- Open access
- Published: 13 November 2019

Evidence-based models of care for the treatment of alcohol use disorder in primary health care settings: protocol for systematic review
- Susan A. Rombouts 1 ,
- James Conigrave 2 ,
- Eva Louie 1 ,
- Paul Haber 1 , 3 &
- Kirsten C. Morley ORCID: orcid.org/0000-0002-0868-9928 1
Systematic Reviews volume 8 , Article number: 275 ( 2019 ) Cite this article
7203 Accesses
3 Citations
Metrics details
Alcohol use disorder (AUD) is highly prevalent and accounts globally for 1.6% of disability-adjusted life years (DALYs) among females and 6.0% of DALYs among males. Effective treatments for AUDs are available but are not commonly practiced in primary health care. Furthermore, referral to specialized care is often not successful and patients that do seek treatment are likely to have developed more severe dependence. A more cost-efficient health care model is to treat less severe AUD in a primary care setting before the onset of greater dependence severity. Few models of care for the management of AUD in primary health care have been developed and with limited implementation. This proposed systematic review will synthesize and evaluate differential models of care for the management of AUD in primary health care settings.
We will conduct a systematic review to synthesize studies that evaluate the effectiveness of models of care in the treatment of AUD in primary health care. A comprehensive search approach will be conducted using the following databases; MEDLINE (1946 to present), PsycINFO (1806 to present), Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials (CENTRAL) (1991 to present), and Embase (1947 to present).
Reference searches of relevant reviews and articles will be conducted. Similarly, a gray literature search will be done with the help of Google and the gray matter tool which is a checklist of health-related sites organized by topic. Two researchers will independently review all titles and abstracts followed by full-text review for inclusion. The planned method of extracting data from articles and the critical appraisal will also be done in duplicate. For the critical appraisal, the Cochrane risk of bias tool 2.0 will be used.
This systematic review and meta-analysis aims to guide improvement of design and implementation of evidence-based models of care for the treatment of alcohol use disorder in primary health care settings. The evidence will define which models are most promising and will guide further research.
Protocol registration number
PROSPERO CRD42019120293.
Peer Review reports
It is well recognized that alcohol use disorders (AUD) have a damaging impact on the health of the population. According to the World Health Organization (WHO), 5.3% of all global deaths were attributable to alcohol consumption in 2016 [ 1 ]. The 2016 Global Burden of Disease Study reported that alcohol use led to 1.6% (95% uncertainty interval [UI] 1.4–2.0) of total DALYs globally among females and 6.0% (5.4–6.7) among males, resulting in alcohol use being the seventh leading risk factor for both premature death and disability-adjusted life years (DALYs) [ 2 ]. Among people aged 15–49 years, alcohol use was the leading risk factor for mortality and disability with 8.9% (95% UI 7.8–9.9) of all attributable DALYs for men and 2.3% (2.0–2.6) for women [ 2 ]. AUD has been linked to many physical and mental health complications, such as coronary heart disease, liver cirrhosis, a variety of cancers, depression, anxiety, and dementia [ 2 , 3 ]. Despite the high morbidity and mortality rate associated with hazardous alcohol use, the global prevalence of alcohol use disorders among persons aged above 15 years in 2016 was stated to be 5.1% (2.5% considered as harmful use and 2.6% as severe AUD), with the highest prevalence in the European and American region (8.8% and 8.2%, respectively) [ 1 ].
Effective and safe treatment for AUD is available through psychosocial and/or pharmacological interventions yet is not often received and is not commonly practiced in primary health care. While a recent European study reported 8.7% prevalence of alcohol dependence in primary health care populations [ 4 ], the vast majority of patients do not receive the professional treatment needed, with only 1 in 5 patients with alcohol dependence receiving any formal treatment [ 4 ]. In Australia, it is estimated that only 3% of individuals with AUD receive approved pharmacotherapy for the disorder [ 5 , 6 ]. Recognition of AUD in general practice uncommonly leads to treatment before severe medical and social disintegration [ 7 ]. Referral to specialized care is often not successful, and those patients that do seek treatment are likely to have more severe dependence with higher levels of alcohol use and concurrent mental and physical comorbidity [ 4 ].
Identifying and treating early stage AUDs in primary care settings can prevent condition worsening. This may reduce the need for more complex and more expensive specialized care. The high prevalence of AUD in primary health care and the chronic relapsing character of AUD make primary care a suitable and important location for implementing evidence-based interventions. Successful implementation of treatment models requires overcoming multiple barriers. Qualitative studies have identified several of those barriers such as limited time, limited organizational capacity, fear of losing patients, and physicians feeling incompetent in treating AUD [ 8 , 9 , 10 ]. Additionally, a recent systematic review revealed that diagnostic sensitivity of primary care physicians in the identification of AUD was 41.7% and that only in 27.3% alcohol problems were recorded correctly in primary care records [ 11 ].
Several models for primary care have been created to increase identification and treatment of patients with AUD. Of those, the model, screening, brief interventions, and referral to specialized treatment for people with severe AUD (SBIRT [ 12 ]) is most well-known. Multiple systematic reviews exist, confirming its effectiveness [ 13 , 14 , 15 ], although implementation in primary care has been inadequate. Moreover, most studies have looked primarily at SBIRT for the treatment of less severe AUD [ 16 ]. In the treatment of severe AUD, efficacy of SBIRT is limited [ 16 ]. Additionally, many patient referred to specialized care often do not attend as they encounter numerous difficulties in health care systems including stigmatization, costs, lack of information about existing treatments, and lack of non-abstinence-treatment goals [ 7 ]. An effective model of care for improved management of AUD that can be efficiently implemented in primary care settings is required.
Review objective
This proposed systematic review will synthesize and evaluate differential models of care for the management of AUD in primary health care settings. We aim to evaluate the effectiveness of the models of care in increasing engagement and reducing alcohol consumption.
By providing this overview, we aim to guide improvement of design and implementation of evidence-based models of care for the treatment of alcohol use disorder in primary health care settings.
The systematic review is registered in PROSPERO international prospective register of systematic reviews (CRD42019120293) and the current protocol has been written according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols (PRISMA-P) recommended for systematic reviews [ 17 ]. A PRISMA-P checklist is included as Additional file 1 .
Eligibility criteria
Criteria for considering studies for this review are classified by the following:
Study design
Both individualized and cluster randomized trials will be included. Masking of patients and/or physicians is not an inclusion criterion as it is often hard to accomplish in these types of studies.
Patients in primary health care who are identified (using screening tools or by primary health care physician) as suffering from AUD (from mild to severe) or hazardous alcohol drinking habits (e.g., comorbidity, concurrent medication use). Eligible patients need to have had formal assessment of AUD with diagnostic tools such as Diagnostic and Statistical Manual of Mental Disorders (DSM-IV/V) or the International Statistical Classification of Diseases and Related Health Problems (ICD-10) and/or formal assessment of hazardous alcohol use assessed by the Comorbidity Alcohol Risk Evaluation Tool (CARET) or the Alcohol Use Disorders Identification test (AUDIT) and/or alcohol use exceeding guideline recommendations to reduce health risks (e.g., US dietary guideline (2015–2020) specifies excessive drinking for women as ≥ 4 standard drinks (SD) on any day and/or ≥ 8 SD per week and for men ≥ 5 SD on any day and/or ≥ 15 SD per week).
Studies evaluating models of care for additional diseases (e.g., other dependencies/mental health) other than AUD are included when they have conducted data analysis on the alcohol use disorder patient data separately or when 80% or more of the included patients have AUD.
Intervention
The intervention should consist of a model of care; therefore, it should include multiple components and cover different stages of the care pathway (e.g., identification of patients, training of staff, modifying access to resources, and treatment). An example is the Chronic Care Model (CCM) which is a primary health care model designed for chronic (relapsing) conditions and involves six elements: linkage to community resources, redesign of health care organization, self-management support, delivery system redesign (e.g., use of non-physician personnel), decision support, and the use of clinical information systems [ 18 , 19 ].
As numerous articles have already assessed the treatment model SBIRT, this model of care will be excluded from our review unless the particular model adds a specific new aspect. Also, the article has to assess the effectiveness of the model rather than assessing the effectiveness of the particular treatment used. Because identification of patients is vital to including them in the trial, a care model that only evaluates either patient identification or treatment without including both will be excluded from this review.
Model effectiveness may be in comparison with the usual care or a different treatment model.
Included studies need to include at least one of the following outcome measures: alcohol consumption, treatment engagement, uptake of pharmacological agents, and/or quality of life.
Solely quantitative research will be included in this systematic review (e.g., randomized controlled trials (RCTs) and cluster RCTs). We will only include peer-reviewed articles.
Restrictions (language/time period)
Studies published in English after 1 January 1998 will be included in this systematic review.
Studies have to be conducted in primary health care settings as such treatment facilities need to be physically in or attached to the primary care clinic. Examples are co-located clinics, veteran health primary care clinic, hospital-based primary care clinic, and community primary health clinics. Specialized primary health care clinics such as human immunodeficiency virus (HIV) clinics are excluded from this systematic review. All studies were included, irrespective of country of origin.
Search strategy and information sources
A comprehensive search will be conducted. The following databases will be consulted: MEDLINE (1946 to present), PsycINFO (1806 to present), Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials (CENTRAL) (1991 to present), and Embase (1947 to present). Initially, the search terms will be kept broad including alcohol use disorder (+synonyms), primary health care, and treatment to minimize the risk of missing any potentially relevant articles. Depending on the number of references attained by this preliminary search, we will add search terms referring to models such as models of care, integrated models, and stepped-care models, to limit the number of articles. Additionally, we will conduct reference searches of relevant reviews and articles. Similarly, a gray literature search will be done with the help of Google and the Gray Matters tool which is a checklist of health-related sites organized by topic. The tool is produced by the Canadian Agency for Drugs and Technologies in Health (CADTH) [ 20 ].
See Additional file 2 for a draft of our search strategy in MEDLINE.
Data collection
The selection of relevant articles is based on several consecutive steps. All references will be managed using EndNote (EndNote version X9 Clarivate Analytics). Initially, duplicates will be removed from the database after which all the titles will be screened with the purpose of discarding clearly irrelevant articles. The remaining records will be included in an abstract and full-text screen. All steps will be done independently by two researchers. Disagreement will lead to consultation of a third researcher.
Data extraction and synthesis
Two researchers will extract data from included records. At the conclusion of data extraction, these two researchers will meet with the lead author to resolve any discrepancies.
In order to follow a structured approach, an extraction form will be used. Key elements of the extraction form are information about design of the study (randomized, blinded, control), type of participants (alcohol use, screening tool used, socio-economic status, severity of alcohol use, age, sex, number of participants), study setting (primary health care setting, VA centers, co-located), type of intervention/model of care (separate elements of the models), type of health care worker (primary, secondary (co-located)), duration of follow-up, outcome measures used in the study, and funding sources. We do not anticipate having sufficient studies for a meta-analysis. As such, we plan to perform a narrative synthesis. We will synthesize the findings from the included articles by cohort characteristics, differential aspects of the intervention, controls, and type of outcome measures.
Sensitivity analyses will be conducted when issues suitable for sensitivity analysis are identified during the review process (e.g., major differences in quality of the included articles).
Potential meta-analysis
In the event that sufficient numbers of effect sizes can be extracted, a meta-analytic synthesis will be performed. We will extract effect sizes from each study accordingly. Two effect sizes will be extracted (and transformed where appropriate). Categorical outcomes will be given in log odds ratios and continuous measures will be converted into standardized mean differences. Variation in effect sizes attributable to real differences (heterogeneity) will be estimated using the inconsistency index ( I 2 ) [ 21 , 22 ]. We anticipate high degrees of variation among effect sizes, as a result moderation and subgroup-analyses will be employed as appropriate. In particular, moderation analysis will focus on the degree of heterogeneity attributable to differences in cohort population (pre-intervention drinking severity, age, etc.), type of model/intervention, and study quality. We anticipate that each model of care will require a sub-group analysis, in which case a separate meta-analysis will be performed for each type of model. Small study effect will be assessed with funnel plots and Egger’s symmetry tests [ 23 ]. When we cannot obtain enough effect sizes for synthesis or when the included studies are too diverse, we will aim to illustrate patterns in the data by graphical display (e.g., bubble plot) [ 24 ].
Critical appraisal of studies
All studies will be critically assessed by two researchers independently using the Revised Cochrane risk-of-bias tool (RoB 2) [ 25 ]. This tool facilitates systematic assessment of the quality of the article per outcome according to the five domains: bias due to (1) the randomization process, (2) deviations from intended interventions, (3) missing outcome data, (4) measurement of the outcome, and (5) selection of the reported results. An additional domain 1b must be used when assessing the randomization process for cluster-randomized studies.
Meta-biases such as outcome reporting bias will be evaluated by determining whether the protocol was published before recruitment of patients. Additionally, trial registries will be checked to determine whether the reported outcome measures and statistical methods are similar to the ones described in the registry. The gray literature search will be of assistance when checking for publication bias; however, completely eliminating the presence of publication bias is impossible.
Similar to article selection, any disagreement between the researchers will lead to discussion and consultation of a third researcher. The strength of the evidence will be graded according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach [ 26 ].
The primary outcome measure of this proposed systematic review is the consumption of alcohol at follow-up. Consumption of alcohol is often quantified in drinking quantity (e.g., number of drinks per week), drinking frequency (e.g., percentage of days abstinent), binge frequency (e.g., number of heavy drinking days), and drinking intensity (e.g., number of drinks per drinking day). Additionally, outcomes such as percentage/proportion included patients that are abstinent or considered heavy/risky drinkers at follow-up. We aim to report all these outcomes. The consumption of alcohol is often self-reported by patients. When studies report outcomes at multiple time points, we will consider the longest follow-up of individual studies as a primary outcome measure.
Depending on the included studies, we will also consider secondary outcome measures such as treatment engagement (e.g., number of visits or pharmacotherapy uptake), economic outcome measures, health care utilization, quality of life assessment (physical/mental), alcohol-related problems/harm, and mental health score for depression or anxiety.
This proposed systematic review will synthesize and evaluate differential models of care for the management of AUD in primary health care settings.
Given the complexities of researching models of care in primary care and the paucity of a focus on AUD treatment, there are likely to be only a few studies that sufficiently address the research question. Therefore, we will do a preliminary search without the search terms for model of care. Additionally, the search for online non-academic studies presents a challenge. However, the Gray Matters tool will be of guidance and will limit the possibility of missing useful studies. Further, due to diversity of treatment models, outcome measures, and limitations in research design, it is possible that a meta-analysis for comparative effectiveness may not be appropriate. Moreover, in the absence of large, cluster randomized controlled trials, it will be difficult to distinguish between the effectiveness of the treatment given and that of the model of care and/or implementation procedure. Nonetheless, we will synthesize the literature and provide a critical evaluation of the quality of the evidence.
This review will assist the design and implementation of models of care for the management of AUD in primary care settings. This review will thus improve the management of AUD in primary health care and potentially increase the uptake of evidence-based interventions for AUD.
Availability of data and materials
Not applicable.
Abbreviations
Alcohol use disorder
Alcohol Use Disorders Identification test
Canadian Agency for Drugs and Technologies in Health
The Comorbidity Alcohol Risk Evaluation
Cochrane Central Register of Controlled Trials
Diagnostic and Statistical Manual of Mental Disorders
Human immunodeficiency virus
10 - International Statistical Classification of Diseases and Related Health Problems
Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols
Screening, brief intervention, referral to specialized treatment
Standard drinks
World Health Organization
WHO. Global status report on alcohol and health: World health organization; 2018.
The global burden of disease attributable to alcohol and drug use in 195 countries and territories, 1990–2016. a systematic analysis for the Global Burden of Disease Study 2016. Lancet Psychiatry. 2018;5(12):987–1012.
Article Google Scholar
WHO. Global strategy to reduce the harmful use of alcohol: World health organization; 2010.
Rehm J, Allamani A, Elekes Z, Jakubczyk A, Manthey J, Probst C, et al. Alcohol dependence and treatment utilization in Europe - a representative cross-sectional study in primary care. BMC Fam Pract. 2015;16:90.
Morley KC, Logge W, Pearson SA, Baillie A, Haber PS. National trends in alcohol pharmacotherapy: findings from an Australian claims database. Drug Alcohol Depend. 2016;166:254–7.
Article CAS Google Scholar
Morley KC, Logge W, Pearson SA, Baillie A, Haber PS. Socioeconomic and geographic disparities in access to pharmacotherapy for alcohol dependence. J Subst Abus Treat. 2017;74:23–5.
Rehm J, Anderson P, Manthey J, Shield KD, Struzzo P, Wojnar M, et al. Alcohol use disorders in primary health care: what do we know and where do we go? Alcohol Alcohol. 2016;51(4):422–7.
Le KB, Johnson JA, Seale JP, Woodall H, Clark DC, Parish DC, et al. Primary care residents lack comfort and experience with alcohol screening and brief intervention: a multi-site survey. J Gen Intern Med. 2015;30(6):790–6.
McLellan AT, Starrels JL, Tai B, Gordon AJ, Brown R, Ghitza U, et al. Can substance use disorders be managed using the chronic care model? review and recommendations from a NIDA consensus group. Public Health Rev. 2014;35(2).
Storholm ED, Ober AJ, Hunter SB, Becker KM, Iyiewuare PO, Pham C, et al. Barriers to integrating the continuum of care for opioid and alcohol use disorders in primary care: a qualitative longitudinal study. J Subst Abus Treat. 2017;83:45–54.
Mitchell AJ, Meader N, Bird V, Rizzo M. Clinical recognition and recording of alcohol disorders by clinicians in primary and secondary care: meta-analysis. Br J Psychiatry. 2012;201:93–100.
Babor TF, Ritson EB, Hodgson RJ. Alcohol-related problems in the primary health care setting: a review of early intervention strategies. Br J Addict. 1986;81(1):23–46.
Kaner EF, Beyer F, Dickinson HO, Pienaar E, Campbell F, Schlesinger C, et al. Effectiveness of brief alcohol interventions in primary care populations. Cochrane Database Syst Rev. 2007;(2):Cd004148.
O'Donnell A, Anderson P, Newbury-Birch D, Schulte B, Schmidt C, Reimer J, et al. The impact of brief alcohol interventions in primary healthcare: a systematic review of reviews. Alcohol Alcohol. 2014;49(1):66–78.
Bertholet N, Daeppen JB, Wietlisbach V, Fleming M, Burnand B. Reduction of alcohol consumption by brief alcohol intervention in primary care: systematic review and meta-analysis. Arch Intern Med. 2005;165(9):986–95.
Saitz R. ‘SBIRT’ is the answer? Probably not. Addiction. 2015;110(9):1416–7.
Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. Bmj. 2015;350:g7647.
Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. Jama. 2002;288(14):1775–9.
Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness: the chronic care model, part 2. Jama. 2002;288(15):1909–14.
CADTH. Grey Matters: a practical tool for searching health-related grey literature Internet. 2018 (cited 2019 Feb 22).
Higgins JPT. Thompson SG. Quantifying heterogeneity in a meta-analysis. 2002;21(11):1539–58.
Google Scholar
Higgins JPT, Thompson SG, Deeks JJ. Altman DG. Measuring inconsistency in meta-analyses. 2003;327(7414):557–60.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical research ed). 1997;315(7109):629–34.
Higgins JPT, López-López JA, Becker BJ, Davies SR, Dawson S, Grimshaw JM, et al. Synthesising quantitative evidence in systematic reviews of complex health interventions. BMJ Glob Health. 2019;4(Suppl 1):e000858–e.
Higgins, J.P.T., Sterne, J.A.C., Savović, J., Page, M.J., Hróbjartsson, A., Boutron, I., Reeves, B., Eldridge, S. (2016). A revised tool for assessing risk of bias in randomized trials. In: Chandler, J., McKenzie, J., Boutron, I., Welch, V. (editors). Cochrane methods. Cochrane database of systematic reviews, 10 (Suppl 1). https://doi.org/10.1002/14651858.CD201601 .
Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html ).
Download references
Acknowledgements
There is no dedicated funding.
Author information
Authors and affiliations.
Discipline of Addiction Medicine, Central Clinical School, Faculty of Medicine and Health, University of Sydney, Sydney, NSW, Australia
Susan A. Rombouts, Eva Louie, Paul Haber & Kirsten C. Morley
NHMRC Centre of Research Excellence in Indigenous Health and Alcohol, Central Clinical School, Faculty of Medicine and Health, University of Sydney, Sydney, NSW, Australia
James Conigrave
Drug Health Services, Royal Prince Alfred Hospital, Camperdown, NSW, Australia
You can also search for this author in PubMed Google Scholar
Contributions
KM and PH conceived the presented idea of a systematic review and meta-analysis and helped with the scope of the literature. KM is the senior researcher providing overall guidance and the guarantor of this review. SR developed the background, search strategy, and data extraction form. SR and EL will both be working on the data extraction and risk of bias assessment. SR and JC will conduct the data analysis and synthesize the results. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Kirsten C. Morley .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1..
PRISMA-P 2015 Checklist.
Additional file 2.
Draft search strategy MEDLINE. Search strategy.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Rombouts, S.A., Conigrave, J., Louie, E. et al. Evidence-based models of care for the treatment of alcohol use disorder in primary health care settings: protocol for systematic review. Syst Rev 8 , 275 (2019). https://doi.org/10.1186/s13643-019-1157-7
Download citation
Received : 25 March 2019
Accepted : 13 September 2019
Published : 13 November 2019
DOI : https://doi.org/10.1186/s13643-019-1157-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Model of care
- Primary health care
- Systematic review
Systematic Reviews
ISSN: 2046-4053
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]

An official website of the United States government
Here's how you know
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.

Alcohol Research: Current Reviews (ARCR)
ARCR, a peer-reviewed scientific journal published by the National Institute on Alcohol Abuse and Alcoholism at the National Institutes of Health, marks its 50th anniversary in 2024. Explore our "News & Notes" webpage for more on this historic accomplishment.
Recent Articles
Liz Simon, Brianna L. Bourgeois, and Patricia E. Molina
Julie A. Kable 1,2 and Kenneth Lyons Jones 3
Grace Chang
News and Notes

25 January 2024
ARCR Celebrates Its 50th Anniversary
2024 marks the 50th anniversary of Alcohol Research: Current Reviews (ARCR), an open-access, peer-reviewed journal published by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) at the National Institutes of Health.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Systematic Review
- Open access
- Published: 25 August 2022
Age-related differences in the effect of chronic alcohol on cognition and the brain: a systematic review
- Lauren Kuhns ORCID: orcid.org/0000-0002-3156-8905 1 , 2 ,
- Emese Kroon ORCID: orcid.org/0000-0003-1803-9336 1 , 2 ,
- Heidi Lesscher 3 ,
- Gabry Mies 1 &
- Janna Cousijn 1 , 2 , 4
Translational Psychiatry volume 12 , Article number: 345 ( 2022 ) Cite this article
4462 Accesses
4 Citations
3 Altmetric
Metrics details
- Human behaviour
Adolescence is an important developmental period associated with increased risk for excessive alcohol use, but also high rates of recovery from alcohol use-related problems, suggesting potential resilience to long-term effects compared to adults. The aim of this systematic review is to evaluate the current evidence for a moderating role of age on the impact of chronic alcohol exposure on the brain and cognition. We searched Medline, PsycInfo, and Cochrane Library databases up to February 3, 2021. All human and animal studies that directly tested whether the relationship between chronic alcohol exposure and neurocognitive outcomes differs between adolescents and adults were included. Study characteristics and results of age-related analyses were extracted into reference tables and results were separately narratively synthesized for each cognitive and brain-related outcome. The evidence strength for age-related differences varies across outcomes. Human evidence is largely missing, but animal research provides limited but consistent evidence of heightened adolescent sensitivity to chronic alcohol’s effects on several outcomes, including conditioned aversion, dopaminergic transmission in reward-related regions, neurodegeneration, and neurogenesis. At the same time, there is limited evidence for adolescent resilience to chronic alcohol-induced impairments in the domain of cognitive flexibility, warranting future studies investigating the potential mechanisms underlying adolescent risk and resilience to the effects of alcohol. The available evidence from mostly animal studies indicates adolescents are both more vulnerable and potentially more resilient to chronic alcohol effects on specific brain and cognitive outcomes. More human research directly comparing adolescents and adults is needed despite the methodological constraints. Parallel translational animal models can aid in the causal interpretation of observed effects. To improve their translational value, future animal studies should aim to use voluntary self-administration paradigms and incorporate individual differences and environmental context to better model human drinking behavior.
Similar content being viewed by others
Yohimbine as a pharmacological probe for alcohol research: a systematic review of rodent and human studies

Consequences of adolescent drug use
Chronic voluntary alcohol consumption causes persistent cognitive deficits and cortical cell loss in a rodent model
Introduction.
Alcohol use disorder (AUD) is the most prevalent substance use disorder worldwide [ 1 ]. Most AUDs remain untreated [ 2 ] and for those seeking treatment, relapse rates are high [ 3 ]. Adolescence marks a rapid increase in AUD and an earlier onset of AUD is associated with worse long-term outcomes, including greater problem severity and more relapses [ 4 , 5 ]. Loss of control over alcohol use is a core aspect of AUD [ 6 ] and the developmentally normative difficulty to control motivational urges in tempting and arousing situations is thought to put adolescents at risk for developing addictive behaviors [ 7 ]. Moreover, neurotoxic consequences of alcohol use may be more severe for a developing brain [ 8 ]. Paradoxically, adolescence is also a period of remarkable behavioral flexibility and neural plasticity [ 9 , 10 , 11 ], allowing adolescents to adapt their goals and behavior to changing situations [ 12 ] and to recover from brain trauma more easily than adults [ 10 ]. In line with this, the transition from adolescence to adulthood is associated with high rates of AUD recovery without formal intervention [ 13 ]. While the adolescent brain may be a vulnerability for the development of addiction, it may also be more resilient to long-term effects compared to adults. Increased neural plasticity during this period could help protect adolescents from longer-term alcohol use-related cognitive impairments across multiple domains, from learning and memory to decision-making and cognitive flexibility. Therefore, the goal of this systematic review was to examine the evidence of age-related differences in the effect of alcohol on the brain and cognitive outcomes, evaluating evidence from both human and animal studies.
In humans, the salience and reinforcement learning network as well as the central executive network are involved in the development and maintenance of AUD [ 7 , 14 ]. The central executive network encompasses fronto-parietal regions and is the main network involved in cognitive control [ 15 ]. The salience network encompasses fronto-limbic regions crucial for emotion regulation, salience attribution, and integration of affective information into decision-making [ 15 , 16 ], which overlaps with fronto-limbic areas of the reinforcement learning network (Fig. 1 ). Relatively early maturation of salience and reinforcement learning networks compared to the central executive network is believed to put adolescents at heightened risk for escalation of alcohol use compared to adults [ 7 ]. Rodent models are regularly used for AUD research and allow in-depth neurobehavioral analyses of the effects of ethanol exposure during different developmental periods while controlling for experimental conditions such as cumulative ethanol exposure in a way that is not possible using human subjects because exposure is inherently confounded with age. For example, animal models allow for detailed neurobiological investigation of the effects of alcohol exposure in a specific age range on neural activation, protein expression, gene expression, epigenetic changes, and neurotransmission in brain regions that are homologous to those that have been implicated in AUD in humans.
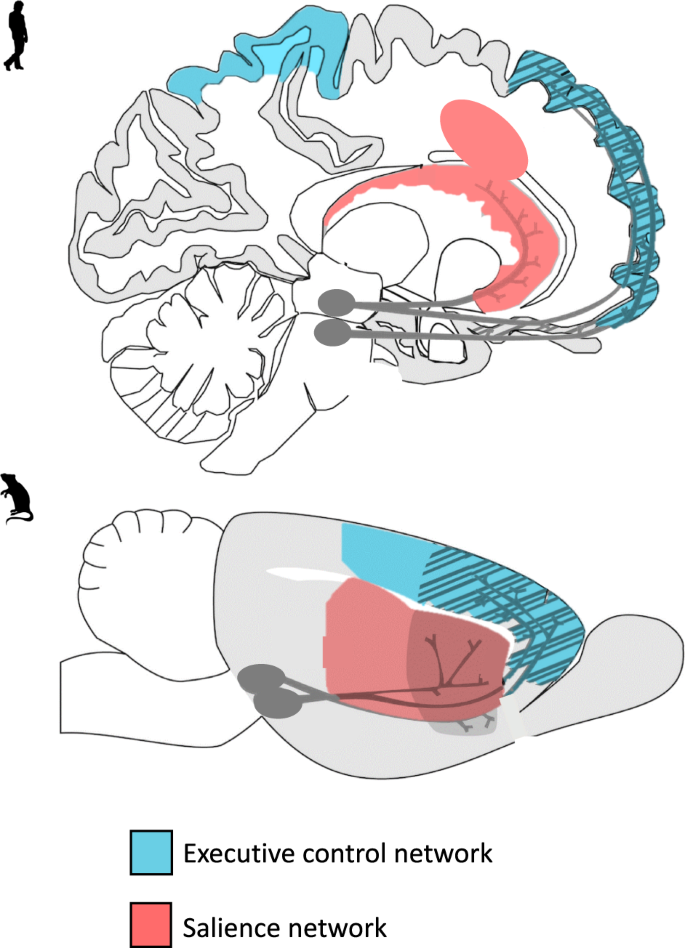
A visual representation of the translational model of the executive control and salience networks in humans and rodents. The executive control and salience are key networks believed to play a part in adolescent vulnerability to alcohol-related problems.
While most of our knowledge on the effects of alcohol on the brain and cognitive outcomes is based on research in adults, several recent reviews have examined the effects of alcohol on the brain and cognition in adolescents and young adults specifically [ 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 ]. Heavy or binge drinking has been associated with reduced gray and white matter. Also, altered task-related brain activity [ 20 ], structural abnormalities [ 25 ], and overlapping behavioral impairment in executive functioning have been identified in adolescent and young adult alcohol users [ 19 ]. While some of the observed neurocognitive differences between drinkers and non-drinkers may be predisposing factors, they may be further exacerbated by heavy and binge drinking [ 21 , 23 ]. Furthermore, reviews of longitudinal studies concluded that adolescent alcohol use is associated with neural and cognitive alterations in a dose-dependent manner [ 17 , 22 ].
Although previous reviews underscore the potential negative consequences of heavy alcohol use on the brain and cognition in adolescence, they do not typically address the question of whether adolescents are differentially vulnerable compared to adults to the effects of alcohol on these outcomes. Explicit comparisons between adolescents and adults are crucial to identify potential risk and resilience factors. In the current review, we aimed to extend previous work by systematically examining this critical question: does the relationship between chronic alcohol use and neurocognitive outcomes differ between adolescents and adults? To address this question, we systematically reviewed human and animal studies that included both age groups and used a factorial design that would allow for the comparison of the effects of chronic alcohol use on cognitive and brain-related outcomes across age groups. We specifically highlight outcomes from voluntary self-administration paradigms when available and discuss the translational quality of the animal evidence base. We conclude with a discussion of prominent knowledge gaps, future research directions, and clinical implications.
Study inclusion criteria and search strategy
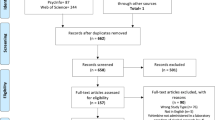
We followed the PRISMA guidelines for the current systematic review (The PRIMSA Group, 2009). An initial MedLine, Cochrane Library, and PsycInfo search was conducted during September of 2018 with terms related to alcohol, cognition, adolescence/adulthood, and study type (see Appendix for full search strategy and syntax). Two search updates using the same search strategy were conducted on 31 March 2020 and 3 February 2021. For all searches, the identified citations were split into batches and at least two of the following assessors (GM, LK, JC, or CG) conducted a blinded review to determine whether articles met the inclusion criteria. In the first phase of screening, only titles and abstracts were screened and articles that clearly did not meet the inclusion criteria were excluded. In the second phase, the remaining articles received a full-text review and those that did not meet all inclusion criteria were excluded. The first inclusion criterion that was not adhered to was recorded as the reason for excluding. If there was a discrepancy between authors after initial and full-text screening process, the reviewing authors discussed the article and a consensus was reached.
The inclusion criteria were: (1) Human samples including both adolescents younger than 18 and adults older than 18 and animal samples including adolescent (Post Natal Day (PND) 25–42 for rodents) and adult [ 8 ] animals (greater than PND 65 for rodents); (2) Exploration of alcohol as the independent variable and cognitive, reward-related, or brain outcomes as the dependent variables; (3) Alcohol and cognitive outcomes must meet our operationalization defined below; (4) Study design comparing adults and adolescents on outcome measures; (5) Administering or measuring alcohol use during adolescence or adulthood, not retrospectively (e.g., no age of onset work in humans using retrospective self-reports of alcohol consumption); (6) Primary quantitative data collection (no case studies, or review papers); (7) Solely looking at alcohol-related factors as the independent variables (e.g., cannot explore alcohol-related factors in individuals with psychosis); (8) Written in English; (9) Published in a peer-reviewed journal before February 3, 2021 (see Fig. 2 for a detailed screening process).
The definitions for adolescence are variable, hampering the direct comparison of human and rodent research. In rodents, the end of early-mid adolescence is considered to be approximately PND 42 when rats reach sexual puberty. By contrast, the boundaries for the onset of early adolescence are less clear. Based on the notion that most age-typical physiological changes that are characteristic of adolescence emerge from PND 28 [ 26 ], the conservative boundary for adolescence has been set at PND 28 (e.g., seminal review on adolescence [ 27 ]). The preceding week (PND 21-PND 28) has been described as the juvenile period (e.g., [ 28 , 29 ]) but these same reports consider PND 21-PND 23 as the lower boundary for early adolescence [ 28 , 29 ], further emphasizing that the boundary of PND28 may be too conservative. Indeed, multiple studies (e.g., [ 30 , 31 ]), have chosen to take PND25 as the boundary for early adolescence. Hence, we have decided to also follow this less conservative approach and include all studies where alcohol was administered between PND 25 and PND 42.
The exact boundaries of human adolescence are similarly nebulous. From a neurodevelopmental perspective, adolescence is now often thought of as continuing until approximately age 25 because of the continuing maturation of the brain [ 32 ]. However, the delineation of adolescence and adulthood is also dependent on societal norms, and is commonly defined as the transitional period between puberty and legal adulthood and independence which typically begins around age eighteen. In light of this, we chose a relatively liberal inclusion criteria for the human studies; studies needed to include at least some adolescents below eighteen, the age at which drinking typically begins, as well as ‘adult’ participants over the age of eighteen. We are careful to interpret the results of human studies within the neurodevelopmental framework of adolescence, such that 18–25-year-olds are considered late adolescents to young adults who are still undergoing cognitive and brain maturation.
Notably, we excluded studies that assessed alcohol exposure retrospectively (primarily early onset alcohol studies) because age of onset variables are often inaccurate, with reported age of alcohol onset increasing with both historical age [ 33 ] and current alcohol use patterns [ 34 ]. In addition, we excluded work that has not undergone peer-review to ensure high-quality papers.
In humans, we defined cognition as any construct that typically falls within the umbrella of neuropsychological testing, as well as brain-based studies. We also included more distal constructs of cognition, like craving and impulsivity, because they play a prominent role in addictive behaviors [ 35 , 36 ]. In rodents, we defined cognition as attention, learning, and memory in line with a seminal review paper [ 37 ]. Given the importance of social cognition in patterns of alcohol use particularly in adolescence [ 38 ] and its proposed role in adolescent risk and resilience to addiction [ 39 ], we included social behavior as an outcome. Furthermore, because many rodent studies assessed anxiety-related behaviors and the high degree of comorbidity between anxiety disorders and alcohol addiction [ 40 ], we also included anxiety as a secondary outcome. On the other hand, locomotor activity was excluded as an outcome because even though behavioral sensitization is considered to reflect neurobiological changes that may underlie certain aspects of addictive behavior [ 36 ], the translational relevance for addictive behavior and human addiction in particular remains unclear [ 41 , 42 ]. Across both rodents and humans, general alcohol metabolization and ethanol withdrawal studies were not included except if they included brain-related outcomes. The relevant reported findings (i.e., the results of an analysis of comparing age groups on the effect of alcohol on an included outcome) were extracted by a one reviewer and then confirmed by at least one other reviewer. In addition, the characteristics of the sample, details of alcohol exposure, and study design were extracted by a single reviewer and then confirmed by at least one other reviewer. No automation tools were used for extraction. Within the included studies, peripheral findings that did not relate to cognition were excluded from review and not extracted. The protocol for this systematic review was not registered and no review protocol can be accessed.
Study search
Our searches identified 7229 studies once duplicates were removed. A total of 6791 studies were excluded after initial review of abstracts. Then, 434 studies received a full-text review and 371 were excluded for failing to meet all inclusion criteria. See Fig. 2 for a flow diagram of the full screening process. At the end of the inclusion process, 59 rodent studies and 4 human studies were included. The characteristics and findings of the final studies are detailed in Table 1 (rodents) and Table 2 (humans). Due to the heterogeneity of outcomes, meta-regression was not suitable for synthesizing results. Results are narratively synthesized and grouped based on forced or voluntary ethanol exposure and by outcome within the tables and by outcome only in text. Two authors independently rated the quality of evidence for human studies (Table 2 ) based on criteria used in a similar systematic review [ 43 ]: (1) strong level of causality: longitudinal design comparing adolescent and adults while adjusting for relevant covariates; (2) moderate level of causality: longitudinal design comparing adolescents and adults without adjusting for relevant covariates or cross-sectional designs with matched groups that considered relevant covariates; (3) weak level of causality: cross-sectional design without matched adolescent and adult groups and/or did not adjust for relevant covariates. A methodological quality assessment was not conducted for the animal studies due to a lack of empirically validated risk of bias tools and lack of standardized reporting requirements in the animal literature.
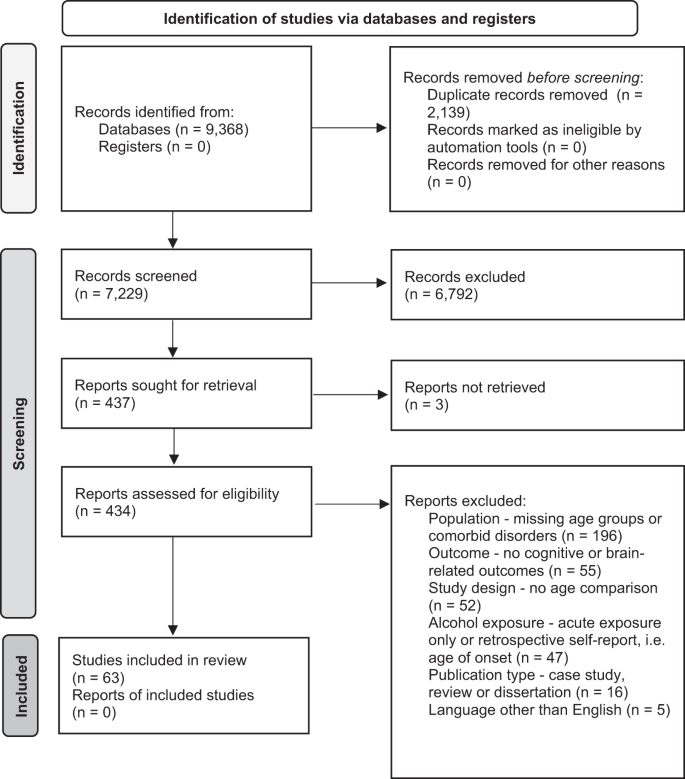
PRIMSA flow diagram detailing the screening process.
Animal studies
Cognitive outcomes, learning and memory.
Human evidence clearly suggests that alcohol is related to learning and memory impairments, both during intoxication [ 44 ] and after sustained heavy use and dependence [ 45 , 46 ]. Paradigms that assess learning and memory provide insight into the negative consequences of alcohol consumption on brain functioning, as well as the processes underlying the development and maintenance of learned addictive behaviors.
Conditioned alcohol aversion or preference: Lower sensitivity to alcohol’s aversive effects (e.g., nausea, drowsiness, motor incoordination) but higher sensitivity to alcohol’s rewarding effects has been hypothesized to underlie the higher levels of alcohol use, especially binge-like behavior, in adolescents compared to adults [ 47 ]. Several conditioning paradigms have been developed to assess the aversive and motivational effects of alcohol exposure.
The conditioned taste aversion (CTA) paradigm is widely used to measure perceived aversiveness of alcohol in animals. Repeated high-dose ethanol injections are paired with a conditioned stimulus (CS, e.g., a saccharin or NaCL solution). The reduction in CS consumption after conditioning is used as an index of alcohol aversion. Two studies examined CTA in mice [ 48 , 49 ] and two in rats [ 50 , 51 ]. Three of the four studies found age-related differences. In all three studies using a standard CTA paradigm, adolescents required a higher ethanol dosage to develop aversion compared to adults [ 48 , 49 , 50 ]. Using a similar second-order conditioning (SOC) paradigm pairing high doses of ethanol (3.0 g/kg) with sucrose (CS), both adolescent and adult rats developed equal aversion to the testing compartment paired with ethanol [ 51 ].
Overall, three studies found support for lower sensitivity to alcohol’s aversive effects in adolescents, whereas one observed no differences. Future research should employ intragastric as opposed intraperitoneal exposure to better mimic human binge-like drinking in order to increase the translational value of the findings.
To measure differences in alcohol’s motivational value, conditioned place preference (CPP) paradigms have been used. This involves repeated pairings of ethanol injections with one compartment and saline injections with another compartment of the testing apparatus. On test days, CPP is assessed by measuring how long the animal stays in the compartment paired with ethanol relative to saline injections. Four studies examined CPP, with two studies observing age-related differences [ 52 , 53 , 54 , 55 ]. In the only mouse study, history of chronic ethanol exposure during adolescence (2.0 g/kg for 15 days) but not adulthood [ 52 ] led to increased CPP after brief abstinence (5 days) before the conditioning procedure (2.0 g/kg, four doses over 8 days). This suggests that early ethanol exposure increases alcohol’s rewarding properties later on. However, two rat studies did not observe either preference or aversion in either age when using lower ethanol doses and a shorter exposure period (0.5 and 1.0 g/kg for 8 days) [ 53 ], nor when using higher doses and intermittent exposure (3.0 g/kg, 2 days on, 2 days off schedule) [ 55 ]. Next to species and exposure-specific factors, environmental factors also play a role [ 54 ], with adolescents raised in environmentally enriched conditions demonstrating CPP (2 g/kg) while adolescents raised in standard conditions did not. In contrast, CPP was insensitive to rearing conditions in adults with both enriched and standard-housed rats showing similar levels of CPP.
Overall, there is inconsistent evidence for age-related differences in the motivational value of ethanol. One study found support for increased sensitivity to the rewarding effects of ethanol in adolescents, whereas one found support for adults being more sensitive and two observed no differences.
Fear conditioning and retention: Pavlovian fear conditioning paradigms are used to investigate associative learning and memory in animals. These paradigms are relevant for addiction because fear and drug-seeking behavior are considered conditioned responses with overlapping neural mechanisms [ 56 ]. Rodents are administered an unconditioned stimulus (US; e.g., foot shock) in the presence of a conditioned stimulus (CS; unique context or cue). Conditioned responses (CR; e.g., freezing behavior) are then measured in the presence of the CS without the US as a measure of fear retention. Contextual fear conditioning is linked to hippocampus and amygdala functioning and discrete cue-based (e.g., tone) fear is linked to amygdala functioning. [ 57 , 58 , 59 ], and fear extinction involves medial PFC functioning [ 60 ]. Five studies investigated fear conditioning, four in rats [ 61 , 62 , 63 , 64 ] and one in mice [ 65 ].
Only one of the four studies observed age-related differences in tone fear conditioning. Bergstrom et al. [ 61 ] found evidence for impaired tone fear conditioning in male and female alcohol-exposed (18d) adolescent compared to adult rats after extended abstinence (30d). However, adolescent rats consumed more ethanol during the one-hour access period than adults, which may explain the observed age differences in fear tone conditioning. Small but significant sex differences in consumption also emerged in the adolescent group, with males showing more persistent impairment across the test sessions compared to females, despite adolescent females consuming more ethanol than males. In contrast, three studies found no evidence of impaired tone fear conditioning in either age group after chronic alcohol exposure (4 g/kg, every other day for 20d) and extended abstinence [ 62 , 63 ] (22d), [ 64 ].
Two of the three studies observed age-related differences in contextual fear conditioning [ 62 , 63 , 64 ]. In two studies with similar exposure paradigms, only adolescents exposed to chronic high dosages of ethanol (4 g/kg) showed disrupted contextual fear conditioning after extended abstinence (22d) [ 62 , 63 ]. Importantly, differences disappeared when the context was also paired with a tone, which is suggestive of a potential disruption in hippocampal-linked contextual fear conditioning specifically [ 64 ]. Furthermore, there may be distinct vulnerability periods during adolescence as contextual fear retention was disrupted after chronic alcohol exposure (4 g/kg, every other day for 20d) during early-mid adolescence but not late adolescence [ 62 ]. In the only study to combine chronic exposure and acute ethanol challenges, contextual conditioning was impaired by the acute challenge (1 g/kg) but there was no effect of pre-exposure history in either age group (4 g/kg, every other day for 20d) [ 63 ].
Only one study examined fear extinction, and found no effect of ethanol exposure (4/kg, every other day for 20d) on extinction after tone conditioning. However, adults had higher levels of contextual fear extinction compared to mid-adolescents while late adolescents performed similar to adults [ 62 ]. Moreover, looking at binge-like exposure in mice (three binges, 3d abstinence), Lacaille et al. [ 65 ] showed comparable impairments in long-term fear memory in adolescents and adults during a passive avoidance task in which one compartment of the testing apparatus was paired with a foot shock once and avoidance of this chamber after a 24 h delay was measured.
In sum, there is limited but fairly consistent evidence for adolescent-specific impairments in hippocampal-linked contextual fear conditioning across two rat studies, while no age differences emerged in context-based fear retention in one study of mice. In contrast, only one of the four studies found evidence of impaired tone fear conditioning in adolescents (that also consumed more alcohol), with most finding no effect of alcohol on tone fear conditioning regardless of age. With only one study examining medial PFC-linked fear extinction, no strong conclusions can be drawn, but initial evidence suggests context-based fear extinction may be diminished in mid-adolescents compared to adults and late adolescents. Research on age-related differences on the effect of alcohol on longer-term fear memory is largely missing.
Spatial learning and memory: The Morris Water Maze (MWM) is commonly used to test spatial learning and memory in rodents. Across trials, time to find the hidden platform in a round swimming pool is used as a measure of spatial learning. Spatial memory can be tested by removing the platform and measuring the time the animal spends in the quadrant where the escape used to be. The sand box maze (SBM) is a similar paradigm in which animals need to locate a buried appetitive reinforcer.
Six rat studies examined spatial learning and memory using these paradigms. Three of the six studies observed age-related differences. Four examined the effects of repeated ethanol challenges 30 minutes prior to MWM training, showing mixed results [ 30 , 66 , 67 , 68 ]. While one found ethanol-induced spatial learning impairments in adolescents only (1.0 and 2.0 g/kg doses) [ 66 ], another found no age-related differences, with both age groups showing impairments after moderate doses (2.5 g/kg) and enhancements in learning after very low doses (0.5 g/kg) [ 67 ]. Sircar and Sircar [ 68 ] also found evidence of ethanol-induced spatial learning and memory impairments in both ages (2.0 g/kg). However, memory impairments recovered after extended abstinence (25d) in adults only. Importantly, MWM findings could be related to thigmotaxis, an anxiety-related tendency to stay close to the walls of the maze. Developmental differences in stress sensitivity may potentially confound ethanol-related age effects in these paradigms. Using the less stress-inducing SBM, adults showed greater impairments in spatial learning compared to adolescents after 1.5 g/kg ethanol doses 30 min prior to training [ 30 ].
Two studies examined the effects of chronic ethanol exposure prior to training with or without acute challenges [ 69 , 70 ]. Matthews et al. [ 70 ] looked at the effect of 20 days binge-like (every other day) pre-exposure and found no effect on spatial learning in either age following an extended abstinence period (i.e., 6–8 weeks). Swartzwelder et al. [ 69 ] examined effects of 5-day ethanol pre-exposure with and without ethanol challenges before MWM training. Ethanol challenges (2.0 g/kg) impaired learning in both age groups regardless of pre-exposure history. Thigmotaxis was also increased in both age groups after acute challenges while pre-exposure increased it in adults only.
In sum, evidence for impaired spatial learning and memory after acute challenges is mixed across six studies. Two studies found support for ethanol having a larger impact in adolescents compared to adults, whereas one study found the opposite and three studies did not observe any differences. Differences in ethanol doses stress responses may partially explain the discrepancies across studies. Importantly, given the sparsity of studies addressing the effects of long-term and voluntary ethanol exposure, no conclusion can be drawn about the impact of age on the relation between chronic alcohol exposure and spatial learning and memory.
Non-spatial learning and memory: Non-spatial learning can also be assessed in the MWM and SBM by marking the target location with a pole and moving it across trials, measuring time and distances traveled to locate the target. By assessing non-spatial learning as well, studies can determine whether learning is more generally impaired by ethanol or whether it is specific to hippocampal-dependent spatial learning processes. A total of six studies assessed facets of non-spatial learning and memory. Two of the six studies observed age-related differences.
In the four studies that examined non-spatial memory using the MWM or SBM in rats, none found an effect of alcohol regardless of dose, duration, or abstinence period in either age group [ 30 , 66 , 67 , 70 ]. Two other studies examined other facets of non-spatial memory in rats [ 65 , 71 ]. Galaj et al. [ 71 ] used an incentive learning paradigm to examine conditioned reward responses and approach behavior towards alcohol after chronic intermittent ethanol (CIE; 4 g/kg; 3d on, 2d off) exposure to mimic binge drinking. To examine reward-related learning and approach behavior, a CS (light) was paired with food pellets and approach behavior to CS only presentation and responses to a lever producing the CS were measured. In both adolescents and adults, the ethanol-exposed rats showed impaired reward-related learning after both short (2d) and extended (21d) abstinence. No effect of alcohol on conditioned approach behavior was observed in either age group during acute (2d) or extended (21d) abstinence. Using a novel object recognition test in mice, Lacaille et al. [ 65 ] assessed non-spatial recognition memory by replacing a familiar object with a novel object in the testing environment. Explorative behavior of the new object was used as an index of recognition. After chronic binge-like exposure (three injections daily at 2 h intervals) and limited abstinence (4d), only adolescents showed reduced object recognition.
Across facets of non-spatial memory, there is little evidence for age-related differences in the effect of chronic alcohol, with four of the six studies finding no age differences. For memory of visually cued target locations in the MWM and SBM paradigms, alcohol does not alter performance in either age. Also, both adolescents and adults appear similarly vulnerable to alcohol-induced impairments in reward-related learning based on the one study. Only in the domain of object memory did any age-related differences emerge, with adolescents and not adults showing reduced novel object recognition after binge-like alcohol exposure in one study. However, more research into object recognition memory and reward-related learning and memory is needed to draw strong conclusions in these domains.
Executive function and higher-order cognition
Executive functions are a domain of cognitive processes underlying higher-order cognitive functions such as goal-directed behavior. Executive functions can include but are not limited to working memory, attentional processes, cognitive flexibility, and impulse control or inhibition [ 72 ]. A core feature of AUD is the transition from goal-directed alcohol use to habitual, uncontrolled alcohol use. Impaired executive functioning, linked to PFC dysfunction [ 73 ], is assumed to be both a risk factor and consequence of chronic alcohol use. A meta-analysis of 62 studies highlighted widespread impairments in executive functioning in individuals with AUD that persisted even after 1-year of abstinence [ 46 ]. Thirteen studies examined facets of executive functioning and higher-order cognition, specifically in the domains of working memory, attentional processes, cognitive flexibility, impulsivity in decision-making, and goal-directed behavior [ 65 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 ].
Working memory: Working memory refers to the limited capacity system for temporarily storing and manipulating information, which is necessary for reasoning and decision-making [ 84 ]. In the Radial Arm Maze test (RAM) [ 85 ], some of the equally spaced arms (typically eight) around a circular platform contain a food reward for animals to find. Spatial working memory is measured by recording the number of revisits to previously visited arms (i.e., working memory error) and first entries into unbaited arms (i.e., reference memory). Alternatively, the hippocampus mediated [ 86 ] spontaneous tendency to alternate arms can be used as a measure of spatial working memory. In this case, revisiting an arm in back-to-back trials in close temporal succession is interpreted as a working memory error. Five studies examined the effects of chronic ethanol exposure on spatial working memory [ 65 , 75 , 79 , 80 , 83 ]. One of the five studies observed age-related differences.
Chronic binge-like alcohol exposure had no effects on spontaneous alterations after prolonged abstinence (2d on, 2d off; 3 weeks abstinence) [ 79 , 80 ] in rats or limited abstinence (three injections daily at 2 h intervals; 24 h abstinence) [ 65 ] in mice, nor on RAM performance in rats (2d on, 2d off) [ 75 , 83 ]. However, acute ethanol challenges (1.5 g/kg) after chronic binge-like exposure (2d on, 2d off) resulted in RAM test impairments in both age groups in rats [ 75 , 83 ], with some evidence for increased working memory errors in adolescents [ 83 ].
In sum, there is little evidence for impairments in working memory function in rats after chronic ethanol exposure, with four of the five studies observing no difference between age groups. While acute intoxication impairs working memory function in both ages, there is evidence from only one study that adolescents may make more working memory errors.
Attentional processes: Attentional processing refers to the selection of information that gains access to working memory [ 87 ]. PPI is a pre-attentional cognitive function which provides an index of sensorimotor gating and measures the ability of a lower intensity sensory stimulus to reduce the magnitude of response to a more intense stimulus presented closely afterward. Reduced sensorimotor gating (reduced PPI) can disrupt information processing and thereby impair cognitive function, while enhanced sensorimotor gating (enhanced PPI) may reflect behavioral inflexibility [ 88 ]. For example, lesions in the medial PFC produce both behavioral inflexibility and enhancements in PPI in rats. Two studies assessed attentional processes by measuring prepulse inhibition (PPI) in rats [ 82 , 89 ]. One study observed age-related differences and one did not.
Slawecki and Ehlers [ 82 ] observed age-related differences in sensorimotor gating following ethanol vapor exposure (2w) and brief abstinence (6d), with adolescents showing enhanced PPI at some decibels reflective of behavioral inflexibility, while adults did not exhibit PPI at any of the intensities tested. Slawecki et al. [ 89 ] did not observe any age-related differences in PPI during the acute phase of ethanol withdrawal (7–10 h abstinence) during a period of chronic ethanol exposure (14d).
In sum, there is limited and mixed evidence from two studies of age-related differences in the pre-attentional process of sensorimotor gating. Only one study found support for adolescent sensitivity to ethanol effects.
Cognitive flexibility: Cognitive flexibility refers to the ability to update information based on environmental factors r changing goals in order to adaptively guide decision-making and is linked to the inability to reduce or abstain from drinking [ 90 ]. Three studies examined facets of cognitive and behavioral flexibility [ 79 , 80 , 81 ]. Two of the three studies observed age-related differences.
In two rat studies, cognitive flexibility was assessed using reversal learning paradigms [ 79 , 80 ]. In the reversal learning paradigm, rats were trained on simple (e.g., visual cue) and more complex discriminations (e.g., visual + scent cue) between rewarded and non-rewarded bowls. After learning the discriminants, the rewards were reversed. Ethanol exposure reduced flexibility in both adolescents and adults for simple discriminations in both studies. Age-related differences emerged for the more complex discriminations in one study, with only adults showing reduced flexibility after prolonged abstinence (21d) following binge-like exposure (5 g/kg, 2d on, 2d off) [ 79 ]. In contrast, both age groups showed reduced flexibility for complex discrimination in the other study after prolonged abstinence (21d) despite adolescents consuming more ethanol orally than adults during the 28 week exposure [ 80 ].
In another study, Labots et al. [ 81 ] used a conditioned suppression of alcohol-seeking task after two months of voluntary ethanol consumption (2 months) in rats to examine flexibility around alcohol-seeking behavior. After stratifying the age groups based on levels of ethanol consumption, medium- and high-consuming, adolescents showed higher levels of conditioned suppression compared to similarly drinking adults, indicating greater behavioral flexibility and control over alcohol-seeking in adolescents after chronic voluntary exposure.
Overall, there is limited evidence for adolescent resilience to the effects of chronic alcohol on cognitive flexibility. Two studies found support for adolescent resilience to ethanol’s effect on behavioral flexibility, whereas another study found no differences between adolescents and adults.
Impulsivity: Impulsivity is a multi-faceted behavioral trait that encompasses impaired response inhibition, preference for an immediate reward over a larger but delayed reward, and premature expression of behaviors which may be maladaptive or in conflict with conscious goals. Impulsivity is a risk-factor for the development of addiction and may also be a consequence of sustained substance use [ 35 ]. Pharmacological evidence points towards overlapping neuronal mechanisms in impulsivity and addictive behavior, particularly within the mesolimbic dopamine system [ 91 ]. Two studies examined impulsive decision-making behavior in rats [ 74 , 78 ]. Both studies observed age-related differences.
One study examined impulsive behavior using a delay-discounting task in which choices are made between immediate small rewards and larger delayed rewards [ 78 ]. Regardless of age, chronic intermittent exposure (2d on, 2d off) had no effect on choice behavior in non-intoxicated rats. Following acute challenges, adolescents but not adults demonstrated a reduced preference for the large reward regardless of ethanol exposure history, reflecting a general adolescent-specific heightened impulsivity during intoxication. Another study examined decision-making under risk conditions using an instrumental training and probability-discounting task [ 74 ]. After prolonged abstinence (20d), rats were trained to press two levers for sucrose rewards and were concurrently trained to choose between two levers with different associated probabilities of reward and reward size, creating a choice between a certain, small reward and an uncertain, large reward (i.e., riskier choice). Ethanol consumption was voluntary and while adolescents initially consumed more ethanol than adults at the beginning of the exposure period, the total amount of consumption was similar by the end of the exposure period. Only adolescents showed increased risky and sub-optimal decision-making compared to age-matched controls, while adults performed similarly to controls.
In sum, both studies found support for ethanol having a larger impact on adolescent compared to adults on impulsive behavior.
Goal-directed behavior: Goal-directed behavior refers to when actions are sensitive to both the outcome value (goal) and contingency between the behavior and the outcome [ 92 ]. Two studies used a sign-tracking and omission contingency learning paradigm to examine goal-directed versus habitual behavior [ 76 , 77 ]. One study observed age-related differences and the other did not. Sign tracking refers to tasks where a cue predicts a reward, but no response is needed for the reward to be delivered. Despite this, after repeated pairings of the cue and reward, animals and humans may respond (e.g., via a lever) when the cue is presented anyway, and even when no reward is known to be available. Sign-directed behavior is considered habitual and has been proposed to underlie the lack of control of alcohol use in addiction [ 93 ]. In humans, sign-tracking behavior is difficult to differentiate from goal-directed behavior based on only the observable behavior, i.e., seeing a cue such as a favorite drink or bar and then having a drink [ 94 ]. In the context of alcohol use, reflexively having a drink when seeing an item that is often associated with the rewarding effects of alcohol (e.g., wine glass, bar, smell of alcohol) despite not consciously desiring the alcohol ‘reward’ is an example of how habitual behavior (possibly driven by sign-tracking) can initiate the behavior as opposed to an intentional goal [ 93 ]. Omission contingency refers to a 2nd phase after sign-tracking when the response is punished and the behavior must be inhibited to avoid punishment. After both forced and voluntary ethanol exposure (6w), no alterations to sign-tracking behavior were observed in adolescent and adult rats [ 76 , 77 ]. One study did observe an age-related difference in omission contingency learning, with adolescents performing better than adults after chronic voluntary ethanol exposure [ 77 ]. This preliminarily suggests that adolescents may be more capable of adapting their behavior to avoid punishment compared to adults after chronic use. However, before behavioral testing began, adolescent rats were abstinent for 17 days, while adults were only abstinence for 10 days which may have influenced the results.
In summary, one study found support for adolescents being less sensitive to ethanol effects on goal-directed behavior compared to adults, whereas one study found no effect of ethanol in either age group.
Across the domains of executive function, there is some evidence that adolescents may be more vulnerable to impairments in certain executive and higher-order cognitive functions following chronic alcohol exposure, with increased risky decision-making after prolonged abstinence [ 74 ], impulsivity during intoxication [ 78 ], and reduced working memory function during intoxication after chronic exposure. In contrast, animals exposed to alcohol during adolescence may better retain cognitive flexibility [ 77 , 79 ] and are better able to regain control over alcohol-seeking in adulthood [ 81 ].
Other behavioral outcomes
Anxiety : AUD is highly comorbid with anxiety disorders [ 95 ], especially in adolescence [ 96 ]. While anxiety is not strictly a cognitive outcome, it is related to altered cognitive functioning [ 97 , 98 ]. Many studies assessing the effects of ethanol on the rodent brain and cognition also include anxiety-related measures. Multiple paradigms have been developed to elicit behaviors thought to reflect anxiety in rodents (e.g., rearing, startle, avoidance, etc.). In the open field test (OFT), anxiety is indexed as the tendency to stay close to perimeter walls as animals have a natural aversion to brightly lit open spaces [ 99 ]. In the elevated plus maze paradigm, rodents are placed at the center of an elevated four-arm maze with two open arms two closed arms [ 100 ]. The open arms elicit unconditioned fear of heights/open spaces and the closed arms elicit the proclivity for enclosed, dark spaces. Anxiety is indexed as entries/duration of time in open vs. closed arms, as well as rearing, freezing, or other postural indices of anxiety. In startle paradigms, the startle response is a defensive mechanism reflecting anxiety which follows a sudden, unpredictable stimulus (e.g., tones, light) [ 101 ]. In light-dark box paradigms, anxiety is elicited using a testing apparatus with a light and dark compartment, relying on the conflict between natural aversions to well-lit spaces and the tendency to explore new areas. Percentage of time spent in the light compartment, latency to return to the dark compartment, movement between compartments (transitions), and rearing-behavior are measured as indices of anxiety [ 102 ]. Anxiety can also be assessed using a social interaction test with an unfamiliar partner, with approach and avoidance behaviors measured to index anxiety [ 103 ]. In the novel object test (NOT) [ 104 ], anxiety is elicited by the introduction of a new object in the rodent’s environment. The amount of contacts and time spent in contact with the object is used as an index of anxiety. Similarly, in the marble-burying test (MBT), novel marbles are placed in an environment and the amount of defensive burying of the objects is used as an index of anxiety [ 105 ].
Eleven studies examined anxiety-like behavior in rodents with mixed results across paradigms [ 70 , 78 , 82 , 83 , 89 , 106 , 107 , 108 , 109 , 110 , 111 ]. Overall, five of the eleven studies observed age-related differences.
Two studies used the OFT, finding no effects of voluntary (2w, 4 h/day access) or forced (12/day vapor) ethanol exposure on anxiety-like behavior in adolescents or adult rats during withdrawal (7–9 h) [ 110 ] or after a brief abstinence period (4 days) [ 107 ]. One study used both the MBT and NOT after voluntary ethanol consumption (2 h/d for 2 weeks; no abstinence) and observed higher anxiety in ethanol-exposed adults and reduced anxiety in ethanol-exposed adolescents compared to controls as indexed by marble burying [ 106 ]. However, no age effects were observed in response to a novel object, with reduced interaction with the novel object in both age groups after chronic exposure.
Four studies used the elevated maze paradigm with mixed results. Only one study observed age-related differences in mice after chronic exposure (8–10w vapor) [ 109 ]. Adolescents showed reduced anxiety compared to adults during the acute withdrawal period, but all mice were kept under chronic social isolation and unpredictable stress conditions, which may have affected the results. Two studies in rats found no effect of intermittent (1 g/kg) or binge-like (5 g/kg) exposure in either age group after short (24 h) [ 70 ] or sustained abstinence (20d) [ 83 ]. A third study observed heightened anxiety in both age groups after intermittent exposure (4 g/kg), with anxiety increasing with prolonged abstinence periods (24 h to 12d) [ 108 ].
Three rat studies used a startle paradigm to assess anxiety. Two observed reduced acoustic startle responses after ethanol exposure (12 h/d vapor) in both age groups during acute withdrawal periods (7–10 h) and following more sustained abstinence (6d) [ 82 , 89 ]. In the other study, light-potentiated startle was also reduced in both ages during days 1–10 of withdrawal after binge-like exposure (2d on, 2d off), but age-related differences emerged when the rats were re-exposed via a 4-day binge (1–4/kg). Then, only adults showed higher levels of light-potentiated startle compared to controls [ 78 ], suggesting that ethanol pre-exposure increases anxiety in adults but not adolescents when re-exposed to ethanol after withdrawal.
Two studies used the light-dark box paradigm with mixed results [ 89 , 111 ]. Only adult rats showed increased mild anxiety-like behaviors during early withdrawal (7–10 h) after chronic vapor exposure 12 h/d) [ 89 ]. In contrast, no age-related differences emerged after voluntary ethanol consumption (18 h/d access; 3d/w for 6 weeks), with male mice showing less anxiety-like behavior in both ages [ 111 ]. In contrast, the one study using the social interaction test observed reduced anxiety in adult mice compared to both adolescents and age-matched controls during early withdrawal (4–6 h) after chronic, unpredictable vapor exposure [ 109 ].
In summary, there is inconsistent evidence for age-related differences in the effect of chronic ethanol exposure on anxiety outcomes in rodents. The substantial differences across studies in how anxiety was elicited and measured make it challenging to draw strong conclusions. In the five studies that found age-related differences, adults tend to show higher levels of anxiety, particularly during early withdrawal; however, the opposite was found in the one study examining anxiety in social interactions. Six studies did not observe any age-related differences. Overall, adolescents may be less sensitive to the anxiety-inducing effects of chronic alcohol exposure.
Social behavior: Two studies were identified that examined the effects of chronic ethanol exposure on social behavior in rats [ 112 , 113 ], with both observing age-related differences. After chronic exposure (1 g/kg, 7d), followed by a brief abstinence period (24–48 h), one study found a decrease in social preference in adolescents only [ 112 ], while the other study found no ethanol-related effects on social behavior (2 g/kg, 10d) [ 113 ]. After acute challenges, age and treatment interactions emerged in both studies, but the directions of the results are inconsistent. In the first study, adolescents showed increased social preference, as indexed by the number of cross-overs between compartments toward and away from a peer, across multiple acute doses (0.5–1.0 g/kg) administered immediately before testing, while adults showed no changes in social preference [ 112 ]. In contrast, Morales et al. [ 113 ] found evidence for age-related temporal differences in social activity after acute challenge, with adults showing decreased social impairment five minutes post injection (1 g/kg) and adolescents (1.25 g/kg) after 25 min compared to age-matched controls.
The findings from these two studies paint a complicated and inconsistent picture of the effects of ethanol on social behavior in adults and adolescents warranting further research. One study found support for a larger effect of chronic ethanol on adolescent social behavior compared to adults, while the other did not observe effects of ethanol in either group. One study found support for a larger effect of chronic plus acute ethanol intoxication on social behavior, with the opposite observed in the other.
Brain outcomes
Neurotransmitter systems.
Glutamate is the brain’s main excitatory neurotransmitter and plays a crucial role in synaptic plasticity (i.e., experience-related strengthening or weakening of synaptic connections). Glutamatergic transmission plays an important role in the formation and maintenance of addictive behaviors and the nucleus accumbens (NAc) is considered an important hub in this, receiving glutamatergic input from cortical-limbic areas and dopaminergic input from the midbrain [ 114 ]. Seven studies investigated glutamate functioning in regions of the brain [ 106 , 107 , 108 , 109 , 115 , 116 , 117 , 118 ]. Four of the seven studies observed age-related differences.
Three studies investigated glutamate-related processes in the NAc [ 106 , 107 , 118 ]. Two weeks of voluntary binge drinking (4-h access, no abstinence) did not affect expression of calcium-dependent kinase II alpha (CaMKIIα) and the AMPA receptor GluA1 subunit in the NAc of mice [ 107 ]. In contrast, Lee et al. [ 106 ] showed that voluntary binge drinking (2-h access, no abstinence) increased mGlu1, mGlu5, and GluN2b expression in the shell of the NAc, as well as PKCε and CAMKII in the core of the NAc in adult mice only. In rats, Pascual et al. [ 118 ] showed reduced NR2B phosphorylation in the NAc of adolescents only after two weeks of chronic intermittent ethanol exposure; an effect that also lasted until 24 h after end of exposure. This indicates that adolescents might be less affected by the effects of ethanol on NAc-related glutamatergic neurotransmission than adults. This may in turn mediate decreased withdrawal symptoms and potentially facilitate increased drinking [ 106 ].
Two studies investigated glutamate-related processes in the (basolateral) amygdala [ 107 , 116 ]. In mice, Agoglia et al. [ 107 ] showed decreased CaMKIIα phosphorylation in adolescents, but increased GluA1 expression in adults after two weeks of voluntary binge drinking (4-h access, no abstinence). Also, drug-induced AMPAR activation resulted in increased binge drinking in adolescents but decreased binge drinking in adults, highlighting the potential importance of glutamatergic signaling in age-related differences in alcohol consumption. However, Falco et al. [ 116 ] reported no difference in NR2A mRNA levels in the basolateral amygdala for either age group after 60-day abstinence.
Alcohol’s effects on frontal cortex functioning is thought to be mediated by alterations in NMDA receptor subunit expression [ 119 , 120 ]. Two studies investigated glutamate-related processes in the frontal cortex of rats [ 115 , 118 ]. Pascual et al. [ 118 ] showed reduced NR2B phosphorylation after two weeks of forced intermittent ethanol exposure in adolescents only. Using a 2-week ethanol vapor paradigm, Pian et al. [ 115 ] found different patterns of NMDAR subunit expression. These patterns were highly dependent on abstinence duration (0 h, 24 h, 2w), however, they only statistically compared results within rather than between age groups. Ethanol exposure was associated with decreased NR1 receptor expression in both age groups, but only the adult group showed a decrease in NR2A and NR2B expression. The NR1 and NR2A expression returned to normal during withdrawal, but in adults NR2B expression increased after two weeks of abstinence.
Conrad and Winder [ 109 ] assessed long-term potentiation (LTP) in the bed nucleus stria terminalis (BNST), a major output pathway of the amygdala towards the hypothalamus and thalamus. Voluntary ethanol exposure resulted in blunted LTP responses in the dorsolateral BNST regardless of age. However, all mice were socially isolated during the experiments to induce anxiety, so it is unclear whether the effects were solely due to ethanol exposure.
Two studies looked at glutamate receptor subunit expression in the hippocampus [ 108 , 115 ]. Pian et al. [ 115 ] observed increased expression of NR1, NR2A, and NR2B in adults after 2 weeks of ethanol exposure. In adolescents, a reduction in NR2A expression was observed. After abstinence, adult levels returned to normal, while in adolescents, decreased NR1 and NR2A expression was seen after 24 h but an increased expression of these subunits was seen after 2 weeks of abstinence. These findings support regional specific effects of age group, with potentially increased sensitivity to the impact of alcohol on glutamatergic mediated hippocampal functioning in adolescents. Unlike expected, van Skike et al. [ 108 ] did not find effects of chronic intermittent ethanol exposure or withdrawal on NMDA receptor subunit expression in the hippocampus and cortex as a whole in adolescent and adult rats. The authors speculate that these null results might be associated with the exposure design (limited exposure and route of administration) and lack of withdrawal periods compared to Pian et al. [ 115 ].
In sum, there is limited and inconsistent evidence for age-related differences in glutamate function across seven studies. The direction of the observed age-related differences varies across regions, with evidence of both increased and decreased sensitivity to ethanol effects in adolescents compared to adults in the four studies that observed age-related differences.
GABA is the brain’s main inhibitory neurotransmitter. GABA A receptors are a primary mediator of alcohol’s pharmacological effects [ 121 ]. A total of four studies looked at GABAergic functioning [ 108 , 116 , 122 , 123 ]. Three of the four studies observed age-related differences.
One study investigated GABA-related processes in the (basolateral) amygdala, showing reduced GABA A α1 and GAD67 (enzyme that converts Glutamate to GABA) mRNA expression in adult rats only, 60 days after 18-days ethanol exposure [ 116 ].
Two studies looked at the rat cortex as a whole [ 108 , 122 ]. Van Skike et al. did not find effects of chronic intermittent ethanol exposure on GABA A receptor expression [ 108 ]. Grobin et al. [ 122 ] showed that, while basal GABA A receptor functioning was not affected by 1 month of chronic intermittent ethanol exposure, GABA A receptors were less sensitive to the neurosteroid THDOC in adolescents. This neuromodulatory effect was not found in adults and did not persist after 33 days of abstinence. However, these results indicate that neurosteroids may play an indirect role in age differences in the GABAA receptor’s response to alcohol.
Two studies focused on the rat hippocampus [ 108 , 124 ]. Fleming et al. [ 124 ] found age-specific effects of chronic intermittent ethanol exposure on hippocampal (dentate gyrus) GABA A receptor functioning. Adolescent rats showed decreased tonic inhibitory current amplitudes after ethanol exposure, which was not the case for young adult and adult rats. Also, only the adolescents showed greater sensitivity to (ex vivo) acute ethanol exposure induced enhanced GABAergic tonic currents. The specificity of these effects to adolescent exposure might indicate adolescent vulnerability to ethanol-induced effects on the hippocampus; however, Van Skike et al. [ 108 ] did not find any effects of chronic intermittent ethanol exposure on GABA A receptor expression in the hippocampus.
In sum, given the limited number of studies and lack of replicated effects, no clear conclusions can be drawn about the role of age on the effects of alcohol on GABAergic neurotransmission. Age-specific effects appear to be regionally distinct. The only available study found support for heightened adult sensitivity to ethanol in the amygdala. In contrast, one study found support for greater adolescent sensitivity in the hippocampus and whole cortex, whereas the other found no age-related differences.
The mesocorticolimbic dopamine system, with dopaminergic neurons in the ventral tegmental area (VTA) projecting to the NAc and prefrontal cortex, plays a key role in AUD, particularly through reward and motivational processes [ 14 ]. Only two studies investigated dopaminergic processes, focusing on the frontal cortex, NAc, and broader striatum [ 118 , 125 ]. Both studies observed age-related differences in certain dopamine outcomes.
Carrara-Nascimento et al. [ 125 ] investigated acute effects of ethanol in adolescent and adult mice 5 days after a 15-day treatment with either ethanol or saline. In the PFC, ethanol pretreated adolescents showed reduced dopamine levels (DA) and related metabolites (DOPAC and HVA) in response to an acute ethanol challenge compared to ethanol pretreated adults and adolescent saline controls. In the NAc, there were no differences between pretreated adolescents and adults, but analyses within each age group revealed that ethanol-pretreatment with an acute challenge decreased DOPAC within the adolescent group. Results from the dorsal striatum also showed no differences between adolescents and adults. However, within the adolescent group, ethanol pre-treatment increased DOPAC and, within the adult group, it increased HVA. Pascual et al. [ 118 ] found similar results looking at the expression of DRD1 and DRD2 dopamine receptors after two weeks of chronic intermittent ethanol exposure in rats. In the NAc and dorsal striatum, DRD2 expression was reduced in adolescent compared to adult exposed rats, while both DRD1 and DRD2 expression were reduced in the frontal cortex.
These results suggest reduced alcohol-induced dopamine reactivity in adolescents in the PFC and NAc based on the two available studies, but more studies are warranted for a more detailed understanding of the relationship between age and dopamine receptor expression following chronic ethanol exposure.
Acetylcholine
Acetylcholine is a known neuromodulator of reward and cognition-related processes [ 126 ]. The composition and expression of nicotinic and muscarinic acetylcholine receptors have been implicated in various alcohol use-related behaviors [ 127 , 128 ]. Only one study investigated cholinergic processes and observed age-related differences. Vetreno et al. [ 129 ] showed global reductions in choline acetyltransferase (ChAT; cholinergic cell marker) expression after adolescent onset, but not adult onset of forced intermittent binge-like exposure (20 days – every other day, 25 days abstinence).
Neuromodulatory processes
Neurodegeneration and neurodevelopment.
Chronic alcohol consumption is thought to lead to brain damage by influencing processes involved in neurodegeneration and neurogenesis. The formation of addictive behaviors is paralleled by the formation of new axons and dendrites, strengthening specific neuronal pathways [ 130 ]. While brain morphology is commonly investigated in humans, it is a proxy of the impact of alcohol on the brain and therefore rarely studied in rodents. Five studies investigated facets of neurodegeneration or development in rodents [ 55 , 65 , 131 , 132 , 133 ]. All five studies observed age-related differences.
Huang et al. [ 131 ] showed reduced cerebral cortex mass in adolescent mice, but shortening of the corpus collosum in adults after 45 days of ethanol injections, suggesting some age-specific regional effects. Using an amino cupric silver staining, significant brain damage was revealed for both adolescent and adult rats after 4 days of binge-like ethanol exposure [ 132 ]. However, adolescents showed more damage in the olfactory-frontal cortex, perirhinal cortex, and piriform cortex.
Looking at hippocampal neurogenesis, ethanol exposure has been shown to initially reduce hippocampal neurogenesis in adult rodents, recovering after 1-month abstinence [ 134 ]. Compared to adults, neurogenesis in the dentate gyrus of the hippocampus was found to be reduced in adolescent exposed mice (Bromodeoxyuridine levels) [ 65 ] and rats (doublecortin levels) [ 133 ]. Lacaille et al. [ 65 ] also measured the expression level of genes involved in oxidative mechanisms after binge-like alcohol exposure. In whole brain samples, they found increased expression of genes involved in brain protection (i.e., gpx3, srxn1) in adults, but increased expression of genes involved in cell death (i.e., casp3) combined with decreased expression of genes involved in brain protection (i.e., gpx7, nudt15) in adolescents. Casp3 protein levels were also higher in the whole brain of adolescent exposed mice [ 65 ] and the adolescent dentate gyrus [ 133 ], suggesting more neurodegeneration and less neurogenesis in adolescents versus adults following ethanol consumption.
Cyclin-dependent kinase 5 (CDK5) is involved in axon, dendrite, and synapse formation and regulation. CDK5 is overexpressed in the prefrontal cortex and the NAc following exposure to substances of abuse including alcohol [ 135 ]. Moreover, CDK5 inhibition has been shown to reduce operant self-administration of alcohol in alcohol-dependent rats [ 136 ]. One study reported higher H4 acetylation of the CDK5 promoter in the PFC of adult versus adolescent ethanol-exposed rats during acute withdrawal, however, CDK5 mRNA expression was control-like after 2 weeks of abstinence [ 55 ].
In sum, strong conclusions cannot be drawn due to the limited number of studies and lack of replicated effects. However, preliminary evidence points to adolescent vulnerability to damage in the cortex, reduced neurogenesis, and increased neurodegeneration in the hippocampus and the cortex as a whole based on four of the five studies. In contrast, one study found support for adult vulnerability to ethanol’s effects axon, dendrite, and synapse formation and regulation.
Growth factors
Brain-derived neurotrophic factor (BNDF) and nerve growth factor (NGF) are involved in brain homeostasis and neural recovery [ 137 , 138 ]. While ethanol exposure initially increases BDNF and NGF, chronic ethanol exposure seems to reduce BDNF and NGF levels and can thereby result in long-term brain damage and related cognitive problems [ 139 , 140 ]. Four studies investigated growth factor expression in the frontal cortex [ 54 , 55 , 79 , 80 ] and two studies also investigated the hippocampus [ 79 , 80 ]. All four studies of the frontal cortex observed age-related differences. Neither study of the hippocampus observed age-related differences.
In rats, 30 weeks of chronic ethanol exposure reduced prefrontal mBDNF and β-NGF regardless of age, despite adolescents consuming more ethanol [ 80 ]. Moreover, the reduction of mBDNF was correlated with higher blood alcohol levels and was persistent up to 6–8 weeks abstinence. Interestingly, during acute withdrawal (48 h) adolescents but not adults temporarily showed control-like mBDNF levels. This might indicate an attempt to counteract neurodegeneration as a result of ethanol exposure in adolescents. These results were partially replicated using a shorter intermittent exposure paradigm (13 doses, 2 days on/off) [ 79 ]. While intoxication after chronic ethanol exposure reduced prefrontal BDNF, levels recovered after 3-weeks abstinence regardless of age. However, during acute withdrawal (24 h), BDNF was still reduced in early-adolescent onset rats, increased in adult-onset rats, but control-like in mid-adolescent onset-rats, suggesting slower recovery in younger animals. Looking at BDNF gene regulation, a similar study (8 doses, 2 days on/off) reported higher H3 demethylation but lower H4 acetylation of the BDNF promoter in the PFC of adult versus adolescent ethanol-exposed rats during acute withdrawal [ 55 ]. However, prefrontal BDNF mRNA expression returned to control levels after 2 weeks of abstinence. Interestingly, social housing may be protective, as reduced prefrontal BDNF was no longer observed in alcohol-exposed adolescent mice housed in environmentally enriched relative to standard conditions [ 54 ]. Two studies investigated hippocampal BDNF expression but reported no significant interactions between alcohol exposure and age group [ 79 , 80 ].
In sum, the results of the four available studies suggest lower prefrontal BDNF during chronic alcohol use that recovers after abstinence regardless of age. However, the rate of recovery may be influenced by age with slower recovery in adolescents. In the two available studies, no age-related differences were observed in BDNF expression in the hippocampus.
Transcription factors
The transcription factors cFos and FosB are transiently upregulated in response to substance use, and ΔFosB accumulates after chronic exposure, particularly in striatal and other reward-related areas [ 141 ]. Two studies investigated cFos and FosB [ 55 , 142 ] and one study ΔFosB related processes [ 111 ]. All three studies observed age-related differences.
After chronic ethanol exposure (8 doses, 2 days on/off), adolescent compared to adult rats showed increased prefrontal H3 and H4 acetylation of the cFos promotor region and increased H4 acetylation and H3 dimethylation of FosB promotor regions after acute abstinence [ 55 ]. Moreover, mRNA expression of FosB was elevated in adolescents but not adults after 2-weeks abstinence. The upregulating effects of an acute ethanol challenge on prefrontal cFos appears to reduce after chronic pre-treatment to a larger extent in adolescent than adult exposed mice [ 142 ]. This pattern of results was similar in the NAc, but desensitization to ethanol’s acute effects on cFos in the hippocampus was more pronounced in adults. Faria et al. [ 142 ] also looked at Egr-1 (transcription factor, indirect marker of neuronal activity and involved in neuroplasticity), showing a stronger reduction in Egr-1 expression in the PFC, NAc, and hippocampus of adolescent versus adults after repeated ethanol exposure. Regarding ∆FosB, Wille-Bille et al. [ 111 ] found increased ∆FosB in adolescent compared to adult rats in the prelimbic PFC, dorsomedial striatum, NAc core and shell, central amygdala nucleus capsular, and basolateral amygdala after 3 days per week 18 h ethanol exposure sessions for 6 weeks. In sum, the three available studies provide preliminary evidence for increased adolescent vulnerability to ethanol-induced long-term genetic (mRNA expression) and epigenetic (methylation) changes in mesocorticolimbic areas.
Immune factors
Ethanol is known to trigger immune responses in the brain (e.g., increase production of hemokines and cytokines), causing inflammation and oxidative stress [ 143 , 144 , 145 ]. Three studies examined immune factors [ 146 , 147 , 148 ]. Two of the three studies observed age-related differences.
Microglia remove damaged brain tissue and infectious agents and are key to the brain’s immune defense. Only one study investigated microglia levels [ 146 ]. Although direct comparisons between age groups were missing, both adolescent and adult rats showed less microglia in the hippocampus (CA and DG) and peri-entorhinal cortex, and more dysmorphic microglia in the hippocampus after 2 and 4 days of binge-like ethanol exposure [ 146 ]. Notably, age groups were matched on intoxication scores, with adolescents needing more ethanol to reach the same level of intoxication. An in silico transcriptome analysis of brain samples from mice after 4 days of 4 h/day drinking in the dark, suggest overexpression of neuroimmune pathways related to microglia action (toll-like receptor signaling, MAPK signaling, Jak-STAT signaling, T-cell signaling, and chemokine signaling) in adults that was not observed in adolescents, while adolescents consumed more ethanol [ 147 ]. Similarly, ethanol-exposed adult mice showed higher chemokine expression (CCL2/MCP-1) in the hippocampus, cerebral cortex, and cerebellum and higher cytokine expression (IL-6, but not TNF-α) in the cerebellum, while no chemokine or cytokine changes were observed in ethanol exposed adolescent mice [ 148 ]. Both adolescents and adults showed increased astrocyte levels in the hippocampus (CA1) and the cerebellum after ethanol exposure, but changes in astrocyte morphology were only observed in the adult hippocampus.
In sum, two of the studies found support for increased immune responses after ethanol exposure in adults compared to adolescents, whereas the one other study found no difference between the age groups.
HPA-axis functionality
Chronic stress and HPA-axis functionality have been associated with the maintenance of AUD (e.g., reinstatement drug seeking, withdrawal) [ 149 ]. Two studies investigated corticotropin-release factor (CRF) expression in rats [ 116 , 150 ]. One study observed age-related differences and the other did not.
Falco et al. [ 116 ] found decreased CRF mRNA expression in the adult but not adolescent basolateral amygdala 2 months after 18-day restricted ethanol exposure. In contrast, Slawecki et al. did not find any interaction between age and treatment on CRF levels in the amygdala, as well as the frontal lobe, hippocampus, hypothalamus, and caudate 7 weeks after 10-days of ethanol vapor exposure.
No conclusions can be drawn. One study observed found support for reduced effects of ethanol on HPA-axis functionality compared to adults, whereas the other observed no difference between the age groups. Future studies using different (voluntary) exposure paradigms are needed to further investigate the effects of alcohol on HPA activity in relation to age of alcohol exposure.
Neuropeptides
Neuropeptides are a diverse class of proteins that have a modulatory function in many different processes, including but not limited to neurotransmission, stress, immune responses, homeostasis, and pain [ 151 , 152 , 153 ]. Only one study investigated neuropeptides in rats and observed age-related differences [ 150 ].
Slawecki et al. [ 150 ] specifically investigated neuropeptide-Y, substance-P, and interleukine expression in the frontal lobe, hippocampus, hypothalamus, dorsal striatum, and amygdala 7 weeks after 10-days of ethanol vapor exposure in rats [ 150 ]. Interactions between age and treatment were found for the hippocampus and caudate only. Ethanol-induced reductions in hippocampal neuropeptide-Y and increases in caudate neurokinine were more pronounced in adults compared to adolescents suggesting long-lasting effects of ethanol in adults but not adolescents.
Ethanol metabolism
The first metabolite of ethanol is acetaldehyde, which has been theorized to mediate the effects of ethanol on both brain and behavior [ 154 ]. Only one study investigated ethanol metabolism in the brain and did not observe age-related differences [ 155 ].
Rhoads et al. showed that despite the fact that adolescent rats consumed more alcohol brain catalase levels after 3-weeks of ethanol exposure (no abstinence) did not differ between adolescents and adults [ 155 ]. Although the general role of catalase in ethanol metabolism is small, catalase can oxidize ethanol to acetaldehyde in the brain, affecting elimination of ethanol after consumption [ 156 , 157 ]. These findings may therefore imply that ethanol metabolism may not differ between adolescent and adult animals, which should be studied in a more direct manner.
Full proteome analysis
While the previously described studies focused on specific factors involved in neurotransmission, brain health, and plasticity, proteomics allows for the study of the full proteome in a specific region or tissue type. One study investigated the impact of age on ethanol-induced changes in the hippocampal proteome, observing age-related differences [ 158 ]. In this study, rats intermittently and voluntarily consumed beer for 1 month and the hippocampal proteome was analyzed after 2 weeks of abstinence. The results point to the involvement of many of the factors described above and imply age-specific effects of alcohol. Adult beer exposure increased citrate synthase (part of the citric acid, or Krebs, cycle) and fatty acid binding proteins (involved in membrane transport) compared to controls. Adolescent beer exposure increased cytoskeletal protein T-complex protein 1 subunit epsilon (TCP-1), involved in ATP-dependent protein folding, and reduced expression of a variety of other proteins involved in glycolysis, glutamate expression, aldehyde detoxification, protein degradation, and synaptogenesis, as well as neurotransmitter release. These more extensive changes suggest that the adolescent hippocampus might be more vulnerable to the effects of ethanol exposure, but more studies are needed to clarify and replicate these findings and extend the focus to different brain areas.
Neuronal activity and functioning
Ethanol-induced molecular changes may eventually change neuronal activity. Three studies investigated neuronal activity and functioning [ 89 , 159 , 160 ] using electrophysiological methods. All three studies observed age-related differences.
Galaj et al. [ 159 ] assessed firing patterns and the structure of pyramidal neurons in the L2 and L5 layers of the prelimbic cortex of the rat brain using ex vivo electrophysiological recordings and morphological staining. Following chronic intermittent ethanol exposure and brief abstinence (2 days), adolescents, but not adults, showed reduced amplitudes of spontaneous excitatory post-synaptic currents (sEPSCs) in L5 neurons compared to controls, indicating reductions in intrinsic excitability. In line with this, Dil staining showed increased thin spine ratios in the L5 layer in adolescents only. Age differences were more pronounced after prolonged abstinence (21 days), with adolescents showing reduced amplitude and frequency of sEPSCs in L5 neurons while adult’s L5 neurons showed augmented firing patterns (i.e., amplitude and frequency). Furthermore, adolescent rats showed decreased total spine density and non-thin spines, indicating less excitatory postsynaptic receptors in the L5 layer. In contrast, adults showed increases in spine density and non-thin spines.
Li et al. [ 160 ] examined the functioning of CA1 interneurons, which are important for learning and memory processes [ 161 ], in the rat hippocampus using ex vivo whole-cell recordings. After prolonged abstinence (20 days), voltage-gated A-type potassium channel ( I A ) conductance was measured. Differences emerged between age groups (although no statistical interaction effect was directly assessed): EtOH-exposed adolescents and adults both showed lower I A mean peak amplitude compared to the respective control groups. However, adolescents also showed reduced I A density and increased mean decay time, which decreased in adults. Furthermore, only adolescents showed increased depolarization required for activation compared to controls, which can result in higher interneuron firing rates in the CA1 region that could affect learning processes. Additional research is needed to connect these findings to behavioral measures of learning and memory.
Slawecki et al. [ 89 ] was the only study to use in vivo electroencephalogram (EEG) recordings with rats to examine function in the frontal and parietal cortex at different times during a 14-day vapor exposure period. During acute withdrawal (7–10 h abstinence period), following daily exposure no effects emerged in frontal cortical regions throughout the exposure period. In parietal regions, only adolescents showed increased high frequency (16–32 Hz and 32–50 Hz) power on days 8 and 12 compared to controls. Adolescent hyperexcitability during withdrawal may indicate increased arousal in adolescents compared to adults during withdrawal, but more studies linking brain activity to behavioral indices of withdrawal will allow for clearer interpretations.
Overall, strong conclusions cannot be drawn given the disparate paradigms and outcomes utilized. While adolescents and adults appear to differ in the effect of ethanol on neuronal firing, the meaning of these differences is not clear given the lack of connection between these findings and behavioral outcomes.
Human studies
Four studies examined age-related differences of the effect of alcohol on brain or cognition in humans [ 162 , 163 , 164 , 165 ].
Müller-Oehring et al. [ 162 ] examined the moderating role of age on resting state functional connectivity and synchrony in the default mode, central executive, salience, emotion, and reward networks of the brain in a sample of no/low and heavier drinkers aged 12–21 years old. While the study did not compare discrete groups of adolescents and adults, analyses investigating the interaction between continuous age and alcohol exposure history were conducted which provide insight into the effect of alcohol use on functional brain networks from early adolescence to emerging adulthood. Regardless of age, no differences were observed between matched subgroups of no/low drinkers and moderate/heavy drinkers in the default mode, salience, or reward networks. However, in the central executive network, connectivity between the superior frontal gyrus (SFG) and insula increased with age in the no/low drinkers but not in heavier drinkers. Age-related strengthening of this fronto-limbic connection correlated with better performance on a delay discounting task in boys, suggesting that adolescent alcohol use may interfere with typical development of higher-level cognitive functions. In the emotion network, amygdala-medial parietal functional synchrony was reduced in the heavier drinkers compared to the no/low drinkers and exploratory analyses suggested that weaker amygdala-precuneus/posterior cingulate connectivity related to later stages of pubertal development in the no/low drinking group only. Interestingly, in the default mode (posterior cingulate-right hippocampus/amygdala) and emotional networks (amygdala, cerebellum), connectivity in regions that exhibited age-related desynchronization was negatively correlated with episodic memory performance in the heavy drinkers. These results give preliminary evidence that alcohol might have age-dependent effects on resting state connectivity and synchronization in the central executive, emotion, and default mode networks that could potentially interfere with normative maturation of these networks during adolescence.
Three studies examined age effects in alcohol-related implicit cognitions, specifically attentional bias [ 163 , 165 ], alcohol approach bias [ 165 ], and implicit memory associations and explicit outcome expectancies [ 164 ]. Attentional bias refers to the preferential automatic allocation or maintenance of attention to alcohol-related cues compared to neutral cues which is correlated with alcohol use severity and craving [ 166 ]. McAteer et al. [ 163 ] measured attentional bias with eye tracking during presentation of alcohol and neutral stimuli in heavy and light drinkers in early adolescents (12–13 yrs), late adolescents (16–17 yrs), and young adults (18–21 yrs). Regardless of age, heavy drinkers spent longer fixating on alcohol cues compared to light drinkers. Cousijn et al. [ 165 ] measured attentional bias with an Alcohol Stroop task [ 167 ], comparing the speed of naming the print color of alcohol-related and control words. Consistent with the findings of McAteer et al. [ 163 ], adults and adolescents matched on monthly alcohol consumption showed similar levels of alcohol attentional bias. In the same study, Cousijn et al. [ 165 ] did not find any evidence for an approach bias towards alcohol cues in any age group.
Rooke and Hine [ 164 ] found evidence for age-related differences in implicit and explicit alcohol cognitions and their relationship with binge drinking. Using a teen-parent dyad design, adolescents (13–19 yrs) showed stronger memory associations in an associative phrase completion task and more positive explicit alcohol expectancies than adults. Interestingly, both explicit positive alcohol expectancies and implicit memory associations were a stronger predictor of binge drinking in adolescents compared to adults. It is important to note that adolescents also had higher levels of binge drinking than adults in the study.
Cousijn et al. [ 165 ] also investigated impulsivity, drinking motives, risky decision-making, interference control, and working memory. No age differences emerged in the cognitive functioning measures including risky decision-making (Columbia Card Task – “hot” version), interference control (Classical Stroop Task), or working memory (Self-Ordered Pointing Task). However, adolescents were more impulsive (Barrett Impulsiveness Scale) than adults and reported more enhancement motives. Importantly, impulsivity as well as social, coping, and enhancement motives of alcohol use correlated with alcohol use in both ages. However, age only moderated the relationship between social drinking motives and alcohol use-related problems (as measured by the Alcohol Use Disorder Identification Test), with a stronger positive association in adolescents compared to adults. Importantly, the adolescent group had a different pattern of drinking, with less drinking days per month but more drinks per episode than the adult group.
In summary, human evidence is largely missing, with no studies comparing more severe and dependent levels of alcohol use between adolescents and adults. The preliminary evidence is too weak and heterogeneous to draw conclusions, warranting future studies investigating the impact of age.
The current systematic review assessed the evidence for the moderating role of age in the effects of chronic alcohol use on the brain and cognition. The identified 59 rodent studies (Table 1 ) and 4 human studies (Table 2 ) provide initial evidence for the presence of age-related differences. Rodents exposed to ethanol during adolescence show both increased risk and resilience to the effects of ethanol depending on the outcome parameter. However, due to the high variability in the outcomes studied and the limited number of studies per outcome, conclusions should be considered preliminary. Moreover, brain and behavioral outcomes were mostly studied separately, with studies focusing on either brain or behavioral outcomes. The behavioral consequences of changes in certain brain outcomes still need to be investigated. Table 3 provides a comprehensive overview of the strength of the evidence for age-related differences for all outcomes. Below, we will discuss the most consistent patterns of results, make connections between the behavioral and neurobiological findings when possible, highlight strengths and limitations of the evidence base, and identify the most prominent research gaps.
Patterns of results
Age-related differences in learning and memory-related processes appear to be highly domain specific. There is limited but fairly consistent evidence for adolescent-specific impairments in contextual fear conditioning, which could be related to hippocampal dysfunction. Results for other hippocampus-related memory processes such as spatial memory are mixed and largely based on forced exposure with acute challenge studies rather than voluntary long-term exposure to alcohol. The evidence base is currently insufficient to draw conclusions about the role of age in alcohol’s effects on non-spatial types of learning and memory. Alcohol generally did not impact performance in the non-spatial variants of the MWM and SBM paradigms or in reward-learning, but the results of the limited studies in the object-learning domain highlight potential impairments and the importance of age therein. For example, adolescents but not adults demonstrated impaired object memory in the only study using the novel object recognition task [ 65 ]. Acute challenges after chronic pre-exposure to alcohol also appear to impair performance in the working memory domain, with one study suggesting heightened adolescent sensitivity to working memory impairment [ 83 ]. Thus, although the domain-specific evidence is limited by the relative lack of research, overall patterns suggest that learning and memory functions that are primarily hippocampus-dependent may be differentially affected by adolescent compared to adult alcohol use. Studies focusing on neural hippocampal processes corroborate these findings, reporting more extensive changes in protein expression [ 158 ], less desensitization of cFos upregulation [ 142 ], larger changes in GABAa receptor subunit expression [ 124 ], longer lasting changes in NMDA receptor expression [ 115 ], and larger reductions in neurogenesis [ 65 , 133 ] in the hippocampus of adolescent compared to adult ethanol-exposed rodents. On the other hand, ethanol-induced changes in the hippocampus recovered more quickly in younger animals after abstinence [ 150 ] and adolescent mice showed less signs of ethanol-induced neuroinflammation compared to adults [ 148 ].
Higher rates of adolescent alcohol use, especially binge drinking, may be facilitated by a heightened sensitivity to the rewarding properties of alcohol in combination with a reduced sensitivity to the negative effects of high doses [ 47 ]. In line with this, there is limited but consistent evidence that adolescents show less CTA in response to chronic ethanol and consequently voluntarily consume more ethanol [ 50 ]. Importantly, distinct vulnerability periods within adolescence for altered CTA may exist [ 168 , 169 ], with early adolescents potentially being least sensitive to aversive effects. Future studies using chronic exposure paradigms comparing different stages of adolescence to adults are needed. In contrast to CTA, there is insufficient evidence of age-related differences in the motivational value of alcohol based on CPP paradigms, with only one of five studies reporting stronger CPP in adolescents than adults [ 52 ]. Adolescents may be more sensitive to the effects of environmental factors on the motivational value of alcohol than adults, as adolescents housed in enriched environments acquired CPP while those in standard housing did not, an effect that was not found in adults [ 54 ]. Evidence for environmentally enriched housing being protective against these changes in adolescents provides an important indication that environmental factors matter and are important factors to consider in future research on the motivational value of ethanol on both the behavioral and neural level. Complementary studies on the functioning of brain regions within the mesolimbic dopamine pathway and PFC, which play an important role in motivated behavior, indicate limited but consistent evidence for age-related differences. Adolescents showed less dopamine reactivity in the PFC and NAc compared to adults after chronic ethanol exposure. Furthermore, there is limited but consistent evidence that adolescents are more vulnerable to epigenetic changes in the frontal cortex and reward-related areas after chronic ethanol exposure. For instance, adolescents may be more sensitive to histone acetylation of transcription factors in motivational circuits underlying the rewarding effects of alcohol [ 55 ], which may contribute to addictive behaviors [ 170 , 171 ]. Chronic alcohol use is also associated with lower BDNF levels in the PFC and subsequent increases in alcohol consumption, implicating BDNF as an important regulator of alcohol intake [ 172 ]. While evidence is limited, chronic alcohol use consistently reduced prefrontal BDNF in both age groups. However, the rate of recovery of BDNF levels after abstinence appears to be slower in adolescents.
Regarding executive functioning, there is limited but fairly consistent evidence from animal studies that adolescents are more vulnerable to long-term effects of chronic exposure on decision-making and are more impulsive than adults during acute intoxication and after prolonged abstinence following chronic exposure. Impulsivity is associated with functional alterations of the limbic cortico-striatal systems [ 91 ], with involvement of both the dopaminergic and serotonergic neurotransmitter systems [ 173 ]. While no studies investigating serotonergic activity were identified, the consistent reduction in dopamine reactivity observed in the PFC and NAc in adolescents compared to adults parallel the behavioral findings. There is also limited but fairly consistent evidence that adolescents are more resilient to impairments in cognitive flexibility than adults following chronic exposure to alcohol, and that adolescents may more easily regain control over their alcohol-seeking behavior than adults. These behavioral findings provide preliminary support for the paradox of adolescent risk and resilience in which adolescents are at once more at risk to develop harmful patterns of drinking, but are also more resilient in that they may be more equipped to flexibly change behavior and with time regain control over alcohol consumption. However, studies assessing processes that might be related to brain recovery provide little conclusive evidence for potential underlying mechanisms of these behavioral findings. While adolescents appear more vulnerable to ethanol-induced brain damage [ 131 , 132 ], show reduced neurogenesis [ 65 , 133 ], and show less changes in gene expression associated with brain recovery [ 65 , 133 ], adults show relatively higher immune responses after repeated ethanol exposure [ 147 , 148 ]. The limited evidence for adolescent resilience to alcohol’s effects on cognitive flexibility diverge from the conclusions of recent reviews that focused mostly on adolescent-specific research. Spear et al. [ 18 ] concluded that adolescents are more sensitive to impairments in cognitive flexibility; however, this was based on adolescent-only animal studies. Similarly, the systematic review of Carbia et al. [ 19 ] on the neuropsychological effects of binge drinking in adolescents and young adults also revealed impairments in executive functions, particularly inhibitory control. However, as pointed out by the authors, the lack of consideration of confounding variables (e.g., other drug use, psychiatric comorbidities, etc.) in the individual studies and the lack of prospective longitudinal studies limit our ability to causally interpret these results. This further highlights the difficulty of conducting human studies which elucidate causal associations of the effects of alcohol, and the need for animal research that directly compares adolescents to adults to bolster interpretation of findings from human research.
Only a few studies have investigated age-related differences in cognitive functioning in humans. These studies focused on mostly non-dependent users and studied different outcomes, including cognitive biases and implicit and explicit alcohol-related cognitions. Overall, there was limited but consistent evidence that age does not affect alcohol attentional or approach biases, with heavy drinkers in both age groups allocating more attention to alcohol cues compared to controls [ 163 , 165 ]. In contrast, in line with a recent meta-analysis of the neurocognitive profile of binge-drinkers aged 10–24 [ 23 ], there is limited evidence that age affects alcohol associations. One study found age effects on implicit (memory associations) and explicit (expectancies) cognition in relation to alcohol use. Adolescents showed stronger memory associations and more positive expectancies than adults [ 164 ]. These expectancies were also predictive of higher binge drinking in adolescents but not adults, highlighting the importance of future research into age differences in alcohol-related cognitions and their consequences on alcohol consumption. However, the quality of the evidence was rated as weak based on the methodological design of the included studies.
Regarding anxiety-related outcomes, results are inconsistent across studies and paradigms. When age-differences are observed, adolescents often show reduced anxiety compared to adults during both acute withdrawal and sustained abstinence following chronic ethanol exposure. However, the direction of age-related effects of alcohol may also be anxiety-domain specific. In social settings, adults show reduced anxiety compared to adolescents. Research on the neurocircuitry of anxiety processes implicates the extended amygdala, especially the BNST, in anxiety behaviors with an emphasis on the role of GABAergic projections to the limbic, hindbrain, and cortical structures in rodents [ 174 ]. Despite adolescents showing less non-social anxiety than adults after ethanol exposure, no age-differences were observed for LTP in the BNST [ 109 ]. Also, GABA receptor expression in the hippocampus and whole cortex was not altered by ethanol exposure in either age group [ 108 ]. However, the anxiolytic effects of NMDA antagonists [ 175 ] also highlight the importance of glutamatergic activity in anxiety processes [ 176 ]. In line with behavioral findings, adolescents were less sensitive to changes in glutamate expression: adults showed heightened expression in the NAc, which has been suggested to underlie the higher levels of anxiety observed in adults compared to adolescents [ 106 ]. Importantly, across the various studies, different paradigms were used to assess anxiety, potentially contributing to the inconsistent results. Furthermore, most of the identified studies used a forced ethanol exposure paradigm. As alcohol-induced anxiety is likely also dependent on individual trait anxiety, voluntary consumption studies in high and low trait anxiety animals are important to further our understanding of the interaction between alcohol use and anxiety. Of note, the observed pattern suggestive of reduced anxiety in adolescents compared to adults diverges from conclusions of previous reviews such as Spear et al. [ 18 ] which concluded that adolescents are more likely to show augmented anxiety after alcohol exposure based on animal studies with adolescent animals only. Importantly, anxiety was included as a secondary outcome in this review because of the high comorbidity between anxiety disorders and alcohol addiction, warranting the inclusion of age-related differences in the relation between alcohol and anxiety. However, the search strategy was not specifically tailored to capturing all studies assessing age-related differences in the effect of alcohol on anxiety.
Translational considerations, limitations, and future directions
The reviewed studies revealed a high degree of variability in study designs and outcomes, hindering integration and evaluation of research findings. We were unable to differentiate our conclusions based on drinking patterns (i.e., comparing binge drinking, heavy prolonged use, AUD). The prevalence of binge-drinking in adolescence is very high and is associated with neurocognitive alterations [ 177 ]. Studies investigating the potential differential impact of binge-drinking compared to non-binge-like heavy alcohol use in adolescence and adulthood are critical for understanding the risks of chronic binge-like exposure in adolescence, even if it does not progress to AUD.
It is also important to acknowledge the limitations of the choice of adolescent and adult age ranges in our inclusion criteria. Rodent studies had to include an adolescent group exposed to alcohol between the ages of PND 25–42 and an adult group exposed after age PND 65. Ontogenetic changes may still be occurring between PND 42–55, and this period may more closely correspond to late adolescence and emerging adulthood in humans (e.g., 18–25 years). Studies that compared animals in this post-pubertal but pre-adulthood age range were not reviewed. Studies investigating age-related differences in the effects of ethanol on brain and cognitive outcomes in emerging adulthood are also translationally valuable given the high rates and risky patterns of drinking observed during this developmental period [ 178 ]. Indeed, an important future direction is to examine whether there are distinct vulnerability periods within adolescence itself for the effects of ethanol on brain and cognitive outcomes. Given that emerging adulthood is a period of continued neurocognitive maturation and heightened neural plasticity, studies comparing this age range to older adults (e.g., over 30) are also necessary for a more thorough understanding of periods of risk and resilience to the effects of alcohol.
Furthermore, we did not conduct a risk of bias assessment to examine the methodological quality of the animal studies. The applicability and validity of the risk of bias tools for general animal intervention studies, such as the SYRCLE risk of bias tool [ 179 ], remain in question at the moment. The lack of standardized reporting in the literature for many of the criteria (e.g., process of randomizing animals into intervention groups) would lead to many studies being labeled with an ‘unclear risk of bias’. Furthermore, there is still a lack of empirical evidence regarding the impact of the criteria in these tools on bias [ 179 , 180 ]. This is a significant limitation in evaluating the strength of the evidence for age-related differences based on the animal studies, which highlights the importance of more rigorous reporting standards in animal studies.
Moreover, most work is done in male rodents and is based on forced ethanol exposure regimes. In a recent opinion article, Field and Kersbergen [ 181 ] question the usefulness of these types of animal models to further our understanding of human substance use disorders (SUD). They argue that animal research has failed to deliver effective SUD treatment and that social, cultural, and other environmental factors crucial to human SUD are difficult, if not impossible, to model in animals. While it is clear that more sophisticated multi-symptom models incorporating social factors are needed to further our understanding of SUD and AUD specifically, a translational approach is still crucial in the context of investigating the more fundamental impact of alcohol use on brain and cognition. In humans, comparing the impact of alcohol use on brain and cognition between adolescents and adults is complicated by associations between age and cumulative exposure to alcohol; i.e., the older the individual, the longer and higher the overall exposure to alcohol. Although animal models may be limited in their ability to model every symptom of AUD, they can still provide critical insights into causal mechanisms underlying AUD by allowing direct control over alcohol exposure and in-depth investigation of brain mechanisms.
The intermittent voluntary access protocol resembles the patterns of alcohol use observed in humans, and also result in physiologically relevant levels of alcohol intake [ 182 , 183 , 184 ]. Only a minority of the studies included in this review employed a voluntary access protocol, with one study using beer instead of ethanol in water [ 158 ], which better accounts for the involvement of additional factors (e.g., sugar, taste) in the appeal of human alcohol consumption. Voluntary access protocols can also model behavioral aspects of addictive behavior such as loss of control over substance use and relapse [ 185 , 186 , 187 ], an important area in which little is known about the role of age. Ideally, one would also investigate choices between ethanol and alternative reinforcers, such as food or social interaction, that better mimic human decision-making processes [ 188 ]. However, studies on the effects of ethanol on social behavior are limited and show inconsistent results and studies assessing reward processes often lack a social reward component as an alternative reinforcer.
On a practical level, rodents mature quickly and choice-based exposure paradigms are more complex and time-consuming than most forced exposure paradigms. Consequently, by the time final behavioral measurements are recorded, both the adolescent and adult exposure groups have reached adulthood. To combat this, many of the included studies use forced ethanol exposure, such as ethanol vapor, to quickly expose rodents to very high doses of ethanol. Although the means and degrees of alcohol exposure may not directly translate to human patterns of alcohol use, such studies do allow for the assessment of the impact of high cumulative doses of ethanol within a relatively short period of time which allows for more time in the developmental window to test age-related differences in the outcomes. When considering the translational value of a study, it is therefore important to evaluate studies based on the goal, while not ignoring the practical constraints.
While human research is challenging due to the lack of experimental control and the inherent confounds in observational studies between age and alcohol exposure history, large-scale prospective longitudinal studies offer a gateway towards a better understanding. Comparisons of different trajectories of drinking from adolescence to adulthood (i.e., heavy drinking to light drinking, light drinking to heavy drinking, continuously heavy drinking, and continuously light drinking) could offer insight into the associated effects on cognitive and brain-related outcomes. Of course, different drinking trajectories are likely confounded with potentially relevant covariates which limits causal inference. Direct comparisons of low and heavy adolescent and adult drinkers, supported by a parallel animal model can help to bolster the causality of observed age-related differences in human studies. In addition, changes in legislation around the minimum age for alcohol consumption in some countries provide a unique opportunity to investigate how delaying alcohol use to later in adolescence or even young adulthood impacts cognitive functioning over time. Importantly, future studies investigating the moderating role of age in humans should carefully consider the impact of psychiatric comorbidities. While adolescence into young adulthood is the period in which mental health issues often emerge [ 189 , 190 ], there is some evidence that the prevalence of comorbidities is higher in adults with AUD [ 95 ]. This is an important to control for when considering age-related differences on cognition and the brain given the evidence of altered cognitive functioning in other common mental illnesses [ 191 , 192 ].
Concluding remarks
The aim of this systematic review was to extend our understanding of adolescent risk and resilience to the effects of alcohol on brain and cognitive outcomes compared to adults. In comparison to recent existing reviews on the impact of alcohol on the adolescent brain and cognition [ 17 , 18 , 19 , 22 , 23 ], a strength of the current review is the direct comparison of the effects of chronic alcohol exposure during adolescence versus adulthood. This approach allows us to uncover both similarities and differences in the processes underlying alcohol use and dependence between adolescents and adults. However, due to the large degree of heterogeneity in the studies included in sample, designs, and outcomes, we were unable to perform meta-analytic synthesis techniques.
In conclusion, while the identified studies used varying paradigms and outcomes, key patterns of results emerged indicating a complex role of age, with evidence pointing towards both adolescent vulnerability and resilience. The evidence suggests adolescents may be more vulnerable than adults in domains that may promote heavy and binge drinking, including reduced sensitivity to aversive effects of high alcohol dosages, reduced dopaminergic neurotransmission in the NAc and PFC, greater neurodegeneration and impaired neurogenesis, and other neuromodulatory processes. At the same time, adolescents may be more resilient than adults to alcohol-induced impairments in domains which may promote recovery from heavy drinking, such as cognitive flexibility. However, in most domains, the evidence was too limited or inconsistent to draw clear conclusions. Importantly, human studies directly comparing adolescents and adults are largely missing. Recent reviews of longitudinal human research in adolescents, however, revealed consistent evidence of alterations to gray matter, and to a lesser extent white matter, structure in drinkers [ 17 , 18 ], but also highlight the limited evidence available in the domains of neural and cognitive functioning in humans [ 17 ]. Future results from ongoing large-scale longitudinal neuroimaging studies like the ABCD study [ 193 ] will likely shed valuable light on the impact of alcohol use on the adolescent brain. However, our results also stress the need for direct comparisons with adult populations. Moreover, while the lack of experimental control and methodological constraints limit interpretations and causal attributions in human research, translational work aimed at connecting findings from animal models to humans is necessary to build upon the current knowledge base. Furthermore, the use of voluntary self-administration paradigms and incorporation of individual differences and environmental contexts are important steps forward in improving the validity of animal models of alcohol use and related problems. A more informed understanding of the effects of alcohol on adolescents compared to adults can further prevention efforts and better inform policy efforts aimed at minimizing harm during a crucial period for both social and cognitive development.
Degenhardt L, Charlson F, Ferrari A, Santomauro D, Erskine H, Mantilla-Herrara A, et al. The global burden of disease attributable to alcohol and drug use in 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Psychiatry. 2018;5:987–1012.
Article Google Scholar
Kohn R, Saxena S, Levav I, Saraceno B. The treatment gap in mental health care. World Health Organization; 2004. https://doi.org/10.1590/S0042-96862004001100011 .
Fleury MJ, Djouini A, Huỳnh C, Tremblay J, Ferland F, Ménard JM, et al. Remission from substance use disorders: a systematic review and meta-analysis. Drug Alcohol Depend. 2016;168:293–306.
Article PubMed Google Scholar
Hingson RW, Heeren T, Winter MR. Age of alcohol-dependence onset: associations with severity of dependence and seeking treatment. Pediatrics. 2006;118:e755–e763.
Hingson RW, Heeren T, Winter MR. Age at drinking onset and alcohol dependence: age at onset, duration, and severity. Arch Pediatr Adolesc Med. 2006;160:739–46.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th edn. Arlington, VA: American Psychiatric Association; 2013. https://doi.org/10.1176/appi.books.9780890425596.dsm04 .
Conrod P, Nikolaou K. Annual Research Review: On the developmental neuropsychology of substance use disorders. J Child Psychol Psychiatry Allied Discip. 2016;57:371–94.
Spear LP. Adolescent alcohol exposure: Are there separable vulnerable periods within adolescence? Physiol Behav. 2015;148:122–30.
Article CAS PubMed PubMed Central Google Scholar
Simon NW, Gregory TA, Wood J, Moghaddam B. Differences in response initiation and behavioral flexibility between adolescent and adult rats. Behav Neurosci. 2013;127:23–32.
Article PubMed PubMed Central Google Scholar
Carroll LJ, Cassidy JD, Peloso PM, Borg J, von Holst H, Holm L, et al. Prognosis for mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med 2004(Suppl. 43):84–105.
Mastwal S, Ye Y, Ren M, Jimenez DV, Martinowich K, Gerfen CR, et al. Phasic dopamine neuron activity elicits unique mesofrontal plasticity in adolescence. J Neurosci. 2014;34:9484–96.
Article PubMed PubMed Central CAS Google Scholar
Crone EA, Dahl RE. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nat Rev Neurosci. 2012;13:636–50.
Article CAS PubMed Google Scholar
Vergés A, Haeny AM, Jackson KM, Bucholz KK, Grant JD, Trull TJ, et al. Refining the notion of maturing out: results from the national epidemiologic survey on alcohol and related conditions. Am J Public Health. 2013;103:e67–e73.
Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology 2010;35:217–38.
Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–56.
Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–9.
de Goede J, van der Mark-Reeuwijk KG, Braun KP, le Cessie S, Durston S, Engels RCME, et al. Alcohol and brain development in adolescents and young adults: a systematic review of the literature and advisory report of the health council of the Netherlands. Adv Nutr. 2021. https://doi.org/10.1093/advances/nmaa170 .
Spear LP. Effects of adolescent alcohol consumption on the brain and behaviour. Nat Rev Neurosci. 2018;19:197–214.
Carbia C, López-Caneda E, Corral M, Cadaveira F. A systematic review of neuropsychological studies involving young binge drinkers. Neurosci Biobehav Rev. 2018;90:332–49.
Feldstein Ewing SW, Sakhardande A, Blakemore SJ. The effect of alcohol consumption on the adolescent brain: a systematic review of MRI and fMRI studies of alcohol-using youth. NeuroImage Clin. 2014;5:420–37.
Article PubMed Central Google Scholar
Squeglia LM, Boissoneault J, Van Skike CE, Nixon SJ, Matthews DB. Age-related effects of alcohol from adolescent, adult, and aged populations using human and animal models. Alcohol Clin Exp Res. 2014;38:2509–16.
Lees B, Meredith LR, Kirkland AE, Bryant BE, Squeglia LM. Effect of alcohol use on the adolescent brain and behavior. Pharm Biochem Behav. 2020;192:172906.
Article CAS Google Scholar
Lees B, Mewton L, Stapinski LA, Squeglia LM, Rae CD, Teesson M. Neurobiological and cognitive profile of young binge drinkers: a systematic review and meta-analysis. Neuropsychol Rev. 2019;29:357–85.
Cservenka A, Brumback T. The burden of binge and heavy drinking on the brain: effects on adolescent and young adult neural structure and function. Front Psychol. 2017;8:1111.
Welch KA, Carson A, Lawrie SM. Brain structure in adolescents and young adults with alcohol problems: systematic review of imaging studies. Alcohol Alcohol. 2013;48:433–44.
Maeda K-I, Satoshi O, Hiroko T. Physiology of reproduction. Academic Press; 2000.
Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–63.
Burke AR, Miczek KA. Stress in adolescence and drugs of abuse in rodent models: role of dopamine, CRF, and HPA axis. Psychopharmacology. 2014;231:1557–80.
Doremus-Fitzwater TL, Spear LP. Reward-centricity and attenuated aversions: an adolescent phenotype emerging from studies in laboratory animals. Neurosci Biobehav Rev. 2016;70:121–34.
Rajendran P, Spear LP. The effects of ethanol on spatial and nonspatial memory in adolescent and adult rats studied using an appetitive paradigm. In: Annals of the New York Academy of Sciences. New York Academy of Sciences; 2004. p. 441–4.
Morales M, Schatz KC, Anderson RI, Spear LP, Varlinskaya EI. Conditioned taste aversion to ethanol in a social context: impact of age and sex. Behav Brain Res. 2014;261:323–7.
Dumontheil I. Adolescent brain development. Curr Opin Behav Sci. 2016;10:39–44.
Shillington AM, Woodruff SI, Clapp JD, Reed MB, Lemus H. Self-reported age of onset and telescoping for cigarettes, alcohol, and marijuana: across eight years of the national longitudinal survey of youth. J Child Adolesc Subst Abus. 2012;21:333–48.
Livingston MD, Xu X, Komro KA. Predictors of recall error in self-report of age at alcohol use onset. J Stud Alcohol Drugs. 2016;77:811–8.
De Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol. 2009;14:22–31.
Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–91.
Rodriguiz RM, Wetsel WC. Assessments of cognitive deficits in mutant mice. In: Levin ED, Buccafusco JJ, editors. Animal models of cognitive impairment. CRC Press; 2006. p. 223–82.
Leung RK, Toumbourou JW, Hemphill SA. The effect of peer influence and selection processes on adolescent alcohol use: a systematic review of longitudinal studies. Health Psychol Rev. 2014;8:426–57.
Cousijn J, Luijten M, Feldstein Ewing SW. Adolescent resilience to addiction: a social plasticity hypothesis. Lancet Child Adolesc Heal. 2018;2:69–78.
Kushner MG, Abrams K, Borchardt C. The relationship between anxiety disorders and alcohol use disorders: a review of major perspectives and findings. Clin Psychol Rev. 2000;20:149–71.
Robinson TE, Berridge KC. Review. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci. 2008;363:3137–46.
Vanderschuren LJMJ, Pierce RC. Sensitization processes in drug addiction. Curr Top Behav Neurosci. 2010;3:179–95.
Gorey C, Kuhns L, Smaragdi E, Kroon E, Cousijn J. Age-related differences in the impact of cannabis use on the brain and cognition: a systematic review. Eur Arch Psychiatry Clin Neurosci. 2019;269:37–58.
Schweizer TA, Vogel-Sprott M, Danckert J, Roy EA, Skakum A, Broderick CE. Neuropsychological profile of acute alcohol intoxication during ascending and descending blood alcohol concentrations. Neuropsychopharmacology. 2006;31:1301–9.
Ambrose ML, Bowden SC, Whelan G. Working memory impairments in alcohol-dependent participants without clinical amnesia. Alcohol Clin Exp Res. 2001;25:185–91.
Stavro K, Pelletier J, Potvin S. Widespread and sustained cognitive deficits in alcoholism: a meta-analysis. Addict Biol. 2013;18:203–13.
Spear LP. Adolescent neurobehavioral characteristics, alcohol sensitivities, and intake: setting the stage for alcohol use disorders? Child Dev Perspect. 2011;5:231–8.
Holstein SE, Spanos M, Hodge CW. Adolescent C57BL/6J mice show elevated alcohol intake, but reduced taste aversion, as compared to adult mice: a potential behavioral mechanism for binge drinking. Alcohol Clin Exp Res. 2011;35:1842–51.
Moore EM, Forrest RD, Boehm SL. Genotype modulates age-related alterations in sensitivity to the aversive effects of ethanol: an eight inbred strain analysis of conditioned taste aversion. Genes, Brain Behav. 2013;12:70–77.
Schramm-Sapyta NL, DiFeliceantonio AG, Foscue E, Glowacz S, Haseeb N, Wang N, et al. Aversive effects of ethanol in adolescent versus adult rats: potential causes and implication for future drinking. Alcohol Clin Exp Res. 2010;34:2061–9.
Pautassi RM, Myers M, Spear LP, Molina JC, Spear NE. Ethanol induces second-order aversive conditioning in adolescent and adult rats. Alcohol. 2011;45:45–55.
Carrara-Nascimento PF, Olive MF, Camarini R. Ethanol pre-exposure during adolescence or adulthood increases ethanol intake but ethanol-induced conditioned place preference is enhanced only when pre-exposure occurs in adolescence. Dev Psychobiol. 2014;56:36–48.
Leichtweis KS, Carvalho M, Morais-Silva G, Marin MT, Amaral VCS. Short and prolonged maternal separation impacts on ethanol-related behaviors in rats: sex and age differences. Stress. 2020;23:162–73.
Pautassi RM, Suárez AB, Hoffmann LB, Rueda AV, Rae M, Marianno P, et al. Effects of environmental enrichment upon ethanol-induced conditioned place preference and pre-frontal BDNF levels in adolescent and adult mice. Sci Rep. 2017;7:1–12.
Pascual M, Do Couto BR, Alfonso-Loeches S, Aguilar MA, Rodriguez-Arias M, Guerri C. Changes in histone acetylation in the prefrontal cortex of ethanol-exposed adolescent rats are associated with ethanol-induced place conditioning. Neuropharmacology. 2012;62:2309–19.
Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem. 2009;16:279–88.
Antoniadis EA, McDonald RJ. Amygdala, hippocampus and discriminative fear conditioning to context. Behav Brain Res. 2000;108:1–19.
Marschner A, Kalisch R, Vervliet B, Vansteenwegen D, Büchel C. Dissociable roles for the hippocampus and the amygdala in human cued versus context fear conditioning. J Neurosci. 2008;28:9030–6.
Orsini CA, Maren S. Neural and cellular mechanisms of fear and extinction memory formation. Neurosci Biobehav Rev. 2012;36:1773–802.
Quirk GJ, Garcia R, González-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry. 2006;60:337–43.
Bergstrom HC, McDonald CG, Smith RF. Alcohol exposure during adolescence impairs auditory fear conditioning in adult Long-Evans rats. Physiol Behav. 2006;88:466–72.
Broadwater M, Spear LP. Consequences of ethanol exposure on cued and contextual fear conditioning and extinction differ depending on timing of exposure during adolescence or adulthood. Behav Brain Res. 2013;256:10–19.
Broadwater M, Spear LP. Consequences of adolescent or adult ethanol exposure on tone and context fear retention: effects of an acute ethanol challenge during conditioning. Alcohol Clin Exp Res. 2014;38:1454–60.
Broadwater M, Spear LP. Tone conditioning potentiates rather than overshadows context fear in adult animals following adolescent ethanol exposure. Dev Psychobiol. 2014;56:1150–5.
Lacaille H, Duterte-Boucher D, Liot D, Vaudry H, Naassila M, Vaudry D. Comparison of the deleterious effects of binge drinking-like alcohol exposure in adolescent and adult mice. J Neurochem. 2015;132:629–41.
Markwiese BJ, Acheson SK, Levin ED, Wilson WA, Swartzwelder HS. Differential effects of ethanol on memory in adolescent and adult rats. In: Chapple L, editors. Alcoholism: clinical and Experimental Research. Blackwell Publishing Ltd; 1998. p. 416–21.
Acheson SK, Ross EL, Swartzwelder HS. Age-independent and dose-response effects of ethanol on spatial memory in rats. Alcohol. 2001;23:167–75.
Sircar R, Sircar D. Adolescent rats exposed to repeated ethanol treatment show lingering behavioral impairments. Alcohol Clin Exp Res. 2005;29:1402–10.
Swartzwelder HS, Hogan A, Risher ML, Swartzwelder RA, Wilson WA, Acheson SK. Effect of sub-chronic intermittent ethanol exposure on spatial learning and ethanol sensitivity in adolescent and adult rats. Alcohol. 2014;48:353–60.
Matthews DB, Watson MR, James K, Kastner A, Schneider A, Mittleman G. The impact of low to moderate chronic intermittent ethanol exposure on behavioral endpoints in aged, adult, and adolescent rats. Alcohol. 2019;78:33–42.
Galaj E, Barrera E, Morris D, Ma YY, Ranaldi R. Aberrations in incentive learning and responding to heroin in male rats after adolescent or adult chronic binge-like alcohol exposure. Alcohol Clin Exp Res. 2020;44:1214–23.
Diamond A. Executive functions. 2013;64:135–68. https://doi.org/10.1146/annurev-psych-113011-143750 .
Funahashi S, Andreau JM. Prefrontal cortex and neural mechanisms of executive function. J Physiol Paris. 2013;107:471–82.
Schindler AG, Tsutsui KT, Clark JJ. Chronic alcohol intake during adolescence, but not adulthood, promotes persistent deficits in risk-based decision making. Alcohol Clin Exp Res. 2014;38:1622–9.
Risher ML, Fleming RL, Boutros N, Semenova S, Wilson WA, Levin ED, et al. Long-term effects of chronic intermittent ethanol exposure in adolescent and adult rats: radial-arm maze performance and operant food reinforced responding. PLoS ONE. 2013;8:e62940.
Pickens CL, Cook A, Gaeddert B. Dose-dependent effects of alcohol injections on omission-contingency learning have an inverted-U pattern. Behav Brain Res. 2020;392:112736.
Pickens CL, Kallenberger P, Pajser A, Fisher H. Voluntary alcohol access during adolescence/early adulthood, but not during adulthood, causes faster omission contingency learning. Behav Brain Res. 2019;370:111918.
Mejia-Toiber J, Boutros N, Markou A, Semenova S. Impulsive choice and anxiety-like behavior in adult rats exposed to chronic intermittent ethanol during adolescence and adulthood. Behav Brain Res. 2014;266:19–28.
Fernandez GM, Lew BJ, Vedder LC, Savage LM. Chronic intermittent ethanol exposure leads to alterations in brain-derived neurotrophic factor within the frontal cortex and impaired behavioral flexibility in both adolescent and adult rats. Neuroscience. 2017;348:324–34.
Fernandez GM, Stewart WN, Savage LM. Chronic drinking during adolescence predisposes the adult rat for continued heavy drinking: neurotrophin and behavioral adaptation after long-term, continuous ethanol exposure. PLoS ONE 2016;11. https://doi.org/10.1371/journal.pone.0149987 .
Labots M, Cousijn J, Jolink LA, Leon Kenemans J, Vanderschuren LJMJ, Lesscher HMB. Age-related differences in alcohol intake and control over alcohol seeking in rats. Front Psychiatry. 2018;9:419.
Slawecki CJ, Ehlers CL. Enhanced prepulse inhibition following adolescent ethanol exposure in Sprague-Dawley rats. Alcohol Clin Exp Res. 2005;29:1829–36.
White AM, Ghia AJ, Levin ED, Scott Swartzwelder H. Binge pattern ethanol exposure in adolescent and adult rats: differential impact on subsequent responsiveness to ethanol. Alcohol Clin Exp Res. 2000;24:1251–6.
Baddeley A. Working memory. Science. 1992;255:556–9.
Olton DS, Samuelson RJ. Remembrance of places passed: spatial memory in rats. J Exp Psychol Anim Behav Process. 1976;2:97–116.
Deacon RMJ, Rawlins JNP. T-maze alternation in the rodent. Nat Protoc. 2006;1:7–12.
Knudsen EI. Fundamental components of attention. Annu Rev Neurosci. 2007;30:57–78.
Koch M, Schnitzler HU. The acoustic startle response in rats—circuits mediating evocation, inhibition and potentiation. Behav Brain Res. 1997;89:35–49.
Slawecki CJ, Roth J, Gilder A. Neurobehavioral profiles during the acute phase of ethanol withdrawal in adolescent and adult Sprague-Dawley rats. Behav Brain Res. 2006;170:41–51.
Cunha PJ, Nicastri S, de Andrade AG, Bolla KI. The frontal assessment battery (FAB) reveals neurocognitive dysfunction in substance-dependent individuals in distinct executive domains: abstract reasoning, motor programming, and cognitive flexibility. Addict Behav. 2010;35:875–81.
Jupp B, Dalley JW. Convergent pharmacological mechanisms in impulsivity and addiction: insights from rodent models. Br J Pharm. 2014;171:4729–66.
Dickinson A, Balleine B. Motivational control of goal-directed action. Anim Learn Behav. 1994;22:1–18.
Tomie A, Sharma N. Pavlovian sign-tracking model of alcohol abuse. Curr Drug Abus Rev. 2013;6:201–19.
Tomie A, Jeffers P, Zito B. Sign-tracking model of the addiction blind spot. In: Tomie JMA, editors. Sign tracking and drug addiction. Maize Books; 2018. p. 8–34.
Castillo-Carniglia A, Keyes KM, Hasin DS, Cerdá M. Psychiatric comorbidities in alcohol use disorder. Lancet Psychiatry. 2019;6:1068–80.
Wolitzky-Taylor K, Bobova L, Zinbarg RE, Mineka S, Craske MG. Longitudinal investigation of the impact of anxiety and mood disorders in adolescence on subsequent substance use disorder onset and vice versa. Addict Behav. 2012;37:982–5.
Park J, Moghaddam B. Impact of anxiety on prefrontal cortex encoding of cognitive flexibility. Neuroscience 2017;345:193–202.
Vytal KE, Cornwell BR, Letkiewicz AM, Arkin NE, Grillon C. The complex interaction between anxiety and cognition: Insight from spatial and verbal working memory. Front Hum Neurosci. 2013;7:93.
Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33.
Pellow S, Chopin P, File SE, Briley M. Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–67.
Elsey JWB, Kindt M. Startle reflex. In: Zeigler-Hill V, Shackelford TK, editors. Encyclopedia of personality and individual differences. Springer International Publishing; 2018. p. 1–5.
Crawley J, Goodwin FK. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharm Biochem Behav. 1980;13:167–70.
File SE, Seth P. A review of 25 years of the social interaction test. Eur J Pharm. 2003;463:35–53.
Misslin R, Ropartz P. Responses in mice to a novel object author. Behavior. 1981;78:169–77.
Njung’e K, Handley SL. Evaluation of marble-burying behavior as a model of anxiety. Pharm Biochem Behav. 1991;38:63–67.
Lee KM, Coelho MA, McGregor HA, Solton NR, Cohen M, Szumlinski KK. Adolescent mice are resilient to alcohol withdrawal-induced anxiety and changes in indices of glutamate function within the nucleus accumbens. Front Cell Neurosci. 2016;10:265.
Agoglia AE, Holstein SE, Reid G, Hodge CW. CaMKIIα-GluA1 activity underlies vulnerability to adolescent binge alcohol drinking. Alcohol Clin Exp Res. 2015;39:1680–90.
Van Skike CE, Diaz-Granados JL, Matthews DB. Chronic intermittent ethanol exposure produces persistent anxiety in adolescent and adult rats. Alcohol Clin Exp Res. 2015;39:262–71.
Conrad KL, Winder DG. Altered anxiety-like behavior and long-term potentiation in the bed nucleus of the stria terminalis in adult mice exposed to chronic social isolation, unpredictable stress, and ethanol beginning in adolescence. Alcohol. 2011;45:585–93.
Slawecki CJ, Roth J. Comparison of the onset of hypoactivity and anxiety-like behavior during alcohol withdrawal adolescent and adult rats. Alcohol Clin Exp Res. 2004;28:598–607.
Wille-Bille A, de Olmos S, Marengo L, Chiner F, Pautassi RM. Long-term ethanol self-administration induces ΔFosB in male and female adolescent, but not in adult, Wistar rats. Prog Neuro-Psychopharmacol Biol Psychiatry. 2017;74:15–30.
Varlinskaya EI, Spear LP. Chronic tolerance to the social consequences of ethanol in adolescent and adult Sprague-Dawley rats. Neurotoxicol Teratol. 2007;29:23–30.
Morales M, Varlinskaya EI, Spear LP. Age differences in the expression of acute and chronic tolerance to ethanol in male and female rats. Alcohol Clin Exp Res. 2011;35:1614–24.
PubMed PubMed Central Google Scholar
Neuhofer D, Kalivas P. Metaplasticity at the addicted tetrapartite synapse: a common denominator of drug induced adaptations and potential treatment target for addiction. Neurobiol Learn Mem. 2018;154:97–111.
Pian JP, Criado JR, Milner R, Ehlers CL. N-methyl-d-aspartate receptor subunit expression in adult and adolescent brain following chronic ethanol exposure. Neuroscience. 2010;170:645–54.
Falco AM, Bergstrom HC, Bachus SE, Smith RF. Persisting changes in basolateral amygdala mRNAs after chronic ethanol consumption. Physiol Behav. 2009;96:169–73.
Chin VS, Van Skike CE, Berry RB, Kirk RE, Diaz-Granados J, Matthews DB. Effect of acute ethanol and acute allopregnanolone on spatial memory in adolescent and adult rats. Alcohol. 2011;45:473–83.
Pascual M, Boix J, Felipo V, Guerri C. Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. J Neurochem. 2009;108:920–31.
Akkus F, Mihov Y, Treyer V, Ametamey SM, Johayem A, Senn S, et al. Metabotropic glutamate receptor 5 binding in male patients with alcohol use disorder. Transl Psychiatry 2018;8. https://doi.org/10.1038/s41398-017-0066-6 .
Leurquin-Sterk G, Ceccarini J, Crunelle CL, De Laat B, Verbeek J, Deman S, et al. Lower limbic metabotropic glutamate receptor 5 availability in alcohol dependence. J Nucl Med. 2018;59:682–90.
Davies M. The role of GABAA receptors in mediating the effects of alcohol in the central nervous system. J Psychiatry Neurosci. 2003;28:263–74.
Grobin AC, Matthews DB, Montoya D, Wilson WA, Morrow AL, Swartzwelder HS. Age-related differences in neurosteroid potentiation of muscimol-stimulated 36Cl- flux following chronic ethanol treatment. Neuroscience. 2001;105:547–52.
Fleming RL, Acheson SK, Moore SD, Wilson WA, Swartzwelder HS. GABA transport modulates the ethanol sensitivity of tonic inhibition in the rat dentate gyrus. Alcohol. 2011;45:577–83.
Fleming RL, Li Q, Risher ML, Sexton HG, Moore SD, Wilson WA, et al. Binge-pattern ethanol exposure during adolescence, but not adulthood, causes persistent changes in GABAA receptor-mediated tonic inhibition in dentate granule cells. Alcohol Clin Exp Res. 2013;37:1154–60.
Carrara-Nascimento PF, Hoffmann LB, Flório JC, Planeta CS, Camarini R. Effects of ethanol exposure during adolescence or adulthood on locomotor sensitization and dopamine levels in the reward system. Front Behav Neurosci. 2020;14:31.
Picciotto MR, Higley MJ, Mineur YS. Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron 2012;76:116–29.
Wu J, Gao M, Taylor DH. Neuronal nicotinic acetylcholine receptors are important targets for alcohol reward and dependence. Acta Pharmacol Sin. 2014;35:311–5.
Walker LC, Berizzi AE, Chen NA, Rueda P, Perreau VM, Huckstep K, et al. Acetylcholine muscarinic M4 receptors as a therapeutic target for alcohol use disorder: converging evidence from humans and rodents. Biol Psychiatry. 2020;88:898–909.
Vetreno RP, Broadwater M, Liu W, Spear LP, Crews FT. Adolescent, but not adult, binge ethanol exposure leads to persistent global reductions of choline acetyltransferase expressing neurons in brain. PLoS ONE. 2014;9:113421.
Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3:760–73.
Huang C, Titus JA, Bell RL, Kapros T, Chen J, Huang R. A mouse model for adolescent alcohol abuse: stunted growth and effects in brain. Alcohol Clin Exp Res. 2012;36:1728–37.
Crews FT, Braun CJ, Hoplight B, Switzer RC, Knapp DJ. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcohol Clin Exp Res. 2000;24:1712–23.
Broadwater MA, Liu W, Crews FT, Spear LP. Persistent loss of hippocampal neurogenesis and increased cell death following adolescent, but not adult, chronic ethanol exposure. Dev Neurosci. 2014;36:297–305.
Nixon K, Kim DH, Potts EN, He J, Crews FT. Distinct cell proliferation events during abstinence after alcohol dependence: microglia proliferation precedes neurogenesis. Neurobiol Dis. 2008;31:218–29.
Camp MC, Mayfield RD, McCracken M, McCracken L, Alcantara AA. Neuroadaptations of Cdk5 in cholinergic interneurons of the nucleus accumbens and prefrontal cortex of inbred alcohol-preferring rats following voluntary alcohol drinking. Alcohol Clin Exp Res. 2006;30:1322–35.
Goulding SP, de Guglielmo G, Carrette LLG, George O, Contet C. Systemic administration of the cyclin-dependent kinase inhibitor (S)-CR8 selectively reduces escalated ethanol intake in dependent rats. Alcohol Clin Exp Res. 2019;43:2079–89.
Joe KH, Kim YK, Kim TS, Roh SW, Choi SW, Kim YB, et al. Decreased plasma brain-derived neurotrophic factor levels in patients with alcohol dependence. Alcohol Clin Exp Res. 2007;31:1833–8.
Huang MC, Chen CH, Chen CH, Liu SC, Ho CJ, Shen WW, et al. Alterations of serum brain-derived neurotrophic factor levels in early alcohol withdrawal. Alcohol Alcohol. 2008;43:241–5.
Miller R, King MA, Heaton MB, Walker DW. The effects of chronic ethanol consumption on neurotrophins and their receptors in the rat hippocampus and basal forebrain. Brain Res. 2002;950:137–47.
Vetreno RP, Crews FT. Binge ethanol exposure during adolescence leads to a persistent loss of neurogenesis in the dorsal and ventral hippocampus that is associated with impaired adult cognitive functioning. Front Neurosci. 2015;9:35.
Robison AJ, Nestler EJ. Transcriptional and epigenetic mechanisms of addiction. Nat Rev Neurosci. 2011;12:623–37.
Faria RR, Lima Rueda AV, Sayuri C, Soares SL, Malta MB, Carrara-Nascimento PF, et al. Environmental modulation of ethanol-induced locomotor activity: Correlation with neuronal activity in distinct brain regions of adolescent and adult Swiss mice. Brain Res. 2008;1239:127–40.
Crews FT, Bechara R, Brown LA, Guidot DM, Mandrekar P, Oak S, et al. Cytokines and alcohol. In: Alcoholism: clinical and experimental research. John Wiley & Sons, Ltd; 2006. p. 720–30.
Davis RL, Syapin PJ. Chronic ethanol inhibits CXC chemokine ligand 10 production in human A172 astroglia and astroglial-mediated leukocyte chemotaxis. Neurosci Lett. 2004;362:220–5.
Knapp DJ, Crews FT. Induction of cyclooxygenase-2 in brain during acute and chronic ethanol treatment and ethanol withdrawal. Alcohol Clin Exp Res. 1999;23:633–43.
Marshall SA, McClain JA, Wooden JI, Nixon K. Microglia dystrophy following binge-like alcohol exposure in adolescent and adult male rats. Front Neuroanat. 2020;14:52.
Agrawal RG, Owen JA, Levin PS, Hewetson A, Berman AE, Franklin SR, et al. Bioinformatics analyses reveal age-specific neuroimmune modulation as a target for treatment of high ethanol drinking. Alcohol Clin Exp Res. 2014;38:428–37.
Kane CJM, Phelan KD, Douglas JC, Wagoner G, Johnson JW, Xu J, et al. Effects of ethanol on immune response in the brain: region-specific changes in adolescent versus adult mice. Alcohol Clin Exp Res. 2014;38:384–91.
Blaine SK, Sinha R. Alcohol, stress, and glucocorticoids: from risk to dependence and relapse in alcohol use disorders. Neuropharmacology 2017;122:136–47.
Slawecki CJ, Jiménez-Vasquez P, Mathé AA, Ehlers CL. Effect of ethanol on brain neuropeptides in adolescent and adult rats. J Stud Alcohol. 2005;66:46–52.
van den Pol AN. Neuropeptide transmission in brain circuits. Neuron. 2012;76:98–115.
Souza-Moreira L, Campos-Salinas J, Caro M, Gonzalez-Rey E. Neuropeptides as pleiotropic modulators of the immune response. Neuroendocrinology. 2011;94:89–100.
Carniglia L, Ramírez D, Durand D, Saba J, Turati J, Caruso C, et al. Neuropeptides and microglial activation in inflammation, pain, and neurodegenerative diseases. Mediators Inflamm. 2017;2017. https://doi.org/10.1155/2017/5048616 .
Hipolito L, Sanchez M, Polache A, Granero L. Brain metabolism of ethanol and alcoholism: an update. Curr Drug Metab. 2007;8:716–27.
Rhoads DE, Contreras C, Fathalla S. Brain levels of catalase remain constant through strain, developmental, and chronic alcohol challenges. Enzyme Res. 2012;2012. https://doi.org/10.1155/2012/572939 .
Vasiliou V, Ziegler TL, Bludeau P, Petersen DR, Gonzalez FJ, Deitrich RA. CYP2E1 and catalase influence ethanol sensitivity in the central nervous system. Pharmacogenet Genomics. 2006;16:51–58.
Zimatkin SM, Buben AI. Ethanol oxidation in the living brain. Alcohol Alcohol. 2007;42:529–32.
Hargreaves GA, Quinn H, Kashem MA, Matsumoto I, McGregor IS. Proteomic analysis demonstrates adolescent vulnerability to lasting hippocampal changes following chronic alcohol consumption. Alcohol Clin Exp Res. 2009;33:86–94.
Galaj E, Guo C, Huang D, Ranaldi R, Ma YY. Contrasting effects of adolescent and early-adult ethanol exposure on prelimbic cortical pyramidal neurons. Drug Alcohol Depend. 2020;216:108309.
Li Q, Fleming RL, Acheson SK, Madison RD, Moore SD, Risher ML, et al. Long-term modulation of A-type K+ conductances in hippocampal CA1 interneurons in rats after chronic intermittent ethanol exposure during adolescence or adulthood. Alcohol Clin Exp Res. 2013;37:2074–85.
Artinian J, Lacaille JC. Disinhibition in learning and memory circuits: new vistas for somatostatin interneurons and long-term synaptic plasticity. Brain Res Bull. 2018;141:20–26.
Müller-Oehring EM, Kwon D, Nagel BJ, Sullivan EV, Chu W, Rohlfing T, et al. Influences of age, sex, and moderate alcohol drinking on the intrinsic functional architecture of adolescent brains. Cereb Cortex. 2018;28:1049–63.
McAteer AM, Hanna D, Curran D. Age-related differences in alcohol attention bias: a cross-sectional study. Psychopharmacology. 2018;235:2387–93.
Rooke SE, Hine DW. A dual process account of adolescent and adult binge drinking. Addict Behav. 2011;36:341–6.
Cousijn J, Green KH, Labots M, Vanderschuren LJMJ, Kenemans JL, Lesscher HMB. Motivational and control mechanisms underlying adolescent versus adult alcohol use. NeuroSci. 2020;1:44–58.
Field M, Cox WM. Attentional bias in addictive behaviors: a review of its development, causes, and consequences. Drug Alcohol Depend. 2008;97:1–20.
Cousijn J, van Benthem P, van der Schee E, Spijkerman R. Motivational and control mechanisms underlying adolescent cannabis use disorders: a prospective study. Dev Cogn Neurosci. 2015;16:36–45.
Saalfield J, Spear L. Consequences of repeated ethanol exposure during early or late adolescence on conditioned taste aversions in rats. Dev Cogn Neurosci. 2015;16:174–82.
Saalfield J, Spear L. The ontogeny of ethanol aversion. Physiol Behav. 2016;156:164–70.
McQuown SC, Wood MA. Epigenetic regulation in substance use disorders. Curr Psychiatry Rep. 2010;12:145–53.
Renthal W, Nestler EJ. Epigenetic mechanisms in drug addiction. Trends Mol Med. 2008;14:341–50.
Logrip ML, Barak S, Warnault V, Ron D. Corticostriatal BDNF and alcohol addiction. Brain Res. 2015;1628:60–67.
Dalley JW, Roiser JP. Dopamine, serotonin and impulsivity. Neuroscience 2012;215:42–58.
Robinson OJ, Pike AC, Cornwell B, Grillon C. The translational neural circuitry of anxiety. J Neurol Neurosurg Psychiatry. 2019;90:1353–60.
PubMed Google Scholar
Martínez G, Ropero C, Funes A, Flores E, Blotta C, Landa AI, et al. Effects of selective NMDA and non-NMDA blockade in the nucleus accumbens on the plus-maze test. Physiol Behav. 2002;76:219–24.
Bergink V, Van Megen HJGM, Westenberg HGM. Glutamate and anxiety. Eur Neuropsychopharmacol. 2004;14:175–83.
Scott AJ, Jordan M, Lueras BJN. Effects of binge drinking on the developing brain. Alcohol Res. 2018;39:87–96.
Google Scholar
Krieger H, Young CM, Anthenien AM, Neighbors C. The epidemiology of binge drinking among college-age individuals in the United States. Alcohol Res. 2018;39:23–30.
Hooijmans CR, Rovers MM, De Vries RBM, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14:1–9.
Krauth D, Woodruff TJ, Bero L. Instruments for assessing risk of bias and other methodological criteria of published animal studies: a systematic review. Environ Health Perspect. 2013;121:985–92.
Field M, Kersbergen I. Are animal models of addiction useful? Addiction. 2020;115:6–12.
Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, et al. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–23.
Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84:53–63.
Spoelder M, Hesseling P, Baars AM, Lozeman-van’t Klooster JG, Rotte MD, Vanderschuren LJMJ, et al. Individual variation in alcohol intake predicts reinforcement, motivation, and compulsive alcohol use in rats. Alcohol Clin Exp Res. 2015;39:2427–37.
Fredriksson I, Venniro M, Reiner DJ, Chow JJ, Bossert JM, Shaham Y. Animal models of drug relapse and craving after voluntary abstinence: a review. Pharm Rev. 2021;73:1050–83.
Vanderschuren LJMJ, Ahmed SH. Animal models of the behavioral symptoms of substance use disorders. Cold Spring Harb Perspect Med. 2021;11:a040287.
Kuhn BN, Kalivas PW, Bobadilla AC. Understanding addiction using animal models. Front Behav Neurosci. 2019;13. https://doi.org/10.3389/fnbeh.2019.00262 .
Venniro M, Shaham Y. An operant social self-administration and choice model in rats. Nat Protoc. 2020;15:1542–59.
Thapar A, Collishaw S, Pine DS, Thapar AK. Depression in adolescence. Lancet 2012;379:1056–67.
Costello EJ, Egger H, Angold A. 10-Year research update review: the epidemiology of child and adolescent psychiatric disorders: I. Methods and public health burden. J Am Acad Child Adolesc Psychiatry. 2005;44:972–86.
Snyder HR, Kaiser RH, Whisman MA, Turner AEJ, Guild RM, Munakata Y. Opposite effects of anxiety and depressive symptoms on executive function: the case of selecting among competing options. 2014;28:893–902. https://doi.org/10.1080/026999312013859568 .
McDermott LM, Ebmeier KP. A meta-analysis of depression severity and cognitive function. J Affect Disord. 2009;119:1–8.
Volkow ND, Koob GF, Croyle RT, Bianchi DW, Gordon JA, Koroshetz WJ, et al. The conception of the ABCD study: from substance use to a broad NIH collaboration. Dev Cogn Neurosci. 2018;32:4–7.
Download references
Acknowledgements
This work was supported by grant 1RO1 DA042490-01A1 awarded to Janna Cousijn and Francesca Filbey from the National Institute on Drug Abuse/National Institutes of Health. The grant supported the salaries of authors Lauren Kuhns, Emese Kroon, and Janna Cousijn. Thank you to Claire Gorey (CG) for running the initial search and aiding in the screening process.
Author information
Authors and affiliations.
Neuroscience of Addiction (NofA) Lab, Department of Psychology, University of Amsterdam, Amsterdam, The Netherlands
Lauren Kuhns, Emese Kroon, Gabry Mies & Janna Cousijn
The Amsterdam Brain and Cognition Center (ABC), University of Amsterdam, Amsterdam, The Netherlands
Lauren Kuhns, Emese Kroon & Janna Cousijn
Department of Animals in Science and Society, Division of Behavioural Neuroscience, Faculty of Veterinary Medicine, Utrecht University, Utrecht, The Netherlands
Heidi Lesscher
Department of Psychology, Education & Child Studies, Erasmus University Rotterdam, Rotterdam, The Netherlands
Janna Cousijn
You can also search for this author in PubMed Google Scholar
Contributions
LK conducted the systematic searches; LK, EK, GM, and JC screened the citations for exclusion and inclusion; LK, EK, HL, and JC wrote the review; LK, EK, HL, GM, and JC revised the manuscript.
Corresponding author
Correspondence to Lauren Kuhns .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Kuhns, L., Kroon, E., Lesscher, H. et al. Age-related differences in the effect of chronic alcohol on cognition and the brain: a systematic review. Transl Psychiatry 12 , 345 (2022). https://doi.org/10.1038/s41398-022-02100-y
Download citation
Received : 26 October 2021
Revised : 21 June 2022
Accepted : 28 July 2022
Published : 25 August 2022
DOI : https://doi.org/10.1038/s41398-022-02100-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

Binge drinking is a growing public health crisis − a neurobiologist explains how research on alcohol use disorder has shifted
Assistant Professor of Biology, Biomedical Engineering and Pharmacology, Penn State
Disclosure statement
Nikki Crowley receives funding from The National Institutes of Health, The Brain and Behavior Research Foundation, and the Penn State Huck Institutes of the Life Sciences endowment funds.
Penn State provides funding as a founding partner of The Conversation US.
View all partners
With the new Amy Winehouse biopic “Back to Black ” in U.S. theaters as of May 17, 2024, the late singer’s relationship with alcohol and drugs is under scrutiny again. In July 2011, Winehouse was found dead in her flat in north London from “death by misadventure” at the age of 27. That’s the official British term used for accidental death caused by a voluntary risk.
Her blood alcohol concentration was 0.416%, more than five times the legal intoxication limit in the U.S. – leading her cause of death to be later adjusted to include “alcohol toxicity” following a second coroner’s inquest.
Nearly 13 years later, alcohol consumption and binge drinking remain a major public health crisis , not just in the U.K. but also in the U.S.
Roughly 1 in 5 U.S. adults report binge drinking at least once a week, with an average of seven drinks per binge episode . This is well over the amount of alcohol thought to produce legal intoxication, commonly defined as a blood alcohol concentration over 0.08% – on average, four drinks in two hours for women, five drinks in two hours for men.
Among women, days of “heavy drinking” increased 41% during the COVID-19 pandemic compared with pre-pandemic levels , and adult women in their 30s and 40s are rapidly increasing their rates of binge drinking , with no evidence of these trends slowing down. Despite efforts to comprehend the overall biology of substance use disorders, scientists’ and physicians’ understanding of the relationship between women’s health and binge drinking has lagged behind.
I am a neurobiologist focused on understanding the chemicals and brain regions that underlie addiction to alcohol . I study how neuropeptides – unique signaling molecules in the prefrontal cortex , one of the key brain regions in decision-making, risk-taking and reward – are altered by repeated exposure to binge alcohol consumption in animal models.
My lab focuses on understanding how things like alcohol alter these brain systems before diagnosable addiction, so that we can better inform efforts toward both prevention and treatment.
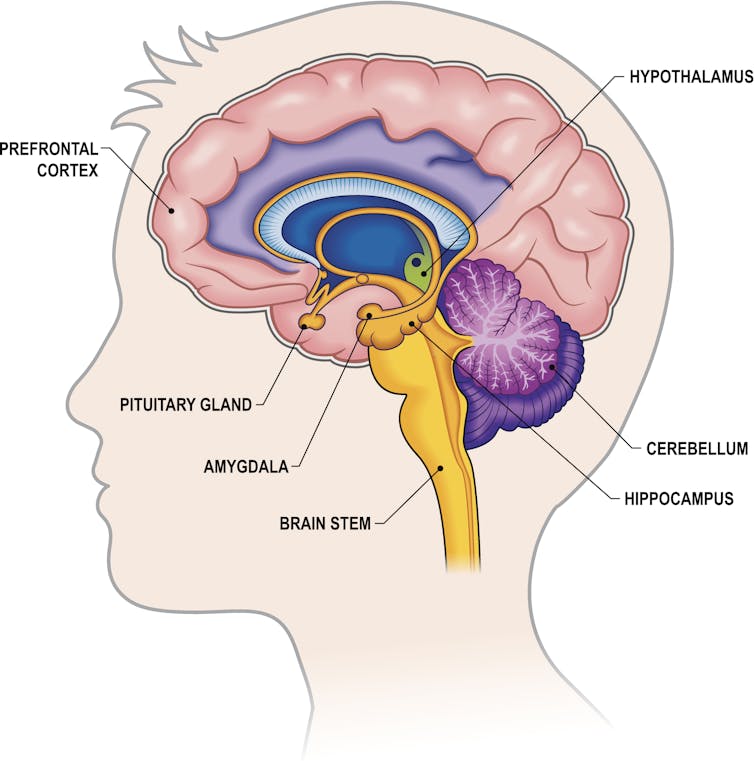
The biology of addiction
While problematic alcohol consumption has likely occurred as long as alcohol has existed, it wasn’t until 2011 that the American Society of Addiction Medicine recognized substance addiction as a brain disorder – the same year as Winehouse’s death. A diagnosis of an alcohol use disorder is now used over outdated terms such as labeling an individual as an alcoholic or having alcoholism.
Researchers and clinicians have made great strides in understanding how and why drugs – including alcohol, a drug – alter the brain. Often, people consume a drug like alcohol because of the rewarding and positive feelings it creates, such as enjoying drinks with friends or celebrating a milestone with a loved one. But what starts off as manageable consumption of alcohol can quickly devolve into cycles of excessive alcohol consumption followed by drug withdrawal.
While all forms of alcohol consumption come with health risks, binge drinking appears to be particularly dangerous due to how repeated cycling between a high state and a withdrawal state affect the brain. For example, for some people, alcohol use can lead to “ hangxiety ,” the feeling of anxiety that can accompany a hangover.
Repeated episodes of drinking and drunkenness, coupled with withdrawal, can spiral, leading to relapse and reuse of alcohol. In other words, alcohol use shifts from being rewarding to just trying to prevent feeling bad.
It makes sense. With repeated alcohol use over time, the areas of the brain engaged by alcohol can shift away from those traditionally associated with drug use and reward or pleasure to brain regions more typically engaged during stress and anxiety .
All of these stages of drinking, from the enjoyment of alcohol to withdrawal to the cycles of craving, continuously alter the brain and its communication pathways . Alcohol can affect several dozen neurotransmitters and receptors , making understanding its mechanism of action in the brain complicated.
Work in my lab focuses on understanding how alcohol consumption changes the way neurons within the prefrontal cortex communicate with each other. Neurons are the brain’s key communicator, sending both electrical and chemical signals within the brain and to the rest of your body.
What we’ve found in animal models of binge drinking is that certain subtypes of neurons lose the ability to talk to each other appropriately. In some cases, binge drinking can permanently remodel the brain. Even after a prolonged period of abstinence, conversations between the neurons don’t return to normal .
These changes in the brain can appear even before there are noticeable changes in behavior . This could mean that the neurobiological underpinnings of addiction may take root well before an individual or their loved ones suspect a problem with alcohol.
Researchers like us don’t yet fully understand why some people may be more susceptible to this shift, but it likely has to do with genetic and biological factors, as well as the patterns and circumstances under which alcohol is consumed.
Women are forgotten
While researchers are increasingly understanding the medley of biological factors that underlie addiction, there’s one population that’s been largely overlooked until now: women.
Women may be more likely than men to have some of the most catastrophic health effects caused by alcohol use, such as liver issues, cardiovascular disease and cancer . Middle-aged women are now at the highest risk for binge drinking compared with other populations.
When women consume even moderate levels of alcohol, their risk for various cancers goes up, including digestive, breast and pancreatic cancer , among other health problems – and even death. So the worsening rates of alcohol use disorder in women prompt the need for a greater focus on women in the research and the search for treatments.
Yet, women have long been underrepresented in biomedical research.
It wasn’t until 1993 that clinical research funded by the National Institutes of Health was required to include women as research subjects. In fact, the NIH did not even require sex as a biological variable to be considered by federally funded researchers until 2016. When women are excluded from biomedical research, it leaves doctors and researchers with an incomplete understanding of health and disease, including alcohol addiction.
There is also increasing evidence that addictive substances can interact with cycling sex hormones such as estrogen and progesterone . For instance, research has shown that when estrogen levels are high, like before ovulation, alcohol might feel more rewarding , which could drive higher levels of binge drinking. Currently, researchers don’t know the full extent of the interaction between these natural biological rhythms or other unique biological factors involved in women’s health and propensity for alcohol addiction.

Looking ahead
Researchers and lawmakers are recognizing the vital need for increased research on women’s health. Major federal investments into women’s health research are a vital step toward developing better prevention and treatment options for women.
While women like Amy Winehouse may have been forced to struggle both privately and publicly with substance use disorders and alcohol, the increasing focus of research on addiction to alcohol and other substances as a brain disorder will open new treatment avenues for those suffering from the consequences.
For more information on alcohol use disorder, causes, prevention and treatments, visit the National Institute on Alcohol Abuse and Alcoholism .
- Amy Winehouse
- Binge drinking
- Neurobiology
- Intoxication
- Alcohol consumption
- Alcohol use
- Alcohol use disorder
- COVID-19 pandemic
Want to write?
Write an article and join a growing community of more than 183,700 academics and researchers from 4,959 institutions.
Register now

Alcohol and Your Brain: The Latest Scientific Insights
Want to protect your brain here's what you need to know about alcohol consumption..
Posted March 18, 2024 | Reviewed by Devon Frye
- What Is Alcoholism?
- Find a therapist to overcome addiction
- Transient memory loss, “blackouts,” and hangovers related to alcohol consumption are brain health risks.
- Alcohol use disorder (alcoholism) is a risk factor for developing dementia.
- Heavy or excessive alcohol consumption is dangerous to the brain for a number of reasons.
- The impact of mild to moderate alcohol consumption (1-3 drinks a day) on brain function is less clear.

Depending on who you ask, you might be told to drink a few glasses of red wine a day or to avoid alcohol altogether. The reasons for such recommendations are many, but, by and large, they tend to stem from a study someone read about or saw reported in the news.
So why is it so hard to know whether alcohol is good or bad for us—especially for our brains? In this post, we’ll explore the current science and some practical ideas on how to approach the topic.
What Is Alcohol Anyway?
When people talk about drinking “alcohol,” they’re almost always referring to the consumption of ethanol. Ethanol is a natural product that is formed from the fermentation of grains, fruits, and other sources of sugar. It’s found in a wide range of alcoholic beverages including beer, wine, and spirits like vodka, whiskey, rum, and gin.
Evidence for human consumption of alcohol dates back over 10,000 years. Consumption of alcohol has and continues to serve major roles in religious and cultural ceremonies around the world. But unlike most food products, in the last century, alcohol has been wrapped up in nearly perpetual controversy over its moral effects and health implications.
How Does Alcohol Impact the Brain?
As anyone who’s consumed alcohol knows, ethanol can directly influence brain function. Ethanol is classified as a “depressant” because it has a generally slowing effect on brain activity through activation of γ-aminobutyric acid (GABA) pathways.
In an acute sense, consumption of alcohol can lead to uninhibited behavior, sedation, lapses in judgment, and impairments in motor function. At higher levels, the effects can progress to coma and even death.
The Known Brain-Damaging Effects of Excess Alcohol
There is no debate here: Excessively high levels of alcohol consumption over short periods of time are toxic and potentially deadly, specifically because of its effects on the brain.
One critical fact to understand about the overall and brain-specific effects of alcohol is that the entirety of the debate around the risk/benefit ratio concerns mild to moderate alcohol consumption. As it relates to the effects of high amounts of alcohol on the body and brain, the research is consistent: It’s a very bad choice.
High amounts of alcohol use are causal risk factors in the development of disease in the heart, liver, pancreas, and brain (including the brains of children in utero). In fact, 1 in 8 deaths in Americans aged 20-64 is attributable to alcohol use. When it comes to adults, excessive alcohol use can cause multiple well-defined brain issues ranging from short-term confusion to dementia .
What Is “Excessive” or “High” Alcohol Use?
Key to the nuance in the conversation about alcohol use are definitions. Across the board, “excessive” or “high” alcohol use is linked to worse overall and brain health outcomes. So what does that mean?
While definitions can be variable, one way to look at this is the consumption of 4 or more drinks on an occasion (for women) and 5 or more for men. Additionally, excess alcohol is defined as drinking more than 8 drinks a week (women) and 15 a week (men), or consuming alcohol if you are pregnant or younger than age 21.
Beyond this, by definition, consuming enough alcohol to cause a “brownout,” “blackout,” hangover, or other overt brain symptomatology is evidence that the alcohol you’ve consumed is creating problems in your brain. Alcohol use disorder (or alcoholism ) is also a clear issue for the brain. It has been linked to a higher risk for dementia, especially early-onset dementia in a study of 262,000 adults, as well as to smaller brain size .
Is There a “Safe” Amount of Alcohol for the Brain?
In a highly publicized article from Nature Communications , researchers looked at brain imaging data from nearly 37,000 middle-aged to older adults and cross-referenced their brain scans with their reported alcohol consumption. The findings were profound: People who drank more alcohol had smaller brains, even in people drinking only one or two alcoholic beverages a day.

Conversely, other recent data suggest a lower risk for dementia in people consuming a few alcoholic beverages a day. This includes a 2022 study showing that in around 27,000 people, consuming up to 40 grams of alcohol (around 2.5 drinks) a day was linked to a lower risk for dementia versus abstinence in adults over age 60. A much larger study of almost 4 million people in Korea noted that mild to moderate alcohol consumption was linked to a lower risk for dementia compared to non-drinking.
How Do We Make Sense of This Data?
When it comes to the bottom line as it relates to alcohol consumption and brain health, the data are rather solid on some fronts, and a bit less so on others. There’s also the potential for confounding variables, including the fact that many people like to drink alcohol to enjoy and enhance social bonds (which we know are beneficial for the brain). Here’s a summary of what the most recent research is telling us.
- Experiencing transient memory loss, “blackouts,” or hangovers related to alcohol consumption is overt evidence of threats to brain health.
- The impact of mild to moderate alcohol consumption (1-3 drinks a day) on brain function is less clear, but it seems unreasonable to start alcohol use for brain health.

Austin Perlmutter, M.D. , is a board-certified internal medicine physician and the co-author of Brain Wash .
- Find a Therapist
- Find a Treatment Center
- Find a Psychiatrist
- Find a Support Group
- Find Online Therapy
- United States
- Brooklyn, NY
- Chicago, IL
- Houston, TX
- Los Angeles, CA
- New York, NY
- Portland, OR
- San Diego, CA
- San Francisco, CA
- Seattle, WA
- Washington, DC
- Asperger's
- Bipolar Disorder
- Chronic Pain
- Eating Disorders
- Passive Aggression
- Personality
- Goal Setting
- Positive Psychology
- Stopping Smoking
- Low Sexual Desire
- Relationships
- Child Development
- Self Tests NEW
- Therapy Center
- Diagnosis Dictionary
- Types of Therapy

At any moment, someone’s aggravating behavior or our own bad luck can set us off on an emotional spiral that threatens to derail our entire day. Here’s how we can face our triggers with less reactivity so that we can get on with our lives.
- Emotional Intelligence
- Gaslighting
- Affective Forecasting
- Neuroscience
Featured Topics
Featured series.
A series of random questions answered by Harvard experts.
Explore the Gazette
Read the latest.

How do you read organization’s silence over rise of Nazism?

Got milk? Does it give you problems?


Cancer risk, wine preference, and your genes
Alcohol is dangerous. so is ‘alcoholic.’.

Harvard Staff Writer
Researcher explains the human toll of language that makes addiction feel worse
When Mass General transplant hepatologist Wei Zhang says he wants his colleagues to think before they speak, he has the tragedy of a recent patient in mind.
Admitted to intensive care for advanced alcohol-associated liver disease, the 36-year-old woman hid the truth when asked about her drinking. “She was like, ‘No, I quit over a year ago, I didn’t drink at all,’” said Zhang, also director of the hospital’s Alcohol-Associated Liver Disease Clinic. “But we have tools that can detect the use of alcohol in the past three, four weeks.”
The patient, who had been traumatized by years of physical abuse, was denied a liver transplant, in part because she withheld information about her alcohol use. Her death days later was “a consequence of stigma,” Zhang said. Patients too often “feel they’re being judged and may fear that their condition is seen as a result of personal failing rather than a medical issue that needs treatment.”
Amid increases in high-risk drinking and alcohol-associated liver disease across the country , he hopes that new research can help complete the years-long work of erasing that stigma, saving lives in the process.
For decades, medical terminology has labeled liver disease and other alcohol-related conditions as “alcoholic”: alcoholic liver disease, alcoholic hepatitis, alcoholic cirrhosis, alcoholic pancreatitis. Meanwhile, clinicians and administrators have described patients as addicts and alcoholics.
More recently, specialists and advocates have sought with some success to revise how we talk about substance use and those struggling to overcome it, not just to reduce stigma but also to combat bias among medical professionals. According to the National Institute on Alcohol Abuse and Alcoholism , the term “alcohol use disorder” is now preferable to “alcohol abuse,” “alcohol dependence,” and “alcoholism.”
“Emphasizing non-stigmatizing language is crucial not only for fostering honesty but also for supporting the overall treatment process and patient outcomes,” Zhang said.

The new study is a step toward that goal. Inspired by his patients, Zhang set out to observe whether the terminology used by institutions that treat alcohol-associated liver disease reflects or rejects stigma. He and his team reviewed messages on more than 100 accredited liver transplant center websites, along with language used by addiction psychiatry sites. They found that almost nine of 10 transplant center websites use stigmatizing language such as “alcoholic.” Less than half of addiction psychiatry websites do the same.
“The gap between professional society recommendations and actual practice is concerning, since patients frequently use these online resources for information which can significantly influence their behavior and perceptions about alcohol-associated liver disease,” Zhang said.
Zhang’s anti-stigma efforts are grounded in strong evidence, according to Harvard Medical School psychiatrist John F. Kelly , who published “Does It Matter How We Refer to Individuals with Substance-Related Conditions?” in 2009.
“Emphasizing non-stigmatizing language is crucial not only for fostering honesty but also for supporting the overall treatment process and patient outcomes.”
“Drug use disorder and alcohol use disorder are among the most stigmatized conditions universally across different societies because people feel that it’s self-induced — that people are to blame because they put it in their body,” said Kelly, also the founder of Mass General’s Recovery Research Institute . “Just because they made that decision initially, doesn’t mean they plan on becoming addicted.”
In the 2009 study, Kelly and his colleagues described patients to more than 600 clinicians, alternating between “substance abuser” and “having a substance use disorder.” Those in the latter category were viewed more sympathetically and as more worthy of treatment.
“I was quite surprised just how susceptible they were,” Kelly said. “These were passionate, dedicated clinicians. They were still susceptible to the negative punitive bias.”
They still are today, Zhang’s findings suggest.
“We are very good at seeing patients with liver disease but if we add this behavioral mental disorder, it is somewhat out of our scope,” he said. “I think education could at least have them be more familiar with this topic and be willing to at least listen to the adoption and use of non-stigmatizing language.”
“I think education could at least have them be more familiar with this topic and be willing to at least listen to the adoption and use of non-stigmatizing language.”
Building on the new study, Zhang has recommended to healthcare institutions and professional societies that they implement website feedback mechanisms and carry out regular content audits to guard against potentially harmful language.
“The steps we are recommending should not only help to align clinical practice with sound language guidelines, but also foster a more empathetic and supportive healthcare environment for patients,” he said.
Zhang also said healthcare institutions should look to leverage technology to support adoption of appropriate standards.
His team is collaborating with Mass General’s Research Patient Data Registry to obtain de-identified patient records, which they plan to review for instances of stigmatizing language. He hopes the process will help researchers quantify the prevalence of such language in clinical notes and identify patterns that can inform interventions. The team will also analyze the association of stigmatizing language with patient outcomes.
Share this article
You might like.
Medical historians look to cultural context, work of peer publications in wrestling with case of New England Journal of Medicine

Biomolecular archaeologist looks at why most of world’s population has trouble digesting beverage that helped shape civilization

Biologist separates reality of science from the claims of profiling firms
Epic science inside a cubic millimeter of brain
Researchers publish largest-ever dataset of neural connections
Pop star on one continent, college student on another
Model and musician Kazuma Mitchell managed to (mostly) avoid the spotlight while at Harvard
Finding right mix on campus speech policies
Legal, political scholars discuss balancing personal safety, constitutional rights, academic freedom amid roiling protests, cultural shifts
- Today's news
- Reviews and deals
- Climate change
- 2024 election
- Fall allergies
- Health news
- Mental health
- Sexual health
- Family health
- So mini ways
- Unapologetically
- Buying guides
Entertainment
- How to Watch
- My watchlist
- Stock market
- Biden economy
- Personal finance
- Stocks: most active
- Stocks: gainers
- Stocks: losers
- Trending tickers
- World indices
- US Treasury bonds
- Top mutual funds
- Highest open interest
- Highest implied volatility
- Currency converter
- Basic materials
- Communication services
- Consumer cyclical
- Consumer defensive
- Financial services
- Industrials
- Real estate
- Mutual funds
- Credit cards
- Balance transfer cards
- Cash back cards
- Rewards cards
- Travel cards
- Online checking
- High-yield savings
- Money market
- Home equity loan
- Personal loans
- Student loans
- Options pit
- Fantasy football
- Pro Pick 'Em
- College Pick 'Em
- Fantasy baseball
- Fantasy hockey
- Fantasy basketball
- Download the app
- Daily fantasy
- Scores and schedules
- GameChannel
- World Baseball Classic
- Premier League
- CONCACAF League
- Champions League
- Motorsports
- Horse racing
- Newsletters
New on Yahoo

- CA Privacy Notice
Binge drinking is a growing public health crisis − a neurobiologist explains how research on alcohol use disorder has shifted
- Oops! Something went wrong. Please try again later. More content below
With the new Amy Winehouse biopic “Back to Black ” in U.S. theaters as of May 17, 2024, the late singer’s relationship with alcohol and drugs is under scrutiny again. In July 2011, Winehouse was found dead in her flat in north London from “death by misadventure” at the age of 27. That’s the official British term used for accidental death caused by a voluntary risk.
Her blood alcohol concentration was 0.416%, more than five times the legal intoxication limit in the U.S. – leading her cause of death to be later adjusted to include “alcohol toxicity” following a second coroner’s inquest.
Nearly 13 years later, alcohol consumption and binge drinking remain a major public health crisis , not just in the U.K. but also in the U.S.
Roughly 1 in 5 U.S. adults report binge drinking at least once a week, with an average of seven drinks per binge episode . This is well over the amount of alcohol thought to produce legal intoxication, commonly defined as a blood alcohol concentration over 0.08% – on average, four drinks in two hours for women, five drinks in two hours for men.
Among women, days of “heavy drinking” increased 41% during the COVID-19 pandemic compared with pre-pandemic levels , and adult women in their 30s and 40s are rapidly increasing their rates of binge drinking , with no evidence of these trends slowing down. Despite efforts to comprehend the overall biology of substance use disorders, scientists’ and physicians’ understanding of the relationship between women’s health and binge drinking has lagged behind.
I am a neurobiologist focused on understanding the chemicals and brain regions that underlie addiction to alcohol . I study how neuropeptides – unique signaling molecules in the prefrontal cortex , one of the key brain regions in decision-making, risk-taking and reward – are altered by repeated exposure to binge alcohol consumption in animal models.
My lab focuses on understanding how things like alcohol alter these brain systems before diagnosable addiction, so that we can better inform efforts toward both prevention and treatment.
The biology of addiction
While problematic alcohol consumption has likely occurred as long as alcohol has existed, it wasn’t until 2011 that the American Society of Addiction Medicine recognized substance addiction as a brain disorder – the same year as Winehouse’s death. A diagnosis of an alcohol use disorder is now used over outdated terms such as labeling an individual as an alcoholic or having alcoholism.
Researchers and clinicians have made great strides in understanding how and why drugs – including alcohol, a drug – alter the brain. Often, people consume a drug like alcohol because of the rewarding and positive feelings it creates, such as enjoying drinks with friends or celebrating a milestone with a loved one. But what starts off as manageable consumption of alcohol can quickly devolve into cycles of excessive alcohol consumption followed by drug withdrawal.
While all forms of alcohol consumption come with health risks, binge drinking appears to be particularly dangerous due to how repeated cycling between a high state and a withdrawal state affect the brain. For example, for some people, alcohol use can lead to “ hangxiety ,” the feeling of anxiety that can accompany a hangover.
Repeated episodes of drinking and drunkenness, coupled with withdrawal, can spiral, leading to relapse and reuse of alcohol. In other words, alcohol use shifts from being rewarding to just trying to prevent feeling bad.
It makes sense. With repeated alcohol use over time, the areas of the brain engaged by alcohol can shift away from those traditionally associated with drug use and reward or pleasure to brain regions more typically engaged during stress and anxiety .
All of these stages of drinking, from the enjoyment of alcohol to withdrawal to the cycles of craving, continuously alter the brain and its communication pathways . Alcohol can affect several dozen neurotransmitters and receptors , making understanding its mechanism of action in the brain complicated.
Work in my lab focuses on understanding how alcohol consumption changes the way neurons within the prefrontal cortex communicate with each other. Neurons are the brain’s key communicator, sending both electrical and chemical signals within the brain and to the rest of your body.
What we’ve found in animal models of binge drinking is that certain subtypes of neurons lose the ability to talk to each other appropriately. In some cases, binge drinking can permanently remodel the brain. Even after a prolonged period of abstinence, conversations between the neurons don’t return to normal .
These changes in the brain can appear even before there are noticeable changes in behavior . This could mean that the neurobiological underpinnings of addiction may take root well before an individual or their loved ones suspect a problem with alcohol.
Researchers like us don’t yet fully understand why some people may be more susceptible to this shift, but it likely has to do with genetic and biological factors, as well as the patterns and circumstances under which alcohol is consumed.
Women are forgotten
While researchers are increasingly understanding the medley of biological factors that underlie addiction, there’s one population that’s been largely overlooked until now: women.
Women may be more likely than men to have some of the most catastrophic health effects caused by alcohol use, such as liver issues, cardiovascular disease and cancer . Middle-aged women are now at the highest risk for binge drinking compared with other populations.
When women consume even moderate levels of alcohol, their risk for various cancers goes up, including digestive, breast and pancreatic cancer , among other health problems – and even death. So the worsening rates of alcohol use disorder in women prompt the need for a greater focus on women in the research and the search for treatments.
Yet, women have long been underrepresented in biomedical research.
It wasn’t until 1993 that clinical research funded by the National Institutes of Health was required to include women as research subjects. In fact, the NIH did not even require sex as a biological variable to be considered by federally funded researchers until 2016. When women are excluded from biomedical research, it leaves doctors and researchers with an incomplete understanding of health and disease, including alcohol addiction.
There is also increasing evidence that addictive substances can interact with cycling sex hormones such as estrogen and progesterone . For instance, research has shown that when estrogen levels are high, like before ovulation, alcohol might feel more rewarding , which could drive higher levels of binge drinking. Currently, researchers don’t know the full extent of the interaction between these natural biological rhythms or other unique biological factors involved in women’s health and propensity for alcohol addiction.
Looking ahead
Researchers and lawmakers are recognizing the vital need for increased research on women’s health. Major federal investments into women’s health research are a vital step toward developing better prevention and treatment options for women.
While women like Amy Winehouse may have been forced to struggle both privately and publicly with substance use disorders and alcohol, the increasing focus of research on addiction to alcohol and other substances as a brain disorder will open new treatment avenues for those suffering from the consequences.
For more information on alcohol use disorder, causes, prevention and treatments, visit the National Institute on Alcohol Abuse and Alcoholism .
This article is republished from The Conversation , a nonprofit, independent news organization bringing you facts and trustworthy analysis to help you make sense of our complex world. It was written by: Nikki Crowley , Penn State
For decades, mothers have borne the brunt of scrutiny for alcohol use during pregnancy − new research points to dad’s drinking as a significant factor in fetal alcohol syndrome
Alcohol use is widely accepted in the US, but even moderate consumption is associated with many harmful effects
Drinking during holidays and special occasions could affect how you parent your kids
Nikki Crowley receives funding from The National Institutes of Health, The Brain and Behavior Research Foundation, and the Penn State Huck Institutes of the Life Sciences endowment funds.
Recommended Stories
The it list: 'bridgerton' returns with a steamy season 3, 'back to black' shows amy winehouse's rise to fame, 'i saw the tv glow' blurs reality and real life.
The beloved Regency-era romance makes a triumphant return to Netflix.
Sam Taylor-Johnson wants to 'celebrate' Amy Winehouse in 'Back to Black,' not judge her: 'I felt like I had a huge responsibility'
Director Sam Taylor-Johnson explains the "careful way" she portrays Amy Winehouse in new biopic as critics take issue with how difficult subject matter is handled.
The 27 best Walmart deals to shop this weekend — save up to 75% on early Memorial Day sales, summer essentials and more
Ready for your pre-summer perusal: A massive patio umbrella for over half off, a 65-inch Hisense smart TV for under $500 and a Keurig hot/iced coffeemaker for nearly $70 off.
Las Vegas tourism arm announces $100,000 sponsorship for every Aces player
That's more than some of the Aces players' salaries.
Report: David Fletcher, former Shohei Ohtani teammate, placed bets with same bookie as Ippei Mizuhara
This scandal refuses to end for MLB.
PGA Championship: Will Zalatoris says group of players considered asking for postponement after death, Scottie Scheffler arrest
It was a surreal day at the PGA Championship.
'Bye, AirPods': Beats Studio earbuds are nearly 50% off for Memorial Day — that's less than Black Friday
'They do the job just as good, and probably even better than Apple,' says one of over 60,000 five-star fans.
Doctor Who: Boom review: All hail the conquering hero
It's the first classic of the Disney+ era.
What is a student credit card and how do you pick one?
Learn how to choose a student card by evaluating the available rates, terms, rewards, and bonus offers.
Giants outfielder Jung Hoo Lee to undergo season-ending shoulder surgery
The Korean star signed a $113 million deal with the Giants last offseason.
Capital One Spark Cash Select for Excellent Credit review: A cash-back business card with simple rewards
This business card comes with a generous welcome bonus and earns a flat 1.5% cash back on every purchase
Memorial Day 2024 deals on road trip essentials
Check out these great road tripping Memorial Day deals on pet harnesses, power stations, coolers and more.
The OpenAI team tasked with protecting humanity is no more
In the summer of 2023, OpenAI created a “Superalignment” team whose goal was to steer and control future AI systems that could be so powerful they could lead to human extinction. Less than a year later, that team is dead.
Meta's latest experiment borrows from BeReal's and Snapchat's core ideas
Meta is once again taking on its competitors by developing a feature that borrows concepts from others -- in this case, BeReal and Snapchat. The company is developing a feature for Instagram called “Peek” that would allow users to post authentic pictures that can only be viewed once. While Snapchat popularized the idea of ephemeral content on social media, BeReal led the trend of posting authentic, unedited content.
Courtroom sketch artists capture history at Trump's hush money trial. Here are some of the best.
Since it’s not being televised, — the only images of testimony from inside the courtroom are portraits being done by sketch artists like Jane Rosenberg, whose sketches depict Trump and other key figures in various states and moods.
'My skin is tighter and softer': Grab this popular anti-aging retinol cream on sale for just $16
Raved a 65-year-old fan: 'I tried this lotion on one arm and did a comparison. The difference was astounding and immediate.'
AI-generated images are running rampant on social media. What are X, TikTok and Meta doing to control them?
While an AI-generated photo of Katy Perry at the Met Gala isn't a major cause for concern, Instagram's fact-checkers taking hours to flag it indicates a larger issue social platforms have to grapple with regarding AI.
Here are all of the Republicans who have shown up in court to support Trump at his hush money trial
GOP lawmakers have been showing up at the Manhattan Criminal Courthouse in waves to give the former president a public show of support, help him circumvent the gag order — and audition for a role in a possible second-term Cabinet.
I'm an interior designer, and these are my top picks from Amazon's outdoor rug Memorial Day sale — save up to 80%
Treat your toes to these elusive deals on Safavieh, Loloi, Amber Lewis, Nourison, NuLoom and more.
Apple is said to be working on a 'significantly thinner' iPhone
The iPhone could be going the way of the iPad Pro by becoming much thinner next year. However, you'll may have to pay quite a lot for this rumored slender model, which may replace the Plus in the annual iPhone lineup.
- Introduction
- Conclusions
- Article Information
a Adjusted for age at survey, sex, race and ethnicity, marital status, educational level, annual household income, insurance status, smoking status, cancer type, age at cancer diagnosis, and currently prescribed medication and/or receiving treatment.
b Included individuals reporting races or ethnicities other than Hispanic, non-Hispanic Black, or non-Hispanic White and individuals with more than 1 race or ethnicity.
c Included breast, colon and rectum, and head and neck cancer. Esophageal cancer was not included because the association with alcohol drinking is confined largely to squamous cell carcinoma, whereas most cases of esophageal cancer were adenocarcinoma in the US. Liver cancer was not included as it was not specifically included in the All of Us Research Program survey.
d Self-reported current medication prescription and/or treatment in the Personal Medical History survey.
a Non-Hispanic White was used as the reference group.
c Included breast, colon and rectum, and head and neck cancer. Esophageal cancer was not included because the association with alcohol drinking is confined largely to squamous cell carcinoma whereas most cases of esophageal cancer were adenocarcinoma in the US. Liver cancer was not included as it was not specifically included in the All of Us Research Program survey.
e Adjusted for age at survey, sex, race and ethnicity, marital status, educational level, annual household income, insurance status, smoking status, cancer type, age at cancer diagnosis, and currently prescribed medication and/or receiving treatment.
eTable 1. Cancer Characteristics According to Sex, All of Us Research Program
eTable 2. Alcohol Use Disorders Identification Test–Consumption (AUDIT-C)
eTable 3. Characteristics of Cancer Survivors Who Underwent Cancer Treatment Within 1 Year Before the Baseline Survey, All of Us Research Program
eTable 4. Adjusted Odds Ratios of Current Drinking Among Cancer Survivors, All of Us Research Program
eTable 5. Adjusted Odds Ratios of Risky Drinking Behaviors Among Current Drinking Cancer Survivors, All of Us Research Program
eTable 6. Prevalence of Alcohol Consumption Patterns Among Survey Participants Without Prior Cancer Diagnosis According to Sex, All of Us Research Program
eFigure 1. Flow Chart of the Study Population
eFigure 2. (A) Mean AUDIT-C Score Among Cancer Survivors According to Sex; (B) Venn Diagram Showing Cancer Survivors Engaged in Exceeding Moderate Drinking, Binge Drinking and Hazardous Drinking Among 11815 Current Drinkers, All of Us Research Program
eFigure 3. Mean AUDIT-C Score Among Cancer Survivors According to Age at Cancer Diagnosis and Smoking Status, All of Us Research Program
Data Sharing Statement
See More About
Sign up for emails based on your interests, select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Get the latest research based on your areas of interest.
Others also liked.
- Download PDF
- X Facebook More LinkedIn
Shi M , Luo C , Oduyale OK , Zong X , LoConte NK , Cao Y. Alcohol Consumption Among Adults With a Cancer Diagnosis in the All of Us Research Program. JAMA Netw Open. 2023;6(8):e2328328. doi:10.1001/jamanetworkopen.2023.28328
Manage citations:
© 2024
- Permissions
Alcohol Consumption Among Adults With a Cancer Diagnosis in the All of Us Research Program
- 1 Division of Public Health Sciences, Department of Surgery, Washington University in St Louis School of Medicine, St Louis, Missouri
- 2 Division of Hematology, Medical Oncology and Palliative Care, Department of Medicine, University of Wisconsin School of Medicine and Public Health, Madison
- 3 University of Wisconsin Carbone Cancer Center, Madison
- 4 Alvin J. Siteman Cancer Center, Washington University in St Louis School of Medicine, St Louis, Missouri
- 5 Division of Gastroenterology, Department of Medicine, Washington University in St Louis School of Medicine, St Louis, Missouri
Question What is the prevalence of current alcohol consumption and of risky alcohol consumption among cancer survivors in the US?
Findings In this cross-sectional study of 15 199 adults with a cancer diagnosis from the All of Us Research Program, 77.7% self-reported as current drinkers, and among these, 13.0% exceeded moderate drinking, 23.8% reported binge drinking, and 38.3% engaged in hazardous drinking. Among 1839 survivors receiving cancer treatment, the prevalence of current drinking and risky drinking were similar to the overall cohort and across treatment types.
Meaning This study suggests that current drinking and risky drinking are common among US cancer survivors even during cancer treatment.
Importance Alcohol consumption is associated with adverse oncologic and treatment outcomes among individuals with a diagnosis of cancer. As a key modifiable behavioral factor, alcohol consumption patterns among cancer survivors, especially during treatment, remain underexplored in the United States.
Objective To comprehensively characterize alcohol consumption patterns among US cancer survivors.
Design, Setting, and Participants This cross-sectional study used data from May 6, 2018, to January 1, 2022, from the National Institutes of Health All of Us Research Program, a diverse US cohort with electronic health record (EHR) linkage, and included 15 199 participants who reported a cancer diagnosis and 1839 patients among a subset with EHR data who underwent treatment within the past year of the baseline survey. Data analysis was performed from October 1, 2022, to January 31, 2023.
Main Outcomes and Measures Prevalence of current drinking and of risky drinking behaviors, including exceeding moderate drinking (>2 drinks on a typical drinking day), binge drinking (≥6 drinks on 1 occasion), and hazardous drinking (Alcohol Use Disorders Identification Test–Consumption [AUDIT-C] score ≥3 for women or ≥4 for men).
Results This study included 15 199 adults (mean [SD] age at baseline, 63.1 [13.0] years; 9508 women [62.6%]) with a cancer diagnosis. Overall, 11 815 cancer survivors (77.7%) were current drinkers. Among current drinkers, 1541 (13.0%) exceeded moderate drinking, 2812 (23.8%) reported binge drinking, and 4527 (38.3%) engaged in hazardous drinking. After multivariable adjustment, survivors who were younger than 65 years, men, or of Hispanic ethnicity or who received a diagnosis before 18 years of age or ever smoked were more likely to exceed moderate drinking (aged <50 years: odds ratio [OR], 2.90 [95% CI, 2.41-3.48]; aged 50-64 years: OR, 1.84 [95% CI, 1.58-2.15]; men: OR, 2.38 [95% CI, 2.09-2.72]; Hispanic ethnicity: OR, 1.31 [95% CI, 1.04-1.64]; aged <18 years at diagnosis: OR, 1.52 [95% CI, 1.04-2.24]; former smokers: OR, 2.46 [95% CI, 2.16-2.79]; current smokers: OR, 4.14 [95% CI, 3.40-5.04]) or binge drink (aged <50 years: OR, 4.46 [95% CI, 3.85-5.15]; aged 50-64 years: OR, 2.15 [95% CI, 1.90-2.43]; men: OR, 2.10 [95% CI, 1.89-2.34]; Hispanic ethnicity: OR, 1.31 [95% CI, 1.09-1.58]; aged <18 years at diagnosis: OR, 1.71 [95% CI, 1.24-2.35]; former smokers: OR, 1.69 [95% CI, 1.53-1.87]; current smokers: OR, 2.27 [95% CI, 1.91-2.71]). Survivors with cancer diagnosed before 18 years of age or who ever smoked were more likely to be hazardous drinkers (aged <18 years at diagnosis: OR, 1.52 [95% CI, 1.11-2.08]; former smokers: OR, 1.83 [95% CI, 1.68-1.99]; current smokers: OR, 2.13 [95% CI, 1.79-2.53]). Of 1839 survivors receiving treatment as captured in the EHR, 1405 (76.4%) were current drinkers, and among these, 170 (12.1%) exceeded moderate drinking, 329 (23.4%) reported binge drinking, and 540 (38.4%) engaged in hazardous drinking, with similar prevalence across different types of cancer treatment.
Conclusions and Relevance This cross-sectional study of a diverse US cohort suggests that alcohol consumption and risky drinking behaviors were common among cancer survivors, even among individuals receiving treatment. Given the adverse treatment and oncologic outcomes associated with alcohol consumption, additional research and implementation studies are critical in addressing this emerging concern among cancer survivors.
With more than 18 million cancer survivors in the United States as of 2022, 1 identifying modifiable behavioral factors that could improve survivorship and quality of life is a clinical and public health priority. Alcohol consumption, which is ubiquitous in the US and causally linked with multiple types of cancer (oral cavity, pharynx, larynx, esophagus, colorectum, liver, and female breast cancer), 2 , 3 is also associated with adverse health outcomes among individuals with a diagnosis of cancer, including higher risks of recurrence 4 , 5 or onset of new primary cancers 5 - 7 as well as death. 4 , 5 , 8 - 12 In addition, alcohol is associated with worsened treatment outcomes, such as decreased effectiveness and increased risk of complications. 13 - 17 Despite these findings, currently, no specific surveillance and counseling guidelines are in place for cancer survivors. Cancer survivors are advised to adhere to the American Cancer Society guideline on nutrition and physical activity for cancer prevention, including (1) that it is best not to drink alcohol and (2) that individuals who choose to drink alcohol should limit alcohol intake to 1 drink or fewer per day for women and 2 drinks or fewer per day for men. 18
A 2018 statement from the American Society of Clinical Oncology (ASCO) reinforces the need to prioritize alcohol consumption as a key modifiable behavioral factor in the cancer control research agenda. 19 However, our understanding of alcohol drinking patterns among cancer survivors in the US is limited. Using the National Health Interview Survey (2000-2017), Sanford et al 20 reported that 35% of cancer survivors who were current drinkers exceeded moderate drinking limits (>1 drink for women and >2 drinks for men) and 21% engaged in binge drinking (≥5 drinks during at least 1 day over the past year). However, to our knowledge, patterns of drinking, including frequency as well as the co-occurrence of multiple risky drinking behaviors, have not been described. 21 , 22 The Alcohol Use Disorders Identification Test–Consumption (AUDIT-C) score, a validated score that incorporates frequency of drinking, quantity of drinking, and binge drinking, has been used in primary care and other settings to identify individuals engaging in hazardous drinking. 23 - 26 One study in 17 European countries and Israel reported that 20% of cancer survivors aged 50 years or older engaged in hazardous drinking, 27 yet such analyses have not been conducted in the US, to our knowledge. More important, although we recently began to recognize the potential adverse effects of drinking during cancer treatment, alcohol consumption patterns during such a critical time window for cancer survivors remain underexplored. To address these knowledge gaps that are critical for short- and long-term survivorship for US cancer survivors, we aimed to comprehensively characterize alcohol consumption patterns among cancer survivors overall and during cancer treatment, using data collected from the All of Us Research Program, a diverse US cohort with electronic health record (EHR) linkage.
We identified cancer survivors enrolled in the National Institutes of Health All of Us Research Program, one of the largest, diverse biomedical cohorts within the US. 28 , 29 The All of Us Research Program collects data using survey responses, EHR data, biospecimen collection, and physical measurements. 28 , 30 The All of Us Research Program institutional review board approved all study procedures. All participants provided written informed consent to share EHRs, surveys, and other study data with qualified investigators for broad-based research. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology ( STROBE ) reporting guideline.
Among 142 100 participants who completed the Basics, Overall Health, Lifestyle, and Personal Medical History surveys, we identified 15 297 cancer survivors who self-reported a cancer diagnosis (excluding individuals with skin cancer and multiple cancers) from May 6, 2018, to January 1, 2022 (eFigure 1 in Supplement 1 ). We categorized the cancers as alcohol-related cancers (breast, colon and rectum, and head and neck) 2 and nonalcohol-related cancers (eTable 1 in Supplement 1 ). Esophageal cancer was categorized as nonalcohol related because the association with alcohol drinking is confined largely to squamous cell carcinoma, 2 whereas most cases of esophageal cancer in the US were adenocarcinoma. 31 Liver cancer was not included in alcohol-related cancers because it was not specifically included in the survey. We also retrieved information on age at cancer diagnosis (child [≤11 years], adolescent [12-17 years], adult [18-64 years], older adult [65-74 years], or elderly adult [≥75 years]) and current treatment status (“Are you currently prescribed medications and/or receiving treatment for this condition?” with an answer of yes or no).
Current alcohol consumption status (never, former, and current drinkers) was defined based on the questions in the Lifestyle survey. Participants were asked “In your entire life, have you had at least 1 drink of any kind of alcohol, not counting small tastes or sips?” which was adapted from the National Epidemiologic Survey on Alcohol and Related Conditions. We defined participants who reported not having at least 1 drink of any kind of alcohol as never drinkers, those who had at least 1 drink in their entire life but never had a drink in the past year as former drinkers, and those who had at least 1 drink in the past year as current drinkers. After excluding 98 participants without adequate information to define their current alcohol consumption status, 15 199 cancer survivors were retained in the analyses.
Among current drinkers, we further characterized risky drinking behaviors based on 3 questions: (1) frequency of drinking: “How often did you have a drink containing alcohol in the past year?” with options of never, monthly or less, 2 to 4 times a month, 2 to 3 times a week, or 4 or more times a week; (2) quantity of drinking: “On a typical day when you drink, how many drinks do you have?” with options of 1 or 2, 3 or 4, 5 or 6, 7 to 9, or 10 or more; and (3) binge drinking: “How often did you have 6 or more drinks on 1 occasion in the past year?” with options of never, less than monthly, monthly, weekly, or daily or almost daily. Exceeding moderate drinking was defined from answers about quantity of drinking as participants who drink more than 2 drinks on a typical day when they drink. Binge drinking was defined from the question about binge drinking as participants who ever had 6 or more drinks on 1 occasion. To create the AUDIT-C score (range, 0-12), we added scores of 3 questions with 5 possible answers, which were scored from 0 (less alcohol use) to 4 points (more alcohol use) (eTable 2 in Supplement 1 ). 24 Hazardous drinkers included women with AUDIT-C scores of 3 or higher and men with scores of 4 or higher. 24 , 32 , 33
We included information on age, sex, race and ethnicity, marital status, educational level, annual household income, and insurance status from the Basics survey and general health condition from the Overall Health survey. Sex was categorized based on the question “What was your biological sex assigned at birth?” as women, men, and other sex (including participants who selected “intersex,” “prefer not to answer,” “none of these,” and “skip”). Data on race and ethnicity were collected because prior research has demonstrated different drinking patterns according to racial and ethnic groups. 34 , 35 Race and ethnicity were categorized as Hispanic, non-Hispanic Black, non-Hispanic White, and other according to participant self-report. Other race included individuals reporting races other than Hispanic, non-Hispanic Black, or non-Hispanic White (Asian, Middle Eastern or North African, Native Hawaiian or Other Pacific Islander, and participants who responded that none of the provided options fully describe them) and individuals with more than 1 race and ethnicity. Smoking status was assessed in the Lifestyle survey: participants who reported not smoking at least 100 cigarettes in their entire life were categorized as never smokers, those smoking at least 100 cigarettes in their entire life but now do not smoke at all were categorized as former smokers, and those smoking at least 100 cigarettes in the entire life and now smoke every day or some days were categorized as current smokers.
After linking with the EHR, 36 we identified 10 892 cancer survivors with a first medical encounter 1 year or more before the baseline surveys and a subset of 1839 patients who underwent treatment within the past year of the baseline survey. Treatment was retrieved based on prior studies, using the Current Procedural Terminology , 4th Edition; Healthcare Common Procedure Coding System; Systematized Nomenclature of Medicine Clinical Terms; International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, Procedure Coding System ; and RxNorm. 37 - 39 We further classified the treatment as surgery, chemotherapy, hormone therapy, radiotherapy, and immunotherapy. We identified treatment modalities that aligned with self-reported cancer type. For surgery, we ensured to include only procedures that matched the specific cancers for which patients received a diagnosis. For instance, we did not count colectomies for any patient without a diagnosis of colorectal cancer.
Statistical analysis was performed from October 1, 2022, to January 31, 2023. We estimated the crude prevalence of current drinking among cancer survivors as well as the crude prevalence of risky drinking behaviors (including exceeding moderate drinking, binge drinking, and hazardous drinking) among current drinkers. Multivariable logistic regression was used to estimate odds ratios (ORs) and 95% CIs of current drinking and risky drinking behaviors among current drinkers, adjusting for age at survey (<50, 50-64, or ≥65 years), sex (women, men, or other), race and ethnicity (Hispanic, non-Hispanic Black, non-Hispanic White, or other), marital status (never or ever), educational level (<high school, high school or General Educational Development certification, some college, or college), annual household income (<$34 999, $35 000-$74 999, $75 000-$149 999, or ≥$150 000), insurance status (yes or no), smoking status (never, former, or current), cancer type (nonalcohol-related cancers or alcohol-related cancers), age at cancer diagnosis (<18, 18-64, or ≥65 years), and medication and/or receiving treatment (yes or no).
Among the subset of cancer survivors with EHR data who underwent treatment, we estimated the crude prevalence of current drinking and risky drinking behaviors overall and according to type of cancer treatment. To compare with the general population, we conducted secondary analyses to estimate the crude prevalence of current and risky drinking behaviors among survey participants without a prior cancer diagnosis. Data were analyzed in the All of Us Research Workbench (R, version 4.0.2 [R Group For Statistical Computing]).
In the overall cohort of 15 199 cancer survivors, the mean (SD) age at baseline was 63.1 (13.0) years, 9508 survivors (62.6%) were women, and 11 633 survivors (76.5%) were non-Hispanic White ( Table 1 ). Most cancers (11 515 [75.8%]) were diagnosed when the patient was between 18 and 64 years of age. Most cancer survivors had a college degree (9291 [61.1%]) and a high annual household income, 5333 (35.1%) were former smokers, and 997 (6.6%) were current smokers. Among 1839 cancer survivors who underwent cancer treatment within the past year of the baseline survey, their characteristics were similar to those in the overall cohort (eTable 3 in Supplement 1 ).
Of 15 199 cancer survivors, 11 815 (77.7%) were current drinkers (women, 7344 of 9508 [77.2%]; men, 3971 of 5049 [78.6%]) ( Table 2 ). After multivariable adjustment, survivors who were non-Hispanic White, with alcohol-related cancers, without self-reported current medication prescription and/or treatment, and who were ever smokers were more likely to be current drinkers ( Figure 1 ; eTable 4 in Supplement 1 ). Compared with non-Hispanic White individuals, survivors who were Hispanic (OR, 0.65; 95% CI, 0.56-0.76), non-Hispanic Black (OR, 0.71; 95% CI, 0.61-0.82), and of other race and ethnicity (OR, 0.49; 95% CI 0.41-0.58) were less likely to be current drinkers. Survivors with alcohol-related cancers were 16% more likely (OR, 1.16; 95% CI, 1.06-1.27) to be current drinkers. Compared with survivors who self-reported they were not currently receiving prescription medication or treatment, those who underwent treatment were less likely to be current drinkers (OR, 0.87; 95% CI, 0.80-0.94). Former smokers (OR, 1.27; 95% CI, 1.16-1.39) and current smokers (OR, 1.44; 95% CI, 1.22-1.70) were also more likely to be current drinkers compared with never smokers.
Of 11 815 survivors who were current drinkers, 1541 (13.0%) exceeded moderate drinking (women, 777 of 7344 [10.6%]; men, 696 of 3971 [17.5%]), and 2812 (23.8%) reported binge drinking (women, 1560 of 7344 [21.2%]; men, 1119 of 3971 [28.2%]) ( Table 2 ; eFigure 2 in Supplement 1 ). After multivariable adjustment, survivors who were younger than 65 years, who were men, who were Hispanic, with cancer diagnosed before 18 years of age, or who ever smoked were more likely to exceed moderate drinking (aged <50 years: odds ratio [OR], 2.90 [95% CI, 2.41-3.48]; aged 50-64 years: OR, 1.84 [95% CI, 1.58-2.15]; men: OR, 2.38 [95% CI, 2.09-2.72]; Hispanic ethnicity: OR, 1.31 [95% CI, 1.04-1.64]; aged <18 years at diagnosis: OR, 1.52 [95% CI, 1.04-2.24]; former smokers: OR, 2.46 [95% CI, 2.16-2.79]; current smokers: OR, 4.14 [95% CI, 3.40-5.04]) and engage in binge drinking (aged <50 years: OR, 4.46 [95% CI, 3.85-5.15]; aged 50-64 years: OR, 2.15 [95% CI, 1.90-2.43]; men: OR, 2.10 [95% CI, 1.89-2.34]; Hispanic ethnicity: OR, 1.31 [95% CI, 1.09-1.58]; aged <18 years at diagnosis: OR, 1.71 [95% CI, 1.24-2.35]; former smokers: OR, 1.69 [95% CI, 1.53-1.87]; current smokers: OR, 2.27 [95% CI, 1.91-2.71]) ( Figure 2 ; eTable 5 in Supplement 1 ). The odds of engaging in more than moderate drinking or binge drinking were similar among current drinkers who reported receiving medication and/or undergoing treatment and those who did not.
A total of 4527 current drinkers (38.3%) engaged in hazardous drinking, defined by an AUDIT-C score of 3 or higher for women and 4 or higher for men, with similar prevalences among women and men. After multivariable adjustment, survivors with cancer diagnosed before 18 years of age were more likely to be hazardous drinkers (OR, 1.52; 95% CI, 1.11-2.08) compared with those diagnosed at 65 years of age or older (eTable 5 in Supplement 1 ). Compared with never smokers, former smokers were 83% more likely (OR, 1.83; 95% CI, 1.68-1.99) to be hazardous drinkers, and current smokers had more than 2-fold the odds (OR, 2.13; 95% CI, 1.79-2.53) of engaging in hazardous drinking. For survivors with the highest risk of hazardous drinking (current smokers who received a cancer diagnosis before 18 years of age), their risky drinking behaviors were associated with more frequent, heavy drinking as well as binge drinking (eFigure 3 in Supplement 1 ). No association was observed between self-reported receipt of medication or treatment and hazardous drinking. Of 119 977 survey participants without a prior cancer diagnosis, 96 058 (80.1%) were current drinkers; among these, 19 949 (20.8%) exceeded moderate drinking, 34 135 (35.5%) reported binge drinking, and 48 090 (50.1%) engaged in hazardous drinking (eTable 6 in Supplement 1 ).
Of 1839 cancer survivors who received treatment within the past year of the baseline survey, 1405 (76.4%) self-reported as current drinkers ( Table 3 ), similar to the prevalence in the overall cohort of patients who self-reported receiving medication and/or treatment and being current drinkers (4211 of 5531 [76.1%]). This prevalence was largely similar for each cancer treatment, with the highest for patients who underwent surgery (329 of 409 [80.4%]) ( Table 3 ). Of 1405 current drinkers who received treatment within the past year of the baseline survey, 170 (12.1%) exceeded moderate drinking, 329 (23.4%) reported binge drinking, and 540 (38.4%) engaged in hazardous drinking.
Our study extends the scope of prior understanding through using a diverse US cohort to characterize risky drinking behaviors comprehensively among cancer survivors. We again highlight that alcohol consumption and risky drinking behaviors are common among cancer survivors, and we found that, among current drinkers, men, Hispanic individuals, those with cancer diagnosed before 18 years of age, and smokers are more likely to engage in risky drinking behaviors. More important, by linking with EHR data to annotate treatment information, we found that drinking and risky drinking behaviors are prevalent even among individuals concurrently receiving treatment for cancer.
Similar to a prior study using a nationally representative survey, 20 we found that most cancer survivors were current drinkers, and non-Hispanic White individuals or ever smokers were more likely to be current drinkers. In addition, we found that survivors with alcohol-related cancers or without self-reported current treatment were more likely to be current drinkers. Also in line with the previous study, 20 we found that, among current drinkers, survivors who were younger, men, Hispanic, and ever smokers were more likely to exceed moderate drinking or binge drink. Comparable with previous findings, 40 our study also suggested that Hispanic individuals are less likely to drink compared with non-Hispanic White individuals, but Hispanic individuals who choose to drink are more likely to consume higher volumes of alcohol, possibly due in part to acculturation. 41 Although adolescent or young adult cancer survivors were reported to be more likely than peers without cancer to drink alcohol, 42 our study found that survivors with cancer diagnosed before 18 years of age were more likely to engage in both heavy and binge drinking. Using validated AUDIT-C scores that incorporate frequency of drinking, quantity of drinking, and binge drinking, we reported for the first time, to our knowledge, that 38.3% of cancer survivors in this diverse US cohort engaged in hazardous drinking. This higher prevalence compared with those reported in Europe by Bosque-Prous et al 27 might be explained in part by using lower cutoff points to define hazardous drinking in our study (AUDIT-C scores of ≥3 for women and ≥4 for men) vs those used by Bosque-Prous et al 27 (AUDIT-C scores of ≥4 for women and ≥5 for men). Although more studies are warranted, the high prevalence of cancer survivors engaged in hazardous drinking highlights the need for immediate interventions to reduce alcohol intake among US cancer survivors.
Alcohol consumption and risky drinking behaviors among cancer survivors are associated with various adverse long-term outcomes, including higher risk of recurrence, 4 , 5 secondary primary tumors, 5 - 7 and increased mortality. 4 , 5 , 8 - 12 In a meta-analysis involving 209 597 cancer survivors, alcohol consumption was associated with a 17% increased risk of cancer recurrence and an 8% increased risk of overall mortality. 4 More studies are warranted to elucidate the role of each risky drinking behavior and the overall pattern in long-term outcomes. Survivors with cancer diagnosed before 18 years of age or ever smokers were more likely to be hazardous drinkers. Because of the persistent excess risks for second primary cancers throughout the life course for childhood cancer survivors 43 - 45 and the elevated risks for alcohol- and tobacco-related secondary primary cancers among drinkers who ever smoke, 6 targeted efforts for alcohol reduction are needed for these 2 groups of survivors who are more susceptible.
As highlighted in the 2018 ASCO statement, 19 in addition to long-term survivorship, accumulating data support the associations between alcohol drinking and treatment outcomes among cancer survivors. For instance, alcohol use worsens postsurgical outcomes, including increased risk of surgical complications, longer hospitalizations, more surgical procedures, prolonged recovery, higher health care costs, 46 - 48 and higher mortality. 19 , 49 Alcohol use during and after radiotherapy is associated with a higher risk of osteonecrosis of the jaw among patients with head and neck cancers. 50 - 53 In addition, alcohol is well known to have neurotoxic, cardiotoxic, and hepatotoxic effects. 54 - 56 Among patients undergoing chemotherapy, alcohol has been suggested to worsen cognition and cardiotoxicity. 57 , 58 Furthermore, alcohol use is associated with hepatic dysfunction and regulates cytochrome enzymatic activity, 54 which is important for the metabolism of chemotherapeutic agents and possibly alters their effectiveness or toxic effects. Although the association of alcohol use with immunotherapy for cancer is unclear, the treatment outcomes may be somewhat affected due to alcohol-induced immune dysfunction. 59
Our understanding of alcohol consumption patterns among cancer survivors receiving treatment has just begun to emerge. In a recent pilot study of 69 patients in Wisconsin, 30% of cancer survivors reported drinking alcohol while receiving chemotherapy, and 38% of these drinkers reported at least some complications. 60 To date, the All of Us Research Program is the only national cohort that allows us to capture alcohol consumption patterns in the context of cancer treatment. Unexpectedly, a large proportion of cancer survivors undergoing cancer treatment were current drinkers (76.4%) or were engaged in risky drinking (exceeding moderate drinking, 12.1%; binge drinking, 23.4%; hazardous drinking, 38.4%); these proportions were similar across different types of cancer treatment as well as in the overall cohort. Taken together, our findings point to the immediate and unmet need to intervene on the behalf of individuals with risky drinking behaviors in oncologic care settings. Clinicians should collect alcohol consumption information while also informing survivors of the potential harms in an effort to reduce risky alcohol use. Given that drinking is deeply ingrained in societal norms and rituals, and considering the limited awareness of how alcohol consumption is associated with cancer outcomes, it is imperative to provide support to patients who are identified as alcohol users and offer them guidance. Our findings also call for large-scale epidemiologic studies to further evaluate the association of alcohol with therapeutic efficacy and treatment outcomes among cancer survivors.
This study has some strengths, including the use of a large and diverse national cohort to comprehensively characterize risky drinking behaviors, including hazardous drinking, whereas previous studies focused on exceeding moderate drinking and binge drinking only. More important, we used the EHR linkages to retrieve information on cancer treatment.
Our study also has several limitations. First, per the Dietary Guidelines for Americans 2020-2025, exceeding moderate drinking was defined as having more than 1 drink per day for women. 61 However, the All of Us Research Program survey only allowed us to define exceeding moderate drinking among women as having more than 2 drinks. Similarly, we characterized patients who consumed 6 or more drinks on 1 occasion as binge drinkers, instead of those who consumed 4 or more drinks for women or 5 or more drinks for men per the National Institute on Alcohol Abuse and Alcoholism guideline. 62 However, with these underestimates, the prevalence of women exceeding moderate drinking was high, as was the prevalence of binge drinking among both women and men, which further highlight the pressing need for reduction of alcohol consumption. Second, because the All of Us Research Program survey asked about average alcohol consumption in the past year, we retrieved cancer treatment information during the same time in the EHR. However, the exact timing of alcohol consumption in association with cancer treatment was not clear. Additional studies are required to validate and refine our findings.
This cross-sectional study found that current and risky drinking (exceeding moderate drinking, binge drinking, and hazardous drinking) were common among US cancer survivors even during cancer treatment. Given the short- and long-term adverse treatment and oncologic outcomes associated with alcohol consumption, additional research and implementation studies are critical to address this emerging concern among cancer survivors.
Accepted for Publication: June 30, 2023.
Published: August 10, 2023. doi:10.1001/jamanetworkopen.2023.28328
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2023 Shi M et al. JAMA Network Open .
Corresponding Author: Yin Cao, ScD, MPH, Division of Public Health Sciences, Department of Surgery, Washington University in St Louis School of Medicine, 660 S Euclid Ave, Campus Box 8100, St Louis, MO 63110 ( [email protected] ).
Author Contributions: Ms Shi and Dr Cao had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Ms Shi and Dr Luo contributed equally.
Concept and design: Shi, Cao.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Shi, Oduyale, Cao.
Critical review of the manuscript for important intellectual content: All authors.
Statistical analysis: Luo, Zong, Cao.
Obtained funding: Cao.
Administrative, technical, or material support: LoConte, Cao.
Supervision: Cao.
Conflict of Interest Disclosures: Dr LoConte reported receiving personal fees from AbbVie and PDGX; and grants from Exact Sciences outside the submitted work. Dr Cao reported receiving personal fees from Geneoscopy outside the submitted work. No other disclosures were reported.
Funding/Support: This work was supported by grants P30 CA091842 and R21 AA027608 from the US National Institutes of Health (Dr Cao). Dr Oduyale was supported by the Foundation for Barnes-Jewish Hospital.
Role of the Funder/Sponsor: The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data Sharing Statement: See Supplement 2 .
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts

An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.

The Healthcare Professional's
Core Resource on Alcohol
Knowledge. Impacts. Strategies.
National Institute on Alcohol Abuse and Alcoholism (NIAAA)
Alcohol use disorder: from risk to diagnosis to recovery.
Step 1 - Read the Article
Step 2 - Complete the Brief CME/CE Post-Test
- Earn CME/CE Credit
- Alcohol use disorder (AUD) is defined by the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) as "a problematic pattern of alcohol use leading to clinically significant impairment or distress," and is diagnosed as mild, moderate, or severe based on the number of symptoms, out of a possible 11, in the past 12 months.
- As it progresses in severity, AUD can cause brain changes that make it difficult to stop drinking, but with prolonged abstinence, at least some AUD-induced brain function changes may improve.
- A combination of genetic and environmental factors contributes to a person’s vulnerability to AUD.
- People with AUD can receive effective, science-backed treatment in a variety of settings, including primary care.
- A small proportion of patients with AUD will need a few days of "detox" to manage potentially dangerous withdrawal symptoms before starting a long-term care plan.
- Individual paths to recovery vary widely and the majority of people with AUD reduce or resolve their drinking problems over time.
Whether you care for youth or adults, you are likely to encounter patients with alcohol use disorder (AUD) regularly in your practice. According to a 2022 national survey, about 1 in 7 men, 1 in 11 women, and 1 in 33 adolescents (aged 12-17) meet the diagnostic criteria for AUD. 1 Thus, it is important to know how to identify this often-undetected condition, to have a plan for managing it, and to encourage patients that they can recover.
Here, we briefly share the basics about AUD, from risk to diagnosis to recovery. This article introduces a number of AUD topics that link to other Core articles for more detail.
What is AUD?
AUD is a medical condition that is characterized by the Diagnostic and Statistical Manual of Mental Disorders (DSM-5), 2 as “a problematic pattern of alcohol use leading to clinically significant impairment or distress.” AUD can be mild, moderate, or severe, depending on the number of symptoms a patient has experienced in the previous 12 months (see next section on symptoms of AUD). As AUD progresses in severity, alcohol-induced changes in the brain can make it very difficult to cut down or quit. 3 With prolonged abstinence, however, at least some AUD-induced brain function changes may improve and even reverse 4 as other neurocircuits compensate for those compromised by alcohol. 5–7 Evidence-based treatment can help people achieve abstinence and facilitate these brain changes. (See Core articles on neuroscience and treatment .)
Previously, AUD has been referred to as alcohol abuse, alcohol dependence, alcohol addiction, and, colloquially, alcoholism. It is important to note that the terms “alcohol abuse” and “alcoholism” may increase stigma, whereas using the diagnostic term “alcohol use disorder” with patients may help reduce stigma. (See Core article on stigma .)
The term “addiction” is widely used but is not a diagnosis. When drinking becomes compulsive, it can be considered an addiction. 8 In the context of addiction, compulsivity can be described as repetitive behaviors that persevere in the face of adverse consequences and are inappropriate to a particular situation. Individuals who suffer from compulsions often recognize that the behaviors are harmful, but they nonetheless perform them anyway to temporarily reduce tension, stress, or anxiety. 9,10
An alcohol addiction aligns symptomatically with the former diagnosis of alcohol dependence (DSM-IV) and the current diagnoses of moderate or severe AUD (DSM-5). 11,12 Alcohol addiction can be framed as a three-stage cycle that serves as a model for translating the brain changes associated with AUD to the clinical domain. 13,14 In this model, dysregulation occurs in three functional domains, including incentive salience, negative emotionality, and executive function, that overlap with the three stages of the addiction cycle.
- The first stage, called the binge/intoxication stage, is associated with the development of incentive salience neurocircuits, which link the pleasurable, rewarding experience of drinking with “cues” such that the cues gain motivational significance. These and other neurocircuits help develop and strengthen habitual drinking and may lay the groundwork for compulsive use of alcohol.
- The second stage, called the withdrawal/negative affect stage, is associated with states such as anxiety, dysphoria, and irritability, and the person feels alcohol is needed for relief from discomfort and emotional pain.
- The third stage, called the preoccupation/anticipation stage, is associated with executive function deficits.
The three stages are hypothesized to be mediated by three major neurocircuitry elements: the basal ganglia, extended amygdala, and prefrontal cortex, respectively. People who drink heavily can enter the addition cycle at any of these stages. (See the Core article on neuroscience .)
What are the symptoms of AUD?
The DSM-5 defines AUD as a problematic pattern of alcohol use leading to clinically significant impairment or distress, as manifested by at least 2 of the following 11 symptoms occurring within a 12-month period. 2 The number of symptoms determines the severity: 2 to 3 symptoms for mild AUD, 4 to 5 for moderate, and 6 or more for severe.
- Alcohol is often taken in larger amounts or over a longer period than was intended.
- There is a persistent desire or unsuccessful efforts to cut down or control alcohol use.
- A great deal of time is spent in activities necessary to obtain alcohol, use alcohol, or recover from its effects.
- Craving, or a strong desire or urge to use alcohol.
- Recurrent alcohol use resulting in a failure to fulfill major role obligations at work, school, or home.
- Continued alcohol use despite having persistent or recurrent social or interpersonal problems caused or exacerbated by the effects of alcohol.
- Important social, occupational, or recreational activities are given up or reduced because of alcohol use.
- Recurrent alcohol use in situations in which it is physically hazardous.
- Alcohol use is continued despite knowledge of having a persistent or recurrent physical or psychological problem that is likely to have been caused or exacerbated by alcohol.
- A need for markedly increased amounts of alcohol to achieve intoxication or desired effect.
- A markedly diminished effect with continued use of the same amount of alcohol.
- The characteristic withdrawal syndrome for alcohol (See the “How is alcohol withdrawal managed?” section for some DSM-5 symptoms of withdrawal).
- Alcohol (or a closely related substance, such as a benzodiazepine) is taken to relieve or avoid withdrawal symptoms.
Healthcare professionals can use an Alcohol Symptom Checklist [PDF – 147.8 KB] based on these criteria to diagnose AUD and determine its level of severity in patients who screen positive for heavy drinking. (See Core article on screening and assessment .) Routinely integrating such a checklist into primary care may make it easier to hold comfortable, patient-centered, non-judgmental conversations about alcohol that help destigmatize AUD and its treatment. 15,16 (See Core article on stigma .)
Whether or not your patients who drink heavily have AUD, you can help motivate them to cut back or quit 17 as needed by providing advice and assistance, to include noting how alcohol may be causing or worsening other health conditions they may have (see Core articles on brief intervention , medical complications , and mental health issues ).
What puts people at risk for developing AUD?
A complex interplay of genetic and environmental factors influences a person’s risk for AUD. (See Core article on risk factors .) Between 50% and 60% of the vulnerability to AUD is inherited. 18,19 This risk is likely due to common variants in many genes, each of small effect. 20 Different genes confer risk by affecting a variety of biological processes and mental states and traits, including, for example, addiction-related neurobiology, physiological responses to alcohol and stress, co-morbid psychiatric conditions, and behavioral tendencies such as impulsivity. 18,19
Among the environmental risk factors for AUD, external stress may be one of the most potent. 20–22 Your patients who experienced trauma, particularly in childhood, or an accumulation of significant stressors throughout life, may be prone to developing AUD and to relapsing in response to stress during recovery. 20,22 The type of stressor combines with a person’s genetic makeup and drinking history to influence the stress response. Furthermore, once moderate to severe AUD is established, the brain’s stress circuits activate during acute and protracted withdrawal, which fuels negative emotional states and helps to maintain the addition cycle. (See Core article on neuroscience .) Indeed, negative emotional states are the leading precipitant of relapse. 23,24
Additional risk factors for AUD include other mental health conditions, heavy drinking, and the age of onset of drinking, each of which can be influenced by a combination of genetic and environmental factors. People with mental health conditions, including anxiety, depression, and PTSD, have a greater risk for AUD, and vice versa. (See Core article on mental health issues .) And the odds of having AUD are markedly increased among those with heavy drinking patterns 25 and those who started drinking in adolescence, with earlier onset of drinking linked with greater risk of AUD. 26,27
How is AUD treated?
One size does not fit all when it comes to treatment for patients with AUD. The good news is, there are more treatment and support options than many people expect. Healthcare professionals offer two evidence-based options—AUD-focused behavioral healthcare and FDA-approved AUD medications. Many patients also benefit from active participation in mutual support groups such as Alcoholics Anonymous (AA) or a number of secular alternatives (see Resources ), either on their own or as a complement to professionally offered treatment. 28
The behavioral health and medication options for AUD offered by healthcare professionals are about equally effective 28 and can be combined and tailored to the needs of each patient:
- Behavioral healthcare for AUD includes cognitive-behavioral, motivational enhancement, mindfulness-based, contingency management, 12-step facilitation, and couples or family therapy.
- Medication options for AUD include newer FDA-approved medications (acamprosate and naltrexone) that some patients may find more appealing than the older medication (disulfiram) that makes people feel sick if they drink alcohol. 29 AUD medications are non-addicting and easy to prescribe in primary care. (See prescribing guides in the Resources , below.)
Healthcare professionals offer AUD care in more settings than just specialty addiction programs. Addiction physicians and therapists in solo or group practices can also provide flexible outpatient care. These and other outpatient options may reduce stigma and other barriers to treatment. Telehealth specialty services and online support groups, for example, can allow people to maintain their routines and privacy and may encourage earlier acceptance of treatment. The NIAAA Alcohol Treatment Navigator can help you connect patients with the full range of evidence–based, professional alcohol treatment providers.
Active participation in a mutual support group can benefit many people as well. 28 Groups vary widely in beliefs and demographics, so advise patients who are interested in joining a group to try different options to find a good fit. In addition to widely recognized 12-step programs with spiritual components such as AA, a number of secular groups promote abstinence as well, such as SMART Recovery, LifeRing, Women for Sobriety, Secular Organizations for Sobriety, and Secular AA (see Resources , below, for links).
See the Core article on treatment for more details.
How is alcohol withdrawal managed?
Alcohol withdrawal can be life threatening if patients who chronically engage in heavy drinking stop drinking suddenly, rather than cutting back gradually or stopping drinking with medical support. (See Core article on treatment .) Up to half of AUD patients will have some withdrawal symptoms when they stop drinking, and a small proportion will need medical care and monitoring, or “detox,” to manage potentially dangerous symptoms. 30,31 Alcohol withdrawal accounts for approximately 260,000 emergency department visits 32 and 850 deaths each year. 33
According to the DSM-5, symptoms of withdrawal include:
- Elevated pulse and blood pressure
- Nausea or vomiting
- Delirium tremens
In addition, many patients with AUD experience dysphoria and irritability when the effects of alcohol are wearing off. (See Core article on neuroscience .)
Some withdrawal symptoms may be managed in an outpatient detox setting, whereas intensive inpatient detox is needed for patients at risk for potentially life-threatening symptoms. Assessment tools are available to help predict which patients will be at high risk for severe withdrawal symptoms. 28,34 Treatment for acute withdrawal symptoms includes benzodiazepines, considered the gold standard with the deepest evidence base, 35 along with other possible adjunct treatments. 28,34 For more information, see (1) Emergency Department Management of Patients with Alcohol Intoxication, Alcohol Withdrawal, and Alcohol Use Disorder , a white paper prepared for the American Academy of Emergency Medicine, and (2) the Alcohol Withdrawal Management Guideline developed by the American Society of Addiction Medicine. 36
Detox can be a critical first step toward recovery but it is not, in itself, “alcohol treatment.” Treatment and continuing care for AUD are measured in months and sometimes years, not just a few days of detox. (See the Core articles on treatment and recovery .)
What does recovery look like?
Recovery is a dynamic, individualized process through which a person pursues two clinical goals, cessation from heavy drinking and remission from AUD symptoms (except craving, see Core article on recovery ). 37 If people achieve both aims and maintain them over time, they are considered clinically recovered from AUD. Importantly, recovery is often marked by additional improvements in physical health, mental health, relationships, spirituality, and other measures of well-being, which in turn, help sustain recovery. NIAAA has developed a recovery definition that reflects these and other aspects of recovery. 37
While individual paths to recovery vary widely, the majority of people with AUD reduce or resolve their drinking problems over time, with studies showing a reliable pattern of improvement that counters views of AUD as an inevitably worsening disorder. 38–40 The first year can be a mix of gains and setbacks, but in the long term, quality of life measures typically increase and psychological distress decreases. 41
Some patients with AUD may be hesitant to commit to abstinence, but they may be willing to set a starting goal to cut down on their drinking. You can encourage them by sharing the benefits of cutting down significantly, at least as a first step, while noting that abstinence is the safest strategy. (See Core article on brief intervention .)
Even people who have some heavy drinking days following treatment often cut their drinking and related problems by more than half 42 and may feel and function as well as those who do not drink heavily. 43,44 It’s important to acknowledge these marked improvements, which may often be overlooked. 45 (See Core article on recovery .)
As mentioned in this article, you can support recovery by offering patients AUD medication in primary care, referring to healthcare professional specialists as needed, and promoting mutual support groups. See the Core article on recovery for additional, effective strategies that can help your patients prevent or recover from a relapse to heavy drinking, including managing stress and negative moods, handling urges to drink, and building drink refusal skills.
In closing , as a healthcare professional, you are in a prime position to make a difference in the lives of your patients who are vulnerable to AUD, may be in the process of developing AUD, or currently have AUD, by identifying the condition through alcohol screening and assessment, recommending evidence-based treatment, and supporting patients on their individual paths to recovery. The NIAAA Core Resource on Alcohol can help you each step of the way.
Alcohol Use Disorder Medication Guides
- Medication for the Treatment of Alcohol Use Disorder: A Brief Guide , NIAAA and the Substance Abuse and Mental Health Services Administration, 2015
- COMBINE Monograph Series Volume 2: Medication Management Treatment Manual [PDF – 1,351 KB], NIAAA, 2004
- Medications for Adults with Alcohol Use Disorder ( Provider-facing and Patient-facing ), Agency for Healthcare Research and Quality, 2016
- Practice Guideline for the Pharmacological Treatment of Patients With Alcohol Use Disorder ( Summary and Full guidelines ), The American Psychiatric Association, 2018
Alcohol SBIRT Resources Related to this Article
- Alcohol Symptom Checklist [PDF – 80 KB]
Mutual Support Groups
- Alcoholics Anonymous (AA) . Phone: 212-870-3400. Meeting finder app for iOS and Android smartphones: Meeting Guide .
- LifeRing . Phone: 800-811-4142
- Moderation Management .
- Secular AA – Calendar of worldwide secular meetings
- Secular Organizations for Sobriety – Find a meeting
- SMART Recovery . Phone: 440-951-5357
- Women for Sobriety . Phone: 215-536-8026
More resources for a variety of healthcare professionals can be found in the Additional Links for Patient Care .
- Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. 2022 National Survey on Drug Use and Health: Table 5.9B – Alcohol Use Disorder in Past Year: Among People Aged 12 or Older; by Age Group and Demographic Characteristics, Percentages, 2021 and 2022. Accessed January 3, 2024. https://www.samhsa.gov/data/report/2022-nsduh-detailed-tables
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th Edition: DSM-5 . 5th edition. American Psychiatric Publishing; 2013. Reprinted with permission.
- Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry . 2016;3(8):760-773. doi:10.1016/S2215-0366(16)00104-8
- Fritz M, Klawonn AM, Zahr NM. Neuroimaging in alcohol use disorder: From mouse to man. J Neurosci Res . Published online April 22, 2019. doi:10.1002/jnr.24423
- Pfefferbaum A, Desmond JE, Galloway C, Menon V, Glover GH, Sullivan EV. Reorganization of Frontal Systems Used by Alcoholics for Spatial Working Memory: An fMRI Study. NeuroImage . 2001;14(1):7-20. doi:10.1006/nimg.2001.0785
- Koob GF, BS MAA, McCracken M, Moal ML. Alcohol: Neurobiology of Addiction . Vol 3. 1st edition. Academic Press; 2021.
- Rosenbloom MJ, Pfefferbaum A. Magnetic Resonance Imaging of the Living Brain. Alcohol Res Health . 2008;31(4):362-376.
- Substance Abuse and Mental Health Services Administration (US), Office of the Surgeon General (US). Facing Addiction in America: The Surgeon General’s Report on Alcohol, Drugs, and Health . US Department of Health and Human Services; 2016. Accessed May 13, 2021. http://www.ncbi.nlm.nih.gov/books/NBK424857/
- Berlin GS, Hollander E. Compulsivity, impulsivity, and the DSM-5 process. CNS Spectr . 2014;19(1):62-68. doi:10.1017/S1092852913000722
- Robbins TW, Gillan CM, Smith DG, de Wit S, Ersche KD. Neurocognitive endophenotypes of impulsivity and compulsivity: towards dimensional psychiatry. Trends Cogn Sci . 2012;16(1):81-91. doi:10.1016/j.tics.2011.11.009
- Goldstein RB, Chou SP, Smith SM, et al. Nosologic Comparisons of DSM-IV and DSM-5 Alcohol and Drug Use Disorders: Results From the National Epidemiologic Survey on Alcohol and Related Conditions-III. J Stud Alcohol Drugs . 2015;76(3):378-388. doi:10.15288/jsad.2015.76.378
- Alcohol Use Disorder: A Comparison Between DSM–IV and DSM–5. National Institute on Alcohol Abuse and Alcoholism (NIAAA). Accessed November 3, 2021. https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/alcoho…
- Kwako LE, Momenan R, Litten RZ, Koob GF, Goldman D. Addictions Neuroclinical Assessment: A Neuroscience-Based Framework for Addictive Disorders. Biol Psychiatry . 2016;80(3):179-189. doi:10.1016/j.biopsych.2015.10.024
- Koob GF, Powell P, White A. Addiction as a Coping Response: Hyperkatifeia, Deaths of Despair, and COVID-19. Am J Psychiatry . 2020;177(11):1031-1037. doi:10.1176/appi.ajp.2020.20091375
- Sayre M, Lapham GT, Lee AK, et al. Routine Assessment of Symptoms of Substance Use Disorders in Primary Care: Prevalence and Severity of Reported Symptoms. J Gen Intern Med . 2020;35(4):1111-1119. doi:10.1007/s11606-020-05650-3
- Hallgren KA, Matson TE, Oliver M, et al. Practical Assessment of Alcohol Use Disorder in Routine Primary Care: Performance of an Alcohol Symptom Checklist. J Gen Intern Med . Published online August 1, 2021. doi:10.1007/s11606-021-07038-3
- Kaner EF, Beyer FR, Muirhead C, et al. Effectiveness of brief alcohol interventions in primary care populations. Cochrane Database Syst Rev . 2018;2:CD004148. doi:10.1002/14651858.CD004148.pub4
- Reilly MT, Noronha A, Goldman D, Koob GF. Genetic studies of alcohol dependence in the context of the addiction cycle. Neuropharmacology . 2017;122:3-21. doi:10.1016/j.neuropharm.2017.01.017
- Goldman D, Oroszi G, Ducci F. The genetics of addictions: uncovering the genes. Nat Rev Genet . 2005;6(7):521-532. doi:10.1038/nrg1635
- Enoch MA. Genetic influences on the development of alcoholism. Curr Psychiatry Rep . 2013;15(11):412. doi:10.1007/s11920-013-0412-1
- Anthenelli R, Grandison L. Effects of Stress on Alcohol Consumption. Alcohol Res Curr Rev . 2012;34(4):381-382.
- Sinha R. How Does Stress Lead to Risk of Alcohol Relapse? Alcohol Res Curr Rev . 2012;34(4):432-440.
- Marlatt GA. Determinants of Relapse: Implications for the Maintenance of Behavior Change. In: Davidson PO, Davidson SM, eds. Behavioral Medicine: Changing Health Lifestyles . Brunner/Mazel; 1980:410-452.
- Lowman C, Allen J, Stout RL. Replication and extension of Marlatt’s taxonomy of relapse precipitants: overview of procedures and results. The Relapse Research Group. Addict Abingdon Engl . 1996;91 Suppl:S51-71.
- Dawson DA, Li TK, Grant BF. A Prospective Study of Risk Drinking: At Risk for What? Drug Alcohol Depend . 2008;95(1-2):62-72. doi:10.1016/j.drugalcdep.2007.12.00
- Hingson RW, Heeren T, Winter MR. Age at drinking onset and alcohol dependence: age at onset, duration, and severity. Arch Pediatr Adolesc Med . 2006;160(7):739-746. doi:10.1001/archpedi.160.7.739
- Hingson R, Heeren T, Zakocs R, Winter M, Wechsler H. Age of first intoxication, heavy drinking, driving after drinking and risk of unintentional injury among U.S. college students. J Stud Alcohol . 2003;64(1):23-31. doi:10.15288/jsa.2003.64.23
- Witkiewitz K, Litten RZ, Leggio L. Advances in the science and treatment of alcohol use disorder. Sci Adv . 2019;5(9):eaax4043. doi:10.1126/sciadv.aax4043
- Wallhed Finn S, Bakshi AS, Andréasson S. Alcohol consumption, dependence, and treatment barriers: perceptions among nontreatment seekers with alcohol dependence. Subst Use Misuse . 2014;49(6):762-769. doi:10.3109/10826084.2014.891616
- Mirijello A, D’Angelo C, Ferrulli A, et al. Identification and management of alcohol withdrawal syndrome. Drugs . 2015;75(4):353-365. doi:10.1007/s40265-015-0358-1
- Hall W, Zador D. The alcohol withdrawal syndrome. Lancet Lond Engl . 1997;349(9069):1897-1900. doi:10.1016/S0140-6736(97)04572-8
- Healthcare Cost and Utilization Project. Agency for Healthcare Research and Quality. Accessed April 1, 2020. https://www.hcup-us.ahrq.gov/
- Multiple Cause of Death Files 1999-2018 from the CDC WONDER Online Database. Centers for Disease Control and Prevention, National Center for Health Statistics. Accessed April 1, 2020. https://wonder.cdc.gov/mcd-icd10.html
- Wood E, Albarqouni L, Tkachuk S, et al. Will This Hospitalized Patient Develop Severe Alcohol Withdrawal Syndrome?: The Rational Clinical Examination Systematic Review. JAMA . 2018;320(8):825-833. doi:10.1001/jama.2018.10574
- Sachdeva A, Choudhary M, Chandra M. Alcohol Withdrawal Syndrome: Benzodiazepines and Beyond. J Clin Diagn Res JCDR . 2015;9(9):VE01-VE07. doi:10.7860/JCDR/2015/13407.6538
- American Society of Addiction Medicine. The ASAM Clinical Practice Guideline on Alcohol Withdrawal Management. Published online January 23, 2020. Accessed November 3, 2021. https://journals.lww.com/10.1097/ADM.0000000000000668
- Hagman B, Falk D, Litten R, Koob GF. Defining Recovery from Alcohol Use Disorder: Development of an NIAAA Research Definition. Am J Psychiatry . In Press
- Kelly JF, Bergman B, Hoeppner BB, Vilsaint C, White WL. Prevalence and pathways of recovery from drug and alcohol problems in the United States population: Implications for practice, research, and policy. Drug Alcohol Depend . 2017;181:162-169. doi:10.1016/j.drugalcdep.2017.09.028
- Fan AZ, Chou SP, Zhang H, Jung J, Grant BF. Prevalence and Correlates of Past-Year Recovery From DSM-5 Alcohol Use Disorder: Results From National Epidemiologic Survey on Alcohol and Related Conditions-III. Alcohol Clin Exp Res . 2019;43(11):2406-2420. doi:10.1111/acer.14192
- Tucker JA, Chandler SD, Witkiewitz K. Epidemiology of Recovery From Alcohol Use Disorder. Alcohol Res Curr Rev . 2020;40(3):02. doi:10.35946/arcr.v40.3.02
- Kelly JF, Greene MC, Bergman BG. Beyond Abstinence: Changes in Indices of Quality of Life with Time in Recovery in a Nationally Representative Sample of U.S. Adults. Alcohol Clin Exp Res . 2018;42(4):770-780. doi:10.1111/acer.13604
- Miller WR, Walters ST, Bennett ME. How effective is alcoholism treatment in the United States? J Stud Alcohol . 2001;62(2):211-220. doi:10.15288/jsa.2001.62.211
- Falk DE, O’Malley SS, Witkiewitz K, et al. Evaluation of Drinking Risk Levels as Outcomes in Alcohol Pharmacotherapy Trials: A Secondary Analysis of 3 Randomized Clinical Trials. JAMA Psychiatry . 2019;76(4):374-381. doi:10.1001/jamapsychiatry.2018.3079
- Witkiewitz K, Pearson MR, Wilson AD, et al. Can Alcohol Use Disorder Recovery Include Some Heavy Drinking? A Replication and Extension up to 9 Years Following Treatment. Alcohol Clin Exp Res . 2020;44(9):1862-1874. doi:10.1111/acer.14413
- Project MATCH Research Group. Matching alcoholism treatments to client heterogeneity: Project MATCH three-year drinking outcomes. Alcohol Clin Exp Res . 1998;22(6):1300-1311. doi:10.1111/j.1530-0277.1998.tb03912.x
We invite healthcare professionals to complete a post-test after reviewing this article to earn FREE continuing education (CME/CE) credit, which is available for physicians, physician assistants, nurses, pharmacists, and psychologists, as well as other healthcare professionals whose licensing boards accept APA or AMA credits. Others may earn a certificate of completion. This CME/CE credit opportunity is jointly provided by the Postgraduate Institute for Medicine and NIAAA.
Correctly Answer 3 of the 4 Post-Test Questions to Earn CME/CE Credit for This Article
Released on 5/6/2022 Expires on 5/10/2025
This activity provides 0.75 CME/CE credits for physicians, physician assistants, nurses, pharmacists, and psychologists, as well as other healthcare professionals whose licensing boards accept APA or AMA credits. Others may earn a certificate of completion. Learn more about credit designations here .
Please note that you will need to log into or create an account on CME University in order to complete this post-test.
Learning Objectives
After completing this activity, the participant should be better able to:
- Describe the DSM-5 diagnosis of AUD, including awareness of major symptoms and levels of severity.
- Identify major risk factors contributing to the development of AUD.
- List evidence-based options for treatment of AUD.
- Describe symptoms of alcohol withdrawal.
Contributors
Contributors to this article for the NIAAA Core Resource on Alcohol include the writers for the full article, content contributors to subsections, reviewers, and editorial staff. These contributors included both experts external to NIAAA as well as NIAAA staff.
NIAAA Writers and Content Contributors
George F. Koob, PhD Director, NIAAA
Rachel I. Anderson, PhD Health Science Policy Analyst, NIAAA
Raye Z. Litten, PhD Editor and Content Advisor for the Core Resource on Alcohol, Director, Division of Treatment and Recovery, NIAAA
Laura E. Kwako, PhD Editor and Content Advisor for the Core Resource on Alcohol, Health Scientist Administrator, Division of Treatment and Recovery, NIAAA
Maureen B. Gardner Project Manager, Co-Lead Technical Editor, and Writer for the Core Resource on Alcohol, Division of Treatment and Recovery, NIAAA
External Reviewers
Louis E. Baxter Sr., MD, DFASAM Assistant Professor Medicine, ADM Fellowship Director, Howard University Hospital, Washington, DC; Assistant Clinical Professor Medicine Rutgers Medical School, Newark, NJ
John H. Krystal, MD Chair, Department of Psychiatry Yale School of Medicine, New Haven, CT
Jessica L. Mellinger, MD MSc Assistant Professor, Gastroenterology, Internal Medicine, Transplant Hepatology, Michigan Medicine, Ann Arbor, MI
Kenneth J. Sher, PhD Curators’ Distinguished Professor of Psychological Sciences, University of Missouri, Columbia, MO
Katie Witkiewitz, PhD Professor, Department of Psychology, University of New Mexico, Albuquerque, NM
NIAAA Reviewers
Patricia Powell, PhD Deputy Director, NIAAA
Lorenzo Leggio, MD, PhD NIDA/NIAAA Senior Clinical Investigator and Section Chief; NIDA Branch Chief; NIDA Deputy Scientific Director; Senior Medical Advisor to the NIAAA Director
Aaron White, PhD Senior Scientific Advisor to the NIAAA Director, NIAAA
Editorial Team
Contractor support.
Elyssa Warner, PhD Co-Lead Technical Editor, Ripple Effect
Daria Turner, MPH Reference and Resource Analyst, Ripple Effect
To learn more about CME/CE credit offered as well as disclosures, visit our CME/CE General Information page . You may also click here to learn more about contributors .
niaaa.nih.gov
An official website of the National Institutes of Health and the National Institute on Alcohol Abuse and Alcoholism
Reducing Alcohol Use May Help Curb Opioid Misuse, Study Finds

Intervening to reduce alcohol use is associated with a lower likelihood that an individual will receive a new opioid prescription or develop an opioid use disorder, according to a study led by researchers at Duke University and the Durham Veterans Affairs (VA) Medical Center.
The research, which appears in the May 6 issue of the American Journal of Psychiatry, suggests that delivering a brief alcohol-related intervention coincided with less opioid use, fewer opioid use disorder diagnoses, and potentially fewer opioid-related emergency department visits and hospitalizations.
“People are still dying. People are still struggling with addiction. People are still struggling to taper down opioid use. We can’t rest on our laurels. Instead, we need to look at different avenues where we can make improvements.” — Dan V. Blalock, PhD

“The opioid epidemic is something we’ve made a lot of progress on in a lot of ways, but there’s still much work to be done,” said lead author Dan V. Blalock, PhD , associate consulting professor in the Department of Psychiatry & Behavioral Sciences and a clinical research psychologist at the Durham VA’s Center of Innovation to Advance Discovery and Practice Transformation. “People are still dying. People are still struggling with addiction. People are still struggling to taper down opioid use. We can’t rest on our laurels. Instead, we need to look at different avenues where we can make improvements.”
The Link between Alcohol and Opioids
Simultaneous overuse of alcohol and opioid misuse is common. According to research from the National Institutes of Health, roughly 26 percent of people with an opioid use disorder consume high levels of alcohol. These individuals are more than two and a half times as likely to also have an alcohol use disorder than those without an opioid use disorder.* Additionally, people who binge drink (consuming four or five drinks in a single setting) are almost twice as likely to misuse prescription opioids, even after accounting for other relevant factors.
Alcohol use also undermines pain relief—the reason why many people take opioids. Initially, alcohol consumption can reduce pain sensations, much like opioids. However, high use of both substances over time can trigger an increase in pain sensitivity. As a result, a person may boost their alcohol and opioid use to achieve the same level of pain relief, increasing the risk of overdose and death.
With these factors in mind, Blalock and his team examined how reducing alcohol use could be a first-line defense to curbing opioid misuse.
“Many people use alcohol to self-medicate for some pain. But this creates a spiral because excessive alcohol use is associated with higher levels of chronic pain,” he said. “Reducing alcohol use may help with some of the pain issues that lead people to seek out opioids in the first place and prompt them to examine other lifestyle factors.”
Examining an Alcohol-Based Intervention
To determine whether addressing alcohol use could reduce opioid use, including new prescriptions, opioid use disorder, and hospitalizations, Blalock’s team studied the impact of an alcohol-based intervention.
“If you’re using opioids and alcohol, that really increases your risk of harm because they’re both depressants on the system. You’re more likely to have an overdose, some other medical event, or death.” — Dan V. Blalock, PhD
“We know that alcohol use disorder and opioid use disorder co-occur at high rates. If you have one, you’re more likely to have the other,” Blalock said. “If you’re using opioids and alcohol, that really increases your risk of harm because they’re both depressants on the system. You’re more likely to have an overdose, some other medical event, or death.”
In a retrospective review study of medical records from almost 500,000 VA patients, the team determined that about 63,800 patients had elevated alcohol use. They also examined how many of those patients received a brief five- to 15-minute intervention in which providers explained the normative trends of drinking and the health effects of excessive drinking. Referrals for any necessary additional treatment may have been placed.
Among all study participants with elevated alcohol screenings, 72 percent were documented to have received the intervention. Within one year, 8.5 percent had a new opioid prescription, 1.1 percent received a new opioid use disorder diagnosis, and 0.8 percent experienced an opioid-related hospitalization. Patients who didn’t receive the intervention had higher rates of new opioid prescriptions and disorder diagnoses, and although not quite statistically significant, a strong trend toward more opioid-related hospitalizations as well.
Based on these results, Blalock and his team want to dive deeper into the relationship between reduced alcohol consumption and opioid use.
“I want to spend the next several years looking further into the impact of pulling back on alcohol. Even if you’re using opioids to the same extent, it makes sense that there would be less risk involved,” he said. “You’re taking away a risk factor, so there’s a chance that reducing alcohol could also reduce opioid-related harms.”
An Overlooked Approach
While the alcohol-based intervention examined in this study isn’t new, Blalock’s team is the first to assess its impact on opioid use.
“We were trying for some outside-the-box thinking. There’s nothing different happening here with this questionnaire and intervention,” he said. “Instead, we’re looking at an existing tool to see if it may have some effects that we’re missing on other problems that are also happening.”
The hope, he says, is that providers will recognize the value of conducting the annual alcohol screening and intervention and consider it to be part of their opioid risk mitigation strategy.
“We’re hoping that providers’ ears might perk up and that they will be sure to not only check these boxes because they have to, but actively look to administer this intervention anytime it might be indicated,” he said. “Doing so can contribute to building a strong alcohol-opioid surveillance system. And more concretely and directly, it might improve patients’ lives in more ways than we previously thought.”
“We’re hoping that providers’ ears might perk up and that they will be sure to not only check these boxes because they have to, but actively look to administer this intervention anytime it might be indicated. Doing so can contribute to building a strong alcohol-opioid surveillance system. And more concretely and directly, it might improve patients’ lives in more ways than we previously thought.” — Dan V. Blalock, PhD
*Statistic derived from two sources: NIAA and “ Co-Occurring Substance Use And Mental Disorders Among Adults With Opioid Use Disorder ”
Funding for the study was provided by a U.S. Department of Veterans Affairs Health Services Research and Development Career Development Award 19-035 (IK2HXOO3085-01A2, K2HX003087), The Duke Endowment (grant 6754-SP SUB #21 P3630024), and the Center of Innovation to Accelerate Discovery and Practice Transformation at the Durham VA Health Care System (CIN 13-410).
CITATION: “Associations Between a Primary-Care Delivered Alcohol-Related Brief Intervention and Subsequent Opioid-Related Outcomes,” David Blalock, Sophia Berlin, Theodore Berkowitz, Valerie Smith, Charlie Wright, Rachel Bachrach, Janet Grubber. American Journal of Psychiatry, May 1, 2024. DOI: 10.1176/appi.ajp.20230683
A .gov website belongs to an official government organization in the United States.
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Alcohol Use and Your Health
- Underage Drinking
- Publications
- About Surveys on Alcohol Use
- About Standard Drink Sizes
- CDC Alcohol Program
- Alcohol Outlet Density Measurement Tools
- Resources to Prevent Excessive Alcohol Use
- Alcohol Applications and Online Tools
- Funding to Prevent Excessive Alcohol Use
- Alcohol-Related Disease Impact (ARDI) Application
- Check Your Drinking. Make a Plan to Drink Less.
- Controle su forma de beber. Haga un plan para beber menos.
- Deaths from Excessive Alcohol Use in the United States
- Addressing Excessive Alcohol Use: State Fact Sheets
- Excessive alcohol use can have immediate and long-term effects.
- Excessive drinking includes binge drinking, heavy drinking, and any drinking during pregnancy or by people younger than 21.
- Drinking less is better for your health than drinking more.
- You can lower your health risks by drinking less or choosing not to drink.

Why it's important
- The rest of the alcohol can harm your liver and other organs as it moves through the body.
- Using alcohol excessively on occasion or over time can have immediate and long-term health risks.
- By drinking less alcohol, you can improve your health and well-being.
Deaths from excessive alcohol use
Understanding alcohol use, excessive alcohol use.
Excessive alcohol use is a term used to describe four ways that people drink alcohol that can negatively impact health. Excessive drinking can also be deadly.
Excessive alcohol use includes:
- Binge drinking—Four or more drinks for women, or five or more drinks for men during an occasion.
- Heavy drinking—Eight or more drinks for women, or 15 or more drinks for men during a week.
- Underage drinking —any alcohol use by people younger than 21.
- Drinking while pregnant—any alcohol use during pregnancy .
Moderate alcohol use
Moderate drinking is having one drink or less in a day for women, or two drinks or less in a day for men.
Keep in mind
Effects of short-term alcohol use.
Drinking excessively on an occasion can lead to these harmful health effects:
- Injuries— motor vehicle crashes , falls, drownings, and burns.
- Violence—homicide, suicide, sexual violence, and intimate partner violence.
- Alcohol poisoning—high blood alcohol levels that affect body functions like breathing and heart rate.
- Overdose—from alcohol use with other drugs , like opioids.
- Sexually transmitted infections or unplanned pregnancy—alcohol use can lead to sex without protection, which can cause these conditions.
- Miscarriage, stillbirth, or fetal alcohol spectrum disorder (FASD) —from any alcohol use during pregnancy.
Effects of long-term alcohol use
Over time, drinking alcohol can have these effects:
- The risk of some cancers increases with any amount of alcohol use. 2 This includes breast cancer (in women). 2 A
- More than 20,000 people die from alcohol-related cancers each year in the United States. 3
Other chronic diseases
Excessive alcohol use can lead to:
- High blood pressure.
- Heart disease.
- Liver disease.
- Alcohol use disorder—this affects both physical and mental health. B
- Digestive problems.
- Weaker immune system—increasing your chances of getting sick.
Social and wellness issues
- Mental health conditions, including depression and anxiety.
- Learning problems, and issues at school or work.
- Memory problems, including dementia.
- Relationship problems with family and friends.
You can take steps to lower your risk of alcohol-related harms.
The less alcohol you drink, the lower your risk for these health effects, including several types of cancer.
Check your drinking
- The risk of alcohol use leading to breast cancer in men has not been established.
- Most people who drink excessively do not have alcohol use disorder (also known as "alcohol dependence" or "alcoholism"). Many people who drink excessively can lower their alcohol use without specialized medical treatment. Facts about alcohol use disorder are available at: https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/understanding-alcohol-use-disorder .
- Esser MB, Sherk A, Liu Y, Naimi TS. Deaths from excessive alcohol use — United States, 2016-2021. MMWR Morb Mortal Wkly Rep . 2024;73:154–161. doi: http://dx.doi.org/10.15585/mmwr.mm7308a1
- Bagnardi V, Rota M, Botteri E, et al. Alcohol consumption and site-specific cancer risk: a comprehensive dose-response meta-analysis. Br J Cancer . 2015;112(3):580-593. doi: 10.1038/bjc.2014.579
- Esser MB, Sherk A, Liu Y, Henley SJ, Naimi TS. Reducing alcohol use to prevent cancer deaths: estimated effects among U.S. adults. Am J Prev Med . 2024;66(4):725–729. doi: 10.1016/j.amepre.2023.12.003
Alcohol Use
Excessive alcohol use can harm people who drink and those around them. You and your community can take steps to improve everyone’s health and quality of life.
For Everyone
Public health.

An official website of the United States government
Here's how you know
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.

How much is too much?
What are the symptoms of alcohol use disorder (aud).
Having even a couple of symptoms—which you might not see as trouble signs—can signal a drinking problem. It helps to know the signs so you can make a change early. Doctors diagnose AUD when a person has two or more of the symptoms listed below. AUD can be mild (the presence of two to three symptoms), moderate (the presence of four to five symptoms), or severe (the presence of six or more symptoms). See if you recognize any of these symptoms—or others, such as feeling low, dysphoria, or malaise—in yourself. And don’t worry—even if you have a symptom, you can take steps on your own or with help to reduce your risk of AUD and other alcohol-related consequences. (For more information about AUD, see What Are the Harms ?)
In the past year, have you:
- Had times when you ended up drinking more, or longer, than you intended?
- More than once wanted to cut down or stop drinking, or tried to, but couldn't?
- Spent a lot of time drinking, being sick from drinking, or getting over other aftereffects?
- Wanted a drink so badly you couldn't think of anything else?
- Found that drinking—or being sick from drinking—often interfered with taking care of your home or family? Or caused job troubles? Or school problems?
- Continued to drink even though it was causing trouble with your family or friends?
- Given up or cut back on activities that were important or interesting to you, or gave you pleasure, in order to drink?
- More than once gotten into situations while or after drinking that increased your chances of getting hurt (such as driving, swimming, using machinery, walking in a dangerous area, or engaging in unsafe sexual behavior)?
- Continued to drink even though it was making you feel depressed or anxious or adding to another health problem? Or after having had an alcohol-related memory blackout?
- Had to drink much more than you once did to get the effect you want? Or found that your usual number of drinks had much less effect than before?
- Found that when the effects of alcohol were wearing off, you had withdrawal symptoms, such as trouble sleeping, shakiness, restlessness, nausea, sweating, a racing heart, dysphoria (feeling uneasy or unhappy), malaise (general sense of being unwell), feeling low, or a seizure? Or sensed things that were not there?
If you don’t have any symptoms, then staying within the limits provided in the 2020–2025 Dietary Guidelines for Americans could reduce your chances of having problems in the future. If you do have any symptoms, then alcohol may already be a cause for concern. The more symptoms you have, the more urgent the need for change. A health care professional can look at the number, pattern, and severity of symptoms to see whether AUD is present and help you decide the best course of action.
Thinking about a change? The next section may help .
Note: The questions listed above are based on symptoms of AUD in the American Psychiatric Association’s Diagnostic and Statistical Manual (DSM) of Mental Disorders, Fifth Edition, Text Revision. The DSM is the most commonly used system in the United States for diagnosing mental health disorders.
Previous What are the Harms?
Next Pros & Cons
Download or order the free 20-page booklet, “Rethinking Drinking: Alcohol & Your Health” .

Disponible en Español Descargue o ordene copias gratis del folleto de 20 páginas ‘Piénselo Antes de Beber: El Alcohol y su Salud’ .

rethinkingdrinking.niaaa.nih.gov
An official website of the U.S. Department of Health and Human Services and the National Institutes of Health
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Subst Abuse Rehabil
- PMC10657770
Neurobiology and the Treatment of Alcohol Use Disorder: A Review of the Evidence Base
Suzanna donato.
1 Department of Psychology, University of California, Los Angeles, Los Angeles, CA, USA
2 Brain Research Institute, University of California, Los Angeles, CA, USA
3 Department of Psychiatry and Biobehavioral Sciences, University of California, Los Angeles, CA, USA
Alcohol use disorder (AUD) is a significant public health concern, accounting for a majority of substance use disorder cases in the United States. Treatment for AUD is complex, with multiple intervention points that may be further complicated by genotype and phenotype, resulting in diverse outcomes. In order to better understand the current landscape of AUD treatment, the present review considers different etiological models of AUD and assesses the evidence base of current treatment options. The first section of this review summarizes various etiological models of AUD and presents different approaches to classifying the disorder. Various theories, including neurobiological models, are discussed. The second section presents a comprehensive analysis of available treatment options for AUD, encompassing behavioral and pharmacological interventions and their current evidence base. Finally, this review discusses the ongoing treatment gap and significant factors contributing to low treatment utilization. Together, this review provides an overview of different etiological processes and mechanisms of AUD, as well as summarizes the literature on key treatment approaches. By integrating historical, theoretical, and empirical data, this review aims to inform both researchers and providers with valuable insights to advance AUD treatment approaches and narrow the treatment gap.
Introduction
Alcohol use disorder (AUD) is one of the most common psychiatric disorders and represents a serious health condition, affecting nearly 14.1 million people in the United States alone. 1 It is estimated that alcohol contributes to 1 in 10 adult deaths in the United States and more than 140,000 Americans die from alcohol-related causes annually, making it a leading risk factor for premature death. 2 Worldwide, the harmful use of alcohol causes approximately 3.0 million deaths every year and accounts for 5.1% of the total global burden of disease. 3 Higher doses of alcohol in the brain (ie, blood alcohol level 0.08%–0.15%) can lead to motor impairment, muscular incoordination, impairments in reaction time, impairments in judgment, impairments in sensory processing, and impairments in cognitive function. Heavy drinking, both continuous and episodic, has been linked to over 200 diseases and injuries. 4 , 5 Even at low doses, alcohol consumption has been linked to health and cancer risks. 6 Within mental health and medical settings, it is estimated that at least 25% of clients are likely to have AUD as part of their presenting problem and AUD is highly comorbid with other psychiatric conditions such as depression, anxiety disorders, and post-traumatic stress disorder. 7–9
Despite its prevalence and consequences, treatment-seeking rates for AUD are surprisingly low. 10 , 11 It is estimated that less than 10% of individuals that meet criteria for current AUD will seek treatment. 12–14 Treatment seeking status is indicated by a desire to enter treatment and does not necessarily indicate that these individuals will receive treatment. Epidemiological data suggest that there is an average lag of 8 years between AUD onset and the decision to seek treatment. 15 Individual- and systemic-level barriers, coupled with the perceptions around natural recovery and the need for treatment, likely impact treatment utilization. Together, the high documented rates of AUD and observed consequences on health demand research attention in order to improve the landscape of AUD treatment.
The Etiology of AUD and Current Diagnostic Practices
To develop effective therapeutic agents for such a complex disorder, it is important to understand the mechanisms related to the development and maintenance of AUD. The NIAAA currently defines AUD as a “chronic relapsing brain disease characterized by an impaired ability to stop or control alcohol use”. 16 Neurobiological models of AUD theorize that alcohol addiction is based on pathological changes to the system produced by chronic alcohol use. 17 , 18 The allostatic model of addiction 19 , 20 emphasizes three primary stages, which are thought to cycle among one another and increase in amplitude with repeated experience. The first stage is characterized as “binge/intoxication”, or loss-of-control drinking. Alcohol activates the reward circuitry of the brain (eg, neurocircuitry in ventral striatum), leading to acute reinforcing effects and increased salience of alcohol cues (ie, incentive salience). The positive reinforcing effects of alcohol are also facilitated at the neurotransmitter level by dopamine, opioid peptides, and γ-aminobutyric acid (GABA). As the dose of alcohol increases, activation in the basal ganglia shifts from the nucleus accumbens to the dorsal striatum, engaging “habit” neurocircuitry (ie, habit formation). In the “withdrawal/negative affect” stage, withdrawal from chronic alcohol use disrupts reward neurotransmitter function (eg, dopamine and opioid peptide function in nucleus accumbens), creating a reward deficit. Further, changes to key neuromodulators of stress reactivity (eg, neuropeptide Y and corticotropin-releasing factor (CRF)) enhance stress reactivity in the amygdala. Excessive drinking at this stage is thought to be maintained by negative reinforcement (ie, drinking to alleviate negative affective and physiological states). Finally, the “preoccupation/anticipation stage” involves adaptations to the prefrontal cortex (ie, the control center of the brain) and is characterized by the return to alcohol-seeking behaviors after a period of abstinence. These alcohol-seeking behaviors are encouraged by prolonged deficits in reward circuitry (ie, dopamine, opioid peptide, and GABA function) and protracted stress effects (eg, corticotropin-releasing factor, norepinephrine, neuroimmune function, neuropeptide Y, and oxytocin). Essentially, AUD is theorized to be the result of deficits in both cognitive control and reward circuitry. 21 Understanding the neurobiological bases behind an individual’s inability to control behavior toward alcohol and increased sensitivity to the saliency of alcohol cues helps to explain why individuals with AUD continue to struggle in controlling their drinking, despite the desire to quit or cut down. This model offers advantages for outlining neural mechanisms relevant to treatment research and shifting the perspective of AUD away from a “lack of willpower.” However, the neuroscience framework is not the only perspective with valuable implications for clinical translation and only represents a piece of the puzzle.
The biological mechanisms discussed above fit within a larger framework known as the biopsychosocial model of addiction. 22 , 23 This model, recently reviewed and updated by Ray and Grodin, 24 is meant to provide a rich multidimensional perspective that addresses the complexity of the disorder and emphasizes the interplay of multiple factors, rather than relying on a single explanatory factor. Support for this model has been shown through work on the interplay of genetics and environment, impacting one’s susceptibility towards AUD. 25 , 26 The psychosocial components of the model include psychological theories and social influences, with both domains lending significant discoveries for treatment development. Psychological models of addiction stress the importance of factors such as reinforcement-based learning, subjective response to alcohol, maladaptive cognitive processes, personality factors (eg, impulsivity), and developmental psychopathology. 27 Social models of addiction investigate factors such as social networks, parent modeling, social identity, and sociocultural context. Taken together, a biopsychosocial approach to alcohol use disorder provides an array of etiological processes and mechanisms for clinicians to consider in the treatment of AUD. Additionally, it can offer rationale towards collaboration between professionals (eg, physicians and psychologists) and more comprehensive care targeting multiple domains of functioning such as work, health, and interpersonal relationships.
As theorized by contemporary models of addiction, each individual develops an AUD based on the complex interaction of underlying genetic and environmental mechanisms. 28 Some of the strongest support for this model is shown through twin and adoption studies. 29–31 Further, recent models theorize that AUD manifests itself in a continuum of severity and phenotypic profiles, ranging from the occasional social drinker to the chronic relapsing heavy drinker. As opposed to a disease model orientation (ie, defined by presence or absence), conceptualizing AUD as existing on a continuum of severity may aid in greater problem recognition among lower severity individuals. Further, research on the prevention paradox confirms that a majority of the overall harm caused by alcohol arises from the large majority of individuals who drink at the light to moderate level, as opposed to the small minority at the severe end. Therefore, it can be suggested that greater health improvements at the population level will come from prevention strategies aimed at the entire population of drinkers. While there is some concern over how the continuum model of severity will affect the treatment landscape, advancing this model may have significant benefits to public beliefs about the nature of alcohol use and potentially improve overall population-level outcomes. Support for these models, including consumption-based models that define AUD in terms of heavy use over time, 32 can be seen through efforts to promote drinking reduction as a viable treatment target. For example, WHO drinking risk levels have been shown to correspond to improvements in health and quality of life and have been suggested as clinically meaningful endpoints to be used in clinical trials. 33
Research has acknowledged the heterogeneity of AUD and attempted to capture different phenotypic profiles through empirically based typology systems that consider multiple factors such as genetics, drinking profiles, motivation for drinking, and personality traits (eg, 34–38 ). However, a crucial limitation to the translation of addiction research into clinical samples lies in the misalignment between research-based models of AUD and the current DSM diagnostic criteria. A major critique of the DSM-5’s AUD criteria is that they are not informative about underlying mechanisms of dysfunction and fail to account for the heterogeneity that exists. If research has given us empirical data to support a wide array of mechanisms involved in the development and maintenance of AUD and identified important phenotypic profiles related to treatment outcomes, should our diagnostic methods not be able to reflect and accurately capture these mechanisms?
Currently, the diagnosis of AUD is set to represent a single disorder, ranging along three categories of severity (DSM-5 ® ; 39 ). To be diagnosed with AUD, an individual must meet at least two of 11 criteria. The 11 diagnostic criteria include (1) drinking larger amounts of alcohol or drinking over longer periods than planned; (2) having the desire to or attempting to cut down or stop without success; (3) spending considerable time drinking alcohol and recovering from its effects; (4) experiencing a persistent craving for alcohol; (5) failing to fulfill major social role obligations, such as those related to work, home, or school; (6) neglect of other activities; (7) continuing to drink despite the fact that drinking is causing social or interpersonal problems; (8) continuing to use despite knowing that alcohol is causing recurrent physical or psychological problems; (9) drinking repeatedly in a way that that has the potential to create physical harm (eg, drinking and driving); (10) exhibiting signs of physical tolerance; and (11) showing signs of physical withdrawal. Based on the number of criteria met, the AUD is then classified as mild (two to three symptoms), moderate (four to five symptoms), or severe (six to 11 symptoms).
The new conceptualization of AUD in DSM-5 marks a change from the previous edition of the DSM (ie, DSM-IV), which discriminated between alcohol abuse and alcohol dependence. Additionally, the new criteria eliminated the previous DSM-IV symptom of “repeated alcohol-related legal consequences”, and added a criterion for craving. The problem with this approach is it oversimplifies the phenotypic profile of individuals with AUD and lumps them into three categories based on a simplified “count” of symptoms. There are over 2000 different symptom profiles possible to meet criteria for AUD, including approximately 55+ ways to configure a “mild” diagnosis, 330+ ways to configure “moderate”, and 462+ ways to configure a “severe” diagnosis. By the terms of the current diagnostic system, an individual presenting with withdrawal symptoms, cravings, and tolerance would be labeled the same as an individual presenting with interpersonal problems and a persistent desire to control their drinking. The DSM is often used to determine treatment programs, despite the fact that the thresholds set by the DSM have often failed to predict treatment response. 40–42 Further, the DSM is often used to determine study inclusion for clinical trials in order to promote external validity, despite the fact that use of the DSM likely limits the enrollment of individuals representing the larger spectrum of functioning. Overreliance on the categorical classifications set by the DSM may present a serious hindrance to the translation and reverse-translation between research findings and clinical decision-making.
To address this issue, there has been a movement toward a new classification framework known as the Research Domain Criteria (RDoC), an initiative funded by the National Institute of Mental Health (NIMH) in 2009. RDoC is intended to be a transdiagnostic, neuroscience-based research framework approach that focuses on specific domains that can help explain psychopathology in terms of varying degrees of dysfunction in fundamental psychology/biological systems. 43 , 44 RDoC is not meant to serve as a diagnostic guide, although it is hoped that research using the RDoC framework can lead to revisions to diagnostic systems, as well as the development of screening tools and treatment interventions. To represent RDoC in the addiction field, the Addictions Neuroclinical Assessment (ANA) was recently proposed as a research framework for neuroscience-informed assessment that captures three functional domains – incentive salience, negative emotionality, and executive dysfunction. 45 , 46 This framework aims to understand the heterogeneity in AUD by leveraging deep phenotyping profiles coupled with factor analytic methods. 47 , 48 The benefit of these research methods over DSM is that they do not rely on disorder-based categories and instead span functional domains, each containing a set of constructs that include elements, processes, mechanisms, and responses informed by various types of information including genetics, brain circuitry, behavior, physiology, and self-report. Once validated, the heuristic framework offered in ANA presents new opportunities whereby dysfunctions in these domains may serve as treatment targets.
In addition to the ANA framework as a translational approach, understanding reward and relief drinking has been advanced in recent years as a clinically relevant marker. These two subtypes of AUD are centered around the underlying motivation for alcohol consumption, specifically categorized as drinking for hedonic pleasure (reward type) or drinking to mitigate negative emotions (relief type). Recent empirical studies have underscored the potential clinical relevance of this typology by demonstrating that individuals exhibiting reward drinking benefit more from naltrexone, a medication known to blunt the rewarding effects of alcohol, compared to other medications. 49 , 50 The identification and differentiation of reward and relief drinking subtypes within the context of AUD may offer valuable insight into tailoring personalized treatment interventions.
By acknowledging AUD as a complex clinical phenomenon, we must inherently accept that there is not a single causal path to this disorder. By creating a translational framework that integrates neurobiological models of addiction with observed clinical phenotypes, we can refine our conceptualization of clinical phenomena and make more precise treatment selection.
Overview of AUD Treatment Options
According to NESARC-III data, which uses AUDADIS, among those with current, past 12-month AUD diagnoses, the most commonly reported treatment modalities included 12-step programs (4.5%), health-care providers (3.6%), outpatient substance abuse treatment (2.0%), emergency departments (1.4%), various family and social services (1.4%), inpatient detoxification (1.3%), and other inpatient programs (1.2%). 51 The available treatment options will be briefly reviewed in two broad categories: behavioral treatments and pharmacological treatments.
Behavioral treatment options for AUD include cognitive-behavioral therapy, motivational interviewing, inpatient and outpatient rehabilitation facilities, and mutual support/12-step groups. Among the behavioral treatments with the best support are brief interventions, motivational interviewing, cognitive-behavioral treatment, 12-step facilitation treatment, behavioral couple therapy, cue exposure treatment, mindfulness-based relapse prevention, and the community reinforcement approach. Figure 1 offers a visual representation of common psychosocial treatments, ranked by AUD disorder stage and strength of evidence base. 52

Summary of available psychological treatments for AUD with the y-axis indicating the strength of the evidence in favor of a particular treatment and the x-axis indicating the recommended placement of that treatment across the continuum of AUD severity.
Although often underutilized, pharmacological treatment options do exist. Since 1949, the United States FDA has approved 3 evidence-based medications for treating AUD. These pharmacotherapies include 1) disulfiram (Antabuse), first introduced in 1951; 2) naltrexone, approved in 1994 as an oral formulation (Revia) and in 2006 as a long-acting injectable formulation (Vivitrol); and 3) acamprosate (Campral), approved in 2004. 53 Disulfiram is an aversion therapy agent that blocks the enzyme aldehyde dehydrogenase and leads to the build-up of acetaldehyde causing facial flushing, headache, hypotension, nausea, and vomiting. Naltrexone works by blocking the endogenous opioid system and decreasing the craving for alcohol, as well as blunting the rewarding effects of alcohol. Finally, acamprosate works by increasing gamma-aminobutyric acid (GABA) and decreasing N- methyl-D-aspartate (NMDA) in order to reduce craving for alcohol and lessen negative emotional states during withdrawal. Overall, these medications target symptomatic improvement (eg, suppression of alcohol-craving) or act to blunt or punish the reinforcing properties of alcohol.
While the number of approved medications for AUD pales in comparison to other psychiatric disorders such as major depression, which shares a similar prevalence with AUD yet boasts more than 20 medications approved by the FDA, there has been progress in medication development in the past few decades. Figure 2 highlights the current repertoire of promising medications, stratified by the strength of evidence base and appropriate AUD disorder stage. 52 Currently, there are several off-label (ie, not FDA-approved for the treatment of AUD) medications with promising findings, including nalmefene, baclofen, gabapentin, ondansetron, topiramate, varenicline, ABT-436, and zonisamide (Litten et al, 2016, Ray et al, 2019). Additionally, other off-label medications with preliminary support include Mifepristone (RU-486); Aripiprazole; Ibudilast; Prazosin; Doxazosin; N -Acetylcysteine (NAC); and Suvorexant. There are also novel medications with promising theoretical support, including: N -[(4-Trifluoromethyl) benzyl] 4-methoxybutyramide (GET73), ASP8062, PF-5190457, and cannabidiol (CBD). Given the importance of translating research findings into clinical settings and the potential burden placed on providers to stay up to date on the most recent clinical trial results, comprehensive summaries on the evidence-base for available treatment methods are routinely published to inform best treatment practices (eg, 52 , 54–56 ). Most recently, the American Psychiatric Association published “Practice Guideline for the Pharmacological Treatment of Patients with Alcohol Use Disorder”, 57 which translates current findings on AUD pharmacotherapies into evidence-based recommendations for medication use. It also includes a discussion on factors relevant to medication success (eg, non-compliance, negative drug interactions, and bothersome side effects) and recommendations.

Summary of available pharmacological treatments for AUD with the y-axis indicating the strength of the evidence in favor of a particular treatment and the x-axis indicating the recommended placement of that treatment across the continuum of AUD severity. Pharmacotherapies are divided into FDA-approved and off-label treatments.
The combination of multiple therapeutic approaches (eg, behavioral and pharmacological) has also been investigated as a potential treatment approach and reviewed in the literature. 58–60 For example, meta-analytic approaches have been used to investigate the effectiveness of combined interventions compared to monotherapy in the treatment of AUD. Most found a benefit in incorporating pharmacotherapy with psychotherapy, as compared to psychotherapy alone 61–63 . However, this pattern of benefit was not found in the landmark US study Project COMBINE. 64 The results of adding psychotherapy to pharmacotherapy treatment are also unclear, with fewer studies demonstrating a possible added value for combined therapy. 61 Other studies have also investigated the benefits of combining multiple psychosocial approaches, such as cognitive-behavioral therapy, motivational interviewing, and multiple pharmacotherapies. In a previous meta-analysis, 62 CBT combined with another psychosocial treatment (ie, motivational interviewing or contingency management) showed a pooled effect size roughly double that of studies testing CBT alone. The literature on combining multiple pharmacological options is difficult to summarize succinctly given differences in co-morbidities, medications, and treatment targets. A systematic review investigating the clinical evidence of combined pharmacological interventions for individuals with AUD without co-morbid conditions found no significant benefit of combination medication over single agents. 59 Further research in the area of combination therapies is warranted in order to clarify the utility of this approach.
Of note, there has been some progress in developing novel treatment options beyond the domains of current behavioral and pharmacological treatment options. One such treatment option is repetitive transcranial magnetic stimulation (rTMS), a non-invasive neurophysiological tool that has the ability to modulate activity in discrete brain regions and has shown therapeutic efficacy in major depression 65 , 66 and tobacco use disorder. 67 , 68 Given the association of brain reward circuitry with alcohol use disorder, stimulation of these neutral systems, such as the dorsal lateral prefrontal cortex (dIPFC), via rTMS may serve as a useful treatment for AUD.
Another innovative approach to the treatment of AUD is technology-based interventions. For example, telemedicine programs that can be accessed through one’s smartphone have shown promise for reducing alcohol use. 69 Other technology-based interventions that have shown preliminary evidence for targeting alcohol consumption include web-based applications (eg, 70 wearable biosensors, 71 smartphone applications (eg, 72 and computer-delivered treatment modules. 73 , 74 Notably, systematic reviews and meta-analyses of technology-based interventions have highlighted limitations on the research support of these interventions, including small effect sizes and limited well-controlled comparisons. 75–77 However, the availability of these interventions is promising, as they reflect a push in the field for innovative treatment options that can improve accessibility and help bridge the alarming “treatment gap” that exists.
Within AUD treatment research, there is a well-documented “treatment gap.” 10 , 78 , 79 This “gap” may be impacted by factors affecting individuals’ motivation to seek treatment, as well as factors affecting individuals’ ability to connect with care. In a recent review by our laboratory, 10 we have discussed various reasons for low treatment seeking rates, including both person-related treatment barriers and treatment-related barriers. Person-related barriers that were identified in the review include attitudes and beliefs (eg, “my drinking isn’t serious enough” and “the problem will get better by itself”), fear of stigma, and socioeconomic status. The treatment-related barriers included “lack of treatment knowledge and options” and “cost of treatment” 10 For treatment utilization, the latest national surveys estimate that less than 8% of adults with AUD receive pharmacotherapy and/or psychotherapy treatment. 1 , 78 , 80 Globally, it is estimated that approximately one in six people with AUD receives treatment. 79 These treatment statistics are even lower when confined to pharmacotherapy utilization. In the United States, it is estimated that only 4% of individuals with AUD receive a Food and Drug Administration (FDA)–approved medication for treatment. 81–83 Recent results from the 2019 National Survey on Drug Use and Health indicate that this rate may be as low as 1.6%. 84 It should be noted that these rates are confined to utilization of FDA-approved medications and there is some evidence that these rates are slightly higher when off-label medications are taken into consideration. 85 , 86
The low rates of medication utilization for AUD may be due to the small-to-moderate effect size of the available medications, further complicated by factors such as non-compliance, co-morbid conditions, and genetic variations impacting response. Data-driven methods, such as machine learning, offer unique opportunities to identify factors affecting treatment response. For example, the first genome-wide association study of AUD treatment outcomes found evidence of a polygenic effect on AUD treatment response and identified certain genetic variants shown to influence medication effects. 87 Other data-driven approaches have identified important factors relevant to treatment response for a number of widely utilized medications and behavioral approaches. 42 , 88 By looking deeper into the low treatment rates and investigating factors that impact treatment success, clinical researchers can more readily identify opportunities to improve the landscape of AUD.
Conclusions
AUD is a complex clinical phenomenon, with multiple potential pathways to the disorder. By refining our conceptualization of AUD and identifying key factors contributing to its development and maintenance, researchers can uncover opportunities for translation and advancements in treatment. Other clinical resources, such as published practice guidelines (eg, “Practice Guideline for the Pharmacological Treatment of Patients with Alcohol Use Disorder”; APA, 2021), further assist providers by providing specific statements and recommendations on evidence-based treatments for AUD.
There are multiple potential intervention points in the treatment of AUD, including withdrawal management, targeting symptomatic improvement (eg, craving), behavioral/cognitive modification, and relapse prevention. The most extensively supported interventions for AUD include behavioral interventions (eg, cognitive-behavioral therapy and motivational interviewing) and pharmacotherapies (naltrexone and acamprosate). Notably, studies demonstrate that treatment outcomes can be further improved when pharmacotherapies are used in combination with psychosocial treatments. 60 , 61 The field has also witnessed growing interest in novel technology-based therapeutic approaches, which have the potential to improve overall accessibility and ease of treatment delivery.
The heterogeneity of AUD and the complexity of treatment, coupled with the additional individual- and systemic-level barriers, likely impacts treatment utilization and treatment efficacy among individuals with AUD. It is well known in the field that a “one size fits all” approach to treatment is unlikely to work given the heterogeneity of AUD. Instead, both health-care providers and patients frequently adopt a multifaceted “more is better” approach, integrating various evidence-based resources concurrently to improve the chances of achieving sustained recovery. Additionally, the demonstrated negative health implications of alcohol consumption, even at light-to-moderate levels, suggest the utility of more population-level interventions and prevention efforts. As the field of AUD treatment continues to prioritize the advancement of novel therapeutic options, this current review aims to establish a foundation by summarizing decades of research on diverse treatment options. In highlighting those interventions with the strongest evidence base, we underscore the scientific basis of treatment methodologies and the significance of translating research findings into advancements in patient care.
The authors report no conflicts of interest in this work.
Advertisement
- Register for Free
- My account Orders Downloads Address Payment methods Account details
- About About Psychiatrist.com About JCP About PCC About CME Institute
Error: Search field were incomplete.

Swiss Scientists Develop Alcohol Neutralizing Gel
by Denis Storey May 16, 2024 at 12:11 PM UTC

Clinical relevance: Swiss researchers developed a protein gel that converts alcohol into harmless acetic acid in the digestive system.
- The gel prevents alcohol’s intoxicating and harmful effects before it enters the bloodstream.
- Testing on mice showed significant reductions in blood alcohol levels and liver damage.
- The gel is made from whey proteins, iron, glucose, and gold, triggering a multi-stage enzymatic reaction.
It probably won’t earn it a spot in any 12-step program, but a team of Swiss researchers announced that they’ve developed a protein gel that helps the body synthesize alcohol by breaking it down in the digestive system.
Researchers at ETH Zurich, in Zurich, Switzerland, published a paper in the journal Nature Nanotechnology , that claims this gel –in mice, at least – “converts alcohol quickly, efficiently, and directly into harmless acetic acid before it enters the bloodstream, where it would normally develop its intoxicating and harmful effects.”
Interrupting Alcohol’s Destructive Path
Alcohol typically makes its way into the bloodstream through the mucous membrane layer of the stomach and the intestinal tract, where it does its well-documented damage:
- In the short term, it hampers concentration and impairs one’s reaction time.
- Over the longer term, it wreaks havoc on the liver and the gastrointestinal tract while increasing cancer risk.
In the United States alone, alcohol-related deaths have jumped more than 29 percent over the last decade, according to the U.S. Centers for Disease Control and Prevention (CDC). Globally, the World Health Organization (WHO) contends that alcohol use contributes to 3 million deaths every year, accounting for 5.3 percent of fatalities.
How Does It Work
“The gel shifts the breakdown of alcohol from the liver to the digestive tract. In contrast to when alcohol is metabolized in the liver, no harmful acetaldehyde is produced as an intermediate product,” Professor Raffaele Mezzenga from the Laboratory of Food & Soft Materials at ETH Zurich, explained in a press release.
Acetaldehyde is a toxin, responsible for multiple health problems stemming from alcohol overconsumption.
In theory, consumers could take the gel orally either before or during consumption to stem rising blood alcohol levels, thereby preventing acetaldehyde from damaging the body. As opposed to so-called “hangover cures” already on the market, this gel, the researchers claim, targets the cause of intoxication rather than its symptoms.
Despite that, the Swiss scientists explain that “the gel is only effective as long as there is still alcohol in the gastrointestinal tract.” As a result, the gel can’t do much once alcohol has made it into the bloodstream. And it certainly doesn’t address the addiction that leads to overconsumption.
“It’s healthier not to drink alcohol at all,” Mezzenga added. “However, the gel could be of particular interest to people who don’t want to give up alcohol completely, but don’t want to put a strain on their bodies and aren’t actively seeking the effects of alcohol.”
Methodology
The researchers tested the gel’s efficacy by giving it to mice that had taken alcohol once, as opposed to those who’d received it for 10 days.
After 30 minutes, “the prophylactic application of the gel reduced the alcohol level in the mice by 40 percent.” Five hours later, their blood alcohol levels tumbled more than 55 percent.
The research team noticed a reduction in the accumulation of acetaldehyde, less damage to the liver, and higher overall blood values.
In the mice that had received alcohol for ten days, the researchers found not only lower alcohol leves,l” but also a lasting therapeutic effect of the gel: the mice that were given the gel daily in addition to alcohol showed significantly less weight loss, less liver damage and hence better fat metabolism in the liver as well as better blood values.”
Simple Ingredients
The researchers relied on everyday whey proteins to produce the gel. After boiling the proteins into long, thin fibrils, the researchers then added salt and water to act as a solvent that converts the fibrils into the gel. But they lacked catalysts.
“We immersed the fibrils in an iron bath, so to speak, so that they can react effectively with the alcohol and convert it into acetic acid,” ETH researcher Jiaqi Su, the first author of the study, explained.
The researchers then relied on hydrogen peroxide – generated by an upstream reaction between glucose and gold nanoparticles – to trigger the necessary reaction.
The scientists turned to gold to trigger hydrogen peroxide since gold can’t be digested, “and therefore stays effective for longer in the digestive tract.”
The researchers took all three substances – iron, glucose, and gold – and worked them into the gel, which “resulted in a multi-stage cascade of enzymatic reactions that ultimately converts alcohol into acetic acid.
Needless to say, the researchers have already applied for a patent, while conceding that regulators will demand more rounds of clinical trials.
Further Reading
Patients With Alcohol Use Disorder Co-Occurring With Depression and Anxiety Symptoms
Decrease in Alcohol Use Disorder Symptoms With Semaglutide
Screening and Referral Algorithm for Substance Use
Clinical and Practical Psychopharmacology

Antipsychotic Medication Continuation vs Taper and Discontinuation in Patients With Schizophrenia and Other Nonaffective Psychotic Disorders
Dr Andrade discusses two studies that examined the outcomes of gradual, individualized antipsychotic dose reduction and discontinuation in patients with psychosis.
Chittaranjan Andrade
Letter to the Editor

Geriatric Depression: What Clinicians Need to Know
The authors discuss what clinicians should be aware of when caring for elderly patients with depression.
Ahmed Naguy and others
Search Articles
Related articles, table of contents.

IMAGES
VIDEO
COMMENTS
Abstract. Alcohol is a major contributor to global disease and a leading cause of preventable death, causing approximately 88,000 deaths annually in the United States alone. Alcohol use disorder is one of the most common psychiatric disorders, with nearly one-third of U.S. adults experiencing alcohol use disorder at some point during their lives.
Only a small percent of individuals with alcohol use disorder contribute to the greatest societal and economic costs ().For example, in the 2015 National Survey on Drug Use and Health survey (total n = 43,561), a household survey conducted across the United States, 11.8% met criteria for an alcohol use disorder (n = 5124) ().Of these 5124 individuals, 67.4% (n = 3455) met criteria for a mild ...
Alcohol Research Resource (R24 and R28) Awards. Resources include biological specimens, animals, data, materials, tools, or services made available to any qualified investigato r to accelerate alcohol-related research in a cost-effective manner. Current and potential alcohol research investigators and trainees are encouraged to subscribe to our ...
Alcohol is the most commonly used substance in the United States, with 84% of people 18 and older reporting lifetime use, according to data from the 2022 National Survey on Drug Use and Health. Alcohol use exists along a spectrum from low risk to alcohol use disorder (AUD). The intervening category, known as risky drinking, includes heavy drinking as well as binge drinking.[1] AUD is a chronic ...
It is well recognized that alcohol use disorders (AUD) have a damaging impact on the health of the population. According to the World Health Organization (WHO), 5.3% of all global deaths were attributable to alcohol consumption in 2016 [].The 2016 Global Burden of Disease Study reported that alcohol use led to 1.6% (95% uncertainty interval [UI] 1.4-2.0) of total DALYs globally among females ...
Pathologic alcohol use affects more than 2 billion people and accounts for nearly 6% of all deaths worldwide. There are three medications approved for the treatment of alcohol use disorder by the US Food and Drug Administration (FDA): disulfiram, naltrexone (oral and long-acting injectable), and acamprosate. Of growing interest is the use of anticonvulsants for the treatment of alcohol use ...
Incorporating harm reduction into alcohol use disorder treatment and recovery. Research Update. ... complete abstinence from alcohol consumption was viewed as the most effective way to recover from alcohol use disorder (AUD) and was a primary outcome of AUD treatment. A large body of evidence, however, suggests that treatment and recovery ...
Alcohol is regularly consumed throughout most of the world, including by nearly half the U.S. population age 12 or older. Heavy drinking, which is also common, contributes to multiple adverse medical, psychiatric, and social outcomes and more than 140,000 deaths annually in the United States. It is the major risk factor for alcohol use disorder (AUD), whose current U.S. prevalence is 11% ...
New research characterizes alcohol use disorder profiles to predict treatment outcomes. Research Update. Friday, March 3, 2023. Image. This article was first published in NIAAA Spectrum Volume 15, Issue 1. Alcohol use disorder (AUD) is a heterogeneous disorder, meaning individuals with AUD differ in their clinical symptoms and in the biological ...
Lifetime incidence of alcohol use disorder (AUD) is as high as 29-30% 1,2, with alcohol use being a leading cause of death [3 million worldwide in 2016 or 5.3% of all deaths worldwide 3].
Sleep-Related Predictors of Risk for Alcohol Use and Related Problems in Adolescents and Young Adults ... 1 February 2024. Gut-Liver-Brain Axis and Alcohol Use Disorder: Treatment Potential of Fecal Microbiota Transplantation. Jennifer T. Wolstenholme, 1,2 Nikki K. Duong, 3,4 ... 2024 marks the 50th anniversary of Alcohol Research: Current ...
Alcohol use disorder (AUD) ... However, more research into object recognition memory and reward-related learning and memory is needed to draw strong conclusions in these domains.
Research on the treatment of alcoholism has gained significant ground over the past 40 years. Studies such as the National Institute on Alcohol Abuse and Alcoholism's Project MATCH, which examined the prospect of tailoring treatments for particular people to better suit their needs, and Project COMBINE, which examined in-depth, cognitive-behavioral therapy and medical management, helped ...
In 2010, the economic burden of alcohol misuse in the United States was estimated at a quarter of a trillion dollars, which is equivalent to $807 per person, or $2.05 per drink. 3. Treatment options for the 30 million individuals with alcohol use disorder (AUD) 4 in the US include behavioral interventions and pharmacotherapy. Naltrexone ...
We would like to feature articles that describe novel approaches to alcohol and substance-related research, practices, and topics that also include findings, thoughts, or recommendations across the continuum of care. There is still a shortage of recovery-focused research. ... where 90% of attendees endorsed alcohol use disorder. A mixed-methods ...
Major federal investments into women's health research are a vital step toward developing better prevention and treatment options for ... For more information on alcohol use disorder, causes, ...
INTRODUCTION. Treatments for alcohol use disorder (AUD) are highly effective (Huebner & Kantor, 2011; Miller et al., 2001), but national surveys in the United States have consistently shown low utilization.For example, the National Longitudinal Alcohol Epidemiology Survey (NLAES; 1991-1992) and Wave 1 of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC; 2001-2002 ...
Alcohol use disorder (or alcoholism) is also a clear issue for the brain. It has been linked to a higher risk for dementia, especially early-onset dementia in a study of 262,000 adults, as well as ...
INCORPORATING HARM REDUCTION INTO ALCOHOL USE DISORDER TREATMENT AND RECOVERY For many years, complete . abstinence from alcohol . consumption was viewed as the most effective way to recover from alcohol use disorder (AUD) and was a primary outcome of A UD treatment. A large body of evidence, however, suggests that treatment and recovery
"Drug use disorder and alcohol use disorder are among the most stigmatized conditions universally across different societies because people feel that it's self-induced — that people are to blame because they put it in their body," said Kelly, also the founder of Mass General's Recovery Research Institute. "Just because they made ...
Alcohol use disorder (AUD) is the most prevalent SUD, with 5% of persons age 12 and older reporting AUD in 2018. Of persons with an SUD in 2018, and excluding those with a tobacco use disorder, 60% had AUD, 27% had an illicit drug use disorder, and 13% had disorders involving alcohol and illicit drugs. On a lifetime basis, almost one-third of ...
Alcohol use disorders (AUDs) ... Research into neuroinflammatory signaling focused on secretory chemokines and cytokines as opposed to intracellular signaling cascades and was also heavily weighted toward pro-inflammatory mediators as opposed to anti-inflammatory mediators. A clear focus for signaling cascades was on extracellular receptors and ...
Binge drinking is a growing public health crisis − a neurobiologist explains how research on alcohol use disorder has shifted Nikki Crowley, Penn State May 13, 2024 at 8:17 AM · 7 min read
Cancer Characteristics According to Sex, All of Us Research Program. eTable 2. Alcohol Use Disorders Identification Test-Consumption (AUDIT-C) eTable 3. Characteristics of Cancer Survivors Who Underwent Cancer Treatment Within 1 Year Before the Baseline Survey, All of Us Research Program. eTable 4.
Takeaways. Alcohol use disorder (AUD) is defined by the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) as "a problematic pattern of alcohol use leading to clinically significant impairment or distress," and is diagnosed as mild, moderate, or severe based on the number of symptoms, out of a possible 11, in the past 12 months.; As it progresses in severity, AUD can cause brain ...
The research, which appears in the May 6 issue of the American Journal of Psychiatry, suggests that delivering a brief alcohol-related intervention coincided with less opioid use, fewer opioid use disorder diagnoses, and potentially fewer opioid-related emergency department visits and hospitalizations.
Cancer. Drinking alcohol increases the risk of several types of cancer. Drinking any alcoholic beverages, including red and white wine, beer, and liquor, is linked with cancer. The risk of some cancers increases with any amount of alcohol use. 2 This includes breast cancer (in women). 2 A. More than 20,000 people die from alcohol-related ...
Or found that your usual number of drinks had much less effect than before? Found that when the effects of alcohol were wearing off, you had withdrawal symptoms, such as trouble sleeping, shakiness, restlessness, nausea, sweating, a racing heart, dysphoria (feeling uneasy or unhappy), malaise (general sense of being unwell), feeling low, or a ...
Alcohol use disorder (AUD) is one of the most common psychiatric disorders and represents a serious health condition, ... However, a crucial limitation to the translation of addiction research into clinical samples lies in the misalignment between research-based models of AUD and the current DSM diagnostic criteria. A major critique of the DSM ...
May 16, 2024 at 12:11 PM UTC. Clinical relevance: Swiss researchers developed a protein gel that converts alcohol into harmless acetic acid in the digestive system. The gel prevents alcohol's intoxicating and harmful effects before it enters the bloodstream. Testing on mice showed significant reductions in blood alcohol levels and liver damage.