- USC Libraries
- Research Guides

Organizing Your Social Sciences Research Paper
- Limitations of the Study
- Purpose of Guide
- Design Flaws to Avoid
- Independent and Dependent Variables
- Glossary of Research Terms
- Reading Research Effectively
- Narrowing a Topic Idea
- Broadening a Topic Idea
- Extending the Timeliness of a Topic Idea
- Academic Writing Style
- Applying Critical Thinking
- Choosing a Title
- Making an Outline
- Paragraph Development
- Research Process Video Series
- Executive Summary
- The C.A.R.S. Model
- Background Information
- The Research Problem/Question
- Theoretical Framework
- Citation Tracking
- Content Alert Services
- Evaluating Sources
- Primary Sources
- Secondary Sources
- Tiertiary Sources
- Scholarly vs. Popular Publications
- Qualitative Methods
- Quantitative Methods
- Insiderness
- Using Non-Textual Elements
- Common Grammar Mistakes
- Writing Concisely
- Avoiding Plagiarism
- Footnotes or Endnotes?
- Further Readings
- Generative AI and Writing
- USC Libraries Tutorials and Other Guides
- Bibliography
The limitations of the study are those characteristics of design or methodology that impacted or influenced the interpretation of the findings from your research. Study limitations are the constraints placed on the ability to generalize from the results, to further describe applications to practice, and/or related to the utility of findings that are the result of the ways in which you initially chose to design the study or the method used to establish internal and external validity or the result of unanticipated challenges that emerged during the study.
Price, James H. and Judy Murnan. “Research Limitations and the Necessity of Reporting Them.” American Journal of Health Education 35 (2004): 66-67; Theofanidis, Dimitrios and Antigoni Fountouki. "Limitations and Delimitations in the Research Process." Perioperative Nursing 7 (September-December 2018): 155-163. .
Importance of...
Always acknowledge a study's limitations. It is far better that you identify and acknowledge your study’s limitations than to have them pointed out by your professor and have your grade lowered because you appeared to have ignored them or didn't realize they existed.
Keep in mind that acknowledgment of a study's limitations is an opportunity to make suggestions for further research. If you do connect your study's limitations to suggestions for further research, be sure to explain the ways in which these unanswered questions may become more focused because of your study.
Acknowledgment of a study's limitations also provides you with opportunities to demonstrate that you have thought critically about the research problem, understood the relevant literature published about it, and correctly assessed the methods chosen for studying the problem. A key objective of the research process is not only discovering new knowledge but also to confront assumptions and explore what we don't know.
Claiming limitations is a subjective process because you must evaluate the impact of those limitations . Don't just list key weaknesses and the magnitude of a study's limitations. To do so diminishes the validity of your research because it leaves the reader wondering whether, or in what ways, limitation(s) in your study may have impacted the results and conclusions. Limitations require a critical, overall appraisal and interpretation of their impact. You should answer the question: do these problems with errors, methods, validity, etc. eventually matter and, if so, to what extent?
Price, James H. and Judy Murnan. “Research Limitations and the Necessity of Reporting Them.” American Journal of Health Education 35 (2004): 66-67; Structure: How to Structure the Research Limitations Section of Your Dissertation. Dissertations and Theses: An Online Textbook. Laerd.com.
Descriptions of Possible Limitations
All studies have limitations . However, it is important that you restrict your discussion to limitations related to the research problem under investigation. For example, if a meta-analysis of existing literature is not a stated purpose of your research, it should not be discussed as a limitation. Do not apologize for not addressing issues that you did not promise to investigate in the introduction of your paper.
Here are examples of limitations related to methodology and the research process you may need to describe and discuss how they possibly impacted your results. Note that descriptions of limitations should be stated in the past tense because they were discovered after you completed your research.
Possible Methodological Limitations
- Sample size -- the number of the units of analysis you use in your study is dictated by the type of research problem you are investigating. Note that, if your sample size is too small, it will be difficult to find significant relationships from the data, as statistical tests normally require a larger sample size to ensure a representative distribution of the population and to be considered representative of groups of people to whom results will be generalized or transferred. Note that sample size is generally less relevant in qualitative research if explained in the context of the research problem.
- Lack of available and/or reliable data -- a lack of data or of reliable data will likely require you to limit the scope of your analysis, the size of your sample, or it can be a significant obstacle in finding a trend and a meaningful relationship. You need to not only describe these limitations but provide cogent reasons why you believe data is missing or is unreliable. However, don’t just throw up your hands in frustration; use this as an opportunity to describe a need for future research based on designing a different method for gathering data.
- Lack of prior research studies on the topic -- citing prior research studies forms the basis of your literature review and helps lay a foundation for understanding the research problem you are investigating. Depending on the currency or scope of your research topic, there may be little, if any, prior research on your topic. Before assuming this to be true, though, consult with a librarian! In cases when a librarian has confirmed that there is little or no prior research, you may be required to develop an entirely new research typology [for example, using an exploratory rather than an explanatory research design ]. Note again that discovering a limitation can serve as an important opportunity to identify new gaps in the literature and to describe the need for further research.
- Measure used to collect the data -- sometimes it is the case that, after completing your interpretation of the findings, you discover that the way in which you gathered data inhibited your ability to conduct a thorough analysis of the results. For example, you regret not including a specific question in a survey that, in retrospect, could have helped address a particular issue that emerged later in the study. Acknowledge the deficiency by stating a need for future researchers to revise the specific method for gathering data.
- Self-reported data -- whether you are relying on pre-existing data or you are conducting a qualitative research study and gathering the data yourself, self-reported data is limited by the fact that it rarely can be independently verified. In other words, you have to the accuracy of what people say, whether in interviews, focus groups, or on questionnaires, at face value. However, self-reported data can contain several potential sources of bias that you should be alert to and note as limitations. These biases become apparent if they are incongruent with data from other sources. These are: (1) selective memory [remembering or not remembering experiences or events that occurred at some point in the past]; (2) telescoping [recalling events that occurred at one time as if they occurred at another time]; (3) attribution [the act of attributing positive events and outcomes to one's own agency, but attributing negative events and outcomes to external forces]; and, (4) exaggeration [the act of representing outcomes or embellishing events as more significant than is actually suggested from other data].
Possible Limitations of the Researcher
- Access -- if your study depends on having access to people, organizations, data, or documents and, for whatever reason, access is denied or limited in some way, the reasons for this needs to be described. Also, include an explanation why being denied or limited access did not prevent you from following through on your study.
- Longitudinal effects -- unlike your professor, who can literally devote years [even a lifetime] to studying a single topic, the time available to investigate a research problem and to measure change or stability over time is constrained by the due date of your assignment. Be sure to choose a research problem that does not require an excessive amount of time to complete the literature review, apply the methodology, and gather and interpret the results. If you're unsure whether you can complete your research within the confines of the assignment's due date, talk to your professor.
- Cultural and other type of bias -- we all have biases, whether we are conscience of them or not. Bias is when a person, place, event, or thing is viewed or shown in a consistently inaccurate way. Bias is usually negative, though one can have a positive bias as well, especially if that bias reflects your reliance on research that only support your hypothesis. When proof-reading your paper, be especially critical in reviewing how you have stated a problem, selected the data to be studied, what may have been omitted, the manner in which you have ordered events, people, or places, how you have chosen to represent a person, place, or thing, to name a phenomenon, or to use possible words with a positive or negative connotation. NOTE : If you detect bias in prior research, it must be acknowledged and you should explain what measures were taken to avoid perpetuating that bias. For example, if a previous study only used boys to examine how music education supports effective math skills, describe how your research expands the study to include girls.
- Fluency in a language -- if your research focuses , for example, on measuring the perceived value of after-school tutoring among Mexican-American ESL [English as a Second Language] students and you are not fluent in Spanish, you are limited in being able to read and interpret Spanish language research studies on the topic or to speak with these students in their primary language. This deficiency should be acknowledged.
Aguinis, Hermam and Jeffrey R. Edwards. “Methodological Wishes for the Next Decade and How to Make Wishes Come True.” Journal of Management Studies 51 (January 2014): 143-174; Brutus, Stéphane et al. "Self-Reported Limitations and Future Directions in Scholarly Reports: Analysis and Recommendations." Journal of Management 39 (January 2013): 48-75; Senunyeme, Emmanuel K. Business Research Methods. Powerpoint Presentation. Regent University of Science and Technology; ter Riet, Gerben et al. “All That Glitters Isn't Gold: A Survey on Acknowledgment of Limitations in Biomedical Studies.” PLOS One 8 (November 2013): 1-6.
Structure and Writing Style
Information about the limitations of your study are generally placed either at the beginning of the discussion section of your paper so the reader knows and understands the limitations before reading the rest of your analysis of the findings, or, the limitations are outlined at the conclusion of the discussion section as an acknowledgement of the need for further study. Statements about a study's limitations should not be buried in the body [middle] of the discussion section unless a limitation is specific to something covered in that part of the paper. If this is the case, though, the limitation should be reiterated at the conclusion of the section.
If you determine that your study is seriously flawed due to important limitations , such as, an inability to acquire critical data, consider reframing it as an exploratory study intended to lay the groundwork for a more complete research study in the future. Be sure, though, to specifically explain the ways that these flaws can be successfully overcome in a new study.
But, do not use this as an excuse for not developing a thorough research paper! Review the tab in this guide for developing a research topic . If serious limitations exist, it generally indicates a likelihood that your research problem is too narrowly defined or that the issue or event under study is too recent and, thus, very little research has been written about it. If serious limitations do emerge, consult with your professor about possible ways to overcome them or how to revise your study.
When discussing the limitations of your research, be sure to:
- Describe each limitation in detailed but concise terms;
- Explain why each limitation exists;
- Provide the reasons why each limitation could not be overcome using the method(s) chosen to acquire or gather the data [cite to other studies that had similar problems when possible];
- Assess the impact of each limitation in relation to the overall findings and conclusions of your study; and,
- If appropriate, describe how these limitations could point to the need for further research.
Remember that the method you chose may be the source of a significant limitation that has emerged during your interpretation of the results [for example, you didn't interview a group of people that you later wish you had]. If this is the case, don't panic. Acknowledge it, and explain how applying a different or more robust methodology might address the research problem more effectively in a future study. A underlying goal of scholarly research is not only to show what works, but to demonstrate what doesn't work or what needs further clarification.
Aguinis, Hermam and Jeffrey R. Edwards. “Methodological Wishes for the Next Decade and How to Make Wishes Come True.” Journal of Management Studies 51 (January 2014): 143-174; Brutus, Stéphane et al. "Self-Reported Limitations and Future Directions in Scholarly Reports: Analysis and Recommendations." Journal of Management 39 (January 2013): 48-75; Ioannidis, John P.A. "Limitations are not Properly Acknowledged in the Scientific Literature." Journal of Clinical Epidemiology 60 (2007): 324-329; Pasek, Josh. Writing the Empirical Social Science Research Paper: A Guide for the Perplexed. January 24, 2012. Academia.edu; Structure: How to Structure the Research Limitations Section of Your Dissertation. Dissertations and Theses: An Online Textbook. Laerd.com; What Is an Academic Paper? Institute for Writing Rhetoric. Dartmouth College; Writing the Experimental Report: Methods, Results, and Discussion. The Writing Lab and The OWL. Purdue University.
Writing Tip
Don't Inflate the Importance of Your Findings!
After all the hard work and long hours devoted to writing your research paper, it is easy to get carried away with attributing unwarranted importance to what you’ve done. We all want our academic work to be viewed as excellent and worthy of a good grade, but it is important that you understand and openly acknowledge the limitations of your study. Inflating the importance of your study's findings could be perceived by your readers as an attempt hide its flaws or encourage a biased interpretation of the results. A small measure of humility goes a long way!
Another Writing Tip
Negative Results are Not a Limitation!
Negative evidence refers to findings that unexpectedly challenge rather than support your hypothesis. If you didn't get the results you anticipated, it may mean your hypothesis was incorrect and needs to be reformulated. Or, perhaps you have stumbled onto something unexpected that warrants further study. Moreover, the absence of an effect may be very telling in many situations, particularly in experimental research designs. In any case, your results may very well be of importance to others even though they did not support your hypothesis. Do not fall into the trap of thinking that results contrary to what you expected is a limitation to your study. If you carried out the research well, they are simply your results and only require additional interpretation.
Lewis, George H. and Jonathan F. Lewis. “The Dog in the Night-Time: Negative Evidence in Social Research.” The British Journal of Sociology 31 (December 1980): 544-558.
Yet Another Writing Tip
Sample Size Limitations in Qualitative Research
Sample sizes are typically smaller in qualitative research because, as the study goes on, acquiring more data does not necessarily lead to more information. This is because one occurrence of a piece of data, or a code, is all that is necessary to ensure that it becomes part of the analysis framework. However, it remains true that sample sizes that are too small cannot adequately support claims of having achieved valid conclusions and sample sizes that are too large do not permit the deep, naturalistic, and inductive analysis that defines qualitative inquiry. Determining adequate sample size in qualitative research is ultimately a matter of judgment and experience in evaluating the quality of the information collected against the uses to which it will be applied and the particular research method and purposeful sampling strategy employed. If the sample size is found to be a limitation, it may reflect your judgment about the methodological technique chosen [e.g., single life history study versus focus group interviews] rather than the number of respondents used.
Boddy, Clive Roland. "Sample Size for Qualitative Research." Qualitative Market Research: An International Journal 19 (2016): 426-432; Huberman, A. Michael and Matthew B. Miles. "Data Management and Analysis Methods." In Handbook of Qualitative Research . Norman K. Denzin and Yvonna S. Lincoln, eds. (Thousand Oaks, CA: Sage, 1994), pp. 428-444; Blaikie, Norman. "Confounding Issues Related to Determining Sample Size in Qualitative Research." International Journal of Social Research Methodology 21 (2018): 635-641; Oppong, Steward Harrison. "The Problem of Sampling in qualitative Research." Asian Journal of Management Sciences and Education 2 (2013): 202-210.
- << Previous: 8. The Discussion
- Next: 9. The Conclusion >>
- Last Updated: Apr 20, 2024 2:57 PM
- URL: https://libguides.usc.edu/writingguide
How to Write Limitations of the Study (with examples)
This blog emphasizes the importance of recognizing and effectively writing about limitations in research. It discusses the types of limitations, their significance, and provides guidelines for writing about them, highlighting their role in advancing scholarly research.
Updated on August 24, 2023

No matter how well thought out, every research endeavor encounters challenges. There is simply no way to predict all possible variances throughout the process.
These uncharted boundaries and abrupt constraints are known as limitations in research . Identifying and acknowledging limitations is crucial for conducting rigorous studies. Limitations provide context and shed light on gaps in the prevailing inquiry and literature.
This article explores the importance of recognizing limitations and discusses how to write them effectively. By interpreting limitations in research and considering prevalent examples, we aim to reframe the perception from shameful mistakes to respectable revelations.
What are limitations in research?
In the clearest terms, research limitations are the practical or theoretical shortcomings of a study that are often outside of the researcher’s control . While these weaknesses limit the generalizability of a study’s conclusions, they also present a foundation for future research.
Sometimes limitations arise from tangible circumstances like time and funding constraints, or equipment and participant availability. Other times the rationale is more obscure and buried within the research design. Common types of limitations and their ramifications include:
- Theoretical: limits the scope, depth, or applicability of a study.
- Methodological: limits the quality, quantity, or diversity of the data.
- Empirical: limits the representativeness, validity, or reliability of the data.
- Analytical: limits the accuracy, completeness, or significance of the findings.
- Ethical: limits the access, consent, or confidentiality of the data.
Regardless of how, when, or why they arise, limitations are a natural part of the research process and should never be ignored . Like all other aspects, they are vital in their own purpose.
Why is identifying limitations important?
Whether to seek acceptance or avoid struggle, humans often instinctively hide flaws and mistakes. Merging this thought process into research by attempting to hide limitations, however, is a bad idea. It has the potential to negate the validity of outcomes and damage the reputation of scholars.
By identifying and addressing limitations throughout a project, researchers strengthen their arguments and curtail the chance of peer censure based on overlooked mistakes. Pointing out these flaws shows an understanding of variable limits and a scrupulous research process.
Showing awareness of and taking responsibility for a project’s boundaries and challenges validates the integrity and transparency of a researcher. It further demonstrates the researchers understand the applicable literature and have thoroughly evaluated their chosen research methods.
Presenting limitations also benefits the readers by providing context for research findings. It guides them to interpret the project’s conclusions only within the scope of very specific conditions. By allowing for an appropriate generalization of the findings that is accurately confined by research boundaries and is not too broad, limitations boost a study’s credibility .
Limitations are true assets to the research process. They highlight opportunities for future research. When researchers identify the limitations of their particular approach to a study question, they enable precise transferability and improve chances for reproducibility.
Simply stating a project’s limitations is not adequate for spurring further research, though. To spark the interest of other researchers, these acknowledgements must come with thorough explanations regarding how the limitations affected the current study and how they can potentially be overcome with amended methods.
How to write limitations
Typically, the information about a study’s limitations is situated either at the beginning of the discussion section to provide context for readers or at the conclusion of the discussion section to acknowledge the need for further research. However, it varies depending upon the target journal or publication guidelines.
Don’t hide your limitations
It is also important to not bury a limitation in the body of the paper unless it has a unique connection to a topic in that section. If so, it needs to be reiterated with the other limitations or at the conclusion of the discussion section. Wherever it is included in the manuscript, ensure that the limitations section is prominently positioned and clearly introduced.
While maintaining transparency by disclosing limitations means taking a comprehensive approach, it is not necessary to discuss everything that could have potentially gone wrong during the research study. If there is no commitment to investigation in the introduction, it is unnecessary to consider the issue a limitation to the research. Wholly consider the term ‘limitations’ and ask, “Did it significantly change or limit the possible outcomes?” Then, qualify the occurrence as either a limitation to include in the current manuscript or as an idea to note for other projects.
Writing limitations
Once the limitations are concretely identified and it is decided where they will be included in the paper, researchers are ready for the writing task. Including only what is pertinent, keeping explanations detailed but concise, and employing the following guidelines is key for crafting valuable limitations:
1) Identify and describe the limitations : Clearly introduce the limitation by classifying its form and specifying its origin. For example:
- An unintentional bias encountered during data collection
- An intentional use of unplanned post-hoc data analysis
2) Explain the implications : Describe how the limitation potentially influences the study’s findings and how the validity and generalizability are subsequently impacted. Provide examples and evidence to support claims of the limitations’ effects without making excuses or exaggerating their impact. Overall, be transparent and objective in presenting the limitations, without undermining the significance of the research.
3) Provide alternative approaches for future studies : Offer specific suggestions for potential improvements or avenues for further investigation. Demonstrate a proactive approach by encouraging future research that addresses the identified gaps and, therefore, expands the knowledge base.
Whether presenting limitations as an individual section within the manuscript or as a subtopic in the discussion area, authors should use clear headings and straightforward language to facilitate readability. There is no need to complicate limitations with jargon, computations, or complex datasets.
Examples of common limitations
Limitations are generally grouped into two categories , methodology and research process .
Methodology limitations
Methodology may include limitations due to:
- Sample size
- Lack of available or reliable data
- Lack of prior research studies on the topic
- Measure used to collect the data
- Self-reported data

The researcher is addressing how the large sample size requires a reassessment of the measures used to collect and analyze the data.
Research process limitations
Limitations during the research process may arise from:
- Access to information
- Longitudinal effects
- Cultural and other biases
- Language fluency
- Time constraints

The author is pointing out that the model’s estimates are based on potentially biased observational studies.
Final thoughts
Successfully proving theories and touting great achievements are only two very narrow goals of scholarly research. The true passion and greatest efforts of researchers comes more in the form of confronting assumptions and exploring the obscure.
In many ways, recognizing and sharing the limitations of a research study both allows for and encourages this type of discovery that continuously pushes research forward. By using limitations to provide a transparent account of the project's boundaries and to contextualize the findings, researchers pave the way for even more robust and impactful research in the future.
Charla Viera, MS
See our "Privacy Policy"
Ensure your structure and ideas are consistent and clearly communicated
Pair your Premium Editing with our add-on service Presubmission Review for an overall assessment of your manuscript.
- Privacy Policy
Buy Me a Coffee

Home » Limitations in Research – Types, Examples and Writing Guide
Limitations in Research – Types, Examples and Writing Guide
Table of Contents

Limitations in Research
Limitations in research refer to the factors that may affect the results, conclusions , and generalizability of a study. These limitations can arise from various sources, such as the design of the study, the sampling methods used, the measurement tools employed, and the limitations of the data analysis techniques.
Types of Limitations in Research
Types of Limitations in Research are as follows:
Sample Size Limitations
This refers to the size of the group of people or subjects that are being studied. If the sample size is too small, then the results may not be representative of the population being studied. This can lead to a lack of generalizability of the results.
Time Limitations
Time limitations can be a constraint on the research process . This could mean that the study is unable to be conducted for a long enough period of time to observe the long-term effects of an intervention, or to collect enough data to draw accurate conclusions.
Selection Bias
This refers to a type of bias that can occur when the selection of participants in a study is not random. This can lead to a biased sample that is not representative of the population being studied.
Confounding Variables
Confounding variables are factors that can influence the outcome of a study, but are not being measured or controlled for. These can lead to inaccurate conclusions or a lack of clarity in the results.
Measurement Error
This refers to inaccuracies in the measurement of variables, such as using a faulty instrument or scale. This can lead to inaccurate results or a lack of validity in the study.
Ethical Limitations
Ethical limitations refer to the ethical constraints placed on research studies. For example, certain studies may not be allowed to be conducted due to ethical concerns, such as studies that involve harm to participants.
Examples of Limitations in Research
Some Examples of Limitations in Research are as follows:
Research Title: “The Effectiveness of Machine Learning Algorithms in Predicting Customer Behavior”
Limitations:
- The study only considered a limited number of machine learning algorithms and did not explore the effectiveness of other algorithms.
- The study used a specific dataset, which may not be representative of all customer behaviors or demographics.
- The study did not consider the potential ethical implications of using machine learning algorithms in predicting customer behavior.
Research Title: “The Impact of Online Learning on Student Performance in Computer Science Courses”
- The study was conducted during the COVID-19 pandemic, which may have affected the results due to the unique circumstances of remote learning.
- The study only included students from a single university, which may limit the generalizability of the findings to other institutions.
- The study did not consider the impact of individual differences, such as prior knowledge or motivation, on student performance in online learning environments.
Research Title: “The Effect of Gamification on User Engagement in Mobile Health Applications”
- The study only tested a specific gamification strategy and did not explore the effectiveness of other gamification techniques.
- The study relied on self-reported measures of user engagement, which may be subject to social desirability bias or measurement errors.
- The study only included a specific demographic group (e.g., young adults) and may not be generalizable to other populations with different preferences or needs.
How to Write Limitations in Research
When writing about the limitations of a research study, it is important to be honest and clear about the potential weaknesses of your work. Here are some tips for writing about limitations in research:
- Identify the limitations: Start by identifying the potential limitations of your research. These may include sample size, selection bias, measurement error, or other issues that could affect the validity and reliability of your findings.
- Be honest and objective: When describing the limitations of your research, be honest and objective. Do not try to minimize or downplay the limitations, but also do not exaggerate them. Be clear and concise in your description of the limitations.
- Provide context: It is important to provide context for the limitations of your research. For example, if your sample size was small, explain why this was the case and how it may have affected your results. Providing context can help readers understand the limitations in a broader context.
- Discuss implications : Discuss the implications of the limitations for your research findings. For example, if there was a selection bias in your sample, explain how this may have affected the generalizability of your findings. This can help readers understand the limitations in terms of their impact on the overall validity of your research.
- Provide suggestions for future research : Finally, provide suggestions for future research that can address the limitations of your study. This can help readers understand how your research fits into the broader field and can provide a roadmap for future studies.
Purpose of Limitations in Research
There are several purposes of limitations in research. Here are some of the most important ones:
- To acknowledge the boundaries of the study : Limitations help to define the scope of the research project and set realistic expectations for the findings. They can help to clarify what the study is not intended to address.
- To identify potential sources of bias: Limitations can help researchers identify potential sources of bias in their research design, data collection, or analysis. This can help to improve the validity and reliability of the findings.
- To provide opportunities for future research: Limitations can highlight areas for future research and suggest avenues for further exploration. This can help to advance knowledge in a particular field.
- To demonstrate transparency and accountability: By acknowledging the limitations of their research, researchers can demonstrate transparency and accountability to their readers, peers, and funders. This can help to build trust and credibility in the research community.
- To encourage critical thinking: Limitations can encourage readers to critically evaluate the study’s findings and consider alternative explanations or interpretations. This can help to promote a more nuanced and sophisticated understanding of the topic under investigation.
When to Write Limitations in Research
Limitations should be included in research when they help to provide a more complete understanding of the study’s results and implications. A limitation is any factor that could potentially impact the accuracy, reliability, or generalizability of the study’s findings.
It is important to identify and discuss limitations in research because doing so helps to ensure that the results are interpreted appropriately and that any conclusions drawn are supported by the available evidence. Limitations can also suggest areas for future research, highlight potential biases or confounding factors that may have affected the results, and provide context for the study’s findings.
Generally, limitations should be discussed in the conclusion section of a research paper or thesis, although they may also be mentioned in other sections, such as the introduction or methods. The specific limitations that are discussed will depend on the nature of the study, the research question being investigated, and the data that was collected.
Examples of limitations that might be discussed in research include sample size limitations, data collection methods, the validity and reliability of measures used, and potential biases or confounding factors that could have affected the results. It is important to note that limitations should not be used as a justification for poor research design or methodology, but rather as a way to enhance the understanding and interpretation of the study’s findings.
Importance of Limitations in Research
Here are some reasons why limitations are important in research:
- Enhances the credibility of research: Limitations highlight the potential weaknesses and threats to validity, which helps readers to understand the scope and boundaries of the study. This improves the credibility of research by acknowledging its limitations and providing a clear picture of what can and cannot be concluded from the study.
- Facilitates replication: By highlighting the limitations, researchers can provide detailed information about the study’s methodology, data collection, and analysis. This information helps other researchers to replicate the study and test the validity of the findings, which enhances the reliability of research.
- Guides future research : Limitations provide insights into areas for future research by identifying gaps or areas that require further investigation. This can help researchers to design more comprehensive and effective studies that build on existing knowledge.
- Provides a balanced view: Limitations help to provide a balanced view of the research by highlighting both strengths and weaknesses. This ensures that readers have a clear understanding of the study’s limitations and can make informed decisions about the generalizability and applicability of the findings.
Advantages of Limitations in Research
Here are some potential advantages of limitations in research:
- Focus : Limitations can help researchers focus their study on a specific area or population, which can make the research more relevant and useful.
- Realism : Limitations can make a study more realistic by reflecting the practical constraints and challenges of conducting research in the real world.
- Innovation : Limitations can spur researchers to be more innovative and creative in their research design and methodology, as they search for ways to work around the limitations.
- Rigor : Limitations can actually increase the rigor and credibility of a study, as researchers are forced to carefully consider the potential sources of bias and error, and address them to the best of their abilities.
- Generalizability : Limitations can actually improve the generalizability of a study by ensuring that it is not overly focused on a specific sample or situation, and that the results can be applied more broadly.
About the author
Muhammad Hassan
Researcher, Academic Writer, Web developer
You may also like

How to Cite Research Paper – All Formats and...

Data Collection – Methods Types and Examples

Delimitations in Research – Types, Examples and...

Research Paper Format – Types, Examples and...

Research Process – Steps, Examples and Tips

Research Design – Types, Methods and Examples
What are the limitations in research and how to write them?
Learn about the potential limitations in research and how to appropriately address them in order to deliver honest and ethical research.
It is fairly uncommon for researchers to stumble into the term research limitations when working on their research paper. Limitations in research can arise owing to constraints on design, methods, materials, and so on, and these aspects, unfortunately, may have an influence on your subject’s findings.
In this Mind The Graph’s article, we’ll discuss some recommendations for writing limitations in research , provide examples of various common types of limitations, and suggest how to properly present this information.
What are the limitations in research?
The limitations in research are the constraints in design, methods or even researchers’ limitations that affect and influence the interpretation of your research’s ultimate findings. These are limitations on the generalization and usability of findings that emerge from the design of the research and/or the method employed to ensure validity both internally and externally.
Researchers are usually cautious to acknowledge the limitations of their research in their publications for fear of undermining the research’s scientific validity. No research is faultless or covers every possible angle. As a result, addressing the constraints of your research exhibits honesty and integrity .
Why should include limitations of research in my paper?
Though limitations tackle potential flaws in research, commenting on them at the conclusion of your paper, by demonstrating that you are aware of these limitations and explaining how they impact the conclusions that may be taken from the research, improves your research by disclosing any issues before other researchers or reviewers do .
Additionally, emphasizing research constraints implies that you have thoroughly investigated the ramifications of research shortcomings and have a thorough understanding of your research problem.
Limits exist in any research; being honest about them and explaining them would impress researchers and reviewers more than disregarding them.
Remember that acknowledging a research’s shortcomings offers a chance to provide ideas for future research, but be careful to describe how your study may help to concentrate on these outstanding problems.
Possible limitations examples
Here are some limitations connected to methodology and the research procedure that you may need to explain and discuss in connection to your findings.
Methodological limitations
Sample size.
The number of units of analysis used in your study is determined by the sort of research issue being investigated. It is important to note that if your sample is too small, finding significant connections in the data will be challenging, as statistical tests typically require a larger sample size to ensure a fair representation and this can be limiting.
Lack of available or reliable data
A lack of data or trustworthy data will almost certainly necessitate limiting the scope of your research or the size of your sample, or it can be a substantial impediment to identifying a pattern and a relevant connection.
Lack of prior research on the subject
Citing previous research papers forms the basis of your literature review and aids in comprehending the research subject you are researching. Yet there may be little if any, past research on your issue.
The measure used to collect data
After finishing your analysis of the findings, you realize that the method you used to collect data limited your capacity to undertake a comprehensive evaluation of the findings. Recognize the flaw by mentioning that future researchers should change the specific approach for data collection.
Issues with research samples and selection
Sampling inaccuracies arise when a probability sampling method is employed to choose a sample, but that sample does not accurately represent the overall population or the relevant group. As a result, your study suffers from “sampling bias” or “selection bias.”
Limitations of the research
When your research requires polling certain persons or a specific group, you may have encountered the issue of limited access to these interviewees. Because of the limited access, you may need to reorganize or rearrange your research. In this scenario, explain why access is restricted and ensure that your findings are still trustworthy and valid despite the constraint.
Time constraints
Practical difficulties may limit the amount of time available to explore a research issue and monitor changes as they occur. If time restrictions have any detrimental influence on your research, recognize this impact by expressing the necessity for a future investigation.
Due to their cultural origins or opinions on observed events, researchers may carry biased opinions, which can influence the credibility of a research. Furthermore, researchers may exhibit biases toward data and conclusions that only support their hypotheses or arguments.
The structure of the limitations section
The limitations of your research are usually stated at the beginning of the discussion section of your paper so that the reader is aware of and comprehends the limitations prior to actually reading the rest of your findings, or they are stated at the end of the discussion section as an acknowledgment of the need for further research.
The ideal way is to divide your limitations section into three steps:
1. Identify the research constraints;
2. Describe in great detail how they affect your research;
3. Mention the opportunity for future investigations and give possibilities.
By following this method while addressing the constraints of your research, you will be able to effectively highlight your research’s shortcomings without jeopardizing the quality and integrity of your research.
Present your research or paper in an innovative way
If you want your readers to be engaged and participate in your research, try Mind The Graph tool to add visual assets to your content. Infographics may improve comprehension and are easy to read, just as the Mind The Graph tool is simple to use and offers a variety of templates from which you can select the one that best suits your information.
Subscribe to our newsletter
Exclusive high quality content about effective visual communication in science.
Unlock Your Creativity
Create infographics, presentations and other scientifically-accurate designs without hassle — absolutely free for 7 days!
About Jessica Abbadia
Jessica Abbadia is a lawyer that has been working in Digital Marketing since 2020, improving organic performance for apps and websites in various regions through ASO and SEO. Currently developing scientific and intellectual knowledge for the community's benefit. Jessica is an animal rights activist who enjoys reading and drinking strong coffee.
Content tags
How to present limitations in research
Last updated
30 January 2024
Reviewed by
Limitations don’t invalidate or diminish your results, but it’s best to acknowledge them. This will enable you to address any questions your study failed to answer because of them.
In this guide, learn how to recognize, present, and overcome limitations in research.
- What is a research limitation?
Research limitations are weaknesses in your research design or execution that may have impacted outcomes and conclusions. Uncovering limitations doesn’t necessarily indicate poor research design—it just means you encountered challenges you couldn’t have anticipated that limited your research efforts.
Does basic research have limitations?
Basic research aims to provide more information about your research topic. It requires the same standard research methodology and data collection efforts as any other research type, and it can also have limitations.
- Common research limitations
Researchers encounter common limitations when embarking on a study. Limitations can occur in relation to the methods you apply or the research process you design. They could also be connected to you as the researcher.
Methodology limitations
Not having access to data or reliable information can impact the methods used to facilitate your research. A lack of data or reliability may limit the parameters of your study area and the extent of your exploration.
Your sample size may also be affected because you won’t have any direction on how big or small it should be and who or what you should include. Having too few participants won’t adequately represent the population or groups of people needed to draw meaningful conclusions.
Research process limitations
The study’s design can impose constraints on the process. For example, as you’re conducting the research, issues may arise that don’t conform to the data collection methodology you developed. You may not realize until well into the process that you should have incorporated more specific questions or comprehensive experiments to generate the data you need to have confidence in your results.
Constraints on resources can also have an impact. Being limited on participants or participation incentives may limit your sample sizes. Insufficient tools, equipment, and materials to conduct a thorough study may also be a factor.
Common researcher limitations
Here are some of the common researcher limitations you may encounter:
Time: some research areas require multi-year longitudinal approaches, but you might not be able to dedicate that much time. Imagine you want to measure how much memory a person loses as they age. This may involve conducting multiple tests on a sample of participants over 20–30 years, which may be impossible.
Bias: researchers can consciously or unconsciously apply bias to their research. Biases can contribute to relying on research sources and methodologies that will only support your beliefs about the research you’re embarking on. You might also omit relevant issues or participants from the scope of your study because of your biases.
Limited access to data : you may need to pay to access specific databases or journals that would be helpful to your research process. You might also need to gain information from certain people or organizations but have limited access to them. These cases require readjusting your process and explaining why your findings are still reliable.
- Why is it important to identify limitations?
Identifying limitations adds credibility to research and provides a deeper understanding of how you arrived at your conclusions.
Constraints may have prevented you from collecting specific data or information you hoped would prove or disprove your hypothesis or provide a more comprehensive understanding of your research topic.
However, identifying the limitations contributing to your conclusions can inspire further research efforts that help gather more substantial information and data.
- Where to put limitations in a research paper
A research paper is broken up into different sections that appear in the following order:
Introduction
Methodology
The discussion portion of your paper explores your findings and puts them in the context of the overall research. Either place research limitations at the beginning of the discussion section before the analysis of your findings or at the end of the section to indicate that further research needs to be pursued.
What not to include in the limitations section
Evidence that doesn’t support your hypothesis is not a limitation, so you shouldn’t include it in the limitation section. Don’t just list limitations and their degree of severity without further explanation.
- How to present limitations
You’ll want to present the limitations of your study in a way that doesn’t diminish the validity of your research and leave the reader wondering if your results and conclusions have been compromised.
Include only the limitations that directly relate to and impact how you addressed your research questions. Following a specific format enables the reader to develop an understanding of the weaknesses within the context of your findings without doubting the quality and integrity of your research.
Identify the limitations specific to your study
You don’t have to identify every possible limitation that might have occurred during your research process. Only identify those that may have influenced the quality of your findings and your ability to answer your research question.
Explain study limitations in detail
This explanation should be the most significant portion of your limitation section.
Link each limitation with an interpretation and appraisal of their impact on the study. You’ll have to evaluate and explain whether the error, method, or validity issues influenced the study’s outcome and how.
Propose a direction for future studies and present alternatives
In this section, suggest how researchers can avoid the pitfalls you experienced during your research process.
If an issue with methodology was a limitation, propose alternate methods that may help with a smoother and more conclusive research project. Discuss the pros and cons of your alternate recommendation.
Describe steps taken to minimize each limitation
You probably took steps to try to address or mitigate limitations when you noticed them throughout the course of your research project. Describe these steps in the limitation section.
- Limitation example
“Approaches like stem cell transplantation and vaccination in AD [Alzheimer’s disease] work on a cellular or molecular level in the laboratory. However, translation into clinical settings will remain a challenge for the next decade.”
The authors are saying that even though these methods showed promise in helping people with memory loss when conducted in the lab (in other words, using animal studies), more studies are needed. These may be controlled clinical trials, for example.
However, the short life span of stem cells outside the lab and the vaccination’s severe inflammatory side effects are limitations. Researchers won’t be able to conduct clinical trials until these issues are overcome.
- How to overcome limitations in research
You’ve already started on the road to overcoming limitations in research by acknowledging that they exist. However, you need to ensure readers don’t mistake weaknesses for errors within your research design.
To do this, you’ll need to justify and explain your rationale for the methods, research design, and analysis tools you chose and how you noticed they may have presented limitations.
Your readers need to know that even when limitations presented themselves, you followed best practices and the ethical standards of your field. You didn’t violate any rules and regulations during your research process.
You’ll also want to reinforce the validity of your conclusions and results with multiple sources, methods, and perspectives. This prevents readers from assuming your findings were derived from a single or biased source.
- Learning and improving starts with limitations in research
Dealing with limitations with transparency and integrity helps identify areas for future improvements and developments. It’s a learning process, providing valuable insights into how you can improve methodologies, expand sample sizes, or explore alternate approaches to further support the validity of your findings.
Get started today
Go from raw data to valuable insights with a flexible research platform
Editor’s picks
Last updated: 21 December 2023
Last updated: 16 December 2023
Last updated: 6 October 2023
Last updated: 5 March 2024
Last updated: 25 November 2023
Last updated: 15 February 2024
Last updated: 11 March 2024
Last updated: 12 December 2023
Last updated: 6 March 2024
Last updated: 10 April 2023
Last updated: 20 December 2023
Latest articles
Related topics, log in or sign up.
Get started for free

Research Limitations: A Comprehensive Guide
Embarking on a research journey is an exciting endeavor, but every study has its boundaries and constraints. Understanding and transparently acknowledging these limitations is a crucial aspect of scholarly work. In this guide, we'll explore the concept of research limitations, why they matter, and how to effectively address and navigate them in your academic endeavors.
1. Defining Research Limitations:
- Definition: Research limitations are the constraints or shortcomings that affect the scope, applicability, and generalizability of a study.
- Inherent in Research: Every research project, regardless of its scale or significance, possesses limitations.
2. Types of Research Limitations:
- Methodological Limitations: Constraints related to the research design, data collection methods, or analytical techniques.
- Sampling Limitations: Issues associated with the representativeness or size of the study sample.
- Contextual Limitations: Restrictions stemming from the specific time, place, or cultural context of the study.
- Resource Limitations: Constraints related to time, budget, or access to necessary resources.
3. Why Acknowledge Limitations?
- Transparency: Acknowledging limitations demonstrates transparency and honesty in your research.
- Robustness of Findings: Recognizing limitations adds nuance to your findings, making them more robust.
- Future Research Directions: Addressing limitations provides a foundation for future researchers to build upon.
4. Identifying Research Limitations:
- Reflect on Methodology: Consider the strengths and weaknesses of your research design, data collection methods, and analysis.
- Examine Sample Characteristics: Evaluate the representativeness and size of your study sample.
- Consider External Factors: Assess external factors that may impact the generalizability of your findings.
5. How to Address Limitations:
- In the Methodology Section: Clearly articulate limitations in the methodology section of your research paper.
- Offer Solutions: If possible, propose ways to mitigate or address identified limitations.
- Future Research Suggestions: Use limitations as a springboard to suggest areas for future research.
6. Common Phrases to Express Limitations:
- "This study is not without limitations."
- "One limitation of our research is..."
- "It is important to acknowledge the constraints of this study, including..."
7. Examples of Addressing Limitations:
- Example 1 (Methodological): "While our survey provided valuable insights, the reliance on self-reported data introduces the possibility of response bias."
- Example 2 (Sampling): "The small sample size of our study limits the generalizability of our findings to a broader population."
- Example 3 (Resource): "Due to budget constraints, our research was limited to a single geographical location, potentially impacting the external validity."
8. Balancing Strengths and Limitations:
- Emphasize Contributions: Highlight the contributions and strengths of your research alongside the limitations.
- Maintain a Positive Tone: Discuss limitations objectively without undermining the significance of your study.
9. Feedback and Peer Review:
- Seek Feedback: Share your research with peers or mentors to gain valuable insights.
- Peer Review: Embrace the feedback received during the peer-review process to enhance the robustness of your work.
10. Continuous Reflection:
- Throughout the Research Process: Continuously reflect on potential limitations during the entire research process.
- Adjust as Needed: Be willing to adjust your approach as you encounter unforeseen challenges.
Conclusion:
Understanding and effectively addressing research limitations is a hallmark of rigorous and responsible scholarship. By openly acknowledging these constraints, you not only enhance the credibility of your work but also contribute to the broader academic discourse. Embrace the nuances of your research journey, navigate its limitations thoughtfully, and pave the way for future investigations.
Related Guides
- How to Create the Outline of a Research Paper?
- Qualitative Research Methods
- How to select the right research topic? : A Step-by-Step Guide
- Tips for Avoiding Plagiarism
- How to Write the Body of a Research Paper?
- Your Ultimate Guide to In-text Citation

Writing Limitations of Research Study — 4 Reasons Why It Is Important!
It is not unusual for researchers to come across the term limitations of research during their academic paper writing. More often this is interpreted as something terrible. However, when it comes to research study, limitations can help structure the research study better. Therefore, do not underestimate significance of limitations of research study.
Allow us to take you through the context of how to evaluate the limits of your research and conclude an impactful relevance to your results.
Table of Contents
What Are the Limitations of a Research Study?
Every research has its limit and these limitations arise due to restrictions in methodology or research design. This could impact your entire research or the research paper you wish to publish. Unfortunately, most researchers choose not to discuss their limitations of research fearing it will affect the value of their article in the eyes of readers.
However, it is very important to discuss your study limitations and show it to your target audience (other researchers, journal editors, peer reviewers etc.). It is very important that you provide an explanation of how your research limitations may affect the conclusions and opinions drawn from your research. Moreover, when as an author you state the limitations of research, it shows that you have investigated all the weaknesses of your study and have a deep understanding of the subject. Being honest could impress your readers and mark your study as a sincere effort in research.

Why and Where Should You Include the Research Limitations?
The main goal of your research is to address your research objectives. Conduct experiments, get results and explain those results, and finally justify your research question . It is best to mention the limitations of research in the discussion paragraph of your research article.
At the very beginning of this paragraph, immediately after highlighting the strengths of the research methodology, you should write down your limitations. You can discuss specific points from your research limitations as suggestions for further research in the conclusion of your thesis.
1. Common Limitations of the Researchers
Limitations that are related to the researcher must be mentioned. This will help you gain transparency with your readers. Furthermore, you could provide suggestions on decreasing these limitations in you and your future studies.
2. Limited Access to Information
Your work may involve some institutions and individuals in research, and sometimes you may have problems accessing these institutions. Therefore, you need to redesign and rewrite your work. You must explain your readers the reason for limited access.
3. Limited Time
All researchers are bound by their deadlines when it comes to completing their studies. Sometimes, time constraints can affect your research negatively. However, the best practice is to acknowledge it and mention a requirement for future study to solve the research problem in a better way.
4. Conflict over Biased Views and Personal Issues
Biased views can affect the research. In fact, researchers end up choosing only those results and data that support their main argument, keeping aside the other loose ends of the research.
Types of Limitations of Research
Before beginning your research study, know that there are certain limitations to what you are testing or possible research results. There are different types that researchers may encounter, and they all have unique characteristics, such as:
1. Research Design Limitations
Certain restrictions on your research or available procedures may affect your final results or research outputs. You may have formulated research goals and objectives too broadly. However, this can help you understand how you can narrow down the formulation of research goals and objectives, thereby increasing the focus of your study.
2. Impact Limitations
Even if your research has excellent statistics and a strong design, it can suffer from the influence of the following factors:
- Presence of increasing findings as researched
- Being population specific
- A strong regional focus.
3. Data or statistical limitations
In some cases, it is impossible to collect sufficient data for research or very difficult to get access to the data. This could lead to incomplete conclusion to your study. Moreover, this insufficiency in data could be the outcome of your study design. The unclear, shabby research outline could produce more problems in interpreting your findings.
How to Correctly Structure Your Research Limitations?
There are strict guidelines for narrowing down research questions, wherein you could justify and explain potential weaknesses of your academic paper. You could go through these basic steps to get a well-structured clarity of research limitations:
- Declare that you wish to identify your limitations of research and explain their importance,
- Provide the necessary depth, explain their nature, and justify your study choices.
- Write how you are suggesting that it is possible to overcome them in the future.
In this section, your readers will see that you are aware of the potential weaknesses in your business, understand them and offer effective solutions, and it will positively strengthen your article as you clarify all limitations of research to your target audience.
Know that you cannot be perfect and there is no individual without flaws. You could use the limitations of research as a great opportunity to take on a new challenge and improve the future of research. In a typical academic paper, research limitations may relate to:
1. Formulating your goals and objectives
If you formulate goals and objectives too broadly, your work will have some shortcomings. In this case, specify effective methods or ways to narrow down the formula of goals and aim to increase your level of study focus.
2. Application of your data collection methods in research
If you do not have experience in primary data collection, there is a risk that there will be flaws in the implementation of your methods. It is necessary to accept this, and learn and educate yourself to understand data collection methods.
3. Sample sizes
This depends on the nature of problem you choose. Sample size is of a greater importance in quantitative studies as opposed to qualitative ones. If your sample size is too small, statistical tests cannot identify significant relationships or connections within a given data set.
You could point out that other researchers should base the same study on a larger sample size to get more accurate results.
4. The absence of previous studies in the field you have chosen
Writing a literature review is an important step in any scientific study because it helps researchers determine the scope of current work in the chosen field. It is a major foundation for any researcher who must use them to achieve a set of specific goals or objectives.
However, if you are focused on the most current and evolving research problem or a very narrow research problem, there may be very little prior research on your topic. For example, if you chose to explore the role of Bitcoin as the currency of the future, you may not find tons of scientific papers addressing the research problem as Bitcoins are only a new phenomenon.
It is important that you learn to identify research limitations examples at each step. Whatever field you choose, feel free to add the shortcoming of your work. This is mainly because you do not have many years of experience writing scientific papers or completing complex work. Therefore, the depth and scope of your discussions may be compromised at different levels compared to academics with a lot of expertise. Include specific points from limitations of research. Use them as suggestions for the future.
Have you ever faced a challenge of writing the limitations of research study in your paper? How did you overcome it? What ways did you follow? Were they beneficial? Let us know in the comments below!
Frequently Asked Questions
Setting limitations in our study helps to clarify the outcomes drawn from our research and enhance understanding of the subject. Moreover, it shows that the author has investigated all the weaknesses in the study.
Scope is the range and limitations of a research project which are set to define the boundaries of a project. Limitations are the impacts on the overall study due to the constraints on the research design.
Limitation in research is an impact of a constraint on the research design in the overall study. They are the flaws or weaknesses in the study, which may influence the outcome of the research.
1. Limitations in research can be written as follows: Formulate your goals and objectives 2. Analyze the chosen data collection method and the sample sizes 3. Identify your limitations of research and explain their importance 4. Provide the necessary depth, explain their nature, and justify your study choices 5. Write how you are suggesting that it is possible to overcome them in the future
Excellent article ,,,it has helped me big
This is very helpful information. It has given me an insight on how to go about my study limitations.
Good comments and helpful
Rate this article Cancel Reply
Your email address will not be published.

Enago Academy's Most Popular Articles

- Diversity and Inclusion
- Trending Now
The Silent Struggle: Confronting gender bias in science funding
In the 1990s, Dr. Katalin Kariko’s pioneering mRNA research seemed destined for obscurity, doomed by…

- Promoting Research
Plain Language Summary — Communicating your research to bridge the academic-lay gap
Science can be complex, but does that mean it should not be accessible to the…

Addressing Barriers in Academia: Navigating unconscious biases in the Ph.D. journey
In the journey of academia, a Ph.D. marks a transitional phase, like that of a…

- Manuscripts & Grants
- Reporting Research
Unraveling Research Population and Sample: Understanding their role in statistical inference
Research population and sample serve as the cornerstones of any scientific inquiry. They hold the…

- Manuscript Preparation
- Publishing Research
Research Problem Statement — Find out how to write an impactful one!
What Is a Research Problem Statement? A research problem statement is a clear, concise, and…
How to Develop a Good Research Question? — Types & Examples
5 Effective Ways to Avoid Ghostwriting for Busy Researchers
Top 5 Key Differences Between Methods and Methodology

Sign-up to read more
Subscribe for free to get unrestricted access to all our resources on research writing and academic publishing including:
- 2000+ blog articles
- 50+ Webinars
- 10+ Expert podcasts
- 50+ Infographics
- 10+ Checklists
- Research Guides
We hate spam too. We promise to protect your privacy and never spam you.
I am looking for Editing/ Proofreading services for my manuscript Tentative date of next journal submission:

What should universities' stance be on AI tools in research and academic writing?
21 Research Limitations Examples
Research limitations refer to the potential weaknesses inherent in a study. All studies have limitations of some sort, meaning declaring limitations doesn’t necessarily need to be a bad thing, so long as your declaration of limitations is well thought-out and explained.
Rarely is a study perfect. Researchers have to make trade-offs when developing their studies, which are often based upon practical considerations such as time and monetary constraints, weighing the breadth of participants against the depth of insight, and choosing one methodology or another.
In research, studies can have limitations such as limited scope, researcher subjectivity, and lack of available research tools.
Acknowledging the limitations of your study should be seen as a strength. It demonstrates your willingness for transparency, humility, and submission to the scientific method and can bolster the integrity of the study. It can also inform future research direction.
Typically, scholars will explore the limitations of their study in either their methodology section, their conclusion section, or both.
Research Limitations Examples
Qualitative and quantitative research offer different perspectives and methods in exploring phenomena, each with its own strengths and limitations. So, I’ve split the limitations examples sections into qualitative and quantitative below.
Qualitative Research Limitations
Qualitative research seeks to understand phenomena in-depth and in context. It focuses on the ‘why’ and ‘how’ questions.
It’s often used to explore new or complex issues, and it provides rich, detailed insights into participants’ experiences, behaviors, and attitudes. However, these strengths also create certain limitations, as explained below.
1. Subjectivity
Qualitative research often requires the researcher to interpret subjective data. One researcher may examine a text and identify different themes or concepts as more dominant than others.
Close qualitative readings of texts are necessarily subjective – and while this may be a limitation, qualitative researchers argue this is the best way to deeply understand everything in context.
Suggested Solution and Response: To minimize subjectivity bias, you could consider cross-checking your own readings of themes and data against other scholars’ readings and interpretations. This may involve giving the raw data to a supervisor or colleague and asking them to code the data separately, then coming together to compare and contrast results.
2. Researcher Bias
The concept of researcher bias is related to, but slightly different from, subjectivity.
Researcher bias refers to the perspectives and opinions you bring with you when doing your research.
For example, a researcher who is explicitly of a certain philosophical or political persuasion may bring that persuasion to bear when interpreting data.
In many scholarly traditions, we will attempt to minimize researcher bias through the utilization of clear procedures that are set out in advance or through the use of statistical analysis tools.
However, in other traditions, such as in postmodern feminist research , declaration of bias is expected, and acknowledgment of bias is seen as a positive because, in those traditions, it is believed that bias cannot be eliminated from research, so instead, it is a matter of integrity to present it upfront.
Suggested Solution and Response: Acknowledge the potential for researcher bias and, depending on your theoretical framework , accept this, or identify procedures you have taken to seek a closer approximation to objectivity in your coding and analysis.
3. Generalizability
If you’re struggling to find a limitation to discuss in your own qualitative research study, then this one is for you: all qualitative research, of all persuasions and perspectives, cannot be generalized.
This is a core feature that sets qualitative data and quantitative data apart.
The point of qualitative data is to select case studies and similarly small corpora and dig deep through in-depth analysis and thick description of data.
Often, this will also mean that you have a non-randomized sample size.
While this is a positive – you’re going to get some really deep, contextualized, interesting insights – it also means that the findings may not be generalizable to a larger population that may not be representative of the small group of people in your study.
Suggested Solution and Response: Suggest future studies that take a quantitative approach to the question.
4. The Hawthorne Effect
The Hawthorne effect refers to the phenomenon where research participants change their ‘observed behavior’ when they’re aware that they are being observed.
This effect was first identified by Elton Mayo who conducted studies of the effects of various factors ton workers’ productivity. He noticed that no matter what he did – turning up the lights, turning down the lights, etc. – there was an increase in worker outputs compared to prior to the study taking place.
Mayo realized that the mere act of observing the workers made them work harder – his observation was what was changing behavior.
So, if you’re looking for a potential limitation to name for your observational research study , highlight the possible impact of the Hawthorne effect (and how you could reduce your footprint or visibility in order to decrease its likelihood).
Suggested Solution and Response: Highlight ways you have attempted to reduce your footprint while in the field, and guarantee anonymity to your research participants.
5. Replicability
Quantitative research has a great benefit in that the studies are replicable – a researcher can get a similar sample size, duplicate the variables, and re-test a study. But you can’t do that in qualitative research.
Qualitative research relies heavily on context – a specific case study or specific variables that make a certain instance worthy of analysis. As a result, it’s often difficult to re-enter the same setting with the same variables and repeat the study.
Furthermore, the individual researcher’s interpretation is more influential in qualitative research, meaning even if a new researcher enters an environment and makes observations, their observations may be different because subjectivity comes into play much more. This doesn’t make the research bad necessarily (great insights can be made in qualitative research), but it certainly does demonstrate a weakness of qualitative research.
6. Limited Scope
“Limited scope” is perhaps one of the most common limitations listed by researchers – and while this is often a catch-all way of saying, “well, I’m not studying that in this study”, it’s also a valid point.
No study can explore everything related to a topic. At some point, we have to make decisions about what’s included in the study and what is excluded from the study.
So, you could say that a limitation of your study is that it doesn’t look at an extra variable or concept that’s certainly worthy of study but will have to be explored in your next project because this project has a clearly and narrowly defined goal.
Suggested Solution and Response: Be clear about what’s in and out of the study when writing your research question.
7. Time Constraints
This is also a catch-all claim you can make about your research project: that you would have included more people in the study, looked at more variables, and so on. But you’ve got to submit this thing by the end of next semester! You’ve got time constraints.
And time constraints are a recognized reality in all research.
But this means you’ll need to explain how time has limited your decisions. As with “limited scope”, this may mean that you had to study a smaller group of subjects, limit the amount of time you spent in the field, and so forth.
Suggested Solution and Response: Suggest future studies that will build on your current work, possibly as a PhD project.
8. Resource Intensiveness
Qualitative research can be expensive due to the cost of transcription, the involvement of trained researchers, and potential travel for interviews or observations.
So, resource intensiveness is similar to the time constraints concept. If you don’t have the funds, you have to make decisions about which tools to use, which statistical software to employ, and how many research assistants you can dedicate to the study.
Suggested Solution and Response: Suggest future studies that will gain more funding on the back of this ‘ exploratory study ‘.
9. Coding Difficulties
Data analysis in qualitative research often involves coding, which can be subjective and complex, especially when dealing with ambiguous or contradicting data.
After naming this as a limitation in your research, it’s important to explain how you’ve attempted to address this. Some ways to ‘limit the limitation’ include:
- Triangulation: Have 2 other researchers code the data as well and cross-check your results with theirs to identify outliers that may need to be re-examined, debated with the other researchers, or removed altogether.
- Procedure: Use a clear coding procedure to demonstrate reliability in your coding process. I personally use the thematic network analysis method outlined in this academic article by Attride-Stirling (2001).
Suggested Solution and Response: Triangulate your coding findings with colleagues, and follow a thematic network analysis procedure.
10. Risk of Non-Responsiveness
There is always a risk in research that research participants will be unwilling or uncomfortable sharing their genuine thoughts and feelings in the study.
This is particularly true when you’re conducting research on sensitive topics, politicized topics, or topics where the participant is expressing vulnerability .
This is similar to the Hawthorne effect (aka participant bias), where participants change their behaviors in your presence; but it goes a step further, where participants actively hide their true thoughts and feelings from you.
Suggested Solution and Response: One way to manage this is to try to include a wider group of people with the expectation that there will be non-responsiveness from some participants.
11. Risk of Attrition
Attrition refers to the process of losing research participants throughout the study.
This occurs most commonly in longitudinal studies , where a researcher must return to conduct their analysis over spaced periods of time, often over a period of years.
Things happen to people over time – they move overseas, their life experiences change, they get sick, change their minds, and even die. The more time that passes, the greater the risk of attrition.
Suggested Solution and Response: One way to manage this is to try to include a wider group of people with the expectation that there will be attrition over time.
12. Difficulty in Maintaining Confidentiality and Anonymity
Given the detailed nature of qualitative data , ensuring participant anonymity can be challenging.
If you have a sensitive topic in a specific case study, even anonymizing research participants sometimes isn’t enough. People might be able to induce who you’re talking about.
Sometimes, this will mean you have to exclude some interesting data that you collected from your final report. Confidentiality and anonymity come before your findings in research ethics – and this is a necessary limiting factor.
Suggested Solution and Response: Highlight the efforts you have taken to anonymize data, and accept that confidentiality and accountability place extremely important constraints on academic research.
13. Difficulty in Finding Research Participants
A study that looks at a very specific phenomenon or even a specific set of cases within a phenomenon means that the pool of potential research participants can be very low.
Compile on top of this the fact that many people you approach may choose not to participate, and you could end up with a very small corpus of subjects to explore. This may limit your ability to make complete findings, even in a quantitative sense.
You may need to therefore limit your research question and objectives to something more realistic.
Suggested Solution and Response: Highlight that this is going to limit the study’s generalizability significantly.
14. Ethical Limitations
Ethical limitations refer to the things you cannot do based on ethical concerns identified either by yourself or your institution’s ethics review board.
This might include threats to the physical or psychological well-being of your research subjects, the potential of releasing data that could harm a person’s reputation, and so on.
Furthermore, even if your study follows all expected standards of ethics, you still, as an ethical researcher, need to allow a research participant to pull out at any point in time, after which you cannot use their data, which demonstrates an overlap between ethical constraints and participant attrition.
Suggested Solution and Response: Highlight that these ethical limitations are inevitable but important to sustain the integrity of the research.
For more on Qualitative Research, Explore my Qualitative Research Guide
Quantitative Research Limitations
Quantitative research focuses on quantifiable data and statistical, mathematical, or computational techniques. It’s often used to test hypotheses, assess relationships and causality, and generalize findings across larger populations.
Quantitative research is widely respected for its ability to provide reliable, measurable, and generalizable data (if done well!). Its structured methodology has strengths over qualitative research, such as the fact it allows for replication of the study, which underpins the validity of the research.
However, this approach is not without it limitations, explained below.
1. Over-Simplification
Quantitative research is powerful because it allows you to measure and analyze data in a systematic and standardized way. However, one of its limitations is that it can sometimes simplify complex phenomena or situations.
In other words, it might miss the subtleties or nuances of the research subject.
For example, if you’re studying why people choose a particular diet, a quantitative study might identify factors like age, income, or health status. But it might miss other aspects, such as cultural influences or personal beliefs, that can also significantly impact dietary choices.
When writing about this limitation, you can say that your quantitative approach, while providing precise measurements and comparisons, may not capture the full complexity of your subjects of study.
Suggested Solution and Response: Suggest a follow-up case study using the same research participants in order to gain additional context and depth.
2. Lack of Context
Another potential issue with quantitative research is that it often focuses on numbers and statistics at the expense of context or qualitative information.
Let’s say you’re studying the effect of classroom size on student performance. You might find that students in smaller classes generally perform better. However, this doesn’t take into account other variables, like teaching style , student motivation, or family support.
When describing this limitation, you might say, “Although our research provides important insights into the relationship between class size and student performance, it does not incorporate the impact of other potentially influential variables. Future research could benefit from a mixed-methods approach that combines quantitative analysis with qualitative insights.”
3. Applicability to Real-World Settings
Oftentimes, experimental research takes place in controlled environments to limit the influence of outside factors.
This control is great for isolation and understanding the specific phenomenon but can limit the applicability or “external validity” of the research to real-world settings.
For example, if you conduct a lab experiment to see how sleep deprivation impacts cognitive performance, the sterile, controlled lab environment might not reflect real-world conditions where people are dealing with multiple stressors.
Therefore, when explaining the limitations of your quantitative study in your methodology section, you could state:
“While our findings provide valuable information about [topic], the controlled conditions of the experiment may not accurately represent real-world scenarios where extraneous variables will exist. As such, the direct applicability of our results to broader contexts may be limited.”
Suggested Solution and Response: Suggest future studies that will engage in real-world observational research, such as ethnographic research.
4. Limited Flexibility
Once a quantitative study is underway, it can be challenging to make changes to it. This is because, unlike in grounded research, you’re putting in place your study in advance, and you can’t make changes part-way through.
Your study design, data collection methods, and analysis techniques need to be decided upon before you start collecting data.
For example, if you are conducting a survey on the impact of social media on teenage mental health, and halfway through, you realize that you should have included a question about their screen time, it’s generally too late to add it.
When discussing this limitation, you could write something like, “The structured nature of our quantitative approach allows for consistent data collection and analysis but also limits our flexibility to adapt and modify the research process in response to emerging insights and ideas.”
Suggested Solution and Response: Suggest future studies that will use mixed-methods or qualitative research methods to gain additional depth of insight.
5. Risk of Survey Error
Surveys are a common tool in quantitative research, but they carry risks of error.
There can be measurement errors (if a question is misunderstood), coverage errors (if some groups aren’t adequately represented), non-response errors (if certain people don’t respond), and sampling errors (if your sample isn’t representative of the population).
For instance, if you’re surveying college students about their study habits , but only daytime students respond because you conduct the survey during the day, your results will be skewed.
In discussing this limitation, you might say, “Despite our best efforts to develop a comprehensive survey, there remains a risk of survey error, including measurement, coverage, non-response, and sampling errors. These could potentially impact the reliability and generalizability of our findings.”
Suggested Solution and Response: Suggest future studies that will use other survey tools to compare and contrast results.
6. Limited Ability to Probe Answers
With quantitative research, you typically can’t ask follow-up questions or delve deeper into participants’ responses like you could in a qualitative interview.
For instance, imagine you are surveying 500 students about study habits in a questionnaire. A respondent might indicate that they study for two hours each night. You might want to follow up by asking them to elaborate on what those study sessions involve or how effective they feel their habits are.
However, quantitative research generally disallows this in the way a qualitative semi-structured interview could.
When discussing this limitation, you might write, “Given the structured nature of our survey, our ability to probe deeper into individual responses is limited. This means we may not fully understand the context or reasoning behind the responses, potentially limiting the depth of our findings.”
Suggested Solution and Response: Suggest future studies that engage in mixed-method or qualitative methodologies to address the issue from another angle.
7. Reliance on Instruments for Data Collection
In quantitative research, the collection of data heavily relies on instruments like questionnaires, surveys, or machines.
The limitation here is that the data you get is only as good as the instrument you’re using. If the instrument isn’t designed or calibrated well, your data can be flawed.
For instance, if you’re using a questionnaire to study customer satisfaction and the questions are vague, confusing, or biased, the responses may not accurately reflect the customers’ true feelings.
When discussing this limitation, you could say, “Our study depends on the use of questionnaires for data collection. Although we have put significant effort into designing and testing the instrument, it’s possible that inaccuracies or misunderstandings could potentially affect the validity of the data collected.”
Suggested Solution and Response: Suggest future studies that will use different instruments but examine the same variables to triangulate results.
8. Time and Resource Constraints (Specific to Quantitative Research)
Quantitative research can be time-consuming and resource-intensive, especially when dealing with large samples.
It often involves systematic sampling, rigorous design, and sometimes complex statistical analysis.
If resources and time are limited, it can restrict the scale of your research, the techniques you can employ, or the extent of your data analysis.
For example, you may want to conduct a nationwide survey on public opinion about a certain policy. However, due to limited resources, you might only be able to survey people in one city.
When writing about this limitation, you could say, “Given the scope of our research and the resources available, we are limited to conducting our survey within one city, which may not fully represent the nationwide public opinion. Hence, the generalizability of the results may be limited.”
Suggested Solution and Response: Suggest future studies that will have more funding or longer timeframes.

How to Discuss Your Research Limitations
1. in your research proposal and methodology section.
In the research proposal, which will become the methodology section of your dissertation, I would recommend taking the four following steps, in order:
- Be Explicit about your Scope – If you limit the scope of your study in your research question, aims, and objectives, then you can set yourself up well later in the methodology to say that certain questions are “outside the scope of the study.” For example, you may identify the fact that the study doesn’t address a certain variable, but you can follow up by stating that the research question is specifically focused on the variable that you are examining, so this limitation would need to be looked at in future studies.
- Acknowledge the Limitation – Acknowledging the limitations of your study demonstrates reflexivity and humility and can make your research more reliable and valid. It also pre-empts questions the people grading your paper may have, so instead of them down-grading you for your limitations; they will congratulate you on explaining the limitations and how you have addressed them!
- Explain your Decisions – You may have chosen your approach (despite its limitations) for a very specific reason. This might be because your approach remains, on balance, the best one to answer your research question. Or, it might be because of time and monetary constraints that are outside of your control.
- Highlight the Strengths of your Approach – Conclude your limitations section by strongly demonstrating that, despite limitations, you’ve worked hard to minimize the effects of the limitations and that you have chosen your specific approach and methodology because it’s also got some terrific strengths. Name the strengths.
Overall, you’ll want to acknowledge your own limitations but also explain that the limitations don’t detract from the value of your study as it stands.
2. In the Conclusion Section or Chapter
In the conclusion of your study, it is generally expected that you return to a discussion of the study’s limitations. Here, I recommend the following steps:
- Acknowledge issues faced – After completing your study, you will be increasingly aware of issues you may have faced that, if you re-did the study, you may have addressed earlier in order to avoid those issues. Acknowledge these issues as limitations, and frame them as recommendations for subsequent studies.
- Suggest further research – Scholarly research aims to fill gaps in the current literature and knowledge. Having established your expertise through your study, suggest lines of inquiry for future researchers. You could state that your study had certain limitations, and “future studies” can address those limitations.
- Suggest a mixed methods approach – Qualitative and quantitative research each have pros and cons. So, note those ‘cons’ of your approach, then say the next study should approach the topic using the opposite methodology or could approach it using a mixed-methods approach that could achieve the benefits of quantitative studies with the nuanced insights of associated qualitative insights as part of an in-study case-study.
Overall, be clear about both your limitations and how those limitations can inform future studies.
In sum, each type of research method has its own strengths and limitations. Qualitative research excels in exploring depth, context, and complexity, while quantitative research excels in examining breadth, generalizability, and quantifiable measures. Despite their individual limitations, each method contributes unique and valuable insights, and researchers often use them together to provide a more comprehensive understanding of the phenomenon being studied.
Attride-Stirling, J. (2001). Thematic networks: an analytic tool for qualitative research. Qualitative research , 1 (3), 385-405. ( Source )
Atkinson, P., Delamont, S., Cernat, A., Sakshaug, J., & Williams, R. A. (2021). SAGE research methods foundations . London: Sage Publications.
Clark, T., Foster, L., Bryman, A., & Sloan, L. (2021). Bryman’s social research methods . Oxford: Oxford University Press.
Köhler, T., Smith, A., & Bhakoo, V. (2022). Templates in qualitative research methods: Origins, limitations, and new directions. Organizational Research Methods , 25 (2), 183-210. ( Source )
Lenger, A. (2019). The rejection of qualitative research methods in economics. Journal of Economic Issues , 53 (4), 946-965. ( Source )
Taherdoost, H. (2022). What are different research approaches? Comprehensive review of qualitative, quantitative, and mixed method research, their applications, types, and limitations. Journal of Management Science & Engineering Research , 5 (1), 53-63. ( Source )
Walliman, N. (2021). Research methods: The basics . New York: Routledge.

Chris Drew (PhD)
Dr. Chris Drew is the founder of the Helpful Professor. He holds a PhD in education and has published over 20 articles in scholarly journals. He is the former editor of the Journal of Learning Development in Higher Education. [Image Descriptor: Photo of Chris]
- Chris Drew (PhD) https://helpfulprofessor.com/author/chris-drew-phd/ 5 Top Tips for Succeeding at University
- Chris Drew (PhD) https://helpfulprofessor.com/author/chris-drew-phd/ 50 Durable Goods Examples
- Chris Drew (PhD) https://helpfulprofessor.com/author/chris-drew-phd/ 100 Consumer Goods Examples
- Chris Drew (PhD) https://helpfulprofessor.com/author/chris-drew-phd/ 30 Globalization Pros and Cons
Leave a Comment Cancel Reply
Your email address will not be published. Required fields are marked *
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Common pitfalls in the research process.
Jacob Shreffler ; Martin R. Huecker .
Affiliations
Last Update: March 6, 2023 .
- Definition/Introduction
Conducting research from planning to publication can be a very rewarding process. However, multiple preventable setbacks can occur within each stage of research. While these inefficiencies are an inevitable part of the research process, understanding common pitfalls can limit those hindrances. Many issues can present themselves throughout the research process. It has been said about academics that “the politics are so harsh because the stakes are so low.” Beyond interpersonal and political / funding concerns, prospective authors may encounter some disenchantment with the publish or perish culture. With a metric of (any) publication, the motivation to contribute meaningfully to science can be overshadowed by a compulsive drive to publish. [1] We believe in quality over quantity and highlight the importance of channeling creativity when pursuing scholarly work.
When considering embarking on a medical research project, one must begin with detailed planning. Do not underestimate the amount of time a project can take, often spanning years from conception to manuscript preparation. Will you conduct a retrospective chart review, a prospective study, or a true clinical trial with randomization and blinding? Will you systematically seek out and remove sources of bias from the study design and interpretation of results? Will you ensure the study is powered properly to justify conclusions? Will you eliminate or explain any conflicts of interest occurring among your author group? Will you fall victim to the temptation of frivolous subgroup analyses, or will you stick with the original plan? Will your study have a realistic chance at publication in a journal within your specialty, or perhaps another subfield? The study results may prove the null hypothesis, a ‘negative study,’ and therefore be difficult to publish. [2] Additionally, the intervention you find beneficial may subsequently be proven unhelpful or even dangerous, leading to prudent medical reversal. [3]
These considerations and more necessitate meticulous planning and vigilant adherence to a sound protocol. Along the way, you will encounter obstacles, pitfalls, some of which are presented in this article. But remain persistent, and your efforts will be rewarded with publication and contribution to science. This review covers common pitfalls researchers encounter and suggested strategies to avoid them.
- Issues of Concern
There are five phases of research: planning phase, data collection/analysis phase, writing phase, journal submission phase, and rejections/revisions/acceptance phase.
Phase I Pitfalls: Planning a Study
The highest yield preempting of pitfalls in the research process occurs in the planning phase. This is when a researcher can set the stage for an optimal research process. Below are pitfalls that can occur during the planning phase.
Pitfall: Underestimating what committing to a research project requires
Conducting a research study and achieving publication sounds fulfilling, right?
Consider the many steps: conducting a literature search, writing an IRB proposal, planning and having research meetings, long and cumbersome data collection processes, working with statisticians or analyzing complex data, having unexpected research setbacks (e.g., subjects drop out, newly published papers on same topic, etc.), the possibility that after data collection you have no statistically (or clinically) significant findings, conducting an updated literature search, writing introduction, methods, results, and discussion sections of a paper, going through the many journal options to determine best fit while aiming for high impact factors, adhering to journal guidelines/fixing drafts, writing cover letters stating importance of the topic to respective journals, creating journal portal accounts, possibly being rejected numerous times, waiting months for journal decisions, working on numerous revisions and being informed by numerous individuals about all of the flaws in your writing and research.
Does it sound, maybe less fulfilling ?
Conducting a research project from inception to publication can be a rewarding experience. Research requires significant time. Setbacks are normal. To produce an important and sought-after research product, an individual must understand the magnitude of commitment required.
Pitfall: Choosing the wrong research pursuit/topic lacks precision
Consider an investigator interested in substance use research. The first challenge is the immense amount of research already published on this topic. Fortunately, there is still a massive amount of uncharted territory in substance use research.
It is important to understand what has been done and what is still undiscovered in your area of research. Do not simply study a topic because you find it interesting; passion is advantageous, but you should ensure that your study will contribute to some field/specialty or research in a significant way.
How does your research differ from what has been done?
How will it impact practice in a way that no previous study has?
Consider these questions when choosing a topic for research. Otherwise, you may struggle to get the work published. It can be demoralizing if you have already written your paper and realize that your paper is not going to get accepted by a reputable journal due to the presence of other papers already describing the same concepts you have.
As always, the first step is a thorough literature search.
Pitfall: Not considering research bias
A common theme noted in literature is that bias can, unfortunately, lead to failure to reproduce results, raising concerns regarding the integrity of science. [4] Bias can be considered various (inadvertent) poor strategies related to data design, analysis, and results reporting that produce spurious results and papers that perhaps should not be published. [5]
While one cannot completely eliminate bias from the research process, researchers should take steps to understand research bias in study endeavors and determine how to minimize bias during the planning phase of the study.
Pitfall: Not focusing on which variables to collect
Researchers often want to collect as much data as possible but should not build a list of variables that includes every single detail about subjects if the variables collected are unlikely to yield insight into the topic of research. The longer the data collection instrument, the higher likelihood of (human) errors (if manually data entry) and the longer duration of the data collection phase. Instead of taking time to build a database with many variables, consider cutting irrelevant variables and use that time to increase the sample size. Determine, based on your own clinical knowledge and published empirical works, which variables are most crucial.
Pitfall: Worrying about the statistics after the data has been collected
A vital part of the research process is ensuring you have a rigorous statistical approach. Involve your statistician very early in the project, preferably in the planning stages. They will have insight into the types of variables to collect and help shape the research methods. Statistical power is an important concept to consider before data collection to avoid false-negative results (Zlowodzki et al., 2006). Furthermore, other concepts, such as covariates, need to be part of the planning phase. Do not wait until after the data collection phase to give data to the statistician who cannot transform the data you have into outputs you want.
Pitfall: Not setting defined author roles
It is important to define who will be declared authors at the beginning of the research process to avoid conflict. Do most people want to be an author? Sure. Does everybody do the work worthy of authorship? No. While placing general comments in a shared document's margin may make the paper slightly better, it probably should not qualify for authorship. Review authorship criteria to determine what constitutes authorship. Clear expectations can ensure that everyone is on the same page and that everyone feels the process is fair, especially for individuals who plan to invest significant time in the project. Clear expectations for each author should occur before any writing begins, including deadlines and specific contributions. [6] [7] [6]
Pitfall: Not considering limitations of work before the paper is written
Avoid this pitfall by reviewing recent manuscripts and reading the limitations sections of these papers. Many of these limitations sections will make notions about generalizability to other populations. Some will discuss low power. Even the best papers in the top journals have many limitations. The best way to avoid or mitigate your work's limitations is to consider them during the planning phase.
How can you set up your project to limit your limitations section?
What (types of) samples should you include in your study?
Were you originally thinking of retrospective design, but it could be prospective?
What steps can you utilize to control baseline characteristics between groups?
Consider all limitations and think about how you can control these before data collection.
Phase II Pitfalls: Data Collection and Analysis
After the planning has occurred, typically after institutional review board (IRB) approval, the data collection and analysis phase can transpire. The entire team should typically stay involved throughout these phases. Below are pitfalls to avoid.
Pitfall: Not being involved in the data collection phase
It is important to be involved with the data collection phase, even if you do not personally collect data. Train the individuals who collect data to ensure all are on the same page and provide periodic oversight to ensure accuracy and quality of the data over time. [8] Do not assume the data collection phase is going smoothly – you may find yourself with a huge dataset riddled with inconsistencies or errors. Schedule periodic meetings to review data.
Pitfall: Not being involved with the statistical analysis phase
If you are not conducting the statistical analysis, do not assume that the person who is analyzing the data is 100% on the same page. Have meetings about the data, how to interpret the data, and the limitations of the data. Ask what other ways the data could be analyzed and how reviewers might negatively critique the data itself or the statistical methods.
The person conducting the analysis will not have the same familiarity with the topic. You are not going to be as familiar with the outputs. By understanding each other, you will a) have clearer, more robust methods and results in sections of the paper, b) limit critiques regarding the statistical approach/data outcomes, c) understand your research better for any presentations, discussion, or future work, and d) develop a positive collaboration for future work.
Phase III Pitfalls: The Writing Phase
The next phase is the writing phase. While this section covers pitfalls during the writing phase, for recommendations on conducting a literature search, writing, and publishing research, see StatPearls Evidence-Base Medicine Chapter: How to Write and Publish a Scientific Manuscript. [9] Below are pitfalls that can occur during the writing phase.
Pitfall: Poor or outdated references
When writing your paper, perform multiple literature searches to ensure all recent, salient references are covered—claims about recent similar work or research that frames your study if the references are outdated. Journals may even ask reviewers to comment on the presence or absence of up-to-date/suitable references. Conduct a literature search prior to data collection and stay on top of references throughout the research process as new papers become available.
Pitfall: No clearly defined purpose of the paper
Many aspects of manuscripts can get overlooked. Lack of a clear purpose statement can doom a paper to futility. Remind the readers of the goal of the project. You do not want consumers of your research to read the results section and forget what the goals/main outcomes are. The purpose statement should be located at the end of the introduction section.
Pitfall: Unclear methods making research hard to reproduce
A common concern in science is the lack of transparency in methods for reproducibility. The methods section should allow a reader to understand exactly what was done and conduct the study. Consider examining the S treng T hening the R eporting of OB servational studies in E pidemiology (STROBE) checklist for the methods (as well as other paper sections) to ensure best reporting practices for reproducibility. [10]
Pitfall: The tables and narratives are the same
Reviewers prefer you not to state findings in narratives that are in tables. Tables focus readers on the most important results and are not redundant with the written content. Make call-outs to the table in the paper's narrative sections, but do not state information found in tables.
Pitfall: Not reporting all data/outcomes
Some authors will state the main outcome of interest or have a statement such as “there were no other statistically significant findings between other groups.” Authors must report all outcomes and statistical analyses for reproducibility of the research. While this may be difficult to do with a broad approach, utilize tables and appendices to report all outcomes to show transparency and limit researcher bias.
Pitfall: Repeating results in discussion
Do not simply restate in the discussion what you already have in the results section. Utilize this section of the paper to link other references to your work and reflect on other empirical investigations' similarities or differences. Explain why your research provides an impactful contribution to the topic.
Pitfall: Making conclusions that do not align with your work
Authors sometimes note in their conclusions how the work impacts a topic due to X reason when X may be too broad a claim and the work doesn’t really support or prove that notion. Researchers should align their conclusions to their own results and highlight the significance of their findings.
Pitfall: Thinking the title is not a big deal
A strong title will help with the impact/readership of your paper. Consider keeping a short title that provides the main takeaway. Papers with more concise titles and present the study conclusion result in a bigger impact/receive more citations. [11]
Pitfall: Completing the abstract last minute
Similar to the title, do not underestimate an abstract. Journal and conference reviewers (and the general audience) may only read your abstract. The abstract must have the key results and contributions of the study and be well-written.
Phase IV Pitfalls: Submitting to a Journal
After the paper has been written, it is time to choose the journal. This phase also has numerous pitfalls. Below are pitfalls that can occur during this phase.
Pitfall: Choosing the wrong journal
Choosing the journal for your work can be overwhelming due to the number of options. Always look at the aims and scope of prospective journals. Look through the author guidelines to ensure that your manuscript adheres. This will save time. Review your reference list for any journals that appear more than once; if so, consider submitting to that journal. You do not want to submit your paper, wait two weeks, and then get a desk rejection because the editors state the paper is not aligned to the journal's aims and scope.
Additionally, researchers can aim too high and spend months (and numerous hours in journal submission portals) trying to publish a manuscript in a journal with a very large impact factor. Though admirable, if the research design and results lacking “gold standard” reporting, authors should consider a journal that is more likely to accept. Find a balance between the quality of your paper and the quality of the journal. Seek feedback from the other authors and/or senior colleagues who can provide honest feedback.
Pitfall: Poor cover letter on journal submission
Do not submit work with a flawed cover letter (errors or lack of clarity in how your work contributes to the body of literature). Spend time writing a detailed cover letter once, have it edited by someone else, and utilize that for all future projects. You can highlight the differences (e.g., the purpose of this work, our results showed) with each project. Use the cover letter to highlight the significance of the study while adhering to the disclosure guidelines (e.g., conflicts of interests, authors contributions, data releases, etc.), which will help the editorial board determine not only the suitability of the paper for the journal but also streamline the review process. [12]
Pitfall: Assuming that after the paper has been submitted to a journal, the work is done
The paper has been submitted! You think you are finished…but, unfortunately, the publishing game may still be far from over. Researchers often do not recognize the amount of time going into the submission/rejection/revisions phases. Revisions can sometimes be total overhauls, more work than writing a whole new paper. Be prepared to continue working.
Phase V Pitfalls: The Rejections, Revisions, and Acceptance Phase
Finally, perhaps the most unpredictable phase, the rejections, revisions, and acceptance phase, has unique pitfalls and other obstacles.
Pitfall: Mourning rejections too long/ “sitting on” a rejected paper
Did you get a desk to reject (i.e., the manuscript was not even sent for blind review)? That is unfortunate but common. You do not have time to sulk. Get that paper submitted somewhere else. The older the data, the less desirable your paper becomes. If the paper went in for a full review and was rejected, that may be even tougher than a desk reject because more time has elapsed. The good news is that (hopefully) you received feedback to incorporate in a revision. Do not spend too much time grieving rejections.
Pitfall: Not laying to rest rejected papers when it is indeed their time to go
Did you write a paper a couple of years ago, and you’ve submitted it to 20 different journals? The data is getting old. The topic wasn’t focused on. The sample size was small. Perhaps the project is not worth pursuing any longer. Do not give in to the sunk cost fallacy. If, however, you are proud of the work and stand by the paper, do not give up. If you believe after the numerous rejections that the topic/project is flawed, you can use this failure as a personal learning/growth opportunity. Do not repeat controllable mistakes on future projects.
Pitfall: Not addressing all of reviewer feedback
Did you get a revise and resubmit? Great news! The reviewers and editors will likely ask you to respond to each comment when you resubmit. Address all of the reviewer feedback. Take your time reading through the feedback, digest it, and re-read it. Carefully respond and decide how to revise your manuscript based on the feedback. Share the reviews and the duties of revision with coauthors. In your response to reviewers, stay professional and address each statement, even if you disagree with what is stated. If you do not respond to each statement, the reviewers often highlight the concern(s) again.
Pitfall: Thinking you know what the reviewers are going to say
Research reviewers are like a box of chocolates. You never know what you are going to get. You may be worried about a section of your paper/research approach, and the reviewers do not mention it at all in their review; instead, they criticize a section of your manuscript that you are most proud of.
In some reviews, you may get feedback like the following:
Reviewer #1
Please change lines 104-108 as I believe they are irrelevant to your study.
Reviewer #2
Please build on lines 104-108, as I believe they are the foundation of your study.
Sometimes, after multiple revisions, there are new concerns presented by the reviewers. This can be disheartening. Should some regulations restrict reviewers from bringing up new ideas/concerns during revision #7? Perhaps. Does any current rule prevent them from doing this? No.
During the review process, we must have faith that the reviewers are knowledgeable and provide fair, insightful, and constructive feedback. While the review process can be arbitrary or frustrating in some cases, peer review remains the gold standard in a scientific publication. Stay positive and persistent. Stay professional in responses to the reviewers. Remember that the review process can be very beneficial as it often leads to feedback that truly elevates your work and makes the product (and you) look better. [13]
Pitfall: Not rewarding yourself for a published paper
You did it! Celebrate your accomplishment. Reflect on the merit of your effort before you move on to other work or re-enter the cycle of IRBs, data coding, journal submissions, etc. Remember and appreciate how remarkable it is that you just contributed knowledge to the world.
- Clinical Significance
Many pitfalls can occur throughout the research process. Researchers should understand these pitfalls and utilize strategies to avoid them to produce high-quality, sought-after research results that are useful for basic science and clinical practice.
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Disclosure: Jacob Shreffler declares no relevant financial relationships with ineligible companies.
Disclosure: Martin Huecker declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Shreffler J, Huecker MR. Common Pitfalls In The Research Process. [Updated 2023 Mar 6]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- Publish or perish: ensuring longevity in nurse education-evaluation of a strategy to engage academics, students, and clinicians in publication activity. [J Prof Nurs. 2013] Publish or perish: ensuring longevity in nurse education-evaluation of a strategy to engage academics, students, and clinicians in publication activity. Wilson A, Sharrad S, Rasmussen P, Kernick J. J Prof Nurs. 2013 Jul-Aug; 29(4):210-6.
- Extent of publishing in predatory journals by academics in higher education institutions in Zimbabwe: A case study of a university. [Account Res. 2023] Extent of publishing in predatory journals by academics in higher education institutions in Zimbabwe: A case study of a university. Jingura RM, Chigwada J, Diver T, Shangwa D. Account Res. 2023 Sep 11; :1-15. Epub 2023 Sep 11.
- Has "Publish or Perish" Become "Publish and Payment"? Navigating Neurosurgical Research in an Innovative Industry. [World Neurosurg. 2017] Has "Publish or Perish" Become "Publish and Payment"? Navigating Neurosurgical Research in an Innovative Industry. Fraser JF. World Neurosurg. 2017 Aug; 104:987-989. Epub 2017 May 17.
- Review [Publish & Perish; research on research and researchers]. [Tijdschr Psychiatr. 2017] Review [Publish & Perish; research on research and researchers]. Tijdink JK. Tijdschr Psychiatr. 2017; 59(7):406-413.
- Review Publication Practices and Responsible Authorship: A Review Article. [J Public Health Afr. 2017] Review Publication Practices and Responsible Authorship: A Review Article. Tarkang EE, Kweku M, Zotor FB. J Public Health Afr. 2017 Jun 23; 8(1):723. Epub 2017 Jun 27.
Recent Activity
- Common Pitfalls In The Research Process - StatPearls Common Pitfalls In The Research Process - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
- Affiliate Program

- UNITED STATES
- 台灣 (TAIWAN)
- TÜRKIYE (TURKEY)
- Academic Editing Services
- - Research Paper
- - Journal Manuscript
- - Dissertation
- - College & University Assignments
- Admissions Editing Services
- - Application Essay
- - Personal Statement
- - Recommendation Letter
- - Cover Letter
- - CV/Resume
- Business Editing Services
- - Business Documents
- - Report & Brochure
- - Website & Blog
- Writer Editing Services
- - Script & Screenplay
- Our Editors
- Client Reviews
- Editing & Proofreading Prices
- Wordvice Points
- Partner Discount
- Plagiarism Checker
APA Citation Generator
MLA Citation Generator
Chicago Citation Generator
Vancouver Citation Generator
- - APA Style
- - MLA Style
- - Chicago Style
- - Vancouver Style
- Writing & Editing Guide
- Academic Resources
- Admissions Resources
How to Present the Limitations of the Study Examples
What are the limitations of a study?
The limitations of a study are the elements of methodology or study design that impact the interpretation of your research results. The limitations essentially detail any flaws or shortcomings in your study. Study limitations can exist due to constraints on research design, methodology, materials, etc., and these factors may impact the findings of your study. However, researchers are often reluctant to discuss the limitations of their study in their papers, feeling that bringing up limitations may undermine its research value in the eyes of readers and reviewers.
In spite of the impact it might have (and perhaps because of it) you should clearly acknowledge any limitations in your research paper in order to show readers—whether journal editors, other researchers, or the general public—that you are aware of these limitations and to explain how they affect the conclusions that can be drawn from the research.
In this article, we provide some guidelines for writing about research limitations, show examples of some frequently seen study limitations, and recommend techniques for presenting this information. And after you have finished drafting and have received manuscript editing for your work, you still might want to follow this up with academic editing before submitting your work to your target journal.
Why do I need to include limitations of research in my paper?
Although limitations address the potential weaknesses of a study, writing about them toward the end of your paper actually strengthens your study by identifying any problems before other researchers or reviewers find them.
Furthermore, pointing out study limitations shows that you’ve considered the impact of research weakness thoroughly and have an in-depth understanding of your research topic. Since all studies face limitations, being honest and detailing these limitations will impress researchers and reviewers more than ignoring them.

Where should I put the limitations of the study in my paper?
Some limitations might be evident to researchers before the start of the study, while others might become clear while you are conducting the research. Whether these limitations are anticipated or not, and whether they are due to research design or to methodology, they should be clearly identified and discussed in the discussion section —the final section of your paper. Most journals now require you to include a discussion of potential limitations of your work, and many journals now ask you to place this “limitations section” at the very end of your article.
Some journals ask you to also discuss the strengths of your work in this section, and some allow you to freely choose where to include that information in your discussion section—make sure to always check the author instructions of your target journal before you finalize a manuscript and submit it for peer review .
Limitations of the Study Examples
There are several reasons why limitations of research might exist. The two main categories of limitations are those that result from the methodology and those that result from issues with the researcher(s).
Common Methodological Limitations of Studies
Limitations of research due to methodological problems can be addressed by clearly and directly identifying the potential problem and suggesting ways in which this could have been addressed—and SHOULD be addressed in future studies. The following are some major potential methodological issues that can impact the conclusions researchers can draw from the research.
Issues with research samples and selection
Sampling errors occur when a probability sampling method is used to select a sample, but that sample does not reflect the general population or appropriate population concerned. This results in limitations of your study known as “sample bias” or “selection bias.”
For example, if you conducted a survey to obtain your research results, your samples (participants) were asked to respond to the survey questions. However, you might have had limited ability to gain access to the appropriate type or geographic scope of participants. In this case, the people who responded to your survey questions may not truly be a random sample.
Insufficient sample size for statistical measurements
When conducting a study, it is important to have a sufficient sample size in order to draw valid conclusions. The larger the sample, the more precise your results will be. If your sample size is too small, it will be difficult to identify significant relationships in the data.
Normally, statistical tests require a larger sample size to ensure that the sample is considered representative of a population and that the statistical result can be generalized to a larger population. It is a good idea to understand how to choose an appropriate sample size before you conduct your research by using scientific calculation tools—in fact, many journals now require such estimation to be included in every manuscript that is sent out for review.
Lack of previous research studies on the topic
Citing and referencing prior research studies constitutes the basis of the literature review for your thesis or study, and these prior studies provide the theoretical foundations for the research question you are investigating. However, depending on the scope of your research topic, prior research studies that are relevant to your thesis might be limited.
When there is very little or no prior research on a specific topic, you may need to develop an entirely new research typology. In this case, discovering a limitation can be considered an important opportunity to identify literature gaps and to present the need for further development in the area of study.
Methods/instruments/techniques used to collect the data
After you complete your analysis of the research findings (in the discussion section), you might realize that the manner in which you have collected the data or the ways in which you have measured variables has limited your ability to conduct a thorough analysis of the results.
For example, you might realize that you should have addressed your survey questions from another viable perspective, or that you were not able to include an important question in the survey. In these cases, you should acknowledge the deficiency or deficiencies by stating a need for future researchers to revise their specific methods for collecting data that includes these missing elements.
Common Limitations of the Researcher(s)
Study limitations that arise from situations relating to the researcher or researchers (whether the direct fault of the individuals or not) should also be addressed and dealt with, and remedies to decrease these limitations—both hypothetically in your study, and practically in future studies—should be proposed.
Limited access to data
If your research involved surveying certain people or organizations, you might have faced the problem of having limited access to these respondents. Due to this limited access, you might need to redesign or restructure your research in a different way. In this case, explain the reasons for limited access and be sure that your finding is still reliable and valid despite this limitation.
Time constraints
Just as students have deadlines to turn in their class papers, academic researchers might also have to meet deadlines for submitting a manuscript to a journal or face other time constraints related to their research (e.g., participants are only available during a certain period; funding runs out; collaborators move to a new institution). The time available to study a research problem and to measure change over time might be constrained by such practical issues. If time constraints negatively impacted your study in any way, acknowledge this impact by mentioning a need for a future study (e.g., a longitudinal study) to answer this research problem.
Conflicts arising from cultural bias and other personal issues
Researchers might hold biased views due to their cultural backgrounds or perspectives of certain phenomena, and this can affect a study’s legitimacy. Also, it is possible that researchers will have biases toward data and results that only support their hypotheses or arguments. In order to avoid these problems, the author(s) of a study should examine whether the way the research problem was stated and the data-gathering process was carried out appropriately.
Steps for Organizing Your Study Limitations Section
When you discuss the limitations of your study, don’t simply list and describe your limitations—explain how these limitations have influenced your research findings. There might be multiple limitations in your study, but you only need to point out and explain those that directly relate to and impact how you address your research questions.
We suggest that you divide your limitations section into three steps: (1) identify the study limitations; (2) explain how they impact your study in detail; and (3) propose a direction for future studies and present alternatives. By following this sequence when discussing your study’s limitations, you will be able to clearly demonstrate your study’s weakness without undermining the quality and integrity of your research.
Step 1. Identify the limitation(s) of the study
- This part should comprise around 10%-20% of your discussion of study limitations.
The first step is to identify the particular limitation(s) that affected your study. There are many possible limitations of research that can affect your study, but you don’t need to write a long review of all possible study limitations. A 200-500 word critique is an appropriate length for a research limitations section. In the beginning of this section, identify what limitations your study has faced and how important these limitations are.
You only need to identify limitations that had the greatest potential impact on: (1) the quality of your findings, and (2) your ability to answer your research question.

Step 2. Explain these study limitations in detail
- This part should comprise around 60-70% of your discussion of limitations.
After identifying your research limitations, it’s time to explain the nature of the limitations and how they potentially impacted your study. For example, when you conduct quantitative research, a lack of probability sampling is an important issue that you should mention. On the other hand, when you conduct qualitative research, the inability to generalize the research findings could be an issue that deserves mention.
Explain the role these limitations played on the results and implications of the research and justify the choice you made in using this “limiting” methodology or other action in your research. Also, make sure that these limitations didn’t undermine the quality of your dissertation .
Step 3. Propose a direction for future studies and present alternatives (optional)
- This part should comprise around 10-20% of your discussion of limitations.
After acknowledging the limitations of the research, you need to discuss some possible ways to overcome these limitations in future studies. One way to do this is to present alternative methodologies and ways to avoid issues with, or “fill in the gaps of” the limitations of this study you have presented. Discuss both the pros and cons of these alternatives and clearly explain why researchers should choose these approaches.
Make sure you are current on approaches used by prior studies and the impacts they have had on their findings. Cite review articles or scientific bodies that have recommended these approaches and why. This might be evidence in support of the approach you chose, or it might be the reason you consider your choices to be included as limitations. This process can act as a justification for your approach and a defense of your decision to take it while acknowledging the feasibility of other approaches.
P hrases and Tips for Introducing Your Study Limitations in the Discussion Section
The following phrases are frequently used to introduce the limitations of the study:
- “There may be some possible limitations in this study.”
- “The findings of this study have to be seen in light of some limitations.”
- “The first is the…The second limitation concerns the…”
- “The empirical results reported herein should be considered in the light of some limitations.”
- “This research, however, is subject to several limitations.”
- “The primary limitation to the generalization of these results is…”
- “Nonetheless, these results must be interpreted with caution and a number of limitations should be borne in mind.”
- “As with the majority of studies, the design of the current study is subject to limitations.”
- “There are two major limitations in this study that could be addressed in future research. First, the study focused on …. Second ….”
For more articles on research writing and the journal submissions and publication process, visit Wordvice’s Academic Resources page.
And be sure to receive professional English editing and proofreading services , including paper editing services , for your journal manuscript before submitting it to journal editors.
Wordvice Resources
Proofreading & Editing Guide
Writing the Results Section for a Research Paper
How to Write a Literature Review
Research Writing Tips: How to Draft a Powerful Discussion Section
How to Captivate Journal Readers with a Strong Introduction
Tips That Will Make Your Abstract a Success!
APA In-Text Citation Guide for Research Writing
Additional Resources
- Diving Deeper into Limitations and Delimitations (PhD student)
- Organizing Your Social Sciences Research Paper: Limitations of the Study (USC Library)
- Research Limitations (Research Methodology)
- How to Present Limitations and Alternatives (UMASS)
Article References
Pearson-Stuttard, J., Kypridemos, C., Collins, B., Mozaffarian, D., Huang, Y., Bandosz, P.,…Micha, R. (2018). Estimating the health and economic effects of the proposed US Food and Drug Administration voluntary sodium reformulation: Microsimulation cost-effectiveness analysis. PLOS. https://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1002551
Xu, W.L, Pedersen, N.L., Keller, L., Kalpouzos, G., Wang, H.X., Graff, C,. Fratiglioni, L. (2015). HHEX_23 AA Genotype Exacerbates Effect of Diabetes on Dementia and Alzheimer Disease: A Population-Based Longitudinal Study. PLOS. Retrieved from https://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1001853

Organizing Academic Research Papers: Limitations of the Study
- Purpose of Guide
- Design Flaws to Avoid
- Glossary of Research Terms
- Narrowing a Topic Idea
- Broadening a Topic Idea
- Extending the Timeliness of a Topic Idea
- Academic Writing Style
- Choosing a Title
- Making an Outline
- Paragraph Development
- Executive Summary
- Background Information
- The Research Problem/Question
- Theoretical Framework
- Citation Tracking
- Content Alert Services
- Evaluating Sources
- Primary Sources
- Secondary Sources
- Tertiary Sources
- What Is Scholarly vs. Popular?
- Qualitative Methods
- Quantitative Methods
- Using Non-Textual Elements
- Limitations of the Study
- Common Grammar Mistakes
- Avoiding Plagiarism
- Footnotes or Endnotes?
- Further Readings
- Annotated Bibliography
- Dealing with Nervousness
- Using Visual Aids
- Grading Someone Else's Paper
- How to Manage Group Projects
- Multiple Book Review Essay
- Reviewing Collected Essays
- About Informed Consent
- Writing Field Notes
- Writing a Policy Memo
- Writing a Research Proposal
- Acknowledgements
The limitations of the study are those characteristics of design or methodology that impacted or influenced the application or interpretation of the results of your study. They are the constraints on generalizability and utility of findings that are the result of the ways in which you chose to design the study and/or the method used to establish internal and external validity.
Importance of...
Always acknowledge a study's limitations. It is far better for you to identify and acknowledge your study’s limitations than to have them pointed out by your professor and be graded down because you appear to have ignored them.
Keep in mind that acknowledgement of a study's limitations is an opportunity to make suggestions for further research. If you do connect your study's limitations to suggestions for further research, be sure to explain the ways in which these unanswered questions may become more focused because of your study.
Acknowledgement of a study's limitations also provides you with an opportunity to demonstrate to your professor that you have thought critically about the research problem, understood the relevant literature published about it, and correctly assessed the methods chosen for studying the problem. A key objective of the research process is not only discovering new knowledge but also to confront assumptions and explore what we don't know.
Claiming limitiations is a subjective process because you must evaluate the impact of those limitations . Don't just list key weaknesses and the magnitude of a study's limitations. To do so diminishes the validity of your research because it leaves the reader wondering whether, or in what ways, limitation(s) in your study may have impacted the findings and conclusions. Limitations require a critical, overall appraisal and interpretation of their impact. You should answer the question: do these problems with errors, methods, validity, etc. eventually matter and, if so, to what extent?
Structure: How to Structure the Research Limitations Section of Your Dissertation . Dissertations and Theses: An Online Textbook. Laerd.com.
Descriptions of Possible Limitations
All studies have limitations . However, it is important that you restrict your discussion to limitations related to the research problem under investigation. For example, if a meta-analysis of existing literature is not a stated purpose of your research, it should not be discussed as a limitation. Do not apologize for not addressing issues that you did not promise to investigate in your paper.
Here are examples of limitations you may need to describe and to discuss how they possibly impacted your findings. Descriptions of limitations should be stated in the past tense.
Possible Methodological Limitations
- Sample size -- the number of the units of analysis you use in your study is dictated by the type of research problem you are investigating. Note that, if your sample size is too small, it will be difficult to find significant relationships from the data, as statistical tests normally require a larger sample size to ensure a representative distribution of the population and to be considered representative of groups of people to whom results will be generalized or transferred.
- Lack of available and/or reliable data -- a lack of data or of reliable data will likely require you to limit the scope of your analysis, the size of your sample, or it can be a significant obstacle in finding a trend and a meaningful relationship. You need to not only describe these limitations but to offer reasons why you believe data is missing or is unreliable. However, don’t just throw up your hands in frustration; use this as an opportunity to describe the need for future research.
- Lack of prior research studies on the topic -- citing prior research studies forms the basis of your literature review and helps lay a foundation for understanding the research problem you are investigating. Depending on the currency or scope of your research topic, there may be little, if any, prior research on your topic. Before assuming this to be true, consult with a librarian! In cases when a librarian has confirmed that there is a lack of prior research, you may be required to develop an entirely new research typology [for example, using an exploratory rather than an explanatory research design]. Note that this limitation can serve as an important opportunity to describe the need for further research.
- Measure used to collect the data -- sometimes it is the case that, after completing your interpretation of the findings, you discover that the way in which you gathered data inhibited your ability to conduct a thorough analysis of the results. For example, you regret not including a specific question in a survey that, in retrospect, could have helped address a particular issue that emerged later in the study. Acknowledge the deficiency by stating a need in future research to revise the specific method for gathering data.
- Self-reported data -- whether you are relying on pre-existing self-reported data or you are conducting a qualitative research study and gathering the data yourself, self-reported data is limited by the fact that it rarely can be independently verified. In other words, you have to take what people say, whether in interviews, focus groups, or on questionnaires, at face value. However, self-reported data contain several potential sources of bias that should be noted as limitations: (1) selective memory (remembering or not remembering experiences or events that occurred at some point in the past); (2) telescoping [recalling events that occurred at one time as if they occurred at another time]; (3) attribution [the act of attributing positive events and outcomes to one's own agency but attributing negative events and outcomes to external forces]; and, (4) exaggeration [the act of representing outcomes or embellishing events as more significant than is actually suggested from other data].
Possible Limitations of the Researcher
- Access -- if your study depends on having access to people, organizations, or documents and, for whatever reason, access is denied or otherwise limited, the reasons for this need to be described.
- Longitudinal effects -- unlike your professor, who can literally devote years [even a lifetime] to studying a single research problem, the time available to investigate a research problem and to measure change or stability within a sample is constrained by the due date of your assignment. Be sure to choose a topic that does not require an excessive amount of time to complete the literature review, apply the methodology, and gather and interpret the results. If you're unsure, talk to your professor.
- Cultural and other type of bias -- we all have biases, whether we are conscience of them or not. Bias is when a person, place, or thing is viewed or shown in a consistently inaccurate way. It is usually negative, though one can have a positive bias as well. When proof-reading your paper, be especially critical in reviewing how you have stated a problem, selected the data to be studied, what may have been omitted, the manner in which you have ordered events, people, or places and how you have chosen to represent a person, place, or thing, to name a phenomenon, or to use possible words with a positive or negative connotation. Note that if you detect bias in prior research, it must be acknowledged and you should explain what measures were taken to avoid perpetuating bias.
- Fluency in a language -- if your research focuses on measuring the perceived value of after-school tutoring among Mexican-American ESL [English as a Second Language] students, for example, and you are not fluent in Spanish, you are limited in being able to read and interpret Spanish language research studies on the topic. This deficiency should be acknowledged.
Brutus, Stéphane et al. Self-Reported Limitations and Future Directions in Scholarly Reports: Analysis and Recommendations. Journal of Management 39 (January 2013): 48-75; Senunyeme, Emmanuel K. Business Research Methods . Powerpoint Presentation. Regent University of Science and Technology.
Structure and Writing Style
Information about the limitations of your study are generally placed either at the beginning of the discussion section of your paper so the reader knows and understands the limitations before reading the rest of your analysis of the findings, or, the limitations are outlined at the conclusion of the discussion section as an acknowledgement of the need for further study. Statements about a study's limitations should not be buried in the body [middle] of the discussion section unless a limitation is specific to something covered in that part of the paper. If this is the case, though, the limitation should be reiterated at the conclusion of the section.
If you determine that your study is seriously flawed due to important limitations , such as, an inability to acquire critical data, consider reframing it as a pilot study intended to lay the groundwork for a more complete research study in the future. Be sure, though, to specifically explain the ways that these flaws can be successfully overcome in later studies.
But, do not use this as an excuse for not developing a thorough research paper! Review the tab in this guide for developing a research topic . If serious limitations exist, it generally indicates a likelihood that your research problem is too narrowly defined or that the issue or event under study is too recent and, thus, very little research has been written about it. If serious limitations do emerge, consult with your professor about possible ways to overcome them or how to reframe your study.
When discussing the limitations of your research, be sure to:
- Describe each limitation in detailed but concise terms;
- Explain why each limitation exists;
- Provide the reasons why each limitation could not be overcome using the method(s) chosen to gather the data [cite to other studies that had similar problems when possible];
- Assess the impact of each limitation in relation to the overall findings and conclusions of your study; and,
- If appropriate, describe how these limitations could point to the need for further research.
Remember that the method you chose may be the source of a significant limitation that has emerged during your interpretation of the results [for example, you didn't ask a particular question in a survey that you later wish you had]. If this is the case, don't panic. Acknowledge it, and explain how applying a different or more robust methodology might address the research problem more effectively in any future study. A underlying goal of scholarly research is not only to prove what works, but to demonstrate what doesn't work or what needs further clarification.
Brutus, Stéphane et al. Self-Reported Limitations and Future Directions in Scholarly Reports: Analysis and Recommendations. Journal of Management 39 (January 2013): 48-75; Ioannidis, John P.A. Limitations are not Properly Acknowledged in the Scientific Literature. Journal of Clinical Epidemiology 60 (2007): 324-329; Pasek, Josh. Writing the Empirical Social Science Research Paper: A Guide for the Perplexed . January 24, 2012. Academia.edu; Structure: How to Structure the Research Limitations Section of Your Dissertation . Dissertations and Theses: An Online Textbook. Laerd.com; What Is an Academic Paper? Institute for Writing Rhetoric. Dartmouth College; Writing the Experimental Report: Methods, Results, and Discussion. The Writing Lab and The OWL. Purdue University.
Writing Tip
Don't Inflate the Importance of Your Findings! After all the hard work and long hours devoted to writing your research paper, it is easy to get carried away with attributing unwarranted importance to what you’ve done. We all want our academic work to be viewed as excellent and worthy of a good grade, but it is important that you understand and openly acknowledge the limitiations of your study. Inflating of the importance of your study's findings in an attempt hide its flaws is a big turn off to your readers. A measure of humility goes a long way!
Another Writing Tip
Negative Results are Not a Limitation!
Negative evidence refers to findings that unexpectedly challenge rather than support your hypothesis. If you didn't get the results you anticipated, it may mean your hypothesis was incorrect and needs to be reformulated, or, perhaps you have stumbled onto something unexpected that warrants further study. Moreover, the absence of an effect may be very telling in many situations, particularly in experimental research designs. In any case, your results may be of importance to others even though they did not support your hypothesis. Do not fall into the trap of thinking that results contrary to what you expected is a limitation to your study. If you carried out the research well, they are simply your results and only require additional interpretation.
Yet Another Writing Tip
A Note about Sample Size Limitations in Qualitative Research
Sample sizes are typically smaller in qualitative research because, as the study goes on, acquiring more data does not necessarily lead to more information. This is because one occurrence of a piece of data, or a code, is all that is necessary to ensure that it becomes part of the analysis framework. However, it remains true that sample sizes that are too small cannot adequately support claims of having achieved valid conclusions and sample sizes that are too large do not permit the deep, naturalistic, and inductive analysis that defines qualitative inquiry. Determining adequate sample size in qualitative research is ultimately a matter of judgment and experience in evaluating the quality of the information collected against the uses to which it will be applied and the particular research method and purposeful sampling strategy employed. If the sample size is found to be a limitation, it may reflect your judgement about the methodological technique chosen [e.g., single life history study versus focus group interviews] rather than the number of respondents used.
Huberman, A. Michael and Matthew B. Miles. Data Management and Analysis Methods. In Handbook of Qualitative Research. Norman K. Denzin and Yvonna S. Lincoln, eds. (Thousand Oaks, CA: Sage, 1994), pp. 428-444.
- << Previous: 8. The Discussion
- Next: 9. The Conclusion >>
- Last Updated: Jul 18, 2023 11:58 AM
- URL: https://library.sacredheart.edu/c.php?g=29803
- QuickSearch
- Library Catalog
- Databases A-Z
- Publication Finder
- Course Reserves
- Citation Linker
- Digital Commons
- Our Website
Research Support
- Ask a Librarian
- Appointments
- Interlibrary Loan (ILL)
- Research Guides
- Databases by Subject
- Citation Help
Using the Library
- Reserve a Group Study Room
- Renew Books
- Honors Study Rooms
- Off-Campus Access
- Library Policies
- Library Technology
User Information
- Grad Students
- Online Students
- COVID-19 Updates
- Staff Directory
- News & Announcements
- Library Newsletter
My Accounts
- Interlibrary Loan
- Staff Site Login
FIND US ON

Research Limitations
It is for sure that your research will have some limitations and it is normal. However, it is critically important for you to be striving to minimize the range of scope of limitations throughout the research process. Also, you need to provide the acknowledgement of your research limitations in conclusions chapter honestly.
It is always better to identify and acknowledge shortcomings of your work, rather than to leave them pointed out to your by your dissertation assessor. While discussing your research limitations, don’t just provide the list and description of shortcomings of your work. It is also important for you to explain how these limitations have impacted your research findings.
Your research may have multiple limitations, but you need to discuss only those limitations that directly relate to your research problems. For example, if conducting a meta-analysis of the secondary data has not been stated as your research objective, no need to mention it as your research limitation.
Research limitations in a typical dissertation may relate to the following points:
1. Formulation of research aims and objectives . You might have formulated research aims and objectives too broadly. You can specify in which ways the formulation of research aims and objectives could be narrowed so that the level of focus of the study could be increased.
2. Implementation of data collection method . Because you do not have an extensive experience in primary data collection (otherwise you would not be reading this book), there is a great chance that the nature of implementation of data collection method is flawed.
3. Sample size. Sample size depends on the nature of the research problem. If sample size is too small, statistical tests would not be able to identify significant relationships within data set. You can state that basing your study in larger sample size could have generated more accurate results. The importance of sample size is greater in quantitative studies compared to qualitative studies.
4. Lack of previous studies in the research area . Literature review is an important part of any research, because it helps to identify the scope of works that have been done so far in research area. Literature review findings are used as the foundation for the researcher to be built upon to achieve her research objectives.
However, there may be little, if any, prior research on your topic if you have focused on the most contemporary and evolving research problem or too narrow research problem. For example, if you have chosen to explore the role of Bitcoins as the future currency, you may not be able to find tons of scholarly paper addressing the research problem, because Bitcoins are only a recent phenomenon.
5. Scope of discussions . You can include this point as a limitation of your research regardless of the choice of the research area. Because (most likely) you don’t have many years of experience of conducing researches and producing academic papers of such a large size individually, the scope and depth of discussions in your paper is compromised in many levels compared to the works of experienced scholars.
You can discuss certain points from your research limitations as the suggestion for further research at conclusions chapter of your dissertation.
My e-book, The Ultimate Guide to Writing a Dissertation in Business Studies: a step by step assistance offers practical assistance to complete a dissertation with minimum or no stress. The e-book covers all stages of writing a dissertation starting from the selection to the research area to submitting the completed version of the work within the deadline. John Dudovskiy

Limited by our limitations
Affiliations.
- 1 Medical School, University of Michigan, Ann Arbor, MI, USA. [email protected].
- 2 Medical School, University of Michigan, Ann Arbor, MI, USA.
- PMID: 31347033
- PMCID: PMC6684501
- DOI: 10.1007/s40037-019-00530-x
Study limitations represent weaknesses within a research design that may influence outcomes and conclusions of the research. Researchers have an obligation to the academic community to present complete and honest limitations of a presented study. Too often, authors use generic descriptions to describe study limitations. Including redundant or irrelevant limitations is an ineffective use of the already limited word count. A meaningful presentation of study limitations should describe the potential limitation, explain the implication of the limitation, provide possible alternative approaches, and describe steps taken to mitigate the limitation. This includes placing research findings within their proper context to ensure readers do not overemphasize or minimize findings. A more complete presentation will enrich the readers' understanding of the study's limitations and support future investigation.
Keywords: Limitations; Research.
- Biomedical Research / standards*
- Education, Medical*
- Reproducibility of Results

Diving Deeper into Limitations and Delimitations

If you are working on a thesis, dissertation, or other formal research project, chances are your advisor or committee will ask you to address the delimitations of your study. When faced with this request, many students respond with a puzzled look and then go on to address what are actually the study’s limitations.
In a previous article , we covered what goes into the limitations, delimitations, and assumptions sections of your thesis or dissertation. Here, we will dive a bit deeper into the differences between limitations and delimitations and provide some helpful tips for addressing them in your research project—whether you are working on a quantitative or qualitative study.
Acknowledging Weaknesses vs. Defining Boundaries
These concepts are easy to get confused because both limitations and delimitations restrict (or limit) the questions you’ll be able to answer with your study, most notably in terms of generalizability.
However, the biggest difference between limitations and delimitations is the degree of control you have over them—that is, how much they are based in conscious, intentional choices you made in designing your study.
Limitations occur in all types of research and are, for the most part, outside the researcher’s control (given practical constraints, such as time, funding, and access to populations of interest). They are threats to the study’s internal or external validity.
Limitations may include things such as participant drop-out, a sample that isn’t entirely representative of the desired population, violations to the assumptions of parametric analysis (e.g., normality, homogeneity of variance), the limits of self-report, or the absence of reliability and validity data for some of your survey measures.
Limitations can get in the way of your being able to answer certain questions or draw certain types of inferences from your findings. Therefore, it’s important to acknowledge them upfront and make note of how they restrict the conclusions you’ll be able to draw from your study. Frequently, limitations can get in the way of our ability to generalize our findings to the larger populations or to draw causal conclusions, so be sure to consider these issues when you’re thinking about the potential limitations of your study.
Delimitations are also factors that can restrict the questions you can answer or the inferences you can draw from your findings. However, they are based on intentional choices you make a priori (i.e., as you’re designing the study) about where you’re going to draw the boundaries of your project. In other words, they define the project’s scope.
Like limitations, delimitations are a part of every research project, and this is not a bad thing. In fact, it’s very important! You can’t study everything at once. If you try to do so, your project is bound to get huge and unwieldy, and it will become a lot more difficult to interpret your results or come to meaningful conclusions with so many moving parts. You have to draw the line somewhere, and the delimitations are where you choose to draw these lines.
One of the clearest examples of a delimitation that applies to almost every research project is participant exclusion criteria. In conducting either a quantitative or a qualitative study, you will have to define your population of interest. Defining this population of interest means that you will need to articulate the boundaries of that population (i.e., who is not included). Those boundaries are delimitations.
For example, if you’re interested in understanding the experiences of elementary school teachers who have been implementing a new curriculum into their classrooms, you probably won’t be interviewing or sending a survey to any of the following people: non-teachers, high-school teachers, college professors, principals, parents of elementary school children, or the children themselves. Furthermore, you probably won’t be talking to elementary school teachers who have not yet had the experience of implementing the curriculum in question. You would probably only choose to gather data from elementary school teachers who have had this experience because that is who you’re interested in for the purposes of your study. Perhaps you’ll narrow your focus even more to elementary school teachers in a particular school district who have been teaching for a particular length of time. The possibilities can go on. These are choices you will need to make, both for practical reasons (i.e., the population you have access to) and for the questions you are trying to answer.
Of course, for this particular example, this does not mean that it wouldn’t be interesting to also know what principals think about the new curriculum. Or parents. Or elementary school children. It just means that, for the purposes of your project and your research questions, you’re interested in the experience of the teachers, so you’re excluding anyone who does not meet those criteria. Having delimitations to your population of interest also means that you won’t be able to answer any questions about the experiences of those other populations; this is ok because those populations are outside of the scope of your project . As interesting as their experiences might be, you can save these questions for another study. That is the part of the beauty of research: there will always be more studies to do, more questions to ask. You don’t have to (and can’t) do it all in one project.
Continuing with the previous example, for instance, let’s suppose that the problem you are most interested in addressing is the fact that we know relatively little about elementary school teachers’ experiences of implementing a new curriculum. Perhaps you believe that knowing more about teachers’ experiences could inform their training or help administrators know more about how to support their teachers. If the identified problem is our lack of knowledge about teachers’ experiences, and your research questions focus on better understanding these experiences, that means that you are choosing not to focus on other problems or questions, even those that may seem closely related. For instance, you are not asking how effective the new curriculum is in improving student test scores or graduation rates. You might think that would be a very interesting question, but it will have to wait for another study. In narrowing the focus of your research questions, you limit your ability to answer other questions, and again, that’s ok. These other questions may be interesting and important, but, again, they are beyond the scope of your project .
Common Examples of Limitations
While each study will have its own unique set of limitations, some limitations are more common in quantitative research, and others are more common in qualitative research.
In quantitative research, common limitations include the following:
– Participant dropout
– Small sample size, low power
– Non-representative sample
– Violations of statistical assumptions
– Non-experimental design, lack of manipulation of variables, lack of controls
– Potential confounding variables
– Measures with low (or unknown) reliability or validity
– Limits of an instrument to measure the construct of interest
– Data collection methods (e.g., self-report)
– Anything else that might limit the study’s internal or external validity
In qualitative research, common limitations include the following:
– Lack of generalizability of findings (not the goal of qualitative research, but still worth mentioning as a limitation)
– Inability to draw causal conclusions (again, not the goal of qualitative research, but still worth mentioning)
– Researcher bias/subjectivity (especially if there is only one coder)
– Limitations in participants’ ability/willingness to share or describe their experiences
– Any factors that might limit the rigor of data collection or analysis procedures
Common Examples of Delimitations
As noted above, the two most common sources of delimitations in both quantitative and qualitative research include the following:
– Inclusion/exclusion criteria (or how you define your population of interest)
– Research questions or problems you’ve chosen to examine
Several other common sources of delimitations include the following:
– Theoretical framework or perspective adopted
– Methodological framework or paradigm chosen (e.g., quantitative, qualitative, or mixed-methods)
– In quantitative research, the variables you’ve chosen to measure or manipulate (as opposed to others)
Whether you’re conducting a quantitative or qualitative study, you will (hopefully!) have chosen your research design because it is well suited to the questions you’re hoping to answer. Because these questions define the boundaries or scope of your project and thus point to its delimitations, your research design itself will also be related to these delimitations.
Questions to Ask Yourself
As you are considering the limitations and delimitations of your project, it can be helpful to ask yourself a few different questions.
Questions to help point out your study’s limitations :
1. If I had an unlimited budget, unlimited amounts of time, access to all possible populations, and the ability to manipulate as many variables as I wanted, how would I design my study differently to be better able to answer the questions I want to answer? (The ways in which your study falls short of this will point to its limitations.)
2. Are there design issues that get in the way of my being able to draw causal conclusions?
3. Are there sampling issues that get in the way of my being able to generalize my findings?
4. Are there issues related to the measures I’m using or the methods I’m using to collect data? Do I have concerns about participants telling the truth or being able to provide accurate responses to my questions?
5. Are there any other factors that might limit my study’s internal or external validity?
Questions that help point out your study’s delimitations :
1. What are my exclusion criteria? Who did I not include in my study, and why did I make this choice?
2. What questions did I choose not to address in my study? (Of course, the possibilities are endless here, but consider related questions that you chose not to address.)
3. In what ways did I narrow the scope of my study in order to hone in on a particular issue or question?
4. What other methodologies did I not use that might have allowed me to answer slightly different questions about the same topic?
How to Write About Limitations and Delimitations
Remember, having limitations and delimitations is not a bad thing. They’re present in even the most rigorous research. The important thing is to be aware of them and to acknowledge how they may impact your findings or the conclusions you can draw.
In fact, writing about them and acknowledging them gives you an opportunity to demonstrate that you can think critically about these aspects of your study and how they impact your findings, even if they were out of your control.
Keep in mind that your study’s limitations will likely point to important directions for future research. Therefore, when you’re getting ready to write about your recommendations for future research in your discussion, remember to refer back to your limitations section!
As you write about your delimitations in particular, remember that they are not weaknesses, and you don’t have to apologize for them. Good, strong research projects have clear boundaries. Also, keep in mind that you are the researcher and you can choose whatever delimitations you want for your study. You’re in control of the delimitations. You just have to be prepared—both in your discussion section and in your dissertation defense itself—to justify the choices you make and acknowledge how these choices impact your findings.
Browse More on PhDStudent

Everything You Need to Know About References and Citations: Part 1
When you conduct your research, it is important to record the details of all the information you find to provide accurate references, …

How to Write a Proposal: For a Master’s Thesis or Dissertation
Note: Many thanks to fellow PhDStudent blogger Ryan Krone for his contributions and insight to this post. Your thesis/dissertation proposal provides an …

How to Find Free Money for Graduate School Part 2
Getting into graduate school is already a challenge on its own, and funding the program once admitted is even harder. Graduate studies …

How to Find Free Money for Graduate School
You’ve finally earned your Bachelor’s degree and have made it into graduate school. Whether you already have massive student loans from undergrad …

Part 3 of How to Pick Your Defense Committee
What strategies can a doctoral student employ to maneuver the trials and tribulations of a dissertation committee? In Part 2, we …
Best Dissertation Proofreading and Editing Tips to Make Your Work Spotless
You’ve heard this statement many times before: “the dissertation is the most important project you’ve ever worked on.” That may sound like …

Part 2 of How to Pick Your Defense Committee
Choosing a committee can be a daunting task for a doctoral student. We’ve already covered two strategies that can help you through this …

Part 1 of How to Pick Your Defense Committee
So you’re ready to pick your committee members; there are a few things to keep in mind first—after all, it is a …

Intro To Series on How to Pick Your Defense Committee
Choosing the right defense committee can potentially be the difference between a smooth transition of receiving your doctoral degree or dodging bullets …

On Babies and Dissertations: Part 3
I recently had the experience of expecting my first baby a month before I graduated. Throughout the process, I accidentally learned several …

On Babies and Dissertations: Part 2

On Babies and Dissertations: Part 1
I recently had the experience of expecting my first baby a month before I graduated and accidentally learned tips on graduating on time with a …
Click here to cancel reply.
You must be logged in to post a comment.
Copyright © 2024 PhDStudent.com. All rights reserved. Designed by Divergent Web Solutions, LLC .
Current progress and limitations of research regarding the therapeutic use of adipose-derived stem cells: literature review
- Open access
- Published: 17 April 2024
Cite this article
You have full access to this open access article
- Maksym Skrypnyk ORCID: orcid.org/0000-0002-9552-4098 1
74 Accesses
Explore all metrics
Adipose tissue has recently become one of the most promising and predominant sources of mesenchymal stem cells owing to its high accessibility, culturing properties, regenerative potential, and relatively fewer ethical considerations. From the time of the adipose-derived stem cells (ADSCs) discovery, many beneficial properties have been found, including their regenerative, anti-inflammatory, immunomodulatory, and antimicrobial effects. The number of publications and clinical trials using ADSCs has increased significantly worldwide, attesting to the promising nature of the therapeutic properties of ADSCs.
Main body of the abstract
In clinical studies, ADSCs are mainly used to treat wounds, multiple sclerosis, soft tissue trauma, aging, diabetes, Parkinson’s disease, bone and cartilage regeneration, strokes, and spinal cord injuries. Few and insignificant adverse effects after ADSC treatment have been documented, suggesting their relative safety for clinical use. Despite significant progress in ADSC-related studies, several issues are yet to be addressed, including a lack of standardization of ADSC-associated protocols and the methods used to obtain them, inconsistent dosages, small numbers of patients in each treatment group, and variable graft purity. This severely complicates our ability to compare these studies, making the results even of similar studies controversial.
Short conclusion
This review described the current stage of ADSCs-based treatment outcomes and their limitations, associated with standardization of ADSCs.
Similar content being viewed by others

Stem cells: past, present, and future
Wojciech Zakrzewski, Maciej Dobrzyński, … Zbigniew Rybak

A Brief Overview of Global Trends in MSC-Based Cell Therapy
Dragomirka Jovic, Yingjia Yu, … Yonglun Luo

Highly Aligned Ternary Nanofiber Matrices Loaded with MXene Expedite Regeneration of Volumetric Muscle Loss
Moon Sung Kang, Yeuni Yu, … Dong-Wook Han
Avoid common mistakes on your manuscript.
1 Background
Regenerative medicine is a relatively new branch of science that aims to replace aged, damaged, and disease- or trauma-affected tissues and organs, and to stimulate organismal regenerative potential.
Stem cell therapy involves several mechanisms of action. One is direct replacement of damaged cells and tissues [ 1 ]. Another is a paracrine mechanism that involves modulation of the microenvironment, activation of the native immunity, anti-inflammatory effect and prevention of fibrosis development, pain relief through the secretion of cytokines, regulation of cell death, and immunomodulatory effect [ 2 , 3 ]. Stem cell therapy is considered one of the most promising and highly effective treatment methods for several inflammatory diseases, infectious diseases, non-communicable diseases, cancer, age-related pathologies, pediatric diseases and rejuvenation [ 4 , 5 , 6 ].
Despite this, stem cell therapy is still not widespread and is even forbidden in some countries. Based on available data, no more than 15 allogeneic mesenchymal stem cell (MSC) products have been approved worldwide [ 7 ]. Implementation rates of stem-cell-based therapeutic products remain low, but they have been gradually increasing; as of 17 August 2022, twenty-four cellular and gene therapy products have been licensed by the Office of Tissues and Advanced Therapies (USA) [ 8 ]. Medical tourism to seek stem-cell-based therapies has increased significantly despite the small number of clinical studies and poor evidence base for such therapies [ 7 , 9 ].
Initially MSCs were first found in bone marrow in 1976. It has been shown that MSCs, which are multipotent, can differentiate into mesenchymal, endodermal, and ectodermal cell lines [ 10 ]. Bone marrow is the gold-standard source of MSCs [ 11 ]. The most common harvesting site for bone marrow is the iliac crest, followed by the proximal femur [ 12 ]. However, bone marrow aspiration has significant drawbacks due to its high invasiveness and low MSCs yield [ 13 ]. Even MSCs harvested from different bones of the same individual differ in terms of their regenerative potential and cell concentration, and their effects vary between in-vivo and in-vitro settings [ 14 ]. Bone marrow biopsy is poorly tolerated by patients because of post-procedure pain, and most patients experience anxiety before and during the procedure, even in the case of experienced bone marrow donors [ 15 , 16 ]. Owing to these limitations, alternative MSC donor sites and new approaches are in high demand. Connective tissue and stromal components of inner organs are graft-rich sources for MSCs isolation. One of these is adipose tissue, which is in abundance in a human body. The high proliferation and differentiation capacity of adipose-derived stem cells (ADSCs) and their more accessible donor sites make them a more promising and less invasive alternative to bone-marrow MSCs (BM-MSCs) for stem-cell-based therapies [ 17 ]. ADSCs and BM-MSCs have similar characteristics in terms of their morphology, properties, and receptors[ 18 ]. There are also other sources of adult tissue-derived MSC such as peripheral blood, endometrium, tooth pulp, and breast milk [ 19 ]. Umbilical cord, cord blood, placenta and amniotic fluid are the neonatal sources of stem cells [ 20 ]. Adult and neonatal stem cells have various clinical applications and their own advantages and disadvantages. Neonatal stem cells have higher proliferative capacity, potential growth by multi-layering due to the absence of contact inhibition, no senescence over passaging and lower immunogenicity, and higher immunosuppressive capacity [ 20 ]. Despite possessing better immunological properties, neonatal stem cells have several disadvantages that limit their clinical application such as low cell amount in a single cord blood unit, single time collection, high storage cost etc. [ 21 ].
In most circumstances, only the allogeneic application of neonatal stem cells is possible, while BM-MSCs, ADSCs and peripheral blood stem cells can also be used in autologous settings, which significantly facilitates ethical issues, prevents infections from spreading, and provides a limitless source of cells [ 22 ]. Moreover, BM-MSCs are not immune-privileged and have immunogenic potential in allogeneic settings [ 23 ]. Two strategies exist for prolonging their persistence and improving the efficacy of stem cell therapy: modifying the host immune system response or modifying the antigen properties of MSCs [ 24 ].
The range of application of stem cells-based treatment in clinical medicine expands every year, especially adipose tissue as one of the favorable sources of stem cells found a broad application in tissues engineering [ 25 , 26 ]. This review aims to summarize current understandings of ADSC biology, to discuss the latest ADSC-based experimental studies and clinical trials, and to highlight the current advantages and limitations of using ADSCs in medicine.
A systematic search in the PubMed and Scopus database was conducted on 12 October 2023 for all studies including ADSCs, BM-MSCs and MSCs. Original articles, review articles, meta-analysis, clinical cases and case series written in English were selected for review. The search strategy included usage of the following terms: “adipose-derived stem cells”, “fat-derived stem cells”, “bone marrow-derived stem cells”, “mesenchymal stem cells” and their synonyms. Retrieved articles, relative to the review topic, were stored in a database and duplicates were removed.
2 Main text
2.1 status of research regarding regenerative medicine using adscs.
ADSCs were first retrieved from lipoaspirates by Zuk et al. in 2001 [ 27 ]. While BM-MSCs were historically discovered earlier than ADSCs, their clinical application is sometimes limited. This is why an alternative source of MSCs is required. One of them is ADSCs that have been intensively studied worldwide owing to their relative ease of isolation, few ethical considerations, non-invasive harvesting procedure, good culturing properties, and promising results in in-vitro and in-vivo research.
Medical stem cell therapy is flourishing worldwide; however, patients sometimes have unsubstantiated expectations regarding stem cell therapy. Sometimes, stem cell treatment is provided without proper indications and has life-threatening consequences [ 28 ]. The high cost of treatment, low quality, long waiting times, jurisdictional legal restrictions, inability to participate in clinical trials, and lack of access to unapproved treatments lead patients to engage in stem cell tourism. The leaders in international MSC tourism are the USA, China, India, Thailand, and Mexico [ 29 ].
In Japan, in addition to laws governing clinical trials conducted under the International Conference on Harmonisation – Good Clinical Practice and the requirement for the approval of regenerative medical products ( Pharmaceutical and Medical Device Act ), the Act on the Safety of Regenerative Medicine governs the implementation of regenerative medicine in clinical trials or as a treatment. Due to the Regenerative Medicine Act all the procedures were classified into risk categories (high, intermediate and low risk, which are Class I, II and III respectively), among which treatment and research using ADSCs are being conducted in various clinical departments, including the orthopedic and dental fields [ 30 ].
Around the world, stem cell therapies, including those using ADSCs, are offered in clinical practice, with the main clinical indications being multiple sclerosis, cellular therapy of cornea injuries, chronic pulmonary disease, rejuvenation, Parkinson's disease, bone and soft tissues augmentation and regeneration which were destroyed due periodontitis, stroke therapy, severe spinal cord injury, cerebral palsy, chronic wound healing, autism, amyotrophic latent arteriosclerosis, Alzheimer's disease, and inflammatory joints disease [ 31 ].
A search of “adipose-derived stem cells” on www.clinicaltrials.gov found that more than 394 clinical trials using ADSCs have been conducted worldwide, 132 of which have already been completed. In clinical trials, ADSCs have been used for face rejuvenation, keloid treatment, reconstructive surgery, alopecia treatment, arthritis therapy, periodontal therapy, diabetic wound healing, and many other purposes.
2.2 Biology of adipose tissue
Adipose tissue is a connective tissue with special properties. Approximately 20–25% percent of a healthy individual’s weight is adipose tissue. Based on morphological differences, adipocytes were distributed into white, brown and bright (beige) adipocytes. Depending on its location, adipose tissue is classified into subcutaneous (located under the skin) or visceral (around inner organs) fat. White adipocytes are found in white adipose tissue (WAT), and cell shape varies from spherical to oval or polyhedral. Almost the entire cell volume is occupied by a unilocular lipid droplet which occupied the central part of an adipocyte and flattens to the periphery nucleus. The adipocyte`s lipid droplets are lost on a histological section during the traditional way of tissues preparation, which gives WAT a thin polygonal mesh appearance [ 32 , 33 ]. Visceral adipose tissue (VAT) presented as abdominal viscera, mesenterium and omentum, has completely different qualities compared to WAT. Adipocytes type, their secretome, endocrine regulation, proliferation rate, lipolytic activity, sensitivity to insulin and other hormones differ between subcutaneous WAT and visceral fat. Macrophages are more prevalent in VAT compared with subcutaneous WAT [ 34 ].
Brown adipose tissue is common in newborns and located in the neck, back and shoulder areas. With maturation, brown fat scatters around the body. In adults it is located around the neck and inner organs such as the kidneys, adrenal glands, aorta and mediastinum. Brown adipocytes are much smaller compared to white and beige adipocytes, and their lipids are distributed into numerous lipid droplets, with their nucleus located at the cell center. These cells are abundant in mitochondria, with a brown appearance. The major function of these cells is to produce heat. There are two types of brown adipocytes: high- and low-thermogenic adipocytes [ 35 ].
Beige adipocytes are a recently discovered type of brown adipocyte located in subcutaneous fat depots, such as the inguinal and anterior subcutaneous WAT; however, a small number can also be found in VAT [ 36 ]. Beige adipocytes have a multilocular morphology. Properties, cultural and functional differences of white, brown and beige adipocytes summarized in Table 1 .
Similar to every connective tissue, adipose tissue presented as cells surrounded by an extracellular matrix. Cells percentage in adipose tissue is significantly prevail under the extracellular matrix component. Adipocyte is a minimal structural and functional unit of adipose tissue. Besides adipocytes, adipose tissue also consists of preadipocytes, fibroblasts, capillary endothelial cells, macrophages, and stem cells, all of which form the stromal vascular fraction (SVF) that supports, supplies, and protect adipocytes [ 36 ]. Adipose tissue has a good blood supply and is innervated by unmyelinated nerves [ 37 ].
In mammals, adipose tissue has the following important functions: energy storage, hormone secretion, metabolism, protection, and thermogenesis. In recent years, adipose tissue has been considered as a powerful endocrine organ because it produces several hormones such as estrogen, leptin, adiponectin, resistin, and biologically active substances such as TNF, IL-6, IL-1, CCL2, MCP1, PAI-1, and complement factors [ 38 , 39 ].
SVF is one of the adipose tissue components that is a mixture of cells contained within adipocytes that is traditionally isolated by enzymatic digestion. After adipocytes extraction, connective tissue and blood from lipoaspirate, come the SVF, a mix including MSC, endothelial precursor, T-reg, adipose tissue macrophages, smooth muscle cells, pericytes and preadipocytes [ 40 , 41 ].
2.3 Adipose tissue as a source of MSC
WAT is a huge source of MSCs with superior culturing properties. In humans that WAT has an abundance of CD-34+ -cells, immunohistochemical analysis has confirmed that CD-34+ cells are evenly distributed among white adipocytes [ 10 ]. It has been shown that about 5 × 10 5 stem cells can be isolated from a few milligrams of adipose tissue with the possibility of continuously culturing in vitro for up to one month without cell passaging [ 42 ]. Adipose tissue is a prospective source of MSCs owing to variable donor sites, the large quantity of biological sources from deceased donors, and routine deceased-donor workups [ 43 , 44 ]. Studies have shown that WAT harvested from the abdomen of deceased, research-consenting donors indicated that the total nucleated cell count was even higher than that in living donors, and the morphology and functional properties (growth potential, gene expression level, and differentiation ability) of the cell culture were similar [ 43 , 44 ]. However, changes in the properties and biology of adipose tissue in obese individuals are a general health condition [ 39 , 45 , 46 , 47 ]. Isolated ADSCs from VAT and subcutaneous WAT had no differences in morphology and had the same expression of CD antigens. However, the growth rate of subcutaneous WAT ADSCs is 1.75 faster than ADSCs isolated from VAT also ADSCs were different in terms of angiogenic and inflammatory cytokines level. ADSCs from subcutaneous WAT have significantly lower concentrations of chitinase 3-like 1, IL-1β, EGF, MCP-1, Cystatin C, IL-6, IL-8, Pentraxin 3, TGF-β, plasminogen activator urokinase receptor and TNF-α [ 48 ].
There are three main criteria for ADSCs. Firstly, MSCs must have adherent growth; trilineage mesenchymal differentiation (adipocytes, osteoblasts, and chondroblasts). Secondly, ADSCs must express surface specific antigens such as expressing MSCs markers like CD44, CD105, CD90, and CD73, which are progenitors in subcutaneous WAT, and their phenotype is similar to BM-MSCs. Thirdly, ADSCs do not express the HLA-DR protein or MHC Class I molecules, which enable the possibility of allogeneic transplantation [ 49 ].
Some scientists considered that ADSCs to be immune-privileged cells [ 41 ]. The concept of immune privilege means that some biological grafts can survive in the recipient’s body for a certain time without triggering a graft-versus-host response or large-scale destructive inflammation in the place of application [ 50 ]. However, other studies have shown that MSCs are not completely immune-privileged, due to the triggering of both humoral and cellular immune responses in vivo, which depends on the microenvironment [ 24 ]. For example, the second transplantation of allogeneic MSCs from the same donor in mice resulted in accelerated rejection of cells, which attests to the formation of T-cell memory [ 51 ]. It was reported that ADSCs have superior immunomodulatory action because of the less MHC class II expression that makes them a prospective graft material for allogenic treatment [ 52 ]. Allogeneic ADSCs have immunological potential and can trigger graft rejection and inflammation in the recipient’s body. Introducing the human cytomegalovirus US2/US3 gene into ADSCs reduced ADSC immunogenicity and graft rejection by decreasing MHC I protein expression [ 53 ]. This method is promising for obtaining the same effect after transplantation of allogeneic ADSCs as autogenetic ADSCs [ 53 ]. Was reported that immunomodulatory effect related to the regenerative capacity has been increasing [ 52 ]. Moreover, it was shown that MSCs are able to produce molecules which have antimicrobial and analgetic properties, making them a prospective therapeutic agent against cytokine storm- infections [ 54 , 55 ].
Several studies also indicate promising clinical results with brown adipocyte transplantation for the treatment of diabetes and obesity [ 56 ]. In experimental research, brown adipocyte transplantation improved the regulation of adipose tissue and glucose homeostasis as well as insulin resistance [ 57 ]. However, the specific mechanisms behind these effects have not yet been discovered [ 35 ].
2.4 Mechanism of ADSCs action
ADSCs therapy is based on direct replacement of damaged cells with differentiated ADSCs or modification of local paracrine signaling by extracellular vesicles (see Fig. 1 ). Studies report that under different conditions in vitro, ADSCs can differentiate into ectodermal, mesodermal, and endodermal progenitors [ 11 , 17 , 58 , 59 ]. However, only several of these studies reported a successful result in in-vivo studies or clinical trials. Differentiation of ADSCs in vivo is challenging due to poor cell survival, mostly because of the transplantation of cells into organs with a hypoxic environment. However, compared with mature adipocytes, ADSCs have higher survival rates because of less sensitivity to ischemia and secretion of angiogenic factors that stimulate local angiogenesis [ 60 ].
Possible mechanism of ADSCs action via direct cell replacement and paracrine signaling
Studies reported the successful usage of ADSCs in endometrial injury treatment. ADSCs underwent differentiation into mature endometrial epithelial cells, which resulted in endometrial structure and function regeneration [ 61 ]. However, most of studies are limited to the in vitro demonstration of ADSCs differentiation such as differentiation of ADSCs into insulin-producing cells, cells with hepatocytic function, osteocytes, adipocytes etc. [ 58 , 60 , 62 , 63 ]. Nowadays, clinical translation of ADSC-based therapy for a direct cell’s replacement is difficult since most of the mechanisms for stem cells differentiation in the in vivo setting remains unclear. Such treatment might possibly result in the initial stages of cancer development and other adverse results [ 64 ]. ADSCs under inflammation regulate the inflammatory stimuli, triggering the synthesis of pro-angiogenic factors such as VEGF-A, hepatocyte growth factors, and IGF-1 as well as that of hematopoietic cytokines such as macrophage-colony stimulating factor, granulocyte-colony stimulating factor, IL-6, TNF-α [ 65 ].
Another more promising implication of ADSCs is via regulation of local tissue homeostasis. ADSCs possess unique paracrine characteristics. It is realized through extracellular vesicles (EVs) which contain products of cell secretion and transport it to the target cells to regulate cell function and change their phenotype via cell signaling. EVs are secreted by many different cell types, including ADSCs. They contain microRNA, mRNA, lipids, and proteins, and are classified as microvesicles (50–1000 nm in size) and exosomes (30–100 nm)[ 66 , 67 ].
Recently, several promising results of treatment using isolated from ADSCs exosomes were shown. Exosomes of ADSCs contain numerous growths regulating cytokines that enhance recovery of damaged tissue and growth factors that mediate tissue regeneration. These growth factors are: basic fibroblast growth factor, VEGF-A, insulin-like growth factor 1, hepatocyte growth factors, and transforming growth factor, brain-derived neurotrophic factor, nerve growth factor, and glial-derived neurotrophic factor, matrix metalloproteinase- (MMP-) 3 and MMP-9 [ 68 , 69 ].
ADSCs exosomes treatment showed promising results in therapy of neurological diseases, liver fibrosis, myocardial ischemic injuries, endocrine diseases, bone and skin regeneration. Isolated ADSCs exosomes were used for the treatment of ischemic brain injury. They reduced brain ischemia caused by the microglial polarization, which was caused by the delivery of microRNA to inhibit the expression of signal transducers and activators of transcription 1 and phosphatase and tensin homolog deleted on chromosome ten (PTEN) [ 70 ]. Metastasis-associated lung adenocarcinoma transcript 1 was identified as one of the ADSCs exosomes component that contributes to increased neuronal survival and proliferation in traumatic brain injury or other neurodegenerative diseases [ 71 , 72 ]. Mouse ADSC EVs reduced apoptosis of motor neurons of in vitro amyotrophic lateral sclerosis model under the condition of oxidative stress alteration [ 73 ].
Further, exosomes of ADSCs decrease hepatic fibrosis development through the suppression of autophagy, PI3K/AKT/mTOR,,TGF-β/smad, Wnt/β-catenin, LPS/TLR4, EMT/ERK1, PPAR-γ, NF-κB signaling pathways and by the changing of lipid metabolism through regulation of choline metabolism [ 74 , 75 ]. ADSCs exosomes also suppress the proliferation rate of stellate cells through stimulation of apoptosis and arrest of G1 phase of the cell cycle, and through the inhibition of profibrogenic proteins and epithelio-mesenchymal transition [ 76 ]. ADSCs exosome therapy reduced liver damage by downregulation of collagen I, vimentin, α-SMA and fibronectin in liver via selectively transfer of miR-181-5p to affected hepatocytes [ 77 ].
Exosomes isolated from ADSCs are used for the therapy of diabetes mellitus associated erectile dysfunction. They enhance the secretion of the endothelial markers and downregulate caspase-3 after the operation [ 78 ]. ADSCs exosomes activate functional recovery and activate endogenous repair mechanisms of corpus cavernosum via micro RNA 126, 130a and 132 that provides angiogenesis and restore erectile function, and inhibit fibrosis in corpus cavernosum by antifibrotic properties of micro RNA-let7b and c [ 79 ]. Zhao et al. showed that ADSCs exosomes-based treatment induces endometrial regeneration and fertility restoration by collagen remodeling and enhancement of integrin-β3, LIF, and VEGF expression [ 74 ]. EV isolated from human ADSCs increase wound healing and restore the function and prevent scar formation via activation of PI3K/AKT pathway in sebocytes on a murine model [ 80 ].
However, ADSCs and their exosomes have very variable biological properties and cytokine content, even if they were harvested from the SCAT of the same donor but from a different anatomical location. Thus, the thigh fat had a significantly higher cytokines profile except for IL-1β and IL-6, compared with abdominal and chin sites [ 81 ]. Nowadays, standardised issue of ADSC-based therapy, that determine their mechanism of action, is one of a several major limitations of its clinical translation.
2.5 Factors impacting the clinical effectiveness of ADSCs treatment
The result of stem cell treatment depends on the general health of the cell donor. Thus, in patients with diabetes mellitus, type II ADSCs exhibit impaired viability and proliferation rate, mitochondrial dysfunction, senescence phenotype, impaired glucose homeostasis, and insulin sensitivity. Significantly low secretion of VEGF, adiponectin, and CXCL-12, in the background of hypo concentration of leptin, were observed among type-II ADSC samples [ 82 ]. General systemic diseases lead to disturbances in the function and morphology of ADSCs and reduce their therapeutic properties.
Co-transplantation of ADSCs and platelet-rich plasma (PRP) resulted in significantly increased alveolar bone and gingiva regeneration [ 83 , 84 ]. Moreover, PRP activates ADSCs by increasing cytokines and growth factors production, and a fibrin network can be used as a scaffold for the stem cells and to create a conducive microenvironment that increases stemness and prolongs cellular survival rate and duration [ 83 , 85 , 86 ]. Mechanical tension significantly enhances osteoblastic ADSCs differentiation; however, the mitotic activity of ADSCs is not affected by mechanical tension [ 85 ]. Li et al. showed that pretreatment of freshly isolated ADSCs with thymosin beta 4 (Tβ) upregulates the expression of genes associated with cell division, decrease cells doubling time and apoptosis [ 87 ].
In reconstructive surgery, transplantation of ADSCs alone for regenerative purposes is not as effective as co-transplantation with a composition of different cells to create a favorable environment for revascularization, preventing graft resorption and necrosis. In particular, transplantation of ADSCs, adipocytes, and endothelial cells implanted into the extracellular matrix has shown a higher cellular survival rate and volume maintenance when compared to non-prevascularized control grafts [ 86 ].
The injection of ADSCs along with intraoral administration of sildenafil citrate, which enhances blood supply and NO synthesis in animal models, significantly improves the healing rate after colon anastomosis and better reduces inflammation when compared with ADSCs alone [ 88 ]. For promoting hair growth ADSCs pretreated with bee venom is reported to increase the release of fibroblast -1 and -6, endothelial and platelet growth factors and enhancement of cells migration [ 89 ].
The actions of ADSCs are determined by their environment. Human ADSCs transferred to non-inflamed mouse lungs resulted in development of mild low-grade inflammation, which can be associated with apoptotic graft or heterotransplant clearance. T-cells that produce IFNγ can activate the immune response to efferocytosis, thus altering lung homeostasis [ 90 ]. The combination of Shilajit (a herbomineral natural substance) and an alginate hydrogel environment induced osteogenic differentiation of ADSCs into osteoblasts in a short period of time [ 91 ]. Thus, a proper microenvironment can significantly enhance the outcome of ADSCs clinical applications. There are still many concerns about safety of ADSCs therapy, thus, EV from ADSCs showed suppression of breast cancer tumor growth meanwhile the components of cell growth medium had an opposite effect of a tumor [ 92 ].
2.6 Standardization of ADSCs
The translation of novel findings in stem cell therapy to clinical practice has been discouragingly limited and ambiguous, with the effectiveness of some forms of stem cell therapies remaining poorly supported by evidence. The main problem that limits the clinical application of stem cells, in addition to many other biological medical products, is poor standardization and a lack of comprehensive guidelines [ 93 ]. Standardization of biological grafts is necessary because it offers an opportunity to compare research outcomes, which leads to the optimization of ADSC-based treatment.
It is impossible to effectively translate the results of basic research to clinical settings due to differences in cell origins, cultivation conditions, obtainment methods, and the number of cell passages. Tragoonlugkana et al. showed that cell culture plates coated with platelet lysate significantly increased properties of ADSCs such as adhesion, proliferation speed and growth as well as the cells’ viability [ 94 ]. Thus, the same method of adipose tissue harvesting, but used by different commercial systems, influences the cellular content and cytokine secretion of ADSCs [ 95 ]. Distinctive changes in gene expression have been observed after a 48-h ADSCs cultivation period. Regulatory genes are involved in cell morphogenesis and metabolism, cell-to-substrate adhesion, glycoprotein metabolic processes, and regulation of fiber molecular structure organization. Downregulated genes were those involved in cell proliferation, differentiation, and transformation [ 96 ].
Cultural, biological, and functional properties of ADSCs depend on the anatomical location of fat, age, gender, and BMI of patients [ 97 , 98 , 99 ]. It is not yet clear whether isolated cells are actually ADSCs or what types of cells they are able to generate. Researchers agree that not all MSCs have identical characteristics, which can depend on the patient’s age, donor site, isolation technique, and growth [ 100 ]. Close attention should also be paid to the origin of the allogeneic graft, since several studies have underlined that donor age, sex, tissue source, and method of isolation have an effect on cellular and molecular variability [ 101 , 102 ]. Another problem is the safety of the graft and its possibility of being infected with diverse latent viruses that do not trigger a manifestation of the disease under normal conditions. ADSCs harvested from a dog`s omentum with canine distemper disease were found to be infected with canine morbillivirus [ 103 ]. In this study, before the clinical use of ADSCs, cells were checked for the presence of latent viruses.
Currently, the major dilemma with fat grafting, as well as with other biological grafts and substances, is inconsistent results of experimental and clinical findings attributable to poor standardization resulting from wide varieties of harvesting methods, donor sites, and patients’ initial state of health, as well as a lack of established, objective methods for assessment of clinical results and a lack of knowledge on the precise mechanism of stem cell action and regenerative mechanisms. There is also a lack of data and evidence from which to draw conclusions regarding the safety, effectiveness, and impact of ADSCs and other adipose tissue grafts on tissue regeneration [ 104 ].
The main issues that should undergo standardization are adipose tissue harvesting and processing, donor`s health condition, age, cryopreservation and storage procedure, freezing media that was used, quantification of ADSCs number and their phenotypical markers, storage duration, dosage used for the treatment of particular disease etc. Moreover, apart from three main widely accepted criteria for ADSCs – such as plastic-adherent during culturing, trilineage mesenchymal differentiation and expression of specific cell-surface antigens – a functional analysis of ADSCs properties (doubling time, specter and quantity of cytokines secretion, migration speed etc.) should be checked and compared to some standard in order to receive a predictable treatment result.
2.7 Latest clinical studies implementing ADSCs
The number of clinical trials using MSCs has recently significantly increased owing to notable successes and breakthroughs in basic research and experimental studies. New properties and clinical actions of MSCs have been discovered, and their clinical applications and indications have broadened.
Clinical studies have shown that infusion of MSCs leads to vigorous anti-inflammatory effects characterized by lymphocytosis and a decrease in levels of overactivated pro-inflammatory immune cells and TNF-α, in contrast to upregulation of IL-10 secretion. MSCs are known to auto-induce and address their microenvironment to promote cell proliferation and tissue regeneration. MSCs act via paracrine effects on cells and the organ environment, reducing cytokine storms and severe inflammation [ 105 ]. MSCs have been shown to demonstrate antimicrobial properties, increasing the immune response through the production of bactericide peptides and proteins, and the expression of indoleamine 2,3-dioxygenase (enzyme that decrease reproduction rate of viruses, some mammalian cells) and IL-17 [ 106 ]. ADSCs have proven efficient in the treatment of pulmonary diseases in vivo targeting a paracrine pathway, through the promotion of the epitheliocytes mitosis and apoptosis suppression [ 107 ]. The outcomes and limitations of clinical and randomized clinical trials with adipose tissue grafting products are shown in Table 2 .
3 Conclusion
The last five years have witnessed a huge breakthrough in the translation of basic research and experimental studies of ADSCs into clinical practice. ADSCs are the most promising and easy-to-obtain cells when compared to other MSCs because of their satisfactory cultural, biological, and clinical properties. The future of ADSC-based therapies likely belongs to allogeneic ADSCs. ADSC-based treatment is a highly promising method that utilizes etiological treatment approaches for diseases that are accompanied by cell death or acute tissue loss such as diabetes mellitus type I, xerostomia, periodontitis, and wound treatment for them stem cell therapy. ADSCs act through their differentiation to the specific type of mature cells which are determined by the particular microenvironment or cell stimuli or via paracrine regulation, such as secretion of growth factors and cytokines.
The clinical translation of ADSCs requires proper validation in large controlled trials, discovery of the exact mechanism of action, research standardization, and the adoption of pre-determined therapeutic guidelines.
The vast majority of pre-clinical in vivo studies showed positive treatment outcomes, however there were only a few clinical trials performed, indicating that enough clinical evidence is not yet available to allow broad ADSCs implementation into clinical practice. Among the limiting factors are: small patient sample sizes, predominantly short-term observation time, a lack of adipose tissue graft standardization (procedure and cite of the graft harvesting, cell culturing protocols), deficit of clinical protocols and guidelines, and subjective scoring methods for clinical study results (such as visual assessment and patients’ response to pain) [ 22 ]. Currently, most preclinical studies and clinical trials reported that ADSCs are relatively safe and effective [ 104 , 115 ]. The issue of dose, quality of the graft and indications remains unresolved and debatable [ 62 ]. According to the reviewed literature analysis, adipose tissue-derived biological products such as (ADSCs, SFV, ADSCs-Ev) showed promising results in clinical and in vivo setting. However, one of the main limitations of ADSCs therapy, as all other biologics-based drugs, is a process of the graft standardization. Such standardization should take into consideration the functional properties of the graft, such as doubling time, specter and quantity of cytokines secretion, migration speed etc., all of which varied based on the procedure of their isolation, localization, and pretreatment used in order to provide predictable and effective treatment outcomes.
Data availability
Not applicable for review paper.
Availability of data and materials
Not applicable.
Abbreviations
- Adipose-derived stem cells
Mesenchymal stem cell
Bone marrow-derived stem cells
- Regenerative medicine
Visceral adipose tissue
White adipose tissue
Stromal vascular fraction
Extracellular vesicles
Vascular endothelial growth factor
Transforming growth factor
Matrix metalloproteinase
Tumor necrosis factor
Platelet-rich plasma
Interferon gamma
Peroxisome proliferator-activated receptor gamma
Nuclear factor kappa-light-chain-enhancer of activated B cells
Yang L, Hu Z-M, Jiang F-X, Wang W (2022) Stem cell therapy for insulin-dependent diabetes: Are we still on the road? WJSC 14:503–512. https://doi.org/10.4252/wjsc.v14.i7.503
Article PubMed PubMed Central Google Scholar
Cheng W, Zeng Y, Wang D (2022) Stem cell-based therapy for pulmonary fibrosis. Stem Cell Res Ther 13:492. https://doi.org/10.1186/s13287-022-03181-8
Article CAS PubMed PubMed Central Google Scholar
Ma J, Lei P, Chen H et al (2022) Advances in lncRNAs from stem cell-derived exosome for the treatment of cardiovascular diseases. Front Pharmacol 13:986683. https://doi.org/10.3389/fphar.2022.986683
Hoang DM, Pham PT, Bach TQ et al (2022) Stem cell-based therapy for human diseases. Signal Transduct Target Ther 7:272. https://doi.org/10.1038/s41392-022-01134-4
Yan S, Campos de Souza S, Xie Z, Bao Y (2023) Research progress in clinical trials of stem cell therapy for stroke and neurodegenerative diseases. Ibrain 9:214–230. https://doi.org/10.1002/ibra.12095
El Sayed R, Shankar KM, Mankame AR, Cox CS Jr (2023) Innovations in cell therapy in pediatric diseases: a narrative review. Transl Pediatr 12:1239–1257. https://doi.org/10.21037/tp-23-92
Orozco-Solares TE, León-Moreno LC, Rojas-Rizo A et al (2022) Allogeneic mesenchymal stem cell-based treatment legislation in Latin America: the need for standardization in a medical tourism context. Stem Cells Dev 31:143–162. https://doi.org/10.1089/scd.2022.0013
Article CAS PubMed Google Scholar
https://www.Fda.Gov/Vaccines-Blood-Biologics/Cellular-Gene-Therapy-Products/Approved-Cellular-and-Gene-Therapy-Products .
Glenn Cohen I, Simana S (2018) Regulation of stem cell therapy travel. Curr Stem Cell Rep 4:220–227. https://doi.org/10.1007/s40778-018-0134-8
Article Google Scholar
Şovrea AS, Boşca AB, Constantin AM et al (2019) State of the art in human adipose stem cells and their role in therapy. Rom J Morphol Embryol 60:7–31
PubMed Google Scholar
Frese L, Dijkman PE, Hoerstrup SP (2016) Adipose tissue-derived stem cells in regenerative medicine. Transfus Med Hemother 43:268–274. https://doi.org/10.1159/000448180
Youn GM, Woodall BM, Elena N et al (2018) Arthroscopic bone marrow aspirate concentrate harvesting from the intercondylar notch of the knee. Arthrosc Tech 7:e1173–e1176. https://doi.org/10.1016/j.eats.2018.07.016
Pittenger MF, Mackay AM, Beck SC et al (1979) (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147. https://doi.org/10.1126/science.284.5411.143
Sivasubramaniyan K, Ilas DC, Harichandan A et al (2018) Bone marrow-harvesting technique influences functional heterogeneity of mesenchymal stem/stromal cells and cartilage regeneration. Am J Sports Med 46:3521–3531. https://doi.org/10.1177/0363546518804807
Tanasale B, Kits J, Kluin PM et al (2013) Pain and anxiety during bone marrow biopsy. Pain Manag Nurs 14:310–317. https://doi.org/10.1016/j.pmn.2011.06.007
Article PubMed Google Scholar
Filbet M, Fawoubo A, Tricou C et al (2020) Management of the procedural pain induced by bone marrow biopsies: an observational study. Ann Clin Oncol. https://doi.org/10.31487/j.ACO.2020.01.06
Mazini L, Rochette L, Amine M, Malka G (2019) Regenerative capacity of adipose derived stem cells (ADSCs), comparison with mesenchymal stem cells (MSCs). IJMS 20:2523. https://doi.org/10.3390/ijms20102523
Bourin P, Bunnell BA, Casteilla L et al (2013) Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 15:641–648. https://doi.org/10.1016/j.jcyt.2013.02.006
Macrin D, Joseph JP, Pillai AA, Devi A (2017) Eminent sources of adult mesenchymal stem cells and their therapeutic imminence. Stem Cell Rev and Rep 13:741–756. https://doi.org/10.1007/s12015-017-9759-8
Hass R, Kasper C, Böhm S, Jacobs R (2011) Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal 9:12. https://doi.org/10.1186/1478-811X-9-12
Sanchez-Petitto G, Rezvani K, Daher M et al (2023) Umbilical cord blood transplantation: connecting its origin to its future. Stem Cells Transl Med. https://doi.org/10.1093/stcltm/szac086
Malhotra A, Thebaud B, Paton MCB et al (2023) Advances in neonatal cell therapies: proceedings of the First Neonatal Cell Therapies Symposium (2022). Pediatr Res 94:1631–1638. https://doi.org/10.1038/s41390-023-02707-x
Zhang J, Huang X, Wang H et al (2015) The challenges and promises of allogeneic mesenchymal stem cells for use as a cell-based therapy. Stem Cell Res Ther 6:234. https://doi.org/10.1186/s13287-015-0240-9
Ankrum JA, Ong JF, Karp JM (2014) Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol 32:252–260. https://doi.org/10.1038/nbt.2816
Biniazan F, Stoian A, Haykal S (2024) Adipose-derived stem cells: angiogenetic potential and utility in tissue engineering. Int J Mol Sci 25:2356. https://doi.org/10.3390/ijms25042356
Nasser RA, Arya SS, Alshehhi KH et al (2024) Conducting polymer scaffolds: a new frontier in bioelectronics and bioengineering. Trends Biotechnol. https://doi.org/10.1016/j.tibtech.2023.11.017
Zuk PA, Zhu M, Mizuno H et al (2001) Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7:211–228. https://doi.org/10.1089/107632701300062859
Madhavan AA, Summerfield D, Hunt CH et al (2020) Polyclonal lymphocytic infiltrate with arachnoiditis resulting from intrathecal stem cell transplantation. Neuroradiol J 33:174–178. https://doi.org/10.1177/1971400920902451
Lyons S, Salgaonkar S, Flaherty GT (2022) International stem cell tourism: a critical literature review and evidence-based recommendations. Int Health 14:132–141. https://doi.org/10.1093/inthealth/ihab050
Tobita M, Konomi K, Torashima Y et al (2016) Japan’s challenges of translational regenerative medicine: Act on the safety of regenerative medicine. Regen Ther 4:78–81. https://doi.org/10.1016/j.reth.2016.04.001
Connolly R, O’Brien T, Flaherty G (2014) Stem cell tourism–a web-based analysis of clinical services available to international travellers. Travel Med Infect Dis 12:695–701. https://doi.org/10.1016/j.tmaid.2014.09.008
Rehfeld A, Nylander M, Karnov K (2017) Adipose tissue. Compendium of histology. Springer International Publishing, Cham, pp 201–207
Chapter Google Scholar
Mescher AL, Junqueira LCU (2013) Junqueira’s basic histology: text and atlas, 13th edn. McGraw-Hill Medical, New York, NY
Google Scholar
Ibrahim MM (2010) Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev 11:11–18. https://doi.org/10.1111/j.1467-789X.2009.00623.x
Shinde AB, Song A, Wang QA (2021) Brown adipose tissue heterogeneity, energy metabolism, and beyond. Front Endocrinol 12:651763. https://doi.org/10.3389/fendo.2021.651763
Tordjman J (2013) Histology of adipose tissue. In: Bastard J-P, Fève B (eds) Physiology and physiopathology of adipose tissue. Springer Paris, Paris, pp 67–75
Blaszkiewicz M, Willows JW, Johnson CP, Townsend KL (2019) The importance of peripheral nerves in adipose tissue for the regulation of energy balance. Biology (Basel) 8:10. https://doi.org/10.3390/biology8010010
Beregova TV, Neporada KS, Skrypnyk M et al (2017) Efficacy of nanoceria for periodontal tissues alteration in glutamate-induced obese rats-Multidisciplinary considerations for personalized dentistry and prevention. EPMA J. https://doi.org/10.1007/s13167-017-0085-7
González-Muniesa P, Mártinez-González M-A, Hu FB et al (2017) Obesity. Nat Rev Dis Primers 3:17034. https://doi.org/10.1038/nrdp.2017.34
Nguyen A, Guo J, Banyard DA et al (2016) Stromal vascular fraction: a regenerative reality? Part 1: current concepts and review of the literature. J Plast Reconstr Aesthet Surg 69:170–179. https://doi.org/10.1016/j.bjps.2015.10.015
Ramakrishnan VM, Boyd NL (2018) The adipose stromal vascular fraction as a complex cellular source for tissue engineering applications. Tissue Eng Part B Rev 24:289–299. https://doi.org/10.1089/ten.teb.2017.0061
Zhu Y, Liu T, Song K et al (2008) Adipose-derived stem cell: a better stem cell than BMSC. Cell Res 18:S165–S165. https://doi.org/10.1038/cr.2008.255
Cieśla J, Tomsia M (2021) Cadaveric stem cells: their research potential and limitations. Front Genet 12:798161. https://doi.org/10.3389/fgene.2021.798161
Rao P, Deo D, Marchioni M et al (2019) 24: deceased donor adipose tissue: an untapped source of mesenchymal stem cells. Transplantation 103:S5–S6. https://doi.org/10.1097/01.tp.0000581296.60427.ad
Kim SM, Lun M, Wang M et al (2014) Loss of white adipose hyperplastic potential is associated with enhanced susceptibility to insulin resistance. Cell Metab 20:1049–1058. https://doi.org/10.1016/j.cmet.2014.10.010
Skrypnyk M, Petrushanko T, Neporada K et al (2022) Colonization resistanCe of oral muCosa in individuals with diverse body mass index. J Stomatol. https://doi.org/10.5114/jos.2022.119168
Skrypnyk M, Petrushanko T, Neporada K et al (2022) Dependence of the dental status of young individuals with different body weights on their eating behavior. Acta Facult Med Naissensis. https://doi.org/10.5937/afmnai39-35901
Wada Y, Ikemoto T, Morine Y et al (2019) The differences in the characteristics of insulin-producing cells using human adipose-tissue derived mesenchymal stem cells from subcutaneous and visceral tissues. Sci Rep 9:13204. https://doi.org/10.1038/s41598-019-49701-0
Yoon H-J, Jung Ho W (2019) Minimum criteria for adipose derived stem cells. Clin Med Rep. https://doi.org/10.15761/CMR.1000145
Hong S, van Kaer L (1999) Immune privilege: keeping an eye on natural killer T cells. J Exp Med 190:1197–1200. https://doi.org/10.1084/jem.190.9.1197
Zangi L, Margalit R, Reich-Zeliger S et al (2009) Direct imaging of immune rejection and memory induction by allogeneic mesenchymal stromal cells. Stem Cells 27:2865–2874. https://doi.org/10.1002/stem.217
Mazini L, Rochette L, Admou B et al (2020) Hopes and limits of adipose-derived stem cells (ADSCs) and mesenchymal stem cells (MSCs) in wound healing. IJMS 21:1306. https://doi.org/10.3390/ijms21041306
Ren M-L, Peng W, Yang Z-L et al (2012) Allogeneic adipose-derived stem cells with low immunogenicity constructing tissue-engineered bone for repairing bone defects in pigs. Cell Transplant 21:2711–2721. https://doi.org/10.3727/096368912X654966
Gentile P, Sterodimas A (2020) Adipose stem cells (ASCs) and stromal vascular fraction (SVF) as a potential therapy in combating (COVID-19)-disease. Aging Dis 11:465. https://doi.org/10.14336/AD.2020.0422
Copcu HE (2020) Potential using of fat-derived stromal cells in the treatment of active disease, and also, in both pre- and post-periods in COVID-19. Aging Dis 11:730. https://doi.org/10.14336/AD.2020.0621
Cypess AM, Weiner LS, Roberts-Toler C et al (2015) Activation of human brown adipose tissue by a β3-adrenergic receptor agonist. Cell Metab 21:33–38. https://doi.org/10.1016/j.cmet.2014.12.009
Liu X, Zheng Z, Zhu X et al (2013) Brown adipose tissue transplantation improves whole-body energy metabolism. Cell Res 23:851–854. https://doi.org/10.1038/cr.2013.64
Khazaei S, Keshavarz G, Bozorgi A et al (2022) Adipose tissue-derived stem cells: a comparative review on isolation, culture, and differentiation methods. Cell Tissue Bank 23:1–16. https://doi.org/10.1007/s10561-021-09905-z
Si Z, Wang X, Sun C et al (2019) Adipose-derived stem cells: sources, potency, and implications for regenerative therapies. Biomed Pharmacother 114:108765. https://doi.org/10.1016/j.biopha.2019.108765
Tsuji W (2014) Adipose-derived stem cells: Implications in tissue regeneration. WJSC 6:312. https://doi.org/10.4252/wjsc.v6.i3.312
Shao X, Ai G, Wang L et al (2019) Adipose-derived stem cells transplantation improves endometrial injury repair. Zygote 27:367–374. https://doi.org/10.1017/S096719941900042X
Mazini L, Ezzoubi M, Malka G (2021) Overview of current adipose-derived stem cell (ADSCs) processing involved in therapeutic advancements: flow chart and regulation updates before and after COVID-19. Stem Cell Res Ther 12:1. https://doi.org/10.1186/s13287-020-02006-w
Raposio E, Simonacci F, Perrotta RE (2017) Adipose-derived stem cells: Comparison between two methods of isolation for clinical applications. Ann Med Surg (Lond) 20:87–91. https://doi.org/10.1016/j.amsu.2017.07.018
Dalerba P, Diehn M, Weissman IL, Clarke MF (2020) Stem Cells, Cell Differentiation, and Cancer. In: Abeloff’s Clinical Oncology. Elsevier, pp 97–107.e5
Xin L, Lin X, Pan Y et al (2019) A collagen scaffold loaded with human umbilical cord-derived mesenchymal stem cells facilitates endometrial regeneration and restores fertility. Acta Biomater 92:160–171. https://doi.org/10.1016/j.actbio.2019.05.012
Wong DE, Banyard DA, Santos PJF et al (2019) Adipose-derived stem cell extracellular vesicles: a systematic review✰. J Plast Reconstr Aesthet Surg 72:1207–1218. https://doi.org/10.1016/j.bjps.2019.03.008
Bazzan E, Tinè M, Casara A et al (2021) Critical review of the evolution of extracellular vesicles’ knowledge: from 1946 to today. Int J Mol Sci 22:6417. https://doi.org/10.3390/ijms22126417
Gokce A, Abd Elmageed ZY, Lasker GF et al (2014) Adipose tissue-derived stem cell therapy for prevention and treatment of erectile dysfunction in a rat model of Peyronie’s disease. Andrology 2:244–251. https://doi.org/10.1111/j.2047-2927.2013.00181.x
Braga Osorio Gomes Salgado JA, Goncalves Reis LR, Jorge Carvalho Sousa N, et al (2010) Adipose Tissue Derived Stem Cells Secretome: Soluble Factors and Their Roles in Regenerative Medicine. CSCR 5:103–110. https://doi.org/10.2174/157488810791268564
Hu X, Pan J, Li Y et al (2022) Extracellular vesicles from adipose-derived stem cells promote microglia M2 polarization and neurological recovery in a mouse model of transient middle cerebral artery occlusion. Stem Cell Res Ther 13:21. https://doi.org/10.1186/s13287-021-02668-0
el Bassit G, Patel RS, Carter G et al (2016) MALAT1 in human adipose stem cells modulates survival and alternative splicing of PKCδII in HT22 cells. Endocrinology. https://doi.org/10.1210/en.2016-1819
Article PubMed Central Google Scholar
Patel NA, Moss LD, Lee J-Y et al (2018) Long noncoding RNA MALAT1 in exosomes drives regenerative function and modulates inflammation-linked networks following traumatic brain injury. J Neuroinflammation 15:204. https://doi.org/10.1186/s12974-018-1240-3
Bonafede R, Scambi I, Peroni D et al (2016) Exosome derived from murine adipose-derived stromal cells: neuroprotective effect on in vitro model of amyotrophic lateral sclerosis. Exp Cell Res 340:150–158. https://doi.org/10.1016/j.yexcr.2015.12.009
Zhao S, Qi W, Zheng J et al (2020) Exosomes derived from adipose mesenchymal stem cells restore functional endometrium in a rat model of intrauterine adhesions. Reprod Sci 27:1266–1275. https://doi.org/10.1007/s43032-019-00112-6
Ma L, Wei J, Zeng Y et al (2022) Mesenchymal stem cell-originated exosomal circDIDO1 suppresses hepatic stellate cell activation by miR-141-3p/PTEN/AKT pathway in human liver fibrosis. Drug Deliv 29:440–453. https://doi.org/10.1080/10717544.2022.2030428
Zhang Z, Shang J, Yang Q et al (2023) Exosomes derived from human adipose mesenchymal stem cells ameliorate hepatic fibrosis by inhibiting PI3K/Akt/mTOR pathway and remodeling choline metabolism. J Nanobiotechnol 21:29. https://doi.org/10.1186/s12951-023-01788-4
Article CAS Google Scholar
Qu Y, Zhang Q, Cai X et al (2017) Exosomes derived from miR-181-5p-modified adipose-derived mesenchymal stem cells prevent liver fibrosis via autophagy activation. J Cell Mol Med 21:2491–2502. https://doi.org/10.1111/jcmm.13170
Chen F, Zhang H, Wang Z et al (2017) Adipose-derived stem cell-derived exosomes ameliorate erectile dysfunction in a rat model of type 2 diabetes. J Sex Med 14:1084–1094. https://doi.org/10.1016/j.jsxm.2017.07.005
Zhu LL, Huang X, Yu W et al (2018) Transplantation of adipose tissue-derived stem cell-derived exosomes ameliorates erectile function in diabetic rats. Andrologia 50:e12871. https://doi.org/10.1111/and.12871
Zhang Y, Zouboulis CC, Xiao Z (2024) Exosomes from adipose-derived stem cells activate sebocytes through the PI3K/AKT/SREBP-1 pathway to accelerate wound healing. Cell Tissue Res. https://doi.org/10.1007/s00441-024-03872-z
Santos J, Dalla P (2021) A molecular analysis of cytokine content across extracellular vesicles, secretions, and intracellular space from different site-specific adipose-derived stem cells. IJMS 23:397. https://doi.org/10.3390/ijms23010397
Alicka M, Major P, Wysocki M, Marycz K (2019) Adipose-derived mesenchymal stem cells isolated from patients with type 2 diabetes show reduced “stemness” through an altered secretome profile, impaired anti-oxidative protection, and mitochondrial dynamics deterioration. JCM 8:765. https://doi.org/10.3390/jcm8060765
Tobita M, Uysal CA, Guo X et al (2013) Periodontal tissue regeneration by combined implantation of adipose tissue-derived stem cells and platelet-rich plasma in a canine model. Cytotherapy 15:1517–1526. https://doi.org/10.1016/j.jcyt.2013.05.007
Tobita M, Uysal AC, Ogawa R et al (2008) Periodontal tissue regeneration with adipose-derived stem cells. Tissue Eng Part A 14:945–953. https://doi.org/10.1089/ten.tea.2007.0048
Wang L, Lu Y, Cai G et al (2022) Polycystin-2 mediates mechanical tension-induced osteogenic differentiation of human adipose-derived stem cells by activating transcriptional co-activator with PDZ-binding motif. Front Physiol 13:917510. https://doi.org/10.3389/fphys.2022.917510
Louis F, Sowa Y, Irie S, Higuchi Y, Kitano S, Mazda O, Matsusaki M (2022) Injectable prevascularized mature adipose tissues (iPAT) to achieve long-term sur-vival in soft tissue regeneration. Adv Healthcare Mater. https://doi.org/10.1002/adhm.202201440
Li W, Yang Y, Lin Y, Mu D (2024) In vitro study of thymosin beta 4 promoting transplanted fat survival by regulating adipose-derived stem cells. Aesthetic Plast Surg. https://doi.org/10.1007/s00266-024-03861-1
Demir M, Yılmaz EM, İpek E et al (2022) Effects of adipose tissue-derived mesenchymal stem cells and/or sildenafil citrate in experimental colon anastomosis model. Ulus Travma Acil Cerrahi Derg 28:1373–1381. https://doi.org/10.14744/tjtes.2021.57500
Kim JH, Kim TY, Goo B, Park Y (2024) Bee venom stimulates growth factor release from adipose-derived stem cells to promote hair growth. Toxins (Basel) 16:84. https://doi.org/10.3390/toxins16020084
Tynecka M, Janucik A, Niemira M et al (2022) The short-term and long-term effects of intranasal mesenchymal stem cell administration to noninflamed mice lung. Front Immunol 13:967487. https://doi.org/10.3389/fimmu.2022.967487
Kangari P, Roshangar L, Iraji A et al (2022) Accelerating effect of Shilajit on osteogenic property of adipose-derived mesenchymal stem cells (ASCs). J Orthop Surg Res 17:424. https://doi.org/10.1186/s13018-022-03305-z
Felthaus O, Vedlin S, Eigenberger A et al (2024) Exosomes from adipose-tissue-derived stem cells induce proapoptotic gene expression in breast tumor cell line. Int J Mol Sci 25:2190. https://doi.org/10.3390/ijms25042190
Lappin T, Cheng T (2021) An urgent need for standardization of stem cells and stem cell-derived products toward clinical applications. Stem Cells Transl Med 10:S1–S3. https://doi.org/10.1002/sctm.21-0269
Tragoonlugkana P, Chitchongyingcharoen N, Pruksapong C et al (2024) The use of human platelet lysate as a coating substance for adipose-derived stem cell expansion. Front Biosci Landmark 29:88. https://doi.org/10.31083/j.fbl2902088
Greenwood V, Clausen P, Matuska AM (2022) Micro-fragmented adipose tissue cellular composition varies by processing device and analytical method. Sci Rep 12:16107. https://doi.org/10.1038/s41598-022-20581-1
Taha S, Akova E, Saller MM et al (2022) Early transcriptional changes of adipose-derived stem cells (ADSCs) in cell culture. Medicina (B Aires) 58:1249. https://doi.org/10.3390/medicina58091249
Schipper BM, Marra KG, Zhang W et al (2008) Regional anatomic and age effects on cell function of human adipose-derived stem cells. Ann Plast Surg 60:538–544. https://doi.org/10.1097/SAP.0b013e3181723bbe
Aksu AE, Rubin JP, Dudas JR, Marra KG (2008) Role of gender and anatomical region on induction of osteogenic differentiation of human adipose-derived stem cells. Ann Plast Surg 60:306–322. https://doi.org/10.1097/SAP.0b013e3180621ff0
van Harmelen V, Skurk T, Röhrig K et al (2003) Effect of BMI and age on adipose tissue cellularity and differentiation capacity in women. Int J Obes Relat Metab Disord 27:889–895. https://doi.org/10.1038/sj.ijo.0802314
Ryu Y, Ha H, Lee C et al (2016) ADSC from younger donors have more abundant initial ADSC yield. Cytotherapy 18:S79. https://doi.org/10.1016/j.jcyt.2016.03.164
Abbo O, Taurand M, Monsarrat P et al (2017) Comparison between pediatric and adult adipose mesenchymal stromal cells. Cytotherapy 19:395–407. https://doi.org/10.1016/j.jcyt.2016.11.012
Ock S-A, Lee Y-M, Park J-S et al (2016) Evaluation of phenotypic, functional and molecular characteristics of porcine mesenchymal stromal/stem cells depending on donor age, gender and tissue source. J Vet Med Sci 78:987–995. https://doi.org/10.1292/jvms.15-0596
Altamirano-Samaniego F, Enciso-Benavides J, Rojas N et al (2022) First report of canine morbillivirus infection of adipose tissue-derived stem cells from dogs with distemper. Vet World. https://doi.org/10.14202/vetworld.2022.1835-1842
Firriolo JM, Condé-Green A, Pu LLQ (2022) Fat grafting as regenerative surgery: a current review. Plast Reconstr Surg 150:1340e–1347e. https://doi.org/10.1097/PRS.0000000000009710
Shetty AK (2020) Mesenchymal stem cell infusion shows promise for combating coronavirus (COVID-19)- induced pneumonia. Aging Dis 11:462–464. https://doi.org/10.14336/AD.2020.0301
Alcayaga-Miranda F, Cuenca J, Khoury M (2017) Antimicrobial activity of mesenchymal stem cells: current status and new perspectives of antimicrobial peptide-based therapies. Front Immunol 8:339. https://doi.org/10.3389/fimmu.2017.00339
Al-Ghadban S, Bunnell BA (2020) Adipose tissue-derived stem cells: immunomodulatory effects and therapeutic potential. Physiology 35:125–133. https://doi.org/10.1152/physiol.00021.2019
Zhou L, Wang H, Yao S et al (2022) Efficacy of human adipose derived mesenchymal stem cells in promoting skin wound healing. J Healthc Eng 2022:6590025. https://doi.org/10.1155/2022/6590025
Randelli PS, Cucchi D, Fossati C et al (2022) Arthroscopic rotator cuff repair augmentation with autologous microfragmented lipoaspirate tissue is safe and effectively improves short-term clinical and functional results: a prospective randomized controlled trial with 24-month follow-up. Am J Sports Med 50:1344–1357. https://doi.org/10.1177/03635465221083324
el Zarif M et al (2020) Corneal stroma cell density evolution in keratoconus corneas following the implantation of adipose mesenchymal stem cells and corneal laminas: an in vivo confocal microscopy study. Investigative Opthalmology & Visual Science 61:22. https://doi.org/10.1167/iovs.61.4.22
Zheng C, Chiu I, Chen Y et al (2022) Allogeneic adipose tissue-derived stem cells ELIXCYTE ® in chronic kidney disease: a phase I study assessing safety and clinical feasibility. J Cell Mol Med 26:2972–2980. https://doi.org/10.1111/jcmm.17310
Ferracini R, Alessio-Mazzola M, Sonzogni B et al (2022) Age and synovitis affect the results of the treatment of knee osteoarthritis with microfragmented autologous fat tissue. Knee Surg Sports Traumatol Arthrosc. https://doi.org/10.1007/s00167-022-07139-4
Behrangi E, Moradi S, Ghassemi M et al (2022) The investigation of the efficacy and safety of stromal vascular fraction in the treatment of nanofat-treated acne scar: a randomized blinded controlled clinical trial. Stem Cell Res Ther 13:298. https://doi.org/10.1186/s13287-022-02957-2
Lynggaard CD, Grønhøj C, Jensen SB et al (2022) Long-term safety of treatment with autologous mesenchymal stem cells in patients with radiation-induced xerostomia: primary results of the MESRIX phase I/II randomized trial. Clin Cancer Res 28:2890–2897. https://doi.org/10.1158/1078-0432.CCR-21-4520
Ceccarelli S, Pontecorvi P, Anastasiadou E et al (2020) Immunomodulatory effect of adipose-derived stem cells: the cutting edge of clinical application. Front Cell Dev Biol. https://doi.org/10.3389/fcell.2020.00236
Download references
Acknowledgements
The author thank Dr. Darren Smith for kindly providing editorial assistance while preparing the manuscript.
Author information
Authors and affiliations.
Department: Centre for Health Informatics, Australian Institute of Health Innovation, Faculty of Medicine, Health and Human Sciences, Macquarie University, Sydney, Australia
Maksym Skrypnyk
You can also search for this author in PubMed Google Scholar
Contributions
Corresponding author.
Correspondence to Maksym Skrypnyk .
Ethics declarations
Conflict of interest.
The corresponding author states that there is no conflict of interest.
Ethics approval and consent to participate
Consent for publication, additional information, publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Skrypnyk, M. Current progress and limitations of research regarding the therapeutic use of adipose-derived stem cells: literature review. J.Umm Al-Qura Univ. Appll. Sci. (2024). https://doi.org/10.1007/s43994-024-00147-9
Download citation
Received : 06 December 2023
Accepted : 26 March 2024
Published : 17 April 2024
DOI : https://doi.org/10.1007/s43994-024-00147-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Cell therapy
- Mesenchymal stem cells
- Adipose tissue
- Find a journal
- Publish with us
- Track your research
- Open access
- Published: 19 April 2024
Applications of peptides in nanosystems for diagnosing and managing bacterial sepsis
- Mohammed A. Gafar 1 , 2 ,
- Calvin A. Omolo 1 , 3 ,
- Eman Elhassan 1 ,
- Usri H. Ibrahim 4 &
- Thirumala Govender 1
Journal of Biomedical Science volume 31 , Article number: 40 ( 2024 ) Cite this article
80 Accesses
Metrics details
Sepsis represents a critical medical condition stemming from an imbalanced host immune response to infections, which is linked to a significant burden of disease. Despite substantial efforts in laboratory and clinical research, sepsis remains a prominent contributor to mortality worldwide. Nanotechnology presents innovative opportunities for the advancement of sepsis diagnosis and treatment. Due to their unique properties, including diversity, ease of synthesis, biocompatibility, high specificity, and excellent pharmacological efficacy, peptides hold great potential as part of nanotechnology approaches against sepsis. Herein, we present a comprehensive and up-to-date review of the applications of peptides in nanosystems for combating sepsis, with the potential to expedite diagnosis and enhance management outcomes. Firstly, sepsis pathophysiology, antisepsis drug targets, current modalities in management and diagnosis with their limitations, and the potential of peptides to advance the diagnosis and management of sepsis have been adequately addressed. The applications have been organized into diagnostic or managing applications, with the last one being further sub-organized into nano-delivered bioactive peptides with antimicrobial or anti-inflammatory activity, peptides as targeting moieties on the surface of nanosystems against sepsis, and peptides as nanocarriers for antisepsis agents. The studies have been grouped thematically and discussed, emphasizing the constructed nanosystem, physicochemical properties, and peptide-imparted enhancement in diagnostic and therapeutic efficacy. The strengths, limitations, and research gaps in each section have been elaborated. Finally, current challenges and potential future paths to enhance the use of peptides in nanosystems for combating sepsis have been deliberately spotlighted. This review reaffirms peptides' potential as promising biomaterials within nanotechnology strategies aimed at improving sepsis diagnosis and management.
Graphical Abstract
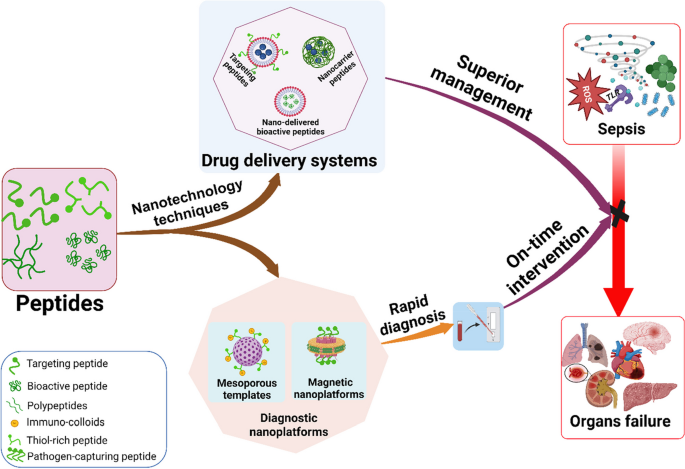
• Due to their unique characteristics, Peptides hold significant promise as part of nanotechnology approaches for diagnosing and treating sepsis, a current leading global killer.
• Various diagnostic nanotools utilizing peptides as pathogen recognition moieties can improve the pathogen capturing efficiency for sepsis diagnosis.
• Nano-delivery can overcome the limitations of bioactive peptides and enhance their antibacterial and anti-inflammatory efficacy in sepsis management.
• Peptides offer significant capabilities as targeting moieties and nanocarriers to augment the effectiveness of antisepsis agents.
• Future research identified can potentiate the applications of peptides for the diagnosis and treatment of sepsis.
Introduction
Bacterial infections are still a major public health concern [ 1 ]. As per WHO reports, infections due to antimicrobial-resistant organisms resulted in 1.27 million deaths in the year 2019 and contributed to 4.95 deaths globally [ 2 ]. It is estimated that 700,000 people die each year worldwide, which is expected to rise to 10 million by 2050 [ 3 ]. The problem of mortality and morbidity of bacterial infections is made worse due to associated complications such as sepsis [ 4 ]. An estimated 48.9 million incident instances of sepsis were reported globally in 2017, leading to 11 million fatalities, accounting for 19.7% of all deaths worldwide [ 5 ]. With the emergence of the COVID-19 pandemic, antimicrobial resistance continues to gain ground and exacerbates bacterial sepsis, which is now the leading cause of death from infections [ 6 ]. If not detected and treated timely, sepsis can progress to septic shock, multiple organ failure, and death due to cardiovascular, coagulation, and endothelial dysfunction [ 7 ]. Accordingly, sepsis is a critical worldwide health problem with life-threatening implications, necessitating immediate attention to developing novel and powerful diagnostic and therapeutic strategies.
Nanoplatforms are providing new avenues for critical illnesses diagnosis and treatment [ 8 ]. These platforms offer cutting-edge approaches for disease diagnosis, enhancing sensitivity and decreasing processing time Without the necessity for specialized expertise [ 9 ]. Moreover, nanoplatforms can be fine-tuned to overcome conventional dosage forms' limitations by enhancing loaded drug pharmacokinetic and pharmacodynamic characteristics through disease site targeting, stimuli responsiveness, and mimicking disease pathophysiology [ 10 , 11 ]. These positive attributes enable the use of lower drug concentrations, co-loading of different drugs in the nanosystems, having multi-responsive systems that respond to different disease environments, hence reducing systemic toxicity and improving therapeutic effectiveness [ 12 ]. These distinguishing characteristics of nanoscale drug formulations make them promising candidates for enhancing the efficiency of existing conventional antibiotics against multidrug-resistant bacteria [ 13 ]. Compared to conventional preparations, nano-antimicrobial formulations have demonstrated superior outcomes in managing sepsis [ 14 , 15 ]. Therefore, nanotechnology-based systems provide efficient tools to decrease the burden of bacterial infections and sepsis.
The advancements and improvement of nanosystems' functionality require synthesizing bio-functional materials that can be employed in formulating them. Peptides are emerging as useful biomaterials for the formulation of nanosystems [ 16 ]. Peptides are a class of biological molecules composed of short chains of around 50 amino acids or less joined together by amide bonds [ 17 , 18 ]. Due to the infinite possibilities of joining amino acids, peptides can serve as pathogens and biomarkers capturing motifs, bioactive agents, or as excipients in making diagnostic biosensors and drug delivery systems. As components in nanotools for sepsis diagnosis, peptides can be designed to have specific binding with high affinity to causative pathogens and sepsis-released inflammatory biomarkers, thus making diagnostic procedures more efficient and prompter [ 19 ]. Furthermore, peptides can be incorporated within these nanoplatforms to improve the stability and binding of sepsis biomarkers-capturing immune-colloids to mesoporous nano-templates for sepsis immunoassays [ 20 ]. Therefore, peptide-based nanoplatforms hold promising potential in advancing sepsis diagnosis, allowing for efficient and rapid interventions that will improve patient outcomes.
In sepsis management, bioactive peptides have been found to exhibit therapeutic and protective properties against sepsis and so provide effective new treatment options for patients suffering from this deadly condition [ 21 , 22 ]. Among different classes of bioactive peptides, antimicrobial peptides (AMPs) are naturally existing peptides that have the ability to fight microbial infections and their related complications, such as sepsis [ 23 ]. Due to their novel antimicrobial modes of action, robust antimicrobial efficacy, minimal drug residual, and simplicity of production and modification, AMPs have held significant potential as a promising alternative to antibiotic therapy over decades [ 24 ]. More importantly, it is shown that antimicrobial resistance levels developed by AMPs are substantially lower as they target a variety of mechanisms that are not targeted by traditional antibiotics [ 25 ]. Additionally, anti-inflammatory peptides (AIPs) demonstrated beneficial effects in bacterial infections and sepsis management. The use of these peptides has been shown to help reduce inflammation by targeting various sites in the sepsis inflammatory cascade, thus reducing the amount of tissue and organ damage associated with sepsis and making the treatment more effective [ 26 , 27 , 28 , 29 , 30 ]. Overall, the unique properties and potential for applying AMPs and AIPs in bacterial infections and sepsis make them a promising area of research for developing new treatments. Nevertheless, bioactive peptides have certain drawbacks regarding bioavailability and tolerability (Teixeira et al. 2020), and research focuses on improving their potency and safety profile.
One of the ways the therapeutic profile of bioactive peptides is being improved is through encapsulation in nano-delivery systems [ 31 ]. Moreover, due to their diversity and ability to produce secondary nanostructures, bioactive peptides may be modified to form nanomaterials with enhanced characteristics [ 32 , 33 ]. Apart from their biological activity and due to their superior physical, chemical, and biological characteristics, peptides have emerged as a potential constituent for the development of nanosystems for targeted delivery of drugs and genes [ 34 , 35 , 36 ]. Peptides can be employed in drug delivery technologies as nanocarriers, cell penetration enhancers, and targeting agents [ 17 , 36 ]. Consequently, peptide-based nano-delivery systems have been developed and applied to treat a wide range of illnesses, such as sepsis, cancer, viral infections, and immune system disorders [ 37 , 38 , 39 , 40 , 41 , 42 ].
Numerous review articles have highlighted the application of peptides in nanotechnology-based bacterial infection management [ 43 , 44 ] and the use of nanotechnology for AMPs delivery against general bacterial infections [ 45 , 46 , 47 , 48 , 49 ]. Additionally, several publications have reviewed the use of nanotechnology to manage sepsis [ 14 , 19 , 50 , 51 ]. To the best of our knowledge, no review has discussed the various applications of peptides in nanosystems for diagnosing and managing bacterial sepsis.
Therefore, this review focuses on the numerous applications of peptides in nanosystems to identify and control bacterial sepsis. Initially, a theoretical background about the pathophysiology of sepsis, challenges associated with sepsis diagnosis and management, drug targets in sepsis, and peptides' physicochemical properties and their potential for application in nanotools against sepsis are presented. In addition, following a thorough search of many scientific databases, we discuss and critically analyze different applications of peptides in nanotechnology for sepsis diagnosis and management. The studies have been organized into two main sections, viz. diagnostic and management peptides-based nanosystems. We have further systematically categorized the nanosystems for sepsis management according to the role of peptides into: (i) nano-delivered bioactive peptides; (ii) peptides as targeting moieties on the surface of nanosystems; (iii) peptides as nanocarriers for antisepsis drug. Finally, this review highlights the challenges, gaps, and future perspectives to maximize the potential of applying peptides in nanotechnology tools to improve sepsis diagnosis and management.
A clear understanding of the pathophysiology of sepsis and potential drug targets is critical for developing new effective sepsis diagnostic and therapeutic tools. This section will discuss the pathophysiological background of sepsis, including the inflammatory pathways triggered by invading microorganisms and the consequences of that on the structure and function of body organs. The current trends in sepsis diagnosis and management and potential drug targets for sepsis management will also be discussed, accompanied by their challenges. Finally, the physicochemical and biological properties that make peptides a potential component of nanotools for sepsis diagnosis and management are covered.
Pathophysiology of sepsis
Sepsis is a medical emergency and a life-threatening condition associated with a global disease burden [ 52 ]. Despite all experimental and clinical research efforts, sepsis remains one of the leading causes of morbidity and mortality in critically ill patients [ 53 ]. In the Third International Consensus (Sepsis-3), sepsis is defined as "organ dysfunction caused by a dysregulated host response to infection", highlighting for the first time the critical role of immune responses in the establishment of the illness [ 7 ]. After the invasion of microorganisms into the body, an immune response is triggered to fight off the invading microorganisms. This causes inflammation, a normal and necessary response to promptly identify, eradicate, and keep the infection localized [ 54 ]. However, as shown in Fig. 1 , the immune response is exaggerated during sepsis, resulting in collateral damage and death of host cells and tissues, compromising both the affected and distant organs and leading to functional abnormalities and life-threatening multiorgan failure [ 55 ]. The pathophysiology of sepsis is generally defined as an early hyperinflammatory state that lasts many days, followed by a longer immunosuppressive state [ 56 ]. These two stages are connected with higher mortality, with the highest death rate in the early phase attributable to an enormous inflammatory response (cytokine storm) [ 14 ].
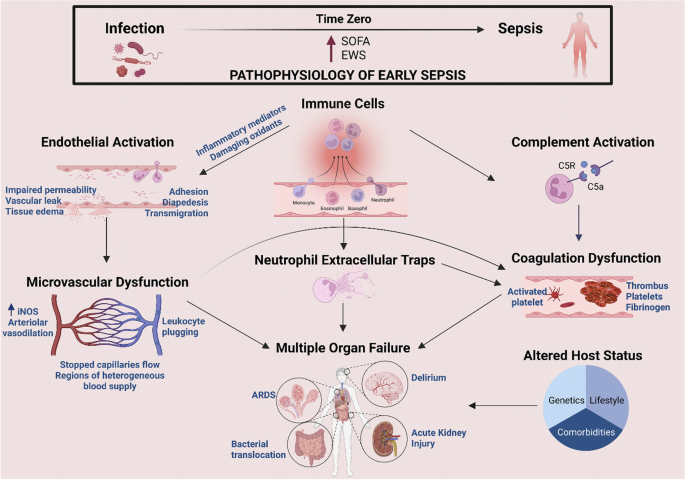
Immune responses in sepsis owing to infection. Illustration of converting to sepsis from infection. Immune cells activation results in the overproduction of inflammatory mediators that induce detrimental changes in cells and tissues, leading to multiorgan dysfunction and failure (SOFA: sequential organ failure assessment; EWS: early warning score; iNOS: inducible nitric oxide synthase; ARDS: acute respiratory distress syndrome) (Adopted with permission from [ 55 ]
The over-released inflammatory mediators during the cytokine storms lead to significant damage to the endothelium and disruption of it is barrier function, vasodilation, activation of coagulation pathways, platelet aggregation and adhesion, and mitochondrial dysfunction [ 57 , 58 ]. Overall, the dysregulated inflammatory-immune responses and their consequences mentioned above eventually lead to the formation of microvascular thrombi, hypotension, impaired cellular functions, local perfusion defects, tissue hypoxia, and progressive tissue damage, which finally cause refractory shock and multiorgan failure [ 59 , 60 , 61 , 62 ]. Cardiovascular Dysfunction, acute lung injury and acute respiratory distress syndrome, acute kidney injury, hepatic dysfunction, and CNS dysfunction and encephalopathy are well-known complications of sepsis, and their underlying mechanisms are reported in the literature [ 63 , 64 , 65 , 66 , 67 , 68 ]. These alterations and dysfunctions in the tissues and organs collectively contribute to much of the morbidity and mortality of sepsis [ 69 ].
Although the advancements in therapeutic approaches have enhanced the survival rate in the early phase of the exaggerated inflammatory response, current patterns in sepsis indicate that mortality arises during the subsequent stage of a compensatory immunosuppressive response when there is a shift toward an overall anti-inflammatory milieu [ 56 , 70 ]. This post-sepsis immune paralysis involves various quantitative and functional defects of immune cells as a result of uncontrolled apoptosis of lymphocytes and decreased immunoglobulin production, which is linked to an increased susceptibility to secondary infections and organ injury and/or failure [ 14 , 60 , 71 , 72 ]. Immunosuppression can last months after the septic event and is associated with increased mortality [ 73 , 74 ]. The detailed sepsis pathophysiology and different involved pathways have been widely discussed in the literature, and readers are referred to them for more details [ 55 , 67 , 69 , 73 , 75 , 76 , 77 ].
Sepsis diagnosis and management
Sepsis is considered a medical emergency that, if not diagnosed in its early stages, will result in a poor prognosis with increased morbidity and mortality [ 78 ]. The current diagnosis of sepsis relies on clinical evaluation, blood or urine cultures, and detection of inflammatory response biomarkers such as C-reactive protein (CRP), procalcitonin (PCT), and interleukin 6 (IL-6) [ 19 ]. However, the currently used biomarkers are not specific, and none have proven to be a specific sepsis indicator [ 79 ]. Moreover, the microbial cultures take a long time, and results may come out after 72 hours, making rapid sepsis diagnosis difficult [ 80 ]. Starting sepsis management as early as possible is critical to avoid complications and multiorgan failure [ 81 ]. As the current diagnostic tools for sepsis have such a delay, the empirical administration of intravenous broad-spectrum antibiotics is a usual initial intervention together with other additional therapies (e.g., anti-inflammatory (corticosteroids) and venous thromboembolism prophylactics) and measures for ventilation and hemodynamic stabilization (e.g., oxygen, albumin, and vasopressors administration and fluid resuscitation) [ 82 ]. However, the empirical use of broad-spectrum antibiotics with the uncertainty of diagnosis results and difficulties in differentiating infectious sepsis from noninfectious inflammations [ 83 ] will result in unwanted side effects for the already stressed patient and increased risk of antimicrobial resistance development [ 81 , 84 ]. Putting all these challenges together raises the urgent need for new and specific sepsis diagnostics and management approaches.
Drug targets in sepsis
As mentioned above, treating sepsis involves a combination of antibiotics to fight the underlying infection and supportive care to address the systemic inflammation and organ dysfunction that can occur because of the condition [ 85 ]. One of the key challenges in treating sepsis is identifying effective drug targets that can help reducing inflammation and tissue damage [ 86 ]. Besides targeting the invading microorganisms with antibiotics, several drug targets have been identified and studied in sepsis management [ 87 , 88 ]. One of the main drug targets in sepsis is the inhibition of inflammatory mediators that play a critical role in developing sepsis, including cytokines, chemokines, and other inflammatory signaling molecules [ 89 ]. Drugs that target these inflammatory mediators have been developed and tested as potential treatments for sepsis [ 90 ]. For example, monoclonal antibodies that neutralize TNF-α, such as infliximab and etanercept, have been shown to improve outcomes in patients with sepsis [ 91 ]. Similarly, drugs that inhibit the activity of IL-1 (e.g., IL-1 receptor antagonist), IL-6, and IL-8 have also been shown to improve outcomes in patients with sepsis [ 92 ].
The coagulation cascade is another critical therapeutic target in sepsis [ 93 ]. As sepsis is associated with a hypercoagulable state, targeting the coagulation process with drugs such as anticoagulants or clotting factor inhibitors can prevent micro-clots formation and improve outcomes in sepsis [ 94 ]. Drugs that target the coagulation cascade, such as activated protein C (APC) and thrombin inhibitors, have been studied as sepsis therapies and demonstrated to enhance sepsis outcomes [ 88 , 95 ]. Furthermore, the endothelial cells that line blood vessels play a crucial role in the body's reaction to inflammation and infection [ 96 ]. As a result, targeting the endothelium to manage sepsis is an active area of research [ 96 ]. In sepsis, the dysfunction of these cells can lead to increased permeability of blood vessels and decreased blood flow and so leakage of fluid and plasma protein into the tissues, resulting in hypotension and organ dysfunction [ 97 ]. One of the key pathways activated in the endothelium during sepsis is the nitric oxide (NO) pathway, leading to excessive vasodilation and decreased blood pressure [ 98 ]. Drugs that target the NO pathway, such as nitric oxide synthase inhibitors, have been investigated as potential treatments for sepsis [ 99 ]. Another promising strategy is the use of drugs that can improve endothelial function and reduce inflammation [ 100 ]. For example, endothelial protective agents, such as statins, have been shown to reduce inflammation and improve blood flow in sepsis [ 101 ]. In addition, new approaches, such as using extracellular vesicles as drug carriers for targeting the endothelium, are also being explored [ 102 ].
The complement system, a part of the immune system that helps identify and eliminate foreign invaders such as bacteria and viruses, is also one of the potential drug targets for sepsis management [ 103 ]. In sepsis, the complement system is overactivated, which can lead to inflammation and tissue damage [ 104 ]. As a result, targeting the complement system has been investigated for managing sepsis [ 105 ]. One approach to target the complement system in sepsis management is using complement inhibitors, which are drugs that block the activation of the complement system [ 106 ]. For example, eculizumab, a monoclonal antibody that targets the complement protein C5, has been shown to reduce the incidence of death and organ failure in patients with sepsis caused by meningococcal infections [ 107 ].
Bacterial toxins such as lipopolysaccharides (LPS) from Gram-negative bacteria, exotoxins from Gram-positive bacteria, and superantigens from both Gram-positive and Gram-negative bacteria are also potential targets in the management of sepsis [ 108 ]. Bacterial toxins can contribute to the development of sepsis by triggering the release of inflammatory cytokines and damaging vital organs and tissue, leading to septic shock and death [ 109 ]. Inhibiting the production or activity of these toxins can prevent toxicity and improve outcomes in sepsis management [ 108 ]. Various strategies are being studied, such as blocking toxins' binding to host cells, inhibiting their production, or neutralizing them using toxin-binding proteins or immunoglobulins [ 90 , 110 ]. Even though developing therapies that target bacterial toxins is a promising area of research for treating sepsis, more research needs to be done to understand how these drugs work fully and if they are safe to use in clinical settings.
Overall, managing sepsis remains a complex and challenging task, and there is currently no single drug that can effectively address all the different pathways and processes involved in the condition. Further research is needed to identify additional drug targets and to develop more specific and effective medicines for sepsis.
Potential of peptides for use in sepsis
Several drugs have failed in the treatment of sepsis. However, continued research and improved understanding of sepsis pathophysiology, including the complex interactions between inflammatory, coagulation, and fibrinolytic systems, has accelerated the development of novel treatments [ 88 , 111 ]. Some of these drugs being researched and developed are peptides that hold great promise as therapeutic agents for treating sepsis [ 112 , 113 , 114 ]. As amino acids can be used in infinite arrangements in peptide synthesis, then peptides can be designed to have a wide range of unique physicochemical and biological properties. These unique properties include: 1) High specificity: Peptides may be designed to specifically target specific pharmacological targets, making their action highly selective with reduced off-target effects [ 115 ]; 2) Ability to target multiple pathways: Peptides can target multiple pathways in the sepsis cascade, which can help reduce the chances of antimicrobial resistance [ 19 ]; 3) Biodegradability: Inside the body, peptides are broken down into smaller biocompatible components, making them biodegradable with fewer side effects than traditional small molecule drugs [ 116 ]; 4) Ease of synthesis: Peptides can be synthesized using modern techniques in a laboratory setting, making it possible to produce cost-effectively large quantities of specific peptides for drug development [ 116 ]; 5) Permeability and bioavailability: some peptides can cross cell membranes, which increases their bioavailability and allows them to target intracellular targets in sepsis pathophysiology [ 117 ].
The properties mentioned above make peptide synthesis a promising approach for developing new diagnostic and management tools that can efficiently diagnose sepsis and effectively target both the bacteria causing sepsis and the pathophysiological pathways involved in the disease. For example, peptides have been designed to have strong and specific binding affinity to certain pathogens and inflammatory biomarkers, making them excellent capturing motifs for the diagnosis of sepsis [ 118 ]. Moreover, antimicrobial peptides have been shown to have potent bactericidal activity against Gram-negative and Gram-positive bacteria that are commonly associated with sepsis [ 119 ]. In addition to their antibacterial properties, peptides can target the pathophysiological pathways involved in sepsis, such as inflammation, oxidative stress, complement system, and coagulation [ 114 , 120 ]. Furthermore, the excellent physicochemical and biological properties of peptides make them hold great potential as drug delivery systems construction materials for antisepsis drug delivery [ 121 ]. For example, peptides for drug delivery can be designed to respond to the characteristic microenvironment of sepsis, including acidity and high concentrations of reactive oxygen species (ROS) [ 121 ]. This allows for site-specific drug release, so good therapeutic outcomes with low side effects can be achieved with small doses of drugs that improve patient compliance and decrease the treatment cost. As a result, the application of peptides in sepsis diagnosis and management is an active area of research with promising outcomes that make them an attractive option in the battle against sepsis.
Application of peptides in nanotools for sepsis diagnosis and management
Sepsis continues to pose a significant global health challenge despite remarkable advancements in medical technology. Its high mortality rates and limited effective diagnosis and treatment approaches underscore the urgency to develop new, efficient, and novel techniques for accurate diagnosis and timely intervention [ 79 , 122 ]. The application of nanotechnology-based tools presents a multitude of opportunities for advancing sepsis diagnosis and management [ 14 ]. With their distinctive properties, peptides have emerged as promising candidates for developing nanotools to combat critical illnesses like sepsis [ 112 , 114 ]. Figure 2 provides a visual representation of the multiple roles peptides play as components within nanosystems for sepsis diagnosis and management. This section aims to explore and critically review the diverse range of research studies that have employed peptides as integral elements of nanotools for sepsis diagnosis and management, encompassing both pharmacological and pharmaceutical applications.
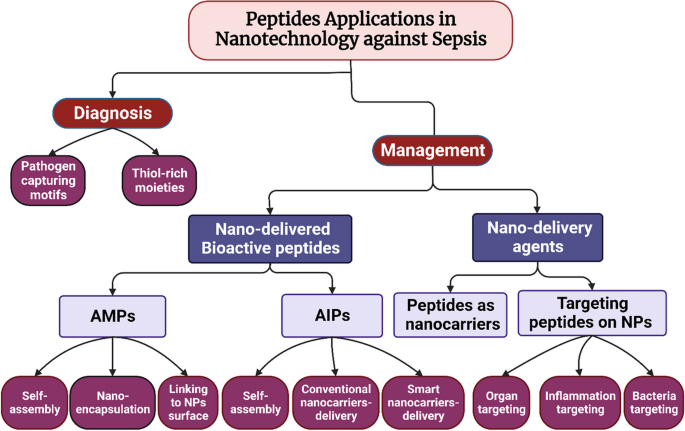
A schematic illustration of various applications of peptides in nanotools for sepsis diagnosis and management (NPs: Nanoparticles; AIPs: Anti-inflammatory Peptides; AMPs: Antimicrobial Peptides) (Created with BioRender.com)
Peptides in nanotechnology for sepsis diagnosis
The timely diagnosis of sepsis is crucial to ensure effective treatment outcomes [ 78 ]. Although they can offer a promising avenue for early detection of sepsis, the use of peptides in developing nanotechnology tools for sepsis diagnosis is still in its infancy. Table 1 summarizes various studies done on the utilization of peptides in nanotools for sepsis diagnosis, highlighting the type of nanosystem, the peptide sequence, the role of peptide in the nanosystem, the targeted microorganisms or biomarkers, the mechanism of detection, the mode of investigation (in vitro and/or in vivo), and the key findings. As shown in the table, peptides have been mainly used for two different roles. The major role was using peptides as pathogen recognition moieties conjugated to the surface of magnetic or fluorescent nanoparticles to allow the capturing of bacteria and then separation or imaging. The other role has been the utilization of peptide as a thiol-rich moiety to enhance the binding of immuno-colloidal metallic nanoparticles to the surface of a mesoporous Surface-enhanced Raman scattering (SERS) template. It is clear that there is great potential for further research to uncover additional roles that peptides can play in nanotools for sepsis diagnosis. Moreover, most studies have focused on Gram-positive bacteria, indicating the need to address Gram-negative bacteria, which significantly influence the development of bacterial sepsis. The studies will be discussed according to the role of peptides in the nanosystem in the following subsections.
Peptides as pathogen capturing motifs on nanoplatforms
To date, various approaches for detecting, capturing, and separating bacteria from blood have been developed to improve the diagnosis and management of bloodstream infections [ 126 , 127 ]. Various pathogen recognition molecules, such as aptamers, antibodies, oligonucleotides, and carbohydrates, have been used to modify nanoplatforms for pathogens detection and separation [ 19 , 128 , 129 ]. Since the bacterial surface displays unique molecular compositions, a well-designed peptide can have a specific and robust interaction with an epitope or a receptor on the bacterial walls. When engineered on the surface of nanoparticles, these peptides will yield an efficient nanotool for pathogen capturing with improved efficacy in sepsis diagnosis [ 130 ]. The subsequent paragraphs will discuss the findings of various studies that have investigated the utilization of peptides as capturing motifs exhibited on magnetic nanoparticles or fluorescent quantum dots to fabricate nanoplatforms for pathogen detection and then isolation or imaging.
Magnetic nanoparticles are promising nanoplatforms that can be effectively engineered with peptides as pathogen-capturing motifs for detecting and separating bacteria from human samples in simple and controllable processes [ 79 , 131 , 132 ]. Among different magnetic nanoparticles, magnetic beads (MBs), known for their superparamagnetic characteristics, have demonstrated their versatility in detecting, purifying, and analyzing analytes from intricate matrices. In order to achieve selectivity, MBs can be complexed with ligands such as peptides, aptamers, and antibodies to target the concerned pathogen selectively [ 133 ]. To this end, Feng et al., as illustrated in Fig. 3 , have developed a vancomycin (Van)-modified magnetic nanoplatform composed of a dendrimer (G4 PAMAM) anchored with biotin and complexed with streptavidin-modified magnetic beads (MBs-S) to detect and isolate L. monocytogenes and S. aureus from human blood samples [ 123 ]. The glycopeptide antibiotic vancomycin (Van) can interact with the surface of the bacterial wall via hydrogen bonding and works as a pathogen recognition molecule [ 134 ].
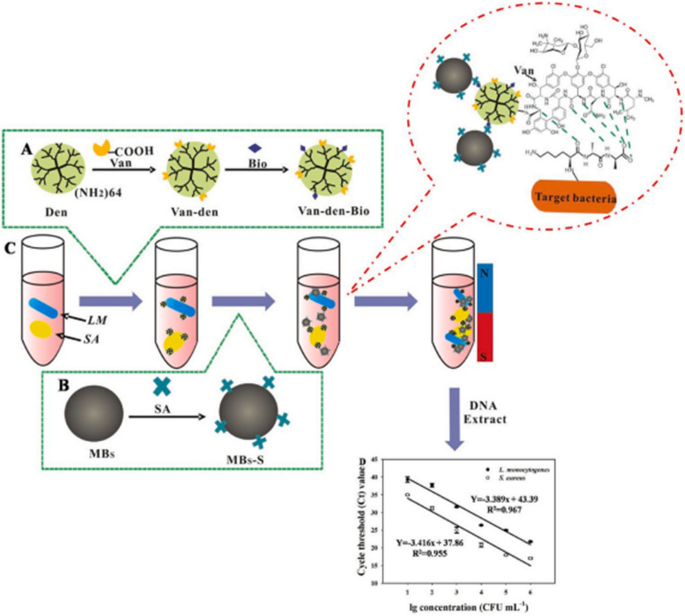
Detection of S. aureus and L. monocytogenes using a two-step approach paired with m-qPCR, utilizing the MBs-S~Bio-den-Van~bacteria complex [ 123 ]
They combined the MBs-S~Bio-den-Van platform with a multiplex quantitative PCR (m-qPCR) to enrich and identify the isolated pathogens. The platform demonstrated rapid bacteria isolation within 2 minutes and exhibited a capturing efficiency of 93.14% for L. monocytogenes and 94.58% for S. aureus from spiked healthy donors’ whole blood. The platform showed high sensitivity with limits of detection of 32 and 41 CFUmL -1 for L. monocytogenes and S. aureus , respectively. Their findings exhibited several potential advantages for bacterial detection in septic patients, including processing simplicity, low cost, high stability and specificity, and low detection limit. However, it should be noted that Van has the ability to bind to different types of Gram-positive bacteria, but it cannot bind to Gram-negative bacteria because of the differences in the components of their outer cell wall. Therefore, we believe this nanoplatform efficiency would be limited in cases of Gram-negative or mixed infections due to the specificity of Van to Gram-positives bacteria.
Another type of magnetic nanoparticles that can be modified with peptides to capture and separate bacteria from human samples are superparamagnetic iron oxide nanoparticles (SPIONs). SPOINs' non-toxicity, controllable size, large surface area-to-volume ratio, and ability to be functionalized with targeting moieties make them promising tools for early detection and diagnosis of diseases [ 135 , 136 ]. For instance, Friedrich and coworkers developed a 3-aminopropyl triethoxysilane (APTES)-coated superparamagnetic iron oxide nanoparticles modified with bacterial cell wall-binding peptides for bloodstream bacterial pathogens separation [ 118 ]. The two peptide sequences (Pep1 (RKQGRVEVLYRASWGTV) and Pep2 (RKQGRVEILYRGSWGTVC)) were obtained from the salivary glycoprotein GP-340, which is known to interact with bacterial cell wall components [ 137 ]. A modified one-step coprecipitation approach was used to produce the SPION-APTES with a hydrodynamic diameter of 166 nm, which formed nanoparticle agglomerates of 1679 nm diameter after peptide functionalization.
The nanoparticles were found to be cyto- and hemo-compatible. As shown in Fig. 4 , whole blood samples from healthy volunteers spiked with Gram-negative ( E. coli , S. marcescens, S. enterica, S. enteritidis , and P. aeruginosa ) and Gram-positive ( S. aureus ) bacteria were used to test the separation efficiency. S. aureus had an above 60% removal rate, E. coli and S. marcescens over 50%, and P. aeruginosa separation was only 35%. Besides the diagnostic role, the system showed a strong inhibition of cytokines (TNF-α, IL-6, IL-1β, Il-10, and IFN-γ) release. It is worth noting that this dual and simple theranostic approach could hasten both diagnosis and management for patients suspected to have sepsis. However, as the platform showed reduced efficiency in Gram-negative bacteria, we are of the view that this will limit its applicability, especially in the cases of mixed infections. Moreover, the separation efficiency was found to be affected by the anticoagulant employed and the concentration of Ca 2+ ions in the blood collection tubes. As a result, it is imperative to underscore that this may affect the system's applicability in emergency situations where blood collection tubes with the suitable anticoagulant are unavailable.
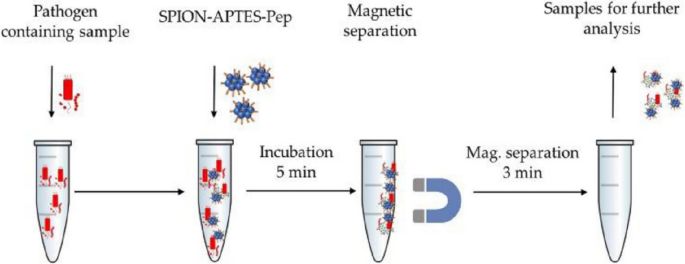
Experimental setup of separation of bacteria from blood using SPION-APTES-Pep (Adopted from [ 118 ])
To further improve the performance of magnetic iron oxide nanoparticles, coating with a layer of polyethylene glycol (PEG) can be performed. PEGylation provides improved stability, biocompatibility, and magnetic properties. PEGylation also reduces non-specific binding and potential immunogenicity, making the magnetic nanoparticles more suitable for various biomedical applications such as diagnosis and targeted drug delivery [ 138 ]. On this point, Pan et al. developed a peptide-modified PEGylated-iron oxide composite nanoclusters (peptide@PEG@MNCs) to isolate and identify S. aureus form blood samples [ 124 ]. The peptide (SA5-1: VPHNPGLISLQG) was chosen from a bacteriophage display library for its ability to selectively attach to the cell surface of S. aureus . SA5-1 was chemically bonded to the PEGylated magnetic nanoclusters (PEG@MNCs), resulting in the formation of the peptide@PEG@MNCs particles with an average diameter of 150.8 ± 1.8 nm and good cytocompatibility and magnetic properties. Human serum spiked with different bacterial strains, including E. coli , P. aeruginosa , S. aureus (susceptible and resistant strain), and S. epidermidis, was utilized to demonstrate the capturing efficacy under conditions simulating sepsis. A capture efficiency of over 70% was achieved for all tested microorganisms within 10 minutes. When a rinsing step was introduced to the process, only S. aureus was detected, notably, indicating the system's ability to selectively capture S. aureus , which was anticipated to the strong affinity of the peptide to S. aureus pathogens. Nevertheless, as the capture efficiency declined with increasing the bacterial concentration, we believe this may affect the applicability of the platform if high bacterial load exists in patients' blood samples.
In summary, the findings from the three studies indicate that peptides-modified magnetic nanoparticles have significant promise as a rapid and effective diagnostic tool for bacterial sepsis. Regarding efficacy, Feng et al.'s system showed a significantly higher capturing efficiency of S. aureus compared to the other two systems by Friedrich et al. and Pan et al. Although the second study by Friedrich et al. included Gram-negative bacteria in their evaluations, their system’s capturing efficacy was again higher for Gram-positive than Gram-negative bacteria. Interestingly, Pan et al. in their study were successfully captured both Gram-positive and Gram-negative bacteria and, at the same time able to enhance the system’s specificity toward Gram-positive bacteria by introducing a rinsing step; consequently, we contend that their approach was more flexible and comprehensive for sepsis diagnosis applications. Notably, Friedrich et al.'s system stood out for its unique ability to inhibit the release of cytokines, which will add value to the therapy of septic patients, in addition to its diagnostic use. Overall, the advantages and limitations of each one of them should be considered when applying them in clinical practice.
While the other three studies have used peptides as capturing motifs expressed on magnetic nanoparticles for bacterial separation from blood samples, another study conducted by Shrivastava and coworkers [ 125 ] applied the pathogen-capturing properties of peptides for in vivo imaging of bacteria inside the body. Imaging techniques for bacteria play an essential role in various aspects of diagnosing infections and understanding their pathogenesis [ 139 ]. Using in vivo imaging techniques, researchers can gain insights into the spatial distribution of bacteria within the host, track their movement, and understand the dynamics of infections [ 140 ]. Various molecular probes have been employed to target biological processes during infections. Nonetheless, visualizing bacteria in vivo remains challenging due to the inherent difficulty of targeting the specified bacteria directly [ 141 ]. Hence, there exists a necessity to identify highly efficient materials capable of precisely targeting specific pathways within bacterial internal processes that play a crucial role in their pathogenicity and so allow for efficient in vivo imaging of bacterial pathogens.
A potential solution to enhance the in vivo imaging of bacteria lies in using biomaterials capable of selectively targeting the quorum sensing (QS) communication employed by many bacteria to coordinate the expression of virulence genes during infections [ 140 ]. Based on that, Shrivastava and colleagues have developed a new fluorescent quorum-based nano-bio probe (QNBP) to monitor the localization of multiple-drug resistant S. aureus (MRSA) bacteria in vivo. An auto-inducing peptide (AIPq) with the ability to target the MRSA accessory gene regulator (AGR) QS system has been conjugated onto a fluorescent quantum dot (QD) surface to develop the QNBP system [ 125 ]. The nano-bio probe was assessed in vitro for bacterial binding characteristics and in vivo for imaging the bacteria in a mouse model. The QNBPs showed higher selectivity in binding to AGR-positive virulent strains than the mutant strain, thus confirming its suitability for in vivo imaging of pathogenic S. aureus. The QNBP was also capable of penetrating MRSA biofilm and effectively image embedded colonies, giving a new approach for identifying MRSA embedded in biofilms [ 142 ]. When employed as a fluorescence probe for in vivo imaging of MRSA in a systemic infection mouse model, QNBP resulted in the detection of robust fluorescent signals in most infected organs and high-quality fluorescence images were acquired post-infection. It is to be noted that this peptide-based nano-bio probe can offer new perspectives into exploration of infection pathways in vivo and aid in the diagnosis and management of life-threatening infections such as MRSA-induced sepsis. However, we argue that the need for advanced instruments such as fluorescence spectrophotometer and confocal laser scanning microscopy for recording the results may restrict the practical application of this system.
Peptides as thiol-rich moiety for binding of metallic colloids to mesoporous templates
Surface-enhanced Raman spectroscopy (SERS) is a surface-sensitive technique that enhances Raman signals of molecules adsorbed on metallic nanostructures such as plasmonic-magnetic silica templates. SERS templates modified with SERS- tags specific to certain disease biomarkers can effectively detect and analyze these biomarkers, aiding in disease diagnosis [ 143 , 144 ]. Metallic colloids, such as gold nanoparticles, when functionalized with Raman dyes and disease biomarkers-specific antibodies and subsequently immobilized on plasmonic mesoporous templates, hold great promise as SERS-based diagnostic tools for the detection of biomarkers levels [ 145 , 146 , 147 ]. Bacteriophages have the potential for synthesizing mesoporous templates; however, to incorporate metallic colloids into them, their surfaces must be chemically modified with thiol donors, which affects their critical properties, such as assembly and binding [ 148 ]. To overcome this, thiol moieties, such as cysteine-rich peptides, can be displayed on the phage during synthesis without chemical modification [ 149 ]. In this regard, Nguyen and colleagues have developed a mesoporous SERS substrate based on M13KE phage displaying a cysteine-rich peptide (243bp: GBS101000616.1) as a template for sepsis biomarkers assay in human serum sample [ 20 ].
As shown in Fig. 5 , the surface of the template was magnetized with gold-coated magnetic nano-stars (Au-MNS) modified with a SERS-tag consisting of specific antibodies for three sepsis biomarkers (soluble Triggering Receptor Expressed on Myeloid cells-1 (sTREM1)), C-reactive protein (CRP), and procalcitonin (PCT) and a RAMAN dye for RAMAN signal amplification. The cysteine-rich peptide allowed strong binding of the Au-MNS when its thiol groups were reduced to active thiols. The SERS-based immunoassay was done on human serum samples, and the SERS spectra of the magnetically separated template exhibited characteristic peaks of the tags corresponding to the three biomarkers. The system demonstrated high sensitivity, excellent specificity, and low detection limits for CRP (27 pM), PCT (103 pM), and sTREM1 (78 pM). We believe this approach offers a potential alternate tool for the initial stages of sepsis monitoring. Despite this, it is to be mentioned that the fabrication complexity and risk of surface contaminants interference due to the high surface area of mesoporous templates could limit the scalability and reproducibility and interfere with the accuracy and reliability of SERS measurements in clinical settings.
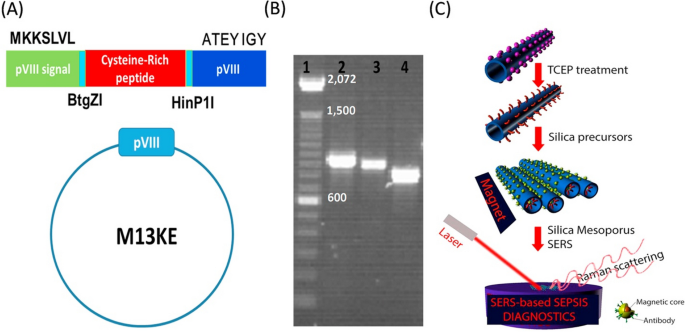
Representation of the process from phage display to manufacturing of the SERS substrate. A Insertion of the cysteine-rich peptide into the major PV111 protein domain of the M13KE phage using two restriction enzymes (BtgZI and HinP1I). B The colony PCR analysis on a 1% agarose gel confirms the effective integration of the cysteine-rich peptide into the pVIII region of the M13KE plasmid. C Utilization of cysteine-rich peptide phage display for the production of SERS substrates. The phage was decorated with an immuno-colloid made of gold-coated magnetic nano-stars (Au-MNS) after treatment with tris (2-carboxyethyl) phosphine hydrochloride solution (TCEP) to activate the thiol groups. The phage was polymerized with silica precursor to give amorphous biomaterial gel and then calcinated to form the mesoporous template. After incubation of the template with a serum sample spiked with sepsis biomarkers, the complexes were separated using a magnet and subjected to Surface-enhanced Raman scattering (SERS) measurement (adopted from [ 20 ])
To conclude, the studies discussed in this section demonstrated the crucial role of peptides in sepsis diagnosis by either offering pathogen recognition capacities or enabling the binding of metallic nanoparticles to mesoporous templates. These approaches offer several advantages, including enhanced selectivity and sensitivity in detecting sepsis-causing pathogens, as well as the ability to provide rapid results. Nevertheless, relying on blood samples from healthy volunteers to assess the developed nanoplatforms may not accurately reflect the complexity of sepsis cases. Therefore, it is imperative to validate the clinical applicability of the developed nanoplatforms by testing them on blood samples obtained from sepsis patients. Such validation would ensure these nanoplatforms' accuracy, reliability, and effectiveness in real-world clinical settings.
Peptides in nanotechnology for sepsis management
Although numerous potential therapeutics for sepsis have been identified, achieving effective treatment remains a formidable challenge, and sepsis continues to threaten healthcare systems worldwide with its high mortality rates and complex pathophysiology [ 150 ]. In recent years, using peptides in nanotechnology has ignited new hopes for developing targeted and efficient therapeutic strategies for complex illnesses such as sepsis [ 151 ]. This section will delve into the developments and transformative potential of peptides in nanosystems to manage sepsis. The multifaceted applications of these peptides will be explored and discussed, including their utilization as bioactive agents with antimicrobial or anti-inflammatory properties delivered through nanosystems. Additionally, their role as nanosystem components, including nanocarriers for antisepsis drug delivery or as surface modifiers for nanosystems to target sepsis microenvironment or causative bacteria, will be highlighted and critically analyzed in the following subsections. An emphasis will be put on the manufacturing processes of nanosystems, key characterization and evaluation, and key findings of the studies.
Nano-delivered bioactive peptides
Antimicrobial peptides nanosystems.
Antimicrobial peptides (AMPs) are short chains of amino acids, typically ranging from 5 to 50 residues, which may be regarded as natural antibiotics synthesized by various organisms, including mammals, plants, protozoa, fungi, and bacteria. They may have amphipathic or cationic structural composition and can display a wide range of antimicrobial activity, targeting both Gram-positive and Gram-negative bacteria, fungi, viruses, and protozoa [ 152 ]. A complete understanding of the mechanism of action of AMPs remains elusive. Nevertheless, many mechanisms have been proposed, suggesting diverse interactions with phospholipid membranes of microorganisms [ 153 , 154 ]. In recent years, exploring AMPs as potential therapeutics has gained substantial momentum, driven by their remarkable antimicrobial activity and ability to overcome antimicrobial resistance [ 155 ]. However, the clinical translation of AMPs into effective sepsis treatments has been hindered by various challenges, including toxicity, limited stability and enzymatic degradation, and ununderstood pharmacokinetic profiles [ 156 ]. Therefore, robust delivery strategies are needed to allow effective use of AMPs in clinical settings.
The utilization of nanotechnology-based delivery systems has emerged as a promising trajectory for mitigating the aforementioned challenges and augmenting the therapeutic efficacy of AMPs. Literature has documented that nano-scaled delivered peptides exhibit diminished cytotoxicity, improved physiological stability, and increased efficiency at the desired target [ 157 ]. Hence, nano-delivery systems could enhance the physicochemical and pharmacological characteristics of AMPs, enabling their use as effective sepsis therapeutics in clinical practice. Table 2 illustrates different studies reported on nano-delivered AMPs for sepsis management, highlighting the type of nanosystem, the peptide sequence, the nano-delivery strategy, the targeted bacteria, the key evaluations, and the key findings. As illustrated, peptides have been either conjugated with other moieties to aid nanoscale self-assembly, encapsulated into nanosystems, or linked to the surface of nanoparticles. Evidently, most of the studies have focused on the self-assembly and nano-encapsulation of AMPs, indicating room for more research on the conjugation of AMPs to the surfaces of metallic and organic nanoparticles. The studies will be discussed in the following subsections according to the strategy of nano-delivery of AMPs.
Self-assembled AMP nanosystems
Designing peptides to self-assemble into nanostructures is a powerful strategy to bolster their stability, biosafety, and therapeutic outcomes, allowing their effective application in disease management [ 166 ]. The self-assembly of peptides is usually achieved by selecting amino acid series encompassing both hydrophilic and hydrophobic amino acids and linking them to yield amphiphilic structures capable of spontaneous self-assembly upon exposure to water. Alternatively, amphiphilic peptides can be engineered by conjugating them with hydrophobic motifs such as alkyl chains and fatty acids [ 166 ].
AMPs are among the bioactive peptides that can be designed to self-assemble and become more stable and effective [ 167 ]. In this regard, Lei et al. have successfully prepared self-assembled nanoparticles of the host-defense antimicrobial peptide, human alpha-defensin 5 (HD5), with improved stability, biosafety, and antibacterial effectiveness [ 158 ]. To introduce hydrophobicity and promote the nano-assembly of HD5, they conjugated myristic acid, a 14-carbon chain saturated fatty acid, to the C-terminus of HD5 to produce myristoylated HD5 (HD5-myr). HD5-myr spontaneously self-assembled in aqueous media, producing spherical-shaped nanoparticles -termed Nanobiotic- that were found to be hemocompatible and unlike the native HD5 resistant to proteolytic enzymes. The Nanobiotic exhibited significantly enhanced broad-spectrum bactericidal activity in vitro against different Gram-positive and Gram-negative bacterial strains, including S. aureus, MRSA , E. coli, A. baumannii, P. aeruginosa, and K. pneumoniae, compared to the free HD5 . The Nanobiotic was stable and retained the antibacterial efficacy even in the presence of proteolytic enzymes and high salt concentration, while the free HD5 underwent extensive hydrolysis within 24 hours. In the in vivo studies, the Nanobiotic exhibited protective effects, effectively rescued mice from E. coli -induced sepsis, and improved their survival rates by reducing the overall bacterial load in the body and preventing organ damage. Their outcomes have shown that the supramolecular assembly of AMP to make nanoparticles has tackled peptides' instability problem and achieved good antibacterial activity both in vitro and in vivo. We believe this work provides a promising candidate for treating bacterial sepsis that can be simply scaled up and manufactured.
Utilizing the same concept of peptide-conjugates assembly into nanoparticles, Tan and coworkers designed self-assembling chimeric peptide nanoparticles to treat bacterial infection and sepsis [ 159 ]. As shown in Fig. 6 , the AMP peptide sequence (PFPFPFP-KPKPKPKPKPKP-NH2) was linked to a 14-carbon alkyl chain to provide hydrophobic properties and modified with PEG domain at various locations to offer stealth effect and biocompatibility. The peptide amphiphiles self-assembled into nanoparticles of around 20-50 nm in diameter, which have shown good in vitro and in vivo biocompatibility. The self-assembled nanoparticles demonstrated broad-spectrum antibacterial activity against various strains of E. coli (MICs: 7.3 to 12.3 μM) and S. aureus (MICs: 5.3 to 10 μM) even in the presence of high concentrations of proteases and different salt conditions. Moreover, no spontaneous antimicrobial resistance to the peptide nanoparticles was detected when the E. coli ATCC25922 strain was subjected to sub-MIC dose treatment. In vivo, the nanoparticles have also demonstrated the ability to alleviate E. coli sepsis in mice and piglets and significantly reduced organs' bacterial load and the concentrations of pro-inflammatory cytokines (TNF-α, IL-6, and IL-1β). We argue that this approach provides an effective strategy to accelerate the clinical translation of newly developed peptides to meet the real need for effective sepsis therapies and to fight the growing antimicrobial resistance.
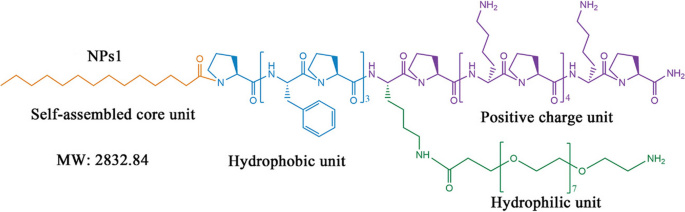
The structural design of self-assembling chimeric peptide. The peptide sequence comprises hydrophobic and cationic amino acids. The peptide is linked to the hydrophobic alkyl chain to enhance the self-assembly and the hydrophilic PEG unit to provide a stealth effect and improve biocompatibility (adopted from [ 159 ])
Carrying on with the application of peptide nano-assembly, Pan and colleagues reported two studies [ 22 , 160 ] on the development of co-assembled antibacterial peptide polymeric nanoparticles (AMPNP) for targeting bacteria and inflammation sites to combat bacterial sepsis. They synthesized antibacterial peptide (KR-12: KRIVKRIKKWLR)-grafted amphiphilic block copolymer and biotin grafted block copolymer, which were co-assembled in aqueous solution to produce the AMPNP. After that, as illustrated in Fig. 7 , they tried two different approaches to achieve AMP-targeted delivery against sepsis. In their first approach [ 22 ], they modified the AMPNP with an antibody against intercellular adhesion molecule-1 (anti-ICAM-1 antibody) to target the inflammation sites with over-expressed ICAM-1 receptor. In their second targeting approach [ 160 ], they coated the AMPNP with a macrophage membrane of the mouse leukemia cells of monocyte-macrophage (M) to achieve specific binding to bacteria through the bacterial recognition molecules (Toll-Like receptors) on the macrophage membrane. The anti-ICAM-1-AMPNP and the M-AMPNP have shown specific targeting and adhesion to the inflamed human cells and bacterial cells, respectively. The in vitro antibacterial activity of the anti-ICAM-1-AMPNP and M-AMPNP was evaluated against E. coli, S. aureus , and MRSA, and they both exhibited good antibacterial efficacy. Moreover, when evaluated in vivo on mice sepsis model, both nanosystems have demonstrated a superior effect over the uncoated AMPNP regarding the reduction in serum cytokines (IL-1β, TNF-α, and IL-6) levels and inflammatory cells tissue infiltration.
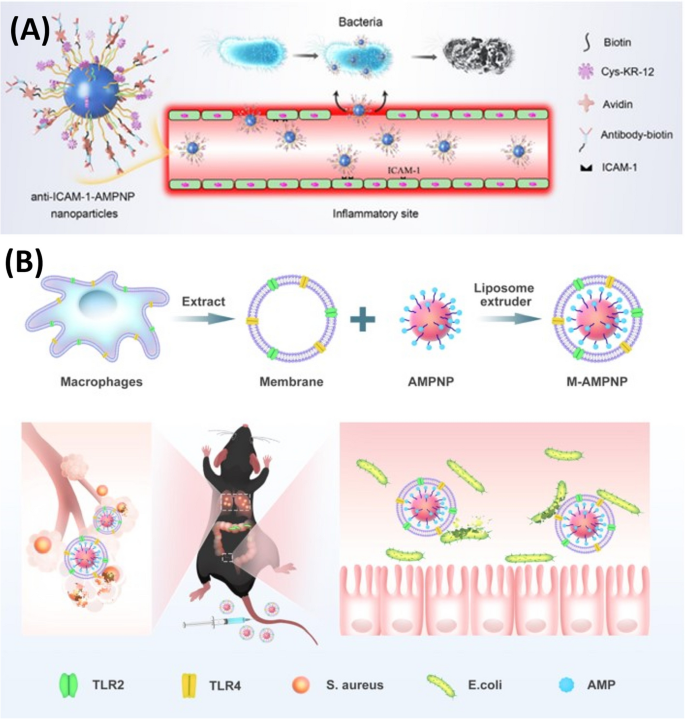
A Preparation of anti-ICAM-1-AMPNP to specifically target inflammation sites with overexpressed ICAM-1 receptor. B Preparation of macrophages membrane-coated AMPNP (M−AMPNP) to specifically target bacteria through the TLR2 and TLR4 on the macrophage membrane (Adopted from [ 22 , 160 ])
Overall, both nanosystems (anti-ICAM-1-AMPNP and M-AMPNP) are promising and suggest a potential efficacy in bacterial sepsis management by explicitly targeting the inflammation sites and the causative bacteria. It is worth noting that, although the in vitro antibacterial activities of anti-ICAM-1-AMPNP and M-AMPNP were comparable to the bare peptide when evaluated in vivo , the nanosystems' efficacies were superior, which could be due to the improved stability of nanoparticles against proteases enzymes and the specific targeting and delivery to the inflammation and bacterial infections sites. However, using macrophage membranes derived from cancerous mouse cells could raise safety and immunogenicity concerns. Therefore, we think this mouse macrophage coating should be carefully considered when the nanosystem is to be further taken for clinical translation.
Nano-encapsulated AMPs
Encapsulation into nanosystems is another effective strategy for nano-delivery of AMPs to fight bacterial sepsis. This nano-encapsulation improves the AMPs' stability, reduces systemic toxicity, and improves their therapeutic efficacy [ 157 ]. In this context, Saúde et al. have encapsulated the antimicrobial peptide Clavanin A in a polymeric matrix for bacterial sepsis control, aiming to improve its stability and therapeutic efficacy [ 161 ]. The Clavanin A peptide (VFQFLGKIIHHVGNFVHGFSHVF-NH2) was nanostructured in a mixture of the methacrylate polymers EUDRAGIT® L 100-55 and EUDRAGIT® RS 30 D to make a nano-antibiotic. The nano-antibiotic demonstrated a sustained release, with 69% of the loaded Clavanin A released after 48 hrs. The in vitro antibacterial assay showed that nanoparticles containing 12 µg of Clavanin A inhibited the growth of S. aureus by 91%, K. pneumoniae by 20%, P. aeruginosa by 39.8%, and no effect on E. coli . In vivo efficacy of the nano-antibiotic evaluated on polymicrobial sepsis model on mice showed a 100% survival rate under a sub-lethal dose of bacteria and a 40% survival rate with a lethal inoculum. It is worth mentioning that, although the peptide loading significantly reduced the stimulation of pro-inflammatory cytokines (TNF-α, IL-12, and IL-10) release in vitro compared to the blank nanoparticles, these nanoparticles-induced cytokines releases were still significant. Therefore, we believe more in vivo evaluations are needed in this regard, and it would be better to avoid using these thiolated methacrylate polymers for antisepsis drug delivery as they are known to induce inflammation and cytokines release [ 168 ].
Another study carried out by Hassan and coworkers also reported the nano-encapsulation of an AMP (Mastoparan (Mast)) in polymeric nanoparticles to improve its stability and efficacy in managing multidrug-resistant bacterial sepsis [ 162 ]. They nano-encapsulated Mast (INLKALAALAKKIL-NH2) by structuring it with chitosan to produce a chitosan–Mast nano-construct (Mast-Cs NC). Mast-Cs-NC's in vitro antibacterial activity against A. baumannii clinical isolates demonstrated a significantly lower MIC of 4 μg/mL compared to the bare-Mast, which got an MIC of 16 μg/mL. When evaluated for in vivo efficacy on an A. baumannii- induced mice sepsis model, Mast-Cs-NC improved the physical activity and significantly decreased the blood bacterial counts compared to the chitosan and bare-Mast treated groups. Their findings showed enhanced in vitro and in vivo activity; however, they didn't report evaluation of the peptide release behavior from the nanosystem, which we believe is a fundamental property that will affect the selection of dosing frequency in clinical settings.
While the other studies reported encapsulation of functional AMPs in nanoparticles, Hou et al. have used an alternative unique approach by encapsulating the mRNA of the AMP-IB367 ( RGGLCYCRGRFCVCVGR CONH2 ) linked to mRNA of cathepsin B (CatB) (AMP-CatB mRNA) in vitamin C lipid nanoparticles (VLNPs) [ 163 ]. They transfected the nanoparticles in macrophages, where the mRNA will be translated to functional AMP-IB367 and CatB. CatB is an endogenous lysosomal protein that assists in translocating AMP-IB367 inside the macrophages' lysosomes, resulting in macrophages containing antimicrobial peptides linked to cathepsin B in the lysosomes (MACs). Upon the adoptive transfer of MACs to animals infected with bacteria, the lysosomes will fuse with phagosomes encapsulating bacteria and effectively kill the bacteria through both AMP-IB367 and lysosomal antimicrobial constituents. In vitro, MACs showed strong bacterial growth inhibition of 87% when evaluated against multi-drug resistant S. aureus (MDRSA) intracellular infection on RAW264.7 cells. MACs also significantly decreased bacterial loads in blood and improved survival rates of MDRSA-induced septic mice. Their findings presented the applicability of using nano-delivered mRNA of AMPs to target intracellular bacterial infections and sepsis. However, this strategy is limited by the possibility of mRNA degradation during loading and transfection, the immunogenicity that may arise from the adoptive transfer of macrophages, and the difficulties of scaling up these complex nanosystems. Therefore, we think these limitations must be carefully addressed before the MACs can be used in clinical practice.
AMPs conjugated to nanoparticles surface
Covalent conjugation of AMPs to the surface of nanoparticles has been applied to achieve nano-delivery of these conjugated AMPs. The conjugation can be accomplished on various types of nanoparticles, including both organic and metallic nanoparticles [ 24 ]. This approach is beneficial, especially in the cases of organic nanoparticles where another antisepsis drug can be encapsulated in the nanosystem and achieve simultaneous multiple drug delivery to target various pathways involved in the complex pathophysiology of sepsis. So far, only two studies have been reported on the covalent conjugation of AMPs to the surface of nanoparticles to enhance the stability and efficacy against bacterial sepsis, that is, one has conjugated the AMP to gold nanoparticles [ 164 ], and the other conjugated AMP to the surface of liposomes loaded with antibiotic [ 165 ]. Therefore, this strategy has not been thoroughly investigated, providing an opportunity to efficiently administer AMPs alone or combined with other drugs to address bacterial sepsis.
Rai and colleagues conjugated Cecropin melittin-cysteine (CM-SH: KWKLFKKIGAVLKVLC) AMP to gold nanoparticles (Au NPs) to improve the physiological stability and therapeutic efficacy against bacterial sepsis [ 164 ]. The optimized AMP-conjugated gold nanoparticles (CM-SH-Au NPs) were produced in one-step synthesis and found to have high AMP concentration (50% per nanoparticle mass). The in vitro antibacterial activity of CM-SH-Au NPs against S. aureus and E. coli showed a 4-fold reduction in MIC compared to the free CM-SH peptide. Unlike the free CM-SH peptide, CM-SH-Au NPs were found to be resistant to degradation, retaining even in the presence of cell culture media, human serum, and proteolytic enzymes such as trypsin, S. aureus V8 protease, and human neutrophil elastase. To evaluate the antimicrobial resistance development, they exposed E. coli to a sub-MIC dose of CM-SH-Au NPs for 28 days and then evaluated their efficacy against the treated strain. CM-SH-Au NPs were found to be still effective against the sub-MIC-exposed E. coli strain with no resistance development, unlike the control drug, chloramphenicol, which developed resistance after only 3 days of exposure. The therapeutic potential of CM-SH-Au NPs was also evaluated against CLP mice model of sepsis and demonstrated a significant reduction in bloodstream bacterial count and IL-10 level compared to unconjugated Au-NPs and free-CM-SH. We contend that their findings are promising and provide an effective strategy for improving the physiological stability and therapeutic efficacy of AMPs. However, the use of metallic nanoparticles needs comprehensive toxicity evaluations as they are known to carry more risk of systemic toxicity [ 169 ] than organic nanoparticles.
In another study, Fan et al. linked the AMP S-thanatin (Ts) (GSKKPVPIIYCNRRSGKCQRM) to the surface of liposomes loaded with levofloxacin (Ts-LPs-LEV) to target K. pneumoniae induced sepsis [ 165 ]. As illustrated in Fig. 8 , Levofloxacin-loaded liposomes were prepared by thin film hydration method with the incorporation of Ts-PEG2000-DSPE to produce positively charged liposomes and Ts anchored on the surface. The incorporation of Ts synergistically improved levofloxacin's antimicrobial activity on sensitive K. pneumoniae and restored its sensitivity on multidrug-resistant clinical isolates. The calculated MICs of Ts-LPs-LEV were 2 to 8 folds less than that of LPs-LEV on different strains of K. pneumoniae. Moreover, when evaluated on mice sepsis model of MDR K. pneumonia clinical isolate, the anchoring of Ts peptide resulted in a significant difference in bacterial clearance from blood and mice's survival rates with a reduction in lethality from 73.3% to 6.7% compared to LPs-LEV. It should be pointed out that while the peptide linking synergistically improved the efficacy of LEV, the Ts-linked liposomes without loading of LEV showed no activity against the majority of tested bacterial isolates (16 out of 18 isolates). However, the free peptide was effective on those isolates; therefore, we think this peptide's activity loss needs to be addressed and investigated. Furthermore, they didn't examine the enzymatic stability of conjugated peptide compared to the free peptide, which we believe is one of the main advantages of incorporating AMPs into nanosystems. Besides, they didn't evaluate the release pattern of the Ts and LEV, which is critical in determining dosing frequencies in clinical settings.
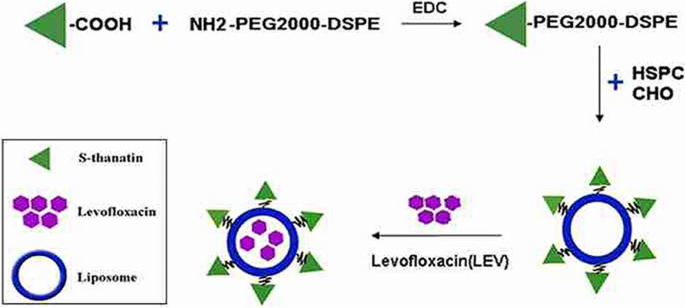
Preparation of Ts-LPs-LEV. Adopted from [ 165 ]
In a nutshell, nano-delivery of AMPs has been proven to be an effective strategy to overcome the shortcomings of antimicrobial peptides, such as physiological instability and systemic toxicity, resulting in improved biosafety and efficacy against bacterial infections and sepsis both in vitro and in vivo. The AMPs have been nano-delivered through various strategies, including conjugation with hydrophobic moieties to allow their self-assembly into nanoparticles, encapsulation in organic nanosystems, and linking the surfaces of metallic and organic nanoparticles. Therefore, we believe that with more investigations, these nanomedicines could be scaled up and become available for clinical use to help combat the life-threatening bacterial sepsis and the growing antimicrobial resistance.
Anti-inflammatory peptides nanosystems
Recently, anti-inflammatory peptides (AIPs) nanosystems have shown excellent properties, making them exceptional therapeutic candidates for sepsis management [ 23 , 170 ]. These characteristics include potent neutralizing effects against pro-inflammatory molecules [ 171 , 172 , 173 ], improved biodegradability and biocompatibility [ 174 ], advanced delivery properties [ 175 ], and multiple actions across various intracellular inflammatory pathways [ 30 , 176 ]. This section covers all AIP nanosystems reported for sepsis management, including self-assembled nanostructured AIPs and other nanocarriers used to deliver AIPs.
Self-assembled nanostructured AIPs
Self-assembled nanostructured peptides have been introduced to improve the anti-inflammatory properties of LPS-binding proteins (Limulus anti-LPS factor, serum amyloid P, and bactericidal permeability-increasing protein) by Mas-Moruno et al. This study successfully synthesized and structurally characterized several N-acylated peptides derived from the above proteins with advanced anti-inflammatory activity against LPS-induced cytokines storm. In vitro investigations have been done to evaluate their biosafety profile against RAW 264.7 macrophages, and most of them were found to be biosafe and tolerable. Compared to their parent peptides, some N-acylated peptides showed up to 10-fold enhancement in the in vitro LPS neutralizing activity within their biosafe concentration ranges. This activity enhancement may be related to their ability to form fibril-like and micellar nanostructures, as shown during TEM imaging. Their findings are promising, and we suggest they can be taken for preclinical and clinical evaluations to prove these results and allow for application in clinical settings [ 171 ].
Later, Tram et al. designed a stimuli-responsive self-assembled nanostructured dual active peptides (anti-inflammatory and antimicrobial) as an effective antisepsis agent with an advanced ability to form amyloid-like nanostructured nets in response to LPS and other bacterial endotoxins contact [ 173 ]. These multifunctional positively charged synthetic β-hairpin peptides could efficiently exert their anti-inflammatory effect through selective entrapping of negatively charged pro-inflammatory molecules and cytokines such as TNF-α, IL-6, LPS, and lipoteichoic acid (LTA) and, therefore, inhibiting bacterial endotoxin-induced cytokine storm. On the other hand, they exerted their antimicrobial activity by physically trapping bacterial cells and lysing bacterial membranes through interaction with the negatively charged bacterial cell walls and membranes. In this study, the selected peptides have shown promising bacterial toxin-neutralizing activity, which has been evaluated in a bacterial toxin-challenged-murine macrophage cell line model (in vitro) and acute lung injury mice model (in vivo). Furthermore, this study has involved various techniques to show the selective trapping of negatively charged pro-inflammatory cytokines (TNF-α and IL-6). In summary, modifications that confer AIPs the ability to self-assemble into nanostructured systems significantly enhanced their neutralizing activity against pro-inflammatory molecules and overall antisepsis outcomes with improved stability and circulation time.
Conventional nanocarriers-delivered AIPs
Several nanocarriers, including polymeric-based [ 30 , 176 ], protein-based [ 174 ], and metallic-based nanosystems [ 172 ], have been employed to deliver AIPs in attempts to improve their antisepsis activity. Table 3 summarizes the studies reported on designing AIP-loaded nanocarriers for sepsis management, highlighting the utilized AIP, the type of nanocarrier, the preparation method, and the key advantages of AIP nano-delivery. As depicted, nano-delivery enhanced the peptide's anti-inflammatory activity, stability, circulation time, biodistribution, biodegradability, and hemocompatibility. Moreover, nanocarriers are involved in the AIPs' co-delivery with antibiotics and other antithrombotic peptides to develop innovative and comprehensive antisepsis therapies with promising clinical outcomes. The studies will be discussed in this subsection based on the type of constituents of the nanocarriers, including polymeric, metallic, and protein-based materials.
Firstly, modified polyethylene glycol and acrylate polymers have been used to fabricate biocompatible nanocarriers to improve AIPs delivery for sepsis treatment [ 30 , 176 , 177 ]. These polymer-based nanocarriers significantly increased the antisepsis activity of AIPs by enhancing their stability, anti-inflammatory activity, and circulation time. For instance, Sadikot et al. utilized distearoyl phosphatidyl-linked PEG (DSPE-PEG 2000 ) to form 15 nm-micelles as an innovative co-delivery system for the two AIPs; human glucagon-like peptide (GLP-1) and triggering receptor expressed on myeloid cells 1 (TREM-1) inhibitor peptide (LP17)) to treat sepsis-related acute lung injury. This phospholipid micellar system stabilized both peptides in their activated alpha helix form and conferred prolonged circulation and in vivo bioactivity time compared to their parent peptides. Similarly, Cheng et al. and colleagues used DSPE-PEG 2000 to develop highly loaded peptide nanoparticles to deliver anti-inflammatory/antithrombotic dual active peptide, MB2mP6 (Myr-FEKEKL), as a new thoughtful approach for sepsis treatment [ 30 ]. MB2mP6 nanoparticles effectively inhibited both thrombosis and inflammation with limited vascular leakage by targeting inflammatory and thrombotic pathways associated with integrin’s G-protein alpha subunit-13 (Gα13) interactions in leukocytes and platelets. Immediate and late administration of MB2mP6 nanoparticles after severe sepsis initiation significantly increased mice survival rate, reduced inflammatory and thrombosis mediators, and prevented tissue and organ damage.
Likewise, utilizing polymeric material for AIP delivery, Novoselova et al. used polyacrylate-modified polybutyl cyanoacrylate polymer to formulate thymulin (an AIP thymic peptide)-bound nanoparticles to efficiently treat chronic inflammation and sepsis [ 177 ]. The developed acrylate-based nanoparticles showed 90% entrapment efficiency, improving the delivery aspect of thymulin against sepsis, such as circulation time and biodegradability. Thymulin-loaded nanoparticles efficiently alleviated sepsis-induced cytokines storm, decreased heat shock proteins and TLR-4 expression, reduced apoptosis, and increased splenic cell counts in mice. In summary, the findings of the three studies [ 30 , 176 , 177 ] demonstrated that utilizing polymeric nanosystems significantly enhanced the therapeutic efficacy of AIPs. However, it is essential to highlight that they didn't report much characterization of the developed nanosystems, such as size, PDI, ZP, and release kinetics measurements, which are highly important in indicating nanomedicines' storage stability and dosing frequencies.
Secondly, two studies by Karawacka et al. and Piktel et al. have used magnetic metallic nanoparticles to immobilize anti-inflammatory peptides via electrostatic interaction to improve their stability, activity, and circulation time [ 172 , 178 ]. In their study, Karawacka and colleagues used a coated superparamagnetic iron oxide nanoparticle to bind and immobilize agglutinating salivary proteins-derived peptides (LPS-neutralizing peptides) via hetero functional linkers. This modification significantly enhanced the in vitro LPS-neutralizing activity of conjugated peptides by more than 3-fold when evaluated by the endotoxin binding assay [ 178 ]. In the second study, Piktel and coworkers developed an innovative iron oxide-based peptide nanosystem with advanced delivery properties. Synthetic pro-inflammatory molecules-neutralizing peptide PBP10 (synthetic rhodamine B-conjugated peptide, bioinspired from the naturally occurring protein human plasma gelsolin) and its derivatives were used in this study to functionalize iron oxide nanoparticles. These fabrications simultaneously enhanced the antibacterial activity of metallic nanoparticles, the anti-inflammatory properties of immobilized peptides, and their biocompatibility properties, promoting the promising potential of AIPs metallic nanosystems as effective antisepsis therapeutic agents [ 172 ].
Finally, ferritin-based nanocages are well-known nanocarriers that improve stability and overall activity for different types of drugs due to their excellent properties, such as inherent cavity sizes and biocompatibility [ 179 ]. With this regard, Wei and coworkers used an emulsification technique to formulate ferritin-based nanocages as an efficient nano drug delivery system for both anti-inflammatory peptide GF9 (a TREM-1 inhibitor) and the antibacterial agent streptomycin as a dual therapy against bacterial-induced sepsis [ 174 ]. Using an E. coli -induced sepsis mice model, this Antibacterial/ anti-inflammatory co-delivery successfully reduced bacterial burden, suppressed harmful inflammatory responses, prevented lungs from sepsis-associated tissue damages, and achieved better overall clinical outcomes and survival rates compared to monotherapies. Thus, we believe ferritin-based co-delivery of AIPs with antibacterial agents could be an efficient therapeutic strategy against sepsis.
Stimuli-responsive nanocarriers- delivered AIPs
Compared to conventional nanocarriers, stimuli-responsive nanocarriers have shown superior clinical outcomes due to their advanced release patterns that accumulate the loaded therapeutic agents in their site of action in response to distinguished pathophysiological changes [ 180 ]. Concerning this, a brilliant study by Lee et al. reported stimuli-responsive ferritin-based nanocages that simultaneously delivered two bioactive peptides, targeting two different intracellular pathways to improve sepsis control and reduce harmful side effects [ 175 ]. In this study, ferritin was Genetically modified by inserting the endothelial protein C receptor-targeting ligand (PC-Gla domain) and protease-activated receptor-1 activator (TRAP peptide) to form ferritin-based nanocarriers, which showed an advanced antisepsis activity. This promising activity has been further improved by inserting a matrix metalloproteinase-sensitive linker to confer PC-Gla domain a stimuli-response release pattern in response to metalloproteinase at the metalloproteinase-rich inflammatory sites. In vitro and in vivo sepsis models confirmed the stimuli-responsive PC-Gla release, significantly reducing inflammatory cells infiltration and lung injury scores and improving mice survival rates after CLP.
Later, Lui et al. reported pH-responsive nanoplexes that could target CD44-overexpressed cells as an efficient stimuli-responsive nanocarrier of SS-31 peptide (an AIP) against sepsis-induced acute kidney injury [ 181 ]. This innovative design perfectly overcame the poor pharmacokinetic characteristics of the loaded peptide SS-31, enhancing its activity and targetability. As presented in Fig. 9 , biocompatible polymers Hyaluronic acid and chitosan (CS) electrostatically interacted to form stable nanoplexes, promoting payload release via low pH condition destabilization. SS-31 loaded nanoplexes were stable at physiological pH with an average size of 53 nm, ZP of -20 mV, and PDI of 0.17. large surface charge conferred nanoplexes high stability to prevent aggregation and enhance accumulation at the site of action. In vitro drug release studies confirmed the pH-responsive release pattern with an approximately 10-fold higher drug release percentage at pH 4.5 compared to pH 7.4. SS-31 loaded nanoplexes showed an enhanced intracellular uptake and higher antioxidant and antiapoptotic properties compared to bare SS-31 peptides in both in vitro and in vivo studies. Furthermore, histopathological analysis revealed that treatment with SS-31-loaded Nanoplexes improved kidney functions and reduced sepsis-associated tissue damage and tubular injury.
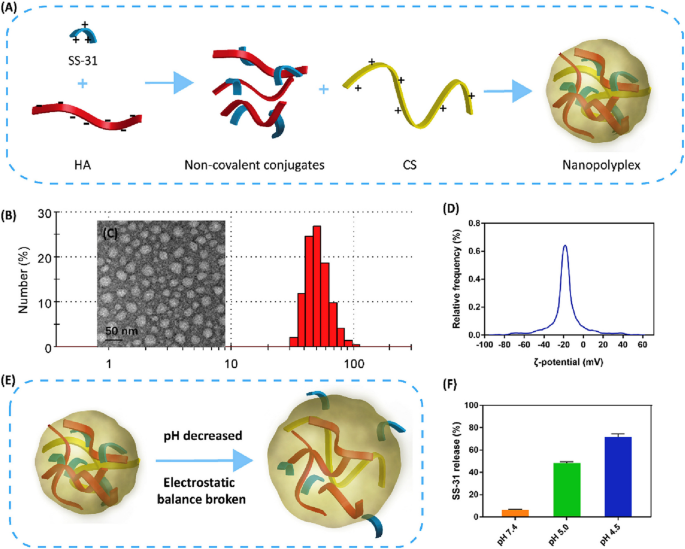
Preparation and evaluation of SS-31 loaded nanoplexes. A The method for fabricating nanoplexes through electrostatic complexation; ( B ) and ( D ) DLS characterization; ( C ) TEM imaging; ( E ) pH-responsiveness; ( F ) release patterns at different pHs. (Taken from [ 181 ])
In conclusion, exploring nano-delivered AIPs to combat sepsis has promising outcomes. The improved neutralizing action of AIPs in nanosystems against pro-inflammatory cytokines opened avenues for further preclinical and clinical evaluations. In addition, as compared to free peptides, nano-delivered AIPs exhibited markedly enhanced stability, circulation time, and therapeutic effectiveness. Furthermore, stimuli-responsive nanocarriers demonstrated superior clinical outcomes with advanced release patterns, offering a targeted and efficient approach to sepsis treatment.
Overall, the studies discussed in the above two sections underscore the potential of nano-delivery systems to enhance bioactive peptides (AMPs and AIPs)' stability and therapeutic efficacy for effectively managing sepsis, presenting a compelling foundation for further exploration and clinical translation.
Peptides as nanocarriers components
Peptides as targeting moieties on nanoparticles’ surface.
The surface of nanoparticles can be modified using various techniques and modifying materials. This surface modification achieves several advantages, including targeted and site-specific drug delivery, improved cellular uptake of loaded drugs, and enhanced nanosystems' physicochemical, biological, and therapeutic properties [ 44 ]. Different materials, such as antibodies [ 182 ], oligonucleotides [ 183 ], polymers [ 184 ], and peptides [ 185 ], have been used for nanoparticles’ surface modifications. Table 4 presents a summary of peptides’ application for surface modification of nanoparticles delivered against bacterial sepsis, showcasing the type of the nanosystem, peptide sequence, biological target, loaded drugs, key characterizations, and key findings. As presented, peptides have been used as targeting moieties for specific delivery to inflammation sites, bacterial cells, and body organs. It is obvious that the most studied application was the use of peptides to target inflammation sites through the overexpressed receptors, enzymes, and other proteins. Within these, ICAM-1 was the most targeted inflammatory site components. This open avenues for more research on the application of peptides to target other sepsis inflammatory-microenvironment’s overexpressed components such as selectins, CD44, TREM-1, and protease-activated receptor-1 (PAR-1) [ 186 ]. Also, the of targeting invading bacteria and specific body organs that are at high risk of damage during sepsis are yet to be more explored. The studies will be discussed in the following subsections based on the biological target that has been utilized to achieve improved sepsis management.
Inflammation sites targeting peptides
Inflammation sites, such as those of sepsis, are characterized by an overexpression of certain proteins involved in the inflammation processes [ 188 , 191 ]. These up-regulated proteins can be exploited to develop smart nanosystems modified with targeting moieties (such as peptides) that bind specifically to these proteins, resulting in targeted drug release at the inflammation sites [ 195 ]. In this subsection, we discuss the utilization of peptides as surface modifiers for nanosystems to target various upregulated proteins at the sepsis’s inflammation sites. The studies will be organized according to the targeted protein.
ICAM-1 is a key adhesion molecule on epithelial cells that acts as a ligand to the integrins receptors on polymorphonuclear leukocytes, mediating their recruitment and migration into tissues. ICAM-1 is typically expressed at low levels; however, its expression is up-regulated during sepsis and inflammatory conditions, increasing the leukocytes adhesion [ 196 ]. Therefore, ICAM-1 targeting ligands such as peptides [ 187 ] and anti-ICAM-1 antibodies [ 22 ] have been used to modify nanoparticles to achieve inflammation sites targeted drug release. To this end, three studies [ 187 , 188 , 189 ] have reported the use of peptides for surface modification of Poly DL-lactic-co-glycolic acid (PLGA) nanoparticles targeting the inflammation-induced overexpression of ICAM-1. In their study, Zhang group reported a proof-of-concept investigation in which they decorated PLGA nanoparticles with cyclo (1,12) PenITDGEATDSGC (cLABL) peptide, which is proven to have a good binding affinity to the D1 domain of ICAM-1 [ 197 ], to target the overexpressed ICAM-1 on inflamed cells. cLABL-PLGA-NPs demonstrated rapid binding and internalization into human umbilical cord vascular endothelial cells (HUVECs) with up-regulated ICAM-1 induced by interferon -γ treatment. They found the binding of cLABL-PLGA-NPs to be inhibited by pretreatment with free cLABL, proving that the binding of nanoparticles was due to the peptide conjugation [ 187 ].
The other two studies by Yang et al. and Liu et al. utilized γ3 peptide (NNQKIVNLKEKVAQLEA) as an ICAM-1 ligand for surface modification of PLGA nanoparticles achieving targeted release of the loaded antisepsis agents at the sepsis’s inflamed sites. Yang and colleagues, as illustrated in Fig. 10 , utilized the γ3-PLGA-NPs for co-delivery of the antibiotic Sparfloxacin (SFX) and the anti-inflammatory/immunosuppressant Tacrolimus (TAC), resulting in γ3-PLGA-NPs@SFX/TAC. Conversely, Liu et al. applied a monotherapy strategy by loading only ciprofloxacin (CIP) in the γ3 modified PLGA-NPs. However, Liu’s group coated the PLGA-NP with red blood cells (RBC) membrane producing γ3-RBCNPs@CIP to avoid immune vigilance and provide prolonged circulation time. Both γ3-PLGA-NPs@SFX/TAC and γ3-RBCNPs@CIP showed good cytocompatibility, hemocompatibility, and low systemic toxicity. When evaluated on TNF-α activated HUVECs cell line, both nanosystems demonstrated significantly higher binding to the stimulated cells than the peptide-unconjugated nanoparticles. Furthermore, they both exhibited superior in vitro antibacterial efficacy.
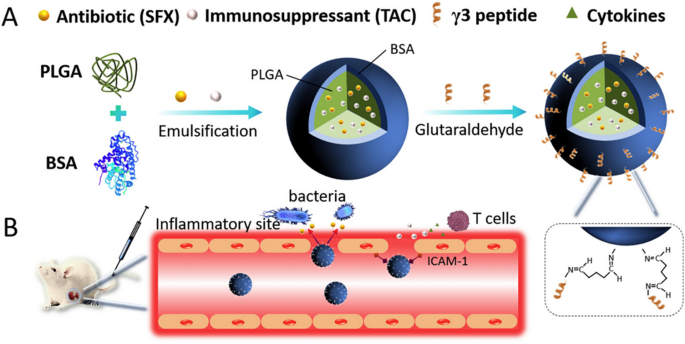
Preparation and in vivo evaluation of γ3-PLGA NPs ( A ) Preparation of γ3-PLGA NPs loaded with Sparfloxacin and Tacrolimus ( B ) Effective treatment of lung-infected mice by specific targeting of the overexpressed ICAM-1 (Taken from [ 188 ])
In the in vivo therapeutic efficacy evaluated against acute lung infection mice models, both γ3-PLGA-NPs@SFX/TAC and γ3-RBCNPs@CIP achieved significantly higher lung tissue accumulation and significantly reduced the bacterial load, inflammatory cytokines level, and inflammatory cells infiltration. As a result, they improved the mice survival rates compared to the peptide-unmodified nanosystems, proving the enhanced release at the inflamed lung tissues due to the ICAM-1 targeting. Both nanosystems have been extensively characterized and evaluated in vitro and in vivo. It should be noted that while the nanosystem developed by Yang group was assessed against both Gram-positive and Gram-negative bacteria ( S. aureus and P. aeruginosa ), the efficacy of the one by Liu et al. was studied only against Gram-negative bacteria (K. pneumoniae). On the other hand, Liu’s group proved the ability of their system to avoid macrophages’ phagocytosis, which we consider an essential feature that improves and prolongs the in vivo activity. Overall, we believe using peptides for surface modification of nanoparticles as targeting ligands to the overexpressed ICAM-1 at the inflammatory sites is a promising strategy for effective sepsis management with minimized systemic toxicities, as evidenced by these reports.
Exploring the same ICAM-1/integrin ligand-receptor pair, Shi and coworkers have also designed a peptide-modified nanosystem for inflammation targeting. However, their approach targeted the integrin receptor on the immune cells instead of the ICAM-1 ligand. They anchored the Arginine-Glycine-Aspartic Acid (RGD) peptide, a well-known integrin receptor ligand, on curcumin (Cur)-loaded liposomes (RGD-lipo/Cur) to achieve macrophages targeted Cur release against sepsis-induced inflammation [ 190 ]. While there was no significant increase in the uptake of RGD-unmodified liposomes after LPS-stimulation, RGD-lipo/Cur demonstrated a significantly higher uptake by LPS-stimulated RAW264.7 cells compared to the unstimulated cells. Also, the fluorescence microscope imaging showed colocalization of the RGD-lipo/Cur and the fluorescently labeled integrin receptors, indicating the liposomes' internalization to be through the RGD/integrin interaction. As a result, RGD-lipo/Cur showed a superior reduction in intracellular ROS levels and significantly inhibited the high inflammatory lytic programmed cell death (pyroptosis) of LPS-activated RAW264.7 cells compared to the peptide-unmodified liposomes. In vivo, RGD-lipo/Cur significantly reduced the release of inflammatory cytokines (TNF-α and IL-6) and prevented sepsis-induced organ damage in the LPS-induced mice sepsis model. Their findings showed the promising potential of RGD modification of Cur-loaded liposomes to improve sepsis management. However, they did not report ZP measurement, which we believe is a critical property in determining the storage and physiological stability of the nanosystem.
Another up-regulated protein during sepsis inflammatory conditions is dipeptidase 1 (DPEP1), expressed on cells of key organs such as the kidney, liver, and lung. Similar to ICAM-1, DPEP1 is an adhesion molecule for the recruitment of leukocytes [ 198 , 199 ]. Therefore, DPEP1 ligands can be utilized for nanoparticles surface modification to accomplish targeted drug release at the inflamed tissues during sepsis. In this respect, Yan and colleagues exploited Cys-LSA peptide (CLSALTPSPSWLKYKAL), a DPEP1 ligand, for surface coating of hollow mesoporous polydopamine nanocarrier (HMPDA) specifically targeting inflammation sites for the management of sepsis. As depicted in Fig. 11 , HMPDA was prepared by soft template method, loaded with NAD + and BAPTA-AM, and grafted with LSA peptide to give HMPDA@BA/NAD + @LSA NPs [ 191 ].
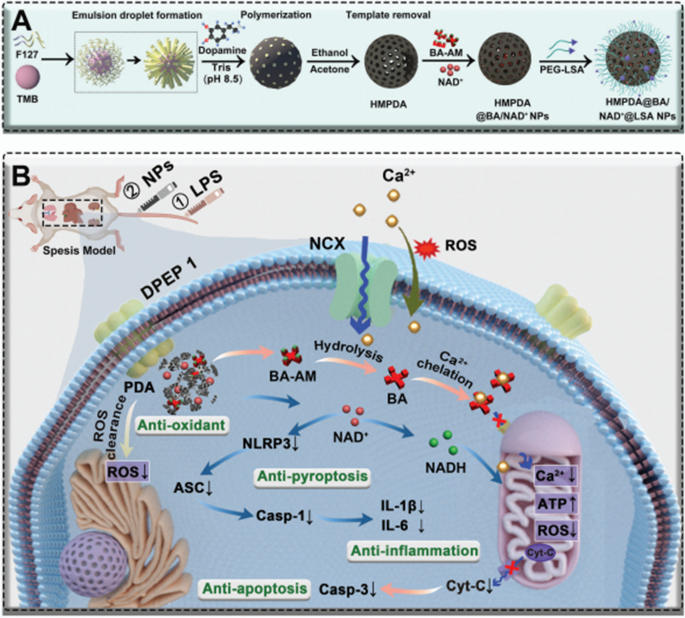
Preparation and mechanism of action of HMPDA@BA/NAD + @LSA NPs ( A ) Preparation of HMPDA@BA/NAD + @LSA NPs. B Pharmacological effects of HMPDA@BA/NAD + @LSA NPs in an LPS-induced sepsis mice model (Adopted from [ 191 ]
Their approach was based on a triple therapy to prevent sepsis-induced cells, tissues, and organs damage with NAD + restoring the energy production and exerting anti-inflammatory effect, BAPTA-AM chelating the overloaded intracellular Ca ++ and PDA scavenging the intracellular ROS. When evaluated in vitro using H 2 O 2 -stimulated liver and kidney cells, HMPDA@BA/NAD + @LSA NPs demonstrated superior ability to restore mitochondrial function, Ca ++ hemostasis, and antioxidant system and so rescued endangered cells. Studied in LPS-induced mice sepsis model, the accumulation of the peptide-modified nanoparticles in the key organs was markedly higher than that of healthy mice. Moreover, peptide modification significantly increased the accumulation of nanoparticles in the liver, kidney, and lung (1.4, 1.6, and 1.5 times) compared to the peptide-unmodified nanoparticles, proving the targeting of the overexpressed DPEP1 at the inflamed organs. As a result, HMPDA@BA/NAD + @LSA NPs significantly reduced the sepsis-induced key organs damage and improved the survival rate of mice. We argue that this multifunctional nanosystem with inflammation-targeted drug release is a promising nanomedicine to help combat sepsis’s multiple pathogenesis with less systemic drug exposure.
In conclusion, these studies underscore the potential of utilizing peptides for surface modification of nanoparticles, serving as selective targeting moieties for the up-regulated proteins at inflammation sites for sepsis management. However, more avenues are available to explore other up-regulated proteins using various peptide sequences.
Organ targeting peptides
Among the strategies for incorporating peptides as targeting moieties on nanosystems surface, a notable approach is targeting specific organs’ tissues and cells. Organ-specific peptides allow for accurate drug delivery to particular organs during sepsis, offering a mechanism to rescue key organs at high risk of damage and failure [ 192 , 200 ]. In this regard, Huang et al. engineered zeolite imidazolate framework-8 nanoparticles coated with renal tubular epithelial cell membrane and modified with a kidney targeting peptide (KCSAVPLC) to promote specific drug uptake by renal tubular cells. This nano-construct, denoted as KMZ@FGF21, was loaded with the antioxidant/anti-inflammatory hormone, fibroblast growth factor 21 (FGF21), against sepsis-induced acute kidney injury (AKI) [ 193 ]. Peptide modification significantly increased the nanoparticles uptake by murine renal tubular epithelial cells (TCMK-1) in vitro and decreased intracellular oxidative stress, inflammation, and apoptosis compared to the peptide-unmodified nanoparticles. Moreover, when injected into mice, the fluorescently labeled KMZ@FGF21 favorably accumulated in kidney tissues. When evaluated in a sepsis-induced AKI mice model, KMZ@FGF21 exhibited superior antioxidant and anti-inflammatory efficacy, alleviated AKI, and improved renal function recovery.
Utilizing a similar approach, Ouyang and coworkers designed hollow mesoporous silica nanoparticles (PCM-MSN@LA) loaded with L-arginine (LA) as nitric oxide (NO)-releasing agent and modified with primary cardiomyocytes specific peptide (PCM) for heart tissues-targeted delivery to combat LPS-induced cardiac injury [ 192 ]. As illustrated in Fig. 12 , cardiac targeting was further increased by applying low-intensity focused ultrasound (LIFU). PCM-MSN@LA were found to have significantly higher localization and affinity to cardiomyocytes compared to the hepatoblastoma cell line (HepG2) as a control, suggesting PCM's cardiac selectivity. Likewise, when evaluated in vivo, the fluorescently labeled PCM-MSN@LA showed 60-fold higher fluorescence intensity in the hearts of mice compared to PCM-unmodified nanoparticles. In contrast, the other organs (kidney, spleen, lung, and liver) showed markedly less nanoparticles distribution. Moreover, the fluorescence intensity of PCM-MSN@LA in mice’s hearts increased by 7-fold upon application of LIFU. In vitro, PCM-MSN@LA and PCM-MSN@LA + LIFU improved the cell viability of LPS-treated cardiomyocytes from 62 to 72% and 80%, respectively. Furthermore, PCM-MSN@LA combined with LIFU significantly reduced inflammatory cells recruitment, mitochondrial dysfunction, and oxidative stress in LPS-induced septic mice's cardiac tissues, thus prevented myocardial injury and cardiac dysfunction. We believe their strategy is promising and holds great potential for preventing sepsis-induced cardiac dysfunction in clinical settings.
Design of heart-targeting L-arginine loaded mesoporous silica nanoparticles (PCM-MSN@LA) and its combined application with low-intensity focused ultrasound (LIFU) to prevent cardiac damage in mice [ 192 ]
Ultimately, the findings of these two studies by Huang et al. and Ouyang et al. show the potential of organs targeting peptides as surface modifiers of nanosystems loaded with cytoprotective agents to prevent organ damage and dysfunction associated with sepsis. However, more avenues are available to explore targeting of other vulnerable organs such as liver, spleen, and lung.
Bacterial cells targeting peptides
Beyond targeting specific human body organs and inflammation sites, nanosystems can be tailored to selectively target the causative bacteria, addressing sepsis at the early levels of the pathogen invasion. It is well demonstrated that short peptides possessing cationic or amphiphilic properties can be adsorbed to the negatively charged bacterial membranes, specifically targeting bacterial cells and surmounting cellular barriers [ 201 , 202 , 203 ]. However, only one study by Lee et al. has been retrieved about using peptides for nanoparticles surface modification to improve bacterial cell affinity for sepsis management [ 194 ]. They utilized the chiral dipeptide D-/L-Cys-Phe (CF) for surface modification of gold nano-bipyramids (GBPs) to improve adsorption to and targeting of bacterial cells.
As depicted in Fig. 13 , D-/L-CF peptides gave the GBPs a spike shape (sea cucumber-like morphology) with higher binding affinity to protein A of the S. aureus cell membrane. Fluorescence imaging showed a higher overlapping of D-GBPs with the fluorescence of S. aureus compared to L-GBPs and DL-GBPs, indicating the higher adsorption and interaction of D-GBPs with bacterial cells. D-/L-GBPs demonstrated good photothermal properties and efficient absorption of near-infrared light irradiation (NIR), raising temperatures above the human body, suggesting they could damage the bacterial cell wall and achieve photothermal antibacterial treatments. Both D-GBPs and L-GBPs showed superior antibacterial activity against S. aureus compared to the peptide-unmodified GBPs, with increased activity upon NIR irradiation. Notably, the activity of D-GBPs was substantially higher compared to that of L-GBPs. Evaluated in vivo using S. aureus -induced mice sepsis model, D-/L-GBPs treatment showed significant reduction in organs’ bacterial counts and recovery of serum white blood cells levels and animal weights, with the D-GBPs + NIR group being the most effective. It is worth mentioning that their findings prove the strong potential of peptides for coating metallic nanoparticles to achieve bacterial targeting and better antibacterial efficacy. Moreover, their findings shed light on the difference in activity between D- and L-chiral isomers of peptides, which we believe is of significant importance when designing peptides for different purposes.
Preparation and characterization of D-/L-GBPs. A Coating of GBPs with D-/L-CF ( B ) TEM images of peptide-unmodified Au NBPs ( C ) TEM and ( D ) SEM images of D-/L-GBPs (adopted from ([ 194 ])
In summary, the studies highlighted in this section contribute valuable insights into applying peptides as targeting moieties modified on the surface of nanoparticles for effective sepsis management. Three biological targets have been utilized, viz. inflammation sites, specific organs, and bacterial cells. With inflammation sites targeting being the most studied strategy, more avenues are available to unlock the full potential of peptides as targeting motifs for nanoparticles against sepsis, especially bacterial cells targeting.
Peptides as nanocarriers for antisepsis agents
Peptides can be designed to have varied surface charges, solubility, and other physicochemical properties that allow them to form stable nanosystems with excellent encapsulation and cargo delivery efficacy [ 204 ]. Therefore, Peptides could be used as nanocarriers to improve the antisepsis drugs' stability, cellular uptake, and therapeutic outcomes. Besides, the nanocarrier peptides can be designed to have adjuvant pharmacological activity toward sepsis management, such as antimicrobial and anti-inflammatory efficacy. So far, only three studies [ 205 , 206 , 207 ] have been reported on using peptides as nanocarriers for antisepsis agents, highlighting the potential for further exploration to leverage these excellent biomaterials for enhanced and targeted drug delivery against bacterial sepsis. This section will discuss the application of peptides as nanocarriers for antisepsis agents based on the therapeutic effect of the loaded agent, whether it was an anti-inflammatory or antibacterial effect.
As for this, He and co-authors designed polypeptide-based hybrid nanoparticles (HNPs) encapsulating the anti-inflammatory TNF-α small interfering RNA (TNF-α siRNA) to provide endosomal escape and cellular internalization against hepatic sepsis [ 205 ]. HNPs were designed from a combination of the cationic helical polypeptide (PPABLG) and the anionic polypeptide (PAOBLG-MPA). The helical structure of PPABLG is supposed to form pores on the cell membrane and provide strong membrane permeability, enhancing the TNF-α siRNA internalization. This intracellular delivery of TNF-α siRNA was evaluated in vitro in LPS-stimulated murine macrophages (RAW 264.7) cells, and the HNPs were found to markedly increase the cellular uptake of TNF-α siRNA, achieving 90% inhibition of TNF-α production, unlike the free TNF-α siRNA which showed a negligible gene-silencing effect. Moreover, HNPs mediated endosomal escape and significantly reduced the colocalization of TNF-α siRNA with the fluorescently labeled endosomes/lysosomes compared to free TNF-α siRNA. When evaluated in an LPS/D-galactosamine (D-GalN)-mice model of hepatic sepsis, the free TNF-α siRNA degraded within 2 hours while the HNPs-encapsulated siRNA remained stable and attained higher accumulation in the macrophage-rich organs such as liver, lung, and spleen. Furthermore, compared to the free siRNA, the HNPs demonstrated a stronger anti-inflammatory efficacy and significantly downregulated TNF-α, rescuing the mice from hepatic sepsis.
While in the previous study, a peptide with cell penetration enhancing properties was used as a nanocarrier for the anti-inflammatory agent, Chen et al. employed an antimicrobial peptide nanogel as a nanocarrier for the anti-inflammatory agent, TNF-Related Apoptosis-Inducing Ligand (TRAIL), for effective sepsis management [ 206 ]. The bactericidal cationic poly(L-lysine)-block-poly(L-threonine) co-polypeptide (PLL-b-PLT) was crosslinked to nanogel encapsulating TRAIL protein to accomplish dual antibacterial and anti-inflammatory efficacy to combat bacterial sepsis. The optimized TRAIL nanogel was found to have a spherical shape with size, PDI, ZP, and EE% of 295.3 ± 5.7 nm, 0.21 ± 0.01, 18.5 ± 2.1 mV, and 98.5 ± 2.1%, respectively. Evaluated in MLE-12 (mouse lung epithelial cell) and Raw264.7 (macrophage) cells, TRAIL nanogel exhibited a good biosafety profile against normal cells. However, it showed superior cytotoxicity and apoptosis induction to LPS-activated cells compared to free TRAIL and blank nanogel. Furthermore, TEM analyses of TRAIL nanogel-treated K. pneumoniae revealed significant cell membrane destruction and loss of integrity. In vivo, TRAIL-encapsulated nanogel significantly reduced TNF-α and IL-6 levels, blood bacterial load, and pulmonary leukocytes accumulation; thus, it protected mice against K. pneumoniae- induced sepsis and LPS-induced lung and kidney injury and prolonged their survival rates. It is noteworthy that their dual therapeutic approach holds great potential in targeting both infecting bacteria and the inflammatory syndrome of sepsis.
In contrast to the other studies in which anti-inflammatory agents have been loaded in peptides-based nanocarriers, Z. Chen and coworkers have encapsulated the antibacterial antisense oligonucleotides (ASOs) in dendritic polypeptides nanoparticles coated with DSPE-mPEG2000 (DP-AD7) against multidrug-resistant bacterial infections and sepsis [ 207 ]. The high molecular weight and hydrophilicity of ASOs hinder them from cellular penetration. However, their encapsulation into cationic nanoparticles can provide a solution [ 208 ]. While the free ASO could not penetrate bacterial cells, DP-AD7 achieved 90% uptake by S. aureus , MRSA, E. coli , and extended-spectrum beta-lactamases producing (ESBLs)- E. coli . As a result, DP-AD7 significantly inhibited the in vitro growth and the expression of target genes in these bacteria. Going further, when evaluated in septic mice model of ESBLs- E. coli, DP-AD7 significantly improved the survival rate and decreased the organs’ bacterial load.
Notably, the findings of the studies in this section underscore peptides as promising nanocarriers for antisepsis agents, including both anti-inflammatory and antibacterial agents. However, none of the discussed studies reported the evaluation of in vitro drug release . We believe it would have been worthwhile to investigate the release profiles as they are critical for the clinical use of the nanosystems in terms of dosing frequency and therapeutic efficacy. Overall, peptides exhibit the potential to overcome the inherent limitations that hinder the clinical translation of antisepsis agents, such as improving their stability and cellular internalization.
Conclusion, challenges and future perspectives
Peptides have been effectively utilized in nanotools against sepsis and have shown enormous potential to improve its diagnosis and management. This review highlighted and critically discussed various reports on using peptides in nanotechnology against sepsis with the capacity to attain prompt diagnosis and efficient management. Based on the reported findings, peptides demonstrated specific and robust interactions with bacterial cell membranes and inflamed tissues, enhancing the bacterial recognition in blood for diagnosis, cell penetration for intracellular nanosystems-payload delivery, membrane disruption for effective bacterial killing, and inflammation-targeted and organ-specific release of nano-delivered antisepsis agents. In addition, engineering within nanosystems significantly overcame the limitation of bioactive peptides (AMPs and AIPs) and enhanced their stability, biosafety, and efficacy. Moreover, peptides have been effectively applied as nanocarriers for antisepsis agents, improving their effectiveness, reducing systemic toxicity, and improving management outcomes. The potentiality of the reviewed nanosystems has been investigated both in vitro and in vivo and found to be superior to that of free bioactive peptides and the peptides-unmodified nanosystems. Cumulatively, these findings reveal diverse and prominent roles peptides can undertake as active agents or excipients in nanosystems for fighting sepsis.
For sepsis diagnosis, it is apparent that peptides were predominantly used as pathogen recognition moieties conjugated to nanoplatforms, focusing mainly on Gram-positive bacteria identification. Thus, more research is recommended to explore using peptides to capture other virulent bacteria that significantly contribute to sepsis development, such as E. coli and K. pneumoniae [ 209 ]. Furthermore, the advancement of molecular sciences and increased understanding of sepsis pathophysiology continue to identify new biomarkers suited for sepsis diagnosis. Henceforth, further investigations to design peptides with high affinity to such biomarkers and engineering them within nanotools could significantly help to advance sepsis diagnosis techniques.
With respect to sepsis management, peptides have been mostly utilized as bioactive peptides that are nano-delivered through self-assembly, encapsulation, or conjugation to surfaces of nanoparticles. With self-assembly and nanoencapsulation being the prevalent explored strategies, linking bioactive peptides to the surface of metallic or organic nanoparticles awaits more investigations as a nano-delivery approach against sepsis. It is perceivable that the application of peptides as targeting moieties on nanosystems has been mostly directed to ICAM-1 targeting to achieve drug release at the inflamed tissues. However, with the development in identifying new proteins and receptors involved in sepsis pathogenesis, peptides could be more investigated to specifically target those proteins such as PAR-1, CD44, and TREM-1 [ 186 ], accomplishing improved management outcomes with less systemic toxicity. Moreover, peptides utilization as nanocarriers for antisepsis drugs was the least studied application in nanotechnology for sepsis management. This fosters opportunities for further utilization of these unique biomaterials to enhance antisepsis drug delivery and improve patients’ management outcomes. Finally, with the aid of molecular dynamics simulation and artificial intelligence, peptide sequences can be harnessed to have excellent and improved properties, such as having a stable nano-assembly and strong binding to biological targets and sepsis biomarkers, reducing the cost and accelerating the clinical translation to improve both diagnosis and management of sepsis.
Even with encouraging advancements in the utilization of peptides in sepsis diagnosis and management nanotools, the field is still emerging, and various challenges are encountering that hinder clinical translation. Primarily, these nanoplatforms' design and efficacy depend on the sepsis pathophysiology, which is very discrepant during different disease stages and from patient to patient. Moreover, all the discussed nanosystems have been evaluated in vitro or in vivo on mice models from which human sepsis pathogenesis, prognosis, and treatment response may vary significantly. Therefore, new sepsis models that mimic human responses and outcomes are of great importance in hastening the clinical adoption of the developed nanotools. Moreover, the fabrication of these nanoplatforms is complex, resulting in concerns regarding their reproducibility, scalability, stability, and cost-effectiveness. Additionally, the absence of official quality control guidance and recommendations for nanoplatforms characterization is further delaying their clinical utilization. Thus, optimization and comprehensive evaluation during development are critical to ensure reproducible and scalable manufacturing. Also, pharmacoeconomic and regulatory assessments are required to evaluate the cost-effectiveness of further clinical application of these nanosystems.
Even so, with the ongoing research and in-depth investigations, we envisage that the aforementioned challenges would be overcome and allow for clinical utilization of peptides-based nanosystems to enhance sepsis diagnosis and management practices, ultimately improving patient outcomes. Consequently, this review provides a foundation and comprehensive background for both academia and industry to advance and scale-up these nanoplatforms for efficient sepsis diagnosis and management. To bring it all together, peptides have shown promising potential as active principals and excipients in nanotools against sepsis, achieving rapid identification and on-time superior interventions to ensure better patient outcomes.
Availability of data and materials
Not applicable.
Romandini A, Pani A, Schenardi PA, Pattarino GAC, De Giacomo C, Scaglione F. Antibiotic resistance in pediatric infections: global emerging threats, predicting the near future. Antibiotics. 2021;10:1–12.
Article Google Scholar
WHO. Antibiotic resistance. https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance . Accessed on 08 Dec 2023.
Mohammed M, Devnarain N, Elhassan E, Govender T. Exploring the applications of hyaluronic acid-based nanoparticles for diagnosis and treatment of bacterial infections. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2022;14:1–29.
Karampela I, Fragkou PC. Future perspectives in the diagnosis and treatment of sepsis and septic shock. Medicina (B Aires). 2022;58:844.
Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395:200–11.
Article PubMed PubMed Central Google Scholar
Ansari S, Hays JP, Kemp A, Okechukwu R, Murugaiyan J, Ekwanzala MD, et al. The potential impact of the COVID-19 pandemic on global antimicrobial and biocide resistance: an AMR Insights global perspective. JAC-AMR. 2021;3:1–12.
Google Scholar
Singer M, Deutschman CS, Seymour C, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315:801–10.
Article CAS PubMed PubMed Central Google Scholar
Sahli C, Moya SE, Lomas JS, Gravier-pelletier C, Briandet R. Recent advances in nanotechnology for eradicating bacterial biofilm. Theranostics. 2022;12:2383–405.
Kumar S, Singh H, Feder-Kubis J, Nguyen DD. Recent advances in nanobiosensors for sustainable healthcare applications: a systematic literature review. Environ Res. 2023;238:117177.
Article CAS PubMed Google Scholar
Tian H, Zhang T, Qin S, Huang Z, Zhou L, Shi J, et al. Enhancing the therapeutic efficacy of nanoparticles for cancer treatment using versatile targeted strategies. J Hematol Oncol. 2022;15:132.
Ibrahim UH, Devnarain N, Govender T. Biomimetic strategies for enhancing synthesis and delivery of antibacterial nanosystems. Int J Pharm. 2021;596:120276.
Shimanovich U, Gedanken A. Nanotechnology solutions to restore antibiotic activity. J Mater Chem B. 2016;4:824–33.
Munir MU, Ahmad MM. Nanomaterials aiming to tackle antibiotic-resistant bacteria. Pharmaceutics. 2022;14:1–17.
Pant A, Mackraj I, Govender T. Advances in sepsis diagnosis and management: a paradigm shift towards nanotechnology. J Biomed Sci. 2021;28:1–30.
Tian S, Van Der Mei HC, Ren Y, Busscher HJ, Shi L. Recent advances and future challenges in the use of nanoparticles for the dispersal of infectious biofilms. J Mater Sci Technol. 2021;84:208–18.
Article CAS Google Scholar
Li T, Lu X-M, Zhang M-R, Hu K, Li Z. Peptide-based nanomaterials: self-assembly, properties and applications. Bioact Mater. 2022;11:268–82.
CAS PubMed Google Scholar
Ghosh D, Peng X, Leal J, Mohanty RP. Peptides as drug delivery vehicles across biological barriers. J Pharm Investig. 2018;48:89–111.
Komin A, Russell LM, Hristova KA, Searson PC. Peptide-based strategies for enhanced cell uptake, transcellular transport, and circulation: mechanisms and challenges. Adv Drug Deliv Rev. 2017;110–111:52–64.
Article PubMed Google Scholar
Papafilippou L, Claxton A, Dark P, Kostarelos K, Hadjidemetriou M. Nanotools for sepsis diagnosis and treatment. Adv Healthc Mater. 2021;10:1–25.
Nguyen AH, Shin Y, Sim SJ. Development of SERS substrate using phage-based magnetic template for triplex assay in sepsis diagnosis. Biosens Bioelectron. 2016;85:522–8.
Kuhlmann N, Nehls C, Heinbockel L, Correa W, Moll R, Gutsmann T, et al. Encapsulation and release of As pidasept peptides in polysaccharide formulation for oral application. Eur J Pharm Sci. 2021;158:105687.
Pan L, Jiang D, Pan L, Meng Z, Zhuang Y, Huang Y. ICAM-1-targeted and antibacterial peptide modified polymeric nanoparticles for specific combating sepsis. Mater Des. 2022;222:111007.
Zou P, Chen W-T, Sun T, Gao Y, Li L-L, Wang H. Recent advances: peptides and self-assembled peptide-nanosystems for antimicrobial therapy and diagnosis. Biomater Sci. 2020;8:4975–96.
Yang Z, He S, Wu H, Yin T, Wang L, Shan A. Nanostructured antimicrobial peptides: crucial steps of overcoming the bottleneck for clinics. Front Microbiol. 2021;12:1–28.
Spohn R, Daruka L, Lázár V, Martins A, Vidovics F, Grézal G, et al. Integrated evolutionary analysis reveals antimicrobial peptides with limited resistance. Nat Commun. 2019;10:1–13.
Son H, Park S-C, Kim Y, Lee J-K, Park S, Guk T, et al. Potent anti-inflammatory effects of a helix-to-helix peptide against pseudomonas aeruginosa endotoxin-mediated sepsis. Antibiotics. 2022;11:1675.
Sun Y, Shang D. Inhibitory effects of antimicrobial peptides on lipopolysaccharide-induced inflammation. Mediators Inflamm. 2015;2015:1–8.
Lee JK, Seo CH, Luchian T, Park Y. Antimicrobial peptide CMA3 derived from the CA-MA hybrid peptide: Antibacterial and anti-inflammatory activities with low cytotoxicity and mechanism of action in Escherichia coli. Antimicrob Agents Chemother. 2016;60:495–506.
Zhang Z, Datta G, Zhang Y, Miller AP, Mochon P, Chen YF, et al. Apolipoprotein A-I mimetic peptide treatment inhibits inflammatory responses and improves survival in septic rats. Am J Physiol - Hear Circ Physiol. 2009;297:866–73.
Cheng N, Zhang Y, Delaney MK, Wang C, Bai Y, Skidgel RA, et al. Targeting Gα13-integrin interaction ameliorates systemic inflammation. Nat Commun. 2021;12:1–14.
Radaic A, de Jesus MB, Kapila YL. Bacterial anti-microbial peptides and nano-sized drug delivery systems: the state of the art toward improved bacteriocins. J Control Release. 2020;321:100–18.
Gazit E. Self-assembled peptide nanostructures: The design of molecular building blocks and their technological utilization. Chem Soc Rev. 2007;36:1263–9.
Mandal D, et al. Self-assembly of peptides to nanostructures. Org Biomol Chem. 2014;12:3544–61.
Eskandari S, Guerin T, Toth I, Stephenson RJ. Recent advances in self-assembled peptides: Implications for targeted drug delivery and vaccine engineering. Adv Drug Deliv Rev. 2017;110–111:169–87.
Vedadghavami A, Zhang C, Bajpayee AG. Overcoming negatively charged tissue barriers: drug delivery using cationic peptides and proteins. Nano Today. 2020;34:100898.
Yang B, Wang TT, Yang YS, Zhu HL, Li JH. The application progress of peptides in drug delivery systems in the past decade. J Drug Deliv Sci Technol. 2021;66:102880.
Tian Y, Zhou S. Advances in cell penetrating peptides and their functionalization of polymeric nanoplatforms for drug delivery. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2021;13:1–12.
Zhang Z, Ai S, Yang Z, Li X. Peptide-based supramolecular hydrogels for local drug delivery q. Adv Drug Deliv Rev. 2021;174:482–503.
Rayatpour A, Javan M. Targeting the brain lesions using peptides: a review focused on the possibility of targeted drug delivery to multiple sclerosis lesions. Pharmacol Res. 2021;167:105441.
Jeong WJ, Bu J, Kubiatowicz LJ, Chen SS, Kim YS, Hong S. Peptide–nanoparticle conjugates: a next generation of diagnostic and therapeutic platforms? Nano Converg. 2018;5:1–18.
Mehrotra N, Kharbanda S, Singh H. Peptide-based combination nanoformulations for cancer therapy. Nanomedicine. 2020;15:2201–17.
Nahhas AF, Nahhas AF, Webster TJ. Nanoscale pathogens treated with nanomaterial-like peptides: a platform technology appropriate for future pandemics. Nanomedicine. 2021;16:1237–54.
Santos RS, Figueiredo C, Azevedo NF, Braeckmans K, De Smedt SC. Nanomaterials and molecular transporters to overcome the bacterial envelope barrier: towards advanced delivery of antibiotics. Adv Drug Deliv Rev. 2018;136–137:28–48.
Osman N, Devnarain N, Omolo CA, Fasiku V, Jaglal Y, Govender T. Surface modification of nano-drug delivery systems for enhancing antibiotic delivery and activity. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2022;14:1–24.
Teixeira MC, Carbone C, Sousa MC, Espina M, Garcia ML, Sanchez-Lopez E, et al. Nanomedicines for the Delivery of Antimicrobial Peptides (AMPs). Nanomaterials. 2020;10:560.
Biswaro LS, Sousa MG d. C, Rezende TMB, Dias SC, Franco OL. Antimicrobial peptides and nanotechnology, recent advances and challenges. Front Microbiol. 2018;9:1–14.
Faya M, Kalhapure RS, Kumalo HM, Waddad AY, Omolo C, Govender T. Conjugates and nano-delivery of antimicrobial peptides for enhancing therapeutic activity. J Drug Deliv Sci Technol. 2018;44:153–71.
Urban P, Jose Valle-Delgado J, Moles E, Marques J, Diez C, Fernandez-Busquets X. Nanotools for the delivery of antimicrobial peptides. Curr Drug Targets. 2012;13:1158–72.
Carratalá JV, Serna N, Villaverde A, Vázquez E, Ferrer-Miralles N. Nanostructured antimicrobial peptides: The last push towards clinics. Biotechnol Adv. 2020;44.
Claxton A, Papafilippou L, Hadjidemetriou M, Kostarelos K, Dark P. The challenge of recognising sepsis: Future nanotechnology solutions. J Intensive Care Soc. 2020;21:241–6.
Sadikot RT. The potential role of nano- and micro-technology in the management of critical illnesses. Adv Drug Deliv Rev. 2014;77:27–31.
Rhee C, Klompas M. Elucidating the spectrum of disease severity encompassed by sepsis. JAMA Netw Open. 2022;5:e2147888.
Jacobi J. The pathophysiology of sepsis - 2021 update: part 2, organ dysfunction and assessment. Am J Health Syst Pharm. 2022;79:424–36.
Delano MJ, Ward PA. The immune system’s role in sepsis progression, resolution, and long-term outcome. Immunol Rev. 2016;274:330–53.
Arora J, Mendelson AA, Fox-Robichaud A. Sepsis: network pathophysiology and implications for early diagnosis. Am J Physiol - Regul Integr Comp Physiol. 2023;324:R613-24.
Cao C, Yu M, Chai Y. Pathological alteration and therapeutic implications of sepsis-induced immune cell apoptosis. Cell Death Dis. 2019;10:1–14.
Pons S, Arnaud M, Loiselle M, Arrii E, Azoulay E, Zafrani L. Immune consequences of endothelial cells’ activation and dysfunction during sepsis. Crit Care Clin. 2020;36:401–13.
Neubauer K, Zieger B. Endothelial cells and coagulation. Cell Tissue Res. 2022;387:391–8.
Tamayo E, Fernández A, Almansa R, Carrasco E, Heredia M, Lajo C, et al. Pro- and anti-inflammatory responses are regulated simultaneously from the first moments of septic shock. Eur Cytokine Netw. 2011;22:82–7.
Choi H, Kim Y, Mirzaaghasi A, Heo J, Kim YN, Shin JH, et al. Exosome-based delivery of super-repressor IκBα relieves sepsis-associated organ damage and mortality. Sci Adv. 2020;6:1–9.
Rossaint J, Zarbock A. Pathogenesis of multiple organ failure in sepsis. Crit Rev Immunol. 2015;35:277–91.
Ryan TAJ, Preston RJS, O’Neill LAJ. Immunothrombosis and the molecular control of tissue factor by pyroptosis: prospects for new anticoagulants. Biochem J. 2022;479:731–50.
Kawaguchi S, Okada M. Cardiac metabolism in sepsis. Metabolites. 2021;11:846.
Matthay MA, Zemans RL, Zimmerman GA, Arabi YM, Beitler JR, Mercat A, et al. Acute respiratory distress syndrome. Nat Rev Dis Prim. 2019;5:18.
Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800.
Peerapornratana S, Manrique-Caballero CL, Gómez H, Kellum JA. Acute kidney injury from sepsis: current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. 2019;96:1083–99.
Gotts JE, Matthay MA. Sepsis: pathophysiology and clinical management. BMJ. 2016;353:i1585.
Ziaja M. Septic encephalopathy. Curr Neurol Neurosci Rep. 2013;13:383.
Gyawali B, Ramakrishna K, Dhamoon AS. Sepsis: The evolution in definition, pathophysiology, and management. SAGE Open Med. 2019;7:205031211983504.
Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13:862–74.
Krautz C, Maier SL, Brunner M, Langheinrich M, Giamarellos-Bourboulis EJ, Gogos C, et al. Reduced circulating B cells and plasma IgM levels are associated with decreased survival in sepsis - A meta-analysis. J Crit Care. 2018;45:71–5.
Luan YY, Yao YM, Xiao XZ, Sheng ZY. Insights into the apoptotic death of immune cells in sepsis. J Interf Cytokine Res. 2015;35:17–22.
Arina P, Singer M. Pathophysiology of sepsis. Curr Opin Anaesthesiol. 2021;34:77–84.
Drewry A, Samra N, Skrupky L, Fuller B, Compton S, Hotchkiss R. Persistent lymphopenia after diagnosis of sepsis predicts mortality. Shock. 2014;42:383–91.
Jarczak D, Kluge S, Nierhaus A. Sepsis—pathophysiology and therapeutic concepts. Front Med. 2021;8:1–22.
Chousterman BG, Swirski FK, Weber GF. Cytokine storm and sepsis disease pathogenesis. Semin Immunopathol. 2017;39:517–28.
Hotchkiss RS, Moldawer LL, Opal SM, Reinhart K, Turnbull IR, Vincent J-L. Sepsis and septic shock. Nat Rev Dis Prim. 2016;2:16045.
Kim HIL, Park S. Sepsis: early recognition and optimized treatment. Tuberc Respir Dis. 2019;82:6.
Durkin TJ, Barua B, Savagatrup S. Rapid detection of sepsis: recent advances in biomarker sensing platforms. ACS Omega. 2021;6:31390–5.
Lim J, Lee YY, Choy Y Bin, Park W, Park CG. Sepsis diagnosis and treatment using nanomaterials. Biomed Eng Lett. 2021:197–210.
Mancuso G, Midiri A, Gerace E, Biondo C. Bacterial antibiotic resistance: the most critical pathogens. Pathogens. 2021;10:1310.
Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47:1181–247.
Herrmann IK, Bertazzo S, O’Callaghan DJP, Schlegel AA, Kallepitis C, Antcliffe DB, et al. Differentiating sepsis from non-infectious systemic inflammation based on microvesicle-bacteria aggregation. Nanoscale. 2015;7:13511–20.
Kollef MH, Shorr AF, Bassetti M, Timsit J-F, Micek ST, Michelson AP, et al. Timing of antibiotic therapy in the ICU. Crit Care. 2021;25:360.
Rello J, Valenzuela-Sánchez F, Ruiz-Rodriguez M, Moyano S. Sepsis: a review of advances in management. Adv Ther. 2017;34:2393–411.
Ulloa L, Brunner M, Ramos L, Deitch E. Scientific and clinical challenges in sepsis. Curr Pharm Des. 2009;15:1918–35.
Fink MP, Warren HS. Strategies to improve drug development for sepsis. Nat Rev Drug Discov. 2014;13:741–58.
Huang M, Cai S, Su J. The pathogenesis of sepsis and potential therapeutic targets. Int J Mol Sci. 2019;20:5376.
Nedeva C, Menassa J, Puthalakath H. Sepsis: inflammation is a necessary evil. Front Cell Dev Biol. 2019;7:1–12.
Shukla P, Rao GM, Pandey G, Sharma S, Mittapelly N, Shegokar R, et al. Therapeutic interventions in sepsis: current and anticipated pharmacological agents. Br J Pharmacol. 2014;171:5011–31.
Qiu P, Cui X, Sun J, Welsh J, Natanson C, Eichacker PQ. Antitumor necrosis factor therapy is associated with improved survival in clinical sepsis trials. Crit Care Med. 2013;41:2419–29.
Grimaldi D, Turcott EWG, Taccone FS. IL-1 receptor antagonist in sepsis: new findings with old data? J Thorac Dis. 2016;8:2379–82.
Simmons J, Pittet J-F. The coagulopathy of acute sepsis. Curr Opin Anaesthesiol. 2015;28:227–36.
Levi M, Schultz M, Van Der Poll T. Sepsis and thrombosis. Semin Thromb Hemost. 2013;39:559–66.
Montinari MR, Minelli S. From ancient leech to direct thrombin inhibitors and beyond: new from old. Biomed Pharmacother. 2022;149:112878.
Dolmatova EV, Wang K, Mandavilli R, Griendling KK. The effects of sepsis on endothelium and clinical implications. Cardiovasc Res. 2021;117:60–73.
Chang JC. Sepsis and septic shock: endothelial molecular pathogenesis associated with vascular microthrombotic disease. Thromb J. 2019;17:1–19.
Singh J, Lee Y, Kellum JA. A new perspective on NO pathway in sepsis and ADMA lowering as a potential therapeutic approach. Crit Care. 2022;26:1–8.
Lambden S. Bench to bedside review: therapeutic modulation of nitric oxide in sepsis—an update. Intensive Care Med Exp. 2019;7:64.
Radenković M, Stojanović M, Potpara T, Prostran M. Therapeutic approach in the improvement of endothelial dysfunction: the current state of the art. Biomed Res Int. 2013;2013.
Dobesh PP, Swahn SM, Peterson EJ, Olsen KM. Statins in sepsis. J Pharm Pract. 2010;23:38–49.
Mathiesen A, Hamilton T, Carter N, Brown M, McPheat W, Dobrian A. Endothelial extracellular vesicles: from keepers of health to messengers of disease. Int J Mol Sci. 2021;22:4640.
Rodrigues PRS, Picco N, Morgan BP, Ghazal P. Sepsis target validation for repurposing and combining complement and immune checkpoint inhibition therapeutics. Expert Opin Drug Discov. 2021;16:537–51.
Charchaflieh J, Wei J, Labaze G, Hou YJ, Babarsh B, Stutz H, et al. The role of complement system in septic shock. Clin Dev Immunol. 2012;2012.
Ward PA, Guo R-F, Riedemann NC. Manipulation of the complement system for benefit in sepsis. Crit Care Res Pract. 2012;2012:1–8.
Lupu F, Keshari RS, Lambris JD, Coggeshall KM. Crosstalk between the coagulation and complement systems in sepsis. Thromb Res. 2014;133:S28-31.
Koelman DLH, Brouwer MC, Van De Beek D. Targeting the complement system in bacterial meningitis. Brain. 2019;142:3325–37.
Seeley EJ, Bernard GR. Therapeutic targets in sepsis: past, present, and future. Clin Chest Med. 2016;37:181–9.
Ramachandran G. Gram-positive and gram-negative bacterial toxins in sepsis. Virulence. 2014;5:213–8.
Munguia J, Nizet V. Pharmacological targeting of the host-pathogen interaction: alternatives to classical antibiotics to combat drug-resistant superbugs. Trends Pharmacol Sci. 2017;38:473–88.
Gopalan PD. Pathophysiology of sepsis. South African J Anaesth Analg. 2022;28:S23-9.
Soni M, Handa M, Singh KK, Shukla R. Recent nanoengineered diagnostic and therapeutic advancements in management of Sepsis. J Control Release. 2022;352:931–45.
Pandompatam G, Kashani K, Vallabhajosyula S. The role of natriuretic peptides in the management, outcomes and prognosis of sepsis and septic shock. Rev Bras Ter Intensiva. 2019;31:368–78.
Martin L, van Meegern A, Doemming S, Schuerholz T. Antimicrobial peptides in human sepsis. Front Immunol. 2015;6:1–7.
Heh E, Allen J, Ramirez F, Lovasz D, Fernandez L, Hogg T, et al. Peptide drug conjugates and their role in cancer therapy. Int J Mol Sci. 2023;24:829.
Wang L, Wang N, Zhang W, Cheng X, Yan Z, Shao G, et al. Therapeutic peptides: current applications and future directions. Signal Transduct Target Ther. 2022;7:48.
Lin L, Chi J, Yan Y, Luo R, Feng X, Zheng Y, et al. Membrane-disruptive peptides/peptidomimetics-based therapeutics: Promising systems to combat bacteria and cancer in the drug-resistant era. Acta Pharm Sin B. 2021;11:2609–44.
Friedrich B, Lyer S, Janko C, Unterweger H, Brox R, Cunningham S, et al. Scavenging of bacteria or bacterial products by magnetic particles functionalized with a broad-spectrum pathogen recognition receptor motif offers diagnostic and therapeutic applications. Acta Biomater. 2022;141:418–28.
Rizzetto G, Gambini D, Maurizi A, Candelora M, Molinelli E, Cirioni O, et al. Our Experience over 20 years: antimicrobial peptides against gram positives, gram negatives, and fungi. Pharmaceutics. 2023;15:1–18.
Kalle M, Papareddy P, Kasetty G, Mörgelin M, van der Plas MJA, Rydengård V, et al. Host defense peptides of thrombin modulate inflammation and coagulation in endotoxin-mediated shock and pseudomonas aeruginosa sepsis. Benarafa C, editor. PLoS One. 2012;7:e51313.
Aluri S, Janib SM, Mackay JA. Environmentally responsive peptides as anticancer drug carriers. Adv Drug Deliv Rev. 2009;61:940–52.
Kharga K, Kumar L, Patel SKS. Recent advances in monoclonal antibody-based approaches in the management of bacterial sepsis. Biomedicines. 2023;11:765.
Feng X, Meng X, Xiao F, Aguilar ZP, Xu H. Vancomycin-dendrimer based multivalent magnetic separation nanoplatforms combined with multiplex quantitative PCR assay for detecting pathogenic bacteria in human blood. Talanta. 2021;225:121953.
Pan F, Altenried S, Scheibler S, Rodriguez Fernandez I, Giovannini G, Ren Q. Ultrafast determination of antimicrobial resistant staphylococcus aureus specifically captured by functionalized magnetic nanoclusters. ACS Sensors. 2022;7:3491–500.
Shrivastava S, Arya R, Kim KK, Lee NE. A quorum-based fluorescent probe for imaging pathogenic bacteria. J Mater Chem B. 2022;10:4491–500.
Afshari A, Schrenzel J, Ieven M, Harbarth S. Bench-to-bedside review: rapid molecular diagnostics for bloodstream infection - a new frontier? Crit Care. 2012;16.
Tjandra KC, Ram-Mohan N, Abe R, Hashemi MM, Lee J-H, Chin SM, et al. Diagnosis of bloodstream infections: an evolution of technologies towards accurate and rapid identification and antibiotic susceptibility testing. Antibiotics. 2022;11:511.
Neely LA, Audeh M, Phung NA, Min M, Suchocki A, Plourde D, et al. T2 magnetic resonance enables nanoparticle-mediated rapid detection of candidemia in whole blood. Sci Transl Med. 2013;5:1–8.
Lv D, Jiao H, Dong J, Sheng L, Liu J, Dong H, et al. Biomimetic octopus-like particles for ultraspecific capture and detection of pathogens. ACS Appl Mater Interfaces. 2019;11:22164–70.
Rao SS, Mohan KVK, Gao Y, Atreya CD. Identification and evaluation of a novel peptide binding to the cell surface of Staphylococcus aureus. Microbiol Res. 2013;168:106–12.
Dulińska-Litewka J, Łazarczyk A, Hałubiec P, Szafrański O, Karnas K, Karewicz A. Superparamagnetic iron oxide nanoparticles-current and prospective medical applications. Materials (Basel). 2019;12:617.
Sabale S, Kandesar P, Jadhav V, Komorek R, Motkuri RK, Yu XY. Recent developments in the synthesis, properties, and biomedical applications of core/shell superparamagnetic iron oxide nanoparticles with gold. Biomater Sci. 2017;5:2212–25.
Modh H, Scheper T, Walter J-G. Aptamer-modified magnetic beads in biosensing. Sensors. 2018;18:1041.
Wang F, Zhou H, Olademehin OP, Kim SJ, Tao P. Insights into key interactions between vancomycin and bacterial cell wall structures. ACS Omega. 2018;3:37–45.
Abdel Aziz OA, Arafa K, Abo Dena AS, El-sherbiny IM. Superparamagnetic Iron Oxide Nanoparticles (SPIONs): preparation and recent applications. J Nanotechnol Adv Mater. 2020;8:21–9.
Dadfar SM, Roemhild K, Drude NI, von Stillfried S, Knüchel R, Kiessling F, et al. Iron oxide nanoparticles: diagnostic, therapeutic and theranostic applications. Adv Drug Deliv Rev. 2019;138:302–25.
Leito JTD, Ligtenberg AJM, Nazmi K, De Blieck-Hogervorst JMA, Veerman ECI, Nieuw Amerongen AV. A common binding motif for various bacteria of the bacteria-binding peptide SRCRP2 of DMBT1/gp-340/salivary agglutinin. Biol Chem. 2008;389:1193–200.
Setia A, Mehata AK, Vikas, Malik AK, Viswanadh MK, Muthu MS. Theranostic magnetic nanoparticles: synthesis, properties, toxicity, and emerging trends for biomedical applications. J Drug Deliv Sci Technol. 2023;81:104295.
Ordonez AA, Sellmyer MA, Gowrishankar G, Ruiz-Bedoya CA, Tucker EW, Palestro CJ, et al. Molecular imaging of bacterial infections: overcoming the barriers to clinical translation. Sci Transl Med. 2019;11:139–48.
LaSarre B, Federle MJ. Exploiting quorum sensing to confuse bacterial pathogens. Microbiol Mol Biol Rev. 2013;77:73–111.
Zhang Q, Song B, Xu Y, Yang Y, Ji J, Cao W, et al. In vivo bioluminescence imaging of natural bacteria within deep tissues via ATP-binding cassette sugar transporter. Nat Commun. 2023;14:2331.
Funari R, Shen AQ. Detection and characterization of bacterial biofilms and biofilm-based sensors. ACS Sensors. 2022;7:347–57.
Cialla D, März A, Böhme R, Theil F, Weber K, Schmitt M, et al. Surface-enhanced Raman spectroscopy (SERS): Progress and trends. Anal Bioanal Chem. 2012;403:27–54.
Xu K, Zhou R, Takei K, Hong M. Toward Flexible Surface-Enhanced Raman Scattering (SERS) sensors for point-of-care diagnostics. Adv Sci. 2019;6:1900925.
Andreiuk B, Nicolson F, Clark LM, Panikkanvalappil SR, Kenry, Rashidian M, et al. Design and synthesis of gold nanostars-based SERS nanotags for bioimaging applications. Nanotheranostics. 2022;6:10–30.
Mousavi SM, Hashemi SA, Rahmanian V, Kalashgrani MY, Gholami A, Omidifar N, et al. Highly sensitive flexible SERS-based sensing platform for detection of COVID-19. Biosensors. 2022;12:466.
Yáñez-Sedeño P, Campuzano S, Pingarrón J. Multiplexed electrochemical immunosensors for clinical biomarkers. Sensors. 2017;17:965.
Blaik RA, Lan E, Huang Y, Dunn B. Gold-coated M13 bacteriophage as a template for glucose oxidase biofuel cells with direct electron transfer. ACS Nano. 2016;10:324–32.
Paczesny J, Bielec K. Application of bacteriophages in nanotechnology. Nanomaterials. 2020;10:1–25.
Walsh TJ, Bright RA, Ahuja A, McCarthy MW, Marfuggi RA, Simpson SQ. Meeting the challenges of sepsis in severe coronavirus disease 2019: a call to arms. Open Forum Infect Dis. 2023;10:1–7.
Liu M, Fang X, Yang Y, Wang C. Peptide-enabled targeted delivery systems for therapeutic applications. Front Bioeng Biotechnol. 2021;9:1–15.
Huan Y, Kong Q, Mou H, Yi H. Antimicrobial peptides: classification, design, application and research progress in multiple fields. Front Microbiol. 2020;11:1–21.
Lee SH, Jun HK, Lee HR, Chung CP, Choi BK. Antibacterial and lipopolysaccharide (LPS)-neutralising activity of human cationic antimicrobial peptides against periodontopathogens. Int J Antimicrob Agents. 2010;35:138–45.
Scocchi M, Mardirossian M, Runti G, Benincasa M. Non-membrane permeabilizing modes of action of antimicrobial peptides on bacteria. Curr Top Med Chem. 2015;16:76–88.
Elibe Mba I, Innocent Nweze E. Antimicrobial peptides therapy: an emerging alternative for treating drug-resistant bacteria. Yale J Biol Med. 2022;95:445–63.
Wang C, Hong T, Cui P, Wang J, Xia J. Antimicrobial peptides towards clinical application: delivery and formulation. Adv Drug Deliv Rev. 2021;175:113818.
Fadaka AO, Sibuyi NRS, Madiehe AM, Meyer M. Nanotechnology-based delivery systems for antimicrobial peptides. Pharmaceutics. 2021;13:1795.
Lei R, Hou J, Chen Q, Yuan W, Cheng B, Sun Y, et al. Self-assembling myristoylated human α-defensin 5 as a next-generation nanobiotics potentiates therapeutic efficacy in bacterial infection. ACS Nano. 2018;12:5284–96.
Tan P, Tang Q, Xu S, Zhang Y, Fu H, Ma X. Designing self-assembling chimeric peptide nanoparticles with high stability for combating piglet bacterial infections. Adv Sci. 2022;9:1–16.
Meng Z, Pan L, Qian S, Yang X, Pan L, Chi R, et al. Antimicrobial peptide nanoparticles coated with macrophage cell membrane for targeted antimicrobial therapy of sepsis. Mater Des. 2023;229:111883.
Franco OL, Saude A, Ombredani A, Silva O, Barbosa J, Moreno S, et al. Clavanin bacterial sepsis control using a novel methacrylate nanocarrier. Int J Nanomed. 2014;9:5055.
Hassan A, Ikram A, Raza A, Saeed S, Paracha RZ, Younas Z, et al. Therapeutic potential of novel mastoparan-chitosan nanoconstructs against clinical MDR Acinetobacter baumannii: In silico, in vitro and in vivo Studies. Int J Nanomedicine. 2021;16:3755–73.
Hou X, Zhang X, Zhao W, Zeng C, Deng B, McComb DW, et al. Vitamin lipid nanoparticles enable adoptive macrophage transfer for the treatment of multidrug-resistant bacterial sepsis. Nat Nanotechnol. 2020;15:41–6.
Rai A, Pinto S, Velho TR, Ferreira AF, Moita C, Trivedi U, et al. One-step synthesis of high-density peptide-conjugated gold nanoparticles with antimicrobial efficacy in a systemic infection model. Biomaterials. 2016;85:99–110.
Fan X, Fan J, Wang X, Wu P, Wu G. S-thanatin functionalized liposome potentially targeting on Klebsiella pneumoniae and its application in sepsis mouse model. Front Pharmacol. 2015;6:1–12.
Lee, Trinh, Yoo, Shin, Lee, Kim, et al. Self-Assembling Peptides and Their Application in the Treatment of Diseases. Int J Mol Sci. 2019;20:5850.
Lombardi L, Falanga A, Del Genio V, Galdiero S. A new hope: self-assembling peptides with antimicrobial activity. Pharmaceutics. 2019;11:166.
Lee WJ, Cha S, Shin M, Islam MA, Cho CS, Yoo HS. Induction of Th1 polarized immune responses by thiolated Eudragit-coated F4 and F18 fimbriae of enterotoxigenic Escherichia coli. Eur J Pharm Biopharm. 2011;79:226–31.
Zhang N, Xiong G, Liu Z. Toxicity of metal-based nanoparticles: challenges in the nano era. Front Bioeng Biotechnol. 2022;10:1–16.
Zhao Y, Pu M, Zhang J, Wang Y, Yan X, Yu L, et al. Recent advancements of nanomaterial-based therapeutic strategies toward sepsis: bacterial eradication, anti-inflammation, and immunomodulation. Nanoscale. 2021;13:10726–47.
Mas-Moruno C, Cascales L, Cruz LJ, Mora P, Pérez-Payá E, Albericio F. Nanostructure formation enhances the activity of LPS-Neutralizing peptides. ChemMedChem. 2008;3:1748–55.
Piktel E, Wnorowska U, Cieśluk M, Deptula P, Pogoda K, Misztalewska-Turkowicz I, et al. Inhibition of inflammatory response in human keratinocytes by magnetic nanoparticles functionalized with PBP10 peptide derived from the PIP2-binding site of human plasma gelsolin. J Nanobiotechnology. 2019;17:1–19.
Tram NDT, Tran QTN, Xu J, Su JCT, Liao W, Wong WSF, et al. Multifunctional antibacterial nanonets attenuate inflammatory responses through selective trapping of endotoxins and pro-inflammatory cytokines. Adv Healthc Mater. 2023;12:1–7.
Wei B, Ma Y. Synergistic effect of GF9 and streptomycin on relieving gram-negative bacteria-induced sepsis. Front Bioeng Biotechnol. 2022;10:1–10.
Lee W, Seo J, Kwak S, Park EJ, Na DH, Kim S, et al. A Double-Chambered Protein Nanocage Loaded with Thrombin Receptor Agonist Peptide (TRAP) and γ-Carboxyglutamic Acid of Protein C (PC-Gla) for Sepsis Treatment. Adv Mater. 2015;27:6637–43.
Sadikot RT, Rubinstein I. Long-acting, multi-targeted nanomedicine: Addressing unmet medical need in acute lung injury. J Biomed Nanotechnol. 2009;5:614–9.
Novoselova EG, Lunin SM, Glushkova OV, Khrenov MO, Parfenyuk SB, Zakharova NM, et al. Thymulin, free or bound to PBCA nanoparticles, protects mice against chronic septic inflammation. PLoS One. 2018;13:1–21.
Karawacka W, Janko C, Unterweger H, Mühlberger M, Lyer S, Taccardi N, et al. SPIONs functionalized with small peptides for binding of lipopolysaccharide, a pathophysiologically relevant microbial product. Colloids Surfaces B Biointerfaces. 2019;174:95–102.
Zhang C, Zhang X, Zhao G. Ferritin nanocage: a versatile nanocarrier utilized in the field of food, nutrition, and medicine. Nanomaterials. 2020;10:1–25.
Devnarain N, Osman N, Fasiku VO, Makhathini S, Salih M, Ibrahim UH, et al. Intrinsic stimuli-responsive nanocarriers for smart drug delivery of antibacterial agents—An in-depth review of the last two decades. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2021;13:1–38.
Liu D, Jin F, Shu G, Xu X, Qi J, Kang X, et al. Enhanced efficiency of mitochondria-targeted peptide SS-31 for acute kidney injury by pH-responsive and AKI-kidney targeted nanopolyplexes. Biomaterials. 2019;211:57–67.
Kocbek P, Obermajer N, Cegnar M, Kos J, Kristl J. Targeting cancer cells using PLGA nanoparticles surface modified with monoclonal antibody. J Control Release. 2007;120:18–26.
Rosi NL, Giljohann DA, Thaxton CS, Lytton-Jean AKR, Han MS, Mirkin CA. Oligonucleotide-modified gold nanoparticles for intracellular gene regulation. Science. 2006;312:1027–30.
Muthiah M, Park IK, Cho CS. Surface modification of iron oxide nanoparticles by biocompatible polymers for tissue imaging and targeting. Biotechnol Adv. 2013;31:1224–36.
Pang L, Pei Y, Uzunalli G, Hyun H, Lyle LT, Yeo Y. Surface modification of polymeric nanoparticles with M2pep peptide for drug delivery to tumor-associated macrophages. Pharm Res. 2019;36:4–15.
d’Arcy R, Tirelli N. Fishing for fire: strategies for biological targeting and criteria for material design in anti-inflammatory therapies. Polym Adv Technol. 2014;25:478–98.
Zhang N, Chittasupho C, Duangrat C, Siahaan TJ, Berkland C. PLGA nanoparticle-peptide conjugate effectively targets intercellular cell-adhesion molecule-1. Bioconjug Chem. 2008;19:145–52.
Yang Y, Ding Y, Fan B, Wang Y, Mao Z, Wang W, et al. Inflammation-targeting polymeric nanoparticles deliver sparfloxacin and tacrolimus for combating acute lung sepsis. J Control Release. 2020;321:463–74.
Liu J, Ding H, Zhao M, Tu F, He T, Zhang L, et al. Functionalized erythrocyte membrane-coated nanoparticles for the treatment of klebsiella pneumoniae-induced sepsis. Front Microbiol. 2022;13:1–12.
Shi Y, Wu Q, Lu Y, Meng L-P, Xu X-L, Wang X-J, et al. Arginine-Glycine-Aspartic Acid-anchored Curcumin-based Nanotherapeutics Inhibit Pyroptosis-induced Cytokine Release Syndrome for In Vivo and In Vitro Sepsis Applications. Curr Pharm Des. 2023;29:283–94.
Yan J, Zhang J, Wang Y, Liu H, Sun X, Li A, et al. Rapidly inhibiting the inflammatory cytokine storms and restoring cellular homeostasis to alleviate sepsis by blocking pyroptosis and mitochondrial apoptosis pathways. Adv Sci. 2023;10:1–20.
Ouyang M, Ouyang X, Peng Z, Liu M, Xu G, Zou Z, et al. Heart-targeted amelioration of sepsis-induced myocardial dysfunction by microenvironment responsive nitric oxide nanogenerators in situ. J Nanobiotechnology. 2022;20:1–19.
Huang Z, Chun C, Li X. Kidney targeting peptide-modified biomimetic nanoplatforms for treatment of acute kidney injury. J Control Release. 2023;358:368–81.
Chen P, Wang G, Hao C, Ma W, Xu L, Kuang H, et al. Peptide-directed synthesis of chiral nano-bipyramids for controllable antibacterial application. Chem Sci. 2022;13:10281–90.
Todaro B, Ottalagana E, Luin S, Santi M. Targeting peptides: the new generation of targeted drug delivery systems. Pharmaceutics. 2023;15:1648.
Zhao Y, Yi W, Wan X, Wang J, Tao T, Li J, et al. Blockade of ICAM-1 improves the outcome of polymicrobial sepsis via modulating neutrophil migration and reversing immunosuppression. Mediators Inflamm. 2014;2014:1–10.
Yusuf-Makagiansar H, Siahaan TJ. Binding and internalization of an LFA-1-derived cyclic peptide by ICAM receptors on activated lymphocyte: a potential ligand for drug targeting to ICAM-1-expressing cells. Pharm Res. 2001;18:329–35.
Choudhury SR, Babes L, Rahn JJ, Ahn BY, Goring KAR, King JC, et al. Dipeptidase-1 is an adhesion receptor for neutrophil recruitment in lungs and liver. Cell. 2019;178:1205–21.
Lau A, Rahn JJ, Chappellaz M, Chung H, Benediktsson H, Bihan D, et al. Dipeptidase-1 governs renal inflammation during ischemia reperfusion injury. Sci Adv. 2022;8:1–11.
Sahagun D, Zahid M. Cardiac-targeting peptide: from discovery to applications. Biomolecules. 2023;13:1690.
Ma B, Hu G, Guo S, Zeng Q, Chen Y, Oh DH, et al. Use of peptide-modified nanoparticles as a bacterial cell targeting agent for enhanced antibacterial activity and other biomedical applications. Food Res Int. 2022;161:111638.
Bahrami A, Delshadi R, Jafari SM. Active delivery of antimicrobial nanoparticles into microbial cells through surface functionalization strategies. Trends Food Sci Technol. 2020;99:217–28.
Kumar M, Tegge W, Wangoo N, Jain R, Sharma RK. Insights into cell penetrating peptide conjugated gold nanoparticles for internalization into bacterial cells. Biophys Chem. 2018;237:38–46.
Habibi N, Kamaly N, Memic A, Shafiee H. Self-assembled peptide-based nanostructures: smart nanomaterials toward targeted drug delivery. Nano Today. 2016;11:41–60.
He H, Zheng N, Song Z, Kim KH, Yao C, Zhang R, et al. Suppression of hepatic inflammation via systemic siRNA delivery by membrane-disruptive and endosomolytic helical polypeptide hybrid nanoparticles. ACS Nano. 2016;10:1859–70.
Chen YF, Chen GY, Chang CH, Su YC, Chen YC, Jiang YS, et al. TRAIL encapsulated to polypeptide-crosslinked nanogel exhibits increased anti-inflammatory activities in Klebsiella pneumoniae-induced sepsis treatment. Mater Sci Eng C. 2019;102:85–95.
Chen Z, Hu Y, Mao X, Nie D, Zhao H, Hou Z, et al. Amphipathic dendritic poly-peptides carrier to deliver antisense oligonucleotides against multi-drug resistant bacteria in vitro and in vivo. J Nanobiotechnology. 2022;20:1–17.
Angrish N, Khare G. Antisense oligonucleotide based therapeutics and its applications against bacterial infections. Med Drug Discov. 2023;20:100166.
Umemura Y, Ogura H, Takuma K, Fujishima S, Abe T, Kushimoto S, et al. Current spectrum of causative pathogens in sepsis: A prospective nationwide cohort study in Japan. Int J Infect Dis. 2021;103:343–51. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1201971220324760 .
Download references
Acknowledgments
The authors are thankful to the University of KwaZulu-Natal (UKZN), the National Research Foundation of South Africa (Grant Nos. 123162, 106040, 103664, and 11665), and the Medical Research Council (MRC) of South Africa for financial support.
Author information
Authors and affiliations.
Discipline of Pharmaceutical Sciences, College of Health Sciences, University of KwaZulu-Natal, Private Bag X54001, Durban, South Africa
Mohammed A. Gafar, Calvin A. Omolo, Eman Elhassan & Thirumala Govender
Department of Pharmaceutics, Faculty of Pharmacy, University of Khartoum, P.O. Box 1996, Khartoum, Sudan
Mohammed A. Gafar
Department of Pharmaceutics and Pharmacy Practice, School of Pharmacy and Health Sciences, United States International University-Africa, P. O. Box 14634-00800, Nairobi, Kenya
Calvin A. Omolo
Discipline of Human Physiology, School of Laboratory Medicine and Medical Sciences, College of Health Sciences, University of KwaZulu-Natal, Durban, South Africa
Usri H. Ibrahim
You can also search for this author in PubMed Google Scholar
Contributions
MAG: Conceptualization; data curation; investigation; methodology; validation; visualization; writing-original draft; writing-review and editing; project administration. CAO: Conceptualization; validation; writing-review and editing; supervision. EE: Conceptualization; date curation; validation; writing-review and editing. UHI: Data curation; methodology; writing-original draft. TG: Conceptualization; validation; writing-review and editing; funding acquisition; project administration; supervision. All authors read and approved the final manuscript.
Corresponding authors
Correspondence to Calvin A. Omolo or Thirumala Govender .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Gafar, M.A., Omolo, C.A., Elhassan, E. et al. Applications of peptides in nanosystems for diagnosing and managing bacterial sepsis. J Biomed Sci 31 , 40 (2024). https://doi.org/10.1186/s12929-024-01029-2
Download citation
Received : 25 February 2024
Accepted : 10 April 2024
Published : 19 April 2024
DOI : https://doi.org/10.1186/s12929-024-01029-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Sepsis diagnosis
- Sepsis management
- Drug delivery
Journal of Biomedical Science
ISSN: 1423-0127
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Current issue
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Online First
- Bad news: how the media reported on an observational study about cardiovascular outcomes of COVID-19
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- http://orcid.org/0000-0002-4166-5450 Camilla Alderighi 1 , 2 ,
- Raffaele Rasoini 1 , 2 ,
- Rebecca De Fiore 2 , 3 ,
- Fabio Ambrosino 2 , 3 ,
- Steven Woloshin 1 , 4
- 1 Lisa Schwartz Foundation for Truth in Medicine , Norwich , Vermont , USA
- 2 Alessandro Liberati Association - Cochrane Affiliate Centre , Potenza , Italy
- 3 Pensiero Scientifico Editore s.r.l , Roma , Italy
- 4 Center for Medicine and the Media, The Dartmouth Institute for Health Policy and Clinical Practice , Dartmouth University , Lebanon , New Hampshire , USA
- Correspondence to Dr Camilla Alderighi, Lisa Schwartz Foundation for Truth in Medicine, Norwich, Vermont, USA; camilla.alderighi{at}gmail.com
https://doi.org/10.1136/bmjebm-2023-112814
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
- Cardiovascular Diseases
- PUBLIC HEALTH
- Cardiovascular Abnormalities
Medical research gets plenty of media attention. Unfortunately, the attention is often problematic, frequently failing to provide readers with information needed to understand findings or decide whether to believe them. 1 Unless journalists highlight study cautions and limitations, avoid spin 2 and overinterpretation of findings, the public may draw erroneous conclusions about the reliability and actionability of the research. Coverage of observational research may be especially challenging given inherent difficulty in inferring causation, a limitation that is rarely mentioned in medical journals articles or corresponding news. 3 We used news coverage of a retrospective cohort study, published in Nature Medicine in 2022, 4 as a case study to assess news reporting quality. The index study used national data from US Department of Veteran Affairs to characterise the post-acute cardiovascular manifestations of COVID-19. We chose this study because of its potential public health impact (ie, reporting increased cardiovascular diseases after even mild COVID-19 infection) and its enormous media attention: one of the highest Altmetric scores ever (>20 k, coverage in over 600 news outlets and 40 000 tweets). Our study supplements a previous analysis limited to Italian news. 5
Supplemental material
Using Altmetric news page, we collected the news stories released in the first month after index study publication. We excluded duplicate articles, articles where the index study was not the main topic, articles<150 words or with unreachable link, paywalled articles and articles aimed at healthcare professionals. We translated articles not in English or Italian into Italian using Google Translate. Four raters (two physicians and two scientific journalists) independently analysed the included news articles using the coding scheme in online supplemental appendix 1 . Outcome was the proportion of news articles failing to meet each of the quality measures. Inter-rater agreement across all items was substantial (Fleiss’ kappa=0.78). Coder disagreements were resolved through discussion.
Almost all news stories (95 of 96, 99%) failed to mention the causal inference limitation or used causal language (eg, “Covid causes substantial long-term cardiovascular risks.”). 69 of 96 (72%) made unsupported recommendations (eg, “Based on the results of this study, I recommend that everyone who has been infected with Covid-19 […] get a cardiovascular workup within 12 months.”). 62 of 88 (70%) employed spin, for example, by reporting only relative risks (eg, “Overall, for all cardiovascular diseases combined, the risk after Covid-19 infection increased by 55%.”). 84 of 96 (87%) employed fear mongering (eg, “The results of the paper have shocked other researchers.”). 75 of 96 (78%) failed to undertake a basic critical evaluation of the study (eg, mention population characteristics and study context). More quality measure details and examples from the news are given in table 1 .
- View inline
Quality measures investigated in the analysis and examples from the news
This case study highlights how uncritical reporting of observational research in the news can result in dissemination of poor-quality information to the public. In this case, a high-impact study described an increased incidence of cardiovascular diseases after COVID-19, including coronary disease, myocarditis, pericarditis, heart failure, dysrhythmias, cerebrovascular disease and thromboembolic disease. Because they were based on observational analyses of US Veterans cohorts, these findings should be interpreted cautiously. Nevertheless, many of the subsequent news reports used inappropriate causal language and made recommendations unsupported by the research.
In this analysis, we focused on issues about reporting, that is what people eventually read. However, upstream sources are part of the problem 8 : for instance, the quality of reporting in the case study press release 9 reflects what we have observed in the news (eg, from an investigator quoted in the press release: “Because of the chronic nature of these conditions, they will likely have long-lasting consequences for patients and health systems and also have broad implications on economic productivity and life expectancy”).
The Nature Medicine paper was timely and of great interest to a public concerned about the sequelae of COVID-19. Not surprisingly, it received extraordinary coverage in the media. Careful, balanced news coverage could have helped the public understand that there might be long-term harms of COVID-19. Unfortunately, instead, as documented in our analysis, most media tended to overstate the certainty of results, likely generating substantial public anxiety about an inevitable epidemic of post-COVID-19 cardiovascular disease, and that is bad news.
Our analysis has limitations, such as, being restricted to a single study, unpaywalled articles and using a subjective selection of quality measures—albeit consistent with minimum quality standards used to judge reporting on observational research. 6 7
Ethics statements
Patient consent for publication.
Not applicable.
Ethics approval
- Pérez Gaxiola G , et al
- Boutron I ,
- Bolland MJ ,
- Bowe B , et al
- Rasoini R ,
- Ambrosino F ,
- De Fiore R , et al
- von Elm E ,
- Altman DG ,
- Egger M , et al
- Schwitzer G
- Schwartz LM ,
- Woloshin S ,
- Andrews A , et al
- Nordemberg T
Supplementary materials
Supplementary data.
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
- Data supplement 1
- Data supplement 2
X @camialderighi
Contributors All authors contributed to conception, planning, design and conduct; acquisition, analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; and administrative, technical or material support and had full access to all the data in the study. CA, FA, RDF and RR: contributed to statistical analysis and take responsibility for the integrity of the data and the accuracy of the data analysis. CA and RR contributed equally to the creation of this manuscript; the order of their authorship is entirely arbitrary. CA, RR and SW: contributed to supervision.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Read the full text or download the PDF:
Michael Skuhersky Thesis Defense: An Integrated Approach for Caenorhabditis elegans Nervous System Simulation
Speaker : Michael Skuhersky
Advisor : Edward Boyden
Defense date/time : April 24th at 2pm
In-person location : Singleton Auditorium, 46-3002
Title : An Integrated Approach for Caenorhabditis elegans Nervous System Simulation
Abstract : The Caenorhabditis elegans (C. elegans) connectome, with its relatively simple structure of approximately 300 neurons and several thousand synaptic connections, presents as the most immediately tractable target for whole-nervous system functional simulation. Despite this potential, research has predominantly focused on isolated neural circuits due to technical limitations such as the field of view of existing microscopes, activity indicator considerations, and the complexity of mapping interneuron connections. Existing whole-brain models of C. elegans often rely on assumptions due to inadequate biophysical data per neuron, limiting their accuracy and utility. We describe the development of a specialized lightsheet microscope capable of capturing isotropic high-resolution, functional activity across the entire worm during various behaviors, automated imaging by use of hybrid microfluidics, alongside novel neuron identification methods to precisely map neural identity, so as to generate a comprehensive dataset ideal for model training. Our goal is to create an accurate and interpretable whole-worm functional model of C. elegans, derived from real-world data under diverse behavioral conditions. The accuracy of the model will be validated by its ability to predict future neural activity based on initial states derived from actual worms, thereby offering a more accurate representation of the organism's neural dynamics. This effort not only advances our understanding of the C. elegans functional connectome but also sets a precedent for whole-brain modeling techniques applicable to broader neuroscientific studies.
Upcoming Events
Sara kornfeld simpson thesis defense: physiology and plasticity of primary visual cortex in wild-type and fragile x syndrome model mice, neurolunch: ruidong chen (jazayeri lab) and iakovos lazaridis (graybiel lab), coglunch: gabor brody "how context shapes what symbols mean".

IMAGES
VIDEO
COMMENTS
Price, James H. and Judy Murnan. "Research Limitations and the Necessity of Reporting Them." American Journal of Health Education 35 (2004): 66-67; Theofanidis, Dimitrios and Antigoni Fountouki. "Limitations and Delimitations in the Research Process." ... However, self-reported data can contain several potential sources of bias that you ...
Common types of limitations and their ramifications include: Theoretical: limits the scope, depth, or applicability of a study. Methodological: limits the quality, quantity, or diversity of the data. Empirical: limits the representativeness, validity, or reliability of the data. Analytical: limits the accuracy, completeness, or significance of ...
Identify the limitations: Start by identifying the potential limitations of your research. These may include sample size, selection bias, measurement error, or other issues that could affect the validity and reliability of your findings. Be honest and objective: When describing the limitations of your research, be honest and objective.
A meaningful presentation of study limitations should describe the potential limitation, explain the implication of the limitation, provide possible alternative approaches, and describe steps taken to mitigate the limitation. ... The limitations of any research study will be rooted in the validity of its results—specifically threats to ...
The ideal way is to divide your limitations section into three steps: 1. Identify the research constraints; 2. Describe in great detail how they affect your research; 3. Mention the opportunity for future investigations and give possibilities. By following this method while addressing the constraints of your research, you will be able to ...
Here's an example of a limitation explained in a research paper about the different options and emerging solutions for delaying memory decline. These statements appeared in the first two sentences of the discussion section: "Approaches like stem cell transplantation and vaccination in AD [Alzheimer's disease] work on a cellular or molecular level in the laboratory.
Throughout the Research Process: Continuously reflect on potential limitations during the entire research process. Adjust as Needed: Be willing to adjust your approach as you encounter unforeseen challenges. Conclusion: Understanding and effectively addressing research limitations is a hallmark of rigorous and responsible scholarship.
Figure 13.1: (1) describe the potential limitation, (2) describe the potential impact of the limitation on your study findings, (3) discuss alternatives and why they were not selected, and (4) describe the methods that you propose to minimize the impact of this limitation. 13.2.1 Step #1: Describe the Potential Limitation
specifically, are already taking steps to address the limitations: "Finally, because our study included only patients with this rare disease, its findings likely are not widely applicable beyond this population. Despite these potential limitations, our study provides the strongest insight yet into effective treatment options for this population.
Analyze the chosen data collection method and the sample sizes. 3. Identify your limitations of research and explain their importance. 4. Provide the necessary depth, explain their nature, and justify your study choices. 5. Write how you are suggesting that it is possible to overcome them in the future.
Research limitations refer to the potential weaknesses inherent in a study. All studies have limitations of some sort, meaning declaring limitations doesn't necessarily need to be a bad thing, so long as your declaration of limitations is well thought-out and explained. Rarely is a study perfect.
1 Answer to this question. Answer: The limitations of a study are its flaws or shortcomings which could be the result of unavailability of resources, small sample size, flawed methodology, etc. No study is completely flawless or inclusive of all possible aspects. Therefore, listing the limitations of your study reflects honesty and transparency ...
Conducting research from planning to publication can be a very rewarding process. However, multiple preventable setbacks can occur within each stage of research. While these inefficiencies are an inevitable part of the research process, understanding common pitfalls can limit those hindrances. Many issues can present themselves throughout the research process. It has been said about academics ...
You only need to identify limitations that had the greatest potential impact on: (1) the quality of your findings, and (2) your ability to answer your research question. Step 1: Identify and describe the limitation. Here, the model's estimates are based on potentially biased observational studies. Step 2.
Limitations generally fall into some common categories, and in a sense we can make a checklist for authors here. Price and Murnan ( 2004) gave an excellent and detailed summary of possible research limitations in their editorial for the American Journal of Health Education. They discussed limitations affecting internal and external validity ...
However, self-reported data contain several potential sources of bias that should be noted as limitations: (1) selective memory (remembering or not remembering experiences or events that occurred at some point in the past); (2) telescoping [recalling events that occurred at one time as if they occurred at another time]; (3) attribution [the act ...
Research Limitations. It is for sure that your research will have some limitations and it is normal. However, it is critically important for you to be striving to minimize the range of scope of limitations throughout the research process. Also, you need to provide the acknowledgement of your research limitations in conclusions chapter honestly.
Acknowledging limitations and making recommendations for future research are often presented in thesis handbooks and rubrics as obligatory moves that demonstrate an author's critical self-evaluation and authority. Published research articles (RAs), however, reflect nuanced variation that challenges this interpretation. Based on two specialized corpora of 100 quantitative and 100 qualitative ...
Abstract. Study limitations represent weaknesses within a research design that may influence outcomes and conclusions of the research. Researchers have an obligation to the academic community to present complete and honest limitations of a presented study. Too often, authors use generic descriptions to describe study limitations.
Perhaps more importantly, pointing out the limitations of a study also allows to think about the potential of improvements and opportunities for further research (Ross and Bibler Zaidi, 2019 ...
While each study will have its own unique set of limitations, some limitations are more common in quantitative research, and others are more common in qualitative research. In quantitative research, common limitations include the following: - Participant dropout. - Small sample size, low power. - Non-representative sample.
Recognizing and understanding research bias is crucial for determining the utility of study results and an essential aspect of evidence-based decision-making in the health professions. ... has written eloquently on the challenges and complexities of the evidence-based movement for understanding the potential contributions of qualitative ...
Jamshed (2014) advocates the use of interviewing and observation as two main methods. to have an in depth and extensive understanding of a complex reality. Qualitative studies ha ve been used in a ...
Background Adipose tissue has recently become one of the most promising and predominant sources of mesenchymal stem cells owing to its high accessibility, culturing properties, regenerative potential, and relatively fewer ethical considerations. From the time of the adipose-derived stem cells (ADSCs) discovery, many beneficial properties have been found, including their regenerative, anti ...
The strengths, limitations, and research gaps in each section have been elaborated. Finally, current challenges and potential future paths to enhance the use of peptides in nanosystems for combating sepsis have been deliberately spotlighted.
Medical research gets plenty of media attention. Unfortunately, the attention is often problematic, frequently failing to provide readers with information needed to understand findings or decide whether to believe them.1 Unless journalists highlight study cautions and limitations, avoid spin2 and overinterpretation of findings, the public may draw erroneous conclusions about the reliability ...
Future research would benefit from exploring other factors that may influence symptom severity and disease progression in PD, such as asymmetric loss of nigrostriatal dopaminergic neurons. ... Potential publication bias and validity were assessed by visually inspecting funnel plots in combination with ... Limitations. Like any investigation ...
Despite this potential, research has predominantly focused on isolated neural circuits due to technical limitations such as the field of view of existing microscopes, activity indicator considerations, and the complexity of mapping interneuron connections. Existing whole-brain models of C. elegans often rely on assumptions due to inadequate ...
Nanomaterials have been widely used in diverse fields of research such as engineering, biomedical science, energy, and environment. At present, chemical and physical methods are the main methods for large-scale synthesis of nanomaterials, but these methods have adverse effects on the environment, and health issues, consume more energy, and are expensive. The green synthesis of nanoparticles is ...