We couldn’t find any results matching your search.
Please try using other words for your search or explore other sections of the website for relevant information.

We’re sorry, we are currently experiencing some issues, please try again later.
Our team is working diligently to resolve the issue. Thank you for your patience and understanding.
News & Insights

3 Cancer-Focused Biotechs That Appear Promising Bets for 2024
December 21, 2023 — 09:23 am EST
Written by Ekta Bagri for Zacks ->
Oncology treatments have been at the forefront this year, with biotech companies looking to capitalize on advancements in technology in this challenging but lucrative space. This has put the spotlight on companies with a solid portfolio of cancer treatments and/or a promising pipeline.
While decades of research and advanced treatments have prolonged the lives of cancer patients in the last 20 years, a possible cure remains elusive. As the world at large continues to grapple with a significant increase in the number of cancer patients, the market for cancer medicines is expected to grow concurrently.
The biotech sector experienced a choppy ride in 2023 amid a challenging environment. Nevertheless, an increase in mergers & acquisition activity boosted investor sentiment in the last couple of months. The recent spate of acquisitions has put the spotlight on oncology-focused companies.
Bristol Myers BMY is set to acquire oncology-focused company Mirati Therapeutics for a total equity value of $5.8 billion and add an approved drug for lung cancer to its portfolio. AbbVie recently announced that it will acquire ImmunoGen, which is developing the next generation of antibody-drug conjugates (ADC) to improve outcomes for cancer patients. The acquisition will add ImmunoGen’s flagship cancer therapy, Elahere, a first-in-class ADC approved for platinum-resistant ovarian cancer. Eli Lilly LLY is set to buy POINT Biopharma, a maker of next-generation radioligand therapies for treating cancers, for approximately $1.4 billion in cash.
The evolving pharma landscape ensures that emerging biotechs with promising oncology treatments in their pipeline using novel mechanisms of action will continue to be the target of pharma/biotech bigwigs.
Here, we discuss three oncology-focused biotech stocks, which have a solid portfolio/promising pipeline. These are BeiGene, Ltd . BGNE , Tango Therapeutics, Inc . TNGX and Allogene Therapeutics, Inc . ALLO .
BeiGene ’s Brukinsa is witnessing rapid uptake across all approved indications, including chronic lymphocytic leukemia. Sales of the drug were strong in the first nine months of 2023. The company is working to expand the drug’s label. The Committee for Health and Medicinal Products of the European Medicines Agency gave a positive opinion for Brukinsa for the treatment of adult patients with relapsed or refractory follicular lymphoma who have received at least two prior systematic treatments.
BeiGene also regained global rights to the development, manufacture and commercialization of Tevimbra, strengthening its global portfolio in solid tumors. The FDA accepted for review the company’s biologics license application for tislelizumab as a first-line treatment for patients with unresectable, recurrent, locally advanced, or metastatic esophageal squamous-cell carcinoma with a target action date in July 2024.
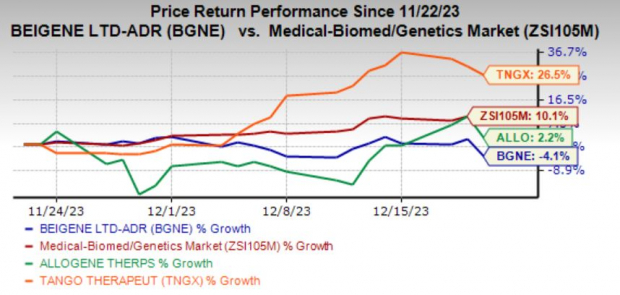
BGNE currently carries a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here .
Loss estimates for 2024 have narrowed by 27.2% over the past 60 days.
Tango Therapeutics is a clinical-stage biotechnology company committed to discovering and delivering the next generation of precision cancer medicines. The company addresses the specific genetic alterations that fuel cancer. Its lead program, TNG908, is a methylthioadenosine (MTA)-cooperative inhibitor of protein arginine methyltransferase 5 (PRMT5) designed to work selectively in cancer cells with a methylthioadenosine phosphorylase (MTAP) deletion. TNG908 demonstrated 15-fold greater potency in MTAP-deleted cancer cells versus normal cells and robust efficacy in vitro and in vivo in preclinical studies. A phase I/II study is ongoing.
The company has also developed a next-generation PRMT5 inhibitor, TNG462, with increased potency, MTAP-deletion selectivity as well as longer target coverage. The company is evaluating its safety and efficacy in multiple tumor types in a phase I/II study. Its other candidates include TNG260 and TNG348.
TNGX currently carries a Zacks Rank #2. Loss estimates for 2024 have narrowed by 17 cents over the past 60 days.
Allogene is focused on developing allogenic CAR T therapies for treating cancer, especially hematologic indications with high unmet needs. The company has six pipeline candidates in the early stage of development, including five CAR T cell product candidates — ALLO-501, ALLO-501A, ALLO-605, ALLO-316 and ALLO-715 — and a monoclonal antibody (mAB), ALLO-647. The company’s most advanced product candidates are ALLO-501 and ALLO-501A, which have been designed to target the CD19 protein that is expressed on the cell surface of B-cells. ALLO-501 is being evaluated in a phase I ALPHA study for r/r non-Hodgkin lymphoma (NHL) subtypes.
A second-generation version of ALLO-501, ALLO-501A, is also being developed for NHL in the phase I/II ALPHA2 study. ALLO-316, the company’s first CAR T candidate for solid tumors, is being evaluated in the phase I TRAVERSE study in adults with advanced or metastatic renal cell carcinoma.
ALLO currently carries a Zacks Rank #2. Loss estimates for 2024 have narrowed by 23 cents over the past 60 days.
Zacks Naming Top 10 Stocks for 2024
Want to be tipped off early to our 10 top picks for the entirety of 2024?
History suggests their performance could be sensational.
From 2012 (when our Director of Research, Sheraz Mian assumed responsibility for the portfolio) through November, 2023, the Zacks Top 10 Stocks gained +974.1% , nearly TRIPLING the S&P 500’s +340.1%. Now Sheraz is combing through 4,400 companies to handpick the best 10 tickers to buy and hold in 2024. Don’t miss your chance to get in on these stocks when they’re released on January 2.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Bristol Myers Squibb Company (BMY) : Free Stock Analysis Report
Eli Lilly and Company (LLY) : Free Stock Analysis Report
BeiGene, Ltd. (BGNE) : Free Stock Analysis Report
Allogene Therapeutics, Inc. (ALLO) : Free Stock Analysis Report
Tango Therapeutics, Inc. (TNGX) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
The views and opinions expressed herein are the views and opinions of the author and do not necessarily reflect those of Nasdaq, Inc.

Stocks mentioned
More related articles.
This data feed is not available at this time.
Sign up for the TradeTalks newsletter to receive your weekly dose of trading news, trends and education. Delivered Wednesdays.
To add symbols:
- Type a symbol or company name. When the symbol you want to add appears, add it to My Quotes by selecting it and pressing Enter/Return.
- Copy and paste multiple symbols separated by spaces.
These symbols will be available throughout the site during your session.
Your symbols have been updated
Edit watchlist.
- Type a symbol or company name. When the symbol you want to add appears, add it to Watchlist by selecting it and pressing Enter/Return.
Opt in to Smart Portfolio
Smart Portfolio is supported by our partner TipRanks. By connecting my portfolio to TipRanks Smart Portfolio I agree to their Terms of Use .
10 new breakthroughs in the fight against cancer

Medical advances are continuing to help the world fight cancer. Image: Unsplash/National Cancer Institute
.chakra .wef-1c7l3mo{-webkit-transition:all 0.15s ease-out;transition:all 0.15s ease-out;cursor:pointer;-webkit-text-decoration:none;text-decoration:none;outline:none;color:inherit;}.chakra .wef-1c7l3mo:hover,.chakra .wef-1c7l3mo[data-hover]{-webkit-text-decoration:underline;text-decoration:underline;}.chakra .wef-1c7l3mo:focus,.chakra .wef-1c7l3mo[data-focus]{box-shadow:0 0 0 3px rgba(168,203,251,0.5);} Victoria Masterson
Madeleine north.

.chakra .wef-9dduvl{margin-top:16px;margin-bottom:16px;line-height:1.388;font-size:1.25rem;}@media screen and (min-width:56.5rem){.chakra .wef-9dduvl{font-size:1.125rem;}} Explore and monitor how .chakra .wef-15eoq1r{margin-top:16px;margin-bottom:16px;line-height:1.388;font-size:1.25rem;color:#F7DB5E;}@media screen and (min-width:56.5rem){.chakra .wef-15eoq1r{font-size:1.125rem;}} Global Health is affecting economies, industries and global issues

.chakra .wef-1nk5u5d{margin-top:16px;margin-bottom:16px;line-height:1.388;color:#2846F8;font-size:1.25rem;}@media screen and (min-width:56.5rem){.chakra .wef-1nk5u5d{font-size:1.125rem;}} Get involved with our crowdsourced digital platform to deliver impact at scale
Stay up to date:, global health.
Listen to the article
This article was originally published in May 2022, and most recently updated in May 2024 .
- Cancer is one of the world’s biggest killers, with around 10 million deaths per year due to the disease.
- Scientists are using artificial intelligence, DNA sequencing, precision oncology and other technologies to improve treatment and diagnosis.
- The Centre for the Fourth Industrial Revolution India, a collaboration with the World Economic Forum, hopes to accelerate 18 cancer interventions.
Cancer kills around 10 million people a year and is a leading cause of death globally, according to the World Health Organization.
Breast, lung and colon cancer are among the most common. Death rates from cancer were falling before the pandemic . But COVID-19 caused a big backlog in diagnosis and treatment .
There is some good news, however. Medical advances are accelerating the battle against cancer. Here are 10 recent developments.
Test to identify 18 early-stage cancers
Researchers in the US have developed a test they say can identify 18 early-stage cancers. Instead of the usual invasive and costly methods, Novelna's test works by analyzing a patient's blood protein. In a screening of 440 people already diagnosed with cancer, the test correctly identified 93% of stage 1 cancers in men and 84% in women. The researchers believe the findings "pave the way for a cost-effective, highly accurate, multi-cancer screening test that can be implemented on a population-wide scale". It's early days, however. With such a small sample screening and a lack of information on co-existing conditions, the test is currently more of "a starting point for developing a new generation of screening tests for the early detection of cancer".
Seven-minute cancer treatment jab
England's National Health Service (NHS) is to be the first in the world to make use of a cancer treatment injection , which takes just seven minutes to administer, rather than the current time of up to an hour to have the same drug via intravenous infusion. This will not only speed up the treatment process for patients, but also free up time for medical professionals. The drug, Atezolizumab or Tecentriq, treats cancers including lung and breast, and it's expected most of the 3,600 NHS patients in England currently receiving it intravenously will now switch to the jab.
Precision oncology
Precision oncology is the “ best new weapon to defeat cancer ”, the chief executive of Genetron Health, Sizhen Wang, says in a blog for the World Economic Forum. This involves studying the genetic makeup and molecular characteristics of cancer tumours in individual patients. The precision oncology approach identifies changes in cells that might be causing the cancer to grow and spread. Personalized treatments can then be developed. The 100,000 Genomes Project, a National Health Service initiative, studied more than 13,000 tumour samples from UK cancer patients , successfully integrating genomic data to more accurately pin-point effective treatment. Because precision oncology treatments are targeted – as opposed to general treatments like chemotherapy – it can mean less harm to healthy cells and fewer side effects as a result.
Artificial intelligence fights cancer
In India, World Economic Forum partners are using emerging technologies like artificial intelligence (AI) and machine learning to transform cancer care. For example, AI-based risk profiling can help screen for common cancers like breast cancer, leading to early diagnosis. AI technology can also be used to analyze X-rays to identify cancers in places where imaging experts might not be available. These are two of 18 cancer interventions that The Centre for the Fourth Industrial Revolution India, a collaboration with the Forum , hopes to accelerate.

Greater prediction capabilities
Lung cancer kills more people in the US yearly than the next three deadliest cancers combined. It's notoriously hard to detect the early stages of the disease with X-rays and scans alone. However, MIT scientists have developed an AI learning model to predict a person's likelihood of developing lung cancer up to six years in advance via a low-dose CT scan. Trained using complex imaging data, 'Sybil' can forecast both short- and long-term lung cancer risk, according to a recent study. "We found that while we as humans couldn't quite see where the cancer was, the model could still have some predictive power as to which lung would eventually develop cancer," said co-author Jeremy Wohlwend.
Clues in the DNA of cancer
At Cambridge University Hospitals in England, the DNA of cancer tumours from 12,000 patients is revealing new clues about the causes of cancer, scientists say. By analyzing genomic data, oncologists are identifying different mutations that have contributed to each person’s cancer. For example, exposure to smoking or UV light, or internal malfunctions in cells. These are like “fingerprints in a crime scene”, the scientists say – and more of them are being found. “We uncovered 58 new mutational signatures and broadened our knowledge of cancer,” says study author Dr Andrea Degasperi, from Cambridge’s Department of Oncology.
Liquid and synthetic biopsies
Biopsies are the main way doctors diagnose cancer – but the process is invasive and involves removing a section of tissue from the body, sometimes surgically, so it can be examined in a laboratory. Liquid biopsies are an easier and less invasive solution where blood samples can be tested for signs of cancer. Synthetic biopsies are another innovation that can force cancer cells to reveal themselves during the earliest stages of the disease.
The application of “precision medicine” to save and improve lives relies on good-quality, easily-accessible data on everything from our DNA to lifestyle and environmental factors. The opposite to a one-size-fits-all healthcare system, it has vast, untapped potential to transform the treatment and prediction of rare diseases—and disease in general.
But there is no global governance framework for such data and no common data portal. This is a problem that contributes to the premature deaths of hundreds of millions of rare-disease patients worldwide.
The World Economic Forum’s Breaking Barriers to Health Data Governance initiative is focused on creating, testing and growing a framework to support effective and responsible access – across borders – to sensitive health data for the treatment and diagnosis of rare diseases.
The data will be shared via a “federated data system”: a decentralized approach that allows different institutions to access each other’s data without that data ever leaving the organization it originated from. This is done via an application programming interface and strikes a balance between simply pooling data (posing security concerns) and limiting access completely.
The project is a collaboration between entities in the UK (Genomics England), Australia (Australian Genomics Health Alliance), Canada (Genomics4RD), and the US (Intermountain Healthcare).
CAR-T-cell therapy
A treatment that makes immune cells hunt down and kill cancer cells was declared a success for leukaemia patients in 2022. Known as CAR-T-cell therapy, it involves removing and genetically altering immune cells, called T cells, from cancer patients. The altered cells then produce proteins called chimeric antigen receptors (CARs), which can recognize and destroy cancer cells. In the journal Nature , scientists at the University of Pennsylvania announced that two of the first people treated with CAR-T-cell therapy were still in remission 12 years on.
However, the US Food and Drug Administration is currently investigating whether the process can in fact cause cancer , after 33 cases of secondary cancer were observed in patients receiving CAR-T therapies. The jury is still out as to whether the therapy is to blame but, as a precaution, the drug packaging now carries a warning.
Fighting pancreatic cancer
Pancreatic cancer is one of the deadliest cancers. It is rarely diagnosed before it starts to spread and has a survival rate of less than 5% over five years. At the University of California San Diego School of Medicine, scientists developed a test that identified 95% of early pancreatic cancers in a study. The research, published in Nature Communications Medicine , explains how biomarkers in extracellular vesicles – particles that regulate communication between cells – were used to detect pancreatic, ovarian and bladder cancer at stages I and II.
Have you read?
Cancer: how to stop the next global health crisis, how to improve access to cancer medicines in low and middle-income countries, why is cancer becoming more common among millennials, a tablet to cut breast cancer risk.
A drug that could halve the chance of women developing breast cancer is being tested out by England's National Health Service (NHS). It will be made available to almost 300,000 women seen as being at most risk of developing breast cancer, which is the most common type of cancer in the UK . The drug, named anastrozole, cuts the level of oestrogen women produce by blocking the enzyme aromatase . It has already been used for many years as a breast cancer treatment but has now been repurposed as a preventive medicine. “This is the first drug to be repurposed through a world-leading new programme to help us realize the full potential of existing medicines in new uses to save and improve more lives on the NHS," says NHS Chief Executive Amanda Pritchard.
Don't miss any update on this topic
Create a free account and access your personalized content collection with our latest publications and analyses.
License and Republishing
World Economic Forum articles may be republished in accordance with the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International Public License, and in accordance with our Terms of Use.
The views expressed in this article are those of the author alone and not the World Economic Forum.
The Agenda .chakra .wef-n7bacu{margin-top:16px;margin-bottom:16px;line-height:1.388;font-weight:400;} Weekly
A weekly update of the most important issues driving the global agenda
.chakra .wef-1dtnjt5{display:-webkit-box;display:-webkit-flex;display:-ms-flexbox;display:flex;-webkit-align-items:center;-webkit-box-align:center;-ms-flex-align:center;align-items:center;-webkit-flex-wrap:wrap;-ms-flex-wrap:wrap;flex-wrap:wrap;} More on Health and Healthcare Systems .chakra .wef-nr1rr4{display:-webkit-inline-box;display:-webkit-inline-flex;display:-ms-inline-flexbox;display:inline-flex;white-space:normal;vertical-align:middle;text-transform:uppercase;font-size:0.75rem;border-radius:0.25rem;font-weight:700;-webkit-align-items:center;-webkit-box-align:center;-ms-flex-align:center;align-items:center;line-height:1.2;-webkit-letter-spacing:1.25px;-moz-letter-spacing:1.25px;-ms-letter-spacing:1.25px;letter-spacing:1.25px;background:none;padding:0px;color:#B3B3B3;-webkit-box-decoration-break:clone;box-decoration-break:clone;-webkit-box-decoration-break:clone;}@media screen and (min-width:37.5rem){.chakra .wef-nr1rr4{font-size:0.875rem;}}@media screen and (min-width:56.5rem){.chakra .wef-nr1rr4{font-size:1rem;}} See all

How midwife mentors are making it safer for women to give birth in remote, fragile areas
Anna Cecilia Frellsen
May 9, 2024

From Athens to Dhaka: how chief heat officers are battling the heat
Angeli Mehta
May 8, 2024

How a pair of reading glasses could increase your income
Emma Charlton
Nigeria is rolling out Men5CV, a ‘revolutionary’ meningitis vaccine

5 conditions that highlight the women’s health gap
Kate Whiting
May 3, 2024

How philanthropy is empowering India's mental health sector
Kiran Mazumdar-Shaw
May 2, 2024
FDA approves groundbreaking treatment for advanced melanoma
The Food and Drug Administration on Friday approved a new cancer therapy that could one day transform the way a majority of aggressive and advanced tumors are treated.
The treatment, called Amtagvi, from Iovance Biotherapeutics , is for metastatic melanoma patients who have already tried and failed other drugs. It’s known as TIL therapy and involves boosting the number of immune cells inside tumors, harnessing their power to fight the cancer.
It’s the first time a cellular therapy has been approved to treat solid tumors. The drug was given a fast-track approval based on the results of a phase 2 clinical trial. The company is conducting a larger phase 3 trial to confirm the treatment’s benefits. The therapy’s list price — the price before insurance and other potential discounts — is $515,000 per patient.
“This is going to be huge,” said Dr. Elizabeth Buchbinder, a senior physician at Dana-Farber Cancer Institute in Boston. Melanoma is “not one of those cancers where there’s like 20 different” possible treatments, she said. “You start running out of options fast.”

Friday’s approval is only for melanoma, the deadliest form of skin cancer , but experts say it holds promise for treating other solid tumors, which account for 90% of all cancers.
“It is our hope that future iterations of TIL therapy will be important for lung cancer, colon cancer , head and neck cancer, bladder cancer and many other cancer types,” said Dr. Patrick Hwu, chief executive of the Moffitt Cancer Center in Tampa, Florida. Moffitt has been involved with Iovance’s clinical trials of TIL therapy.
TIL stands for tumor-infiltrating lymphocytes, which are immune cells that exist within tumors . But there are nowhere nearly enough of those cells to effectively fight off cancer cells. TIL therapy involves, in part, extracting some of those immune cells from the patient’s tumor and replicating them billions of times in a lab, then reinfusing them back into the patient.
It’s similar to CAR-T cell therapy, where healthy cells are taken out of a person’s body and then modified in a lab to fight cancers. That’s usually used for hard-to-treat blood cancers such as leukemia and lymphoma. With TIL therapy, the cells used are already programmed to recognize cancer — no lab modifications needed — they just need a boost in numbers to fight it.
Like CAR-T, TIL therapy is a one-time treatment, though the entire process can take up to eight weeks. The TIL cells are first harvested from the tumor through a minimally invasive procedure and then grown and multiplied in the lab, a process that takes 22 days, according to Iovance.
While that’s happening, patients are given chemotherapy to clear out their immune cells to make room for the billions of new melanoma-fighting TIL cells. Once the TIL cells are reinfused back into the body, patients get a drug called interleukin-2 to further stimulate those cells.
Hwu said that most side effects in patients undergoing TIL therapy are not from the reinfusion of cells, but from the chemotherapy and the interleukin-2. These can include nausea and extreme fatigue, and patients are also vulnerable to other illnesses because the body is depleted of disease-fighting white blood cells.
Putting billions of cells back into the body is not entirely risk-free, however, said Dr. William Dahut, chief scientific officer of the American Cancer Society. It’s possible that the body’s immune system could overreact in what’s known as a cytokine storm, which can cause flu-like symptoms, low blood pressure and organ damage. “There are risks for immune-related side effects, which could be serious,” he said.
Common side effects associated with Amtagvi can include abnormally fast heart rate, fluid buildup, rash, hair loss and feeling short of breath, the FDA said.
Those side effects can be managed, said Dr. Steven Rosenberg, chief of the surgery branch at the National Cancer Institute. “They’re a small price to pay for a growing cancer that would otherwise be lethal.”
Overall, Dahut said the approval of TIL therapy is “meaningful.”
“What’s nice about this is that patients will receive a wide variety of tumor fighting lymphocytes that will be able to have the capacity to overcome resistance and actually be a living therapy over time, too, to target additional cancer cells should they develop,” Dahut said.
In addition to melanoma, Dahut said that TIL therapy is most likely to be useful in cancers that respond to drugs that “take the brakes off the immune system,” called checkpoint inhibitors .
“Those would be things like non-small cell lung cancer, kidney cancer, maybe bladder cancer, that we know are responsive to immune-based therapies to begin with,” he said. “Many of those patients relapse, so another immune-based therapy that works in a different way, seems to me, the most likely way for this to be effective.”
Much more research is needed, and it may be years before TIL therapy is approved for other types of cancer.
One of Iovance’s clinical trials investigating TIL therapy for non-small cell lung cancer was forced to pause when a participant died. While the death is under investigation, the company said it may have been the result of either chemotherapy or interleukin 2 — therapies meant to knock down each patients’ immune system before they can get the reinfusion of their TIL cells.
The therapy is not expected to work for every metastatic melanoma patient. Clinical trial data that Iovance submitted to the FDA showed that tumors shrank in about a third of patients who received TIL therapy.
Of those patients, about half saw their tumors shrink for at least one year, Dr. Friedrich Graf Finckenstein, chief medical officer of Iovance Biotherapeutics. “Some of these patients even had their tumor completely disappear,” he said.
Another study, conducted in the Netherlands , did a head-to-head analysis of TIL therapy and another form of immunotherapy, called ipilimumab. Twenty percent of the patients who received TIL had complete remissions, compared with 7% of patients who got ipilimumab. Iovance was not involved with the Dutch trial.
The goal of the therapy, Hwu said, “is to get rid of the cancer and have it stay away. These immune cells stay in the body and live in the body for decades.”
The technology has been in development and studied for nearly 40 years. It was Rosenberg who pioneered TIL therapy — first describing how it could shrink melanoma tumors in the New England Journal of Medicine in 1988 .
“I’ve been waiting for a very long time to see this given to patients, because I know that it can cure some patients that have metastatic melanoma that cannot be affected by any other treatment,” Rosenberg said.
It’s worked so far for Dan Bennett, 59, of Clermont, Florida. Bennett was diagnosed with melanoma in 2011 after his daughter noticed a suspicious mole on his neck that had changed color.
Despite surgery, chemotherapy and radiation, his cancer kept returning. In 2014, his doctors at Moffitt recommended he try TIL therapy.
“At first, we were pretty leery about it because it was unproven,” Bennett said. Ten years later, Bennett is convinced the TIL therapy is the reason he has survived so long with stage 4 melanoma, which usually has a five-year survival rate of 22.5% .
“I would recommend any experimental drug if it’s your last opportunity,” he said. “You owe it to yourself and your family to do whatever you can to stay alive and to be a productive member of society.”
Buchbinder, the Dana-Faber doctor, was not involved with Iovance’s TIL therapy trial for melanoma, but she is scheduled to begin similar trials with other drugmakers.
“We literally have patients right now waiting for approval because they are hoping they’ll be able to go on it,” Buchbinder said. “It is definitely a practice-changing therapy.”
Erika Edwards is a health and medical news writer and reporter for NBC News and "TODAY."
Anne Thompson is NBC News’ chief environmental affairs correspondent.
Marina Kopf is an associate producer with the NBC News Health and Medical Unit.
- See us on facebook
- See us on twitter
- See us on youtube
- See us on linkedin
- See us on instagram
Stanford Medicine delivers first FDA-approved cell-based therapy for solid tumors
The FDA recently approved the first cell-based therapy — widely used in treating blood cancers — for solid tumors. Stanford Medicine treated the first patient with advanced melanoma.
May 6, 2024 - By Krista Conger
Allison Betof Warner speaks with a patient who received CAR-T therapy for melanoma. Stanford Medicine
In March, Stanford Medicine became the first center in the nation to treat a patient with metastatic melanoma using a new cell-based therapy approved by the Food and Drug Administration. The therapy, which offers hope for people with advanced melanoma whose cancers have resisted immunotherapies, is the first cell-based therapy approved by the FDA to treat solid tumors.
Cell-based therapies — in which immune cells are collected from a patient and treated or genetically modified to enhance their cancer-fighting ability — are not new. Chimeric antigen receptor T-cell therapies, or CAR-T therapies, have been used for several years to fight blood cancers such as leukemias.
The new treatment, which uses immune cells harvested from a patient’s tumor called tumor-infiltrating lymphocytes, exploits the body’s own natural cancer-fighting ability. Once the tumor-infiltrating lymphocytes, or T cells, are harvested, they are encouraged in the laboratory to multiply into billions of cancer-fighting cells, then returned to the patient about a month later.
“These cells are naturally existing T cells that target multiple aspects of the existing tumor,” said assistant professor of medicine Allison Betof Warner , MD, PhD. “Before now, there was no approved therapy for people with melanoma whose cancers had progressed after immunotherapy and/or targeted therapy. Now we can bring the promise of cell therapy to these patients.”
In contrast to tumor-infiltrating lymphocytes, which are not genetically modified, CAR-T therapies use genetic engineering to modify a patient’s T cells to recognize and respond to a specific molecule or molecules on the surfaces of cancer cells. Doing so requires researchers to predict which molecules will serve as the best targets — easier for blood cancers than for solid tumors.
“We are very excited to move cell-based therapies beyond blood cancers,” said David Miklos , MD, PhD, professor of medicine and chief of bone marrow transplantation and cellular therapy. “This has been a long time coming, but now we have a new standard of care for these patients.”
Stanford Medicine is one of fewer than 30 centers around the country offering the treatment.
The therapy, called lifileucel and commercially known as Amtagvi, is marketed by San Carlos-based Iovance Biotherapeutics.
Clinical trials of lifileucel for metastatic melanoma that worsened while on standard treatment found that about 30% of 153 patients who received the therapy had their tumors shrink or disappear. Of those, 40% experienced no progression of their cancers for at least 18 months after the one-time infusion.
A quarter-century of research
Tumor-infiltrating lymphocyte therapy was first conceived of in the late 1980s by Steven Rosenberg, MD, PhD, at the National Cancer Institute. In the intervening decades, he and others optimized the treatment and conducted clinical trials exploring its promise. In October, Rosenberg was awarded the National Medal of Technology and Innovation, in part for his role in bringing tumor-infiltrating lymphocyte therapies to the clinic.
For the treatment, surgeons first remove a sample of the tumor and collect any T cells it contains. These cells have already infiltrated and started to fight the tumor, presumably by recognizing a variety of molecules on the surfaces of the cancer cells. These T cells are grown in the laboratory for about one month in the presence of a growth-promoting immune molecule called IL-2.
IL-2 stimulates the cells to divide repeatedly, generating an army of billions. While the cells are growing in the laboratory, the patient undergoes chemotherapy to deplete existing lymphocytes — creating a biological niche for the newly energized cells to further expand when they are returned to the patient.
“Normally, when you remove a tumor from a patient with cancer, you throw it away,” Miklos said. “But these trials have shown that the lymphocytes that tumor contains are gold. Your body is already trying to fight these cancers. We’re just helping it out.”
After the cells are returned, the patient is given several doses of IL-2 to further encourage the expansion of the cancer-fighting cells.
The technique works particularly well with melanomas, which are flush with lymphocytes. But Betof Warner and Miklos feel that improvements in the cell-expansion technique may make it useful even for tumors such as lung and colorectal cancers that tend to have fewer infiltrating immune cells — a class of tumor known as “immune cold.”
Researchers worldwide are now conducting clinical trials of tumor-infiltrating lymphocytes in other solid cancers including non-small cell lung cancer, advanced colorectalm and breast cancer, while other trials are investigating the effect of combining lifileucel with immunotherapy as a first-line treatment for advanced melanoma.
Not every person with metastatic melanoma will qualify for the therapy under the terms of the FDA approval. People need to be relatively healthy with good heart, kidney, and lung function and able to withstand the preparative immune-depleting chemotherapy given before the infusion. They also must have seen their cancers worsen while on immunotherapies or targeted treatment that are currently the standard of care for these cancers. Betof Warner estimates that, of the 8,000 to 10,000 or so people diagnosed with advanced melanomas each year in the United States, about 2,000 people will qualify if patients are identified appropriately and referred to authorized treatment centers.
The researchers are also investigating which molecules the tumor-infiltrating lymphocytes are targeting, with the hopes of making even more targeted treatments.
“We study the tumor that we remove from the patient, as well as the cells we return to the patient and compare that with the patient’s clinical response,” Betof Warner said. “Eventually we hope to predict and improve the responses based on the lymphocytes found in the tumor. Are they healthy? What molecules on the cancer cells are they targeting? Although there is more to learn, we are excited to have another option, another line of treatment for these advanced cancers.”
See also: The promise of TIL Therapy

About Stanford Medicine
Stanford Medicine is an integrated academic health system comprising the Stanford School of Medicine and adult and pediatric health care delivery systems. Together, they harness the full potential of biomedicine through collaborative research, education and clinical care for patients. For more information, please visit med.stanford.edu .
Hope amid crisis
Psychiatry’s new frontiers

Scientists make breakthrough for 'next generation' cancer treatment
Scientists at the University of East Anglia are a step closer to creating a new generation of light-activated cancer treatments.
The futuristic sounding treatment would work by switching on LED lights embedded close to a tumour, which would then activate biotherapeutic drugs.
These new treatments would be highly targeted and more effective than current state-of-the-art cancer immunotherapies.
New research published today reveals the science behind this innovative idea.
It shows how the UEA team have engineered antibody fragments -- which not only 'fuse' with their target but are also light activated.
It means that in future, immunotherapy treatments could be engineered to attack tumours more precisely than ever before.
The principal scientist for this study, Dr Amit Sachdeva, from UEA's School of Chemistry, said: "Current cancer treatments like chemotherapy kill cancer cells, but they can also damage healthy cells in your body such as blood and skin cells.
"This means that they can cause side effects including hair loss, feeling tired and sick, and they also put patients at increased risk of picking up infections.
"There has therefore been a very big drive to create new treatments that are more targeted and don't have these unwanted side-effects.
"Several antibodies and antibody fragments have already been developed to treat cancer. These antibodies are much more selective than the cytotoxic drugs used in chemotherapy, but they can still cause severe side effects, as antibody targets are also present on healthy cells."
Now, the UEA team has engineered one of the first antibody fragments that binds to, and forms a covalent bond with, its target -- upon irradiation with UV light of a specific wavelength.
Dr Sachdeva said: "A covalent bond is a bit like melting two pieces of plastic and fusing them together. It means that drug molecules could for example be permanently fixed to a tumour.
"We hope that our work will lead to the development of a new class of highly targeted light-responsive biotherapeutics. This would mean that antibodies could be activated at the site of a tumour and covalently stick to their target upon light activation.
"In other words, you could activate antibodies to attack tumour cells by shining light - either directly on to the skin, in the case of skin cancer, or using small LED lights that could be implanted at the site of a tumour inside the body.
"This would allow cancer treatment to be more efficient and targeted because it means that only molecules in the vicinity of the tumour would be activated, and it wouldn't affect other cells.
"This would potentially reduce side effects for patients, and also improve antibody residence time in the body."
"It would work for cancers like skin cancer, or where there is a solid tumour - but not for blood cancers like leukaemia.
"Development of these antibody fragments would not have been possible without pioneering work from several other research groups across the globe who developed and optimised methods for site-specific incorporation of non-natural amino acids into proteins expressed in live cells.
"We employed some of these methods to site-specifically install unique light-sensitive amino acids into antibody fragments."
If the researchers are successful in the next stages of their work, they hope to see the 'next generation' light-activated immunotherapies being used to treat cancer patients within five to 10 years.
This research was funded by the Biotechnology and Biological Sciences Research Council (BBSRC) and the Wellcome Trust. It was led by the University of East Anglia with assistance from the proteomics facility at the John Innes Centre.
- Colon Cancer
- Lung Cancer
- Breast Cancer
- Skin Cancer
- Brain Tumor
- Stem cell treatments
- Breast cancer
- Cervical cancer
- Colorectal cancer
- Prostate cancer
- Ultraviolet
- Ovarian cancer
Story Source:
Materials provided by University of East Anglia . Note: Content may be edited for style and length.
Journal Reference :
- Thomas Bridge, Udo Wegmann, Jason C. Crack, Kate Orman, Saher A. Shaikh, William Farndon, Carlo Martins, Gerhard Saalbach, Amit Sachdeva. Site-specific encoding of photoactivity and photoreactivity into antibody fragments . Nature Chemical Biology , 2023; DOI: 10.1038/s41589-022-01251-9
Cite This Page :
Explore More
- Controlling Shape-Shifting Soft Robots
- Brain Flexibility for a Complex World
- ONe Nova to Rule Them All
- AI Systems Are Skilled at Manipulating Humans
- Planet Glows With Molten Lava
- A Fragment of Human Brain, Mapped
- Symbiosis Solves Long-Standing Marine Mystery
- Surprising Common Ideas in Environmental ...
- 2D All-Organic Perovskites: 2D Electronics
- Generative AI That Imitates Human Motion
Trending Topics
Strange & offbeat.
MIT Technology Review
- Newsletters
Cancer vaccines are having a renaissance
After years of lackluster results, cancer vaccines seem poised for success. Finally.
- Cassandra Willyard archive page
This article first appeared in The Checkup, MIT Technology Review’s weekly biotech newsletter. To receive it in your inbox every Thursday, and read articles like this first, sign up here .
Last week, Moderna and Merck launched a large clinical trial in the UK of a promising new cancer therapy: a personalized vaccine that targets a specific set of mutations found in each individual’s tumor. This study is enrolling patients with melanoma. But the companies have also launched a phase III trial for lung cancer. And earlier this month BioNTech and Genentech announced that a personalized vaccine they developed in collaboration shows promise in pancreatic cancer, which has a notoriously poor survival rate.
Drug developers have been working for decades on vaccines to help the body’s immune system fight cancer, without much success. But promising results in the past year suggest that the strategy may be reaching a turning point. Will these therapies finally live up to their promise?
This week in The Checkup, let’s talk cancer vaccines. (And, you guessed it, mRNA.)
Long before companies leveraged mRNA to fight covid, they were developing mRNA vaccines to combat cancer. BioNTech delivered its first mRNA vaccines to people with treatment-resistant melanoma nearly a decade ago. But when the pandemic hit, development of mRNA vaccines jumped into warp drive. Now dozens of trials are underway to test whether these shots can transform cancer the way they did covid.
Recent news has some experts cautiously optimistic. In December, Merck and Moderna announced results from an earlier trial that included 150 people with melanoma who had undergone surgery to have their cancer removed. Doctors administered nine doses of the vaccine over about six months, as well as what’s known as an immune checkpoint inhibitor. After three years of follow-up, the combination had cut the risk of recurrence or death by almost half compared with the checkpoint inhibitor alone.
The new results reported by BioNTech and Genentech, from a small trial of 16 patients with pancreatic cancer, are equally exciting. After surgery to remove the cancer, the participants received immunotherapy, followed by the cancer vaccine and a standard chemotherapy regimen. Half of them responded to the vaccine, and three years after treatment, six of those people still had not had a recurrence of their cancer. The other two had relapsed. Of the eight participants who did not respond to the vaccine, seven had relapsed. Some of these patients might not have responded because they lacked a spleen, which plays an important role in the immune system. The organ was removed as part of their cancer treatment.
The hope is that the strategy will work in many different kinds of cancer. In addition to pancreatic cancer, BioNTech’s personalized vaccine is being tested in colorectal cancer, melanoma, and metastatic cancers.
The purpose of a cancer vaccine is to train the immune system to better recognize malignant cells, so it can destroy them. The immune system has the capacity to clear cancer cells if it can find them. But tumors are slippery. They can hide in plain sight and employ all sorts of tricks to evade our immune defenses. And cancer cells often look like the body’s own cells because, well, they are the body’s own cells.
There are differences between cancer cells and healthy cells, however. Cancer cells acquire mutations that help them grow and survive, and some of those mutations give rise to proteins that stud the surface of the cell—so-called neoantigens.
Personalized cancer vaccines like the ones Moderna and BioNTech are developing are tailored to each patient’s particular cancer. The researchers collect a piece of the patient’s tumor and a sample of healthy cells. They sequence these two samples and compare them in order to identify mutations that are specific to the tumor. Those mutations are then fed into an AI algorithm that selects those most likely to elicit an immune response. Together these neoantigens form a kind of police sketch of the tumor, a rough picture that helps the immune system recognize cancerous cells.
“A lot of immunotherapies stimulate the immune response in a nonspecific way—that is, not directly against the cancer,” said Patrick Ott, director of the Center for Personal Cancer Vaccines at the Dana-Farber Cancer Institute, in a 2022 interview . “Personalized cancer vaccines can direct the immune response to exactly where it needs to be.”
How many neoantigens do you need to create that sketch? “We don’t really know what the magical number is,” says Michelle Brown, vice president of individualized neoantigen therapy at Moderna. Moderna’s vaccine has 34. “It comes down to what we could fit on the mRNA strand, and it gives us multiple shots to ensure that the immune system is stimulated in the right way,” she says. BioNTech is using 20.
The neoantigens are put on an mRNA strand and injected into the patient. From there, they are taken up by cells and translated into proteins, and those proteins are expressed on the cell’s surface, raising an immune response
mRNA isn’t the only way to teach the immune system to recognize neoantigens. Researchers are also delivering neoantigens as DNA, as peptides, or via immune cells or viral vectors. And many companies are working on “off the shelf” cancer vaccines that aren’t personalized, which would save time and expense. Out of about 400 ongoing clinical trials assessing cancer vaccines last fall, roughly 50 included personalized vaccines.
There’s no guarantee any of these strategies will pan out. Even if they do, success in one type of cancer doesn’t automatically mean success against all. Plenty of cancer therapies have shown enormous promise initially, only to fail when they’re moved into large clinical trials.
But the burst of renewed interest and activity around cancer vaccines is encouraging. And personalized vaccines might have a shot at succeeding where others have failed. The strategy makes sense for “a lot of different tumor types and a lot of different settings,” Brown says. “With this technology, we really have a lot of aspirations.”
Now read the rest of The Checkup
Read more from mit technology review’s archive.
mRNA vaccines transformed the pandemic. But they can do so much more. In this feature from 2023, Jessica Hamzelou covered the myriad other uses of these shots , including fighting cancer.
This article from 2020 covers some of the background on BioNTech’s efforts to develop personalized cancer vaccines. Adam Piore had the story .
Years before the pandemic, Emily Mullin wrote about early efforts to develop personalized cancer vaccines—the promise and the pitfalls.
From around the web
Yes, there’s bird flu in the nation’s milk supply. About one in five samples had evidence of the H5N1 virus. But new testing by the FDA suggests that the virus is unable to replicate. Pasteurization works! ( NYT )
Studies in which volunteers are deliberately infected with covid—so-called challenge trials—have been floated as a way to test drugs and vaccines, and even to learn more about the virus. But it turns out it’s tougher to infect people than you might think. ( Nature )
When should women get their first mammogram to screen for breast cancer? It’s a matter of hot debate. In 2009, an expert panel raised the age from 40 to 50. This week they lowered it to 40 again in response to rising cancer rates among younger women. Women with an average risk of breast cancer should get screened every two years, the panel says. ( NYT )
Wastewater surveillance helped us track covid. Why not H5N1? A team of researchers from New York argues it might be our best tool for monitoring the spread of this virus. ( Stat )
Biotechnology and health
How scientists traced a mysterious covid case back to six toilets.
When wastewater surveillance turns into a hunt for a single infected individual, the ethics get tricky.
An AI-driven “factory of drugs” claims to have hit a big milestone
Insilico is part of a wave of companies betting on AI as the "next amazing revolution" in biology
- Antonio Regalado archive page
The quest to legitimize longevity medicine
Longevity clinics offer a mix of services that largely cater to the wealthy. Now there’s a push to establish their work as a credible medical field.
- Jessica Hamzelou archive page
There is a new most expensive drug in the world. Price tag: $4.25 million
But will the latest gene therapy suffer the curse of the costliest drug?
Stay connected
Get the latest updates from mit technology review.
Discover special offers, top stories, upcoming events, and more.
Thank you for submitting your email!
It looks like something went wrong.
We’re having trouble saving your preferences. Try refreshing this page and updating them one more time. If you continue to get this message, reach out to us at [email protected] with a list of newsletters you’d like to receive.
- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español
You are here
News releases.
News Release
Thursday, October 12, 2017
NIH partners with 11 leading biopharmaceutical companies to accelerate the development of new cancer immunotherapy strategies for more patients
Supports Cancer Moonshot goal to bring immunotherapy success to more patients in half the time.

The National Institutes of Health and 11 leading biopharmaceutical companies today launched the Partnership for Accelerating Cancer Therapies (PACT), a five-year public-private research collaboration totaling $215 million as part of the Cancer Moonshot. PACT will initially focus on efforts to identify, develop and validate robust biomarkers — standardized biological markers of disease and treatment response — to advance new immunotherapy treatments that harness the immune system to attack cancer. The partnership will be managed by the Foundation for the National Institutes of Health (FNIH), with the Food and Drug Administration serving in an advisory role.
“This new public-private partnership is a significant step forward in the battle against cancer and a real boost to the potential of immunotherapy,” said Acting Health and Human Services Secretary Eric Hargan. “We are excited for this partnership, which will strengthen efforts already underway across HHS.”
New immunotherapies have resulted in dramatic responses in certain cancer cases. They have also been the focus of intense investment by biopharmaceutical companies seeking to provide new options for patients who do not respond to other cancer therapies, but they don’t work for all patients. Development and standardization of biomarkers to understand how immunotherapies work in some patients, and predict their response to treatment, are urgently needed for these therapies to provide benefit to the maximum number of people.
“We have seen dramatic responses from immunotherapy, often eradicating cancer completely for some cancer patients,” said NIH Director Francis S. Collins, M.D., Ph.D. “We need to bring that kind of success — and hope — for more people and more types of cancers, and we need to do it quickly. A systematic approach like PACT will help us to achieve success faster.”
PACT will facilitate systematic and uniform clinical testing of biomarkers to advance our understanding of the mechanisms of response and resistance to cancer therapy. The research conducted under the partnership will also integrate immune and other related oncology biomarkers into clinical trials by defining a set of standardized biomarkers to be tested across a variety of studies. This approach will allow for consistent generation of data, uniform and harmonized assays to support data reproducibility, comparability of data across trials, and discovery and validation of new biomarkers for immunotherapy and related combinations. PACT will also facilitate information sharing by all stakeholders to better coordinate clinical efforts, align investigative approaches, reduce duplication and enable more high-quality trials to be conducted.
“A scientific and organizational challenge this complex cannot be addressed effectively by any one organization acting alone,” said Maria C. Freire, Ph.D., President and Executive Director of the FNIH. “Instead, it requires the energies and resources of public and private partners working in close collaboration.”
PACT partners include AbbVie, North Chicago, Illinois; Amgen, Thousand Oaks, California; Boehringer Ingelheim Pharma GmbH & Co. KG, Ingelheim, Germany; Bristol-Myers Squibb, New York; Celgene Corporation, Summit, New Jersey; Genentech, a member of the Roche Group, San Francisco; Gilead Sciences, Foster City, California; GlaxoSmithKline plc, Brentford, United Kingdom; Janssen Pharmaceutical Companies of Johnson & Johnson, New Jersey; Novartis, Basel, Switzerland; and Pfizer, Inc., New York. ( See what the partner organizations are saying about PACT ). Additional support has been provided by the Pharmaceutical Research and Manufacturers Association (PhRMA). The 11 partner organizations will contribute up to $1 million per year for five years through the FNIH for a total private sector contribution of $55 million. NIH will contribute $160 million over the five years of the partnership, pending availability of funds.
NIH’s National Cancer Institute (NCI) recently awarded cooperative agreements to support four Cancer Immune Monitoring and Analysis Centers (CIMACs) and a Cancer Immunologic Data Commons (CIDC) with a total of $53.6 million in funding over five years. The four CIMACs and one CIDC will form a network of laboratory centers that will support both adult and pediatric immunotherapy trials. Researchers at the CIMACs will perform deep tumor and immune profiling. The resulting data will be collected in the CIDC database for exploration of biomarkers of immune response. This network will also provide a foundation for the core laboratory, assay development and database functions required by PACT.
“NCI’s long-term support for basic and translational research in immunotherapy paved the way for the recent dramatic clinical successes in this area,” said Douglas R. Lowy, M.D., Acting Director of NCI. “This partnership, and the data the partners have committed to making publicly accessible to the broader research community, will facilitate our continued progress in helping to find the cancer treatments that benefit the greatest number of patients.”
The NCI cooperative agreements have been awarded to Dana-Farber Cancer Institute, Boston (CIMAC and CIDC); Stanford Cancer Institute, Stanford, California (CIMAC); Precision Immunology Institute and the Tisch Cancer Institute at Icahn School of Medicine at Mount Sinai, New York (CIMAC) and University of Texas MD Anderson Cancer Center, Houston (CIMAC).
About the Foundation for the National Institutes of Health (FNIH): The FNIH creates and manages alliances with public and private institutions in support of the mission of the NIH. The FNIH works with its partners to accelerate biomedical research and strategies against diseases and health concerns in the United States and across the globe. Established by Congress in 1990, the FNIH is a not-for-profit 501(c)(3) charitable organization. For additional information about the FNIH, please visit fnih.org .
About the National Cancer Institute (NCI): NCI leads the National Cancer Program and NIH’s efforts to dramatically reduce the prevalence of cancer and improve the lives of cancer patients and their families, through research into prevention and cancer biology, the development of new interventions, and the training and mentoring of new researchers. For more information about cancer, please visit the NCI website at cancer.gov or call NCI’s Contact Center (formerly known as the Cancer Information Service) at 1-800-4-CANCER (1-800-422-6237).
About the National Institutes of Health (NIH): NIH, the nation's medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit www.nih.gov .
NIH…Turning Discovery Into Health ®
What PACT partners are saying
Multimedia: Partnership for Accelerating Cancer Therapies
Connect with Us
- More Social Media from NIH
Biotech startup using AI to take guesswork out of cancer drug selection raises $70M
Xilis is developing technology that creates a living model of a patient’s tumor, then applies artificial intelligence to determine the best drug or drug combinations to treat the cancer. Clinical trials are planned to test this precision medicine approach and the biotech startup has raised $70 million to fund its research.
- Copy Link Copy Link

From the moment cancer is diagnosed, the clock is ticking. Early treatment can lead to better outcomes, but physicians must first choose a drug. It can take months to assess how the selected drug is working, which takes time away from another one that might be better. Biotechnology company Xilis aims to replace the drug selection guesswork with precision medicine technology that simultaneously assesses multiple therapies against a patient’s tumor, then predicts the ones that will work best. The startup is pressing forward with clinical trials, and it has raised $70 million for its research.

With the Rise of AI, What IP Disputes in Healthcare Are Likely to Emerge?
Munck Wilson Mandala Partner Greg Howison shared his perspective on some of the legal ramifications around AI, IP, connected devices and the data they generate, in response to emailed questions.
The Series A round of funding announced Thursday was led by Mubadala Capital, the asset management arm of Mubadala Investment Company, a sovereign investor based in Abu Dhabi.
Testing a patient’s tumor to determine the proper treatment isn’t a new concept; many of the newly approved targeted cancer therapies come with companion diagnostics. But each companion diagnostic only tests the suitability of its counterpart drug. Durham, North Carolina-based Xilis (pronounced “ZY-lus”) aims to test the spectrum of potential cancer therapies.
Xilis creates a living model of a patient’s cancer, said co-founder and CEO Xiling Shen. The process starts with a biopsy of the tumor. The company’s proprietary hardware then encapsulates cancer cells in tiny droplets, each one retaining the tissue structure, genetic alterations, and immune system characteristics of the original tumor. These droplets, which Xilis calls MicroOrganoSpheres, represent thousands of copies of the tumor.
The droplets are dispensed into wells where they are tested against individual drugs or various drug combinations. Xilis’s artificial intelligence-driven algorithms assess response to a panel of cancer therapies, identifying the most promising cancer treatment. Compared to the months it can take to assess how a single drug is working inside of a patient, Shen said that Xilis’s technology can provide answers for multiple drugs within two weeks.

A Personalized Approach to Medication Nonadherence
At the Abarca Forward conference earlier this year, George Van Antwerp, managing director at Deloitte, discussed how social determinants of health and a personalized member experience can improve medication adherence and health outcomes.
“Our technology provides, for the first time, a scalable, functional assay to test a patient’s tumor against many different drugs and then go back with AI to predict more precisely the patient’s response to the treatment,” Shen said.
Xilis isn’t the first company to develop organoids, tiny representations of a patient, as a way of testing drugs. About one hour’s drive west of Xilis, Wake Forest Institute for Regenerative Medicine scientists are researching the use of patient tissue to create tumors on a chip , which is then used to test different cancer drugs. Startup Emulate is collaborating with the FDA on research that is assessing the Boston-based company’s organ-on-a-chip technology as an alternative to the cell-based and animal tests used to assess toxicology.
One startup that, like Xilis, is taking an organoid approach to informing cancer drug selection is SEngine . The test requires an oncologist to send a biopsy or specimen to the Seattle company’s labs. SEngine isolates the cancer cells, then tests more than 200 cancer drugs against organoids to determine the most suitable therapies. The company says it can generate results within a week, which are then shared via a report sent to the oncologist.
Shen said Xilis’s approach differs from other testing technologies in that it incorporates a patient’s immune cells, providing a more complete representation of a person’s response to a drug. During the annual meeting of the American Society of Clinical Oncology (ASCO) in May, Xilis submitted an abstract describing how its MicroOrganoSpheres were able to preserve the original tumor as well as a patient’s immune cells.
“Because we can do things so fast, do things while a patient’s immune environment is still intact, that allows you to test against the patient’s tumor and the patient’s immune environment,” Shen said.
In addition to providing a new way to help oncologists select cancer drugs, Xilis sees its technology as a way to guide the cancer drug research of pharmaceutical companies. The technology can help them determine whether to advance an experimental therapy to clinical trials, Shen said. When a clinical trial begins, a pharma company can then use the technology to select the patients most likely to respond to treatment.
Xilis already has some research showing how its technology could help pharma companies. At ASCO, Xilis submitted an abstract describing use of MicroOrganoSpheres to assess the efficacy of tumor infiltrating lymphocytes, a type of cancer immunotherapy, in advance of a non-small cell lung cancer clinical trial. Shen said that Xilis has several pharmaceutical partners, none of which have been disclosed.
Xilis launched in 2019, co-founded by Shen and David Hsu, both Duke University professors, and Hans Clevers, principal investigator at the Hubrecht Institute in the Netherlands. Clevers pioneered organoids and organoid research. The startup was backed at its start by $3 million in seed funding. In addition to Mubadala Capital, Xilis’s new financing added new investors GV, European healthcare investment firm LSP, Catalio Capital Management, and Duke Angel Network. Earlier investors Felicis Ventures, Two Sigma Ventures, Pear VC, KdT Ventures, and Alix Ventures also participated.
Ayman AlAbdallah, venture capital investor at Mubadala, said his fund was impressed by the Xilis technology, as well as its broad applicability to both clinical and research use.
“There is a critical need for a technology like Xilis to help validate and accelerate clinical development and give clinicians certainty that next-generation therapies will work in patients,” he said.
The new financing comes as Xilis moves forward with clinical trial plans. The initial tests will be in colon and breast cancers, followed by a study in lung cancer. Shen said those cancers were selected because of their prevalence. As for the company’s name, Shen said it’s a reference to Xilinx, a semiconductor company that has commercialized programmable technologies used in a wide range of products. The Xilis name is meant to evoke the accomplishments of the semiconductor company, but applied to biology: functional testing that’s flexible, programmable, automated, and scalable, Shen said.
Photo by Xilis
More From MedCity News

‘Two-Sided Green’: How 4 Health Systems Are Saving Money With Renewable Energy

Healthcare’s Next Void to Fill: Digital Primary Care

Mental Health Platform Wave Gains First Payer Contract

This Chip Seeks to Transform Drug Manufacturing by Learning More About Intracellular Dynamics
Five AI-driven Drug Discovery Companies Enabling Precision Oncology
Artificial Intelligence (AI) is steadily making its presence felt across various sectors, including the pharmaceutical industry. Among the multiple applications of AI in this field, immunotherapy - a treatment method that utilizes the body's immune system to combat diseases, notably cancer - is seeing a significant influence. The integration of AI can potentially enhance treatments and patient care by making it more precise and personalized.
Making sense of complex biological systems
The human immune system is a densely interconnected network involving numerous immune cells, signaling molecules, and genes. The interactions within this network are intricate, dynamic, and largely unpredictable. The vast amount of biological data often surpasses the comprehension ability of humans and the scope of traditional statistical methods.
This is where AI, with its capacity to understand and predict from data, comes into play. Deep learning algorithms can handle enormous amounts of genetic, proteomic, and clinical data, discern patterns and correlations, and contribute to the identification of new immunotherapeutic targets.
What is immunotherapy?
Immunotherapy refers to a set of medical treatments that use the body's natural defenses – the immune system – to identify, fight, and eliminate diseases, particularly cancer. Instead of directly attacking the disease, immunotherapies equip the immune system to recognize disease cells and stimulate an immune response against them. There are several types of immunotherapies, each with a different mechanism of action - checkpoint inhibitors, Chimeric Antigen Receptor T-cell (CAR-T cel)l therapy, cancer vaccines, monoclonal antibodies, Immune system modulators.
Enhancing personalized immunotherapies
Personalized immunotherapies, such as CAR-T cell therapy, have shown promise in cancer treatment. In this therapy, T cells are removed from a patient's body, genetically modified to produce receptors (CARs) that target specific proteins on cancer cells, and then infused back into the patient.
However, predicting which patients will respond favorably to these therapies has been a persistent challenge. AI algorithms can process data from various sources, including genomics, clinical trials, and real-world evidence. This helps to discover biomarkers and predict patients' responses to treatment. Thus, AI can assist in selecting patients who are likely to benefit most from the therapy, leading to improved treatment outcomes and cost-effectiveness.
Refining Combination Therapies
Immunotherapies often yield better results when combined with other treatments. Yet, determining the optimal combination can be a time-consuming process. AI can aid in identifying effective drug combinations, potentially speeding up the process and the development of more efficient treatment strategies.
Improving Clinical Trials
Clinical trials play a pivotal role in the development of new immunotherapies. However, they can be expensive and lengthy. AI can potentially expedite the clinical trial process, right from patient selection to data collection and analysis. Predictive models powered by AI can identify suitable patients more accurately, reduce drop-out rates, and enable real-time data analysis.
Below is an alphabetically-ordered list of 5 notable AI-driven drug discovery companies pushing the boundaries of what can be done to treat cancer:
Achilles Therapeutics
Achilles Therapeutics is a London-based biopharmaceutical company that focuses on the development of next-generation, patient-specific therapies to treat cancer. Achilles Therapeutics has created PELEUS platform designed to predict the immunogenicity of neoantigens. The PELEUS platform is an integral part of Achilles' “Target-to-T Cell” approach that aims to revolutionize precision therapies, including TIL-based clonal neoantigen-reactive T cells (cNeT) and personalized neoantigen methods. The platform's predictive capabilities have the potential to transform precision treatments by focusing on the most potent antigens for robust, long-lasting responses. PELEUS's predictive success is attributed to its training on high-quality proprietary data sets. Furthermore, the platform's superiority is patent-protected, further enhancing its value and potential for Achilles Therapeutics.
A neoantigen is a type of antigen, a substance that stimulates an immune response, that is formed by a novel protein sequence generated through mutations in tumor cells. These mutations lead to changes in the protein sequences that are not present in normal cells. Because they are unique to cancer cells, they can act as ideal targets for immune-based cancer therapies.
However, not all neoantigens are equal; some are more likely to generate a strong immune response, known as being more "immunogenic". A large number of potential neoantigen can be identified foe a tumor sample, but the challenge is to determine which of these are most likely to elicit a potent immune response, thus being effective for personalized therapy.
PELEUS platform uses machine learning algorithms trained on extensive proprietary datasets to predict which identified neoantigens are most likely to be immunogenic. By predicting the most potent neoantigens, the platform supports the development of personalized cancer therapies, including TIL-based (Tumor-Infiltrating Lymphocytes) therapies and clonal neoantigen cancer vaccines. It's a critical tool for implementing precision medicine in cancer treatment, making therapy more efficient and potentially more effective.
The company's innovative work extends to various clinical trials, including the ongoing CHIRON and THETIS trials, investigating advanced non-small cell lung cancer and recurrent or metastatic melanoma respectively. Achilles Therapeutics received a U.S. patent last year for treating patients with an immunotherapy targeting clonal neoantigens identified using their Achilles Clonality Engine (ACE). This ongoing, innovative research positions Achilles Therapeutics at the forefront of personalized cancer therapy development.
Achilles Therapeutics is a public company with total funding of $213M, with $175M raised as a result of the IPO in 2021.
Immunai is a New-York-based artificial intelligence startup specializing in cutting-edge multiomics technology. They focus on enhancing the effectiveness of cell-based therapies and other immune-altering treatments, not just in oncology but also in a broad spectrum of inflammatory diseases. Their technology allows for the precise measurement of gene expression changes in individual cells, a capability that has broad-ranging applications in medicine and therapy design.
Recently, Immunai started a partnership with Baylor College of Medicine (BCM) to uncover the role of a new molecular target, BTG1. This finding, drawn from clinical samples of an ongoing BCM trial, promises to enhance the efficacy of T and natural killer T (NKT) cell-based cancer immunotherapies.
Their advanced single-cell RNA sequencing technology played a critical role in this discovery, demonstrating the potential of Immunai's platform to drive novel treatment options. With the revelation that targeting BTG1 could amplify CAR-NKT cells' anti-tumor function, Immunai has paved the way for the development of more effective cancer immunotherapies.
In 2021 ImmuneAI secured an impressive $215 million in a Series B funding round led by Koch Disruptive Technologies, taking its total funding to an impressive $295 million.
iBio, Inc. is an innovator in the field of biotechnology, specializing in the application of artificial intelligence to develop precision antibody immunotherapies. Based in Bryan, Texas, the company utilizes a combination of its proprietary AI-based antibody optimization and mammalian display technologies to discover and develop novel therapeutic antibodies.
The latest news from iBio is the discovery of a panel of CD3 T-cell binding antibodies. These novel antibodies are designed to bind to both T cells and tumor cells, inducing the T cells to kill the tumor cells. Earlier research into CD3-based T-cell engagers had shown promise, but was hindered by high toxicity levels and lack of cross-reactivity with non-human primates, slowing down their clinical development.
iBio has addressed these challenges using its patented epitope steering technology, directing antibodies towards specific CD3 epitopes. By combining AI-based antibody optimization with mammalian display technology, the company has widened the range of CD3 affinities, identified antibodies with cross-reactivity to non-human primates, and improved the humanness of the antibody sequences.
iBio, Inc. first went public through an initial public offering (IPO) in 2008.
Evaxion Biotech
Evaxion Biotech is a public clinical-stage biotechnology company, headquartered in Copenhagen, Denmark, that specializes in developing AI-powered immunotherapies for the treatment of cancer, bacterial diseases, and viral infections. Evaxion's core strength lies in its proprietary and scalable AI technologies, designed to decode the human immune system and thereby innovate new immunotherapies.
Evaxion has recently announced the development of a new AI platform technology called ObsERV™. This technology is capable of identifying endogenous retroviruses (ERVs) - remnants of ancient viruses that are often overexpressed in cancer cells. By targeting these ERVs, Evaxion aims to develop personalized cancer immunotherapies for patients who are typically unresponsive to existing treatments.
The company's preclinical studies have shown promising results, including complete tumor eradication in animal models. CEO Per Norlén has expressed enthusiasm for this discovery, highlighting its potential to significantly expand the scope of cancer patient treatment.
Evaxion's EVX-02 Showcases the Potential of DNA-based Personalized Cancer Immunotherapy
DNA-based personalized cancer immunotherapy uses the patient's tumor DNA to create a tailored treatment. Scientists identify cancer-specific mutations and predict which mutated proteins, or neoantigens, will trigger the strongest immune response. They then encode the genetic information for these neoantigens into DNA, which is incorporated into a delivery vector. This customized DNA treatment is administered to the patient, where it triggers cells to produce these neoantigens and present them on their surface. This stimulates the immune system to recognize and destroy the cancer cells carrying these neoantigens.
Evaxion has shown impressive results with its DNA-based personalized cancer immunotherapy, EVX-02. In its Phase 1/2a study, EVX-02, in combination with the checkpoint inhibitor nivolumab, was found to be well-tolerated and all participating patients who completed the treatment were relapse-free at their last assessment. The study affirmed the ability of Evaxion's AI platform, PIONEERTM, to select appropriate neoantigens matched to each patient's specific cancer.
These results have given Evaxion the confidence to fast-track EVX-03, its next-generation DNA-based personalized cancer immunotherapy, towards clinical trials. The company aims to transform these technological advancements into effective patient care.
Lantern Pharma
Lantern Pharma Inc., an innovative biopharmaceutical company, based in Dellas, USA, that is focused on transforming oncology drug discovery and development, stands out with its proprietary RADR® artificial intelligence and machine learning platform. Using over 25 billion oncology-focused data points and a collection of advanced ML algorithms, Lantern aims to solve real-world, billion-dollar challenges in the oncology field. With a number of Phase 1 and two Phase 2 clinical programs and several anticipated Phase 1 clinical trials scheduled for 2023, Lantern is pushing boundaries in the race to find effective cancer therapies. The company has established a wholly-owned subsidiary, Starlight Therapeutics Inc., dedicated to the clinical execution of promising treatments for CNS and brain cancers.
RELATED: Unveiling Lantern Pharma's Success Story in AI-powered Precision Oncology
Recently, Lantern Pharma announced an important collaboration with Bielefeld University. This partnership is set to develop next-generation antibody-drug conjugates (ADCs), which, while not classified as immunotherapy, represent a significant advancement in targeted cancer therapy. The focus will initially be on synthesizing and evaluating novel ADCs linked to cryptophycins, highly potent antitumor molecules. These cryptophycin-ADCs will be tested across multiple cancer cell lines, with initial results expected in 2023.
Beyond the initial phase, Lantern plans to leverage its RADR® AI platform and the AI ADC development module to launch multiple ADCs, utilizing cryptophycins or other promising payloads. This move underscores the company's commitment to harnessing the power of AI for more targeted and effective cancer treatments. Furthermore, Lantern has secured an exclusive worldwide option to license intellectual property (IP) from Bielefeld University related to the collaboration.
In summary, Lantern Pharma is not just at the cutting edge of oncology research and treatment development; it's shaping the future with its forward-thinking AI-driven approach and significant discoveries in ADC development.
Lantern Pharma has successfully secured $95M in investment across seven funding rounds. Their most recent capital influx occurred on January 14, 2021, stemming from a Post-IPO secondary financing round.
In summary , artificial intelligence offers a set of tools that can help in understanding complex biological systems, refining personalized treatments, enhancing combination therapies, and improving clinical trials. As this field continues to mature, collaborations between different sectors - academia, industry, healthcare providers, and policy makers - will be vital to responsibly navigate potential challenges and make a substantial difference in patients' lives.
AI's integration into immunotherapy marks a significant shift in pharmaceutical research and treatment. As we delve deeper into this intersection, it is apparent that it holds numerous possibilities that could shape the future of medical treatments.
Topics: Biotech Companies
Get Exclusive Insights Into Your Inbox join 5000+ BPT insiders
There are no comments yet. You can be the first.
Leave a Reply cancel reply
Your email address will not be published. Required fields are marked *
IP Intelligence
R&d intelligence, life sciences intelligence, other services.
- Visit the User Community
Patent Analytics
Eureka Materials
Data Service
Professional Services
Global Innovation Report
Success Stories
Help center
Al-Powered Innovation Intelligence
5 companies pioneering breakthrough cancer research .
In 2022, the global investment in cancer research reached a staggering $193 billion, reflecting the immense dedication and resources committed to unraveling the mysteries of this complex disease. And for good reason — every sixth death worldwide is attributed to cancer.
In this article, we’ll explore what cancer research is and highlight five companies pioneering the future of this devastating disease.
Jump to . . .
- What is Cancer Research?
- How Many Types of Cancer Exist?
- What are the Current Treatments for Cancer?
- 5 Companies Pioneering Cancer Research
- Bristol Myers Squibb
- Curtis, Inc.
What is Cancer Research?
Cancer research is a multidisciplinary field dedicated to understanding the complexities of cancer, exploring its causes, mechanisms, and patterns, and developing innovative strategies to prevent, detect, diagnose, and treat this disease. It involves scientific investigations conducted by researchers, oncologists, biologists, geneticists, and other experts who collaborate to advance our knowledge of cancer at various levels, from cellular and molecular processes to clinical trials and population studies.
Cancer research encompasses a wide range of disciplines, including genetics, biochemistry, immunology, pharmacology, epidemiology, and computational biology. Through extensive laboratory experiments, clinical trials, data analysis, and translational research, scientists strive to unravel the fundamental mechanisms of cancer development, identify potential risk factors, discover new biomarkers, and develop novel therapies that can effectively target and eliminate cancer cells while minimizing harm to healthy tissues.
How Many Types of Cancer Exist?
The number of recognized types of cancer is not fixed and can evolve as our understanding of the disease advances. However, numerous known types of cancer are classified based on the organ or tissue in which they originate. Some common types of cancer include breast cancer, lung cancer, colorectal cancer, prostate cancer, skin cancer (such as melanoma), ovarian cancer, pancreatic cancer, and leukemia.
Additionally, there are less common and rare types of cancer that affect specific organs or tissues. The diversity of cancer types reflects the complexity of the disease and underscores the need for ongoing research and advancements in cancer detection, diagnosis, and treatment tailored to each specific type.
What are the Current Treatments for Cancer?
Current cancer treatments encompass a range of modalities aimed at combating the disease. These treatments include surgery, where tumors or cancerous tissues are physically removed through precise procedures. Radiation therapy utilizes high-energy beams to target and destroy cancer cells, while chemotherapy employs powerful medications to kill or control the growth of cancer cells throughout the body.
Immunotherapy boosts the body’s immune response to recognize and attack cancer cells, while targeted therapy utilizes drugs that specifically target molecules or pathways involved in tumor growth. Hormone therapy is employed for hormone-sensitive cancers, while precision medicine tailors treatment based on an individual’s genetic profile and specific biomarkers.
These treatment approaches are often used in combination, with personalized treatment plans designed in collaboration between patients and a multidisciplinary healthcare team.
5 Companies Pioneering Cancer Research
1.) genentech (roche) : .
Genentech, a leading biotechnology company, is spearheading cancer research with its groundbreaking advancements in personalized medicine and targeted therapies.
Through extensive genomic analysis and precision medicine approaches, Genentech focuses on developing therapies tailored to specific cancer types and individual patients. Genentech’s commitment to understanding the molecular basis of cancer enables the development of effective treatments that can directly target cancer cells while minimizing damage to healthy tissues.
Through analysis on Patsnap’s platform , Genentech is focusing on Antiendomysial antibodies in 2023. Last year the company’s primary research objective revolved around mitigating the severity of the disease.
2.) Novartis
Novartis, a global pharmaceutical company, is revolutionizing cancer treatment through innovative therapies, including targeted therapies, immunotherapies, and cellular therapies.
Novartis has made significant strides in the field of CAR-T cell therapy, a groundbreaking approach that harnesses the power of a patient’s immune system to recognize and destroy cancer cells. Novartis’ commitment to pushing the boundaries of science and collaboration has led to remarkable advancements in cancer research and treatment options.
Over the last three years, the company has been focused on improving security, stability, and other effects. Novartis AG is also increasing its focus on solving problems that increase the risk of its pharmaceuticals.
3.) Bristol Myers Squibb
Bristol Myers Squibb (BMS) is a renowned pharmaceutical company known for its pioneering research in oncology. BMS focuses on developing innovative immunotherapies that activate the immune system to fight cancer.
The company’s checkpoint inhibitors, which target proteins that suppress the immune response, have transformed the treatment landscape for various types of cancer. BMS continues to explore new therapeutic avenues, aiming to enhance patient outcomes and improve survival rates.
In 2022, BMS directed significant IP attention towards multiple areas, including reducing tumor quantity, tumor shrinkage, stabilizing disease progression, achieving durable clinical responses, and attaining complete responses.
4.) Moderna
Moderna, a pioneering biotechnology company, has made disruptive contributions to cancer research through its mRNA technology platform.
While mRNA vaccines have gained prominence in recent times, Moderna has also been harnessing this technology for cancer immunotherapy. Moderna’s personalized cancer vaccines, which leverage the body’s immune response to target tumor-specific antigens, hold great potential in treating a wide range of cancers. Moderna’s innovative mRNA-based therapies are reshaping the landscape of cancer treatment.
This year, Moderna is intensifying its R&D efforts in the fields of polynucleotides, nucleotides, and fusion proteins, with a keen awareness of their potential implications for specific health concerns. Additionally, the company is actively dedicated to facilitating broader accessibility to RNA therapeutics, aiming to simplify its acquisition process and ultimately extend its benefits to a larger population.
Curis is a biotechnology company focused on the development of first-in-class and innovative therapeutics for the treatment of cancer. The company has identified several promising drug candidates that have shown significant efficacy in preclinical and clinical trials.
Its portfolio includes small molecule inhibitors and monoclonal antibodies designed to selectively target and inhibit the activity of proteins involved in cancer cell proliferation and survival. The company has also demonstrated a commitment to precision medicine by incorporating genomic profiling and biomarker analysis into its clinical trials. By identifying specific genetic alterations and biomarkers associated with treatment response, Curis aims to personalize treatment decisions and maximize the chances of success for individual patients.
“With Patsnap email alerts, I can get weekly updates on competitor patent filings and specific technology spaces sent straight to my inbox. This has saved us a lot of valuable time which would have otherwise been spent searching and sorting through patent documents. Overall, PatSnap makes our innovation process much more efficient.” -Mark Noel, Vice President of Technology and IP, Curis Inc.
In the quest to conquer cancer, these five companies are disrupting cancer research. Driven by a relentless pursuit of breakthroughs, they are reshaping our understanding of cancer, redefining diagnostics, and reimagining treatments.
- Analytics Eureka Discovery Synapse Bio Chemical
- AI Customer Stories Global Innovation Report Webinars Newsletter About us
- Book a demo
© 2023 Patsnap. All rights reserved Legal | Privacy policy | Modern Slavery Act Transparency Statement
Top 10 Cancer treatment startups


Top 30 Innovative Immuno-Oncology Companies in the US: The future of Immunotherapy for Cancer Patients
Defining the field of immuno-oncology and the potential of immunotherapy for cancer patients.
Immunotherapy for cancer, also known as immuno-oncology, has emerged as a game-changing approach in the field of cancer treatment. Unlike traditional cancer therapies that directly target cancer cells, immunotherapy leverages the power of the immune system to recognize and attack cancer cells more effectively. It offers remarkable advancements in cancer care, with notable modalities such as immune checkpoint inhibitors, CAR-T cell therapy, and cancer vaccines.
Immune checkpoint inhibitors release the brakes on the immune system, reinvigorating its ability to identify and eliminate cancer cells. CAR-T cell therapy involves modifying a patient's T cells to express receptors that specifically target cancer cells. Cancer vaccines educate the immune system to recognize and attack cancer cells by introducing cancer-specific antigens. These immunotherapies have demonstrated exceptional success, leading to durable responses and even complete remissions in some cases.
Immunotherapy has transformed the landscape of cancer treatment, offering new hope and improved outcomes for patients. Ongoing research aims to enhance response rates and broaden the applications of immuno-oncology. As scientists continue to explore innovative strategies and combination therapies, the future of cancer treatment looks promising, with immunotherapy playing a pivotal role in the fight against cancer.
The current state of the immuno-oncology market
The immuno-oncology market has grown at a compound rate of 10%, from $57 billion to $69 billion in 2021 and is expected to reach around $98 billion by 2025.
This is a highly competitive market, so companies in this sector have to innovate rapidly by creating novel drugs and therapies.

These are the top 30 immuno-oncology companies in the US
Dragonfly Therapeutics
CEO & Co-Founder - William Haney
Located in Massachusetts, Dragon Therapeutics is developing cancer treatments that promote immune responses against cancer are being developed. The company's platform is developing revolutionary first-in-class therapies targeted at natural killer cells and other innate immune system cells.
CEO - Kenneth Galbraith
Located in British Columbia, Zymeworks is dedicated to the development, commercialization, and discovery of next-generation multifunctional biotherapeutics. Zanidatamab, the company's flagship clinical candidate, is a novel Azymetric HER2-targeted bispecific antibody that is now being tested in several Phase 1, Phase 2, and pivotal clinical trials around the world as a targeted therapeutic option for patients with HER2-positive solid tumors.
Verastem Oncology
CEO - Brian Stuglik
Located in Massachusetts, Verastem Oncology is developing novel treatments for cancer patients. COPIKTRA (duvelisib), the company's first commercial product, is for the treatment of adult patients with relapsed or refractory chronic lymphocytic leukemia/small lymphocytic lymphoma after two or more prior therapies, as well as relapsed or refractory follicular lymphoma after two or more prior systemic therapies.
Gritstone bio
CEO & President & Co-Founder - Andrew Allen
Located in California, Gritstone bio is developing tailored cancer immunotherapies to combat a variety of cancers and infectious disorders.
Pieris Pharmaceuticals
CEO & President - Stephen Yoder
Located in Massachusetts, Pieris Pharmaceuticals is focused on discovering and developing biotherapeutics in the Anticalin class.
Anticalin proteins are modified forms of lipocalins, which are human proteins that bind, store, and transport a wide range of chemicals naturally.
Alaunos Therapeutics
CMO - Francois Lebel
Located in Texas, Alaunos Therapeutics Alaunos is focused on adoptive TCR-T cell therapy for the treatment of solid malignancies. It attacks cancer at its source by developing cell treatments that target Neoantigens (neoAg), which are produced as a result of genomic alterations.
CTO - Raymond Stapleton, Jr.
Located in Massachusetts, Genocea focuses on creating innovative vaccines and immunotherapies to treat diseases with high unmet medical needs. Using it's ATLAS™ technology can profile each patient's T cell responses to possible tumor targets, or antigens, in great detail. ATLAS targets antigens that trigger anti-tumor T cell responses as well as inhibitory antigens, known as Inhibigens™, that induce pro-tumor immune responses.
ImmunityBio
CEO - Richard Adcock
Located in California, ImmunityBio is developing next-generation treatments that stimulate immunogenic pathways in cancer and infectious disease patients. To develop long-term immunological memory, the company's immunotherapy platform engages both the innate (natural killer cell and macrophage) and adaptive (T cell) immune systems.
Galectin Therapeutics
CEO & President - Joel Lewis
Located in Georgia, Galectin Therapeutics focuses on drug development to find novel treatments for fibrotic illness, cancer, and a few other ailments.
CEO - Michael A. Metzger
Located in Massachusetts, Syndax is working on a pipeline of combination medicines for a variety of cancers. Entinostat, which has direct effects on both cancer cells and immune regulatory cells, and SNDX-6352, an anti-CSF-1R monoclonal antibody, are two of the company's product prospects.
Allogene Therapeutics
CEO & President - David Chang
Located in California, Allogene Therapeutics is a clinical-stage biotechnology company specializing in developing allogeneic chimeric antigen receptor T cell (AlloCAR T™) therapies for cancer treatment. The company's pipeline includes several allogeneic CAR T therapies and UCART19 which is intended for the treatment of acute lymphoblastic leukemia (ALL).
Asher Biotherapeutics
CEO - Craig Gibbs
Located in California, Asher Biotherapeutics develops targeted immunotherapies to treat cancer and other diseases. Their cis-targeting platform enables the activation of immune cell types to take on cancer cells with minimum side effects.
Rubius Therapeutics
CEO - Pablo Cagnoni
Located in Massachusetts, Rubius Therapeutics is a pioneer in developing Red Cell Therapeutics™ (RCTs) using their proprietary RED PLATFORM™. The RCTs are potent, selective, and ready-to-use cellular therapies that can be used to treat autoimmune diseases and cancer.
Marengo Therapeutics
CEO - Zhen Su
Located in Massachusetts, Marengo Therapeutics is a pioneer in developing therapies that boost the body's immune system to fight cancer. Using their proprietary therapeutic platform, the company boosts the patient's T cells to amplify their anti-tumor activity while promoting long-lasting immunity.
Catamaran Bio
CEO & President - Alvin Shih
Located in Massachusetts, Catamaran Bio develops off-the-shelf, allogeneic immune cell therapies that are safe and effective using their proprietary TAILWIND™ platform to treat cancer and immune disorders.
Obsidian Therapeutics
CEO - Paul Wotton
Located in Massachusetts, Obsidian Therapeutics is developing next-generation controlled cell and gene therapies are being developed to bring adoptive immunotherapy to every cancer patient.
Odyssey Therapeutics
CEO & Founder - Gary D. Glick
Located in Massachusetts, Odyssey Therapeutics aims to create next-generation precision medicine for patients with cancer and inflammatory diseases. The Odyssey team can quickly identify relevant targets and optimize therapeutic assets for clinical development using their in-house intellectual capital.
CEO & President - Xiaodong Yang
Located in California, Apexigen is a clinical-stage biopharmaceutical company that uses its proprietary antibody-drug discovery platform to develop drugs that enhance tumor-specific immunity.
Artiva Biotherapeutics
CEO & President - Fred Aslan
Located in California, Artiva develops off-the-shelf therapies like allogeneic NK cell therapies, including CAR-NK cell therapies designed to treat solid tumors or hematologic malignancies. Its mission is to deliver safe and easily accessible cancer therapies to patients.
Fate Therapeutics
CEO & President - Scott Wolchko
Located in California, Fate Therapeutics is a clinical-stage biopharmaceutical company developing cellular immunotherapies for cancer and immune disorders. Their therapy pipeline includes natural killer (NK) cell and T-cell cancer immunotherapies, which are designed to work in conjunction with current cancer therapies such as immune checkpoint inhibitors and monoclonal antibodies.
ALX Oncology
CEO & President - Jaume Pons
Located in California, ALX Oncology is developing a pipeline of pharmaceutical candidates based on expertise in protein engineering and oncology, led by the CD47 blocker evorpacept, which is currently in phase 1 and 2 clinical trials. To avoid detection by the immune system, cancer cells use CD47, a cell surface protein, as a don't eat me signal.
CEO - John Chant
Located in California, Filtricine has developed targeted nutrient deprivation (TND) therapy, which focuses on taking away nutrients critical for the growth of cancer cells. This helps to exploit metabolic dependencies to stop cancer from spreading.
OncoMyx Therapeutics
CSO - Leslie Sharp
Located in Arizona, OncoMyx Therapeutics develops immunotherapy for cancer research and treatments. Its proprietary myxoma platform targets hematologic and solid tumors with a unique oncolytic virus called myxoma and a novel patented systemic delivery approach.
CEO & Co-Founder - Ken Drazan
Located in California, ArsenalBio is a programmable cell therapy company that uses CRISPR-enabled genome editing to engineer living medicines that attack cancer and improve patient lives.
Pyxis Oncology
CEO & President - Lara S. Sullivan
Located in Massachusetts, Pyxis Oncology develops biologics that overcome suppression and target tumor cells. They believe that antibody-drug candidates (ADCs) and immunotherapies for cancer hold the potential to improve patient lives and target difficult-to-treat cancers.
Arch Oncology
CEO & President - Laurence Blumberg
Located in California, Arch Oncology is a clinical-stage company that develops antibody therapies to treat hematologic malignancies and solid tumors. Their lead candidate AO-176 is used to treat patients with solid tumors and is currently going through Phase 1/2 clinical trials. AO-176 can directly kill tumor cells and induce DAMPs (Damage Associated Molecular Patterns), resulting in Immunogenic Cell Death.
Rakuten Medical
CSO - Miguel Garcia-Guzman
Located in California, Rakuten Medical is developing cell-targeting investigational therapies on its Illuminox™ technology platform. These therapies are known to demonstrate selective cell killing and tumor necrosis in preclinical studies.
Triumvira Immunologics
CEO & President - Paul Lammers
Located in Texas, Triumvira Immunologics is developing a novel and proprietary T cell Antigen Coupler (TAC) technology that is thought to be safer and more effective than some of the current cancer treatments like antigen receptor (CAR) and engineered T cell receptor (TCR) therapies.
CEO & President - Evans Jay
Located in Montana, Inimmune is at the forefront of advancing the field of immunotherapy for cancer and autoimmunity. With a focus on developing next-generation therapies, Inimmune is dedicated to harnessing the power of the immune system to combat these diseases. By leveraging innovative approaches and cutting-edge research, Inimmune aims to revolutionize treatment options and improve outcomes for patients battling cancer and autoimmune disorders.
Arcus Biosciences
CEO - Terry Rosen
Located in California, Arcus Biosciences is a clinical-stage biopharmaceutical company focused on developing therapies for cancer using the ATP-adenosine pathway, a crucial pathway associated with immunosuppression in a tumor microenvironment. The company's product pipeline includes Etrumadenant, AB680, and Domvanalimab.
Lab Management Software for Immuno-Oncology Companies: Scispot.io
Scispot has become the primary lab management software of choice for numerous modern biotech companies, including immuno-oncology startups.
Scispot creates a connected digital replica of these innovative bioscience companies. It centralizes their company-wide data, templatizes routine research and automates non-scientific tasks. It makes other lab software such as electronic lab notebooks (ELNs) and lab information management systems (LIMS) redundant.
Immuno-Oncology Companies use Scispot's operating platform to manage their research projects, inventory, samples, and partners all in one spot. Request a demo to learn how you can accelerate your research using Scispot.
What’s a Rich Text element?
The rich text element allows you to create and format headings, paragraphs, blockquotes, images, and video all in one place instead of having to add and format them individually. Just double-click and easily create content.
Static and dynamic content editing
A rich text element can be used with static or dynamic content. For static content, just drop it into any page and begin editing. For dynamic content, add a rich text field to any collection and then connect a rich text element to that field in the settings panel. Voila!
How to customize formatting for each rich text
Headings, paragraphs, blockquotes, figures, images, and figure captions can all be styled after a class is added to the rich text element using the "When inside of" nested selector system.
Check Out Our Other Blog Posts
.png)
How to Make Your Lab CFR Part 11 Compliant
Ensuring your laboratory adheres to CFR Part 11 is crucial in the era of digital record-keeping. The FDA issued a regulation that sets standards for using electronic records and signatures in research and development. Here's a straightforward guide to understanding and achieving compliance with these FDA regulations.

Universal Lab Instrument Integration with Scispot’s Agents
Do you need an efficient method to transfer data from laboratory instruments to the cloud? Try Scispot GLUE for immediate use without any implementation delays.

Choosing the Right Path for Laboratory Instrument Integration: A Hybrid Model
When it comes to integrating laboratory instruments, the question often arises: should labs create their own custom solutions or opt for pre-existing software?

Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- NATURE INDEX
- 06 September 2023
Global leaders in science’s battle against cancer
Cancer research attracts billions of dollars in funding every year from governments, charities and other institutions. This helps to establish the field as a cornerstone of scientific activity in many countries, especially the United States.
Special focus
Cancer research represents a large slice of overall research output in the Nature Index for many institutions, although this does not always translate to a high Share in this field. This is shown in the chart below, which plots Share against the proportion of an institution’s Nature Index output represented by cancer research for the leading 200 institutions in the topic.
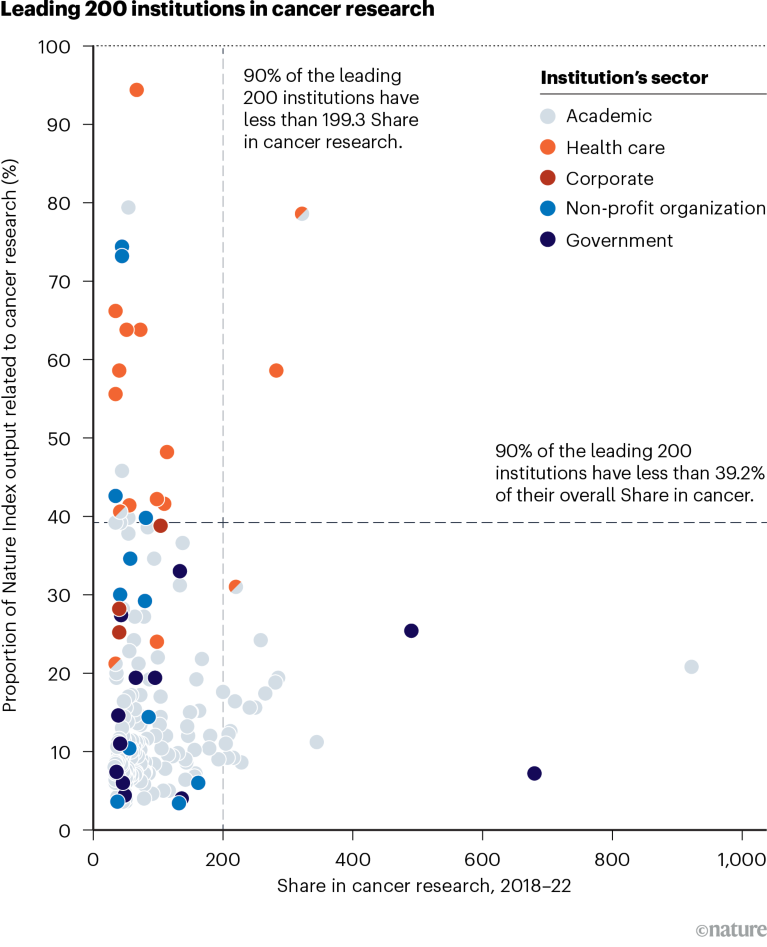
Source: Nature Index. Data analysis by Bo Wu. Infographic by Simon Baker, Bec Crew and Tanner Maxwell
Some outliers are highlighted in the chart below. Specialist cancer centres unsurprisingly have a high proportion of Nature Index output related to cancer research. But two in the United States, the Memorial Sloan Kettering Cancer Center in New York City and University of Texas MD Anderson Cancer Center in Houston, Texas, are clear of other specialist institutions on Share.
The Chinese Academy of Sciences, in Beijing, has a relatively low proportion of Nature Index output focused on cancer research, but it still has the second highest Share for the period 2018–22.
Harvard University in Cambridge, Massachusetts, has the world’s leading Share of cancer research, and more than one-fifth of its total Nature Index output is in this area. The National Institutes of Health, a major funder of cancer research in the United States, also has a high Share. Stanford University in California has a Share of 343, despite just 11% of its overall Nature Index output for the period 2018–22 being related to cancer research.
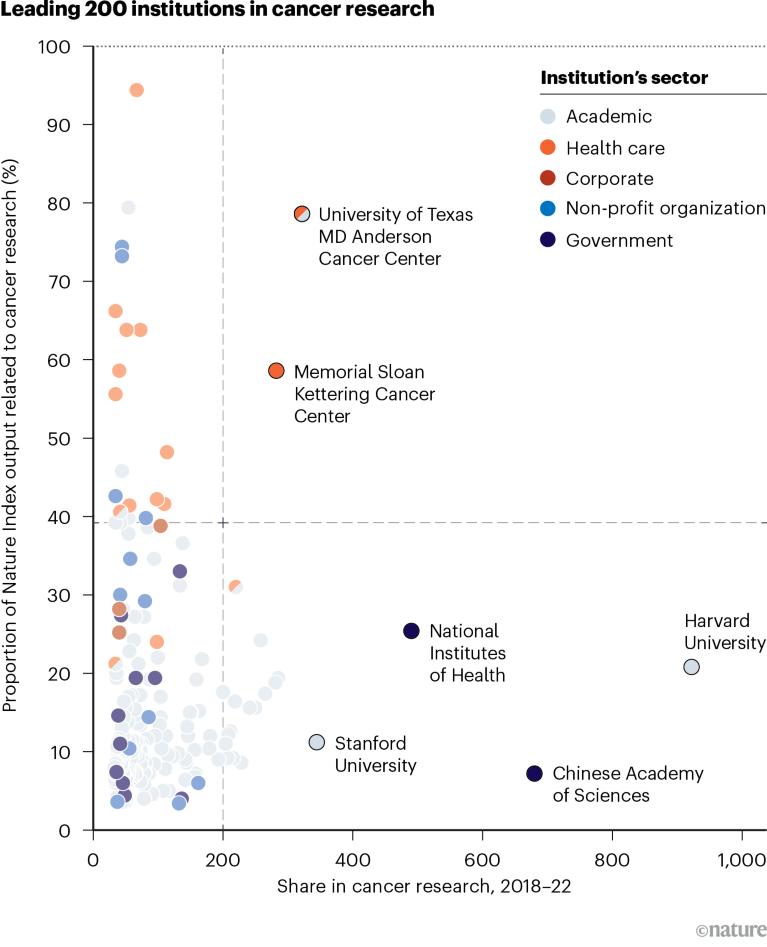
North American giant
With 10,280 grants worth a total of US$13.1 billion awarded in 2018–22, the US National Cancer Institute (NCI) dwarfs all other major North American funders of cancer research. Among the 27 institutes and centres of the US National Institutes of Health, the NCI is the largest, with a federally allocated budget of $7.3 billion for 2023, up $408 million on last year.
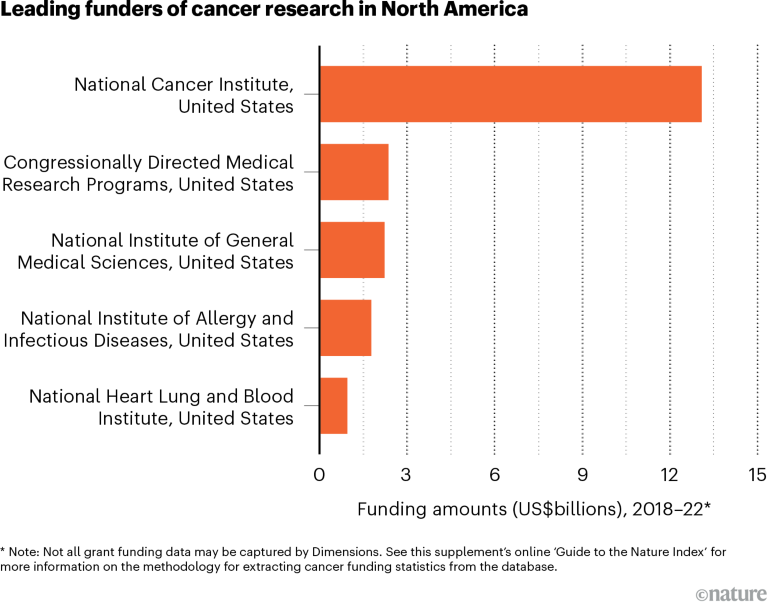
Source: Dimensions. Data analysis by Bo Wu. Infographic by Simon Baker, Bec Crew and Tanner Maxwell
Europe’s key spenders
The European Commission, which invests in science through the European Union’s ‘Horizon’ funding programmes, allocated almost 1,300 cancer research grants from 2018 to 2022, totalling $2.5 billion, according to Dimensions data. The European Research Council, another beneficiary of Horizon money, funded grants worth around $850 million.
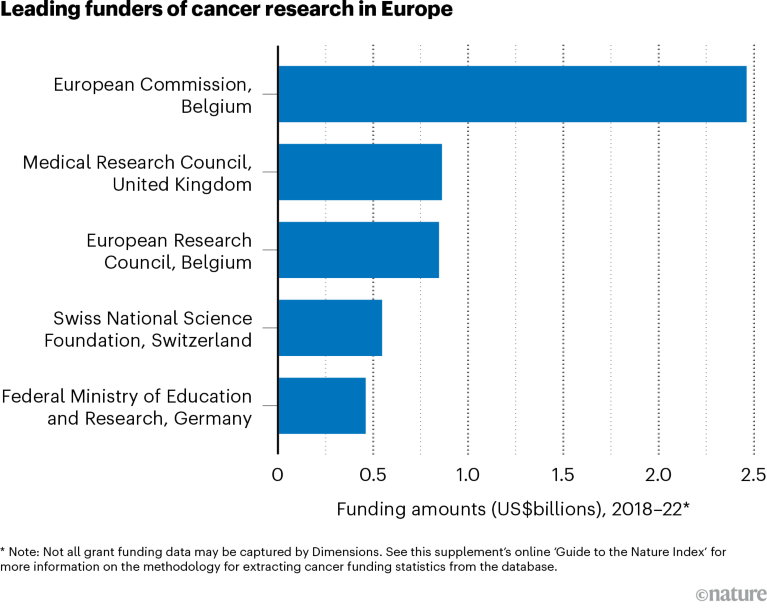
Asia-Pacific spread
The Japan Society for the Promotion of Science allocated the most money for cancer research from 2018 to 2022 among Asia-Pacific funders listed in the Dimensions database. Its 15,665 grants were worth a total of around $860 million. The National Natural Science Foundation of China was close behind, distributing 11,910 grants worth about $810 million.
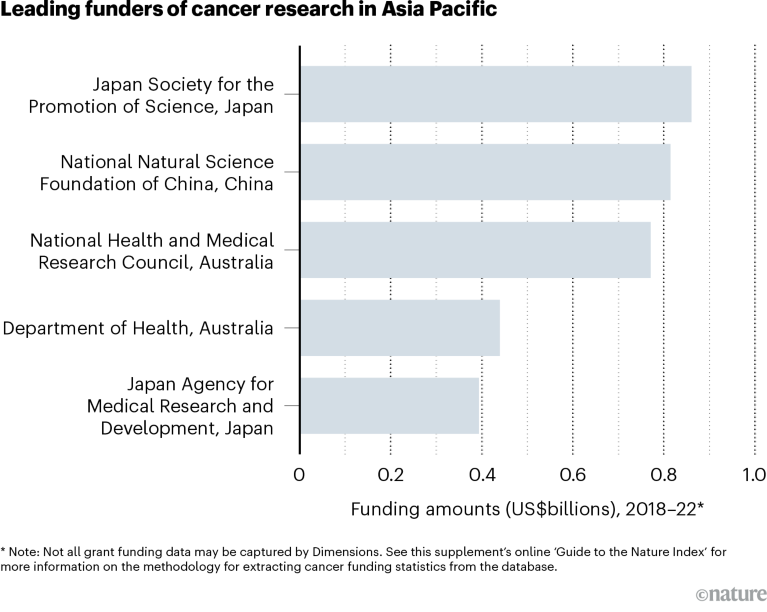
Lost connections
The United States is a force in international cancer research: it helps form the five leading bilateral country collaborations in the Nature Index for 2018–22. The United States and China had the most prolific partnership by some margin. But a steep decline since 2018, probably reflecting the impact of rising political tensions and the COVID-19 pandemic, has hugely reduced the gap with other US partnerships.
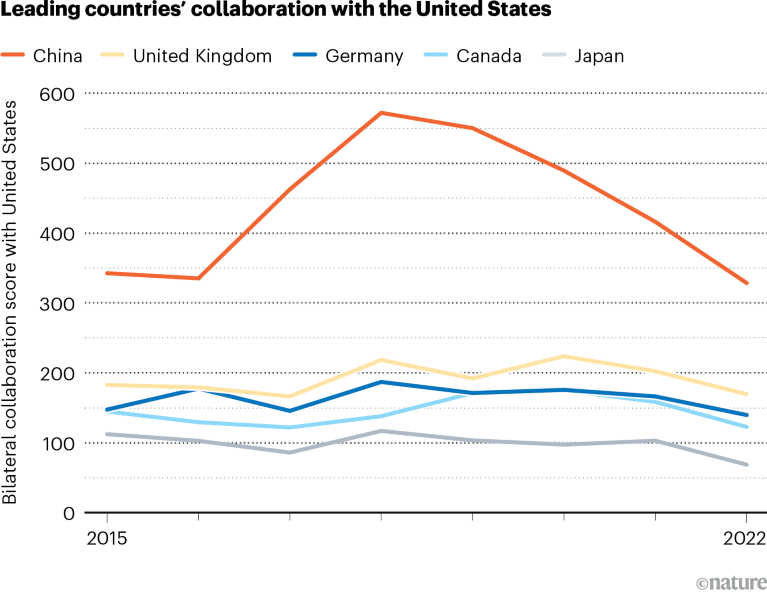
Prolific partners
The leading five institutional pairs in cancer-related research for 2018–22 are all domestic partnerships. A rapid growth in collaborative articles between the University of Chinese Academy of Sciences and the Chinese Academy of Sciences since 2016 is the most striking trend.
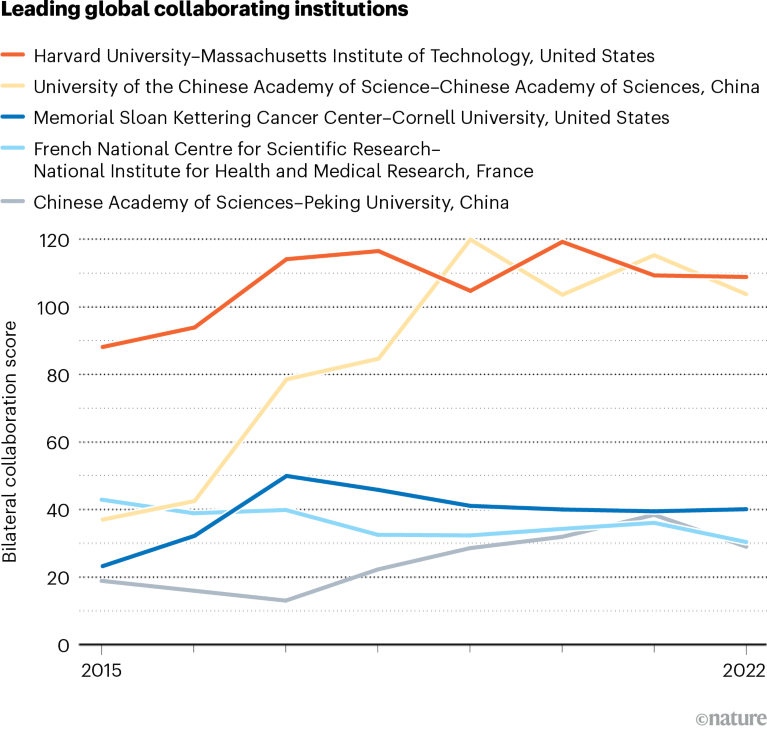
Nature 621 , S12-S13 (2023)
doi: https://doi.org/10.1038/d41586-023-02608-3
This article is part of Nature Index 2023 Cancer , an editorially independent supplement. Advertisers have no influence over the content.
Related Articles

Partner content: Training immune cells to catch up with cancer
Partner content: Cancer research beyond personalized medicine
Partner content: Delivering a direct hit to tumours
Partner content: Tumour profiling offers new clinical trial options
Partner content: Fighting deadly diseases on multiple fronts
Partner content: Gaining ground in the war on cancer
Partner content: A kidney disease drug helps fight cancer and ageing
Partner content: How surgical robots and tiny lab-based organs are defeating urologic and gastrointestinal cancers
Partner content: How collaboration is accelerating cancer insight
Partner content: Triple-threat approach packs a punch against hard-to-hit tumours
Partner content: Precision medicine built in Zurich keeps patients in the LOOP
Partner content: New frontiers in cancer therapy: from blood cancers to solid tumours
Partner content: Alert, avert, advise: how genetic testing is transforming hereditary cancer care
- Medical research
- Health care
- Institutions

Mapping genotypes to chromatin accessibility profiles in single cells
Article 08 MAY 24
Targetable leukaemia dependency on noncanonical PI3Kγ signalling

Genomics reveal unknown mutation-promoting agents at global sites
News & Views 01 MAY 24

Hacking the immune system could slow ageing — here’s how
News Feature 07 MAY 24

‘Orangutan, heal thyself’: First wild animal seen using medicinal plant
News 02 MAY 24

Is the Internet bad for you? Huge study reveals surprise effect on well-being
News 12 MAY 24

How ignorance and gender inequality thwart treatment of a widespread illness
Outlook 09 MAY 24

Bird flu in US cows: where will it end?
News 08 MAY 24
Clinician Researcher/Group Leader in Cancer Cell Therapies
An excellent opportunity is available for a Group Leader with expertise in cellular therapies to join the Cancer Research program at QIMR Berghofer.
Herston, Brisbane (AU)
QIMR Berghofer
Faculty Positions at the Center for Machine Learning Research (CMLR), Peking University
CMLR's goal is to advance machine learning-related research across a wide range of disciplines.
Beijing, China
Center for Machine Learning Research (CMLR), Peking University
Faculty Positions at SUSTech Department of Biomedical Engineering
We seek outstanding applicants for full-time tenure-track/tenured faculty positions. Positions are available for both junior and senior-level.
Shenzhen, Guangdong, China
Southern University of Science and Technology (Biomedical Engineering)
Southeast University Future Technology Institute Recruitment Notice
Professor openings in mechanical engineering, control science and engineering, and integrating emerging interdisciplinary majors
Nanjing, Jiangsu (CN)
Southeast University
Staff Scientist
A Staff Scientist position is available in the laboratory of Drs. Elliot and Glassberg to study translational aspects of lung injury, repair and fibro
Maywood, Illinois
Loyola University Chicago - Department of Medicine
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- Today's news
- Reviews and deals
- Climate change
- 2024 election
- Fall allergies
- Health news
- Mental health
- Sexual health
- Family health
- So mini ways
- Unapologetically
- Buying guides
Entertainment
- How to Watch
- My Portfolio
- Latest News
- Stock Market
- Premium News
- Biden Economy
- EV Deep Dive
- Stocks: Most Actives
- Stocks: Gainers
- Stocks: Losers
- Trending Tickers
- World Indices
- US Treasury Bonds
- Top Mutual Funds
- Highest Open Interest
- Highest Implied Volatility
- Stock Comparison
- Advanced Charts
- Currency Converter
- Basic Materials
- Communication Services
- Consumer Cyclical
- Consumer Defensive
- Financial Services
- Industrials
- Real Estate
- Mutual Funds
- Credit cards
- Balance Transfer Cards
- Cash-back Cards
- Rewards Cards
- Travel Cards
- Personal Loans
- Student Loans
- Car Insurance
- Morning Brief
- Market Domination
- Market Domination Overtime
- Opening Bid
- Stocks in Translation
- Lead This Way
- Good Buy or Goodbye?
- Fantasy football
- Pro Pick 'Em
- College Pick 'Em
- Fantasy baseball
- Fantasy hockey
- Fantasy basketball
- Download the app
- Daily fantasy
- Scores and schedules
- GameChannel
- World Baseball Classic
- Premier League
- CONCACAF League
- Champions League
- Motorsports
- Horse racing
- Newsletters
New on Yahoo
- Privacy Dashboard
Yahoo Finance
14 best cancer stocks to buy now.
In this article, we discuss 14 best cancer stocks to buy. If you want to skip our detailed discussion on the oncology industry, head directly to 5 Best Cancer Stocks To Buy Now .
The global oncology landscape continues to evolve, with ongoing efforts to discover, develop, and provide innovative treatments aimed at improving outcomes for a growing number of patients. However, the accessibility and utilization of these new cancer medications vary significantly worldwide. According to IQVIA , the number of oncology clinical trials initiated in 2022 remained high, marking a 22% increase since 2018. Over the past five years, there has been a consistent annual rise of 5% in the global population of patients receiving cancer treatment. Projections indicate that global spending on cancer drugs is set to reach $375 billion by 2027, a significant increase from $196 billion in 2022. The oncology portfolio is expanding rapidly, with more than 2,000 products currently in development. A substantial portion of these products, nearly 71%, is being developed by emerging biopharmaceutical companies. These companies have significantly increased their contributions to the development of cancer therapies, up from 51% in 2017. Globally, 237 new active substances have been introduced for cancer treatment over the past two decades, with almost 115 launched within the last five years. The United States has seen 78 NAS launches in the past five years and a total of 189 over the last two decades.
The steady rise in cancer treatment can be attributed to aging populations and improved access to healthcare services in developed markets. In lower-income markets, efforts to expand healthcare access and longer treatment durations have led to a higher number of patients receiving cancer treatment each year.
As per Fortune Business Insights ’ findings, the global market for oncology drugs reached a value of $184.95 billion in 2022. Projections indicate significant growth, with the market expected to increase from $205.52 billion in 2023 to an estimated $484.32 billion by 2030, representing a compound annual growth rate of 13% during the forecast period. Oncology drugs include a wide range of treatments, including targeted therapies, chemotherapy agents, immunotherapies, and hormone therapies, all developed to combat cancer. Several factors contribute to the growth of this market, including the increasing prevalence of different types of cancer, the approval and launch of new drugs, and improved research efforts by pharmaceutical companies in the oncology field. For instance, as per data released by the American Cancer Society in January, 2023 will see a rise in cancer patients in the United States, with an anticipated 28% increase compared to 2010, resulting in approximately 1.96 million cancer cases by the end of 2023.
Faced with a severe shortage of over a dozen cancer medications, the US Food and Drug Administration is considering the temporary importation of chemotherapy drugs from international manufacturers not currently approved for distribution in the United States, an FDA spokesperson told CNBC . This potential move aims to address a pressing issue highlighted by medical professionals at hospitals across the country, particularly concerning two drugs – cisplatin and carboplatin, which play a critical role in cancer treatment and are widely utilized. The World Health Organization has recognized the significance of these drugs in basic healthcare. Shortages of these drugs have compelled hospitals to ration their supply by reducing doses and prioritizing patients who stand to gain the most from treatment. Dr. Abdul Rafeh Naqash, a doctor at the Stephenson Cancer Center at the University of Oklahoma, stated:
“The lawmakers in the country need to understand that this is a big problem at this point, where unless something changes in the next few weeks, this can lead to a big national emergency from a patient and health care standpoint.”
The shortages of cisplatin and carboplatin are the result of a temporary production halt for the U.S. market at an Intas Pharmaceuticals facility in India. Dr. Karen Knudsen, CEO of the American Cancer Society, pointed out that these shortages highlight a persistent economic issue in the generic drug market. She also noted that the demand for these drugs is expected to increase in the coming years due to an aging population, as older individuals face a higher risk of cancer.
In this article, we discuss some of the top cancer stocks to invest in, which include Pfizer Inc. (NYSE: PFE ), Merck & Co., Inc. (NYSE: MRK ), and Johnson & Johnson (NYSE: JNJ ).
Our Methodology
We selected the following cancer stocks based on the hedge fund sentiment toward each stock. We have assessed the hedge fund sentiment from Insider Monkey’s database of 910 elite hedge funds tracked as of the end of the second quarter of 2023. The list is arranged in ascending order of the number of hedge fund investors in each firm.
Photo by National Cancer Institute on Unsplash
Best Cancer Stocks To Buy Now
14. kura oncology, inc. (nasdaq: kura ).
Number of Hedge Fund Holders: 29
Kura Oncology, Inc. (NASDAQ:KURA) is a clinical-stage biopharmaceutical firm based in San Diego, California. The company's focus revolves around a range of small molecule product candidates designed to combat cancer. On August 11, BofA Securities started coverage of Kura Oncology, Inc. (NASDAQ:KURA) with a Buy rating. They expressed optimism about the stock, seeing it as undervalued considering the promising revenue prospects of its primary drug candidate, ziftomenib, currently in development for treating acute myeloid leukemia.
According to Insider Monkey’s second quarter database, 29 hedge funds were bullish on Kura Oncology, Inc. (NASDAQ:KURA). This number increased from the previous quarter when 24 funds had invested in the stock. Aaron Cowen’s Suvretta Capital Management is the leading position holder in the company, with 7.03 million shares worth $74.36 million.
In addition to Pfizer Inc. (NYSE:PFE), Merck & Co., Inc. (NYSE:MRK), and Johnson & Johnson (NYSE:JNJ), Kura Oncology, Inc. (NASDAQ:KURA) is one of the best cancer stocks to buy.
13. Exelixis, Inc. (NASDAQ: EXEL )
Number of Hedge Fund Holders: 31
Exelixis, Inc. (NASDAQ:EXEL) is a biotechnology company with a primary focus on oncology. The company is dedicated to discovering, developing, and bringing new cancer treatments to the market in the United States. It is one of the best cancer stocks to buy. On September 26, H.C. Wainwright began coverage of Exelixis, Inc. (NASDAQ:EXEL) with a Buy rating, highlighting the company's strong portfolio of targeted oncology treatments. The firm has set a price target of $28 for the stock.
According to Insider Monkey’s second quarter database, 31 hedge funds were bullish on Exelixis, Inc. (NASDAQ:EXEL), compared to 28 funds in the preceding quarter. Thomas Steyer’s Farallon Capital held the largest position in the company, with 25.86 million shares worth $494.2 million.
12. Guardant Health, Inc. (NASDAQ: GH )
Number of Hedge Fund Holders: 36
Guardant Health, Inc. (NASDAQ:GH), one of the best cancer stocks, is a precision oncology company offering blood tests, data analysis, and insights globally. The company has a research collaboration with the Parker Institute for Cancer Immunotherapy, focusing on understanding how molecular cancer biomarkers relate to patient responses to immunotherapy treatments across different cancer types. On August 3, Guardant Health, Inc. (NASDAQ:GH) announced a Q2 non-GAAP EPS of -$0.82 and a revenue of $137.15 million, outperforming Wall Street estimates by $0.11 and $7.73 million, respectively.
According to Insider Monkey’s second quarter database, 36 hedge funds were bullish on Guardant Health, Inc. (NASDAQ:GH), up from 27 funds in the previous quarter. Steve Cohen’s Point72 Asset Management held a significant position in the company, with 2.7 million shares valued at $97.17 million.
Here is what Aristotle Atlantic Partners has to say about Guardant Health, Inc. (NASDAQ:GH) in its Q2 2023 investor letter:
“Guardant shares ended the quarter strong, recovering to some extent from the previous weakness in the quarter, as the company reported accelerating clinical volume growth and raised earnings guidance. In addition, the company announced additional U.S. commercial insurance coverage for their Guardant 360 test in the U.S. and government reimbursement in Japan. Lasty, the company completed a successful secondary offering, raising approximately $250 million.”
11. Moderna, Inc. (NASDAQ: MRNA )
Number of Hedge Fund Holders: 40
Ranked 11th on our list of the best cancer stocks, Moderna, Inc. (NASDAQ:MRNA) is a biotechnology company involved in the development and distribution of messenger RNA therapeutics and vaccines. The company focuses on treating immuno-oncology, infectious diseases, rare diseases, autoimmune conditions, and cardiovascular diseases across the United States, Europe, and other regions. Moderna, Inc. (NASDAQ:MRNA)’s portfolio includes a range of therapeutic options, from systemic treatments to cancer vaccines, immuno-oncology products, and multiple therapeutics for different medical conditions.
On September 13, during its R&D Day event, Moderna, Inc. (NASDAQ:MRNA) announced plans for its future growth. The company anticipates generating between $10 billion to $15 billion in annual sales five years after introducing new products in oncology, rare diseases, and latent diseases by 2028. To support this organic expansion, Moderna, Inc. (NASDAQ:MRNA) also plans to invest approximately $25 billion in research and development between 2024 and 2028.
According to Insider Monkey’s second quarter database, 40 hedge funds were bullish on Moderna, Inc. (NASDAQ:MRNA), same as the last quarter. Philippe Laffont’s Coatue Management held the largest position in the company, with 6.55 million shares worth $795.35 million.
Baron Health Care Fund made the following comment about Moderna, Inc. (NASDAQ:MRNA) in its Q1 2023 investor letter :
“Moderna, Inc. (NASDAQ:MRNA) is a leader in the emerging field of mRNA-based vaccines and therapeutics and was one of the three main producers of the COVID vaccine. Shares fell during the quarter. We believe as COVID shifts away from pandemic status and becomes an increasingly commercial market (rather than government funded), there is increasing investor uncertainty around what a booster market could look like, which is pressuring shares. Looking beyond COVID, we think Moderna has the potential to disrupt the biopharmaceutical industry, from infectious disease vaccines to oncology, and we remain shareholders.”

10. AstraZeneca PLC (NASDAQ: AZN )
Number of Hedge Fund Holders: 41
AstraZeneca PLC (NASDAQ:AZN), a biopharmaceutical company, engages in the research, development, manufacturing, and distribution of prescription medications. The company's portfolio of marketed products includes medications such as Calquence, Enhertu, Faslodex, Imfinzi, Iressa, Koselugo, Lumoxiti, Lynparza, Orpathys, Tagrisso, and Zoladex for oncology. It is one of the best cancer stocks to buy. AstraZeneca PLC (NASDAQ:AZN) is also involved in the development of treatments for a range of conditions, including cardiovascular, renal, and metabolic diseases, respiratory and immunological disorders, rare diseases, influenza, and COVID-19. On August 9, AstraZeneca PLC (NASDAQ:AZN) declared a $0.93 per share interim dividend. The dividend was paid on September 11.
According to Insider Monkey’s second quarter database, 41 hedge funds were bullish on AstraZeneca PLC (NASDAQ:AZN), compared to the last quarter when 39 funds had invested in the stock. Rajiv Jain’s GQG Partners is the largest stakeholder of the company, with 21.2 million shares valued at $1.52 billion.
Baron Health Care Fund made the following comment about AstraZeneca PLC (NASDAQ:AZN) in its Q1 2023 investor letter :
“We reduced our position in AstraZeneca PLC (NASDAQ:AZN) ahead of a clinical data read-out of a competitor drug that would compete with one of the company’s important drugs.”
9. Illumina, Inc. (NASDAQ: ILMN )
Number of Hedge Fund Holders: 43
Illumina, Inc. (NASDAQ:ILMN) is a company involved in the creation, production, and distribution of tools and systems used in the extensive examination of genetic differences and biological functions. It operates through two segments, Core Illumina and GRAIL. The company offers a multi-cancer early detection test called Galleri, in addition to a range of instruments and materials for sequencing and genetic analysis. On August 9, Illumina, Inc. (NASDAQ:ILMN) announced results for the second quarter of 2023. The company reported a non-GAAP EPS of $0.32 and a revenue of $1.18 billion, exceeding estimates by $0.30 and $11.11 million, respectively.
According to Insider Monkey’s second quarter database, 43 hedge funds were bullish on Illumina, Inc. (NASDAQ:ILMN). 44 funds had invested in the stock during the prior quarter. Select Equity Group is the largest position holder in the company, with 1.5 million shares worth $280 million.
RiverPark Large Growth Fund made the following comment about Illumina, Inc. (NASDAQ:ILMN) in its Q2 2023 investor letter :
“Illumina, Inc. (NASDAQ:ILMN): Illumina was our top detractor in the quarter despite reporting first quarter results that were generally in line with expectations and reaffirming full-year guidance. Uncertainty around activist investor Carl Icahn’s impact on the business, the change of CEO and the possible forced divestiture of liquid biopsy subsidiary Grail (early-stage cancer screening via blood samples) all weighed on Illumina’s stock price. We continue to view the company’s core genomics industry as offering one of the larger total addressable markets that we cover, and ILMN is the clear innovation leader in sequencing and array-based solutions for genetic analysis. With less than 0.02% of humans having been sequenced and 99% of the variants discovered in the genome having not yet been deciphered, Illumina, at less than $5 billion of TTM revenue, is still in its infancy in what is potentially a greater than $50 billion genetics analysis tools market opportunity. We believe Carl Icahn’s involvement is neutral to slightly positive to the extent he can help the company be more disciplined on expenses. We are cautiously optimistic that EU regulators’ push to force Illumina to divest Grail will lead to either or both 1) much higher core earnings or 2) a big valuation for Grail in a sale. We added to our ILMN position during the quarter; it is a core holding.”
8. Jazz Pharmaceuticals plc (NASDAQ: JAZZ )
Number of Hedge Fund Holders: 44
Next on our list of the best cancer stocks is Jazz Pharmaceuticals plc (NASDAQ:JAZZ), which is a biopharmaceutical firm engaged in the discovery, advancement, and distribution of pharmaceutical products to address unmet medical demands worldwide. The company's product portfolio is primarily focused on two areas – oncology, covering hematologic and solid tumors; and neuroscience, which includes sleep medicine and movement disorders. On August 9, Jazz Pharmaceuticals plc (NASDAQ:JAZZ) reported a Q2 non-GAAP EPS of $4.51 and a revenue of $957.32 million, outperforming Street consensus by $0.08 and $15.16 million, respectively.
According to Insider Monkey’s second quarter database, 44 hedge funds were bullish on Jazz Pharmaceuticals plc (NASDAQ:JAZZ), up from 40 funds in the last quarter. Robert Pohly’s Samlyn Capital held the largest position in the company, with 1.35 million shares worth $166.9 million.
7. Regeneron Pharmaceuticals, Inc. (NASDAQ: REGN )
Number of Hedge Fund Holders: 56
Regeneron Pharmaceuticals, Inc. (NASDAQ:REGN) is one of the best cancer stocks. It is a global biopharmaceutical company dedicated to the discovery, innovation, development, manufacturing, and distribution of medications designed to combat a range of diseases. The company’s product portfolio includes ZALTRAP, an intravenous infusion for metastatic colorectal cancer, and it is working on developing new treatments for different medical conditions, including cancer, hematologic disorders, eye disorders, allergies, inflammation, cardiovascular ailments, infectious diseases, rare disorders, and pain management, among others. On August 3, Regeneron Pharmaceuticals, Inc. (NASDAQ:REGN) announced a Q2 non-GAAP EPS of $10.24 and a revenue of $3.16 billion, outperforming market estimates by $0.34 and $140 million, respectively.
According to Insider Monkey’s second quarter database, 56 hedge funds were bullish on Regeneron Pharmaceuticals, Inc. (NASDAQ:REGN), compared to 54 funds in the prior quarter. John Overdeck and David Siegel’s Two Sigma Advisors is a significant stakeholder of the company, with a position comprising 299,300 shares worth $215.06 million.
Bronte Capital made the following comment about Regeneron Pharmaceuticals, Inc. (NASDAQ:REGN) in its Q3 2022 investor letter :
“There have been some bright spots in our long book. Regeneron Pharmaceuticals, Inc. (NASDAQ:REGN), a major position and a stock we wrote up in our June 2021 letter, has been one of the best performing stocks in the S&P 500 this year. Alas it has not been enough to offset some of our weaker stocks, let alone our overweight exposure to the UK (and Europe) which have suffered from both stock and currency weakness. We do not think we are bad at picking stocks on the long side and hope – reasonably we think – for better relative results in the future. Prior to COVID, our longs were markedly better than the index. Unfortunately, if you look at our long book this quarter and since the onset of the COVID pandemic, there is scant evidence that we have added any value by picking stocks to go long.”
6. Amgen Inc. (NASDAQ: AMGN )
Number of Hedge Fund Holders: 57
Amgen Inc. (NASDAQ:AMGN) is a global biopharmaceutical company involved in the research, development, manufacturing, and distribution of human therapeutics. The company mainly focuses on oncology/hematology, inflammation, bone health, cardiovascular diseases, nephrology, and neuroscience. Amgen Inc. (NASDAQ:AMGN) primarily serves healthcare providers, which include physicians, clinics, dialysis centers, hospitals, and pharmacies. The products are distributed through pharmaceutical wholesale distributors and direct-to-consumer channels. On September 1, Horizon Therapeutics Public Limited Company (HZNP) saw a 3% increase in premarket trading following the settlement between Amgen Inc. (NASDAQ:AMGN) and the Federal Trade Commission concerning Amgen's proposed $28 billion acquisition of Horizon Therapeutics Public Limited Company (HZNP).
According to Insider Monkey’s second quarter database, 57 hedge funds were bullish on Amgen Inc. (NASDAQ:AMGN), same as the preceding quarter. John Overdeck and David Siegel’s Two Sigma Advisors is the largest stakeholder of the company, with 1.6 million shares valued at $355.9 million.
Like Pfizer Inc. (NYSE:PFE), Merck & Co., Inc. (NYSE:MRK), and Johnson & Johnson (NYSE:JNJ), Amgen Inc. (NASDAQ:AMGN) is one of the best cancer stocks to buy.
ClearBridge Large Cap Value Strategy made the following comment about Amgen Inc. (NASDAQ:AMGN) in its Q2 2023 investor letter :
“In health care we exited global biotechnology company Amgen Inc. (NASDAQ:AMGN). Similar to many of its large cap pharma/biotech peers, Amgen is facing patent cliffs for its major drugs in the second half of the decade, while the company’s pipeline has disappointed. In addition, Amgen is facing above-average pricing pressure impacting a number of its current products that compete in crowded therapeutic areas. Amgen is now relying more heavily on acquisitions to address its patent cliff and weak internal pipeline. However, Amgen’s recently proposed $28 billion acquisition of Horizon Therapeutics is being challenged by the FTC, creating further uncertainty.”
Click to continue reading and see 5 Best Cancer Stocks To Buy Now .
Suggested articles:
12 Best Booming Stocks to Buy Now According to Hedge Funds
10 Best Small-Cap Casino Stocks Hedge Funds Are Buying
11 Best Stocks to Buy in Falling Markets According to Hedge Funds
Disclosure: None. 14 Best Cancer Stocks To Buy Now is originally published on Insider Monkey.
- Get Benzinga Pro
- Data & APIs
- Our Services
- News Earnings Guidance Dividends M&A Buybacks Legal Interviews Management Offerings IPOs Insider Trades Biotech/FDA Politics Government Healthcare
- Markets Pre-Market After Hours Movers ETFs Forex Cannabis Commodities Binary Options Bonds Futures CME Group Global Economics Previews Small-Cap Real Estate Cryptocurrency Penny Stocks Digital Securities Volatility
- Ratings Analyst Color Downgrades Upgrades Initiations Price Target
- Ideas Trade Ideas Covey Trade Ideas Long Ideas Short Ideas Technicals From The Press Jim Cramer Rumors Best Stocks & ETFs Best Penny Stocks Best S&P 500 ETFs Best Swing Trade Stocks Best Blue Chip Stocks Best High-Volume Penny Stocks Best Small Cap ETFs Best Stocks to Day Trade Best REITs
- Money Investing Cryptocurrency Mortgage Insurance Yield Personal Finance Forex Startup Investing Real Estate Investing Credit Cards
- Cannabis Cannabis Conference News Earnings Interviews Deals Regulations Psychedelics
10 Biopharmaceutical Companies Trying To Cure Cancer
Cancer. Research shows nearly every sixth death in the world is due to the disease, making it the second leading cause of death behind cardiovascular diseases.
The global pharmaceutical industry is estimated at an astounding $1.11 trillion in size. Every year, billions are spent on the research and development of cancer drugs, with numerous trials conducted in an attempt to find a cure for various types of cancer. Only a small percentage will gain regulatory approval to be used by patients to treat disease and improve quality of life.
A number of well-known global pharmaceutical giants such as AstraZeneca plc AZN , Pfizer Inc. PFE , Roche Holdings AG Basel ADR RHHBY and Novartis AG NVS are huge players.
The difference between biopharmaceuticals and traditional pharmaceuticals is the method by which the drugs are produced.
Biopharmaceuticals are manufactured in living organisms such as bacteria, yeast and mammalian cells, while pharmaceuticals are manufactured through chemical synthesis.
AstraZeneca’s new cancer research chief José Baselga has said he wants the company to prioritize early stage cancers over advanced disease when developing new cancer drugs, according to The Wall Street Journal .
The following is a look at biopharmaceutical companies working on cures for cancer.
Can-Fite Biopharma Ltd CANF develops treatments for autoimmune inflammatory diseases and cancer. The company's liver cancer study of the drug namodenoson was recently selected by the International Liver Cancer Association conference.
A Phase 3 trial for namodenoson in advanced liver cancer is now in the works.
ESSA Pharma Inc. EPIX engages in the development of small-molecule drugs for prostate cancer. The company recently acquired the U.S. biopharmaceutical company Realm Therapeutics plc RLM .
Merck & Co., Inc . MRK is recognized for its cancer research and recently announced it will acquire the clinical-stage biopharmaceutical company Peloton Therapeutics for $1.05 billion cash upfront and a further $1.15 billion in if certain regulatory and sales milestones are reached. The company is committed to accelerating the development of candidates targeting HIF-2α to help patients with advanced cancers and other diseases.
Genocea Biosciences GNCA is a biopharmaceutical company developing personalized cancer immunotherapies. The company recently announced that the Journal of Clinical Oncology has selected the company’s GEN-009 clinical results presentation at the 2019 ASCO Annual Meeting as a top 10 featured abstract in immuno-oncology.
Heat Biologics Inc. HTBX is a biopharmaceutical company developing therapies designed to activate a patient's immune system against cancer.
Synlogic, Inc. SYBX is a clinical-stage company applying synthetic biology to beneficial microbes to develop novel, living medicines. The company recently announced a platform collaboration to speed up the development of Synlogic’s pipeline of synthetic biotic medicines using Ginkgo’s cell programming platform.
Synthetic biotic medicines are microbes genetically engineered to counter the biology that drives diseases such as hyperammonemia and forms of cancer.
Xynomic Pharmaceuticals Holdings, Inc. XYN is a clinical stage Sino-American oncology drug development company. The company recently held a pre-IND meeting with the Food and Drug Administration for its pan-PAF inhibitor XP-102 for the treatment of cancer.
Xynomic said it's on track to file an Investigational New Drug application in the second half of 2019. XP-102 holds potential as an innovative therapy against B-RAF V600 mutated solid tumors including CRC and non-small cell lung cancer and hairy cell leukemia.
BridgeBio Pharma BBIO focuses on identifying and advancing transformative medicines to treat diseases that arise from defects in a single gene, and for cancers with clear genetic drivers.
Pharmaceutical company Array BioPharma Inc’s two drugs, Braftovi and Mektovi, are approved to treat skin cancer patients with certain genetic alterations. The company recently announced that investors may have to wait longer for results from Array's BeaconCRC colon cancer study.
Pfizer is a well-known American multinational pharmaceutical corporation. The company recently received approval from the European Commission for TALZENNA, a breast cancer drug.
The medicine was approved by the Food and Drug Administration in October 2018. In June, Pfizer announced it would acquire Array BioPharma for $48 per share in cash for a total of around $11.4 billion.
These are only a handful of the companies tackling the disease. Cancer is a global issue, and remedies are being developed in Asia, the Middle East, Europe and South America.
The cure for cancer is possible, and for the optimistic observer, it's not a question of if, but when.
Related Links:
TrovaGene Shares Volatile Ahead of Oncology Presentation
AstraZeneca Success On Prostate Cancer Drug Lowers Enthusiasm For Clovis
© 2024 Benzinga.com. Benzinga does not provide investment advice. All rights reserved.
Trade confidently with insights and alerts from analyst ratings, free reports and breaking news that affects the stocks you care about.

Select "Patients / Caregivers / Public" or "Researchers / Professionals" to filter your results. To further refine your search, toggle appropriate sections on or off.
American Association for Cancer Research (AACR)
Read inspiring stories from survivors benefiting from research-driven advances against cancer, like brain cancer survivor Lanette Veres of Cave Creek, Arizona.

Support Lifesaving Cancer Research. Donate Now.

Thanks to spectacular research-driven advances, more than 18 million people in the United States and millions more worldwide are cancer survivors living with, through, and beyond their disease.
What is Cancer Precision Medicine? Research has powered an explosion in our understanding of the biology of cancer genomics and is leading to tailored treatments for patients.

More than 106,000 people in the U.S. are expected to be diagnosed with melanoma this year. May is Melanoma and Skin Cancer Awareness Month. Learn more about the prevention, screening, and treatment options.

Making Treatment Decisions: Abigail Johnston shares how she has approached decisions about her cancer care since she was diagnosed with metastatic breast cancer in 2017.

The Week in Cancer News: A roundup of significant cancer research news from the past week, selected by the staff of Cancer Today magazine.

Whether honoring a special person or a special day, a donation to the American Association for Cancer Research has a lasting impact.

The official news website of the AACR Annual Meeting 2024. Stay up to date on the latest developments from the most important cancer meeting in the world.
The AACR Cancer Progress Report 2023 provides a comprehensive overview of the latest research-driven advances against the collection of devastating diseases called cancer.
The AACR and its more than 58,000 members worldwide are advancing a scientifically bold agenda against the collection of diseases we call cancer.
A new wave of research-driven discoveries and technological innovations are delivering – and will propel additional – transformative advances to save more lives from cancer..
By the Numbers
percent decrease of the overall age-adjusted cancer death rate in the U.S. from 1991 to 2020
therapeutics were approved for new or expanded uses by the FDA from Aug. 1, 2022, to July 31, 2023
million cancer survivors in the U.S. are living with, through, and beyond their disease thanks to research
cancer diagnoses in the United States are associated with preventable risk factors
Your donation to the American Association for Cancer Research helps our more than 58,000 members worldwide drive progress against cancer.

- The Progression of Cancer
- Patient Advocacy
- Ductal Carcinoma In Situ: The Weight of the...
- Australia North America World
Investing News Network Your trusted source for investing success
- Top Weekly TSX Stocks
- Top Weekly TSXV Stocks
- Top Gold Stocks
- Top Junior Gold Stocks
- Top Copper Stocks
- Top Lithium Stocks
- Top Rare Earth Stocks
- Top Graphite Stocks
- Top Cobalt Stocks
- Top Oil and Gas Stocks
- Biggest Silver Stocks
- Biggest Hydrogen Stocks
- Top Generative AI Stocks
- Top EV Stocks
- Biggest AI Companies
- Biggest Blockchain Stocks
- Biggest Cryptocurrency-mining Stocks
- Biggest Cybersecurity Companies
- Biggest Robotics Companies
- Biggest Social Media Companies
- Biggest Technology ETFs
- Artificial Intellgience ETFs
- Robotics ETFs
- Canadian Cryptocurrency ETFs
- Cannabis Weekly Round-Up
- Top Alzheimer's Treatment Stocks
- Top Biotech Stocks
- Top Plant-based Food Stocks
- Biggest Cannabis Stocks
- Biggest Pharma Stocks
- Biggest Stem Cell Stocks
- Longevity Stocks to Watch
- Psychedelics Stocks to Watch
- Small Biotech ETFs to Watch
- Top Life Science ETFs
- Biggest Pharmaceutical ETFs
- Market Outlook Reports
- Outlook Reports (Australia Ed.)
- Investing Guides
- Investing Guides (Australia Ed.)
How Would a New BRICS Currency Affect the US Dollar? (Updated 2024)
Could the silver price really hit us$100 per ounce (updated 2024), top 10 copper-producing companies (updated 2024), investing in graphene companies, top 10 countries for natural gas production (updated 2024), can you invest in elon musk’s neuralink, 7 biggest lithium-mining companies, where does tesla get its lithium (updated 2024), rare earths reserves: top 8 countries (updated 2024), osisko metals announces updated mineral resource estimate at gaspé copper - indicated resource of 495 mt grading 0.37% copper equivalent, next generation dle provider electralith produces 99.9% pure battery-grade lithium hydroxide from mandrake brine, energy fuels announces q1-2024 results, including continued net income, continued successful uranium ramp-up, commissioning rare earth oxides production, and steps to secure world-scale sources of heavy mineral sands and monazite, correction - brunswick exploration drills 93.45 meters at 1.55% li2o at mr-6, far northern resources: newly listed australia-based gold, copper explorer, western copper and gold announces completion of c$5 million financing with rio tinto, firebird metals, osisko metals, element79 gold corp., energy fuels, lithium outlook for australia, 2024 gold outlook report, 2024 battery metals outlook: australia edition, 2024 lithium market outlook (updated for q2).
- Precious Metals
- Battery Metals
- Base Metals
- Oil and Gas
- Critical Metals
- Rare Earths
- Industrial Metals
- Agriculture
- Resource Companies
- Private Placements
- Press Releases
- CEO Interviews
- Uranium Stocks Index
- Lithium Outlook
- Oil and Gas Outlook
- Gold Outlook Report
- Uranium Outlook
- Rare Earths Outlook
- All Outlook Reports
- Start Here: Investing in Gold
- Start Here: Investing in Silver
- Start Here: Investing in Uranium
- Start Here: Investing in Lithium
- Start Here: Investing in Oil & Gas
- All Investing Guides
- Artificial Intelligence
- Blockchain & Crypto
- Cybersecurity
- Emerging Tech
- Gaming & Esports
- Nanoscience
- Tech Companies
- Artificial Intelligence Outlook
- Cleantech Outlook
- Crypto Outlook
- Tech Outlook
- All Market Outlook Reports
- Start Here: Investing in AI
- Start Here: Investing in Cleantech
- Start Here: Robotics
- Start Here: Investing in Emerging Tech
- Start Here: Investing in Esports
- Pharmaceutical
- Psychedelics
- Medical Devices
- Life Science Companies
- Life Science Outlook
- Biotech Outlook
- Cannabis Outlook
- Pharma Outlook
- Psychedelics Outlook
- Start Here: Investing in Biotech
- Start Here: Investing in Pharma
- Start Here: Investing in Psychedelics
- Start Here: Investing in Cannabis
- Start Here: Investing in Genetics
- Oil & Gas
Oncology Stocks: 8 Biggest NASDAQ Companies in 2023
Investors interested in the life science sector should know about key oncology companies. Here the Investing News Network highlights eight on the NASDAQ.

The wide-ranging oncology market covers every area of cancer care, from diagnosis to treatment.
Coming in only after cardiovascular disease, cancer is the second leading cause of death worldwide; unsurprisingly, oncology is one of the biggest sectors in the life science space. With that in mind, biotechnology and pharmaceutical companies alike are working to develop best-in-class therapeutics for the treatment of various cancers, including lung, breast and prostate cancer.
At this point, their work is far from finished — Precedence Research projects that the global oncology market will increase at a compound annual growth rate of 8.2 percent to reach US$581.25 billion in 2030.
As the global oncology market grows, investors who want exposure to companies working to treat cancer should consider taking a look at biotech and pharma companies with a focus on oncology drugs and testing.
This list of the biggest oncology stocks on the NASDAQ was generated using TradingViews's stock screener . The companies are listed in order of market cap, and all figures below were current as of January 31, 2023.
1. AstraZeneca (NASDAQ:AZN)
Market cap: US$204.99 billion; current share price: US$65.37
First on this list of the top NASDAQ oncology companies by market cap is multinational pharma and biotech firm AstraZeneca, which also specializes in several other therapeutic areas, including cardiovascular, respiratory, central nervous system and pain control. The company is aiming to strengthen its position in the oncology market by more than doubling its cancer drug offerings by 2030.
AstraZeneca has several partnerships with other pharma companies, including Merck (NYSE: MRK ). The pair are currently working on a broad clinical trial development program for LYNPARZA to better understand how the drug may affect multiple PARP-dependent tumors as a monotherapy and in combination across multiple cancer types, including metastatic castration-resistant prostate cancer.
2. Amgen (NASDAQ:AMGN)
Market cap: US$134.68 billion; current share price: US$252.4
One of the world's leading independent biotechnology companies, Amgen uses advanced human genetics to develop and manufacture therapeutics targeting oncological diseases, including a range of solid tumors and hematologic malignancies.
Amgen's oncology portfolio includes LUMAKRAS, which has demonstrated a positive benefit-risk profile in patients with locally advanced or metastatic non-small cell lung cancer harboring the KRAS G12C mutation. The company is advancing a robust pipeline with several mid- to late-stage candidates, including drug candidates targeting leukemia, colorectal cancer and solid tumors.
3. Sanofi (NASDAQ:SNY)
Market cap: US$122.01 billion; current share price: US$49.14
Based in France, Sanofi is developing new technologies based on molecular oncology, immuno-oncology and genomic medicine platforms targeting some of the most difficult-to-treat cancers. The company's oncology strategy encompasses four disease areas: blood cancers, including multiple myeloma; skin cancers; lung cancers; and breast cancer and other hormone-positive cancers.
Sanofi's oncology pipeline includes 10 drug candidates in clinical development and four additional new molecular entities set to enter clinical development in the next few years. The company's tusamitamab ravtansine is the most clinically advanced treatment to target anti-carcinoembryonic antigen-related cell adhesion molecule 5, which plays a key role in a variety of cancer cell types.
4. Gilead Sciences (NASDAQ:GILD)
Market cap: US$105.28 billion; current share price: US$83.94
Global biopharmaceutical company Gilead Sciences is in the business of developing breakthrough medicines to prevent and treat serious conditions such as HIV, viral hepatitis and cancer. One of the company's biggest successes is Yescarta, a CAR-T cell therapy for blood cancer and the first such therapy for certain types of non-Hodgkin's lymphoma.
Yestcarta recently garnered approval in Japan for the initial treatment of relapsed/refractory large B-cell lymphoma. The European Medicines Agency has also validated the Marketing Authorization Application for Gilead’s second-line cancer treatment Trodelvy for pre-treated HR+/HER2- metastatic breast cancer.
5. Regeneron Pharmaceuticals (NASDAQ:REGN)
Market cap: US$82.59 billion; current share price: US$758.47
Biotech leader Regeneron Pharmaceuticals develops and commercializes medicines targeting cancer, pain and a wide variety of diseases, including inflammatory, cardiovascular, metabolic, hematologic and rare diseases. Its drug candidate portfolio includes 17 clinical-stage programs targeting various cancers, including solid tumors, prostate cancer, cervical cancer and metastatic melanoma.
In 2022, Regeneron acquired immuno-oncology company Checkmate Pharmaceuticals, whose lead investigational candidate, vidutolimod, has demonstrated clinical responses as a monotherapy in patients with PD-1 refractory melanoma.
6. Moderna (NASDAQ:MRNA)
Market cap: US$67.86 billion; current share price: US$176.06
Moderna is a leader in applied mRNA science with a diverse clinical portfolio of vaccines and therapeutics. Its mRNA platform harnesses the body's immune system to identify and kill cancer cells, including individualized mRNA-based personalized cancer vaccines.
Through its partnership with Merck , pharma giant Moderna is advancing a novel investigational mRNA-based personalized cancer vaccine consisting of a single synthetic mRNA coding for up to 34 neoantigens that is designed and produced based on the unique mutational signature of the DNA sequence of the patient's tumor.
7. Illumina (NASDAQ:ILMN)
Market cap: US$33.69 billion; current share price: US$214.20
Illumina develops, manufactures and markets life science tools and integrated systems that enable the implementation of genomic solutions for the healthcare sector with a focus on oncology testing, genetic disease testing, reproductive health and research. The company is expanding its next-generation sequencing oncology portfolio to help clinical cancer researchers estimate tumor mutational burden, identify neoantigens and study innovative therapies to boost the immune response.
Illumina has a partnership with Bristol-Meyers Squibb (NYSE: BMY ) to collaborate to develop and commercialize companion diagnostics for oncology immunotherapies. More recently, the company partnered with AstraZeneca on a drug discovery project that harnesses the power of artificial intelligence technology.
8. BeiGene (NASDAQ:BGNE)
Market cap: US$27.2 billion; current share price: US$256.00
Last on this list of top NASDAQ oncology companies by market cap is BeiGene, a biotechnology company that specializes in the development of drugs for cancer treatment. The company's clinical development pipeline includes 12 advanced Phase 3 programs across a broad range of cancer areas, including esophageal squamous cell carcinoma, non-small cell lung cancer, mantle cell lymphoma, non-Hodgkin's lymphoma and ovarian cancer.
BeiGene's bruton tyrosine kinase inhibitor Brukinsa recently gained US Food and Drug Administration approval for patients with chronic lymphocytic leukemia or small lymphocytic leukemia, both forms of non-Hodgkin's lymphoma.
This is an updated version of an article first published by the Investing News Network in 2018.
Don’t forget to follow us @INN_LifeScience for real-time updates!
Securities Disclosure: I, Melissa Pistilli, hold no direct investment interest in any company mentioned in this article.
- 5 Small Biotech ETFs ›
- 5 Biggest Biotechnology ETFs in 2022 ›
- Biotech Stocks: 5 Biggest Companies in 2022 ›
- How to Invest in Biotechnology ›
- Biotech Market Forecast: 3 Top Trends That Will Affect Biotech in 2023 ›
- 'The Top Line': Inflation Reduction Act, and biotech's outlook ›
- Pharmaceutical & life sciences: US Deals 2023 outlook: PwC ›

Melissa Pistilli
Educational Content Specialist
Melissa Pistilli has been reporting on the markets and educating investors since 2006. She has covered a wide variety of industries in the investment space including mining, cannabis, tech and pharmaceuticals. She helps to educate investors about opportunities in a variety of growth markets. Melissa holds a bachelor's degree in English education as well as a master's degree in the teaching of writing, both from Humboldt State University, California.
Latest News
Sirona biochem engages stonegate healthcare partners, outlook reports.
- Pharmaceuticals
Featured Biotech Investing Stocks
Sirona biochem, hydralyte international, browse companies, commodities.
Investing News Network websites or approved third-party tools use cookies. Please refer to the cookie policy for collected data, privacy and GDPR compliance. By continuing to browse the site, you agree to our use of cookies.
Learn about our editorial policies.
New on NCI’s Websites for April 2024
April 12, 2024 , by Daryl McGrath
The Cancer Screening Research Network will conduct rigorous, multicenter cancer screening trials.
NCI’s collection of cancer information products is constantly growing, so periodically we provide updates on new and updated content of interest to the cancer community.
NCI Launches Research Network to Evaluate Emerging Cancer Screening Technologies
The Cancer Screening Research Network (CSRN) will support studies that investigate new ways to identify cancers earlier , when they may be easier to treat. In late 2024, CSRN plans to launch the Vanguard Study on Multi-Cancer Detection, a pilot study to inform the design of a larger trial of multi-cancer detection tests. Multi-cancer detection tests measure biomarkers, such as pieces of DNA, that cancer cells release into the blood and can potentially be used to detect many kinds of cancer.
For Childhood Cancer Survivors, Inherited Genetic Factors Influence Risk of Cancers Later in Life
Common inherited genetic factors that predict cancer risk in the general population may also predict elevated risk of new cancers among childhood cancer survivors , according to a study led by researchers at NCI.
NCI Launches Virtual Clinical Trials Office
NCI has launched the Virtual Clinical Trials Office , a centralized team of support staff—including research nurses, clinical research associates, and clinical data specialists—who will work remotely to assist NCI-Designated Comprehensive Cancer Centers and community practices with their clinical trials activities.
Meet the NCI Director Video
During a live social media event that took place February 22, NCI Director Kimryn Rathmell, M.D., Ph.D., introduced herself and answered live questions about the future of cancer research.
President’s Cancer Panel Issues Progress Report on the National Cancer Plan
On February 28, the President’s Cancer Panel released its I nitial Assessment of the National Cancer Plan . This report examines the National Cancer Plan as a road map for the National Cancer Program and offers recommendations in five priority areas to accelerate progress toward the plan’s goals.
Small Business Innovation Research Program
NCI’s Small Business Innovation Research (SBIR) and Small Business Technology Transfer (STTR) programs support small businesses across the United States to develop innovative cancer technologies. The SBIR Development Center has a new website that features the center’s SBIR Innovation Lab podcast , highlights success stories , and showcases companies chosen to pitch for funding at investor events.
Progress in Basic Cancer Research
NCI’s Division of Cancer Biology (DCB) features recent progress in advancing basic cancer research . A new infographic shows progress made in fiscal year 2023, highlights of recent research, and examples of DCB-sponsored research intended to help achieve the goals of the National Cancer Plan.
Center for Cancer Research Updates
NCI’s Center for Cancer Research (CCR) has added and updated several web pages. CCR’s training page for postdoctoral researchers features a new video explaining the benefits of postdoc training with NCI tenure-track investigators.
CCR also added a new chromosome biology research section to its website. The section includes a new web page for the NCI Center of Excellence in Chromosome Biology , information about specific researchers working on chromosome biology, and related fellowships and jobs in CCR.
CCR also has released its annual Milestones publication. The 10 stories highlighted in this year’s publication illustrate the breadth, creativity, and impact of CCR research.
Why We Love Diverse Data
NCI’s Center for Biomedical Informatics and Information Technology celebrated “Love Data Week!” (February 12–16) with a blog post featuring six scientists talking about why they love diverse data .
Inside Cancer Careers Podcast
NCI’s Center for Cancer Training has launched the second season of the Inside Cancer Careers podcast . The February 1 episode was a “fireside chat” with NCI Director Dr. Kimryn Rathmell , who shared her career journey and the importance of supporting the next generation of cancer researchers. The February 15 episode featured Ophira Ginsburg, M.D. , senior scientific officer and senior advisor for clinical research in NCI’s Center for Global Health, who gave her thoughts on the importance of women's leadership and representation in cancer care.
World Cancer Day 2024 Blog
NCI’s Center for Global Health (CGH) marked World Cancer Day with a blog post by CGH Director Satish Gopal, M.D., M.P.H. Dr. Gopal highlighted important accomplishments and activities in 2023, including CGH’s ongoing NCI Global Cancer Research and Control Seminar Series , NCI support and participation in the November 2023 African Organization for Research and Training in Cancer (AORTIC) meeting in Senegal, initiation of cancer health disparities minilabs to develop transdisciplinary approaches to advance scientific progress globally, and leadership and launch of the Lancet Commission on Women, Cancer, and Power .
NCI Office of Cancer Survivorship – New Resources
The NCI’s Office of Cancer Survivorship (OCS) has revamped its Cancer Survivor and Caregiver Stories page . Along with adding caregiver stories, the page also enables readers to filter stories—such as by cancer type and specific populations—to select those that will be most meaningful to them. Also new is a video library that includes videos sharing the words of advocates on survivorship-related topics and an FAQ with OCS Director Emily Tonorezos, M.D.
HINTS Brief 53 on Telehealth Use Among US Adults
The Health Information National Trends Survey (HINTS) Management Team has released HINTS Brief 53, “Patterns and Predictors of Telehealth Use among US Adults in 2022 .” This brief discusses the impact of the COVID-19 pandemic on the expansion of telehealth; the prevalence of, and factors associated with, telehealth use; and more.
Updated: Catchment Areas of NCI-Designated Cancer Centers
NCI’s Division of Cancer Control and Population Sciences updated its page on the Catchment Areas of NCI-Designated Cancer Centers , including designation status and catchment area updates for several cancer enters.
Co-Use of Tobacco with Alcohol and Cannabis
The NCI’s Behavioral Research Program has launched a new web page on the co-use of tobacco with alcohol and cannabis . This page, created by program directors in the Health Behaviors Research Branch and the Tobacco Control Research Branch, discusses the importance of addressing co-use of tobacco with alcohol and tobacco with cannabis as targets for cancer prevention and control.
Smokefree.gov Launches SmokefreeNATIVE
NCI’s Smokefree.gov initiative has partnered with the Indian Health Service to launch a new, free text messaging resource to help American Indian and Alaska Native adults and adolescents quit smoking commercial tobacco. People who smoke and are ready to set a date to quit can enroll online or by texting NATIVE to 47848.
Participate in Cancer Research
NCI has enhanced its information about participating in cancer research studies, including how to join clinical trials or observational studies and how to donate medical data and biological samples. The new Participate in Cancer Research section of NCI’s website includes information about cancer research studies , a clinical trials search tool , personal stories from people who have participated in studies, and more.
Lymphedema, the buildup of lymph fluid in tissues causing swelling, affects people’s ability to do certain activities and their quality of life. NCI has revised and consolidated its expert-reviewed content about ways to prevent and treat lymphedema .
The ALCHEMIST Lung Cancer Trials
The ALCHEMIST clinical trials are a group of randomized clinical trials for people with early-stage non-small cell lung cancer whose tumors have been completely removed by surgery. People entering the ALCHEMIST study will have their tumor tissue genetically sequenced to determine if one of the drugs being studied targets a specific biomarker, including certain genetic mutations, found in their tumors. This revised and streamlined page describes the different trials and which are currently recruiting participants.
Updated Fact Sheets
Tumor Markers : This updated fact sheet defines tumor markers and describes how they can be used to help diagnose and treat cancer. This revised and streamlined page also includes a list of tumor marker tests in common use .
Computed Tomography (CT) Scans and Cancer : This updated page explains what CT scans are, how they're used for cancer screening, diagnosis, and treatment, and what people can expect during the procedure.
New and Updated Patient Information Summaries
Skin Cancer Prevention : Skin cancer prevention includes avoiding risk factors like ultraviolet radiation that comes from the sun, sun lamps, and tanning beds. This updated expert-reviewed summary discusses the risks and possible protective factors for skin cancer.
Childhood Colorectal Cancer Treatment : Colorectal cancer is defined as a cancer that starts anywhere along the colon or rectum. Learn about risk factors, symptoms, and tests to diagnose colorectal cancer in children and how it is treated.
Depression : Depression is a treatable medical problem that can affect adults and children with cancer. This updated page describes symptoms of depression, risk factors, diagnosis, and treatment for adults and children with cancer.
Childhood Brain Tumors: NCI’s pages on DIPG (diffuse intrinsic pontine glioma) , childhood ependymoma , and childhood glioma (including astrocytoma) have a new look. The pages cover causes and risk factors, symptoms, screening, diagnosis, prognosis, stages, and treatment.
Spirituality and Cancer Care : Religion and spirituality in cancer care are very personal matters that can affect treatment decisions and the ability to cope. This updated page explains ways a person’s health care team can support their spiritual and religious well-being and how this can lead to improved health and quality of life.
Oral Complications of Cancer Therapies : Mouth and throat problems are common problems of cancer treatment. This updated page explains ways to prevent and manage problems like dry mouth, taste changes, pain, and infection.
Acute Myeloid Leukemia Treatment : Acute myeloid leukemia (AML) is a cancer that causes the rapid growth of abnormal white blood cells. These abnormal cells can replace healthy blood cells, leading to symptoms such as bleeding, anemia, and infection. This recently updated page includes information about the causes, symptoms, diagnosis, and treatment options for AML.
New Health Professional Summary: Hospice
Health care professionals and health care systems face many challenges when caring for patients at the end of life. This new page offers expert-reviewed information about many aspects of hospice care .
Chemotherapy and You : Support for People with Cancer
Chemotherapy and You is a booklet for people who are about to receive or are now receiving chemotherapy for cancer. It includes facts about chemotherapy and its side effects and highlights ways people can care for themselves before, during, and after treatment.
Infographic: Chances of Developing Breast Cancer by Age 70 to 80
Women who have harmful changes in certain genes have a higher risk of some cancers. This updated infographic helps explain the risk of breast cancer for women with harmful BRCA1 and BRCA2 mutations.
Featured Posts
March 27, 2024, by Edward Winstead
March 21, 2024, by Elia Ben-Ari
March 5, 2024, by Carmen Phillips
- Biology of Cancer
- Cancer Risk
- Childhood Cancer
- Clinical Trial Results
- Disparities
- FDA Approvals
- Global Health
- Leadership & Expert Views
- Screening & Early Detection
- Survivorship & Supportive Care
- February (6)
- January (6)
- December (7)
- November (6)
- October (7)
- September (7)
- February (7)
- November (7)
- October (5)
- September (6)
- November (4)
- September (9)
- February (5)
- October (8)
- January (7)
- December (6)
- September (8)
- February (9)
- December (9)
- November (9)
- October (9)
- September (11)
- February (11)
- January (10)
Explore CRI’s 2023 Cancer Research Impact
For a World Immune to Cancer
We established immunotherapy as the fourth pillar in treating cancer for one reason only – to save more lives.
Inventing the Next Wave in Immunotherapy
Breakthrough science has given researchers, clinicians, and patients new hope in the fight against cancer. With recent discoveries in immunotherapy treatments and an increased understanding of the immune system’s role in fighting disease, we are closer than ever before to transforming all cancers into curable diseases.
4M+ PATIENTS
$515m+ invested, the best minds.
Supporting and training scientists today for breakthrough discoveries in immunotherapy. We fund both open-ended laboratory research and data-driven clinical studies and turn discoveries into real-world results that save lives.
Promoting the Power of Our Immune System
CRI funds scientists from around the world to advance new avenues of discovery, new technologies, and new ways to analyze and share data about our immune system and its power to outsmart cancer — with the shared goal of making immunotherapy a lifesaving treatment for all cancers.
The IO Research Intelligence Center
As the science of cancer immunotherapy advances and the amount of data generated in clinical and laboratory studies grows exponentially, CRI is harnessing this information and putting it into the hands of our global community of research scientists to help speed the discovery and development of cancer cures.
Data on its own is not enough. We need to make sense of data within what we already know about cancer immunology. We need to marry data science, bioinformatics, and cancer immunology if we are going to take the next big leap of developing immunotherapies that are effective for all cancers.
Take Action

Make a gift today to make an impact now. Memorial and Honor donations also welcome. Your tax-deductible gift will fuel the discovery and development of powerful immunotherapies for all cancers.

Other Ways to Support CRI
We offer many convenient ways to support our mission. Be a fundraiser. Make a planned gift. Donate a vehicle. Explore all the many ways you can join in our lifesaving work.
Conquering Cancer Together
We cultivate lasting strategic partnerships with research institutions, cancer treatment centers, biopharmaceutical companies, and nonprofits to facilitate innovation in immuno-oncology and advance new lifesaving immunotherapies for more patients.
Annual Reports
CRI’s annual reports recount our accomplishments in the previous fiscal year.

Charity Ratings & Reporting
The Cancer Research Institute is a top-rated nonprofit organization.

Stay current on the latest cancer immunotherapy and CRI developments.

Browse CRI press releases, news articles, and subject matter experts.

Patient Summits
Patient Summits connect cancer patients with scientific and medical experts.

View immunotherapy webinars and how CRI’s work is impacting cancer research.
Bring to Life More Cures, Together
Become part of CRI’s mission to create a world immune to cancer
This website uses tracking technologies, such as cookies, to provide a better user experience. If you continue to use this site, then you acknowledge our use of tracking technologies. For additional information, review our Privacy Policy .
Triomics raises $15M Series A to automate cancer clinical trials matching

For cancer patients, medicines administered in clinical trials can help save or extend lives.
But despite thousands of trials in the United States each year, only 3% to 5% of eligible patients enroll in investigations of new treatments.
Triomics , a generative AI startup, claims it can significantly reduce the time it takes doctors to match patients with trials.
Doctors’ recommendations are often key to getting patients enrolled. However, busy oncologists and nurses often lack the time to learn about all the clinical trials that may be right for their patients.
I am not a doctor, so I don’t know about the day-to-day challenges of oncology medical staff. But I unfortunately know from personal experience how hard it is to find clinical trials for cancer patients. When my father was sick, I spent countless hours poring over clinicaltrials.gov, a website and database that lists thousands of ongoing trials. And just in March, I spent half a Saturday trying to find a clinical trial for a friend who has stage IV cancer. Her doctor only offered one trial, so she asked me if there were other options.
Since most clinical trials have complex criteria, there are often dozens of factors such as stage of cancer, mutations and previous treatments for eligibility. Medical staff often need hours to manually review a patient’s medical record to find a fitting clinical trial. But due to a shortage of oncology professionals, many cancer patients aren’t offered to participate or they miss their eligibility window.
Triomics was founded by former MIT biotech researcher Sarim Khan and AI scientist at Adobe Hrituraj Singh. The pair, who have been friends since college, decided to build Triomics in 2021 after realizing that advances in generative AI and LLMs could help extract data from electronic health records (EHR) to help find appropriate clinical trials for cancer patients in minutes instead of hours.
Khan and Singh entered Y Combinator in the winter of 2021 and proceeded to work on an LLM built specifically for cancer centers and oncology departments in hospital systems.
Three years later, Triomics says six cancer centers and hospitals are actively using or piloting its LLM, and it plans to double that number by the end of the year. And now the company has raised a $15 million Series A from Lightspeed, Nexus Venture Partners, General Catalyst and Y Combinator to help it continue to develop its platform and roll it out to new customers.
While reducing how long it takes for patients to be matched with clinical trials may seem like the most immediately valuable application of Triomics software, Khan says that Triomics is a lot more than a clinical trials company. “Doctors use it for several different use cases that I could just go on and on about,” he said.
After Triomics’ LLM, which the company is calling OncoLLM, “reads” the patient’s medical record, the data could be used to help prepare doctors and other medical staff for patient visits or to help submit cancer data with details of organs affected and stage of progressions to state regulatory agencies.
Of course, Triomics isn’t alone in tackling this area. Other startups doing AI clinical trial matching include Deep 6 AI, QuantHealth , Trajectory, among others .
But Khan believes that Triomics is one of the few startups processing large swaths of datasets specifically for cancer centers.
More TechCrunch
Get the industry’s biggest tech news, techcrunch daily news.
Every weekday and Sunday, you can get the best of TechCrunch’s coverage.
Startups Weekly
Startups are the core of TechCrunch, so get our best coverage delivered weekly.
TechCrunch Fintech
The latest Fintech news and analysis, delivered every Sunday.
TechCrunch Mobility
TechCrunch Mobility is your destination for transportation news and insight.
Women in AI: Rep. Dar’shun Kendrick wants to pass more AI legislation
To give AI-focused women academics and others their well-deserved — and overdue — time in the spotlight, TechCrunch has been publishing a series of interviews focused on remarkable women who’ve contributed to…

A reckoning is coming for emerging venture funds, and that, VCs say, is a good thing
We took the pulse of emerging fund managers about what it’s been like for them during these post-ZERP, venture-capital-winter years.

Workers at a Maryland Apple store authorize strike
It’s been a busy weekend for union organizing efforts at U.S. Apple stores, with the union at one store voting to authorize a strike, while workers at another store voted…

Alora Baby aims to push baby gear away from the ‘landfill economy’
Alora Baby is not just aiming to manufacture baby cribs in an environmentally friendly way but is attempting to overhaul the whole lifecycle of a product

Go on, let bots date other bots
Bumble founder and executive chair Whitney Wolfe Herd raised eyebrows this week with her comments about how AI might change the dating experience. During an onstage interview, Bloomberg’s Emily Chang…

Why Apple’s ‘Crush’ ad is so misguided
Welcome to Week in Review: TechCrunch’s newsletter recapping the week’s biggest news. This week Apple unveiled new iPad models at its Let Loose event, including a new 13-inch display for…

U.K. agency releases tools to test AI model safety
The U.K. Safety Institute, the U.K.’s recently established AI safety body, has released a toolset designed to “strengthen AI safety” by making it easier for industry, research organizations and academia…

At the AI Film Festival, humanity triumphed over tech
AI startup Runway’s second annual AI Film Festival showcased movies that incorporated AI tech in some fashion, from backgrounds to animations.

Women in AI: Rachel Coldicutt researches how technology impacts society
Rachel Coldicutt is the founder of Careful Industries, which researches the social impact technology has on society.

SAP’s chief sustainability officer isn’t interested in getting your company to do the right thing
SAP Chief Sustainability Officer Sophia Mendelsohn wants to incentivize companies to be green because it’s profitable, not just because it’s right.

Tesla’s profitable Supercharger network is in limbo after Musk axed the entire team
Here’s what one insider said happened in the days leading up to the layoffs.

StrictlyVC London welcomes Phoenix Court and WEX
StrictlyVC events deliver exclusive insider content from the Silicon Valley & Global VC scene while creating meaningful connections over cocktails and canapés with leading investors, entrepreneurs and executives. And TechCrunch…
Meesho, an Indian social commerce platform with 150M transacting users, raises $275M
Meesho, a leading e-commerce startup in India, has secured $275 million in a new funding round.

Scammers found planting online betting ads on Indian government websites
Some Indian government websites have allowed scammers to plant advertisements capable of redirecting visitors to online betting platforms. TechCrunch discovered around four dozen “gov.in” website links associated with Indian states,…

Motional cut about 550 employees, around 40%, in recent restructuring, sources say
Around 550 employees across autonomous vehicle company Motional have been laid off, according to information taken from WARN notice filings and sources at the company. Earlier this week, TechCrunch reported…

OpenAI’s ChatGPT announcement: What we know so far
The company is describing the event as “a chance to demo some ChatGPT and GPT-4 updates.”

Pitch Deck Teardown: Cloudsmith’s $15M Series A deck
The deck included some redacted numbers, but there was still enough data to get a good picture.

Anthropic’s Claude sees tepid reception on iOS compared with ChatGPT’s debut
Unlike ChatGPT, Claude did not become a new App Store hit.

Startups Weekly: Trouble in EV land and Peloton is circling the drain
Welcome to Startups Weekly — Haje‘s weekly recap of everything you can’t miss from the world of startups. Sign up here to get it in your inbox every Friday. Look,…

Founders Fund leads financing of composites startup Layup Parts
Scarcely five months after its founding, hard tech startup Layup Parts has landed a $9 million round of financing led by Founders Fund to transform composites manufacturing. Lux Capital and Haystack…

Anthropic now lets kids use its AI tech — within limits
AI startup Anthropic is changing its policies to allow minors to use its generative AI systems — in certain circumstances, at least. Announced in a post on the company’s official…

The buzziest EV IPO of the year is a Chinese automaker
Zeekr’s market hype is noteworthy and may indicate that investors see value in the high-quality, low-price offerings of Chinese automakers.

VC fund performance is down sharply — but it may have already hit its lowest point
Venture capital has been hit hard by souring macroeconomic conditions over the past few years and it’s not yet clear how the market downturn affected VC fund performance. But recent…
Threat actor says he scraped 49M Dell customer addresses before the company found out
The person who claims to have 49 million Dell customer records told TechCrunch that he brute-forced an online company portal and scraped customer data, including physical addresses, directly from Dell’s…

Bluesky now lets you personalize main Discover feed using new controls
The social network has announced an updated version of its app that lets you offer feedback about its algorithmic feed so you can better customize it.

Microsoft is launching its mobile game store in July
Microsoft will launch its own mobile game store in July, the company announced at the Bloomberg Technology Summit on Thursday. Xbox president Sarah Bond shared that the company plans to…

Oura launches two new heart health features
Smart ring maker Oura is launching two new features focused on heart health, the company announced on Friday. The first claims to help users get an idea of their cardiovascular…

This Week in AI: OpenAI considers allowing AI porn
Keeping up with an industry as fast-moving as AI is a tall order. So until an AI can do it for you, here’s a handy roundup of recent stories in the world…

Garena is quietly making India-themed games even as Free Fire’s relaunch remains doubtful
Garena is quietly developing new India-themed games even though Free Fire, its biggest title, has still not made a comeback to the country.

Fisker Ocean faces fourth federal safety probe
The U.S.’ NHTSA has opened a fourth investigation into the Fisker Ocean SUV, spurred by multiple claims of “inadvertent Automatic Emergency Braking.”


- Adolescent and Young Adult Cancer
- Bile Duct Cancer
- Bladder Cancer
- Brain Cancer
- Breast Cancer
- Cervical Cancer
- Childhood Cancer
- Colorectal Cancer
- Endometrial Cancer
- Esophageal Cancer
- Head and Neck Cancer
- Kidney Cancer
- Liver Cancer
- Lung Cancer
- Mouth Cancer
- Mesothelioma
- Multiple Myeloma
- Neuroendocrine Tumors
- Ovarian Cancer
- Pancreatic Cancer
- Prostate Cancer
- Skin Cancer/Melanoma
- Stomach Cancer
- Testicular Cancer
- Throat Cancer
- Thyroid Cancer
- Prevention and Screening
- Diagnosis and Treatment
- Research and Clinical Trials
- Survivorship

Request an appointment at Mayo Clinic

Clinical trials: A significant part of cancer care
Share this:.
Editor's note: May is National Cancer Research Month.
By Mayo Clinic staff
A cancer diagnosis is an emotional experience. Learning that you have cancer can create feelings of hopelessness, fear and sadness. This is especially true if your cancer is advanced or available treatments are unable to stop or slow its growth.
"Often, when patients are diagnosed with cancer , they feel hopeless and scared. Clinical trials are one way patients can be proactive. They can make a choice in how their care is going to be," says Matthew Block, M.D., Ph.D. , a Mayo Clinic medical oncologist.
Cancer clinical trials help physician-scientists test new and better ways to control and treat cancer. During a clinical trial, participants receive specific interventions, and researchers determine if those interventions are safe and effective. Interventions studied in clinical trials might be new cancer drugs or new combinations of drugs, new medical procedures, new surgical techniques or devices, new ways to use existing treatments, and lifestyle or behavior changes.
Clinical trials provide access to potential treatments under investigation, giving options to people who otherwise may face limited choices. "Clinical trials open the door to a new hope that maybe we can fight their cancer back and give them a better quality of life," says Geoffrey Johnson, M.D., Ph.D. , a Mayo Clinic radiologist, nuclear medicine specialist and co-chair of the Mayo Clinic Comprehensive Cancer Center Experimental and Novel Therapeutics Disease Group.
You will receive cancer treatment if you participate in a clinical trial. "I think one common misperception about clinical trials is that if you enter a clinical trial, you may not get treatment (receive a placebo). And that's actually very much not true. Most clinical trials are looking at one treatment compared to another treatment," says Judy C. Boughey, M.D. , a Mayo Clinic surgical oncologist, chair of Breast and Melanoma Surgical Oncology at Mayo Clinic in Rochester, Minnesota, and chair of the Mayo Clinic Comprehensive Cancer Center Breast Cancer Disease Group.
"I think one common misperception about clinical trials is that if you enter a clinical trial, you may not get treatment (receive a placebo). And that's actually very much not true. Most clinical trials are looking at one treatment compared to another treatment." Judy C. Boughey, M.D.
Watch this video to hear the experiences of people who have participated in cancer clinical trials and to hear Drs. Block, Johnson and Boughey discuss the importance of clinical trials in cancer care:
Clinical trials are a significant part of cancer care at Mayo Clinic Comprehensive Cancer Center. Cancer care teams work together across specialties to make sure the right clinical trials are available to serve the needs of people with cancer who come to Mayo Clinic.
"We are very particular in how we select the clinical trials that we have available for patients," says Dr. Boughey. "We want to have the best trials available for our patients. Some of the clinical trials are evaluating drugs — we are so excited about those drugs, but we can't prescribe those drugs for patients without having that trial. And so we will actually fight to try to get that trial open here to have it available as an opportunity for our patients."
If you choose to participate in a clinical trial, you will continue to receive cancer care. "For most patients that we evaluate, there's always the standard of care treatment option for those patients. And then, in many situations, there's also a clinical trial that the patient can participate in," says Dr. Boughey.
People who participate in clinical trials help make new and better cancer care available for future patients. The treatments available for cancer patients today exist because of the clinical trial participants of yesterday. "We couldn't advance medicine if it wasn't for people volunteering for trials. And the promise from our side is to say we're not going to put patients on trials or offer trials for them to consider unless we think there's a good chance that they'll get a benefit or that society at large will get a benefit," says Dr. Johnson.
"We couldn't advance medicine if it wasn't for people volunteering for trials. And the promise from our side is to say we're not going to put patients on trials or offer trials for them to consider unless we think there's a good chance that they'll get a benefit or that society at large will get a benefit." Geoffrey Johnson, M.D., Ph.D.
Participating in a clinical trial may give you access to cutting-edge treatment, improve your quality of life and extend your time with loved ones.
"It's definitely worth reaching out to your healthcare provider and asking, 'What clinical trials could I be a potential candidate for?'" says Dr. Boughey. "And remember, you can ask this of your surgical oncologist, your medical oncologist, your radiation oncologist, or any of the physicians you're seeing because there are trials in all disciplines. There are also ongoing trials that require the collection of tissue or the donation of blood. They can also be important in trying to help future generations as we continue to work to end cancer."
Participating in a clinical trial is an important decision with potential risks and benefits. Explore these FAQ about cancer clinical trials, and ask your care team if a clinical trial might be right for you.
Learn more about cancer clinical trials and find a clinical trial at Mayo Clinic.
Join the Cancer Support Group on Mayo Clinic Connect , an online community moderated by Mayo Clinic for patients and caregivers.
Read these articles about people who have participated in clinical trials at Mayo Clinic:
- A silent tumor, precancerous polyps and the power of genetic screening
- Mayo Clinic’s DNA study reveals BRCA1 mutations in 3 sisters, prompts life-changing decisions
Read more articles about Mayo Clinic cancer research made possible by people participating in clinical trials.
Related Posts

Dr. Sujay Vora is studying a new approach to glioblastoma treatment that is improving health outcomes and quality of life for elderly people like Richard Casper.

Dr. S. John Weroha discusses new treatments and research that are helping more people survive ovarian cancer.

Hypothesis-driven AI offers an innovative way to use massive datasets to help discover the complex causes of diseases such as cancer and improve treatment strategies.

Pfizer Agrees to Settle More than 10,000 Zantac Cancer Suits
(Bloomberg) -- Pfizer Inc. has agreed to settle more than 10,000 cases accusing it of hiding the cancer risks of its Zantac heartburn drug, according to people familiar with the deal, the biggest of the litigation.
The agreements cover cases in state courts across the US but don’t completely resolve the company’s exposure to Zantac claims, according to the people, who spoke on the condition of anonymity because they weren’t authorized to discuss the settlement publicly. Financial details of the accords weren’t immediately available.
The deal is likely to reassure investors, who have seen other Zantac makers, including GSK Plc and Sanofi, sign settlements. Concerns about the drugmakers’ exposure to Zantac suits helped wipe out about $45 billion in combined market value in the summer of 2022. The shares have since recovered and have risen on news of the earlier deals.
Pfizer shares fell 1.5% in trading before US markets opened Thursday. They’ve lost 27% in the past 12 months through Wednesday’s close.
“Pfizer has explored and will continue to explore opportunistic settlements of certain cases if appropriate, and has settled certain cases,” the New York-based company said in an emailed statement. “The company has not sold a Zantac product in more than 15 years and did so only for a limited period of time.”
Settling and Fighting
Bloomberg News reported last month that Sanofi agreed to pay more than $100 million to resolve about 4,000 Zantac cases. Zantac has been owned by different drugmakers in its more than 30-year run as one of the most popular antacids in the US.
The settlements come as GSK defends itself in its first US jury trial over claims it knew Zantac posed a serious risk. In opening statements May 2 in Chicago, a lawyer for the plaintiff blamed corporate greed for his client’s colorectal cancer, while an attorney for GSK told the jurors no scientific studies have linked Zantac to the disease, which affects millions of Americans every year.
GSK has also settled some Zantac cases before they could go to trial.
News of the Pfizer settlement surfaced in a filing in state court in Delaware tied to the Chicago trial. More than 70,000 Zantac suits have been filed in Delaware, where a judge is mulling whether scientific evidence underlying those cases is sufficiently robust to allow them to go to trial.
Telltale Filing
Zantac plaintiffs’ lawyers filed an April 29 notice that the Chicago judge had reviewed the underlying science and cleared the case for trial. They noted that the order applied only to GSK and Boehringer Ingelheim GmbH, which also made Zantac at one point, because “Pfizer had already settled.”
Zantac, developed by GSK and Warner-Lambert, hit the US market as a prescription drug in 1983 before becoming an over-the-counter heartburn treatment in 1996. Sanofi, which acquired it in 2017, recalled it in 2019, about a month after an independent lab released tests showing the likely carcinogen NDMA in the drug and its generics. The lab’s research indicated the drug’s active ingredient, ranitidine, formed NDMA over time or at higher temperatures.
The US Food and Drug Administration confirmed the findings in 2020 and ordered drugmakers to take all versions of the medicine off the market. Sanofi has since returned Zantac to store shelves but without ranitidine. It is now made with famotidine, the active ingredient in competitor Pepcid.
The companies got a big win in 2022 when a federal judge threw out more than 5,000 suits in Florida, saying the science behind the cancer claims was flawed. That decision also applied to about 50,000 unfiled cases covered by a multi-district litigation, according to court filings. Many of those cases later were filed in Delaware.
The main Delaware case is In Re Zantac, N22C-109-101, Delaware Superior Court (Wilmington).
(Updates with premarket trading in 4th paragraph.)
Most Read from Bloomberg
- Microsoft’s Xbox Is Planning More Cuts After Studio Closings
- Americans Are Racking Up ‘Phantom Debt’ That Wall Street Can’t Track
- ‘Seriously Underwater’ Home Mortgages Tick Up Across the US
- Arm Slides as Tepid Outlook Fuels Concerns Over AI Slowdown
©2024 Bloomberg L.P.

- MSKCC.org external-link
- Employee login
- Talent Community
Career Areas
- Saved Jobs (0)
- Jobs Search
- Equality, Diversity & Inclusion
- Innovation & Growth
- Our Culture
- Our Locations
- Experienced Professionals
- Administrative
- Advanced Practice Providers
- Allied Health
- Data Science & Engineering
- Digital Informatics & Technology Solutions
- Environmental Health & Safety
- Facilities Management
- Nurse Anesthetists
- Professionals
- Rehabillitation
- Support Services
- Other Career Areas
- Students & Graduates
- Ready To Work
- Anesthesiology & Critical Care
- Pathology & Laboratory Medicine
- Medical Physics
- Neurosurgery
- Psychiatry & Behavioral Sciences
- Search jobs
- 2024-76979 Research Histology Technician I
Research Histology Technician I
Salary: $34.11-$52.88
Company overview
The people of Memorial Sloan Kettering Cancer Center (MSK) are united by a singular mission: ending cancer for life. Our specialized care teams provide personalized, compassionate, expert care to patients of all ages. Informed by basic research done at our Sloan Kettering Institute, scientists across MSK collaborate to conduct innovative translational and clinical research that is driving a revolution in our understanding of cancer as a disease and improving the ability to prevent, diagnose, and treat it. MSK is dedicated to training the next generation of scientists and clinicians, who go on to pursue our mission at MSK and around the globe.
Please review important announcements about vaccination requirements and our upcoming EHR implementation by clicking here .
Important Note for MSK Employees:
Your Career Hub profile is submitted to the hiring team as your internal resume. Please be sure your profile is fully complete with your skills, relevant experience and education (if required). Click here to learn more. Please note, this link is only accessible for MSK employees.
Job details
The Precision Pathology Biobanking Center (PPBC) is a newly established research center at Memorial Sloan Kettering Cancer Center. A central pillar of the Center is the collection, database annotation, and banking of human biospecimens. Our team of professionals procures surgical and biopsy research samples from over 8,000 patients per year. Our samples are a key prerequisite for high-impact cutting-edge basic, translational, and clinical trial research at MSK.
The PPBC Histology Core Laboratory provides research histology support for a range of MSK investigators. We provide project specific consultation and expertise in tissue based experimentation and help with appropriate tissue selection and processing of fresh frozen and paraffin embedded tissues. Our team of technicians is experienced in a wide range of routine and special histology techniques, including sectioning of frozen and paraffin blocks.
We are looking to recruit a highly motivated Research Histology Technician/Technologist to join our active high-volume Research Pathology Core Facility within the PPBC.
- Embed tissue specimen using orientation techniques appropriate to the type of tissue
- Cut paraffin and frozen sections
- Stain and coverslip (manual and automated methods)
- Label slides, verify patient and specimen information
- Prepare and maintain special solutions and reagents
- Perform routine stains
- Work on tissue microarrays and other technologies
- Participate in Center Quality Assurance and Quality Improvement activities
- Help develop new research histology protocols and methods; develops SOPs
- Work well in a large team and communicate well
- Ideal for highly motivated individuals with opportunity for academic development, participation in individual research projects, and growing responsibilities
Comfortable with computer use.
Formal licensing as a ASCP Histotechnician (HT) or Histotechnologist (HTL) preferred but not required .
New York State certification and licensing is not required .
Pay Range $34.11-$52.88
Please click to learn more about MSK’s compensation philosophy.
Application Process
Complete an Online Application
Interview Process
Provide References
Extension of Job Offer
New Employee Orientation
Department:
Find out more
Lead, digital ventures.
Location: New York, NY
Your next opportunity could be right here.
Search Jobs and Apply Now

COMMENTS
At first glance, the list of cancer drug makers predicted to head the field by 2024 may not look so different from the list of top-selling cancer drug makers in 2017. The cast of characters is ...
Here, we discuss three oncology-focused biotech stocks, which have a solid portfolio/promising pipeline. These are BeiGene, Ltd. BGNE, Tango Therapeutics, Inc. TNGX and Allogene Therapeutics, Inc ...
Precision oncology is the "best new weapon to defeat cancer", the chief executive of Genetron Health, Sizhen Wang, says in a blog for the World Economic Forum. This involves studying the genetic makeup and molecular characteristics of cancer tumours in individual patients. The precision oncology approach identifies changes in cells that might be causing the cancer to grow and spread.
Like Moderna, Inc. (NASDAQ:MRNA), BeiGene, Ltd. (NASDAQ:BGNE), and AstraZeneca PLC (NASDAQ:AZN), Bristol-Myers Squibb Company (NYSE:BMY) is one of the most promising cancer stocks to monitor. RGA ...
The Food and Drug Administration on Friday approved a new cancer therapy that could one day transform the way a majority of aggressive and advanced tumors are treated. The treatment, called ...
Led by haematologist Philipp Staber at the Medical University of Vienna, the study is exploring an innovative treatment strategy in which drugs are tested on the patient's own cancer cells ...
The new treatment, which uses immune cells harvested from a patient's tumor called tumor-infiltrating lymphocytes, exploits the body's own natural cancer-fighting ability. Once the tumor-infiltrating lymphocytes, or T cells, are harvested, they are encouraged in the laboratory to multiply into billions of cancer-fighting cells, then ...
Experts in several leading fields of cancer research discussed potential new advances against cancer in the coming year. ... where many pharmaceutical and biotechnology companies are actively developing a lot of novel tumor-specific targets, better linkers, and new payloads that have dramatic antitumor activity." These developments, Siu ...
Deal value: $6.15 billion. Deal background: • Sanofi and IGM Biosciences will develop a new class of antibody therapeutics against three oncology targets and three immunology/inflammation ...
These new treatments would be highly targeted and more effective than current state-of-the-art cancer immunotherapies. New research published today reveals the science behind this innovative idea.
The new results reported by BioNTech and Genentech, from a small trial of 16 patients with pancreatic cancer, are equally exciting. After surgery to remove the cancer, the participants received ...
The National Institutes of Health and 11 leading biopharmaceutical companies today launched the Partnership for Accelerating Cancer Therapies (PACT), a five-year public-private research collaboration totaling $215 million as part of the Cancer Moonshot.
In addition to providing a new way to help oncologists select cancer drugs, Xilis sees its technology as a way to guide the cancer drug research of pharmaceutical companies. The technology can ...
This ongoing, innovative research positions Achilles Therapeutics at the forefront of personalized cancer therapy development. Achilles Therapeutics is a public company with total funding of $213M, with $175M raised as a result of the IPO in 2021.
Further establishes Pfizer as a leading oncology company poised to accelerate the next generation of breakthrough treatments for people with cancer To address U.S. Federal Trade Commission concerns, Pfizer has chosen to irrevocably donate the rights of royalties from sales of Bavencio® (avelumab) in the U.S. to the American Association for Cancer Research (AACR) Pfizer Inc. (NYSE: PFE) today ...
5 Companies Pioneering Cancer Research. 1.) Genentech (Roche) : Genentech, a leading biotechnology company, is spearheading cancer research with its groundbreaking advancements in personalized medicine and targeted therapies. Through extensive genomic analysis and precision medicine approaches, Genentech focuses on developing therapies tailored ...
Country: USA | Funding: $967.1M. Arcus discovers and develops innovative cancer immunotherapies based on known but under-exploited biology. Arcus's lead program targets the adenosine pathway, which has been shown to play a significant role in driving immuno-suppression in the tumor micro-environment. 15.
Immunotherapy has transformed the landscape of cancer treatment, offering new hope and improved outcomes for patients. Ongoing research aims to enhance response rates and broaden the applications of immuno-oncology. ... therapies for cancer treatment. The company's pipeline includes several allogeneic CAR T therapies and UCART19 which is ...
The European Commission, which invests in science through the European Union's 'Horizon' funding programmes, allocated almost 1,300 cancer research grants from 2018 to 2022, totalling $2.5 ...
John Overdeck and David Siegel's Two Sigma Advisors is the largest stakeholder of the company, with 1.6 million shares valued at $355.9 million. Like Pfizer Inc. (NYSE:PFE), Merck & Co., Inc ...
Cancer immunotherapy Opdivo is on track to move ahead of Revlimid in Bristol Myers Squibb's lineup. The drug is expected to achieve peak sales of $11.75 billion by 2026, almost 50% more than the ...
MRK is recognized for its cancer research and recently announced it will acquire the clinical-stage biopharmaceutical company Peloton Therapeutics for $1.05 billion cash upfront and a further $1. ...
Your donation to the American Association for Cancer Research helps our more than 58,000 members worldwide drive progress against cancer. The AACR is the first and largest cancer research organization. Our mission is to prevent and cure cancer through research, education, communication, collaboration, funding, and advocacy. Our 58,000 members ...
3. Sanofi (NASDAQ:SNY) Company Profile. Market cap: US$122.01 billion; current share price: US$49.14. Based in France, Sanofi is developing new technologies based on molecular oncology, immuno ...
April 12, 2024 , by Daryl McGrath. The Cancer Screening Research Network will conduct rigorous, multicenter cancer screening trials. Credit: NCI. NCI's collection of cancer information products is constantly growing, so periodically we provide updates on new and updated content of interest to the cancer community.
Conquering Cancer Together. We cultivate lasting strategic partnerships with research institutions, cancer treatment centers, biopharmaceutical companies, and nonprofits to facilitate innovation in immuno-oncology and advance new lifesaving immunotherapies for more patients.
Three years later, Triomics says six cancer centers and hospitals are actively using or piloting its LLM, and it plans to double that number by the end of the year. And now the company has raised ...
Cancer clinical trials help physician-scientists test new and better ways to control and treat cancer. During a clinical trial, participants receive specific interventions, and researchers determine if those interventions are safe and effective. Interventions studied in clinical trials might be new cancer drugs or new combinations of drugs, new ...
Pfizer Inc. has agreed to settle more than 10,000 cases accusing it of hiding the cancer risks of its Zantac heartburn drug, according to people familiar with the deal, the biggest of the litigation.
The Precision Pathology Biobanking Center (PPBC) is a newly established research center at Memorial Sloan Kettering Cancer Center. A central pillar of the Center is the collection, database annotation, and banking of human biospecimens. Our team of professionals procures surgical and biopsy research samples from over 8,000 patients per year.