- Program Finder
- Admissions Services
- Course Directory
- Academic Calendar
- Hybrid Campus
- Lecture Series
- Convocation
- Strategy and Development
- Implementation and Impact
- Integrity and Oversight
- In the School
- In the Field
- In Baltimore
- Resources for Practitioners
- Articles & News Releases
- In The News
- Statements & Announcements
- At a Glance
- Student Life
- Strategic Priorities
- Inclusion, Diversity, Anti-Racism, and Equity (IDARE)
- What is Public Health?

Is the Hygiene Hypothesis True?
Did Covid shutdowns stunt kids' immune systems?
Caitlin Rivers
The hygiene hypothesis is the idea that kids need to be exposed to germs in order to develop healthy immune systems. We know that many common viruses did not circulate as widely during the pandemic, thanks to social distancing, masking, and other COVID mitigation measures. Are there downsides to those missed infections?
In this Q&A, Caitlin Rivers speaks with Marsha Wills-Karp, PhD, MHS , professor and chair of Environmental Health and Engineering , about the role of household microbiomes, birth, and vaccines in the development of kids’ immune systems—and whether early exposure really is the best medicine.
This Q&A is adapted from Rivers’ Substack blog, Force of Infection .
I think there’s some concern among parents who have heard about the hygiene hypothesis that there is a downside to all those stuffy noses that didn’t happen [during the COVID-19 pandemic]. Are there any upsides to viral infections? Do they help the immune system in some meaningful way?
I don’t think so.
You mentioned the hygiene hypothesis, which was postulated back in the ‘80s. German scientists noticed that families with fewer children tended to have more allergic disease. This was interpreted [to mean] that allergic disease was linked to experiencing fewer infections. I have explored this idea in my research for a couple of decades now.
This phenomenon has helped us to understand the immune system, but our interpretation of it has grown and expanded—particularly with respect to viruses. Almost no virus is protective against allergic disease or other immune diseases. In fact, infections with viruses mostly either contribute to the development of those diseases or worsen them.
The opposite is true of bacteria. There are good bacteria and there are bad bacteria. The good bacteria we call commensals . Our bodies actually have more bacterial cells than human cells. What we’ve learned over the years is that the association with family life and the environment probably has more to do with the microbiome. So one thing I would say is sanitizing every surface in your home to an extreme is probably not a good thing. Our research team showed in animals that sterile environments don’t allow the immune system to develop at all. We don’t want that.
What does contribute to the development of the immune system, if not exposure to viruses?
There are a number of factors that we’ve associated with the hygiene hypothesis over the last 20 years, and these exposures start very early in life. Cesarean sections, which do not allow the baby to travel through the birth canal and get exposed to the mother’s really healthy bacterial content, is a risk factor for many different immune diseases. Getting that early seeding with good bacteria is critical for setting up the child going forward. Breastfeeding also contributes to the development of a healthy immune system.
There are other factors. Our diets have changed dramatically over the years. We eat a lot of processed food that doesn’t have the normal components of a healthy microbiome, like fiber. These healthy bacteria in our gut need that fiber to maintain themselves. They not only are important for our immune system but they’re absolutely critical to us deriving calories and nutrients from our food. All these things contribute to a healthy child.
We’ve also noticed that people who live on farms have fewer of these diseases because they’re exposed to—for lack of a better term—the fecal material of animals. And what we have found is that it’s due to these commensal bacteria. That is one of the components that help us keep a healthy immune system. Most of us will probably not adopt farm life. But we can have a pet, we can have a dog.
I think all the pet lovers out there will be pleased to hear that.
There’s a lot of evidence that owning a pet in early childhood is very protective.
What about the idea that you need to be exposed to viruses in early life because if you get them as an adult, you’ll get more severely ill? We know that’s true for chickenpox, for example. Do you have any concerns about that?
We should rely on vaccines for those exposures because we can never predict who is going to be susceptible to severe illness, even in early childhood. If we look back before vaccines, children under 4 often succumbed to infections. I don’t think we want to return to that time in history.
Let me just give you one example. There’s a virus called RSV, it’s a respiratory virus. Almost all infants are positive for it by the age of 2. But those who get severe disease are more likely to develop allergic disease and other problems. So this idea that we must become infected with a pathogenic virus to be healthy is not a good one.
Even rhinovirus, which is the common cold, most people recover fine. But there’s a lot of evidence that for somebody who is allergic, rhinovirus exposures make them much worse. In fact, most allergic or asthmatic kids suffer through the winter months when these viruses are more common.
And that’s particularly salient because there is a lot of rhinovirus and enterovirus circulating right now.
From my point of view, right now, avoiding flu and COVID-19 is a priority. Those are not going to help you develop a healthy immune response, and in fact, they can do a lot of damage to the lungs during that critical developmental time. Data [show] that children that have more infections in the first 6 months to a year of life go on to have more problems.
It’s always surprising to me when I look at the data of the fraction of time that young children spend with these common colds—and this is pre-pandemic—it’s not uncommon for kids to be sick 50% of the time. That feels right as a parent, but it’s startling.
The other thing people don’t know is that the GI tract is where you get tolerized to all of your foods, allergens and things. Without those healthy bacteria in your gut, you can’t tolerate common allergens.
How does that relate to the guidance that’s changed over the years—that you should withhold peanuts in early life and now you’re supposed to offer them in early life?
The guidance to delay exposure to peanuts didn’t consider the fact that oral exposure to peanuts was not the only exposure kids were getting. There were peanut oils in all kinds of skin creams and other things. So kids got exposed through their skin, but they had no gut protection—and the GI tract is important for a tolerant system. If you have a healthy immune response, you get tolerized in early life.
This concept is a little bit different for those families who may already have a predisposition to allergies. But for the general public, exposure is key to protecting them in early life.
I think some parents look at the guidance that you should now offer peanuts in early life and say, “Are we not doing that with rhinovirus by masking kids or improving ventilation?” How should people think about the development of the immune system for food allergies compared to infections?
The thing about rhinoviruses is that after recovering, you’re not protected from the next infection. There is no real immune protection there. Most of us suffer from colds throughout our whole life. Like I said, bacterial exposure is what’s key to priming the immune response.
Also, we forget that a lot of kids die from the flu. Unlike COVID-19, where younger kids are not quite as susceptible to severe illness, that’s not true for flu. RSV, too, can be quite severe in young children and older adults.
Caitlin Rivers, PhD, MPH , is a senior scholar at the Johns Hopkins Center for Health Security and an assistant professor in Environmental Health and Engineering at the Johns Hopkins Bloomberg School of Public Health.
- Study Finds That Children’s Antibody Responses to COVID-19 Are Stronger Than Adults’
- Back to School: COVID, CDC Guidance, Monkeypox, and More
- A New Shot Prevents Serious Illness from RSV
Related Content

Outbreak Preparedness for All

The Public Health Strategy Behind Baltimore’s Record-Low Infant Mortality Rate

What’s Happening With Dairy Cows and Bird Flu
Decisive Action Needed to Stop Cervical Cancer Deaths

How Dangerous is Dengue?
What Is the Hygiene Hypothesis?
Many parents believe that their children must be kept in an environment that is as clean as possible, but some research suggests that being exposed to what many would call unclean conditions is good for a child's immune system. Research has indicated that children who are kept in very clean environments have a higher rate of hay fever, asthma and a wide range of other conditions. This is what is called the hygiene hypothesis.
The hygiene hypothesis was first introduced in the late 1980s by David P. Strachan, a professor of epidemiology, in the British Medical Journal. Strachan found that children in larger households had fewer instances of hay fever because they are exposed to germs by older siblings. This finding led to further research that suggests a lack of early childhood exposure to less than pristine conditions can increase the individual's susceptibility to disease.
For example, in the late 1990s, Dr. Erika von Mutius, a health researcher, compared the rates of allergies and asthma in East Germany and West Germany, which unified in 1999. Her initial hypothesis was that East German children, who grew up in dirtier and generally less healthful conditions, would have more allergies and suffer more from asthma than their Western counterparts. However, her research found the opposite: children in the polluted areas of East Germany had lower allergic reactions and fewer cases of asthma than children in West Germany.
Further research has found that children in developing areas of the world are less likely to develop allergies and asthma compared with children in the developed world.
Building the immune system
The idea is simple. When babies are inside the womb they have a very weak immune system because they are given protection by their mother's antibodies. When they exit the womb, though, the immune system must start working for itself. For the immune system to work properly, it is thought that the child must be exposed to germs so that it has a chance to strengthen, according to the U.S. Food and Drug Administration (FDA).
The idea is similar to the training of a body builder. For a body builder to be able to lift heavy objects, the muscles must be trained by lifting heavier and heavier objects. If the body builder never trains, then he will be unable to lift a heavy object when asked. The same is thought to be true for the immune system. In able to fight off infection, the immune system must train by fighting off contaminants found in everyday life. Systems that aren't exposed to contaminants have trouble with the heavy lifting of fighting off infections.
Mutius hypothesized that the reason children who are not exposed to germs and bacteria are sicklier is due to how the human immune system evolved. She thinks there are two types of biological defenses. If one of the defense systems isn't trained or practiced enough to fight off illness, the other system overcompensates and creates an allergic reaction to harmless substances like pollen.
Research by other scientists has found similar results. Exposure to germs triggered an internal inflammatory response in children who were raised in cleaner environments, leading to ailments such as asthma, according to a 2002 article in Science magazine.
One researcher has personal experience has leads him to back the hygiene hypothesis. "I believe that there is a role in the development of a child's immunity exposure to various germs and a vast microbiome diversity," said Dr. Niket Sonpal, an assistant professor of clinical medicine at Touro College of Osteopathic Medicine, Harlem Campus. "I was born in India but moved to the U.S. and went to college in Virginia and medical school in Europe. I am sure that the vast change in environment has played a role in my immunity. How has it? I don't think we know just yet."
In 1997, some began to question if there is a correlation between the hygiene hypothesis and vaccinations. The number of children getting vaccinations was going up, but so were the number of children afflicted with allergies, eczema and other problems. Could depriving the developing immune system of infections using vaccines cause the immune system to eventually attack itself and cause autoimmune diseases like asthma and diabetes? This is a highly contested issue.
Three studies conducted in the 1990s showed that vaccines had no correlation with children developing allergies and other ailments later in life. In fact, vaccinations may help prevent asthma and other health problems other than the diseases they were intended to prevent, according to The National Center for Immunization Research and Surveillance . The idea that vaccinations can cause health problems does not consider the fact that children, whether vaccinated or not, are still exposed to pathogens that help build the immune system. These pathogens also have no relation to the diseases that the vaccines prevent.
The conflict between cleanliness and exposure can leave parents feeling confused. There are many microbes that can make children very sick, such as such as respiratory syncytial virus (RSV), E.coli and salmonella. So cleaning the home is still very important. What should children be exposed to and what should they be protected from?
The CDC recommends regularly cleaning and disinfecting surfaces in the home, especially when surfaces have been contaminated by fecal matter or meat or have come in contact with those who have a virus. Children are also encouraged, though, to play outside , even if they may get dirty in the process. This balancing act may prove to help children stay healthy while still developing a healthy immune system.
Sonpal thinks that the healthy growth of the immune system isn't just about coming in contact with dirt. It also has to do with what foods are consumed, what kind of environments the person grows up in and intrinsic genetics coupled with physical activity levels. Harvard Medical School noted that getting plenty of sleep, avoiding cigarette smoke, drinking in moderation and controlling blood pressure also all play a part in a healthy immune system.
Additional Resources
- Clinical & Experimental Immunology: The 'Hygiene Hypothesis' for Autoimmune and Allergic Diseases: An Update
- Mayo Clinic: Early germ exposure prevents asthma?
- U.S. National Library of Medicine: The Hygiene Hypothesis and home hygiene
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.

'Vampire' bacteria thirst for human blood — and cause deadly infections as they feed
Scientists uncover the cells that save you when water goes down the wrong pipe
'Unprecedented' discovery of mysterious circular monument near 2 necropolises found in France
Most Popular
- 2 'Gambling with your life': Experts weigh in on dangers of the Wim Hof method
- 3 'Exceptional' prosthesis of gold, silver and wool helped 18th-century man live with cleft palate
- 4 NASA spacecraft snaps mysterious 'surfboard' orbiting the moon. What is it?
- 5 AI pinpoints where psychosis originates in the brain
- 2 2,500-year-old skeletons with legs chopped off may be elites who received 'cruel' punishment in ancient China
- 3 Modern Japanese people arose from 3 ancestral groups, 1 of them unknown, DNA study suggests
- 4 Giant, 82-foot lizard fish discovered on UK beach could be largest marine reptile ever found
- 5 The universe may be dominated by particles that break causality and move faster than light, new paper suggests
- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
U.S. Food and Drug Administration
- Search
- Menu
- Vaccines, Blood & Biologics
- Resources for You (Biologics)
- Consumers (Biologics)
Asthma: The Hygiene Hypothesis
What do clean houses have in common with childhood infections.
One of the many explanations for asthma being the most common chronic disease in the developed world is the “hygiene hypothesis.” This hypothesis suggests that the critical post-natal period of immune response is derailed by the extremely clean household environments often found in the developed world. In other words, the young child’s environment can be “too clean” to pose an effective challenge to a maturing immune system.
According to the “hygiene hypothesis,” the problem with extremely clean environments is that they fail to provide the necessary exposure to germs required to “educate” the immune system so it can learn to launch its defense responses to infectious organisms. Instead, its defense responses end up being so inadequate that they actually contribute to the development of asthma.
Scientists based this hypothesis in part on the observation that, before birth, the fetal immune system’s “default setting” is suppressed to prevent it from rejecting maternal tissue. Such a low default setting is necessary before birth—when the mother is providing the fetus with her own antibodies. But in the period immediately after birth the child’s own immune system must take over and learn how to fend for itself.
The “hygiene hypothesis” is supported by epidemiologic studies demonstrating that allergic diseases and asthma are more likely to occur when the incidence and levels of endotoxin (bacterial lipopolysaccharide, or LPS) in the home are low. LPS is a bacterial molecule that stimulates and educates the immune system by triggering signals through a molecular “switch” called TLR4, which is found on certain immune system cells.
The science behind the hygiene hypothesis
The Inflammatory Mechanisms Section of the Laboratory of Immunobiochemistry is working to better understand the hygiene hypothesis, by looking at the relationship between respiratory viruses and allergic diseases and asthma, and by studying the respiratory syncytial virus (RSV) in particular.
What does RSV have to do with the hygiene hypothesis?
- RSV is often the first viral pathogen encountered by infants.
- RSV pneumonia puts infants at higher risk for developing childhood asthma. (Although children may outgrow this type of asthma, it can account for clinic visits and missed school days.)
- RSV carries a molecule on its surface called the F protein, which flips the same immune system “switch” (TLR4) as do bacterial endotoxins.
It may seem obvious that, since both the RSV F protein and LPS signal through the same TLR4 “switch,” they both would educate the infant’s immune system in the same beneficial way. But that may not be the case.
The large population of bacteria that normally lives inside humans educates the growing immune system to respond using the TLR4 switch. When this education is lacking or weak, the response to RSV by some critical cells in the immune system’s defense against infections—called “T-cells”—might inadvertently trigger asthma instead of protecting the infant and clearing the infection. How this happens is a mystery that we are trying to solve.
In order to determine RSV’s role in triggering asthma, our laboratory studied how RSV blocks T-cell proliferation.
Studying the effect of RSV on T-cells in the laboratory, however, has been very difficult. That’s because when RSV is put into the same culture as T-cells, it blocks them from multiplying as they would naturally do when they are stimulated. To get past this problem, most researchers kill RSV with ultraviolet light before adding the virus to T-cell cultures. However we did not have the option of killing the RSV because that would have prevented us from determining the virus’s role in triggering asthma.
Our first major discovery was that RSV causes the release from certain immune system cells of signaling molecules called Type I and Type III interferons that can suppress T-cell proliferation (Journal of Virology 80:5032-5040; 2006).
The hygiene hypothesis suggests that a newborn baby’s immune system must be educated so it will function properly during infancy and the rest of life. One of the key elements of this education is a switch on T cells called TLR4. The bacterial protein LPS normally plays a key role by flipping that switch into the “on” position.
Prior research suggested that since RSV flips the TLR4 switch, RSV should “educate” the child’s immune system to defend against infections just like LPS does.
But it turns out that RSV does not flip the TLR switch in the same way as LPS. This difference in switching on TLR, combined with other characteristics of RSV, can prevent proper education of the immune system.
One difference in the way that RSV flips the TLR4 switch may be through the release of interferons, which suppresses the proliferation of T-cells. We still do not know whether these interferons are part of the reason the immune system is not properly educated or simply an indicator of the problem. Therefore, we plan to continue our studies about how RSV can contribute to the development of asthma according to the hygiene hypothesis.
Further research
This finding that Type I and Type III interferons can mediate the suppression of T-cells caused by RSV generated two significant questions that our laboratory is now addressing:
- Interferons are important molecules that enhance inflammation, so why--in the context of RSV--do they suppress T-cells?
- Interferons are clearly not the only way RSV suppresses T-cells. What are the other mechanisms that may depend upon T-cells coming in direct contact and communicating with other immune cells?
Related Research
- Assessing the Mechanism of Immunotherapy for Allergy and Allergic Asthma: Effect of Viral Respiratory Infections on Pathogenesis and Clinical Course of Asthma and Allergy Ronald Rabin, MD
MINI REVIEW article
The hygiene hypothesis and new perspectives—current challenges meeting an old postulate.

- 1 Translational Inflammation Research Division & Core Facility for Single Cell Multiomics, Medical Faculty, Biochemical Pharmacological Center (BPC), Philipps University of Marburg, Marburg, Germany
- 2 German Center for Lung Research (DZL), Marburg, Germany
- 3 Comprehensive Biobank Marburg (CBBMR), Medical Faculty, Philipps University of Marburg, Marburg, Germany
- 4 German Biobank Alliance (GBA), Marburg, Germany
During its 30 years history, the Hygiene Hypothesis has shown itself to be adaptable whenever it has been challenged by new scientific developments and this is a still a continuously ongoing process. In this regard, the mini review aims to discuss some selected new developments in relation to their impact on further fine-tuning and expansion of the Hygiene Hypothesis. This will include the role of recently discovered classes of innate and adaptive immune cells that challenges the old Th1/Th2 paradigm, the applicability of the Hygiene Hypothesis to newly identified allergy/asthma phenotypes with diverse underlying pathomechanistic endotypes, and the increasing knowledge derived from epigenetic studies that leads to better understanding of mechanisms involved in the translation of environmental impacts on biological systems. Further, we discuss in brief the expansion of the Hygiene Hypothesis to other disease areas like psychiatric disorders and cancer and conclude that the continuously developing Hygiene Hypothesis may provide a more generalized explanation for health burden in highly industrialized countries also relation to global changes.
Introduction
Throughout its history, the Hygiene Hypothesis has shown itself to be adaptable and flexible whenever it has been challenged by innovation in science ( 1 ). A number of new findings need to be considered in this ongoing revisiting process: The originally proposed Th1/Th2 paradigm is challenged by currently elucidated new classes of effector and regulating immune cells pointing out to a more complex immune network involved in allergy development ( 2 ). Studies on biomarkers and deep phenotyping techniques changed our understanding of asthma as a uniform disease in favor of distinct phenotypes that are driven by different causations ( 3 ). The emerging field of epigenetics enables us to fill the black box of “gene-by-environment interactions” with conveying mechanisms ( 4 ). Currently recognized epigenetic pathways overlapping between chronic inflammatory diseases and other disorders such as psychiatric conditions or cancer might extend the Hygiene Hypothesis toward a model explaining in a broader sense the rise of health burdens in westernized societies ( 5 ). Finally, the world-wide challenge caused by the climate changes will not leave the consequences of Hygiene Hypothesis unaffected. Changing life-styles are closely related to measures implemented to slow down CO 2 emissions and to stabilize the world climate ( 6 ).
Challenges From Immunology – The Immune System Becomes More Complex
Parallel to the revisions that Hygiene Hypothesis has undergone over time ( 7 ), our perception of the mechanisms underlying cellular and humoral immune responses has changed fundamentally over the last decades. High-resolution flow cytometry and cell sorting and, most recently, single cell multiomics-based analyses provided a deeper insight into the phenotypic characterization, function, and development of diverse classes of hematopoietic cell types. The dichotomous model of divergent Th1 and Th2 responses was significantly expanded by the discovery that T lymphocytes represent a branched network of subsets, characterized by a high level of plasticity and adaptability ( 8 ). Namely, Sakaguchi’s discovery of regulatory T-cell (Treg) subsets provided a significant new impetus to researchers investigating the immunological origin of allergic and autoimmune diseases and their prevention under healthy conditions and pointed out new strategies to combat those maladies ( 9 , 10 ). Moreover, the discovery of new classes of effector cells and their cellular interactions added relevant evidence to the field. As one example, innate lymphoid cells (ILC) became of main interest as they have been shown to be both directly and indirectly associated with and involved in the development of allergic responses ( 11 ). This unique class of effector cells lacks a clonally distributed antigen receptor which thus resemble innate immune cells characterized by (antigen) unspecific activation, however, they exert T helper (Th)-like effector cell activities ( 12 ). According to their expression of effector cytokines and transcription factors ILC have been classified into three groups: ILC1, ILC2, and ILC3 ( 13 ). While ILC1 produce interferon-gamma (IFNγ) and tumor necrosis factor α (TNFα) and, similarly to Th1 cells, express T-bet, ILC2 are able to produce Th2 cytokines such as IL-5 and IL-13, like Th2 cells under the control of the transcription factor GATA-3. ILC3 are similar to Th17 cells and release IL-17A and IL-22 as well as granulocyte macrophage colony stimulating factor (GM-CSF). In animal models of allergic airway inflammation as well as in human allergic asthmatics ILC2 are present at elevated frequencies within the lung and airways epithelial compartments where they were found to produce high amounts of the type-2 cytokines IL-5 and IL-13 ( 14 ). Within the last years, ILC2 have been recognized as early promoters to establish and maintain allergic airway inflammatory responses but also as protectors promoting repair processes of the lung epithelium ( 15 , 16 ).
A potential link between the Hygiene Hypothesis and the function of ILC lineages comes from the gut. The symbiotic interaction between immune cells and the microbiota in the gut is principally decisive for the development of tolerance or pathogenicity. The ILC3 lineage is essential in the development of lymphoid follicles and Peyer’s patches in the gut and was shown to be crucial for the maintenance of a well-balanced symbiosis with the microbiota ( 17 ). The host microbiota itself might play an important role in determining ILC subsets specificity as indicated by results coming from experimental approaches. Sepahi et al. very recently reported that short chain fatty acids (SCFA) arising from dietary fibers by microbial fermentation in the intestine induced expansion of prevailing ILC subsets. By triggering ILC subset expansion via G-protein-coupled receptors (GPCR) those dietary metabolites contribute to the homeostasis in the local compartment ( 18 ). Another mechanism to induce repair and homeostatic conditions at epithelial surfaces is mediated via IL-22-producing ILC3 in response to the microbiota. In interaction with IL-18 produced by the epithelial cells, IL-22 is involved in the promotion of repair and remodeling processes as well as in the maintenance of the gut homeostasis ( 19 ). By acting as mediators between the microbiota and the host ILC are recognized as crucial in the early host response to microbial stimuli.
Challenges From Changing Environments – Can Epigenetics Provide the Missing Link to Explain “Gene-by-Environment Interactions”?
Very recently, damaging factors that jeopardize the normal development or disturb the balance of an established immune system have come into the focus of research on allergic diseases. Environmental changes caused by in- and outdoor pollution ( 20 , 21 ) and the global warming impact the atopic epidemic and some attempts were undertaken recently to integrate these scenarios into the concept of the Hygiene Hypothesis on the basis of epigenetic changes driven by gene-by-environment interactions ( 22 ).
In contrast to our ancestors who spent most of their life time outdoors and thus close to a natural environment, post-modern and mainly urban life-styles are characterized by a significantly higher proportion of indoor activities. These changing habits underline the potential importance of indoor air composition on the development of allergic diseases and further emphasize the role of the environmental microbiota ( 23 ). Indoor air in urban homes is often burdened with elevated levels of molds which are found to be harmful to the airways and favor the development of airway inflammation and asthma ( 24 ). Against the background of growing climate awareness and the resulting increased efforts to reduce energy consumption and CO 2 emissions, current research on these indoor exposures in homes with improved house insulation points out to an up-coming health problem. Enrichment of volatile organic compounds released from furniture or brought in by tobacco smoke as well perennial allergens and molds will jeopardize mainly infants as the developing immune system and the growing lung are highly susceptible to these damage factors ( 25 ). Already the fetus might become affected by these components ( 26 ). This was exemplarily shown for tobacco smoke in a transgenerational case control study conducted to assess the risk for asthma by prenatal smoking. Grandmothers and mothers of asthmatic and non-asthmatic children were asked about smoking habits during their own pregnancy. The study reported an odds ratio twice as high for children to develop asthma in families where grandmothers frequently smoked during the mother’s fetal period ( 27 ).
At that point the Hygiene Hypothesis was in line with an upcoming general idea that non-inherited/non communicable diseases like allergies and asthma develop on the background of an inappropriate interaction between environmental exposures and a given genotype to shape a specific (disease) phenotype. Though based on the concept of a so-called epigenetic landscape postulated by Waddington already in the 50ties of the last century, the underlying molecular mechanisms of epigenetic programming had still been the “missing link” in the scenario of gene-by-environment-interactions ( 28 ). By discovering mechanisms such as DNA methylation, diverse histone modifications and microRNA regulation as molecular mechanisms underlying epigenetic regulation of gene expression, an exciting new field of research was opened that currently has a strong impact on research aiming to unravel the still existing mysteries of allergy development and prevention ( 29 – 31 ).
Indeed, epigenetic mechanisms have meanwhile clearly been demonstrated to be involved in mediating the effects of environmental factors increasing or decreasing the risk of allergy development ( 4 ). Pro-allergic environmental influences can be exemplified by pollution. For instance, higher in utero exposure to polycyclic aromatic hydrocarbons (PAH) has been shown to be associated with increased cord blood leukocyte DNA methylation at the promoter of the IFNγ-encoding gene ( 32 , 33 ). Moreover, in Treg isolated from peripheral blood mononuclear cells, higher PAH exposure has been correlated with elevated DNA methylation at the promoter of the gene encoding FOXP3, a master regulator of Treg development and activities, with the effect being stronger in asthmatic than in non-asthmatic children ( 34 ).
After epidemiological studies had demonstrated an association between spending early life time in specific agricultural environments and protection against the development of allergies in childhood ( 35 , 36 ), functional investigations of various types started to clarify which elements of farming, such as contact with farm animals, consumption of raw cow’s milk, exposure to so-called farm-dust, and others, mechanistically underlie this observation. DNA demethylation at the FOXP3-encoding locus related to higher expression of the gene and activation of Treg ( 37 ) has been associated in cord blood with maternal consumption of raw cow’s milk ( 38 ) and in children’s whole blood with early-life ingestion of raw cow’s milk ( 39 ). Compared to processed shop milk, pretreatment with raw cow’s milk reduced features of the disease in mice subjected to a model of food allergy and this effect was mediated by changes in histone acetylation patterns at crucial T cell-related genes ( 40 , 41 ). Interestingly enough, unprocessed cow’s milk has been shown to contain miRNAs potentially affecting the expression of important allergy-related immune genes, which might contribute to its protective effects against asthma ( 42 ). Several bacteria have been isolated from the farming environment, for instance Acinetobacter lwoffii ( A. lwoffii ), which were demonstrated to diminish the development of allergic symptoms in murine models ( 43 ). A. lwoffii -mediated protection against allergic airway inflammation has been observed in mouse models also transmaternally and shown to be IFNγ−dependent, with this effect being at least partly mediated by preservation of histone H4 acetylation at the promoter of the IFNγ-encoding gene as observed in CD4 + T cells isolated from spleens of the offspring ( 44 , 45 ).
Challenges From the Clinics and Lessons From Animal Models – Asthma Phenotypes and the Hygiene Hypothesis
A recurrent debate flared up in the field of asthma research excellently summarized at the time being in a review by Wenzel in 2012 ( 46 ). Coming from clinical heterogeneity of asthma patients she highlighted that basic inflammation patterns differ in asthma patients which in turn determines the success of the applied therapeutic strategy. As an early diagnosis and adequate treatment may prevent the development of a severe asthma phenotype later on, novel strategies to discriminate children at risk from those who will not develop asthma are required ( 47 ). Following the clinical definition of a phenotype as a result of an interaction between a given genotype and the environment Wenzel and colleagues expressed the strong medical need for novel molecular and genetic biomarkers indicative for the characterization of such phenotypes and defining the specific requirements for stratified therapies. Based on differences between Th2-driven atopic asthma and non-atopic asthma a number of subtypes were defined that evolve and differ with age and respond differentially to standard drug treatment regimes. It quickly became clear that the search for a specific biomarker that clearly identifies a respective phenotype would not be successful. Rather, the synopsis of all data collected from a subject known as “deep phenotyping” may lead to better understanding of complex asthmatic conditions ( 48 ). Deep phenotyping in the era of OMICS goes along with a tremendous increase in data that needs to be analyzed. To handle these big data-sets new approaches become increasingly employed involving models of statistical data dimension reduction and machine-learning strategies ( 49 , 50 ). The idea behind these data-driven approaches is to mine data collections and classify them based on so far hidden patterns behind the data. The hypothesis-free latent class analysis (LCA) approach represent one of the most promising tools to identify new or verify proposed asthma (and other allergic disease) phenotypes. A first LCA approach was carried out in two cohorts of adult asthmatics. Based on clinical and personal characteristics Siroux et al. described two distinct phenotypes in two independent cohorts, a severe phenotype in which asthma is already established in childhood and a second type that starts in adulthood with milder outcomes ( 51 ). In line with the Hygiene Hypothesis, these results pointed out specific preconditions in infant age which pave the pathway to severe asthma later in life. LCA analyses in children substantiated the link between early onset and later disease since early clinical signs such as current unremitting wheezing episodes are ascribed to indicate a higher risk for asthma development later in life while transient wheezing seems to have no pathological consequences ( 52 ).
LCA approaches using data from patient studies elucidated that there might be phenotypic asthmatic manifestations that could be explained by the Hygiene Hypothesis while other phenotypes that might have different pathomechanistic origins failed to be covered by this supposition ( 53 ). Among others, this discrepancy led to new approaches in pre-clinical animal-based experimental set-ups as well as investigations based on human data. New animal models were employed to prove the postulate of such phenotypes that can be discriminated on the immunological and histological levels. By switching from the well-established Ovalbumin (OVA) model, where the sensitization was mainly achieved by a rather artificial intraperitoneal allergen sensitization in the presence of the type-2 driving adjuvant alum, to a more flexible administration of standardized house dust mite extracts (HDM) via the nasal route, it was feasible to induce a more natural and broader spectrum of inflammatory phenotypes ranging from typical allergic eosinophil-dominated respiratory inflammation to airway inflammatory conditions almost exclusively dominated by the influx of neutrophils ( 54 – 56 ). Such more flexible model systems allow deeper and more precise investigations of the mechanisms underlying the development of different phenotypes and a much better characterization of the orchestration of different regulatory and effector T cell subsets in dependence of allergen administration on a continuum between Th2 and Th1/Th17-driven inflammation. In addition, these mouse models mimic the natural situation more closely by using common allergens and a potential natural route of sensitization and thus became helpful for understanding the diverse clinical phenotypes of allergic and non-allergic as well as mild and severe asthma ( 57 , 58 ). By switching between different effector T cell responses in these experimental set-ups substantial knowledge is currently added to our understanding of clinical manifestations in asthma. In combination with LCA helping to elucidate clinical phenotypes these recent research developments strongly boosted a better discrimination between transient and persistent pediatric allergic conditions as well as allergic and non-allergic asthma later in life. This new evidence might lead us to the current limits of the Hygiene Hypothesis. While IgE-driven allergic asthma undoubtedly fits to the Hygiene Hypothesis, it is still unclear whether this holds true also for non-atopic asthma phenotypes the development of which is much more strongly determined by factors different from a missing (microbial) education of the immune system. Thus and to further fine-tune the Hygiene Hypothesis, continuous efforts are required to distinguish between environmental conditions (such as early life infection with pathogenic viruses) that are either associated with the induction of a disease phenotype and/or just contribute to a shift between distinct inflammatory manifestations of allergic disease phenotypes ( 59 , 60 ) and those that really result in a general or a phenotype/endotype-specific prevention of disease in line with the Hygiene Hypothesis ( 43 , 61 ).
Challenges From a View Over the Fence – The Hygiene Hypothesis in Psychiatric Disorders and Cancer
The French scientist Bach was the first who made the principal observation of a general inverse correlation in the prevalences of infectious versus non-communicable chronic inflammatory diseases within the last seven decades ( 62 ). Meanwhile we know that abundant exposure to a high diversity of infectious or even harmless microbes resulting in repeated, low-grade acute inflammatory episodes in early life, associates with lower prevalence of chronic inflammatory disorders accompanied by low levels of inflammatory markers in adulthood. Conversely, high levels of hygiene during perinatal and early childhood developmental periods characteristic for Western countries corresponds to higher levels of inflammatory markers correlating with a higher prevalence of chronic inflammatory disorders later in life. Based on these facts, it has been hypothesized that frequent episodes of low-grade, in most cases clinically symptom-free inflammation in infancy may balance responses to inflammatory stimuli and thus reduce the rate of continuation of chronic inflammation into adulthood, most probably by adequately shaping the adaptive immunity-dependent regulation ( 23 ).
Interestingly, this observation considers a broader spectrum of chronic inflammatory conditions beyond allergies that might fit under the umbrella of the Hygiene Hypothesis such as multiple sclerosis, irritable bowel disease or diabetes type 1 ( 63 ). Moreover, within the last years a similar approach emerged to explain the tremendous increase in psychiatric disorders in westernized countries. Mainly affective disorders such as major depression and bipolar disorder are increasingly diagnosed in the westernized world. Patients suffering from affective and anxiety disorders depict an array of features that mirror inflammatory conditions such as pro-inflammatory cytokines in the blood and the central nervous system accompanied by elevated levels of circulating C-reactive protein (CRP), activation of lymphocytes and inflammatory cellular signaling pathways (MAPK and NF-κB), with the question of causality remaining a chicken or egg problem ( 64 ). Nevertheless, based on genetic predispositions and epigenetic modifications in the brain (nervous system) and the periphery (immune system), both kinds of pathologies, mood and inflammatory disorders, might become established on the basis of a disturbed homeostasis of otherwise tightly balanced adaptive systems of the body. Interestingly but fitting to the hypothesis, the microbiota of the gut seems to play a critical role also in the development of psychiatric disorders as shown by recently conducted studies ( 65 ). Based on an interplay between the gut and the central nervous system, persistent stress and maltreatment modifies the nervous system and thereby the endocrine hypothalamic pituitary axis (HPA) which in turn alters gut microbiota by cortisol release ( 66 ). Dysbiosis in the gut might lead to a compromised cytokine balance in the blood followed by an activation of the microglia in the brain after transfer of inflammatory mediators/cytokines through the blood-brain barrier ( 67 ). Further, degradation of beneficial bacteria in the gut microbiota might result in a loss of microbiota-derived products such as butyrate which directly results in the downregulation of γ-aminobutyrate, serotonin and dopamine, all factors directly involved in the neurological regulation circuits and thus in the genesis of neuropsychiatric disorders when dysregulated ( 68 ).
Finally, to add another example to this collection, there is increasing evidence that similar mechanisms as involved in the protection from allergies might also play a role in the prevention of oncologic diseases ( 69 ). There is no doubt that preceding infections with certain pathogens may favor initiation and further development of several tumor disease entities. However, a variety of recent studies also demonstrated positive effects of pathogen-induced “benign” inflammatory processes on cancer development, even though the underlying mechanisms of this dichotomous influence of microbial exposure-mediated immune modulation on carcinogenesis are not well understood so far ( 70 ). As one example, the origins of childhood leukemia have long been discussed in the context of microbial stimuli in early childhood. Already at the end of 20 th century the question emerged whether early infections in childhood may act protectively against childhood acute leukemia by eliminating expanding aberrant leukocyte clones through well-trained and established immune mechanisms. In concordance with the Hygiene Hypothesis, Greaves propagated the “Delayed Infection Hypothesis” as an explanation for the development of childhood acute (lymphoblastic/myeloid) leukemia (ALL/AML) that peaks at the age of 2-5 years of life in affluent countries ( 71 , 72 ). In his two hit model, Greaves proposed that based on a prenatally occurred chromosomal translocation or hyperdiploidy a pre-leukemic clone is already established around birth (first hit). A second hit event beyond the toddler age then leads to gene deletion or mutation and subsequent transformation to ALL/AML. While children suffering from infections and/or exposed to a rich microbial environment early in life might be ready to prevent that second aberration, predisposed children with an insufficiently educated immune system due to missing “old friends” contacts in the early postnatal life might not be able to eliminate expanding malignant cell clones ( 72 ). A number of studies aimed to prove this hypothesis by exploiting “day care attendance” before the third year of life as a proxy for infection. This concept is still a matter of debate. While the vast majority of these studies could add evidence to the Greaves hypothesis, some well-conducted studies could not support his assumptions ( 73 , 74 ). Recently, a meta-analysis investigated the farm effect with regard to childhood leukemia and confirmed that contact to livestock provides protection not only against allergies but also against childhood leukemia ( 75 ). This study might point out to microbiota as a crucial player in both prevention of allergies and childhood cancer.
The challenges outlined in this mini review are intended to stimulate further exciting debates that might result in continuing revisions and adaptations of the Hygiene Hypothesis. We are aware that the examples reported in this review may only describe a limited subjective selection of the scientific topics currently discussed in context of the Hygiene Hypothesis. However, it is common to all topics that the explanations to unravel the underlying mechanisms refer to the close and beneficial relationship between man and microbes as established on the mucosal surfaces of our body. These interactions result in adequate shaping of adaptive systems of the body (mainly the immune system) that enables the whole organisms to appropriately handle diverse adverse influences. Without exaggeration, this finding might be considered one of the most fundamental insights of the life sciences within the last thirty years.
Author Contributions
All authors contributed by writing and editing of the manuscript. All authors contributed to the article and approved the submitted version.
Funded by the German Center for Lung Research (DZL). Open Access was funded by the Library of the Philipps University Marburg, Germany.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Haahtela T. A biodiversity hypothesis. Allergy (2019) 74:1445–56. doi: 10.1111/all.13763
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Gelfand EW, Joetham A, Wang M, Takeda K, Schedel M. Spectrum of T-lymphocyte activities regulating allergic lung inflammation. Immunol Rev (2017) 278:63–86. doi: 10.1111/imr.12561
3. Breiteneder H, Peng Y-Q, Agache I, Diamant Z, Eiwegger T, Fokkens WJ, et al. Biomarkers for diagnosis and prediction of therapy responses in allergic diseases and asthma. Allergy (2020) 75:3039–68. doi: 10.1111/all.14582
4. Alashkar Alhamwe B, Miethe S, Pogge von Strandmann E, Potaczek DP, Garn H. Epigenetic Regulation of Airway Epithelium Immune Functions in Asthma. Front Immunol (2020) 11:1747. doi: 10.3389/fimmu.2020.01747
5. Langie SA, Timms JA, de Boever P, McKay JA. DNA methylation and the hygiene hypothesis: connecting respiratory allergy and childhood acute lymphoblastic leukemia. Epigenomics (2019) 11:1519–37. doi: 10.2217/epi-2019-0052
6. D’Amato G, Akdis CA. Global warming, climate change, air pollution and allergies. Allergy (2020) 75:2158–60. doi: 10.1111/all.14527
7. Liu AH. Revisiting the hygiene hypothesis for allergy and asthma. J Allergy Clin Immunol (2015) 136:860–5. doi: 10.1016/j.jaci.2015.08.012
8. Zhu J. T Helper Cell Differentiation, Heterogeneity, and Plasticity. Cold Spring Harb Perspect Biol (2018) 10:a030338. doi: 10.1101/cshperspect.a030338
9. Sakaguchi S, Toda M, Asano M, Itoh M, Morse SS, Sakaguchi N. T cell-mediated maintenance of natural self-tolerance: its breakdown as a possible cause of various autoimmune diseases. J Autoimmun (1996) 9:211–20. doi: 10.1006/jaut.1996.0026
10. Sakaguchi S, Mikami N, Wing JB, Tanaka A, Ichiyama K, Ohkura N. Regulatory T Cells and Human Disease. Annu Rev Immunol (2020) 38:541–66. doi: 10.1146/annurev-immunol-042718-041717
11. Gurram RK, Zhu J. Orchestration between ILC2s and Th2 cells in shaping type 2 immune responses. Cell Mol Immunol (2019) 16:225–35. doi: 10.1038/s41423-019-0210-8
12. Bal SM, Golebski K, Spits H. Plasticity of innate lymphoid cell subsets. Nat Rev Immunol (2020) 20:552–65. doi: 10.1038/s41577-020-0282-9
13. Borger JG, Le Gros G, Kirman JR. Editorial: The Role of Innate Lymphoid Cells in Mucosal Immunity. Front Immunol (2020) 11:1233. doi: 10.3389/fimmu.2020.01233
14. Martinez-Gonzalez I, Steer CA, Takei F. Lung ILC2s link innate and adaptive responses in allergic inflammation. Trends Immunol (2015) 36:189–95. doi: 10.1016/j.it.2015.01.005
15. Messing M, Jan-Abu SC, McNagny K. Group 2 Innate Lymphoid Cells: Central Players in a Recurring Theme of Repair and Regeneration. Int J Mol Sci (2020) 21:1350. doi: 10.3390/ijms21041350
CrossRef Full Text | Google Scholar
16. Ealey KN, Moro K, Koyasu S. Are ILC2s Jekyll and Hyde in airway inflammation? Immunol Rev (2017) 278:207–18. doi: 10.1111/imr.12547
17. Sawa S, Lochner M, Satoh-Takayama N, Dulauroy S, Bérard M, Kleinschek M, et al. RORγt+ innate lymphoid cells regulate intestinal homeostasis by integrating negative signals from the symbiotic microbiota. Nat Immunol (2011) 12:320–6. doi: 10.1038/ni.2002
18. Sepahi A, Liu Q, Friesen L, Kim CH. Dietary fiber metabolites regulate innate lymphoid cell responses. Mucosal Immunol (2020) 4:317–30. doi: 10.1038/s41385-020-0312-8
19. Gonçalves P, Di Santo JP. An Intestinal Inflammasome - The ILC3-Cytokine Tango. Trends Mol Med (2016) 22:269–71. doi: 10.1016/j.molmed.2016.02.008
20. Jendrossek M, Standl M, Koletzko S, Lehmann I, Bauer C-P, Schikowski T, et al. Residential Air Pollution, Road Traffic, Greenness and Maternal Hypertension: Results from GINIplus and LISAplus. Int J Occup Environ Med (2017) 8:131–42. doi: 10.15171/ijoem.2017.1073
21. Burte E, Leynaert B, Marcon A, Bousquet J, Benmerad M, Bono R, et al. Long-term air pollution exposure is associated with increased severity of rhinitis in 2 European cohorts. J Allergy Clin Immunol (2020) 145:834–42.e6. doi: 10.1016/j.jaci.2019.11.040
22. Eguiluz-Gracia I, Mathioudakis AG, Bartel S, Vijverberg SJ, Fuertes E, Comberiati P, et al. The need for clean air: The way air pollution and climate change affect allergic rhinitis and asthma. Allergy (2020) 75:2170–84. doi: 10.1111/all.14177
23. McDade TW. Early environments and the ecology of inflammation. Proc Natl Acad Sci U S A (2012) 109(Suppl 2):17281–8. doi: 10.1073/pnas.1202244109
24. Caillaud D, Leynaert B, Keirsbulck M, Nadif R. Indoor mould exposure, asthma and rhinitis: findings from systematic reviews and recent longitudinal studies. Eur Respir Rev (2018) 27:170137. doi: 10.1183/16000617.0137-2017
25. Kwon J-W, Park H-W, Kim WJ, Kim M-G, Lee S-J. Exposure to volatile organic compounds and airway inflammation. Environ Health (2018) 17:65. doi: 10.1186/s12940-018-0410-1
26. Gallant MJ, Ellis AK. Prenatal and early-life exposure to indoor air-polluting factors and allergic sensitization at 2 years of age. Ann Allergy Asthma Immunol (2020) 124:283–7. doi: 10.1016/j.anai.2019.11.019
27. Li Y-F, Langholz B, Salam MT, Gilliland FD. Maternal and grandmaternal smoking patterns are associated with early childhood asthma. Chest (2005) 127:1232–41. doi: 10.1378/chest.127.4.1232
28. Waddington CH. The strategies of the genes; a dsicussion of some aspects of theoretical biology . London: Allen & Unwin (1957).
Google Scholar
29. Holliday R, Pugh JE. DNA modification mechanisms and gene activity during development. Science (1975) 187:226–32.
PubMed Abstract | Google Scholar
30. Compere SJ, Palmiter RD. DNA methylation controls the inducibility of the mouse metallothionein-I gene lymphoid cells. Cell (1981) 25:233–40. doi: 10.1016/0092-8674(81)90248-8
31. Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T. Identification of novel genes coding for small expressed RNAs. Science (2001) 294:853–8. doi: 10.1126/science.1064921
32. Tang W-y, Levin L, Talaska G, Cheung YY, Herbstman J, Tang D, et al. Maternal Exposure to Polycyclic Aromatic Hydrocarbons and 5’-CpG Methylation of Interferon-γ in Cord White Blood Cells. Environ Health Perspect (2012) 120:1195–200. doi: 10.1289/ehp.1103744
33. Potaczek DP, Miethe S, Schindler V, Alhamdan F, Garn H. Role of airway epithelial cells in the development of different asthma phenotypes. Cell Signal (2020) 69:109523. doi: 10.1016/j.cellsig.2019.109523
34. Hew KM, Walker AI, Kohli A, Garcia M, Syed A, McDonald-Hyman C, et al. Childhood exposure to ambient polycyclic aromatic hydrocarbons is linked to epigenetic modifications and impaired systemic immunity in T cells. Clin Exp Allergy (2015) 45:238–48. doi: 10.1111/cea.12377
35. Renz H, Conrad M, Brand S, Teich R, Garn H, Pfefferle PI. Allergic diseases, gene-environment interactions. Allergy (2011) 66 Suppl 95:10–2. doi: 10.1111/j.1398-9995.2011.02622.x
36. Smits HH, Hiemstra PS, Da Prazeres Costa C, Ege M, Edwards M, Garn H, et al. Microbes and asthma: Opportunities for intervention. J Allergy Clin Immunol (2016) 137:690–7. doi: 10.1016/j.jaci.2016.01.004
37. Potaczek DP, Harb H, Michel S, Alhamwe BA, Renz H, Tost J. Epigenetics and allergy: from basic mechanisms to clinical applications. Epigenomics (2017) 9:539–71. doi: 10.2217/epi-2016-0162
38. Schaub B, Liu J, Höppler S, Schleich I, Huehn J, Olek S, et al. Maternal farm exposure modulates neonatal immune mechanisms through regulatory T cells. J Allergy Clin Immunol (2009) 123:774–82.e5. doi: 10.1016/j.jaci.2009.01.056
39. Lluis A, Depner M, Gaugler B, Saas P, Casaca VI, Raedler D, et al. Increased regulatory T-cell numbers are associated with farm milk exposure and lower atopic sensitization and asthma in childhood. J Allergy Clin Immunol (2014) 133:551–9. doi: 10.1016/j.jaci.2013.06.034
40. Abbring S, Ryan JT, Diks MA, Hols G, Garssen J, van Esch BC. Suppression of Food Allergic Symptoms by Raw Cow’s Milk in Mice is Retained after Skimming but Abolished after Heating the Milk-A Promising Contribution of Alkaline Phosphatase. Nutrients (2019) 11:1499. doi: 10.3390/nu11071499
41. Abbring S, Wolf J, Ayechu-Muruzabal V, Diks MAP, Alashkar Alhamwe B, Alhamdan F, et al. Raw Cow’s Milk Reduces Allergic Symptoms in a Murine Model for Food Allergy—A Potential Role for Epigenetic Modifications. Nutrients (2019) 11:1721. doi: 10.3390/nu11081721
42. Kirchner B, Pfaffl MW, Dumpler J, von Mutius E, Ege MJ. microRNA in native and processed cow’s milk and its implication for the farm milk effect on asthma. J Allergy Clin Immunol (2016) 137:1893–95.e13. doi: 10.1016/j.jaci.2015.10.028
43. Debarry J, Garn H, Hanuszkiewicz A, Dickgreber N, Blümer N, von Mutius E, et al. Acinetobacter lwoffii and Lactococcus lactis strains isolated from farm cowsheds possess strong allergy-protective properties. J Allergy Clin Immunol (2007) 119:1514–21. doi: 10.1016/j.jaci.2007.03.023
44. Brand S, Teich R, Dicke T, Harb H, Yildirim AÖ, Tost J, et al. Epigenetic regulation in murine offspring as a novel mechanism for transmaternal asthma protection induced by microbes. J Allergy Clin Immunol (2011) 128:618–25.e1-7. doi: 10.1016/j.jaci.2011.04.035
45. Conrad ML, Ferstl R, Teich R, Brand S, Blümer N, Yildirim AÖ, et al. Maternal TLR signaling is required for prenatal asthma protection by the nonpathogenic microbe Acinetobacter lwoffii F78. J Exp Med (2009) 206:2869–77. doi: 10.1084/jem.20090845
46. Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med (2012) 18:716–25. doi: 10.1038/nm.2678
47. Ray A, Oriss TB, Wenzel SE. Emerging molecular phenotypes of asthma. Am J Physiol Lung Cell Mol Physiol (2015) 308:L130–40. doi: 10.1152/ajplung.00070.2014
48. Szefler SJ, Wenzel S, Brown R, Erzurum SC, Fahy JV, Hamilton RG, et al. Asthma outcomes: biomarkers. J Allergy Clin Immunol (2012) 129:S9–23. doi: 10.1016/j.jaci.2011.12.979
49. Oksel C, Haider S, Fontanella S, Frainay C, Custovic A. Classification of Pediatric Asthma: From Phenotype Discovery to Clinical Practice. Front Pediatr (2018) 6:258. doi: 10.3389/fped.2018.00258
50. Saglani S, Custovic A. Childhood Asthma: Advances Using Machine Learning and Mechanistic Studies. Am J Respir Crit Care Med (2019) 199:414–22. doi: 10.1164/rccm.201810-1956CI
51. Siroux V, Basagaña X, Boudier A, Pin I, Garcia-Aymerich J, Vesin A, et al. Identifying adult asthma phenotypes using a clustering approach. Eur Respir J (2011) 38:310–7. doi: 10.1183/09031936.00120810
52. Depner M, Fuchs O, Genuneit J, Karvonen AM, Hyvärinen A, Kaulek V, et al. Clinical and epidemiologic phenotypes of childhood asthma. Am J Respir Crit Care Med (2014) 189:129–38. doi: 10.1164/rccm.201307-1198OC
53. Ajdacic-Gross V, Mutsch M, Rodgers S, Tesic A, Müller M, Seifritz E, et al. A step beyond the hygiene hypothesis-immune-mediated classes determined in a population-based study. BMC Med (2019) 17:75. doi: 10.1186/s12916-019-1311-z
54. Debeuf N, Haspeslagh E, van Helden M, Hammad H, Lambrecht BN. Mouse Models of Asthma. Curr Protoc Mouse Biol (2016) 6:169–84. doi: 10.1002/cpmo.4
55. Tan H-TT, Hagner S, Ruchti F, Radzikowska U, Tan G, Altunbulakli C, et al. Tight junction, mucin, and inflammasome-related molecules are differentially expressed in eosinophilic, mixed, and neutrophilic experimental asthma in mice. Allergy (2019) 74:294–307. doi: 10.1111/all.13619
56. Hagner S, Keller M, Raifer H, Tan H-TT, Akdis CA, Buch T, et al. T cell requirement and phenotype stability of house dust mite-induced neutrophil airway inflammation in mice. Allergy (2020) 75:2970–3. doi: 10.1111/all.14424
57. Marqués-García F, Marcos-Vadillo E. Review of Mouse Models Applied to the Study of Asthma. Methods Mol Biol (2016) 1434:213–22. doi: 10.1007/978-1-4939-3652-6_15
58. Maltby S, Tay HL, Yang M, Foster PS. Mouse models of severe asthma: Understanding the mechanisms of steroid resistance, tissue remodelling and disease exacerbation. Respirology (2017) 22:874–85. doi: 10.1111/resp.13052
59. McCann JR, Mason SN, Auten RL, St Geme JW, Seed PC. Early-Life Intranasal Colonization with Nontypeable Haemophilus influenzae Exacerbates Juvenile Airway Disease in Mice. Infect Immun (2016) 84:2022–30. doi: 10.1128/IAI.01539-15
60. Malinczak C-A, Fonseca W, Rasky AJ, Ptaschinski C, Morris S, Ziegler SF, et al. Sex-associated TSLP-induced immune alterations following early-life RSV infection leads to enhanced allergic disease. Mucosal Immunol (2019) 12:969–79. doi: 10.1038/s41385-019-0171-3
61. Wickens K, Barthow C, Mitchell EA, Kang J, van Zyl N, Purdie G, et al. Effects of Lactobacillus rhamnosus HN001 in early life on the cumulative prevalence of allergic disease to 11 years. Pediatr Allergy Immunol (2018) 29:808–14. doi: 10.1111/pai.12982
62. Bach J-F. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med (2002) 347:911–20. doi: 10.1056/NEJMra020100
63. Bach J-F. The hygiene hypothesis in autoimmunity: the role of pathogens and commensals. Nat Rev Immunol (2018) 18:105–20. doi: 10.1038/nri.2017.111
64. Bauer ME, Teixeira AL. Inflammation in psychiatric disorders: what comes first? Ann N Y Acad Sci (2019) 1437:57–67. doi: 10.1111/nyas.13712
65. Petra AI, Panagiotidou S, Hatziagelaki E, Stewart JM, Conti P, Theoharides TC. Gut-Microbiota-Brain Axis and Its Effect on Neuropsychiatric Disorders With Suspected Immune Dysregulation. Clin Ther (2015) 37:984–95. doi: 10.1016/j.clinthera.2015.04.002
66. Langgartner D, Lowry CA, Reber SO. Old Friends, immunoregulation, and stress resilience. Pflugers Arch (2019) 471:237–69. doi: 10.1007/s00424-018-2228-7
67. Willyard C. How gut microbes could drive brain disorders. Nature (2021) 590:22–5. doi: 10.1038/d41586-021-00260-3
68. Cenit MC, Sanz Y, Codoñer-Franch P. Influence of gut microbiota on neuropsychiatric disorders. World J Gastroenterol (2017) 23:5486–98. doi: 10.3748/wjg.v23.i30.5486
69. Untersmayr E, Bax HJ, Bergmann C, Bianchini R, Cozen W, Gould HJ, et al. AllergoOncology: Microbiota in allergy and cancer-A European Academy for Allergy and Clinical Immunology position paper. Allergy (2019) 74:1037–51. doi: 10.1111/all.13718
70. Oikonomopoulou K, Brinc D, Kyriacou K, Diamandis EP. Infection and cancer: revaluation of the hygiene hypothesis. Clin Cancer Res (2013) 19:2834–41. doi: 10.1158/1078-0432.CCR-12-3661
71. Greaves M. Infection, immune responses and the aetiology of childhood leukaemia. Nat Rev Cancer (2006) 6:193–203. doi: 10.1038/nrc1816
72. Greaves M. A causal mechanism for childhood acute lymphoblastic leukaemia. Nat Rev Cancer (2018) 18:471–84. doi: 10.1038/s41568-018-0015-6
73. Cardwell CR, McKinney PA, Patterson CC, Murray LJ. Infections in early life and childhood leukaemia risk: a UK case-control study of general practitioner records. Br J Cancer (2008) 99:1529–33. doi: 10.1038/sj.bjc.6604696
74. Simpson J, Smith A, Ansell P, Roman E. Childhood leukaemia and infectious exposure: a report from the United Kingdom Childhood Cancer Study (UKCCS). Eur J Cancer (2007) 43:2396–403. doi: 10.1016/j.ejca.2007.07.027
75. Orsi L, Magnani C, Petridou ET, Dockerty JD, Metayer C, Milne E, et al. Living on a farm, contact with farm animals and pets, and childhood acute lymphoblastic leukemia: pooled and meta-analyses from the Childhood Leukemia International Consortium. Cancer Med (2018) 7:2665–81. doi: 10.1002/cam4.1466
Keywords: hygiene hypothesis, allergy, asthma, non-communicable inflammatory diseases, chronic inflammation
Citation: Garn H, Potaczek DP and Pfefferle PI (2021) The Hygiene Hypothesis and New Perspectives—Current Challenges Meeting an Old Postulate. Front. Immunol. 12:637087. doi: 10.3389/fimmu.2021.637087
Received: 02 December 2020; Accepted: 04 March 2021; Published: 18 March 2021.
Reviewed by:
Copyright © 2021 Garn, Potaczek and Pfefferle. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Holger Garn, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
The Hygiene Hypothesis - Learning From but Not Living in the Past
Affiliations.
- 1 Comprehensive Biobank Marburg, Medical Faculty, Philipps University of Marburg, Comprehensive Biobank Marburg, Marburg, Germany.
- 2 German Center for Lung Research (DZL), Marburg, Germany.
- 3 German Biobank Alliance, Marburg, Germany.
- 4 Institute for Pathology, Medical Faculty, Institute for Pathology, Philipps University of Marburg, Marburg, Germany.
- 5 Translational Inflammation Research Division & Core Facility for Single Cell Multiomics, Medical Faculty, Biochemical Pharmacological Center, Philipps University of Marburg, Marburg, Germany.
- PMID: 33796103
- PMCID: PMC8007786
- DOI: 10.3389/fimmu.2021.635935
Postulated by Strachan more than 30 years ago, the Hygiene Hypothesis has undergone many revisions and adaptations. This review journeys back to the beginnings of the Hygiene Hypothesis and describes the most important landmarks in its development considering the many aspects that have refined and generalized the Hygiene Hypothesis over time. From an epidemiological perspective, the Hygiene Hypothesis advanced to a comprehensive concept expanding beyond the initial focus on allergies. The Hygiene Hypothesis comprise immunological, microbiological and evolutionary aspects. Thus, the original postulate developed into a holistic model that explains the impact of post-modern life-style on humans, who initially evolved in close proximity to a more natural environment. Focusing on diet and the microbiome as the most prominent exogenous influences we describe these discrepancies and the resulting health outcomes and point to potential solutions to reestablish the immunological homeostasis that frequently have been lost in people living in developed societies.
Keywords: T cell-response; allergy; asthma; hygiene hypothesis; immune tolerance; microbiome.
Copyright © 2021 Pfefferle, Keber, Cohen and Garn.
Publication types
- Historical Article
- Research Support, Non-U.S. Gov't
- Adaptive Immunity*
- Asthma / immunology
- Asthma / microbiology
- Bacteria / immunology*
- Bacteria / pathogenicity
- Diet / adverse effects
- Evolution, Molecular
- Gastrointestinal Microbiome / immunology*
- History, 20th Century
- History, 21st Century
- Host-Pathogen Interactions
- Hygiene Hypothesis* / history
- Immune Tolerance
- Immunity, Innate*
- T-Lymphocytes / immunology*
- T-Lymphocytes / metabolism
- T-Lymphocytes / microbiology
Is Being Too Clean Bad for Your Health?

From taking a shower to brushing your teeth to washing your hands, you practice good personal hygiene on the daily. And it’s not just because you like the way your new shampoo smells, either. You know these habits keep you clean and, in some cases, can even help prevent you from getting sick.
But after all that lathering, rinsing and scrubbing, can you actually be too clean for your own good?
That’s what supporters of the so-called hygiene hypothesis think, saying that the rising rates of allergies, asthma and other autoimmune disorders in children is linked to our increasingly hygienic surroundings. And while statistics appear to back this up, experts in the fields of immunology and infectious disease say, not so fast.
“To say that being clean means you’re at a higher risk of allergies or asthma is not quite right,” agrees Dr. John Lynch , an associate microbiology professor at the University of Washington School of Medicine and medical director of Harborview Medical Center’s Infection Control, Antibiotic Stewardship and Employee Health programs.
The problem, he says, is that the hygiene hypothesis doesn’t tell the full story.
What is the hygiene hypothesis?
Largely popularized by British epidemiologist David Strachan in 1989, the hygiene hypothesis theorizes that because modern parents are able to clean their children and households more effectively, kids these days just aren’t exposed to the same level of germs as previous generations.
That excessively sterile upbringing — hand sanitizer, anyone? — means children’s immune systems aren’t able to develop properly and, as a result, malfunction.
When you look at the statistics, they seem to support this idea. In developed countries, the number of kids who have asthma and allergies has been going up.
Washington state has some of the highest incidences of asthma in the nation, and it’s only increasing. More than 600,000 Washingtonians have asthma, and nearly 120,000 of them are children.
Research from the Centers for Disease Control and Prevention shows that the same trend applies to kids who have food allergies. Now 1 in 13 children in the United States has a food allergy, a 50% increase between 1997 and 2011. To put it in perspective, that means in every American classroom, there are two kids who may have a food-related allergic reaction.
Is the hygiene hypothesis true?
Don’t toss out your hand soap or quit bathing your kids just yet. Remember, while data appears to back up the hygiene hypothesis, it’s not a complete picture.
“There was no randomized control study to determine the hygiene hypothesis,” Lynch explains. “It ends up being observations of populations, biased by our ability to detect diseases. You have less likelihood of being diagnosed with asthma or even diabetes in a developing country versus a developed country.”
What Lynch means is that as the field of medicine has advanced in recent decades, so has our ability to detect and diagnose conditions like the aforementioned food allergies and asthma. And the reason why much of the evidence to support the hygiene hypothesis comes from industrialized countries is because these nations have greater medical infrastructure and resources to detect autoimmune dysfunctions than the developing world.
So while the number of children with asthma and food allergies is higher than in decades past, there’s no way to know if that’s because more kids actually have those conditions or if it’s because doctors are more able to recognize and diagnose those conditions.
Another problem with the hygiene hypothesis, Lynch notes, is that while getting exposed to some germs does help build up your immune systems, other types of bacteria and viruses can actually cause asthma or serious diseases.
That’s why researchers and medical professionals in Lynch’s field of infectious disease and immunology cringe at the name “hygiene hypothesis,” he says. It implies that good personal hygiene is related to higher rates of disease when, in fact, it’s the opposite.
Think about it this way: If the hygiene hypothesis is really accurate and being overly clean makes our immune systems malfunction, children who don’t wash their hands, are exposed to pathogens on a regular basis and live in unclean conditions would be the healthiest.
“Unfortunately, we know that people who live in places that lack access to hygiene die more frequently,” Lynch says.
How do you build up a child’s immune system?
It’s not that the hygiene hypothesis gets it all wrong. Children do need to be exposed to certain microorganisms in order to influence the right immune response and develop a robust immune system. And having a too-clean environment can hinder that in some ways.
“We don’t need to sterilize things with antibacterial products or create an incredibly hygienic environment,” Lynch says. “You don’t want to put any extra chemicals or agents in anything because that’s how you create antibiotic-resistant bacteria.”
In fact, your body is full of trillions of bacteria, fungi and viruses — an entire community called your microbiota — which are critical to your immune response and overall health.
Now here’s where glimmers of the hygiene hypothesis come in: Children develop a healthy microbiota by acquiring bacteria in a variety of ways, from vaginal birth and breastfeeding to getting kissed by their parents and sticking their fingers in their mouths as babies.
“That’s all normal,” Lynch says. “We don’t want women washing their breasts before breastfeeding or parents washing their lips before kissing their children.”
What also matters for immune development, though, is what you’re exposed to and how that affects your body. Getting a common cold virus is a totally normal part of childhood. But being exposed to an antibiotic-resistant superbug is a much more serious issue.
“When you’re talking about the hygiene hypothesis, the point of contention is that the focus should be less about hygiene and more about access to the right microbiota,” Lynch explains.
What’s the takeaway from the hygiene hypothesis controversy?
So while the hygiene hypothesis isn’t totally correct, going in the opposite direction to an overly sterilized childhood isn’t exactly healthy either. It can feel like the balance between exposing children to good bacteria and keeping them safe from the bad stuff is pretty much out of your control.
Just try to keep everything in perspective, Lynch says. Use common sense — and maybe go easy on the hand sanitizer.
“I like to think of it like this: Hand washing is important if you’re around someone who’s sick or if your kid is rolling around on the floor at a restaurant, but maybe not so much if they’re just playing outside at the park,” he says.
Recommended for you
Plus, what to do, where to go and who to call if you have an exposure.

- Load more stories
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 16 October 2017
The hygiene hypothesis in autoimmunity: the role of pathogens and commensals
- Jean-François Bach 1 , 2 , 3
Nature Reviews Immunology volume 18 , pages 105–120 ( 2018 ) Cite this article
19k Accesses
298 Citations
264 Altmetric
Metrics details
- Autoimmune diseases
- Toll-like receptors
The initial application of the hygiene hypothesis for autoimmune diseases proposed in the early 2000s has been confirmed and consolidated by a wealth of published data in both animal models and human autoimmune conditions.
The hygiene hypothesis probably explains the uneven geographical distribution of autoimmune diseases in the world. Individuals migrating from countries with low incidence of autoimmune diseases to countries with high incidence develop the disease with the frequency of the host country, provided that migration occurred at a young age and under a threshold that varies according to the disease.
Pathogenic bacteria, viruses and parasites are often endowed with strong protective effects on autoimmunity even when infection occurs late after birth.
Gut commensal bacteria may also have a protective role in autoimmunity when administered early in life.
Pathogens, parasites and commensals essentially act by stimulating immune regulatory pathways, implicating the innate and the adaptive immune system. Importantly, the effect is seen with both living organisms and their derivatives or purified extracts.
Both pathogens and commensals stimulate pattern recognition receptors, including Toll-like receptors (TLRs) to protect against autoimmunity. This effect may be mimicked by TLR agonists acting through pharmacological stimulation or desensitization of the target receptor.
The incidence of autoimmune diseases has been steadily rising. Concomitantly, the incidence of most infectious diseases has declined. This observation gave rise to the hygiene hypothesis, which postulates that a reduction in the frequency of infections contributes directly to the increase in the frequency of autoimmune and allergic diseases. This hypothesis is supported by robust epidemiological data, but the underlying mechanisms are unclear. Pathogens are known to be important, as autoimmune disease is prevented in various experimental models by infection with different bacteria, viruses and parasites. Gut commensal bacteria also play an important role: dysbiosis of the gut flora is observed in patients with autoimmune diseases, although the causal relationship with the occurrence of autoimmune diseases has not been established. Both pathogens and commensals act by stimulating immunoregulatory pathways. Here, I discuss the importance of innate immune receptors, in particular Toll-like receptors, in mediating the protective effect of pathogens and commensals on autoimmunity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others

Interaction between microbiota and immunity in health and disease
Danping Zheng, Timur Liwinski & Eran Elinav
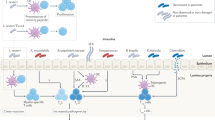
The impact of the gut microbiome on extra-intestinal autoimmune diseases
Eiji Miyauchi, Chikako Shimokawa, … Hiroshi Ohno
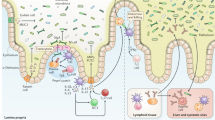
Host–microbiota interactions in inflammatory bowel disease
Roberta Caruso, Bernard C. Lo & Gabriel Núñez
Strachan, D. P. Hay fever, hygiene, and household size. BMJ 299 , 1259–1260 (1989). This is a visionary epidemiological study that paved the way for the hygiene hypothesis in atopic diseases.
CAS PubMed PubMed Central Google Scholar
Strachan, D. P. Family size, infection and atopy: the first decade of the “hygiene hypothesis”. Thorax 55 (Suppl. 1), S2–S10 (2000).
PubMed PubMed Central Google Scholar
Ege, M. J. et al. Exposure to environmental microorganisms and childhood asthma. N. Engl. J. Med. 364 , 701–709 (2011).
CAS PubMed Google Scholar
Greenwood, B. M., Herrick, E. M. & Voller, A. Suppression of autoimmune disease in NZB and (NZB x NZW) F1 hybrid mice by infection with malaria. Nature 226 , 266–267 (1970).
Greenwood, B. M., Herrick, E. M. & Voller, A. Can parasitic infection suppress autoimmune disease? Proc. R. Soc. Med. 63 , 19–20 (1970).
Rook, G. A. & Stanford, J. L. Give us this day our daily germs. Immunol. Today 19 , 113–116 (1998).
Sewell, D. L., Reinke, E. K., Hogan, L. H., Sandor, M. & Fabry, Z. Immunoregulation of CNS autoimmunity by helminth and mycobacterial infections. Immunol. Lett. 82 , 101–110 (2002).
Oldstone, M. B. Prevention of type I diabetes in nonobese diabetic mice by virus infection. Science 239 , 500–502 (1988). This seminal study demonstrates the protective effect of a viral infection on the development of spontaneous autoimmune IDDM in NOD mice.
Oldstone, M. B., Ahmed, R. & Salvato, M. Viruses as therapeutic agents. II. Viral reassortants map prevention of insulin-dependent diabetes mellitus to the small RNA of lymphocytic choriomeningitis virus. J. Exp. Med. 171 , 2091–2100 (1990).
Bach, J. F. The effect of infections on susceptibility to autoimmune and allergic diseases. N. Engl. J. Med. 347 , 911–920 (2002).
PubMed Google Scholar
Bach, J. F. Protective role of infections and vaccinations on autoimmune diseases. J. Autoimmun. 16 , 347–353 (2001).
Deckers, I. A. et al. Investigating international time trends in the incidence and prevalence of atopic eczema 1990-2010: a systematic review of epidemiological studies. PLoS ONE 7 , e39803 (2012).
Kotz, D., Simpson, C. R. & Sheikh, A. Incidence, prevalence, and trends of general practitioner-recorded diagnosis of peanut allergy in England, 2001 to 2005. J. Allergy Clin. Immunol. 127 , 623–630.e1 (2011).
Patterson, C. C. et al. Trends in childhood type 1 diabetes incidence in Europe during 1989-2008: evidence of non-uniformity over time in rates of increase. Diabetologia 55 , 2142–2147 (2012).
Karvonen, M., Pitkaniemi, J. & Tuomilehto, J. The onset age of type 1 diabetes in Finnish children has become younger. The Finnish Childhood Diabetes Registry Group. Diabetes Care 22 , 1066–1070 (1999).
Koch-Henriksen, N. & Sorensen, P. S. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol. 9 , 520–532 (2010).
Mackenzie, I. S., Morant, S. V., Bloomfield, G. A., MacDonald, T. M. & O'Riordan, J. Incidence and prevalence of multiple sclerosis in the UK 1990-2010: a descriptive study in the General Practice Research Database. J. Neurol. Neurosurg. Psychiatry 85 , 76–84 (2014).
Grytten, N., Torkildsen, O. & Myhr, K. M. Time trends in the incidence and prevalence of multiple sclerosis in Norway during eight decades. Acta Neurol. Scand. 132 , 29–36 (2015).
Houzen, H. et al. Increased prevalence, incidence, and female predominance of multiple sclerosis in northern Japan. J. Neurol. Sci. 323 , 117–122 (2012).
Li, X. H. et al. A nine-year prospective study on the incidence of childhood type 1 diabetes mellitus in China. Biomed. Environ. Sci. 13 , 263–270 (2000).
Handel, A. E., Handunnetthi, L., Ebers, G. C. & Ramagopalan, S. V. Type 1 diabetes mellitus and multiple sclerosis: common etiological features. Nat. Rev. Endocrinol. 5 , 655–664 (2009).
Stewart, A. W., Mitchell, E. A., Pearce, N., Strachan, D. P. & Weiland, S. K. The relationship of per capita gross national product to the prevalence of symptoms of asthma and other atopic diseases in children (ISAAC). Int. J. Epidemiol. 30 , 173–179 (2001).
Patterson, C. C., Carson, D. J. & Hadden, D. R. Epidemiology of childhood IDDM in Northern Ireland 1989-1994: low incidence in areas with highest population density and most household crowding. Diabetologia 39 , 1063–1069 (1996).
Paalanen, L., Prattala, R., Palosuo, H., Helakorpi, S. & Laatikainen, T. Socio-economic differences in the use of dairy fat in Russian and Finnish Karelia, 1994–2004. Int. J. Publ. Health 55 , 325–337 (2010).
Google Scholar
Kondrashova, A. et al. A six-fold gradient in the incidence of type 1 diabetes at the eastern border of Finland. Ann. Med. 37 , 67–72 (2005).
Laatikainen, T. et al. Allergy gap between Finnish and Russian Karelia on increase. Allergy 66 , 886–892 (2011).
Kondrashova, A. et al. Signs of beta-cell autoimmunity in nondiabetic schoolchildren: a comparison between Russian Karelia with a low incidence of type 1 diabetes and Finland with a high incidence rate. Diabetes Care 30 , 95–100 (2007).
Kuehni, C. E., Strippoli, M. P., Low, N. & Silverman, M. Asthma in young south Asian women living in the United Kingdom: the importance of early life. Clin. Exp. Allergy 37 , 47–53 (2007).
Bodansky, H. J., Staines, A., Stephenson, C., Haigh, D. & Cartwright, R. Evidence for an environmental effect in the aetiology of insulin dependent diabetes in a transmigratory population. BMJ 304 , 1020–1022 (1992).
Feltbower, R. G. et al. Trends in the incidence of childhood diabetes in south Asians and other children in Bradford. UK. Diabet. Med. 19 , 162–166 (2002).
Dean, G. & Elian, M. Age at immigration to England of Asian and Caribbean immigrants and the risk of developing multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 63 , 565–568 (1997).
Gale, C. R. & Martyn, C. N. Migrant studies in multiple sclerosis. Prog. Neurobiol. 47 , 425–448 (1995).
Kostic, A. D. et al. The dynamics of the human infant gut microbiome in development and in progression toward type 1 diabetes. Cell Host Microbe 17 , 260–273 (2015). This is a remarkable study reporting the detailed follow-up of the gut microbiota composition in children at risk of developing IDDM from birth to the onset of hyperglycaemia.
Ball, T. M. et al. Siblings, day-care attendance, and the risk of asthma and wheezing during childhood. N. Engl. J. Med. 343 , 538–543 (2000).
Cardwell, C. R. et al. Birth order and childhood type 1 diabetes risk: a pooled analysis of 31 observational studies. Int. J. Epidemiol. 40 , 363–374 (2011).
Almeida, M. C. et al. The effect of antihelminthic treatment on subjects with asthma from an endemic area of schistosomiasis: a randomized, double-blinded, and placebo-controlled trial. J. Parasitol. Res. 2012 , 296856 (2012).
Fleming, J. O. et al. Probiotic helminth administration in relapsing-remitting multiple sclerosis: a phase 1 study. Mult. Scler. 17 , 743–754 (2011).
Larson, J. D. et al. Murine gammaherpesvirus 68 infection protects lupus-prone mice from the development of autoimmunity. Proc. Natl Acad. Sci. USA 109 , E1092–E1100 (2012).
Alyanakian, M. A. et al. Transforming growth factor-beta and natural killer T-cells are involved in the protective effect of a bacterial extract on type 1 diabetes. Diabetes 55 , 179–185 (2006).
Finlay, C. M., Walsh, K. P. & Mills, K. H. Induction of regulatory cells by helminth parasites: exploitation for the treatment of inflammatory diseases. Immunol. Rev. 259 , 206–230 (2014).
Gause, W. C. & Maizels, R. M. Macrobiota -— helminths as active participants and partners of the microbiota in host intestinal homeostasis. Curr. Opin. Microbiol. 32 , 14–18 (2016). This study reports that the helminth H. polygyrus produces a TGFβ mimic that fully reproduces the effect of this immunoregulatory cytokine on the host immune system.
Arpaia, N. et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504 , 451–455 (2013).
De Filippo, C. et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl Acad. Sci. USA 107 , 14691–14696 (2010).
Lin, A. et al. Distinct distal gut microbiome diversity and composition in healthy children from Bangladesh and the United States. PLoS ONE 8 , e53838 (2013).
David, L. A. et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505 , 559–563 (2014).
Schmidt, B. et al. Establishment of normal gut microbiota is compromised under excessive hygiene conditions. PLoS ONE 6 , e28284 (2011). This study provides direct confirmation of the role of hygiene on gut microbiota composition in an original experimental model using piglets reared in conventional or clean conditions.
Vatanen, T. et al. Variation in microbiome LPS immunogenicity contributes to autoimmunity in humans. Cell 165 , 842–853 (2016). This is a comparative study of the gut microbiota composition in individuals from Finland and Karelia, two neighbouring countries with a substantial difference in the incidence of IDDM. The study demonstrates structural differences in LPS produced by 'non-protective' versus 'protective' commensals in the Finnish and the Karelian microbiota, respectively.
Alam, C. et al. Effects of a germ-free environment on gut immune regulation and diabetes progression in non-obese diabetic (NOD) mice. Diabetologia 54 , 1398–1406 (2011).
Candon, S. et al. Antibiotics in early life alter the gut microbiome and increase disease incidence in a spontaneous mouse model of autoimmune insulin-dependent diabetes. PLoS ONE 10 , e0125448 (2015).
Yurkovetskiy, L. et al. Gender bias in autoimmunity is influenced by microbiota. Immunity 39 , 400–412 (2013).
Brown, K. et al. Prolonged antibiotic treatment induces a diabetogenic intestinal microbiome that accelerates diabetes in NOD mice. ISME J. 10 , 321–332 (2016).
Berer, K. et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 479 , 538–541 (2011).
Lee, Y. K., Menezes, J. S., Umesaki, Y. & Mazmanian, S. K. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc. Natl Acad. Sci. USA 108 (Suppl. 1), 4615–4622 (2011).
Goverman, J. et al. Transgenic mice that express a myelin basic protein-specific T cell receptor develop spontaneous autoimmunity. Cell 72 , 551–560 (1993).
Ivanov, I. I. et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139 , 485–498 (2009).
Gaboriau-Routhiau, V. et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity 31 , 677–689 (2009).
Barclay, W. & Shinohara, M. L. Inflammasome activation in multiple sclerosis and experimental autoimmune encephalomyelitis (EAE). Brain Pathol. 27 , 213–219 (2017).
Dumas, A. et al. The inflammasome pyrin contributes to pertussis toxin-induced IL-1β synthesis, neutrophil intravascular crawling and autoimmune encephalomyelitis. PLoS Pathog. 10 , e1004150 (2014).
Aumeunier, A. et al. Systemic Toll-like receptor stimulation suppresses experimental allergic asthma and autoimmune diabetes in NOD mice. PLoS ONE 5 , e11484 (2010). This is the first systematic comparative study of the protective effect of different TLR agonists on autoimmunity and experimental asthma, which shows that distinct mechanisms underlie the therapeutic activity, depending on the TLR agonist used.
Calcinaro, F. et al. Oral probiotic administration induces interleukin-10 production and prevents spontaneous autoimmune diabetes in the non-obese diabetic mouse. Diabetologia 48 , 1565–1575 (2005).
Falcone, M. et al. Prevention of onset in an insulin-dependent diabetes mellitus model, NOD mice, by oral feeding of Lactobacillus casei . J. Diabetes Res. 105 , 643–649 (1997).
Lavasani, S. et al. A novel probiotic mixture exerts a therapeutic effect on experimental autoimmune encephalomyelitis mediated by IL-10 producing regulatory T cells. PLoS ONE 5 , e9009 (2010).
Markle, J. G. et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 339 , 1084–1088 (2013).
Wen, L. et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature 455 , 1109–1113 (2008).
Peng, J. et al. Long term effect of gut microbiota transfer on diabetes development. J. Autoimmun 53 , 85–94 (2014).
Cardwell, C. R. et al. Caesarean section is associated with an increased risk of childhood-onset type 1 diabetes mellitus: a meta-analysis of observational studies. Diabetologia 51 , 726–735 (2008).
Clausen, T. D. et al. Prelabor cesarean section and risk of childhood type 1 diabetes: a nationwide register-based cohort study. Epidemiology 27 , 547–555 (2016).
Maghzi, A. H. et al. Cesarean delivery may increase the risk of multiple sclerosis. Mult. Scler. 18 , 468–471 (2012).
Knights, D. et al. Use of antibiotics in childhood and risk of Type 1 diabetes: a population-based case-control study. Nat. Microbiol. 34 , 272–277 (2017).
Boursi, B., Mamtani, R., Haynes, K. & Yang, Y. X. The effect of past antibiotic exposure on diabetes risk. Eur. J. Endocrinol. 172 , 639–648 (2015).
Clausen, T. D. et al. Broad-spectrum antibiotic treatment and subsequent childhood type 1 diabetes: a nationwide Danish cohort study. PLoS ONE 11 , e0161654 (2016).
Hviid, A. & Svanstrom, H. Antibiotic use and type 1 diabetes in childhood. Am. J. Epidemiol. 169 , 1079–1084 (2009).
Livanos, A. E. et al. Antibiotic-mediated gut microbiome perturbation accelerates development of type 1 diabetes in mice. Nat. Microbiol. 1 , 16140 (2016).
Alonso, A., Jick, S. S., Jick, H. & Hernan, M. A. Antibiotic use and risk of multiple sclerosis. Am. J. Epidemiol. 163 , 997–1002 (2006).
Ljungberg, M., Korpela, R., Ilonen, J., Ludvigsson, J. & Vaarala, O. Probiotics for the prevention of beta cell autoimmunity in children at genetic risk of type 1 diabetes — the PRODIA study. Ann. NY Acad. Sci. 1079 , 360–364 (2006).
Uusitalo, U. et al. Association of early exposure of probiotics and islet autoimmunity in the TEDDY study. JAMA Pediatr. 170 , 20–28 (2016).
Pelucchi, C. et al. Probiotics supplementation during pregnancy or infancy for the prevention of atopic dermatitis: a meta-analysis. Epidemiology 23 , 402–414 (2012).
Liacopoulos, P. & Ben-Efraim, S. Antigenic competition. Prog. Allergy 18 , 97–204 (1975).
Pross, H. F. & Eidinger, D. Antigenic competition: a review of nonspecific antigen-induced suppression. Adv. Immunol. 18 , 133–168 (1974).
Buus, S., Sette, A., Colon, S. M., Miles, C. & Grey, H. M. The relation between major histocompatibility complex (MHC) restriction and the capacity of Ia to bind immunogenic peptides. Science 235 , 1353–1358 (1987).
Guillet, J. G. et al. Immunological self, nonself discrimination. Science 235 , 865–870 (1987).
Almeida, A. R., Rocha, B., Freitas, A. A. & Tanchot, C. Homeostasis of T cell numbers: from thymus production to peripheral compartmentalization and the indexation of regulatory T cells. Semin. Immunol. 17 , 239–249 (2005).
Surh, C. D. & Sprent, J. Homeostasis of naive and memory T cells. Immunity 29 , 848–862 (2008).
Qin, H. Y., Sadelain, M. W., Hitchon, C., Lauzon, J. & Singh, B. Complete Freund's adjuvant-induced T cells prevent the development and adoptive transfer of diabetes in nonobese diabetic mice. J. Immunol. 150 , 2072–2080 (1993).
Qin, H. Y. & Singh, B. BCG vaccination prevents insulin-dependent diabetes mellitus (IDDM) in NOD mice after disease acceleration with cyclophosphamide. J. Autoimmun. 10 , 271–278 (1997).
Lee, I. F., Qin, H., Trudeau, J., Dutz, J. & Tan, R. Regulation of autoimmune diabetes by complete Freund's adjuvant is mediated by NK cells. J. Immunol. 172 , 937–942 (2004).
Tian, B. et al. Upregulating CD4 + CD25 + FOXP3 + regulatory T cells in pancreatic lymph nodes in diabetic NOD mice by adjuvant immunotherapy. Transplantation 87 , 198–206 (2009).
Serreze, D. V. et al. Th1 to Th2 cytokine shifts in nonobese diabetic mice: sometimes an outcome, rather than the cause, of diabetes resistance elicited by immunostimulation. J. Immunol. 166 , 1352–1359 (2001).
Mori, Y., Kodaka, T., Kato, T., Kanagawa, E. M. & Kanagawa, O. Critical role of IFN-γ in CFA-mediated protection of NOD mice from diabetes development. Int. Immunol. 21 , 1291–1299 (2009).
Salomon, B. et al. B7/CD28 costimulation is essential for the homeostasis of the CD4 + CD25 + immunoregulatory T cells that control autoimmune diabetes. Immunity 12 , 431–440 (2000).
Caramalho, I. et al. Regulatory T cells contribute to diabetes protection in lipopolysaccharide-treated non-obese diabetic mice. Scand. J. Immunol. 74 , 585–595 (2011).
Tian, J. et al. Lipopolysaccharide-activated B cells down-regulate Th1 immunity and prevent autoimmune diabetes in nonobese diabetic mice. J. Immunol. 167 , 1081–1089 (2001).
Fillatreau, S., Sweenie, C. H., McGeachy, M. J., Gray, D. & Anderton, S. M. B cells regulate autoimmunity by provision of IL-10. Nat. Immunol. 3 , 944–950 (2002).
Mauri, C., Gray, D., Mushtaq, N. & Londei, M. Prevention of arthritis by interleukin 10-producing B cells. J. Exp. Med. 197 , 489–501 (2003).
Mizoguchi, A., Mizoguchi, E., Takedatsu, H., Blumberg, R. S. & Bhan, A. K. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity 16 , 219–230 (2002).
Shen, P. & Fillatreau, S. Antibody-independent functions of B cells: a focus on cytokines. Nat. Rev. Immunol. 15 , 441–451 (2015).
Shen, P. et al. IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature 507 , 366–370 (2014).
Shen, P. & Fillatreau, S. Suppressive functions of B cells in infectious diseases. Int. Immunol. 27 , 513–519 (2015).
Filippi, C. M., Estes, E. A., Oldham, J. E. & von Herrath, M. G. Immunoregulatory mechanisms triggered by viral infections protect from type 1 diabetes in mice. J. Clin. Invest. 119 , 1515–1523 (2009).
Cooke, A. et al. Infection with Schistosoma mansoni prevents insulin dependent diabetes mellitus in non-obese diabetic mice. Parasite Immunol. 21 , 169–176 (1999).
Zaccone, P. et al. Schistosoma mansoni antigens modulate the activity of the innate immune response and prevent onset of type 1 diabetes. Eur. J. Immunol. 33 , 1439–1449 (2003).
Grainger, J. R. et al. Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-β pathway. J. Exp. Med. 207 , 2331–2341 (2010).
Ince, M. N. et al. Role of T cell TGF-β signaling in intestinal cytokine responses and helminthic immune modulation. Eur. J. Immunol. 39 , 1870–1878 (2009).
Liu, Q. et al. Helminth infection can reduce insulitis and type 1 diabetes through CD25- and IL-10-independent mechanisms. Infect. Immun. 77 , 5347–5358 (2009).
Walk, S. T., Blum, A. M., Ewing, S. A., Weinstock, J. V. & Young, V. B. Alteration of the murine gut microbiota during infection with the parasitic helminth Heligmosomoides polygyrus . Inflamm. Bowel Dis. 16 , 1841–1849 (2010).
Ramanan, D. et al. Helminth infection promotes colonization resistance via type 2 immunity. Science 352 , 608–612 (2016).
Hubner, M. P., Stocker, J. T. & Mitre, E. Inhibition of type 1 diabetes in filaria-infected non-obese diabetic mice is associated with a T helper type 2 shift and induction of FoxP3 + regulatory T cells. Immunology 127 , 512–522 (2009).
Hubner, M. P. et al. Helminth protection against autoimmune diabetes in nonobese diabetic mice is independent of a type 2 immune shift and requires TGF-β. J. Immunol. 188 , 559–568 (2012).
Lund, M. E. et al. Secreted proteins from the helminth Fasciola hepatica inhibit the initiation of autoreactive T cell responses and prevent diabetes in the NOD mouse. PLoS ONE 9 , e86289 (2014).
Finlay, C. M. et al. Helminth products protect against autoimmunity via innate type 2 cytokines IL-5 and IL-33, which promote eosinophilia. J. Immunol. 196 , 703–714 (2016).
Cording, S., Medvedovic, J., Aychek, T. & Eberl, G. Innate lymphoid cells in defense, immunopathology and immunotherapy. Nat. Immunol. 17 , 755–757 (2016).
Dolpady, J. et al. Oral probiotic VSL#3 prevents autoimmune diabetes by modulating microbiota and promoting indoleamine 2,3-dioxygenase-enriched tolerogenic intestinal environment. J. Diabetes Res. 2016 , 7569431 (2016).
Kobayashi, T. et al. Probiotic upregulation of peripheral IL-17 responses does not exacerbate neurological symptoms in experimental autoimmune encephalomyelitis mouse models. Immunopharmacol. Immunotoxicol. 34 , 423–433 (2012).
Ochoa-Reparaz, J. et al. Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis. J. Immunol. 183 , 6041–6050 (2009).
Ochoa-Reparaz, J. et al. A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal Immunol. 3 , 487–495 (2010). This is an important study describing a constituent of the commensal organism B. fragilis (that is, PSA) with remarkable protective activity in models of EAE and colitis.
Mazmanian, S. K., Round, J. L. & Kasper, D. L. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature 453 , 620–625 (2008).
Round, J. L. & Mazmanian, S. K. Inducible Foxp3 + regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl Acad. Sci. USA 107 , 12204–12209 (2010).
Chinen, T., Volchkov, P. Y., Chervonsky, A. V. & Rudensky, A. Y. A critical role for regulatory T cell-mediated control of inflammation in the absence of commensal microbiota. J. Exp. Med. 207 , 2323–2330 (2010).
Ivanov, I. I. et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe 4 , 337–349 (2008).
Atarashi, K. et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331 , 337–341 (2011).
Nagano, Y., Itoh, K. & Honda, K. The induction of Treg cells by gut-indigenous Clostridium . Curr. Opin. Immunol. 24 , 392–397 (2012).
Gagliani, N. et al. Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nat. Med. 19 , 739–746 (2013).
Apetoh, L. et al. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Brain Pathol. 11 , 854–861 (2010).
CAS Google Scholar
Weiner, H. L., da Cunha, A. P., Quintana, F. & Wu, H. Oral tolerance. Immunol. Rev. 241 , 241–259 (2011).
Pistoia, V. & Raffaghello, L. Mesenchymal stromal cells and autoimmunity. Int. Immunol. 29 , 49–58 (2017).
Uccelli, A., Moretta, L. & Pistoia, V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 8 , 726–736 (2008).
Sica, A. & Massarotti, M. Myeloid suppressor cells in cancer and autoimmunity. J. Autoimmun. http://dx.doi.org/10.1016/j.jaut.2017.07.010 (2017).
Karumuthil-Melethil, S., Perez, N., Li, R. & Vasu, C. Induction of innate immune response through TLR2 and dectin 1 prevents type 1 diabetes. J. Immunol. 181 , 8323–8334 (2008).
Karumuthil-Melethil, S. et al. TLR2- and Dectin 1-associated innate immune response modulates T-cell response to pancreatic β-cell antigen and prevents type 1 diabetes. Diabetes 64 , 1341–1357 (2015).
Serreze, D. V., Hamaguchi, K. & Leiter, E. H. Immunostimulation circumvents diabetes in NOD/Lt mice. J. Autoimmun. 2 , 759–776 (1989).
Quintana, F. J., Rotem, A., Carmi, P. & Cohen, I. R. Vaccination with empty plasmid DNA or CpG oligonucleotide inhibits diabetes in nonobese diabetic mice: modulation of spontaneous 60-kDa heat shock protein autoimmunity. J. Immunol. 165 , 6148–6155 (2000).
Round, J. L. et al. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science 332 , 974–977 (2011).
Wang, Y. et al. An intestinal commensal symbiosis factor controls neuroinflammation via TLR2-mediated CD39 signalling. Nat. Commun. 5 , 4432 (2014).
Filippi, C. M. et al. TLR2 signaling improves immunoregulation to prevent type 1 diabetes. Eur. J. Immunol. 41 , 1399–1409 (2011).
Xiong, Y. et al. Endotoxin tolerance inhibits Lyn and c-Src phosphorylation and association with Toll-like receptor 4 but increases expression and activity of protein phosphatases. J. Innate Immun. 8 , 171–184 (2016).
Freudenberg, M. A. & Galanos, C. Induction of tolerance to lipopolysaccharide (LPS)-D-galactosamine lethality by pretreatment with LPS is mediated by macrophages. Infect. Immun. 56 , 1352–1357 (1988).
Medvedev, A. E., Kopydlowski, K. M. & Vogel, S. N. Inhibition of lipopolysaccharide-induced signal transduction in endotoxin-tolerized mouse macrophages: dysregulation of cytokine, chemokine, and Toll-like receptor 2 and 4 gene expression. J. Immunol. 164 , 5564–5574 (2000). This is a comprehensive work on changes in intracellular signalling in macrophages that lead to LPS (endotoxin) tolerance, which highlights the fundamental role of the activation of various phosphatases.
Kim, D. H. et al. Inhibition of autoimmune diabetes by TLR2 tolerance. J. Immunol. 187 , 5211–5220 (2011).
Anstadt, E. J., Fujiwara, M., Wasko, N., Nichols, F. & Clark, R. B. TLR tolerance as a treatment for central nervous system autoimmunity. J. Immunol. 197 , 2110–2118 (2016). This is an interesting report demonstrating that low doses of two different TLR2 ligands attenuate adoptively transferred EAE through receptor desensitization. One of these TLR2 ligands is a human microbiome product, which has significantly decreased serum levels in patients with multiple sclerosis compared with unaffected controls.
Hayashi, T. et al. Treatment of autoimmune inflammation by a TLR7 ligand regulating the innate immune system. PLoS ONE 7 , e45860 (2012).
Schuijs, M. J. et al. Farm dust and endotoxin protect against allergy through A20 induction in lung epithelial cells. Science 349 , 1106–1110 (2015). This is a study highlighting the potential in vivo relevance of TLR desensitization by LPS in humans. The novel finding is that the LPS-induced TLR4 desensitization targets the lung epithelium and requires the ubiquitin-modifying enzyme A20.
Siebeneicher, S. et al. Epicutaneous immune modulation with Bet v 1 plus R848 suppresses allergic asthma in a murine model. Allergy 69 , 328–337 (2014).
Aryan, Z. & Rezaei, N. Toll-like receptors as targets for allergen immunotherapy. Curr. Opin. Allergy Clin. Immunol. 15 , 568–574 (2015).
Burrows, M. P., Volchkov, P., Kobayashi, K. S. & Chervonsky, A. V. Microbiota regulates type 1 diabetes through Toll-like receptors. Proc. Natl Acad. Sci. USA 112 , 9973–9977 (2015). This is a unique study that uses a genetic approach and proposes a distinct role for single TLRs in their capacity to modulate autoimmunity.
Bras, A. & Aguas, A. P. Diabetes-prone NOD mice are resistant to Mycobacterium avium and the infection prevents autoimmune disease. Immunology 89 , 20–25 (1996).
Lee, J., Reinke, E. K., Zozulya, A. L., Sandor, M. & Fabry, Z. Mycobacterium bovis bacille Calmette-Guerin infection in the CNS suppresses experimental autoimmune encephalomyelitis and Th17 responses in an IFN-γ-independent manner. J. Immunol. 181 , 6201–6212 (2008).
Newland, S. A. et al. PD-L1 blockade overrides Salmonella typhimurium -mediated diabetes prevention in NOD mice: no role for Tregs. Eur. J. Immunol. 41 , 2966–2976 (2011).
Drescher, K. M., Kono, K., Bopegamage, S., Carson, S. D. & Tracy, S. Coxsackievirus B3 infection and type 1 diabetes development in NOD mice: insulitis determines susceptibility of pancreatic islets to virus infection. Virology 329 , 381–394 (2004).
Tracy, S. et al. Toward testing the hypothesis that group B coxsackieviruses (CVB) trigger insulin-dependent diabetes: inoculating nonobese diabetic mice with CVB markedly lowers diabetes incidence. J. Virol. 76 , 12097–12111 (2002).
Davydova, B. et al. Coxsackievirus immunization delays onset of diabetes in non-obese diabetic mice. J. Med. Virol. 69 , 510–520 (2003).
Richer, M. J., Straka, N., Fang, D., Shanina, I. & Horwitz, M. S. Regulatory T-cells protect from type 1 diabetes after induction by coxsackievirus infection in the context of transforming growth factor-β. Diabetes 57 , 1302–1311 (2008).
Hermitte, L. et al. Paradoxical lessening of autoimmune processes in non-obese diabetic mice after infection with the diabetogenic variant of encephalomyocarditis virus. Eur. J. Immunol. 20 , 1297–1303 (1990).
Takei, I. et al. Suppression of development of diabetes in NOD mice by lactate dehydrogenase virus infection. J. Autoimmun. 5 , 665–673 (1992).
Wilberz, S., Partke, H. J., Dagnaes-Hansen, F. & Herberg, L. Persistent MHV (mouse hepatitis virus) infection reduces the incidence of diabetes mellitus in non-obese diabetic mice. Diabetologia 34 , 2–5 (1991).
Smith, K. A., Efstathiou, S. & Cooke, A. Murine gammaherpesvirus-68 infection alters self-antigen presentation and type 1 diabetes onset in NOD mice. J. Immunol. 179 , 7325–7333 (2007).
Mishra, P. K., Patel, N., Wu, W., Bleich, D. & Gause, W. C. Prevention of type 1 diabetes through infection with an intestinal nematode parasite requires IL-10 in the absence of a Th2-type response. Mucosal Immunol. 6 , 297–308 (2013).
Saunders, K. A., Raine, T., Cooke, A. & Lawrence, C. E. Inhibition of autoimmune type 1 diabetes by gastrointestinal helminth infection. Infect. Immun. 75 , 397–407 (2007).
Broz, P. & Dixit, V. M. Inflammasomes: mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 16 , 407–420 (2016).
Inoue, M., Williams, K. L., Gunn, M. D. & Shinohara, M. L. NLRP3 inflammasome induces chemotactic immune cell migration to the CNS in experimental autoimmune encephalomyelitis. Proc. Natl Acad. Sci. USA 109 , 10480–10485 (2012).
Inoue, M. et al. Interferon-β therapy against EAE is effective only when development of the disease depends on the NLRP3 inflammasome. Sci. Signal. 5 , ra38 (2012).
Cuda, C. M., Pope, R. M. & Perlman, H. The inflammatory role of phagocyte apoptotic pathways in rheumatic diseases. Nat. Rev. Rheumatol. 12 , 543–558 (2016).
de Souza, H. S. & Fiocchi, C. Immunopathogenesis of IBD: current state of the art. Nat. Rev. Gastroenterol. Hepatol. 13 , 13–27 (2016).
von Moltke, J., Ayres, J. S., Kofoed, E. M., Chavarria-Smith, J. & Vance, R. E. Recognition of bacteria by inflammasomes. Annu. Rev. Immunol. 31 , 73–106 (2013).
Rzepecka, J. et al. Prophylactic and therapeutic treatment with a synthetic analogue of a parasitic worm product prevents experimental arthritis and inhibits IL-1beta production via NRF2-mediated counter-regulation of the inflammasome. J. Autoimmun. 60 , 59–73 (2015).
Patterson, C. et al. Diabetes in the young — a global view and worldwide estimates of numbers of children with type 1 diabetes. Diabetes Res. Clin. Pract. 103 , 161–175 (2013).
Giongo, A. et al. Toward defining the autoimmune microbiome for type 1 diabetes. ISME J. 5 , 82–91 (2011).
Brown, C. T. et al. Gut microbiome metagenomics analysis suggests a functional model for the development of autoimmunity for type 1 diabetes. PLoS ONE 6 , e25792 (2011).
de Goffau, M. C. et al. Fecal microbiota composition differs between children with beta-cell autoimmunity and those without. Diabetes 62 , 1238–1244 (2013).
Murri, M. et al. Gut microbiota in children with type 1 diabetes differs from that in healthy children: a case-control study. BMC Med. 11 , 46 (2013).
Soyucen, E. et al. Differences in the gut microbiota of healthy children and those with type 1 diabetes. Pediatr. Int. 56 , 336–343 (2014).
Davis-Richardson, A. G. et al. Bacteroides dorei dominates gut microbiome prior to autoimmunity in Finnish children at high risk for type 1 diabetes. Front. Microbiol. 5 , 678 (2014).
Endesfelder, D. et al. Compromised gut microbiota networks in children with anti-islet cell autoimmunity. Diabetes 63 , 2006–2014 (2014).
Mejia-Leon, M. E., Petrosino, J. F., Ajami, N. J., Dominguez-Bello, M. G. & de la Barca, A. M. Fecal microbiota imbalance in Mexican children with type 1 diabetes. Sci. Rep. 4 , 3814 (2014).
Alkanani, A. K. et al. Alterations in intestinal microbiota correlate with susceptibility to type 1 diabetes. Diabetes 64 , 3510–3520 (2015).
Qi, C. J. et al. Imbalance of fecal microbiota at newly diagnosed type 1 diabetes in Chinese children. Chin. Med. J. 129 , 1298–1304 (2016).
Maffeis, C. et al. Association between intestinal permeability and faecal microbiota composition in Italian children with beta cell autoimmunity at risk for type 1 diabetes. Diabetes Metab. Res. Rev. 32 , 700–709 (2016).
Stewart, C. J. et al. Gut microbiota of Type 1 diabetes patients with good glycaemic control and high physical fitness is similar to people without diabetes: an observational study. Diabet Med. 34 , 127–134 (2017).
Miyake, S. et al. Dysbiosis in the gut microbiota of patients with multiple sclerosis, with a striking depletion of species belonging to Clostridia XIVa and IV clusters. PLoS ONE 10 , e0137429 (2015).
Tremlett, H. et al. Gut microbiota in early pediatric multiple sclerosis: a case-control study. Eur. J. Neurol. 23 , 1308–1321 (2016).
Chen, J. et al. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci. Rep. 6 , 28484 (2016).
Hevia, A. et al. Intestinal dysbiosis associated with systemic lupus erythematosus. mBio 5 , e01548–e01514 (2014).
Wilson, C. S., Elizer, S. K., Marshall, A. F., Stocks, B. T. & Moore, D. J. Regulation of B lymphocyte responses to Toll-like receptor ligand binding during diabetes prevention in non-obese diabetic (NOD) mice. J. Diabetes 8 , 120–131 (2016).
Zhang, Y. et al. TLR9 blockade inhibits activation of diabetogenic CD8 + T cells and delays autoimmune diabetes. J. Immunol. 184 , 5645–5653 (2010).
Manicassamy, S. et al. Toll-like receptor 2-dependent induction of vitamin A-metabolizing enzymes in dendritic cells promotes T regulatory responses and inhibits autoimmunity. Nat. Med. 15 , 401–409 (2009).
Manoharan, I. et al. TLR2-dependent activation of beta-catenin pathway in dendritic cells induces regulatory responses and attenuates autoimmune inflammation. J. Immunol. 193 , 4203–4213 (2014).
Li, H. et al. Low dose zymosan ameliorates both chronic and relapsing experimental autoimmune encephalomyelitis. J. Neuroimmunol. 254 , 28–38 (2013).
Dillon, S. et al. Yeast zymosan, a stimulus for TLR2 and dectin-1, induces regulatory antigen-presenting cells and immunological tolerance. J. Clin. Invest. 116 , 916–928 (2006).
Barrat, F. J. & Coffman, R. L. Development of TLR inhibitors for the treatment of autoimmune diseases. Immunol. Rev. 223 , 271–283 (2008).
Barrat, F. J., Meeker, T., Chan, J. H., Guiducci, C. & Coffman, R. L. Treatment of lupus-prone mice with a dual inhibitor of TLR7 and TLR9 leads to reduction of autoantibody production and amelioration of disease symptoms. Eur. J. Immunol. 37 , 3582–3586 (2007).
Wong, F. S. et al. The role of Toll-like receptors 3 and 9 in the development of autoimmune diabetes in NOD mice. Ann. NY Acad. Sci. 1150 , 146–148 (2008).
Gulden, E. et al. Toll-like receptor 4 deficiency accelerates the development of insulin-deficient diabetes in non-obese diabetic mice. PLoS ONE 8 , e75385 (2013).
Prinz, M. et al. Innate immunity mediated by TLR9 modulates pathogenicity in an animal model of multiple sclerosis. J. Clin. Invest. 116 , 456–464 (2006).
Cohen, S. J., Cohen, I. R. & Nussbaum, G. IL-10 mediates resistance to adoptive transfer experimental autoimmune encephalomyelitis in MyD88 −/− mice. J. Immunol. 184 , 212–221 (2010).
Marta, M., Andersson, A., Isaksson, M., Kampe, O. & Lobell, A. Unexpected regulatory roles of TLR4 and TLR9 in experimental autoimmune encephalomyelitis. Eur. J. Immunol. 38 , 565–575 (2008).
Miranda-Hernandez, S. et al. Role for MyD88, TLR2 and TLR9 but not TLR1, TLR4 or TLR6 in experimental autoimmune encephalomyelitis. J. Immunol. 187 , 791–804 (2011). This is a comprehensive study that examines the impact of TLR invalidation in the development of EAE.
Reynolds, J. M. et al. Toll-like receptor 2 signaling in CD4 + T lymphocytes promotes T helper 17 responses and regulates the pathogenesis of autoimmune disease. Immunity 32 , 692–702 (2010).
Christensen, S. R. et al. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity 25 , 417–428 (2006).
Atlas of MS 2013. https://www.msif.org/wp-content/uploads/2014/09/Atlas-of-MS.pdf
Global tuberculosis report 2016. http://apps.who.int/iris/bitstream/10665/250441/1/9789241565394-eng.pdf?ua=1
Screening for hepatitis during the domestic medical examination for newly arrived refugees. https://www.cdc.gov/immigrantrefugeehealth/pdf/domestic-hepatitis-screening-guidelines.pdf
Caisse des Français de l'Étranger. https://www.cfe.fr/pages/votre-sante/guidespatho.php?id=126 [French]
International Monetary Fund. World Economic Outlook Database. https://www.imf.org/external/pubs/ft/weo/2015/01/weodata/index.aspx
Download references
Acknowledgements
The laboratory of the author was supported by an advanced grant from the European Research Council (ERC, Hygiene N°: 250290).
Author information
Authors and affiliations.
Université Paris Descartes, Sorbonne Paris Cité, Paris, France
Jean-François Bach
INSERM U1151, Institut Necker-Enfants Malades, Hôpital Necker-Enfants Malades, Paris, France
CNRS UMR 8253, Institut Necker-Enfants Malades, Hôpital Necker-Enfants Malades, Paris, France
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Jean-François Bach .
Ethics declarations
Competing interests.
The author declares no competing financial interests.
PowerPoint slides
Powerpoint slide for fig. 1, powerpoint slide for fig. 2, powerpoint slide for table 1, powerpoint slide for table 2, powerpoint slide for table 3.
A genetic predisposition to the cumulative development of common allergies, for example, atopic dermatitis and allergic asthma. Atopy involves phenomena of cutaneous or general hypersensitivity to allergens.
A hypothesis that postulates that an increased frequency of infections contributes to a decrease in autoimmune and allergic diseases.
An inbred mouse line that spontaneously develops an autoimmune syndrome including insulin-dependent diabetes mellitus (IDDM or type 1 diabetes).
A digestive tract disorder provoked by eating contaminated food or drinking contaminated water. In the context of our discussion, it is a self-limited pathology that illustrates the presence of a basic health environment.
Autoantibodies to various β-cell-specific autoantigens that are markers of the destruction of insulin-producing β-cells, which is the hallmark of insulin-dependent diabetes mellitus (IDDM or type 1 diabetes).
An imbalance of the microbial flora that most frequently affects the digestive tract. Dysbiosis can also be detected in other 'barrier' organs such as the skin, the lungs or the vagina.
The metabolome consists of all signalling molecules (for example, metabolites and hormones) detected in a biological sample. The metabolome thus defines a given physiological or pathological state and is therefore dynamic.
Mice born by hysterectomy under sterile conditions and raised in isolators to guarantee an environment totally devoid of pathogenic and commensal germs.
(EAE). A demyelinating allergic encephalomyelitis produced by the injection of brain tissue or purified proteins of the nervous system or their derived peptides in the presence of an adjuvant.
Germ-free mice whose intestinal microflora is reconstituted by a single commensal bacterium (monocolonized mice).
Gut commensal bacteria available as single or combined species delivered orally and putatively endowed with a health benefit.
The competition for recognition of the cognate antigen for soluble factors (cytokines) driving the proliferation and differentiation of antigen-specific lymphocytes.
Islet transplants between syngeneic (genetically identical) donor and recipient individuals, which therefore does not give rise to allograft rejection. These grafts performed in diabetic non-obese diabetic mice provide a robust model to test for recurrence of the autoimmune disease.
Rights and permissions
Reprints and permissions

About this article
Cite this article.
Bach, JF. The hygiene hypothesis in autoimmunity: the role of pathogens and commensals. Nat Rev Immunol 18 , 105–120 (2018). https://doi.org/10.1038/nri.2017.111
Download citation
Published : 16 October 2017
Issue Date : February 2018
DOI : https://doi.org/10.1038/nri.2017.111
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Galectin from trichinella spiralis alleviates dss-induced colitis in mice by regulating the intestinal microbiota.
- Jianqing Li
- Xiangjiang Wang
Veterinary Research (2024)
The burden of dermatitis from 1990–2019 in the Middle East and North Africa region
- Saeid Safiri
- Mehran Jaberinezhad
- Ali-Asghar Kolahi
BMC Public Health (2024)
Efficacy and safety of gut microbiota-based therapies in autoimmune and rheumatic diseases: a systematic review and meta-analysis of 80 randomized controlled trials
- Liuting Zeng
- Kailin Yang
- Lingyun Sun
BMC Medicine (2024)
Immunologic derangement caused by intestinal dysbiosis and stress is the intrinsic basis of reactive arthritis
- Weiqing Qian
Zeitschrift für Rheumatologie (2024)
Childhood and adolescence factors and multiple sclerosis: results from the German National Cohort (NAKO)
- Heiko Becher
BMC Neurology (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Newsletters
Site search
- Israel-Hamas war
- 2024 election
- Solar eclipse
- Supreme Court
- All explainers
- Future Perfect
Filed under:
- Science of Everyday Life
The hygiene hypothesis: How being too clean might be making us sick
Share this story.
- Share this on Facebook
- Share this on Twitter
- Share this on Reddit
- Share All sharing options
Share All sharing options for: The hygiene hypothesis: How being too clean might be making us sick
/cdn.vox-cdn.com/uploads/chorus_image/image/45568666/shutterstock_162745193.0.0.jpg)
Over the past few decades, doctors have arrived at a counterintuitive hypothesis about our modern, ultra-sanitized world. Too much cleanliness may be causing us to develop allergies, asthma, inflammatory bowel diseases, and other autoimmune disorders.
The idea is that for many children in the wealthy world, a lack of exposure to bacteria, viruses, and allergens prevents the normal development of the immune system, ultimately increasing the chance of disorders within this system down the road. This is called the hygiene hypothesis .
a lack of exposure to bacteria, viruses, and allergens may prevent the normal development of the immune system
" A child's immune system needs education, just like any other growing organ in the human body," says Erika von Mutius, a pediatric allergist at the University of Munich and one of the first doctors to research the idea. "The hygiene hypothesis suggests that early life exposure to microbes helps in the education of an infant's developing immune system." Without this education, your immune system may be more prone to attacking the wrong target — in the case of autoimmune diseases, yourself.
It's still a matter of active debate among scientists, but e vidence for the idea has been slowly accumulating over time, both in humans and animal subjects. It's been cited as an explanation for why allergy and asthma rates are so much higher in wealthy countries, and m ost recently, a study published last year found that babies who grow up in houses with higher levels of certain bacteria — carried on cockroach, mouse, and cat dander — are less likely to develop wheezing and asthma by the age of three.
(However, it's important to note that despite the claims of some anti-vaccine activists, there's absolutely no evidence that not getting vaccinated has similar benefits.)
How could this kind of filth possibly make us healthier? Here's an explanation of the hygiene hypothesis.
How doctors got the idea that dirt could make us healthy
(REMY GABALDA/AFP/Getty Images)
Obviously, the basic sanitary practices we've developed as a society over the past few centuries — such as building infrastructure to remove garbage and sewage from cities — have provided all sorts of benefits. They're a huge part of the reason so few Americans get infectious diseases like cholera or typhoid nowadays.
asthma, hay fever, and other allergies have become much more common as we've become more sanitary
But researchers have found that a few specific autoimmune diseases — asthma, hay fever , inflammatory bowel diseases, and various allergies — have become much more common as we've become more sanitary, and are much more prevalent in the wealthy world than the developing one.
In the late 1980s, when studying childhood allergies in East and West Germany, British epidemiologist David Strachan began to suspect there was a connection. In the dirtier, more polluted, less wealthy cities of East Germany, he found, children had much lower rates of hay fever and asthma than in the cleaner, richer cities of West Germany.
To explain this, he looked at all sorts of lifestyle differences — and found that West German children were much less likely to spend time in day care centers, around other kids, than East German children. He proposed that their reduced exposure to bacteria and other antigens, normally acquired from other children, somehow affected their immune systems, leading to their increased chance of developing the autoimmune diseases.
The evidence for the hygiene hypothesis
Children who grow up on farms have lower rates of allergies. (John Moore/Getty Images)
In the decades since, all sorts of epidemiological evidence has been collected that supports Strachan's idea. He initially found that in Britain, children who grew up in larger families also had lower chances of developing asthma and hay fever , presumably because they were exposed to more bacteria from their siblings.
Other doctors have found that, on the whole, people in wealthy, more heavily sanitized nations have much higher rates of asthma and allergies than those in the developing world. This could be a function of natural variations among the populations, but more recently, doctors have found that people who move from a developing country to a wealthier one have a higher chance of developing these diseases than people who stay in their country of origin.
kids who grow up on farms or have pets have lower rates of allergies and asthma
Even within a developing country like Ghana, wealthy urban children have higher rates of these autoimmune diseases than poorer or rural children. In the wealthy world, adults who clean their houses with antibacterial sprays have higher asthma rates, and people who are more often exposed to triclosan (the active ingredient in antibacterial soap) have higher rates of allergies and hay fever . Kids who grow up on farms or have pets, meanwhile, have lower rates of allergies and asthma .
These are all correlations — not causations — but they suggest that something about the relatively clean, modern urban environment makes these autoimmune diseases more likely to develop. And the handful of controlled studies conducted on the topic have provided further support — such as one, conducted recently in Uganda , in which babies born to mothers who were given drugs to treat parasitic worm infections during pregnancy ended up having higher rates of eczema and asthma.
Controlled studies with animals have also provided compelling evidence for the idea. "In experimental studies with germ-free mice raised in a sterile environment, researchers have found they're extremely prone to developing colitis and asthma, among many other problems," von Mutius says. But interestingly, if during childhood, these ultra-sanitized mice are inoculated with the stomach bacteria present in normal mice, they no longer have an increased autoimmune disease risk . Somehow, not being exposed to bacteria during childhood seems to increase the risk of autoimmune diseases, for both mice and humans.
How bacteria might prevent disease
A human T cell, shown under a microscope. (NAID)
Increased evidence for the hygiene hypothesis has come as scientists in general have awakened to the importance of "good" bacteria in our bodies in general. The particular species living inside your body — collectively called the microbiome — may be involved in preventing obesity , diabetes , and perhaps even depression .
Scientists have proposed several different mechanisms for how limited exposure to bacteria could lead autoimmune disorders to develop in particular. The most likely one, at the moment, involves specialized cells that are part of your immune system called T cells .
without being exposed to enough bacteria, our immune systems may not be able to learn to properly recognize harmful invaders
As part of the same mouse experiments, scientists found that the bacteria-free mice had exceptionally high numbers of these cells present in their stomachs and lungs. Normally, T cells serve a number of roles in the immune system — among other things, they recognize and eliminate harmful viruses and bacteria — but in some cases, certain types of T cells have previously been found to play a role in the development of colitis and asthma in mice . That seemed to be the case in the disease-stricken, ultra-clean mice as well — because when the scientists dosed them with a chemical that deactivated these T cells, they no longer developed the autoimmune diseases at such high rates.
If the same mechanism exists in humans, it would help explain all these epidemiological findings about autoimmune diseases — and strongly support the hygiene hypothesis.
But why would abnormal T cell behavior occur in the absence of bacteria? One theory, called the "Old Friends" hypothesis , is that our immune systems as a whole evolved in the presence of bacteria, viruses, and small animals that naturally inhabit our bodies .
We still don't fully understand how the immune system develops as we grow up, but the idea is that this exposure is actually necessary for it to develop properly. Without being regularly exposed to bacteria, it can't learn to properly recognize the few harmful invaders that need to be eliminated. As a result, autoimmune diseases — in which the immune system erroneously turns on our own bodies, effectively attacking ourselves — become more common.
But there's still some disagreement among scientists
(Media for Medical/UIG via Getty Images)
At the moment, the hygiene hypothesis is still a hypothesis: a working theory, subject to change.
One major caveat is that no scientists believe it can account for all cases of allergies and asthma. Autoimmune disorders have a clear genetic component, so interactions between a person's environment and genes contribute to rates of autoimmune diseases.
no scientists believe this can account for all cases of allergies and asthma
Additionally, there are some who believe that the theory can explain increases in some sorts of allergies, but not asthma , partly because asthma rates in the wealthy world didn't begin increasing until the 1980s, decades after present-day levels of sanitation were largely established. It's possible that there are varieties of asthma triggered by allergic reactions, and other types that aren't — and are actually exacerbated by exposure to dust and other less sanitary conditions.
Even regarding allergies, there are all sorts of other epidemiological questions that can't be answered by the hygiene hypothesis — such as why, in some European cities, the children of migrants from other countries have lower rates of allergies than other children, even though they basically live in the same conditions. Clearly, we're still in the early stages of understanding the development of the immune system, and don't fully know how bacteria exposure affects it.
Perhaps most importantly, all scientists agree that basic sanitary practices have brought us enormous benefits: they've saved millions of lives by cutting down on all sorts of infectious diseases, and are probably the most important health advances we've made as a species so far.
So the key is using research to figure out the proper balance of sanitation and bacteria exposure, in order to limit the spread of infectious diseases without prompting increases in autoimmune disorders.
So what does this mean for you?
:no_upscale()/cdn.vox-cdn.com/assets/4664559/498868201.jpg)
(Getty Images)
None of this means that you should stop cleaning your house or washing yourself, or begin drinking potentially sewage-contaminated water.
none of this means you should stop cleaning your house or washing yourself
For one, most of these findings involve bacteria exposure during childhood — not for adults. Additionally, most of the reduction in bacteria exposure we have in modern society comes from broader trends (like antibiotic overuse and sewage treatment plants) rather than personal choices.
So, at the moment, the practical applications of this research on a personal level are relatively limited. It might make you think twice before having your kid use antibiotic soap (which you really shouldn't be using anyway ). More importantly, it provides some evidence that vaginal births and breastfeeding are important for the development of a healthy microbiome in infants.
But what's more important is how the hygiene hypothesis will guide doctors' thinking about the growth of autoimmune diseases. In the future, if scientists are able to better understand the mechanisms of the hygiene hypothesis at the cellular level, we might be able to figure out how to balance basic sanitation with bacteria exposure — and the right kind of exposure to prevent allergies, inflammatory bowel diseases, and asthma from developing.
Further reading
- An interview with Kathleen Barnes , a Johns Hopkins researcher who studies the hygiene hypothesis
- The science of seasonal allergies — and why they're so awful
- Michael Pollan's deep look at the importance of the microbiome
Will you support Vox today?
We believe that everyone deserves to understand the world that they live in. That kind of knowledge helps create better citizens, neighbors, friends, parents, and stewards of this planet. Producing deeply researched, explanatory journalism takes resources. You can support this mission by making a financial gift to Vox today. Will you join us?
We accept credit card, Apple Pay, and Google Pay. You can also contribute via
Next Up In Science
Sign up for the newsletter today, explained.
Understand the world with a daily explainer plus the most compelling stories of the day.
Thanks for signing up!
Check your inbox for a welcome email.
Oops. Something went wrong. Please enter a valid email and try again.

Will AI mean the end of liberal democracy?

Israel and Iran’s conflict enters a new, dangerous phase

Trump’s jury doesn’t have to like him to be fair to him

What’s behind the latest right-wing revolt against Mike Johnson

Taylor Swift seems sick of being everyone’s best friend

Are there really more things going wrong on airplanes?
April 7, 2011
Can It Be Bad to Be Too Clean?: The Hygiene Hypothesis
Johns Hopkins School of Medicine researcher Kathleen Barnes talks about the hygiene hypothesis, which raises the possibility that our modern sterile environment may contribute to conditions such as asthma and eczema
By Steve Mirsky

Johns Hopkins School of Medicine researcher Kathleen Barnes talks about the hygiene hypothesis , which raises the possibility that our modern sterile environment may contribute to conditions such as asthma and eczema.
Podcast Transcription
Steve: Welcome to Science Talk , the more of less weekly podcast of Scientific American , posted on April 6th, 2011. I am Steve Mirsky. This week on the podcast:
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing . By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Barnes: The hypothesis is that as we make the shift from dirt to sterile that you're changing the direction of your immune response. This causes diseases.
Steve: That's Kathleen Barnes. We'll hear from her, and we'll test your knowledge of some recent science in the news. Kathleen Barnes studies what's called the hygiene hypothesis at the Johns Hopkins School of Medicine in Baltimore. She presented some of her research at the recent meeting of the American Association for the Advancement of Science in Washington, D.C., after which we sat down to chat.
Steve: First, tell me what is the hygiene hypothesis, for people who haven't heard of it?
Barnes: So, the hygiene hypothesis, I guess, simply stated is this notion that as human society has morphed from developing environment, or what we consider a developing world environment, into the developed world, there have been radical changes in our environment; changes associated with the size of families, so going from many to fewer siblings. The idea being that with fewer children in the house, there's less opportunity for exposure to viruses. The idea that we move from a rural to an urban environment; that we have moved from that situation where we're exposed to microbes—one of the best examples in asthma being the idea that we're exposed to endotoxins that is a byproduct of the livestock and farms—to moving to an environment that's more sterile, where we don't have such exposures. The notion that as we move from a developing to a developed environment, we have less exposure to microbes in general. We treat every symptom with antibiotics; we've changed our gut microflora with the diets that we eat.
Steve: We use antibacterial soap and antibacterial surface cleaners in the house.
Barnes: Exactly, yeah, the idea is sterile is good. Sterile is healthy.
Steve: That's the hygiene; what's the hypothesis part?
Barnes: The hypothesis is that as we make the shift from dirt to sterile that you are changing the direction of your immune response. And so in the context of asthma, and frankly in other autoimmune diseases and diseases of inflammation, it's this imbalance from that side of our immune response that we believe evolved to protect us against things like bacteria and viruses and malarial parasites to the other side of our immune system that, frankly, when it's revved up causes diseases like allergies and some of these other diseases of inflammation. So it's really this imbalance between these two sides of our immune system, both which were designed to do something good for us; but when it's not equal, when it's imbalanced, we're going to have too much of one disease versus another.
Steve: So without the exposure to these environmental challenges, we wind up trading one set of conditions for a different set of conditions or illnesses.
Barnes: Exactly. And the whole, the notion of the epidemiological transition is that we've gone from a situation in our distant past, where we're exposed to lots of microbes—but we also, to be frank, we died from a lot of these diseases; so, it's not to romanticize our past, certainly these microbes were able to kill us and cause great consternation in city-state populations. But the idea is that some balance protects you on the one hand from some of those.
Steve: Yeah, I mean, nobody wants to go back to the days when we didn't have clean drinking water. Arguably clean drinking water is the single most important public health development in the history of humanity. And so we're not dying so much in the developed world of dysentery and other conditions of unsanitary drinking water, and we don't have to drink alcohol all day to make sure that we're not drinking dangerous liquids; but you know, dangerous in a different way and people used to die younger because they were getting infection and you would die from it. And, I mean, that still those happen today but, you know, fortunately we do have antibiotics and other things. So we're not arguing to go back to that. But we have realized that people have teased out the fact that maybe some of the now epidemic asthma rates, for example, are related to the fact that kids when they are growing up and even before they're born are not being challenged, their immune systems are not being challenged the way that we evolved.
Barnes: So I was going to throw that very example out, sort of, the classic example or the classic notion is that an individual's propensity to be more upregulated on one side of their immune system—say Th1 versus their Th2, the Th1 side being that side of our immune system that we believe evolved to protect us against things like the bacteria and the viruses and microbes; versus Th2 which served a very fundamental purpose in an environment where one was exposed to worms. But we're not exposed to worms anymore, so that side doesn't serve as much of a purpose, and we know from very sophisticated studies that infants that are born to mothers in the, sort of, with the, sort of, sterile environment, infants are born with the predisposition to be Th2 skewed. And there is a reason for that. During the neonatal period, it's important for the mother not to reject the fetus as the fetus is developing and so the mother's immune response is slightly tilted towards this Th2 to not treat the fetus as a microbe, if I can put it that bluntly.
Steve: Which is really a good thing.
Barnes: (laughter) At the time that when the infant is born, so the infant is born with a slight Th2 preference over the Th1, because that was the intrauterine environment. The thinking is that when exposed to some bacteria, some viruses, it sort of shifts this back into an equilibrium. But unfortunately in our current environment, where everything is sterile, we tend to forego breastfeeding and feed our infants formula from sterile water and so on and so forth, there are fewer siblings at home, we're not really giving these infants the chance to equalize, if you will, that immune response.
Steve: So what are some of the actual studies? What are your research interests that have confirmed that this situation exists out there?
Barnes: So, there have been a number of studies in the field of asthma, studies that have not necessarily been our own certainly include the German farming studies by Erika von Mutius and others showing that children who lived in very close proximity to livestock, and we know are exposed to lots of bacteria, for example, from the livestock are less likely to have asthma and allergies. There have been beautiful studies showing that children who go to daycare very early in life, who are therefore exposed to more rhinovirus and virus in general tend to have fewer asthma and allergies as they grow up. In our own work, we've taken a closer look at that exposure to that bacteria, the gram-negative bacteria we call endotoxin, which is ubiquitous, it's around us all the time; but it's certainly higher in some places than others and endotoxin we know doesn't just come from livestock, endotoxin comes from diesel exhaust. So there have been some various nice studies showing elevated levels of endotoxins in regions where folks live in close proximity to traffic. Right up the road in the Baltimore tunnel, there's some of the highest endotoxin levels that have ever been recorded.
Steve: How does that it get in the diesel exhaust?
Barnes: It's part of the particulate matter.
Steve: It's just picking it up from the environment and pluming it out?
Barnes: Exactly, we were interested in testing that theory that with higher levels of endotoxin, there would be lower levels of asthma and allergy. When we measure endotoxin in the tropical environment it looks very different than it does in a developed environment such as here. It's much higher and it's probably higher for a variety of reasons. In our one particular study that we've done in Barbados, folks live very close to the road, and they're exposed to very high levels of diesel exhaust, and we believe that pollution has contributed over time to an ever increasing prevalence of asthma in that society. When we measure endotoxin in the homes of these folks, it's very, very high but they have a lot of asthma and allergy, so I think that's telling us that the hygiene hypothesis is not a black and white hypothesis, that there are a lot of complexities to this. And for me personally it tells me that there are also genetic underpinnings. So it's not just about exposure to the environment, just as it's not just about having a particular mutation that puts you at risk of developing disease, but it's the interaction of those two factors—genes and environment that will probably help us have a better understanding of the Hygiene hypothesis.
Steve: It's definitely multifactorial, but when you deal with large enough populations you can start to tease out these kinds of relationships.
Barnes: Absolutely. Having the opportunity to study very large populations allows you to stratify folks based on exposure to factor A and B; allows you to stratify on not just one genetic polymorphism but many polymorphisms. So there is much to be gained from these very large population studies. And I frankly think that to get a better handle on the role of the hygiene hypothesis not just an asthma and allergic disease but any of these other complex diseases, the real key also are longitudinal studies, birth cohort studies, where we've been able to track an individual from the time he or she is born, measure various environmental exposures along the way, and then compare that to their genetic background.
Steve: So, we're not going to advise people to, oh go get the helminth worm infection, or you know, go roll around in the dirt on a farm nearby with your kids; nobody is going to advise that. So what is the practical application going to be of this kind of knowledge?
Barnes: Right, so it's very tempting, it's very tempting to come up with a conclusion that being exposed to a lot of dirt is good for your health or that being exposed to and infested with worms is good for your health. We wouldn't advocate either of those, but what I think the real value in this science really is, is if we can understand what it is about those microbes, and in the case of the parasite, what is it about the protein within the parasite that elicits this response to the parasite, either protective or conferring risk of the disease? If we could put our finger on that particular molecule we could develop better therapeutics for people. We could, you're not going to advocate giving an individual worms to cure their asthma or to cure their autoimmune disease. But you could come up with a drug that has some part of that protein that elicits this biological response and give that to the individual therapeutically.
Steve: How did you wind up working on this subject? Did you start way back in grad school? Or I don't mean way back.
Barnes: (laughter) No, you can say that because it's true.
Steve: Okay, or was it something that came along in the middle of your academic career?
Barnes: My work has really evolved truly over several decades. So as a graduate student, I was very interested, as a graduate student in medical anthropology, in differences across human populations and our response to environmental factors that confer risk of disease; and schistosomiasis was one disease I was particularly interested in.
Steve: This is a worm-borne disease.
Barnes: Schistosomiasis is a worm-borne disease; it's referred to as a helminth and it's spread to the human host through infested waters. Schistosomiasis typically doesn't carry a very high mortality rate. It can be debilitating for those individuals who can't mount an appropriate response against the worm. But my interest really started at that point in time with this interest in how and why we respond to different factors such as parasites, and truly that dovetailed in to what was a growing interest in the mid '80s of why there was an increase in asthma and allergic disease; and the hypothesis had been put forth that the IgE antibody that we all make but typically in very low quantities, had only been discovered in 1968. So, it was a relatively new immunological molecule that we knew was important in our immune system, and we knew it was very important in causing risk to asthma and other allergies like eczema and hay fever. At the same time, we began to appreciate that this IgE molecule was also protective against extracellular parasites. So, I was interested in that co-association with this molecule IgE. Over time, my research really focused more on identifying genetic determinants for asthma and allergies. I put the schistosomiasis studies aside. And then about 10 years ago, I had the opportunity to join forces with colleagues and immunologists in Brazil where schistosomiasis is still quite endemic and; in fact it's one of the last strongholds for schistosomiasis in the western hemisphere. And it just provided a really unique opportunity to test the hypothesis that some of these asthma genes that we had identified might also be important in schistosomiasis.
Steve: This is, you know, the hackneyed question, but where do you think things might be in another 10 or 15 years?
Barnes: I think that the hygiene hypothesis particularly and a sort of Darwinian approach in the way we think about medicine in general; that is how did we develop heart disease? How did we develop allergic diseases? How did we develop inflammatory bowel disease? We know as anthropologists that traditional foraging societies didn't have these diseases, and we know that it is not just because they didn't live past the age of 40. They simply didn't develop many of these complex chronic diseases that we experience now. So, I think this new way of approaching the pathology behind these different diseases is opening our minds and understanding, or elucidating new pathways that we didn't think about before. And I am very hopeful that with the better understanding of the pathologies that contribute to these diseases, with a better understanding of how a genetic mutation long ago that protected us against one disease now coincidentally causes another disease, will absolutely help us to develop better therapeutic targets; and not just treat these diseases but also predict who is going to be at greatest risk for disease. And once we know enough about all the environmental factors that go into causing a disease, we as medical folk can give better advise to patients about what they need to avoid or how they need to modify their lifestyle so that they can live longer and healthier lives.
Steve: This host-pathogen interaction is really so fascinating to study because you're dealing with this co-evolutionary situation but the rates are so different. So, we're practically standing still compared to the rates at which the pathogens get to evolve. And it's just I think, it's one of the most fascinating fields to be studying right now.
Barnes: It's indeed an exciting field, and I think it's a very important perspective that where we are now in the 21st century with the diseases we face, is such a recent moment in our human evolutionary time. And frankly that's one of the reasons why we've been particularly interested in focusing on diseases for which there is tremendous ethnic and racial disparities. Because it really isn't until very recently in time that we see the admixture, the mixing of individuals of different ancestral backgrounds. And it actually provides us a great opportunity to try to tease out at least from a genetic epidemiology perspective what mutations might have evolved that were selective or advantageous in one particular environment that individuals have carried with them as they've migrated to new parts of the world; and basically admix with other populations for which there might not have been selective advantage for having a mutation and therefore no mutation at all. And so by studying populations from a more ancient background, if I could, we believe that because our gene pool simply hasn't had enough time to change this rapidly, it's a great test tube experiment, if you will.
Steve: Natural experiment….
Barnes: A natural experiment to say, okay just yesterday in the time clock of human evolution you lived in an environment where if you have this mutation you will better off than the next guy who didn't have it, because it protected you against, for example, parasites. But now you've moved to another part of the world, and you're not exposed to those parasites anymore, but you still have that mutation that was adaptive back in that environment. Since you're not there anymore and you're exposed to new environmental factors, does that place you at risk for diseases that your ancestors wouldn't have thought about?
Steve: It's really fascinating. And we may be living in a unique window of time to do those studies, because with the mixing of all the different human population groups, in another couple of hundred years, it may be impossible to do those kinds of studies.
Barnes: So this is a really important point that right now it's a really terrific opportunity for us to try to tease out what about our human genome, based on our bio-geographical past, can we look at to try to explain why in contemporary times we have disease X. But this will change over time because with that mixture, there are simply diminishing of the benefit of having these mutations that might have been adaptive in a previous time. So yes it is a unique opportunity. And I guess I will just add to that the other opportunity, if we can call it that, is that with globalization and rapid changes in these places that we perform these studies where we are looking at more traditional ways of living and then comparing that to the way we live here in the United States, for example; so even within our Brazil study where we've studied parasitic disease, certainly the public health goal is to eradicate parasitic disease in these populations. And that is the first and foremost priority. But as that happens, we will have less and less opportunity to understand what it is about our past that brought us to where we are now in terms of these genetic polymorphisms that might have served some beneficial purpose.
Steve: Right. We're in no way saying not to try to make this situation better. We're just saying that we better do these studies now before we do accomplish what we hope to accomplish.
Barnes: Exactly. We can learn so much from our past by comparing people who live in different environments under different conditions and in different degrees of development, and I think that comes back to the hygiene hypothesis—that it is a unique opportunity to compare populations with different degrees of development to try to hone in on those specific factors that were emblematic of our past but are simply not part of our modern lifestyle.
Steve: Now it's time to play TOTALL……. Y BOGUS. Here are four science stories; only three are true. See if you know which story is TOTALL……. Y BOGUS.
Story 1: In the newest version of the popular Madden NFL football video game players can get concussions.
Story 2: The late Elizabeth Taylor probably had a FOXC2 gene mutation that affects embryonic development. Story 3: Wind turbines are becoming a huge killer of birds, taking down some 20 million annually.
And story 4: Growing salamander embryos have been found to have algae living happily inside their tissues. While you think about those stories: Do you believe in the effectiveness of subliminal messages? I think the idea that such messages can really work has pretty much been discredited, but who knows.
Hey, you're time is up.
Story 1 is true. In the new Madden Football game, players can get concussed and will be unavailable for the remainder of the game. So as in real football the object of the game will be to give the opposing team's quarterback a concussion.
Story 2 is true. Liz Taylor probably did have the FOXC2 gene mutation which conferred upon her a double role of her famous thick eyelashes.
And story 4 is true. Growing salamander embryos have been found to harbor algae within their tissues. Researchers think the algae get nitrogen rich waste products and the salamanders get oxygen. It's a winner-winner salamander dinner situation. For more check out the April 5th episode of the daily SciAm, podcast, 60-Second Science .
All of which means that Story 3 about wind turbines killing 20 million birds annually is TOTALL……. Y BOGUS. Now what is true is that the bird death toll from turbines is significant—about 440,000 birds per year according to the US Fish and Wildlife Service, and it's an issue that needs to be addressed as wind power hopefully becomes more of an energy contributor. But the real bird killer is on your couch. The American Bird Conservancy says that domestic cats kill about 250 million birds each year, a figure matched by feral cats; which means that wind turbines right now get less than a 10th of a percent as many birds as cats do.
That's it for this episode. Get your science news at www.ScientificAmerican.com, where you can check out the Daily Science Agenda, which features what you need to know now. For example: How does a 737 lose its fuselage in mid-flight? Find out at our Web site safely on the ground and follow us on Twitter, where you'll get a tweet about each new article posted to our Web site. Our Twitter handle is @sciam. For Science Talk , the podcast of Scientific American , I'm Steve Mirsky. Thanks for clicking on us. Try the Scientific American smart phone app.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Clin Exp Immunol
- v.160(1); 2010 Apr

The ‘hygiene hypothesis’ for autoimmune and allergic diseases: an update
According to the ‘hygiene hypothesis’, the decreasing incidence of infections in western countries and more recently in developing countries is at the origin of the increasing incidence of both autoimmune and allergic diseases. The hygiene hypothesis is based upon epidemiological data, particularly migration studies, showing that subjects migrating from a low-incidence to a high-incidence country acquire the immune disorders with a high incidence at the first generation. However, these data and others showing a correlation between high disease incidence and high socio-economic level do not prove a causal link between infections and immune disorders. Proof of principle of the hygiene hypothesis is brought by animal models and to a lesser degree by intervention trials in humans. Underlying mechanisms are multiple and complex. They include decreased consumption of homeostatic factors and immunoregulation, involving various regulatory T cell subsets and Toll-like receptor stimulation. These mechanisms could originate, to some extent, from changes in microbiota caused by changes in lifestyle, particularly in inflammatory bowel diseases. Taken together, these data open new therapeutic perspectives in the prevention of autoimmune and allergic diseases.
Introduction
Changes of lifestyle in industrialized countries have led to a decrease of the infectious burden and are associated with the rise of allergic and autoimmune diseases, according to the ‘hygiene hypothesis’. The hypothesis was first proposed by Strachan, who observed an inverse correlation between hay fever and the number of older siblings when following more than 17 000 British children born in 1958 [ 1 ]. The original contribution of our group to the field was to propose for the first time that it was possible to extend the hypothesis from the field of allergy, where it was formulated, to that of autoimmune diseases such as type 1 diabetes (T1D) or multiple sclerosis (MS) [ 2 ]. The leading idea is that some infectious agents – notably those that co-evolved with us – are able to protect against a large spectrum of immune-related disorders. This review summarizes in a critical fashion recent epidemiological and immunological data as well as clinical studies that corroborate the hygiene hypothesis.
The strongest evidence for a causal relationship between the decline of infections and the increase in immunological disorders originates from animal models and a number of promising clinical studies, suggesting the beneficial effect of infectious agents or their composites on immunological diseases.
In this review, we shall attempt to evaluate the arguments in favour of the hygiene hypothesis with particular interest on the underlying mechanisms.
Evolving epidemiology of allergic and autoimmune diseases
The rising incidence of atopic and autoimmune diseases.
In 1998, about one in five children in industrialized countries suffered from allergic diseases such as asthma, allergic rhinitis or atopic dermatitis [ 3 ]. This proportion has tended to increase over the last 10 years, asthma becoming an ‘epidemic’ phenomenon [ 4 ]. The increasing prevalence of asthma is important in developed countries (more than 15% in United Kingdom, New Zealand and Australia) but also in developing countries, as illustrated by a prevalence greater than 10% in Peru, Costa Rica and Brazil. In Africa, South Africa is the country with the highest incidence of asthma (8%) [ 5 ]. Unfortunately, data from most other African countries are unavailable [ 6 ]. The prevalence of atopic dermatitis has doubled or tripled in industrialized countries during the past three decades, affecting 15–30% of children and 2–10% of adults [ 7 ]. In parallel, there is also an increase in the prevalence of autoimmune diseases such as T1D, which now occurs earlier in life than in the past, becoming a serious public health problem in some European countries, especially Finland, where an increasing number of cases in children of 0–4 years of age has been reported [ 8 ]. The incidence of inflammatory bowel diseases (IBD), such as Crohn's disease or ulcerative colitis [ 2 ] and primary biliary cirrhosis [ 9 ] is also rising. Part of the increased incidence of these diseases may be attributed to better diagnosis or improved access to medical facilities in economically developed countries. However, this cannot explain the marked increase in immunological disorder prevalence that has occurred over such a short period of time in those countries, particularly for diseases which can be diagnosed easily, such as T1D or MS [ 10 – 12 ].
The decreasing incidence of infectious diseases
Public health measures were taken after the industrial revolution by western countries to limit the spread of infections. These measures comprised decontamination of the water supply, pasteurization and sterilization of milk and other food products, respect of the cold chain procedure, vaccination against common childhood infections and the wide use of antibiotics. The decline is particularly clear for hepatitis A (HAV), childhood diarrhoea and perhaps even more spectacular for parasitic diseases such as filariasis, onchocercosis, schistosomiasis or other soil-transmitted helminthiasis [ 13 ]. In countries where good health standards do not exist, people are chronically infected by those various pathogens. In those countries, the prevalence of allergic diseases remains low. Interestingly, several countries that have eradicated those common infections see the emergence of allergic and autoimmune diseases.
Uneven distribution
The geographical distribution of allergic and autoimmune diseases is a mirror image of the geographical distribution of various infectious diseases, including HAV, gastrointestinal infections and parasitic infections. There is an overall North–South gradient for immune disorders in North America [ 14 ], Europe [ 2 ] and also in China [ 15 ] with intriguing exceptions such as asthma in South America or T1D and MS in Sardinia. There is also a West–East gradient in Europe: the incidence of T1D in Bulgaria or Romania is lower compared to western Europe, but is increasing fast [ 16 ]. This gradient cannot be fully explained by genetic differences. Indeed, the incidence of diabetes is sixfold higher in Finland compared to the adjacent Karelian republic of Russia, although the genetic background is the same [ 17 ].
Additionally, migration studies have shown that offspring of immigrants coming from a country with a low incidence acquire the same incidence as the host country, as rapidly as the first generation for T1D [ 18 ] and MS [ 19 , 20 ]. This is well illustrated by the increasing frequency of diabetes in families of immigrants from Pakistan to the United Kingdom [ 21 ] or the increasing risk of MS in Asian immigrants moving to the United States [ 22 ]. The prevalence of systemic lupus erythematosus (SLE) is also much higher in African Americans compared to West Africans [ 23 ].
These data do not exclude the importance of genetic factors for those immunological disorders, as assessed by the high concordance of asthma, T1D or IBD in monozygotic twins: for example, the concordance rate for atopic dermatitis among monozygotic twins is high (77%) compared to dizygotic twins (15%) [ 7 ]. The difference in some genetic factors according to ethnicity [human leucocyte antigen (HLA) gene difference between Caucasian and Asian, for example] is well documented, but probably plays a minor role in geographical distribution in view of migrant data.
Search for risk factors at the origin of the increase of immunological disorders
Several factors, such as socio-economic indices, may explain the difference in the prevalence of immunological disorders according to time and geographical distribution. In fact, there is a positive correlation between gross national product and incidence of asthma, T1D and MS in Europe [ 2 ]. This is true at the country level, but also at that of smaller regions, such as Northern Ireland, where the low incidence of T1D is correlated with low average socio-economic level, as evaluated by conventional indices [ 24 ]. Similar results have been obtained in the province of Manitoba in Canada for Crohn's disease [ 25 ]. This correlation has even been demonstrated at the individual level for atopic dermatitis, as family income is correlated directly with the incidence of the disease [ 26 ]. However, this does not pinpoint which factor within the socio-economic indices is directly responsible for the immunological disorder. Several epidemiological studies have indicated a positive correlation between sanitary conditions and T1D [ 24 ] or MS [ 27 ], suggesting a possible role of infections. Other factors are often incriminated, such as air pollution for asthma [ 28 ], but their role has not been demonstrated convincingly. For example, it has been shown that in East Germany before the fall of the Berlin Wall, where the air pollution was greater, the incidence of asthma was lower than in West Germany [ 29 ].
Vitamin D production is linked to sun exposure, and has been shown recently to have immunomodulatory effects [ 30 ]. However, this does not explain the West–East gradient of T1D in Europe, or the huge difference between Finland and its neighbouring Karelian region, where people have the same sun exposure level [ 31 ].
Epidemiological data indicating a direct link between the decreasing level of infectious burden and the rising incidence of immunological disorders
Several epidemiological studies have investigated the protective effect of infectious agents in allergic and autoimmune diseases. The presence of one or more older siblings protects against development of hay fever and asthma [ 1 ], of MS [ 32 ] and also of T1D [ 33 ], as does attendance at day care during the first 6 months of life in the case of atopic dermatitis and asthma [ 34 ].
Interestingly, exposure to farming and cowsheds early in life prevents atopic diseases, especially if the mother is exposed during pregnancy [ 35 , 36 ]. It has also been shown that prolonged exposure to high levels of endotoxin during the first year of life protects from asthma and atopy [ 37 ]. However, these data have been contradicted by other studies showing an increased prevalence of asthma correlated with higher levels of endotoxins in urban housing [ 38 , 39 ]. The level of endotoxins is higher in farms as compared to cities, and subjects are in contact with a greater variety of microbial compounds in farms, which could explain this discrepancy.
Do helminth parasites protect against atopy? Epidemiological data of cross-sectional studies revealed that Schistosoma infections have a strong protective effect against atopy, as reviewed recently [ 40 ]. Hookworms such as Necator americanus also seem to protect from asthma. In contrast, Ascaris lumbricoides and Trichuris trichiura have no significant effect on disease. Parasitic infections have been almost eradicated in European countries since the Second World War, concomitant with the increase of atopy and allergy. This trend can explain part of the epidemiological difference between Europe and Africa, but cannot explain readily the intra-European North–South gradient.
Proof of principle of the causal relationship between decline of infectious diseases and increase of immunological disorders
We have seen that there is a strong correlation between changes in lifestyle and modifications of the incidence of allergic or autoimmune diseases, but this does not prove a causal relationship between these two observations. This is a crucial question, as many factors unrelated to infections are a consequence of lifestyle, such as food habits, quality of medical care or dinner time gradient from North to South Europe. The answer to this question comes from animal models of autoimmune and allergic diseases and, to a lesser degree, from clinical intervention studies.
Animal models
The incidence of spontaneous T1D is directly correlated with the sanitary conditions of the animal facilities, for both the non-obese diabetic (NOD) mouse [ 2 ] and the bio-breeding diabetes-prone (BB-DP) rat [ 41 ]: the lower the infectious burden, the higher the disease incidence. Diabetes has a very low incidence and may even be absent in NOD mice bred in ‘conventional’ facilities, whereas the incidence is close to 100% in female mice bred in specific pathogen-free (SPF) conditions. Conversely, infection of NOD mice with a wide variety of bacteria, virus and parasites protects completely (‘clean’ NOD mice) from diabetes [ 2 ]. Similarly, mycobacteria (e.g. complete Freund's adjuvant) prevent induction of experimental autoimmune encephalomyelitis [ 42 ] and ovalbumin-induced allergic asthma [ 43 ]. Data obtained in our laboratory show that living pathogens are not required, as bacterial extracts are sufficient to afford protection [ 44 ].
Increased atopy after anti-parasitic treatments
It has been shown that helminth eradication increases atopic skin sensitization in Venezuela [ 45 ], in Gabon [ 46 ] and in Vietnam [ 40 ]. However, in a small study of 89 Venezuelan adults and children with asthma there was a clinical improvement, and specific immunoglobulin E (IgE) levels decreased after anti-helminthic treatment [ 47 ], while a similar deworming treatment showed no effect in Ecuador [ 48 ]. It is difficult to explain these contradictory data, which may relate to the complexity of asthma pathophysiology. In the same vein, one might also mention the increased atopy observed after vaccination with Streptococcus pneumoniae in South Africa [ 49 ].
Prevention of allergic and autoimmune diseases by infections
In a prospective study in Argentina, 12 patients with MS with high peripheral blood eosinophilia were followed. These patients presented parasitic infections and showed a lower number of MS exacerbations, increased interleukin (IL)-10 and transforming growth factor (TGF)-β secretion by peripheral blood mononuclear cells (PBMC) [ 50 ].
Similarly, deliberate administration of ova from the swine-derived parasite Trichuris suis , every 3 weeks for 6 months to 29 patients with active Crohn's disease, improved symptoms in 21 of 29 patients (72%) with no adverse events [ 51 ]. T. suis ova were also given to patients with active ulcerative colitis, with significant improvement (43% improvement versus 17% for placebo) [ 52 ].
Another helminth, Necator americanus , has also been used in Crohn's disease, patients being inoculated subcutaneously with infective larvae. There was a slight improvement of symptoms, but the disease reactivated when immunosuppressive drugs were reduced [ 53 ].
Probiotics are non-pathogenic microorganisms that are assumed to exert a positive influence on host health and physiology [ 54 ]. Encouraging results were first shown in a double-blind randomized placebo-controlled trial in Finland, where Lactobacillus GG taken daily by expectant mothers for 2–4 weeks before delivery and postnatally for 6 months could decrease significantly the incidence of atopic dermatitis [ 55 ]. Perinatal protection lasted up to 7 years [ 56 ]. Another trial showed improvement of atopic dermatitis using other strains of probiotics [ 57 ]. However, a German group using the same protocol did not find any protective effect after 2 years [ 58 ]. Additionally, a recent study of 445 pregnant women in Finland who were treated with the same protocol as the initial Finnish study, but with freeze-dried Lactobacillus GG , failed to show any significant effect on eczema, allergic rhinitis or asthma 5 years after treatment. The only difference observed was a decreased IgE-associated allergic disease in caesarean-delivered children [ 59 ].
In T1D, only experimental data are available. The protective effect of a probiotic [ 60 ] and a bacterial extract [ 44 ] was reported on the onset of diabetes in NOD mice. A pilot study in humans, the PRODIA study (probiotics for the prevention of beta cell autoimmunity in children at genetic risk of type 1 diabetes), was begun in 2003 in Finland in children carrying genes associated with disease predisposition [ 61 ].
The case of probiotics in IBD is more complex because of the possible local anti-inflammatory effect, which could explain the relief of symptoms without changes in disease progression, as implicated in the hygiene hypothesis. Following a number of uncontrolled studies in a small cohort of 14 paediatric patients with newly diagnosed ulcerative colitis, probiotic treatment induced a significant rate of remission compared to the control group (93% versus 36%) and a lower relapse rate [ 62 ].
In brief, there are data suggesting that probiotics may have a favourable role in immune disorders, but the case is far from proven and requires further investigation. Additionally, although side effects are very low they might not be non-existent, as shown in a set of patients with acute pancreatitis [ 63 ]. Thus, probiotics should not be considered as totally harmless, particularly in the immunodeficient host, and more safety studies are needed. As mentioned by Sharp et al ., ‘probiotics may have unpredictable behaviour like all microorganisms, such as unanticipated gene expression in non-native host environment, or acquired mutations occurring spontaneously via bacterial DNA-transfer mechanisms’.
Is there a role for microbiota changes in the hygiene hypothesis?
The human gut is the natural niche for more than 10 14 bacteria of more than 1000 different species [ 64 ]. Immediately after birth, the human gut is colonized with different strains of bacteria. This commensal microbiota is important in shaping the immune system, for other basic physiological functions [ 65 ] as well as for the integrity of the intestinal barrier [ 66 ]. Interestingly, the intestinal flora was different in a small group of allergic Estonian and Swedish children compared to the control group, with a higher count of aerobic bacteria such as coliforms and Staphylocccus aureus and a decreased proportion of Lactobacilli , or anaerobes such as Bifidobacterium or Bacteroides [ 67 , 68 ]. However, this difference was not seen in a larger birth cohort study comparing three European baby populations [ 69 ]. Additionally, this study showed a slower acquisition of typical faecal bacteria such as Escherichia coli , especially in children delivered by caesarian section or children without siblings. It should be noted that all these studies were based on the analysis of culturable bacteria, and only atopic dermatitis and skin prick test were evaluated.
In autoimmune diseases the microbiota also seems to modulate the immune response. In NOD mice deficient for the myeloid differentiation primary response gene 88 (MyD88) signalling molecule it has been shown that microbiota protect mice from diabetes via a MyD88-independent pathway [ 70 ]. Using the metagenomic approach, it has been demonstrated that the biodiversity of the faecal microbiota of patients with Crohn's disease is diminished, especially for the Firmicutes phylum [ 71 , 72 ]. Faecalibacterium prautsnitzii is one of the Firmicutes that was particularly depleted, and it has been shown that this deficient commensal bacterium could improve IBD in a murine model of the disease [ 73 ]. This protective effect was also obtained with the supernatant of F. prautsnitzii culture, demonstrating the importance of one of the secreted molecules for its anti-inflammatory effect. Another bacterium, Bacteroides fragilis , has also been shown to protect animals from experimental colitis, and this protective effect was linked to a single microbial molecule, polysaccharide A [ 74 ]. As mentioned above, with regard to IBD these data must be interpreted with caution before extrapolating to other autoimmune disorders where the disease site is extra-intestinal. First, the respective anti-inflammatory and immunomodulatory effects of protective bacteria remain to be determined. Secondly, this protective effect should be discussed in the context of disease-promoting bacteria such as Helicobacter hepaticus .
In brief, there is an increasing amount of data showing that microbiota changes could contribute to the modulation of immune disorders but evidence is still slim, except in IBD. It is to be hoped that studies which provide a fair description of the molecular changes following intestinal infections will help in analysing the question further. The recent report by Fumagalli et al . is a good illustration of this new approach [ 75 ].
Mechanisms of the hygiene hypothesis
When considering the multitude of infectious agents that can induce protection from various immunological disorders, it is not surprising that more than one single mechanism has been found.
T helper type 1 (Th1)–Th2 deviation
Th1–Th2 deviation was the first major candidate mechanism for explaining the protective influence of infectious agents from immunological disorders. Th1 T cells produce inflammatory cytokines such as IL-2, interferon (IFN)-γ and tumour necrosis factor (TNF)-α that are operational in cell-mediated immunity (including autoimmune diabetes). In contrast, Th2 T cells that produce IL-4, IL-5, IL-6 and IL-13 contribute to IgE production and allergic responses. Given the reciprocal down-regulation of Th1 and Th2 cells, some authors suggested initially that in developed countries the lack of microbial burden in early childhood, which normally favours a strong Th1-biased immunity, redirects the immune response towards a Th2 phenotype and therefore predisposes the host to allergic disorders. The problem with such an explanation is that autoimmune diseases, which in most cases are Th1 cell-mediated, are protected by infections leading to a Th1 response and that atopy may be protected, as seen above, by parasites which induce a Th2 response. These observations fit with the concept of a common mechanism underlying infection-mediated protection against allergy and autoimmunity. Several hypotheses may explain these common mechanisms.
Antigenic competition /homeostasis
It has been known for several decades that two immune responses elicited by distinct antigens occurring simultaneously tend to inhibit each other. Numerous mechanisms were evoked to explain antigenic competition that might be pertinent to the hygiene hypothesis. The development of strong immune responses against antigens from infectious agents could inhibit responses to ‘weak’ antigens such as autoantigens and allergens. Among the mechanisms that explain antigenic competition, attention has been drawn recently to lymphocyte competition for cytokines, recognition for major histocompatibility complex (MHC)/self-peptide complexes and growth factors necessary to the differentiation and proliferation of B and T cells during immune responses within the frame of lymphocyte homeostasis. Similarly to red blood cell mass, which is restored to normal levels after a haemorrhage with the help of erythropoietin, CD4 and CD8 T lymphocytes are restored to normal levels after a lymphopenia. Homeostatic factors that play an equivalent role to that of erythropoietin have not been elucidated completely; however, cytokines such as IL-2, IL-7, and IL-15 are known to play a crucial role. Regulatory T cells that we discuss below may also be implicated in the mechanism of antigenic competition.
Immunoregulation
Another mechanism involves regulatory T cells which can suppress immune responses distinct from reponses against the antigen in question, here antigens expressed by infectious agents (a phenomenon called bystander suppression). The problem is complicated by the multiplicity of regulatory lymphocytes involving diverse cytokines that mediate their differentiation or their regulatory effects. The role of CD4 + CD25 + forkhead box P3 (FoxP3 + ) T cells has been suggested by transfer experiments performed in a murine parasite model [ 76 ]. The role of such cells is also suggested by the observation that CD28 –/– NOD mice devoid of CD4 + CD25 + FoxP3 + regulatory T cells (T regs ) lose their sensitivity to the protective effect of bacterial extract (our unpublished data). It has also been reported that in cord blood from newborns of mothers exposed to farming, CD25 + FoxP3 cells were up-regulated [ 77 ]. This observation should be interpreted with caution because of the uncertain specificity of these markers in man.
Other data suggest a role for IL-10-producing B cells [ 78 ], natural killer (NK) T cells [ 79 ] and more generally cytokines such as IL-10 [ 80 ] and TGF-β[ 81 ] whatever the cell type producing these cytokines. More work is needed in experimental models to delineate further the involvement of regulatory mechanisms in the protective effects of the various infections relevant to the hygiene hypothesis. It might emerge that different mechanisms are operational according to the protective infection.
Non-antigenic ligands
All the mechanisms mentioned previously are based on the notion that the hygiene effect is due to the decrease of immunological responses elicited against infectious agents. A number of experiments indicate that infectious agents can promote protection from allergic diseases through mechanisms independent of their constitutive antigens, leading to stimulation of non-antigen specific receptors. This concept is well illustrated by the example of Toll-like receptors (TLRs). Knowing the capacity of TLRs to stimulate cytokine production and immune responses, it might be predicted that TLR stimulation by infectious ligands should trigger or exacerbate allergic and autoimmune responses. This has indeed been demonstrated in some experimental models [ 82 , 83 ].
Surprisingly, and paradoxically, it has also been observed that TLR stimulation could prevent the onset of spontaneous autoimmune diseases such as T1D in NOD mice, an observation made for TLR-2, -3, -4, -7 and -9 [ 84 ] (and our unpublished data). In this model, treatment with TLR agonists before disease onset prevents disease progression completely. The mechanisms underlying such protections are still ill defined, but could involve production of immunoregulatory cytokines and the induction of regulatory T cells or NK T cells. Similar data have been observed in an ovalbumin-induced model of asthma [ 85 ].
Concerning HAV, it was shown initially that atopic diseases were less common in subjects that have been exposed to the virus [ 86 ]. It was difficult to say whether this association was due to a direct protective effect of HAV infection or explained only by the fact that HAV exposure is a matter of poor hygiene. Data obtained by Umetsu et al . have shown that HAV could influence T cells directly, notably Th2 cells that express the HAV receptor [ 87 ], a finding corroborated by the observation that atopy prevalence is associated with HAV receptor gene polymorphisms in anti-HAV antibody-positive subjects. In fact, recent data indicate that the HAV receptor, the TIM-1 protein (T cell, immunoglobulin domain and mucin domain), could play an important role in the severity of HAV and its putative effect on atopic diseases.
Gene–environment interactions
An interesting approach to identify mechanisms underlying allergic and autoimmune diseases consists in searching for associations between these diseases and polymorphisms of various genes, notably those coding for molecules involved in immune responses. It is interesting to note that such an association has been found for genes implicated in the control of infection. Among them, polymorphism in genes of the innate immune response such as CD14 , TLR2 , TLR4 , TLR6 or TLR10 , and intracellular receptors such as NOD1 and NOD 2 [also known as caspase-recruitment domain (CARD)4 and CARD15, respectively], appears to be important [ 88 , 89 ]. Mouse studies have shown that these gene–environment interactions explain a proportion of the phenotypic variance. One of those genes is CD14 , which is important in lipopolysaccharide (LPS)/TLR-4 signalling. Many association studies have highlighted the role of the CD14–159CT polymorphism and allergic inflammation [ 90 ].
Therapeutic strategies
The notions presented above open new, interesting, therapeutic perspectives for the prevention of allergic and autoimmune diseases. Of course, contaminating children or adults at high risk of developing these diseases by infectious agents cannot be envisioned, at a time when medical progress has allowed the reduction of major infectious diseases. It should be mentioned, however, that even if we do not believe that this is not the best strategy for the future, some groups have used living parasites such as T. suis in the prevention of IBD, as mentioned above, or living Lactobacilli in the prevention of atopic dermatitis. These approaches present the obvious limitation of insufficient standardization, and hazards linked to unpredictable disease course in subjects presenting an unknown immunodeficiency by contamination with xenogeneic virus in the case of swine-derived parasites.
Conversely, the use of bacterial extracts, already shown to be efficacious in a number of experimental models and in the clinic, such as OM-85 in T1D, should be envisioned seriously [ 44 ]. These extracts, which represent the mixture of a wide spectrum of chemically ill-defined components, are also submitted to the criticism of poor standardization. On the other hand, they are a better representation of the various components of bacteria known for their protective effects. The same comments apply to parasitic extracts, shown to be effective in T1D [ 91 ]. In the long-term future, one would like to use chemically defined components of protective infectious agents, such as TLR agonists, polysaccharide A or the active substance secreted by F. prautsnitzii . In any event, the use of bacterial extracts or chemically defined products will be confronted with the double problem of the timing of administration (sufficiently early in the natural history of the disease), and of safety. Indeed, any side effects are unacceptable in young subjects who are apparently healthy and whose risk of developing the disease in question is not demonstrated absolutely.
None of the authors has conflicts of interest to declare, or any relevant financial interest, in any company or institution that might benefit from this publication.

COMMENTS
Hygiene hypothesis. In medicine, the hygiene hypothesis states that early childhood exposure to particular microorganisms (such as the gut flora and helminth parasites) protects against allergies by strengthening the immune system. [1] [2] In particular, a lack of such exposure is thought to lead to poor immune tolerance. [1]
The hygiene hypothesis is the idea that kids need to be exposed to germs in order to develop healthy immune systems. We know that many common viruses did not circulate as widely during the pandemic, thanks to social distancing, masking, and other COVID mitigation measures. ... This was interpreted [to mean] that allergic disease was linked to ...
The hygiene hypothesis says a child's environment can be "too clean," and the lack of exposure to germs does not give the immune system a chance to develop resistance to diseases.(Image credit ...
Conclusion. The hygiene hypothesis suggests that a newborn baby's immune system must be educated so it will function properly during infancy and the rest of life. One of the key elements of this ...
In 1989, Strachan proposed the hygiene hypothesis of allergic disease after observing that hay fever was less common among children with older siblings. 8 He reasoned that children growing up in larger families may experience increased exposure to microbes in early childhood due to inevitable unhygienic contact with older siblings or prenatal exposure from the mother infected by similar ...
The 'hygiene hypothesis' as originally formulated by Strachan, proposes that a cause of the recent rapid rise in atopic disorders could be a lower incidence of infection in early childhood, transmitted by unhygienic contact with older siblings. ... The epidemiological evidence supporting the hygiene hypothesis as an explanation for the ...
The hygiene hypothesis emerged as an explanation for the epidemic of asthma and allergies. It was originally based on the observation that hay fever was inversely associated with sibship size and was probably influenced by the notion of an early origin of chronic disease and several preexisting ideas of an antagonism between infections and ...
The Hygiene Hypothesis and the Bacterial World. Even before high-throughput sequencing techniques were established that allow a deeper view into the microbial world on our body surfaces, Noverr and Hufnagle proclaimed the "Microbiota Hypothesis" by which they claimed the microbiota to be indispensable for developing and maintaining a tolerogenic immune status ().
The Hygiene Hypothesis and New Perspectives—Current Challenges Meeting an Old Postulate. During its 30 years history, the Hygiene Hypothesis has shown itself to be adaptable whenever it has been challenged by new scientific developments and this is a still a continuously ongoing process.
The Hygiene Hypothesis comprise immunological, microbiological and evolutionary aspects. Thus, the original postulate developed into a holistic model that explains the impact of post-modern life-style on humans, who initially evolved in close proximity to a more natural environment. Focusing on diet and the microbiome as the most prominent ...
That's what supporters of the so-called hygiene hypothesis think, saying that the rising rates of allergies, asthma and other autoimmune disorders in children is linked to our increasingly hygienic surroundings. And while statistics appear to back this up, experts in the fields of immunology and infectious disease say, not so fast.
The hygiene hypothesis postulates that an increased frequency of infections contributes to a decrease in autoimmune and allergic diseases. Here, Bach summarizes the epidemiological and ...
The hygiene hypothesis was proposed almost three decades ago. Nevertheless, its mechanism still remains with relevant controversies. Some studies defend that early exposures during childhood to microbes and parasites are key determinants to prevent allergies and autoimmune diseases; however, other studies demonstrated that these early exposures can even potentiate the clinical scenario of the ...
"The hygiene hypothesis suggests that early life exposure to microbes helps in the education of an infant's developing immune system." Without this education, your immune system may be more prone ...
Barnes: The hypothesis is that as we make the shift from dirt to sterile that you're changing the direction of your immune response. This causes diseases. Steve: That's Kathleen Barnes. We'll hear ...
Keywords: hygiene hypothesis, allergy, asthma, non-communicable inflammatory diseases, chronic inflammation. Go to: Throughout its history, the Hygiene Hypothesis has shown itself to be adaptable and flexible whenever it has been challenged by innovation in science ( 1 ). A number of new findings need to be considered in this ongoing revisiting ...
The hygiene hypothesis emerged as a tentative explanation to a massive shift in the human disease spectrum from infections to allergies ().It borrowed its name from a hypothesis originally proposed in the 1980s to explain the appendicitis epidemic of the early 20th century by an immune system not adequately trained for infections ().Against the then prevailing idea that viruses triggered ...
They decided the name has to go ( 15 ). "The trouble is, as soon as you use the words 'hygiene hypothesis,' the word hygiene prejudges what the cause is," says Bloomfield. To the public, "hygiene" is interpreted as personal cleanliness: washing hands, keeping food clean and fresh, sanitizing the home.
It's not that adherents to the offshoots of the hygiene hypothesis are urging us to live in dirty homes. Rather, it's about striking a reasonable balance between cleanliness and zero tolerance. (Send your questions to [email protected], or write: Ask the Doctors, c/o UCLA Health Sciences Media Relations, 10880 Wilshire Blvd ...
The Hygiene Hypothesis. The "Hygiene Hypothesis" is a theory that suggests a young child's environment can be "too clean" to effectively stimulate or challenge the child's immune system to respond to various threats during the time a child's immune system is maturing. As a fetus, the immune system is thought to be repressed to avoid rejecting ...
The hygiene hypothesis, it was suggested, would mean that with continued improvements in hygiene, exposure to infections during childhood and early adult life falls and rates of appendicitis fall with it. "Unhygienic contact with older siblings" stated, but term "hygiene hypothesis" not used in the paper.
The hygiene hypothesis is a theory that suggests exposing children to pathogens early in life could help them gain immunity from infectious diseases, ...
The hygiene hypothesis is based upon epidemiological data, particularly migration studies, showing that subjects migrating from a low-incidence to a high-incidence country acquire the immune disorders with a high incidence at the first generation. ... The problem with such an explanation is that autoimmune diseases, which in most cases are Th1 ...