- Case report
- Open access
- Published: 15 January 2021

Beyond the guidelines management of juvenile idiopathic arthritis: a case report of a girl with polyarticular disease refractory to multiple treatment options and Leri Weill syndrome
- Vana Vukić 1 ,
- Ana Smajo 1 ,
- Mandica Vidović 2 ,
- Rudolf Vukojević 3 ,
- Miroslav Harjaček 1 , 2 &
- Lovro Lamot 1 , 2
BMC Pediatrics volume 21 , Article number: 40 ( 2021 ) Cite this article
2247 Accesses
4 Citations
1 Altmetric
Metrics details
The last two decades brought new treatment options and high quality guidelines into the paediatric rheumatologic practice. Nevertheless, a number of patients still present a diagnostic and therapeutic challenge due to combination of vague symptoms and unresponsiveness to available treatment modalities.
Case presentation
We report a case of sixteen years old girl suffering from polyarticular type of juvenile idiopathic arthritis refractory to multiple treatment options. She first presented at the age of 4 with swelling and contractures of both knees. Her symptoms were initially unresponsive to nonsteroidal anti-inflammatory drugs and progressed despite treatment with intraarticular and systemic glucocorticoids and methotrexate. Throughout the years, she received several biologics together with continuous administration of nonsteroidal anti-inflammatory drugs and disease modifying anti-rheumatic drugs as well as intraarticular and systemic glucocorticoids in disease flares. However, none of this options provided a permanent remission, so various other modalities, as well as other possible diagnoses were constantly being considered. Eventually she became dependent on a daily dose of systemic glucocorticoids. In 2018, the treatment with Janus kinase inhibitor tofacitinib was initiated, which led to gradual amelioration of musculoskeletal symptoms, improvement of inflammatory markers and overall well-being, as well as to the weaning of systemic glucocorticoids. As the swelling of the wrists subsided for the first time in many years, Madelung’s deformity was noticed, first clinically, and later radiographically as well. Genetic analysis revealed short-stature homeobox gene deficiency and confirmed the diagnosis of Leri Weill syndrome.
Conclusions
This case report emphasizes the need for reporting refractory, complicated cases from everyday clinical practice in order to build-up the overall knowledge and share experience which is complementary to available guidelines. Individual reports of difficult to treat cases, especially when additional diagnoses are involved, can be helpful for physicians treating patients with common rheumatological diseases such as juvenile idiopathic arthritis.
Peer Review reports
Joint pain and/or swelling with limited range of motion is a common manifestation of many paediatric diseases, most notably wide range of rheumatic conditions. If both of these symptoms are present for longer than 6 weeks in a patient younger than 16 years of age, a diagnosis of juvenile idiopathic arthritis (JIA), the most common childhood rheumatic disease, should be considered [ 1 ]. JIA is heterogenous disease that encompasses different subtypes of childhood arthritis defined depending on the number of affected joints and/or presence of the enthesitis and/or sacroiliitis. Nevertheless, alongside JIA, there is a wide range of loosely related noninflammatory causes of a swollen joint in children, especially in the absence of clinical signs of inflammation. Lysosomal storage diseases (LSD) such as mucopolysaccharidosis type I (MPS I), Gaucher disease type I and Fabry disease all have prominent musculoskeletal symptoms early in the course of the disease, and are often first seen by a pediatric rheumatologist [ 2 , 3 , 4 , 5 , 6 , 7 , 8 ]. However, the underlying mechanism of those disorders does not directly involve the immune mediated inflammatory response, but rather an inflammation caused by genetic defects and subsequent perturbations at the protein level. More specifically, in Gaucher disease, bone marrow infiltration with histiocytes causes acute attacks of pain, which may be mistaken for arthritis in the vicinity of a joint [ 5 , 8 ]. In Fabry disease, episodes of neuropathic pain in hands, feet, wrists, ankles (acroparesthesias), often associated with fever, malaise and elevated inflammation markers, can mimic a rheumatic condition, such as an inflammatory arthritis [ 3 , 9 ]. Finally, joint stiffness and contractures are characteristic for some types of MPS, the so called „attenuated“ forms like Hurler-Scheie syndrome, which have a less severe presentation and progress silently over the years, making the diagnosis a challenge [ 3 , 10 , 11 ]. On the other hand, some congenital conditions, such as Madelung and Madelung-type deformities resulting from the premature closure of the medial volar aspect of the distal radial physis, might cause similar symptoms [ 12 ]. Hence, it is not a rarity that some of the children with LSD and congenital deformities are treated for prolonged periods of time as having an inflammatory arthritis, despite the lack of appropriate response [ 13 ].
With regard to the treatment of JIA, various modalities emerged over the last two decades, revolutionizing the pediatric rheumatology practice [ 14 ]. Majority of the presently available guidelines recommend a step-up approach, starting with nonsteroidal anti-inflammatory drugs (NSAID) and intraarticular glucocorticoid injections (IAGI), followed by conventional and biologic disease modifying anti-rheumatic drugs (cDMARD and bDMARD), respectively [ 15 ]. Moreover, despite many known adverse effects, systemic glucocorticoids (GC) are still being used as an important therapeutic option for wide range of complications associated with JIA (e.g., macrophage activation syndrome, myocarditis, pericarditis, pleuritis, peritonitis, uveitis and severe anaemia), as well as a bridge therapy in severe forms of JIA before the full effect of other treatment modalities has been achieved [ 16 ]. Those modalities nowadays primarily involve tumor necrosis factor alpha (TNFα) inhibitors (TNFi), such as etanercept, adalimumab and infliximab, and non-TNFi, such as anti-interleukin-6 (anti-IL-6) agent tocilizumab and a selective T-cell co-stimulation modulator abatacept [ 17 , 18 ]. Furthermore, new medications are continuously being investigated [ 19 , 20 ].
Together with the expansion of new treatment modalities, efforts have been made to introduce the treat-to-target (T2T) model in pediatric rheumatologic practice [ 21 ]. Signs and symptoms control, prevention of structural damage of joints and optimization of linear growth and pubertal development, as well as abolition of inflammation, have all been set as treatment goals. Essentially, this model advocates that therapy should be revised and adjusted based on regular disease activity assessments to reach and maintain the treatment target. Special attention should be paid to preventing or minimizing the side effects of systemic GC given their negative effect on growth and pubertal development. Consequently, their long-term use to maintain treatment target should be avoided, especially considering that GC dependence demonstrates the inadequacy of chosen treatment. The shared decision making, as well as multidisciplinary approach, have been recognized as exceptionally valuable for assurance of better adherence to treatment and subsequently improvement of outcome and overall prognosis.
Unfortunately, despite all these achievements, only 14 % of patients with rheumatoid factor (RF) negative and 0 % with RF positive polyarticular JIA achieves the remission off medications within five years, implying that JIA treatment requires a long-lasting commitment [ 22 ]. Hence, it comes as no surprise that every clinician involved in the care of children with JIA patients eventually sees one with disease not responding to acclaimed treatment options (i.e. NSAIDs, cDMARDS, bDMARDS) [ 15 ]. At that point, the consideration of additional treatment modalities seems like a valid course of action, but sometimes alternative diagnosis should be considered as well. Here, we present one such case, a girl with long standing polyarticular JIA refractory to many standard treatment modalities, with symptoms suggestive of other diseases.
A four-year-old first came to our attention in February 2008 due to painful swelling and contractures of both knees. Her symptoms started a year earlier and were not associated with any discernible trigger such as infection or trauma. Moreover, the symptoms were not responding to NSAIDs and she soon developed a severe morning stiffness lasting for up to three hours. Her birth history as well as psychomotor development prior to disease evolution was unremarkable. She was born as a first child into a family of non-consanguine parents, with no relatives having the similar symptoms.
The initial laboratory findings showed persistently elevated inflammatory markers (erythrocyte sedimentation rate (ESR) up to 100 mm/h and C-reactive protein (CRP) up to 100 mg/dL), with negative RF and antinuclear antibody (ANA) screen, and normal immunoglobulin levels. Despite the initial treatment with GC and methotrexate (MTX), her symptoms progressed affecting elbows, wrists, ankles and small joints of both hands. Moreover, she developed a severe uveitis of the left eye. In November 2008 biologic therapy with infliximab was started, with initially good response. Unfortunately, this lasted only for a few months and frequent relapses necessitated switching to adalimumab in April 2011. Again, there was an initial period of remission followed by progressive exacerbation characterized by swelling and pain in some joints, and persistent contracture of others. Other diagnosis, such as mucopolysaccharidosis and systemic lupus erythematosus (SLE) were suspected, but metabolic and immunological screening were negative, respectively. Beside ESR and CRP, the increased values of IL-6 (up to 75 pg/mL) and TNF-alfa (up to 20 pg/mL) were measured.
In November 2012 therapy was cycled to non-TNFi, tocilizumab, which led only to a short period of remission. Finally, in September 2014, etanercept was introduced, again with the lack of permanent response. Along with four different bDMARDs she continuously received cDMARD methotrexate, and for a short period of time leflunomide. During the periods of disease flare, bridge therapy with intraarticular and/or systemic GC was used, and soon she was dependent on a daily dose of GC. Eventually, this led to the development of iatrogenic Cushing syndrome with characteristic appearance, growth retardation and low bone mineral density, regardless of the vitamin D and ibandronic acid therapy. Since every attempt to wean off GC inevitably led to disease flare, from June to December 2016 she received cyclophosphamide (6x) and rituximab (3x), again without achieving a sustained remission. Afterwards, for a short period of time she was given metformin but without an appropriate improvement in musculoskeletal symptoms (Fig. 1 ).
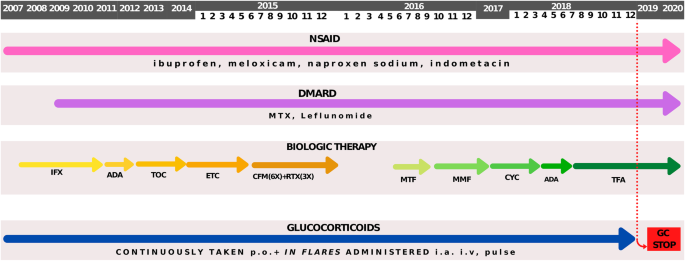
Schematic representation of treatment modalities during the time. 2007 – 2020 - the period of treatment, NSAID - nonsteroidal anti-inflammatory drug, IFX - infliximab, ADA - adalimumab, TOC - tocilizumab, ETC - etanercept, DMARD - disease modifying anti-rheumatic drug, MTX - methotrexate, CFM - cyclophosphamide, RTX - rituximab, MTF - metformin, MMF - mycophenolate mofetil, CYC - cyclosporine, TFA - tofacitinib, p.o. - per os, i.a. - intraarticular, i.v. - intravenous, GC - glucocorticoids
During the 2017, at the age of 13, the trial of mycophenolate mofetil (MMF) followed by the trial of cyclosporin was initiated, but the patient nevertheless remained GC dependent. Both shoulders, elbows, radiocarpal joints, metacarpophalangeal (MCP), proximal interphalangeal (PIP), distal interphalangeal (DIP) joints and both knees had restricted range of movement, and repeated IAGI were necessary to alleviate the symptoms. Finally, in 2018, the treatment with Janus kinase (JAK) inhibitor tofacitinib was initiated, which lead to gradual amelioration of musculoskeletal symptoms and improvement of inflammatory markers and overall well-being, as well as to the weaning of systemic GC. Moreover, as the swelling of the wrists subsided for the first time in many years, Madelung deformity was noticed by clinical examination. Interestingly, it was only then, in 2019, that the deformity was for the first time described on x-ray (Fig. 2 ), although x-ray and MRI imaging of both hands were previously performed on many occasions in order to assess the inflammation (Figs. 3 and 4 ). Nevertheless, subsequent analysis by experienced musculoskeletal radiologist revealed characteristic bilateral signs of Madelung deformity dating back in 2015 and 2017 (Fig. 3 ), with the MRI showing the Vickers and radiotriquetral ligament (Fig. 4 ). The finding of those two ligaments allowed the distinguishing between Madelung deformity and pseudo-Madelung deformity, which includes post-traumatic and post-infective forms, forms associated with Turner syndrome, multiple hereditary exostoses and Ollier disease [ 23 ]. Unfortunately, due to the parent’s refusal, no further radiological assessment was performed, so we have not documented the other important aspects of Madelung deformity, such as radial shortening and diaphysis bowing, nor the mesolimbic shortening of limbs characteristic for Leri Weill syndrome.
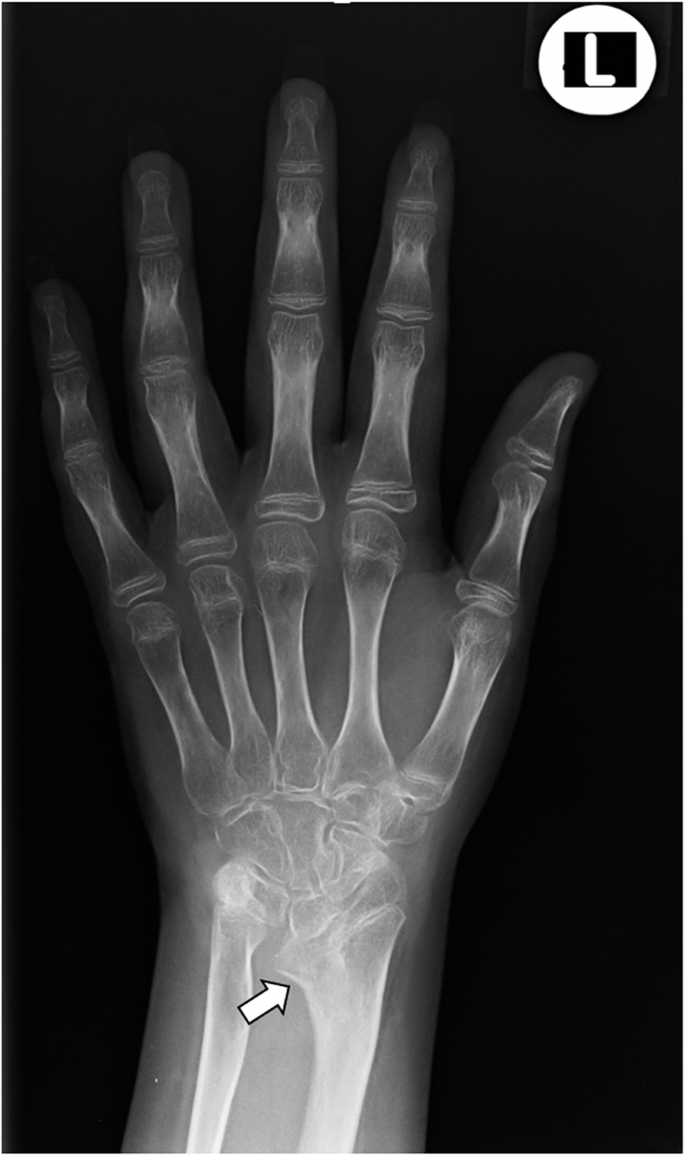
The anteroposterior radiograph of left hand at the age of 14. Note the increased volar angulation of distal radius, wedge shaped carpus with proximally positioned lunate and a characteristic notch on the distal radius (white arrow), which are the features of the Madelung deformity [ 12 ]
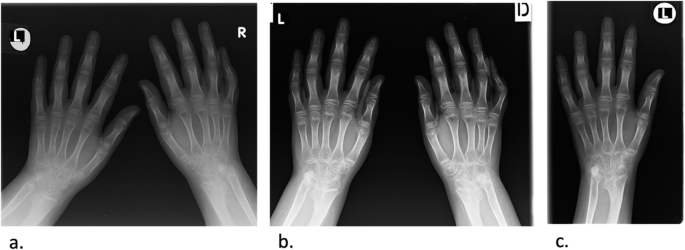
The anteroposterior radiograph of both hands at the age of 10 ( a ) and 13 ( b ), and of the left hand at the age of 14 ( c ). Note the bowing of the distal radius, an increased radial inclination (~ 30°) with the deformation of the carpus that acquired a triangular appearance and widening of the distal radial-ulnar joint bilaterally, which are the typical features of Madelung deformity. Dorsal subluxation of the ulnar head is not seen as lateral images of the wrist were not taken. Osteopenia of carpal bones and periarticular osteopenia of MCP, PIP and DIP joints related to JIA are present. No relevant changes are observed during the time
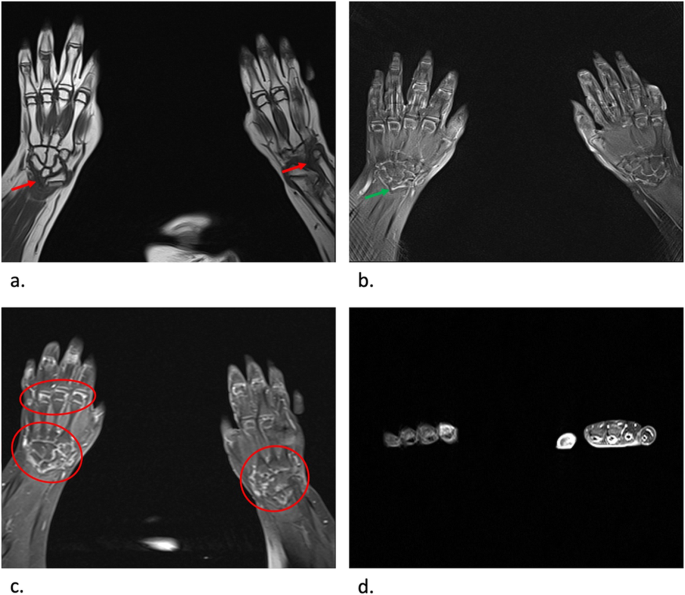
MRI coronal T1-weighted ( a ), proton density BLADE fluid sensitive sequence ( b ), post contrast T1-weighted coronal fat sat sequence ( c ) and post contrast T1-weighted axial fat sat sequence ( d ) images of both hands at the age of 13. Note the radiotriquetral ligament (red arrow) and Vickers ligament (green arrow) ( a , b ). Note the inflammatory changes characterized by postcontrast imbibition in carpal joints and MCP joints (red circle), as well as tenosynovitis of flexor tendons related to JIA, more prominent on the left hand ( c , d )
Although our patient was simultaneously followed by pediatric endocrinologist from age of 11, her short stature, along with delayed menarche, and Cushingoid appearance, was attributed to the prolonged use of GC. It was only after the Madelung’s deformity was observed that genetic causes, primarily Leri Weill syndrome, were taken into consideration. Genetic analysis was performed by commercially available SALSA MLPA Probemix P018 SHOX (MRC-Holland, Amsterdam, The Netherland) according to the manufacturer’s recommendations. The MLPA mix included probes for each exon of SHOX, one probe just before the promoter region as well as probes covering a region downstream of the gene. The results revealed one copy of sixteen probes (10 probes for Xp22-PAR1 from CNE2 to CNE9, 4 probes for SHOX area downstream, 1 probe for CRLF2 in PAR1 region and 1 probe for CSF2RA in PAR1 region), with the size of the smallest deletion of 766,5 kb. Based on the genetic testing and imaging findings of Madelung deformity, the diagnosis of Leri Weill syndrome was established, and parents were advised to undergo further genetic testing of both the patient and themselves, which they rejected.
Currently, our patient has many consequences of the adverse course of the disease and prolonged GC treatment, such as joint contractures of elbows, wrists, DIPs, PIPs, hips, knees and MCPs and low bone density, respectively. She developed secondary sex characteristics only after the therapy with estradiol was initiated at the age of 14. Nevertheless, after the discontinuation of GC and subsequent discontinuation of estradiol, she finally had a menarche at the age of 15, along with a long-awaited growth spurt.
Discussion and Conclusion
Children with rheumatic diseases, their families, as well as their treating physicians are dealt with numerous issues and dilemmas regarding either the disease itself or ongoing treatment modalities. Besides, the diagnosis of rheumatic diseases in children is regularly made by excluding wide range of other diseases, with no pathognomonic tests and/or criteria. Therefore, even after the classification criteria are fulfilled, the diagnosis should be revised if new symptoms emerge or if the recommended treatment options are failing.
In our patient, a variety of steroid-sparing agents with different mechanisms of action have been employed with limited or no clinical success. Some of these agents were used in line with current treatment recommendations, but many were used based on anecdotal reports [ 24 , 25 , 26 , 27 , 28 ]. Finally, due to signs of systemic inflammation characterized by increased inflammatory markers (CRP and ESR) and cytokines (IL-6 and TNF-alfa), which is indicative for the activation of JAK/STAT pathway, treatment with tofacitinib, a first generation JAK inhibitor, was initiated with a good clinical response. Several clinical trials in adults with rheumatoid and psoriatic arthritis have given solid evidence about the use of tofacitinib, while the results of a phase 3 randomized double blind placebo controlled withdrawal study in patients with polyarticular JIA showed improvement in symptoms, less disease flares and improved functional ability, together with a clinical amelioration of disease activity [ 29 , 30 ]. Moreover, tofacitinib is an oral agent, and the challenge of using biologics requiring injection or infusion for an extended length of time, especially in children, should not be overlooked.
Along with the various treatment modalities, the different diagnosis was constantly being considered in our patient. Firstly, due to persistent contracture in some joints with little or no signs of swelling, LSDs such as mucopolysaccharidosis type I, Gaucher disease type I and Fabry disease were investigated. Besides, other inflammatory causes like systemic lupus erythematosus were also excluded. Lastly, the diagnosis of Leri Weill syndrome characterized by deletions in SHOX gene and Madelung deformity was established. This painful deformity of the wrist was first described in 1878 by the German surgeon Otto Madelung in adolescents between the ages 8 and 14 [ 12 ]. Although initially asymptomatic, the patients often went on to develop pain, loss of grip strength and reduced mobility, which were the symptoms present in our patient even after the inflammation was tackled with tofacitinib. Moreover, the features of Leri Weill syndrome include the short stature, which was also one of the dominant finding in the presented patient. Yet, the growth deficit caused by SHOX haploinsufficiency in Leri Weill syndrome is around 2 standard deviation scores (SDS) [ 31 ], while our patient had a SDS of -5,5. Besides, due to a prolonged use of GC, our patient had a full blown Cushingoid appearance, low bone density, delayed puberty and growth retardation. Therefore, the possible explanation for the short stature and growth delay in our patient includes multifactorial aetiology. Firstly, it is well known that extended GC treatment leads to a defect in bone turnover (and formation) due to impaired osteoblastogenesis and osteoclastogenesis, and may have direct effects on the growth plate [ 32 ]. Additionally, higher prepubertal glucocorticoid level appears to delay early and late pubertal timing of healthy girls, particularly the onset of pubertal growth spurt and menarche [ 33 ]. Moreover, it has been shown that girls with polyarticular juvenile idiopathic arthritis are significantly more likely to present with short stature even 6 months after stopping the steroid therapy [ 34 ]. Finally, the product of SHOX gene is implicated in bone development and regulation of chondrocyte differentiation, which clarifies the association of SHOX gene haploinsufficiency with idiopathic short stature, as well as short stature in Turner syndrome and Leri Weill dyschondrosteosis [ 31 ].
In the presented case, the diagnosis of the Madelung deformity and Leri Weill syndrome was delayed due to the concomitant active inflammation caused by JIA taking the focus from other possible causes of pain in the wrists. However, as subsequent analysis by experienced musculoskeletal radiologist has shown, the characteristic signs of the Madelung deformity were present few years before the final diagnosis was reached, emphasizing once again the importance of multidisciplinary approach and close collaboration of many subspecialists in the care of children with rheumatic diseases. Nevertheless, this lag probably did not influence the therapeutic management in our particular patient; although positive effect on final height was observed with growth hormone therapy in patients with Leri Weill syndrome, due to the matching influence of GC and lack of agreement with her parents, this treatment option was avoided in our patient [ 35 ].
In conclusion, this case report emphasizes the difficulties and challenges in management of patient with long-standing polyarticular JIA refractory to wide range of treatment modalities. Although many high-quality guidelines are available for treatment of JIA patients, there is still need for individual reports of difficult to treat cases, especially when additional diagnosis are involved. While Leri Weill syndrome is extensively reported in the literature, to the best of our knowledge, our case report describes it for the first time along with JIA. Taking all these into account, we strongly encourage the aggregation of similar patients and establishment of the common ground that will help clinician to decide upon the introduction of treatment options outside of the contemporary guidelines.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study. / Not applicable.
Abbreviations
- Juvenile idiopathic arthritis
Lysosomal storage disease
Mucopolysaccharidosis
Nonsteroidal anti-inflammatory drug
Conventional disease modifying anti-rheumatic drug
Biologic disease modifying anti-rheumatic drug
Intraarticular glucocorticoid injection
Glucocorticoids
Macrophage activation syndrome
Tumor necrosis factor inhibitor
Treat-to-target
Rheumatoid factor
Erythrocyte sedimentation rate
C-reactive protein
Antinuclear antibody
Methotrexate
Systemic lupus erythematosus
Tumor necrosis factor alpha
Anti-interleukin-6
Mycophenolate mofetil
Metacarpophalangeal
Proximal interphalangeal
Distal interphalangeal
Janus kinase
Magnetic resonance imaging
Short-stature homeobox
Janus kinase/signal transducer and activator of transcription proteins
Standard deviation score
Gonadotropin-releasing hormone
Hypothalamic-pituitary-gonadal
Tocilizumab
Cyclophosphamide
Cyclosporine
Tofacitinib
Prakken B, Albani S, Martini A. Juvenile idiopathic arthritis. Lancet. 2011;377:2138–49. doi: https://doi.org/10.1016/S0140-6736(11)60244-4 .
Article PubMed Google Scholar
Manger B, Mengel E, Schaefer RM. Rheumatologic aspects of lysosomal storage diseases. Clin Rheumatol. 2007;26:335–41.
Article Google Scholar
Paira SO, Roverano S, Iribas JL, Barceló HA. Joint manifestations of Fabry’s disease. Clin Rheumatol. 1992;11:562–5.
Article CAS Google Scholar
Rosa Neto NS, Bento JCDB, Pereira RMR. Higher rate of rheumatic manifestations and delay in diagnosis in Brazilian Fabry disease patients. Adv Rheumatol. 2020;60:1–8.
Michels H, Mengel E. Lysosomal storage diseases as differential diagnoses to rheumatic disorders. Curr Opin Rheumatol. 2008;20:76–81.
Politei J, Remondino G, Heguilen R, Wallace E, Durand C, Schenone A. When arthralgia is not arthritis. Eur J Rheumatol. 2017;3:182–4.
Mahoney D. Lysosomal Storage Disorders: Awareness, Early Action Are Key. Rheumatol News. 7:17. doi: https://doi.org/10.1016/S1541-9800(08)70584-2 .
James RA, Singh-Grewal D, Lee SJ, McGill J, Adib N. Lysosomal storage disorders: A review of the musculoskeletal features. J Paediatr Child Health. 2016;52:262–71.
Moiseev S, Karovaikina E, Novikov PI, Ismailova D, Moiseev A, Bulanov N. What rheumatologist should know about Fabry disease. Ann Rheum Dis. 2019;79:6–7.
Google Scholar
Vijay S, Wraith JE. Clinical presentation and follow-up of patients with the attenuated phenotype of mucopolysaccharidosis type I. Acta Paediatr Int J Paediatr. 2005;94:872–7.
Cimaz R, Coppa GV, Koné-Paut I, Link B, Pastores GM, Elorduy MR, et al. Joint contractures in the absence of inflammation may indicate mucopolysaccharidosis. Pediatr Rheumatol. 2009;7:1–8.
Ali S, Kaplan S, Kaufman T, Fenerty S, Kozin S, Zlotolow DA. Madelung deformity and Madelung-type deformities: a review of the clinical and radiological characteristics. Pediatr Radiol. 2015;45:1856–63.
Bruni S, Lavery C, Broomfield A. The diagnostic journey of patients with mucopolysaccharidosis I: A real-world survey of patient and physician experiences. Mol Genet Metab Reports. 2016;8:67–73.
Giancane G, Ravelli AC. SL. SD. BS. A, Received: Juvenile Idiopathic Arthritis: Diagnosis and Treatment. Rheumatol Ther. 2016;3:187–207.
Ringold S, Angeles-Han ST, Beukelman T, Lovell D, Cuello CA, Becker ML, et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Treatment of Juvenile Idiopathic Arthritis: Therapeutic Approaches for Non-Systemic Polyarthritis, Sacroiliitis, and Enthesitis. Arthritis Care Res. 2019;71:717–34.
Batu ED. Glucocorticoid treatment in juvenile idiopathic arthritis. Rheumatol Int. 2019;39:13–27. doi: https://doi.org/10.1007/s00296-018-4168-0 .
Giancane G, Alongi A, Rosina S, Tibaldi J, Consolaro A, Ravelli A. Recent therapeutic advances in juvenile idiopathic arthritis. Best Pract Res Clin Rheumatol. 2017;31:476–87. doi: https://doi.org/10.1016/j.berh.2018.01.001 .
Blazina Š, Markelj G, Avramovič MZ, Toplak N, Avčin T. Management of Juvenile Idiopathic Arthritis: A Clinical Guide. Pediatr Drugs. 2016;18:397–412.
Kearsley-Fleet L, Sampath S, McCann LJ, Baildam E, Beresford MW, Davies R, et al. Use and effectiveness of rituximab in children and young people with juvenile idiopathic arthritis in a cohort study in the United Kingdom. Rheumatol (United Kingdom). 2019;58:331–5.
CAS Google Scholar
Mauro A, Rigante D, Cimaz R. Investigational drugs for treatment of juvenile idiopathic arthritis. Expert Opin Investig Drugs. 2017;26:381–7. doi: https://doi.org/10.1080/13543784.2017.1301929 .
Article CAS PubMed Google Scholar
Ravelli A, Consolaro A, Horneff G, Laxer RM, Lovell DJ, Wulffraat NM, et al. Treating juvenile idiopathic arthritis to target: Recommendations of an international task force. Ann Rheum Dis. 2018;77:819–28.
PubMed Google Scholar
Guzman J, Oen K, Tucker LB, Huber AM, Shiff N, Boire G, et al. The outcomes of juvenile idiopathic arthritis in children managed with contemporary treatments: Results from the reacch-out cohort. Ann Rheum Dis. 2015;74:1854–60.
De Leucio A, Castelein S, Bellemans M, Simoni P. Radiotriquetral Ligament in Madelung’s Deformity Associated with Leri-Weill’s Dyschondrosteosis. Cureus. 2020;12.
Sakamoto AP, Pinheiro MM, Barbosa CMPL, Fraga MM, Len CA, Terreri MT. Uso de rituximabe em adultos jovens com diagnóstico de artrite idiopática juvenil refratária ao tratamento convencional: relato de 6 casos. Rev Bras Reumatol. 2015;55:536–41. doi: https://doi.org/10.1016/j.rbr.2014.12.015 .
Berrada K, Abourazzak FE, Mezouar I, El, Lazrak F, Aradoini N, Tahiri L, et al. A successful treatment of juvenile idiopathic arthritis with rituximab: A report of two cases. Eur J Rheumatol. 2014;1:164–6.
Monteiro de Castro TC, Terreri MT, Len C, Esteves Hilário MO. Treatment of refractory juvenile idiopathic arthritis via pulse therapy using methylprednisolone and cyclophosphamide. Sao Paulo Med J. 2003;121:117–20.
Ishikawa S, Tasaki M, Kuroda T, Kobayashi D, Saito K, Nakagawa Y, et al. Management of Juvenile Idiopathic Arthritis in ABO-incompatible Kidney Transplantation: A Case Report. Transplant Proc. 2018;50:869–72. doi: https://doi.org/10.1016/j.transproceed.2017.12.052 .
Semo Oz R, Tesher MS. Arthritis in children with LRBA deficiency - Case report and literature review. Pediatr Rheumatol. 2019;17:1–6.
Kerrigan SA, Mcinnes IB. JAK Inhibitors in Rheumatology: Implications for Paediatric Syndromes ? 2018;:1–9.
Brunner HI. Tofacitinib for the treatment of polyarticular course juvenile idiopathic arthritis: results of a Phase 3 randomized, double-blind, placebo-controlled withdrawal study Disclosures (continued). 2019;:1–14.
Leka SK, Kitsiou-tzeli S, Kalpini-mavrou A, Kanavakis E. Short stature and dysmorphology associated with defects in the SHOX gene. 2006;5:107–18.
Weinstein RS, Jilka RL, Michael Parfitt A, Manolagas SC. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts end osteocytes by glucocorticoids potential mechanisms of their deleterious effects on bone. J Clin Invest. 1998;102:274–82.
Shi L, Wudy SA, Buyken AE, Maser-Gluth C, Hartmann MF, Remer T. Prepubertal glucocorticoid status and pubertal timing. J Clin Endocrinol Metab. 2011;96:891–8.
Machado SH, Xavier RM, Lora PS, Gonçalves LMK, Trindade LR, Marostica PJC. Height and sexual maturation in girls with juvenile idiopathic arthritis. J Pediatr (Rio J). 2020;96:100–7.
Benabbad I, Rosilio M, Child CJ, Carel JC, Ross JL, Deal CL, et al. Safety Outcomes and Near-Adult Height Gain of Growth Hormone-Treated Children with SHOX Deficiency: Data from an Observational Study and a Clinical Trial. Horm Res Paediatr. 2017;87:42–50.
Download references
Acknowledgements
We thank parents for availability of publishing medical history of their daughter.
Author information
Authors and affiliations.
Department of Pediatrics, University of Zagreb School of Medicine, Zagreb, Croatia
Vana Vukić, Ana Smajo, Miroslav Harjaček & Lovro Lamot
Division of Clinical Immunology and Rheumatology, Department of Pediatrics, Sestre milosrdnice University Hospital Center, Zagreb, Croatia
Mandica Vidović, Miroslav Harjaček & Lovro Lamot
Department of Diagnostic and Interventional Radiology, Sestre milosrdnice University Hospital Center, University of Zagreb, Zagreb, Croatia
Rudolf Vukojević
You can also search for this author in PubMed Google Scholar
Contributions
VV and AS have equally contributed to the paper. VV: medical charts review, literature search, creation of figures and tables and manuscript draft preparation. AS: medical charts review, literature search, creation of figures and tables and manuscript draft preparation RV: radiographic images interpretation, manuscript draft preparation. MV: clinical care and the final revision of the manuscript. MH: clinical care and the final revision of the manuscript. LL: clinical care, medical charts review, literature search, creation of figures and tables, manuscript draft preparation, final revision of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Lovro Lamot .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Written informed consent was obtained from the parents for publication of this case report and any accompanying images.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Vukić, V., Smajo, A., Vidović, M. et al. Beyond the guidelines management of juvenile idiopathic arthritis: a case report of a girl with polyarticular disease refractory to multiple treatment options and Leri Weill syndrome. BMC Pediatr 21 , 40 (2021). https://doi.org/10.1186/s12887-021-02494-6
Download citation
Received : 07 September 2020
Accepted : 05 January 2021
Published : 15 January 2021
DOI : https://doi.org/10.1186/s12887-021-02494-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Leri Weill syndrome
- Madelung deformity
- Tofacitinib.
BMC Pediatrics
ISSN: 1471-2431
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 06 February 2024
Treatment of non-systemic juvenile idiopathic arthritis
- Susan Shenoi ORCID: orcid.org/0000-0002-2495-594X 1 ,
- Gerd Horneff 2 , 3 ,
- Amita Aggarwal 4 &
- Angelo Ravelli ORCID: orcid.org/0000-0001-9658-0385 5 , 6
Nature Reviews Rheumatology volume 20 , pages 170–181 ( 2024 ) Cite this article
914 Accesses
1 Citations
3 Altmetric
Metrics details
- Juvenile idiopathic arthritis
- Therapeutics
In the past two decades, the treatment of juvenile idiopathic arthritis (JIA) has evolved markedly, owing to the availability of a growing number of novel, potent and relatively safe therapeutic agents and the shift of management strategies towards early achievement of disease remission. However, JIA encompasses a heterogeneous group of diseases that require distinct treatment approaches. Furthermore, some old drugs, such as methotrexate, sulfasalazine and intraarticular glucocorticoids, still maintain an important therapeutic role. In the past 5 years, information on the efficacy and safety of drug therapies for JIA has been further enriched through the accomplishment of several randomized controlled trials of newer biologic and synthetic targeted DMARDs. In addition, a more rational therapeutic approach has been fostered by the promulgation of therapeutic recommendations and guidelines. A multinational collaborative effort has led to the development of the recommendations for the treat-to-target strategy in JIA. There is currently increasing interest in establishing the optimal time and modality for discontinuation of treatment in children with JIA who achieve sustained clinical remission. The aim of this Review is to summarize the current evidence and discuss the therapeutic approaches to the management of non-systemic phenotypes of JIA, including oligoarthritis, polyarthritis, enthesitis-related arthritis and psoriatic arthritis.
In the past two decades, important progress has been made in the management of juvenile idiopathic arthritis, including the availability of new therapeutic agents and the shift towards early aggressive interventions.
Several randomized controlled trials, therapeutic recommendations and consensus treatment plans have facilitated a more rational approach to therapy.
Contemporary therapeutic goals include early achievement of disease control, sparing use of glucocorticoids and the prevention of disease-related and treatment-related morbidity.
The application of the treat-to-target strategy, an innovative treatment modality that has already been explored successfully in pivotal therapeutic studies, is now garnering increased interest.
The variability in clinical presentation and course of juvenile idiopathic arthritis implies that the therapeutic choices, optimal targets and treatment strategy might be different across disease categories.
The research agenda calls for innovative trials that improve remission rates and pave the way for refined precision studies, personalized medicine and, ultimately, future prevention.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others

Bispecific T cell engager therapy for refractory rheumatoid arthritis

An autoantibody signature predictive for multiple sclerosis

Therapeutic application of circular RNA aptamers in a mouse model of psoriasis
Levinson, J. E. & Wallace, C. A. Dismantling the pyramid. J. Rheumatol. 33 , 6–10 (1992).
CAS Google Scholar
Tambralli, A. et al. High doses of infliximab in the management of juvenile idiopathic arthritis. J. Rheumatol. 40 , 1749–55 (2013).
Article CAS PubMed Google Scholar
Ravelli, A. Treating juvenile idiopathic arthritis to target: recommendations of an international task force. Ann. Rheum. Dis. 77 , 819–828 (2018).
PubMed Google Scholar
Giancane, G. et al. Disease activity and damage in juvenile idiopathic arthritis: methotrexate era versus biologic era. Arthritis Res. Ther. 21 , 168 (2019).
Article PubMed PubMed Central Google Scholar
Petty, R. E. et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J. Rheumatol. 31 , 390–392 (2004).
Martini, A. It is time to rethink juvenile idiopathic arthritis classification and nomenclature. Ann. Rheum. Dis. 71 , 1437–1439 (2012).
Article PubMed Google Scholar
Martini, A. et al. Juvenile idiopathic arthritis. Nat. Rev. Dis. Prim. 8 , 5 (2022).
Martini, A. Toward new classification criteria for juvenile idiopathic arthritis: first steps, Pediatric Rheumatology International Trials Organization international consensus. J. Rheumatol. 46 , 190–197 (2019).
Beukelman, T. & Nigrovic, P. Juvenile idiopathic arthritis: an idea whose time has gone? J. Rheumatol. 46 , 124–126 (2019).
Nigrovic, P. A. et al. Biological classification of childhood arthritis: roadmap to a molecular nomenclature. Nat. Rev. Rheumatol. 17 , 257–269 (2021).
Hinze, C. H., Foell, D. & Kessel, C. Treatment of systemic juvenile idiopathic arthritis. Nat. Rev. Rheumatol. 19 , 778–789 (2023).
Ravelli, A. et al. Antinuclear antibody-positive patients should be grouped as a separate category in the classification of juvenile idiopathic arthritis. Arthritis Rheum. 63 , 267–275 (2011).
Ringold, S. et al. 2019 American College of Rheumatology/Arthritis Foundation guideline for the treatment of juvenile idiopathic arthritis: therapeutic approaches for non-systemic polyarthritis, sacroiliitis, and enthesitis. Arthritis Care Res. 71 , 717–734 (2019).
Article Google Scholar
Onel, K. B. et al. 2021 American College of Rheumatology guideline for the treatment of juvenile idiopathic arthritis: therapeutic approaches for oligoarthritis, temporomandibular joint arthritis, and systemic juvenile idiopathic arthritis. Arthritis Rheumatol. 74 , 553–569 (2022).
Scott, C. et al. A reappraisal of intra-articular corticosteroid therapy in juvenile idiopathic arthritis. Clin. Exp. Rheumatol. 28 , 774–781 (2010).
CAS PubMed Google Scholar
Klein, A. et al. Efficacy and safety of oral and parenteral methotrexate therapy in children with juvenile idiopathic arthritis: an observational study with patients from the German Methotrexate Registry. Arthritis Care Res. 64 , 1349–1356 (2012).
Article CAS ADS Google Scholar
Raab, A. et al. Outcome of children with oligoarticular juvenile idiopathic arthritis compared to polyarthritis on methotrexate- data of the German BIKER registry. Pediatr. Rheumatol. Online J. 19 , 41 (2021).
Article CAS PubMed PubMed Central Google Scholar
Bakry, R., Klein, M. A. & Horneff, G. Oral or parenteral methotrexate for the treatment of polyarticular juvenile idiopathic arthritis. Eur. J. Rheumatol. 9 , 197–205 (2022).
Dupuis, L. L., Koren, G., Silverman, E. D. & Laxer, R. M. Influence of food on the bioavailability of oral methotrexate in children. J. Rheumatol. 22 , 1570–1573 (1995).
Jundt, J. W., Browne, B. A., Fiocco, G. P., Steele, A. D. & Mock, D. A comparison of low dose methotrexate bioavailability: oral solution, oral tablet, subcutaneous and intramuscular dosing. J. Rheumatol. 20 , 1845–1849 (1993).
Hissink Muller, P. et al. Treat to target (drug-free) inactive disease in DMARD-naive juvenile idiopathic arthritis: 24-month clinical outcomes of a three-armed randomised trial. Ann. Rheum. Dis. 78 , 51–59 (2019).
Hinze, C., Gohar, F. & Foell, D. et al. Management of juvenile idiopathic arthritis: hitting the target. Nat. Rev. Rheumatol. 11 , 290–300 (2015).
Ter Haar, N. M. et al. Treatment to target using recombinant interleukin-1 receptor antagonist as first-line monotherapy in new-onset systemic juvenile Idiopathic arthritis: results from a five-year follow-up study. Arthritis Rheumatol. 71 , 1163–1173 (2019).
Klein, A. et al. Treat-to-target study for improved outcome in polyarticular juvenile idiopathic arthritis. Ann. Rheum. Dis. 79 , 969–974 (2020).
Rosina, S., Rebollo-Giménez, A. I., Consolaro, A. & Ravelli, A. Treat-to-target in pediatric rheumatic diseases. Curr. Rheumatol. Rep. 25 , 226–235 (2023).
Wallace, C., Giannini, E., Huang, B., Itert, L. & Ruperto, N. American College of Rheumatology provisional criteria for defining clinical inactive disease in select categories of juvenile idiopathic arthritis. Arthritis Care Res. 63 , 929–936 (2011).
Magni-Manzoni, S. et al. Development and validation of a preliminary definition of minimal disease activity in patients with juvenile idiopathic arthritis. Arthritis Rheum. 59 , 1120–1127 (2008).
Consolaro, A. et al. Remission, minimal disease activity, and acceptable symptom state in juvenile idiopathic arthritis: defining criteria based on the juvenile arthritis disease activity score. Arthritis Rheum. 64 , 2366–2374 (2012).
Trincianti, C. et al. Definition and validation of the American College of Rheumatology 2021 Juvenile Arthritis Disease Activity Score Cutoffs for disease activity states in juvenile idiopathic arthritis. Arthritis Rheumatol. 73 , 1966–1975 (2021).
Sherry, D. D., Stein, L. D., Reed, A. M., Schanberg, L. E. & Kredich, D. W. Prevention of leg length discrepancy in young children with pauciarticular juvenile rheumatoid arthritis by treatment with intraarticular steroids. Arthritis Rheum. 42 , 2330–2334 (1999).
Ravelli, A. et al. Intra-articular corticosteroids versus intra-articular corticosteroids plus methotrexate in oligoarticular juvenile idiopathic arthritis: a multicentre, prospective, randomised, open-label trial. Lancet 389 , 909–916 (2017).
Stoustrup, P. et al. Management of orofacial manifestations of juvenile idiopathic arthritis: interdisciplinary consensus-based recommendations. Arthritis Rheumatol. 75 , 4–14 (2023).
Horneff, G. et al. Efficacy and safety of open-label etanercept on extended oligoarticular juvenile idiopathic arthritis, enthesitis-related arthritis and psoriatic arthritis: part 1 (week 12) of the CLIPPER study. Ann. Rheum. Dis. 73 , 1114–1122 (2014).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03841357 (2024).
O’Dell, J. R. et al. Treatment of rheumatoid arthritis with methotrexate alone, sulfasalazine and hydroxychloroquine, or a combination of all three medications. N. Engl. J. Med. 334 , 1287–1291 (1996).
Tynjälä, P. et al. Aggressive combination drug therapy in very early polyarticular juvenile idiopathic arthritis (ACUTE-JIA): a multicentre randomised open-label clinical trial. Ann. Rheum. Dis. 70 , 1605–1612 (2011).
Alexeeva, E. I. et al. Efficacy and safety of repeat courses of rituximab treatment in patients with severe refractory juvenile idiopathic arthritis. Clin. Rheumatol. 30 , 1163–1172 (2011).
Kearsley-Fleet, L. et al. Use and effectiveness of rituximab in children and young people with juvenile idiopathic arthritis in a cohort study in the United Kingdom. Rheumatology 58 , 331–335 (2019).
Marino, A., Orsini, F., Pregnolato, F. & Cimaz, R. Tumor necrosis factor-α inhibition before and after rituximab treatment in juvenile idiopathic arthritis: what shall we expect? A pilot study. J. Rheumatol. 49 , 654–656 (2022).
De Benedetti, F. et al. Sarilumab, a human monoclonal antibody to the interleukin-6 receptor, in polyarticular-course juvenile idiopathic arthritis: a 12-week, multinational, open-label, dose-finding study. Arthritis Rheumatol . 71 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02776735 (2023).
Amarilyo, G. et al. Biological agents in polyarticular juvenile idiopathic arthritis: a meta-analysis of randomized withdrawal trials. Semin. Arthritis Rheum. 46 , 312–318 (2016).
Kerrigan, S. A. & McInnes, I. B. JAK inhibitors in rheumatology: implications for paediatric syndromes? Curr. Rheumatol. Rep. 20 , 83–87 (2018).
Ruperto, N. et al. Tofacitinib in juvenile idiopathic arthritis: a double-blind, placebo-controlled, withdrawal phase 3 randomised trial. Lancet 398 , 1984–1996 (2021).
Ramanan, A. V. et al. Baricitinib in juvenile idiopathic arthritis: an international, phase 3, randomised, double-blind, placebo-controlled, withdrawal, efficacy, and safety trial. Lancet 402 , 555–570 (2023).
Brunner, H. I. et al. Safety and efficacy of upadacitinib for pediatric patients with polyarticular course juvenile idiopathic arthritis: an interim analysis of an open-label, phase 1 trial [abstract]. Ann. Rheum. Dis. 82 , 108–109 (2023).
Google Scholar
Qian, Y. et al. Pharmacokinetics of upadacitinib in pediatric patients with polyarticular course juvenile idiopathic arthritis [abstract]. Ann. Rheum. Dis. 82 , 666–667 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03725007 (2023).
Ringold, S. et al. Childhood Arthritis and Rheumatology Research Alliance consensus treatment plans for new-onset polyarticular juvenile idiopathic arthritis. Arthritis Care. Res 66 , 1063–1072 (2014).
Kimura, Y. et al. Optimizing the start time of biologics in polyarticular juvenile idiopathic arthritis: a comparative effectiveness study of Childhood Arthritis and Rheumatology Research Alliance consensus treatment plans. Arthritis Rheumatol. 73 , 1898–1909 (2021).
Ong, M. S. et al. Improved disease course associated with early initiation of biologics in polyarticular juvenile idiopathic arthritis: trajectory analysis of a Childhood Arthritis and Rheumatology Research Alliance consensus treatment plans study. Arthritis Rheumatol. 73 , 1910–1920 (2021).
Kimura, Y. et al. The Childhood Arthritis and Rheumatology Research Alliance Start Time Optimization of Biologic Therapy in Polyarticular JIA (STOP-JIA) study: three-year outcomes [abstract]. Arthritis Rheumatol . 74 , (2022).
Weiss, P. F. et al. Children with enthesitis-related arthritis and possible benefits from treatments for adults with spondyloarthritis. Arthritis Care Res. 74 , 1058–1064 (2022).
Chamlati, R. et al. Image guided sacroiliac joint corticosteroid injections in children: an 18-year single-center retrospective study. Pediatr. Rheumatol. Online J. 18 , 52 (2020).
Oliver, M., Simard, J. F., Lee, T., Gerstbacher, D. & Sandborg, C. Determinants of tumor necrosis factor inhibitor use in juvenile spondyloarthropathy and impact on clinical disease outcomes. ACR Open. Rheumatol. 4 , 19–26 (2022).
Burgos-Vargas, R. et al. A randomized, double-blind, placebo-controlled 12-week trial of infliximab in patients with juvenile-onset spondyloarthritis. Arthritis Res. Ther. 24 , 187 (2022).
Horneff, G. et al. Efficacy and safety of etanercept in patients with the enthesitis-related arthritis category of juvenile idiopathic arthritis: results from a phase III randomized, double-blind study. Arthritis Rheumatol. 67 , 2240–2249 (2015).
Foeldvari, I. et al. Etanercept treatment for extended oligoarticular juvenile idiopathic arthritis, enthesitis-related arthritis, or psoriatic arthritis: 6-year efficacy and safety data from an open-label trial. Arthritis Res. Ther. 21 , 125 (2019).
Burgos-Vargas, R. et al. A randomized, double-blind, placebo-controlled multicenter study of adalimumab in pediatric patients with enthesitis-related arthritis. Arthritis Care Res. 67 , 1503–1512 (2015).
Article CAS Google Scholar
Favalli, E. G. et al. Real-life 10-year retention rate of first-line anti-TNF drugs for inflammatory arthritides in adult- and juvenile-onset populations: similarities and differences. Clin. Rheumatol. 36 , 1747–1755 (2017).
Gaur, P., Misra, R. & Aggarwal, A. Natural killer cells and gamma-delta T cells alterations in enthesitis related arthritis category of juvenile idiopathic arthritis. Clin. Immunol. 161 , 163–169 (2015).
Mahendra, A., Misra, R. & Aggarwal, A. Th1 and Th17 predominance in enthesitis related arthritis form of juvenile idiopathic arthritis. J. Rheumatol. 36 , 1730–1736 (2009).
Braun, J., Baraliakos, X. & Kiltz, U. Secukinumab (AIN457) in the treatment of ankylosing spondylitis. Expert. Opin. Biol. Ther. 16 , 711–722 (2016).
Brunner, H. I. et al. Secukinumab in enthesitis-related arthritis and juvenile psoriatic arthritis: a randomised, double-blind, placebo-controlled, treatment withdrawal, phase 3 trial. Ann. Rheum. Dis. 82 , 154–160 (2023).
Baer, J., Klotsche, J. & Foeldvari, I. Secukinumab in the treatment for patients with juvenile enthesitis related arthritis non-responsive to anti-TNF treatment according the Juvenile Spondyloarthritis Disease Activity Index. Clin. Exp. Rheumatol. 40 , 620–624 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04527380 (2024).
Rahman, M. M., Laila, K. & Rahman, S. A. Efficacy and safety of tofacitinib in the treatment of refractory cases of polyarticular course juvenile idiopathic arthritis: a study from Bangladesh. Int. J. Rheum. Dis. 25 , 678–684 (2022).
Stoll, M. L. & Mellins, E. D. Psoriatic arthritis in childhood: a commentary on the controversy. Clin. Immunol. 214 , 108396 (2020).
Naddei, R. et al. Juvenile psoriatic arthritis: myth or reality? An unending debate. J. Clin. Med. 12 , 367 (2023).
Ravelli, A., Consolaro, A., Schiappapietra, B. & Martini, A. The conundrum of juvenile psoriatic arthritis. Clin. Exp. Rheumatol. 33 , S40–S43 (2015).
Constantin, T. et al. Two-year efficacy and safety of etanercept in pediatric patients with extended oligoarthritis, enthesitis-related arthritis, or psoriatic arthritis. J. Rheumatol. 43 , 816–824 (2016).
Leu, J. H. et al. Intravenous golimumab in patients with polyarticular juvenile idiopathic arthritis and juvenile psoriatic arthritis and subcutaneous ustekinumab in patients with juvenile psoriatic arthritis: extrapolation of data from studies in adults and adjacent pediatric populations. Paediatr. Drugs 24 , 699–714 (2022).
Wang, E. A., Suzuki, E., Maverakis, E. & Adamopoulos, I. E. Targeting IL-17 in psoriatic arthritis. Eur. J. Rheumatol. 4 , 272–277 (2017).
Navarro-Compan, V. et al. The paradigm of IL-23-independent production of IL-17F and IL-17A and their role in chronic inflammatory diseases. Front. Immunol. 14 , 1191782 (2023).
Schinocca, C. et al. Role of the IL-23/IL-17 pathway in rheumatic diseases: an overview. Front. Immunol. 12 , 637829 (2021).
Philipp, S. et al. Ustekinumab for the treatment of moderate-to-severe plaque psoriasis in paediatric patients (≥6 to <12 years of age): efficacy, safety, pharmacokinetic and biomarker results from the open-label CADMUS Jr study. Br. J. Dermatol. 183 , 664–672 (2020).
Landells, I. et al. Ustekinumab in adolescent patients age 12 to 17 years with moderate-to-severe plaque psoriasis: results of the randomized phase 3 CADMUS study. J. Am. Acad. Dermatol. 73 , 594–603 (2015).
McInnes, I. B. et al. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet 382 , 780–789 (2013).
Halyabar, O., Mehta, J., Ringold, S., Rumsey, D. G. & Horton, D. B. Treatment withdrawal following remission in juvenile idiopathic arthritis: a systematic review of the literature. Paediatr. Drugs 21 , 469–492 (2019).
Gerss, J. et al. Prevention of disease flares by risk-adapted stratification of therapy withdrawal in juvenile idiopathic arthritis: results from the PREVENT-JIA trial. Ann. Rheum. Dis. 81 , 990–997 (2022).
Gieling, J., van den Bemt, B., Hoppenreijs, E. & Schatorjé, E. Discontinuation of biologic DMARDs in non-systemic JIA patients: a scoping review of relapse rates and associated factors. Pediatr. Rheumatol. Online J. 20 , 109 (2022).
Ringold, S. et al. Disease recapture rates after medication discontinuation and flare in juvenile idiopathic arthritis: an observational study within the Childhood Arthritis and Rheumatology Research Alliance registry. Arthritis Care. Res. 75 , 715–723 (2023).
Schanberg, L. E. et al. Therapeutic development in polyarticular course juvenile idiopathic arthritis: extrapolation, dose selection, and clinical trial design. Arthritis Rheumatol. 75 , 1856–1866 (2023).
Burrone, M. et al. Looking for the best strategy to treat children with new onset juvenile idiopathic arthritis: presentation of the “comparison of STep-up and step-down therapeutic strategies in childhood ARthritiS” (STARS) trial. Pediatr. Rheumatol. Online J. 20 , 80 (2022).
Wedderburn, L. R. et al. Towards molecular-pathology informed clinical trials in childhood arthritis to achieve precision medicine in juvenile idiopathic arthritis. Ann. Rheum. Dis. 82 , 449–456 (2022).
Patient-Centered Outcomes Research Institute (PCORI). Trial of Sequential Medications after TNF Failure in Juvenile Idiopathic Arthritis (SMART-JIA). PCORI http://www.pcori.org/research-results/2023/trial-sequential-medications-after-tnf-failure-juvenile-idiopathic-arthritis-smart-jia#project_summary (2023).
Scott, C. et al. Juvenile arthritis management in less resourced countries (JAMLess): consensus recommendations from the Cradle of Humankind. Clin. Rheumatol. 38 , 563–575 (2019).
Consolaro, A. et al. Phenotypic variability and disparities in treatment and outcomes of childhood arthritis throughout the world: an observational cohort study. Lancet Child. Adolesc. Health 3 , 255–263 (2019).
Slamang, W., Smith, N., Scott, C. & Foster, H. Revising the WHO Essential Medicines List for paediatric rheumatology update. Pediatr. Rheumatol. 20 , 89 (2022).
Stoll, M. L. & Cron, R. Q. Treatment of juvenile idiopathic arthritis: a revolution in care. Pediatr. Rheumatol. Online J. 12 , 13 (2014).
Ahmed, W. et al. Dual biologic or small molecule therapy for treatment of inflammatory bowel disease: a systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 20 , e361–e379 (2022).
Record, J. L., Beukelman, T. & Cron, R. Q. Combination therapy of abatacept and anakinra in children with refractory systemic juvenile idiopathic arthritis: a retrospective case series. J. Rheumatol. 38 , 180–181 (2011).
Dolinger, M. T., Spencer, E. A., Lai, J., Dunkin, D. & Dubinsky, M. C. Dual biologic and small molecule therapy for the treatment of refractory pediatric inflammatory bowel disease. Inflamm. Bowel Dis. 27 , 1210–1214 (2021).
Furer, V. & Elkayam, O. Dual biologic therapy in patients with rheumatoid arthritis and psoriatic arthritis. Rambam Maimonides Med. J. 14 , e0007 (2023).
Brunner, H. I. et al. New medications are needed for children with juvenile idiopathic arthritis. Arthritis Rheumatol. 72 , 1945–1951 (2020).
Bava, C. et al. Analysis of arthritis flares after achievement of inactive disease with methotrexate monotherapy in juvenile idiopathic arthritis. Clin. Exp. Rheumatol. 39 , 426–433 (2021).
Mannion, M. L. & Cron, R. Q. To wean or not to wean: that is the question. Arthritis Care Res. 75 , 712–714 (2023).
Onel, K.B. et al. 2021 American College of Rheumatology Guideline for the treatment of juvenile idiopathic arthritis: recommendations for nonpharmacologic therapies, medication monitoring, immunizations, and imaging. Arthritis Care Res. 74 , 505–520 (2022).
Brunner, H. I. et al. Subcutaneous golimumab for children with active polyarticular-course juvenile idiopathic arthritis: results of a multicentre, double-blind, randomised withdrawal trial. Ann. Rheum. Dis. 77 , 21–29 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT05083182 (2024).
Download references
Author information
Authors and affiliations.
Seattle Children’s Hospital and Research Centre, University of Washington, Seattle, WA, USA
Susan Shenoi
Department of General Paediatrics, Asklepios Clinic Sankt Augustin, Sankt Augustin, Germany
Gerd Horneff
Department of Paediatric and Adolescents Medicine, University Hospital of Cologne, Cologne, Germany
Department of Clinical Immunology and Rheumatology, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India
Amita Aggarwal
Direzione Scientifica, IRCCS Istituto Giannina Gaslini, Genoa, Italy
Angelo Ravelli
Dipartimento di Neuroscienze, Riabilitazione, Oftalmologia, Genetica e Scienze Materno-Infantili (DINOGMI), Università degli Studi di Genova, Genoa, Italy
You can also search for this author in PubMed Google Scholar
Contributions
The authors contributed equally to all aspects of the article
Corresponding author
Correspondence to Angelo Ravelli .
Ethics declarations
Competing interests.
S.S. declares that she has received consulting fees from Amgen, Novartis and Pfizer, all unrelated to this manuscript. G.H. declares that has he has received grants from MSD, Novartis, Pfizer, Roche and consulting and/or speaker’s fees from AbbVie, Boehringer, Celgene, Chugai, GSK, Janssen, MSD, Novartis, Pfizer, Roche, Sanofi and Sobi, all unrelated to this manuscript. A.R. declares that has he has received grants from Novartis and Pfizer and consulting and/or speaker’s fees from AbbVie, Alexion, Angelini, Galapagos, Novartis, Pfizer, Reckitt-Benkiser, Roche, BMS and SOBI, all unrelated to this manuscript. A.A. declares no competing interests.
Peer review
Peer review information.
Nature Reviews Rheumatology thanks Yukiko Kimura and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Cluster Consortium: https://www.clusterconsortium.org.uk/
IMID-Bio-UK: https://www.gla.ac.uk/research/az/imid/
UCAN-Can DU: https://www.ucancandu.com
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Shenoi, S., Horneff, G., Aggarwal, A. et al. Treatment of non-systemic juvenile idiopathic arthritis. Nat Rev Rheumatol 20 , 170–181 (2024). https://doi.org/10.1038/s41584-024-01079-8
Download citation
Accepted : 05 January 2024
Published : 06 February 2024
Issue Date : March 2024
DOI : https://doi.org/10.1038/s41584-024-01079-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Anti-tumor necrosis factor (atnf) weaning strategy in juvenile idiopathic arthritis (jia): does duration matter.
- Kai Liang Teh
- Thaschawee Arkachaisri
Clinical Rheumatology (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Research article
- Open access
- Published: 19 April 2024
Bone health and physical activity in adolescents with juvenile idiopathic arthritis: a cross-sectional case-control study
- Egi Vasil 1 , 2 ,
- Colleen M. Nesbitt 3 ,
- Clodagh Toomey 3 , 4 ,
- Gregor Kuntze 5 ,
- Shane Esau 3 ,
- Carolyn A. Emery 2 , 3 , 6 , 7 &
- Leigh Gabel ORCID: orcid.org/0000-0002-7429-2750 1 , 2 , 7
Pediatric Rheumatology volume 22 , Article number: 45 ( 2024 ) Cite this article
318 Accesses
Metrics details
Adolescents with juvenile idiopathic arthritis (JIA) tend to engage in less physical activity than their typically developing peers. Physical activity is essential for bone development and reduced physical activity may detrimentally effect bone health. Thus, we examined differences in total body bone mineral content (BMC) and areal bone mineral density (aBMD) between adolescents with JIA and adolescent controls without JIA. We also examined associations between moderate-to-vigorous physical activity (MVPA), lean mass, and bone outcomes.
Participants included 21 adolescents with JIA (14 females, 7 males) and 21 sex- and age-matched controls aged 10–20 years. Assessments included: height; weight; triple-single-leg-hop distance (TSLH); MVPA by accelerometry; and total body BMC, aBMD, and lean mass measured using dual X-ray absorptiometry. Height-adjusted z-scores were calculated for BMC and aBMD and used for all analyses. Multiple linear mixed effects models examined group differences in BMC and aBMD, adjusting for sex, maturity, MVPA, TSLH, and lean mass. Participants clusters, based on sex and age (within 18 months), were considered random effects.
Adolescents with JIA had lower total body aBMD z-scores [β (95% CI); -0.58 (-1.10 to -0.07), p = 0.03] and BMC z-scores [-0.47 (-0.91 to -0.03), p = 0.04] compared with controls. Mean daily MVPA was 22.0 min/day lower in adolescents with JIA than controls; however, MVPA was not associated with aBMD [-0.01 (-0.01 to 0.01), p = 0.32] or BMC [0.00 (-0.01 to 0.00), p = 0.39]. Lean mass was positively associated with aBMD [0.05 (0.01 to 0.09) g/cm 2 , p = 0.03] and BMC [0.06 (0.03 to 0.10) g, p < 0.001].
Adolescents with JIA had lower total body aBMD and BMC compared with sex- and age-matched controls without JIA. Group differences in bone outcomes were not associated with the lower MVPA participation of adolescents with JIA. Despite this, physical activity should still be encouraged as it promotes physical well-being.
Introduction
Juvenile idiopathic arthritis (JIA) is an autoimmune disease acquired during childhood. JIA results from a disturbed balance between proinflammatory effector cells and anti-inflammatory regulating cells [ 1 ]. In Canada, 1 in 1000 children suffer from JIA which affects 0.07–4.01 per 1000 youth worldwide [ 2 ]. Children with JIA suffer from a range of symptoms including joint pain and swelling which can make it difficult to complete daily activities of living [ 1 ]. Youth with JIA may find it difficult to use the stairs, sit for long periods of time, and play outside due to pain [ 3 ]. The joint pain and swelling that JIA causes reduces range of motion which can result in reduced physical activity participation [ 4 , 5 , 6 , 7 ]. Youth with JIA have also reported hesitating to participate in physical activity as they believe it will be painful and others may judge their reduced ability [ 3 ]. Common treatments for JIA include various anti-rheumatic drugs that seek to reduce inflammation and symptoms [ 8 , 9 , 10 , 11 , 12 ]. Effectiveness of drugs varies between individuals and not all types of JIA respond positively to drug therapies [ 8 , 9 , 10 , 11 , 12 ]. Physical activity and exercise are important non-pharmacological treatments for JIA that help build bone and muscle [ 13 , 14 ] and are used in conjunction with pharmacological therapies to treat individuals with JIA.
Childhood onset of arthritis has been shown to increase fracture incidence by 1.5-4.0 times that of non-arthritic healthy controls across the lifespan [ 15 ]. Since children and adolescents with JIA are less likely to engage in recommended levels of physical activity compared with their healthy peers [ 16 , 17 ], they are at greater risk of compromised bone health. Weight-bearing physical activity during the critical periods of childhood and adolescence is important for optimal bone mass accrual [ 18 , 19 ] and is positively associated with total body bone mineral content (BMC) in both children with JIA and typically developing (TD) youth [ 8 ]. Physical activity and exercise are promising therapies for managing JIA symptoms and improving bone health.
Accrual and consolidation of bone mineral density (BMD) is mediated by lean mass as muscle transmits forces to bone [ 20 ]. In a two-year longitudinal study, children and adolescents with JIA performed significantly less self-reported leisure time weight bearing physical activity and had less gains in lean mass and BMC compared to TD controls [ 13 ]. Weight bearing physical activity was significantly associated with increases in total body BMC in both children with JIA and TD children [ 13 ]. While supervised weight bearing exercise interventions have proved beneficial in improving quality of life and bone health in youth with JIA [ 4 , 13 ], a recent study found that an at home exercise intervention had low adherence and minimal effect on bone mass, structure, and strength [ 21 ]. By better understanding the factors that are associated with poor bone health in children and adolescents with JIA, including physical activity, we may be able to develop programs to improve their bone health.
The primary aim of this study is to examine differences in BMC and areal BMD (aBMD) between adolescents with JIA and healthy adolescents. The secondary aim is to determine the relationship between free-living physical activity and bone outcomes and lean mass and bone outcomes. We hypothesize that adolescents with JIA will have significantly reduced BMC and aBMD compared with their TD peers. We further expect that adolescents engaging in more moderate to vigorous physical activity (MVPA) will have greater BMC and aBMD.
Study design
This is a secondary analysis of previously collected cross-sectional data [ 22 ]. Ethics approval was granted by the University of Calgary Conjoint Health Research Ethics Board (REB15-312) [ 22 ].
Participants
Participants with JIA were recruited by their clinician between July 2016 and November 2017 in collaboration with two pediatric rheumatology outpatient clinics [ 22 ]. Inclusion criteria were: 10–20 years old, a diagnosis of JIA, experiencing knee joint involvement (with or without other joint involvement other than the ankle), and active or inactive disease at time of testing [ 22 ]. Participants with JIA were excluded if systemic symptoms were present, if changes in medication occurred within the last three weeks, or if they had active ankle involvement [ 22 ]. We included knee involvement and excluded ankle involvement to assess knee joint biomechanics in previous studies [ 5 , 7 ]. Participants with JIA were age and sex matched (within 18 months) with TD controls who were recruited via an online research portal by convenience [ 22 ]. Exclusion criteria for all participants included: pregnancy, diagnosis of other arthritides, lower extremity musculoskeletal injury within the past three months prior to testing that resulted in time loss from work, school, or sport, and contraindications as assessed through the Physical Activity Readiness Questionnaire for Everyone [ 22 ]. We conducted a sample size estimation using G*Power software [ 23 ] based on total body aBMD (g/cm2) by DXA for individuals with JIA and TD controls by Brabnikova Maresova et al. [JIA group mean (SD) 1.07 (0.19), TD group 1.21 (0.08)] [ 24 ]. Based on a paired t-test, due to the paired study design, and assuming a correlation between groups of 0.5, this equates to an effect size of 0.85 and a sample size of at least 17 pairs for a significance level of 0.05 and a power of 90%.
Measurements
Data were collected in two sessions, one week apart [ 22 ]. Measurements included: anthropometrics (height, weight, and leg length), disease activity, and functional performance through right leg triple-single-leg-hop distance normalized to leg length (TSLH, three maximal consecutive hops forward with one leg– the distance measured being from the starting line to the point the heel lands on the third hop). Pain was assessed using the Child Health Assessment Questionnaire (CHAQ), which uses a visual analogue scale for disease-related pain and is converted into a continuous score of 0 to 3 [ 25 ]. Physical activity was measured using accelerometry (ActiGraph GT3X+, ActiGraph Inc., USA) with a 10-second epoch and worn for seven days including at least one weekend day [ 22 ]. Data were analyzed using ActiLife (v6.13.3, ActiGraph Inc.) and MVPA (minutes/day) was defined using the Evenson cut points as ≥ 2296 counts/minute [ 22 ]. Wear time was validated using the Choi algorithm [ 26 ] and data were included if participants wore the accelerometers for at least 10 waking hours per day on at least 5 days, including at least 1 weekend day [ 22 ]. Total body DXA (QDR 4500 A, Hologic Inc., USA) measured BMC, aBMD, and lean mass [ 22 ] with calibration procedures following the official recommendations of the International Society of Clinical Densitometry [ 27 ]. Height adjusted z-scores (HAZ) for BMC and aBMD were calculated as described by Zemel et al. [ 28 ]. In brief, sex-specific z-scores for BMC and aBMD were calculated relative to age from a reference dataset [ 28 ] and were then adjusted for height z-score using the Centre for Disease Control growth data [ 29 ]. Maturity offset (years from age at peak height velocity) was estimated using the approach described by Moore et al. [ 30 ]. To calculate height adjusted z-scores and maturity offset, exact chronological age was used.
Data analysis
Participants with valid DXA and accelerometry data were included in analyses. R software was used to perform statistical analyses (2023.03.1 + 446, R Core Team, Austria). We summarized participant data by group and sex using median (min, max). We assessed group differences in participant characteristics and bone outcomes using multiple linear mixed effects models using the LMER package [ 31 ]. Base model covariates included group, sex, and maturity offset, except for the model with maturity offset as the dependent variable which was only adjusted for group and sex. Subsequent models evaluated the additional contributions of MVPA, TSLH max, and total body lean mass. We assessed model assumptions of normality of residuals using QQ plots and plots of residuals against fitted values. Significance was set at p < 0.05. We explored interactions between covariates, including effect modification by pain; however, none were significant; thus, we only retained models without interactions. Participant clusters based on sex and age matched pairs were considered as random effects.
Participant characteristics
Of 32 initial participants with JIA, a subset of 21 ( n = 7 males, n = 14 females) had valid DXA and accelerometry measures and were age and sex-matched with TD adolescents (Table 1 ). We excluded 11 of the 32 participants with JIA due to incomplete DXA data ( n = 3), incomplete accelerometry data ( n = 4), or no age and sex matched pair ( n = 4). Adolescents with JIA were diagnosed between 0.0 and 3.3 years before assessment with a median of 1.2 years since diagnosis. Oligoarthritis was the most prevalent type of JIA in this sample ( n = 12), followed by polyarticular arthritis ( n = 7), and enthesis related arthritis ( n = 2). 80% of participants with JIA for which medication data were collected (missing data for 1 participant) used at least two different classes of arthritis medications including: corticosteroids, biologics, disease-modifying antirheumatic drugs (DMARDs), and non-steroidal anti-inflammatory drugs (NSAIDS). Adolescents with JIA had a median of zero joints affected and range of motion impaired (range 0–3) and low physician global assessment of disease activity [female, n = 12, median 0.0 (0.0–1.0); male, n = 6, 0.6 (range 0.0-2.5) out of 10] and parent global assessment of disease activity [female, n = 10, 0.2 (0.0–2.0); male, n = 3, 1.7 (0.0–8.0) out of 10].Pain ranged from 0 to 2.3 [JIA, n = 21, median 0.15 (range 0.0-2.3); TD, n = 21, 0.0 (0.0 to 2.0) out of 3]. No differences between groups were observed for height [B (95% CI); 0.8 (-2.4 to 4.0) cm], body mass [0.3, (-5.5 to 6.2) kg), or maturity offset [-0.1, (-1.8 to 1.7) years].
Bone mineral content and density
Adolescents with JIA had lower unadjusted aBMD [β (95% CI); -0.04 (-0.08 to -0.002) g/cm 2 , p = 0.04] and HAZ aBMD compared with their TD peers [β (95% CI); -0.58 (-1.10 to -0.07), p = 0.03 (Fig. 1 ; Table 2 ). Adolescents with JIA also had lower HAZ BMC compared with their TD peers [-0.47 (-0.91 to -0.03), p = 0.04] (Fig. 1 ; Table 2 ), but not unadjusted for height BMC (-1 (-225 to 43) g, p = 0.18). Two adolescents with JIA had low HAZ aBMD (z-score < -2.0). All participants had HAZ BMC within the normal range compared with reference data (z-score > -2.0).
MVPA, lean Mass, and TSLH
Adolescents with JIA engaged in 22 min less MVPA day than their TD peers [-22.0 (-38.7 to -5.3) min, p = 0.01] (Fig. 2 ). However, MVPA was not associated with either HAZ aBMD [-0.01 (-0.01 to 0.01) g/cm 2 , p = 0.32] or HAZ BMC [0.00 (-0.01 to 0.00) g, p = 0.39]. No differences between groups were observed for lean mass [-0.4, (-3.2 to 2.5) kg or TSLH [-10 (-61 to 42) % leg length]. Lean mass was positively associated with both HAZ aBMD [0.05 (0.01 to 0.09) g/cm 2 , p = 0.03] and HAZ BMC [0.06 (0.03 to 0.10) g, p < 0.001] (Table 2 ). TSLH was positively associated with HAZ aBMD [0.00 (0.00 to 0.01) g/cm 2 , p = 0.04] (Table 2 ) and a similar trend was indicated in HAZ BMC [0.00 (-0.00 to 0.00) g, p = 0.09] (Table 2 ).
Participants with JIA in this study had lower HAZ aBMD and HAZ BMC compared to age and sex matched peers. Our findings are supported by several studies in adolescents with JIA [ 32 , 33 , 34 ]. Despite individuals with JIA having lower HAZ aBMD and HAZ BMC than their TD counterparts, most participants with JIA had HAZ aBMD and HAZ BMC values within a healthy range (95% and 100%, respectively). This concurs with findings from Galindo Zavala and colleagues who found that fewer than 5% of children and adolescents with JIA experience low BMC and aBMD [ 35 ]. In our study cohort, this may have been due to low joint involvement and range of motion impairment (94% of participants had zero or only one joint with impaired ROM), as the relatively good disease status of participants likely facilitated bone accrual.
MVPA and bone outcomes
Participants with JIA performed substantially less MVPA per day than adolescents in the control group, which is consistent with other reports in youth with JIA [ 36 ]. Considering the relatively good disease status of the cohort, it was interesting that we observed such large group discrepancies in MVPA. Canadian physical activity guidelines recommend that youth attain 60 min per day of MVPA [ 37 ]. Only 29% of adolescents with JIA achieved this participation compared with 62% of TD adolescents in our cohort. The proportion of TD adolescents in our study who achieved the recommended daily physical activity is comparable to the 51% of Canadian youth achieving 60 min per day of MVPA pre-pandemic [ 38 ]. Individuals with JIA experience many barriers to movement that may influence lifetime bone accrual and other health benefits associated with physical activity, such as reduced pain and improved emotional well-being [ 16 , 39 ]. It may be important to consider barriers that children and adolescents with JIA face that prevent physical activity participation, including joint pain and fear of being in pain, fatigue, embarrassment about not being able to participate in sport fully, and lack of accommodations to reduce anxiety around movement [ 3 , 40 ]. Effective education strategies for children with chronic disease (and their caregivers) are needed to provide the tools to become more physically active.
It was surprising that despite group differences in MVPA and in contrast to our hypothesis, we did not observe a relationship between MVPA and HAZ aBMD or HAZ BMC. However, bone accrual is complex and influenced by several factors other than physical activity, including genetics, the endocrine environment, pharmacotherapy, and inflammation. For example, in patients with JIA, synovial macrophages produce inflammatory cytokines which increase production and activation of osteoclasts and leads to bone resorption [ 41 ]. It is possible that participants engaged in other forms of bone strengthening activities that may not have been captured by accelerometry. For example, a recent 3-month supervised lumbar spine and pelvic-core strengthening and stability program in conjunction with physical therapy (e.g., isometric strength, weight bearing, stretching, range of motion exercises) significantly improved BMC and aBMD of the femoral neck and lumbar spine compared with the control group which received only conventional physical therapy [ 4 ]. Likely neither these types of lumbar spine nor pelvic-core strengthening activities would be captured as MVPA via accelerometry as accelerometers are typically worn around the waist which is stationary and would not detect vertical acceleration during these types of exercises. It is also possible that participants with JIA decreased their MVPA after experiencing the onset of arthritis symptoms. As median years since diagnosis was short (1.2 years), a longer duration of disease may be needed to detect changes in bone health in relation to decreased MVPA. Longitudinal studies examining changes in MVPA would allow for a better understanding of the effect of physical activity on bone health in children and adolescents with JIA.
Lean mass, functional performance, and bone outcomes
Muscle mass and strength are important determinants of bone accrual [ 19 ] and TSLH is a functional test that reflects muscular strength and power of the lower limbs [ 42 ]. Consistent with the functional muscle-bone unit theory [ 43 ], we found that both greater lean mass and TSLH were related to accruing greater HAZ aBMD and HAZ BMC. Our findings reflect current literature showing that lean mass is a correlate of greater absolute and z-score aBMD in adolescents with JIA [ 44 ]. The relationships we observed between lean mass and HAZ aBMD and HAZ BMC are weaker than previously reported in children and adolescents with JIA [ 4 ]. We suspect this is because our bone outcomes were adjusted for height (body size) in addition to lean body mass (another surrogate for body size). The taller an individual is, the more lean mass and bone mass they likely have [ 45 , 46 ]. As most previous studies of bone health in adolescents with JIA did not adjust for height, lean mass would have been a stronger surrogate for body size than it was in our analyses.
Strengths and limitations
Strengths of our study include assessment of device-based physical activity and adjusting DXA bone outcomes for age and height. We acknowledge several limitations of our study. Our study sample was small and total body DXA data were collected opposed to total body less head [ 22 ], which is recommended by the International Society for Clinical Densitometry [ 27 ] due to the change in contribution of the skull to aBMD and BMC during growth [ 28 ]. Given the age-matched nature of the study, we suspect differences in bone mass (with head vs. no head) did not bias findings. A primary limitation of DXA is that it is a two-dimensional assessment and cannot account for bone depth. Therefore, aBMD is systematically underestimated in smaller individuals [ 47 ]. Adjusting bone outcomes for height helps alleviate these limitations. Future work should consider a three-dimensional imaging modality, such as peripheral quantitative computed tomography (pQCT). Further, we did not collect data regarding dose or duration of medication or duration of active disease. Finally, due to the small sample size, we were unable to stratify the participants into specific subtypes of JIA or active vs. inactive disease status. While a strength of this study is considering both height adjusted z-scores and maturity offset to minimize the influence of body size and maturation on bone outcomes, a limitation is that race/ethnicity data were not collected so all participants were designated as white for height adjusted z-score calculations [ 22 ]. Z-scores may have differed slightly from those reported if participants were not white. Future studies should account for race/ethnicity and the lack of normative DXA data beyond white and black ethnicities must be addressed.
Adolescents with JIA had significant deficits in bone outcomes compared with their TD peers. Despite substantial group differences in MVPA participation, we did not see a relationship between physical activity and bone outcomes. We found that adolescents who had more lean mass also had greater bone accrual. Physical activity promotes physical, emotional, and mental well-being and can prevent secondary consequences later in life; thus, physical activity should still be encouraged for children and adolescents with JIA as it is for their healthy peers.
Bone outcomes by group and sex: aBMD (top), BMC (bottom), females (left), males (right). aBMD = areal bone mineral density, BMC = bone mineral content, CON = typically developing controls
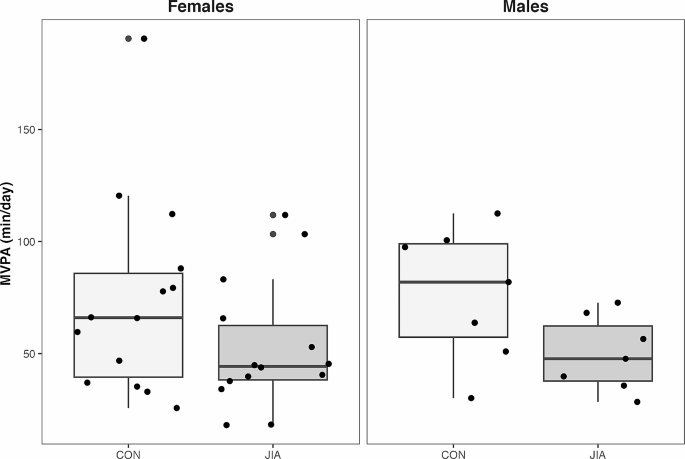
Moderate-to-vigorous physical activity (MVPA; min/day) by group for females (left) and males (right). CON = typically developing controls

Data availability
The data analysed during the current study are only available from the corresponding author on reasonable request.
Abbreviations
juvenile idiopathic arthritis
areal bone mineral density
bone mineral density
bone mineral content
typically developing
triple single leg hop
height adjusted z-scores
dual X-ray absorptiometry
moderate-vigorous physical activity
disease-modifying antirheumatic drugs
non-steroidal anti-inflammatory drugs
Swart JF, Roock S, Prakken BJ. Understanding inflammation in juvenile idiopathic arthritis: how immune biomarkers guide clinical strategies in the systemic onset subtype. Eur J Immunol. 2016;46(9):2068–77. https://doi.org/10.1002/eji.201546092 .
Article CAS PubMed Google Scholar
Saurenmann RK, Levin AV, Feldman BM, Rose JB, Laxer RM, Schneider R, et al. Prevalence, risk factors, and outcome of uveitis in juvenile idiopathic arthritis: a long-term followup study. Arthritis Rheum. 2007;56(2):647–57. https://doi.org/10.1002/art.22381 .
Chomistek K, Johnson N, Stevenson R, Luca N, Miettunen P, Benseler SM, et al. Patient-reported barriers at School for children with juvenile idiopathic arthritis. ACR Open Rheumatol. 2019;1(3):182–7. https://doi.org/10.1002/acr2.1023 .
Article PubMed Central PubMed Google Scholar
Elnaggar RK, Mahmoud WS, Moawd SA, Azab AR. Impact of core stability exercises on bone mineralization and functional capacity in children with polyarticular juvenile idiopathic arthritis: a randomized clinical trial. Clin Rheumatol. 2021;40(1):245–53. https://doi.org/10.1007/s10067-020-05219-9 .
Article PubMed Google Scholar
Kuntze G, Nesbitt C, Nettel-Aguirre A, Esau S, Scholz R, Brooks J, et al. Gait adaptations in Youth with Juvenile Idiopathic Arthritis. Arthritis Care Res. 2020;72(7):917–24. https://doi.org/10.1002/acr.23919 .
Article Google Scholar
Kuntze G, Nettel-Aguirre A, Brooks J, Esau S, Nesbitt C, Mosher D, et al. Consequences of juvenile idiopathic arthritis on single Leg Squat Performance in Youth. Arthritis Care Res. 2021;73(8):1187–93. https://doi.org/10.1002/acr.24254 .
Kuntze G, Nettel-Aguirre A, Brooks J, Esau S, Nesbitt C, Mosher D, et al. Vertical Drop Jump performance in Youth with Juvenile Idiopathic Arthritis. Arthritis Care Res. 2021;73(7):955–63. https://doi.org/10.1002/acr.24219 .
Sura A, Failing C, Sturza J, Stannard J, Riebschleger M. Patient characteristics associated with response to NSAID monotherapy in children with systemic juvenile idiopathic arthritis. Pediatr Rheumatol. 2018;16(1):2. https://doi.org/10.1186/2Fs12969-017-0219-4
Sobel RE, Lovell DJ, Brunner HI, Weiss JE, Morris PW, Gottlieb BS, et al. Safety of celecoxib and nonselective nonsteroidal anti-inflammatory drugs in juvenile idiopathic arthritis: results of the phase 4 registry. Pediatr Rheumatol. 2014;12(1):29. https://doi.org/10.1186/1546-0096-12-29 .
Li S, Zhang W, Lin Y. Application of intra-articular corticosteroid injection in Juvenile Idiopathic Arthritis. Front Pediatr. 2022;10:822009. https://doi.org/10.3389/2Ffped.2022.822009
Ferrara G, Mastrangelo G, Barone P, La Torre F, Martino S, Pappagallo G, et al. Methotrexate in juvenile idiopathic arthritis: advice and recommendations from the MARAJIA expert consensus meeting. Pediatr Rheumatol. 2018;16(1):46. https://doi.org/10.1186/s12969-018-0255-8 .
Giancane G, Muratore V, Marzetti V, Quilis N, Benavente BS, Bagnasco F, et al. Disease activity and damage in juvenile idiopathic arthritis: methotrexate era versus biologic era. Arthritis Res Ther. 2019;21(1):168. https://doi.org/10.1186/2Fs13075-09-1950-7
Lien G, Selvaag AM, Flatø B, Haugen M, Vinje O, Sørskaar D, et al. A two-year prospective controlled study of bone mass and bone turnover in children with early juvenile idiopathic arthritis: bone Mass and turnover in children with early JIA. Arthritis Rheum. 2005;52(3):833–40. https://doi.org/10.1002/art.20963 .
Kuntze G, Nesbitt C, Whittaker JL, Nettel-Aguirre A, Toomey C, Esau S, et al. Exercise Therapy in Juvenile Idiopathic Arthritis: a systematic review and Meta-analysis. Arch Phys Med Rehabil. 2018;99(1):178–e1931. https://doi.org/10.1016/j.apmr.2017.05.030 .
Burnham JM. Childhood onset arthritis is associated with an increased risk of fracture: a population based study using the General Practice Research Database. Ann Rheum Dis. 2006;65(8):1074–9. https://doi.org/10.1136/ard.2005.048835 .
Article CAS PubMed Central PubMed Google Scholar
Bos GJFJ, Lelieveld OTHM, Armbrust W, Sauer PJJ, Geertzen JHB, Dijkstra PU. Physical activity in children with juvenile idiopathic arthritis compared to controls. Pediatr Rheumatol Online J. 2016;14(1):42. https://doi.org/10.1186/s12969-016-0102-8 .
Lelieveld OTHM, Armbrust W, van Leeuwen MA, Duppen N, Geertzen JHB, Sauer PJJ, et al. Physical activity in adolescents with juvenile idiopathic arthritis. Arthritis Rheum. 2008;59(10):1379–84. https://doi.org/10.1002/art.24102 .
Bailey DA. The Saskatchewan Pediatric Bone Mineral Accrual Study: bone mineral acquisition during the growing years. Int J Sports Med. 1997;18(Suppl 3):S191–194. https://doi.org/10.1055/s-2007-972713 .
Tan VPS, Macdonald HM, Kim S, Nettlefold L, Gabel L, Ashe MC, et al. Influence of physical activity on bone strength in children and adolescents: a systematic review and narrative synthesis. J Bone Min Res off J Am Soc Bone Min Res. 2014;29(10):2161–81. https://doi.org/10.1002/jbmr.2254 .
Gerosa L, Lombardi G, Bone-to-Brain. A round trip in the adaptation to mechanical stimuli. Front Physiol. 2021;12:623893. https://doi.org/10.3389/fphys.2021.623893 .
Houghton KM, Macdonald HM, McKay HA, Guzman J, Duffy C, Tucker L. Feasibility and safety of a 6-month exercise program to increase bone and muscle strength in children with juvenile idiopathic arthritis. Pediatr Rheumatol. 2018;16(1):67. https://doi.org/10.1186/s12969-018-0283-4 .
Nesbitt C, Kuntze G, Toomey C, Esau S, Brooks J, Mosher D, et al. Secondary consequences of juvenile idiopathic arthritis in children and adolescents with knee involvement: physical activity, adiposity, fitness, and functional performance. Rheumatol Int. 2022;42(2):319–27. https://doi.org/10.1007/s00296-021-04920-5 .
Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41(4):1149–60. https://doi.org/10.3758/brm.41.4.1149 .
Brabnikova Maresova K, Jarosova K, Pavelka K, Stepan JJ. The association between lean mass and bone mineral content in the high disease activity group of adult patients with juvenile idiopathic arthritis. BMC Musculoskelet Disord. 2014;15:51. https://doi.org/10.1186/1471-2474-15-51 .
Andersson Gäre B, Ruperto N, Berg S, et al. The Swedish version of the Childhood Health Assessment Questionnaire (CHAQ) and the Child Health Questionnaire (CHQ). Clin Exp Rheumatol. 2001;19:S146–50.
PubMed Google Scholar
Choi L, Liu Z, Matthews CE, Buchowski MS. Validation of accelerometer wear and nonwear time classification algorithm. Med Sci Sports Exerc. 2011;43(2):357–64. https://doi.org/10.1249/mss.0b013e3181ed61a3 .
Hangartner TN, Warner S, Braillon P, Jankowski L, Shepherd J. The Official positions of the International Society for Clinical Densitometry: Acquisition of Dual-Energy X-Ray Absorptiometry Body composition and considerations regarding analysis and repeatability of measures. J Clin Densitom. 2013;16(4):520–36. https://doi.org/10.1016/j.jocd.2013.08.007 .
Zemel BS, Leonard MB, Kelly A, Lappe JM, Gilsanz V, Oberfield S, et al. Height Adjustment in assessing Dual Energy X-Ray Absorptiometry measurements of bone Mass and Density in Children. J Clin Endocrinol Metab. 2010;95(3):1265–73. https://doi.org/10.1210/jc.2009-2057 .
Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. CDC Growth Charts for the United States: methods and development. Vital Health Stat 11. 2000;2002(246):1–190.
Google Scholar
Moore SA, Mckay HA, Macdonald H, Nettlefold L, Baxter-Jones ADG, Cameron N, et al. Enhancing a somatic maturity prediction model. Med Sci Sports Exerc. 2015;47(8):1755–64. https://doi.org/10.1249/mss.0000000000000588 .
Bates D, Mächler M, Bolker B, Walker S. Fitting Linear mixed-effects models using lme4. J Stat Softw. 2015;67(1). https://doi.org/10.18637/jss.v067.i01 .
Brabnikova Maresova K. Secondary osteoporosis in patients with juvenile idiopathic arthritis. J Osteoporos. 2011;2011:1–7. https://doi.org/10.4061/2F2011/2F569417
Shin J, Kang MJ, Kim KN. Prevalence of Lower Bone Mineral density and its Associated factors in Korean children and adolescents with juvenile idiopathic arthritis. J Rheum Dis. 2018;25(4):248. https://doi.org/10.4078/jrd.2018.25.4.248 .
Stagi S, Cavalli L, Signorini C, Bertini F, Cerinic M, Brandi M, et al. Bone mass and quality in patients with juvenile idiopathic arthritis: longitudinal evaluation of bone-mass determinants by using dual-energy x-ray absorptiometry, peripheral quantitative computed tomography, and quantitative ultrasonography. Arthritis Res Ther. 2014;16(2):R83. https://doi.org/10.1186/2Far4525
Galindo Zavala R, Núñez Cuadros E, Martín Pedraz L, Díaz-Cordovés Rego G, Sierra Salinas C, Urda Cardona A. Low bone mineral density in juvenile idiopathic arthritis: prevalence and related factors. Pediatría Engl Ed. 2017;87(4):218–25. https://doi.org/10.1016/j.anpedi.2016.12.005 .
Nørgaard M, Twilt M, Andersen L, Herlin T. Accelerometry-based monitoring of daily physical activity in children with juvenile idiopathic arthritis. Scand J Rheumatol. 2016;45(3):179–87. https://doi.org/10.3109/03009742.2015.1057862 .
Tremblay MS, Carson V, Chaput JP, Connor Gorber S, Dinh T, Duggan M, et al. Canadian 24-Hour Movement Guidelines for Children and Youth: an integration of physical activity, sedentary Behaviour, and Sleep. Appl Physiol Nutr Metab Physiol Appl Nutr Metab. 2016;41(6 Suppl 3):S311–327. https://doi.org/10.1139/apnm-2016-0151 .
Colley R, Watt J. Youth - but not adults - reported less physical activity during the COVID-19 pandemic. StatCan. 2017; 2021001.
Sandstedt E, Fasth A, Eek MN, Beckung E. Muscle strength, physical fitness and well-being in children and adolescents with juvenile idiopathic arthritis and the effect of an exercise programme: a randomized controlled trial. Pediatr Rheumatol. 2013;11(1):7. https://doi.org/10.1186/1546-0096-11-7 .
Bourdier P, Birat A, Rochette E, Doré É, Courteix D, Dutheil F, et al. Muscle function and architecture in children with juvenile idiopathic arthritis. Acta Paediatr. 2021;110(1):280–7. https://doi.org/10.1111/apa.15335 .
Epsley S, Tadros S, Farid A, Kargilis D, Mehta S, Rajapakse CS. The effect of inflammation on bone. Front Physiol. 2021;11:511799. https://doi.org/10.3389/fphys.2020.511799 .
Hamilton RT, Shultz SJ, Schmitz RJ, Perrin DH. Triple-hop Distance as a valid predictor of Lower Limb Strength and Power. J Athl Train. 2008;43(2):144–51. https://doi.org/10.4085/1062-6050-43.2.144 .
Schoenau E. From mechanostat theory to development of the functional muscle-bone-unit. J Musculoskelet Neuronal Interact. 2005;5(3):232–8.
CAS PubMed Google Scholar
Burnham JM, Shults J, Dubner SE, Sembhi H, Zemel BS, Leonard MB. Bone density, structure, and strength in juvenile idiopathic arthritis: importance of disease severity and muscle deficits. Arthritis Rheum. 2008;58(8):2518–27. https://doi.org/10.1002/2Fart.23683
Hume R. Prediction of lean body mass from height and weight. J Clin Pathol. 1966;19(4):389–91. https://doi.org/10.1136/jcp.19.4.389 .
Forbes GB. Relation of lean body Mass to height in children and adolescents. Pediatr Res. 1972;6(1):32–7. https://doi.org/10.1203/00006450-197201000-00005 .
Leonard MB, Shults J, Elliott DM, Stallings VA, Zemel BS. Interpretation of whole body dual energy X-ray absorptiometry measures in children: comparison with peripheral quantitative computed tomography. Bone. 2004;34:1044–52.
Download references
Acknowledgements
We would like to thank the research team for data collection. We would also like to thank all the participants of this study and their families for giving their time so we can better understand the relationship between bone outcomes in JIA and physical activity.
This project was supported by the Vi Riddell Pediatric Rehabilitation Research Program. No specific funding was received from any bodies in the public, commercial, or not-for-profit sectors to carry out the work described in this article.
Author information
Authors and affiliations.
Human Performance Laboratory, Faculty of Kinesiology, University of Calgary, 2500 University Dr NW, T2N 1N4, Calgary, AB, Canada
Egi Vasil & Leigh Gabel
McCaig Institute for Bone and Joint Health, University of Calgary, Calgary, Canada
Egi Vasil, Carolyn A. Emery & Leigh Gabel
Sport Injury Prevention Research Center, Faculty of Kinesiology, University of Calgary, Calgary, Canada
Colleen M. Nesbitt, Clodagh Toomey, Shane Esau & Carolyn A. Emery
School of Allied Health, Faculty of Education and Health Sciences, University of Limerick, Limerick, Ireland
Clodagh Toomey
Alberta Bone and Joint Health Institute, Calgary, AB, Canada
Gregor Kuntze
Departments of Community Health Sciences and Paediatrics, Cumming School of Medicine, University of Calgary, Calgary, Canada
Carolyn A. Emery
Alberta Children’s Hospital Research Institute, University of Calgary, Calgary, Canada
Carolyn A. Emery & Leigh Gabel
You can also search for this author in PubMed Google Scholar
Contributions
Study design and conduct: CE, CN, CT, GK, and SE. Data analysis: EV and LG. Data interpretation: EV and LG. Drafting, revising, and approving the final manuscript: EV, CE, CN, CT, GK, SE and LG. EV takes responsibility for the integrity of the data analysis.
Corresponding author
Correspondence to Leigh Gabel .
Ethics declarations
Ethics approval and consent.
Ethics approvalwas granted by the University of Calgary Conjoint Health Research Ethics Board (REB15-312).
Consent for publication
Not applicable.
Competing interests
The authors report no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Vasil, E., M. Nesbitt, C., Toomey, C. et al. Bone health and physical activity in adolescents with juvenile idiopathic arthritis: a cross-sectional case-control study. Pediatr Rheumatol 22 , 45 (2024). https://doi.org/10.1186/s12969-024-00982-4
Download citation
Received : 19 December 2023
Accepted : 04 April 2024
Published : 19 April 2024
DOI : https://doi.org/10.1186/s12969-024-00982-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Juvenile idiopathic arthritis
- Physical activity
Pediatric Rheumatology
ISSN: 1546-0096
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Juvenile idiopathic arthritis: recognition, diagnosis and conventional pharmacological management
Shutterstock.com
Juvenile idiopathic arthritis (JIA) is an autoimmune disease whereby the body’s immune system fails to distinguish between self and non-self. The cells of the synovium — connective soft-tissue membrane that lines the inner surface of synovial joint capsules — are recognised as foreign, leading to an immune cell attack and inflammation, causing arthritis [1] . The impact of a JIA diagnosis can have a profound effect on the person and their family; it can affect emotional wellbeing and educational achievements, particularly in adolescence [2] . JIA is the leading cause of musculoskeletal disability in children and is distinct from adult rheumatoid arthritis [1] . JIA affects approximately 1 in 1,000 children in the UK and is defined as persistent joint inflammation that lasts for at least 6 weeks with onset before the age of 16 years [3] . As suggested by its name, JIA includes all types of arthritis with no apparent cause, with its aetiology not fully described [4] . Data suggest that 10–15% of children with JIA will also have uveitis [5] .
The International League of Associations for Rheumatology (ILAR) has defined subtypes based on clinical presentations (including the type and number of joints involved in conjunction with symptoms) and biochemical markers, such as rheumatoid factor (see Table 1) [6–8] .
The involvement of systemic inflammation makes systemic JIA (sJIA) distinct from other subtypes owing to its association with macrophage activation syndrome (MAS), a severe life-threatening complication [9] . MAS (sometimes called haemophagocytic lymphohistiocytosis [HLH]) is the resultant uncontrolled activation and proliferation of T lymphocytes and macrophages, which typically presents as fever, seizures, hypotension, purpura, hepatitis, splenomegaly and, occasionally, multi-system organ failure [4] . MAS occurs during active disease phases or at disease onset.
Advances in JIA treatments with novel agents, such as biologics, enable patients with JIA to live not only with suppressed symptoms but also with immunologically inactive disease. In one-third of patients, JIA symptoms can persist into adulthood [6] . The mortality rate is 0.3% to 1.0%, with 5.0% of patients having a physical disability of Class III (limited to few or no activities of the patient’s usual occupation) or Class IV (bedridden with little or no self-care), according to the four-level Stein-Brocker functional classification scale [6] .
This article will cover classification of the different types of JIA, recognition and diagnosis and conventional pharmacological management of the condition, information on management of JIA with biologic disease modifying anti-rheumatic drugs can be found in ‘Juvenile idiopathic arthritis: biologic disease modifying anti-rheumatic drugs’ .
Recognition and diagnosis
The aetiology of JIA is still poorly understood and has been associated with the influence of immunogenic mechanisms secondary to genetic, environmental and infection factors [8] . T-lymphocytes are found in the synovial fluid of JIA patients, causing the activation of macrophages to produce interleukin (IL)-1, IL-6 and tumour necrosis factor-alpha (TNF-ɑ) [10] .
There is no diagnostic test for JIA and a clinical diagnosis is made based on history and examination. The British Society for Rheumatology (BSR) provides a statement of the standards of care for children and young people with JIA [2] . Paediatric musculoskeletal assessment tools like the Gait Arms Legs Spine (pGALS), ultrasound, X-rays and MRI are commonly used to help diagnose and detect early inflammation and monitor subsequent joint diseases [11] . The Paediatric Musculoskeletal Matters website provides useful online learning information on the clinical assessment of children with musculoskeletal problems [12] .
Laboratory results can aid the diagnosis of MAS. Laboratory features include hyperferritinemia, thrombocytopenia, anaemia, leukopenia, coagulopathy and elevated levels of C-reactive protein and D-dimer [6] . Regular specialist ophthalmology reviews reviews are also required because uveitis, despite often being asymptomatic, can be sight-threatening [5] .
Management of JIA
The multidisciplinary team (MDT) is essential in managing a child in the children’s rheumatology service and enables holistic and equitable access to care. The team should comprise consultant paediatric rheumatologists and ophthalmologists, paediatric clinical nurse specialists, physiotherapists, occupational therapists, psychologists and colleagues in community care [1,2] . Pharmacists can provide advice on complex medicines, including funding and prescribing roles (in some cases). All members of the MDT should have received appropriate training and experience in paediatric rheumatology.
The MDT can help to manage and provide:
- Information on specialist medicines, which can include unlicensed medicines;
- Advice on procedural anxiety;
- Advice for people with needle phobia for both injectable medicines and blood monitoring;
- Advice on alternative formulations of medicines to improve patient concordance;
- Advice for techniques on administration of injectable and oral medicines;
- Lifestyle interventions for optimising disease control [1,2] .
Drug management and best practice in JIA
The goals of medical management of JIA is to eliminate active disease, to normalise joint function, to preserve normal growth and to prevent long-term damage of joints [13,14] . In recent years, two major trends have emerged in the drug treatment of auto-inflammatory conditions. First, the concept of a ‘window of opportunity’ suggests that targeting the disease early and aggressively to ‘switch off’ the immune process can lead to better long-term outcomes [15] . Second, ‘treat-to-target’ is now being applied to the management of JIA. This approach states that tighter disease control can be achieved by formally measuring and tracking patient outcomes, looking to achieve not just clinical but also immunological remission. Immunological remission is becoming measurable using increasingly sensitive biochemical and radiological markers, even in the absence of clinically apparent disease [16] .
Non-steroidal anti-inflammatory drugs
At initial diagnosis and at any point during a flare-up, non-steroidal anti-inflammatory drugs (NSAIDs) can be used to bridge the starting effect of other therapies, but they are no longer used alone to treat JIA [6] . NSAIDs can control inflammation if used regularly at optimised doses but will not induce remission in the long term. An individual may fail to respond to one type of NSAID but have a good therapeutic response to another [17] . Preparation, side effects and dosing frequency need to be taken into account when prescribing NSAIDs. For patients regularly taking NSAIDs, co-prescription of a gastro-protective agent should be considered, particularly if there is any history of indigestion, stomach ulcers or bleeding [18] . Active gastrointestinal bleeding is a contraindication for NSAID use [19] . Both NSAIDs and PPIs have the potential to interact with methotrexate reducing its clearance, which may increase the risk of methotrexate-related adverse drug events [18] . Concomitant use is not contraindicated but they should be used with caution alongside appropriate blood monitoring to detect methotrexate-related toxicity (see monitoring of methotrexate) [18] .
Specific JIA recommended doses of NSAIDs should be prescribed [19] . For agents such as ibuprofen, the JIA recommended dose can be twice as much as an over-the-counter dose, so the patient and parent/carer education should include that caveat. Ibuprofen is the only NSAID of choice licensed for use in JIA in children aged under five years and it is also widely available as a liquid formulation [19,20] . Diclofenac is available as a liquid formulation unlicensed special [21] . Naproxen is licensed for children aged five years and older for JIA and is available as a liquid formulation, but it does contain significant amounts of sorbitol, which can cause diarrhoea [22] . While naproxen is expensive in comparison to ibuprofen, it offers the advantage of twice-daily dosing [22] . Slow-release preparations can help with morning pain and stiffness symptoms until other systemic therapies start affecting the disease course [17] .
Intra-articular corticosteroids
Some cases of oligo-JIA can be controlled by intra-articular corticosteroids (IACs) on their own without the need to expose the patient to systemic immunosuppressants [23] . They can also be used for ‘bridging’ in extended oligo and poly-JIA flare ups, providing relief until the systemic treatment becomes fully effective [23] . The IAC of choice in JIA is triamcinolone hexacetonide, which can be used alongside intra-articular lidocaine 1% or normal saline (used to flush the needle track after the IAC injection to avoid skin changes caused by leakage) at different doses depending on the size of the joint [24] . The hexacetonide salt is less soluble than other salts, attaining synovial levels for a longer period of time. There is no stipulated maximum number of joints that can be injected in one visit with this product; however, from a practical point of view, consideration for systemic corticosteroids should take place if there are more than eight affected joints [25,26] . There has been a worldwide shortage of triamcinolone hexacetonide since early 2022 [27] . If this product cannot be sourced, triamcinolone acetonide (not equipotent, so different doses will apply), which is the first line treatment for inflamed temporomandibular joints, can be considered [19] .
Systemic corticosteroids
Given orally, intramuscularly or intravenously, systemic corticosteroids (SyC) have immediate, potent anti-inflammatory properties mediated by the inhibition of several cytokines. However, prolonged use should be avoided in children owing to their effects on bone homeostasis, growth, weight gain and neuropsychiatric symptoms [19,28] . Any child started on a course of SyC should be given a blue steroid card, which contains general advice for patients receiving steroids and can be used to record the current steroid treatment regime. It should be used for children receiving IV or IM methylprednisolone pulses (short, high-dose SyC therapy) but might not be necessary when sporadic IAC injections are used [29] .
The UK Health Security Agency’s Green Book for immunisations, which has the latest information on vaccines and vaccination procedures, suggests that significant immunosuppression should be expected in children on doses of prednisolone equivalent to 2mg/kg/day for over 1 week, OR more than 40mg/day for over 1 week, OR 1mg/kg/day for over 2 weeks [30] . Live vaccines should be avoided in patients who have received any such courses in the past three months [29] . Prednisolone (available as 1mg, 5mg and 25mg tablets, oral solution and soluble tablets) is the usual oral SyC of choice in the UK. Once daily dosing (preferably in the morning to mimic physiological cortisol production) and a variety of available formulations can aid with patient treatment adherence [28] . The usual maximum oral dose used in JIA is 60mg once daily (if weight allows) [19] . IV methylprednisolone sodium succinate can be given as ‘pulsed therapy’. IV methylprednisolone pulses can range from 10–30mg/kg (ceiling dose of 1,000mg), given on three to five consecutive days, sometimes over one to two weeks. Monthly pulses are also sometimes recommended alongside biologic therapy [14,26] . Methylprednisolone administration may vary from centre to centre. The Medusa Guidelines can be used to recommend infusion strategies and suggested monitoring [31] .
Despite SyC being used for decades in the management of childhood auto-immune rheumatological conditions, there is very little evidence with regards to the best SyC of choice or type of treatment regimens, including method of weaning high doses of SyC [32] . Many clinical trials have tried to address this evidence gap in recent years: the SIRJIA trial established a clear need for the development of a randomised controlled trial to compare different corticosteroid induction regimens in children and young people with JIA using a mixed methods approach (qualitative questionnaires alongside critical appraisal of the literature) [31] . The first randomised controlled clinical trial to compare IV methylprednisolone versus oral prednisolone for induction of remission in poly course JIA (STAR-JIA) has opened for recruitment in 2024 [33] .
Conventional disease modifying anti-rheumatic drugs
Methotrexate.
Methotrexate is the first-line disease modifying anti-rheumatic drugs (DMARD) for children with JIA. It is a cytotoxic agent with immunosuppressive properties that acts to antagonise folic acid in the body [34] . Dosing is based on body surface area and is usually 10-15mg/m 2 by subcutaneous injection or mouth once a week. Low-dose once weekly methotrexate has been the subject of several drug safety alerts. Most recently in 2020, continuing reports of inadvertent overdose prompted a series of measures including recording the day of the week on which the dose is to be taken in an attempt to prevent fatal overdose [34] . Communication with families to prevent daily dosing is essential [35] .
Optimal dosing of methotrexate for at least 12 weeks is needed before assessment of efficacy, although improvement in symptoms can occur sooner than this in some patients [36] . Most patients should trial methotrexate before consideration of a biologic therapy; however, patients with systemic JIA and associated HLH, those presenting with axial JIA or sacroiliitis do not need to trial methotrexate before starting a biologic [37] .
Methotrexate is structurally very similar to folic acid and competes with folic acid for the synthesis of purine nucleotides essential for the synthesis of DNA and hence for cell division; the most rapidly dividing cells are the ones that are affected to the greatest extent [37,38] . These are the cells of the bone marrow, GI tract, skin and hair, hence the side effects of bone marrow suppression and consequent immunosuppression, nausea and vomiting, mouth ulcers and hair thinning. Hair loss has been reported with both high dose and low dose methotrexate; however, it is also possible that hair loss may be a consequence of untreated inflammation and so it may be possible to reassure patients and their families that any hair thinning is likely to reduce as the JIA becomes better controlled [33,39,40] .
Nausea and vomiting may occur following and prior to methotrexate doses (anticipatory nausea) [3] . Various actions can help, including the addition of folic acid and giving ondansetron at least one hour before the methotrexate dose and regularly for the following 24-48 hours, if nausea persists. Giving the dose at night can be helpful. Psychological support may be needed for those suffering from anticipatory nausea [3] .
Another principal side effect of methotrexate is hepatotoxicity and careful monitoring, including full blood count, liver and kidney function, is required [33] . There have been some reports of a significant reduction in hepatic dysfunction when methotrexate is supplemented with folic acid [41] . Folic acid has also been found to help with methotrexate induced mouth ulcers. Dosing varies widely but 5mg once a week or 1mg every day, other than the day of methotrexate, are common regimens and appear not to reduce the efficacy of methotrexate [41] . Some centres routinely use higher folic acid doses. Addition of folic acid or folinic acid to methotrexate has been shown to reduce the number of patients discontinuing treatment and is often started routinely alongside methotrexate [38,39] . Folic acid and folinic acid have both been found to be effective, however folic acid is a more cost-effective folate supplement [41] .
Vaccination, both live and non-live, may be less effective when a patient is immunosuppressed. The immunosuppression associated with methotrexate does not require the avoidance of live vaccines if the dose is kept below 15mg/m 2 per week [29] . Methotrexate is a known teratogen and must be avoided in pregnancy [42] . Caution around handling cytotoxic medications should be discussed with patients and their carers if they will be administering the medication [43] .
Sulfasalazine
Used off-label, sulfasalazine can be a useful alternative DMARD for children who cannot tolerate methotrexate [44] . The disadvantages include a twice-daily dosage regimen and relatively large tablets or relatively large volume liquid preparations. Despite these issues most children appear to manage well on sulfasalazine and it is an effective drug for JIA [43] .
Dosage is usually increased over a few weeks until a maintenance dose of 20–25mg/kg per day in two divided doses (maximum 1g twice daily) is reached. Sulfasalazine can cause profound bone marrow suppression and appropriate monitoring for haematological abnormalities should be undertaken. The risk is greatest in the first three to six months of treatment [19] . Patients and their families should be warned that sulfasalazine can cause tears, urine and other body secretions to be tinged orange. This may be of particular concern for contact lens wearers who may find that their contact lenses become stained [19] .
Sulfasalazine is less immunosuppressive than methotrexate and children can receive live vaccines if they are receiving no other immunosuppression (and have not had immunosuppressive doses of steroid in the recent past). It does not usually need to be stopped during intercurrent illness; however, if a child is likely to be dehydrated (for example because of pyrexia or severe gastric upset), it is advised to withhold sulfasalazine until the child is better. Sulfasalazine causes crystalluria and kidney stone formation, which can be potentiated by inadequate hydration [45] .
Sulfasalazine should not be used by patients with aspirin (or any other salicylate) or sulfonamide allergy [43] .
Leflunomide
Leflunomide is not licensed for children. It has been shown to be effective in JIA, although methotrexate has shown a greater response when the two are directly compared [45–47] . It is an inhibitor of pyrimidine synthesis, which blocks lymphocyte activation and inflammatory response and is available as 10mg or 20mg tablets [19] .
It is generally better tolerated than methotrexate and can be a useful alternative when patients have discontinued methotrexate because of side effects. The manufacturer recommends a loading dose over the first three days [48] ; however, this has been found to be associated with more side effects, particularly gastrointestinal and liver effects, and many prescribers prefer to omit the loading doses.
Leflunomide has a very long elimination half-life (one to four weeks) and is suspected to cause birth defects if taken during pregnancy [19] . Women of childbearing potential are required to use effective contraception both during leflunomide use and for two years after stopping leflunomide. This is a conversation that should be had with young females before they start taking leflunomide and at regular intervals, and effective contraception arranged if necessary [19] .
Live vaccines should not be given while on leflunomide [47] .
Choice of conventional disease-modifying anti-rheumatic drugs
Methotrexate is the preferred first line cDMARD and is effective against both arthritis and uveitis [49] . Sulfasalazine although generally less effective than methotrexate for arthritis has been shown to reduce attacks of HLAB27 associated uveitis in adults and children [43,50] . Leflunomide, while having efficacy against JIA, appears to be less effective than methotrexate for JIA associated uveitis [45,46] . Leflunomide is associated with a higher flare rate compared to methotrexate in the treatment of chronic uveitis in juvenile idiopathic arthritis [51] .
Monitoring of conventional disease-modifying anti-rheumatic drugs
Monitoring schedules for methotrexate, sulfasalazine and leflunomide have been recommended by the British Society of Rheumatology (BSR) for adult patients with rheumatoid arthritis [52] . Centres that treat children generally adapt these guidelines for the use in children [53] . The BSR recommends the following tests: full blood count, creatinine/calculated glomerular filtration rate, liver transaminases and albumin every two weeks until on stable dose for six weeks. The same tests should be carried out monthly for three months and then at least every twelve weeks for the duration of treatment. Additional monitoring is recommended when doses are increased [52] .
Blood pressure and weight at each monitoring visit is additionally recommended for leflunomide. Sulfasalazine monitoring does not need to continue past 12 months if the patient’s previous results have not shown abnormalities [52] .
In practice, children often find having blood tests traumatic and so regimens for monitoring may be pragmatically adjusted unless the child has significant comorbidity that pre-disposes them to liver or haematological abnormalities.
Patient advice and counselling
Patients and their families should be given clear advice about risks of infection when starting any immunomodulating therapy. This should include:
- What to do if they develop or are in contact with infectious diseases including specific advice about chicken pox, measles and COVID-19 [54–56] ;
- Advice around live and non-live vaccines: this advice will depend on both the current medication and medication received in the recent past (e.g. a recent course of oral steroids may preclude live vaccine despite current medication being insufficiently immunosuppressive to warrant avoidance of live vaccines [29] );
- Travel advice: families may need letters to allow them to travel with medication in hand luggage and advice may be needed about travel vaccines;
- General advice about simple hygiene measures to reduce infection, such as hand washing may also be given;
- Patients should be given a contact number of their specialist team and should also carry any patient alert card issued by the manufacturer of their medication.
Useful resources
- Pediatric Rheumatology INternational Trials Organisation (PRINTO) ;
- Versus Arthriti: ‘Juvenile idiopathic arthritis ‘;
- British Society for Rheumatology : (paediatric and adolescence sections).
- Versus Arthritis: ‘Young people’ ;
- Versus Arthritis ;
- National Rheumatoid Arthritis Society ;
- Kids with Arthritis (CCAA) .
- 1 Scott C, Brice N. Juvenile idiopathic arthritis – an update on its diagnosis and management. S Afr Med J. 2015;105:1077. https://doi.org/10.7196/samj.2015.v105i12.10223
- 2 Davies K, Cleary G, Foster H, et al. BSPAR Standards of Care for children and young people with juvenile idiopathic arthritis. Rheumatology. 2010;49:1406–8. https://doi.org/10.1093/rheumatology/kep460
- 3 Foster HE, Brogan PA, editors. Paediatric Rheumatology . Oxford University Press 2018. https://doi.org/10.1093/med/9780198738756.001.0001
- 4 Petty R, Southwood T, Manners P, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol . 2004;31:390–2.
- 5 Rypdal V, Glerup M, Songstad NT, et al. Uveitis in Juvenile Idiopathic Arthritis. Ophthalmology. 2021;128:598–608. https://doi.org/10.1016/j.ophtha.2020.08.024
- 6 Martini A, Ravelli A, Avcin T, et al. Toward New Classification Criteria for Juvenile Idiopathic Arthritis: First Steps, Pediatric Rheumatology International Trials Organization International Consensus. J Rheumatol. 2018;46:190–7. https://doi.org/10.3899/jrheum.180168
- 7 Momah T, Ray L. Juvenile idiopathic arthritis: Old disease, new tactics. J Fam Pract . 2019;68:E8–13.
- 8 Rigante D, Bosco A, Esposito S. The Etiology of Juvenile Idiopathic Arthritis. Clinic Rev Allerg Immunol. 2014;49:253–61. https://doi.org/10.1007/s12016-014-8460-9
- 9 Bracaglia C, Prencipe G, De Benedetti F. Macrophage Activation Syndrome: different mechanisms leading to a one clinical syndrome. Pediatr Rheumatol. 2017;15. https://doi.org/10.1186/s12969-016-0130-4
- 10 Zaripova LN, Midgley A, Christmas SE, et al. Juvenile idiopathic arthritis: from aetiopathogenesis to therapeutic approaches. Pediatr Rheumatol. 2021;19. https://doi.org/10.1186/s12969-021-00629-8
- 11 Foster HE, Jandial S. pGALS – paediatric Gait Arms Legs and Spine: a simple examination of the musculoskeletal system. Pediatr Rheumatol. 2013;11. https://doi.org/10.1186/1546-0096-11-44
- 12 Clinical assessment. Paediatric Musculoskeletal Matters Online. 2024. https://www.pmmonline.org/doctor/clinical-assessment (accessed April 2024)
- 13 Hayward K, Wallace CA. Recent developments in anti-rheumatic drugs in pediatrics: treatment of juvenile idiopathic arthritis. Arthritis Res Ther. 2009;11:216. https://doi.org/10.1186/ar2619
- 14 James RA, Wedderburn LR. Modern management of juvenile idiopathic arthritis. Prescriber. 2016;27:37–43. https://doi.org/10.1002/psb.1472
- 15 Wallace CA, Giannini EH, Spalding SJ, et al. Trial of early aggressive therapy in polyarticular juvenile idiopathic arthritis. Arthritis & Rheumatism. 2012;64:2012–21. https://doi.org/10.1002/art.34343
- 16 Consolaro A, Negro G, Lanni S, et al. Toward a treat-to-target approach in the management of juvenile idiopathic arthritis. Clin Exp Rheumatol . 2012;30:S157-62.
- 17 Ardoin SP, Sundy JS. Update on nonsteriodal anti-inflammatory drugs. Current Opinion in Rheumatology. 2006;18:221–6. https://doi.org/10.1097/01.bor.0000218940.04613.cc
- 18 Dowd JE, Cimaz R, Fink CW. Nonsteroidal antiinflammatory drug‐induced gastroduodenal injury in children. Arthritis & Rheumatism. 1995;38:1225–31. https://doi.org/10.1002/art.1780380908
- 19 Paediatric Formulary Committee. BNF for Children . Medicines Complete. http://www.medicinescomplete.com (accessed April 2024)
- 20 Pinewood Healthcare. Ibuprofen 100mg/5mL Oral Suspension SmPC. Electronic Medicines Compendium. 2023. https://www.medicines.org.uk/emc/product/4560/smpc#gref (accessed April 2024)
- 21 Diclofenac sodium. BNF-C. https://bnfc.nice.org.uk/drugs/diclofenac-sodium/ (accessed April 2024)
- 22 Thornton & Ross Ltd. Naproxen 50mg/1mL Oral Suspension SmPC. Electronic Medicines Compendium. 2022. https://www.medicines.org.uk/emc/product/11105/smpc#gref (accessed April 2024)
- 23 Cleary AG. Intra-articular corticosteroid injections in juvenile idiopathic arthritis. Archives of Disease in Childhood. 2003;88:192–6. https://doi.org/10.1136/adc.88.3.192
- 24 Job-Deslandre C, Menkes C. Complications of intra-articular injections of triamcinolone hexacetonide in chronic arthritis in children. Clin Exp Rheumatol . 1990;8:413–6.
- 25 Sherry DD, Stein LD, Reed AM, et al. Prevention of leg length discrepancy in young children with pauciarticular juvenile rheumatoid arthritis by treatment with intraarticular steroids. Arthritis & Rheumatism. 1999;42:2330–4. 3.0.co;2-b”>https://doi.org/10.1002/1529-0131(199911)42:11<2330::aid-anr11>3.0.co;2-b
- 26 Zulian F. Triamcinolone acetonide and hexacetonide intra-articular treatment of symmetrical joints in juvenile idiopathic arthritis: a double-blind trial. Rheumatology. 2004;43:1288–91. https://doi.org/10.1093/rheumatology/keh313
- 27 UK drug shortages list. Clinigen Direct UK. 2024. https://www.clinigengroup.com/direct/en/shortages-list/?parentId=&page=3&countryCode=en&sortType=0&prodName= (accessed April 2024)
- 28 Prieur A. The place of corticosteroid therapy in juvenile chronic arthritis in 1992. J Rheumatol Suppl . 1993;37:32–4.
- 29 Position statement 2021-02 Use of Steroid Medication Warning Cards for Children and Young People. Neonatal and Paediatric Pharmacist Group. 2021. https://nppg.org.uk/wp-content/uploads/2021/12/Position-Statement-Steroid-Cards-V1.pdf (accessed April 2024)
- 30 Contraindications and special considerations. UK Health Security Agency. 2017. https://www.gov.uk/government/publications/contraindications-and-special-considerations-the-green-book-chapter-6 (accessed April 2024)
- 31 Medusa – the Injectable Medicines Guide. Medusa – the Injectable Medicines Guide. https://www.medusaimg.nhs.uk (accessed April 2024)
- 32 Jones AP, Clayton D, Nkhoma G, et al. Different corticosteroid induction regimens in children and young people with juvenile idiopathic arthritis: the SIRJIA mixed-methods feasibility study. Health Technol Assess. 2020;24:1–152. https://doi.org/10.3310/hta24360
- 33 Steroid TreAtment TRial in JIA (STAR-JIA): a randomised trial to compare effectiveness, safety and cost-effectiveness of intravenous versus oral corticosteroid induction regimens for children and young people with juvenile idiopathic arthritis. National Institute for Health and Care Research. 2023. https://fundingawards.nihr.ac.uk/award/NIHR134350 (accessed April 2024)
- 34 Martindale, the complete drug reference. Pharmaceutical Press. 2017. https://www.pharmaceuticalpress.com/products/martindale-the-complete-drug-reference (accessed April 2024)
- 35 Cronstein BN, Aune TM. Methotrexate and its mechanisms of action in inflammatory arthritis. Nat Rev Rheumatol. 2020;16:145–54. https://doi.org/10.1038/s41584-020-0373-9
- 36 Ringold S, Angeles‐Han ST, Beukelman T, et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Treatment of Juvenile Idiopathic Arthritis: Therapeutic Approaches for Non‐Systemic Polyarthritis, Sacroiliitis, and Enthesitis. Arthritis & Rheumatology. 2019;71:846–63. https://doi.org/10.1002/art.40884
- 37 NHS England Clinical commissioning policy statement: biologic therapies for the treatment of juvenile idiopathic arthritis. NHS England. 2015. https://www.england.nhs.uk/commissioning/wp-content/uploads/sites/12/2015/10/e03pd-bio-therapies-jia-oct15.pdf (accessed April 2024)
- 38 Rang H. Rang & Dale’s pharmacology . 8th ed. Philadelphia : Elsevier Churchill Livingstone 2016. https://search.worldcat.org/title/Rang-and-Dale’s-pharmacology/oclc/904420215 (accessed April 2024)
- 39 Methotrexate once-weekly for autoimmune diseases: new measures to reduce risk of fatal overdose due to inadvertent daily instead of weekly dosing. Medicines and Healthcare products Regulatory Agency. 2020. https://www.gov.uk/drug-safety-update/methotrexate-once-weekly-for-autoimmune-diseases-new-measures-to-reduce-risk-of-fatal-overdose-due-to-inadvertent-daily-instead-of-weekly-dosing (accessed April 2024)
- 40 Harrison S, Bergfeld W. Diffuse hair loss: Its triggers and management. CCJM. 2009;76:361–7. https://doi.org/10.3949/ccjm.76a.08080
- 41 Shea B, Swinden MV, Ghogomu ET, et al. Folic Acid and Folinic Acid for Reducing Side Effects in Patients Receiving Methotrexate for Rheumatoid Arthritis. J Rheumatol. 2014;41:1049–60. https://doi.org/10.3899/jrheum.130738
- 42 Russell MD, Dey M, Flint J, et al. British Society for Rheumatology guideline on prescribing drugs in pregnancy and breastfeeding: immunomodulatory anti-rheumatic drugs and corticosteroids. Rheumatology. 2022;62:e48–88. https://doi.org/10.1093/rheumatology/keac551
- 43 Administering subcutaneous methotrexate for inflammatory arthritis. Royal College of Nursing . 2021. https://www.rcn.org.uk/-/media/Royal-College-Of-Nursing/Documents/Publications/2021/October/009-675.pdf (accessed April 2024)
- 44 Brooks C. Sulfasalazine for the management of juvenile rheumatoid arthritis. J Rheumatol . 2001;28:845–53.
- 45 Rosemont Pharmaceuticals limited Sulfasalazine 250mg/5ml Oral SmPC. Electronic Medicines Compendium. 2023. https://www.medicines.org.uk/emc/product/413/smpc (accessed April 2024)
- 46 Bichler J, Benseler S, Krumrey-Langkammerer M, et al. Leflunomide is associated with a higher flare rate compared to methotrexate in the treatment of chronic uveitis in juvenile idiopathic arthritis. Scandinavian Journal of Rheumatology. 2015;44:280–3. https://doi.org/10.3109/03009742.2015.1013983
- 47 FOELDVARI I, WIERK A. Effectiveness of Leflunomide in Patients with Juvenile Idiopathic Arthritis in Clinical Practice. J Rheumatol. 2010;37:1763–7. https://doi.org/10.3899/jrheum.090874
- 48 Sanofi. Arava 10mg tablets SmPC. Electronic Medicines Compendium. 2022. https://www.medicines.org.uk/emc/product/4056/smpc (accessed April 2024)
- 49 Constantin T, Foeldvari I, Anton J, et al. Consensus-based recommendations for the management of uveitis associated with juvenile idiopathic arthritis: the SHARE initiative. Ann Rheum Dis. 2018;annrheumdis-2018-213131. https://doi.org/10.1136/annrheumdis-2018-213131
- 50 Heiligenhaus A, Minden K, Tappeiner C, et al. Update of the evidence based, interdisciplinary guideline for anti-inflammatory treatment of uveitis associated with juvenile idiopathic arthritis. Seminars in Arthritis and Rheumatism. 2019;49:43–55. https://doi.org/10.1016/j.semarthrit.2018.11.004
- 51 Silverman E, Mouy R, Spiegel L, et al. Leflunomide or Methotrexate for Juvenile Rheumatoid Arthritis. N Engl J Med. 2005;352:1655–66. https://doi.org/10.1056/nejmoa041810
- 52 Ledingham J, Gullick N, Irving K, et al. BSR and BHPR guideline for the prescription and monitoring of non-biologic disease-modifying anti-rheumatic drugs. Rheumatology. 2017;56:865–8. https://doi.org/10.1093/rheumatology/kew479
- 53 Paediatric and adolescent guidance. British Society for Rheumatology. 2022. https://www.rheumatology.org.uk/practice-quality/guidelines/paediatric-adolescent-guidance (accessed April 2024)
- 54 Guidelines on post exposure prophylaxis (PEP) for varicella or shingles. UK Health Secure Agency. 2023. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1134812/UKHSA-guidelines-on-VZ-post-exposure-prophylaxis-january-2023.pdf (accessed April 2024)
- 55 Guidelines on Post-Exposure Prophylaxis for measles. Public Health England. . 2019. https://www.gov.uk/government/publications/measles-post-exposure-prophylaxis (accessed April 2024)
- 56 COVID-19: guidance for people whose immune system means they are at higher risk. Department of Health and Social Care, UK Health Security Agency. 2023. https://www.gov.uk/government/publications/covid-19-guidance-for-people-whose-immune-system-means-they-are-at-higher-risk/covid-19-guidance-for-people-whose-immune-system-means-they-are-at-higher-risk (accessed April 2024)
Test your knowledge by completing the questions associated with this article.
More CPD modules are available in the 'My CPD' section of your account.
Please leave a comment Cancel reply
You must be logged in to post a comment.
You might also be interested in…
Juvenile idiopathic arthritis: biologic disease modifying anti-rheumatic drugs
First drug for severe alopecia recommended by NICE could benefit 14,000 patients
Community pharmacy could detect unknown cases of coeliac disease
Caregiver burden in families of children with juvenile idiopathic arthritis in India
- ORIGINAL ARTICLE
- Published: 25 April 2024
Cite this article

- Nikhil C. Gowda 1 ,
- Rudrarpan Chatterjee ORCID: orcid.org/0000-0002-3558-0178 1 ,
- Anu Balakrishnan ORCID: orcid.org/0000-0003-4021-0654 1 ,
- Able Lawrence ORCID: orcid.org/0000-0003-3439-8048 1 &
- Amita Aggarwal ORCID: orcid.org/0000-0002-2187-5186 1
39 Accesses
Explore all metrics
Juvenile Idiopathic Arthritis (JIA) causes caregiver burden on families with children affected with it. Our study aimed to explore this multifaceted burden in the Indian context. In this cross-sectional study, we administered the Hindi translated CAREGIVER questionnaire to adult caregivers in the families of JIA patients ≤ 18 years. The responses to the 28 items were used to calculate the burden scores in various dimensions. The relationship of the global burden scores with demographic and socioeconomic factors were analysed. Non parametric tests were used. Two hundred twenty-one caregivers participated with a median age of 39 years (IQR 32–45). This included 116 fathers, 50 mothers, 32 brothers, 18 uncles, three grandfathers, one sister, and one grandmother. The JIA patients had a median age of 15 (12–17) years, and the male-to-female ratio was 3.2:1. Enthesitis-related arthritis was the predominant subtype (72.4%). Most caregivers (70.6%) expressed sadness at diagnosis, and 29.9% continued to express sadness. Nearly two-thirds (65.6%) had to borrow money from others. More than half (59.3%) of the caregivers neglected their health, and 9.0% became sick. Male gender of the child, systemic JIA subtype, low socioeconomic status, high disease activity, extra-articular damage, high parent-reported disease activity and poor quality of life were associated with higher global caregiver burden. JIA has a significant emotional, social, economic, and labour impact on caregivers. Economic and psychosocial support needs to be given to family caregivers caring for children with JIA.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others
Elevating the Standard of Care for Patients with Axial Spondyloarthritis: ‘Calls to Action’ from Rheumacensus, a Multistakeholder Pan-European Initiative

Dementia Caregiver Burden: a Research Update and Critical Analysis
Rethinking disability: the social model of disability and chronic disease, data availability.
The raw data underlying this article cannot be shared publicly to protect the privacy of the participants. The data will be shared on reasonable request to the corresponding author.
Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, He X, Maldonado-Cocco J, Orozco-Alcala J, Prieur AM, Suarez-Almazor ME, Woo P (2004) International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol 31:390–392
PubMed Google Scholar
Thierry S, Fautrel B, Lemelle I, Guillemin F (2014) Prevalence and incidence of juvenile idiopathic arthritis: a systematic review. Joint Bone Spine 81:112–117. https://doi.org/10.1016/j.jbspin.2013.09.003
Article PubMed Google Scholar
Abujam B, Mishra R, Aggarwal A (2014) Prevalence of musculoskeletal complaints and juvenile idiopathic arthritis in children from a developing country: a school-based study. Int J Rheum Dis 17:256–260. https://doi.org/10.1111/1756-185x.12276
Moorthy LN, Peterson MG, Hassett AL, Lehman TJ (2010) Burden of childhood-onset arthritis. Pediatr Rheumatol Online J 8:20. https://doi.org/10.1186/1546-0096-8-20
Article PubMed PubMed Central Google Scholar
Foster HE, Marshall N, Myers A, Dunkley P, Griffiths ID (2003) Outcome in adults with juvenile idiopathic arthritis: a quality of life study. Arthritis Rheum 48:767–775. https://doi.org/10.1002/art.10863
Street RL Jr, Elwyn G, Epstein RM (2012) Patient preferences and healthcare outcomes: an ecological perspective. Expert Rev Pharmacoecon Outcomes Res 12:167–180. https://doi.org/10.1586/erp.12.3
Brandelli YN, Tutelman PR, Chambers CT, Parker JA, Stinson JN, Huber AM, Stirling Cameron E, Wilson JP (2022) “Every Little Furrow of Her Brow Makes Me Want To Stop”: An Interpretative Phenomenologic Analysis of Mothers’ Experiences With Juvenile Idiopathic Arthritis Treatments. Arthritis Care Res (Hoboken) 74:1761–1769. https://doi.org/10.1002/acr.24735
Wells DK, James K, Stewart JL, Moore IM, Kelly KP, Moore B, Bond D, Diamond J, Hall B, Mahan R, Roll L, Speckhart B (2002) The care of my child with cancer: a new instrument to measure caregiving demand in parents of children with cancer. J Pediatr Nurs 17:201–210. https://doi.org/10.1053/jpdn.2002.124113
Ryan JL, Mullins LL, Ramsey RR, Bonner MS, Jarvis JN, Gillaspy SR, Chaney JM (2002) The care of my child with cancer: a new instrument to measure caregiving demand in parents of children with cancer. J Pediatr Nurs 17:201–210. https://doi.org/10.1053/jpdn.2002.124113
Article Google Scholar
Knafl K, Leeman J, Havill NL, Crandell JL, Sandelowski M (2015) The Contribution of Parent and Family Variables to the Well-Being of Youth With Arthritis. J Fam Nurs 21:579–616. https://doi.org/10.1177/1074840715601475
Yuwen W, Lewis FM, Walker AJ, Ward TM (2017) Struggling in the Dark to Help My Child: Parents’ Experience in Caring for a Young Child with Juvenile Idiopathic Arthritis. J Pediatr Nurs 37:e23–e29. https://doi.org/10.1016/j.pedn.2017.07.007
Gómez-Ramírez O, Gibbon M, Berard R, Jurencak R, Green J, Tucker L, Shiff N, Guzman J (2016) A recurring rollercoaster ride: a qualitative study of the emotional experiences of parents of children with juvenile idiopathic arthritis. Pediatr Rheumatol Online J 14:13. https://doi.org/10.1186/s12969-016-0073-9
Mawani N, Amine B, Rostom S, El Badri D, Ezzahri M, Moussa F, Shyen S, Gueddari S, Wabi M, Shkirat B, Hassouni NH (2013) Moroccan parents caring for children with juvenile idiopathic arthritis: positive and negative aspects of their experiences. Pediatr Rheumatol Online J 11:39. https://doi.org/10.1186/1546-0096-11-39
Torres-Made MD, Peláez-Ballestas I, García-Rodríguez F, Villarreal-Treviño AV, Fortuna-Reyna BJ, de la O-Cavazos ME, Rubio-Pérez NE, (2020) Development and validation of the CAREGIVERS questionnaire: multi-assessing the impact of juvenile idiopathic arthritis on caregivers. Pediatr Rheumatol Online J 18:3. https://doi.org/10.1186/s12969-020-0400-z
Fortuna-Reyna BJ, Peláez-Ballestas I, García-Rodríguez F, Faugier-Fuentes E, Mendieta-Zerón S, Villarreal-Treviño AV, Rosiles-De la Garza SG, Reyes-Cordero G, Jiménez-Hernández S, Guadarrama-Orozco JH, de la O-Cavazos ME, Rubio-Pérez N (2021) Psychosocial and economic impact of rheumatic diseases on caregivers of Mexican children. Pediatr Rheumatol Online J 19:30. https://doi.org/10.1186/s12969-021-00524-2
Aggarwal A, Misra R (1994) Juvenile chronic arthritis in India: is it different from that seen in Western countries? Rheumatol Int 14:53–56. https://doi.org/10.1007/bf00300247
Article CAS PubMed Google Scholar
Kunjir V, Venugopalan A, Chopra A (2010) Profile of Indian patients with juvenile onset chronic inflammatory joint disease using the ILAR classification criteria for JIA: a community-based cohort study. J Rheumatol 37:1756–1762. https://doi.org/10.3899/jrheum.090937
Aggarwal A, Khubchandani R, Sawhney S, Rahman MT, Agarwal M, Consolaro A, Bovis F, Ruperto N; Paediatric Rheumatology International Trials Organisation (PRINTO) (2018) The Hindi version of the Juvenile Arthritis Multidimensional Assessment Report (JAMAR). Rheumatol Int 38(Suppl 1):235-242. https://doi.org/10.1007/s00296-018-3957-9
Filocamo G, Consolaro A, Schiappapietra B, Dalprà S, Lattanzi B, Magni-Manzoni S, Ruperto N, Pistorio A, Pederzoli S, Civino A, Guseinova D, Masala E, Viola S, Martini A, Ravelli A (2011) A new approach to clinical care of juvenile idiopathic arthritis: the Juvenile Arthritis Multidimensional Assessment Report. J Rheumatol 38:938–953. https://doi.org/10.3899/jrheum.100930
Consolaro A, Giancane G, Schiappapietra B, Davì S, Calandra S, Lanni S, Ravelli A (2016) Clinical outcome measures in juvenile idiopathic arthritis. Pediatr Rheumatol Online J 14:23. https://doi.org/10.1186/s12969-016-0085-5
Viola S, Felici E, Magni-Manzoni S, Pistorio A, Buoncompagni A, Ruperto N, Rossi F, Bartoli M, Martini A, Ravelli A (2005) Development and validation of a clinical index for assessment of long-term damage in juvenile idiopathic arthritis. Arthritis Rheum 52:2092–2102. https://doi.org/10.1002/art.21119
Cavallo S, Feldman DE, Swaine B, Meshefedjian G, Malleson PN, Duffy CM (2009) Is parental coping associated with quality of life in juvenile idiopathic arthritis? Pediatr Rheumatol Online J 7:7. https://doi.org/10.1186/1546-0096-7-7
Raina P, O’Donnell M, Rosenbaum P, Brehaut J, Walter SD, Russell D, Swinton M, Zhu B, Wood E (2005) The Health and Well-Being of Caregivers of Children With Cerebral Palsy. Pediatrics 115:e626–e636. https://doi.org/10.1542/peds.2004-1689
Talarico R, Marinello D, Manzo A, Cannizzo S, Palla I, Ticciati S, Gaglioti A, Trieste L, Pisa L, Badalamenti L, Randisi G, Del Bianco A, Lorenzoni V, Turchetti G, Mosca M (2021) Being a caregiver of a Behçet’s syndrome patient: challenges and perspectives during a complex journey. Orphanet J Rare Dis 16:436. https://doi.org/10.1186/s13023-021-02070-2
Livermore P, Ainsworth S, Beesley R, Douglas S, Earle E, Wilson D, Woolley L, Clinch J (2023) ‘The current mental health status of children and young people with JIA, and their wider family’: a charity partner collaboration survey. Pediatr Rheumatol Online J 21:111. https://doi.org/10.1186/s12969-023-00898-5
Chausset A, Gominon AL, Montmaneix N, Echaubard S, Guillaume-Czitrom S, Cambon B, Miele C, Rochette E, Merlin E (2016) Why we need a process on breaking news of Juvenile Idiopathic Arthritis: a mixed methods study. Pediatr Rheumatol Online J 14:31. https://doi.org/10.1186/s12969-016-0092-6
García-Rodríguez F, Gamboa-Alonso A, Jiménez-Hernández S, Ochoa-Alderete L, Barrientos-Martínez VA, Alvarez-Villalobos NA, Luna-Ruíz GA, Peláez-Ballestas I, Villarreal-Treviño AV, de la O-Cavazos ME, Rubio-Pérez N (2021) Economic impact of Juvenile Idiopathic Arthritis: a systematic review. Pediatr Rheumatol Online J 19:152. https://doi.org/10.1186/s12969-021-00641-y
Kip MMA, Currie G, Marshall DA, Grazziotin Lago L, Twilt M, Vastert SJ, Swart JF, Wulffraat N, Yeung RSM, Benseler SM, IJzerman MJ; UCAN CAN-DU Health Economics Working Group (2019) Seeking the state of the art in standardized measurement of health care resource use and costs in juvenile idiopathic arthritis: a scoping review. Pediatric Rheumatology 17:20. https://doi.org/10.1186/s12969-019-0321-x
Angelis A, Tordrup D, Kanavos P (2015) Socio-economic burden of rare diseases: A systematic review of cost of illness evidence. Health Policy 119:964–979. https://doi.org/10.1016/j.healthpol.2014.12.016
Sangar S, Dutt V, Thakur R (2019) Comparative Assessment of Economic Burden of Disease in Relation to Out of Pocket Expenditure. Front Public Health 7:9. https://doi.org/10.3389/fpubh.2019.00009
Chivukula U, Kota S, Nandinee D (2018) Burden Experience of Caregivers of Acute Lymphoblastic Leukemia: Impact of Coping and Spirituality. Indian J Palliat Care 24:189–195. https://doi.org/10.4103/ijpc.ijpc_209_17
Popp JM, Robinson JL, Britner PA, Blank TO (2014) Parent adaptation and family functioning in relation to narratives of children with chronic illness. J Pediatr Nurs 29:58–64. https://doi.org/10.1016/j.pedn.2013.07.004
Stinson JN, Feldman BM, Duffy CM, Huber AM, Tucker LB, McGrath PJ, Tse SM, Hetherington R, Spiegel LR, Campillo S, Benseler S, Gill N, White ME, Baker N, Vijenthira A (2012) Jointly managing arthritis: information needs of children with juvenile idiopathic arthritis (JIA) and their parents. J Child Health Care 16:124–140. https://doi.org/10.1177/1367493511430679
Kuhlmann A, Schmidt T, Treskova M, López-Bastida J, Linertová R, Oliva-Moreno J, Serrano-Aguilar P, Posada-de-la-Paz M, Kanavos P, Taruscio D, Schieppati A, Iskrov G, Péntek M, Delgado C, von der Schulenburg JM, Persson U, Chevreul K; BURQOL-RD Research Network (2016) Social/economic costs and health-related quality of life in patients with juvenile idiopathic arthritis in Europe. Eur J Health Econ 17 Suppl 1:79-87. https://doi.org/10.1007/s10198-016-0786-1
Bruns A, Hilário MOE, Jennings F, Silva CA, Natour J (2008) Quality of life and impact of the disease on primary caregivers of juvenile idiopathic arthritis patients. Joint Bone Spine 75:149–154. https://doi.org/10.1016/j.jbspin.2007.06.016
Angelis A, Kanavos P, López-Bastida J, Linertová R, Serrano-Aguilar P; BURQOL-RD Research Network (2016) Socioeconomic costs and health-related quality of life in juvenile idiopathic arthritis: a cost-of-illness study in the United Kingdom. BMC Musculoskelet Disord 17:321. https://doi.org/10.1186/s12891-016-1129-1
Shenoi S, Horneff G, Cidon M, Ramanan AV, Kimura Y, Quartier P, Foeldvari I, Zeft A, Lomax KG, Gregson J, Abma T, Campbell-Hill S, Weiss J, Patel D, Marinsek N, Wulffraat N (2018) The burden of systemic juvenile idiopathic arthritis for patients and caregivers: an international survey and retrospective chart review. Clin Exp Rheumatol 36:920–928
Roper SO, Allred DW, Mandleco B, Freeborn D, Dyches T (2014) Caregiver burden and sibling relationships in families raising children with disabilities and typically developing children. Fam Syst Health 32:241–246. https://doi.org/10.1037/fsh0000047
Mulligan K, Etheridge A, Kassoumeri L, Wedderburn LR, Newman S (2009) Do mothers and fathers hold similar views about their child’s arthritis? Arthritis Rheum 61:1712–1718. https://doi.org/10.1002/art.25008
Shoop-Worrall SJW, Wu Q, Davies R, Hyrich KL, Wedderburn LR (2019) Predicting disease outcomes in juvenile idiopathic arthritis: challenges, evidence, and new directions. Lancet Child Adolesc Health 3:725–733. https://doi.org/10.1016/s2352-4642(19)30188-9
Bramanti SM, Manippa V, Babore A, Dilillo A, Marcellino A, Martucci V, Mallardo S, Isoldi S, Bloise S, Sanseviero M, Iorfida D, De Luca E, Trumello C, D'Alleva F, Ventriglia F, Lubrano R, Del Giudice E (2022) Comparing parental distress and children’s difficulties between parents of children with rheumatic diseases and parents of healthy children in families facing the COVID-19 pandemic. Curr Psychol. https://doi.org/10.1007/s12144-022-03589-8
Download references
Acknowledgements
The authors would like to thank Mr. Vinod, Mr. Arvind and Mr. Abhishek for help with the forward translations, Ms. Namisha, Mr. Neeraj and Mrs. Rashmi for help with back translations, and Mr. Akash for assisting the caregivers in filling the forms. The authors would like to thank all the families recruited in the study for their valuable time.
No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this article.
Author information
Authors and affiliations.
Department of Clinical Immunology and Rheumatology, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India, 226014
Nikhil C. Gowda, Rudrarpan Chatterjee, Anu Balakrishnan, Able Lawrence & Amita Aggarwal
You can also search for this author in PubMed Google Scholar
Contributions
AA conceived and designed the study. NCG and AB collected the data. AA and AL contributed the data. NCG and RC performed the analysis. AA and NCG wrote the paper. All authors critically reviewed the manuscript. All co-authors have read the final version, approved it and take full responsibility for the integrity of the study and the final manuscript.
Corresponding author
Correspondence to Amita Aggarwal .
Ethics declarations
Disclosures, informed consent.
Written informed consent from the participants has been obtained from all the participants. Patient confidentiality is maintained.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (PDF 177 KB)
Supplementary file2 (pdf 164 kb), rights and permissions.
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Gowda, N.C., Chatterjee, R., Balakrishnan, A. et al. Caregiver burden in families of children with juvenile idiopathic arthritis in India. Clin Rheumatol (2024). https://doi.org/10.1007/s10067-024-06975-8
Download citation
Received : 20 February 2024
Revised : 16 April 2024
Accepted : 19 April 2024
Published : 25 April 2024
DOI : https://doi.org/10.1007/s10067-024-06975-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Childhood arthritis
- Economic impact
- Family relationship
- Multidimensional impact
- Find a journal
- Publish with us
- Track your research

IMAGES
VIDEO
COMMENTS
Introduction: Juvenile idiopathic arthritis (JIA) is an umbrella term for inflammatory arthritis occurring before the age of 16 years. JIA is associated with several extra-articular manifestations including uveitis, macrophage activation syndrome, inflammatory bowel disease and psoriasis. JIA affects 1:1000 children and young people (CYP) and ...
Systemic juvenile idiopathic arthritis (sJIA, also called Still's disease) is a rare childhood auto-inflammatory disease with significant morbidity. This case report illustrates the clinical course and highlights diagnostic challenges.
Juvenile idiopathic arthritis (JIA) is not a disease but an umbrella term for arthritis of unknown origin, lasting for >6 weeks and with onset at <16 years of age 1, 2. Two classification systems ...
We report a case of sixteen years old girl suffering from polyarticular type of juvenile idiopathic arthritis refractory to multiple treatment options. ... et al. Use and effectiveness of rituximab in children and young people with juvenile idiopathic arthritis in a cohort study in the United Kingdom. ... Vidović, M. et al. Beyond the ...
E-mail: [email protected], Ph: 0091 9975188055. ABSTRACT: The prevalence of Juvenile idiopathic arthritis (JIA) is 0.86 per 1000 children. Subcutaneous nodules have been reported in 5% to 10 ...
Extrapolation in juvenile i diopathic arthritis (JIA) 2 . Certolizumab pegol (CZP) case-study . 1. CZP overview 2. JIA overview 3. CZP JIA clinical trial programs overview 4. Evidence to support efficacy extrapolation 5. Extrapolation of exposure, allometric scaling 6. Planned interim review of data 7. Analysis performed (1) 8. Analysis ...
Joint pain is a common complaint in pediatrics and is most often attributed to overuse or injury. In the face of persistent, severe, or recurrent symptoms, the differential typically expands to include bony or structural causes versus rheumatologic conditions. Rarely, a child has two distinct causes for joint pain. In this case, an obese 15-year-old male was diagnosed with gout, a disease ...
JIA Case Study Meet Elizabeth, an 8-year-old girl with a new diagnosis of polyarticular juvenile idiopathic arthritis (JIA). Follow Elizabeth from the initial presentation of her symptoms through diagnosis, treatment and the overall management of her case. Children with JIA can have widely varying experiences of the disease, but this case study ...
Background Juvenile idiopathic arthritis (JIA) describes heterogenous categories of chronic inflammatory rheumatic conditions of unknown origin in children and adolescents. Epidemiological data in the literature vary, depending on geographic location, ethnicity and the case definition used. We evaluated epidemiology, especially that of the categories defined by the International League of ...
Abstract. Systemic juvenile idiopathic arthritis (sJIA) is an inflammatory disease with hallmarks of severe systemic inflammation, which can be accompanied by arthritis. Contemporary scientific ...
Juvenile idiopathic arthritis treatment has evolved with new therapies, early remission goals and global efforts, including randomized trials and a treat-to-target strategy. This Review summarizes ...
Juvenile idiopathic arthritis (JIA) is an autoimmune disease acquired during childhood. JIA results from a disturbed balance between proinflammatory effector cells and anti-inflammatory regulating cells [].In Canada, 1 in 1000 children suffer from JIA which affects 0.07-4.01 per 1000 youth worldwide [].Children with JIA suffer from a range of symptoms including joint pain and swelling which ...
Treatment for juvenile idiopathic arthritis has undergone substantial changes in recent decades. These changes are partly due to the availability of new treatments, mainly biological agents, as well as developments in treatment strategies, including a focus on concepts such as treat-to-target. In addition, the creation of large paediatric research networks has improved patient access to, and ...
Juvenile idiopathic arthritis (JIA) is the most common rheumatic disease of childhood, presenting clinically as inflammatory arthritis in children younger than 16 years. ... Does sport negatively influence joint scores in patients with juvenile rheumatoid arthritis: an 8-year prospective study. Rheumatol Int. 1993;12(6):239-242. https://doi ...
Juvenile idiopathic Arthritis (JIA) is a common rheumatic disorder in children that can cause multiple systems to be affected simultaneously, leading to severe clinical symptoms and a high mortality rate in those with pulmonary involvement. ... A case study by Luciana et al. reported the use of IL-1 inhibitor canakinumab to treat a patient with ...
BACKGROUND AND OBJECTIVE:. Recent evidence has linked childhood antibiotic use and microbiome disturbance to autoimmune conditions. This study tested the hypothesis that antibiotic exposure was associated with newly diagnosed juvenile idiopathic arthritis (JIA).METHODS:. We performed a nested case-control study in a population-representative medical records database from the United Kingdom ...
Juvenile idiopathic arthritis (JIA) is an autoimmune disease whereby the body's immune system fails to distinguish between self and non-self. The cells of the synovium — connective soft-tissue membrane that lines the inner surface of synovial joint capsules — are recognised as foreign, leading to an immune cell attack and inflammation, causing arthritis [1] . The impact of […]
Results . Median age of juvenile idiopathic arthritis-associated uveitis diagnosis was 11 (Interquartile Range: 8 to 15). In the Cox models adjusting for sociodemographic and insurance factors, the hazard ratios of best corrected visual acuity 20/200 or worse were higher in males compared to females (HR 2.15; 95% CI: 1.45-3.18), in Black or African American patients compared to White ...
Prior studies indicated that women with rheumatoid arthritis (RA) and juvenile idiopathic arthritis (JIA) are at twice higher risk of developing adverse pregnancy outcomes, this include preterm births and infants with low birth weight. A wide knowledge gap exists in our current understanding of how RA and JIA affect fetal growth during pregnancy.
Objective Our study was designed to investigate the reasons for starting the conventional disease-modifying anti-rheumatic drugs (DMARDs) and the variables that impact the response to DMARD treatment in oligoarticular juvenile idiopathic arthritis (JIA) patients. Methods Oligoarticular JIA patients (n = 187) were categorized into two groups: Group A consisted of patients who achieved remission ...
Juvenile Idiopathic Arthritis (JIA) causes caregiver burden on families with children affected with it. Our study aimed to explore this multifaceted burden in the Indian context. In this cross-sectional study, we administered the Hindi translated CAREGIVER questionnaire to adult caregivers in the families of JIA patients ≤ 18 years. The responses to the 28 items were used to calculate the ...