An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Diagnostics (Basel)


A Review of the Recent Advances in Alzheimer’s Disease Research and the Utilization of Network Biology Approaches for Prioritizing Diagnostics and Therapeutics
1 Department of Pharmacy, Faculty of Pharmacy, Al-Zaytoonah University of Jordan, P.O. Box 130, Amman 11733, Jordan
2 Laboratory for Molecular Modeling, Division of Chemical Biology and Medicinal Chemistry, Eshelman School of Pharmacy, The University of North Carlina at Chapel Hill, Chapel Hill, NC 27599, USA
3 National Center for Epidemics and Communicable Disease Control, Amman 11118, Jordan
Dima A. Sabbah
Osama h. abusara, abdel qader al bawab, associated data.
Data supporting the reported results can be requested by contacting the corresponding author directly.
Alzheimer’s disease (AD) is a polygenic multifactorial neurodegenerative disease that, after decades of research and development, is still without a cure. There are some symptomatic treatments to manage the psychological symptoms but none of these drugs can halt disease progression. Additionally, over the last few years, many anti-AD drugs failed in late stages of clinical trials and many hypotheses surfaced to explain these failures, including the lack of clear understanding of disease pathways and processes. Recently, different epigenetic factors have been implicated in AD pathogenesis; thus, they could serve as promising AD diagnostic biomarkers. Additionally, network biology approaches have been suggested as effective tools to study AD on the systems level and discover multi-target-directed ligands as novel treatments for AD. Herein, we provide a comprehensive review on Alzheimer’s disease pathophysiology to provide a better understanding of disease pathogenesis hypotheses and decipher the role of genetic and epigenetic factors in disease development and progression. We also provide an overview of disease biomarkers and drug targets and suggest network biology approaches as new tools for identifying novel biomarkers and drugs. We also posit that the application of machine learning and artificial intelligence to mining Alzheimer’s disease multi-omics data will facilitate drug and biomarker discovery efforts and lead to effective individualized anti-Alzheimer treatments.
1. Introduction
Alzheimer’s disease (AD) is a polygenic and multifactorial disease characterized by the deposition of amyloid-β (Aβ) fibrils in the brain, leading to the formation of plaques and neurofibrillary tangles (NFTs), and ultimately resulting in dendritic dysfunction, neuronal cell death, memory loss, behavioral changes, and organ shutdown [ 1 , 2 , 3 , 4 , 5 ]. It is estimated that 6.5 million Americans, 65 years and older, are living with Alzheimer’s dementia, and this number is likely to grow rapidly [ 6 ], reaching 13.8 million by 2060 [ 6 , 7 ]. Additionally, approximately 10 million Americans are currently living with mild cognitive impairment (MCI). In fact, 50% of MCI cases are due to AD based on biomarker evidence [ 8 ]. Eventually, 15% of MCI patients develop dementia after two years, and one-third develop dementia due to AD within five years [ 6 ]. The number of Americans with AD-MCI and AD dementia is approximately 11.2 million [ 6 ]. The healthcare cost for AD patients in 2022 is expected to reach USD 321 billion [ 6 ]. Furthermore, as the global population ages, the number of people suffering from dementia is expected to triple from 50 million to 152 million by 2050 [ 9 ]. Therefore, there is an urgent need to impede or slow down the onset and progress of the disease and ameliorate the disease-debilitating symptoms in hopes of putting an end to this disease and preventing a growing public health crisis [ 10 ].
Multifactorial mechanisms are involved in AD pathogenesis, including genetic, epigenetic, biological, and environmental factors networking with each other [ 11 ]. However, genomic experiments affirmed that no particular gene could be assigned as a potential target for AD pathogenesis. In fact, multiple genetic and non-genetic factors contribute to disease development [ 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 ]. In the past decade, there has been an increased understanding of potential targets for disease-modifying therapies that delayed or slowed down the clinical course of AD. According to the U.S. National Library of Medicine’s (NLM) ClinicalTrials.gov Beta (beta.clinicaltrials.gov) [ 19 ], there have been 1943 clinical trials of different phases and different designs to investigate new potential molecules in treating AD. Among these studies, 151 clinical trials progressed to Phase III clinical trials, in which safety and monitoring of side effects were investigated.
After decades of AD drug discovery research and billions of dollars spent on clinical trials, we still do not have a single effective anti-AD drug. Promising novel strategies to developing anti-AD drugs keep failing in clinical trials [ 20 , 21 ]. The exact cause of the disease remains the subject of ongoing debate and investigation. At this time, the most plausible hypothesis is that AD is a multifactorial disorder in which genetic and environmental risk factors interact, leading to an acceleration in the rate of normal aging. More than 600 genes contribute to AD pathogenesis, along with environmental factors and epigenetic changes [ 4 , 5 ]. The genetic deficiencies in AD consist of germline mutations, mitochondrial DNA mutations, and single-DNA nucleotide polymorphisms [ 22 , 23 ].
Therapeutic clinical treatment targets of AD aim to enhance behavioral, cognitive, and non-cognitive symptoms of the diseases. In the last two decades, there were no new medication approvals for the treatment or the prevention of AD. Currently, the aim of developing new anti-AD agents is to utilize new disease-modifying agents that delay the onset or slow down the progression of an established disease. Aβ, tau protein, and cell oxidation are the most currently promising targets to modify the pathologic status of Alzheimer’s disease [ 24 ].
In this review, we aim to provide a comprehensive overview of AD pathophysiological hypotheses, classifications, diagnostic biomarkers, and currently approved pharmacological and non-pharmacological treatment options. Moreover, we discuss the emerging role of the microbiome as well as epigenetics in the pathogenesis of AD and discuss the potential of epigenetic and genetic treatment options. We also provide a summary on drugs being developed or currently investigated in clinical trials, their success, failures or drawbacks, newer target approaches, and most important considerations for conducting effective future clinical trials. In addition, we discuss novel approaches for the proper diagnosis and drug-development for AD through exploiting network biology, artificial intelligence (AI), and machine learning (ML).
2. Alzheimer’s Disease Pathophysiology
There are two main pathological hallmarks of AD: (1) the presence of increased extracellular Aβ plaques, formed due to the aggregation and impaired clearance of Aβ oligomers (hydrophobic Aβ aggregates) [ 25 , 26 ], and (2) the formation of NFTs [ 27 ] that are composed of an insoluble intracellular hyperphosphorylated microtubule-associated protein tau [ 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 ]. MetaCore’s [ 39 ] pathway map presented in Figure 1 summarizes the most important processes, genes, and proteins linked to AD pathophysiology. We further highlighted which network objects on the map that are known AD biomarkers according the MetaCore TM , and which ones are drug targets for known drugs (i.e., as a proof of their druggability).

Alzheimer’s disease (AD) pathway map highlighting validated diagnostic/prognostic AD biomarkers in addition to genes/gene products involved in the tau pathology pathway. Connections between network objects on the map are referred to as links (or edges). A link identifies an interaction or a logical relation between two nodes. The type of interaction or relation is reflected by an appropriate symbol placed in the middle of the link. B = binding (i.e., physical interaction between molecules); C = cleavage; CM = covalent modification; +P = phosphorylation; −P = dephosphorylation; Tn = transport; TR = transcription regulation; red arrows = inhibition; green arrows = activation; grey arrows = unspecified action; solid purple arrows = emergence in disease; dashed purple arrows = enhancement in disease; arrows with purple x = disruption in disease; light violet text box = normal process; pink text box = pathological processes; white text box with blue outline = notes; grey block = reaction; blue block = normal process; pink block = pathological process; solid purple hexagon = compound; CS = complex subunit; GR = group relation; starred network objects = groups or complex processes; thermometers on pathway map = network object is also a validated diagnostic or prognostic disease biomarker from the Cortellis Drug Discovery Intelligence (CDDI) database [ 40 ]; thermometer 1 is for late-onset AD (LOAD); thermometer 2 is for early-onset AD (EOAD); a pink hexagon with a capital D on the upper left side of the network object indicates an AD biomarker according to MetaCore TM ; a blue hexagon on the upper left side of the network object with a capital R indicates that the network object (i.e., gene/gene product) is a drug target (not necessarily for AD). Map generated using MetaCore TM version 21.4. MetaCore TM , a Cortellis™ solution, 14 October 2022, © 2022 Clarivate. All rights reserved.
2.1. The Amyloid Hypothesis
It was long thought that polymorphism in the amyloid precursor protein (APP) gene ( APP ) causes alterations within and around the Aβ domain of translated APP proteins, altering APP proteolytic processing, and leading to changes in Aβ cleavage and aggregation [ 41 ]. This would favor the amyloidogenic pathway, causing the accumulation of Aβ, which contributes to AD pathogenesis [ 42 ].
The APP protein contains the Aβ domain, which, in healthy individuals, is cleaved and processed into Aβ and other Aβ-related peptides [ 43 , 44 , 45 , 46 , 47 , 48 , 49 ] by α-secretase followed by γ-secretase and later cleared [ 26 , 50 , 51 ]. The APP protein is encoded by the APP gene ( Figure 2 ), which has 17 transcripts (splice variants), 279 orthologues, 2 paralogues, and is associated with 9 phenotypes (ABeta amyloidosis Arctic type, ABeta amyloidosis Dutchr type, ABeta amyloidosis Iowa type, ABeta amyloidosis Italian type, ABetaA21G amyloidosis, ABetaL34V amyloidosis, ADs, cerebral amyloid angiopathy APP-related, and early-onset autosomal dominant AD).

Visualizing APP gene information using ( a ) Ensembl genome browser 107 (July 2022, GRCh38) and ( b ) NCBI’s gene browser.
APP genetic variants are often observed in Familial Alzheimer’s disease (FAD) [ 52 , 53 , 54 , 55 , 56 , 57 , 58 ]. Such mutations in the amino terminus of the Aβ domain result in the production of better substrates for β-secretase (BACE1) activity [ 55 ], leading to significant increase in Aβ production and Aβ fragments consisting of 40 or 42 amino acid residues (Aβ40 and Aβ42, respectively) [ 51 , 59 , 60 ]. These Aβ fragments are formed via the amyloidogenic pathway in AD patients, as BACE1 cleaves APP followed by γ-secretase [ 50 ]. Enhanced aggregation of altered Aβ is observed due to mutations affecting the mid region of Aβ [ 57 , 58 ]. Amino acid mutations located beyond the carboxyl terminus region of Aβ increase the production of Aβ42 [ 55 , 56 ], which is considered the major neurotoxic Aβ species [ 25 , 42 , 50 , 61 ]. Aβ42 is of particular interest; it is more hydrophobic, more pathogenic, and it aggregates faster than Aβ-40, resulting in an earlier onset of AD.
Furthermore, APP has a substrate inhibitory domain (ASID) that negatively regulates the activity of γ-secretase by decreasing Aβ production [ 62 ]. Alterations of ASID region by deletion or single-nucleotide polymorphism were reported, in which an inhibitory effect is reduced, causing an increase in Aβ generation [ 50 , 62 ]. Presenilin (PSEN), the catalytic subunit of γ-secretase [ 63 , 64 , 65 , 66 ], may also be modified via mutations in PSEN1 and PSEN2 genes ( PSEN1 and PSEN2 , respectively) [ 67 ], resulting in the destabilization of the γ-secretase–APP interactions [ 68 ], causing the generation of longer and more hydrophobic Aβ peptides that contribute to the pathogenesis of AD [ 41 , 68 , 69 , 70 ].
Tau is a microtubule-associated protein, found in axons of neurons [ 71 ], and it functions as a microtubule stabilizer that enables cytoplasmic extensions for the eventual formation of the axon and the dendrites [ 72 , 73 ]. Once tau is phosphorylated, its ability to bind to microtubules is reduced [ 74 , 75 , 76 , 77 ] and self-assembly into tangles/filaments is induced [ 78 ], hence affecting its normal function. It is considered as a main pathological lesion in the brains of patients with AD in addition to Aβ fibrils. In fact, AD is considered one of the neurodegenerative diseases that are termed “tauopathies” [ 79 , 80 , 81 ], where tauopathy indicates a tau-related pathology (i.e., dysfunction and/or tangle formation that contributes to the pathogenesis of AD and other neurodegenerative diseases) [ 27 ]. As for AD, this is driven by the intracellular aggregation of tau proteins via different mechanisms. Tau’s phosphorylation can lead to aggregation via liquid–liquid phase separation mechanism [ 82 ]. Cleavage of tau by several proteases can also lead to aggregation [ 83 , 84 , 85 ]. It is also suggested that tau pathology could be a downstream effect of Aβ aggregation or both could enhance each other’s toxic effects [ 41 , 86 , 87 ]. In vivo, Aβ plaques enhanced the aggregation of human-derived pathological tau injected into mouse brains [ 88 ]. In autopsy specimens from deceased AD patients, the number of NFT-positive cells is positively correlated to AD stages [ 89 , 90 , 91 , 92 , 93 ].
2.1.3. APOE, TERM2, SORL1, and ABCA7 Mutations
Mutations of genes, such as apolipoprotein E ( APOE ) and triggering receptor expressed on myeloid cells 2 ( TREM2 ) [ 70 ], would affect the microglial clearance of Aβ. Briefly, APOE protein, which is a lipoprotein, binds to Aβ plaques forming Aβ-lipoprotein complexes that are engulfed into microglia via TREM2 receptors [ 94 ]. Mutations of APOE and TREM2, resulting in mutated proteins, such as APOE2, APOE4, or TREM2 mutations (R47H, R62H, and D87N), would contribute to AD development [ 94 , 95 , 96 , 97 ]. Additionally, genetic mutations of SORL1 and ABCA7 are also considered as causative or strong risk-increasing variants for AD [ 70 ]. Sortilin-related receptor L1 (SORL1) controls APP cleavage and APOE uptake, while increasing ATP-binding cassette transporter A7 (ABCA7) increases Aβ phagocytosis [ 67 ]. Hence, mutations resulting in alterations of SORL1 and ABCA7 would act as a risk factor for AD [ 67 ]. Other genetic mutations that have low, medium, or high risk for AD are summarized by Scheltens et al. [ 41 , 70 ].
2.2. The Cholinergic Hypothesis
In addition to the amyloid hypothesis described above, other hypotheses have been suggested such as the impairment of cholinergic function, also known as the cholinergic hypothesis [ 98 ]. The cholinergic deficit in AD patients was confirmed in various studies, which highlighted a major deficit in the enzyme responsible for acetylcholine (ACh) synthesis (choline acetyltransferase) [ 99 , 100 , 101 ], reduced choline uptake [ 102 ], reduced ACh release [ 103 ], and loss of ACh (cholinergic) neurons [ 104 ]. Along with the role of ACh in learning and memory [ 105 , 106 ], degeneration of cholinergic neurons and loss of cholinergic neurotransmission contributes to the worsening of cognitive function in patients with AD [ 106 , 107 ].
2.3. The Mitochondrial Cascade (Oxidative Stress) Hypothesis
Neuronal mitochondria are considered the main organelles responsible for the neuronal oxidative stress via the generation of free radicals through their electron transport chain [ 108 ]. In the case of high levels of ATP and diminishing electron transport effect, superoxide would be formed from oxygen via mitochondrial respiration [ 109 ]. Superoxide would then be converted to hydrogen peroxide by superoxide dismutase and later to hydroxyl radicals and anions by the Fenton reaction [ 110 ]. These reactive oxygen species (ROS) would affect redox imbalance, cause neurotoxicity and genomic instability, transcription of pro-inflammatory genes, and cytokine release [ 111 ]. ROS would further damage and inactivate parts of the mitochondrial electron transport chain, leading to the formation of superoxide from the electron reduction of oxygen in a positive feedback cycle [ 109 , 111 , 112 ].
ROS damage mitochondrial DNA (mtDNA) that leads to neuronal functional impairments and damaged mitochondria due to this oxidative stress will not be degraded by mitophagy [ 108 , 111 , 113 ]. Usually, oxidative stress acts as a signal for mitophagy process to degrade damaged mitochondria, as oxidative stress reduces mitochondrial membrane potential [ 108 ]. However, ROS alter parkin, which is a key mitophagy regulator, and inhibits its function, causing the continual presence of mitochondria [ 113 ]. Collectively, ROS, DNA damage, and mitochondria contribute to the aging process [ 114 , 115 ]. Briefly, following DNA damage via ROS, kinases and PARP are activated, leading to the decrease of NAD+ production, which is essential for metabolic pathways and ATP production. Thus, oxygen consumption and ATP production would be required to increase to meet high energy demand. Mitochondrial coupling would occur, which increases membrane potential, increases free radical formation, and decreases mitophagy. Furthermore, as discussed above, free radicals would further cause DNA damage.
Denham Harman proposed the free radical theory of aging, in which free radicals are involved in the changes associated with the aging process [ 116 ]. It was later confirmed that free radicals are involved in the aging process and advanced age diseases, such as AD [ 115 , 117 , 118 ]. Furthermore, the effect of ROS on mitochondria supported the theory that relates mitochondria to the aging process and neurodegenerative diseases, such as AD [ 108 ]. This theory is associated mostly with the central nervous system since it consumes 20% of the body’s oxygen and is susceptible to oxidative stress [ 119 ]. Neurons would have high sensitivity to free radicals since they are non-dividing and post-mitotic cells and cannot be replaced in the event of damage, leading to mitochondrial dysfunction with aging [ 118 , 120 ].
It is observed that mitochondrial dysfunction is prevalent in the aging process [ 108 ]. In addition, healthy aging results in reduced mitochondrial metabolism in terms of α subunit reduction of the mitochondrial F1 ATP synthase, thus interfering with ATP production [ 108 ]. Eventually, ATP production decreases, ROS production increase, and this will cause an increase in DNA, protein, and lipid oxidation [ 118 , 121 , 122 ]. Moreover, apart from mtDNA damage caused by mitochondrial dysfunction, nuclear DNA is also damaged, leading to impairments in vesicular function, synaptic plasticity, and mitochondrial function [ 121 ].
Excess ROS and bioactive metals, such as copper, iron, zinc, and magnesium are present in AD brains that promote Aβ aggregations and NFT formation [ 123 , 124 , 125 , 126 ]. Moreover, the elevated levels of mtDNA oxidation are considered as one of the early markers of AD pathogenesis [ 118 , 122 ]. Moreover, late onset AD pathogenesis may be linked to age-associated mitochondrial decline [ 108 ]. The expression and processing of APP would be altered with age-associated mitochondrial decline, leading to the production of Aβ oligomers that aggregate into plaques in AD [ 127 , 128 ]. These Aβ oligomers are associated with neuronal toxicities and ROS generation. Studies have shown that the hydrophobic 25–35 region of Aβ leads to neuronal toxicity and generates ROS, showing that Aβ itself is a source of oxidative stress [ 108 , 129 , 130 ]. Aβ42 is hydrophobic in nature, and it can reside within the neuronal membrane lipid bilayer and cause lipid peroxidation, identified through 4-hydroxy-2-trans-nonenal (HNE) production that is bound to neuronal proteins [ 131 ]. Studies have also shown that the production of HNE—due to lipid peroxidation associated with the residing of hydrophobic Aβ protein in lipid bilayer—and HNE’s neuronal protein binding are linked to cell death [ 132 , 133 , 134 ]. This are related to neurodegenerative diseases’ pathogenesis, including AD. Furthermore, the oxidative stress triggered by Aβ is also likely to be as result of complexation with redox active metals, such as copper, zinc, and iron [ 108 ], which are highly present in AD brains [ 123 , 124 , 125 , 126 ], promoting Aβ aggregation into plaques [ 108 ]. Copper forms the most stable complex and can generate superoxide and hydrogen peroxide that contribute to AD pathogenesis [ 127 , 135 ].
2.4. Other Factors Affecting Disease Pathogenesis
Other factors that affect the clinical development of AD include vascular pathology of blood–brain barrier that result in leakage and cause dementia [ 136 , 137 ]. Moreover, elevated iron levels in brain and dysregulation of its metabolism are also present in AD patients as it is linked with cellular damage and oxidative stress [ 138 ]. Exosomes may play a part in the spreading of Aβ plaques and NFTs through the brain [ 139 ].
3. Alzheimer’s Disease Classifications
AD is usually classified on the basis of two criteria: age and heredity ( Table 1 ). Based on age, there are two main forms of AD: late onset AD (LOAD) and early onset AD (EOAD). LOAD is diagnosed at age 65 or older [ 140 ], although the degenerative process may begin damaging the brain 20 years before symptoms appear [ 141 ]. This is the most prevalent form of the disease, comprising 95% of patients [ 142 ]. Polymorphism in APOE is a major risk determinant of LOAD, contributing to 60–80% of the disease pathogenesis [ 140 , 143 , 144 ]. APOE encodes for the principal lipid transport protein in the central nervous system (CNS), which has three alleles (E2, E3, and E4). APOE4 surges the risk of LOAD development [ 11 ]. Genome-wide association studies (GWAS) identified >20 LOAD risk genes linked to endocytosis, inborn immunity, and lipid metabolism [ 143 , 145 ]. EOAD is typically diagnosed between 40 and 50 years of age, comprises 5% of the total AD cases [ 146 ], is linked to a defect in chromosome 14 and myoclonus [ 147 , 148 ], and, therefore, it is referred to as a distinctive autosomal dominant inherited form of AD [ 140 ]. Usually, people with Down’s syndrome are more prone to EOAD [ 149 ]. In fact, mutations in APP genes, particularly PSEN1 and PSEN2 , are associated with EOAD development [ 140 ]. These APP variants explain 5–10% of the autosomal dominant inherited cases, leaving the majority of cases totally unexplained [ 150 , 151 ].
Based on heredity, AD can be either familial or sporadic [ 152 ]. Compiling the two diagnostic criteria (i.e., age and hereditability), AD can be classified into EOAD, LOAD, early onset familial (eFAD), late-onset familial, early onset sporadic, and late-onset sporadic. Yet, these classifications overlap in terms of nomenclature, diagnosis, and treatment. FAD is linked to genes [ 153 ].
Main types of Alzheimer’s disease based on age and heredity.
| Classification | Genetic Factors | Age Onset | Clinical Features | Risk Factors | Top Treatments | References |
|---|---|---|---|---|---|---|
| Early-onset | Yes | 40s–50s | Plaques of amyloid and tau proteins | Family history | Acetylcholinesterase inhibitors (Donepezil, Galantamine, and Rivastigmine) | [ ] |
| Late-onset | Yes (APOE) | ≥65 | (APOE) ε4 allele | Age ≥ 65 years, genetic and environmental factors | Acetylcholinesterase inhibitors (Donepezil, Galantamine, and Rivastigmine) and treatment of vascular risk factors and sleep and mood disorders | [ ] |
| Familial | Yes (PSEN1, PSEN2, APP) | 40s–50s | Mutations in PSEN1, PSEN2, and APP | Family history | Acetylcholinesterase inhibitors (Donepezil, Galantamine, and Rivastigmine) | [ , , ] |
4. Alzheimer’s Disease Diagnosis
AD is a dual clinicopathological condition, which means that two requirements must be met for the definite diagnosis: (1) the presence of a clinical phenotype characterized by symptoms, such as episodic memory impairment or involvement of other cognitive, behavioral, and neuropsychiatric domains and (2) the development of neurological changes, such as the accumulation of NFTs and Aβ plaques in the brain. NFTs and Aβ plaques can be detected only through autopsy; as such, clinical diagnosis relies heavily on the observation of behaviors that are compatible with known clinical features of AD [ 41 , 158 ] and the exclusion of other potential causes [ 9 ]. The guidelines of neuropathological assessment of autopsy samples for the definitive diagnosis of AD have been published by the National Institute on Aging and the Alzheimer’s Association in 2012 [ 159 ].
In fact, it remains challenging to discriminate AD from other neuropathological dementia despite the advances in research protocols and current diagnostic tools [ 11 ]. Currently, AD diagnosis is based on confirming memory loss and cognitive impairments using neurological tests, such as the Montreal Cognitive Assessment (MOCA) [ 160 ] and Mini-Mental Status Examination (MMSE) [ 161 ]. However, the ultimate AD diagnostic protocol can only be performed post-mortem to detect Aβ and tau NFTs in brains of deceased patients [ 11 ]. The current limitations in AD diagnostics burden the development of effective AD treatments since it depends on signs and symptoms, whereas the accurate status of the brain can only be assessed post-death [ 162 ]. Currently, scientists are suggesting epigenetics alterations could be exploited as diagnostic surrogates for AD [ 7 , 11 , 12 , 13 , 14 , 15 , 16 , 18 , 163 , 164 , 165 , 166 , 167 , 168 , 169 , 170 , 171 ].
5. Epigenetic Changes in Alzheimer’s Disease
Epigenetic modifications have emerged as significant contributors in AD pathogenesis, mediating promises for AD treatment [ 7 , 11 , 12 , 13 , 14 , 15 , 16 , 18 , 163 , 164 , 165 , 166 , 167 , 168 , 169 , 170 , 171 ]. Various epigenetic changes, including mitochondrial epigenetics (i.e., mitoepigenetics), DNA methylation and hydroxymethylation, noncoding RNA translation, and histone post-translational modifications have been implicated in AD development [ 11 ]. Disruption of both DNA methylation and DNA hydroxymethylation processes has been implicated in many diseases that are classified as neuropathologies, including AD [ 172 ]. Interestingly, the key genes involved in AD pathogenesis are regulated by miRNAs and DNA methylation [ 11 , 13 ]. Other studies reported that there is an overlap between distinctively methylated DNA spots in AD and histone signatures in H3K27me3 and H3K4me3 in the Polycomb-repressed (poised) promoter [ 173 ]. Further studies declared that indistinguishable 5-methylcytosine (5mC) models are observed in AD patients associated or not associated with schizophrenia [ 174 ].
5.1. DNA Methylation
DNA methylation retains fundamental cellular functions and synaptic elasticity in the CNS, and it influences cognitive processes [ 175 ]. DNA hydroxymethylation is essential for neurodevelopment and is concentrated in the CNS, which further signifies the importance of DNA methylation [ 175 ]. Some studies showed that overall DNA methylation is decreased in AD patients [ 176 , 177 , 178 , 179 ], while other studies recorded no significant differences in DNA methylation between AD and age-matched healthy individuals [ 180 , 181 ]. DNA methylation patterns that were in connection with AD were investigated for the following genes: glycogen synthase kinase 3 beta ( GSK3b ) [ 182 , 183 ], ankyrin 1 ( ANK1 ) [ 184 ], TREM2 [ 17 ], and brain-derived neurotrophic factor ( BDNF ) [ 185 ]. ANK1 methylation has been increased in AD patients [ 186 , 187 , 188 , 189 ]. An increase in DNA methylation has been also recorded in the dorsolateral prefrontal cortex [ 187 ], entorhinal cortex [ 190 ], temporal cortex [ 190 ], temporal gyrus [ 191 ], and the hippocampus. Contrarily, a decrease in DNA methylation has been reported in locus coeruleus, prefrontal cortex [ 192 , 193 ], and blood samples [ 194 ]. Additionally, studies showed that 13% of noncoding RNA CpG motifs were methylated in AD patients, leading to a significant increase in 5mC levels in these genetic loci in particular [ 190 ].
5.2. Mitochondrial DNA Methylation
Mitochondria generate the energy (ATP) required to vitalize the cell’s reactions, and AD has been postulated to be associated with energetic decrease arising from mitochondrial disorder. Dysfunction of the mitochondrial oxidative phosphorylation and energy-producing cascade increases in reactive oxygen species (ROS) generation and apoptosis such that both are implicated in neurodegeneration and disease development [ 11 , 12 , 13 , 16 , 195 , 196 , 197 ]. Studies reported that multiple considerable deletions are detected in mitochondrial DNA (mtDNA) and are linked with AD pathogenesis [ 198 ]. In addition, mutations of mitochondrial rRNA and tRNAs [ 199 , 200 ], cytochrome C oxidase [ 200 , 201 ], and the regulatory D-loop influence mtDNA copy number, transcription, and translation [ 202 ]. Low levels of mtDNA were observed in AD patients having low Aβ and high tau in the cerebrospinal fluid (CSF) and in presymptomatic patients having PSEN1 mutation [ 203 ]. Low mtDNA copy number and abnormal propagation of mitochondria were associated with low level of mtDNA in CSF that might act as a biomarker for AD in the preclinical phase [ 203 ]. Another study demonstrated a positive correlation between Aβ and CSF mtDNA content but a negative correlation between phosphorylated tau protein and CSF mtDNA levels [ 204 ]. Low levels of CSF mtDNA accompanied by low Aβ and high phosphorylated tau assist in distinctive AD diagnosis against other neurological disabilities [ 204 ]. Studies revealed an increase in methylation of mtDNA at CpG and non-CpG repeats of D-loop of AD entorhinal cortex with Braak stages I to II and III to IV [ 192 , 205 ]. However, a significant decrease in mtDNA methylation in AD blood samples was detected [ 197 ].
5.3. DNA Hydroxymethylation
Studies revealed that thousands of distinguishable hydroxymethylated regions (DhMRs) in AD brains are associated with an increase of 5hmC levels in intragenic regions [ 206 , 207 , 208 ]. Genomic studies reported an increase in 5-hydroxymethylcytosine (5hmC) levels in the F-box and leucine rich motif protein 16 (FBXL16) gene [ 186 ]. FBXL16 was reported as a potential AD-associated gene, showing an encoding decrease in microglia cells of mouse AD models [ 209 ]. Another study reported a decrease in 5hmC levels in four CPG repeats in ANK1 [ 186 ]. Diverse studies confirmed a decrease in 5hmC levels in the AD entorhinal cortex, cerebellum [ 173 ], and CA3 region of the hippocampus [ 210 ] but an increase in 5hmC levels in AD brains’ parahippocampal gyrus [ 211 ], middle frontal gyrus, and middle temporal gyrus [ 212 ]. Further studies reported an increase in tau protein deposition and a decrease in astrocytes location [ 213 ], whereas one investigation recorded that 5hmC is not localized in AD cerebellum and entorhinal cortex [ 214 ]. Another study declared a decrease in 5hmC deposition in AD glial cells of hippocampus CA1 region [ 210 ]. Further study investigated the effect of TREM2 on AD pathogenesis and found there is a positive association between 5hmC repeats in exon 2 of TREM2 and TREM2 expression, postulating that an increase in gene expression might assist in tissue repair [ 215 ]. TREM2 is encoded in microglia cells and is required in tissue repair, homeostasis, and natural immunity reaction [ 215 ].
5.4. Histone Modifications
Histones (H1, H2A, H2B, H3, and H4) are biochemically highly basic proteins rich in arginine and lysine residues. Histones serves as a scaffold, assisting DNA to wrap and condense in eukaryotic nucleus forming nucleosomes [ 216 , 217 ]. Histone modifications are involved in neuronal differentiation and growth, older individuals’ brains homeostasis, and in AD pathology [ 7 , 11 , 12 , 13 , 14 , 16 , 18 , 164 , 165 , 169 , 171 , 204 , 218 ]. A prevalent lack of heterochromatin was detected in human AD, tau transgenic Drosophila, and mice [ 219 ]. Oxidative stress and DNA deterioration were associated with transgenic tau expression and heterochromatin relaxation [ 219 ].
Histone modifications, such as abnormal acetylation, were linked with aberrant signaling, apoptosis, inflammation, immunity, and neuroplasticity [ 220 ]. Histone acetylation was detected in postmortem AD brains [ 221 , 222 , 223 ]. A decrease in histone acetylation was observed in AD temporal lobes [ 224 ]. Acetylation of lysine 16 on histone H4 (H4K16ac) is implicated in aging and DNA damage, and such deterioration was previously observed in AD cortex patients [ 218 , 225 ]. However, acetylation of lysine 12 on histone H4 (H4K12ac) was accompanied with memory disturbance [ 218 ]. Higher H4K12ac content was detected in MCI but not detected in AD, confirming its role in an infant stage of disease development and aggregation deposition [ 15 ]. High levels of acetylated histone as well as H3 and H4 were detected in human post-mortem AD brains [ 225 ]. In addition, higher levels of histone deacetylases (HDACs), particularly class I (HDAC2 and HDAC3), were observed in AD brains’ regions that are involved in memory, learning, and neural plasticity. HDACs are linked with cognitive impairments and synaptic functions [ 171 , 218 ]. On the contrary, other investigations declared a decrease in HDACs in dysfunction brains’ regions that are associated with MCI symptoms [ 11 ]. In addition, class II HDACs are implicated in AD pathogenesis [ 226 ]. An increase in HDAC6 level was discovered in AD brains’ cortex and hippocampus and in AD animal models [ 226 ]. HDAC6 influences tau phosphorylation and degradation as well as tubulin acetylation, and it mediates inflammatory processes [ 169 , 227 ]. A decrease in HDAC6 level results in higher clearance and decrease of tau aggregation and consequently assists in nerve survival [ 220 , 228 ], while an increase in HDAC6 level leads to a decrease in α-tubulin acetylation and subsequently disrupts microtubules’ homeostasis and mitochondrial as well as vesicular transport [ 220 , 228 ]. A decrease in HDAC4 content, another member of class II HDACs, adversely influences learning and memory development [ 229 ]. HDAC4 is involved in neural function, and its increase results in apoptosis, whereas its decrease inhibits nerve cell death [ 229 ]. Class III HDACs, sirtuins (SIRTs), are involved in synaptic elasticity and memory functions as well as AD pathogenesis [ 227 ]. Studies reported that levels of SIRT1 are reduced in the parietal cortex, whereas SIRT1 levels in AD cerebellum are not reduced [ 171 ]. Such expression aberrations, probing Aβ and tau deposition, as well as acetylation of lysine 28 of tau protein, lead to tau aggregation [ 171 , 220 ]. Aberrations in histone methylation have been detected in AD patients as well [ 230 ]. The levels of histone methyltransferases (HMT) and histone demethylases are significant for brain vitality and memory function [ 230 ]. Studies declared that an increase in trimethylation of lysine residue on histone H3 (H3K9), a biomarker of gene silencing and heterochromatin condensation [ 231 ], and overexpression of histone lysine methyltransferase 1 (EHMT1) are observed in post-mortem AD brains [ 232 ]. The G9a HMT, an enzyme responsible for demethylation of lysine 9 on H3 (H3K9), is involved in cognitive function in mice; however, H3K4 demethylase contributes to human memory deficiency [ 230 ]. Studies observed that an increase in phosphorylation of serine 10 on H3 (H3S10), detected in AD hippocampal neurons [ 233 ], and an increase in phosphorylation of serine 139 on H2AX, detected in AD astrocytes, might serve as indicators of DNA damage [ 234 ]. ADP ribosylation of H1 was detected in AD brains [ 235 ]. Altogether, these results shed light on histone aberration in AD pathogenesis and motivate more researchers to explore the complexity of such factor.
5.5. MicroRNA
Diverse microRNAs (miRNAs) target genes are implicated in AD pathogenesis [ 11 , 12 , 13 , 14 , 16 , 18 , 169 , 171 ]. There are approximately 161 miRNAs that could contribute to AD pathogenesis, while ten miRNAs have been linked to AD, including miRNA-9, miRNA-29, miRNA-34, miRNA-107, miRNA-125, miRNA-132/-212, miRNA-146, miRNA-155, miRNA-181, and miRNA-206 [ 190 ]. Additionally, specific miRNAs were related to myelin sheath formation and others were involved in AD development, such as SIRT1, BACE1, and APP [ 190 ]. miRNAs are also involved in APP degradation and Aβ metabolism by modulating the activity of APP-degrading enzymes, such as BACE1 [ 236 ]. Furthermore, many miRNAs were found to regulate BACE1 expression, such as miRNA-124, miRNA-135b, miRNA-195, miRNA-15b, miRNA-29c, and miRNA-399-5p [ 237 , 238 , 239 , 240 ]. Other miRNAs, such as miRNA-219, regulate microtubule-associated protein tau (MAPT) gene ( MAPT ), while others, such as miRNA-124-3p and miRNA-125b, modulate the activity of kinases that are involved in the phosphorylation of tau protein [ 183 , 241 , 242 , 243 ].
BDNF, or abrineurin, expressed by BDNF , is a potential regulator of synaptic elasticity and transmission that induces miRNA-132 expression [ 244 ]. Studies reported that miRNA-132 and miRNA-212 encoding is suppressed in the early AD stage [ 245 , 246 ]. Other studies declared that miRNA-9 modulates neural progenitor cells’ growth, differentiation, and migration [ 247 , 248 ]. In addition, miRNA-9 upregulates ACE1 [ 249 ], and subsequently, increases Aβ formation and accumulation [ 250 ].
It was found that downregulation of miRNA-9 modulates calcium/calmodulin-dependent protein kinase kinase 2 (CAMKK2) expression [ 251 ], resulting in an increase in phosphorylated tau and Aβ deposition through CAMKK2-cyclic adenosine monophosphate-activated protein kinase (AMPK) cascade [ 183 , 252 ]. Modulation of BACE1 encoding is also carried by miRNA-29, implying that an increased level of BACE1 is associated with a decrease in the level of miRNA-29 [ 253 , 254 ]. Studies declared that miRNA-29 regulates neuron navigator 3 ( NAV3 ) that is overexpressed in AD frontal cortexes [ 255 ]. Studies showed that both miRNA-34 and tau mRNA are upregulated in AD, suggesting a linked mechanism for AD pathogenesis [ 256 ]. Studies demonstrated that miRNA-107 expression is suppressed in AD CNS and blood, particularly at the begging of AD. Further studies exhibited negative association between miRNA-107 expression and BACE1, inferring that BACE1 mRNA could modulate miRNA-107 [ 257 ]. In addition, miRNA-107 regulates cyclin-dependent kinase 5 ( CDK5 ) that is responsible for CNS integrity and function [ 258 ]. Studies showed that higher levels of miRNA-125 stimulate tau hyperphosphorylation, resulting in promoting mitogen-activated protein kinase/extracellular signal-regulated kinases (MAPK/ERK) signaling and increasing p53 expression [ 183 , 241 ]. Studies revealed that miRNA-132/-212 was linked with cognitive function and was suppressed in AD brains [ 259 ]. Studies reported that miRNA-146 expression is modulated by nuclear factor kappa-B (NF-κB), and the overexpression of miRNA-146 paves the way for NF-κB to downregulate the translation of complement factor H (CFH) and subsequently influence the inflammatory reaction in CNS [ 260 ].
Studies showed that the overexpression of some microRNAs (miRNA-155, miRNA-146, and miRNA-124) is associated with over production of APP and Aβ [ 261 ]. Studies revealed that miRNA-181 was suppressed in AD CNS [ 262 ]. Further investigations showed that the downregulation of miRNA-181 is associated with higher level of Aβ expression [ 262 ]. Furthermore, the downregulation of miRNA-181 influences MAPK signaling cascade [ 262 ]. Other investigations reported that miRNA-206 is overexpressed in AD CSF and blood [ 51 , 263 ].
6. Alzheimer’s Disease Biomarkers
Biomarkers are important tools for the accurate diagnosis of many diseases, including AD. Despite the recent advances in diagnostic methodology for Alzheimer’s disease, differentiation of Alzheimer’s dementia from other forms of dementia remains challenging. The analysis of Aβ-42, total tau protein, and phosphorylated tau (p-tau) from cerebrospinal fluid (CSF) is currently considered the best-established biological marker for the diagnosis of AD as well as differentiation from mild cognitive impairment and other types of dementia. The familiar AD biomarkers are the reduced levels of Aβ in CSF and the appearance of Aβ or tau depositions in the brains of AD patients [ 264 , 265 , 266 ]. Additionally, biomarker evidence obtained through PET can be used to attribute the clinical syndrome of dementia or MCI to underlying AD pathology, with varying probability [ 264 ]. In most cases, AD diagnosis in living patients continues to rely on the patient’s clinical history, family members with neuropsychological conditions, and the observance of symptom progression over time [ 41 ].
Before the early 2000s, the only sure way to know whether a person had AD or another form of dementia was after death through autopsy. Today, we have 12,073 biomarkers linked to AD. An overview of these biomarkers is provided in Figure 3 a–d. Approximately 441 biomarkers are either approved or in late-stage clinical studies for AD diagnosis, prognosis, staging, and monitoring of disease progression. The most widely used AD biomarkers are Aβ42 (the major component of amyloid plaques in the brain), tau, and phospho-tau (major components of tau tangles in the brain) [ 267 ]. These biomarkers are measured in CSF, which is the clear fluid that surrounds the brain and spinal cord, providing protection and insulation.

An overview of Alzheimer’s disease biomarkers in all stages of clinical development. Biomarkers are presented by ( a ) type of biomarker, ( b ) highest phase of biomarker development, ( c ) clinical phase of development for clinical biomarkers, and ( d ) according to the biomarker use. Data source: Cortellis Drug Discovery Intelligence, 10 October 2022, https://www.cortellis.com/drugdiscovery/ © 2022 Clarivate. All rights reserved.
In May 2022, the US FDA authorized the use of Lumipulse G beta-Amyloid Ratio (1-42/1-40) in vitro diagnostic test for the assessment of beta-amyloid pathology in CSF samples [ 268 ]. The ratio of these two proteins in CSF is indicative of the presence of amyloid plaques. The test is minimally invasive, and it is the first FDA-authorized in vitro diagnostic biomarker for use in individuals being evaluated for AD and other causes of cognitive decline. However, results of the test must be interpreted in conjunction with other patient clinical information. Additionally, Aβ42 levels measured in plasma have been evaluated as a potential biomarker for AD since it is less invasive to sample plasm than CSF. All recommended/approved AD biomarkers for disease diagnosis and prognosis are list in Table 2 .
Highest validity Alzheimer’s disease biomarkers listed according to their biomarker uses in Alzheimer’s disease.
| Biomarker Name | Population | Role | Highest Use Validity | Gene Symbol | |
|---|---|---|---|---|---|
| 1 | Amyloid beta A4 protein | Mild Cognitive Impairment | Risk Factor | Recommended/Approved | APP |
| 2 | Amyloid beta A4 protein | All | Diagnosis | Recommended/Approved | APP |
| 3 | Amyloid beta A4 protein | Early Onset | Diagnosis | Recommended/Approved | APP |
| 4 | Apolipoprotein E | Mild Cognitive Impairment | Risk Factor | Recommended/Approved | APOE |
| 5 | beta-amyloid protein 42 | Mild Cognitive Impairment | Risk Factor | Recommended/Approved | |
| 6 | beta-amyloid protein 42 | All | Diagnosis | Recommended/Approved | |
| 7 | Glucose transporters and hexokinases | Mild Cognitive Impairment | Risk Factor | Recommended/Approved | |
| 8 | Glucose transporters and hexokinases | All | Diagnosis | Recommended/Approved | |
| 9 | Microtubule-associated protein tau | Mild Cognitive Impairment | Risk Factor | Recommended/Approved | MAPT |
| 10 | Microtubule-associated protein tau | All | Diagnosis | Recommended/Approved | MAPT |
| 11 | Presenilin-1 | All | Diagnosis | Recommended/Approved | PSEN1 |
| 12 | Presenilin-1 | Early Onset | Diagnosis | Recommended/Approved | PSEN1 |
| 13 | Presenilin-2 | All | Diagnosis | Recommended/Approved | PSEN2 |
| 14 | Presenilin-2 | Early Onset | Diagnosis | Recommended/Approved | PSEN2 |
7. Anti-Alzheimer’s Drugs
There are currently 868 anti-Alzheimer’s drugs in different stages of development. However, only 273 drugs are currently under active development by biotech and/or pharma with evidence of active development in the last 6 months, according to Cortellis Drug Discovery Intelligence [ 269 ]. The most effective drugs currently approved for AD management are listed in Table 3 , and they comprise cholinesterase inhibitors (donepezil, rivastigmine, and galantamine) and the N -methyl-D-aspartate (NMDA) receptor antagonist (glutamate antagonist) memantine [ 41 , 70 ]. All of these drugs offer symptomatic treatments.
Approved symptomatic pharmacological treatments for patients with AD.
| Drug | Drug Targets | Managed Symptoms | Mechanism of Action | Disease Stage |
|---|---|---|---|---|
| Donepezil [ , , , , ] | AChE | Improves cognition and behavior | Cholinesterase inhibitor; inhibition of various aspects of glutamate-induced excitotoxicity; the reduction of early expression of inflammatory cytokines; the induction of a neuroprotective isoform of AChE; the reduction of oxidative stress-induced effects | Mild to moderate AD |
| Rivastigmine [ , , , ] | AChE; BChE | Improves cognitive functions and daily life activities | Cholinesterase inhibitor; increases cholinergic function | Mild to moderate AD |
| Galantamine [ , , ] | AChE; nicotinic ACh receptor | Improves behavioral symptoms, daily life activities, and cognitive functions | Cholinesterase inhibitor; binds to α-subunit of nicotinic ACh receptors and activates them | Mild to moderate AD |
| Memantine [ , ] | NMDA receptor | Improves learning and memory | NMDA receptor antagonist (prevents over-activation of glutaminergic system that is involved in neurotoxicity in AD patients) | Moderate to severe AD |
AChE: acetylcholine esterase; AD: Alzheimer’s disease; BChE: butyrylcholinesterase; ACh: acetylcholine; and NMDA: N -methyl-D-aspartate.
7.1. Drugs under Active Development
There are currently 273 drugs under active development for the treatment of AD, including small molecules, biotechnology products, peptides, combinations, and herbal materials ( Figure 4 a). The majority of these drugs target Aβ42 precursor protein (24.9%), followed by APP (18.7%), MAPT (10.6%), acetylcholinesterase (AChE) (3.3%), cholinergic receptor muscarinic 1 (CHRM1) (3.3%), NMDA receptor (2.2%), tumor necrosis factor (TNF) (2.2%), 5-hydroxytryptamine receptor 6 (5-HTR6), cholinergic receptor muscarinic 4 (CHRM4) (1.8%), glucagon-like peptide 1 receptor (GLP1R), insulin (1.5%), sigma non-opioid intracellular receptor 1 (SIGMAR1) (1.5%), sodium channel (1.5%), and 5-hydroxytryptamine receptor 4 (5-HTR4) (1.1%). Drug count details are shown in Figure 4 b. Additionally, the top organizations developing these drugs as well as the development status of these drugs are shown in Figure 4 c and Figure 4 d, respectively.

An overview of Alzheimer’s disease drug discovery pipeline under active development by ( a ) drug type, ( b ) top targets, ( c ) top organizations, and ( d ) development status. Under active development, according to Cortellis Drug Discovery Intelligence (CDDI) [ 40 ] database as of 10 October 2022. Data source: Cortellis Drug Discovery Intelligence, 10 October 2022, https://www.cortellis.com/drugdiscovery/ © 2022 Clarivate. All rights reserved.
Recently, monoclonal antibodies (mAbs) have revived the hope for AD treatments. Aducanumab, an mAb, targets Aβ aggregates in AD patients’ brains to decrease their formation [ 10 , 277 ]. In 2021, aducanumab was approved for AD and prescribed for individuals with AD-MCI and mild AD dementia [ 10 , 277 ]. It is a humanized recombinant monoclonal antibody to Aβ. In a clinical study on 165 patients, aducanumab demonstrated significant reduction of soluble and insoluble Aβ. Furthermore, aducanumab reduced AD clinical decline measured by Mini-Mental State Examination scores. At 12-month follow-up, cerebral Aβ disappeared from almost 50% of patients diagnosed with mild AD. A Phase III clinical trial on 1638 patients of aducanumab has been terminated due to safety and efficacy issues [ 278 ]. In addition, two monoclonal antibodies, donanemab and lecanemab, are currently under US Food and Drug Administration (FDA) investigations [ 277 , 279 , 280 , 281 ].
The pro-drug of methylene blue, leuco-methylthioninium, is a second-generation tau aggregation inhibitor (TAI) and the only tau-specific agent to undergo Phase III clinical trials. Two Phase III clinical trials were conducted in 2016 to demonstrate the efficacy of different doses of leuco-methylthioninium and to compare the efficacy of monotherapy compared with combination with cholinesterase inhibitors or memantine. A third clinical trial to demonstrate the efficacy of low dose leuco-methylthioninium is still active and recruiting to date [ 282 ]. Anti-tau monoclonal antibody (tau vaccine) is an IgG4 antibody that targets aggregated tau protein. Preclinical and Phase I clinical trial data demonstrated that it was safe and might present a potential agent for treating AD. A 96-week Phase II safety and efficacy trial (453 participants with AD) was conducted. Recruitment has completed (in August 2022), but the final study report is not yet published [ 283 ]. Gosuranemab is a therapeutic mAb for the N-terminal of extracellular tau. Gosuranemab was demonstrated to be safe and effective in a single ascending dose study. Gosuranemab has been investigated through a Phase II clinical trial (654 participants with MCI or mild AD). However, the study was terminated due to lack of efficacy following the placebo-controlled period readout [ 284 ]. Semorinemab is another antibody that targets the extracellular tau. Promising results were concluded from a pilot safety study, and currently, a Phase II clinical trial (272 patients with moderate AD) to investigate cognitive function and functional capacities of patients is still active [ 285 ]. Zagotenemab, an mAb, binds to tau aggregates. Phase I clinical trial (single dose) was conducted on zagotenemab in patients with mild AD [ 277 ].
7.2. Withdrawn, Discontinued, or Suspended Drugs
There are 108 drugs that have been either withdrawn, discontinued, or suspended from use for AD. The majority of these drugs were small molecules, but there were some biotechnology products and few peptides ( Figure 5 a). Many of the drug targets ( Figure 5 b) are similar to drug targets under active development for AD, which may give the impression that those drug targets may not be successful for the treatment of the disease, especially since many of the big pharmaceutical companies have abandoned them, including Pfizer, Sanofi, Lilly, AstraZeneca, and others ( Figure 5 c). Table 4 summarizes the major failures and suggests hypotheses explaining them.

An overview of Alzheimer’s disease drugs that were either suspended, withdrawn, or discontinued by ( a ) drug type, ( b ) top targets, and ( c ) top organizations. Under active development, according to Cortellis Drug Discovery Intelligence (CDDI) [ 40 ] database as of 10 October 2022. Data source: Cortellis Drug Discovery Intelligence, 10 October 2022, https://www.cortellis.com/drugdiscovery/ © 2022 Clarivate. All rights reserved.
Most important drug classes that failed as anti-AD treatments in different stages of clinical trials.
| Drug Category | Classification | Why Suggested | Why Failed |
|---|---|---|---|
| Monoclonal Antibodies (mABs) | Disease-modifying | These antibodies target the amyloid protein, and they predominate drug discovery efforts [ ]. Amyloid has been considered a promising drug target since it is located outside the nerve cells, and it is toxic to the brain’s tissues [ ]. | The mABs have not succeeded in eradicating AD because cognitive impairment predisposing dementia does not associate with amyloid precipitation [ ]. |
| Gamma (γ-) Secretase Inhibitors | Disease-modifying | It was proposed that targeting γ-secretase might reduce amyloid production, particularly Aβ42 isoform [ , , , ]. Phase II trials showed a dose-dependent decrease in both Aβ isoforms (Aβ40 and Aβ42) without significant decrease in tau protein, though the magnetic resonance imaging (MRI) recorded a cerebral atrophy following such treatment [ , ]. Patients showed some improvement at the beginning of treatment. | No distinct response of improvement nor worsening could be traced after 3 months of treatment [ , ]. Side effects were reported with higher doses, such as skin rashes, nausea, and diarrhea, accompanied by higher rate of skin cancer [ , ]. Furthermore, the narrow therapeutic window impeded their proceeding to Phase III [ , ]. |
| Tau Inhibitors | Disease-modifying | The tau protein appeared as a potential target for AD dementia since an irregular phosphorylation of tau results in neurofibrillary tangle formation [ , , ]. Clinical studies reported that AD progress is related to tangle formation more than that of Aβ [ ]. Initially, tau aggregation inhibitors (TAIs) showed better response. | After long-term treatment (approximately 15 months), TAIs failed in AD treatment. Moreover, 15% of patients showed minor improvement without any co-administered therapy [ ]. |
| Neurochemical Enhancers | Symptomatic | Idalopiridine that inhibits 5-hydroxytryptamine 6 (5-HT6) receptors and consequently enhances the release of acetylcholine in the brain, i.e., pro-cholinergic effector [ , ]. Encenicline incites cholinergic response through activating α-7 nicotinic acetylcholine receptors [ , , ]. | Further clinical studies declared that Idalopiridine does not show any promising effect in AD treatment [ , ]. Side effects of Encenicline were observed in Phase II trials at the maximum dose (2 mg) [ , , ]. In addition, the Phase III trials, with doses of 2–3 mg, were terminated due to GI toxicity and eventually discontinued because there was no improvement in cognitive function [ , , ]. |
| Miscellaneous | Symptomatic | Dimebon is a histamine (H1) antagonist [ ]. It affects α-adrenergic and serotonergic receptors, AMPA and NMDA glutamate receptors, and L-type voltage-gated calcium channels [ ]. | It exerted a better response in AD patients and one Phase II trial in Russia [ ], but it failed in Phase III trials in Austria, Europe, New Zealand, and the US [ ]. |
8. Exploiting Network Biology Approaches in Alzheimer’s Disease Research
Network biology approaches have been suggested as paradigm-changing approaches for the discovery of disease biomarkers, drug targets, and effective drugs for polygenic multifactorial diseases, including cancer, diabetes, psychological disorders, and AD. However, the typical focus on one single type of omics has been a limiting factor for the success of previous systems biology studies because the findings were explaining only a modest portion of the complex disease, and AD was no exception. Therefore, future studies should study multiple omics data simultaneously and apply new technologies, including machine leaning (ML) and artificial intelligence (AI) to derive novel multi-system and multi-target hypotheses.
8.1. Previous Alzheimer’s Disease Drug Discovery Failures
Misunderstanding of the disease mechanisms coupled with inconsistent drug development protocols that relied on single-target approaches, in addition to the improper management of drug discovery projects, led to the inopportune nomination of drug targets which contributed to many drug failures [ 3 , 286 ]. Additionally, clinical trial design utilized in drug discovery failed due to many reasons, including the delay in initiation of treatments, incorrect drug doses, or lack of good drug-monitoring biomarkers [ 287 , 288 ]. The success rate in progressing AD clinical trials from one phase to the next has been poor, and the number of therapeutic agents approaching FDA approval is low [ 289 ]. Failures in clinical trials might be due to ineffective treatments, drug side effects, or misconducted trials [ 289 ]. The improper selection of methodological parameters in clinical trial design [ 290 ] impeded the success of previous clinical trials [ 287 , 288 , 289 , 291 ]. In fact, the clinical trials dilemma in psychiatry, neurology, and AD has been discussed elsewhere by many researchers [ 292 , 293 , 294 ]. Issues including inaccuracy, incorrectness, and bias hindered clinical trials success [ 295 , 296 ]. Other factors included personal errors, drawbacks in rating scales, and limitations in neuropsychological tests leading to errors regarding the underestimation of the clinical outcome in clinical trials [ 290 , 297 ]. Increasing the number of clinical trials investigating drug effects has been associated with better treatment outcome [ 289 ].
Additionally, limitations in cell-based models to probe neurodegenerative diseases, such as AD, contributes to AD failure treatment [ 298 ]. The complexity of CNS motivates researchers to integrate the molecular basis of neurodegenerative diseases with the unique organization and construction of brain tissue [ 298 ]. This combined approach is displayed via 3D cell models accompanied by microfluidic technology, which are in their early stages and ready for improvement [ 298 ]. Subsequently, this integrative system should enrich the preclinical drug development pipeline [ 298 ]. The biodiversity of AD-drug design and development needs to unify healthcare workers’ and scientists’ efforts [ 289 ]. Other factors that played an important part in the failure of many AD drug development programs were improper diagnostic evaluations, elusive genetic factors, and/or concomitant diseases [ 299 , 300 ].
8.2. Network Biology Approaches Hold the Promise to Revolutionize Alzhiemer’s Disease Research
AD is a complex disease associated with multiple perturbations in biological networks and functional network connectivity that are fundamental for normal physiological function; hence, multi-target treatment approaches seem imperative to treat the disease [ 301 , 302 , 303 ]. Studies have reported that numerous brain functional networks are significantly impaired in AD patients, including the control network (CON), default mode network (DMN), dorsal attention network (DAN), salience network (SAL), and sensory–motor network (SMN) [ 304 ]. In mild AD patients, there is evidence indicating reduced functional network connectivity in the brain is a predisposing factor. Additionally, the DMN is impaired in very mild to mild AD patients, while severe AD patients suffer from disrupted network crosstalk [ 304 ]. Thus, network and systems biology approaches that target multiple disease networks and pathways hold great promise to revolutionize AD drug discovery research.
Furthermore, network biology approaches enable the identification of novel disease biomarkers, including quantitative diagnostic and prognostic biomarkers, imaging, and biochemical tests. Novel validated disease biomarkers could potentially equip AD researchers with the proper tools to accurately differentiate between AD and non-AD dementias, which can positively impact drug discovery efforts, clinical trial design, and patient selection for clinical trials [ 305 ]. There is agreement among scientists [ 1 , 2 , 3 , 4 , 5 , 22 , 306 ] that future AD research should focus on the following: (1) reassessing previous and current prevalent AD pathogenesis hypotheses, (2) identifying effective disease-specific biomarkers, (3) re-evaluating previous disease diagnostic standards, (4) considering new guidelines and procedures for disease control, (5) reorienting drug discovery efforts toward employing approved multi-target approaches and pharmacogenetic hypotheses, (6) updating the managerial requirements for drug design and development, (7) applying pharmacogenomics approaches in biomarker and drug discovery and development, and (8) implementing disease-prevention strategies for susceptible individuals.
8.3. Current Network Biology Efforts
The underlying hypothesis of network medicine has been recruited in the development of multi-target ligands and combination drugs [ 21 ]. The multi-target ligands and combination drugs are considered promising network medicines for challenging and complex diseases [ 307 , 308 ]. Clinical studies showed that multi-target ligands and combined drugs are more effective than single-target drugs in complex diseases treatment, including depression, cancer, and infectious diseases, such as the acquired immunodeficiency syndrome (AIDS) [ 309 , 310 , 311 , 312 ]. Combining donepezil and memantine improve the brain’s cognition function, and patient’s overall status in mild and advanced AD. Additionally, such drugs decrease the rate of clinical decay and are safe and tolerable [ 307 ].
The idea of network medicine is based on the hypothesis that diseases occur due to the disruption of biological networks responsible for homeostasis as a result of activation or deactivation of certain proteins or biochemical reactions, which eventually disturb the balance of normal physiology pathways [ 313 , 314 , 315 ]. Hence, disease networks are complex disease processes that are caused by irregular diverse genes, proteins, and signaling cascades [ 308 ]. Therefore, network medicines intend to restore disrupted disease networks to their default normal physiology status by targeting multiple key effectors in disease pathways [ 308 ].
Recent advances in multi-omics data analysis coupled with advancements in computational chemical biology methods led to better disease understanding. As a result, network medicines have been suggested as potential surrogates for identifying effective treatments for complex diseases, including AD [ 301 , 302 , 303 , 316 ]. Additionally, the application of network approaches to AD research projects has shed light on a crosstalk among diverse signaling pathways involved in AD pathogenesis [ 21 ]. Further work is required to lay the groundwork for the development of the next-generation anti-AD drugs. Furthermore, the diverse disease networks could not be revived through targeting of a single protein and/or signaling cascade because there are numerous active and spare cellular mechanisms in biological systems [ 317 ].
8.4. Multi-Target-Directed Ligands as Network Biology Treatments
The Multi-Target-Directed Ligands (MTDLs) approach is one of the most promising therapeutic interventions for AD patients as well as other complex multifactorial diseases, including cancer, diabetes, and other psychological disorders [ 318 , 319 , 320 ]. The design of MTDL hypothesizes that successful disease-modifying treatments of AD should target systems biology pathways rather than selectively targeting individual proteins or drug targets [ 321 , 322 ]. As such, MTDLs can be defined as drugs and/or technologies designed to interact with more than one target involved in the pathogenesis of a defined disease [ 322 , 323 , 324 , 325 , 326 , 327 , 328 ], surpassing the “one-molecule, one-target” model [ 313 , 319 ]. It has been theorized that potent MTDL should simultaneously target the typical signs of AD, such as the irregular accumulation of Aβ peptides [ 329 , 330 , 331 , 332 , 333 ], tauopathies [ 334 , 335 , 336 , 337 ], and the cholinergic insufficiency in CNS [ 106 , 338 , 339 ]. In addition, the effective MTDL should consider other AD features, such as the oxidative and nitrosative stresses [ 117 , 340 , 341 ], inflammatory response of brain and spinal cord, excitotoxicity [ 342 ], mitochondrial dysfunction [ 327 , 343 , 344 ], aberrances in calcium [ 345 , 346 ] and other metals [ 19 , 267 , 347 ], and irregularities in apolipoproteins [ 348 , 349 ].
It is suggested that the rational design of MTDLs can be achieved by two approaches: (1) drug repurposing, considering drug design methods that take into account the biological fingerprints (or biological spectra) of familiar active drugs against other therapeutic receptors where one or more drugs can modulate several targets [ 269 , 350 , 351 , 352 , 353 ] and (2) fragment-based drug design, which is based on the core structures of active compounds against specific targets to generate a new merged scaffold with dual or multiple activity against two or more targets [ 269 ].
In the first approach, compounds are screened against multiple proteins/drug targets to retrieve hits with the desired biological profiles [ 350 , 351 , 352 ]. The main advantage of this approach is that the investigated compounds are often commercially available and clinically proven to be safe, thus reducing development time and costs [ 269 , 350 , 351 ], and most importantly, the proposed lead might act as a synergistic effector, modulating the disease pathway effectively [ 354 ]. However, the optimization protocol of the biological activity of the lead compound to fit the new disease application has been limited [ 269 , 350 , 351 ]. Therefore, more work is required to improve hit identification and lead optimization. Sometimes the pharmacokinetics properties of the lead hinder the application to new diseases such as AD where drugs have to meet the criteria for CNS drug design [ 355 , 356 ]. The latter, fragment-based, MTDL approach can be designed using three main methods: (1) linking active fragments/compounds using a linker/spacer and keeping known pharmacophoric features [ 269 ], (2) fusing or integrating the active compounds to generate a new chemical entity that shares identical features [ 269 ], and (3) merging/mixing the selected bioactive compounds to yield a scaffold that has the key functionalities of the pharmacophore [ 269 ].
Studies indicated that the major impedance of the MTDL success is the need to maintain or boost the biological activity of the prioritized compounds while preserving drug-like properties [ 40 ]. Many MTDLs may have limitations due to lower selectivity towards some drug targets [ 357 , 358 , 359 , 360 , 361 , 362 ], while drug development efforts focusing on increasing the biological activity of MTDL may increase the risk of drug toxicity [ 357 , 358 , 359 , 360 , 361 , 362 ]. Therefore, MTDLs should be optimized by improving the selectivity towards certain protein targets while reducing drug toxicity [ 359 , 360 , 361 , 362 ]. Additionally, the designed chimeric entities using the fragment approach have higher molecular weights than the parent compounds, which may affect drug-like properties, while at other times, the merging protocol might be a promising solution for developing oral bioavailable drugs [ 363 , 364 , 365 ].
Finally, when considering MTDL, it is crucial to pay special attention to the required physicochemical properties, including pharmacokinetics, pharmacodynamics, hydrophilicity, and hydrophobicity [ 269 ]. MTDL design against neurodegenerative disorders should take into account the drug’s blood–brain barrier permeability [ 355 , 356 ].
8.5. Suggested Disease Biomarkers and Disease Modifying Drugs
Known diagnostic and prognostic biomarkers for AD [ 269 ] significantly enrich pathways involved in inflammation and immune regulation. AD biomarkers can be divided into two groups: 168 EOAD biomarkers [ 154 , 366 ] and 932 LOAD biomarkers [ 367 , 368 ]. There are 69 biomarkers that overlap between EOAD and LOAD: ACO2, ACTB, ACTG1, ADAM10, ADIPOQ, ADRA1A, AIF1, APP, ANG, ACE, APOE, ABCA7, ATP6V1B2, ATP2A2, AURKC, AXL, BACE1, CACNA1G, CD33, CLP1, CLU, CR1, DICER1, DUSP13, DNMBP, FNDC5, GRK5, GBA1, GRN, H3C1, H3C10, H3C11, H3C12, H3C2, H3C3, H3C4, H3C6, H3C7, H3C8, IL1B, IL6, IL6R, KIF5A, HLA-DRA, MTHFR, MAPT, MBP, NSF, NDRG4, NRGN, NCSTN, NSUN2, PAK1, PLD3, PSEN1, RTN3, SLC10A3, SLC12A5, SLC24A4, SORBS2, SORL1, SPARCL1, TCIRG1, TYROBP, TREM2, TNF, YWHAG, VSNL1, and VWA2.
In order to get a better idea of these 69 overlapping biomarkers, we used the compared experiment workflow in Metacore [ 39 ] to compare enrichments results in pathway maps for EOAD and LOAD biomarkers. We found that the top enriched pathway map by common disease biomarkers for EOAD and LOAD is “protein-folding and maturation related to angiotensin system maturation” ( Figure 6 ).

Top enriched pathway map for ‘common’ biomarkers of EOAD and LOAD. The pathway map representing protein-folding and maturation for the angiotensin system maturation. Connections between network objects on the map are referred to as links (or edges). A link identifies an interaction or a logical relation between two nodes. The type of interaction or relation is reflected by an appropriate symbol placed in the middle of the link. B = binding (i.e., physical interaction between molecules); C = cleavage of a protein at a specific site, yielding distinctive peptide fragments and carried out by enzymes or compounds; red arrows = inhibition; green arrows = activation; grey arrows = unspecified action; solid purple arrows = emergence in disease; light violet text box = normal process; pink text box = pathological processes; white text box with blue outline = notes; grey block = reaction; blue block = normal process; pink block = pathological process; starred network objects = groups or complex processes; red thermometers on pathway map = network object is a validated biomarker for AD, according to the Cortellis Drug Discovery Intelligence (CDDI) database [ 40 ]; thermometer 1 for EOAD, and thermometer 2 for LOAD; a pink hexagon with a capital D on the upper left side of the network object indicates an AD biomarker according to MetaCore TM ; a green hexagon on the upper left side of the network object with a capital T indicates that the network object is expressed in the brain. Map generated using MetaCore TM version 21.4. MetaCore TM , a Cortellis™ solution, 14 October 2022, © 2022 Clarivate. All rights reserved.
LOAD biomarkers led to more significant enrichments of immune system and allergic response pathways, apoptosis, tissue remodeling and repair, cell differentiation, cell cycle regulation, and neurofibromatosis [ 366 ], while EOAD led to more significant enrichments of heart failure pathway maps, stem cells, spermatogenesis, lipid biosynthesis regulation, and blood clotting pathways [ 366 ].
9. Artificial Intelligence and Machine Learning Approaches
Machine learning (ML) and artificial intelligence (AI) have been used successfully to extract insight from ‘big’ biological data [ 369 , 370 , 371 ]. Domain expertise from biology, genetics, elderly medicine, psychiatry, psychology, neurology, and neuroscience could be combined with new bioinformatics and statistical analytical tools to gain insight from multi-omics data. Such insight is valuable for providing answers for challenging research questions, and it can be achieved through the use of theoretical modeling [ 372 , 373 ]. In AD research, ML and AI can answer critical questions about combination diagnostic biomarkers, AD patient subgroups, and disease pathogenesis, thus supporting the identification of a personalized treatments for AD patients [ 374 , 375 ]. In fact, the use of AI has been suggested to probe the pathogenesis mechanisms of AD by analyzing big multi-omics data in parallel [ 376 , 377 ]. Additionally, AI has the capability to differentiate AD patients from other patients suffering from non-AD cognition impairment. It can also anticipate the progression from MCI to AD dementia and assign a tailored treatment for each individual patient [ 376 ]. Furthermore, the application of ML and AI approaches to AD research data, can lead to novel hypotheses regarding efficient interventions for AD patients [ 376 ]. AI can also aid in the diagnosis of the early stage of dementia [ 268 ].
Many research efforts focused on utilizing ML and AI approaches to mine data from clinicaltrials.gov records to evaluate anti-AD therapeutics in different stages of clinical development to study their mechanisms of action and important clinical trial characteristics [ 10 , 372 , 378 , 379 , 380 , 381 , 382 ]. AI and ML approaches can lead to important discoveries by learning from the recent advances in clinical trials and anti-Alzheimer’s drug development pipelines [ 383 , 384 , 385 , 386 , 387 , 388 ]. Complex AI-based models could be exploited to inform researchers and health care providers about diverse disease etiologies, effective diagnostic biomarkers [ 375 ], and individualized treatments based on network biology approaches [ 389 , 390 ].
10. Exploring Epigenetic Treatments
Studies showed that DNA methylation/hydroxymethylation is dysregulated in AD patients prior the onset of clinical symptoms [ 11 ]. These were presented in a prospective study on autopsied brains, as level of methylation, in terms of 5mC levels, in presymptomatic patients is similar to those with AD patients [ 189 ]. The levels of 5mC, 5hmC, and ten–eleven translocation 1 (TET1) proteins were elevated in preclinical AD patients and AD patients compared with the control group [ 171 , 211 ]. Although further validation is required, DNA methylation/hydroxymethylation may be used as a biomarker for AD diagnosis [ 11 ].
Histone modifications, particularly acetylation, deacetylation, and methylation dysregulation, play a role AD pathogenesis [ 11 ]. HDACs are highly expressed in patients with AD [ 171 , 218 ], affecting learning, memory, and cognition; hence, HDAC inhibitors (HDACi) are considered a potential treatment option [ 391 ]. Studies on AD patients showing low histone acetylation were reported [ 211 , 224 , 225 ], allowing the potential use of histone acetyltransferases (HATs) [ 211 ]. Increased levels of histone methylation and histone methyltransferase enzyme mRNA were reported in postmortem brains of AD patients [ 11 , 218 ]. Although the loss of histone methyltransferase function would affect learning capabilities in AD patients [ 11 ], the use of partial histone methyltransferase inhibitors [ 211 ] would restore the balance between histone methylation and demethylation in patients with AD to maintain brain integrity and memory [ 230 ]. Inhibitor of histone acetyltransferases (INHAT) is reported to bind to histones and block their access to HATs [ 392 ]. Studies showed that ANP32A, which is a component of INHAT and inhibitor of protein phosphatase-2A, is upregulated in AD patients [ 393 , 394 ]. In an in vivo study, the down regulation of ANP32A would reduce INHAT formation and allow for histone acetylation [ 395 ]. Collectively, drugs from those classes would comprise potential therapeutic options for AD treatment.
HDACi are considered to be non-selective [ 220 ], but they are beneficial, as they reduce AD hallmarks [ 225 ]. The use of HDACi that selectively inhibits HDAC2 and HDAC3 would improve cognition, in contrast to inhibiting HDAC1 that would result in neurotoxicity [ 11 , 225 ]. HDAC6 selective inhibitors were also shown to have neuroprotective effects [ 228 , 229 ]. Sirtuins, which are a class of HDACs, contribute to AD pathogenesis and selective inhibitors would also be beneficial [ 227 , 229 ]. Although some HATs showed better response than non-selective HDACi, their low membrane permeability and solubility limit their use in AD treatment [ 11 ].
The miRNAs are responsible for the regulation of gene expression through post-transcriptional gene silencing [ 396 ]. In relation to AD, several studies summarized by Nikolac Perkovic et al., 2021 [ 11 ] showed that miRNAs would be either downregulated or upregulated, altering proteins and enzymes expression responsible for AD pathology. Hence, the use of miRNA mimics to downregulate the expression of genes or proteins [ 397 ] or anti-miRNA therapies to alter the function of a specific miRNA [ 398 ] are also considered potential treatment options for AD patients.
11. Genetic Treatments
Targeting genetic alterations in AD patients and consequent gene editing and correction is another potential treatment strategy. These include the use of programmable nucleases, such as zinc finger proteins (ZFP), transcription activator-like effectors (TALE), and RNA-guided clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9) [ 399 ]. The latter showed more promising results for AD treatment and other neurological diseases than did ZFP and TALE [ 400 , 401 , 402 ]. The presence of the mutant Cas9 protein, dead Cas9 (dCas9), advanced the CRISPR/Cas9 editing tool, resulting in the emergence of CRISPR interference (CRISPRi) and CRISPR activation (CRISPRa) technologies, in which dCas9 is fused or interacts with transcriptional repressors or activators, respectively [ 403 ]. With regard to epigenetics, AD, and dCas9 protein, studies showed promising results with targeting histone demethylase [ 404 ], histone acetyltransferase [ 405 ], and histone methyltransferases [ 406 , 407 ].
12. Non-Pharmacological Treatment Options and Preventive Measures
Non-pharmacological treatments encompass several recommendations for various lifestyle modifications, including physical and social activity, tobacco cessation, alcohol consumption, weight management, nutrition, and regular exercise. Other interventions include underlying-disease management (e.g., hypertension, diabetes, dyslipidemia, depression, and hearing loss), as stated in WHO guidelines [ 408 ]. More studies should assess the relationship between vaccines and AD; it was found that flu vaccines reduce the risk of AD development [ 409 ]. However, the protective mechanisms have not yet been elucidated.
Aberrations in the ecosystem of microbiome have been implicated in diverse gastrointestinal and metabolic dysfunction, such as diabetes, insulin resistance, obesity, and inflammatory bowel disease [ 410 ]. In addition, studies showed that changes in gut microbiome is associated with neurological disorders, such as multiple sclerosis (MS), autism, and Parkinson’s disease [ 411 , 412 , 413 ]. Studies recorded a decrease in microbial diversity in gut microbiome of AD patients [ 414 , 415 , 416 , 417 , 418 , 419 , 420 , 421 ]. Further studies in rats suggested that alterations in gut microbiome might proceed Aβ deposition [ 422 ].
13. Special Considerations for Clinical Trials
Aspects to be considered when designing a clinical trial include trial rationale, outcomes of interest, statistical analysis design, sample size and recruitment, and interim monitoring [ 423 ]. Common clinical trial designs include single-arm trials, placebo-controlled trials, crossover trials, and factorial trials [ 424 ]. In AD-related clinical trials, infrastructure and technology, cultures and linguistics, regulatory and reimbursement issues, academia and industry harmonization, availability, and access were considered to be the ultimate challenges that limit the conducting of successful clinical trials [ 425 ].
According to NLM’s ClinicalTrials.gov Beta (beta.clinicaltrials.gov), 109 clinical trials related to AD were terminated in the last ten years [ 19 ]. AD clinical trials were terminated due to the following reasons: unavailability of further funding, halted visits due to COVID-19, feasibility of enrolment, safety issues, slow recruitment of eligible participants (patients), inappropriate study design to achieve the trial’s endpoint, new safety or efficacy data from other studies, unfavourable risk–benefit ratio, and inappropriate dosage settings. Yet, patient recruitment remains the ultimate determinant in AD clinical trials.
Therefore, there is a need for new and advanced clinical trials designs to accelerate passage through the legal authorities’ requirements to register new promising molecules for treatment and/or prevention of AD. However, new investigation approaches need to be fully validated before they can be implemented in clinical trials [ 426 ].
14. Conclusions
AD is a multifactorial and polygenetic disease. Novel disease diagnostic biomarkers and disease-modifying treatments are required to halt or slow the onset and disease progression, decrease behavioral aberrations, and ameliorate cognition in AD patients. The recent advances in network biology approaches coupled with the advances in clinical trial design and protocols, in additional to the availability of powerful machine learning and artificial intelligence algorithms, hold promise to identify novel diagnostic biomarkers, better drug targets, and effective disease-modifying drugs. Herein, we provide a comprehensive review on Alzheimer’s disease highlighting the mainstream hypotheses explaining disease pathophysiology as well as current disease treatments and drug discovery projects. We also emphasize the recent scientific evidence implicating epigenetic mechanisms and the microbiome in AD pathogenesis and progression. We suggest that the application of Al and ML approaches in analyzing AD network biology derived from AD data, including genetic, transcriptomic, epigenetic, and metagenomic data would revolutionize our understanding of the disease pathways and will lead to the discovery of novel biomarkers and drug targets. Ultimately, these studies will increase our chances of identifying validated diagnostic biomarkers and effective disease-modifying cures. Hence, breakthrough discoveries in AD research are more likely to occur in the near future. This review provides a summary of the current hypotheses regarding AD pathogenesis in addition to the most recent advances in the search of effective disease biomarkers and drug targets. This review also details AD drugs in various stages of development and highlights technologies that are expected to accelerate AD drug and biomarker discoveries.
Acknowledgments
The authors acknowledge funding from the Deanship of Scientific Research at Al-Zaytoonah University of Jordan (Grant number 2020-2019/17/03).
Funding Statement
R.H. and D.A.S. acknowledge support from the Deanship of Scientific Research at Al-Zaytoonah University of Jordan (Grant number 2020-2019/17/03).
Author Contributions
Conceptualization, R.H.; software, R.H.; formal analysis, R.H.; investigation, R.H., D.A.S., O.H.A. and A.Q.A.B.; resources, R.H., D.A.S., O.H.A. and A.Q.A.B.; biomarker data curation, R.H.; writing—original draft preparation, R.H., D.A.S., O.H.A. and A.Q.A.B.; writing—review and editing, R.H., D.A.S., O.H.A. and A.Q.A.B.; visualization, R.H.; supervision, R.H.; project administration, R.H.; funding acquisition, R.H. and D.A.S. All authors have read and agreed to the published version of the manuscript.
Informed Consent Statement
Not applicable.
Data Availability Statement
Conflicts of interest.
The authors declare no conflict of interest.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
- Search by keyword
- Search by citation
Page 1 of 34
Central autonomic network dysfunction and plasma Alzheimer’s disease biomarkers in older adults
Higher order regulation of autonomic function is maintained by the coordinated activity of specific cortical and subcortical brain regions, collectively referred to as the central autonomic network (CAN). Auto...
- View Full Text
Evaluating the inter-species transmission risk of amyloid beta peptide aggregates via ingestion
Recent reports suggest that amyloid beta (Aβ) peptides can exhibit prion-like pathogenic properties. Transmission of Aβ peptide and the development of associated pathologies after surgeries with contaminated i...
Effects of intensive lifestyle changes on the progression of mild cognitive impairment or early dementia due to Alzheimer’s disease: a randomized, controlled clinical trial
Evidence links lifestyle factors with Alzheimer’s disease (AD). We report the first randomized, controlled clinical trial to determine if intensive lifestyle changes may beneficially affect the progression of ...
Intermittent hypoxia training enhances Aβ endocytosis by plaque associated microglia via VPS35-dependent TREM2 recycling in murine Alzheimer’s disease
Beta-amyloid (Aβ) deposition in the brain parenchyma is a crucial initiating step in the amyloid cascade hypothesis of Alzheimer’s disease (AD) pathology. Furthermore, dysfunction of plaque-associated microgli...

Bayesian genome-wide TWAS with reference transcriptomic data of brain and blood tissues identified 141 risk genes for Alzheimer’s disease dementia
Transcriptome-wide association study (TWAS) is an influential tool for identifying genes associated with complex diseases whose genetic effects are likely mediated through transcriptome. TWAS utilizes referenc...
Locus coeruleus integrity and left frontoparietal connectivity provide resilience against attentional decline in preclinical alzheimer’s disease
Autopsy work reported that neuronal density in the locus coeruleus (LC) provides neural reserve against cognitive decline in dementia. Recent neuroimaging and pharmacological studies reported that left frontop...
Integrating a multimodal lifestyle intervention with medical food in prodromal Alzheimer’s disease: the MIND-AD mini randomized controlled trial
The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) showed cognitive benefits from a multidomain lifestyle intervention in at-risk older people. The LipiDiDiet tria...
Cholinesterase inhibitors and memantine are associated with a reduced mortality in nursing home residents with dementia: a longitudinal observational study
A large proportion of nursing home (NH) residents suffer from dementia and effects of conventional anti-dementia drugs on their health is poorly known. We aimed to investigate the associations between exposure...
Combining plasma Aβ and p-tau217 improves detection of brain amyloid in non-demented elderly
Maximizing the efficiency to screen amyloid-positive individuals in asymptomatic and non-demented aged population using blood-based biomarkers is essential for future success of clinical trials in the early st...
Experimental colitis in young Tg2576 mice accelerates the onset of an Alzheimer’s-like clinical phenotype
Systemic inflammation and neuroinflammation affect the natural course of the sporadic form of Alzheimer’s disease (AD), as supported by epidemiological and preclinical data, and several epidemiological studies...
Photobiomodulation in experimental models of Alzheimer’s disease: state-of-the-art and translational perspectives
Alzheimer’s disease (AD) poses a significant public health problem, affecting millions of people across the world. Despite decades of research into therapeutic strategies for AD, effective prevention or treatm...
Plasma trimethylamine N-oxide (TMAO): associations with cognition, neuroimaging, and dementia
The gut-derived metabolite Trimethylamine N-oxide (TMAO) and its precursors - betaine, carnitine, choline, and deoxycarnitine – have been associated with an increased risk of cardiovascular disease, but their ...
Blood biomarkers of neurodegeneration associate differently with amyloid deposition, medial temporal atrophy, and cerebrovascular changes in APOE ε4-enriched cognitively unimpaired elderly
Alzheimer’s disease (AD) is characterized by the accumulation of amyloid-β (Aβ) plaques, neurofibrillary tau tangles, and neurodegeneration in the brain parenchyma. Here, we aimed to (i) assess differences in ...
The impact of incident stroke on cognitive trajectories in later life
Cognitive impairment is common after stroke, and a large proportion of stroke patients will develop dementia. However, there have been few large prospective studies which have assessed cognition both prior to ...
Comparison of plasma and neuroimaging biomarkers to predict cognitive decline in non-demented memory clinic patients
Plasma biomarkers of Alzheimer’s disease (AD) pathology, neurodegeneration, and neuroinflammation are ideally suited for secondary prevention programs in self-sufficient persons at-risk of dementia. Plasma bio...
Cholecystokinin B receptor agonists alleviates anterograde amnesia in cholecystokinin-deficient and aged Alzheimer's disease mice
As one major symptom of Alzheimer’s disease (AD), anterograde amnesia describes patients with an inability in new memory formation. The crucial role of the entorhinal cortex in forming new memories has been we...
Proximity extension assay in cerebrospinal fluid identifies neurofilament light chain as biomarker of neurodegeneration in sporadic cerebral amyloid angiopathy
Sporadic cerebral amyloid angiopathy (sCAA) is a disease characterised by the progressive deposition of the amyloid beta (Aβ) in the cerebral vasculature, capable of causing a variety of symptoms, from (mild) ...
Plasma biomarkers increase diagnostic confidence in patients with Alzheimer’s disease or frontotemporal lobar degeneration
The recent development of techniques to assess plasma biomarkers has changed the way the research community envisions the future of diagnosis and management of Alzheimer’s disease (AD) and other neurodegenerat...
Serum urate levels and neurodegenerative outcomes: a prospective cohort study and mendelian randomization analysis of the UK Biobank
Previous studies on the associations between serum urate levels and neurodegenerative outcomes have yielded inconclusive results, and the causality remains unclear. This study aimed to investigate whether urat...
Updated safety results from phase 3 lecanemab study in early Alzheimer’s disease
Alzheimer disease (AD) is a major health problem of aging, with tremendous burden on healthcare systems, patients, and families globally. Lecanemab, an FDA-approved amyloid beta (Aβ)-directed antibody indicate...
Association between untreated and treated blood pressure levels and cognitive decline in community-dwelling middle-aged and older adults in China: a longitudinal study
Optimal blood pressure (BP) levels to reduce the long-term risk of cognitive decline remains controversial. We aimed to investigate the association between BP and anti-hypertensive treatment status with cognit...
Cerebrospinal fluid α-synuclein adds the risk of cognitive decline and is associated with tau pathology among non-demented older adults
The role of α-synuclein in dementia has been recognized, yet its exact influence on cognitive decline in non-demented older adults is still not fully understood.
Older adults at greater risk for Alzheimer’s disease show stronger associations between sleep apnea severity in REM sleep and verbal memory
Obstructive sleep apnea (OSA) increases risk for cognitive decline and Alzheimer’s disease (AD). While the underlying mechanisms remain unclear, hypoxemia during OSA has been implicated in cognitive impairment...
Selective targeting and modulation of plaque associated microglia via systemic hydroxyl dendrimer administration in an Alzheimer’s disease mouse model
In Alzheimer’s disease (AD), microglia surround extracellular plaques and mount a sustained inflammatory response, contributing to the pathogenesis of the disease. Identifying approaches to specifically target...
Association between retinal microvascular abnormalities and late-life brain amyloid-β deposition: the ARIC-PET study
Retinal microvascular signs are accessible measures of early alterations in microvascular dysregulation and have been associated with dementia; it is unclear if they are associated with AD (Alzheimer’s disease...
The relation of a cerebrospinal fluid profile associated with Alzheimer’s disease with cognitive function and neuropsychiatric symptoms in sporadic cerebral amyloid angiopathy
Patients with sporadic cerebral amyloid angiopathy (sCAA) frequently report cognitive or neuropsychiatric symptoms. The aim of this study is to investigate whether in patients with sCAA, cognitive impairment a...
Clinical validity of the Italian adaptation of the Uniform Data Set Neuropsychological Test Battery (I-UDSNB) in Mild Cognitive Impairment and Alzheimer’s Disease
The identification and staging of Alzheimer’s Disease (AD) represent a challenge, especially in the prodromal stage of Mild Cognitive Impairment (MCI), when cognitive changes can be subtle. Worldwide efforts w...
Combined in vivo MRI assessment of locus coeruleus and nucleus basalis of Meynert integrity in amnestic Alzheimer’s disease, suspected-LATE and frontotemporal dementia
The locus coeruleus (LC) and the nucleus basalis of Meynert (NBM) are altered in early stages of Alzheimer’s disease (AD). Little is known about LC and NBM alteration in limbic-predominant age-related TDP-43 e...
Irregular word reading as a marker of semantic decline in Alzheimer’s disease: implications for premorbid intellectual ability measurement
Irregular word reading has been used to estimate premorbid intelligence in Alzheimer’s disease (AD) dementia. However, reading models highlight the core influence of semantic abilities on irregular word readin...
N, N-Dimethyltryptamine, a natural hallucinogen, ameliorates Alzheimer’s disease by restoring neuronal Sigma-1 receptor-mediated endoplasmic reticulum-mitochondria crosstalk
Aberrant neuronal Sigma-1 receptor (Sig-1r)-mediated endoplasmic reticulum (ER)- mitochondria signaling plays a key role in the neuronal cytopathology of Alzheimer’s disease (AD). The natural psychedelic N, N-...
Alzheimer’s and neurodegenerative disease biomarkers in blood predict brain atrophy and cognitive decline
Although blood-based biomarkers have been identified as cost-effective and scalable alternatives to PET and CSF markers of neurodegenerative disease, little is known about how these biomarkers predict future b...
Utilization of fluid-based biomarkers as endpoints in disease-modifying clinical trials for Alzheimer’s disease: a systematic review
Clinical trials in Alzheimer’s disease (AD) had high failure rates for several reasons, including the lack of biological endpoints. Fluid-based biomarkers may present a solution to measure biologically relevan...
Effects of risk factors on the development and mortality of early- and late-onset dementia: an 11-year longitudinal nationwide population-based cohort study in South Korea
Early-onset dementia (EOD, onset age < 65) and late-onset dementia (LOD, onset age ≥ 65) exhibit distinct features. Understanding the risk factors for dementia development and mortality in EOD and LOD respecti...
Association of midlife body-weight variability and cycles with earlier dementia onset: a nationwide cohort study
Given the rising awareness of health-related lifestyle modifications, the impact of changes in body weight (BW) on cognitive function and dementia generates significant concern. This study aimed to investigate...
Associations of plasma neurofilament light chain with cognition and neuroimaging measures in community-dwelling early old age men
Plasma neurofilament light chain (NfL) is a promising biomarker of neurodegeneration with potential clinical utility in monitoring the progression of neurodegenerative diseases. However, the cross-sectional as...
Alteration of medial temporal lobe metabolism related to Alzheimer’s disease and dementia with lewy bodies
Association of medial temporal lobe (MTL) metabolism with Alzheimer’s disease (AD) and dementia with Lewy bodies (DLB) has not been evaluated considering their mixed disease (MD).
Exploring morphological similarity and randomness in Alzheimer’s disease using adjacent grey matter voxel-based structural analysis
Alzheimer’s disease is characterized by large-scale structural changes in a specific pattern. Recent studies developed morphological similarity networks constructed by brain regions similar in structural featu...
Longitudinal validation of cognitive reserve proxy measures: a cohort study in a rural Chinese community
While evidence supports cognitive reserve (CR) in preserving cognitive function, longitudinal validation of CR proxies, including later-life factors, remains scarce. This study aims to validate CR’s stability ...
Serum and cerebrospinal fluid neurofilament light chain and glial fibrillary acid protein levels in early and advanced stages of cerebral amyloid Angiopathy
Neurofilament light chain (NFL) is a biomarker for neuroaxonal damage and glial fibrillary acidic protein (GFAP) for reactive astrocytosis. Both processes occur in cerebral amyloid angiopathy (CAA), but studie...
Who am I with my Lewy bodies? The insula as a core region of the self-concept networks
Dementia with Lewy bodies (DLB) is characterized by insular atrophy, which occurs at the early stage of the disease. Damage to the insula has been associated with disorders reflecting impairments of the most f...
Pathophysiology characterization of Alzheimer’s disease in South China’s aging population: for the Greater-Bay-Area Healthy Aging Brain Study (GHABS)
The Guangdong-Hong Kong-Macao Greater-Bay-Area of South China has an 86 million population and faces a significant challenge of Alzheimer’s disease (AD). However, the characteristics and prevalence of AD in th...
Prefrontal intra-individual ERP variability and its asymmetry: exploring its biomarker potential in mild cognitive impairment
The worldwide trend of demographic aging highlights the progress made in healthcare, albeit with health challenges like Alzheimer’s Disease (AD), prevalent in individuals aged 65 and above. Its early detection...
Synaptic vesicle glycoprotein 2 A in serum is an ideal biomarker for early diagnosis of Alzheimer’s disease
Previous studies have demonstrated that early intervention was the best plan to inhibit the progression of Alzheimer’s disease (AD), which relied on the discovery of early diagnostic biomarkers. In this study,...
Risk of conversion to mild cognitive impairment or dementia among subjects with amyloid and tau pathology: a systematic review and meta-analysis
Measurement of beta-amyloid (Aβ) and phosphorylated tau (p-tau) levels offers the potential for early detection of neurocognitive impairment. Still, the probability of developing a clinical syndrome in the pre...
Subclinical epileptiform discharges in Alzheimer’s disease are associated with increased hippocampal blood flow
In epilepsy, the ictal phase leads to cerebral hyperperfusion while hypoperfusion is present in the interictal phases. Patients with Alzheimer’s disease (AD) have an increased prevalence of epileptiform discha...
Biophysical models applied to dementia patients reveal links between geographical origin, gender, disease duration, and loss of neural inhibition
The hypothesis of decreased neural inhibition in dementia has been sparsely studied in functional magnetic resonance imaging (fMRI) data across patients with different dementia subtypes, and the role of social...
Neuroinflammation increases oxygen extraction in a mouse model of Alzheimer’s disease
Neuroinflammation, impaired metabolism, and hypoperfusion are fundamental pathological hallmarks of early Alzheimer’s disease (AD). Numerous studies have asserted a close association between neuroinflammation ...
Impact of amyloid and tau positivity on longitudinal brain atrophy in cognitively normal individuals
Individuals on the preclinical Alzheimer's continuum, particularly those with both amyloid and tau positivity (A + T +), display a rapid cognitive decline and elevated disease progression risk. However, limite...
Equine-assisted services for people living with dementia: a systematic review
Dementia has a significant impact on the social, physical, and psychological wellbeing of people living with dementia, their families and society. Animal-assisted interventions can have positive effects on the...
Use of a digital tool to support the diagnostic process in memory clinics–a usability study
Both memory clinic professionals and patients see value in digital tools, yet these hardly find their way to clinical practice. We explored the usability of a digital tool to support the diagnostic work-up in ...
- Editorial Board
- Instructions for Editors
- Sign up for article alerts and news from this journal
- Follow us on Twitter
- ISSN: 1758-9193 (electronic)
Alzheimer's Research & Therapy
ISSN: 1758-9193
- General enquiries: [email protected]
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Innovative therapeutic strategies in alzheimer’s disease: a synergistic approach to neurodegenerative disorders.

1. Introduction
2. alzheimer’s disease (ad), 3. multi-target drugs, 3.1. chemical-based drugs, 3.2. immune system-modulating drugs, 3.3. nanobodies, 3.3.1. fab fragments, 3.3.2. domain antibodies, 3.3.3. single-chain variable fragments (scfv), 3.4. antibody targeting, 3.5. mrna-based antibodies, 4. ai-driven multi-target drugs, 5. drug delivery across the bbb, strategies that aid drugs cross the blood–brain barrier, 6. conclusions, author contributions, conflicts of interest.
- Ciurea, A.V.; Mohan, A.G.; Covache-Busuioc, R.A.; Costin, H.P.; Glavan, L.A.; Corlatescu, A.D.; Saceleanu, V.M. Unraveling molecular and genetic insights into neurodegenerative diseases: Advances in understanding Alzheimer’s, Parkinson’s, and Huntington’s diseases and amyotrophic lateral sclerosis. Int. J. Mol. Sci. 2023 , 24 , 10809. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lamptey, R.N.L.; Chaulagain, B.; Trivedi, R.; Gothwal, A.; Layek, B.; Singh, J. A review of the common neurodegenerative disorders: Current therapeutic approaches and the potential role of nanotherapeutics. Int. J. Mol. Sci. 2022 , 23 , 1851. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Brett, B.L.; Gardner, R.C.; Godbout, J.; Dams-O’Connor, K.; Keene, C.D. Traumatic brain injury and risk of neurodegenerative disorder. Biol. Psychiatry 2022 , 91 , 498–507. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kakoti, B.B.; Bezbaruah, R.; Ahmed, N. Therapeutic drug repositioning with special emphasis on neurodegenerative diseases: Threats and issues. Front. Pharmacol. 2022 , 13 , 1007315. [ Google Scholar ] [ CrossRef ]
- Han, C.; Chaineau, M.; Chen, C.X.; Beitel, L.K.; Durcan, T.M. Open science meets stem cells: A new drug discovery approach for neurodegenerative disorders. Front. Neurosci. 2018 , 12 , 47. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Cummings, J.; Lee, G.; Nahed, P.; Kambar, M.; Zhong, K.; Fonseca, J.; Taghva, K. Alzheimer’s disease drug development pipeline: 2022. Alzheimer’s Dement. 2022 , 8 , e12295. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Doherty, T.; Yao, Z.; Khleifat, A.A.L.; Tantiangco, H.; Tamburin, S.; Albertyn, C.; Thakur, L.; Llewellyn, D.J.; Oxtoby, N.P.; Lourida, I.; et al. Artificial intelligence for dementia drug discovery and trials optimization. Alzheimer’s Dement. 2023 , 19 , 5922–5933. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Owen, M.; Bose, N.; Nisenbaum, L.; Partrick, K.A.; Fillit, H.M. The critical role of biomarkers for drug development targeting the biology of aging. J. Prev. Alzheimer’s Dis. 2023 , 10 , 729–742. [ Google Scholar ] [ CrossRef ]
- Li, S.; Yi, Y.; Cui, K.; Zhang, Y.; Chen, Y.; Han, D.; Sun, L.; Zhang, X.; Chen, F.; Zhang, Y.; et al. A single-chain variable fragment antibody inhibits aggregation of phosphorylated tau and ameliorates tau toxicity in vitro and in vivo. J. Alzheimer’s Dis. 2021 , 79 , 1613–1629. [ Google Scholar ] [ CrossRef ]
- Medeiros, R.; Baglietto-Vargas, D.; LaFerla, F.M. The role of tau in Alzheimer’s disease and related disorders. CNS Neurosci. Ther. 2011 , 17 , 514–524. [ Google Scholar ] [ CrossRef ]
- Kumari, S.; Mehta, S.L.; Li, P.A. Glutamate induces mitochondrial dynamic imbalance and autophagy activation: Preventive effects of selenium. PLoS ONE 2012 , 7 , e39382. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Xie, D.; Song, C.; Qin, T.; Zhai, Z.; Cai, J.; Dai, J.; Sun, T.; Xu, Y. Moschus ameliorates glutamate-induced cellular damage by regulating autophagy and apoptosis pathway. Sci. Rep. 2023 , 13 , 18586. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Glenner, G.G. The pathobiology of Alzheimer’s disease. Annu. Rev. Med. 1989 , 40 , 45–51. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Roher, A.E.; Lowenson, J.D.; Clarke, S.; Woods, A.S.; Cotter, R.J.; Gowing, E.; Ball, M.J. beta-amyloid-(1-42) is a major component of cerebrovascular amyloid deposits: Implications for the pathology of Alzheimer disease. Proc. Natl. Acad. Sci. USA 1993 , 90 , 10836–10840. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ow, S.Y.; Dunstan, D.E. A brief overview of amyloids and Alzheimer’s disease. Protein Sci. 2014 , 23 , 1315–1331. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Mathur, S.; Gawas, C.; Ahmad, I.Z.; Wani, M.; Tabassum, H. Neurodegenerative disorders: Assessing the impact of natural vs drug-induced treatment options. Aging Med. (Milton). 2023 , 6 , 82–97. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Wareham, L.K.; Liddelow, S.A.; Temple, S.; Benowitz, L.I.; Di Polo, A.; Wellington, C.; Goldberg, J.L.; He, Z.; Duan, X.; Bu, G.; et al. Solving neurodegeneration: Common mechanisms and strategies for new treatments. Mol. Neurodegener. 2022 , 17 , 23. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Verma, A.; Waiker, D.K.; Bhardwaj, B.; Saraf, P.; Shrivastava, S.K. The molecular mechanism, targets, and novel molecules in the treatment of Alzheimer’s disease. Bioorganic Chem. 2022 , 119 , 105562. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Cheong, S.L.; Tiew, J.K.; Fong, Y.H.; Leong, H.W.; Chan, Y.M.; Chan, Z.L.; Kong, E.W.J. Current pharmacotherapy and multi-target approaches for Alzheimer’s disease. Pharmaceuticals 2022 , 15 , 1560. [ Google Scholar ] [ CrossRef ]
- Medina-Franco, J.L.; Giulianotti, M.A.; Welmaker, G.S.; Houghten, R.A. Shifting from the single to the multitarget paradigm in drug discovery. Drug Discov. Today 2013 , 18 , 495–501. [ Google Scholar ] [ CrossRef ]
- Talevi, A. Multi-target pharmacology: Possibilities and limitations of the "skeleton key approach" from a medicinal chemist perspective. Front. Pharmacol. 2015 , 6 , 205. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Makhoba, X.H.; Viegas, C., Jr.; Mosa, R.A.; Viegas, F.P.D.; Pooe, O.J. Potential impact of the multi-target drug approach in the treatment of some complex diseases. Drug Des. Devel Ther. 2020 , 14 , 3235–3249. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Löscher, W. Single-target versus multi-target drugs versus combinations of drugs with multiple targets: Preclinical and clinical evidence for the treatment or prevention of epilepsy. Front. Pharmacol. 2021 , 12 , 730257. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kieburtz, K. Treating neurodegenerative disease before illness: A challenge for the 21st century. Lancet Neurol. 2016 , 15 , 540–541. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Sheikh, S.; Safia; Haque, E.; Mir, S.S. Neurodegenerative diseases: Multifactorial conformational diseases and their therapeutic interventions. J. Neurodegener. Dis. 2013 , 2013 , 563481. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Gu, X.; Zhang, G.; Qin, Z.; Yin, M.; Chen, W.; Zhang, Y.; Liu, X. Safinamide protects against amyloid β (Aβ)-induced oxidative stress and cellular senescence in M17 neuronal cells. Bioengineered 2022 , 13 , 1921–1930. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Dodge, H.H.; Arnold, S.E. One step forward to personalized medicine? Alzheimer’s Dement 2023 , 9 , e12435. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Alqahtani, T.; Deore, S.L.; Kide, A.A.; Shende, B.A.; Sharma, R.; Dadarao Chakole, R.; Nemade, L.S.; Kale, N.K.; Borah, S.; Deokar, S.S.; et al. Mitochondrial dysfunction and oxidative stress in Alzheimer’s disease, and Parkinson’s disease, Huntington’s disease and amyotrophic lateral sclerosis: An updated review. Mitochondrion 2023 , 71 , 83–92. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Cummings, J.; Aisen, P.S.; DuBois, B.; Frölich, L.; Jack, C.R., Jr.; Jones, R.W.; Morris, J.C.; Raskin, J.; Dowsett, S.A.; Scheltens, P. Drug development in Alzheimer’s disease: The path to 2025. Alzheimer’s Res. Ther. 2016 , 8 , 39. [ Google Scholar ] [ CrossRef ]
- Kabir, M.T.; Sufian, M.A.; Uddin, M.S.; Begum, M.M.; Akhter, S.; Islam, A.; Amran, M.S.; Md Ashraf, G. NMDA receptor antagonists: Repositioning of memantine as a multitargeting agent for Alzheimer’s therapy. Curr. Pharm. Des. 2019 , 25 , 3506–3518. [ Google Scholar ] [ CrossRef ]
- Uddin, M.S.; Kabir, M.T.; Tewari, D.; Mathew, B.; Aleya, L. Emerging signal regulating potential of small molecule biflavonoids to combat neuropathological insults of Alzheimer’s disease. Sci. Total Environ. 2020 , 700 , 134836. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Deardorff, W.J.; Grossberg, G.T. A fixed-dose combination of memantine extended-release and donepezil in the treatment of moderate-to-severe Alzheimer’s disease. Drug Des. Devel Ther. 2016 , 10 , 3267–3279. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kabir, M.T.; Uddin, M.S.; Mamun, A.A.; Jeandet, P.; Aleya, L.; Mansouri, R.A.; Ashraf, G.M.; Mathew, B.; Bin-Jumah, M.N.; Abdel-Daim, M.M. Combination drug therapy for the management of Alzheimer’s disease. Int. J. Mol. Sci. 2020 , 21 , 3272. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Morphy, R.; Kay, C.; Rankovic, Z. From magic bullets to designed multiple ligands. Drug Discov. Today 2004 , 9 , 641–651. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ma, X.H.; Shi, Z.; Tan, C.; Jiang, Y.; Go, M.L.; Low, B.C.; Chen, Y.Z. In-silico approaches to multi-target drug discovery: Computer aided multi-target drug design, multi-target virtual screening. Pharm. Res. 2010 , 27 , 739–749. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Chow, V.W.; Savonenko, A.V.; Melnikova, T.; Kim, H.; Price, D.L.; Li, T.; Wong, P.C. Modeling an anti-amyloid combination therapy for Alzheimer’s. Sci. Transl. Med. 2010 , 2 , 13ra1. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Pepeu, G.; Giovannini, M.G. Cholinesterase inhibitors and beyond. Curr. Alzheimer Res. 2009 , 6 , 86–96. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Pohanka, M. Acetylcholinesterase inhibitors: A patent review (2008-present). Expert. Opin. Ther. Pat. 2012 , 22 , 871–886. [ Google Scholar ] [ CrossRef ]
- Zheng, H.; Fridkin, M.; Youdim, M. From single target to multitarget/network therapeutics in Alzheimer’s therapy. Pharmaceuticals 2014 , 7 , 113–135. [ Google Scholar ] [ CrossRef ]
- Plazas, E.; Hagenow, S.; Avila Murillo, M.; Stark, H.; Cuca, L.E. Isoquinoline alkaloids from the roots of Zanthoxylum rigidum as multi-target inhibitors of cholinesterase, monoamine oxidase A and Aβ(1-42) aggregation. Bioorg Chem. 2020 , 98 , 103722. [ Google Scholar ] [ CrossRef ]
- Fang, Y.; Zhou, H.; Gu, Q.; Xu, J. Synthesis and evaluation of tetrahydroisoquinoline-benzimidazole hybrids as multifunctional agents for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2019 , 167 , 133–145. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- González-Naranjo, P.; Pérez-Macias, N.; Pérez, C.; Roca, C.; Vaca, G.; Girón, R.; Sánchez-Robles, E.; Martín-Fontelles, M.I.; de Ceballos, M.L.; Martin-Requero, A.; et al. Indazolylketones as new multitarget cannabinoid drugs. Eur. J. Med. Chem. 2019 , 166 , 90–107. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ivanova, L.; Karelson, M.; Dobchev, D.A. Multitarget approach to drug candidates against Alzheimer’s disease related to AChE, SERT, BACE1 and GSK3β protein targets. Molecules 2020 , 25 , 1846. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Garland, E.F.; Hartnell, I.J.; Boche, D. Microglia and astrocyte function and communication: What do we know in humans? Front. Neurosci. 2022 , 16 , 824888. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ji, K.; Miyauchi, J.; Tsirka, S.E. Microglia: An active player in the regulation of synaptic activity. Neural Plast. 2013 , 2013 , 627325. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Gao, C.; Jiang, J.; Tan, Y.; Chen, S. Microglia in neurodegenerative diseases: Mechanism and potential therapeutic targets. Signal Transduct. Target. Ther. 2023 , 8 , 359. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Toader, C.; Dobrin, N.; Brehar, F.M.; Popa, C.; Covache-Busuioc, R.A.; Glavan, L.A.; Costin, H.P.; Bratu, B.G.; Corlatescu, A.D.; Popa, A.A.; et al. From recognition to remedy: The significance of biomarkers in neurodegenerative disease pathology. Int. J. Mol. Sci. 2023 , 24 , 16119. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kempuraj, D.; Thangavel, R.; Natteru, P.A.; Selvakumar, G.P.; Saeed, D.; Zahoor, H.; Zaheer, S.; Iyer, S.S.; Zaheer, A. Neuroinflammation induces neurodegeneration. J. Neurol. Neurosurg. Spine 2016 , 1 , 1003. [ Google Scholar ] [ PubMed ]
- Doty, K.R.; Guillot-Sestier, M.V.; Town, T. The role of the immune system in neurodegenerative disorders: Adaptive or maladaptive? Brain Res. 2015 , 1617 , 155–173. [ Google Scholar ] [ CrossRef ]
- Ransohoff, R.M.; Schafer, D.; Vincent, A.; Blachère, N.E.; Bar-Or, A. Neuroinflammation: Ways in which the immune system affects the brain. Neurotherapeutics 2015 , 12 , 896–909. [ Google Scholar ] [ CrossRef ]
- Strzelec, M.; Detka, J.; Mieszczak, P.; Sobocińska, M.K.; Majka, M. Immunomodulation-a general review of the current state-of-the-art and new therapeutic strategies for targeting the immune system. Front. Immunol. 2023 , 14 , 1127704. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Miao, J.; Ma, H.; Yang, Y.; Liao, Y.; Lin, C.; Zheng, J.; Yu, M.; Lan, J. Microglia in Alzheimer’s disease: Pathogenesis, mechanisms, and therapeutic potentials. Front. Aging Neurosci. 2023 , 15 , 1201982. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Yeh, F.L.; Hansen, D.V.; Sheng, M. TREM2, microglia, and neurodegenerative diseases. Trends Mol. Med. 2017 , 23 , 512–533. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Gratuze, M.; Leyns, C.E.G.; Holtzman, D.M. New insights into the role of TREM2 in Alzheimer’s disease. Mol. Neurodegener. 2018 , 13 , 66. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Burmeister, A.R.; Marriott, I. The interleukin-10 family of cytokines and their role in the CNS. Front. Cell Neurosci. 2018 , 12 , 458. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lobo-Silva, D.; Carriche, G.M.; Castro, A.G.; Roque, S.; Saraiva, M. Balancing the immune response in the brain: IL-10 and its regulation. J. Neuroinflamm. 2016 , 13 , 297. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kwon, H.S.; Koh, S.H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020 , 9 , 42. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Food and Drug Administration. FDA Grants Accelerated Approval for Alzheimer’s Disease Treatment. Available online: https://www.fda.gov/news-events/press-announcements/fda-grants-accelerated-approval-alzheimers-disease-treatment (accessed on 24 February 2024).
- Silva, J.D.; Taglialatela, G.; Jupiter, D.C. Reduced prevalence of dementia in patients prescribed tacrolimus, sirolimus, or cyclosporine. J. Alzheimer’s Dis. 2023 , 95 , 585–597. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Hill, J.; Zawia, N.H. Fenamates as potential therapeutics for neurodegenerative disorders. Cells 2021 , 10 , 702. [ Google Scholar ] [ CrossRef ]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 2020 , 180 , 114147. [ Google Scholar ] [ CrossRef ]
- Song, C.; Shi, J.; Zhang, P.; Zhang, Y.; Xu, J.; Zhao, L.; Zhang, R.; Wang, H.; Chen, H. Immunotherapy for Alzheimer’s disease: Targeting β-amyloid and beyond. Transl. Neurodegener. 2022 , 11 , 18. [ Google Scholar ] [ CrossRef ]
- Khorassani, F.; Hilas, O. Bapineuzumab, an investigational agent for Alzheimer’s disease. Pharm. Ther. 2013 , 38 , 89–91. [ Google Scholar ]
- Abushouk, A.I.; Elmaraezy, A.; Aglan, A.; Salama, R.; Fouda, S.; Fouda, R.; AlSafadi, A.M. Bapineuzumab for mild to moderate Alzheimer’s disease: A meta-analysis of randomized controlled trials. BMC Neurol. 2017 , 17 , 66. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Gugliandolo, A.; Bramanti, P.; Mazzon, E. Role of vitamin E in the treatment of Alzheimer’s disease: Evidence from animal models. Int. J. Mol. Sci. 2017 , 18 , 2504. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Browne, D.; McGuinness, B.; Woodside, J.V.; McKay, G.J. Vitamin E and Alzheimer’s disease: What do we know so far? Clin. Interv. Aging 2019 , 14 , 1303–1317. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Grundman, M. Vitamin E and Alzheimer disease: The basis for additional clinical trials. Am. J. Clin. Nutr. 2000 , 71 , 630s–636s. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Numakawa, T.; Kajihara, R. Neurotrophins and other growth factors in the pathogenesis of Alzheimer’s disease. Life 2023 , 13 , 647. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Budni, J.; Bellettini-Santos, T.; Mina, F.; Garcez, M.L.; Zugno, A.I. The involvement of BDNF, NGF and GDNF in aging and Alzheimer’s disease. Aging Dis. 2015 , 6 , 331–341. [ Google Scholar ]
- Bathina, S.; Das, U.N. Brain-derived neurotrophic factor and its clinical implications. Arch. Med. Sci. 2015 , 11 , 1164–1178. [ Google Scholar ] [ CrossRef ]
- Gao, L.; Zhang, Y.; Sterling, K.; Song, W. Brain-derived neurotrophic factor in Alzheimer’s disease and its pharmaceutical potential. Transl. Neurodegener. 2022 , 11 , 4. [ Google Scholar ] [ CrossRef ]
- Muyldermans, S. Nanobodies: Natural single-domain antibodies. Annu. Rev. Biochem. 2013 , 82 , 775–797. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Abskharon, R.; Pan, H.; Sawaya, M.R.; Seidler, P.M.; Olivares, E.J.; Chen, Y.; Murray, K.A.; Zhang, J.; Lantz, C.; Bentzel, M.; et al. Structure-based design of nanobodies that inhibit seeding of Alzheimer’s patient-extracted tau fibrils. Proc. Natl. Acad. Sci. USA 2023 , 120 , e2300258120. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Zheng, F.; Pang, Y.; Li, L.; Pang, Y.; Zhang, J.; Wang, X.; Raes, G. Applications of nanobodies in brain diseases. Front. Immunol. 2022 , 13 , 978513. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Hamers-Casterman, C.; Atarhouch, T.; Muyldermans, S.; Robinson, G.; Hamers, C.; Songa, E.B.; Bendahman, N.; Hammers, R. Naturally occurring antibodies devoid of light chains. Nature 1993 , 363 , 446–448. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Moos, T.; Thomsen, M.S.; Burkhart, A.; Hede, E.; Laczek, B. Targeted transport of biotherapeutics at the blood-brain barrier. Expert. Opin. Drug Deliv. 2023 , 20 , 1823–1838. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Harmsen, M.M.; De Haard, H.J. Properties, production, and applications of camelid single-domain antibody fragments. Appl. Microbiol. Biotechnol. 2007 , 77 , 13–22. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Van Audenhove, I.; Gettemans, J. Nanobodies as versatile tools to understand, diagnose, visualize and treat cancer. EBioMedicine 2016 , 8 , 40–48. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Zhao, Z.; Liu, Y.; Ruan, S.; Hu, Y. Current anti-amyloid-β therapy for Alzheimer’s disease treatment: From clinical research to nanomedicine. Int. J. Nanomed. 2003 , 18 , 7825–7845. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Paudel, Y.N.; Angelopoulou, E.; Piperi, C.; Othman, I.; Aamir, K.; Shaikh, M.F. Impact of HMGB1, RAGE, and TLR4 in Alzheimer’s disease (AD): From risk factors to therapeutic targeting. Cells 2020 , 9 , 383. [ Google Scholar ] [ CrossRef ]
- Saeed, A.F.; Wang, R.; Ling, S.; Wang, S. Antibody engineering for pursuing a healthier future. Front. Microbiol. 2017 , 8 , 495. [ Google Scholar ] [ CrossRef ]
- Nilvebrant, J.; Tessier, P.M.; Sidhu, S.S. Engineered autonomous human variable domains. Curr. Pharm. Des. 2016 , 22 , 6527–6537. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Carter, P.J.; Lazar, G.A. Next generation antibody drugs: Pursuit of the ‘high-hanging fruit’. Nat. Rev. Drug Discov. 2018 , 17 , 197–223. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Amano, A.; Sanjo, N.; Araki, W.; Anraku, Y.; Nakakido, M.; Matsubara, E.; Tomiyama, T.; Nagata, T.; Tsumoto, K.; Kataoka, K.; et al. Peripheral administration of nanomicelle-encapsulated anti-Aβ oligomer fragment antibody reduces various toxic Aβ species in the brain. J. Nanobiotechnol. 2023 , 21 , 36. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Tammer, A.H.; Coia, G.; Cappai, R.; Fuller, S.; Masters, C.L.; Hudson, P.; Underwood, J.R. Generation of a recombinant Fab antibody reactive with the Alzheimer’s disease-related Abeta peptide. Clin. Exp. Immunol. 2002 , 129 , 453–463. [ Google Scholar ] [ CrossRef ]
- Krah, S.; Schröter, C.; Zielonka, S.; Empting, M.; Valldorf, B.; Kolmar, H. Single-domain antibodies for biomedical applications. Immunopharmacol. Immunotoxicol. 2016 , 38 , 21–28. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Sun, Z.T.; Ma, C.; Li, G.J.; Zheng, X.Y.; Hao, Y.T.; Yang, Y.; Wang, X. Application of antibody fragments against Aβ with emphasis on combined application with nanoparticles in Alzheimer’s disease. Front. Pharmacol. 2021 , 12 , 654611. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Si, Z.Z.; Zou, C.J.; Mei, X.; Li, X.F.; Luo, H.; Shen, Y.; Hu, J.; Li, X.X.; Wu, L.; Liu, Y. Targeting neuroinflammation in Alzheimer’s disease: From mechanisms to clinical applications. Neural Regen. Res. 2023 , 18 , 708–715. [ Google Scholar ] [ PubMed ]
- Satheeshkumar, P.K. Expression of single chain variable fragment (scFv) molecules in plants: A comprehensive update. Mol. Biotechnol. 2020 , 62 , 151–167. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Montoliu-Gaya, L.; Villegas, S. Production of therapeutic single-chain variable fragments (ScFv) in Pichia pastoris. Methods Mol. Biol. 2022 , 2313 , 151–167. [ Google Scholar ]
- Fan, X.; Xu, L.; Zhang, J.; Wang, Y.; Wu, Z.; Sun, W.; Yao, X.; Wang, X.; Guan, S.; Shan, Y. Mechanism exploration of amyloid-β-42 disaggregation by single-chain variable fragments of Alzheimer’s disease therapeutic antibodies. Int. J. Mol. Sci. 2023 , 24 , 8371. [ Google Scholar ] [ CrossRef ]
- Martin-Peña, A.; Rincon-Limas, D.E.; Fernandez-Funez, P. Anti-Aβ single-chain variable fragment antibodies restore memory acquisition in a Drosophila model of Alzheimer’s disease. Sci. Rep. 2017 , 7 , 11268. [ Google Scholar ] [ CrossRef ]
- Logovinsky, V.; Satlin, A.; Lai, R.; Swanson, C.; Kaplow, J.; Osswald, G.; Basun, H.; Lannfelt, L. Safety and tolerability of BAN2401--a clinical study in Alzheimer’s disease with a protofibril selective Abeta antibody. Alzheimer’s Res. Ther. 2016 , 8 , 14. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Dominy, S.S.; Lynch, C.; Ermini, F.; Benedyk, M.; Marczyk, A.; Konradi, A.; Nguyen, M.; Haditsch, U.; Raha, D.; Griffin, C.; et al. Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 2019 , 5 , eaau3333. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Im, D.; Heo, C.E.; Son, M.K.; Park, C.R.; Kim, H.I.; Choi, J.M. Kinetic modulation of amyloid-beta (1-42) aggregation and toxicity by structure-based rational design. J. Am. Chem. Soc. 2022 , 144 , 1603–1611. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Thacker, D.; Willas, A.; Dear, A.J.; Linse, S. Role of hydrophobicity at the N-terminal region of Abeta42 in secondary nucleation. ACS Chem. Neurosci. 2022 , 13 , 3477–3487. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ravetch, J.V.; Bolland, S. IgG Fc receptors. Annu. Rev. Immunol. 2001 , 19 , 275–290. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Janeway, C.; Janeway, C. Immunobiology: The Immune System in Health and Disease , 5th ed.; Garland Pub: New York, NY, USA, 2001; p. xviii. [ Google Scholar ]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990 , 215 , 403–410. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Benson, D.A.; Cavanaugh, M.; Clark, K.; Karsch-Mizrachi, I.; Lipman, D.J.; Ostell, J.; Sayers, E.W. GenBank. Nucleic Acids Res. 2013 , 41 , D36–D42. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Mooers, B.H.M. Shortcuts for faster image creation in PyMOL. Protein Sci. 2020 , 29 , 268–276. [ Google Scholar ] [ CrossRef ]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Meng, E.C.; Couch, G.S.; Croll, T.I.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci. 2021 , 30 , 70–82. [ Google Scholar ] [ CrossRef ]
- Pal, A.; Pyne, N.; Paul, S. In-silico designing of a multi-epitope vaccine against SARS-CoV2 and studying the interaction of the vaccine with alpha, beta, delta and Omicron variants of concern. Curr. Drug Discov. Technol. 2023 , 20 , e090922208713. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kozakov, D.; Hall, D.R.; Xia, B.; Porter, K.A.; Padhorny, D.; Yueh, C.; Beglov, D.; Vajda, S. The ClusPro web server for protein-protein docking. Nat. Protoc. 2017 , 12 , 255–278. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Mauger, D.M.; Cabral, B.J.; Presnyak, V.; Su, S.V.; Reid, D.W.; Goodman, B.; Link, K.; Khatwani, N.; Reynders, J.; Moore, M.J.; et al. mRNA structure regulates protein expression through changes in functional half-life. Proc. Natl. Acad. Sci. USA 2019 , 116 , 24075–24083. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Wu, Y.; Mao, M.; Wang, L.J. Integrated clustering signature of genomic heterogeneity, stemness and tumor microenvironment predicts glioma prognosis and immunotherapy response. Aging (Albany NY) 2023 , 15 , 9086–9104. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lührs, T.; Ritter, C.; Adrian, M.; Riek-Loher, D.; Bohrmann, B.; Döbeli, H.; Schubert, D.; Riek, R. 3D structure of Alzheimer’s amyloid-beta(1-42) fibrils. Proc. Natl. Acad. Sci. USA 2005 , 102 , 17342–17347. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002 , 297 , 353–356. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lambert, M.P.; Barlow, A.K.; Chromy, B.A.; Edwards, C.; Freed, R.; Liosatos, M.; Morgan, T.E.; Rozovsky, I.; Trommer, B.; Viola, K.L.; et al. Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc. Natl. Acad. Sci. USA 1998 , 95 , 6448–6453. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Hartley, D.M.; Walsh, D.M.; Ye, C.P.; Diehl, T.; Vasquez, S.; Vassilev, P.M.; Teplow, D.B.; Selkoe, D.J. Protofibrillar intermediates of amyloid beta-protein induce acute electrophysiological changes and progressive neurotoxicity in cortical neurons. J. Neurosci. 1999 , 19 , 8876–8884. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kayed, R.; Head, E.; Thompson, J.L.; McIntire, T.M.; Milton, S.C.; Cotman, C.W.; Glabe, C.G. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 2003 , 300 , 486–489. [ Google Scholar ] [ CrossRef ]
- Lim, C.M.; González Díaz, A.; Fuxreiter, M.; Pun, F.W.; Zhavoronkov, A.; Vendruscolo, M. Multiomic prediction of therapeutic targets for human diseases associated with protein phase separation. Proc. Natl. Acad. Sci. USA 2023 , 120 , e2300215120. [ Google Scholar ] [ CrossRef ]
- Merchant, J.P.; Zhu, K.; Henrion, M.Y.R.; Zaidi, S.S.A.; Lau, B.; Moein, S.; Alamprese, M.L.; Pearse, R.V., 2nd; Bennett, D.A.; Ertekin-Taner, N.; et al. Predictive network analysis identifies JMJD6 and other potential key drivers in Alzheimer’s disease. Commun. Biol. 2023 , 6 , 503. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Silva-Spínola, A.; Baldeiras, I.; Arrais, J.P.; Santana, I. The road to personalized medicine in Alzheimer’s disease: The use of artificial intelligence. Biomedicines 2022 , 10 , 315. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Arrué, L.; Cigna-Méndez, A.; Barbosa, T.; Borrego-Muñoz, P.; Struve-Villalobos, S.; Oviedo, V.; Martínez-García, C.; Sepúlveda-Lara, A.; Millán, N.; Márquez Montesinos, J.C.E.; et al. New drug design avenues targeting Alzheimer’s disease by pharmacoinformatics-aided tools. Pharmaceutics 2022 , 14 , 1914. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kumar, S.; Chowdhury, S.; Kumar, S. In silico repurposing of antipsychotic drugs for Alzheimer’s disease. BMC Neurosci. 2017 , 18 , 76. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Pardridge, W.M. The blood-brain barrier: Bottleneck in brain drug development. NeuroRx 2005 , 2 , 3–14. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Whittlesey, K.J.; Shea, L.D. Nerve growth factor expression by PLG-mediated lipofection. Biomaterials 2006 , 27 , 2477–2486. [ Google Scholar ] [ CrossRef ]
- Gärtner, A.; Collin, L.; Lalli, G. Nucleofection of primary neurons. Methods Enzymol. 2006 , 406 , 374–388. [ Google Scholar ] [ PubMed ]
- Luo, D.; Saltzman, W.M. Enhancement of transfection by physical concentration of DNA at the cell surface. Nat. Biotechnol. 2000 , 18 , 893–895. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Pardridge, W.M. Drug targeting to the brain. Pharm. Res. 2007 , 24 , 1733–1744. [ Google Scholar ] [ CrossRef ]
- Fortin, D.; Gendron, C.; Boudrias, M.; Garant, M.P. Enhanced chemotherapy delivery by intraarterial infusion and blood-brain barrier disruption in the treatment of cerebral metastasis. Cancer 2007 , 109 , 751–760. [ Google Scholar ] [ CrossRef ]
- Jones, A.R.; Shusta, E.V. Blood-brain barrier transport of therapeutics via receptor-mediation. Pharm. Res. 2007 , 24 , 1759–1771. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Bradley, M.O.; Swindell, C.S.; Anthony, F.H.; Witman, P.A.; Devanesan, P.; Webb, N.L.; Baker, S.; Wolff, A.; Donehower, R. Tumor targeting by conjugation of DHA to paclitaxel. J. Control Release 2001 , 74 , 233–236. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Moghimi, S.M.; Szebeni, J. Stealth liposomes and long circulating nanoparticles: Critical issues in pharmacokinetics, opsonization and protein-binding properties. Prog. Lipid Res. 2003 , 42 , 463–478. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Yu, Y.J.; Zhang, Y.; Kenrick, M.; Hoyte, K.; Luk, W.; Lu, Y.; Atwal, J.; Elliott, J.M.; Prabhu, S.; Watts, R.J.; et al. Boosting brain uptake of a therapeutic antibody by reducing its affinity for a transcytosis target. Sci. Transl. Med. 2011 , 3 , 84ra44. [ Google Scholar ] [ CrossRef ]
- Niewoehner, J.; Bohrmann, B.; Collin, L.; Urich, E.; Sade, H.; Maier, P.; Rueger, P.; Stracke, J.O.; Lau, W.; Tissot, A.C.; et al. Increased brain penetration and potency of a therapeutic antibody using a monovalent molecular shuttle. Neuron 2014 , 81 , 49–60. [ Google Scholar ] [ CrossRef ]
- Biden, J. FACT SHEET: The United States Announces New Investments and Resources to Advance President Biden’s National Biotechnology and Biomanufacturing Initiative. White House. 2022. Available online: https://www.whitehouse.gov/briefing-room/statements-releases/2022/09/14/fact-sheet-the-united-states-announces-new-investments-and-resources-to-advance-president-bidens-national-biotechnology-and-biomanufacturing-initiative/ (accessed on 24 February 2024).
| Drug Name | Target(s) | Function(s) | Stage of Development |
|---|---|---|---|
| Aβ oligomer inhibitors (e.g., BAN2401, aducanumab) | Amyloid-β oligomers | Prevent or disassemble toxic clumps of amyloid-β | Clinical trials (aducanumab recently received FDA approval) |
| BACE1 inhibitors (e.g., verubecestat, MK-8931) | β-Secretase 1 (BACE1) | Reduce production of amyloid-β by inhibiting the enzyme that cleaves its precursor | Clinical trials (some promising results, others halted due to lack of efficacy) |
| Tau aggregates inhibitors (e.g., P-tau217 PET tracers, LMTX) | Tau protein aggregates | Prevent or remove tangles of misfolded tau protein | Preclinical/early clinical trials (imaging agents more advanced than therapeutic agents) |
| Cholinesterase inhibitors (e.g., donepezil, rivastigmine, galantamine) | Acetylcholinesterase (AChE) | Increase levels of the neurotransmitter acetylcholine, which is depleted in AD | Approved for symptomatic treatment of mild-to-moderate AD |
| NMDA receptor modulators (e.g., memantine) | N-methyl-D-aspartate (NMDA) receptors | Protect neurons from excitotoxicity and improve cognitive function | Approved for moderate-to-severe AD |
| Multi-target drugs (e.g., J147, AV-1750, CTS-5559) | Combinations of targets from above (e.g., AChE + NMDA, BACE1 + tau) | Address multiple aspects of AD pathology for potentially greater efficacy | Preclinical/early clinical trials (potentially more effective but require careful design and validation) |
| Type of Nanobodies | Description | Mechanism of Action | Advantage | Disadvantage |
|---|---|---|---|---|
| Fab fragments | Modified antigen-binding fragments of conventional antibodies | Bind to specific targets, trigger immune response | High affinity, good specificity | Large size, limited tissue penetration |
| Domain antibodies | Single variable domains from antibodies with only the heavy chain (VH) | Bind to specific targets, inhibit specific pathways | Smaller than Fab fragments, they have potentially better tissue penetration | Less potent than Fab fragments, limited repertoire |
| Single-chain variable fragments (scFv) | Engineered fusion of heavy and light chain variable domains | Bind to specific targets, can be engineered for additional functions | Smaller than Fab fragments, customizable | Lower affinity than Fab fragments, limited potential stability |
| Element | Description | Position |
|---|---|---|
| cap | A modified 5′-cap 1 structure (m7G+m3′-5′-ppp-5′-Am) | 1–2 |
| 5′-UTR | The 5′-untranslated region derived from human alpha globin RNA with an optimized Kozak sequence. | 3–54 |
| sig | S glycoprotein signal peptide (extended leader sequence) guides translocation of the nascent polypeptide chain into the endoplasmic reticulum. | 55–102 |
| ORF | Codon-optimized sequence: GAAΨΨ ΨCGCC AΨGAΨ AGCGG CΨAΨG AAGΨG CAΨCA ΨGGCA GCGGC AGCGG CAGCG GCAGC GAGAΨ GΨGG GCAGC AACAA AGGC | 103–187 |
| 3′-UTR | The 3′ untranslated region comprises two sequence elements derived from the amino-terminal enhancer of split (AES) mRNA and the mitochondrial encoded 12S ribosomal RNA to confer RNA stability and high total protein expression: GCΨAG CΨGCC CCΨΨΨ CCCGΨ CCΨGG GΨACC CCGAG ΨCΨCC CCCGA CCΨCG GGΨCC CAGGΨ AΨGC ΨCCCA CCΨCC ACCΨG CCCCA CΨCAC CACCΨ CΨGCΨ AGΨΨC CAGAC ACCΨCC CAAGC ACGCA GCAAΨ GCAGC ΨCAAA ACGCΨ ΨAGCC ΨAGCC ACACC CCCAC GGGAA ACAGC AGΨGA ΨΨAAC CΨΨΨA GCAAΨ AAACG AAAGΨ ΨΨAAC ΨAAGC ΨAΨAC ΨAACC CCAGG GΨΨGG ΨCAAΨ ΨΨCGΨ GCCAG CCACA CCCΨG GAGCΨ AGC | 188–456 |
| poly(A) | A 110-nucleotide poly(A)-tail consisting of a stretch of 30 adenosine residues, followed by a 10-nucleotide linker sequence and another 70 adenosine residues: AAAAA AAAAA AAAAA AAAAA AAAAA AAAAA GCAΨA ΨGACΨ AAAAA AAAAA AAAAA AAAAA AAAAA AAAAA AAAAAA AAAAA AAAAA AAAAA AAAAA AAAAA AAAAA AAAA | 457–566 |
| Aβ42 | DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVVIA | 42 amino acids | ||||
| ( ) | No. | Start | End | Peptide | Length | EFRHDSGYEVHH -GSGSGSGS- EDVGSNKG |
| 1 | 3 | 14 | EFRHDSGYEVHH | 12 | ||
| 2 | 22 | 29 | EDVGSNKG | 8 | ||
| The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Share and Cite
Niazi, S.K.; Magoola, M.; Mariam, Z. Innovative Therapeutic Strategies in Alzheimer’s Disease: A Synergistic Approach to Neurodegenerative Disorders. Pharmaceuticals 2024 , 17 , 741. https://doi.org/10.3390/ph17060741
Niazi SK, Magoola M, Mariam Z. Innovative Therapeutic Strategies in Alzheimer’s Disease: A Synergistic Approach to Neurodegenerative Disorders. Pharmaceuticals . 2024; 17(6):741. https://doi.org/10.3390/ph17060741
Niazi, Sarfaraz K., Matthias Magoola, and Zamara Mariam. 2024. "Innovative Therapeutic Strategies in Alzheimer’s Disease: A Synergistic Approach to Neurodegenerative Disorders" Pharmaceuticals 17, no. 6: 741. https://doi.org/10.3390/ph17060741
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
- Get new issue alerts Get alerts
Secondary Logo
Journal logo.
Colleague's E-mail is Invalid
Your message has been successfully sent to your colleague.
Save my selection
Current Alzheimer disease research highlights: evidence for novel risk factors
Editor(s): Ji, Yuan-Yuan
1 Department of Psychiatry and Behavioral Sciences, University of California, San Francisco, CA, USA
2 Department of Epidemiology and Biostatistics, University of California, San Francisco, CA, USA
3 Institute of Neurology, Sichuan Provincial People's Hospital, University of Electronic Science and Technology of China, Chengdu, Sichuan 610072, China
4 Chinese Academy of Sciences Sichuan Translational Medicine Research Hospital, Chengdu, Sichuan, China
5 Centre for Public Health, Queen's University Belfast, Belfast, UK
6 Global Brain Health Institute, University of California, San Francisco, San Francisco, CA, USA
7 Department of Neurology, University of California, San Francisco, CA, USA.
Correspondence to: Dr. Wei-Dong Le, Institute of Neurology, Sichuan Provincial People's Hospital, University of Electronic Science and Technology of China, Chengdu, Sichuan 610072, ChinaE-Mail: [email protected] ; Dr. Yue Leng, Department of Psychiatry and Behavioral Sciences, University of California, San Francisco, CA, USAE-Mail: [email protected]
How to cite this article: Brenowitz WD, Xiang Y, McEvoy CT, Yang C, Yaffe K, Le WD, Leng Y. Current Alzheimer disease research highlights: evidence for novel risk factors. Chin Med J 2021;134:2150–2159. doi: 10.1097/CM9.0000000000001706
Received 2 February, 2021
Willa D. Brenowitz and Yang Xiang contributed equally to the work.
This is an open access article distributed under the terms of the Creative Commons Attribution-Non Commercial-No Derivatives License 4.0 (CCBY-NC-ND), where it is permissible to download and share the work provided it is properly cited. The work cannot be changed in any way or used commercially without permission from the journal. http://creativecommons.org/licenses/by-nc-nd/4.0
Alzheimer disease (AD) is the most common type of dementia characterized by the progressive cognitive and social decline. Clinical drug targets have heavily focused on the amyloid hypothesis, with amyloid beta (Aβ), and tau proteins as key pathophysiologic markers of AD. However, no effective treatment has been developed so far, which prompts researchers to focus on other aspects of AD beyond Aβ, and tau proteins. Additionally, there is a mounting epidemiologic evidence that various environmental factors influence the development of dementia and that dementia etiology is likely heterogenous. In the past decades, new risk factors or potential etiologies have been widely studied. Here, we review several novel epidemiologic and clinical research developments that focus on sleep, hypoxia, diet, gut microbiota, and hearing impairment and their links to AD published in recent years. At the frontiers of AD research, these findings and updates could be worthy of further attention.
Introduction
As one of the most common neurodegenerative diseases and causes of dementia, Alzheimer disease (AD) is a critical topic for biomedical research. The main clinical manifestation of AD is the progressive decline of cognitive function and activities of daily living, and the pivotal pathological features of AD are amyloid beta (Aβ) deposition, neurofibrillary tangles, neuroinflammation, synaptic degeneration, and neuronal loss. [1] While research on AD has been ongoing for >100 years, our understanding of AD is also constantly enriched by the new research directions. However, there are still no effective treatments to delay, halt, or even reverse the process of AD.
In the past few decades, researchers have gradually shifted their attention from treatments to the early diagnosis and prevention of AD. [2] There is an interest in identifying the novel risk factors for AD as well as the novel biomarkers to help detect AD before the symptom onset. Several potential risk factors for AD have been studied extensively, including cardiovascular disease (CVD), diabetes, obesity, low education, social isolation, and depression. [3] However, recent epidemiologic and clinical studies are expanding our understanding of potential AD markers and risk factors to other health behaviors and conditions, such as sleep, diet, and hearing loss. For example, sleep disturbance has a complex association with AD and may be either a preclinical biomarker or potential modifiable risk factor for AD. In particular, hypoxia, often caused by severe obstructive sleep apnea syndrome (OSAS), significantly promotes the development of AD, inspiring the attempts to treat AD using the hyperbaric oxygen treatment (HBOT). [4] Dietary patterns are associated with cognition in older adults, and the Mediterranean-style diet (MD) is associated with reduced risk of AD. Gut microbiota may be an intriguing potential mediator between diet and AD. [5] Also, hearing impairment which is often ignored in clinical practice has strong associations with the risk of AD.
Here, we review the latest developments and especially the epidemiological evidence on sleep, hypoxia, diet, gut microbiota, and hearing impairment in the research field of AD published in recent years. These topics are receiving increasing research interest and may point to novel areas for intervention in the treatment and prevention of AD. As the frontiers of AD research, these findings and updates could be worthy of further attention.
Recent Progress in the Research of AD
In this section, we will review and summarize the recent progress in the research of AD focusing on five novel potential risk factors or early disease indicators such as sleep, hypoxia, diet, gut microbiota, and hearing impairment. As these factors are relatively novel, this review focuses on the epidemiologic evidence with some discussion of potential mechanisms as well as areas for future research.
Sleep and AD
One of the most exciting recent highlights in Alzheimer research is the bi-directional relationship between sleep disturbances and the risk of AD. Patients with AD frequently experience sleep disturbances, including insomnia, abnormal sleep duration, poor nighttime sleep quality, excessive daytime sleepiness, and disrupted circadian rhythms. [6] These sleep problems subsequently reduce patient quality of life and increase the risk for premature institutionalization.
Importantly, growing evidence from the epidemiological studies has suggested that 50% to 80% increased risk of dementia is associated with sleep disturbances, including insomnia, sleep-disordered breathing (SDB), disrupted circadian rhythms, and sleep-related movement disorders. [7–12] Excessive daytime napping has also been associated with an increased risk of cognitive impairment in older men, although the underlying mechanisms are less clear. [13] Several studies have found a U-shaped association between sleep duration and risk of dementia, [14,15] indicating the effects of both short and long sleep duration on cognitive aging. Furthermore, one recent study discovered 23 macro- and micro-physiological architecture metrics of sleep, including rapid eye movement sleep duration, features of the electroencephalography power spectra derived from multivariate analysis, and spindle and slow oscillation morphology and coupling, which were all strongly linked with cognitive performance in older adults. [16] Sleep disturbances are increasingly recognized as a preclinical marker or potential modifiable risk factor for AD.
Over the past decade, emerging evidence from animal and human studies has begun to uncover the nature of the association between sleep disturbances and AD. Since the earlier animal studies that identified a close link between sleep-wake cycle and the pathogenesis of AD, [17,18] a growing body of research has suggested sleep deprivation as both a result and trigger of Aβ, the hallmark pathological feature of AD. [19] Prospective analysis also showed that baseline measures of non-rapid eye movement (NREM) sleep slow-wave activity and sleep quality are sensitive in predicting longitudinal trajectory of Aβ deposition in healthy older adults, indicating the role of sleep as a useful biomarker for forecasting Aβ pathological progression before the clinical cognitive impairment. [20] Furthermore, sleep–wake cycle was found to regulate brain interstitial fluid (ISF) tau, and chronic sleep deprivation might increase ISF and cerebrospinal fluid (CSF) tau as well as tau pathology spreading. [21] It was also suggested that SDB might lead to increased tau levels over time in those with normal cognition or mild cognitive impairment (MCI). [22] Interestingly, one recent study found a coherent pattern of electrophysiological, hemodynamic, and CSF oscillations during human NREM sleep, suggesting a link in the neurophysiology of sleep and waste clearance in the brain. [23]
In addition to the use of neuroimaging and biomarkers, recent studies are also starting to use novel statistical approaches, such as Mendelian randomization (MR), to overcome the limitations of observational epidemiological studies and to reveal the causal relationship between sleep and AD. For instance, using the MR approach, it was found that higher genetic risk for AD might predict shorter sleep duration, suggesting that short sleep duration could be part of the AD disease process and thus serve as an early marker for AD. [24] Meanwhile, another MR study showed no causal effect of self-reported or accelerometer-measured sleep traits on AD risk. Given the growing evidence that indicates a bi-directional relationship between sleep disturbances and AD, emerging studies are underway to examine the use of sleep interventions in the prevention and treatment of AD. One recent review summarized the effects of several sleep interventions that have been studied in patients with MCI or mild dementia, including Cognitive Behavioral Therapy-Insomnia (CBT-I), a structured limbs exercise program, aromatherapy, phase locked loop acoustic stimulation, transcranial stimulation, suvorexant, melatonin, donepezil, galantamine, rivastigmine, tetrahydroaminoacridine, and Continuous Positive Airway Pressure (CPAP) and concluded that CBT-I, melatonin, suvorexant, and CPAP for OSA hold the most promises. [25] Since medications might further impair cognition, non-pharmacological interventions are of particular interest for older adults who are at high risk for dementia. Cordone et al [26] highlighted several promising techniques to enhance NREM sleep oscillations that have solid scientific basis for preventing or slowing down AD pathology but remain to be tested in clinical settings.
Overall, while it remains inconclusive whether sleep disturbances are early signs or risk factors for AD, recent research highlights the importance of sleep among older adults. Changes in sleep architecture and electroencephalogram might be considered as a valuable marker of AD before the onset of cognitive symptoms and help with the early detection of the disease. [27] Future research is needed to test whether sleep disturbances could be the risk factors for AD and to explore the use of sleep interventions in patients at high risk for AD. [25]
Hypoxia and AD
Hypoxia can be caused by CVD, hematological diseases, chronic kidney diseases (CKD), respiratory dysfunction, and environmental conditions, which could influence the central nervous system and induce neurodegeneration. [28–33] Acute hypoxia can be induced by stroke, while OSAS, capillary dysfunction, and CKD may lead to chronic hypoxia. Cognitive impairment may also occur in normal adults after hypoxia. [34,35]
Hypoxia is associated with AD. [28–33] Both acute and chronic hypoxia intervention in experimental animals can result in the aggravation of cognitive dysfunction and the AD-type pathological alterations including Aβ deposition, hyperphosphorylation of tau protein, synaptic degeneration, and neuronal loss. [36] Increasing evidence suggests that hypoxia facilitates the pathogenesis of AD through multiple pathways including increasing the production and accelerating the accumulation of Aβ, [36] decreasing the degradation of Aβ, [37] reducing the clearance of Aβ, [38] elevating the hyperphosphorylation of tau, [39] inhibiting the autophagic function, [40] aggravating neuroinflammation [41] and oxidation stress, [42] ruining the mitochondria function, [43] and causing the stress of endoplasmic reticulum. [44] As mentioned above, it is rational to propose that hypoxia is one of the essential factors contributing to the pathogenesis of AD.
Given that hypoxia contributes to the pathogenesis of AD, the development of prevention and treatment targeting hypoxia is promising. HBOT is a safe, effective, and routinely used medical procedure. Growing evidence suggests that HBOT can induce the neuroplasticity and improve the cognitive function in patients suffering from neurocognitive impairment due to stroke and brain injuries. [45,46] Moreover, HBOT cannot only improve cognitive functions and ameliorate the brain glucose metabolism in AD and amnestic MCI (aMCI) patients [4,47] but also induce significant senolytic effects including significantly increasing telomere length and clearance of senescent cells in the aging individuals. [48]
Besides, HBOT is capable of improving the cognitive behavioral performance, reducing Aβ burden and tau hyperphosphorylation, alleviating neuroinflammation by decreasing astrogliosis and microgliosis, reducing proinflammatory cytokines, and elevating phagocytic markers in mouse mole of AD. [49] More recently, HBOT was shown to be able to reduce Aβ accumulation and hippocampal neuritic atrophy, increase hippocampal neurogenesis, and profoundly improve the cognitive deficits through the upregulation of neurotrophic factors. [50] Moreover, HBOT has been proved to inhibit Aβ25–35-induced toxicity, oxidative stress, and neuronal apoptosis. [51,52] In addition, both the cognitive impairment and hippocampal damage can be attenuated by HBOT via NF-κB signaling pathway [53] or p38 mitogen-activated protein kinase (MAPK) in the animal model of AD. [54] However, there is still no research report on whether HBOT has the capacity of preventing or delaying the occurrence of AD in high-risk groups at an early stage. Besides, further experiments are still warranted because of the possibility of oxygen toxicity, even though HBOT itself seems to be beneficial to cognition.
Diet and AD
As poor diet contributes to several AD risk factors including obesity, hypertension, and diabetes, modifying the dietary behavior could be an effective public health strategy to protect against age-related neurodegeneration and AD in late life.
A growing body of evidence has linked several foods (eg, green vegetables, berries, fish, and olive oil), nutrients (eg, B-vitamins, vitamin E, and omega-3 fatty acids), and plant bioactives (eg, flavanoids) to reduce dementia risk. Consuming these nutrients and foods in combination as a dietary pattern is likely to exert greater synergistic effects on the physiological processes underlying neurodegeneration. The MD rich in antioxidants and flavonoids and characterized by high intake of fruits, vegetables, whole grains, olive oil, nuts, and legumes; moderate intake of fish, poultry, and alcohol, and low intake of red meat have proven cardiometabolic benefits [55] and remain the most frequently studied dietary pattern for neuroprotection during ageing. Evidence from prospective studies indicate beneficial associations among MD adherence, slower rate of cognitive decline, and reduced risk of cognitive impairment, in Western [56,57] and Asian [58] populations. However, findings have not been consistent likely because of the differences in populations studied, measures of MD and cognition, length of follow-up, and adjustment for important confounders such as cardiovascular morbidity and baseline cognitive function. To date, only a small number of studies have examined the relationship between diet and incident AD. [59] Among older cognitively healthy U.S. adults, those in the highest tertile of MD adherence had 40% to 54% reduced AD risk compared to those in the lowest MD tertile, but results have not been replicated in French or Swedish populations [59] making it difficult to draw firm conclusions.
The neuroprotective mechanisms of a healthy diet are not fully elucidated, but multiple antioxidants, anti-inflammatory, and vascular pathways are likely to be important. The MD improves vascular function and insulin resistance [55,60] that contribute to the cognitive decline and AD. Experimental and preclinical studies have shown that dietary antioxidants and flavonoids have a direct effect on the brain by inhibiting oxidative stress, cytokine production and pro-inflammatory cell signaling pathways, and suppressing neuroinflammatory processes implicated in AD. Emerging data suggest that diets rich in fruit, vegetables, whole grains, and fiber promote biodiversity of the gut microbiome and decreased pro-inflammatory gut-derived bacteria and toxins are shown to contribute to early neuroinflammatory changes, and AD pathology. [5,60] Evidence from observational studies report a link between greater MD adherence and favorable brain structures and functions that protect against neurodegeneration as well as less Aβ accumulation in AD-vulnerable regions of the brain. [61] Furthermore, dietary restriction [62] achieved by calorie restriction (30%–40%) or intermittent fasting may provide neuroprotection by attenuating neuroinflammation and insulin resistance and promoting synaptic plasticity and neurogenesis. [63] Beneficial effects of DR on AD pathology have been demonstrated in some [64,65] but not all [66] transgenic animal models, and it is not yet clear on how the findings translate to humans. [63]
The effect of diet on AD risk is not yet known; however, randomized controlled trial data evaluating the effect of diet on cognitive performance demonstrated less decline in global cognition, memory, and executive function in response to a MD >4 to 6 years [60] with no convincing benefit for shorter-term MD interventions (up to 12 months) [59] in cognitively healthy older adults, suggesting that several years of dietary exposure may be needed to detect changes in intermediate cognitive tests of AD risk in general populations.
Overall, accumulating data suggest a role for diet in AD prevention but larger adequately powered intervention and prospective studies in diverse populations with clinically relevant endpoints incorporating incident AD, MCI as well as sensitive neurocognitive tests and brain biomarkers associated with preclinical AD risk are required to understand the effect of diet on AD from the earliest to later stages of disease.
Gut microbiota and AD
Given the complex bidirectional communication system that exists between the gut and brain, there is a growing interest in the gut microbiome as a novel and potentially modifiable risk factor for cognitive impairment and AD. Gut dysbiosis has been implicated in the pathogenesis and progression of AD.
Compared with the normal controls, the Bacteroidetes , Actinomyces , Ruminococcus , and Selenomonas in AD patients are significantly different. [67] The cognitively normal elderly do not have an AD-type pattern of gut microbiota compared with patients at the early stage of AD, [68] and specific gut microbiota, especially enriched Enterobacteriaceae , are associated with AD patients compared with aMCI and cognitively healthy controls, [69] indicating the potential of gut microbiota in the differential diagnosis of AD. Besides, the alteration of gut microbiota tends to occur several years before the onset of dementia, even at the early stage of MCI. [68] A cross-sectional study showed that an increase in Bacteroidetes in non-dementia patients is independently associated with the presence of MCI. [70] The abundance increase of Enterobacteriaceae , Akkermansia , Slackia , Christensenellaceae , and Erysipelotriaceae in MCI patients suggests that this special gut microbiota composition may indicate the presence of MCI. [71]
To investigate the causal relationship between gut microbiota and AD, a clinical study found that gamma-aminobutyric acid (GABA) and serotonin may play an important role in the gut microbiota–host interaction in AD patients. [72] A pilot study revealed the characteristics of the MCI-specific gut fungi (mycobiome) signatures and elucidated that the diet-regulated mycobiome are associated with AD markers and fungal–bacterial co-regulation networks in MCI patients. [73] A clinical study showed that Proteobacteria is positively correlated with Aβ42:Aβ40 ratio, but the fecal propionic acid and butyric acid are negatively correlated with Aβ42 level in MCI patients with the MD. [71] Intriguingly, the gut microbiota composition is strongly correlated with apolipoprotein E (ApoE) genotype. The relative abundance of different bacterial groups is significantly different under the influence of ApoE genotype, [74] which is also related to the specific gut microbiota composition of human and ApoE-targeted replacement mice, especially the Prevotellaceae and Ruminococcaceae and several butyrate-producing genera. [75] Moreover, the relative abundance of Prevotella and Ruminococcus in female ApoE4-familial Alzheimers disease (FAD) mice is higher than that of female ApoE3-FAD mice, whereas the relative abundance of Sutterella in male ApoE4-FAD mice is significantly higher than that of female ApoE3-FAD mice, implying a synergistic effect of ApoE and sex on gut microbiota of AD. [76]
The metabolites of gut microbiota are particularly critical in the mechanism of the gut–brain axis. Trimethylamine-n-oxide (TMAO), a kind of gut microbiota metabolites, can be found in human CSF. [77] It was suggested that the gut microbiota metabolites, such as lipopolysaccharide (LPS) and short chain fatty acids, could mediate the systemic inflammation and intracerebral amyloidosis through endothelial dysfunction. [78] A multicenter clinical study found that the serum concentration of primary bile acid (BA) is significantly decreased, whereas the concentrations of secondary BA, deoxycholic acid, and its conjugated form of glycine and taurine are increased in AD patients compared with cognitively normal elderly. [79] Moreover, it was found that the certain blood BA-related indicators are associated with CSF-Aβ, CSF-p-tau 181, CSF-t-tau, glucose metabolism, and brain atrophy in patients with MCI and AD, respectively. [80]
Additionally, there are also many studies exploring the effect and mechanism of different types of intervention on AD using gut microbiota as a potential mediator. High dose of Jatrorrhizine, [81] an essential component of coptidis rhizome, a Chinese traditional herb, is capable of improving the learning and memory ability, reducing Aβ deposition, and altering the abundance of certain gut microbiota composition such as Firmicutes and Bacteroidetes in APP/PS1 mice. [82] Besides, 27-hydroxycholesterol can aggravate AD-type pathological alterations, gut microbiota dysbiosis, and intestinal barrier dysfunction. [83] The fructooligosaccharides derived from Morinda officinalis improves the learning and memory abilities of rats by regulating the interaction between intestinal ecosystem and brain. [84] Xanthoceraside can alleviate the symptoms of AD by affecting the composition and endogenous metabolites of gut microbiota in rats. [85] GV-971, an oligosaccharide sodium, has the capacity of inhibiting gut microbiota dysbiosis and related phenylalanine/isoleucine accumulation, alleviating neuroinflammation, and reversing cognitive dysfunction. [86]
Environmental factors may also influence the pathogenesis of AD through gut microbiota. Long-term exposure to noise alters gut microbiota composition and accelerates age-related neurochemical and inflammatory regulation disorders, resulting in the aggravated AD-type pathological changes in the brain of senescence-accelerated prone mice. [87] Treatment of mid infrared light of peak wavelength 7.7 to 10 mm can attenuate the decline of learning and memory, reduce Aβ deposition, and alter the gut microbiota composition. [88]
Due to the importance of gut microbiota in the pathogenesis of AD, many studies assessed its potential therapeutic value. Clinical studies have found that supplement of multiple probiotics alters the gut microbiota composition and serum tryptophan metabolism in AD patients, [89] and promotes the mental plasticity and stress relief in healthy elderly. [90] A multicenter study published recently has also shown that the MD can alter the composition of the gut microbiota and improve cognitive function in older adults. [5]
In animal study, it revealed that the transplantation of feces from normal wild type mice to AD transgenic mice significantly reduces Aβ burden and tau pathology, attenuates the glial activation, learning and memory impairment, and abnormal expression of genes related to intestinal macrophage activity, and restores the circulating inflammatory monocytes and synaptic plasticity in AD mice. [91,92] The gut microbiota alteration can promote Aβ deposition by activating the MAPK signaling pathway and C/enhancer binding protein β (EBP)/asparagine endopeptidase (AEP) signaling pathway in the brain of AD transgenic mice. [93,94] In addition, the probiotics have the capacity of improving the maze navigation, restoring the long-term potential, and balancing the antioxidant/oxidative biomarkers in mice. [95] In detail, Lactobacillus plantarum inhibits the synthesis of TMAO and reduces the clusterin level. [96] Clostridium butyricum and its metabolites inhibit microglia-mediated neuroinflammation. [97] Bifidobacterium longum regulates NF-κB activation via inhibiting LPS production. [98] Of note, however, clinical studies have found that probiotics supplement does not significantly improve the cognitive and biochemical indicators in patients with severe AD. [99]
Unlike the intestinal probiotics, the effects of antibiotics on AD are more complicated. Antibiotics, such as streptozotocin, can promote the growth of proinflammatory gut microbiota sub-types in animals, leading to learning and memory impairment, as has been used to establish sporadic AD models. [100] Rifampicin and minocycline could decrease the levels of Aβ, glial activation, and inflammatory cytokines in the brain of AD mice. [101,102] Rapamycin not only reduces the level of Aβ and the activation of microglia but also decreases the phosphorylation of tau protein. [103] Nevertheless, despite some encouraging results in the animal studies, the clinical efficacy of antibiotics in patients with AD remains controversial so far.
Hearing impairment and AD
There is an emerging interest in the role of age-related hearing impairment on development of AD and other dementias. Hearing impairments can be caused by changes in the inner ear (eg, peripheral hearing) and/or dysfunction in auditory processing (eg, central hearing). Hearing impairments are common among older adults: affecting up to 40% of adults aged 65 and up to 90% of adults aged >90. [104] Hearing difficulty is commonly reported by patients with AD. [105] Observational studies have found a consistent association between hearing loss and risk of dementia and cognitive decline. [106,107] This work raises the question whether hearing loss may cause AD and dementia; however, alternative mechanisms could also explain this association. [108]
Hearing impairments may directly affect dementia risk through brain atrophy by impairing cognitive processing abilities or by increasing cognitive load. [108] In animal studies, noise-induced (peripheral) hearing loss is associated with increased neurodegeneration in the hippocampus, decreased neurogenesis, and poor memory function. [109] Hearing impairments may also affect “psychosocial wellbeing” including social engagement, mental health, and physical activity, [109] which could lead to increased dementia risk. [110] In epidemiologic studies, peripheral hearing loss measured by pure tone audiometry may offer some of the stronger evidence that hearing loss may cause dementia, since pure tones are less affected by AD-neurodegeneration than central hearing. [110] Peripheral hearing impairment has also been associated with decreased whole brain volumes, reduced temporal lobe or auditory cortex volumes, [111,112] or reduced hippocampal volume. [113] But there are conflicting results. [114]
Hearing loss can often be corrected or mitigated, which could in turn also reduce dementia burden if hearing loss causes dementia. The Lancet commission on dementia prevention in 2020 suggested that treating hearing loss may reduce dementia burden by up to 8%. [110] However, this estimate is based on observational studies which may be biased. Evidence from several small clinical trials has been mixed; some studies are suggestive that treatment of hearing impairments may improve cognition in non-demented patients, [115] but this was not found in AD patients. [116] Although studies often include important confounders in statistical models, unmeasured or residual confounder may remain. Both hearing and cognitive impairments are strongly associated with age, tend to have a gradual onset, and may have shared etiologies, including neurodegeneration, vascular and metabolic diseases, and aging processes. [117] Some studies even question a biologic between hearing and cognition, as many cognitive tests rely on hearing and poor hearing may lead to more errors in hearing-based cognitive tests. [118]
Relatively few studies have examined associations between hearing and AD specifically; one study on dementia sub-types have found associations between hearing impairment and clinical AD but not vascular dementia. [119] One neuroimaging study found an association with pure tone and word recognition hearing loss and in vivo Aβ deposition, [120] while an autopsy study found an association between clinician rated hearing loss and tau neurofibrillary degeneration stage but not Aβ plaque frequency. [121] Higher genetic risk for AD also is associated with increased difficulty hearing in noise in older adults, suggesting a shared biologic pathway and that central hearing loss such as difficulty hearing in noise may be a preclinical marker for AD. [122] Neurodegeneration in AD affects anatomical structures including the auditory pathways: neuritic plaques and tangles have been found in auditory association cortex as well as subcortical auditory pathways, which includes the medial temporal lobe. Several studies find that central auditory processing dysfunction is strongly associated with AD and precedes dementia diagnosis. [123]
Older adults with hearing impairments are a higher risk for dementia and may be an important subgroup for referral for dementia evaluation. Treating hearing impairment may also help to prevent dementia; however, further research is needed to clarify the relationships between hearing impairments, AD, and dementia. Regardless, treatment for hearing impairments should be prioritized to improve quality of life of older adults with hearing loss.
Perspectives
There is no denying that the clinical treatment of AD is currently facing significant bottlenecks. The failure of many clinical trials suggests the importance of early diagnosis and prevention. Therefore, in recent years, researchers have been interested in thinking about AD from a broad perspective and in evaluating novel potential risk factors beyond those traditionally associated with AD such as CVD, diabetes, and education. These findings of the effects of oxygen metabolism, inflammation, and gut microbiota provide novel evidence that systemic effects may impact brain aging. Furthermore, our review highlights the potential importance of underappreciated health factors to healthy aging such as sleep, diet, and hearing. This new research adds further evidence to support a shift from amyloid focused drug targets to multi-domain interventions that may help prevent AD and slow cognitive decline.
However, there is still a lot of work to be done in these areas. In Table 1 , we present main evidence for each topic in our review as well as list key next steps for research. In particular, studies are needed to clarify the causal directions of the association between these potential novel risk factors and AD and to understand the underlying mechanisms and how they are related to AD neuropathogenesis. The clinical application of the study of oxygen metabolism and AD, such as the attempt to treat AD with HBOT; development of new intestinal probiotics; the prevention and treatment effect of specific diet components on AD. As the future direction of AD research, these works will require more multidisciplinary collaboration and the use of innovative research methods.
| Items | Summary of main evidence | Priorities for future research |
| Sleep | There exists a bi-directional relationship between sleep disturbances and dementia, but it remains unclear whether sleep disturbances are early signs or risk factors for AD. | Future research to uncover potential mechanisms and to explore the use of sleep interventions for the prevention and treatment of AD among high-risk older adults. |
| Hypoxia | Chronic hypoxia is one of the important environmental factors contributing to the pathogenesis of AD. | Further research is needed to determine whether prospective prevention and treatment of hypoxia may be helpful to delay or ameliorate the progression of AD by any mechanism. |
| Diet | Certain nutrients (eg, antioxidants) and dietary patterns (eg, Mediterranean diet) might have neuroprotective effects, but results have been inconsistent. | Larger adequately powered intervention and prospective studies in diverse populations with clinically relevant endpoints as well as sensitive neurocognitive tests and brain biomarkers associated with preclinical AD risk are required to understand the effect of diet on AD from the earliest to later stages of disease. |
| Gut microbiome | Numerous evidences have been obtained on the relationship between gut microbiota and AD from clinical studies, animal experiments, and mechanism exploration. | Whether some specific bacteria or combinations of bacteria in the gut microbiota have a role in the prevention and treatment of AD remains to be further clarified. |
| Hearing loss | Peripheral and central hearing loss are associated with lower regional brain volumes and dementia risk | Studies to determine mechanisms and direction of associations. |
- Cited Here |
- View Full Text | PubMed | CrossRef
- PubMed | CrossRef
- View Full Text | PubMed
Alzheimer disease; Sleep; Hypoxia; Diet; Gut microbiota
- + Favorites
- View in Gallery
Readers Of this Article Also Read
Relationship between depression and lifestyle factors in chinese adults using..., association between common cardiovascular drugs and depression, limited value of recovery phase-limited st segment depression of treadmill exercise test</strong>', 'yang hongbo; huang, zheyong; lou, yi; shen, yunli; qian, juying; ge, junbo', 'chinese medical journal', 'february 20, 2014', '127', '4' , 'p 742-746');" onmouseout="javascript:tooltip_mouseout()" class="ejp-uc__article-title-link"> limited value of recovery phase-limited st segment depression of treadmill....
ORIGINAL RESEARCH article
Early-stage alzheimer's disease prediction using machine learning models.

- 1 Department of Computer Science and Engineering, Sathyabama Institute of Science and Technology, Chennai, India
- 2 Al-Nahrain Nanorenewable Energy Research Center, Al-Nahrain University, Baghdad, Iraq
- 3 COMBA R&D Laboratory, Faculty of Engineering, Universidad Santiago de Cali, Cali, Colombia
Alzheimer's disease (AD) is the leading cause of dementia in older adults. There is currently a lot of interest in applying machine learning to find out metabolic diseases like Alzheimer's and Diabetes that affect a large population of people around the world. Their incidence rates are increasing at an alarming rate every year. In Alzheimer's disease, the brain is affected by neurodegenerative changes. As our aging population increases, more and more individuals, their families, and healthcare will experience diseases that affect memory and functioning. These effects will be profound on the social, financial, and economic fronts. In its early stages, Alzheimer's disease is hard to predict. A treatment given at an early stage of AD is more effective, and it causes fewer minor damage than a treatment done at a later stage. Several techniques such as Decision Tree, Random Forest, Support Vector Machine, Gradient Boosting, and Voting classifiers have been employed to identify the best parameters for Alzheimer's disease prediction. Predictions of Alzheimer's disease are based on Open Access Series of Imaging Studies (OASIS) data, and performance is measured with parameters like Precision, Recall, Accuracy, and F1-score for ML models. The proposed classification scheme can be used by clinicians to make diagnoses of these diseases. It is highly beneficial to lower annual mortality rates of Alzheimer's disease in early diagnosis with these ML algorithms. The proposed work shows better results with the best validation average accuracy of 83% on the test data of AD. This test accuracy score is significantly higher in comparison with existing works.
Introduction
Alzheimer's Disease (AD) is a progressive neurological condition that leads to short-term memory loss, paranoia, and delusional ideas that are mistaken for the effects of stress or aging. In the United States, this Disease affects about 5.1 million people. AD does not have proper medical treatment. In order to control AD, continuous medication is necessary. AD ( 1 ) is chronic so that it can last for years or the rest of your life. Therefore, it is most important to prescribe medication at the appropriate stage so that the brain is not damaged to a great extent. Early detection of this Disease is a tedious and costly process since we must collect a lot of data and use sophisticated tools for prediction and have an experienced doctor involved. Automated systems are more accurate than human assessment and can be used in medical decision support systems because they are not subject to human errors. Based on previous research on AD, researchers have applied images (MRI scans), biomarkers (chemicals, blood flow), and numerical data extracted from the MRI scans to study this Disease. As such, they were able to determine whether a person was demented or not. In addition to shortening diagnosis time, more human interaction will be reduced by automating Alzheimer's diagnosis. In addition, automation reduces overall costs and provides more accurate results. For example, we can predict whether a patient is demented by analyzing MRI scans and applying prediction techniques. If a person has early-stage Alzheimer's Disease, they are considered demented. By doing so, we can achieve better accuracy.
When a person has Alzheimer's Disease in the early stages, they can usually function without any assistance. In some cases, the person can still work, drive, and partake in social activities. Although this is the case, the person may still feel uneasy or suffer from memory loss, such as not remembering familiar words and locations. People close to the individual notice that they have difficulty remembering their names. By conducting a detailed medical interview, a doctor may identify problems with memory and concentration in the patient. Common challenges in early stage of Alzheimer's Disease include,
• It's hard to remember the right word or name.
• Having difficulty remembering names when meeting new people.
• Working in social settings or the workplace every day can be challenging.
• Having forgotten something that you have just read in a book or something else.
• Having trouble finding or misplacing a valuable object.
• Tasks and activities are becoming increasingly difficult to plan or organize.
Alzheimer's symptoms become more persistent as the Disease progresses. When people suffer from dementia, their ability to communicate, adapt to their environment, and eventually move is lost. It becomes much more difficult for them to communicate pain through words or phrases. Individuals may need substantial assistance with daily activities as their memory, and cognitive skills continue to decline. At this stage, individuals may:
• Personal care and daily activities require 24/7 assistance.
• The consciousness of their surroundings, as well as recent experiences, is lost.
• As you age, you may experience changes in your physical abilities and walking, sitting, and eventually swallowing.
• Communication with others is becoming increasingly difficult.
• Infections, specifically pneumonia, become more prevalent.
Under the current conditions, human instinct and standard measurements do not often coincide. In order to solve this problem, we need to leverage innovative approaches such as machine learning, which are computationally intensive and non-traditional. Machine learning techniques are increasingly being used in disease prediction and visualization to offer prescient and customized prescriptions. In addition to improving patients' quality of life, this drift aids physicians in making treatment decisions and health economists in making their analyses. Viewing medical reports may lead radiologists to miss other disease conditions. As a result, it only considers a few causes and conditions. The goal here is to identify the knowledge gaps and potential opportunities associated with ML frameworks and EHR derived data.
Contribution
In our research work, people affected by Alzheimer's Disease are identified and we aims at finding individuals who potentially have Alzheimer's at an early stage. The datasets for Alzheimer's Disease is available on both OASIS and Kaggle which is used for training all patient's data using various machine learning algorithms such as SVM, Random Forest classifier, Decision tree classifier, XGBoost and Voting classifier to effectively distinguish the affected individuals with high degree of efficiency and speed. Finally, an overview of how the Disease has affected the population according to various criteria is analyzed.

Organization
Following are the different sections of our work: Section Related Works address the recent papers on detecting Alzheimer's Disease using Machine learning and Deep learning models. Section Materials and Methods discusses the exploratory data analysis, and different Machine learning classifier models. Results and Discussion section address the performance measures of different Machine Learning models. Finally Section Conclusion concludes the work and discusses the future work.
Related Works
Alzheimer's Disease is predicted using ML algorithms by using a feature selection and extraction technique, and the classification is conducted based on the oasis longitudinal dataset. The different techniques ( 2 ) involved in analyzing brain images for diagnosing diseases of the brain to provide a brief overview. Several major issues are discussed in this article relating to machine learning and deep learning-based brain disease diagnostics based on the results of the reviewed articles. The most accurate method of detecting brain disorders was found in this study and can be used to improve future techniques. Using machine learning and deep learning platforms, this study aims to combine recent research on four brain diseases: Alzheimer's disease, brain tumors, epilepsy, and Parkinson's disease. By using 22 brain disease databases that are used most during the reviews, the authors can determine the most accurate diagnostic method.
Martinez-Murcia et al. ( 3 ) uses deep convolutional autoencoders to explore data analysis of AD. Data-driven decomposition of MRI images allows us to extract MRI features that represent an individual's cognitive symptoms as well as the underlying neurodegeneration process. A regression and classification analysis are then performed to examine the distribution of the extracted features in a wide variety of combinations, and the influence of each coordinate of the autoencoder manifold on the brain is calculated. MMSE or ADAS11 scores, along with imaging-derived markers, can be used for over 80% accuracy to predict AD diagnosis.
A deep neural network is used with layers ( 4 , 5 )) that are all connected to perform binary classification. Each hidden layer is activated by a different activation function. A model with the best performance is chosen after k-folds validation Researchers at the Lancet Commission found that about 35% of Alzheimer's risk factors can be modified. The following factors can contribute to these risks: a lack of education, hypertension, obesity, hearing loss, depression, diabetes, lack of physical activity, smoking, and social isolation. Regardless of the impact of these factors at any stage of life, it is beneficial to eliminate them. Studies have suggested ( 6 ) that early intervention and treatment of modifiable Alzheimer's risk factors can prevent or delay 30% of cases of Alzheimer's ( 7 ). According to the Innovative Midlife Intervention for Alzheimer's Deterrence (In-MINDD) project ( 8 ) one way to calculate Alzheimer's risk based on risk factors is by using the Lifestyle for Brain Health (LIBRA) index ( 9 – 12 ). According to the National Academy of Medicine ( 13 , 14 ) cognitive training, hypertension management, and increased physical activity were the three main categories of dementia intervention. The most common type of Alzheimer's is Alzheimer's Disease (AD). Among the types of Alzheimer's, Vascular Alzheimer's (VaD) is the second most common, followed by Alzheimer's with Lewy bodies. A few other types of Alzheimer's are associated with brain injuries, infections, and alcohol abuse. Tatiq and Barber ( 15 ) in their study suggested that Alzheimer's can be prevented by targeting modifiable vascular risk factors because these two types often co-exist in the brain and share some modifiable risk factors. Williams et al. ( 16 ) obtained predictions of cognitive functioning based on neuropsychological and demographic data using four different models: SVM, Decision Tree, NN, and Naïve-Bayes. In this case, average values were substituted for the missing values; the accuracy of Naive Bayes was the highest. Data from ADNI study are applied using ten-fold cross-validation ( 17 , 18 ) and show high correlation between genetic, imaging, biomarker, and neuropsychological outcomes. MRI images from the OASIS dataset ( 19 , 20 ) are analyzed using voxel-based morphometry. Table 1 summarizes the recent work on prediction of Alzheimer's disease.
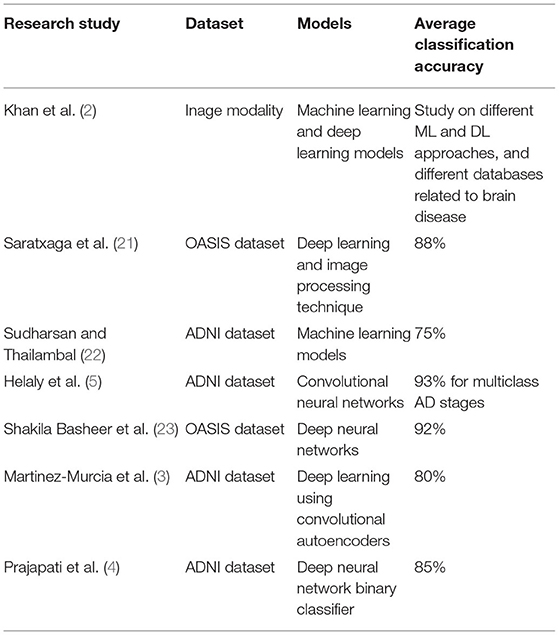
Table 1 . Summary of recent work related to AD.
Materials and Methods
The proposed approach consists of three basic steps. Firstly, the Alzheimer's disease dataset ( 24 – 26 ) was loaded into pandas for data preprocessing. This study utilized a longitudinal dataset, so a timeline of the study was necessary to gain further insight into the data. Our first step was to determine how cross-sectional the data appear to be, if either at a baseline or at a particular time. A complete analysis of the data was then conducted, including a comparison of the main study parts and the corresponding data collected during each visit. In this work, longitudinal MRI data is our primary data source. MRI data from 150 patients aged from 60 to 96 were included in the study. We scanned each patient at least once. Everyone is right-handed. Throughout the study, 72 of the patients were classified as “non-demented”. At the time of their initial visits, 64 patients were classified as being “Demented,” and they remained in this category throughout the study. Table 2 shows the dataset description of MRI data.
Table 2 . Dataset description.
The Machine Learning techniques ( 26 , 27 ) were applied to Alzheimer's disease datasets to bring a new dimension to predict Disease at an early stage. The raw Alzheimer's disease datasets are inconsistent and redundant, which affects the accuracy of algorithms ( 28 , 29 ). Before evaluating machine-learning algorithms, data must be effectively prepared for analysis by removing unwanted attributes, missing values, and redundant records. Building a machine-learning model requires splitting the data into training and testing sets. In the following data preparation step, the training data were used to create a model, which was then applied to test data to predict Alzheimer's Disease ( 28 , 30 , 31 ). The model was trained from training set data, and test set data were used to test unseen data. Cross-validation was carried out by dividing the dataset into three subsets. Model predictions are made using one subset of the data (test data) and model performance is evaluated using the other subsets (training and validation) of the data. The data had been preprocessed, and we randomly divided it into an 80:20 ratio, with 80% going to training and 20% gone to testing. Figure 1 describes the system workflow for predicting the Alzheimer's Disease at early stage ( 32 , 33 ).
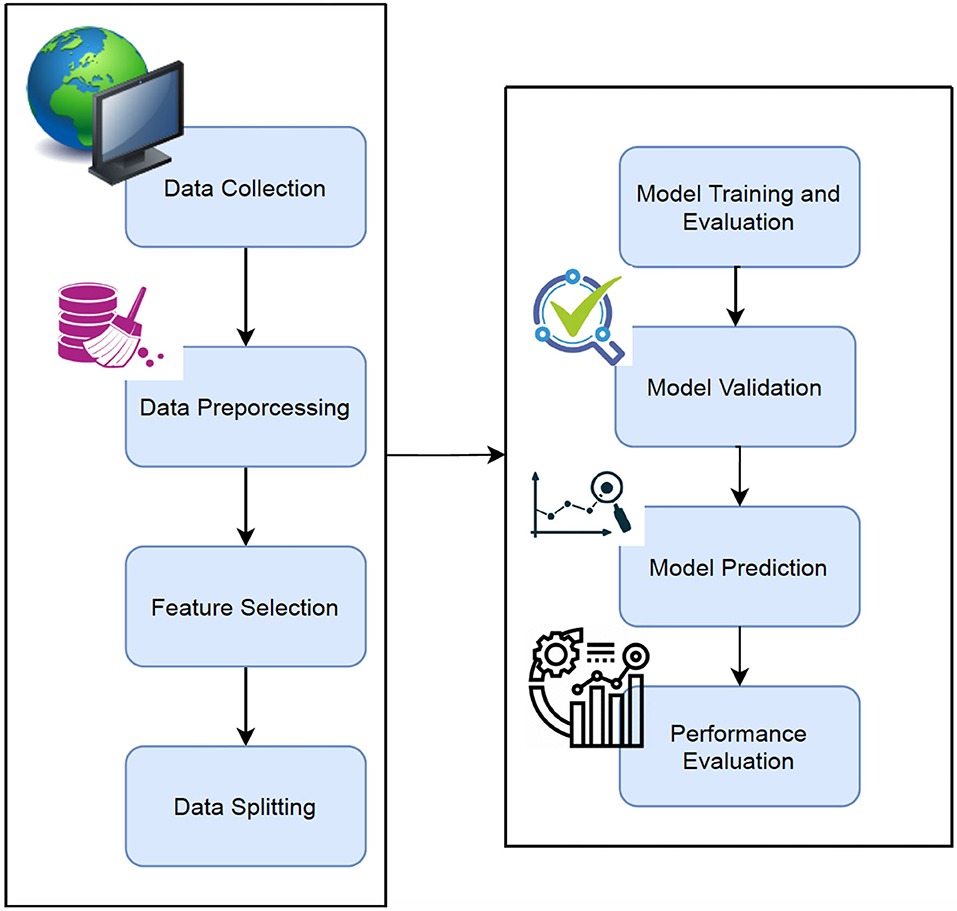
Figure 1 . Proposed workflow.
Data Preparation
Various data-mining techniques were used to clean and preprocess the data in this phase. As part of this, missing values are handled, features are extracted, and features are transformed, and so on. In the SES column, we identified 9 rows with missing values ( 34 , 35 ). This issue is addressed in two ways. The simplest solution is to drop the rows with missing values. The other way to fill in missing values is by Imputation ( 21 ), which refers to replacing them with their corresponding values. The model should perform better if we impute since we only have 140 measurements. The 9 rows with missing values are removed in the SES attribute and the median value is used for the imputation.
Data Analysis
We have discussed the relationships between each feature of an MRI test and dementia in this section. In order to formulate the relationship of data explicitly using a graph, we conducted this Exploratory Data Analysis process ( 36 , 37 ) to estimate the correlations before extracting data or analyzing it. The information could be used to interpret the nature of the data later on and to determine what method to use to analyze it. Table 3 shows the Min, Max and median values of each attribute.
Table 3 . Min, max, and median values of each attribute.
Feature Selection
Feature selection is very important in machine learning. In this work, feature selection is applied to the clinical data of Alzheimer's disease where we have thousands of samples. Feature selection ( 22 ) has three methods such as: Filter methods, Wrapper methods, and embedded methods. Filter method is a common method used in the stage of pre-processing. Wrapper methods is another method which core the feature subset. Finally, Embedded method combines the filter and wrapper methods.
The most common and popular feature selection methods are chosen in this work are Correlation coefficient, Information gain, and Chi-Square.
Correlation Coefficient
The covariance between the two variables X and Y is defined as
The covariance between two variables measures the linear relationship between them. Using correlation coefficients, it is easy to find a correlation between the various stages of Alzheimer's. The problem with this method is that the data is collected from a broad range of sources, so it becomes very sensitive to outliers.
Information Gain
The entropy of the lower node is subtracted from the entropy of upper node to obtain the Information gain value when the attribute D is selected.
Chi-Square: Using this method, we can examine categorical variables such as the relationship between food and obesity.
Preparation and Splitting the Data
Figure 2 shows the schematic representation of data splitting stage
1. Select Data: M.F, Age, EDUC, SES, MMSE, eTIV, nWBV, ASF, CDR
2. Train_Data < - round(0.8 * nrow(data)) #Select 80% of train data
3. TrainData_indices < - sample(1:nrow(data), Train_Data). #Vector is created with random indeces
4. TrainML < - data[TrainData_indices, ] #Training dataset is generated
5. SplitFormula < - CDR ~ M.F + Age + EDUC + SES + MMSE + eTIV + nWBV
7. Split < - nWayCrossValidation(nrow(data), n). #5-fold cross validation is generated
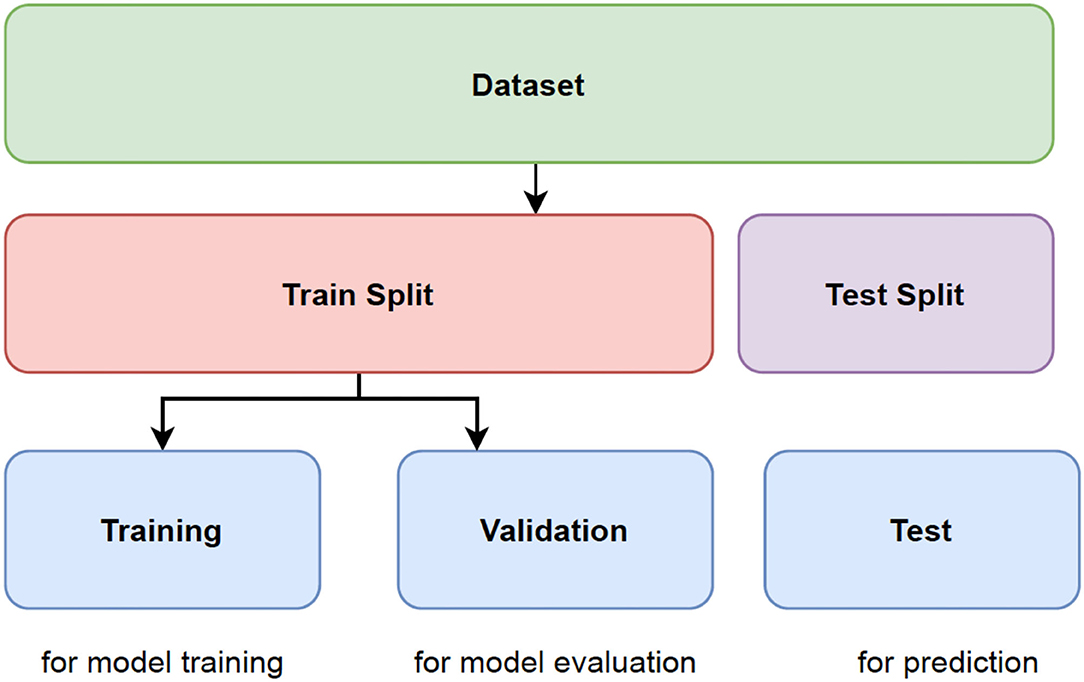
Figure 2 . Representation of data splitting.
Classifier Models
Decision tree (dt).
An overview of the decision tree gives a tree-based model for dividing the data repeatedly based on the cutoff values of the features. Splitting creates subsets by separating instances into subsets. Intermediate subsets are referred to as internal nodes, while leaves are referred to as leaf nodes. A decision tree is most useful when there is significant interaction between the features and the target.
Random Forest (RF)
A random forest model performs better than a decision tree because it avoids the problem of overfitting. Models based on random forests consist of various decision trees, each slightly different from the others. Using the majority voting algorithm, the ensemble makes predictions based on each individual decision tree model (bagging). As a result, the amount of overfitting is reduced while maintaining the predictive ability of each tree.
Support Vector Machine (SVM)
This method involves determining the class of data points by appropriate hyper planes in a multidimensional space. By using SVM ( 25 ), we aim to find a hyperplane that separates cases of two categories of variables that take up neighboring clusters of vectors, one on one side, the other on the other side. Support vectors are those that are closer to the hyperplane. Training and test data are used in SVM. Training data is broken up into target values and attributes. SVM produces a model for predicting target values for test data.
XGBoost stands for eXtreme Gradient BOOSTting. It refers to the process of implementing gradient-boosted decision trees for maximum speed and performance. Due to the sequential nature of model training, gradient boosting machines are generally slow in implementation and not very scalable. XGBoost is focused on speed and performance.
Voting is one of the simplest ways of combining the predictions from multiple earning algorithms. Voting classifiers aren't actually classifiers but are more like wrappers for multiple ones that are trained and evaluated concurrently in order to benefit from their specific characteristics. We can train data sets using different algorithms and ensembles then to predict the final output. There are two ways to reach a majority vote on a prediction:
Hard voting: The simplest form of majority voting is hard voting. The class with the most votes (Nc) will be chosen in this case. Our prediction is based on the majority vote of each classifier.
Soft voting: This involves adding up the probability vectors for each predicted class (for all classifiers) and choosing the one that represents the highest value (recommended only when the classifiers are well calibrated).
Model Validation
Model validation reduces the overfitting problem. Cross Validation is done to train the ML model and are used to calculate the accuracy of the model. It is a challenging task to make the ML model from noise free. Hence, in this research work, Cross validation is performed which divides the whole dataset into n divisions which is of equal in size. The ML model is trained for every iteration with the n-1 divisions. The performance of the method is analyzed by the mean of all n -folds. In this work, the ML model was trained and tested 10 times by applying ten-fold cross validation to the model.
Results and Discussions
We evaluate various performance metrics like accuracy, precision, recall and F1 score. To determine the best parameters for each model, we perform 5-fold cross-validation: Decision Tree, SVM, Random Forests, XGBoost and Voting. Finally, we compare accuracy of each model. Several metrics and techniques were used to identify overfitting and parameter tuning issues after the models were developed. Performance evaluations can either be binary or multiclass and are described using the confusion matrix. A learning model was developed to distinguish true Alzheimer's disease affected people from a given population and a novel Machine Learning classifier was developed and validated to predict and separate true Alzheimer's disease affected people. The following evaluation measures were calculated using these components: precision, recall, accuracy, and F-score. Based on this study, recall (sensitivity) is the proportion of people accurately classified as having Alzheimer's. The precision of Alzheimer's diagnosis is the rate of people correctly classified as not having the disease. Alternatively, F1 represents the weighted average of recall and precision, while accuracy represents the proportion of people correctly classified. According to the results, the patient receives a report that tells him or her what stage of Alzheimer's Disease he or she is currently in. It is very important to detect the stages because the stages are based on the responses of the patients. In addition, knowing the stage helps doctors better understand how the Disease is affecting them. This research used these environments, tools, and libraries to conduct its experiments and analysis:
a) Environments Used: Python 3
b) Scikit-learn libraries for machine learning
The Figure 3 indicates that men are more likely than women to have dementia. Figure 4 that the non-demented group had much higher MMSE (Mini-Mental State Examination) scores than those with dementia.
Figure 3 . Analysis of demented and non-demented rate based on gender, Gender group Female = 0, Male = 1.
Figure 4 . Analysis of MMSE scores for demented and non-demented group of patients.
The Figures 5A–C shows the analyzed value of ASF, eTIV and nWBV for Demented and Non-demented group of people. As indicated by the graph in Figure 5 , the Non-demented group has a higher brain volume ratio than the Demented group. The reason for this is that the diseases influence the brain tissues causing them to shrink. Figure 6 shows the analyzed results of EDUC for Demented and Non-demented people.
Figure 5. (A–C) Analysis of ASF, eTIV and nWBV for Demented and Non-demented group.
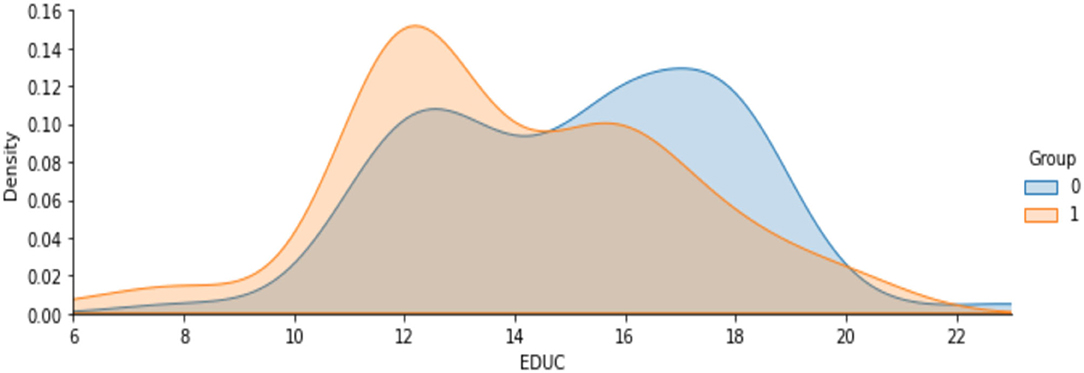
Figure 6 . Analysis on years of education.
Figure 7 shows the analysis on age attribute to find the percentage of people affected based on the demented and non-demented group. It is observed that a higher percentage of Demented patients are 70-80 years old than non-demented patients. It is likely that people with that kind of Disease have a low survival rate. Only a few people are over 90 years old.
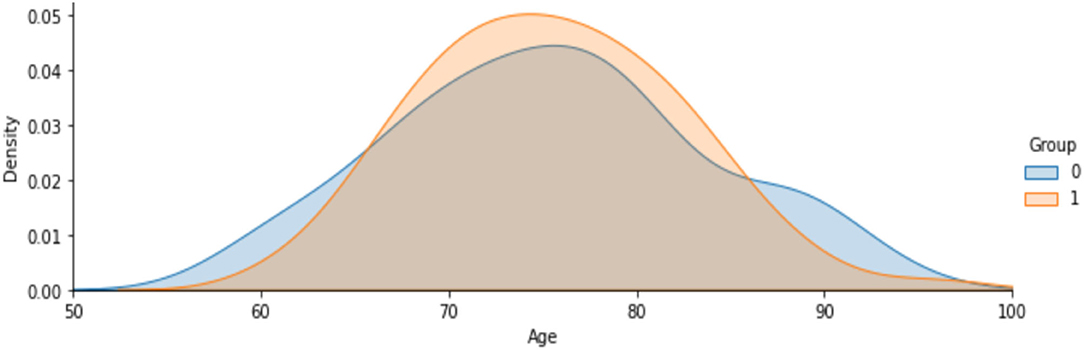
Figure 7 . Analysis on people affected by demented and non-demented group based on age.
From the above all analysis on the attributes, the following are the summary on intermediate results.
1. It is more likely for men to have demented, or Alzheimer's Disease, than for women.
2. In terms of years of education, demented patients were less educated.
3. Brain volume in non-demented groups is greater than in demented groups.
4. Among the demented group there is a higher concentration of 70-80-year-olds than in the non-demented patients.
Table 4 shows the performance comparison of accuracy, precision, recall, and F1 score for different ML models. The performance measures are defined as,
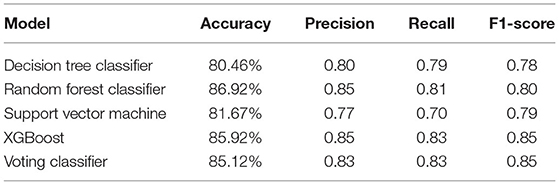
Table 4 . Performance comparison of different ML models.
Accuracy: It is the measure of finding the proportion of correctly classified result from the total instances.
Precision: This measures the number of correctly predicted positive rate divided by the total predicted positive rates. If the Precision value is 1, it is meant as a good classifier.
Recall: Recall is a true positive rate. If the recall is 1, it is meant as a good classifier.
F1 Score: It is a measure which considers both Recall and Precision parameters. F1 score becomes 1 only when both the measure such as Recall and Precision is 1.
The most common metrics are the conversions of the True Positive (TP), the False Positive (FP), the True Negative (TN), and the False Negative (FN) metrics. Figures 8 – 13 shows the confusion matrix for Decision tree, Random Forest, SVM, XG boost, Soft, and Hard Voting classifier ML models.
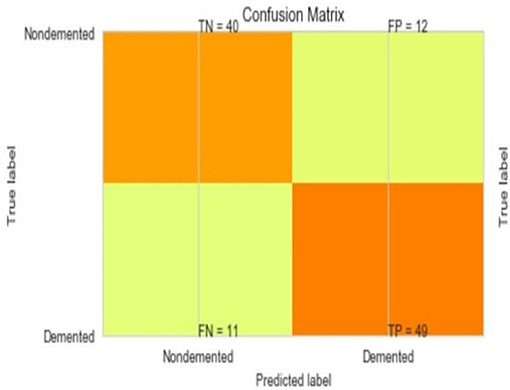
Figure 8 . Confusion matrix for decision tree.
Figure 9 . Confusion matrix for random forest.
Figure 10 . Confusion matrix for SVM.
Figure 11 . Confusion matrix for XGBoost.
Figure 12 . Confusion matrix for soft voting classifier.
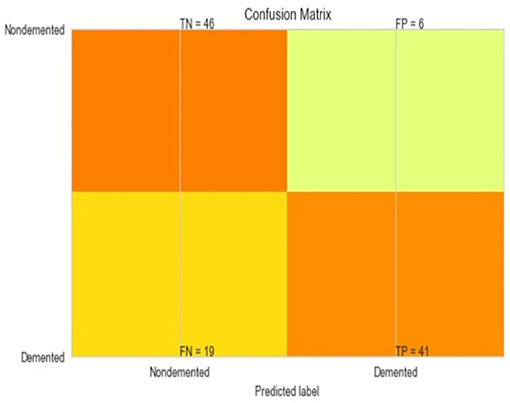
Figure 13 . Confusion matrix for hard voting classifier.
A comparison of training and testing accuracy has been conducted for each model to eliminate overfitting. For each model, precision, recall, accuracy, and F1-score are shown in Table 3 . Based on the analysis showed in the Table 3 , the results approved that the best and ideal techniques, which have a good performance, are random forest, and XGBoost. The accuracy value of Voting classifier model is also closer to the random forest, and XGBoost models. All the experimental results (the average accuracy, precision, recall, and F measure of each model) were collected for extra analysis. The comparative analyses among all the Machine Learning models in terms of accuracy, precision, recall, and F1 score are presented graphically in Figures 14 – 17 respectively.
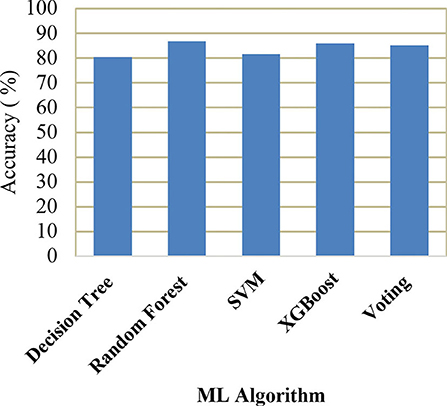
Figure 14 . Comparison of accuracy.
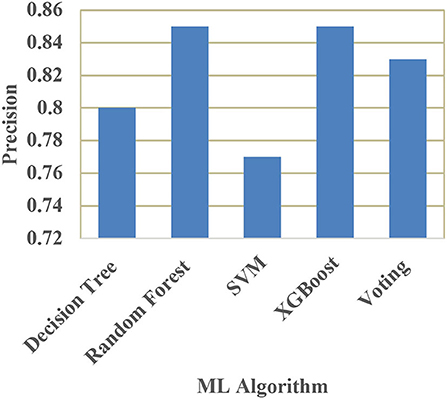
Figure 15 . Comparison of precision.
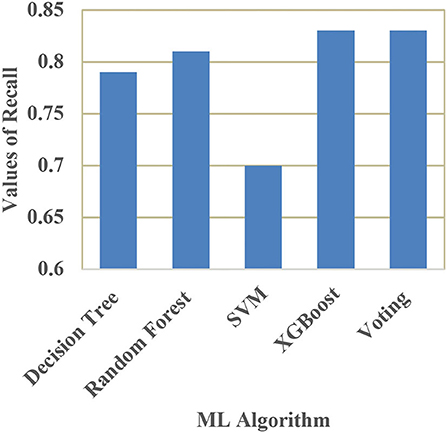
Figure 16 . Comparison of recall.
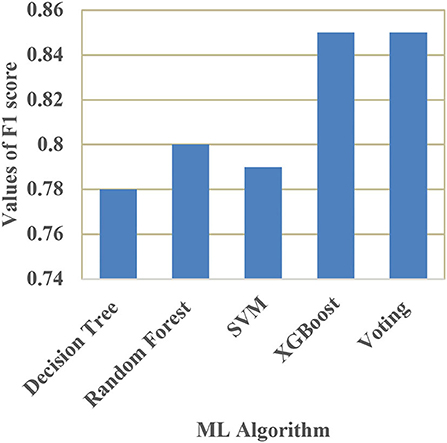
Figure 17 . Comparison of F1 score.
Conclusions
Alzheimer's is a major health concern, and rather than offering a cure, it is more important to reduce risk, provide early intervention, and diagnose symptoms early and accurately. As seen in the literature survey there have been a lot of efforts made to detect Alzheimer's Disease with different machine learning algorithms and micro-simulation methods; however, it remains a challenging task to identify relevant attributes that can detect Alzheimer's very early. The future work will focus on the extraction and analysis of new features that will be more likely to aid in the detection of Alzheimer's Disease, and on eliminating redundant and irrelevant features from existing feature sets to improve the accuracy of detection techniques. By adding metrics like MMSE and Education to our model, we'll be able to train it to distinguish between healthy adults and those with Alzheimer's.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found at: https://www.kaggle.com/jboysen/mri-and-alzheimers?select=oasis_cross-sectional.csv .
Author Contributions
CK: research concept and methodology and writing—original draft preparation. VM: review and editing. SS: supervision. OK and CT: validation. All authors contributed to the article and approved the submitted version.
This research has been funded by Dirección General de Investigaciones of Universidad Santiago de Cali under call No. 01-2021.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sivakani GA, Ansari R. Machine learning framework for implementing Alzheimer's disease. Int Conferen Commun Signal Process. (2020) 12:588–92. doi: 10.1109/ICCSP48568.2020.9182220
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Khan P, Kader MF, Islam SR, Rahman AB, Kamal MS, Toha MU, et al. Machine learning and deep learning approaches for brain disease diagnosis: principles and recent advances. IEEE Access. (2021) 9:37622–55. doi: 10.1109/ACCESS.2021.3062484
CrossRef Full Text | Google Scholar
3. Martinez-Murcia FJ, Ortiz A, Gorriz JM, Ramirez J, Castillo-Barnes D. Studying the manifold structure of Alzheimer's disease: a deep learning approach using convolutional autoencoders. IEEE J Biomed Health Inform. (2020) 24:17–26. doi: 10.1109/JBHI.2019.2914970
4. Prajapati R, Khatri U, Kwon GR. “An efficient deep neural network binary classifier for alzheimer's disease classification,” In: International Conference on Artificial Intelligence in Information and Communication (ICAIIC) . (2021), p. 231–234.
Google Scholar
5. Helaly HA, Badawy M, Haikal AY. Deep learning approach for early detection of Alzheimer's disease. Cogn Computing. (2021) 21:1–17. doi: 10.1007/s12559-021-09946-2
6. Yaffe K. Modifiable risk factors and prevention of dementia: what is the latest evidence. JAMA Intern Med. (2018) 178:281–2. doi: 10.1001/jamainternmed.2017.7299
7. Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley D, et al. Dementia prevention, intervention, and care. The Lancet. (2017) 390:2673–73. doi: 10.1016/S0140-6736<17>31363-6
8. O'Donnell CA, Manera V, Köhler S, Irving K. Promoting modifiable risk factors for dementia: is there a role for general practice? British J General Pract. (2015) 65:567–8. doi: 10.3399/bjgp15X687241
9. Sulaiman N, Abdulsahib G, Khalaf O, Mohammed MN. “Effect of Using Different Propagations of OLSR and DSDV Routing Protocols”, In Proceedings of the IEEE International Conference on Intelligent Systems Structureing and Simulation . (2014), pp. 540–5.
10. Deckers K, van Boxtel MP, Schiepers OJ, de Vugt M, Muñoz Sánchez JL, Anstey KJ, et al. Target risk factors for dementia prevention: a systematic review and Delphi consensus study on the evidence from observational studies. Int J Geriatric Psychiatry. (2015) 30:234–46. doi: 10.1002/gps.4245
11. Schiepers OJ, Köhler S, Deckers K, Irving K, O'donnell CA, Van den Akker, et al. Lifestyle for Brain Health (LIBRA): a new model for dementia prevention. Int J Geriatric Psychiatry. (2018) 33:167–75. doi: 10.1002/gps.4700
12. Vos SJ, Van Boxtel MP, Schiepers OJ, Deckers K, De Vugt M, Carrière I, et al. Modifiable risk factors for prevention of dementia in midlife, late life and the oldest-old: validation of the LIBRA Index. J Alzheimer's Dis. (2017) 58:537–47. doi: 10.3233/JAD-161208
13. Osamh Khalaf I, Ghaida M, Abdulsahib D. Energy efficient routing and reliable data transmission protocol in WSN. Int J Adv Soft Comput Applicat. (2020) 12:45–53.
14. National National Academies of Sciences Engineering and Medicine. Preventing cognitive decline and dementia: A way forward . London: The National Academies Press (2018).
15. Tariq S, Barber PA. Dementia risk and prevention by targeting modifiable vascular risk factors. J Neurochemistr. (2018) 144:565–81. doi: 10.1111/jnc.14132
16. Williams, Jennifer A, Weakley A, Cook MS, Edgecombe DJ. “Machine learning techniques for diagnostic differentiation of mild cognitive impairment and dementia,” In Workshops at the Twenty-Seventh AAAI Conference on Artificial Intelligence . (2018), pp. 71–6.
17. Khalaf OI, Sabbar BM. A modified algorithm for improving lifetime WSN. J Eng Appl Sci. (2018) 13:9277–82.
18. Khalaf OI, Abdulsahib GM, Sabbar BM. Optimization of wireless sensor network coverage using the Bee Algorithm. J Inf Sci Eng. (2020) 36:377–86.
PubMed Abstract | Google Scholar
19. Chi CL Oh, Borson WS. “Feasibility Study of a Machine Learning Approach to Predict Dementia Progression,” in International Conference: In Health care Informatics (ICHI) . (2015), p. 450.
20. Chyzhyk A, Savio D. Feature extraction from structural MRI images based on VBM: data from OASIS database, University of The Basque Country, Internal research publication (2010).
21. Saratxaga CL, Moya I, Picón A, Acosta M, Moreno-Fernandez-de-Leceta A, Garrote E, et al. MRIDeep learning-based solution forAlzheimer's Disease Prediction. J.Pers. Med. (2021) 11:902. doi: 10.3390/jpm11090902
22. Sudharsan M, Thailambal G. Alzheimer's disease prediction using machine learning techniques and principal component analysis (PCA), Materials Today: Proceedings (2021).
23. Basheer S, Bhatia S, Sakri SB. “Computational Modeling of Dementia Prediction Using Deep Neural Network: Analysis on OASIS Dataset,” in IEEE Access . (2021) 9:42449–42462. doi: 10.1109/ACCESS.2021.3066213
24. Khalaf OI, Abdulsahib GM. Frequency estimation by the method of minimum mean squared error and P-value distributed in the wireless sensor network. J Informat Sci Eng. (2019) 35:1099–112.
25. Ogudo KA, Muwawa J, Ibrahim Khalaf O, Daei Kasmaei H. A device performance and data analytics concept for smartphones' IoT services and machine-type communication in cellular networks. Symmetry. (2019) 11:593–609. doi: 10.3390/sym11040593
26. Reddy GT, Reddy MP, Lakshmanna K, Rajput DS, Kaluri R, Srivastava G. Hybrid genetic algorithm and a fuzzy logic classifier for heart disease diagnosis. Evol Intel. (2020) 13:185–96. doi: 10.1007/s12065-019-00327-1
27. Abdulsahib GM, Khalaf OI. Accurate and effective data collection with minimum energy path selection in wireless sensor networks using mobile sinks. J Informat Technol Manage. (2020) 13:139–53.
28. Gadekallu TR, Khare N, Bhattacharya S, Singh S, Maddikunta PK, Srivastava G. Deep neural networks to predict diabetic retinopathy. J Ambient Intell Human Computing. (2020) 21:1–4. doi: 10.1007/s12652-020-01963-7
29. Salman AD, Khalaf OI, Abdulsahib GM. An adaptive intelligent alarm system for wireless sensor network. Indonesian J Electric Eng Comput Sci. (2019) 15:142–7. doi: 10.11591/ijeecs.v15.i1.pp142-147
30. Srinivasu PN, Bhoi AK, Jhaveri RH, Reddy GT, Bilal M. Probabilistic Deep Q Network for real-time path planning in censorious robotic procedures using force sensors. J Real-Time Image Proc. (2021) 18:773–1785. doi: 10.1007/s11554-021-01122-x
31. Khalaf OI, Abdulsahib GM, Kasmaei HD, Ogudo KA. A new algorithm on application of blockchain technology in live stream video transmissions and telecommunications. Int J e-Collaboration (IJeC). (2020) 16:16–32. doi: 10.4018/IJeC.2020010102
32. Abdulsahib GM, Khalaf OI. An improved algorithm to fire detection in forest by using wireless sensor networks. Int J Civil Eng Technol (IJCIET). (2018) 9:369–77.
33. Abdulsahib GM, Khalaf IO. Comparison and evaluation of cloud processing models in cloud-based networks. Int J Simul Syst Sci Technol. (2018) 19:5. doi: 10.5013/IJSSST.a.19.05.26
34. Khalaf OI, Abdulsahib GM. Optimized dynamic storage of data (ODSD) in IoT based on blockchain for wireless sensor networks. Peer-to-Peer Netw. (2021) 21:255. doi: 10.1007/s12083-021-01115-4
35. Javed AR, Fahad LG, Farhan AA, Abbas S, Srivastava G, Parizi RM, et al. Automated cognitive health assessment in smart homes using machine learning. Sustain Cities Soc. (2020) 65:102572. doi: 10.1016/j.scs.2020.102572
36. Maddikunta PR, Gadekallu TR, Iwendi C. Identification of malnutrition and prediction of BMI from facial images using real-time image processing and machine learning. IET Image Process. (2021) 21:1–12. doi: 10.1049/ipr2.12222
37. Javed AR, Sarwar MU, ur Rehman S, Khan HU, Al-Otaibi YD, Alnumay WS. PP-SPA: privacy preserved smartphone-based personal assistant to improve routine life functioning of cognitive impaired individuals. Neural Process Lett. (2021) 21:1–8. doi: 10.1007/s11063-020-10414-5
Keywords: healthcare, prediction, Alzheimer's disease (AD), machine learning, feature selection
Citation: Kavitha C, Mani V, Srividhya SR, Khalaf OI and Tavera Romero CA (2022) Early-Stage Alzheimer's Disease Prediction Using Machine Learning Models. Front. Public Health 10:853294. doi: 10.3389/fpubh.2022.853294
Received: 12 January 2022; Accepted: 26 January 2022; Published: 03 March 2022.
Reviewed by:
Copyright © 2022 Kavitha, Mani, Srividhya, Khalaf and Tavera Romero. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: C. Kavitha, kavitha4cse@gmail.com
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
A Research Paper on Alzheimer’s Disease
This research paper will delve into Alzheimer’s Disease, covering its causes, symptoms, progression, and current treatments. It will also explore ongoing research in finding a cure and ways to manage this debilitating condition. On PapersOwl, there’s also a selection of free essay templates associated with Alzheimers Disease.
How it works
In this paper, Alzheimer’s disease will be delved into, investigated and dissected. This will include all that is known about the disease as much of it is unknown still, despite increasing efforts from the medical community to uncover its origin. The disease’s causes, symptoms and stages will be discussed and illuminated. The effects on other body systems, its signs and symptoms and any other complications will be highlighted as well. Additionally, advancements in treating this disease are carefully examined.
In this paper, I will be giving an overview of Alzheimer’s disease. This will include its history, prognosis and treatment available and recent advancements made towards finding a cure. Alzheimer’s disease is a somewhat recently discovered phenomenon. It is a specific type of dementia, a disease that impairs a person’s cognitive functioning and behavioral abilities to the point that it interferes with that person’s daily life. The disease was discovered in 1906 by Dr. Alois Alzheimer who noted shrinkage around nerve cells in his patient’s brain who had also reported,” symptoms of memory loss, paranoia and psychological changes.”, according to the National Institute of Aging. After the patient passed, Dr. Alzheimer dissected her brain, finding strange clumps which we now know are amyloid plaques as well as tangled fibers now called neurofibrillary. Alzheimer’s disease is characterized by the symptoms of the aforementioned patient’s, however the disease is a very slowly progressing one, so most afflicted don’t know until the symptoms become obvious to those around them. “Alzheimer’s disease is the most frequent cause of irreversible dementia in adults. The intellectual impairment progresses gradually from forgetfulness to total impairment.” (Mace, Rabins 15) Symptoms usually appear in a person’s mid-60’s, however there are rare cases of early on-set Alzheimer’s where symptoms are exhibited in a person’s 30’s and 40’s. The disease usually progresses to the point that a person afflicted is unable to take care of themselves due to severe memory loss and loss of motor skills, requiring full time assistance. They may also experience forms of delusion such as hallucinations or paranoia that cause them to act impulsively in the moderate stage of dementia, according to the National Institute of Aging. Most diagnosed with this disease will reach this point sadly, as they usually have an average of eight years left to live after the diagnosis as there is no cure, only treatments. The difficulty in treating Alzheimer’s is highlighted by the fact that the first FDA approved drug to treat it wasn’t available until 1993, almost a full century after its discovery. As of today, there are a total of five FDA approved drugs for treating Alzheimer’s disease, none of which truly treat the disease but only prolong the symptoms that will eventually surface. “If we had a drug or other intervention that made people with Alzheimer’s disease even a little better, nevermind curing the disease, I’d sing its praises to the rooftops.[…] But there is not.” (Dedsen 4)
Alzheimer’s disease affects every body system in humans due to the fact that it destroys the brain. It atrophizes, or shrinks, the brain’s neurons and their networks die off, resulting in shrinking of various brain regions. There is no cure as of yet because there is no known way to reverse deterioration of these precious cells. Warning signs of the disease include symptoms of memory loss, severe enough that it affects job performance, difficulty with familiar tasks, issues with language, difficulty with keeping track of time or place, decreased judgment skills, severe mood changes and inability to recognize loved ones, especially in the late stages of the disease, according to the Alzheimer’s Association. Even a change of a person’s sense of humor can be a warning sign. Most individuals who reach the late stages of this disease will require full time assistance such as live in nurses.
On the bright side, specialists typically accurately diagnose Alzheimer’s at a rate of 95%. The only true way to confirm Alzheimer’s disease is through autopsy, however there are a multitude of tests specialists utilize to differentiate Alzheimer’s from other forms of dementia. These include genetic testing, magnetic resonance imaging, urinalysis, blood tests, electroencephalogram, spinal tap, computed tomography scan, chest X-ray and a mental status test, according to the Alzheimer’s Association. In contrast, the prognosis with treatment for those affected is currently bleak. There are few medications available to those with Alzheimer’s and none prevent or cure the disease. Average life expectancy is eight years after diagnosis, but it can range from one to twenty years for some, all according to the Alzheimer’s Association.
Currently, there are hundreds of studies being conducted on treating and preventing Alzheimer’s disease. Most medications being proposed are modifying therapies, meaning that they could alter how the disease progresses. others include cognitive enhancers for improving memory or attentiveness and lastly symptomatic agents which may lessen symptoms such as hallucinations. The focus areas of research currently being conducted are clinical and laboratory research. Clinical research at the Mayo Clinic Study of Aging focus on normal aging, mild cognitive impairment and dementia disorders. This process is used in the hopes of discovering patterns or signs that may help specialists discover risk of Alzheimer’s even sooner than previously possible. Laboratory research includes studying amyloid and tau proteins. Both of these proteins have strong associations with those at risk for Alzheimer’s and other forms of dementia. Amyloid proteins are being studied with both human and mouse models to determine any genetic factors that might predispose people to this disease. Additionally, tau proteins are being studied to ascertain the possibility of preventing the build up of this protein that causes neurons to malfunction and die, according to the Alzheimer’s Association. Currently, there is no prevention of the disease itself, only medication that may slow down the progress of the disease in certain individuals. There are tests available to help determine if you or a loved one may be at risk, but no prevention. Alzheimer’s is a devastating disease due to the fact that it’s largely out of our control. Later generations may see improvements in treating it or preventing it or ideally finding a cure. However, the fact that it’s been known for over a century and there has yet to be substantial slowing of the progress of the disease through medication, no available prevention and no cure whatsoever is depressing to say the least. It is “the only one of the nation’s leading 10 causes of death for which there is no effective treatment.” (Dedsen 4) However, having said this, there has been a greater push and call for urgency in discovering a cure for the disease. President Obama signed The National Plan to Address Alzheimer’s Disease into effect in January of 2011. This initiative gave greater funding for new research projects, better tools for clinicians, easier access to information to help caregivers and created an awareness campaign, according to the National Institute of Aging. There has even been news about its awareness efforts in pop culture thanks to Seth Rogen and his wife Lauren MIller Rogen, who’s mother passed away due to complications from being diagnosed with Alzheimer’s disease, creating the program, “Hilarity for Charity”. This program is described as being,” a non-profit movement dedicated to raising awareness, inspiring change and accelerating progress in Alzheimer’s care, research and support through the engagement of millenials.” For six years in a row now, the Rogens have put on a stand-up special grouping together various comedians to help raise funds for Alzheimer’s research, the most recent of which can be streamed on netflix. While there is no cure, seeing such a push for progress in understanding and fighting this disease can only give one hope that major advancements will be made in the future.
- About Hilarity for Charity. (n.d.). Retrieved from https://hilarityforcharity.org/about/
- Alzheimer’s Disease Fact Sheet. (2016, August). Retrieved from https://www.nia.nih.gov/health/alzheimers-disease-fact-sheet
- Bredesen, D. (2017) The End of Alzheimer’s: The First Program to Prevent and Reverse Cognitive Decline. New York, NY: Penguin Random House.
- R. C. (n.d.). Research and Prognosis on Alzheimer’s Disease. Retrieved from https://www.gulfbend.org/poc/view_doc.php?type=doc&id=3249&cn=231
- Mace, N. L., & Rabins, P. V. (2017). The 36-hour day: A family guide to caring for people who have Alzheimer disease, related dementias and memory loss. New York: Grand Central.
- Obama administration presents national plan to fight Alzheimer’s disease. (n.d.). Retrieved from https://www.nia.nih.gov/news/obama-administration-presents-national-plan-fight-alzheimers-disease
- What Is Alzheimer’s? (n.d.). Retrieved from https://www.alz.org/alzheimers-dementia/what-is-alzheimers
Cite this page
A Research Paper on Alzheimer's Disease. (2019, Aug 31). Retrieved from https://papersowl.com/examples/a-research-paper-on-alzheimers-disease/
"A Research Paper on Alzheimer's Disease." PapersOwl.com , 31 Aug 2019, https://papersowl.com/examples/a-research-paper-on-alzheimers-disease/
PapersOwl.com. (2019). A Research Paper on Alzheimer's Disease . [Online]. Available at: https://papersowl.com/examples/a-research-paper-on-alzheimers-disease/ [Accessed: 10 Jun. 2024]
"A Research Paper on Alzheimer's Disease." PapersOwl.com, Aug 31, 2019. Accessed June 10, 2024. https://papersowl.com/examples/a-research-paper-on-alzheimers-disease/
"A Research Paper on Alzheimer's Disease," PapersOwl.com , 31-Aug-2019. [Online]. Available: https://papersowl.com/examples/a-research-paper-on-alzheimers-disease/. [Accessed: 10-Jun-2024]
PapersOwl.com. (2019). A Research Paper on Alzheimer's Disease . [Online]. Available at: https://papersowl.com/examples/a-research-paper-on-alzheimers-disease/ [Accessed: 10-Jun-2024]
Don't let plagiarism ruin your grade
Hire a writer to get a unique paper crafted to your needs.

Our writers will help you fix any mistakes and get an A+!
Please check your inbox.
You can order an original essay written according to your instructions.
Trusted by over 1 million students worldwide
1. Tell Us Your Requirements
2. Pick your perfect writer
3. Get Your Paper and Pay
Hi! I'm Amy, your personal assistant!
Don't know where to start? Give me your paper requirements and I connect you to an academic expert.
short deadlines
100% Plagiarism-Free
Certified writers

- June 9, 2024 | Mayo Clinic Study Reveals Startling Connection Between Energy Drinks and Sudden Cardiac Arrest
- June 9, 2024 | Quantum Theory Unveils Surprising Black Hole Shortage
- June 9, 2024 | Cracking the Code: Scientists Solve Reynolds’ Century-Old Fluid Flow Mystery
- June 9, 2024 | A Sustainable Solution to Fighting Global Warming – New Catalyst Efficiently Converts CO2 to Natural Gas
- June 9, 2024 | Beware the Rise of Superweeds: Mowing’s Unintended Consequences
Alzheimer’s Breakthrough: Researchers Discover Novel Way To Potentially Halt Disease Progression
By The Mount Sinai Hospital / Mount Sinai School of Medicine June 1, 2024
Mount Sinai researchers have discovered a potential new method to treat Alzheimer’s by targeting the plexin-B1 protein to improve plaque clearance in the brain, opening avenues for future therapeutic strategies. Credit: SciTechDaily.com
Innovative research from Mount Sinai has also identified new pathways for research.
Researchers at the Icahn School of Medicine at Mount Sinai have achieved a major breakthrough in Alzheimer’s disease research. Their study identifies a promising method that could potentially slow or even stop the progression of the disease. Focusing on the role of reactive astrocytes and the plexin-B1 protein in Alzheimer’s disease, the research offers vital insights into how brain cells communicate. This opens up new avenues for innovative treatment approaches. The findings were published on May 27 in the journal Nature Neuroscience .
This groundbreaking work is centered on the manipulation of the plexin-B1 protein to enhance the brain’s ability to clear amyloid plaques, a hallmark of Alzheimer’s disease. Reactive astrocytes, a type of brain cell that becomes activated in response to injury or disease, were found to play a crucial role in this process. They help control the spacing around amyloid plaques, affecting how other brain cells can access and clear these harmful deposits.
“Our findings offer a promising path for developing new treatments by improving how cells interact with these harmful plaques,” said Roland Friedel, PhD, Associate Professor of Neuroscience, and Neurosurgery, at Icahn Mount Sinai and a senior author of the study. The research was driven by the analysis of complex data comparing healthy individuals to those with Alzheimer’s, aiming to understand the disease’s molecular and cellular foundations.
Icahn Mount Sinai researchers find PLXNB1, a hub gene predicted to drive a gene subnetwork causally linked to human AD, is upregulated in reactive astrocytes surrounding amyloid plaques. Credit: Bin Zhang, PhD, Icahn Mount Sinai
Broad Implications and Validation of Gene Network Models
Hongyan Zou, PhD, Professor of Neurosurgery, and Neuroscience, at Icahn Mount Sinai and one of the study’s lead authors, highlighted the broader implications of their findings: “Our study opens new pathways for Alzheimer’s research, emphasizing the importance of cellular interactions in developing neurodegenerative disease treatments.”
One of the study’s most significant achievements is its validation of multiscale gene network models of Alzheimer’s disease. “This study not only confirms one of the most important predictions from our gene network models but also significantly advances our understanding of Alzheimer’s. It lays a solid foundation for developing novel therapeutics targeting such highly predictive network models,” said Bin Zhang, PhD, Willard T.C. Johnson Research Professor of Neurogenetics at Icahn Mount Sinai and one of the study’s lead authors. By demonstrating the critical role of plexin-B1 in Alzheimer’s disease, the research underscores the potential of targeted therapies to disrupt the disease’s progression.
The research team emphasizes that while their findings mark a significant advance in the fight against Alzheimer’s, more research is needed to translate these discoveries into treatments for human patients.
“Our ultimate goal is to develop treatments that can prevent or slow down Alzheimer’s progression,” Dr. Zhang added, outlining the team’s commitment to further exploring the therapeutic potential of plexin-B1.
Reference: “Regulation of cell distancing in peri-plaque glial nets by Plexin-B1 affects glial activation and amyloid compaction in Alzheimer’s disease” by Yong Huang, Minghui Wang, Haofei Ni, Jinglong Zhang, Aiqun Li, Bin Hu, Chrystian Junqueira Alves, Shalaka Wahane, Mitzy Rios de Anda, Lap Ho, Yuhuan Li, Sangjo Kang, Ryan Neff, Ana Kostic, Joseph D. Buxbaum, John F. Crary, Kristen J. Brennand, Bin Zhang, Hongyan Zou and Roland H. Friedel, 27 May 2024, Nature Neuroscience . DOI: 10.1038/s41593-024-01664-w
This study is supported by the NIH National Institute on Aging (NIA) grants U01AG046170 and RF1AG057440 and is part of the NIA-led Accelerating Medicines Partnership – Alzheimer’s Disease (AMP-AD) Target Discovery and Preclinical Validation program. This public-private partnership aims to shorten the time between the discovery of potential drug targets and the development of new drugs for Alzheimer’s disease treatment and prevention.
More on SciTechDaily

Omega-3 Supplementation Linked to 30% Reduction in Aggression Across Age and Gender
New RNA Tool Can Illuminate Brain Circuits and Edit Specific Cells

NASA’s Solar Dynamics Observatory Caught the Sun “Smiling”

Suppressing NgR1 Returns Brain to Adolescent Levels of Plasticity

High Contrast Imaging Reveals Unknown Structure in Galaxy

Rewiring Children’s Anxious Minds With Cognitive Behavioral Therapy

Don’t Worry, Research Suggests Birds Won’t Become Dependent on You Feeding Them
Why We Can’t “Boost” Our Way Out of the COVID-19 Pandemic
Be the first to comment on "alzheimer’s breakthrough: researchers discover novel way to potentially halt disease progression", leave a comment cancel reply.
Email address is optional. If provided, your email will not be published or shared.
Save my name, email, and website in this browser for the next time I comment.
Researchers identify novel way to potentially slow down or halt Alzheimer’s progression
- Download PDF Copy
Researchers at the Icahn School of Medicine at Mount Sinai have made a significant breakthrough in Alzheimer's disease research by identifying a novel way to potentially slow down or even halt disease progression. The study, which focuses on the role of reactive astrocytes and the plexin-B1 protein in Alzheimer's pathophysiology, provides crucial insights into brain cell communication and opens the door to innovative treatment strategies. It was published in Nature Neuroscience (DOI 10.1038/s41593-024-01664-w) on May 27.
This groundbreaking work is centered on the manipulation of the plexin-B1 protein to enhance the brain's ability to clear amyloid plaques, a hallmark of Alzheimer's disease. Reactive astrocytes, a type of brain cell that becomes activated in response to injury or disease, were found to play a crucial role in this process. They help control the spacing around amyloid plaques, affecting how other brain cells can access and clear these harmful deposits.
Our findings offer a promising path for developing new treatments by improving how cells interact with these harmful plaques." Roland Friedel, PhD, Associate Professor of Neuroscience, and Neurosurgery, at Icahn Mount Sinai and senior author of the study
The research was driven by the analysis of complex data comparing healthy individuals to those with Alzheimer's, aiming to understand the disease's molecular and cellular foundations.
Hongyan Zou, PhD, Professor of Neurosurgery, and Neuroscience, at Icahn Mount Sinai and one of the study's lead authors, highlighted the broader implications of their findings: " Our study opens new pathways for Alzheimer's research, emphasizing the importance of cellular interactions in developing neurodegenerative disease treatments."
One of the study's most significant achievements is its validation of multiscale gene network models of Alzheimer's disease. "This study not only confirms one of the most important predictions from our gene network models but also significantly advances our understanding of Alzheimer's. It lays a solid foundation for developing novel therapeutics targeting such highly predictive network models," said Bin Zhang, PhD, Willard T.C. Johnson Research Professor of Neurogenetics at Icahn Mount Sinai and one of the study's lead authors. By demonstrating the critical role of plexin-B1 in Alzheimer's disease, the research underscores the potential of targeted therapies to disrupt the disease's progression.
Related Stories
- Novel Concepts Medical's plant-based formula achieves 99% reduction in cancer cell viability
- New algorithm enhances deep brain stimulation for Parkinson's by targeting specific symptoms
- Research reveals light's impact on metabolism beyond circadian rhythms
The research team emphasizes that while their findings mark a significant advance in the fight against Alzheimer's, more research is needed to translate these discoveries into treatments for human patients.
"Our ultimate goal is to develop treatments that can prevent or slow down Alzheimer's progression ," Dr. Zhang added, outlining the team's commitment to further exploring the therapeutic potential of plexin-B1.
This study is supported by the NIH National Institute on Aging (NIA) grants U01AG046170 and RF1AG057440 and is part of the NIA-led Accelerating Medicines Partnership - Alzheimer's Disease (AMP-AD) Target Discovery and Preclinical Validation program. This public private partnership aims to shorten the time between the discovery of potential drug targets and the development of new drugs for Alzheimer's disease treatment and prevention.
The paper is titled "Regulation of cell distancing in peri-plaque glial nets by Plexin-B1 affects glial activation and amyloid compaction in Alzheimer's disease."
Mount Sinai Health System
Huang, Y., et al . (2024). Regulation of cell distancing in peri-plaque glial nets by Plexin-B1 affects glial activation and amyloid compaction in Alzheimer’s disease. Nature Neuroscience . doi.org/10.1038/s41593-024-01664-w .
Posted in: Medical Science News | Medical Research News | Medical Condition News
Tags: Aging , Alzheimer's Disease , Brain , Brain Cell , Cardiology , Cell , Drugs , Ear , Education , Eye , Gene , Genomics , Geriatrics , Health Systems , Healthcare , Hospital , Liver , Medicine , Neurodegenerative Disease , Neuroscience , Neurosurgery , Pathophysiology , Preclinical , Protein , Research , students , Technology , Therapeutics , Virology
Suggested Reading

Cancel reply to comment
- Trending Stories
- Latest Interviews
- Top Health Articles

Discover how SciY empowers scientists
Santi Dominguez
In this interview, NewsMedical talks to Santi Dominguez about the genesis of SCiY, and how the consortium intends to empower scientists and researchers.

Why use light scattering to analyze proteins and viral vectors?
Dr. Michelle Chen
n this interview, Dr. Michelle Chen, Senior Director of Analytical Sciences at Wyatt Technology, talks to NewsMedical about how to use light scattering techniques to analyze proteins for their multi-attribute quantification (MAQ).

Changing the landscape of R&D to build clinical success from the ground up
Amanda Jones
In this interview, NewsMedical talks to Amanda Jones at Revvity about the strategies and solutions available to revolutionize the landscape of R&D in clinical research.

Latest News

Newsletters you may be interested in
Your AI Powered Scientific Assistant
Hi, I'm Azthena, you can trust me to find commercial scientific answers from News-Medical.net.
A few things you need to know before we start. Please read and accept to continue.
- Use of “Azthena” is subject to the terms and conditions of use as set out by OpenAI .
- Content provided on any AZoNetwork sites are subject to the site Terms & Conditions and Privacy Policy .
- Large Language Models can make mistakes. Consider checking important information.
Great. Ask your question.
Azthena may occasionally provide inaccurate responses. Read the full terms .
While we only use edited and approved content for Azthena answers, it may on occasions provide incorrect responses. Please confirm any data provided with the related suppliers or authors. We do not provide medical advice, if you search for medical information you must always consult a medical professional before acting on any information provided.
Your questions, but not your email details will be shared with OpenAI and retained for 30 days in accordance with their privacy principles.
Please do not ask questions that use sensitive or confidential information.
Read the full Terms & Conditions .
Provide Feedback
129 Alzheimer’s Disease Essay Topics & Examples
If you’re writing about patients with memory loss or dementia care and treatment, this article will be of use. Our team has prepared Alzheimer’s disease essay examples and topics below.
🏆 Best Alzheimer’s Disease Essay Examples & Topics
💡 most interesting alzheimer’s disease topics to write about, 📌 simple & easy alzheimer’s disease research topics, 👍 good research topics about alzheimer’s disease, ❓ research questions about alzheimer’s disease.
- Managing Dementia and Alzheimer’s Disease The PICOT question is “In the care of Alzheimer’s and dementia patients, does integrated community-based care as compared to being in a long-term care facility improve outcome throughout the remainder of their lives”.
- The Case Study of Patient With Late-Stage Alzheimer’s Disease In the majority of cases of Alzheimer’s, it has been shown that patients are unable to make decisions on their own and are also unable to communicate their assent verbally.
- Pathophysiology of Alzheimer’s Disease The study will discuss the pathophysiology of Alzheimer’s disease, such as risk factors, cellular involvement, genetic influences, and the interventions of the available therapy’s pharmacological Interventions.
- Therapeutic Dogs, Dementia, Alzheimer’s and Fluid Intelligence It is worth noting that with dementia, the patient has a speech disorder and a personality change in the early stages of the pathology.
- The Alzheimer’s Association Dementia Care Practice Therefore, achieving the philosophy and recommendations of the association is a shared responsibility between doctors, patients, and caregivers. Ultimately, CAPD tests the functionalities of the patient ranging from the psychomotor activities, perceptions, awareness, and orientations, […]
- Dementia, Alzheimer, and Delirium in an Elderly Woman Additionally, she struggles with identifying the appropriate words to use in dialogue and changes the topic. Timing: While in the middle of conversations and public places like supermarkets.
- Alzheimer’s Disease Diagnosis and Intervention The accumulation of plaques and tangles in the brain is a hallmark of the disease, resulting in the death of neurons and a decline in mental capacity.
- Alzheimer’s Disease: Assessment and Intervention The caregiver is recommended to install safety locks and alarms on all doors and windows to prevent the patient from leaving the apartment without supervision.
- Management of a Patient With Alzheimer’s: Case Study The correlation between this issue and the probability of the emergence of AD in elderly citizens is proved by the scholars who examined the impact of the quality of air on a person’s health.
- Bilinguals’ Cognitive-Linguistic Abilities and Alzheimer’s Disease This irregularity is reflected in the preserved linguistic abilities, including code-switching and semantic fluency, and the declined functions in translation, picture naming, and phonemic fluency, calling for improved therapy and testing practices.
- Alzheimer’s Disease: Definition, Stages, Diagnosis Alzheimer’s disease is the most common type of dementia, and it is a condition in which the brain stops appropriately performing its functions.
- Fall Risk Assessment of Alzheimer’s Patient The nurse answers questions about the old lady helps fill the Stay Independent brochure and assists the observing physician in carrying the various clinical tests on the patient.
- Alzheimer’s Disease in an Iranian Patient The patient in the company of his son returns to the clinic after four weeks. Since the patient shows no side effects of the disease and an increase in Exelon to 6 mg orally BID […]
- Mr. Akkad and Alzheimer’s Disease: Case Study The onset of the symptoms is reported to have been within the past two years, but the situation has begun to deteriorate, prompting Mr.
- Alzheimer’s Disease: History, Mechanisms and Treatment Nevertheless, researchers state that the development of Alzheimer’s is impacted by the formation of protein plaques and tangles in the brain.
- Alzheimer’s Disease: Causes and Treatment AD is associated with different changes, both cognitive and behavioral. A patient can observe some or all of them depending on the development of the disease.
- Frontotemporal Dementia vs. Alzheimer’s Disease in a Patient Moreover, Alzheimer’s disease affects hypertrophies in the hippocampus as the initial part is involved in the brain’s memory areas and spatial orientation.
- Alzheimer’s Disease: Diagnostic and Treatment Alzheimer’s disease is a progressive degenerative disorder that causes a deterioration of mental and cognitive abilities.
- The Effect of Music on People With Alzheimer’s Disease The evidence suggests that one of the most prominent effects of music on patients with Alzheimer’s disease is autobiographical memory preservation alongside the stimulation of both sympathetic and parasympathetic nervous systems.
- Community Health: Alzheimer’s Disease The community nurse’s role is to develop and participate in primary, secondary, and tertiary preventive strategies and to provide a wide range of nursing care services while maintaining the health and wellbeing of individuals with […]
- Challenges of Living With Alzheimer Disease The medications make the condition of the patient better during the first stages of the disease. During the middle stage of the disease, the symptoms worsen.
- The Burden of Alzheimer’s Disease Assessing the appropriateness and effectiveness of reducing the cost of providing care for patients with Alzheimer remains a major issue that needs to be addressed.
- Chronic Care For Alzheimer’s Disease The application of the Chronic Care Model, in its turn, will serve as the foundation for building the patient’s awareness about their condition, thus, improving the patient’s quality of life and creating the environment, in […]
- Synopsis of Research Studies of Individuals Afflicted by Mild Alzheimer’s Disease The research questions in the articles were tailored along the various physical activities that can assist patients affected by Alzheimer Disease.
- Alzheimer’s Disease and Naturopathic Medicine The main feature of AD is the aggregation of -amyloid. However, application of natural therapies to prohibit the process of the pathways can slow the progress of AD.
- Brain Reduction and Presence of Alzheimer’s Disease The purpose of the study was to examine the correlation between brain reduction and the presence of Alzheimer’s disease. The researchers wanted to examine the nature of such changes in elderly individuals at low risk […]
- Alzheimer Related Morbidity and Death Among New Yorkers Generally, Alzheimer disease is a form of dementia, which inflicts a loss of memory, thinking and behavior. The proportion of ethnic and racial diversity in the US is increasing.
- Environmental Interview on a Patient With Alzheimer Disease In the 1980s, delusions and hallucinations were added as signs of the disease. Researches in the 1960’s show a link between cognitive reduction and the number of ailments in the brain.
- Alzheimer’s Disease Article and Clinical Trial This study shows that environmental hazards, in this case lead, increase the risk of developing Alzheimer’s disease and that the development period is crucial for determining future vulnerability to neurodegeneration and Alzheimer’s disease.
- Alzheimer’s Disease: Regarding Physiology However, one clear aspect of the development of this disease arises from a very complex chain of activities taking place in the brain over a long period of time.
- Mapping the Neurofibrillary Degeneration From Alzheimer’s Disease Patient This is an analytic review of the studies elaborating on the relationship of hyperphosphorylated tau proteins to the development of Alzheimer’s disease and focusing on the antigen capture ELISA specific for p-tau proteins.
- Role of Alzheimer’s Disease Advanced in Our Understanding of the Aging Process Aging on the hand can be defined as the accumulation of different harmful changes in the tissues and cells that raises the possibility of disease and death.
- Depression and Alzheimer’s Disease Moretti et al have studied the relationship between depression and Alzheimer’s disease and explored whether depression is a symptom of AD or comorbidity.
- Alzheimer’s Disease: Medical Analysis Such gene-associated markers have been characterized, in particular the apolipoprotein E gene, which was linked to chromosome# 19, and was responsible for accumulation of A by way of binding to this protein.
- Diabetic Teaching Plan for Alzheimer’s Patient He knows the purposes and some of the steps and needs to be taught again to regain his independence in monitoring his blood glucose level.
- Comparing Alzheimer’s Disease and Parkinson’s Disease There are many superficial similarities between Alzheimer’s disease and Parkinson’s disease primarily in some symptoms and age-group of persons afflicted by these two diseases.
- Alzheimer’s Disease and Long Term Care Alzheimer’s disease is a progressive disease in which memory impairment and disturbances in reasoning and perception are the primary symptoms. Also, well-known skills and recognition of objects and person is diminished in this stage of […]
- The Effects of Alzheimer’s Disease on Family Members The disease develops gradually and is said to be a disease of the old because it relates to the inability to remember.
- Alzheimer’s Disease in Science Daily News Article The news article accurately reports the focus of the study in the diagnosis of AD. Hence, the news article accurately presents that the diagnostic method is important in the diagnosis and prognosis of AD among […]
- Dancing and Risk of Alzheimer’s Disease Despite the fact that there is no effective treatment for Alzheimer’s disease, scientists discovered that dancing could help reduce the severity of the disorder as this activity involves simultaneous brain functioning, which helps to affect […]
- Alzheimer’s Disease Prevalence and Prevention The estimated global prevalence of Alzheimer’s disease is 50 million and is projected to triple by 2050 due to growth in the older generation. According to Alzheimer’s Association, AD is the fifth-ranking killer of persons […]
- Alzheimer’s Disease: Managing Cognitive Dysfunction In the majority of cases, Alzheimer’s disease turns out to be the cause of this problem. Alzheimer’s disease can be caused by different risk factors, but in the majority of cases, it is associated with […]
- Alzheimer’s Disease in Newspaper Articles The number of patients diagnosed with Alzheimer’s and diabetes in the United States, and indeed globally, has increased significantly in the last few years. This means that the main interest of such collaboration is to […]
- Alzheimer’s and Cardiovascular Diseases Progress While the design of the study involves a review of the existing papers and a compilation of their key results, the information provided by the authors is nonetheless crucial to the understanding of the issue.
- Heart Disease and Alzheimer’s in Adult Women Education and Employment History: The patient reported she is a college graduate and has a master’s degree in Victorian Literature. The patient is currently working full-time as a Literature professor at UC Berkeley, in a […]
- The Alzheimer’s Disease Concept In simple words, it is the condition caused by the negative changes in the human brain that, as the end result, leads to memory loss and some behavioral issues that worsen the quality of patient’s […]
- Alzheimer’s Disease, Its Nature and Diagnostics According to the Alzheimer’s Association, this condition is the sixth leading cause of lethal outcomes in the United States. The most frequent symptoms of Alzheimer’s disease include problems with memory, reasoning, thinking processes, perception, and […]
- Alzheimer’s Disease in Medical Research The existing data proposes that if the illness is distinguished before the commencement of evident warning signs, it is probable that the treatments founded on the facts of fundamental pathogenesis will be of assistance in […]
- Alzheimer’s Disease and Antisocial Personality Disorder Since there is currently no cure for Alzheimer’s disease, the future of the nursing care for the people that have the identified disorder concerns mostly maintaining the patient’s quality of life.
- Plasma Amyloid-Beta and Alzheimer’s Disease The impact of AD on public health includes increased rates of informal care and the direct charges of communal care. The aim of this study is to find the precise relationship between plasma amyloid beta […]
- Age Ailment: Dementia and Alzheimer’s Disease It is a time for one to clean the mind and take time to do what matters most in life. With an increased level of technological advancements, a digital sabbatical is mandatory to lower the […]
- Psychology Issues: Alzheimer’s Disease Alzheimer’s disease is a psychological disorder that involves the progressive destruction of brain cells and reduction in the proper functioning of the brain.
- Importance of Drug Therapy in Management of Alzheimer’s Disease The effects of Alzheimer’s disease can be controlled by early detection. Most studies are based on the effects of drug therapy mild Alzheimer’s patients.
- The Development of Alzheimer’s Disease and It’s Effect on the Brain Research studies have revealed that prevalence of the Alzheimer’s disease is increasing exponentially due to change in lifestyles and the incurable nature of the disease.
- Treatment of Alzheimer’s Disease According to documented research, Alzheimer’s disease is the primary cause of dementia affecting close to half a million people in the United Kingdom and five million in the United States.
- Health Care for Elderly People With Alzheimer’s Disease C’s condition is not likely to affect the relationship between her and her relatives if they are sensible toward her. C is to take her to a nursing home for the elderly.
- Diagnosis of Alzheimer’s Disease The most remarkable feature of the disease is the loss of ability to remember events in an individual’s life. According to the latter hypothetical medical study, it has been exemplified that the presence of deposits […]
- Concept and Treatment of the Alzheimer Disorder This implies that cognitive and natural therapies are highly perceived to be effective as opposed to pharmacological treatments. One cannot ignore the fact that both cognitive and natural therapies have become widely accepted in treating […]
- Understanding Alzheimer’s Disease Among Older Population After the 65 years, it has been found that the probability of developing Alzheimer’s disease doubles after every 5 years and as a result, by the age of 85 years, the risk of acquiring the […]
- Concepts of Alzheimer’s Disease The brain changes are the same in both men and women suffering from Alzheimer’s disease. There is also a significant increase in the death of the neurons leading to the shrinking of the affected regions.
- Alzheimer’s Association Of Neurological Disorders And Stroke
- The Potential Treatment of Alzheimer’s Disease: Through CRISPR-Cas9 Genome Editing
- Alzheimer’s Condition as an Enemy of Mental Health
- Vitamin A as a Potential Therapy to Prevent Alzheimer’s Disease
- The Relationship Between Gender And Alzheimer’s Disease
- The Stages and Treatments of Alzheimer’s Disease
- The Clinical Description of the Causes, Symptoms and Treatment of Alzheimer’s Disease
- The Description of Alzheimer’s Disease and Its Statistics in America
- The Psychological Symptoms Of Alzheimer’s The Cognitive Symptoms
- Varying Aspects of Alzheimer’s Disease and Implementations
- The Effects Of Alzheimer’s And Dementia Among Elderly
- The Early Symptoms and Progression of Alzheimer’s Disease
- Watching a Loved One Slip Away from Alzheimer’s Disease
- The Differences Between Dementia And Alzheimer’s Dementia
- A History of Alzheimer’s Disease and Why it is Still One of the Most Researched Diseases Today
- A Healthy Lifestyle Might Help Combat Parkinson’s Disease And Alzheimer’s Disease
- The Studies Of Music And How It May Not Help The Alzheimer’s Disease
- The Trials of Caring For A Loved One With Alzheimer’s Disease
- Alzheimer’s Disease A Progressive And Fatal Disease Of The Brain
- The Effects of Dementia and Alzheimer’s Disease on Caregivers and the Care Needed for Suffering Patients
- The Psychologist’s Role in Addressing Family and Community Problems for Families with Alzheimer’s Disease
- Alzheimer’s Disease and Its Effect on the Patient and Care Giver
- The Statistics of Prevalence of Alzheimer’s Disease in the 21st Century
- The Link Between Down Syndrome and Alzheimer’s Disease
- The Pathophysiology Of Alzheimer’s Disease
- The Causes, Symptoms and Treatment of Alzheimer’s Disease
- The Focus on Alzheimer’s Disease in the Documentary Black Daises for the Bride
- The Physiology and Genetics Behind Alzheimer’s Disease
- The Early Manifestations of Alzheimer’s Disease
- The Role Of Gamma Secretase In Alzheimer’s Disease
- The Lack Of Early Detection Of Alzheimer’s Disease
- The Representation of Alzheimer’s Disease and Its Impact in the Film Still Alice
- The Possible Link of the Human Immune System to Alzheimer’s Disease
- The Study of Alzheimer’s Disease and Its Affect on the Elderly
- The Characteristics, History, Symptoms, Statistics, and Treatment of Alzheimer’s Disease, a Degenerative Brain Disease
- The Triggers, Progression, and Treatment of Alzheimer’s Disease
- Traumatic Brain Injury and Alzheimer’s Disease
- The Positive Impact of Exercise in Protecting the Brain from Alzheimer’s Disease
- Three Primary Types of Dementia: Alzheimer’s Disease, Vascular Dementia
- The Causes, Risks, Factors, and Stages of Alzheimer’s Disease
- The Contingent Valuation Method in Health Care: An Economic Evaluation of Alzheimer’s Disease
- What Is the Difference Between Dementia and Alzheimer’s Disease?
- What Is the Main Cause of Alzheimer’s Disease?
- How Do You Prevent Alzheimer’s Disease?
- Who Is at High Risk for Alzheimer’s Disease?
- What Foods Cause Alzheimer’s Disease?
- Do Alzheimer’s Disease Patients Sleep a Lot?
- Do Alzheimer’s Disease Patients Know They Have It?
- Do Alzheimer’s Disease Patients Feel Pain?
- What Is the Best Treatment for Alzheimer’s Disease?
- How Long Do Alzheimer’s Disease Patients Live?
- What Do Alzheimer’s Disease Patients Think?
- Do People with Alzheimer’s Disease Have Trouble Walking?
- Is End Stage Alzheimer’s Disease Painful?
- What Are the Final Stages of Alzheimer’s Disease Before Death?
- Does Alzheimer’s Disease Run in Families?
- Should You Tell Alzheimer’s Disease Patients the Truth?
- Why Do Alzheimer’s Disease Patients Stop Talking?
- How Do You Know When an Alzheimer’s Disease Patient Is Dying?
- Which Is Worse: Dementia or Alzheimer’s Disease?
- What to Say to Someone Who Has Alzheimer’s Disease?
- How Does Alzheimer’s Disease Affect Eyes?
- Are Alzheimer’s Disease Patients Happy?
- What Are the Warning Signs of Alzheimer’s Disease?
- What Is the Best Way to Help Someone with Alzheimer’s Disease?
- What Are Good Activities for Alzheimer’s Disease Patients?
- Disease Questions
- Disorders Ideas
- Nervous System Research Topics
- Pathogenesis Research Ideas
- Caregiver Topics
- Health Promotion Research Topics
- Neuropsychology Topics
- Therapeutics Research Ideas
- Chicago (A-D)
- Chicago (N-B)
IvyPanda. (2024, February 21). 129 Alzheimer’s Disease Essay Topics & Examples. https://ivypanda.com/essays/topic/alzheimers-disease-essay-topics/
"129 Alzheimer’s Disease Essay Topics & Examples." IvyPanda , 21 Feb. 2024, ivypanda.com/essays/topic/alzheimers-disease-essay-topics/.
IvyPanda . (2024) '129 Alzheimer’s Disease Essay Topics & Examples'. 21 February.
IvyPanda . 2024. "129 Alzheimer’s Disease Essay Topics & Examples." February 21, 2024. https://ivypanda.com/essays/topic/alzheimers-disease-essay-topics/.
1. IvyPanda . "129 Alzheimer’s Disease Essay Topics & Examples." February 21, 2024. https://ivypanda.com/essays/topic/alzheimers-disease-essay-topics/.
Bibliography
IvyPanda . "129 Alzheimer’s Disease Essay Topics & Examples." February 21, 2024. https://ivypanda.com/essays/topic/alzheimers-disease-essay-topics/.
Official websites use .gov
A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
Alzheimer’s Disease and Related Dementias
- What is Alzheimer's Disease?
- Who has it?
- What we know?
- Recognizing signs of Alzheimer's
- What to do?
- How is Alzheimer's disease treated?
Support for family and friends
- Burden of Alzheimer's
What is Alzheimer’s Disease?
- Alzheimer’s disease is the most common type of dementia.
- It is a progressive disease beginning with mild memory loss and possibly leading to loss of the ability to carry on a conversation and respond to the environment.
- Alzheimer’s disease involves parts of the brain that control thought, memory, and language.
- It can seriously affect a person’s ability to carry out daily activities.
Who has Alzheimer’s Disease?
Alzheimer's Disease and Racial and Ethnic Disparities infographic
All Alzheimer-related infographics
- In 2020, as many as 5.8 million Americans were living with Alzheimer’s disease. 1
- Younger people may get Alzheimer’s disease, but it is less common.
- The number of people living with the disease doubles every 5 years beyond age 65.
- This number is projected to nearly triple to 14 million people by 2060. 1
- Symptoms of the disease can first appear after age 60, and the risk increases with age.
What is known about Alzheimer’s Disease?
Scientists do not yet fully understand what causes Alzheimer’s disease. There likely is not a single cause but rather several factors that can affect each person differently.
- Age is the best known risk factor for Alzheimer’s disease.
- Family history—researchers believe that genetics may play a role in developing Alzheimer’s disease. However, genes do not equal destiny. A healthy lifestyle may help reduce your risk of developing Alzheimer’s disease. Two large, long term studies indicate that adequate physical activity, a nutritious diet, limited alcohol consumption, and not smoking may help people. To learn more about the study, you can listen to a short podcast.
- Changes in the brain can begin years before the first symptoms appear.
- Researchers are studying whether education, diet, and environment play a role in developing Alzheimer’s disease.
- There is growing scientific evidence that healthy behaviors, which have been shown to prevent cancer, diabetes, and heart disease, may also reduce risk for subjective cognitive decline. Here’s 8 ways .
What are the warning signs of Alzheimer’s disease?

Watch this video “Memory Loss is Not a Normal Part of Aging”
Alzheimer’s disease is not a normal part of aging. Memory problems are typically one of the first warning signs of Alzheimer’s disease and related dementias.
In addition to memory problems, someone with symptoms of Alzheimer’s disease may experience one or more of the following:
- Memory loss that disrupts daily life, such as getting lost in a familiar place or repeating questions.
- Trouble handling money and paying bills.
- Difficulty completing familiar tasks at home, at work or at leisure.
- Decreased or poor judgment.
- Misplacing things and being unable to retrace steps to find them.
- Changes in mood, personality, or behavior.
Even if you or someone you know has several or even most of these signs, it doesn’t mean it’s Alzheimer’s disease. Know the 10 warning signs (also available in Spanish ).
What to do if you suspect Alzheimer’s disease

Getting checked by your healthcare provider can help determine if the symptoms you are experiencing are related to Alzheimer’s disease, or a more treatable conditions such as a vitamin deficiency or a side effect from medication. Early and accurate diagnosis also provides opportunities for you and your family to consider financial planning, develop advance directives, enroll in clinical trials, and anticipate care needs.
How is Alzheimer’s disease treated?
Medical management can improve quality of life for individuals living with Alzheimer’s disease and for their caregivers. There is currently no known cure for Alzheimer’s disease. Treatment addresses several areas:
- Helping people maintain brain health.
- Managing behavioral symptoms.
- Slowing or delaying symptoms of the disease.

Currently, many people living with Alzheimer’s disease are cared for at home by family members. Caregiving can have positive aspects for the caregiver as well as the person being cared for. It may bring personal fulfillment to the caregiver, such as satisfaction from helping a family member or friend, and lead to the development of new skills and improved family relationships.
Although most people willingly provide care to their loved ones and friends, caring for a person with Alzheimer’s disease at home can be a difficult task and may become overwhelming at times. Each day brings new challenges as the caregiver copes with changing levels of ability and new patterns of behavior. As the disease gets worse, people living with Alzheimer’s disease often need more intensive care.
You can find more information about caring for yourself and access a helpful care planning form .
What is the burden of Alzheimer’s disease in the United States?
- Alzheimer’s disease is one of the top 10 leading causes of death in the United States. 2
- The 6th leading cause of death among US adults.
- The 5th leading cause of death among adults aged 65 years or older. 3
In 2020, an estimated 5.8 million Americans aged 65 years or older had Alzheimer’s disease. 1 This number is projected to nearly triple to 14 million people by 2060. 1
In 2010, the costs of treating Alzheimer’s disease were projected to fall between $159 and $215 billion. 4 By 2040, these costs are projected to jump to between $379 and more than $500 billion annually. 4
Death rates for Alzheimer’s disease are increasing, unlike heart disease and cancer death rates that are on the decline. 5 Dementia, including Alzheimer’s disease, has been shown to be under-reported in death certificates and therefore the proportion of older people who die from Alzheimer’s may be considerably higher. 6
What is known about reducing your risk of Alzheimer’s Disease?
The science on risk reduction is quickly evolving, and major breakthroughs are within reach. For example, there is growing evidence that people who adopt healthy lifestyle habits — like regular exercise and blood pressure management — can lower their risk of dementia. There is growing scientific evidence that healthy behaviors, which have been shown to prevent cancer, diabetes, and heart disease, may also reduce risk for subjective cognitive decline . To learn more about the current state of evidence on dementia risk factors and the implications for public health, please read the following summaries on Cardiovascular Health , Exercise , Diabetes and Obesity , Traumatic Brain Injury (TBI) , Tobacco and Alcohol , Diet and Nutrition , Sleep , Sensory Impairment , and Social Engagement or the Compiled Report (includes all reports in this list).
- CDC Healthy Aging Program
- National Institute on Aging
- The National Library of Medicine
- Administration for Community Living
CDC Healthy Brain Initiative
- The Healthy Brain Initiative
- State and Local Public Health Partnerships to Address Dementia: The 2018-2023 Road Map [PDF – 19.3 MB]
- Road Map for Indian Country [PDF – 10.3 MB]
Other Alzheimer’s Disease and Cognitive Health Resources
- Alzheimer’s Association
- Alzheimer’s Disease Public Health Curriculum [PDF – 10 MB]
- Alzheimer’s Foundation
- Alzheimer’s.gov
- Brain Health as You Age [PDF – 371 KB]
- Us Against Alzheimer’s
- Alzheimer’s Association Public Health Efforts
- Matthews, K. A., Xu, W., Gaglioti, A. H., Holt, J. B., Croft, J. B., Mack, D., & McGuire, L. C. (2018). Racial and ethnic estimates of Alzheimer’s disease and related dementias in the United States (2015–2060) in adults aged≥ 65 years. Alzheimer’s & Dementia . https://doi.org/10.1016/j.jalz.2018.06.3063
- Xu J, Kochanek KD, Sherry L, Murphy BS, Tejada-Vera B. Deaths: final data for 2007. National vital statistics reports; vol. 58, no. 19. Hyattsville, MD: National Center for Health Statistics. 2010.
- Heron M. Deaths: leading causes for 2010. National vital statistics reports; vol. 62, no 6. Hyattsville, MD: National Center for Health Statistics. 2013.
- Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM. Monetary costs of dementia in the United States. NEJM . 2013;368(14):1326-34.
- Tejada-Vera B. Mortality from Alzheimer’s disease in the United States: data for 2000 and 2010. NCHS data brief, no 116. Hyattsville, MD: National Center for Health Statistics. 2013.
- James BD. Leurgans SE, Hebert LE, et al. Contribution of Alzheimer disease to mortality in the United States. Neurology . 2014;82:1-6.
To receive email updates about Alzheimer's Disease and Healthy Aging, enter your email address:
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 06 April 2023
A global view of the genetic basis of Alzheimer disease
- Christiane Reitz 1 , 2 , 3 , 4 ,
- Margaret A. Pericak-Vance 5 , 6 ,
- Tatiana Foroud ORCID: orcid.org/0000-0002-5549-2212 7 , 8 &
- Richard Mayeux ORCID: orcid.org/0000-0001-6519-9696 1 , 2 , 3 , 4
Nature Reviews Neurology volume 19 , pages 261–277 ( 2023 ) Cite this article
5582 Accesses
23 Citations
52 Altmetric
Metrics details
- Alzheimer's disease
- Genetics research
The risk of Alzheimer disease (AD) increases with age, family history and informative genetic variants. Sadly, there is still no cure or means of prevention. As in other complex diseases, uncovering genetic causes of AD could identify underlying pathological mechanisms and lead to potential treatments. Rare, autosomal dominant forms of AD occur in middle age as a result of highly penetrant genetic mutations, but the most common form of AD occurs later in life. Large-scale, genome-wide analyses indicate that 70 or more genes or loci contribute to AD. One of the major factors limiting progress is that most genetic data have been obtained from non-Hispanic white individuals in Europe and North America, preventing the development of personalized approaches to AD in individuals of other ethnicities. Fortunately, emerging genetic data from other regions — including Africa, Asia, India and South America — are now providing information on the disease from a broader range of ethnicities. Here, we summarize the current knowledge on AD genetics in populations across the world. We predominantly focus on replicated genetic discoveries but also include studies in ethnic groups where replication might not be feasible. We attempt to identify gaps that need to be addressed to achieve a complete picture of the genetic and molecular factors that drive AD in individuals across the globe.
The genetic variation underlying Alzheimer disease (AD) differs across ethnic groups.
Large-scale genomic studies have identified over 70 genes or genetic loci associated with AD risk, but these data have largely been obtained from populations in Europe and North America, which hinders our understanding of the molecular mechanism(s) underlying the disease in under-represented populations and the development of a personalized therapeutic approach.
Expansion of efforts to sequence and analyse the genomes of people from under-studied areas of the world, combined with acquisition of additional multiomics data and appropriate development of infrastructure, resources, training and ethical guidelines, will be essential to improve our understanding of global genetic variation profiles underlying dementia.
Pathological heterogeneity is the norm in AD and efforts to incorporate this information into genetic studies is underway.
Incorporation of improved biomarkers that can be obtained in low-resource countries will be critical to increase diagnostic accuracy in these efforts.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
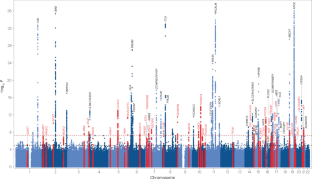
Similar content being viewed by others

Step by step: towards a better understanding of the genetic architecture of Alzheimer’s disease
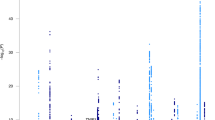
A genome-wide association study with 1,126,563 individuals identifies new risk loci for Alzheimer’s disease
Genetics of Alzheimer’s disease: an East Asian perspective
2022 Alzheimer’s disease facts and figures. Alzheimers Dement. 18 , 700–789 (2022).
Gatz, M. et al. Role of genes and environments for explaining Alzheimer disease. Arch. Gen. Psychiatry 63 , 168–174 (2006).
Article PubMed Google Scholar
Chartier-Harlin, M. C. et al. Early-onset Alzheimer’s disease caused by mutations at codon 717 of the β-amyloid precursor protein gene. Nature 353 , 844–846 (1991).
Article CAS PubMed Google Scholar
Goate, A. et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature 349 , 704–706 (1991).
Sherrington, R. et al. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature 375 , 754–760 (1995).
Sherrington, R. et al. Alzheimer’s disease associated with mutations in presenilin 2 is rare and variably penetrant. Hum. Mol. Genet. 5 , 985–988 (1996).
Cacace, R., Sleegers, K. & Van Broeckhoven, C. Molecular genetics of early-onset Alzheimer’s disease revisited. Alzheimers Dement. 12 , 733–748 (2016).
Jarmolowicz, A. I., Chen, H. Y. & Panegyres, P. K. The patterns of inheritance in early-onset dementia: Alzheimer’s disease and frontotemporal dementia. Am. J. Alzheimers Dis. Other Demen 30 , 299–306 (2015).
Wallon, D. et al. The French series of autosomal dominant early onset Alzheimer’s disease cases: mutation spectrum and cerebrospinal fluid biomarkers. J. Alzheimers Dis. 30 , 847–856 (2012).
Cruchaga, C. et al. Rare variants in APP , PSEN1 and PSEN2 increase risk for AD in late-onset Alzheimer’s disease families. PLoS One 7 , e31039 (2012).
Article CAS PubMed PubMed Central Google Scholar
Arboleda-Velasquez, J. F. et al. Resistance to autosomal dominant Alzheimer’s disease in an APOE3 Christchurch homozygote: a case report. Nat. Med. 25 , 1680–1683 (2019).
Pericak-Vance, M. A. et al. Linkage studies in familial Alzheimer disease: evidence for chromosome 19 linkage. Am. J. Hum. Genet. 48 , 1034–1050 (1991).
CAS PubMed PubMed Central Google Scholar
Wingo, T. S., Lah, J. J., Levey, A. I. & Cutler, D. J. Autosomal recessive causes likely in early-onset Alzheimer disease. Arch. Neurol. 69 , 59–64 (2012).
Wightman, D. P. et al. A genome-wide association study with 1,126,563 individuals identifies new risk loci for Alzheimer’s disease. Nat. Genet. 53 , 1276–1282 (2021).
Kunkle, B. W. et al. Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nat. Genet. 51 , 414–430 (2019).
Bellenguez, C. et al. New insights into the genetic etiology of Alzheimer’s disease and related dementias. Nat. Genet. 54 , 412–436 (2022).
Jansen, I. E. et al. Genome-wide meta-analysis for Alzheimer’s disease cerebrospinal fluid biomarkers. Acta Neuropathol. 144 , 821–842 (2022).
Rubinski, A. et al. Polygenic effect on tau pathology progression in Alzheimer’s disease. Ann. Neurol. https://doi.org/10.1002/ana.26588 (2022).
Article Google Scholar
Vigilant, L., Stoneking, M., Harpending, H., Hawkes, K. & Wilson, A. C. African populations and the evolution of human mitochondrial DNA. Science 253 , 1503–1507 (1991).
Schlebusch, C. M. et al. Genomic variation in seven Khoe-San groups reveals adaptation and complex African history. Science 338 , 374–379 (2012).
Gronau, I., Hubisz, M. J., Gulko, B., Danko, C. G. & Siepel, A. Bayesian inference of ancient human demography from individual genome sequences. Nat. Genet. 43 , 1031–1034 (2011).
Rasmussen, M. et al. Ancient human genome sequence of an extinct Palaeo-Eskimo. Nature 463 , 757–762 (2010).
Green, R. E. et al. A draft sequence of the Neandertal genome. Science 328 , 710–722 (2010).
Ramachandran, S. et al. Support from the relationship of genetic and geographic distance in human populations for a serial founder effect originating in Africa. Proc. Natl Acad. Sci. USA 102 , 15942–15947 (2005).
Jakobsson, M. et al. Genotype, haplotype and copy-number variation in worldwide human populations. Nature 451 , 998–1003 (2008).
Li, J. Z. et al. Worldwide human relationships inferred from genome-wide patterns of variation. Science 319 , 1100–1104 (2008).
Rosenberg, N. A. et al. Genetic structure of human populations. Science 298 , 2381–2385 (2002).
Gurdasani, D. et al. The African Genome Variation Project shapes medical genetics in Africa. Nature 517 , 327–332 (2015).
Beltrame, M. H., Rubel, M. A. & Tishkoff, S. A. Inferences of African evolutionary history from genomic data. Curr. Opin. Genet. Dev. 41 , 159–166 (2016).
Tishkoff, S. A. et al. The genetic structure and history of Africans and African Americans. Science 324 , 1035–1044 (2009).
Duncan, L. et al. Analysis of polygenic risk score usage and performance in diverse human populations. Nat. Commun. 10 , 3328 (2019).
The 1000 Genomes Project Consortium. A global reference for human genetic variation. Nature 526 , 68–74 (2015).
Rotimi, C. N. & Adeyemo, A. A. From one human genome to a complex tapestry of ancestry. Nature 590 , 220–221 (2021).
Hong, S. et al. TMEM106B and CPOX are genetic determinants of cerebrospinal fluid Alzheimer’s disease biomarker levels. Alzheimers Dement. 17 , 1628–1640 (2021).
Rotimi, C. N. et al. The genomic landscape of African populations in health and disease. Hum. Mol. Genet. 26 , R225–R236 (2017).
Kunkle, B. W. et al. Novel Alzheimer disease risk loci and pathways in African American individuals using the African genome resources panel: a meta-analysis. JAMA Neurol. 78 , 102–113 (2021).
Hohman, T. J. et al. Global and local ancestry in African-Americans: implications for Alzheimer’s disease risk. Alzheimers Dement. 12 , 233–243 (2016).
Reitz, C. et al. Variants in the ATP-binding cassette transporter (ABCA7), apolipoprotein E ε4, and the risk of late-onset Alzheimer disease in African Americans. JAMA 309 , 1483–1492 (2013).
Bussies, P. L. et al. Use of local genetic ancestry to assess TOMM40-523’ and risk for Alzheimer disease. Neurol. Genet. 6 , e404 (2020).
Vardarajan, B. N. et al. Whole genome sequencing of Caribbean Hispanic families with late-onset Alzheimer’s disease. Ann. Clin. Transl. Neurol. 5 , 406–417 (2018).
Vardarajan, B. N. et al. Ultra-rare mutations in SRCAP segregate in Caribbean Hispanic families with Alzheimer disease. Neurol. Genet. 3 , e178 (2017).
Feliciano-Astacio, B. E. et al. The Puerto Rico Alzheimer Disease Initiative (PRADI): a multisource ascertainment approach. Front. Genet. 10 , 538 (2019).
Article PubMed PubMed Central Google Scholar
Tosto, G. et al. F-box/LRR-repeat protein 7 is genetically associated with Alzheimer’s disease. Ann. Clin. Transl. Neurol. 2 , 810–820 (2015).
Tosto, G. et al. Association of variants in PINX1 and TREM2 with late-onset Alzheimer disease. JAMA Neurol. 76 , 942–948 (2019).
Farrer, L. A. et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA 278 , 1349–1356 (1997).
Maestre, G. et al. Apolipoprotein E and Alzheimer’s disease: ethnic variation in genotypic risks. Ann. Neurol. 37 , 254–259 (1995).
Tang, M. X. et al. The APOE-ε4 allele and the risk of Alzheimer disease among African Americans, whites, and Hispanics. JAMA 279 , 751–755 (1998).
Jonsson, T. et al. Variant of TREM2 associated with the risk of Alzheimer’s disease. N. Engl. J. Med. 368 , 107–116 (2013).
Raghavan, N. S. et al. Whole-exome sequencing in 20,197 persons for rare variants in Alzheimer’s disease. Ann. Clin. Transl. Neurol. 5 , 832–842 (2018).
Griswold, A. J. et al. Increased APOE ε4 expression is associated with the difference in Alzheimer’s disease risk from diverse ancestral backgrounds. Alzheimers Dement. 17 , 1179–1188 (2021).
Jun, G. R. et al. Transethnic genome-wide scan identifies novel Alzheimer’s disease loci. Alzheimers Dement. 13 , 727–738 (2017).
Cukier, H. N. et al. ABCA7 frameshift deletion associated with Alzheimer disease in African Americans. Neurol. Genet. 2 , e79 (2016).
Bryc, K. et al. Genome-wide patterns of population structure and admixture in West Africans and African Americans. Proc. Natl Acad. Sci. USA 107 , 786–791 (2010).
Kunkle, B. W. et al. Targeted sequencing of ABCA7 identifies splicing, stop-gain and intronic risk variants for Alzheimer disease. Neurosci. Lett. 649 , 124–129 (2017).
De Roeck, A. et al. Deleterious ABCA7 mutations and transcript rescue mechanisms in early onset Alzheimer’s disease. Acta Neuropathol. 134 , 475–487 (2017).
Cuyvers, E. et al. Mutations in ABCA7 in a Belgian cohort of Alzheimer’s disease patients: a targeted resequencing study. Lancet Neurol. 14 , 814–822 (2015).
Luukkainen, L. et al. Mutation analysis of the genes linked to early onset Alzheimer’s disease and frontotemporal lobar degeneration. J. Alzheimers Dis. 69 , 775–782 (2019).
Thordardottir, S. & Graff, C. Findings from the Swedish study on familial Alzheimer’s disease including the APP Swedish double mutation. J. Alzheimers Dis. 64 , S491–S496 (2018).
Al-Thani, H. F., Ahmad, M. N., Younes, S. & Zayed, H. Genetic variants associated with Alzheimer disease in the 22 Arab countries: a systematic review. Alzheimer Dis. Assoc. Disord. 35 , 178–186 (2021).
Eryilmaz, I. E. et al. Evaluation of the clinical features accompanied by the gene mutations: the 2 novel PSEN1 variants in a Turkish early-onset Alzheimer disease cohort. Alzheimer Dis. Assoc. Disord. 35 , 214–222 (2021).
Lee, J. H. et al. Disease-related mutations among Caribbean Hispanics with familial dementia. Mol. Genet. Genom. Med. 2 , 430–437 (2014).
Article CAS Google Scholar
Athan, E. S. et al. A founder mutation in presenilin 1 causing early-onset Alzheimer disease in unrelated Caribbean Hispanic families. JAMA 286 , 2257–2263 (2001).
Bertoli Avella, A. M. et al. A novel presenilin 1 mutation (L174 M) in a large Cuban family with early onset Alzheimer disease. Neurogenetics 4 , 97–104 (2002).
Arnold, S. E. et al. Frequency and clinicopathological characteristics of presenilin 1 Gly206Ala mutation in Puerto Rican Hispanics with dementia. J. Alzheimers Dis. 33 , 1089–1095 (2013).
Ramos, C., Aguillon, D., Cordano, C. & Lopera, F. Genetics of dementia: insights from Latin America. Dement. Neuropsychol. 14 , 223–236 (2020).
Itzcovich, T. et al. A novel mutation in PSEN1 (p.T119I) in an Argentine family with early- and late-onset Alzheimer’s disease. Neurobiol. Aging 85 , 155.e9–155.e12 (2020).
Abdala, B. B. et al. Influence of low frequency PSEN1 variants on familial Alzheimer’s disease risk in Brazil. Neurosci. Lett. 653 , 341–345 (2017).
El Kadmiri, N. et al. Novel mutations in the amyloid precursor protein gene within Moroccan patients with Alzheimer’s disease. J. Mol. Neurosci. 53 , 189–195 (2014).
CAS PubMed Google Scholar
El Kadmiri, N. et al. Novel presenilin mutations within Moroccan patients with early-onset Alzheimer’s disease. Neuroscience 269 , 215–222 (2014).
Heckmann, J. M. et al. Novel presenilin 1 mutation with profound neurofibrillary pathology in an indigenous Southern African family with early-onset Alzheimer’s disease. Brain 127 , 133–142 (2004).
Guerreiro, R. J. et al. Genetic screening of Alzheimer’s disease genes in Iberian and African samples yields novel mutations in presenilins and APP. Neurobiol. Aging 31 , 725–731 (2010).
Joshi, P., Gardner, M., Lintott, C. & Anderson, T. Novel presenilin-1 mutation (Ala275Ser) associated with clinical features of dementia with Lewy bodies. Alzheimer Dis. Assoc. Disord. 35 , 350–352 (2021).
Bechara, J. A., Brooks, W. S., Kwok, J. B., Halliday, G. M. & Schofield, P. R. Familial Alzheimer’s disease in Australia. Alzheimer’s Dement. 16 , e040062 (2020).
Syama, A. et al. Mutation burden profile in familial Alzheimer’s disease cases from India. Neurobiol. Aging 64 , 158.e7–158.e13 (2018).
Li, H. et al. The identification of PSEN1 p.Tyr159Ser mutation in a non-canonic early-onset Alzheimer’s disease family. Mol. Cell Neurosci. 120 , 103715 (2022).
Qin, Q. et al. Gene mutations associated with early onset familial Alzheimer’s disease in China: an overview and current status. Mol. Genet. Genom. Med. 8 , e1443 (2020).
CAS Google Scholar
Bagyinszky, E., Youn, Y. C., An, S. S. & Kim, S. Mutations, associated with early-onset Alzheimer’s disease, discovered in Asian countries. Clin. Interv. Aging 11 , 1467–1488 (2016).
Ikeuchi, T. et al. Mutational analysis in early-onset familial dementia in the Japanese population. The role of PSEN1 and MAPT R406W mutations. Dement. Geriatr. Cogn. Disord. 26 , 43–49 (2008).
Hsu, J. L., Lin, C. H., Chen, P. L., Lin, K. J. & Chen, T. F. Genetic study of young-onset dementia using targeted gene panel sequencing in Taiwan. Am. J. Med. Genet. B Neuropsychiatr. Genet. 186 , 67–76 (2021).
Bagyinszky, E., Ch’ng, G. S., Chan, M. Y., An, S. S. A. & Kim, S. A pathogenic presenilin-1 Val96Phe mutation from a Malaysian family. Brain Sci. 11 , 1328 (2021).
Alzheimer’s Disease International. Dementia in Sub-Saharan Africa: Challenges and Opportunities (Alzheimer’s Disease International, 2017).
Landoulsi, Z. et al. Genetic analysis of TREM2 variants in Tunisian patients with Alzheimer’s disease. Med. Princ. Pract. 27 , 317–322 (2018).
Hendrie, H. C. et al. APOE ε4 and the risk for Alzheimer disease and cognitive decline in African Americans and Yoruba. Int. Psychogeriatr. 26 , 977–985 (2014).
Hall, K. et al. Cholesterol, APOE genotype, and Alzheimer disease: an epidemiologic study of Nigerian Yoruba. Neurology 66 , 223–227 (2006).
Chen, C. H. et al. A comparative study to screen dementia and APOE genotypes in an ageing East African population. Neurobiol. Aging 31 , 732–740 (2010).
Gureje, O. et al. APOE ε4 is not associated with Alzheimer’s disease in elderly Nigerians. Ann. Neurol. 59 , 182–185 (2006).
Haithem, H. et al. Association between dementia and vascular disease-associated polymorphisms in a Tunisian population. Int. J. Neurosci. 128 , 32–41 (2018).
Corder, E. H. et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 261 , 921–923 (1993).
Mayeux, R. et al. The apolipoprotein epsilon 4 allele in patients with Alzheimer’s disease. Ann. Neurol. 34 , 752–754 (1993).
McKhann, G. et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services task force on Alzheimer’s disease. Neurology 34 , 939–944 (1984).
World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines (WHO, 1992).
Blue, E. E., Horimoto, A., Mukherjee, S., Wijsman, E. M. & Thornton, T. A. Local ancestry at APOE modifies Alzheimer’s disease risk in Caribbean Hispanics. Alzheimers Dement. 15 , 1524–1532 (2019).
Rajabli, F. et al. Ancestral origin of ApoE ε4 Alzheimer disease risk in Puerto Rican and African American populations. PLoS Genet. 14 , e1007791 (2018).
Rajabli, F. et al. A locus at 19q13.31 significantly reduces the ApoE ε4 risk for Alzheimer’s disease in African ancestry. PLoS Genet. 18 , e1009977 (2022).
Hendrie, H. C. et al. Incidence of dementia and Alzheimer disease in 2 communities: Yoruba residing in Ibadan, Nigeria, and African Americans residing in Indianapolis, Indiana. JAMA 285 , 739–747 (2001).
Adhikari, K., Chacon-Duque, J. C., Mendoza-Revilla, J., Fuentes-Guajardo, M. & Ruiz-Linares, A. The genetic diversity of the Americas. Annu. Rev. Genomics Hum. Genet. 18 , 277–296 (2017).
Adhikari, K., Mendoza-Revilla, J., Chacon-Duque, J. C., Fuentes-Guajardo, M. & Ruiz-Linares, A. Admixture in Latin America. Curr. Opin. Genet. Dev. 41 , 106–114 (2016).
Salzano, F. M. & Bortolini, M. C. The Evolution and Genetics of Latin American Populations (Cambridge Univ. Press, 2001).
Prince, M. et al. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 9 , 63–75.e2 (2013).
Ferri, C. P. et al. Global prevalence of dementia: a Delphi consensus study. Lancet 366 , 2112–2117 (2005).
Ramirez Aguilar, L. et al. Genetic origin of a large family with a novel PSEN1 mutation (Ile416Thr). Alzheimers Dement. 15 , 709–719 (2019).
Lopera, F. et al. Clinical features of early-onset Alzheimer disease in a large kindred with an E280A presenilin-1 mutation. JAMA 277 , 793–799 (1997).
Tariot, P. N. et al. The Alzheimer’s Prevention Initiative Autosomal-Dominant Alzheimer’s Disease Trial: a study of crenezumab versus placebo in preclinical PSEN1 E280A mutation carriers to evaluate efficacy and safety in the treatment of autosomal-dominant Alzheimer’s disease, including a placebo-treated noncarrier cohort. Alzheimers Dement. 4 , 150–160 (2018).
Guerreiro, R. et al. TREM2 variants in Alzheimer’s disease. N. Engl. J. Med. 368 , 117–127 (2013).
Vardarajan, B. N. et al. Rare coding mutations identified by sequencing of Alzheimer disease genome-wide association studies loci. Ann. Neurol. 78 , 487–498 (2015).
Vardarajan, B. N. et al. Inbreeding among Caribbean Hispanics from the Dominican Republic and its effects on risk of Alzheimer disease. Genet. Med. 17 , 639–643 (2015).
An, F. et al. MiR-124 acts as a target for Alzheimer’s disease by regulating BACE1. Oncotarget 8 , 114065–114071 (2017).
Sims, R. et al. Rare coding variants in PLCG2 , ABI3 , and TREM2 implicate microglial-mediated innate immunity in Alzheimer’s disease. Nat. Genet. 49 , 1373–1384 (2017).
Kizil, C. et al. Admixture mapping of Alzheimer’s disease in Caribbean Hispanics identifies a new locus on 22q13.1. Mol. Psychiatry 27 , 2813–2820 (2022).
Toral-Rios, D. et al. SORL1 polymorphisms in Mexican patients with Alzheimer’s disease. Genes 13 , 587 (2022).
Jia, L. et al. Dementia in China: epidemiology, clinical management, and research advances. Lancet Neurol. 19 , 81–92 (2020).
Gan, C. L., Zhang, T. & Lee, T. H. The genetics of Alzheimer’s disease in the Chinese population. Int. J. Mol. Sci. 21 , 2381 (2020).
Miyashita, A., Kikuchi, M., Hara, N. & Ikeuchi, T. Genetics of Alzheimer’s disease: an East Asian perspective. J. Hum. Genet. 68 , 115–124 (2022).
Jiang, T. et al. A rare coding variant in TREM2 increases risk for Alzheimer’s disease in Han Chinese. Neurobiol. Aging 42 , 217 (2016).
Jiang, T. et al. TREM2 p.H157Y variant and the risk of Alzheimer’s disease: a meta-analysis involving 14,510 subjects. Curr. Neurovasc Res. 13 , 318–320 (2016).
Hirano, A. et al. A genome-wide association study of late-onset Alzheimer’s disease in a Japanese population. Psychiatr. Genet. 25 , 139–146 (2015).
Jia, L. et al. Prediction of Alzheimer’s disease using multi-variants from a Chinese genome-wide association study. Brain 144 , 924–937 (2021).
Kang, S. et al. Potential novel genes for late-onset Alzheimer’s disease in East-Asian descent identified by APOE-stratified genome-wide association study. J. Alzheimers Dis. 82 , 1451–1460 (2021).
Park, J. H. et al. Novel Alzheimer’s disease risk variants identified based on whole-genome sequencing of APOE ε4 carriers. Transl. Psychiatry 11 , 296 (2021).
Shigemizu, D. et al. Ethnic and trans-ethnic genome-wide association studies identify new loci influencing Japanese Alzheimer’s disease risk. Transl. Psychiatry 11 , 151 (2021).
Zhou, X. et al. Identification of genetic risk factors in the Chinese population implicates a role of immune system in Alzheimer’s disease pathogenesis. Proc. Natl Acad. Sci. USA 115 , 1697–1706 (2018).
Wang, Y., Lu, D., Chung, Y. J. & Xu, S. Genetic structure, divergence and admixture of Han Chinese, Japanese and Korean populations. Hereditas 155 , 19 (2018).
Dogan, M. et al. Clinical and molecular findings in a Turkish family who had a (c.869-1G>A) splicing variant in PSEN1 gene with a rare condition: the variant Alzheimer’s disease with spastic paraparesis. Curr. Alzheimer Res. 19 , 223–235 (2022).
El Bitar, F. et al. Genetic study of Alzheimer’s disease in Saudi population. J. Alzheimers Dis. 67 , 231–242 (2019).
Guven, G. et al. A novel PSEN2 p.Ser175Phe variant in a family with Alzheimer’s disease. Neurol. Sci. 42 , 2497–2504 (2021).
Kalfon, L. et al. Familial early-onset Alzheimer’s caused by novel genetic variant and APP duplication: a cross-sectional study. Curr. Alzheimer Res. 19 , 694–707 (2022).
Lohmann, E. et al. Identification of PSEN1 and PSEN2 gene mutations and variants in Turkish dementia patients. Neurobiol. Aging 33 , 1850.e17–1850.e27 (2012).
Bazrgar, M. et al. Apolipoprotein E polymorphism in Southern Iran: E4 allele in the lowest reported amounts. Mol. Biol. Rep. 35 , 495–499 (2008).
Abdi, S. et al. Association of Alzheimer’s disease with genetic variants of apolipoprotein E, clusterin, TNF-α, and IL-6 among elderly Saudis. Curr. Pharm. Biotechnol. 23 , 1893–1902 (2022).
Abyadeh, M., Djafarian, K., Heydarinejad, F., Alizadeh, S. & Shab-Bidar, S. Association between apolipoprotein E gene polymorphism and Alzheimer’s disease in an Iranian population: a meta-analysis. J. Mol. Neurosci. 69 , 557–562 (2019).
Amer, M. S. et al. Interaction between apolipoprotein E genotyping, serum inflammatory biomarkers and cognitive functions in Egyptian elderly. Egypt. J. Immunol. 28 , 1–11 (2021).
Durmaz, A. et al. Genetic factors associated with the predisposition to late onset Alzheimer’s disease. Gene 707 , 212–215 (2019).
El Shamieh, S., Costanian, C., Kassir, R., Visvkis-Siest, S. & Bissar-Tadmouri, N. APOE genotypes in Lebanon: distribution and association with hypercholesterolemia and Alzheimer’s disease. Per Med. 16 , 15–23 (2019).
Isbir, T. et al. Interaction between apolipoprotein-E and angiotensin-converting enzyme genotype in Alzheimer’s disease. Am. J. Alzheimers Dis. Other Demen. 16 , 205–210 (2001).
Gharesouran, J., Rezazadeh, M., Khorrami, A., Ghojazadeh, M. & Talebi, M. Genetic evidence for the involvement of variants at APOE, BIN1, CR1, and PICALM loci in risk of late-onset Alzheimer’s disease and evaluation for interactions with APOE genotypes. J. Mol. Neurosci. 54 , 780–786 (2014).
Kleinberger, G. et al. TREM2 mutations implicated in neurodegeneration impair cell surface transport and phagocytosis. Sci. Transl. Med. 6 , 243ra286 (2014).
Manzali, S. B. et al. Association of the CD2AP locus with cognitive functioning among middle-aged individuals with a family history of Alzheimer’s disease. Neurobiol. Aging 101 , 50–56 (2021).
Mehdizadeh, E. et al. Association of MS4A6A, CD33, and TREM2 gene polymorphisms with the late-onset Alzheimer’s disease. Bioimpacts 9 , 219–225 (2019).
Mehrjoo, Z. et al. Association study of the TREM2 gene and identification of a novel variant in exon 2 in Iranian patients with late-onset Alzheimer’s disease. Med. Princ. Pract. 24 , 351–354 (2015).
Rezazadeh, M. et al. Genetic factors affecting late-onset Alzheimer’s disease susceptibility. Neuromolecular Med. 18 , 37–49 (2016).
Talebi, M. et al. ABCA7 and EphA1 genes polymorphisms in late-onset Alzheimer’s disease. J. Mol. Neurosci. 70 , 167–173 (2020).
Masri, I., Salami, A., El Shamieh, S. & Bissar-Tadmouri, N. rs3851179G>A in PICALM is protective against Alzheimer’s disease in five different countries surrounding the Mediterranean. Curr. Aging Sci. 13 , 162–168 (2020).
Akinyemi, R. O. et al. Dementia in Africa: current evidence, knowledge gaps, and future directions. Alzheimers Dement. 18 , 790–809 (2022).
Bis, J. C. et al. Whole exome sequencing study identifies novel rare and common Alzheimer’s-associated variants involved in immune response and transcriptional regulation. Mol. Psychiatry 25 , 1859–1875 (2020).
Mena, P. R. et al. The Alzheimer’s disease sequencing project–follow up study (ADSP-FUS): increasing ethnic diversity in Alzheimer’s genetics research with the addition of potential new cohorts. Alzheimer’s Dement. 16 , e046400 (2020).
John P. Hussman Institute to lead international genetic study of Alzheimer’s disease in people of Hispanic and African ancestry. Inventum https://physician-news.umiamihealth.org/john-p-hussman-institute-to-lead-international-genetic-study-of-alzheimers-disease-in-people-of-hispanic-and-african-ancestry/ (2022).
Weiner, M. W. et al. Increasing participant diversity in AD research: plans for digital screening, blood testing, and a community-engaged approach in the Alzheimer’s Disease Neuroimaging Initiative 4. Alzheimers Dement. 19 , 307–317 (2022).
McKhann, G. M. et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7 , 263–269 (2011).
Ayodele, T., Rogaeva, E., Kurup, J. T., Beecham, G. & Reitz, C. Early-onset Alzheimer’s disease: what is missing in research? Curr. Neurol. Neurosci. Rep. 21 , 4 (2021).
Reitz, C., Rogaeva, E. & Beecham, G. W. Late-onset vs nonmendelian early-onset Alzheimer disease: a distinction without a difference? Neurol. Genet. 6 , e512 (2020).
Bateman, R. J. et al. Autosomal-dominant Alzheimer’s disease: a review and proposal for the prevention of Alzheimer’s disease. Alzheimers Res. Ther. 3 , 1 (2011).
van der Flier, W. M., Pijnenburg, Y. A., Fox, N. C. & Scheltens, P. Early-onset versus late-onset Alzheimer’s disease: the case of the missing APOE ε4 allele. Lancet Neurol. 10 , 280–288 (2011).
Lee, S. et al. White matter hyperintensities are a core feature of Alzheimer’s disease: evidence from the dominantly inherited Alzheimer network. Ann. Neurol. 79 , 929–939 (2016).
Moller, C. et al. Different patterns of gray matter atrophy in early- and late-onset Alzheimer’s disease. Neurobiol. Aging 34 , 2014–2022 (2013).
Marshall, G. A., Fairbanks, L. A., Tekin, S., Vinters, H. V. & Cummings, J. L. Early-onset Alzheimer’s disease is associated with greater pathologic burden. J. Geriatr. Psychiatry Neurol. 20 , 29–33 (2007).
Lowe, V. J. et al. Widespread brain tau and its association with ageing, Braak stage and Alzheimer’s dementia. Brain 141 , 271–287 (2018).
Scholl, M. et al. Distinct 18F-AV-1451 tau PET retention patterns in early- and late-onset Alzheimer’s disease. Brain 140 , 2286–2294 (2017).
Josephs, K. A. et al. Rates of hippocampal atrophy and presence of post-mortem TDP-43 in patients with Alzheimer’s disease: a longitudinal retrospective study. Lancet Neurol. 16 , 917–924 (2017).
Attems, J. & Jellinger, K. A. The overlap between vascular disease and Alzheimer’s disease – lessons from pathology. BMC Med. 12 , 206 (2014).
Kapasi, A., DeCarli, C. & Schneider, J. A. Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol. 134 , 171–186 (2017).
Schneider, J. A., Arvanitakis, Z., Bang, W. & Bennett, D. A. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 69 , 2197–2204 (2007).
DeTure, M. A. & Dickson, D. W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 14 , 32 (2019).
Schneider, J. A., Wilson, R. S., Bienias, J. L., Evans, D. A. & Bennett, D. A. Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology 62 , 1148–1155 (2004).
Barnes, D. E. & Yaffe, K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 10 , 819–828 (2011).
Barker, W. W. et al. Relative frequencies of Alzheimer disease, Lewy body, vascular and frontotemporal dementia, and hippocampal sclerosis in the State of Florida Brain Bank. Alzheimer Dis. Assoc. Disord. 16 , 203–212 (2002).
Galvin, J. E., Pollack, J. & Morris, J. C. Clinical phenotype of Parkinson disease dementia. Neurology 67 , 1605–1611 (2006).
Lippa, C. F. et al. Lewy bodies contain altered α-synuclein in brains of many familial Alzheimer’s disease patients with mutations in presenilin and amyloid precursor protein genes. Am. J. Pathol. 153 , 1365–1370 (1998).
Chung, E. J. et al. Clinical features of Alzheimer disease with and without Lewy bodies. JAMA Neurol. 72 , 789–796 (2015).
Azar, M. et al. Cognitive tests aid in clinical differentiation of Alzheimer’s disease versus Alzheimer’s disease with Lewy body disease: evidence from a pathological study. Alzheimers Dement. 16 , 1173–1181 (2020).
Kraybill, M. L. et al. Cognitive differences in dementia patients with autopsy-verified AD, Lewy body pathology, or both. Neurology 64 , 2069–2073 (2005).
Forstl, H., Burns, A., Luthert, P., Cairns, N. & Levy, R. The Lewy-body variant of Alzheimer’s disease. Clinical and pathological findings. Br. J. Psychiatry 162 , 385–392 (1993).
Merdes, A. R. et al. Influence of Alzheimer pathology on clinical diagnostic accuracy in dementia with Lewy bodies. Neurology 60 , 1586–1590 (2003).
Ferreira, D. et al. Distinct subtypes of Alzheimer’s disease based on patterns of brain atrophy: longitudinal trajectories and clinical applications. Sci. Rep. 7 , 46263 (2017).
Moreno-Grau, S. et al. Genome-wide association analysis of dementia and its clinical endophenotypes reveal novel loci associated with Alzheimer’s disease and three causality networks: the GR@ACE project. Alzheimers Dement. 15 , 1333–1347 (2019).
Lee, A. J. et al. FMNL2 regulates gliovascular interactions and is associated with vascular risk factors and cerebrovascular pathology in Alzheimer’s disease. Acta Neuropathol. 144 , 59–79 (2022).
Jack, C. R. Jr. et al. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 14 , 535–562 (2018).
Jack, C. R. Jr. et al. A/T/N: an unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology 87 , 539–547 (2016).
Balogun, W. G., Zetterberg, H., Blennow, K. & Karikari, T. K. Plasma biomarkers for neurodegenerative disorders: ready for prime time? Curr. Opin. Psychiatry 36 , 112–118 (2023).
Apostolova, L. G. et al. Associations of the top 20 Alzheimer disease risk variants with brain amyloidosis. JAMA Neurol. 75 , 328–341 (2018).
Shi, Z. et al. Amyloid PET in dementia syndromes: a Chinese multicenter study. J. Nucl. Med. 61 , 1814–1819 (2020).
Raghavan, N. S. et al. Association between common variants in RBFOX1, an RNA-binding protein, and brain amyloidosis in early and preclinical Alzheimer disease. JAMA Neurol. 77 , 1288–1298 (2020).
Kim, S. et al. Genome-wide association study of CSF biomarkers Aβ 1-42 , t-tau, and p-tau 181p in the ADNI cohort. Neurology 76 , 69–79 (2011).
Babapour Mofrad, R. et al. Sex differences in CSF biomarkers vary by Alzheimer disease stage and APOE ε4 genotype. Neurology 95 , e2378–e2388 (2020).
Deming, Y. et al. Genome-wide association study identifies four novel loci associated with Alzheimer’s endophenotypes and disease modifiers. Acta Neuropathol. 133 , 839–856 (2017).
Deming, Y. et al. The MS4A gene cluster is a key modulator of soluble TREM2 and Alzheimer’s disease risk. Sci. Transl. Med. 11 , eaau2291 (2019).
Hong, S. et al. Genome-wide association study of Alzheimer’s disease CSF biomarkers in the EMIF-AD multimodal biomarker discovery dataset. Transl. Psychiatry 10 , 403 (2020).
Li, W. W. et al. Association of polygenic risk score with age at onset and cerebrospinal fluid biomarkers of Alzheimer’s disease in a Chinese cohort. Neurosci. Bull. 36 , 696–704 (2020).
Zhao, B. et al. Common genetic variation influencing human white matter microstructure. Science 372 , eabf3736 (2021).
Zhao, B. et al. Large-scale GWAS reveals genetic architecture of brain white matter microstructure and genetic overlap with cognitive and mental health traits ( n = 17,706). Mol. Psychiatry 26 , 3943–3955 (2021).
Homann, J. et al. Genome-wide association study of Alzheimer’s disease brain imaging biomarkers and neuropsychological phenotypes in the European medical information framework for Alzheimer’s disease multimodal biomarker discovery dataset. Front. Aging Neurosci. 14 , 840651 (2022).
Li, J. Q. et al. GWAS-linked loci and neuroimaging measures in Alzheimer’s disease. Mol. Neurobiol. 54 , 146–153 (2017).
Squillario, M. et al. A telescope GWAS analysis strategy, based on SNPs-genes-pathways ensamble and on multivariate algorithms, to characterize late onset Alzheimer’s disease. Sci. Rep. 10 , 12063 (2020).
Park, J. Y. et al. A missense variant in SHARPIN mediates Alzheimer’s disease-specific brain damages. Transl. Psychiatry 11 , 590 (2021).
Janelidze, S. et al. Plasma P-tau181 in Alzheimer’s disease: relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer’s dementia. Nat. Med. 26 , 379–386 (2020).
Thijssen, E. H. et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Nat. Med. 26 , 387–397 (2020).
Stevenson-Hoare, J. et al. Plasma biomarkers and genetics in the diagnosis and prediction of Alzheimer’s disease. Brain https://doi.org/10.1093/brain/awac128 (2022).
Article PubMed Central Google Scholar
Wang, Z. T. et al. Genome-wide association study identifies CD1A associated with rate of increase in plasma neurofilament light in non-demented elders. Aging 11 , 4521–4535 (2019).
Jiao, B. et al. Associations of risk genes with onset age and plasma biomarkers of Alzheimer’s disease: a large case-control study in mainland China. Neuropsychopharmacology 47 , 1121–1127 (2022).
Kim, J. et al. miR-27a and miR-27b regulate autophagic clearance of damaged mitochondria by targeting PTEN-induced putative kinase 1 (PINK1). Mol. Neurodegener. 11 , 55 (2016).
Garcia-Aranda, M., Serrano, A. & Redondo, M. Regulation of clusterin gene expression. Curr. Protein Pept. Sci. 19 , 612–622 (2018).
Kumar, P. et al. Circulating miRNA biomarkers for Alzheimer’s disease. PLoS One 8 , e69807 (2013).
Hampel, H. et al. Omics sciences for systems biology in Alzheimer’s disease: state-of-the-art of the evidence. Ageing Res. Rev. 69 , 101346 (2021).
Joyce, A. R. & Palsson, B. O. The model organism as a system: integrating ‘omics’ data sets. Nat. Rev. Mol. Cell Biol. 7 , 198–210 (2006).
Ritchie, M. D., Holzinger, E. R., Li, R., Pendergrass, S. A. & Kim, D. Methods of integrating data to uncover genotype-phenotype interactions. Nat. Rev. Genet. 16 , 85–97 (2015).
Choudhury, A. et al. High-depth African genomes inform human migration and health. Nature 586 , 741–748 (2020).
Mez, J. et al. Two novel loci, COBL and SLC10A2, for Alzheimer’s disease in African Americans. Alzheimers Dement. 13 , 119–129 (2017).
Miyashita, A. et al. SORL1 is genetically associated with late-onset Alzheimer’s disease in Japanese, Koreans and Caucasians. PLoS One 8 , e58618 (2013).
Nielsen, R. et al. Tracing the peopling of the world through genomics. Nature 541 , 302–310 (2017).
Tosto, G. et al. Polygenic risk scores in familial Alzheimer disease. Neurology 88 , 1180–1186 (2017).
DeMichele-Sweet, M. A. A. et al. Genome-wide association identifies the first risk loci for psychosis in Alzheimer disease. Mol. Psychiatry 26 , 5797–5811 (2021).
Kunkle, B. W. et al. Genome-wide linkage analyses of non-Hispanic white families identify novel loci for familial late-onset Alzheimer’s disease. Alzheimers Dement. 12 , 2–10 (2016).
Barral, S. et al. Linkage analyses in Caribbean Hispanic families identify novel loci associated with familial late-onset Alzheimer’s disease. Alzheimers Dement. 11 , 1397–1406 (2015).
Lanoiselee, H. M. et al. APP, PSEN1, and PSEN2 mutations in early-onset Alzheimer disease: a genetic screening study of familial and sporadic cases. PLoS Med. 14 , e1002270 (2017).
Golan, M. P. et al. Early-onset Alzheimer’s disease with a de novo mutation in the presenilin 1 gene. Exp. Neurol. 208 , 264–268 (2007).
Kukull, W. A. et al. Dementia and Alzheimer disease incidence: a prospective cohort study. Arch. Neurol. 59 , 1737–1746 (2002).
Frenzel, S. et al. A biomarker for Alzheimer’s disease based on patterns of regional brain atrophy. Front. Psychiatry 10 , 953 (2019).
Dickerson, B. C. et al. The cortical signature of Alzheimer’s disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb. Cortex 19 , 497–510 (2009).
Killiany, R. J. et al. MRI measures of entorhinal cortex vs hippocampus in preclinical AD. Neurology 58 , 1188–1196 (2002).
Delaby, C. et al. Clinical reporting following the quantification of cerebrospinal fluid biomarkers in Alzheimer’s disease: an international overview. Alzheimers Dement. 18 , 1868–1879 (2021).
Morris, J. C. et al. Assessment of racial disparities in biomarkers for Alzheimer disease. JAMA Neurol. 76 , 264–273 (2019).
Chaudhry, A. & Rizig, M. Comparing fluid biomarkers of Alzheimer’s disease between African American or Black African and white groups: a systematic review and meta-analysis. J. Neurol. Sci. 421 , 117270 (2021).
Leuzy, A. et al. Blood-based biomarkers for Alzheimer’s disease. EMBO Mol. Med. 14 , e14408 (2022).
Zetterberg, H. et al. Hypoxia due to cardiac arrest induces a time-dependent increase in serum amyloid β levels in humans. PLoS One 6 , e28263 (2011).
Gaiottino, J. et al. Increased neurofilament light chain blood levels in neurodegenerative neurological diseases. PLoS One 8 , e75091 (2013).
Khalil, M. et al. Neurofilaments as biomarkers in neurological disorders. Nat. Rev. Neurol. 14 , 577–589 (2018).
Ashton, N. J. et al. Increased plasma neurofilament light chain concentration correlates with severity of post-mortem neurofibrillary tangle pathology and neurodegeneration. Acta Neuropathol. Commun. 7 , 5 (2019).
Feinberg, A. P. Phenotypic plasticity and the epigenetics of human disease. Nature 447 , 433–440 (2007).
Fransquet, P. D. & Ryan, J. Micro RNA as a potential blood-based epigenetic biomarker for Alzheimer’s disease. Clin. Biochem. 58 , 5–14 (2018).
Lister, R. et al. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature 462 , 315–322 (2009).
Gertz, J. et al. Analysis of DNA methylation in a three-generation family reveals widespread genetic influence on epigenetic regulation. PLoS Genet. 7 , e1002228 (2011).
Shoemaker, R., Deng, J., Wang, W. & Zhang, K. Allele-specific methylation is prevalent and is contributed by CpG-SNPs in the human genome. Genome Res. 20 , 883–889 (2010).
Zhi, D. et al. SNPs located at CpG sites modulate genome-epigenome interaction. Epigenetics 8 , 802–806 (2013).
Dehghani, R., Rahmani, F. & Rezaei, N. MicroRNA in Alzheimer’s disease revisited: implications for major neuropathological mechanisms. Rev. Neurosci. 29 , 161–182 (2018).
Newgard, C. B. Metabolomics and metabolic diseases: where do we stand? Cell Metab. 25 , 43–56 (2017).
Mittelstrass, K. et al. Discovery of sexual dimorphisms in metabolic and genetic biomarkers. PLoS Genet. 7 , e1002215 (2011).
Shah, S. H. et al. Branched-chain amino acid levels are associated with improvement in insulin resistance with weight loss. Diabetologia 55 , 321–330 (2012).
Shah, S. H., Kraus, W. E. & Newgard, C. B. Metabolomic profiling for the identification of novel biomarkers and mechanisms related to common cardiovascular diseases: form and function. Circulation 126 , 1110–1120 (2012).
Vardarajan, B. et al. Differences in plasma metabolites related to Alzheimer’s disease, APOE epsilon4 status, and ethnicity. Alzheimers Dement. 6 , e12025 (2020).
Google Scholar
Wang, T. J. et al. Metabolite profiles and the risk of developing diabetes. Nat. Med. 17 , 448–453 (2011).
Heath, L. et al. Manifestations of Alzheimer’s disease genetic risk in the blood are evident in a multiomic analysis in healthy adults aged 18 to 90. Sci. Rep. 12 , 6117 (2022).
Walker, D. I. et al. High-resolution metabolomics of occupational exposure to trichloroethylene. Int. J. Epidemiol. 45 , 1517–1527 (2016).
Download references
Acknowledgements
The authors acknowledge support from the National Institute on Aging of the National Institutes of Health in the USA. C.R. (U24AG056270, P30AG066462, U19AG074865, RO1AG064614); M.A.P.-V. (R56AG072547, RO1AG070864, UO1AG057659, UO1AG062943, UO1AG076482, U19AG074865); T.F. (U24AG021886, P30AG072976, U24AG056270), R.M. (U24AG056270, R01AG072474, RF1AG066107, R01AG067501). The authors also thank R. Akinyemi for his help with the review of genetics in Africa and M. Miller for continued encouragement and support of the investigation of AD worldwide.
Author information
Authors and affiliations.
The Taub Institute for Research on Alzheimer’s Disease and the Aging Brain, Columbia University, New York, NY, USA
Christiane Reitz & Richard Mayeux
The Gertrude H. Sergievsky Center, Columbia University, New York, NY, USA
Department of Neurology, Columbia University, New York, NY, USA
Department of Epidemiology, Columbia University, New York, NY, USA
The John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, Miami, FL, USA
Margaret A. Pericak-Vance
The Dr. John T. Macdonald Foundation Department of Human Genetics, University of Miami Miller School of Medicine, Miami, FL, USA
Department of Medical and Molecular Genetics, Indiana University School of Medicine, Indianapolis, IN, USA
Tatiana Foroud
National Centralized Repository for Alzheimer’s Disease and Related Dementias, Indiana University School of Medicine, Indianapolis, IN, USA
You can also search for this author in PubMed Google Scholar
Contributions
All authors researched data for the article. All authors contributed substantially to discussion of the content. All authors wrote the article, and reviewed and/or edited the manuscript before submission.
Corresponding author
Correspondence to Richard Mayeux .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Nature Reviews Neurology thanks Ricardo Nitrini, who co-reviewed with Leonel Takada; and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Alzheimer’s Disease Neuroimaging Initiative: https://adni.loni.usc.edu/
Alzheimer’s Disease Sequencing Project-Follow-Up Study (ADSP-FUS): https://adsp.niagads.org/
Alzheimer’s Disease Sequencing Project-Follow-Up Study: https://www.nia.nih.gov/research/ad-genetics
Asian Cohort for Alzheimer’s Disease: https://acadstudy.org/
Estudio Familiar de Influencia Genetica en Alzheimer (Puerto Rican Alzheimer Disease Initiative; EFIGA): https://dss.niagads.org/cohorts/estudio-familiar-de-influencia-genetica-en-alzheimer-efiga/
Gwangju Alzheimer’s and Related Dementias (GARD) Study: https://dss.niagads.org/cohorts/gwangju-alzheimers-and-related-dementia-gard/
Longitudinal Aging Study in India: https://lasi-india.org/
Mexican Health and Aging Study: https://www.mhasweb.org/Home/index.aspx
Research in African American Alzheimer’s Disease Initiative (REAAADI): https://med.miami.edu/centers-and-institutes/hihg/research-programs/alzheimers-disease-and-related-dementias/research-in-african-american-alzheimer-disease-initiative
WHO dementia fact sheet: https://www.who.int/news-room/fact-sheets/detail/dementia
An aggregate of genetically related individuals.
Principal components analysis is a statistical method commonly used in population genetics to identify substructure in the distribution of genetic variation within populations.
Regions of DNA each associated with a particular quantitative phenotypic trait.
Genetic recombination is the exchange of genetic material between different individuals which leads to offspring with combinations of traits that differ from those in either parent.
Genetic variants for which association with a specific trait is unclear.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Reitz, C., Pericak-Vance, M.A., Foroud, T. et al. A global view of the genetic basis of Alzheimer disease. Nat Rev Neurol 19 , 261–277 (2023). https://doi.org/10.1038/s41582-023-00789-z
Download citation
Accepted : 21 February 2023
Published : 06 April 2023
Issue Date : May 2023
DOI : https://doi.org/10.1038/s41582-023-00789-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Immunological aspects of central neurodegeneration.
- Mireia Niso-Santano
- José M. Fuentes
- Lorenzo Galluzzi
Cell Discovery (2024)
Alzheimer’s disease risk reduction in clinical practice: a priority in the emerging field of preventive neurology
- Kellyann Niotis
- Corey Saperia
- Richard S. Isaacson
Nature Mental Health (2024)
Rare genetic variation in fibronectin 1 (FN1) protects against APOEε4 in Alzheimer’s disease
- Prabesh Bhattarai
- Tamil Iniyan Gunasekaran
- Badri N. Vardarajan
Acta Neuropathologica (2024)
Nerve growth factor receptor (Ngfr) induces neurogenic plasticity by suppressing reactive astroglial Lcn2/Slc22a17 signaling in Alzheimer’s disease
- Tohid Siddiqui
- Mehmet Ilyas Cosacak
- Caghan Kizil
npj Regenerative Medicine (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, generate accurate citations for free.
- Knowledge Base
Methodology
- How to Write a Literature Review | Guide, Examples, & Templates
How to Write a Literature Review | Guide, Examples, & Templates
Published on January 2, 2023 by Shona McCombes . Revised on September 11, 2023.
What is a literature review? A literature review is a survey of scholarly sources on a specific topic. It provides an overview of current knowledge, allowing you to identify relevant theories, methods, and gaps in the existing research that you can later apply to your paper, thesis, or dissertation topic .
There are five key steps to writing a literature review:
- Search for relevant literature
- Evaluate sources
- Identify themes, debates, and gaps
- Outline the structure
- Write your literature review
A good literature review doesn’t just summarize sources—it analyzes, synthesizes , and critically evaluates to give a clear picture of the state of knowledge on the subject.
Instantly correct all language mistakes in your text
Upload your document to correct all your mistakes in minutes

Table of contents
What is the purpose of a literature review, examples of literature reviews, step 1 – search for relevant literature, step 2 – evaluate and select sources, step 3 – identify themes, debates, and gaps, step 4 – outline your literature review’s structure, step 5 – write your literature review, free lecture slides, other interesting articles, frequently asked questions, introduction.
- Quick Run-through
- Step 1 & 2
When you write a thesis , dissertation , or research paper , you will likely have to conduct a literature review to situate your research within existing knowledge. The literature review gives you a chance to:
- Demonstrate your familiarity with the topic and its scholarly context
- Develop a theoretical framework and methodology for your research
- Position your work in relation to other researchers and theorists
- Show how your research addresses a gap or contributes to a debate
- Evaluate the current state of research and demonstrate your knowledge of the scholarly debates around your topic.
Writing literature reviews is a particularly important skill if you want to apply for graduate school or pursue a career in research. We’ve written a step-by-step guide that you can follow below.
Here's why students love Scribbr's proofreading services
Discover proofreading & editing
Writing literature reviews can be quite challenging! A good starting point could be to look at some examples, depending on what kind of literature review you’d like to write.
- Example literature review #1: “Why Do People Migrate? A Review of the Theoretical Literature” ( Theoretical literature review about the development of economic migration theory from the 1950s to today.)
- Example literature review #2: “Literature review as a research methodology: An overview and guidelines” ( Methodological literature review about interdisciplinary knowledge acquisition and production.)
- Example literature review #3: “The Use of Technology in English Language Learning: A Literature Review” ( Thematic literature review about the effects of technology on language acquisition.)
- Example literature review #4: “Learners’ Listening Comprehension Difficulties in English Language Learning: A Literature Review” ( Chronological literature review about how the concept of listening skills has changed over time.)
You can also check out our templates with literature review examples and sample outlines at the links below.
Download Word doc Download Google doc
Before you begin searching for literature, you need a clearly defined topic .
If you are writing the literature review section of a dissertation or research paper, you will search for literature related to your research problem and questions .
Make a list of keywords
Start by creating a list of keywords related to your research question. Include each of the key concepts or variables you’re interested in, and list any synonyms and related terms. You can add to this list as you discover new keywords in the process of your literature search.
- Social media, Facebook, Instagram, Twitter, Snapchat, TikTok
- Body image, self-perception, self-esteem, mental health
- Generation Z, teenagers, adolescents, youth
Search for relevant sources
Use your keywords to begin searching for sources. Some useful databases to search for journals and articles include:
- Your university’s library catalogue
- Google Scholar
- Project Muse (humanities and social sciences)
- Medline (life sciences and biomedicine)
- EconLit (economics)
- Inspec (physics, engineering and computer science)
You can also use boolean operators to help narrow down your search.
Make sure to read the abstract to find out whether an article is relevant to your question. When you find a useful book or article, you can check the bibliography to find other relevant sources.
You likely won’t be able to read absolutely everything that has been written on your topic, so it will be necessary to evaluate which sources are most relevant to your research question.
For each publication, ask yourself:
- What question or problem is the author addressing?
- What are the key concepts and how are they defined?
- What are the key theories, models, and methods?
- Does the research use established frameworks or take an innovative approach?
- What are the results and conclusions of the study?
- How does the publication relate to other literature in the field? Does it confirm, add to, or challenge established knowledge?
- What are the strengths and weaknesses of the research?
Make sure the sources you use are credible , and make sure you read any landmark studies and major theories in your field of research.
You can use our template to summarize and evaluate sources you’re thinking about using. Click on either button below to download.
Take notes and cite your sources
As you read, you should also begin the writing process. Take notes that you can later incorporate into the text of your literature review.
It is important to keep track of your sources with citations to avoid plagiarism . It can be helpful to make an annotated bibliography , where you compile full citation information and write a paragraph of summary and analysis for each source. This helps you remember what you read and saves time later in the process.
Prevent plagiarism. Run a free check.
To begin organizing your literature review’s argument and structure, be sure you understand the connections and relationships between the sources you’ve read. Based on your reading and notes, you can look for:
- Trends and patterns (in theory, method or results): do certain approaches become more or less popular over time?
- Themes: what questions or concepts recur across the literature?
- Debates, conflicts and contradictions: where do sources disagree?
- Pivotal publications: are there any influential theories or studies that changed the direction of the field?
- Gaps: what is missing from the literature? Are there weaknesses that need to be addressed?
This step will help you work out the structure of your literature review and (if applicable) show how your own research will contribute to existing knowledge.
- Most research has focused on young women.
- There is an increasing interest in the visual aspects of social media.
- But there is still a lack of robust research on highly visual platforms like Instagram and Snapchat—this is a gap that you could address in your own research.
There are various approaches to organizing the body of a literature review. Depending on the length of your literature review, you can combine several of these strategies (for example, your overall structure might be thematic, but each theme is discussed chronologically).
Chronological
The simplest approach is to trace the development of the topic over time. However, if you choose this strategy, be careful to avoid simply listing and summarizing sources in order.
Try to analyze patterns, turning points and key debates that have shaped the direction of the field. Give your interpretation of how and why certain developments occurred.
If you have found some recurring central themes, you can organize your literature review into subsections that address different aspects of the topic.
For example, if you are reviewing literature about inequalities in migrant health outcomes, key themes might include healthcare policy, language barriers, cultural attitudes, legal status, and economic access.
Methodological
If you draw your sources from different disciplines or fields that use a variety of research methods , you might want to compare the results and conclusions that emerge from different approaches. For example:
- Look at what results have emerged in qualitative versus quantitative research
- Discuss how the topic has been approached by empirical versus theoretical scholarship
- Divide the literature into sociological, historical, and cultural sources
Theoretical
A literature review is often the foundation for a theoretical framework . You can use it to discuss various theories, models, and definitions of key concepts.
You might argue for the relevance of a specific theoretical approach, or combine various theoretical concepts to create a framework for your research.
Like any other academic text , your literature review should have an introduction , a main body, and a conclusion . What you include in each depends on the objective of your literature review.
The introduction should clearly establish the focus and purpose of the literature review.
Depending on the length of your literature review, you might want to divide the body into subsections. You can use a subheading for each theme, time period, or methodological approach.
As you write, you can follow these tips:
- Summarize and synthesize: give an overview of the main points of each source and combine them into a coherent whole
- Analyze and interpret: don’t just paraphrase other researchers — add your own interpretations where possible, discussing the significance of findings in relation to the literature as a whole
- Critically evaluate: mention the strengths and weaknesses of your sources
- Write in well-structured paragraphs: use transition words and topic sentences to draw connections, comparisons and contrasts
In the conclusion, you should summarize the key findings you have taken from the literature and emphasize their significance.
When you’ve finished writing and revising your literature review, don’t forget to proofread thoroughly before submitting. Not a language expert? Check out Scribbr’s professional proofreading services !
This article has been adapted into lecture slides that you can use to teach your students about writing a literature review.
Scribbr slides are free to use, customize, and distribute for educational purposes.
Open Google Slides Download PowerPoint
If you want to know more about the research process , methodology , research bias , or statistics , make sure to check out some of our other articles with explanations and examples.
- Sampling methods
- Simple random sampling
- Stratified sampling
- Cluster sampling
- Likert scales
- Reproducibility
Statistics
- Null hypothesis
- Statistical power
- Probability distribution
- Effect size
- Poisson distribution
Research bias
- Optimism bias
- Cognitive bias
- Implicit bias
- Hawthorne effect
- Anchoring bias
- Explicit bias
A literature review is a survey of scholarly sources (such as books, journal articles, and theses) related to a specific topic or research question .
It is often written as part of a thesis, dissertation , or research paper , in order to situate your work in relation to existing knowledge.
There are several reasons to conduct a literature review at the beginning of a research project:
- To familiarize yourself with the current state of knowledge on your topic
- To ensure that you’re not just repeating what others have already done
- To identify gaps in knowledge and unresolved problems that your research can address
- To develop your theoretical framework and methodology
- To provide an overview of the key findings and debates on the topic
Writing the literature review shows your reader how your work relates to existing research and what new insights it will contribute.
The literature review usually comes near the beginning of your thesis or dissertation . After the introduction , it grounds your research in a scholarly field and leads directly to your theoretical framework or methodology .
A literature review is a survey of credible sources on a topic, often used in dissertations , theses, and research papers . Literature reviews give an overview of knowledge on a subject, helping you identify relevant theories and methods, as well as gaps in existing research. Literature reviews are set up similarly to other academic texts , with an introduction , a main body, and a conclusion .
An annotated bibliography is a list of source references that has a short description (called an annotation ) for each of the sources. It is often assigned as part of the research process for a paper .
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the “Cite this Scribbr article” button to automatically add the citation to our free Citation Generator.
McCombes, S. (2023, September 11). How to Write a Literature Review | Guide, Examples, & Templates. Scribbr. Retrieved June 10, 2024, from https://www.scribbr.com/dissertation/literature-review/
Is this article helpful?
Shona McCombes
Other students also liked, what is a theoretical framework | guide to organizing, what is a research methodology | steps & tips, how to write a research proposal | examples & templates, "i thought ai proofreading was useless but..".
I've been using Scribbr for years now and I know it's a service that won't disappoint. It does a good job spotting mistakes”
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
June 4, 2024
PLOS ONE
An inclusive journal community working together to advance science by making all rigorous research accessible without barriers
Calling all experts!
Plos one is seeking talented individuals to join our editorial board. .
Cancer Epidemiology
Impact of aging on acute myeloid leukemia epidemiology and survival outcomes: A real-world, population-based longitudinal cohort study
Han and colleagues report an association between aging and incidence of acute myeloid leukemia diagnoses in South Korea, with recommendations to expand treatment options for older patients.
Image credit: Couple by Mabel Amber, Pixabay
Agriculture
Persistence of genetically engineered canola populations in the U.S. and the adventitious presence of transgenes in the environment
Travers and colleagues research reveal that escaped GMO canola plants persist long-term outside farms but may be losing their herbicide resistant transgenes.
Image credit: Canola field in Manitoba, Canada by Ethan Sahagun, Wikimedia Commons
Climate Change
Uncertainty reduction for precipitation prediction in North America
Lou and colleagues investigated the uncertainties across 27 CMIP6 models for projecting future annual precipitation increases in North America. They captured emergent constraint relationships between annual growth rates of simulated historical temperature and future precipitation. This reduced precipitation prediction uncertainties and improved temperature trend accuracy.
Image credit: Puddle by pictures101, Pixabay
Neuroscience
Orienteering combines vigorous-intensity exercise with navigation to improve human cognition and increase brain-derived neurotrophic factor
Waddington and colleagues report the benefits of the sport of orienteering, which combines vigorous exercise with spatial navigation, on memory and molecular markers of cognition such as BDNF.
Image credit: Fig 7 by Waddington et al., CC BY 4.0
Official PLOS Blog
Driving Open Science adoption with a global framework: the Open Science Monitoring Initiative
PLOS discusses the recent launch of the Open Science Monitoring Initiative.
Image credit: Lighthouse by Masami, CC BY 4.0
Editor Spotlight: Frank Kyei-Arthur
In this interview, PLOS ONE Academic Editor Dr Frank Kyei-Arthur discusses assessing reviewers' comments, his research interest in diverse populations, and the importance of Open Science in population health research.
Image credit: Dr. Frank Kyei-Arthur by Dr. Frank Kyei-Arthur, CC BY 4.0
Editor Spotlight: Bogdan Cristescu
In this interview, PLOS ONE Academic Editor Dr Bogdan Cristescu shares his experiences with PLOS ONE as author, reviewer and editor, his research in wildlife conservation ecology, and memorable places from his fieldwork.
Image credit: Dr. Bogdan Cristescu by Dr. Bogdan Cristescu, CC BY 4.0
Biochemistry
Formamide denaturation of double-stranded DNA for fluorescence in situ hybridization (FISH) distorts nanoscale chromatin structure
Shim and colleagues compare DNA labelling methods.
Image credit: Bottoms spiral string by Qimono, Pixabay
Archaeology
Pottery spilled the beans: Patterns in the processing and consumption of dietary lipids in Central Germany from the Early Neolithic to the Bronze Age
Breu and colleagues analyzed pottery vessels to study ancient culinary traditions in Germany.
Image credit: Fig 2 by Breu et al., CC BY 4.0
Rapid respiratory microbiological point-of-care-testing and antibiotic prescribing in primary care: Protocol for the RAPID-TEST randomised controlled trial
Abbs and colleagues report the protocol for the RAPID-TEST randomised controlled trial.
Image credit: Healthcare worker taking PCR test by Drazen Zigic, Freepik
Animal behaviour
Eurasian jays ( Garrulus glandarius ) show episodic-like memory through the incidental encoding of information
Davies and colleagues show how Eurasian jays can use mental time travel like humans
Image credit: Eurasian Jay (Garrulus glandarius) by Zeynel Cebeci, Wikimedia Commons
Collections
Browse the lastest collections of papers from across PLOS
Watch this space for future collections of papers in PLOS ONE
RCPSYCH International Congress 2024
Associate Editor Annesha Sil will be representing PLOS ONE at this conference in Edinburgh, UK, June 17-20, 2024.
Sunbelt 2024
Senior Editor Hanna Landenmark will be representing PLOS ONE at this conference in Edinburgh, UK, June 24-30, 2024.
UK Alliance for Disaster Reduction (UKADR) 2024 Conference
Associate Editor Joanna Tindall will be representing PLOS ONE at this conference in London, UK, June 26-27, 2024.
Publish with PLOS ONE
- Submission Instructions
- Submit Your Manuscript
Connect with Us
- PLOS ONE on Twitter
- PLOS on Facebook
Get new content from PLOS ONE in your inbox
Thank you you have successfully subscribed to the plos one newsletter., sorry, an error occurred while sending your subscription. please try again later..

IMAGES
VIDEO
COMMENTS
1. Introduction. Alzheimer's disease (AD) is a polygenic and multifactorial disease characterized by the deposition of amyloid-β (Aβ) fibrils in the brain, leading to the formation of plaques and neurofibrillary tangles (NFTs), and ultimately resulting in dendritic dysfunction, neuronal cell death, memory loss, behavioral changes, and organ ...
The inexorable progression of Alzheimer's disease exerts a huge toll on patients, families, and society, costing approximately $1 trillion annually, an amount that is likely to increase with the ...
Alzheimer's disease is a progressive neurodegenerative disease that impairs memory and cognitive judgment and is often accompanied by mood swings, disorientation and eventually delirium. It is the ...
Late-onset Alzheimer's disease is an irreversible neurodegenerative disorder which accounts for the majority of dementia cases 1.Despite major private and public investments in research, there ...
Alzheimer disease is a neurodegenerative disorder that causes cognitive impairment. This Primer by Knopman et al. reviews the epidemiology of cognitive manifestations and risk factors, summarizes ...
Alzheimer's disease (AD) is a progressive neurodegenerative disorder that is the sixth leading cause of death and the most common cause of dementia worldwide. ... Neuropsychological tools with relevance for humans are also emerging from NHP research. For example, ... This paper results from the Arthur M. Sackler Colloquium of the National ...
Alzheimer's disease (AD) poses a significant public health problem, affecting millions of people across the world. Despite decades of research into therapeutic strategies for AD, effective prevention or treatm... Zhihai Huang, Michael R. Hamblin and Quanguang Zhang. Alzheimer's Research & Therapy 2024 16 :114.
Alzheimer's disease (AD) remains a significant challenge in the field of neurodegenerative disorders, even nearly a century after its discovery, due to the elusive nature of its causes. The development of drugs that target multiple aspects of the disease has emerged as a promising strategy to address the complexities of AD and related conditions. The immune system's role, particularly in ...
As one of the most common neurodegenerative diseases and causes of dementia, Alzheimer disease (AD) is a critical topic for biomedical research. The main clinical manifestation of AD is the progressive decline of cognitive function and activities of daily living, and the pivotal pathological features of AD are amyloid beta (Aβ) deposition ...
The Machine Learning techniques (26, 27) were applied to Alzheimer's disease datasets to bring a new dimension to predict Disease at an early stage.The raw Alzheimer's disease datasets are inconsistent and redundant, which affects the accuracy of algorithms (28, 29).Before evaluating machine-learning algorithms, data must be effectively prepared for analysis by removing unwanted attributes ...
Alzheimer's disease is a progressive neurodegenerative disease that causes brain cells to waste away and die. It is characterized by progressive cognitive deterioration and continuous decline in ...
The Nature paper has been cited in about 2300 scholarly articles—more than all but four other Alzheimer's basic research reports published since 2006, according to the Web of Science database. Since then, annual NIH support for studies labeled "amyloid, oligomer, and Alzheimer's" has risen from near zero to $287 million in 2021.
A Research Paper on Alzheimer's Disease. Abstract. In this paper, Alzheimer's disease will be delved into, investigated and dissected. This will include all that is known about the disease as much of it is unknown still, despite increasing efforts from the medical community to uncover its origin. The disease's causes, symptoms and stages ...
Meta-analysis of genome-wide association studies on Alzheimer's disease and related dementias identifies new loci and enables generation of a new genetic risk score associated with the risk of ...
Researchers at the Icahn School of Medicine at Mount Sinai have achieved a major breakthrough in Alzheimer's disease research. Their study identifies a promising method that could potentially slow or even stop the progression of the disease. Focusing on the role of reactive astrocytes and the plexin-B1 protein in Alzheimer's disease, the ...
Datasets. The database encompasses 277 integrated datasets from 67 Alzheimer's disease-related scRNA-seq & snRNA-seq studies, totaling 7,332,202 cells. The repository also includes 381 spatial ...
May 27 2024 Mount Sinai Health System. Researchers at the Icahn School of Medicine at Mount Sinai have made a significant breakthrough in Alzheimer's disease research by identifying a novel way to ...
A version of this story appeared in Science, Vol 384, Issue 6700. Authors of a landmark Alzheimer's disease research paper published in Nature in 2006 have agreed to retract the study in response to allegations of image manipulation. University of Minnesota (UMN) Twin Cities neuroscientist Karen Ashe, the paper's senior author, acknowledged ...
Alzheimer's Disease: History, Mechanisms and Treatment. Nevertheless, researchers state that the development of Alzheimer's is impacted by the formation of protein plaques and tangles in the brain. Alzheimer's Disease: Causes and Treatment. AD is associated with different changes, both cognitive and behavioral.
Alzheimer's disease is the most common type of dementia. It is a progressive disease beginning with mild memory loss and possibly leading to loss of the ability to carry on a conversation and respond to the environment. Alzheimer's disease involves parts of the brain that control thought, memory, and language. It can seriously affect a ...
Abstract. The risk of Alzheimer disease (AD) increases with age, family history and informative genetic variants. Sadly, there is still no cure or means of prevention. As in other complex diseases ...
Examples of literature reviews. Step 1 - Search for relevant literature. Step 2 - Evaluate and select sources. Step 3 - Identify themes, debates, and gaps. Step 4 - Outline your literature review's structure. Step 5 - Write your literature review.
In this interview, PLOS ONE Academic Editor Dr Bogdan Cristescu shares his experiences with PLOS ONE as author, reviewer and editor, his research in wildlife conservation ecology, and memorable places from his fieldwork. Image credit: Dr. Bogdan Cristescu by Dr. Bogdan Cristescu, CC BY 4.0. 05/28/2024.