

Get Started
Take the first step and invest in your future.

Online Programs
Offering flexibility & convenience in 51 online degrees & programs.

Prairie Stars
Featuring 15 intercollegiate NCAA Div II athletic teams.

Find your Fit
UIS has over 85 student and 10 greek life organizations, and many volunteer opportunities.

Arts & Culture
Celebrating the arts to create rich cultural experiences on campus.

Give Like a Star
Your generosity helps fuel fundraising for scholarships, programs and new initiatives.

Bragging Rights
UIS was listed No. 1 in Illinois and No. 3 in the Midwest in 2023 rankings.
- Quick links Applicants & Students Important Apps & Links Alumni Faculty and Staff Community Admissions How to Apply Cost & Aid Tuition Calculator Registrar Orientation Visit Campus Academics Register for Class Programs of Study Online Degrees & Programs Graduate Education International Student Services Study Away Student Support Bookstore UIS Life Dining Diversity & Inclusion Get Involved Health & Wellness COVID-19 United in Safety Residence Life Student Life Programs UIS Connection Important Apps UIS Mobile App Advise U Canvas myUIS i-card Balance Pay My Bill - UIS Bursar Self-Service Email Resources Bookstore Box Information Technology Services Library Orbit Policies Webtools Get Connected Area Information Calendar Campus Recreation Departments & Programs (A-Z) Parking UIS Newsroom Connect & Get Involved Update your Info Alumni Events Alumni Networks & Groups Volunteer Opportunities Alumni Board News & Publications Featured Alumni Alumni News UIS Alumni Magazine Resources Order your Transcripts Give Back Alumni Programs Career Development Services & Support Accessibility Services Campus Services Campus Police Facilities & Services Registrar Faculty & Staff Resources Website Project Request Web Services Training & Tools Academic Impressions Career Connect CSA Reporting Cybersecurity Training Faculty Research FERPA Training Website Login Campus Resources Newsroom Campus Calendar Campus Maps i-Card Human Resources Public Relations Webtools Arts & Events UIS Performing Arts Center Visual Arts Gallery Event Calendar Sangamon Experience Center for Lincoln Studies ECCE Speaker Series Community Engagement Center for State Policy and Leadership Illinois Innocence Project Innovate Springfield Central IL Nonprofit Resource Center NPR Illinois Community Resources Child Protection Training Academy Office of Electronic Media University Archives/IRAD Institute for Illinois Public Finance
Request Info

How to Review a Journal Article

- Request Info Request info for.... Undergraduate/Graduate Online Study Away Continuing & Professional Education International Student Services General Inquiries
For many kinds of assignments, like a literature review , you may be asked to offer a critique or review of a journal article. This is an opportunity for you as a scholar to offer your qualified opinion and evaluation of how another scholar has composed their article, argument, and research. That means you will be expected to go beyond a simple summary of the article and evaluate it on a deeper level. As a college student, this might sound intimidating. However, as you engage with the research process, you are becoming immersed in a particular topic, and your insights about the way that topic is presented are valuable and can contribute to the overall conversation surrounding your topic.
IMPORTANT NOTE!!
Some disciplines, like Criminal Justice, may only want you to summarize the article without including your opinion or evaluation. If your assignment is to summarize the article only, please see our literature review handout.
Before getting started on the critique, it is important to review the article thoroughly and critically. To do this, we recommend take notes, annotating , and reading the article several times before critiquing. As you read, be sure to note important items like the thesis, purpose, research questions, hypotheses, methods, evidence, key findings, major conclusions, tone, and publication information. Depending on your writing context, some of these items may not be applicable.
Questions to Consider
To evaluate a source, consider some of the following questions. They are broken down into different categories, but answering these questions will help you consider what areas to examine. With each category, we recommend identifying the strengths and weaknesses in each since that is a critical part of evaluation.
Evaluating Purpose and Argument
- How well is the purpose made clear in the introduction through background/context and thesis?
- How well does the abstract represent and summarize the article’s major points and argument?
- How well does the objective of the experiment or of the observation fill a need for the field?
- How well is the argument/purpose articulated and discussed throughout the body of the text?
- How well does the discussion maintain cohesion?
Evaluating the Presentation/Organization of Information
- How appropriate and clear is the title of the article?
- Where could the author have benefited from expanding, condensing, or omitting ideas?
- How clear are the author’s statements? Challenge ambiguous statements.
- What underlying assumptions does the author have, and how does this affect the credibility or clarity of their article?
- How objective is the author in his or her discussion of the topic?
- How well does the organization fit the article’s purpose and articulate key goals?
Evaluating Methods
- How appropriate are the study design and methods for the purposes of the study?
- How detailed are the methods being described? Is the author leaving out important steps or considerations?
- Have the procedures been presented in enough detail to enable the reader to duplicate them?
Evaluating Data
- Scan and spot-check calculations. Are the statistical methods appropriate?
- Do you find any content repeated or duplicated?
- How many errors of fact and interpretation does the author include? (You can check on this by looking up the references the author cites).
- What pertinent literature has the author cited, and have they used this literature appropriately?
Following, we have an example of a summary and an evaluation of a research article. Note that in most literature review contexts, the summary and evaluation would be much shorter. This extended example shows the different ways a student can critique and write about an article.
Chik, A. (2012). Digital gameplay for autonomous foreign language learning: Gamers’ and language teachers’ perspectives. In H. Reinders (ed.), Digital games in language learning and teaching (pp. 95-114). Eastbourne, UK: Palgrave Macmillan.
Be sure to include the full citation either in a reference page or near your evaluation if writing an annotated bibliography .
In Chik’s article “Digital Gameplay for Autonomous Foreign Language Learning: Gamers’ and Teachers’ Perspectives”, she explores the ways in which “digital gamers manage gaming and gaming-related activities to assume autonomy in their foreign language learning,” (96) which is presented in contrast to how teachers view the “pedagogical potential” of gaming. The research was described as an “umbrella project” consisting of two parts. The first part examined 34 language teachers’ perspectives who had limited experience with gaming (only five stated they played games regularly) (99). Their data was recorded through a survey, class discussion, and a seven-day gaming trial done by six teachers who recorded their reflections through personal blog posts. The second part explored undergraduate gaming habits of ten Hong Kong students who were regular gamers. Their habits were recorded through language learning histories, videotaped gaming sessions, blog entries of gaming practices, group discussion sessions, stimulated recall sessions on gaming videos, interviews with other gamers, and posts from online discussion forums. The research shows that while students recognize the educational potential of games and have seen benefits of it in their lives, the instructors overall do not see the positive impacts of gaming on foreign language learning.
The summary includes the article’s purpose, methods, results, discussion, and citations when necessary.
This article did a good job representing the undergraduate gamers’ voices through extended quotes and stories. Particularly for the data collection of the undergraduate gamers, there were many opportunities for an in-depth examination of their gaming practices and histories. However, the representation of the teachers in this study was very uneven when compared to the students. Not only were teachers labeled as numbers while the students picked out their own pseudonyms, but also when viewing the data collection, the undergraduate students were more closely examined in comparison to the teachers in the study. While the students have fifteen extended quotes describing their experiences in their research section, the teachers only have two of these instances in their section, which shows just how imbalanced the study is when presenting instructor voices.
Some research methods, like the recorded gaming sessions, were only used with students whereas teachers were only asked to blog about their gaming experiences. This creates a richer narrative for the students while also failing to give instructors the chance to have more nuanced perspectives. This lack of nuance also stems from the emphasis of the non-gamer teachers over the gamer teachers. The non-gamer teachers’ perspectives provide a stark contrast to the undergraduate gamer experiences and fits neatly with the narrative of teachers not valuing gaming as an educational tool. However, the study mentioned five teachers that were regular gamers whose perspectives are left to a short section at the end of the presentation of the teachers’ results. This was an opportunity to give the teacher group a more complex story, and the opportunity was entirely missed.
Additionally, the context of this study was not entirely clear. The instructors were recruited through a master’s level course, but the content of the course and the institution’s background is not discussed. Understanding this context helps us understand the course’s purpose(s) and how those purposes may have influenced the ways in which these teachers interpreted and saw games. It was also unclear how Chik was connected to this masters’ class and to the students. Why these particular teachers and students were recruited was not explicitly defined and also has the potential to skew results in a particular direction.
Overall, I was inclined to agree with the idea that students can benefit from language acquisition through gaming while instructors may not see the instructional value, but I believe the way the research was conducted and portrayed in this article made it very difficult to support Chik’s specific findings.
Some professors like you to begin an evaluation with something positive but isn’t always necessary.
The evaluation is clearly organized and uses transitional phrases when moving to a new topic.
This evaluation includes a summative statement that gives the overall impression of the article at the end, but this can also be placed at the beginning of the evaluation.
This evaluation mainly discusses the representation of data and methods. However, other areas, like organization, are open to critique.
Page Content
Overview of the review report format, the first read-through, first read considerations, spotting potential major flaws, concluding the first reading, rejection after the first reading, before starting the second read-through, doing the second read-through, the second read-through: section by section guidance, how to structure your report, on presentation and style, criticisms & confidential comments to editors, the recommendation, when recommending rejection, additional resources, step by step guide to reviewing a manuscript.
When you receive an invitation to peer review, you should be sent a copy of the paper's abstract to help you decide whether you wish to do the review. Try to respond to invitations promptly - it will prevent delays. It is also important at this stage to declare any potential Conflict of Interest.
The structure of the review report varies between journals. Some follow an informal structure, while others have a more formal approach.
" Number your comments!!! " (Jonathon Halbesleben, former Editor of Journal of Occupational and Organizational Psychology)
Informal Structure
Many journals don't provide criteria for reviews beyond asking for your 'analysis of merits'. In this case, you may wish to familiarize yourself with examples of other reviews done for the journal, which the editor should be able to provide or, as you gain experience, rely on your own evolving style.
Formal Structure
Other journals require a more formal approach. Sometimes they will ask you to address specific questions in your review via a questionnaire. Or they might want you to rate the manuscript on various attributes using a scorecard. Often you can't see these until you log in to submit your review. So when you agree to the work, it's worth checking for any journal-specific guidelines and requirements. If there are formal guidelines, let them direct the structure of your review.
In Both Cases
Whether specifically required by the reporting format or not, you should expect to compile comments to authors and possibly confidential ones to editors only.

Following the invitation to review, when you'll have received the article abstract, you should already understand the aims, key data and conclusions of the manuscript. If you don't, make a note now that you need to feedback on how to improve those sections.
The first read-through is a skim-read. It will help you form an initial impression of the paper and get a sense of whether your eventual recommendation will be to accept or reject the paper.
Keep a pen and paper handy when skim-reading.
Try to bear in mind the following questions - they'll help you form your overall impression:
- What is the main question addressed by the research? Is it relevant and interesting?
- How original is the topic? What does it add to the subject area compared with other published material?
- Is the paper well written? Is the text clear and easy to read?
- Are the conclusions consistent with the evidence and arguments presented? Do they address the main question posed?
- If the author is disagreeing significantly with the current academic consensus, do they have a substantial case? If not, what would be required to make their case credible?
- If the paper includes tables or figures, what do they add to the paper? Do they aid understanding or are they superfluous?
While you should read the whole paper, making the right choice of what to read first can save time by flagging major problems early on.
Editors say, " Specific recommendations for remedying flaws are VERY welcome ."
Examples of possibly major flaws include:
- Drawing a conclusion that is contradicted by the author's own statistical or qualitative evidence
- The use of a discredited method
- Ignoring a process that is known to have a strong influence on the area under study
If experimental design features prominently in the paper, first check that the methodology is sound - if not, this is likely to be a major flaw.
You might examine:
- The sampling in analytical papers
- The sufficient use of control experiments
- The precision of process data
- The regularity of sampling in time-dependent studies
- The validity of questions, the use of a detailed methodology and the data analysis being done systematically (in qualitative research)
- That qualitative research extends beyond the author's opinions, with sufficient descriptive elements and appropriate quotes from interviews or focus groups
Major Flaws in Information
If methodology is less of an issue, it's often a good idea to look at the data tables, figures or images first. Especially in science research, it's all about the information gathered. If there are critical flaws in this, it's very likely the manuscript will need to be rejected. Such issues include:
- Insufficient data
- Unclear data tables
- Contradictory data that either are not self-consistent or disagree with the conclusions
- Confirmatory data that adds little, if anything, to current understanding - unless strong arguments for such repetition are made
If you find a major problem, note your reasoning and clear supporting evidence (including citations).
After the initial read and using your notes, including those of any major flaws you found, draft the first two paragraphs of your review - the first summarizing the research question addressed and the second the contribution of the work. If the journal has a prescribed reporting format, this draft will still help you compose your thoughts.
The First Paragraph
This should state the main question addressed by the research and summarize the goals, approaches, and conclusions of the paper. It should:
- Help the editor properly contextualize the research and add weight to your judgement
- Show the author what key messages are conveyed to the reader, so they can be sure they are achieving what they set out to do
- Focus on successful aspects of the paper so the author gets a sense of what they've done well
The Second Paragraph
This should provide a conceptual overview of the contribution of the research. So consider:
- Is the paper's premise interesting and important?
- Are the methods used appropriate?
- Do the data support the conclusions?
After drafting these two paragraphs, you should be in a position to decide whether this manuscript is seriously flawed and should be rejected (see the next section). Or whether it is publishable in principle and merits a detailed, careful read through.
Even if you are coming to the opinion that an article has serious flaws, make sure you read the whole paper. This is very important because you may find some really positive aspects that can be communicated to the author. This could help them with future submissions.
A full read-through will also make sure that any initial concerns are indeed correct and fair. After all, you need the context of the whole paper before deciding to reject. If you still intend to recommend rejection, see the section "When recommending rejection."
Once the paper has passed your first read and you've decided the article is publishable in principle, one purpose of the second, detailed read-through is to help prepare the manuscript for publication. You may still decide to recommend rejection following a second reading.
" Offer clear suggestions for how the authors can address the concerns raised. In other words, if you're going to raise a problem, provide a solution ." (Jonathon Halbesleben, Editor of Journal of Occupational and Organizational Psychology)
Preparation
To save time and simplify the review:
- Don't rely solely upon inserting comments on the manuscript document - make separate notes
- Try to group similar concerns or praise together
- If using a review program to note directly onto the manuscript, still try grouping the concerns and praise in separate notes - it helps later
- Note line numbers of text upon which your notes are based - this helps you find items again and also aids those reading your review
Now that you have completed your preparations, you're ready to spend an hour or so reading carefully through the manuscript.
As you're reading through the manuscript for a second time, you'll need to keep in mind the argument's construction, the clarity of the language and content.
With regard to the argument’s construction, you should identify:
- Any places where the meaning is unclear or ambiguous
- Any factual errors
- Any invalid arguments
You may also wish to consider:
- Does the title properly reflect the subject of the paper?
- Does the abstract provide an accessible summary of the paper?
- Do the keywords accurately reflect the content?
- Is the paper an appropriate length?
- Are the key messages short, accurate and clear?
Not every submission is well written. Part of your role is to make sure that the text’s meaning is clear.
Editors say, " If a manuscript has many English language and editing issues, please do not try and fix it. If it is too bad, note that in your review and it should be up to the authors to have the manuscript edited ."
If the article is difficult to understand, you should have rejected it already. However, if the language is poor but you understand the core message, see if you can suggest improvements to fix the problem:
- Are there certain aspects that could be communicated better, such as parts of the discussion?
- Should the authors consider resubmitting to the same journal after language improvements?
- Would you consider looking at the paper again once these issues are dealt with?
On Grammar and Punctuation
Your primary role is judging the research content. Don't spend time polishing grammar or spelling. Editors will make sure that the text is at a high standard before publication. However, if you spot grammatical errors that affect clarity of meaning, then it's important to highlight these. Expect to suggest such amendments - it's rare for a manuscript to pass review with no corrections.
A 2010 study of nursing journals found that 79% of recommendations by reviewers were influenced by grammar and writing style (Shattel, et al., 2010).
1. The Introduction
A well-written introduction:
- Sets out the argument
- Summarizes recent research related to the topic
- Highlights gaps in current understanding or conflicts in current knowledge
- Establishes the originality of the research aims by demonstrating the need for investigations in the topic area
- Gives a clear idea of the target readership, why the research was carried out and the novelty and topicality of the manuscript
Originality and Topicality
Originality and topicality can only be established in the light of recent authoritative research. For example, it's impossible to argue that there is a conflict in current understanding by referencing articles that are 10 years old.
Authors may make the case that a topic hasn't been investigated in several years and that new research is required. This point is only valid if researchers can point to recent developments in data gathering techniques or to research in indirectly related fields that suggest the topic needs revisiting. Clearly, authors can only do this by referencing recent literature. Obviously, where older research is seminal or where aspects of the methodology rely upon it, then it is perfectly appropriate for authors to cite some older papers.
Editors say, "Is the report providing new information; is it novel or just confirmatory of well-known outcomes ?"
It's common for the introduction to end by stating the research aims. By this point you should already have a good impression of them - if the explicit aims come as a surprise, then the introduction needs improvement.
2. Materials and Methods
Academic research should be replicable, repeatable and robust - and follow best practice.
Replicable Research
This makes sufficient use of:
- Control experiments
- Repeated analyses
- Repeated experiments
These are used to make sure observed trends are not due to chance and that the same experiment could be repeated by other researchers - and result in the same outcome. Statistical analyses will not be sound if methods are not replicable. Where research is not replicable, the paper should be recommended for rejection.
Repeatable Methods
These give enough detail so that other researchers are able to carry out the same research. For example, equipment used or sampling methods should all be described in detail so that others could follow the same steps. Where methods are not detailed enough, it's usual to ask for the methods section to be revised.
Robust Research
This has enough data points to make sure the data are reliable. If there are insufficient data, it might be appropriate to recommend revision. You should also consider whether there is any in-built bias not nullified by the control experiments.
Best Practice
During these checks you should keep in mind best practice:
- Standard guidelines were followed (e.g. the CONSORT Statement for reporting randomized trials)
- The health and safety of all participants in the study was not compromised
- Ethical standards were maintained
If the research fails to reach relevant best practice standards, it's usual to recommend rejection. What's more, you don't then need to read any further.
3. Results and Discussion
This section should tell a coherent story - What happened? What was discovered or confirmed?
Certain patterns of good reporting need to be followed by the author:
- They should start by describing in simple terms what the data show
- They should make reference to statistical analyses, such as significance or goodness of fit
- Once described, they should evaluate the trends observed and explain the significance of the results to wider understanding. This can only be done by referencing published research
- The outcome should be a critical analysis of the data collected
Discussion should always, at some point, gather all the information together into a single whole. Authors should describe and discuss the overall story formed. If there are gaps or inconsistencies in the story, they should address these and suggest ways future research might confirm the findings or take the research forward.
4. Conclusions
This section is usually no more than a few paragraphs and may be presented as part of the results and discussion, or in a separate section. The conclusions should reflect upon the aims - whether they were achieved or not - and, just like the aims, should not be surprising. If the conclusions are not evidence-based, it's appropriate to ask for them to be re-written.
5. Information Gathered: Images, Graphs and Data Tables
If you find yourself looking at a piece of information from which you cannot discern a story, then you should ask for improvements in presentation. This could be an issue with titles, labels, statistical notation or image quality.
Where information is clear, you should check that:
- The results seem plausible, in case there is an error in data gathering
- The trends you can see support the paper's discussion and conclusions
- There are sufficient data. For example, in studies carried out over time are there sufficient data points to support the trends described by the author?
You should also check whether images have been edited or manipulated to emphasize the story they tell. This may be appropriate but only if authors report on how the image has been edited (e.g. by highlighting certain parts of an image). Where you feel that an image has been edited or manipulated without explanation, you should highlight this in a confidential comment to the editor in your report.
6. List of References
You will need to check referencing for accuracy, adequacy and balance.
Where a cited article is central to the author's argument, you should check the accuracy and format of the reference - and bear in mind different subject areas may use citations differently. Otherwise, it's the editor’s role to exhaustively check the reference section for accuracy and format.
You should consider if the referencing is adequate:
- Are important parts of the argument poorly supported?
- Are there published studies that show similar or dissimilar trends that should be discussed?
- If a manuscript only uses half the citations typical in its field, this may be an indicator that referencing should be improved - but don't be guided solely by quantity
- References should be relevant, recent and readily retrievable
Check for a well-balanced list of references that is:
- Helpful to the reader
- Fair to competing authors
- Not over-reliant on self-citation
- Gives due recognition to the initial discoveries and related work that led to the work under assessment
You should be able to evaluate whether the article meets the criteria for balanced referencing without looking up every reference.
7. Plagiarism
By now you will have a deep understanding of the paper's content - and you may have some concerns about plagiarism.
Identified Concern
If you find - or already knew of - a very similar paper, this may be because the author overlooked it in their own literature search. Or it may be because it is very recent or published in a journal slightly outside their usual field.
You may feel you can advise the author how to emphasize the novel aspects of their own study, so as to better differentiate it from similar research. If so, you may ask the author to discuss their aims and results, or modify their conclusions, in light of the similar article. Of course, the research similarities may be so great that they render the work unoriginal and you have no choice but to recommend rejection.
"It's very helpful when a reviewer can point out recent similar publications on the same topic by other groups, or that the authors have already published some data elsewhere ." (Editor feedback)
Suspected Concern
If you suspect plagiarism, including self-plagiarism, but cannot recall or locate exactly what is being plagiarized, notify the editor of your suspicion and ask for guidance.
Most editors have access to software that can check for plagiarism.
Editors are not out to police every paper, but when plagiarism is discovered during peer review it can be properly addressed ahead of publication. If plagiarism is discovered only after publication, the consequences are worse for both authors and readers, because a retraction may be necessary.
For detailed guidelines see COPE's Ethical guidelines for reviewers and Wiley's Best Practice Guidelines on Publishing Ethics .
8. Search Engine Optimization (SEO)
After the detailed read-through, you will be in a position to advise whether the title, abstract and key words are optimized for search purposes. In order to be effective, good SEO terms will reflect the aims of the research.
A clear title and abstract will improve the paper's search engine rankings and will influence whether the user finds and then decides to navigate to the main article. The title should contain the relevant SEO terms early on. This has a major effect on the impact of a paper, since it helps it appear in search results. A poor abstract can then lose the reader's interest and undo the benefit of an effective title - whilst the paper's abstract may appear in search results, the potential reader may go no further.
So ask yourself, while the abstract may have seemed adequate during earlier checks, does it:
- Do justice to the manuscript in this context?
- Highlight important findings sufficiently?
- Present the most interesting data?
Editors say, " Does the Abstract highlight the important findings of the study ?"
If there is a formal report format, remember to follow it. This will often comprise a range of questions followed by comment sections. Try to answer all the questions. They are there because the editor felt that they are important. If you're following an informal report format you could structure your report in three sections: summary, major issues, minor issues.
- Give positive feedback first. Authors are more likely to read your review if you do so. But don't overdo it if you will be recommending rejection
- Briefly summarize what the paper is about and what the findings are
- Try to put the findings of the paper into the context of the existing literature and current knowledge
- Indicate the significance of the work and if it is novel or mainly confirmatory
- Indicate the work's strengths, its quality and completeness
- State any major flaws or weaknesses and note any special considerations. For example, if previously held theories are being overlooked
Major Issues
- Are there any major flaws? State what they are and what the severity of their impact is on the paper
- Has similar work already been published without the authors acknowledging this?
- Are the authors presenting findings that challenge current thinking? Is the evidence they present strong enough to prove their case? Have they cited all the relevant work that would contradict their thinking and addressed it appropriately?
- If major revisions are required, try to indicate clearly what they are
- Are there any major presentational problems? Are figures & tables, language and manuscript structure all clear enough for you to accurately assess the work?
- Are there any ethical issues? If you are unsure it may be better to disclose these in the confidential comments section
Minor Issues
- Are there places where meaning is ambiguous? How can this be corrected?
- Are the correct references cited? If not, which should be cited instead/also? Are citations excessive, limited, or biased?
- Are there any factual, numerical or unit errors? If so, what are they?
- Are all tables and figures appropriate, sufficient, and correctly labelled? If not, say which are not
Your review should ultimately help the author improve their article. So be polite, honest and clear. You should also try to be objective and constructive, not subjective and destructive.
You should also:
- Write clearly and so you can be understood by people whose first language is not English
- Avoid complex or unusual words, especially ones that would even confuse native speakers
- Number your points and refer to page and line numbers in the manuscript when making specific comments
- If you have been asked to only comment on specific parts or aspects of the manuscript, you should indicate clearly which these are
- Treat the author's work the way you would like your own to be treated
Most journals give reviewers the option to provide some confidential comments to editors. Often this is where editors will want reviewers to state their recommendation - see the next section - but otherwise this area is best reserved for communicating malpractice such as suspected plagiarism, fraud, unattributed work, unethical procedures, duplicate publication, bias or other conflicts of interest.
However, this doesn't give reviewers permission to 'backstab' the author. Authors can't see this feedback and are unable to give their side of the story unless the editor asks them to. So in the spirit of fairness, write comments to editors as though authors might read them too.
Reviewers should check the preferences of individual journals as to where they want review decisions to be stated. In particular, bear in mind that some journals will not want the recommendation included in any comments to authors, as this can cause editors difficulty later - see Section 11 for more advice about working with editors.
You will normally be asked to indicate your recommendation (e.g. accept, reject, revise and resubmit, etc.) from a fixed-choice list and then to enter your comments into a separate text box.
Recommending Acceptance
If you're recommending acceptance, give details outlining why, and if there are any areas that could be improved. Don't just give a short, cursory remark such as 'great, accept'. See Improving the Manuscript
Recommending Revision
Where improvements are needed, a recommendation for major or minor revision is typical. You may also choose to state whether you opt in or out of the post-revision review too. If recommending revision, state specific changes you feel need to be made. The author can then reply to each point in turn.
Some journals offer the option to recommend rejection with the possibility of resubmission – this is most relevant where substantial, major revision is necessary.
What can reviewers do to help? " Be clear in their comments to the author (or editor) which points are absolutely critical if the paper is given an opportunity for revisio n." (Jonathon Halbesleben, Editor of Journal of Occupational and Organizational Psychology)
Recommending Rejection
If recommending rejection or major revision, state this clearly in your review (and see the next section, 'When recommending rejection').
Where manuscripts have serious flaws you should not spend any time polishing the review you've drafted or give detailed advice on presentation.
Editors say, " If a reviewer suggests a rejection, but her/his comments are not detailed or helpful, it does not help the editor in making a decision ."
In your recommendations for the author, you should:
- Give constructive feedback describing ways that they could improve the research
- Keep the focus on the research and not the author. This is an extremely important part of your job as a reviewer
- Avoid making critical confidential comments to the editor while being polite and encouraging to the author - the latter may not understand why their manuscript has been rejected. Also, they won't get feedback on how to improve their research and it could trigger an appeal
Remember to give constructive criticism even if recommending rejection. This helps developing researchers improve their work and explains to the editor why you felt the manuscript should not be published.
" When the comments seem really positive, but the recommendation is rejection…it puts the editor in a tough position of having to reject a paper when the comments make it sound like a great paper ." (Jonathon Halbesleben, Editor of Journal of Occupational and Organizational Psychology)
Visit our Wiley Author Learning and Training Channel for expert advice on peer review.
Watch the video, Ethical considerations of Peer Review
- PRO Courses Guides New Tech Help Pro Expert Videos About wikiHow Pro Upgrade Sign In
- EDIT Edit this Article
- EXPLORE Tech Help Pro About Us Random Article Quizzes Request a New Article Community Dashboard This Or That Game Popular Categories Arts and Entertainment Artwork Books Movies Computers and Electronics Computers Phone Skills Technology Hacks Health Men's Health Mental Health Women's Health Relationships Dating Love Relationship Issues Hobbies and Crafts Crafts Drawing Games Education & Communication Communication Skills Personal Development Studying Personal Care and Style Fashion Hair Care Personal Hygiene Youth Personal Care School Stuff Dating All Categories Arts and Entertainment Finance and Business Home and Garden Relationship Quizzes Cars & Other Vehicles Food and Entertaining Personal Care and Style Sports and Fitness Computers and Electronics Health Pets and Animals Travel Education & Communication Hobbies and Crafts Philosophy and Religion Work World Family Life Holidays and Traditions Relationships Youth
- Browse Articles
- Learn Something New
- Quizzes Hot
- This Or That Game New
- Train Your Brain
- Explore More
- Support wikiHow
- About wikiHow
- Log in / Sign up
- Education and Communications
How to Review & Evaluate a Journal Publication
Last Updated: January 8, 2024 Fact Checked
Active Reading
Critical evaluation, final review.
This article was co-authored by Richard Perkins . Richard Perkins is a Writing Coach, Academic English Coordinator, and the Founder of PLC Learning Center. With over 24 years of education experience, he gives teachers tools to teach writing to students and works with elementary to university level students to become proficient, confident writers. Richard is a fellow at the National Writing Project. As a teacher leader and consultant at California State University Long Beach's Global Education Project, Mr. Perkins creates and presents teacher workshops that integrate the U.N.'s 17 Sustainable Development Goals in the K-12 curriculum. He holds a BA in Communications and TV from The University of Southern California and an MEd from California State University Dominguez Hills. This article has been fact-checked, ensuring the accuracy of any cited facts and confirming the authority of its sources. This article has been viewed 149,390 times.
Whether you’re publishing a journal article review or completing one for a class, your critique should be fair, thorough, and constructive. Don't worry—this article will walk you through exactly how to review a journal article step-by-step. Keep reading for tips on how to analyze the article, assess how successful it is, and put your thoughts into words.

- Familiarizing yourself with format and style guidelines is especially important if you haven’t published with that journal in the past. For example, a journal might require you to recommend an article for publication, meet a certain word count, or provide revisions that the authors should make.
- If you’re reviewing a journal article for a school assignment, familiarize yourself the guidelines your instructor provided.

- While giving the article a closer read, gauge whether and how well the article resolves its central problem. Ask yourself, “Is this investigation important, and does it uniquely contribute to its field?”
- At this stage, note any terminological inconsistencies, organizational problems, typos, and formatting issues.

- How well does the abstract summarize the article, the problem it addresses, its techniques, results, and significance? For example, you might find that an abstract describes a pharmaceutical study's topic and skips to results without discussing the experiment's methods with much detail.
- Does the introduction map out the article’s structure? Does it clearly lay out the groundwork? A good introduction gives you a clear idea of what to expect in the coming sections. It might state the problem and hypothesis, briefly describe the investigation's methods, then state whether the experiment proved or disproved the hypothesis.

- If necessary, spend some time perusing copies of the article’s sources so you can better understand the topic’s existing literature.
- A good literature review will say something like, "Smith and Jones, in their authoritative 2015 study, demonstrated that adult men and women responded favorably to the treatment. However, no research on the topic has examined the technique's effects and safety in children and adolescents, which is what we sought to explore in our current work."

- For example, you might observe that subjects in medical study didn’t accurately represent a diverse population.

- For example, you might find that tables list too much undigested data that the authors don’t adequately summarize within the text.

- For example, if you’re reviewing an art history article, decide whether it analyzes an artwork reasonably or simply leaps to conclusions. A reasonable analysis might argue, “The artist was a member of Rembrandt’s workshop, which is evident in the painting’s dramatic light and sensual texture.”

- Is the language clear and unambiguous, or does excessive jargon interfere with its ability to make an argument?
- Are there places that are too wordy? Can any ideas be stated in a simpler way?
- Are grammar, punctuation, and terminology correct?

- Your thesis and evidence should be constructive and thoughtful. Point out both strengths and weaknesses, and propose alternative solutions instead of focusing only on weaknesses.
- A good, constructive thesis would be, “The article demonstrates that the drug works better than a placebo in specific demographics, but future research that includes a more diverse subject sampling is necessary.”

- The introduction summarizes the article and states your thesis.
- The body provides specific examples from the text that support your thesis.
- The conclusion summarizes your review, restates your thesis, and offers suggestion for future research.

- Make sure your writing is clear, concise, and logical. If you mention that an article is too verbose, your own writing shouldn’t be full of unnecessarily complicated terms and sentences.
- If possible, have someone familiar with the topic read your draft and offer feedback.
Community Q&A
You Might Also Like

- ↑ https://www.science.org/content/article/how-review-paper
- ↑ https://www.uis.edu/learning-hub/writing-resources/handouts/learning-hub/how-to-review-a-journal-article
- ↑ http://library.queensu.ca/inforef/criticalreview.htm
About This Article

If you want to review a journal article, you’ll need to carefully read it through and come up with a thesis for your piece. Read the article once to get a general idea of what it says, then read it through again and make detailed notes. You should focus on things like whether the introduction gives a good overview of the topic, whether the writing is concise, and whether the results are presented clearly. When you write your review, present both strengths and weaknesses of the article so you’re giving a balanced assessment. Back up your points with examples in the main body of your review, which will make it more credible. You should also ensure your thesis about the article is clear by mentioning it in the introduction and restating it in the conclusion of your review. For tips on how to edit your review before publication, keep reading! Did this summary help you? Yes No
- Send fan mail to authors
Reader Success Stories
Jun 30, 2019
Did this article help you?

Laura Drawls
Aug 26, 2017
Oct 29, 2019
Sep 27, 2018
Sarah Corduroy
Dec 5, 2022

Featured Articles

Trending Articles

Watch Articles

- Terms of Use
- Privacy Policy
- Do Not Sell or Share My Info
- Not Selling Info
Get all the best how-tos!
Sign up for wikiHow's weekly email newsletter
- Technical Support
- Find My Rep
You are here
How to review articles.
Become a reviewer | Things to consider | What to include | Your recommendation | Webinar | Web of Science Academy
Peer review is essential for filtering out poor quality articles by assessing the validity and integrity of the research. We value the work done by peer reviewers in the academic community, who facilitate the process of publication and drive research within their fields of expertise. Please visit the Reviewer Rewards page to learn more about discounts and free journal access offered to reviewers of articles for Sage journals.
If you are an inexperienced or first-time reviewer, the peer review process may seem daunting. In fact, peer review can be a very rewarding process that allows you to contribute to the development of your field and hone your own research and writing skills. The resources below will explain what peer review involves and help you to write useful reviews.
How to become a reviewer
There are three ways to register as a reviewer.
1. Create a journal-specific reviewer account on Sage Track. Search for the journal’s name here and then click the ‘Submit paper’ link. This will take you to the peer review system where you can create an account.
Why create an account on a specific journal Sage Track site?
- You will be part of the journal’s reviewer database.
- You can make your profile more attractive by adding keywords related to your areas of expertise to boost your chances of being invited to review.
- Editors can rate your reviews which may increase your chances of being invited to review again.
2. Contact the journal editor or editorial office directly. In your communication, express your interest, summarize your expertise, and present yourself as a valuable reviewer.
Why contact the editor or editorial office directly?
- If you’re unsure if your area of expertise fits the journal's scope you can double-check with the editor or editorial office directly before registering your details on the Web of Science or the journal’s Sage Track site.
- Editors get a firsthand look at your experience and expertise.
- This is an opportunity for you to introduce yourself and your interests to the editor to increase your chances of being invited to review.
3. Sign up to the Web of Science Reviewer Recognition Service . Indicate your interest in reviewing for a journal by clicking on the journal’s Reviewer Recognition page. Editors use Reviewer Recognition to find suitable reviewers for their journals and may contact you directly via the Reviewer Recognition site.
Why sign up to WoS Reviewer Recognition?
- Editors can clearly see your interest in reviewing for their journal.
- Editors can look into your reviewer insights, including which other journals you are reviewing for.
- Editors can rate reviewers as ‘excellent’.
- You can review your satisfaction of Reviewer Recognition.
- You can receive weekly email updates summarizing your reviewer activity on the site.
- For Sage Journals, a claimed review will result in the individual’s name being listed as a reviewer for that journal.
- Co-reviewers can also receive credit on Reviewer Recognition.
Once you are registered as a reviewer, the editors will send you an invitation to review if a manuscript in your area of expertise is submitted.
We understand that our reviewers are busy, so it may not always be possible for you to accept an invitation to review. To avoid delays, please inform the editor as soon as possible if you are unable to accept an invitation to review or encounter any issues after accepting. If you cannot review a manuscript, we appreciate if you can suggest an alternative reviewer.
Tip: To further increase your chances of receiving review invitations, see our comprehensive blog post on Steps to Strengthen Your Reviewer Profile .
Watch the Video Tutorial
Things to consider before you begin a review
- Timing Inform the editor immediately if you will not be able to meet the deadline and keep your availability updated in Sage Track to avoid receiving invitations to review when you are unavailable.
- Suitability Do you have any reason why you should not review the submission? If in doubt, check with the journal’s editor. Learn more about your ethical responsibilities as a reviewer.
- Individual Journal Reviewer Guidelines Some journals ask reviewers to answer specific questions, so check what is expected before beginning your review.
- Confidentiality You must not share the content of a paper you have been invited to review, unless you have permission from the journal’s editor. If you suspect that author misconduct has taken place, only discuss this with the editor.
- Co-reviewing Inform the journal editor if you wish to collaborate on a review with a colleague or student. See the Ethics and Responsibility page for further instructions on this.
You may also wish to refer to COPE’s guide on what to consider when asked to peer review a manuscript before beginning a review .
What to include in a review
Watch our short video, How to Conduct a Peer Review , for a step-by-step walkthrough of the review process. Alternatively, you can download our Reviewer's Guide for written instructions on how to assess a manuscript and what to include in a review.
Watch the video tutorial
DOWNLOAD REVIEWER'S GUIDE PDF
Making your recommendation
In addition to your review comments, you will likely be expected to select an overall recommendation to the editor. Sage’s most common recommendation types are:
Accept: No further revision required. The manuscript is publishable in its current form.
The majority of articles require revision before reaching this stage.
Minor Revision: The paper is mostly sound but will be sent back to the authors for minor corrections and clarifications such as the addition of minor citations or the tweaking of arguments.
These revisions should not involve any major changes. However, changes should be clearly marked for the attention of the previous reviewers. The paper may be subject to re-review.
Major Revision: The principle of the article is sound and it has a chance of being accepted but requires substantial change to be made. This may include further experiments or analysis, the inclusion of additional literature or theory, or an improvement of arguments and conclusions. The authors are required to submit a point-by-point response to the reviewers and the paper will be subject to a re-review.
If issues of quality, novelty and/or contribution* cannot be addressed through revision, the reviewer should recommend rejection rather than revision. Editors withhold the right to reject the paper should revisions be insufficient.
Reject: The manuscript is of insufficient quality, novelty or significance to warrant publication.
Even when recommending rejection, the reviewer is encouraged to share their suggestions for improvement in the Comments to the Authors field.
If you would like to give us feedback on your experience of reviewing for a Sage journal to help us to improve our systems, please contact [email protected] .
*Check the journal Aims and Scope for any specific requirements with regard to levels of novelty and/or contribution.
How to Be a Peer Reviewer webinar
Considering becoming a reviewer or getting more involved with peer review? Our free webinar will guide you through the process of conducting peer review, including how to get started, basic principles of reviewing articles, what journal editors expect from reviewers, and important considerations such as research ethics and reviewer responsibilities. Learn more here .
Learn more with the Web of Science Academy
The Web of Science Academy offers free-of-charge short courses providing researchers with the skills and experience required to become an expert peer reviewer. Courses cover:
- What's expected of you as a reviewer
- What to look for in a manuscript
- How to write a review
- Co-reviewing with a mentor
A certificate is awarded on completion of the course.
- Journal Author Gateway
- Journal Editor Gateway
- What is Peer Review?
- How to Be a Peer Reviewer Webinar
- How to Review Plain Language Summaries
- How to Review Registered Reports
- Using Sage Track
- Peer Review Ethics
- Resources for Reviewers
- Reviewer Rewards
- Ethics & Responsibility
- Sage Editorial Policies
- Publication Ethics Policies
- Sage Chinese Author Gateway 中国作者资源
- Open Resources & Current Initiatives
- Discipline Hubs
- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- The PRISMA 2020...
The PRISMA 2020 statement: an updated guideline for reporting systematic reviews
PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews
- Related content
- Peer review
- Matthew J Page , senior research fellow 1 ,
- Joanne E McKenzie , associate professor 1 ,
- Patrick M Bossuyt , professor 2 ,
- Isabelle Boutron , professor 3 ,
- Tammy C Hoffmann , professor 4 ,
- Cynthia D Mulrow , professor 5 ,
- Larissa Shamseer , doctoral student 6 ,
- Jennifer M Tetzlaff , research product specialist 7 ,
- Elie A Akl , professor 8 ,
- Sue E Brennan , senior research fellow 1 ,
- Roger Chou , professor 9 ,
- Julie Glanville , associate director 10 ,
- Jeremy M Grimshaw , professor 11 ,
- Asbjørn Hróbjartsson , professor 12 ,
- Manoj M Lalu , associate scientist and assistant professor 13 ,
- Tianjing Li , associate professor 14 ,
- Elizabeth W Loder , professor 15 ,
- Evan Mayo-Wilson , associate professor 16 ,
- Steve McDonald , senior research fellow 1 ,
- Luke A McGuinness , research associate 17 ,
- Lesley A Stewart , professor and director 18 ,
- James Thomas , professor 19 ,
- Andrea C Tricco , scientist and associate professor 20 ,
- Vivian A Welch , associate professor 21 ,
- Penny Whiting , associate professor 17 ,
- David Moher , director and professor 22
- 1 School of Public Health and Preventive Medicine, Monash University, Melbourne, Australia
- 2 Department of Clinical Epidemiology, Biostatistics and Bioinformatics, Amsterdam University Medical Centres, University of Amsterdam, Amsterdam, Netherlands
- 3 Université de Paris, Centre of Epidemiology and Statistics (CRESS), Inserm, F 75004 Paris, France
- 4 Institute for Evidence-Based Healthcare, Faculty of Health Sciences and Medicine, Bond University, Gold Coast, Australia
- 5 University of Texas Health Science Center at San Antonio, San Antonio, Texas, USA; Annals of Internal Medicine
- 6 Knowledge Translation Program, Li Ka Shing Knowledge Institute, Toronto, Canada; School of Epidemiology and Public Health, Faculty of Medicine, University of Ottawa, Ottawa, Canada
- 7 Evidence Partners, Ottawa, Canada
- 8 Clinical Research Institute, American University of Beirut, Beirut, Lebanon; Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, Ontario, Canada
- 9 Department of Medical Informatics and Clinical Epidemiology, Oregon Health & Science University, Portland, Oregon, USA
- 10 York Health Economics Consortium (YHEC Ltd), University of York, York, UK
- 11 Clinical Epidemiology Program, Ottawa Hospital Research Institute, Ottawa, Canada; School of Epidemiology and Public Health, University of Ottawa, Ottawa, Canada; Department of Medicine, University of Ottawa, Ottawa, Canada
- 12 Centre for Evidence-Based Medicine Odense (CEBMO) and Cochrane Denmark, Department of Clinical Research, University of Southern Denmark, Odense, Denmark; Open Patient data Exploratory Network (OPEN), Odense University Hospital, Odense, Denmark
- 13 Department of Anesthesiology and Pain Medicine, The Ottawa Hospital, Ottawa, Canada; Clinical Epidemiology Program, Blueprint Translational Research Group, Ottawa Hospital Research Institute, Ottawa, Canada; Regenerative Medicine Program, Ottawa Hospital Research Institute, Ottawa, Canada
- 14 Department of Ophthalmology, School of Medicine, University of Colorado Denver, Denver, Colorado, United States; Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA
- 15 Division of Headache, Department of Neurology, Brigham and Women's Hospital, Harvard Medical School, Boston, Massachusetts, USA; Head of Research, The BMJ , London, UK
- 16 Department of Epidemiology and Biostatistics, Indiana University School of Public Health-Bloomington, Bloomington, Indiana, USA
- 17 Population Health Sciences, Bristol Medical School, University of Bristol, Bristol, UK
- 18 Centre for Reviews and Dissemination, University of York, York, UK
- 19 EPPI-Centre, UCL Social Research Institute, University College London, London, UK
- 20 Li Ka Shing Knowledge Institute of St. Michael's Hospital, Unity Health Toronto, Toronto, Canada; Epidemiology Division of the Dalla Lana School of Public Health and the Institute of Health Management, Policy, and Evaluation, University of Toronto, Toronto, Canada; Queen's Collaboration for Health Care Quality Joanna Briggs Institute Centre of Excellence, Queen's University, Kingston, Canada
- 21 Methods Centre, Bruyère Research Institute, Ottawa, Ontario, Canada; School of Epidemiology and Public Health, Faculty of Medicine, University of Ottawa, Ottawa, Canada
- 22 Centre for Journalology, Clinical Epidemiology Program, Ottawa Hospital Research Institute, Ottawa, Canada; School of Epidemiology and Public Health, Faculty of Medicine, University of Ottawa, Ottawa, Canada
- Correspondence to: M J Page matthew.page{at}monash.edu
- Accepted 4 January 2021
The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement, published in 2009, was designed to help systematic reviewers transparently report why the review was done, what the authors did, and what they found. Over the past decade, advances in systematic review methodology and terminology have necessitated an update to the guideline. The PRISMA 2020 statement replaces the 2009 statement and includes new reporting guidance that reflects advances in methods to identify, select, appraise, and synthesise studies. The structure and presentation of the items have been modified to facilitate implementation. In this article, we present the PRISMA 2020 27-item checklist, an expanded checklist that details reporting recommendations for each item, the PRISMA 2020 abstract checklist, and the revised flow diagrams for original and updated reviews.
Systematic reviews serve many critical roles. They can provide syntheses of the state of knowledge in a field, from which future research priorities can be identified; they can address questions that otherwise could not be answered by individual studies; they can identify problems in primary research that should be rectified in future studies; and they can generate or evaluate theories about how or why phenomena occur. Systematic reviews therefore generate various types of knowledge for different users of reviews (such as patients, healthcare providers, researchers, and policy makers). 1 2 To ensure a systematic review is valuable to users, authors should prepare a transparent, complete, and accurate account of why the review was done, what they did (such as how studies were identified and selected) and what they found (such as characteristics of contributing studies and results of meta-analyses). Up-to-date reporting guidance facilitates authors achieving this. 3
The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement published in 2009 (hereafter referred to as PRISMA 2009) 4 5 6 7 8 9 10 is a reporting guideline designed to address poor reporting of systematic reviews. 11 The PRISMA 2009 statement comprised a checklist of 27 items recommended for reporting in systematic reviews and an “explanation and elaboration” paper 12 13 14 15 16 providing additional reporting guidance for each item, along with exemplars of reporting. The recommendations have been widely endorsed and adopted, as evidenced by its co-publication in multiple journals, citation in over 60 000 reports (Scopus, August 2020), endorsement from almost 200 journals and systematic review organisations, and adoption in various disciplines. Evidence from observational studies suggests that use of the PRISMA 2009 statement is associated with more complete reporting of systematic reviews, 17 18 19 20 although more could be done to improve adherence to the guideline. 21
Many innovations in the conduct of systematic reviews have occurred since publication of the PRISMA 2009 statement. For example, technological advances have enabled the use of natural language processing and machine learning to identify relevant evidence, 22 23 24 methods have been proposed to synthesise and present findings when meta-analysis is not possible or appropriate, 25 26 27 and new methods have been developed to assess the risk of bias in results of included studies. 28 29 Evidence on sources of bias in systematic reviews has accrued, culminating in the development of new tools to appraise the conduct of systematic reviews. 30 31 Terminology used to describe particular review processes has also evolved, as in the shift from assessing “quality” to assessing “certainty” in the body of evidence. 32 In addition, the publishing landscape has transformed, with multiple avenues now available for registering and disseminating systematic review protocols, 33 34 disseminating reports of systematic reviews, and sharing data and materials, such as preprint servers and publicly accessible repositories. To capture these advances in the reporting of systematic reviews necessitated an update to the PRISMA 2009 statement.
Summary points
To ensure a systematic review is valuable to users, authors should prepare a transparent, complete, and accurate account of why the review was done, what they did, and what they found
The PRISMA 2020 statement provides updated reporting guidance for systematic reviews that reflects advances in methods to identify, select, appraise, and synthesise studies
The PRISMA 2020 statement consists of a 27-item checklist, an expanded checklist that details reporting recommendations for each item, the PRISMA 2020 abstract checklist, and revised flow diagrams for original and updated reviews
We anticipate that the PRISMA 2020 statement will benefit authors, editors, and peer reviewers of systematic reviews, and different users of reviews, including guideline developers, policy makers, healthcare providers, patients, and other stakeholders
Development of PRISMA 2020
A complete description of the methods used to develop PRISMA 2020 is available elsewhere. 35 We identified PRISMA 2009 items that were often reported incompletely by examining the results of studies investigating the transparency of reporting of published reviews. 17 21 36 37 We identified possible modifications to the PRISMA 2009 statement by reviewing 60 documents providing reporting guidance for systematic reviews (including reporting guidelines, handbooks, tools, and meta-research studies). 38 These reviews of the literature were used to inform the content of a survey with suggested possible modifications to the 27 items in PRISMA 2009 and possible additional items. Respondents were asked whether they believed we should keep each PRISMA 2009 item as is, modify it, or remove it, and whether we should add each additional item. Systematic review methodologists and journal editors were invited to complete the online survey (110 of 220 invited responded). We discussed proposed content and wording of the PRISMA 2020 statement, as informed by the review and survey results, at a 21-member, two-day, in-person meeting in September 2018 in Edinburgh, Scotland. Throughout 2019 and 2020, we circulated an initial draft and five revisions of the checklist and explanation and elaboration paper to co-authors for feedback. In April 2020, we invited 22 systematic reviewers who had expressed interest in providing feedback on the PRISMA 2020 checklist to share their views (via an online survey) on the layout and terminology used in a preliminary version of the checklist. Feedback was received from 15 individuals and considered by the first author, and any revisions deemed necessary were incorporated before the final version was approved and endorsed by all co-authors.
The PRISMA 2020 statement
Scope of the guideline.
The PRISMA 2020 statement has been designed primarily for systematic reviews of studies that evaluate the effects of health interventions, irrespective of the design of the included studies. However, the checklist items are applicable to reports of systematic reviews evaluating other interventions (such as social or educational interventions), and many items are applicable to systematic reviews with objectives other than evaluating interventions (such as evaluating aetiology, prevalence, or prognosis). PRISMA 2020 is intended for use in systematic reviews that include synthesis (such as pairwise meta-analysis or other statistical synthesis methods) or do not include synthesis (for example, because only one eligible study is identified). The PRISMA 2020 items are relevant for mixed-methods systematic reviews (which include quantitative and qualitative studies), but reporting guidelines addressing the presentation and synthesis of qualitative data should also be consulted. 39 40 PRISMA 2020 can be used for original systematic reviews, updated systematic reviews, or continually updated (“living”) systematic reviews. However, for updated and living systematic reviews, there may be some additional considerations that need to be addressed. Where there is relevant content from other reporting guidelines, we reference these guidelines within the items in the explanation and elaboration paper 41 (such as PRISMA-Search 42 in items 6 and 7, Synthesis without meta-analysis (SWiM) reporting guideline 27 in item 13d). Box 1 includes a glossary of terms used throughout the PRISMA 2020 statement.
Glossary of terms
Systematic review —A review that uses explicit, systematic methods to collate and synthesise findings of studies that address a clearly formulated question 43
Statistical synthesis —The combination of quantitative results of two or more studies. This encompasses meta-analysis of effect estimates (described below) and other methods, such as combining P values, calculating the range and distribution of observed effects, and vote counting based on the direction of effect (see McKenzie and Brennan 25 for a description of each method)
Meta-analysis of effect estimates —A statistical technique used to synthesise results when study effect estimates and their variances are available, yielding a quantitative summary of results 25
Outcome —An event or measurement collected for participants in a study (such as quality of life, mortality)
Result —The combination of a point estimate (such as a mean difference, risk ratio, or proportion) and a measure of its precision (such as a confidence/credible interval) for a particular outcome
Report —A document (paper or electronic) supplying information about a particular study. It could be a journal article, preprint, conference abstract, study register entry, clinical study report, dissertation, unpublished manuscript, government report, or any other document providing relevant information
Record —The title or abstract (or both) of a report indexed in a database or website (such as a title or abstract for an article indexed in Medline). Records that refer to the same report (such as the same journal article) are “duplicates”; however, records that refer to reports that are merely similar (such as a similar abstract submitted to two different conferences) should be considered unique.
Study —An investigation, such as a clinical trial, that includes a defined group of participants and one or more interventions and outcomes. A “study” might have multiple reports. For example, reports could include the protocol, statistical analysis plan, baseline characteristics, results for the primary outcome, results for harms, results for secondary outcomes, and results for additional mediator and moderator analyses
PRISMA 2020 is not intended to guide systematic review conduct, for which comprehensive resources are available. 43 44 45 46 However, familiarity with PRISMA 2020 is useful when planning and conducting systematic reviews to ensure that all recommended information is captured. PRISMA 2020 should not be used to assess the conduct or methodological quality of systematic reviews; other tools exist for this purpose. 30 31 Furthermore, PRISMA 2020 is not intended to inform the reporting of systematic review protocols, for which a separate statement is available (PRISMA for Protocols (PRISMA-P) 2015 statement 47 48 ). Finally, extensions to the PRISMA 2009 statement have been developed to guide reporting of network meta-analyses, 49 meta-analyses of individual participant data, 50 systematic reviews of harms, 51 systematic reviews of diagnostic test accuracy studies, 52 and scoping reviews 53 ; for these types of reviews we recommend authors report their review in accordance with the recommendations in PRISMA 2020 along with the guidance specific to the extension.
How to use PRISMA 2020
The PRISMA 2020 statement (including the checklists, explanation and elaboration, and flow diagram) replaces the PRISMA 2009 statement, which should no longer be used. Box 2 summarises noteworthy changes from the PRISMA 2009 statement. The PRISMA 2020 checklist includes seven sections with 27 items, some of which include sub-items ( table 1 ). A checklist for journal and conference abstracts for systematic reviews is included in PRISMA 2020. This abstract checklist is an update of the 2013 PRISMA for Abstracts statement, 54 reflecting new and modified content in PRISMA 2020 ( table 2 ). A template PRISMA flow diagram is provided, which can be modified depending on whether the systematic review is original or updated ( fig 1 ).
Noteworthy changes to the PRISMA 2009 statement
Inclusion of the abstract reporting checklist within PRISMA 2020 (see item #2 and table 2 ).
Movement of the ‘Protocol and registration’ item from the start of the Methods section of the checklist to a new Other section, with addition of a sub-item recommending authors describe amendments to information provided at registration or in the protocol (see item #24a-24c).
Modification of the ‘Search’ item to recommend authors present full search strategies for all databases, registers and websites searched, not just at least one database (see item #7).
Modification of the ‘Study selection’ item in the Methods section to emphasise the reporting of how many reviewers screened each record and each report retrieved, whether they worked independently, and if applicable, details of automation tools used in the process (see item #8).
Addition of a sub-item to the ‘Data items’ item recommending authors report how outcomes were defined, which results were sought, and methods for selecting a subset of results from included studies (see item #10a).
Splitting of the ‘Synthesis of results’ item in the Methods section into six sub-items recommending authors describe: the processes used to decide which studies were eligible for each synthesis; any methods required to prepare the data for synthesis; any methods used to tabulate or visually display results of individual studies and syntheses; any methods used to synthesise results; any methods used to explore possible causes of heterogeneity among study results (such as subgroup analysis, meta-regression); and any sensitivity analyses used to assess robustness of the synthesised results (see item #13a-13f).
Addition of a sub-item to the ‘Study selection’ item in the Results section recommending authors cite studies that might appear to meet the inclusion criteria, but which were excluded, and explain why they were excluded (see item #16b).
Splitting of the ‘Synthesis of results’ item in the Results section into four sub-items recommending authors: briefly summarise the characteristics and risk of bias among studies contributing to the synthesis; present results of all statistical syntheses conducted; present results of any investigations of possible causes of heterogeneity among study results; and present results of any sensitivity analyses (see item #20a-20d).
Addition of new items recommending authors report methods for and results of an assessment of certainty (or confidence) in the body of evidence for an outcome (see items #15 and #22).
Addition of a new item recommending authors declare any competing interests (see item #26).
Addition of a new item recommending authors indicate whether data, analytic code and other materials used in the review are publicly available and if so, where they can be found (see item #27).
PRISMA 2020 item checklist
- View inline
PRISMA 2020 for Abstracts checklist*

PRISMA 2020 flow diagram template for systematic reviews. The new design is adapted from flow diagrams proposed by Boers, 55 Mayo-Wilson et al. 56 and Stovold et al. 57 The boxes in grey should only be completed if applicable; otherwise they should be removed from the flow diagram. Note that a “report” could be a journal article, preprint, conference abstract, study register entry, clinical study report, dissertation, unpublished manuscript, government report or any other document providing relevant information.
- Download figure
- Open in new tab
- Download powerpoint
We recommend authors refer to PRISMA 2020 early in the writing process, because prospective consideration of the items may help to ensure that all the items are addressed. To help keep track of which items have been reported, the PRISMA statement website ( http://www.prisma-statement.org/ ) includes fillable templates of the checklists to download and complete (also available in the data supplement on bmj.com). We have also created a web application that allows users to complete the checklist via a user-friendly interface 58 (available at https://prisma.shinyapps.io/checklist/ and adapted from the Transparency Checklist app 59 ). The completed checklist can be exported to Word or PDF. Editable templates of the flow diagram can also be downloaded from the PRISMA statement website.
We have prepared an updated explanation and elaboration paper, in which we explain why reporting of each item is recommended and present bullet points that detail the reporting recommendations (which we refer to as elements). 41 The bullet-point structure is new to PRISMA 2020 and has been adopted to facilitate implementation of the guidance. 60 61 An expanded checklist, which comprises an abridged version of the elements presented in the explanation and elaboration paper, with references and some examples removed, is available in the data supplement on bmj.com. Consulting the explanation and elaboration paper is recommended if further clarity or information is required.
Journals and publishers might impose word and section limits, and limits on the number of tables and figures allowed in the main report. In such cases, if the relevant information for some items already appears in a publicly accessible review protocol, referring to the protocol may suffice. Alternatively, placing detailed descriptions of the methods used or additional results (such as for less critical outcomes) in supplementary files is recommended. Ideally, supplementary files should be deposited to a general-purpose or institutional open-access repository that provides free and permanent access to the material (such as Open Science Framework, Dryad, figshare). A reference or link to the additional information should be included in the main report. Finally, although PRISMA 2020 provides a template for where information might be located, the suggested location should not be seen as prescriptive; the guiding principle is to ensure the information is reported.
Use of PRISMA 2020 has the potential to benefit many stakeholders. Complete reporting allows readers to assess the appropriateness of the methods, and therefore the trustworthiness of the findings. Presenting and summarising characteristics of studies contributing to a synthesis allows healthcare providers and policy makers to evaluate the applicability of the findings to their setting. Describing the certainty in the body of evidence for an outcome and the implications of findings should help policy makers, managers, and other decision makers formulate appropriate recommendations for practice or policy. Complete reporting of all PRISMA 2020 items also facilitates replication and review updates, as well as inclusion of systematic reviews in overviews (of systematic reviews) and guidelines, so teams can leverage work that is already done and decrease research waste. 36 62 63
We updated the PRISMA 2009 statement by adapting the EQUATOR Network’s guidance for developing health research reporting guidelines. 64 We evaluated the reporting completeness of published systematic reviews, 17 21 36 37 reviewed the items included in other documents providing guidance for systematic reviews, 38 surveyed systematic review methodologists and journal editors for their views on how to revise the original PRISMA statement, 35 discussed the findings at an in-person meeting, and prepared this document through an iterative process. Our recommendations are informed by the reviews and survey conducted before the in-person meeting, theoretical considerations about which items facilitate replication and help users assess the risk of bias and applicability of systematic reviews, and co-authors’ experience with authoring and using systematic reviews.
Various strategies to increase the use of reporting guidelines and improve reporting have been proposed. They include educators introducing reporting guidelines into graduate curricula to promote good reporting habits of early career scientists 65 ; journal editors and regulators endorsing use of reporting guidelines 18 ; peer reviewers evaluating adherence to reporting guidelines 61 66 ; journals requiring authors to indicate where in their manuscript they have adhered to each reporting item 67 ; and authors using online writing tools that prompt complete reporting at the writing stage. 60 Multi-pronged interventions, where more than one of these strategies are combined, may be more effective (such as completion of checklists coupled with editorial checks). 68 However, of 31 interventions proposed to increase adherence to reporting guidelines, the effects of only 11 have been evaluated, mostly in observational studies at high risk of bias due to confounding. 69 It is therefore unclear which strategies should be used. Future research might explore barriers and facilitators to the use of PRISMA 2020 by authors, editors, and peer reviewers, designing interventions that address the identified barriers, and evaluating those interventions using randomised trials. To inform possible revisions to the guideline, it would also be valuable to conduct think-aloud studies 70 to understand how systematic reviewers interpret the items, and reliability studies to identify items where there is varied interpretation of the items.
We encourage readers to submit evidence that informs any of the recommendations in PRISMA 2020 (via the PRISMA statement website: http://www.prisma-statement.org/ ). To enhance accessibility of PRISMA 2020, several translations of the guideline are under way (see available translations at the PRISMA statement website). We encourage journal editors and publishers to raise awareness of PRISMA 2020 (for example, by referring to it in journal “Instructions to authors”), endorsing its use, advising editors and peer reviewers to evaluate submitted systematic reviews against the PRISMA 2020 checklists, and making changes to journal policies to accommodate the new reporting recommendations. We recommend existing PRISMA extensions 47 49 50 51 52 53 71 72 be updated to reflect PRISMA 2020 and advise developers of new PRISMA extensions to use PRISMA 2020 as the foundation document.
We anticipate that the PRISMA 2020 statement will benefit authors, editors, and peer reviewers of systematic reviews, and different users of reviews, including guideline developers, policy makers, healthcare providers, patients, and other stakeholders. Ultimately, we hope that uptake of the guideline will lead to more transparent, complete, and accurate reporting of systematic reviews, thus facilitating evidence based decision making.
Acknowledgments
We dedicate this paper to the late Douglas G Altman and Alessandro Liberati, whose contributions were fundamental to the development and implementation of the original PRISMA statement.
We thank the following contributors who completed the survey to inform discussions at the development meeting: Xavier Armoiry, Edoardo Aromataris, Ana Patricia Ayala, Ethan M Balk, Virginia Barbour, Elaine Beller, Jesse A Berlin, Lisa Bero, Zhao-Xiang Bian, Jean Joel Bigna, Ferrán Catalá-López, Anna Chaimani, Mike Clarke, Tammy Clifford, Ioana A Cristea, Miranda Cumpston, Sofia Dias, Corinna Dressler, Ivan D Florez, Joel J Gagnier, Chantelle Garritty, Long Ge, Davina Ghersi, Sean Grant, Gordon Guyatt, Neal R Haddaway, Julian PT Higgins, Sally Hopewell, Brian Hutton, Jamie J Kirkham, Jos Kleijnen, Julia Koricheva, Joey SW Kwong, Toby J Lasserson, Julia H Littell, Yoon K Loke, Malcolm R Macleod, Chris G Maher, Ana Marušic, Dimitris Mavridis, Jessie McGowan, Matthew DF McInnes, Philippa Middleton, Karel G Moons, Zachary Munn, Jane Noyes, Barbara Nußbaumer-Streit, Donald L Patrick, Tatiana Pereira-Cenci, Ba’ Pham, Bob Phillips, Dawid Pieper, Michelle Pollock, Daniel S Quintana, Drummond Rennie, Melissa L Rethlefsen, Hannah R Rothstein, Maroeska M Rovers, Rebecca Ryan, Georgia Salanti, Ian J Saldanha, Margaret Sampson, Nancy Santesso, Rafael Sarkis-Onofre, Jelena Savović, Christopher H Schmid, Kenneth F Schulz, Guido Schwarzer, Beverley J Shea, Paul G Shekelle, Farhad Shokraneh, Mark Simmonds, Nicole Skoetz, Sharon E Straus, Anneliese Synnot, Emily E Tanner-Smith, Brett D Thombs, Hilary Thomson, Alexander Tsertsvadze, Peter Tugwell, Tari Turner, Lesley Uttley, Jeffrey C Valentine, Matt Vassar, Areti Angeliki Veroniki, Meera Viswanathan, Cole Wayant, Paul Whaley, and Kehu Yang. We thank the following contributors who provided feedback on a preliminary version of the PRISMA 2020 checklist: Jo Abbott, Fionn Büttner, Patricia Correia-Santos, Victoria Freeman, Emily A Hennessy, Rakibul Islam, Amalia (Emily) Karahalios, Kasper Krommes, Andreas Lundh, Dafne Port Nascimento, Davina Robson, Catherine Schenck-Yglesias, Mary M Scott, Sarah Tanveer and Pavel Zhelnov. We thank Abigail H Goben, Melissa L Rethlefsen, Tanja Rombey, Anna Scott, and Farhad Shokraneh for their helpful comments on the preprints of the PRISMA 2020 papers. We thank Edoardo Aromataris, Stephanie Chang, Toby Lasserson and David Schriger for their helpful peer review comments on the PRISMA 2020 papers.
Contributors: JEM and DM are joint senior authors. MJP, JEM, PMB, IB, TCH, CDM, LS, and DM conceived this paper and designed the literature review and survey conducted to inform the guideline content. MJP conducted the literature review, administered the survey and analysed the data for both. MJP prepared all materials for the development meeting. MJP and JEM presented proposals at the development meeting. All authors except for TCH, JMT, EAA, SEB, and LAM attended the development meeting. MJP and JEM took and consolidated notes from the development meeting. MJP and JEM led the drafting and editing of the article. JEM, PMB, IB, TCH, LS, JMT, EAA, SEB, RC, JG, AH, TL, EMW, SM, LAM, LAS, JT, ACT, PW, and DM drafted particular sections of the article. All authors were involved in revising the article critically for important intellectual content. All authors approved the final version of the article. MJP is the guarantor of this work. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: There was no direct funding for this research. MJP is supported by an Australian Research Council Discovery Early Career Researcher Award (DE200101618) and was previously supported by an Australian National Health and Medical Research Council (NHMRC) Early Career Fellowship (1088535) during the conduct of this research. JEM is supported by an Australian NHMRC Career Development Fellowship (1143429). TCH is supported by an Australian NHMRC Senior Research Fellowship (1154607). JMT is supported by Evidence Partners Inc. JMG is supported by a Tier 1 Canada Research Chair in Health Knowledge Transfer and Uptake. MML is supported by The Ottawa Hospital Anaesthesia Alternate Funds Association and a Faculty of Medicine Junior Research Chair. TL is supported by funding from the National Eye Institute (UG1EY020522), National Institutes of Health, United States. LAM is supported by a National Institute for Health Research Doctoral Research Fellowship (DRF-2018-11-ST2-048). ACT is supported by a Tier 2 Canada Research Chair in Knowledge Synthesis. DM is supported in part by a University Research Chair, University of Ottawa. The funders had no role in considering the study design or in the collection, analysis, interpretation of data, writing of the report, or decision to submit the article for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at http://www.icmje.org/conflicts-of-interest/ and declare: EL is head of research for the BMJ ; MJP is an editorial board member for PLOS Medicine ; ACT is an associate editor and MJP, TL, EMW, and DM are editorial board members for the Journal of Clinical Epidemiology ; DM and LAS were editors in chief, LS, JMT, and ACT are associate editors, and JG is an editorial board member for Systematic Reviews . None of these authors were involved in the peer review process or decision to publish. TCH has received personal fees from Elsevier outside the submitted work. EMW has received personal fees from the American Journal for Public Health , for which he is the editor for systematic reviews. VW is editor in chief of the Campbell Collaboration, which produces systematic reviews, and co-convenor of the Campbell and Cochrane equity methods group. DM is chair of the EQUATOR Network, IB is adjunct director of the French EQUATOR Centre and TCH is co-director of the Australasian EQUATOR Centre, which advocates for the use of reporting guidelines to improve the quality of reporting in research articles. JMT received salary from Evidence Partners, creator of DistillerSR software for systematic reviews; Evidence Partners was not involved in the design or outcomes of the statement, and the views expressed solely represent those of the author.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient and public involvement: Patients and the public were not involved in this methodological research. We plan to disseminate the research widely, including to community participants in evidence synthesis organisations.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/ .
- Gurevitch J ,
- Koricheva J ,
- Nakagawa S ,
- Liberati A ,
- Tetzlaff J ,
- Altman DG ,
- PRISMA Group
- Tricco AC ,
- Sampson M ,
- Shamseer L ,
- Leoncini E ,
- de Belvis G ,
- Ricciardi W ,
- Fowler AJ ,
- Leclercq V ,
- Beaudart C ,
- Ajamieh S ,
- Rabenda V ,
- Tirelli E ,
- O’Mara-Eves A ,
- McNaught J ,
- Ananiadou S
- Marshall IJ ,
- Noel-Storr A ,
- Higgins JPT ,
- Chandler J ,
- McKenzie JE ,
- López-López JA ,
- Becker BJ ,
- Campbell M ,
- Sterne JAC ,
- Savović J ,
- Sterne JA ,
- Hernán MA ,
- Reeves BC ,
- Whiting P ,
- Higgins JP ,
- ROBIS group
- Hultcrantz M ,
- Stewart L ,
- Bossuyt PM ,
- Flemming K ,
- McInnes E ,
- France EF ,
- Cunningham M ,
- Rethlefsen ML ,
- Kirtley S ,
- Waffenschmidt S ,
- PRISMA-S Group
- ↵ Higgins JPT, Thomas J, Chandler J, et al, eds. Cochrane Handbook for Systematic Reviews of Interventions : Version 6.0. Cochrane, 2019. Available from https://training.cochrane.org/handbook .
- Dekkers OM ,
- Vandenbroucke JP ,
- Cevallos M ,
- Renehan AG ,
- ↵ Cooper H, Hedges LV, Valentine JV, eds. The Handbook of Research Synthesis and Meta-Analysis. Russell Sage Foundation, 2019.
- IOM (Institute of Medicine)
- PRISMA-P Group
- Salanti G ,
- Caldwell DM ,
- Stewart LA ,
- PRISMA-IPD Development Group
- Zorzela L ,
- Ioannidis JP ,
- PRISMAHarms Group
- McInnes MDF ,
- Thombs BD ,
- and the PRISMA-DTA Group
- Beller EM ,
- Glasziou PP ,
- PRISMA for Abstracts Group
- Mayo-Wilson E ,
- Dickersin K ,
- MUDS investigators
- Stovold E ,
- Beecher D ,
- Noel-Storr A
- McGuinness LA
- Sarafoglou A ,
- Boutron I ,
- Giraudeau B ,
- Porcher R ,
- Chauvin A ,
- Schulz KF ,
- Schroter S ,
- Stevens A ,
- Weinstein E ,
- Macleod MR ,
- IICARus Collaboration
- Kirkham JJ ,
- Petticrew M ,
- Tugwell P ,
- PRISMA-Equity Bellagio group
How to Write an Article Review: Tips and Examples

Did you know that article reviews are not just academic exercises but also a valuable skill in today's information age? In a world inundated with content, being able to dissect and evaluate articles critically can help you separate the wheat from the chaff. Whether you're a student aiming to excel in your coursework or a professional looking to stay well-informed, mastering the art of writing article reviews is an invaluable skill.
Short Description
In this article, our research paper writing service experts will start by unraveling the concept of article reviews and discussing the various types. You'll also gain insights into the art of formatting your review effectively. To ensure you're well-prepared, we'll take you through the pre-writing process, offering tips on setting the stage for your review. But it doesn't stop there. You'll find a practical example of an article review to help you grasp the concepts in action. To complete your journey, we'll guide you through the post-writing process, equipping you with essential proofreading techniques to ensure your work shines with clarity and precision!
What Is an Article Review: Grasping the Concept
A review article is a type of professional paper writing that demands a high level of in-depth analysis and a well-structured presentation of arguments. It is a critical, constructive evaluation of literature in a particular field through summary, classification, analysis, and comparison.
If you write a scientific review, you have to use database searches to portray the research. Your primary goal is to summarize everything and present a clear understanding of the topic you've been working on.
Writing Involves:
- Summarization, classification, analysis, critiques, and comparison.
- The analysis, evaluation, and comparison require the use of theories, ideas, and research relevant to the subject area of the article.
- It is also worth nothing if a review does not introduce new information, but instead presents a response to another writer's work.
- Check out other samples to gain a better understanding of how to review the article.
Types of Review
When it comes to article reviews, there's more than one way to approach the task. Understanding the various types of reviews is like having a versatile toolkit at your disposal. In this section, we'll walk you through the different dimensions of review types, each offering a unique perspective and purpose. Whether you're dissecting a scholarly article, critiquing a piece of literature, or evaluating a product, you'll discover the diverse landscape of article reviews and how to navigate it effectively.
.webp)
Journal Article Review
Just like other types of reviews, a journal article review assesses the merits and shortcomings of a published work. To illustrate, consider a review of an academic paper on climate change, where the writer meticulously analyzes and interprets the article's significance within the context of environmental science.
Research Article Review
Distinguished by its focus on research methodologies, a research article review scrutinizes the techniques used in a study and evaluates them in light of the subsequent analysis and critique. For instance, when reviewing a research article on the effects of a new drug, the reviewer would delve into the methods employed to gather data and assess their reliability.
Science Article Review
In the realm of scientific literature, a science article review encompasses a wide array of subjects. Scientific publications often provide extensive background information, which can be instrumental in conducting a comprehensive analysis. For example, when reviewing an article about the latest breakthroughs in genetics, the reviewer may draw upon the background knowledge provided to facilitate a more in-depth evaluation of the publication.
Need a Hand From Professionals?
Address to Our Writers and Get Assistance in Any Questions!
Formatting an Article Review
The format of the article should always adhere to the citation style required by your professor. If you're not sure, seek clarification on the preferred format and ask him to clarify several other pointers to complete the formatting of an article review adequately.
How Many Publications Should You Review?
- In what format should you cite your articles (MLA, APA, ASA, Chicago, etc.)?
- What length should your review be?
- Should you include a summary, critique, or personal opinion in your assignment?
- Do you need to call attention to a theme or central idea within the articles?
- Does your instructor require background information?
When you know the answers to these questions, you may start writing your assignment. Below are examples of MLA and APA formats, as those are the two most common citation styles.
Using the APA Format
Articles appear most commonly in academic journals, newspapers, and websites. If you write an article review in the APA format, you will need to write bibliographical entries for the sources you use:
- Web : Author [last name], A.A [first and middle initial]. (Year, Month, Date of Publication). Title. Retrieved from {link}
- Journal : Author [last name], A.A [first and middle initial]. (Publication Year). Publication Title. Periodical Title, Volume(Issue), pp.-pp.
- Newspaper : Author [last name], A.A [first and middle initial]. (Year, Month, Date of Publication). Publication Title. Magazine Title, pp. xx-xx.
Using MLA Format
- Web : Last, First Middle Initial. “Publication Title.” Website Title. Website Publisher, Date Month Year Published. Web. Date Month Year Accessed.
- Newspaper : Last, First M. “Publication Title.” Newspaper Title [City] Date, Month, Year Published: Page(s). Print.
- Journal : Last, First M. “Publication Title.” Journal Title Series Volume. Issue (Year Published): Page(s). Database Name. Web. Date Month Year Accessed.
Enhance your writing effortlessly with EssayPro.com , where you can order an article review or any other writing task. Our team of expert writers specializes in various fields, ensuring your work is not just summarized, but deeply analyzed and professionally presented. Ideal for students and professionals alike, EssayPro offers top-notch writing assistance tailored to your needs. Elevate your writing today with our skilled team at your article review writing service !
.jpg)
The Pre-Writing Process
Facing this task for the first time can really get confusing and can leave you unsure of where to begin. To create a top-notch article review, start with a few preparatory steps. Here are the two main stages from our dissertation services to get you started:
Step 1: Define the right organization for your review. Knowing the future setup of your paper will help you define how you should read the article. Here are the steps to follow:
- Summarize the article — seek out the main points, ideas, claims, and general information presented in the article.
- Define the positive points — identify the strong aspects, ideas, and insightful observations the author has made.
- Find the gaps —- determine whether or not the author has any contradictions, gaps, or inconsistencies in the article and evaluate whether or not he or she used a sufficient amount of arguments and information to support his or her ideas.
- Identify unanswered questions — finally, identify if there are any questions left unanswered after reading the piece.
Step 2: Move on and review the article. Here is a small and simple guide to help you do it right:
- Start off by looking at and assessing the title of the piece, its abstract, introductory part, headings and subheadings, opening sentences in its paragraphs, and its conclusion.
- First, read only the beginning and the ending of the piece (introduction and conclusion). These are the parts where authors include all of their key arguments and points. Therefore, if you start with reading these parts, it will give you a good sense of the author's main points.
- Finally, read the article fully.
These three steps make up most of the prewriting process. After you are done with them, you can move on to writing your own review—and we are going to guide you through the writing process as well.
Outline and Template
As you progress with reading your article, organize your thoughts into coherent sections in an outline. As you read, jot down important facts, contributions, or contradictions. Identify the shortcomings and strengths of your publication. Begin to map your outline accordingly.
If your professor does not want a summary section or a personal critique section, then you must alleviate those parts from your writing. Much like other assignments, an article review must contain an introduction, a body, and a conclusion. Thus, you might consider dividing your outline according to these sections as well as subheadings within the body. If you find yourself troubled with the pre-writing and the brainstorming process for this assignment, seek out a sample outline.
Your custom essay must contain these constituent parts:
- Pre-Title Page - Before diving into your review, start with essential details: article type, publication title, and author names with affiliations (position, department, institution, location, and email). Include corresponding author info if needed.
- Running Head - In APA format, use a concise title (under 40 characters) to ensure consistent formatting.
- Summary Page - Optional but useful. Summarize the article in 800 words, covering background, purpose, results, and methodology, avoiding verbatim text or references.
- Title Page - Include the full title, a 250-word abstract, and 4-6 keywords for discoverability.
- Introduction - Set the stage with an engaging overview of the article.
- Body - Organize your analysis with headings and subheadings.
- Works Cited/References - Properly cite all sources used in your review.
- Optional Suggested Reading Page - If permitted, suggest further readings for in-depth exploration.
- Tables and Figure Legends (if instructed by the professor) - Include visuals when requested by your professor for clarity.
Example of an Article Review
You might wonder why we've dedicated a section of this article to discuss an article review sample. Not everyone may realize it, but examining multiple well-constructed examples of review articles is a crucial step in the writing process. In the following section, our essay writing service experts will explain why.
Looking through relevant article review examples can be beneficial for you in the following ways:
- To get you introduced to the key works of experts in your field.
- To help you identify the key people engaged in a particular field of science.
- To help you define what significant discoveries and advances were made in your field.
- To help you unveil the major gaps within the existing knowledge of your field—which contributes to finding fresh solutions.
- To help you find solid references and arguments for your own review.
- To help you generate some ideas about any further field of research.
- To help you gain a better understanding of the area and become an expert in this specific field.
- To get a clear idea of how to write a good review.
View Our Writer’s Sample Before Crafting Your Own!
Why Have There Been No Great Female Artists?
Steps for Writing an Article Review
Here is a guide with critique paper format on how to write a review paper:
.webp)
Step 1: Write the Title
First of all, you need to write a title that reflects the main focus of your work. Respectively, the title can be either interrogative, descriptive, or declarative.
Step 2: Cite the Article
Next, create a proper citation for the reviewed article and input it following the title. At this step, the most important thing to keep in mind is the style of citation specified by your instructor in the requirements for the paper. For example, an article citation in the MLA style should look as follows:
Author's last and first name. "The title of the article." Journal's title and issue(publication date): page(s). Print
Abraham John. "The World of Dreams." Virginia Quarterly 60.2(1991): 125-67. Print.
Step 3: Article Identification
After your citation, you need to include the identification of your reviewed article:
- Title of the article
- Title of the journal
- Year of publication
All of this information should be included in the first paragraph of your paper.
The report "Poverty increases school drop-outs" was written by Brian Faith – a Health officer – in 2000.
Step 4: Introduction
Your organization in an assignment like this is of the utmost importance. Before embarking on your writing process, you should outline your assignment or use an article review template to organize your thoughts coherently.
- If you are wondering how to start an article review, begin with an introduction that mentions the article and your thesis for the review.
- Follow up with a summary of the main points of the article.
- Highlight the positive aspects and facts presented in the publication.
- Critique the publication by identifying gaps, contradictions, disparities in the text, and unanswered questions.
Step 5: Summarize the Article
Make a summary of the article by revisiting what the author has written about. Note any relevant facts and findings from the article. Include the author's conclusions in this section.
Step 6: Critique It
Present the strengths and weaknesses you have found in the publication. Highlight the knowledge that the author has contributed to the field. Also, write about any gaps and/or contradictions you have found in the article. Take a standpoint of either supporting or not supporting the author's assertions, but back up your arguments with facts and relevant theories that are pertinent to that area of knowledge. Rubrics and templates can also be used to evaluate and grade the person who wrote the article.
Step 7: Craft a Conclusion
In this section, revisit the critical points of your piece, your findings in the article, and your critique. Also, write about the accuracy, validity, and relevance of the results of the article review. Present a way forward for future research in the field of study. Before submitting your article, keep these pointers in mind:
- As you read the article, highlight the key points. This will help you pinpoint the article's main argument and the evidence that they used to support that argument.
- While you write your review, use evidence from your sources to make a point. This is best done using direct quotations.
- Select quotes and supporting evidence adequately and use direct quotations sparingly. Take time to analyze the article adequately.
- Every time you reference a publication or use a direct quotation, use a parenthetical citation to avoid accidentally plagiarizing your article.
- Re-read your piece a day after you finish writing it. This will help you to spot grammar mistakes and to notice any flaws in your organization.
- Use a spell-checker and get a second opinion on your paper.
The Post-Writing Process: Proofread Your Work
Finally, when all of the parts of your article review are set and ready, you have one last thing to take care of — proofreading. Although students often neglect this step, proofreading is a vital part of the writing process and will help you polish your paper to ensure that there are no mistakes or inconsistencies.
To proofread your paper properly, start by reading it fully and checking the following points:
- Punctuation
- Other mistakes
Afterward, take a moment to check for any unnecessary information in your paper and, if found, consider removing it to streamline your content. Finally, double-check that you've covered at least 3-4 key points in your discussion.
And remember, if you ever need help with proofreading, rewriting your essay, or even want to buy essay , our friendly team is always here to assist you.
Need an Article REVIEW WRITTEN?
Just send us the requirements to your paper and watch one of our writers crafting an original paper for you.
What Is A Review Article?
How to write an article review, how to write an article review in apa format, related articles.
%20(2).webp)
How to Write an Article Review: Template & Examples
An article review is an academic assignment that invites you to study a piece of academic research closely. Then, you should present its summary and critically evaluate it using the knowledge you’ve gained in class and during your independent study. If you get such a task at college or university, you shouldn’t confuse it with a response paper, which is a distinct assignment with other purposes (we’ll talk about it in detail below).
Our specialists will write a custom essay specially for you!
In this article, prepared by Custom-Writing experts, you’ll find:
- the intricacies of article review writing;
- the difference between an article review and similar assignments;
- a step-by-step algorithm for review composition;
- a couple of samples to guide you throughout the writing process.
So, if you wish to study our article review example and discover helpful writing tips, keep reading.
❓ What Is an Article Review?
- ✍️ Writing Steps
📑 Article Review Format
🔗 references.
An article review is an academic paper that summarizes and critically evaluates the information presented in your selected article.

The first thing you should note when approaching the task of an article review is that not every article is suitable for this assignment. Let’s have a look at the variety of articles to understand what you can choose from.
Popular Vs. Scholarly Articles
In most cases, you’ll be required to review a scholarly, peer-reviewed article – one composed in compliance with rigorous academic standards. Yet, the Web is also full of popular articles that don’t present original scientific value and shouldn’t be selected for a review.
Just in 1 hour! We will write you a plagiarism-free paper in hardly more than 1 hour
Not sure how to distinguish these two types? Here is a comparative table to help you out.
Article Review vs. Response Paper
Now, let’s consider the difference between an article review and a response paper:
- If you’re assigned to critique a scholarly article , you will need to compose an article review .
- If your subject of analysis is a popular article , you can respond to it with a well-crafted response paper .
The reason for such distinctions is the quality and structure of these two article types. Peer-reviewed, scholarly articles have clear-cut quality criteria, allowing you to conduct and present a structured assessment of the assigned material. Popular magazines have loose or non-existent quality criteria and don’t offer an opportunity for structured evaluation. So, they are only fit for a subjective response, in which you can summarize your reactions and emotions related to the reading material.
All in all, you can structure your response assignments as outlined in the tips below.
✍️ How to Write an Article Review: Step by Step
Here is a tried and tested algorithm for article review writing from our experts. We’ll consider only the critical review variety of this academic assignment. So, let’s get down to the stages you need to cover to get a stellar review.
Receive a plagiarism-free paper tailored to your instructions. Cut 20% off your first order!
Read the Article
As with any reviews, reports, and critiques, you must first familiarize yourself with the assigned material. It’s impossible to review something you haven’t read, so set some time for close, careful reading of the article to identify:
- Its topic.
- Its type.
- The author’s main points and message.
- The arguments they use to prove their points.
- The methodology they use to approach the subject.
In terms of research type , your article will usually belong to one of three types explained below.
Summarize the Article
Now that you’ve read the text and have a general impression of the content, it’s time to summarize it for your readers. Look into the article’s text closely to determine:
- The thesis statement , or general message of the author.
- Research question, purpose, and context of research.
- Supporting points for the author’s assumptions and claims.
- Major findings and supporting evidence.
As you study the article thoroughly, make notes on the margins or write these elements out on a sheet of paper. You can also apply a different technique: read the text section by section and formulate its gist in one phrase or sentence. Once you’re done, you’ll have a summary skeleton in front of you.
Evaluate the Article
The next step of review is content evaluation. Keep in mind that various research types will require a different set of review questions. Here is a complete list of evaluation points you can include.
Get an originally-written paper according to your instructions!

Write the Text
After completing the critical review stage, it’s time to compose your article review.
The format of this assignment is standard – you will have an introduction, a body, and a conclusion. The introduction should present your article and summarize its content. The body will contain a structured review according to all four dimensions covered in the previous section. The concluding part will typically recap all the main points you’ve identified during your assessment.
It is essential to note that an article review is, first of all, an academic assignment. Therefore, it should follow all rules and conventions of academic composition, such as:
- No contractions . Don’t use short forms, such as “don’t,” “can’t,” “I’ll,” etc. in academic writing. You need to spell out all those words.
- Formal language and style . Avoid conversational phrasing and words that you would naturally use in blog posts or informal communication. For example, don’t use words like “pretty,” “kind of,” and “like.”
- Third-person narrative . Academic reviews should be written from the third-person point of view, avoiding statements like “I think,” “in my opinion,” and so on.
- No conversational forms . You shouldn’t turn to your readers directly in the text by addressing them with the pronoun “you.” It’s vital to keep the narrative neutral and impersonal.
- Proper abbreviation use . Consult the list of correct abbreviations , like “e.g.” or “i.e.,” for use in your academic writing. If you use informal abbreviations like “FYA” or “f.i.,” your professor will reduce the grade.
- Complete sentences . Make sure your sentences contain the subject and the predicate; avoid shortened or sketch-form phrases suitable for a draft only.
- No conjunctions at the beginning of a sentence . Remember the FANBOYS rule – don’t start a sentence with words like “and” or “but.” They often seem the right way to build a coherent narrative, but academic writing rules disfavor such usage.
- No abbreviations or figures at the beginning of a sentence . Never start a sentence with a number — spell it out if you need to use it anyway. Besides, sentences should never begin with abbreviations like “e.g.”
Finally, a vital rule for an article review is properly formatting the citations. We’ll discuss the correct use of citation styles in the following section.
When composing an article review, keep these points in mind:
- Start with a full reference to the reviewed article so the reader can locate it quickly.
- Ensure correct formatting of in-text references.
- Provide a complete list of used external sources on the last page of the review – your bibliographical entries .
You’ll need to understand the rules of your chosen citation style to meet all these requirements. Below, we’ll discuss the two most common referencing styles – APA and MLA.
Article Review in APA
When you need to compose an article review in the APA format , here is the general bibliographical entry format you should use for journal articles on your reference page:
- Author’s last name, First initial. Middle initial. (Year of Publication). Name of the article. Name of the Journal, volume (number), pp. #-#. https://doi.org/xx.xxx/yyyy
Horigian, V. E., Schmidt, R. D., & Feaster, D. J. (2021). Loneliness, mental health, and substance use among US young adults during COVID-19. Journal of Psychoactive Drugs, 53 (1), pp. 1-9. https://doi.org/10.1080/02791072.2020.1836435
Your in-text citations should follow the author-date format like this:
- If you paraphrase the source and mention the author in the text: According to Horigian et al. (2021), young adults experienced increased levels of loneliness, depression, and anxiety during the pandemic.
- If you paraphrase the source and don’t mention the author in the text: Young adults experienced increased levels of loneliness, depression, and anxiety during the pandemic (Horigian et al., 2021).
- If you quote the source: As Horigian et al. (2021) point out, there were “elevated levels of loneliness, depression, anxiety, alcohol use, and drug use among young adults during COVID-19” (p. 6).
Note that your in-text citations should include “et al.,” as in the examples above, if your article has 3 or more authors. If you have one or two authors, your in-text citations would look like this:
- One author: “According to Smith (2020), depression is…” or “Depression is … (Smith, 2020).”
- Two authors: “According to Smith and Brown (2020), anxiety means…” or “Anxiety means (Smith & Brown, 2020).”
Finally, in case you have to review a book or a website article, here are the general formats for citing these source types on your APA reference list.
Article Review in MLA
If your assignment requires MLA-format referencing, here’s the general format you should use for citing journal articles on your Works Cited page:
- Author’s last name, First name. “Title of an Article.” Title of the Journal , vol. #, no. #, year, pp. #-#.
Horigian, Viviana E., et al. “Loneliness, Mental Health, and Substance Use Among US Young Adults During COVID-19.” Journal of Psychoactive Drugs , vol. 53, no. 1, 2021, pp. 1-9.
In-text citations in the MLA format follow the author-page citation format and look like this:
- According to Horigian et al., young adults experienced increased levels of loneliness, depression, and anxiety during the pandemic (6).
- Young adults experienced increased levels of loneliness, depression, and anxiety during the pandemic (Horigian et al. 6).
Like in APA, the abbreviation “et al.” is only needed in MLA if your article has 3 or more authors.
If you need to cite a book or a website page, here are the general MLA formats for these types of sources.
✅ Article Review Template
Here is a handy, universal article review template to help you move on with any review assignment. We’ve tried to make it as generic as possible to guide you in the academic process.
📝 Article Review Examples
The theory is good, but practice is even better. Thus, we’ve created three brief examples to show you how to write an article review. You can study the full-text samples by following the links.
📃 Men, Women, & Money
This article review examines a famous piece, “Men, Women & Money – How the Sexes Differ with Their Finances,” published by Amy Livingston in 2020. The author of this article claims that men generally spend more money than women. She makes this conclusion from a close analysis of gender-specific expenditures across five main categories: food, clothing, cars, entertainment, and general spending patterns. Livingston also looks at men’s approach to saving to argue that counter to the common perception of women’s light-hearted attitude to money, men are those who spend more on average.
📃 When and Why Nationalism Beats Globalism
This is a review of Jonathan Heidt’s 2016 article titled “When and Why Nationalism Beats Globalism,” written as an advocacy of right-wing populism rising in many Western states. The author illustrates the case with the election of Donald Trump as the US President and the rise of right-wing rhetoric in many Western countries. These examples show how nationalist sentiment represents a reaction to global immigration and a failure of globalization.
📃 Sleep Deprivation
This is a review of the American Heart Association’s article titled “The Dangers of Sleep Deprivation.” It discusses how the national organization concerned with the American population’s cardiovascular health links the lack of high-quality sleep to far-reaching health consequences. The organization’s experts reveal how a consistent lack of sleep leads to Alzheimer’s disease development, obesity, type 2 diabetes, etc.
✏️ Article Review FAQ
A high-quality article review should summarize the assigned article’s content and offer data-backed reactions and evaluations of its quality in terms of the article’s purpose, methodology, and data used to argue the main points. It should be detailed, comprehensive, objective, and evidence-based.
The purpose of writing a review is to allow students to reflect on research quality and showcase their critical thinking and evaluation skills. Students should exhibit their mastery of close reading of research publications and their unbiased assessment.
The content of your article review will be the same in any format, with the only difference in the assignment’s formatting before submission. Ensure you have a separate title page made according to APA standards and cite sources using the parenthetical author-date referencing format.
You need to take a closer look at various dimensions of an assigned article to compose a valuable review. Study the author’s object of analysis, the purpose of their research, the chosen method, data, and findings. Evaluate all these dimensions critically to see whether the author has achieved the initial goals. Finally, offer improvement recommendations to add a critique aspect to your paper.
- Scientific Article Review: Duke University
- Book and Article Reviews: William & Mary, Writing Resources Center
- Sample Format for Reviewing a Journal Article: Boonshoft School of Medicine
- Research Paper Review – Structure and Format Guidelines: New Jersey Institute of Technology
- Article Review: University of Waterloo
- Article Review: University of South Australia
- How to Write a Journal Article Review: University of Newcastle Library Guides
- Writing Help: The Article Review: Central Michigan University Libraries
- Write a Critical Review of a Scientific Journal Article: McLaughlin Library
- Share to Facebook
- Share to Twitter
- Share to LinkedIn
- Share to email

Short essays answer a specific question on the subject. They usually are anywhere between 250 words and 750 words long. A paper with less than 250 words isn’t considered a finished text, so it doesn’t fall under the category of a short essay. Essays of such format are required for...

High school and college students often face challenges when crafting a compare-and-contrast essay. A well-written paper of this kind needs to be structured appropriately to earn you good grades. Knowing how to organize your ideas allows you to present your ideas in a coherent and logical manner This article by...

If you’re a student, you’ve heard about a formal essay: a factual, research-based paper written in 3rd person. Most students have to produce dozens of them during their educational career. Writing a formal essay may not be the easiest task. But fear not: our custom-writing team is here to guide...

Narrative essays are unlike anything you wrote throughout your academic career. Instead of writing a formal paper, you need to tell a story. Familiar elements such as evidence and arguments are replaced with exposition and character development. The importance of writing an outline for an essay like this is hard...

A précis is a brief synopsis of a written piece. It is used to summarize and analyze a text’s main points. If you need to write a précis for a research paper or the AP Lang exam, you’ve come to the right place. In this comprehensive guide by Custom-Writing.org, you’ll...

A synthesis essay requires you to work with multiple sources. You combine the information gathered from them to present a well-rounded argument on a topic. Are you looking for the ultimate guide on synthesis essay writing? You’ve come to the right place! In this guide by our custom writing team,...

Do you know how to make your essay stand out? One of the easiest ways is to start your introduction with a catchy hook. A hook is a phrase or a sentence that helps to grab the reader’s attention. After reading this article by Custom-Writing.org, you will be able to...

Critical thinking is the process of evaluating and analyzing information. People who use it in everyday life are open to different opinions. They rely on reason and logic when making conclusions about certain issues. A critical thinking essay shows how your thoughts change as you research your topic. This type...

Process analysis is an explanation of how something works or happens. Want to know more? Read the following article prepared by our custom writing specialists and learn about: process analysis and its typesa process analysis outline tipsfree examples and other tips that might be helpful for your college assignment So,...

A visual analysis essay is an academic paper type that history and art students often deal with. It consists of a detailed description of an image or object. It can also include an interpretation or an argument that is supported by visual evidence. In this article, our custom writing experts...

Want to know how to write a reflection paper for college or school? To do that, you need to connect your personal experiences with theoretical knowledge. Usually, students are asked to reflect on a documentary, a text, or their experience. Sometimes one needs to write a paper about a lesson...

A character analysis is an examination of the personalities and actions of protagonists and antagonists that make up a story. It discusses their role in the story, evaluates their traits, and looks at their conflicts and experiences. You might need to write this assignment in school or college. Like any...
Purdue Online Writing Lab Purdue OWL® College of Liberal Arts
Writing a Literature Review

Welcome to the Purdue OWL
This page is brought to you by the OWL at Purdue University. When printing this page, you must include the entire legal notice.
Copyright ©1995-2018 by The Writing Lab & The OWL at Purdue and Purdue University. All rights reserved. This material may not be published, reproduced, broadcast, rewritten, or redistributed without permission. Use of this site constitutes acceptance of our terms and conditions of fair use.
A literature review is a document or section of a document that collects key sources on a topic and discusses those sources in conversation with each other (also called synthesis ). The lit review is an important genre in many disciplines, not just literature (i.e., the study of works of literature such as novels and plays). When we say “literature review” or refer to “the literature,” we are talking about the research ( scholarship ) in a given field. You will often see the terms “the research,” “the scholarship,” and “the literature” used mostly interchangeably.
Where, when, and why would I write a lit review?
There are a number of different situations where you might write a literature review, each with slightly different expectations; different disciplines, too, have field-specific expectations for what a literature review is and does. For instance, in the humanities, authors might include more overt argumentation and interpretation of source material in their literature reviews, whereas in the sciences, authors are more likely to report study designs and results in their literature reviews; these differences reflect these disciplines’ purposes and conventions in scholarship. You should always look at examples from your own discipline and talk to professors or mentors in your field to be sure you understand your discipline’s conventions, for literature reviews as well as for any other genre.
A literature review can be a part of a research paper or scholarly article, usually falling after the introduction and before the research methods sections. In these cases, the lit review just needs to cover scholarship that is important to the issue you are writing about; sometimes it will also cover key sources that informed your research methodology.
Lit reviews can also be standalone pieces, either as assignments in a class or as publications. In a class, a lit review may be assigned to help students familiarize themselves with a topic and with scholarship in their field, get an idea of the other researchers working on the topic they’re interested in, find gaps in existing research in order to propose new projects, and/or develop a theoretical framework and methodology for later research. As a publication, a lit review usually is meant to help make other scholars’ lives easier by collecting and summarizing, synthesizing, and analyzing existing research on a topic. This can be especially helpful for students or scholars getting into a new research area, or for directing an entire community of scholars toward questions that have not yet been answered.
What are the parts of a lit review?
Most lit reviews use a basic introduction-body-conclusion structure; if your lit review is part of a larger paper, the introduction and conclusion pieces may be just a few sentences while you focus most of your attention on the body. If your lit review is a standalone piece, the introduction and conclusion take up more space and give you a place to discuss your goals, research methods, and conclusions separately from where you discuss the literature itself.
Introduction:
- An introductory paragraph that explains what your working topic and thesis is
- A forecast of key topics or texts that will appear in the review
- Potentially, a description of how you found sources and how you analyzed them for inclusion and discussion in the review (more often found in published, standalone literature reviews than in lit review sections in an article or research paper)
- Summarize and synthesize: Give an overview of the main points of each source and combine them into a coherent whole
- Analyze and interpret: Don’t just paraphrase other researchers – add your own interpretations where possible, discussing the significance of findings in relation to the literature as a whole
- Critically Evaluate: Mention the strengths and weaknesses of your sources
- Write in well-structured paragraphs: Use transition words and topic sentence to draw connections, comparisons, and contrasts.
Conclusion:
- Summarize the key findings you have taken from the literature and emphasize their significance
- Connect it back to your primary research question
How should I organize my lit review?
Lit reviews can take many different organizational patterns depending on what you are trying to accomplish with the review. Here are some examples:
- Chronological : The simplest approach is to trace the development of the topic over time, which helps familiarize the audience with the topic (for instance if you are introducing something that is not commonly known in your field). If you choose this strategy, be careful to avoid simply listing and summarizing sources in order. Try to analyze the patterns, turning points, and key debates that have shaped the direction of the field. Give your interpretation of how and why certain developments occurred (as mentioned previously, this may not be appropriate in your discipline — check with a teacher or mentor if you’re unsure).
- Thematic : If you have found some recurring central themes that you will continue working with throughout your piece, you can organize your literature review into subsections that address different aspects of the topic. For example, if you are reviewing literature about women and religion, key themes can include the role of women in churches and the religious attitude towards women.
- Qualitative versus quantitative research
- Empirical versus theoretical scholarship
- Divide the research by sociological, historical, or cultural sources
- Theoretical : In many humanities articles, the literature review is the foundation for the theoretical framework. You can use it to discuss various theories, models, and definitions of key concepts. You can argue for the relevance of a specific theoretical approach or combine various theorical concepts to create a framework for your research.
What are some strategies or tips I can use while writing my lit review?
Any lit review is only as good as the research it discusses; make sure your sources are well-chosen and your research is thorough. Don’t be afraid to do more research if you discover a new thread as you’re writing. More info on the research process is available in our "Conducting Research" resources .
As you’re doing your research, create an annotated bibliography ( see our page on the this type of document ). Much of the information used in an annotated bibliography can be used also in a literature review, so you’ll be not only partially drafting your lit review as you research, but also developing your sense of the larger conversation going on among scholars, professionals, and any other stakeholders in your topic.
Usually you will need to synthesize research rather than just summarizing it. This means drawing connections between sources to create a picture of the scholarly conversation on a topic over time. Many student writers struggle to synthesize because they feel they don’t have anything to add to the scholars they are citing; here are some strategies to help you:
- It often helps to remember that the point of these kinds of syntheses is to show your readers how you understand your research, to help them read the rest of your paper.
- Writing teachers often say synthesis is like hosting a dinner party: imagine all your sources are together in a room, discussing your topic. What are they saying to each other?
- Look at the in-text citations in each paragraph. Are you citing just one source for each paragraph? This usually indicates summary only. When you have multiple sources cited in a paragraph, you are more likely to be synthesizing them (not always, but often
- Read more about synthesis here.
The most interesting literature reviews are often written as arguments (again, as mentioned at the beginning of the page, this is discipline-specific and doesn’t work for all situations). Often, the literature review is where you can establish your research as filling a particular gap or as relevant in a particular way. You have some chance to do this in your introduction in an article, but the literature review section gives a more extended opportunity to establish the conversation in the way you would like your readers to see it. You can choose the intellectual lineage you would like to be part of and whose definitions matter most to your thinking (mostly humanities-specific, but this goes for sciences as well). In addressing these points, you argue for your place in the conversation, which tends to make the lit review more compelling than a simple reporting of other sources.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- PLoS Comput Biol
- v.9(7); 2013 Jul

Ten Simple Rules for Writing a Literature Review
Marco pautasso.
1 Centre for Functional and Evolutionary Ecology (CEFE), CNRS, Montpellier, France
2 Centre for Biodiversity Synthesis and Analysis (CESAB), FRB, Aix-en-Provence, France
Literature reviews are in great demand in most scientific fields. Their need stems from the ever-increasing output of scientific publications [1] . For example, compared to 1991, in 2008 three, eight, and forty times more papers were indexed in Web of Science on malaria, obesity, and biodiversity, respectively [2] . Given such mountains of papers, scientists cannot be expected to examine in detail every single new paper relevant to their interests [3] . Thus, it is both advantageous and necessary to rely on regular summaries of the recent literature. Although recognition for scientists mainly comes from primary research, timely literature reviews can lead to new synthetic insights and are often widely read [4] . For such summaries to be useful, however, they need to be compiled in a professional way [5] .
When starting from scratch, reviewing the literature can require a titanic amount of work. That is why researchers who have spent their career working on a certain research issue are in a perfect position to review that literature. Some graduate schools are now offering courses in reviewing the literature, given that most research students start their project by producing an overview of what has already been done on their research issue [6] . However, it is likely that most scientists have not thought in detail about how to approach and carry out a literature review.
Reviewing the literature requires the ability to juggle multiple tasks, from finding and evaluating relevant material to synthesising information from various sources, from critical thinking to paraphrasing, evaluating, and citation skills [7] . In this contribution, I share ten simple rules I learned working on about 25 literature reviews as a PhD and postdoctoral student. Ideas and insights also come from discussions with coauthors and colleagues, as well as feedback from reviewers and editors.
Rule 1: Define a Topic and Audience
How to choose which topic to review? There are so many issues in contemporary science that you could spend a lifetime of attending conferences and reading the literature just pondering what to review. On the one hand, if you take several years to choose, several other people may have had the same idea in the meantime. On the other hand, only a well-considered topic is likely to lead to a brilliant literature review [8] . The topic must at least be:
- interesting to you (ideally, you should have come across a series of recent papers related to your line of work that call for a critical summary),
- an important aspect of the field (so that many readers will be interested in the review and there will be enough material to write it), and
- a well-defined issue (otherwise you could potentially include thousands of publications, which would make the review unhelpful).
Ideas for potential reviews may come from papers providing lists of key research questions to be answered [9] , but also from serendipitous moments during desultory reading and discussions. In addition to choosing your topic, you should also select a target audience. In many cases, the topic (e.g., web services in computational biology) will automatically define an audience (e.g., computational biologists), but that same topic may also be of interest to neighbouring fields (e.g., computer science, biology, etc.).
Rule 2: Search and Re-search the Literature
After having chosen your topic and audience, start by checking the literature and downloading relevant papers. Five pieces of advice here:
- keep track of the search items you use (so that your search can be replicated [10] ),
- keep a list of papers whose pdfs you cannot access immediately (so as to retrieve them later with alternative strategies),
- use a paper management system (e.g., Mendeley, Papers, Qiqqa, Sente),
- define early in the process some criteria for exclusion of irrelevant papers (these criteria can then be described in the review to help define its scope), and
- do not just look for research papers in the area you wish to review, but also seek previous reviews.
The chances are high that someone will already have published a literature review ( Figure 1 ), if not exactly on the issue you are planning to tackle, at least on a related topic. If there are already a few or several reviews of the literature on your issue, my advice is not to give up, but to carry on with your own literature review,

The bottom-right situation (many literature reviews but few research papers) is not just a theoretical situation; it applies, for example, to the study of the impacts of climate change on plant diseases, where there appear to be more literature reviews than research studies [33] .
- discussing in your review the approaches, limitations, and conclusions of past reviews,
- trying to find a new angle that has not been covered adequately in the previous reviews, and
- incorporating new material that has inevitably accumulated since their appearance.
When searching the literature for pertinent papers and reviews, the usual rules apply:
- be thorough,
- use different keywords and database sources (e.g., DBLP, Google Scholar, ISI Proceedings, JSTOR Search, Medline, Scopus, Web of Science), and
- look at who has cited past relevant papers and book chapters.
Rule 3: Take Notes While Reading
If you read the papers first, and only afterwards start writing the review, you will need a very good memory to remember who wrote what, and what your impressions and associations were while reading each single paper. My advice is, while reading, to start writing down interesting pieces of information, insights about how to organize the review, and thoughts on what to write. This way, by the time you have read the literature you selected, you will already have a rough draft of the review.
Of course, this draft will still need much rewriting, restructuring, and rethinking to obtain a text with a coherent argument [11] , but you will have avoided the danger posed by staring at a blank document. Be careful when taking notes to use quotation marks if you are provisionally copying verbatim from the literature. It is advisable then to reformulate such quotes with your own words in the final draft. It is important to be careful in noting the references already at this stage, so as to avoid misattributions. Using referencing software from the very beginning of your endeavour will save you time.
Rule 4: Choose the Type of Review You Wish to Write
After having taken notes while reading the literature, you will have a rough idea of the amount of material available for the review. This is probably a good time to decide whether to go for a mini- or a full review. Some journals are now favouring the publication of rather short reviews focusing on the last few years, with a limit on the number of words and citations. A mini-review is not necessarily a minor review: it may well attract more attention from busy readers, although it will inevitably simplify some issues and leave out some relevant material due to space limitations. A full review will have the advantage of more freedom to cover in detail the complexities of a particular scientific development, but may then be left in the pile of the very important papers “to be read” by readers with little time to spare for major monographs.
There is probably a continuum between mini- and full reviews. The same point applies to the dichotomy of descriptive vs. integrative reviews. While descriptive reviews focus on the methodology, findings, and interpretation of each reviewed study, integrative reviews attempt to find common ideas and concepts from the reviewed material [12] . A similar distinction exists between narrative and systematic reviews: while narrative reviews are qualitative, systematic reviews attempt to test a hypothesis based on the published evidence, which is gathered using a predefined protocol to reduce bias [13] , [14] . When systematic reviews analyse quantitative results in a quantitative way, they become meta-analyses. The choice between different review types will have to be made on a case-by-case basis, depending not just on the nature of the material found and the preferences of the target journal(s), but also on the time available to write the review and the number of coauthors [15] .
Rule 5: Keep the Review Focused, but Make It of Broad Interest
Whether your plan is to write a mini- or a full review, it is good advice to keep it focused 16 , 17 . Including material just for the sake of it can easily lead to reviews that are trying to do too many things at once. The need to keep a review focused can be problematic for interdisciplinary reviews, where the aim is to bridge the gap between fields [18] . If you are writing a review on, for example, how epidemiological approaches are used in modelling the spread of ideas, you may be inclined to include material from both parent fields, epidemiology and the study of cultural diffusion. This may be necessary to some extent, but in this case a focused review would only deal in detail with those studies at the interface between epidemiology and the spread of ideas.
While focus is an important feature of a successful review, this requirement has to be balanced with the need to make the review relevant to a broad audience. This square may be circled by discussing the wider implications of the reviewed topic for other disciplines.
Rule 6: Be Critical and Consistent
Reviewing the literature is not stamp collecting. A good review does not just summarize the literature, but discusses it critically, identifies methodological problems, and points out research gaps [19] . After having read a review of the literature, a reader should have a rough idea of:
- the major achievements in the reviewed field,
- the main areas of debate, and
- the outstanding research questions.
It is challenging to achieve a successful review on all these fronts. A solution can be to involve a set of complementary coauthors: some people are excellent at mapping what has been achieved, some others are very good at identifying dark clouds on the horizon, and some have instead a knack at predicting where solutions are going to come from. If your journal club has exactly this sort of team, then you should definitely write a review of the literature! In addition to critical thinking, a literature review needs consistency, for example in the choice of passive vs. active voice and present vs. past tense.
Rule 7: Find a Logical Structure
Like a well-baked cake, a good review has a number of telling features: it is worth the reader's time, timely, systematic, well written, focused, and critical. It also needs a good structure. With reviews, the usual subdivision of research papers into introduction, methods, results, and discussion does not work or is rarely used. However, a general introduction of the context and, toward the end, a recapitulation of the main points covered and take-home messages make sense also in the case of reviews. For systematic reviews, there is a trend towards including information about how the literature was searched (database, keywords, time limits) [20] .
How can you organize the flow of the main body of the review so that the reader will be drawn into and guided through it? It is generally helpful to draw a conceptual scheme of the review, e.g., with mind-mapping techniques. Such diagrams can help recognize a logical way to order and link the various sections of a review [21] . This is the case not just at the writing stage, but also for readers if the diagram is included in the review as a figure. A careful selection of diagrams and figures relevant to the reviewed topic can be very helpful to structure the text too [22] .
Rule 8: Make Use of Feedback
Reviews of the literature are normally peer-reviewed in the same way as research papers, and rightly so [23] . As a rule, incorporating feedback from reviewers greatly helps improve a review draft. Having read the review with a fresh mind, reviewers may spot inaccuracies, inconsistencies, and ambiguities that had not been noticed by the writers due to rereading the typescript too many times. It is however advisable to reread the draft one more time before submission, as a last-minute correction of typos, leaps, and muddled sentences may enable the reviewers to focus on providing advice on the content rather than the form.
Feedback is vital to writing a good review, and should be sought from a variety of colleagues, so as to obtain a diversity of views on the draft. This may lead in some cases to conflicting views on the merits of the paper, and on how to improve it, but such a situation is better than the absence of feedback. A diversity of feedback perspectives on a literature review can help identify where the consensus view stands in the landscape of the current scientific understanding of an issue [24] .
Rule 9: Include Your Own Relevant Research, but Be Objective
In many cases, reviewers of the literature will have published studies relevant to the review they are writing. This could create a conflict of interest: how can reviewers report objectively on their own work [25] ? Some scientists may be overly enthusiastic about what they have published, and thus risk giving too much importance to their own findings in the review. However, bias could also occur in the other direction: some scientists may be unduly dismissive of their own achievements, so that they will tend to downplay their contribution (if any) to a field when reviewing it.
In general, a review of the literature should neither be a public relations brochure nor an exercise in competitive self-denial. If a reviewer is up to the job of producing a well-organized and methodical review, which flows well and provides a service to the readership, then it should be possible to be objective in reviewing one's own relevant findings. In reviews written by multiple authors, this may be achieved by assigning the review of the results of a coauthor to different coauthors.
Rule 10: Be Up-to-Date, but Do Not Forget Older Studies
Given the progressive acceleration in the publication of scientific papers, today's reviews of the literature need awareness not just of the overall direction and achievements of a field of inquiry, but also of the latest studies, so as not to become out-of-date before they have been published. Ideally, a literature review should not identify as a major research gap an issue that has just been addressed in a series of papers in press (the same applies, of course, to older, overlooked studies (“sleeping beauties” [26] )). This implies that literature reviewers would do well to keep an eye on electronic lists of papers in press, given that it can take months before these appear in scientific databases. Some reviews declare that they have scanned the literature up to a certain point in time, but given that peer review can be a rather lengthy process, a full search for newly appeared literature at the revision stage may be worthwhile. Assessing the contribution of papers that have just appeared is particularly challenging, because there is little perspective with which to gauge their significance and impact on further research and society.
Inevitably, new papers on the reviewed topic (including independently written literature reviews) will appear from all quarters after the review has been published, so that there may soon be the need for an updated review. But this is the nature of science [27] – [32] . I wish everybody good luck with writing a review of the literature.
Acknowledgments
Many thanks to M. Barbosa, K. Dehnen-Schmutz, T. Döring, D. Fontaneto, M. Garbelotto, O. Holdenrieder, M. Jeger, D. Lonsdale, A. MacLeod, P. Mills, M. Moslonka-Lefebvre, G. Stancanelli, P. Weisberg, and X. Xu for insights and discussions, and to P. Bourne, T. Matoni, and D. Smith for helpful comments on a previous draft.
Funding Statement
This work was funded by the French Foundation for Research on Biodiversity (FRB) through its Centre for Synthesis and Analysis of Biodiversity data (CESAB), as part of the NETSEED research project. The funders had no role in the preparation of the manuscript.
Journal Article Review in APA Style
Journal article reviews refer to the appraisal of potencies and limitations of an article’s opinion and subject matter. The article reviews offer the readers with an explanation, investigation and clarification to evaluate the importance of the article. A journal article review usually follows the APA style, which is in itself an exceptional mode of writing. Writing a journal article review in APA style requires a thorough reading of an article and then present our personal opinions on its subject matter.
In order to write a journal article review in APA style, one must necessarily conform to the detailed guidelines of APA style of writing. As such, a few tips for writing a journal article review in APA style have been provided in details below.
Tips for Writing Journal Article Review in APA Style
Getting started.
Read the complete article. Most journal articles use highly complicated and difficult language and wording. Thus, it is suggested to read the article thoroughly several times to understand it perfectly. Select a statement that effectively conveys the main idea of your review. Present the ideas in a rational order, keeping in mind that all opinions must sustain the main idea.
Start with a header with citation
Journal article reviews start with a header, including citation of the sources being reviewed. This citation is mentioned at the top of the review, following the APA style (refer to the APA style manual for more information). We will need the author’s name for the article, title of the article, journal of the published article, volume and issue number, publication date, and page numbers for the article.
Write a summary
The introductory paragraph of the review should provide a brief summary of the article, strictly limiting it to one to three paragraphs depending on the article length. The summary should discuss only the most imperative details about the article, like the author’s intention in writing the article, how the study was conducted, how the article relates to other work on the same subject, the results and other relevant information from the article.
Body of the review
The succeeding paragraphs of the review should present your ideas and opinions on the article. Discuss the significance and suggestion of the results of the study. The body of the article review should be limited to one to two paragraphs, including your understanding of the article, quotations from the article demonstrating your main ideas, discussing the article’s limitations and how to overcome them.
Concluding the review
The concluding paragraphs of the review should provide your personal appraisal of the journal article. Discuss whether the article is well-written or not, whether any information is missing, or if further research is necessary on the subject. Also, write a paragraph on how the author could develop the study results, what the information means on a large scale, how further investigation can develop the subject matter, and how the knowledge of this field can be extended further.
Citation and Revision
In-text citation of direct quotes or paraphrases from the article can be done using the author’s name, year of publication and page numbers (refer to the APA-style manual for citation guidelines). After finishing the writing of journal article review in APA style, it would be advised to re-visit the review after a few days and then re-read it altogether. By doing this, you will be able to view the review with a new perspective and may detect mistakes that were previously left undetected.
The above mentioned tips will help and guide you for writing a journal article review in APA style. However, while writing a journal article review, remember that you are undertaking more than just a narrative review. Thus, the article review should not merely focus on discussing what the article is about, but should reveal your personal ideas and opinions on the article.
Related Posts
Formatting quotations in mla style.
MLA style of formatting is one of the most commonly used styles for writing and formatting papers. The present article on ‘Formatting Quotations in MLA Style’ presents some effective tips to help you learn the ways and techniques essential for formatting quotations in MLA style. Some useful tips for formatting quotations in MLA style are […]
MLA style citation: In-text
The basic purpose of using in-text citations and references is to enable the reader to easily locate/access the sources from which data/information has been collected. The MLA style citations are formatted according to the guidelines of the Modern Language Association. A paper with MLA style citation should include in-text citations with a corresponding reference list, […]
Formatting tables, graphs, and other visuals in your research paper
The format in which you present your research data is very important because it helps you communicate your data to your reader and editors in the best possible way. We always recommend to use a good Data Management in order to keep all your date organized. Although there are many formats in which tables, graphs, and […]
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
Guidelines for tuberculosis screening and preventive treatment among pregnant and breastfeeding women living with HIV in PEPFAR-supported countries
Contributed equally to this work with: Yael Hirsch-Moverman, Allison Hsu
Roles Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliations ICAP at Columbia University, New York, New York, United States of America, Department of Epidemiology, Mailman School of Public Health Columbia University, New York, New York, United States of America
Roles Data curation, Formal analysis, Project administration, Writing – original draft, Writing – review & editing
Affiliation ICAP at Columbia University, New York, New York, United States of America
Roles Conceptualization, Formal analysis, Funding acquisition, Methodology, Writing – review & editing
¶ ‡ EJA and AAH also contributed equally to this work.
Affiliations ICAP at Columbia University, New York, New York, United States of America, Department of Epidemiology, Mailman School of Public Health Columbia University, New York, New York, United States of America, Department of Pediatrics, Vagelos College of Physicians & Surgeons, Columbia University, New York, New York, United States of America
Roles Conceptualization, Writing – review & editing
Affiliation Division of Global HIV and TB, U.S. Centers for Disease Control and Prevention, Atlanta, Georgia
Roles Conceptualization, Formal analysis, Funding acquisition, Methodology, Supervision, Writing – review & editing
- Yael Hirsch-Moverman,
- Allison Hsu,
- Elaine J. Abrams,
- William P. Killam,
- Brittany Moore,
- Andrea A. Howard

- Published: April 16, 2024
- https://doi.org/10.1371/journal.pone.0296993
- Reader Comments
Tuberculosis (TB) preventive treatment (TPT) is recommended by the World Health Organization (WHO) for persons living with HIV, including pregnant and breastfeeding women. Given the President’s Emergency Plan for AIDS Relief (PEPFAR)’s investment in TPT services for persons living with HIV as a strategy to prevent TB as well as uncertainty in guidelines and policy regarding use of TPT during pregnancy and the postpartum period, we conducted a review of current relevant national guidelines among PEPFAR-supported countries.
Our review included 44/49 PEPFAR-supported countries to determine if TB screening and TPT are recommended specifically for pregnant and breastfeeding women living with HIV (WLHIV). National guidelines reviewed and abstracted included TB, HIV, prevention of vertical HIV transmission, TPT, and any other relevant guidelines. We abstracted information regarding TB screening, including screening tools and frequency; and TPT, including timing, regimen, frequency, and laboratory monitoring.
Of 44 PEPFAR-supported countries for which guidelines were reviewed, 66% were high TB incidence countries; 41% were classified by WHO as high TB burden countries, and 43% as high HIV-associated TB burden countries. We found that 64% (n = 28) of countries included TB screening recommendations for pregnant WLHIV in their national guidelines, and most (n = 35, 80%) countries recommend TPT for pregnant WLHIV. Fewer countries included recommendations for breastfeeding as compared to pregnant WLHIV, with only 32% (n = 14) mentioning TB screening and 45% (n = 20) specifically recommending TPT for this population; most of these recommend isoniazid-based TPT regimens for pregnant and breastfeeding WLHIV. However, several countries also recommend isoniazid combined with rifampicin (3RH) or rifapentine (3HP).
Conclusions
Despite progress in the number of PEPFAR-supported countries that specifically include TB screening and TPT recommendations for pregnant and breastfeeding WLHIV in their national guidelines, many PEPFAR-supported countries still do not include specific screening and TPT recommendations for pregnant and breastfeeding WLHIV.
Citation: Hirsch-Moverman Y, Hsu A, Abrams EJ, Killam WP, Moore B, Howard AA (2024) Guidelines for tuberculosis screening and preventive treatment among pregnant and breastfeeding women living with HIV in PEPFAR-supported countries. PLoS ONE 19(4): e0296993. https://doi.org/10.1371/journal.pone.0296993
Editor: Dickens Otieno Onyango, Kisumu County, KENYA
Received: August 3, 2023; Accepted: December 21, 2023; Published: April 16, 2024
This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Data Availability: All relevant data are within the paper and its Supporting information files. The dashboard mentioned can be found at https://icap.columbia.edu/tools_resources/tpt-recommendations-dashboard-for-pepfar-supported-countries/ .
Funding: This work was supported by a grant from the US Centers for Disease Control and Prevention (CDC) under PEPFAR, Cooperative Agreement #NU2GGH002216. The funders assisted in study design, decision to publish, and preparation of the manuscript. Authors AAH, EJA, AH, and YHM were supported by the funders.
Competing interests: The authors have declared that no competing interests exist.
Introduction
Tuberculosis (TB) is the leading cause of morbidity and mortality among persons living with HIV (PLHIV) who reside in low- and middle-income countries (LMIC) with a high TB burden [ 1 ]. Among women, TB incidence peaks during reproductive age, irrespective of HIV status. The World Health Organization (WHO) reports incidence rate ratios of TB in pregnant and postpartum women as compared to non-pregnant women of 1.4 and 1.9, respectively [ 2 – 5 ]. The End TB Strategy calls for provision of TB preventive treatment (TPT) for persons at high risk for TB [ 6 , 7 ]. Studies have shown that provision of TPT to PLHIV reduced TB incidence and mortality by 37%, independent of antiretroviral therapy (ART) status [ 8 , 9 ].
Of the 38.4 million PLHIV, 19.7 million are women aged ≥15 years, and an estimated 1.3 million women living with HIV (WLHIV) became pregnant in 2021 [ 10 – 12 ]. Both HIV and pregnancy are associated with immune suppression and an increased risk of progression to active TB disease [ 13 – 15 ]. Maternal TB disease can have devastating consequences for both mothers and infants including increased maternal and infant mortality; adverse pregnancy outcomes including prematurity, low birth weight, and intrauterine growth restriction; and infant TB [ 5 , 16 – 20 ]. TB in pregnant women increases the risk of maternal mortality three-fold (odds ratio [OR] = 2.8, 95% confidence interval [CI] 1.7–4.6) and perinatal death four-fold (OR = 4.2; 95% CI 1.5–11.8) [ 20 ]. Infants infected with TB perinatally have a very high risk of death [ 18 , 19 ]. Pregnant WLHIV have a high risk of acquiring TB, which can have severe consequences for both mother and infant [ 17 ]. Maternal TB more than doubles the risk of perinatal HIV transmission and significantly increases mortality for all children in the household [ 17 , 21 – 24 ].
The 2011 WHO guidelines on isoniazid preventive therapy (IPT) for PLHIV in resource-constrained settings recommend IPT for all PLHIV regardless of pregnancy status [ 7 ]. However, these guidelines as well as the 2018 WHO guidelines on latent TB infection advise caution and clinical judgement when deciding the best time to start TPT in pregnant women in light of emerging safety concerns of TPT during pregnancy [ 25 ]. The International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) P1078 TB APPRISE study, a phase 4 randomized controlled trial evaluating the safety and timing of IPT initiation among pregnant WLHIV in high TB burden settings, reported no difference in maternal hepatotoxicity but found a higher risk of a composite adverse pregnancy outcome (fetal demise, prematurity, low birth weight, and congenital anomaly) among women who received IPT during pregnancy compared to those who delayed IPT initiation until the postpartum period [ 26 ]. However, observational sub-studies of TPT trials assessing the use of daily and weekly isoniazid during pregnancy or at conception found no association between IPT use and adverse pregnancy outcomes [ 27 , 28 ]. A recent systematic review and meta-analysis of nine studies found inconsistent associations between IPT and adverse pregnancy outcomes [ 27 , 28 ]. The authors concluded that considering the grave consequences of active TB in pregnancy, current evidence does not support systematic deferral of IPT until the postpartum period [ 29 ]. This conclusion was echoed in the 2020 WHO Consolidated Guidelines on Tuberculosis module on prevention, which strongly encouraged, when feasible, baseline liver function tests (LFTs) when IPT is given in pregnancy or within three months of delivery [ 30 ]. No specific guidance on TPT for breastfeeding women was provided despite countries recommending deferral of TPT to the postpartum period, which may raise concerns for providers and patients about the infant’s exposure to TPT drugs through breast milk.
Implementation and scale-up of TB case finding, prevention, and treatment services have been a major focus in the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR) programming. In 2018, the Department of State’s Office of the U.S. Global AIDS Coordinator and Health Diplomacy announced a goal to provide TPT to all 13.6 million PLHIV who are on ART in PEPFAR-supported countries by 2021. In 2019, the Department of State’s Office of the U.S. Global AIDS Coordinator and Health Diplomacy included provision of TPT for all PLHIV as one of 13 minimum program requirements for PEPFAR funding [ 31 ]. The Technical Considerations in the PEPFAR 2022 Country and Regional Operational Plan (COP/ROP) Guidance for all PEPFAR-Supported Countries stated that it is imperative for PMTCT programs to screen for active TB during clinical encounters and ensure linkage to diagnostic testing and treatment, however given the uncertainties around the safety, efficacy and appropriate timing of TPT in pregnant WLHIV, country programs should consider the benefits and risks of deferring TPT initiation for pregnant WLHIV based on their ART history, clinical presentation, and documentation of close contact with a person with active TB disease [ 32 ]. More specific guidance for breastfeeding WLHIV was not provided.
Given PEPFAR’s investment in TPT services for PLHIV, and the lack of clarity in guidelines and policy regarding use of TPT during pregnancy or breastfeeding, we conducted a review of current relevant national TB and HIV guidelines among PEPFAR-supported countries. Our objectives were to determine: (1) if TB screening is currently recommended specifically for pregnant and breastfeeding WLHIV; (2) whether TPT is currently recommended during pregnancy or the postpartum period; and (3) whether any laboratory or radiologic tests are recommended when evaluating WLHIV for TPT eligibility during pregnancy or the postpartum period.
Materials and methods
We obtained current national guidelines from countries supported by PEPFAR through CDC in 2021 (n = 49), including TB, HIV, prevention of maternal-to-child HIV transmission (PMTCT), TPT, and any other relevant guidelines in these countries. Guidelines were collected between November 2021 and June 2022.
We created a data abstraction form to record information on recommendations regarding: TB screening, including screening tools and frequency; and TPT, including timing, regimen, frequency, and laboratory testing for eligibility and monitoring, for the following populations: (1) PLHIV, (2) pregnant WLHIV, and (3) breastfeeding WLHIV. The data abstraction form was piloted and modified to ensure consistency and clarity.
Data abstraction was conducted between December 2021 and June 2022, with the exception of the updated South African 2022 TPT guidelines, which were published and abstracted in September 2022 and the Vietnam and Thailand guidelines, which were abstracted in December 2022. Two trained data abstractors fluent in the language of the guidelines abstracted information from all guideline documents for a given country independently except for Thailand where the abstraction was conducted by a single data abstractor. The two abstractors then met to discuss their findings and resolve any discrepancies. Where discrepancies could not be resolved, an investigator was consulted.
Abstracted data for each country were compiled into a comprehensive table containing key information for each country. This table contained information for PLHIV, pregnant WLHIV, and breastfeeding WLHIV and was used to create a dashboard with filtering capabilities that allowed for a quick visual summary of recommendations [ 33 ]. In instances where a country had multiple guidelines and TB screening was recommended for a specific population in one guideline and another guideline did not include this information, the country was recorded as “TB screening recommended.” The same rule was applied to TPT recommendations. In the one instance where the guidelines for a particular country were contradictory, (i.e., TPT was recommended for a population in one guideline and not recommended in another guideline), we used the guideline that was published more recently to categorize the country. We defined high TB incidence countries as >100 TB cases per 100,000 population reported in 2020, and defined countries as high TB, TB/HIV or MDR/RR-TB burden based on WHO’s classification [ 1 , 34 ].
We obtained and reviewed guidelines from 90% (44/49) of PEPFAR-supported countries. Both TB and HIV guidelines were reviewed for all countries except Cambodia and Senegal, where we only obtained HIV guidelines. Some countries also had PMTCT and/or TPT guidelines available for review. Guideline publication year varied greatly within and across countries, with Haiti having the oldest guideline (TB guidelines from 2009) and Ukraine and South Africa having the most recent guidelines in place (PMTCT and HIV guidelines from 2022, TPT guidelines from 2022, respectively).
Of the 44 countries for which guidelines were reviewed, 66% (29/44) were high TB incidence countries; 18 countries (41%) were classified by WHO as high TB burden countries, 19 (43%) were classified as high HIV-associated TB burden countries, and 14 (32%) were classified as high multi-drug resistant/rifampicin-resistant TB (MDR/RR-TB) burden countries [ 1 ]. More than half (27/44) of countries were from the WHO African Region (see Table 1 ).
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0296993.t001
As shown in Fig 1 , TB screening and TPT recommendations for PLHIV were included in guidelines from all 44 countries reviewed. With regards to pregnant WLHIV, we found that 64% (n = 28) of countries included recommendations for TB screening in at least one of their national guidelines ( Fig 1a ) and 84% (n = 37) included recommendations regarding TPT, with 80% (n = 35) recommending TPT for pregnant WLHIV and 5% (n = 2) not recommending TPT for pregnant WLHIV ( Fig 1b ). Fewer countries had specific recommendations for breastfeeding WLHIV in their national guidelines, with only 32% (n = 14) of countries including TB screening recommendations ( Fig 1a ) and 48% (n = 21) including TPT recommendations, with 45% (n = 20) recommending TPT and 2% (n = 1) not recommending TPT for breastfeeding women ( Fig 1b ).
https://doi.org/10.1371/journal.pone.0296993.g001
S1 Table summarizes guidelines for all countries reviewed, outlining TB screening and TPT recommendations, TPT regimens, and any special considerations, with a focus on pregnant and breastfeeding WLHIV.
Recommendations for pregnant women living with HIV
In all 28 countries that specifically mention TB screening for pregnant WLHIV in their guidelines, a symptom screen is recommended; however, five of these countries (Brazil, Kenya, Nigeria, Thailand, Zimbabwe) also recommend a chest x-ray. Zimbabwe additionally recommends using body mass index as a TB screening tool for pregnant WLHIV and a molecular WHO-recommended rapid diagnostic test (mWRD) for individuals newly diagnosed with HIV in their 2017 TB guidelines. South Africa included a recommendation that pregnant WLHIV receive a mWRD at the first antenatal care visit in their 2022 TPT guidelines.
TPT during pregnancy is recommended in guidelines from 36 countries; for five of these countries, the guidelines did not specify a TPT regimen. Of the 31 countries that specify a TPT regimen for pregnant WLHIV, all recommend an isoniazid-based regimen. Among these, four countries also recommend an alternate regimen: Lesotho only specifies three months of isoniazid and rifampicin (3RH) in their 2021 PMTCT guidelines, while in their older guidelines six months of isoniazid (6H) is recommended; Nigeria specifies 3RH as an alternative to 6H and nine months of isoniazid (9H) in their 2020 HIV guidelines; Uganda recommends three months of isoniazid and rifapentine (3HP) as an equally viable alternative to 6H if the patient is not on protease inhibitors in their 2020 HIV guidelines; and Zimbabwe recommends 3HP over 6H –the 2020 TPT guidelines only recommend 6H if contraindications to 3HP exist. For Burundi, TPT for pregnant WLHIV is not mentioned in the 2020 HIV guidelines. However, in the 2020 TB guidelines, TPT is not recommended for pregnant WLHIV, stating that pregnant women should defer TPT until the postpartum period. Malawi’s 2018 HIV guidelines recommend TPT for pregnant WLHIV, but this recommendation was reversed in their 2019 addendum to the 2018 HIV guidelines, where it is stated that the TPT regimens recommended in Malawi (6H and 3HP) are not suitable for pregnant WLHIV. In Uganda, the 2020 HIV guidelines state that pregnant WLHIV should defer TPT until three months post-delivery unless there is a history of TB exposure or advanced HIV disease (WHO Stage 3/4 or CD4 count <200 cells/mm 3 ), in which case either 6H or 3HP are recommended. South Africa also conditionally recommends deferring TPT until 6 weeks after delivery for pregnant WLHIV with a CD4 count >350 cells/mm 3 in their 2020 HIV guidelines and 2022 TPT guidelines. The 2021 HIV guidelines from Thailand recommend TPT if the pregnant WLHIV was exposed to TB within the last year, otherwise it is recommended to defer TPT until 12 weeks postpartum.
LFTs prior to TPT initiation are required for pregnant WLHIV in three country guidelines: Cambodia (HIV 2020), Vietnam (TPT 2021), and Zambia (TPT 2019); Vietnam also requires monthly LFTs during TPT. Five other country guidelines strongly encourage baseline LFTs in pregnant WLHIV: Eswatini (TB 2019), India (TPT 2021), Kenya (TB 2021), Uganda (HIV 2020), and Zimbabwe (TB 2017). Uganda recommends doing LFTs both at baseline and after three months of treatment if possible, as well as closely monitoring WLHIV who get pregnant while on TPT for side effects.
Recommendations for breastfeeding women living with HIV.
All 14 countries that mention TB screening for breastfeeding WLHIV in their national guidelines recommend a symptom screen; none recommend other TB screening tools specifically for breastfeeding WLHIV.
Only 20 countries specifically recommend TPT for breastfeeding WLHIV, including 18 countries that specify the TPT regimen and two countries that do not specify the regimen (Nigeria and Zambia). All regimens recommended for breastfeeding WLHIV are isoniazid-based, except Thailand, where only 3HP is recommended in their 2021 HIV guidelines. Eswatini includes 3HP as an equally viable alternative to 6H as long as ART medications are not contraindicated in their 2019 TB guidelines. Eswatini also recommends that TPT be initiated after one month of ART in both their 2019 TB guidelines and 2018 HIV guidelines, and within three months of delivery in their 2019 TB guidelines. Burundi recommends the postponement of TPT until the cessation of breastfeeding in their 2020 TB guidelines. The Thailand 2021 HIV guidelines recommend that unless the breastfeeding woman was recently exposed to TB (i.e., in the past year), TPT should be deferred until 12 weeks postpartum.
LFTs prior to TPT initiation in breastfeeding WLHIV are required in three country guidelines: Cambodia (HIV 2020), Vietnam (TPT 2021), and Zambia (TPT 2019); Vietnam also requires monthly LFTs during TPT. Three other country guidelines strongly encourage baseline LFTs: Eswatini (TB 2019), India (TPT 2021), and Zimbabwe (TB 2017); Zimbabwe guidelines refer specifically to the period within 3 months of delivery.
Recommendations in high vs. low TB burden countries.
Table 2 illustrates the differences in TB screening and TPT recommendations for pregnant and breastfeeding WLHIV between high and low TB burden countries. Countries classified as high TB incidence, high TB burden and/or high TB/HIV burden are more likely to include recommendations on TB screening and TPT specifically for pregnant and breastfeeding WLHIV in their national guidelines.
https://doi.org/10.1371/journal.pone.0296993.t002
In this review we found that nearly two thirds of PEPFAR-supported countries include TB screening recommendations for pregnant WLHIV in their national guidelines, while the remainder do not specifically address pregnancy in their TB screening guidelines for PLHIV. While most (80%) countries recommend TPT for pregnant WLHIV, two countries (Burundi and Malawi) recommend not administering TPT during pregnancy, and three countries (South Africa, Thailand, and Uganda) only recommend TPT for pregnant WLHIV in specific circumstances that place them at increased risk for TB. Fewer PEPFAR-supported countries include recommendations for breastfeeding WLHIV in their national guidelines, with less than a third (32%) mentioning TB screening tools and less than half (45%) specifically recommending TPT for this population. One country (Burundi) recommends not administering TPT until the cessation of breastfeeding, and one country (Thailand) only recommends TPT for breastfeeding WLHIV in specific circumstances that place them at increased risk for TB. Eight countries require or recommend LFTs prior to TPT during pregnancy, while six countries recommend baseline LFTs during the postpartum period.
A review of TB screening and TPT recommendations for pregnant WLHIV in national guidelines published before 2017 from 38 high TB and TB/HIV burden countries found a lower proportion of countries that included TB screening (39%) and TPT recommendations (64%) than was found in our review [ 35 ]. Over half (22/38) of the included countries in this recent review overlapped with countries included in our review. Our findings suggest that there has been an increase in the proportion of high TB and TB/HIV burden countries that include TB screening and TPT recommendations for pregnant WLHIV in their national guidelines since 2017 [ 35 ]. This increase may be attributable to promotion of the End TB Strategy by the United Nations, WHO, and PEPFAR, which includes collaborative TB/HIV activities and increasing TPT provision for persons at high risk, as well as the Department of State’s Office of the U.S. Global AIDS Coordinator and Health Diplomacy’s inclusion of TPT provision for all PLHIV as a minimum program requirement for PEPFAR funding [ 6 , 31 ]. Despite the apparent increase in the proportion of high TB and TB/HIV burden countries that include TB screening and TPT recommendations for pregnant WLHIV in their national guidelines, this is not evident in low TB and TB/HIV burden countries that receive PEPFAR support, where there is a substantially lower proportion of countries that include specific recommendations on TB screening or TPT for pregnant WLHIV. This could be because countries with a higher burden of disease have a larger population at risk for TB and a need for clear guidance to be able to meet WHO TPT targets in the End TB Strategy.
To our knowledge, this is the first report that summarizes TB screening and TPT recommendations for breastfeeding WLHIV in national guidelines. Although WHO in 2011 recommended TPT for pregnant and breastfeeding women, we found that only 45% of PEPFAR-supported countries specifically recommend TPT for breastfeeding women, which is particularly relevant given that for many WLHIV, TPT will have been deferred during pregnancy. It is imperative that country guidelines routinely indicate whether their recommendations for adults apply to breastfeeding WLHIV and provide specific recommendations for this population when indicated. The postpartum period potentially represents an important time when women are still in care and should be receiving TPT that was postponed during pregnancy, and yet it is also a period when providers and patients may have concerns about the infant’s exposure to TPT drugs through breast milk.
While most countries recommend isoniazid-only TPT regimens for pregnant and breastfeeding WLHIV, several also recommend isoniazid combined with rifampicin or rifapentine. Current WHO guidelines suggest that more evidence is needed regarding use of rifamycins, and in particular rifapentine, in pregnant WLHIV even if pregnancy should not disqualify WLHIV from receiving 3RH [ 30 ]. Nigeria and Lesotho recommend 3RH along with isoniazid-based regimens in pregnant WLHIV. 3HP is currently not recommended during pregnancy by the CDC owing to limited data on rifapentine pharmacokinetics, dosing, and safety during pregnancy [ 25 , 30 , 36 ]. More data is needed on the rifamycin agents as well as new agents being studied for TPT in nonpregnant adults [ 37 ]. Despite this, both Zimbabwe and Uganda recommend 3HP in addition to isoniazid-based regimens in pregnant WLHIV, and both Thailand and Eswatini recommend 3HP in breastfeeding WLHIV. IMPAACT 2001, which studied the pharmacokinetics and safety of 3HP among pregnant women with indications for TPT, found no drug-related serious adverse events [ 38 ]. The authors concluded that 3HP use in pregnant women was safe and tolerable. There is an urgent need for timely safety and pharmacokinetic studies of new TPT regimens during the pregnancy and breastfeeding periods, and for strong pharmacovigilance systems and targeted cohort studies to further assess safety in pregnancy and breastfeeding without delaying drug access to women [ 37 – 40 ].
The routine exclusion of pregnant and breastfeeding women from clinical trials of new treatments, including treatment for TB, results in no clear dosing information and safety considerations when the drug is approved and moved into programmatic settings. Additionally, because birth and pregnancy outcomes are rarely studied in controlled trials, resulting data are difficult to interpret. These factors may be contributing to countries’ hesitancy to include TPT recommendations for these vulnerable populations in their guidelines.
Because of the evolving science, WHO frequently updates its TPT recommendations, and countries must in turn revise their national guidelines to incorporate the latest WHO recommendations. The importance of this review is that it provides a summary of current TB screening and TPT recommendations for pregnant and breastfeeding WLHIV, two highly vulnerable groups, specifically comparing high and low TB and TB/HIV burden countries in different regions of the globe that receive PEPFAR support. The accompanying dashboard allows for a quick understanding of the gaps in TB screening and TPT policy for pregnant and breastfeeding WLHIV compared to PLHIV overall [ 33 ].
A limitation of our review is that there are some inconsistencies among guidelines from the same country, whereby the TB screening and/or TPT recommendations for pregnant and breastfeeding WLHIV in the TB guidelines may differ from those in the HIV guidelines. This is most likely a reflection of changes in WHO guidance and the time it takes to update national guidelines. Another limitation was that we were unable to identify publicly available guidelines for all countries supported by PEPFAR through CDC, and did not include four countries supported by PEPFAR through another U.S. government agency. Despite these limiting factors, we were able to include most of the original list of countries in our review (44/49, 90%). It is important to note that our findings do not necessarily reflect the extent to which pregnant and breastfeeding WLHIV have access to TB screening and TPT services in PEPFAR-supported countries, as recommendations for “all PLHIV” might be relevant in some countries. While guidelines are important to inform policy, there is often a long lag between guideline development and implementation in the field. A strength of our review is that we included data from the most recent guidelines that were available during our data collection period from a wide geographic area. Additionally, we abstracted and analyzed data specific to breastfeeding WLHIV, a population that is not usually disaggregated from the general population of PLHIV, as well as abstracted data regarding guidance on obtaining LFTs prior to TPT during pregnancy and the postpartum period.
Despite progress in the number of PEPFAR-supported countries that specifically include TB screening and TPT recommendations for pregnant and breastfeeding WLHIV in their national guidelines, many PEPFAR-supported countries still do not provide screening or TPT recommendations specifically for pregnant and breastfeeding WLHIV or provide guidance on the operationalization and implementation of these guidelines in programmatic settings. This may impact WLHIV’s access to these potentially life-saving services.
Supporting information
S1 table. tb screening and tpt recommendations for pregnant and breastfeeding women living with hiv, listed alphabetically within who region..
https://doi.org/10.1371/journal.pone.0296993.s001
Acknowledgments
We would like to thank individuals who helped abstract data from guidelines in different languages: Indira Garcia, Kyaw Soe Htet, Almat Juvashev, Herve Kambale, Aung Myin Ko Ko, Yelena Kudussova, Axel Martinez, Monica Negrete, Nicole Nguessan-Adonis, Kateryna Ruda, Natalia Ruda, Pich Seekaew, Marzio Steffanuto, and Alla Volokha.
- 1. World Health Organization. Global tuberculosis control: WHO report 2021. Geneva, Switzerland World Health Organization; 2021. https://www.who.int/publications/i/item/9789240037021 . Accessed Nov 23, 2021.
- View Article
- PubMed/NCBI
- Google Scholar
- 5. World Health Organization. WHO consolidated guidelines on tuberculosis. Module 2: screening—systematic screening for tuberculosis disease. Geneva: World Health Organization; 2021. https://www.who.int/publications/i/item/9789240022676 . Accessed on 10 January 2023.
- 6. World Health Organization. The End TB Strategy. Geneva, Switzerland: World Health Organization; 2015. WHO/HTM/TB/2015.19. http://www.who.int/tb/End_TB_brochure.pdf?ua=1 . Accessed on Jun 22, 2017.
- 7. World Health Organization. Guidelines for Intensifed Tuberculosis Case-Finding and Isoniazid Preventive Therapy for People Living with HIV in Resource-Constrained Settings. Geneva, Switzerland. 2010.
- 10. Fact Sheet 2022—Latest global and regional statistics on the status of the AIDS epidemic: UNAIDS; [cited 2022]. https://www.unaids.org/en/resources/fact-sheet .
- 11. 20.2 million girls and women living with HIV: UNAIDS; 2022 [updated 08 March 2022]. https://www.unaids.org/en/resources/infographics/girls-and-women-living-with-HIV .
- 12. World Health Organization. Data on the HIV response: World Health Organization. https://www.who.int/data/gho/data/themes/hiv-aids/data-on-the-hiv-aids-response .
- 25. World Health Organization. Latent tuberculosis infection: updated and consolidated guidelines for programmatic management. Geneva: World Health Organization; 2018. http://apps.who.int/iris/bitstream/10665/260233/1/9789241550239-eng.pdf?ua=1 . Accessed on 28 February 2018.
- 30. World Health Organization. WHO consolidated guidelines on tuberculosis. Module 1: prevention—tuberculosis preventive treatment. Geneva: World Health Organization; 2020. https://www.who.int/publications/i/item/9789240001503 . Accessed on 10 January 2023.
- 31. US President’s Emergency Plan for AIDS Relief. 2019 Country operational plan guidance for all PEPFAR countries. Washington, DC: US President’s Emergency Plan for AIDS Relief; 2019.
- 32. US President’s Emergency Plan for AIDS Relief. PEPFAR 2022 Country and Regional Operational Plan Guidance for all PEPFAR-supported countries. Washington DC: US President’s Emergency Plan for AIDS Relief; 2022.
- 33. ICAP. TPT Recommendations Dashboard for PEPFAR-Supported Countries: ICAP; 2023. https://icap.columbia.edu/tools_resources/tpt-recommendations-dashboard-for-pepfar-supported-countries/ .
- 34. World Health Organization. WHO releases new global lists of high-burden countries for TB, HIV-associated TB and drug-resistant TB: World Health Organization; 2021 [cited 2023]. https://www.who.int/news/item/17-06-2021-who-releases-new-global-lists-of-high-burden-countries-for-tb-hiv-associated-tb-and-drug-resistant-tb .
- 36. Treatment for TB during Pregnancy: Centers for Disease Control and Prevention; 2020. https://www.cdc.gov/tb/topic/treatment/pregnancy.htm .
Management of Gastrointestinal Bleeding and Resumption of Oral Anticoagulant Therapy in Patients with Atrial Fibrillation: A Multidisciplinary Discussion
- Therapy in Practice
- Open access
- Published: 05 May 2023
- Volume 23 , pages 407–418, ( 2023 )
Cite this article
You have full access to this open access article
- Anne-Céline Martin ORCID: orcid.org/0000-0002-6148-3523 1 , 2 ,
- Robert Benamouzig 3 ,
- Isabelle Gouin-Thibault 4 &
- Jeannot Schmidt 5 , 6
11k Accesses
2 Citations
Explore all metrics
Direct oral anticoagulants (DOACs) are recommended for the prevention of thromboembolism in patients with atrial fibrillation (AF), and are now preferred over vitamin K antagonists due to their beneficial efficacy and safety profile. However, all oral anticoagulants carry a risk of gastrointestinal (GI) bleeding. Although the risk is well documented and acute bleeding well codified, there is limited high-quality evidence and no guidelines to guide physicians on the optimal management of anticoagulation after a GI bleeding event. The aim of this review is to provide a multidisciplinary critical discussion of the optimal management of GI bleeding in patients with AF receiving oral anticoagulants to help physicians provide individualized treatment for each patient and optimize outcomes. It is important to perform endoscopy when a patient presents with bleeding manifestations or hemodynamic instability to determine the bleed location and severity of bleeding and then perform initial resuscitation. Administration of all anticoagulants and antiplatelets should be stopped and bleeding allowed to resolve with time; however, anticoagulant reversal should be considered for patients who have life-threatening bleeding or when the bleeding is not controlled by the initial resuscitation. Anticoagulation needs to be timely resumed considering that bleeding risk outweighs thrombotic risk when anticoagulation is resumed early after the bleeding event. To prevent further bleeding, physicians should prescribe anticoagulant therapy with the lowest risk of GI bleeding, avoid medications with GI toxicity, and consider the effect of concomitant medications on potentiating the bleeding risk.
Similar content being viewed by others

Perioperative Guidelines on Antiplatelet and Anticoagulant Agents: 2022 Update
Michael Moster & Daniel Bolliger

Revisiting the Pharmacology of Unfractionated Heparin
Abdallah Derbalah, Stephen Duffull, … Hesham Al-Sallami

Unfractionated Heparin and Low-Molecular-Weight Heparin
Avoid common mistakes on your manuscript.
1 Introduction
Atrial fibrillation (AF) is the most common type of cardiac arrhythmia that increases the risk of cardiovascular events such as stroke, systemic embolism, and heart failure and promotes the worsening of cardiac and noncardiac conditions [ 1 , 2 ]. According to the Global Burden of Disease Study, there were 37.57 million [95% uncertainty interval (UI) 32.55–42.59] prevalent cases of AF and 3.05 million (95% UI 2.61–3.51) incident cases of AF worldwide in 2017 [ 2 ]. The prevalence of AF has nearly doubled between 1990 and 2017, and rates are expected to keep increasing due to the aging population [ 2 ].
European guidelines recommend the use of direct oral anticoagulants (DOACs) such as dabigatran, rivaroxaban, apixaban, or edoxaban for the prevention of stroke and systemic embolism in AF [ 3 , 4 ]. Their mechanism of action is based on the direct inhibition of activated coagulation factors; dabigatran inhibits thrombin (factor IIa), while rivaroxaban, apixaban, and edoxaban inhibit factor Xa [ 5 , 6 , 7 , 8 , 9 , 10 ]. Overall, DOACs are preferred over vitamin K antagonists (VKA) such as warfarin in adult patients, except in the context of pregnancy and in patients with mechanical valve prosthesis, triple-positive antiphospholipid syndrome, or end-stage kidney disease [ 3 , 11 ]. DOACs are being increasingly used due to their improved efficacy/safety ratio, predictable anticoagulant effect without need for routine coagulation monitoring, fixed dose regimens, and fewer food and drug interactions compared with VKAs [ 12 ]. However, gastrointestinal (GI) bleeding remains a serious and challenging complication of any anticoagulant medication [ 13 ]. The management of acute major bleeding in patients treated with anticoagulants is well codified [ 14 ], but there is a lack of standardized protocols as to how and when to resume anticoagulant therapy after GI bleeding. As such, international guidelines recommend the development of a hospital-based multidisciplinary approach including cardiologists, gastroenterologists, emergency physicians/intensive care specialists, hemostasis experts, and others to optimally treat patients with GI bleeding [ 3 ].
This article aims to critically discuss the optimal approach to the multidisciplinary management of GI bleeding in patients with AF receiving anticoagulants.
2 Risk of GI Bleeding Depending on the Type of Oral Anticoagulant
All anticoagulants may promote or potentiate bleeding from a preexisting GI lesion. Although the pathophysiology remains unclear [ 15 ], several mechanisms by which anticoagulant agents may contribute to GI bleeding have been suggested [ 16 ]. For example, warfarin has been associated with GI bleeding via a systemic decrease in vitamin-K-dependent clotting factors; because warfarin is not directly active, 95% of a dose is absorbed in the GI tract and the unabsorbed 5% does not have anticoagulant activity [ 13 , 17 , 18 ]. In contrast, factor Xa inhibitors are directly active and are not completely absorbed, so the unabsorbed drug may have a direct topical effect on GI tissues, potentially increasing the risk of bleeding [ 17 ]. The dabigatran prodrug has only 6% oral bioavailability, and the unabsorbed prodrug could be activated intraluminally during transit through the GI tract. Factor Xa inhibitors have higher (50–80%) oral bioavailability than dabigatran [ 19 ] and are reported to have different GI bleeding safety profiles. It is also possible that anticoagulants induce bleeding by compromising GI mucosal integrity and/or inhibiting its healing [ 17 , 20 ].
The comparative bleeding risk of VKAs (warfarin) and DOACs in patients with AF has been evaluated in several clinical trials. In the RE-LY trial, the risk of major GI bleeding with dabigatran was dose dependent, and only higher doses of dabigatran [150 mg twice daily (BID) but not 110 mg BID] increased bleeding risk compared with warfarin [1.51% versus 1.02% per year, respectively; relative risk (RR) 1.50; 95% confidence interval (CI) 1.19–1.89; p < 0.001] [ 21 ]. Similarly, in the ENGAGE AF-TIMI 48 trial, edoxaban doses of 60 mg once daily (QD) but not 30 mg QD significantly increased GI major bleeding risk in patients versus warfarin [60 mg QD of edoxaban, 1.51% versus warfarin, 1.23% per year; hazard ratio (HR) 1.23; 95% CI 1.02–1.50 per year; p = 0.03; and 30 mg QD of edoxaban, 0.82% versus warfarin, 1.23%; HR 0.67; 95% CI 0.53–0.83; p < 0.001] [ 22 ]. The ROCKET AF trial showed that major GI bleeding was significantly higher in patients treated with 20 mg rivaroxaban QD compared with warfarin (2.00% versus 1.24% per year, respectively; HR 1.66; 95% CI 1.34–2.05; p < 0.0001) [ 23 ]. Similar rates of major GI bleeding were found in the ARISTOTLE trial between 5 mg apixaban BID and warfarin (0.76% versus 0.86% per year, respectively; HR 0.89; 95% CI 0.70–1.15; p = 0.37) [ 24 ]. In the absence of direct head-to-head comparisons between DOACs, no conclusions can be drawn regarding which drug has the lowest GI bleeding risk [ 25 ]. Differences between DOACs could be due to differences in dosage, reporting of GI bleeding, or the study population (e.g., compared with the RE-LY and ARISTOTLE trial populations, the ROCKET-AF trial population was older and had more comorbidities at baseline) [ 21 , 23 , 24 ]. Whether the different chemical structures and differences in the pharmacodynamic and pharmacokinetic characteristics have an impact on the GI bleeding risk between DOACs is still unknown [ 16 , 26 ].
3 Clinical Factors that Predict Bleeding and Poor Prognosis in Anticoagulant-Treated Patients
Advanced age, intestinal ischemia, multiple comorbidities, blood cell transfusion, and in-hospital bleeding in the lower GI tract have been reported as risk factors for in-hospital mortality, post-discharge mortality, and 30-day hospital readmissions [ 27 ]. Hypertension [systolic blood pressure (SBP) > 160 mmHg], stroke, low hemoglobin (< 13 g/dL in men and < 12 g/dL in women), coexisting hepatic or renal diseases, and concomitant use of medications that affect hemostasis (e.g., antiplatelet therapy) increase morbidity and worsen outcomes in patients treated with anticoagulants [ 28 ].
The GI bleeding risk with DOACs depends on the dosage and type of DOAC used, on patient characteristics [ethnicity, older age (> 75 years), and comorbidities such as chronic kidney disease (CKD) and cirrhosis], and on concomitant use of other medications such as proton pump inhibitors (PPIs) or histamine H 2 -receptor antagonists [ 13 , 29 , 30 ]. Acute coronary syndrome has also been related to an increased risk of GI bleeding [odds ratio (OR) 5.21] in patients treated with DOACs, especially those who are co-prescribed antiplatelet agents [ 13 ]. Another risk factor for DOAC-related GI bleeding is renal impairment. AF affects about 18% of patients with CKD and > 25% of patients with CKD older than 70 years [ 31 ]; compared with the general population, patients with CKD receiving DOACs have an increased risk of thromboembolism and bleeding due to altered pharmacokinetics, decreased clearance, and altered volume of distribution because of reduced kidney function and limited protein binding [ 32 ]. However, the benefit–risk profile of DOACs has been reported to be superior to that of VKAs in the early stages of CKD [ 32 , 33 ]. Patients with cirrhosis are also at an increased risk of bleeding compared with the general population. No DOACs are recommended in Child–Pugh score C. In Child–Pugh score B, rivaroxaban is not recommended, while dabigatran, apixaban, and edoxaban should be used with caution [ 3 ]; however, more data are needed to evaluate how cirrhosis increases the bleeding risk in patients treated with DOACs [ 34 ].
Elderly patients present a particular challenge because of coexisting comorbidities, frailty, and concomitant medications increasing the risk of drug interactions [ 35 ]. A study comparing the risk of GI bleeding with dabigatran, rivaroxaban, or apixaban in patients with AF showed that rates of events for all DOACs increased among patients 75 years or older. However, apixaban had a lower risk of association with GI bleeding in the very elderly than dabigatran or rivaroxaban [ 36 ].
Several scores have been described to assess bleeding risk, especially in patients with AF exposed to long-term anticoagulation therapy. The HAS-BLED scale identifies patients at higher risk of bleeding by assessing the following risk factors: hypertension (SBP > 160 mmHg), abnormal renal and/or liver function, history of stroke or thromboembolism, history of bleeding or bleeding diathesis (severe anemia), age > 65 years, use of aspirin or nonsteroidal antiinflammatory drugs, and alcohol abuse [ 37 ]. The use of this tool may help to design individualized anticoagulant therapy on the basis of patient characteristics, especially in patients with an elevated bleeding risk [ 28 ]. In a systematic review commissioned by the PCORI including 38 studies on bleeding risk prediction, the HAS-BLED score had the best evidence for predicting bleeding risk [ 38 ]. However, this score and other bleeding scores (i.e., ATRIA) yielded only moderate discrimination ( c = 0.60, 95% CI 0.59–0.62 for the HAS-BLED score and c = 0.63, 95% CI 0.61–0.65 for the ATRIA bleeding risk score in the ORBIT-AR Registry) and should be critically used [ 39 ].
4 Key Steps to Triage Patients on Active Anticoagulant Treatment and GI Bleeding in the Emergency Department
When a patient enters the emergency department with a suspected clinically relevant GI bleed, it is important to evaluate the patient’s medical history, dosage, and timing of last DOAC intake [ 13 ], concomitant medications, and presenting/underlying conditions along with severity, location, and potential source of the bleeding [ 40 ] (Table 1 , Fig. 1 ). Additionally, vital signs such as blood pressure, temperature, and cardiac and respiratory frequency as well as the status of hemorrhagic shock should be assessed [ 41 , 42 ], and history of prior digestive system bleeding episodes should be investigated [ 42 ]. Possible bleeding locations other than the GI tract should be excluded [ 43 ]. Simultaneously, hemodynamic and cardiorespiratory stabilization must be performed. Resuscitation measures must be applied according to the Airway, Breathing, Circulation, Disability, Exposure (ABCDE) approach. Additionally, consider oxygen supplementation and orotracheal intubation to protect the patient’s airway in case of persistent hematemesis or change in consciousness level [ 42 , 44 ]. Oxygen supplementation should be administered with caution due to the potential release of free oxygen radicals that may negatively impact myocardium and cardiac function [ 45 ]. One of the immediate priorities in a patient with GI bleeding is to establish intravenous (IV) access in order to provide volume resuscitation. The European Society of Gastrointestinal Endoscopy recommends a restrictive red blood cell (RBC) transfusion with a target hemoglobin level between 7 and 9 g/dL; a higher target hemoglobin should be considered in patients with significant comorbidity, such as a history of myocardial infarction [ 42 ]. Several scales have been developed to define bleeding severity, including the Thrombolysis in Myocardial Infarction (TIMI), Global Usage of Strategies to Open Occluded Arteries (GUSTO), and Bleeding Academic Research Consortium (BARC) scales, and these are widely used in clinical trials.
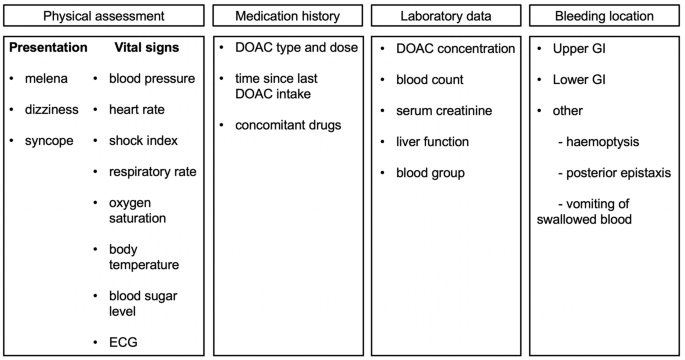
Parameters to consider in the management of a patient at the emergency department with suspected clinically relevant GI bleeding. DOAC direct oral anticoagulant, ECG electrocardiogram, GI gastrointestinal, INR international normalized ratio
The International Society on Thrombosis and Hemostasis (ISTH) defines major bleeding as symptomatic bleeding in a critical organ or area, and/or causing a ≥ 20 g/L fall in hemoglobin or requiring a transfusion of ≥ 2 units of whole blood or red cells [ 46 , 47 ]. In addition to these parameters, the American College of Cardiology includes hemodynamic instability [increased heart rate, SBP < 90 mmHg, decrease in SBP > 40 mmHg, or orthostatic blood pressure changes, SBP drop of ≥ 20 mmHg or diastolic blood pressure (DBP) drop of ≥ 10 mmHg upon standing] [ 14 ]. The Haute Autorité de Santé (HAS) defines severe bleeding as bleeding that requires urgent and specific management; any other bleeding situation is classified as nonsevere. Severe bleeding events are those that are accompanied by one or more of the following criteria: externalized bleeding that cannot be stopped by application of conventional methods, hemodynamic instability (SBP < 90 mmHg or ≥ 40 mmHg lower than usual, or mean arterial pressure < 65 mmHg, or signs of shock), need for an emergency procedure to stop the bleeding (e.g., endoscopy, interventional radiology, or surgery), need for packed RBC transfusion, and bleeding that is life-threatening or compromises function (including acute GI bleeding) [ 48 , 49 ].
In patients with upper GI hemorrhage, the Glasgow-Blatchford score (GBS) has been useful for stratifying the risk of needing treatment to manage bleeding in patients treated with anticoagulants and includes the following parameters: hemoglobin and blood urea nitrogen levels, initial SBP, heart rate, presence of melena or syncope, and presence of heart failure or hepatic disease [ 50 ]. If a peptic ulcer is the likely cause of upper GI bleeding, IV PPIs (e.g., omeprazole bolus of 80 mg then 8 mg/h infusion) may be administered for 72 h, followed by early transition to oral PPI therapy. The platelet plug is best stabilized if the gastric pH is greater than 5.4, that can be achieved with various PPIs infusion regimen or an oral route when possible. In patients with cirrhosis, esophageal varices are the likely source of bleeding, and these patients should receive somatostatin analogs (e.g., octreotide) and antibiotics (e.g., ceftriaxone or fluoroquinolones) [ 51 ]. Somatostatin infusion decreases arterial blood flow to the stomach and duodenum and portal blood flow. Prophylactic antibiotherapy decreases infection, infection mortality rate and all-cause mortality in cirrhotic patients with gastrointestinal bleeding.
An upper endoscopy should be performed if a patient presents with melena, hematemesis, or hematochezia or hemodynamic instability (signs of hypovolemia and iron-deficiency anemia) to allow for the determination of bleeding cause and location [ 41 , 52 ].
5 Role of Endoscopy in the Assessment and Management of Patients with GI Bleeding
It is often challenging to distinguish between upper and lower GI bleeding on the basis of initial symptoms. Endoscopies aid in determining the location and cause of bleeding to optimize patient management, stop bleeding, and prevent recurrence [ 53 ]. Physicians may consider administration of IV erythromycin before endoscopy, with ECG assessment of the QT interval and careful monitoring of potential arrhythmia in patients with cardiovascular disease or taking antiarrhythmic drugs. No alternative drug with such a prokinetic effect is currently available. Glycoprotein (P-gp) and CYP3A4/5 inhibitors such as erythromycin interact with DOACs [ 53 , 54 , 55 , 56 ], resulting in an increase in DOAC plasma concentration, which can potentially worsen or prolong bleeding events [ 57 ].
In emergency situations, the recommended timing of the endoscopy varies depending on whether the patient is suspected of having an upper or lower GI bleeding event, and it also differs between guidelines [ 52 ]. Different scores can be used in this scenario; the GBS is often used to determine the need for endoscopic treatment and transfusion, and to predict re-bleeding rate and prognosis [ 50 ]. This score has been validated as a predictive tool of in-hospital mortality and therapeutic endoscopic need, even prior to the identification of the bleeding source [ 58 ]. Patients with a GBS of 0 are considered to be at low risk and can be managed conservatively without the need for endoscopic investigation. The Forrest classification is used to categorize endoscopy findings such as active bleeding (Forrest Ia), high-risk lesion (Forrest Ib–IIc), and low-risk lesions without signs of active bleeding or recent hemorrhage (Forrest III) [ 59 ].
The Forrest classification can also be helpful for assessing the probability of bleeding recurrence, and can be used with other risk scores to weigh the benefit/risk of resuming anticoagulation [ 60 ]. The Rockall score system predicts the likelihood of death within 30 days by using patient age; accompanying shock; comorbidities such as heart, liver, and kidney disease; causative diseases of bleeding; and endoscopic bleeding stigmata [ 61 ]. It has been validated and is recommended by international guidelines [ 62 ].
Patients with suspected lower GI bleeding may require a colonoscopy to locate the bleeding site [ 51 ], for which bowel preparation is recommended [ 52 ]. Colonoscopies can detect diverticular bleeding or angiodysplastic bleeding [ 63 ]. Endoscopic methods of hemostasis for acute upper or lower GI bleeding include injection (usually diluted epinephrine or a special sclerosing agent), contact and noncontact thermal devices (unipolar or bipolar electrocoagulation, heater probes, and argon plasma coagulation), and mechanical devices (endoscopic clips and band ligation) [ 64 , 65 ]. Patients who have had these procedures may undergo a repeat endoscopy 24 h later.
6 Use of Reversal Agents in Patients with GI Bleeding
Anticoagulants and antiplatelets should be stopped on admission in patients with GI bleeding. The pharmacokinetic profile (half-life) of DOACs makes time the best antidote for bleeding in most situations. However, physicians should consider anticoagulant reversal in patients who have life-threatening bleeding or when the bleeding is not controlled by the initial resuscitation methods described above [ 66 ]. For VKA antagonization, prothrombin complex concentrate (PCC) 25 IU/kg is recommended. DOAC reversal is indicated for a concentration over 50 ng/mL. Below this threshold, bleeding is not considered to be related to DOACs [ 67 ].
The US Food and Drug Administration and the European Medicines Agency approved idarucizumab as a specific reversal agent for dabigatran in 2015. Idarucizumab is a humanized monoclonal antibody fragment that can be used for the emergency reversal of dabigatran’s anticoagulant effect [ 68 , 69 , 70 , 71 ]. However, prohemostatic agents, namely PCC or activated PCC (aPCC) 30–50 U/kg IV, can be administered if a specific antidote is not available. Few data support the use of oral activated charcoal within 6 h of drug intake, especially in the context of overdose [ 66 ]. Regarding factor Xa inhibitors, the US Food and Drug Administration approved andexanet alfa for the reversal of apixaban and rivaroxaban in life-threatening or uncontrolled bleeding under its accelerated approval program in 2018 [ 72 ]. The approval was conditional on performance of an ongoing randomized clinical trial (ANNEXA-I NCT03661528). The European Medicines Agency also gave conditional approval in April 2019, and full marketing approval was granted in Japan in March 2022, including for the reversal of edoxaban in patients with life-threatening or uncontrolled bleeding. Andexanet alfa is a recombinant inactive form of factor Xa that binds to the factor Xa inhibitors. Andexanet alfa is administered as a bolus followed by a continuous infusion, with the dosage dependent on the DOAC dose and the time of last drug intake [ 72 ]. PCC 50 IU/kg or aPCC 30–50 IU/kg are recommended if a specific antidote is not available. A recent observational study suggested that aPCC (25 IU/kg or 50 IU/kg for intracerebral hemorrhage or 30 IU/kg for GI bleeding) could be an option in patients with life-threatening bleeding associated with apixaban or rivaroxaban. Indeed, a clinical hemostasis was achieved in 24/35 patients, including 10/10 patients with GI bleeding after aPCC administration [ 73 ].
However, given the methodological limitations of open-label single-cohort and observational studies, the contribution of idarucizumab, andexanet alfa, PCC, or aPCC to promoting and maintaining hemostasis in case of life-threatening bleeding remains uncertain. In the absence of a control group, it is unclear whether DOAC reversion leads to improved clinical outcomes and whether use of specific antidotes provides more efficacy and safety than prohemostatic agents; all the more so as most GI bleeding can be managed through drug clearance (i.e., short half-life) and maximum supportive measures (e.g., transfusion, procedural/surgical intervention).
7 Timing for Resuming Anticoagulant Therapy after GI Bleeding
The decision to resume anticoagulant therapy after a bleeding event is critically important and should balance the risk of re-bleeding in the case of resumption, and the risk of thromboembolism if anticoagulation is not resumed. This decision needs to be made on a case-by-case basis after thorough assessment of the risks and benefits [ 74 ] by a multidisciplinary team including a gastroenterologist, a cardiologist, and others if needed [ 3 ].
Because of the overlap in risk factors for bleeding and thrombotic events, patients who are suffering from anticoagulation-induced bleeding are also at higher risk of thrombotic events. Discontinuation of anticoagulation, a prothrombotic inflammatory response to bleeding, and RBC transfusions may lead to increased rates of thrombotic events. Clearly, balancing the risks of further bleeding versus potentially fatal thrombotic events is critical for decisions about if and when to resume antithrombotic therapy after bleeding.
Resuming treatment was associated with an overall positive effect on the clinical course of patients with AF after the occurrence of a major bleeding event compared with not resuming oral anticoagulants [ 75 ]. A systematic review and meta-analysis on the risk of resuming oral anticoagulants after an episode of GI bleeding concluded that resuming treatment seemed to be associated with a reduced risk of thromboembolism (70%) and mortality 235 (49%) despite an increased risk of recurrent GI bleeding (91%) [ 76 ]. Another systematic review and meta-analysis using data from > 5000 patients showed similar recurrent GI bleeding risk, and significantly reduced risks of any thromboembolic event in patients resuming oral anticoagulant therapy compared with those who did not. The mortality rate in patients who resumed anticoagulation (21.3%) was lower compared with patients who discontinued anticoagulation (31%), with a significantly lower risk of all-cause mortality (OR 0.499; 95% CI 0.419–0.595; p < 0.0001) associated with the resumption of anticoagulation [ 77 ]. However, several biases and confounding hinder the interpretation of these two meta-analyses. All the studies included were observational rather than randomized control trials, there was a substantial amount of heterogeneity among them, the timing of anticoagulant resumption varied widely, and outcomes were not reported on the basis of when anticoagulant was resumed. Kido and colleagues have shown a decreased risk of mortality and thromboembolic events without an increased risk of a recurrent GI bleeding event when resuming warfarin within 7–15 days of a GI bleed [ 78 ]. A prospective cohort study by Sengupta and colleagues and two review articles by Witt and Radaelly and colleagues, respectively, on the benefit/risk associated with resuming anticoagulation after a GI bleed recommended resuming anticoagulation therapy after no more than 2 weeks to reduce the risk of bleeding, thromboembolism, and mortality [ 75 , 79 , 80 ].
Currently, there are no tools designed to specifically assess the risk of bleeding recurrence at anticoagulation resumption and to assess whether the risk of re-bleeding is higher than the risk of thrombosis.
Anticoagulation needs to be resumed in a timely manner, bearing in mind that bleeding risk outweighs thrombotic risk when anticoagulation is resumed early after the bleeding event [ 3 ]. This is well illustrated in the perioperative setting, where resuming anticoagulation early postoperatively increases the risk of bleeding since it compromises hemostasis [ 81 ].
However, the specific time when to resume anticoagulation is not well defined. Majeed et al. demonstrated that bleeding risk decreases over time after a GI bleeding event, especially after 21 days, whereas the risk of thromboembolism is stable over time and is often lower than the bleeding risk [ 82 ]. Data suggest that there is a threefold increase in the risk of bleeding if anticoagulation is resumed within 7 days of a hemorrhage compared with after 7 days, but there is no difference in the risk of bleeding if anticoagulants are resumed within 21 days versus after 21 days [ 82 , 83 , 84 ]. In the ARISTOTLE trial, resumption occurred at a median time of 15 days [ 85 ]. Similarly, in the REVERSE trial, 66% of the patients who had experienced a bleed resumed anticoagulation within 16 days [ 86 ].
The former version of the European Heart Rhythm Association (EHRA) guidelines on anticoagulants in patients with AF based the decision-making process on the assessment of factors that favor withholding anticoagulation (such as an unidentifiable site of bleeding, multiple angiodysplasia in the GI tract, no identifiable treatable cause, and older age) and those that favor resuming anticoagulation. The recommendation was to resume DOACs within 4–7 days following a major GI bleed but only if clinical benefits outweighed the risk of developing recurrent GI bleeding [ 87 ].
In the latest version of their guidelines, the EHRA changed their point of view, with the decision-making process suggesting a net assessment in favor of resuming anticoagulation and a recommendation to resume DOACs as early as clinically feasible (Fig. 2 ) [ 3 ].
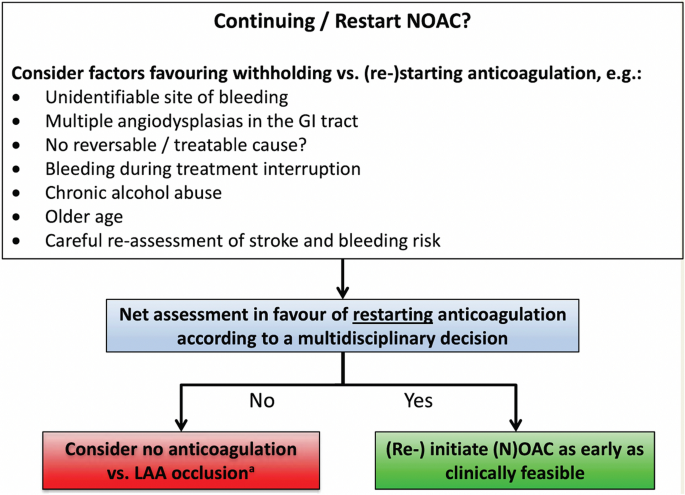
Algorithm for the resumption of direct oral anticoagulants after a gastrointestinal hemorrhage according to the European Heart Rhythm Association guidelines [ 3 ]. GI gastrointestinal, LAA left atrial appendage, NOAC novel oral anticoagulants. Reproduced from Steffel et al. [ 3 ] by permission of Oxford University Press
The American College of Cardiology guidelines recommend determining the optimal timing for oral anticoagulant resumption on the basis of whether there is a greater risk of thromboembolism or bleeding. In conditions with high thrombotic risk, the recommendation is for early resumption of anticoagulation once hemostasis is achieved and the patient is clinically stable; for patients with moderate or high re-bleeding risk, individualized strategies are more appropriate [ 14 ].
Further studies and randomized controlled trials are urgently needed to establish optimal timing of DOAC resumption in patients after a GI hemorrhage according to baseline patient characteristics (age, comorbidities, indication for anticoagulants, source and severity of bleeding, risk of re-bleeding or thrombosis).
8 Minimizing the Risk of Recurrences in Patients Resuming Anticoagulants after GI Bleeding
As previously described, resuming anticoagulant treatment after a GI bleeding event generally provides clinical benefit. To prevent recurrent bleeding after a GI bleeding episode, it is important to evaluate the main risk factors favoring the occurrence of GI bleeding [ 88 ], such as the presence of a digestive luminal disease, older age, renal or liver dysfunction, hypertension, anemia, history of hemorrhage or stroke, genetic factors, malignancy, and concomitant treatments and diseases [ 11 , 28 , 30 , 74 ]. Overall, despite a potential 23% increase in GI bleeding, DOACs have a 14% trend toward a relative risk reduction in major bleeding relative to warfarin [ 89 ]. Since the risk of bleeding appears to be higher with VKAs than with DOACs, it may be advisable to resume with a DOAC after a significant GI bleed.
However, several retrospective cohort studies of real-world patients starting DOAC therapy for AF have shown that, after adjustment for potential confounders, apixaban was associated with a significantly lower risk of major bleeding and GI bleeding compared with rivaroxaban or dabigatran [ 90 , 91 , 92 ].
Guidelines stress the need to minimize bleeding risk for all patients on oral anticoagulants by addressing modifiable risk factors such as concomitant use of aspirin, which increases the hazard for major bleeding events by at least 50% [ 12 ].
In summary, to prevent further bleeding, physicians should ensure the following steps are taken in a patient resuming anticoagulant therapy:
Preferentially prescribe DOACs with the lowest risk of GI bleeding rather than VKAs. The choice of DOAC cannot be determined by evidence-based medicine but should be determined by the risk of GI bleeding.
Comply with all guidelines and prescribing information, especially avoiding DOAC accumulation related to kidney disease.
Consider the effect of concomitant medications on potentiating the bleeding risk (e.g., CYP3A4 or P-gp inhibitors, antiplatelet agents) [ 93 ].
Avoid medications with GI toxicity (nonsteroidal antiinflammatory drugs).
Initiate treatment with PPIs to reduce the risk of bleeding (although be cognizant of a possible interaction between PPIs and dabigatran).
Test patients with peptic ulcers for Helicobacter pylori and initiate eradication therapy as needed.
9 Conclusions
Guidelines now recommend DOACs over VKAs for the prevention of thromboembolism in patients with AF due to their safety and efficacy profiles. However, the major complication with DOAC treatment is the increased risk of bleeding, particularly GI bleeding. Management of GI bleeding and resumption of oral anticoagulants in patients with AF are associated with increased survival rates. The optimal approach for patients with GI bleeding who are taking anticoagulants involves multidisciplinary care to provide an individualized optimal balance of benefit and risk for each patient.
Bassand J-P, Apenteng PN, Atar D, Camm AJ, Cools F, Corbalan R, et al. GARFIELD-AF: a worldwide prospective registry of patients with atrial fibrillation at risk of stroke. Future Cardiol. 2021;17(1):19–38. https://doi.org/10.2217/fca-2020-0014 .
Article CAS PubMed Google Scholar
Dai H, Zhang Q, Much AA, Maor E, Segev A, Beinart R, et al. Global, regional, and national prevalence, incidence, mortality, and risk factors for atrial fibrillation, 1990–2017: results from the Global Burden of Disease Study 2017. Eur Heart J Qual Care Clin Outcomes. 2020;7(6):574–82. https://doi.org/10.1093/ehjqcco/qcaa061 .
Article PubMed Central Google Scholar
Steffel J, Collins R, Antz M, Cornu P, Desteghe L, Haeusler KG, et al. 2021 European Heart Rhythm Association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Europace. 2021;23(10):1612–76. https://doi.org/10.1093/europace/euab065 .
Article PubMed Google Scholar
Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42(5):373–498. https://doi.org/10.1093/eurheartj/ehaa612 .
Bathala MS, Masumoto H, Oguma T, He L, Lowrie C, Mendell J. Pharmacokinetics, biotransformation, and mass balance of edoxaban, a selective, direct factor Xa inhibitor, in humans. Drug Metab Dispos. 2012;40(12):2250–5. https://doi.org/10.1124/dmd.112.046888 .
Chan NC, Hirsh J, Ginsberg JS, Eikelboom JW. Betrixaban (PRT054021): pharmacology, dose selection and clinical studies. Future Cardiol. 2014;10(1):43–52. https://doi.org/10.2217/fca.13.98 .
Gulseth MP, Michaud J, Nutescu EA. Rivaroxaban: an oral direct inhibitor of factor Xa. Am J Health Syst Pharm. 2008;65(16):1520–9. https://doi.org/10.2146/ajhp070624 .
He K, Luettgen JM, Zhang D, He B, Grace JE, Xin B, et al. Preclinical pharmacokinetics and pharmacodynamics of apixaban, a potent and selective factor Xa inhibitor. Eur J Drug Metab Pharmacokinet. 2011;36(3):129–39. https://doi.org/10.1007/s13318-011-0037-x .
Shantsila E, Lip GY. Apixaban, an oral, direct inhibitor of activated factor Xa. Curr Opin Investig Drugs. 2008;9(9):1020–33.
CAS PubMed Google Scholar
Stangier J, Clemens A. Pharmacology, pharmacokinetics, and pharmacodynamics of dabigatran etexilate, an oral direct thrombin inhibitor. Clin Appl Thromb Hemost. 2009;15(Suppl 1):9s–16s. https://doi.org/10.1177/1076029609343004 .
Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893–962. https://doi.org/10.1093/eurheartj/ehw210 .
January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74(1):104–32. https://doi.org/10.1016/j.jacc.2019.01.011 .
Cheung K-S, Leung WK. Gastrointestinal bleeding in patients on novel oral anticoagulants: risk, prevention and management. World J Gastroenterol. 2017;23(11):1954. https://doi.org/10.3748/wjg.v23.i11.1954 .
Article CAS PubMed PubMed Central Google Scholar
Tomaselli GF, Mahaffey KW, Cuker A, Dobesh PP, Doherty JU, Eikelboom JW, et al. 2020 ACC expert consensus decision pathway on management of bleeding in patients on oral anticoagulants. J Am Coll Cardiol. 2020;76(5):594–622. https://doi.org/10.1016/j.jacc.2020.04.053 .
Bouget J, Viglino D, Yvetot Q, Oger E. Major gastrointestinal bleeding and antithrombotics: characteristics and management. World J Gastroenterol. 2020;26(36):5463–73. https://doi.org/10.3748/wjg.v26.i36.5463 .
Raymond J, Imbert L, Cousin T, Duflot T, Varin R, Wils J, et al. Pharmacogenetics of direct oral anticoagulants: a systematic review. J Pers Med. 2021;11(1):37. https://doi.org/10.3390/jpm11010037 .
Article PubMed PubMed Central Google Scholar
Desai J, Granger CB, Weitz JI, Aisenberg J. Novel oral anticoagulants in gastroenterology practice. Gastrointest Endosc. 2013;78(2):227–39. https://doi.org/10.1016/j.gie.2013.04.179 .
Vaduganathan M, Bhatt DL. Gastrointestinal bleeding with oral anticoagulation: understanding the scope of the problem. Clin Gastroenterol Hepatol. 2017;15(5):691–3. https://doi.org/10.1016/j.cgh.2016.12.033 .
Hellenbart EL, Faulkenberg KD, Finks SW. Evaluation of bleeding in patients receiving direct oral anticoagulants. Vasc Health Risk Manag. 2017;13:325–42. https://doi.org/10.2147/VHRM.S121661 .
Thapa N, Shatzel J, Deloughery TG, Olson SR. Direct oral anticoagulants in gastrointestinal malignancies: is the convenience worth the risk? J Gastrointest Oncol. 2019;10(4):807–9. https://doi.org/10.21037/jgo.2019.02.07 .
Eikelboom JW, Wallentin L, Connolly SJ, Ezekowitz M, Healey JS, Oldgren J, et al. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: an analysis of the randomized evaluation of long-term anticoagulant therapy (RE-LY) trial. Circulation. 2011;123(21):2363–72. https://doi.org/10.1161/circulationaha.110.004747 .
Ruff CT, Giugliano RP, Antman EM, Crugnale SE, Bocanegra T, Mercuri M, et al. Evaluation of the novel factor Xa inhibitor edoxaban compared with warfarin in patients with atrial fibrillation: design and rationale for the Effective aNticoaGulation with factor xA next GEneration in Atrial Fibrillation-Thrombolysis In Myocardial Infarction study 48 (ENGAGE AF-TIMI 48). AM Heart J. 2010;160(4):635-41.e2. https://doi.org/10.1016/j.ahj.2010.06.042 .
Sherwood MW, Nessel CC, Hellkamp AS, Mahaffey KW, Piccini JP, Suh E-Y, et al. Gastrointestinal bleeding in patients with atrial fibrillation treated with rivaroxaban or warfarin: ROCKET AF trial. J Am Coll Cardiol. 2015;66(21):2271–81. https://doi.org/10.1016/j.jacc.2015.09.024 .
Granger CB, Lopes RD, Hanna M, Ansell J, Hylek EM, Alexander JH, et al. Clinical events after transitioning from apixaban versus warfarin to warfarin at the end of the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial. Am Heart J. 2015;169(1):25–30. https://doi.org/10.1016/j.ahj.2014.09.006 .
Cannon CP, Kohli P. Danger ahead: watch out for indirect comparisons! J Am Coll Cardiol. 2012;60(8):747–8. https://doi.org/10.1016/j.jacc.2012.05.012 .
Desai JC, Chatterjee P, Friedman K, Aisenberg J. Incidence and clinical presentation of gastrointestinal bleeding in atrial fibrillation patients taking direct oral anticoagulants. Am J Gastroenterol Suppl. 2016;3(1):13. https://doi.org/10.1038/ajgsup.2016.3 .
Article CAS Google Scholar
Sengupta N, Tapper EB, Patwardhan VR, Ketwaroo GA, Thaker AM, Leffler DA, et al. Risk factors for adverse outcomes in patients hospitalized with lower gastrointestinal bleeding. Mayo Clin Proc. 2015;90(8):1021–9. https://doi.org/10.1016/j.mayocp.2015.04.024 .
Undas A, Drabik L, Potpara T. Bleeding in anticoagulated patients with atrial fibrillation: practical considerations. Kardiol Pol. 2020;78(2):105–16. https://doi.org/10.33963/KP.15205 .
White EM, Coons JC. Direct oral anticoagulant use in special populations: elderly, obesity, and renal failure. Curr Cardiol Rep. 2021;23(4):27. https://doi.org/10.1007/s11886-021-01456-9 .
Gunasekaran K, Rajasurya V, Devasahayam J, Singh Rahi M, Chandran A, Elango K, et al. A review of the incidence diagnosis and treatment of spontaneous hemorrhage in patients treated with direct oral anticoagulants. J Clin Med. 2020;9(9):2984. https://doi.org/10.3390/jcm9092984 .
Soliman EZ, Prineas RJ, Go AS, Xie D, Lash JP, Rahman M, et al. Chronic kidney disease and prevalent atrial fibrillation: the Chronic Renal Insufficiency Cohort (CRIC). Am Heart J. 2010;159(6):1102–7. https://doi.org/10.1016/j.ahj.2010.03.027 .
Padrini R. Clinical pharmacokinetics and pharmacodynamics of direct oral anticoagulants in patients with renal failure. Eur J Drug Metab Pharmacokinet. 2019;44(1):1–12. https://doi.org/10.1007/s13318-018-0501-y .
Ha JT, Neuen BL, Cheng LP, Jun M, Toyama T, Gallagher MP, et al. Benefits and harms of oral anticoagulant therapy in chronic kidney disease: a systematic review and meta-analysis. Ann Intern Med. 2019;171(3):181–9. https://doi.org/10.7326/m19-0087 .
Weinberg EM, Palecki J, Reddy KR. Direct-acting oral anticoagulants (DOACs) in cirrhosis and cirrhosis-associated portal vein thrombosis. Semin Liver Dis. 2019;39(2):195–208. https://doi.org/10.1055/s-0039-1679934 .
Lobraico-Fernandez J, Baksh S, Nemec E. Elderly bleeding risk of direct oral anticoagulants in nonvalvular atrial fibrillation: a systematic review and meta-analysis of cohort studies. Drugs R D. 2019;19(3):235–45. https://doi.org/10.1007/s40268-019-0275-y .
Abraham NS, Noseworthy PA, Yao X, Sangaralingham LR, Shah ND. Gastrointestinal safety of direct oral anticoagulants: a large population-based study. Gastroenterology. 2017;152(5):1014-22.e1. https://doi.org/10.1053/j.gastro.2016.12.018 .
Lip GYH. Implications of the CHA2DS2-VASc and HAS-BLED scores for thromboprophylaxis in atrial fibrillation. Am J Med. 2011;124(2):111–4. https://doi.org/10.1016/j.amjmed.2010.05.007 .
Borre ED, Goode A, Raitz G, Shah B, Lowenstern A, Chatterjee R, et al. Predicting thromboembolic and bleeding event risk in patients with non-valvular atrial fibrillation: a systematic review. Thromb Haemost. 2018;118(12):2171–87. https://doi.org/10.1055/s-0038-1675400 .
Steinberg BA, Shrader P, Kim S, Thomas L, Fonarow GC, Ansell J, et al. How well does physician risk assessment predict stroke and bleeding in atrial fibrillation? Results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Am Heart J. 2016;181:145–52. https://doi.org/10.1016/j.ahj.2016.07.026 .
Srygley FD, Gerardo CJ, Tran T, Fisher DA. Does this patient have a severe upper gastrointestinal bleed? JAMA. 2012;307(10):1072–9. https://doi.org/10.1001/jama.2012.253 .
Barnert J, Messmann H. Diagnosis and management of lower gastrointestinal bleeding. Nat Rev Gastroenterol Hepatol. 2009;6(11):637–46. https://doi.org/10.1038/nrgastro.2009.167 .
Gralnek IM, Dumonceau J-M, Kuipers EJ, Lanas A, Sanders DS, Kurien M, et al. Diagnosis and management of nonvariceal upper gastrointestinal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy. 2015;47(10):a1–46. https://doi.org/10.1055/s-0034-1393172 .
Gaiani F, De’Angelis N, Kayali S, Manfredi M, Di Mario F, Leandro G, et al. Clinical approach to the patient with acute gastrointestinal bleeding. Acta Biomed Ateneo Parmense. 2018;89(8-S):12–9. https://doi.org/10.23750/abm.v89i8-S.7861 .
Article Google Scholar
Rodrigues A, Carrilho A, Almeida N, Baldaia C, Alves Â, Gomes M, et al. Interventional algorithm in gastrointestinal bleeding-an expert consensus multimodal approach based on a multidisciplinary team. Clin Appl Thromb Hemost. 2020;26:1076029620931943. https://doi.org/10.1177/1076029620931943 .
McNulty PH, King N, Scott S, Hartman G, McCann J, Kozak M, et al. Effects of supplemental oxygen administration on coronary blood flow in patients undergoing cardiac catheterization. Am J Physiol Heart Circ Physiol. 2005;288(3):H1057–62. https://doi.org/10.1152/ajpheart.00625.2004 .
Schulman S, Angeras U, Bergqvist D, Eriksson B, Lassen MR, Fisher W, et al. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. J Thromb Haemost. 2010;8(1):202–4. https://doi.org/10.1111/j.1538-7836.2009.03678.x .
Schulman S, Kearon C, Subcommittee on Control of Anticoagulation of the of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692–4. https://doi.org/10.1111/j.1538-7836.2005.01204.x
Pernod G, Godier A, Gozalo C, Tremey B, Sie P. French National Authority for Health French clinical practice guidelines on the management of patients on vitamin K antagonists in at-risk situations (overdose, risk of bleeding, and active bleeding). Thromb Res. 2010;126(3):e167–74. https://doi.org/10.1016/j.thromres.2010.06.017
Haute Autorité de Santé. Oral anticoagulants. Saint-Denis La Plaine. 2018. https://www.has-sante.fr/jcms/c_2851086/fr/les-anticoagulants-oraux . Accessed 22 July 2021.
Blatchford O, Murray WR, Blatchford M. A risk score to predict need for treatment for upper-gastrointestinal haemorrhage. Lancet. 2000;356(9238):1318–21. https://doi.org/10.1016/S0140-6736(00)02816-6 .
Piazza VM, Popenko NA, Winograd S. An evidence-based review of gastrointestinal bleeding evaluation and management in the emergency department. Emerg Med Rep Relias Media. 2019. https://www.reliasmedia.com/articles/144557-an-evidence-based-review-of-gastrointestinal-bleeding-evaluation-and-management-in-the-emergency-department . Accessed 27 Apr 27.
Jung K, Moon W. Role of endoscopy in acute gastrointestinal bleeding in real clinical practice: an evidence-based review. World J Gastrointest Endosc. 2019;11(2):68–83. https://doi.org/10.4253/wjge.v11.i2.68 .
U.S. Food and Drug Administration. SAVAYSA™ (edoxaban) prescribing information. 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/206316s015lbl.pdf . Accessed 2 Aug 2021.
U.S. Food and Drug Administration. PRADAXA ® (dabigatran etexilate) prescribing information. 2021. https://docs.boehringer-ingelheim.com/Prescribing%20Information/PIs/Pradaxa/Pradaxa.pdf . Accessed 4 Aug 2021.
U.S. Food and Drug Administration. XARELTO (rivaroxaban) prescribing information. 2021. https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/XARELTO-pi.pdf . Accessed 4 Aug 2021.
U.S. Food and Drug Administration. ELIQUIS ® (apixaban) prescribing information. 2021. https://packageinserts.bms.com/pi/pi_eliquis.pdf . Accessed 4 Aug 2021.
Chen A, Stecker E, Bruce AW. Direct oral anticoagulant use: a practical guide to common clinical challenges. J Am Heart Assoc. 2020;9(13):e017559. https://doi.org/10.1161/jaha.120.017559 .
Ur-Rahman A, Guan J, Khalid S, Munaf A, Sharbatji M, Idrisov E, et al. Both full Glasgow–Blatchford score and modified Glasgow-Blatchford score predict the need for intervention and mortality in patients with acute lower gastrointestinal bleeding. Dig Dis Sci. 2018;63(11):3020–5. https://doi.org/10.1007/s10620-018-5203-4 .
Forrest JA, Finlayson ND, Shearman DJ. Endoscopy in gastrointestinal bleeding. Lancet. 1974;2(7877):394–7. https://doi.org/10.1016/s0140-6736(74)91770-x .
Lee S, Ahn JY, Jung HY, Jung KW, Lee JH, Kim DH, et al. Effective endoscopic treatment of Mallory-Weiss syndrome using Glasgow-Blatchford score and Forrest classification. J Dig Dis. 2016;17(10):676–84. https://doi.org/10.1111/1751-2980.12409 .
Rockall TA, Logan RF, Devlin HB, Northfield TC. Risk assessment after acute upper gastrointestinal haemorrhage. Gut. 1996;38(3):316–21. https://doi.org/10.1136/gut.38.3.316 .
Barkun AN, Bardou M, Kuipers EJ, Sung J, Hunt RH, Martel M, et al. International consensus recommendations on the management of patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2010;152(2):101–13. https://doi.org/10.7326/0003-4819-152-2-201001190-00009 .
Strate LL, Gralnek IM. ACG clinical guideline: management of patients with acute lower gastrointestinal bleeding. Am J Gastroenterol. 2016;111(4):459–74. https://doi.org/10.1038/ajg.2016.41 .
Pasha SF, Shergill A, Acosta RD, Chandrasekhara V, Chathadi KV, Early D, et al. The role of endoscopy in the patient with lower GI bleeding. Gastrointest Endosc. 2014;79(6):875–85. https://doi.org/10.1016/j.gie.2013.10.039 .
Conway JD, Adler DG, Diehl DL, Farraye FA, Kantsevoy SV, Kaul V, et al. Endoscopic hemostatic devices. Gastrointest Endosc. 2009;69(6):987–96. https://doi.org/10.1016/j.gie.2008.12.251 .
Cuker A, Burnett A, Triller D, Crowther M, Ansell J, Van Cott EM, et al. Reversal of direct oral anticoagulants: guidance from the anticoagulation forum. Am J Hematol. 2019;94(6):697–709. https://doi.org/10.1002/ajh.25475 .
Levy JH, Ageno W, Chan NC, Crowther M, Verhamme P, Weitz JI, et al. When and how to use antidotes for the reversal of direct oral anticoagulants: guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14(3):623–7. https://doi.org/10.1111/jth.13227 .
U.S. Food and Drug Administration. PRAXBIND ® (idarucizumab) prescribing information. 2015. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/761025lbl.pdf . Accessed 2 Aug 2021.
Marano G, Vaglio S, Pupella S, Liumbruno GM, Franchini M. How we treat bleeding associated with direct oral anticoagulants. Blood Transfus. 2016;14(5):465–73. https://doi.org/10.2450/2016.0180-15 .
Sheikh-Taha M. Idarucizumab for reversal of dabigatran: single-center real-world experience. Am J Cardiovasc Drugs. 2019;19(1):59–64. https://doi.org/10.1007/s40256-018-0300-5 .
Chaudhary R, Sharma T, Garg J, Sukhi A, Bliden K, Tantry U, et al. Direct oral anticoagulants: a review on the current role and scope of reversal agents. J Thromb Thrombolysis. 2020;49(2):271–86. https://doi.org/10.1007/s11239-019-01954-2 .
U.S. Food and Drug Administration. ANDEXXA ® (coagulation factor Xa) prescribing information. 2018. https://www.fda.gov/media/113279/download . Accessed 02 Aug 2021.
Sheikh-Taha M, Crawley RM. Reversal of apixaban and rivaroxaban using activated prothrombin complex concentrates in patients with major bleeding. Am J Cardiovasc Drugs. 2020;20(3):295–9. https://doi.org/10.1007/s40256-019-00383-z .
Sengupta N, Feuerstein JD, Patwardhan VR, Tapper EB, Ketwaroo GA, Thaker AM, et al. The risks of thromboembolism vs recurrent gastrointestinal bleeding after interruption of systemic anticoagulation in hospitalized inpatients with gastrointestinal bleeding: a prospective study. Am J Gastroenterol. 2015. https://doi.org/10.1038/ajg.2014.398 .
Proietti M, Romiti GF, Romanazzi I, Farcomeni A, Staerk L, Nielsen PB, et al. Restarting oral anticoagulant therapy after major bleeding in atrial fibrillation: a systematic review and meta-analysis. Int J Cardiol. 2018;261:84–91. https://doi.org/10.1016/j.ijcard.2018.03.053 .
Little D, Chai-Adisaksopha C, Hillis C, Witt DM, Monreal M, Crowther MA, et al. Resumption of anticoagulant therapy after anticoagulant-related gastrointestinal bleeding: a systematic review and meta-analysis. Thromb Res. 2019;175:102–9. https://doi.org/10.1016/j.thromres.2019.01.020 .
Tapaskar N, Pang A, Werner DA, Sengupta N. Resuming anticoagulation following hospitalization for gastrointestinal bleeding is associated with reduced thromboembolic events and improved mortality: results from a systematic review and meta-analysis. Dig Dis Sci. 2021;66(2):554–66. https://doi.org/10.1007/s10620-020-06248-9 .
Kido K, Scalese MJ. Management of oral anticoagulation therapy after gastrointestinal bleeding: whether to, when to, and how to restart an anticoagulation therapy. Ann Pharmacother. 2017;51(11):1000–7. https://doi.org/10.1177/1060028017717019 .
Radaelli F, Fuccio L, Paggi S, Bono CD, Dumonceau JM, Dentali F. What gastroenterologists should know about direct oral anticoagulants. Dig Liver Dis. 2020;52(10):1115–25. https://doi.org/10.1016/j.dld.2020.04.032 .
Witt DM. What to do after the bleed: resuming anticoagulation after major bleeding. Hematology. 2016;2016(1):620–4. https://doi.org/10.1182/asheducation-2016.1.620 .
Albaladejo P, Pernod G, Godier A, de Maistre E, Rosencher N, Mas JL, et al. Management of bleeding and emergency invasive procedures in patients on dabigatran: updated guidelines from the French Working Group on perioperative haemostasis (GIHP)—September 2016. Anaesth Crit Care Pain Med. 2018;37(4):391–9. https://doi.org/10.1016/j.accpm.2018.04.009 .
Majeed A, Wallvik N, Eriksson J, Höijer J, Bottai M, Holmström M, et al. Optimal timing of vitamin k antagonist resumption after upper gastrointestinal bleeding a risk modelling analysis. Thromb Haemost. 2017;117(3):491–9. https://doi.org/10.1160/TH16-07-0498 .
Qureshi W, Mittal C, Patsias I, Garikapati K, Kuchipudi A, Cheema G, et al. Restarting anticoagulation and outcomes after major gastrointestinal bleeding in atrial fibrillation. Am J Cardiol. 2014;113(4):662–8. https://doi.org/10.1016/j.amjcard.2013.10.044 .
Witt DM, Delate T, Garcia DA, Clark NP, Hylek EM, Ageno W, et al. Risk of thromboembolism, recurrent hemorrhage, and death after warfarin therapy interruption for gastrointestinal tract bleeding. Arch Intern Med. 2012;172(19):1484–91. https://doi.org/10.1001/archinternmed.2012.4261 .
Held C, Hylek EM, Alexander JH, Hanna M, Lopes RD, Wojdyla DM, et al. Clinical outcomes and management associated with major bleeding in patients with atrial fibrillation treated with apixaban or warfarin: insights from the ARISTOTLE trial. Eur Heart J. 2015;36(20):1264–72. https://doi.org/10.1093/eurheartj/ehu463 .
Van der Wall SJ, Lopes RD, Aisenberg J, Reilly P, van Ryn J, Glund S, et al. Idarucizumab for dabigatran reversal in the management of patients with gastrointestinal bleeding. Circulation. 2019;139(6):748–56. https://doi.org/10.1161/CIRCULATIONAHA.118.036710 .
Steffel J, Verhamme P, Potpara TS, Albaladejo P, Antz M, Desteghe L, et al. The 2018 European Heart Rhythm Association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation: executive summary. Europace. 2018;20(8):1231–42. https://doi.org/10.1093/europace/euy054 .
Pipilis A, Makrygiannis S, Chrisanthopoulou E, Sourlas N, Kaliambakos S, Ntailianas P. Gastrointestinal bleeding in patients receiving antiplatelet and anticoagulant therapy: practical guidance for restarting therapy and avoiding recurrences. Hellenic J Cardiol. 2014;55(6):499–509.
PubMed Google Scholar
Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955–62. https://doi.org/10.1016/S0140-6736(13)62343-0 .
Lip GYH, Keshishian AV, Zhang Y, Kang A, Dhamane AD, Luo X, et al. Oral anticoagulants for nonvalvular atrial fibrillation in patients with high risk of gastrointestinal bleeding. JAMA Netw Open. 2021;4(8):e2120064. https://doi.org/10.1001/jamanetworkopen.2021.20064 .
Ray WA, Chung CP, Stein CM, Smalley W, Zimmerman E, Dupont WD, et al. Association of rivaroxaban vs apixaban with major ischemic or hemorrhagic events in patients with atrial fibrillation. JAMA. 2021;326(23):2395–404. https://doi.org/10.1001/jama.2021.21222 .
Souverein PC, van den Ham HA, Huerta C, Merino EM, Montero D, León-Muñoz LM, et al. Comparing risk of major bleeding between users of different oral anticoagulants in patients with nonvalvular atrial fibrillation. Br J Clin Pharmacol. 2021;87(3):988–1000. https://doi.org/10.1111/bcp.14450 .
Sheikh-Taha M, Deeb ME. Assessment of non-vitamin K oral anticoagulants use in a tertiary care center in the USA: a chart review of 909 patients. Am J Cardiovasc Drugs. 2019;19(2):195–201. https://doi.org/10.1007/s40256-018-0310-3 .
Download references
Acknowledgements
We would like to thank Catherine Rees and Alma Orts-Sebastien of Springer Healthcare Communications who wrote the outline and first draft, respectively. This medical writing assistance was funded by Pfizer and Bristol Myers Squibb.
Author information
Authors and affiliations.
Advanced Heart Failure Unit, AP-HP, Cardiology Department, European Hospital Georges Pompidou, Paris, France
Anne-Céline Martin
INSERM UMRS_1140, Innovative Therapies in Haemostasis, Université Paris Cité, 75006, Paris, France
Service de Gastroentérologie, Hôpital Avicenne, AP-HP, Université Paris-Nord-La Sorbonne, Bobigny, France
Robert Benamouzig
Laboratory of Hematology, IRSET-INSERM UMRS 1085, Rennes University Hospital, Rennes, France
Isabelle Gouin-Thibault
LaPSCo, Physiological and Psychosocial Stress, CNRS, Université Clermont Auvergne, Clermont-Ferrand, France
Jeannot Schmidt
Emergency Department, CHU Clermont-Ferrand, University Hospital Gabriel Montpied, Clermont-Ferrand, France
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Anne-Céline Martin .
Ethics declarations
Conflicts of interest.
Anne-Céline Martin: BMS/Pfizer, Bayer, Boehringer Ingelheim, Astra Zeneca, Novartis. Robert Benamouzig: no relevant conflicts of interest to declare. Isabelle Gouin-Thibault: Alliance BMS/Pfizer, Bayer, Leo Pharma, Abbvie. Jeannot Schmidt: no relevant conflicts of interest to declare.
Medical writing assistance was funded by Pfizer and Bristol Myers Squibb.
Ethics approval
Not applicable.
Consent to participate
Consent for publication, availability of data and material.
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Code availability
Author contributions.
The content of this manuscript was originally based on discussions during expert meetings including all the authors. All authors critically reviewed the outline and first draft of the manuscript and have read and approved the final manuscript for submission.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/ .
Reprints and permissions
About this article
Martin, AC., Benamouzig, R., Gouin-Thibault, I. et al. Management of Gastrointestinal Bleeding and Resumption of Oral Anticoagulant Therapy in Patients with Atrial Fibrillation: A Multidisciplinary Discussion. Am J Cardiovasc Drugs 23 , 407–418 (2023). https://doi.org/10.1007/s40256-023-00582-9
Download citation
Accepted : 05 April 2023
Published : 05 May 2023
Issue Date : July 2023
DOI : https://doi.org/10.1007/s40256-023-00582-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Find a journal
- Publish with us
- Track your research

Tropical Journal of Pharmaceutical Research Journal / Tropical Journal of Pharmaceutical Research / Vol. 23 No. 3 (2024) / Articles (function() { function async_load(){ var s = document.createElement('script'); s.type = 'text/javascript'; s.async = true; var theUrl = 'https://www.journalquality.info/journalquality/ratings/2404-www-ajol-info-tjpr'; s.src = theUrl + ( theUrl.indexOf("?") >= 0 ? "&" : "?") + 'ref=' + encodeURIComponent(window.location.href); var embedder = document.getElementById('jpps-embedder-ajol-tjpr'); embedder.parentNode.insertBefore(s, embedder); } if (window.attachEvent) window.attachEvent('onload', async_load); else window.addEventListener('load', async_load, false); })();
Article sidebar.

Article Details
Submission of a manuscript to this journal is a representation that the manuscript has not been published previously and is not under consideration for publication elsewhere.
All authors named in each manuscript would be required to sign a form (to be supplied by the Editor) so that they may retain their copyright in the article but to assign to us (the Publishers) and its licensees in perpetuity, in all forms, formats and media (whether known or created in the future) to (i) publish, reproduce, distribute, display and store the contribution, (ii) translate the contribution into other languages, create adaptations, reprints, include within collections and create summaries, extracts and/or abstracts of the contribution, (iii) create any other derivative works(s) based on the contribution, (iv) to exploit all subsidiary rights in the contribution, (v) the inclusion of electronic links from the contribution to third party material where-ever it may be located, and (vi) license any thrid party to do any or all of the above.
Main Article Content
Safety and tolerability of moxifloxacin in pediatric population: a systematic review of evidence based practice, mohammed kanan alshammari, noura abdullah alatwi, wejdan mohammed alshehri, shatha khaled altwijri, sama abdulmohsen alsheehe, abdulaziz khalaf alshammari, rehab raja alroqi, raghad othman alsharafi, razan ali alshehri, khalid mokhlef alenazi, faisal shouick alenazi, nasser hamdan alenazy, faisal saleem alanazi, mujtaba abbas jasim aljasim, fatimah raja alahmari.
Purpose : To evaluate the adverse events (AEs) associated with moxifloxacin (MFX) use in children below the age of 18 years.
Methods: This review was performed in conformity with the preferred items for systematic reviews and meta-analysis (PRISMA) guidelines using different databases. Articles meeting the inclusion criteria were screened and the studies were selected for the qualitative synthesis.
Results : A total of 21 studies were included in the systematic review. Among these, 7 retrospective cohort studies, 6 case reports, 3 prospective cohort studies, 2 randomized clinical trials (RCT) and the remainder utilized other methodologies. The variability in studies allowed for an assessment of the safety and tolerability of both short-term and long-term MFX administration in pediatric patients.
Conclusion: Although MFX use is associated with AEs, the majority were mild and resolved on their own. The reason for QTc prolongation and elevated liver enzymes remain a question for clinicians in prescribing MFX in pediatric patients.
AJOL is a Non Profit Organisation that cannot function without donations. AJOL and the millions of African and international researchers who rely on our free services are deeply grateful for your contribution. AJOL is annually audited and was also independently assessed in 2019 by E&Y.
Your donation is guaranteed to directly contribute to Africans sharing their research output with a global readership.
- For annual AJOL Supporter contributions, please view our Supporters page.
Journal Identifiers


IMAGES
VIDEO
COMMENTS
If you don't spot any major flaws, take a break from the manuscript, giving you time to think. Consider the article from your own perspective. When you sit down to write the review, again make sure you familiarize yourself with any journal-specific guidelines (these will be noted in the journal's guide for authors). 3.
Author Hub | A Guide to Peer Reviewing Journal Articles 9/12 4. Writing your review Once you have read the article and made notes on both your broad and detailed impressions, you have the raw material for writing your review. Many reviewers choose to summarise their thoughts in the first paragraphs of the review, and then, in the second half
For many kinds of assignments, like a literature review, you may be asked to offer a critique or review of a journal article.This is an opportunity for you as a scholar to offer your qualified opinion and evaluation of how another scholar has composed their article, argument, and research.That means you will be expected to go beyond a simple summary of the article and evaluate it on a deeper ...
Step by step. guide to reviewing a manuscript. When you receive an invitation to peer review, you should be sent a copy of the paper's abstract to help you decide whether you wish to do the review. Try to respond to invitations promptly - it will prevent delays. It is also important at this stage to declare any potential Conflict of Interest.
Identify the article. Start your review by referring to the title and author of the article, the title of the journal, and the year of publication in the first paragraph. For example: The article, "Condom use will increase the spread of AIDS," was written by Anthony Zimmerman, a Catholic priest. 4.
2. Benefits of Review Articles to the Author. Analysing literature gives an overview of the "WHs": WHat has been reported in a particular field or topic, WHo the key writers are, WHat are the prevailing theories and hypotheses, WHat questions are being asked (and answered), and WHat methods and methodologies are appropriate and useful [].For new or aspiring researchers in a particular ...
Think about structuring your review like an inverted pyramid. Put the most important information at the top, followed by details and examples in the center, and any additional points at the very bottom. Here's how your outline might look: 1. Summary of the research and your overall impression. In your own words, summarize what the manuscript ...
Clinicians frequently benefit from review articles to update their knowledge in their field of specialization, and use these articles as a starting point for formulating guidelines. [ 1 , 2 ] The institutions which provide financial support for further investigations resort to these reviews to reveal the need for these researches. [ 3 ]
The most critical characteristics of an effective review are clarity, specificity, constructiveness, and thoroughness (Hyman, 1995 ). A journal article review should inform the managing editor and author of the primary strengths and weaknesses of a manuscript in a focused way (see Table 11.1 ).
Hames, I. Peer Review and Manuscript Management in Scientific Journals: Guidelines for Good Practice. Wiley-Blackwell: Oxford, UK, 2007. Writing a journal article review. Australian National University: Canberra, Australia, 2010. Available online. Golash-Boza, T. How to write a peer review for an academic journal: Six steps from start to finish.
A well-written review article must summarize key research findings, reference must-read articles, describe current areas of agreement as well as controversies and debates, point out gaps in current knowledge, depict unanswered questions, and suggest directions for future research ( 1 ). During the last decades, there has been a great expansion ...
2. Skim the article to get a feel for its organization. First, look through the journal article and try to trace its logic. Read the title, abstract, and headings to get a feel for how the article is organized. In this initial, quick skim, identify the question or problem that the article addresses. 3.
How to become a reviewer. There are three ways to register as a reviewer. 1. Create a journal-specific reviewer account on Sage Track. Search for the journal's name here and then click the 'Submit paper' link. This will take you to the peer review system where you can create an account.
The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement, published in 2009, was designed to help systematic reviewers transparently report why the review was done, what the authors did, and what they found. Over the past decade, advances in systematic review methodology and terminology have necessitated an update to the guideline. The PRISMA 2020 statement ...
A review article can also be called a literature review, or a review of literature. It is a survey of previously published research on a topic. It should give an overview of current thinking on the topic. And, unlike an original research article, it will not present new experimental results. Writing a review of literature is to provide a ...
Regularly update journal author instructions with the latest systematic review guidelines, methods, and tools. - Include the latest update date in the author instructions. - Set a regular updating schedule (e.g., yearly or when new guidelines, methods, or tools emerge). - Involve an information specialist in updating the author instructions.
As mentioned previously, there are a number of existing guidelines for literature reviews. Depending on the methodology needed to achieve the purpose of the review, all types can be helpful and appropriate to reach a specific goal (for examples, please see Table 1).These approaches can be qualitative, quantitative, or have a mixed design depending on the phase of the review.
Journal Article Review. Just like other types of reviews, a journal article review assesses the merits and shortcomings of a published work. ... In the body of the review, use APA style guidelines for formatting citations and references. Include an introduction, summary of the article, critical evaluation, and conclusion. Make sure to follow ...
Ethical guidelines for peer reviewers. Peer reviewers must follow these ethical guidelines when reviewing for Taylor & Francis journal articles: Reviewers must give unbiased consideration to each manuscript submitted. They should judge each on its merits, without regard to race, religion, nationality, gender, seniority, or institutional ...
Article Review vs. Response Paper . Now, let's consider the difference between an article review and a response paper: If you're assigned to critique a scholarly article, you will need to compose an article review.; If your subject of analysis is a popular article, you can respond to it with a well-crafted response paper.; The reason for such distinctions is the quality and structure of ...
Writing a Literature Review. A literature review is a document or section of a document that collects key sources on a topic and discusses those sources in conversation with each other (also called synthesis ). The lit review is an important genre in many disciplines, not just literature (i.e., the study of works of literature such as novels ...
Literature reviews are in great demand in most scientific fields. Their need stems from the ever-increasing output of scientific publications .For example, compared to 1991, in 2008 three, eight, and forty times more papers were indexed in Web of Science on malaria, obesity, and biodiversity, respectively .Given such mountains of papers, scientists cannot be expected to examine in detail every ...
Writing a journal article review in APA style requires a thorough reading of an article and then present our personal opinions on its subject matter. In order to write a journal article review in APA style, one must necessarily conform to the detailed guidelines of APA style of writing. As such, a few tips for writing a journal article review ...
Methods Our review included 44/49 PEPFAR-supported countries to determine if TB screening and TPT are recommended specifically for pregnant and breastfeeding women living with HIV (WLHIV). National guidelines reviewed and abstracted included TB, HIV, prevention of vertical HIV transmission, TPT, and any other relevant guidelines.
Direct oral anticoagulants (DOACs) are recommended for the prevention of thromboembolism in patients with atrial fibrillation (AF), and are now preferred over vitamin K antagonists due to their beneficial efficacy and safety profile. However, all oral anticoagulants carry a risk of gastrointestinal (GI) bleeding. Although the risk is well documented and acute bleeding well codified, there is ...
Purpose: To evaluate the adverse events (AEs) associated with moxifloxacin (MFX) use in children below the age of 18 years. Methods: This review was performed in conformity with the preferred items for systematic reviews and meta-analysis (PRISMA) guidelines using different databases. Articles meeting the inclusion criteria were screened and the studies were selected for the qualitative synthesis.