- Affiliate Program

- UNITED STATES
- 台灣 (TAIWAN)
- TÜRKIYE (TURKEY)
- Academic Editing Services
- - Research Paper
- - Journal Manuscript
- - Dissertation
- - College & University Assignments
- Admissions Editing Services
- - Application Essay
- - Personal Statement
- - Recommendation Letter
- - Cover Letter
- - CV/Resume
- Business Editing Services
- - Business Documents
- - Report & Brochure
- - Website & Blog
- Writer Editing Services
- - Script & Screenplay
- Our Editors
- Client Reviews
- Editing & Proofreading Prices
- Wordvice Points
- Partner Discount
- Plagiarism Checker
- APA Citation Generator
- MLA Citation Generator
- Chicago Citation Generator
- Vancouver Citation Generator
- - APA Style
- - MLA Style
- - Chicago Style
- - Vancouver Style
- Writing & Editing Guide
- Academic Resources
- Admissions Resources

How to Write the Results/Findings Section in Research
What is the research paper Results section and what does it do?
The Results section of a scientific research paper represents the core findings of a study derived from the methods applied to gather and analyze information. It presents these findings in a logical sequence without bias or interpretation from the author, setting up the reader for later interpretation and evaluation in the Discussion section. A major purpose of the Results section is to break down the data into sentences that show its significance to the research question(s).
The Results section appears third in the section sequence in most scientific papers. It follows the presentation of the Methods and Materials and is presented before the Discussion section —although the Results and Discussion are presented together in many journals. This section answers the basic question “What did you find in your research?”
What is included in the Results section?
The Results section should include the findings of your study and ONLY the findings of your study. The findings include:
- Data presented in tables, charts, graphs, and other figures (may be placed into the text or on separate pages at the end of the manuscript)
- A contextual analysis of this data explaining its meaning in sentence form
- All data that corresponds to the central research question(s)
- All secondary findings (secondary outcomes, subgroup analyses, etc.)
If the scope of the study is broad, or if you studied a variety of variables, or if the methodology used yields a wide range of different results, the author should present only those results that are most relevant to the research question stated in the Introduction section .
As a general rule, any information that does not present the direct findings or outcome of the study should be left out of this section. Unless the journal requests that authors combine the Results and Discussion sections, explanations and interpretations should be omitted from the Results.
How are the results organized?
The best way to organize your Results section is “logically.” One logical and clear method of organizing research results is to provide them alongside the research questions—within each research question, present the type of data that addresses that research question.
Let’s look at an example. Your research question is based on a survey among patients who were treated at a hospital and received postoperative care. Let’s say your first research question is:
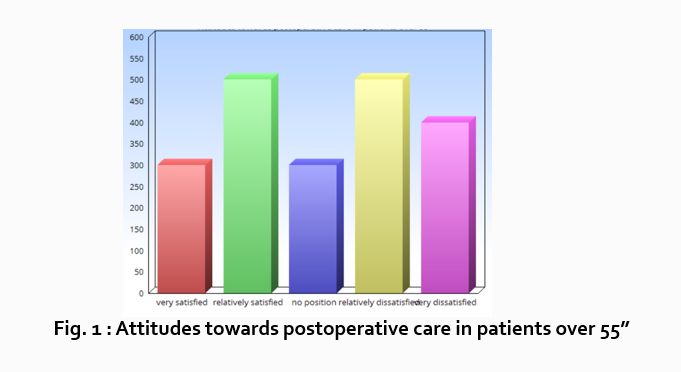
“What do hospital patients over age 55 think about postoperative care?”
This can actually be represented as a heading within your Results section, though it might be presented as a statement rather than a question:
Attitudes towards postoperative care in patients over the age of 55
Now present the results that address this specific research question first. In this case, perhaps a table illustrating data from a survey. Likert items can be included in this example. Tables can also present standard deviations, probabilities, correlation matrices, etc.
Following this, present a content analysis, in words, of one end of the spectrum of the survey or data table. In our example case, start with the POSITIVE survey responses regarding postoperative care, using descriptive phrases. For example:
“Sixty-five percent of patients over 55 responded positively to the question “ Are you satisfied with your hospital’s postoperative care ?” (Fig. 2)
Include other results such as subcategory analyses. The amount of textual description used will depend on how much interpretation of tables and figures is necessary and how many examples the reader needs in order to understand the significance of your research findings.
Next, present a content analysis of another part of the spectrum of the same research question, perhaps the NEGATIVE or NEUTRAL responses to the survey. For instance:
“As Figure 1 shows, 15 out of 60 patients in Group A responded negatively to Question 2.”
After you have assessed the data in one figure and explained it sufficiently, move on to your next research question. For example:
“How does patient satisfaction correspond to in-hospital improvements made to postoperative care?”
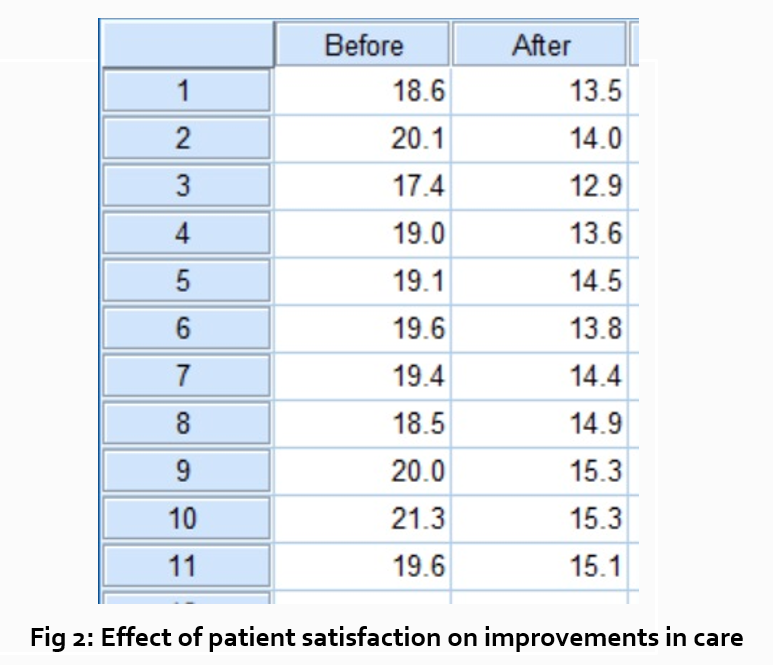
This kind of data may be presented through a figure or set of figures (for instance, a paired T-test table).
Explain the data you present, here in a table, with a concise content analysis:
“The p-value for the comparison between the before and after groups of patients was .03% (Fig. 2), indicating that the greater the dissatisfaction among patients, the more frequent the improvements that were made to postoperative care.”
Let’s examine another example of a Results section from a study on plant tolerance to heavy metal stress . In the Introduction section, the aims of the study are presented as “determining the physiological and morphological responses of Allium cepa L. towards increased cadmium toxicity” and “evaluating its potential to accumulate the metal and its associated environmental consequences.” The Results section presents data showing how these aims are achieved in tables alongside a content analysis, beginning with an overview of the findings:
“Cadmium caused inhibition of root and leave elongation, with increasing effects at higher exposure doses (Fig. 1a-c).”
The figure containing this data is cited in parentheses. Note that this author has combined three graphs into one single figure. Separating the data into separate graphs focusing on specific aspects makes it easier for the reader to assess the findings, and consolidating this information into one figure saves space and makes it easy to locate the most relevant results.
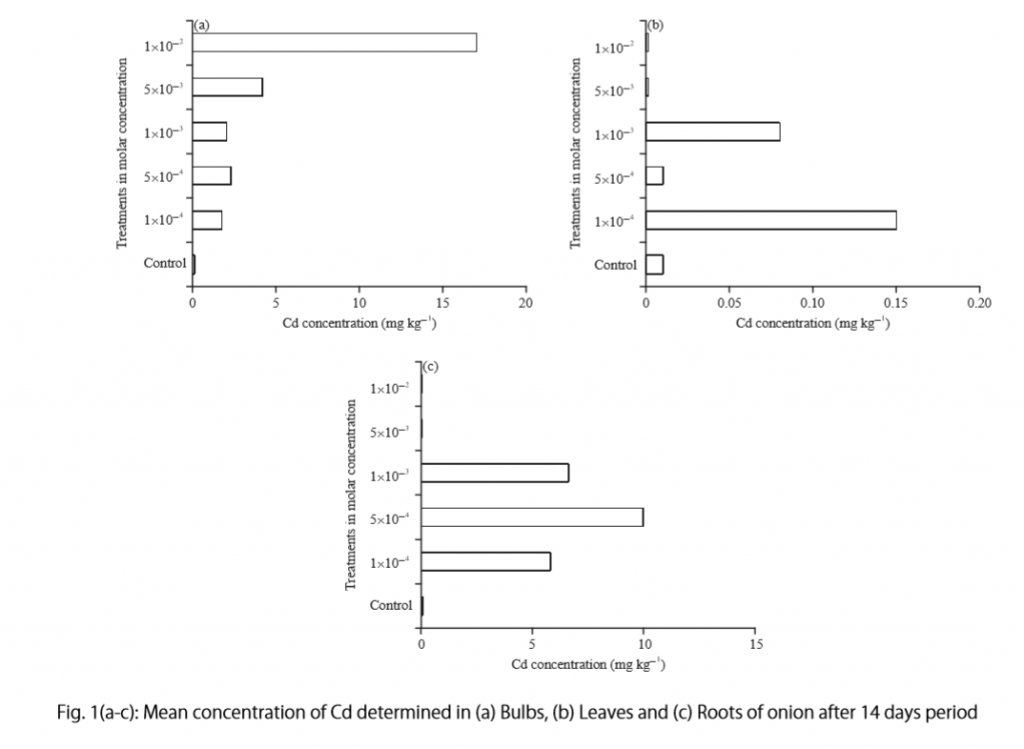
Following this overall summary, the relevant data in the tables is broken down into greater detail in text form in the Results section.
- “Results on the bio-accumulation of cadmium were found to be the highest (17.5 mg kgG1) in the bulb, when the concentration of cadmium in the solution was 1×10G2 M and lowest (0.11 mg kgG1) in the leaves when the concentration was 1×10G3 M.”
Captioning and Referencing Tables and Figures
Tables and figures are central components of your Results section and you need to carefully think about the most effective way to use graphs and tables to present your findings . Therefore, it is crucial to know how to write strong figure captions and to refer to them within the text of the Results section.
The most important advice one can give here as well as throughout the paper is to check the requirements and standards of the journal to which you are submitting your work. Every journal has its own design and layout standards, which you can find in the author instructions on the target journal’s website. Perusing a journal’s published articles will also give you an idea of the proper number, size, and complexity of your figures.
Regardless of which format you use, the figures should be placed in the order they are referenced in the Results section and be as clear and easy to understand as possible. If there are multiple variables being considered (within one or more research questions), it can be a good idea to split these up into separate figures. Subsequently, these can be referenced and analyzed under separate headings and paragraphs in the text.
To create a caption, consider the research question being asked and change it into a phrase. For instance, if one question is “Which color did participants choose?”, the caption might be “Color choice by participant group.” Or in our last research paper example, where the question was “What is the concentration of cadmium in different parts of the onion after 14 days?” the caption reads:
“Fig. 1(a-c): Mean concentration of Cd determined in (a) bulbs, (b) leaves, and (c) roots of onions after a 14-day period.”
Steps for Composing the Results Section
Because each study is unique, there is no one-size-fits-all approach when it comes to designing a strategy for structuring and writing the section of a research paper where findings are presented. The content and layout of this section will be determined by the specific area of research, the design of the study and its particular methodologies, and the guidelines of the target journal and its editors. However, the following steps can be used to compose the results of most scientific research studies and are essential for researchers who are new to preparing a manuscript for publication or who need a reminder of how to construct the Results section.
Step 1 : Consult the guidelines or instructions that the target journal or publisher provides authors and read research papers it has published, especially those with similar topics, methods, or results to your study.
- The guidelines will generally outline specific requirements for the results or findings section, and the published articles will provide sound examples of successful approaches.
- Note length limitations on restrictions on content. For instance, while many journals require the Results and Discussion sections to be separate, others do not—qualitative research papers often include results and interpretations in the same section (“Results and Discussion”).
- Reading the aims and scope in the journal’s “ guide for authors ” section and understanding the interests of its readers will be invaluable in preparing to write the Results section.
Step 2 : Consider your research results in relation to the journal’s requirements and catalogue your results.
- Focus on experimental results and other findings that are especially relevant to your research questions and objectives and include them even if they are unexpected or do not support your ideas and hypotheses.
- Catalogue your findings—use subheadings to streamline and clarify your report. This will help you avoid excessive and peripheral details as you write and also help your reader understand and remember your findings. Create appendices that might interest specialists but prove too long or distracting for other readers.
- Decide how you will structure of your results. You might match the order of the research questions and hypotheses to your results, or you could arrange them according to the order presented in the Methods section. A chronological order or even a hierarchy of importance or meaningful grouping of main themes or categories might prove effective. Consider your audience, evidence, and most importantly, the objectives of your research when choosing a structure for presenting your findings.
Step 3 : Design figures and tables to present and illustrate your data.
- Tables and figures should be numbered according to the order in which they are mentioned in the main text of the paper.
- Information in figures should be relatively self-explanatory (with the aid of captions), and their design should include all definitions and other information necessary for readers to understand the findings without reading all of the text.
- Use tables and figures as a focal point to tell a clear and informative story about your research and avoid repeating information. But remember that while figures clarify and enhance the text, they cannot replace it.
Step 4 : Draft your Results section using the findings and figures you have organized.
- The goal is to communicate this complex information as clearly and precisely as possible; precise and compact phrases and sentences are most effective.
- In the opening paragraph of this section, restate your research questions or aims to focus the reader’s attention to what the results are trying to show. It is also a good idea to summarize key findings at the end of this section to create a logical transition to the interpretation and discussion that follows.
- Try to write in the past tense and the active voice to relay the findings since the research has already been done and the agent is usually clear. This will ensure that your explanations are also clear and logical.
- Make sure that any specialized terminology or abbreviation you have used here has been defined and clarified in the Introduction section .
Step 5 : Review your draft; edit and revise until it reports results exactly as you would like to have them reported to your readers.
- Double-check the accuracy and consistency of all the data, as well as all of the visual elements included.
- Read your draft aloud to catch language errors (grammar, spelling, and mechanics), awkward phrases, and missing transitions.
- Ensure that your results are presented in the best order to focus on objectives and prepare readers for interpretations, valuations, and recommendations in the Discussion section . Look back over the paper’s Introduction and background while anticipating the Discussion and Conclusion sections to ensure that the presentation of your results is consistent and effective.
- Consider seeking additional guidance on your paper. Find additional readers to look over your Results section and see if it can be improved in any way. Peers, professors, or qualified experts can provide valuable insights.
One excellent option is to use a professional English proofreading and editing service such as Wordvice, including our paper editing service . With hundreds of qualified editors from dozens of scientific fields, Wordvice has helped thousands of authors revise their manuscripts and get accepted into their target journals. Read more about the proofreading and editing process before proceeding with getting academic editing services and manuscript editing services for your manuscript.
As the representation of your study’s data output, the Results section presents the core information in your research paper. By writing with clarity and conciseness and by highlighting and explaining the crucial findings of their study, authors increase the impact and effectiveness of their research manuscripts.
For more articles and videos on writing your research manuscript, visit Wordvice’s Resources page.
Wordvice Resources
- How to Write a Research Paper Introduction
- Which Verb Tenses to Use in a Research Paper
- How to Write an Abstract for a Research Paper
- How to Write a Research Paper Title
- Useful Phrases for Academic Writing
- Common Transition Terms in Academic Papers
- Active and Passive Voice in Research Papers
- 100+ Verbs That Will Make Your Research Writing Amazing
- Tips for Paraphrasing in Research Papers
Get science-backed answers as you write with Paperpal's Research feature
How to Write a Conclusion for Research Papers (with Examples)

The conclusion of a research paper is a crucial section that plays a significant role in the overall impact and effectiveness of your research paper. However, this is also the section that typically receives less attention compared to the introduction and the body of the paper. The conclusion serves to provide a concise summary of the key findings, their significance, their implications, and a sense of closure to the study. Discussing how can the findings be applied in real-world scenarios or inform policy, practice, or decision-making is especially valuable to practitioners and policymakers. The research paper conclusion also provides researchers with clear insights and valuable information for their own work, which they can then build on and contribute to the advancement of knowledge in the field.
The research paper conclusion should explain the significance of your findings within the broader context of your field. It restates how your results contribute to the existing body of knowledge and whether they confirm or challenge existing theories or hypotheses. Also, by identifying unanswered questions or areas requiring further investigation, your awareness of the broader research landscape can be demonstrated.
Remember to tailor the research paper conclusion to the specific needs and interests of your intended audience, which may include researchers, practitioners, policymakers, or a combination of these.
Table of Contents
What is a conclusion in a research paper, summarizing conclusion, editorial conclusion, externalizing conclusion, importance of a good research paper conclusion, how to write a conclusion for your research paper, research paper conclusion examples.
- How to write a research paper conclusion with Paperpal?
Frequently Asked Questions
A conclusion in a research paper is the final section where you summarize and wrap up your research, presenting the key findings and insights derived from your study. The research paper conclusion is not the place to introduce new information or data that was not discussed in the main body of the paper. When working on how to conclude a research paper, remember to stick to summarizing and interpreting existing content. The research paper conclusion serves the following purposes: 1
- Warn readers of the possible consequences of not attending to the problem.
- Recommend specific course(s) of action.
- Restate key ideas to drive home the ultimate point of your research paper.
- Provide a “take-home” message that you want the readers to remember about your study.

Types of conclusions for research papers
In research papers, the conclusion provides closure to the reader. The type of research paper conclusion you choose depends on the nature of your study, your goals, and your target audience. I provide you with three common types of conclusions:
A summarizing conclusion is the most common type of conclusion in research papers. It involves summarizing the main points, reiterating the research question, and restating the significance of the findings. This common type of research paper conclusion is used across different disciplines.
An editorial conclusion is less common but can be used in research papers that are focused on proposing or advocating for a particular viewpoint or policy. It involves presenting a strong editorial or opinion based on the research findings and offering recommendations or calls to action.
An externalizing conclusion is a type of conclusion that extends the research beyond the scope of the paper by suggesting potential future research directions or discussing the broader implications of the findings. This type of conclusion is often used in more theoretical or exploratory research papers.
Align your conclusion’s tone with the rest of your research paper. Start Writing with Paperpal Now!
The conclusion in a research paper serves several important purposes:
- Offers Implications and Recommendations : Your research paper conclusion is an excellent place to discuss the broader implications of your research and suggest potential areas for further study. It’s also an opportunity to offer practical recommendations based on your findings.
- Provides Closure : A good research paper conclusion provides a sense of closure to your paper. It should leave the reader with a feeling that they have reached the end of a well-structured and thought-provoking research project.
- Leaves a Lasting Impression : Writing a well-crafted research paper conclusion leaves a lasting impression on your readers. It’s your final opportunity to leave them with a new idea, a call to action, or a memorable quote.

Writing a strong conclusion for your research paper is essential to leave a lasting impression on your readers. Here’s a step-by-step process to help you create and know what to put in the conclusion of a research paper: 2
- Research Statement : Begin your research paper conclusion by restating your research statement. This reminds the reader of the main point you’ve been trying to prove throughout your paper. Keep it concise and clear.
- Key Points : Summarize the main arguments and key points you’ve made in your paper. Avoid introducing new information in the research paper conclusion. Instead, provide a concise overview of what you’ve discussed in the body of your paper.
- Address the Research Questions : If your research paper is based on specific research questions or hypotheses, briefly address whether you’ve answered them or achieved your research goals. Discuss the significance of your findings in this context.
- Significance : Highlight the importance of your research and its relevance in the broader context. Explain why your findings matter and how they contribute to the existing knowledge in your field.
- Implications : Explore the practical or theoretical implications of your research. How might your findings impact future research, policy, or real-world applications? Consider the “so what?” question.
- Future Research : Offer suggestions for future research in your area. What questions or aspects remain unanswered or warrant further investigation? This shows that your work opens the door for future exploration.
- Closing Thought : Conclude your research paper conclusion with a thought-provoking or memorable statement. This can leave a lasting impression on your readers and wrap up your paper effectively. Avoid introducing new information or arguments here.
- Proofread and Revise : Carefully proofread your conclusion for grammar, spelling, and clarity. Ensure that your ideas flow smoothly and that your conclusion is coherent and well-structured.
Write your research paper conclusion 2x faster with Paperpal. Try it now!
Remember that a well-crafted research paper conclusion is a reflection of the strength of your research and your ability to communicate its significance effectively. It should leave a lasting impression on your readers and tie together all the threads of your paper. Now you know how to start the conclusion of a research paper and what elements to include to make it impactful, let’s look at a research paper conclusion sample.

How to write a research paper conclusion with Paperpal?
A research paper conclusion is not just a summary of your study, but a synthesis of the key findings that ties the research together and places it in a broader context. A research paper conclusion should be concise, typically around one paragraph in length. However, some complex topics may require a longer conclusion to ensure the reader is left with a clear understanding of the study’s significance. Paperpal, an AI writing assistant trusted by over 800,000 academics globally, can help you write a well-structured conclusion for your research paper.
- Sign Up or Log In: Create a new Paperpal account or login with your details.
- Navigate to Features : Once logged in, head over to the features’ side navigation pane. Click on Templates and you’ll find a suite of generative AI features to help you write better, faster.
- Generate an outline: Under Templates, select ‘Outlines’. Choose ‘Research article’ as your document type.
- Select your section: Since you’re focusing on the conclusion, select this section when prompted.
- Choose your field of study: Identifying your field of study allows Paperpal to provide more targeted suggestions, ensuring the relevance of your conclusion to your specific area of research.
- Provide a brief description of your study: Enter details about your research topic and findings. This information helps Paperpal generate a tailored outline that aligns with your paper’s content.
- Generate the conclusion outline: After entering all necessary details, click on ‘generate’. Paperpal will then create a structured outline for your conclusion, to help you start writing and build upon the outline.
- Write your conclusion: Use the generated outline to build your conclusion. The outline serves as a guide, ensuring you cover all critical aspects of a strong conclusion, from summarizing key findings to highlighting the research’s implications.
- Refine and enhance: Paperpal’s ‘Make Academic’ feature can be particularly useful in the final stages. Select any paragraph of your conclusion and use this feature to elevate the academic tone, ensuring your writing is aligned to the academic journal standards.
By following these steps, Paperpal not only simplifies the process of writing a research paper conclusion but also ensures it is impactful, concise, and aligned with academic standards. Sign up with Paperpal today and write your research paper conclusion 2x faster .
The research paper conclusion is a crucial part of your paper as it provides the final opportunity to leave a strong impression on your readers. In the research paper conclusion, summarize the main points of your research paper by restating your research statement, highlighting the most important findings, addressing the research questions or objectives, explaining the broader context of the study, discussing the significance of your findings, providing recommendations if applicable, and emphasizing the takeaway message. The main purpose of the conclusion is to remind the reader of the main point or argument of your paper and to provide a clear and concise summary of the key findings and their implications. All these elements should feature on your list of what to put in the conclusion of a research paper to create a strong final statement for your work.
A strong conclusion is a critical component of a research paper, as it provides an opportunity to wrap up your arguments, reiterate your main points, and leave a lasting impression on your readers. Here are the key elements of a strong research paper conclusion: 1. Conciseness : A research paper conclusion should be concise and to the point. It should not introduce new information or ideas that were not discussed in the body of the paper. 2. Summarization : The research paper conclusion should be comprehensive enough to give the reader a clear understanding of the research’s main contributions. 3 . Relevance : Ensure that the information included in the research paper conclusion is directly relevant to the research paper’s main topic and objectives; avoid unnecessary details. 4 . Connection to the Introduction : A well-structured research paper conclusion often revisits the key points made in the introduction and shows how the research has addressed the initial questions or objectives. 5. Emphasis : Highlight the significance and implications of your research. Why is your study important? What are the broader implications or applications of your findings? 6 . Call to Action : Include a call to action or a recommendation for future research or action based on your findings.
The length of a research paper conclusion can vary depending on several factors, including the overall length of the paper, the complexity of the research, and the specific journal requirements. While there is no strict rule for the length of a conclusion, but it’s generally advisable to keep it relatively short. A typical research paper conclusion might be around 5-10% of the paper’s total length. For example, if your paper is 10 pages long, the conclusion might be roughly half a page to one page in length.
In general, you do not need to include citations in the research paper conclusion. Citations are typically reserved for the body of the paper to support your arguments and provide evidence for your claims. However, there may be some exceptions to this rule: 1. If you are drawing a direct quote or paraphrasing a specific source in your research paper conclusion, you should include a citation to give proper credit to the original author. 2. If your conclusion refers to or discusses specific research, data, or sources that are crucial to the overall argument, citations can be included to reinforce your conclusion’s validity.
The conclusion of a research paper serves several important purposes: 1. Summarize the Key Points 2. Reinforce the Main Argument 3. Provide Closure 4. Offer Insights or Implications 5. Engage the Reader. 6. Reflect on Limitations
Remember that the primary purpose of the research paper conclusion is to leave a lasting impression on the reader, reinforcing the key points and providing closure to your research. It’s often the last part of the paper that the reader will see, so it should be strong and well-crafted.
- Makar, G., Foltz, C., Lendner, M., & Vaccaro, A. R. (2018). How to write effective discussion and conclusion sections. Clinical spine surgery, 31(8), 345-346.
- Bunton, D. (2005). The structure of PhD conclusion chapters. Journal of English for academic purposes , 4 (3), 207-224.
Paperpal is a comprehensive AI writing toolkit that helps students and researchers achieve 2x the writing in half the time. It leverages 21+ years of STM experience and insights from millions of research articles to provide in-depth academic writing, language editing, and submission readiness support to help you write better, faster.
Get accurate academic translations, rewriting support, grammar checks, vocabulary suggestions, and generative AI assistance that delivers human precision at machine speed. Try for free or upgrade to Paperpal Prime starting at US$19 a month to access premium features, including consistency, plagiarism, and 30+ submission readiness checks to help you succeed.
Experience the future of academic writing – Sign up to Paperpal and start writing for free!
Related Reads:
- 5 Reasons for Rejection After Peer Review
- Ethical Research Practices For Research with Human Subjects
7 Ways to Improve Your Academic Writing Process
- Paraphrasing in Academic Writing: Answering Top Author Queries
Preflight For Editorial Desk: The Perfect Hybrid (AI + Human) Assistance Against Compromised Manuscripts
You may also like, ai in education: it’s time to change the..., is it ethical to use ai-generated abstracts without..., what are journal guidelines on using generative ai..., quillbot review: features, pricing, and free alternatives, what is an academic paper types and elements , should you use ai tools like chatgpt for..., publish research papers: 9 steps for successful publications , what are the different types of research papers, how to make translating academic papers less challenging, self-plagiarism in research: what it is and how....
- How it works
How to Write the Dissertation Findings or Results – Steps & Tips
Published by Grace Graffin at August 11th, 2021 , Revised On October 9, 2023
Each part of the dissertation is unique, and some general and specific rules must be followed. The dissertation’s findings section presents the key results of your research without interpreting their meaning .
Theoretically, this is an exciting section of a dissertation because it involves writing what you have observed and found. However, it can be a little tricky if there is too much information to confuse the readers.
The goal is to include only the essential and relevant findings in this section. The results must be presented in an orderly sequence to provide clarity to the readers.
This section of the dissertation should be easy for the readers to follow, so you should avoid going into a lengthy debate over the interpretation of the results.
It is vitally important to focus only on clear and precise observations. The findings chapter of the dissertation is theoretically the easiest to write.
It includes statistical analysis and a brief write-up about whether or not the results emerging from the analysis are significant. This segment should be written in the past sentence as you describe what you have done in the past.
This article will provide detailed information about how to write the findings of a dissertation .
When to Write Dissertation Findings Chapter
As soon as you have gathered and analysed your data, you can start to write up the findings chapter of your dissertation paper. Remember that it is your chance to report the most notable findings of your research work and relate them to the research hypothesis or research questions set out in the introduction chapter of the dissertation .
You will be required to separately report your study’s findings before moving on to the discussion chapter if your dissertation is based on the collection of primary data or experimental work.
However, you may not be required to have an independent findings chapter if your dissertation is purely descriptive and focuses on the analysis of case studies or interpretation of texts.
- Always report the findings of your research in the past tense.
- The dissertation findings chapter varies from one project to another, depending on the data collected and analyzed.
- Avoid reporting results that are not relevant to your research questions or research hypothesis.
Does your Dissertation Have the Following?
- Great Research/Sources
- Perfect Language
- Accurate Sources
If not, we can help. Our panel of experts makes sure to keep the 3 pillars of the Dissertation strong.

1. Reporting Quantitative Findings
The best way to present your quantitative findings is to structure them around the research hypothesis or questions you intend to address as part of your dissertation project.
Report the relevant findings for each research question or hypothesis, focusing on how you analyzed them.
Analysis of your findings will help you determine how they relate to the different research questions and whether they support the hypothesis you formulated.
While you must highlight meaningful relationships, variances, and tendencies, it is important not to guess their interpretations and implications because this is something to save for the discussion and conclusion chapters.
Any findings not directly relevant to your research questions or explanations concerning the data collection process should be added to the dissertation paper’s appendix section.
Use of Figures and Tables in Dissertation Findings
Suppose your dissertation is based on quantitative research. In that case, it is important to include charts, graphs, tables, and other visual elements to help your readers understand the emerging trends and relationships in your findings.
Repeating information will give the impression that you are short on ideas. Refer to all charts, illustrations, and tables in your writing but avoid recurrence.
The text should be used only to elaborate and summarize certain parts of your results. On the other hand, illustrations and tables are used to present multifaceted data.
It is recommended to give descriptive labels and captions to all illustrations used so the readers can figure out what each refers to.
How to Report Quantitative Findings
Here is an example of how to report quantitative results in your dissertation findings chapter;
Two hundred seventeen participants completed both the pretest and post-test and a Pairwise T-test was used for the analysis. The quantitative data analysis reveals a statistically significant difference between the mean scores of the pretest and posttest scales from the Teachers Discovering Computers course. The pretest mean was 29.00 with a standard deviation of 7.65, while the posttest mean was 26.50 with a standard deviation of 9.74 (Table 1). These results yield a significance level of .000, indicating a strong treatment effect (see Table 3). With the correlation between the scores being .448, the little relationship is seen between the pretest and posttest scores (Table 2). This leads the researcher to conclude that the impact of the course on the educators’ perception and integration of technology into the curriculum is dramatic.
Paired Samples
Paired samples correlation, paired samples test.
Also Read: How to Write the Abstract for the Dissertation.
2. Reporting Qualitative Findings
A notable issue with reporting qualitative findings is that not all results directly relate to your research questions or hypothesis.
The best way to present the results of qualitative research is to frame your findings around the most critical areas or themes you obtained after you examined the data.
In-depth data analysis will help you observe what the data shows for each theme. Any developments, relationships, patterns, and independent responses directly relevant to your research question or hypothesis should be mentioned to the readers.
Additional information not directly relevant to your research can be included in the appendix .
How to Report Qualitative Findings
Here is an example of how to report qualitative results in your dissertation findings chapter;
How do I report quantitative findings?
The best way to present your quantitative findings is to structure them around the research hypothesis or research questions you intended to address as part of your dissertation project. Report the relevant findings for each of the research questions or hypotheses, focusing on how you analyzed them.
How do I report qualitative findings?
The best way to present the qualitative research results is to frame your findings around the most important areas or themes that you obtained after examining the data.
An in-depth analysis of the data will help you observe what the data is showing for each theme. Any developments, relationships, patterns, and independent responses that are directly relevant to your research question or hypothesis should be clearly mentioned for the readers.
Can I use interpretive phrases like ‘it confirms’ in the finding chapter?
No, It is highly advisable to avoid using interpretive and subjective phrases in the finding chapter. These terms are more suitable for the discussion chapter , where you will be expected to provide your interpretation of the results in detail.
Can I report the results from other research papers in my findings chapter?
NO, you must not be presenting results from other research studies in your findings.
You May Also Like
How to Structure a Dissertation or Thesis Need interesting and manageable Finance and Accounting dissertation topics? Here are the trending Media dissertation titles so you can choose one most suitable to your needs.
Writing a dissertation can be tough if this is the first time you are doing it. You need to look into relevant literature, analyze past researches, conduct surveys, interviews etc.
A literature review is a survey of theses, articles, books and other academic sources. Here are guidelines on how to write dissertation literature review.
USEFUL LINKS
LEARNING RESOURCES

COMPANY DETAILS

- How It Works
How To Write The Results/Findings Chapter
For qualitative studies (dissertations & theses).
By: Jenna Crossley (PhD Cand). Expert Reviewed By: Dr. Eunice Rautenbach | August 2021
So, you’ve collected and analysed your qualitative data, and it’s time to write up your results chapter – exciting! But where do you start? In this post, we’ll guide you through the qualitative results chapter (also called the findings chapter), step by step.
Overview: Qualitative Results Chapter
- What (exactly) the qualitative results chapter is
- What to include in your results chapter
- How to write up your results chapter
- A few tips and tricks to help you along the way
What exactly is the results chapter?
The results chapter in a dissertation or thesis (or any formal academic research piece) is where you objectively and neutrally present the findings of your qualitative analysis (or analyses if you used multiple qualitative analysis methods ). This chapter can sometimes be combined with the discussion chapter (where you interpret the data and discuss its meaning), depending on your university’s preference. We’ll treat the two chapters as separate, as that’s the most common approach.
In contrast to a quantitative results chapter that presents numbers and statistics, a qualitative results chapter presents data primarily in the form of words . But this doesn’t mean that a qualitative study can’t have quantitative elements – you could, for example, present the number of times a theme or topic pops up in your data, depending on the analysis method(s) you adopt.
Adding a quantitative element to your study can add some rigour, which strengthens your results by providing more evidence for your claims. This is particularly common when using qualitative content analysis. Keep in mind though that qualitative research aims to achieve depth, richness and identify nuances , so don’t get tunnel vision by focusing on the numbers. They’re just cream on top in a qualitative analysis.
So, to recap, the results chapter is where you objectively present the findings of your analysis, without interpreting them (you’ll save that for the discussion chapter). With that out the way, let’s take a look at what you should include in your results chapter.

What should you include in the results chapter?
As we’ve mentioned, your qualitative results chapter should purely present and describe your results , not interpret them in relation to the existing literature or your research questions . Any speculations or discussion about the implications of your findings should be reserved for your discussion chapter.
In your results chapter, you’ll want to talk about your analysis findings and whether or not they support your hypotheses (if you have any). Naturally, the exact contents of your results chapter will depend on which qualitative analysis method (or methods) you use. For example, if you were to use thematic analysis, you’d detail the themes identified in your analysis, using extracts from the transcripts or text to support your claims.
While you do need to present your analysis findings in some detail, you should avoid dumping large amounts of raw data in this chapter. Instead, focus on presenting the key findings and using a handful of select quotes or text extracts to support each finding . The reams of data and analysis can be relegated to your appendices.
While it’s tempting to include every last detail you found in your qualitative analysis, it is important to make sure that you report only that which is relevant to your research aims, objectives and research questions . Always keep these three components, as well as your hypotheses (if you have any) front of mind when writing the chapter and use them as a filter to decide what’s relevant and what’s not.
Need a helping hand?
How do I write the results chapter?
Now that we’ve covered the basics, it’s time to look at how to structure your chapter. Broadly speaking, the results chapter needs to contain three core components – the introduction, the body and the concluding summary. Let’s take a look at each of these.
Section 1: Introduction
The first step is to craft a brief introduction to the chapter. This intro is vital as it provides some context for your findings. In your introduction, you should begin by reiterating your problem statement and research questions and highlight the purpose of your research . Make sure that you spell this out for the reader so that the rest of your chapter is well contextualised.
The next step is to briefly outline the structure of your results chapter. In other words, explain what’s included in the chapter and what the reader can expect. In the results chapter, you want to tell a story that is coherent, flows logically, and is easy to follow , so make sure that you plan your structure out well and convey that structure (at a high level), so that your reader is well oriented.
The introduction section shouldn’t be lengthy. Two or three short paragraphs should be more than adequate. It is merely an introduction and overview, not a summary of the chapter.
Pro Tip – To help you structure your chapter, it can be useful to set up an initial draft with (sub)section headings so that you’re able to easily (re)arrange parts of your chapter. This will also help your reader to follow your results and give your chapter some coherence. Be sure to use level-based heading styles (e.g. Heading 1, 2, 3 styles) to help the reader differentiate between levels visually. You can find these options in Word (example below).
Section 2: Body
Before we get started on what to include in the body of your chapter, it’s vital to remember that a results section should be completely objective and descriptive, not interpretive . So, be careful not to use words such as, “suggests” or “implies”, as these usually accompany some form of interpretation – that’s reserved for your discussion chapter.
The structure of your body section is very important , so make sure that you plan it out well. When planning out your qualitative results chapter, create sections and subsections so that you can maintain the flow of the story you’re trying to tell. Be sure to systematically and consistently describe each portion of results. Try to adopt a standardised structure for each portion so that you achieve a high level of consistency throughout the chapter.
For qualitative studies, results chapters tend to be structured according to themes , which makes it easier for readers to follow. However, keep in mind that not all results chapters have to be structured in this manner. For example, if you’re conducting a longitudinal study, you may want to structure your chapter chronologically. Similarly, you might structure this chapter based on your theoretical framework . The exact structure of your chapter will depend on the nature of your study , especially your research questions.
As you work through the body of your chapter, make sure that you use quotes to substantiate every one of your claims . You can present these quotes in italics to differentiate them from your own words. A general rule of thumb is to use at least two pieces of evidence per claim, and these should be linked directly to your data. Also, remember that you need to include all relevant results , not just the ones that support your assumptions or initial leanings.
In addition to including quotes, you can also link your claims to the data by using appendices , which you should reference throughout your text. When you reference, make sure that you include both the name/number of the appendix , as well as the line(s) from which you drew your data.
As referencing styles can vary greatly, be sure to look up the appendix referencing conventions of your university’s prescribed style (e.g. APA , Harvard, etc) and keep this consistent throughout your chapter.

Section 3: Concluding summary
The concluding summary is very important because it summarises your key findings and lays the foundation for the discussion chapter . Keep in mind that some readers may skip directly to this section (from the introduction section), so make sure that it can be read and understood well in isolation.
In this section, you need to remind the reader of the key findings. That is, the results that directly relate to your research questions and that you will build upon in your discussion chapter. Remember, your reader has digested a lot of information in this chapter, so you need to use this section to remind them of the most important takeaways.
Importantly, the concluding summary should not present any new information and should only describe what you’ve already presented in your chapter. Keep it concise – you’re not summarising the whole chapter, just the essentials.
Tips and tricks for an A-grade results chapter
Now that you’ve got a clear picture of what the qualitative results chapter is all about, here are some quick tips and reminders to help you craft a high-quality chapter:
- Your results chapter should be written in the past tense . You’ve done the work already, so you want to tell the reader what you found , not what you are currently finding .
- Make sure that you review your work multiple times and check that every claim is adequately backed up by evidence . Aim for at least two examples per claim, and make use of an appendix to reference these.
- When writing up your results, make sure that you stick to only what is relevant . Don’t waste time on data that are not relevant to your research objectives and research questions.
- Use headings and subheadings to create an intuitive, easy to follow piece of writing. Make use of Microsoft Word’s “heading styles” and be sure to use them consistently.
- When referring to numerical data, tables and figures can provide a useful visual aid. When using these, make sure that they can be read and understood independent of your body text (i.e. that they can stand-alone). To this end, use clear, concise labels for each of your tables or figures and make use of colours to code indicate differences or hierarchy.
- Similarly, when you’re writing up your chapter, it can be useful to highlight topics and themes in different colours . This can help you to differentiate between your data if you get a bit overwhelmed and will also help you to ensure that your results flow logically and coherently.
If you have any questions, leave a comment below and we’ll do our best to help. If you’d like 1-on-1 help with your results chapter (or any chapter of your dissertation or thesis), check out our private dissertation coaching service here or book a free initial consultation to discuss how we can help you.

Psst… there’s more (for free)
This post is part of our dissertation mini-course, which covers everything you need to get started with your dissertation, thesis or research project.
You Might Also Like:

20 Comments
This was extremely helpful. Thanks a lot guys
Hi, thanks for the great research support platform created by the gradcoach team!
I wanted to ask- While “suggests” or “implies” are interpretive terms, what terms could we use for the results chapter? Could you share some examples of descriptive terms?
I think that instead of saying, ‘The data suggested, or The data implied,’ you can say, ‘The Data showed or revealed, or illustrated or outlined’…If interview data, you may say Jane Doe illuminated or elaborated, or Jane Doe described… or Jane Doe expressed or stated.
I found this article very useful. Thank you very much for the outstanding work you are doing.
What if i have 3 different interviewees answering the same interview questions? Should i then present the results in form of the table with the division on the 3 perspectives or rather give a results in form of the text and highlight who said what?
I think this tabular representation of results is a great idea. I am doing it too along with the text. Thanks
That was helpful was struggling to separate the discussion from the findings
this was very useful, Thank you.
Very helpful, I am confident to write my results chapter now.
It is so helpful! It is a good job. Thank you very much!
Very useful, well explained. Many thanks.
Hello, I appreciate the way you provided a supportive comments about qualitative results presenting tips
I loved this! It explains everything needed, and it has helped me better organize my thoughts. What words should I not use while writing my results section, other than subjective ones.
Thanks a lot, it is really helpful
Thank you so much dear, i really appropriate your nice explanations about this.
Thank you so much for this! I was wondering if anyone could help with how to prproperly integrate quotations (Excerpts) from interviews in the finding chapter in a qualitative research. Please GradCoach, address this issue and provide examples.
what if I’m not doing any interviews myself and all the information is coming from case studies that have already done the research.
Very helpful thank you.
This was very helpful as I was wondering how to structure this part of my dissertation, to include the quotes… Thanks for this explanation
This is very helpful, thanks! I am required to write up my results chapters with the discussion in each of them – any tips and tricks for this strategy?
Submit a Comment Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
- Print Friendly
When you choose to publish with PLOS, your research makes an impact. Make your work accessible to all, without restrictions, and accelerate scientific discovery with options like preprints and published peer review that make your work more Open.
- PLOS Biology
- PLOS Climate
- PLOS Complex Systems
- PLOS Computational Biology
- PLOS Digital Health
- PLOS Genetics
- PLOS Global Public Health
- PLOS Medicine
- PLOS Mental Health
- PLOS Neglected Tropical Diseases
- PLOS Pathogens
- PLOS Sustainability and Transformation
- PLOS Collections
- How to Write Discussions and Conclusions

The discussion section contains the results and outcomes of a study. An effective discussion informs readers what can be learned from your experiment and provides context for the results.
What makes an effective discussion?
When you’re ready to write your discussion, you’ve already introduced the purpose of your study and provided an in-depth description of the methodology. The discussion informs readers about the larger implications of your study based on the results. Highlighting these implications while not overstating the findings can be challenging, especially when you’re submitting to a journal that selects articles based on novelty or potential impact. Regardless of what journal you are submitting to, the discussion section always serves the same purpose: concluding what your study results actually mean.
A successful discussion section puts your findings in context. It should include:
- the results of your research,
- a discussion of related research, and
- a comparison between your results and initial hypothesis.
Tip: Not all journals share the same naming conventions.
You can apply the advice in this article to the conclusion, results or discussion sections of your manuscript.
Our Early Career Researcher community tells us that the conclusion is often considered the most difficult aspect of a manuscript to write. To help, this guide provides questions to ask yourself, a basic structure to model your discussion off of and examples from published manuscripts.
Questions to ask yourself:
- Was my hypothesis correct?
- If my hypothesis is partially correct or entirely different, what can be learned from the results?
- How do the conclusions reshape or add onto the existing knowledge in the field? What does previous research say about the topic?
- Why are the results important or relevant to your audience? Do they add further evidence to a scientific consensus or disprove prior studies?
- How can future research build on these observations? What are the key experiments that must be done?
- What is the “take-home” message you want your reader to leave with?
How to structure a discussion
Trying to fit a complete discussion into a single paragraph can add unnecessary stress to the writing process. If possible, you’ll want to give yourself two or three paragraphs to give the reader a comprehensive understanding of your study as a whole. Here’s one way to structure an effective discussion:
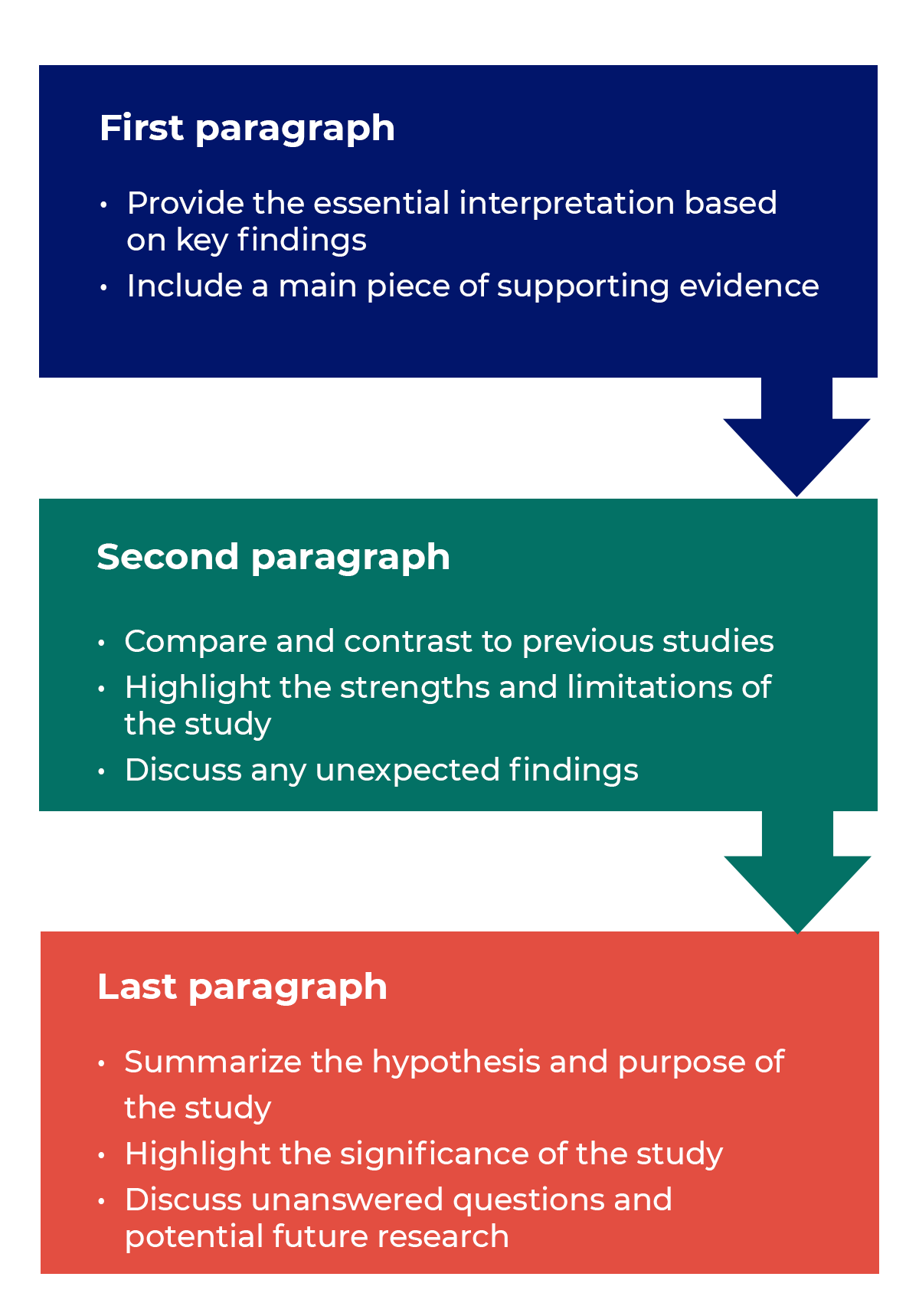
Writing Tips
While the above sections can help you brainstorm and structure your discussion, there are many common mistakes that writers revert to when having difficulties with their paper. Writing a discussion can be a delicate balance between summarizing your results, providing proper context for your research and avoiding introducing new information. Remember that your paper should be both confident and honest about the results!

- Read the journal’s guidelines on the discussion and conclusion sections. If possible, learn about the guidelines before writing the discussion to ensure you’re writing to meet their expectations.
- Begin with a clear statement of the principal findings. This will reinforce the main take-away for the reader and set up the rest of the discussion.
- Explain why the outcomes of your study are important to the reader. Discuss the implications of your findings realistically based on previous literature, highlighting both the strengths and limitations of the research.
- State whether the results prove or disprove your hypothesis. If your hypothesis was disproved, what might be the reasons?
- Introduce new or expanded ways to think about the research question. Indicate what next steps can be taken to further pursue any unresolved questions.
- If dealing with a contemporary or ongoing problem, such as climate change, discuss possible consequences if the problem is avoided.
- Be concise. Adding unnecessary detail can distract from the main findings.

Don’t
- Rewrite your abstract. Statements with “we investigated” or “we studied” generally do not belong in the discussion.
- Include new arguments or evidence not previously discussed. Necessary information and evidence should be introduced in the main body of the paper.
- Apologize. Even if your research contains significant limitations, don’t undermine your authority by including statements that doubt your methodology or execution.
- Shy away from speaking on limitations or negative results. Including limitations and negative results will give readers a complete understanding of the presented research. Potential limitations include sources of potential bias, threats to internal or external validity, barriers to implementing an intervention and other issues inherent to the study design.
- Overstate the importance of your findings. Making grand statements about how a study will fully resolve large questions can lead readers to doubt the success of the research.
Snippets of Effective Discussions:
Consumer-based actions to reduce plastic pollution in rivers: A multi-criteria decision analysis approach
Identifying reliable indicators of fitness in polar bears
- How to Write a Great Title
- How to Write an Abstract
- How to Write Your Methods
- How to Report Statistics
- How to Edit Your Work
The contents of the Peer Review Center are also available as a live, interactive training session, complete with slides, talking points, and activities. …
The contents of the Writing Center are also available as a live, interactive training session, complete with slides, talking points, and activities. …
There’s a lot to consider when deciding where to submit your work. Learn how to choose a journal that will help your study reach its audience, while reflecting your values as a researcher…
- Discoveries
- Right Journal
- Journal Metrics
- Journal Fit
- Abbreviation
- In-Text Citations
- Bibliographies
- Writing an Article
- Peer Review Types
- Acknowledgements
- Withdrawing a Paper
- Form Letter
- ISO, ANSI, CFR
- Google Scholar
- Journal Manuscript Editing
- Research Manuscript Editing
Book Editing
- Manuscript Editing Services
Medical Editing
- Bioscience Editing
- Physical Science Editing
- PhD Thesis Editing Services
- PhD Editing
- Master’s Proofreading
- Bachelor’s Editing
- Dissertation Proofreading Services
- Best Dissertation Proofreaders
- Masters Dissertation Proofreading
- PhD Proofreaders
- Proofreading PhD Thesis Price
- Journal Article Editing
- Book Editing Service
- Editing and Proofreading Services
- Research Paper Editing
- Medical Manuscript Editing
- Academic Editing
- Social Sciences Editing
- Academic Proofreading
- PhD Theses Editing
- Dissertation Proofreading
- Proofreading Rates UK
- Medical Proofreading
- PhD Proofreading Services UK
- Academic Proofreading Services UK
Medical Editing Services
- Life Science Editing
- Biomedical Editing
- Environmental Science Editing
- Pharmaceutical Science Editing
- Economics Editing
- Psychology Editing
- Sociology Editing
- Archaeology Editing
- History Paper Editing
- Anthropology Editing
- Law Paper Editing
- Engineering Paper Editing
- Technical Paper Editing
- Philosophy Editing
- PhD Dissertation Proofreading
- Lektorat Englisch
- Akademisches Lektorat
- Lektorat Englisch Preise
- Wissenschaftliches Lektorat
- Lektorat Doktorarbeit
PhD Thesis Editing
- Thesis Proofreading Services
- PhD Thesis Proofreading
- Proofreading Thesis Cost
- Proofreading Thesis
- Thesis Editing Services
- Professional Thesis Editing
- Thesis Editing Cost
- Proofreading Dissertation
- Dissertation Proofreading Cost
- Dissertation Proofreader
- Correção de Artigos Científicos
- Correção de Trabalhos Academicos
- Serviços de Correção de Inglês
- Correção de Dissertação
- Correção de Textos Precos
- 定額 ネイティブチェック
- Copy Editing
- FREE Courses
- Revision en Ingles
- Revision de Textos en Ingles
- Revision de Tesis
- Revision Medica en Ingles
- Revision de Tesis Precio
- Revisão de Artigos Científicos
- Revisão de Trabalhos Academicos
- Serviços de Revisão de Inglês
- Revisão de Dissertação
- Revisão de Textos Precos
- Corrección de Textos en Ingles
- Corrección de Tesis
- Corrección de Tesis Precio
- Corrección Medica en Ingles
- Corrector ingles
Select Page
How To Write the Findings Section of a Research Paper
Posted by Rene Tetzner | Sep 2, 2021 | Paper Writing Advice | 0 |

How To Write the Findings Section of a Research Paper Each research project is unique, so it is natural for one researcher to make use of somewhat different strategies than another when it comes to designing and writing the section of a research paper dedicated to findings. The academic or scientific discipline of the research, the field of specialisation, the particular author or authors, the targeted journal or other publisher and the editor making the decisions about publication can all have a significant impact. The practical steps outlined below can be effectively applied to writing about the findings of most advanced research, however, and will prove especially helpful for early-career scholars who are preparing a research paper for a first publication.

Step 1 : Consult the guidelines or instructions that the targeted journal (or other publisher) provides for authors and read research papers it has already published, particularly ones similar in topic, methods or results to your own. The guidelines will generally outline specific requirements for the results or findings section, and the published articles will provide sound examples of successful approaches. Watch particularly for length limitations and restrictions on content. Interpretation, for instance, is usually reserved for a later discussion section, though not always – qualitative research papers often combine findings and interpretation. Background information and descriptions of methods, on the other hand, almost always appear in earlier sections of a research paper. In most cases it is appropriate in a findings section to offer basic comparisons between the results of your study and those of other studies, but knowing exactly what the journal wants in the report of research findings is essential. Learning as much as you can about the journal’s aims and scope as well as the interests of its readers is invaluable as well.

Step 2 : Reflect at some length on your research results in relation to the journal’s requirements while planning the findings section of your paper. Choose for particular focus experimental results and other research discoveries that are particularly relevant to your research questions and objectives, and include them even if they are unexpected or do not support your ideas and hypotheses. Streamline and clarify your report, especially if it is long and complex, by using subheadings that will help you avoid excessive and peripheral details as you write and also help your reader understand and remember your findings. Consider appendices for raw data that might interest specialists but prove too long or distracting for other readers. The opening paragraph of a findings section often restates research questions or aims to refocus the reader’s attention, and it is always wise to summarise key findings at the end of the section, providing a smooth intellectual transition to the interpretation and discussion that follows in most research papers. There are many effective ways in which to organise research findings. The structure of your findings section might be determined by your research questions and hypotheses or match the arrangement of your methods section. A chronological order or hierarchy of importance or meaningful grouping of main themes or categories might prove effective. It may be best to present all the relevant findings and then explain them and your analysis of them, or explaining the results of each trial or test immediately after reporting it may render the material clearer and more comprehensible for your readers. Keep your audience, your most important evidence and your research goals in mind.

Step 3 : Design effective visual presentations of your research results to enhance the textual report of your findings. Tables of various styles and figures of all kinds such as graphs, maps and photos are used in reporting research findings, but do check the journal guidelines for instructions on the number of visual aids allowed, any required design elements and the preferred formats for numbering, labelling and placement in the manuscript. As a general rule, tables and figures should be numbered according to first mention in the main text of the paper, and each one should be clearly introduced and explained at least briefly in that text so that readers know what is presented and what they are expected to see in a particular visual element. Tables and figures should also be self-explanatory, however, so their design should include all definitions and other information necessary for a reader to understand the findings you intend to show without returning to your text. If you construct your tables and figures before drafting your findings section, they can serve as focal points to help you tell a clear and informative story about your findings and avoid unnecessary repetition. Some authors will even work on tables and figures before organising the findings section (Step 2), which can be an extremely effective approach, but it is important to remember that the textual report of findings remains primary. Visual aids can clarify and enrich the text, but they cannot take its place.
Step 4 : Write your findings section in a factual and objective manner. The goal is to communicate information – in some cases a great deal of complex information – as clearly, accurately and precisely as possible, so well-constructed sentences that maintain a simple structure will be far more effective than convoluted phrasing and expressions. The active voice is often recommended by publishers and the authors of writing manuals, and the past tense is appropriate because the research has already been done. Make sure your grammar, spelling and punctuation are correct and effective so that you are conveying the meaning you intend. Statements that are vague, imprecise or ambiguous will often confuse and mislead readers, and a verbose style will add little more than padding while wasting valuable words that might be put to far better use in clear and logical explanations. Some specialised terminology may be required when reporting findings, but anything potentially unclear or confusing that has not already been defined earlier in the paper should be clarified for readers, and the same principle applies to unusual or nonstandard abbreviations. Your readers will want to understand what you are reporting about your results, not waste time looking up terms simply to understand what you are saying. A logical approach to organising your findings section (Step 2) will help you tell a logical story about your research results as you explain, highlight, offer analysis and summarise the information necessary for readers to understand the discussion section that follows.
Step 5 : Review the draft of your findings section and edit and revise until it reports your key findings exactly as you would have them presented to your readers. Check for accuracy and consistency in data across the section as a whole and all its visual elements. Read your prose aloud to catch language errors, awkward phrases and abrupt transitions. Ensure that the order in which you have presented results is the best order for focussing readers on your research objectives and preparing them for the interpretations, speculations, recommendations and other elements of the discussion that you are planning. This will involve looking back over the paper’s introductory and background material as well as anticipating the discussion and conclusion sections, and this is precisely the right point in the process for reviewing and reflecting. Your research results have taken considerable time to obtain and analyse, so a little more time to stand back and take in the wider view from the research door you have opened is a wise investment. The opinions of any additional readers you can recruit, whether they are professional mentors and colleagues or family and friends, will often prove invaluable as well.
You might be interested in Services offered by Proof-Reading-Service.com
Journal editing.
Journal article editing services
PhD thesis editing services
Scientific Editing
Manuscript editing.
Manuscript editing services
Expert Editing
Expert editing for all papers
Research Editing
Research paper editing services
Professional book editing services
How To Write the Findings Section of a Research Paper These five steps will help you write a clear & interesting findings section for a research paper
Related Posts

How To Write a Journal Article
September 6, 2021

Tips on How To Write a Journal Article
August 30, 2021

How To Write Highlights for an Academic or Scientific Paper
September 7, 2021

Tips on How To Write an Effective Figure Legend
August 27, 2021
Our Recent Posts

Our review ratings
- Examples of Research Paper Topics in Different Study Areas Score: 98%
- Dealing with Language Problems – Journal Editor’s Feedback Score: 95%
- Making Good Use of a Professional Proofreader Score: 92%
- How To Format Your Journal Paper Using Published Articles Score: 95%
- Journal Rejection as Inspiration for a New Perspective Score: 95%
Explore our Categories
- Abbreviation in Academic Writing (4)
- Career Advice for Academics (5)
- Dealing with Paper Rejection (11)
- Grammar in Academic Writing (5)
- Help with Peer Review (7)
- How To Get Published (146)
- Paper Writing Advice (17)
- Referencing & Bibliographies (16)
Jump to navigation

Cochrane Training
Chapter 14: completing ‘summary of findings’ tables and grading the certainty of the evidence.
Holger J Schünemann, Julian PT Higgins, Gunn E Vist, Paul Glasziou, Elie A Akl, Nicole Skoetz, Gordon H Guyatt; on behalf of the Cochrane GRADEing Methods Group (formerly Applicability and Recommendations Methods Group) and the Cochrane Statistical Methods Group
Key Points:
- A ‘Summary of findings’ table for a given comparison of interventions provides key information concerning the magnitudes of relative and absolute effects of the interventions examined, the amount of available evidence and the certainty (or quality) of available evidence.
- ‘Summary of findings’ tables include a row for each important outcome (up to a maximum of seven). Accepted formats of ‘Summary of findings’ tables and interactive ‘Summary of findings’ tables can be produced using GRADE’s software GRADEpro GDT.
- Cochrane has adopted the GRADE approach (Grading of Recommendations Assessment, Development and Evaluation) for assessing certainty (or quality) of a body of evidence.
- The GRADE approach specifies four levels of the certainty for a body of evidence for a given outcome: high, moderate, low and very low.
- GRADE assessments of certainty are determined through consideration of five domains: risk of bias, inconsistency, indirectness, imprecision and publication bias. For evidence from non-randomized studies and rarely randomized studies, assessments can then be upgraded through consideration of three further domains.
Cite this chapter as: Schünemann HJ, Higgins JPT, Vist GE, Glasziou P, Akl EA, Skoetz N, Guyatt GH. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane, 2023. Available from www.training.cochrane.org/handbook .
14.1 ‘Summary of findings’ tables
14.1.1 introduction to ‘summary of findings’ tables.
‘Summary of findings’ tables present the main findings of a review in a transparent, structured and simple tabular format. In particular, they provide key information concerning the certainty or quality of evidence (i.e. the confidence or certainty in the range of an effect estimate or an association), the magnitude of effect of the interventions examined, and the sum of available data on the main outcomes. Cochrane Reviews should incorporate ‘Summary of findings’ tables during planning and publication, and should have at least one key ‘Summary of findings’ table representing the most important comparisons. Some reviews may include more than one ‘Summary of findings’ table, for example if the review addresses more than one major comparison, or includes substantially different populations that require separate tables (e.g. because the effects differ or it is important to show results separately). In the Cochrane Database of Systematic Reviews (CDSR), all ‘Summary of findings’ tables for a review appear at the beginning, before the Background section.
14.1.2 Selecting outcomes for ‘Summary of findings’ tables
Planning for the ‘Summary of findings’ table starts early in the systematic review, with the selection of the outcomes to be included in: (i) the review; and (ii) the ‘Summary of findings’ table. This is a crucial step, and one that review authors need to address carefully.
To ensure production of optimally useful information, Cochrane Reviews begin by developing a review question and by listing all main outcomes that are important to patients and other decision makers (see Chapter 2 and Chapter 3 ). The GRADE approach to assessing the certainty of the evidence (see Section 14.2 ) defines and operationalizes a rating process that helps separate outcomes into those that are critical, important or not important for decision making. Consultation and feedback on the review protocol, including from consumers and other decision makers, can enhance this process.
Critical outcomes are likely to include clearly important endpoints; typical examples include mortality and major morbidity (such as strokes and myocardial infarction). However, they may also represent frequent minor and rare major side effects, symptoms, quality of life, burdens associated with treatment, and resource issues (costs). Burdens represent the impact of healthcare workload on patient function and well-being, and include the demands of adhering to an intervention that patients or caregivers (e.g. family) may dislike, such as having to undergo more frequent tests, or the restrictions on lifestyle that certain interventions require (Spencer-Bonilla et al 2017).
Frequently, when formulating questions that include all patient-important outcomes for decision making, review authors will confront reports of studies that have not included all these outcomes. This is particularly true for adverse outcomes. For instance, randomized trials might contribute evidence on intended effects, and on frequent, relatively minor side effects, but not report on rare adverse outcomes such as suicide attempts. Chapter 19 discusses strategies for addressing adverse effects. To obtain data for all important outcomes it may be necessary to examine the results of non-randomized studies (see Chapter 24 ). Cochrane, in collaboration with others, has developed guidance for review authors to support their decision about when to look for and include non-randomized studies (Schünemann et al 2013).
If a review includes only randomized trials, these trials may not address all important outcomes and it may therefore not be possible to address these outcomes within the constraints of the review. Review authors should acknowledge these limitations and make them transparent to readers. Review authors are encouraged to include non-randomized studies to examine rare or long-term adverse effects that may not adequately be studied in randomized trials. This raises the possibility that harm outcomes may come from studies in which participants differ from those in studies used in the analysis of benefit. Review authors will then need to consider how much such differences are likely to impact on the findings, and this will influence the certainty of evidence because of concerns about indirectness related to the population (see Section 14.2.2 ).
Non-randomized studies can provide important information not only when randomized trials do not report on an outcome or randomized trials suffer from indirectness, but also when the evidence from randomized trials is rated as very low and non-randomized studies provide evidence of higher certainty. Further discussion of these issues appears also in Chapter 24 .
14.1.3 General template for ‘Summary of findings’ tables
Several alternative standard versions of ‘Summary of findings’ tables have been developed to ensure consistency and ease of use across reviews, inclusion of the most important information needed by decision makers, and optimal presentation (see examples at Figures 14.1.a and 14.1.b ). These formats are supported by research that focused on improved understanding of the information they intend to convey (Carrasco-Labra et al 2016, Langendam et al 2016, Santesso et al 2016). They are available through GRADE’s official software package developed to support the GRADE approach: GRADEpro GDT (www.gradepro.org).
Standard Cochrane ‘Summary of findings’ tables include the following elements using one of the accepted formats. Further guidance on each of these is provided in Section 14.1.6 .
- A brief description of the population and setting addressed by the available evidence (which may be slightly different to or narrower than those defined by the review question).
- A brief description of the comparison addressed in the ‘Summary of findings’ table, including both the experimental and comparison interventions.
- A list of the most critical and/or important health outcomes, both desirable and undesirable, limited to seven or fewer outcomes.
- A measure of the typical burden of each outcomes (e.g. illustrative risk, or illustrative mean, on comparator intervention).
- The absolute and relative magnitude of effect measured for each (if both are appropriate).
- The numbers of participants and studies contributing to the analysis of each outcomes.
- A GRADE assessment of the overall certainty of the body of evidence for each outcome (which may vary by outcome).
- Space for comments.
- Explanations (formerly known as footnotes).
Ideally, ‘Summary of findings’ tables are supported by more detailed tables (known as ‘evidence profiles’) to which the review may be linked, which provide more detailed explanations. Evidence profiles include the same important health outcomes, and provide greater detail than ‘Summary of findings’ tables of both of the individual considerations feeding into the grading of certainty and of the results of the studies (Guyatt et al 2011a). They ensure that a structured approach is used to rating the certainty of evidence. Although they are rarely published in Cochrane Reviews, evidence profiles are often used, for example, by guideline developers in considering the certainty of the evidence to support guideline recommendations. Review authors will find it easier to develop the ‘Summary of findings’ table by completing the rating of the certainty of evidence in the evidence profile first in GRADEpro GDT. They can then automatically convert this to one of the ‘Summary of findings’ formats in GRADEpro GDT, including an interactive ‘Summary of findings’ for publication.
As a measure of the magnitude of effect for dichotomous outcomes, the ‘Summary of findings’ table should provide a relative measure of effect (e.g. risk ratio, odds ratio, hazard) and measures of absolute risk. For other types of data, an absolute measure alone (such as a difference in means for continuous data) might be sufficient. It is important that the magnitude of effect is presented in a meaningful way, which may require some transformation of the result of a meta-analysis (see also Chapter 15, Section 15.4 and Section 15.5 ). Reviews with more than one main comparison should include a separate ‘Summary of findings’ table for each comparison.
Figure 14.1.a provides an example of a ‘Summary of findings’ table. Figure 15.1.b provides an alternative format that may further facilitate users’ understanding and interpretation of the review’s findings. Evidence evaluating different formats suggests that the ‘Summary of findings’ table should include a risk difference as a measure of the absolute effect and authors should preferably use a format that includes a risk difference .
A detailed description of the contents of a ‘Summary of findings’ table appears in Section 14.1.6 .
Figure 14.1.a Example of a ‘Summary of findings’ table
Summary of findings (for interactive version click here )
a All the stockings in the nine studies included in this review were below-knee compression stockings. In four studies the compression strength was 20 mmHg to 30 mmHg at the ankle. It was 10 mmHg to 20 mmHg in the other four studies. Stockings come in different sizes. If a stocking is too tight around the knee it can prevent essential venous return causing the blood to pool around the knee. Compression stockings should be fitted properly. A stocking that is too tight could cut into the skin on a long flight and potentially cause ulceration and increased risk of DVT. Some stockings can be slightly thicker than normal leg covering and can be potentially restrictive with tight foot wear. It is a good idea to wear stockings around the house prior to travel to ensure a good, comfortable fit. Participants put their stockings on two to three hours before the flight in most of the studies. The availability and cost of stockings can vary.
b Two studies recruited high risk participants defined as those with previous episodes of DVT, coagulation disorders, severe obesity, limited mobility due to bone or joint problems, neoplastic disease within the previous two years, large varicose veins or, in one of the studies, participants taller than 190 cm and heavier than 90 kg. The incidence for the seven studies that excluded high risk participants was 1.45% and the incidence for the two studies that recruited high-risk participants (with at least one risk factor) was 2.43%. We have used 10 and 30 per 1000 to express different risk strata, respectively.
c The confidence interval crosses no difference and does not rule out a small increase.
d The measurement of oedema was not validated (indirectness of the outcome) or blinded to the intervention (risk of bias).
e If there are very few or no events and the number of participants is large, judgement about the certainty of evidence (particularly judgements about imprecision) may be based on the absolute effect. Here the certainty rating may be considered ‘high’ if the outcome was appropriately assessed and the event, in fact, did not occur in 2821 studied participants.
f None of the other studies reported adverse effects, apart from four cases of superficial vein thrombosis in varicose veins in the knee region that were compressed by the upper edge of the stocking in one study.
Figure 14.1.b Example of alternative ‘Summary of findings’ table
14.1.4 Producing ‘Summary of findings’ tables
The GRADE Working Group’s software, GRADEpro GDT ( www.gradepro.org ), including GRADE’s interactive handbook, is available to assist review authors in the preparation of ‘Summary of findings’ tables. GRADEpro can use data on the comparator group risk and the effect estimate (entered by the review authors or imported from files generated in RevMan) to produce the relative effects and absolute risks associated with experimental interventions. In addition, it leads the user through the process of a GRADE assessment, and produces a table that can be used as a standalone table in a review (including by direct import into software such as RevMan or integration with RevMan Web), or an interactive ‘Summary of findings’ table (see help resources in GRADEpro).
14.1.5 Statistical considerations in ‘Summary of findings’ tables
14.1.5.1 dichotomous outcomes.
‘Summary of findings’ tables should include both absolute and relative measures of effect for dichotomous outcomes. Risk ratios, odds ratios and risk differences are different ways of comparing two groups with dichotomous outcome data (see Chapter 6, Section 6.4.1 ). Furthermore, there are two distinct risk ratios, depending on which event (e.g. ‘yes’ or ‘no’) is the focus of the analysis (see Chapter 6, Section 6.4.1.5 ). In the presence of a non-zero intervention effect, any variation across studies in the comparator group risks (i.e. variation in the risk of the event occurring without the intervention of interest, for example in different populations) makes it impossible for more than one of these measures to be truly the same in every study.
It has long been assumed in epidemiology that relative measures of effect are more consistent than absolute measures of effect from one scenario to another. There is empirical evidence to support this assumption (Engels et al 2000, Deeks and Altman 2001, Furukawa et al 2002). For this reason, meta-analyses should generally use either a risk ratio or an odds ratio as a measure of effect (see Chapter 10, Section 10.4.3 ). Correspondingly, a single estimate of relative effect is likely to be a more appropriate summary than a single estimate of absolute effect. If a relative effect is indeed consistent across studies, then different comparator group risks will have different implications for absolute benefit. For instance, if the risk ratio is consistently 0.75, then the experimental intervention would reduce a comparator group risk of 80% to 60% in the intervention group (an absolute risk reduction of 20 percentage points), but would also reduce a comparator group risk of 20% to 15% in the intervention group (an absolute risk reduction of 5 percentage points).
‘Summary of findings’ tables are built around the assumption of a consistent relative effect. It is therefore important to consider the implications of this effect for different comparator group risks (these can be derived or estimated from a number of sources, see Section 14.1.6.3 ), which may require an assessment of the certainty of evidence for prognostic evidence (Spencer et al 2012, Iorio et al 2015). For any comparator group risk, it is possible to estimate a corresponding intervention group risk (i.e. the absolute risk with the intervention) from the meta-analytic risk ratio or odds ratio. Note that the numbers provided in the ‘Corresponding risk’ column are specific to the ‘risks’ in the adjacent column.
For the meta-analytic risk ratio (RR) and assumed comparator risk (ACR) the corresponding intervention risk is obtained as:
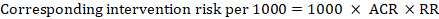
As an example, in Figure 14.1.a , the meta-analytic risk ratio for symptomless deep vein thrombosis (DVT) is RR = 0.10 (95% CI 0.04 to 0.26). Assuming a comparator risk of ACR = 10 per 1000 = 0.01, we obtain:
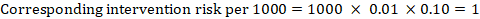
For the meta-analytic odds ratio (OR) and assumed comparator risk, ACR, the corresponding intervention risk is obtained as:
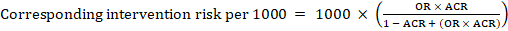
Upper and lower confidence limits for the corresponding intervention risk are obtained by replacing RR or OR by their upper and lower confidence limits, respectively (e.g. replacing 0.10 with 0.04, then with 0.26, in the example). Such confidence intervals do not incorporate uncertainty in the assumed comparator risks.
When dealing with risk ratios, it is critical that the same definition of ‘event’ is used as was used for the meta-analysis. For example, if the meta-analysis focused on ‘death’ (as opposed to survival) as the event, then corresponding risks in the ‘Summary of findings’ table must also refer to ‘death’.
In (rare) circumstances in which there is clear rationale to assume a consistent risk difference in the meta-analysis, in principle it is possible to present this for relevant ‘assumed risks’ and their corresponding risks, and to present the corresponding (different) relative effects for each assumed risk.
The risk difference expresses the difference between the ACR and the corresponding intervention risk (or the difference between the experimental and the comparator intervention).
For the meta-analytic risk ratio (RR) and assumed comparator risk (ACR) the corresponding risk difference is obtained as (note that risks can also be expressed using percentage or percentage points):
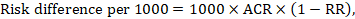
As an example, in Figure 14.1.b the meta-analytic risk ratio is 0.41 (95% CI 0.29 to 0.55) for diarrhoea in children less than 5 years of age. Assuming a comparator group risk of 22.3% we obtain:
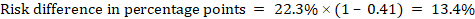
For the meta-analytic odds ratio (OR) and assumed comparator risk (ACR) the absolute risk difference is obtained as (percentage points):
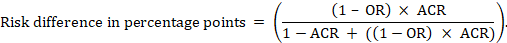
Upper and lower confidence limits for the absolute risk difference are obtained by re-running the calculation above while replacing RR or OR by their upper and lower confidence limits, respectively (e.g. replacing 0.41 with 0.28, then with 0.55, in the example). Such confidence intervals do not incorporate uncertainty in the assumed comparator risks.
14.1.5.2 Time-to-event outcomes
Time-to-event outcomes measure whether and when a particular event (e.g. death) occurs (van Dalen et al 2007). The impact of the experimental intervention relative to the comparison group on time-to-event outcomes is usually measured using a hazard ratio (HR) (see Chapter 6, Section 6.8.1 ).
A hazard ratio expresses a relative effect estimate. It may be used in various ways to obtain absolute risks and other interpretable quantities for a specific population. Here we describe how to re-express hazard ratios in terms of: (i) absolute risk of event-free survival within a particular period of time; (ii) absolute risk of an event within a particular period of time; and (iii) median time to the event. All methods are built on an assumption of consistent relative effects (i.e. that the hazard ratio does not vary over time).
(i) Absolute risk of event-free survival within a particular period of time Event-free survival (e.g. overall survival) is commonly reported by individual studies. To obtain absolute effects for time-to-event outcomes measured as event-free survival, the summary HR can be used in conjunction with an assumed proportion of patients who are event-free in the comparator group (Tierney et al 2007). This proportion of patients will be specific to a period of time of observation. However, it is not strictly necessary to specify this period of time. For instance, a proportion of 50% of event-free patients might apply to patients with a high event rate observed over 1 year, or to patients with a low event rate observed over 2 years.
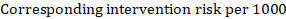
As an example, suppose the meta-analytic hazard ratio is 0.42 (95% CI 0.25 to 0.72). Assuming a comparator group risk of event-free survival (e.g. for overall survival people being alive) at 2 years of ACR = 900 per 1000 = 0.9 we obtain:
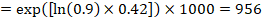
so that that 956 per 1000 people will be alive with the experimental intervention at 2 years. The derivation of the risk should be explained in a comment or footnote.
(ii) Absolute risk of an event within a particular period of time To obtain this absolute effect, again the summary HR can be used (Tierney et al 2007):
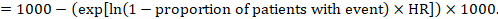
In the example, suppose we assume a comparator group risk of events (e.g. for mortality, people being dead) at 2 years of ACR = 100 per 1000 = 0.1. We obtain:
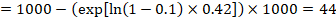
so that that 44 per 1000 people will be dead with the experimental intervention at 2 years.
(iii) Median time to the event Instead of absolute numbers, the time to the event in the intervention and comparison groups can be expressed as median survival time in months or years. To obtain median survival time the pooled HR can be applied to an assumed median survival time in the comparator group (Tierney et al 2007):
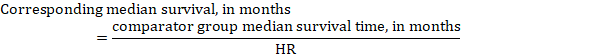
In the example, assuming a comparator group median survival time of 80 months, we obtain:
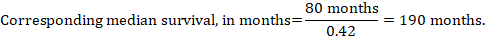
For all three of these options for re-expressing results of time-to-event analyses, upper and lower confidence limits for the corresponding intervention risk are obtained by replacing HR by its upper and lower confidence limits, respectively (e.g. replacing 0.42 with 0.25, then with 0.72, in the example). Again, as for dichotomous outcomes, such confidence intervals do not incorporate uncertainty in the assumed comparator group risks. This is of special concern for long-term survival with a low or moderate mortality rate and a corresponding high number of censored patients (i.e. a low number of patients under risk and a high censoring rate).
14.1.6 Detailed contents of a ‘Summary of findings’ table
14.1.6.1 table title and header.
The title of each ‘Summary of findings’ table should specify the healthcare question, framed in terms of the population and making it clear exactly what comparison of interventions are made. In Figure 14.1.a , the population is people taking long aeroplane flights, the intervention is compression stockings, and the control is no compression stockings.
The first rows of each ‘Summary of findings’ table should provide the following ‘header’ information:
Patients or population This further clarifies the population (and possibly the subpopulations) of interest and ideally the magnitude of risk of the most crucial adverse outcome at which an intervention is directed. For instance, people on a long-haul flight may be at different risks for DVT; those using selective serotonin reuptake inhibitors (SSRIs) might be at different risk for side effects; while those with atrial fibrillation may be at low (< 1%), moderate (1% to 4%) or high (> 4%) yearly risk of stroke.
Setting This should state any specific characteristics of the settings of the healthcare question that might limit the applicability of the summary of findings to other settings (e.g. primary care in Europe and North America).
Intervention The experimental intervention.
Comparison The comparator intervention (including no specific intervention).
14.1.6.2 Outcomes
The rows of a ‘Summary of findings’ table should include all desirable and undesirable health outcomes (listed in order of importance) that are essential for decision making, up to a maximum of seven outcomes. If there are more outcomes in the review, review authors will need to omit the less important outcomes from the table, and the decision selecting which outcomes are critical or important to the review should be made during protocol development (see Chapter 3 ). Review authors should provide time frames for the measurement of the outcomes (e.g. 90 days or 12 months) and the type of instrument scores (e.g. ranging from 0 to 100).
Note that review authors should include the pre-specified critical and important outcomes in the table whether data are available or not. However, they should be alert to the possibility that the importance of an outcome (e.g. a serious adverse effect) may only become known after the protocol was written or the analysis was carried out, and should take appropriate actions to include these in the ‘Summary of findings’ table.
The ‘Summary of findings’ table can include effects in subgroups of the population for different comparator risks and effect sizes separately. For instance, in Figure 14.1.b effects are presented for children younger and older than 5 years separately. Review authors may also opt to produce separate ‘Summary of findings’ tables for different populations.
Review authors should include serious adverse events, but it might be possible to combine minor adverse events as a single outcome, and describe this in an explanatory footnote (note that it is not appropriate to add events together unless they are independent, that is, a participant who has experienced one adverse event has an unaffected chance of experiencing the other adverse event).
Outcomes measured at multiple time points represent a particular problem. In general, to keep the table simple, review authors should present multiple time points only for outcomes critical to decision making, where either the result or the decision made are likely to vary over time. The remainder should be presented at a common time point where possible.
Review authors can present continuous outcome measures in the ‘Summary of findings’ table and should endeavour to make these interpretable to the target audience. This requires that the units are clear and readily interpretable, for example, days of pain, or frequency of headache, and the name and scale of any measurement tools used should be stated (e.g. a Visual Analogue Scale, ranging from 0 to 100). However, many measurement instruments are not readily interpretable by non-specialist clinicians or patients, for example, points on a Beck Depression Inventory or quality of life score. For these, a more interpretable presentation might involve converting a continuous to a dichotomous outcome, such as >50% improvement (see Chapter 15, Section 15.5 ).
14.1.6.3 Best estimate of risk with comparator intervention
Review authors should provide up to three typical risks for participants receiving the comparator intervention. For dichotomous outcomes, we recommend that these be presented in the form of the number of people experiencing the event per 100 or 1000 people (natural frequency) depending on the frequency of the outcome. For continuous outcomes, this would be stated as a mean or median value of the outcome measured.
Estimated or assumed comparator intervention risks could be based on assessments of typical risks in different patient groups derived from the review itself, individual representative studies in the review, or risks derived from a systematic review of prognosis studies or other sources of evidence which may in turn require an assessment of the certainty for the prognostic evidence (Spencer et al 2012, Iorio et al 2015). Ideally, risks would reflect groups that clinicians can easily identify on the basis of their presenting features.
An explanatory footnote should specify the source or rationale for each comparator group risk, including the time period to which it corresponds where appropriate. In Figure 14.1.a , clinicians can easily differentiate individuals with risk factors for deep venous thrombosis from those without. If there is known to be little variation in baseline risk then review authors may use the median comparator group risk across studies. If typical risks are not known, an option is to choose the risk from the included studies, providing the second highest for a high and the second lowest for a low risk population.
14.1.6.4 Risk with intervention
For dichotomous outcomes, review authors should provide a corresponding absolute risk for each comparator group risk, along with a confidence interval. This absolute risk with the (experimental) intervention will usually be derived from the meta-analysis result presented in the relative effect column (see Section 14.1.6.6 ). Formulae are provided in Section 14.1.5 . Review authors should present the absolute effect in the same format as the risks with comparator intervention (see Section 14.1.6.3 ), for example as the number of people experiencing the event per 1000 people.
For continuous outcomes, a difference in means or standardized difference in means should be presented with its confidence interval. These will typically be obtained directly from a meta-analysis. Explanatory text should be used to clarify the meaning, as in Figures 14.1.a and 14.1.b .
14.1.6.5 Risk difference
For dichotomous outcomes, the risk difference can be provided using one of the ‘Summary of findings’ table formats as an additional option (see Figure 14.1.b ). This risk difference expresses the difference between the experimental and comparator intervention and will usually be derived from the meta-analysis result presented in the relative effect column (see Section 14.1.6.6 ). Formulae are provided in Section 14.1.5 . Review authors should present the risk difference in the same format as assumed and corresponding risks with comparator intervention (see Section 14.1.6.3 ); for example, as the number of people experiencing the event per 1000 people or as percentage points if the assumed and corresponding risks are expressed in percentage.
For continuous outcomes, if the ‘Summary of findings’ table includes this option, the mean difference can be presented here and the ‘corresponding risk’ column left blank (see Figure 14.1.b ).

14.1.6.6 Relative effect (95% CI)
The relative effect will typically be a risk ratio or odds ratio (or occasionally a hazard ratio) with its accompanying 95% confidence interval, obtained from a meta-analysis performed on the basis of the same effect measure. Risk ratios and odds ratios are similar when the comparator intervention risks are low and effects are small, but may differ considerably when comparator group risks increase. The meta-analysis may involve an assumption of either fixed or random effects, depending on what the review authors consider appropriate, and implying that the relative effect is either an estimate of the effect of the intervention, or an estimate of the average effect of the intervention across studies, respectively.
14.1.6.7 Number of participants (studies)
This column should include the number of participants assessed in the included studies for each outcome and the corresponding number of studies that contributed these participants.
14.1.6.8 Certainty of the evidence (GRADE)
Review authors should comment on the certainty of the evidence (also known as quality of the body of evidence or confidence in the effect estimates). Review authors should use the specific evidence grading system developed by the GRADE Working Group (Atkins et al 2004, Guyatt et al 2008, Guyatt et al 2011a), which is described in detail in Section 14.2 . The GRADE approach categorizes the certainty in a body of evidence as ‘high’, ‘moderate’, ‘low’ or ‘very low’ by outcome. This is a result of judgement, but the judgement process operates within a transparent structure. As an example, the certainty would be ‘high’ if the summary were of several randomized trials with low risk of bias, but the rating of certainty becomes lower if there are concerns about risk of bias, inconsistency, indirectness, imprecision or publication bias. Judgements other than of ‘high’ certainty should be made transparent using explanatory footnotes or the ‘Comments’ column in the ‘Summary of findings’ table (see Section 14.1.6.10 ).
14.1.6.9 Comments
The aim of the ‘Comments’ field is to help interpret the information or data identified in the row. For example, this may be on the validity of the outcome measure or the presence of variables that are associated with the magnitude of effect. Important caveats about the results should be flagged here. Not all rows will need comments, and it is best to leave a blank if there is nothing warranting a comment.
14.1.6.10 Explanations
Detailed explanations should be included as footnotes to support the judgements in the ‘Summary of findings’ table, such as the overall GRADE assessment. The explanations should describe the rationale for important aspects of the content. Table 14.1.a lists guidance for useful explanations. Explanations should be concise, informative, relevant, easy to understand and accurate. If explanations cannot be sufficiently described in footnotes, review authors should provide further details of the issues in the Results and Discussion sections of the review.
Table 14.1.a Guidance for providing useful explanations in ‘Summary of findings’ (SoF) tables. Adapted from Santesso et al (2016)
14.2 Assessing the certainty or quality of a body of evidence
14.2.1 the grade approach.
The Grades of Recommendation, Assessment, Development and Evaluation Working Group (GRADE Working Group) has developed a system for grading the certainty of evidence (Schünemann et al 2003, Atkins et al 2004, Schünemann et al 2006, Guyatt et al 2008, Guyatt et al 2011a). Over 100 organizations including the World Health Organization (WHO), the American College of Physicians, the American Society of Hematology (ASH), the Canadian Agency for Drugs and Technology in Health (CADTH) and the National Institutes of Health and Clinical Excellence (NICE) in the UK have adopted the GRADE system ( www.gradeworkinggroup.org ).
Cochrane has also formally adopted this approach, and all Cochrane Reviews should use GRADE to evaluate the certainty of evidence for important outcomes (see MECIR Box 14.2.a ).
MECIR Box 14.2.a Relevant expectations for conduct of intervention reviews
For systematic reviews, the GRADE approach defines the certainty of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the quantity of specific interest. Assessing the certainty of a body of evidence involves consideration of within- and across-study risk of bias (limitations in study design and execution or methodological quality), inconsistency (or heterogeneity), indirectness of evidence, imprecision of the effect estimates and risk of publication bias (see Section 14.2.2 ), as well as domains that may increase our confidence in the effect estimate (as described in Section 14.2.3 ). The GRADE system entails an assessment of the certainty of a body of evidence for each individual outcome. Judgements about the domains that determine the certainty of evidence should be described in the results or discussion section and as part of the ‘Summary of findings’ table.
The GRADE approach specifies four levels of certainty ( Figure 14.2.a ). For interventions, including diagnostic and other tests that are evaluated as interventions (Schünemann et al 2008b, Schünemann et al 2008a, Balshem et al 2011, Schünemann et al 2012), the starting point for rating the certainty of evidence is categorized into two types:
- randomized trials; and
- non-randomized studies of interventions (NRSI), including observational studies (including but not limited to cohort studies, and case-control studies, cross-sectional studies, case series and case reports, although not all of these designs are usually included in Cochrane Reviews).
There are many instances in which review authors rely on information from NRSI, in particular to evaluate potential harms (see Chapter 24 ). In addition, review authors can obtain relevant data from both randomized trials and NRSI, with each type of evidence complementing the other (Schünemann et al 2013).
In GRADE, a body of evidence from randomized trials begins with a high-certainty rating while a body of evidence from NRSI begins with a low-certainty rating. The lower rating with NRSI is the result of the potential bias induced by the lack of randomization (i.e. confounding and selection bias).
However, when using the new Risk Of Bias In Non-randomized Studies of Interventions (ROBINS-I) tool (Sterne et al 2016), an assessment tool that covers the risk of bias due to lack of randomization, all studies may start as high certainty of the evidence (Schünemann et al 2018). The approach of starting all study designs (including NRSI) as high certainty does not conflict with the initial GRADE approach of starting the rating of NRSI as low certainty evidence. This is because a body of evidence from NRSI should generally be downgraded by two levels due to the inherent risk of bias associated with the lack of randomization, namely confounding and selection bias. Not downgrading NRSI from high to low certainty needs transparent and detailed justification for what mitigates concerns about confounding and selection bias (Schünemann et al 2018). Very few examples of where not rating down by two levels is appropriate currently exist.
The highest certainty rating is a body of evidence when there are no concerns in any of the GRADE factors listed in Figure 14.2.a . Review authors often downgrade evidence to moderate, low or even very low certainty evidence, depending on the presence of the five factors in Figure 14.2.a . Usually, certainty rating will fall by one level for each factor, up to a maximum of three levels for all factors. If there are very severe problems for any one domain (e.g. when assessing risk of bias, all studies were unconcealed, unblinded and lost over 50% of their patients to follow-up), evidence may fall by two levels due to that factor alone. It is not possible to rate lower than ‘very low certainty’ evidence.
Review authors will generally grade evidence from sound non-randomized studies as low certainty, even if ROBINS-I is used. If, however, such studies yield large effects and there is no obvious bias explaining those effects, review authors may rate the evidence as moderate or – if the effect is large enough – even as high certainty ( Figure 14.2.a ). The very low certainty level is appropriate for, but is not limited to, studies with critical problems and unsystematic clinical observations (e.g. case series or case reports).
Figure 14.2.a Levels of the certainty of a body of evidence in the GRADE approach. *Upgrading criteria are usually applicable to non-randomized studies only (but exceptions exist).
14.2.2 Domains that can lead to decreasing the certainty level of a body of evidence
We now describe in more detail the five reasons (or domains) for downgrading the certainty of a body of evidence for a specific outcome. In each case, if no reason is found for downgrading the evidence, it should be classified as 'no limitation or not serious' (not important enough to warrant downgrading). If a reason is found for downgrading the evidence, it should be classified as 'serious' (downgrading the certainty rating by one level) or 'very serious' (downgrading the certainty grade by two levels). For non-randomized studies assessed with ROBINS-I, rating down by three levels should be classified as 'extremely' serious.
(1) Risk of bias or limitations in the detailed design and implementation
Our confidence in an estimate of effect decreases if studies suffer from major limitations that are likely to result in a biased assessment of the intervention effect. For randomized trials, these methodological limitations include failure to generate a random sequence, lack of allocation sequence concealment, lack of blinding (particularly with subjective outcomes that are highly susceptible to biased assessment), a large loss to follow-up or selective reporting of outcomes. Chapter 8 provides a discussion of study-level assessments of risk of bias in the context of a Cochrane Review, and proposes an approach to assessing the risk of bias for an outcome across studies as ‘Low’ risk of bias, ‘Some concerns’ and ‘High’ risk of bias for randomized trials. Levels of ‘Low’. ‘Moderate’, ‘Serious’ and ‘Critical’ risk of bias arise for non-randomized studies assessed with ROBINS-I ( Chapter 25 ). These assessments should feed directly into this GRADE domain. In particular, ‘Low’ risk of bias would indicate ‘no limitation’; ‘Some concerns’ would indicate either ‘no limitation’ or ‘serious limitation’; and ‘High’ risk of bias would indicate either ‘serious limitation’ or ‘very serious limitation’. ‘Critical’ risk of bias on ROBINS-I would indicate extremely serious limitations in GRADE. Review authors should use their judgement to decide between alternative categories, depending on the likely magnitude of the potential biases.
Every study addressing a particular outcome will differ, to some degree, in the risk of bias. Review authors should make an overall judgement on whether the certainty of evidence for an outcome warrants downgrading on the basis of study limitations. The assessment of study limitations should apply to the studies contributing to the results in the ‘Summary of findings’ table, rather than to all studies that could potentially be included in the analysis. We have argued in Chapter 7, Section 7.6.2 , that the primary analysis should be restricted to studies at low (or low and unclear) risk of bias where possible.
Table 14.2.a presents the judgements that must be made in going from assessments of the risk of bias to judgements about study limitations for each outcome included in a ‘Summary of findings’ table. A rating of high certainty evidence can be achieved only when most evidence comes from studies that met the criteria for low risk of bias. For example, of the 22 studies addressing the impact of beta-blockers on mortality in patients with heart failure, most probably or certainly used concealed allocation of the sequence, all blinded at least some key groups and follow-up of randomized patients was almost complete (Brophy et al 2001). The certainty of evidence might be downgraded by one level when most of the evidence comes from individual studies either with a crucial limitation for one item, or with some limitations for multiple items. An example of very serious limitations, warranting downgrading by two levels, is provided by evidence on surgery versus conservative treatment in the management of patients with lumbar disc prolapse (Gibson and Waddell 2007). We are uncertain of the benefit of surgery in reducing symptoms after one year or longer, because the one study included in the analysis had inadequate concealment of the allocation sequence and the outcome was assessed using a crude rating by the surgeon without blinding.
(2) Unexplained heterogeneity or inconsistency of results
When studies yield widely differing estimates of effect (heterogeneity or variability in results), investigators should look for robust explanations for that heterogeneity. For instance, drugs may have larger relative effects in sicker populations or when given in larger doses. A detailed discussion of heterogeneity and its investigation is provided in Chapter 10, Section 10.10 and Section 10.11 . If an important modifier exists, with good evidence that important outcomes are different in different subgroups (which would ideally be pre-specified), then a separate ‘Summary of findings’ table may be considered for a separate population. For instance, a separate ‘Summary of findings’ table would be used for carotid endarterectomy in symptomatic patients with high grade stenosis (70% to 99%) in which the intervention is, in the hands of the right surgeons, beneficial, and another (if review authors considered it relevant) for asymptomatic patients with low grade stenosis (less than 30%) in which surgery appears harmful (Orrapin and Rerkasem 2017). When heterogeneity exists and affects the interpretation of results, but review authors are unable to identify a plausible explanation with the data available, the certainty of the evidence decreases.
(3) Indirectness of evidence
Two types of indirectness are relevant. First, a review comparing the effectiveness of alternative interventions (say A and B) may find that randomized trials are available, but they have compared A with placebo and B with placebo. Thus, the evidence is restricted to indirect comparisons between A and B. Where indirect comparisons are undertaken within a network meta-analysis context, GRADE for network meta-analysis should be used (see Chapter 11, Section 11.5 ).
Second, a review may find randomized trials that meet eligibility criteria but address a restricted version of the main review question in terms of population, intervention, comparator or outcomes. For example, suppose that in a review addressing an intervention for secondary prevention of coronary heart disease, most identified studies happened to be in people who also had diabetes. Then the evidence may be regarded as indirect in relation to the broader question of interest because the population is primarily related to people with diabetes. The opposite scenario can equally apply: a review addressing the effect of a preventive strategy for coronary heart disease in people with diabetes may consider studies in people without diabetes to provide relevant, albeit indirect, evidence. This would be particularly likely if investigators had conducted few if any randomized trials in the target population (e.g. people with diabetes). Other sources of indirectness may arise from interventions studied (e.g. if in all included studies a technical intervention was implemented by expert, highly trained specialists in specialist centres, then evidence on the effects of the intervention outside these centres may be indirect), comparators used (e.g. if the comparator groups received an intervention that is less effective than standard treatment in most settings) and outcomes assessed (e.g. indirectness due to surrogate outcomes when data on patient-important outcomes are not available, or when investigators seek data on quality of life but only symptoms are reported). Review authors should make judgements transparent when they believe downgrading is justified, based on differences in anticipated effects in the group of primary interest. Review authors may be aided and increase transparency of their judgements about indirectness if they use Table 14.2.b available in the GRADEpro GDT software (Schünemann et al 2013).
(4) Imprecision of results
When studies include few participants or few events, and thus have wide confidence intervals, review authors can lower their rating of the certainty of the evidence. The confidence intervals included in the ‘Summary of findings’ table will provide readers with information that allows them to make, to some extent, their own rating of precision. Review authors can use a calculation of the optimal information size (OIS) or review information size (RIS), similar to sample size calculations, to make judgements about imprecision (Guyatt et al 2011b, Schünemann 2016). The OIS or RIS is calculated on the basis of the number of participants required for an adequately powered individual study. If the 95% confidence interval excludes a risk ratio (RR) of 1.0, and the total number of events or patients exceeds the OIS criterion, precision is adequate. If the 95% CI includes appreciable benefit or harm (an RR of under 0.75 or over 1.25 is often suggested as a very rough guide) downgrading for imprecision may be appropriate even if OIS criteria are met (Guyatt et al 2011b, Schünemann 2016).
(5) High probability of publication bias
The certainty of evidence level may be downgraded if investigators fail to report studies on the basis of results (typically those that show no effect: publication bias) or outcomes (typically those that may be harmful or for which no effect was observed: selective outcome non-reporting bias). Selective reporting of outcomes from among multiple outcomes measured is assessed at the study level as part of the assessment of risk of bias (see Chapter 8, Section 8.7 ), so for the studies contributing to the outcome in the ‘Summary of findings’ table this is addressed by domain 1 above (limitations in the design and implementation). If a large number of studies included in the review do not contribute to an outcome, or if there is evidence of publication bias, the certainty of the evidence may be downgraded. Chapter 13 provides a detailed discussion of reporting biases, including publication bias, and how it may be tackled in a Cochrane Review. A prototypical situation that may elicit suspicion of publication bias is when published evidence includes a number of small studies, all of which are industry-funded (Bhandari et al 2004). For example, 14 studies of flavanoids in patients with haemorrhoids have shown apparent large benefits, but enrolled a total of only 1432 patients (i.e. each study enrolled relatively few patients) (Alonso-Coello et al 2006). The heavy involvement of sponsors in most of these studies raises questions of whether unpublished studies that suggest no benefit exist (publication bias).
A particular body of evidence can suffer from problems associated with more than one of the five factors listed here, and the greater the problems, the lower the certainty of evidence rating that should result. One could imagine a situation in which randomized trials were available, but all or virtually all of these limitations would be present, and in serious form. A very low certainty of evidence rating would result.
Table 14.2.a Further guidelines for domain 1 (of 5) in a GRADE assessment: going from assessments of risk of bias in studies to judgements about study limitations for main outcomes across studies
Table 14.2.b Judgements about indirectness by outcome (available in GRADEpro GDT)
Intervention:
Comparator:
Direct comparison:
Final judgement about indirectness across domains:
14.2.3 Domains that may lead to increasing the certainty level of a body of evidence
Although NRSI and downgraded randomized trials will generally yield a low rating for certainty of evidence, there will be unusual circumstances in which review authors could ‘upgrade’ such evidence to moderate or even high certainty ( Table 14.3.a ).
- Large effects On rare occasions when methodologically well-done observational studies yield large, consistent and precise estimates of the magnitude of an intervention effect, one may be particularly confident in the results. A large estimated effect (e.g. RR >2 or RR <0.5) in the absence of plausible confounders, or a very large effect (e.g. RR >5 or RR <0.2) in studies with no major threats to validity, might qualify for this. In these situations, while the NRSI may possibly have provided an over-estimate of the true effect, the weak study design may not explain all of the apparent observed benefit. Thus, despite reservations based on the observational study design, review authors are confident that the effect exists. The magnitude of the effect in these studies may move the assigned certainty of evidence from low to moderate (if the effect is large in the absence of other methodological limitations). For example, a meta-analysis of observational studies showed that bicycle helmets reduce the risk of head injuries in cyclists by a large margin (odds ratio (OR) 0.31, 95% CI 0.26 to 0.37) (Thompson et al 2000). This large effect, in the absence of obvious bias that could create the association, suggests a rating of moderate-certainty evidence. Note : GRADE guidance suggests the possibility of rating up one level for a large effect if the relative effect is greater than 2.0. However, if the point estimate of the relative effect is greater than 2.0, but the confidence interval is appreciably below 2.0, then some hesitation would be appropriate in the decision to rate up for a large effect. Another situation allows inference of a strong association without a formal comparative study. Consider the question of the impact of routine colonoscopy versus no screening for colon cancer on the rate of perforation associated with colonoscopy. Here, a large series of representative patients undergoing colonoscopy may provide high certainty evidence about the risk of perforation associated with colonoscopy. When the risk of the event among patients receiving the relevant comparator is known to be near 0 (i.e. we are certain that the incidence of spontaneous colon perforation in patients not undergoing colonoscopy is extremely low), case series or cohort studies of representative patients can provide high certainty evidence of adverse effects associated with an intervention, thereby allowing us to infer a strong association from even a limited number of events.
- Dose-response The presence of a dose-response gradient may increase our confidence in the findings of observational studies and thereby enhance the assigned certainty of evidence. For example, our confidence in the result of observational studies that show an increased risk of bleeding in patients who have supratherapeutic anticoagulation levels is increased by the observation that there is a dose-response gradient between the length of time needed for blood to clot (as measured by the international normalized ratio (INR)) and an increased risk of bleeding (Levine et al 2004). A systematic review of NRSI investigating the effect of cyclooxygenase-2 inhibitors on cardiovascular events found that the summary estimate (RR) with rofecoxib was 1.33 (95% CI 1.00 to 1.79) with doses less than 25mg/d, and 2.19 (95% CI 1.64 to 2.91) with doses more than 25mg/d. Although residual confounding is likely to exist in the NRSI that address this issue, the existence of a dose-response gradient and the large apparent effect of higher doses of rofecoxib markedly increase our strength of inference that the association cannot be explained by residual confounding, and is therefore likely to be both causal and, at high levels of exposure, substantial. Note : GRADE guidance suggests the possibility of rating up one level for a large effect if the relative effect is greater than 2.0. Here, the fact that the point estimate of the relative effect is greater than 2.0, but the confidence interval is appreciably below 2.0 might make some hesitate in the decision to rate up for a large effect
- Plausible confounding On occasion, all plausible biases from randomized or non-randomized studies may be working to under-estimate an apparent intervention effect. For example, if only sicker patients receive an experimental intervention or exposure, yet they still fare better, it is likely that the actual intervention or exposure effect is larger than the data suggest. For instance, a rigorous systematic review of observational studies including a total of 38 million patients demonstrated higher death rates in private for-profit versus private not-for-profit hospitals (Devereaux et al 2002). One possible bias relates to different disease severity in patients in the two hospital types. It is likely, however, that patients in the not-for-profit hospitals were sicker than those in the for-profit hospitals. Thus, to the extent that residual confounding existed, it would bias results against the not-for-profit hospitals. The second likely bias was the possibility that higher numbers of patients with excellent private insurance coverage could lead to a hospital having more resources and a spill-over effect that would benefit those without such coverage. Since for-profit hospitals are likely to admit a larger proportion of such well-insured patients than not-for-profit hospitals, the bias is once again against the not-for-profit hospitals. Since the plausible biases would all diminish the demonstrated intervention effect, one might consider the evidence from these observational studies as moderate rather than low certainty. A parallel situation exists when observational studies have failed to demonstrate an association, but all plausible biases would have increased an intervention effect. This situation will usually arise in the exploration of apparent harmful effects. For example, because the hypoglycaemic drug phenformin causes lactic acidosis, the related agent metformin was under suspicion for the same toxicity. Nevertheless, very large observational studies have failed to demonstrate an association (Salpeter et al 2007). Given the likelihood that clinicians would be more alert to lactic acidosis in the presence of the agent and over-report its occurrence, one might consider this moderate, or even high certainty, evidence refuting a causal relationship between typical therapeutic doses of metformin and lactic acidosis.
14.3 Describing the assessment of the certainty of a body of evidence using the GRADE framework
Review authors should report the grading of the certainty of evidence in the Results section for each outcome for which this has been performed, providing the rationale for downgrading or upgrading the evidence, and referring to the ‘Summary of findings’ table where applicable.
Table 14.3.a provides a framework and examples for how review authors can justify their judgements about the certainty of evidence in each domain. These justifications should also be included in explanatory notes to the ‘Summary of Findings’ table (see Section 14.1.6.10 ).
Chapter 15, Section 15.6 , describes in more detail how the overall GRADE assessment across all domains can be used to draw conclusions about the effects of the intervention, as well as providing implications for future research.
Table 14.3.a Framework for describing the certainty of evidence and justifying downgrading or upgrading
14.4 Chapter information
Authors: Holger J Schünemann, Julian PT Higgins, Gunn E Vist, Paul Glasziou, Elie A Akl, Nicole Skoetz, Gordon H Guyatt; on behalf of the Cochrane GRADEing Methods Group (formerly Applicability and Recommendations Methods Group) and the Cochrane Statistical Methods Group
Acknowledgements: Andrew D Oxman contributed to earlier versions. Professor Penny Hawe contributed to the text on adverse effects in earlier versions. Jon Deeks provided helpful contributions on an earlier version of this chapter. For details of previous authors and editors of the Handbook , please refer to the Preface.
Funding: This work was in part supported by funding from the Michael G DeGroote Cochrane Canada Centre and the Ontario Ministry of Health.
14.5 References
Alonso-Coello P, Zhou Q, Martinez-Zapata MJ, Mills E, Heels-Ansdell D, Johanson JF, Guyatt G. Meta-analysis of flavonoids for the treatment of haemorrhoids. British Journal of Surgery 2006; 93 : 909-920.
Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, Guyatt GH, Harbour RT, Haugh MC, Henry D, Hill S, Jaeschke R, Leng G, Liberati A, Magrini N, Mason J, Middleton P, Mrukowicz J, O'Connell D, Oxman AD, Phillips B, Schünemann HJ, Edejer TT, Varonen H, Vist GE, Williams JW, Jr., Zaza S. Grading quality of evidence and strength of recommendations. BMJ 2004; 328 : 1490.
Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, Vist GE, Falck-Ytter Y, Meerpohl J, Norris S, Guyatt GH. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011; 64 : 401-406.
Bhandari M, Busse JW, Jackowski D, Montori VM, Schünemann H, Sprague S, Mears D, Schemitsch EH, Heels-Ansdell D, Devereaux PJ. Association between industry funding and statistically significant pro-industry findings in medical and surgical randomized trials. Canadian Medical Association Journal 2004; 170 : 477-480.
Brophy JM, Joseph L, Rouleau JL. Beta-blockers in congestive heart failure. A Bayesian meta-analysis. Annals of Internal Medicine 2001; 134 : 550-560.
Carrasco-Labra A, Brignardello-Petersen R, Santesso N, Neumann I, Mustafa RA, Mbuagbaw L, Etxeandia Ikobaltzeta I, De Stio C, McCullagh LJ, Alonso-Coello P, Meerpohl JJ, Vandvik PO, Brozek JL, Akl EA, Bossuyt P, Churchill R, Glenton C, Rosenbaum S, Tugwell P, Welch V, Garner P, Guyatt G, Schünemann HJ. Improving GRADE evidence tables part 1: a randomized trial shows improved understanding of content in summary of findings tables with a new format. Journal of Clinical Epidemiology 2016; 74 : 7-18.
Deeks JJ, Altman DG. Effect measures for meta-analysis of trials with binary outcomes. In: Egger M, Davey Smith G, Altman DG, editors. Systematic Reviews in Health Care: Meta-analysis in Context . 2nd ed. London (UK): BMJ Publication Group; 2001. p. 313-335.
Devereaux PJ, Choi PT, Lacchetti C, Weaver B, Schünemann HJ, Haines T, Lavis JN, Grant BJ, Haslam DR, Bhandari M, Sullivan T, Cook DJ, Walter SD, Meade M, Khan H, Bhatnagar N, Guyatt GH. A systematic review and meta-analysis of studies comparing mortality rates of private for-profit and private not-for-profit hospitals. Canadian Medical Association Journal 2002; 166 : 1399-1406.
Engels EA, Schmid CH, Terrin N, Olkin I, Lau J. Heterogeneity and statistical significance in meta-analysis: an empirical study of 125 meta-analyses. Statistics in Medicine 2000; 19 : 1707-1728.
Furukawa TA, Guyatt GH, Griffith LE. Can we individualize the 'number needed to treat'? An empirical study of summary effect measures in meta-analyses. International Journal of Epidemiology 2002; 31 : 72-76.
Gibson JN, Waddell G. Surgical interventions for lumbar disc prolapse: updated Cochrane Review. Spine 2007; 32 : 1735-1747.
Guyatt G, Oxman A, Vist G, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann H. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336 : 3.
Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, DeBeer H, Jaeschke R, Rind D, Meerpohl J, Dahm P, Schünemann HJ. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011a; 64 : 383-394.
Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, Devereaux PJ, Montori VM, Freyschuss B, Vist G, Jaeschke R, Williams JW, Jr., Murad MH, Sinclair D, Falck-Ytter Y, Meerpohl J, Whittington C, Thorlund K, Andrews J, Schünemann HJ. GRADE guidelines 6. Rating the quality of evidence--imprecision. Journal of Clinical Epidemiology 2011b; 64 : 1283-1293.
Iorio A, Spencer FA, Falavigna M, Alba C, Lang E, Burnand B, McGinn T, Hayden J, Williams K, Shea B, Wolff R, Kujpers T, Perel P, Vandvik PO, Glasziou P, Schünemann H, Guyatt G. Use of GRADE for assessment of evidence about prognosis: rating confidence in estimates of event rates in broad categories of patients. BMJ 2015; 350 : h870.
Langendam M, Carrasco-Labra A, Santesso N, Mustafa RA, Brignardello-Petersen R, Ventresca M, Heus P, Lasserson T, Moustgaard R, Brozek J, Schünemann HJ. Improving GRADE evidence tables part 2: a systematic survey of explanatory notes shows more guidance is needed. Journal of Clinical Epidemiology 2016; 74 : 19-27.
Levine MN, Raskob G, Landefeld S, Kearon C, Schulman S. Hemorrhagic complications of anticoagulant treatment: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004; 126 : 287S-310S.
Orrapin S, Rerkasem K. Carotid endarterectomy for symptomatic carotid stenosis. Cochrane Database of Systematic Reviews 2017; 6 : CD001081.
Salpeter S, Greyber E, Pasternak G, Salpeter E. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database of Systematic Reviews 2007; 4 : CD002967.
Santesso N, Carrasco-Labra A, Langendam M, Brignardello-Petersen R, Mustafa RA, Heus P, Lasserson T, Opiyo N, Kunnamo I, Sinclair D, Garner P, Treweek S, Tovey D, Akl EA, Tugwell P, Brozek JL, Guyatt G, Schünemann HJ. Improving GRADE evidence tables part 3: detailed guidance for explanatory footnotes supports creating and understanding GRADE certainty in the evidence judgments. Journal of Clinical Epidemiology 2016; 74 : 28-39.
Schünemann HJ, Best D, Vist G, Oxman AD, Group GW. Letters, numbers, symbols and words: how to communicate grades of evidence and recommendations. Canadian Medical Association Journal 2003; 169 : 677-680.
Schünemann HJ, Jaeschke R, Cook DJ, Bria WF, El-Solh AA, Ernst A, Fahy BF, Gould MK, Horan KL, Krishnan JA, Manthous CA, Maurer JR, McNicholas WT, Oxman AD, Rubenfeld G, Turino GM, Guyatt G. An official ATS statement: grading the quality of evidence and strength of recommendations in ATS guidelines and recommendations. American Journal of Respiratory and Critical Care Medicine 2006; 174 : 605-614.
Schünemann HJ, Oxman AD, Brozek J, Glasziou P, Jaeschke R, Vist GE, Williams JW, Jr., Kunz R, Craig J, Montori VM, Bossuyt P, Guyatt GH. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ 2008a; 336 : 1106-1110.
Schünemann HJ, Oxman AD, Brozek J, Glasziou P, Bossuyt P, Chang S, Muti P, Jaeschke R, Guyatt GH. GRADE: assessing the quality of evidence for diagnostic recommendations. ACP Journal Club 2008b; 149 : 2.
Schünemann HJ, Mustafa R, Brozek J. [Diagnostic accuracy and linked evidence--testing the chain]. Zeitschrift für Evidenz, Fortbildung und Qualität im Gesundheitswesen 2012; 106 : 153-160.
Schünemann HJ, Tugwell P, Reeves BC, Akl EA, Santesso N, Spencer FA, Shea B, Wells G, Helfand M. Non-randomized studies as a source of complementary, sequential or replacement evidence for randomized controlled trials in systematic reviews on the effects of interventions. Research Synthesis Methods 2013; 4 : 49-62.
Schünemann HJ. Interpreting GRADE's levels of certainty or quality of the evidence: GRADE for statisticians, considering review information size or less emphasis on imprecision? Journal of Clinical Epidemiology 2016; 75 : 6-15.
Schünemann HJ, Cuello C, Akl EA, Mustafa RA, Meerpohl JJ, Thayer K, Morgan RL, Gartlehner G, Kunz R, Katikireddi SV, Sterne J, Higgins JPT, Guyatt G, Group GW. GRADE guidelines: 18. How ROBINS-I and other tools to assess risk of bias in nonrandomized studies should be used to rate the certainty of a body of evidence. Journal of Clinical Epidemiology 2018.
Spencer-Bonilla G, Quinones AR, Montori VM, International Minimally Disruptive Medicine W. Assessing the Burden of Treatment. Journal of General Internal Medicine 2017; 32 : 1141-1145.
Spencer FA, Iorio A, You J, Murad MH, Schünemann HJ, Vandvik PO, Crowther MA, Pottie K, Lang ES, Meerpohl JJ, Falck-Ytter Y, Alonso-Coello P, Guyatt GH. Uncertainties in baseline risk estimates and confidence in treatment effects. BMJ 2012; 345 : e7401.
Sterne JAC, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JPT. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016; 355 : i4919.
Thompson DC, Rivara FP, Thompson R. Helmets for preventing head and facial injuries in bicyclists. Cochrane Database of Systematic Reviews 2000; 2 : CD001855.
Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007; 8 .
van Dalen EC, Tierney JF, Kremer LCM. Tips and tricks for understanding and using SR results. No. 7: time‐to‐event data. Evidence-Based Child Health 2007; 2 : 1089-1090.
For permission to re-use material from the Handbook (either academic or commercial), please see here for full details.

Plasma Processing of Materials: Scientific Opportunities and Technological Challenges (1991)
Chapter: 1 summary, findings, conclusions, and recommendations, 1 summary, findings, conclusions, and recommendations.
This study focuses on the plasma processing of materials, a technology that impacts and is of vital importance to several of the largest manufacturing industries in the world. Foremost among these industries is the electronics industry, in which plasma-based processes are indispensable for the manufacture of very large-scale integrated (VLSI) microelectronic circuits (or chips). Plasma processing of materials is also a critical technology in the aerospace, automotive, steel, biomedical, and toxic waste management industries. Because plasma processing is an integral part of the infrastructure of so many American industries, it is important for both the economy and the national security that America maintain a strong leadership role in this technology.
A plasma is a partially or fully ionized gas containing electrons, ions, and neutral atoms or molecules. In Chapter 2 , the panel categorizes different kinds of plasmas and focuses on properties of man-made low-energy, highly collisional plasmas that are particularly useful in materials processing applications. The outstanding properties of most plasmas applied to processing of materials are associated with nonequilibrium conditions. These properties present a challenge to the plasma scientist and an opportunity to the technologist. The opportunities for materials processing stem from the ability of a plasma to provide a highly excited medium that has no chemical or physical counterpart in a natural, equilibrium environment. Plasmas alter the normal pathways through which chemical systems evolve from one stable state to another, thus providing the potential to produce materials with properties that are not attainable by any other means.
Applications of plasma-based systems used to process materials are diverse because of the broad range of plasma conditions, geometries, and excitation methods that may be used. The scientific underpinnings of plasma applications are multidisciplinary and include elements of electrodynamics, atomic science, surface science, computer science, and industrial process control. Because of the diversity of applications and the multidisciplinary nature of the science, scientific understanding lags technology. This report highlights this critical issue.
A summary of the many industrial applications of plasma-based systems for processing materials is included in Chapter 2 . Electronics and aerospace are the two major industries that are served by plasma processing technologies, although the automotive industry is likely to become a significant user of plasma-processed materials like those now in widespread use in the aerospace industry. The critical role of plasma processing technology in industry is illustrated in Chapter 2 .
For the electronics industry more than for any other considered by the panel, the impact of—and the critical and urgent need for—plasma-based materials processing is overwhelming. Thus Chapter 3 further elucidates plasma processing of electronic materials and, in particular, the use of plasmas in fabricating microelectronic components. The plasma equipment industry is an integral part of the electronics industry and has experienced dramatic growth in recent years because of the increasing use of plasma processes to meet the demands of fabricating devices with continually shrinking dimensions. In this country, the plasma equipment industry
is composed of many small companies loosely connected to integrated circuit manufacturers. In Japan, on the other hand, equipment vendors and device manufacturers are tightly linked and are often parts of the same company.
Plasma processes used today in fabricating microelectronic devices have been developed largely by time-consuming, costly, empirical exploration. The chemical and physical complexity of plasma-surface interactions has so far eluded the accurate numerical simulation that would enable process design. Similarly, plasma reactors have also been developed by trial and error. This is due, in part, to the fact that reactor design is intimately intertwined with the materials process for which it will be used. Nonetheless, fundamental studies of surface processes and plasma phenomena—both experimental and numerical—have contributed to process development by providing key insights that enable limitation of the broad process-variable operating space. The state of the science that underpins plasma processing technology in the United States is outlined in Chapter 4 . Although an impressive arsenal of both experimental and numerical tools has been developed, significant gaps in understanding and lack of instrumentation limit progress.
The broad interdisciplinary nature of plasma processing is highlighted in the discussion of education issues outlined in Chapter 5 , which addresses the challenges and opportunities associated with providing a science education in the area of plasma processing. For example, graduate programs specifically focused on plasma processing are rare because of insufficient funding of university research programs in this field. By contrast, both Japan and France have national initiatives that support education and research in plasma processing.
FINDINGS, CONCLUSIONS, AND RECOMMENDATIONS
Finding and Conclusion : In recent years, the number of applications requiring plasmas in the processing of materials has increased dramatically. Plasma processing is now indispensable to the fabrication of electronic components and is widely used in the aerospace industry and other industries. However, the United States is seeing a serious decline in plasma reactor development that is critical to plasma processing steps in the manufacture of VLSI microelectronic circuits. In the interest of the U.S. economy and national defense, renewed support for low-energy plasma science is imperative.
Finding and Conclusion : The demand for technology development is outstripping scientific understanding of many low-energy plasma processes. The central scientific problem underlying plasma processing concerns the interaction of low-energy collisional plasmas with solid surfaces. Understanding this problem requires knowledge and expertise drawn from plasma physics, atomic physics, condensed matter physics, chemistry, chemical engineering, electrical engineering, materials science, computer science, and computer engineering. In the absence of a coordinated approach, the diversity of the applications and of the science tends to diffuse the focus of both.
Finding : Technically, U.S. laboratories have made many excellent contributions to plasma processing research—making fundamental discoveries, developing numerical algorithms, and inventing new diagnostic techniques. However, poor coordination and inefficient transfer of insights gained from this research have inhibited its use in the design of new plasma reactors and processes.
Finding : The Panel on Plasma Processing of Materials finds that plasma processing of materials is a critical technology that is necessary to implement key recommendations contained in the National Research Council report Materials Science and Engineering for the 1990s (National Academy Press, Washington, D.C., 1989) and to enhance the health of technologies as identified in Report of the National Critical Technologies Panel (U.S. Government Printing Office, Washington, D.C., 1991). Specifically, plasma processing is an essential element in the synthesis and processing arsenal for manufacturing electronic, photonic, ceramic, composite, high-performance metal, and alloy materials.
Accordingly, the panel recommends:
Plasma processing should be identified as a component program of the Federal Initiative on advanced materials synthesis and processing that currently is being developed by the Office of Science and Technology Policy.
Through such a Plasma Processing Program, federal funds should be allocated specifically to stimulate focused research in plasma processing, both basic and applied, consistent with the long-term economic and defense goals of the nation.
The Plasma Processing Program should not only provide focus on common goals and promote coordination of the research performed by the national laboratories, universities, and industrial laboratories, but also integrate plasma equipment suppliers into the program.
Finding and Conclusion : Currently, computer-based modeling and plasma simulation are inadequate for developing plasma reactors. As a result, the detailed descriptions required to guide the transfer of processes from one reactor to another or to scale processes from a small to a large reactor are not available. Until we understand how geometry, electromagnetic design, and plasma-surface interactions affect material properties, the choice of plasma reactor for a given process will not be obvious, and costly trial-and-error methods will continue to be used. Yet there is no fundamental obstacle to improved modeling and simulation nor to the eventual creation of computer-aided design (CAD) tools for designing plasma reactors. The key missing ingredients are the following:
A reliable and extensive plasma data base against which the accuracy of simulations of plasmas can be compared . Plasma measurement technologies are sophisticated, but at present experiments are performed on a large variety of different reactors under widely varying conditions. A coordinated effort to diagnose simple, reference reactors is necessary to generate the necessary data base for evaluation of simulation results and to test new and old experimental methodology.
A reliable and extensive input data base for calculating plasma generation, transport, and surface interaction . The dearth of basic data needed for simulation of plasma generation, transport, and surface reaction processes results directly from insufficient generation of data, insufficient data compilation, insufficient distribution of data, and insufficient funding of these activities. The critical basic data needed for simulations and experiments have not been prioritized. For plasma-surface interactions, in particular, lack of data has precluded the formation of mechanistic models on which simulation tools are based. Further experimental studies are needed to elucidate these mechanisms.
Efficient numerical algorithms and supercomputers for simulating magnetized plasmas in three dimensions . The advent of unprecedented supercomputer capability in the next 5 to 10 years will have a major impact in this area, provided that current simulation methods are expanded to account for multidimensional effects in magnetized plasmas.
The Plasma Processing Program should include a thrust toward development of computer-aided design tools for developing and designing new plasma reactors.
The Plasma Processing Program should emphasize a coordinated approach toward generating the diagnostic and basic data needed for improved plasma and plasma-surface simulation capability.
A program to extend current algorithms for plasma reactor simulation should be included among the activities funded under the umbrella of the federal High
Performance Computing and Communications program 1 developed by the Office of Science and Technology Policy and started in FY92.
Finding and Conclusion : In the coming decade, custom-designed and custom-manufactured chips, i.e ., application-specific integrated circuits (ASICs), will gain an increasing fraction of the world market in microelectronic components. This market, in turn, will belong to the flexible manufacturer who uses a common set of processes and equipment to fabricate many different circuit designs. Such flexibility in processing will result only from real understanding of processes and reactors. On the other hand, plasma processes in use today have been developed using a combination of intuition, empiricism, and statistical optimization. Although it is unlikely that detailed, quantitative, first-principles-based simulation tools will be available for process design in the near future, design aids such as expert systems, which can be used to guide engineers in selecting initial conditions from which the final process is derived, could be developed if gaps in our fundamental understanding of plasma chemistry were filled.
Finding and Conclusion : Three areas are recognized by the panel as needing concerted, coordinated experimental and theoretical research: surface processes, plasma generation and transport, and plasma-surface interactions. For surface processes, studies using well-controlled reactive beams impinging on well-characterized surfaces are essential for enhancing our understanding and developing mechanistic models. For plasma generation and transport, chemical kinetic data and diagnostic data are needed to augment the basic plasma reactor CAD tool. For studying plasma-surface interactions, there is an urgent need for in situ analytical tools that provide information on surface composition, electronic structure, and material properties.
Finding and Conclusion : Breakthroughs in understanding the science will be paced by development of tools for the characterization of the systems. To meet the coming demands for flexible device manufacturing, plasma processes will have to be actively and precisely controlled. But today no diagnostic techniques exist that can be used unambiguously to determine material properties related to device yield. Moreover, the parametric models needed to relate diagnostic data to process variables are also lacking.
According, the panel recommends:
The Plasma Processing Program should be dedicated in part to the development of plasma process expert systems.
A coordinated program should be supported to generate basic data and simulation of surface processes, plasma generation and transport, and plasma-surface interactions.
A program should be supported that focuses on development of new instrumentation for real-time, in situ monitoring for control and analysis.
Finding : Research resources in low-energy plasma science in the United States are eroding at an alarming rate. U.S. scientists trained in this area in the 1950s and early 1960s are retiring or are moving to other areas of science for which support is more forthcoming. When compared to those in Japan and France, the U.S. educational infrastructure in plasma processing lacks focus, coordination, and funding. As a result, the United States will not be prepared to maintain its leading market position in plasma processing, let alone capture more market share as the plasma process industry grows into the 21st century.
Finding : Graduate programs are not offering adequate educational opportunities in the science of weakly ionized, highly collisional plasmas. An informal survey by the panel indicated that only a few U.S. universities offer formal course work in this science and that there are
insufficient texts on collisional plasmas and plasma processing. These deficiencies are a direct result of low-level funding for graduate research in plasma processing and low-energy plasmas.
Finding and Conclusion : The most serious need in undergraduate education is adequate, modern teaching laboratories. Due to the largely empirical nature of many aspects of plasma processing, proper training in the traditional scientific method, as provided in laboratory classes, is a necessary component of undergraduate education. The Instrumentation and Laboratory Improvement Program sponsored by the National Science Foundation has been partly successful in fulfilling these needs, but it is not sufficient.
Finding and Conclusion : Research experiences for undergraduates made available through industrial cooperative programs or internships are essential for high-quality technical education. But teachers and professors themselves must first be educated in low-energy plasma science and plasma processing before they can be expected to educate students. Industrial-university links can also help to impart a much needed, longer-term view to industrial research efforts.
As part of the Plasma Processing Program, government and industry together should support cooperative programs specific to plasma processing with universities and national laboratories.
A program should be established to provide industrial internships for teachers and professors in the area of plasma processing.
Plasma processing of materials is a critical technology to several of the largest manufacturing industries in the world—electronics, aerospace, automotive, steel, biomedical, and toxic waste management. This book describes the relationship between plasma processes and the many industrial applications, examines in detail plasma processing in the electronics industry, highlights the scientific foundation underlying this technology, and discusses education issues in this multidisciplinary field.
The committee recommends a coordinated, focused, and well-funded research program in this area that involves the university, federal laboratory, and industrial sectors of the community. It also points out that because plasma processing is an integral part of the infrastructure of so many American industries, it is important for both the economy and the national security that America maintain a strong leadership role in this technology.
READ FREE ONLINE
Welcome to OpenBook!
You're looking at OpenBook, NAP.edu's online reading room since 1999. Based on feedback from you, our users, we've made some improvements that make it easier than ever to read thousands of publications on our website.
Do you want to take a quick tour of the OpenBook's features?
Show this book's table of contents , where you can jump to any chapter by name.
...or use these buttons to go back to the previous chapter or skip to the next one.
Jump up to the previous page or down to the next one. Also, you can type in a page number and press Enter to go directly to that page in the book.
Switch between the Original Pages , where you can read the report as it appeared in print, and Text Pages for the web version, where you can highlight and search the text.
To search the entire text of this book, type in your search term here and press Enter .
Share a link to this book page on your preferred social network or via email.
View our suggested citation for this chapter.
Ready to take your reading offline? Click here to buy this book in print or download it as a free PDF, if available.
Get Email Updates
Do you enjoy reading reports from the Academies online for free ? Sign up for email notifications and we'll let you know about new publications in your areas of interest when they're released.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Dental Press J Orthod
- v.19(4); Jul-Aug 2014
Language: English | Portuguese
How sample size influences research outcomes
Jorge faber.
1 Adjunct professor, Department of Orthodontics, University of Brasília.
Lilian Martins Fonseca
2 Invited Professor, Department of Orthodontics, University of Brasília.
Sample size calculation is part of the early stages of conducting an epidemiological, clinical or lab study. In preparing a scientific paper, there are ethical and methodological indications for its use. Two investigations conducted with the same methodology and achieving equivalent results, but different only in terms of sample size, may point the researcher in different directions when it comes to making clinical decisions. Therefore, ideally, samples should not be small and, contrary to what one might think, should not be excessive. The aim of this paper is to discuss in clinical language the main implications of the sample size when interpreting a study.
O cálculo amostral faz parte dos estágios iniciais de realização de um estudo epidemiológico, clínico ou laboratorial. Há indicações éticas e metodológicas para o seu emprego na elaboração de um trabalho científico. Duas pesquisas, realizadas com a mesma metodologia obtendo resultados equivalentes, e que diferem apenas no tamanho da amostra, podem apontar para diferentes direções no processo de tomada de decisão clínica. Portanto, as amostras estudadas idealmente não devem ser pequenas e, ao contrário do que pode-se pensar, não devem ser excessivas. O objetivo desse artigo é discutir, numa linguagem clínica, as principais implicações do tamanho das amostras na interpretação de um estudo.
In recent years a growing concern has overwhelmed the scientific community in the healthcare area: Sample size calculation. Although at first blush it may seem like an overriding concern over methodological issues, notably to clinicians, such concern is utterly justifiable. This issue is of paramount importance.
Samples should not be either too big or too small since both have limitations that can compromise the conclusions drawn from the studies. Too small a sample may prevent the findings from being extrapolated, whereas too large a sample may amplify the detection of differences, emphasizing statistical differences that are not clinically relevant. 1 We will discuss in this article the major impacts of sample size on orthodontic studies.
FACTORS THAT AFFECT SAMPLE SIZE
The purpose of estimating the appropriate sample size is to produce studies capable of detecting clinically relevant differences. Bearing this point in mind, there are different formulas to calculate sample size. 2 , 3 These formulas comprise several aspects which are listed below. Most sample size calculators available on the web have limited validity because they use a single formula - which is usually not divulged - to generate sample sizes for the studies.
The first aspect is the type of variable being studied. For example, it should be determined if the variable is categorical like the Angle classification (Class I, II or III), or continuous like the length of the dental arch (usually measured in millimeters).
It is then necessary to determine the relationship between the groups that will be evaluated and the statistical analysis that will be employed. Are we going to evaluate groups that are independent, i.e., the measurements of one group do not influence the other? Are they dependent groups like the measurements taken before and after treatment? Are we going to use a split-mouth design, whereby treatment is performed on one quadrant and a different therapy on another quadrant? Will we be using t-test or chi-square test? All these questions lead to different sample size calculation formulas.
Subsequently, we have to answer the question concerning which results we envisage if a standard treatment is performed. What is the mean value or the expected ratio? The answer to this question is usually obtained from the literature or by means of pilot studies.
It is also important to determine what is the smallest magnitude of the effect and the extent to which it is clinically relevant. For example, how many degrees of difference in the ANB angle can be considered relevant? It is vital that we address this issue. The smaller the difference that we wish to identify, the greater the number of cases in a study. If researchers wish to detect a difference as small as 0.1° in an ANB angle, they will probably need thousands of patients in their study. If this value rises to 1°, the number of cases required falls drastically.
Finally, it is essential that the researcher determine the level of significance and the type II error, which is the probability of not rejecting the null hypothesis, although the hypothesis is actually false, which the study will accept as reasonable.
With this information in hand, we will apply the appropriate formula according to the study design in question, and determine the sample size. Today, this calculation is typically carried out with the aid of a computer program. For example, Pocock's formula 2 for continuous variables is frequently used in our specialty. It is used in studies where one wishes to examine the difference between data means with normal distribution and equal-size, independent groups.
PROBLEMS WITH VERY SMALL SAMPLES
Try to envision the following scenario. A researcher conducts a study on patients who are being treated with a new device which although very uncomfortable has the potential to improve treatment of Class II malocclusions. The researcher wishes to compare the new functional device with the Herbst appliance. Patients will be randomly assigned to each group. The researcher is not aware, but we are, that s/he needs 60 subjects (30 patients in each group) to ensure sufficient power to be able to extrapolate the statistical analysis results to the overall population. In other words, so that we can feel confident that these results will serve as a parameter on which to base the proposed treatment. Furthermore, we also know, although the researcher does not, that this new therapy is less effective than the traditional method.
However, the researcher used only 15 patients in each group. The results of the study showed that the new device is inferior to conventional treatment. What are the implications?
The first is that using a sample smaller than the ideal increases the chance of assuming as true a false premise. Thus, chances are that the proposed device has no disadvantage compared to traditional therapy. Furthermore, it is assumed that people were subjected to a study, and had to undergo in vain all additional suffering associated with the therapy, given that the goals of the study were not achieved. In addition, financial and time resources were squandered since ultimately it will contribute absolutely nothing to improve clinical practice or quality of life. The situation becomes even worse if the research involves public funding: A total waste of taxpayer money.
PROBLEMS WITH VERY LARGE SAMPLES
There is a widespread belief that large samples are ideal for research or statistical analysis. However, this is not always true. Using the above example as a case study, very large samples that exceed the value estimated by sample size calculation present different hurdles.
The first is ethical. Should a study be performed with more patients than necessary? This means that more people than needed are exposed to the new therapy. Potentially, this implies increased hassle and risk. Obviously the problem is compounded if the new protocol is inferior to the traditional method: More patients are involved in a new, uncomfortable therapy that yields inferior results.
The second obstacle is that the use of a larger number of cases can also involve more financial and human resources than necessary to obtain the desired response.
In addition to these factors, there is another noteworthy issue that has to do with statistics. Statistical tests were developed to handle samples, not populations. When numerous cases are included in the statistics, analysis power is substantially increased. This implies an exaggerated tendency to reject null hypotheses with clinically negligible differences. What is insignificant becomes significant. Thus, a potential statistically significant difference in the ANB angle of 0.1° between the groups cited in the previous example would obviously produce no clinical difference in the effects of wearing an appliance.
When very large samples are available in a retrospective study, the researcher needs first to collect subsamples randomly, and only then perform the statistical test. If it is a prospective study, the researcher should collect only what is necessary, and include a few more individuals to compensate for subjects that leave the study.
CONCLUSIONS
In designing a study, sample size calculation is important for methodological and ethical reasons, as well as for reasons of human and financial resources. When reading an article, the reader should be on the alert to ascertain that the study they are reading was subjected to sample size calculation. In the absence of this calculation, the findings of the study should be interpreted with caution.
An appropriate sample renders the research more efficient: Data generated are reliable, resource investment is as limited as possible, while conforming to ethical principles. The use of sample size calculation directly influences research findings. Very small samples undermine the internal and external validity of a study. Very large samples tend to transform small differences into statistically significant differences - even when they are clinically insignificant. As a result, both researchers and clinicians are misguided, which may lead to failure in treatment decisions.
How to cite this article: Faber J, Fonseca LM. How sample size influences research outcomes. Dental Press J Orthod. 2014 July-Aug;19(4):27-9. DOI: http://dx.doi.org/10.1590/2176-9451.19.4.027-029.ebo
- Privacy Policy
Buy Me a Coffee

Home » Research Paper Conclusion – Writing Guide and Examples
Research Paper Conclusion – Writing Guide and Examples
Table of Contents

Research Paper Conclusion
Definition:
A research paper conclusion is the final section of a research paper that summarizes the key findings, significance, and implications of the research. It is the writer’s opportunity to synthesize the information presented in the paper, draw conclusions, and make recommendations for future research or actions.
The conclusion should provide a clear and concise summary of the research paper, reiterating the research question or problem, the main results, and the significance of the findings. It should also discuss the limitations of the study and suggest areas for further research.
Parts of Research Paper Conclusion
The parts of a research paper conclusion typically include:
Restatement of the Thesis
The conclusion should begin by restating the thesis statement from the introduction in a different way. This helps to remind the reader of the main argument or purpose of the research.
Summary of Key Findings
The conclusion should summarize the main findings of the research, highlighting the most important results and conclusions. This section should be brief and to the point.
Implications and Significance
In this section, the researcher should explain the implications and significance of the research findings. This may include discussing the potential impact on the field or industry, highlighting new insights or knowledge gained, or pointing out areas for future research.
Limitations and Recommendations
It is important to acknowledge any limitations or weaknesses of the research and to make recommendations for how these could be addressed in future studies. This shows that the researcher is aware of the potential limitations of their work and is committed to improving the quality of research in their field.
Concluding Statement
The conclusion should end with a strong concluding statement that leaves a lasting impression on the reader. This could be a call to action, a recommendation for further research, or a final thought on the topic.
How to Write Research Paper Conclusion
Here are some steps you can follow to write an effective research paper conclusion:
- Restate the research problem or question: Begin by restating the research problem or question that you aimed to answer in your research. This will remind the reader of the purpose of your study.
- Summarize the main points: Summarize the key findings and results of your research. This can be done by highlighting the most important aspects of your research and the evidence that supports them.
- Discuss the implications: Discuss the implications of your findings for the research area and any potential applications of your research. You should also mention any limitations of your research that may affect the interpretation of your findings.
- Provide a conclusion : Provide a concise conclusion that summarizes the main points of your paper and emphasizes the significance of your research. This should be a strong and clear statement that leaves a lasting impression on the reader.
- Offer suggestions for future research: Lastly, offer suggestions for future research that could build on your findings and contribute to further advancements in the field.
Remember that the conclusion should be brief and to the point, while still effectively summarizing the key findings and implications of your research.
Example of Research Paper Conclusion
Here’s an example of a research paper conclusion:
Conclusion :
In conclusion, our study aimed to investigate the relationship between social media use and mental health among college students. Our findings suggest that there is a significant association between social media use and increased levels of anxiety and depression among college students. This highlights the need for increased awareness and education about the potential negative effects of social media use on mental health, particularly among college students.
Despite the limitations of our study, such as the small sample size and self-reported data, our findings have important implications for future research and practice. Future studies should aim to replicate our findings in larger, more diverse samples, and investigate the potential mechanisms underlying the association between social media use and mental health. In addition, interventions should be developed to promote healthy social media use among college students, such as mindfulness-based approaches and social media detox programs.
Overall, our study contributes to the growing body of research on the impact of social media on mental health, and highlights the importance of addressing this issue in the context of higher education. By raising awareness and promoting healthy social media use among college students, we can help to reduce the negative impact of social media on mental health and improve the well-being of young adults.
Purpose of Research Paper Conclusion
The purpose of a research paper conclusion is to provide a summary and synthesis of the key findings, significance, and implications of the research presented in the paper. The conclusion serves as the final opportunity for the writer to convey their message and leave a lasting impression on the reader.
The conclusion should restate the research problem or question, summarize the main results of the research, and explain their significance. It should also acknowledge the limitations of the study and suggest areas for future research or action.
Overall, the purpose of the conclusion is to provide a sense of closure to the research paper and to emphasize the importance of the research and its potential impact. It should leave the reader with a clear understanding of the main findings and why they matter. The conclusion serves as the writer’s opportunity to showcase their contribution to the field and to inspire further research and action.
When to Write Research Paper Conclusion
The conclusion of a research paper should be written after the body of the paper has been completed. It should not be written until the writer has thoroughly analyzed and interpreted their findings and has written a complete and cohesive discussion of the research.
Before writing the conclusion, the writer should review their research paper and consider the key points that they want to convey to the reader. They should also review the research question, hypotheses, and methodology to ensure that they have addressed all of the necessary components of the research.
Once the writer has a clear understanding of the main findings and their significance, they can begin writing the conclusion. The conclusion should be written in a clear and concise manner, and should reiterate the main points of the research while also providing insights and recommendations for future research or action.
Characteristics of Research Paper Conclusion
The characteristics of a research paper conclusion include:
- Clear and concise: The conclusion should be written in a clear and concise manner, summarizing the key findings and their significance.
- Comprehensive: The conclusion should address all of the main points of the research paper, including the research question or problem, the methodology, the main results, and their implications.
- Future-oriented : The conclusion should provide insights and recommendations for future research or action, based on the findings of the research.
- Impressive : The conclusion should leave a lasting impression on the reader, emphasizing the importance of the research and its potential impact.
- Objective : The conclusion should be based on the evidence presented in the research paper, and should avoid personal biases or opinions.
- Unique : The conclusion should be unique to the research paper and should not simply repeat information from the introduction or body of the paper.
Advantages of Research Paper Conclusion
The advantages of a research paper conclusion include:
- Summarizing the key findings : The conclusion provides a summary of the main findings of the research, making it easier for the reader to understand the key points of the study.
- Emphasizing the significance of the research: The conclusion emphasizes the importance of the research and its potential impact, making it more likely that readers will take the research seriously and consider its implications.
- Providing recommendations for future research or action : The conclusion suggests practical recommendations for future research or action, based on the findings of the study.
- Providing closure to the research paper : The conclusion provides a sense of closure to the research paper, tying together the different sections of the paper and leaving a lasting impression on the reader.
- Demonstrating the writer’s contribution to the field : The conclusion provides the writer with an opportunity to showcase their contribution to the field and to inspire further research and action.
Limitations of Research Paper Conclusion
While the conclusion of a research paper has many advantages, it also has some limitations that should be considered, including:
- I nability to address all aspects of the research: Due to the limited space available in the conclusion, it may not be possible to address all aspects of the research in detail.
- Subjectivity : While the conclusion should be objective, it may be influenced by the writer’s personal biases or opinions.
- Lack of new information: The conclusion should not introduce new information that has not been discussed in the body of the research paper.
- Lack of generalizability: The conclusions drawn from the research may not be applicable to other contexts or populations, limiting the generalizability of the study.
- Misinterpretation by the reader: The reader may misinterpret the conclusions drawn from the research, leading to a misunderstanding of the findings.
About the author
Muhammad Hassan
Researcher, Academic Writer, Web developer
You may also like

How to Cite Research Paper – All Formats and...

Data Collection – Methods Types and Examples

Delimitations in Research – Types, Examples and...

Research Paper Format – Types, Examples and...

Research Process – Steps, Examples and Tips

Research Design – Types, Methods and Examples
Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, generate accurate citations for free.
- Knowledge Base
- Starting the research process
- How to Write a Research Proposal | Examples & Templates
How to Write a Research Proposal | Examples & Templates
Published on October 12, 2022 by Shona McCombes and Tegan George. Revised on November 21, 2023.
A research proposal describes what you will investigate, why it’s important, and how you will conduct your research.
The format of a research proposal varies between fields, but most proposals will contain at least these elements:
Introduction
Literature review.
- Research design
Reference list
While the sections may vary, the overall objective is always the same. A research proposal serves as a blueprint and guide for your research plan, helping you get organized and feel confident in the path forward you choose to take.
Table of contents
Research proposal purpose, research proposal examples, research design and methods, contribution to knowledge, research schedule, other interesting articles, frequently asked questions about research proposals.
Academics often have to write research proposals to get funding for their projects. As a student, you might have to write a research proposal as part of a grad school application , or prior to starting your thesis or dissertation .
In addition to helping you figure out what your research can look like, a proposal can also serve to demonstrate why your project is worth pursuing to a funder, educational institution, or supervisor.
Research proposal length
The length of a research proposal can vary quite a bit. A bachelor’s or master’s thesis proposal can be just a few pages, while proposals for PhD dissertations or research funding are usually much longer and more detailed. Your supervisor can help you determine the best length for your work.
One trick to get started is to think of your proposal’s structure as a shorter version of your thesis or dissertation , only without the results , conclusion and discussion sections.
Download our research proposal template
Here's why students love Scribbr's proofreading services
Discover proofreading & editing
Writing a research proposal can be quite challenging, but a good starting point could be to look at some examples. We’ve included a few for you below.
- Example research proposal #1: “A Conceptual Framework for Scheduling Constraint Management”
- Example research proposal #2: “Medical Students as Mediators of Change in Tobacco Use”
Like your dissertation or thesis, the proposal will usually have a title page that includes:
- The proposed title of your project
- Your supervisor’s name
- Your institution and department
The first part of your proposal is the initial pitch for your project. Make sure it succinctly explains what you want to do and why.
Your introduction should:
- Introduce your topic
- Give necessary background and context
- Outline your problem statement and research questions
To guide your introduction , include information about:
- Who could have an interest in the topic (e.g., scientists, policymakers)
- How much is already known about the topic
- What is missing from this current knowledge
- What new insights your research will contribute
- Why you believe this research is worth doing
Prevent plagiarism. Run a free check.
As you get started, it’s important to demonstrate that you’re familiar with the most important research on your topic. A strong literature review shows your reader that your project has a solid foundation in existing knowledge or theory. It also shows that you’re not simply repeating what other people have already done or said, but rather using existing research as a jumping-off point for your own.
In this section, share exactly how your project will contribute to ongoing conversations in the field by:
- Comparing and contrasting the main theories, methods, and debates
- Examining the strengths and weaknesses of different approaches
- Explaining how will you build on, challenge, or synthesize prior scholarship
Following the literature review, restate your main objectives . This brings the focus back to your own project. Next, your research design or methodology section will describe your overall approach, and the practical steps you will take to answer your research questions.
To finish your proposal on a strong note, explore the potential implications of your research for your field. Emphasize again what you aim to contribute and why it matters.
For example, your results might have implications for:
- Improving best practices
- Informing policymaking decisions
- Strengthening a theory or model
- Challenging popular or scientific beliefs
- Creating a basis for future research
Last but not least, your research proposal must include correct citations for every source you have used, compiled in a reference list . To create citations quickly and easily, you can use our free APA citation generator .
Some institutions or funders require a detailed timeline of the project, asking you to forecast what you will do at each stage and how long it may take. While not always required, be sure to check the requirements of your project.
Here’s an example schedule to help you get started. You can also download a template at the button below.
Download our research schedule template
If you are applying for research funding, chances are you will have to include a detailed budget. This shows your estimates of how much each part of your project will cost.
Make sure to check what type of costs the funding body will agree to cover. For each item, include:
- Cost : exactly how much money do you need?
- Justification : why is this cost necessary to complete the research?
- Source : how did you calculate the amount?
To determine your budget, think about:
- Travel costs : do you need to go somewhere to collect your data? How will you get there, and how much time will you need? What will you do there (e.g., interviews, archival research)?
- Materials : do you need access to any tools or technologies?
- Help : do you need to hire any research assistants for the project? What will they do, and how much will you pay them?
If you want to know more about the research process , methodology , research bias , or statistics , make sure to check out some of our other articles with explanations and examples.
Methodology
- Sampling methods
- Simple random sampling
- Stratified sampling
- Cluster sampling
- Likert scales
- Reproducibility
Statistics
- Null hypothesis
- Statistical power
- Probability distribution
- Effect size
- Poisson distribution
Research bias
- Optimism bias
- Cognitive bias
- Implicit bias
- Hawthorne effect
- Anchoring bias
- Explicit bias
Once you’ve decided on your research objectives , you need to explain them in your paper, at the end of your problem statement .
Keep your research objectives clear and concise, and use appropriate verbs to accurately convey the work that you will carry out for each one.
I will compare …
A research aim is a broad statement indicating the general purpose of your research project. It should appear in your introduction at the end of your problem statement , before your research objectives.
Research objectives are more specific than your research aim. They indicate the specific ways you’ll address the overarching aim.
A PhD, which is short for philosophiae doctor (doctor of philosophy in Latin), is the highest university degree that can be obtained. In a PhD, students spend 3–5 years writing a dissertation , which aims to make a significant, original contribution to current knowledge.
A PhD is intended to prepare students for a career as a researcher, whether that be in academia, the public sector, or the private sector.
A master’s is a 1- or 2-year graduate degree that can prepare you for a variety of careers.
All master’s involve graduate-level coursework. Some are research-intensive and intend to prepare students for further study in a PhD; these usually require their students to write a master’s thesis . Others focus on professional training for a specific career.
Critical thinking refers to the ability to evaluate information and to be aware of biases or assumptions, including your own.
Like information literacy , it involves evaluating arguments, identifying and solving problems in an objective and systematic way, and clearly communicating your ideas.
The best way to remember the difference between a research plan and a research proposal is that they have fundamentally different audiences. A research plan helps you, the researcher, organize your thoughts. On the other hand, a dissertation proposal or research proposal aims to convince others (e.g., a supervisor, a funding body, or a dissertation committee) that your research topic is relevant and worthy of being conducted.
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the “Cite this Scribbr article” button to automatically add the citation to our free Citation Generator.
McCombes, S. & George, T. (2023, November 21). How to Write a Research Proposal | Examples & Templates. Scribbr. Retrieved April 11, 2024, from https://www.scribbr.com/research-process/research-proposal/
Is this article helpful?
Shona McCombes
Other students also liked, how to write a problem statement | guide & examples, writing strong research questions | criteria & examples, how to write a literature review | guide, examples, & templates, "i thought ai proofreading was useless but..".
I've been using Scribbr for years now and I know it's a service that won't disappoint. It does a good job spotting mistakes”
- Open supplemental data
- Reference Manager
- Simple TEXT file
People also looked at
Original research article, associations between monitor-independent movement summary (mims) and fall risk appraisal combining fear of falling and physiological fall risk in community-dwelling older adults.

- 1 Department of Mechanical Engineering, University of Central Florida, Orlando, FL, United States
- 2 Disability, Aging and Technology Cluster, University of Central Florida, Orlando, FL, United States
- 3 College of Medicine, University of Central Florida, Orlando, FL, United States
- 4 School of Kinesiology and Rehabilitation Sciences, College of Health Professions and Sciences, University of Central Florida, Orlando, FL, United States
- 5 Department of Statistics and Data Science, University of Central Florida, Orlando, FL, United States
- 6 College of Nursing, University of Central Florida, Orlando, FL, United States
Introduction: Fall Risk Appraisal (FRA), a process that integrates perceived and objective fall risk measures, serves as a crucial component for understanding the incongruence between fear of falling (FOF) and physiological fall risk in older adults. Despite its importance, scant research has been undertaken to investigate how habitual physical activity (PA) levels, quantified in Monitor-Independent Movement Summary (MIMS), vary across FRA categories. MIMS is a device-independent acceleration summary metric that helps standardize data analysis across studies by accounting for discrepancies in raw data among research-grade and consumer devices.
Objective: This cross-sectional study explores the associations between MIMS (volume and intensity) and FRA in a sample of older adults in the United States.
Methods: We assessed FOF (Short Falls Efficacy Scale-International), physiological fall risk (balance: BTrackS Balance, leg strength: 30-s sit-to-stand test) and 7-day free-living PA (ActiGraph GT9X) in 178 community-dwelling older adults. PA volume was summarized as average daily MIMS (MIMS/day). PA intensity was calculated as peak 30-min MIMS (average of highest 30 non-consecutive MIMS minutes/day), representing a PA index of higher-intensity epochs. FRA categorized participants into following four groups: Rational (low FOF-low physiological fall risk), Irrational (high FOF-low physiological fall risk), Incongruent (low FOF-high physiological fall risk) and Congruent (high FOF-high physiological fall risk).
Results: Compared to rational group, average MIMS/day and peak 30-min MIMS were, respectively, 15.8% ( p = .025) and 14.0% ( p = .004) lower in irrational group, and 16.6% ( p = .013) and 17.5% ( p < .001) lower in congruent group. No significant differences were detected between incongruent and rational groups. Multiple regression analyses showed that, after adjusting for age, gender, and BMI (reference: rational), only irrational FRA was significantly associated with lower PA volume (β = −1,452.8 MIMS/day, p = .034); whereas irrational and congruent FRAs were significantly associated with lower “peak PA intensity” (irrational: β = −5.40 MIMS/day, p = .007; congruent: β = −5.43 MIMS/day, p = .004).
Conclusion: These findings highlight that FOF is a significant barrier for older adults to participate in high-intensity PA, regardless of their balance and strength. Therefore, PA programs for older adults should develop tailored intervention strategies (cognitive reframing, balance and strength exercises, or both) based on an individual’s FOF and physiological fall risk.
Introduction
In the United States (US), over 14 million adults aged 65 years or older fall each year ( Moreland et al., 2020 ; Kakara et al., 2023 ). According to the US Centers for Disease Control and Prevention, about 20% of falls in older adults cause serious injuries, which results in limited functional mobility, loss of independence, reduced quality of life, and premature death ( Ambrose et al., 2013 ). Fear of falling (FOF) has been recognized as an important psychological aspect associated with falls in older adults ( Jansen et al., 2021 ). However, studies report that many older adults might show a discrepancy between their FOF and physiological fall risk, known as maladaptive fall risk appraisal (FRA) ( Thiamwong et al., 2021a ), and such discrepancies can lead to adverse consequences. For example, individuals with low physiological fall risk but high FOF may overestimate their actual fall risk and restrict their daily activities, which can further lead to physical deconditioning and loss of muscle strength ( Deshpande et al., 2008 ). On the contrary, those with high physiological fall risk but low FOF may underestimate their actual fall risk and engage in unnecessary risky behavior beyond their physical capacity, making them even more vulnerable to falling ( Delbaere et al., 2010 ).
Therefore, FRA combining subjective and objective fall risk measures is important for understanding the discrepancy between FOF and physiological fall risk in older adults to inform more targeted interventions for fall prevention ( Thiamwong et al., 2020a ; Thiamwong et al., 2020b ). FRA is a two-dimensional fall risk assessment matrix that classifies older adults into four groups based on their FOF and physiological fall risk status ( Thiamwong, 2020 ). In FRA matrix, as shown in Figure 1 , two groups have their FOF level aligned with their physiological fall risk status, which are denoted as Rational (low FOF-low physiological fall risk) and Congruent (high FOF-high physiological fall risk). The other two groups show a mismatch between their FOF level and physiological fall risk status and are denoted as Incongruent (low FOF-high physiological fall risk) and Irrational (high FOF-low physiological fall risk).
Figure 1 . Fall Risk Appraisal (FRA) based on Fear of Falling (FOF) and physiological fall risk. Maladaptive FRA = mismatch between FOF and physiological fall risk; Adaptive FRA = FOF aligned with physiological fall risk.
Prior research has mostly focused on exploring the independent associations of FOF and objective fall risk measures with physical activity (PA) participation in older adults ( Gregg et al., 2000 ; Chan et al., 2007 ; Zijlstra et al., 2007 ; Heesch et al., 2008 ; Mendes da Costa et al., 2012 ). To date, only a small number of studies have investigated the combined effects of FOF and objective fall risk on PA engagement. For example, one study examined the joint associations of FOF and objective fall risk with everyday walking activities in older adults. This study used a four-group categorization from ( Delbaere et al., 2010 ), and found that the number of steps/day in their study sample was in accordance with objective fall risk rather than FOF ( Jansen et al., 2021 ). Another study examined accelerometry-based PA levels between FRA categories using the intensity cut-point approach and found that participants with high FOF accumulated significantly less time in moderate-to-vigorous PA (MVPA) compared to those with rational FRA, regardless of their balance performance ( Thiamwong et al., 2023 ). However, there exists a lack of evidence on how habitual PA levels, expressed in Monitor-Independent Movement Summary (MIMS) units, differ between FRA categories in older adults.
MIMS is used to summarize the acceleration measurements obtained on the x-, y-, and z-axes of wrist-worn activity monitors. This PA metric was first introduced in 2019 to summarize participant-level PA data for the 2011-2012 and 2013-2014 cycles of the US National Health and Nutrition Examination Survey (NHANES) ( John et al., 2019 ). The major benefit of using MIMS is that it is generated by a nonproprietary device–independent universal algorithm, allowing us to compare the total movement across studies regardless of the heterogeneity introduced by different brands, models and device types (such as consumer vs. research-grade) ( John et al., 2019 ). Similar to other traditional PA metrics such as steps/day or daily activity counts, PA volume can be expressed as daily MIMS (i.e., total MIMS unit accumulated per day) across valid days of assessment, where larger MIMS/day indicates higher daily PA volume ( Wolff-Hughes et al., 2014 ).
Traditionally, quantification of accelerometer-measured PA intensity has been predominantly based on minutes/day (or minutes/week) spent in MVPA, using either manufacturer-specific or device-specific cut points corresponding with ≥3 Metabolic Equivalents of Task (METs) ( Troiano et al., 2008 ). Recently, to establish an intensity-based expression for MIMS units, the concept of peak 30-min MIMS has been introduced ( Zheng et al., 2023 ). It is analogous to the concept of peak 30-min cadence, i.e., the average of 30 highest cadence (steps/minutes) values within a day, representing an individual’s best efforts ( Tudor-Locke et al., 2012 ). Similar to cadence (steps/minutes), MIMS/minutes values were shown to have a strong correlation with higher PA intensity ( John et al., 2019 ). Therefore, peak 30-min MIMS (i.e., the average of the highest 30 non-consecutive MIMS [minutes/day] values within a day) can be used as a measure of higher-intensity epochs across the PA monitoring period ( Zheng et al., 2023 ). Evaluating daily MIMS (volume) and peak 30-min MIMS (intensity) can facilitate a more comprehensive assessment of PA and its relationship with FRA.
Thus, the aim of this study is to investigate the associations between wrist-worn accelerometer-measured PA (expressed as daily MIMS and peak 30-min MIMS) and FRA in a sample of community-dwelling older adults. We are particularly interested in the question: “Which of the maladaptive FRA groups, i.e., Incongruent (low FOF-high physiological fall risk) and Irrational (high FOF-low physiological fall risk), differ more from the Rational (low FOF-low physiological fall risk) group in terms of habitual PA level?.” This will allow us to understand which of the two factors—FOF or physiological fall risk—has a stronger relationship with reduced PA participation among older adults.
Materials and methods
Study design and participants.
In this cross-sectional study, purposive sampling was used to recruit 178 community-dwelling older adults from the region of Central Florida, United States, between February 2021 and March 2023. The inclusion criteria were: i) 60 years of age or older; ii) being able to walk with or without an assistive device (but without the assistance of another person); iii) no marked cognitive impairment [i.e., Memory Impairment Screen score ≥5 ( Buschke et al., 1999 )], iv) fluency in English or Spanish, and v) living in their own homes or apartments. The exclusion criteria were: i) medical conditions that prevent PA engagement (e.g., shortness of breath, tightness in the chest, dizziness, or unusual fatigue at light exertion), ii) unable to stand on the balance plate, iii) currently receiving treatment from a rehabilitation facility, and iv) having medical implants (e.g., pacemakers). This study was approved by the Institutional Review Board at the University of Central Florida (Protocol No: 2189; 10 September 2020). All subjects provided written informed consent to participate. This cross-sectional assessment required one visit to the study site during which participants completed a demographic survey and anthropometric measurements, followed by assessments of FOF and physiological fall risk. At the end of the visit, each participant was fitted with a wrist-worn accelerometer for 7-day PA monitoring in free-living conditions.
Measurements
Fear of falling (fof).
FOF was assessed using the Short Falls Efficacy Scale-International (FES-I) questionnaire ( Yardley et al., 2005 ; Kempen et al., 2008 ). It is a 7-item, self-administered tool that uses a 4-point Likert scale to measure the level of concern about falling while performing seven activities (1 = not at all concerned to 4 = very concerned). The total scores ranged from 7 to 28. Short FES-I scores of 7–10 indicated low FOF, while scores of 11–28 indicated high FOF.
Physiological fall risk
Physiological fall risk was assessed using balance test and lower limb strength assessment. BTrackS Balance System (Balance Tracking Systems, San Diego, CA, United States) was used to measure static balance. This system includes a portable BTrackS Balance Plate and BTrackS Assess Balance Software running on a computer. It has shown high test–retest reliability (intraclass correlation coefficient, ICC = 0.83) and excellent validity (Pearson’s product-moment correlations, r > 0.90) in evaluating static balance ( Levy et al., 2018 ). The test protocol included four trials (each trial taking 20 s) with less than 10 s of inter-trial delays. During the trials, participants were asked to stand still on the BTrackS Balance Plate with their eyes closed, hands on their hips, and feet placed shoulder-width apart. BTrackS balance plate is an FDA-registered, lightweight force plate that measures center of pressure (COP) excursions during the static stance. The first trial was done for familiarization only. Results from the remaining three trials were used to calculate the average COP path length (in cm) across trials. COP path length is considered as a proxy measure for postural sway magnitude; thus, the larger the COP path length, the greater the postural sway is ( Goble et al., 2017 ). COP path length of 0–30 cm was used to indicate normal balance, while ≥31 cm indicated poor balance ( Thiamwong et al., 2021b ).
Lower limb strength was assessed using the 30-s sit-to-stand (STS) test, in accordance with the established protocol ( Yee et al., 2021 ; Choudhury et al., 2023 ). Participants were instructed to keep their arms folded across their chest, rise from a seated position on a chair to a standing posture and return to the sitting position as many times as possible within 30 s. The number of chair stands completed was counted and recorded. If a participant used his/her arms to stand, the test was stopped, and the score was recorded as zero. Age- and gender-specific STS normative scores were used as cut-offs to classify participants into below-average and average STS scores, as shown in Table 1 ( Rikli and Jones, 1999 ). A below-average STS score was indicative of a higher risk of fall. Meeting both normal balance and average STS score criteria was defined as low physiological fall risk, while not meeting either or both criteria was defined as high physiological fall risk.
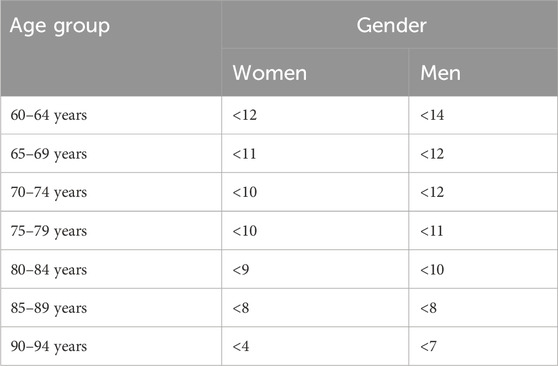
Table 1 . Age and gender-specific below average scores for 30-s sit-to-stand test.
Fall risk appraisal (FRA) matrix
The FRA matrix was obtained using a combination of FOF and physiological fall risk status. Participants were grouped into the following four categories based on their FOF and physiological fall risk according to existing literature ( Thiamwong et al., 2020a ): i) Rational (low FOF-low physiological fall risk), ii) Irrational (high FOF-low physiological fall risk), iii) Incongruent (low FOF-high physiological fall risk), and iv) Congruent (high FOF-high physiological fall risk).
Physical activity (PA)
PA was assessed using ActiGraph GT9X Link (ActiGraph LLC., Pensacola, FL, United States), which contains a triaxial accelerometer with a dynamic range of ±8 gravitational units (g). The device was initialized to record acceleration data at 30 Hz sampling frequency. Participants wore the ActiGraph on their non-dominant wrists for seven consecutive days in free-living conditions. They were given instructions to wear it during waking hours and remove it only during sleeping, showering, swimming and medical imaging tests. After 7-day of PA monitoring, ActiGraph devices were collected from participants. Participants with ≥4 valid days were included in the analysis, and a day was considered valid if participants wore the device for at least 14 h or more ( Choudhury et al., 2023 ).
Raw acceleration data were downloaded as “.csv” files using ActiLife software v6.13.4 (ActiGraph LLC, Pensacola, FL, United States) and converted into MIMS units using MIMSunit package ( John et al., 2019 ) in R statistical software (R Core Team, Vienna, Austria). The data processing steps included: i) interpolating data to a consistent sampling rate (i.e., 100 Hz ) to account for inter-device variability in sampling rate, ii) extrapolating data to extend maxed-out signals to account for inter-device variability in dynamic range, iii) band-pass filtering to remove artifacts from acceleration signals that do not pertain to voluntary human movement, and iv) aggregation of processed signals from each axis into a sum of MIMS-units that represents the total amount of movement activity [details on MIMS-unit algorithm are published elsewhere ( John et al., 2019 )].
PA volume, denoted by daily MIMS (MIMS/day), was calculated by summing up all triaxial MIMS/minutes accumulated throughout a day and averaged across all valid days. PA intensity, expressed as peak 30-min MIMS, was obtained by (a) first rank ordering a participant’s triaxial MIMS/minutes values within each valid day, (b) calculating the average of the highest 30 MIMS/minutes values within each day, and (c) finally taking the average of the resulting MIMS/minutes values across all valid wear days.
Anthropometric measurements
Height (in cm) was measured using a stadiometer. Body mass (in kilograms) was measured using a digital scale with no shoes. Body mass index (BMI) was calculated as the weight (kg) divided by the square of height (m 2 ).
Statistical analyses
All statistical analyses were performed in R statistical software (version 4.1.2, R Core Team, Vienna, Austria) with statistical significance level set at .05. Descriptive characteristics of participants were summarized as mean (standard deviation, SD) for normally distributed continuous variables, as median (Interquartile Range, IQR) for non-normally distributed continuous variables, and as frequency (percentage) for categorical variables, stratified by FRA categories. The Shapiro-Wilk test was performed to check if a continuous variable followed a normal distribution. Differences across groups were examined using one-way analysis of variance (ANOVA) for normally distributed data and Kruskal–Wallis test for non-normally distributed data, with Bonferroni adjustment for post hoc comparisons.
Multiple linear regression was conducted for each outcome variable (i.e., daily MIMS and peak 30-min MIMS) using the four FRA groups—“Rational,” “Irrational,” “Incongruent” and “Congruent”—as explanatory variables, controlled by age, gender and BMI. A priori sample size calculation for multiple linear regression revealed that the minimum number of samples for 8 explanatory variables at a statistical power level of 0.8, α = 0.05, and a medium effect size (Cohen f 2 = 0.15) would be 108; therefore, our sample size (i.e., N = 178) had sufficient statistical power for multiple regression. The rational group (i.e., low FOF-low physiological fall risk) was selected as the reference group in the regression analysis.
Among 178 participants, 163 samples were included in the analyses, after retaining only those who had at least 4 days of valid PA data and completed both FOF and physiological fall risk assessments. The mean (SD) age of participants was 75.3 (7.1) years, and 73.6% of participants were in 60–79 years of age group ( n = 120) and 26.4% were above 80 years of age ( n = 43). Figure 2 shows the scatterplot of participants’ age (years) and FOF scores, stratified by physiological fall risk status. The proportion of participants with low FOF was 71.7% ( n = 86) in the 60–79 years of age group and 48.8% ( n = 21) in the ≥80 years of age group. The median (IQR) BMI of participants was 26.6 (6.3) kg/m 2 and majority of participants were female (79.1%). The median (IQR) Short FES-I score was 9 (5) and 34.4% of participants had high FOF. The median (IQR) COP path length was 27 (15) cm , and the median (IQR) sit-to-stand score was 13 (6) reps. 38.0% of participants had poor balance, 27.0% had below average lower limb strength, and 48.5% showed both poor balance and below average lower limb strength. Finally, 37.4% of participants were screened as rational ( n = 61), 14.2% were irrational ( n = 23), 28.2% were incongruent ( n = 46) and 20.2% were congruent ( n = 37). Table 2 summarizes the characteristics of study participants according to FRA categories.
Figure 2 . Scatterplot of Age (years) across Fear of Falling scores, stratified by physiological fall risk status. Low physiological fall risk = meeting both normal static balance cut-off and average sit-to-stand score cut-off. High physiological fall risk = not meeting normal static balance cut-off or average sit-to-stand score cut-off or both.
Table 2 . Participant characteristics stratified by Fall Risk Appraisal matrix.
In Figure 3A , the variations in average MIMS (MIMS/hours) over 24-h by FRA categories are shown. The average MIMS across all groups was in general low at night, then substantially increased during morning hours and gradually decreased as the day progressed and evening approached. In Figure 3B , the mean (line) and standard error (shaded area) of MIMS/hours for each FRA group is shown. Overall, rational group showed the highest average MIMS/hours across the day hours, while congruent had the lowest average MIMS/hours. Among maladaptive FRA groups, the peak was higher in incongruent group than their irrational counterparts, which indicates the potential role of FOF in limiting high-intensity PA participation.
Figure 3 . (A) Daily patterns of average MIMS per hour by Fall Risk Appraisal (FRA) groups. (B) Mean (line) and standard error (shaded area) of MIMS per hour for each FRA group.
The mean (SD) age in congruent group was 78.8 (7.6) years, which was higher than both rational (74.3 [5.8] years, p = .005) and incongruent (74.3 [7.0] years, p = .010) groups, as shown in Supplementary Figure S1 . This suggests that prevalence of high FOF, irrespective of balance performance and lower limb strength, may increase with advanced age. Also, the median (IQR) BMI in congruent group (28.9 [5.8]) kg/m 2 ) was higher in comparison to rational (24.9 [6.4] kg/m 2 , p = .001) and incongruent (26.9 [4.7] kg/m 2 , p = .018) groups (shown in Supplementary Figure S2 ), indicating that higher BMI in older adults may result in high FOF. However, no significant group differences were observed between rational and irrational groups in terms of age and BMI.
The mean (SD) daily MIMS in rational group was 10,408 (2,439) MIMS/day, which was 15.8% higher than irrational ( p = .025) and 16.6% higher than congruent ( p = .013) groups, as shown in Figure 4 . Also, the mean (SD) peak 30-min MIMS in rational group was 39.9 (8.3) MIMS/day, which was 14.0% higher than irrational ( p = .004) and 17.5% higher than congruent ( p < .001) groups ( Figure 5 ). Compared to rational group, incongruent participants showed no significant differences in PA volume and intensity, despite having poor balance and below average lower limb strength.
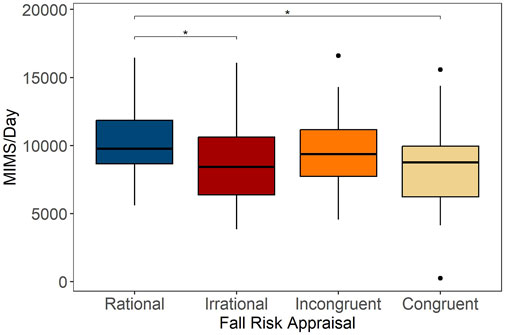
Figure 4 . Average daily MIMS (MIMS/day) across categories of Fall Risk Appraisal combining FOF and physiological fall risk, * p < .05.
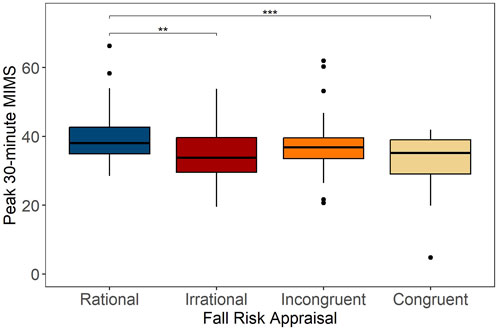
Figure 5 . Peak 30-min MIMS per day across categories of Fall Risk Appraisal combining FOF and physiological fall risk, ** p < .01, *** p < .001.
Table 2 presents the regression models for daily MIMS. In comparison to reference group (i.e., rational), lower PA volume was associated with irrational ( β [SE] = −1,463.2 [687.7] MIMS/day, p = .035) and congruent ( β [SE] = −1,579.5 [582.9] MIMS/day, p = .007) FRAs in Model 1 (unadjusted). In model 2, after adjusting for age, gender and BMI, only irrational FRA was significantly associated with lower PA volume ( β [SE] = −1,476.41 [582.26] MIMS/day, p = .025; regression coefficients of covariates are presented in Supplementary Table S1 ).
Results for regression analysis for peak 30-min MIMS are presented in Table 3 . In model 1 (unadjusted), lower ‘peak PA intensity’ was associated with irrational ( β [SE] = −5.63 [1.99] MIMS/day, p = .005) and congruent FRAs ( β [SE] = −7.06 [1.76] MIMS/day, p < .001) compared to the reference group. In Model 2 ( Table 4 ), after adjusting for age, gender and BMI, both irrational and congruent FRAs were still significantly associated with lower “peak PA intensity”(irrational: β [SE] = −5.40 [1.97] MIMS/day, p = .007; congruent: β [SE] = −5.43 [1.86] MIMS/day, p = .004; regression coefficients of covariates are presented in Supplementary Table S2 ).
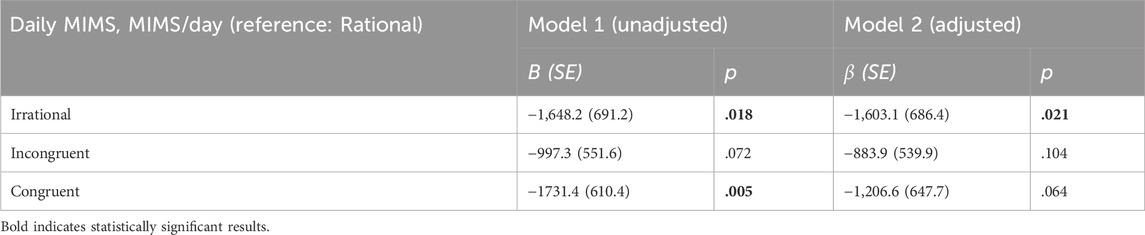
Table 3 . Association between Fall Risk Appraisal groups and average daily MIMS (MIMS/day) using linear regression. Model 2 was adjusted for age, gender and BMI.
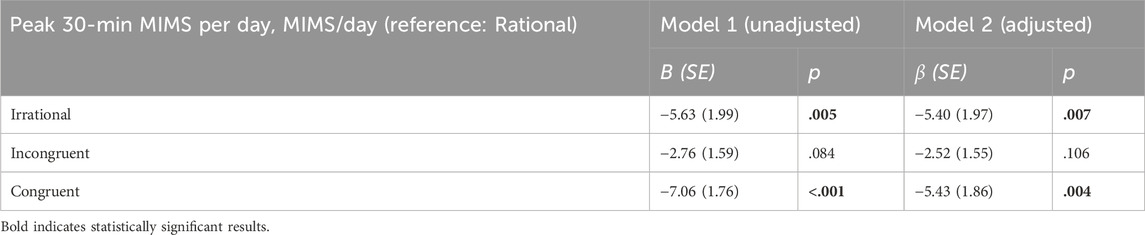
Table 4 . Association between Fall Risk Appraisal groups and peak 30-min MIMS per day (MIMS/day) using linear regression. Model 2 was adjusted for age, gender and BMI.
This is the first study, to our knowledge, to evaluate the associations of FRA with daily MIMS and peak 30-min MIMS in a sample of community-dwelling US older adults. In general, both the volume and intensity of PA were highest in the rational group and lowest in the congruent group. In maladaptive FRA groups, high FOF (i.e., irrational FRA) was associated with lower PA volume and intensity compared to the reference group (i.e., rational FRA), but no significant differences were observed for high physiological fall risk (i.e., incongruent FRA).
Prior research has shown that FOF is associated with reduced PA levels in community-dwelling older adults using objectively measured PA data ( Jefferis et al., 2014 ; Choudhury et al., 2022 ). Our results broadly agree with it, showing that total daily PA volume was significantly lower in two high FOF groups (i.e., irrational and congruent) than the rational group. This suggests that regardless of balance performance and lower limb strength, low FOF was associated with high PA volume in our study sample. In linear regression analysis, after accounting for age, gender and BMI, reduced daily MIMS was significantly associated with irrational FRA, but not with congruent FRA. It can be attributed to the fact that the average age and BMI of congruent participants were higher than all other groups, and evidence suggests that that increasing older age and higher BMI contribute to lower PA levels in older adults ( Smith et al., 2015 ).
We did not observe any significant difference between two low FOF groups (i.e., rational and incongruent) in terms of daily PA volume. This suggests that, for maladaptive FRA, high physiological fall risk (not high FOF) had a stronger association with reduced daily PA accumulation in our study sample. In contrast to our findings, a recent study found that low physiological fall risk was more strongly associated with increased walking activity (steps/day) than low perceived fall risk in a sample of community-dwelling German older adults ( n = 294) ( Jansen et al., 2021 ). However, it should be noted that Jansen et al. used multiple independent risk factors (i.e., previous falls, balance impairment, gait impairment, and multimedication) to distinguish between high and low physiological fall risk, whereas they used only one tool (Short FES-I) to assess perceived fall risk. Furthermore, participants with low FOF and high physiological fall risk in that German older adult cohort ( Jansen et al., 2021 ) were relatively older than those in our study sample [mean (SD) age: 81.6 (5.5) years vs. 74.3 (7.0) years in our study]. Previous studies indicate that the likelihood of reduced participation in PA gradually increases with advanced age, because of age-related declines in muscle mass, muscle strength, and functional fitness (i.e., the physical capacity to perform activities of daily living independently and without the early onset of fatigue) ( Milanović et al., 2013 ; Westerterp, 2018 ; Suryadinata et al., 2020 ). Therefore, future research should examine how age-related functional declines mediate the relationship between maladaptive FRA and daily PA volume in older adults.
In our study, the peak PA intensity in both high FOF groups (i.e., irrational and congruent) was significantly lower than the rational group. Despite the differences in the PA metrics, this is in general agreement with the previous findings that showed older adults with irrational and congruent FRAs were more likely to spend less time in MVPA ( Thiamwong et al., 2023 ). Interestingly, after adjusting for confounders, the decrease in peak 30-min MIMS for irrational and congruent groups was almost equivalent in our study. This suggests that older adults with high FOF may restrict their participation in high intensity PA, irrespective of their physiological fall risk status. Our findings extend the previously reported association between PA intensity and FOF in older adults ( Sawa et al., 2020 ), highlighting the need to integrate cognitive behavioral therapy to reduce FOF in fall intervention programs.
For peak 30-min MIMS, we did not find any significant difference between two low FOF groups (i.e., rational and incongruent). This suggests that, similar to total PA volume, peak PA intensity was more strongly associated with high FOF (rather than high physiological fall risk) for maladaptive FRA in our sample. Unlike MVPA cut points that exclude PA intensities ≤3 METs or equivalent, peak 30-min MIMS considers acceleration magnitudes ranging from lower to higher peak efforts within a day, enabling comparison over the whole spectrum of PA intensity levels (e.g., light vs. vigorous) ( Zheng et al., 2023 ). Further research should investigate domains of peak PA efforts across different FRA groups, so that informed strategies can be developed to promote high-intensity PA participation according to the perceived and physiological risk of fall.
Based on the findings of our study, it can be conferred that the FRA assessment may be useful in designing customized PA interventions to promote an active lifestyle in older adults. For example, to increase PA participation in older adults with irrational FRA, cognitive behavioral therapy can be integrated into PA programs to improve their self-efficacy and sense of control over falling ( Tennstedt et al., 1998 ). For incongruent FRA, PA recommendations should include exercise regimens specifically designed to reduce physiological fall risk, such as high intensity balance and strength training, in addition to aerobic activities ( Sherrington et al., 2008 ). On the other hand, older adults with congruent FRA may benefit from PA programs that combine both balance and strength exercises, and cognitive behavioral therapy ( Brouwer et al., 2003 ).
A strength of our study is the use of MIMS metric to provide a comprehensive PA assessment (volume and intensity) enabling reliable, cross-study comparisons of our findings with other MIMS-based studies regardless of the device type, model or manufacturer. Furthermore, we used evidence-based cut-off points to determine FOF level (low vs. high FOF), balance status (poor vs. normal balance) and lower limb strength (below average vs. average strength) to categorize participants into FRA groups. However, our study has several limitations. First, to determine physiological fall risk status, we didn’t use the Physiological Profile Assessment ( Delbaere et al., 2010 ) or multiple independent risk factors ( Jansen et al., 2021 ), which might have led to different group formations than those studies. Instead, we used static balance and lower limb strength as physiological fall risk indicators. While balance and strength deficits are important predictors of falls in older adults, they might not account for all aspects of physiological fall risk (such as gait impairment, visual and sensory deficits, use of multi-medications etc.) ( Fabre et al., 2010 ). Second, it is to be noted that the balance performance measure (i.e., static balance) used in this study may not capture the full spectrum of an individual’s balance capabilities. There are different measures of balance performance, including static steady-state balance (i.e., the ability to maintain a steady position while standing or sitting), dynamic steady-state balance (i.e., the ability to maintain a steady position while performing postural transitions and walking), proactive balance (i.e., the ability to anticipate and mitigate a predicted postural disturbance), and reactive balance (i.e., the ability to recover a stable position following an unexpected postural disturbance) ( Shumway-Cook and Woollacott, 2007 ). Therefore, future studies may consider using more comprehensive assessments of balance performance in older adults to define physiological fall risk in FRA. Third, our study only considered FOF as the psychological fall risk measure in FRA and did not investigate other psychological constructs such as falls efficacy or balance confidence ( Moore et al., 2011 ). FOF and falls efficacy are two major fall-related psychological constructs in preventing and managing fall risks in older adults. It is to be noted that, though FOF and falls efficacy are correlated, they represent theoretically distinct concepts ( Hadjistavropoulos et al., 2011 ). FOF is defined as “the lasting concerns about falling that leads to an individual avoiding activities that one remains capable of performing.” Some common instruments for FOF measurement include FES-I, Short FES-I, Iconographical Falls Efficacy Scale (ICON-FES), Geriatric Fear of Falling Measure (GFFM), Survey of Activities and Fear of Falling in the Elderly (SAFE), Fear of Falling Avoidance Behaviour Questionnaire (FFABQ) etc., ( Soh et al., 2021 ). On the other hand, falls efficacy is defined as the perceived confidence in one’s ability to carry out activities of daily living without experiencing a fall ( Moore and Ellis, 2008 ). Existing instruments for measuring falls efficacy include Falls Efficacy Scale (FES), modified FES (MFES), Perceived Ability to Prevent and Manage Fall Risks (PAPMFR), and Perceived Ability to Manage Risk of Falls or Actual Falls (PAMF) ( Soh et al., 2021 ). Prior research has reported that, compared to FOF, falls efficacy shows stronger relationship with measures of basic and instrumental activities of daily living (ADL-IADL), and physical and social functioning ( Tinetti et al., 1994 ). Therefore, future studies should consider exploring the combined effects of falls efficacy and physiological fall risk measures on habitual PA level to determine whether FOF or fall efficacy should be considered as a target for PA interventions in older adults. Fourth, to date, there exists no established cut-offs for the MIMS metric to categorize total PA volume and intensity that correspond to meeting national PA guidelines, and it is still unknown how well MIMS/minute can estimate energy expenditure ( Vilar-Gomez et al., 2023 ). Our study just provided a first step toward the use of a standardized metric to associate PA behavior with FRA in a community-dwelling older adult sample in the US. Future studies should examine such associations in large, nationally representative populations to establish benchmark values for daily MIMS and peak 30-min MIMS in different FRA categories. Fifth, the cross-sectional design of the study didn’t allow us to determine a causal relationship between FRA and PA, so reverse and/or bidirectional causality might still be present. Sixth, although we controlled for age, gender, and BMI in the regression analyses, there remains the possibility of additional residual confounding [such as neuropsychological constructs that have been associated with FOF, which include depression, anxiety, neuroticism, attention, and executive function ( Delbaere et al., 2010 )]. Finally, our sample size was relatively small and 79% of participants were female. The generalizability of our findings might be restricted by the small, female dominant nature of our sample.
In conclusion, compared to rational FRA, the habitual PA level (daily MIMS and peak 30-min MIMS) was lower in both high FOF groups (i.e., irrational and congruent), but not in incongruent group. This suggests that, for maladaptive FRA in our study sample, high perceived fall risk had a stronger association with reduced PA level, rather than high physiological fall risk. When controlled for covariates, decrease in peak PA intensity remained significantly associated with irrational and congruent FRAs, indicating that older adults with high FOF performed PA at lower peak efforts, irrespective of their physiological fall status. Future prospective studies should focus on identifying the optimal habitual PA level (total PA volume and peak PA intensity) in accordance with an older adult’s FOF and physiological fall risk to better inform public health policies for sustainable, effective PA framework.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Institutional Review Board, University of Central Florida. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
RC: Data curation, Formal Analysis, Investigation, Methodology, Software, Visualization, Writing–original draft. J-HP: Conceptualization, Funding acquisition, Investigation, Methodology, Resources, Supervision, Writing–review and editing. CB: Formal Analysis, Visualization, Writing–review and editing. MC: Data curation, Investigation, Writing–review and editing. DF: Conceptualization, Funding acquisition, Writing–review and editing. RX: Conceptualization, Funding acquisition, Writing–review and editing. JS: Conceptualization, Funding acquisition, Supervision, Writing–review and editing. LT: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The research was funded by the National Institute on Aging (R03AG06799) and the National Institute on Minority Health and Health Disparities (R01MD018025) of National Institutes of Health. This research also received financial support from the University of Central Florida CONNECT CENTRAL (Interdisciplinary research seed grant; AWD00001720 and AWD00005378).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fragi.2024.1284694/full#supplementary-material
Ambrose, A. F., Paul, G., and Hausdorff, J. M. (2013). Risk factors for falls among older adults: a review of the literature. Maturitas 75 (1), 51–61. doi:10.1016/j.maturitas.2013.02.009
PubMed Abstract | CrossRef Full Text | Google Scholar
Brouwer, B. J., Walker, C., Rydahl, S. J., and Culham, E. G. (2003). Reducing fear of falling in seniors through education and activity programs: a randomized trial. J. Am. Geriatr. Soc. 51 (6), 829–834. doi:10.1046/j.1365-2389.2003.51265.x
Buschke, H., Kuslansky, G., Katz, M., Stewart, W. F., Sliwinski, M. J., Eckholdt, H. M., et al. (1999). Screening for dementia with the memory impairment screen. Neurology 52 (2), 231–238. doi:10.1212/wnl.52.2.231
Chan, B. K., Marshall, L. M., Winters, K. M., Faulkner, K. A., Schwartz, A. V., and Orwoll, E. S. (2007). Incident fall risk and physical activity and physical performance among older men: the Osteoporotic Fractures in Men Study. Am. J. Epidemiol. 165 (6), 696–703. doi:10.1093/aje/kwk050
Choudhury, R., Park, J. H., Banarjee, C., Thiamwong, L., Xie, R., and Stout, J. R. (2023). Associations of mutually exclusive categories of physical activity and sedentary behavior with body composition and fall risk in older women: a cross-sectional study. Int. J. Environ. Res. Public Health 20 (4), 3595. doi:10.3390/ijerph20043595
Choudhury, R., Park, J. H., Thiamwong, L., Xie, R., and Stout, J. R. (2022). Objectively measured physical activity levels and associated factors in older US women during the COVID-19 pandemic: cross-sectional study. JMIR Aging 5 (3), e38172. doi:10.2196/38172
Delbaere, K., Close, J. C., Brodaty, H., Sachdev, P., and Lord, S. R. (2010). Determinants of disparities between perceived and physiological risk of falling among elderly people: cohort study. Bmj 341, c4165. doi:10.1136/bmj
Deshpande, N., Metter, E. J., Lauretani, F., Bandinelli, S., Guralnik, J., and Ferrucci, L. (2008). Activity restriction induced by fear of falling and objective and subjective measures of physical function: a prospective cohort study. J. Am. Geriatr. Soc. 56 (4), 615–620. doi:10.1111/j.1532-5415.2007.01639.x
Fabre, J. M., Ellis, R., Kosma, M., and Wood, R. H. (2010). Falls risk factors and a compendium of falls risk screening instruments. J. Geriatr. Phys. Ther. 33 (4), 184–197. doi:10.1519/jpt.0b013e3181ff2a24
Goble, D. J., Hearn, M. C., and Baweja, H. S. (2017). Combination of BTrackS and Geri-Fit as a targeted approach for assessing and reducing the postural sway of older adults with high fall risk. Clin. Interv. Aging 12, 351–357. doi:10.2147/cia.S131047
Gregg, E. W., Pereira, M. A., and Caspersen, C. J. (2000). Physical activity, falls, and fractures among older adults: a review of the epidemiologic evidence. J. Am. Geriatr. Soc. 48 (8), 883–893. doi:10.1111/j.1532-5415.2000.tb06884.x
Hadjistavropoulos, T., Delbaere, K., and Fitzgerald, T. D. (2011). Reconceptualizing the role of fear of falling and balance confidence in fall risk. J. Aging Health 23 (1), 3–23. doi:10.1177/0898264310378039
Heesch, K. C., Byles, J. E., and Brown, W. J. (2008). Prospective association between physical activity and falls in community-dwelling older women. J. Epidemiol. Community Health 62 (5), 421–426. doi:10.1136/jech.2007.064147
Jansen, C. P., Klenk, J., Nerz, C., Todd, C., Labudek, S., Kramer-Gmeiner, F., et al. (2021). Association between everyday walking activity, objective and perceived risk of falling in older adults. Age Ageing 50 (5), 1586–1592. doi:10.1093/ageing/afab037
Jefferis, B. J., Iliffe, S., Kendrick, D., Kerse, N., Trost, S., Lennon, L. T., et al. (2014). How are falls and fear of falling associated with objectively measured physical activity in a cohort of community-dwelling older men? BMC Geriatr. 14, 114. doi:10.1186/1471-2318-14-114
John, D., Tang, Q., Albinali, F., and Intille, S. (2019). An open-source monitor-independent movement summary for accelerometer data processing. J. Meas. Phys. Behav. 2 (4), 268–281. doi:10.1123/jmpb.2018-0068
Kakara, R., Bergen, G., Burns, E., and Stevens, M. (2023). Nonfatal and fatal falls among adults aged ≥65 Years - United States, 2020-2021. MMWR Morb. Mortal. Wkly. Rep. 72 (35), 938–943. doi:10.15585/mmwr.mm7235a1
Kempen, G. I., Yardley, L., van Haastregt, J. C., Zijlstra, G. A., Beyer, N., Hauer, K., et al. (2008). The Short FES-I: a shortened version of the falls efficacy scale-international to assess fear of falling. Age Ageing 37 (1), 45–50. doi:10.1093/ageing/afm157
Levy, S. S., Thralls, K. J., and Kviatkovsky, S. A. (2018). Validity and reliability of a portable balance tracking system, BTrackS, in older adults. J. Geriatr. Phys. Ther. 41 (2), 102–107. doi:10.1519/jpt.0000000000000111
Mendes da Costa, E., Pepersack, T., Godin, I., Bantuelle, M., Petit, B., and Levêque, A. (2012). Fear of falling and associated activity restriction in older people. results of a cross-sectional study conducted in a Belgian town. Arch. Public Health 70 (1), 1. doi:10.1186/0778-7367-70-1
Milanović, Z., Pantelić, S., Trajković, N., Sporiš, G., Kostić, R., and James, N. (2013). Age-related decrease in physical activity and functional fitness among elderly men and women. Clin. Interv. Aging 8, 549–556. doi:10.2147/cia.S44112
Moore, D. S., and Ellis, R. (2008). Measurement of fall-related psychological constructs among independent-living older adults: a review of the research literature. Aging Ment. Health 12 (6), 684–699. doi:10.1080/13607860802148855
Moore, D. S., Ellis, R., Kosma, M., Fabre, J. M., McCarter, K. S., and Wood, R. H. (2011). Comparison of the validity of four fall-related psychological measures in a community-based falls risk screening. Res. Q. Exerc. Sport 82 (3), 545–554. doi:10.1080/02701367.2011.10599787
Moreland, B., Kakara, R., and Henry, A. (2020). Trends in nonfatal falls and fall-related injuries among adults aged ≥65 Years - United States, 2012-2018. MMWR Morb. Mortal. Wkly. Rep. 69 (27), 875–881. doi:10.15585/mmwr.mm6927a5
Rikli, R. E., and Jones, C. J. (1999). Functional fitness normative scores for community-residing older adults, ages 60-94. J. Aging Phys. Act. 7 (2), 162–181. doi:10.1123/japa.7.2.162
CrossRef Full Text | Google Scholar
Sawa, R., Asai, T., Doi, T., Misu, S., Murata, S., and Ono, R. (2020). The association between physical activity, including physical activity intensity, and fear of falling differs by fear severity in older adults living in the community. J. Gerontol. B Psychol. Sci. Soc. Sci. 75 (5), 953–960. doi:10.1093/geronb/gby103
Sherrington, C., Whitney, J. C., Lord, S. R., Herbert, R. D., Cumming, R. G., and Close, J. C. (2008). Effective exercise for the prevention of falls: a systematic review and meta-analysis. J. Am. Geriatr. Soc. 56 (12), 2234–2243. doi:10.1111/j.1532-5415.2008.02014.x
Shumway-Cook, A., and Woollacott, M. H. (2007). Motor control: translating research into clinical practice . United States: Lippincott Williams & Wilkins .
Google Scholar
Smith, L., Gardner, B., Fisher, A., and Hamer, M. (2015). Patterns and correlates of physical activity behaviour over 10 years in older adults: prospective analyses from the English Longitudinal Study of Ageing. BMJ Open 5 (4), e007423. doi:10.1136/bmjopen-2014-007423
Soh, S. L., Tan, C. W., Thomas, J. I., Tan, G., Xu, T., Ng, Y. L., et al. (2021). Falls efficacy: extending the understanding of self-efficacy in older adults towards managing falls. J. Frailty Sarcopenia Falls 6 (3), 131–138. doi:10.22540/jfsf-06-131
Suryadinata, R. V., Wirjatmadi, B., Adriani, M., and Lorensia, A. (2020). Effect of age and weight on physical activity. J. Public Health Res. 9 (2), 1840. doi:10.4081/jphr.2020.1840
Tennstedt, S., Howland, J., Lachman, M., Peterson, E., Kasten, L., and Jette, A. (1998). A randomized, controlled trial of a group intervention to reduce fear of falling and associated activity restriction in older adults. J. Gerontol. B Psychol. Sci. Soc. Sci. 53 (6), P384–P392. doi:10.1093/geronb/53b.6.p384
Thiamwong, L. (2020). A hybrid concept analysis of fall risk appraisal: integration of older adults' perspectives with an integrative literature review. Nurs. Forum 55 (2), 190–196. doi:10.1111/nuf.12415
Thiamwong, L., Huang, H. J., Ng, B. P., Yan, X., Sole, M. L., Stout, J. R., et al. (2020a). Shifting maladaptive fall risk appraisal in older adults through an in-home physio-fEedback and exercise pRogram (peer): a pilot study. Clin. Gerontol. 43 (4), 378–390. doi:10.1080/07317115.2019.1692120
Thiamwong, L., Ng, B. P., Kwan, R. Y. C., and Suwanno, J. (2021a). Maladaptive fall risk appraisal and falling in community-dwelling adults aged 60 and older: implications for screening. Clin. Gerontol. 44 (5), 552–561. doi:10.1080/07317115.2021.1950254
Thiamwong, L., Sole, M. L., Ng, B. P., Welch, G. F., Huang, H. J., and Stout, J. R. (2020b). Assessing fall risk appraisal through combined physiological and perceived fall risk measures using innovative Technology. J. Gerontol. Nurs. 46 (4), 41–47. doi:10.3928/00989134-20200302-01
Thiamwong, L., Stout, J. R., Park, J. H., and Yan, X. (2021b). Technology-based fall risk assessments for older adults in low-income settings: protocol for a cross-sectional study. JMIR Res. Protoc. 10 (4), e27381. doi:10.2196/27381
Thiamwong, L., Xie, R., Park, J. H., Choudhury, R., Malatyali, A., Li, W., et al. (2023). Levels of accelerometer-based physical activity in older adults with a mismatch between physiological fall risk and fear of falling. J. Gerontol. Nurs. 49 (6), 41–49. doi:10.3928/00989134-20230512-06
Tinetti, M. E., Mendes de Leon, C. F., Doucette, J. T., and Baker, D. I. (1994). Fear of falling and fall-related efficacy in relationship to functioning among community-living elders. J. Gerontol. 49 (3), M140–M147. doi:10.1093/geronj/49.3.m140
Troiano, R. P., Berrigan, D., Dodd, K. W., Mâsse, L. C., Tilert, T., and McDowell, M. (2008). Physical activity in the United States measured by accelerometer. Med. Sci. Sports Exerc. 40 (1), 181–188. doi:10.1249/mss.0b013e31815a51b3
Tudor-Locke, C., Brashear, M. M., Katzmarzyk, P. T., and Johnson, W. D. (2012). Peak stepping cadence in free-living adults: 2005-2006 NHANES. J. Phys. Act. Health 9 (8), 1125–1129. doi:10.1123/jpah.9.8.1125
Vilar-Gomez, E., Vuppalanchi, R., Gawrieh, S., Pike, F., Samala, N., and Chalasani, N. (2023). Significant dose-response association of physical activity and diet quality with mortality in adults with suspected NAFLD in a population study. Am. J. Gastroenterol. 118 (9), 1576–1591. doi:10.14309/ajg.0000000000002222
Westerterp, K. R. (2018). Changes in physical activity over the lifespan: impact on body composition and sarcopenic obesity. Obes. Rev. 19 (1), 8–13. doi:10.1111/obr.12781
Wolff-Hughes, D. L., Bassett, D. R., and Fitzhugh, E. C. (2014). Population-referenced percentiles for waist-worn accelerometer-derived total activity counts in U.S. youth: 2003 - 2006 NHANES. PLoS One 9 (12), e115915. doi:10.1371/journal.pone.0115915
Yardley, L., Beyer, N., Hauer, K., Kempen, G., Piot-Ziegler, C., and Todd, C. (2005). Development and initial validation of the falls efficacy scale-international (FES-I). Age Ageing 34 (6), 614–619. doi:10.1093/ageing/afi196
Yee, X. S., Ng, Y. S., Allen, J. C., Latib, A., Tay, E. L., Abu Bakar, H. M., et al. (2021). Performance on sit-to-stand tests in relation to measures of functional fitness and sarcopenia diagnosis in community-dwelling older adults. Eur. Rev. Aging Phys. Act. 18 (1), 1. doi:10.1186/s11556-020-00255-5
Zheng, P., Pleuss, J. D., Turner, D. S., Ducharme, S. W., and Aguiar, E. J. (2023). Dose-response association between physical activity (daily MIMS, peak 30-minute MIMS) and cognitive function among older adults: NHANES 2011-2014. J. Gerontol. A Biol. Sci. Med. Sci. 78 (2), 286–291. doi:10.1093/gerona/glac076
Zijlstra, G. A., van Haastregt, J. C., van Eijk, J. T., van Rossum, E., Stalenhoef, P. A., and Kempen, G. I. (2007). Prevalence and correlates of fear of falling, and associated avoidance of activity in the general population of community-living older people. Age Ageing 36 (3), 304–309. doi:10.1093/ageing/afm021
Keywords: falls, physical activity, accelerometry, aging, fear of falling, fall risk, MIMS
Citation: Choudhury R, Park J-H, Banarjee C, Coca MG, Fukuda DH, Xie R, Stout JR and Thiamwong L (2024) Associations between monitor-independent movement summary (MIMS) and fall risk appraisal combining fear of falling and physiological fall risk in community-dwelling older adults. Front. Aging 5:1284694. doi: 10.3389/fragi.2024.1284694
Received: 28 August 2023; Accepted: 20 March 2024; Published: 09 April 2024.
Reviewed by:
Copyright © 2024 Choudhury, Park, Banarjee, Coca, Fukuda, Xie, Stout and Thiamwong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joon-Hyuk Park, [email protected]
This article is part of the Research Topic
Insights into Falls Efficacy and Fear of Falling

Research shows how manufacturing choices can reduce microfibre shed
A ccording to the research, washing synthetic fabrics can release millions of microfibres, posing a significant threat to the environment. Microfibres are particularly problematic for marine life and the fibres can take hundreds of years to degrade.
The objective of the research was to shed slight on the impact of manufacturing choices on microfibre release in textiles as they are washed.
To conduct the study, Alice Hazlehurst, a doctoral researcher at University of Leeds' School of Design, led a research team that used a washing simulator, called a gyrowash, to test various densities of yarns in unknitted hanks and knitted forms.
The researchers compared how much microfibre each sample released when washed.
Key findings on microfibre release:
- Unknitted polyester released more microfibre than unknitted cotton, but similar amounts were released when polyester was knitted. This suggests that polyester suffered less damage than cotton during the knitting process.
- Microfibre release in tightly knitted fabrics during laundering was reduced, but manufacturers should also consider "fibre fly" – the visible fluff that comes off fabrics as they are knitted in factories, which is higher when garments are tightly knitted.
- Vortext-sun yarns (where fibres are twisted using jets of air in a vortex) had a lower microfibre release than ring-spun yarns (where fibres are twisted in a metal ring). Ring-spun yarns tend to be ‘hairier’ than vortex-spun yarns, which has been shown to increase microfibre release in washing.
The researchers concluded that changes to the fibre composition and the yarn spinning system would have the greatest influence in terms of reducing microfibre release.
However, the researchers added, these details are not currently included in product specifications, which makes it more difficult for brands to make informed choices about which garments will release more or less microfibre.
University of Leeds' Hazlehurst believes that their findings show the entire process of textile production, down to the way yarn is spun, is important in the effort to limit microfibre release.
Hazlehurst said: "Our findings show that the entire process of textile production, down to the way yarn is spun, is important in the effort to limit microfibre release. Manufacturers should contain these details in specifications of yarn spinning, as well as the fibre type, to help clothing designers make more informed choices."
Dr Mark Taylor, research fellow at Leeds' school of design, added: "We know cotton produces more microfibre than polyester and people assume that cellulose (from cotton) is less worrying than microplastic because it’s natural. But the truth is that we don’t have enough information about the impacts of these fibres on humans, aside from knowing they can take hundreds of years to biodegrade and can have a negative impact on marine life."
The academics are calling for more research into the effects of microfibre release on the environment and human health so measures can be targeted towards reducing harm.
In August 2023, another research paper ‘Impact of sewing on microfiber release from polyester fabric during laundry’ revealed that laser and ultrasonic cutting methods can reduce microfibre release from garments by up to 20 times compared to using conventional scissor-cut edges.
"Research shows how manufacturing choices can reduce microfibre shed" was originally created and published by Just Style , a GlobalData owned brand.
The information on this site has been included in good faith for general informational purposes only. It is not intended to amount to advice on which you should rely, and we give no representation, warranty or guarantee, whether express or implied as to its accuracy or completeness. You must obtain professional or specialist advice before taking, or refraining from, any action on the basis of the content on our site.


A systematic review of sample size estimation accuracy on power in malaria cluster randomised trials measuring epidemiological outcomes
- Find this author on Google Scholar
- Find this author on PubMed
- Search for this author on this site
- For correspondence: [email protected]
- Info/History
- Supplementary material
- Preview PDF
Introduction Cluster randomised trials (CRTs) are the gold standard for measuring the community-wide impacts of malaria control tools. CRTs rely on well-defined sample size estimations to detect statistically significant effects of trialled interventions; however these are often predicted poorly by investigators. Here, we review the accuracy of predicted parameters used in sample size calculations for malaria CRTs with epidemiological outcomes.
Methods We searched for published malaria CRTs using four online databases in March 2022. Eligible trials included those with malaria-specific epidemiological outcomes which randomised at least six geographical clusters to study arms. Predicted and observed sample size parameters were extracted by reviewers for each trial. Pair-wise correlation coefficients were calculated to assess the accuracy of predicted control-arm outcome estimates and desired relative reductions (effect sizes) between arms compared to what was observed. Among trials which retrospectively calculated an estimate of heterogeneity in cluster outcomes, we recalculated study power according to observed trial estimates.
Results Of the 1889 records identified and screened, 108 articles were eligible and comprised of 71 malaria CRTs. Among trials that included sample size calculations (91.5%, 65/71), most estimated cluster heterogeneity using the coefficient of variation (k) (80%, 52/65) which were often predicted without using prior data (67.7%, 44/65). Predicted control-arm epidemiological outcomes correlated weakly with those observed, with 61.2% (19/31) of prevalence estimates overestimated. Among the minority of trials which retrospectively calculated cluster heterogeneity (20%, 13/65), empirical values contrasted with those used in sample size estimations and often compromised study power. Observed effect sizes were often smaller than had been predicted at the sample size stage (72.9%, 51/70) and were typically higher in the first, compared to the second, year of trials. Overall, effect sizes achieved by malaria interventions tested in trials decreased between 1995 and 2021.
Conclusions Study findings reveal sample size parameters in malaria CRTs were often inaccurate and resulted in underpowered studies. Future trials must strive to obtain more representative epidemiological sample size inputs to ensure interventions against malaria are adequately evaluated.
Registration This review is registered with PROSPERO (CRD42022315741).
Competing Interest Statement
The authors have declared no competing interest.
Funding Statement
This research is supported by a grant to the London School of Hygiene and Tropical Medicine from the Bill & Melinda Gates Foundation (INV-038132). JDC, JH, and TC also acknowledge funding from the MRC Centre for Global Infectious Disease Analysis (reference MR/X020258/1), funded by the UK Medical Research Council (MRC). This UK funded award is carried out in the frame of the Global Health EDCTP3 Joint Undertaking. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author Declarations
I confirm all relevant ethical guidelines have been followed, and any necessary IRB and/or ethics committee approvals have been obtained.
I confirm that all necessary patient/participant consent has been obtained and the appropriate institutional forms have been archived, and that any patient/participant/sample identifiers included were not known to anyone (e.g., hospital staff, patients or participants themselves) outside the research group so cannot be used to identify individuals.
I understand that all clinical trials and any other prospective interventional studies must be registered with an ICMJE-approved registry, such as ClinicalTrials.gov. I confirm that any such study reported in the manuscript has been registered and the trial registration ID is provided (note: if posting a prospective study registered retrospectively, please provide a statement in the trial ID field explaining why the study was not registered in advance).
I have followed all appropriate research reporting guidelines, such as any relevant EQUATOR Network research reporting checklist(s) and other pertinent material, if applicable.
Email contacts j.challenger{at}imperial.ac.uk , joel{at}ebi.ac.uk , thomas.churcher{at}imperial.ac.uk , jackie.cook{at}lshtm.ac.uk
Data Availability
All data produced in the present work are contained in the manuscript
List of abbreviations
View the discussion thread.
Supplementary Material
Thank you for your interest in spreading the word about medRxiv.
NOTE: Your email address is requested solely to identify you as the sender of this article.

Citation Manager Formats
- EndNote (tagged)
- EndNote 8 (xml)
- RefWorks Tagged
- Ref Manager
- Tweet Widget
- Facebook Like
- Google Plus One
Subject Area
- Epidemiology
- Addiction Medicine (316)
- Allergy and Immunology (620)
- Anesthesia (160)
- Cardiovascular Medicine (2284)
- Dentistry and Oral Medicine (280)
- Dermatology (201)
- Emergency Medicine (370)
- Endocrinology (including Diabetes Mellitus and Metabolic Disease) (805)
- Epidemiology (11593)
- Forensic Medicine (10)
- Gastroenterology (681)
- Genetic and Genomic Medicine (3600)
- Geriatric Medicine (337)
- Health Economics (618)
- Health Informatics (2311)
- Health Policy (916)
- Health Systems and Quality Improvement (865)
- Hematology (335)
- HIV/AIDS (753)
- Infectious Diseases (except HIV/AIDS) (13170)
- Intensive Care and Critical Care Medicine (758)
- Medical Education (360)
- Medical Ethics (100)
- Nephrology (391)
- Neurology (3369)
- Nursing (191)
- Nutrition (508)
- Obstetrics and Gynecology (652)
- Occupational and Environmental Health (647)
- Oncology (1764)
- Ophthalmology (526)
- Orthopedics (210)
- Otolaryngology (284)
- Pain Medicine (223)
- Palliative Medicine (66)
- Pathology (441)
- Pediatrics (1008)
- Pharmacology and Therapeutics (422)
- Primary Care Research (407)
- Psychiatry and Clinical Psychology (3077)
- Public and Global Health (6005)
- Radiology and Imaging (1227)
- Rehabilitation Medicine and Physical Therapy (715)
- Respiratory Medicine (811)
- Rheumatology (367)
- Sexual and Reproductive Health (356)
- Sports Medicine (318)
- Surgery (390)
- Toxicology (50)
- Transplantation (171)
- Urology (142)

IMAGES
VIDEO
COMMENTS
Following is a Research Findings Example sample for students: Title: The Effects of Exercise on Mental Health. Sample: 500 participants, both men and women, between the ages of 18-45. Methodology: Participants were divided into two groups. The first group engaged in 30 minutes of moderate intensity exercise five times a week for eight weeks.
A two-sample t test was used to test the hypothesis that higher social distance from environmental problems would reduce the intent to donate to environmental organizations, with donation intention (recorded as a score from 1 to 10) as the outcome variable and social distance (categorized as either a low or high level of social distance) as the predictor variable.Social distance was found to ...
Step 1: Consult the guidelines or instructions that the target journal or publisher provides authors and read research papers it has published, especially those with similar topics, methods, or results to your study. The guidelines will generally outline specific requirements for the results or findings section, and the published articles will ...
Research Summary. Definition: A research summary is a brief and concise overview of a research project or study that highlights its key findings, main points, and conclusions. It typically includes a description of the research problem, the research methods used, the results obtained, and the implications or significance of the findings.
Provide a brief description of your study: Enter details about your research topic and findings. This information helps Paperpal generate a tailored outline that aligns with your paper's content. Generate the conclusion outline: After entering all necessary details, click on 'generate'.
Research Results. Research results refer to the findings and conclusions derived from a systematic investigation or study conducted to answer a specific question or hypothesis. These results are typically presented in a written report or paper and can include various forms of data such as numerical data, qualitative data, statistics, charts, graphs, and visual aids.
1. Reporting Quantitative Findings. The best way to present your quantitative findings is to structure them around the research hypothesis or questions you intend to address as part of your dissertation project. Report the relevant findings for each research question or hypothesis, focusing on how you analyzed them.
The results chapter (also referred to as the findings or analysis chapter) is one of the most important chapters of your dissertation or thesis because it shows the reader what you've found in terms of the quantitative data you've collected. It presents the data using a clear text narrative, supported by tables, graphs and charts.
The Results (also sometimes called Findings) section in an empirical research paper describes what the researcher(s) found when they analyzed their data. Its primary purpose is to use the data collected to answer the ... • Make sure to review examples of Results sections from sample papers or journal articles in your discipline, as ...
The results chapter in a dissertation or thesis (or any formal academic research piece) is where you objectively and neutrally present the findings of your qualitative analysis (or analyses if you used multiple qualitative analysis methods ). This chapter can sometimes be combined with the discussion chapter (where you interpret the data and ...
Begin with a clear statement of the principal findings. This will reinforce the main take-away for the reader and set up the rest of the discussion. Explain why the outcomes of your study are important to the reader. Discuss the implications of your findings realistically based on previous literature, highlighting both the strengths and ...
Having summed up your key arguments or findings, the conclusion ends by considering the broader implications of your research. This means expressing the key takeaways, practical or theoretical, from your paper—often in the form of a call for action or suggestions for future research. Argumentative paper: Strong closing statement
Instead, you select a sample. The sample is the group of individuals who will actually participate in the research. To draw valid conclusions from your results, you have to carefully decide how you will select a sample that is representative of the group as a whole. This is called a sampling method. There are two primary types of sampling ...
The results (or findings) section is one of the most important parts of a research paper, in which an author reports the findings of their study in connection to their research question(s). The results section should not attempt to interpret or analyze the findings, only state the facts. In this handout, you will find a description of a results ...
The discussion section is one of the final parts of a research paper, in which an author describes, analyzes, and interprets their findings. They explain the significance of those results and tie everything back to the research question(s). In this handout, you will find a description of what a discussion section does, explanations of how to ...
Step 4: Write your findings section in a factual and objective manner. The goal is to communicate information - in some cases a great deal of complex information - as clearly, accurately and precisely as possible, so well-constructed sentences that maintain a simple structure will be far more effective than convoluted phrasing and expressions.
Indicate where the sample size or number of events does not meet the optimal information size as calculated, or the 'rules of thumb' (e.g. 400 events). Avoid reference to the number of studies as a reason for imprecision. Indicate whether the confidence intervals include the possibility of a small or no effect AND important benefit or harm.
Translating research findings into practice requires understanding how to meet communication and dissemination needs and preferences of intended audiences including past research participants (PSPs) who want, but seldom receive, information on research findings during or after participating in research studies. ... The sample was drawn from ...
Finding: The Panel on Plasma Processing of Materials finds that plasma processing of materials is a critical technology that is necessary to implement key recommendations contained in the National Research Council report Materials Science and Engineering for the 1990s (National Academy Press, Washington, D.C., 1989) and to enhance the health of ...
An appropriate sample renders the research more efficient: Data generated are reliable, resource investment is as limited as possible, while conforming to ethical principles. The use of sample size calculation directly influences research findings. Very small samples undermine the internal and external validity of a study.
The conclusions are as stated below: i. Students' use of language in the oral sessions depicted their beliefs and values. based on their intentions. The oral sessions prompted the students to be ...
Research Paper Conclusion. Definition: A research paper conclusion is the final section of a research paper that summarizes the key findings, significance, and implications of the research. It is the writer's opportunity to synthesize the information presented in the paper, draw conclusions, and make recommendations for future research or ...
3). Research Questions as Headings . You can also present your findings using your research questions as the headings in the findings section. This is a useful strategy that ensures you're answering your research questions and also allows the reader to quickly ascertain where the answers to your research questions are.
6.2.1 Findings based on the questionnaires completed by teachers (quantitative data) In the sampled schools most of the variables that could have been expected, according to the literature, to have an effect on Grade 12 results, in fact had had no effect, at least among the sampled schools. The most significant variables which emerged from the ...
Writing a research proposal can be quite challenging, but a good starting point could be to look at some examples. We've included a few for you below. Example research proposal #1: "A Conceptual Framework for Scheduling Constraint Management" Example research proposal #2: "Medical Students as Mediators of Change in Tobacco Use" Title page
Prior research has mostly focused on exploring the independent associations of FOF and objective fall risk measures with physical activity (PA) participation in older adults (Gregg et al., 2000; Chan et al., 2007; Zijlstra et al., 2007; Heesch et al., 2008; Mendes da Costa et al., 2012).To date, only a small number of studies have investigated the combined effects of FOF and objective fall ...
In comparison to in-person GKT, findings demonstrated online GKT is as effective on the outcomes tested. Our results corroborate previous research comparing modes of delivering another GKT (Question, Persuade, and Refer), finding online as effective as in-person training (Lancaster et al., Citation 2014). The use of online learning environments ...
New research from the University of Leeds' School of Design shows how changes to fibre composition and the systems used to spin yarn could reduce the volume of microfibres released from textiles ...
Introduction Cluster randomised trials (CRTs) are the gold standard for measuring the community-wide impacts of malaria control tools. CRTs rely on well-defined sample size estimations to detect statistically significant effects of trialled interventions; however these are often predicted poorly by investigators. Here, we review the accuracy of predicted parameters used in sample size ...