Systematic foodborne disease prevention and risk management based on infection mechanisms
- Published: 15 March 2024

Cite this article

- Ran Liu 1 &
- Lindu Zhao ORCID: orcid.org/0000-0003-4902-3679 1
65 Accesses
Explore all metrics
A dynamic system with 3 levels of risk management was developed to prevent the spread of foodborne diseases in populations while considering secondary infections. The effects of factors such as information quality, time delay and periodicity were compared. The results indicated that the infection mechanism of foodborne diseases exacerbated the severity of epidemics. This severity manifested as a greater number of dormant and visible populations at each stage. The risk of susceptible and exposed populations was significantly amplified during infection, which promotes the probability of dormant populations transforming into diseased populations. The infection mechanism reduced the risk of infection in the population. An increase in the time delay and periodicity increased the risk of foodborne disease, while an increase in information quality reduced the risk. By preventing infections, reducing information delay times and cycle times, and improving information quality, the prevalence of foodborne diseases can be controlled or prevented.
A dynamic system with 3 levels: exposed, susceptible and diseased population.
Emphasize the secondary infection.
Distinguish the influence of time-delay, periodic and information quality factors.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others

Software and Data Bases: Use and Application

Microbiological Quality Systems and Microbial Risk Analysis

Application of Omics Technologies and Computational Approaches for Control of Foodborne Pathogens in Foods
Data availability.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
The setting of the time range was mainly based on the population change equation. This article set the incidence function of the sudden increase in the disease after the 40th period. Therefore, the setting value of the total research time needed to be greater than 40 periods, but above the 40th period, the setting was modified. A certain range had no essential influence on the conclusion.
The setting of the population base to 0 was to better respond to the characteristics of sudden diseases, that is, there has been a sudden increase in such diseases before, so the population base of each disease stage was 0; however, from a mathematical perspective, the adjustment for base population had no essential impact on the conclusions.
These basic parameters could be modified within a certain range without affecting the main conclusion. To ensure that the simulation results were valid, the setting of each parameter was mainly based on the function setting of the main equations in the appendix. Under the condition of ensuring that the function was meaningful, modifying the parameters would not change the research conclusion.
The setting of the population base to 0 was to better respond to the characteristics of sudden diseases, that is, there has been a sudden increase in such diseases before, so the population base of each disease stage was 0; however, from a mathematical perspective, the adjustment for the base population had no essential impact on the conclusions.
Augustin, J. C., Kooh, P., Bayeux, T., et al. (2020). Contribution of foods and poor food-handling practices to the burden of food-borne infectious diseases in France. Foods , 9 (1644), 1644.
Article PubMed PubMed Central Google Scholar
Bertolini, M., Bevilacqua, M., & Massini, R. (2006). FMECA approach to product traceability in the food industry. Food Control , 2 (17), 137–145. https://doi.org/10.1016/j.foodcont.2004.09.013
Bisht, A., Kamble, M. P., Choudhary, P., et al. (2021). A surveillance of food borne disease outbreaks in India: 2009–2018. Food Control , 121 , 107630.
Article Google Scholar
Cai, Y., Lian, X., Peng, Z., et al. (2019). Spatiotemporal transmission dynamics for influenza disease in a heterogenous environment. Nonlinear Analysis: Real World Applications , 46 , 178–194.
MathSciNet Google Scholar
Cao, M., & Zhang, Q. (2011). Supply chain collaboration: Impaction collaborative advantage and firm performance. Journal of Operations Management , 29 (3), 163–180.
Chang, L., Duan, M., Sun, G., et al. (2020). Cross-diffusion-induced patterns in an SIR epidemic model on complex networks. Chaos: An Interdisciplinary Journal of Nonlinear Science , 30 (1), 013147.
Article MathSciNet Google Scholar
Chen, J., Chen, L., Liao, N., et al. (2021). Practice and consideration of foodborne disease surveillance in Zhejiang Province. Chinese Journal of Food Hygiene , 33 (01), 47–52.
CAS Google Scholar
Czarnecki, D. (2014). The Incidence of Melanoma is increasing in the susceptible young Australian population. Acta dermato-venereologica , 94 (5), 539–411.
Article MathSciNet PubMed Google Scholar
Fitzgibbon, W. E., Morgan, J. J., Webb, G. F., et al. (2020). Analysis of a reaction–diffusion epidemic model with asymptomatic transmission. Journal of Biological Systems , 28 (03), 561–587.
Article MathSciNet CAS Google Scholar
Ge, Y. (2021). Quality control in food supply chain with dynamic quality characteristics. Journal of Control and Decision . https://doi.org/10.1080/23307706.2021.1960646 .
Huang, C., Zhang, H., Cao, J., et al. (2019). Stability and Hopf bifurcation of a delayed prey–predator model with disease in the predator. International Journal of Bifurcation and Chaos , 29 (07), 1950091.
Article ADS MathSciNet Google Scholar
Kermack, W. O., & Mckendrick, A. G. (1927). A contribution to the mathematical theory of epidemics. Mathematical Physical and Engineering Sciences , 115 (772), 700–721.
Google Scholar
Lai, Y. H., Chung, Y. A., Wu, Y. C., et al. (2020). Disease burden from foodborne illnesses in Taiwan, 2012–2015. Journal of the Formosan Medical Association , 119 (9), 1372–1381.
Article PubMed Google Scholar
Li, C. H., Tsai, C. C., & Yang, S. Y. (2014). Analysis of epidemic spreading of an SIRS model in complex heterogeneous networks. Communications in Nonlinear Science and Numerical Simulation , 19 (04), 1042–1054.
Loukieh, M., Mouannes, E., Abou, J. C., et al. (2018). Street foods in Beirut city: An assessment of the food safety practices and of the microbiological quality. Journal of Food Safety , 38 (3), 1–11.
Machiela, M. J., & Chanock, S. J. (2015). Dlink a web-based application for exploring population-specific haplotype structure and linking correlated alleles of possible functional variants. Bioinformatics , 31 (21), 3555–3557.
Article CAS PubMed PubMed Central Google Scholar
>Nayak, R. (2016). P. Waterson (Ed.), ‘When Food kills’: A socio-technical systems analysis of the UK Pennington 1996 and 2005 E. Coli O157 Outbreak reports. Safety Science 86 36–47.
Ozdemir, Berna, C., & Dotto, G. P. (2017). Racial differences in cancer susceptibility and survival: More than the color of the skin? Trends in Cancer , 3 (3), 181–207.
Park, J. M., Kim, J. M., Hong, J. W., et al. (2020). Introduction of highly effective proactive food safety management programs into food distribution channels: For safe food labeling and safe advertisements. Journal of Food Safety , 40 (2), 1–12.
Petrignani, M., Verhoef, L., Vennema, H., et al. (2014). Underdiagnosis of food-borne hepatitis A, the Netherlands, 2008–2010. Emerging Infectious Diseases , 20 , 596–602.
Pogreba-Brown, K., Austhof, E., Armstrong, A., et al. (2020). Chronic gastrointestinal and joint-related sequelae associated with common foodborne illnesses: A scoping review. Foodborne Pathogens and Disease , 17 (2), 67–86.
Prajogo, D., & Olhager, J. (2012). Supply chain integration and performance: The effects of long-term relationships, information technology and sharing, and logistics integration. International Journal of Production Economics , 135 (1), 514–522.
Rihan, F. A., Al-Mdalla, Q. M., Alsakaji, H. J., et al. (2019). A fractional-order epidemic model with time-delay and nonlinear incidence rate. Chaos, Solitons, and Fractals , 126 (3), 97–105.
Ruey, S., Chen, C., Yan, B., et al. (2008). Using RFID technology in food produce traceability. WSEAS Transactions on Information Science and Applications , 5 (11), 1551–1560.
Scalia, G. L., Nasca, A., Corona, O., et al. (2017). An innovative shelf-life model based on smart logistic unit for an efficient management of the perishable food supply chain. Journal of Food Process Engineering , 40 (1), 1–13.
Shashi, C., Rajwinder, S., Piera, C., et al. (2018). Food cold chain management: From a structured literature review to a conceptual framework and research agenda. The International Journal of Logistics Management , 29 (3), 792–821.
Song, L., Yu, Z., & He, Q. (2023). Evolutionary game theory and simulations based on doctor and patient medical malpractice. Plos One , 18 (3), e0282434.
Sundstrom, K. (2018). Cost of illness for five major foodborne illnesses and sequelae in Sweden. Applied Health Economics and Health Policy , 16 (2), 243–257.
Syahrul, F., Wahyuni, C. U., Notobroto, H. B., et al. (2020). Transmission media of foodborne diseases as an Index prediction of Diarrheagenic Escherichia coli: Study at Elementary School, Surabaya, Indonesia. International Journal of Environmental Research and Public Health , 17 (21), 8227.
Tack, D. M., Ray, L., Griffin, P. M., et al. (2020). Preliminary incidence and trends of infections with pathogens transmitted commonly through food-foodborne diseases active surveillance network, 10 U.S. Sites, 2016–2019. Mmwr. Morbidity and Mortality Weekly Report , 69 (17), 509–514.
Taylor, D. A. (2011). Laws, regulations and policy: New food safety law brings opportunities amid hurdles. Environmental Health Perspectives, 119 (3), A119. https://doi.org/10.2307/41203198
Ushadevi, N., Vidyasagar, P., & Arshinder, K. (2015). A case study to explore influence of traceability factors on Australian food supply chain performance. Procedia - Social and Behavioral Sciences , 15 (189), 17–32.
Van, C., Le, D., Sommen, S. Y. C., et al. (2017). Estimated annual numbers of food-borne pathogen–associated illnesses, hospitalizations, and deaths, France, 2008–2013. Emerging Infectious Diseases , 23 , 1486–1492.
Verhoef, L., Hewitt, J., Barclay, L., et al. (2015). Norovirus genotype profiles associated with food-borne transmission, 1999–2012. Emerging Infectious Diseases , 21 , 592–599.
Walsh, C., & Leva, M. C. (2019). A review of human factors and food safety in Ireland. Safety Science , 119 , 399–411.
Wang, J., Wu, X., Guo, C., et al. (2018). An empirical study on the transparency of food safety logistics in China: A sampling survey based on 151 food logistics enterprises in 31 provinces. Science and Technology Management Research , 38 (23), 219–227. https://doi.org/10.3969/j.issn.1000-7695.2018.23.032
Wang, Y., Cao, J., Alsaedi, A., et al. (2017). Edge-based SEIR dynamics with or without infectious force in latent period on random networks. Communications in Nonlinear Science and Numerical Simulation , 45 , 35–54.
Wolfert, J., Verdouw, C. N., Verloop, C. M., et al. (2010). Organizing information integration in architecture food: A method based on a service-oriented architecture and living lab approach. Computers & Electronics in Agriculture , 70 (2), 389–405.
Yan, S., Zhang, Y., Ma, J., et al. (2018). An edge-based SIR model for sexually transmitted diseases on the contact network. Journal of Theoretical Biology , 439 , 216–225.
Article ADS MathSciNet PubMed Google Scholar
Yu, J. H., & Fu, R. (2015). Study on application of food safety management in cold chain logistics process based on HACCP. Logistics Technology , 34 (15), 45–47.
Yu, W., Lv, Y., Hu, C., et al. (2018). Research of an emergency medical system for mass casualty incidents in Shanghai, China: A system dynamics model. Patient Preference and Adherence , 12 , 207–222.
Zhang, B., Lin, J., & Liu, R. (2016). Factors affecting the food firm’s intention to control quality safety in China: The moderating effect of government regulation. Chinese Management Studies , 10 (2), 256–271.
Download references
Acknowledgements
For useful feedback on previous versions, we thank the editor and reviewers.This work is partially supported by the National Natural Science Foundation of China (No. 71390333), the National Key Technology RD Program of China during the 12th Five-Year Plan Period (No. 2013BAD19B05). The authors also gratefully acknowledge the helpful comments and suggestions of the reviewers, which have improved the presentation.
This work is partially supported by the National Natural Science Foundation of China (No. 71390333), the National Key Technology RD Program of China during the 12th Five-Year Plan Period (No. 2013BAD19B05).
Author information
Authors and affiliations.
Institute of Systems Engineering, School of Economics and Management, Southeast University, Nanjing, 210096, China
Ran Liu & Lindu Zhao
You can also search for this author in PubMed Google Scholar
Contributions
All authors contributed to the study conception and design, material preparation, data collection, methodology, analysis and investigation.
Corresponding author
Correspondence to Lindu Zhao .
Ethics declarations
Ethics approval and consent to participate.
Not applicable. This manuscript does not report on or involve the use of any animal or human data or tissue.
Consent to publish
Not applicable. This manuscript does not contain data from any individual person.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Liu, R., Zhao, L. Systematic foodborne disease prevention and risk management based on infection mechanisms. Environ Dev Sustain (2024). https://doi.org/10.1007/s10668-024-04732-0
Download citation
Received : 14 October 2022
Accepted : 03 March 2024
Published : 15 March 2024
DOI : https://doi.org/10.1007/s10668-024-04732-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Foodborne diseases
- Infection mechanism
- Risk management
- Information traceability
- System dynamics
- Find a journal
- Publish with us
- Track your research
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 13 October 2020
An analecta of visualizations for foodborne illness trends and seasonality
- Ryan B. Simpson 1 ,
- Bingjie Zhou 1 ,
- Tania M. Alarcon Falconi 1 &
- Elena N. Naumova ORCID: orcid.org/0000-0002-9562-4734 1
Scientific Data volume 7 , Article number: 346 ( 2020 ) Cite this article
3601 Accesses
8 Citations
Metrics details
- Disease prevention
- Epidemiology
- Infectious diseases
- Research management
Disease surveillance systems worldwide face increasing pressure to maintain and distribute data in usable formats supplemented with effective visualizations to enable actionable policy and programming responses. Annual reports and interactive portals provide access to surveillance data and visualizations depicting temporal trends and seasonal patterns of diseases. Analyses and visuals are typically limited to reporting the annual time series and the month with the highest number of cases per year. Yet, detecting potential disease outbreaks and supporting public health interventions requires detailed spatiotemporal comparisons to characterize spatiotemporal patterns of illness across diseases and locations. The Centers for Disease Control and Prevention’s (CDC) FoodNet Fast provides population-based foodborne-disease surveillance records and visualizations for select counties across the US. We offer suggestions on how current FoodNet Fast data organization and visual analytics can be improved to facilitate data interpretation, decision-making, and communication of features related to trend and seasonality. The resulting compilation, or analecta, of 436 visualizations of records and codes are openly available online.
Similar content being viewed by others

CovidCounties is an interactive real time tracker of the COVID19 pandemic at the level of US counties

EpiVECS: exploring spatiotemporal epidemiological data using cluster embedding and interactive visualization

Using digital surveillance tools for near real-time mapping of the risk of infectious disease spread
Introduction.
Disease surveillance systems worldwide face increasing pressure to maintain and distribute data in usable formats with clearly communicated visualizations to promote actionable policy and programming responses 1 . Decade-long efforts to sustain surveillance systems improve early outbreak detection, infection containment, and mobilization of health resources 1 , 2 , 3 , 4 and create adaptive, near-time forecasts for disease outbreaks 5 , 6 . Web-based platforms provide access to more accurate, timely, and frequent surveillance data. The World Health Organization’s (WHO) FluNet, for example, provides time-referenced data on worldwide influenza 7 . Publicly available downloads increase the flexibility for analyses and enables adaptive research due to frequent and timely reporting.
The pandemic of 2019 novel coronavirus disease (COVID-19) serves as a vivid demonstration of how limited access to publicly available high-quality data can stymy research. As the quantity and diversity of data available for processing, synthesizing, and communicating increases, new visual analytics, including complex multi-panel plots, must be considered to monitor trends, investigate seasonality, and support public health planning 8 . These visualizations, and the methodologies used to generate them, must be standardized to enable comparability across time periods, locations, at-risk populations, and pathogens. However, current surveillance systems, including foodborne disease surveillance in the United States, often compress time series records to simplistic annual trends 9 , 10 , 11 , 12 , 13 and describe seasonality by the month(s) with the highest cases per year or the first month of outbreak onset 14 , 15 , 16 , 17 , 18 , 19 . Visualizations using these annual trends or broad assessments of seasonality fail to utilize the full complexity of surveillance data and in some cases may be misleading. More specifically, these visualizations fail to provide detailed examination of how long-term trends change over time, how seasonality estimates vary by year or across locations, or how peak timing and amplitude estimates could change over time.
The CDC Foodborne Disease Active Surveillance Network (FoodNet) provides preprocessed population-based foodborne-disease surveillance records and visualizations via FoodNet Fast, a publicly available data portal 20 , 21 . The FoodNet Fast platform contains rich demographic data, including age group, gender, and ethnic group, valuable for a broad spectrum of analyses. The visualizations aim to aid users in identifying trends of nine laboratory-confirmed foodborne diseases in select counties from ten US states and nationally. However, in the present form and due to substantial data compression, the available data and visualizations provided are limited in scope preventing the characterization of inter-annual trends and seasonality characteristics and comparisons across locations, diseases, and time 14 , 15 , 17 , 22 , 23 , 24 . The data portal allows researchers to download time series from a public domain. After downloading, processing, and analyzing the data, we created an analecta of visualization techniques enabling inter-annual and sub-annual comparisons between diseases and locations with respect to long-term trends and seasonal oscillations.
Analects, or analecta, is defined as a compilation of select passages from the work of an author 25 . Our analecta includes two compilations: (1) a collection of FoodNet Fast datasets collated into usable and analyzable time series data, and (2) an anthology of effective and informative visualizations for communicating information on disease trends and seasonality. By merging FoodNet Fast datasets and calculating disease rates from counts, we facilitate the comparison of spatial and temporal patterns of foodborne diseases, and detection of potential outbreaks and their synchronization. The presented anthology eases data interpretation, decision-making, and communication of features related to trend and seasonality and can be replicated for other longitudinal disease surveillance databases to support public health interventions.
In this paper, we describe the process of compiling, organizing, and visualizing surveillance time series data using FoodNet Fast as an example 21 . We demonstrate how current FoodNet Fast visual analytics can be improved to provide a clear yet comprehensive description of trends and seasonality features. We emphasize the utility of multi-panel graphs 8 for describing trends over time and features of seasonality, including disease peak timing and amplitude. We also provide guides on how to explore and compare trends and seasonality between multiple diseases and geographic locations. As surveillance systems offer a time-referenced data repository, analects of data visualizations can show how diseases change over time. Such a tool can help ensure the longevity of data and information to better understand and evaluate the efficacy of public health programming over time. The resulting analecta of 436 visualizations for foodborne diseases, original and processed data, and the codes used to produce visualizations are available at our website ( https://sites.tufts.edu/naumovalabs/analecta/ ).
FoodNet fast data compilation
The FoodNet Fast platform provides publicly available data for laboratory-confirmed diseases caused by seven bacteria [ Campylobacter , Listeria , Salmonella , Shigella , Shiga toxin-producing Escherichia coli O157 and non-O157 (STEC), Vibrio , and Yersinia enterocolitica ] and two protozoa ( Cryptosporidium and Cyclospora ) in counties from 10 states: California (CA), Connecticut (CT), Georgia (GA), Minnesota (MN), and Oregon (OR) since 1996; Maryland (MD) and New York (NY) since 1998; Tennessee (TN) since 2000; Colorado (CO) since 2001; and New Mexico (NM) since 2004. National estimates are generated from the sum of data across these 10 states, which represent approximately 15% of the US population 20 , 21 .
FoodNet Fast allows data download and visualization of these diseases for a user-specified time period. Data downloads include information on the incidence of confirmed cases, monthly percentage of confirmed cases, distribution of cases by pathogen, and totals of cases, hospitalizations, and deaths. For multi-year periods, the portal aggregates totals and monthly percentages into single statistics for the full time period selected rather than showing individual years. This aggregation ensures case anonymity but monthly time units minimize the refinement of trend and seasonality analyses. To calculate monthly percentages of confirmed cases for all diseases in one year and one location, we had to download each state-year combination individually, for a total of 221 files in MS Excel format.
To create a time series of total monthly cases by pathogen and location, we used data from two tables in each data download: annual counts of confirmed cases (long format) and monthly percentage of confirmed cases (wide format). We transposed the monthly percentages of confirmed cases from wide to long format and then multiplied them by the annual counts of confirmed cases (Supplementary Figure S1 ). Since the provided monthly percentages are rounded to 1 digit in the data download, calculated counts slightly under- or over-estimate annual totals. We did not round non-integer cases in our calculated time series to best preserve the monthly distribution of cases from the original data download. A monthly time series of confirmed cases of hospitalizations or deaths could not be reconstructed as described because no information is provided on their monthly percentages. We next calculated disease rates using confirmed monthly cases and annual population data. Rates are preferred over counts since changes in counts could be a direct result of changes in the population catchment area of a surveillance system. The number of counties and states monitored in FoodNet increased between 1996 and 2004 and has remained constant to date since (Supplementary Table S1 ).
We downloaded county-level population estimates from the 1990, 2000, and 2010 US Census Bureau interannual census reports, which provide annual population estimates 26 , 27 , 28 . We then estimated state-level FoodNet population catchment area by adding all mid-year (July 1 st ) populations of surveyed counties monitored in each year. Next, we calculated the United States population catchment area by adding all state-level estimates for all surveyed counties for each year. Finally, we developed a time series of monthly rates per 1,000,000 persons for each pathogen and location by dividing monthly counts by annual population estimates and multiplying this quotient by 1,000,000. In addition to monthly rates, we calculated yearly rates by adding all monthly counts each year, dividing by the annual population, and multiplying this quotient by 1,000,000.
Modeling trends and seasonality
We estimated trend and seasonality characteristics using Negative Binomial Harmonic Regression (NBHR) models, which are commonly used to analyse count-based time series records with periodic fluctuations 29 , 30 , 31 . These models include harmonic terms representing sine and cosine functions, which allow us to fit periodic oscillations. The regression parameters for these harmonic terms serve as a base for estimating important characteristics of seasonality: when the maximum rate occurs (peak timing) and the magnitude at that peak (amplitude). We calculated peak timing, amplitude, and their confidence intervals from NBHR model coefficients using the δ-method, which allow us to transform the regression coefficients of the model to seasonality characteristics based on the properties of the basic trigonometric functions (Supplementary Table S2 ) 29 , 30 . To estimate annualized seasonality characteristics, we applied a NBHR model for each study year and location with the length of the time series set to 12 to represent the months of the year. We also estimated seasonality characteristics for the full time period. To show average trends across the entire 22-year period, we fit a NBHR model with three trend terms (linear, quadratic, and cubic) where the length of the time series varied according to when FoodNet began surveying that location from 168 to 264 months. The selection of three polynomial terms was driven by the clarity of interpretation as a monthly increase and the potential for overall acceleration or deceleration, although other ways of assessing the trend such moving averages and spline functions could be also explored.
Plot terminology
We develop multi-panel visualization techniques using the best practices of current data visualization resources 32 , 33 and our own research 8 , 34 , 35 . A multi-panel plot, as defined by our earlier work, “involves the strategic positioning of two or more graphs sharing at least one common axis on a single canvas 8 .” These plots can effectively illustrate multiple dimensions of information including different time units (e.g. yearly, monthly), disease statistics (e.g. pathogens, rates, counts), seasonality characteristics (e.g. peak timing, amplitude), and locations (e.g. state-level, national). We use the following common, standardized terminology across visualizations to ensure comprehension:
Disease – each of the nine reported FoodNet infections, including campylobacteriosis (Camp), listeriosis (List), salmonellosis (Salm), shigellosis (Shig), infection due to Shiga toxin-producing Escherichia coli O157 and non-O157 (Ecol), vibriosis (Vibr), infection due to Yersinia enterocolitica (Yers), cryptosporidiosis (Cryp) and cyclosporiasis (Cycl)
Monthly Rate – monthly confirmed cases per 1,000,000 persons
Yearly Rate – total confirmed cases in a year divided by the mid-year population of all surveyed counties in that location (cases per 1,000,000 persons)
Frequency – the number of months reporting the disease rates in the same range
Peak Timing – the time of year according to the Gregorian calendar that a disease reaches its maximal rate; for monthly time series, peak timing ranges in [1, 13[, i.e. from 1.0 (beginning of January) to 12.9 (end of December)
Amplitude – the mathematical amplitude, or the midpoint of relative intensity; for NBHR models, the amplitude estimate reflects the ratio between the disease rate at the peak (maximum rate) and the disease rate at the midpoint (median rate)
FoodNet Surveyed County – the counties under FoodNet surveillance as of 2017
Non-Surveyed County – all remaining counties within a surveillance state as of 2017.
We present our analecta of visualizations allowing to describe trend, examine seasonal signatures, curves depicting characteristic variations in disease incidence over the course of one year, and understand features of seasonality, such as peak timing and amplitude across locations and diseases. We illustrate all visualizations using salmonellosis for the United States from 1996–2017. The full analecta with time series data and code are available on our website ( https://sites.tufts.edu/naumovalabs/analecta/ ) with data and code also available on figshare 36 .
Describing trend
The interpretability of trends in a time series plot is greatly affected by the length and units of the time series. FoodNet Fast aggregates data annually, as shown in Supplementary Figure S2 , which provides clear, concise information on annual rates. In this example, the rate of salmonellosis remains largely unchanging over time with distinct outbreaks seen in 1999 and 2010. As expected, by compressing data to annual rates, Supplementary Figure S2 masks within-year trends of disease rates. FoodNet reports and publications similarly tend to show only inter-annual changes in disease counts or rates 9 , 10 , 11 , 12 , 13 , 37 , 38 . Without more granular within-year variations, the viewer cannot determine if increased yearly rates are driven by erratic outbreaks in a specific month or higher rates across all months of the year.
To capture within-year trends, we propose a multi-panel plot that combines information on monthly rates, inter-annual trends, and the frequency distribution of rates by utilizing the shared axes of individual plots (Fig. 1 ). The right panel of Fig. 1 provides a time series of monthly rates with a NBHR model fit with three trend terms (linear, quadratic, and cubic). The inclusion of polynomial terms allows us to capture long-term trends (linear term) and their acceleration and deceleration over time (quadratic and cubic terms). The predicted trend line is shown in blue and its 95% confidence interval is in grey shades. The estimated median monthly rate is shown in red. The left panel depicts a rotated histogram of rate frequencies indicating the right-skewness of the monthly rate distribution. The histogram shares the vertical monthly rate-axis with the time series plot and is essential for connecting two concepts: the distribution of monthly counts on the base of their frequency and the distribution of monthly counts over time. Two pictograms refer to the selected pathogen and location.
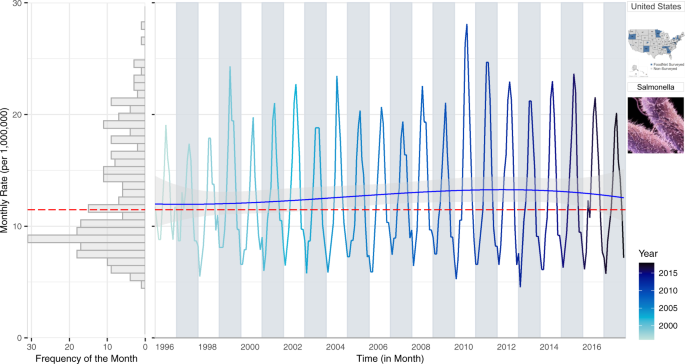
A multi-panel plot: a rotated histogram of monthly rate frequency (left panel) sharing the vertical monthly rate-axis with the time series of monthly rates (right panel) for salmonellosis in the US from 1996–2017. The red line indicates the median rates while the blue line is a NBHR model fit with seasonal oscillators and three (linear, quadratic, and cubic) trend terms.
Figure 1 shows the stability of seasonal oscillation in salmonellosis over time series with increased rates from 1998–2010 followed by a gradual decrease in rates through 2017. While preserving the within-year seasonal fluctuations, the plot provides additional information. Alternating background colours help distinguish differences in the shape of seasonal curves between adjacent years. An increasingly darker hue for the monthly rate values distinguishes more recent data from more historic data. Contrasting background colours mixed with a gradual intensity of line hues, saturation, brightness, and transparency allow for greater focus and attention to trends in the data 32 , 33 , 34 .
The rotated histogram in the left panel of Fig. 1 shows the distribution of monthly rates and its degree of skewness due to months with high counts. We include the red median line to provide the most appropriate measure of central tendency for the skewed distribution. The shared vertical axis helps readers track those high values to a specific month in the time series. The distribution also justifies the use of negative binomial regression models to evaluate temporal patterns. By supplementing the time series plot with the distribution of monthly rates, we show a visual rationale for using appropriate analytical tools (negative binomial model, in this case) for calculating inter-annual trends.
Examining seasonal signatures
FoodNet reports describe seasonal patterns with the month with the highest cases per year 13 . Supplementary Figure S3 provides an example of a typical seasonal curve from the FoodNet Fast portal. The estimates are the average percentage of confirmed cases per month for salmonellosis in the US from 1996–2017. This visualization provides easily computed and interpreted information on seasonality: on average, salmonellosis cases peak in August for this 22-year period. However, the data compression and use of relative measurements, such as average percentages, masks variability in monthly values across years. Supplementary Figure S3 leaves certain questions unanswered. Do seasonal patterns vary by year? Is this gradual incline and decline in the signature stable over the 22-years or masking frequent erratic outbreaks? What is the variability in cases at each time of year? What are the actual rates for each month of the year for each year?
To better understand annual differences in seasonal behaviors, we propose a multi-panel plot that incorporates annual seasonal signatures, summary statistics of monthly rates, and radar plots (Fig. 2 ). Given varying visual perceptions of these three ways of presenting seasonal patterns, we offer side-by-side comparisons that aim to increase comprehension. The top-left panel provides an overlay of all annual seasonal signatures, a set of curves depicting characteristic variations in disease incidence over the course of one year, where line hues become increasingly darker with more recent data and a red line indicates median monthly rates, as in Fig. 1 . The bottom-left panel provides a set of box plots for each month that aggregates information over the study period and provides essential summary statistics, including the median rate values and the measures of spread. The shared horizontal axis allows the two plots to be compared across the years using identical scales. To provide visual context, background colours were used to indicate the four seasons (winter, spring, summer and autumn). The right panel provides overlaying monthly rates using a radar plot where time is indicated on the rotational axis and rates are indicated on the radial axis. The radar plot emphasizes the periodic nature of seasonal variations in one continuous line with graduating colours. The colour hue of the lines, background colour, median line colour and the axis scales are uniform across all three panels. We also repeat the pictograms to refer to the selected pathogen and location.
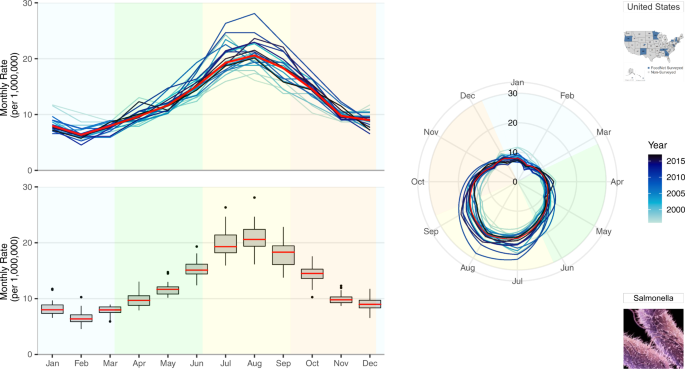
A multi-panel plot for visualizing seasonal signatures of salmonellosis monthly rates in the US from 1996–2017. This includes overlaid annual time series plots of monthly rates, a box plot of average monthly rates for the 22-year period, and overlaid annual radar plots of monthly rates. Background colours indicate the four seasons defined by solar solstices and equinoxes: winter (blue), spring (green), summer (yellow), and autumn (orange).
For salmonellosis, disease rates are highest in the summertime (with peaks in July and August) and lowest during the wintertime (with a well-defined February nadir). Rate increases and decreases during equinox periods indicate bacterial growth rates due to more and less favourable climate conditions, respectively.
The top-left panel of Fig. 2 disaggregates annual seasonal signatures to show the stable seasonal peak timing of salmonellosis across all years. This stable behavior reflects the average monthly percentages shown in Supplementary Figure S3 . This panel provides the seasonal signatures of salmonellosis rates and helps to better understand the annual variations hidden in Supplementary Figure S3 . Supplementary Figures S4 and S5 provide examples of how occasional irregular outbreak behaviors could be identified using this annual overlay plotting technique.
The bottom-left panel of Fig. 2 overcomes another deficiency of Supplementary Figure S3 of masking variations. This panel provides box plots depicting the median monthly rate (red), interquartile range (box), 95% confidence interval (whisker), and outliers or potentially influential observations (markers) over the 22-year period. Measures of distribution spread provide an insight for the dispersion of rates in each month: the variability of salmonellosis rates decreases in winter months closer to the February nadir but increases in summer months of July and August closer to the seasonal peak. Unusually high values are indicative of erratic behavior characterized by spikes in specific months and years.
The right-hand panel of Fig. 2 further emphasizes the periodic nature and the positioning of the seasonal peaks and nadirs. Radar or spider plots describe time using a rotational axis where the radial distance from the centre of the plot depicts rate magnitude 39 , 40 , 41 , 42 . Radial axes, compared to perpendicular axes, show annual fluctuations as a continuous flow. This more clearly demonstrates declines of salmonellosis rates during nadir months (November to March) without the visual discontinuity of left panel visuals.
To capture the advantage of a multi-panel plot (Fig. 3 ), we incorporate the boxplot from Fig. 2 (lower left panel) with a calendar heatmap containing 264 monthly rate values. In the heatmap, information for each individual year is shown as stacked rows of width 12 (for each month of the year) where cell colour intensity represents the magnitude of monthly rates. Like Fig. 2 , the heatmap illustrates the highest rates (shown as the darker cells) are in July and August. Compared to stacked line plots, however, Fig. 3 provides an individual row for each year of the time series, allowing for greater decomposition, differentiation, and comparison of seasonal signatures across years. In this plot, seasonal changes are shown horizontally from left to right - from January to December and the yearly trend transition can be observed in a vertical view from bottom to top-from year 1996 to 2017 in the right panel.
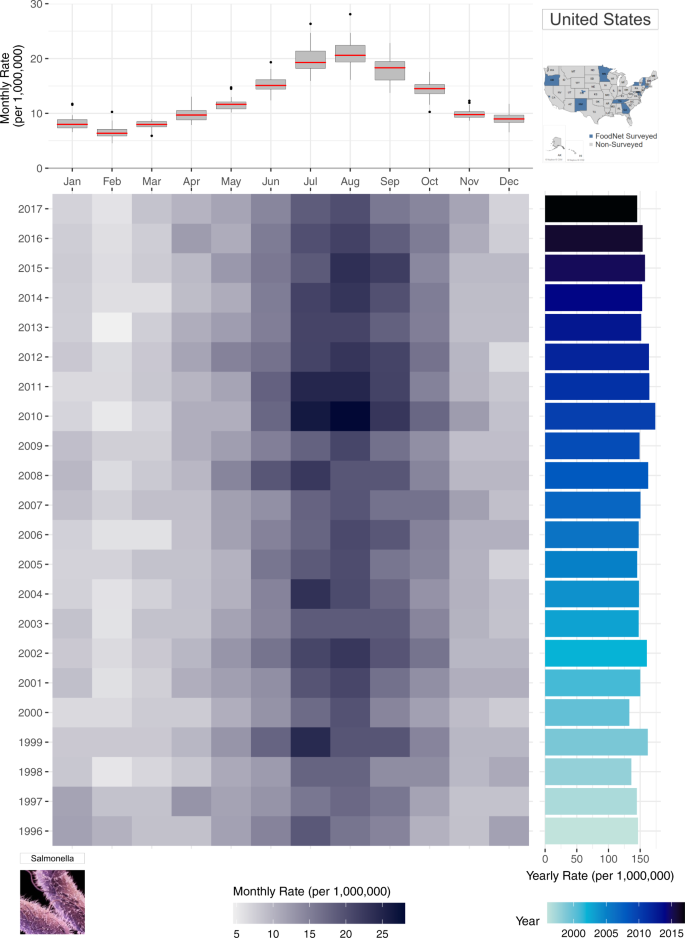
A multi-panel plot for improved visualization of the annual seasonal signatures of monthly rates of salmonellosis in the US from 1996–2017. The top panel provides a box plot of monthly rates for each month of year. The bottom heatmap shows the distribution of monthly rates for each year where darker hues indicate greater rates. The right panel provides a rotated bar graph of yearly rates.
While Fig. 2 provides the annual variability of seasonal patterns, monthly rate values for each year are difficult to ascertain. Instead, the emphasis is placed on similarities and differences of the seasonal curvature over time. In Fig. 3 , the attention shifts to comparing the intensity of rates per month of the year across years. Here, we evaluate which months of the year are most intense across years using the intensity of each cell’s colour hue to describe the intensity of rates. The Fig. 3 panel integrates information on both trends and seasonality along with the individual monthly values unlike any of the previously shown visualizations. Yearly rates provide a bar graph for comparing fluctuations in inter-annual rates while the adjacent heatmap indicates the month(s) driving these fluctuations. In doing so, the calendar heatmap identifies whether inter-annual changes are driven by sporadic outbreaks or increased seasonal magnitude of rates. At the same time, the shared axis box plot provides an overview of the average seasonal signature for the entire time series, as emphasized in Fig. 2 .
Understanding seasonal features
Detailed characterization of the timing and intensity of seasonal peaks requires a standardized estimation of peak timing and amplitude. This standardization improves upon implemented techniques of comparing months with the highest cases in a given year by applying the δ–methods to NBHR model parameters 29 , 30 . Average seasonality characteristics can be estimated across the full time series while annual estimates allow for more granular comparisons between years. To depict point estimates and confidence intervals of seasonality characteristics, we use forest plots - a technique commonly used in meta-analyses 18 , 43 , 44 . We develop a multi-panel forest plot to depict annual peak timing, annual amplitude, and their joint distribution, to better understand the relationship among the seasonal features and how it changes over time (Fig. 4 ).
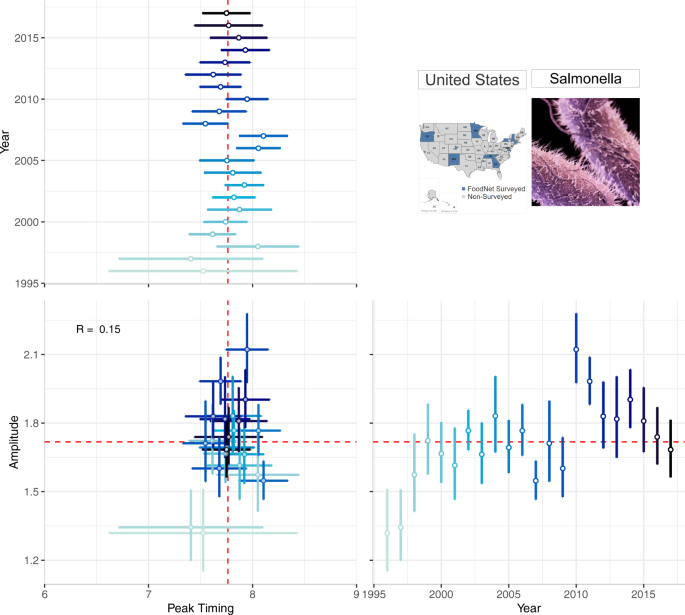
A multi-panel plot for visualizing the annual peak timing and amplitude of salmonellosis in the US from 1996–2017. The top-left panel shows the peak timing of salmonellosis by year; the bottom-right panel shows the annual amplitude by year. The bottom-left panel shows their combination: a scatterplot between peak timing and amplitude. Marker colour intensity indicates more historic vs. more recent data, horizontal and vertical whiskers provide measures of uncertainty, and red lines indicate median peak timing and amplitude across 22 years.
Figure 4 is a multi-panel plot that incorporates two forest plots (one each for annual peak timing and amplitude estimates) and one scatterplot (for peak timing and amplitude) to describe seasonality features. The top-left panel shows peak timing estimates (as month of the year, ranging from 1.0 (beginning of January) to 12.9 (end of December) - horizontal axis) for each study year (vertical axis). The bottom-right panel shows amplitude estimates where the horizontal axis indicates the study year and the vertical axis shows the amplitude (ratio between the disease rate at peak and the median rate). The bottom-left corner shows the scatterplot of peak timing (horizontal axis) and amplitude (vertical axis) with markers representing each pair of annual estimates. Measures of uncertainty (95% confidence intervals) are reflected in error bars of each marker; dashed red lines show median peak timing and amplitude estimates.
Forest plots in Fig. 4 provide a compact, clear, and comprehensive visual describing the stability of peak timing and amplitude, even without showing the entire seasonal signature. For example, salmonellosis peak timing and amplitude vary little each year indicating strong, stable seasonal peaks in July and August. Consistent peak timing means practitioners could time preventive strategies, increase awareness for foodborne illnesses to prevent transmission, and inform food retailers of when food safety inspections should be in higher demand within their supply chains. Consistent amplitude estimates show that the intensity of salmonellosis varies little over time, suggesting that federal food safety regulations have not greatly influenced the number of salmonellosis cases annually. This type of information is likely to benefit FoodNet Fast users.
Supplementary Figure S6 provides an example of how a sporadic outbreak behavior can be depicted by forest plots of peak timing and amplitude estimates for shigellosis in NY. The lack of seasonality for shigellosis is shown by the broad confidence intervals for peak timing, spanning the entire year and beyond.
Drawing comparisons across locations
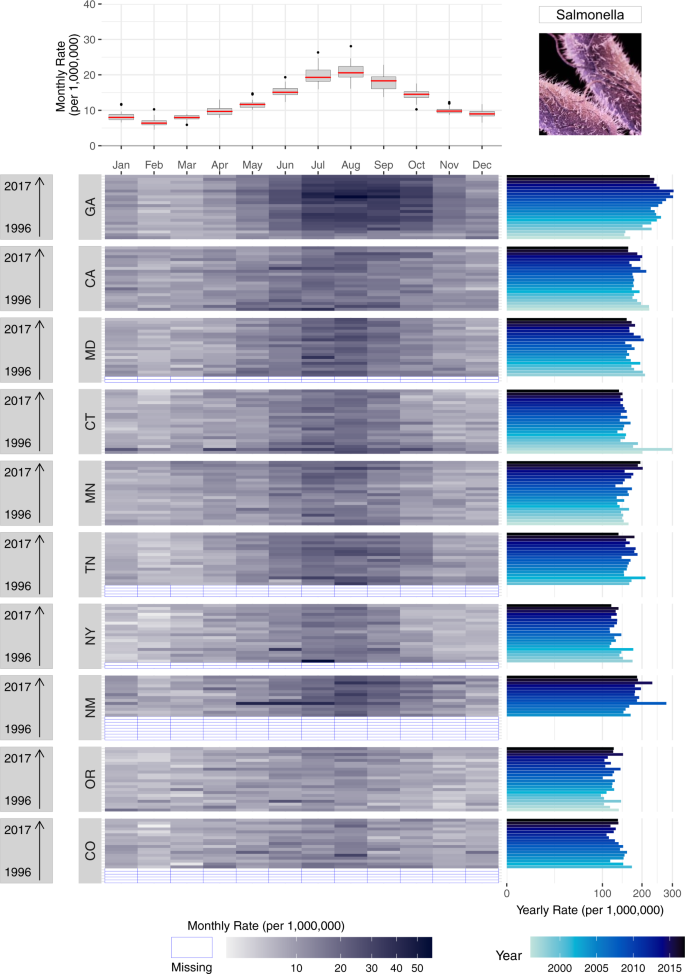
Supplementary Figure S7 provides an example bar chart showing differences in the average annual incidence of salmonellosis for the ten FoodNet-surveyed states. As with other FoodNet visualizations, data has been compressed to show only average annual estimates. Like in Fig. 1 , annual rates mask within-year seasonal variations, calling into question if differences in states are driven by single year outbreaks. The alphabetical organization of the horizontal axis makes states ranking and comparison more difficult than if they were ordered from highest to lowest rates. To ease comparisons of a single disease across geographic locations, we generated two multi-panel plots (Figs. 5 and 6 ). These plots mirror the same techniques shown above but include multiple shared axes and multiple locations to draw spatial comparisons.
A multi-panel plot for comparing seasonal signatures and yearly rates of salmonellosis in the US from 1996–2017 disaggregated by the ten FoodNet-surveyed states. The top panel provides a box plot of monthly rates for each month of year for the US. The calendar heatmap uses shared horizontal axes to show the distribution of monthly rates for each year and each state. Darker hues indicate higher rates while empty cells with blue borders indicate years when FoodNet surveillance was not conducted. The right panel provides a rotated bar graph of yearly rates. Given sizable differences in rates across states we applied a high-order calibration colour scheme.
A multi-panel plot for visualizing the annual peak timing and amplitude of salmonellosis in ten FoodNet-reporting states and the US from 1996–2017. The top-left panel shows the average peak timing of salmonellosis per location while the bottom-right panel shows the average amplitude per location. The bottom-left panel shows a combined scatterplot between peak timing and amplitude estimates.
Supplementary Figure S8 follows the same design as Fig. 3 ; we replicate this design for salmonellosis in all FoodNet-surveyed states. We present all states in one plot in a descending order by the sum of yearly rates in each state and display all available data so that state level patterns can be compared. The box plot in the top panel provides an overview of the seasonal signature for the entire US. The bottom panel disaggregates the entire US by states. As shown, all states share similar peak timing in July and August for almost every surveillance year from 1996–2017. For some states, like GA and CA, rates are densely concentrated from July to September with rapid decline from September to February and gradual incline from February to July. For other states, like NY and OR, seasonal peaks are much less pronounced and rate differences are smaller between months. Clear indication of missing data provides additional information on differences in reporting completeness not captured by previous figures.
While heatmaps provide information on seasonal signatures, yearly rate bar graphs (right panel) capture state-level trends over time. States are stacked in the order of total cases from 1996–2017, showing differences in the intensity of salmonellosis infection across states. Comparisons within states between years help identify inter-annual rate changes over time. For example, while MD and CA have generally declined in annual rates over the 22-year period, GA rates increased from 1996–2012 and steadily declined from 2012–2017. In combination with heatmaps, yearly rates also allow for detailed assessment of sporadic outbreaks. For example, erratic outbreaks came from two monthly spikes in April and June for CT in 1997 while for NM in 2000 a multi-month outbreak lasted from May to July.
By using shared horizontal and vertical axes, this plot eases the comparison of disease rates across months, years and states. It also helps to determine hotspots and detect potential co-occurrences of infection in different states. Moreover, the plot can be periodically updated by adding new information, offering a sustainable approach to make consistent comparisons between historical data and data captured in the future.
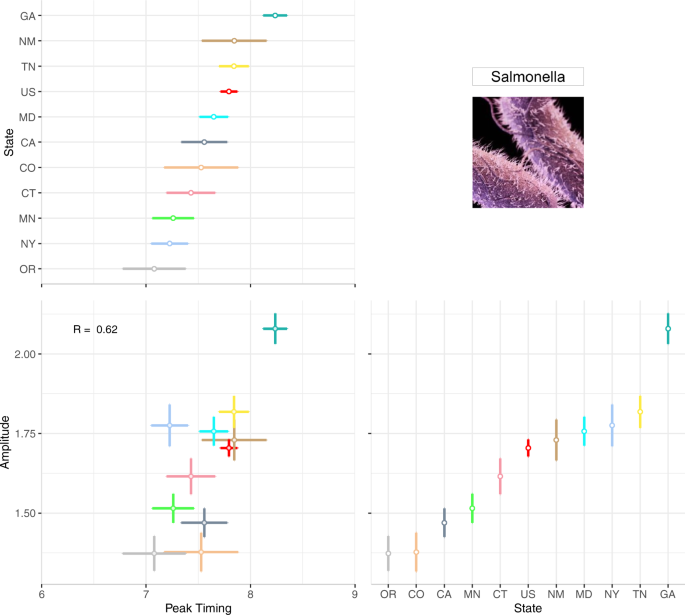
To compare seasonality features across locations, we designed a multi-panel plot similar to Fig. 4 to show average peak timing and amplitude estimates over the 22-year period for each state. In Fig. 6 the top-left panel plots peak timing estimates ordered from the earliest (OR) to latest (GA) peak timing while the bottom-right panel plots amplitude estimates in order of magnitude. Marker and line colours are used to differentiate the seasonality feature estimate and its measure of uncertainty between states. The bottom-left panel shows the relationship between peak timing and amplitude across states.
Comparisons across diseases
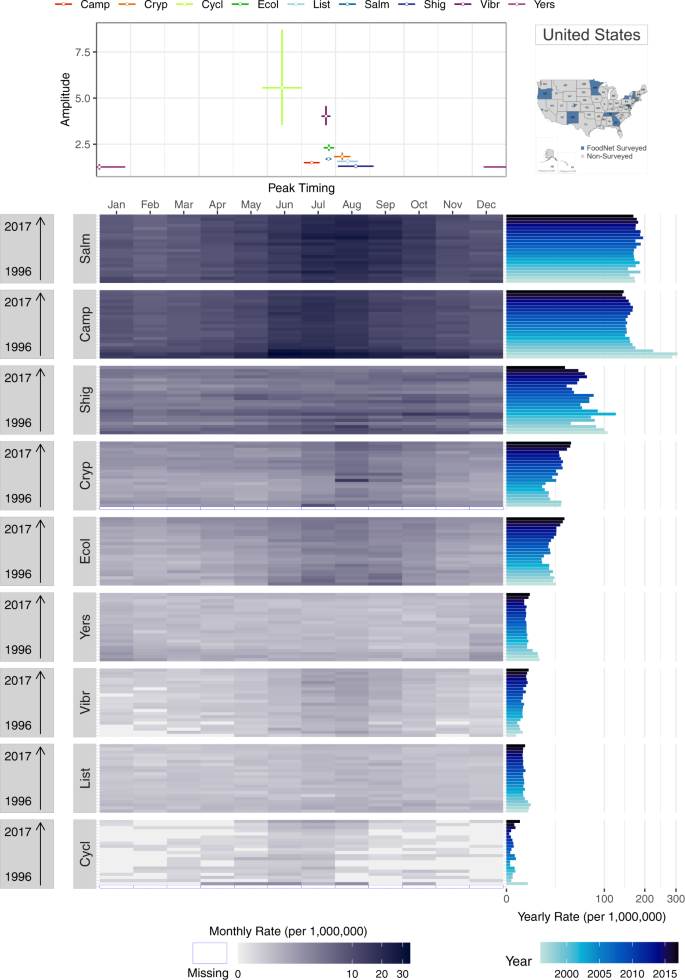
Supplementary Figure S8 provides an example of FoodNet Fast bar chart showing differences in the total confirmed infections for each of the nine surveyed pathogens in the US from 1996–2017. The visual shows that infections due to Campylobacter and Salmonella have the highest cumulative counts of infections while Cyclospora has the lowest counts. While depicting these differences clearly, this visual lacks sufficient specificity for drawing more intricate comparisons between infections. How are counts or rates distributed by year? What are the within-year variations of rates by pathogen? How do seasonal signatures and their variability differ by pathogen? Can axes be reordered or recalculated for easier comparisons between pathogen counts or rates? We propose two multi-panel plots (Figs. 7 and 8 ) that improve the comparisons of multiple diseases for a given geographic location.
A multi-panel plot for comparing seasonal signatures and yearly rates of nine FoodNet-reported infections in the US from 1996–2017. The top panel provides a scatterplot of average peak timing and amplitude estimates per pathogen across the 22-year period. The bottom heatmap uses shared horizontal axes to show the distribution of monthly rates for each year and each disease (campylobacteriosis (Camp), listeriosis (List), salmonellosis (Salm), shigellosis (Shig), infections caused by Shiga toxin-producing Escherichia coli O157 and non-O157 (Ecol), vibriosis (Vibr), infections caused by Yersinia enterocolitica (Yers), cryptosporidiosis (Cryp) and cyclosporiasis (Cycl)). Darker hues indicate greater rates while empty cells with blue borders indicate years when FoodNet surveillance was not conducted. The right panel provides a rotated bar graph of yearly rates.
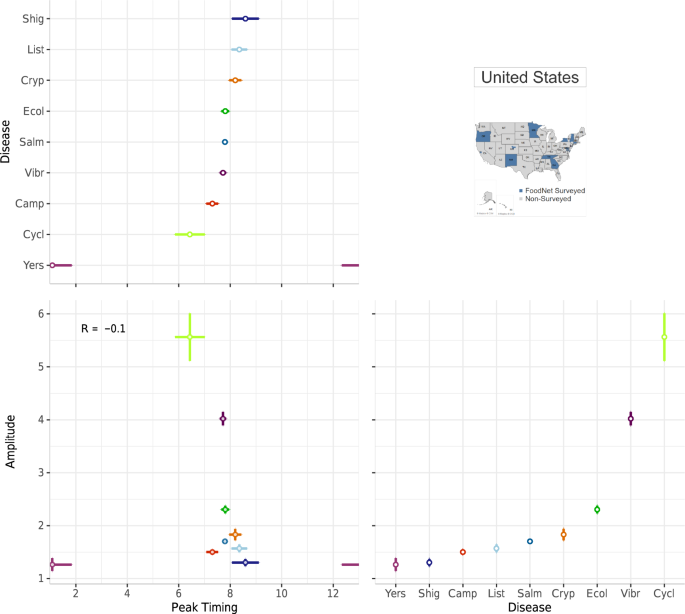
A multi-panel plot for visualizing the average peak timing and amplitude of nine FoodNet-reported pathogens in the US from 1996–2017. The top-left panel shows the average peak timing of each disease while the bottom-right panel shows the average amplitude per disease (campylobacteriosis (Camp), listeriosis (List), salmonellosis (Salm), shigellosis (Shig), infections caused by Shiga toxin-producing Escherichia coli O157 and non-O157 (Ecol), vibriosis (Vibr), infections caused by Yersinia enterocolitica (Yers), cryptosporidiosis (Cryp) and cyclosporiasis (Cycl)). The bottom-left panel shows a combined scatterplot between peak timing and amplitude estimates.
Figure 7 replicates the plot design of Fig. 5 but emphasizes comparisons between pathogens for a single location. Instead of a seasonal signature box plot, the top panel provides a scatterplot to illustrate the peak timing and amplitude of each pathogen. In combination with the heatmap in the bottom panel, these plots illustrate the strong seasonality of salmonellosis, campylobacteriosis, and STEC in July and August and cryptosporidiosis in August. These seasonal peaks are consistent across almost all years suggesting a stable seasonal periodicity and strong alignment between infections. In contrast, infections caused by Yersinia enterocolitica , vibriosis, listeriosis, and cyclosporiasis have much less pronounced seasonality and monthly rates much lower than salmonellosis or campylobacteriosis. Yearly rates, shown in the right panel, indicate erratic outbreak behaviors for cyclosporiasis. Given sizable differences in rates across diseases we applied a high-order calibration colour scheme.
We also provide the same multi-panel, shared-axis visualization design seen in Fig. 6 for comparisons across pathogens. Figure 8 includes a forest plot of peak timing by disease pathogen (top-left panel), a forest plot of amplitude by pathogen (bottom-right panel), and a scatterplot between peak timing and amplitude estimates (bottom-left panel). As in Fig. 6 , average peak timing and amplitude estimates are calculated using NBHR models for the entire 22-year time series. Comparisons between diseases allow for understanding the alignment of seasonal processes across pathogens as well as shared relative magnitudes in a specific location. In our case, most of the pathogens peak during the summertime except cyclosporiasis. However, if the selected diseases peak during winter months, we recommend adjusting the starting and ending months to center these peaks in the figure.
In this study we offered ways of thinking on how public data platforms can be improved by using visual analytics to provide a comprehensive description of trends and seasonality features in reported infectious diseases. We emphasize the utility of multi-panel graphs by showing side-by-side different methods of depicting trends over time and features of seasonality, including disease peak timing and amplitude. We provided visual tools to show trends (Fig. 1 ), examine seasonal signatures (Figs. 2 and 3 ) and their characteristics (Fig. 4 ), compare diseases across locations for trends (Fig. 5 ) and seasonal signatures (Fig. 6 ), and drawing comparisons across pathogens for trends (Fig. 7 ) and seasonal signatures (Fig. 8 ). We also provide guides on how to explore and compare trends and seasonality between multiple diseases and geographic locations using FoodNet Fast data. Given varying visual perceptions, we offer side-by-side comparison of different tools aiming to increase comprehension and faster adoption of efficient graphical depictions.
We developed a time series of monthly rates by reconstructing a time series of monthly counts (see Fig. 1 ) then dividing counts by the sum of all FoodNet-surveyed counties’ mid-year populations per state per 1,000,000 persons. In this calculation, we recognize that average monthly percentages are rounded in the raw data file and do not sum to 100% annually for downloaded years. This rounding resulted in obtaining non-integer counts within our time series. To prevent modification of raw data files, we did not round counts to integers before or after calculating rates. No information is provided on the FoodNet Fast website for the definition of confirmed cases, and data downloads provide no metadata for distinguishing cases from hospitalizations and deaths. Although the case definition is provided on the CDC website as “laboratory-confirmed cases (defined as isolation for bacteria or identification for parasites of an organism from a clinical specimen) and cases diagnosed using culture-independent methods” 45 , it forces the user to assume that a confirmed case is any person with laboratory confirmed cultures of a specific pathogen who may or may not have been hospitalized or died from infection. FoodNet also collects information on hospitalizations and deaths, but does not provide information on the monthly percentage of hospitalizations or deaths, so users are unable to reconstruct a monthly time series for deaths or hospitalizations.
The FoodNet Fast platform states all confirmed diseases as “incidence” calculations. Technical documentation on the FoodNet website shows that the term incidence reflects cases per 100,000 persons (used interchangeably with a disease rate) with no distinction of whether these are newly introduced within the population (i.e. incidence) or the total persons diagnosed with a disease (i.e. prevalence) 46 . We found that monthly rates can similarly be calculated by multiplying annual incidence rates and the monthly percentage of confirmed cases for each disease-state pair. Differences between our calculations and this alternative method are no more than ± 2%. We suspect that rounding errors of average monthly percentages and differential population catchment areas for rate calculations cause these differences. As shown in Supplemental Table S1 , population of the surveillance catchment area is changing over time. Oftentimes, publicly available surveillance datasets, including FoodNet Fast, do not include location- and year-specific population catchment area estimates, which are needed for calculating rates from diseases counts. As FoodNet does not provide population catchment areas for calculating rates, it forces the user to assume that FoodNet surveillance reaches the total population of a surveyed county (likely an overestimate), yet such oversight is easy to fix. Three collaborators confirmed our monthly rate calculations for quality control.
We applied the negative binomial harmonic regression NBHR models, commonly used in the time series analysis of counts and cases. While the use of NBHR models, specifically the inclusion of trigonometric harmonic oscillations, is similar to existing works on foodborne illnesses, these studies often incorporate harmonic oscillators only to adjust for or remove seasonal oscillations 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 . We have extended the use of harmonic terms and develop the tools to estimate peak timing and amplitude 8 , 29 , 30 . The developed δ-method provides a systematic calculation of confidence intervals for peak timing and amplitude estimates based on the results of harmonic regression models. In the proposed approach, we present the amplitude as the ratio of seasonal peak to seasonal median, which offers robust estimation even for rare or highly sporadic infections. These features are not available when traditional models, like Auto-Regressive Integrated Moving Average (ARIMA), are applied 56 . Measures of uncertainty enable formal testing and comparisons across diseases in the same location or locations for the same disease. In our previous works, we have demonstrated the broad utility of the δ-method and applications of peak timing and amplitude estimation in the context of epidemiological studies 6 , 29 , 56 , 57 , 58 , 59 , 60 , 61 , 62 .
We evaluate each state’s cases individually as well as all national cases as the sum of all states’ cases. Our analysis evaluated all cases reported to FoodNet Fast irrespective of demographic factors such as age group, sex, or ethnic group. Future analyses can consider conducting analyses using demographic factors available on the FoodNet Fast platform such as age group (<5, 5–9, 10–19, 20–29, 30–39, 40–49, 50–59, 60–69, 70 + years), sex (male and female), and ethnic group (American Indian and Alaskan Native, Asian and Pacific Islander, Black, Multiple, White). To incorporate this information, our methodology for data extraction would need to be repeated for each subcategory or combination of categories desired (e.g. download 221 files for males and 221 files for females). Future analyses can also consider differences in pathogen strain, which can only be obtained if extracting data for each pathogen-location-year combination (e.g. 221 files for each of the 9 diseases for each of 11 locations or 21,879 files).
FoodNet Fast, like many global disease surveillance databases, has no metadata describing missing data. FoodNet Fast reports missing counts using “N/A” for years when pathogens or locations were not under surveillance. However, there are also years when FoodNet surveillance was live in a state, but a pathogen is missing from the data download. We believe that this missing data comes when, for a given year, a pathogen has 0 total cases. However, we cannot specify whether absences of surveillance reporting came due to a breakdown in reporting or 0 annual counts. Without specification, we have set any year with “N/A” as missing due to no reported case information.
When calculating peak timing and amplitude using the δ-methods, we applied NBHR models adjusted for harmonic seasonal oscillators and three trends (linear, quadratic, and cubic). We selected the polynomial terms as an example, yet researchers can consider alternative techniques for measuring seasonality such as splines, nonparametric regression, ARIMA models, or their extensions. Additionally, the CDC recommends using a mixed effects model when conducting time series analyses on FoodNet Fast data to account for differential population catchment areas and laboratory culture confirmation techniques pre- and post-2004 1 , 2 , 46 . We focus on the analysis of individual states and diseases and adjust for population catchment variations by calculating monthly rates using county-level population estimates. Future analyses could include detailed assessments between peak timing and amplitude across diseases, locations, and time periods. Such analyses will help determine whether a synchronization of outbreak peaks occurs or if social, economic, or environmental factors influence peak timing and amplitude.
Future applications
This analecta of visualizations intends to communicate detailed information on foodborne outbreak trends and seasonality suitable for a general audience, public health professionals, stakeholders, and policymakers. Future applications would involve the development of an interactive web-based platform allowing users to select the outcome, timeframe, and location of interest for educational training and research purposes. For example, public health researchers and practitioners could use this tool to generate insights related to long-term trends, changes in disease dynamics, or changes in populations at risk 62 . Information on when and where outbreaks are most common enable producers, distributors, and retailers to improve food safety practices to prevent these outbreaks. Finally, this platform could aid policymakers in shaping public understanding of outbreak dynamics and using scientific evidence to refine public health policies.
Data availability
The analecta of our time series of monthly rates, 436 data visualizations, and code used for all calculations and visualizations are available on our website ( https://sites.tufts.edu/naumovalabs/analecta/ ). Data and code can be directly downloaded from the website while visualizations are linked on the website to an external visualization repository. Time series data and code are also available on figshare 36 . Visualizations on our website are provided in the same order as presented here: describing trends (Fig. 1 ), examining seasonal signatures with the three standard techniques: line graphs, boxplots, and radar plots (Fig. 2 ) and heatmaps (Fig. 3 ), characterizing features of seasonality (Fig. 4 ), drawing comparisons across locations for trends (Fig. 5 ) and seasonal signatures (Fig. 6 ), and drawing comparisons across pathogens for trends (Fig. 7 ) and seasonal signatures (Fig. 8 ). File downloads are available for trend, seasonal signature, and annual time series visualizations. For images examining a single disease in a single location, downloads are formatted where the prefix abbreviates the location and the suffix abbreviates the pathogen (see Supplementary Table S3 ). For visualizations comparing multiple locations or diseases, the prefix “LOC” indicates comparisons across locations while the prefix “DIS” indicates comparisons across pathogens (see Supplementary Tables S4 , S5 ).
Code availability
All statistical analyses were conducted using STATA (SE 15.1) software. All visualizations were created using R Version 3.6.2 and Tableau Professional 2019.1 software. All software code is open access on our website ( https://sites.tufts.edu/naumovalabs/analecta/ ) and figshare, and is available for public reuse with proper citation of this manuscript 36 .
Choi, J., Cho, Y., Shim, E. & Woo, H. Web-based infectious disease surveillance systems and public health perspectives: a systematic review. BMC Public Health. 16 , 1238 (2016).
Article PubMed PubMed Central Google Scholar
Ginsberg, J. et al . Detecting influenza epidemics using search engine query data. Nature. 457 , 1012–1014 (2009).
Article ADS CAS PubMed Google Scholar
Fefferman, N. & Naumova, E. N. Innovation in observation: a vision for early outbreak detection. Emerg. Health Threat. J. 3 , 7103 (2010).
Article Google Scholar
Keller, M. et al . Use of unstructured event-based reports for global infectious disease surveillance. Emerg. Infect. Dis. 15 , 689 (2009).
Buckeridge, D. L., Burkom, H., Campbell, M., Hogan, W. R. & Moore, A. W. Algorithms for rapid outbreak detection: a research synthesis. J. Biomed. Inform. 38 , 99–113 (2005).
Article PubMed Google Scholar
Lofgren, E., Fefferman, N. H., Naumov, Y. N., Gorski, J. & Naumova, E. N. Influenza seasonality: underlying causes and modeling theories. J. Virol. 81 , 5429–5436 (2007).
Article CAS PubMed Google Scholar
World Health Organization (WHO). FluNet. Global Influenza Surveillance and Response Systems (GISRS) https://www.who.int/influenza/gisrs_laboratory/flunet/en/ (2020).
Chui, K. K., Wenger, J. B., Cohen, S. A. & Naumova, E. N. Visual analytics for epidemiologists: understanding the interactions between age, time, and disease with multi-panel graphs. PLoS One. 6 , e14683 (2011).
Article ADS CAS PubMed PubMed Central Google Scholar
Crim, S. M. et al . Incidence and trends of disease with pathogens transmitted commonly through food — Foodborne Diseases Active Surveillance Network, 10 U.S. sites, 2006–2013. Morb. Mortal. Wkly. Rep. 63 , 328–332 (2014).
Google Scholar
Crim, S. M. et al . Preliminary incidence and trends of disease with pathogens transmitted commonly through food—Foodborne Diseases Active Surveillance Network, 10 US sites, 2006–2014. Morb. Mortal. Wkly. Rep. 64 , 495–499 (2015).
Marder, E. P. et al . Incidence and trends of diseases with pathogens transmitted commonly through food and the effect of increasing use of culture-independent diagnostic tests on surveillance—Foodborne Diseases Active Surveillance Network, 10 U.S. sites, 2013–2016. Morb. Mortal. Wkly. Rep. 66 , 397–403 (2017).
Huang, J. Y. et al . Disease with pathogens transmitted commonly through food and the effect of increasing use of culture-independent diagnostic tests on surveillance — Foodborne Diseases Active Surveillance Network, 10 U.S. sites, 2012–2015. Morb. Mortal. Wkly. Rep. 65 , 368–371 (2016).
Centers for Disease Control and Prevention (CDC). Foodborne Diseases Active Surveillance Network: FoodNet 2013 surveillance report. National Center for Emerging and Zoonotic Diseases https://www.cdc.gov/foodnet/pdfs/508-Compliant-2013-FoodNet-Annual-Report.pdf (2013).
Bennett, S. D. et al . Produce-associated foodborne disease outbreaks, USA, 1998-2013. Epidemiol. Infect. 146 , 1397–1406 (2018).
Ebel, E. D. W. et al . Comparing characteristics of sporadic and outbreak-associated foodborne illnesses, United States, 2004–2011. Emerg. Infect. Dis. 22 , 1193–1200 (2016).
Article CAS PubMed PubMed Central Google Scholar
Geissler, A. L., et al . Increasing Campylobacter infections, outbreaks, and antimicrobial resistance in the United States, 2004-2012. Clin. Infect. Dis . 65, 1624-1631 (2017).
Jiang, C. et al . Climate change, extreme events and increased risk of salmonellosis in Maryland, USA: Evidence for coastal vulnerability. Environ. Int. 83 , 58–62 (2015).
Keithlin, J., Sargeant, J., Thomas, M. K. & Fazil, A. Systematic review and meta-analysis of the proportion of Campylobacter cases that develop chronic sequelae. BMC Public Health 14 , 1203 (2014).
Moffatt, C. R., Glass, K., Stafford, R., D’Este, C. & Kirk, M. D. The campylobacteriosis conundrum - examining the incidence of infection with Campylobacter sp. in Australia, 1998-2013. Epidemiol. Infect. 145 , 839–847 (2017).
Centers for Disease Control and Prevention (CDC). FoodNet Fast: pathogen surveillance tool. Atlanta, Georgia: U.S. Department of Health and Human Services http://wwwn.cdc.gov/foodnetfast (2020).
Centers for Disease Control and Prevention (CDC). FoodNet surveillance. U.S. Department of Health and Human Services https://www.cdc.gov/foodnet/surveillance.html (2020).
Scallan, E. et al . Bacterial enteric diseases among older adults in the United States: Foodborne Diseases Active Surveillance Network, 1996-2012. Foodborne Pathog. Dis. 12 , 492–499 (2015).
Taylor, E. V. et al . Common source outbreaks of Campylobacter disease in the USA, 1997-2008. Epidemiol. Infect. 141 , 987–996 (2013).
Williams, M. S., Golden, N. J., Ebel, E. D., Crarey, E. T. & Tate, H. P. Temporal patterns of Campylobacter contamination on chicken and their relationship to campylobacteriosis cases in the United States. Int. J. Food Microbiol. 208 , 114–121 (2015).
Merriam-Webster Dictionary. Analects. Merriam-Webster https://www.merriam-webster.com/dictionary/analecta (2020).
United States Census Bureau. 1990s: county tables. United States Department of Commerce https://www.census.gov/data/tables/time-series/demo/popest/1990s-county.html#statelist_6 (2016)
United States Census Bureau. County intercensal tables: 2000-2010. United States Department of Commerce https://www.census.gov/content/census/en/data/tables/time-series/demo/popest/intercensal-2000-2010-counties.html (2017).
United States Census Bureau. Annual estimates of the resident population: April 1, 2010 to July 1, 2017. United States Department of Commerce https://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?pid=PEP_2017_PEPANNRES&prodType=table (2018).
Simpson, R. B. et al . Incorporating calendar effects to predict influenza seasonality in Milwaukee, Wisconsin. Epidemiol. Infect. 147 , 1–13 (2019).
Falconi, T. A., Cruz, M. S. & Naumova, E. N. The shift in seasonality of legionellosis in the USA. Epidemiol. Infect. 146 , 1824–1833 (2018).
Davis, R. A. & Rongning, W. A negative binomial model for time series of counts. Biometrika. 96 , 735–749 (2009).
Article MathSciNet MATH Google Scholar
Munzner, T. Visualization analysis and design. CRC press (2014).
Fey, S. Information Dashboard Design: The Effective Visual Communication of Data. O’Reilly (2006).
Castronovo, D. A., Chui, K. K. H. & Naumova, E. N. Dynamic maps: a visual-analytic methodology for exploring spatio-temporal disease patterns. J. Environ. Health. 8 , 1–9 (2009).
Alarcon Falconi, T. M., Estrella, B., Sempértegui, F. & Naumova, E. N. Effects of Data Aggregation on Time Series Analysis of Seasonal Infections. Int. J. Environ. Res. Public Health. 17 , 5887 (2020).
Article PubMed Central Google Scholar
Simpson, R. B., Zhou, B., Alarcon Falconi, T. M., & Naumova, E. N. An analecta of visualizations for foodborne illness trends and seasonality. figshare https://doi.org/10.6084/m9.figshare.c.4904736 (2020).
Henao, O. L., Jones, T. F., Vugia, D. J. & Griffin, P. M. Foodborne Diseases Active Surveillance Network - 2 decades of achievements, 1996–2015. Emerg. Infect. Dis. 21 , 1529–1536 (2015).
Scallan, E. Activities, achievements, and lessons learned during the first 10 years of the Foodborne Diseases Active Surveillance Network: 1996–2005. Clin. Infect. Dis. 44 , 718–725 (2007).
Smallman-Raynor, M. & Trevelyan, B. The spatial structure of epidemic emergence: geographical aspects of poliomyelitis in north-eastern USA, July –October 2016. J. R. Stat. Soc. Ser. A. Stat. Soc. 168 , 701–722 (2006).
Tunstall-Pedoe, H. et al . Prime mover or fellow traveller: 25-hydroxy vitamin D’s seasonal variation, cardiovascular disease and death in the Scottish Heart Health Extended Cohort (SHHEC). Int. J. Epidemiol. 44 , 1602–1612 (2015).
Su, D. et al . Season and outdoor temperature in relation to detection and control of hypertension in a large rural Chinese population. Int. J. Epidemiol. 43 , 1835–1845 (2014).
Semenza, J. C. et al . Climate change impact assessment of food- and waterborne diseases. Crit. Rev. Environ. Sci. Technol. 42 , 857–890 (2012).
Pintar, K. D. et al . A systematic review and meta-analysis of the Campylobacter spp. prevalence and concentration in household pets and petting zoo animals for use in exposure assessments. PLoS One. 10 , e0144976 (2015).
Article PubMed PubMed Central CAS Google Scholar
Qi, R. et al . Global prevalence of asymptomatic norovirus disease: a meta-analysis. EClinicalMedicine. 2 , 50–58 (2018).
Centers for Disease Control and Prevention (CDC). FoodNet Surveillance. U.S. Department of Health and Human Services https://www.cdc.gov/foodnet/surveillance.html#:~:text=FoodNet%20collects%20information%20on%20laboratory,diagnosed%20using%20culture-independent%20methods (2015).
Centers for Disease Control and Prevention (CDC). FoodNet Fast: pathogen surveillance tool FAQ. U.S. Department of Health and Human Services https://www.cdc.gov/foodnet/foodnet-fast/faq-pathogen-surveillance.html (2019).
Brankston, G. et al . Assessing the impact of environmental exposures and Cryptosporidium disease in cattle on human incidence of cryptosporidiosis in southwestern Ontario, Canada. PLoS One. 13 , e0196573 (2018).
David, J. M. et al . Do contamination of and exposure to chicken meat and water drive the temporal dynamics of Campylobacter cases? Epidemiol. Infect. 145 , 3191–3203 (2017).
De Roos, A. J. et al . Review of epidemiological studies of drinking-water turbidity in relation to acute gastrointestinal illness. Environ. Health Perspect. 125 , 086003 (2017).
Djennad, A. et al . Seasonality and the effects of weather on Camylobacter diseases. BMC Infect. Dis. 19 , 255 (2019).
Hulland, E. et al . Increase in reported cholera cases in Haiti following Hurricane Matthew: an interrupted time series model. Am. J. Trop. Med. Hyg. 100 , 368–373 (2019).
Morris, A. et al . Complex temporal climate signals drive the emergence of human water-borne disease. Emerg. Microbes Infect. 3 , e56 (2014).
Rosenberg Goldstein, R. E. et al . Association between community socioeconomic factors, animal feeding operations, and campylobacteriosis incidence rates: Foodborne Diseases Active Surveillance Network (FoodNet), 2004–2010. BMC Infect. Dis. 16 , 354 (2016).
Rushton, S. P. et al . Climate, human behaviour or environment: individual-based modelling of Campylobacter seasonality and strategies to reduce disease burden. J. Transl. Med. 17 , 34 (2019).
Louis, V. R. et al . Temperature-driven Campylobacter seasonality in England and Wales. Appl. Environ. Microbiol. 71 , 85–92 (2005).
Alsova, O. K., Loktev, V. B. & Naumova, E. N. Rotavirus seasonality: an application of singular spectrum analysis and polyharmonic modeling. Int. J. Environ. Res. Public Health. 16 , 4309 (2019).
Naumova, E. N. Mystery of seasonality: getting the rhythm of nature. J. Public Health Policy. 27 , 2–12 (2006).
Wenger, J. B. & Naumova, E. N. Seasonal synchronization of influenza in the United States older adult population. PLoS One. 5 , e10187 (2010).
Article ADS PubMed PubMed Central CAS Google Scholar
Chui, K. K., Webb, P., Russell, R. M. & Naumova, E. N. Geographic variations and temporal trends of Salmonella-associated hospitalization in the U.S. elderly, 1991–2004: a time series analysis of the impact of HACCP regulation. BMC Public Health. 9 , 447 (2009).
Chui, K. K., Jagai, J. S., Griffiths, J. K. & Naumova, E. N. Hospitalization of the elderly in the United States for nonspecific gastrointestinal diseases: a search for etiological clues. Am. J. Public Health. 101 , 2082–2086 (2011).
Ramanathan, K. et al . Assessing seasonality variation with harmonic regression: accommodations for sharp peaks. Int. J Environ. Res. Public Health. 17 , 1318 (2020).
Stratton, M., Ehrlich, H., Mor, S. & Naumova, E. N. A comparative analysis of three vector-borne diseases across Australia using seasonal and meteorological models. Sci. Rep. 7 , 40186 (2017).
Download references
Acknowledgements
This research is based upon work supported in part by the Office of the Director of National Intelligence (ODNI), Intelligence Advanced Research Projects Activity (IARPA), via 2017-17072100002. The views and conclusions contained herein are those of the authors and should not be interpreted as necessarily representing the official policies, either expressed or implied, of ODNI, IARPA, or the U.S. Government. The U.S. Government is authorized to reproduce and distribute reprints for governmental purposes notwithstanding any copyright annotation therein. The research was in part supported by the National Science Foundation (NSF) Innovations in Graduate Education (IGE) Program, via grant award 1855886 and by the United States Department of Agriculture (USDA) National Institute of Food and Agriculture (NIFA) Cooperative State Research, Education, and Extension Service Fellowship, via grant award 2020-38420-30724. The authors would also like to thank Dr. Meghan Hartwick for editorial and technical assistance.
Author information
Authors and affiliations.
Tufts University Friedman School of Nutrition Science and Policy, Boston, USA
Ryan B. Simpson, Bingjie Zhou, Tania M. Alarcon Falconi & Elena N. Naumova
You can also search for this author in PubMed Google Scholar
Contributions
R.S. contributed to data extraction, formal analysis, and writing. B.Z. contributed to data validation, conceptualization of visual aids, and visualization creation. T.M.A.F. contributed to data validation, review, and editing. E.N.N. contributed to methodology development, review and editing, supervision, project administration and funding acquisition.
Corresponding author
Correspondence to Elena N. Naumova .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Simpson, R.B., Zhou, B., Alarcon Falconi, T.M. et al. An analecta of visualizations for foodborne illness trends and seasonality. Sci Data 7 , 346 (2020). https://doi.org/10.1038/s41597-020-00677-x
Download citation
Received : 30 March 2020
Accepted : 16 September 2020
Published : 13 October 2020
DOI : https://doi.org/10.1038/s41597-020-00677-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Review of visual analytics methods for food safety risks.
npj Science of Food (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Open access
- Published: 28 September 2022

Descriptive study of foodborne disease using disease monitoring data in Zhejiang Province, China, 2016–2020
- Xiaojuan Qi 1 ,
- Xialidan Alifu 2 , 3 ,
- Jiang Chen 1 ,
- Wenliang Luo 3 ,
- Jikai Wang 1 ,
- Yunxian Yu 2 , 3 &
- Ronghua Zhang 1
BMC Public Health volume 22 , Article number: 1831 ( 2022 ) Cite this article
4889 Accesses
9 Citations
Metrics details
This study aimed to identify the epidemiology, seasonality, aetiology and clinical characteristics of sporadic foodborne diseases in Zhejiang province during 2016–2020.
Descriptive statistical methods were used to analyze the data from surveillance network established by the Zhejiang Provincial Center for Disease Control and Prevention. There were 31 designated hospitals in all 11 cities which were selected using probability proportionate to size sampling method.
During the study period, the surveillance system received 75,124 cases with 4826 (6.42%) hospitalizations from 31 hospitals. The most common cause was Norovirus, 6120 cases (42.56%), followed by Salmonella, 3351 cases (23.30%). A significant seasonal trend was observed for the V. parahaemolyticus, with the highest rates over the summer period, peaking in August, 1171 cases (38.75%), a similar trend was also observed with Salmonella and Diarrheagenic E. coli. Norovirus infections showed the highest rate in November (904, 14.77%) and March (660,10.78%), the lowest in August, 215 cases (3.51%). Patients between 19 ~ 40 years were more likely to infected by Norovirus, V. parahaemolyticus and Diarrheagenic E. coli, patients below 1 year were the highest among patients with Salmonella infection, 881 cases (26.3%). The Norovirus, V. parahaemolyticus and Diarrheagenic E. coli infection with the highest positive detection rates among the workers were observed. The largest number cases of food categories were from aquatic product infection. The private home was the most common exposure setting.
Our study highlighted the necessity for conducting an active, comprehensive surveillance for pathogens in all age groups, to monitor the changing dynamics in the epidemiology and aetiology of foodborne diseases to guide policies that would reduce related illnesses.
Peer Review reports
Introduction
Foodborne illnesses are usually infectious or virulent and caused by bacteria, viruses, parasites or chemicals that enter the body through contaminated food or water. Although, food science and related technologies are developing rapidly, but still, it remains a challenge to prevent foodborne diseases completely [ 1 ]. An estimated 600 million in the world (almost 1 in 10 people), fall ill after eating contaminated food and 420 000 die every year, resulting in the loss of 33 million healthy life years in terms of Disability Adjusted Life Years (DALYs) according to an estimate based on the 2015 data [ 2 ]. Diarrhoeal diseases account for more than 50% of foodborne diseases, according to the data released by World Health Organization (WHO), foodborne or water-borne diarrhea alone causes about 2.2 million deaths worldwide every year [ 3 ]. As in other countries, foodborne diseases characterized by acute gastrointestinal diseases are the largest food safety problem as well as the most distressing food-related threat to public health in China [ 4 , 5 , 6 ]. In order to reduce the disease burden, China has established a web-based foodborne disease surveillance system since 2011, which has gradually played a role in food safety incidence prevention. The surveillance contents include hygiene indicator bacteria, pathogenic bacteria, viruses, and parasites in many food categories. Moreover, sampling points are no more limited to retail and catering sites, and have been extended to processing, and sales locations.
The studies discussed the characteristics of food contamination by pathogens according to surveillance data and reflects the contamination and distribution trend of foodborne pathogens in different regions. A wide range of representative agents (including pathogenic bacteria, viruses and etc.) are covered to understand their contamination in meat and meat products [ 7 ], milk and dairy products [ 8 ], eggs and egg products [ 9 ], children’s foods [ 10 ] and ready-to-eat foods [ 11 ]. Norovirus, Salmonella spp., Vibrio parahaemolyticus (V. parahaemolyticus), Shigella and Diarrheagenic E. coli have been identified as the most common pathogens responsible for foodborne diseases in China [ 12 , 13 ]. The surveillance data showed that occurrence of V. parahaemolyticus in aquatic products tended to increase over the period from 2015 to 2018 [ 11 , 14 ].
Safe food supplies support national economies, trade and tourism, contribute to food and nutrition security, and underpin sustainable development. As there are a limited number of existing epidemiological studies and reports on the foodborne diseases in Zhejiang province, the need for researches has become important. The aim of this study was to summarize epidemiological characteristics of foodborne disease cases and provide effective interventions to prevent foodborne disease illnesses in Zhejiang province, we analyzed the surveillance data of foodborne disease cases caused by Norovirus, Salmonella spp., Vibrio parahaemolyticus (V. parahaemolyticus), Shigella and Diarrheagenic E. coli in Zhejiang province from 2016 to 2020.
Geographical position, climatic and socio-demographic feature of study site
Zhejiang Province, one of the southeastern coastal provinces of China, is located at 27°02’N to 31°11’N and 118°01’E to 123°10’E [ 15 ], the 11 cities and their subordinate counties are listed in Supplementary Table 1. Zhejiang experience a subtropical humid climate. During summer the weather is hot and humid and the temperature is around 27 to 30 °C (81 to 86 °F). During winter the temperature falls down to a minimum temperature of 2℃ to 8℃ (36 to 46 °F). Rainfall and typhoons are a common phenomenon in summers. Zhejiang province has a permanent population of 65.4 million at the end of 2021, and GDP grew 8.5% year-on-year to 7.35 trillion yuan ($1.16 trillion) in 2021 [ 15 ]. Most of Zhejiang’s wealth derives from light industry and mostly located in rural villages [ 16 ].
Data source
Zhejiang Provincial Center for Disease Control and Prevention (ZJCDC) has collected foodborne disease relevant data through the China National Foodborne Diseases Surveillance Network (NFDSN) since 2012. 31 hospitals were inquired to detect 5 major pathogens and corresponding subtypes, including Salmonella, Norovirus, V. parahaemolyticus, Diarrheagenic E. coli and Shigella for all suspected foodborne disease cases, and reported illnesses through NFDSN since 2016. In this study the cases reported by 31 hospitals in Zhejiang province during the period 2016–2020 were included. Epidemiologists from the health departments first conducted the investigation to ascertain the full extent of the foodborne illness and the information collected for each case includes reporting region, date of occurrence, setting, etiology, food categories, number of illnesses / hospitalizations, and some other details. Unknown etiology refers to those foodborne disease cases where the confirmed etiology has not been identified. Foods was identified as the sources of disease through epidemiologic or laboratory methods and was classified into 13 categories. The food that cannot be determined was classified as “Unknown”. The GIS map data of Zhejiang Province is downloaded by the national basic geographic information center of China ( http://bzdt.ch.mnr.gov.cn/ ).
Statistical analysis
Total positive detection rate and hospitalization rate were calculated for each pathogen and linear trend test was used to test the change of positive detection rate and hospitalization rate annually for each pathogen. Chi-square test was used to compare the demographic characteristics, contaminated food category and food settings among four pathogens, including Salmonella, Norovirus, V. parahaemolyticus, Diarrheagenic E. coli while Shigella was not included due to limited sample sizes. Fisher exact test was used if the conditions were not met for Chi-square test. Post-hoc test was used for pairwise comparisons. Comparison was only programmed within illnesses with single etiology. Open-source software QGIS (Quantum GIS version 3.22.9) was used to map the spatial distribution of cases with positive detection rate caused by five pathogens for the period between 2016 and 2020. All statistical analyses were performed using R 3.6.2 and P -value was considered as significant at < 0.05.
General epidemiological characteristics
During the study period (2016–2020), the surveillance system received 75,124 cases with 4826 (6.42%) hospitalizations from 31 hospitals. As shown in Table 1 , total positive detection rate was 14,381(3.97%). The most common cause was Norovirus, 6120 cases (42.56%), followed by Salmonella, 3351 cases (23.30%), V. parahaemolyticus, 3022 cases (21.01%), Diarrheagenic E. coli,1849 cases (12.86%) and Shigella, 39 cases (0.27%). The positive detection rate increased in Salmonella and E. coli (from 3.37 to 6.59% and from 1.14 to 2.38%, respectively), while the rate for V. parahaemolyticus and Norovirus decreased during 2016–2020 (from 6.29 to 2.39% and from 10.62 to 6.62%, respectively); the rate in Shigella remained low level (Fig. 1 .A). As for hospitalization rate, a significant decrease of Norovirus and Salmonella was observed during the study period as well ( P < 0.001), with the highest in 2016 (from 12.62 to 6.55% and from 8.21 to 6.24%, respectively) (Fig. 1 .B). Among all cases with positive detection, which were being hospitalized, the most common cause was Salmonella (Table 1 ).
The change of positive detection rate (A) and hospitalization rate (B) of major pathogens during 2016–2020
The regional distribution of cases with positive caused by five pathogens among 11 cities, as shown in Fig. 2 : 2028 cases with 5.34% detection rate in Huzhou city, 1636 (4.89%) cases in Taizhou city, 1073 (4.88%) cases in Lishui city (Fig. 2 ).
The regional distribution of cases with positive detection rate caused by five pathogens
Characteristics for four pathogens
For this analysis, only the highest contributing pathogens were included (Salmonella, Norovirus, V. parahaemolyticus, and Diarrheagenic E. coli).
Trend and seasonality
A significant seasonal trend was observed for the V. parahaemolyticus, with the highest rates over the summer period, peaking in August, 1171 cases (38.75%). A similar trend was also observed with Salmonella and Diarrheagenic E. coli, with the peak in August, 612 cases (18.26%) and 335 cases (18.12%), respectively. Norovirus infections showed the highest rate in November (904 cases, 14.77%) and March (660 cases,10.78%) and the lowest in August, 215 cases (3.51%) (Fig. 3 ).
Monthly trends of selected foodborne diseases
Age, gender and occupational differences
A significant difference was observed between different age groups ( P < 0.01), with the majority of reported cases affecting young people aged 19–40 years, as shown in Table 2 . Among Salmonella infections, illnesses below one year old accounted for 26.30%, significantly higher than other three pathogens. V. parahaemolyticus showed much lower proportion for illnesses in population under 18 years old. As for gender distribution, though significantly different among four pathogens, all showed higher proportion in males ( P < 0.05). A significant occupational difference was observed. For Norovirus, V. parahaemolyticus and Diarrheagenic E. coli infection with the highest proportion among the workers. Salmonella infections showed the highest proportion in kids living scattered,1180 cases (35.21%) (Table 2 ).
Implicated foods and settings
In this study, four type of foodborne cases were reported due to certain food vehicles, as shown in Fig. 4 . Aquatic products were the most common cause for Norovirus, V. parahaemolyticus and Diarrheagenic E. coli infection (17.73%, 39.34% and 15.84%, respectively), followed by cooked meat products (17.04%, 15.57% and 15.73% respectively). The top three food vehicles in Salmonella infection were fruits (16.25%), aquatic products (12.36%) and cereals (12.29%). The places with more cases caused by four pathogens were household settings, followed by restaurants, data shown in Table 3 .
Food categories between foodborne disease cases
Among the Norovirus cases: 52.81% with abdominal cramps, 38.35% with vomiting, 38.28% with nausea; Salmonella caused 49.93% abdominal cramps, 28.20% fever, 19.04% nausea cases; V. parahaemolyticus caused 76.15% abdominal cramps, 46.92% nausea, 37.62% vomiting cases; Diarrheagenic E. coli caused 60.57% abdominal cramps, 25.26% nausea, 19.47% vomiting cases. Watery diarrhea was the most common symptom for four pathogens (Table 4 ).
Foodborne diseases impede socioeconomic development by straining health care systems, and harming national economies, tourism and trade. This study described the epidemiology of foodborne diseases caused by different pathogens in Zhejiang Province during the period 2016–2020. Over the 5 years, 75,124 cases with 4826 (6.42%) hospitalizations caused by Norovirus, Salmonella, V. parahaemolyticus, Diarrheagenic E. coli and Shigella from 31 hospitals were reported. Among 11 cities, 2028 cases in Huzhou city (14.33%), 1933 cases in Wenzhou city (13.66%), 1636 cases in Taizhou city (11.56%). The results were quite different from Sun Liang’s report, in which Wenzhou city accounts for the largest percentage of illnesses [ 17 ].
The number of illnesses caused by Norovirus ranks first among all etiologies, which is consistent with Shanghai, in which Norovirus was the most common pathogen (43.10%) [ 18 ], but quite different from the studies in China’s coastal provinces such as Hainan [ 19 ]. Wang [ 20 ] et al. reviewed 2447 papers in China that reported 1082 foodborne disease cases occurring between 1994 and 2005, in which V. parahaemolyticus caused the most events in littoral provinces, whereas in inland provinces, the largest percentage of cases were caused by Salmonella. Thus, there are regional differences in the distribution of pathogenic bacteria in China. These studies suggests that region-specific policies on foodborne disease control should be established.
Seasonality of foodborne illnesses was observed in this study. A seasonal trend was found for the V. parahaemolyticus, Salmonella and Diarrheagenic E. coli with the highest rates during summer period, peaking in August, this was similar in Enserink’s [ 21 ] and Gong’s [ 18 ] reports. However, the seasonal peak of infection attributed to some foodborne pathogens isn’t in the summer. For instance, Norovirus infections showed the highest rate in November and March and the lowest in summer, which was in line with previous studies [ 18 , 22 , 23 ]. Seasonality related to the temperature, humidity and rainfall, all of which may affect exposure frequency and host immune status. These findings indicated that temperature is an important factor in foodborne illnesses, and investigation of the reasons for the seasonal dominance on foodborne diseases should be the focus of surveillance.
This study showed the distinctive differences between four main pathogens with age groups. In general, the positive detection rate was higher in people aged 19 ~ 30 and 31 ~ 40 years than that in those aged < 18 and 40 + years, which were infected by Norovirus, V. parahaemolyticus and Diarrheagenic E. coli. This was partly consistent with a study in China which found incidence of foodborne diseases in youth group was higher than that in elderly group [ 14 ]. Also, a study in France which found incidence of foodborne diseases in young was higher than that in elders, in which, elders (≥ 60 years) were at least likely to get infected with V. parahaemolyticus, whereas people aged 30 ~ 44 years were the most likely get infected [ 24 ]. Similar results were observed in a Shanghai study [ 25 ]. In contrast to previous studies which found children (< 5 years) and elder people more likely to get infected with Norovirus [ 26 , 27 ], our study found that the highest proportion in Norovirus infections was people aged 19–30 years old. Among Salmonella infections, cases among children aged under 1 year old accounted for 26.30%, significantly higher than other age groups. Similar findings reported in Guangdong Province that children aged < 5 years were the group most affected by Salmonella (73%), of whom the infants under 1 year old were 81.5% [ 28 ]. As for gender distribution, though significantly different among four pathogens, all showed higher proportion in male. The Norovirus, V. parahaemolyticus and Diarrheagenic E. coli infection with the highest positive detection rates in the workers were observed. Foodborne illnesses among workers are liable to occur frequently because poor hygienic conditions at workers’ camps and work situations, in the meantime, high summer temperatures impacting the transportation, distribution and storing of foods [ 29 ]. The related knowledge on what is safe should be handed down through families, work sites and credible institutions.
Analysis of exposed foods of foodborne illnesses in this study, the cases caused by Norovirus, V. parahaemolyticus and Diarrheagenic E. coli, the largest number of food categories involved were aquatic product infection (17.73%, 39.34% and 15.84%, respectively). On the contrary, a study showed the analysis of exposed foods of reported cases in Shandong Province, multiple foods (meaning more than two kinds of food) were the most commonly reported classification [ 30 ]. The reason for the different findings may be that Zhejiang is a coastal province with a vast sea area and various aquatic products. Therefore, consumers would be advised to separate raw and cooked foods, cook thoroughly as much as possible and keep food at safe temperatures to reduce the risk of foodborne diseases. However, avoiding all raw seafood should be difficult for those who are in the habit of eating seafood. As for cases infected by Salmonella, fruits, aquatic products and cooked meat products were identified as the most frequent food vehicles in the present study. Conversely, eggs have been reported as the most common classification for Salmonella infection in the US [ 31 ]. The main reason for this difference was cultural differences in eating habits. Yet it’s worth noting that, the reported classification of multiple foods relatively high as well. That means people eat more and more diverse foods, on the other hand, the category of exposed foods in national foodborne disease surveillance system is not specified in enough detail.
Analysis of the settings, according to our analysis, private home was the most common exposure setting, followed by restaurant. However, the average annual case ratios in the Republic of Korea were the highest at restaurant (57%) [ 32 ]. Among cases reported in US, restaurants also the most common settings of preparation [ 31 ]. On the contrary, Wu et al. [ 33 ] from CDC of China found that, foodborne illnesses most frequently occurred in household (32%). Similar results were observed in a EU study [ 34 ]. These findings consistent with present results, this means a large proportion of foodborne diseases caused by foods improperly prepared or mishandled at home. The effective actions can include the following aspects: know the food they use, for example, read labels on food packages, make informed choices, become familiar with common food hazards; furthermore, government should focus on home settings to reduce infections.
In regard to clinical symptoms in general, results showed similar clinical symptoms, such as nausea, abdominal pain and watery diarrhea between patients caused by four pathogens, respectively. The proportion of fever was the highest in Salmonella while lowest in Diarrheagenic E. coli. The proportion of fever in Salmonella infections in our findings was close to that in another research [ 35 ]. As Most foodborne pathogens can cause acute gastroenteritis with gastrointestinal symptoms, it is difficult to distinguish the cases infected by different pathogens by symptoms.
The limitations of this study need to be explained. First, for many reported cases, information on certain aspects, such as food category, settings and etc. were missing or incomplete, so the conclusions might not be representative of unknown classifications. Second, information and detection data were collected from 31 hospitals and several laboratories. Though detection methods were unified and regular trainings were held, there was a chance of bias caused by the different conditions and levels of hospitals and laboratories. Third, inability to conduct an epidemiological investigation due to lack of patient cooperation, there were still some missing information.
Norovirus was the most common enteric pathogen detected in our surveillance during 2016–2020. Since the different epidemiological characteristics of foodborne diseases caused by different pathogens, we suggest that targeted measures be taken according to the characteristics of different etiologies and food vehicles to improve the prevention and control efficiency. The Norovirus, V. parahaemolyticus and Diarrheagenic E. coli infection with the highest positive detection rates over the workers were observed. Foodborne illnesses among workers are liable to occur frequently because hygienic conditions at workers’ camps and work situations are not always at the same standard. The related knowledge on what is safe should be handed down through families, work sites and credible institutions. Most foodborne diseases are preventable, we should further improve the identification rate of the causes of the epidemic, carry out attribution analysis for “precise prevention and control”.
Data availability
The data that support the findings of this study are available from the Foodborne Disease Case Surveillance Reporting System of the China National Center for Food Safety Risk Assessment, and these data are not publicly available.
The data that support the findings of this study are available from the Foodborne Disease Case Surveillance Reporting System ( https://sppt.cfsa.net.cn/goto ), and these data are not publicly available.
Kim JJ, Ryu S, Lee H. Foodborne illness outbreaks in Gyeonggi province, Korea, following seafood consumption potentially caused by Kudoa septempunctata between 2015 and 2016. Osong Public Health Res Perspect. 2018;9:66–72.
Article Google Scholar
World Health Organization. WHO estimates of the global burden of foodborne diseases: foodborne diseases burden epidemiology reference group 2007–2015. https://www.who.int/publications/i/item/9789241565165 . Accessed 3 December 2015.
Food Standards Agency. The FSA food borne disease strategy 2010-15. London: Food Standards Agency; 2011.
Google Scholar
Chung SS, Wong CK. Regulatory and policy control on food safety in China. J Epidemiol Community Health. 2013;67(6):476–7.
Akhtar S, Sarker MR, Hossain A. Microbiological food safety: a dilemma of developing societies. Crit Rev Microbiol. 2014;40(4):348–59.
Article CAS Google Scholar
Wu YN, Liu P, Chen JS. Food safety risk assessment in China: Past, present and future. Food Control. 2018;90:212–21.
Yang S, Yan L, Pei X, Yang D. Study on the contamination status of foodborne pathogens in frozen minced meat products on China market. Chin J Food Hyg. 2020;32:180–3.
Yan L, Li Y, Yang D, et al. Contamination of pathogens in milk powder available in retail markets. Chin J Public Health. 2016;32:602–4.
Li Y, Yang X, Zhang H, et al. Prevalence and antimicrobial susceptibility of Salmonella in the commercial eggs in China. Int J Food Microbiol. 2020;325:108623.
Hu J, Pei X, Li N, Yang D. Survey of microbiological contamination of commercial available infant formula sheep milk powder. Chin J Health Lab Tec 2016;26:1942–1943, 1946.
Kim HW, Hong YJ, Jo JI, et al. Raw ready-to-eat seafood safety: microbiological quality of the various seafood species available in fishery, hyper and online markets. Lett Appl Microbiol. 2017;64(1):27–34.
Li W, Cui Q, Bai L, Fu P, Han H, Liu J, Guo Y. Application of Whole-Genome Sequencing in the National Molecular Tracing Network for Foodborne Disease Surveillance in China. Foodborne Pathog Dis. 2021;18(8):538–46.
Kim SS, Kang DH. Combination treatment of ohmic heating with various essential oil components for inactivation of food-borne pathogens in buffered peptone water and salsa. Food Cont. 2017;80:29–36.
Yan L, Hu J, Pei X, Yang D, Zhang X. Contamination of foodborne pathogens in frozen surimi products sold in China in 2014. J Hyg Res. 2017;46:328–30.
Zhejiang Provincial Bureau of Statistics. http://tjj.zj.gov.cn/ .
Official site of Zhejiang province. China. http://www.ezhejiang.gov.cn/index.html .
Sun L, Chen Ll, Liao NB, Chen J. Analysis of foodborne disease outbreak surveillance data in Zhejiang Province,2006–2017. Chin J Health Lab Tec. 2019;29(15):1874–7.
Gong XH, Wu HY, Li J, Xiao WJ, Zhang X, Chen M, Teng Z, Pan H, Yuan ZA. Epidemiology, aetiology and seasonality of infectious diarrhoea in adult outpatients through active surveillance in Shanghai, China, 2012–2016: a cross-sectional study. BMJ Open. 2018;8(9):e019699.
Wang JX, He J, Wang S, et al. Incidence of foodborne diseases in Hainan province, 2007–2016. Chin J Public Health. 2018;34(9):1288–91.
Wang S, Duan H, Zhang W, Li JW. Analysis of bacterial foodborne disease outbreaks in China between 1994 and 2005. FEMS Immunol Med Microbiol. 2007;51(1):8–13.
Enserink R, van den Wijngaard C, Bruijning-Verhagen P, et al. Gastroenteritis attributable to 16 enteropathogens in children attending day care: significant effects of rotavirus, norovirus, astrovirus, Cryptosporidium and Giardia. Pediatr Infect Dis J. 2015;34(1):5–10.
Karsten C, et al. Incidence and risk factors for community-acquired acute gastroenteritis in north-west Germany in 2004. Eur J Clin Microbiol Infect Dis. 2009;28(8):935–43.
Zhang Z, Lai S, Yu J, et al. Etiology of acute diarrhea in the elderly in China: A six-year observational study. PLoS ONE. 2017;12(3):e0173881.
Arena C, Amoros JP, Vaillant V, et al. Acute diarrhea in adults consulting a general practitioner in France during winter: incidence, clinical characteristics, management and risk factors. BMC Infect Dis. 2014;14:574.
Zhang Y, Zhao Y, Ding K, Wang X, Chen X, Liu Y, Chen Y. Analysis of bacterial pathogens causing acute diarrhea on the basis of sentinel surveillance in Shanghai, China, 2006–2011. Jpn J Infect Dis. 2014;67(4):264–8.
Hossain ME, Islam MM, Miah M, Haque W, Vinjé J, Rahman MZ, Faruque ASG, Khan AI, Ahmed T, Rahman M. Corrigendum to: Viral Etiology of Acute Gastroenteritis Among Forcibly Displaced Myanmar Nationals and Adjacent Host Population in Bangladesh. J Infect Dis. 2022;225(6):1114.
Patel MM, Widdowson MA, Glass RI, Akazawa K, Vinjé J, Parashar UD. Systematic literature review of role of noroviruses in sporadic gastroenteritis. Emerg Infect Dis. 2008;14(8):1224–31.
Liang Z, Ke B, Deng X, Liang J, Ran L, Lu L, He D, Huang Q, Ke C, Li Z, Yu H, Klena JD, Wu S. Serotypes, seasonal trends, and antibiotic resistance of non-typhoidal Salmonella from human patients in Guangdong Province, China, 2009–2012. BMC Infect Dis. 2015;15:53.
Todd ECD. Foodborne disease and food control in the Gulf States. Food Control. 2017;73:341–66.
Wu G, Wang L, Wang Q, et al. Descriptive Study of Foodborne Disease Using Case Monitoring Data in Shandong Province, China, 2016–2017. Iran J Public Health. 2019;48(4):722–9.
PubMed PubMed Central Google Scholar
Dewey-Mattia D, Manikonda K, Hall AJ, Wise ME, Crowe SJ. Surveillance for Foodborne Disease Outbreaks - United States, 2009–2015. MMWR Surveill Summ. 2018;67(10):1–11.
Kim JJ, Ryu S, Lee H. Foodborne Illness Outbreaks in Gyeonggi Province, Korea, Following Seafood Consumption Potentially Caused by Kudoa septempunctata between 2015 and 2016. Osong Public Health Res Perspect. 2018;9(2):66–72.
Wu YN, Liu XM, Chen Q, et al. Surveillance for foodborne disease outbreaks in China, 2003 to 2008. Food Control. 2018;84:382–8.
Schlinkmann KM, Razum O, Werber D. Characteristics of foodborne outbreaks in which use of analytical epidemiological studies contributed to identification of suspected vehicles, European Union, 2007 to 2011. Epidemiol Infect. 2017;145(6):1231–8.
Qi XL, Wang HX, Bu SR, Xu XG, Wu XY, Lin DF. Incidence rates and clinical Symptoms of Salmonella, Vibrio parahaemolyticus, and Shigella infections in China, 1998–2013. J Infect Dev Ctries. 2016;10(2):127–33.
Download references
Acknowledgements
The authors thank the food safety staff of the CDC at all levels of the province for their positive role and responsible handling of foodborne cases and timely submitting accurate reports, which enabled current study to have a large number of data to analyze the epidemiological characteristics of which in our province and put forward targeted intervention measures for further prevention and control.
This research was sponsored by Medical and Health Science and Technology Project of Zhejiang Province (No.2022KY712), Chinese National Natural Science Foundation (81973055), the National Key Research and Development Programme of China (No.2021YFC2701901), Major research and development projects of the Zhejiang Science and Technology Department (2018C03010), Key Laboratory of Intelligent Preventive Medicine of Zhejiang Province (2020E10004), and Leading Innovative and Entrepreneur Team Introduction Program of Zhejiang (2019R01007).
Author information
Authors and affiliations.
Department of Nutrition and Food Safety, Zhejiang Provincial Center for Disease Control and Prevention, 3399 Binsheng Road, Binjiang District, 310051, Hangzhou City, Zhejiang Province, China
Xiaojuan Qi, Jiang Chen, Jikai Wang & Ronghua Zhang
Department of Epidemiology & Health Statistics, School of Public Health, School of Medicine, Zhejiang University, 310058, Hangzhou City, Zhejiang Province, China
Xialidan Alifu & Yunxian Yu
Department of Public Health, Department of Anesthesiology, Second Affiliated Hospital of Zhejiang University School of Medicine, 310003, Hangzhou City, Zhejiang Province, China
Xialidan Alifu, Wenliang Luo & Yunxian Yu
You can also search for this author in PubMed Google Scholar
Contributions
X.J.Q. and X.A. made substantial contributions to the design of the work. X.J.Q. and X.A. drafted the work, Y.X.Y. and R.H.Z. substantively revised it. All authors made substantial contributions to the acquisition, analysis, and interpretation of data. All authors read and approved the final manuscript.
Corresponding authors
Correspondence to Yunxian Yu or Ronghua Zhang .
Ethics declarations
Ethics approval and consent to participate.
This study was approved by the Ethics Committee of Zhejiang Provincial Center for Disease Control and Prevention (CDC). The study protocol was performed in accordance with the relevant guidelines. The ethics committee approved the procedure for verbal consent because Zhejiang CDC has the authority of the Zhejiang provincial government to collect and utilize information on foodborne disease cases, which is part of disease surveillance scope in Zhejiang CDC. All participants were informed that they had the right to reject or terminate the study at any time during the interview. Since we have obtained verbal consent, documentation of consent was not required. The information provided by each participant is confidential in Zhejiang CDC. The China’s National Center for Food Safety Risk Assessment (CFSA) is responsible for maintaining and managing the foodborne disease case surveillance system, and our use of the data has been verbally approved by CFSA.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiaojuan Qi and Xialidan Alifu contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Qi, X., Alifu, X., Chen, J. et al. Descriptive study of foodborne disease using disease monitoring data in Zhejiang Province, China, 2016–2020. BMC Public Health 22 , 1831 (2022). https://doi.org/10.1186/s12889-022-14226-1
Download citation
Received : 07 May 2022
Revised : 23 August 2022
Accepted : 19 September 2022
Published : 28 September 2022
DOI : https://doi.org/10.1186/s12889-022-14226-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Food safety
- Microbial hazard
- Foodborne pathogen
- Surveillance network
BMC Public Health
ISSN: 1471-2458
- Submission enquiries: [email protected]
- General enquiries: [email protected]
EDITORIAL article
Editorial: foodborne pathogens: hygiene and safety.

- Faculty of Bioscience and Technology for Food, Agriculture and Environment, University of Teramo, Teramo, Italy
Editorial on the Research Topic Foodborne Pathogens: Hygiene and Safety
Introduction
The foodborne outbreaks occurred in last decades highlight the importance of the development and implementation of preventive measures and programs aiming at ensuring food safety on one hand and constituting a common basis for the hygienic production of food on the other hand. In particular, a farm to fork approach has been applied in all sectors of food production chain in order to improve hygiene and reduce all potential biological hazards. The food supply chain is very complex because of the differences in food composition and processing and this can result in emergence and re-emergence of foodborne pathogens. However, many factors related to an increase in foodborne illness have been reported, such as the change in eating habits and consumer preferences, increased international travels, change in food processing, production and distribution, pathogen adaptation to new environments, acquisition of virulence factors and antimicrobial drug resistance by microorganisms, advances in pathogen detection methods, inadequate sanitation and vector control measures, inadequate public health services, including consumer information ( Smith and Fratamico, 2018 ). This Research Topic titled “Foodborne Pathogens: Hygiene and Safety” focuses on important food safety concerns such as the potential presence of pathogens in food as well as their toxins/metabolites, the resistance to antibiotics or sanitizers, and other virulence characteristics. It includes four reviews and 44 original research papers. The main foodborne pathogens studied herein are: Campylobacter jejuni, Cronobacter sakazakii, Escherichia coli, Listeria monocytogenes, Salmonella spp., and Staphyloccus aureus , but some other researches deal with Helicobacter pilori, Klebsiella pneumoniae, Vibrio parahaemolyticus , mycobacteria, and molds as well. Studies on characterization and genetic typing of foodborne pathogens, detection methods and inactivation of these microorganisms by natural preservatives derived from plant sources, essential oils and biocontrol, and influence of probiotics are also reported.
Prevalence and Monitoring of Pathogens in Food
Foodborne diseases represent one of the most important public health troubles worldwide. The potential of foodborne pathogens to cause illness or even death in consumers highlights the importance of such events and consequent need of their monitoring and prevention. Millions of cases of foodborne illnesses and/or chronic complications are reported in many countries every year ( Heredia and García, 2018 ). Li S. et al. studied the prevalence and characteristics of Non-typhoidal Salmonella isolated from poultry meat (broilers and spent hens) from supermarkets in China. Three serotypes were identified in 40 Salmonella strains and Salmonella Enteritidis resulted as dominant. The antibiotic resistance was tested as well, showing the highest rates to ampicillin for the strains isolated from commercial broilers, and to nalidixic acid for those isolated from spent hence. Thung et al. investigated the prevalence of Salmonella spp. in different beef meat samples from retail markets in Malaysia as well as the virulence genes and antimicrobial resistance. Eight different serovars were identified and Salmonella Agona was the predominant one. All 23 isolates were resistant at least to three antibiotics. Colello et al. determined the prevalence of Salmonella spp. in 764 samples collected from swine farms, slaughterhouses, boning rooms, and retail markets. The strains were classified into five serotypes (i.e., Salmonella Typhimurium, Salmonella Kentucky, Salmonella Brandenburg, Salmonella Livingstone, and Salmonella Agona) and showed different resistance to antibiotics.
The microbiological quality (mesophilic aerobic bacteria, total coliforms, yeasts, and molds) and safety level ( E. coli O157:H7, Shiga toxin-producing E. coli, Salmonella Enteritidis, Salmonella Typhimurium, Listeria spp., and L. monocytogenes ) of organic and conventional vegetables from Malaysia were evaluated. Salmonella spp., L. monocytogenes , and Listeria spp. were the most representatives, with no trend between organically or conventionally grown vegetables ( Kuan et al. ). The presence of total and pathogenic V. parahaemolyticus strains was detected in short mackerel samples collected from different retail markets in Malaysia. The antimicrobial susceptibility profiles were also studied, showing a resistance to penicillin G and ampicillin ( Tan et al. ).
The genetic diversity as well as the antibiotic resistance and biofilm formation of Cronobacter spp. recovered from spices and cereals were studied by Li Y. et al. Cronobacter sakazakii was the most common species, and 62.5% of 40 Cr. sakazakii strains were non-biofilm producers. Parra-Flores et al. evaluated the presence of Cr. sakazakii , microbiological levels of aerobic plate count and Enterobacteriaceae in dairy product batches associated with a recent food alert in Chile.
Sevilla et al. investigated the presence of members of the genus Mycobacterium by culture and PCR-based methods in raw dairy and meat products purchased at different supermarkets in Spain. Mycobacterial DNA was detected in 23 out of 257 samples, corresponding to Mycobacterium avium, Mycobacterium tuberculosis , and other non-tuberculous mycobacteria.
Wang W et al. submitted two papers to this Research Topic, the first one concerned the complete genomic analysis of a Salmonella Typhimurium isolate from ready-to-eat pork samples in China, the second dealt with the prevalence of S. aureus among raw milk from dairy cows with clinical mastitis.
Lipophilic marine biotoxins belonging to okadaic acid, pectenotoxin, yessotoxin, and azaspiracid groups were determined in specimens of mussels collected along the coasts of the Central Adriatic Sea (Italy) by LC-MS/MS. The concentrations exceeded the maximum regulatory limits only for 11 out of 400 samples, and some samples showed a multi-toxin mixture contamination ( Schirone et al. ).
Antimicrobial Resistance and Virulence Factors
Microbial interactions can show beneficial or detrimental effects that influence the fate of pathogenic species contaminating foods. The study of such interactions can provide a new knowledge about the different activities of the microorganisms from proliferation and metabolism to pathogenicity and virulence ( Zilelidou and Skandamis, 2018 ).
Dairy products can host microorganisms belonging to Enterobacteriaceae family showing multidrug resistance to antibiotics and other virulence factors such as production of biofilm and synthesis of proteolytic and lipolytic enzymes responsible for spoilage. Their presence can be reduced or avoided through good hygiene conditions during processing and manufacturing, as well as storage and distribution ( Amorim et al. ). Chagnot et al. investigated the adhesion of E. coli O157:H7 to well-defined types of skeletal muscle and demonstrated that such microorganism mainly adhered to the extracellular matrix of muscle cells, with no significant differences among the different constituent myofibres, whereas the influence of post-mortem structural modifications of muscle tissues was substantial.
The adhesion capacity of 40 C. jejuni strains to abiotic surfaces was studied. All C . jejuni strains were shown to be capable of forming strong biofilms when Mueller Hinton medium was supplemented with chicken juice. However, the use of biocides was effective in controlling viable cells of strains in biofilm ( Melo et al. ). Oh et al. demonstrated that ferrous and ferric iron stimulated biofilm formation in C. jejuni through oxidative stress. Premarathne et al. determined the prevalence and antibiotic resistance of Campylobacter spp. in the beef food system in Malaysia. Most isolates were identified as C. jejuni , with a high percentage resistant to tetracycline and ampicillin.
The effect of cold stress on the adhesion to abiotic surfaces and biofilm formation of 22 L. monocytogenes strains from different serogroups and origins was studied by Lee et al. Such study demonstrated the increase of the adhesion capacity, whereas the cold-adapted cells remained in planktonic form. Pasquali et al. studied the persistence and physiological adaptation to food-processing environmental stress of L. monocytogenes strains from a rabbit meat processing plant. While some strains showed a resistance to sanitizers, some others were biofilm producers and these specific characteristics could contribute to their high prevalence. The nucleotide diversity of L. monocytogenes strains from human clinical cases—as well as food or food-related environments originating from three different geographical locations (i.e., Australia, Greece, and Ireland)—was studied by Poimenidou et al. The authors demonstrated that virulence genes showed different evolutionary pathways affected by the origin and serotype of the specific strain.
Lang et al. demonstrated that drying of milk powder increased the Caco-2 cell invasion capacity of two pathogens, i.e., Salmonella enterica and Cr. sakazakii , probably due to the activation of stress response transcriptional factors, and a subsequent heat treatment did not offset the loss of cultivability that was observed in the experimental design.
Javed et al. described the characteristics, prevalence, survival, and transmission, as well as pathogenesis and virulence determinants of Helicobacter pullorum . Such microorganism causes gastroenteritis in poultry, but it is also an emerging zoonotic bacterium associated with enteric infections in humans with colitis, hepatitis, and recurrent diarrhea.
Detection Methods Applicable in Food Industry
A microfluid system combining loop-mediated isothermal amplification with gold nanoparticles for rapid detection of Salmonella spp. in food samples was performed. Such method showed relative sensitivity, specificity and accuracy of 100% and could be used in the food industry as a simple, inexpensive and fast analytical approach ( Garrido-Maestu et al. ). A new standard operating procedure for multiple-locus variable number tandem repeat analysis (MLVA) of Salmonella Dublin was proposed by Vignaud et al. The MLVA scheme was applied to a foodborne outbreak occurred in France in 2012, in order to discriminate between epidemiologically related strains and sporadic case strains. Fong et al. characterized four Salmonella phages isolated from irrigation water, cattle feces, and sediment from irrigation ditches, based on their phenotypic and genotypic determinants, and assessed their infectivity against various Salmonella strains in vitro . Among them, the phage isolate SI1 was the most effective in control of Salmonella Enteritidis in sprouting alfalfa seeds artificially contaminated.
The study of Ogrodzki and Forsythe described the application of three genotyping methods (Multilocus Sequence Typing, MLST, capsular profiling of the K -antigen and colanic acid byosinthesis regions and CRISPR- cas array profiling) to discriminate different species belonging to Cronobacter genus. Chase et al. found a Cr. sakazakii isolate, H322, in a batch of powdered infant formula (PIF) and two other isolates showing indistinguishable Pulsed Field Gel Electrophoresis patterns with H322, during routine testing of these products ready for distribution. Therefore, whole genome sequencing, as well as microarray analysis, was applied to these strains, showing a phylogenetic relation among them. This study confirmed that the pathogen could persist within the PIF manufacturing facility for years.
Wang J. et al. developed a novel approach to predict the growth kinetics of S. aureus on rice cake under different environmental conditions. These probability models could be useful for food safety management and microbiological risk assessment of such pathogen.
Listeria monocytogenes encodes a functional ArgR, a transcriptional regulator with specific functions in arginine metabolism regulation and acid tolerance. Cheng et al. showed that a single ArgR regulator could have opposite regulatory effects on the arginine deiminase pathway in an arginine-independent and dependent manner under neutral and acidic conditions, respectively.
Henri et al. compared different genomic methods, i.e., MLST, Whole Genome Sequencing (WGS), and Single Nucleotide Polymorphism (SNP), used to cluster L. monocytogenes strains. This study revealed high concordance between MLST and SNP approaches for diagnostic laboratories responsible for outbreak detection and surveillance.
Williams et al. described a rapid flow cytometric method for determining E. coli O157:H7 contamination in raw spinach. This method could be used as a screening tool to detect such microorganism in food. The presence of two distinct loci of heat resistance on a plasmid encoding type three fimbriae and three bacteriocins, in 1 out 90 E. coli raw milk cheese strains, was investigated. Such plasmid was transferable to other E. coli strains including Shiga-toxin-producing strains, posing great concern in food production environments ( Boll et al. ). Hussain et al. evaluated the contamination with pathogenic and/or multiresistant E. coli among broiler free-range chicken specimens (ceca and meat). The isolates were characterized using both conventional typing and WGS and compared with human E. coli pathotypes. The results showed that the poultry E. coli strains shared closer genetic identity to human E. coli . Zhang B. et al. demonstrated that a specific genetic marker (named fimbrial gene z3276 ) of Enterohemorrhagic E. coli O157:H7 encoded multifunctional structures with properties contributing to host colonization and bacterial survival in the environment.
The regulatory mechanism of secondary metabolism by comparative transcriptomic in Aspergillus flavus was studied by Yao et al. Such approach allowed the authors to identify known and novel regulators required for aflatoxins biosynthesis.
Zhang S. et al. determined biotypes, serotypes, virulence genes, and antimicrobial resistance patterns of K. pneumoniae strains from retail foods in China. The authors reported that some strains from the same geographic area had a closer relationship and they showed high levels of resistance to ampicillin.
Yang et al. utilized a proteomic approach involving anti-acetyl lysine-based enrichment and highly sensitive mass spectrometry to identify the global acetylated proteome and investigate lysine acetylation in Trichinella spiralis .
Zhao et al. described the surface enhanced Raman spectroscopy as testing technology used for the detection of pathogenic bacteria in food. Such method can be considered fast, simple, specific, and sensitive.
Promising Strategies for Food Preservation
Preservation technologies are applied to extend the shelf-life, improve the hygienic quality, and ensure the safety of food. In food industry bacteriocins or other natural preservatives such as herbal extracts and essential oils are used as alternative to prevent the growth of both pathogenic and spoiling microorganisms ( Martínez et al., 2019 ; Nazari et al., 2019 ).
Gray et al. described novel biocontrol methods such as bacteriophages, endolysins, bacteriocins, and plant derived products (essential oils) for the prevention of biofilm formation by L. monocytogenes in food production facilities. The inhibitory effect of Hedychium spicatum L. essential oil and radiation on production of deoxynivalenol and zearalenone by Fusarium graminearum in maize grains was studied by response surface methodology. The results showed a reduction of fungal growth rate as well as mycotoxin content ( Kalagatur et al. ).
Bajpai et al. described a significant antibacterial activity of a quinoline compound (jineol) isolated from the insect Scolopendra subspinipes mutilans against two selected foodborne pathogens (i.e. E. coli O157:H7 and S. aureus KCTC-1621). Such compound could be used as alternative means of antimicrobial in pharma and food industries.
The study of García and Cabo focused on the optimization of E. coli inactivation by a quaternary ammonium compound based on a mathematical model. The results showed that the optimal disinfectant dose increased exponentially with the initial bacterial concentration.
Different pressure-temperature combinations were applied to investigate the inactivation kinetics of E. coli, Listeria innocua , and S. aureus in black tiger shrimp. Staphylococcus aureus was the most baro-resistant species among the three bacteria. Such study could be used to predict non-linear survival curves of other microorganisms in foods ( Kaur and Rao ).
In their study, Kiran et al. isolated an actinobacterial strain from a marine sponge producing a lipopeptide that was demonstrated to be an effective emulsifier as well as good antioxidant and protective agent against S. aureus . The authors used this lipopeptide as food additive in muffin production with good results in organoleptic qualities of such food.
Kollanoor Johny et al. evaluated the antimicrobial effects of subinhibitory concentrations of two plant-derived compounds (i.e., trans -cinnamaldehyde and eugenol) on different genes of S. enterica serotype Enteritidis phage type 8 associated with virulence, colonization, motility, and invasion capability of such pathogen.
Mohanta et al. described the biological synthesis of silver nanoparticles using a cell-free aqueous leaf extract of plant Protium serratum and their antibacterial activity against some foodborne pathogens, i.e., S. aureus, E. coli , and Pseudomonas aeruginosa . The authors suggested the application of such nanoparticles in food packaging materials as well as disinfectant and cleaning agents.
Nair and Kollanoor Johny submitted two papers to the present Research Topic, the first study described the potential of pimenta leaf essential oil in reducing Salmonella Heidelberg attachment on to turkey skin during poultry processing, whereas the second work studied the antimicrobial function of a dairy-originated probiotic strain against multidrug resistant Salmonella Heidelberg in poults, i.e., young turkeys. The cecal colonization, dissemination to internal organs and potential for skeletal muscle deposition of multidrug resistant strains of Salmonella Heidelberg were studied by a challenge experimental design in poults and adult turkey hens. The results showed the highest recovery in the cecum followed by spleen, liver, thigh, and breast, and could be used to better control this microorganism at farm level and improve the safety of turkey products ( Nair et al. ).
Conclusions
The high number of studies collected in this Research Topic confirms the importance of foodborne pathogens as a global issue and provides a robust and up-to-date scientific advice. It has been highlighted how much important and essential is a rapid detection of foodborne pathogens by sensitive culture independent methods and by new technologies such as WGS or other biomarkers assay analysis. The outbreak investigations play also key roles in the prevention of foodborne pathogens growth and diffusion, such as their food vehicles and how the contamination can occur in the food supply chain. The positive results of this Research Topic suggest to collect additional and new data for the future on this topic “Foodborne pathogens: hygiene and safety.”
Author Contributions
MS and PV drafted the editorial. RT and GS contributed to editorial revision. All authors approved the final paper.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Heredia, N., and García, S. (2018). Animals as sources of food-borne pathogens: a review. Anim. Nutr . 4, 250–255. doi: 10.1016/j.aninu.2018.04.006
PubMed Abstract | CrossRef Full Text | Google Scholar
Martínez, B., García, P., and Rodríguez, A. (2019). Swapping the roles of bacteriocins and bacteriophages in food biotechnology. Curr. Opin. Biotechnol . 56, 1–6. doi: 10.1016/j.copbio.2018.07.007
Nazari, M., Ghanbarzadeh, B., Kafil, H. S., Zeinali, M., and Hamishehkar, H. (2019). Garlic essential oil nanophytosomes as a natural food preservatives: its application in yogurt as food model. Colloid Interface Sci. Commun . 30:100176. doi: 10.1016/j.colcom.2019.100176
CrossRef Full Text | Google Scholar
Smith, J. L., and Fratamico, P. M. (2018). Emerging and re-emerging foodborne pathogens. Foodborne Pathog. Dis. 15, 737–757. doi: 10.1089/fpd.2018.2493
Zilelidou, E. A., and Skandamis, P. N. (2018). Growth, detection and virulence of Listeria monocytogenes in the presence of other microorganisms: microbial interactions from species to strain level. Int. J. Food Microbiol . 277, 10–25. doi: 10.1016/j.ijfoodmicro.2018.04.011
Keywords: food, microorganisms, virulence, illness, preservatives
Citation: Schirone M, Visciano P, Tofalo R and Suzzi G (2019) Editorial: Foodborne Pathogens: Hygiene and Safety. Front. Microbiol. 10:1974. doi: 10.3389/fmicb.2019.01974
Received: 29 June 2019; Accepted: 12 August 2019; Published: 27 August 2019.
Reviewed by:
Copyright © 2019 Schirone, Visciano, Tofalo and Suzzi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria Schirone, mschirone@unite.it
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
U.S. Food and Drug Administration
- Search
- Menu
- Recalls, Outbreaks & Emergencies
- Outbreaks of Foodborne Illness
Investigations of Foodborne Illness Outbreaks

Note: Not all recalls and alerts result in an outbreak of foodborne illness . Check recent Food Recalls and Safety Alerts .
The following is a list of outbreak and adverse event investigations primarily being managed by FDA’s CORE Response Teams . The investigations are in a variety of stages, meaning that some have limited information, while others may be near completion. If you think you have symptoms of foodborne illness, talk to your healthcare provider and public health officials to provide them with details of what you ate before becoming sick. This often aids in helping solve emerging or ongoing outbreaks.
A public health advisory will be issued for investigations that have resulted in specific, actionable steps for consumers to take to protect themselves. Please direct your attention to those pages for the most up to date information on the investigation and for consumer protection information.
Outbreak and adverse event investigations that do not result in specific, actionable steps for consumers may or may not conclusively identify a source or reveal any contributing factors. Adverse event investigations rely on self-reported data. Although these reports may name a particular product, FDA will only indicate a product category in the table and will not publicly name a specific product until there is sufficient evidence to implicate that product as a cause of illnesses or adverse events. If a cause and/or contributing factors are identified that could inform future prevention, FDA commits to providing a summary of those findings.
- For the outbreak of Salmonella Typhimurium (ref #1218), Infinite Herbs, LLC, voluntarily recalled 2.5-oz packages of Infinite Herbs-brand fresh organic basil and on April 19, 2024, Infinite Herbs, LLC, expanded the recall to include 2.0- and 4.0-oz packages of Melissa’s-brand organic basil. The outbreak advisory has been updated to include additional information about recalled products. FDA’s investigation is ongoing.
- For the outbreak of E. coli in a not yet identified product (ref #1221) the case count increased from nine to 12. FDA’s investigation is ongoing.
Active Investigations
Closed investigations, 2024 investigations, 2023 investigations, 2022 investigations, 2021 investigations.
1 This cluster represents a subset of the total number of domestically-acquired cases of cyclosporiasis cases in the U.S.
2 Based on CDC’s epidemiological investigation of two large multistate outbreaks of cyclosporiasis , ill people reported eating a variety of leafy greens before becoming sick. For both investigations, CDC, FDA, and state and local partners conducted epidemiologic and traceback investigations and collected and analyzed product and environmental samples. All samples collected were reported as negative for Cyclospora . Due to the lack of additional detail in the epidemiological data and the absence of supporting evidence collected from traceback and sample collection, FDA could not identify a specific product as the source of either outbreak.
2020 Investigations
Related links.
CDC Investigations
FSIS Investigations
Table Definitions
Date Posted : Date the investigation is posted to the table. This happens once CORE begins to actively coordinate an investigation. In collaboration with federal and state partners, CORE initiates response activities to control the outbreak or adverse events.
Reference Number : This number is assigned to incidents that CORE is working on. Each foodborne illness investigation on the table will have a unique reference number and this is provided to help users of this table differentiate between investigations. Those reference numbers beginning with an “E” have carried over from an older numbering system that will not be used by CORE in the future.
Pathogen or Cause of Illness : A bacterium, virus, other microorganism, toxin, or other contaminant that can cause disease.
Product(s) Linked to Illnesses (if any) : During an outbreak or adverse event investigation, the FDA and CDC, along with state and local authorities collect and analyze three types of information: epidemiological information, laboratory analyses of food and/or samples taken from food production environments, and traceback investigation findings. Each outbreak or adverse event is unique and the information available to investigators varies from outbreak to outbreak – however, through rigorous analysis of the information collected, investigators are often able to identify a likely or confirmed food source of an outbreak or adverse events. Additionally, adverse event investigations rely on self-reported data, which may not include all necessary information to fully investigate the product or event. It is important to note that before a specific food is linked to an outbreak or adverse events, the investigation of a commodity or a specific food by the FDA, CDC and state and local partners does not mean that the food is the cause of an outbreak or adverse events. In many cases the investigation is also looking to rule out specific foods even as it identifies the particular suspect. If there is evidence that a specific food is linked to illnesses, it will be reflected here and health authorities will warn the public about that food.
Total Case Count : Updated weekly. For outbreak investigations, the case count is provided to the FDA by the CDC. Case counts are dynamic and the exact number of illnesses constantly changes during an investigation. This number is provided in order to provide an estimate of the size of an outbreak each week. In the case of adverse event investigations, FDA will provide the number of adverse events that have been self-reported by consumers to FDA consumer complaint coordinators and the CFSAN Adverse Event Reporting System (CAERS), which could include duplicate reports. More formalized data will be published in CDC Investigation Notices or in FDA and CDC advisories, should they be posted.
Investigation Status : Communicates whether this outbreak is still under investigation by CORE or the investigational activities have ended. Options for this column would be either “Active” or “Closed”. At times an FDA investigation may be active after an outbreak has ended.
Outbreak/Event Status : Communicates whether this outbreak or series of adverse event reports is ongoing or has ended.
Recall Initiated : A recall occurs when a firm takes a product off the market because there is reason to believe that it may cause consumers to become ill. In some situations, FDA may request the company recall a potentially contaminated food. In other situations, FDA may issue a mandatory recall if there is a reasonable probability that the food is adulterated under certain FDA authorities, and that the food could cause serious illnesses or death.
FDA Traceback Initiated : Used to identify the source and distribution of the implicated food and remove the contaminated product from the marketplace, to distinguish between two or more implicated food products, and to determine potential routes and/or sources of contamination in order to help prevent future illnesses. For additional information, see How the FDA Uses Traceback to Respond to Foodborne Illness Outbreaks .
FDA Inspection Initiated : An official examination by FDA of the operational processes of a facility to determine its compliance with federal law, which may include, among other things, record and sample collection. Activities reported on the table are limited to those conducted by FDA; however, state and local partners work in coordination with FDA and may also conduct inspectional activities. Additional information on the different types of inspections conducted by FDA can be found on the FDA website .
FDA Sampling Initiated : Collection of samples for the presence or absence of a pathogen in a food or in the environment surrounding the food. Samples reported on the table include those collected by the FDA or state collected samples that are analyzed by the FDA. Significant sample findings are reviewed by FDA and are reported in Public Health Advisories .
Who to Contact if you Have Symptoms of Foodborne Illness
Consumers who have symptoms of foodborne illness should contact their health care provider to report their symptoms and receive care.
To report a complaint or adverse event (illness or serious allergic reaction), you have three choices:
- Call an FDA Consumer Complaint Coordinator if you wish to speak directly to a person about your problem.
- Complete an electronic Voluntary MedWatch form online.
- Complete a paper Voluntary MedWatch form that can be mailed to FDA.
Visit www.fda.gov/fcic for additional consumer and industry assistance.
Get email updates delivered to your inbox.
Get regular FDA email updates delivered on this topic to your inbox.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Elsevier - PMC COVID-19 Collection

Perspectives in Foodborne Illness
This article provides a historical framework on food safety for more contemporary details to rest on, focusing primarily on the past 100 years or so (with a touch of ancient history) as particular issues that affect how the safety of the food we eat have been appreciated, have evolved or at times have been successfully dealt with, or have newly emerged or reemerged, in large part because of the impact of technology, trade, and travel.
- • Foodborne illness has undoubtedly plagued humans from the beginning, as long as we have existed.
- • The transition from hunter-gathering societies to settlements with agriculture and domesticated animals improved food security but increased the need for food safety and the opportunities for foodborne illnesses.
- • Technology, from simple systems for sanitary disposal of human and animal waste, protection of water from fecal contamination, refrigeration, freezing, and other methods to inhibit microbial growth in food, for example pasteurization of milk, has contributed to the safety of the food supply chain and the reduction in foodborne illness.
- • The globalization and increased magnitude of transport of food across the world, partly in response to the growth in population and in part to changes in the way that people obtain, prepare, and consume food, provides new ways for pathogens to be transported and transmitted.
- • In addition to travel of the food supply, travel of people may expose them to foodborne illness to which they would not otherwise be exposed.
- • Factory farming, the large-scale growth and processing especially of food animals, can easily promote unhygienic conditions and contamination in unprecedented scale of food products with pathogens, toxins, antibiotics, and other potentially dangerous substances.
- • The scale of these factors has diminished the ability of regulatory agencies to monitor the safety of the products being consumed on a daily basis.
- • Nonetheless, there are many opportunities to improve both technology and practice and, in turn, help to prevent or reduce future disease incidence.
Introduction
To many people in affluent nations, mere mention of the word food conjures up visions of heaped-up and sometimes well-presented piles of carbohydrate-laden and fat-laden items on a plate, daring to be consumed in 1 sitting. Thoughts may drift to nutritional value and health, or perhaps to weight control, but these thoughts usually do not linger. Rarely is there a concern for the safety of the food in front of the consumer. When this safety is considered, myths, superstitions, and urban legends abound. In contrast, to the poor, hungry, and destitute, food is a luxury in whatever form it takes (myths, superstitions, or urban legends notwithstanding). Therefore, perhaps it is best to start this perspective on foodborne illness with a fundamental and undeniable truth: to live we must eat, for that is where it all begins. What we eat, and whether or not it sustains and promotes our growth and our health, or if it might just kill us (whether that be sooner or later), is not on the table. Where food is scarce, or famines prevail, safety is definitely not an issue, for to live one must eat.
In the articles in this issue, the causes and consequences, the diagnosis and disposition, tracking and transmission, and treatment and travel aspects of foodborne illness are presented in depth. This introductory article has a different purpose; it is intended to provide a framework for the contemporary details to rest on, a perspective over the past 100 years or so (with a touch of ancient history) as particular issues that affect the safety of the food we eat have been appreciated, have evolved or at times been successfully dealt with, or have newly emerged or reemerged, in large part because of the impact of 3 critical and rapidly changing T words: technology, trade, and travel. The intent is to provide context for the details to follow in other articles, to avoid the possibility that the forest is missed because attention is entirely focused on the individual trees within it.
Without written records, it is difficult to reconstruct what life was really like for early prehumans, except that the search for food must surely have been central to daily life and survival. What impact climate change had on the environment and the availability of things to eat, and how this affected the evolution of early hominids into Homo sapiens , dropping from the trees to the ground and over time becoming bipedal, developing functional hands capable of crafting and using tools, including those for more efficient hunting and for cultivation, can only be inferred from the anthropologic, archeological, and paleontologic evidence that has accumulated in the recent past. This evidence includes hard evidence, based on the study of fossils and the use of new biochemical and isotopic methods (for example, carbon and nitrogen stable isotope concentrations in collagen), 1 about the prehistoric diet, which consisted primarily of terrestrial animal meat, predominantly deer and to a lesser extent a few other animals. By the time Homo erectus appeared about half a million years ago, this basic diet was supplemented by edible plants, initially seeds, 2 and in coastal areas by marine life as well. 3 We can only imagine how many times decayed meat contaminated with microbial pathogens or plants that contained rapidly lethal poisons, such as the ricin in castor plant seeds or the alkaloid coniine in hemlock leaves, or the more gradually lethal constituents such as heavy metals in plants grown in soil or water containing high levels of these natural elements, were consumed, and how many times the safe practice lessons had to be learned and at what cost in health or life. The dramatic events related to consuming toxic foods were the stuff to build myths on, without the science or the ability to preserve experience in writing, as occurred later in human development. As a consequence, also as a result of nutritional deficits related to limited dietary options, unsafe food, injury, and disease, life was short 4 : for men, who also risked injury or death in hunting, and for women, who had the added burden of mortality during pregnancy and childbirth to contend with.
Food historians, such as Reay Tannahill, 5 describe the discovery of cooking and preservation methods as a way of making some inedible food stuffs edible, enhancing taste (is this just a relatively modern imperative, the result of greater choice?), improving nutritive value, and preserving quality and safety. These innovations no doubt helped hunters of large animals to kill, butcher, cook, and haul the meat back to their camp or village, making it more efficient for those groups to settle down, rather than constantly moving to follow the animal herds on which they depended between winter caves in the lowlands and summer camps at the higher altitude pastures. Somewhere along the way, the discovery was made that plants could be cultivated, given the right soil and growing conditions, which further encouraged settlement rather than a migratory lifestyle. Tannahill comments on the likelihood that at some point it was known that animals regularly visited salt licks, which suggests that early human communities could have used salt to lure animals closer to their homes, especially in winter, making hunting even more efficient. With the domestication of the dog, herding of cattle and sheep undoubtedly became easier as well, and dogs proved to be more valuable alive than as a source of food. So, although populations could grow, other challenges awaited them.
The story of pigbel, the name in Papua New Guinea (PNG) Pidgin English for a necrotizing enteritis associated with traditional PNG pig feasts, and similar diseases in other societies elsewhere in the world, 6 is illustrative of some of the foodborne hazards for early humans. In PNG, common rituals and festivals involve pig feasts, in which pigs are slaughtered in a manner that often results in spillage of intestinal contents over the carcasses. These carcasses are slow cooked at low temperature using heated stones in earthen pits between layers of fruits and leaves to enhance flavor and aroma. Water is also added and the oven is then further sealed with large leaves and dirt so that the meat and offal are steamed anaerobically, essentially in the juices of the pig tissues. These events are sometimes followed after several days by acute intestinal syndromes in participants, varying from mild diarrheas to acute fulminating lethal diseases. 7 However, it is the fulminant lethal ones that really demand attention. Sharing of remnants of the pig feast over a few days to a few weeks with other clans and other villages, often the payback for previous debts or as part of celebrations like weddings, results in the geographic spread of these illnesses. The lack of consistency between feast and disease would have affected the appreciation of the relationship between the 2. From this description, it is not so surprising that soon after its clinical “discovery” in 1963 microbiological studies demonstrated the presence of β-toxin–producing Clostridium perfringens type C in bowel contents and stool of patients. This finding helped to make the etiologic connection with Darmbrand (gangrene of the bowel), a similar acute disease that appeared in Germany at the end of World War II, also associated with β-toxin–producing C perfringens . 8 Some have suggested that the effects of the toxin are enhanced by diets containing foods with large amounts of trypsin inhibitors, such as sweet potatoes, which limit the breakdown of the microbial toxin protein and increase the likelihood that it remain enzymatically active in those consuming the tainted feast, especially children, who are most at risk of the consequences. This syndrome is to be distinguished from food poisoning caused by type A C perfringens , which produces α-toxin, often associated with meat stews allowed to remain warm for hours or reheated after some time, although that too might have been a risk for these early human populations. Because societies in which food scarcity prevails are likely to be cultures in which all parts of an animal that can be eaten are eaten, contemporary examples of foodborne diseases suggest that primitive societies would also be subject to similar outbreaks. Given the barriers to good hygiene in primitive conditions, fecal-oral transmission of enteric pathogens introduced into foods would have been a frequent consequence of daily life.
The agricultural revolution
Although Tannahill cautions that food history is no better than informed speculation, the prevailing understanding is that the climate change associated with the end of the ice age some 10,000 to 12,000 years ago, as the Paleolithic period gave way to the Neolithic period, enhanced the growth of grains such as wheat and barley across more areas across the globe. Slowly, the gathering of wild grains morphed into the deliberate spreading of seeds to cultivate those same plants, and so modern agriculture had its beginnings. With this development, it became feasible to harvest and store grains in sufficient quantities to provide for families during lean times, serving as an additional factor in population increase. This population increase, in turn, was necessary to rapidly harvest the mature plants before the grains exploded and spread to reseed the earth. Although this event would reseed the fields, it would also waste the food that the same seeds could provide. Which came first, planting or population growth, is difficult to know for certain. Learning how to plant and harvest meant that the food supply could more effectively be moved adjacent to human habitats rather than requiring humans to go to where the food could be found. Clusters of individuals could now become fixed communities adjacent to the fields of grain, and the conditions under which they lived meant sharing not only chores and the resulting food supply but also hazards to health, including microbial pathogens. As gathering food slowly gave way to cultivating food, and gatherers became farmers, building fixed dwellings where the food was growing, the intimacies with pathogens also deepened, and toxins affecting some harvested bounty (ie, heavy metals accumulated during growth or aflatoxins during storage) became equal-opportunity poisons for the whole community. Community, let alone individual sanitation, is a recent advance.
The change to an agricultural society also meant that lessons were learned regarding the ill effects of eating raw grain, with its high and poorly digestible content of starch, along with methods for cooking the inner nutritionally valuable germ, or allowing for the sprouting of the seeds, which converted the starches into digestible sugars, increasing the content of vitamins, and partially digesting the proteins for better utilization. Cultivating and harvesting also required the creation of methods of threshing and winnowing in preparation for cooking, or otherwise preparing and storing edible foods. Along the way, the development of pottery allowed greater variation in preparation of food, because this made cooking and roasting in fires possible.
As fields were developed surrounding clusters of households, these settlements would have attracted wild ruminants also looking for food for survival. Tannahill posits that it was more effective to domesticate sheep and goats (and later on, cattle) than to constantly have to fend them off. Herding would also have allowed the addition of milk and products derived from milk into the diet, the use of animal fat for cooking (and as medicinal salves), as well as tallow for preparation of primitive candles (rush lights), the fabrication of containers from the skins for storage of solids and liquids, and the wool as well as clothing, and even the use of dried dung for fuel. Humans and animals must have lived in proximity, as they do in many rural societies to this day, sharing microbial flora and sometimes the resulting illnesses caused by commensals in one were or became pathogens in the other. As time passed, the variety of foods increased, sometimes locally depending on specific conditions, sometimes more universally, and sometimes moving with migrating people: other grains such as corn, rye, and oats, honey, pulses, olives, figs, dates, grapes, pomegranates, tomatoes, potatoes, and more. Technology improved yields, for example irrigation, the use of bulls and oxen as beasts of burden to help to till fields, thresh grain, and, with the development of the wheel, also to serve as the engines for transporting goods and materials. So, recognizable civilizations came into being, and with advances came adversities and new threats. Insects and parasites affecting plants and animals could become vectors for disease as well as direct threats to humans and domesticated animals. Ground waters used for irrigation could become contaminated with microbes and ova from the feces of infected individuals, both human and animal in origin, and so threaten the health and vitality of the whole of a village population.
Early civilizations
Records in art and texts, in addition to archeological finds, attest to the growth of civilizations in different parts of the world. As technology improved, diets became more varied, breads, and ale produced from the same grains, became staples, and fruits with high sugar content, such as dates or figs in the Middle East and North African countries where they originated, became popular. Drying and salting (another way to dry food, in addition to the salinity per se) for preservation were introduced. Awareness of medicinal plants grew. With increased yields of grains, storage in silos began, perhaps as early as the fifth millennium bc in the fertile crescent of the Middle East. 9
In some settings, dietary laws were formulated, for example as codified in Deuteronomy, the fifth book of the Old Testament, defining what may and what may not be consumed. Although the latter are often thought of as principles in an early public health textbook, for example the separation of animals for food into clean and unclean categories, which certainly suggests a health rationale, the translation of the original terms and the context used may be misleading. Other interpretations at least as logical for prohibiting the consumption of certain items as food, even although in the case of pork, it may have been true with respect to transmission of trichinosis or the tapeworm Taenia solium , or the proscriptions against eating the flesh of animals found injured or dead of natural causes may have prevented some cases of intestinal anthrax or, from a modern perspective, the spillover of an animal infection to a human host. Specifically, social and cultural imperatives may also have played a major and perhaps even more important role in the development of Mosaic law, as the Hebrew tribes worked at creating an identity for themselves distinct from the Egyptians in as many ways as possible, including their food habits. For example, in Deuteronomy 14:21, we find the following: “You are not to eat any animal that dies naturally; although you may let a stranger staying with you eat it, or sell it to a foreigner; because you are a holy people for Adonai your God.” 10 It is difficult to believe that it would have been considered holy to provide something known to be potentially harmful to a stranger or foreigner, suggesting that something other than health concerns must have underlain this, and potentially other dietary laws.
The rationale for the Mosaic rules about avoiding the consumption of fish without scales or fins is more difficult to interpret in terms of health consequences, with the exception of known toxin-producing fish of the order Tetraodontiformes, such as pufferfish (also known as blowfish, or in Japan, fugu ). These creatures produce a potent neurotoxin (tetrodotoxin), which acts by blocking sodium channels in nerve cell membranes, interrupting the propagation of impulses along the axon and resulting in various dramatic neurologic manifestations, essentially causing progressive muscle paralysis, which interferes with breathing, often leading to respiratory death. Where these fish are consumed, for example Japan, preparation of the flesh for eating is highly regulated, only permitted at special restaurants by chefs specifically trained for the task. As a consequence, instances of fugu poisoning have become uncommon in Japan, especially in licensed restaurants, although cases continue to occur, especially among fishermen. However, this subject is not relevant to Egypt and The Levant.
The Mosaic dietary laws also preclude consumption of bottom feeders, such as catfish and eels, filter-feeding shellfish, shrimp, even swordfish. These creatures have become increasingly unhealthy to humans because they live in pesticide-polluted river beds, or bodies of water contaminated by heavy metals released into the air from coal-burning power plants and certain other industries, or concentrate the bacterial cause of cholera to an infectious dose. Such considerations would not have been so compelling when these laws were laid down, and their apparent prescient warning for our contemporary society, when several health hazards have been identified with these otherwise tasty seafoods, was at best serendipitous.
On the other hand, specific instructions to improve sanitation among inhabitants living in a community are also presented in the Old Testament, and these could be an attempt to implement practices that reduce disease incidence. In Deuteronomy 23:12–13, for example, the sanitary disposition of human feces is succinctly described: “You shall also have a place outside the camp and go out there, and you shall have a spade among your tools, and it shall be when you sit down outside, you shall dig with it and shall turn to cover up your excrement.” 10 Because a major source of foodborne illness is contamination of food by pathogens excreted in human feces, any means to reduce the introduction of feces into foods by improving sanitation practices reduces the incidence of such infections. However, the early Israelites did not know about microorganisms, and the relationship between illness and deposits of feces close to where food was prepared or eaten, or where water was stored in cisterns, may not have been obvious to them. However, the associated smells would have been strong and perhaps unpleasant enough to make it worth the extra effort to deposit and bury fecal material in designated spots away from the home. For the system be effective, everybody would need to follow the same practice, hence codification as a rule would have made great sense. Later on, during the Roman Empire, elaborate systems for water supply and sewage disposal were implemented, but these did not last after the Empire fell, being replaced by a simpler method: chamber pots, which were simply emptied into the streets outside the closely clustered homes of the people, to be carried away in the gutters (or more widely dispersed) by the rains, because water was too precious to use.
Let us fast forward to London in the middle of the nineteenth century: a major urban center, a rapidly growing population hub, and a thriving center of the economic and industrial revolution. It was also a city of filth, covered with a veneer of human and animal feces, fetid, and lacking even a minimally effective system to remove human waste (usually just tossed out of the windows of the homes on to the streets below, and anybody unfortunate enough to be in the wrong place). Charles Dickens describes London as follows.
A dirtier or more wretched place he had never seen. The street was very narrow and muddy, and the air was impregnated with filthy odors…Covered ways and yards, which here and there diverged from the main street, disclosed little knots of houses, where drunken men and women were positively wallowing in filth. 11
It was not atypical of the major cities around the world. The cholera epidemic of 1831 and 1832 drew new “attention to the deplorable lack of sanitation in the industrial cities. It was obvious that cholera was concentrated in the poorest districts, where sanitation was most neglected and the slum housing most befouled by excremental filth and other dirt. The relationship between disease, dirt and destitution clarified the need for sanitary reform as, in the crowded and congested cities, disease could fairly readily spread from the homes of the poor to the homes of the wealthy.” 12
In part, the realization that disease, dirt, and destitution were fellow travelers, all contributing to the pungent smells of filth and decay, supported and promoted the miasmic theory of disease, which really remained in vogue to the beginning of the twentieth century, well beyond the discovery of the microbial world as the cause of many diseases. Cholera also stimulated the movement to improve sanitation, exemplified by laws promulgated in London in 1848. During the cholera epidemic in London in 1854, Dr John Snow unraveled the role of contaminated water in cholera transmission, but it was the Great London Stink of 1858, when the polluted Thames was so foul that Parliament was forced into recess, that finally galvanized action, led by the Chief Engineer of the newly created Metropolitan Board of Works, Sir Joseph Bazalgette, to build an adequate sewage system, completed in 1875. Additional laws were put in to effect that prevented companies supplying drinking water from using the most contaminated Thames waters as a source, and requiring as well the use of some type of filtration. In conjunction with advances in the mechanics and uptake in the use of flush toilets, promoted by John Crapper, a nineteenth-century English plumbing entrepreneur and businessman who is immortalized in its common name, 13 significant improvements in environmental sanitation ultimately followed. Ultimately is the operative word, for an unintended consequence of the water closet was an initial increase in the amount of human excreta reaching the river, 14 until other reforms and administrative improvements in implementation finally had an impact and the incidence of foodborne infections could begin to diminish. This is an important point, for it is rarely the rule that single public health interventions have major impacts; more often than not, it is a combination of approaches, technical, legal, and administrative, that finally achieves the desired result.
Another technological advance affecting food safety was the development of reliable refrigeration for the storage of food (fresh or cooked) for consumption at a later date. Although earlier societies recognized the use of snow and ice as winter season refrigerants, this evolved later on (relatively recently) into the harvesting and long-term storage of ice obtained from lakes and rivers for use in domestic ice boxes year round and for shipment of goods around the globe. 15 Root cellars, as a reverse geothermal approach, became common in rural settings to safely store certain fruits, vegetables, and cooked preserves at a cool constant temperature below ground, and were especially accessible in rural settings. However, the major advance came with the identification of the microbial causes of foodborne illness and the effect of low temperature on their growth on the one hand, and on the other hand, the science behind evaporation and the development of mechanical refrigeration systems that could be used in commercial settings to produce ice. By 1882, a system to refrigerate a ship was developed by William Soltau Davidson, a Canadian entrepreneur working in Australia, leading to a global trade in refrigerated meat and dairy products, which has escalated to the present time. 16 With further innovation of the technology, home refrigerators became available around the time of World War I, but they were expensive and often used toxic chemicals as a refrigerant. 17 The development of Freon as a safer refrigerant (albeit subsequently identified as harmful to the atmospheric ozone layer and abandoned in favor of newer less harmful chemicals) and the economic boom after World War II led to a dramatic increase in the use of home refrigerators, with freezer compartments as a standard as well, after Clarence Birdseye’s flash-freezing methods developed in the 1920s to preserve fish (and subsequently, a whole variety of foods) became a commercial enterprise, preserving food for later cooking and consumption that resembled the fresh item in texture and taste. 18 Refrigeration allowed longer shelf life for a variety of foods without spoilage, although freezing kept foods safe for a long time, although the desirable fresh quality was lost after variable periods of storage, depending on the food involved.
The Food Safety and Inspection Service of the US Department of Agriculture regularly publishes recommendations for the duration of safe storage of refrigerated foods kept at temperatures between 2.7°C (37°F) and 4.4°C (40°F). 19 However, it is likely that most households keep food considerably longer than recommended (for example, how many toss out cooked meat, poultry, or fish after the recommended 3–4 days is reached?); for some, the refrigerator temperature exceeds 4.4°C (40°F), and in some cases, food is kept until mold is obvious, foods smell off, or there is liquefaction or other evidence of spoilage. This situation sometimes but does not necessarily lead to intestinal illness of 1 sort or another. In addition, some organisms, such as Listeria monocytogenes , grow at refrigerator temperature, and can reach a level high enough to cause illness, particularly in highly susceptible individuals such as pregnant women, the immunocompromised, and the elderly. 20 There are no practical devices, such as the temperature indicators to monitor cold chain storage of vaccines, in use to indicate the safety of refrigerated foods. Without refrigeration, especially in warm and tropical climates, food spoilage occurs rapidly and the practical rule in such settings, but not necessarily followed, should be eat it or toss it.
Pasteurization, a process involving heating followed by rapid cooling to reduce microbial populations to levels that do not cause illness and delay spoilage, was developed by Louis Pasteur and subsequently has been highly successfully applied to milk and milk products, and has virtually eliminated milk transmission of infections. Implementation of pasteurization, and other initiatives to improve milk safety from “1870 to 1940 [launched] a vigorous public health movement to prevent the bacterial contamination of milk…In this period, the market milk supply gradually became safer, with improved sanitary conditions in dairy farms, pasteurization of milk, keeping milk at low temperatures during shipping and delivery, and prohibition of the sale of loose milk (unpackaged bulk milk stored in a large canister and sold using a dipper) in grocery stores.” 21 There are now several methods to improve milk safety. The classic method, referred to as high-temperature, short-time (HTST), involves heating to 71.7°C (161°F) for 15 to 20 seconds, and extends the refrigerated shelf life to 2 to 3 weeks. The introduction of ultrapasteurized milk, which involves heating milk to a temperature of 135°C (275°F) for a minimum of 1 second, has increased refrigerated stored life to 2 to 3 months. When ultrapasteurization is coupled with sterile handling and container technology, a shelf life of 6 to 9 months can be achieved, even without refrigeration. A third technology, referred to as extended shelf life (ESL), processes HTST milk through a microbial filtration step that further increases the useful shelf life of the product, although a lack of established standards for its production results in variable shelf life among products labeled as ESL.
Another technology, tube wells, aimed at providing clean water for developing countries, has been extensively applied since the 1970s, especially in Bangladesh and India. By obtaining water from underground rather than surface sources, microbial contamination from human or animal feces deposited in the environment and present in ground water is precluded. This development has had a major impact on the incidence of cholera and dysentery in areas where it has been used. By the early 1990s, nearly all rural populations in Bangladesh and India had access to tube well water. However, in many locales, tube well water drawn from shallow sources, 10 to 70 m below ground, contained high concentrations of arsenic, leached from sediments via biogeochemical processes that promote reducing environments. 22 An unanticipated consequence, widespread evidence of chronic arsenic poisoning, had become evident. Presenting initially with nonspecific abdominal pain, diarrhea, weight loss, and skin changes consisting of hyperpigmented areas with diffuse or nodular keratotic thickening of the palms and soles, and hepatomegaly, over time involvement of the intestinal, cardiac, respiratory, genitourinary, neurologic, endocrine, and hematologic systems became evident. A significant increase in cancers of the skin, lung, liver, kidney, and bladder has since been documented, 23 with an excess in mortality compared with populations not affected by arsenic. 24 It has been difficult to mitigate this problem, although attempts have been made to test individual tube wells, taking those with high levels of arsenic out of service, and creating shared water resources using clean wells. To find a simple, cheap, readily maintained system, the US National Academy of Engineering offered a prize, supported by the Grainger Foundation, to develop a method to remove arsenic from contaminated tube well water, allowing its continued and safe use. A winning team was identified in 2007, 25 and by 2012, the investigators reported its successful field testing in more than 200 localities in West Bengal, India, with each unit serving around 150 families, who were able to monitor and maintain the system once trained. 26 Technology may solve 1 problem and in so doing create another, as is the case with tube wells, but when the secondary problem is recognized and addressed, it is often possible to identify technological fixes to solve it.
The technological revolution not only provided options for the safe storage of perishable foods for increasing periods, but coupled with the emergence of economically viable rapid air freight systems and the development of genetic food variants that reduced damage in shipping (often at the cost of flavor and taste), has allowed a global food transportation system to develop, with an unprecedented increase in volume in the past 2 decades. The food markets, at least in affluent countries, are no longer seasonal; you can more or less consume the foods you want when you want them. Raspberries in December? Done! All sorts of tropical fruits can be obtained, at a price of course, in the dead of winter in New York, London, Paris, and most developed world centers. Bananas in January? Done! Although some warm weather fruits and vegetables can be grown in hot-house conditions in the northern hemisphere, the ability to transport them from the southern hemisphere and tropical agricultural regions to the north is now big business. The value of this global trade escalated from an estimated $50 billion in 1960, to $438 billion in 1998, to nearly $1060 billion in 2008. 27 The North American Free Trade Agreement area, the European Union, and Asia (much of it representing food exported from China to Japan) are the major destinations. All 3 regions depend on southern hemisphere countries for imports of juices and off-season fresh fruits, and on equatorial regions for bananas, the leading global fresh fruit import. 28 In the United States, in the first 10 months of 2012 alone, nearly 32 million metric tonnes of food and agricultural products were imported, legally, exclusive of wine and beer. 29 To conceptualize this statistic, to deliver a load of that magnitude would require nearly 800 Boeing 747–400 cargo planes landing every day. Now think of conducting comprehensive food safety inspections on that number of aircraft. Of course, foods are moved by every means of transportation, from ships, to railroads, to trucks, making it even more complicated as the entry points and routes of transport are so numerous and diverse; if only it were confined to the airports! Moreover, it is not just fresh foods that are involved, because increasingly, frozen and processed or at least partially processed foods are being imported. The US Food and Drug Administration (FDA) estimates that 10% to 15% of all food consumed in the United States is produced elsewhere, and that 75% of processed foods in the United States contain ingredients that originated in other countries, almost impossible to fully trace. 30 The FDA recognizes that in the future “(p)roducts entering the U.S. will come from new and different markets and will flow through long, multi-step processes to convert globally sourced materials into finished goods. As global product flows change, many individuals will encounter the growing dangers of fraud and economic or other intentional adulteration of both foods and medical products.” 30 It is not without reason that the international food trade network has been characterized as “a perfect platform to spread potential contaminants with practically untraceable origins.” 27
As we move forward in a period of climate change, which will have a variety of impacts on food production, at the same time that growth in populations will require an increasing volume of food trade, there may be additional impacts on food safety. Among the more likely of these impacts are an increase in contamination of food products by mycotoxins in a variety of crops, a likely increase in the use of pesticides at higher concentrations, and variable effects on the transfer of trace elements (some necessary and some potentially toxic). 31 On top of these effects, the deliberate adulteration of foods for animals and humans for economic gain (eg, the recent scandals in China in which melamine has been added to milk in order to boost the measurement of protein content) has resulted in an international outbreak of severe kidney disease in young children consuming the tainted products. 32 As food becomes more precious with population growth, the likelihood of adulteration of foods for economic gain will undoubtedly increase. Regulatory and inspection services will be stressed in technology, resources, and human capacity, which can only partially be ameliorated by point-of-use technology to identify pathogens or toxins in foodstuff, or drugs, pesticides, or adulterants, which, of course, is still to be developed.
Another aspect of this problem is the trade in wildlife intended for consumption. 33 The magnitude of this trade and its relationship to food safety is uncertain, especially because a considerable proportion is illicit, and some of it relates to trade in ivory, skins, hair, or parts reputed to have medicinal or aphrodisiac properties, that is, not for food. However, all the indications are that the volume is big, and potentially dangerous. 34 In an era when we recognize the global problem of emerging and reemerging infectious diseases of humans, most of which originate in animals that infect humans 35 and, in the nightmarish scenario, can successfully transmit from human to human, there is great concern about the potential of this trade to introduce new diseases, particularly when it is illicit, underground, and difficult to monitor. Given the likely origins of human immunodeficiency virus/AIDS in nonhuman primates consumed as food in the bush in Africa, 36 an agent that only subsequently became capable of transmitting from human to human, it can be thought about as an originally foodborne disease, and a dead-end infection as well. How many more like it are out there? How many can make the species jump to humans and to efficient transmission between humans? Can these possibly be monitored? And if they can, will it be possible to intervene in time to prevent the next global pandemic? The increasing global trade in bush meat for consumption suggests the virtual impossibility of accomplishing that goal, and (very) creative thinking will be essential. Another issue, generally overlooked, is the role of wildlife in the transmission of disease at the wildlife-livestock interface. 37 Because the agents that may be found are often capable of being transmitted to humans, this relationship is of potential importance. It may not be simply transmission of organisms from wildlife to livestock. In 1 study conducted in Spain, cattle were identified as the source of transmission of serovars of Salmonella to wildlife, in this case, wild boars. 38 In environments in which wildlife, livestock, and humans interact frequently and closely, disease transmission among them must be considered in developing models for disease control. The concept of One Health, the interrelated health of animals and humans, is even broader than is generally considered, and these more complex relationships will need to be considered as an appropriate and effective One Health research, surveillance, and response policy is developed. 39 As this development happens, and wildlife are increasingly seen as contributing to the risk of human disease, the message needs to be carefully crafted in order to avoid serious effects on wildlife conservation programs. 40
Two other brief comments: first, although we are paying attention to viruses and bacteria, we should not forget about parasitic infections of humans. It has recently been noted that “(c)hanging eating habits, population growth and movements, global trade of foodstuff, changes in food production systems, climate change, increased awareness and better diagnostic tools are some of the main drivers affecting the emergence or reemergence of many foodborne parasitic diseases in recent years. In particular, the increasing demand for exotic and raw food is one of the reasons why reports of foodborne infections, and especially water-borne parasitosis, have increased in the last years.” 41 This concern demands greater attention. Second, considered as a domestic US issue, the twin movements toward huge commercial farms and, for livestock, the crowded and often filthy conditions in which the animals are sometimes kept on the one hand, and the desire for small, local, organic, and not necessarily well-run farms on the other, may each in their own way increase the risk of marketing unsafe foods. The risks in factory farming are already known, including runoff of pathogen-containing waste, which can contaminate nearby fields of vegetables, not to mention the effect of the use of antimicrobials to promote animal growth and earlier marketing on the selection of antibiotic-resistant human pathogens, and the large-scale processing that goes along with it, which may put many people at risk when 1 contaminated carcass is processed with many clean carcasses, resulting in a lot of contaminated meat. The potential risks in small farms, struggling to compete and perhaps leading to cutting corners, is a still poorly assessed risk. 42 Proposed legislation to tighten up regulatory and inspection oversight for large farms is meeting resistance from small farmers, who fear that the costs of implementation may make them uncompetitive. 43
If the discussion on trade is seen as moving potentially unsafe foods across the globe and within countries to the consumer, travel of people, especially to low-income and middle-income countries, can move them to risky foods, an equally problematic situation made potentially more risky where the regulatory environment is worse than in their home country, and access to good medical care, if necessary, may be problematic. Although not all travel-related illness is foodborne, many disease episodes, in particular diarrhea, result from eating food contaminated with bacterial, protozoal, and viral pathogens. 44 Younger travelers, on limited budgets, extended trips, and more likely to engage in risky behavior, experience frequent episodes of diarrhea and other foodborne infections. 45 Cruise ships have been the scene and source of outbreaks of diarrheal disease affecting the more affluent, often older traveler, frequently caused by norovirus but also associated with a variety of bacterial and other enteric pathogens. 46
It has been known for a long time that disease may spread along travel and trade routes. The movement of cholera is an excellent example of this. Even before the cause was identified, evidence emerged in New York in 1832 that cholera spread with human movement along the newly constructed Erie Canal. 47 It is ironic that the author of the report did not believe in his own data, succumbing to the lure of the miasmic theory of disease. Contemporary proof of this theory is provided by the origins of the cholera epidemic in Haiti, after the devastating earthquake of January 2010, introduced by Nepalese soldiers who had joined the UN Peacekeeping Force to deal with security aspects of the response to the earthquake. 48 Transport of pathogens by travelers can be an efficient way to distribute an agent widely across the globe. Albeit not foodborne, the severe acute respiratory syndrome outbreak of 2003 shows the rapid dissemination of an agent carried by airline passengers, which in this instance led to a pandemic within a few days of the transmission of the infection from the index case to secondary cases. 49
Foodborne illnesses have been a part of the human experience from the beginning. Over time, keen observation and deduction have identified particular illnesses associated with particular foods or food-related behavior. Such observations have stimulated attempts to mitigate these risks, often with significant success when generally applied. However, such lessons are hard won, and many have suffered and many have succumbed over time until, in a Darwinian fashion, lessons learned with positive survival value have been incorporated into cultural practices.
With the growth of science, and the ability to identify specific causes of foodborne illnesses, several technological solutions have been developed that have significantly reduced the burden of disease. Not only are they technological (eg, pasteurization), but sometimes, they are in the form of regulatory oversight to ensure good practice. However, in the real world, technology and optimal practice can break down from time to time, resulting in periodic outbreaks of illnesses that could have been avoided. The scale of contemporary food production, preservation, trade, and storage in the modern era not only makes these incidents more likely but has also introduced many new ways for foodborne disease to be transmitted, sometimes in outbreak or epidemic form. These new opportunities for transmission of illness must be identified, unraveled, and addressed, often in a manner unique to each setting. The future presents us with opportunities to improve both technology and practice and, in turn, to help to reduce disease incidence. The magnitude of the food trade, partly in response to the growth in population and in part to changes in the way that people obtain, prepare, and consume food, provides new ways for pathogens to be transported and transmitted. The emergence of new agents, and the economic incentives for some to put others at risk through poor practice or adulteration of food, ensures that the incidence of foodborne diseases will continue to be high.
In the articles that follow in this issue, the agents, the epidemiology, the clinical aspects and treatment, and the prevention of foodborne illnesses are more thoroughly examined. Although it remains a significant problem even in the United States and in all other developed countries, it also results in a major burden of disease and death in low-income and middle-income countries as well. Although some of the potential approaches to reduce these burdens are common across all environments, many will need to be targeted to the specific conditions that create the risk. For this goal, we need a continued vigorous research agenda, attention to improving practices in the food industry and culturally sensitive customs in different populations, and sensible and affordable regulation in individual nations and in the international setting. There is the need to understand how human health, animal health, and wildlife health are linked together in order to develop policies that address systemic issues that can only be, or are best, tackled together. This approach is termed One Health, and although taken in the abstract, it makes great sense, until the scientific and political leadership truly buy in, and until the general public is engaged, based on knowledge and fact, progress will be slow and avoidable illnesses will, all too often, continue to occur. This issue of Infectious Diseases Clinics of North America is a timely presentation of the most contemporary information, and is of use to all interested in the issue, regardless of their background.

IMAGES
VIDEO
COMMENTS
"Food-borne Disease Prevention and Risk Assessment" is a Special Issue of the International Journal of Environmental Research and Public Health on understanding how food-borne disease is still a global threat to health today and to be able to target strategies to reduce its prevalence. Despite decades of government and industry interventions, food-borne disease remains unexpectedly high in ...
Foodborne diseases can be both acute and chronic, and stem from three sources: biological, chemical, and physical. Bacteria, viruses, and parasites, are the main biological hazards causing acute foodborne diseases. Certain biological toxins can also be considered as causing acute effects, such as most seafood toxins, and these are discussed ...
Introduction. Foodborne diseases (FBD) still cause a substantial public health, economic and social burden worldwide. Recognizing the need to measure the burden and distribution of FBD and encourage evidence-informed policies, in 2015 the World Health Organization (WHO) reported the first estimates of global and regional disease burden due to 31 foodborne hazards [1].
His lectures and papers are arguably some of the most significant illustrations of the foundation of public health prevention philosophy and practice today. ... The CDC established FoodCORE (Foodborne Diseases Centers for Outbreak Response Enhancement), improving foodborne disease outbreak response capacity in ... VA Medicinal Cannabis Research ...
Generate hypotheses and describe the outbreak in terms of person, time and place 22 Step 5. Test hypotheses through analytical studies and food, environmental and laboratory investigations 30 Step 6. Identify the point of contamination and the original source of the outbreak 43 Step 7. Control the outbreak 45.
Foodborne illnesses pose a substantial public health concern in the United States, with 48 million cases reported leading to economic costs of up to $90 billion (Scallan et al., 2011, Scharff, 2020).Although research has been conducted to estimate the incidence and economic burden of foodborne illnesses, for policy purposes, it is also crucial to understand the attribution of foodborne ...
31 hazards was 33 (95% UI 25-46) million Disability Adjusted Life Y ears (DAL Ys) in 2010; 40% of. the food-borne disease burden was among children under five years of age. Since we know that ...
A dynamic system with 3 levels of risk management was developed to prevent the spread of foodborne diseases in populations while considering secondary infections. The effects of factors such as information quality, time delay and periodicity were compared. The results indicated that the infection mechanism of foodborne diseases exacerbated the severity of epidemics. This severity manifested as ...
Foodborne disease (also referred to as foodborne illness or food poisoning) is any illness that results from the consumption of contaminated food, contaminated with pathogenic bacteria, viruses, or parasites. ... Technology and Education, vol. 1, 978-84-939843-9-7, Formatex Research Center, Badajoz, Spain (2013), pp. 216-226. Google Scholar.
The CDC Foodborne Disease Active Surveillance Network (FoodNet) provides preprocessed population-based foodborne-disease surveillance records and visualizations via FoodNet Fast, a publicly ...
In Europe, 3,166 foodborne disease outbreaks occurred during the same period, resulting in 22,010 illnesses, 1,838 hospitalizations, and 48 deaths (EFSA, 2022). Since the late 1990s, food safety certification has emerged as a prominent and influential regulatory mechanism in both the private and public spheres of the contemporary agri-food system.
Foodborne Illness: Pathogens and Diseases. June 2019. In book: Research Trends in Food Technology and Nutrition Vol-8 (pp.1-20) Chapter: First. Publisher: AkiNik Publications. Authors: Ena Gupta ...
ABSTRACT. Foodborne illness is a major public health concern, often approached by focusing on socio-demographic groups who are considered most 'vulnerable' to foodborne disease such as elderly people or pregnant women. Based on a review of existing literature and original research with UK consumers, this paper proposes an alternative ...
Foodborne infection is a major health issue and its impact on global social and economic development remains unknown ().A paper published in the journal Nature estimates 30% of all infections over the last 60 years were Foodborne ().The Centre for Disease Control in the United States estimates that 76 million people per year are affected by Foodborne illness, with 300,000 requiring hospital ...
Foodborne diseases and outbreaks are significant threats to public health, resulting in millions of illnesses and deaths worldwide each year. Traditional foodborne disease surveillance systems rely on data from healthcare facilities, laboratories, and government agencies to monitor and control outbreaks. Recently, there is a growing recognition of the potential value of incorporating social ...
Background This study aimed to identify the epidemiology, seasonality, aetiology and clinical characteristics of sporadic foodborne diseases in Zhejiang province during 2016-2020. Methods Descriptive statistical methods were used to analyze the data from surveillance network established by the Zhejiang Provincial Center for Disease Control and Prevention. There were 31 designated hospitals ...
This Research Topic titled "Foodborne Pathogens: Hygiene and Safety" focuses on important food safety concerns such as the potential presence of pathogens in food as well as their toxins/metabolites, the resistance to antibiotics or sanitizers, and other virulence characteristics. It includes four reviews and 44 original research papers.
Foodborne illnesses caused by bacteria are a worldwide occurrence. For exam- ple, between 128,000 and 325,000 hospitalizations and 3000 to 5000 deaths, and at least
Additionally, to ensure food safety and prevent foodborne disease outbreaks, the development of novel methods for the microbiological detection and identification of foodborne pathogens is of great importance and, therefore, welcomed as a topic in this Special Issue, taking both original research papers and reviews into consideration for ...
consumption of contaminated food, pathogenic. bacteria, viruses, or p arasites that contaminate food, as well as chemical or natural toxins such as. poisonous mushrooms. It also r eferred to food ...
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications. ... Foodborne disease caused by ...
The disease course for C. perfringens foodborne illness tends to be self- limited, requiring only supportive care, and resolving within 12 to 24 hours. As previously noted, there is some thought ...
Each year, an estimated one in six people (or 48 million people) gets sick, 128,000 persons are hospitalized, and 3,000 deaths occur as a result of foodborne diseases. 1 In addition to the pain and suffering, foodborne-related illnesses are estimated to cost $35 billion annually in medical expenses, lost productivity, and related mortality. 2
2.1. Bacteria. Foodborne illnesses caused by bacteria are a worldwide occurrence. For example, between 128,000 and 325,000 hospitalizations and 3000 to 5000 deaths, and at least 76 million illnesses per year are estimated to be associated with foodborne illnesses in the USA, costing the economy up to $83 billion per year [4,18,19].In the EU, specifically in 2020, there were 20,017 human cases ...
Product (s) Linked to Illnesses (if any): During an outbreak or adverse event investigation, the FDA and CDC, along with state and local authorities collect and analyze three types of information ...
Perspectives in Foodborne Illness. This article provides a historical framework on food safety for more contemporary details to rest on, focusing primarily on the past 100 years or so (with a touch of ancient history) as particular issues that affect how the safety of the food we eat have been appreciated, have evolved or at times have been ...