
Deep Venous Thrombosis (DVT)
- Pathophysiology |
- Symptoms and Signs |
- Diagnosis |
- Treatment |
- Prognosis |
- Prevention |
- Key Points |
Deep venous thrombosis (DVT) is clotting of blood in a deep vein of an extremity (usually calf or thigh) or the pelvis. DVT is the primary cause of pulmonary embolism. DVT results from conditions that impair venous return, lead to endothelial injury or dysfunction, or cause hypercoagulability. DVT may be asymptomatic or cause pain and swelling in an extremity; pulmonary embolism is an immediate complication. Diagnosis is by history and physical examination and is confirmed by objective testing, typically with duplex ultrasonography. D-Dimer testing is sometimes used when DVT is suspected; a negative result helps to exclude DVT, whereas a positive result is nonspecific and requires additional testing to confirm DVT. Treatment is with anticoagulants. Prognosis is generally good with prompt, adequate treatment. Common long-term complications include venous insufficiency with or without the post-thrombotic syndrome.
DVT occurs most commonly in the lower extremities or pelvis (see figure Deep Veins of the Legs ). DVT is less common in deep veins of the upper extremities ( < 5% of DVT cases) ( 1 ).
Deep Veins of the Legs
Lower extremity DVT is much more likely to cause pulmonary embolism (PE), possibly because of the higher clot burden. Approximately 90% of proximal DVTs involve the femoral or popliteal veins and 10% extend more proximally to involve the iliofemoral veins ( 2 ). DVT of the distal or calf veins usually involve the posterior tibial or peroneal veins. Distal or calf vein DVT is less likely to be a source of large emboli but can propagate to the proximal thigh veins and from there cause PE. About 50% of patients with DVT have occult PE, and at least 30% of patients with PE have demonstrable DVT ( 3 ).
Pearls & Pitfalls
General references.
1. Yamashita Y, Morimoto T, Amano H, et al . Deep vein thrombosis in upper extremities: Clinical characteristics, management strategies and long-term outcomes from the COMMAND VTE Registry. Thromb Res 2019;177:1-9. doi:10.1016/j.thromres.2019.02.029
2. Douketis JD, Kearon C, Bates S, Duku EK, Ginsberg JS . Risk of fatal pulmonary embolism in patients with treated venous thromboembolism. JAMA 1998;279(6):458-462. doi:10.1001/jama.279.6.458
3. Stevens SM, Woller SC, Kreuziger LB, et al . Antithrombotic Therapy for VTE Disease: Second Update of the CHEST Guideline and Expert Panel Report [published correction appears in Chest. 2022 Jul;162(1):269]. Chest 2021;160(6):e545-e608. doi:10.1016/j.chest.2021.07.055
Etiology of Deep Venous Thrombosis
Many factors can contribute to DVT (see table Risk Factors for Deep Venous Thrombosis and Pulmonary Embolism ). Cancer is a risk factor for DVT, particularly in older patients and in patients with recurrent thrombosis. The association is strongest for lung, ovarian, gastric, brain or pancreatic cancers where 10 to 15% of patients can develop VTE ( 1 ). Occult cancers may be present in patients with apparently idiopathic DVT, but extensive workup of patients for tumors is not recommended unless patients have major risk factors for cancer or symptoms suggestive of an occult cancer.
Etiology reference
1. Farge D, Frere C, Connors JM, et al . 2022 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer, including patients with COVID-19. Lancet Oncol 2022;23(7):e334-e347. doi:10.1016/S1470-2045(22)00160-7
Pathophysiology of Deep Venous Thrombosis
Lower extremity DVT most often results from
Impaired venous return (eg, in immobilized patients)
Endothelial injury or dysfunction (eg, after leg fractures)
Hypercoagulability
Upper extremity DVT most often results from
Endothelial injury due to central venous catheters, pacemakers, or injection drug use
Upper extremity DVT occasionally occurs as part of superior vena cava (SVC) syndrome (compression or invasion of the superior vena cava by a tumor and causing symptoms such as facial swelling, dilated neck veins, and facial flushing) or results from a hypercoagulable state or subclavian vein compression at the thoracic outlet ( 1 ). The compression may be due to a normal or an accessory first rib or fibrous band ( thoracic outlet syndrome ) or occur during strenuous arm activity (effort thrombosis, or Paget-Schroetter syndrome, which is rare).
Deep venous thrombosis usually begins in venous valve cusps. Thrombi consist of thrombin , fibrin, and red blood cells with relatively few platelets (red thrombi); without treatment, thrombi may propagate proximally or travel to the lungs.
Complications
Common complications of DVT include
Chronic venous insufficiency
Post-thrombotic syndrome
Pulmonary embolism
Much less commonly, acute massive DVT lead to phlegmasia alba dolens or phlegmasia cerulea dolens, both of which, unless promptly diagnosed and treated, can result in venous gangrene.
In phlegmasia alba dolens, a rare complication of DVT during pregnancy, the leg turns milky white. Pathophysiology is unclear, but edema may increase soft-tissue pressure beyond capillary perfusion pressures, resulting in tissue ischemia and venous gangrene. Phlegmasia alba dolens may progress to phlegmasia cerulea dolens.
In phlegmasia cerulea dolens, massive iliofemoral venous thrombosis causes near-total venous occlusion; the leg becomes ischemic, extremely painful, and cyanotic. Pathophysiology may involve complete stasis of venous and arterial blood flow in the lower extremity because venous return is occluded or massive edema cuts off arterial blood flow. Venous gangrene may result.
Infection rarely develops in venous clots. Jugular vein suppurative thrombophlebitis (Lemierre syndrome), a bacterial (usually anaerobic) infection of the internal jugular vein and surrounding soft tissues, may follow tonsillopharyngitis and is often complicated by bacteremia and sepsis. In septic pelvic thrombophlebitis, pelvic thromboses develop postpartum and become infected, causing intermittent fever. Suppurative (septic) thrombophlebitis, a bacterial infection of a superficial peripheral vein, comprises infection and clotting and usually is caused by venous catheterization.
Pathophysiology reference
1. Bosch FTM, Nisio MD, Büller HR, van Es N . Diagnostic and Therapeutic Management of Upper Extremity Deep Vein Thrombosis. J Clin Med 2020;9(7):2069. doi:10.3390/jcm9072069
Symptoms and Signs of Deep Venous Thrombosis
DVT may occur in ambulatory patients or as a complication of surgery or major medical illness. Among patients who are hospitalized and at high risk, most deep vein thrombi occur in the small calf veins, are asymptomatic, and may not be detected.
When present, symptoms and signs of DVT (eg, vague aching pain, tenderness along the distribution of the veins, edema, erythema) are nonspecific, vary in frequency and severity, and are similar in arms and legs. Dilated collateral superficial veins may become visible or palpable. Calf discomfort elicited by ankle dorsiflexion with the knee extended (Homans sign) occasionally occurs with distal leg DVT but is neither sensitive nor specific. Tenderness, swelling of the whole leg, > 3 cm difference in circumference between calves, pitting edema, and collateral superficial veins may be most specific; DVT is likely with a combination of ≥ 3 in the absence of another likely diagnosis (see table Probability of Deep Venous Thrombosis Based on Clinical Factors ).
Low-grade fever may be present; DVT may be the cause of fever without an obvious source, especially in postoperative patients. Symptoms of pulmonary embolism , if it occurs, may include shortness of breath and pleuritic chest pain.
Common causes of asymmetric leg swelling that mimic DVT are
Soft-tissue trauma
Compression of a pelvic vein
Obstruction of a lymphatic vessel in the pelvis
Popliteal cyst ( Baker cyst ) that obstructs venous return
Less common causes include abdominal or pelvic tumors that obstruct venous or lymphatic return.
Symmetric bilateral leg swelling is the typical result of use of medications that cause dependent edema (eg, dihydropyridine calcium channel blockers, estrogen , high-dose opioids), venous hypertension (usually due to right heart failure), and hypoalbuminemia; however, such swelling may be asymmetric if venous insufficiency coexists and is worse in one leg.
Common causes of calf pain that mimic acute DVT include
Venous insufficiency and post-thrombotic syndrome
Cellulitis that causes painful erythema of the calf
Ruptured popliteal (Baker) cyst (pseudo-DVT), which causes calf swelling, pain, and sometimes bruising in the region of the medial malleolus
Partial or complete tears of the calf muscles or tendons
Diagnosis of Deep Venous Thrombosis
Ultrasonography.
Sometimes D-dimer testing
History and physical examination help determine probability of DVT before testing (see table Probability of Deep Venous Thrombosis Based on Clinical Factors ). Diagnosis is typically by ultrasonography with Doppler flow studies (duplex ultrasonography). The need for additional tests (eg, D-dimer testing) and their choice and sequence depend on pretest probability and sometimes ultrasonography results. No single testing protocol is best; one approach is described in the figure One Approach to Testing for Suspected Deep Venous Thrombosis .
One Approach to Testing for Suspected Deep Venous Thrombosis
© 2017 Elliot K. Fishman, MD.
Ultrasonography identifies thrombi by directly visualizing the venous lining and by demonstrating abnormal vein compressibility or, with Doppler flow studies, impaired venous flow. The test is > 90% sensitive and > 95% specific for femoral and popliteal vein thrombosis but is less accurate for iliac or calf vein thrombosis ( 1 ).
D-Dimer is a byproduct of fibrinolysis; elevated levels suggest recent presence and lysis of thrombi. D-Dimer assays vary in sensitivity and specificity; however, most are sensitive and not specific. A positive test result is nonspecific because levels can be elevated by other conditions (eg, liver disease, trauma, pregnancy, positive rheumatoid factor, inflammation, recent surgery, cancer), and further testing is necessary. Only the most accurate tests should be used. For example, a highly sensitive test is enzyme-linked immunosorbent assay (ELISA), which has a sensitivity of about 95% ( 2 ). D-dimer levels also increase with age, which further decreases the specificity in older patients ( 3 ).
The Pulmonary Embolism Graduated D-dimer (PEGeD) strategy is a diagnostic approach for pulmonary embolism that adjusts for D-dimer levels according to the patient's clinical pretest probability( 4 ):
If pretest probability of DVT is low, DVT can generally be excluded in patients with a D-dimer level < 1000 ng/mL( < 5476 nmol/L) on a sensitive test.
If pretest probability of DVT is moderate , DVT can be excluded in patients with a normal D-dimer level (ie,
If pretest probability of DVT is high, D-dimer testing can be done at the same time as duplex ultrasonography. A positive ultrasound result confirms the diagnosis regardless of the D-dimer level. If ultrasonography does not reveal evidence of DVT, a normal D-dimer level helps exclude DVT. Patients with an elevated D-dimer level should have repeat ultrasonography in a few days or additional imaging, such as venography, depending on clinical suspicion.
Additional testing
If symptoms and signs suggest PE, additional imaging (eg, CT pulmonary angiography or, less often, ventilation/perfusion [V/Q] scanning) is required.
Alternative imaging
Contrast-enhanced computed tomographic venography and magnetic resonance venography are other imaging modalities rarely used in the diagnosis of DVT. They are generally reserved for cases in which ultrasonography results are negative or indeterminate and the clinical suspicion for DVT remains high. These imaging tests are less well validated for DVT, are more costly, and may be associated with other complications (eg, related to exposure to radiation and contrast agents).
Contrast venography was the definitive test for the diagnosis of DVT in the past but has been largely replaced by ultrasonography, which is noninvasive, more readily available, and almost equally accurate for detecting DVT.
Determination of cause
Patients with confirmed DVT and an obvious cause (eg, immobilization, surgical procedure, leg trauma) need no further testing. Testing to detect hypercoagulability is controversial but is sometimes done in selected patients who have idiopathic (or unprovoked) DVT or recurrent DVT, in patients who have a personal or family history of other thromboses, and in young patients with no obvious predisposing factors. Some evidence suggests that testing for the presence of hypercoagulability in patients with or without clinical risk factors does not predict DVT recurrence ( 5, 6, 7 ) .
Screening patients with DVT for cancer has a low yield. Selective testing guided by complete history and physical examination and basic "routine" tests (complete blood count, chest x-ray, urinalysis, liver enzymes, and serum electrolytes, blood urea nitrogen [BUN], creatinine) aimed at detecting cancer is probably adequate. In addition, patients should have any appropriate cancer screening (eg, mammography, colonoscopy) that is due.
Diagnosis references
1. Lensing AW, Prandoni P, Brandjes D, et al . Detection of deep-vein thrombosis by real-time B-mode ultrasonography. N Engl J Med 1989;320(6):342-345. doi:10.1056/NEJM198902093200602
2. Di Nisio M, Squizzato A, Rutjes AW, Büller HR, Zwinderman AH, Bossuyt PM . Diagnostic accuracy of D-dimer test for exclusion of venous thromboembolism: a systematic review [published correction appears in J Thromb Haemost 2013 Oct;11(10):1942]. J Thromb Haemost 2007;5(2):296-304. doi:10.1111/j.1538-7836.2007.02328.x
3. Righini M, Van Es J, Den Exter PL, et al . Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: the ADJUST-PE study [published correction appears in JAMA. 2014 Apr 23-30;311(16):1694]. JAMA 2014;311(11):1117-1124. doi:10.1001/jama.2014.2135
4. Kearon C, de Wit K, Parpia S, et al . Diagnosis of Pulmonary Embolism with d-Dimer Adjusted to Clinical Probability. N Engl J Med 2019;381(22):2125-2134. doi:10.1056/NEJMoa1909159
5. Coppens M, Reijnders JH, Middeldorp S, Doggen CJ, Rosendaal FR . Testing for inherited thrombophilia does not reduce the recurrence of venous thrombosis. J Thromb Haemost 2008;6(9):1474-1477. doi:10.1111/j.1538-7836.2008.03055.x
6. Lijfering WM, Middeldorp S, Veeger NJ, et al . Risk of recurrent venous thrombosis in homozygous carriers and double heterozygous carriers of factor V Leiden and prothrombin G20210A. Circulation 2010;121(15):1706-1712. doi:10.1161/CIRCULATIONAHA.109.906347
7. Segal JB, Brotman DJ, Necochea AJ, et al . Predictive value of factor V Leiden and prothrombin G20210A in adults with venous thromboembolism and in family members of those with a mutation: a systematic review. JAMA 2009;301(23):2472-2485. doi:10.1001/jama.2009.853
Treatment of Deep Venous Thrombosis
Anticoagulation
Sometimes inferior vena cava filter, thrombolytic therapy, or surgery
Treatment is aimed primarily at pulmonary embolism prevention and secondarily at symptom relief and prevention of DVT recurrence, chronic venous insufficiency , and post-thrombotic syndrome. Treatment of lower and upper extremity DVT is generally the same.
General supportive measures include pain control with analgesics, which may include short (3- to 5-day) courses of a nonsteroidal anti-inflammatory drug (NSAID). Extended treatment with NSAIDs and aspirin should be avoided because their antiplatelet effects may increase the risk of bleeding complications. In addition, elevation of legs (supported by a pillow or other soft surface to avoid venous compression) is recommended during periods of inactivity. Patients may be as physically active as they can tolerate; there is no evidence that early activity increases risk of clot dislodgement and PE and may help to reduce the risk of the post-thrombotic syndrome.
Anticoagulants
(For details on medications and their complications, see Medications for Deep Venous Thrombosis )
Almost all patients with DVT are treated with anticoagulants ( 1 , 2 ). Various anticoagulants are suitable for initial therapy, and the choice of agent is influenced by patient comorbidities (eg, renal dysfunction, cancer), preferences, cost, and convenience.
heparin . Although heparin acts rapidly and provides immediate anticoagulation, warfarin takes about 5 days to achieve a therapeutic effect; hence, heparin factor Xa thrombin inhibitor), the oral agent is started after 5 days of injectable heparin .
heparin . Starting rivaroxaban or apixaban without heparin factor Xa inhibitor, is sometimes substituted for low molecular weight heparin and can also be used to treat acute DVT.
For selected patients (eg, with extensive iliofemoral DVT or cancer), continued treatment with a low-molecular-weight heparin rather than switching to an oral agent may be preferred.
Inadequate anticoagulation in the first 24 to 48 hours may increase risk of recurrence or of PE. Acute DVT can be treated on an outpatient basis unless severe symptoms require parenteral analgesics, other disorders preclude safe outpatient discharge, or other factors (eg, functional, socioeconomic) might prevent the patient from adhering to prescribed treatments.
Inferior vena cava (IVC) filter
An IVC filter may help prevent PE in patients with lower extremity DVT who have contraindications to anticoagulant therapy or in patients with recurrent DVT (or emboli) despite adequate anticoagulation. An IVC filter is placed in the inferior vena cava just below the renal veins via catheterization of an internal jugular or femoral vein. Some IVC filters are removable and can be used temporarily (eg, until contraindications to anticoagulation subside or resolve).
IVC filters reduce the risk of acute embolic complications but can have longer-term complications (eg, venous collaterals can develop, providing a pathway for emboli to circumvent the filter) There is also an increased risk of recurrent DVT). Also, IVC filters can dislodge or become obstructed by a clot. Thus, patients with recurrent DVT or nonmodifiable risk factors for DVT may still require anticoagulation despite the presence of an IVC filter.
A clotted filter may cause bilateral lower extremity venous congestion (including acute phlegmasia cerulea dolens), lower body ischemia, and acute kidney injury . Treatment for a dislodged filter is removal, using angiographic or, if necessary, surgical methods. Despite widespread use of IVC filters, efficacy in preventing PE is understudied and unproven ( 3 ). IVC filters should be removed whenever possible.
Thrombolytic (fibrinolytic) therapy
< 60 years with extensive iliofemoral DVT who have evolving or existing limb ischemia (eg, phlegmasia cerulea dolens) and do not have risk factors for bleeding ( 4 ).
Catheter-directed thrombolysis has largely replaced systemic administration when used for DVT.
Surgery is rarely needed. However, thrombectomy, fasciotomy, or both are mandatory for phlegmasia alba dolens or phlegmasia cerulea dolens unresponsive to thrombolytics to try to prevent limb-threatening gangrene.
Treatment references
1. Ortel TL, Neumann I, Ageno W, et al : American Society of Hematology 2020 guidelines for management of venous thromboembolism: treatment of deep vein thrombosis and pulmonary embolism. Blood Adv 4(19):4693-4738, 2020. doi: 10.1182/bloodadvances.2020001830
2. Stevens SM, Woller SC, Kreuziger LB, et al : Antithrombotic Therapy for VTE Disease: Second Update of the CHEST Guideline and Expert Panel Report [published correction appears in Chest 2022 Jul;162(1):269]. Chest 160(6):e545-e608, 2021. doi:10.1016/j.chest.2021.07.055
3. Turner TE, Saeed MJ, Novak E, Brown DL : Association of Inferior Vena Cava Filter Placement for Venous Thromboembolic Disease and a Contraindication to Anticoagulation With 30-Day Mortality. JAMA Netw Open 1(3):e180452, 2018. Published 2018 Jul 6. doi:10.1001/jamanetworkopen.2018.0452
4. Kearon C, Akl EA, Comerota AJ, et al . Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines [published correction appears in Chest 2012 Dec;142(6):1698-1704]. Chest 2012;141(2 Suppl):e419S-e496S. doi:10.1378/chest.11-2301
Prognosis for Deep Venous Thrombosis
Without adequate treatment, lower extremity DVT has a 3% risk of fatal PE ( 1, 2 ); death due to upper extremity DVT is very rare. Risk of recurrent DVT is lowest for patients with transient risk factors (eg, surgery, trauma, temporary immobility) and greatest for patients with persistent risk factors (eg, cancer), idiopathic DVT, or incomplete resolution of past DVT (residual thrombus). A normal D-dimer level obtained after anticoagulation is stopped for 3 to 4 weeks may help predict a relatively low risk of DVT or PE recurrence, more so in women than in men. Risk of venous insufficiency is difficult to predict. Risk factors for post-thrombotic syndrome include proximal thrombosis, recurrent ipsilateral DVT, and body mass index (BMI) ≥ 22 kg/m 2 .
Prognosis references
1. Yamashita Y, Murata K, Morimoto T, et al . Clinical outcomes of patients with pulmonary embolism versus deep vein thrombosis: From the COMMAND VTE Registry. Thromb Res 2019;184:50-57. doi:10.1016/j.thromres.2019.10.029
Prevention of Deep Venous Thrombosis
It is preferable and safer to prevent DVT than to treat it, particularly in patients who are at high risk. The following modalities are used (for a more complete discussion, see DVT Prevention ).
Prevention of immobility
Intermittent pneumatic compression
Patients who should not receive anticoagulants may benefit from intermittent pneumatic compression devices, elastic stockings, or both.
Inferior vena cava (IVC) filters do not prevent DVT but are sometimes placed in an attempt to prevent PE. An IVC filter may help prevent PE in patients with lower extremity DVT who have contraindications to anticoagulant therapy or in patients with recurrent DVT (or emboli) despite adequate anticoagulation. IVC filters are also sometimes used for the primary prevention of PE after certain types of surgery or in patients with multiple severe injuries; however, their use is not routinely recommended for these indications given the lack of evidence of efficacy ( 1 ).
Prevention reference
1. Ho KM, Rao S, Honeybul S, et al . A Multicenter Trial of Vena Cava Filters in Severely Injured Patients. N Engl J Med 2019;381(4):328-337. doi:10.1056/NEJMoa1806515
Symptoms and signs are nonspecific, so clinicians must be alert, particularly in high-risk patients.
Low-risk patients may have D-dimer testing, as a normal result essentially excludes deep venous thrombosis (DVT); others should have ultrasonography.
heparin factor Xa inhibitor) or a LMWH; alternatively, the oral factor Xa
Duration of treatment is typically 3 or 6 months, depending on the presence and nature of risk factors; certain patients require lifelong treatment.
Preventive treatment is required for bedbound patients with major illness and/or those undergoing certain surgical procedures.
Early mobilization, leg elevation, and an anticoagulant are the recommended preventive measures; patients who should not receive anticoagulants may benefit from intermittent pneumatic compression devices, elastic stockings, or both.

- Cookie Preferences

Copyright © 2024 Merck & Co., Inc., Rahway, NJ, USA and its affiliates. All rights reserved.
Deep vein thrombosis (DVT)
On this page, preparing for your appointment.
To diagnose deep vein thrombosis (DVT), your health care provider will do a physical exam and ask questions about your symptoms. The provider will check the legs for swelling, tenderness or changes in skin color.
The tests you have depend on whether your provider thinks you are at a low or a high risk of DVT .
Tests used to diagnose or rule out DVT include:
- D-dimer blood test. D dimer is a type of protein produced by blood clots. Almost all people with severe DVT have increased blood levels of D dimer. This test often can help rule out pulmonary embolism (PE).
- Duplex ultrasound. This noninvasive test uses sound waves to create pictures of how blood flows through the veins. It's the standard test for diagnosing DVT . For the test, a care provider gently moves a small hand-held device (transducer) on the skin over the body area being studied. Additional ultrasounds may be done over several days to check for new blood clots or to see if an existing one is growing.
- Venography. This test uses X-rays and dye to create a picture of the veins in the legs and feet. The dye is injected into a large vein in the foot or ankle. It helps blood vessels show up more clearly on X-rays. The test is invasive, so it's rarely done. Other tests, such as ultrasound, often are done first.
- Magnetic resonance imaging (MRI) scan. This test may be done to diagnose DVT in veins of the belly (abdomen).
More Information
There are three main goals to DVT treatment.
- Prevent the clot from getting bigger.
- Prevent the clot from breaking loose and traveling to the lungs.
- Reduce the chances of another DVT .
DVT treatment options include:
Blood thinners. These medicines, also called anticoagulants, help prevent blood clots from getting bigger. Blood thinners reduce the risk of developing more clots.
Blood thinners may be taken by mouth or given by intravenous (IV) or an injection under the skin. There are many different types of blood-thinning drugs used to treat DVT . Together, you and your health care provider will discuss their benefits and risks to determine the best one for you.
You might need to take blood thinner pills for three months or longer. It's important to take them exactly as prescribed to prevent serious side effects.
People who take a blood thinner called warfarin (Jantoven) need regular blood tests to monitor levels of the drug in the body. Certain blood-thinning medications are not safe to take during pregnancy.
Clot busters (thrombolytics). These drugs are used for more-serious types of DVT or PE , or if other medications aren't working.
Clot busters are given by or through a tube (catheter) placed directly into the clot. They can cause serious bleeding, so they're usually only used for people with severe blood clots.
- Filters. If you can't take medicines to thin your blood, a filter may be placed into a large vein — the vena cava — in your belly (abdomen). A vena cava filter prevents clots that break loose from lodging in the lungs.
- Support stockings (compression stockings). These special knee socks help prevent blood from pooling in the legs. They help reduce leg swelling. Wear them on your legs from your feet to about the level of your knees. For DVT , you typically wear these stockings during the day for a few years, if possible.

Compression stockings
Compression stockings, also called support stockings, press on the legs, improving blood flow. A stocking butler may help with putting on the stockings.
- Warfarin side effects
- Blood thinners: Can I still get blood clots?
From Mayo Clinic to your inbox
Clinical trials.
Explore Mayo Clinic studies testing new treatments, interventions and tests as a means to prevent, detect, treat or manage this condition.
After DVT treatment, follow these tips to manage the condition and prevent complications or more blood clots:
- Ask about your diet. Foods high in vitamin K, such as spinach, kale, other leafy greens and Brussels sprouts, can interfere with the blood thinner warfarin.
- Take medications as directed. Your provider will tell you how long you need treatment. If you're taking certain blood thinners, you'll need regular blood tests to see how well your blood is clotting.
- Watch for excessive bleeding. This can be a side effect of blood thinners. Ask your care provider about the warning signs. Know what to do if bleeding happens. Also ask your provider if you have activity restrictions. Minor injuries that cause bruising or even a simple cut may become serious if you're taking blood thinners.
- Move. If you've been on bed rest because of surgery or other reasons, the sooner you get moving, the lower the chance that blood clots will develop.
- Wear support stockings. Wear these to help prevent blood clots in the legs if your provider recommends them.
DVT is considered a medical emergency. It's important to get treated quickly. If there's time before your appointment, here's some information to help you get ready.
What you can do
Make a list of:
- Your symptoms, including any that seem unrelated to deep vein thrombosis, and when they began
- Important personal information, including notes about travel, hospital stays, any illness, surgery or trauma in the past three months, and any personal or family history of blood-clotting disorders
- All medications, vitamins or other supplements you take, including doses
- Questions to ask your health care provider
If possible, take a family member or friend with you to help you remember the information you're given.
For DVT , questions to ask your health care provider include:
- What's the most likely cause of my symptoms?
- What tests do I need?
- What's the best treatment?
- What are the options other than the main treatment that you're suggesting?
- Will I need to restrict travel or activities?
- I have other health conditions. How can I best manage these conditions together?
- Are there brochures or other printed material I can have? What websites do you recommend?
What to expect from your doctor
Your health care provider is likely to ask you questions, such as:
- Have you been inactive lately, such as sitting or lying down for long periods?
- Do you always have symptoms, or do they come and go?
- How severe are your symptoms?
- What, if anything, makes your symptoms improve?
- What, if anything, makes your symptoms worse?
Jun 11, 2022
- Venous thromboembolism. National Heart, Lung, and Blood Institute. https://www.nhlbi.nih.gov/health/venous-thromboembolism. Accessed April 5, 2022.
- Bauer KA, et al. Clinical presentation and diagnosis of the nonpregnant adult with suspected deep vein thrombosis of the lower extremity. https://www.uptodate.com/contents/search. Accessed April 5, 2022.
- Libby P, et al., eds. Cardiovascular disease in older adults. In: Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. 12th ed. Elsevier; 2022. https://www.clinicalkey.com. Accessed April 5, 2022.
- Lip GYH, et al. Overview of the treatment of lower extremity deep vein thrombosis (DVT). https://www.uptodate.com/contents/search. Accessed April 5, 2022.
- What is venous thromboembolism? Centers for Disease Control and Prevention. https://www.cdc.gov/ncbddd/dvt/facts.html. Accessed April 5, 2022.
- Diagnosis and treatment of venous thromboembolism. Centers for Disease Control and Prevention. https://www.cdc.gov/ncbddd/dvt/diagnosis-treatment.html. Accessed April 5, 2022.
- Jameson JL, et al., eds. Pulmonary thromboembolism and deep-vein thrombosis. In: Harrison's Manual of Medicine. 20th ed. McGraw Hill; 2020. https://accessmedicine.mhmedical.com. Accessed April 5, 2022.
- Hull RD, et al. Biology of warfarin and modulators of INR control. https://www.uptodate.com/contents/search. Accessed April 5, 2022.
- Blood thinner pills: Your guide to using them safely. Agency for Healthcare Research and Quality. https://www.ahrq.gov/patients-consumers/diagnosis-treatment/treatments/btpills/btpills.html. Accessed April 5, 2022.
- Pruthi RK (expert opinion). Mayo Clinic. Sept. 22, 2020.
- Physical Activity Guidelines for Americans. 2nd ed. U.S. Department of Health and Human Services. https://health.gov/our-work/physical-activity/current-guidelines. Accessed April 5, 2022.
- Symptoms & causes
- Doctors & departments
- Diseases & Conditions
- Deep vein thrombosis (DVT) diagnosis & treatment
Associated Procedures
Products & services.
- A Book: Mayo Clinic Family Health Book, 5th Edition
- Assortment of Compression Products at Mayo Clinic Store
- Newsletter: Mayo Clinic Health Letter — Digital Edition
CON-XXXXXXXX
Your gift holds great power – donate today!
Make your tax-deductible gift and be a part of the cutting-edge research and care that's changing medicine.
- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
Deep vein thrombosis
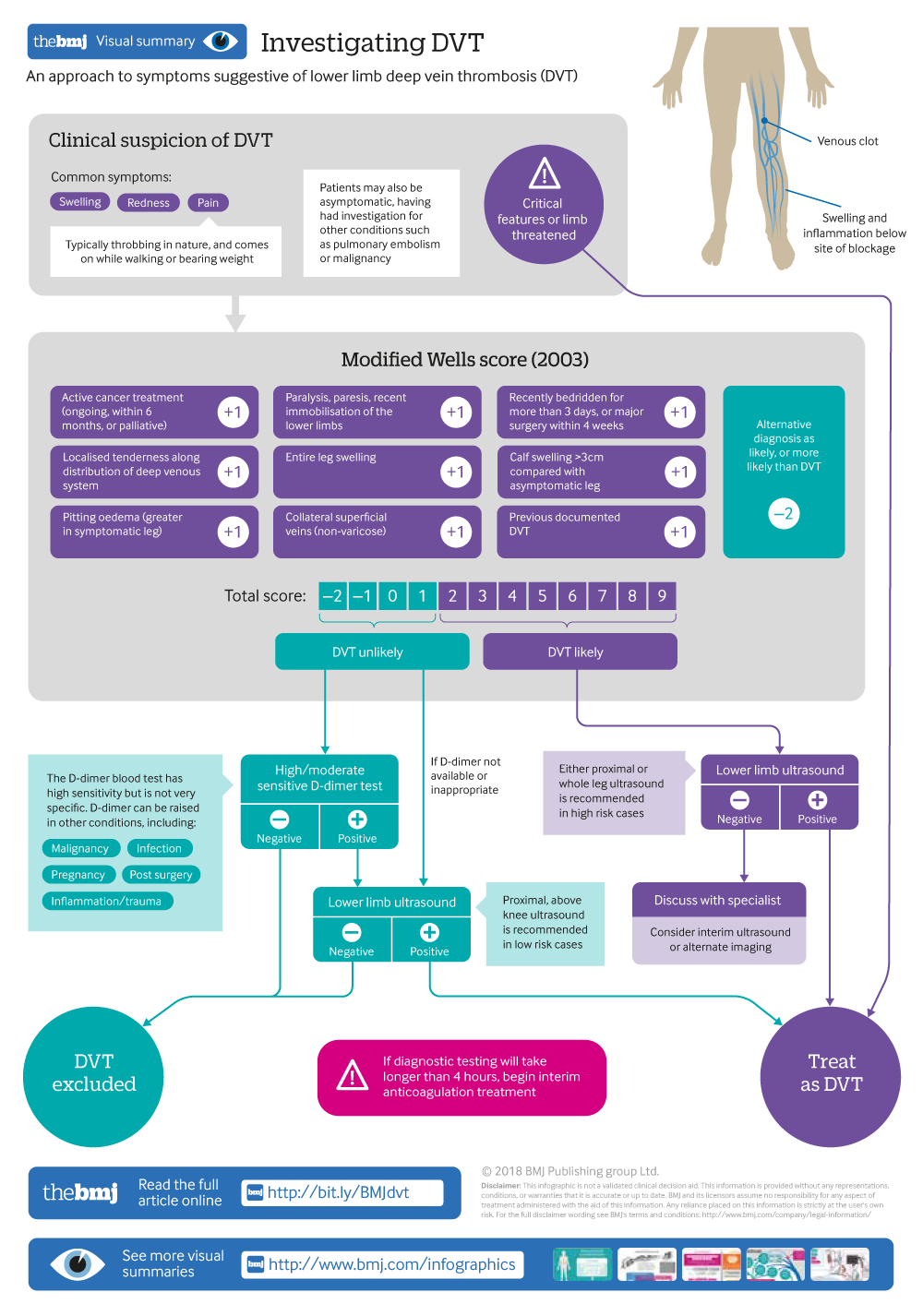
Investigating DVT
An approach to symptoms suggestive of lower deep vein thrombosis (DVT)
- Related content
- Peer review
This article has a correction. Please see:
- Deep vein thrombosis - March 21, 2018
- M J Stubbs , clinical research fellow and haematology registrar 1 ,
- Maria Mouyis , consultant rheumatologist 2 ,
- Mari Thomas , consultant haematologist 1
- 1 University College London Hospital, London, UK
- 2 North West London Hospitals NHS Trust, London, UK
- Correspondence to M Stubbs m.stubbs{at}doctors.org.uk
What you need to know
Pain, swelling, and redness of the affected limb are common symptoms of deep vein thrombosis (DVT)
Assess patients’ clinical risk of DVT using the Wells score
Refer urgently patients with suspected DVT for D-dimer test and/or proximal leg ultrasound
Anticoagulation to prevent clot extension and embolisation is initiated in secondary care, ideally within four hours of presentation
A direct oral anticoagulant is now first line for anticoagulation in patients with DVT not associated with cancer
Deep vein thrombosis (DVT) commonly affects the lower limb, with clot formation beginning in a deep calf vein and propagating proximally. 1 It is a common venous thromboembolic (VTE) disorder with an incidence of nearly 1.6 per 1000 inhabitants a year. 2 3 4 The rate of involvement of particular sites varies: distal veins 40%, popliteal 16%, femoral 20%, common femoral 20%, and iliac veins 4%. 1 Certain medical conditions listed in box 1 increase the likelihood of clot formation in the deep veins. Upper limb DVT represents less than 10% of all DVT, and central venous catheters are the main risk factor. 7 Venocaval thromboses are rare and are associated with malignancy, compression, and vascular abnormalities. 8 This article provides an overview for non-specialists on initial approach to patients with suspected DVT.
DVT risk factors 5 6
Transient risk factors.
Surgery with general anaesthetic (increased if >30 minutes)*
Hospitalisation (increased if >3 days with “bed rest”)*
Caesarean section*
Oestrogen therapy
Pregnancy or puerperium
Leg injury with reduced mobility for at least three days
Persistent risk factors
Active cancer
Medical condition with increased risk of recurrent VTE (inflammatory bowel disease, systemic lupus erythematosus)
Unprovoked VTE
If the above “Transient” and “Persistent” criteria are not met
*10 fold increase in VTE risk
Sources and selection criteria
We searched Medline and Cochrane databases for clinical trials, systematic reviews, and meta-analyses relevant to the diagnosis and management of DVT. Search terms included “deep vein thrombosis,” “venous thromboembolism,” “direct oral anticoagulants,” “thrombolysis,” and “post-thrombotic syndrome.” We reviewed guidelines from the British Society of Haematology, American College of Chest Physicians, and National Institute for Health and Care Excellence (NICE).
How do patients present?
Early recognition and referral for further investigation of DVT is likely to happen in primary care, however, diagnosis and initiation of anticoagulant treatment usually takes place in secondary care or hospital settings.
Pain, swelling, and redness in the affected limb are common symptoms. Pain is typically throbbing in nature, and comes on while walking or bearing weight. Skin changes include erythema, warmth, and oedema ( fig 1 ). 9 10 Some patients are asymptomatic, having had investigation for other conditions such as pulmonary embolism or malignancy 11 (although data on how many is unavailable). Alternative diagnoses to consider include cellulitis, ruptured Baker’s cyst, chronic venous insufficiency, and lymphoedema. 2 3 4
Deep vein thrombosis in the right leg of a patient, with leg swelling and erythema visible
- Download figure
- Open in new tab
- Download powerpoint
How is it diagnosed?
Refer patients with symptoms suggestive of DVT to acute/emergency services for further evaluation. 12 Diagnosis is based on clinical assessment and investigations (ultrasound or D-dimer) being conducted ideally within four hours of presentation as per NICE recommendations. 12 If a delay is expected, interim anticoagulation can be offered in hospital or a secondary care setting (discussed below under ‘How is it treated?’ ) to avoid clot progression and the risk of pulmonary embolism. 12 Ultrasound investigation is recommended within 24 hours in suspected cases. 12
Clinical score
The pre-test probability of DVT can be calculated using a validated score, such as the Wells score ( box 2 ), which combines assessment for risk factors and clinical features of DVT. 12 NICE recommends using the modified two-tier Wells score, whereas the American College of Chest Physicians (ACCP) guidelines cite the three-tiered Wells score. 9 10 Both scores are clinically acceptable, and local departmental guidance determines which is used in practice. 12 14
Pre-test probability scores for DVT 10 13
Wells score (1997).
Active cancer (treatment ongoing or within 6 months, or palliative) +1 point
Paralysis, paresis, recent immobilisation of the lower limbs +1 point
Recently bedridden for >3 days, or major surgery within 4 weeks +1 point
Localised tenderness along distribution of deep venous system +1 point
Entire leg swelling +1 point
Calf swelling, >3 cm, compared with asymptomatic leg +1 point
Pitting oedema (greater in symptomatic leg) +1 point
Collateral superficial veins (non-varicose) +1 point
Alternative diagnosis as likely, or more likely, than DVT −2 points
Modified Wells score (2003)
Scoring criteria as for Wells Score, with the addition of
Previous documented DVT +1 point
Interpretation
Wells score ≥3 high, 1-2 moderate, 0 low probability
Modified Wells score ≥2 likely DVT, <2 DVT unlikely
In clinical trials, 10 13 the Wells score has shown a high negative predictive value in patients with a low probability score for DVT (negative predictive value 99.7%, 95% confidence interval 98.3% to 100%). It is thus effective to exclude DVT in these patients. However, the score had a lower negative predictive value in high risk patients (82%, 95% confidence interval 98.3% to 100%), and should not be used to rule out DVT in these patients. 10 13 Patients were excluded if they were under 18, had less than three months’ life expectancy, were pregnant, or had already started anticoagulant treatment. The score is not appropriate in these groups. Investigation with D-dimer or ultrasound is required after calculating the pre-test probability in all patients, but the investigation pathway varies (infographic). 14
D-dimer test
The D-dimer blood test measures degraded fibrinogen, which is raised in patients with a clot. The reference range varies and is set by the laboratory. This test is recommended in patients with a low or moderate clinical probability of DVT, as calculated by the Wells score. 13 Patients with a high clinical probability of DVT need not undergo the D-dimer test and should directly have ultrasonography. 13 The test is easy to perform and readily available in secondary care. It has high sensitivity but is not very specific. Thus, a negative D-dimer can be used to exclude DVT in patients with a low clinical probability of DVT. However, it cannot confirm DVT, as D-dimer can be raised in other conditions including malignancy, infection, pregnancy, post-surgery, inflammation/trauma, disseminated intravascular coagulopathy, and renal impairment. 14 15 An ultrasound is needed to confirm DVT. 14
Ultrasonography
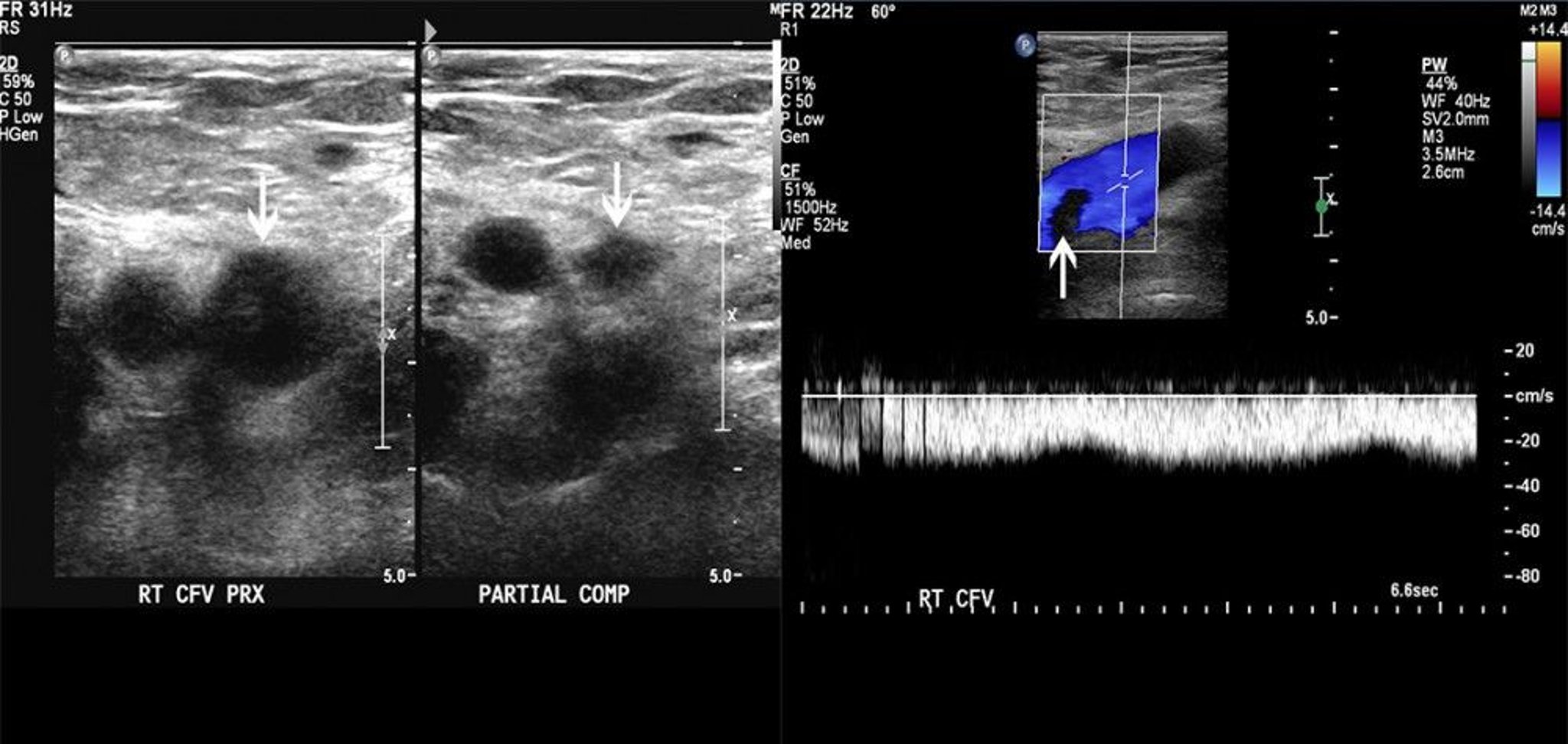
Request an ultrasound scan of the leg in patients with a high pre-test probability of DVT or with a low/moderate probability and a positive D-dimer test ( fig 2 ). Guidelines recommend performing an ultrasound (when indicated) within four hours of presentation, otherwise interim anticoagulation should be initiated. If not possible within four hours, ultrasound should be performed within 24 hours. In practice, substantial delays in ultrasound scanning for DVT should not occur.
Ultrasound image of the lower limb veins. (A) Normal deep vein, with patent vessel visible. (B) Thrombosed deep vein, with occluded vein apparent within dashed line
Proximal, above knee ultrasound is recommended in low risk cases, and either proximal or whole leg in high risk cases. 12 14 Rarely, a repeat scan after one week might be required to ensure DVT is not missed, for example in patients with a moderate/high probability and an initial negative ultrasound scan. Initiate anticoagulation during this window period. 12 14
Alternative imaging modalities, such as venography (computed tomography or magnetic resonance imaging, where radio-opaque contrast is injected to visualise the patency of vasculature) can be used if there is diagnostic uncertainty (eg, an inconclusive ultrasound report that might be caused by chronic venous scarring) or if ultrasound is impractical (eg, plaster cast). 14 These modalities are not routinely recommended, and should be discussed with a specialist before requesting them. 14
What additional investigations might be considered?
Cancer is a recognised risk factor for developing DVT. Up to 10% of DVT patients are subsequently diagnosed with a malignancy. 16 17 However, recent trials have found a low yield from screening for occult malignancy in patients with unprovoked DVT. 18 19 20 21 NICE now recommends only a limited cancer screen in patients with unprovoked DVT (ie, history, examination, basic blood tests, and age appropriate national cancer screening investigation, eg, in UK, mammography in women who are 50 to 70). 12
Inherited and acquired thrombophilias contribute to DVT. There is conflicting guidance on the role of thrombophilia testing, 22 and this should be discussed with a haematologist. 23
What are the complications?
Common chronic complications after DVT include post-thrombotic syndrome (25%-38%) and venous ulceration (9.8%), whereas pulmonary embolism (6%-32%) is more acute and can be fatal in 5%-10% of cases. 1 24 25 26 27 Complications can occur immediately following an acute DVT or several months to years later.
Patients with post-thrombotic syndrome might present with pain, swelling/oedema, leg heaviness, aching, skin discolouration, or venous ulceration. Venous ulcers are typically located medially above the ankle, with an irregular outline, and can be discoloured, oedematous, and exudative. The main risk factors include recurrent ipsilateral DVT and non-therapeutic international normalised ratio (>50% of the time). 28 Other risks include advanced age, increased body mass index, female sex, and size and location of thrombosis. 28 29
Rarer complications include chronic thromboembolic pulmonary hypertension, sudden death, and loss of limb. 1
How is it treated?
Anticoagulation to prevent clot extension and embolisation is the standard treatment, where bleeding risk permits. It is started in hospital or secondary care settings after a diagnosis of DVT is established, preferably within four hours of presentation. 12 30
Direct oral anticoagulants
Guidelines from NICE and ACCP recommend direct oral anticoagulants (DOACs) as first line treatment for DVT. 12 14 DOACs include direct factor Xa inhibitors apixaban, rivaroxaban, and edoxaban, and a direct thrombin inhibitor, dabigatran. Randomised controlled trials have shown DOACs to be at least as effective as vitamin K antagonists in treating thromboembolic events (see supplemental file for details of these trials). 31 32 33 34 Dabigatran and edoxaban require initial treatment with low molecular weight heparin (LMWH) (>5 days) before commencement of the DOAC, whereas rivaroxaban and apixaban do not 31 32 33 34 35 36 (see supplementary file). Typically patients are anticoagulated between five and 17 hours of taking these drugs. Caution is advised, however, in patients with chronic renal impairment, particularly with a glomerular filtration rate <30 ml/min, and in patients taking other drugs that have possible risk of interaction. 37
Low molecular weight heparin and warfarin
These are established anticoagulants that are preferred in certain people, such as those with liver and renal dysfunction, extremes of body weight (<50 kg or >120 kg), and DVT associated with cancer.
Warfarin, a vitamin K antagonist, is an effective and cheap oral anticoagulant. However, it requires frequent monitoring with blood tests, and has a narrow therapeutic window, with bleeding events being not uncommon. 30 LMWH is delivered daily or twice daily as a subcutaneous injection. It has a rapid onset of action, predictable anticoagulation effect, and does not require routine monitoring. 30
LMWH is recommended in patients with cancer-associated VTE, because VTE recurrence rates are lower than in patients taking vitamin K antagonists. 18 38 39 40 41 However, in a randomised controlled trial (1050 patients with cancer-associated VTE) oral edoxaban was shown to be non-inferior to daltaparin in terms of VTE recurrence (non-inferiority P=0.006, 95% confidence interval 0.70 to 1.36), but with a higher rate of major bleeding (P=0.04, 95% confidence interval 1.03 to 3.04). 42 More investigation is needed before recommending DOACs in cancer-associated VTE.
How long is anticoagulation continued?
The optimal duration of anticoagulation depends on what provoked the DVT, bleeding risk, patient preference, and thrombophilia status. Consensus expert opinion is to offer three months of anticoagulation treatment for patients with a DVT provoked by surgery or with a non-surgical transient risk factor. 38 Patients with a proximal DVT and a persistent risk factor or high risk of DVT recurrence might be offered lifelong anticoagulation. 43 Scoring systems such as the DASH prediction score and HERD002 score (in women) help predict recurrence after an unprovoked VTE event. 43 44 These might be used for risk stratification of patients to guide duration of anticoagulation.
What other treatments are available?
Some patients, such as those with extensive DVT, might require escalation of treatment beyond simple anticoagulation to reduce complications and recurrence of DVT. Box 3 lists other treatment options for DVT clot dissolution. These might be offered in specialist settings.
Other treatment options for patients with DVT 45
Percutaneous endovascular venous thrombolysis
Endovascular mechanical thrombectomy
Ultrasonic destruction of thrombus (+ thrombolysis)
Endovascular stenting (including iliac vein stenting)
Systemic thrombolysis (rarely used)
Surgical thrombectomy (rarely used, reserved for failed thrombolysis)
Inferior vena cava filter (rarely used and with limited/controversial evidence)
A Cochrane review (17 trials, 1103 patients) found that thrombolysis with anticoagulation reduced the incidence of post-thrombotic syndrome by a third compared with anticoagulation alone in patients with lower limb DVT. 46 47 On follow-up at >5 years, the rate of post-thrombotic syndrome was 390/1000 in the thrombolysis group, compared with 658/1000 in the control group (relative risk 0.58, 95% confidence interval 0.45 to 0.77; P<0.0001). No difference in mortality was observed between the two groups. Bleeding complications were increased in the thrombolysis group compared with controls (relative risk 2.23; 95% confidence interval 1.41 to 3.52, P=0.0006). There is limited data on the effect of thrombolysis in preventing leg ulceration, pulmonary embolism, and recurrence.
NICE guidelines suggest thrombolysis can be considered in an acute proximal DVT (<14 days) in a patient with low bleeding risk, with good performance status, and life expectancy >1 year. 47 Selection of patients must be guided by a multidisciplinary approach, with inputs from a vascular surgeon and interventional radiologist, and must consider patient preferences. 28 45 46 47 48
Results from the large multicentre ATTRACT study comparing catheter-directed thrombolysis with standard anticoagulation treatment in 692 DVT patients are expected soon, and will further inform thrombolysis decisions. 41
Is there a role for compression stockings?
Historically, it was believed that wearing compression stockings after DVT could reduce the risk of developing post-thrombotic syndrome. However, trials and meta-analyses have found no evidence of this, 49 50 51 and it is no longer recommended to wear compression stockings to prevent post-thrombotic syndrome. 38
How does management of superficial vein thrombosis differ?
Patients with superficial vein thrombosis can present with leg pain, erythema, and swelling, which are often indistinguishable from DVT. Patients with a recent superficial vein thrombosis have a four- to sixfold increased risk for DVT/pulmonary embolism. Risk factors for extension of superficial vein thrombosis into DVT include a superficial vein thrombosis <10 cm from the saphenofemoral junction, male sex, history of VTE, cancer, absence of varicose veins, and severe venous insufficiency. 8 Management is directed at reducing the risk of DVT, and has been discussed in depth elsewhere. 52
Questions for future research
What is the efficacy and safety of DOACs for treatment of DVT in patients with cancer?
Additional educational resources
British Society of Haematology haemostasis guidelines ( www.b-s-h.org.uk/guidelines )
Royal College of Obstetricians and Gynaecologists guidelines ( www.rcog.org.uk/guidelines )
American College of Chest Physicians ( www.chestnet.org/Guidelines-and-Resources )
BMJ review—BMJ clinical review. Diagnosis and management of heritable thrombophilias 14
BMJ Practice Pointer—Superficial vein thrombosis: http://www.bmj.com/content/350/bmj.h2039
Information resources for patients
Thrombosis UK Charity: www.thrombosisuk.org
Education into practice
Describe how you would investigate a patient with suspected DVT
How would you draw up a protocol for management of DVT in hospital?
What are the different treatment options for DVT, including indications for bridging therapy and duration of treatment?
How patients were involved in the creation of this article
A patient and carer kindly reviewed an earlier draft of this article. The patient highlighted the importance of making a timely diagnosis of DVT. Based on his suggestion, we have included the timescales suggested by NICE for urgent investigations and initiation of treatment.
Contributors M J Stubbs, M Mouyis, M Thomas
We have read and understood The BMJ policy on declaration of interests and declare that we have no competing interests.
Patients were not involved in the creation of this article.
Provenance and peer review: Commissioned; externally peer reviewed.
- Strijkers RH ,
- Cate-Hoek AJ ,
- Bukkems SF ,
- Nordström M ,
- Lindblad B ,
- Bergqvist D ,
- Kjellström T
- Julian JA ,
- Newman TE ,
- Ginsberg JS
- van Rooden CJ ,
- Westerbeek RE ,
- Cannegieter SC ,
- Geersing GJ ,
- Subcommittees on Control of Anticoagulation, and Predictive and Diagnostic Variables in Thrombotic Disease
- Flinterman LE ,
- Van Der Meer FJ ,
- Rosendaal FR ,
- British Committee for Standards in Haematology
- Lensing AW ,
- Koopman MM ,
- Anderson DR ,
- Bormanis J ,
- Yamashita Y ,
- Morimoto T ,
- ↵ National Institute for Health and Care Excellence. Deep vein thrombosis. 2013. https://cks.nice.org.uk/deep-vein-thrombosis
- Jaeschke R ,
- Stevens SM ,
- Righini M ,
- Den Exter PL ,
- Carrier M ,
- Fergusson D ,
- Gheshmy A ,
- Watson HG ,
- Keeling DM ,
- Van Doormaal FF ,
- Terpstra W ,
- Van Der Griend R ,
- Le Roux PY ,
- Planquette B ,
- MVTEP study group
- Prandoni P ,
- Bernardi E ,
- MacCallum P ,
- Greaves M ,
- Eichlisberger R ,
- Frauchiger B ,
- Widmer MT ,
- Widmer LK ,
- Lapner ST ,
- Rodgers A ,
- Birchall N ,
- Vedantham S ,
- Kaufman JA ,
- Arnoldussen CW ,
- ↵ National Institute for Clinical Excellence. Guidelines on licensing for DOACs. 2015 https://cks.nice.org.uk/anticoagulation-oral
- Bauersachs R ,
- Berkowitz SD ,
- Brenner B ,
- EINSTEIN Investigators
- Agnelli G ,
- Buller HR ,
- AMPLIFY Investigators
- Schulman S ,
- Kakkar AK ,
- Goldhaber SZ ,
- RE-COVER II Trial Investigators
- Connolly SJ ,
- Ezekowitz MD ,
- RE-LY Steering Committee and Investigators
- Büller HR ,
- Décousus H ,
- Grosso MA ,
- Hokusai-VTE Investigators
- Gómez-Outes A ,
- Terleira-Fernández AI ,
- Lecumberri R ,
- Suárez-Gea ML ,
- Vargas-Castrillón E
- Ornelas J ,
- Levine MN ,
- Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) Investigators
- Kamphuisen PW ,
- CATCH Investigators
- Raskob GE ,
- Verhamme P ,
- Hokusai VTE Cancer Investigators
- Tosetto A ,
- Marcucci M ,
- Rodger MA ,
- REVERSE II Study Investigators
- ↵ Behravesh S, Hoang P, Nanda A, et al. Pathogenesis of thromboembolism and endovascular management. Thrombosis 2017;2017:3039713.
- Broderick C ,
- ↵ National Institute for Health and Care Excellence. Guidelines on ultrasound-enhanced, catheter-directed thrombolysis for deep vein thrombosis. 2015. https://www.nice.org.uk/guidance/ipg523
- Shapiro S ,
- SOX trial investigators
- Subbiah R ,
- Aggarwal V ,
- Kolluri R ,
- Chatterjee S ,

An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Heart-Healthy Living
- High Blood Pressure
- Sickle Cell Disease
- Sleep Apnea
- Information & Resources on COVID-19
- The Heart Truth®
- Learn More Breathe Better®
- Blood Diseases and Disorders Education Program
- Publications and Resources
- Blood Disorders and Blood Safety
- Sleep Science and Sleep Disorders
- Lung Diseases
- Health Disparities and Inequities
- Heart and Vascular Diseases
- Precision Medicine Activities
- Obesity, Nutrition, and Physical Activity
- Population and Epidemiology Studies
- Women’s Health
- Research Topics
- Clinical Trials
- All Science A-Z
- Grants and Training Home
- Policies and Guidelines
- Funding Opportunities and Contacts
- Training and Career Development
- Email Alerts
- NHLBI in the Press
- Research Features
- Past Events
- Upcoming Events
- Mission and Strategic Vision
- Divisions, Offices and Centers
- Advisory Committees
- Budget and Legislative Information
- Jobs and Working at the NHLBI
- Contact and FAQs
- NIH Sleep Research Plan
- < Back To Home
- Deep Vein Thrombosis (DVT)
- What Is Venous Thromboembolism?
- Pulmonary Embolism (PE)
- Causes and Risk Factors
- Preventing Blood Clots
- Women and venous thromboembolism (VTE)
MORE INFORMATION
Venous Thromboembolism Deep Vein Thrombosis (DVT)
Language switcher.
Call your healthcare provider right away if you think you may have symptoms of deep vein thrombosis, or DVT. DVT should be taken seriously, as it may lead to a life-threatening pulmonary embolism (PE) .
What is DVT?
Learn about DVT and steps you can take to prevent it. Medical Animation Copyright © 2022 Nucleus Medical Media, All rights reserved .
DVT is the most common type of venous thromboembolism (VTE). It occurs when a blood clot forms in a deep vein, usually in the lower leg, thigh, or pelvis.
How does blood clot?
Learn about the normal blood clotting process and how problems in this process can lead to dangerous blood clots such as DVT.
What are the symptoms of DVT?
You may notice these symptoms of DVT around the area of a blood clot in your leg:
- Pain or tenderness
- Cramping, aching, or increased warmth
- Red or discolored skin
How is DVT diagnosed?
Your provider will diagnose DVT based on your symptoms, medical history, a physical exam, and various imaging or blood test results.
- D-dimer tests measure a substance in the blood that is released when the fibrin protein (proteins that help stop bleeding) in a blood clot dissolve. If the test shows high levels of the substance, you may have DVT. These tests may be used as a first step to look for signs of a blood clot in otherwise healthy people.
- Compression ultrasound looks for blood clots in the deep veins of your legs. This test uses sound waves to create pictures of blood flowing in your veins. The person doing the test may press on your veins to see whether the veins compress normally or are stiff with blood clots.
- Magnetic resonance venography uses a specialized magnet to take images of your veins. Your provider will need to give you a special dye through an intravenous tube (IV) before the test. This test is usually only used if your provider cannot diagnose DVT from the compression ultrasonography results.
What causes DVT?
DVT may occur if the flow of blood slows down in your body’s deep veins, if something damages the blood vessel lining, or if the makeup of the blood itself changes so that blood clots form more easily.
Many factors can raise the likelihood of blood clotting in the deep veins of the legs.
- Age: DVT can occur at any age, but the chances rise as you get older.
- Family history: Some genes you inherit may raise your likelihood of developing blood clots.
- Not moving for long periods of time: DVT can develop during a long flight or when a person is on bed rest in a nursing home, hospital setting, or after surgery. The chance of developing a blood clot is highest in the first 3 months after surgery and lowers with time. Ask your healthcare provider about prevention plans if you are scheduled for major surgery.
- Medical conditions: A blood clotting disorder , immune illnesses such as lupus, heart problems, Cancer , or serious illness such as getting infected with SARS-CoV-2, the virus that causes COVID-19, can raise the likelihood of DVT.
- Sex: Women in their childbearing years are more likely than men to develop blood clots. The chance is higher for pregnant women and women who take birth control pills or get hormone therapy. After menopause, women’s risk is lower than men’s.
How is DVT treated?
Most people can treat DVT with medicines at home. Sometimes, more serious blood clots require you to stay in the hospital for treatment.
Your healthcare provider will likely prescribe blood-thinning medicine to keep blood clots from getting larger and prevent a DVT from becoming a life-threatening pulmonary embolism. If you are unable to take blood thinners, other medicines or procedures can help. Learn more about treatments for DVT .
As you recover from DVT, talk to your provider about what you can do to stay healthy.
- Be aware of possible complications. A condition called post-thrombotic syndrome can develop following DVT. If you experience pain, itchiness, or swelling, tell your healthcare provider.
- Prevent a repeat DVT. Talk with your provider about your risk , get regular checkups, and take all medicines as prescribed to help lower your chance of having repeat blood clots.
- Make healthy lifestyle changes. Talk to your provider about changes you may need to make, including choosing heart-healthy foods, getting physically active, aiming for a healthy weight, and quitting smoking.
- Take care of your mental health. Anxiety, fear, and stress can be common after a blood clot. Reach out to your healthcare provider if you need support.
Advertisement
Summary of recommendations
Introduction, recommendations, what are others saying, and what is new, limitations of these guidelines, plans for updating these guidelines, adapting recommendations locally, acknowledgment, american society of hematology 2020 guidelines for management of venous thromboembolism: treatment of deep vein thrombosis and pulmonary embolism.
- Split-Screen
- Request Permissions
- Cite Icon Cite
- Search Site
- Open the PDF for in another window
Thomas L. Ortel , Ignacio Neumann , Walter Ageno , Rebecca Beyth , Nathan P. Clark , Adam Cuker , Barbara A. Hutten , Michael R. Jaff , Veena Manja , Sam Schulman , Caitlin Thurston , Suresh Vedantham , Peter Verhamme , Daniel M. Witt , Ivan D. Florez , Ariel Izcovich , Robby Nieuwlaat , Stephanie Ross , Holger J. Schünemann , Wojtek Wiercioch , Yuan Zhang , Yuqing Zhang; American Society of Hematology 2020 guidelines for management of venous thromboembolism: treatment of deep vein thrombosis and pulmonary embolism. Blood Adv 2020; 4 (19): 4693–4738. doi: https://doi.org/10.1182/bloodadvances.2020001830
Download citation file:
- Ris (Zotero)
- Reference Manager
Venous thromboembolism (VTE), which includes deep vein thrombosis (DVT) and pulmonary embolism (PE), occurs in ∼1 to 2 individuals per 1000 each year, corresponding to ∼300 000 to 600 000 events in the United States annually.
These evidence-based guidelines from the American Society of Hematology (ASH) intend to support patients, clinicians, and others in decisions about treatment of VTE.
ASH formed a multidisciplinary guideline panel balanced to minimize potential bias from conflicts of interest. The McMaster University GRADE Centre supported the guideline development process, including updating or performing systematic evidence reviews. The panel prioritized clinical questions and outcomes according to their importance for clinicians and adult patients. The Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach was used to assess evidence and make recommendations, which were subject to public comment.
The panel agreed on 28 recommendations for the initial management of VTE, primary treatment, secondary prevention, and treatment of recurrent VTE events.
Strong recommendations include the use of thrombolytic therapy for patients with PE and hemodynamic compromise, use of an international normalized ratio (INR) range of 2.0 to 3.0 over a lower INR range for patients with VTE who use a vitamin K antagonist (VKA) for secondary prevention, and use of indefinite anticoagulation for patients with recurrent unprovoked VTE. Conditional recommendations include the preference for home treatment over hospital-based treatment for uncomplicated DVT and PE at low risk for complications and a preference for direct oral anticoagulants over VKA for primary treatment of VTE.
Initial management
Recommendation 1..
For patients with uncomplicated deep vein thrombosis (DVT), the American Society of Hematology (ASH) guideline panel suggests offering home treatment over hospital treatment (conditional recommendation based on low certainty in the evidence of effects ⨁⨁○○).
Remarks: This recommendation does not apply to patients who have other conditions that would require hospitalization, have limited or no support at home, and cannot afford medications or have a history of poor compliance. Patients with limb-threatening DVT or a high risk for bleeding and those requiring IV analgesics may benefit from initial treatment in the hospital.
Recommendation 2.
For patients with pulmonary embolism (PE) with a low risk for complications, the ASH guideline panel suggests offering home treatment over hospital treatment (conditional recommendation based on very low certainty in the evidence of effects ⨁○○○).
Remarks: Clinical prediction scores have, at best, a moderate ability to predict patient outcomes and, therefore, do not replace clinical judgment. However, they may help to select patients at low risk for complications. The Pulmonary Embolism Severity Index (PESI) 1 and simplified PESI 2 have been most widely validated. This recommendation does not apply to patients who have other conditions that would require hospitalization, have limited or no support at home, and cannot afford medications or have a history of poor adherence. Patients with submassive (ie, intermediate-high risk) or massive PE or at high risk for bleeding and those requiring IV analgesics may benefit from initial treatment in the hospital.
Recommendation 3.
For patients with DVT and/or PE, the ASH guideline panel suggests using direct oral anticoagulants (DOACs) over vitamin K antagonists (VKAs) (conditional recommendation based on moderate certainty in the evidence of effects ⨁⨁⨁○).
Remarks: This recommendation may not apply to certain subgroups of patients, such as those with renal insufficiency (creatinine clearance <30 mL/min), moderate to severe liver disease, or antiphospholipid syndrome.
Recommendation 4.
For patients with DVT and/or PE, the ASH guideline panel does not suggest 1 DOAC over another (conditional recommendation based on very low certainty in the evidence of comparative effects ⨁○○○).
Remarks: Factors, such as a requirement for lead-in parenteral anticoagulation, once- vs twice-daily dosing, and out-of-pocket cost may drive the selection of specific DOACs. Other factors, such as renal function, concomitant medications (eg, need for a concomitant drug metabolized through the CYP3A4 enzyme or P-glycoprotein), and the presence of cancer, may also impact DOAC choice.
Recommendation 5.
In most patients with proximal DVT, the ASH guideline panel suggests anticoagulation therapy alone over thrombolytic therapy in addition to anticoagulation (conditional recommendation based on low certainty in the evidence of effects ⨁⨁○○).
Remarks : Thrombolysis is reasonable to consider for patients with limb-threatening DVT (phlegmasia cerulea dolens) and for selected younger patients at low risk for bleeding with symptomatic DVT involving the iliac and common femoral veins (higher risk for more severe postthrombotic syndrome [PTS] 3 ). Patients in these categories who value rapid resolution of symptoms, are averse to the possibility of PTS, and accept the added risk of major bleeding may prefer thrombolysis. The use of thrombolysis should be rare for patients with DVT limited to veins below the common femoral vein.
Recommendation 6.
For patients with PE and hemodynamic compromise, the ASH guideline panel recommends using thrombolytic therapy followed by anticoagulation over anticoagulation alone (strong recommendation despite low certainty in the evidence of effects ⨁⨁○○).
Remarks: Strong recommendations based on low certainty in the evidence are exceptional. In this case, the high mortality of patients with PE and hemodynamic compromise, as well as the potential lifesaving effect of thrombolytics, warranted a strong recommendation. This exception is in accordance with the exceptional circumstances that allow strong recommendations based on low-certainty evidence in the Grading of Recommendations Assessment, Development and Evaluation (GRADE) ASH rules.
Recommendation 7.
For patients with PE with echocardiography and/or biomarkers compatible with right ventricular dysfunction but without hemodynamic compromise (submassive PE), the ASH guideline panel suggests anticoagulation alone over the routine use of thrombolysis in addition to anticoagulation (conditional recommendation based on low certainty in the evidence of effects ⨁⨁○○).
Remarks: Thrombolysis is reasonable to consider for submassive PE and low risk for bleeding in selected younger patients or for patients at high risk for decompensation due to concomitant cardiopulmonary disease. Patients with submassive PE should be monitored closely for the development of hemodynamic compromise.
Recommendation 8.
For patients with extensive DVT in whom thrombolysis is considered appropriate, the ASH guideline panel suggests using catheter-directed thrombolysis over systemic thrombolysis (conditional recommendation based on very low certainty in the evidence of effects ⨁○○○).
Remarks: Given the very-low-certainty evidence (uncertainty regarding the benefits and harms of catheter-directed thrombolysis compared with systemic thrombolysis), the panel followed the GRADE ASH rules and issued a conditional recommendation. However, 4 panel members believed the recommendation should have been graded as strong based on the lack of evidence showing meaningful clinical benefits outweighing the known bleeding risks associated with systemic thrombolysis.
Recommendation 9.
For patients with PE in whom thrombolysis is considered appropriate, the ASH guideline panel suggests using systemic thrombolysis over catheter-directed thrombolysis (conditional recommendation based on very low certainty in the evidence of effects ⨁○○○).
Remarks : This recommendation reflects uncertainty about catheter-directed thrombolysis for PE rooted in the paucity of randomized trial data and variability in procedural experience across centers. In centers with the appropriate infrastructure, clinical staff, and procedural experience, catheter-directed thrombolysis may be an alternative to systemic thrombolysis, especially for patients with an intermediate to high risk for bleeding, because the total dose and duration of administration of thrombolytic agents are lower when delivered by catheter.
Recommendations 10 and 11.
For patients with proximal DVT and significant preexisting cardiopulmonary disease, as well as for patients with PE and hemodynamic compromise, the ASH guideline panel suggests anticoagulation alone rather than anticoagulation plus insertion of an inferior vena cava (IVC) filter (conditional recommendations based on low certainty in the evidence of effects ⨁⨁○○).
Remarks : These recommendations apply to patients who are eligible to receive anticoagulation. For patients with a contraindication to anticoagulation, insertion of a retrievable IVC filter may be indicated with retrieval as soon as the patient is able to receive anticoagulation.
Primary treatment
Primary treatment refers to the minimal length of time a patient must be on therapeutic anticoagulation to treat the initial venous thromboembolism (VTE) before consideration is given to discontinuing anticoagulation or switching to a long-term anticoagulation regimen aimed at preventing VTE recurrence (secondary prevention) ( Figure 1 ). Recommendations 12 through 14 refer to the length of time for primary treatment of the initial VTE in 3 patient populations.

Time frame of the decisions. Initial management (yellow box) spans the first 5 to 21 days following diagnosis of a new VTE and includes issues concerning whether the patient can be treated at home or requires admission to the hospital, use of thrombolytic therapy, whether an IVC filter needs to be placed, and initial anticoagulant therapy. Primary treatment continues anticoagulant therapy for 3 to 6 months total and represents the minimal duration of treatment for the VTE. After completion of primary treatment, the next decision concerns whether anticoagulant therapy will be discontinued or if it will be continued for secondary prevention of recurrent VTE. Typically, secondary prevention is continued indefinitely, although patients should be reevaluated on a regular basis to review the benefits and risks of continued anticoagulant therapy. Our choice of terminology reflects the distinct clinical intentions of the different phases of VTE management, linking them to important clinical decisions addressed in the guidelines, rather than using terms reflecting the relative duration of therapy.
Recommendations 12, 13, and 14 .
For primary treatment of patients with DVT and/or PE, whether provoked by a transient risk factor (recommendation 12) or by a chronic risk factor (recommendation 13) or unprovoked (recommendation 14), the ASH guideline panel suggests using a shorter course of anticoagulation for primary treatment (3-6 months) over a longer course of anticoagulation for primary treatment (6-12 months) (conditional recommendations based on moderate certainty in the evidence of effects ⨁⨁⨁○).
Remarks: These recommendations are intended to address the duration of primary anticoagulant treatment for all patients with DVT and/or PE, defined as the minimal length of time for treatment of the initial VTE ( Figure 1 ). Most patients with DVT and/or PE provoked by temporary risk factors will discontinue anticoagulant therapy after completion of the primary treatment. In contrast, many patients with DVT and/or PE provoked by chronic risk factors, as well as patients with unprovoked DVT and/or PE, may continue anticoagulant therapy indefinitely for secondary prevention after completion of the primary treatment ( Figure 1 ). However, if patients and clinicians decide to stop anticoagulation, the ASH guideline panel suggests against using a longer course of primary anticoagulant therapy (6-12 months). For selected patients with a chronic risk factor for which some improvement is expected over time (eg, improved mobility with rehabilitation), a longer course of anticoagulation for the primary treatment phase (eg, 6-12 months) could be justified.
Secondary prevention
Following completion of primary treatment for the initial VTE, providers must decide whether to discontinue anticoagulant therapy or continue with long-term anticoagulation with the intent to prevent VTE recurrence, referred to as secondary prevention. Recommendations 15 through 19 address which patients should be considered for indefinite secondary prevention, and recommendations 20 through 22 address which antithrombotic therapies could be chosen for patients continuing indefinite secondary prevention.
Recommendations 15, 16, and 17 .
For patients with unprovoked DVT and/or PE, the ASH guideline panel suggests against routine use of prognostic scores (recommendation 15), D-dimer testing (recommendation 16), or ultrasound to detect residual vein thrombosis (recommendation 17) to guide the duration of anticoagulation (conditional recommendations based on very low certainty in the evidence of effects ⨁○○○).
Remarks: Indefinite anticoagulation is probably appropriate for the majority of patients with unprovoked VTE. However, in certain circumstances, such as when patients are undecided or the balance between risks and benefits is uncertain, clinicians and patients may use prognostic scores, D-dimer testing, or ultrasound assessment for residual thrombosis from an initial DVT to aid in reaching a final decision.
Recommendation 18 .
After completion of primary treatment for patients with DVT and/or PE provoked by a chronic risk factor, the ASH guideline panel suggests indefinite antithrombotic therapy over stopping anticoagulation (conditional recommendation based on moderate certainty in the evidence of effects ⨁⨁⨁○).
Remarks: Patients with DVT and/or PE provoked by a transient risk factor typically do not require antithrombotic therapy after completion of primary treatment. This recommendation refers to patients with DVT and/or PE provoked by a chronic persistent risk factor. However, this recommendation does not apply to patients who have a high risk for bleeding complications. For guidance on selection of antithrombotic therapy after completion of primary treatment, see Recommendation 20. Decisions regarding anticoagulation in individuals with cancer are discussed in a separate ASH guideline.

Recommendation 19 .
After completion of primary treatment for patients with unprovoked DVT and/or PE, the ASH guideline panel suggests indefinite antithrombotic therapy over stopping anticoagulation (conditional recommendation based on moderate certainty in the evidence of effects ⨁⨁⨁○).
Remarks: This recommendation does not apply to patients who have a high risk for bleeding complications. For guidance on selection of antithrombotic therapy after completion of primary treatment, see Recommendation 20.
Recommendation 20 .
For patients with DVT and/or PE who have completed primary treatment and will continue to receive secondary prevention, the ASH guideline panel suggests using anticoagulation over aspirin (conditional recommendation based on moderate certainty in the evidence of effects ⨁⨁⨁○).
Recommendation 21 .
For patients with DVT and/or PE who have completed primary treatment and will continue VKA therapy as secondary prevention, the ASH guideline panel recommends using an international normalized ratio (INR) range of 2.0 to 3.0 over a lower INR range (eg, 1.5-1.9) (strong recommendation based on moderate certainty in the evidence of effects ⨁⨁⨁○).
Recommendation 22 .
For patients with DVT and/or PE who have completed primary treatment and will continue with a DOAC for secondary prevention, the ASH guideline panel suggests using a standard-dose DOAC or a lower-dose DOAC (conditional recommendation based on moderate certainty in the evidence of effects ⨁⨁⨁○).
Remarks: Lower-dose DOAC regimens that may be considered for patients who have completed primary treatment and will continue with a DOAC include rivaroxaban, 10 mg daily, or apixaban, 2.5 mg twice daily.
Treatment of recurrent events
Recommendation 23 ..
For patients with breakthrough DVT and/or PE during therapeutic VKA treatment, the ASH guideline panel suggests using low-molecular-weight heparin (LMWH) over DOAC therapy (conditional recommendation based on very low certainty in the evidence of effects ⨁○○○).
Remarks: Patients who present with a new VTE event during therapeutic VKA treatment should be further investigated to identify potential underlying causes. This recommendation does not apply to patients who develop breakthrough VTE in the setting of poor INR control, in whom a DOAC may be a reasonable option.
Recommendation 24a .
For patients who develop DVT and/or PE provoked by a transient risk factor and have a history of previous unprovoked VTE or VTE provoked by a chronic risk factor, the ASH guideline panel suggests indefinite antithrombotic therapy over stopping anticoagulation after completing primary treatment (conditional recommendation based on moderate certainty in the evidence of effects ⨁⨁⨁○).
Recommendation 24b .
For patients who develop DVT and/or PE provoked by a transient risk factor and have a history of a previous VTE also provoked by a transient risk factor, the ASH guideline panel suggests stopping anticoagulation after completion of primary treatment over indefinite antithrombotic therapy (conditional recommendation based on moderate certainty in the evidence of effects ⨁⨁⨁○).
Recommendation 25 .
For patients with a recurrent unprovoked DVT and/or PE, the ASH guideline panel recommends indefinite antithrombotic therapy over stopping anticoagulation after completion of primary treatment (strong recommendation based on moderate certainty in the evidence of effects ⨁⨁⨁○).
Remarks ( Recommendations 24a , 24b , and 25 ): For guidance on selection of antithrombotic therapy after completion of primary treatment, see Recommendation 20.
Additional management issues
Recommendation 26 ..
For patients with DVT and/or PE with stable cardiovascular disease (CVD) who initiate anticoagulation and were previously taking aspirin for cardiovascular risk modification, the ASH guideline panel suggests suspending aspirin over continuing it for the duration of anticoagulation therapy (conditional recommendation based on very low certainty in the evidence of effects ⨁○○○).
Remarks: A critical review of the indication for aspirin therapy is needed at the time anticoagulant therapy is initiated, considering the increased risk of bleeding vs the potential benefit in terms of cardiovascular prevention. This recommendation does not apply to patients with a recent acute coronary event or coronary intervention.
Recommendations 27 and 28 .
For patients with DVT, with (Recommendation 27) or without (Recommendation 28) an increased risk for PTS, the ASH guideline panel suggests against the routine use of compression stockings (conditional recommendations based on very low certainty in the evidence of effects ⨁○○○).
Remarks: Although the majority of patients may not benefit from the use of stockings to reduce the risk of PTS, stockings may help to reduce edema and pain associated with acute DVT in selected patients.
Aim of the guideline and specific objectives
The purpose of this guideline is to provide evidence-based recommendations about the treatment of DVT and PE for patients without cancer. The target audience includes patients, hematologists, general practitioners, internists, hospitalists, vascular interventionalists, intensivists, other clinicians, pharmacists, and decision makers. Policy makers interested in these guidelines include those involved in developing local, national, or international programs aiming to reduce the incidence of VTE or to evaluate direct and indirect harms and costs related to VTE. This document may also serve as the basis for adaptation by local, regional, or national guideline panels.
Description of the health problem
VTE, which includes DVT and PE, occurs in ∼1 to 2 individuals per 1000 each year, or ∼300 000 to 600 000 events in the United States annually. 4 DVT most commonly occurs in the lower extremities but also affects the upper extremities. 5,6 Approximately one third of all patients with a new diagnosis of VTE have PE, with or without DVT, 7-9 and it is estimated that up to a quarter of all patients with PE present with sudden death. 4
The risk for recurrent VTE varies according to whether the initial event was associated with an acquired risk factor, referred to as a provoked event, or in the absence of any provoking risk factors, referred to as an unprovoked event. 10 For patients with unprovoked VTE, the risks of recurrent VTE after completing a course of anticoagulant therapy have been estimated to be 10% by 2 years and >30% by 10 years. 11,12 Long-term complications include PTS, which develops in 20% to 50% of patients after DVT and is severe in up to 5% of cases, 13 and chronic thromboembolic pulmonary hypertension, which may develop in up to 5% of patients with PE. 14
Anticoagulant therapy is very effective at preventing recurrent VTE but is associated with an increased frequency of bleeding complications. Major bleeding events may occur in ∼1% to 3% of patients on VKAs each year, compared to an ∼30% lower relative risk for major bleeding with DOACs. 15
Description of the target populations
The incidence of VTE increases with age, ranging from ∼1 in 10 000 in individuals younger than 20 years of age to as high as ∼1 in 100 in individuals who are 80 years of age and older. 16 VTE affects all races and ethnicities, with black persons having a higher incidence than white persons in most studies and individuals of Asian descent having a lower incidence than other races. 17-19 Certain acquired characteristics identify subsets of individuals at higher risk for VTE, including individuals who are currently or were recently hospitalized, residents in long-term care facilities, and patients undergoing surgical procedures. 4
Time frame of the decisions
Conceptually, the therapeutic management of patients with a new diagnosis of VTE can be divided into 3 phases: (1) initial management, which occurs from the time of diagnosis through the first 3 weeks of therapy; (2) primary treatment, which is a time-limited phase that typically runs for a minimum of 3 months; and (3) secondary prevention, which begins after completion of the primary treatment phase and extends for a prolonged, usually indefinite, period of time ( Figure 1 ). The specific questions addressed by the guideline committee are most relevant at specific points in time during treatment, as summarized below.
Home treatment vs hospital treatment (Recommendations 1 and 2)
Choice of anticoagulant therapy (Recommendations 3 and 4)
Use of fibrinolytic therapy (Recommendations 5-9)
Use of IVC filters (Recommendations 10 and 11)
Duration of primary treatment (Recommendations 12-14)
Choice between stopping anticoagulation and indefinite therapy (Recommendations 15-19)
Choice of treatment for secondary prevention (Recommendations 20-22)
The following sections represent topics that may occur during any phase of treatment
Management of breakthrough and recurrent DVT/PE (Recommendations 23-25)
Decision concerning use of aspirin while on anticoagulant therapy (Recommendation 26)
Decision concerning use of compression stockings (Recommendations 27 and 28)
The guideline panel assessed the certainty in the supporting evidence and developed and graded the recommendations following the GRADE approach. 20-24 The overall guideline-development process, including funding of the work, panel formation, management of conflicts of interest, internal and external review, and organizational approval, was guided by ASH policies and procedures derived from the Guideline International Network–McMaster Guideline Development Checklist ( http://cebgrade.mcmaster.ca/guidecheck.html ). We developed our recommendations using the principles outlined by the Institute of Medicine and Guideline International Network. 25-27 An article detailing the methods used to develop these guidelines has been published. 360
Organization, panel composition, planning, and coordination
The work of this panel was coordinated with 9 other guideline panels (addressing other aspects of VTE) by ASH and the McMaster GRADE Centre (funded by ASH under a paid agreement). Project oversight was provided initially by a coordination panel, which reported to the ASH Committee on Quality, and then by the coordination panel chair (A.C.) and vice chair (H.J.S.). ASH vetted and appointed individuals to the guideline panel. The McMaster GRADE Centre vetted and retained researchers to conduct systematic reviews of evidence and to coordinate the guideline-development process. The membership of the panel and the GRADE Centre team is described in Supplement 1.
The panel included hematologists, internists, specialists in vascular medicine, an interventional radiologist, a cardiologist, and pharmacists who all had clinical and research expertise in VTE treatment; methodologists with expertise in evidence appraisal and guideline development; and 2 patient representatives. The panel chair was a hematologist with content expertise, whereas the vice chair was an internist with expertise in guideline development methodology.
In addition to synthesizing evidence systematically, the McMaster GRADE Centre supported the guideline-development process, including determining methods, preparing agendas and meeting materials, and facilitating panel discussions. The panel’s work was done using Web-based tools ( https://www.surveymonkey.com and https://www.gradepro.org ) and face-to-face and online meetings.
Guideline funding and management of conflicts of interest
Development of these guidelines was wholly funded by ASH, a nonprofit medical specialty society that represents hematologists. Most members of the panel were members of ASH. ASH staff supported panel appointments and coordinated meetings but had no role in choosing the guideline questions or determining the recommendations.
Members of the guideline panel received travel reimbursement for attendance at in-person meetings but received no other payments. The patient representative was offered, but declined, an honorarium of $200. Through the McMaster GRADE Centre, some researchers who contributed to the systematic evidence reviews received salary or grant support. Other researchers participated to fulfill requirements of an academic degree or program.
Conflicts of interest of all participants were managed according to ASH policies based on recommendations of the Institute of Medicine and Guideline International Network. 26,27 At the time of appointment, a majority of the guideline panel, including the chair and the vice chair, had no conflicts of interest, as defined and judged by ASH (ie, no current material interest in any commercial entity with a product that could be affected by the guidelines). Some panelists disclosed new interests or relationships during the development process, but the balance of the majority was maintained.
Before appointment of the panel and during the development process, panelists disclosed financial and nonfinancial interests. Members of the VTE Guideline Coordination Panel reviewed the disclosures and judged which interests were conflicts and should be managed. Supplement 2 provides the complete “Disclosure of Interests” forms of all panel members. In Part A of the forms, individuals disclosed material interests for 2 years prior to appointment. In Part B, they disclosed interests that were not primarily financial. Part C summarizes ASH decisions about which interests were judged to be conflicts. Part D describes new interests disclosed by individuals after appointment.
Recusal was used to manage conflicts of interest. During deliberations, panel members with a current direct financial conflict of interest in a commercial entity with any product that could be affected by the guidelines were recused from making judgments about relevant recommendations. The evidence-to-decision (EtD) framework for each recommendation describes which individuals were recused from making judgments about each recommendation.
None of the McMaster University–affiliated researchers who contributed to the systematic evidence reviews or who supported the guideline-development process had any current material interest in a commercial entity with any product that could be affected by the guidelines. Supplement 3 provides the complete “Disclosure of Interests” forms of researchers who contributed to these guidelines.
Formulating specific clinical questions and determining outcomes of interest
We initially brainstormed clinical issues relevant for VTE management. Then, using an on-line survey developed with SurveyMonkey ( https://www.surveymonkey.com ) and in an online meeting, we prioritized 32 clinical questions. We addressed 28 such questions in this guideline. Three questions related to clinical issues were seen more frequently for cancer patients and, therefore, will be addressed in the chapter about management of VTE for patients with cancer. One additional question was dropped at the in-person panel meeting because we considered that the clinical issue was sufficiently addressed by another recommendation ( Table 1 ).
Patient populations and interventions in VTE treatment
CVC, central venous catheter.
Panelists then selected outcomes of interest for each question a priori, following an approach described in detail elsewhere. 28 In brief, the panel first brainstormed all possible outcomes before rating the relative importance for decision making of each. During this rating process, the panel used definitions of the outcomes (“marker states”) that were developed for these guidelines. The panel rated the following outcomes as critical for clinical decision making across questions: mortality, PE, proximal DVT, and major bleeding. Additionally, for the questions related to thrombolysis in DVT and the use of compression stockings, the panel also rated the incidence of PTS as critical.
Evidence review and development of recommendations
For each guideline question, the McMaster GRADE Centre prepared a GRADE EtD framework, using the GRADEpro Guideline Development Tool ( www.gradepro.org ). The EtD table summarized the results of systematic reviews of the literature that were conducted for these guidelines. Each EtD table addressed the effects of interventions, resource use (cost effectiveness), values and preferences (relative importance of outcomes), equity, acceptability, and feasibility. The guideline panel reviewed draft EtD tables before, during, or after the guideline panel meeting and made suggestions for correction and identified missing evidence.
To estimate the effect of the interventions covered in this guideline, we conducted a search for systematic reviews on MEDLINE, Embase, the Cochrane Library, and Epistemonikos from their respective dates of inception to January 2017. We also conducted a search of potentially missed trials in MEDLINE and Embase from January 2014 to January 2017. Before the publication of this guideline, we updated the searches to January 2019 (detailed search strategies are described in Supplement 4). Additionally, panel members were asked to suggest any studies that may have been missed and fulfilled the inclusion criteria for the individual questions. We excluded trials evaluating the effects of the direct thrombin inhibitor ximelagatran, given that this drug was withdrawn from the market because of safety concerns in those countries where it had received approval.
We used existing systematic reviews as a way to identify relevant trials, but we conducted our own meta-analyses for all of the questions following the principles outlined in the Cochrane Handbook for Systematic Reviews of Interventions ( https://training.cochrane.org/handbook ). We meta-analyzed the data using a random effects model according to the method of Mantel-Haenszel. We explored heterogeneity with the χ 2 test and with the I 2 statistic. When significant heterogeneity was detected (I 2 ≥ 50%), we explored differences among trials in the included population, the way that interventions were used, outcomes measurement, and risk of bias.
All of the meta-analyses were conducted using RevMan (version 5.3 Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). Publication bias was assessed graphically by evaluating symmetry in the funnel plots. To estimate the absolute effect of the intervention, we calculated the risk difference by multiplying the pooled risk ratio and the baseline risk of each outcome. As baseline risk, we used the median of the risks observed in control groups of the included trials. Additionally, when possible, we used the baseline risk observed in large observational studies.
Certainty in the body of evidence was assessed (also known as quality of the evidence or confidence in the estimated effects) following the GRADE approach. We made judgments regarding risk of bias, precision, consistency, directness, and likelihood of publication bias and categorized the certainty in the evidence into 4 levels ranging from very low to high. 22,23 In addition, we conducted systematic searches to identify evidence related to baseline risks, values, preferences, and costs and summarized findings within the EtD tables.
During a 2-day in-person meeting, followed by online communication and conference calls, the panel developed clinical recommendations based on the evidence summarized in the EtD tables. For each recommendation, the panel took a population perspective and came to consensus on the following: the certainty in the evidence, the balance of benefits and harms of the compared management options, and the assumptions about the values and preferences associated with the decision. The guideline panel also explicitly took into account the extent of resource use associated with alternative management options.
The panel agreed on recommendations (including direction and strength), remarks, and qualifications by consensus or, in rare instances, by voting (an 80% majority was required for a strong recommendation) based on the balance of all desirable and undesirable consequences. In such circumstances, the result of the voting was recorded on the respective EtD table. The final guidelines, including recommendations, were reviewed and approved by all members of the panel.
Interpretation of strong and conditional recommendations
The recommendations are labeled as “strong” or “conditional” according to the GRADE approach. The words “the guideline panel recommends” are used for strong recommendations and “the guideline panel suggests” are used for conditional recommendations. Table 2 provides GRADE’s interpretation of strong and conditional recommendations by patients, clinicians, health care policy makers, and researchers.
Document review
All panel members reviewed the recommendations and remarks. The full EtD tables (including recommendations) were made available from 30 November 2018 to 19 January 2019 for external review by stakeholders, including allied organizations, medical professionals, patients, and the general public. We received comments and additional references from 17 individuals and organizations. The final document and supplemental material were revised to address pertinent inputs, but no changes were made to recommendations. The guidelines were approved by the ASH Guideline Oversight Subcommittee and Committee on Quality on 18 February 2020 and by the ASH Executive Committee on 26 February 2020 and then subjected to peer review.
How to use these guidelines
These guidelines are primarily intended to help clinicians make decisions about treatment alternatives. Other purposes are to inform policy, to promote education and advocacy, and to state future research needs. They may also be used by patients. These guidelines are not intended to serve or be construed as a standard of care. Clinicians must make decisions on the basis of the clinical presentation of each individual patient, ideally through a shared decision-making process that considers the patient’s values and preferences with respect to the anticipated outcomes of the chosen option. Decisions may be constrained by the realities of a specific clinical setting and local resources, including, but not limited to, institutional policies, time limitations, or availability of treatments.
These guidelines may not include all appropriate methods of care for the clinical scenarios described. As science advances and new evidence becomes available, recommendations may become outdated. Following these guidelines cannot guarantee successful outcomes. ASH does not warrant or guarantee any products described in these guidelines.
Statements about the underlying values and preferences, as well as qualifying remarks accompanying each recommendation, are its integral parts and serve to facilitate more accurate interpretation. They should never be omitted when recommendations from these guidelines are quoted or translated. The use of these guidelines is also facilitated by the links to the EtD frameworks and interactive summary of findings tables in each section.
Initial management: up through the first week
For patients with uncomplicated DVT, the ASH guideline panel suggests offering home treatment over hospital treatment (conditional recommendation based on low certainty in the evidence of effects ⨁⨁◯◯).
Remarks: This recommendation does not apply to patients who have other conditions that would require hospitalization, have limited or no support at home, and cannot afford medications or have a history of poor compliance. Patients with limb-threatening DVT or at high risk for bleeding and those requiring IV analgesics may benefit from initial treatment in the hospital.
Summary of the evidence
We identified 1 systematic review 29 and 7 randomized controlled trials (RCTs) (n = 1922). 30-36 Trials included individuals with an objectively confirmed symptomatic DVT. Participants were randomized to home or hospital management with LMWH, warfarin (both can be used in either setting), or unfractionated heparin (UFH) (which is generally used in hospital only). Four RCTs 30 , 33-35 had partial hospital treatment for some participants in the home group, ranging from a mean of 1 to 3 days, compared with 6.5 to 9.6 days in the hospital treatment arm. Trials with a hospital stay ≥3 days prior to home treatment were excluded. All seven trials reported the effect of antithrombotic therapy on mortality and PE and assessed the risk of major bleeding. Six trials reported the effect on proximal DVT. The EtD framework is shown online at: https://guidelines.gradepro.org/profile/A269DB76-A3AE-4994-A718-6F1E493D0A75 .
Treating patients with DVT at home, rather than in the hospital, reduced the risk of PE (relative risk [RR], 0.64; 95% confidence interval [CI], 0.44-0.93; absolute risk reduction [ARR], 25 fewer per 1000 patients; 95% CI, 38 fewer to 5 fewer; moderate-certainty evidence) and the risk of subsequent DVT (RR, 0.61; 95% CI, 0.42-0.90; ARR, 29 fewer per 1000 patients; 95% CI, 43 fewer to 7 fewer; moderate-certainty evidence). In a low-risk population, 37 home treatment reduces the risk of PE (2 fewer per 1000 patients with 95% CI of 4 fewer to 0 fewer; moderate-certainty evidence) and proximal DVT (4 fewer per 1000 patients with 95% CI of 6 fewer to 1 fewer; moderate-certainty evidence) as well. Home treatment was associated with a reduction in long-term mortality (RR, 0.72; 95% CI, 0.45-1.15; ARR, 13 fewer per 1000 patients; 95% CI, 25 fewer to 7 more; low-certainty evidence), although this was not statistically significant. When considering the mortality at 90 days for patients with DVT treated in the hospital as the baseline risk, 37 treating at home instead of treating in the hospital may lead to a reduction of 19 fewer deaths per 1000 patients (95% CI, 37 fewer to 10 more; low-certainty evidence).
Harms and burden
The risk of major bleeding may be lower when treating patients at home rather than in the hospital (RR, 0.67; 95% CI, 0.33-1.36; ARR, 6 fewer per 1000 patients; 95% CI, 13 fewer to 7 more; low-certainty evidence). In populations with a low bleeding risk, 37 treating at home instead of treating in the hospital may lead to a reduction of 5 fewer bleeding events per 1000 patients (95% CI, 11 fewer to 6 more; low-certainty evidence).
Different types of burdens are associated with both interventions. Hospital stay is associated with procedures, risk, and burden for patients. Home treatment is associated with increased burden on patients and family (eg, self-injection of LMWH and/or clinic visits for INR monitoring).
Certainty in the evidence of effects
The certainty in the evidence was judged low for mortality because of the serious risk of bias and imprecision and moderate for PE and proximal DVT because of the serious risk of bias. Of 7 RCTs, allocation was clearly concealed in 3 (unclear in 3 and probably unconcealed in 1 with unspecified opaque envelope), outcome adjudicators were clearly blinded in the 2 largest RCTs (unclear in remaining 5), and missing data were significant in 1 small RCT. Considering that the CIs around the absolute estimates likely include values suggesting substantial benefit and substantial harm, we also rated down the certainty in the evidence because of imprecision for mortality. For major bleeding, the certainty in the evidence was judged low because of serious risk of bias and imprecision.
Other EtD criteria and considerations
We considered that avoidance of PE, DVT, and bleeding was critical for patients. However, there are likely important variations in how individual patients may value thrombosis vs bleeding risk.
We identified 5 reports based on real-world data that compared the treatment cost of home management vs hospital management. 30,32 , 38-40 All reports showed that home management is cost saving compared with inpatient management. LMWH was used in these reports for home management. We also identified 5 reports that compared the cost and effectiveness of home treatment and hospital treatment for patients with DVT or for VTE patients in general. One economic evaluation in a Canadian setting based on a decision tree suggests home treatment as cost effective compared with hospital management. 41 The other 4 reports suggest that home management leads to cost savings without compromising outcome effects and safety. LMWH was used for home management in all of these studies, whereas UFH was primarily used in hospital-based management. 42-45
Health equity may decrease in rural areas or settings with limited health care access. In health systems with good primary care, home treatment is feasible and safe. In health systems with poor primary care, home treatment may reduce equity.
The panel considered home treatment acceptable and feasible in most cases, although economic incentives might favor in-hospital treatment in fee-for-service systems.
Health equity may be reduced for selected groups of patients based on observational studies evaluating outcomes after VTE treatment, including uninsured patients, 46 African American patients, 47 female patients, 48 and older patients. 49 Reductions in health equity for ≥1 of these groups of patients may be present for all of the recommendations considered in this guideline document.
Conclusions and implementation considerations
Although our analysis suggests that patients with uncomplicated DVT treated at home rather than in the hospital have a lower risk for PE and DVT, as well as a lower risk for major bleeding, the evidence in support of these observations is of low quality, making the recommendation conditional. The decision to treat a patient with an isolated DVT at home needs to be individualized, and certain patients would be more appropriately treated in the hospital, including patients with massive DVT (defined as being associated with severe pain, swelling of the entire limb, phlegmasia cerulea dolens, or limb ischemia), at high risk for anticoagulant-related bleeding, or with major comorbidities. 50 Social factors, such as limited home support, history of noncompliance, and limited financial resources, may also favor the hospital setting for the initial phase of treatment.
For patients with PE with low risk for complications, the ASH guideline panel suggests offering home treatment over hospital treatment (conditional recommendation based on very low certainty in the evidence of effects ⨁○○○).
Remarks : Clinical prediction scores for PE severity have, at best, a moderate ability to predict patient outcomes and, therefore, do not replace clinical judgment. However, they may help to select patients with PE at low risk for complications. The PESI 1 and simplified PESI 2 have been most widely validated. This recommendation does not apply to patients who have other conditions that would require hospitalization, have limited or no support at home, and cannot afford medications or have a history of poor adherence. Patients with submassive or massive PE or a high risk for bleeding or requiring IV analgesics may benefit from initial treatment in the hospital.
We identified 5 systematic reviews, 51-55 2 RCTs 56,57 (n = 451), and 3 observational studies (n = 451). 58-60 The RCTs included participants who had an objectively confirmed low-risk acute PE. In 1 trial, participants were randomized to home or hospital management; regardless of the treatment arm, all participants received subcutaneous enoxaparin, 1 mg/kg twice daily, followed by VKA. 56 In another trial, 57 patients were randomized to early discharge on 15 mg of oral rivaroxaban twice daily, followed by 20 mg of oral rivaroxaban once daily for 90 days, whereas the inpatient group received local standard of care, which included any US Food and Drug Administration–approved anticoagulant strategy. In both trials, the outpatient treatment groups were discharged within 24 hours after randomization. These trials reported the effect of antithrombotic therapy on mortality, VTE, and major bleeding. Additionally, 3 observational studies reported mortality and major bleeding at 3 months follow-up, and 1 reported PE at 3 months. The EtD framework is shown online at: https://guidelines.gradepro.org/profile/BC081756-C49E-138F-A6D3-7C40D5A6EB57 .
Analysis of RCTs showed that treating patients with PE and a low risk for complications at home, rather than in the hospital, may reduce the risk of mortality at 30 days (RR, 0.33; 95% CI, 0.01-7.98; ARR, 2 fewer per 1000 patients, 95% CI, 2 fewer to 16 more for low-risk PE patients treated in the hospital 51 ; low-certainty evidence) and 90 days (RR, 0.98; 95% CI, 0.06-15.58; ARR, 0 fewer per 1000 patients, 95% CI, 7 fewer to 108 more for low-risk PE patients treated in the hospital 51 ; low-certainty evidence), although CIs included significant benefit and harm. The analyses of observational studies also suggested a possible small reduction in long-term mortality at 90 days of follow-up (RR, 0.81; 95% CI, 0.42-1.58; ARR, 18 fewer per 1000 patients; 95% CI, 56 fewer to 56 more; very-low-certainty evidence) or PE (RR, 0.72; 95% CI, 0.07-7.70; ARR, 9 fewer per 1000 patients; 95% CI, 30 fewer to 216 more; very-low-certainty evidence).
Evidence from the included RCTs demonstrates that treating patients with PE at low risk for complications at home, rather than in the hospital, may increase the risk of subsequent PE (RR, 2.95; 95% CI, 0.12-71.85; ARR, 23 more per 1000 patients; 95% CI, 11 fewer to 850 more; low-certainty evidence) and major bleeding (RR, 6.88; 95% CI, 0.36-132.14; ARR, 59 more per 1000 patients; 95% CI, 6 fewer to 1000 more; low-certainty evidence), although CI included significant benefit and harm. Observational studies also demonstrated a potential increase in major bleeding risk (RR, 2.68; 95% CI, 0.11-63.45; ARR could not be calculated; very-low-certainty evidence).
The certainty in the evidence from the included RCTs was judged low for short-term and long-term mortality, PE, DVT, and major bleeding because of the small number of events and wide CI that covered appreciable benefit and harm. The certainty in the evidence from observational studies was judged very low for PE and major bleeding because of the inappropriate adjustment for additional factors, the lack of reporting for the assessment of outcomes and adequacy of follow-up in most studies, the small number of events among the included studies, and wide CIs that covered appreciable benefit and harm. The certainty in the evidence from observational studies was judged very low for long-term mortality for the same reasons as well as a high degree of inconsistency among the pooled estimates. In addition, 1 of the studies included patients who had active or palliative cancer and may have had a higher risk for dying than the other patient populations included in the systematic review.
We considered that avoidance of PE, DVT, and major bleeding was critical for patients. However, there is likely important variation in how individual patients may value the risk of thrombosis vs the risk of bleeding.
We identified 5 reports based on real-world data that compared the cost of home management vs hospital management. 30,32 , 38-40 All reports showed that home management is cost saving compared with inpatient management. LMWH was used in these reports for home management. We also identified 5 reports that compared the cost and effectiveness of home treatment and hospital treatment for patients with DVT or for VTE patients, in general. One economic evaluation in a Canadian setting based on a decision tree suggests that home treatment is cost effective compared with hospital management. 41 The other 4 reports suggest that home management leads to cost savings without compromising effects and safety. In all of these studies, LMWH was used for home management, whereas UFH was primarily used in hospital management. 42-45
Several studies have demonstrated that patients with PE who are at low risk for complications can be effectively and safely treated at home; however, the quality of evidence in support of this recommendation is of very low certainty, making this a conditional recommendation. Most patients with PE continue to be admitted to the hospital for treatment initiation, including a significant proportion of individuals who could be treated at home. 61 Multiple factors likely contribute to the selection of the treatment setting, and implementation of an outpatient treatment program for PE requires several steps. First, there needs to be a systematic approach to determine which individuals with PE can be considered for outpatient management. 61 Several assessment tools that use baseline clinical information to identify patients at low risk for adverse events during the first few months after diagnosis of PE have been developed, but these prognostic risk scores have not been evaluated prospectively for identification of patients with PE who can be safely treated at home. Clinical assessment and judgment are still required for identifying patients with PE who are appropriate for home management. Second, it is essential that risk stratification be performed quickly, shortly after the patient has been diagnosed with PE, to facilitate discharge from the emergency department and avoid hospitalization. Lastly, and most importantly, an infrastructure to provide outpatient PE treatment needs to be established to ensure that patients can be followed closely. As with outpatient DVT treatment, social factors, such as limited home support, history of nonadherence, and limited financial resources, would favor the hospital setting for the initial phase of treatment. Well-designed prospective studies that confirm the safety and efficacy of home treatment for selected patients with PE would be helpful, but the barriers listed above would need to be addressed prior to more widespread adoption of this recommendation.
For patients with DVT and/or PE, the ASH guideline panel suggests using DOACs over VKAs (conditional recommendation based on moderate certainty in the evidence of effects ⨁⨁⨁○).
We identified 24 systematic reviews 62-85 and 12 randomized trials 86-97 (n = 28 876). Trials included individuals with an objectively confirmed symptomatic proximal DVT or PE. Participants were randomized to DOACs or to an initial treatment with LMWH (5-10 days) with dose-adjusted warfarin (INR range, 2.0-3.0). Dabigatran and edoxaban were also administered after an initial treatment of 5 to 10 days with LMWH, whereas rivaroxaban and apixaban were administered without initial parenteral anticoagulants. The length of the anticoagulation varied across trials from 3 to 12 months. Individuals with significant renal impairment, as indicated by an estimated creatinine clearance <25 mL/min (apixaban) or 30 mL/min (all other DOACs) and patients at high risk for bleeding were excluded. The EtD framework is shown online at: https://guidelines.gradepro.org/profile/B7293C21-767F-B3F8-8BB2-A4E5173CDAC3 .
The use of a DOAC instead of a VKA for patients with VTE does not impact mortality (RR, 0.99; 95% CI, 0.85-0.15; ARR, 0 fewer per 1000 patients; 95% CI, 6 fewer to 6 more; moderate-certainty evidence) or the risk of PE (RR, 0.97; 95% CI, 0.77-1.23; ARR, 1 fewer per 1000 patients; 95% CI, 5 fewer to 5 more; moderate-certainty evidence). However, we did observe a reduction in the risk of DVT (RR, 0.80; 95% CI, 0.59-1.09; ARR, 5 fewer per 1000 patients; 95% CI, 11 fewer to 2 more; moderate-certainty evidence), although this was not statistically significant.
The use of a DOAC was associated with a reduction in the risk of major bleeding (RR, 0.63; 95% CI, 0.47-0.84; ARR, 6 fewer per 1000 patients; 95% CI, 9 fewer to 3 fewer; high-certainty evidence). In populations with a high risk for bleeding, 98 the use of a DOAC instead of a VKA may lead to a reduction of 8 fewer bleeding events per 1000 (95% CI, 11 fewer to 3 fewer; high-certainty evidence).
Additionally, given that the DOACs do not require frequent dose adjustment, monitoring of the INR, or dietary restrictions, they are probably associated with a lower burden for patients, particularly during anticoagulant initiation.
The certainty in the evidence was judged moderate for mortality, PE, and DVT because of imprecision, given that the CI around the absolute estimates likely crossed the thresholds that patients would consider important. Therefore, it was not possible to completely rule out a small difference between the alternatives on such outcomes. For major bleeding, the certainty in the evidence was judged high.
We considered that avoidance of PE, DVT, and major bleeding was critical for patients. However, there is likely an important variation in how individual patients value the risk of thrombosis vs the risk of bleeding.
We identified 5 cost comparisons between DOACs and VKA for patients with VTE. Four reports suggested that a DOAC is cost saving compared with warfarin, 99-102 and 1 study found an equivalent cost between a DOAC and a VKA. 103 Also, we identified 14 economic evaluations comparing the cost and effectiveness of DOACs vs VKA. All of them suggested that DOACs are cost-effective relative to VKA. 100 , 104-116
Finally, we considered DOACs to be acceptable and feasible to implement in most scenarios. However, given their cost, some patients might not be able to afford them.
The ASH VTE treatment guideline panel has provided a conditional recommendation for the use of DOACs over VKAs as treatment for patients with a new diagnosis of VTE. Although the evidence supporting a reduced risk for bleeding with the use of a DOAC compared with a VKA was of high certainty, the lack of benefit for the VTE outcomes resulted in the conditional recommendation.
Several additional variables need to be taken into consideration when selecting an anticoagulant for an individual patient. For example, patients who require medications that are inhibitors or inducers of P-glycoprotein, or strong inhibitors or inducers of cytochrome P450 3A4 (CYP3A4) enzymes, should consider treatment with a VKA or LMWH rather than a DOAC, given the interactions of these medications with DOACs. Renal and/or hepatic insufficiency also needs to be taken into consideration prior to selecting an anticoagulant. Other variables that may impact the choice of anticoagulant therapy for individual patients include the cost of the DOACs and patient preference for once- or twice-daily dosing. Finally, patients with antiphospholipid antibody syndrome, bariatric surgery, short gut, or other conditions that may influence medication absorption, as well as patients at extremes of body weight, are not optimal candidates for DOACs. See the ASH guideline on optimal anticoagulant therapy for additional details. 117
Anticoagulant therapy needs to be started during the initial management phase of VTE treatment and continued through the primary treatment phase for all patients with VTE who do not have a contraindication to anticoagulant therapy ( Figure 1 ). For patients who will be treated with a VKA, initiation must be overlapped with UFH or LMWH for a minimum of 5 days and a therapeutic INR is achieved for 24 hours, at which time the heparin is discontinued. For patients who will be treated with dabigatran or edoxaban, pretreatment with UFH or LMWH for up to 5 to 10 days is needed before switching to the DOAC. For patients treated with rivaroxaban or apixaban, there is no need for pretreatment with UFH or LMWH. In contrast, a higher dose is administered for the 3 three weeks of therapy with rivaroxaban and for the first week of therapy with apixaban. These differences can be particularly important for those patients being considered for treatment at home rather than in the hospital.
Remarks : Factors such as a requirement for lead-in parenteral anticoagulation, once- vs twice-daily dosing, and out-of-pocket cost may drive the selection of specific DOACs. Other factors, such as renal function, concomitant medications (eg, need for a concomitant drug metabolized through CYP3A4 enzymes or P-glycoprotein), and the presence of cancer, may also impact DOAC choice.
We did not find any systematic reviews or randomized trials comparing different DOACs head to head. We conducted a subgroup analysis of the evidence of DOACs vs VKAs 86-97 and found no interaction between the specific agent used and the risk of mortality, PE, symptomatic DVT, or major bleeding. The EtD framework is shown online at: https://guidelines.gradepro.org/profile/FFEF27C2-5C33-BB1B-B096-9624FCBB0456 .
Benefits, harms, and burden
Given the lack of evidence of the comparative effectiveness of different DOACs, we were unable to estimate the benefits and harms of specific agents.
The certainty in the evidence was judged very low for all of the relevant outcomes, given that only indirect evidence was available.
Given the lack of direct evidence, economic evaluation assessing the cost utility of different DOACs is based on assumptions and indirect observations.
In addition to cost, factors like coverage by health insurers, dosing (once vs twice daily), and requirement of initial use of LMWH will probably influence patient preferences.
For patients who will be treated with a DOAC, the ASH guideline panel does not suggest 1 medication over another given the very low certainty in the evidence on comparative effects. However, for patients who will be taking a DOAC, there are differences that should be taken into consideration. 118 Renal insufficiency is a variable that needs to be taken into consideration when selecting a DOAC, because the 4 agents that are currently available differ in the proportion of drug that is cleared by the kidneys, ranging from ∼80% for dabigatran to 25% for apixaban. 118 Ongoing studies evaluating apixaban use for patients with end-stage renal disease will further clarify its safety in this population. DOACs should be avoided as a class for patients with severe hepatic disease associated with coagulopathy; however, there are differences in which drugs can be used for patients with milder hepatic insufficiency, and dabigatran is least reliant on hepatic clearance. Other variables that may be important for the individual patient include whether the medication must be taken with food, preference for once-daily vs twice-daily dosing, the need to use a pill box, or the need to crush tablets prior to administration. Prospective research studies comparing different DOACs would be valuable in selected patient populations, such as individuals with renal insufficiency, liver disease, or morbid obesity.
Remarks : Thrombolysis is reasonable to consider for patients with limb-threatening DVT (phlegmasia cerulea dolens) and for selected younger patients at low risk for bleeding with symptomatic DVT involving the iliac and common femoral veins (higher risk of more severe PTS). Patients in these categories who value rapid resolution of symptoms, are averse to the possibility of PTS, and accept the added risk of major bleeding may prefer thrombolysis. The use of thrombolysis should be rare for patients with DVT limited to veins below the common femoral vein.
We identified 11 systematic reviews 119-129 and 19 randomized trials (n = 1944). 130-148 Trials included individuals with objectively confirmed symptomatic proximal DVT. Participants were randomized to thrombolytic therapy in addition to anticoagulation or to anticoagulation alone. In general, thrombolytics were systemically infused, except in 4 trials, including the recently published ATTRACT trial, in which thrombolytics were catheter directed 133,134,146,148 and 2 trials in which thrombolytics were locoregionally infused. 141,142 The EtD framework is shown online at: https://guidelines.gradepro.org/profile/8C3F2B15-9D6F-8618-8A41-444E83A9B780 .
The use of thrombolytics for patients with DVT may reduce the risk of PTS (RR, 0.70; 95% CI, 0.59-0.83; ARR, 169 fewer per 1000 patients; 95% CI, 96 fewer to 231 fewer; low-certainty evidence) without significantly impacting mortality (RR, 0.77; 95% CI, 0.26-2.28; ARR, 0 fewer per 1000 patients; 95% CI, 1 fewer to 1 more; low-certainty evidence), the risk of PE (RR, 1.33; 95% CI, 0.71-2.46; ARR, 5 more per 1000 patients; 95% CI, 4 fewer to 21 more; low-certainty evidence), or the risk of DVT (RR, 0.99; 95% CI, 0.56-1.76; ARR, 1 fewer per 1000 patients; 95% CI, 57 fewer to 99 more; low-certainty evidence).
The use of thrombolytics for patients with VTE (PE or DVT) was associated with an increase in the risk of major bleeding (RR, 1.89; 95% CI, 1.46-2.46; ARR, 31 more per 1000 patients; 95% CI, 16 more to 51 more; high-certainty evidence) and intracranial bleeding (RR, 3.17; 95% CI, 1.19-8.41; ARR, 7 more per 1000 patients; 95% CI, 1 more to 22 more; moderate-certainty evidence).
We tested whether the risk of major bleeding varied with the different routes of administration (ie, systemic vs locoregional vs catheter directed) and found that major bleeding was increased, regardless of the strategy used (RR for systemic infusion, 1.74; RR for catheter-directed infusion, 3.77; RR for locoregional infusion, 4.14).
The certainty in the evidence was judged as low for mortality, PE, and DVT because of risk of bias (none of the included trials were blinded) and imprecision (CI around the absolute estimates likely crossed the thresholds that patients would consider important).
The effect on PTS was considered precise, but as before, we rated it down by risk of bias. Additionally, we also rated down the certainty in the evidence by inconsistency, given that 7 of the included studies reported significant PTS reduction, whereas 1 single trial (ATTRACT trial) 146 reported the absence of a significant effect (I 2 = 57%).
Finally, the certainty in the evidence for major bleeding was judged as high.
The panel considered that avoidance of PE, DVT, PTS, and major bleeding was critical for patients. However, the more relevant trade-off for patients may be between the risk of PTS and the risk of major bleeding. We judged that there is probably a large variation in what informed patients may choose.
Because only catheter-directed thrombolysis is available in the United States, implementing the procedure would probably result in large costs, which, in turn, will probably reduce equity and limit its acceptability and feasibility.
PTS may develop in up to 30% to 50% of patients following the development of a proximal DVT, 149,150 and this may be severe in 5% to 10% of patients. 3,150 Thrombolytic therapy has been shown to result in a more rapid and complete lysis of thrombus than anticoagulant therapy alone, but relatively few studies have linked radiographic improvements to clinical outcomes. Based on the low certainty in the evidence, the ASH guideline panel has suggested against the addition of thrombolytic therapy to anticoagulation for patients with proximal DVT. However, as noted above, certain patients with acute DVT might benefit from the addition of thrombolytic therapy, as determined by the severity of symptoms, location and extent of the thrombosis, and/or initial response to anticoagulant therapy. The decision to proceed with thrombolytic therapy needs to take into consideration the potential bleeding risk for the individual patient, as well as the potential benefits from early clot lysis. For patients with DVT who will be treated with thrombolytic therapy, the decision about whether to use catheter-directed thrombolysis or systemic thrombolysis is addressed in Recommendation 8. Additional research is necessary to facilitate the identification of which patients with DVT would benefit most from thrombolytic therapy.
Remarks : Strong recommendations based on low certainty in the evidence of effects are exceptional. In this case, the high mortality of patients with PE and hemodynamic compromise, as well as the potential lifesaving effect of thrombolytics, warranted a strong recommendation. This exception is in accordance with the exceptional circumstances that allow strong recommendations based on low-certainty evidence in the GRADE ASH rules.
We identified 29 systematic reviews 151-179 and 26 RCTs (n = 2787). 130 , 180-204 Trials included individuals with an objectively confirmed symptomatic PE. Most trials included patients without hemodynamic compromise but with ultrasonography or biomarkers compatible with right ventricular dysfunction (submassive PE). Participants were randomized to thrombolytic therapy in addition to anticoagulation or to anticoagulation alone. Thrombolytics were systemically infused in all of the trials with the exception of 1, 190 in which it was catheter directed. The EtD framework is shown online at: https://guidelines.gradepro.org/profile/073BA619-F572-CFBA-9A07-8DC73ADB05FD .
The use of thrombolytics for patients with PE and hemodynamic compromise may reduce mortality (RR, 0.61; 95% CI, 0.40-0.94; ARR, 58 fewer per 1000 patients; 95% CI, 9 fewer to 90 fewer; low-certainty evidence).
Additionally, thrombolytic therapy might reduce the risk of subsequent PE (RR, 0.56; 95% CI, 0.35-0.91; ARR, 7 fewer per 1000 patients; 95% CI, 10 fewer to 2 fewer; very-low-certainty evidence) and DVT (RR, 0.92; 95% CI, 0.14-6.03; ARR, 1 fewer per 1000 patients; 95% CI, 8 fewer to 46 more; very-low-certainty evidence).
The use of thrombolytics for patients with VTE (PE or DVT) was associated with an increase in the risk of major bleeding (RR, 1.89; 95% CI, 1.46-2.46; ARR, 31 more per 1000 patients; 95% CI, 16 more to 51 more; high-certainty evidence) and intracranial bleeding (RR, 3.17; 95% CI, 1.19-8.41; ARR, 7 more per 1000 patients; 95% CI, 1 more to 21 more; moderate-certainty evidence).
We tested whether the risk of major bleeding varied with the different routes of administration (ie, systemic vs locoregional vs catheter directed) and found that the effects were similar, regardless of the strategy used (RR for systemic infusion, 1.74; RR for catheter-directed infusion, 3.77; RR for locoregional infusion, 4.14).
The certainty in the evidence was judged as low for mortality because of indirectness and imprecision. The trials identified primarily included patients without hemodynamic compromise, and the panel judged that thrombolytic effect may be different in such patients. Also, the number of patients studied was relatively small compared with the optimal information size, and the CIs around the absolute effect likely crossed the thresholds that patients would consider important. The same was true for the outcomes PE and DVT, but in addition to indirectness and imprecision, the panel also rated this down by risk of bias, given that none of the included trials was blinded.
The panel considered that most informed patients would place more value in avoiding death than in the risk of bleeding associated with thrombolysis. Also, although no direct evidence was identified, in the context of hemodynamically unstable patients, the potential benefit of thrombolytic therapy on survival would probably result in the intervention being cost-effective. Finally, the panel considered that thrombolysis is acceptable and feasible to implement in most scenarios.
Approximately 3% to 5% of patients with an acute PE present with hemodynamic compromise, defined as a systolic blood pressure <90 mm Hg or a decrease in systolic blood pressure ≥40 mm Hg from baseline. 205,206 These patients are at a significantly greater risk for mortality, as high as 50% by 90 days, 205 compared with patients with acute PE who do not present with hemodynamic compromise. As documented above, although thrombolytic therapy may reduce mortality for patients with PE and hemodynamic compromise, it is also associated with an increased risk for major bleeding and intracranial bleeding. Nevertheless, because of the high risk of mortality in this small subset of patients with PE, the ASH guideline panel provided a strong recommendation in favor of the use of thrombolytic therapy (the decision as to whether this should be systemic or catheter-directed thrombolysis is addressed in Recommendation 9). Implementation of this recommendation depends on the ability to rapidly evaluate patients, confirm the diagnosis of PE and associated hemodynamic compromise, and initiate appropriate therapy. Multidisciplinary PE response teams have recently been implemented at several institutions to expedite rapid assessment and decision-making for these patients 207,208 ; however, there has not been a demonstrated improvement in mortality with this approach. 208,209 Additional research with clinical outcomes is needed to confirm the role of thrombolytic therapy for patients with PE and hemodynamic compromise, including the optimal strategy for administration of the thrombolytic.
Remarks : Thrombolysis is reasonable to consider for younger patients with submassive PE at low risk for bleeding. Patients with submassive PE should be monitored closely for the development of hemodynamic compromise.
We identified 29 systematic reviews 151-179 and 26 RCTs (n = 2787). 130 , 180-204 Trials included individuals with an objectively confirmed symptomatic PE. Most trials included patients without hemodynamic compromise but with ultrasonography or biomarkers compatible with right ventricular dysfunction (submassive PE). Participants were randomized to thrombolytic therapy in addition to anticoagulation or to anticoagulation alone. Thrombolytics were systemically infused in all of the trials with the exception of 1, 190 in which it was administered through a catheter-directed approach. The EtD framework is shown online at: https://guidelines.gradepro.org/profile/536D4434-B897-EEEE-A831-DF6C4FBE4DA3 .
The use of thrombolytics for patients with VTE (PE or DVT) was associated with an increased risk for major bleeding (RR, 1.89; 95% CI, 1.46-2.46; ARR, 31 more per 1000 patients; 95% CI, 16 more to 51 more; high-certainty evidence) and intracranial bleeding (RR, 3.17; 95% CI, 1.19-8.41; ARR, 7 more per 1000 patients; 95% CI, 1 more to 21 more; moderate-certainty evidence).
The certainty in the evidence was judged as low for mortality because of indirectness and imprecision. The trials identified primarily included patients without hemodynamic compromise, and the panel judged that thrombolytic effect may be different in such patients. Also, the number of patients studied was relatively small compared with the optimal information size, and the CIs around the absolute effect likely crossed the thresholds that patients would consider important. The same was true for the outcomes PE and DVT, but in addition to indirectness and imprecision, the panel also rated these outcomes down by risk of bias, given that none of the included trials was blinded.
We considered that most informed patients would place more value in avoiding death than in the risk of bleeding associated with the intervention. Finally, we considered that thrombolysis is acceptable and feasible to implement in most scenarios.
Patients with acute PE who do not have evidence of hemodynamic compromise, defined as a systolic blood pressure <90 mm Hg or a decrease in systolic blood pressure ≥40 mm Hg from baseline, but who do have evidence of right ventricular strain by echocardiography or elevated cardiac biomarker levels (eg, elevated troponins or natriuretic peptides), have a higher mortality than do patients without these findings. 210,211 However, the mortality risk is much less than for those patients with hemodynamic compromise. Consequently, because of this lower risk for mortality and the low certainty in the evidence of effects, the ASH guideline panel has provided a conditional recommendation against the routine use of thrombolytic therapy in these patients. This decision needs to be individualized, however, because some patients with acute PE may be assessed as being at higher risk for mortality (eg, patients with comorbid cardiopulmonary conditions) than others. Implementation of this recommendation depends on the ability to rapidly evaluate patients and initiate appropriate therapy. Additional research should target whether certain subsets of patients with acute PE and evidence of right ventricular strain, but without hemodynamic compromise, would benefit from thrombolytic therapy.
Remarks: Given the very-low-certainty evidence (uncertainty regarding the benefits and harms of catheter-directed thrombolysis compared with systemic thrombolysis), the panel followed the GRADE rules and issued a conditional recommendation. However, 4 panel members believed that the recommendation should have been graded as strong based on the uncertain benefit of catheter-directed thrombolysis over systemic thrombolysis and the certain and serious bleeding risks associated with systemic thrombolysis.
We identified 3 systematic reviews 119,212,213 and 5 controlled trials (n = 427). 142 , 214-217 Trials included individuals with objectively confirmed symptomatic proximal DVT. Participants were randomized to directed therapy or systemic thrombolytic therapy. In 4 trials, 214-217 thrombolysis was catheter directed, whereas in 1, 142 it was locoregionally infused. The EtD framework is shown online at: https://guidelines.gradepro.org/profile/67E4FA59-335A-6713-860C-06FCE17BAE15 .
For patients with DVT, catheter-directed thrombolysis might reduce the risk of PE (RR, 0.26; 95% CI, 0.05-1.43; ARR, 11 fewer per 1000 patients; 95% CI, 14 fewer to 6 more; very-low-certainty evidence) and of major bleeding (RR, 0.35; 95% CI, 0.12-1.06; ARR, 29 fewer per 1000 patients; 95% CI, 40 fewer to 3 more; very-low-certainty evidence). However, there is considerable uncertainty regarding the comparative effectiveness of catheter-directed thrombolysis vs systemic thrombolysis given that CIs include evidence for benefit and harm.
Catheter-directed thrombolysis might increase the risk of PTS (RR, 2.59; 95% CI, 1.42-4.74; ARR, 223 more per 1000 patients; 95% CI, 76 more to 369 more; very-low-certainty evidence). However, there is considerable uncertainty given the wide CIs surrounding the effect.
The certainty in the evidence was judged as very low for all of the relevant outcomes. None of the trials were blinded, increasing the possibility of bias. However, the most serious limitation of the evidence supporting this decision was the small number of patients studied. The number of events in the trials was very small, which led to wide CIs around the absolute estimates. Additionally, the certainty in the evidence was rated down because of indirectness in the outcome of PTS, because the only trial that informed this outcome used locoregional thrombolysis instead of catheter-directed thrombolysis.
Given the small body of evidence supporting this decision, there is likely to be variability in what informed patients may choose.
Also, catheter-directed thrombolysis is an expensive procedure, and its implementation would probably result in an increment of direct costs. In the absence of certainty of its effects, it is not possible to reliably estimate its cost-effectiveness.
Finally, catheter-directed thrombolysis is not universally available, given that specialized laboratory support and trained personnel are required, and it might not be acceptable for some stakeholders.
It is important to note that systemic thrombolysis is not offered as an option for DVT management in the United States. Therefore, this recommendation does not fully apply to the US setting, but the panel considered the knowledge gap underlying this practice important to note.
The ASH guideline panel suggested that most patients with proximal DVT do not need thrombolytic therapy in addition to anticoagulation in Recommendation 5. However, for those patients with DVT for whom thrombolytic therapy is considered appropriate, the ASH guideline panel provided a conditional recommendation in favor of catheter-directed thrombolysis over systemic thrombolysis, based on the very low certainty in the level of evidence. As noted above, 4 panel members believed that this should be a strong recommendation because systemic thrombolysis is not considered appropriate therapy in the United States. Regardless of the strength of the recommendation, implementation is contingent upon the local availability of appropriate technical expertise and infrastructure. Future research studies need to focus on the patient populations with DVT in whom thrombolytic therapy is considered most appropriate, to identify the optimal approach for administration of thrombolytic therapy.
Remarks: In centers with the appropriate infrastructure, clinical staff, and procedural experience, catheter-directed thrombolysis may be an alternative to systemic thrombolysis, especially for patients with an intermediate risk for bleeding, because the total dose of thrombolytic agents is lower when delivered by catheter. In arriving at this recommendation, the panel acknowledged that the reduced dose of thrombolytic drug used for catheter-directed thrombolysis might confer a safety advantage. However, estimates of the bleeding rate associated with catheter-directed thrombolysis are very imprecise because of the paucity of quality studies and the diversity of methods used. The single published randomized trial that evaluated efficacy was small and did not demonstrate clinical outcome improvements beyond cardiac hemodynamic parameters. Hence, there remains substantial uncertainty surrounding the actual safety and efficacy of catheter-directed thrombolysis. In contrast, estimates of the safety and efficacy of systemic thrombolysis are more confident, having been derived from many randomized trials comprising a much larger number of patients.
We identified 1 systematic review, 218 1 relevant controlled trial (n = 52), 219 and 3 relevant observational studies (matched population = 7502). 220-222 All of the studies included individuals with an objectively confirmed PE and compared catheter-directed thrombolysis with systemic thrombolysis. The EtD framework is shown online at: https://guidelines.gradepro.org/profile/A7BFDBC4-6A3F-D87D-928A-7ADA50ADED1A .
There is considerable uncertainty regarding the comparative effect of systemic thrombolysis and catheter-directed thrombolysis. Based on 1 very small trial and 3 observational studies, the use of catheter-directed thrombolysis might reduce mortality (RCT estimate: RR, 0.06; 95% CI, 0-0.96; ARR, 157 fewer per 1000 patients; 95% CI, 7 fewer to 167 fewer; very-low-certainty evidence; observational studies estimate: odds ratio [OR], 0.59; 95% CI, 0.33-1.04; ARR during hospitalization, 48 fewer per 1000 patients; 95% CI, 81 fewer to 4 more; very low certainty). Also, catheter-directed thrombolysis might reduce major bleeding (RCT estimate: RR, 0.69; 95% CI, 0.21-2.27; ARR, 24 fewer per 1000 patients; 95% CI, 62 fewer to 99 more; very-low-certainty evidence; observational studies estimate: OR, 0.87; 95% CI, 0.7-1.09; ARR, 7 fewer per 1000 patients; 95% CI, 16 fewer to 5 more; very low certainty).
PE recurrence and DVT were not reported in any of the identified studies.
The certainty in the evidence was judged as very low for mortality and major bleeding because of the risk of bias and imprecision. The only randomized trial was not blinded, and the randomization process was not adequately described. The observational study did adjust for baseline characteristics using propensity scores, but observational studies have a residual selection bias due to unadjusted or unmeasured differences in the groups under comparison.
The other main limitation of the evidence supporting this decision was imprecision of the estimates. In the randomized trial, only 54 patients were studied, yielding a very wide CI. Although the observational studies did include more patients and events, the CIs around the absolute estimates were also wide and probably crossed the thresholds that patients would consider important.
We did not identify relevant economic evaluations, although catheter-directed thrombolysis is an expensive procedure, and its implementation probably would result in an increment of direct costs.
Because catheter-directed thrombolysis requires a specialized laboratory and trained personnel, it is not universally available. Also, given its costs and the uncertainty regarding its effects, it might not be acceptable for some stakeholders.
Thrombolytic therapy can be an appropriate intervention in selected patients with PE, as described in Recommendations 6 and 7, and can be administered systemically or using a catheter-directed approach. Based on the very low level of certainty in the evidence, as outlined above, the ASH guideline panel has provided a conditional recommendation favoring systemic thrombolysis over catheter-directed thrombolysis for those patients with PE in whom thrombolysis is considered clinically appropriate. The panel did recognize the potential safety advantage of using a catheter-directed approach, but the imprecision of the data limited any conclusions that would favor this approach. Future research studies need to be conducted in the appropriate patient populations and designed to answer these questions surrounding optimal administration of thrombolytic therapy for patients with PE.
For patients with proximal DVT and significant preexisting cardiopulmonary disease, as well as for patients with PE and hemodynamic compromise, the ASH guideline panel suggests anticoagulation alone rather than anticoagulation plus insertion of an IVC filter (conditional recommendations based on low certainty in the evidence of effects ⨁⨁○○).
Remarks: These recommendations apply to patients who are eligible to receive anticoagulation. For patients with a contraindication to anticoagulation, insertion of a retrievable IVC filter may be indicated, with retrieval as soon as the patient is able to receive anticoagulation.
We identified 7 systematic reviews 223-229 and 2 randomized trials 230,231 (n = 799). The PREPIC 230 trial included 400 patients with proximal DVT with or without concomitant PE. Participants were randomized to insertion of a nonretrievable IVC filter in addition to anticoagulation or to anticoagulation alone. Patients were followed for 2 years. The PREPIC 2 trial 231 included 399 patients with PE and acute deep or superficial vein thrombosis. Participants were randomized to insertion of a retrievable IVC filter in addition to anticoagulation or to anticoagulation alone. Follow-up was for 6 months. The majority of patients included in the PREPIC trials did not have significant preexisting cardiopulmonary disease, and no patient had PE with hemodynamic failure. The EtD frameworks are shown online at: https://guidelines.gradepro.org/profile/86ED15E4-C608-F07D-9AA7-5F3B5AE994B0 and https://guidelines.gradepro.org/profile/15281C02-EE9F-4E90-B895-5A8EEA854AB9 .
A nonsignificant reduction in the risk of PE with IVC filter was observed (RR, 0.54; 95% CI, 0.22-1.33; ARR, 2 fewer per 1000 patients; 95% CI, 4 fewer to 2 more; low-certainty evidence). Using the baseline risk of PE observed in a cohort of 4036 patients with chronic obstructive pulmonary disease and VTE, 232 we estimated that the use of an IVC filter may lead to 13 fewer PEs (95% CI, 23 fewer to 10 more; low-certainty evidence).
We observed a nonsignificant mortality increase for patients randomized to receive IVC filters (RR, 1.12; 95% CI, 0.83-1.60; ARR, 9 more per 1000 patients; 95% CI, 10 fewer to 36 more; low-certainty evidence). Using the mortality observed in a cohort of 4036 patients with chronic obstructive pulmonary disease and VTE as baseline risk, 232 we estimated that the use of IVC filters for patients with significant preexisting cardiopulmonary disease may lead to 16 more deaths per 1000 (95% CI, 19 fewer to 66 more; low-certainty evidence). Also, using the baseline risk of mortality observed for patients with PE and systolic blood pressure <90 mmHg in the RIETE registry (n = 6599), 233 we estimated that the use of an IVC filter for patients with PE and hemodynamic compromise may lead to 22 more deaths per 1000 (95% CI, 26 fewer to 90 more; low-certainty evidence).
Additionally, we observed a nonsignificant increase in the incidence of subsequent DVT in the group randomized to IVC filters (RR, 1.64; 95% CI, 0.93-2.90; ARR, 3 more per 1000 patients; 95% CI, 0 to 10 more; low-certainty evidence).
Finally, IVC filter insertion was associated with local and mechanical complications. In the PREPIC 2 trial, 231 among the 193 patients who received filters, 5 (2.6%) experienced access site hematoma, 3 (1.6%) experienced filter thrombosis, and 11 (5.7%) experienced retrieval failure for mechanical reasons.
The certainty in the evidence was judged low for all of the relevant outcomes. One of the limitations of the available evidence was that the populations included in the PREPIC trials were different from our populations of interest. Specifically, the risk of death and PE observed in the trials probably underestimated the real risk of patients with significant preexisting cardiopulmonary disease and with PE and hemodynamic failure. The panel took this factor into consideration by using baseline risks observed in the relevant populations in observational studies and by rating down by indirectness.
Additionally, the panel rated down the certainty in the evidence by imprecision, given that the CI around the absolute estimates likely crossed the thresholds that patients would consider important.
The panel considered that the balance between the risks of PE vs death and subsequent DVT episodes was critical for patients, although there likely is important variation in how individual patients may value the different outcomes.
The panel did not find economic evaluations assessing the cost-effectiveness of IVC filters; however, the panel considered that the costs associated with the insertion and removal of IVC filters are at least moderate.
IVC filters were designed almost 50 years ago to trap blood clots originating from the veins in the pelvis and lower extremities, preventing them from occluding the pulmonary vasculature while maintaining caval patency. 234 Recommendations 10 and 11 consider patients who would be considered most likely to benefit from this type of device, specifically those with significant preexisting cardiopulmonary disease and those with hemodynamic compromise related to preexisting PE. If these patients can be safely treated with anticoagulant therapy, however, the ASH guideline panel conditionally recommends against the use of IVC filters, based on the low certainty in the evidence of their effects. Importantly, these recommendations are not intended to apply to patients with VTE who have a contraindication to anticoagulant therapy, in whom placement of an IVC filter may be an important alternative. If an IVC filter is going to be deployed, the panel recommends use of a retrievable filter, with removal once the patient is able to be safely treated with anticoagulant therapy.
Recommendations 12, 13, and 14 address the question of the appropriate duration of time that should be used for primary treatment of the acute event, as defined in Figure 2 . The individual recommendations reference 3 patient populations: those with VTE provoked by transient risk factors (Recommendation 12), those with VTE provoked by chronic (persistent) risk factors (Recommendation 13), and those with VTE not associated with any provoking risk factors (ie, unprovoked VTE; Recommendation 14). Common examples of major and minor transient risk factors and chronic risk factors for VTE are provided in Table 3 . Several studies have demonstrated that primary treatment should continue for a minimum of 3 to 6 months for all patients with VTE. 235,236 The recommendations in this section address whether the 3 patient populations described above would benefit from a longer period for primary treatment of the acute thromboembolic event. Of note, these recommendations do not address whether patients should continue antithrombotic therapy indefinitely to prevent recurrent events, referred to as secondary prevention in Figure 2 , which is covered in the section on secondary prevention below.

Relationships of Recommendations 12 to 22 with primary treatment and secondary prevention phases of VTE treatment. Recommendations 12 to 14 address the duration of the primary treatment phase of therapy. Recommendations 15 to 17 address strategies to decide whether to discontinue anticoagulant therapy or continue with secondary prevention. Recommendations 18 to 22 address which patients should receive secondary prevention and with what antithrombotic therapies.
Risk factors and venous thromboembolism
Patients may present with >1 transient risk factor or a combination of transient and chronic risk factors. Nonenvironmental risk factors for VTE include hereditary thrombophilia, older age, and male sex. These variables typically exhibit a low relative risk for VTE but may be useful in combination with acquired risk factors when considering an individual patient’s risk for recurrence. Other acquired variables that confer a very weak risk for recurrence (OR < 2), such as obesity, varicose veins, or laparoscopic surgery, are not considered significant risk factors individually, but they may have an additive effect when combined with other risk factors listed above. Adapted from Kearon et al 237 and Konstantinides et al 238 with permission.
For patients with VTE and a major transient risk factor >3 months prior to the VTE or a single minor transient risk factor >2 months prior to the VTE, clinical judgment is essential when considering the contribution of this variable to the initial VTE and the risk of recurrence.
Chronic risk factors may fluctuate over time (eg, curative treatment of cancer or clinical waxing and waning of an autoimmune disorder), which may impact the relative risk of recurrent VTE. Active cancer is addressed in a future guideline document from ASH and is not considered in this article.
For primary treatment of patients with DVT and/or PE, whether provoked by a transient risk factor (Recommendation 12) or a chronic risk factor (Recommendation 13) or unprovoked (Recommendation 14), the ASH guideline panel suggests using a shorter course of anticoagulation for primary treatment (3-6 months) over a longer course of anticoagulation for primary treatment (6-12 months) (conditional recommendations based on moderate certainty in the evidence of effects ⨁⨁⨁○).
Remarks: These recommendations are intended to address the duration of primary anticoagulant treatment for all patients with DVT and/or PE, defined as the minimal length of time for treatment of the initial VTE ( Figure 2 ). Most patients with DVT and/or PE provoked by temporary risk factors will discontinue anticoagulant therapy after completion of the primary treatment. In contrast, many patients with DVT and/or PE provoked by chronic risk factors, as well as patients with unprovoked DVT and/or PE, may continue anticoagulant therapy indefinitely for secondary prevention after completion of the primary treatment ( Figure 2 ). However, if patients and clinicians decide to stop anticoagulation after primary treatment, the ASH guideline panel suggests against using a longer course of primary anticoagulant therapy (6-12 months). For selected patients with a chronic risk factor for which some improvement is expected over time (eg, improved mobility with rehabilitation), a longer course of anticoagulation for the primary treatment phase (eg, 6-12 months) could be justified. Recommendations 15 through 22 address decisions concerning which patients should indefinitely continue anticoagulant therapy for secondary prevention and which therapeutic options should be considered.
Recommendation 12: primary treatment for patients with DVT and/or PE provoked by a transient risk factor
Summary of the evidence..
We identified 19 systematic reviews 239-257 and 10 RCTs (n = 2857). 258-267 One set of trials included adults with objectively confirmed DVT and/or PE at the time of diagnosis, who were randomized to a shorter course (3-6 months) or a longer course (6-12 months) of anticoagulant therapy for primary treatment. A second set of trials included adults with objectively confirmed DVT and/or PE who had completed treatment with anticoagulants for 3 to 6 months without recurrence and who were then randomized to receive placebo or continue for ≥6 months of additional treatment. The longer course of therapy varied from 6 months to 24 months. 262 Patients were continuously followed up until the end of the longer course of anticoagulation. The outcomes were measured in both groups at the end of the follow-up. For baseline risks of VTE, we used a meta-analysis of 10 cohort studies and 5 randomized trials 268 that reported a VTE recurrence rate of 4.2 per 100 patient-years for patients with a transient risk factor. Assuming that 45% of the VTE events are PEs and 55% are DVTs, 269 we estimated annualized risks of PE recurrence of 1.89 and of DVT recurrence of 2.31 per 100 patient-years for patients with a nonsurgical transient risk factor. For the baseline risk of major bleeding, we used a meta-analysis of 13 prospective cohort studies and 56 randomized trials 98 in VTE patients showing a 2.1% risk for major bleeding during a 6-month treatment with anticoagulants. We estimated an annualized risk for major bleeding of 2.1%, assuming a risk for major bleeding close to 0 after anticoagulant discontinuation. The EtD framework is shown online at: https://guidelines.gradepro.org/profile/68333EE3-3DBA-5D42-A7AC-B4A3258F08E0 .
The meta-analysis showed that, compared with a shorter course of anticoagulation, treating patients with a longer course of anticoagulation reduced the risk of DVT (RR, 0.50; 95% CI, 0.27-0.95; ARR, 59 fewer per 1000 patients; 95% CI, 86 fewer to 6 fewer; high-certainty evidence). In a low-risk population, 268 a longer course of anticoagulation also reduced the risk of DVT (ARR, 18 fewer per 1000 patients; 95% CI, 21 fewer to 14 fewer). A longer course of anticoagulation also showed a potential reduction in the risk of PE in the study population, without statistical significance (RR, 0.66; 95% CI, 0.29-1.51; ARR, 17 fewer per 1000 patients; 95% CI, 35 fewer to 25 more; moderate-certainty evidence) and likely a small reduction in a low-risk population 268 (ARR, 6 fewer per 1000 patients; 95% CI, 13 fewer to 10 more; moderate-certainty evidence). Using a DOAC for the longer course of anticoagulation reduced the risk of DVT in the study population (RR, 0.21; 95% CI, 0.11-0.41; ARR, 62 fewer per 1000 patients; 95% CI, 70 fewer to 46 fewer; moderate-certainty evidence), as well as in a low-risk population 268 (ARR, 18 fewer per 1000 patients; 95% CI, 21 fewer to 14 fewer; high-certainty evidence). A longer course of a DOAC also reduced the risk of PE for the study population (RR, 0.13; 95% CI, 0.03-0.58; ARR, 21 fewer per 1000 patients; 95% CI, 24 fewer to 10 fewer), as well as for a low-risk population (ARR, 16 fewer per 1000 patients, 95% CI, 18 fewer to 8 fewer; moderate-certainty evidence).
Using a VKA or LMWH for the longer course of anticoagulation resulted in a reduction in the risk of DVT without statistical significance for the study population (RR, 0.60; 95% CI, 0.32-1.11; ARR, 64 fewer per 1000 patients; 95% CI, 109 fewer to 18 more), as well as for a low-risk population (ARR, 9 fewer per 1000 patients; 95% CI, 16 fewer to 3 more; moderate-certainty evidence). There was no significant impact on the risk of PE in the study population (RR, 0.84; 95% CI, 0.43- 1.66; ARR, 13 fewer per 1000 patients; 95% CI, 47 fewer to 55 more) or for a low-risk population 268 (ARR, 3 fewer per 1000 patients; 95% CI, 11 fewer to 12 more; moderate-certainty evidence).
Harms and burden.
Our analysis showed a potential increase in mortality when using a longer course of anticoagulation than with a shorter course of anticoagulation, without statistical significance (RR, 1.38; 95% CI, 0.85-2.23; ARR, 7 more per 1000 patients; 95% CI, 3 fewer to 22 more; moderate-certainty evidence).
The use of a longer course of anticoagulation may increase the risk of major bleeding (RR, 1.46; 95% CI, 0.78-2.73; ARR, 6 more per 1000 patients; 95% CI, 3 fewer to 22 more; moderate-certainty evidence). In populations with a low risk for bleeding, 98 the use of a longer course, instead of a shorter course, of anticoagulation may lead to an increase of 10 more bleeding events per 1000 patients (95% CI, 5 fewer to 36 more; moderate-certainty evidence).
Certainty in the evidence of effects.
The certainty in the evidence was judged moderate for mortality, PE, and major bleeding because of imprecision, given that the CI around the absolute estimates likely crossed the thresholds that patients would consider important. Therefore, it was not possible to completely rule out a small difference between the alternatives on such outcomes. For DVT, the certainty in the evidence was judged high. In the subgroup analysis performed, there are 2 cases in which the quality of the evidence differed from the original analysis. Both of these are for DVT outcome when using a VKA, LMWH, or a DOAC. The certainties of the evidence were judged moderate because of imprecision.
Other EtD criteria and considerations.
We considered that avoidance of PE, DVT, and bleeding was critical for patients. However, there may be important variability in how individual patients value the risk of thrombosis vs the risk of bleeding. We did not identify direct evidence of a cost-effectiveness comparison for nonsurgical-provoked DVT/PE. Four Markov model analyses of cost-effectiveness for a longer course of antithrombotic therapy vs a shorter course of antithrombotic therapy for VTE treatment were identified. Three analyses showed that the longer course was cost-effective compared with the shorter course of antithrombotic therapy, 112,270,271 whereas 1 analysis suggested that a longer course of anticoagulation with warfarin was cost-effective in younger patients and 3 months of anticoagulation was preferred in elderly patients (80-year-old subgroup). 272
The panel considered that a longer course of treatment was probably acceptable and feasible. Observational studies suggested a higher level of patient satisfaction with a DOAC and a lower treatment burden compared with LMWH or a VKA. 273
Conclusions and implementation considerations.
The risk for recurrent VTE is low following completion of a course of anticoagulant therapy as primary treatment for patients who sustain a thromboembolism in the setting of a transient risk factor. 268 Transient risk factors may be surgical or nonsurgical events (eg, hospitalization for an acute illness, estrogen therapy, or pregnancy), and, by definition, they resolve or can be discontinued ( Table 3 ). The risk for a recurrent VTE is lower following a thromboembolism provoked by a surgical procedure or trauma compared with a nonsurgical risk factor, but the risk is low for both groups overall. 268 A longer course of therapeutic anticoagulation for the primary treatment phase may decrease the risk of recurrent VTE while on treatment, but this is offset by an increased risk for bleeding complications. In addition, several of the studies identified above observed that any benefit associated with a longer finite course of therapy is lost after anticoagulation is discontinued. 257,261 The ASH guideline panel provided a conditional recommendation supporting a shorter course (3-6 months) of therapy over a longer duration (6-12 months) for this phase of treatment as a result of the moderate certainty in the evidence of effects. After completion of the primary treatment phase, anticoagulant therapy is typically discontinued for patients with VTE provoked by transient risk factors, and secondary prevention does not need to be considered ( Figure 2 ).
It should be noted that this recommendation is based primarily on data obtained from trials using VKA as the anticoagulant therapy. It is possible that newer studies using DOACs could alter the balance of benefits and harms associated with a longer course of therapy.
Recommendation 13: primary treatment for patients with DVT and/or PE provoked by a chronic risk factor
We identified 19 systematic reviews 239-257 and 10 RCTs 258-267 (n = 2857). One set of trials included adults with objectively confirmed DVT and/or PE at the time of diagnosis, who were randomized to a shorter course (3-6 months) or a longer course (6-12 months) of anticoagulant therapy. A second set of trials included adults with objectively confirmed DVT and/or PE who had been treated with anticoagulants for 3 to 6 months without recurrence; they were randomized to receive placebo or continue treatment for ≥6 more months. The longer course of therapy varied from 6 months to 24 months. 262 Patients were followed after the end of the extended anticoagulation treatment. The outcomes were measured in both groups at the end of the follow-up period. For baseline risks of VTE, we used a multicenter prospective cohort study 274 that included 646 participants reporting a VTE recurrence rate of 9.7% per patient-year for patients with a chronic risk factor. Assuming that 45% of the VTE events are PEs and 55% are DVTs, 269 we estimated annualized risks of recurrent PE of 4.4 and of recurrent DVT of 5.3 per 100 patient-years for patients with a chronic risk factor. For the baseline risk of major bleeding, we used a meta-analysis of 13 prospective cohort studies and 56 randomized trials 98 in VTE patients showing a 2.1% risk for major bleeding during a 6-month treatment with anticoagulants. We estimated an annualized risk for major bleeding of 2.1%, assuming a risk for major bleeding close to 0 after anticoagulant discontinuation. The EtD framework is shown online at: https://guidelines.gradepro.org/profile/55B22415-DEB8-D1B7-9512-224BE01DCC76 .
The meta-analysis showed that, compared with a shorter course of anticoagulation, treating patients with a longer course of anticoagulation reduced the risk of DVT (RR, 0.50; 95% CI, 0.27-0.95; ARR, 59 fewer per 1000 patients; 95% CI, 86 fewer to 6 fewer; high-certainty evidence). In a low-risk population, 274 a longer course of anticoagulant therapy reduced the risk of DVT as well (ARR, 27 fewer per 1000 patients; 95% CI, 39 fewer to 3 fewer). A longer course of anticoagulation also showed a potential reduction in the risk of PE in the study population, without statistical significance (RR, 0.66; 95% CI, 0.29-1.51; ARR, 17 fewer per 1000 patients; 95% CI, 35 fewer to 25 more; moderate-certainty evidence), and likely a small reduction in the low-risk population 274 (ARR, 15 fewer per 1000 patients; 95% CI, 31 fewer to 22 more; moderate-certainty evidence). When using a DOAC for a longer course of anticoagulation, the risk of DVT was reduced in the study population (RR, 0.21; 95% CI, 0.11-0.41; ARR, 62 fewer per 1000 patients; 95% CI, 70 fewer to 46 fewer; moderate-certainty evidence), as well as in the low-risk population 274 (ARR, 42 fewer per 1000 patients; 95% CI, 47 fewer to 31 fewer; moderate-certainty evidence). A longer course of therapy with a DOAC also reduced the risk of PE (RR, 0.13; 95% CI, 0.03-0.58; ARR, 21 fewer per 1000 patients; 95% CI, 24 fewer to 10 fewer for study population; ARR, 38 fewer per 1000 patients; 95% CI, 42 fewer to 18 fewer for low-risk population; moderate-certainty evidence).
When using a VKA or LMWH for a longer course of anticoagulation, there was a reduction in the risk of DVT without statistical significance for the study population (RR, 0.60; 95% CI, 0.32 to 1.11; ARR, 64 fewer per 1000 patients; 95% CI, 109 fewer to 18 more), as well as for a low-risk population (ARR, 21 fewer per 1000 patients; 95% CI, 36 fewer to 6 more; moderate-certainty evidence). Similar outcomes were seen for the risk of PE for the study population (RR, 0.84; 95% CI, 0.43-1.66; ARR, 13 fewer per 1000 patients; 95% CI, 47 fewer to 55 more), as well as for a low-risk population 274 (ARR, 7 fewer per 1000 patients; 95% CI, 25 fewer to 29 more; moderate-certainty evidence).
Our analysis showed a potential increase in mortality when using a longer course of anticoagulation compared with a shorter course for primary treatment, without statistical significance (RR, 1.38; 95% CI, 0.85-2.23; ARR, 7 more per 1000 patients; 95% CI, 3 fewer to 22 more; moderate-certainty evidence).
The use of a longer course of anticoagulant therapy may increase the risk of major bleeding (RR, 1.46; 95% CI, 0.78-2.73; ARR, 6 more per 1000 patients; 95% CI, 3 fewer to 22 more; moderate-certainty evidence). In populations with a low risk for bleeding, 98 the use of a longer course of anticoagulation instead of a shorter course may lead to an increase of 10 more bleeding events per 1000 patients (95% CI, 5 fewer to 36 more; moderate-certainty evidence).
The certainty in the evidence was judged moderate for mortality, PE, and major bleeding because of imprecision, given that the CI around the absolute estimates likely crossed the thresholds that patients would consider important. Therefore, it was not possible to completely rule out a small difference between the alternatives on such outcomes. For DVT, the certainty in the evidence was judged high. In the subgroup analysis performed, there were 2 cases in which the quality of the evidence differed from the original analysis. Both of these were on the outcome when using VKA, LMWH, or DOAC. The certainties of the evidence were judged moderate because of imprecision.
We considered that avoidance of PE, DVT, and bleeding was critical for patients. However, there may be important variability in how individual patients value the risk of thrombosis vs the risk of bleeding.
We did not identify direct evidence on a cost-effectiveness comparison for nonsurgical provoked DVT/PE. Four Markov model analyses of cost-effectiveness for extended antithrombotic therapy vs limited antithrombotic therapy for VTE treatment were identified. Three analyses showed that a longer course of anticoagulation was cost-effective compared with a shorter course of antithrombotic therapy, 112,270,271 whereas 1 analysis suggested that a longer course of anticoagulation with warfarin was cost-effective in younger patients, and 3 months of anticoagulation was preferred in elderly patients (age ≥80 years). 272
The panel considered that a longer course of anticoagulation was probably acceptable and feasible. Observational studies suggested a higher level of patient satisfaction with a DOAC and a lower treatment burden than with LMWH or a VKA. 273
Acquired (environmental) risk factors for DVT and/or PE that are considered chronic include cancer (discussed in a future guideline document from ASH), certain autoimmune disorders (eg, inflammatory bowel disease or antiphospholipid syndrome), and chronic immobility ( Table 3 ). 237 Some of these risk factors may fluctuate over time (eg, the autoimmune disorders), but many of these patients are considered to be at a higher risk for recurrent thromboembolism if anticoagulant therapy is discontinued. As noted in the previous recommendation, any benefit associated with a longer finite course of therapy is lost after anticoagulation is discontinued. For primary treatment of the thromboembolic event, the ASH guideline panel has provided a conditional recommendation for a shorter course (3-6 months) of therapeutic anticoagulation over a longer course (6-12 months) of therapy, based on moderate certainty in the evidence of effects. After completion of the primary treatment phase, subsequent decisions (discussed in Recommendation 18) would determine whether to discontinue anticoagulant therapy or continue it indefinitely for secondary prevention of recurrent VTE ( Figure 2 ).
It should be noted that patients with chronic risk factors for VTE may also have 1 (or more) transient risk factor (eg, surgery) or other nonenvironmental risk factors, such as an inherited thrombophilia, older age, or male sex. 237 These additional risk factors do not change this recommendation for duration of the primary treatment phase for the thromboembolic event.
Recommendation 14, primary treatment for patients with unprovoked DVT and/or PE
We identified 19 systematic reviews 239-257 and 10 RCTs 258-267 (n = 2857). One set of trials included adults with objectively confirmed DVT and/or PE at the time of diagnosis who were randomized to receive a shorter course (3-6 months) or a longer course (>6 months) of anticoagulation. A second set of trials included adults with objectively confirmed DVT and/or PE who had been treated with anticoagulants for 3 to 6 months without recurrence; they were randomized to receive placebo or continue with ≥6 months of additional treatment. The longer course of therapy varied from 6 months to 24 months. 262 Patients were continuously followed up after completion of the longer course of anticoagulation treatment. The outcomes were measured in both groups at the end of the follow-up. For baseline risks of VTE, we used a meta-analysis of 10 cohort studies and 5 randomized trials 268 that reported a risk of recurrent VTE of 7.4 per 100 patient-years for patients with unprovoked VTE. Assuming that 45% of the VTE events are PEs and 55% are DVTs, 269 we estimated annualized risks of recurrent PE of 3.3 and of recurrent DVT of 4.1 per 100 patient-years for patients with unprovoked VTE. For the baseline risk of major bleeding, we used a meta-analysis of 13 prospective cohort studies and 56 randomized trials 98 in VTE patients showing a 2.1% risk for major bleeding during a 6-month period of treatment with anticoagulants. We estimated an annualized risk for major bleeding of 2.1% assuming a risk for major bleeding close to 0 after anticoagulant discontinuation. The EtD framework is shown online at: https://guidelines.gradepro.org/profile/ADBCBA97-1E09-37C6-B664-D6FD9A489DC3 .
The meta-analysis showed that, compared with a shorter course of anticoagulation, treating patients with a longer course of anticoagulation reduced the risk of DVT (RR, 0.50; 95% CI, 0.27-0.95; ARR, 59 fewer per 1000 patients; 95% CI, 86 fewer to 6 fewer; high-certainty evidence). In a low-risk population, 268 a longer course of anticoagulant therapy reduced the risk of DVT as well (ARR, 20 fewer per 1000 patients; 95% CI, 30 fewer to 2 fewer). A longer course of anticoagulation also showed a potential reduction in the risk of PE in the study population, without statistical significance (RR, 0.66; 95% CI, 0.29-1.51; ARR, 17 fewer per 1000 patients; 95% CI, 35 fewer to 25 more; moderate-certainty evidence), and likely a small reduction in a low-risk population 268 (ARR, 11 fewer per 1000 patients; 95% CI, 24 fewer to 17 more; moderate-certainty evidence). When using a DOAC for a longer course of anticoagulation, the risk of DVT was reduced in the study population (RR, 0.21; 95% CI, 0.11-0.41; ARR, 62 fewer per 1000 patients; 95% CI, 70 fewer to 46 fewer; high-certainty evidence), as well as in a low-risk population 268 (ARR, 32 fewer per 1000 patients; 95% CI, 36 fewer to 24 fewer; moderate-certainty evidence). A longer course of therapy with a DOAC also reduced the risk of PE for the study population (RR, 0.13; 95% CI, 0.03-0.58; ARR, 21 fewer per 1000 patients; 95% CI, 24 fewer to 10 fewer), as well as for a low-risk population (ARR, 29 fewer per 1000 patients; 95% CI, 32 fewer to 14 fewer; moderate-certainty evidence).
When using a VKA or LMWH for a longer course of anticoagulation, there was a reduction in the risk of DVT without statistical significance for the study population (RR, 0.60; 95% CI, 0.32-1.11; ARR, 64 fewer per 1000 patients; 95% CI, 109 fewer to 18 more), as well as for a low-risk population (ARR, 16 fewer per 1000 patients; 95% CI, 28 fewer to 4 more; moderate-certainty evidence), and likely a small reduction in the risk of PE for the study population (RR, 0.84; 95% CI, 0.43-1.66; ARR, 13 fewer per 1000 patients; 95% CI, 47 fewer to 55 more), as well as for a low-risk population 268 (ARR, 5 fewer per 1000 patients; 95% CI, 19 fewer to 22 more; moderate-certainty evidence).
Our analysis showed a potential increase in mortality when using a longer course of anticoagulation compared with a shorter course of anticoagulation, without statistical significance (RR, 1.38; 95% CI, 0.85-2.23; ARR, 7 more per 1000 patients; 95% CI, 3 fewer to 22 more; moderate-certainty evidence).
The certainty in the evidence was judged moderate for mortality, PE, and major bleeding because of imprecision, given that the CI around the absolute estimates likely crossed the thresholds that patients would consider important. Therefore, it was not possible to completely rule out a small difference between the alternatives on such outcomes. For DVT, the certainty in the evidence was judged high. In the subgroup analysis performed, there were 2 cases in which the quality of the evidence differed from the original analysis. Both of these involved the DVT outcome when using VKAs/LMWH or DOACs. The certainties in the evidence were judged moderate because of imprecision.
We considered that avoidance of PE, DVT, and bleeding was critical for patients. However, there is important variability in how individual patients may value the risk of thrombosis vs the risk of bleeding.
We did not identify direct evidence for a cost-effectiveness comparison for unprovoked VTE. Four Markov model analyses of cost-effectiveness for a longer course of anticoagulant therapy vs a shorter course of anticoagulant therapy for VTE treatment were identified. Three analyses showed that the longer course of anticoagulant therapy was cost-effective compared with the shorter course of therapy, 112,270,271 whereas 1 analysis suggested that a longer course of anticoagulation with warfarin was cost-effective in younger patients, and 3 months of anticoagulation was preferred in elderly patients (≥80 years). 272
The panel considered that a longer course of anticoagulant therapy was probably acceptable and feasible. An observational study suggested a higher level of patient satisfaction with a DOAC and a lower treatment burden than with LMWH or a VKA. 273
A DVT and/or PE that occurs in the absence of any transient or chronic environmental risk factors for VTE is considered unprovoked. These patients are considered to be at a higher risk for recurrent thromboembolism if anticoagulant therapy is discontinued. In addition, as noted above, any benefit associated with a longer finite course of therapy is lost after anticoagulation is discontinued. For primary treatment of the thromboembolic event, the ASH guideline panel has provided a conditional recommendation for a shorter course (3-6 months) of therapeutic anticoagulation over a longer course (6-12 months) of therapy, based on moderate certainty in the evidence of effects. These patients may have ≥1 nonenvironmental risk factor for recurrent VTE, such as inherited thrombophilia, older age, and/or male sex, but these variables would not affect this recommendation concerning the duration of the primary treatment phase for the thromboembolic event. After completion of the primary treatment phase, subsequent decisions (discussed in Recommendation 19) would determine whether to discontinue anticoagulant therapy or continue indefinitely for secondary prevention of recurrent VTE ( Figure 2 ).
Secondary prevention.
This section covers the phase of treatment identified as secondary prevention in Figures 1 and 2 . This phase occurs after the patient has completed an initial course of anticoagulant therapy, referred to as primary treatment, at which time the patient will discontinue anticoagulation or continue without a predefined stop date. Recommendations 15 to 17 address the use of various tools to assist in the decision-making process concerning whether to discontinue anticoagulant therapy. Recommendations 18 and 19 address whether patients with VTE associated with chronic risk factors and patients with unprovoked VTE, who have completed primary treatment, should discontinue anticoagulation or consider an indefinite course of therapy. Recommendations 20 to 22 address the antithrombotic therapies that might be considered for patients who continue indefinite therapy.
For patients with unprovoked DVT and/or PE, the ASH guideline panel suggests against routine use of prognostic scores (Recommendation 15), D-dimer testing (Recommendation 16), or ultrasound to detect residual vein thrombosis (Recommendation 17) to guide the duration of anticoagulation (conditional recommendations based on very low certainty in the evidence of effects ⨁○○○).
Remarks: Indefinite anticoagulation is probably appropriate for the majority of patients with unprovoked VTE. However, in certain circumstances, such as when patients are undecided or the balance between risks and benefits is uncertain, clinicians and patients may use prognostic scores, the D-dimer test, or ultrasound assessment for residual thrombosis from an initial DVT to aid in reaching a final decision. Recommendations 15 to 17 address the routine use of these strategies.
We identified 1 systematic review evaluating prognostic scores, 275 which included 20 observational studies. 276-293 Additionally, we identified 4 systematic reviews 294-297 and 1 relevant randomized trial 298 assessing the role of D-dimer testing, as well as 5 systematic reviews 299-303 and 1 relevant trial 304 evaluating the use of ultrasound assessment for residual thrombosis from an initial DVT to guide the duration of anticoagulation.
The ideal way to measure the impact of various tools on patient-important outcomes would be to randomize patients to a decision guided by the tool or to a decision guided by specific guidelines without knowledge of the tool prediction. Unfortunately, such evidence is rare. We identified only 1 randomized trial 304 that was designed this way; after the completion of 3 months of anticoagulation, 538 patients with DVT were randomized to anticoagulation for a fixed period of time or to ultrasonography-guided anticoagulation (no further anticoagulation for patients with recanalized veins and continued anticoagulation for patients with residual thrombosis). Another trial, 298 close to the ideal design, included 223 individuals with an elevated D-dimer 1 month after completing 3 to 6 months of anticoagulation. In this study, participants were randomized to stop anticoagulation or to continue it for up to 18 months.
We did find studies evaluating the prognostic performance of the different tools: an individual patient meta-analysis of observational studies and 1 RCT (n = 2527) 300 showed an independent association of residual vein thrombosis and recurrent VTE (HR, 1.32; 95% CI, 1.06-1.65). Another individual patient meta-analysis of 7 observational studies (n = 1818) 296 showed that, after an initial period of anticoagulation, individuals with persistently elevated D-dimer levels have an increased risk for recurrent VTE (HR, 2.59; 95% CI, 1.90-3.52). Finally, the systematic review of prognostic models 275 identified 3 scores: HERDOO2, Vienna, and DASH. The 3 models include D-dimer testing but differ with regard to the additional clinical characteristics considered. The Vienna score has been studied more and has showed moderate discrimination (c-statistic, 0.6) and a tendency to underestimate the true risk of VTE. Further details are provided in the EtD frameworks: https://guidelines.gradepro.org/profile/CC2C2AC0-F4AC-F0A6-BC09-58996B7C1BC3 , https://guidelines.gradepro.org/profile/859646ED-448E-8518-8B15-2CC804FBA8F3 , and https://guidelines.gradepro.org/profile/6731C8B4-1AD1-1582-BA08-6FC54CDFC4B7 .
In the trial assessing the role of residual vein thrombosis, 304 participants randomized to ultrasonography received anticoagulation for an average of 4 to 5 months longer than did individuals randomized to the control group. Consequently, the investigators observed a nonsignificant reduction in the risk of PE (RR, 0.75; 95% CI, 0.21-2.60; ARR, 6 fewer per 1000 patients; 95% CI, 17 fewer to 35 more; low-certainty evidence) and DVT (RR, 0.64; 95% CI, 0.37-1.12; ARR, 16 fewer per 1000 patients; 95% CI, 28 fewer to 5 more; low-certainty evidence) in the intervention group.
In the trial that randomized individuals with high D-dimer levels 298 to continue or to stop anticoagulation, the use of extended anticoagulation was associated with a reduction in PEs (RR, 0.16; 95% CI, 0.02-1.33; ARR, 8 fewer per 1000 patients; 95% CI, 10 fewer to 3 more; very-low-certainty evidence) and DVTs (RR, 0.07; 95% CI, 0.01-0.58; ARR, 9 fewer per 1000 patients; 95% CI, 4 to 10 fewer; very-low-certainty evidence).
In the trial evaluating residual vein thrombosis by ultrasonography, participants in the intervention group received anticoagulation for an average of 4 to 5 months longer than did controls. Hence, they had a higher risk for bleeding (RR, 1.99; 95% CI, 0.37-10.7; ARR, 2 more per 1000 patients; 95% CI, 1 fewer to 20 more; low-certainty evidence).
Also, in the trial randomizing individuals with high D-dimer levels to continue or to stop anticoagulation, extended anticoagulation was associated with a higher risk for bleeding (RR, 3.49; 95% CI, 0.14-84.76; ARR, 24 more per 1000 patients; 95% CI, 8 fewer to 813 more; very-low-certainty evidence).
We did not find any randomized clinical trials investigating the prognostic scores that compared patient-important outcomes.
We judged the certainty in the evidence as low for the use of ultrasonography and as very low for the use of D-dimer and prognostics scores. In the first case, we only found 1 trial comparing fixed periods of anticoagulation with ultrasonography-guided duration for patients without cancer. We rated down the certainty in the evidence for risk of bias, given that the trial was open label, and for imprecision, because the CIs around the absolute estimates include benefit and harm.
In the case of D-dimer, we also rated down the certainty in the evidence for risk of bias (unblinded study) and imprecision (wide CIs around absolute estimates). However, we rated down the certainty in the evidence 1 additional step because of indirectness, given that the trial identified did not really test the use of the D-dimer to make the decision whether to stop or continue anticoagulation. Rather, the study assessed the effect of anticoagulation in individuals with a high D-dimer level, which is a related question but not the specific question addressed by the panel.
As noted above, in the case of prognostic scores, at the time of our systematic review, we did not find any trial assessing their impact in patient-important outcomes, and the evidence regarding their discrimination ability and their validation was limited. Subsequently, a study investigating the HERDOO2 rule showed that women with a first unprovoked VTE and 0 or 1 of the HERDOO2 criteria could safely discontinue anticoagulant therapy after completing 5 to 12 months of therapeutic anticoagulation as primary treatment. 305
We did not identify any relevant economic evaluation; however, we considered the cost of using ultrasonography or D-dimer as moderate. Both tests are generally available, but ultrasonography is operator dependent and, therefore, results might vary in different settings.
For the individual patient who has completed primary treatment of their VTE, information from 1 of the prognostic tools, a D-dimer, and/or an ultrasound assessment may be valuable for the provider and/or the patient in the decision-making process. The ASH guideline panel provides a conditional recommendation against the routine use of any of these modalities for all patients with VTE but acknowledges the potential utility of 1 (or more) of these approaches for management of individual patients.
The panel felt that an important research question concerning the use of prognostic scores, D-dimer testing, and/or ultrasound centered around the identification of which patient populations would benefit most from the incorporation of ≥1 of these strategies into the decision-making process concerning whether anticoagulant therapy should be continued after completion of the primary treatment phase of therapy.
Remarks: Patients with DVT and/or PE provoked by a transient risk factor typically do not require antithrombotic therapy after completion of primary treatment. This recommendation refers to patients with DVT and/or PE provoked by a chronic persistent risk factor (eg, inflammatory bowel disease or autoimmune disease). However, this recommendation does not apply to patients who have a high risk for bleeding complications. For guidance on selection of antithrombotic therapy after completion of primary treatment, see Recommendation 20. Decisions regarding anticoagulation in individuals with cancer are discussed in a future guideline from ASH.
We identified 19 systematic reviews 239-257 and 13 RCTs 88,258,259,261,262,265,267,298 , 306-310 (n = 8593) to inform this recommendation. Trials included adults with objectively confirmed DVT and/or PE who had been treated with anticoagulants for ≥3 months without recurrence. Patients were randomized to receive placebo or continue treatment for ≥6 months. The mean follow-up time varied from 24 to 28 months for different outcomes. The outcomes were measured in both groups immediately at the end of the extended-duration treatment. For baseline risks of VTE, we used a multicenter prospective cohort study 274 that included 646 participants and reported a 9.7% per patient-year VTE recurrence rate for patients with a chronic risk factor. Assuming that 45% of the initial VTE events are PEs and 55% are DVTs, 269 we estimated annualized risks of 4.4 and 5.3 per 100 patient-years for PE and DVT recurrence, respectively, for patients with a chronic risk factor. For the baseline risk of major bleeding, we used data from 2 randomized trials on people with VTE, showing that the risk of major bleeding with placebo during 18 or 24 months of follow-up was as low as 0.5% 306 and as high as 1.5% in 18 months. 259 The EtD framework is shown online at: https://guidelines.gradepro.org/profile/86361A15-ECB8-E636-8A66-7B5713A17FEB .
The meta-analysis showed that, compared with discontinuation of anticoagulation, treating patients with indefinite antithrombotic therapy reduced the risk of PE in the study population (RR, 0.29; 95% CI, 0.15-0.56; ARR, 21 fewer per 1000 patients; 95% CI, 25 fewer to 13 fewer; high-certainty evidence), as well as for patients with chronic risk factors 269,274 (ARR, 31 fewer per 1000 patients; 95% CI, 37 fewer to 19 fewer; high-certainty evidence). Indefinite antithrombotic therapy also showed a reduced risk for DVT in the study population (RR, 0.20; 95% CI, 0.12-0.34; ARR, 50 fewer per 1000 patients; 95% CI, 56 fewer to 42 fewer; high-certainty evidence), as well as for patients with chronic risk factors at 1 year 269,274 (ARR, 45 fewer per 1000 patients; 95% CI, 48 fewer to 41 fewer).
There were significant subgroup effects with different antithrombotic interventions on DVT outcome. When using DOACs for indefinite anticoagulation, the risk of DVT in the study population was reduced (RR, 0.15; 95% CI, 0.10-0.23; ARR, 49 fewer per 1000 patients; 95% CI, 51 fewer to 44 fewer; high-certainty evidence), as well as for patients with chronic risk factors (ARR, 45 fewer per 1000 patients; 95% CI, 48 fewer to 41 fewer; high-certainty evidence). 269,274 When using a VKA or LMWH for indefinite anticoagulation, a reduction in the risk of DVT for the study population was observed (RR, 0.17; 95% CI, 0.05-0.53; ARR, 54 fewer per 1000 patients; 95% CI, 61 fewer to 30 fewer), as well as for patients with chronic risk factors 269,274 (ARR, 44 fewer per 1000 patients; 95% CI, 51 fewer to 25 fewer; high-certainty evidence). Aspirin reduced the risk of DVT in the study population (RR, 0.55; 95% CI, 0.31-0.98; ARR, 64 fewer per 1000 patients; 95% CI, 98 fewer to 3 fewer), as well as for patients with chronic risk factors 269,274 (ARR, 24 fewer per 1000 patients; 95% CI, 37 fewer to 1 fewer; moderate-certainty evidence). Our analysis showed a potential decrease in mortality when using indefinite antithrombotic therapy compared to a defined duration of anticoagulation, but without statistical significance (RR, 0.75; 95% CI, 0.49-1.13; ARR, 4 fewer per 1000 patients; 95% CI, 8 fewer to 2 more; moderate-certainty evidence).
The use of indefinite antithrombotic therapy increased the risk of major bleeding (RR, 2.17; 95% CI, 1.40-3.35; ARR, 6 more per 1000 patients; 95% CI, 2 more to 12 more; high-certainty evidence). In populations with a high risk for bleeding, the use of indefinite therapy instead of a defined duration of anticoagulation led to an increase of 18 more bleeding events per 1000 patients (95% CI, 6 more to 35 more; high-certainty evidence).
The certainty in the evidence was judged high for PE, DVT, and major bleeding but moderate for mortality because of imprecision, given that the CI around the absolute estimates likely crossed the thresholds that patients would consider important. Therefore, it was not possible to completely rule out a small difference between the alternatives on mortality. In the subgroup analysis performed for DVT when using aspirin, the certainty in the evidence was judged moderate because of imprecision.
We considered that avoidance of PE, DVT, and major bleeding was critical for patients. Patients placed a high value on the benefits of risk reduction in VTE recurrence and PTS. 311 However, there is important variability in how individual patients may value the risk of recurrent VTE vs the risk of bleeding.
We did not identify direct evidence on a cost-effectiveness comparison for VTE provoked by a chronic risk factor. Four Markov model analyses of cost-effectiveness for extended antithrombotic therapy vs limited antithrombotic therapy for VTE treatment were identified. Three analyses showed cost-effectiveness for the extended strategy compared with the limited antithrombotic strategy, 112,270,271 whereas 1 analysis suggested that longer initial conventional-intensity anticoagulation with warfarin was cost-effective in younger patients and 3 months of anticoagulation was preferred in elderly patients (≥80 years old). 272 The panel considered that cost-effectiveness varies with patients, the chronic risk factor(s) contributing to the increased risk of recurrent VTE, and the antithrombotic used.
The panel considered that indefinite treatment was probably feasible, but that acceptability varies.
Patients with a chronic (“persistent”) risk factor, such as inflammatory bowel disease or an autoimmune disorder, who sustain a VTE are considered to be at higher risk for recurrence if anticoagulation is discontinued after completion of the primary treatment phase compared with patients who have a transient risk factor. For patients with a chronic risk factor, the ASH guideline panel has provided a conditional recommendation for continuing antithrombotic therapy indefinitely after completion of primary treatment. Additional factors that may be useful for evaluation of the individual patient would include whether a transient risk factor was also present prior to the event and whether the patient has comorbid conditions that may predispose toward an increased risk for bleeding complications. Risk factors for bleeding with anticoagulant therapy include, but are not limited to, older age, history of prior bleeding, cancer, hepatic and/or renal insufficiency, hypertension, thrombocytopenia, prior stroke, need for antiplatelet therapy, anemia, alcohol abuse, and frequent falls. 312 An individual patient’s risk for bleeding will be affected by the severity of the risk factor (eg, degree of thrombocytopenia, location and extent of metastatic cancer), the number of risk factors present, and the presence of additional comorbid conditions. All patients who choose indefinite antithrombotic therapy for secondary prevention of recurrent VTE should be reevaluated at least annually to review their clinical course and reassess the clinical indication for continued indefinite therapy and bleeding risk factors.
The panel felt that additional research was needed to better define the impact of different chronic risk factors on the rate of recurrent VTE, particularly if the risk might vary over time.
After completion of primary treatment for patients with unprovoked DVT or PE, the ASH guideline panel suggests indefinite antithrombotic therapy over stopping anticoagulation (conditional recommendation based on moderate certainty in the evidence of effects ⨁⨁⨁○).
We identified 19 systematic reviews 239-257 and 13 RCTs 88,258,259,261,262,265,267,298 , 306-310 (n = 8593) to inform this recommendation. Trials included adults with objectively confirmed DVT or PE who had been treated with anticoagulants for ≥3 months without recurrence. Patients were randomized to receive placebo or continue with extended treatment for ≥6 months. The mean follow-up varied from 24 months to 28 months for different outcomes. The outcomes were measured in both groups immediately at the end of the extended-duration treatment. For baseline risks of VTE, we used a meta-analysis of 10 cohort studies and 5 randomized trials 268 that reported a risk for recurrent VTE of 7.4% per patient-year for patients with unprovoked VTE. Assuming that 45% of the initial VTE events are PEs and 55% are DVTs, 269 we estimated annualized risks of 3.3 and 4.1 per 100 patient-years for PE and DVT recurrence, respectively, for patients with an unprovoked VTE. For the baseline risk of major bleeding, we used data from 2 randomized trials on people with VTE, which showed that the risk of major bleeding with placebo during 18 months or 24 months of follow-up was as low as 0.5% 306 and as high as 1.5% in 18 months. 259 The EtD framework is shown online at: https://guidelines.gradepro.org/profile/B4FEBCC9-DEB2-C7FE-9420-79D262F2AB0F .
The meta-analysis showed that, compared with discontinuation of anticoagulation, treating patients with indefinite antithrombotic therapy reduced the risk of PE in the study population (RR, 0.29; 95% CI, 0.15-0.56; ARR, 21 fewer per 1000 patients; 95% CI, 25 fewer to 13 fewer; high-certainty evidence), as well as for patients with unprovoked VTE 269,274 (ARR, 24 fewer per 1000 patients; 95% CI, 28 fewer to 15 fewer; high-certainty evidence). Indefinite antithrombotic therapy also showed a risk for reduction of DVT in the study population (RR, 0.20; 95% CI, 0.12-0.34; ARR, 50 fewer per 1000 patients; 95% CI, 56 fewer to 42 fewer; high-certainty evidence), as well as for patients with unprovoked VTE at 1 year 269,274 (ARR, 33 fewer per 1000 patients; 95% CI, 36 fewer to 27 fewer).
There were significant subgroup effects of different antithrombotic interventions on DVT outcome. When using a DOAC for indefinite anticoagulation, the risk of DVT was reduced in the study population (RR, 0.15; 95% CI, 0.10-0.23; ARR, 49 fewer per 1000 patients; 95% CI, 51 fewer to 44 fewer; high-certainty evidence), as well as for patients with unprovoked VTE 269,274 (ARR, 35 fewer per 1000 patients; 95% CI, 37 fewer to 31 fewer; high-certainty evidence). When using a VKA or LMWH for indefinite anticoagulation, the risk of DVT was likely reduced in the study population (RR, 0.17; 95% CI, 0.05-0.53; ARR, 54 fewer per 1000 patients; 95% CI, 61 fewer to 30 fewer; moderate-certainty evidence), as well as for patients with unprovoked VTE (ARR, 34 fewer per 1000 patients, 95% CI, 39 fewer to 19 fewer; high-certainty evidence). 269,274 Aspirin also likely reduced the risk of DVT for the study population (RR, 0.55; 95% CI, 0.31-0.98; ARR, 64 fewer per 1000 patients, 95% CI, 98 fewer to 3 fewer; moderate-certainty evidence), as well as for patients with unprovoked VTE (ARR, 18 fewer per 1000 patients; 95% CI, 28 fewer to 1 fewer; moderate-certainty evidence). 269,274 Our analysis showed a nonsignificant decrease in mortality when using indefinite antithrombotic therapy compared with a defined duration of anticoagulation (RR, 0.75; 95% CI, 0.49-1.13; ARR, 5 fewer per 1000 patients; 95% CI, 9 fewer to 2 more; moderate-certainty evidence).
Indefinite antithrombotic therapy increased the risk of major bleeding (RR, 2.17; 95% CI, 1.40-3.35; ARR, 6 more per 1000 patients; 95% CI, 2 more to 12 more; high-certainty evidence). In populations with a high risk for bleeding, the use of indefinite antithrombotic therapy instead of a defined duration of anticoagulation led to an increase of 18 more bleeding events per 1000 patients (95% CI, 6 more to 35 more; high-certainty evidence).
The certainty in the evidence was judged high for PE, DVT, and major bleeding but moderate for mortality because of imprecision, given the CI around the absolute estimates likely crossed the thresholds that patients would consider important. Therefore, it was not possible to completely rule out a small difference between the alternatives on mortality. In the subgroup analysis performed for recurrent DVT when using aspirin, the certainty in the evidence was judged moderate because of imprecision.
We did not identify direct evidence on a cost-effectiveness comparison for unprovoked VTE. Four Markov model analyses of cost-effectiveness for extended antithrombotic therapy vs limited antithrombotic therapy for VTE treatment were identified. Three analyses showed that the extended strategy was cost-effective compared with limited antithrombotic therapy, 112,270,271 whereas 1 analysis suggested that longer initial conventional-intensity anticoagulation with warfarin was cost-effective in younger patients and 3 months of anticoagulation was preferred in elderly patients (80-year-old subgroup). 272 The panel considered that cost-effectiveness varies with patients, any risk factor(s) contributing to the increased risk of recurrent VTE, and the specific anticoagulant used.
The panel considered that indefinite treatment was probably feasible but that the acceptability varies.
Patients with unprovoked VTE, defined as occurring in the absence of any identifiable transient or chronic acquired risk factors, have the highest risk for recurrent VTE if anticoagulation is discontinued after the primary treatment phase. This risk has been estimated to be as high as 10% by 1 year and up to 30% by 5 to 10 years. 10,11,313 The ASH guideline panel has provided a conditional recommendation for continuing antithrombotic therapy indefinitely after completion of primary treatment for patients with unprovoked VTE, based on moderate certainty in the evidence of effects. All patients who are recommended to take indefinite antithrombotic therapy for secondary prevention of recurrent VTE should be reevaluated at least annually to review the clinical indication for indefinite therapy and any bleeding complications that the patient may have sustained or new bleeding risk factors that the patient may have acquired.
As noted above in Recommendation 18, risk factors for bleeding with anticoagulant therapy include, but are not limited to, older age, history of prior bleeding, cancer, hepatic and/or renal insufficiency, hypertension, thrombocytopenia, prior stroke, need for antiplatelet therapy, anemia, alcohol abuse, and frequent falls. 312 An individual patient’s risk for bleeding will be affected by the severity of the risk factor (eg, degree of thrombocytopenia, location and extent of metastatic cancer), the number of risk factors present, and the presence of additional comorbid conditions.
We identified 1 RCT 317 to inform this recommendation. This trial included adults with objectively confirmed DVT and/or PE who had been treated with a DOAC or VKA for 6 to 12 months and had not interrupted therapy for more than 7 days prior to randomization. Patients were randomized to receive 20 mg of rivaroxaban or 100 mg of aspirin for 12 months. For the purpose of this question, aspirin was considered the intervention, and rivaroxaban was the comparator. The mean follow-up time was 351 days. The EtD framework is shown online at: https://guidelines.gradepro.org/profile/355350CB-41FE-119C-8907-3B646789C1A5 .
The analysis showed that, compared with a standard dose of anticoagulation, treating patients with aspirin may reduce the risk of major bleeding, but these results are not statistically significant (RR, 0.49; 95% CI, 0.12-1.95; ARR, 3 fewer per 1000 patients; 95% CI, 5 fewer to 5 more; moderate-certainty evidence).
The use of aspirin compared with a standard dose of anticoagulation increased the risk of nonfatal PE (RR, 3.10; 95% CI, 1.24-7.73; ARR, 11 more per 1000 patients; 95% CI, 1 more to 36 more; moderate-certainty evidence) or DVT (RR, 3.15; 95% CI, 1.50-6.63; ARR, 17 more per 1000 patients; 95% CI, 4 more to 46 more; moderate-certainty evidence).
The certainty in the evidence was judged moderate for mortality, major bleeding, PE, and DVT because of imprecision, given a small number of events in both treatment arms that did not meet the optimal information size and the fact that the CIs around the absolute estimates likely crossed the thresholds that patients would consider important.
We considered that the avoidance of PE, DVT, and major bleeding was critical for patients. Patients placed a high value on the benefits of risk reduction in VTE recurrence and PTS. 311 However, there is important variability in how individual patients may value the risk of recurrent VTE vs the risk of bleeding. The panel considered the variability of the cost of drugs across different countries and felt that the cost of anticoagulants, specifically DOACs, compared with aspirin would place at least a moderate burden on patients.
The panel considered that switching patients to aspirin at the completion of primary therapy was probably feasible and probably acceptable to relevant stakeholders.
Extending anticoagulant therapy beyond the primary treatment phase reduces the risk of recurrent VTE but is associated with an increased risk for bleeding complications. Consequently, several studies have investigated the role of aspirin for the secondary prevention of VTE. 309,314,315 The WARFASA 309 and ASPIRE 318 trials compared aspirin, 100 mg daily, with placebo for secondary prevention of recurrent VTE for patients with an initial unprovoked event, and the pooled results of the 2 trials showed a decrease in recurrent VTE, as well as major vascular events, without an increased risk for clinically relevant bleeding. 309,315 However, a systematic review and meta-analysis comparing extended anticoagulant therapy and aspirin found that anticoagulant therapy was more effective than aspirin in preventing recurrent VTE, 246 and a single study comparing 2 doses of the direct oral anticoagulant rivaroxaban and aspirin found that anticoagulation was more effective than aspirin without an increase in bleeding rates. 314 Consequently, the ASH guideline panel has provided a conditional recommendation supporting the use of anticoagulation over aspirin for secondary prevention of VTE. For patients who are going to discontinue anticoagulant therapy after completion of the primary treatment phase, the role of aspirin can be considered but needs to be individualized. The panel did not address other nonanticoagulant options for secondary prevention of recurrent VTE.
For patients with DVT and/or PE who have completed primary treatment and will continue VKA therapy as secondary prevention, the ASH guideline panel recommends using an INR range of 2.0 to 3.0 over a lower INR range (eg, 1.5-1.9) (strong recommendation based on moderate certainty in the evidence of effects ⨁⨁⨁○).
We identified 1 RCT 316 to inform this recommendation. This trial included adults with objectively confirmed unprovoked DVT and/or PE who had been treated with oral anticoagulants for ≥at least 3 months. Patients were randomized to receive low-intensity warfarin therapy (target INR, 1.5-1.9) or conventional-intensity warfarin therapy (target INR, 2.0-3.0) after completion of the primary treatment phase of therapy. Patients were followed for an average of 2.4 years. The EtD framework is shown online at: https://guidelines.gradepro.org/profile/09FEF3FA-8317-EA74-8F3D-5106CF1B80EB .
We did not identify any benefits associated with use of a lower INR range.
The use of warfarin with an INR range lower than 2.0 to 3.0 may increase the risk of DVT (RR, 3.25; 95% CI, 1.07-9.87; ARR, 24 more per 1000 patients; 95% CI, 1 more to 96 more; moderate-certainty evidence) and may increase the risk of nonfatal PE (RR, 5.0; 95% CI, 0.24-103.79; ARR could not be estimated; moderate-certainty evidence), although without statistical significance. Use of a lower INR range may also result in a nonsignificant increase in the risk of mortality (RR, 2.00; 95% CI, 0.86-4.47; ARR, 22 more per 1000 patients; 95% CI, 3 fewer to 75 more; moderate-certainty evidence) and major bleeding (RR, 1.13; 95% CI, 0.44-2.88; ARR, 3 more per 1000 patients; 95% CI, 12 fewer to 41 more; moderate-certainty evidence).
The certainty in the evidence was judged moderate for mortality, major bleeding, PE, and DVT due to imprecision, given the small number of events in both arms not meeting optimal information size and the fact that the CI around the absolute estimates likely crossed the thresholds that patients would consider important. Therefore, it was not possible to completely rule out a small difference between the alternatives on such outcomes.
We considered that avoidance of PE, DVT, and major bleeding was critical for patients. Patients placed a high value on the benefits of risk reduction in VTE recurrence and PTS. 311 However, the panel considered the existence of an important variability in how individual patients may value the risk of recurrent VTE vs the risk of bleeding. The panel considered a negligible cost and savings between the interventions. One Markov model 272 compared an unlimited duration of conventional-intensity anticoagulation (INR range, 2.0-3.0) vs low-intensity anticoagulation (INR range, 1.5-2.0) with warfarin. The analysis suggested that an unlimited duration of standard-intensity anticoagulation was always more cost-effective.
The panel thought that there was no impact on health equity when choosing either intervention. The panel considered that a low target INR with warfarin was probably feasible but not acceptable to key stakeholders.
This recommendation specifically applies to patients who are going to be treated with VKAs for secondary prevention of VTE. The ASH guideline panel provided a strong recommendation for VKAs with an INR range of 2.0 to 3.0 over an INR range of 1.5 to 1.9. However, it should be noted that patients considered to have a “high risk of bleeding” were excluded from the randomized trial described in the analysis above. 316 Decisions concerning anticoagulant therapy with warfarin for patients with a significant bleeding risk need to be individualized, and a VKA may not be the optimal anticoagulant in this setting.
For patients with DVT and/or PE who have completed primary treatment and will continue with a DOAC for secondary prevention, the ASH guideline panel suggests using standard-dose DOAC or lower-dose DOAC (conditional recommendation based on moderate certainty in the evidence of effects ⨁⨁⨁○).
Remarks: Lower-dose DOAC regimens that may be considered for patients who have completed primary treatment and will continue with a DOAC include rivaroxaban at 10 mg daily and apixaban at 2.5 mg twice daily.
We identified 2 RCTs 306,314 to inform this recommendation. Trials included adults with objectively confirmed DVT and/or PE who had been treated with anticoagulants for 6 to 12 months. Subsequently, patients were randomized to receive 20 mg or 10 mg of rivaroxaban daily in 1 trial 314 or 5 mg or 2.5 mg of apixaban twice daily in another trial 306 for 12 months. The follow-up time ranged from 351 to 365 days. The EtD framework is shown online at: https://guidelines.gradepro.org/profile/011FBE1F-7460-20AC-A8BB-E3E0B4647907 .
The analysis showed that, compared with a standard dose of rivaroxaban or apixaban, treating patients with a lower DOAC dose was associated with a nonsignificant reduction in the risk of mortality (RR, 0.68; 95% CI, 0.10-4.57; ARR, 2 fewer per 1000 patients; 95% CI, 6 fewer to 22 more; low-certainty evidence). The lower DOAC dose had little impact on the risk of DVT (RR, 0.75; 95% CI, 0.36-1.53; ARR, 2 fewer per 1000 patients; 95% CI, 6 fewer to 5 more; moderate-certainty evidence) or the risk of major bleeding (RR, 0.97; 95% CI, 0.12-1.95; ARR, 0 fewer per 1000 patients; 95% CI, 2 fewer to 7 more; moderate-certainty evidence).
The use of a lower-dose DOAC compared with a standard-dose DOAC was associated with a nonsignificant increase in the risk of nonfatal PE (RR, 1.25; 95% CI, 0.54-2.91; ARR, 1 more per 1000 patients; 95% CI, 2 fewer to 10 more; moderate-certainty evidence).
The certainty in the evidence was judged moderate for major bleeding, nonfatal PE, and DVT because of imprecision, given the small number of events in both arms not meeting optimal information size and the fact that the CI around the absolute estimates likely crossed the thresholds that patients would consider important. Therefore, it was not possible to completely rule out a small difference between the alternatives on such outcomes. The certainty in the evidence was judged low for mortality because of the reasons mentioned above, as well as the large unexplained heterogeneity.
The panel considered that there was important variability in how individual patients might value the risk of thrombosis vs the risk of bleeding. The panel estimated that the cost of a DOAC does not vary significantly with the dose.
The panel considered that both interventions were probably acceptable to relevant stakeholders and feasible to implement.
Three DOACs have been studied for secondary prevention of recurrent VTE after completion of the primary treatment phase. 306,310,314 Two of these agents, rivaroxaban and apixaban, have been studied at a reduced dose: from 20 mg daily to 10 mg daily for rivaroxaban and 5 mg to 2.5 mg twice daily for apixaban. 306,314 The investigation into the use of the lower doses of these 2 anticoagulants for secondary prevention was prompted by the desire to reduce the risk of bleeding, as well as the documented efficacy of the lower doses to prevent VTE after elective hip or knee arthroplasty. 317-321 However, neither study was powered for the comparisons between the standard and low doses. In addition, patients with a higher risk of recurrence were excluded from the studies (eg, multiple prior unprovoked VTE, indication for therapeutic dose anticoagulation, or antiphospholipid syndrome). Given the moderate certainty in the evidence of effects, the ASH guideline panel has provided a conditional recommendation that the standard dose or the lower dose of rivaroxaban or apixaban may be used for the secondary prevention of VTE. For patients who are treated with dabigatran for secondary prevention, only the standard regimen of 150 mg twice daily has been studied. 310
Additional research is necessary to identify which subsets of patients who are going to continue anticoagulant therapy indefinitely for secondary prevention can safely use a lower-dose DOAC and which patients should be maintained on a standard dose (eg, obese individuals and patients at higher risk for recurrence).
For patients with breakthrough DVT and/or PE during therapeutic VKA treatment, the ASH guideline panel suggests using LMWH over DOAC therapy (conditional recommendation based on very low certainty in the evidence of effects ⨁○○○).
Remarks: Patients who present with a new VTE during therapeutic treatment with a VKA should be further investigated to identify potential underlying causes. This recommendation does not include patients who develop breakthrough VTE in the setting of poor INR control, in whom a DOAC may be a reasonable option.
We did not find any systematic review or randomized trial comparing DOACs vs LMWH for patients with recurrent DVT and/or PE during treatment with VKA. DOACs vs LMWH have been compared only in the setting of VTE prophylaxis, which we considered too indirect to make judgments about VTE treatment. The online EtD framework is available here: https://guidelines.gradepro.org/profile/4D133D47-A600-EC68-85E9-5221E45B47F9 .
Given the lack of evidence, we were unable to estimate the benefits and harms.
The certainty in the evidence was judged very low for all of the relevant outcomes.
We did not find any economic evaluation assessing the cost utility of a DOAC vs LMWH for patients with DVT and/or PE during treatment with VKA. Both interventions are widely available, and factors such as the route of administration (subcutaneous vs oral), cost, and coverage by health insurance will probably influence patients’ preferences.
The risk of recurrent VTE while on anticoagulant therapy with a VKA was 1.6% in a Cochrane meta-analysis. 245 Frequent reasons associated with breakthrough thromboembolic events include the underlying condition or disease (eg, cancer, antiphospholipid syndrome, or vasculitis) or inappropriate selection and/or dosing of the anticoagulant (eg, noncompliance, drug-drug interactions, and drug-food interactions). 322 An initial assessment of any patient with an apparent breakthrough VTE while on therapeutic anticoagulation includes confirmation of compliance with the therapy being administered and confirmation that the medication and dosing regimen are appropriate for the individual patient. Initial laboratory testing includes an INR to confirm the patient is therapeutically anticoagulated with a VKA.
A recurrent or breakthrough event for patients receiving a VKA who were recently being treated with UFH or, less commonly, a LMWH may be suggestive of heparin-induced thrombocytopenia (HIT). In this setting, the VKA should be discontinued, the anticoagulant effect should be reversed with vitamin K, and the patient should be started on a nonheparin anticoagulant. The diagnosis and treatment of patients with suspected HIT are discussed in a separate ASH guideline document. 323
For patients who sustain a breakthrough thrombotic event while taking a VKA who do not have HIT, there are minimal data available concerning which anticoagulant to select. The ASH guideline panel has provided a conditional recommendation favoring anticoagulation with LMWH over a DOAC, but this recommendation is based on very low certainty in the evidence of effects. These patients need to be carefully evaluated for underlying conditions and potential contraindications to individual anticoagulant agents, such as antiphospholipid syndrome, in which LMWH may be preferred over a DOAC. In addition, these patients need to be reevaluated when clinically stable to determine whether they need to continue LMWH or switch to an oral agent.
The evaluation and management of patients who sustain recurrent thromboembolic events while taking therapeutic anticoagulation constitute an area needing well-designed prospective studies to provide guidance.
For patients who develop DVT and/or PE provoked by a transient risk factor and have a history of previous unprovoked VTE or VTE provoked by a chronic risk factor, the ASH guideline panel suggests continuing antithrombotic therapy over stopping anticoagulation after completing primary treatment (conditional recommendation based on moderate certainty in the evidence of effects ⨁⨁⨁○).
For patients who develop a DVT and/or PE provoked by a transient risk factor and have a history of a previous thrombotic event also provoked by a transient risk factor, the ASH guideline panel suggests stopping anticoagulation after completion of the primary treatment phase of therapy over indefinite duration therapy (conditional recommendation based on moderate certainty in the evidence of effects ⨁⨁⨁○).
Remarks: For guidance on selection of antithrombotic therapy after completion of primary treatment, see Recommendation 20.
We identified 19 systematic reviews 239-257 and 13 RCTs 88,258,259,261,262,265,267,298 , 306-310 (n = 8593) to inform this recommendation. Trials included adults with objectively confirmed DVT and/or PE who had been treated with anticoagulants for ≥3 months without recurrence, referred to as the “study population” below. Patients were subsequently randomized to placebo or extended anticoagulation for ≥6 months of additional treatment. The mean follow-up time varied from 24 to 28 months for different outcomes. The outcomes were measured in both groups immediately at the end of the extended-duration treatment. For baseline risks of VTE, we used a prospective cohort study 324 that reported an incidence rate for recurrent VTE of 5.6 per 100 patient-years for patients with a provoked VTE who had discontinued anticoagulation after completion of primary treatment. For patients with recurrent provoked VTE, assuming that 45% of the VTE events are PEs and 55% are DVTs, 269 we estimated the risks of PE recurrence to be 2.5 per 100 patient-years and the risks of DVT recurrence to be 3.1 per 100 patient-years. These estimates, however, do not differentiate the risk of recurrence according to the nature of the first thrombotic episode (ie, provoked or unprovoked). For the baseline risk of major bleeding, we used data from 2 randomized trials on people with VTE; the risk of major bleeding with placebo during an 18- or 24-month treatment with anticoagulants was as low as 0.5% 306 and as high as 1.5% in 18 months. 259 The EtD framework is shown online at: https://guidelines.gradepro.org/profile/D9F5564D-D7EE-97A3-8AAB-24FF9D0C32F4 .
The meta-analysis showed that, compared with discontinuation of anticoagulation, treating patients with indefinite antithrombotic therapy reduced the risk of PE in the study population (RR, 0.29; 95% CI, 0.15-0.56; ARR, 21 fewer per 1000 patients; 95% CI, 25 fewer to 13 fewer; high-certainty evidence), as well as for patients with recurrent provoked VTE 269,324 (ARR, 18 fewer per 1000 patients; 95% CI, 21 fewer to 11 fewer; high-certainty evidence). Indefinite antithrombotic therapy also showed a risk reduction in DVT (RR, 0.20; 95% CI, 0.12-0.34; ARR, 50 fewer per 1000 patients; 95% CI, 56 fewer to 42 fewer; high-certainty evidence). Indefinite antithrombotic therapy also reduced the risk of DVT for patients with recurrent provoked VTE at 1 year 269,324 (ARR, 25 fewer per 1000 patients; 95% CI, 27 fewer to 20 fewer).
There were significant subgroup effects with the different antithrombotic interventions on DVT outcome. When using a DOAC for indefinite anticoagulation, the risk of DVT was reduced in the study population (RR, 0.15; 95% CI, 0.10-0.23; ARR, 49 fewer per 1000 patients; 95% CI 51 fewer to 44 fewer; high-certainty evidence), as well as for patients with recurrent provoked VTE 269,324 (ARR, 26 fewer per 1000 patients; 95% CI, 28 fewer to 24 fewer; high-certainty evidence). When using a VKA or LMWH for indefinite anticoagulation, we observed a reduction in the risk of recurrent DVT in the study population (RR, 0.17; 95% CI, 0.05-0.53; ARR, 54 fewer per 1000 patients; 95% CI, 61 fewer to 30 fewer), as well as for patients with recurrent provoked VTE (ARR, 26 fewer per 1000 patients; 95% CI, 29 fewer to 14 fewer; high-certainty evidence). 269,324 Aspirin reduced the risk of DVT in the study population (RR, 0.55; 95% CI, 0.31-0.98; ARR, 64 fewer per 1000 patients; 95% CI, 98 fewer to 3 fewer), as well as for patients with recurrent provoked VTE (ARR, 14 fewer per 1000 patients; 95% CI, 21 fewer to 1 fewer; moderate-certainty evidence). 269,324 Our analysis showed a potential decrease in mortality when using indefinite antithrombotic therapy compared with a defined duration of anticoagulation, without statistical significance (RR, 0.75; 95% CI, 0.49-1.13; ARR, 5 fewer per 1000 patients; 95% CI, 9 fewer to 2 more; moderate-certainty evidence).
The use of indefinite antithrombotic therapy increased the risk of major bleeding (RR, 2.17; 95% CI, 1.40-3.35; ARR, 6 more per 1000 patients; 95% CI, 2 more to 12 more; high-certainty evidence). In populations with a high risk for bleeding, 259 the use of indefinite antithrombotic therapy instead of a defined duration of anticoagulation led to an increase of 18 more major bleeding events per 1000 patients (95% CI, 6 more to 35 more; high-certainty evidence).
The certainty in the evidence was judged high for PE, DVT, and major bleeding but moderate for mortality because of imprecision, given that the CI around the absolute estimates crossed thresholds that patients would likely consider important. Therefore, it was not possible to completely rule out a small difference between the alternatives on mortality. In the subgroup analysis performed for DVT, when using aspirin, the certainty in the evidence was judged moderate because of imprecision.
We considered that avoidance of PE, DVT, and major bleeding was critical for patients. Patients placed a high value on the benefits of risk reduction in VTE recurrence and PTS. 311 However, there is important variability in how individual patients may value the risk of thrombosis vs the risk of bleeding.
We did not identify a cost-effectiveness comparison for nonsurgical provoked VTE. Four Markov model analyses of cost-effectiveness for extended antithrombotic therapy vs limited antithrombotic therapy for VTE treatment were identified. Three analyses showed that the extended strategy was cost-effective compared with limited antithrombotic therapy, 112,270,271 whereas 1 analysis suggested that longer initial conventional-intensity anticoagulation with warfarin was cost-effective in younger patients, and 3 months of anticoagulation was preferred in elderly patients (≥80 years old). 272 The panel considered that cost-effectiveness varies with patients, the chronic risk factor(s) contributing to risk of recurrent VTE, and the antithrombotic therapy used.
The panel considered that indefinite treatment was probably feasible but that acceptability varied.
This recommendation applies to the patient who sustains a VTE related to a transient risk factor, who also has a history of VTE that was unprovoked or provoked by a chronic risk factor (Recommendation 24a) or who has a history of VTE that was provoked by a transient risk factor (Recommendation 24b). These patients need to undergo decisions about initial management (Recommendations 1 to 11) and primary treatment (Recommendations 12 to 14), just as with their first event. If the first event carried a high risk for recurrence (eg, was unprovoked), the ASH guideline panel provided a conditional recommendation in support of indefinite antithrombotic therapy for secondary prevention after completion of the primary treatment phase. In contrast, if the first event carried a lower risk for recurrence (ie, was provoked by a transient risk factor), the panel provided a conditional recommendation favoring discontinuation of anticoagulation after completion of the primary treatment phase. Variables that may impact on decision making for the individual patient, particularly the patient with a first event considered high risk for recurrence, might include whether the second event occurred in the same vascular distribution as the first event, the presence (or absence) of an underlying hypercoagulable state, the development of hemorrhagic complications while on anticoagulant therapy, and/or the clinical severity of the second event (eg, massive PE vs popliteal DVT). For those patients continued on indefinite antithrombotic therapy for secondary prevention, decisions about the optimal antithrombotic strategy for secondary prevention are addressed in Recommendations 20 to 22.
For patients with a recurrent unprovoked DVT or PE, the ASH guideline panel recommends indefinite antithrombotic therapy over stopping anticoagulation after completion of primary treatment (strong recommendation based on moderate certainty in the evidence of effects ⨁⨁⨁○).
We identified 19 systematic reviews 239-257 and 13 RCTs 88,258,259,261,262,265,267,298 , 306-310 (n = 8593) to inform this recommendation. Trials included adults with objectively confirmed DVT and/or PE who had been treated with anticoagulants for ≥3 months without recurrence, referred to as the “study population” below. Patients were subsequently randomized to receive placebo or continue antithrombotic therapy for ≥6 additional months. The mean follow-up varied from 24 to 28 months for different outcomes. The outcomes were measured in both groups immediately at the end of the longer course of anticoagulation. For baseline risks of VTE, we used a prospective cohort study 324 that reported a risk for patients with a recurrent unprovoked VTE of 12 per 100 patient-years. Assuming that 45% of the VTE events are PEs and 55% are DVTs, 269 we estimated the risks of PE recurrence to be 5.4 per 100 patient-years and the risks of DVT recurrence to be 6.6 per 100 patient-years for patients with recurrent unprovoked VTE. For the baseline risk of major bleeding, we used data from 2 randomized trials on people with VTE; the risk of major bleeding with placebo during an 18-month or 24-month treatment with anticoagulants was as low as 0.5% 306 and as high as 1.5% in 18 months. 259 The EtD framework is shown online at: https://guidelines.gradepro.org/profile/C151C2DC-8A88-9E05-9D73-2FEB6B917C00 .
The meta-analysis showed that, compared with discontinuation of anticoagulation, treating patients with indefinite antithrombotic therapy reduced the risk of PE in the study population (RR, 0.29; 95% CI, 0.15-0.56; ARR, 21 fewer per 1000 patients; 95% CI, 25 fewer to 13 fewer; high-certainty evidence), as well as for patients with recurrent unprovoked VTE 269,324 (ARR, 38 fewer per 1000 patients; 95% CI, 46 fewer to 24 fewer; high-certainty evidence). Indefinite antithrombotic therapy also showed a risk reduction in DVT (RR, 0.20; 95% CI, 0.12-0.34; ARR, 50 fewer per 1000 patients; 95% CI, 56 fewer to 42 fewer; high-certainty evidence). For patients with recurrent unprovoked VTE at 1 year, 269,324 indefinite antithrombotic therapy also reduced the risk of DVT (ARR, 53 fewer per 1000 patients; 95% CI, 58 fewer to 44 fewer).
There were significant subgroup effects associated with the different antithrombotic interventions on DVT outcome. When using a DOAC for indefinite anticoagulation, the risk of DVT was reduced in the study population (RR, 0.15; 95% CI, 0.10-0.23; ARR, 49 fewer per 1000 patients; 95% CI, 51 fewer to 44 fewer; high-certainty evidence) as well as for patients with recurrent unprovoked VTE 269,324 (ARR, 56 fewer per 1000 patients; 95% CI, 59 fewer to 51 fewer; high-certainty evidence). When a VKA or LMWH was used for indefinite anticoagulation, we observed a reduction in the risk of DVT in the study population (RR, 0.17; 95% CI, 0.05-0.53; ARR, 54 fewer per 1000 patients; 95% CI, 61 fewer to 30 fewer), as well as for patients with recurrent unprovoked VTE (ARR, 55 fewer per 1000 patients; 95% CI, 63 fewer to 31 fewer; high-certainty evidence). 269,324 Aspirin also reduced the risk of recurrent DVT in the study population (RR, 0.55; 95% CI, 0.31-0.98; ARR, 64 fewer per 1000 patients; 95% CI, 98 fewer to 3 fewer), as well as for patients with recurrent unprovoked VTE (ARR, 30 fewer per 1000 patients; 95% CI, 46 fewer to 1 fewer; moderate-certainty evidence). 269,324 Our analysis showed a potential decrease in mortality when using indefinite antithrombotic therapy compared with a defined duration of anticoagulation, without statistical significance (RR, 0.75; 95% CI, 0.49-1.13; ARR, 5 fewer per 1000 patients; 95% CI, 9 fewer to 2 more; moderate-certainty evidence).
The certainty in the evidence was judged high for PE, DVT, and major bleeding but moderate for mortality because of imprecision given that the CI around the absolute estimates crossed thresholds that patients would likely consider important. Therefore, it was not possible to completely rule out a small difference between the alternatives on mortality. In the subgroup analysis performed for DVT, when using aspirin the certainty in the evidence was judged moderate because of imprecision.
We considered that avoidance of PE, DVT, and major bleeding was critical for patients. Patients placed a high value on the benefits of risk reduction in VTE recurrence and PTS. 310 However, there is important variability in how individual patients may value the risk of thrombosis vs the risk of bleeding.
We did not identify direct evidence on a cost-effectiveness comparison for nonsurgical provoked VTE. Four Markov model analyses of cost-effectiveness for extended antithrombotic therapy vs limited antithrombotic therapy for VTE treatment were identified. Three analyses showed that the extended strategy was cost-effective compared with the limited antithrombotic therapy, 112,270,271 whereas 1 analysis suggested that longer initial conventional-intensity anticoagulation with warfarin was cost-effective in younger patients, and 3 months of anticoagulation was preferred in elderly patients (≥80 years old). 272 The panel considered that cost-effectiveness varies with patients, the chronic risk factor(s) contributing to risk for recurrent VTE, and drug used.
The panel considered that indefinite treatment was probably feasible but that acceptability varies.
This recommendation applies to the patient who sustains an unprovoked VTE and who also has a history of an unprovoked VTE that was treated with a time-limited course of therapy that had been discontinued prior to the current event. These patients need to undergo decisions about initial management (Recommendations 1 to 11) and primary treatment (Recommendations 12 to 14), just as with their initial event. The ASH guideline panel provided a strong recommendation in favor of indefinite antithrombotic therapy for secondary prevention of recurrent thromboembolism in light of the very high risk of recurrence off anticoagulation. Decisions about the optimal antithrombotic strategy for secondary prevention would be similar to those for a first unprovoked VTE (Recommendations 20 to 22).
For patients with DVT and/or PE with stable CVD who initiate anticoagulation and were previously taking aspirin for cardiovascular risk modification, the ASH guideline panel suggests suspending aspirin over continuing it for the duration of anticoagulation therapy (conditional recommendation based on very low certainty in the evidence of effects ⨁○○○).
We identified 13 RCTs (n = 7928) to inform this recommendation. 325-337 Trials included PE patients who were previously receiving aspirin for prevention of CVD and had initiated anticoagulation therapy. Patients who received an initial course of anticoagulants were randomized to anticoagulant with aspirin or anticoagulants alone. The mean follow-up time varied from 1 to 2.5 years. The EtD framework is shown online at: https://guidelines.gradepro.org/profile/64AF970C-9665-2F07-BFD3-EB4E658C5706 .
An assessment of the benefits of aspirin was not relevant to a guideline of VTE treatment and, therefore, was considered outside the scope of this analysis.
Evidence from included RCTs suggested that treating PE patients who had previously received aspirin for the prevention of CVD with anticoagulation with aspirin increased the risk of major bleeding compared with anticoagulation alone (RR, 1.26; 95% CI, 0.92-1.72; ARR, 7 more per 1000 patients; 95% CI, 2 fewer to 21 more; very-low-certainty evidence). In patient populations with any indication for anticoagulation and/or aspirin, 98 the use of anticoagulation with aspirin relative to anticoagulation alone led to an increase of 5 more major bleeding events per 1000 patients (95% CI, 2 fewer to 15 more; very-low-certainty evidence).
The certainty in the evidence from the included RCTs was judged very low for major bleeding because of a serious risk for bias, indirectness, and imprecision. First, there was a lack of allocation concealment and blinding of study participants and personnel across the different studies. Second, the pooled CIs included the null, as well as appreciable benefit and harm. Third, the included trials were conducted for patients without VTE.
The panel considered that aspirin discontinuation was probably feasible but that acceptability varies.
Patients may be taking aspirin daily for prevention of CVD at the time of diagnosis with a VTE. For patients taking aspirin for primary prevention of CVD or for stable coronary artery disease, the ASH guideline panel provides a conditional recommendation in favor of suspending aspirin while taking anticoagulant therapy, based on a very low level of certainty in the evidence. For secondary prevention for patients with stable coronary artery disease, studies have found oral anticoagulants to be effective, with a decreased risk for bleeding compared with the combination of an anticoagulant and aspirin. 330-38 This recommendation does not apply to patients with a recent acute coronary event or coronary intervention. A similar approach has been advocated for patients who are taking anticoagulant therapy for atrial fibrillation who have concomitant CVD. 339
Research needs relevant to this recommendation include studies to determine which patients should continue antiplatelet therapy when anticoagulant therapy is initiated for the treatment of VTE. In addition, research is needed to determine which anticoagulant agent(s) and dose(s) are safest when coadministered with antiplatelet therapy.
For patients with DVT, with (Recommendation 27) or without (Recommendation 28) increased risk for PTS, the ASH guideline panel suggests against the routine use of compression stockings (conditional recommendation based on very low certainty in the evidence of effects ⨁○○○).
Remarks: Although the majority of patients may not benefit from the use of stockings to reduce the risk of PTS, stockings may help to reduce edema and pain associated with DVT in selected patients.
We identified 10 systematic reviews 340-349 and 6 relevant randomized trials 260 , 350-354 (n = 1393). All of the identified trials, with the exception of the SOX trial, compared the use of stockings vs no stockings for patients with proximal DVT. Typically, the intervention group received an elastic stocking on the affected leg with an ankle pressure of 30 to 40 mm Hg for 6 to 24 months. Participants were followed for a period of 2 to 5 years and were periodically assessed for the development of PTS. The definition of PTS was variable in the different trials, although the Villalta scale 355 was used most frequently. The sample size in the SOX trial 353 (n = 806) was larger than in the rest of the trials, and patients with proximal DVT were randomized to elastic stockings with an ankle pressure of 30 to 40 mm Hg or to placebo stockings with an ankle pressure ≤5 mm Hg for 2 years. The investigators reported the incidence of PTS at the end of follow-up using different definitions, including the Villalta criteria. The EtD framework is shown online at: https://guidelines.gradepro.org/profile/88899593-89FA-D803-95A0-B9E113F2B50D (Recommendation 27) and https://guidelines.gradepro.org/profile/77202CC8-4CE2-DE7B-8EFF-96F7C0E80DFD (Recommendation 28).
The use of compression stockings has a negligible effect on mortality (RR, 0.99; 95% CI, 0.72-1.36; ARR, 0 fewer per 1000 patients; 95% CI, 13 fewer to 17 more; moderate-certainty evidence). We did observe a nonsignificant reduction in the risk of PE (RR, 0.72; 95% CI, 0.31-1.70; ARR, 4 fewer per 1000 patients; 95% CI, 10 fewer to 10 more; low-certainty evidence); however, this outcome was reported in only 1 trial, and the number of events was very small.
Pooling all identified trials, we observed a nonsignificant reduction in the risk of PTS (RR, 0.62; 95% CI, 0.38-1.01; ARR, 81 fewer per 1000 patients; 95% CI, 132 fewer to 2 more; very-low certainty evidence). However, when we considered only the trials with a low risk for bias, this potential benefit was not observed (RR, 1.01; 95% CI, 0.76-1.33). The same occurred with the risk of DVT. Pooling all trials, we observed a nonsignificant reduction in DVT (RR, 0.56; 95% CI, 0.12-2.70; ARR, 18 fewer per 1000 patients; 95% CI, 35 fewer to 68 more; low-certainty evidence), although this did not hold in the subgroup of trials with a low risk for bias (RR, 1.08; 95% CI, 0.69-1.71).
We did not identify harmful effects of stockings. However, some patients may experience discomfort, skin breakdown, allergic reaction, or significant cost to acquire the stockings.
The main limitations of the evidence identified were the risk of bias and the small number of patients studied (imprecision). The effect of compression stockings on PTS and DVT observed in the SOX trial was significantly different from the results of unblinded trials, as demonstrated by the tests for interaction.
Additionally, for all of the outcomes, the number of patients studied and number of events were relatively small, and the CI around the absolute estimates likely crossed the thresholds that patients would consider important.
There is probably important variability in patients’ preferences: although some may experience relief of pain and edema, compression of the leg may be associated with discomfort in others.
We did not find any economic evaluation assessing the cost utility of compression stockings, although we considered that maintaining stockings for a long period of time implied a moderate cost.
Finally, stockings are generally available, although they may not be acceptable for some patients and providers given the uncertainty regarding their effect.
PTS may develop in up to 30% to 50% of patients following the development of proximal DVT, 149,150 and it may be severe in 5% to 10% of patients. 3,150 Some patients may experience symptomatic benefit from wearing compression stockings but, as noted above, there was inconsistent evidence that compression stockings can decrease the risk of developing PTS. Consequently, given the very low level of certainty in the evidence, the ASH guideline panel suggests against the routine use of compression stockings for patients with DVT, with or without an increased risk for PTS. Research priorities should focus on the identification of the subsets of patients who would potentially benefit from the use of compression stockings.
There are 4 recent guideline documents concerning the management of patients with VTE. The 2016 guideline and expert panel report on antithrombotic therapy for VTE from the American College of Chest Physicians (ACCP) 356 is an update of the 2012 ACCP guidelines. 312 A second guidance document on the treatment of VTE, endorsed by the Anticoagulation Forum (ACF) Board of Directors, was also published in 2016. 357 The European Society of Cardiology has published separate consensus documents on the diagnosis and management of DVT 358 and PE. 238 Differences between the ASH guidelines and these documents include the consistent use of systematic reviews and EtD frameworks, which increase transparency, and the use of marker states to estimate the relative importance to patients of key outcomes of treatment.
As with prior guidelines, the ASH guideline panel has given considerable thought to distinguishing the primary treatment phase of VTE (first 3-6 months) from the secondary prevention phase (indefinite duration following the primary treatment phase; Figure 2 ). Important decisions concerning which patients should receive indefinite preventive therapy following completion of the primary treatment phase, as well as what antithrombotic therapy should be administered, must have these unique phases of treatment clearly defined. Prior terminology has been confusing, with the primary treatment phase described as “long term” in the 2016 ACCP guidelines and “short term” in the 2016 ACF guidance document; the secondary prevention phase is referred to as “extended” in the 2016 ACCP guidelines and “long term” in the 2016 ACF guidance document. Our choice of terminology reflects the distinct clinical intention of the 2 phases of VTE management, rather than terms reflecting the relative duration of therapy.
New questions addressed in the ASH guidelines include recommendations concerning whether 1 DOAC should be preferred over another for the primary treatment phase (Recommendation 4), whether prognostic scores, D-dimer testing, and/or ultrasound testing should be routinely used to guide decision making concerning continuing therapy after completion of the primary phase of treatment (Recommendations 15 to 17), whether patients receiving rivaroxaban or apixaban for secondary prevention therapy should receive standard-dose or lower-dose therapy (Recommendation 22), and whether patients who have previously sustained a VTE and completed a course of primary treatment, who now sustain a recurrent event associated with a transient risk factor, should receive secondary prevention after completion of the primary treatment phase of therapy (Recommendations 24a and 24b). The ASH guidelines also address the question of whether aspirin should be continued or discontinued during anticoagulation in those patients who sustain a VTE while taking aspirin (Recommendation 26).
The ASH guidelines incorporate the most recent systematic reviews, RCTs, and observational studies, as well as information concerning cost of interventions and health equity, in the final recommendations that have been generated. When appropriate, the panel has also provided suggestions for areas in which future research is needed to address questions important to patients with VTE and their providers.
Treatment of VTE in day-to-day practice poses many challenges to clinicians. We acknowledge that not all of them are covered in this guideline. However, the guideline model implemented by ASH can be easily updated in the future, adding new recommendations to those already published.
Panelists recommended or suggested courses of action based on the evidence available at the moment of development of this guideline. However, new evidence may change the recommendations in the future, especially those based on low- or very-low-certainty evidence.
Finally, the recommendations are meant to inform the decisions of clinicians and patients. They do not, however, replace the careful consideration of the specific clinical circumstances and patients’ values and preferences.
After publication of these guidelines, ASH will maintain them through surveillance for new evidence, ongoing review by experts, and regular revisions.
Although ASH guidelines have a global scope, the recommendations in this article were developed primarily for North America. Likely, resource considerations, feasibility, and acceptability of interventions may vary in different regions.
The “Adolopment” model of guideline adoption, adaptation, and development 359 offers an approach to adapt the recommendations of this article to a specific context, taking advantage of the EtD frameworks accompanying each recommendation. Local guideline groups may reuse the evidence collected and appraised on the frameworks. By adding relevant local information, they can generate local recommendations with fewer resources than those required to develop a guideline de novo.
The authors thank Andrew Kirkman for contributions to the guideline document.
Contribution: T.L.O., I.N., and Yuqing Zhang wrote the first draft of this manuscript and revised the manuscript based on authors’ suggestions; guideline panel members W.A., R.B., N.P.C., A.C., B.A.H., M.R.J., V.M., S.S., C.T., S.V., P.V., and D.M.W., critically reviewed the manuscript and provided suggestions for improvement; members of the knowledge synthesis team, I.D.F., A.I., R.N., S.R., H.J.S., W.W., Yuan Zhang, and Yuqing Zhang, contributed evidence summaries to the guidelines; all authors approved of the content; and T.L.O. was chair and I.N. was vice chair of the panel and led the panel meeting.
Conflict-of-interest disclosure: All authors were members of the guideline panel, the systematic review team, or both. They completed disclosure-of interest-forms, which were reviewed by ASH and are available as Supplements 2 and 3. The following disclosure was added after the finalization of the guideline panel’s disclosure of interest forms: In January 2020, M.R.J. became part-time chief medical officer for Boston Scientific, for which he receives salary and equity. Embolitech was sold to Surmodics, for which M.R.J. received equity.
Correspondence: Thomas L. Ortel, Duke University, 201 Trent Dr, Room 0563, Stead Building Box 3422, Durham, NC 27710; e-mail: [email protected] .
Author notes
The full-text version of this article contains a data supplement.
Supplemental data
- Previous Article
- Next Article
Email alerts
Affiliations.
- Current Issue
- Latest Articles
- Collections
- Community Conversations
- Blood Advance Talks
- Permissions
- Submit to Blood Advances
- Order Reprints
- Advertising in Blood Advances
American Society of Hematology
- 2021 L Street NW, Suite 900
- Washington, DC 20036
- TEL +1 202-776-0544
- FAX +1 202-776-0545
ASH Publications
- Blood Advances
- Blood Neoplasia
- Blood Vessels, Thrombosis & Hemostasis
- Hematology, ASH Education Program
- ASH Clinical News
- The Hematologist
- Publications
- Privacy Policy
- Cookie Policy
- Terms of Use
This Feature Is Available To Subscribers Only
Sign In or Create an Account
Clinical presentation of deep vein thrombosis and pulmonary embolism
Affiliation.
- 1 Vascular Medicine, Medical Department IV, Klinikum Darmstadt GmbH, Darmstadt, Germany. [email protected]
- PMID: 22959541
- DOI: 10.1016/j.beha.2012.07.004
Background: In the past, the clinical diagnosis of venous thromboembolism (VTE), which comprises deep vein thrombosis (DVT) and pulmonary embolism (PE) had been very challenging, because clinical presentation is non-specific and inaccurate.
Objective: To review and assess clinical signs, symptoms and risk factors of DVT and PE and identify most common differential diagnoses.
Results: Important components for the clinical diagnosis of VTE include risk factors such as immobilization, presence of cancer, confinement to bed, previous major surgery, prior VTE and - specific for DVT - whole limb enlargement, one-sided calf enlargement and dilatation of superficial veins. Additional items specific for PE include tachycardia, dyspnea chest pain and hemoptysis. Many of these clinical characteristics are included into clinical prediction rules, such as the Wells pre-test probability score for DVT or PE or the Geneva score for PE. These scores are used to determine the pre-test probability for VTE and they constitute the basis for a diagnostic algorithm. Various clinical prediction rules for DVT or PE show comparable accuracy.
Conclusion: Even though the clinical presentation of DVT and PE varies substantially in individual patients and settings and may be misleading, diagnostic prediction rules based on clinical presentation and risk factors are very useful to assess pre-test probability, which is a very important concept for the diagnosis of DVT and PE.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Publication types
- Chest Pain / pathology
- Diagnosis, Differential
- Dyspnea / pathology
- Hemoptysis / pathology
- Immobilization
- Pulmonary Embolism / diagnosis*
- Pulmonary Embolism / diagnostic imaging
- Pulmonary Embolism / pathology
- Research Design
- Risk Factors
- Sex Factors
- Tachycardia / pathology
- Ultrasonography
- Venous Thrombosis / diagnosis*
- Venous Thrombosis / diagnostic imaging
- Venous Thrombosis / pathology
Learn how UpToDate can help you.
Select the option that best describes you
- Medical Professional
- Resident, Fellow, or Student
- Hospital or Institution
- Group Practice
- Patient or Caregiver
- Find in topic
RELATED TOPICS
INTRODUCTION
An overview of the treatment of lower extremity DVT (distal and proximal), including treatment of special populations of patients with DVT, is discussed in this topic. Initial, long-term, and extended (indefinite) anticoagulation for DVT, as well as the treatment of PE and upper extremity DVT, are discussed in detail separately.
● (See "Venous thromboembolism: Initiation of anticoagulation" .)
● (See "Venous thromboembolism: Anticoagulation after initial management" .)
● (See "Selecting adult patients with lower extremity deep venous thrombosis and pulmonary embolism for indefinite anticoagulation" .)
What is Venous Thromboembolism?
Deep Vein Thrombosis and Pulmonary Embolism (DVT/PE) are often underdiagnosed and serious, but preventable medical conditions.
Deep vein thrombosis (DVT) is a medical condition that occurs when a blood clot forms in a deep vein. These clots usually develop in the lower leg, thigh, or pelvis, but they can also occur in the arm.
It is important to know about DVT because it can happen to anybody and can cause serious illness, disability, and in some cases, death. The good news is that DVT is preventable and treatable if discovered early.
Venous thromboembolism (VTE), a term referring to blood clots in the veins, is an underdiagnosed and serious, yet preventable medical condition that can cause disability and death.
The American Society of Hematology (ASH) recognizes the need for a comprehensive set of guidelines on the treatment of VTE to help the medical community better manage this serious condition. In partnership with the McMaster University GRADE Centre , ASH brought together experts to address this challenge, including hematologists, other clinicians, guideline development specialists, and patient representatives. In November 2018, ASH announced the results of their collective efforts – the 2018 ASH Clinical Practice Guidelines on Venous Thromboembolism . Access the new guidelines .
Complications of DVT
The most serious complication of DVT happens when a part of the clot breaks off and travels through the bloodstream to the lungs, causing a blockage called pulmonary embolism (PE). If the clot is small, and with appropriate treatment, people can recover from PE. However, there could be some damage to the lungs. If the clot is large, it can stop blood from reaching the lungs and is fatal.
In addition, one-third to one-half of people who have a DVT will have long-term complications caused by the damage the clot does to the valves in the vein called post-thrombotic syndrome (PTS). People with PTS have symptoms such as swelling, pain, discoloration, and in severe cases, scaling or ulcers in the affected part of the body. In some cases, the symptoms can be so severe that a person becomes disabled.
For some people, DVT and PE can become a chronic illness; about 30% of people who have had a DVT or PE are at risk for another episode.
Risk Factors for DVT
Almost anyone can have a DVT. However, certain factors can increase the chance of having this condition. The chance increases even more for someone who has more than one of these factors at the same time.
View the full infographic
Following is a list of factors that increase the risk of developing DVT:
- Severe muscle injury, or
- Major surgery (particularly involving the abdomen, pelvis, hip, or legs).
- Confinement to bed (e.g., due to a medical condition or after surgery);
- Limited movement (e.g., a cast on a leg to help heal an injured bone);
- Sitting for a long time, especially with crossed legs; or
- Birth control pills
- Hormone replacement therapy, sometimes used after menopause
- Pregnancy, for up to 3 months after giving birth
- Heart disease
- Lung disease
- Cancer and its treatment
- Inflammatory bowel disease (Crohn’s disease or ulcerative colitis)
- Previous DVT or PE
- Family history of DVT or PE
- Age (risk increases as age increases)
- A catheter located in a central vein
- Inherited clotting disorders
Preventing DVT

The following tips can help prevent DVT:
- Move around as soon as possible after having been confined to bed, such as after surgery, illness, or injury.
- Graduated compression stockings (sometimes called “medical compression stockings”)
- Medication (anticoagulants) to prevent DVT.
- Get up and walk around every 1 to 2 hours.
- Raising and lowering your heels while keeping your toes on the floor
- Raising and lowering your toes while keeping your heels on the floor
- Tightening and releasing your leg muscles
- Wear loose-fitting clothes.
- You can reduce your risk by maintaining a healthy weight, avoiding a sedentary lifestyle, and following your doctor’s recommendations based on your individual risk factors.
Everybody should know the signs and symptoms of DVT/PE, their risk for DVT/PE, to talk to their health care provider about their risk, and to seek care immediately if they have any sign or symptom of DVT/PE.
About half of people with DVT have no symptoms at all. The following are the most common symptoms of DVT that occur in the affected part of the body:
- Redness of the skin
If you have any of these symptoms, you should see your doctor as soon as possible.
You can have a PE without any symptoms of a DVT.
Signs and symptoms of PE can include:
- Difficulty breathing
- Faster than normal or irregular heart beat
- Chest pain or discomfort, which usually worsens with a deep breath or coughing
- Coughing up blood
- Very low blood pressure, lightheadedness, or fainting
If you have any of these symptoms, you should seek medical help immediately.
Diagnosis of DVT and PE
The diagnosis of DVT or PE requires special tests that can only be performed by a doctor. That is why it is important for you to seek medical care if you experience any of the symptoms of DVT or PE.
Learn more about diagnosis »
Treatments for DVT and PE
Medication is used to prevent and treat DVT. Compression stockings (also called graduated compression stockings) are sometimes recommended to prevent DVT and relieve pain and swelling. These might need to be worn for 2 years or more after having DVT. In severe cases, the clot might need to be removed surgically.
Immediate medical attention is necessary to treat PE. In cases of severe, life-threatening PE, there are medicines called thrombolytics that can dissolve the clot. Other medicines, called anticoagulants, may be prescribed to prevent more clots from forming. Some people may need to be on medication long-term to prevent future blood clots.
Learn more about treatments »
DVT does not cause heart attack or stroke. There are two main types of blood clots. How a clot affects the body depends on the type and location of the clot:
- A blood clot in a deep vein of the leg, pelvis, and sometimes arm, is called deep vein thrombosis (DVT). This type of blood clot does not cause heart attack or stroke.
- A blood clot in an artery, usually in the heart or brain, is called arterial thrombosis. This type of blood clot can cause heart attack or stroke.
Both types of clots can cause serious health problems, but the causes and steps you can take to protect yourself are different. To learn more about arterial thrombosis, visit CDC’s information about heart disease and stroke prevention .
Join the Public Health Webinar Series on Blood Disorders
- Upcoming webinar
- View past webinars
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.

StarsInsider
What is deep vein thrombosis, and how can you reduce your risk?
Posted: October 12, 2023 | Last updated: October 12, 2023

Deep vein thrombosis (DVT) is a medical condition that occurs when a blood clot forms in a deep vein. The blood clots may partially or completely block blood flow through your vein. Most DVTs happen in your lower legs, but they can also occur in other parts of your body, including your thighs or pelvis. A commonly occurring medical condition, these clots can break free and travel to major organs, such as your lungs , which can be very dangerous. Life factors such as pregnancy and long flights can increase your risk of DVT, but there are steps you can take to minimize it.
Want to know how? Then check out the following gallery to discover more.
You may also like: Actors who didn't make it until later in their lives

Common DVT symptoms include leg swelling, pain, cramping, and soreness, change in skin color, and warmth in the affected area. Do note that DVT can also occur without noticeable symptoms.
Follow us and access great exclusive content every day
When to see a doctor
If you develop any of the aforementioned symptoms, contact your healthcare provider immediately.
You may also like: Celebs who've been fired from major movie and TV show roles
The main causes of DVT are damage to a vein from surgery or inflammation, and also damage due to infection or injury. However, anything that prevents the blood from flowing or properly clotting can cause a blood clot .

Risk factors
Many things can increase the risk of developing DVT. The more risk factors you have, the greater the risk.
You may also like: Fascinating photos of World War II

Being older than 60 increases the risk of DVT. But it can occur at any age.

Lack of movement
When the legs don't move for a long time, the calf muscles don't contract. Muscle contractions help blood flow. Sitting or laying down for a long time increases the risk of DVT.
You may also like: Elvis Presley: the untold story of the King
Injury or surgery
Injury to the veins or surgery can increase the risk of blood clots.

As pregnancy increases the pressure in the veins in the pelvis and legs, DVT is a risk. The risk of blood clots from pregnancy can continue for up to six weeks after a baby is born.
You may also like: 100 of the famous faces who've passed away in 2019
Birth control pills and hormone replacement therapy
Both birth control pills and hormone replacement therapy can increase the blood's ability to clot.

Being overweight or obese
Being overweight increases the pressure in the veins in the pelvis and legs.
You may also like: Mind-blowing book predictions that actually came true

Smoking affects how blood flows, which can increase the risk of DVT.

Some cancers increase substances in the blood that cause the blood to clot. The same goes for some cancer treatments.
You may also like: Warning signs to look out for if you are over 40

Heart failure
Heart failure increases the risk of DVT and pulmonary embolism.

Inflammatory bowel disease
Crohn's disease or ulcerative colitis increase the risk of DVT.
You may also like: The best whiskey cocktails you just have to try

Family history
If you or someone in your family has had DVT, you might be at greater risk of developing it. Sometimes a blood clot can occur with no identifiable risk factor. This is called an unprovoked VTE.
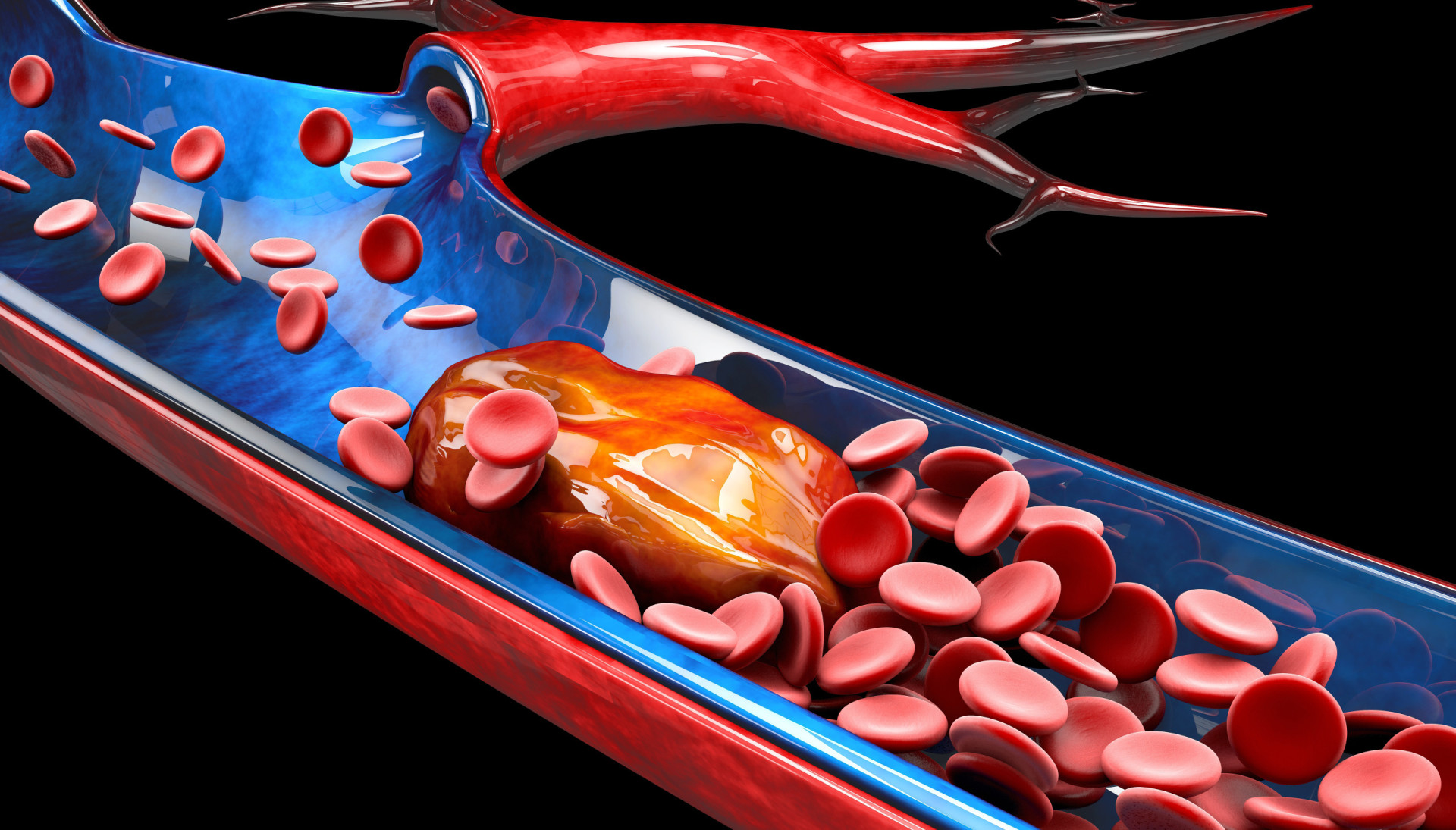
Complications
Unfortunately, some complications can occur following a DVT.
You may also like: Hacks to keep food fresh for longer
Pulmonary embolism (PE)
PE is a potentially life-threatening complication associated with DVT. It occurs when a blood clot in the leg or other area of the body gets stuck in a blood vessel in a lung. Symptoms include sudden shortness of breath, chest pain, fainting, and coughing up blood.

Post-phlebitic syndrome
Damage to the veins from the blood clot reduces blood flow in the affected areas. Symptoms include leg pain and swelling, skin color changes, and skin sores.
You may also like: Celebrities whose ancestors made history

Treatment complications
Blood thinners are often used to treat DVT, however hemorrhage is a worrisome side effect. It's important to have regular blood tests done while taking blood-thinning drugs.

Luckily, even if you're at risk, you can take steps to prevent DVT. Lifestyle changes are major factors.
You may also like: Absurdly short reigns in royal history

Avoid sitting for long periods of time while traveling
No matter your mode of transportation, it's important to stand up and stretch every once in a while when you're taking a long trip.

The same goes for when you're not traveling
Avoid sitting for a long period of time in everyday life, too. Get up to stretch periodically while you’re working or watching TV.
You may also like: Signs that someone will grow up to be a millionaire

Stay hydrated
Dehydration is a significant risk factor for DVT, so make sure you're drinking enough water. Keep in mind that alcohol and large quantities of caffeinated beverages can increase dehydration.

Wear compression stockings
You may also like: How to deal with passive-aggressive behavior

Stop smoking
Smoking affects blood clotting and circulation, which in turn increases the risk of DVT. If you're a smoker, the best thing you can do is to stop.

Maintain a healthy weight
Obesity is a risk factor for DVT. But regular exercise and avoiding a sedentary lifestyle lowers the risk. As a general goal, aim for at least 30 minutes of moderate physical activity every day.
You may also like: The bizarre and gruesome deaths of ancient Greece

Keep up with meds on your vacation
When you take a well-deserved vacation from your daily routine, make sure you don't take a break from your medications!

Keep moving if you're pregnant
When you're pregnant, certain changes that occur in your body reduce blood flow and make your blood more likely to clot. Therefore, it's important to keep moving.
You may also like: What would happen to Earth if humans went extinct?

Check your blood pressure
Check your blood pressure at least once a year, or more often if your doctor says so.
Sources: (CDC) (Everyday Health) (Mayo Clinic)
See also: The health risks of each blood type
More for You
19 Things People Treat As Safe That Actually Are Pretty Dangerous
Bethenny Frankel, fiancé Paul Bernon reportedly split after nearly six years together
20 Loyal Dog Breeds That Will Never Leave Your Side
Donald Trump in 'Best Case Scenario' With Trial: Ex-Aide
Your senses will shut down in a specific order when you’re about to die
Who wore Met Gala’s ‘Garden of Time’ theme best?
What Is the Most Poisonous Spider in the World?
I drove the Tesla Cybertruck. These 7 design flaws surprised me.
You're Going to Want to Buy Your Stamps ASAP. Here's What to Know About USPS' Price Hike
6 Myths About Mexican Food That Mexican Chefs Want Americans To Stop Believing
Doctor shares what happens to our bodies moments before we die
The 11 Smells That Squirrels and Chipmunks Hate
17 Uniquely American Things That Non-Americans Are Utterly Fascinated By
I Lost White Friends When I Finally Spoke Out
Russia Threatens ‘Highly Dangerous’ Conflict After Petition Pushing For NATO Troops Circulates On Ukrainian Government Site
Why do I wake up at 3 a.m. every night?
NFL Schedule Predictions: Thanksgiving Games, Top Prime-Time Showdowns, Christmas Matchups
15 Dog Breeds Most Similar to Pit Bulls
The Most Visited Attraction in Every US State
Is the Economy As Bad as Americans Think?
- Reference Manager
- Simple TEXT file
People also looked at
Case report article, case report: popliteal artery entrapment syndrome as a cause of deep vein thrombosis and subsequent popliteal artery occlusion.

- 1 Division of Vascular and Endovascular Surgery, Department of Surgery, Kyungpook National University Hospital, School of Medicine, Kyungpook National University, Daegu, Republic of Korea
- 2 Division of Vascular and Endovascular Surgery, Department of Surgery, Kyungpook National University Chilgok Hospital, School of Medicine, Kyungpook National University, Daegu, Republic of Korea
Background: Popliteal artery entrapment syndrome (PAES) is a relatively rare cause of arterial insufficiency in young and physically active individuals; however, deep vein thrombosis (DVT) can develop in association with PAES.
Case report: A 47-year-old man presented with a 6-day history of left leg swelling and discomfort which was diagnosed as DVT extending to the distal femoral vein and pulmonary embolism on computed tomography (CT). PAES was not suspected at this time, and the patient was administered anticoagulants for 1 year. Two years after the DVT diagnosis, the patient developed sudden-onset left calf claudication for 1 week. Repeat CT angiography showed popliteal artery (PA) occlusion caused by PA displacement from an abnormally lateral insertion of the medial gastrocnemius head. A retrospective review of the initial CT scan confirmed this, as well as compression of the popliteal vein between the displaced medial head and the normal lateral head of the gastrocnemius. The patient eventually underwent myotomy and resection of the PA with an interposition graft.
Conclusion: This case underscores the potential of PAES as a rare etiology of DVT, emphasizing the importance of considering it in the differential diagnosis of DVT in younger patients lacking common predisposing factors.
1 Introduction
Popliteal artery entrapment syndrome (PAES) is a rare vascular disorder that involves compression of the popliteal artery (PA) from the surrounding musculotendinous structures ( 1 ). PAES can be anatomical or functional—anatomical PAES results from PA compression or entrapment occurring due to anatomical anomalies that are often present at birth and developed over time, whereas in functional PAES, the compression is not due to a fixed anatomical anomaly but rather due to dynamic factors, such as muscle contraction during physical activity ( 1 ).
Although the incidence of functional PAES is not well characterized, the most common symptoms are intermittent claudication and pain in the feet and calves after exercise due to external compression of a normal artery. In anatomic PAES, the claudication could result from stenosis or occlusion of an injured PA by repeated trauma ( 2 , 3 ). However, other clinical manifestations, such as chronic pain, acute limb ischemia, and pulsating mass due to aneurysmal changes in the PA, have also been reported in the literature ( 3 , 4 ). Additionally, a few cases of deep vein thrombosis (DVT) have been reported in association with PAES ( 5 ). Since both DVT and subsequent PA occlusion are rarely associated with PAES, clinical suspicion is crucial, especially in young patients without precipitating factors for DVT.
In this report, we present a case of PAES complicated by DVT and subsequent PA occlusion occurring 2 years later. The patient was ultimately managed surgically with myotomy and interposition graft for the arterial occlusion.
2 Case description
A 47-year-old man presented with a 6-day history of left leg swelling and discomfort. He reported a history of hypertension for 5 years and had undergone gamma knife radiosurgery for an intracranial dural arteriovenous fistula 4 years ago. The patient denied any history of precipitating factors for DVT, including recent trauma, surgery, or immobilization.
Duplex ultrasonography (DUS) conducted at the time of presentation revealed DVT extending to the distal femoral vein; this was confirmed in the subsequent contrast-enhanced computed tomography (CT), which also revealed pulmonary embolism in the right segmental pulmonary artery ( Figure 1 ). Laboratory findings at initial presentation did not show any abnormalities (hemoglobin, 15.1 g/dl; platelet count, 180 K/µl, prothrombin time, 10.9 s; activated partial thromboplastin time, 26.2 s). Additionally, laboratory tests for hypercoagulability revealed normal values for natural anticoagulants, such as protein C, S, and antithrombin III; factor V Leiden, prothrombin, and JAK2 V617F mutations were not found. Accordingly, the patient was diagnosed with idiopathic venous thromboembolism; anticoagulation therapy with rivaroxaban was administered for 1 year, with a dosage of 20 mg for the initial 6 months and 10 mg for the subsequent 6 months for extended therapy, which was eventually discontinued. At this time, the follow-up DUS demonstrated partial recanalization of the popliteal vein (PV) with residual hyperechoic fibrotic material ( Figure 1D ). The waveform of PV venous flow showed continuous waveform and the axial reflux of PV was also noted. The patient complained of swelling in the left calf worsening in the afternoon or with exercise.
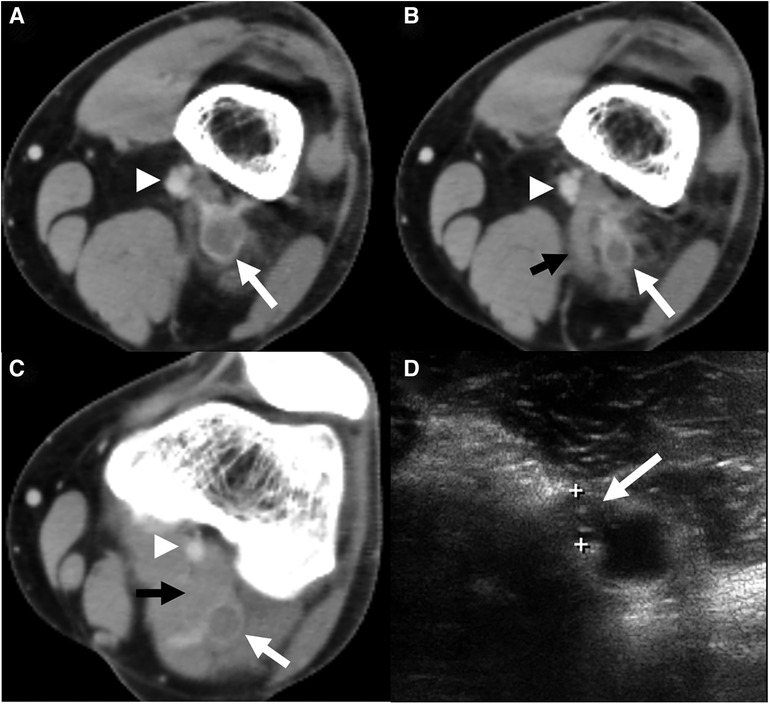
Figure 1 . A computed tomography (CT) scan was conducted at the index visit. ( A ) Axial CT section revealed deep vein thrombosis ( white arrow ) of the popliteal vein (PV) with a patent popliteal artery ( arrowhead ). ( B ) Axial CT imaging of a point 1 cm distal showed the same findings with an abnormally inserted medial head of gastrocnemius muscle ( black arrow ) and thrombosed popliteal vein with most stenosis. ( C ) Axial CT of the mid portion of the popliteal fossa demonstrated thrombosed PV ( white arrow ) between both heads of the gastrocnemius muscle. ( D ) Duplex ultrasonography 1 year after anticoagulation demonstrated partial recanalization of the popliteal vein with residual hyperechoic fibrotic material ( white arrow ).
Two years after this event, the patient again visited our outpatient clinic with sudden-onset claudication in the left calf for 1 week. On physical examination, the left ankle pulse was not palpable compared to a normal pulse in the right ankle [the ankle-brachial index (ABI) decreased to 0.62 on the left ankle]. Repeat DUS revealed occlusion of the PA with distal embolization to the dorsalis pedis artery. Subsequent CT angiography (CTA) showed occlusion of PA caused by type 2 PAES, i.e., medial deviation of the PA due to abnormal lateral attachment of the medial head of the gastrocnemius muscle between PA and PV ( Figure 2 ) ( 6 ). A careful review of the CT conducted during the previous DVT event also confirmed this abnormal gastrocnemius insertion, and PV was thought to be compressed by both heads of the gastrocnemius muscle ( Figure 1C ).
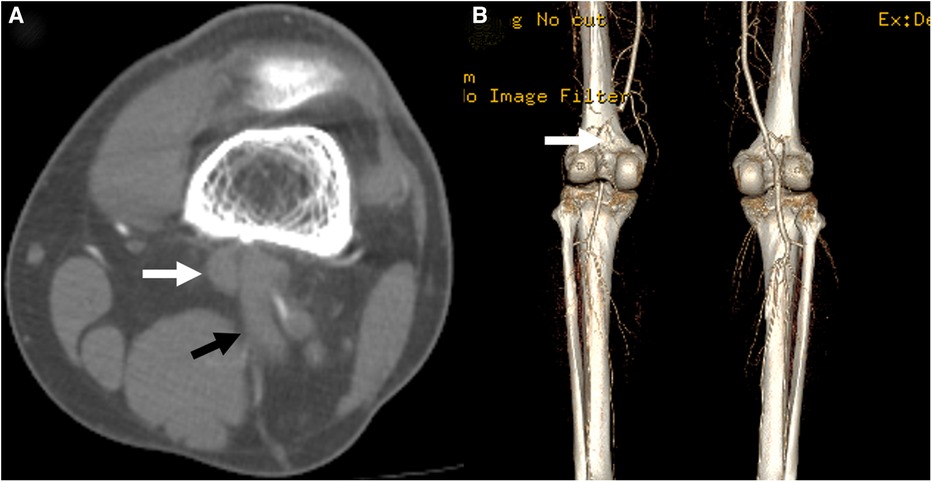
Figure 2 . A computed tomography scan conducted at the time of the second presentation showed occlusion of the popliteal artery ( white arrow ) in the axial ( A ) and 3-dimensional reconstruction ( B , posterior view) images. The anomalous insertion of the medial head of the gastrocnemius muscle between the popliteal artery and vein was also seen ( black arrow ).
Consequently, the patient was diagnosed with PAES with DVT and subsequent PA occlusion, for which he underwent surgical correction three days after presenting with PA occlusion. A myotomy of the medial head of the gastrocnemius was performed using a posterior S-shaped incision. Next, the injured part of the PA was resected and interposition grafting with the great saphenous vein harvested from the medial thigh was done ( Figure 3A ). The PV was not explored because the patient did not exhibit severe symptoms associated with chronic venous insufficiency. The postoperative course was uneventful, and the follow-up ABI before discharge was normalized to 1.10. The follow-up CTA before discharge also demonstrated a patent interposition graft with a typical course of PA ( Figure 3B ), so the patient was discharged a week after the operation. The patient received aspirin 100 mg monotherapy indefinitely following interposition grafting, and anticoagulation therapy was not restarted. During the subsequent 6 years of follow-up, he remained asymptomatic with a patent interposition graft which was monitored using DUS ( Figure 4 ).
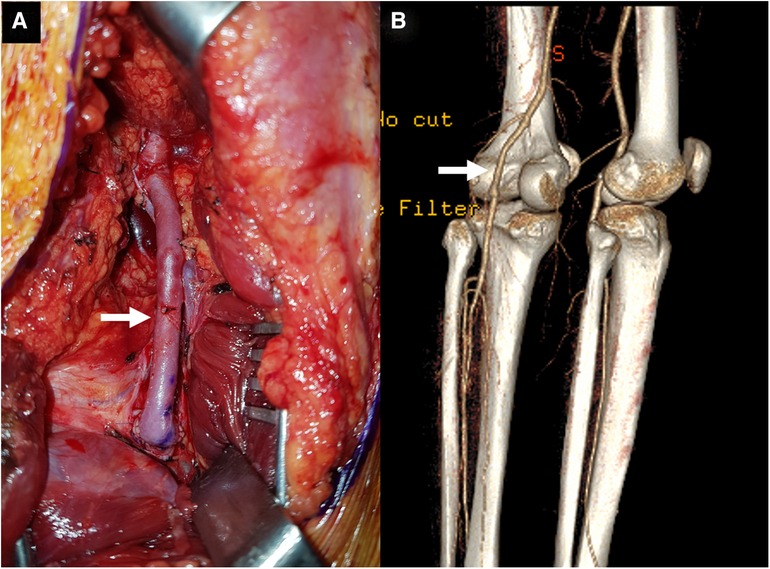
Figure 3 . ( A ) The operative photograph showing a patent interposition graft ( arrow ) made using a reversed great saphenous vein at the distal thigh. ( B ) The follow-up computed tomography angiography showed a sufficiently patent graft ( arrow ) (posterior view).
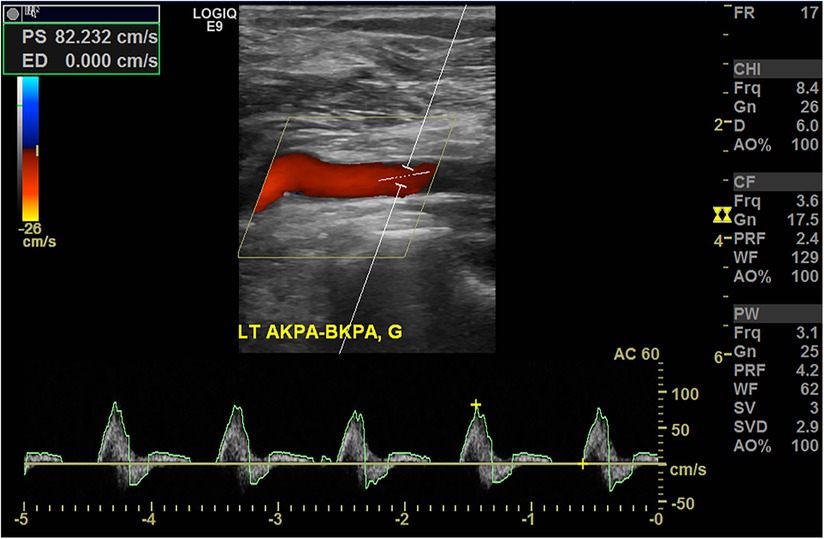
Figure 4 . Duplex ultrasonography conducted 5 years after the operation demonstrating a patent interposition graft without stenosis.
This study, serving as a care report, adhered to the CARE (CAse REport) guidelines. This study was approved by the institutional review board; informed consent was obtained from the patient (approval number: 2024-01-0033).
3 Discussion
DVT associated with PAES is a rare clinical presentation. The first case of PA and PV entrapment was reported in 1967 ( 7 ). At present, approximately 10%–15% of PAES cases are believed to involve the PV ( 5 ). While the most common cause of PV entrapment is an anomaly of the medial head of gastrocnemius ( 5 , 8 ), other causative factors, including bone tumor, cysts within the popliteal fossa, PA aneurysm, and anomalous course of the nerve and short saphenous vein, have been reported ( 9 – 11 ). Even in patients without definite anatomic anomalies around the popliteal vessels, PV entrapment can be caused by hypertrophy of the gastrocnemius and popliteus muscles, similar to functional PAES ( 12 ).
In the standard classification system for PAES, type 1–4 PAES represent the isolated entrapment of PA due to the medial head or aberrant slip of the gastrocnemius and plantaris muscles ( 6 , 13 ); any PV entrapment along with the PA is classified as a type 5 anomaly ( 1 ). Furthermore, functional PAES due to hypertrophy of surrounding muscles in the absence of anatomical anomalies of surrounding musculotendinous structures is classified as type 6. Our patient had type 2 PAES, which involves medial deviation of the PA due to abnormally lateral attachment of the medial gastrocnemius head between the PA and PV. In this case, the PV was not exactly trapped by the medial gastrocnemius head but was rather compressed between the two heads of the gastrocnemius ( Figure 1 ) because of the abnormally lateral course of the medial head. A careful review of the CT scan conducted at the time of the index presentation demonstrated a stenotic segment within the thrombosed PV. Additionally, the patient had negative results for hypercoagulability; therefore, we presume that the abnormally inserted medial head of the gastrocnemius and compression between medial and lateral heads may have caused DVT in this patient.
The treatment of DVT associated with PAES is determined by the severity of DVT and nature of the presenting symptoms. Patients with thrombosis should be treated with anticoagulants, and if indicated like iliofemoral DVT and severe symptoms, catheter-directed thrombolysis may be considered ( 14 ). In patients with concomitant PA entrapment, surgical decompression and thrombectomy with or without popliteal vein reconstruction may be contemplated ( 14 ).
In our case, PAES was not suspected as the cause of DVT at initial presentation and the proximal extent of DVT was located in the distal femoral vein; therefore, only anticoagulant therapy was administered to him for a year. However, the patient developed PA occlusion associated with PAES 1 year after discontinuing the anticoagulants, for which he eventually underwent PA resection with an interposition graft. In a review of the index CT scan conducted at the time of the DVT event, the PA did not show any stenotic changes, thrombus formation, aneurysmal changes, or distal embolization to the tibial arteries. Therefore, if PAES is suspected as the cause of DVT in this patient, a less invasive operation, such as simple myotomy without PA reconstruction, may have been sufficient during the index admission. Furthermore, the possibility of performing venous thrombectomy alongside the myotomy could be considered for the prompt resolution of venous thrombosis.
In summary, this case underscores the potential of PAES as a rare etiology of DVT, emphasizing the importance of considering it in the differential diagnosis of venous diseases in younger patients lacking common predisposing factors. Moreover, if CT imaging is available, meticulous evaluation of anatomical anomalies should be considered in young patients presenting with unprovoked DVT.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Kyungpook National University Hospital (IRB No. 2023-01-033). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SL: Data curation, Writing – original draft, Writing – review & editing. DH: Data curation, Formal Analysis, Writing – review & editing. WY: Data curation, Formal Analysis, Writing – review & editing. SH: Conceptualization, Supervision, Writing – review & editing. HK: Conceptualization, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors would like to thank Enago ( www.enago.co.kr ) for the English language review.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Love JW, Whelan TJ. Popliteal artery entrapment syndrome. Am J Surg . (1965) 109:620–4. doi: 10.1016/s0002-9610(65)80016-2
PubMed Abstract | Crossref Full Text | Google Scholar
2. Fujimura N, Obara H, Takahashi A, Miyata H, Hosaka A, Obitsu Y, et al. Surgical treatment for popliteal artery entrapment syndrome in Japan: a retrospective, multicentre study using a national clinical registry. Eur J Vasc Endovasc Surg . (2023) 66(3):381–8. doi: 10.1016/j.ejvs.2023.05.031
3. Bradshaw S, Habibollahi P, Soni J, Kolber M, Pillai AK. Popliteal artery entrapment syndrome. Cardiovasc Diagn Ther . (2021) 11(5):1159–67. doi: 10.21037/cdt-20-186
4. Kim HJ, Huh S, Kim HK. Popliteal artery entrapment syndrome presented with popliteal artery pseudoaneurysm: a case report. Vasc Specialist Int . (2023) 39:1. doi: 10.5758/vsi.230077
5. Gerkin TM, Beebe HG, Williams DM, Bloom JR, Wakefield TW. Popliteal vein entrapment presenting as deep venous thrombosis and chronic venous insufficiency. J Vasc Surg . (1993) 18(5):760–6. doi: 10.1067/mva.1993.48846
6. Levien LJ. Popliteal artery entrapment syndrome. Semin Vasc Surg . (2003) 16(3):223–31. doi: 10.1016/s0895-7967(03)00028-0
7. Rich NM, Hughes CW. Popliteal artery and vein entrapment. Am J Surg . (1967) 113(5):696–8. doi: 10.1016/0002-9610(67)90323-6
8. Raju S, Neglen P. Popliteal vein entrapment: a benign venographic feature or a pathologic entity? J Vasc Surg . (2000) 31(4):631–41. doi: 10.1067/mva.2000.103786
9. Sanchez JE, Conkling N, Labropoulos N. Compression syndromes of the popliteal neurovascular bundle due to baker cyst. J Vasc Surg . (2011) 54(6):1821–9. doi: 10.1016/j.jvs.2011.07.079
10. Holzapfel BM, Seppel G, Wagner R, Kenn W, Meffert R. Popliteal entrapment syndrome caused by fibular osteochondroma. Ann Vasc Surg . (2011) 25(7):982.e5–10. doi: 10.1016/j.avsg.2011.02.044
11. Sanjay P, Lewis MH. Deep vein thrombosis and pulmonary embolus associated with a ruptured popliteal aneurysm—a cautionary note. World J Emerg Surg . (2007) 2:34. doi: 10.1186/1749-7922-2-34
12. Misselbeck T, Dangleben D, Celani V. Isolated popliteal vein entrapment by the popliteus muscle: a case report. Vasc Med . (2008) 13(1):37–9. doi: 10.1177/1358863X07085109
13. Kwon YJ, Kwon TW, Um EH, Shin S, Cho YP, Kim JM, et al. Anatomical popliteal artery entrapment syndrome caused by an aberrant plantaris muscle. Vasc Specialist Int . (2015) 31(3):95–101. doi: 10.5758/vsi.2015.31.3.95
14. Chen CK, Kolber M. Venous popliteal entrapment syndrome. Cardiovasc Diagn Ther . (2021) 11(5):1168–71. doi: 10.21037/cdt-20-292
Keywords: case report, popliteal artery entrapment syndrome, popliteal vein, deep vein thrombosis, gastrocnemius muscle
Citation: Lee S, Hwang D, Yun W-S, Huh S and Kim H-K (2024) Case Report: Popliteal artery entrapment syndrome as a cause of deep vein thrombosis and subsequent popliteal artery occlusion. Front. Surg. 11:1384331. doi: 10.3389/fsurg.2024.1384331
Received: 9 February 2024; Accepted: 25 April 2024; Published: 6 May 2024.
Reviewed by:
© 2024 Lee, Hwang, Yun, Huh and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hyung-Kee Kim [email protected]
† ORCID Sangho Lee orcid.org/0009-0009-7999-4899 Deokbi Hwang orcid.org/0000-0003-0050-6434 Woo-Sung Yun orcid.org/0000-0001-8956-8310 Seung Huh orcid.org/0000-0002-0275-4960 Hyung-Kee Kim orcid.org/0000-0002-4436-7424

IMAGES
VIDEO
COMMENTS
Deep venous thrombosis (DVT) is a manifestation of venous thromboembolism (VTE). Although most DVT is occult and resolves spontaneously without complication, death from DVT-associated massive pulmonary embolism (PE) causes as many as 300,000 deaths annually in the United States. ... Presentations are similar, with pain and edema. However, the ...
Deep vein thrombosis (DVT) is an obstructive disease with hindering venous reflux mechanism.[1] DVT usually involves the lower limb venous system, with clot formation originating in a deep calf vein and propagating proximally.[2] ... The clinical presentation of acute lower extremity DVT varies with the anatomic distribution, extent, and degree ...
Deep venous thrombosis (DVT) is clotting of blood in a deep vein of an extremity (usually calf or thigh) or the pelvis. DVT is the primary cause of pulmonary embolism. DVT results from conditions that impair venous return, lead to endothelial injury or dysfunction, or cause hypercoagulability. DVT may be asymptomatic or cause pain and swelling ...
Deep vein thrombosis can be serious because blood clots in the veins can break loose. The clots can then travel through the bloodstream and get stuck in the lungs, blocking blood flow (pulmonary embolism). ... Bauer KA, et al. Clinical presentation and diagnosis of the nonpregnant adult with suspected deep vein thrombosis of the lower extremity ...
Deep vein thrombosis (DVT) is a major preventable cause of morbidity and mortality worldwide. Venous thromboembolism (VTE), which includes DVT and pulmonary embolism (PE), affects an estimated 1 per 1,000 people and contributes to 60,000-100,000 deaths annually. ... The clinical presentation of DVT varies with the extent and location of a ...
To diagnose deep vein thrombosis (DVT), your health care provider will do a physical exam and ask questions about your symptoms. The provider will check the legs for swelling, tenderness or changes in skin color. ... Bauer KA, et al. Clinical presentation and diagnosis of the nonpregnant adult with suspected deep vein thrombosis of the lower ...
Venous thrombosis is a condition in which a blood clot (thrombus) forms in a vein. This clot can limit blood flow through the vein, causing swelling and pain. Most commonly, venous thrombosis occurs in the "deep veins" in the legs, thighs, or pelvis ( figure 1 ). This is called a deep vein thrombosis, or DVT.
INTRODUCTION. Lower extremity deep venous thrombosis (DVT) and pulmonary embolism are two manifestations of venous thromboembolism. Given the risks associated with untreated lower extremity DVT (eg, [fatal] pulmonary emboli) and the risk of anticoagulation (eg, major or life-threatening bleeding), accurate diagnosis of DVT is essential.
Deep vein thrombosis (DVT) commonly affects the lower limb, with clot formation beginning in a deep calf vein and propagating proximally. 1 It is a common venous thromboembolic (VTE) disorder with an incidence of nearly 1.6 per 1000 inhabitants a year. 2 3 4 The rate of involvement of particular sites varies: distal veins 40%, popliteal 16%, femoral 20%, common femoral 20%, and iliac veins 4% ...
Deep Vein Thrombosis (DVT) is the most common type of venous thromboembolism (VTE). It occurs when a blood clot forms in a deep vein, usually in the lower leg, thigh, or pelvis. Learn about DVT symptoms, how to prevent it if you are at risk, and what treatment you may need.
Deep-vein thrombosis is a common and important disease. It is part of the venous thromboembolism disorders which represent the third most common cause of death from cardiovascular disease after heart attacks and stroke. ... This activity reviews the etiology, presentation, evaluation, and management of deep vein thrombosis and reviews the role ...
Venous thromboembolism (VTE), which includes deep vein thrombosis (DVT) and pulmonary embolism (PE), occurs in ∼1 to 2 individuals per 1000 each year, corresponding to ∼300 000 to 600 000 events in the United States annually.
Deep vein thrombosis is a blood clot in a vein located deep within your body, usually in your leg. Get treatment right away so you can prevent serious complications. Treatments include medicines, compression stockings and surgery. Be patient. You may need to take medicine for a few months and wear compression stockings for two years.
Venous thromboembolism (VTE) includes deep vein thrombosis (DVT), when a blood clot forms in a deep vein, usually in the leg or pelvis. And it includes pulmonary embolism (PE), when the clot breaks off and travels from the leg up to the lungs. DVT and PE are potentially serious, life-threatening conditions that require immediate medical attention.
Background: In the past, the clinical diagnosis of venous thromboembolism (VTE), which comprises deep vein thrombosis (DVT) and pulmonary embolism (PE) had been very challenging, because clinical presentation is non-specific and inaccurate. Objective: To review and assess clinical signs, symptoms and risk factors of DVT and PE and identify most ...
Catheter-related upper extremity venous thrombosis in adults; Clinical presentation and diagnosis of heparin-induced thrombocytopenia; ... Deep vein thrombosis (DVT) and acute pulmonary embolism (PE) are two manifestations of venous thromboembolism. The mainstay of therapy for DVT is anticoagulation, provided there is no contraindication.
Deep vein thrombosis (DVT) is a medical condition that occurs when a blood clot forms in a deep vein. These clots usually develop in the lower leg, thigh, or pelvis, but they can also occur in the arm. It is important to know about DVT because it can happen to anybody and can cause serious illness, disability, and in some cases, death.
Researchers have extensively studied deep vein thrombosis (DVT) of the lower extremities. With the increased use of central venous catheters, cardiac pacemakers/defibrillators, and peripherally inserted central catheter (PICC) lines. ... The treatment of upper extremity DVTs depends on the clinical presentation. The majority of patients present ...
Symptoms of DVT (deep vein thrombosis) in the leg are: throbbing pain in 1 leg (rarely both legs), usually in the calf or thigh, when walking or standing up. swelling in 1 leg (rarely both legs) warm skin around the painful area. red or darkened skin around the painful area - this may be harder to see on brown or black skin.
The presentation of deep vein thrombosis (DVT) and pulmonary embolism (PE) can vary greatly and clinical signs and symptoms are non-specific, which makes their diagnosis challenging. Nevertheless, the clinical presentation is very important as it first triggers the suspicion of DVT or PE, and commences a diagnostic algorithm (see chapter 7 and ...
Deep vein thrombosis (DVT), is the formation of a blood clot in a deep vein, most commonly the legs. [2] [a] Symptoms may include pain, swelling, redness, or warmth of the affected area. About half of cases have no symptoms. Complications may include pulmonary embolism, as a result of detachment of a clot which travels to the lungs, and post ...
DVT occurs when a blood clot forms in one of the deep veins. It can happen if circulation in the deep veins slows down or if the lining of the vein becomes damaged. Factors that increase the risk ...
Venous thromboembolism (VTE) (deep vein thrombosis and its complication, pulmonary embolism) is a major cause of morbidity and mortality in hospitalized patients and about 7% of these cases are due to immobility secondary to a neurological impairment. Acquired brain injury (ABI) has also been recognized as one of the main risk factors for VTE. Numerous epidemiological studies have been ...
PE is a potentially life-threatening complication associated with DVT. It occurs when a blood clot in the leg or other area of the body gets stuck in a blood vessel in a lung. Symptoms include ...
Deep venous thrombosis (DVT) is a common condition that appears in the emergency department and outpatient settings. Clinical diagnosis is unreliable due to the infrequency of the classic findings of edema, warmth, erythema, pain, and tenderness, which are present only in 23% to 50% of patients.[1] When a patient presents with findings consistent with DVT, it is important to make an accurate ...
BackgroundPopliteal artery entrapment syndrome (PAES) is a relatively rare cause of arterial insufficiency in young and physically active individuals; however, deep vein thrombosis (DVT) can develop in association with PAES.Case reportA 47-year-old man presented with a 6-day history of left leg swelling and discomfort which was diagnosed as DVT extending to the distal femoral vein and ...
Portal vein thrombosis (PVT) is a medical condition marked by the partial or complete obstruction of blood flow in the portal vein, responsible for supplying 75% of the blood flow to the liver due to the presence of a thrombus 1,2. While rare in the general population, its prevalence among cirrhotic patients is significantly higher, ranging ...
Abstract. Venous thromboembolism (VTE) is the third most common cause of vascular mortality worldwide and comprises deep-vein thrombosis (DVT) and pulmonary embolism (PE). In this review, we discuss how an understanding of VTE epidemiology and the results of thromboprophylaxis trials have shaped the current approach to VTE prevention.
Eight presentations showcase the broad applicability of HSC gene therapy to address rare neurometabolic diseases and beyond. TOKYO, LONDON and BOSTON, May 07, 2024 (GLOBE NEWSWIRE) -- Orchard Therapeutics, recently acquired by Kyowa Kirin with the goal of accelerating the delivery of new gene therapies to patients around the globe, today announced four oral and four poster presentations from ...