARS-CoV-2 as well as by endothelial dysfunction of blood brain barrier (BBB). Virus can gain access to CNS via hematogenous spread or retrograde movement across the olfactory bulb. The virus can be detected in CSF by RT-PCR and on brain parenchyma during autopsy. The fact that most patients develop anosmia or hyposmia during illness support this theory ( 45 ). After entry, the virus can cause reactive gliosis with activation of the inflammatory cascade. The combination of systemic inflammation, cytokine storm, and coagulation dysfunction can impair BBB function and alter brain equilibrium causing neuronal death ( 42 ).
Ocular manifestations can vary from conjunctival injection to frank conjunctivitis. In a Chinese cohort of 38 patients, 31.6% had ocular symptoms consisting primarily of conjunctivitis while conjunctival hyperemia, foreign body sensation in eye, chemosis, tearing or epiphora were more common among severe COVID-19 patients. Among them SARS-CoV-2 can be demonstrated in conjunctival as well as nasopharyngeal swab in 5.2% of patients, indicating a potential route for viral transmission ( 46 ). Conjunctivitis or tearing can be the initial presenting symptoms of COVID-19. Despite this fact, there is no documented case of severe ocular features relating to COVID-19.
Similar to other viral infections, SARS-CoV-2 can also produce varied dermatological features. A study of 88 patients from Italy showed that about 20.4% had some form of skin manifestations with 44.4% developing features at onset and duration of the disease progression ( 47 ). Maculopapular exanthem (36.1%) was identified as most common dermatological features followed by papulovesicular rash (34.7%), painful acral red purple papules (15.3%), urticaria (9.7%), livedo reticularis (2.8%), and petechiae (1.4%) ( 48 ). A study of 375 COVID-19 cases in Spain identified five different patterns of cutaneous manifestations in patients: acral areas of erythema with vesicles or pustules (pseudo-chilblain) (19%), other vesicular eruptions (9%), urticarial lesions (19%), maculopapular eruptions (47%), and livedo or necrosis (6%) ( 49 ). Majority of patients had lesions on the trunk with some experiencing lesions on hands and feet. There are case reports of COVID-19 associated with erythema multiforme and Kawasaki Disease in children ( 50 , 51 ). Pathogenesis behind skin involvement remains unclear with some features explained by small vessel vasculitis, thrombotic events like DIC, hyaline thrombus formation, acral ischemia, or the direct effect of the virus like other viral illnesses ( 52 ).
Musculoskeletal
The initial report from China revealed 14.8% of patients had myalgia or arthralgia among 55,924 COVID-19 patients. A review article reports that of 12,046 patients, fatigue was identified in 25.6% and myalgia and/or arthralgia in 15.5% with most patients reporting symptoms from the start of illness ( 53 ). There are reports suggesting myositis and rhabdomyolysis with markedly elevated creatinine kinase can occur during COVID-19 illness especially in patients with severe diseases and multi organ failure. Additionally, in some patients, rhabdomyolysis has been documented as the initial presentation of COVID-19 illness without typical respiratory symptoms ( 54 , 55 ). A case series of four patients developing acute arthritis during hospital admission for COVID-19 has been reported with exacerbation of crystal arthropathy (gout and calcium pyrophosphate diseases) but negative for SARS-CoV-2 RT-PCR in synovial fluid ( 56 ). Treatment with steroids and colchicine was used in all four cases. An important consideration to note was that all four patients developed arthritis despite previous treatment with immunomodulatory therapy (hydroxychloroquine, tocilizumab, and pulse methylprednisolone).
Hematological
As stated, COVID-19 is a systemic disease inducing systemic inflammation and occasionally cytokine storm. This can significantly impact the process of hematopoiesis and hemostasis. During early disease, normal or decreased leukocyte and lymphocyte counts were documented with marked lymphopenia as the diseases progressed, especially in those with cytokine storms and severe disease. In a study of 1,099 patients, lymphopenia, thrombocytopenia, and leukopenia were present in 83.2, 36.2, and 33.7%, respectively, with findings more marked in those with severe diseases ( 18 ). Leukocytosis in COVID-19 patients might suggest a bacterial infection or a superinfection with leukocytosis found more commonly in severe cases (11.4%) as compared to mild and moderate cases (4.8%) ( 18 ). Similarly, thrombocytopenia has been found to be more common (57.7%) in severe cases in contrast to mild and moderate cases (31.6%) ( 18 ). Lymphopenia was also linked with an increased necessity for ICU admission and the risk of ARDS. Thrombocytosis with elevated platelet to lymphocyte ratio may indicate a more marked cytokine storm ( 57 ).
Also, coagulation abnormality can manifest in the form of thrombocytopenia, prolonged prothrombin time (PT), low serum fibrinogen level, and raised D-dimer suggesting Disseminated Intravascular Coagulation (DIC) with these changes more marked in those with severe diseases ( 58 ). Raised lactate dehydrogenase (LDH) and serum ferritin were also present and correlated with the degree of systemic inflammation. In a study of 426 COVID-19 patients, C-Reactive Protein (CRP) was noted to be increased in 75–93% of patients, more commonly in patients with severe disease. Serum procalcitonin levels might not be altered at admission, but progressive increase in its value can suggest a worsening prognosis. Severe disease is linked to increased ALT, bilirubin, serum urea, creatinine, and lowered serum albumin ( 59 ). A study of 1,426 patients showed that Interleukin-6 (IL-6) were raised more in patients with severe COVID-19 than non-severe COVID-19 with progressive rise indicating an increased risk of mortality. Thus, its levels could be regarded as an important prognostic indicator for the extensive inflammation and cytokine storm in COVID-19 patients ( 60 ). Other plasma cytokines and chemokines like IL1B, IFNγ, IP10, MCP, etc. have also been found to be elevated in patients with COVID-19 both in severe and non-severe diseases. Additionally, GCSF, IP10, IL2, IL7, IL10, MCP1, MIP1A, and TNFα were increased in patients who require ICU admission which indicates that cytokine storm is associated with a severe disease ( 61 ).
Endocrine and Reproductive
From the available literature there is no doubt that diabetes mellitus is an important risk factor for COVID-19 illness and is associated with increased risk of development of severe disease. Additionally, there are case reports of subacute thyroiditis linked to SARS-CoV-2 infection ( 62 , 63 ). Based on the statement released from European Society of Endocrinology, patients with primary adrenal failure and congenital adrenal hyperplasia may have theoretically increased susceptibility to infection with higher risk of complications and ultimately mortality but there is no current evidence to support this ( 64 ). The dose of steroids may need to be doubled if there is a clinical suspicion of infection in these patients.
Several claims have been made regarding the impact of COVID-19 on male reproductive function, hypothesizing that COVID-19 can cause potential testicular damage either by binding directly to testicular ACE2 receptors, which are highly expressed in the testicles or by damaging the testis indirectly by exciting local immune system ( 65 ). A study comparing 81 male COVID-19 patients with 100 age matched healthy adults highlighted the presence of low testosterone levels, high levels of luteinizing hormone (LH), low testosterone/LH ratios, low Follicle stimulating hormone (FSH) to LH ratio, and raised serum prolactin. This may suggest a potential COVID-19 testicular damage affecting the Leydig cells in the testis ( 66 ). COVID-19 infected male patients may have reduced sperm count and decreased motility leading to diminished male fertility for 3 months post-infection ( 67 ).
Clinical Presentation in Specific Population
In children.
A case series of 72,314 cases published by the Chinese Center for Disease Control and Prevention reported that 0.9% of the total patients were between 0 and 9 years of age, and 1.2% of the total patients were between 10 and 19 years of age ( 68 ). The most common symptoms found in children are fever, (59%), cough (46%), few cases (12%) of gastrointestinal symptoms, and some cases (26%) showed no specific symptoms initially with patchy consolidation and ground glass opacities in CT chest findings ( 69 ). Chilblain-like acral eruptions, purpuric, and erythema multiforme-like lesions have been found to be more common in children and young adult patients mainly with asymptomatic or mild disease ( 70 ). Lymphopenia in children is relatively less common which is in direct contrast in cases of SARS in children where lymphopenia was more commonly noted ( 69 ).
Multisystem inflammatory syndrome (MIS) is another feared complication of Covid-19 seen in children. Abrams et al. systematically summarized the clinical evidence of 8 studies reporting MIS in 440 children. The median age of patients ranged from 7.3 to 10 years with 59% of all patients being male. The greatest proportion of patients had gastrointestinal symptoms (87%) followed by mucocutaneous symptoms (73%) and cardiovascular symptoms (71%) while fewer patients reported respiratory (47%), neurologic (22%), and musculoskeletal (21%) symptoms. Ferritin and d-dimer were elevated in 50% of patients, and C-reactive protein, interleukin-6, and fibrinogen were elevated in at least 75% of patients. Additionally, 100% of children with cardiovascular involvement reported elevated cardiac-damage markers such as Troponin. Although respiratory manifestation is most frequently expressed in adults, children with MIS exhibited less pulmonary symptoms and more of the other manifestations ( 71 ).
In Pregnant Women
The most common symptoms reported in pregnant women are fever (61.96%), cough (38.04%), malaise (30.49%), myalgia (21.43%), sore throat (12%), and dyspnea (12.05%). Other symptoms found in pregnant women are diarrhea and nasal congestion ( 72 ). In a systematic review including 92 patients, 67.4% manifested diseases at presentation with 31.7% having negative RT-PCR though they had features of viral pneumonia. Only one patient required admission to intensive care and 0% mortality. Fetal outcomes were reported as: 63.8% preterm delivery, 61.1% fetal distress, 80% Cesarean section delivery, 76.92% neonatal intensive care admission, 42.8% low birth weight, and 66.67% had lymphopenia ( 72 ). There was no evidence of vertical transmission. A study of 41 pregnant women with COVID-19 showed that consolidation was more commonly found in CT of pregnant women in contrast to ground-glass opacities in CT of non-pregnant adults ( 73 ). WHO also recommends encouraging lactating mothers with confirmed or suspected COVID-19 to begin or continue breastfeeding including 24-h rooming in, skin to skin contact, and kangaroo mother care especially in immediate postnatal period ( 74 ). On July 14th, 2020, Vivanti et al. published the first case of transplacental transmission of COVID-19 from a 23-years-old pregnant woman to her baby ( 75 ). Thereafter, more studies reported the possibility of the vertical transmission of COVID-19. In this context, Kotlayer et al. published a systematic review of 38 studies. Out of 936 neonates from COVID-19 mothers, 27 tested positive for the virus indicating a pooled proportion of 3.2% (2.2–4.3) for vertical transmission ( 7 ).
In Immuno-Compromised Population
Due to their impaired immune response, it is not surprising that immunocompromised patients with COVID-19 infection might be at greater risk of developing severe forms of the disease and co-infections in comparison to normal populations. Nevertheless, recent studies showed the association between cytokine storm syndrome and the overreaction of the immune system with COVID-19 raising the possibility that immunodeficient states might alleviate the overexpression of the host immune system and thereby prevent deadly forms of the disease ( 76 ). After the RECOVERY trial ( 77 ) that showed the efficacy of dexamethasone in lowering the mortality in severe forms of the disease, many questions were raised regarding whether immunocompromised patients have a greater or lower risk of developing severe forms of the disease. In order to address these questions, Minotti et al. recently published a systematic review that included 16 studies with 110 patients presenting mostly with cancer along with transplantation and immunodeficiency. Out of the 110 patients, 72 (65.5%) recovered without being admitted to the intensive care unit while 23 (20.9%) died ( 76 ). The authors concluded that immunosuppression in both children and adults seem to have a better disease course in comparison to normal population. One of the limitations of this study is that the conclusion was made only based on qualitative synthesis and no meta-analysis was performed. On the other hand, Gao et al. performed a meta-analysis on 8 relevant studies with 4,007 patients. The study showed that immunosuppression and immunodeficiency were associated with non-statistically significant increased risk of severe COVID-19 disease ( 78 ). Additionally, Mirzaei et al. summarized the clinical evidence of 252 HIV positive patients co-infected with COVID-19. The clinical manifestation did not differ from that of the general population. However, out of the 252 patients, 204 (80.9%) were male. Low CD4 count (<200 cells/mm 3 ) were reported for 23 of 176 patients (13.1%). COVID-19 symptoms were present in 223 patients with the most common symptoms of fever in 165 (74.0%) patients, cough in 130 (58.3%), headache in 44 (19.7%), arthralgia and myalgia in 33 (14.8%), gastrointestinal symptoms in 29 (13.0%) followed by sore throat in 18 (8.1%) patients ( 79 ). The number of deaths accounted for 36 (14.3%). Similar to the general population, immunocompromised, and HIV patients were no different in terms of clinical manifestation or severity. However, the results from these studies should be interpreted with caution and more studies are recommended to establish the link between this particular group of patients with severity of the disease.
Multisystem Involvement in COVID-19
As evident from the discussion above, SARS-CoV-2 can affect multiple organ systems and produce a wide array of clinical presentation of COVID-19. Certain studies conducted in Europe and United States have shown that COVID-19 can also have a multi-systemic presentation in individuals in form of a multi-system inflammatory syndrome (MIS) which has been found in both children and adults and is known as MIS-C and MIS-A, respectively ( 80 – 83 ).
According to a recent CDC report about MIS-A, it was found that only half of the patients with MIS-A had preceding respiratory symptoms of COVID-19 ~2–5 weeks before ( 80 ). The most common clinical signs and symptoms included fever, chest pain, palpitations, diarrhea, abdominal pain, vomiting, skin rash, etc. Nearly all patients had electro-cardiological abnormalities like arrythmias, elevated troponin levels, and electrocardiography evidence of left or right ventricular dysfunction. Even though most patients had minimal respiratory symptoms, chest imaging had features of ground glass opacity and pleural effusion. All patients had signs of elevated laboratory markers of inflammation, coagulation markers, and lymphopenia ( 80 ).
MIS-C can clinically mimic Kawasaki Disease ( 81 ). By the end of July, about 570 cases of MIS-C with COVID-19 were found in the United States ( 81 ). In MIS-C, there is involvement of at least four organ systems, most commonly the gastrointestinal system followed by cardiovascular and dermatological systems ( 81 ). Prominent signs and symptoms found in children with MIS-C were abdominal pain, vomiting, skin rash, diarrhea, hypotension, and conjunctival injection. The majority of the children needed ICU admission due to the development of severe complications including cardiac dysfunction, shock, myocarditis, coronary artery aneurysm, and acute kidney injury ( 81 ).
Association Between Clinical Presentations, COVID-19 Severity and Prognosis
Evaluation of 55,924 laboratory confirmed COVID-19 cases in China, the presence of dyspnea, respiratory rate ≥ 30/min, blood saturation levels ≤ 93%, PaO2/FiO2 ratio ≤ 300, lung infiltrates ≥ 50% of the lung fields between 12 and 48 h were associated with severe COVID-19 infection ( 1 ). Clinical signs suggestive of respiratory failure, septic shock, or multiple organ dysfunction/failure were associated with critical disease and poor prognosis ( 1 ). Individuals at highest risk of severe disease and deaths were patients with age > 80 years and associated co-morbidities such as underlying cardiovascular disease, diabetes, hypertension, chronic respiratory disease, and cancer ( 1 ). Another study done with 418 patients in Catalonia (Spain) showed that dyspnea was an important predictor of severe disease while confusion was an important predictor of death, and the presence of cough was strongly associated with good prognosis ( 84 ). Advanced age, male sex, and obesity were independent markers of poor prognosis while eosinophilia was a marker of less severe disease ( 84 ). The mortality was lower in patients with symptoms of diarrhea, arthromyalgia, headache, and loss of smell and taste sensations while low oxygen saturation, high CRP levels, and higher number of lung quadrants affected on Xray were found to be associated with severe disease and death ( 84 ).
COVID-19 is a viral illness which can cause multi-systemic manifestations. Review of existing literature concludes that SARS-CoV-2 can affect any organ system either directly or indirectly leading to a myriad of clinical presentation. The most commonly affected system is the respiratory system with presenting symptoms of fever, cough, and shortness of breath, etc. Other systems which can be affected in COVID-19 include ENT (sore throat, loss of taste, smell, and sensations, and rhinorrhea), cardiovascular system (chest pain, chest tightness, palpitations, and arrhythmias), gastrointestinal system (anorexia, diarrhea, vomiting, nausea, and abdominal pain), renal (proteinuria, hematuria, and acute kidney injury), neurological (headache, confusion, delirium, and altered mental status), ocular (conjunctival hyperemia, foreign body sensation in the eye, chemosis, and tearing), cutaneous (rash, papules, and urticaria), musculoskeletal system (myalgia and arthralgia), hematological (lymphopenia, thrombocytopenia, leukopenia, elevated inflammatory markers, and elevated coagulation markers), endocrine (low testosterone, low FSH, and high LH) and reproductive system (decreased sperm count and decreased sperm motility). Clinical presentation in specific populations like children, pregnant women, and immunocompromised people may vary which emphasizes the importance of further investigation in order to avoid late diagnosis of COVID-19. Severe multi-systemic involvement in COVID-19 in the form of MIS-C and MIS-A can cause significant morbidity and mortality if undiagnosed. The clinical presentations of respiratory failure, acute kidney injury, septic shock, cardiovascular arrest is associated with severe COVID-19 disease and can result in poor prognosis. In the light of exponentially growing pandemic, every patient presenting to hospital must be tested for SARS-CoV-2 by RT-PCR if resources are available to detect early presentations of diseases even if the features are atypical. Understanding of the various clinical presentations of COVID-19 will help the clinicians in early detection, treatment, and isolation of patients in order to contain the virus and slow down the pandemic.
Author Contributions
All authors have contributed equally to the work, and all agreed to be accountable for the content of the work.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Ms. Sairah Zia (American University of Caribbean, School of Medicine, Sint Maarten), a native speaker of English, for proofreading the manuscript.
Abbreviations
ACC/AHA/HFSA, American College of Cardiology/American Heart Association/Heart Failure Society of America; IL1B, Interleukin 1B; IFNγ, Interferon Gamma; IP10, Interferon-inducible Protein 10; MCP1, Monocyte Chemoattractant Protein 1; GCSF, Granulocyte Colony Stimulating Factor; IL2, Interleukin 2; IL7, Interleukin 7; IL10, Interleukin 10; MIP1A, Macrophage Inflammatory Protein-1 alpha; TNFα, Tumor Necrosis Factor alpha.
1. Who-China-Joint-Mission-on-Covid-19-Final-Report.pdf . Available online at: https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf (accessed June 1, 2020).
2. Jin Y, Yang H, Ji W, Wu W, Chen S, Zhang W, et al. Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses. (2020) 12:372. doi: 10.3390/v12040372
CrossRef Full Text | Google Scholar
3. Naming the Coronavirus Disease (COVID-19) and the Virus That Causes it . (2020). Available online at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it (accessed June 7, 2020).
Google Scholar
4. Harapan H, Itoh N, Yufika A, Winardi W, Keam S, Te H, et al. Coronavirus disease 2019 (COVID-19): a literature review. J Infect Public Health. (2020) 13:667–73. doi: 10.1016/j.jiph.2020.03.019
PubMed Abstract | CrossRef Full Text | Google Scholar
5. WHO Director-General's opening remarks at the media briefing on COVID-19 . (2020). Available online at: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19-−11-march-2020 (accessed June 7, 2020).
6. Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. (2020) 109:102433. doi: 10.1016/j.jaut.2020.102433
7. Kotlyar AM, Grechukhina O, Chen A, Popkhadze S, Grimshaw A, Tal O, et al. Vertical transmission of coronavirus disease 2019: a systematic review and meta-analysis. Am J Obstet Gynecol. (2020). doi: 10.1016/j.ajog.2020.07.049. [Epub ahead of print].
8. Tian S, Hu N, Lou J, Chen K, Kang X, Xiang Z, et al. Characteristics of COVID-19 infection in Beijing. J Infect. (2020) 80:401–6. doi: 10.1016/j.jinf.2020.02.018
9. Cave E. COVID-19 super-spreaders: definitional quandaries and implications. Asian Bioeth Rev. (2020) 12:235–42. doi: 10.1007/s41649-020-00118-2
10. Kakodkar P, Kaka N, Baig M. A comprehensive literature review on the clinical presentation, and management of the pandemic coronavirus disease 2019 (COVID-19). Cureus. (2020) 12:e7560. doi: 10.7759/cureus.7560
11. Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. (2020) 20:363–74. doi: 10.1038/s41577-020-0311-8
PubMed Abstract | CrossRef Full Text
12. Veerdonk F, van de Netea MG, Deuren M, van Meer JWM, van der Mast Q, de Bruggemann RJ, et al. Kinins and cytokines in COVID-19: a comprehensive pathophysiological approach. eLife. (2020) 9:e57555. doi: 10.7554/eLife.57555
13. Ye Q, Wang B, Mao J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J Infect. (2020) 80:607–13. doi: 10.1016/j.jinf.2020.03.037
14. Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. (2020) 220, 1–13. doi: 10.1016/j.trsl.2020.04.007
15. Lin L, Lu L, Cao W, Li T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection–a review of immune changes in patients with viral pneumonia. Emerg Microbes Infect. (2020) 9:727–32. doi: 10.1080/22221751.2020.1746199
16. Ottestad W, Seim M, Mæhlen JO. COVID-19 with silent hypoxemia. Tidsskr Den NorLegeforening. (2020) 140. doi: 10.4045/tidsskr.20.0299
17. Couzin-Frankel J. The mystery of the pandemic's “happy hypoxia.” Science . (2020) 368:455–6. doi: 10.1126/science.368.6490.455
18. Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. (2020) 382:1708–20. doi: 10.1056/NEJMoa2002032
19. Ye Z, Zhang Y, Wang Y, Huang Z, Song B. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): a pictorial review. Eur Radiol. (2020) 30:4381–9. doi: 10.1007/s00330-020-06801-0
20. Tong JY, Wong A, Zhu D, Fastenberg JH, Tham T. The prevalence of olfactory and gustatory dysfunction in COVID-19 patients: a systematic review and meta-analysis. Otolaryngol Neck Surg. (2020) 163:3–11. doi: 10.1177/0194599820926473
CrossRef Full Text
21. Krajewska J, Krajewski W, Zub K, Zatoński T. COVID-19 in otolaryngologist practice: a review of current knowledge. Eur Arch Otorhinolaryngol. (2020) 277:1885–97. doi: 10.1007/s00405-020-05968-y
22. Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. (2020) 12:8. doi: 10.1038/s41368-020-0074-x
23. Akhmerov A, Marbán E. COVID-19 and the Heart. Circ Res. (2020) 126:1443–55. doi: 10.1161/CIRCRESAHA.120.317055
24. Clerkin Kevin J, Fried Justin A, Raikhelkar J, Sayer G, Griffin JM, Masoumi A, et al. COVID-19 and cardiovascular disease. Circulation. (2020) 141:1648–55. doi: 10.1161/CIRCULATIONAHA.120.046941
25. Bansal M. Cardiovascular disease and COVID-19. Diabetes Metab Syndr Clin Res Rev. (2020) 14:247–50. doi: 10.1016/j.dsx.2020.03.013
26. Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. (2020) 5:802–10. doi: 10.1001/jamacardio.2020.0950
27. Kochi AN, Tagliari AP, Forleo GB, Fassini GM, Tondo C. Cardiac and arrhythmic complications in patients with COVID-19. J Cardiovasc Electrophysiol. (2020) 31:1003–8. doi: 10.1111/jce.14479
28. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. (2020) 323:1061–9. doi: 10.1001/jama.2020.1585
29. Zheng Y-Y, Ma Y-T, Zhang J-Y, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. (2020) 17:259–60. doi: 10.1038/s41569-020-0360-5
30. Patients Taking ACE-i and ARBs who Contract COVID-19 Should Continue Treatment Unless Otherwise Advised by Their Physician . American Heart Association (2020). Available online at: https://newsroom.heart.org/news/patients-taking-ace-i-and-arbs-who-contract-covid-19-should-continue-treatment-unless-otherwise-advised-by-their-physician (accessed June 27, 2020).
31. Hasan SS, Kow CS, Hadi MA, Zaidi STR, Merchant HA. Mortality and disease severity among COVID-19 patients receiving renin-angiotensin system inhibitors: a systematic review and meta-analysis. Am J Cardiovasc Drugs. (2020). doi: 10.22541/au.158880148.84250526. [Epub ahead of print].
32. Tian Y, Rong L, Nian W, He Y. Review article: gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment Pharmacol Ther. (2020) 51:843–51. doi: 10.1111/apt.15731
33. An P, Chen H, Jiang X, Su J, Xiao Y, Ding Y, et al. Clinical Features of 2019 Novel Coronavirus Pneumonia Presented Gastrointestinal Symptoms But Without Fever Onset . Rochester, NY: Social Science Research Network (2020). Available online at: https://papers.ssrn.com/abstract=3532530 (accessed June 27, 2020). doi: 10.2139/ssrn.3532530
34. Pan L, Mu M, Yang P, Sun Y, Wang R, Yan J, et al. Clinical characteristics of covid-19 patients with digestive symptoms in hubei, china: a descriptive, cross-sectional, multicenter study. Am J Gastroenterol. (2020) 115, 766–73. doi: 10.14309/ajg.0000000000000620
35. Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. (2020) 158:1831–3.e3. doi: 10.1053/j.gastro.2020.02.055
36. Feng G, Zheng KI, Yan Q-Q, Rios RS, Targher G, Byrne CD, et al. COVID-19 and liver dysfunction: current insights and emergent therapeutic strategies. J Clin Transl Hepatol. (2020) 8:18–24. doi: 10.14218/JCTH.2020.00018
37. Su H, Yang M, Wan C, Yi L-X, Tang F, Zhu H-Y, et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. (2020) 98:219–27. doi: 10.1016/j.kint.2020.04.003
38. Ronco C, Reis T. Kidney involvement in COVID-19 and rationale for extracorporeal therapies. Nat Rev Nephrol. (2020) 16:308–10. doi: 10.1038/s41581-020-0284-7
39. Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. (2020) 97:829–38. doi: 10.1016/j.kint.2020.03.005
40. Hirsch JS, Ng JH, Ross DW, Sharma P, Shah HH, Barnett RL, et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. (2020) 98:209–18. doi: 10.1016/j.kint.2020.05.006
41. Akalin E, Azzi Y, Bartash R, Seethamraju H, Parides M, Hemmige V, et al. Covid-19 and kidney transplantation. N Engl J Med. (2020) 382:2475–7. doi: 10.1056/NEJMc2011117
42. Sheraton M, Deo N, Kashyap R, Surani S. A review of neurological complications of COVID-19. Cureus. (2020) 12:e8192. doi: 10.7759/cureus.8192
43. Bridwell R, Long B, Gottlieb M. Neurologic complications of COVID-19. Am J Emerg Med. (2020) 38:1549.e3–7. doi: 10.1016/j.ajem.2020.05.024
44. Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. (2020) 77:683–90. doi: 10.1001/jamaneurol.2020.1127
45. Ghannam M, Alshaer Q, Al-Chalabi M, Zakarna L, Robertson J, Manousakis G. Neurological involvement of coronavirus disease 2019: a systematic review. J Neurol. (2020). doi: 10.21203/rs.3.rs-31183/v1. [Epub ahead of print].
46. Dockery DM, Rowe SG, Murphy MA, Krzystolik MG. The ocular manifestations and transmission of COVID-19; recommendations for prevention. J Emerg Med. (2020) 59:137–40. doi: 10.1016/j.jemermed.2020.04.060
47. Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. (2020) 34:e212–3. doi: 10.1111/jdv.16387
48. Sachdeva M, Gianotti R, Shah M, Lucia B, Tosi D, Veraldi S, et al. Cutaneous manifestations of COVID-19: Report of three cases and a review of literature. J Dermatol Sci. (2020) 98:75–81. doi: 10.1016/j.jdermsci.2020.04.011
49. Casas CG, Català A, Hernández GC, Rodríguez-Jiménez P, Fernández-Nieto D, Lario AR-V, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. (2020) 183:71–7. doi: 10.1111/bjd.19163
50. Jones VG, Mills M, Suarez D, Hogan CA, Yeh D, Segal JB, et al. COVID-19 and kawasaki disease: novel virus and novel case. Hosp Pediatr. (2020) 10:537–40. doi: 10.1542/hpeds.2020-0123
51. Labé P, Ly A, Sin C, Nasser M, Chapelon-Fromont E, Saïd PB, et al. Erythema multiforme and Kawasaki disease associated with COVID-19 infection in children. J Eur Acad Dermatol Venereol. (2020) 34:e539–41. doi: 10.1111/jdv.16666
52. Tang K, Wang Y, Zhang H, Zheng Q, Fang R, Sun Q. Cutaneous manifestations of the Coronavirus Disease 2019 (COVID-19): a brief review. Dermatol Ther. (2020) 33:e13528. doi: 10.1111/dth.13528
53. Cipollaro L, Giordano L, Padulo J, Oliva F, Maffulli N. Musculoskeletal symptoms in SARS-CoV-2 (COVID-19) patients. J Orthop Surg. (2020) 15:178. doi: 10.1186/s13018-020-01702-w
54. Suwanwongse K, Shabarek N. Rhabdomyolysis as a presentation of 2019 novel coronavirus disease. Cureus. (2020) 12:e7561. doi: 10.7759/cureus.7561
55. Chan KH, Farouji I, Hanoud AA, Slim J. Weakness and elevated creatinine kinase as the initial presentation of coronavirus disease 2019 (COVID-19). Am J Emerg Med. (2020) 38:1548.e1–3. doi: 10.1016/j.ajem.2020.05.015
56. López-González M-C, Peral-Garrido ML, Calabuig I, Tovar-Sugrañes E, Jovani V, Bernabeu P, et al. Case series of acute arthritis during COVID-19 admission. Ann Rheum Dis. (2020) doi: 10.1136/annrheumdis-2020-217914. [Epub ahead of print].
57. Qu R, Ling Y, Zhang Y, Wei L, Chen X, Li X, et al. Platelet-to-lymphocyte ratio is associated with prognosis in patients with coronavirus disease-19. J Med Virol. (2020) 92:1533–41. doi: 10.1002/jmv.25767
58. Levi M, Thachil J, Iba T, Levy JH. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. (2020) 7:e438–40. doi: 10.1016/S2352-3026(20)30145-9
59. Lippi G, Plebani M. Laboratory abnormalities in patients with COVID-2019 infection. Clin Chem Lab Med CCLM. (2020) 58:1131–4. doi: 10.1515/cclm-2020-0198
60. Aziz M, Fatima R, Assaly R. Elevated interleukin-6 and severe COVID-19: a meta-analysis. J Med Virol. (2020) 92:2283–5. doi: 10.1002/jmv.25948
61. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet Lond Engl. (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5
62. Brancatella A, Ricci D, Viola N, Sgrò D, Santini F, Latrofa F. Subacute thyroiditis after Sars-COV-2 infection. J Clin Endocrinol Metab. (2020) 105:dgaa276. doi: 10.1210/clinem/dgaa276
63. AsfurogluKalkan E, Ates I. A case of subacute thyroiditis associated with Covid-19 infection. J Endocrinol Invest. (2020) 43:1173–4. doi: 10.1007/s40618-020-01316-3
64. Puig-Domingo M, Marazuela M, Giustina A. COVID-19 and endocrine diseases a statement from the European society of endocrinology. Endocrine. (2020) 68:2–5. doi: 10.1007/s12020-020-02294-5
65. Illiano E, Trama F, Costantini E. Could COVID-19 have an impact on male fertility? Andrologia. (2020) 52:e13654. doi: 10.1111/and.13654
66. Ma L, Xie W, Li D, Shi L, Mao Y, Xiong Y, et al. Effect of SARS-CoV-2 infection upon male gonadal function: a single center-based study. medRxiv [Preprint]. (2020). doi: 10.1101/2020.03.21.20037267
67. Segars J, Katler Q, McQueen DB, Kotlyar A, Glenn T, Knight Z, et al. Prior and novel coronaviruses, Coronavirus disease 2019 (COVID-19), and human reproduction: what is known? FertilSteril. (2020) 113:1140–9. doi: 10.1016/j.fertnstert.2020.04.025
68. CDC Weekly C, The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) — China, 2020. China CDC Wkly . (2020) 2:113–22. doi: 10.46234/ccdcw2020.032
69. Chang T-H, Wu J-L, Chang L-Y. Clinical characteristics and diagnostic challenges of pediatric COVID-19: a systematic review and meta-analysis. J Formos Med Assoc. (2020) 119:982–9. doi: 10.1016/j.jfma.2020.04.007
70. Wollina U, Karadag AS, Rowland-Payne C, Chiriac A, Lotti T. Cutaneous signs in COVID-19 patients: a review. Dermatol Ther. (2020) 33:e13549. doi: 10.1111/dth.13549
71. Abrams JY, Godfred-Cato SE, Oster ME, Chow EJ, Koumans EH, Bryant B, et al. Multisystem inflammatory syndrome in children associated with severe acute respiratory syndrome coronavirus 2: a systematic review. J Pediatr. (2020) 226:45–54.e1. doi: 10.1016/j.jpeds.2020.08.003
72. Smith V, Seo D, Warty R, Payne O, Salih M, Chin KL, et al. Maternal and neonatal outcomes associated with COVID-19 infection: a systematic review. PLoS ONE. (2020) 15:e0234187. doi: 10.1371/journal.pone.0234187
73. Liu H, Liu F, Li J, Zhang T, Wang D, Lan W. Clinical and CT imaging features of the COVID-19 pneumonia: Focus on pregnant women and children. J Infect. (2020) 80:e7–13. doi: 10.1016/j.jinf.2020.03.007
74. Breastfeeding and COVID-19 . (2020). Available online at: https://www.who.int/news-room/commentaries/detail/breastfeeding-and-covid-19 (accessed Jun 25, 2020).
75. Vivanti AJ, Vauloup-Fellous C, Prevot S, Zupan V, Suffee C, Do Cao J, et al. Transplacental transmission of SARS-CoV-2 infection. Nat Commun. (2020) 11:3572. doi: 10.1038/s41467-020-17436-6
76. Minotti C, Tirelli F, Barbieri E, Giaquinto C, Donà D. How is immunosuppressive status affecting children and adults in SARS-CoV-2 infection? a systematic review. J Infect. (2020) 81:e61–6. doi: 10.1016/j.jinf.2020.04.026
77. Europe PMC . (2020). Available online at: https://europepmc.org/articles/pmc7383595/bin/nejmoa2021436_appendix.pdf (accessed November 20, 2020).
78. Gao Y, Chen Y, Liu M, Shi S, Tian J. Impacts of immunosuppression and immunodeficiency on COVID-19: a systematic review and meta-analysis. J Infect. (2020) 81:e93–5. doi: 10.1016/j.jinf.2020.05.017
79. Mirzaei H, McFarland W, Karamouzian M, Sharifi H. COVID-19 among people living with HIV: a systematic review. AIDS Behav. (2020) 1–8. doi: 10.1007/s10461-020-02983-2. [Epub ahead of print].
80. Morris SB, Schwartz NG, Patel P, Abbo L, Beauchamps L, Balan S, et al. Case series of multisystem inflammatory syndrome in adults associated with SARS-CoV-2 infection — United Kingdom and United States, March–August 2020. Morb Mortal Wkly Rep. (2020) 69:1450–6. doi: 10.15585/mmwr.mm6940e1
81. Godfred-Cato S, Bryant B, Leung J, Oster ME, Conklin L, Abrams J, et al. COVID-19–Associated Multisystem Inflammatory Syndrome in Children — United States, March–July 2020. Morb Mortal Wkly Rep. (2020) 69:1074–80. doi: 10.15585/mmwr.mm6932e2
82. Belot A, Antona D, Renolleau S, Javouhey E, Hentgen V, Angoulvant F, et al. SARS-CoV-2-related paediatric inflammatory multisystem syndrome, an epidemiological study, France, 1 March to 17 May (2020. Eurosurveillance. (2020) 25:2001010. doi: 10.2807/1560-7917.ES.2020.25.22.2001010
83. Whittaker E, Bamford A, Kenny J, Kaforou M, Jones CE, Shah P, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. (2020) 324:259. doi: 10.1001/jama.2020.10369
84. Rodríguez-Molinero A, Gálvez-Barrón C, Miñarro A, Macho O, López GF, Robles MT, et al. Association between COVID-19 prognosis and disease presentation, comorbidities and chronic treatment of hospitalized patients. PLoS ONE. (2020) 15:e0239571. doi: 10.1371/journal.pone.0239571
Keywords: SARS-CoV-2, Covid-19, symptomatology, clinical presentation, signs and symptoms, clinical features, coronavirus
Citation: Mehta OP, Bhandari P, Raut A, Kacimi SEO and Huy NT (2021) Coronavirus Disease (COVID-19): Comprehensive Review of Clinical Presentation. Front. Public Health 8:582932. doi: 10.3389/fpubh.2020.582932
Received: 13 July 2020; Accepted: 15 December 2020; Published: 15 January 2021.
Reviewed by:
Copyright © 2021 Mehta, Bhandari, Raut, Kacimi and Huy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nguyen Tien Huy, tienhuy@nagasaki-u.ac.jp
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Impact of COVID-19 on the social, economic, environmental and energy domains: Lessons learnt from a global pandemic
Im rizwanul fattah, md asraful alam, abm saiful islam, hwai chyuan ong, sm ashrafur rahman, md alhaz uddin.
- Author information
- Article notes
- Copyright and License information
Corresponding authors.
Received 2020 Jul 26; Revised 2020 Oct 8; Accepted 2020 Oct 12; Issue date 2021 Apr.
Since January 2020 Elsevier has created a COVID-19 resource centre with free information in English and Mandarin on the novel coronavirus COVID-19. The COVID-19 resource centre is hosted on Elsevier Connect, the company's public news and information website. Elsevier hereby grants permission to make all its COVID-19-related research that is available on the COVID-19 resource centre - including this research content - immediately available in PubMed Central and other publicly funded repositories, such as the WHO COVID database with rights for unrestricted research re-use and analyses in any form or by any means with acknowledgement of the original source. These permissions are granted for free by Elsevier for as long as the COVID-19 resource centre remains active.
COVID-19 has heightened human suffering, undermined the economy, turned the lives of billions of people around the globe upside down, and significantly affected the health, economic, environmental and social domains. This study aims to provide a comprehensive analysis of the impact of the COVID-19 outbreak on the ecological domain, the energy sector, society and the economy and investigate the global preventive measures taken to reduce the transmission of COVID-19. This analysis unpacks the key responses to COVID-19, the efficacy of current initiatives, and summarises the lessons learnt as an update on the information available to authorities, business and industry. This review found that a 72-hour delay in the collection and disposal of waste from infected households and quarantine facilities is crucial to controlling the spread of the virus. Broad sector by sector plans for socio-economic growth as well as a robust entrepreneurship-friendly economy is needed for the business to be sustainable at the peak of the pandemic. The socio-economic crisis has reshaped investment in energy and affected the energy sector significantly with most investment activity facing disruption due to mobility restrictions. Delays in energy projects are expected to create uncertainty in the years ahead. This report will benefit governments, leaders, energy firms and customers in addressing a pandemic-like situation in the future.
Keywords: Environmental pollution, Waste generation, Coronavirus vaccine, PM emission, NO 2 emission, Global Pandemic, SARS-CoV-2
1. Introduction
The newly identified infectious coronavirus (SARS-CoV-2) was discovered in Wuhan and has spread rapidly since December 2019 within China and to other countries around the globe ( Zhou et al., 2020 ; Kabir et al., 2020 ). The source of SARS-CoV-2 is still unclear ( Gorbalenya et al., 2020 ). Fig. 1 demonstrates the initial timeline of the development of SARS-CoV-2 ( Yan et al., 2020 ). The COVID-19 pandemic has posed significant challenges to global safety in public health ( Wang et al., 2020 ). On 31 st January 2020, the World Health Organization (WHO), due to growing fears about the rapid spread of coronavirus, announced a global epidemic and on 11 th March, the disease was recognised as a pandemic ( Chowdhury et al., 2021 ). COVID-19 clinical trials indicate that almost all patients admitted to hospital have trouble breathing and pneumonia-like symptoms ( Holshue et al., 2020 ). Clinical diagnosis has identified that COVID-19 (disease caused by SARS-CoV-2) patients have similar indications to other coronavirus affected patients, e.g. Middle East Respiratory Syndrome (MERS) and Severe Acute Respiratory Syndrome (SARS) ( Wang and Su, 2020 ). The initial indication of a COVID-19 infection is coughing, fever, and short breath, and in the later stages, it can damage the kidney, cause pneumonia, and unexpected death ( Mofijur et al., 2020 ). The vulnerability of the elderly (>80 years of age) is high, with a fatality rate of ~22% of cases infected by COVID-19 ( Abdullah et al., 2020 ). The total number of confirmed COVID-19 cases has reached over 33 million as of 29 th September 2020, with more than 213 countries and regions affected by the pandemic ( Worldometer, 2020 ). Over 1,003,569 people have already passed away ( Worldometer, 2020 ) due to COVID-19. Most countries are currently trying to combat the virus spread by screening for COVID-19 in large numbers and maintaining social distancing policies with an emphasis on the health of human beings.
The initial stage development timeline for COVID-19 ( Yan et al., 2020 ).
Fig. 2 shows infections and replication cycle of the coronavirus. In extreme cases, the lungs are the most severely damaged organ of a SARS-CoV-2 infected person (host). The alveoli are porous cup-formed small cavities located in the structure of the lungs where the gas exchange of the breathing process take place. The most common cells on the alveoli are the type II cells.
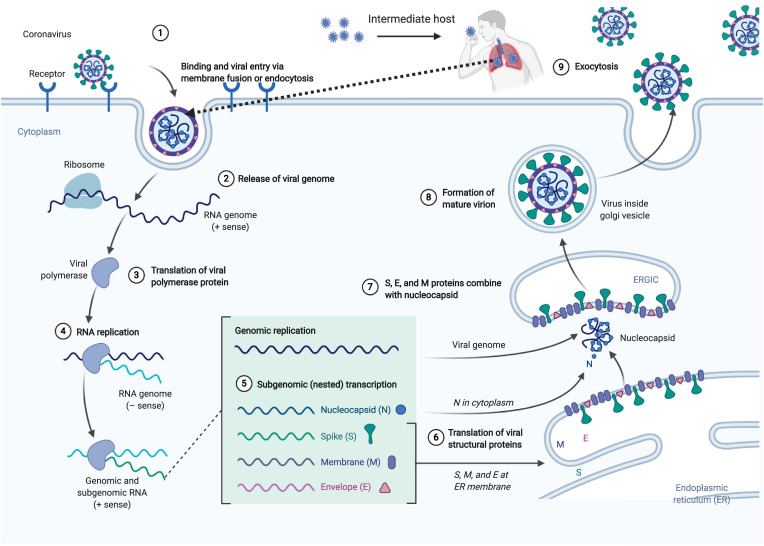
Infections and replication cycle of the coronavirus ( Acter et al., 2020 ).
It has been reported that travel restrictions play a significant role in controlling the initial spread of COVID-19 ( Chinazzi et al., 2020 ; Aldila et al., 2020 ; Beck and Hensher, 2020 ; Bruinen de Bruin et al., 2020 ; de Haas et al., 2020 ). It has been reported that staying at home is most useful in controlling both the initial and last phase of infectious diseases ( de Haas et al., 2020 ; Cohen, 2020 , Pirouz et al., 2020 ). However, since the start of the COVID-19 pandemic, quarantines, entry bans, as well as other limitations have been implemented for citizens in or recent travellers to several countries in the most affected areas ( Sohrabi et al., 2020 ). Also, most of the industries were shutdown to lower mobility. A potential benefit of these measures is the reduction of pollution by the industrial and transportation sector, improving urban sustainability ( Jiang et al., 2021 ). Fig. 3 shows the global responses to lower the impact of the COVID-19 outbreak. There have been negative economic and social implications due to restrictions and decreased travel readiness worldwide ( Leal Filho et al., 2020 ). A fall in the volume of business activity and international events and an increase in online measures could have a long-term impact. The status of global transport and air activity as a result of the COVID-19 pandemic is shown in Fig. 4 ( International Energy Agency (IEA), 2020 ). By March 2020, the average global road haulage activity in regions with lockdowns had declined to almost 50% of the 2019 standard. Air travel has almost completely stopped in certain regions with aviation activity decreasing by over 90% in some European countries. Air activity in China recovered slightly from a low in late February, with lockdown measures somewhat eased. Nevertheless, as lockdowns spread, by the end of Q1 2020, global aviation activity decreased by a staggering 60%.
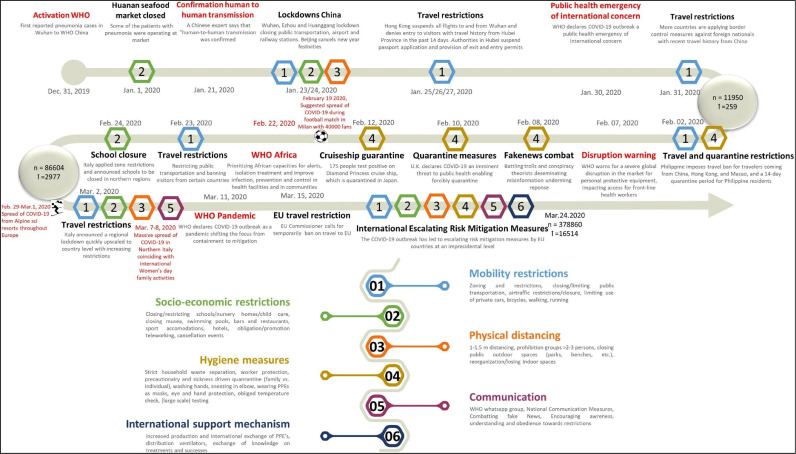
Initial preventive measures to lower the COVID-19 outbreak ( Bruinen de Bruin et al., 2020 ).
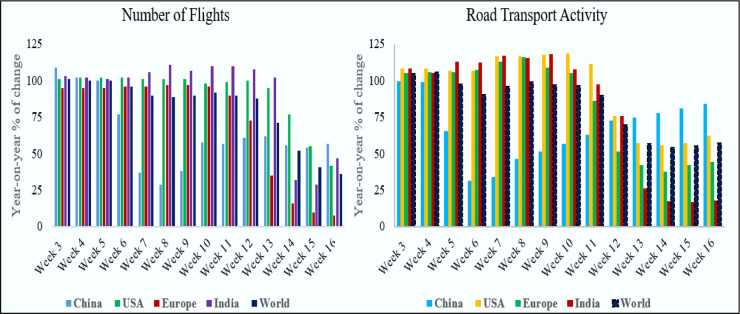
Global transport and aviation activity in the first quarter of the year 2020 ( International Energy Agency (IEA), 2020 ).
The spread of COVID-19 continues to threaten the public health situation severely ( Chinazzi et al., 2020 ) and greatly affect the global economy. Labour displacement, business closures and stock crashes are just some of the impacts of this global lockdown during the pandemic. According to the International Monetary Fund (IMF), the effect of COVID-19 will result in a worldwide economic decline in 2020 and a decline in the economic growth to 3% ( International Monetary Fund (IMF) ). COVID-19 has a detrimental impact on economic growth due to two primary factors. In the beginning, the exponential growth of the global epidemic directly contributed to considerable confusion about instability in the financial and capital markets. Secondly, countries have strictly regulated human movement and transport to monitor the growth of the epidemic and significantly reduced economic activity, putting pressure on both consumer and productive economic activity.
Since the 1970s, the link between economic growth and pollution has been an important global concern. The assessment of energy and financial efficiency is usually connected to environmental pollution research. Green practices at a national level, the inclusion of renewable energy, regulatory pressure and the sustainable use of natural resources are associated with environmental sustainability ( Khan et al., 2020 ). One study has shown that environmental pollution increases with economic growth and vice versa ( Cai et al., 2020 ). The strict control over movement and business activity due to COVID-19 has led to an economic downturn, which is in turn, expected to reduce environmental pollution. This paper systematically assesses how the novel coronavirus has had a global effect on society, the energy sector and the environment. This study presents data compiled from the literature, news sources and reports (from February 2020 to July 2020) on the management steps implemented across the globe to control and reduce the impact of COVID-19. The study will offer guidelines for nations to assess the overall impact of COVID-19 in their countries.
2. Impact of COVID-19 on the environmental domain
2.1. waste generation.
The generation of different types of waste indirectly creates a number of environmental concerns ( Schanes et al., 2018 ). The home isolation and pop-up confinement services in countries that have experienced major impacts of COVID-19 are standard practise, as hospitals are given priority to the most serious cases. In some countries, hotels are being used to isolate travellers for at least two weeks on entry. In several countries, such quarantine measures have resulted in consumers increasing their domestic online shopping activity that has increased domestic waste. In addition, food bought online is packaged, so inorganic waste has also increased. Medical waste has also increased. For instance, Wuhan hospitals produced an average of 240 metric tonnes of medical waste during the outbreak compared to their previous average of fewer than 50 tonnes ( Zambrano-Monserrate et al., 2020 ). This unusual situation poses new and major obstacles in the implementation of waste collection services, thus creating a new challenge for waste collection and recycling groups. With the global adaptation to exponential behavioural and social shifts in the face of COVID-19 challenges, municipal services such as waste collection and management need to alter their operations to play an important role in reducing the spread of infectious diseases.
2.1.1. Lifespan of COVID-19 on different waste media
SARS-CoV-2′s transmission activity has major repercussions for waste services. SARS-CoV-2 attacks host cells with ACE2 proteins directly. ACE2 is a cell membrane-associated enzyme in the lungs, heart and kidneys. When all the resources in the host cell are infected and depleted, the viruses leave the cell in the so-called shedding cycle ( Nghiem et al., 2020 ). Clinical and virological evidence suggests that the elimination of the SARS-CoV-2 virus is most relevant early on, right before and within a couple of days of the onset of the illness ( AEMO, 2020 ). Fomites are known as major vectors for the replication of other infectious viruses during the outbreak ( Park et al., 2015 ). Evidence from SARS-CoV-2 and other coronaviruses show that they remain effective for up to a few days in the atmosphere and on a variety of surfaces ( Fig. 5 ). The survival time of SARS-CoV-2 on hard and plastic surfaces is up to three days indicating that waste materials from COVID-19 patients may contain coronavirus and be a source of infection spread ( Chin et al., 2020 ). During the early stages of this epidemic, updated waste disposal methods to tackle COVID-19 were not implemented on the broader community. The concept of clinical waste essentially also applies to waste from contaminated homes and quarantine facilities. Throughout this pandemic, huge volumes of domestic and hospital waste, particularly plastic waste, has been generated. This has already impeded current efforts to reduce plastic waste and decrease its disposal in the environment. More effort should be made to find alternatives to heavily used plastics.
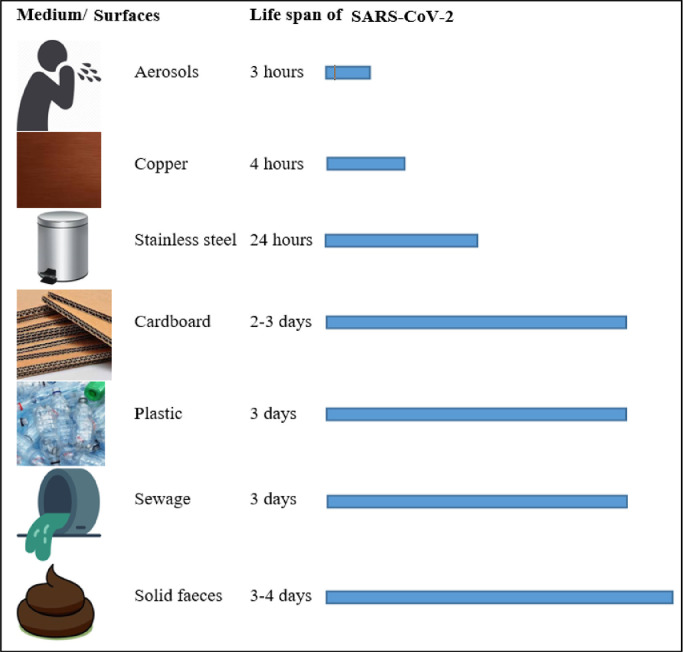
The lifespan of SARS-CoV-2 on different media ( Chin et al., 2020 ; van Doremalen et al.; 2020 ; Ye et al., 2016 )
2.1.2. Waste recycling service
COVID-19 has already had significant effects on waste recycling. Initially, as the outbreak spread and lockdowns were implemented in several countries, both public authorities and municipal waste management officials had to adjust to the situation quickly. Waste disposal has also been a major environmental problem for all technologically advanced nations, as no clear information was available about the retention time of SARS-CoV-2 ( Liu et al., 2020 ). Recycling is a growing and efficient means of pollution control, saving energy and conserving natural resources ( Ma et al., 2019 ). Recycling projects in various cities have been put on hold due to the pandemic, with officials worried about the possibility of COVID-19 spreading to recycling centres. Waste management has been limited in affected European countries. For example, Italy prohibited the sorting of waste by infected citizens. Extensive waste management during the pandemic is incredibly difficult because of the scattered nature of the cases and the individuals affected. The value of implementing best management practises for waste handling and hygiene to minimise employee exposure to potentially hazardous waste, should be highlighted at this time. Considering the possible role of the environment in the spread of SARS-CoV-2 ( Qu et al., 2020 ), the processing of both household and quarantine facility waste is a crucial point of control. Association of Cities and Regions for sustainable Resource management (ACR+) has reported on the provision of separate collection services to COVID-19 contaminated households and quarantine facilities to protect frontline waste workers in Europe, as shown in Fig. 6 . ACR+ also suggests a 72-hour delay in waste disposal (the possible lifespan of COVID-19 in the environment) ( Nghiem et al., 2020 ). Moreover, the collected waste should be immediately transported to waste incinerators or sites without segregation.
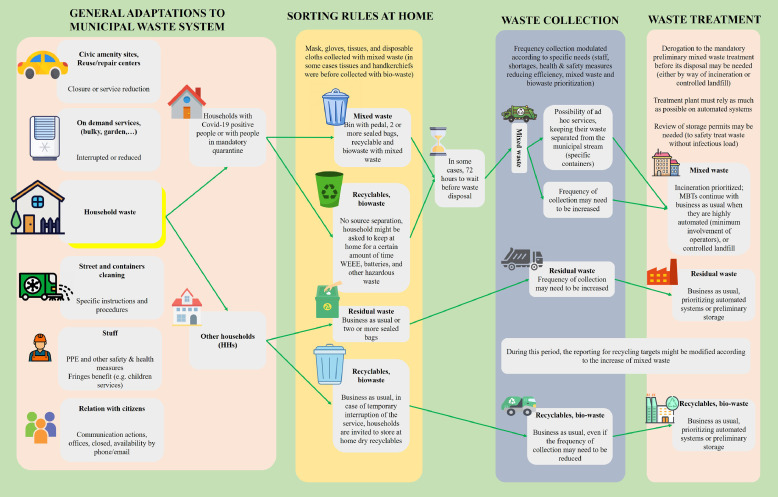
Recommended waste management during COVID-19 ( ACR+ 2020 ).
2.2. NO 2 emissions
Without the global pandemic, we had naively anticipated that in 2020 global emissions would rise by around 1% on a five-year basis. Instead, the sharp decline in economic activity in response to the current crisis will most probably lead to a modest drop in global greenhouse emissions. The European Space Agency (ESA), with its head office in Paris, France, is an intergovernmental body made up of 22 European countries committed to exploring the international space. To monitor air pollution in the atmosphere, the ESA uses the Copernicus Sentinel-5P Satellite. In addition to the compound contents measurement, the Copernicus Sentinel-5P troposphere monitor (TROPOMI) and other specified precision equipment measure ozone content, sulphur dioxide, carbon monoxide, and methane. Table 1 shows NO 2 emissions data acquisition by ESA using Sentinel-5P across different regions of Europe ( Financial Times, 2020 ).
NO 2 emissions data acquisition by ESA using Sentinel-5P across different regions of Europe ( Financial Times, 2020 ).
Burning fossil fuels, such as coal, oil, gas and other fuels, is the source of atmospheric nitrogen dioxide ( Munawer, 2018 ). The bulk of the NO 2 in cities, however, comes from emissions from motor vehicles (approximately 80%). Other NO 2 sources include petroleum and metal refining, coal-fired electricity, other manufacturing and food processing industries. Some NO 2 is naturally produced by lightning in the atmosphere and from the soil, water, and plants, which, taken together, constitutes not even 1% of the total NO 2 found in the air of our localities. Due to pollution variations as well as changes in weather conditions, the levels of the NO 2 in our atmosphere differ widely every day. Anthropogenic pollution is estimated to contain around 53 million tonnes of NO 2 annually. Nitrogen dioxide, together with nitrogen oxide (NO), are considered the major components of oxides of nitrogen (NOx) ( M Palash et al., 2013 ; Fattah et al., 2013 ). NO, and NO 2 are susceptible to other chemicals and form acid rain that is toxic to the environment ( Mofijur et al., 2013 ; Ashraful et al., 2014 ), WHO lists NO 2 as one of the six typical air contaminants in the atmosphere. For this reason, the amount of NO 2 in the atmosphere is used as a precise measure for determining whether the COVID-19 outbreak affects environmental pollution.
NO 2 is an irritating reddish-brown gas with an unpleasant smell, and when cooled or compressed, it becomes a yellowish-brown liquid ( Wang and Su, 2020 ). NO 2 inflames the lung linings and can decrease lung infection immunity. High levels of NO 2 in the air we breathe can corrode our body's lung tissues . Nitrogen dioxide is a problematic air pollutant because it leads to brown photochemical smog formation, which can have significant impacts on human health ( Huang et al., 2020 ). Brief exposure to high concentrations of NO 2 can lead to respiratory symptoms such as coughing, wheezing, bronchitis, flu, etc., and aggravate respiratory illnesses such as asthma. Increased NO 2 levels can have major effects on individuals with asthma, sometimes leading to frequent and intense attacks ( Munawer, 2018 ). Asthmatic children and older individuals with cardiac illness are most vulnerable in this regard. However, its main drawback is that it produces two of the most harmful air pollutants, ozone and airborne particles. Ozone gas affects our lungs and the crops we eat.
2.2.1. NO₂ emissions across different countries
According to the ESA ( European Space Agency (ESA), 2020 ), average levels of NO 2 declined by 40% between 13 th March 2020 to 13 th April 2020. The reduction was 55% compared to the same period in 2019. Fig. 7 compares the 2019-2020 NO 2 concentration ( European Space Agency (ESA), 2020 ). The displayed satellite image was captured with the TROPOMI by ESA satellite Sentinel-5P. The percentage reductions in average NO 2 emissions in European countries during the COVID-19 outbreak from 1 st April to 30 th April 2020 can be seen in Fig. 8 ( Myllyvirta, 2020 ). Portugal, Spain, Norway, Croatia, France, Italy, and Finland are the countries that experienced the largest decrease in NO 2 levels, with 58%, 48%, 47%, 43% and 41%, respectively.
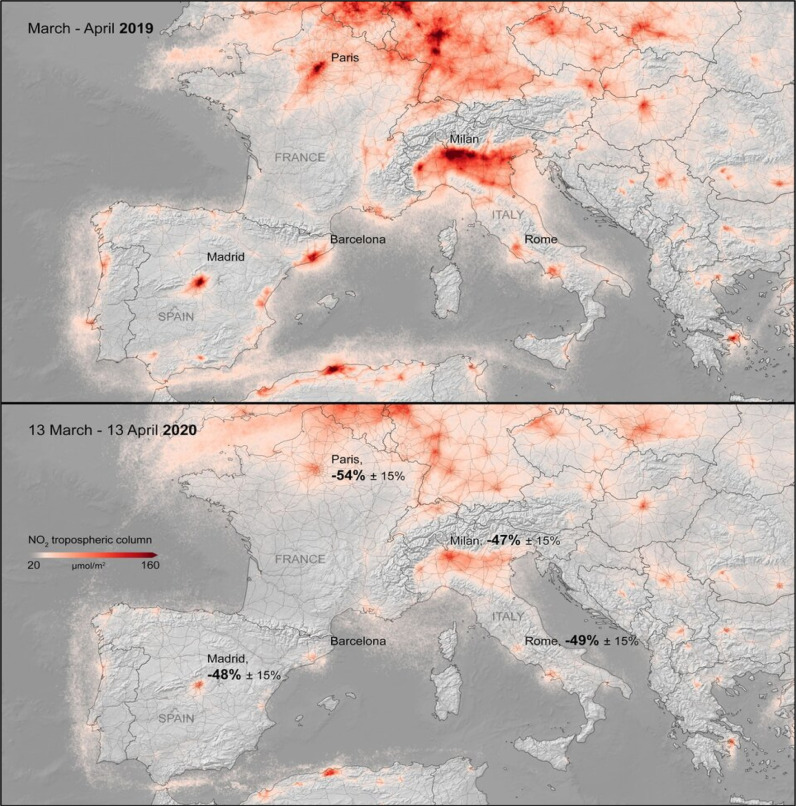
Comparison of the NO 2 concentration between 2019 and 2020 in Europe ( European Space Agency (ESA), 2020 ).
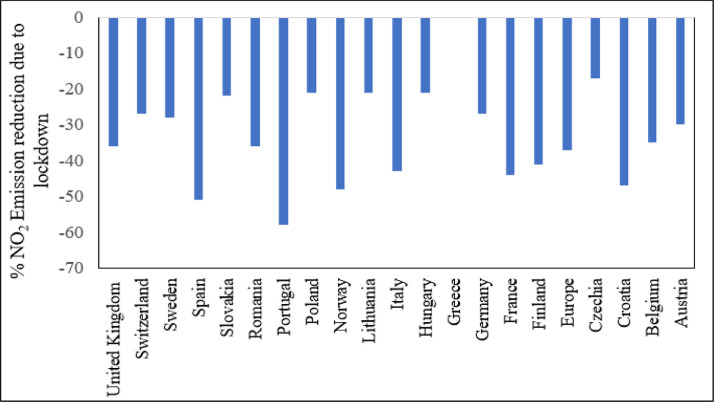
Changes in average NO 2 emission in different countries ( Myllyvirta, 2020 ).
The average 10-day animation of NO 2 emissions throughout Europe (from 1 st January to 11 th March 2020), demonstrated the environmental impact of Italy's economic downturn, see Fig. 9 ( European Space Agency (ESA), 2020 ). In the recent four weeks (Last week of February 2020 to the third week of March 2020) the average concentration of NO 2 in Milan, Italy, has been at least 24% less than the previous four weeks. In the week of 16 – 22 March, the average concentration was 21% lower than in 2019 for the same week. Over the last four weeks of January 2020, NO 2 emissions in Bergamo city has been gradually declining. During the week of 16–22 March, the average concentration was 47% less than in 2019. In Rome, NO 2 rates were 26–35% lower than average in the last four weeks (third week of January 2020 to the third week of February 2020) than they were during the same week of 2019 ( Atmosphere Monitoring Service, 2020 ).
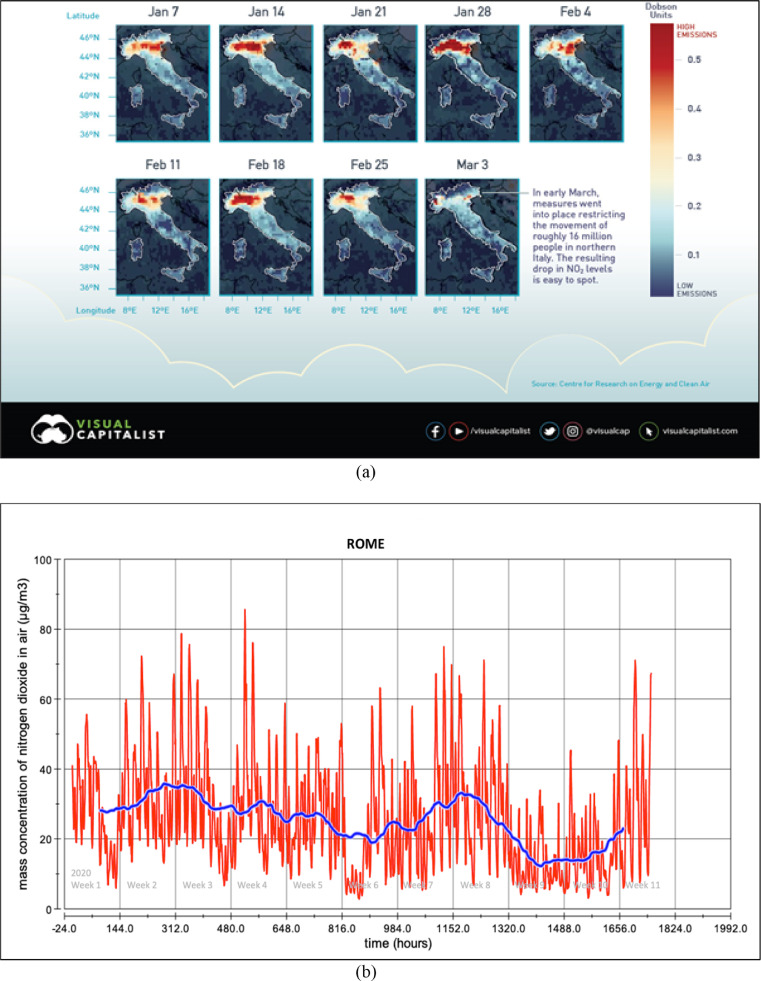
Changes of NO 2 emission (a) over entire Italy (b) capital city (c) other cities ( European Space Agency (ESA), 2020 ; Atmosphere Monitoring Service, 2020 ).
Fig. 10 shows a comparison of NO 2 volumes in Spain in March 2019 and 2020. As per ( European Space Agency (ESA), 2020 ), Spain's NO 2 pollutants decreased by up to 20–30% due to lockdown, particularly across big cities like Madrid, Barcelona, and Seville. ESA Sentinel-5P captured the satellite image using TROPOMI. Satellite images of the 10 days between 14 th and 25 th March 2020 show that NO 2 tropospheric concentration in the areas of Madrid, Barcelona, Valencia, and Murcia ranges from 0–90 mg/m 3 . The NO 2 tropospheric concentration for Seville is almost 0 mg/m 3 for the same time. For March 2019, the average NO 2 tropospheric concentration for the Madrid area was between 90 and 160 mg/m 3 . At the same time, the range of NO 2 tropospheric concentration for Barcelona, Valencia, and Seville area was between 90–140 mg/m 3 , 90-130 mg/m 3 , and 30–50 mg/m 3 , respectively.
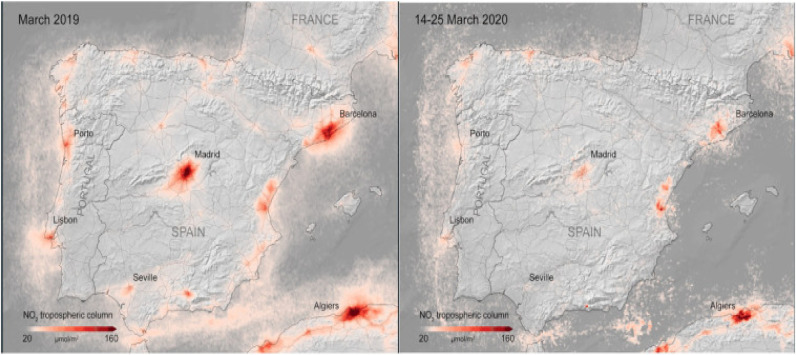
Comparison between before and after lockdown NO 2 emissions in Spain ( European Space Agency (ESA), 2020 ).
Fig. 11 shows the reduction in the amount of NO 2 emissions in France in March 2019 and 2020 ( European Space Agency (ESA), 2020 ). In France, levels of NO 2 have been reduced by 20% to 30%. The ESA Sentinel-5P satellite image was captured with the TROPOMI. In Paris and other major cities, the emission levels of NO 2 considerably lowered due to lockdown. The three major areas of France where NO 2 tropospheric concentration was significant are Paris, Lyon, Marseille and their surroundings. Satellite images of the ten days between 14 th and 25 th March 2020 show that NO 2 tropospheric concentration of the Paris, Lyon, Marseille areas ranges 30–90 mg/m 3 , 20–40 mg/m 3 and 40–80 mg/m 3 , respectively. For March 2019, the average NO 2 tropospheric concentration for the same areas was reported as 100–160 mg/m 3 , 30–60 mg/m 3, and 90–140 mg/m 3 , respectively.
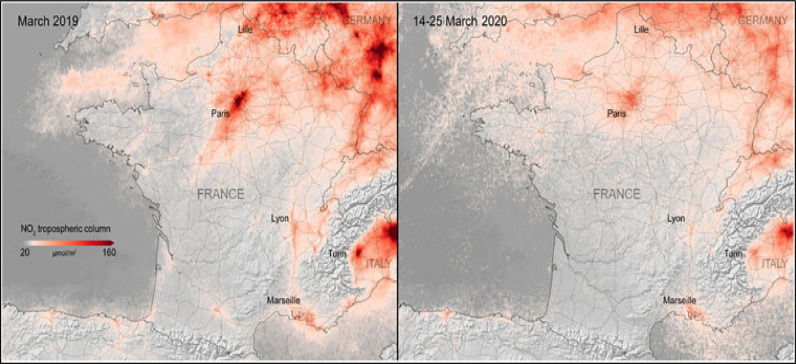
Comparison of NO 2 emissions in France before and after lockdown ( European Space Agency (ESA), 2020 ).
Various industries across the UK have been affected by COVID-19, which has influenced air contamination. As shown in Fig. 12 , there were notable drops in the country's NO 2 emissions on the first day of quarantine ( Khoo, 2020 ). Edinburgh showed the most significant reduction. The average NO 2 emissions on 26 th March 2020, were 28 μg/m 3 while on the same day of 2019, this was 74 μg/m 3 ( Khoo, 2020 ). The second biggest reduction was observed in London Westminster where emissions reduced from 58 µg/m 3 to 30 µg/m 3 . Not all cities have seen such a significant decrease, with daily air pollution reducing by 7 μg/m 3 compared to the previous year in Manchester Piccadilly, for example ( Statista, 2020 ).
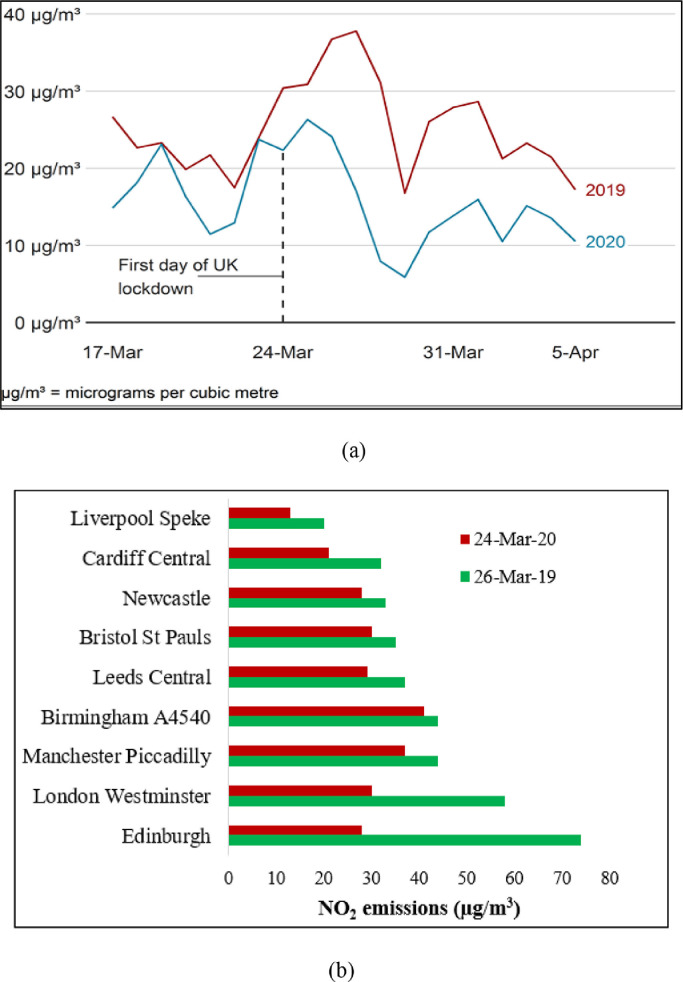
(a) Changes in NO 2 emissions in the UK during lockdown ( European Space Agency (ESA), 2020 ); (b) comparison of NO 2 emissions in 2019 and 2020 ( Khoo, 2020 ).
2.3. PM emission
The term particulate matter, referred to as PM, is used to identify tiny airborne particles. PM forms in the atmosphere when pollutants chemically react with each other. Particles include pollution, dirt, soot, smoke, and droplets. Pollutants emitted from vehicles, factories, building sites, tilled areas, unpaved roads and the burning of fossil fuels also contribute to PM in the air ( Baensch-Baltruschat et al., 2020 ). Grilling food (by burning leaves or gas grills), smoking cigarettes, and burning wood on a fireplace or stove also contribute to PM. The aerodynamic diameter is considered a simple way to describe PM's particle size as these particles occur in various shapes and densities. Particulates are usually divided into two categories, namely, PM 10 that are inhalable particles with a diameter of 10 μm or less and PM 2.5 which are fine inhalable particle with a diameter of 2.5 μm or less. PM 2.5 exposure causes relatively severe health problems such as non-fatal heart attacks, heartbeat irregularity, increased asthma, reduced lung function, heightened respiratory symptoms, and premature death ( Weitekamp et al., 2020 ).
PM 2.5 also poses a threat to the environment, including lower visibility (haze) in many parts of the globe. Particulates can be transported long distances then settle on the ground or in water sources. In these contexts and as a function of the chemical composition, PM 2.5 may cause acidity in lakes and stream water, alter the nutrient balance in coastal waters and basins, deplete soil nutrients and damage crops on farms, affect the biodiversity in the ecosystem, and contribute to acid rain. This settling of PM, together with acid rain, can also stain and destroy stones and other materials such as statues and monuments, which include valuable cultural artefacts ( Awad et al., 2020 ).
2.3.1. PM emission in different countries
Due to the COVID-19 outbreak, PM emission in most countries has been reduced ( Chatterjee et al., 2020 ; Ghahremanloo et al., 2021 ; Gualtieri et al., 2020 ; Sharifi and Khavarian-Garmsir, 2020 ; Srivastava, 2020 ). Fig. 13 shows the impact of COVID19 on PM emission in a number of some countries around the world ( Myllyvirta, 2020 ). The largest reductions in PM pollution took place in Portugal, with 55%, followed by Norway, Sweden, and Poland with reductions of 32%, 30%, and 28%, respectively. Spain, Poland, and Finland recorded PM emission reductions of 19%, 17% and 16%, respectively. Both Romania and Croatia recorded no changes in PM level, with Switzerland and Hungary recording about a 3% increase in PM emission.
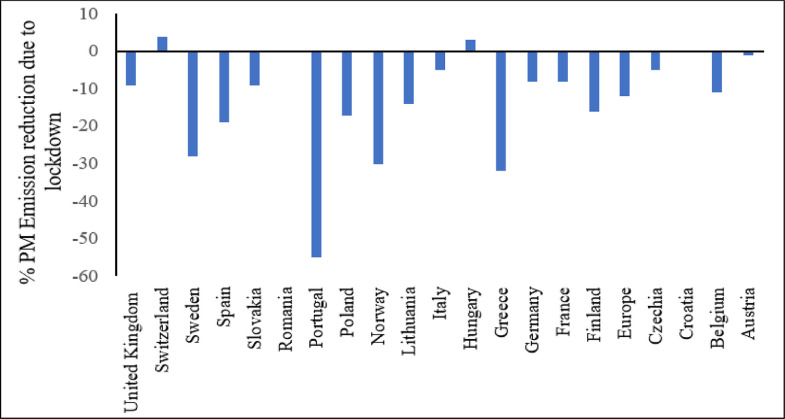
Reduction of PM emission in different countries ( Myllyvirta, 2020 ).
PM emissions have been significantly reduced during the epidemic in most regions of Italy. Fig. 14 illustrates the changes in COVID-19 containment emissions before and after a lockdown in major cities in Italy. According to a recent study by Sicard et al. ( Sicard et al., 2020 ), lockdown interventions have had a greater effect on PM emission. They found that confinement measures reduce PM 10 emissions in all major cities by “around 30% to 53%” and “around 35% to 56%”.
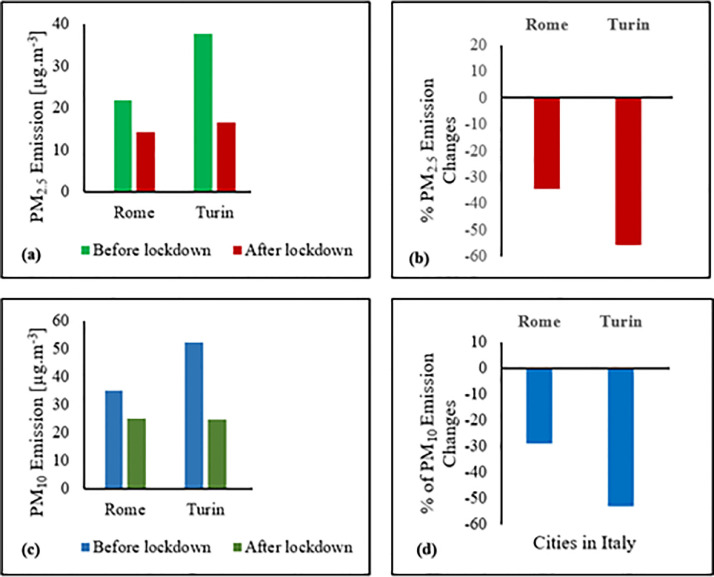
Comparison of PM emission in Italy (a) PM 2.5 emission (b) Changes of PM 2.5 emission (c) PM 10 emission (d) Changes of PM 10 emission ( Sicard et al., 2020 ).
2.4. Noise emission
Noise is characterised as an undesirable sound that may be produced from different activities, e.g. transit by engine vehicles and high volume music. Noise can cause health problems and alter the natural condition of ecosystems. It is among the most significant sources of disruption in people and the environment ( Zambrano-Monserrate and Ruano, 2019 ). The European Environment Agency (EEA) states that traffic noise is a serious environmental problem that negatively affects the health and security of millions of citizens in Europe. The consequences of long-term exposure to noise include sleep disorders, adverse effects on the heart and metabolic systems, and cognitive impairment in children. The EEA estimates that noise pollution contributes to 48,000 new cases of heart disease and 12,000 early deaths per year. They also reported chronic high irritation for 22 million people and a chronic high level of sleep disorder for 6.5 million people ( Lillywhite, 2020 ).
Most governments have imposed quarantine measures that require people to spend much more time at home. This has considerably reduced the use of private and public transport. Commercial activities have almost completely stopped. In most cities in the world, these changes have caused a significant decline in noise levels. This was followed by a significant decline in pollution from contaminants and greenhouse gas emissions. Noise pollution from sources like road, rail or air transport has been linked to economic activity. Consequently, we anticipate that the levels of transport noise will decrease significantly due to the decreased demand for mobility in the short term ( Ro, 2020 ).
For example, it was obvious that environmental noise in Italy was reduced after 8 th March 2020 (the lockdown start date) due to a halt in commercial and recreational activities. A seismograph facility in Lombardy city in Italy that was severely affected by the COVID-19 pandemic indicated how the quarantine measures reduce both traffic and noise emissions. The comparison of the 24-hour seismic noise data before and after the lockdown period indicates a considerable drop in environmental noise in Italy ( Bressan, 2020 ).
3. Impact of COVID-19 on the socio-economic domain
COVID-19 has created a global health crisis where countless people are dying, human suffering is spreading, and people's lives are being upended ( Nicola et al., 2020 ). It is not only just a health crisis but also a social and economic crisis, both of which are fundamental to sustainable development ( Pirouz et al., 2020 ). On 11 th March 2020, when WHO declared a global pandemic, 118,000 reported cases spanning 114 countries with over 4,000 fatalities had been reported. It took 67 days from the first reported case to reach 100,000 cases, 11 days for the second 100,000, and just four days for the third ( United Nations Development Programme (UNDP), 2020 ). This has overwhelmed the health systems of even the richest countries with doctors being forced to make the painful decision of who lives and who dies. The COVID-19 pandemic has pushed the world into uncertainty and countries do not have a clear exit strategy in the absence of a vaccine. This pandemic has affected all segments of society. However, it is particularly damaging to vulnerable social groups, including people living in poverty, older persons, persons with disabilities, youths, indigenous people and ethnic minorities. People with no home or shelter such as refugees, migrants, or displaced persons will suffer disproportionately, both during the pandemic and in its aftermath. This might occur in multiple ways, such as experiencing limited movement, fewer employment opportunities, increased xenophobia, etc. The social crisis created by the COVID-19 pandemic may also increase inequality, discrimination and medium and long-term unemployment if not properly addressed by appropriate policies.
The protection measures taken to save lives are severely affecting economies all over the world. As discussed previously, the key protection measure adopted universally is the lockdown, which has forced people to work from home wherever possible. Workplace closures have disrupted supply chains and lowered productivity. In many instances, governments have closed borders to contain the spread. Other measures such as travel bans and the prohibition of sporting events and other mass gatherings are also in place. In addition, measures such as discouraging the use of public transport and public spaces, for example, restaurants, shopping centres and public attractions are also in place in many parts of the world. The situation is particularly dire in hospitality-related sectors and the global travel industry, including airlines, cruise companies, casinos and hotels which are facing a reduction in business activity of more than 90% ( Fernandes, 2020 ). The businesses that rely on social interactions like entertainment and tourism are suffering severely, and millions of people have lost their jobs. Layoffs, declines in personal income, and heightened uncertainty have made people spend less, triggering further business closures and job losses ( Ghosh, 2020 ).
A key performance indicator of economic health is Gross Domestic Product (GDP), typically calculated on a quarterly or annual basis. IMF provides a GDP growth estimate per quarter based on global economic developments during the near and medium-term. According to its estimate, the global economy is projected to contract sharply by 3% in 2020, which is much worse than the 2008 global financial crisis ( International Monetary Fund (IMF), 2020 ). The growth forecast was marked down by 6% in the April 2020 World Economic Outlook (WEO) compared to that of the October 2019 WEO and January 2020 WEO. Most economies in the advanced economy group are expected to contract in 2020, including the US, Japan, the UK, Germany, France, Italy and Spain by 5.9%, 5.4%, 6.5%, 7.0%, 7.2%, 9.1%, and 8.0% respectively. Fig. 15 a shows the effect of COVID-19 on the GDP of different countries around the globe. On the other hand, economies of emerging market and developing economies, excluding China, are projected to contract by only 1.0% in 2020. The economic recovery in 2021 will depend on the gradual rolling back of containment efforts in the latter part of 2020 that will restore consumer and investor confidence. According to the April 2020 WEO, the level of GDP at the end of 2021 in both advanced and emerging market and developing economies is expected to remain below the pre-virus baseline (January 2020 WEO Update), as shown in Fig. 15 b.
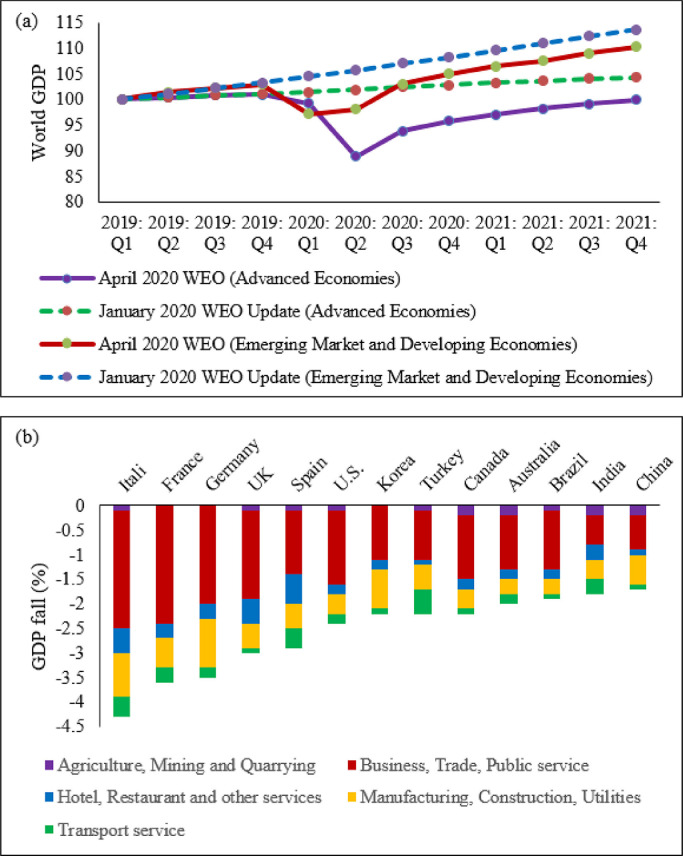
(a) Quarterly World GDP. 2019:Q1 =100, dashed line indicates estimates from January 2020 WEO; (b) GDP fall due to lockdown in selected countries.
A particular example of a country hardest hit by COVID-19 is Italy. During the early days of March, the Italian government imposed quarantine orders in major cities that locked down more than seventeen million people ( Andrews, 2020 ). The mobility index data by Google for Italy shows there has been a significant reduction in mobility (and therefore economic activity) across various facets of life. The reported decline of mobility in retail and recreation, grocery and pharmacy, transit stations and workplaces were 35%, 11%, 45% and 34% respectively ( Rubino, 2020 ). The Italian economy suffered great financial damage from the pandemic. The tourism, and hospitality sectors were among those most severely affected by foreign countries prohibiting travel to and from Italy, and by the government's national lockdowns in early March ( Brunton, 2020 ). A March 2020 study in Italy showed that about 99% of the companies in the housing and utility sector said the epidemic had affected their industry. In addition, transport and storage was the second most affected sector. Around 83% of companies operating in this sector said that their activities had been affected by the coronavirus ( Statista, 2020 ) pandemic. In April 2020, Italian Minister Roberto Gualtieri estimated a 6% reduction in the GDP for the year 2020 ( Bertacche et al., 2020 ). The government of Italy stopped all unnecessary companies, industries and economic activities on 21 st March 2020. Therefore The Economist estimates a 7% fall in GDP in 2020 ( Horowitz, 2020 ). The Economist predicted that the Italian debt-to-GDP ratio would grow from 130% to 180% by the end of 2020 ( Brunton, 2020 ) and it is also assumed that Italy will have difficulty repaying its debt ( Bertacche et al., 2020 ).
4. Impact of COVID-19 on the energy domain
COVID-19 has not only impacted health, society and the economy but it has also had a strong impact on the energy sector ( Chakraborty and Maity, 2020 ; Abu-Rayash and Dincer, 2020 ). World energy demand fell by 3.8% in the first quarter (Q1) of 2020 compared with Q1 2019. In Q1 of 2020, the global coal market was heavily impacted by both weather conditions and the downturn in economic activity resulting in an almost 8% fall compared to Q1 2019. The fall was primarily in the electricity sector as a result of substantial declines in demand (-2.5%) and competitive advantages from predominantly low-cost natural gas. The market for global oil has plummeted by almost 5%. Travel bans, border closures, and changes in work routines significantly decreased the demand for the use of personal vehicles and air transport. Thus rising global economic activity slowed down the use of fuel for transportation ( Madurai Elavarasan et al., 2020 ). In Q1 2020, the output from nuclear energy plants decreased worldwide, especially in Europe and the US, as they adjusted for lower levels of demand. Demand for natural gas dropped significantly, by approximately 2% in Q1 2020, with the biggest declines in China, Europe, and the United States. In the Q1 2020, the need for renewable energy grew by around 1.5%, driven in recent years by the increasing output of new wind and solar plants. Renewable energy sources substantially increased in the electricity generation mix, with record hourly renewable energy shares in Belgium, Italy, Germany, Hungary, and East America. The share of renewable energy sources in the electricity generation mix has increased. Table 2 shows the effect of COVID-19 outbreak on the energy demand around the world.
Impact of COVID-19 on global energy sector ( AEMO, 2020 ; CIS Editorial, 2020 ; Eurelectric, 2020 ; Livemint, 2020 ; Renewable Energy World, 2020 ; S&P Global, 2020 ; Madurai Elavarasan et al., 2020 ).
Different areas have implemented lockdown of various duration. Therefore, regional energy demand depends on when lockdowns were introduced and how lockdowns influence demand in each country. In Korea and Japan, the average impact on demand is reduced to less than 10%, with lower restrictions. In China, where the first COVID-19 confinement measures were introduced, not all regions faced equally stringent constraints. Nevertheless, virus control initiatives have resulted in a decline of up to 15% in weekly energy demand across China. In Europe, moderate to complete lockdowns were more radical. On average, a 17% reduction in weekly demand was experienced during temporary confinement periods. India's complete lockdown has cut energy requirements by approximately 30%, which indicates yearly energy needs are lowered by 0.6% for each incremental lockdown week ( International Energy Agency (IEA) 2020 ).
The International Energy Agency (IEA) has predicted an annual average decline in oil production of 9% in 2020, reflecting a return to 2012 levels. Broadly, as electricity demand has decreased by about 5% throughout the year, coal production may fall by 8%, and the output of coal-fired electricity generation could fall by more than 10%. During the entire year, gas demand may fall far beyond Q1 2020 due to a downward trend in power and industrial applications. Nuclear energy demand will also decrease in response to reduced electricity demand. The demand for renewable energies should grow due to low production costs and the choice of access to many power systems. Khan et al. (2020) reported that international trade is significantly and positively dependent on renewable energy. In addition, sustainable growth can be facilitated through the consumption of renewable energy which improves the environment, enhances national image globally and opens up international trade opportunities with environmentally friendly countries ( Khan et al., 2021 ). As such, policies that promote renewables can result in economic prosperity, create a better environment as well as meet critical goals for sustainable development ( Khan et al., 2020 ).
5. Preventive measures to control COVID-19 outbreak
COVID-19 is a major crisis needing an international response. Governments will ensure reliable information is provided to assist the public in combating this pandemic. Community health and infection control measures are urgently needed to reduce the damage done by COVID-19 and minimise the overall spread of the virus. Self-defence techniques include robust overall personal hygiene, face washing, refraining from touching the eyes, nose or mouth, maintaining physical distance and avoiding travel. In addition, different countries have already taken preventive measures, including the implementation of social distancing, medicine, forestation and a worldwide ban on wildlife trade. A significant aim of the community health system is to avoid SARS-CoV-2 transmission by limiting large gatherings. COVID-19 is transmitted by direct communication from individual to individual. Therefore, the key preventive technique is to limit mass gatherings. Table 3 shows the impact of lockdown measures on the recovery rate of COVID-19 infections. The baseline data for this table is the median value, for the corresponding day of the week, during the 5-week period 3 rd January to 6 th February 2020.
Mobility index report of different countries ( Ghosh, 2020 ; Johns Hopkins University (JHU), 2020 ; Worldometer, 2020 ).
As of today, no COVID-19 vaccine is available. Worldwide scientists are racing against time to develop the COVID-19 vaccine, and WHO is now monitoring more than 140 vaccine candidates. As of 29 th September 2020, about 122 candidates have been pre-clinically checked, i.e. determining whether an immune response is caused when administering the vaccine to animals ( Biorender, 2020 ). About 45 candidates are in stage I where tests on a small number of people are conducted to decide whether it is effective ( Biorender, 2020 ). About 29 candidates are in Phase II where hundreds of people are tested to assess additional health issues and doses ( Biorender, 2020 ). Only 14 candidates are currently in Phase III, where thousands of participants are taking a vaccine to assess any final safety concerns, especially with regard to side effects ( Biorender, 2020 ). 3 candidates are in Phase IV, where long-term effects of the vaccines on a larger population is observed ( Biorender, 2020 ). The first generation of COVID-19 vaccines is expected to gain approval by the end of 2020 or in early 2021 ( Peiris and Leung, 2020 ). It is anticipated that these vaccines will provide immunity to the population. These vaccines can also reduce the transmission of SARS-CoV-2 and lead to a resumption of a pre-COVID-19 normal. Table 4 shows the list of vaccines that have been passed in the pre-clinical stage. In addition, according to the COVID-19 vaccine and therapeutics tracker, there are 398 therapeutic drugs in development. Of these, 83 are in the pre-clinical phase, 100 in Phase I, 224 in Phase II, 119 in Phase III and 46 in Phase IV ( Biorender, 2020 ).
List of vaccines that have passed the pre-clinical stage ( Biorender, 2020 ).
In addition to the above, forestation and a worldwide ban on wildlife trade can also play a significant role in reducing the spread of different viruses. More than 30% of the ground area is covered with forests. The imminent increase in population contributes to deforestation in agriculture or grazing for food, industries and property. The rise in ambient temperature, sea levels and extreme weather events affects not only the land and environment but also public health ( Ruscio et al., 2015 ; Arora and Mishra, 2020 ). Huge investment has been made into treatments, rehabilitation and medications to avoid the impact of this epidemic. However, it is important to focus on basic measures, e.g. forestation and wildlife protection. The COVID-19 infection was initially spread from the Seafood Market, Wuhan, China. Therefore, China temporarily banned wildlife markets in which animals are kept alive in small cages. It has been reported that 60% of transmittable diseases are animal-borne, 70% of which are estimated to have been borne by wild animals ( Chakraborty and Maity, 2020 ). Deforestation is also related to various kinds of diseases caused by birds, bats, etc. ( Afelt et al., 2018 ). For example, COVID-19 is a bat-borne disease that is transmitted to humans. Therefore, several scientists have advised various countries to ban wildlife trade indefinitely so that humans can be protected from new viruses and global pandemics like COVID-19.
6. Conclusion
In this article, comprehensive analyses of energy, environmental pollution, and socio-economic impacts in the context of health emergency events and the global responses to mitigate the effects of these events have been provided. COVID-19 is a worldwide pandemic that puts a stop to economic activity and poses a severe risk to overall wellbeing. The global socio-economic impact of COVID-19 includes higher unemployment and poverty rates, lower oil prices, altered education sectors, changes in the nature of work, lower GDPs and heightened risks to health care workers. Thus, social preparedness, as a collaboration between leaders, health care workers and researchers to foster meaningful partnerships and devise strategies to achieve socio-economic prosperity, is required to tackle future pandemic-like situations. The impact on the energy sector includes increased residential energy demand due to a reduction in mobility and a change in the nature of work. Lockdowns across the globe have restricted movement and have placed people primarily at home, which has, in turn, decreased industrial and commercial energy demand as well as waste generation. This reduction in demand has resulted in substantial decreases in NO 2, PM, and environmental noise emissions and as a consequence, a significant reduction in environmental pollution. Sustainable urban management that takes into account the positive benefits of ecological balance is vital to the decrease of viral infections and other diseases. Policies that promote sustainable development, ensuring cities can enforce recommended measures like social distancing and self-isolation will bring an overall benefit very quickly. The first generation of COVID-19 vaccines is expected to gain approval by the end of 2020 or in early 2021, which will provide immunity to the population. It is necessary to establish preventive epidemiological models to detect the occurrence of viruses like COVID-19 in advance. In addition, governments, policymakers, and stakeholders around the world need to take necessary steps, such as ensuring healthcare services for all citizens, supporting those who are working in frontline services and suffering significant financial impacts, ensuring social distancing, and focussing on building a sustainable future. It is also recommended that more investment is required in research and development to overcome this pandemic and prevent any similar crisis in the future.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Editor: Dr. Syed Abdul Rehman Khan
- Abdullah S., et al. Air quality status during 2020 Malaysia movement control order (MCO) due to 2019 novel coronavirus (2019-nCoV) pandemic. Sci. Total Environ. 2020;729 doi: 10.1016/j.scitotenv.2020.139022. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Abu-Rayash A., Dincer I. Analysis of the electricity demand trends amidst the COVID-19 coronavirus pandemic. Energy Res. Soc. Sci. 2020;68 doi: 10.1016/j.erss.2020.101682. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- ACR+, Municipal waste management and COVID-19. URL: https://www.acrplus.org/en/municipal-waste-management-covid-19 . Date accessed: 22nd September, 2020. 2020.
- Acter T., et al. Evolution of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as coronavirus disease 2019 (COVID-19) pandemic: a global health emergency. Sci. Total Environ. 2020;730 doi: 10.1016/j.scitotenv.2020.138996. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- AEMO, COVID-19 demand impact in Australia. https://aemo.com.au/en/news/demand-impact-australia-covid19 . Date accessed: 21st September, 2020. 2020.
- Afelt A., Frutos R., Devaux C. Bats, coronaviruses, and deforestation: toward the emergence of novel infectious diseases? Front. Microbiol. 2018;9 doi: 10.3389/fmicb.2018.00702. 702-702. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Aldila D., et al. A mathematical study on the spread of COVID-19 considering social distancing and rapid assessment: the case of Jakarta, Indonesia. Chaos Solitons Fractals. 2020;139 doi: 10.1016/j.chaos.2020.110042. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Andrews, F., Before and after: Italy's tourist attractions left deserted amid coronavirus lockdown. https://www.thenational.ae/lifestyle/travel/before-and-after-italy-s-tourist-attractions-left-deserted-amid-coronavirus-lockdown-1.991274 . Date accessed: 21st September, 2020, in The National. 2020: Abu Dhabi.
- Arora N.K., Mishra J. COVID-19 and importance of environmental sustainability. Environ. Sustain. 2020;3(2):117–119. doi: 10.1007/s42398-020-00107-z. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ashraful A.M., et al. Production and comparison of fuel properties, engine performance, and emission characteristics of biodiesel from various non-edible vegetable oils: a review. Energy Convers. Manage. 2014;80:202–228. [ Google Scholar ]
- Atmosphere Monitoring Service. Air quality information confirms reduced activity levels due to lockdown in Italy. https://atmosphere.copernicus.eu/air-quality-information-confirms-reduced-activity-levels-due-lockdown-italy . Date accessed: 21st September, 2020. 2020.
- Awad O.I., et al. Particulate emissions from gasoline direct injection engines: a review of how current emission regulations are being met by automobile manufacturers. Sci. Total Environ. 2020;718 doi: 10.1016/j.scitotenv.2020.137302. [ DOI ] [ PubMed ] [ Google Scholar ]
- Baensch-Baltruschat B., et al. Tyre and road wear particles (TRWP) - a review of generation, properties, emissions, human health risk, ecotoxicity, and fate in the environment. Sci. Total Environ. 2020;733 doi: 10.1016/j.scitotenv.2020.137823. [ DOI ] [ PubMed ] [ Google Scholar ]
- Beck M.J., Hensher D.A. Insights into the impact of COVID-19 on household travel and activities in Australia – the early days under restrictions. Transp. Policy. 2020;96:76–93. doi: 10.1016/j.tranpol.2020.07.001. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bertacche, M., Orihuela, R., and Colten, J., Italy struck by deadliest day as virus prompts industry shutdown. https://www.bloomberg.com/news/articles/2020-03-21/germany-plans-extra-spending-of-eu150-billion-scholz-says . Date accessed: 21st September, 2020, in Bloomberg. 2020.
- Biorender, COVID-19 vaccine & therapeutics tracker. URL: https://biorender.com/covid-vaccine-tracker . Date accessed: 28th September, 2020. 2020.
- Bressan, D., Coronavirus lockdowns cause worldwide decrease in man-made seismic noise. https://www.forbes.com/sites/davidbressan/2020/04/04/coronavirus-lockdowns-cause-worldwide-decrease-in-man-made-seismic-noise/#1643453464da . Date accessed: 21st September, 2020, in Forbes. 2020.
- Bruinen de Bruin Y., et al. Initial impacts of global risk mitigation measures taken during the combatting of the COVID-19 pandemic. Saf. Sci. 2020;128 doi: 10.1016/j.ssci.2020.104773. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Brunton, J., Nothing less than a catastrophe': Venice left high and dry by coronavirus. https://www.theguardian.com/travel/2020/mar/17/nothing-less-than-a-catastrophe-venice-left-high-and-dry-by-coronavirus . date accessed: 21st September, 2020, in The Guardian. 2020.
- Cai C., et al. Temperature-responsive deep eutectic solvents as green and recyclable media for the efficient extraction of polysaccharides from Ganoderma lucidum. J. Cleaner Prod. 2020;274 [ Google Scholar ]
- Chakraborty I., Maity P. COVID-19 outbreak: migration, effects on society, global environment and prevention. Sci. Total Environ. 2020;728 doi: 10.1016/j.scitotenv.2020.138882. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chatterjee A., et al. High rise in carbonaceous aerosols under very low anthropogenic emissions over eastern Himalaya, India: impact of lockdown for COVID-19 outbreak. Atmos. Environ. 2020 doi: 10.1016/j.atmosenv.2020.117947. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chin A.W.H., et al. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe. 2020;1(1):e10. doi: 10.1016/S2666-5247(20)30003-3. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chinazzi M., et al. The effect of travel restrictions on the spread of the 2019 novel coronavirus (COVID-19) outbreak. Science. 2020;368(6489):395–400. doi: 10.1126/science.aba9757. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- CIS Editorial, Coronavirus impact on energy markets. URL: https://www.icis.com/explore/resources/news/2020/04/08/10482507/topic-page-coronavirus-impact-on-energy-markets . Date accessed: 2nd September, 2020. 2020.
- Cohen M.J. Does the COVID-19 outbreak mark the onset of a sustainable consumption transition? Sustainability. 2020;16(1):1–3. [ Google Scholar ]
- Chowdhury, M. A., M. B. A. Shuvho, M. A. Shahid, A. K. M. M. Haque, M. A. Kashem, S. S. Lam, H. C. Ong, M. A. Uddin and M. Mofijur (2021). "Prospect of biobased antiviral face mask to limit the coronavirus outbreak." Environmental Research 192: 110294. [ DOI ] [ PMC free article ] [ PubMed ]
- de Haas M., Faber R., Hamersma M. How COVID-19 and the Dutch ‘intelligent lockdown’ change activities, work and travel behaviour: evidence from longitudinal data in the Netherlands. Transp. Res. Interdiscip. Perspect. 2020;6 doi: 10.1016/j.trip.2020.100150. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Eurelectric, Impact of COVID 19 on customers and society. URL: https://cdn.eurelectric.org/media/4313/impact_of_covid_19_on_customers_and_society-2020-030-0216-01-e-h-584D2757.pdf . Date accessed: 28th Spetember, 2020. 2020.
- European Space Agency (ESA). Sentinel-5P. https://www.esa.int/Applications/Observing_the_Earth/Copernicus/Sentinel-5P . Date accessed: 21st September, 2020. 2020.
- Fattah I.M.R., et al. Impact of various biodiesel fuels obtained from edible and non-edible oils on engine exhaust gas and noise emissions. Renew. Sustain. Energy Rev. 2013;18:552–567. [ Google Scholar ]
- Fernandes, N., Economic effects of coronavirus outbreak (COVID-19) on the world economy. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3557504 . Date accessed: 21st September, 2020. 2020.
- Financial Times, Coronavirus: is Europe losing Italy? https://www.ft.com/content/f21cf708-759e-11ea-ad98-044200cb277f . Date accessed: 21st September, 2020, in Financial Times. 2020.
- Ghahremanloo M., et al. Impact of the COVID-19 outbreak on air pollution levels in East Asia. Sci. Total Environ. 2021;754 doi: 10.1016/j.scitotenv.2020.142226. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ghosh, I. The road to recovery: which economies are reopening? URL: https://www.visualcapitalist.com/the-road-to-recovery-which-economies-are-reopening-covid-19/ . Date accessed: 22nd September, 2020. 2020 [cited 2020 25th July, 2020]; Available from: https://www.visualcapitalist.com/the-road-to-recovery-which-economies-are-reopening-covid-19/.
- Gorbalenya A.E., et al. The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;5(4):536–544. doi: 10.1038/s41564-020-0695-z. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Gualtieri G., et al. Quantifying road traffic impact on air quality in urban areas: a Covid19-induced lockdown analysis in Italy. Environ. Pollut. 2020 doi: 10.1016/j.envpol.2020.115682. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Holshue M.L., et al. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020;382(10):929–936. doi: 10.1056/NEJMoa2001191. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Horowitz, J., Italy locks down much of the country's north over the coronavirus. URL: https://www.nytimes.com/2020/03/07/world/europe/coronavirus-italy.html . Date accessed: 21st September, 2020, in The New York Times. 2020.
- Huang, Y., W.-c. Mok, Y.-s. Yam, J. L. Zhou, N. C. Surawski, B. Organ, E. F. C. Chan, M. Mofijur, T. M. I. Mahlia and H. C. Ong (2020). Evaluating in-use vehicle emissions using air quality monitoring stations and on-road remote sensing systems. Science of The Total Environment 740: 139868. [ DOI ] [ PubMed ]
- International Energy Agency (IEA) IEA; Paris: 2020. Global Energy Review. https://www.iea.org/reports/global-energy-review-2020 Date accessed: 21st September, 2020. 2020. [ Google Scholar ]
- International Monetary Fund (IMF), World Economic Outlook, April 2020: The Great Lockdown. https://www.imf.org/en/Publications/WEO/Issues/2020/04/14/weo-april-2020 . Date accessed: 22nd September, 2020. 2020.
- International Monetary Fund (IMF), World economic outlook, April 2020: The Great Lockdown. URL: https://www.imf.org/en/Publications/WEO/Issues/2020/04/14/weo-april-2020 . Date accessed: 21st September, 2020. 2020.
- Jiang P., et al. Spatial-temporal potential exposure risk analytics and urban sustainability impacts related to COVID-19 mitigation: a perspective from car mobility behaviour. J. Cleaner Prod. 2021;279 doi: 10.1016/j.jclepro.2020.123673. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Johns Hopkins University (JHU). COVID-19 dashboard by the center for systems science and engineering (CSSE) at Johns Hopkins University (JHU). https://coronavirus.jhu.edu/map.html . Date accessed: 25th July, 2020. 2020.
- Kabir M.T., et al. nCOVID-19 pandemic: from molecular pathogenesis to potential investigational therapeutics. Front. Cell Dev. Biol. 2020;8 doi: 10.3389/fcell.2020.00616. 616-616. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Khan S.A.R., et al. Measuring the impact of renewable energy, public health expenditure, logistics, and environmental performance on sustainable economic growth. Sustain. Dev. 2020;28(4):833–843. [ Google Scholar ]
- Khan S.A.R., et al. Investigating the effects of renewable energy on international trade and environmental quality. J. Environ. Manage. 2020;272 doi: 10.1016/j.jenvman.2020.111089. [ DOI ] [ PubMed ] [ Google Scholar ]
- Khan S.A.R., et al. Determinants of economic growth and environmental sustainability in South Asian association for regional cooperation: evidence from panel ARDL. Environ. Sci. Pollut. Res. 2020 doi: 10.1007/s11356-020-10410-1. [ DOI ] [ PubMed ] [ Google Scholar ]
- Khan S.A.R., et al. A state-of-the-art review and meta-analysis on sustainable supply chain management: future research directions. J. Cleaner Prod. 2021;278 [ Google Scholar ]
- Khoo, A., Coronavirus lockdown sees air pollution plummet across UK. https://www.bbc.com/news/uk-england-52202974 . Date accessed: 21st September, 2020, in BBC News. 2020.
- Leal Filho W., et al. COVID-19 and the UN sustainable development goals: threat to solidarity or an opportunity? Sustainability. 2020;12(13):5343. [ Google Scholar ]
- Lillywhite, R., Air quality and wellbeing during COVID-19 lockdown. https://www.newswise.com/coronavirus/air-quality-and-wellbeing-during-covid-19-lockdown/?article_id=730455 . Date accessed: 21st September, 2020, in Newswise. 2020: USA.
- Liu M., et al. Waste paper recycling decision system based on material flow analysis and life cycle assessment: a case study of waste paper recycling from China. J. Environ. Manage. 2020;255 doi: 10.1016/j.jenvman.2019.109859. [ DOI ] [ PubMed ] [ Google Scholar ]
- Livemint. India's energy demand falls by 30% due to COVID-19 lockdown. URL: https://www.livemint.com/news/world/lockdown-cuts-india-s-energy-demand-by-30-says-iea-11588235067694.html . Date accessed: 28th September, 2020. 2020.
- M Palash S., et al. Impacts of biodiesel combustion on NOx emissions and their reduction approaches. Renewable Sustainable Energy Rev. 2013;23(0):473–490. [ Google Scholar ]
- Ma B., et al. Recycle more, waste more? When recycling efforts increase resource consumption. J. Cleaner Prod. 2019;206:870–877. [ Google Scholar ]
- Madurai Elavarasan R., et al. COVID-19: impact analysis and recommendations for power sector operation. Appl. Energy. 2020;279 doi: 10.1016/j.apenergy.2020.115739. 115739-115739. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Madurai Elavarasan R., et al. COVID-19: impact analysis and recommendations for power sector operation. Appl. Energy. 2020 doi: 10.1016/j.apenergy.2020.115739. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Mofijur M., et al. A study on the effects of promising edible and non-edible biodiesel feedstocks on engine performance and emissions production: a comparative evaluation. Renew. Sustain. Energy Rev. 2013;23(0):391–404. [ Google Scholar ]
- Munawer M.E. Human health and environmental impacts of coal combustion and post-combustion wastes. J. Sustain. Min. 2018;17(2):87–96. [ Google Scholar ]
- Myllyvirta, L., 11,000 air pollution-related deaths avoided in Europe as coal, oil consumption plummet. https://energyandcleanair.org/air-pollution-deaths-avoided-in-europe-as-coal-oil-plummet/ . Date accessed: 21st September, 2020. 2020.
- Mofijur, M., I. M. Rizwanul Fattah, A. B. M. Saiful Islam, M. N. Uddin, S. M. Ashrafur Rahman, M. A. Chowdhury, M. A. Alam and M. A. Uddin (2020). "Relationship between Weather Variables and New Daily COVID-19 Cases in Dhaka, Bangladesh." Sustainability 12(20): 8319.
- Nghiem L.D., et al. The COVID-19 pandemic: considerations for the waste and wastewater services sector. Case Stud. Chem. Environ. Eng. 2020;1 doi: 10.1016/j.cscee.2020.100006. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Nicola M., et al. The socio-economic implications of the coronavirus pandemic (COVID-19): a review. Int. J. Surg. 2020;78:185–193. doi: 10.1016/j.ijsu.2020.04.018. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Park G.W., et al. Evaluation of a new environmental sampling protocol for detection of human norovirus on inanimate surfaces. Appl. Environ. Microbiol. 2015;81(17):5987–5992. doi: 10.1128/AEM.01657-15. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Peiris M., Leung G.M. What can we expect from first-generation COVID-19 vaccines? Lancet North Am. Ed. 2020 doi: 10.1016/S0140-6736(20)31976-0. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Pirouz B., et al. Investigating a serious challenge in the sustainable development process: analysis of confirmed cases of COVID-19 (new type of coronavirus) through a binary classification using artificial intelligence and regression analysis. Sustainability. 2020;12(6):2427. [ Google Scholar ]
- Qu G., et al. An imperative need for research on the role of environmental factors in transmission of novel coronavirus (COVID-19) Environ. Sci. Technol. 2020;54(7):3730–3732. doi: 10.1021/acs.est.0c01102. [ DOI ] [ PubMed ] [ Google Scholar ]
- Renewable Energy World, Renewables achieve clean energy record as COVID-19 hits demand. URL: https://www.renewableenergyworld.com/2020/04/06/renewables-achieve-clean-energy-record-as-covid-19-hits-demand/ . Date accessed: 28th September, 2020. 2020.
- Ro, C., Is coronavirus reducing noise pollution? https://www.forbes.com/sites/christinero/2020/04/19/is-coronavirus-reducing-noise-pollution/#2fe787d5766f . Date accessed: 21st September, 2020, in Forbes. 2020.
- Rubino, d.M., Coronavirus, il decreto del governo: tutte le misure per la zona arancione e quelle per il resto d'Italia. URL: https://www.repubblica.it/cronaca/2020/03/08/news/coronavirus_i_decreti_del_governo-250617415/ . Date accessed: 21st September, 2020, in la Repubblica. 2020.
- Ruscio B.A., et al. One health - a strategy for resilience in a changing arctic. Int. J. Circumpolar Health. 2015;74:27913. doi: 10.3402/ijch.v74.27913. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- S&P Global, Japan, Singapore lockdowns to stifle Asian gas, power demand further. https://www.spglobal.com/platts/en/market-insights/latest-news/natural-gas/040720-japan-singapore-lockdowns-to-stifle-asian-gas-power-demand-further . Date accessed: 21st September, 2020. 2020.
- Schanes K., Dobernig K., Gözet B. Food waste matters - a systematic review of household food waste practices and their policy implications. J. Cleaner Prod. 2018;182:978–991. [ Google Scholar ]
- Sharifi A., Khavarian-Garmsir A.R. The COVID-19 pandemic: impacts on cities and major lessons for urban planning, design, and management. Sci. Total Environ. 2020 doi: 10.1016/j.scitotenv.2020.142391. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sicard P., et al. Amplified ozone pollution in cities during the COVID-19 lockdown. Sci. Total Environ. 2020;735 doi: 10.1016/j.scitotenv.2020.139542. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sohrabi C., et al. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19) Int. J. Surg. 2020;76:71–76. doi: 10.1016/j.ijsu.2020.02.034. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Srivastava A. COVID-19 and air pollution and meteorology-an intricate relationship: a review. Chemosphere. 2020 doi: 10.1016/j.chemosphere.2020.128297. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Statista, Perceived impact of coronavirus (COVID-19) among Italian companies in March 2020, by macro sector. https://www.statista.com/statistics/1103017/perceived-impact-of-coronavirus-covid-19-among-italian-companies-by-sector/ . Date accessed: 21st September, 2020. 2020.
- Statista, COVID-19 lockdown affect on nitrogen dioxide (NO2) emissions in UK cities in March 2020 compared with March 2019. https://www.statista.com/statistics/1111519/no2-emissions-decrease-due-to-lockdown-united-kingdom/ . Date accessed: 21st September, 2020. 2020.
- United Nations Development Programme (UNDP), The social and economic impact of COVID-19 in the Asia-Pacific region. https://www.undp.org/content/undp/en/home/librarypage/crisis-prevention-and-recovery/the-social-and-economic-impact-of-covid-19-in-asia-pacific.html . Date accessed: 21st September, 2020. 2020.
- van Doremalen N., et al. Aerosol and Surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wang D., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wang Q., Su M. A preliminary assessment of the impact of COVID-19 on environment – a case study of China. Sci. Total Environ. 2020;728 doi: 10.1016/j.scitotenv.2020.138915. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Weitekamp C.A., et al. Health effects from freshly emitted versus oxidatively or photochemically aged air pollutants. Sci. Total Environ. 2020;704 doi: 10.1016/j.scitotenv.2019.135772. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Worldometer, Reported cases and deaths by country, territory, or conveyance. https://www.worldometers.info/coronavirus/ . Date accessed: 25th July, 2020. 2020.
- Worldometer, Reported cases and deaths by country, territory, or conveyance. https://www.worldometers.info/coronavirus/ . Date accessed: 22nd September, 2020. 2020.
- Yan Y., et al. The first 75 days of novel coronavirus (SARS-CoV-2) outbreak: recent advances, prevention, and treatment. Int. J. Environ. Res. Public Health. 2020;17(7):2323. doi: 10.3390/ijerph17072323. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ye Y., et al. Survivability, partitioning, and recovery of enveloped viruses in untreated municipal wastewater. Environ. Sci. Technol. 2016;50(10):5077–5085. doi: 10.1021/acs.est.6b00876. [ DOI ] [ PubMed ] [ Google Scholar ]
- Zambrano-Monserrate M.A., Ruano M.A. Does environmental noise affect housing rental prices in developing countries? Evidence from Ecuador. Land Use Policy. 2019;87 [ Google Scholar ]
- Zambrano-Monserrate M.A., Ruano M.A., Sanchez-Alcalde L. Indirect effects of COVID-19 on the environment. Sci. Total Environ. 2020;728 doi: 10.1016/j.scitotenv.2020.138813. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Zhou P., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (7.7 MB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections

IMAGES
COMMENTS
Background The pandemic of Coronavirus Disease 2019 (COVID-19) is a timely reminder of the nature and impact of Public Health Emergencies of International Concern. As of 12 January 2022, there were over 314 million cases and over 5.5 million deaths notified since the start of the pandemic. The COVID-19 pandemic takes variable shapes and forms, in terms of cases and deaths, in different regions ...
The spread of the "Severe Acute Respiratory Coronavirus 2" (SARS-CoV-2), the causal agent of COVID-19, was characterized as a pandemic by the World Health Organization (WHO) in March 2020 and has triggered an international public health emergency [].The numbers of confirmed cases and deaths due to COVID-19 are rapidly escalating, counting in millions [], causing massive economic strain ...
The impact on research in progress prior to COVID-19 was rapid, dramatic, and no doubt will be long term. The pandemic curtailed most academic, industry, and government basic science and clinical ...
The severity of acute COVID-19 is a major risk factor for poor functional outcomes, but even people with mild initial illness can experience long-term functional impairments. Increased number and severity of long-term symptoms correlate with decreased quality of life, physical functioning, and ability to work or perform in school.
5. Conclusion. This review has collated the 100 most influential COVID-19 papers and assessed trends within them. We have established that in the early phases of a pandemic new and ground-breaking research surfaces regardless of the evidence level and can gain high levels of citation.
Conclusions. The COVID-19 pandemic has affected the world in various ways. The deficiency of information, the need for accurate information, and the rapidity of its dissemination are important, as this pandemic requires the cooperation of entire populations. ... "Your research proposal 'Response of the public and health care providers to a ...
This essay examines key aspects of social relationships that were disrupted by the COVID-19 pandemic. It focuses explicitly on relational mechanisms of health and brings together theory and emerging evidence on the effects of the COVID-19 pandemic to make recommendations for future public health policy and recovery. We first provide an overview of the pandemic in the UK context, outlining the ...
COVID-19, the disease caused by SARS-CoV-2, has claimed approximately 5 million lives and 257 million cases reported globally. ... Conclusion. The causative agent of the COVID-19 the pandemic ...
Conclusion. COVID-19 is a viral illness which can cause multi-systemic manifestations. Review of existing literature concludes that SARS-CoV-2 can affect any organ system either directly or indirectly leading to a myriad of clinical presentation. ... The authors declare that the research was conducted in the absence of any commercial or ...
COVID-19 is a worldwide pandemic that puts a stop to economic activity and poses a severe risk to overall wellbeing. The global socio-economic impact of COVID-19 includes higher unemployment and poverty rates, lower oil prices, altered education sectors, changes in the nature of work, lower GDPs and heightened risks to health care workers.