An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Res Involv Engagem


Research Buddy partnership in a MD–PhD program: lessons learned
Daniel j. gould.
1 Department of Surgery, St. Vincent’s Hospital, University of Melbourne, Melbourne, VIC Australia
Marion Glanville-Hearst
Samantha bunzli.
2 School of Health Sciences and Social Work, Griffith University, Nathan Campus, Brisbane, QLD Australia
3 Physiotherapy Department, Royal Brisbane and Women’s Hospital, Brisbane, QLD Australia
Peter F. M. Choong
4 Department of Orthopaedics, St. Vincent’s Hospital, Melbourne, VIC Australia
Michelle M. Dowsey
Associated data.
Not applicable.
Background and aims
There is increasing recognition of the importance of patient involvement in research. In recent years, there has also been growing interest in patient partnerships with doctoral studies students. However, it can be difficult to know where to start and how to go about such involvement activities. The purpose of this perspective piece was to share experiential insight of the experience of a patient involvement program such that others can learn from this experience.
This is a co-authored perspective piece centred on the experience of MGH, a patient who has had hip replacement surgery, and DG, a medical student completing a PhD, participating in a Research Buddy partnership over the course of over 3 years. The context in which this partnership took place was also described to facilitate comparison with readers’ own circumstances and contexts. DG and MGH met regularly to discuss, and work together on, various aspects of DG’s PhD research project. Reflexive thematic analysis was conducted on reflections from DG and MGH regarding their experience in the Research Buddy program to synthesise nine lessons which were then corroborated with reference to published literature on patient involvement in research. These lessons were: learn from experience; tailor the program; get involved early; embrace uniqueness; meet regularly; build rapport; ensure mutual benefit; broad involvement; regularly reflect and review.
Conclusions
In this perspective piece, a patient and a medical student completing a PhD reflected upon their experience co-designing a Research Buddy partnership within a patient involvement program. A series of nine lessons was identified and presented to inform readers seeking to develop or enhance their own patient involvement programs. Researcher-patient rapport is foundational to all other aspects of the patient’s involvement.
Plain English summary
The importance of patient involvement in research is gaining recognition. Existing research centres, as well as those that are just getting started, need to find their own way to involve patients and community members. However, learning from the experience of others is crucial to ensure every effort is made to do this in a fruitful way. Therefore, we aimed to share our experience and provide a list of lessons learned to help other researchers and patients get started and work together effectively. Our research centre developed a framework for involving patients in joint replacement research. Part of this framework is a ‘Research Buddy’ program, where a research student partners with a patient so that the research they conduct is more relevant and applicable to the target population. In our case, the research student partnered with someone who had a hip replacement to develop and test a questionnaire for an interview study about artificial intelligence in shared decision-making. The student and patient worked together and wrote this perspective piece outlining nine lessons so readers can learn from their experience of this program. The lessons were: learn from experience, tailor the program, get involved early, embrace uniqueness, meet regularly, build rapport, ensure mutual benefit, broad involvement, regularly reflect and review. People interested in starting, or improving, their own patient involvement activities can learn from our experience. These lessons will need to be adapted to fit the purpose and unique situation of other researchers and patients who have different needs and circumstances.
Introduction
There is growing recognition of the importance of patient and public involvement and engagement in research [ 1 , 2 ]. However, it can be difficult to know where to begin and how to go about it. Sharing experiential knowledge is critical for different groups seeking to develop and deploy their own programs and strategies [ 3 ]. This is because a one-size-fits-all approach will not address the culture and context of the unique settings in which these programs take place [ 4 – 7 ]. Rather, strategies should be tailored to each unique setting [ 8 ]. An in-depth description of the experience of the patient involvement program in a particular context, including the nature of the program and the challenges overcome, provides others with ideas and examples they can modify to suit their own needs [ 1 , 3 , 9 ]. These other groups can read about the experience of others and possibly avoid some pitfalls while harnessing the strengths of the program. This is not intended to restrict creativity nor to provide a prescriptive roadmap, but rather to provide an experiential account of the nature of engagement in a particular setting and offer suggestions for ways in which engagement can take place.
In recent years, there has also been growing interest in efforts to involve patients in doctoral studies [ 5 , 10 – 17 ]. This presents a unique opportunity to instil recognition of the importance of patient involvement early in the career of researchers and equip them with the skills and experience to facilitate meaningful involvement. It simultaneously offers patients an opportunity to partner with researchers in this formative stage of their career as they develop their research interests and approach to research.
Aims and rationale
The aim of this perspective piece was for the first two members (DG and MGH) of a Research Buddy partnership (described below in the ‘Context’ section) to critically reflect on their experience of this involvement activity throughout the course of a MD–PhD program.
Through this reflective process, lessons learned through experience were consolidated such that they can be of benefit to others seeking to learn from this experiential insight as they establish or improve their own patient involvement programs [ 1 , 3 , 18 – 20 ]. We also provided a detailed description of the context in which this Research Buddy partnership took place, to provide readers with a more complete understanding of the way in which this context influenced the partnership such that readers can consider the nature of their own unique situation and how this might impact upon patient involvement efforts [ 21 ].
Overview of structure of this perspective piece
Definitions.
Theoretical Underpinnings.
- Method of Patient Involvement throughout MD–PhD program
Method of reflection in this perspective piece
Findings—Lessons Learned.
Definitions
The body of literature in the patient involvement space is fraught with complex and nuanced terminology [ 22 ]. Therefore, the purpose of this section is to clarify the terminology and definitions used. Influential stakeholder involvement literature, as well as recent publications, were reviewed to select widely-used and functionally relevant terminology for this perspective piece [ 1 , 22 – 33 ]. The other critical aspect of the rationale for selecting these terms and definitions was that they were agreed upon with the patient partner, MGH, as they accurately described the nature of her involvement.
Terms used in this paper include the following: patient, involvement, partnership, and co-design.
The term ‘patient’ was used because this perspective piece concerns involvement with patients as distinct from members of the public. While this term may invoke the image of an individual seeking care, in the context of involvement it also refers to individuals with experience of a health condition and its management [ 28 ], which in this case is total joint replacement surgery.
Building upon this is the definition of ‘involvement’, which is commonly accepted to refer to research done ‘with’ or ‘by’ patients rather than ‘for’, ‘to’, or ‘about’ them [ 30 ]. One specific type of involvement is the ‘patient partnership’, in which patients are embedded in various research activities at different levels and in multiple stages of the research process [ 1 ]. A particularly powerful example of a patient partnership is the Research Buddy program, which has been described previously as a program in which a doctoral student works closely with a patient throughout their project [ 13 ] and which was also described and reflected upon later in this perspective piece in the section ‘Method of Patient Involvement throughout MD–PhD program’.
Finally, the term ‘co-design’ was used in this piece to describe the active, voluntary process of researcher and patient partner working together on the design of a PhD project and its constituent studies, as this terminology is appropriate for the timeframe in which doctoral studies typically take place [ 22 , 32 – 35 ].
Theoretical underpinnings
Theory development was not an aim of this perspective piece. However, it is important to describe the theoretical underpinnings of the patient involvement program.
There are three main approaches to patient involvement in research: epistemological, consequentialist, and emancipatory or moral [ 33 , 36 , 37 ]. The epistemological approach recognises the fact that patients have lived experience of a health condition that can be of benefit to the research by broadening the perspective of researchers. The emancipatory approach recognises that patients have a right to be involved in publicly funded research that impacts upon them or the care they receive, and consequently researchers have a responsibility to involve patients. Finally, the consequentialist argument states that involvement can improve the design, conduct, and reporting of research.
Primarily, the emancipatory argument motivated the formation of the involvement program described in this perspective piece. Furthermore, patient involvement can lead to more ethically sound research [ 38 ], and this was recognised in the work reflected upon in this piece as MGH assisted DG in the ethics application process for the PhD research projects through document review. However, it was also recognised that the quality and relevance of research conducted at the research centre could be improved through involvement of a patient with lived experience of joint replacement surgery. Therefore, the consequentialist and epistemological arguments also underpinned the involvement program.
Recent literature has also advocated for a pragmatic approach to patient-oriented research by utilising patient involvement to ultimately improve health outcomes for affected individuals [ 39 ]. These authors recognised that achieving such impact is a complex task and, as such, mixed methods approaches to research are encouraged to harness the strength of both quantitative and qualitative research methodologies. This is particularly relevant to the current perspective piece, which describes patient involvement in a mixed methods PhD project on a topic of clinical relevance aimed to improve outcomes for patients.
Finally, the word ‘perspective’ was intentionally selected instead of the term ‘representative’ because it is unrealistic and unfair to ask a single patient with their own unique experience to generalise their experience to represent that of all patients with the same health condition. Rather, patient involvement was incorporated to broaden the perspective of the researchers while they co-designed research with the patient [ 36 , 40 ].
A detailed description of the context in which the patient involvement program took place facilitates comparison to one’s own context such that the potential applicability of a framework and experience can be considered in detail [ 6 , 7 ]. This is not intended to limit the generalisability of the findings reflected upon in this perspective piece. Rather, the specific context in which the patient involvement partnership and activities took place is described in detail such that readers can accurately compare this context to their own.
The patient involvement program described in this perspective piece took place in an Australian National Health and Medical Research Council Centre for Research Excellence for Total Joint Replacement, seeking to O ptimise P atient o U tcomes and S election for Total Joint Replacement (OPUS). OPUS is embedded in a hospital in Melbourne, Victoria, which is a tertiary referral centre for total joint replacement (TJR), providing care for people from a broad range of geographical regions, socioeconomic backgrounds, and cultures [ 41 ]. OPUS brings together clinician-researchers with backgrounds in orthopaedic surgery, nursing, and physiotherapy, as well as trialists, epidemiologists, health economists, statisticians and qualitative researchers. It comprises researchers at various stages of their careers, including graduate research students.
The patient involvement program at OPUS was developed and launched in 2020 and has been described previously [ 42 ]. The program involves a four-tiered framework of involvement (see Fig. 1 ). Table 3 of this prior publication also outlines the remuneration fee schedule according to which MGH was remunerated for her time. This prior publication used the term ‘consumer’, therefore, for consistency, this terminology is used below when describing the framework.

Proposed level of involvement for consumers and community members presents different opportunities of participation and the relevant benefits to researchers
This Figure was made available under the Creative Commons Attribution 4.0 ( http://creativecommons.org/licenses/by/4.0/ ).
The Consumer and Community Advisory Group (bottom of Fig. 1 ) oversees the operations of the CCI program. It is chaired by a consumer (MGH, co-author on this paper).
This perspective piece details the experience of a Research Buddy, Tier 3 as shown in Fig. 1 , and an MD–PhD candidate at OPUS. An MD–PhD candidate is a medical student who takes time out from their clinical studies to complete a PhD. The details of this Research Buddy program are described in the next section.
Method of patient involvement throughout MD–PhD program
The Research Buddy program reflected upon in this piece took the approach of broadening the perspective of the research team by including the voice a patient with lived experience of a health condition [ 43 ]. This is distinct from an approach which would seek to achieve representativeness from one patient partner on behalf of all patients with a shared lived experience, which would be unrealistic [ 31 , 44 ].
The focus of this piece is on the Research Buddy partnership comprising authors DG and MGH. In this section, DG and MGH reflected upon their expectations prior to the commencement of the Research Buddy program and how their actual experience played out.
DG was a final-year postgraduate medical student in 2019, carrying out a research project on risk factors for readmission following total knee replacement (TKR) surgery. The opportunity arose to enrol in a PhD to expand upon this work, which led to the deferment of DG’s final year of medical school in order to complete a PhD as part of an MD–PhD program [ 45 ]. Building upon the research into risk factors for readmission following TKR, the PhD centred around the development of a clinical risk prediction tool utilising machine learning. DG, along with his PhD supervisors (authors MD, SB, and PC) felt that the strong clinical focus of the MD–PhD program would position it well for close involvement with a Research Buddy. MGH had previously participated, as a subject, in a study conducted by MD and PC investigating the impact of a mindfulness program on recovery post-surgery for patients undergoing total hip replacement (THR) [ 46 ]. MGH reached out to MD regarding the findings of the mindfulness study, coincidentally while MD was establishing the patient involvement framework at OPUS and seeking volunteers for the Research Buddy role. MGH subsequently embraced the opportunity and volunteered to be DG’s Research Buddy approximately 6 months after the official commencement of the PhD. MGH decided to take part specifically in DG’s project because she wanted to learn more about research, had a keen interest in artificial intelligence and machine learning and its application to lower limb joint replacement surgery, and has had a lifelong interest and involvement in academia. This presented a tangible opportunity to make a substantial impact on the direction of the research and cement its clinical, stakeholder-focused approach, and MGH felt her interests and experience aligned most closely with DG’s project [ 47 ].
During this formative stage of the PhD project, MGH and DG worked together to define the scope and structure of the Research Buddy program. There was a shared expectation of open-ended discussions and regular contact with updates on the research. Neither DG nor MGH had been involved in such a patient involvement partnership before, therefore they did not have rigid expectations about the manner in which the partnership would unfold. It was decided that regular monthly meetings would facilitate the development of rapport, ongoing discussion of ideas for the PhD project and opportunities for involvement its constituent studies, and the opportunity for regular updates on progress.
The first part of DG’s PhD project discussed in these meetings was the systematic review, which was in the manuscript drafting stage and benefited greatly from MGH’s input on the clarity of the language [ 48 , 49 ] and ensuring the pertinence and importance of the topic were clearly communicated. Patient involvement in systematic reviews has been reported extensively in the literature [ 32 , 50 – 52 ], and it was an opportunity for MGH to gain familiarity with the topic at the foundation of the PhD project. More broadly, the regular monthly meetings were critical in defining the overall scope and purpose of the PhD project. Specifically, during the early stages it was difficult to determine how to make the research more clinically relevant. MGH had a background in occupational therapy and medical anthropology and had previously been involved in qualitative interview projects in which she interviewed participants and coded transcripts. The idea of a qualitative study exploring the views of TKR patients on the use of artificial intelligence and machine learning for risk prediction in shared clinical decision-making was already being developed, and MGH felt this was a fitting opportunity to be involved given her personal journey as a patient and her prior academic and research experience. MGH was involved as a co-author on the qualitative study and made a substantial contribution by pilot-testing the interview guide, contributing to the reflexive thematic analysis, and preparing and editing the manuscript for publication.
Next, MGH reviewed all of the ethics approval application documents for the studies comprising DG’s PhD project. Not only did this improve the clarity of the documents in a similar way to MGH’s review of the systematic review manuscript, but it also led to the development of more ethically conscious research by incorporating a patient’s perspective into the application [ 38 ] and helped DG to clarify the way these studies related to one another and formed a cohesive program of research.
Beyond the specific elements in which MGH made a direct contribution, DG’s reflection upon the stakeholder involvement in the form of the Research Buddy program prompted a shift in focus from machine learning to enhance predictive performance of the TKR readmission prediction model, to stakeholder involvement and engagement in the whole process of developing clinical risk prediction models, including not only patients but also clinicians [ 29 , 53 ]. In light of this, thanks to MGH’s Research Buddy input, DG designed the PhD project with stakeholder involvement embedded throughout.
At the time of writing this perspective piece, the main studies of the PhD project had been completed and DG was in the final stages of thesis write-up following feedback provided by MGH on the first full draft. The overall focus of the PhD project is on co-design with stakeholders, inspired by the Research Buddy partnership with MGH and cemented by exploration of the literature [ 24 , 54 – 56 ].
The PhD project centres around the development of a machine learning model to predict 30-day readmission [ 57 ] in people undergoing TKA surgery years [ 58 , 59 ] for osteoarthritis (OA) [ 60 ], co-designed with clinicians and patients [ 61 ]. The project is divided into four components, each comprising at least one main study. While MGH’s involvement in the project overall has been outlined above, what follows is a brief description of each part of the PhD project as well as the level [ 29 ] and phase [ 62 ] at which MGH was involved. While a high level of involvement was aimed at from the early phases of each part of the PhD project, it is recognised that higher levels of involvement are not automatically more desirable [ 27 ] and, since some aspects of the project were already underway when MGH and DG commenced the Research Buddy program, flexibility and responsiveness to the realities of the circumstances were of utmost importance in deciding with MGH at what level she wanted to be involved.
- i. Level = Involve
- ii. Phase = Design and preparation, dissemination
- ii. Phase = Dissemination
- i. Level = Collaborate
- ii. Phase = Design and preparation, conduct and implementation, and dissemination
Rather than speaking for the patient, it is important for the patient to speak for themselves. Prior reflective work on patient involvement in doctoral research adopted this approach to great effect [ 5 , 14 ]. This piece was therefore co-written by MGH and DG, the two members of the inaugural Research Buddy partnership at OPUS.
Pertinent elements of the GRIPP2 (Guidance for Reporting Involvement of Patients and the Public) checklist [ 70 ] were adhered to while writing this piece.
The experiential insight gained by DG and MGH through their involvement in the Research Buddy program was consolidated in regular meetings throughout DG’s PhD project. Additionally, DG and MGH conducted an informal interview using questions informed by prior literature in which patient advocates from various patient involvement programs in the UK were interviewed [ 9 ]. This interview was audio recorded and transcribed. The transcript was then analysed using reflective thematic analysis [ 71 ] in which themes were generated inductively by DG and MGH based on MGH’s responses to the questions in the interview, informed by their shared experience in the Research Buddy partnership. These themes were then synthesised and refined into lessons, inspired by prior patient involvement literature which has used thematic analysis in a similar way to generate lessons and recommendations [ 1 , 3 , 55 , 62 , 72 , 73 ].
A literature search was then carried out to identify literature reviews which contained lessons from, or recommendations for, patient involvement [ 1 , 5 , 13 , 15 , 28 , 35 , 62 , 74 , 75 ]. These reviews were used to corroborate the findings from our own inductive analysis and reflection.
DG and MGH derived these lessons by reflecting primarily on their experience in the Research Buddy program. However, MGH was embedded in various other patient involvement activities at the research centre (OPUS), therefore some of these lessons will be broadly applicable beyond Research Buddy partnerships. This will depend on the patient’s desired level of involvement, and it is advised that readers are flexible in their interpretation and implementation of these findings.
Findings: lessons learned
Lesson (1) learn from experience.
Don’t go in completely blind. Learn from the experience of others as much as possible. Bring in experienced patient partners, and researchers experienced in patient involvement, who can offer advice on promising avenues to explore as well as flagging those which are less likely to be fruitful. For example, as part of the Research Buddy program reflected upon in this perspective piece, Carol Vleeskens was consulted to conduct a training session attended by both MGH and DG. Carol was a co-author on the publication outline OPUS’ patient involvement framework [ 42 ] and has extensive experience in patient involvement [ 76 ]. MGH was then involved in preparing an orientation day for prospective patient partners at OPUS, which she also attended.
Another powerful resource is published work on the experience and appraisal of other researcher-patient partnerships sharing their experience [ 5 ].
This lesson is pertinent to consider both in preparation for partnering with patients in order to maximise the likelihood of success prior to launching involvement programs [ 8 , 13 , 48 , 77 ], as well as for ongoing appraisal and improvement of existing programs based on patient partner feedback [ 1 ].
Lesson (2) tailor the program
A one-size-fits-all approach does not work. Patient involvement programs should be bespoke. They should be tailored to the unique context of the research centre engaging the patients [ 5 , 78 ]. Learning from the work of others is critical for generating ideas about how one’s own program could take shape [ 78 ], but it is equally important to modify these programs so they are fit for purpose.
Tailoring the patient involvement program has been emphasised in prior literature as a critical component of fruitful involvement through the translation of general principles into context-specific systems and actions [ 4 , 5 , 8 , 15 , 35 , 74 ].
Lesson (3) get involved early
If possible, involve patients in discussions where ideas are generated for research projects. Allow ample time to include patients on ethics applications and governance approvals to ensure they can make a meaningful contribution to the research.
Integrating patient involvement in earlier stages of research helps to facilitate truly patient-led research. Based on the experience outlined in this perspective piece, there are two main ways in which this can be done. First, patient involvement can be embedded in the ongoing work of established research centres as well as the commencement of work at new research centres in order to identify research priorities and steer the direction of research activities [ 4 , 7 ]. Second, in the style of Research Buddy partnerships, patients can be partnered with PhD students in order to co-design the program of research in a meaningful way by embedding engagement and co-design at the beginning of the student’s project [ 19 ]. It is not difficult to imagine a pipeline in which researchers collaborate with patients to generate ideas and then these ideas are translated into PhD research projects with patients.
Having patients engaged at these different stages of idea-generation and research project design could have the added benefit of anticipating and addressing challenges likely to arise in complex research projects such that solutions can be suggested proactively [ 29 ].
Early involvement has been demonstrated or recommended numerous times in a diverse range of contexts and for different levels of involvement [ 5 , 7 , 15 , 28 , 35 , 43 , 75 ].
Lesson (4) embrace uniqueness
Patients bring a wealth of unique experience. They should be treated as individuals with their own unique characteristics, experience, and insight [ 79 ]. Embrace their unique skills and experience. Throughout regular meetings and correspondence, various aspects of the patient’s experience may enable them to bring a unique perspective to the research which researchers could not have anticipated.
Embracing uniqueness of patient partners has been found to encourage more inclusive stakeholder involvement by demonstrating a flexibility in the researchers and responsiveness to the individual needs, experiences, and preferences of people seeking to be involved [ 1 , 5 , 35 , 48 , 77 ].
Lesson (5) meet regularly
Meet regularly, right from the start, even in the absence of a clear idea of what to do in terms of exactly how the patient will be involved. The level and nature of involvement can be collaboratively defined through these regular meetings.
Be open to a variety of forms the patient’s involvement could take and be willing to engage in some trial and error. Some ideas will not pan out, and that is just part of the process. Be willing to persevere and adapt. For example, during the qualitative study conducted as part of DG’s PhD project, MGH attempted cross-coding of the qualitative interview transcripts using the coding framework being developed by DG and author SB. However, MGH found that her style of coding based her prior experience was not functioning as well as it had for previous projects. DG and MGH attempted different styles and continued to discuss their findings in their regular monthly meetings, however ultimately this cross-coding exercise was abandoned as it was becoming quite convoluted and MGH felt more comfortable discussing the transcripts and contributing to the discussions around the development of themes through reflexive thematic analysis.
Both DG and MGH had an openness that resulted in these meetings being fruitful beyond the pragmatic aspects of setting the research agenda and tracking progress; they were critical in building rapport. Even when there were no specific research updates, the regular meeting time was maintained and utilised for an opportunity to discuss topics related to the research.
Regular meetings are a critical component of impactful patient involvement programs reported in the literature [ 15 , 35 , 62 , 75 ] as they facilitates ongoing communication, continuous feedback, and strong rapport-building as expanded upon in Lesson 6.
Lesson (6) build rapport
Establishing trust and rapport is critical to the success of the program. Put in the time. Create an environment in which frank and open discussions are encouraged. Follow through on action items from meetings and email discussions. Respect the patient’s autonomy while supporting them in their role. Listen to the patient and take their feedback into account. Demonstrate to the patient how their input is influencing the research. This designated time to meet and talk openly was critical for building rapport, which is arguably the most important component of patient involvement to increase the likelihood of success [ 37 , 80 ]. The centrality of rapport to the overall success of patient involvement in research is reflected in the large volume of diverse literature recognising its importance [ 1 , 5 , 17 , 28 , 30 , 35 , 62 , 73 , 75 , 81 – 84 ].
Lesson (7) ensure mutual benefit
Patients are more engaged when there is something in it for them. Ensure the interaction is mutually beneficial. For example, patients might learn more about their condition, or the condition of those they care for, through the patient involvement program [ 85 ].
This pragmatic aspect of involvement has been recognised in the literature detailing meaningful and impactful involvement activities that recognise the importance of ensuring the involvement benefits the research, the researchers, and the patient partners themselves [ 1 , 19 , 35 , 62 , 75 , 83 ].
The nature of a successful patient involvement program, in which there is rapport, mutual respect, and open discussion, is associated with a certain degree of passive learning simply by the two parties being in contact with one another [ 85 ]. Without any specific educational component to the program, regular and open communication regarding a common focus in the context of a research centre or program can have the effect of educating the patient about research [ 53 ], and the researcher about the patient’s lived experience of their condition [ 72 , 86 ]. Patients may also learn more about their own condition, its management, and current research being done to further understanding about it [ 85 ].
Lesson (8) broad involvement
It is beneficial to have the patient embedded in the broader patient involvement activities at the research centre, not just the Research Buddy (or equivalent) program. This may enable the patient to participate in broad discussions about current research and setting the direction of future research.
This is important for contextualising the Research Buddy’s work and enhancing their understanding of the way in which it fits into the broader body of work being conducted at the research centre in which the program is taking place. This is not to say patient partners should be stretched beyond their limit, but rather that they are not forced to limit themselves to one project and instead are encouraged to broaden their perspective through opportunities to be involved in varying capacities in other research projects and activities depending on their interests and capacity [ 28 , 35 , 82 ].
Lesson (9) regularly reflect and review
It is not enough to have good intentions. Having a framework is also critical, but the patient’s role may evolve over time and ethical considerations related to their involvement also evolve. It is easy for the patient’s role to devolve into tokenism despite the best intentions [ 22 ]. Regular meetings incorporating feedback from patient partners on their involvement in the research are critical to ensuring the patient’s role as partner in the research is respected and maintained. This requires transparency on the part of the researcher regarding how the patient’s involvement is influencing the research. Researchers must also be open to feedback from patients that may indicate they are not satisfied with their involvement and adjustments to their involvement may be required.
One potential way in which the patient partnership can break down is for researchers to treat them less like partners in the research process and more like participants in quasi-qualitative research by simply calling upon patients to complete surveys or answer questions as if in a qualitative interview. This is problematic because patient involvement in research is distinct from qualitative research and must be treated as such, but vigilance and effort is required to maintain the boundaries between the two [ 22 ].
Beyond this specific problem, regular reflection and review are broadly applicable to every aspect of patient involvement to ensure the patient’s involvement is meeting expectations as these expectations evolve throughout the research project alongside changes in the patient’s capacity and interests.
Regular review is crucial to ensure ethical principles are being adhered to at all times as the patient’s role evolves. Circumstances change, both intentionally and unintentionally, so it is critical to have discussions specifically dedicated to reviewing the patient’s role and ensuring they are still meaningfully involved and ethical principles are adhered to [ 87 ]. There may be occasions on which the patient’s preconceived ideas need to be discussed and worked through.
Building upon the other lessons outlined in this perspective piece, rapport based on mutual respect, coupled with regular meetings, provide the structure and nurture the culture necessary to facilitate continuous feedback as well as regular designated touch points at which reflection and review can take place [ 5 , 13 , 28 , 35 , 62 , 74 , 75 , 83 , 87 ].
Summary of findings
In this perspective piece, a patient partner with lived experience of total joint replacement surgery (MGH) and a MD–PhD candidate (DG) reflected on their experience in a Research Buddy program throughout a PhD project. Their aim was to provide experiential insight such that other researchers and patient partners can learn from their experience as they develop and refine their own patient involvement programs. Inductive thematic analysis was applied to the transcript of an informal interview carried out between DG and MGH, informed by reflections from regular meetings throughout their Research Buddy partnership. Through this process, nine lessons were synthesised from their experience: Learn from experience, Tailor the program, Get involved early, Embrace uniqueness, Meet regularly, Build rapport, Ensure mutual benefit, Broad involvement, and Regularly reflect and review. These lessons were corroborated by published literature, and rapport was found to be the most important factor in successful patient involvement. Each of the remaining lessons either stem from and/or contribute to the development of rapport.
Contribution to the literature
To the best of the authors’ knowledge, this is the first perspective piece on patient partnership in a MD–PhD program. This builds upon prior work highlighting the positive impact of partnering medical students with patients to further their clinical education and broaden their perspective, while empowering patients through the opportunity to learn more about their own health condition and health service experience while sharing their experiential insight with future clinicians in a formative stage of their career [ 88 , 89 ]. It builds upon this by demonstrating how MD–PhD students, being situated at the interface between the research and clinical domains, can partner with patients in a fruitful way that has a meaningful impact upon their clinical development while also influencing the design, conduct, and reporting of the research. This was particularly noteworthy during DG’s PhD journey, most of which was undertaken during lockdowns imposed in light of the COVID-19 pandemic. Regular contact with MGH was, for long periods of time, DG’s only patient interaction and therefore was crucial to maintaining patient contact [ 88 ] from a clinical perspective as well as working with MGH to develop solutions to convert various research activities to be entirely online [ 15 , 16 ].
Through regular meetings based on mutual trust and respect, largely comprising open discussion especially when there were no major research progress updates, building rapport was prioritised from the outset of the Research Buddy program. Since MGH and DG had the freedom to define the scope and nature of the program, they were able to emphasise the importance of this reciprocal relationship both through conversation as well as through document review, providing comments alongside DG’s PhD supervisors, which enabled MGH to provide a critical perspective on DG’s work. With strong rapport as the foundation and critical feedback encouraged throughout the research journey, MGH’s role as ‘critical friend’ was characterised and strengthened [ 10 , 17 ].
This perspective piece also builds upon exemplary prior work reporting a Research Buddy program [ 13 ] by being co-authored with the Research Buddy. This perspective piece conveyed lessons learned through a program which drew on elements of embedded consultation [ 5 , 13 ] and demonstrated flexibility in the level of involvement in various aspects of the PhD project in response to changes in MGH’s interests and capacity throughout the partnership [ 16 ].
Furthermore, while the PhD project reflected upon in this perspective piece did not strictly pertain to a digital health innovation nor to a full translational research innovation, a predictive model was developed which was intended for use in the clinical setting for shared clinical decision-making in orthopaedics. Therefore, MGH’s involvement throughout the project was critical for in laying a solid foundation for the development of a clinically relevant digital health innovation [ 90 , 91 ] primed for translation into the clinical setting [ 27 ] in orthopaedics, which is a clinical discipline in which patient involvement is gaining recognition and appreciation [ 26 ].
Implications of findings
The most impactful implication of the findings of this paper is that the description of the context in which the researcher-patient partnership took place, coupled with the co-authored lessons learned and experiential insight [ 3 ] offered through reflection by both members of the Research Buddy partnership, can be utilised by others [ 92 , 93 ]. Learning through experience and reporting these lessons along with a detailed description of the context in which they were learned [ 6 , 18 ] is an impactful outcome of its own [ 92 ]. This enables readers to inform their own involvement efforts, both when establishing involvement programs and refining them.
Through involvement, patients like MGH learn more about their health condition as well as the research that goes into the developments in management approaches and the way in which patients experience the healthcare system [ 85 ].
It is hoped that these findings can be of assistance to ongoing [ 11 , 12 ] and future patient involvement efforts to bring researchers and patients onto the same page from the outset of research projects [ 49 ].
Future directions
Evaluating impact.
The question of how best to evaluate impact of patient involvement is longstanding and complicated [ 48 , 77 ]. There have been many attempts over the years to answer this question [ 11 , 12 , 23 , 73 , 78 , 79 , 94 – 99 ], but it appears there is no single best approach to measuring the effectiveness, success, or impact of involvement [ 100 ], and perhaps such a measure will never exist. However, ongoing efforts to develop and validate consistent patient involvement evaluation measures are encouraged to facilitate the comparison of different involvement strategies and gain some indication of which might be the most promising in a given context, when resources are limited and ought to be allocated to the most promising strategy [ 101 ].
Exploring patient involvement in decision-making non-research patient involvement and empowerment
The research project reflected upon in this perspective piece centred around the development of a risk prediction tool intended to be used in the process of shared clinical decision-making. This perspective piece explored patient involvement in clinically-oriented research being carried out by a MD–PhD candidate, which positions it well for ongoing work exploring the role patients play in the shared clinical decision-making process [ 102 – 104 ], particularly as it pertains to the use of decision aids including predictive tools.
Further reading
Further reading is recommended for those seeking to expand their knowledge of different types of patient involvement in varied contexts in order to better understand how to initiate, or improve, their own patient involvement programs [ 32 , 40 , 105 – 148 ].
Strengths and limitations
Strengths of this perspective piece include the consistent and frequent contact between DG and MGH throughout the course of the PhD program, which facilitated widespread and deep involvement in various aspects of the research project and its constituent studies. This also facilitated continuous feedback and reflection through open discussion, which laid the foundation for the writing of this piece.
One potential limitation is that MGH may be regarded as a ‘professional’ patient partner, given her academic background. This may raise concerns about whether her perspective as a patient is diminished by her familiarity with academia and the research process. This was certainly an important consideration, especially considering MGH’s involvement spanned over 3 years and involved close and regular contact with DG. However, it was not a concern because regardless of how familiar MGH becomes with the research process, she will never lose her perspective as a patient who has lived experience of total joint replacement surgery [ 149 ].
In this perspective piece, a patient and a medical student completing a PhD reflected upon their experience co-designing a Research Buddy partnership within a patient involvement program. To the authors’ knowledge, this is the first time such a partnership has been written about within the context of a MD–PhD program, which is uniquely placed at the interface between the research and clinical domains. A series of nine lessons was identified and presented to inform readers seeking to develop or enhance their own patient involvement programs: learn from experience, tailor the program, get involved early, embrace uniqueness, meet regularly, build rapport, ensure mutual benefit, broad involvement, regularly reflect and review. Researcher-patient rapport is foundational to all other aspects of the patient’s involvement.
Acknowledgements
We acknowledge the work of Dr Tilini Gunatillake and Dr Michelle Lam in establishing the OPUS CCI program. We also acknowledge the work of Dr Elizabeth Nelson, Claire Weeden and Lauren Patten in leading, administering, maintaining, and expanding the program. MMD is supported by a University of Melbourne Dame Kate Campbell Fellowship. PFC holds a National Health & Medical Research Council Practitioner Fellowship (GNT1154203)
Abbreviations
| PhD | Doctor of philosophy |
| MD | Doctor of medicine |
| GRIPP2 | Guidance for reporting Involvement of Patients and the Public |
| OPUS | OPtimise Patient oUtcomes and Selection for Total Joint Replacement |
| TKR | Total knee replacement |
| THR | Total hip replacement |
Author contributions
DG and MGH conceptualised the manuscript and wrote the initial draft, with PFC, MMD, and SB providing intellectual content. All authors contributed to discussions in which the recommendations were synthesised and refined. All authors contributed to revising the manuscript prior to submission, and have all reviewed and approved the final manuscript. All authors agree to be accountable for all aspects of the manuscript and will work together to ensure questions relating to the accuracy and integrity of any part of it are appropriately investigated and resolved. All authors read and approved the final manuscript.
No funding was received directly for this study. DG, MGH, and SB receive no funding. PFC had the following funding sources to declare: Royalties from Johnson and Johnson, Consultancy with Johnson & Johnson, Consultancy with Stryker Corportation (paid personally); Australian National Health & Medical Research Council Practitioner Fellowship (paid to institution), HCF Foundation, BUPA Foundation, St. Vincents Health Australia, Australian Research Council, (Grant support provided to institution for research unrelated to the current manuscript); Axcelda cartilage regeneration project, Patent applied for device, composition of matter and process (institution and personally). MMD had the following funding sources to declare: National Health and Medical Research Council, HCF Foundation, BUPA Foundation, St. Vincents Health Australia, Australian Research Council, (Grant support provided to my institution for research unrelated to the current manuscript).
Availability of data and materials
Declarations.
DG, MGH, and SB have no competing interests. PFC has the following to declare: Chair, Research Committee, Australian Orthopaedic Association (now completed term); Emeritus Board Member Musculoskeletal Australia. MMD has the following to declare: Chair, Australian Orthopaedic Association Research Foundation, Research Advisory Committee.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Research Buddy
- Writing Assistant
Pricing Model
Ai tool description.
Research Buddy is an innovative AI-powered tool designed to generate comprehensive Academic Literature Reviews in minutes.
With Research Buddy, users can quickly and easily type in a subject or research question, and generate a comprehensive literature review with Harvard referencing in minutes. The platform is ideal for those looking to stay up to date on a subject or issue before a meeting or class or generate a literature review for a research paper or thesis.
Research Buddy outputs to Word or PDF format, providing convenient export options for easy sharing and editing. The one-page executive summary is ideal for getting up-to-date on any subject or issue quickly before a meeting or class.
- Harvard referencing
- Comprehensive literature reviews
- One-page executive summary
- Outputs to Word or PDF format
Other related tools
Facial recognition and reverse image search for privacy and security protection.
Perplexity AI
AI-driven answer engine for complex questions.
Metaphor Systems
Search the internet creatively with large language models using Metaphor.
Selected AI Tools Direct to Your Inbox.
Join 100,000+ other Executives, Marketers, Developers & Founders
Spotlight: Nailedit (LLM Comparison) .
- My Saved AIs
Research Buddy
ResearchBuddy is an AI tool that automates the process of literature reviews. The tool helps researchers streamline their literature review process by presenting the most relevant information in a concise and easily digestible format.
Its intelligent algorithms help identify the most pertinent information in a given research field, saving hours of time and effort. Through its user-friendly interface, ResearchBuddy enables researchers to quickly and efficiently analyze large amounts of information, allowing them to focus on more critical aspects of their research.
The tool is designed to work with a wide range of research topics and is particularly useful in the fields of Science, Humanities and Social Sciences.
Its fully automated approach eliminates the need for manual intervention, reducing the possibility of errors or biases common in traditional literature reviews.
The tool can be accessed from anywhere, making it convenient for researchers to conduct virtual collaborations from different locations.ResearchBuddy helps researchers stay up-to-date with the latest research trends and findings.
The tool is backed by a team of experts who continuously update its algorithms and databases to ensure that the information presented is accurate and relevant.
Overall, ResearchBuddy provides an efficient and reliable method for conducting literature reviews and offers significant benefits to researchers in terms of saving time, effort and resources.
Community ratings
How would you rate research buddy.
Help other people by letting them know if this AI was useful.
Feature requests
54 alternatives to Research Buddy for Academic research
Most impacted jobs

Pros and Cons
If you liked research buddy, featured matches.
Other matches
People also searched
- Builder dashboard
Subscribe to our exclusive newsletter, coming out 3 times per week with the latest AI tools. Join over 470,000 readers.
To prevent spam, some actions require being signed in. It's free and only takes a few seconds.

Research Buddy
What is research buddy.
Research Buddy is an innovative AI-powered research assistant designed to help users efficiently gather information, analyze data, and generate insights. This powerful tool leverages advanced natural language processing and machine learning algorithms to streamline the research process and provide users with a comprehensive understanding of their topics of interest.
Top Features:
- Intelligent Search: Research Buddy's search functionality goes beyond traditional keyword matching, using semantic analysis to deliver highly relevant and contextual results.
- Summarization and Synthesis: The tool can quickly summarize large amounts of information, identifying key points and synthesizing insights to provide users with a concise understanding of their research topic.
- Collaboration and Sharing: Research Buddy allows users to easily share their findings and collaborate with others, facilitating seamless teamwork and knowledge sharing.
Pros and Cons
- Time-saving: Research Buddy significantly reduces the time and effort required to conduct research, allowing users to focus on analysis and decision-making.
- Comprehensive Results: The tool's advanced search capabilities and ability to synthesize information from multiple sources provide users with a well-rounded understanding of their research topic.
- Ease of Use: Research Buddy has a user-friendly interface and intuitive features, making it accessible to users of all skill levels.
- Potential Bias: Like any AI system, Research Buddy may be subject to biases in its training data or algorithms, which could influence the accuracy of its results.
- Dependence on Internet Connectivity: The tool requires a stable internet connection to function, which could be a limitation for users in areas with unreliable connectivity.
- Limited Customization: While Research Buddy offers a range of features, some users may desire more customization options to tailor the tool to their specific research needs.
- Academic Research: Students and researchers can use Research Buddy to quickly gather information, synthesize findings, and generate insights for their academic projects and papers.
- Business Intelligence: Professionals in various industries can leverage Research Buddy to gather market data, analyze trends, and make informed business decisions.
- Personal Knowledge Management: Individuals can use the tool to organize and synthesize information on topics they are passionate about, expanding their knowledge and understanding.
Who Can Use Research Buddy?
- Students: Research Buddy can greatly benefit students at all levels, from high school to graduate school, by streamlining their research process and helping them generate high-quality insights.
- Researchers: Academics and researchers in various fields can use the tool to efficiently gather information, collaborate with colleagues, and produce well-researched papers and reports.
- Professionals: Individuals working in fields such as business, marketing, consulting, and journalism can leverage Research Buddy to gather market intelligence, analyze trends, and make informed decisions.
- Free Trial: Research Buddy offers a free trial period, allowing users to explore the tool's features and functionality before committing to a paid plan.
- Pricing Plan: The tool offers various pricing plans based on the user's needs, with options for individuals, teams, and enterprises. Prices are competitive and offer good value for the features and functionality provided.
Our Review Rating Score:
- Functionality and Features: 4.5/5
- User Experience (UX): 4.2/5
- Performance and Reliability: 4.3/5
- Scalability and Integration: 4.1/5
- Security and Privacy: 4.4/5
- Cost-Effectiveness and Pricing Structure: 4.2/5
- Customer Support and Community: 4.0/5
- Innovation and Future Proofing: 4.6/5
- Data Management and Portability: 4.1/5
- Customization and Flexibility: 3.9/5
- Overall Rating: 4.3/5
Final Verdict:
Research Buddy is a powerful and innovative AI-powered research assistant that can significantly streamline the research process for users across various domains. Its intelligent search capabilities, summarization and synthesis features, and collaboration tools make it an excellent choice for anyone looking to efficiently gather information, analyze data, and generate insights. While it may have some limitations, such as potential biases and limited customization options, Research Buddy's overall performance, user-friendliness, and cost-effectiveness make it a valuable tool for students, researchers, and professionals alike.
1) What data sources does Research Buddy use?
Research Buddy accesses a wide range of online data sources, including academic databases, news articles, industry reports, and social media platforms. The tool uses advanced algorithms to gather and synthesize information from these sources, providing users with comprehensive and up-to-date research results.
2) Can Research Buddy be used for team collaboration?
Yes, Research Buddy offers collaboration features that allow users to share their findings, comment on each other's work, and work together on research projects. The tool's sharing capabilities make it easy for teams to stay organized and aligned throughout the research process.
3) How accurate are Research Buddy's results?
While Research Buddy's results are generally accurate and reliable, it's important to note that the tool's accuracy depends on the quality and accuracy of the data sources it uses. Users should always critically evaluate the information provided by Research Buddy and cross-reference it with other reliable sources before relying on it for important decisions.
4) Can Research Buddy be used for non-English languages?
At the moment, Research Buddy primarily supports English language research. However, the tool's developers are working on expanding its language capabilities to support other major languages in the near future.
5) Is Research Buddy secure and private?
Research Buddy takes user privacy and data security very seriously. The tool uses advanced encryption and security protocols to protect user data, and has a strict privacy policy that ensures user information is never shared or sold to third parties. However, users should always exercise caution when sharing sensitive information online.
Become the AI Expert of Your Office
Join 200,000 professionals adopting AI tools for work
- Bookmark 100s of AI tools that interest you
- Get personalized AI tool recommendations every week
- Free weekly newsletter with practical news, trending tools, tutorials and more
Research Buddy alternatives
Thumbmachine
Crypto Co-Pilot
Join 40,000+ subscribers including Amazon, Apple, Google, and Microsoft employees reading our free newsletter.
Remember Me
Please enter your email address or username. You will receive a link to create a new password via email.
- I agree with the privacy policy
- Best AI Tools

Research Buddy
- Matthew Edwards
- Read reviews
- Create collection
- --> {{ item.title }}
- {{ item.label }}
About Research Buddy
Introducing ResearchBuddy, the ultimate AI-powered solution for hassle-free literature reviews. Designed to streamline the research process, our user-friendly platform grants researchers seamless access to a vast array of articles and papers. With ResearchBuddy, conducting literature reviews has never been easier.
Our cutting-edge technology enables ResearchBuddy to swiftly search and categorize an extensive range of scholarly content. By utilizing advanced algorithms, our tool effectively analyzes and extracts crucial information from articles, empowering researchers to swiftly pinpoint relevant sources. Furthermore, ResearchBuddy offers customization options through filters and tags, ensuring effortless organization and tracking of research findings.
Whether you are an aspiring student, a distinguished professor, or a seasoned industry professional, ResearchBuddy is an invaluable companion for comprehensive literature reviews. Our tool keeps you updated with the latest research in your field, ensuring you never overlook any seminal studies. Invest in ResearchBuddy today and revolutionize the way you conduct literature reviews.
Research Buddy image gallery
Research Buddy core features
❤ Automate literature review ❤ Search and categorize scholarly content ❤ Track research findings
Research Buddy use cases
#️⃣Keep yourself informed about the most recent research. #️⃣Reduce the amount of time and energy spent on literature reviews. #️⃣Easily access and evaluate articles that are relevant to your field. #️⃣Streamline the process of conducting literature reviews with automation. #️⃣Efficiently organize and monitor your research findings.
Authentic Links 🌍
- Official Website
Research Buddy pricing 👀
Dubformer.ai
- Audio Generation

- Advertising
- Customer Support
AI Job Description Generator
- Human Resources

- Image Generation
- Developer tools
GummySearch
Storydoc Pitch Deck
- Startup tools
There are no results matching your search.
Similar AI tools like Research Buddy

Mirrorthink
Lumina Chat
All Search AI
AlphaInquire
History Timelines
ContextClue
AcademicGPT
Color Pop AI
Paperade Startup Idea Generator
Branchminds
Connected Papers

PaperClipapp

AI Text Classifier
Research Buddy Reviews
- {{ order.label }}
No reviews available
Excellent 33%
Very good 67%
- Promote your tool
- Privacy Policy
- Terms and Conditions
- Refund Policy
Research Articles
Enhancing Student-Student Online Interaction: Exploring the Study Buddy Peer Review Activity
- Colin Madland and
- Griff Richards
…more information
Colin Madland Thompson Rivers University, Canada [email protected]
Griff Richards Athabasca University, Canada [email protected]

Online publication: Dec. 6, 2019
An article of the journal International Review of Research in Open and Distributed Learning
Volume 17, Number 3, April 2016 , p. 157–175
Copyright (c) Colin Madland, Griff Richards, 2016

The study buddy is a learning strategy employed in a graduate distance course to promote informal peer reviewing of assignments before submission. This strategy promotes student-student interaction and helps break the social isolation of distance learning. Given the concern by Arum and Roksa (2011) that student-student interaction may be distracting from instead of contributing to academic achievement it was felt important to examine the way peer interaction can contribute to learning in a well-structured collaborative learning activity. This mixed-methods study (n=31) examined both quantitative and qualitative aspects of student perceptions of the study buddy activity. While quantitative findings regarding depth of processing were inconclusive due to the small and homogeneous sample, qualitative analysis showed very high levels of learner support for the activity as well as evidence that the activity encouraged learners to approach their learning with greater depth. 88% of study buddies said they found the activity well worth their time, and would recommend it for other graduate courses. It is thought with greater scaffolding, the quality of buddy feedback might be improved. The few who did not appreciate the activity felt let down by a lack of buddy commitment to the process.
- interaction,
- cooperative learning,
- critical thinking,
- study buddy,
- approaches to learning,
- learning design
Download the article in PDF to read it. Download
Citation Tools
Cite this article, export the record for this article.
RIS EndNote, Papers, Reference Manager, RefWorks, Zotero
ENW EndNote (version X9.1 and above), Zotero
BIB BibTeX, JabRef, Mendeley, Zotero

Research Buddy
What is Research Buddy?
Research Buddy is an AI tool that automates the process of literature reviews, helping researchers streamline their literature review process by identifying the most pertinent information in a given research field. It uses intelligent algorithms to present the most relevant information, saving hours of time and effort. The tool is designed to work with a wide range of research topics and is particularly useful in the fields of Science, Humanities, and Social Sciences. Research Buddy is accessible from anywhere, enabling researchers to collaborate virtually from different locations. The tool is backed by experts who continuously update its algorithms and databases to ensure accuracy and reliability.
⚡Top 5 Research Buddy Features:
- Automated Literature Reviews: Research Buddy uses AI technology to automate the process of literature reviews, helping researchers save time and effort.
- Intelligent Algorithms: The tool’s algorithms identify the most pertinent information in a given research field, ensuring accuracy and relevance.
- User-Friendly Interface: A simple and intuitive design, enabling users to easily navigate and analyze large amounts of information.
- Accessible Anywhere: The platform allows researchers to conduct virtual collaborations from different locations, increasing convenience and flexibility.
- Continuous Updates: Research Buddy’s team of experts regularly updates the algorithms and databases to maintain the quality and reliability of the information provided.
⚡Top 5 Research Buddy Use Cases:
- Academic Research: Research Buddy assists researchers in various fields by generating comprehensive literature reviews and providing valuable insights.
- Staying Updated: Users can stay informed about a subject or issue before a meeting or class by using ResearchBuddy’s one-page executive summary feature.
- Literature Review Generation: Research Buddy simplifies creating literature reviews for research papers or theses by automatically generating relevant information.
- Collaboration: The platform facilitates virtual collaboration among researchers, allowing them to share and discuss findings from different locations.
- Harvard Referencing: Get literature reviews in either Word or PDF formats, which can be easily shared and edited while maintaining proper citation styles.
View Research Buddy Alternatives:
CoolMindMaps
Collaborate on custom mind maps to organize ideas visually.
Stellaris AI
Advanced language models and analytics for pioneering solutions.
MonkeyLearn
contact for pricing
No-code text analytics platform for insights from feedback, surveys.
Generate insights and extract valuable information from data instantly.
Securely track, store documents in the cloud, cite sources easily.
Intelligent assistant for instant answers, knowledge gaps.
Simplify & distill lengthy documents to boost productivity.
Experience PDFs with light mode, login, bug reports, and more.
Organize digital life, capture ideas, manage tasks, and share with peers
Uncover customer problems, decide what to build, strategize.
Predictive algorithms for designing improved proteins.

Comprehensive resource hub for exploring AI advances.
© 2023 Powerusers, All Rights Reserved
Some of the links on this site may be affiliate links, and we may earn a commission if you purchase any of the products at no extra cost to you.
We use cookies to improve user experience. By using powerusers.ai you accept cookies
- Cambridge Dictionary +Plus
Meaning of buddy in English
Your browser doesn't support HTML5 audio
- friend We've been friends for years.
- buddy He's one of my dad's old war buddies.
- pal The heartthrob was spotted hanging with his Hollywood pals in L.A.
- mate UK He's out with his mates.
- acquaintance
- acquaintanceship
- acquainted with someone
- contact list
- contemporary
- fair-weather friend
- non-contemporary
- office spouse
- on further acquaintance
- with friends like you, who needs enemies? idiom
You can also find related words, phrases, and synonyms in the topics:
buddy | American Dictionary
Examples of buddy, translations of buddy.
Get a quick, free translation!

Word of the Day
knock someone down
to cause someone or something to fall to the ground by hitting him, her, or it

Fakes and forgeries (Things that are not what they seem to be)

Learn more with +Plus
- Recent and Recommended {{#preferredDictionaries}} {{name}} {{/preferredDictionaries}}
- Definitions Clear explanations of natural written and spoken English English Learner’s Dictionary Essential British English Essential American English
- Grammar and thesaurus Usage explanations of natural written and spoken English Grammar Thesaurus
- Pronunciation British and American pronunciations with audio English Pronunciation
- English–Chinese (Simplified) Chinese (Simplified)–English
- English–Chinese (Traditional) Chinese (Traditional)–English
- English–Dutch Dutch–English
- English–French French–English
- English–German German–English
- English–Indonesian Indonesian–English
- English–Italian Italian–English
- English–Japanese Japanese–English
- English–Norwegian Norwegian–English
- English–Polish Polish–English
- English–Portuguese Portuguese–English
- English–Spanish Spanish–English
- English–Swedish Swedish–English
- Dictionary +Plus Word Lists
- English Noun
- American Noun
- Translations
- All translations
To add buddy to a word list please sign up or log in.
Add buddy to one of your lists below, or create a new one.
{{message}}
Something went wrong.
There was a problem sending your report.
Calculate for all schools
Your chance of acceptance, your chancing factors, extracurriculars, what's a study buddy.
I've heard people mention having a 'study buddy' to help them with schoolwork. What exactly does that mean? Is this something I should look into?
A 'study buddy' is a term commonly used to describe a friend or classmate with whom you study regularly. The main idea behind having a study buddy is to enhance the learning experience by providing mutual support, sharing resources, and holding each other accountable. Study buddies can help you stay focused, motivated, and organized throughout the academic year.
There are several benefits to having a study buddy. Some of these include:
1. Improved understanding of the material: By explaining concepts to each other and working through problems together, you can gain a better grasp of the subject matter.
2. Keeping each other on track: Study buddies can help with time management by establishing study schedules and ensuring you stick to them.
3. Emotional support: A study buddy can offer encouragement during challenging times, like exam season or when tackling difficult assignments.
4. Learning new strategies: Study buddies often share effective study techniques, skills, or strategies that you may not have considered using before.
5. Networking: Forming a bond with a study buddy may lead to new connections and friendships both inside and outside the classroom.
You might consider finding a study buddy if you enjoy collaborative learning, find it hard to stay focused when studying alone, or just want to expand your academic support network. To maximize the benefits, try to find someone with a similar work ethic, learning style, and commitment to academics. That being said, every student is different, and what works for one person may not work for another. Ultimately, the decision to get a study buddy should be based on your personal preferences and learning style.
About CollegeVine’s Expert FAQ
CollegeVine’s Q&A seeks to offer informed perspectives on commonly asked admissions questions. Every answer is refined and validated by our team of admissions experts to ensure it resonates with trusted knowledge in the field.
Advertisement
Making Meaning Together: Buddy Reading in a First Grade Classroom
- Published: 26 September 2010
- Volume 38 , pages 289–297, ( 2010 )
Cite this article

- Tori K. Flint 1 , 2
2088 Accesses
Explore all metrics
This study uses a Vygotskian approach and a socio-cultural lens, as well as the Transactional Reading Theory to investigate how social interactions and literary transactions can combine through buddy reading to empower young readers and promote literacy in a first grade classroom. The research focuses on how literary transaction and social interaction work together to facilitate emergent and early readers in a ‘partner/buddy’ reading approach. The research question asked whether or not ‘partner/buddy’ reading can promote literacy through social interaction, and yielded three major themes, including the use of reading strategies to scaffold learning, making connections with and to the text in order to construct meaning, and using play as a type of social interaction and motivational method. The findings suggest that buddy reading as a classroom tool can effectively promote literacy and learning in a cooperative setting.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or Ebook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions

Similar content being viewed by others

Facilitating Reading Habits and Creating Peer Culture in Shared Book Reading: An Exploratory Case Study in a Toddler Classroom
“reading is social”: dialogic responses to interactive read-alouds with nonfiction picturebooks.

How Second-Grade English Learners Experienced Dyad Reading with Fiction and Nonfiction Texts
Bodrova, E., & Leong, D. (1996). Tools of the mind: The Vygotskian approach to early childhood education . Columbus, OH: Merrill/Prentice Hall.
Google Scholar
Christie, J. F., & Roskos, K. A. (2009). Play’s potential in early literacy development. In: R. E. Tremblay, R. G. Barr, R. De V. Peters, M. Boivin (Eds.), Encyclopedia on early childhood development [online]. Montreal, Quebec: Centre of Excellence for Early Childhood Development:1–6. Available at: http://www.child-encyclopedia.com/documents/Christie-RoskosANGxp.pdf . Accessed [April 2010].
Cochran-Smith, M. (1984). The making of a reader . Norwood, NJ: Ablex Publishing.
Cronin, D. (2000). Click, clack, moo: Cows that type . New York, NY: Simon & Schuster.
Griffin, M. L. (2002). Why don’t you use your finger? Paired reading in first grade. The Reading Teacher, 55 (8), 766–774.
Hubbard, R. S. & Power, B. M. (1993, 2003). The art of classroom inquiry: A handbook for teacher researchers. Portsmouth, NH: Heinemann.
MacGillivray, L., & Hawes, S. (1994). “ I don’t know what I’m doing-they all start with B”: First graders negotiate peer reading interactions. The Reading Teacher, 48 (3), 210–217.
Masurel, C. (2000). Ten dogs in the window: A countdown book . Croton on Hudson, NY: North-South Books (Houghton Mifflin Big Book Series).
Rosenblatt, L. M. (2001). The literary transaction: Evocation and response. Theory into Practice, XXI (4), 268–277.
Shannon, D. (1998). No David! New York, NY: Scholastic Publishing.
Vygotsky, L. S. (1978). Mind in society: Development of higher psychological processes . Cambridge, MA: Harvard University Press.
Download references

Author information
Authors and affiliations.
Skyline Ranch K-8, 1084 W. San Tan Hills Drive, San Tan Valley, AZ, 85143, USA
Tori K. Flint
Arizona State University, Tempe, AZ, 85287, USA
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Tori K. Flint .
Rights and permissions
Reprints and permissions
About this article
Flint, T.K. Making Meaning Together: Buddy Reading in a First Grade Classroom. Early Childhood Educ J 38 , 289–297 (2010). https://doi.org/10.1007/s10643-010-0418-9
Download citation
Published : 26 September 2010
Issue Date : December 2010
DOI : https://doi.org/10.1007/s10643-010-0418-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Social interactions
- Literary transactions
- Buddy reading
- Find a journal
- Publish with us
- Track your research

- The Oxford Review DEI (Diversity, Equity and Inclusion) Dictionary /
Buddy System – Definition and Explanation

Understanding the Buddy System in the Workplace: Fostering DEI
In the realm of Diversity, Equity, and Inclusion (DEI), the buddy system emerges as a powerful tool for fostering a supportive and inclusive workplace culture.
Definition:
The buddy system in the workplace refers to a structured approach where new employees are paired with existing employees, often referred to as “buddies” or mentors, to facilitate their integration into the company culture, processes, and workflows. This system aims to provide support, guidance, and a sense of belonging to newcomers, thereby enhancing their overall experience within the organisation.
Significance:
The buddy system plays a pivotal role in advancing DEI initiatives within organisations. By pairing individuals from diverse backgrounds as buddies, companies create opportunities for cross-cultural exchange, empathy building, and understanding. This fosters an inclusive environment where differences are celebrated, and employees feel valued irrespective of their background, ethnicity, or identity.
Implementation:
Implementing a successful buddy system requires careful planning and execution. Companies can start by assigning buddies based on shared interests, experiences, or backgrounds to facilitate meaningful connections. Training sessions for both mentors and mentees can be conducted to set expectations, establish boundaries, and equip participants with effective communication and mentoring skills.
Imagine a scenario in a UK-based tech firm where a new hire, Sarah, joins the team. As part of the buddy system, Sarah is paired with an experienced employee, James, who shares similar professional interests. James welcomes Sarah on her first day, gives her a tour of the office, introduces her to colleagues, and provides insights into the company culture. Over the following weeks, James continues to support Sarah by offering guidance on projects, facilitating networking opportunities, and addressing any challenges she encounters. Through this buddy relationship, Sarah feels empowered, supported, and valued within the organisation.
Conclusion:
Incorporating the buddy system into the workplace not only aids in the seamless integration of new hires but also serves as a catalyst for DEI efforts. By fostering meaningful connections and promoting mutual respect and understanding, the buddy system contributes to building a more inclusive and supportive work environment. Embracing this approach underscores a company’s commitment to diversity, equity, and inclusion, ultimately driving employee engagement, retention, and organisational success.
References:
Peterson, J. L., & Norman, T. A. (1977). Buddy systems. Communications of the ACM, 20(6), 421-431. https://dl.acm.org/doi/abs/10.1145/359605.359626
O’Donnell, C. R., Lydgate, T., & Fo, W. S. (1979). The buddy system: Review and follow-up. Child Behavior Therapy, 1(2), 161-169. https://www.tandfonline.com/doi/abs/10.1300/J473v01n02_03
Lopez, P. J., & Gillespie, K. (2016). A love story: for ‘Buddy System’research in the academy. Gender, Place & Culture, 23(12), 1689-1700. https://www.tandfonline.com/doi/abs/10.1080/0966369X.2016.1249354
Fo, W. S., & O’Donnell, C. R. (1975). The buddy system: Effect of community intervention on delinquent offenses. Behavior Therapy, 6(4), 522-524. https://www.sciencedirect.com/science/article/abs/pii/S0005789475800086
Be impressively well informed

Get the very latest research intelligence briefings, video research briefings, infographics and more sent direct to you as they are published
Be the most impressively well-informed and up-to-date person around...
Success! Now check your email to confirm that we got your email right. If you don't get an email in the next 4-5 minutes something went wrong: 1. Check your junk folder just in case 🙁 2. If it's not there either, you may have accidentally mistyped your email address (it happens). Have another go. Many thanks
There was an error submitting your subscription. Please try again.
- Open access
- Published: 18 February 2023
Research Buddy partnership in a MD–PhD program: lessons learned
- Daniel J. Gould ORCID: orcid.org/0000-0002-0423-5822 1 ,
- Marion Glanville-Hearst 1 ,
- Samantha Bunzli ORCID: orcid.org/0000-0002-5747-9361 2 , 3 ,
- Peter F. M. Choong ORCID: orcid.org/0000-0002-3522-7374 1 , 4 &
- Michelle M. Dowsey ORCID: orcid.org/0000-0002-9708-5308 1 , 4
Research Involvement and Engagement volume 9 , Article number: 4 ( 2023 ) Cite this article
1888 Accesses
2 Altmetric
Metrics details
Background and aims
There is increasing recognition of the importance of patient involvement in research. In recent years, there has also been growing interest in patient partnerships with doctoral studies students. However, it can be difficult to know where to start and how to go about such involvement activities. The purpose of this perspective piece was to share experiential insight of the experience of a patient involvement program such that others can learn from this experience.
This is a co-authored perspective piece centred on the experience of MGH, a patient who has had hip replacement surgery, and DG, a medical student completing a PhD, participating in a Research Buddy partnership over the course of over 3 years. The context in which this partnership took place was also described to facilitate comparison with readers’ own circumstances and contexts. DG and MGH met regularly to discuss, and work together on, various aspects of DG’s PhD research project. Reflexive thematic analysis was conducted on reflections from DG and MGH regarding their experience in the Research Buddy program to synthesise nine lessons which were then corroborated with reference to published literature on patient involvement in research. These lessons were: learn from experience; tailor the program; get involved early; embrace uniqueness; meet regularly; build rapport; ensure mutual benefit; broad involvement; regularly reflect and review.
Conclusions
In this perspective piece, a patient and a medical student completing a PhD reflected upon their experience co-designing a Research Buddy partnership within a patient involvement program. A series of nine lessons was identified and presented to inform readers seeking to develop or enhance their own patient involvement programs. Researcher-patient rapport is foundational to all other aspects of the patient’s involvement.
Plain English summary
The importance of patient involvement in research is gaining recognition. Existing research centres, as well as those that are just getting started, need to find their own way to involve patients and community members. However, learning from the experience of others is crucial to ensure every effort is made to do this in a fruitful way. Therefore, we aimed to share our experience and provide a list of lessons learned to help other researchers and patients get started and work together effectively. Our research centre developed a framework for involving patients in joint replacement research. Part of this framework is a ‘Research Buddy’ program, where a research student partners with a patient so that the research they conduct is more relevant and applicable to the target population. In our case, the research student partnered with someone who had a hip replacement to develop and test a questionnaire for an interview study about artificial intelligence in shared decision-making. The student and patient worked together and wrote this perspective piece outlining nine lessons so readers can learn from their experience of this program. The lessons were: learn from experience, tailor the program, get involved early, embrace uniqueness, meet regularly, build rapport, ensure mutual benefit, broad involvement, regularly reflect and review. People interested in starting, or improving, their own patient involvement activities can learn from our experience. These lessons will need to be adapted to fit the purpose and unique situation of other researchers and patients who have different needs and circumstances.
Peer Review reports
Introduction
There is growing recognition of the importance of patient and public involvement and engagement in research [ 1 , 2 ]. However, it can be difficult to know where to begin and how to go about it. Sharing experiential knowledge is critical for different groups seeking to develop and deploy their own programs and strategies [ 3 ]. This is because a one-size-fits-all approach will not address the culture and context of the unique settings in which these programs take place [ 4 , 5 , 6 , 7 ]. Rather, strategies should be tailored to each unique setting [ 8 ]. An in-depth description of the experience of the patient involvement program in a particular context, including the nature of the program and the challenges overcome, provides others with ideas and examples they can modify to suit their own needs [ 1 , 3 , 9 ]. These other groups can read about the experience of others and possibly avoid some pitfalls while harnessing the strengths of the program. This is not intended to restrict creativity nor to provide a prescriptive roadmap, but rather to provide an experiential account of the nature of engagement in a particular setting and offer suggestions for ways in which engagement can take place.
In recent years, there has also been growing interest in efforts to involve patients in doctoral studies [ 5 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 ]. This presents a unique opportunity to instil recognition of the importance of patient involvement early in the career of researchers and equip them with the skills and experience to facilitate meaningful involvement. It simultaneously offers patients an opportunity to partner with researchers in this formative stage of their career as they develop their research interests and approach to research.
Aims and rationale
The aim of this perspective piece was for the first two members (DG and MGH) of a Research Buddy partnership (described below in the ‘Context’ section) to critically reflect on their experience of this involvement activity throughout the course of a MD–PhD program.
Through this reflective process, lessons learned through experience were consolidated such that they can be of benefit to others seeking to learn from this experiential insight as they establish or improve their own patient involvement programs [ 1 , 3 , 18 , 19 , 20 ]. We also provided a detailed description of the context in which this Research Buddy partnership took place, to provide readers with a more complete understanding of the way in which this context influenced the partnership such that readers can consider the nature of their own unique situation and how this might impact upon patient involvement efforts [ 21 ].
Overview of structure of this perspective piece
Definitions.
Theoretical Underpinnings.
Method of Patient Involvement throughout MD–PhD program
Method of reflection in this perspective piece
Findings—Lessons Learned.
Definitions
The body of literature in the patient involvement space is fraught with complex and nuanced terminology [ 22 ]. Therefore, the purpose of this section is to clarify the terminology and definitions used. Influential stakeholder involvement literature, as well as recent publications, were reviewed to select widely-used and functionally relevant terminology for this perspective piece [ 1 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 ]. The other critical aspect of the rationale for selecting these terms and definitions was that they were agreed upon with the patient partner, MGH, as they accurately described the nature of her involvement.
Terms used in this paper include the following: patient, involvement, partnership, and co-design.
The term ‘patient’ was used because this perspective piece concerns involvement with patients as distinct from members of the public. While this term may invoke the image of an individual seeking care, in the context of involvement it also refers to individuals with experience of a health condition and its management [ 28 ], which in this case is total joint replacement surgery.
Building upon this is the definition of ‘involvement’, which is commonly accepted to refer to research done ‘with’ or ‘by’ patients rather than ‘for’, ‘to’, or ‘about’ them [ 30 ]. One specific type of involvement is the ‘patient partnership’, in which patients are embedded in various research activities at different levels and in multiple stages of the research process [ 1 ]. A particularly powerful example of a patient partnership is the Research Buddy program, which has been described previously as a program in which a doctoral student works closely with a patient throughout their project [ 13 ] and which was also described and reflected upon later in this perspective piece in the section ‘Method of Patient Involvement throughout MD–PhD program’.
Finally, the term ‘co-design’ was used in this piece to describe the active, voluntary process of researcher and patient partner working together on the design of a PhD project and its constituent studies, as this terminology is appropriate for the timeframe in which doctoral studies typically take place [ 22 , 32 , 33 , 34 , 35 ].
Theoretical underpinnings
Theory development was not an aim of this perspective piece. However, it is important to describe the theoretical underpinnings of the patient involvement program.
There are three main approaches to patient involvement in research: epistemological, consequentialist, and emancipatory or moral [ 33 , 36 , 37 ]. The epistemological approach recognises the fact that patients have lived experience of a health condition that can be of benefit to the research by broadening the perspective of researchers. The emancipatory approach recognises that patients have a right to be involved in publicly funded research that impacts upon them or the care they receive, and consequently researchers have a responsibility to involve patients. Finally, the consequentialist argument states that involvement can improve the design, conduct, and reporting of research.
Primarily, the emancipatory argument motivated the formation of the involvement program described in this perspective piece. Furthermore, patient involvement can lead to more ethically sound research [ 38 ], and this was recognised in the work reflected upon in this piece as MGH assisted DG in the ethics application process for the PhD research projects through document review. However, it was also recognised that the quality and relevance of research conducted at the research centre could be improved through involvement of a patient with lived experience of joint replacement surgery. Therefore, the consequentialist and epistemological arguments also underpinned the involvement program.
Recent literature has also advocated for a pragmatic approach to patient-oriented research by utilising patient involvement to ultimately improve health outcomes for affected individuals [ 39 ]. These authors recognised that achieving such impact is a complex task and, as such, mixed methods approaches to research are encouraged to harness the strength of both quantitative and qualitative research methodologies. This is particularly relevant to the current perspective piece, which describes patient involvement in a mixed methods PhD project on a topic of clinical relevance aimed to improve outcomes for patients.
Finally, the word ‘perspective’ was intentionally selected instead of the term ‘representative’ because it is unrealistic and unfair to ask a single patient with their own unique experience to generalise their experience to represent that of all patients with the same health condition. Rather, patient involvement was incorporated to broaden the perspective of the researchers while they co-designed research with the patient [ 36 , 40 ].
A detailed description of the context in which the patient involvement program took place facilitates comparison to one’s own context such that the potential applicability of a framework and experience can be considered in detail [ 6 , 7 ]. This is not intended to limit the generalisability of the findings reflected upon in this perspective piece. Rather, the specific context in which the patient involvement partnership and activities took place is described in detail such that readers can accurately compare this context to their own.
The patient involvement program described in this perspective piece took place in an Australian National Health and Medical Research Council Centre for Research Excellence for Total Joint Replacement, seeking to O ptimise P atient o U tcomes and S election for Total Joint Replacement (OPUS). OPUS is embedded in a hospital in Melbourne, Victoria, which is a tertiary referral centre for total joint replacement (TJR), providing care for people from a broad range of geographical regions, socioeconomic backgrounds, and cultures [ 41 ]. OPUS brings together clinician-researchers with backgrounds in orthopaedic surgery, nursing, and physiotherapy, as well as trialists, epidemiologists, health economists, statisticians and qualitative researchers. It comprises researchers at various stages of their careers, including graduate research students.
The patient involvement program at OPUS was developed and launched in 2020 and has been described previously [ 42 ]. The program involves a four-tiered framework of involvement (see Fig. 1 ). Table 3 of this prior publication also outlines the remuneration fee schedule according to which MGH was remunerated for her time. This prior publication used the term ‘consumer’, therefore, for consistency, this terminology is used below when describing the framework.
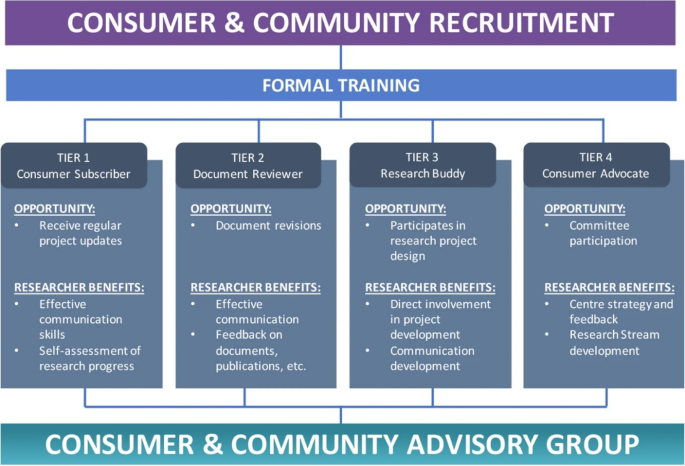
Proposed level of involvement for consumers and community members presents different opportunities of participation and the relevant benefits to researchers
This Figure was made available under the Creative Commons Attribution 4.0 ( http://creativecommons.org/licenses/by/4.0/ ).
The Consumer and Community Advisory Group (bottom of Fig. 1 ) oversees the operations of the CCI program. It is chaired by a consumer (MGH, co-author on this paper).
This perspective piece details the experience of a Research Buddy, Tier 3 as shown in Fig. 1 , and an MD–PhD candidate at OPUS. An MD–PhD candidate is a medical student who takes time out from their clinical studies to complete a PhD. The details of this Research Buddy program are described in the next section.
Method of patient involvement throughout MD–PhD program
The Research Buddy program reflected upon in this piece took the approach of broadening the perspective of the research team by including the voice a patient with lived experience of a health condition [ 43 ]. This is distinct from an approach which would seek to achieve representativeness from one patient partner on behalf of all patients with a shared lived experience, which would be unrealistic [ 31 , 44 ].
The focus of this piece is on the Research Buddy partnership comprising authors DG and MGH. In this section, DG and MGH reflected upon their expectations prior to the commencement of the Research Buddy program and how their actual experience played out.
DG was a final-year postgraduate medical student in 2019, carrying out a research project on risk factors for readmission following total knee replacement (TKR) surgery. The opportunity arose to enrol in a PhD to expand upon this work, which led to the deferment of DG’s final year of medical school in order to complete a PhD as part of an MD–PhD program [ 45 ]. Building upon the research into risk factors for readmission following TKR, the PhD centred around the development of a clinical risk prediction tool utilising machine learning. DG, along with his PhD supervisors (authors MD, SB, and PC) felt that the strong clinical focus of the MD–PhD program would position it well for close involvement with a Research Buddy. MGH had previously participated, as a subject, in a study conducted by MD and PC investigating the impact of a mindfulness program on recovery post-surgery for patients undergoing total hip replacement (THR) [ 46 ]. MGH reached out to MD regarding the findings of the mindfulness study, coincidentally while MD was establishing the patient involvement framework at OPUS and seeking volunteers for the Research Buddy role. MGH subsequently embraced the opportunity and volunteered to be DG’s Research Buddy approximately 6 months after the official commencement of the PhD. MGH decided to take part specifically in DG’s project because she wanted to learn more about research, had a keen interest in artificial intelligence and machine learning and its application to lower limb joint replacement surgery, and has had a lifelong interest and involvement in academia. This presented a tangible opportunity to make a substantial impact on the direction of the research and cement its clinical, stakeholder-focused approach, and MGH felt her interests and experience aligned most closely with DG’s project [ 47 ].
During this formative stage of the PhD project, MGH and DG worked together to define the scope and structure of the Research Buddy program. There was a shared expectation of open-ended discussions and regular contact with updates on the research. Neither DG nor MGH had been involved in such a patient involvement partnership before, therefore they did not have rigid expectations about the manner in which the partnership would unfold. It was decided that regular monthly meetings would facilitate the development of rapport, ongoing discussion of ideas for the PhD project and opportunities for involvement its constituent studies, and the opportunity for regular updates on progress.
The first part of DG’s PhD project discussed in these meetings was the systematic review, which was in the manuscript drafting stage and benefited greatly from MGH’s input on the clarity of the language [ 48 , 49 ] and ensuring the pertinence and importance of the topic were clearly communicated. Patient involvement in systematic reviews has been reported extensively in the literature [ 32 , 50 , 51 , 52 ], and it was an opportunity for MGH to gain familiarity with the topic at the foundation of the PhD project. More broadly, the regular monthly meetings were critical in defining the overall scope and purpose of the PhD project. Specifically, during the early stages it was difficult to determine how to make the research more clinically relevant. MGH had a background in occupational therapy and medical anthropology and had previously been involved in qualitative interview projects in which she interviewed participants and coded transcripts. The idea of a qualitative study exploring the views of TKR patients on the use of artificial intelligence and machine learning for risk prediction in shared clinical decision-making was already being developed, and MGH felt this was a fitting opportunity to be involved given her personal journey as a patient and her prior academic and research experience. MGH was involved as a co-author on the qualitative study and made a substantial contribution by pilot-testing the interview guide, contributing to the reflexive thematic analysis, and preparing and editing the manuscript for publication.
Next, MGH reviewed all of the ethics approval application documents for the studies comprising DG’s PhD project. Not only did this improve the clarity of the documents in a similar way to MGH’s review of the systematic review manuscript, but it also led to the development of more ethically conscious research by incorporating a patient’s perspective into the application [ 38 ] and helped DG to clarify the way these studies related to one another and formed a cohesive program of research.
Beyond the specific elements in which MGH made a direct contribution, DG’s reflection upon the stakeholder involvement in the form of the Research Buddy program prompted a shift in focus from machine learning to enhance predictive performance of the TKR readmission prediction model, to stakeholder involvement and engagement in the whole process of developing clinical risk prediction models, including not only patients but also clinicians [ 29 , 53 ]. In light of this, thanks to MGH’s Research Buddy input, DG designed the PhD project with stakeholder involvement embedded throughout.
At the time of writing this perspective piece, the main studies of the PhD project had been completed and DG was in the final stages of thesis write-up following feedback provided by MGH on the first full draft. The overall focus of the PhD project is on co-design with stakeholders, inspired by the Research Buddy partnership with MGH and cemented by exploration of the literature [ 24 , 54 , 55 , 56 ].
The PhD project centres around the development of a machine learning model to predict 30-day readmission [ 57 ] in people undergoing TKA surgery years [ 58 , 59 ] for osteoarthritis (OA) [ 60 ], co-designed with clinicians and patients [ 61 ]. The project is divided into four components, each comprising at least one main study. While MGH’s involvement in the project overall has been outlined above, what follows is a brief description of each part of the PhD project as well as the level [ 29 ] and phase [ 62 ] at which MGH was involved. While a high level of involvement was aimed at from the early phases of each part of the PhD project, it is recognised that higher levels of involvement are not automatically more desirable [ 27 ] and, since some aspects of the project were already underway when MGH and DG commenced the Research Buddy program, flexibility and responsiveness to the realities of the circumstances were of utmost importance in deciding with MGH at what level she wanted to be involved.
Identify and appraise risk factors for 30-day readmission following TKA.
As previously mentioned, MGH was involved in reviewing the final draft of the systematic review manuscript [ 63 ] and provided feedback on the clarity of the language. The next stage of this part of the project involved a modified Delphi and focus group study involving clinicians who care for TKR patients to gain their insight on the clinical relevance of risk factors identified in the systematic review and meta-analysis, as well as giving them the opportunity to suggest novel risk factors which had not been identified in prior literature. MGH helped by observing the pilot test of the focus group and providing feedback on its structure and conduct. This study has also been published [ 64 ].
Level = Involve
Phase = Design and preparation, dissemination
Develop and internally validate the model.
The findings of the systematic review and meta-analysis, as well as the Delphi and focus group study, were utilised alongside machine learning to develop a clinically applicable risk prediction tool for 30-day readmission following TKR which harnessed both clinical reasoning and statistical learning techniques. The manuscript is currently under review in an academic journal. MGH and DG discussed the project in their regular meetings as DG designed the study, particularly the purpose of the predictive tool and the way in which it could be used in the live clinical setting to facilitate shared clinical decision-making between patient and surgeon. and MGH reviewed several drafts of the manuscript ahead of submission for publication.
Phase = Dissemination
Explore the understanding of AI for risk prediction in shared clinical decision-making among people with TKA.
This is the qualitative semi-structured interview study, involving interviews with 20 total knee replacement patients, in which MGH had the most direct involvement, being included as a co-author. Patient and stakeholder involvement in qualitative studies is a longstanding practice [ 5 , 7 , 65 , 66 , 67 , 68 , 69 ]. MGH was involved in refining the research question and interview guideline through two rounds of pilot-testing. MGH was also actively involved in the reflexive thematic analysis and in drafting the manuscript for submission to an academic journal, where it is currently under review.
Level = Collaborate
Phase = Design and preparation, conduct and implementation, and dissemination
Compare the performance of the predictive model to that of clinicians involved in the care of people receiving TKA surgery.
Preliminary findings for this study have been analysed and recruitment is almost complete at the time of writing this perspective piece. MGH was involved in a similar way to the predictive model development study, however this comparison study involved recruiting clinicians to participate in a survey. MGH reviewed recruitment material and discussed with DG how to structure the online survey to ensure the instructions were clear and straightforward and the length of the survey was manageable for busy clinicians.
Rather than speaking for the patient, it is important for the patient to speak for themselves. Prior reflective work on patient involvement in doctoral research adopted this approach to great effect [ 5 , 14 ]. This piece was therefore co-written by MGH and DG, the two members of the inaugural Research Buddy partnership at OPUS.
Pertinent elements of the GRIPP2 (Guidance for Reporting Involvement of Patients and the Public) checklist [ 70 ] were adhered to while writing this piece.
The experiential insight gained by DG and MGH through their involvement in the Research Buddy program was consolidated in regular meetings throughout DG’s PhD project. Additionally, DG and MGH conducted an informal interview using questions informed by prior literature in which patient advocates from various patient involvement programs in the UK were interviewed [ 9 ]. This interview was audio recorded and transcribed. The transcript was then analysed using reflective thematic analysis [ 71 ] in which themes were generated inductively by DG and MGH based on MGH’s responses to the questions in the interview, informed by their shared experience in the Research Buddy partnership. These themes were then synthesised and refined into lessons, inspired by prior patient involvement literature which has used thematic analysis in a similar way to generate lessons and recommendations [ 1 , 3 , 55 , 62 , 72 , 73 ].
A literature search was then carried out to identify literature reviews which contained lessons from, or recommendations for, patient involvement [ 1 , 5 , 13 , 15 , 28 , 35 , 62 , 74 , 75 ]. These reviews were used to corroborate the findings from our own inductive analysis and reflection.
DG and MGH derived these lessons by reflecting primarily on their experience in the Research Buddy program. However, MGH was embedded in various other patient involvement activities at the research centre (OPUS), therefore some of these lessons will be broadly applicable beyond Research Buddy partnerships. This will depend on the patient’s desired level of involvement, and it is advised that readers are flexible in their interpretation and implementation of these findings.
Findings: lessons learned
Lesson (1) learn from experience.
Don’t go in completely blind. Learn from the experience of others as much as possible. Bring in experienced patient partners, and researchers experienced in patient involvement, who can offer advice on promising avenues to explore as well as flagging those which are less likely to be fruitful. For example, as part of the Research Buddy program reflected upon in this perspective piece, Carol Vleeskens was consulted to conduct a training session attended by both MGH and DG. Carol was a co-author on the publication outline OPUS’ patient involvement framework [ 42 ] and has extensive experience in patient involvement [ 76 ]. MGH was then involved in preparing an orientation day for prospective patient partners at OPUS, which she also attended.
Another powerful resource is published work on the experience and appraisal of other researcher-patient partnerships sharing their experience [ 5 ].
This lesson is pertinent to consider both in preparation for partnering with patients in order to maximise the likelihood of success prior to launching involvement programs [ 8 , 13 , 48 , 77 ], as well as for ongoing appraisal and improvement of existing programs based on patient partner feedback [ 1 ].
Lesson (2) tailor the program
A one-size-fits-all approach does not work. Patient involvement programs should be bespoke. They should be tailored to the unique context of the research centre engaging the patients [ 5 , 78 ]. Learning from the work of others is critical for generating ideas about how one’s own program could take shape [ 78 ], but it is equally important to modify these programs so they are fit for purpose.
Tailoring the patient involvement program has been emphasised in prior literature as a critical component of fruitful involvement through the translation of general principles into context-specific systems and actions [ 4 , 5 , 8 , 15 , 35 , 74 ].
Lesson (3) get involved early
If possible, involve patients in discussions where ideas are generated for research projects. Allow ample time to include patients on ethics applications and governance approvals to ensure they can make a meaningful contribution to the research.
Integrating patient involvement in earlier stages of research helps to facilitate truly patient-led research. Based on the experience outlined in this perspective piece, there are two main ways in which this can be done. First, patient involvement can be embedded in the ongoing work of established research centres as well as the commencement of work at new research centres in order to identify research priorities and steer the direction of research activities [ 4 , 7 ]. Second, in the style of Research Buddy partnerships, patients can be partnered with PhD students in order to co-design the program of research in a meaningful way by embedding engagement and co-design at the beginning of the student’s project [ 19 ]. It is not difficult to imagine a pipeline in which researchers collaborate with patients to generate ideas and then these ideas are translated into PhD research projects with patients.
Having patients engaged at these different stages of idea-generation and research project design could have the added benefit of anticipating and addressing challenges likely to arise in complex research projects such that solutions can be suggested proactively [ 29 ].
Early involvement has been demonstrated or recommended numerous times in a diverse range of contexts and for different levels of involvement [ 5 , 7 , 15 , 28 , 35 , 43 , 75 ].
Lesson (4) embrace uniqueness
Patients bring a wealth of unique experience. They should be treated as individuals with their own unique characteristics, experience, and insight [ 79 ]. Embrace their unique skills and experience. Throughout regular meetings and correspondence, various aspects of the patient’s experience may enable them to bring a unique perspective to the research which researchers could not have anticipated.
Embracing uniqueness of patient partners has been found to encourage more inclusive stakeholder involvement by demonstrating a flexibility in the researchers and responsiveness to the individual needs, experiences, and preferences of people seeking to be involved [ 1 , 5 , 35 , 48 , 77 ].
Lesson (5) meet regularly
Meet regularly, right from the start, even in the absence of a clear idea of what to do in terms of exactly how the patient will be involved. The level and nature of involvement can be collaboratively defined through these regular meetings.
Be open to a variety of forms the patient’s involvement could take and be willing to engage in some trial and error. Some ideas will not pan out, and that is just part of the process. Be willing to persevere and adapt. For example, during the qualitative study conducted as part of DG’s PhD project, MGH attempted cross-coding of the qualitative interview transcripts using the coding framework being developed by DG and author SB. However, MGH found that her style of coding based her prior experience was not functioning as well as it had for previous projects. DG and MGH attempted different styles and continued to discuss their findings in their regular monthly meetings, however ultimately this cross-coding exercise was abandoned as it was becoming quite convoluted and MGH felt more comfortable discussing the transcripts and contributing to the discussions around the development of themes through reflexive thematic analysis.
Both DG and MGH had an openness that resulted in these meetings being fruitful beyond the pragmatic aspects of setting the research agenda and tracking progress; they were critical in building rapport. Even when there were no specific research updates, the regular meeting time was maintained and utilised for an opportunity to discuss topics related to the research.
Regular meetings are a critical component of impactful patient involvement programs reported in the literature [ 15 , 35 , 62 , 75 ] as they facilitates ongoing communication, continuous feedback, and strong rapport-building as expanded upon in Lesson 6.
Lesson (6) build rapport
Establishing trust and rapport is critical to the success of the program. Put in the time. Create an environment in which frank and open discussions are encouraged. Follow through on action items from meetings and email discussions. Respect the patient’s autonomy while supporting them in their role. Listen to the patient and take their feedback into account. Demonstrate to the patient how their input is influencing the research. This designated time to meet and talk openly was critical for building rapport, which is arguably the most important component of patient involvement to increase the likelihood of success [ 37 , 80 ]. The centrality of rapport to the overall success of patient involvement in research is reflected in the large volume of diverse literature recognising its importance [ 1 , 5 , 17 , 28 , 30 , 35 , 62 , 73 , 75 , 81 , 82 , 83 , 84 ].
Lesson (7) ensure mutual benefit
Patients are more engaged when there is something in it for them. Ensure the interaction is mutually beneficial. For example, patients might learn more about their condition, or the condition of those they care for, through the patient involvement program [ 85 ].
This pragmatic aspect of involvement has been recognised in the literature detailing meaningful and impactful involvement activities that recognise the importance of ensuring the involvement benefits the research, the researchers, and the patient partners themselves [ 1 , 19 , 35 , 62 , 75 , 83 ].
The nature of a successful patient involvement program, in which there is rapport, mutual respect, and open discussion, is associated with a certain degree of passive learning simply by the two parties being in contact with one another [ 85 ]. Without any specific educational component to the program, regular and open communication regarding a common focus in the context of a research centre or program can have the effect of educating the patient about research [ 53 ], and the researcher about the patient’s lived experience of their condition [ 72 , 86 ]. Patients may also learn more about their own condition, its management, and current research being done to further understanding about it [ 85 ].
Lesson (8) broad involvement
It is beneficial to have the patient embedded in the broader patient involvement activities at the research centre, not just the Research Buddy (or equivalent) program. This may enable the patient to participate in broad discussions about current research and setting the direction of future research.
This is important for contextualising the Research Buddy’s work and enhancing their understanding of the way in which it fits into the broader body of work being conducted at the research centre in which the program is taking place. This is not to say patient partners should be stretched beyond their limit, but rather that they are not forced to limit themselves to one project and instead are encouraged to broaden their perspective through opportunities to be involved in varying capacities in other research projects and activities depending on their interests and capacity [ 28 , 35 , 82 ].
Lesson (9) regularly reflect and review
It is not enough to have good intentions. Having a framework is also critical, but the patient’s role may evolve over time and ethical considerations related to their involvement also evolve. It is easy for the patient’s role to devolve into tokenism despite the best intentions [ 22 ]. Regular meetings incorporating feedback from patient partners on their involvement in the research are critical to ensuring the patient’s role as partner in the research is respected and maintained. This requires transparency on the part of the researcher regarding how the patient’s involvement is influencing the research. Researchers must also be open to feedback from patients that may indicate they are not satisfied with their involvement and adjustments to their involvement may be required.
One potential way in which the patient partnership can break down is for researchers to treat them less like partners in the research process and more like participants in quasi-qualitative research by simply calling upon patients to complete surveys or answer questions as if in a qualitative interview. This is problematic because patient involvement in research is distinct from qualitative research and must be treated as such, but vigilance and effort is required to maintain the boundaries between the two [ 22 ].
Beyond this specific problem, regular reflection and review are broadly applicable to every aspect of patient involvement to ensure the patient’s involvement is meeting expectations as these expectations evolve throughout the research project alongside changes in the patient’s capacity and interests.
Regular review is crucial to ensure ethical principles are being adhered to at all times as the patient’s role evolves. Circumstances change, both intentionally and unintentionally, so it is critical to have discussions specifically dedicated to reviewing the patient’s role and ensuring they are still meaningfully involved and ethical principles are adhered to [ 87 ]. There may be occasions on which the patient’s preconceived ideas need to be discussed and worked through.
Building upon the other lessons outlined in this perspective piece, rapport based on mutual respect, coupled with regular meetings, provide the structure and nurture the culture necessary to facilitate continuous feedback as well as regular designated touch points at which reflection and review can take place [ 5 , 13 , 28 , 35 , 62 , 74 , 75 , 83 , 87 ].
Summary of findings
In this perspective piece, a patient partner with lived experience of total joint replacement surgery (MGH) and a MD–PhD candidate (DG) reflected on their experience in a Research Buddy program throughout a PhD project. Their aim was to provide experiential insight such that other researchers and patient partners can learn from their experience as they develop and refine their own patient involvement programs. Inductive thematic analysis was applied to the transcript of an informal interview carried out between DG and MGH, informed by reflections from regular meetings throughout their Research Buddy partnership. Through this process, nine lessons were synthesised from their experience: Learn from experience, Tailor the program, Get involved early, Embrace uniqueness, Meet regularly, Build rapport, Ensure mutual benefit, Broad involvement, and Regularly reflect and review. These lessons were corroborated by published literature, and rapport was found to be the most important factor in successful patient involvement. Each of the remaining lessons either stem from and/or contribute to the development of rapport.
Contribution to the literature
To the best of the authors’ knowledge, this is the first perspective piece on patient partnership in a MD–PhD program. This builds upon prior work highlighting the positive impact of partnering medical students with patients to further their clinical education and broaden their perspective, while empowering patients through the opportunity to learn more about their own health condition and health service experience while sharing their experiential insight with future clinicians in a formative stage of their career [ 88 , 89 ]. It builds upon this by demonstrating how MD–PhD students, being situated at the interface between the research and clinical domains, can partner with patients in a fruitful way that has a meaningful impact upon their clinical development while also influencing the design, conduct, and reporting of the research. This was particularly noteworthy during DG’s PhD journey, most of which was undertaken during lockdowns imposed in light of the COVID-19 pandemic. Regular contact with MGH was, for long periods of time, DG’s only patient interaction and therefore was crucial to maintaining patient contact [ 88 ] from a clinical perspective as well as working with MGH to develop solutions to convert various research activities to be entirely online [ 15 , 16 ].
Through regular meetings based on mutual trust and respect, largely comprising open discussion especially when there were no major research progress updates, building rapport was prioritised from the outset of the Research Buddy program. Since MGH and DG had the freedom to define the scope and nature of the program, they were able to emphasise the importance of this reciprocal relationship both through conversation as well as through document review, providing comments alongside DG’s PhD supervisors, which enabled MGH to provide a critical perspective on DG’s work. With strong rapport as the foundation and critical feedback encouraged throughout the research journey, MGH’s role as ‘critical friend’ was characterised and strengthened [ 10 , 17 ].
This perspective piece also builds upon exemplary prior work reporting a Research Buddy program [ 13 ] by being co-authored with the Research Buddy. This perspective piece conveyed lessons learned through a program which drew on elements of embedded consultation [ 5 , 13 ] and demonstrated flexibility in the level of involvement in various aspects of the PhD project in response to changes in MGH’s interests and capacity throughout the partnership [ 16 ].
Furthermore, while the PhD project reflected upon in this perspective piece did not strictly pertain to a digital health innovation nor to a full translational research innovation, a predictive model was developed which was intended for use in the clinical setting for shared clinical decision-making in orthopaedics. Therefore, MGH’s involvement throughout the project was critical for in laying a solid foundation for the development of a clinically relevant digital health innovation [ 90 , 91 ] primed for translation into the clinical setting [ 27 ] in orthopaedics, which is a clinical discipline in which patient involvement is gaining recognition and appreciation [ 26 ].
Implications of findings
The most impactful implication of the findings of this paper is that the description of the context in which the researcher-patient partnership took place, coupled with the co-authored lessons learned and experiential insight [ 3 ] offered through reflection by both members of the Research Buddy partnership, can be utilised by others [ 92 , 93 ]. Learning through experience and reporting these lessons along with a detailed description of the context in which they were learned [ 6 , 18 ] is an impactful outcome of its own [ 92 ]. This enables readers to inform their own involvement efforts, both when establishing involvement programs and refining them.
Through involvement, patients like MGH learn more about their health condition as well as the research that goes into the developments in management approaches and the way in which patients experience the healthcare system [ 85 ].
It is hoped that these findings can be of assistance to ongoing [ 11 , 12 ] and future patient involvement efforts to bring researchers and patients onto the same page from the outset of research projects [ 49 ].
Future directions
Evaluating impact.
The question of how best to evaluate impact of patient involvement is longstanding and complicated [ 48 , 77 ]. There have been many attempts over the years to answer this question [ 11 , 12 , 23 , 73 , 78 , 79 , 94 , 95 , 96 , 97 , 98 , 99 ], but it appears there is no single best approach to measuring the effectiveness, success, or impact of involvement [ 100 ], and perhaps such a measure will never exist. However, ongoing efforts to develop and validate consistent patient involvement evaluation measures are encouraged to facilitate the comparison of different involvement strategies and gain some indication of which might be the most promising in a given context, when resources are limited and ought to be allocated to the most promising strategy [ 101 ].
Exploring patient involvement in decision-making non-research patient involvement and empowerment
The research project reflected upon in this perspective piece centred around the development of a risk prediction tool intended to be used in the process of shared clinical decision-making. This perspective piece explored patient involvement in clinically-oriented research being carried out by a MD–PhD candidate, which positions it well for ongoing work exploring the role patients play in the shared clinical decision-making process [ 102 , 103 , 104 ], particularly as it pertains to the use of decision aids including predictive tools.
Further reading
Further reading is recommended for those seeking to expand their knowledge of different types of patient involvement in varied contexts in order to better understand how to initiate, or improve, their own patient involvement programs [ 32 , 40 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 , 144 , 145 , 146 , 147 , 148 ].
Strengths and limitations
Strengths of this perspective piece include the consistent and frequent contact between DG and MGH throughout the course of the PhD program, which facilitated widespread and deep involvement in various aspects of the research project and its constituent studies. This also facilitated continuous feedback and reflection through open discussion, which laid the foundation for the writing of this piece.
One potential limitation is that MGH may be regarded as a ‘professional’ patient partner, given her academic background. This may raise concerns about whether her perspective as a patient is diminished by her familiarity with academia and the research process. This was certainly an important consideration, especially considering MGH’s involvement spanned over 3 years and involved close and regular contact with DG. However, it was not a concern because regardless of how familiar MGH becomes with the research process, she will never lose her perspective as a patient who has lived experience of total joint replacement surgery [ 149 ].
In this perspective piece, a patient and a medical student completing a PhD reflected upon their experience co-designing a Research Buddy partnership within a patient involvement program. To the authors’ knowledge, this is the first time such a partnership has been written about within the context of a MD–PhD program, which is uniquely placed at the interface between the research and clinical domains. A series of nine lessons was identified and presented to inform readers seeking to develop or enhance their own patient involvement programs: learn from experience, tailor the program, get involved early, embrace uniqueness, meet regularly, build rapport, ensure mutual benefit, broad involvement, regularly reflect and review. Researcher-patient rapport is foundational to all other aspects of the patient’s involvement.
Availability of data and materials
Not applicable.
Abbreviations
Doctor of philosophy
Doctor of medicine
Guidance for reporting Involvement of Patients and the Public
OPtimise Patient oUtcomes and Selection for Total Joint Replacement
Total knee replacement
Total hip replacement
McCarron TL, Clement F, Rasiah J, Moran C, Moffat K, Gonzalez A, et al. Patients as partners in health research: a scoping review. Health Expect. 2021;24(4):1378–90.
Article PubMed PubMed Central Google Scholar
Holzer JK, Ellis L, Merritt MW. Why we need community engagement in medical research. J Investig Med. 2014;62(6):851–5.
Staley K, Doherty C. It’s not evidence, it’s insight: bringing patients’ perspectives into health technology appraisal at NICE. Res Involv Engagem. 2016;2(1):1–12.
Article Google Scholar
Manafò E, Petermann L, Vandall-Walker V, Mason-Lai P. Patient and public engagement in priority setting: a systematic rapid review of the literature. PLoS ONE. 2018;13(3):e0193579.
Dawson S, Ruddock A, Parmar V, Morris R, Cheraghi-Sohi S, Giles S, et al. Patient and public involvement in doctoral research: reflections and experiences of the PPI contributors and researcher. Res Involv Engagem. 2020;6(1):1–13.
Staley K, Buckland SA, Hayes H, Tarpey M. ‘The missing links’: understanding how context and mechanism influence the impact of public involvement in research. Health Expect. 2014;17(6):755–64.
Article PubMed Google Scholar
Ní Shé É, Cassidy J, Davies C, De Brún A, Donnelly S, Dorris E, et al. Minding the gap: identifying values to enable public and patient involvement at the pre-commencement stage of research projects. Res Involv Engagem. 2020;6(1):1–10.
Concannon T, Grant S, Welch V. Practical guidance for involving stakeholders in 626 health research. J Gen Intern Med. 2018;34:458–63.
Bec Hanley KS. User and carer involvement: sharing our experience. London: London Multicultural Community Association (LMCA); 2005.
Google Scholar
Tomlinson J, Medlinskiene K, Cheong V-L, Khan S, Fylan B. Patient and public involvement in designing and conducting doctoral research: the whys and the hows. Res Involv Engagem. 2019;5(1):1–12.
Horgan F, Lennon O, Hickey A, Sorensen J, Kroll T, McCartan D, et al. A protocol to evaluate the impact of embedding Public and Patient Involvement in a structured PhD programme for stroke care. Front Rehabilit Sci. 2022. https://doi.org/10.3389/fresc.2022.877598/pdf .
Foley L, Kiely B, Croke A, Larkin J, Smith SM, Clyne B, et al. A protocol for the evaluation of the process and impact of embedding formal and experiential public and patient involvement training in a structured PhD programme. J Multimorb Comorb. 2021;11:26335565211024790.
PubMed PubMed Central Google Scholar
Coupe N, Mathieson A. Patient and public involvement in doctoral research: impact, resources and recommendations. Health Expect. 2020;23(1):125–36.
Troya MI, Chew-Graham CA, Babatunde O, Bartlam B, Higginbottom A, Dikomitis L. Patient and public involvement and engagement in a doctoral research project exploring self-harm in older adults. Health Expect. 2019;22(4):617–31.
Manikandan M, Foley K, Gough J, Harrington S, Wall É, Weldon F, et al. Public and patient involvement in doctoral research during the COVID-19 pandemic: reflections on the process, challenges, impact and experiences from the perspectives of adults with cerebral palsy and the doctoral researcher. 2022.
Rees L, Sherwood M, Shields N. Consumer engagement in doctoral research–what difference does it make? Spinal Cord. 2022. https://doi.org/10.1038/s41393-022-00871-1 .
Jones B, Hunt A. Collaboration between doctoral researchers and patient research partners: reflections and considerations. Re All. 2022;6(1):1–9.
Staley K. Changing what researchers’ think and do’: is this how involvement impacts on research? Res All. 2017. https://doi.org/10.18546/RFA.01.1.13 .
Forsythe LP, Carman KL, Szydlowski V, Fayish L, Davidson L, Hickam DH, et al. Patient engagement in research: early findings from the Patient-Centered Outcomes Research Institute. Health Aff. 2019;38(3):359–67.
Tierney E, McEvoy R, O’Reilly-de Brún M, de Brún T, Okonkwo E, Rooney M, et al. A critical analysis of the implementation of service user involvement in primary care research and health service development using normalization process theory. Health Expect. 2016;19(3):501–15.
Telford R, Beverley CA, Cooper CL, Boote JD. Consumer involvement in health research: fact or fiction? Br J Clin Gov. 2002;7(2):92–103.
Locock L, Boaz A. Drawing straight lines along blurred boundaries: qualitative research, patient and public involvement in medical research, co-production and co-design. Evid Policy. 2019;15(3):409–21.
Barber R, Boote JD, Parry GD, Cooper CL, Yeeles P, Cook S. Can the impact of public involvement on research be evaluated? A mixed methods study. Health Expect. 2012;15(3):229–41.
Boote J, Baird W, Beecroft C. Public involvement at the design stage of primary health research: a narrative review of case examples. Health Policy (Amsterdam, Netherlands). 2010;95(1):10–23.
Madden M, Speed E. Beware zombies and unicorns: toward critical patient and public involvement in health research in a neoliberal context. Front Sociol. 2017;2:7.
Owyang D, Bakhsh A, Brewer D, Boughton OR, Cobb JP. Patient and public involvement within orthopaedic research: a systematic review. JBJS. 2021;103(13):e51.
Van der Scheer L, Garcia E, van der Laan AL, van der Burg S, Boenink M. The benefits of patient involvement for translational research. Health Care Anal. 2017;25:225–41.
Manafo E, Petermann L, Mason-Lai P, Vandall-Walker V. Patient engagement in Canada: a scoping review of the ‘how’and ‘what’of patient engagement in health research. Health Res Policy Syst. 2018;16(1):1–11.
Bammer G. Key issues in co-creation with stakeholders when research problems are complex. Evid Policy. 2019;15(3):423–35.
Carroll P, Dervan A, Maher A, McCarthy C, Woods I, Kavanagh R, et al. Applying patient and public involvement in preclinical research: a co-created scoping review. Health Expect. 2022;25(6):2680–99.
Ward PR, Thompson J, Barber R, Armitage CJ, Boote JD, Cooper CL, et al. Critical perspectives on ‘consumer involvement’in health research: epistemological dissonance and the know-do gap. J Sociol. 2010;46(1):63–82.
Boden C, Edmonds AM, Porter T, Bath B, Dunn K, Gerrard A, et al. Patient partners’ perspectives of meaningful engagement in synthesis reviews: a patient-oriented rapid review. Health Expect. 2021;24(4):1056–71.
Greenhalgh T, Hinton L, Finlay T, Macfarlane A, Fahy N, Clyde B, et al. Frameworks for supporting patient and public involvement in research: systematic review and co-design pilot. Health Expect. 2019;22(4):785–801.
Osborne SP, Radnor Z, Strokosch K. Co-production and the co-creation of value in public services: a suitable case for treatment? Public Manag Rev. 2016;18(5):639–53.
Bird M, Ouellette C, Whitmore C, Li L, Nair K, McGillion MH, et al. Preparing for patient partnership: a scoping review of patient partner engagement and evaluation in research. Health Expect. 2020;23(3):523–39.
Boote J, Baird W, Sutton A. Public involvement in the systematic review process in health and social care: a narrative review of case examples. Health Policy (Amsterdam, Netherlands). 2011;102(2–3):105–16.
Gregory S, Wells K, Forsyth K, Latto C, Szyra H, Saunders S, et al. Research participants as collaborators: background, experience and policies from the PREVENT Dementia and EPAD programmes. Dementia. 2018;17(8):1045–54.
Staley K, Minogue V. User involvement leads to more ethically sound research. Clin Eth. 2006;1(2):95–100.
Allemang B, Sitter K, Dimitropoulos G. Pragmatism as a paradigm for patient-oriented research. Health Expect. 2022;25(1):38–47.
Goel N. Enhancing patient research partner engagement: Research in psoriatic arthritis. Best Pract Res Clin Rheumatol. 2021;35(2):101685.
Gould D, Thuraisingam S, Shadbolt C, Knight J, Young J, Schilling C, et al. Cohort profile: the St Vincent’s melbourne arthroplasty outcomes (SMART) registry, a pragmatic prospective database defining outcomes in total hip and knee replacement patients. BMJ Open. 2021;11(1):e040408.
Gunatillake T, Shadbolt C, Gould D, Lam M, Hearst MG, Vleeskens C, et al. Embedding consumer and community involvement within an established research centre: moving from general recommendations to an actionable framework. Res Involv Engagem. 2020;6(1):1–8.
Kretchy IA, Okoibhole LO, Sanuade OA, Jennings H, Strachan DL, Blandford A, et al. Scoping review of community health participatory research projects in Ghana. Glob Health Act. 2022;15(1):2122304.
Thompson J, Barber R, Ward PR, Boote JD, Cooper CL, Armitage CJ, et al. Health researchers’ attitudes towards public involvement in health research. Health Expect. 2009;12(2):209–20.
Ng E, Jones AA, Sivapragasam M, Nath S, Mak LE, Rosenblum ND. The integration of clinical and research training: How and why MD–PhD programs work. Acad Med. 2019;94(5):664–70.
Dowsey M, Castle D, Knowles S, Monshat K, Salzberg M, Nelson E, et al. The effect of mindfulness training prior to total joint arthroplasty on post-operative pain and physical function: a randomised controlled trial. Complement Ther Med. 2019;46:195–201.
Staley K, Cockcroft E, Shelly A, Liabo K. ‘What can I do that will most help researchers?’A different approach to training the public at the start of their involvement in research. Res Involv Engagem. 2019;5(1):1–6.
Telford R, Boote JD, Cooper CL. What does it mean to involve consumers successfully in NHS research? A consensus study. Health Expect. 2004;7(3):209–20.
Staley K, Ashcroft J, Doughty L, Szmukler G. Making it clear and relevant: patients and carers add value to studies through research document reviews. Mental Health Soc Incl. 2016;20(1):36–43.
Boote J, Baird W, Sutton A. Involving the public in systematic reviews: a narrative review of organizational approaches and eight case examples. J Comp Effect Res. 2012;1(5):409–20.
Pollock A, Campbell P, Struthers C, Synnot A, Nunn J, Hill S, et al. Stakeholder involvement in systematic reviews: a scoping review. Syst Rev. 2018;7:1–26.
Bayliss K, Starling B, Raza K, Johansson EC, Zabalan C, Moore S, et al. Patient involvement in a qualitative meta-synthesis: lessons learnt. Res involv Engagem. 2016;2(1):1–19.
Synnot AJ, Cherry CL, Summers MP, Stuckey R, Milne CA, Lowe DB, et al. Consumer engagement critical to success in an Australian research project: reflections from those involved. Aust J Prim Health. 2018;24(3):197–203.
Pandya-Wood R, Barron DS, Elliott J. A framework for public involvement at the design stage of NHS health and social care research: time to develop ethically conscious standards. Res Involv Engagem. 2017;3(1):1–21.
McCarron TL, Moffat K, Wilkinson G, Zelinsky S, Boyd JM, White D, et al. Understanding patient engagement in health system decision-making: a co-designed scoping review. Syst Rev. 2019;8(1):1–10.
Price A, Albarqouni L, Kirkpatrick J, Clarke M, Liew SM, Roberts N, et al. Patient and public involvement in the design of clinical trials: an overview of systematic reviews. J Eval Clin Pract. 2018;24(1):240–53.
ACSQHC. Avoidable Hospital Readmissions: Report on Australian and International indicators, their use and the efficacy of interventions to reduce readmissions. Sydney: Australian Commission on Safety and Quality in Health Care. 2019.
Inacio MCS, Graves SE, Pratt NL, Roughead EE, Nemes S. Increase in total joint arthroplasty projected from 2014 to 2046 in Australia: a conservative local model with international implications. Clin Orthop Relat Res. 2017;475(8):2130–7.
Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780–5.
Carr AJ, Robertsson O, Graves S, Price AJ, Arden NK, Judge A, et al. Knee replacement. The Lancet. 2012;379(9823):1331–40.
Greenhalgh T, Jackson C, Shaw S, Janamian T. Achieving research impact through co-creation in community-based health services: literature review and case study. Milbank Q. 2016;94(2):392–429.
Harrison JD, Auerbach AD, Anderson W, Fagan M, Carnie M, Hanson C, et al. Patient stakeholder engagement in research: a narrative review to describe foundational principles and best practice activities. Health Expect. 2019;22(3):307–16.
Gould D, Dowsey MM, Spelman T, Jo O, Kabir W, Trieu J, et al. Patient-related risk factors for unplanned 30-day hospital readmission following primary and revision total knee arthroplasty: a systematic review and meta-analysis. J Clin Med. 2021;10(1):134.
Gould D, Dowsey M, Spelman T, Bailey J, Bunzli S, Rele S, et al. Established and novel risk factors for 30-day readmission following total knee arthroplasty: a modified delphi and focus group study to identify clinically important predictors. J Clin Med. 2023;12(3):747.
Gillard S, Simons L, Turner K, Lucock M, Edwards C. Patient and public involvement in the coproduction of knowledge: reflection on the analysis of qualitative data in a mental health study. Qual Health Res. 2012;22(8):1126–37.
Garfield S, Jheeta S, Husson F, Jacklin A, Bischler A, Norton C, et al. Lay involvement in the analysis of qualitative data in health services research: a descriptive study. Res Involv Engagem. 2016;2(1):1–12.
Frost J, Gibson A, Harris-Golesworthy F, Harris J, Britten N. Patient involvement in qualitative data analysis in a trial of a patient-centred intervention: reconciling lay knowledge and scientific method. Health Expect. 2018;21(6):1111–21.
Hemming L, Pratt D, Bhatti P, Shaw J, Haddock G. Involving an individual with lived-experience in a co-analysis of qualitative data. Health Expect. 2021;24(3):766–75.
Boote J, Wong R, Booth A. ‘Talking the talk or walking the walk?’A bibliometric review of the literature on public involvement in health research published between 1995 and 2009. Health Expect. 2015;18(1):44–57.
Staniszewska S, Brett J, Simera I, Seers K, Mockford C, Goodlad S, et al. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. BMJ (Clin Res Ed). 2017;358:j3453.
Article CAS Google Scholar
Braun V, Clarke V. Reflecting on reflexive thematic analysis. Qual Res Sport Exer Health. 2019;11(4):589–97.
Staley K, Abbey-Vital I, Nolan C. The impact of involvement on researchers: a learning experience. Res Involv Engagem. 2017;3(1):1–9.
Staniszewska S, Denegri S, Matthews R, Minogue V. Reviewing progress in public involvement in NIHR research: developing and implementing a new vision for the future. BMJ Open. 2018;8(7):e017124.
Preston J, Nafria B, Ohmer A, Gaillard S, Dicks P, West L, et al. Developing a more tailored approach to patient and public involvement with children and families in pediatric clinical research: lessons learned. Ther Innov Regul Sci. 2022;56(6):948–63.
Article CAS PubMed PubMed Central Google Scholar
Chudyk AM, Horrill T, Waldman C, Demczuk L, Shimmin C, Stoddard R, et al. Scoping review of models and frameworks of patient engagement in health services research. BMJ Open. 2022;12(8):e063507.
Vleeskens C. Researchers and the community in partnership- a consumer perspective. health beyond research & innovation showcase; Liverpool, New South Wales, Australia. RESEARCH4ME: Sydney Partnership for Health, Education, Research and Enterprise (SPHERE) Musculoskeletal Health Clinical Academic Group (MSK CAG); 2019.
Boote J, Barber R, Cooper C. Principles and indicators of successful consumer involvement in NHS research: results of a Delphi study and subgroup analysis. Health Policy (Amsterdam, Netherlands). 2006;75(3):280–97.
Staley K. ‘Is it worth doing?’Measuring the impact of patient and public involvement in research. Res Involv Engagem. 2015;1(1):1–10.
Staley K. An evaluation of a pilot project of PPI in research at Parkinson's UK. Edited by Parkinson's UK. Parkinson’s UK London; 2016.
Brett J, Staniszewska S, Mockford C, Herron-Marx S, Hughes J, Tysall C, et al. A systematic review of the impact of patient and public involvement on service users, researchers and communities. Pat-Pat-Center Outcomes Res. 2014;7(4):387–95.
Wilson P, Mathie E, Keenan J, McNeilly E, Goodman C, Howe A, et al. ReseArch with patient and Public invOlvement: a realisT evaluation: the RAPPORT study. Health Serv Deliv Res 2015.
Turner G, Aiyegbusi OL, Price G, Skrybant M, Calvert M. Moving beyond project-specific patient and public involvement in research. J R Soc Med. 2020;113(1):16–23.
Shippee ND, Domecq Garces JP, Prutsky Lopez GJ, Wang Z, Elraiyah TA, Nabhan M, et al. Patient and service user engagement in research: a systematic review and synthesized framework. Health Expect. 2015;18(5):1151–66.
Brett J, Staniszewska S, Mockford C, Herron-Marx S, Hughes J, Tysall C, et al. Mapping the impact of patient and public involvement on health and social care research: a systematic review. Health Expect. 2014;17(5):637–50.
Desborough J, Parkinson A, Lewis F, Ebbeck H, Banfield M, Phillips C. A framework for involving coproduction partners in research about young people with type 1 diabetes. Health Expect. 2022;25(1):430–42.
Bahadori S, Collard S, Williams JM, Swain I. Why do people undergo THR and what do they expect to gain: a comparison of the views of patients and health care professionals. J Pat Exp. 2020;7(6):1778–87.
Montreuil M, Martineau JT, Racine E. Exploring ethical issues related to patient engagement in healthcare: patient, clinician and researcher’s perspectives. J Bioeth Inq. 2019;16(2):237–48.
Dijk SW, Duijzer EJ, Wienold M. Role of active patient involvement in undergraduate medical education: a systematic review. BMJ Open. 2020;10(7):e037217.
Barr J, Bull R, Rooney K. Developing a patient focussed professional identity: an exploratory investigation of medical students’ encounters with patient partnership in learning. Adv Health Sci Educ. 2015;20(2):325–38.
Street J, Stafinski T, Lopes E, Menon D. Defining the role of the public in health technology assessment (HTA) and HTA-informed decision-making processes. Int J Technol Assess Health Care. 2020;36(2):87–95.
Baines R, Bradwell H, Edwards K, Stevens S, Prime S, Tredinnick-Rowe J, et al. Meaningful patient and public involvement in digital health innovation, implementation and evaluation: a systematic review. Health Expect. 2022;25(4):1232–45.
Staley K, Barron D. Learning as an outcome of involvement in research: what are the implications for practice, reporting and evaluation? Res Involv Engagem. 2019;5(1):1–9.
Staley K. The BMJ Opinion [Internet] 2018. https://blogs.bmj.com/bmj/2018/11/28/researchers-dont-know-what-theyre-missing-the-impact-of-patient-involvement-in-research/ . Accessed 01 Feb 2023.
Edelman N, Barron D. Evaluation of public involvement in research: time for a major re-think? J Health Serv Res Policy. 2016;21(3):209–11.
Esmail L, Moore E, Rein A. Evaluating patient and stakeholder engagement in research: moving from theory to practice. J Comparative Effect Res. 2015;4(2):133–45.
Boivin A, L’Espérance A, Gauvin FP, Dumez V, Macaulay AC, Lehoux P, et al. Patient and public engagement in research and health system decision making: a systematic review of evaluation tools. Health Expect. 2018;21(6):1075–84.
Barber R, Beresford P, Boote J, Cooper C, Faulkner A. Evaluating the impact of service user involvement on research: a prospective case study. Int J Consum Stud. 2011;35(6):609–15.
Stergiopoulos S, Michaels DL, Kunz BL, Getz KA. Measuring the impact of patient engagement and patient centricity in clinical research and development. Ther Innov Regul Sci. 2020;54:103–16.
Pizzo E, Doyle C, Matthews R, Barlow J. Patient and public involvement: how much do we spend and what are the benefits? Health Expect. 2015;18(6):1918–26.
Russell J, Fudge N, Greenhalgh T. The impact of public involvement in health research: what are we measuring? Why are we measuring it? Should we stop measuring it? Res Involv Engagem. 2020;6(1):1–8.
Domecq JP, Prutsky G, Elraiyah T, Wang Z, Nabhan M, Shippee N, et al. Patient engagement in research: a systematic review. BMC Health Serv Res. 2014;14(1):1–9.
Fumagalli LP, Radaelli G, Lettieri E, Masella C. Patient empowerment and its neighbours: clarifying the boundaries and their mutual relationships. Health Policy (Amsterdam, Netherlands). 2015;119(3):384–94.
Hyde C, Dunn KM, Higginbottom A, Chew-Graham CA. Process and impact of patient involvement in a systematic review of shared decision making in primary care consultations. Health Expect. 2017;20(2):298–308.
Silberberg M, Martinez-Bianchi V. Community and stakeholder engagement. Prim Care: Clin Office Pract. 2019;46(4):587–94.
Nilsen ES, Myrhaug HT, Johansen M, Oliver S, Oxman AD. Methods of consumer involvement in developing healthcare policy and research, clinical practice guidelines and patient information material. Cochrane Database Syst Rev. 2006. https://doi.org/10.1002/14651858.CD004563.pub2/abstract .
Article PubMed Central Google Scholar
Wright MT. What is participatory health research? A position paper of the international collaboration for participatory health research. Prävention und Gesundheitsförderung. 2013;8:122–31.
Staley K, Sandvei M, Horder M. A problem shared…’The challenges of public involvement for researchers in Denmark and the UK. UK: University of Southern Denmark and TwoCan Associates; 2019.
Staley K, Crowe S. More than a top 10: How James Lind alliance priority setting partnerships transform research, people and organisations. NIHR Oxford Biomedical Research Centre, UK-September. 2019.
Staley K. A series of case studies illustrating the impact of service user and carer involvement on research. London: MHRN; 2013.
Barber R, Boote JD, Cooper CL. Involving consumers successfully in NHS research: a national survey. Health Expect. 2007;10(4):380–91.
Staley K, Elliott J. Public involvement could usefully inform ethical review, but rarely does: what are the implications? Res Involv Engagem. 2017;3(1):1–17.
Muir R, Carlini JJ, Harbeck EL, Gillespie BM, Tuffaha HW, Walker RM, et al. Patient involvement in surgical wound care research: a scoping review. Int Wound J. 2020;17(5):1462–82.
Chambers E, Gardiner C, Thompson J, Seymour J. Patient and carer involvement in palliative care research: an integrative qualitative evidence synthesis review. Palliat Med. 2019;33(8):969–84.
Camelo Castillo W, Heath N, Kim J, Yang K, Ritchey ME, dosReis S, et al. Engaging stakeholders in pharmacoepidemiology research: Current state and recommendations. Pharmacoepidemiol Drug Saf. 2019;28(6):766–76.
Miah J, Dawes P, Edwards S, Leroi I, Starling B, Parsons S. Patient and public involvement in dementia research in the European Union: a scoping review. BMC Geriatr. 2019;19(1):1–20.
Dadich A, Wyer M. Patient involvement in healthcare-associated infection research: a lexical review. Infect Control Hosp Epidemiol. 2018;39(6):710–7.
Kaisler RE, Kulnik ST, Klager E, Kletecka-Pulker M, Schaden E, Stainer-Hochgatterer A. Introducing patient and public involvement practices to healthcare research in Austria: strategies to promote change at multiple levels. BMJ Open. 2021;11(8):e045618.
Cook N, Siddiqi N, Twiddy M, Kenyon R. Patient and public involvement in health research in low and middle-income countries: a systematic review. BMJ Open. 2019;9(5):e026514.
Oldfield BJ, Harrison MA, Genao I, Greene AT, Pappas ME, Glover JG, et al. Patient, family, and community advisory councils in health care and research: a systematic review. J Gen Intern Med. 2019;34:1292–303.
Guise J-M, O’Haire C, McPheeters M, Most C, LaBrant L, Lee K, et al. A practice-based tool for engaging stakeholders in future research: a synthesis of current practices. J Clin Epidemiol. 2013;66(6):666–74.
Castro EM, Van Regenmortel T, Vanhaecht K, Sermeus W, Van Hecke A. Patient empowerment, patient participation and patient-centeredness in hospital care: a concept analysis based on a literature review. Pat Educ Couns. 2016;99(12):1923–39.
Del Gaizo V, Kohlheim M. Patient engagement in pediatric rheumatology research. Rheum Dis Clin. 2022;48(1):1–13.
Harris J, Haltbakk J, Dunning T, Austrheim G, Kirkevold M, Johnson M, et al. How patient and community involvement in diabetes research influences health outcomes: a realist review. Health Expect. 2019;22(5):907–20.
Wiering B, de Boer D, Delnoij D. Patient involvement in the development of patient-reported outcome measures: a scoping review. Health Expect. 2017;20(1):11–23.
Burton A, Ogden M, Cooper C. Planning and enabling meaningful patient and public involvement in dementia research. Curr Opin Psychiatry. 2019;32(6):557–62.
Lee DJ, Avulova S, Conwill R, Barocas DA, editors. Patient engagement in the design and execution of urologic oncology research. Urologic Oncology: Seminars and Original Investigations; 2017: Elsevier.
Bethell J, Commisso E, Rostad HM, Puts M, Babineau J, Grinbergs-Saull A, et al. Patient engagement in research related to dementia: a scoping review. Dementia. 2018;17(8):944–75.
Tivey A, Huddar P, Shotton R, Cheese I, Daniels S, Lorigan P, et al. Patient engagement in melanoma research: from bench to bedside. Future Oncol. 2021;17(28):3705–16.
Article CAS PubMed Google Scholar
Davies-Teye BB, Medeiros M, Chauhan C, Baquet CR, Mullins CD. Pragmatic patient engagement in designing pragmatic oncology clinical trials. Fut Oncol. 2021;17(28):3691–704.
Harting J, Kruithof K, Ruijter L, Stronks K. Participatory research in health promotion: a critical review and illustration of rationales. Health Promot Int. 2022;37(Supplement_2):ii7–20.
Puts MT, Sattar S, Ghodraty-Jabloo V, Hsu T, Fitch M, Szumacher E, et al. Patient engagement in research with older adults with cancer. J Geriatr Oncol. 2017;8(6):391–6.
Cook T, Boote J, Buckley N, Vougioukalou S, Wright M. Accessing participatory research impact and legacy: developing the evidence base for participatory approaches in health research. Educ Act Res. 2017;25(4):473–88.
Slåtsveen R-E, Wibe T, Halvorsrud L, Lund A. Needs-led research: a way of employing user involvement when devising research questions on the trust model in community home-based health care services in Norway. Res Involvd Engagem. 2021;7(1):1–10.
Entwistle VA, Renfrew MJ, Yearley S, Forrester J, Lamont T. Lay perspectives: advantages for health research. BMJ (Clin Res Ed). 1998;316(7129):463–6.
Chalmers I. What do I want from health research and researchers when I am a patient? BMJ (Clin Res Ed). 1995;310(6990):1315–8.
Oliver S, Liabo K, Stewart R, Rees R. Public involvement in research: making sense of the diversity. J Health Serv Res Policy. 2015;20(1):45–51.
Fudge N, Wolfe C, McKevitt C. Involving older people in health research. Age Ageing. 2007;36(5):492–500.
Crockett LK, Shimmin C, Wittmeier KD, Sibley KM. Engaging patients and the public in health research: experiences, perceptions and training needs among Manitoba health researchers. Res Involv Engagem. 2019;5(1):1–11.
Parkinson B. Patient and public involvement for mental health researchers. Mental Health Pract. 2021;24(2):22–6.
Blair C, Best P, Burns P, Campbell A, Davidson G, Duffy J, et al. ‘Getting involved in research’: a co-created, co-delivered and co-analysed course for those with lived experience of health and social care services. Res Involv Engagem. 2022;8(1):1–16.
Cox R, Kendall M, Molineux M, Miller E, Tanner B. Consumer engagement in occupational therapy health-related research: a scoping review of the Australian occupational therapy journal and a call to action. Aust Occup Ther J. 2021;68(2):180–92.
Dudley L, Gamble C, Preston J, Buck D, Group EPA, Hanley B, et al. What difference does patient and public involvement make and what are its pathways to impact? Qualitative study of patients and researchers from a cohort of randomised clinical trials. PLoS ONE. 2015;10(6):e128817.
Troya MI, Bartlam B, Chew-Graham CA. Involving the public in health research in Latin America: making the case for mental health. Rev Panam Salud Publica. 2018;42:e45.
Jinks C, Carter P, Rhodes C, Taylor R, Beech R, Dziedzic K, et al. Patient and public involvement in primary care research-an example of ensuring its sustainability. Res Involv Engagem. 2016;2(1):1–12.
Wiggins A, Wilbanks J. The rise of citizen science in health and biomedical research. Am J Bioeth. 2019;19(8):3–14.
Staley K. An evaluation of service user involvement in studies adopted by the mental health research network. Methods. 2012;10(1):1–82.
Staley K. Lay REC members: patient or public? J Med Eth. 2013;39(12):780–2.
Hamilton CB, Leese JC, Hoens AM, Li LC. Framework for advancing the reporting of patient engagement in rheumatology research projects. Curr Rheumatol Rep. 2017;19:1–10.
Staley K. There is no paradox with PPI in research. J Med Eth. 2013;39(3):186–7.
Download references
Acknowledgements
We acknowledge the work of Dr Tilini Gunatillake and Dr Michelle Lam in establishing the OPUS CCI program. We also acknowledge the work of Dr Elizabeth Nelson, Claire Weeden and Lauren Patten in leading, administering, maintaining, and expanding the program. MMD is supported by a University of Melbourne Dame Kate Campbell Fellowship. PFC holds a National Health & Medical Research Council Practitioner Fellowship (GNT1154203)
No funding was received directly for this study. DG, MGH, and SB receive no funding. PFC had the following funding sources to declare: Royalties from Johnson and Johnson, Consultancy with Johnson & Johnson, Consultancy with Stryker Corportation (paid personally); Australian National Health & Medical Research Council Practitioner Fellowship (paid to institution), HCF Foundation, BUPA Foundation, St. Vincents Health Australia, Australian Research Council, (Grant support provided to institution for research unrelated to the current manuscript); Axcelda cartilage regeneration project, Patent applied for device, composition of matter and process (institution and personally). MMD had the following funding sources to declare: National Health and Medical Research Council, HCF Foundation, BUPA Foundation, St. Vincents Health Australia, Australian Research Council, (Grant support provided to my institution for research unrelated to the current manuscript).
Author information
Authors and affiliations.
Department of Surgery, St. Vincent’s Hospital, University of Melbourne, Melbourne, VIC, Australia
Daniel J. Gould, Marion Glanville-Hearst, Peter F. M. Choong & Michelle M. Dowsey
School of Health Sciences and Social Work, Griffith University, Nathan Campus, Brisbane, QLD, Australia
Samantha Bunzli
Physiotherapy Department, Royal Brisbane and Women’s Hospital, Brisbane, QLD, Australia
Department of Orthopaedics, St. Vincent’s Hospital, Melbourne, VIC, Australia
Peter F. M. Choong & Michelle M. Dowsey
You can also search for this author in PubMed Google Scholar
Contributions
DG and MGH conceptualised the manuscript and wrote the initial draft, with PFC, MMD, and SB providing intellectual content. All authors contributed to discussions in which the recommendations were synthesised and refined. All authors contributed to revising the manuscript prior to submission, and have all reviewed and approved the final manuscript. All authors agree to be accountable for all aspects of the manuscript and will work together to ensure questions relating to the accuracy and integrity of any part of it are appropriately investigated and resolved. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Daniel J. Gould .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
DG, MGH, and SB have no competing interests. PFC has the following to declare: Chair, Research Committee, Australian Orthopaedic Association (now completed term); Emeritus Board Member Musculoskeletal Australia. MMD has the following to declare: Chair, Australian Orthopaedic Association Research Foundation, Research Advisory Committee.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Gould, D.J., Glanville-Hearst, M., Bunzli, S. et al. Research Buddy partnership in a MD–PhD program: lessons learned. Res Involv Engagem 9 , 4 (2023). https://doi.org/10.1186/s40900-023-00414-9
Download citation
Received : 19 October 2022
Accepted : 14 February 2023
Published : 18 February 2023
DOI : https://doi.org/10.1186/s40900-023-00414-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Partnership
- Involvement
- Research Buddy
Research Involvement and Engagement
ISSN: 2056-7529
- General enquiries: [email protected]
Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, generate accurate citations for free.
- Knowledge Base
Methodology
Research Methods | Definitions, Types, Examples
Research methods are specific procedures for collecting and analyzing data. Developing your research methods is an integral part of your research design . When planning your methods, there are two key decisions you will make.
First, decide how you will collect data . Your methods depend on what type of data you need to answer your research question :
- Qualitative vs. quantitative : Will your data take the form of words or numbers?
- Primary vs. secondary : Will you collect original data yourself, or will you use data that has already been collected by someone else?
- Descriptive vs. experimental : Will you take measurements of something as it is, or will you perform an experiment?
Second, decide how you will analyze the data .
- For quantitative data, you can use statistical analysis methods to test relationships between variables.
- For qualitative data, you can use methods such as thematic analysis to interpret patterns and meanings in the data.
Table of contents
Methods for collecting data, examples of data collection methods, methods for analyzing data, examples of data analysis methods, other interesting articles, frequently asked questions about research methods.
Data is the information that you collect for the purposes of answering your research question . The type of data you need depends on the aims of your research.
Qualitative vs. quantitative data
Your choice of qualitative or quantitative data collection depends on the type of knowledge you want to develop.
For questions about ideas, experiences and meanings, or to study something that can’t be described numerically, collect qualitative data .
If you want to develop a more mechanistic understanding of a topic, or your research involves hypothesis testing , collect quantitative data .
| Qualitative | to broader populations. . | |
|---|---|---|
| Quantitative | . |
You can also take a mixed methods approach , where you use both qualitative and quantitative research methods.
Primary vs. secondary research
Primary research is any original data that you collect yourself for the purposes of answering your research question (e.g. through surveys , observations and experiments ). Secondary research is data that has already been collected by other researchers (e.g. in a government census or previous scientific studies).
If you are exploring a novel research question, you’ll probably need to collect primary data . But if you want to synthesize existing knowledge, analyze historical trends, or identify patterns on a large scale, secondary data might be a better choice.
| Primary | . | methods. |
|---|---|---|
| Secondary |
Descriptive vs. experimental data
In descriptive research , you collect data about your study subject without intervening. The validity of your research will depend on your sampling method .
In experimental research , you systematically intervene in a process and measure the outcome. The validity of your research will depend on your experimental design .
To conduct an experiment, you need to be able to vary your independent variable , precisely measure your dependent variable, and control for confounding variables . If it’s practically and ethically possible, this method is the best choice for answering questions about cause and effect.
| Descriptive | . . | |
|---|---|---|
| Experimental |
Prevent plagiarism. Run a free check.
| Research method | Primary or secondary? | Qualitative or quantitative? | When to use |
|---|---|---|---|
| Primary | Quantitative | To test cause-and-effect relationships. | |
| Primary | Quantitative | To understand general characteristics of a population. | |
| Interview/focus group | Primary | Qualitative | To gain more in-depth understanding of a topic. |
| Observation | Primary | Either | To understand how something occurs in its natural setting. |
| Secondary | Either | To situate your research in an existing body of work, or to evaluate trends within a research topic. | |
| Either | Either | To gain an in-depth understanding of a specific group or context, or when you don’t have the resources for a large study. |
Your data analysis methods will depend on the type of data you collect and how you prepare it for analysis.
Data can often be analyzed both quantitatively and qualitatively. For example, survey responses could be analyzed qualitatively by studying the meanings of responses or quantitatively by studying the frequencies of responses.
Qualitative analysis methods
Qualitative analysis is used to understand words, ideas, and experiences. You can use it to interpret data that was collected:
- From open-ended surveys and interviews , literature reviews , case studies , ethnographies , and other sources that use text rather than numbers.
- Using non-probability sampling methods .
Qualitative analysis tends to be quite flexible and relies on the researcher’s judgement, so you have to reflect carefully on your choices and assumptions and be careful to avoid research bias .
Quantitative analysis methods
Quantitative analysis uses numbers and statistics to understand frequencies, averages and correlations (in descriptive studies) or cause-and-effect relationships (in experiments).
You can use quantitative analysis to interpret data that was collected either:
- During an experiment .
- Using probability sampling methods .
Because the data is collected and analyzed in a statistically valid way, the results of quantitative analysis can be easily standardized and shared among researchers.
| Research method | Qualitative or quantitative? | When to use |
|---|---|---|
| Quantitative | To analyze data collected in a statistically valid manner (e.g. from experiments, surveys, and observations). | |
| Meta-analysis | Quantitative | To statistically analyze the results of a large collection of studies. Can only be applied to studies that collected data in a statistically valid manner. |
| Qualitative | To analyze data collected from interviews, , or textual sources. To understand general themes in the data and how they are communicated. | |
| Either | To analyze large volumes of textual or visual data collected from surveys, literature reviews, or other sources. Can be quantitative (i.e. frequencies of words) or qualitative (i.e. meanings of words). |
If you want to know more about statistics , methodology , or research bias , make sure to check out some of our other articles with explanations and examples.
- Chi square test of independence
- Statistical power
- Descriptive statistics
- Degrees of freedom
- Pearson correlation
- Null hypothesis
- Double-blind study
- Case-control study
- Research ethics
- Data collection
- Hypothesis testing
- Structured interviews
Research bias
- Hawthorne effect
- Unconscious bias
- Recall bias
- Halo effect
- Self-serving bias
- Information bias
Quantitative research deals with numbers and statistics, while qualitative research deals with words and meanings.
Quantitative methods allow you to systematically measure variables and test hypotheses . Qualitative methods allow you to explore concepts and experiences in more detail.
In mixed methods research , you use both qualitative and quantitative data collection and analysis methods to answer your research question .
A sample is a subset of individuals from a larger population . Sampling means selecting the group that you will actually collect data from in your research. For example, if you are researching the opinions of students in your university, you could survey a sample of 100 students.
In statistics, sampling allows you to test a hypothesis about the characteristics of a population.
The research methods you use depend on the type of data you need to answer your research question .
- If you want to measure something or test a hypothesis , use quantitative methods . If you want to explore ideas, thoughts and meanings, use qualitative methods .
- If you want to analyze a large amount of readily-available data, use secondary data. If you want data specific to your purposes with control over how it is generated, collect primary data.
- If you want to establish cause-and-effect relationships between variables , use experimental methods. If you want to understand the characteristics of a research subject, use descriptive methods.
Methodology refers to the overarching strategy and rationale of your research project . It involves studying the methods used in your field and the theories or principles behind them, in order to develop an approach that matches your objectives.
Methods are the specific tools and procedures you use to collect and analyze data (for example, experiments, surveys , and statistical tests ).
In shorter scientific papers, where the aim is to report the findings of a specific study, you might simply describe what you did in a methods section .
In a longer or more complex research project, such as a thesis or dissertation , you will probably include a methodology section , where you explain your approach to answering the research questions and cite relevant sources to support your choice of methods.
Is this article helpful?
Other students also liked, writing strong research questions | criteria & examples.
- What Is a Research Design | Types, Guide & Examples
- Data Collection | Definition, Methods & Examples
More interesting articles
- Between-Subjects Design | Examples, Pros, & Cons
- Cluster Sampling | A Simple Step-by-Step Guide with Examples
- Confounding Variables | Definition, Examples & Controls
- Construct Validity | Definition, Types, & Examples
- Content Analysis | Guide, Methods & Examples
- Control Groups and Treatment Groups | Uses & Examples
- Control Variables | What Are They & Why Do They Matter?
- Correlation vs. Causation | Difference, Designs & Examples
- Correlational Research | When & How to Use
- Critical Discourse Analysis | Definition, Guide & Examples
- Cross-Sectional Study | Definition, Uses & Examples
- Descriptive Research | Definition, Types, Methods & Examples
- Ethical Considerations in Research | Types & Examples
- Explanatory and Response Variables | Definitions & Examples
- Explanatory Research | Definition, Guide, & Examples
- Exploratory Research | Definition, Guide, & Examples
- External Validity | Definition, Types, Threats & Examples
- Extraneous Variables | Examples, Types & Controls
- Guide to Experimental Design | Overview, Steps, & Examples
- How Do You Incorporate an Interview into a Dissertation? | Tips
- How to Do Thematic Analysis | Step-by-Step Guide & Examples
- How to Write a Literature Review | Guide, Examples, & Templates
- How to Write a Strong Hypothesis | Steps & Examples
- Inclusion and Exclusion Criteria | Examples & Definition
- Independent vs. Dependent Variables | Definition & Examples
- Inductive Reasoning | Types, Examples, Explanation
- Inductive vs. Deductive Research Approach | Steps & Examples
- Internal Validity in Research | Definition, Threats, & Examples
- Internal vs. External Validity | Understanding Differences & Threats
- Longitudinal Study | Definition, Approaches & Examples
- Mediator vs. Moderator Variables | Differences & Examples
- Mixed Methods Research | Definition, Guide & Examples
- Multistage Sampling | Introductory Guide & Examples
- Naturalistic Observation | Definition, Guide & Examples
- Operationalization | A Guide with Examples, Pros & Cons
- Population vs. Sample | Definitions, Differences & Examples
- Primary Research | Definition, Types, & Examples
- Qualitative vs. Quantitative Research | Differences, Examples & Methods
- Quasi-Experimental Design | Definition, Types & Examples
- Questionnaire Design | Methods, Question Types & Examples
- Random Assignment in Experiments | Introduction & Examples
- Random vs. Systematic Error | Definition & Examples
- Reliability vs. Validity in Research | Difference, Types and Examples
- Reproducibility vs Replicability | Difference & Examples
- Reproducibility vs. Replicability | Difference & Examples
- Sampling Methods | Types, Techniques & Examples
- Semi-Structured Interview | Definition, Guide & Examples
- Simple Random Sampling | Definition, Steps & Examples
- Single, Double, & Triple Blind Study | Definition & Examples
- Stratified Sampling | Definition, Guide & Examples
- Structured Interview | Definition, Guide & Examples
- Survey Research | Definition, Examples & Methods
- Systematic Review | Definition, Example, & Guide
- Systematic Sampling | A Step-by-Step Guide with Examples
- Textual Analysis | Guide, 3 Approaches & Examples
- The 4 Types of Reliability in Research | Definitions & Examples
- The 4 Types of Validity in Research | Definitions & Examples
- Transcribing an Interview | 5 Steps & Transcription Software
- Triangulation in Research | Guide, Types, Examples
- Types of Interviews in Research | Guide & Examples
- Types of Research Designs Compared | Guide & Examples
- Types of Variables in Research & Statistics | Examples
- Unstructured Interview | Definition, Guide & Examples
- What Is a Case Study? | Definition, Examples & Methods
- What Is a Case-Control Study? | Definition & Examples
- What Is a Cohort Study? | Definition & Examples
- What Is a Conceptual Framework? | Tips & Examples
- What Is a Controlled Experiment? | Definitions & Examples
- What Is a Double-Barreled Question?
- What Is a Focus Group? | Step-by-Step Guide & Examples
- What Is a Likert Scale? | Guide & Examples
- What Is a Prospective Cohort Study? | Definition & Examples
- What Is a Retrospective Cohort Study? | Definition & Examples
- What Is Action Research? | Definition & Examples
- What Is an Observational Study? | Guide & Examples
- What Is Concurrent Validity? | Definition & Examples
- What Is Content Validity? | Definition & Examples
- What Is Convenience Sampling? | Definition & Examples
- What Is Convergent Validity? | Definition & Examples
- What Is Criterion Validity? | Definition & Examples
- What Is Data Cleansing? | Definition, Guide & Examples
- What Is Deductive Reasoning? | Explanation & Examples
- What Is Discriminant Validity? | Definition & Example
- What Is Ecological Validity? | Definition & Examples
- What Is Ethnography? | Definition, Guide & Examples
- What Is Face Validity? | Guide, Definition & Examples
- What Is Non-Probability Sampling? | Types & Examples
- What Is Participant Observation? | Definition & Examples
- What Is Peer Review? | Types & Examples
- What Is Predictive Validity? | Examples & Definition
- What Is Probability Sampling? | Types & Examples
- What Is Purposive Sampling? | Definition & Examples
- What Is Qualitative Observation? | Definition & Examples
- What Is Qualitative Research? | Methods & Examples
- What Is Quantitative Observation? | Definition & Examples
- What Is Quantitative Research? | Definition, Uses & Methods
"I thought AI Proofreading was useless but.."
I've been using Scribbr for years now and I know it's a service that won't disappoint. It does a good job spotting mistakes”

Clinical Research
NIH defines human clinical research as research with human subjects that is:
(1) Patient-Oriented Research. Research Conducted with human subjects (or on material of human origin such as tissues, specimens, and cognitive phenomena) for which an investigator (or colleague) directly interacts with human subjects. Excluded from this definition are in vitro studies that utilize human tissues that cannot be linked to a living individual. Patient-oriented research includes:
(a) mechanisms of human disease,
(b) therapeutic interventions,
(c) clinical studies, or
(d) development of new technologies.
(2) Epidemiologic and Behavioral Studies.
(3) Outcomes Research and Health Services Research.
Note: Studies falling under Exemption 4 for human subjects research are not considered clinical research by this definition.
Clinical Trial*
The NIH defines a clinical trial as a research study in which one or more human subjects are prospectively assigned to one or more interventions (which may include placebo or other control) to evaluate the effects of those interventions on health-related biomedical or behavioral outcomes.
- The term "prospectively assigned" refers to a pre-defined process (e.g., randomization) specified in an approved protocol that stipulates the assignment of research subjects (individually or in clusters) to one or more arms (e.g., intervention, placebo or other control) of the clinical trial.
- An intervention is defined as a manipulation of the subject or subject's environment for the purpose of modifying one or more health-related processes and/or endpoints. Examples include, but are not limited, to: drugs/small molecules/compounds, biologics, devices; procedures (e.g., surgical techniques); delivery systems (e.g., telemedicine, face-to-face); strategies to change health-related behavior (e.g., diet, cognitive therapy, exercise, development of new habits); and, treatment, prevention, and diagnostic strategies.
- A health-related biomedical or behavioral outcome is defined as the pre-specified effect of an intervention on the study subjects. Examples include positive or negative changes to physiological or biological parameters (e.g., improvement of lung capacity, gene expression); psychological or neurodevelopmental parameters (e.g., mood management intervention for smokers; reading comprehension and/or information retention); disease processes; health-related behavior; and, well-being or quality of life.
- Biomedical clinical trials of an experimental drug, treatment, device, or behavioral intervention may proceed through four phases: Phase I. Tests a new biomedical intervention in a small group of people (e.g. 20-80) for the first time to determine efficacy and evaluate safety (e.g., determine a safe dosage range and identify side effects). Phase II. Study the biomedical or behavioral intervention in a larger group of people (several hundred) to determine efficacy and further evaluate safety. Phase III . Study to determine efficacy of the biomedical or behavioral intervention in large groups of people (from several hundred to several thousand) by comparing the intervention to other standard or experimental interventions as well as to monitor adverse effects, and to collect information that will allow the interventions to be used safely. NIH-Defined Phase III Clinical Trial. For the purpose of the Guidelines an NIH-defined Phase III clinical trial is a broadly based prospective Phase III clinical investigation, usually involving several hundred or more human subjects, for the purpose of evaluating an experimental intervention in comparison with a standard or controlled intervention or comparing two or more existing treatments. Often the aim of such investigation is to provide evidence leading to a scientific basis for consideration of a change in health policy or standard of care. The definition includes pharmacologic, non-pharmacologic, and behavioral interventions given for disease prevention, prophylaxis, diagnosis, or therapy. Community trials and other population-based intervention trials are also included. Phase IV. Studies conducted after the intervention has been marketed. These studies are designed to monitor the effectiveness of the approved intervention in the general population and to collect information about any adverse effects associated with widespread use.
* Reminder: Ensure compliance with clinicaltrials.gov registration requirements for applicable clinical trials
Is your study a Clinical Trial?
See the Clinical Trials page to determine if your study is a clinical trial and what you need to know when conducting a clinical trial. [ HTML ]
Investigational Drug Service [ HTML ] Phone: 859-2178-0630
Office of Sponsored Projects Administration (OSPA) [ HTML ] Email: [email protected]
Proposal Development Office (PDO) [ HTML ] Email: [email protected]
Center for Clinical and Translational Science (CCTS) [ HTML ] Email: [email protected]
Clinical Research Support Office (CRSO) [ HTML ] Email: [email protected]
- Dictionaries home
- American English
- Collocations
- German-English
- Grammar home
- Practical English Usage
- Learn & Practise Grammar (Beta)
- Word Lists home
- My Word Lists
- Recent additions
- Resources home
- Text Checker
Definition of buddy noun from the Oxford Advanced Learner's Dictionary
- an old college buddy of mine
- Howard and Mick were drinking buddies.
- We were good buddies.
- I’d like you to meet an old college buddy of mine.
- acquaintance
Take your English to the next level
The Oxford Learner’s Thesaurus explains the difference between groups of similar words. Try it for free as part of the Oxford Advanced Learner’s Dictionary app

Research Buddy - new update in June
Thank you for all the great feedback we received. We literally went through them all, there were alot of great ideas and feature requests aswell as a few bugs that needs ironing out. We are currently working on the next update, which will include most of the top requested features. We are very excited for the release in June. Until then hold your research pappers and stay curious.
An official website of the United States Government
- Kreyòl ayisyen
- Search Toggle search Search Include Historical Content - Any - No Include Historical Content - Any - No Search
- Menu Toggle menu
- INFORMATION FOR…
- Individuals
- Business & Self Employed
- Charities and Nonprofits
- International Taxpayers
- Federal State and Local Governments
- Indian Tribal Governments
- Tax Exempt Bonds
- FILING FOR INDIVIDUALS
- How to File
- When to File
- Where to File
- Update Your Information
- Get Your Tax Record
- Apply for an Employer ID Number (EIN)
- Check Your Amended Return Status
- Get an Identity Protection PIN (IP PIN)
- File Your Taxes for Free
- Bank Account (Direct Pay)
- Payment Plan (Installment Agreement)
- Electronic Federal Tax Payment System (EFTPS)
- Your Online Account
- Tax Withholding Estimator
- Estimated Taxes
- Where's My Refund
- What to Expect
- Direct Deposit
- Reduced Refunds
- Amend Return
Credits & Deductions
- INFORMATION FOR...
- Businesses & Self-Employed
- Earned Income Credit (EITC)
- Child Tax Credit
- Clean Energy and Vehicle Credits
- Standard Deduction
- Retirement Plans
Forms & Instructions
- POPULAR FORMS & INSTRUCTIONS
- Form 1040 Instructions
- Form 4506-T
- POPULAR FOR TAX PROS
- Form 1040-X
- Circular 230
Audit techniques guide: Credit for Increasing Research Activities (i.e. Research Tax Credit) IRC § 41* - Qualified research activities
More in file.
- Business tax account
- Small business and self-employed
- Large business
- Corporations
- Partnerships
- Charities and nonprofits
- International taxpayers
- Governmental liaisons
- Federal, state and local governments
- Indian tribal governments
- Tax exempt bonds
Publication date: June 2005
* Unless otherwise indicated, all section references are to the Internal Revenue Code of 1986, as amended, and the treasury regulations.
Note: This guide is current through the publication date. Since changes may have occurred after the publication date that would affect the accuracy of this document, no guarantees are made concerning the technical accuracy after the publication date.
Chapter 4 | Table of contents | Chapter 6
5. Qualified research activities
A. in general.
In order for an activity to qualify for the research credit, the taxpayer must show that it meets all the requirements as described in section 41(d). Under section 41(d), the term "qualified research" means research:
- With respect to which expenditures may be treated as expenses under section 174, (also known as the section 174 test);
- Which is undertaken for the purpose of discovering information which is technological in nature, (also known as the discovering technological information test);
- The application of which is intended to be useful in the development of a new or improved business component of the taxpayer (also known as the business component test); and
- Substantially all of the activities of which constitutes elements of a process of experimentation for a qualified purpose (also known as the process of experimentation test).
To be considered “qualified research”, the taxpayer must be able to establish that the research activity being performed meets ALL four of the above tests. 11 These tests must be applied separately to each business component of the taxpayer. Activities listed in section 41(d)(4) are not qualified research. Infra.
(1). The Section 174 test
In order to meet the section 174 test, the expenditure must (1) be incurred in connection with the taxpayer’s trade or business, and (2) represent a research and development cost in the experimental or laboratory sense.
Expenditures represent research and development costs in the experimental or laboratory sense if they are for activities intended to discover information that would eliminate uncertainty concerning the development or improvement of a product. Uncertainty exists if the information available to the taxpayer does not establish the capability or method for developing or improving the product or the appropriate design of the product.
Whether expenditures qualify as research or experimental expenditures depends on the nature of the activity to which the expenditures relate, not the nature of the product or improvement being developed or the level of technological advancement the product or improvement represents.
Section 174 treatment is allowed only to the extent that the amount is reasonable under the circumstances. Expenditures for land and depreciable property are not allowed under section 174, although in certain cases, depreciation may be treated as a section 174 expense. (Depreciation is not a QRE under section 41). Exploration expenditures do not qualify as section 174 expenses. Furthermore, the provisions of section 174 are not applicable to any expenditure paid or incurred for the purpose of ascertaining the existence, location, extent, or quality of any deposit of ore, oil, gas, or other mineral. Refer to the regulations under section 174 for further explanation on specific expense disallowances.
Treasury Regulation section 1.174-2(a)(3) disallows section 174 treatment for certain activities, including:
- The ordinary testing or inspection of materials or products for quality control;
- Efficiency surveys;
- Management studies;
- Consumer surveys;
- Advertising or promotions;
- The acquisition of another’s patent, model, production or process; or
- Research in connection with literary, historical, or similar projects.
Since section 41 is more restrictive than section 174, expenses allowable under section 174 will still have to meet the other requirements of section 41(b) and (d) to be a QRE. For example, patent procurement expenses generally qualify under section 174 but would not qualify under section 41.
(2). The discovering technological information test
Final regulations, issued in January 2004 (TD 9104), 12 mirror the 2001 proposed regulations with respect to the discovering technological information test. There is no “discovery” requirement under section 41 separate and apart from that already required under Treasury Regulation section 1.174-2(a)(1) (i.e., was the research undertaken to eliminate uncertainty concerning the development or improvement of a business component). The final regulations, like the proposed regulations, abandon the requirement that the research activities be undertaken to obtain knowledge that exceeds, expands or refines the common knowledge of skilled professionals in a particular field of science or engineering.
Research is undertaken for the purpose of discovering information if it is intended to eliminate uncertainty concerning the development or improvement of a business component. Uncertainty exists if the information available to the taxpayer does not establish the capability or method for developing or improving the business component, or the appropriate design of the business component.
In order to satisfy the technological in nature requirement for qualified research, the process of experimentation used to discover information must fundamentally rely on principles of the physical or biological sciences, engineering, or computer science. A taxpayer may employ existing technologies and may rely on existing principles of the physical or biological sciences, engineering, or computer science to satisfy this requirement.
The final regulations state that the issuance of a patent by the Patent and Trademark Office under 35 USC sections 51 is conclusive evidence that a taxpayer has discovered information that is technological in nature that is intended to eliminate uncertainty concerning the development or improvement of a business component. This is known as the “patent safe-harbor”. Be aware that the issuance of a patent is not conclusive evidence of qualified research, as the taxpayer still has to meet all the other activity requirements of section 41(d). Examiners should note that the securing of a patent usually occurs sometime after the actual research year(s).
(3). The business component test
The taxpayer must intend to apply the information being discovered to develop a new or improved business component of the taxpayer. A business component is any product, process, computer software, technique, formula, or invention, which is to be held for sale, lease, license, or used in a trade or business of the taxpayer. Often times, taxpayers group all research in one broad category and do not identify the specific business component to which the business relates. A taxpayer must be able to tie the research it is claiming for the credit to the relevant business component. The ‘substantially all’ test is applied at the business component level.
(4). The process of experimentation test
The final research credit regulations provide rules on the “process of experimentation test”, which requires that qualified research be research “substantially all of the activities of which constitute elements of a process of experimentation”.
The final regulations clarify the requirement that a process of experimentation is a process designed to evaluate one or more alternatives to achieve a result where the capability or the method of achieving that result, or the appropriate design of that result, is uncertain as of the beginning of the taxpayer’s research activities. Examiners are encouraged to read the preamble to these regulations to get a better understanding of the changes made. A taxpayer may undertake a process of experimentation if there is no uncertainty concerning the taxpayer's capability or method of achieving the desired result, so long as the appropriate design of the desired result is uncertain as of the beginning of the taxpayer's research activities. Uncertainty exists if the information available to the taxpayer does not establish the capability or method for developing or improving the business component, or the appropriate design of the business component.
The final regulations articulate the core elements of a process of experimentation. In addition to requiring that the research be undertaken for the purpose of discovering information that is technological in nature, the taxpayer must:
- Identify the uncertainty regarding the development or improvement of a business component that is the object of the taxpayer’s research activities;
- Identify one or more alternatives intended to eliminate that uncertainty; and
- Identify and conduct a process of evaluating the alternatives.
The key difference regarding “uncertainty” in sections 41 and 174 is that, under section 41, uncertainly must relate to a qualified purpose, and must be resolved through a 3-element process of experimentation, fundamentally relying on the principles of the hard sciences, engineering, or computer science. The regulations clarify that merely demonstrating that uncertainty has been eliminated is insufficient to satisfy the process of experimentation test. Focus upon developing facts necessary to determine whether the taxpayer’s activities meet these requirements and the core elements.
The preamble to the final regulations states that because of the clarifications made, the readily discernible and applicable provision in the 2001 proposed regulations is no longer necessary, because those activities do not constitute a process of experimentation under the final regulations. Accordingly, examiners who properly applied the “readily discernible and applicable” rule as a basis for disallowing the research credit have made proper adjustments. In pending and future examinations, however, the readily discernible and applicable standard should not be applied to a taxpayer’s activities.
In order for activities to constitute qualified research under section 41(d)(1), 80 percent or more of taxpayer’s research activities, measured on a cost or other consistently applied reasonable basis (and without regard to Treasury Regulation section 1.41-2(d)(2)), must constitute elements of a process of experimentation for a qualified purpose. The regulations provide that, if this substantially all requirement is met, then the balance of the research activities may qualify, if the remaining balance meets the requirements of section 41(d)(1)(A) (with respect to which expenditures may be treated as expenses under section 174), and if they are not excluded activities under section 41(d)(4) (such as research after commercial production, adaptation or duplication of an existing business component, etc.).
Although the final regulations are effective for taxable years ending after December 31, 2003, the Service will not challenge return positions that are consistent with the final regulations. As these final regulations merely clarify the proposed regulations upon which taxpayers are already relying, the Service’s administrative approach will follow these final rules for all open years.
The process of experimentation must be conducted for a “qualified purpose”, i.e., it must relate to a new or improved function, performance, reliability, or quality of the business component. The process of experimentation is not for a qualified purpose if it relates to style, taste, cosmetic, or seasonal design factors. I.R.C. § 41(d)(3)(B). Accordingly, be alert to claimed QREs for research related to non-functional aspects of the business component.
b. Shrink back
The requirements of section 41(d) are to be applied first at the level of the discrete business component, i.e., the product, process, computer software, technique, formula, or invention to be held for sale, lease, or license, or used by the taxpayer in its trade or business.
If the requirements for credit eligibility are met at that first level, then some, or all, of the taxpayer's research activities are eligible for the credit. If all aspects of such requirements are not met at that level, the test applies at the most significant subset of elements of the product, process, computer software, technique, formula, or invention to be held for sale, lease, or license. This “shrinking back” is to continue until either a subset of elements of the business component that satisfies the requirements is reached, or the most basic element of the business component is reached and such element fails to satisfy the test.
The burden is on the taxpayer to establish that all of the section 41(d)(1) requirements have been met. The examiner should issue an IDR requesting a list of each qualifying project or activity, along with a complete description of that activity or project as a starting point in the evaluation, including the business component to which each research activity relates. As with the evaluation of wages, interviews should be considered to supplement and corroborate information obtained from the review of existing records.
c. Exclusions
There are certain research activities that are specifically excluded from qualified research under section 41(d)(4). It is critical to look at the underlying facts to see if the exclusions apply. Taxpayer labels are not controlling. The following activities are not qualified research:
1. Exclusion for research after commercial production
Section 41(d) (4) states that qualified research does not include any research conducted after the beginning of commercial production. A business component is considered ready for commercial production when it is developed to the point where it is ready for use or meets the basic functional and economic requirements of the taxpayer. In some cases, there may be “product release” documents where all responsible managers sign off that the new product and or new production method is now released for production, which may be helpful in the application of this exclusion.
The following activities are deemed to occur after the commencement of commercial production:
- Preproduction planning for a finished business component,
- Tooling up for production,
- Trial production runs,
- Troubleshooting involving detecting faults in production equipment or processes,
- Accumulating data relating to production processes, and
- Debugging flaws in a business component.
This per se list includes “debugging” activities, but not “correction of flaws”. Treasury Regulation section 1.41 4(c)(10), Examples 1 and 2, illustrate the application of the exclusion for research after commercial production.
2. Exclusion for adaptation
This exclusion applies if the taxpayer's activities relate to adapting an existing business component to a particular customer's requirement or need. This exclusion does not apply merely because a business component is intended for a specific customer. A contractor’s adaptation of an existing business component to a taxpayer’s particular requirement or need is not qualified research.
Treasury Regulation section 1.41 4(c)(10), Examples 3 7, illustrates the application of the adaptation exclusion.
3. Exclusion for duplication
This exclusion applies if the taxpayer reproduced an existing business component, in whole or in part, from a physical examination of the business component, plans, blueprints, detailed specifications, or publicly available information with respect to such component. This exclusion does not apply merely because the taxpayer evaluates another's business component in the course of developing its own business component.
Treasury Regulation section 1.41 4(c)(10), Example 8, illustrates the application of the duplication exclusion.
4. Exclusion for surveys, studies, research relating to management functions
The following activities are excluded under this provision:
- Management functions or techniques, including such items as preparation of financial data and analysis, development of employee training programs and management organization plans, and management based changes in production processes (such as rearranging work stations on an assembly line);
- Market research, testing, or development (including advertising or promotions);
- Routine data collections; or
- Routine or ordinary testing or inspections for quality control.
Treasury Regulation section 41 4(c)(10), Example 9, illustrates the application of this exclusion.
Note that it is the activity which governs, not the intended end result. For example, the development of a new production process, which met all the tests for qualified research, would not be excluded simply because the activity was preceded by a management efficiency survey.
5. Exclusion for internal-use software
This exclusion is beyond the scope of this ATG.
6. Exclusion for foreign research
Qualified research does not include any research conducted outside the United States, Puerto Rico, or any possession of the United States. 13 This exclusion applies to in-house, as well as contract research. The foreign research disallowance applies even if the research is done by American researchers, or performed for an American taxpayer.
7. Exclusion for research in the social sciences, etc.
Qualified research does not include research in the social sciences (including economics, business management, and behavioral sciences, arts, or humanities).
Treasury Regulation section 1.41 4(c)(10), Example 10, illustrates the application of this exclusion. Note that the process, not the end result, governs. The development of new formulation of artists’ paint would not be excluded simply because it benefited the arts, while research into Van Gogh’s life would be excluded under this rule.
8. Exclusion for funded research
The exclusion for "funded research" under section 41(d)(4)(H) provides that the credit shall not be available for qualified research to the extent funded by a contract, grant, or otherwise by another person (or governmental entity).
All agreements (not only research contracts) entered into between the taxpayer performing the research and other persons are to be considered in determining the extent to which the research is funded. As a result, the examiner should request a complete copy of all contracts (including modifications), agreements, letters of understanding or similar documents where funding is an issue. These contracts and similar documents will need to be reviewed to determine whether, and to what, extent the research is to be considered funded. A “fixed-price” contract, where the customer agrees to pay a set price for a deliverable, and a “cost-plus” contract, where the customer agrees to pay the actual costs incurred by the contractor in acquiring/constructing the deliverable plus an additional amount for profit, are examples of the different contracts you may encounter. Counsel can be helpful in securing and interpreting these agreements. In the case of documents that are “classified” by a government agency, contact the Classified Contract Technical Advisor or a Research Credit Technical Advisor for further assistance.
In order to determine if the contractor’s research expenditures are “funded”, you must resolve the following issues:
- Is payment for the contractor’s research activities “contingent upon the success of the research” under Treasury Regulation section 1.41-4A(d)(1)?
- Does the contractor retain “substantial rights” in the results of the research activities within the meaning of Treasury Regulation section 1.41-4A(d)(2)?
If the answer to either question is no, then the research is treated as funded. Amounts payable under any agreements that are contingent on the success of the research (thus considered to be paid for the product or result of the research) are treated as funded research. If a contractor retains substantial rights in the results of the research, and if payment to him is contingent on the success of the research, then the contract is not funded and the contractor is eligible to claim the credit.
Note that, if the contractor performing research for another person does not retain substantial rights in the research, and if the research payments are contingent on the contractor’s success, neither the contractor nor the person paying for the research is eligible to claim the credit.
If a taxpayer performing qualified research for another person retains substantial rights in the research under the agreement providing for the research, the research is funded to the extent of the payments (and fair market value of any property) to which the taxpayer becomes entitled by performing the research. A taxpayer does not retain substantial rights in the research if the taxpayer must pay for the right to use the results of the research.
Frequently, taxpayers make some sort of funding allocation between “qualified research” and “non-qualified research” expenditures incurred in certain types of contracts, e.g., cost-share or cost overrun situations. In so doing, taxpayers often overlook the “pro rata allocation” requirements of Treasury Regulation section 1.41-4A(d)(3)(ii).
The general rule is that funding is to be allocated 100 percent to otherwise qualified research expenses (as provided by Treasury Regulation section 1.41-4A(d)(3)(i)) unless the taxpayer can meet the pro rata allocation requirements of Treasury Regulation section 1.41-4A(d)(3)(ii).
Pursuant to Treasury Regulation section 1.41-4A(d)(3)(ii), the taxpayer may allocate funding pro rata to nonqualified, and otherwise qualified research expenses, rather than allocating it 100 percent to otherwise qualified research expenses, if the taxpayer can establish to the satisfaction of the Service:
- the total amount of research expenses,
- that the total amount of research expenses exceed the funding, and
- that the otherwise qualified research expenses (that is, the expenses that would be qualified research expenses if there were no funding) exceed 65 percent of the funding.
In no event, however, shall less than 65 percent of the funding be applied against the otherwise qualified research expenses. Material adjustments may be warranted if the specific requirements of Treasury Regulation section 1.41-4A(d)(3)(ii) have not been met.
Funding is determinable only in the subsequent taxable year. Treasury Regulation section 1.41-4A(d)(5) states that if, at the time the taxpayer files its return for a taxable year, it is impossible to determine to what extent particular research performed by the taxpayer during the year may be funded, then the taxpayer shall treat the research as completely funded for purposes of completing that return. When the amount of funding is finally determined, the taxpayer should amend the return and any interim returns to reflect the proper amount of funding.
11 In the case of certain software developed for internal use, taxpayers must meet the requirements of an additional three-part “high threshold of innovation” test. See Prop. Treas. Reg. § 1.41-4(c)(6)(vi). See also the ANPRM relating to the section 41(d)(4)(E) internal use software exclusion.
12 Final Regulations for the Definition of Qualified Research under section 41(d) (doc, 90kb), also in HTML (htm, 137kb) and Adobe (pdf, 65kb), T.D. 9104.
13 Section 41(d)(4)(F) was modified by P.L. 106-170 section 502(c)(1) which added the Commonwealth of Puerto Rico and any possession of the United States for amounts paid or incurred after June 30, 1999. Prior to amendment, section 41(d)(4)(F) applied only to the United States.
Chapter 4 | Table of Contents | Chapter 6
Look up a word, learn it forever.
Buddy-buddy, /ˌbʌdi ˌbʌdi/.
- adjective (used informally) associated on close terms synonyms: chummy , thick close close in relevance or relationship
Sign up now (it’s free!)
Whether you’re a teacher or a learner, vocabulary.com can put you or your class on the path to systematic vocabulary improvement..
Watch CBS News
Giant salamander-like predator with fangs existed 40 million years before dinosaurs, research reveals
July 3, 2024 / 1:07 PM EDT / CBS/AP
Scientists have revealed fossils of a giant salamander-like beast with sharp fangs that ruled waters before the first dinosaurs arrived . The animal, researchers say, is roughly 272-million-year-old.
The findings were published Wednesday in the journal Nature . The researchers dubbed the species Gaiasia jennyae , an hommage to Gai-as Formation in Namibia, where the fossil was found, and to Jenny Clack, a paleontologist who studied how vertebrates moved from water to land .
"Gaiasia jennyae was considerably larger than a person, and it probably hung out near the bottom of swamps and lakes," said Jason Pardo, an NSF postdoctoral fellow at the Field Museum in Chicago and the co-lead author of the study, in a news release .

Pardo added that the species had a "big, flat, toilet seat-shaped head," "huge fangs" and "giant teeth."
The predator likely used its wide, flat head and front teeth to suck in and chomp unsuspecting prey, researchers said. Its skull was about 2 feet (60 centimeters) long.
"It's acting like an aggressive stapler," said Michael Coates, a biologist at the University of Chicago who was not involved with the work.
Fossil remnants of four creatures collected about a decade ago were analyzed in the Nature study, including a partial skull and backbone. The creature existed some 40 million years before dinosaurs evolved.

While Gaiasia jennyae was an aquatic animal, it could move on land, albeit slowly . The species belonged to a superclass of animals called tetrapods: four-legged vertebrates that clambered onto land with fingers instead of fins and evolved to amphibians, birds and mammals including humans.
Most early tetrapod fossils hail from hot, prehistoric coal swamps along the equator in what's now North America and Europe. But these latest remnants, dating back to about 280 million years ago, were found in modern-day Namibia, an area in Africa that was once encrusted with glaciers and ice.
The discovery of Gaiasia was a big victory for paleontologists who continue to piece together how the world was evolving during the Permian period.
"The fact that we found Gaiasia in the far south tells us that there was a flourishing ecosystem that could support these very large predators," said Pardo. "The more we look, we might find more answers about these major animal groups that we care about, like the ancestors of mammals and modern reptiles."
More from CBS News

American detained after "So I raped you" message can be extradited

Huge dinosaur skeleton unearthed in Wyoming makes it to museum in Europe

These cannibal baby sharks eat their siblings in the womb

Israel brings journalists into Rafah amid devastating assault on Hamas
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 01 July 2024
Resolution in super-resolution microscopy — definition, trade-offs and perspectives
- Kirti Prakash ORCID: orcid.org/0000-0002-0325-9988 1 , 2 ,
- David Baddeley 3 ,
- Christian Eggeling 4 , 5 ,
- Reto Fiolka 6 ,
- Rainer Heintzmann 5 , 7 ,
- Suliana Manley ORCID: orcid.org/0000-0002-4755-4778 8 ,
- Aleksandra Radenovic ORCID: orcid.org/0000-0001-8194-2785 9 ,
- Carlas Smith 10 ,
- Hari Shroff 11 &
- Lothar Schermelleh ORCID: orcid.org/0000-0002-1612-9699 12
Nature Reviews Molecular Cell Biology ( 2024 ) Cite this article
840 Accesses
14 Altmetric
Metrics details
- Single-molecule biophysics
- Super-resolution microscopy
Super-resolution microscopy (SRM) is gaining popularity in biosciences; however, claims about optical resolution are contested and often misleading. In this Viewpoint, experts share their views on resolution and common trade-offs, such as labelling and post-processing, aiming to clarify them for biologists and facilitate deeper understanding and best use of SRM.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
176,64 € per year
only 14,72 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Heintzmann, R. & Huser, T. Super-resolution structured illumination microscopy. Chem. Rev. 117 , 13890–13908 (2017).
Article CAS PubMed Google Scholar
Vicidomini, G., Bianchini, P. & Diaspro, A. STED super-resolved microscopy. Nat. Methods 15 , 173–182 (2018).
Lelek, M. et al. Single-molecule localization microscopy. Nat. Rev. Methods Primers 1 , 39 (2021).
Article CAS PubMed PubMed Central Google Scholar
Nieuwenhuizen, R. P. J. et al. Measuring image resolution in optical nanoscopy. Nat. Methods 10 , 557–562 (2013).
Descloux, A., Grußmayer, K. S. & Radenovic, A. Parameter-free image resolution estimation based on decorrelation analysis. Nat. Methods 16 , 918–924 (2019).
Smith, C. S. et al. Structured illumination microscopy with noise-controlled image reconstructions. Nat. Methods 18 , 821–828 (2021).
Stelzer, E. Contrast, resolution, pixelation, dynamic range and signal‐to‐noise ratio: fundamental limits to resolution in fluorescence light microscopy. J. Microsc. 189 , 15–24 (1998).
Article Google Scholar
Prakash, K. & Curd, A. P. Assessment of 3D MINFLUX data for quantitative structural biology in cells. Nat. Methods 20 , 48–51 (2023).
Kalisvaart, D., Hung, S.-T. & Smith, C. S. Quantifying the minimum localization uncertainty of image scanning localization microscopy. Biophys. Rep. 4 , 100143 (2024).
Google Scholar
Schermelleh, L. et al. Super-resolution microscopy demystified. Nat. Cell Biol. 21 , 72–84 (2019).
Reinhardt, S. C. M. et al. Ångström-resolution fluorescence microscopy. Nature 617 , 711–716 (2023).
Grimm, J. B. & Lavis, L. D. Caveat fluorophore: an insiders’ guide to small-molecule fluorescent labels. Nat. Methods 19 , 149–158 (2022).
Strauss, S. et al. Modified aptamers enable quantitative sub-10-nm cellular DNA-PAINT imaging. Nat. Methods 15 , 685–688 (2018).
Cordell, P. et al. Affimers and nanobodies as molecular probes and their applications in imaging. J. Cell Sci. 135 , jcs259168 (2022).
Beliu, G. et al. Bioorthogonal labeling with tetrazine-dyes for super-resolution microscopy. Commun. Biol. 2 , 261 (2019).
Article PubMed PubMed Central Google Scholar
Download references
Author information
Authors and affiliations.
Division of Molecular Pathology, The Institute of Cancer Research, London, UK
Kirti Prakash
The Royal Marsden NHS Foundation Trust, London, UK
Auckland Bioengineering Institute, University of Auckland, Auckland, New Zealand
David Baddeley
Institute of Applied Optics and Biophysics and Abbe Center of Photonics, Friedrich-Schiller-University Jena, Jena, Germany
Christian Eggeling
Leibniz Institute of Photonic Technology, Jena, Germany
Christian Eggeling & Rainer Heintzmann
Lyda Hill Department of Bioinformatics, University of Texas Southwestern Medical Center, Dallas, TX, USA
Reto Fiolka
Institute of Physical Chemistry and Abbe Center of Photonics, Friedrich-Schiller-University Jena, Jena, Germany
Rainer Heintzmann
Laboratory of Experimental Biophysics, School of Basic Sciences, Institute of Physics, Interfaculty Institute of Bioengineering, EPFL SB-LEB, Lausanne, Switzerland
Suliana Manley
Laboratory of Nanoscale Biology, School of Engineering, Institute of Bioengineering, EPFL STI IBI-STI LBEN, Lausanne, Switzerland
Aleksandra Radenovic
Delft Center for Systems and Control, Faculty of Mechanical, Maritime, and Materials Engineering, Technische Universiteit Delft, Delft, The Netherlands
Carlas Smith
Janelia Research Campus, Howard Hughes Medical Institute (HHMI), Ashburn, VA, USA
Hari Shroff
Department of Biochemistry, University of Oxford, Oxford, UK
Lothar Schermelleh
You can also search for this author in PubMed Google Scholar
Contributions
Kirti Prakash has degrees in computer science and biology. His expertise lies in single-molecule super-resolution microscopy, mathematical modelling and machine learning. He is dedicated to developing pioneering tools for advanced microscopy, with a research focus on epigenetics and chromatin structure.
David Baddeley is trained as a physicist and started working on super-resolution imaging problems in his doctorate. He has expertise in a range of methodologies including SMLM and a particular interest in the quantitative analysis of super-resolution data.
Christian Eggeling holds a degree in physics and, after gaining industry experience in single-molecule microscopy, joined the super-resolution microscopy group of Stefan Hell. He now leads an independent group advancing super-resolution microscopy and spectroscopy, focusing on molecular diffusion dynamics studies of cell membranes. He also manages a microscope facility.
Reto Fiolka , originally trained as a mechanical engineer in computational fluid dynamics, switched focus to optical microscopy during his doctorate. His laboratory is dedicated to developing new imaging techniques for biomedical research to be applied in 3D environments, ex vivo and in vivo, offering improved spatiotemporal resolution and multi-scale capabilities.
Rainer Heintzmann studied physics and computer science and develops super-resolution fluorescence microscopy methods such as linear and nonlinear structured illumination, pointillism and image inversion interferometry. He has a strong interest in computational optics and inverse problems such as deconvolution and in extracting multidimensional information from biological structures.
Suliana Manley studied physics and mathematics and became fascinated by complex biological systems. Her group studies the biophysical principles of organelle structure and dynamics and develops smart, automated and multi-modal microscopy methods. Their work is enriched by interplay between fundamental discovery and microscopy development.
Aleksandra Radenovic studied physics and is an expert in single-molecule biophysics and nanofluidics. Her research focuses on developing biosensors and optical imaging techniques for observing individual molecules and complexes, enhancing our understanding of their behaviour in various environments.
Carlas Smith studied aerospace engineering and applied physics. He now leads a group focusing on developing advanced computational microscopy techniques and the combination of opto-mechatronics and information processing algorithms, particularly for super-resolution imaging.
Hari Shroff has degrees in bioengineering and biophysics and entered the microscopy field as a postdoctoral researcher working on super-resolution imaging. He now leads a group focused on developing new optical and computational methods that offer the ability to interrogate biological structure and function across diverse spatiotemporal scales.
Lothar Schermelleh is a trained cell biologist who has specialized in the biological application of super-resolution structured illumination microscopy. His research focuses on studying chromatin organization and functional nuclear architecture. To this end, his group developed fluorescent labelling protocols and software tools for image data quality control.
Corresponding authors
Correspondence to Kirti Prakash , David Baddeley , Christian Eggeling , Reto Fiolka , Rainer Heintzmann , Suliana Manley , Aleksandra Radenovic , Carlas Smith , Hari Shroff or Lothar Schermelleh .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Prakash, K., Baddeley, D., Eggeling, C. et al. Resolution in super-resolution microscopy — definition, trade-offs and perspectives. Nat Rev Mol Cell Biol (2024). https://doi.org/10.1038/s41580-024-00755-7
Download citation
Accepted : 11 June 2024
Published : 01 July 2024
DOI : https://doi.org/10.1038/s41580-024-00755-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Human Subjects Office
Medical terms in lay language.
Please use these descriptions in place of medical jargon in consent documents, recruitment materials and other study documents. Note: These terms are not the only acceptable plain language alternatives for these vocabulary words.
This glossary of terms is derived from a list copyrighted by the University of Kentucky, Office of Research Integrity (1990).
For clinical research-specific definitions, see also the Clinical Research Glossary developed by the Multi-Regional Clinical Trials (MRCT) Center of Brigham and Women’s Hospital and Harvard and the Clinical Data Interchange Standards Consortium (CDISC) .
Alternative Lay Language for Medical Terms for use in Informed Consent Documents
A B C D E F G H I J K L M N O P Q R S T U V W X Y Z
ABDOMEN/ABDOMINAL body cavity below diaphragm that contains stomach, intestines, liver and other organs ABSORB take up fluids, take in ACIDOSIS condition when blood contains more acid than normal ACUITY clearness, keenness, esp. of vision and airways ACUTE new, recent, sudden, urgent ADENOPATHY swollen lymph nodes (glands) ADJUVANT helpful, assisting, aiding, supportive ADJUVANT TREATMENT added treatment (usually to a standard treatment) ANTIBIOTIC drug that kills bacteria and other germs ANTIMICROBIAL drug that kills bacteria and other germs ANTIRETROVIRAL drug that works against the growth of certain viruses ADVERSE EFFECT side effect, bad reaction, unwanted response ALLERGIC REACTION rash, hives, swelling, trouble breathing AMBULATE/AMBULATION/AMBULATORY walk, able to walk ANAPHYLAXIS serious, potentially life-threatening allergic reaction ANEMIA decreased red blood cells; low red cell blood count ANESTHETIC a drug or agent used to decrease the feeling of pain, or eliminate the feeling of pain by putting you to sleep ANGINA pain resulting from not enough blood flowing to the heart ANGINA PECTORIS pain resulting from not enough blood flowing to the heart ANOREXIA disorder in which person will not eat; lack of appetite ANTECUBITAL related to the inner side of the forearm ANTIBODY protein made in the body in response to foreign substance ANTICONVULSANT drug used to prevent seizures ANTILIPEMIC a drug that lowers fat levels in the blood ANTITUSSIVE a drug used to relieve coughing ARRHYTHMIA abnormal heartbeat; any change from the normal heartbeat ASPIRATION fluid entering the lungs, such as after vomiting ASSAY lab test ASSESS to learn about, measure, evaluate, look at ASTHMA lung disease associated with tightening of air passages, making breathing difficult ASYMPTOMATIC without symptoms AXILLA armpit
BENIGN not malignant, without serious consequences BID twice a day BINDING/BOUND carried by, to make stick together, transported BIOAVAILABILITY the extent to which a drug or other substance becomes available to the body BLOOD PROFILE series of blood tests BOLUS a large amount given all at once BONE MASS the amount of calcium and other minerals in a given amount of bone BRADYARRHYTHMIAS slow, irregular heartbeats BRADYCARDIA slow heartbeat BRONCHOSPASM breathing distress caused by narrowing of the airways
CARCINOGENIC cancer-causing CARCINOMA type of cancer CARDIAC related to the heart CARDIOVERSION return to normal heartbeat by electric shock CATHETER a tube for withdrawing or giving fluids CATHETER a tube placed near the spinal cord and used for anesthesia (indwelling epidural) during surgery CENTRAL NERVOUS SYSTEM (CNS) brain and spinal cord CEREBRAL TRAUMA damage to the brain CESSATION stopping CHD coronary heart disease CHEMOTHERAPY treatment of disease, usually cancer, by chemical agents CHRONIC continuing for a long time, ongoing CLINICAL pertaining to medical care CLINICAL TRIAL an experiment involving human subjects COMA unconscious state COMPLETE RESPONSE total disappearance of disease CONGENITAL present before birth CONJUNCTIVITIS redness and irritation of the thin membrane that covers the eye CONSOLIDATION PHASE treatment phase intended to make a remission permanent (follows induction phase) CONTROLLED TRIAL research study in which the experimental treatment or procedure is compared to a standard (control) treatment or procedure COOPERATIVE GROUP association of multiple institutions to perform clinical trials CORONARY related to the blood vessels that supply the heart, or to the heart itself CT SCAN (CAT) computerized series of x-rays (computerized tomography) CULTURE test for infection, or for organisms that could cause infection CUMULATIVE added together from the beginning CUTANEOUS relating to the skin CVA stroke (cerebrovascular accident)
DERMATOLOGIC pertaining to the skin DIASTOLIC lower number in a blood pressure reading DISTAL toward the end, away from the center of the body DIURETIC "water pill" or drug that causes increase in urination DOPPLER device using sound waves to diagnose or test DOUBLE BLIND study in which neither investigators nor subjects know what drug or treatment the subject is receiving DYSFUNCTION state of improper function DYSPLASIA abnormal cells
ECHOCARDIOGRAM sound wave test of the heart EDEMA excess fluid collecting in tissue EEG electric brain wave tracing (electroencephalogram) EFFICACY effectiveness ELECTROCARDIOGRAM electrical tracing of the heartbeat (ECG or EKG) ELECTROLYTE IMBALANCE an imbalance of minerals in the blood EMESIS vomiting EMPIRIC based on experience ENDOSCOPIC EXAMINATION viewing an internal part of the body with a lighted tube ENTERAL by way of the intestines EPIDURAL outside the spinal cord ERADICATE get rid of (such as disease) Page 2 of 7 EVALUATED, ASSESSED examined for a medical condition EXPEDITED REVIEW rapid review of a protocol by the IRB Chair without full committee approval, permitted with certain low-risk research studies EXTERNAL outside the body EXTRAVASATE to leak outside of a planned area, such as out of a blood vessel
FDA U.S. Food and Drug Administration, the branch of federal government that approves new drugs FIBROUS having many fibers, such as scar tissue FIBRILLATION irregular beat of the heart or other muscle
GENERAL ANESTHESIA pain prevention by giving drugs to cause loss of consciousness, as during surgery GESTATIONAL pertaining to pregnancy
HEMATOCRIT amount of red blood cells in the blood HEMATOMA a bruise, a black and blue mark HEMODYNAMIC MEASURING blood flow HEMOLYSIS breakdown in red blood cells HEPARIN LOCK needle placed in the arm with blood thinner to keep the blood from clotting HEPATOMA cancer or tumor of the liver HERITABLE DISEASE can be transmitted to one’s offspring, resulting in damage to future children HISTOPATHOLOGIC pertaining to the disease status of body tissues or cells HOLTER MONITOR a portable machine for recording heart beats HYPERCALCEMIA high blood calcium level HYPERKALEMIA high blood potassium level HYPERNATREMIA high blood sodium level HYPERTENSION high blood pressure HYPOCALCEMIA low blood calcium level HYPOKALEMIA low blood potassium level HYPONATREMIA low blood sodium level HYPOTENSION low blood pressure HYPOXEMIA a decrease of oxygen in the blood HYPOXIA a decrease of oxygen reaching body tissues HYSTERECTOMY surgical removal of the uterus, ovaries (female sex glands), or both uterus and ovaries
IATROGENIC caused by a physician or by treatment IDE investigational device exemption, the license to test an unapproved new medical device IDIOPATHIC of unknown cause IMMUNITY defense against, protection from IMMUNOGLOBIN a protein that makes antibodies IMMUNOSUPPRESSIVE drug which works against the body's immune (protective) response, often used in transplantation and diseases caused by immune system malfunction IMMUNOTHERAPY giving of drugs to help the body's immune (protective) system; usually used to destroy cancer cells IMPAIRED FUNCTION abnormal function IMPLANTED placed in the body IND investigational new drug, the license to test an unapproved new drug INDUCTION PHASE beginning phase or stage of a treatment INDURATION hardening INDWELLING remaining in a given location, such as a catheter INFARCT death of tissue due to lack of blood supply INFECTIOUS DISEASE transmitted from one person to the next INFLAMMATION swelling that is generally painful, red, and warm INFUSION slow injection of a substance into the body, usually into the blood by means of a catheter INGESTION eating; taking by mouth INTERFERON drug which acts against viruses; antiviral agent INTERMITTENT occurring (regularly or irregularly) between two time points; repeatedly stopping, then starting again INTERNAL within the body INTERIOR inside of the body INTRAMUSCULAR into the muscle; within the muscle INTRAPERITONEAL into the abdominal cavity INTRATHECAL into the spinal fluid INTRAVENOUS (IV) through the vein INTRAVESICAL in the bladder INTUBATE the placement of a tube into the airway INVASIVE PROCEDURE puncturing, opening, or cutting the skin INVESTIGATIONAL NEW DRUG (IND) a new drug that has not been approved by the FDA INVESTIGATIONAL METHOD a treatment method which has not been proven to be beneficial or has not been accepted as standard care ISCHEMIA decreased oxygen in a tissue (usually because of decreased blood flow)
LAPAROTOMY surgical procedure in which an incision is made in the abdominal wall to enable a doctor to look at the organs inside LESION wound or injury; a diseased patch of skin LETHARGY sleepiness, tiredness LEUKOPENIA low white blood cell count LIPID fat LIPID CONTENT fat content in the blood LIPID PROFILE (PANEL) fat and cholesterol levels in the blood LOCAL ANESTHESIA creation of insensitivity to pain in a small, local area of the body, usually by injection of numbing drugs LOCALIZED restricted to one area, limited to one area LUMEN the cavity of an organ or tube (e.g., blood vessel) LYMPHANGIOGRAPHY an x-ray of the lymph nodes or tissues after injecting dye into lymph vessels (e.g., in feet) LYMPHOCYTE a type of white blood cell important in immunity (protection) against infection LYMPHOMA a cancer of the lymph nodes (or tissues)
MALAISE a vague feeling of bodily discomfort, feeling badly MALFUNCTION condition in which something is not functioning properly MALIGNANCY cancer or other progressively enlarging and spreading tumor, usually fatal if not successfully treated MEDULLABLASTOMA a type of brain tumor MEGALOBLASTOSIS change in red blood cells METABOLIZE process of breaking down substances in the cells to obtain energy METASTASIS spread of cancer cells from one part of the body to another METRONIDAZOLE drug used to treat infections caused by parasites (invading organisms that take up living in the body) or other causes of anaerobic infection (not requiring oxygen to survive) MI myocardial infarction, heart attack MINIMAL slight MINIMIZE reduce as much as possible Page 4 of 7 MONITOR check on; keep track of; watch carefully MOBILITY ease of movement MORBIDITY undesired result or complication MORTALITY death MOTILITY the ability to move MRI magnetic resonance imaging, diagnostic pictures of the inside of the body, created using magnetic rather than x-ray energy MUCOSA, MUCOUS MEMBRANE moist lining of digestive, respiratory, reproductive, and urinary tracts MYALGIA muscle aches MYOCARDIAL pertaining to the heart muscle MYOCARDIAL INFARCTION heart attack
NASOGASTRIC TUBE placed in the nose, reaching to the stomach NCI the National Cancer Institute NECROSIS death of tissue NEOPLASIA/NEOPLASM tumor, may be benign or malignant NEUROBLASTOMA a cancer of nerve tissue NEUROLOGICAL pertaining to the nervous system NEUTROPENIA decrease in the main part of the white blood cells NIH the National Institutes of Health NONINVASIVE not breaking, cutting, or entering the skin NOSOCOMIAL acquired in the hospital
OCCLUSION closing; blockage; obstruction ONCOLOGY the study of tumors or cancer OPHTHALMIC pertaining to the eye OPTIMAL best, most favorable or desirable ORAL ADMINISTRATION by mouth ORTHOPEDIC pertaining to the bones OSTEOPETROSIS rare bone disorder characterized by dense bone OSTEOPOROSIS softening of the bones OVARIES female sex glands
PARENTERAL given by injection PATENCY condition of being open PATHOGENESIS development of a disease or unhealthy condition PERCUTANEOUS through the skin PERIPHERAL not central PER OS (PO) by mouth PHARMACOKINETICS the study of the way the body absorbs, distributes, and gets rid of a drug PHASE I first phase of study of a new drug in humans to determine action, safety, and proper dosing PHASE II second phase of study of a new drug in humans, intended to gather information about safety and effectiveness of the drug for certain uses PHASE III large-scale studies to confirm and expand information on safety and effectiveness of new drug for certain uses, and to study common side effects PHASE IV studies done after the drug is approved by the FDA, especially to compare it to standard care or to try it for new uses PHLEBITIS irritation or inflammation of the vein PLACEBO an inactive substance; a pill/liquid that contains no medicine PLACEBO EFFECT improvement seen with giving subjects a placebo, though it contains no active drug/treatment PLATELETS small particles in the blood that help with clotting POTENTIAL possible POTENTIATE increase or multiply the effect of a drug or toxin (poison) by giving another drug or toxin at the same time (sometimes an unintentional result) POTENTIATOR an agent that helps another agent work better PRENATAL before birth PROPHYLAXIS a drug given to prevent disease or infection PER OS (PO) by mouth PRN as needed PROGNOSIS outlook, probable outcomes PRONE lying on the stomach PROSPECTIVE STUDY following patients forward in time PROSTHESIS artificial part, most often limbs, such as arms or legs PROTOCOL plan of study PROXIMAL closer to the center of the body, away from the end PULMONARY pertaining to the lungs
QD every day; daily QID four times a day
RADIATION THERAPY x-ray or cobalt treatment RANDOM by chance (like the flip of a coin) RANDOMIZATION chance selection RBC red blood cell RECOMBINANT formation of new combinations of genes RECONSTITUTION putting back together the original parts or elements RECUR happen again REFRACTORY not responding to treatment REGENERATION re-growth of a structure or of lost tissue REGIMEN pattern of giving treatment RELAPSE the return of a disease REMISSION disappearance of evidence of cancer or other disease RENAL pertaining to the kidneys REPLICABLE possible to duplicate RESECT remove or cut out surgically RETROSPECTIVE STUDY looking back over past experience
SARCOMA a type of cancer SEDATIVE a drug to calm or make less anxious SEMINOMA a type of testicular cancer (found in the male sex glands) SEQUENTIALLY in a row, in order SOMNOLENCE sleepiness SPIROMETER an instrument to measure the amount of air taken into and exhaled from the lungs STAGING an evaluation of the extent of the disease STANDARD OF CARE a treatment plan that the majority of the medical community would accept as appropriate STENOSIS narrowing of a duct, tube, or one of the blood vessels in the heart STOMATITIS mouth sores, inflammation of the mouth STRATIFY arrange in groups for analysis of results (e.g., stratify by age, sex, etc.) STUPOR stunned state in which it is difficult to get a response or the attention of the subject SUBCLAVIAN under the collarbone SUBCUTANEOUS under the skin SUPINE lying on the back SUPPORTIVE CARE general medical care aimed at symptoms, not intended to improve or cure underlying disease SYMPTOMATIC having symptoms SYNDROME a condition characterized by a set of symptoms SYSTOLIC top number in blood pressure; pressure during active contraction of the heart
TERATOGENIC capable of causing malformations in a fetus (developing baby still inside the mother’s body) TESTES/TESTICLES male sex glands THROMBOSIS clotting THROMBUS blood clot TID three times a day TITRATION a method for deciding on the strength of a drug or solution; gradually increasing the dose T-LYMPHOCYTES type of white blood cells TOPICAL on the surface TOPICAL ANESTHETIC applied to a certain area of the skin and reducing pain only in the area to which applied TOXICITY side effects or undesirable effects of a drug or treatment TRANSDERMAL through the skin TRANSIENTLY temporarily TRAUMA injury; wound TREADMILL walking machine used to test heart function
UPTAKE absorbing and taking in of a substance by living tissue
VALVULOPLASTY plastic repair of a valve, especially a heart valve VARICES enlarged veins VASOSPASM narrowing of the blood vessels VECTOR a carrier that can transmit disease-causing microorganisms (germs and viruses) VENIPUNCTURE needle stick, blood draw, entering the skin with a needle VERTICAL TRANSMISSION spread of disease
WBC white blood cell
The National Archives Catalog

Contribution Type: Person Names
| Mandatory | Repeatable | Edit Type | Data Type | Source | Level Available | Public Element |
|---|---|---|---|---|---|---|
| No | Yes | Read Only | Variable Character Length (13 characters per element) | Catalog Community Member; NARA Staff; NARA Partner; AI/Machine Generated | File Unit Item Item AV Digital Object | Yes |
| The Person Name Elements are used to identify a person in a record. precedes a person’s name (for example, Ms. or Dr.). is a person’s personal name. It can be the name given to a person at birth or a name legally chosen by the person. is a personal name or names used by a person in addition to their first personal name. It can be the name given to a person at birth or a name legally chosen by the person. It might be a middle initial if a full middle name is not provided. is the name identified on the document as the family name of a person. In English it is also referred to as the last name due to it commonly being used following a person’s first or given name. However in other languages, surnames may commonly be written before a person’s given name. Surnames may consist of one name (for example, Smith) or multiple names which may or may not be hyphenated (for example, Beth Smith Jones or Beth Smith-Jones). follows a person’s name (for example, Jr. or IV).
|
| To allow contributors to identify people named in records thereby providing additional context to descriptions and make digitized and born digital content more discoverable. |
| Each of the Name Elements are independent from each other but are connected as one instance of a name. They can be added in any combination. Name Elements have an attribution type modifier and may have a related contributor name. |
| Name Elements are added to Catalog descriptions and digital objects voluntarily by contributors including Citizen Archivists, staff, and NARA partners following the guidance provided on the page and the on Archives.gov. Name elements may be suggested or generated by AI / Machine tools. |
IMAGES
VIDEO
COMMENTS
Building upon this is the definition of 'involvement', which is commonly accepted to refer to research done 'with' or 'by' patients rather ... The Research Buddy program reflected upon in this piece took the approach of broadening the perspective of the research team by including the voice a patient with lived experience of a ...
Research Buddy is an innovative AI-powered tool designed to generate comprehensive Academic Literature Reviews in minutes. With Research Buddy, users can quickly and easily type in a subject or research question, and generate a comprehensive literature review with Harvard referencing in minutes. The platform is ideal for those looking to stay ...
International Review of Research in Open and Distributed Learning Volume 17, Number 3 April - 2016 Enhancing Student-Student Online Interaction: Exploring the Study Buddy Peer Review Activity Colin Madland1 and Griff Richards2 1Thompson Rivers University, Canada, 2Athabasca University, Canada Abstract The study buddy is a learning strategy employed in a graduate distance course to promote ...
Say goodbye to tedious literature reviews with ResearchBuddy.app. Our smart app streamlines the process and presents the most relevant information to you.
ResearchBuddy is an AI tool that automates the process of literature reviews. The tool helps researchers streamline their literature review process by presenting the most relevant information in a concise and easily digestible format. Its intelligent algorithms help identify the most pertinent information in a given research field, saving hours of time and effort. Through its user-friendly ...
Research Buddy is a powerful and innovative AI-powered research assistant that can significantly streamline the research process for users across various domains. Its intelligent search capabilities, summarization and synthesis features, and collaboration tools make it an excellent choice for anyone looking to efficiently gather information ...
Research Buddy use cases. #️⃣Keep yourself informed about the most recent research. #️⃣Reduce the amount of time and energy spent on literature reviews. #️⃣Easily access and evaluate articles that are relevant to your field. #️⃣Streamline the process of conducting literature reviews with automation.
An article from International Review of Research in Open and Distributed Learning, on Érudit. Main Menu. Publication types. Journals; Theses and dissertations; Books and proceedings; Research reports; ... The study buddy is a learning strategy employed in a graduate distance course to promote informal peer reviewing of assignments before ...
Research Buddy is an AI tool that automates the process of literature reviews, helping researchers streamline their literature review process by identifying the most pertinent information in a given research field. It uses intelligent algorithms to present the most relevant information, saving hours of time and effort. ...
BUDDY definition: 1. a friend: 2. someone who provides friendly help to someone with an illness or a problem: 3…. Learn more.
Effective research instruments are more important than ever in the growing digital world. Reliable tools that expedite the research process are in greater demand as people, organizations, and scholars attempt to remain ahead in their respective sectors. Out of all the options accessible, ResearchBuddy is a solid and versatile platform with many features to
A 'study buddy' is a term commonly used to describe a friend or classmate with whom you study regularly. The main idea behind having a study buddy is to enhance the learning experience by providing mutual support, sharing resources, and holding each other accountable. Study buddies can help you stay focused, motivated, and organized throughout the academic year.
This study uses a Vygotskian approach and a socio-cultural lens, as well as the Transactional Reading Theory to investigate how social interactions and literary transactions can combine through buddy reading to empower young readers and promote literacy in a first grade classroom. The research focuses on how literary transaction and social interaction work together to facilitate emergent and ...
The 'buddy reading' or 'partner reading' approach (these. terms are used interchangeably throughout this inquiry and. are defined as two or three students reading and discussing. one book ...
Definition: The buddy system in the workplace refers to a structured approach where new employees are paired with existing employees, often referred to as "buddies" or mentors, to facilitate their integration into the company culture, processes, and workflows. This system aims to provide support, guidance, and a sense of belonging to ...
Part of this framework is a 'Research Buddy' program, where a research student partners with a patient so that the research they conduct is more relevant and applicable to the target population. ... Building upon this is the definition of 'involvement', which is commonly accepted to refer to research done 'with' or 'by' patients ...
Research methods are specific procedures for collecting and analyzing data. Developing your research methods is an integral part of your research design. When planning your methods, there are two key decisions you will make. First, decide how you will collect data. Your methods depend on what type of data you need to answer your research question:
Research Buddie, a state-of-the-art artefact powered by generative Artificial Intelligence tools, takes this phase to new heights by significantly streamlining and enhancing the entire process. Through the utilization of Research Buddie, students are provided with expert guidance across the four key steps of planning: defining the need for ...
This paper presents initial research conducted by the Department of Languages at the Open University to evaluate the efficacy of a peer-support initiative on level 1 modules where the drop-out ...
Definition of buddy noun in Oxford Advanced American Dictionary. Meaning, pronunciation, picture, example sentences, grammar, usage notes, synonyms and more. ... 3 a partner who does an activity with you so that you can help each other The school uses a buddy system to pair newcomers with older ... It furthers the University's objective of ...
Clinical Trial* The NIH defines a clinical trial as a research study in which one or more human subjects are prospectively assigned to one or more interventions (which may include placebo or other control) to evaluate the effects of those interventions on health-related biomedical or behavioral outcomes.. The term "prospectively assigned" refers to a pre-defined process (e.g., randomization ...
Definition of buddy noun in Oxford Advanced Learner's Dictionary. Meaning, pronunciation, picture, example sentences, grammar, usage notes, synonyms and more. ... The school uses a buddy system to pair newcomers with older students. ... It furthers the University's objective of excellence in research, scholarship, and education by publishing ...
ResearchBuddy - new update in June. Thank you for all the great feedback we received. We literally went through them all, there were alot of great ideas and feature requests aswell as a few bugs that needs ironing out. We are currently working on the next update, which will include most of the top requested features. We are very excited for the ...
The foreign research disallowance applies even if the research is done by American researchers, or performed for an American taxpayer. 7. Exclusion for research in the social sciences, etc. Qualified research does not include research in the social sciences (including economics, business management, and behavioral sciences, arts, or humanities).
(used informally) associated on close terms. DISCLAIMER: These example sentences appear in various news sources and books to reflect the usage of the word 'buddy-buddy'.Views expressed in the examples do not represent the opinion of Vocabulary.com or its editors. Send us feedback
Scientists have revealed fossils of a giant salamander-like beast with sharp fangs that ruled waters before the first dinosaurs arrived. The animal, researchers say, is roughly 272-million-year ...
K.P. and C.S.: For SMLM methods, the main trade-offs are emitter density, localization precision and sample quality. For instance, there is an interplay between emitter density and localization ...
For clinical research-specific definitions, see also the Clinical Research Glossary developed by the Multi-Regional Clinical Trials (MRCT) Center of Brigham and Women's Hospital and Harvard and the Clinical Data Interchange Standards Consortium (CDISC). Alternative Lay Language for Medical Terms for use in Informed Consent Documents
Last week's U.S. District Court ruling ordering the NFL to pay more than $4.7 billion in damages after ruling that the league violated antitrust laws in distributing out-of-market Sunday afternoon games on a premium subscription service known as the Sunday Ticket is an "unprecedented" decision with far-reaching implications, says Helen A. "Nellie" Drew, director of the Center for the ...
Definition: The Person Name Elements are used to identify a person in a record. Prefix precedes a person's name (for example, Ms. or Dr.). First name is a person's personal name. It can be the name given to a person at birth or a name legally chosen by the person.