An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Behav Sci (Basel)


What We Know About the Brain Structure–Function Relationship
Karla batista-garcía-ramó.
1 Images Processing Group, Basic Division, International Center for Neurological Restoration (Ciren), La Habana 11300, Cuba
Caridad Ivette Fernández-Verdecia
2 Biomodels Laboratory, Basic Division, International Center for Neurological Restoration (Ciren), La Habana 11300, Cuba; uc.neric.oruen@fettevi
How the human brain works is still a question, as is its implication with brain architecture: the non-trivial structure–function relationship. The main hypothesis is that the anatomic architecture conditions, but does not determine, the neural network dynamic. The functional connectivity cannot be explained only considering the anatomical substrate. This involves complex and controversial aspects of the neuroscience field and that the methods and methodologies to obtain structural and functional connectivity are not always rigorously applied. The goal of the present article is to discuss about the progress made to elucidate the structure–function relationship of the Central Nervous System, particularly at the brain level, based on results from human and animal studies. The current novel systems and neuroimaging techniques with high resolutive physio-structural capacity have brought about the development of an integral framework of different structural and morphometric tools such as image processing, computational modeling and graph theory. Different laboratories have contributed with in vivo, in vitro and computational/mathematical models to study the intrinsic neural activity patterns based on anatomical connections. We conclude that multi-modal techniques of neuroimaging are required such as an improvement on methodologies for obtaining structural and functional connectivity. Even though simulations of the intrinsic neural activity based on anatomical connectivity can reproduce much of the observed patterns of empirical functional connectivity, future models should be multifactorial to elucidate multi-scale relationships and to infer disorder mechanisms.
1. Introduction
How the human brain works is still an open question, as is its implication with brain architecture: the non-trivial structure–function relationship. The development of neuroimaging techniques, the improvement on their processing methods and the unfolding of computational neuroscience field have driven brain research focused on the relationship between anatomical and functional interactions. The principal hypothesis is that the anatomic architecture determines, but not strictly, the network dynamics [ 1 , 2 , 3 , 4 ], meaning that part of functional connectivity cannot be explained by considering only anatomical connectivity. This hypothesis is based not only on human studies but also on animal models, with the advantage that they can be correlated with genetic, histological and molecular methodologies. Network theory, computational modeling and complex system analysis have played pivotal roles in elucidating structure–function relationship.
Studies on connectivity patterns, both functional and anatomical, in humans and animals [ 5 , 6 , 7 , 8 , 9 , 10 ], and their relationship with neurodegenerative conditions such as Alzheimer’s and Parkinson’s diseases [ 11 , 12 , 13 , 14 , 15 , 16 ] and multiple sclerosis [ 17 , 18 ] has been published in recent years. The brain is considered the most complex system in the Animal kingdom, with complex space-time patterns, and where the degree of correspondence between structural and functional connectivity depends on spatial resolution and time scales [ 19 ]. These elements in turn are continuously modified based on the sensorial inputs that enter the system and the feedback that emerges from the interaction between the processing of these signals and the emergence of eferences whose result is functional and adaptive remodeling at different organizational levels.
Computational models allow inferring the network dynamics from empirical anatomical connectivity obtained from diffusion weighted imaging. Several authors showed that the similarities between the simulated functional matrix, by implementing different types of computational models, and the empirical functional matrix, obtained from functional neuroimaging techniques, are maximum when the dynamics of the global network operates on a critical point of state transition [ 20 , 21 , 22 ]. This finding is supported by empirical evidence, from functional neuroimaging techniques, that show that the dynamical regime of resting state corresponds to critical point of state transition [ 23 , 24 ]. Critical dynamics are crucial from the functional point of view given that in this critical state the system presents an optimal sensitivity to possible inputs and a maximization of the number of different functional states available. This allows the system to rapidly compute a specific brain function according to the stimulus received. These findings suggest that this is an optimized state for information transmission and for early adaptation to external perturbation.
However, the high specificity of the mechanisms that condition the structure–function relationship of neural systems is today an incompletely answered question. There is evidence of the modulation of this interrelation by activity and behavior in its broadest sense, but how this feedback works in the time-space context of neural systems is still a big question. This involves complex and controversial aspects of the neurosciences and that the methods and methodologies to obtain both structural and functional connectivity are not always rigorously applied. The heterogeneity regarding methodological approaches and polemic points such as the mechanism driving functional connectivity and spatio-temporal scales make the comprehension and interpretation of the structure–function relationship difficult.
There are questions involving structure–function relationship without a definitive answer: How can the functional activation patterns be changed? How do plasticity mechanisms become involved? In what way and under what conditions do different favorable plastic responses, neuroprotective and successful from the adaptive point of view, happen to be aberrant events and therefore an expression of maladaptation? A deeper understanding of structure–function relationship is still difficult today, as is how this relationship is modified in some neuropathologies.
There are reviews of this topic from different perspectives [ 19 , 25 , 26 , 27 , 28 ]. We have organized this review summarizing those concepts related to the structure–function relationship within the framework of neuroimaging; that is, updated definitions of anatomic and functional connectivity, the methods used to extract these measures, and their interpretation under different spatial-temporal scales. The following section deals with the network theory and computational modeling related to brain function. Afterwards, we discuss the main findings on the structure–function relationship under neurodiscapacity conditions. Finally, we expose limitations identified in current studies to be considered in future research on this topic.
2. Definitions
2.1. structural connectivity.
Anatomical or structural connectivity (SC) refers to physical synaptic connections between neural elements or the white matter fibers connecting gray matter regions, depending on the spatial scale. Tract tracing technology constitutes an invasive method for estimating the weight of axonal connectivity between a pair of pre-defined brain regions at micro-scale. This technique allows a deep characterization of synaptic termination of connections at cellular level and an assessment of the directionality of white matter projections. Tract-tracing studies have allowed collecting connectomes of the mouse [ 29 , 30 ], rat [ 31 ] and the macaque [ 32 ]. On the other hand, diffusion weighted imaging (DWI), an MRI technique, is a non-invasive method to record in vivo white matter’s architecture [ 33 ]. The present review focuses on this non-invasive technique that corresponds to the macro-scale, but its correspondence with the micro-scale is analyzed below. DWI really provides information about the restrictions on the diffusion of water molecules, which is used to infer the orientation of white matter tracts (for review, see [ 34 ]). The main limitations of SC based on DWI are that the connections are non-directed because tractography algorithms cannot determine the direction of axonal projections, and they cannot differentiate between excitatory or inhibitory connections. These limitations are due to intrinsic features of neuroimaging technique.
Different tractography algorithms and software packages have been developed to estimate the likelihood of the existence of connections between two regions from DWI data. In general, these methods can be classified into two types considering how the white matter tracts are modeled within the voxel: probabilistic tractography based methods [ 35 , 36 , 37 ] and deterministic fiber tracking tractography [ 38 , 39 , 40 , 41 ]. The streamline approach is advantageous to characterize long white matter tracts. However, the probabilistic tractography is more effective in resolving crossing fibers [ 42 ]. In any case, using both methods, it is possible to construct structural networks. Bastiani et al. [ 43 ] argued the advantages and disadvantages of tractography algorithms considering not only deterministic or probabilistic distinction but also whether the model is a single-direction or multiple-direction intra-voxel diffusion model and comparing local versus global tractography.
Several measures are used to quantify anatomical connectivity: the probability connection, the fiber count and fiber’s length. Based on these, the density and strength of connection has been defined. These measures have often been used interchangeably in the study of structure–function relationship. There is no consensus on a measure of anatomic connectivity, hampering a comprehensive understanding of the findings obtained by diverse studies.
2.2. Functional Connectivity
Functional magnetic resonance imaging (fMRI), electroencephalography (EEG), magnetoencephalography (MEG) and positron emission tomography (PET) are imaging methods of macroscopic brain activity. Each of these techniques is sensitive to different physiological processes; therefore, they have different spatio-temporal resolutions. For instance, the non-invasive fMRI technique has high spatial resolution (~1 mm) and poor temporal resolution (seconds). In contrast, EEG/MEG measures neuronal activity with excellent temporal resolution and low spatial resolution. Functional connectivity (FC) has been defined as statistical dependencies between distinct and distant regions of information processing neuronal populations [ 44 , 45 ] and can be extracted from anyone of these techniques including the so-called “resting-state” fMRI (rs-fMRI) [ 46 , 47 ]. Regarding structure–function relationship, the activity at slow time scale of the rs-fMRI signals seems more constrained by structural configuration than the faster time scales of the EEG. Functional connections estimated at larger time windows strongly overlap with the underlying structural connections, while, for smaller time windows, there can be a structural–functional network discrepancy due to distributed delays between neuronal populations that cause transient phase (de-)synchronization [ 1 , 7 ]. For this reason, this review focuses on the findings based essentially in fMRI, specifically rs-fMRI. There is still much debate about the neuronal basis of rs-fMRI, although it has been accepted that the rs-fMRI signal is due to the intrinsic activity dynamics that reflects, at least in part, aspects of the functional capacity of neural systems [ 48 ]. Various authors established a relationship between time-varying functional resting-state connectivity and the network topology using large-scale models, which is discussed below.
The blood-oxygen-level dependent (BOLD) signal of fMRI is an indirect measure of brain neuronal activity. Although what it really reflects are the differences in blood oxygen level in brain tissue. The methods proposed to study FC from fMRI have been classified in two general categories: model-based methods and data-driven methods. Model-based methods such as general linear model [ 49 ], cross-correlation analysis [ 50 ] and coherence analysis [ 51 ] are based on prior knowledge. In contradistinction to these methods, data-driven methods are independent of prior information; some of these are principal component analysis (PCA), independent component analysis (ICA) and clustering analysis. Each method has own advantages and limitations (for review, see [ 52 ]).
A variety of measures have been used to describe FC: correlation, covariance, coherence, Granger causality, and mutual information. Pearson correlation coefficient between the time series of different regions is the most used functional measure. The interpretation of functional connectivity metrics is a crucial point in the comprehension of the relation between structure and function [ 53 ] because the FC may reflect direct interaction between two regions but also may exist between anatomically unconnected regions, in contradistinction to SC that reflect white matter fibers that physically connect two regions. The FC is dealt with as a purely spatial measure and this static description is a moot point given that the non-stationary of resting-state connectivity turns around the way that functional interactions are examined and simulated.
The definitions and some limitations to be considered before starting any connectivity study have been reviewed, but an important point that has not been mentioned in obtaining the anatomical and functional connectivity matrices is the parcellation scheme. In connectivity matrices, the columns and rows (targets and sources) represent the neural elements i andj, and c ij entries represent neural connections. The number of neural elements depends on the parcellation schemes of the brain into regions utilized (for review, see [ 54 ]). There are different anatomical brain atlases that are used to define the nodes of the connectivity matrices. The most widely used anatomical atlas is the Automated Anatomical Labeling (AAL) that comprises 116 ROIs (nodes) [ 55 ], which uses the parcellation proposed by Hagmann et al. [ 2 ]. The various parcellation schemes differ in the number, shape and location of ROIs which can alter connectivity profiles and obstruct the comparison of results across studies. In the particular case of FC, it is likely that they include signals from different functional regions due to the usually large size of the ROIs derived from most parcellation schemes.
2.3. Spatio-Temporal Scales
The brain has been described as a complex network on multiple spatial scales, from synaptic circuits to whole-brain system to process and integrate information. Essentially, it is split into three levels: macroscopic, mesoscopic or microscopic scale. At macroscale, we handle with brain regions and white matter fibers connected these regions. The microscopic scale is extended to single neurons, the dendrites, axons and synaptic activity. However, how these scales are related is still an open question. Even though this review focuses on empirical connectivity obtained from neuroimaging techniques, which corresponds to the macroscale, a comprehension of structural correspondence and functional interplay between these two scales is imposed. In this sense, a significant correlation between microscale cellular metrics with macro-cale network properties of macaque cortex has been reported. For instance, highly connected regions show more neural complexity in terms of dendritic branching, soma size and spine density compared with lowly connected regions [ 56 ]. A correlation between cytoarchitectonic properties, such as neuron size, and macroscale connectivity, reflecting an association between local and global organizational features of human cortex, has also been found [ 57 ]. Likewise, a relationship between the functional connectivity and the underlying chemoarchitecturehas been shown [ 10 , 58 , 59 ]. Even though there is evidence of the correspondence between micro- and macroscale, it has not been possible to explain the physiological mechanisms of many of the phenomena experimentally observable at macroscale. Thus, a third scale has been defined, the mesoscopic level, which represents the transitional state between the macro- and microscales. Mesoscopic refers to neuronal populations under the assumption that the neurons of a population have similar properties with the aim of explaining macroscopic spatio-temporal dynamics from mesoscopic approximations.
The brain has been studied essentially in two time-scales: short-term (seconds to minutes) and long-term (days to years) time-scales. The structural configuration is relatively stable across time but can change due to the development and neuroplastic processes, such that the SC (at macroscale) remain stable on short-term time scale while neuroplasticity can be observed on long-term time scale. On the other hand, we have already mentioned that FC is not time-invariant; relationships between neural elements can quickly vary across time. FC from fMRI studies corresponds to macroscopic scale due to the spatial resolution that is not enough to directly represent the neuronal dynamic activity at microscopic level.
3. What We Know About Structure–Function Relationship
Neuroimage processing, network theory and computational modeling have played essential roles in the study of structure–function interactions. Empirical studies obtained the structural and functional matrices where nodes represent regions and the edges represent the connections between these regions (connections can be anatomical measurements or functional correlations), and the correlation between them, structural and functional connectivity, is calculated to evaluate the closeness of their relationships. In the cases where the SC derived from the DWI studies and the FC derived from the rs-fMRI of the same subjects have been compared, different correlation coefficients between functional and structural connectivity matrices have been reported in approximately a range of 0.4–0.7. However, these studies use different methodologies, namely different neuroimaging processing methods, different subdivision schemes and different connectivity measures.
Some papers implement computational models to infer the FC from SC obtained from DWI, and compare the inferred FC with empirical FC obtained with functional neuroimaging techniques, e.g., rs-fMRI or EEG. A consistent result of these studies of the structure–function relationship is that resting-state network reflects at least in part the underlying SC. This has also been demonstrated in mice [ 60 ]. This relationship between structural network and network of dynamic couplings has been demonstrated at macroscale [ 3 , 40 ], mesoscale [ 61 ], and microscale [ 62 ]. However, part of the FC is not supported by the underlying SC and this may be due to several factors. For example, the FC between two regions can be given in the absence of a structural connection between these regions, that is, it can be mediated by third areas. On the other hand, another factor that influences the non-total correspondence between SC and FC is related to the dynamic aspects of the FC, that is, the non-stationary nature of FC. A dependence on the SC–FC relationship with the time scale considered in the study has been described [ 1 , 7 ]. Mišić et al. [ 6 ] illustrates that, at network-level, a particular structural configuration supports a different non-overlapping functional configuration, which means that functional network does not necessarily correspond node-to-node to the underlying structural network.
The brain has been studied using network analysis and a common architectural characteristic has been found in both anatomical and functional networks. A full description and interpretation of graph-theoretical approach is beyond the scope of this review (for review, see [ 63 ]). A group of measures that reflect the integration and segregation processes allow us to characterize structural and functional organization of the brain, but interpretation of these metrics depends on the choice of nodes and edges, again the parcellation problem (to define node).
Even when different experimental methodologies and different parcellation schemes were used, there is evidence that the brain has small-world properties, which means a tendency to clustering nodes into modules reflecting an efficient topological integration and economic wiring, for both functional [ 64 , 65 ] and structural networks [ 2 , 40 , 64 , 66 , 67 ]. This “small-world” network property has been associated with optimal communication efficiency and high-speed information transmission due to the coexistence of high clustering and short average distance, which facilitates the integration and spreading of signals [ 68 ]. Despite all this preliminary evidence, the assumptions of the small-worldness have been questioned [ 69 ]. Another feature that has been demonstrated in humans is the rich-club organization [ 70 , 71 ], which means a presence of highly interconnected regions (hubs), beyond what is expected considering only the node degree, which represents the number of connections that link the node to the rest of the network. This rich-club organization has been associated with the shape and route that global processes follow in neural system and that contributes to the integration process. Rich-club structure is essential in cross-linking of functional modules in the human brain [ 72 ]. These two properties, the modular structure and the rich-club organization, seem to be determinants in the dynamic activity of the brain that is affected when one of these properties is destroyed [ 9 ]. Other network measures have been directly related to the brain’s activity. For example, a previous study found that the shortest paths of the structural network were strong predictors for functional connections [ 68 ].Some of these large-scale topological features are conserved in some species. Small-world network organization, the modular structure and central rich club organization have been shown in rat [ 31 ], mouse [ 60 ], cat [ 73 ] and macaque monkey [ 1 , 74 , 75 ]. These studies allow us to conclude that there are similar topological organizational features of neural network architecture among species.
As mentioned before, the relationship between SC and FC does not exhibit a simple one-to-one mapping, thus modular structure helps clarify this relationship. Diez et al. [ 76 ] proposed a brain partition based on structural–functional modules. They hypothesized that there is a brain partition common to both structure and function networks. They found a strong correspondence between brain structure and resting state dynamics by implementing a standard hierarchical agglomerative clustering algorithm. Their work confirmed that both rs-FC and SC networks display a high modularity, and that there is an excellent matching between functional and structural modules.
In addition to the graph-based approaches that have helped to clarify structure–function relationship, important contributions have also been made by computational modeling. In the last decade, many mathematical and computational models have been proposed that can generate detailed neuronal dynamics to make inferences about brain functionality and its complex behavior. These models have contributed to bridge the gap between anatomy and brain dynamics by making inferences to what extent the anatomical configuration predicts neural dynamics, for instance by comparing simulated functional connectivity with empirical one. A crucial point of computational models is the trade-off between complexity and realism. In general, the large-scale brain network models that incorporate realistic anatomical connectivity to simulate neuronal population activity in each region allow a balance between biophysical realism and model complexity owing to its low-dimensionality. These models are based on mesoscopic approximations: neural-mass and mean-field reduction [ 77 ], and a group of neurons (neuronal population) that shares the same physiological properties at a given physical location is only influenced by the mean activity (activity modeled by a set of dynamical equations) of another population. These neural populations can be coupled together according to empirical measures. These mesoscopic approximations allow a close up image to comprehend the underlying physiological mechanisms (microscale) that give rise to spatial-temporal macroscopic dynamics in the healthy and diseased brain. In this context, several works have utilized computational models to generate neural dynamics from the empirical structural connectivity matrix [ 1 , 3 , 7 , 20 , 21 , 22 , 73 , 78 , 79 , 80 ]. The general methodology followed by many of these articles consist of: (1) obtain structural and functional connectivity matrices from DWI and rs-fMRI, respectively; (2) simulate activity from structural connectivity matrix using a computational model; (3)estimate BOLD signal from the simulated neural activity; and (4) compare empirical versus simulated FC. Following this general methodology, all these works have elucidated and reinforced key points about the relationship between resting state networks and the underlying anatomical connectivity. First, there is a tendency towards high FC between regions strongly anatomically connected, as well as between regions without direct structural connections. Second, there is a dependence on this relationship of the time scales: st a slow time scale (minutes), the FC reflects part of the underlying structural network, snd not the FC fluctuations that take places on shorter time scales. Third, it is suggested that these non-stationarities could explain the part of FC not supported by the underlying SC. Fourth, the best fit between simulated (using SC matrix) and empirical (measured experimentally using fMRI) FC is when the network state is at the edge of a dynamical bifurcation point. This last finding reflects that there is a functionally significant dynamic repertoire that is inherent of the structural configuration. That the brain network operates at the edge of instability is functionally relevant because it indicates the existence of a set of available brain states that can be activated and stabilized quickly and easily when necessary, for example before a given task or for a given function.
The structure–function relationship has not only been studied in humans, similar results have also been obtained in animal studies. An advantage of animal models is that they allow studies at the cellular and molecular level, on either a micro-or a sub-microscale. A correspondence between anatomical and functional connectivity in somatosensory cortex has been demonstrated for anesthetized monkeys [ 28 ] and in mice [ 5 , 60 ]. Díaz-Parra et al. [ 8 ] simulated FC using rat connectome derived from tract tracing experiments to compare with empirical FC obtaining from rs-fMRI obtaining a Pearson correlation of 0.53, which intensifies the hypothesis that anatomical topology shapes, at least in part, functional networks. They also found that functional modules are enriched in densely connected anatomical motifs.
4. Structure–Function Relationship in Neurological Disorders
The objective of this section is to cite some examples where the structure–function relationship has helped to elucidate more about neural mechanisms; for example, in the normal aging process and in some pathologies, particularly those that have been described as network disorders such as epilepsy, schizophrenia and autism.
Considering what has been said up to now, the correspondence between SC and FC would be a better biomarker (greater sensitivity) of brain disorders than those biomarkers based only on imaging modalities. Why some functions are preserved after similar structural brain damage in some patients and not in others is an open question. In this framework, inter-individual variability in the evolution, behavior and neurological profile among patients with the same pathology has been associated with different structure–function relationship between patients. That is, more than diseases, there are patients and, therefore, the personalized medicine forms the basis of the future clinical-therapeutic approach.
Aging is associated with changes in brain morphology as well as with a decline in cognitive performance; in fact, several authors reported anatomical and functional impairment in different brain regions. Persson et al. [ 81 ] reported anatomical and functional differences associated with cognitive decline between two groups with different levels of episodic memory performance over time. They found a reduction in anatomical connectivity in the anterior part of the corpus callosum and an increase in the recruitment of frontal regions in the group with a declining memory performance. In fact, they found a negative correlation between anatomical measurement and the increment of the activation pattern (fMRI), in the form of an increase in the number of activated regions during a cognitive task performance. They argued that this result suggests that the additional activation pattern during task performance could be a compensatory mechanism for the structural disruption associated with memory decline. On the other hand, Nakagawa et al. [ 27 ] demonstrated, based on computational modeling, that the decrease in structural connectivity in aged people led to lower complexity of BOLD signals. The complexity allows quantifying temporal patterns of BOLD signal; higher complexity of brain activity leads to richer and more integrated information in the network. They argued that it could reflect changes in processing speed which would explain the cognitive performance decline distinctive of aging. Although the results may seem contradictory, they are not, since the first study is based on the activation pattern during the execution of a certain task, while the second is based on “resting-state” fMRI, in the absence of an explicit task. These results seem to indicate that deterioration in the structural network during aging causes a decline to information processing so that the system needs higher overall activation to cognitive performance. These two results are examples of how the structure–function relationship can be used as biomarkers, in this case of aging.
4.2. Epilepsy
Studies from epileptic patients have reported alterations in both structural and functional connectivity as well as a negative correlation between both parameters. Liao et al. reported a decrease in both FC and SC in mesial temporal lobe epilepsy (TLE) patients when compared with controls [ 82 ]. Zhang and coworkers corroborated these findings in patients suffering idiopathic generalized epilepsy (IGE) [ 83 ]. Furthermore, the correlation between SC and FC shows a tendency to decrease in TLE patients vs. controls [ 84 ] similar to that described in IGE patients [ 84 ]. Nevertheless, all these studies referred to an interesting finding: the SC–FC correlation increased during the epileptic crisis. It could be suggesting that the SC–FC correlation is a possible biomarker for epilepsy monitoring.
A network topology study in children with frontal lobe epilepsy compared to age-matched controls revealed a relationship between functional networks impairment with an increment of the cluster coefficient and path length of the epileptic patients group. This finding represents the existence of networks with tightly clustered modules but with limited inter-modular connectivity. Because this study did not find significant differences between groups for structural networks, it has been suggested that the functional alteration precedes structural changes [ 85 ].
4.3. Schizophrenia
Schizophrenia has been described as a disorder of brain connectivity [ 86 , 87 ]. Numerous topological disturbances have been reported on structural and functional networks [ 86 , 87 , 88 ]. Skudlarski et al. [ 86 ] found a diminished SC accompanied by either decrement or increment of FC in different regions (hypo- and hyper-connectivity) when comparing connectivity maps of schizophrenic patient group with control group. Some of these functional abnormalities are supported by anatomical changes; the complex nature of the structure–function interaction is also evident in schizophrenia [ 88 ]. Lastly, Stephan et al. [ 89 ] reviewed computational models based on neuroimaging data and focused on schizophrenia as a spectrum disease. In cases such as schizophrenia, where a spectrum is described, individual analyses are imposed for each patient (for review, see [ 87 ]).
4.4. Autism
Autism spectrum disorder (ASD) has also been characterized as a network disorder with abnormal anatomical and functional connectivity. One of the reported findings is a decrease in inter-hemispheric FC [ 90 ] that seems to be related to reduced inter-hemispheric anatomic connectivity [ 91 , 92 ]. However, an SC increased in young children [ 93 ] and adolescents [ 94 ] with ASD has also been reported. Thus, there are questions such as: Is connectivity disorganization part of the primary pathogenesis in ASD? Are there differences in connectivity patterns between distinct syndromes of ASD? Basic and clinical studies will shed light on these issues.
5. Limitations
In this review, the most relevant methodologies in neuroimage processing, network topology analysis and computational modeling used to study of structure–function relationship are discussed. However, the images acquisition and processing per se offer inherent limitations of the methodology. For instance, when structural connectivity is based on DTI, it can ignore long-distance and fiber-cross connections and does not provide information about the directionality of the connections [ 2 , 42 ]. In the case of fMRI, it is important to note some aspects: neuronal activity is not directly a measure of the BOLD signal of each voxel; it is an integration of a variety of neuronal activities and the increase of excitatory or inhibitory synaptic activity can cause an increase of metabolic activity. Such limitations, properties of neuroimaging techniques, may lead to imprecise brain network representations, affecting the analysis of network properties such as the study of the structure–function relationship.
Regarding the methodology followed for image processing, there are two points that limit the interpretation of the results as well as make comparison between the different studies difficult: parcellation schemes and connectivity measures. The definition of nodes and edges is a critical step to obtain connectivity matrices; in fact, the limitations on the application of graph theory and the validity in its interpretation depend on graph representation itself: nodes and edges. Until now, there is no universally accepted parcellation scheme: the diverse anatomical brain atlases exhibit remarkable differences in the number, shape, and location of ROIs. The results of anatomical and functional connectivity depend on the algorithm and parcellation scheme considered. This dependency of the results of anatomical/functional connectivity on parcellation schemes and the non-standardization of connectivity metrics cause inconsistencies between studies. Taylor et al. referred to the use of a high-resolution parcellation scheme (50,000 nodes), focusing the analysis on modularity within and between brain areas [ 95 ]. They described a modular architecture within brain areas that could be the structural mechanism to implement different functions.
In addition, the variety of connectivity measures for both structure and function make comparison across studies difficult. Huang and Ding argued that node weight, a measure that considers the number and length of fibers and the ROI size, and conditional Granger causality are more appropriate measures to link functional and structural connectivity [ 53 ].
In summary, the extent to which the different methodologies and procedures (connectivity measure and graph construction) faithfully reproduce the biological phenomenon itself is something that requires additional studies to establish guidelines in this regard.
In the case of computational modeling, models with many variables make parameter estimation very complex. In addition, it is not clear to what extent the values of some parameters affect how well models predict FC. Some models do not differentiate neural regions and are based on certain global parameters, coupling weight, transmission speed and noise, which determine the global network activity. All these elements must to be carefully considered when a computational model is used to study SC–FC relationship.
6. Conclusions
From surveying the current literature, what is clear, despite all methodological limitations, is that resting-state functional connectivity is constrained by the large-scale brain anatomical configuration, but other factors also influence it. Multi-modal neuroimaging techniques and improved methodologies are needed to obtain structural and functional connectivity matrices. Another consistent finding is that simulations of intrinsic neural activity based on anatomical connectivity can reproduce much of the patterns observed in empirical functional connectivity. Computational modeling will continue to be an increasingly essential scientific endeavor, playing an increasing role in elucidating multi-scale relationship sand inferring disorder mechanisms.
Clearly, more research is imperative to deepen the understanding of the structure–function relationship. Longitudinal investigations can be fruitful in future works to evaluate neuroplasticity processes. In addition, the wide variability among patients with the neurological disorders imposes personalized individual treatment as health-care options.
Author Contributions
K.B.G.R. wrote the paper. C.I.F.V. participated in the writing and revision of the paper.
Conflicts of Interest
The authors declare no conflict of interest.
Suggestions or feedback?
MIT News | Massachusetts Institute of Technology
- Machine learning
- Social justice
- Black holes
- Classes and programs
Departments
- Aeronautics and Astronautics
- Brain and Cognitive Sciences
- Architecture
- Political Science
- Mechanical Engineering
Centers, Labs, & Programs
- Abdul Latif Jameel Poverty Action Lab (J-PAL)
- Picower Institute for Learning and Memory
- Lincoln Laboratory
- School of Architecture + Planning
- School of Engineering
- School of Humanities, Arts, and Social Sciences
- Sloan School of Management
- School of Science
- MIT Schwarzman College of Computing
Studies of unusual brains reveal critical insights into brain organization, function
Press contact :.
Previous image Next image
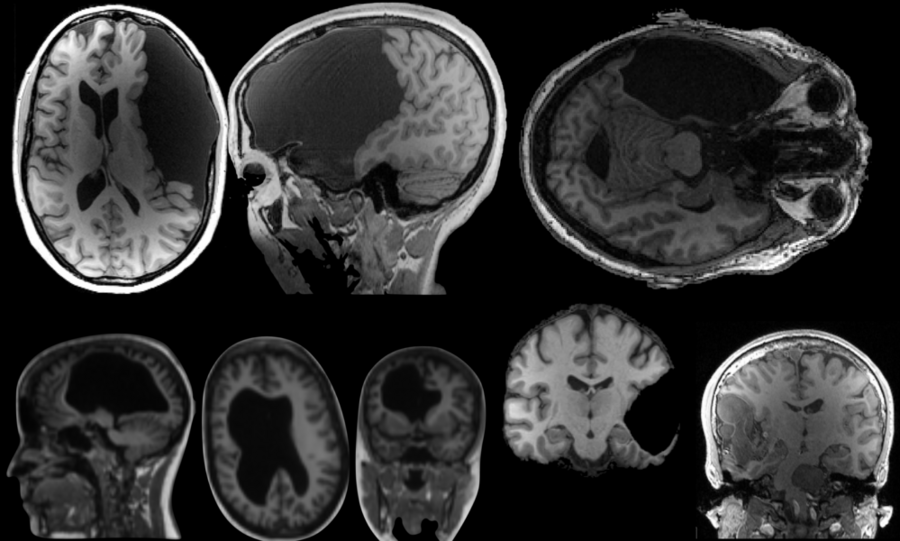
E.G. (a pseudonym) is an accomplished woman in her early 60s: She is a college graduate and has an advanced professional degree. She has a stellar vocabulary — in the 98th percentile, according to tests — and has mastered a foreign language (Russian) to the point that she sometimes dreams in it.
She also has, likely since birth, been missing her left temporal lobe, a part of the brain known to be critical for language.
In 2016, E.G. contacted McGovern Institute for Brain Research Investigator Evelina Fedorenko , who studies the computations and brain regions that underlie language processing, to see if her team might be interested in including her in their research.
“E.G. didn’t know about her missing temporal lobe until age 25, when she had a brain scan for an unrelated reason,” says Fedorenko, the Frederick A. (1971) and Carole J. Middleton Career Development Associate Professor of Neuroscience at MIT. “As with many cases of early brain damage, she had no linguistic or cognitive deficits, but brains like hers are invaluable for understanding how cognitive functions reorganize in the tissue that remains. I told her we definitely wanted to study her brain.”
Previous studies have shown that language processing relies on an interconnected network of frontal and temporal regions in the left hemisphere of the brain. E.G.’s unique brain presented an opportunity for Fedorenko’s team to explore how language develops in the absence of the temporal part of these core language regions.
Their results appeared recently in the journal Neuropsychologia. They found, for the first time, that temporal language regions appear to be critical for the emergence of frontal language regions in the same hemisphere — meaning, without a left temporal lobe, E.G.’s intact frontal lobe did not develop a capacity for language.
They also reveal much more: E.G.’s language system resides happily in her right hemisphere. “Our findings provide both visual and statistical proof of the brain’s remarkable plasticity, its ability to reorganize, in the face of extensive early damage,” says Greta Tuckute, a graduate student in the Fedorenko lab and first author of the paper.
In an introduction to the study, E.G. herself puts the social implications of the findings starkly. “Please do not call my brain abnormal, that creeps me out,” she writes. “My brain is atypical. If not for accidentally finding these differences, no one would pick me out of a crowd as likely to have these, or any other differences that make me unique.”
How we process language
The frontal and temporal lobes are part of the cerebrum, the largest part of the brain. The cerebrum controls many functions, including the five senses, language, working memory, personality, movement, learning, and reasoning. It is divided into two hemispheres, the left and the right, by a deep longitudinal fissure. The two hemispheres communicate via a thick bundle of nerve fibers called the corpus callosum. Each hemisphere comprises four main lobes — frontal, parietal, temporal, and occipital. Core parts of the language network reside in the frontal and temporal lobes.
In most individuals, the language system develops in both the right and left hemispheres, with the left side dominant from an early age. The frontal lobe develops slower than the temporal lobe. Together, the interconnected frontal and temporal language areas enable us to understand and produce words, phrases, and sentences.
How, then, did E.G., with no left temporal lobe, come to speak, comprehend, and remember verbal information (even a foreign language) with such proficiency?
Simply put, the right hemisphere took over: “E.G. has a completely well-functioning neurotypical-like language system in her right hemisphere,” says Tuckute. “It is incredible that a person can use a single hemisphere — and the right hemisphere at that, which in most people is not the dominant hemisphere where language is processed — and be perfectly fine.”
Journey into E.G.’s brain
In the study, the researchers conducted two scans of E.G.’s brain using functional magnetic resonance imaging (fMRI), one in 2016 and one in 2019, and had her complete a range of behavioral tests. fMRI measures the level of blood oxygenation across the brain and can be used to make inferences about where neural activity is taking place. The researchers also scanned the brains of 151 “neurotypical” people. The large number of participants, combined with robust task paradigms and rigorous statistical analyses made it possible to draw conclusions from a single case such as E.G.
Fedorenko is a staunch advocate of the single case study approach — common in medicine, but not currently in neuroscience. “Unusual brains — and unusual individuals more broadly — can provide critical insights into brain organization and function that we simply cannot gain by looking at more typical brains.” Studying individual brains with fMRI, however, requires paradigms that work robustly at the single-brain level. This is not true of most paradigms used in the field, which require averaging many brains together to obtain an effect. Developing individual-level fMRI paradigms for language research has been the focus of Fedorenko’s early work, although the main reason for doing so had nothing to do with studying atypical brains: individual-level analyses are simply better — they are more sensitive and their results are more interpretable and meaningful.
“Looking at high-quality data in an individual participant versus looking at a group-level map is akin to using a high-precision microscope versus looking with a naked myopic eye, when all you see is a blur,” she wrote in an article published in Current Opinion in Behaviorial Sciences in 2021 . Having developed and validated such paradigms, though, is now allowing Fedorenko and her group to probe interesting brains .
While in the scanner, each participant performed a task that Fedorenko began developing more than a decade ago. They were presented with a series of words that form real, meaningful sentences, and with a series of “non-words” — strings of letters that are pronounceable, but without meaning. In typical brains, language areas respond more strongly when participants read sentences compared to when they read non-word sequences.
Similarly, in response to the real sentences, the language regions in E.G.’s brain were bursting with activity while the left frontal lobe regions remained silent. In the neurotypical participants, the language regions in both the left and right frontal and temporal lobes lit up, with the left areas outshining the right.
“E.G. showed a very strong response in the right temporal and frontal regions that process language,” says Tuckute. “And if you look at the controls, whose language-dominant hemisphere is in the left, E.G.’s response in her right hemisphere was similar — or even higher — compared to theirs, just on the opposite side.”
Leaving no stone unturned, the researchers next asked whether the lack of language responses in E.G.’s left frontal lobe might be due to a general lack of response to cognitive tasks, rather than just to language. So they conducted a non-language, working-memory task: they had E.G. and the neurotypical participants perform arithmetic addition problems while in the scanner. In typical brains, this task elicits responses in frontal and parietal areas in both hemispheres.
Not only did regions of E.G.’s right frontal lobe light up in response to the task, those in her left frontal lobe did, too. “Both E.G.’s language-dominant (right) hemisphere, and her non-language-dominant (left) hemisphere, showed robust responses to this working-memory task,” says Tuckute. “So, yes, there’s definitely cognitive processing going on there. This selective lack of language responses in E.G.’s left frontal lobe led us to conclude that, for language, you need the temporal language region to ‘wire up’ the frontal language region.”
In science, the answer to one question opens the door to untold more. “In E.G., language took over a large chunk of the right frontal and temporal lobes,” says Fedorenko. “So what happens to the functions that in neurotypical individuals generally live in the right hemisphere?”
Many of those, she says, are social functions. The team has already tested E.G. on social tasks and is currently exploring how those social functions cohabit with the language ones in her right hemisphere. How can they all fit? Do some of the social functions have to migrate to other parts of the brain? They are also working with E.G.’s family; they have now scanned E.G.’s three siblings (one of whom is missing most of her right temporal lobe; the other two are neurotypical) and her father (also neurotypical).
The project has now grown to include many other individuals with interesting brains, who contacted Fedorenko after some of this work was covered by news outlets. The Interesting Brains Project promises to provide unique insights into how our plastic brains reorganize and adapt to various circumstances.
Share this news article on:
Related links.
- Interesting Brains Project
- Ev Fedorenko
- McGovern Institute for Brain Research
- Department of Brain and Cognitive Sciences
Related Topics
- Brain and cognitive sciences
- Neuroscience
- Magnetic resonance imaging (MRI)
- McGovern Institute
Related Articles
Whether speaking Turkish or Norwegian, the brain’s language network looks the same

What words can convey
Artificial intelligence sheds light on how the brain processes language
Previous item Next item
More MIT News

H2 underground
Read full story →

2024 MAD Design Fellows announced

School of Engineering first quarter 2024 awards

From NASA to MIT to Formlabs

An expansive approach to making new compounds

Q&A: A graduating student looks back on his MIT experience
- More news on MIT News homepage →
Massachusetts Institute of Technology 77 Massachusetts Avenue, Cambridge, MA, USA
- Map (opens in new window)
- Events (opens in new window)
- People (opens in new window)
- Careers (opens in new window)
- Accessibility
- Social Media Hub
- MIT on Facebook
- MIT on YouTube
- MIT on Instagram
Introductory essay
Written by the educators who created Mapping and Manipulating the Brain, a brief look at the key facts, tough questions and big ideas in their field. Begin this TED Study with a fascinating read that gives context and clarity to the material.
Here is this mass of jelly, three-pound mass of jelly you can hold in the palm of your hand, and it can contemplate the vastness of interstellar space. It can contemplate the meaning of infinity and it can contemplate itself contemplating on the meaning of infinity. VS Ramachandran
The brain may well be our body's most mysterious organ. Unbelievably complex, utterly fascinating, and notoriously difficult to study, we're left wondering: What exactly does the brain do and how does it do it?
Despite two centuries of intensive research, supported in recent decades by impressive technological advances, answers to many of our questions about the brain are still distant. The reason is easy to appreciate: the brain contains more than ten billion cells — a number equivalent to the total human population on Earth — interacting with each other through about 1,000 times as many connections. Imagine that what's going on in your brain is like a shrunk-down version of the global human population interacting through the Internet. The Internet is hard enough to understand even though we created it; now imagine trying to understand a process of similar complexity without the benefit of knowing how it was generated!
As you listen to these TEDTalks and expand your study of neuroscience through other sources, remember that although we might now know a great deal more about the brain than we did at the start of the 19th century, it's a tiny fraction of what there is to know. Bear in mind that many current ideas may prove wrong. Indeed, it's the excitement of generating and testing, and trying to prove or disprove ideas that might explain the great unknown inside our heads that motivates many research neuroscientists around the world.
A brief history of brain science
The Egyptians wrote the first known descriptions of the brain and its anatomy about 3700 years ago, but another 1200 years elapsed before Greek philosophers of the Hippocratic School identified the brain as the organ responsible for our cognitive functions. Around 400 B.C., Hippocrates declared, "Men ought to know that from the brain, and from the brain only, arise our pleasures, joy, laughter and jests, as well as our sorrows, pains, griefs, and tears." However, not everyone agreed: although Plato and Hippocrates thought that the brain was responsible for sensation, intelligence and mental processes, Aristotle believed it was the heart.
Over the next 2500 years, the work of great European intellectuals including Galen of Bergama, Leonardo da Vinci and Rene Descartes improved our understanding of the brain. By the start of the 19th century, the brain's importance as the organ of perception and higher mental function was beyond doubt.
In the early 1800s, scientists made an important conceptual breakthrough when they hypothesized that different brain functions are carried out in specific and distinct brain regions. Brain regionalization, a concept central to several of the TEDTalks we'll watch, remains an important though controversial component of modern neuroscience.
Some of the initial models of brain regionalization were severely misguided, mainly because they were built on little or no evidence. For example, the Viennese physician Franz Joseph Gall (1758-1828) became convinced for the flimsiest of reasons that each of mankind's mental faculties, including our moral and intellectual capabilities, are each controlled by a separate "organ" within the cerebral hemispheres of the brain. The pseudo-science of phrenology that grew out of Gall's claims gained an enormous popular following in the 19th century; advocates believed that skilled practitioners could feel the lumps and bumps on an individual's skull to gain information about the underlying "organs" and thus fully describe the individual's personality and mental abilities.
Although phrenology is now discredited, the fundamental idea that different functions are localized to different areas of the brain turned out to have merit — even if Gall got the details wrong. The story of phrenology also provides a salutary lesson on the dangers of accepting popular beliefs about aspects of brain function and dysfunction that are difficult to critically evaluate through scientific experimentation. Even today, it's common to find that people think they know more than it's currently possible to know about how and why brains work or go wrong; for example, the causes and cures for various types of mental illness, which may contribute to the social stigma that surrounds these conditions.
Through the late 19th and early 20th centuries, scientists including Pierre Paul Broca, Carl Wernicke, Korbinian Brodmann and Wilder Penfield found credible scientific evidence supporting the subdivision of the brain into discrete areas with different specific functions. Their work was based on studies of patients with localized lesions of the brain, of the anatomical differences between different parts of the brain and of the effects of stimulating discrete brain regions on bodily actions. Together, scientists such as these laid the foundations of modern neuroscience. As you watch the TEDTalks in Mapping and Manipulating the Brain , notice how the speakers reference some of the same approaches used by Broca, Wernicke, Brodmann and Penfield, and how they apply the concepts of brain regionalization and localization of function . Bear in mind, however, that although these concepts are useful, they're also controversial -- more on this below.
How brains are built
Spanish scientist Santiago Ramón Y Cajal (1852-1934) is often thought of as the father of modern neuroscience. Through his extensive and beautiful studies of the microscopic structure of the brain, he discovered that the neuron is the fundamental unit of the nervous system. Since Ramón Y Cajal's breakthrough, scientists have sought to understand how the billions of neurons in the brain are organized to support so many complex functions.
This daunting task would likely be easier if we could follow the process by which the brain is generated, but following brain development is very difficult to do in humans. Thus, we often have to infer how the human brain develops by studying the developing brains of other species, so-called "model organisms" selected for their particular advantages in certain experimental procedures. Aside from helping us to work out how the adult brain functions, research on brain development is a major area in neuroscience for other reasons as well. For example, many conditions like schizophrenia and autism can be traced back to abnormalities in earlier brain development.
The great molecular, structural and functional diversity of brain cells, along with their specializations and precise interactions, are acquired in an organized way through processes that build on differences between the relatively small numbers of cells in the early embryo. As more and more cells are generated in a growing organism, new cells diversify in specific ways as a result of interactions with pre-existing cells, continually adding to the organism's complexity in a highly regulated manner. To understand how brains develop we need to know how their cells develop in specific and reproducible ways as a result of their own internal mechanisms interacting with an expanding array of stimuli from outside the cell.
Since, as discussed above, regionalization is a prominent organizing feature in mature brains, when and how is it established during brain development? Some of the most exciting research on brain development in recent years has focused on this question.
For neurons to develop regional identities, they must possess or acquire information on where they are located within the brain so that they can take on the appropriate specializations. How neurons gain positional information has been one of the most prominent themes in developmental neuroscience in the last 50 years or so, as indeed it has in the broader field of developmental biology (positional identity is required not only by brain cells).
The model that has dominated current thinking was famously elaborated in the 1960s by Lewis Wolpert in his French flag analogy. Here, a signal produced by a group of organizer cells diffuses from its source through a surrounding field of cells. In so doing, it forms a concentration gradient with more of the signal present in areas closer to the source. Cells respond to the concentration of this signal. In Wolpert's French Flag analogy, they become blue, white or red (in reality, they would become cells of different types, not different colors). Close to the source, cells receive signals above the highest threshold (to become blue, or type 1). Beyond this, cells respond to a lower dose (to become white, or type 2) while farther still cells do not receive enough of the signal to respond (and become red, or type 3). Here the model is expressed in terms of three outcomes, but there might be a different number of outcomes depending on the locations and/ or stages of development. The important point is that cells can work out where they are based on the level of signal they receive and they respond accordingly by developing different attributes.
Beyond Wolpert's basic model, the issue of how brain regionalization develops is an important question and we have relatively few answers. Regional specification is a prerequisite for the development of the connections that must link each region of the brain in a stereotypical and highly precise way (but allowing room for plasticity at a fine level). How these trillions of connections are made is another of life's great mysteries.
The connectome and connectionism
Since Ramón Y Cajal's first description of the neuron, scientists have vastly expanded our understanding of the structure and function of these individual building blocks of the brain. However, as Tim Berners-Lee comments, this is just the first step in understanding how our brains really work: "There are billions of neurons in our brains, but what are neurons? Just cells. The brain has no knowledge until connections are made between neurons. All that we know, all that we are, comes from the way our neurons are connected."
You'll hear about the "connectome" in Sebastian Seung's TEDTalk. The suffix "–ome" is used with increasing frequency to indicate a complete collection of whatever units are specified in the first part of the word, such as genes (hence genome), proteins (proteome) or connections (connectome). The connectome of the human brain is bewildering in its complexity, but the development of new brain imaging methods has catalyzed the first serious attempts to map it in living brains. At present, the resolution of imaging methods that can be applied to living brains isn't sufficient to follow individual connections (called axons). In these TEDTalks you'll hear about an attempt to come at the problem from the other direction, using very high resolution imaging of non-living brain tissue to reconstruct the ultramicroscopic anatomy of connections around individual cells. The extent to which these approaches are likely to succeed remains controversial.
The theory known as connectionism addresses a somewhat different matter within the field of brain organization: the relationship between connectivity and function. Essentially, the idea is that higher mental processes such as object recognition, memory and language result from the activity of the connections between areas of the brain rather than the activity of specific discrete regions. Whereas connectionists would agree that primary sensory and motor functions (i.e. responses to sensory stimuli and the activation of movements) are strongly localized to defined areas within the brain, they argue that this applies less clearly at higher cognitive levels. The theory emphasizes the relationship between connected brain areas and the function of the brain as a whole, with all parts having the potential to contribute to cognitive function. You should appreciate, therefore, that there is as yet no accepted view of the extent to which our higher mental functions are localized to particular parts of the brain. It is worth remembering this as you listen to the TEDTalks; keep an open mind on these truly fascinating issues.
Ways of studying brain function
In these TEDTalks, you're going to hear about some of the ways in which we can work out what the human brain does and how it does it. One longstanding approach is to examine what happens when people suffer brain lesions. Phineas Gage, a Vermont railroad worker, provides one spectacular historical example from 1848. Gage was packing gunpowder into a hole when it exploded, blowing the tamping rod through the front of his brain. Astonishingly, he survived and recovered, but those closest to him claimed that he had a very different personality. From this example, scientists hypothesized that elements of human personality are localized to the frontal lobes.
In Jill Bolte Taylor's TEDTalk, you'll hear how Taylor's own stroke provides further evidence for localization of brain function. A few words of caution, however: when we study the effects of a lesion on the brain, we're really learning about what the rest of the brain does without the damaged part, which is not quite the same as what the damaged structure itself does. Maybe this seems rather subtle, but in some cases it becomes important, for example if a lesion causes other parts of the brain to alter what they do.
You'll also hear about powerful techniques for observing the activity of living brains, for example using functional magnetic resonance imaging (FMRI; see the TEDTalk by Oliver Sacks). And you'll hear about methods for looking at the fine structure of neurons in post-mortem material, as in Sebastian Seung's TEDTalk. All have advantages and limitations, but together they give ever- increasing insight into the workings of the human mind.
Let's begin the TEDTalks with neuroanatomist Jill Bolte Taylor, who provides a basic overview of the brain and describes what she learned firsthand about its structure and function when at age 37 she suffered a massive hemorrhage in the left hemisphere of her brain.

Jill Bolte Taylor
My stroke of insight, relevant talks.

VS Ramachandran
3 clues to understanding your brain.

Oliver Sacks
What hallucination reveals about our minds.
Sebastian Seung
I am my connectome.

Christopher deCharms
A look inside the brain in real time.

A light switch for neurons


Rebecca Saxe
How we read each other's minds.
- Future Students
- Current Students
- Faculty/Staff

News and Media
- News & Media Home
- Research Stories
- School's In
- In the Media
You are here
Stanford-led study links school environment to brain development.

For decades, researchers have linked differences in school-age children’s brain development to their out-of-school environment, using indirect socioeconomic factors such as parental income and neighborhood characteristics.
In a new paper , researchers from Stanford Graduate School of Education (GSE) demonstrate for the first time that, even when controlling for those other factors, there is a direct link between a child’s school environment and the development of their white matter, or the network of nerve fibers that allows different parts of the brain to communicate.
In other words, schools that do better than average at promoting learning are showing greater year-by-year advances in brain development, even for students coming from a wide range of socioeconomic environments.
For their study, the authors, including GSE doctoral candidate Ethan Roy , Professor Bruce McCandliss , and Associate Professor Jason Yeatman , leveraged data from the Adolescent Brain Cognitive Development (ABCD) Study, the largest long-term study of brain development and child health in the United States, and the Stanford Education Data Archive (SEDA), a national database of academic performance developed by the Educational Opportunity Project at Stanford University.
Their findings show that children who attend higher-performing schools have accelerated white matter development, including in an area of the brain closely associated with reading skills.
Roy said the results, published in Developmental Cognitive Neuroscience on April 26, were “striking.”
“What jumped off the page for us is that, even when controlling for things like parental income, parental education, neighborhood context, and household conflict levels, we were still able to observe a significant relationship between the school environment of an individual and growth properties of their brain,” he said.
Filling a gap in learning science research
Yeatman, who along with McCandliss serves as an advisor to Roy, said the study is the first to show how variation in the educational opportunities afforded to children is related to brain development.
“Essentially, two children from similar families who are born on two sides of a school boundary have measurable differences in how their brains wire together,” said Yeatman, who holds a joint faculty appointment at the GSE and Stanford Medicine, is a faculty affiliate of the Stanford Accelerator for Learning , and directs the Brain Development & Education Lab and Rapid Online Assessment of Reading .
The study looked at fractional anisotropy, a measure of how water moves through brain tissue and an indication of how insulated, or myelinated, a neuron’s axons are (higher myelination increases the speed of transmission between neurons and is associated with improved learning). The observational results show that fractional anisotropy is directly linked to a school’s national grade equivalence score, or a measure of how third graders from that school perform compared with the national average.
The paper fills a gap in learning science research. Although past studies have linked socioeconomic status to white matter development, they have not been able to focus in on specific attributes of a child’s development, such as the school they attend. Other research — including from Yeatman’s lab — has shown that educational interventions can lead to changes in white matter, but those have been relatively small-scale studies with participants who are not representative of the broader population.
“This is one of the first cases where we can measure the thing we actually care about at the population level,” Yeatman said.
The authors also trained a deep learning model to conduct a global analysis of white matter, finding that children who attend schools with higher SEDA scores had brains that appeared developmentally “more mature” than their chronological age.
A measurable impact
The implications are “potentially game-changing,” said McCandliss, who directs the Stanford Educational Neuroscience Initiative (SENSI) and is a faculty affiliate of the Stanford Accelerator for Learning.
“National discussions of the importance of elementary school quality have never before been framed in terms of having a measurable impact on physical brain development of our young children,” he said. “I think this changes the frame of the discussion and decision-making around the impact of inequity.”
The study was only possible because of the comprehensive data included in the ABCD Study and SEDA, the researchers said. McCandliss, an investigator in the ABCD Study, first approached the ABCD team leaders about linking the SEDA data with the ABCD data in 2018, and his SENSI team spent about two years creating the resulting “crosswalk.”
McCandliss called the ABCD study a “dream come true,” and the linked data a way to “finally” answer “elusive questions about how inequities in educational opportunities may actually be changing the course of physical and functional brain development during the vulnerable elementary school years across the nation.”
To analyze the brain white matter from the MRI data included in the ABCD study, the authors used pyAFQ , an open-source software developed by Yeatman’s lab. “It was a really fruitful collaboration across both labs,” Roy said.
The authors hope their methods and the newly linked ABCD and SEDA data, which is now freely available to a community of registered researchers around the world, will allow other scholars to pursue their own ideas and hypotheses at the intersection of education and neuroscience.
Yeatman said the methods and data used in the study will allow researchers to be more precise about environmental factors linked to brain development and the mechanisms behind those connections.
“The environment influences brain development,” he said. “That’s obvious. But what about the environment influences brain development? This is the first step in actually unraveling that specificity.”
More Stories

⟵ Go to all Research Stories
Get the Educator
Subscribe to our monthly newsletter.
Stanford Graduate School of Education
482 Galvez Mall Stanford, CA 94305-3096 Tel: (650) 723-2109
- Contact Admissions
- GSE Leadership
- Site Feedback
- Web Accessibility
- Career Resources
- Faculty Open Positions
- Explore Courses
- Academic Calendar
- Office of the Registrar
- Cubberley Library
- StanfordWho
- StanfordYou
Improving lives through learning
- Stanford Home
- Maps & Directions
- Search Stanford
- Emergency Info
- Terms of Use
- Non-Discrimination
- Accessibility
© Stanford University , Stanford , California 94305 .
- Share full article
Advertisement
Supported by
Loneliness Can Change the Brain
Feeling chronically disconnected from others can affect the brain’s structure and function, and it raises the risk for neurodegenerative diseases.

By Dana G. Smith
Everyone feels lonely from time to time — after, say, a move to a new school or city, when a child leaves for college, or following the loss of a spouse.
Some people, though, experience loneliness not just transiently but chronically. It becomes “a personality trait, something that’s pretty sticky,” said Dr. Ellen Lee, an associate professor of psychiatry at the University of California, San Diego. These individuals seem to have “this persistent emotion that then shapes their behavior.”
Research is mounting that this type of entrenched loneliness is bad for our health and can even change our brains, raising the risk for neurodegenerative diseases. Here’s what experts know about how chronic loneliness affects the brain, and some strategies to address it.
How does loneliness change the brain?
Humans evolved to be social creatures probably because, for our ancient ancestors, being alone could be dangerous and reduce the odds of survival. Experts think loneliness may have emerged as a unique type of stress signal to prompt us to seek companionship.
With chronic loneliness, that stress response gets stuck and becomes disadvantageous — similar to the way in which anxiety can shift a helpful fear response to a maladaptive mental illness.
“Small, transient episodes of loneliness really motivate people to then seek out social connection,” said Anna Finley, a postdoctoral research fellow at the Institute on Aging at the University of Wisconsin-Madison. “But in chronic episodes of loneliness, that seems to kind of backfire” because people become especially attuned to social threats or signals of exclusion, which can then make it scary or unpleasant for them to interact with others.
Research has shown that lonely people are hypersensitive to negative social words, like “disliked” or “rejected,” and to faces expressing negative emotions. What’s more, they show a blunted response to images of strangers in pleasant social situations, suggesting that even positive encounters may be less rewarding for them. In the brain , chronic loneliness is associated with changes in areas important for social cognition, self-awareness and processing emotions.
How could a subjective feeling have such a profound effect on the brain’s structure and functions? Scientists aren’t sure, but they think that when loneliness triggers the stress response , it also activates the immune system , increasing levels of some inflammatory chemicals. When they’re experienced for long periods of time, stress and inflammation can be detrimental for brain health, damaging neurons and the connections between them.
How does loneliness affect long-term brain health?
For years, scientists have known about a connection between loneliness and Alzheimer’s disease and other types of dementia . A study published late last year suggested that loneliness is associated with Parkinson’s disease , as well.
“Even low levels of loneliness increase risk, and higher levels are associated with higher risk” for dementia, said Dr. Nancy Donovan, director of the division of geriatric psychiatry at Brigham and Women’s Hospital. Dr. Donovan has shown that people who score higher on a measure of loneliness have higher levels of the proteins amyloid and tau — two of the hallmarks of Alzheimer’s disease — in their brains even before they show signs of cognitive decline.
Scientists think that the stress and inflammation caused by loneliness most likely contribute to the onset or acceleration of neurodegenerative diseases in older adults. The toll loneliness takes on the cardiovascular system, increasing blood pressure and heart rate, can also have a detrimental effect on the brain and probably plays a role, as well, Dr. Donovan said.
The more general way in which loneliness affects mental and physical health may also factor into cognitive decline. The feeling is closely linked to depression, another condition that increases the risk for dementia . And people who are lonely are less likely to be physically active and more likely to smoke cigarettes. “All those different things can affect how our brains age,” Dr. Lee said. “I think there are many paths to get from loneliness to cognitive decline.”
Most research on loneliness and neurodegeneration has been conducted on middle-aged and older adults, so experts don’t know if loneliness in childhood or young adulthood carries the same risk. However, Dr. Wendy Qiu, a professor of psychiatry and experimental pharmacology and therapeutics at Boston University School of Medicine, has found that if people in midlife feel lonely only transiently, not chronically, there is no increased risk for dementia.
With transient loneliness, the brain has the “ability to recover,” Dr. Qiu said. But if people “don’t have help to pull them out of the loneliness, and for a long time they feel lonely, it will be toxic for the brain.”
How can you combat chronic loneliness?
One of the most common recommendations is a little obvious: Try to make new friends . Whether that’s through art classes, sports teams, support groups or volunteer opportunities, the goal is to put yourself in places where people come together.
These types of engineered social situations have mixed results. Dr. Lee said they tend to work best if there is a “shared identity” among the people involved, like groups specifically for widows or for people with diabetes, so they have something to connect over.
The other side of the equation is addressing a person’s attitudes and thought patterns about social interactions through cognitive behavioral therapy. These approaches tend to be a little more effective, Dr. Lee said, because they “get to the root” of the problem, exploring what makes it hard for a person to interact with others.
The strategies may sound simple, but they’re easier said than done. “It’s a thorny problem,” Dr. Finley said. “Otherwise, I don’t think we would have the report from the Surgeon General saying we need to figure this out.”
Dana G. Smith is a Times reporter covering personal health, particularly aging and brain health. More about Dana G. Smith
- Skip to main content
- Keyboard shortcuts for audio player
Your Health
- Treatments & Tests
- Health Inc.
- Public Health
Why writing by hand beats typing for thinking and learning
Jonathan Lambert

If you're like many digitally savvy Americans, it has likely been a while since you've spent much time writing by hand.
The laborious process of tracing out our thoughts, letter by letter, on the page is becoming a relic of the past in our screen-dominated world, where text messages and thumb-typed grocery lists have replaced handwritten letters and sticky notes. Electronic keyboards offer obvious efficiency benefits that have undoubtedly boosted our productivity — imagine having to write all your emails longhand.
To keep up, many schools are introducing computers as early as preschool, meaning some kids may learn the basics of typing before writing by hand.
But giving up this slower, more tactile way of expressing ourselves may come at a significant cost, according to a growing body of research that's uncovering the surprising cognitive benefits of taking pen to paper, or even stylus to iPad — for both children and adults.
Is this some kind of joke? A school facing shortages starts teaching standup comedy
In kids, studies show that tracing out ABCs, as opposed to typing them, leads to better and longer-lasting recognition and understanding of letters. Writing by hand also improves memory and recall of words, laying down the foundations of literacy and learning. In adults, taking notes by hand during a lecture, instead of typing, can lead to better conceptual understanding of material.
"There's actually some very important things going on during the embodied experience of writing by hand," says Ramesh Balasubramaniam , a neuroscientist at the University of California, Merced. "It has important cognitive benefits."
While those benefits have long been recognized by some (for instance, many authors, including Jennifer Egan and Neil Gaiman , draft their stories by hand to stoke creativity), scientists have only recently started investigating why writing by hand has these effects.
A slew of recent brain imaging research suggests handwriting's power stems from the relative complexity of the process and how it forces different brain systems to work together to reproduce the shapes of letters in our heads onto the page.
Your brain on handwriting
Both handwriting and typing involve moving our hands and fingers to create words on a page. But handwriting, it turns out, requires a lot more fine-tuned coordination between the motor and visual systems. This seems to more deeply engage the brain in ways that support learning.

Shots - Health News
Feeling artsy here's how making art helps your brain.
"Handwriting is probably among the most complex motor skills that the brain is capable of," says Marieke Longcamp , a cognitive neuroscientist at Aix-Marseille Université.
Gripping a pen nimbly enough to write is a complicated task, as it requires your brain to continuously monitor the pressure that each finger exerts on the pen. Then, your motor system has to delicately modify that pressure to re-create each letter of the words in your head on the page.
"Your fingers have to each do something different to produce a recognizable letter," says Sophia Vinci-Booher , an educational neuroscientist at Vanderbilt University. Adding to the complexity, your visual system must continuously process that letter as it's formed. With each stroke, your brain compares the unfolding script with mental models of the letters and words, making adjustments to fingers in real time to create the letters' shapes, says Vinci-Booher.
That's not true for typing.
To type "tap" your fingers don't have to trace out the form of the letters — they just make three relatively simple and uniform movements. In comparison, it takes a lot more brainpower, as well as cross-talk between brain areas, to write than type.
Recent brain imaging studies bolster this idea. A study published in January found that when students write by hand, brain areas involved in motor and visual information processing " sync up " with areas crucial to memory formation, firing at frequencies associated with learning.
"We don't see that [synchronized activity] in typewriting at all," says Audrey van der Meer , a psychologist and study co-author at the Norwegian University of Science and Technology. She suggests that writing by hand is a neurobiologically richer process and that this richness may confer some cognitive benefits.
Other experts agree. "There seems to be something fundamental about engaging your body to produce these shapes," says Robert Wiley , a cognitive psychologist at the University of North Carolina, Greensboro. "It lets you make associations between your body and what you're seeing and hearing," he says, which might give the mind more footholds for accessing a given concept or idea.
Those extra footholds are especially important for learning in kids, but they may give adults a leg up too. Wiley and others worry that ditching handwriting for typing could have serious consequences for how we all learn and think.
What might be lost as handwriting wanes
The clearest consequence of screens and keyboards replacing pen and paper might be on kids' ability to learn the building blocks of literacy — letters.
"Letter recognition in early childhood is actually one of the best predictors of later reading and math attainment," says Vinci-Booher. Her work suggests the process of learning to write letters by hand is crucial for learning to read them.
"When kids write letters, they're just messy," she says. As kids practice writing "A," each iteration is different, and that variability helps solidify their conceptual understanding of the letter.
Research suggests kids learn to recognize letters better when seeing variable handwritten examples, compared with uniform typed examples.
This helps develop areas of the brain used during reading in older children and adults, Vinci-Booher found.
"This could be one of the ways that early experiences actually translate to long-term life outcomes," she says. "These visually demanding, fine motor actions bake in neural communication patterns that are really important for learning later on."
Ditching handwriting instruction could mean that those skills don't get developed as well, which could impair kids' ability to learn down the road.
"If young children are not receiving any handwriting training, which is very good brain stimulation, then their brains simply won't reach their full potential," says van der Meer. "It's scary to think of the potential consequences."
Many states are trying to avoid these risks by mandating cursive instruction. This year, California started requiring elementary school students to learn cursive , and similar bills are moving through state legislatures in several states, including Indiana, Kentucky, South Carolina and Wisconsin. (So far, evidence suggests that it's the writing by hand that matters, not whether it's print or cursive.)
Slowing down and processing information
For adults, one of the main benefits of writing by hand is that it simply forces us to slow down.
During a meeting or lecture, it's possible to type what you're hearing verbatim. But often, "you're not actually processing that information — you're just typing in the blind," says van der Meer. "If you take notes by hand, you can't write everything down," she says.
The relative slowness of the medium forces you to process the information, writing key words or phrases and using drawing or arrows to work through ideas, she says. "You make the information your own," she says, which helps it stick in the brain.
Such connections and integration are still possible when typing, but they need to be made more intentionally. And sometimes, efficiency wins out. "When you're writing a long essay, it's obviously much more practical to use a keyboard," says van der Meer.
Still, given our long history of using our hands to mark meaning in the world, some scientists worry about the more diffuse consequences of offloading our thinking to computers.
"We're foisting a lot of our knowledge, extending our cognition, to other devices, so it's only natural that we've started using these other agents to do our writing for us," says Balasubramaniam.
It's possible that this might free up our minds to do other kinds of hard thinking, he says. Or we might be sacrificing a fundamental process that's crucial for the kinds of immersive cognitive experiences that enable us to learn and think at our full potential.
Balasubramaniam stresses, however, that we don't have to ditch digital tools to harness the power of handwriting. So far, research suggests that scribbling with a stylus on a screen activates the same brain pathways as etching ink on paper. It's the movement that counts, he says, not its final form.
Jonathan Lambert is a Washington, D.C.-based freelance journalist who covers science, health and policy.
- handwriting

- Liberty University
- Jerry Falwell Library
- Special Collections
- < Previous Event
- Next Event >
Home > Conferences and Events > Research Week > 2024 > Oral Presentations > 58

Oral Presentations
The Psychology Behind Storytelling
Presenter Information
Stephanie Russak , Liberty University Follow
Oral - Creative and Artistic
Description
This paper will examine the art of storytelling and how humanity is psychologically wired to crave stories. While humans used to tell stories to point to a food supply, ward off danger, or preserve family history, today, people gather in front of movie screens, theater stages, and concert halls. This paper will explore that evolutionary process and how stories remain prevalent in the modern era. It will explore the question “What compels our brain to capture its waning attention span and listen to a story?” This paper looks deeper into what psychologists call “narrative transportation” and how the brain engages in seven different regions to help immerse itself. Not only will it analyze what happens in the brain when a story begins, transpires and ends, but also how a storyteller and listener connect on a cerebral level. This paper will also contribute the Theory of Mind and how it is displayed in storytelling. This paper will prove that it is because of the existence of stories that humanity tries to explain how the world works and provides order. It will conclude that it is through the power of storytelling that people connect with each other through shared experiences as it provides a narrative structure that is familiar, engages emotions, and satisfies. Stories have the ability to pass along emotions of happiness, anger, remorse, or sadness through countless generations and open a window into the lives of hardship, joy, passion, and love. Humanity is psychologically wired to receive such stories which is what makes them delectable and necessary in a fast-paced modern world.
Undergraduate
Since May 20, 2024
To view the content in your browser, please download Adobe Reader or, alternately, you may Download the file to your hard drive.
NOTE: The latest versions of Adobe Reader do not support viewing PDF files within Firefox on Mac OS and if you are using a modern (Intel) Mac, there is no official plugin for viewing PDF files within the browser window.
- Collections
- Faculty Expert Gallery
- Theses and Dissertations
- Conferences and Events
- Open Educational Resources (OER)
- Explore Disciplines
Advanced Search
- Notify me via email or RSS .
Faculty Authors
- Submit Event
- Expert Gallery Login
Student Authors
- Undergraduate Submissions
- Graduate Submissions
- Honors Submissions
Home | About | FAQ | My Account | Accessibility Statement
Privacy Copyright
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 18 March 2015
The neuroscience of mindfulness meditation
- Yi-Yuan Tang 1 , 2 na1 ,
- Britta K. Hölzel 3 , 4 na1 &
- Michael I. Posner 2
Nature Reviews Neuroscience volume 16 , pages 213–225 ( 2015 ) Cite this article
100k Accesses
1427 Citations
919 Altmetric
Metrics details
- Cognitive neuroscience
An Erratum to this article was published on 10 April 2015
It is proposed that the mechanism through which mindfulness meditation exerts its effects is a process of enhanced self-regulation, including attention control, emotion regulation and self-awareness.
Research on mindfulness meditation faces a number of important challenges in study design that limit the interpretation of existing studies.
A number of changes in brain structure have been related to mindfulness meditation.
Mindfulness practice enhances attention. The anterior cingulate cortex is the region associated with attention in which changes in activity and/or structure in response to mindfulness meditation are most consistently reported.
Mindfulness practice improves emotion regulation and reduces stress. Fronto-limbic networks involved in these processes show various patterns of engagement by mindfulness meditation.
Meditation practice has the potential to affect self-referential processing and improve present-moment awareness. The default mode networks — including the midline prefrontal cortex and posterior cingulate cortex, which support self-awareness — could be altered following mindfulness training.
Mindfulness meditation has potential for the treatment of clinical disorders and might facilitate the cultivation of a healthy mind and increased well-being.
Future research into mindfulness meditation should use randomized and actively controlled longitudinal studies with large sample sizes to validate previous findings.
The effects of mindfulness practice on neural structure and function need to be linked to behavioural performance, such as cognitive, affective and social functioning, in future research.
The complex mental state of mindfulness is likely to be supported by the large-scale brain networks; future work should take this into account rather than being restricted to activations in single brain areas.
Research over the past two decades broadly supports the claim that mindfulness meditation — practiced widely for the reduction of stress and promotion of health — exerts beneficial effects on physical and mental health, and cognitive performance. Recent neuroimaging studies have begun to uncover the brain areas and networks that mediate these positive effects. However, the underlying neural mechanisms remain unclear, and it is apparent that more methodologically rigorous studies are required if we are to gain a full understanding of the neuronal and molecular bases of the changes in the brain that accompany mindfulness meditation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
176,64 € per year
only 14,72 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
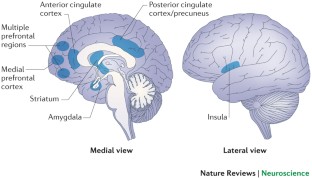
Similar content being viewed by others
Mindfulness meditation increases default mode, salience, and central executive network connectivity

Focused attention meditation changes the boundary and configuration of functional networks in the brain
Meta-analytic evidence that mindfulness training alters resting state default mode network connectivity
Ospina, M. B. et al. Meditation practices for health: state of the research. Evid. Rep. Technol. Assess. (Full Rep.) 155 , 1–263 (2007).
Google Scholar
Tang, Y.-Y. & Posner, M. I. Theory and method in mindfulness neuroscience. Soc. Cogn. Affect. Neurosci. 8 , 118–120 (2013).
Article PubMed Google Scholar
Hart, W. The Art of Living: Vipassana Meditation (Harper and Row, 1987).
Ivanovski, B. & Malhi, G. S. The psychological and neurophysiological concomitants of mindfulness forms of meditation. Acta Neuropsychiatr. 19 , 76–91 (2007).
Chiesa, A. & Malinowski, P. Mindfulness-based approaches: are they all the same? J. Clin. Psychol. 67 , 404–424 (2011).
Baer, R. A. Mindfulness training as a clinical intervention: a conceptual and empirical review. Clin. Psychol. Sci. Practice 10 , 125–143 (2003).
Article Google Scholar
Grossman, P. Defining mindfulness by how poorly I think I pay attention during everyday awareness and other intractable problems for psychology's (re)invention of mindfulness: comment on Brown et al . Psychol. Assess. 23 , 1034–1040 (2011).
Kabat-Zinn, J. Full Catastrophe Living: Using the Wisdom of Your Body and Mind to Face Stress, Pain, and Illness (Delta Trade Paperbacks, 1990).
Lutz, A., Slagter, H. A., Dunne, J. D. & Davidson, R. J. Attention regulation and monitoring in meditation. Trends Cogn. Sci. 12 , 163–169 (2008).
Article PubMed PubMed Central Google Scholar
Hölzel, B. K. et al. How does mindfulness meditation work? Proposing mechanisms of action from a conceptual and neural perspective. Perspect. Psychol. Sci. 6 , 537–559 (2011). A review of the mechanisms of meditation.
Tang, Y.Y., Rothbart, M. K. & Posner, M. I. Neural correlates of establishing, maintaining and switching brain states. Trends Cogn. Sci. 16 , 330–337 (2012). A review of the mechanisms of brain states associated with mental training.
Zeidan, F., Johnson, S. K., Diamond, B. J., David, Z. & Goolkasian, P. Mindfulness meditation improves cognition: evidence of brief mental training. Conscious. Cogn. 19 , 597–605 (2010).
Ding, X. et al. Short-term meditation modulates brain activity of insight evoked with solution cue. Soc. Cogn. Affect. Neurosci. 10 , 43–49 (2014).
Tang, Y. Y. et al. Short-term meditation training improves attention and self-regulation. Proc. Natl Acad. Sci. USA 104 , 17152–17156 (2007). The first longitudinal, randomized study to document that brief training improves executive attention, mood and immune function, and reduces levels of stress hormones.
Manna, A. et al. Neural correlates of focused attention and cognitive monitoring in meditation. Brain Res. Bull. 82 , 46–56 (2010).
Tomasino, B., Fregona, S., Skrap, M. & Fabbro, F. Meditation-related activations are modulated by the practices needed to obtain it and by the expertise: an ALE meta-analysis study. Front. Hum. Neurosci. 6 , 346 (2012).
PubMed Google Scholar
Fox, K. C. et al. Is meditation associated with altered brain structure? A systematic review and meta-analysis of morphometric neuroimaging in meditation practitioners. Neurosci. Biobehav. Rev. 43 , 48–73 (2014). A review of structural alterations in the brain associated with meditation.
Brefczynski-Lewis, J. A., Lutz, A., Schaefer, H. S., Levinson, D. B. & Davidson, R. J. Neural correlates of attentional expertise in long-term meditation practitioners. Proc. Natl Acad. Sci. USA 104 , 11483–11488 (2007). One of the first cross-sectional studies to document the neural correlates of focused meditation.
Article CAS PubMed Google Scholar
Davidson, R. J. Empirical explorations of mindfulness: conceptual and methodological conundrums. Emotion 10 , 8–11 (2010).
MacCoon, D. G. et al. The validation of an active control intervention for Mindfulness Based Stress Reduction (MBSR). Behav. Res. Ther. 50 , 3–12 (2012). One of the first studies to validate the active control conditions in mindfulness training.
Rosenkranz, M. A. et al. A comparison of mindfulness-based stress reduction and an active control in modulation of neurogenic inflammation. Brain Behav. Immun. 27 , 174–184 (2013).
MacCoon, D. G., MacLean, K. A., Davidson, R. J., Saron, C. D. & Lutz, A. No sustained attention differences in a longitudinal randomized trial comparing mindfulness based stress reduction versus active control. PLoS ONE 9 , e97551 (2014).
Tang, Y. Y. et al. Central and autonomic nervous system interaction is altered by short-term meditation. Proc. Natl Acad. Sci. USA 106 , 8865–8870 (2009).
Erisman, S. M. & Roemer, L. The effects of experimentally induced mindfulness on emotional responding to film clips. Emotion 10 , 72–82 (2010).
Leiberg, S., Klimecki, O. & Singer, T. Short-term compassion training increases prosocial behaviour in a newly developed prosocial game. PLoS ONE 6 , e17798 (2011).
Article CAS PubMed PubMed Central Google Scholar
Hoge, E. A. et al. Randomized controlled trial of mindfulness meditation for generalized anxiety disorder: effects on anxiety and stress reactivity. J. Clin. Psychiatry 74 , 786–792 (2013).
Tang, Y. Y., Yang, L., Leve, L. D. & Harold, G. T. Improving executive function and its neurobiological mechanisms through a mindfulness-based intervention: advances within the field of developmental neuroscience. Child Dev. Perspect. 6 , 361–366 (2012).
PubMed PubMed Central Google Scholar
Zeidan, F., Johnson, S. K., Gordon, N. S. & Goolkasian, P. Effects of brief and sham mindfulness meditation on mood and cardiovascular variables. J. Altern. Complement. Med. 16 , 867–873 (2010).
Goldin, P., Ziv, M., Jazaieri, H., Hahn, K. & Gross, J. J. MBSR versus aerobic exercise in social anxiety: fMRI of emotion regulation of negative self-beliefs. Soc. Cogn. Affect. Neurosci. 8 , 65–72 (2013). One of the first randomized mindfulness studies to document the neural mechanisms in social anxiety.
Zeidan, F. et al. Brain mechanisms supporting the modulation of pain by mindfulness meditation. J. Neurosci. 31 , 5540–5548 (2011).
Hölzel, B. K. et al. Investigation of mindfulness meditation practitioners with voxel-based morphometry. Soc. Cogn. Affect. Neurosci. 3 , 55–61 (2008).
Lazar, S. W. et al. Meditation experience is associated with increased cortical thickness. Neuroreport 16 , 1893–1897 (2005). The first cross-sectional study to document that meditation is associated with structural changes in the brain.
Vestergaard-Poulsen, P. et al. Long-term meditation is associated with increased grey matter density in the brain stem. Neuroreport 20 , 170–174 (2009).
Pagnoni, G. & Cekic, M. Age effects on grey matter volume and attentional performance in Zen meditation. Neurobiol. Aging 28 , 1623–1627 (2007).
Grant, J. A., Courtemanche, J. & Rainville, P. A non-elaborative mental stance and decoupling of executive and pain-related cortices predicts low pain sensitivity in Zen meditators. Pain 152 , 150–156 (2010).
Grant, J. A. et al. Cortical thickness, mental absorption and meditative practice: possible implications for disorders of attention. Biol. Psychol. 92 , 275–281 (2013).
Fayed, N. et al. Brain changes in long-term zen meditators using proton magnetic resonance spectroscopy and diffusion tensor imaging: a controlled study. PLoS ONE 8 , e58476 (2013).
Tang, Y. Y. et al. Short-term meditation induces white matter changes in the anterior cingulate. Proc. Natl Acad. Sci. USA 107 , 15649–15652 (2010). The first longitudinal study to document that brief mindfulness training induces white-matter changes.
Tang, Y. Y., Lu, Q., Fan, M., Yang, Y. & Posner, M. I. Mechanisms of white matter changes induced by meditation. Proc. Natl Acad. Sci. USA 109 , 10570–10574 (2012).
Hölzel, B. K. et al. Mindfulness practice leads to increases in regional brain grey matter density. Psychiatry Res. 191 , 36–43 (2011).
Wells, R. E. et al. Meditation's impact on default mode network and hippocampus in mild cognitive impairment: a pilot study. Neurosci. Lett. 556 , 15–19 (2013).
Pickut, B. A. et al. Mindfulness based intervention in Parkinson's disease leads to structural brain changes on MRI: a randomized controlled longitudinal trial. Clin. Neurol. Neurosurg. 115 , 2419–2425 (2013).
Luders, E., Toga, A. W., Lepore, N. & Gaser, C. The underlying anatomical correlates of long-term meditation: larger hippocampal and frontal volumes of grey matter. Neuroimage 45 , 672–678 (2009).
Luders, E., Clark, K., Narr, K. L. & Toga, A. W. Enhanced brain connectivity in long-term meditation practitioners. Neuroimage 57 , 1308–1316 (2011).
Luders, E. et al. Bridging the hemispheres in meditation: thicker callosal regions and enhanced fractional anisotropy (FA) in long-term practitioners. Neuroimage 61 , 181–187 (2012).
Luders, E. et al. Global and regional alterations of hippocampal anatomy in long-term meditation practitioners. Hum. Brain Mapp. 34 , 3369–3375 (2013).
Singleton, O. et al. Change in brainstem grey matter concentration following a mindfulness-based intervention is correlated with improvement in psychological well-being. Front. Hum. Neurosci. 8 , 33 (2014).
Hölzel, B. K. et al. Stress reduction correlates with structural changes in the amygdala. Soc. Cogn. Affect. Neurosci. 5 , 11–17 (2010).
Luders, E., Kurth, F., Toga, A. W., Narr, K. L. & Gaser, C. Meditation effects within the hippocampal complex revealed by voxel-based morphometry and cytoarchitectonic probabilistic mapping. Front. Psychol. 4 , 398 (2013).
Luders, E. et al. The unique brain anatomy of meditation practitioners: alterations in cortical gyrification. Front. Hum. Neurosci. 6 , 34 (2012).
Grant, J. A., Courtemanche, J., Duerden, E. G., Duncan, G. H. & Rainville, P. Cortical thickness and pain sensitivity in zen meditators. Emotion 10 , 43–53 (2010).
Farb, N. A., Segal, Z. V. & Anderson, A. K. Mindfulness meditation training alters cortical representations of interoceptive attention. Soc. Cogn. Affect. Neurosci. 8 , 15–26 (2013).
Tang, Y.-Y. & Posner, M. I. Attention training and attention state training. Trends Cogn. Sci. 13 , 222–227 (2009).
Tang, Y. Y. & Posner, M. I. in Handbook of Mindfulness: Theory, Research, and Practice Ch. 5 (eds Brown, K. W., Creswell, J. D. & Ryan, R. M.) 81–89 (Guildford Press, 2014).
Posner, M. I. & Petersen, S. E. The attention system of the human brain. Annu. Rev. Neurosci. 13 , 25–42 (1990).
Petersen, S. E. & Posner, M. I. The attention system of the human brain: 20 years after. Annu. Rev. Neurosci. 35 , 73–89 (2012).
Fan, J., McCandliss, B. D., Sommer, T., Raz, A. & Posner, M. I. Testing the efficiency and independence of attentional networks. J. Cogn. Neurosci. 14 , 340–347 (2002).
Raz, A. & Buhle, J. Typologies of attentional networks. Nature Rev. Neurosci. 7 , 367–379 (2006).
Article CAS Google Scholar
Posner, M. I. & Rothbart, M. K. Research on attention networks as a model for the integration of psychological science. Annu. Rev. Psychol. 58 , 1–23 (2007).
Chiesa, A., Calati, R. & Serretti, A. Does mindfulness training improve cognitive abilities? A systematic review of neuropsychological findings. Clin. Psychol. Rev. 31 , 449–464 (2011).
Chan, D. & Woollacott, M. Effects of level of meditation experience on attentional focus: is the efficiency of executive or orientation networks improved? J. Altern. Complement. Med. 13 , 651–657 (2007).
Moore, A. & Malinowski, P. Meditation, mindfulness and cognitive flexibility. Conscious. Cogn. 18 , 176–186 (2009).
Wenk-Sormaz, H. Meditation can reduce habitual responding. Altern. Ther. Health Med. 11 , 42–58 (2005).
Slagter, H. A. et al. Mental training affects distribution of limited brain resources. PLoS Biol. 5 , e138 (2007).
Pashler, H. Overlapping mental operations in serial performance with preview. Q. J. Exp. Psychol. A. 47 , 161–191 (discussion 193–199, 201–205) (1994).
Posner, M. I. Measuring alertness. Ann. NY Acad. Sci. 1129 , 193–199 (2008).
Van Leeuwen, S., Willer, N. G. & Melloni, L. Age effects on attentional blink performance in meditation. Conscious. Cogn. 18 , 593–599 (2009).
Van den Hurk, P. A., Giommi, F., Gielen, S. C., Speckens, A. E. M. & Barendregt, H. P. Greater efficiency in attentional processing related to mindfulness meditation. Q. J. Exp. Psychol. (Hove) 63 , 1168–1180 (2010).
Anderson, N. D., Lau, M. A., Segal, Z. V. & Bishop, S. R. Mindfulness-based stress reduction and attentional control. Clin. Psychol. Psychother. 14 , 449–463 (2007).
Jha, A. P., Krompinger, J. & Baime, M. J. Mindfulness training modifies subsystems of attention. Cogn. Affect. Behav. Neurosci. 7 , 109–119 (2007).
MacLean, K. A. et al. Intensive meditation training improves perceptual discrimination and sustained attention. Psychol Sci , 21 , 829–839 (2010).
Tang, Y. Y. & Posner, M. I. Training brain networks and states. Trends Cogn. Sci. 18 , 345–350 (2014).
Tang, Y. Y., Tang, R. & Posner, M. I. Brief meditation training induces smoking reduction. Proc. Natl Acad. Sci. USA 110 , 13971–13975 (2013).
Cahn, B. R. & Polich, J. Meditation states and traits: EEG, ERP, and neuroimaging studies. Psychol. Bull. 132 , 180–211 (2006).
Hölzel, B. K. et al. Differential engagement of anterior cingulate and adjacent medial frontal cortex in adept meditators and non-meditators. Neurosci. Lett. 421 , 16–21 (2007).
Van Veen, V. & Carter, C. S. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiol. Behav. 77 , 477–482 (2002).
Posner, M. I., Sheese, B., Rothbart, M. & Tang, Y. Y. The anterior cingulate gyrus and the mechanism of self-regulation. Cogn. Affect. Behav. Neurosci. 7 , 391–395 (2007).
Tang, Y. Y. & Tang, R. Ventral-subgenual anterior cingulate cortex and self-transcendence. Front. Psychol. 4 , 1000 (2014).
Sridharan, D., Levitin, D. J. & Menon, V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc. Natl Acad. Sci. USA 105 , 12569–12574 (2008).
Gard, T. et al. Pain attenuation through mindfulness is associated with decreased cognitive control and increased sensory processing in the brain. Cereb. Cortex 22 , 2692–2702 (2012).
Allen, M. et al. Cognitive-affective neural plasticity following active-controlled mindfulness intervention. J. Neurosci. 32 , 15601–15610 (2012). One of the first studies to document the effects of mindfulness using active controls.
Goldin, P. R. & Gross, J. J. Effects of mindfulness-based stress reduction (MBSR) on emotion regulation in social anxiety disorder. Emotion 10 , 83–91 (2010).
Deckersbach, T., Hölzel, B. K., Eisner, L. R., Lazar, S. W. & Nierenberg, A. A. Mindfulness-Based Cognitive Therapy for Bipolar Disorder (Guildford Press, 2014).
Book Google Scholar
Passarotti, A. M., Sweeney, J. A. & Pavuluri, M. N. Emotion processing influences working memory circuits in pediatric bipolar disorder and attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry 49 , 1064–1080 (2010).
Gross, J. J. in Handbook of Emotion Regulation 2nd edn (ed. Gross, J. J.) 3–20 (Guildford Press, 2014).
Ortner, C. N. M., Kilner, S. J. & Zelazo, P. D. Mindfulness meditation and reduced emotional interference on a cognitive task. Motiv. Emot. 31 , 271–283 (2007).
Goleman, D. J. & Schwartz, G. E. Meditation as an intervention in stress reactivity. J. Consult. Clin. Psychol. 44 , 456–466 (1976).
Robins, C. J., Keng, S.-L., Ekblad, A. G. & Brantley, J. G. Effects of mindfulness-based stress reduction on emotional experience and expression: a randomized controlled trial. J. Clin. Psychol. 68 , 117–131 (2012).
Chambers, R., Lo, B. C. Y. & Allen, N. B. The impact of intensive mindfulness training on attentional control, cognitive style, and affect. Cogn. Ther. Res. 32 , 303–322 (2008).
Ding, X., Tang, Y. Y., Tang, R. & Posner, M. I. Improving creativity performance by short-term meditation. Behav. Brain Funct. 10 , 9 (2014).
Jain, S. et al. A randomized controlled trial of mindfulness meditation versus relaxation training: effects on distress, positive states of mind, rumination, and distraction. Ann. Behav. Med. 33 , 11–21 (2007).
Desbordes, G. et al. Effects of mindful-attention and compassion meditation training on amygdala response to emotional stimuli in an ordinary, non-meditative state. Front. Hum. Neurosci. 6 , 292 (2012).
Lutz, J. et al. Mindfulness and emotion regulation — an fMRI study. Soc. Cogn. Affect. Neurosci. 9 , 776–785 (2014).
Taylor, V. A. et al. Impact of mindfulness on the neural responses to emotional pictures in experienced and beginner meditators. Neuroimage 57 , 1524–1533 (2011).
Westbrook, C. et al. Mindful attention reduces neural and self-reported cue-induced craving in smokers. Soc. Cogn. Affect. Neurosci. 8 , 73–84 (2013).
Hölzel, B. K. et al. Neural mechanisms of symptom improvements in generalized anxiety disorder following mindfulness training. Neuroimage Clin. 2 , 448–458 (2013). One of the first longitudinal, randomized mindfulness studies to document the neural mechanisms in generalized anxiety disorder.
Farb, N. A. S. et al. Attending to the present: mindfulness meditation reveals distinct neural modes of self-reference. Soc. Cogn. Affect. Neurosci. 2 , 313–322 (2007).
Teper, R., Segal, Z. V. & Inzlicht, M. Inside the mindful mind: how mindfulness enhances emotion regulation through improvements in executive control. Curr. Dir. Psychol. 22 , 449–454 (2013).
Chiesa, A., Serretti, A. & Jakobsen, J. C. Mindfulness: top-down or bottom-up emotion regulation strategy? Clin. Psychol. Rev. 33 , 82–96 (2013).
Malinowski, P. Neural mechanisms of attentional control in mindfulness meditation. Front. Hum. Neurosci. 7 , 8 (2013).
Jensen, C. G. et al. Mindfulness training affects attention — or is it attentional effort? J. Exp. Psychol. Gen. 141 , 106–123 (2012).
Posner, M. I., Rothbart, M. K., Reuda, M. R. & Tang, Y. Y. in Effortless Attention: A New Perspective in the Cognitive Science of Attention and Action (ed. Bruya, B.) 410–424 (MIT Press, 2010).
Banks, S. J., Eddy, K. T., Angstadt, M., Nathan, P. J. & Phan, K. L. Amygdala-frontal connectivity during emotion-regulation. Soc. Cogn. Affect Neurosci. 2 , 303–312 (2007).
Etkin, A., Egner, T., Peraza, D. M., Kandel, E. R. & Hirsch, J. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron 51 , 871–882 (2006).
Kirk, U., Brown, K. W. & Downar, J. Adaptive neural reward processing during anticipation and receipt of monetary rewards in mindfulness meditators. Soc. Cogn. Affect. Neurosci. http://dx.doi.org/10.1093/scan/nsu112 (2014).
Olendzki, A. Unlimiting Mind: The Radically Experiential Psychology of Buddhism (Wisdom Publications, 2010).
Sperduti, M., Martinelli, P. & Piolino, P. A neurocognitive model of meditation based on activation likelihood estimation (ALE) meta-analysis. Conscious. Cogn. 21 , 269–276 (2012).
Fresco, D. M. et al. Initial psychometric properties of the experiences questionnaire: validation of a self-report measure of decentering. Behav. Ther. 38 , 234–246 (2007).
Shapiro, S. L., Carlson, L. E., Astin, J. A. & Freedman, B. Mechanisms of mindfulness. J. Clin. Psychol. 62 , 373–386 (2006).
Josipovic, Z. Neural correlates of nondual awareness in meditation. Ann. NY Acad. Sci. 1307 , 9–18 (2014).
Kerr, C. E., Josyula, K. & Littenberg, R. Developing an observing attitude: an analysis of meditation diaries in an MBSR clinical trial. Clin. Psychol. Psychother. 18 , 80–93 (2011).
Dor-Ziderman, Y., Berkovich-Ohana, A., Glicksohn, J. & Goldstein, A. Mindfulness-induced selflessness: a MEG neurophenomenological study. Front. Hum. Neurosci. 7 , 582 (2013).
Emavardhana, T. & Tori, C. D. Changes in self-concept, ego defense mechanisms, and religiosity following seven-day Vipassana meditation retreats. J. Sci. Stud. Relig. 36 , 194–206 (1997).
Haimerl, C. J. & Valentine, E. R. The effect of contemplative practice on intrapersonal, interpersonal, and transpersonal dimensions of the self-concept. J. Transpers. Psychol. 33 , 37–52 (2001).
Sahdra, B. K., Shaver, P. R. & Brown, K. W. A scale to measure nonattachment: a Buddhist complement to Western research on attachment and adaptive functioning. J. Pers. Assess. 92 , 116–127 (2010).
Brewer, J. A. et al. Meditation experience is associated with differences in default mode network activity and connectivity. Proc. Natl Acad. Sci. USA 108 , 20254–20259 (2011). One of the first studies to document the alteration of the DMN by meditation.
Hasenkamp, W. & Barsalou, L. W. Effects of meditation experience on functional connectivity of distributed brain networks. Front. Hum. Neurosci. 6 , 38 (2012).
Buckner, R. L., Andrews-Hanna, J. R. & Schacter, D. L. The brain's default network: anatomy, function, and relevance to disease. Ann. NY Acad. Sci. 1124 , 1–38 (2008).
Raichle, M. E. et al. A default mode of brain function. Proc. Natl Acad. Sci. USA 98 , 676–682 (2001).
Northoff, G. et al. Self-referential processing in our brain: a meta-analysis of imaging studies on the self. Neuroimage 31 , 440–457 (2006).
Sajonz, B. et al. Delineating self-referential processing from episodic memory retrieval: common and dissociable networks. Neuroimage 50 , 1606–1617 (2010).
Buckner, R. L. & Carroll, D. C. Self-projection and the brain. Trends Cogn. Sci. 11 , 49–57 (2007).
Khalsa, S. S. et al. Interoceptive awareness in experienced meditators. Psychophysiology 45 , 671–677 (2008).
Nielsen, L. & Kaszniak, A. W. Awareness of subtle emotional feelings: a comparison of long-term meditators and nonmeditators. Emotion 6 , 392–405 (2006).
Sze, J. A., Gyurak, A., Yuan, J. W. & Levenson, R. W. Coherence between emotional experience and physiology: does body awareness training have an impact? Emotion 10 , 803–814 (2010).
Fox, K. C. R. et al. Meditation experience predicts introspective accuracy. PLoS ONE 7 , e45370 (2012).
Lutz, A., Brefczynski-Lewis, J., Johnstone, T. & Davidson, R. J. Regulation of the neural circuitry of emotion by compassion meditation: effects of meditative expertise. PLoS ONE 3 , e1897 (2008).
Craig, A. D. How do you feel — now? The anterior insula and human awareness. Nature Rev. Neurosci. 10 , 59–70 (2009).
Monti, D. A. et al. Changes in cerebral blood flow and anxiety associated with an 8-week mindfulness programme in women with breast cancer. Stress Health 28 , 397–407 (2012).
Grey, J. D., Milner, T. A. & McEwen, B. S. Dynamic plasticity: the role of glucocorticoids, brain-derived neurotrophic factor and other trophic factors. Neuroscience 239 , 214–227 (2013).
Creswell, J. D., Pacilio, L. E., Lindsay, E. K. & Brown, K. W. Brief mindfulness meditation training alters psychological and neuroendocrine responses to social evaluative stress. Psychoneuroendocrinology 44 , 1–12 (2014).
Tang, Y. Y., Tang, R., Jiang, C. & Posner, M. I. Short-term meditation intervention improves self-regulation and academic performance. J. Child Adolesc. Behav. 2 , 4 (2014).
Chiesa, A., Serretti, A. Mindfulness-based stress reduction for stress management in healthy people: a review and meta-analysis. J. Altern. Complement. Med. 15 , 593–600 (2009).
Jacobs, T. L. et al. Self-reported mindfulness and cortisol during a Shamatha meditation retreat. Health Psychol. 32 , 1104–1109 (2013).
Fan, Y., Tang, Y. Y. & Posner, M. I. Cortisol level modulated by integrative meditation in a dose-dependent fashion. Stress Health 30 , 65–70 (2013).
Fan, Y., Tang, Y. Y., Ma, Y. & Posner, M. I. Mucosal immunity modulated by integrative meditation in a dose-dependent fashion. J. Altern. Complement. Med. 16 , 151–155 (2010).
Kang, D. H. et al. The effect of meditation on brain structure: cortical thickness mapping and diffusion tensor imaging. Soc. Cogn. Affect. Neurosci. 8 , 27–33 (2013).
Bressler, S. L. & Menon, V. Large-scale brain networks in cognition: emerging methods and principles. Trends Cogn. Sci. 14 , 277–290 (2010).
Menon, V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn. Sci. 15 , 483–506 (2011).
Xue, S., Tang, Y. Y. & Posner, M. I. Short-term meditation increases network efficiency of the anterior cingulate cortex. Neuroreport 22 , 570–574 (2011).
Gard, T. et al. Fluid intelligence and brain functional organization in aging yoga and meditation practitioners. Front. Aging Neurosci. 6 , 76 (2014).
Lane, R. D. & Wager, T. D. The new field of brain-body medicine: what have we learned and where are we headed? Neuroimage 47 , 1135–1140 (2009).
Garrison, K. M. et al. Real-time fMRI links subjective experience with brain activity during focused attention. Neuroimage 81 , 110–118 (2013).
LaConte, S. M. Decoding fMRI brain states in real-time. Neuroimage 56 , 440–454 (2011).
Zotev, V. et al. Self-regulation of amygdala activation using real-time fMRI neurofeedback. PLoS ONE 6 , e24522 (2011).
Haynes, J. D. & Rees, G. Decoding mental states from brain activity in humans. Nature Rev. Neurosci. 7 , 523–534 (2006).
van I. Jendoorn, M. H. et al. Gene-by-environment experiments: a new approach to finding the missing heritability. Nature Rev. Genet. 12 , 881 (2011).
Jung, Y. H. et al. Influence of brain-derived neurotrophic factor and catechol O-methyl transferase polymorphisms on effects of meditation on plasma catecholamines and stress. Stress 15 , 97–104 (2012).
Ding, X., Tang, Y. Y., Deng, Y., Tang, R. & Posner, M. I. Mood and personality predict improvement in creativity due to meditation training. Learn. Individ. Differ. 37 , 217–221 (2014).
Rothbart, M. K. Becoming Who We Are (Guilford Press, 2011).
Takahashi, T. et al. Changes in EEG and autonomic nervous activity during meditation and their association with personality traits. Int. J. Psychophysiol. 55 , 199–207 (2005).
Moffitt, T. E. et al. A gradient of childhood self-control predicts health, wealth, and public safety. Proc. Natl Acad. Sci. USA 108 , 2693–2698 (2011).
Hofmann, S. G., Sawyer, A. T., Witt, A. A. & Oh, D. The effect of mindfulness-based therapy on anxiety and depression: a meta-analytic review. J. Consult. Clin. Psychol. 78 , 169–183 (2010). A review of the effect of mindfulness-based therapy on anxiety and mood symptoms.
Bowen, S. et al. Relative efficacy of mindfulness-based relapse prevention, standard relapse prevention, and treatment as usual for substance use disorders: a randomized clinical trial. JAMA Psychiatry 71 , 547–556 (2014). One of the first longitudinal studies to document the effects of mindfulness on drug use and heavy drinking.
Schoenberg, P. L. A. et al. Effects of mindfulness-based cognitive therapy on neurophysiological correlates of performance monitoring in adult attention-deficit/hyperactivity disorder. Clin. Neurophysiol. 125 , 1407–1416 (2014).
Zeidan, F., Martucci, K. T., Kraft, R. A., McHaffie, J. G. & Coghill, R. C. Neural correlates of mindfulness meditation-related anxiety relief. Soc. Cogn. Affect. Neurosci. 9 , 751–759 (2014).
Desbordes, G. et al. Moving beyond mindfulness: defining equanimity as an outcome measure in meditation and contemplative research. Mindfulness http://dx.doi.org/10.1007/s12671-013-0269-8 (2014).
Smith, J. C. Alterations in brain and immune function produced by mindfulness meditation: three caveats. Psychosom. Med. 66 , 148–152 (2004).
Davidson, R. J., Kabat-Zinn, J. Response to Smith, J. C. Psychosom. Med. 66 , 148–152 (2004).
Lippelt, D. P., Hommel, B. & Colzato, L. S. Focused attention, open monitoring and loving kindness meditation: effects on attention, conflict monitoring, and creativity — a review. Front. Psychol. 5 , 1083 (2014).
Hasenkamp, W., Wilson-Mendenhall, C. D., Duncan, E. & Barsalou, L. W. Mind wandering and attention during focused meditation: a fine-grained temporal analysis of fluctuating cognitive states. Neuroimage 59 , 750–760 (2012). One of the first studies to document brain activity during different phases of focused-attention meditation.
Pagnoni, G. Dynamical properties of BOLD activity from the ventral posteromedial cortex associated with meditation and attentional skills. J. Neurosci. 32 , 5242–5249 (2012).
Öst, L. G. in Phobias: A Handbook of Theory, Research, and Treatment (ed. Davey, G. C. L.) 227–247 (John Wiley, 1997).
Milad, M. R. & Quirk, G. J. Fear extinction as a model for translational neuroscience: ten years of progress. Annu. Rev. Psychol. 63 , 129–151 (2012).
Milad, M. R. et al. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol. Psychiatry. 62 , 446–454 (2007).
LeDoux, J. E. Emotion circuits in the brain. Annu. Rev. Neurosci. 23 , 155–184 (2000).
Davidson, R. J., Jackson, D. C. & Kalin, N. H. Emotion, plasticity, context, and regulation: perspectives from affective neuroscience. Psychol. Bull. 126 , 890–909 (2000).
Phelps, E. A., Delgado, M. R., Nearing, K. I. & LeDoux, J. E. Extinction learning in humans: role of the amygdala and vmPFC. Neuron 43 , 897–905 (2004).
Holt, D. J. et al. Extinction memory is impaired in schizophrenia. Biol. Psychiatry 65 , 455–463 (2009).
Milad, M. R. et al. Presence and acquired origin of reduced recall for fear extinction in PTSD: results of a twin study. J. Psychiatr. Res. 42 , 515–520 (2008).
McEwen, B. S. & Morrison, J. H. The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron 79 , 16–29 (2013).
McEwen, B. S. & Gianaros, P. J. Stress- and allostasis-induced brain plasticity. Annu. Rev. Med. 62 , 431–445 (2011).
Liston, C., McEwen, B. S. & Casey, B. J. Psychossocial stress sreversibly disrupts prefrontal processing and attentional control. Proc. Natl Acad. Sci. USA 106 , 912–917 (2009).
Davidson, R. J. & McEwen, B. S. Social influences on neuroplasticity: stress and interventions to promote well-being. Nature Neurosci. 15 , 689–695 (2012).
McEwen, B. S. The brain on stress: toward an integrative approach to brain, body and behaviour. Perspect. Psychol. Sci. 8 , 673–675 (2013).
Thayer, J. F. & Lane, R. D. A model of neurovisceral integration in emotion regulation and dysregulation. J. Affective Disord. 61 , 201–216 (2000).
Creswell, J. D. in Handbook of Mindfulness: Theory, Research, and Practice Ch. 23 (eds Brown, K. W., Creswell, J. D. & Ryan, R. M.) (Guildford Press, 2014).
Ditto, B., Eclache, M. & Goldman, N. Short-term autonomic and cardiovascular effects of mindfulness body scan meditation. Ann. Behav. Med. 32 , 227–234 (2006).
Xiong, G. L. & Doraiswamy, P. M. Does meditation enhance cognition and brain plasticity? Ann. NY Acad. Sci. 1172 , 63–69 (2009).
Download references
Acknowledgements
This work was supported by the US Office of Naval Research. We thank E. Luders for her contributions to an earlier version of this manuscript. We benefited from discussions with R. Davidson and A. Chiesa. We thank four anonymous reviewers for their constructive comments and R. Tang for manuscript preparation.
Author information
Yi-Yuan Tang and Britta K. Hölzel: These authors contributed equally to this work.
Authors and Affiliations
Department of Psychological Sciences, Texas Tech University, Lubbock, 79409, Texas, USA
Yi-Yuan Tang
Department of Psychology, University of Oregon, Eugene, 97403, Oregon, USA
Yi-Yuan Tang & Michael I. Posner
Department of Neuroradiology, Technical University of Munich, Munich, 81675, Germany
Britta K. Hölzel
Massachusetts General Hospital, Charlestown, 02129, Massachusetts, USA
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Yi-Yuan Tang .
Ethics declarations
Competing interests.
The authors declare no competing financial interests.
PowerPoint slides
Powerpoint slide for fig. 1, powerpoint slide for table 1, powerpoint slide for table 2.
Study designs that compare data from one or more groups at several time points and that ideally include a (preferably active) control condition and random assignment to conditions.
Study designs that compare data from an experimental group with those from a control group at one point in time.
Studies that assess the co-variation between two variables: for example, co-variation of functional or structural properties of the brain and a behavioural variable, such as reported stress.
(BOLD contrasts). Signals that can be extracted with functional MRI and that reflect the change in the amount of deoxyhaemoglobin that is induced by changes in the activity of neurons and their synapses in a region of the brain. The signals thus reflect the activity in a local brain region.
(ASL). An MRI technique that is capable of measuring cerebral blood flow in vivo . It provides cerebral perfusion maps without requiring the administration of a contrast agent or the use of ionizing radiation because it uses magnetically labelled endogenous blood water as a freely diffusible tracer.
The reliable patterns of brain activity that involve the activation and/or connectivity of multiple large-scale brain networks.
A parameter in diffusion tensor imaging, which images brain structures by measuring the diffusion properties of water molecules. It provides information about the microstructural integrity of white matter.
Derived from the eigenvalues of the diffusion tensor, their underlying biophysical properties are associated with axonal density and myelination, respectively.
A technique for coordinate-based meta-analysis of neuroimaging data. It determines the convergence of foci reported from different experiments, weighted by the number of participants in each study.
A method of analysing functional MRI data that is capable of detecting and characterizing information represented in patterns of activity distributed within and across multiple regions of the brain. Unlike univariate approaches, which only identify magnitudes of activity in localized parts of the brain, this approach can monitor multiple areas at once.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Tang, YY., Hölzel, B. & Posner, M. The neuroscience of mindfulness meditation. Nat Rev Neurosci 16 , 213–225 (2015). https://doi.org/10.1038/nrn3916
Download citation
Published : 18 March 2015
Issue Date : April 2015
DOI : https://doi.org/10.1038/nrn3916
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Attentional bias to threat: an investigation of psychological predictors beyond trait anxiety.
- Monique Williams
- Cynthia Honan
- Allison J. Matthews
Current Psychology (2024)
The Acute Effects of Focused Attention and Open Monitoring Meditations on Prospective Memory in Older Adults
- Alex Pak Lik Tsang
- Herman Hay Ming Lo
Mindfulness (2024)
Psychological Outcomes and Mechanisms of Mindfulness-Based Training for Generalised Anxiety Disorder: A Systematic Review and Meta-Analysis
Meta-awareness, mind wandering and negative mood in the context of the continuity hypothesis of dreaming.
- Reza Maleeh
- Shaghayegh Konjedi
Phenomenology and the Cognitive Sciences (2024)
Resting Heart Rate Variability and Emotion Dysregulation in Adolescents with Autism Spectrum Disorder
- Hey Tou Chiu
- Isaac Nam Ip
- Savio Wai Ho Wong
Journal of Autism and Developmental Disorders (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

IMAGES
VIDEO
COMMENTS
1. Introduction. How the human brain works is still an open question, as is its implication with brain architecture: the non-trivial structure-function relationship. The development of neuroimaging techniques, the improvement on their processing methods and the unfolding of computational neuroscience field have driven brain research focused ...
The brain. This supremely complex organ is slowly giving up its valuable secrets. In the hand, the human brain is a jelly-like mass, easily deformed by touch. However, its unassuming appearance ...
Abstract. The brain undergoes profound development across childhood and adolescence, including continuous changes in brain morphology, connectivity, and functioning that are, in part, dependent on ...
The brain is the part of the central nervous system that is contained within the skull. It is responsible for executive and cognitive functions and regulates the functioning of the other parts of ...
Despite these new data on lateralization of microRNA in the youngest regions of the human cortex, the research domain of the working brain molecular machinery remains largely underexplored. As a whole, this is probably the next “terra incognita†of cognitive neuroscience. Boris Velichkovsky et al. / Procedia Computer Science 169 (2020 ...
From basic brain research to treating human brain disorders. The human brain is the most complex entity we know. Disorders of the human brain are embedded in this complexity. Potential advances in treating these disorders result from the growing understanding of this complex organization. The brains of monkeys have some important similarities ...
research at the intersection of cognitive neuroscience and learning, and maps research and policy implications for the next decade. Part I "The Learning Brain" is the main report, which is the distillation from all the analyses and events over the past seven years of the OECD/CERI "Learning Sciences and Brain Research" project.
It takes "novel informatics approaches to constructing databases and database tools for collecting and analyzing neuroscience information, using the olfactory system as a model, with extension to other brain systems." It contains ten related databases that fall into three categories: Neuronal, Olfactory, and Disease.
Brain Research is dedicated to publishing the highest quality and greatest impact articles within the ever-evolving field of Neuroscience. We recognize how technology has changed the way scientific breakthroughs are communicated and Brain Research is committed to serving as a dynamic journal …. Between 1981 and 1988 Brain Research shared its ...
In 2016, E.G. contacted McGovern Institute for Brain Research Investigator Evelina Fedorenko, ... a graduate student in the Fedorenko lab and first author of the paper. In an introduction to the study, E.G. herself puts the social implications of the findings starkly. "Please do not call my brain abnormal, that creeps me out," she writes.
The brain and its function. comprise a central nervous system. A brainstem, which in part relays information between the peripheral nerves and spinal. cord to the upper parts of the brain consists ...
Top 100 in Neuroscience. This collection highlights our most downloaded* neuroscience papers published in 2021. Featuring authors from around the world, these papers showcase valuable research ...
Introductory essay. Written by the educators who created Mapping and Manipulating the Brain, a brief look at the key facts, tough questions and big ideas in their field. Begin this TED Study with a fascinating read that gives context and clarity to the material. Here is this mass of jelly, three-pound mass of jelly you can hold in the palm of ...
Google Research & Lichtman Lab/Harvard University. Next up, the team behind the project aims to create a full map of the brain of a mouse, which would require between 500 and 1,000 times the ...
Love and the Brain. Spring 2015. The Harvard Mahoney Neuroscience Institute has published On The Brain from 1992 to 2020 before transitioning to a public lecture format beginning in spring 2021. Subscribe to receive updates about the On The Brain Lecture Series ».
For decades, researchers have linked differences in school-age children's brain development to their out-of-school environment, using indirect socioeconomic factors such as parental income and neighborhood characteristics. In a new paper, researchers from Stanford Graduate School of Education (GSE) demonstrate for the first time that, even when controlling for those other
The lines of evidence described in the current papers are from the BRAIN Initiative Cell Census Network (BICCN). ... Organoids merge to model the blood-brain barrier. Research Highlight 15 MAY 24.
As a result, research on the teenage brain is finally starting to catch up with studies of other age groups, complete with the level of detail it deserves. "The shift from childhood to adulthood is not a linear one. ... of about 250 papers published using the survey's data so far, half were from investigators outside the consortium.
The toll loneliness takes on the cardiovascular system, increasing blood pressure and heart rate, can also have a detrimental effect on the brain and probably plays a role, as well, Dr. Donovan ...
Credit: Google Research & Lichtman Lab (Harvard University). Renderings by D. Berger (Harvard University) Researchers have mapped a tiny piece of the human brain in astonishing detail.
So far, research suggests that scribbling with a stylus on a screen activates the same brain pathways as etching ink on paper. It's the movement that counts, he says, not its final form.
This paper will examine the art of storytelling and how humanity is psychologically wired to crave stories. While humans used to tell stories to point to a food supply, ward off danger, or preserve family history, today, people gather in front of movie screens, theater stages, and concert halls. This paper will explore that evolutionary process and how stories remain prevalent in the modern era.
A brain state can be defined as a reliable pattern of activity and/or connectivity in multiple large-scale brain networks 11,73.Meditation training involves obtaining a meditative state, and ...