
- SUNY Oswego, Penfield Library
- Resource Guides

Biological Sciences Research Guide
Primary research vs review article.
- Research Starters
- Citing Sources
- Open Educational Resources
- Peer Review
- How to Read a Scientific Article
- Conducting a Literature Review
- Interlibrary Loan
Quick Links
- Penfield Library
- Research Guides
- A-Z List of Databases & Indexes
Characteristics of a Primary Research Article
- Goal is to present the result of original research that makes a new contribution to the body of knowledge
- Sometimes referred to as an empirical research article
- Typically organized into sections that include: Abstract, Introduction, Methods, Results, Discussion/Conclusion, and References.
Example of a Primary Research Article:
Flockhart, D.T.T., Fitz-gerald, B., Brower, L.P., Derbyshire, R., Altizer, S., Hobson, K.A., … Norris, D.R., (2017). Migration distance as a selective episode for wing morphology in a migratory insect. Movement Ecology , 5(1), 1-9. doi: doi.org/10.1186/s40462-017-0098-9
Characteristics of a Review Article
- Goal is to summarize important research on a particular topic and to represent the current body of knowledge about that topic.
- Not intended to provide original research but to help draw connections between research studies that have previously been published.
- Help the reader understand how current understanding of a topic has developed over time and identify gaps or inconsistencies that need further exploration.
Example of a Review Article:
https://www-sciencedirect-com.ezproxy.oswego.edu/science/article/pii/S0960982218302537
- << Previous: Plagiarism
- Next: Peer Review >>

- Master Your Homework
- Do My Homework
Comparing Research and Review Papers: Key Differences
Research papers and review papers are two distinct forms of academic writing that are often confused. In this article, we provide a detailed analysis of the key differences between research and review papers, with an emphasis on helping researchers to better understand these distinctions. Specifically, we will explore the defining features of each paper type, discuss their individual purpose and scope in detail, consider when one should be used over another for certain topics or disciplines, and finally offer practical strategies for crafting effective research and review pieces. With this comprehensive overview of the fundamental distinctions between these two types of writing projects as our guidepost—we hope readers will come away from this piece having gained a clearer sense about how to effectively compare them within their own practice.
I. Introduction
Ii. definition of research and review papers, iii. differences in purpose and scope, iv. key features of research paper writing, v. formatting requirements for review papers, vi. conclusion: distinguishing between the two types of documents, vii. references.
The world of academic writing is vast and varied. From research papers to reviews, there are many genres available for an author to explore. Understanding the differences between these two types of works is essential in order to produce effective scholarly pieces.
- Research Paper : Research papers present evidence gathered from data analysis or experimentation; they also discuss theoretical conclusions based on those results. A successful research paper requires thorough background knowledge, a clear focus, and logical arguments that support your claims.
- Review Paper : Review papers provide an overview of existing literature on a given topic. They should be composed with careful attention paid to detail, as well as offering insights into patterns found within related sources of information. Reviews typically do not include new primary research but rather summarize other studies while synthesizing important themes across multiple works.
Understanding the distinction between research and review papers is critical to a successful academic career. While both seek to explore and synthesize current knowledge on a subject, there are key differences in their purpose.
- Research Papers:
Research papers involve independent investigation into existing literature that strives to add something new or significant to the field of study. The core objective is to use evidence-based methodology as an analytical tool for exploring ideas and hypotheses while establishing scholarly credentials within one’s chosen discipline. It also requires rigorous fact checking, accurate source citation, methodological consistency, adherence to ethical standards of conduct with regard to human subjects, etc.
- Review Papers:
Purpose and Scope: Research papers and review papers both present the findings of an author’s study, but there is a fundamental difference between them. Research papers focus on new research in a particular field or subject, while reviews take an existing body of literature and summarize it for readers to get up-to-date with the topic. While research articles often investigate something that has not been studied before, review articles provide insight into past studies done in various fields. Reviews typically contain fewer details than full research reports due to their summarizing nature; thus they are usually shorter in length as well. Furthermore, different styles of writing may be used depending on which type of paper is being written – narrative for reviews vs analytical for original works.
The scope also differs when comparing these two types of documents – academic journals tend to favor more thorough pieces such as those found in research articles whereas magazines generally prefer short opinion pieces based off other authors’ work (i.e., reviews). Additionally, peer reviewers have differing roles when evaluating each respective article; they examine specific criteria including accuracy and depth when looking at research reports versus precision and clarity within reviews. The primary purpose remains unchanged regardless though – providing valuable information to its audience through thoughtful analysis backed by evidence from scholarly sources!
Research paper writing has its own set of distinct characteristics that render it unique from other genres. This section outlines the key features of research papers and also provides a brief comparison to review papers.
- Formalism: Research papers generally maintain a more formal style than most types of writing, often containing complex vocabulary and thorough explanations in an effort to best communicate ideas. Review papers are slightly less formal, providing summary rather than detailed analyses.
- Structure: A well-structured research paper will typically contain several elements such as an introduction with background information on the topic, multiple body sections covering different aspects related to the topic at hand, and conclusions synthesizing all major points made throughout. In contrast, reviews commonly have fewer structured components but may be organized around themes or topics discussed.
Consistent Formatting Across Review Papers Review papers, like research papers, come with a set of formatting requirements that must be followed to make them look presentable and clear. For starters, the general paper format should be maintained throughout – 1-inch margins on all sides and double spacing are most commonly accepted as standards for any academic writing. Font size may vary depending on the journal but should generally remain between 10–12 pt; Times New Roman is considered an acceptable font choice.
A review paper differs from its research counterparts in two key ways: Firstly, it does not include primary data or raw results which would usually need to accompany experimental work (in fact these findings can be referenced rather than detailed within). Secondly, each section’s content needs to focus more heavily on summarizing existing literature sources instead of discussing interpretations or drawing conclusions based off your own experiments.
- Table titles must align directly above their respective tables.
- Figures should also follow similar conventions as far as alignment goes.
. A comprehensive list of references at the end – including both those cited in body text and otherwise consulted during preparation — is required too. In many cases this will take up significantly more space compared to what you’d find in a comparable research article due to the number of publications discussed therein!
Distinguishing Between Research and Review Papers
Both research and review papers are used for academic purposes, however they differ in purpose and writing style. The primary difference between the two is that a research paper involves original investigation of a subject, while a review paper summarises already-existing information about it.
- A research paper typically includes an introduction outlining the author’s hypothesis or aims; a literature review with citations; methodology describing how data was collected to test hypotheses or answer questions posed in the introduction section; results showing what was found out during analysis of gathered data; discussion interpreting findings from results; conclusion summarising whether study met its goals.
Locating Sources The research process is only as strong as the references it builds upon. To ensure accuracy and relevance, it is important to track down a variety of sources when undertaking an academic task. For this purpose, research papers and review papers are valuable contributions which can be tapped for relevant insights.
Research Papers provide direct accounts from field experts on their experiments or findings in a particular area; these are typically peer-reviewed before they become available publicly. Meanwhile Review Papers synthesize multiple primary documents into one unified viewpoint; here authors can assess past efforts while offering insight into future directions for further inquiry. Both types of paper offer researchers insightful perspectives that aid in understanding complex topics, but each serve different roles depending on the project’s needs.
English: The comparison between research and review papers is an important one, as it can help to inform readers on the differences between two types of academic writing. By highlighting key points such as the purpose, content, structure and language used in each type of paper we have seen that there are distinct advantages and disadvantages to both approaches. It is clear that this understanding is invaluable for those looking to improve their own skills in writing either form of scholarly article or those wanting a better appreciation when reading others’ work.

Science Research: Primary Sources and Original Research vs. Review Articles
- Additional Web Resources
- Health & Science Databases
- Primary Sources and Original Research vs. Review Articles
- Citation Guides, Generators, and Tools
Original Research vs. Review Articles. How can I tell the Difference?
Research vs review articles.
It's often difficult to tell the difference between original research articles and review articles. Here are some explanations and tips that may help: "Review articles are often as lengthy or even longer that original research articles. What the authors of review articles are doing in analysing and evaluating current research and investigations related to a specific topic, field, or problem. They are not primary sources since they review previously published material. They can be of great value for identifying potentially good primary sources, but they aren't primary themselves. Primary research articles can be identified by a commonly used format. If an article contains the following elements, you can count on it being a primary research article. Look for sections titled:
Methods (sometimes with variations, such as Materials and Methods) Results (usually followed with charts and statistical tables) Discussion
You can also read the abstract to get a good sense of the kind of article that is being presented.
If it is a review article instead of a research article, the abstract should make that pretty clear. If there is no abstract at all, that in itself may be a sign that it is not a primary resource. Short research articles, such as those found in Science and similar scientific publications that mix news, editorials, and forums with research reports, however, may not include any of those elements. In those cases look at the words the authors use, phrases such as "we tested" and "in our study, we measured" will tell you that the article is reporting on original research."*
*Taken from Ithca College Libraries
Primary and Secondary Sources for Science
In the Sciences, primary sources are documents that provide full description of the original research. For example, a primary source would be a journal article where scientists describe their research on the human immune system. A secondary source would be an article commenting or analyzing the scientists' research on the human immune system.
EXAMPLES OF PRIMARY AND SECONDARY SOURCES
Source: The Evolution of Scientific Information (from Encyclopedia of Library and Information Science , vol. 26).
Primary Vs. Secondary Vs. Tertiary Sources
- << Previous: Books
- Next: Citation Guides, Generators, and Tools >>
- Last Updated: Nov 16, 2023 1:58 PM
- URL: https://andersonuniversity.libguides.com/ScienceResearch
Thrift Library | (864) 231-2050 | [email protected] | Anderson University 316 Boulevard Anderson, SC 29621
- Study resources
- Calendar - Graduate
- Calendar - Undergraduate
- Class schedules
- Class cancellations
- Course registration
- Important academic dates
- More academic resources
- Campus services
- IT services
- Job opportunities
- Safety & prevention
- Mental health support
- Student Service Centre (Birks)
- All campus services
- Calendar of events
- Latest news
- Media Relations
- Faculties, Schools & Colleges
- Arts and Science
- Gina Cody School of Engineering and Computer Science
- John Molson School of Business
- School of Graduate Studies
- All Schools, Colleges & Departments.
- Directories
- My Library account Renew books and more
- Book a study room or scanner Reserve a space for your group online
- Interlibrary loans (Colombo) Request books from external libraries
- Zotero (formerly RefWorks) Manage your citations and create bibliographies
- Article/Chapter Scan & Deliver Request a PDF of an article/chapter we have in our physical collection
- Contactless Book Pickup Request books, DVDs and more from our physical collection while the Library is closed
- WebPrint Upload documents to print on campus
- Course reserves Online course readings
- Spectrum Deposit a thesis or article
- Sofia Discovery tool
- Databases by subject
- Course Reserves
- E-journals via Browzine
- E-journals via Sofia
- Article/chapter scan
- Intercampus delivery of bound periodicals/microforms
- Interlibrary loans
- Spectrum Research Repository
- Special Collections
- Additional resources & services
- Subject & course guides
- Open Educational Resources Guide
- Borrowing & renewing
- General guides for users
- Evaluating...
- Ask a librarian
- Research Skills Tutorial
- Bibliometrics & research impact guide
- Concordia University Press
- Copyright guide
- Copyright guide for thesis preparation
- Digital scholarship
- Digital preservation
- Open Access
- ORCiD at Concordia
- Research data management guide
- Scholarship of Teaching & Learning
- Systematic Reviews
- Borrow (laptops, tablets, equipment)
- Connect (netname, Wi-Fi, guest accounts)
- Desktop computers, software & availability maps
- Group study, presentation practice & classrooms
- Printers, copiers & scanners
- Technology Sandbox
- Visualization Studio
- Webster Library
- Vanier Library
- Grey Nuns Reading Room
- Study spaces
- Floor plans
- Book a group study room/scanner
- Room booking for academic events
- Exhibitions
- Librarians & staff
- Work with us
- Memberships & collaborations
- Indigenous Student Librarian program
- Wikipedian in residence
- Researcher in residence
- Feedback & improvement
- Annual reports & fast facts
- Strategic Plan 2016/21
- Library Services Fund
- Giving to the Library
- Policies & Code of Conduct
- My Library account
- Book a study room or scanner
- Interlibrary loans (Colombo)
- Zotero (formerly RefWorks)
- Article/Chapter Scan & Deliver
- Contactless Book Pickup
- Course reserves
Review vs. Research Articles
How can you tell if you are looking at a research paper, review paper or a systematic review examples and article characteristics are provided below to help you figure it out., research papers.
A research article describes a study that was performed by the article’s author(s). It explains the methodology of the study, such as how data was collected and analyzed, and clarifies what the results mean. Each step of the study is reported in detail so that other researchers can repeat the experiment.
To determine if a paper is a research article, examine its wording. Research articles describe actions taken by the researcher(s) during the experimental process. Look for statements like “we tested,” “I measured,” or “we investigated.” Research articles also describe the outcomes of studies. Check for phrases like “the study found” or “the results indicate.” Next, look closely at the formatting of the article. Research papers are divided into sections that occur in a particular order: abstract, introduction, methods, results, discussion, and references.
Let's take a closer look at this research paper by Bacon et al. published in the International Journal of Hypertension :

Review Papers
Review articles do not describe original research conducted by the author(s). Instead, they give an overview of a specific subject by examining previously published studies on the topic. The author searches for and selects studies on the subject and then tries to make sense of their findings. In particular, review articles look at whether the outcomes of the chosen studies are similar, and if they are not, attempt to explain the conflicting results. By interpreting the findings of previous studies, review articles are able to present the current knowledge and understanding of a specific topic.
Since review articles summarize the research on a particular topic, students should read them for background information before consulting detailed, technical research articles. Furthermore, review articles are a useful starting point for a research project because their reference lists can be used to find additional articles on the subject.
Let's take a closer look at this review paper by Bacon et al. published in Sports Medicine :

Systematic Review Papers
A systematic review is a type of review article that tries to limit the occurrence of bias. Traditional, non-systematic reviews can be biased because they do not include all of the available papers on the review’s topic; only certain studies are discussed by the author. No formal process is used to decide which articles to include in the review. Consequently, unpublished articles, older papers, works in foreign languages, manuscripts published in small journals, and studies that conflict with the author’s beliefs can be overlooked or excluded. Since traditional reviews do not have to explain the techniques used to select the studies, it can be difficult to determine if the author’s bias affected the review’s findings.
Systematic reviews were developed to address the problem of bias. Unlike traditional reviews, which cover a broad topic, systematic reviews focus on a single question, such as if a particular intervention successfully treats a medical condition. Systematic reviews then track down all of the available studies that address the question, choose some to include in the review, and critique them using predetermined criteria. The studies are found, selected, and evaluated using a formal, scientific methodology in order to minimize the effect of the author’s bias. The methodology is clearly explained in the systematic review so that readers can form opinions about the quality of the review.
Let's take a closer look this systematic review paper by Vigano et al. published in Lancet Oncology :

Finding Review and Research Papers in PubMed
Many databases have special features that allow the searcher to restrict results to articles that match specific criteria. In other words, only articles of a certain type will be displayed in the search results. These “limiters” can be useful when searching for research or review articles. PubMed has a limiter for article type, which is located on the left sidebar of the search results page. This limiter can filter the search results to show only review articles.
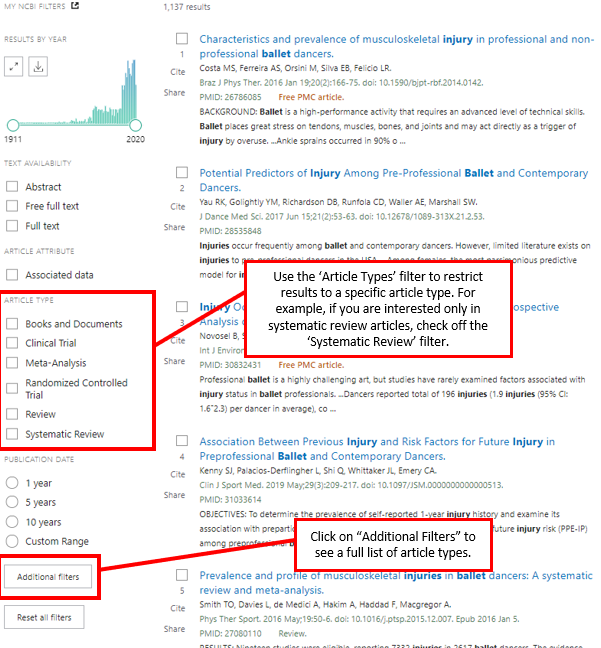
© Concordia University
Main Navigation Menu
Peer-review and primary research.
- Getting Started With Peer-Reviewed Literature
Primary Research
Identifying a primary research article.
- Finding Peer-Reviewed Journal Articles
- Finding Randomized Controlled Trials (RCTs)
- Evaluating Scholarly Articles
- Google Scholar
- Tips for Reading Journal Articles
STEM Librarian

Primary research or a primary study refers to a research article that is an author’s original research that is almost always published in a peer-reviewed journal. A primary study reports on the details, methods and results of a research study. These articles often have a standard structure of a format called IMRAD, referring to sections of an article: Introduction, Methods, Results and Discussion. Primary research studies will start with a review of the previous literature, however, the rest of the article will focus on the authors’ original research. Literature reviews can be published in peer-reviewed journals, however, they are not primary research.
Primary studies are part of primary sources but should not be mistaken for primary documents. Primary documents are usually original sources such as a letter, a diary, a speech or an autobiography. They are a first person view of an event or a period. Typically, if you are a Humanities major, you will be asked to find primary documents for your paper however, if you are in Social Sciences or the Sciences you are most likely going to be asked to find primary research studies. If you are unsure, ask your professor or a librarian for help.
A primary research or study is an empirical research that is published in peer-reviewed journals. Some ways of recognizing whether an article is a primary research article when searching a database:
1. The abstract includes a research question or a hypothesis, methods and results.

2. Studies can have tables and charts representing data findings.

3. The article includes a section for "methods” or “methodology” and "results".

4. Discussion section indicates findings and discusses limitations of the research study, and suggests further research.

5. Check the reference section because it will refer you to the studies and works that were consulted. You can use this section to find other studies on that particular topic.

The following are not to be confused with primary research articles:
- Literature reviews
- Meta-analyses or systematic reviews (these studies make conclusions based on research on many other studies)
- << Previous: Getting Started With Peer-Reviewed Literature
- Next: Finding Peer-Reviewed Journal Articles >>
- Last Updated: Feb 15, 2024 2:45 PM
- URL: https://guides.library.ucmo.edu/peerreview
We've launched our redesigned Learning Commons website. Our former site remains available until 12/16/2022.
BIO 110 - Primary Research Articles vs. Review Articles
- Getting Help
The Big Picture
The type of articles discussed in this guide ( primary research and review ) appear in publications called journals . Journals are similar to magazines, but are written by and for experts. Journals have been around for hundreds of years, and allow experts to share and increase knowledge for their particular fields. There are journals for trades, industries, sciences, humanities, and so on.
Just like magazines, an issue of a journal can contain a variety of content - for example, letters from readers, editorials and opinion pieces, brief articles on new developments, primary research articles, review articles, and so on.
The journals where you will find an article suitable for your BIO 110 assignment fall into a grouping called scholarly journals . Some other names for scholarly journals include academic journals or peer reviewed journals .
An important thing to understand: a journal can be scholarly without being peer reviewed. Peer review is a higher standard which only some scholarly journals meet.
Important features that make a journal scholarly :
Written by experts who usually hold advanced degrees in academic fields
Written for other experts
Frequently plain in appearance and without advertisements
Use language specific to the area of expertise
Sources of information are documented and verifiable by the reader
Important features that make a journal peer reviewed :
Meet all conditions to be considered scholarly
Before being published, articles are reviewed by other experts in the relevant field to ensure quality
The Difference Between Primary Research Articles and Review Articles
What makes a journal article "primary research" rather than a "review"? To answer this question, we need to spend a little more time looking at the big picture.
Information sources of all types (books, newspaper, magazines, journals, blogs, social media, tangible objects, and much much more), about just about anything, can be categorized as primary or secondary .
According to the Library of Congress , primary sources are "the raw materials of history" - the originals which people respond to. Some examples of primary sources - The Autobiography of Benjamin Franklin ; the football in use when the Eagles won Super Bowl LII; the last tweet made by Barack Obama as President of the United States; Vincent van Gogh's painting The Starry Night.
Secondary sources are responses to primary sources. For example, if you wrote a term paper about The Autobiography of Benjamin Franklin , your term paper could be considered a secondary source.
Primary research articles (also called original research or empirical ) are primary sources. Such articles describe newly done (though not necessarily unique) experiments executed with the objective of generating new evidence on a particular topic. All primary research articles taken together are often referred to by scientists as "the literature."
Review articles (also called literature reviews ) are secondary sources. Such articles provide an overview and discussion of primary research done on a particular topic, and can point out problems and suggest needed additional research. Review articles help experts keep up to date in their fields.
Imagine these examples of a primary research article and a review article - let's say you are a scientist who is an expert in your field. You have read extensively in your area of expertise, and the writings of others give you a new idea, which inspires you to perform an experiment to generate evidence in support of your idea. You decide to share your experiment with other experts by writing a paper, which is published in a peer reviewed journal. So, you have contributed primary research to the literature of science; or in more generic terms, you have created a new primary source.
Over time let's say your paper is widely recognized as a landmark in your field. Subsequently other experts write a review article to provide an overview of the important topic you helped to define; this article includes a discussion of your primary research. Since this review article mainly discusses original work created by others, such as your paper about your experiment, it is a secondary source.
- Next: Examples >>
- Last Updated: Sep 10, 2018 10:15 AM
- URL: https://learningcommons.dccc.edu/BIO110articles
"How Do I?" @JWULibrary

Sample Question
- JWU-Providence Library
Q. What's the difference between a research article and a review article?
- 35 about the library
- 29 articles & journals
- 1 Borrowing
- 9 citing sources
- 17 company & industry
- 11 computers
- 1 copyright compliance
- 5 countries & travel
- 2 course registration
- 10 culinary
- 51 databases
- 3 education
- 2 Interlibrary loan
- 5 job search
- 6 libguides
- 9 market research
- 25 my library account
- 12 requests
- 26 research basics
- 20 research topics
- 2 study rooms
- 16 technology
- 7 textbooks
- 42 university
- 3 video tutorial
- 1 writing_help
Answered By: Sarah Naomi Campbell Last Updated: Sep 07, 2018 Views: 214469
Watch this short video to learn about types of scholarly articles, including research articles and literature reviews!
Not in the mood for a video? Read on!
What's the difference between a research article and a review article?
Research articles , sometimes referred to as empirical or primary sources , report on original research. They will typically include sections such as an introduction, methods, results, and discussion.
Here is a more detailed explanation of research articles .
Review articles , sometimes called literature reviews or secondary sources , synthesize or analyze research already conducted in primary sources. They generally summarize the current state of research on a given topic.
Here is a more detailed explanation of review articles .
The video above was created by the Virginia Commonwealth University Libraries .
The defintions, and the linked detailed explanations, are paraphrased from the Publication Manual of the American Psychological Association , 6th ed .
The linked explanations are provided by the Mohawk Valley Community College Libraries .
Links & Files
- How do I find empirical articles in the library databases?
- Share on Facebook
Was this helpful? Yes 63 No 19
Comments (0)
Related topics.
- about the library
- articles & journals
- citing sources
- company & industry
- copyright compliance
- countries & travel
- course registration
- Interlibrary loan
- market research
- my library account
- research basics
- research topics
- study rooms
- video tutorial
- writing_help
Downcity Library:
111 Dorrance Street Providence, Rhode Island 02903
401-598-1121
Harborside Library:
321 Harborside Boulevard Providence, RI 02905
401-598-1466
- Location and Directions
- Off-Campus Access
- Staff Directory
- Student Employment
- Pay Bills and Fines
- Chat with a Librarian
- Course Reserves
- Interlibrary Loan (ILL)
- Study Rooms
- Research Appointment
- Culinary Museum
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Reumatologia
- v.59(1); 2021

Peer review guidance: a primer for researchers
Olena zimba.
1 Department of Internal Medicine No. 2, Danylo Halytsky Lviv National Medical University, Lviv, Ukraine
Armen Yuri Gasparyan
2 Departments of Rheumatology and Research and Development, Dudley Group NHS Foundation Trust (Teaching Trust of the University of Birmingham, UK), Russells Hall Hospital, Dudley, West Midlands, UK
The peer review process is essential for quality checks and validation of journal submissions. Although it has some limitations, including manipulations and biased and unfair evaluations, there is no other alternative to the system. Several peer review models are now practised, with public review being the most appropriate in view of the open science movement. Constructive reviewer comments are increasingly recognised as scholarly contributions which should meet certain ethics and reporting standards. The Publons platform, which is now part of the Web of Science Group (Clarivate Analytics), credits validated reviewer accomplishments and serves as an instrument for selecting and promoting the best reviewers. All authors with relevant profiles may act as reviewers. Adherence to research reporting standards and access to bibliographic databases are recommended to help reviewers draft evidence-based and detailed comments.
Introduction
The peer review process is essential for evaluating the quality of scholarly works, suggesting corrections, and learning from other authors’ mistakes. The principles of peer review are largely based on professionalism, eloquence, and collegiate attitude. As such, reviewing journal submissions is a privilege and responsibility for ‘elite’ research fellows who contribute to their professional societies and add value by voluntarily sharing their knowledge and experience.
Since the launch of the first academic periodicals back in 1665, the peer review has been mandatory for validating scientific facts, selecting influential works, and minimizing chances of publishing erroneous research reports [ 1 ]. Over the past centuries, peer review models have evolved from single-handed editorial evaluations to collegial discussions, with numerous strengths and inevitable limitations of each practised model [ 2 , 3 ]. With multiplication of periodicals and editorial management platforms, the reviewer pool has expanded and internationalized. Various sets of rules have been proposed to select skilled reviewers and employ globally acceptable tools and language styles [ 4 , 5 ].
In the era of digitization, the ethical dimension of the peer review has emerged, necessitating involvement of peers with full understanding of research and publication ethics to exclude unethical articles from the pool of evidence-based research and reviews [ 6 ]. In the time of the COVID-19 pandemic, some, if not most, journals face the unavailability of skilled reviewers, resulting in an unprecedented increase of articles without a history of peer review or those with surprisingly short evaluation timelines [ 7 ].
Editorial recommendations and the best reviewers
Guidance on peer review and selection of reviewers is currently available in the recommendations of global editorial associations which can be consulted by journal editors for updating their ethics statements and by research managers for crediting the evaluators. The International Committee on Medical Journal Editors (ICMJE) qualifies peer review as a continuation of the scientific process that should involve experts who are able to timely respond to reviewer invitations, submitting unbiased and constructive comments, and keeping confidentiality [ 8 ].
The reviewer roles and responsibilities are listed in the updated recommendations of the Council of Science Editors (CSE) [ 9 ] where ethical conduct is viewed as a premise of the quality evaluations. The Committee on Publication Ethics (COPE) further emphasizes editorial strategies that ensure transparent and unbiased reviewer evaluations by trained professionals [ 10 ]. Finally, the World Association of Medical Editors (WAME) prioritizes selecting the best reviewers with validated profiles to avoid substandard or fraudulent reviewer comments [ 11 ]. Accordingly, the Sarajevo Declaration on Integrity and Visibility of Scholarly Publications encourages reviewers to register with the Open Researcher and Contributor ID (ORCID) platform to validate and publicize their scholarly activities [ 12 ].
Although the best reviewer criteria are not listed in the editorial recommendations, it is apparent that the manuscript evaluators should be active researchers with extensive experience in the subject matter and an impressive list of relevant and recent publications [ 13 ]. All authors embarking on an academic career and publishing articles with active contact details can be involved in the evaluation of others’ scholarly works [ 14 ]. Ideally, the reviewers should be peers of the manuscript authors with equal scholarly ranks and credentials.
However, journal editors may employ schemes that engage junior research fellows as co-reviewers along with their mentors and senior fellows [ 15 ]. Such a scheme is successfully practised within the framework of the Emerging EULAR (European League Against Rheumatism) Network (EMEUNET) where seasoned authors (mentors) train ongoing researchers (mentees) how to evaluate submissions to the top rheumatology journals and select the best evaluators for regular contributors to these journals [ 16 ].
The awareness of the EQUATOR Network reporting standards may help the reviewers to evaluate methodology and suggest related revisions. Statistical skills help the reviewers to detect basic mistakes and suggest additional analyses. For example, scanning data presentation and revealing mistakes in the presentation of means and standard deviations often prompt re-analyses of distributions and replacement of parametric tests with non-parametric ones [ 17 , 18 ].
Constructive reviewer comments
The main goal of the peer review is to support authors in their attempt to publish ethically sound and professionally validated works that may attract readers’ attention and positively influence healthcare research and practice. As such, an optimal reviewer comment has to comprehensively examine all parts of the research and review work ( Table I ). The best reviewers are viewed as contributors who guide authors on how to correct mistakes, discuss study limitations, and highlight its strengths [ 19 ].
Structure of a reviewer comment to be forwarded to authors
Some of the currently practised review models are well positioned to help authors reveal and correct their mistakes at pre- or post-publication stages ( Table II ). The global move toward open science is particularly instrumental for increasing the quality and transparency of reviewer contributions.
Advantages and disadvantages of common manuscript evaluation models
Since there are no universally acceptable criteria for selecting reviewers and structuring their comments, instructions of all peer-reviewed journal should specify priorities, models, and expected review outcomes [ 20 ]. Monitoring and reporting average peer review timelines is also required to encourage timely evaluations and avoid delays. Depending on journal policies and article types, the first round of peer review may last from a few days to a few weeks. The fast-track review (up to 3 days) is practised by some top journals which process clinical trial reports and other priority items.
In exceptional cases, reviewer contributions may result in substantive changes, appreciated by authors in the official acknowledgments. In most cases, however, reviewers should avoid engaging in the authors’ research and writing. They should refrain from instructing the authors on additional tests and data collection as these may delay publication of original submissions with conclusive results.
Established publishers often employ advanced editorial management systems that support reviewers by providing instantaneous access to the review instructions, online structured forms, and some bibliographic databases. Such support enables drafting of evidence-based comments that examine the novelty, ethical soundness, and implications of the reviewed manuscripts [ 21 ].
Encouraging reviewers to submit their recommendations on manuscript acceptance/rejection and related editorial tasks is now a common practice. Skilled reviewers may prompt the editors to reject or transfer manuscripts which fall outside the journal scope, perform additional ethics checks, and minimize chances of publishing erroneous and unethical articles. They may also raise concerns over the editorial strategies in their comments to the editors.
Since reviewer and editor roles are distinct, reviewer recommendations are aimed at helping editors, but not at replacing their decision-making functions. The final decisions rest with handling editors. Handling editors weigh not only reviewer comments, but also priorities related to article types and geographic origins, space limitations in certain periods, and envisaged influence in terms of social media attention and citations. This is why rejections of even flawless manuscripts are likely at early rounds of internal and external evaluations across most peer-reviewed journals.
Reviewers are often requested to comment on language correctness and overall readability of the evaluated manuscripts. Given the wide availability of in-house and external editing services, reviewer comments on language mistakes and typos are categorized as minor. At the same time, non-Anglophone experts’ poor language skills often exclude them from contributing to the peer review in most influential journals [ 22 ]. Comments should be properly edited to convey messages in positive or neutral tones, express ideas of varying degrees of certainty, and present logical order of words, sentences, and paragraphs [ 23 , 24 ]. Consulting linguists on communication culture, passing advanced language courses, and honing commenting skills may increase the overall quality and appeal of the reviewer accomplishments [ 5 , 25 ].
Peer reviewer credits
Various crediting mechanisms have been proposed to motivate reviewers and maintain the integrity of science communication [ 26 ]. Annual reviewer acknowledgments are widely practised for naming manuscript evaluators and appreciating their scholarly contributions. Given the need to weigh reviewer contributions, some journal editors distinguish ‘elite’ reviewers with numerous evaluations and award those with timely and outstanding accomplishments [ 27 ]. Such targeted recognition ensures ethical soundness of the peer review and facilitates promotion of the best candidates for grant funding and academic job appointments [ 28 ].
Also, large publishers and learned societies issue certificates of excellence in reviewing which may include Continuing Professional Development (CPD) points [ 29 ]. Finally, an entirely new crediting mechanism is proposed to award bonus points to active reviewers who may collect, transfer, and use these points to discount gold open-access charges within the publisher consortia [ 30 ].
With the launch of Publons ( http://publons.com/ ) and its integration with Web of Science Group (Clarivate Analytics), reviewer recognition has become a matter of scientific prestige. Reviewers can now freely open their Publons accounts and record their contributions to online journals with Digital Object Identifiers (DOI). Journal editors, in turn, may generate official reviewer acknowledgments and encourage reviewers to forward them to Publons for building up individual reviewer and journal profiles. All published articles maintain e-links to their review records and post-publication promotion on social media, allowing the reviewers to continuously track expert evaluations and comments. A paid-up partnership is also available to journals and publishers for automatically transferring peer-review records to Publons upon mutually acceptable arrangements.
Listing reviewer accomplishments on an individual Publons profile showcases scholarly contributions of the account holder. The reviewer accomplishments placed next to the account holders’ own articles and editorial accomplishments point to the diversity of scholarly contributions. Researchers may establish links between their Publons and ORCID accounts to further benefit from complementary services of both platforms. Publons Academy ( https://publons.com/community/academy/ ) additionally offers an online training course to novice researchers who may improve their reviewing skills under the guidance of experienced mentors and journal editors. Finally, journal editors may conduct searches through the Publons platform to select the best reviewers across academic disciplines.
Peer review ethics
Prior to accepting reviewer invitations, scholars need to weigh a number of factors which may compromise their evaluations. First of all, they are required to accept the reviewer invitations if they are capable of timely submitting their comments. Peer review timelines depend on article type and vary widely across journals. The rules of transparent publishing necessitate recording manuscript submission and acceptance dates in article footnotes to inform readers of the evaluation speed and to help investigators in the event of multiple unethical submissions. Timely reviewer accomplishments often enable fast publication of valuable works with positive implications for healthcare. Unjustifiably long peer review, on the contrary, delays dissemination of influential reports and results in ethical misconduct, such as plagiarism of a manuscript under evaluation [ 31 ].
In the times of proliferation of open-access journals relying on article processing charges, unjustifiably short review may point to the absence of quality evaluation and apparently ‘predatory’ publishing practice [ 32 , 33 ]. Authors when choosing their target journals should take into account the peer review strategy and associated timelines to avoid substandard periodicals.
Reviewer primary interests (unbiased evaluation of manuscripts) may come into conflict with secondary interests (promotion of their own scholarly works), necessitating disclosures by filling in related parts in the online reviewer window or uploading the ICMJE conflict of interest forms. Biomedical reviewers, who are directly or indirectly supported by the pharmaceutical industry, may encounter conflicts while evaluating drug research. Such instances require explicit disclosures of conflicts and/or rejections of reviewer invitations.
Journal editors are obliged to employ mechanisms for disclosing reviewer financial and non-financial conflicts of interest to avoid processing of biased comments [ 34 ]. They should also cautiously process negative comments that oppose dissenting, but still valid, scientific ideas [ 35 ]. Reviewer conflicts that stem from academic activities in a competitive environment may introduce biases, resulting in unfair rejections of manuscripts with opposing concepts, results, and interpretations. The same academic conflicts may lead to coercive reviewer self-citations, forcing authors to incorporate suggested reviewer references or face negative feedback and an unjustified rejection [ 36 ]. Notably, several publisher investigations have demonstrated a global scale of such misconduct, involving some highly cited researchers and top scientific journals [ 37 ].
Fake peer review, an extreme example of conflict of interest, is another form of misconduct that has surfaced in the time of mass proliferation of gold open-access journals and publication of articles without quality checks [ 38 ]. Fake reviews are generated by manipulating authors and commercial editing agencies with full access to their own manuscripts and peer review evaluations in the journal editorial management systems. The sole aim of these reviews is to break the manuscript evaluation process and to pave the way for publication of pseudoscientific articles. Authors of these articles are often supported by funds intended for the growth of science in non-Anglophone countries [ 39 ]. Iranian and Chinese authors are often caught submitting fake reviews, resulting in mass retractions by large publishers [ 38 ]. Several suggestions have been made to overcome this issue, with assigning independent reviewers and requesting their ORCID IDs viewed as the most practical options [ 40 ].
Conclusions
The peer review process is regulated by publishers and editors, enforcing updated global editorial recommendations. Selecting the best reviewers and providing authors with constructive comments may improve the quality of published articles. Reviewers are selected in view of their professional backgrounds and skills in research reporting, statistics, ethics, and language. Quality reviewer comments attract superior submissions and add to the journal’s scientific prestige [ 41 ].
In the era of digitization and open science, various online tools and platforms are available to upgrade the peer review and credit experts for their scholarly contributions. With its links to the ORCID platform and social media channels, Publons now offers the optimal model for crediting and keeping track of the best and most active reviewers. Publons Academy additionally offers online training for novice researchers who may benefit from the experience of their mentoring editors. Overall, reviewer training in how to evaluate journal submissions and avoid related misconduct is an important process, which some indexed journals are experimenting with [ 42 ].
The timelines and rigour of the peer review may change during the current pandemic. However, journal editors should mobilize their resources to avoid publication of unchecked and misleading reports. Additional efforts are required to monitor published contents and encourage readers to post their comments on publishers’ online platforms (blogs) and other social media channels [ 43 , 44 ].
The authors declare no conflict of interest.

- Reference Books and eBooks
- Research Databases
- Primary and Review Articles
- Open Access Web Resources
- Citations This link opens in a new window
- Careers and Professional Organizations
Primary Articles
- In a Primary Science Article, authors report on the results of their own experiment or investigation
- Articles will often include a methods and results section which describes their specific study
- Title, authors, and author affiliation
- Introduction
- Materials and Methods
- Results (with figures)
Review Articles
Review articles serve a different purpose than primary articles. They are written to summarize and synthesize studies by others on the same topic. This provides the research community with one article that summarizes the background and context as well as an overview of what is already known about a specific topic.
There are four types of review articles:
- Identify gaps or weaknesses in scholarly literature
- Systematic review articles include a specific time frame and research scope that can be very narrow
- evaluate and synthesize findings
- Form of systematic review
- Uses statistical analysis of the findings from several studies on the same subject to identify common themes in the research
- Authors use non-statistical methods to analyze quantitative findings
Anatomy of a Review Article:
Review articles look like research papers. They contain:
When in doubt, read the methods section to see if the authors are reporting on their personal research. If they are, it is a primary article.
Peer Reviewed or Scholarly Articles
Academic research articles are also called peer-reviewed or scholarly articles. To be considered a peer-reviewed article, it must be read and approved by other scholars in the same field.
A scholar writes an article and submits it to a scholarly journal. Before the article can be published it is sent out by the journal to a group of scholars in the same field. Those scholars read the article and either approve it or request changes before publication. Only after it has been approved can it be published by the journal.
This process helps ensure that information published in scholarly journals is accurate and reliable.
- << Previous: Books
- Next: Open Access Web Resources >>
- Last Updated: Jan 16, 2024 4:09 PM
- URL: https://guides.beloit.edu/biology
- Student Services
- Faculty Services
Peer Review and Primary Literature: An Introduction: Is it Primary Research? How Do I Know?
- Scholarly Journal vs. Magazine
- Peer Review: What is it?
- Finding Peer-Reviewed Articles
- Primary Journal Literature
- Is it Primary Research? How Do I Know?
Components of a Primary Research Study
As indicated on a previous page, Peer-Reviewed Journals also include non -primary content. Simply limiting your search results in a database to "peer-reviewed" will not retrieve a list of only primary research studies.
Learn to recognize the parts of a primary research study. Terminology will vary slightly from discipline to discipline and from journal to journal. However, there are common components to most research studies.
When you run a search, find a promising article in your results list and then look at the record for that item (usually by clicking on the title). The full database record for an item usually includes an abstract or summary--sometimes prepared by the journal or database, but often written by the author(s) themselves. This will usually give a clear indication of whether the article is a primary study. For example, here is a full database record from a search for family violence and support in SocINDEX with Full Text :
Although the abstract often tells the story, you will need to read the article to know for sure. Besides scanning the Abstract or Summary, look for the following components: (I am only capturing small article segments for illustration.)
Look for the words METHOD or METHODOLOGY . The authors should explain how they conducted their research.
NOTE: Different Journals and Disciplines will use different terms to mean similar things. If instead of " Method " or " Methodology " you see a heading that says " Research Design " or " Data Collection ," you have a similar indicator that the scholar-authors have done original research.
Look for the section called RESULTS . This details what the author(s) found out after conducting their research.
Charts , Tables , Graphs , Maps and other displays help to summarize and present the findings of the research.
A Discussion indicates the significance of findings, acknowledges limitations of the research study, and suggests further research.
References , a Bibliography or List of Works Cited indicates a literature review and shows other studies and works that were consulted. USE THIS PART OF THE STUDY! If you find one or two good recent studies, you can identify some important earlier studies simply by going through the bibliographies of those articles.
A FINAL NOTE: If you are ever unclear about whether a particular article is appropriate to use in your paper, it is best to show that article to your professor and discuss it with them. The professor is the final judge since they will be assigning your grade.
Subject Guide

- << Previous: Primary Journal Literature
- Last Updated: Nov 16, 2022 12:46 PM
- URL: https://suffolk.libguides.com/PeerandPrimary
BIO 218: Cellular and Molecular Biology
- Purpose of this Guide
- Find Articles
- Get Ready to Search
What is a Primary Research Article?
How do you identify primary research articles, review articles.
- Reminder: Scholarly vs. Popular Articles
- Reminder: What is a Peer-Reviewed Article?
- Cite Your Sources
Librarian for the Sciences

In a primary research article, author(s) present a new set of findings from original research after conducting an original experiment. Think of what you do in any of your various lab activities. If you were to write a scholarly paper on any of your biology labs (like the Flowers and Pollinators lab from BIO 191), it would be a primary research article.
Primary research articles are also referred to as original research or research articles.
How to Identify Primary Research Article
- Did the author(s) of the paper conduct the experiment themselves? This is the most important thing to look for in order to identify primary research. Look for language that indicates that the author(s) devised the experiment, carried it out, and analyzed the resulting data themselves.
- "Methods"/"Materials and Methods"/"Experimental Methods"(different journals title this section in different ways)
- "Results"
- "Discussion"
Here is One Example of a Primary Research Article and How to Determine that it is a Primary Research Article
"Effects of Salinity Stress on Survival, Metabolism, Limb Regeneration, and Ecdysis in UCA PUGNAX"
Read the Abstract
If you read the abstract, you can see that the author(s) themselves conducted an experiment:
- "This study investigated physiological and metabolic changes in the molt cycle of U. pugnax..."
- "For this study, a limb was removed and its regenerative growth was photographed every two days"
- "...crabs were dissected, and the tissues collected were analyzed for their protein and carbohydrate contents."
Read the Headings
- The article has Materials and Methods, Results, and Discussion sections, all which indicate that the authors conducted an experiment and then analyzed the data they found.
Skim the Article
If you skim the article, it is clear that the authors tested a hypothesis using the scientific method. They are only really talking about research that was conducted by others in the "Introduction" section of the article, which is what you would expect for a primary research article.
Look for Textual Evidence
If you skim the article, you can easily find additional evidence that an experiment was conducted by the authors themselves.
- [They collected their sample i.e. crabs.]
- [They exposed their sample to different variables.]
- [They used statistical methods to analyze their data.]
- [They reported the results of their experiment.]
- [They drew a conclusion from their experimental results.]
What is a Review Article?
Review articles do not report new experiments. Rather, they attempt to provide a thorough review of a specific subject by assessing either all or the best available scholarly literature on that topic. By considering the findings of many primary research articles, review articles can provide a comprehensive background and analysis of all the available evidence on a subject. A single review article will summarize, analyze, and discuss the results of numerous primary research articles all at once, and often will provide comparisons with respect to each other.
For a review article, the authors do not design an experiment and carry it out in a lab. Rather, they search for, find, and read numerous primary research articles on a particular topic. Then, they organize them into a cohesive narrative that provides an overall summary and analysis of a topic.
Review articles can help explain the basics of a particular area of science, provide an overview of all of the research that has been conducted on a particular topic, and/or provide insight into current topics of scholarly disagreement. They can also help identify where there are gaps in research and scientific knowledge.
How to Identify a Review Article
- Author(s) summarize and analyze previously published research. NOTE: While primary research articles provide a background on their subject by summarizing previously conducted research, this typically occurs only in the "Introduction" section of the article. Review articles, however, will summarize previously conducted research throughout the entire paper.
- Author(s) did NOT do original research. Instead, they summarize and discuss the work of others.
- The article might attempt to (1) explain the basics of, (2) provide an overview of, or (3) shed light on aspects of disagreement or confusion regarding a topic.
- In order to provide insight into aspects of disagreement or confusion surrounding a research topic, the article might focus on a specific research question that has been investigated many times by other researchers. Here, they compare and contrast primary research articles in an attempt to answer a complicated question.
- Do not typically contain sections such as Methods (and Materials) or Results because the author(s) did not do any original research!
Practice: An Example of a Review Article
" Stem Cells, Cancer, and Cancer Stem Cells "
Step 1: Read the Abstract
If you read the abstract, there is nothing to indicate that the authors of this paper conducted an experiment themselves. The authors do not list their hypothesis, methods used to conduct an experiment, or specific results. Rather, it sounds like the authors are trying to provide the reader with an overview of recent research.
Step 2: Read the Headings
If you look through the headings of the different sections of this article (the words that are bolded), they do not indicate that an experiment was conducted by the authors. This article does not have those specific section headings commonly found in primary research, such as "Materials and Methods," "Results," or "Discussion."
Step 3: Skim the Article
If you skim through the article, there is nothing to indicate that the authors tested a hypothesis in a lab or in the field. Instead, the article weaves together the findings from many primary research articles and then considers what the results could mean when viewed collectively.
Step 4: Look for Textual Evidence
If you skim through the article, there is more evidence that it is a review:
- [The article actually tells you that it is a review!]
- [The article is summarizing the findings from several studies. REMINDER: While primary research articles provide a background on their subject by summarizing previously conducted research, this typically occurs only in the Introduction section of the article. Review articles, however, will summarize previously conducted research throughout the entire paper. This is demonstrated throughout the entire article, not just in an Introduction section, which helps identify it as a review.]
- << Previous: Get Ready to Search
- Next: Reminder: Scholarly vs. Popular Articles >>
- Last Updated: Jan 18, 2024 2:01 PM
- URL: https://resources.library.lemoyne.edu/courses/bio218
- Privacy Policy

Home » Review Article vs Research Article
Review Article vs Research Article
Table of Contents

Review articles and Research Articles are two different types of scholarly publications that serve distinct purposes in the academic literature.
Research Articles
A Research Article is a primary source that presents original research findings based on a specific research question or hypothesis. These articles typically follow a standard format that includes an introduction, literature review, methodology, results, discussion, and conclusion sections. Research articles often include detailed descriptions of the research design, data collection and analysis procedures, and the results of statistical tests. These articles are typically peer-reviewed to ensure that they meet rigorous scientific standards before publication.
Review Articles
A Review Article is a secondary source that summarizes and analyzes existing research on a particular topic or research question. These articles provide an overview of the current state of knowledge on a particular topic, including a critical analysis of the strengths and limitations of previous research. Review articles often include a meta-analysis of the existing literature, which involves combining and analyzing data from multiple studies to draw more general conclusions about the research question or topic. Review articles are also typically peer-reviewed to ensure that they are comprehensive, accurate, and up-to-date.
Difference Between Review Article and Research Article
Here are some key differences between review articles and research articles:
In summary, research articles and review articles serve different purposes in the academic literature. Research articles present original research findings based on a specific research question or hypothesis, while review articles summarize and analyze existing research on a particular topic or research question. Both types of articles are typically peer-reviewed to ensure that they meet high standards of scientific rigor and accuracy.
Also see Research Methods
About the author
Muhammad Hassan
Researcher, Academic Writer, Web developer
You may also like

Inductive Vs Deductive Research

Exploratory Vs Explanatory Research

Basic Vs Applied Research

Generative Vs Evaluative Research

Reliability Vs Validity

Longitudinal Vs Cross-Sectional Research
Identifying Primary and Secondary Research Articles
- Primary and Secondary

Primary Research Articles
Primary research articles report on a single study. In the health sciences, primary research articles generally describe the following aspects of the study:
- The study's hypothesis or research question
- Some articles will include information on how participants were recruited or identified, as well as additional information about participants' sex, age, or race/ethnicity
- A "methods" or "methodology" section that describes how the study was performed and what the researchers did
- Results and conclusion section
Secondary Research Articles
Review articles are the most common type of secondary research article in the health sciences. A review article is a summary of previously published research on a topic. Authors who are writing a review article will search databases for previously completed research and summarize or synthesize those articles, as opposed to recruiting participants and performing a new research study.
Specific types of review articles include:
- Systematic Reviews
- Meta-Analysis
- Narrative Reviews
- Integrative Reviews
- Literature Reviews
Review articles often report on the following:
- The hypothesis, research question, or review topic
- Databases searched-- authors should clearly describe where and how they searched for the research included in their reviews
- Systematic Reviews and Meta-Analysis should provide detailed information on the databases searched and the search strategy the authors used.Selection criteria-- the researchers should describe how they decided which articles to include
- A critical appraisal or evaluation of the quality of the articles included (most frequently included in systematic reviews and meta-analysis)
- Discussion, results, and conclusions
Determining Primary versus Secondary Using the Database Abstract
Information found in PubMed, CINAHL, Scopus, and other databases can help you determine whether the article you're looking at is primary or secondary.
Primary research article abstract
- Note that in the "Objectives" field, the authors describe their single, individual study.
- In the materials and methods section, they describe the number of patients included in the study and how those patients were divided into groups.
- These are all clues that help us determine this abstract is describing is a single, primary research article, as opposed to a literature review.
- Primary Article Abstract

Secondary research/review article abstract
- Note that the words "systematic review" and "meta-analysis" appear in the title of the article
- The objectives field also includes the term "meta-analysis" (a common type of literature review in the health sciences)
- The "Data Source" section includes a list of databases searched
- The "Study Selection" section describes the selection criteria
- These are all clues that help us determine that this abstract is describing a review article, as opposed to a single, primary research article.
- Secondary Research Article

- Primary vs. Secondary Worksheet
Full Text Challenge
Can you determine if the following articles are primary or secondary?
- Last Updated: Feb 17, 2024 5:25 PM
- URL: https://library.usfca.edu/primary-secondary
2130 Fulton Street San Francisco, CA 94117-1080 415-422-5555
- Facebook (link is external)
- Instagram (link is external)
- Twitter (link is external)
- YouTube (link is external)
- Consumer Information
- Privacy Statement
- Web Accessibility
Copyright © 2022 University of San Francisco

Animal Science
How to identify peer reviewed journals, how to identify primary research articles.
- Reference Sources
- Key Journals
- Writing & Citing
- Self Checkout
- Anatomy Study Resources
- Peer Reviewed Journals Quiz How do I know if a journal is peer reviewed? What is peer review, anyway? Take this short quiz to test your knowledge and perhaps learn something new!
- Primary Research Articles Quiz How do I know if an article is a primary or secondary research article? Are there search techniques that will help me find them? Take this short quiz to test your knowledge and perhaps learn something new!
You must get all answers correct to submit the quiz!
Peer review is defined as “a process of subjecting an author’s scholarly work, research or ideas to the scrutiny of others who are experts in the same field” ( 1 ). Peer review is intended to serve two purposes:
- It acts as a filter to ensure that only high quality research is published, especially in reputable journals, by determining the validity, significance and originality of the study.
- Peer review is intended to improve the quality of manuscripts that are deemed suitable for publication. Peer reviewers provide suggestions to authors on how to improve the quality of their manuscripts, and also identify any errors that need correcting before publication.
How do you determine whether an article qualifies as being a peer-reviewed journal article?
- If you're searching for articles in certain databases, you can limit your search to peer-reviewed sources simply by selecting a tab or checking a box on the search screen.
- If you have an article, an indication that it has been through the peer review process will be the publication history , usually at the beginning or end of the article.
- If you're looking at the journal itself, go to the editorial statement or instructions to authors (usually in the first few pages of the journal or at the end) for references to the peer-review process.
- Lookup the journal by title or ISSN in the ProQuest Source Evaluation Aid .
- Careful! Not all information in a peer-reviewed journal is actually reviewed. Editorials, letters to the editor, book reviews, and other types of information don't count as articles, and may not be accepted by your professor.
What about preprint sites and ResearchGate?
- A preprint is a piece of research that has not yet been peer reviewed and published in a journal. In most cases, they can be considered final drafts or working papers. Preprint sites are great sources of current research - and most preprint sites will provide a link to a later, peer-reviewed version of an article.
- ResearchGate is a commercial social networking site for scientists and researchers to share papers, ask and answer questions, and find collaborators. Members can upload research output including papers, chapters, negative results, patents, research proposals, methods, presentations, etc. Researchers can access these materials, and also contact members to ask for access to material that has not been shared, usually because of copyright restrictions. There is a filter to limit results to articles, but it can be difficult to determine the publication history of ResearchGate items and whether they have been published in peer reviewed sources.
A primary research article reports on an empirical research study conducted by the authors. The goal of a primary research article is to present the result of original research that makes a new contribution to the body of knowledge.
Characteristics:
- Almost always published in a peer-reviewed journal
- Asks a research question or states a hypothesis or hypotheses
- Identifies a research population
- Describes a specific research method
- Tests or measures something
- Often (but not always) structured in a standard format called IMRAD: Introduction, Methods, Results, and Discussion
- Words to look for as clues include: analysis, study, investigation, examination, experiment, numbers of people or objects analyzed, content analysis, or surveys.
To contrast, the following are not primary research articles (i.e., they are secondary sources):
- Literature reviews/Review articles
- Meta-Analyses (studies that arrive at conclusions based on research from many other studies)
- Editorials & Letters
- Dissertations
Articles that are NOT primary research articles may discuss the same research, but they are not reporting on original research, they are summarizing and commenting on research conducted and published by someone else. For example, a literature review provides commentary and analysis of research done by other people, but it does not report the results of the author's own study and is not primary research.
- << Previous: Home
- Next: Reference Sources >>
- Last Updated: Aug 24, 2023 2:38 PM
- URL: https://libguides.berry.edu/ans
Tutorial: Evaluating Information: Primary vs. Secondary Articles
- Evaluating Information
- Scholarly Literature Types
- Primary vs. Secondary Articles
- Peer Review
- Systematic Reviews & Meta-Analysis
- Gray Literature
- Evaluating Like a Boss
- Evaluating AV

Primary vs. Secondary Research Articles
In the sciences, primary (or empirical) research articles :
- are original scientific reports of new research findings (Please note that an original scientific article does not include review articles, which summarize the research literature on a particular subject, or articles using meta-analyses, which analyze pre-published data.)
- usually include the following sections: Introduction , Methods , Results , Discussion, References
- are usually peer-reviewed (examined by expert(s) in the field before publication). Please note that a peer-reviewed article is not the same as a review article, which summarizes the research literature on a particular subject
You may also choose to use some secondary sources (summaries or interpretations of original research) such as books (find these through the library catalog) or review articles (articles which organize and critically analyze the research of others on a topic). These secondary sources, particularly review articles, are often useful and easier-to-read summaries of research in an area. Additionally, you can use the listed references to find useful primary research articles.
Anatomy of a Scholarly Article

from NCSU Libraries' Anatomy of a Scholarly Article
Types of health studies
In the sciences, particularly the health sciences, there are a number of types of primary articles (the gold standard being randomized controlled trials ) and secondary articles (the gold standard being systematic reviews and meta-analysis ). The chart below summarizes their differences and the linked article gives more information.

Searching for Primary vs. Secondary Articles

Some scholarly databases will allow you to specific what kind of scholarly literature you're looking for. However, be careful! Sometimes, depending on the database, the Review article type may mean book review instead of or as well as review article. You may also have to look under more or custom options to find these choices.
- << Previous: Scholarly Literature Types
- Next: Peer Review >>
- Last Updated: Oct 20, 2021 11:11 AM
- URL: https://guides.library.cornell.edu/evaluate

Peer-Reviewed Literature: Peer-Reviewed Research: Primary vs. Secondary
- Peer-Reviewed Research: Primary vs. Secondary
- Types of Peer Review
- Identifying Peer-Reviewed Research
Peer Reviewed Research
Published literature can be either peer-reviewed or non-peer-reviewed. Official research reports are almost always peer reviewed while a journal's other content is usually not. In the health sciences, official research can be primary, secondary, or even tertiary. It can be an original experiment or investigation (primary), an analysis or evaluation of primary research (secondary), or findings that compile secondary research (tertiary). If you are doing research yourself, then primary or secondary sources can reveal more in-depth information.
Primary Research
Primary research is information presented in its original form without interpretation by other researchers. While it may acknowledge previous studies or sources, it always presents original thinking, reports on discoveries, or new information about a topic.
Health sciences research that is primary includes both experimental trials and observational studies where subjects may be tested for outcomes or investigated to gain relevant insight. Randomized Controlled Trials are the most prominent experimental design because randomized subjects offer the most compelling evidence for the effectiveness of an intervention. See the below graphic and below powerpoint for further information on primary research studies.

- Research Design
Secondary Research
Secondary research is an account of original events or facts. It is secondary to and retrospective of the actual findings from an experiment or trial. These studies may be appraised summaries, reviews, or interpretations of primary sources and often exclude the original researcher(s). In the health sciences, meta-analysis and systematic reviews are the most frequent types of secondary research.
- A meta-analysis is a quantitative method of combining the results of primary research. In analyzing the relevant data and statistical findings from experimental trials or observational studies, it can more accurately calculate effective resolutions regarding certain health topics.
- A systematic review is a summary of research that addresses a focused clinical question in a systematic, reproducible manner. In order to provide the single best estimate of effect in clinical decision making, primary research studies are pooled together and then filtered through an inclusion/exclusion process. The relevant data and findings are then compiled and synthesized to arrive at a more accurate conclusion about a specific health topic. Only peer-reviewed publications are used and analyzed in a methodology which may or may not include a meta-analysis.

- << Previous: Home
- Next: Types of Peer Review >>
- Last Updated: Sep 29, 2023 10:05 AM
- URL: https://ttuhsc.libguides.com/PeerReview


Cultural Relativity and Acceptance of Embryonic Stem Cell Research
Article sidebar.
Main Article Content
There is a debate about the ethical implications of using human embryos in stem cell research, which can be influenced by cultural, moral, and social values. This paper argues for an adaptable framework to accommodate diverse cultural and religious perspectives. By using an adaptive ethics model, research protections can reflect various populations and foster growth in stem cell research possibilities.
INTRODUCTION
Stem cell research combines biology, medicine, and technology, promising to alter health care and the understanding of human development. Yet, ethical contention exists because of individuals’ perceptions of using human embryos based on their various cultural, moral, and social values. While these disagreements concerning policy, use, and general acceptance have prompted the development of an international ethics policy, such a uniform approach can overlook the nuanced ethical landscapes between cultures. With diverse viewpoints in public health, a single global policy, especially one reflecting Western ethics or the ethics prevalent in high-income countries, is impractical. This paper argues for a culturally sensitive, adaptable framework for the use of embryonic stem cells. Stem cell policy should accommodate varying ethical viewpoints and promote an effective global dialogue. With an extension of an ethics model that can adapt to various cultures, we recommend localized guidelines that reflect the moral views of the people those guidelines serve.
Stem cells, characterized by their unique ability to differentiate into various cell types, enable the repair or replacement of damaged tissues. Two primary types of stem cells are somatic stem cells (adult stem cells) and embryonic stem cells. Adult stem cells exist in developed tissues and maintain the body’s repair processes. [1] Embryonic stem cells (ESC) are remarkably pluripotent or versatile, making them valuable in research. [2] However, the use of ESCs has sparked ethics debates. Considering the potential of embryonic stem cells, research guidelines are essential. The International Society for Stem Cell Research (ISSCR) provides international stem cell research guidelines. They call for “public conversations touching on the scientific significance as well as the societal and ethical issues raised by ESC research.” [3] The ISSCR also publishes updates about culturing human embryos 14 days post fertilization, suggesting local policies and regulations should continue to evolve as ESC research develops. [4] Like the ISSCR, which calls for local law and policy to adapt to developing stem cell research given cultural acceptance, this paper highlights the importance of local social factors such as religion and culture.
I. Global Cultural Perspective of Embryonic Stem Cells
Views on ESCs vary throughout the world. Some countries readily embrace stem cell research and therapies, while others have stricter regulations due to ethical concerns surrounding embryonic stem cells and when an embryo becomes entitled to moral consideration. The philosophical issue of when the “someone” begins to be a human after fertilization, in the morally relevant sense, [5] impacts when an embryo becomes not just worthy of protection but morally entitled to it. The process of creating embryonic stem cell lines involves the destruction of the embryos for research. [6] Consequently, global engagement in ESC research depends on social-cultural acceptability.
a. US and Rights-Based Cultures
In the United States, attitudes toward stem cell therapies are diverse. The ethics and social approaches, which value individualism, [7] trigger debates regarding the destruction of human embryos, creating a complex regulatory environment. For example, the 1996 Dickey-Wicker Amendment prohibited federal funding for the creation of embryos for research and the destruction of embryos for “more than allowed for research on fetuses in utero.” [8] Following suit, in 2001, the Bush Administration heavily restricted stem cell lines for research. However, the Stem Cell Research Enhancement Act of 2005 was proposed to help develop ESC research but was ultimately vetoed. [9] Under the Obama administration, in 2009, an executive order lifted restrictions allowing for more development in this field. [10] The flux of research capacity and funding parallels the different cultural perceptions of human dignity of the embryo and how it is socially presented within the country’s research culture. [11]
b. Ubuntu and Collective Cultures
African bioethics differs from Western individualism because of the different traditions and values. African traditions, as described by individuals from South Africa and supported by some studies in other African countries, including Ghana and Kenya, follow the African moral philosophies of Ubuntu or Botho and Ukama , which “advocates for a form of wholeness that comes through one’s relationship and connectedness with other people in the society,” [12] making autonomy a socially collective concept. In this context, for the community to act autonomously, individuals would come together to decide what is best for the collective. Thus, stem cell research would require examining the value of the research to society as a whole and the use of the embryos as a collective societal resource. If society views the source as part of the collective whole, and opposes using stem cells, compromising the cultural values to pursue research may cause social detachment and stunt research growth. [13] Based on local culture and moral philosophy, the permissibility of stem cell research depends on how embryo, stem cell, and cell line therapies relate to the community as a whole. Ubuntu is the expression of humanness, with the person’s identity drawn from the “’I am because we are’” value. [14] The decision in a collectivistic culture becomes one born of cultural context, and individual decisions give deference to others in the society.
Consent differs in cultures where thought and moral philosophy are based on a collective paradigm. So, applying Western bioethical concepts is unrealistic. For one, Africa is a diverse continent with many countries with different belief systems, access to health care, and reliance on traditional or Western medicines. Where traditional medicine is the primary treatment, the “’restrictive focus on biomedically-related bioethics’” [is] problematic in African contexts because it neglects bioethical issues raised by traditional systems.” [15] No single approach applies in all areas or contexts. Rather than evaluating the permissibility of ESC research according to Western concepts such as the four principles approach, different ethics approaches should prevail.
Another consideration is the socio-economic standing of countries. In parts of South Africa, researchers have not focused heavily on contributing to the stem cell discourse, either because it is not considered health care or a health science priority or because resources are unavailable. [16] Each country’s priorities differ given different social, political, and economic factors. In South Africa, for instance, areas such as maternal mortality, non-communicable diseases, telemedicine, and the strength of health systems need improvement and require more focus [17] Stem cell research could benefit the population, but it also could divert resources from basic medical care. Researchers in South Africa adhere to the National Health Act and Medicines Control Act in South Africa and international guidelines; however, the Act is not strictly enforced, and there is no clear legislation for research conduct or ethical guidelines. [18]
Some parts of Africa condemn stem cell research. For example, 98.2 percent of the Tunisian population is Muslim. [19] Tunisia does not permit stem cell research because of moral conflict with a Fatwa. Religion heavily saturates the regulation and direction of research. [20] Stem cell use became permissible for reproductive purposes only recently, with tight restrictions preventing cells from being used in any research other than procedures concerning ART/IVF. Their use is conditioned on consent, and available only to married couples. [21] The community's receptiveness to stem cell research depends on including communitarian African ethics.
c. Asia
Some Asian countries also have a collective model of ethics and decision making. [22] In China, the ethics model promotes a sincere respect for life or human dignity, [23] based on protective medicine. This model, influenced by Traditional Chinese Medicine (TCM), [24] recognizes Qi as the vital energy delivered via the meridians of the body; it connects illness to body systems, the body’s entire constitution, and the universe for a holistic bond of nature, health, and quality of life. [25] Following a protective ethics model, and traditional customs of wholeness, investment in stem cell research is heavily desired for its applications in regenerative therapies, disease modeling, and protective medicines. In a survey of medical students and healthcare practitioners, 30.8 percent considered stem cell research morally unacceptable while 63.5 percent accepted medical research using human embryonic stem cells. Of these individuals, 89.9 percent supported increased funding for stem cell research. [26] The scientific community might not reflect the overall population. From 1997 to 2019, China spent a total of $576 million (USD) on stem cell research at 8,050 stem cell programs, increased published presence from 0.6 percent to 14.01 percent of total global stem cell publications as of 2014, and made significant strides in cell-based therapies for various medical conditions. [27] However, while China has made substantial investments in stem cell research and achieved notable progress in clinical applications, concerns linger regarding ethical oversight and transparency. [28] For example, the China Biosecurity Law, promoted by the National Health Commission and China Hospital Association, attempted to mitigate risks by introducing an institutional review board (IRB) in the regulatory bodies. 5800 IRBs registered with the Chinese Clinical Trial Registry since 2021. [29] However, issues still need to be addressed in implementing effective IRB review and approval procedures.
The substantial government funding and focus on scientific advancement have sometimes overshadowed considerations of regional cultures, ethnic minorities, and individual perspectives, particularly evident during the one-child policy era. As government policy adapts to promote public stability, such as the change from the one-child to the two-child policy, [30] research ethics should also adapt to ensure respect for the values of its represented peoples.
Japan is also relatively supportive of stem cell research and therapies. Japan has a more transparent regulatory framework, allowing for faster approval of regenerative medicine products, which has led to several advanced clinical trials and therapies. [31] South Korea is also actively engaged in stem cell research and has a history of breakthroughs in cloning and embryonic stem cells. [32] However, the field is controversial, and there are issues of scientific integrity. For example, the Korean FDA fast-tracked products for approval, [33] and in another instance, the oocyte source was unclear and possibly violated ethical standards. [34] Trust is important in research, as it builds collaborative foundations between colleagues, trial participant comfort, open-mindedness for complicated and sensitive discussions, and supports regulatory procedures for stakeholders. There is a need to respect the culture’s interest, engagement, and for research and clinical trials to be transparent and have ethical oversight to promote global research discourse and trust.
d. Middle East
Countries in the Middle East have varying degrees of acceptance of or restrictions to policies related to using embryonic stem cells due to cultural and religious influences. Saudi Arabia has made significant contributions to stem cell research, and conducts research based on international guidelines for ethical conduct and under strict adherence to guidelines in accordance with Islamic principles. Specifically, the Saudi government and people require ESC research to adhere to Sharia law. In addition to umbilical and placental stem cells, [35] Saudi Arabia permits the use of embryonic stem cells as long as they come from miscarriages, therapeutic abortions permissible by Sharia law, or are left over from in vitro fertilization and donated to research. [36] Laws and ethical guidelines for stem cell research allow the development of research institutions such as the King Abdullah International Medical Research Center, which has a cord blood bank and a stem cell registry with nearly 10,000 donors. [37] Such volume and acceptance are due to the ethical ‘permissibility’ of the donor sources, which do not conflict with religious pillars. However, some researchers err on the side of caution, choosing not to use embryos or fetal tissue as they feel it is unethical to do so. [38]
Jordan has a positive research ethics culture. [39] However, there is a significant issue of lack of trust in researchers, with 45.23 percent (38.66 percent agreeing and 6.57 percent strongly agreeing) of Jordanians holding a low level of trust in researchers, compared to 81.34 percent of Jordanians agreeing that they feel safe to participate in a research trial. [40] Safety testifies to the feeling of confidence that adequate measures are in place to protect participants from harm, whereas trust in researchers could represent the confidence in researchers to act in the participants’ best interests, adhere to ethical guidelines, provide accurate information, and respect participants’ rights and dignity. One method to improve trust would be to address communication issues relevant to ESC. Legislation surrounding stem cell research has adopted specific language, especially concerning clarification “between ‘stem cells’ and ‘embryonic stem cells’” in translation. [41] Furthermore, legislation “mandates the creation of a national committee… laying out specific regulations for stem-cell banking in accordance with international standards.” [42] This broad regulation opens the door for future global engagement and maintains transparency. However, these regulations may also constrain the influence of research direction, pace, and accessibility of research outcomes.
e. Europe
In the European Union (EU), ethics is also principle-based, but the principles of autonomy, dignity, integrity, and vulnerability are interconnected. [43] As such, the opportunity for cohesion and concessions between individuals’ thoughts and ideals allows for a more adaptable ethics model due to the flexible principles that relate to the human experience The EU has put forth a framework in its Convention for the Protection of Human Rights and Dignity of the Human Being allowing member states to take different approaches. Each European state applies these principles to its specific conventions, leading to or reflecting different acceptance levels of stem cell research. [44]
For example, in Germany, Lebenzusammenhang , or the coherence of life, references integrity in the unity of human culture. Namely, the personal sphere “should not be subject to external intervention.” [45] Stem cell interventions could affect this concept of bodily completeness, leading to heavy restrictions. Under the Grundgesetz, human dignity and the right to life with physical integrity are paramount. [46] The Embryo Protection Act of 1991 made producing cell lines illegal. Cell lines can be imported if approved by the Central Ethics Commission for Stem Cell Research only if they were derived before May 2007. [47] Stem cell research respects the integrity of life for the embryo with heavy specifications and intense oversight. This is vastly different in Finland, where the regulatory bodies find research more permissible in IVF excess, but only up to 14 days after fertilization. [48] Spain’s approach differs still, with a comprehensive regulatory framework. [49] Thus, research regulation can be culture-specific due to variations in applied principles. Diverse cultures call for various approaches to ethical permissibility. [50] Only an adaptive-deliberative model can address the cultural constructions of self and achieve positive, culturally sensitive stem cell research practices. [51]
II. Religious Perspectives on ESC
Embryonic stem cell sources are the main consideration within religious contexts. While individuals may not regard their own religious texts as authoritative or factual, religion can shape their foundations or perspectives.
The Qur'an states:
“And indeed We created man from a quintessence of clay. Then We placed within him a small quantity of nutfa (sperm to fertilize) in a safe place. Then We have fashioned the nutfa into an ‘alaqa (clinging clot or cell cluster), then We developed the ‘alaqa into mudgha (a lump of flesh), and We made mudgha into bones, and clothed the bones with flesh, then We brought it into being as a new creation. So Blessed is Allah, the Best of Creators.” [52]
Many scholars of Islam estimate the time of soul installment, marked by the angel breathing in the soul to bring the individual into creation, as 120 days from conception. [53] Personhood begins at this point, and the value of life would prohibit research or experimentation that could harm the individual. If the fetus is more than 120 days old, the time ensoulment is interpreted to occur according to Islamic law, abortion is no longer permissible. [54] There are a few opposing opinions about early embryos in Islamic traditions. According to some Islamic theologians, there is no ensoulment of the early embryo, which is the source of stem cells for ESC research. [55]
In Buddhism, the stance on stem cell research is not settled. The main tenets, the prohibition against harming or destroying others (ahimsa) and the pursuit of knowledge (prajña) and compassion (karuna), leave Buddhist scholars and communities divided. [56] Some scholars argue stem cell research is in accordance with the Buddhist tenet of seeking knowledge and ending human suffering. Others feel it violates the principle of not harming others. Finding the balance between these two points relies on the karmic burden of Buddhist morality. In trying to prevent ahimsa towards the embryo, Buddhist scholars suggest that to comply with Buddhist tenets, research cannot be done as the embryo has personhood at the moment of conception and would reincarnate immediately, harming the individual's ability to build their karmic burden. [57] On the other hand, the Bodhisattvas, those considered to be on the path to enlightenment or Nirvana, have given organs and flesh to others to help alleviate grieving and to benefit all. [58] Acceptance varies on applied beliefs and interpretations.
Catholicism does not support embryonic stem cell research, as it entails creation or destruction of human embryos. This destruction conflicts with the belief in the sanctity of life. For example, in the Old Testament, Genesis describes humanity as being created in God’s image and multiplying on the Earth, referencing the sacred rights to human conception and the purpose of development and life. In the Ten Commandments, the tenet that one should not kill has numerous interpretations where killing could mean murder or shedding of the sanctity of life, demonstrating the high value of human personhood. In other books, the theological conception of when life begins is interpreted as in utero, [59] highlighting the inviolability of life and its formation in vivo to make a religious point for accepting such research as relatively limited, if at all. [60] The Vatican has released ethical directives to help apply a theological basis to modern-day conflicts. The Magisterium of the Church states that “unless there is a moral certainty of not causing harm,” experimentation on fetuses, fertilized cells, stem cells, or embryos constitutes a crime. [61] Such procedures would not respect the human person who exists at these stages, according to Catholicism. Damages to the embryo are considered gravely immoral and illicit. [62] Although the Catholic Church officially opposes abortion, surveys demonstrate that many Catholic people hold pro-choice views, whether due to the context of conception, stage of pregnancy, threat to the mother’s life, or for other reasons, demonstrating that practicing members can also accept some but not all tenets. [63]
Some major Jewish denominations, such as the Reform, Conservative, and Reconstructionist movements, are open to supporting ESC use or research as long as it is for saving a life. [64] Within Judaism, the Talmud, or study, gives personhood to the child at birth and emphasizes that life does not begin at conception: [65]
“If she is found pregnant, until the fortieth day it is mere fluid,” [66]
Whereas most religions prioritize the status of human embryos, the Halakah (Jewish religious law) states that to save one life, most other religious laws can be ignored because it is in pursuit of preservation. [67] Stem cell research is accepted due to application of these religious laws.
We recognize that all religions contain subsets and sects. The variety of environmental and cultural differences within religious groups requires further analysis to respect the flexibility of religious thoughts and practices. We make no presumptions that all cultures require notions of autonomy or morality as under the common morality theory , which asserts a set of universal moral norms that all individuals share provides moral reasoning and guides ethical decisions. [68] We only wish to show that the interaction with morality varies between cultures and countries.
III. A Flexible Ethical Approach
The plurality of different moral approaches described above demonstrates that there can be no universally acceptable uniform law for ESC on a global scale. Instead of developing one standard, flexible ethical applications must be continued. We recommend local guidelines that incorporate important cultural and ethical priorities.
While the Declaration of Helsinki is more relevant to people in clinical trials receiving ESC products, in keeping with the tradition of protections for research subjects, consent of the donor is an ethical requirement for ESC donation in many jurisdictions including the US, Canada, and Europe. [69] The Declaration of Helsinki provides a reference point for regulatory standards and could potentially be used as a universal baseline for obtaining consent prior to gamete or embryo donation.
For instance, in Columbia University’s egg donor program for stem cell research, donors followed standard screening protocols and “underwent counseling sessions that included information as to the purpose of oocyte donation for research, what the oocytes would be used for, the risks and benefits of donation, and process of oocyte stimulation” to ensure transparency for consent. [70] The program helped advance stem cell research and provided clear and safe research methods with paid participants. Though paid participation or covering costs of incidental expenses may not be socially acceptable in every culture or context, [71] and creating embryos for ESC research is illegal in many jurisdictions, Columbia’s program was effective because of the clear and honest communications with donors, IRBs, and related stakeholders. This example demonstrates that cultural acceptance of scientific research and of the idea that an egg or embryo does not have personhood is likely behind societal acceptance of donating eggs for ESC research. As noted, many countries do not permit the creation of embryos for research.
Proper communication and education regarding the process and purpose of stem cell research may bolster comprehension and garner more acceptance. “Given the sensitive subject material, a complete consent process can support voluntary participation through trust, understanding, and ethical norms from the cultures and morals participants value. This can be hard for researchers entering countries of different socioeconomic stability, with different languages and different societal values. [72]
An adequate moral foundation in medical ethics is derived from the cultural and religious basis that informs knowledge and actions. [73] Understanding local cultural and religious values and their impact on research could help researchers develop humility and promote inclusion.
IV. Concerns
Some may argue that if researchers all adhere to one ethics standard, protection will be satisfied across all borders, and the global public will trust researchers. However, defining what needs to be protected and how to define such research standards is very specific to the people to which standards are applied. We suggest that applying one uniform guide cannot accurately protect each individual because we all possess our own perceptions and interpretations of social values. [74] Therefore, the issue of not adjusting to the moral pluralism between peoples in applying one standard of ethics can be resolved by building out ethics models that can be adapted to different cultures and religions.
Other concerns include medical tourism, which may promote health inequities. [75] Some countries may develop and approve products derived from ESC research before others, compromising research ethics or drug approval processes. There are also concerns about the sale of unauthorized stem cell treatments, for example, those without FDA approval in the United States. Countries with robust research infrastructures may be tempted to attract medical tourists, and some customers will have false hopes based on aggressive publicity of unproven treatments. [76]
For example, in China, stem cell clinics can market to foreign clients who are not protected under the regulatory regimes. Companies employ a marketing strategy of “ethically friendly” therapies. Specifically, in the case of Beike, China’s leading stem cell tourism company and sprouting network, ethical oversight of administrators or health bureaus at one site has “the unintended consequence of shifting questionable activities to another node in Beike's diffuse network.” [77] In contrast, Jordan is aware of stem cell research’s potential abuse and its own status as a “health-care hub.” Jordan’s expanded regulations include preserving the interests of individuals in clinical trials and banning private companies from ESC research to preserve transparency and the integrity of research practices. [78]
The social priorities of the community are also a concern. The ISSCR explicitly states that guidelines “should be periodically revised to accommodate scientific advances, new challenges, and evolving social priorities.” [79] The adaptable ethics model extends this consideration further by addressing whether research is warranted given the varying degrees of socioeconomic conditions, political stability, and healthcare accessibilities and limitations. An ethical approach would require discussion about resource allocation and appropriate distribution of funds. [80]
While some religions emphasize the sanctity of life from conception, which may lead to public opposition to ESC research, others encourage ESC research due to its potential for healing and alleviating human pain. Many countries have special regulations that balance local views on embryonic personhood, the benefits of research as individual or societal goods, and the protection of human research subjects. To foster understanding and constructive dialogue, global policy frameworks should prioritize the protection of universal human rights, transparency, and informed consent. In addition to these foundational global policies, we recommend tailoring local guidelines to reflect the diverse cultural and religious perspectives of the populations they govern. Ethics models should be adapted to local populations to effectively establish research protections, growth, and possibilities of stem cell research.
For example, in countries with strong beliefs in the moral sanctity of embryos or heavy religious restrictions, an adaptive model can allow for discussion instead of immediate rejection. In countries with limited individual rights and voice in science policy, an adaptive model ensures cultural, moral, and religious views are taken into consideration, thereby building social inclusion. While this ethical consideration by the government may not give a complete voice to every individual, it will help balance policies and maintain the diverse perspectives of those it affects. Embracing an adaptive ethics model of ESC research promotes open-minded dialogue and respect for the importance of human belief and tradition. By actively engaging with cultural and religious values, researchers can better handle disagreements and promote ethical research practices that benefit each society.
This brief exploration of the religious and cultural differences that impact ESC research reveals the nuances of relative ethics and highlights a need for local policymakers to apply a more intense adaptive model.
[1] Poliwoda, S., Noor, N., Downs, E., Schaaf, A., Cantwell, A., Ganti, L., Kaye, A. D., Mosel, L. I., Carroll, C. B., Viswanath, O., & Urits, I. (2022). Stem cells: a comprehensive review of origins and emerging clinical roles in medical practice. Orthopedic reviews , 14 (3), 37498. https://doi.org/10.52965/001c.37498
[2] Poliwoda, S., Noor, N., Downs, E., Schaaf, A., Cantwell, A., Ganti, L., Kaye, A. D., Mosel, L. I., Carroll, C. B., Viswanath, O., & Urits, I. (2022). Stem cells: a comprehensive review of origins and emerging clinical roles in medical practice. Orthopedic reviews , 14 (3), 37498. https://doi.org/10.52965/001c.37498
[3] International Society for Stem Cell Research. (2023). Laboratory-based human embryonic stem cell research, embryo research, and related research activities . International Society for Stem Cell Research. https://www.isscr.org/guidelines/blog-post-title-one-ed2td-6fcdk ; Kimmelman, J., Hyun, I., Benvenisty, N. et al. Policy: Global standards for stem-cell research. Nature 533 , 311–313 (2016). https://doi.org/10.1038/533311a
[4] International Society for Stem Cell Research. (2023). Laboratory-based human embryonic stem cell research, embryo research, and related research activities . International Society for Stem Cell Research. https://www.isscr.org/guidelines/blog-post-title-one-ed2td-6fcdk
[5] Concerning the moral philosophies of stem cell research, our paper does not posit a personal moral stance nor delve into the “when” of human life begins. To read further about the philosophical debate, consider the following sources:
Sandel M. J. (2004). Embryo ethics--the moral logic of stem-cell research. The New England journal of medicine , 351 (3), 207–209. https://doi.org/10.1056/NEJMp048145 ; George, R. P., & Lee, P. (2020, September 26). Acorns and Embryos . The New Atlantis. https://www.thenewatlantis.com/publications/acorns-and-embryos ; Sagan, A., & Singer, P. (2007). The moral status of stem cells. Metaphilosophy , 38 (2/3), 264–284. http://www.jstor.org/stable/24439776 ; McHugh P. R. (2004). Zygote and "clonote"--the ethical use of embryonic stem cells. The New England journal of medicine , 351 (3), 209–211. https://doi.org/10.1056/NEJMp048147 ; Kurjak, A., & Tripalo, A. (2004). The facts and doubts about beginning of the human life and personality. Bosnian journal of basic medical sciences , 4 (1), 5–14. https://doi.org/10.17305/bjbms.2004.3453
[6] Vazin, T., & Freed, W. J. (2010). Human embryonic stem cells: derivation, culture, and differentiation: a review. Restorative neurology and neuroscience , 28 (4), 589–603. https://doi.org/10.3233/RNN-2010-0543
[7] Socially, at its core, the Western approach to ethics is widely principle-based, autonomy being one of the key factors to ensure a fundamental respect for persons within research. For information regarding autonomy in research, see: Department of Health, Education, and Welfare, & National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research (1978). The Belmont Report. Ethical principles and guidelines for the protection of human subjects of research.; For a more in-depth review of autonomy within the US, see: Beauchamp, T. L., & Childress, J. F. (1994). Principles of Biomedical Ethics . Oxford University Press.
[8] Sherley v. Sebelius , 644 F.3d 388 (D.C. Cir. 2011), citing 45 C.F.R. 46.204(b) and [42 U.S.C. § 289g(b)]. https://www.cadc.uscourts.gov/internet/opinions.nsf/6c690438a9b43dd685257a64004ebf99/$file/11-5241-1391178.pdf
[9] Stem Cell Research Enhancement Act of 2005, H. R. 810, 109 th Cong. (2001). https://www.govtrack.us/congress/bills/109/hr810/text ; Bush, G. W. (2006, July 19). Message to the House of Representatives . National Archives and Records Administration. https://georgewbush-whitehouse.archives.gov/news/releases/2006/07/20060719-5.html
[10] National Archives and Records Administration. (2009, March 9). Executive order 13505 -- removing barriers to responsible scientific research involving human stem cells . National Archives and Records Administration. https://obamawhitehouse.archives.gov/the-press-office/removing-barriers-responsible-scientific-research-involving-human-stem-cells
[11] Hurlbut, W. B. (2006). Science, Religion, and the Politics of Stem Cells. Social Research , 73 (3), 819–834. http://www.jstor.org/stable/40971854
[12] Akpa-Inyang, Francis & Chima, Sylvester. (2021). South African traditional values and beliefs regarding informed consent and limitations of the principle of respect for autonomy in African communities: a cross-cultural qualitative study. BMC Medical Ethics . 22. 10.1186/s12910-021-00678-4.
[13] Source for further reading: Tangwa G. B. (2007). Moral status of embryonic stem cells: perspective of an African villager. Bioethics , 21(8), 449–457. https://doi.org/10.1111/j.1467-8519.2007.00582.x , see also Mnisi, F. M. (2020). An African analysis based on ethics of Ubuntu - are human embryonic stem cell patents morally justifiable? African Insight , 49 (4).
[14] Jecker, N. S., & Atuire, C. (2021). Bioethics in Africa: A contextually enlightened analysis of three cases. Developing World Bioethics , 22 (2), 112–122. https://doi.org/10.1111/dewb.12324
[15] Jecker, N. S., & Atuire, C. (2021). Bioethics in Africa: A contextually enlightened analysis of three cases. Developing World Bioethics, 22(2), 112–122. https://doi.org/10.1111/dewb.12324
[16] Jackson, C.S., Pepper, M.S. Opportunities and barriers to establishing a cell therapy programme in South Africa. Stem Cell Res Ther 4 , 54 (2013). https://doi.org/10.1186/scrt204 ; Pew Research Center. (2014, May 1). Public health a major priority in African nations . Pew Research Center’s Global Attitudes Project. https://www.pewresearch.org/global/2014/05/01/public-health-a-major-priority-in-african-nations/
[17] Department of Health Republic of South Africa. (2021). Health Research Priorities (revised) for South Africa 2021-2024 . National Health Research Strategy. https://www.health.gov.za/wp-content/uploads/2022/05/National-Health-Research-Priorities-2021-2024.pdf
[18] Oosthuizen, H. (2013). Legal and Ethical Issues in Stem Cell Research in South Africa. In: Beran, R. (eds) Legal and Forensic Medicine. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-32338-6_80 , see also: Gaobotse G (2018) Stem Cell Research in Africa: Legislation and Challenges. J Regen Med 7:1. doi: 10.4172/2325-9620.1000142
[19] United States Bureau of Citizenship and Immigration Services. (1998). Tunisia: Information on the status of Christian conversions in Tunisia . UNHCR Web Archive. https://webarchive.archive.unhcr.org/20230522142618/https://www.refworld.org/docid/3df0be9a2.html
[20] Gaobotse, G. (2018) Stem Cell Research in Africa: Legislation and Challenges. J Regen Med 7:1. doi: 10.4172/2325-9620.1000142
[21] Kooli, C. Review of assisted reproduction techniques, laws, and regulations in Muslim countries. Middle East Fertil Soc J 24 , 8 (2020). https://doi.org/10.1186/s43043-019-0011-0 ; Gaobotse, G. (2018) Stem Cell Research in Africa: Legislation and Challenges. J Regen Med 7:1. doi: 10.4172/2325-9620.1000142
[22] Pang M. C. (1999). Protective truthfulness: the Chinese way of safeguarding patients in informed treatment decisions. Journal of medical ethics , 25(3), 247–253. https://doi.org/10.1136/jme.25.3.247
[23] Wang, L., Wang, F., & Zhang, W. (2021). Bioethics in China’s biosecurity law: Forms, effects, and unsettled issues. Journal of law and the biosciences , 8(1). https://doi.org/10.1093/jlb/lsab019 https://academic.oup.com/jlb/article/8/1/lsab019/6299199
[24] Wang, Y., Xue, Y., & Guo, H. D. (2022). Intervention effects of traditional Chinese medicine on stem cell therapy of myocardial infarction. Frontiers in pharmacology , 13 , 1013740. https://doi.org/10.3389/fphar.2022.1013740
[25] Li, X.-T., & Zhao, J. (2012). Chapter 4: An Approach to the Nature of Qi in TCM- Qi and Bioenergy. In Recent Advances in Theories and Practice of Chinese Medicine (p. 79). InTech.
[26] Luo, D., Xu, Z., Wang, Z., & Ran, W. (2021). China's Stem Cell Research and Knowledge Levels of Medical Practitioners and Students. Stem cells international , 2021 , 6667743. https://doi.org/10.1155/2021/6667743
[27] Luo, D., Xu, Z., Wang, Z., & Ran, W. (2021). China's Stem Cell Research and Knowledge Levels of Medical Practitioners and Students. Stem cells international , 2021 , 6667743. https://doi.org/10.1155/2021/6667743
[28] Zhang, J. Y. (2017). Lost in translation? accountability and governance of Clinical Stem Cell Research in China. Regenerative Medicine , 12 (6), 647–656. https://doi.org/10.2217/rme-2017-0035
[29] Wang, L., Wang, F., & Zhang, W. (2021). Bioethics in China’s biosecurity law: Forms, effects, and unsettled issues. Journal of law and the biosciences , 8(1). https://doi.org/10.1093/jlb/lsab019 https://academic.oup.com/jlb/article/8/1/lsab019/6299199
[30] Chen, H., Wei, T., Wang, H. et al. Association of China’s two-child policy with changes in number of births and birth defects rate, 2008–2017. BMC Public Health 22 , 434 (2022). https://doi.org/10.1186/s12889-022-12839-0
[31] Azuma, K. Regulatory Landscape of Regenerative Medicine in Japan. Curr Stem Cell Rep 1 , 118–128 (2015). https://doi.org/10.1007/s40778-015-0012-6
[32] Harris, R. (2005, May 19). Researchers Report Advance in Stem Cell Production . NPR. https://www.npr.org/2005/05/19/4658967/researchers-report-advance-in-stem-cell-production
[33] Park, S. (2012). South Korea steps up stem-cell work. Nature . https://doi.org/10.1038/nature.2012.10565
[34] Resnik, D. B., Shamoo, A. E., & Krimsky, S. (2006). Fraudulent human embryonic stem cell research in South Korea: lessons learned. Accountability in research , 13 (1), 101–109. https://doi.org/10.1080/08989620600634193 .
[35] Alahmad, G., Aljohani, S., & Najjar, M. F. (2020). Ethical challenges regarding the use of stem cells: interviews with researchers from Saudi Arabia. BMC medical ethics, 21(1), 35. https://doi.org/10.1186/s12910-020-00482-6
[36] Association for the Advancement of Blood and Biotherapies. https://www.aabb.org/regulatory-and-advocacy/regulatory-affairs/regulatory-for-cellular-therapies/international-competent-authorities/saudi-arabia
[37] Alahmad, G., Aljohani, S., & Najjar, M. F. (2020). Ethical challenges regarding the use of stem cells: Interviews with researchers from Saudi Arabia. BMC medical ethics , 21 (1), 35. https://doi.org/10.1186/s12910-020-00482-6
[38] Alahmad, G., Aljohani, S., & Najjar, M. F. (2020). Ethical challenges regarding the use of stem cells: Interviews with researchers from Saudi Arabia. BMC medical ethics , 21(1), 35. https://doi.org/10.1186/s12910-020-00482-6
Culturally, autonomy practices follow a relational autonomy approach based on a paternalistic deontological health care model. The adherence to strict international research policies and religious pillars within the regulatory environment is a great foundation for research ethics. However, there is a need to develop locally targeted ethics approaches for research (as called for in Alahmad, G., Aljohani, S., & Najjar, M. F. (2020). Ethical challenges regarding the use of stem cells: interviews with researchers from Saudi Arabia. BMC medical ethics, 21(1), 35. https://doi.org/10.1186/s12910-020-00482-6), this decision-making approach may help advise a research decision model. For more on the clinical cultural autonomy approaches, see: Alabdullah, Y. Y., Alzaid, E., Alsaad, S., Alamri, T., Alolayan, S. W., Bah, S., & Aljoudi, A. S. (2022). Autonomy and paternalism in Shared decision‐making in a Saudi Arabian tertiary hospital: A cross‐sectional study. Developing World Bioethics , 23 (3), 260–268. https://doi.org/10.1111/dewb.12355 ; Bukhari, A. A. (2017). Universal Principles of Bioethics and Patient Rights in Saudi Arabia (Doctoral dissertation, Duquesne University). https://dsc.duq.edu/etd/124; Ladha, S., Nakshawani, S. A., Alzaidy, A., & Tarab, B. (2023, October 26). Islam and Bioethics: What We All Need to Know . Columbia University School of Professional Studies. https://sps.columbia.edu/events/islam-and-bioethics-what-we-all-need-know
[39] Ababneh, M. A., Al-Azzam, S. I., Alzoubi, K., Rababa’h, A., & Al Demour, S. (2021). Understanding and attitudes of the Jordanian public about clinical research ethics. Research Ethics , 17 (2), 228-241. https://doi.org/10.1177/1747016120966779
[40] Ababneh, M. A., Al-Azzam, S. I., Alzoubi, K., Rababa’h, A., & Al Demour, S. (2021). Understanding and attitudes of the Jordanian public about clinical research ethics. Research Ethics , 17 (2), 228-241. https://doi.org/10.1177/1747016120966779
[41] Dajani, R. (2014). Jordan’s stem-cell law can guide the Middle East. Nature 510, 189. https://doi.org/10.1038/510189a
[42] Dajani, R. (2014). Jordan’s stem-cell law can guide the Middle East. Nature 510, 189. https://doi.org/10.1038/510189a
[43] The EU’s definition of autonomy relates to the capacity for creating ideas, moral insight, decisions, and actions without constraint, personal responsibility, and informed consent. However, the EU views autonomy as not completely able to protect individuals and depends on other principles, such as dignity, which “expresses the intrinsic worth and fundamental equality of all human beings.” Rendtorff, J.D., Kemp, P. (2019). Four Ethical Principles in European Bioethics and Biolaw: Autonomy, Dignity, Integrity and Vulnerability. In: Valdés, E., Lecaros, J. (eds) Biolaw and Policy in the Twenty-First Century. International Library of Ethics, Law, and the New Medicine, vol 78. Springer, Cham. https://doi.org/10.1007/978-3-030-05903-3_3
[44] Council of Europe. Convention for the protection of Human Rights and Dignity of the Human Being with regard to the Application of Biology and Medicine: Convention on Human Rights and Biomedicine (ETS No. 164) https://www.coe.int/en/web/conventions/full-list?module=treaty-detail&treatynum=164 (forbidding the creation of embryos for research purposes only, and suggests embryos in vitro have protections.); Also see Drabiak-Syed B. K. (2013). New President, New Human Embryonic Stem Cell Research Policy: Comparative International Perspectives and Embryonic Stem Cell Research Laws in France. Biotechnology Law Report , 32 (6), 349–356. https://doi.org/10.1089/blr.2013.9865
[45] Rendtorff, J.D., Kemp, P. (2019). Four Ethical Principles in European Bioethics and Biolaw: Autonomy, Dignity, Integrity and Vulnerability. In: Valdés, E., Lecaros, J. (eds) Biolaw and Policy in the Twenty-First Century. International Library of Ethics, Law, and the New Medicine, vol 78. Springer, Cham. https://doi.org/10.1007/978-3-030-05903-3_3
[46] Tomuschat, C., Currie, D. P., Kommers, D. P., & Kerr, R. (Trans.). (1949, May 23). Basic law for the Federal Republic of Germany. https://www.btg-bestellservice.de/pdf/80201000.pdf
[47] Regulation of Stem Cell Research in Germany . Eurostemcell. (2017, April 26). https://www.eurostemcell.org/regulation-stem-cell-research-germany
[48] Regulation of Stem Cell Research in Finland . Eurostemcell. (2017, April 26). https://www.eurostemcell.org/regulation-stem-cell-research-finland
[49] Regulation of Stem Cell Research in Spain . Eurostemcell. (2017, April 26). https://www.eurostemcell.org/regulation-stem-cell-research-spain
[50] Some sources to consider regarding ethics models or regulatory oversights of other cultures not covered:
Kara MA. Applicability of the principle of respect for autonomy: the perspective of Turkey. J Med Ethics. 2007 Nov;33(11):627-30. doi: 10.1136/jme.2006.017400. PMID: 17971462; PMCID: PMC2598110.
Ugarte, O. N., & Acioly, M. A. (2014). The principle of autonomy in Brazil: one needs to discuss it ... Revista do Colegio Brasileiro de Cirurgioes , 41 (5), 374–377. https://doi.org/10.1590/0100-69912014005013
Bharadwaj, A., & Glasner, P. E. (2012). Local cells, global science: The rise of embryonic stem cell research in India . Routledge.
For further research on specific European countries regarding ethical and regulatory framework, we recommend this database: Regulation of Stem Cell Research in Europe . Eurostemcell. (2017, April 26). https://www.eurostemcell.org/regulation-stem-cell-research-europe
[51] Klitzman, R. (2006). Complications of culture in obtaining informed consent. The American Journal of Bioethics, 6(1), 20–21. https://doi.org/10.1080/15265160500394671 see also: Ekmekci, P. E., & Arda, B. (2017). Interculturalism and Informed Consent: Respecting Cultural Differences without Breaching Human Rights. Cultura (Iasi, Romania) , 14 (2), 159–172.; For why trust is important in research, see also: Gray, B., Hilder, J., Macdonald, L., Tester, R., Dowell, A., & Stubbe, M. (2017). Are research ethics guidelines culturally competent? Research Ethics , 13 (1), 23-41. https://doi.org/10.1177/1747016116650235
[52] The Qur'an (M. Khattab, Trans.). (1965). Al-Mu’minun, 23: 12-14. https://quran.com/23
[53] Lenfest, Y. (2017, December 8). Islam and the beginning of human life . Bill of Health. https://blog.petrieflom.law.harvard.edu/2017/12/08/islam-and-the-beginning-of-human-life/
[54] Aksoy, S. (2005). Making regulations and drawing up legislation in Islamic countries under conditions of uncertainty, with special reference to embryonic stem cell research. Journal of Medical Ethics , 31: 399-403.; see also: Mahmoud, Azza. "Islamic Bioethics: National Regulations and Guidelines of Human Stem Cell Research in the Muslim World." Master's thesis, Chapman University, 2022. https://doi.org/10.36837/ chapman.000386
[55] Rashid, R. (2022). When does Ensoulment occur in the Human Foetus. Journal of the British Islamic Medical Association , 12 (4). ISSN 2634 8071. https://www.jbima.com/wp-content/uploads/2023/01/2-Ethics-3_-Ensoulment_Rafaqat.pdf.
[56] Sivaraman, M. & Noor, S. (2017). Ethics of embryonic stem cell research according to Buddhist, Hindu, Catholic, and Islamic religions: perspective from Malaysia. Asian Biomedicine,8(1) 43-52. https://doi.org/10.5372/1905-7415.0801.260
[57] Jafari, M., Elahi, F., Ozyurt, S. & Wrigley, T. (2007). 4. Religious Perspectives on Embryonic Stem Cell Research. In K. Monroe, R. Miller & J. Tobis (Ed.), Fundamentals of the Stem Cell Debate: The Scientific, Religious, Ethical, and Political Issues (pp. 79-94). Berkeley: University of California Press. https://escholarship.org/content/qt9rj0k7s3/qt9rj0k7s3_noSplash_f9aca2e02c3777c7fb76ea768ba458f0.pdf https://doi.org/10.1525/9780520940994-005
[58] Lecso, P. A. (1991). The Bodhisattva Ideal and Organ Transplantation. Journal of Religion and Health , 30 (1), 35–41. http://www.jstor.org/stable/27510629 ; Bodhisattva, S. (n.d.). The Key of Becoming a Bodhisattva . A Guide to the Bodhisattva Way of Life. http://www.buddhism.org/Sutras/2/BodhisattvaWay.htm
[59] There is no explicit religious reference to when life begins or how to conduct research that interacts with the concept of life. However, these are relevant verses pertaining to how the fetus is viewed. (( King James Bible . (1999). Oxford University Press. (original work published 1769))
Jerimiah 1: 5 “Before I formed thee in the belly I knew thee; and before thou camest forth out of the womb I sanctified thee…”
In prophet Jerimiah’s insight, God set him apart as a person known before childbirth, a theme carried within the Psalm of David.
Psalm 139: 13-14 “…Thou hast covered me in my mother's womb. I will praise thee; for I am fearfully and wonderfully made…”
These verses demonstrate David’s respect for God as an entity that would know of all man’s thoughts and doings even before birth.
[60] It should be noted that abortion is not supported as well.
[61] The Vatican. (1987, February 22). Instruction on Respect for Human Life in Its Origin and on the Dignity of Procreation Replies to Certain Questions of the Day . Congregation For the Doctrine of the Faith. https://www.vatican.va/roman_curia/congregations/cfaith/documents/rc_con_cfaith_doc_19870222_respect-for-human-life_en.html
[62] The Vatican. (2000, August 25). Declaration On the Production and the Scientific and Therapeutic Use of Human Embryonic Stem Cells . Pontifical Academy for Life. https://www.vatican.va/roman_curia/pontifical_academies/acdlife/documents/rc_pa_acdlife_doc_20000824_cellule-staminali_en.html ; Ohara, N. (2003). Ethical Consideration of Experimentation Using Living Human Embryos: The Catholic Church’s Position on Human Embryonic Stem Cell Research and Human Cloning. Department of Obstetrics and Gynecology . Retrieved from https://article.imrpress.com/journal/CEOG/30/2-3/pii/2003018/77-81.pdf.
[63] Smith, G. A. (2022, May 23). Like Americans overall, Catholics vary in their abortion views, with regular mass attenders most opposed . Pew Research Center. https://www.pewresearch.org/short-reads/2022/05/23/like-americans-overall-catholics-vary-in-their-abortion-views-with-regular-mass-attenders-most-opposed/
[64] Rosner, F., & Reichman, E. (2002). Embryonic stem cell research in Jewish law. Journal of halacha and contemporary society , (43), 49–68.; Jafari, M., Elahi, F., Ozyurt, S. & Wrigley, T. (2007). 4. Religious Perspectives on Embryonic Stem Cell Research. In K. Monroe, R. Miller & J. Tobis (Ed.), Fundamentals of the Stem Cell Debate: The Scientific, Religious, Ethical, and Political Issues (pp. 79-94). Berkeley: University of California Press. https://escholarship.org/content/qt9rj0k7s3/qt9rj0k7s3_noSplash_f9aca2e02c3777c7fb76ea768ba458f0.pdf https://doi.org/10.1525/9780520940994-005
[65] Schenker J. G. (2008). The beginning of human life: status of embryo. Perspectives in Halakha (Jewish Religious Law). Journal of assisted reproduction and genetics , 25 (6), 271–276. https://doi.org/10.1007/s10815-008-9221-6
[66] Ruttenberg, D. (2020, May 5). The Torah of Abortion Justice (annotated source sheet) . Sefaria. https://www.sefaria.org/sheets/234926.7?lang=bi&with=all&lang2=en
[67] Jafari, M., Elahi, F., Ozyurt, S. & Wrigley, T. (2007). 4. Religious Perspectives on Embryonic Stem Cell Research. In K. Monroe, R. Miller & J. Tobis (Ed.), Fundamentals of the Stem Cell Debate: The Scientific, Religious, Ethical, and Political Issues (pp. 79-94). Berkeley: University of California Press. https://escholarship.org/content/qt9rj0k7s3/qt9rj0k7s3_noSplash_f9aca2e02c3777c7fb76ea768ba458f0.pdf https://doi.org/10.1525/9780520940994-005
[68] Gert, B. (2007). Common morality: Deciding what to do . Oxford Univ. Press.
[69] World Medical Association (2013). World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA , 310(20), 2191–2194. https://doi.org/10.1001/jama.2013.281053 Declaration of Helsinki – WMA – The World Medical Association .; see also: National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. (1979). The Belmont report: Ethical principles and guidelines for the protection of human subjects of research . U.S. Department of Health and Human Services. https://www.hhs.gov/ohrp/regulations-and-policy/belmont-report/read-the-belmont-report/index.html
[70] Zakarin Safier, L., Gumer, A., Kline, M., Egli, D., & Sauer, M. V. (2018). Compensating human subjects providing oocytes for stem cell research: 9-year experience and outcomes. Journal of assisted reproduction and genetics , 35 (7), 1219–1225. https://doi.org/10.1007/s10815-018-1171-z https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6063839/ see also: Riordan, N. H., & Paz Rodríguez, J. (2021). Addressing concerns regarding associated costs, transparency, and integrity of research in recent stem cell trial. Stem Cells Translational Medicine , 10 (12), 1715–1716. https://doi.org/10.1002/sctm.21-0234
[71] Klitzman, R., & Sauer, M. V. (2009). Payment of egg donors in stem cell research in the USA. Reproductive biomedicine online , 18 (5), 603–608. https://doi.org/10.1016/s1472-6483(10)60002-8
[72] Krosin, M. T., Klitzman, R., Levin, B., Cheng, J., & Ranney, M. L. (2006). Problems in comprehension of informed consent in rural and peri-urban Mali, West Africa. Clinical trials (London, England) , 3 (3), 306–313. https://doi.org/10.1191/1740774506cn150oa
[73] Veatch, Robert M. Hippocratic, Religious, and Secular Medical Ethics: The Points of Conflict . Georgetown University Press, 2012.
[74] Msoroka, M. S., & Amundsen, D. (2018). One size fits not quite all: Universal research ethics with diversity. Research Ethics , 14 (3), 1-17. https://doi.org/10.1177/1747016117739939
[75] Pirzada, N. (2022). The Expansion of Turkey’s Medical Tourism Industry. Voices in Bioethics , 8 . https://doi.org/10.52214/vib.v8i.9894
[76] Stem Cell Tourism: False Hope for Real Money . Harvard Stem Cell Institute (HSCI). (2023). https://hsci.harvard.edu/stem-cell-tourism , See also: Bissassar, M. (2017). Transnational Stem Cell Tourism: An ethical analysis. Voices in Bioethics , 3 . https://doi.org/10.7916/vib.v3i.6027
[77] Song, P. (2011) The proliferation of stem cell therapies in post-Mao China: problematizing ethical regulation, New Genetics and Society , 30:2, 141-153, DOI: 10.1080/14636778.2011.574375
[78] Dajani, R. (2014). Jordan’s stem-cell law can guide the Middle East. Nature 510, 189. https://doi.org/10.1038/510189a
[79] International Society for Stem Cell Research. (2024). Standards in stem cell research . International Society for Stem Cell Research. https://www.isscr.org/guidelines/5-standards-in-stem-cell-research
[80] Benjamin, R. (2013). People’s science bodies and rights on the Stem Cell Frontier . Stanford University Press.
Mifrah Hayath
SM Candidate Harvard Medical School, MS Biotechnology Johns Hopkins University
Olivia Bowers
MS Bioethics Columbia University (Disclosure: affiliated with Voices in Bioethics)
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License .
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 08 May 2024
Effects of a personalized nutrition program on cardiometabolic health: a randomized controlled trial
- Kate M. Bermingham 1 , 2 na1 ,
- Inbar Linenberg 1 , 2 na1 ,
- Lorenzo Polidori 2 ,
- Francesco Asnicar ORCID: orcid.org/0000-0003-3732-1468 3 ,
- Alberto Arrè 2 ,
- Jonathan Wolf ORCID: orcid.org/0000-0002-0530-2257 2 ,
- Fatema Badri 2 ,
- Hannah Bernard 2 ,
- Joan Capdevila ORCID: orcid.org/0000-0003-1658-1076 2 ,
- William J. Bulsiewicz ORCID: orcid.org/0009-0005-8752-3422 2 , 4 ,
- Christopher D. Gardner ORCID: orcid.org/0000-0002-7596-1530 5 ,
- Jose M. Ordovas ORCID: orcid.org/0000-0002-7581-5680 6 , 7 , 8 ,
- Richard Davies ORCID: orcid.org/0000-0003-2050-3994 2 ,
- George Hadjigeorgiou ORCID: orcid.org/0000-0001-7647-8471 2 ,
- Wendy L. Hall 1 ,
- Linda M. Delahanty 9 ,
- Ana M. Valdes ORCID: orcid.org/0000-0003-1141-4471 10 , 11 ,
- Nicola Segata ORCID: orcid.org/0000-0002-1583-5794 3 na2 ,
- Tim D. Spector ORCID: orcid.org/0000-0002-9795-0365 1 , 12 na2 &
- Sarah E. Berry ORCID: orcid.org/0000-0002-5819-5109 1 na2
Nature Medicine ( 2024 ) Cite this article
19k Accesses
306 Altmetric
Metrics details
- Disease prevention
Machine learning
Large variability exists in people’s responses to foods. However, the efficacy of personalized dietary advice for health remains understudied. We compared a personalized dietary program (PDP) versus general advice (control) on cardiometabolic health using a randomized clinical trial. The PDP used food characteristics, individual postprandial glucose and triglyceride (TG) responses to foods, microbiomes and health history, to produce personalized food scores in an 18-week app-based program. The control group received standard care dietary advice (US Department of Agriculture Guidelines for Americans, 2020–2025) using online resources, check-ins, video lessons and a leaflet. Primary outcomes were serum low-density lipoprotein cholesterol and TG concentrations at baseline and at 18 weeks. Participants ( n = 347), aged 41–70 years and generally representative of the average US population, were randomized to the PDP ( n = 177) or control ( n = 170). Intention-to-treat analysis ( n = 347) between groups showed significant reduction in TGs (mean difference = −0.13 mmol l −1 ; log-transformed 95% confidence interval = −0.07 to −0.01, P = 0.016). Changes in low-density lipoprotein cholesterol were not significant. There were improvements in secondary outcomes, including body weight, waist circumference, HbA1c, diet quality and microbiome (beta-diversity) ( P < 0.05), particularly in highly adherent PDP participants. However, blood pressure, insulin, glucose, C-peptide, apolipoprotein A1 and B, and postprandial TGs did not differ between groups. No serious intervention-related adverse events were reported. Following a personalized diet led to some improvements in cardiometabolic health compared to standard dietary advice. ClinicalTrials.gov registration: NCT05273268 .
Similar content being viewed by others

Long-term weight loss effects of semaglutide in obesity without diabetes in the SELECT trial

Two-year effects of semaglutide in adults with overweight or obesity: the STEP 5 trial

Associations of dietary patterns with brain health from behavioral, neuroimaging, biochemical and genetic analyses
Chronic diseases underpinned by diet and lifestyle exposures are among the leading causes of death globally. Diet and lifestyle strategies can be an effective approach to reduce risk for many chronic diseases 1 , 2 . However, despite evidence for the effectiveness of such approaches, rates of diet-related diseases continue to increase. This may in part be due to poor adherence to population guidelines and because of the large variability in how people respond to foods 3 , 4 , such that a single dietary approach is not the most effective for everyone. Indeed, in the United States, adherence to the Dietary Guidelines for Americans is well below the recommended levels for health 5 ; less than 1% of UK individuals follow all core nine dietary recommendations 6 . Furthermore, we now know the large intraindividual and interindividual variability observed in individual health responses to food are associated with multiple factors 3 . Therefore, personalized nutrition programs that are based on biological, phenotypic and lifestyle advice offer promise to improve both adherence and efficacy.
Observational research supports the application of personalized nutrition 7 , 8 but there are few randomized controlled trials designed to test the efficacy of personalized nutrition programs compared to standard dietary advice on health outcomes. Overall, dietary quality is improved by personalized nutrition programs tailored on baseline dietary information, phenotypic, genotypic or lifestyle factors, compared to nonpersonalized advice 9 . Personalization of dietary advice can assist and motivate individuals to follow a healthier diet and lifestyle 10 . Furthermore, a personalized diet integrating glycemic response, blood parameters, dietary habits, anthropometrics, physical activity and gut microbiota, resulted in greater improvements in markers of glycemic and lipemic control compared to a Mediterranean diet 11 . Personalized nutrition approaches and corresponding studies typically use a single axis of personalization but reported low correlations between biomarkers, for example, triglycerides (TGs) and glucose, suggesting that a prediction algorithm using a multilevel approach to personalization may yield superior results.
Therefore, we hypothesized that a multilevel approach to personalization encompassing multiple factors contributing to intraindividual and interindividual variability in nutritional responses to diet will improve the efficacy of advice to elicit a meaningful impact on health outcomes. This 18-week randomized controlled trial (the ZOE Measuring Efficacy THrough Outcomes of Diet (METHOD) study) assessed a personalized dietary program (PDP) underpinned by multiple biological inputs (glucose, TGs, microbiome and health history) and overlaid with generalized dietary and lifestyle advice (Fig. 1 ) versus the United States Department of Agriculture (USDA) recommended diet (control) on cardiometabolic risk and microbiome composition in a generally representative adult US population.
n = 177 participants were allocated to the PDP intervention group and n = 170 participants were allocated to the control group. DBS, dried blood spot finger-prick test. CGM, continuous glucose monitor. A Food Frequency Questionnaire (FFQ), accompanied by a dietary behavior survey, was administered. Anthropometry measures included waist circumference, hip circumference, height and body weight.
Study participant disposition
Between 1 March 2022 and 10 August 2022, 3,709 participants were screened for enrollment; 347 participants were randomly assigned to the PDP ( n = 177) or control ( n = 170) group and were included in the full analysis set (all randomized participants according to the intention-to-treat (ITT) principle). Of the 347 participants, n = 225 were included in the per-protocol analysis. Recruitment, randomization and follow-up numbers are summarized in the Consolidated Standards of Reporting Trials (CONSORT) diagram in Fig. 2 .
CONSORT, Consolidated Standards of Reporting Trials.
Participant characteristics are shown in Table 1 and were similar between groups at baseline. In total, 86% of participants were female and had a mean ± s.d. age of 52 ± 7.5 years, body mass index (BMI) of 34 ± 5.8 kg m − 2 , fasting serum glucose of 5.32 mmol l −1 (95% confidence interval (CI) = 5.25 to 5.40), fasting total cholesterol of 5.41 mmol l −1 (95% CI = 5.32 to 5.49), TG concentrations of 1.35 mmol l −1 (95% CI = 1.29 to 1.41) and low-density lipoprotein cholesterol (LDL-C) concentrations of 3.37 mmol l −1 (95% CI = 3.30 to 3.44). Compared to a US representative population (National Health and Nutrition Examination Survey 2017–2018), ZOE METHOD study participants had a similar waist circumference (104 cm versus 101 cm); in similarly aged individuals (40.0–69.9 years) they had a slightly higher BMI (BMI > 30 kg m − 2 ; 52% versus 41%) 12 .
Dietary intake
The composition of the participants’ habitual diets at baseline is shown in Supplementary Table 1 . Participants in both groups had a mean (95% CI) change in energy intake from baseline, with the PDP group reducing energy intake versus the control group (mean difference in change between groups 162 kcal per day (95% CI = 22.0 to 302), P < 0.001 for the interaction between diet group and time, adjusted for age and sex). In the PDP versus control diet, the mean 18-week macronutrient distributions were 39% versus 41% for carbohydrates, 46% versus 44% for fat and 16% versus 16% for protein. There were significant between-group differences at week 18 (all P ≤ 0.05) for the percentage of energy from carbohydrates, fat, polyunsaturated fatty acids, fiber and energy density (Supplementary Table 1 ). The PDP was a lower energy density diet compared to the control at week 18 (mean ± s.d., 1.67 ± 0.38 versus 1.87 ± 0.38 kcal g −1 , P < 0.001) (Fig. 3a ).
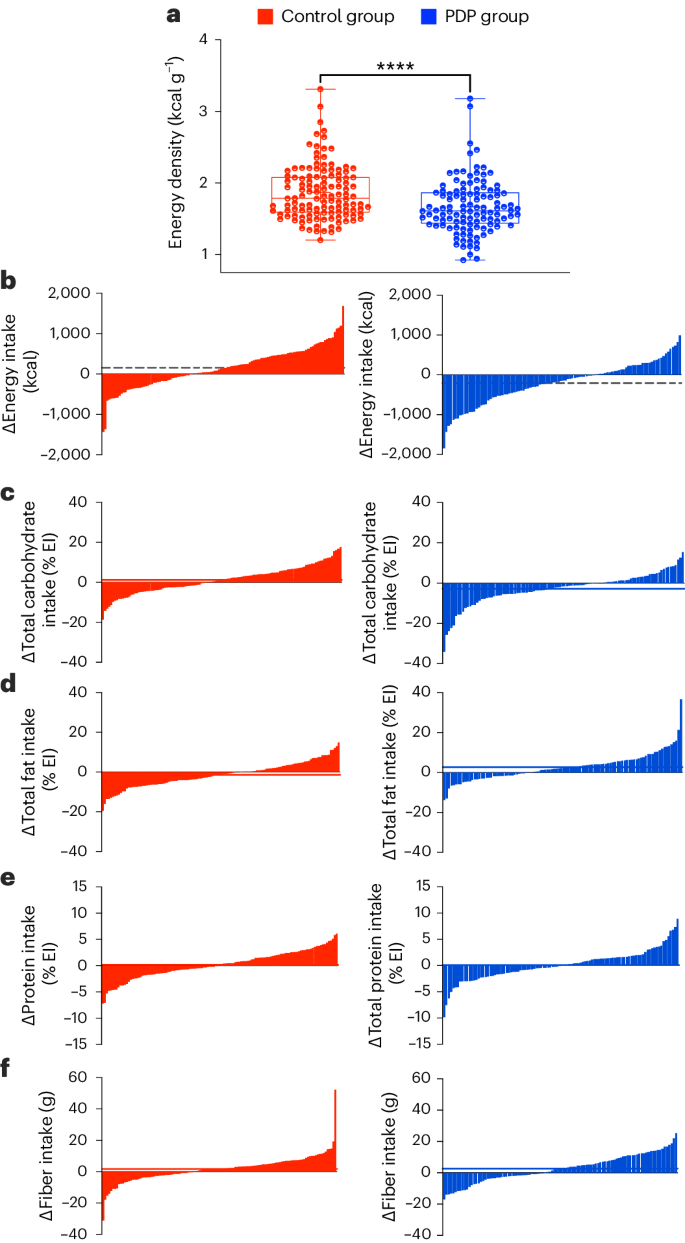
a , Mean energy density (kcal g −1 ) of the diet at the study endpoint for the control group (red) ( n = 120 participants with dietary data available) and PDP group (blue) ( n = 111 participants with dietary data available). An unpaired, two-sided, between-group t -test was used ( P < 0.001). Data presented include the first quartile, median and third quartile. b – f , Individual change in energy and nutrient intake before and after the intervention for energy intake (kcal) ( b ), carbohydrate (% EI) ( c ), fat (% EI) ( d ), protein (% EI) ( e ) and fiber (g) ( f ) intake across the control (red) and PDP (blue) groups.
To demonstrate interindividual variability in dietary intake achieved through personalized and general advice, we assessed the variability in nutrient and food intake. The individual changes in energy and nutrient intake before and after intervention were highly variable between participants following both interventions (Fig. 3b–f ). There was also large variability for individual foods and food groups at the study endpoint, as measured using the coefficient of variation (CV), following the control (mean CV = 262%) and PDP (mean CV = 248%).
Adherence to the advice
Participants in both interventions were asked to self-report adherence to the dietary advice using a questionnaire; 30% more participants reported high or very high subjective adherence (scores ≥ 8, respectively, on a 0–10 scale) to the dietary advice in the PDP versus control group. Participants in both the PDP and control groups with the greatest achieved improvement in overall diet quality (top 30th percentile of the Healthy Eating Index (HEI) score) increased diet quality by 12.9% (mean ± s.d.; 8.41 ± 8.47) and 6.15% (4.0 ± 6.59), respectively. In the PDP group, adherence to the program was also assessed through logging metrics and personalized day scores derived from logged diet data (Supplementary Table 2 ).
Primary outcomes
In the ITT cohort ( n = 347), there was a larger decrease in TGs after the PDP compared to controls at 18 weeks; the mean difference in changes between the groups was −0.13 mmol l −1 (log-transformed, 95% CI = −0.07 to −0.01, P = 0.016 for the interaction between diet group, time-adjusted for age and sex (unadjusted model P = 0.018)). The mean change from baseline after the PDP was −0.21 mmol l −1 (95% CI = −0.33 to −0.10); after the control diet, it was −0.07 mmol l −1 (95% CI = −0.15 to 0.02). Differences in LDL-C concentrations between groups were not significant: −0.04 mmol l −1 (95% CI = −0.16 to 0.08, P = 0.521 for the interaction between diet group and time, adjusted for age and sex (unadjusted model P = 0.504)). The mean change in LDL-C from baseline after the PDP diet was −0.01 mmol l −1 (95% CI = −0.08 to 0.09) and 0.04 mmol l −1 (95% CI = −0.05 to 0.13) for the control (Supplementary Tables 3 and 5 ). The changes in primary outcomes and weight and waist circumference over time are shown in Fig. 4a–d .
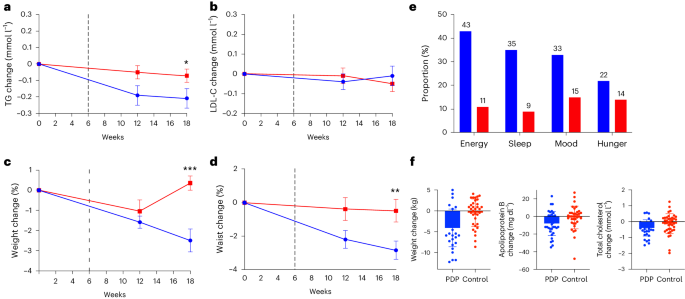
a – d , Mean ± s.e.m. changes from baseline values in TG (mmol l −1 ) ( P = 0.016) ( a ), LDL-C (mmol l −1 ) ( b ), weight (%) ( P < 0.001) ( c ) and waist circumference (%) ( P = 0.008) ( d ), in participants allocated to the PDP (blue line) ( n = 177) or control (red line) ( n = 172) group. Repeated measures model between groups. e , Proportion (%) of participants in the PDP and control groups with subjective improvements in energy level, sleep quality, mood, and hunger levels. f , Changes in weight (kg), apolipoprotein B (mg dl −1 ) and total cholesterol (mmol l −1 ) for highly adherent PDP ( n = 35) and controls ( n = 39) (mean and s.e.m. shown). * P < 0.05, ** P < 0.01, *** P < 0.001.
Secondary outcomes
Changes in secondary outcomes at the 18-week endpoint in the ITT cohort are shown in Fig. 4c,d (weight and waist circumference). Reductions in body weight, waist circumference and glycated hemoglobin (HbA1c), and increases in diet quality (HEI score), were significantly greater after the PDP than with the control diet; differences between treatments were as follows: body weight: −2.46 kg (95% CI = −3.67 to −1.25); waist circumference: −2.35 cm (95% CI = −4.07 to −0.63); HbA1c: −0.05% (95% CI = −0.01 to −0.001); and diet quality (HEI score): 7.08 (95% CI = 5.02 to 9.15). Hip circumference, blood pressure, insulin, glucose, C-peptide, apolipoproteins A1 and B, and postprandial TGs did not differ between the groups (Supplementary Table 3 ).
Within-group analysis of changes in PDP versus control were as follows: weight: −2.17 kg (95% CI = −3.03 to −1.31) versus 0.30 kg (95% CI = −0.56 to 1.15); waist circumference: −2.94 cm (95% CI = −4.17 to −1.71) versus −0.59 cm (95% CI = −1.81 to 0.63); HbA1c: −0.02% (95% CI = −0.05 to 0.01) versus 0.03% (95% CI = −0.01 to 0.07); and diet quality (HEI score): 7.01 (95% CI = 5.51 to 8.51) versus −0.08 (95% CI = −1.35 to 1.50) in the PDP and control group, respectively. Changes were not different for hip circumference, blood pressure, insulin, glucose, C-peptide, apolipoproteins A1 and B, and postprandial TGs.
Changes in total protein, albumin, globulin, bilirubin, alkaline phosphatase, aspartate aminotransferase, alanine aminotransferase, C-reactive protein, tumor necrosis factor alpha and full blood count were also compared between groups at 18 weeks. None of these blood measures differed between groups, apart from mean platelet volume and absolute lymphocyte concentrations (Supplementary Table 4 ).
Impact of dietary intervention on the gut microbiome
The Bray–Curtis dissimilarity index (beta-diversity) was used to assess the impact of the dietary interventions on the whole microbial composition in the two groups. Bray–Curtis dissimilarities were computed in individuals with longitudinal microbiome samples available. At week 12, individuals from both control and PDP groups showed higher beta-diversity with respect to their baseline microbiome composition (Fig. 5a , Wilcoxon rank-sum test, Wp < 0.01). This suggests that regardless of the assigned intervention group, a change in diet composition with respect to the individuals’ habitual diet impacted the whole microbiome composition (Table 1 and Fig. 3 ). Moreover, beta-diversity comparisons in the same individuals at weeks 12 and 18 suggested an increasing trend in the PDP group but not in the control group (Fig. 5a ); the median fold change of beta-diversity was greater at both weeks 12 and 18 in the PDP group than in the control group (Supplementary Table 5 ). Comparing beta-diversity dissimilarities across the control and PDP groups at week 18 showed a statistically significant difference (Kolmogorov–Smirnov stochasticity parameter, KSp = 0.04). In summary, the PDP intervention group showed a greater effect on the whole microbiome composition of different individuals, who were diverging more over time than the control group.
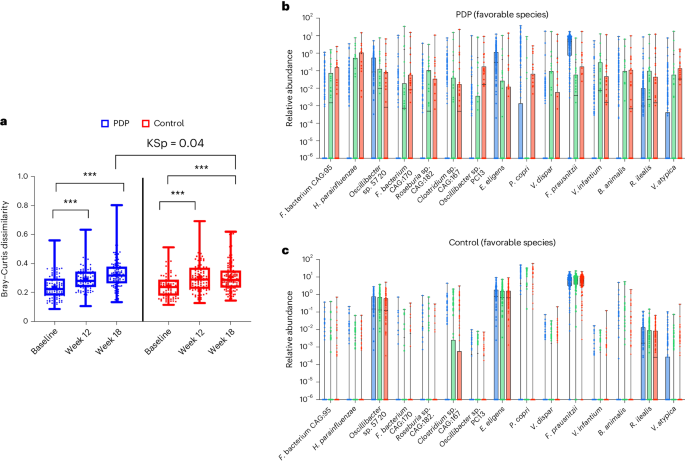
a , Bray–Curtis dissimilarity at baseline, week 12 and week 18, for the control (red) ( n = 118) and PDP (blue) ( n = 112) groups. Data presented include the first quartile, median and third quartile. KSp between treatment groups ( P = 0.04). *** P < 0.001 determined using a paired, one-sided Wilcoxon rank-sum test for within-individual change in Bray–Curtis dissimilarity. b , c , Relative abundance of favorable microbial species at baseline (blue), week 12 (green) and week 18 (red) for PDP ( n = 112) ( b ) and control ( n = 118) ( c ) groups (minimum to maximum shown).
To evaluate the impact of the dietary interventions on the whole microbiome composition, we used machine learning to assess the level of associations between changes at the species level with changes in the measured health markers at the endpoint ( Methods ). For this analysis, we used the same machine learning framework that we developed in our previous work 13 . The results showed that variations in the relative abundance of microbiome species effectively discriminated individuals based on their changes in weight and hip circumference in the PDP intervention group, but not in the control group (area under the curve (AUC) = 0.65 and 0.59 in PDP for weight and hip circumference, respectively, and 0.49 for both in control) (Supplementary Table 6 ).
Finally, we examined differences in terms of relative abundances for the 30 microbial species we previously identified associated with either ‘favorable’ or ‘unfavorable’ cardiometabolic health 13 between the two intervention groups. Notably, among the 15 favorable species, we found eight species in the PDP group showing an increase in terms of relative abundance at the endpoint (difference from baseline greater than 0); conversely, in the control group, none of the 15 favorable species showed an increased relative abundance at the endpoint (summed abundance change: 0.48 ± 9.05 versus −0.73 ± 8.63) (Mann–Whitney–Wilcoxon test, MWWp = 0.015) (Supplementary Table 7 ). For each of the 15 favorable species, we calculated the average fold change. We observed that 11 of the 15 favorable species showed a positive fold change in the PDP intervention group, while only four in the control group (Fig. 5b,c and Supplementary Table 7 ). In contrast, of the 15 previously identified unfavorable species, participants in the PDP or control intervention group did not exhibit differences in terms of changes in relative abundances (summed abundance change; 0.01 ± 3.67 versus 0.50 ± 3.43; Extended Data Fig. 1 and Supplementary Table 7 ). We also found that five of the 15 unfavorable species showed a decrease in fold change between the endpoint and baseline in the PDP group and four in the control group. As basic gut microbiome information, we calculated both species richness and Shannon alpha diversity measures and did not observe significant differences in the ITT group compared to the control group at week 18 (Supplementary Tables 3 ); because of increased taxonomic resolution availability, the value of these measures when interpreting the impact of a dietary modification or for host health is now unclear 14 .
Adverse events were reported to the study coordinator; they were reviewed by the principal investigator and medical director. All adverse events were documented in line with institutional review board (IRB) guidelines. There were four adverse events during the study. None were classified as severe. There were no withdrawals resulting from injury. There was one withdrawal due to an undisclosed food allergy (nut allergy) that precluded further participation in the study. After consuming the test muffin, the participant experienced mild itching of the tongue and throat, nausea and an upset stomach that resolved with oral diphenhydramine. Tree nuts are not an ingredient in the test muffins but they are produced in a facility that handles tree nuts. Symptoms were graded Common Terminology Criteria for Adverse Events Toxicity grade 1 and attributed as a probable allergic reaction to the muffin. The participant had to withdraw because they were unable to complete the test meals due to their undisclosed food allergy. The other three adverse events were bruising after a blood draw (toxicity grade 1), light-headedness at the time of blood draw (toxicity grade 1) and mild bleeding from the continuous glucose monitor (CGM) (toxicity grade 2) that quickly resolved on its own.
Energy, sleep quality, mood and hunger
As well as showing differences in clinical markers of cardiometabolic health, participants reported subjective changes in energy level, sleep quality, mood and hunger. On average, a greater proportion of PDP participants reported improvements in energy level (43% versus 11%), sleep quality (35% versus 9%), general mood (33% versus 15%) and reduced hunger levels (22% versus 14%) compared with controls ( P < 0.01 for all) (Fig. 4e ).
Post-hoc analyses
Per-protocol analysis revealed a larger change in TGs after the PDP intervention ( n = 108) compared to controls ( n = 117) at 18 weeks; the mean difference in changes between groups was −0.17 mmol l −1 (log-transformed 95% CI = −0.07 to −0.01; P = 0.032 for the interaction between diet group and time, adjusted for age and sex (unadjusted model P = 0.032)). The mean change in TGs from baseline after the PDP intervention was −0.23 mmol l −1 (95% CI = −0.33 to −0.12); after the control diet, it was −0.06 mmol l −1 (95% CI = −0.16 to 0.05). Differences in LDL-C concentration between groups remained nonsignificant at 0.05 mmol l −1 (95% CI = −0.08 to 0.19; P = 0.430 for the interaction between diet group and time, adjusted for age and sex (unadjusted model P = 0.43)). For the secondary outcomes, there was a greater difference in change between diet groups in the PDP cohort. Further reductions were observed in the PDP cohort for body weight −2.51 kg (95% CI = −3.79 to −1.23) as well as increases in diet quality (HEI score = 7.32 (95% CI = 5.08 to 9.55)) (Supplementary Table 8 ).
We performed subgroup analysis based on dietary adherence to determine whether highly adherent participants differed across treatment. We identified adherent control participants (top 30th percentile of participants based on the HEI score, a measure of adherence to USDA dietary guidelines) and compared them to adherent PDP participants (top 30th percentile of participants based on a personalized diet quality score). Greater changes in outcomes were observed in adherent PDP versus adherent control groups for weight (−4.09 ± 4.51 versus −0.44 ± 3.27 kg, P = 0.002), apolipoprotein B (−7.94 ± 13.7 versus −1.14 ± 12.8 mg dl −1 , P = 0.025) and total cholesterol (−0.40 ± 0.51 versus −0.13 ± 0.63 mmol l −1 , P = 0.047) (Supplementary Table 9 and Fig. 4f ). We also identified participants with low versus high baseline diet quality (HEI-derived top 30th percentile). When we compared changes in outcomes between these two groups, we saw no difference in any outcomes in the PDP group (high versus low baseline diet quality) or in the control group (high versus low baseline diet quality).
Given the variability in adherence, we also compared PDP participants across tertiles of adherence based on their average personalized diet quality scores throughout the intervention period. Higher adherence to the PDP was associated with greater change in several health outcomes versus low adherence to the PDP (Supplementary Table 9 ); greater reductions in LDL-C (−0.20 ± 0.48 versus 0.07 ± 0.56 mmol l −1 , P = 0.019), waist circumference (−6.31 ± 5.35 versus −1.42 ± 5.95 cm, P = 0.001), diastolic blood pressure (−4.08 ± 8.56 versus 2.71 ± 9.23 mmHg), HbA1c (−0.06 ± 0.20 versus 0.02 ± 0.12%, P = 0.024), total cholesterol (−0.4 ± 0.51 versus −0.01 ± 0.58 mmol l −1 , P = 0.002) and apolipoprotein A1 (−12.74 ± 26.2 versus 3.39 ± 15.6 mg dl −1 , P = 0.001) were observed between highly adherent ( n = 35) and low adherent ( n = 33) participants. The proportion of participants reporting improvements in subjective hunger levels (88.6% versus 66.7%, P = 0.015) was also greater in highly adherent PDP participants versus low adherent participants. Highly adherent PDP participants had an average weight loss of 4.7% versus 2.4% compared to low adherent participants ( P > 0.05). When PDP participants were stratified based on their baseline LDL-C concentration (unhealthy, 3.4 mmol l −1 or greater; healthy, less than 3.4 mmol l −1 ), those with unhealthy baseline levels showed decreasing trends in LDL-C across both adherence groups (low, −0.07 ± 0.18; high, −0.07 ± 0.13 mmol l −1 , P = 0.866), whereas in those with healthy baseline levels, only highly adherent participants had a significantly greater mean decrease (high, −0.03 ± 0.15 mmol l −1 , P = 0.008).
In this randomized, controlled trial of an 18-week dietary intervention in adults, when compared with US standard care dietary advice, a PDP intervention resulted in greater improvements in diet quality, which also resulted in greater reductions in TG concentration, weight, waist circumference and HbA1c, but not LDL-C. It also favorably shifted the gut microbiome composition, as well as subjective feelings of hunger, energy and mood, demonstrating another potential benefit of a PDP in improving overall health and well-being. Overall, these findings suggest that a dietary program focused on personalized advice is more effective in reducing central adiposity and TG concentrations than standard dietary advice in generally healthy individuals.
The PDP led to greater reductions in TG levels versus a control diet. While TGs improved, LDL-C did not differ between the groups at 18 weeks, similar to previous personalized nutrition evidence 11 . However, LDL-C was reduced in highly adherent PDP participants. When participants were further stratified based on their baseline LDL-C, those with unhealthy baseline levels (3.4 mmol l −1 or greater) showed decreasing trends in LDL-C across all adherence groups. While in participants with healthy baseline levels (less than 3.4 mmol l −1 ), only highly adherent participants had a significantly greater mean decrease. These findings suggest that high adherence to a PDP may reduce LDL-C in most participants; clearer effects may have been observed if conducted in participants with hyperlipidemia. These findings are not surprising as recent evidence showed that TG levels are more sensitive to nutritional intervention; additionally, LDL-C levels may not change with weight loss induced by dietary modification 4 , 15 . Livingstone et al. 16 demonstrated the efficacy of personalized nutrition in modifying dietary intakes depending on the clustering of adherence to dietary recommendations. Individuals with the poorest diets benefited the most from a personalized nutrition intervention. Conversely, in this study, we did not observe greater health improvements in participants with lower baseline diet quality. Additionally, we saw greater improvements in adherent PDP versus control participants, which may further support the effect of a personalized nutrition-based treatment independent of adherence and baseline diet.
Our findings support the application of this PDP over generalized guidance for the purpose of improving body weight and waist circumference, despite favorable dietary changes including increased fiber intake in the control group. Previous personalized approaches have not reported greater improvements in body weight on a personalized diet versus control. For example, the Food4Me European study of personalized nutrition 10 showed improved dietary behaviors (that is, HEI), but no significant differences in body weight at 6 months when compared with a nonpersonalized diet group. In addition, Ben-Yacov et al. 11 showed no differences in body weight between a postprandial targeting diet and a Mediterranean diet at 6 months, although both groups experienced weight loss. The Personal Diet Study 17 leveraged the predictive machine learning algorithm developed by Zeevi et al. 18 ; when compared to a standardized low-fat diet, they did not report differences for weight. The PREVENTOMICS study demonstrated no additional benefit of personalizing dietary plans based on metabolic clusters, over a control group, on the change in fat mass or body weight 19 . In our multilevel approach to personalization, the weight loss observed was moderate and below proposed clinically meaningful thresholds (5%) 20 ; however, moderate weight loss of this magnitude has been reported to improve health outcomes 21 . Additionally, evidence shows that the rate of weight loss observed, despite no calorie restriction advice, is likely to be sustained and meaningfully contribute to long-term health 22 . Furthermore, in highly adherent PDP participants weight loss was greater and closer to clinically meaningful levels (4.7%). Our waist circumference reduction was consistent with a magnitude associated with a reduction in cardiometabolic risk factors 23 , 24 , 25 , 26 , 27 . The small but statistically significant positive effect on body weight and waist circumference may reflect the impact of reducing multiple postprandial responses personalized to an individual and the greater satiating capacity reported by participants or lower energy density of the diet 28 .
The gut microbiome has a central role in human health and disease, specifically cardiometabolic health 29 , 30 . A bidirectional relationship exists between the microbiome and diet, whereby the gut microbiome affects host metabolism and response to foods 31 , and diet affects gut microbiome composition and functionality, which in turn exerts downstream effects on human health 32 . We demonstrated that the PDP diet had a greater and more sustained impact in shaping the whole gut microbiome composition. This change in microbiome composition was consistent with the greater change in diet quality (HEI) in the PDP group compared with controls. More specifically, we showed that the PDP diet induced favorable changes in species previously associated with favorable cardiometabolic health and diet 13 compared to controls. At the same time, it did not impact the contribution in the microbiome of previously reported unfavorable species that were instead increased in the control group. In agreement with previous evidence 33 , we showed that changes in microbiome composition of participants after the PDP were more predictive of weight loss and hip circumference than controls. We did not see clear differences in changes of measured gut richness; however, with the increased taxonomic resolution available from MetaPhlAn 3.0, previous research questioned whether this is a valid measure of host health 14 , 34 .
One criticism of personalized advice is that the resulting variance in nutrient intake is low and the advice pushes all individuals toward the same dietary pattern and similar changes in nutrient intake. For example, the study by Ben-Yacov et al. 11 , in adults with prediabetes, demonstrated that a personalized diet resulted in most participants adopting lower carbohydrate and higher protein and fat intakes compared with those randomized to a Mediterranean diet. In the PDP group, while we observed small average decreases in carbohydrate intake and increases in healthy fat (polyunsaturated fats) intake, we saw a large variation in nutrient intake and individual foods, which is not captured by the mean cohort intake. Because this PDP was developed using multiple inputs, including postprandial fat and glucose as well as the microbiome, it does not push all participants toward a low carbohydrate diet. However, we also acknowledge that the composition of foods is more nuanced than their nutrient composition, such that the matrix 35 , 36 and processing level of foods 37 can have major effects on health.
This study tested the first version of a prediction algorithm, developed in 2022, which could be further advanced by personalizing the generalized lifestyle and dietary habit advice that complement personalized diet scores. Evidence showed that health is affected by interlinked factors, including dietary intake, underlying physiological status and the interaction between diet and behaviors such as lifestyle, meal context, time of day, exercise and sleep. For example, we showed that poor sleep efficiency, later bedtimes (midpoint) and deviation from habitual sleep patterns are associated with poorer postprandial glycemic control 38 . Time of day or eating window duration also has implications for dietary responses 39 , 40 , 41 , such that eating later induces nocturnal glucose intolerance and reduces fatty acid oxidation and mobilization, independently of sleep 39 . Food and meal order, including consuming carbohydrates before protein and vegetables in a meal, contributes to elevated glycemic variability 42 . The protective effects of physical activity on responses are well established 43 , with evening exercise eliciting lower lipemic responses to high-sugar breakfasts the next day in postmenopausal females 44 . All of these factors present modifiable behavioral strategies and show the interaction between diet and behaviors. This suggests that a future PDP based on a more representative cohort (more than 100,000 participants) with personalization on lifestyle (for example, physical activity, sleep) and dietary behaviors might deliver even greater improvements in outcomes.
The strengths of this study include it being conducted in generally healthy middle-aged and older males and females broadly representative of the US population, not young healthy individuals. Although the average BMI for this cohort was 34 kg m − 2 , central obesity (using sex and ethnicity-specific waist circumference cutoffs) was representative of the average US population and participants were not receiving lipid-lowering or blood glucose-lowering medications (that is, statins and antidiabetic medications), which is a strength because evidence is lacking for prevention in populations without diabetes. The study was run remotely and participants were free-living, so the result is more reflective of real-world settings than a traditional clinical trial design approach.
Limitations include that we could not accurately capture changes in physical activity status. Furthermore, although reflective of how the advice is delivered in real life, the USDA recommended diet was delivered via leaflet and video, and was intentionally not matched for contact or intensity with the PDP group. These differences between treatments should be considered when interpreting the results. Future studies would benefit from assessing the impact of a personalized program versus personalized food scores. However, the control group slightly improved fiber intake and reduced fat consumption, and were aware that they were in a trial, which we know influences behavior. Although the PDP was well received by participants, and study participants were recruited from the general population, a larger study is required to capture more diverse ethnicities and for better gender representation. This study is also not applicable to children or old adults.
In conclusion, a personalized nutrition program that addresses metabolic heterogeneity is effective in improving cardiometabolic health in generally healthy individuals. The results demonstrates that a PDP underpinned by multiple biological inputs (glucose, TGs, microbiome, cardiovascular disease risk and health history) and overlaid with generalized dietary and lifestyle advice improves TG concentrations substantially more than a standard USDA diet and may contribute to the overall reduction in risk of cardiometabolic diseases.
Study design
The ZOE METHOD study was an 18-week parallel-design, randomized controlled trial. The trial was registered on ClinicalTrials.gov (ClinicalTrials.gov registration: NCT05273268 ) and listed as the ZOE METHOD Study: Comparing Personalized versus Generalized Nutrition Guidelines. The remote trial carried out in the US compared standard care dietary advice (control) versus a PDP in a cohort generally representative of the US adult population. Standard care dietary advice (United States Dietary Guidelines for Americans, 2020–2025) was delivered in the form of an USDA dietary recommendations digital leaflet, a short video lesson, access to online resources and regular check-ins. The PDP provided dietary advice using the ZOE 2022 algorithm, incorporating food characteristics, individuals’ glucose control and postprandial TG concentrations 3 , individuals’ microbiomes 13 , atherosclerotic cardiovascular disease risk and health history, to produce personalized food scores delivered during an 18-week program alongside more generalized nutrition and lifestyle education through a remote mobile phone application (the ZOE app). Ethical approval for the trial was obtained through the Advarra IRB (IRB no. 00000971; protocol no. 00044316). All participants provided written informed consent and the study was carried out in accordance with good clinical practice and the Declaration of Helsinki (2013). Outcome measurements were made at baseline and after randomization to their respective treatments.
Participant selection and randomization
Males and females reflective of the average US adult population (aged 40–70 years; waist circumference greater than ethnicity-specific and sex-specific 25th percentile values; fruit and vegetable intake below 450 g per day (to capture 75% of the population)) living in the US were recruited (1 March 2022 to 10 August 2022) by electronic advertisement (e-mail to the Stanford Nutrition Studies Research Cohort, the Empowered Gut newsletter and the ZOE Ltd mailing lists). Both sexes were eligible for recruitment and sex was determined using self-reported questionnaires with the following question: ‘What sex were you assigned at birth?’ Through the recruitment channels (e-mail and website), participants were invited to complete an online screening questionnaire and then invited to attend a primary baseline clinical visit (described in detail below) where all eligibility criteria were assessed. After this two-step screening process, participant eligibility was confirmed and a minimization-randomization program (MinimPy v.0.3, Python Package Index; pypi.org/project/MinimPy/ ) was used for treatment allocation. Participants were randomly and equally allocated to one of the two treatments based on the following minimization factors: (1) sex, male or female; (2) waist circumference, above or below their ethnicity-specific median; and (3) fruit and vegetable intake, above or below the median US adult intake of 234 g per day. Trained study coordinators enrolled, assigned and informed participants about their allocation to treatment via e-mail. Participants were informed of all study procedures before providing electronic consent. Participants were excluded from the study if any of the following criteria applied: had taken part in the ZOE product or any PREDICT study beforehand; were unable to read and write in English, as the ZOE app is only available in English; did not complete the first Quest visit successfully; had an iOS/Android device not compatible with the app; used medications affecting lipids (lipid-lowering drugs, for example, statins; antidiabetic medications, for example, metformin and insulin), and supplements including fish oil (unless willing to safely come off these for 4 weeks before the start of the study, and for the duration of study); had ongoing inflammatory disease, for example, rheumatoid arthritis, systemic lupus erythematosus, polymyalgia and other connective tissue diseases; had cancer in the last 3 years, excluding skin cancer; had chronic gastrointestinal disorders, including inflammatory bowel disease or celiac disease (gluten allergy), but not including irritable bowel syndrome; were taking the following daily medications: immunosuppressants, corticosteroids or antibiotics in the last 3 months, not including inhalers; were users of prescription proton pump inhibitors, such as omeprazole and pantoprazol, unless they were able to stop 2 weeks before the start of the study and remained off them for the entire duration of the study (provided their treating physician deemed it safe for them to do so); were currently suffering from acute clinically diagnosed depression or anxiety disorder; had a heart attack (myocardial infarction) or stroke in the last 6 months; were pregnant or planning pregnancy in next 12 months, or were breastfeeding; were vegan, had an eating disorder or were unwilling to take foods that were part of the study; had an allergy to adhesives, which would prevent proper attachment of the CGM.
Interventions and procedures
The study design is summarized in Fig. 1 .
Primary baseline testing (week −1)
Baseline clinical visit.
Participants attended a baseline clinical visit at the Quest Diagnostic Patient Service Center, where baseline measures were assessed, including a fasted venous blood draw, and anthropometric measurement of height, body weight, hip circumference, waist circumference and blood pressure. Participants who did not attend a clinic visit within 1 week of their visit date were withdrawn from the study.
Health questionnaire
Participants remotely completed two questionnaires administered through an online survey before randomization. These questionnaires included (1) a primary questionnaire capturing baseline health status and medical health history and (2) a secondary questionnaire capturing information on anthropometrics, sleep, energy level, mood, hunger, skin, female health (menopause) and current medication use.
Participant survey
A survey where participants confirmed completion of the primary baseline study tasks was administered at the end of week −1 to assess participant compliance.
Stool sample collection
Stool samples for microbiome analysis (required for the algorithm predictions) were collected by participants at home using the DNA/RNA SheildTM Fecal Collection Tube (Zymo Research) containing buffer (catalog no. R1101, Zymo Research). Once collected, the sample was stored at room temperature before being shipped to the analyzing laboratory inside a prepaid return kit.
Secondary baseline testing (week 0)
Baseline measures.
After allocation to treatment, both PDP and control groups completed a secondary set of baseline measurements, including fasted venous blood tests, questionnaires and stool collection as described in the primary baseline testing section. Approximately 1 week after their primary clinical visit, participants completed a secondary visit to the Quest center. Non-completion of this second visit within the required time period resulted in participant withdrawal from the study. In addition to this, participants completed an FFQ. The PREDICT FFQ, which captured information on 264 foods, food groups and beverages over the previous month was administered via an online survey 3 . For the control group, links to the FFQ were provided via e-mail. For the PDP group, links to the FFQ were provided via e-mail or via the ZOE app.
ZOE test kit
PDP participants were additionally asked to complete the ZOE test kit. This included (1) a CGM, (2) standardized test meals (three muffins) and (3) a DBS. Participants applied and wore a CGM (Freestyle Libre 2, Abbott) on their upper arm for up to 14 days. Two days after CGM application, participants completed 2 days of standardized meal intervention. Meals consisted of muffins with mixed macronutrient composition and were consumed for breakfast and lunch (day 1, as a sequential mixed meal intervention) and for breakfast only (day 2). Breakfast meals were consumed after an overnight fast of at least 8 h. Participants were asked to consume the entire portion of the meal provided within 15 min. The consumption of their meal was scanned in the app using the unique barcode labeled on each meal. The time participants started and completed eating their meal was recorded. They were asked to report any deviations from this protocol to study staff.
After the sequential test meal, a finger-prick DBS test was completed (6 h after breakfast to measure postprandial responses). Blood test cards were stored at room temperature until shipping to the analyzing laboratory via a prepaid return mailing kit. Finally, after completion of their test meals, participants were asked to log their habitual diet through the ZOE app. This app provided the functionality of a weighed food diary as well as a log of all the study tasks required of the participant during the ZOE test kit phase.
Dietary advice
Participants in the control group were e-mailed a PDF file containing a digital leaflet from the USDA Dietary Guidelines for Americans (2020–2025) accompanied by a video verbalizing the dietary advice, in accordance with a typical general consultation. In addition, participants were provided with online resources. Study coaches were available by e-mail to answer questions and provide support. The USDA guidelines recommend daily or weekly amounts from different food groups to maintain a healthy lifestyle. Participants were advised to follow this dietary advice for the study duration (weeks 2–18). Each week they received an e-mail from a study nutrition coach to check in.
PDP participants received generalized nutrition and lifestyle advice through the ZOE app, which they followed for 4 weeks (weeks 2–6), while personalized results were being generated via the ZOE 2022 algorithm (see Fig. 1 for more details). Generalized advice was presented via the app in the form of interactive ‘lessons’ as part of a program of learning. The lessons covered basic nutritional and dietary health concepts, including dietary diversification, increasing plant food consumption, increasing fiber intake, replacing refined carbohydrates with wholegrains and consumption of fermented foods.
At week 6, PDP participants received a personalized ‘Insights’ study report, including a personalized blood sugar score, blood fat score, gut diversity score, gut microbiome score and presence or absence of several microbial species 2 . These reports also included results from the ZOE 2022 algorithm, specifically information about person-specific food scores.
The interventions were not matched for contact or intensity to test the efficacy of the PDP, which involves personalized diet scores overlaid with generalized dietary and lifestyle advice delivered as a set of program lessons.
Personalized food quality scores
A personalized ZOE food quality score was computed using the ZOE 2022 algorithm for each food item consumed by the PDP participants. Food quality scores were based on both the macronutrients of a food item and further food metadata, including glycemic load, fat quality, level of processing and food group (for example meat, fruit, vegetables and fermented foods). They were personalized to an individual’s glucose control, postprandial TG concentration, atherosclerotic cardiovascular disease risk, health history and microbiome composition (abundance of specific health-promoting and health-reducing microbial taxa and the associations of these taxa with food items). The ZOE 2022 algorithm was trained using expert input on appropriate food quality scores for different individual phenotypes for a small number of foundational foods, and was used to predict personalized food quality scores for all individual phenotypes and all food items, which were then further personalized for detailed microbiome composition.
The food quality scores ranged from 0 to 100, with higher values indicating more healthful meals. Based on this food quality score, personalized recommendations could be made, that is, consume foods with a quality score of 0–24 once in a while, enjoy in moderation foods with a score of 25–49, enjoy foods regularly with a score of 50–74 and enjoy foods freely with a score of 75–100. A participant’s personalized meal scores throughout the day were combined by further algorithms to generate personalized day scores also ranging from 0 to 100. Throughout the study, participants were instructed to consume a diet (and record it in the app) reaching a certain day score threshold, which increased throughout the study duration, to the best of their ability. These day scores were accessible to participants, aiming to motivate them and convey to them their compliance to their dietary advice. The diet did not involve calorie restriction or calorie counting.
From week 6, PDP participants received personalized food scores and meal recommendations within the ZOE app. PDP participants were asked to attend a single phone or video call with a study staff member to discuss their results and to make these results immediately accessible to and actionable by the participant. Following this, a set of program lessons was administered in the app for 12 weeks (termed the ‘action plan’) during which participants were taught how to engage with and adhere to their personalized plan. Contact with study coaches was available via the app.
Week 12 measures
PDP and control groups completed a set of measures at week 12, including fasted venous blood tests (Quest visit), questionnaires, stool collection and FFQ as described in the primary and secondary baseline testing sections above.
Endpoint measures (week 18)
Endpoint data collection was completed in the 19th week of the study, at which point both groups had been allocated to their respective treatments for 18 weeks. PDP and control groups completed a set of endpoint measures, including fasted venous blood tests (Quest visit), questionnaires, stool collection and FFQ as described in the primary and secondary baseline testing sections above.
PDP participants were provided a second ZOE test kit to retest their nutritional responses, including application of a second CGM, consumption of the standardized meal intervention and completion of DBS.
Additional follow-ups
PDP participants were followed up at 8 and 12 months with a clinical visit, including fasted venous blood tests, questionnaires, stool collection and FFQ as described in the primary and secondary baseline testing sections above. Control participants were given the option to join a nested cross-over arm on completion of the 18-week endpoint measures. These participants completed the PDP arm protocol and completed the 6-, 12- and 18-week measures. Alternatively, control participants were offered the ZOE nutrition commercial product.
Participants were recruited from March 2022 to August 2022. The core intervention period took place from April 2022 to February 2023, and follow-ups were completed by September 2023.
As part of the study design, participants in both arms were asked to self-report adherence (scale 0–10) to the dietary advice given by the questionnaire administered every 6 weeks (week 7, week 12 and week 18 for the control group; week 12 and week 18 for the PDP group) during the study period. As part of the PDP only, participants were asked to record their dietary intake in real time on a minimum of four consecutive days (including one weekend day and 1,200 kcal or more per day) per month using a designated smartphone app (ZOE app). Each food item was recorded along with weight or portion units by selecting the food from a database (the USDA compositional database and a commercial database) containing approximately 900,000 items. Adherence to the PDP was evaluated through logging metrics and self-recorded dietary intake in the logging app.
Specified primary outcomes were serum TG concentration and direct LDL-C concentration. The primary outcome was the 18-week change from baseline. Therefore, secondary outcomes were changes in weight, waist circumference, hip circumference, systolic blood pressure and diastolic blood pressure, blood HbA1c, serum insulin, serum glucose, serum C-peptide, serum apolipoprotein A1, serum apolipoprotein B, fecal gut microbiome (species richness, Shannon diversity and Bray–Curtis dissimilarity), postprandial blood TG concentration, habitual diet quality (HEI) and self-reported energy level. Other outcomes included self-reported mood, hunger, total protein, albumin, globulin, bilirubin, alkaline phosphatase, aspartate aminotransferase, alanine aminotransferase, C-reactive protein, tumor necrosis factor alpha and full blood count.
DBS collection and processing
Postprandial TG (mmol l −1 ), high-density lipoprotein cholesterol (mmol l − 1 ) and cholesterol (mmol l −1 ) were quantified from finger-prick DBS (Clinical Reference Laboratory) tests completed by PDP participants in weeks 0 and 18 of the study (during completion of the ZOE test kit). DBS tests were completed 360 min after consuming the breakfast test meal. After washing their hands, participants pricked a finger with a sterile lancet and placed 3–4 drops of blood on their test card. Study staff assessed test validity using a photo and time point of testing logged by the participant in the app. Test cards not meeting the quality protocol (multiple small spots or inadequate coverage) were not included in the analysis. Participants were encouraged to complete the sequential test meal and DBS test again when either of these was inadequately completed. Each test card was stored in a foil pouch with a desiccant packet once completed and mailed to the analyzing laboratory in a prepaid kit within 24 h of completion.
Analysis was done at the Clinical Reference Laboratory. Advance Dx100 Technology DBS cards were analyzed for lipemic metabolites by the Clinical Reference Laboratory. Portions of test cards were taken from the sample, from which the dried blood was extracted and analyzed using standard quantification methods.
Fasted venous blood collection and processing
Fasted venous blood draws were performed at Quest Diagnostic Patient Service Centers and processed by Quest Diagnostics; 500 μl of venous blood was collected in serum separator tubes (SSTs). Then, 250 μl of venous blood was collected in EDTA tubes. SSTs and EDTA tubes were left at room temperature for 30 min (or up to 1 h) and centrifuged at 1,600 g for 15 min at 4 °C. Direct LDL-C, TG, glucose, insulin, C-peptide, apolipoprotein A1 and apolipoprotein B were quantified in serum (SST), and HbA1c was quantified in whole blood (EDTA). The full list of clinical blood chemistry measures quantified in this study are shown in Supplementary Table 10 .
Continuous glucose monitoring
Interstitial glucose was measured every minute and aggregated into 15-min readings, using the Freestyle Libre 14-day CGM (Abbott Diabetes Care). Participants randomized to the PDP group were instructed to apply the CGM two days before starting their standardized meal intervention, to the upper, nondominant arm and to cover the monitor with an adhesive patch (Sourceful) for improved durability. CGMs were worn for up to 14 days and participants were unblinded to the results. Given that the CGM device requires time to calibrate once applied, CGM data collected 12 h and onwards after activating the device was used for the analysis.
Fecal sampling and microbiome testing
Dna extraction and sequencing.
On receipt in the laboratory, samples were homogenized, aliquoted and stored at −80 °C in QIAGEN PowerBeads 1.5-ml tubes and used to extract bacterial DNA. All 815 stool samples were processed and analyzed using a Shotgun Metagenomic Sequencing Service (Zymo Research). The DNA was first isolated using the ZymoBIOMICS 96 MagBead DNA Kit (Zymo Research). Then, the sequencing libraries were prepared using the Illumina DNA Library Prep Kit with up to 500 ng DNA input according to the manufacturer’s protocol, using unique dual-index 10-bp barcodes with Nextera adapters (Illumina). The libraries were pooled in equal abundance and the final pools were quantified using quantitative PCR and a TapeStation (Agilent Technologies). The final libraries were sequenced using the NovaSeq 6000 platform (Illumina) according to the manufacturer’s protocols, generating 150-bp paired-end reads. The NovaSeq control software NCS v.1.5 was used. Image analysis, base calling and quality checking were performed with the Illumina data analysis pipeline RTA3.3.5 and bcl2fastq v.2.20.
Metagenome quality control and preprocessing
All sequenced metagenomes were preprocessed using the pipeline implemented in github.com/SegataLab/preprocessing . Briefly, the pipeline consisted of three steps: the first step involved read-level quality control and removed low-quality reads ( Q < 20), too-short reads (less than 75-bp long) and reads with more than two ambiguous nucleotides. The second step screened for contaminant DNA using Bowtie 2 (ref. 45 ) with the ‘--sensitive-local’ parameter, allowing confident removal of the phi X 174 Illumina spike-in and human-associated reads (hg19 reference human genome release). The last step consisted of splitting and sorting the cleaned reads to create standard forward, reverse and unpaired read output files for each metagenome (average: 35 ± 13 million reads per sample).
Microbiome taxonomic profiling
Species-level profiling of the 815 samples was performed with both MetaPhlAn 3.0 (ref. 34 ) and MetaPhlAn 4.0 (ref. 46 ). Default parameters were used for both versions of MetaPhlAn, while specific databases to each version were used, mpa_v30_CHOCOPhlAn_201901 and mpa_vJan21_CHOCOPhlAnSGB_202103 for version 3 and 4, respectively. MetaPhlAn 3.0 taxonomic profiles were used to assess the presence and contribution of the previously identified 15 positively associated and 15 negatively associated species with dietary and cardiometabolic health markers 2 . MetaPhlAn 4.0 taxonomic profiles were analyzed to compare microbial compositions between participants and to determine alpha diversity indices, the number of detected species (observed richness). Microbiome taxonomic profiles were also analyzed to compare between-microbiome-sample dissimilarity (beta-diversity) using the Bray–Curtis dissimilarity measure.
We used the same machine learning framework developed by Asnicar et al. 13 to assess the link of the microbiome compositions with the different dietary and metabolomic outcomes. Briefly, the machine learning framework is based on the random forest classification and regression algorithms and a 100-fold cross-validation approach with a 80/20 random splitting of the dataset. As training data, we used the differences in relative abundance between the 18-week and baseline time points of only microbial species. The classification task was evaluated using the area under the receiver operating characteristic curve, while the regression was evaluated by correlating the predicted values with the target values using the Spearman correlation coefficient.
Diet information
Participants completed the PREDICT FFQ online, at three separate time points throughout the study (0 weeks, 12 weeks and 18 weeks) to capture habitual dietary intake over the preceding month. The FFQ included 264 food and beverage items for which the participant selected frequency of consumption over the last month. Each survey item was accompanied by an USDA standard portion size, a textual description of the portion and a photograph of the item displayed on standard size tableware. The nutritional composition of each item was allocated according to the matching, or equivalent, item composition in the USDA database 47 ; US nutrient intake, including macronutrient and micronutrient data, was calculated per participant. Submitted FFQs were excluded if more than ten food items were left unanswered, or if the total energy intake estimate derived from the FFQ as a ratio of the individual’s estimated basal metabolic rate (determined using the Schofield et al.’s equation 48 ) was more than 2 s.d. outside the mean of this ratio (less than 0.15 or more than 2.04). Food energy density was calculated as the ratio between food energy (kcal) and food weight (g), excluding caloric (such as milk and juices) and noncaloric beverages 28 .
Adverse events were reported to the study coordinator, and were reviewed by the principal investigator and medical director. All adverse events were documented in line with IRB guidelines. The dietary intervention was anticipated to cause none to minimal discomfort. Some people may be affected by a small change in diet, for example, they may experience gas or bloating after eating the standardized test meals.
Sample size calculations
The study was powered on a sample size of 150 participants per group ( n = 300) at 90% power and P < 0.05, to detect a 0.21 mmol l −1 between-group difference in TG (endpoint change from baseline). An s.d. of 0.55 mmol l −1 was assumed on the basis of earlier data 49 . The same sample size was also powered to detect a 0.30 mmol l −1 change in LDL-C at 90% power and P < 0.05, assuming an s.d. of 0.8 mmol l −1 (ref. 49 ). Given two primary outcomes, statistical significance was defined by P < 0.025.
Statistical analysis
Analyses were carried out using v.4.0.2 of R and Python v.3.9.7. Pandas v.1.1.3, NumPy v.1.23.5 and SciPy v.1.11.1 were used to manage and preprocess data. Analyses of 18-week changes in primary and secondary outcomes were conducted based on an ITT ( n = 347). We conducted a per-protocol analysis using the data collected from participants who returned to their endpoint visit as prespecified in the protocol (18 ± 2 weeks) ( n = 225; 65% of the ITT cohort). An average of the two clinical blood chemistry baseline samples was used as the baseline measure for each participant. The primary outcome was the 18-week changes from baseline. The comparison between treatments in continuous variables over time was performed using repeated measures analysis ensuring that all ITT participants randomized with baseline information were included in the analysis and analyzed according to the original treatment assignment. The model evaluates the interaction between time (within-subject factor) and diet treatment (between-subject factor) with diet treatment, time, age and sex included as fixed effects along with a random effect for participants. The intervention effect was the coefficient for the interaction term in the model and the associated 95% CIs. The simple main effects of differences between the two diet groups were also assessed. For outcomes that were not normally distributed, outcomes were log 10 -transformed and tested for normality using the Shapiro–Wilk test. Given two primary outcomes, statistical significance was defined by P < 0.025. The between-group analysis was performed by a blinded researcher. Group allocation was concealed by labeling the groups with nonidentifying terms.
We assessed gut microbiome composition using species-level taxonomic profiles of participants with longitudinal sampling available. The ITT cohort was restricted to 118 and 112 individuals for the control and PDP groups, respectively. For each individual, we calculated the within beta-diversity using the Bray–Curtis dissimilarity index between the longitudinal samples available. For the baselines (week −1 or week 0), when two samples were available for the same individual, we considered the one with the highest number of preprocessed reads. As reference beta-diversity variability for comparison with the week 12 and week 18 samples, we considered the values calculated in each individual with the two baseline samples available (both week −1 and week 0). Bray–Curtis dissimilarities of the longitudinal samples of the same individuals between control and PDP groups were tested using a paired, one-sided Wilcoxon rank-sum test, while across-intervention groups were tested using a Kolmogorov–Smirnov stochasticity parameter (KSp). As we previously identified microbial bacterial species associated with favorable and unfavorable cardiometabolic risk markers 13 , we tested differences between the two intervention groups. We tested statistically significant differences in terms of relative abundance values for favorable and unfavorable species between groups using a Mann–Whitney–Wilcoxon test (MWWp) and reported the magnitude and direction of change using a log 2 fold change.
We performed a subgroup analysis based on dietary adherence to determine whether highly adherent participants differed across treatments. We identified adherent control participants (top 30% of participants based on the HEI score, a measure of adherence to USDA dietary guidelines) and compared them to adherent PDP participants (top 30% of participants based on a personalized diet quality score). Adherence to the ZOE program was classified based on a mean personalized diet score throughout the study duration. A minimum of 4 days of logged diet data meeting sex-specific caloric cutoffs (females, 500–5,000 kcal or more per day; males, 500–8,000 kcal or more per day) was required per month to ensure high quality and quantity logging. Low adherent participants were classified as the bottom 30th percentile of participants (mean personalized day score of 58 or lower); highly adherent participants were the top 30th percentile (mean personalized day scores of 67 or greater); moderately adherent participants fell in the middle (mean personalized day scores of 59–66). We also conducted a within-PDP analysis to investigate whether participants with good adherence (top 30%) to the PDP personalized dietary advice showed greater improvements in health outcomes compared to those with poor adherence (bottom 30%). Sex-based analysis was not performed because of small sample sizes. Excel v.16.82 and Microsoft Office were used for data and table formatting.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The study data can be released to bona fide researchers submitting a research proposal approved by a subpanel of our scientific advisory board. We have meetings once per month with independent members to assess proposals. The data will be anonymized and conform to UK General Data Protection Regulation standards. Access request proposals should be sent to [email protected]. The microbiome data will be uploaded onto the EBI website ( www.ebi.ac.uk/ ).
Code availability
The scripts for the statistical analysis are freely available upon request to ZOE Ltd. Application is via [email protected]. Code will be made available within 2 months of the request.
Micha, R. et al. Etiologic effects and optimal intakes of foods and nutrients for risk of cardiovascular diseases and diabetes: systematic reviews and meta-analyses from the Nutrition and Chronic Diseases Expert Group (NutriCoDE). PLoS ONE 12 , e0175149 (2017).
Article PubMed PubMed Central Google Scholar
Murray, C. J. L. et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396 , 1223–1249 (2020).
Article Google Scholar
Berry, S. E. et al. Human postprandial responses to food and potential for precision nutrition. Nat. Med. 26 , 964–973 (2020).
Article CAS PubMed PubMed Central Google Scholar
Gardner, C. D. et al. Effect of low-fat vs low-carbohydrate diet on 12-month weight loss in overweight adults and the association with genotype pattern or insulin secretion: the DIETFITS randomized clinical trial. JAMA 319 , 667–679 (2018).
What We Eat in America, National Health and Nutrition Examination Survey (2017–2018) (National Center for Health Statistics, 2021); https://www.ars.usda.gov/ARSUserFiles/80400530/pdf/1718/Key%20Points%20Using%20WWEIA%20NHANES%202017-2018.pdf
Scheelbeek, P. et al. Health impacts and environmental footprints of diets that meet the Eatwell Guide recommendations: analyses of multiple UK studies. BMJ Open 10 , e037554 (2020).
Ordovas, J.M., Ferguson, L. R., Tai, E. S. & Mathers, J. C. Personalised nutrition and health. BMJ 361 , bmj.k2173 (2018).
Betts, J. A. & Gonzalez, J. T. Personalised nutrition: what makes you so special? Nutr. Bull. 41 , 353–359 (2016).
Jinnette, R. et al. Does personalized nutrition advice improve dietary intake in healthy adults? A systematic review of randomized controlled trials. Adv. Nutr. 12 , 657–669 (2021).
Article CAS PubMed Google Scholar
Celis-Morales, C., Lara, J. & Mathers, J. C. Personalising nutritional guidance for more effective behaviour change. Proc. Nutr. Soc. 74 , 130–138 (2015).
Article PubMed Google Scholar
Ben-Yacov, O. et al. Personalized postprandial glucose response-targeting diet versus Mediterranean diet for glycemic control in prediabetes. Diabetes Care 44 , 1980–1991 (2021).
Li, M., Gong, W., Wang, S. & Li, Z. Trends in body mass index, overweight and obesity among adults in the USA, the NHANES from 2003 to 2018: a repeat cross-sectional survey. BMJ Open 12 , e065425 (2022).
Asnicar, F. et al. Microbiome connections with host metabolism and habitual diet from 1,098 deeply phenotyped individuals. Nat. Med. 27 , 321–332 (2021).
Thomas, A. M. et al. Metagenomic analysis of colorectal cancer datasets identifies cross-cohort microbial diagnostic signatures and a link with choline degradation. Nat. Med. 25 , 667–678 (2019).
Chawla, S., Tessarolo Silva, F., Amaral Medeiros, S., Mekary, R. A. & Radenkovic, D. The effect of low-fat and low-carbohydrate diets on weight loss and lipid levels: a systematic review and meta-analysis. Nutrients 12 , 3774 (2020).
Livingstone, K. M. et al. Clustering of adherence to personalised dietary recommendations and changes in healthy eating index within the Food4Me study. Public Health Nutr. 19 , 3296–3305 (2016).
Popp, C. J. et al. Effect of a personalized diet to reduce postprandial glycemic response vs a low-fat diet on weight loss in adults with abnormal glucose metabolism and obesity: a randomized clinical trial. JAMA Netw. Open 5 , e2233760 (2022).
Zeevi, D. et al. Personalized nutrition by prediction of glycemic responses. Cell 163 , 1079–1094 (2015).
Aldubayan, M. A. et al. A double-blinded, randomized, parallel intervention to evaluate biomarker-based nutrition plans for weight loss: the PREVENTOMICS study. Clin. Nutr. 41 , 1834–1844 (2022).
Williamson, D. A., Bray, G. A. & Ryan, D. H. Is 5% weight loss a satisfactory criterion to define clinically significant weight loss? Obesity 23 , 2319–2320 (2015).
Macek, P. et al. A two-year follow-up cohort study-improved clinical control over CVD risk factors through weight loss in middle-aged and older adults. J. Clin. Med. 9 , 2904 (2020).
Jensen, M. D. et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society. Circulation 129 , S102–S138 (2014).
O’Donoghue, G., Blake, C., Cunningham, C., Lennon, O. & Perrotta, C. What exercise prescription is optimal to improve body composition and cardiorespiratory fitness in adults living with obesity? A network meta‐analysis. Obes. Rev. 22 , e13137 (2021).
Wewege, M., van den Berg, R., Ward, R. E. & Keech, A. The effects of high‐intensity interval training vs. moderate‐intensity continuous training on body composition in overweight and obese adults: a systematic review and meta‐analysis. Obes. Rev. 18 , 635–646 (2017).
Thorogood, A. et al. Isolated aerobic exercise and weight loss: a systematic review and meta-analysis of randomized controlled trials. Am. J. Med. 124 , 747–755 (2011).
Schwingshackl, L., Dias, S., Strasser, B. & Hoffmann, G. Impact of different training modalities on anthropometric and metabolic characteristics in overweight/obese subjects: a systematic review and network meta-analysis. PLoS ONE 8 , e82853 (2013).
Morze, J. et al. Impact of different training modalities on anthropometric outcomes in patients with obesity: a systematic review and network meta‐analysis. Obes. Rev. 22 , e13218 (2021).
Ello-Martin, J. A., Roe, L. S., Ledikwe, J. H., Beach, A. M. & Rolls, B. J. Dietary energy density in the treatment of obesity: a year-long trial comparing 2 weight-loss diets. Am. J. Clin. Nutr. 85 , 1465–1477 (2007).
Cani, P. D. Human gut microbiome: hopes, threats and promises. Gut 67 , 1716–1725 (2018).
Talmor-Barkan, Y. et al. Metabolomic and microbiome profiling reveals personalized risk factors for coronary artery disease. Nat. Med. 28 , 295–302 (2022).
Sonnenburg, J. L. & Bäckhed, F. Diet–microbiota interactions as moderators of human metabolism. Nature 535 , 56–64 (2016).
David, L. A. et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505 , 559–563 (2014).
Ben-Yacov, O. et al. Gut microbiome modulates the effects of a personalised postprandial-targeting (PPT) diet on cardiometabolic markers: a diet intervention in pre-diabetes. Gut 72 , 1486–1496 (2023).
Beghini, F. et al. Integrating taxonomic, functional, and strain-level profiling of diverse microbial communities with bioBakery 3. eLife 10 , e65088 (2021).
Sanders, M. E. & Marco, M. L. Food formats for effective delivery of probiotics. Annu. Rev. Food Sci. Technol. 1 , 65–85 (2010).
Aguilera, J. M. The food matrix: implications in processing, nutrition and health. Crit. Rev. Food Sci. Nutr. 59 , 3612–3629 (2019).
Forde, C. G. & Bolhuis, D. Interrelations between food form, texture, and matrix influence energy intake and metabolic responses. Curr. Nutr. Rep. 11 , 124–132 (2022).
Tsereteli, N. et al. Impact of insufficient sleep on dysregulated blood glucose control under standardised meal conditions. Diabetologia 65 , 356–365 (2022).
Gu, C. et al. Metabolic effects of late dinner in healthy volunteers—a randomized crossover clinical trial. J. Clin. Endocrinol. Metab. 105 , 2789–2802 (2020).
Isherwood, C. M., van der Veen, D. R., Hassanin, H., Skene, D. J. & Johnston, J. D. Human glucose rhythms and subjective hunger anticipate meal timing. Curr. Biol. 33 , 1321–1326 (2023).
Hutchison, A. T. et al. Time‐restricted feeding improves glucose tolerance in men at risk for type 2 diabetes: a randomized crossover trial. Obesity 27 , 724–732 (2019).
Shukla, A. P. et al. The impact of food order on postprandial glycaemic excursions in prediabetes. Diabetes Obes. Metab. 21 , 377–381 (2019).
Edinburgh, R. M., Betts, J. A., Burns, S. F. & Gonzalez, J. T. Concordant and divergent strategies to improve postprandial glucose and lipid metabolism. Nutr. Bull. 42 , 113–122 (2017).
Shah, M. et al. Effect of a late afternoon/early evening bout of aerobic exercise on postprandial lipid and lipoprotein particle responses to a high-sugar meal breakfast the following day in postmenopausal women: a randomized cross-over study. J. Sports Sci. 40 , 175–184 (2022).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9 , 357–359 (2012).
Blanco-Míguez, A. et al. Extending and improving metagenomic taxonomic profiling with uncharacterized species using MetaPhlAn 4. Nat. Biotechnol. 41 , 1633–1644 (2023).
Pehrsson P. et al. USDA Branded Food Products Database (USDA Agriculture Research Service, 2018). Accessed October 2022; https://data.nal.usda.gov/dataset/usda-branded-food-products-database
Schofield, W. N., Schofield, C. & James, W. P. T Basal Metabolic Rate: Review and Prediction, Together with an Annotated Bibliography of Source Material (J. Libbey, 1985) .
Reidlinger, D. P. et al. How effective are current dietary guidelines for cardiovascular disease prevention in healthy middle-aged and older men and women? A randomized controlled trial. Am. J. Clin. Nutr. 101 , 922–930 (2015).
Download references
Acknowledgements
We thank the participants of the METHOD study. We also thank D. Sleeper, M. Reardon, A. Platts, A. Houston, C. Jennings and R. Donnellan for their valuable work on data collection. This research was funded by ZOE Ltd; the study funder contributed, as part of the scientific advisory board, to study design, data collection and analysis, and the writing of the manuscript.
Author information
These authors contributed equally: Kate M. Bermingham, Inbar Linenberg.
These authors jointly supervised this work: Nicola Segata, Tim D. Spector, Sarah E. Berry.
Authors and Affiliations
Department of Nutritional Sciences, King’s College London, London, UK
Kate M. Bermingham, Inbar Linenberg, Wendy L. Hall, Tim D. Spector & Sarah E. Berry
Zoe Ltd, London, UK
Kate M. Bermingham, Inbar Linenberg, Lorenzo Polidori, Alberto Arrè, Jonathan Wolf, Fatema Badri, Hannah Bernard, Joan Capdevila, William J. Bulsiewicz, Richard Davies & George Hadjigeorgiou
Department of Cellular, Computational and Integrative Biology, University of Trento, Trento, Italy
Francesco Asnicar & Nicola Segata
Emory University School of Medicine, Atlanta, GA, USA
William J. Bulsiewicz
Stanford University, Stanford, CA, USA
Christopher D. Gardner
Jean Mayer USDA Human Nutrition Research Center on Aging, Tufts University, Boston, MA, USA
Jose M. Ordovas
IMDEA Food Institute, Campus of International Excellence, Universidad Autónoma de Madrid, Consejo Superior de Investigaciones Científicas, Madrid, Spain
Universidad Camilo José Cela, Madrid, Spain
Diabetes Center, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA
Linda M. Delahanty
School of Medicine, University of Nottingham, Nottingham, UK
Ana M. Valdes
Nottingham National Institute for Health and Care Research Biomedical Research Centre, Nottingham, UK
Department of Twin Research and Genetic Epidemiology, King’s College London, London, UK
Tim D. Spector
You can also search for this author in PubMed Google Scholar
Contributions
J.W., G.H. and T.D.S. obtained the funding. I.L., K.B., C.D.G., J.M.O., S.E.B., A.M.V., J.W., G.H., R.D., N.S. and T.D.S. contributed to study design and developed the study concept. S.E.B., I.L., H.B. and F.B. collected the data. K.M.B., L.P., F.A., A.A., R.D., J.C. and N.S. analyzed the data. S.E.B., W.J.B., T.D.S., G.H. and J.W. coordinated the study. K.M.B., S.E.B., A.M.V., J.W., J.C., I.L., J.M.O., C.D.G., W.L.H., L.M.D., N.S. and T.D.S. wrote the manuscript. All authors reviewed and revised the final manuscript.
Corresponding author
Correspondence to Sarah E. Berry .
Ethics declarations
Competing interests.
T.D.S., J.W. and G.H. are co-founders of ZOE Ltd. F.A., L.M.D., A.M.V., W.L.H., N.S., T.D.S. and S.E.B. are consultants to ZOE Ltd. K.M.B., I.L., L.P., A.A., J.W., F.B., H.B., J.C., W.J.B., R.D. and G.H. are or have been employees of ZOE Ltd. K.M.B., I.L., L.P., A.A., J.W., F.B., H.B., J.C., W.J.B., R.D., G.H., L.M.D., A.M.V., N.S., T.D.S. and S.E.B. have received options in ZOE Ltd. A.M.V., C.D.G., L.M.D., J.M.O. and N.S. are members of the Scientific Advisory Board of ZOE Ltd.
Peer reviews
Peer review information.
Nature Medicine thanks Josef Neu and the other anonymous reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Ming Yang, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended data fig. 1 relative abundance of microbial species..
Relative abundance of the 15 favourable and unfavourable microbial species at baseline (blue), week-12 (green) and week-18 (red) for A) PDP (favourable species), B) Control (favourable species), C) PDP (unfavourable species), D) Control (unfavourable species). PDP, n = 112 and Control, n = 118 (Min to Max presented).
Supplementary information
Supplementary information.
Supplementary Tables 1–10.
Reporting Summary
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Bermingham, K.M., Linenberg, I., Polidori, L. et al. Effects of a personalized nutrition program on cardiometabolic health: a randomized controlled trial. Nat Med (2024). https://doi.org/10.1038/s41591-024-02951-6
Download citation
Received : 18 October 2023
Accepted : 26 March 2024
Published : 08 May 2024
DOI : https://doi.org/10.1038/s41591-024-02951-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

IMAGES
VIDEO
COMMENTS
Characteristics of a Primary Research Article. Goal is to present the result of original research that makes a new contribution to the body of knowledge; Sometimes referred to as an empirical research article; Typically organized into sections that include: Abstract, Introduction, Methods, Results, Discussion/Conclusion, and References.
Dec 11, 2017. There are different types of scholarly literature. Some of these require researchers to conduct an original study, whereas others can be based on previously published research. Understanding each of these types and also how they differ from one another can be rather confusing for researchers, especially early career researchers.
In a primary article, the authors are the same people who conducted the research and are writing about what they learned firsthand. In a review article, the authors are asking a question and answering it by reading primary articles written on this topic - they report on the results of experiments/studies secondhand, which is why review articles ...
Distinguishing Between Research and Review Papers. Both research and review papers are used for academic purposes, however they differ in purpose and writing style. The primary difference between the two is that a research paper involves original investigation of a subject, while a review paper summarises already-existing information about it.
Research vs Review Articles. It's often difficult to tell the difference between original research articles and review articles. Here are some explanations and tips that may help: "Review articles are often as lengthy or even longer that original research articles. What the authors of review articles are doing in analysing and evaluating current research and investigations related to a ...
Here are four key differences between research papers and review papers: Purpose: Review papers evaluate existing research, identify trends, and discuss the current state of knowledge on a specific topic; they are based on the study of previously published literature. On the other hand, research paperscontain original research work undertaken ...
An ideal review article should be logically structured and efficiently utilise illustrations, in the form of tables and figures, to convey the key findings and relationships in the study. According to Tay , illustrations often take a secondary role in review papers when compared to primary research papers which are focused on illustrations ...
Finding Review and Research Papers in PubMed. Many databases have special features that allow the searcher to restrict results to articles that match specific criteria. In other words, only articles of a certain type will be displayed in the search results. These "limiters" can be useful when searching for research or review articles.
A primary research or study is an empirical research that is published in peer-reviewed journals. Some ways of recognizing whether an article is a primary research article when searching a database: 1. The abstract includes a research question or a hypothesis, methods and results. 2. Studies can have tables and charts representing data findings. 3.
The Difference Between Primary Research Articles and Review Articles. ... Imagine these examples of a primary research article and a review article - let's say you are a scientist who is an expert in your field. ... Over time let's say your paper is widely recognized as a landmark in your field. Subsequently other experts write a review article ...
Review articles, sometimes called literature reviews or secondary sources, synthesize or analyze research already conducted in primary sources. They generally summarize the current state of research on a given topic. Here is a more detailed explanation of review articles. The video above was created by the Virginia Commonwealth University ...
The peer review process is essential for evaluating the quality of scholarly works, suggesting corrections, and learning from other authors' mistakes. The principles of peer review are largely based on professionalism, eloquence, and collegiate attitude. As such, reviewing journal submissions is a privilege and responsibility for 'elite ...
Review articles serve a different purpose than primary articles. They are written to summarize and synthesize studies by others on the same topic. This provides the research community with one article that summarizes the background and context as well as an overview of what is already known about a specific topic.
Simply limiting your search results in a database to "peer-reviewed" will not retrieve a list of only primary research studies. Learn to recognize the parts of a primary research study. Terminology will vary slightly from discipline to discipline and from journal to journal. However, there are common components to most research studies. STEP ONE:
What is a Review Article? Review articles do not report new experiments. Rather, they attempt to provide a thorough review of a specific subject by assessing either all or the best available scholarly literature on that topic. By considering the findings of many primary research articles, review articles can provide a comprehensive background and analysis of all the available evidence on a ...
Here are some key differences between review articles and research articles: In summary, research articles and review articles serve different purposes in the academic literature. Research articles present original research findings based on a specific research question or hypothesis, while review articles summarize and analyze existing ...
Review articles are the most common type of secondary research article in the health sciences. A review article is a summary of previously published research on a topic. Authors who are writing a review article will search databases for previously completed research and summarize or synthesize those articles, as opposed to recruiting ...
Peer review is defined as "a process of subjecting an author's scholarly work, research or ideas to the scrutiny of others who are experts in the same field" ( 1 ). Peer review is intended to serve two purposes: It acts as a filter to ensure that only high quality research is published, especially in reputable journals, by determining the ...
Research articles follow a particular format. Look for: A brief introduction will often include a review of the existing literature on the topic studied, and explain the rationale of the author's study.; A methods section, where authors describe how they collected and analyzed data.Statistical analysis are included. A results section describes the outcomes of the data analysis.
Primary vs. Secondary Research Articles. In the sciences, primary (or empirical) research articles: are original scientific reports of new research findings (Please note that an original scientific article does not include review articles, which summarize the research literature on a particular subject, or articles using meta-analyses, which ...
The research paper will be based on the analysis and interpretation of this data. A review article or review paper is based on other published articles. It does not report original research. Review articles generally summarize the existing literature on a topic in an attempt to explain the current state of understanding on the topic.
Primary research is any research that you conduct yourself. It can be as simple as a 2-question survey, or as in-depth as a years-long longitudinal study. The only key is that data must be collected firsthand by you. Primary research is often used to supplement or strengthen existing secondary research.
A systematic review is a summary of research that addresses a focused clinical question in a systematic, reproducible manner. In order to provide the single best estimate of effect in clinical decision making, primary research studies are pooled together and then filtered through an inclusion/exclusion process. The relevant data and findings ...
Voices in Bioethics is currently seeking submissions on philosophical and practical topics, both current and timeless. Papers addressing access to healthcare, the bioethical implications of recent Supreme Court rulings, environmental ethics, data privacy, cybersecurity, law and bioethics, economics and bioethics, reproductive ethics, research ethics, and pediatric bioethics are sought.
Differences in LDL-C concentration between groups remained nonsignificant at 0.05 mmol l −1 (95% CI = −0.08 to 0.19; P = 0.430 for the interaction between diet group and time, adjusted for age ...
Currently, climate change and global warming have significantly impacted human life. In the context of sustainable development, achieving the goals of the Paris Agreement is both urgent and complex. This paper presents a comprehensive review of climate policies worldwide. Based on the global comprehensive climate policy database that we constructed and using global panel data from 1990 to 2019 ...