Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article-Invited
- Open access
- Published: 03 April 2019

Prognosis and improved outcomes in major depression: a review
- Christoph Kraus ORCID: orcid.org/0000-0002-7144-2282 1 , 2 ,
- Bashkim Kadriu ORCID: orcid.org/0000-0002-3809-9451 2 ,
- Rupert Lanzenberger ORCID: orcid.org/0000-0003-4641-9539 1 ,
- Carlos A. Zarate Jr. 2 &
- Siegfried Kasper ORCID: orcid.org/0000-0001-8278-191X 1
Translational Psychiatry volume 9 , Article number: 127 ( 2019 ) Cite this article
71k Accesses
237 Citations
26 Altmetric
Metrics details
- Human behaviour
- Predictive markers
- Scientific community
Treatment outcomes for major depressive disorder (MDD) need to be improved. Presently, no clinically relevant tools have been established for stratifying subgroups or predicting outcomes. This literature review sought to investigate factors closely linked to outcome and summarize existing and novel strategies for improvement. The results show that early recognition and treatment are crucial, as duration of untreated depression correlates with worse outcomes. Early improvement is associated with response and remission, while comorbidities prolong course of illness. Potential biomarkers have been explored, including hippocampal volumes, neuronal activity of the anterior cingulate cortex, and levels of brain-derived neurotrophic factor (BDNF) and central and peripheral inflammatory markers (e.g., translocator protein (TSPO), interleukin-6 (IL-6), C-reactive protein (CRP), tumor necrosis factor alpha (TNFα)). However, their integration into routine clinical care has not yet been fully elucidated, and more research is needed in this regard. Genetic findings suggest that testing for CYP450 isoenzyme activity may improve treatment outcomes. Strategies such as managing risk factors, improving clinical trial methodology, and designing structured step-by-step treatments are also beneficial. Finally, drawing on existing guidelines, we outline a sequential treatment optimization paradigm for selecting first-, second-, and third-line treatments for acute and chronically ill patients. Well-established treatments such as electroconvulsive therapy (ECT) are clinically relevant for treatment-resistant populations, and novel transcranial stimulation methods such as theta-burst stimulation (TBS) and magnetic seizure therapy (MST) have shown promising results. Novel rapid-acting antidepressants, such as ketamine, may also constitute a paradigm shift in treatment optimization for MDD.
Similar content being viewed by others

Psilocybin microdosers demonstrate greater observed improvements in mood and mental health at one month relative to non-microdosing controls

The serotonin theory of depression: a systematic umbrella review of the evidence

Major depressive disorder: hypothesis, mechanism, prevention and treatment
Depression: a major and relentless burden.
Major depressive disorder (MDD) is the most common psychiatric disease and a worldwide leading cause of years lived with disability 1 , 2 . In addition, the bulk of suicides are linked to a diagnosis of MDD. Despite the high prevalence rate of MDD and ongoing efforts to increase knowledge and skills for healthcare providers, the illness remains both underdiagnosed and undertreated 3 . Many novel strategies with potentially broad impact are not yet ready for ‘prime time’, as they are either in early experimental stages or undergoing regulatory processes for approval. This review sought to: (1) provide a synopsis of key factors associated with outcomes in MDD, and (2) synthesize the existing literature on novel treatment strategies for depression. A literature search was conducted using the search terms ‘depression’, ‘antidepressant’, ‘outcome’, ‘predictor’, ‘(bio)marker’, ‘treatment-resistant depression (TRD)’, and ‘chronic depression’ in addition to combinations of these terms. The search was conducted in PubMed, Scopus, and Google Scholar with no restrictions on time period and concluded in October 2018. Notably, we defined ‘outcomes’ loosely, as either disease course (i.e., treatment resistance, chronic depression) or response/remission to treatment.
Prognostic variables for treatment outcomes in MDD
Clinical variables.
Clear evidence of an inverse relationship between duration of episode and treatment outcome (either response or remission) underscores the importance of early intervention in MDD 4 (Table 1 ). In particular, replicable prospective and retrospective studies indicate that shorter duration of untreated disease—both in terms of first and recurrent episodes—is a prognostic factor indicating better treatment response and better long-term outcomes 5 , 6 , 7 , 8 , 9 , 10 , although not all studies have found such an association 11 . Another important clinical variable is time to antidepressant response. For instance, one meta-analysis found that early improvement was positively linked to antidepressant treatment outcome in 15 of 16 studies 9 . Early response to antidepressant treatment appears to occur independently of treatment modality 12 , 13 or outcome parameters 14 , 15 . Another study found that early improvement in work productivity was a significant positive predictor of higher remission rates after three and seven months of treatment 16 . Similarly, imaging studies found that early response to treatment correlated with default mode network deactivation in the posterior cingulate 17 , as well as thickening of gray matter in the anterior cingulate cortex (ACC) 18 . Interestingly, two recent meta-analyses found that initial improvement was linked to response and outcome but failed to be associated with treatment resistance 19 , 20 . This suggests that TRD—defined loosely here as non-response to at least two adequate antidepressant trials—and chronic depression (roughly defined here as non-response to any treatment) may have similar response slopes in the earliest treatment stages.
In addition, lower baseline function and quality of life—including longer duration of the current index episode—have been associated with lower remission rates to various types of antidepressant treatments 21 , 22 . This is in line with results from a previous study that found that baseline function predicted antidepressant response in TRD patients 23 . Worse outcomes in more severely ill patients at baseline were also reported in elderly patients treated in primary-care settings 24 . In contrast, several controlled clinical studies found that elevated baseline severity correlated with improved response and remission rates 25 . Two naturalistic studies with broad inclusion criteria similarly found that remission correlated with higher baseline scores 4 , 26 . However, this discrepancy might be explained by variations in outcome according to parameter. It was noted earlier that studies that defined remission as percent change of baseline values might be biased in favor of higher baseline scores, while absolute endpoints (e.g., remission defined below a cutoff score) favor less sick patients 4 .
Psychosocial variables
The influence of sociodemographic factors such as age, age of onset, gender, and number of previous episodes on treatment outcome has been investigated with mixed results 4 , 27 , 28 . One study found that females had higher remission rates 21 , but this was not confirmed by another prospective study 27 . Others have found that stress related to high occupational levels might impair outcomes 29 . The European “Group for the Study of Resistant Depression” (GSRD) multi-site study found that age at first treatment (i.e., early-onset and early treatment), age, timespan between first and last episode (i.e., duration of illness), suicidality, and education level were all important variables for outcome 30 . Notably, authors of long-lasting longitudinal studies have suggested that recall bias may influence the age of onset variable 31 , 32 ; given the cognitive deficits associated with acute episodes of MDD, retrospective studies must hence address the factor of memory bias in data collection.
Environmental stress and stressful life events (SLEs)
High stress levels significantly influence outcomes in MDD patients who are prone to vulnerable states, such as those with high levels of neuroticism 33 , 34 . A meta-analysis found that history of childhood maltreatment was associated with elevated risk of developing recurrent and persistent depressive episodes, as well as with lack of response or remission during treatment 35 . Another meta-analysis confirmed the detrimental impact of childhood maltreatment (emotional physical or sexual maltreatment or neglect) as a predisposing risk factor for severe, early-onset, and treatment-resistant depression 36 , 37 . Studies also found gender-specific effects; in particular, at lower stress levels females were at higher risk of MDD than males 34 . Moreover, twin studies have suggested a differential reactivity of gender in response to type of SLE 38 . For instance, a treatment study using escitalopram and nortriptyline investigated the association between number of SLEs (e.g., job loss, psychological trauma, loss of a loved one) and antidepressant treatment. Subjects with more SLEs exhibited greater cognitive symptoms at baseline but not significantly more mood or neurovegetative symptoms. These patients also had greater cognitive symptom reduction in response to escitalopram but not nortriptyline 39 . This suggests that SLEs may have a cognitive domain-specific impact in MDD, but more data are needed to elucidate this issue.
Psychiatric and physical comorbidities
Psychiatric comorbidity has been shown to influence outcome in both treated and untreated patients 40 , 41 . Studies have found that elevated baseline anxiety symptoms or comorbid anxiety disorder are associated with worse antidepressant response to first-line selective serotonin reuptake inhibitors (SSRIs) or second-line treatment strategies 42 , 43 . Worse outcomes have also been reported for MDD patients with comorbid drug or alcohol use disorders, post-traumatic stress disorder (PTSD), and “double depression” (depression and dysthymia) 26 , 41 . Data from the Sequential Treatment Alternatives to Relieve Depression (STAR*D) study, which included patients who were seeking medical care in routine medical or psychiatric outpatient treatment, indicate that roughly one-third (34.8%) of all MDD patients are free of any comorbidity; the most frequent comorbid Axis-I disorders are social phobia (31.3%), generalized anxiety disorder (23.6%), PTSD (20.6%), and obsessive-compulsive disorder (14.3%) 21 . A large recent study found that clinically diagnosed personality disorder was associated with negative outcomes (with regard to remission and persistent depressive symptoms) six months after diagnosis in MDD subjects enrolled in primary care 44 . Moreover, meta-analytic studies indicate that comorbid personality disorder increases the likelihood of poorer outcomes 45 , 46 ; it should be noted, though, that negative studies have also been reported 40 .
MDD and several physical diseases—including cardiovascular disease and diabetes—appear to have bidirectional effects on disease trajectory 47 , 48 , yet pathophysiologic links are most likely complex and have to be elucidated. In addition, depression appears to be linked to hormonal diseases, including hypothyroidism 49 . A number of physical disabilities and medical comorbidities have been shown to significantly impact outcome measures in MDD 50 , particularly in elderly subjects 51 . This connection appears to be relevant at any stage of the disease, as number of physical comorbidities did not separate TRD from non-TRD patients 52 . Links between MDD and pain have also been noted; subjects with elevated levels of baseline pain due to chronic conditions had longer depressive episodes, delayed remission 53 and, most importantly, elevated suicide risk 54 , 55 . Interestingly, a prospective, 12-month study of older patients found that elderly patients with atrial fibrillation exhibited better remission rates 56 . Patients with chronic pulmonary diseases had worse outcomes in uncontrolled treatment settings than those without these diseases. This difference was absent in the intervention group, in which depression care managers helped physicians with guideline-concordant recommendations and helped patients adhere to treatment 56 . Further longitudinal studies on shared pathophysiology with physical diseases are needed to confirm such associations.
Neuroimaging markers of treatment outcomes
Structural markers of antidepressant treatment outcomes suggest that hippocampal volumes are related to response and remission 57 , 58 . One study found that low baseline hippocampal volumes were related to impaired treatment outcomes after 3 years 59 ; a meta-analysis confirmed that low baseline hippocampal volumes are associated with negative outcomes 60 . However, negative studies have also been reported 61 , 62 . The volume of other brain regions, including the anterior cingulate or orbitofrontal cortices, have also been shown to be decreased in MDD subjects 63 , but more longitudinal neuroimaging trials with antidepressants are needed to clarify this association. Interestingly, several studies, including one meta-analysis 64 , found significant hippocampal volume increases after ECT 65 , 66 , 67 , although the relationship to antidepressant response has yet to be confirmed 64 , 68 .
The largest functional magnetic resonance imaging (fMRI) study of MDD patients conducted to date reported neurophysiological subtypes based on connectivity patterns within limbic and frontostriatal brain areas 69 . In subset analyses, connectivity patterns plus subtype classifications predicted response to repetitive transcranial magnetic stimulation (rTMS) treatment with higher accuracy (89.6%) than clinical characteristics alone. Other task-based and resting-state fMRI studies found that ACC activity (including pregenual activity) predicted treatment response 70 , a finding corroborated by an expanded electroencephalography study 71 as well as a meta-analysis 60 . While these interesting results suggest that fMRI measures could ultimately help classify biological subtypes of depression, these methods are far from ready for clinical application and results will have to be reproduced. However, given its easy implementation and the short time needed to acquire measurements, fMRI appears to be a promising tool for identifying imaging biomarkers.
Positron emission tomography (PET) studies have identified altered serotonin-1A (5-HT 1A ) receptor and 5-HT transporter (SERT) binding potentials, an index of protein concentration, at baseline and in TRD patients 72 , 73 , 74 , 75 . Most of these results found reduced baseline SERT levels and elevated baseline 5-HT 1A heteroreceptors in MDD patients (depending on PET methodology for 5-HT 1A ); non-remitters had lower 5-HT 1A autoreceptor binding in the serotonergic raphe nuclei 75 , as well as lower SERT 76 . Reduced global 5-HT 1A receptor binding has also been observed after ECT 77 . High costs, technical and methodological challenges, lack of dedicated PET centers with 11 C-radiochemistry, small sample sizes, small effect sizes, and unclear cutoff values have heretofore prevented the broader clinical application of these tools in MDD compared to disorders such as Alzheimer’s and Parkinson’s disease. An earlier [ 18 F]FDG PET study of unmedicated MDD patients was consistent with the aforementioned fMRI results, demonstrating increased glucose turnover in the orbitofrontal and posterior cingulate cortices and amygdala and decreased turnover in the subgenual ACC and dorsolateral prefrontal cortex 78 . A later study corroborated these results and found that glucose turnover was differentially affected by cognitive behavioral therapy or venlafaxine 79 . Interestingly, several studies detected microglial activation by labeling translocator protein (TSPO) with PET, using TSPO radioligands like 18 F-FEPPA. Microglial activation is closely linked to brain tissue damage, traumatic brain injury, neuroinflammation, and increased metabolic demands. Increased TSPO binding in MDD patients has been observed in the ACC, insula, and prefrontal cortex 80 . In addition, TSPO binding has also been shown to positively correlate with length of illness and time without antidepressant treatment, and to negatively correlate with SSRI treatment 80 . Elevated TSPO levels in unmedicated, acutely ill MDD patients have now been reported in at least two independent datasets 81 , 82 . However, TSPO-positive MDD patients may reflect a specific subtype (i.e., associated with neuroinflammation) and may, thus, respond better to treatments that target neuroinflammation. For a graphical summary of these findings see Fig. 1 .
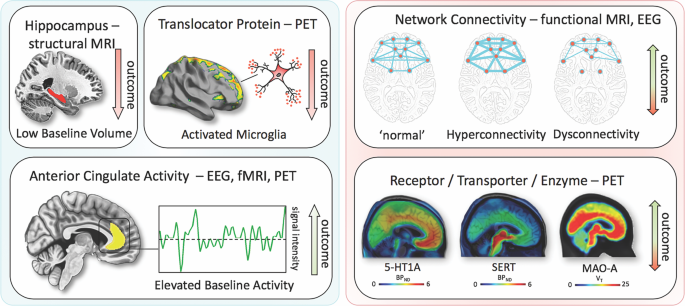
Imaging findings exhibiting unidirectional (left) relationships with outcome in MDD vs. bidirectional (right). fMRI, functional magnetic resonance imaging; PET, positron emission tomography; EEG electroencephalography; 5-HT1A, serotonin-1A receptor; SERT, serotonin transporter; MAO-A monoamine oxidase-A; BP ND , nondisplaceable binding potential; V T , volume of distribution
Blood-based markers of disease outcomes
Consistent with neuroinflammatory processes, elevated levels of C-reactive protein (CRP), tumor necrosis factor alpha (TNFα), and interleukin-6 (IL-6) have been reported in a subset of MDD patients. In particular, elevated levels of CRP, a well-established marker of increased proinflammatory state in blood, was shown to be associated with MDD and increased risk for psychological distress in cross-sectional samples of the general population 83 . A longitudinal study found that lower CRP levels were associated with quicker response to SSRIs, an association not observed for SSRI-bupropion combination therapy 84 . Interestingly, elevated CRP levels have been shown to be more pronounced in female versus male MDD patients 85 . Similar findings have been observed for IL-6 and TNFα. One meta-analysis found that all three were significantly elevated at baseline in MDD patients, but their treatment trajectories differed 86 ; IL-6 levels decreased with antidepressant treatment, but outcomes were indistinguishable. In the same meta-analysis, persistingly high TNFα levels identified TRD patients 86 . Notably, heterogeneity was high within the pooled studies. Another study noted that levels of acute phase protein complement C3 significantly differentiated between atypical and melancholic MDD subtypes 87 . MDD patients have also been shown to have altered levels of peripheral adipokines and bone inflammatory markers; these deficits were corrected with ketamine treatment 88 , 89 .
Given the importance of neuroplasticity in the pathophysiology and treatment of depression, interest has grown in studying brain-derived neurotrophic factor (BDNF), a neurotrophin involved in the structural adaptation of neuronal networks and a prerequisite for neuronal reactions to stressors. BDNF blood levels most likely stem from peripheral tissue. While these peripheral levels are linked to central levels, the question of whether BDNF is actively transported through the blood–brain barrier remains controversial 90 . Compelling evidence suggests that BDNF levels are decreased at baseline in MDD patients and elevated in response to pharmacological 90 , 91 treatments as well as ECT 92 . A meta-analysis found that increased BDNF levels in response to treatment successfully stratified responders and remitters compared to non-responders 93 .
Outcome and genetic and epigenetic links
Heritable risk for MDD is between 30 and 40%, with higher rates in women. A large, collaborative genome-wide association study (GWAS) detected 44 significant loci associated with MDD 94 . Specific analyses identified neuronal genes (but not microglia or astrocytes), gene-expression regulating genes (such as RBFOX1 ), genes involved in gene-splicing, as well as genes that are the targets of antidepressant treatment. The authors suggested that alternative splicing could lead to shifts in the proportion of isoforms and altered biological functions of these proteins 94 .
Hypothesis-driven approaches with candidate genes have provided initial insights into the influence of single-nucleotide polymorphisms (SNPs). It is beyond the scope of this manuscript to review the large number of candidate genes; here, we outline only several representative genes (see Table 1 for meta-analytic evidence of treatment outcomes). These include synaptic proteins involved in stress response, antidepressant binding structures, or neuroplasticity (e.g., CRH receptor 1 ( CRHR1 )), the sodium-dependent serotonin transporter ( SLC6A4 ), and BDNF 95 . The aforementioned multicenter GSRD study also found that combining clinical and genetic variables explained antidepressant response better than SNPs alone in a random forest algorithm 96 . In that study, regulatory proteins such as ZNF804A (associated with response) and CREB1 (associated with remission), as well as a cell adhesion molecule (CHL1, associated with lower risk of TRD), were linked to antidepressant treatment outcomes. Another interesting candidate gene is FK506 binding protein 5 ( FKBP5 ), which was found to moderate the influence of standard treatments in an algorithm lasting up to 14 weeks 97 ; FKBP5 is known to influence HPA axis reactivity 98 , treatment response 99 , and epigenetic mechanisms in response to environmental stressors 100 . Another relevant avenue of research is drug-drug interactions and gene isoforms in the cytochrome P450 pathway (CYP450), which could account for insufficient amounts of a given drug reaching the brain or, conversely, result in exceedingly high plasma values, making subjects more vulnerable to treatment side effects 101 , 102 . Several commercially available kits categorize patients according to their phenotypic status (e.g., CYP2D6, 2C19, CYP3A4). This led to the introduction of phenotype categories—“poor”, “intermediate”, “extensive (normal)”, and “ultrarapid” metabolizers—based on CYP450 isoenzyme status and their relationship to plasma levels at fixed doses 102 . A large naturalistic study of CYP2C19 isoforms found that treatment success with escitalopram was less frequent in “poor” (CYP2C19Null/Null) and “ultrarapid” metabolizers (CYP2C19*1/*17 or CYP2C19*17/*17) 103 .
Clinical subgroups, TRD, and treatment outcomes
While some studies have suggested that depressive subtypes in MDD—including anxious, mixed, melancholic, atypical, and psychotic depression—respond differently to antidepressant treatment, this literature is mixed. For instance, some studies found that melancholic patients initially present with high levels of severity and may respond less well to SSRI treatment than to venlafaxine or tricyclic antidepressants 104 , but other studies did not support this finding 105 . No association was found between subgroups and clinical outcomes in a parallel design, uncontrolled study investigating sertraline, citalopram, and venlafaxine 106 , which found that near equal percentages of patients who met criteria for a pure-form subtype (39%) also had more than one subtype (36%), making these psychopathological subtypes difficult to classify.
It should be noted that treatment success might have more discriminatory power for identifying subgroups than psychopathological subgroup stratification. Although a wide range of definitions exists specifying the number of failed trials necessary to diagnose TRD 107 , the core definition of TRD centers around a lack of improvement in response to consecutive, adequate antidepressant treatments. Resistance occurs at alarmingly high rates and is thought to affect 50–60% of all treated patients 107 . Unsurprisingly, this group of patients has dramatically worse outcomes than those who respond to antidepressants, and factors that are associated with TRD overlap with many of those presented above 28 . Cross-sectional data from the GSRD 108 identified a number of risk factors linked to TRD, including comorbidity (particularly anxiety and personality disorders), suicide risk, episode severity, number of hospitalizations, episode recurrence, early-onset, melancholic features, and non-response at first treatment 28 . Most importantly, TRD is life-threatening, and associated with a two- to threefold increased risk of suicide attempts compared to responding patients, and a 15-fold increased risk compared to the general population 109 . Taken together, the evidence indicates that TRD patients need special attention, as outcomes in these individuals are significantly worse.
Novel and existing strategies to improve treatment outcomes
Early identification, prevention, and early treatment.
Numerous programs for suicide prevention exist 110 , and recognizing acute depressive symptoms is just one of many important facets of such work. Screening tools for early identification of depressed patients can be helpful 111 , and such instruments can start with as few as two items—for instance, the Patient Health Questionnaire-2 112 or Ask Suicide-Screening Questions (asQ’em) 113 —and proceed to more detailed instruments if initial screens are positive. Positive screening should be followed by a diagnostic interview to determine whether patients meet criteria for MDD 111 . In the general population, two large independent studies that used only clinical variables were nevertheless able to accurately predict depression within 1–3 years 114 . In addition, long-term monitoring of vulnerable subjects with known SLEs may further improve the ability to identify at-risk individuals early in their course of illness. As noted above, duration of untreated disease is a negative predictor of treatment outcomes. Because the advantages of early intervention in MDD have been demonstrated 115 , efforts to achieve early treatment might also help slow disease progression in individuals with TRD; however, this hypothesis has not been sufficiently tested.
Modeling environmental impact on predisposition
As noted above, severe SLEs constitute an important risk factor. Elegantly designed studies have demonstrated that genetic predisposition, in concert with SLEs, might account for increased vulnerability to MDD 100 . In this manner, the presence of ‘weak alleles’ in candidate genes such as BDNF, SERT , and others would be increasingly detrimental in the presence of SLEs 116 , 117 . However, studies have been quite inconsistent and yielded small effect sizes, including a negative result in 252 patients enrolled in the GSRD study 118 . It should be noted that counter-regulatory mechanisms or resilience factors, such as social support, may exist that counter SLEs. Nevertheless, preliminary research suggests that the impact of SLEs on MDD may depend on measurable factors such as gender and the timing of exposure 119 . Both genes and the environment are complex systems with frequent opportunity for interaction and elaborate compensatory mechanisms. While the complexity of genetic susceptibility in MDD can be tackled through enormous collaborative projects 94 , the interactions between genetic susceptibility and environmental factors have yet to be determined. Properly powered gene×environment interaction projects may exceed current research capabilities, and large longitudinal studies will certainly be needed 120 .
Developing markers for subgroup identification and disease course
Pioneering research on biological differences—for instance, between patients with atypical versus melancholic depression—suggests differential HPA axis or autonomous nervous system reactivity 121 , 122 , though the subtype results have been only moderately consistent across time and are prone to low group specificity 123 , 124 , 125 . However, at least one study demonstrated the more reliable stability of extreme types over a 2-year period 87 . Interestingly, one study found that individuals with atypical depression had significantly higher body-mass index, waist circumference, levels of inflammatory markers, and triglyceride levels, and lower levels of high-density lipid cholesterol than those with melancholic depression or controls 126 . Using fMRI and biological variables, another study found that MDD subjects could be divided into low/high appetite groups with distinctive correlations between neuronal activity and endocrine, metabolic, and immune states 127 . Other research groups have tried to overcome conventional psychopathological subgroups and model biotypes using resting-state fMRI 69 . Molecular and functional neuroimaging, as well as epigenetic studies, are promising approaches for separating subgroups and may be better suited to identifying screening markers (see Fig. 2 ) that are exclusively valid in certain subgroups with higher predictive power.
These approaches highlight the feasibility of linking and stratifying psychopathological categories with biological variables, a goal further supported by the Research Domain Criteria (RDoc), which seek to link dimensions of observable behavior with neurobiological systems 128 . In the search for biomarkers, subgroup- or domain-specific classifications using unidimensional variables might improve subgroup stratification 129 . Moreover, applying markers to other categories could boost the utility of existing markers that have failed in any given category (see Fig. 2 for established markers). As a field, the focus is largely on staging and prediction markers, but ‘predisposition’ or ‘recurrence’ markers may equally be worth investigating. Presently, however, the relative lack of biologically defined MDD subgroups and their stratification are key obstacles to finding and establishing treament outcome predictors appropriate for broader clinical applications.
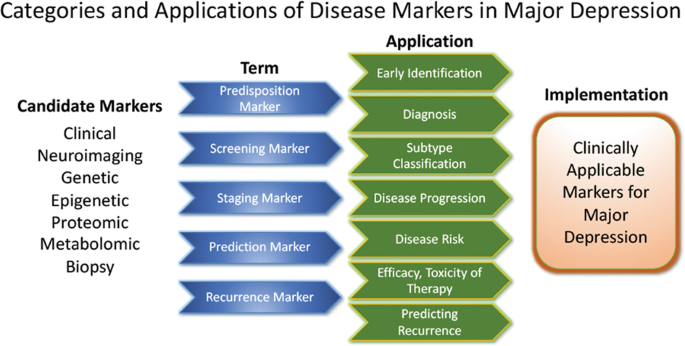
Candidate disease markers can be applied in clinically meaningful ways. While only candidate markers are presently available, sorting these according to their potential applications may facilitate the development of clinically applicable disease markers. The outline follows the classification of markers as suggested by others 200 (modified and reprinted with permission from Springer)
The most important outcome of successful subgroup stratification and staging markers would be that patients and their relatives would receive valuable information at treatment onset about how their disease is likely to improve or worsen. Toward this end, the development of staging methods provides promising solutions. Currently, at least five different methods exist 130 that, to date, have not been evaluated thoroughly enough for clinical implementation. Continuous variables—as obtained by the Maudsley Method and Massachusetts General Hospital Staging Model—appear to provide greater staging advantages than categorical variables. It should be noted here that data indicate that research in severely ill, suicidal, and TRD subjects is safe to conduct in controlled inpatient settings 131 . Presently, patients in various stages of disease and/or treatment history are lumped together and compared in statistical analyses. We propose that staging should be more thoroughly integrated into clinical trial design.
Algorithm- and guideline-based treatments
Despite the availability and distribution of a variety of expert-based guidelines, only a fraction of patients are actually treated according to guidelines 132 (see Table 2 for current guidelines (≤10 years)). New guidelines – particularly for TRD – and more rigorous implementation of guideline-based care are needed. Improvements in currently available treatments have been conducted using treatment algorithms and following sequential treatment strategies, with standardized instructions for therapeutic decision-making. In the past two decades, large, collaborative studies using treatment-based algorithms have introduced standardized, sequential treatments; these include the Texas Medication Algorithm Project 133 , the STAR*D trial 21 , and the German algorithm project 134 . Indeed, evidence suggests that algorithm-based treatments improve treatment outcomes 135 and are cost effective 136 . Here, we considered current clinical treatment guidelines to create a sequential treatment optimization scheme of recommended treatments. While there is no fixed time-frame, first- and second-line treatments are recommended sequentially during the first episode and within 3 months (see Fig. 3 , which also illustrates the need for more third- and fourth-stage treatment options). Figure 4 , illustrates potential reasons for “pseudoresistance” 42 that should be ruled out during this time-frame.
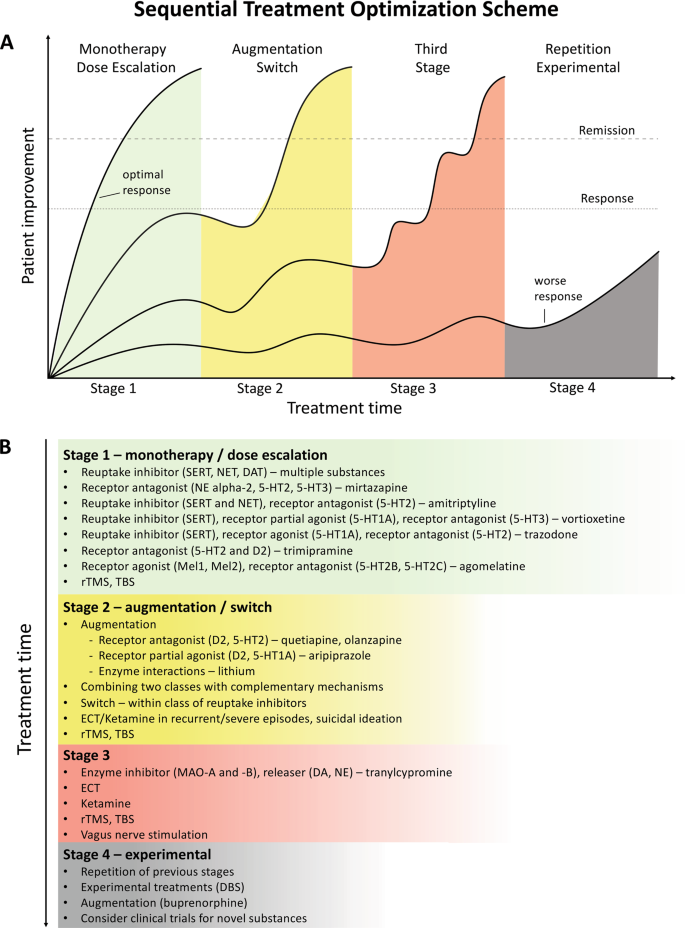
A sequential treatment optimization scheme was generated based on antidepressant treatment guidelines (see Table 2 ). Treatment optimization is possible for patients being treated for the first time but also for patients with insufficient response to first- or second-stage therapies. a Treatment response curves for four common types of patients highlight the importance of sequentially introducing the next step upon non-response to previous steps. b Currently available treatments are listed in neuroscience-based nomenclature 201 with treatment lines corresponding to improvement curves in a . Although current classifications vary, patients classified as having treatment-resistant depression (TRD) are eligible for second- or third-stage therapies. 5-HT1A and similar: serotonin receptor subtypes; DBS: deep brain stimulation; DAT: dopamine transporter; D2: dopamine receptor D2; ECT: electroconvulsive therapy; MAO: monoamine oxidase; NET: noradrenaline transporter; SERT: serotonin transporter; TBS: theta-burst stimulation; rTMS: repetitive transcranial magnetic stimulation; DA: dopamine; NE: norepinephrine.
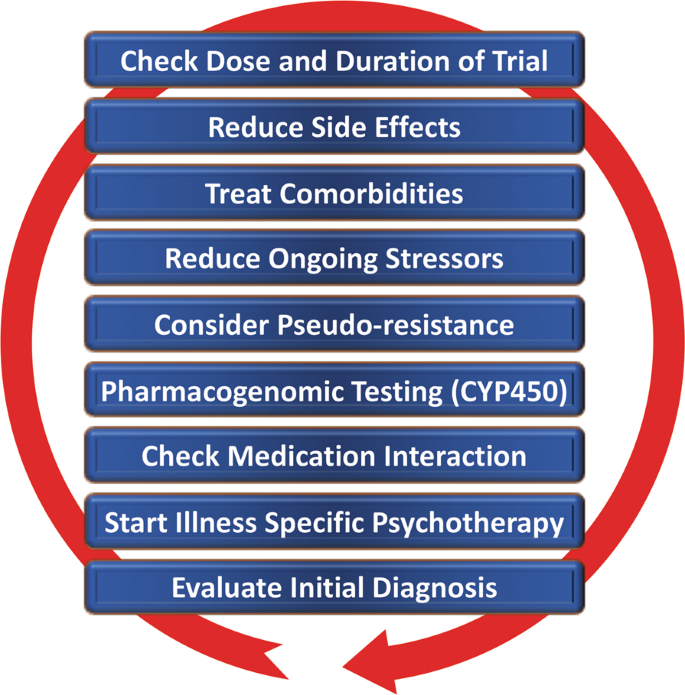
Points—in random order—follow earlier suggestions by Dold and Kasper (2017) 202
Reducing placebo response in clinical trials while harnessing placebo effects in clinical treatment
The issue of placebo response in antidepressant trials has become increasingly important 137 , 138 . Indeed, the contribution of placebo effects to early response needs to be systematically studied in order to disentangle biological therapy-induced effects from psychologically induced effects. Strikingly, in the brain, anatomically similar regions that mediate placebo response are affected by MDD (for a comprehensive review, see ref. 139 ). Several mechanisms contribute to placebo response, including patients’ expectations of benefits, behavioral conditions, and the quality of patient-physician interactions 139 . Strategies for reducing placebo response could lead to better discrimination between effective treatments in clinical trials; such strategies include extending trial duration, excluding placebo responders by including a placebo run-in, or using randomized run-in and withdrawal periods 138 , 139 . Others have suggested using more thorough criteria to select study participants 140 . On the other hand, when antidepressant agents are used clinically, placebo effects must be taken advantage of by harnessing patients’ expectations and learning mechanisms to improve treatment outcomes 141 .
Novel antidepressant treatments
The recent discovery that glutamatergic-based drugs are uniquely capable of rapidly and robustly treating mood disorders has ushered in a new era in the quest to develop novel and effective antidepressants 142 , 143 , 144 . In this regard, the prototypic glutamatergic modulator ketamine has catalyzed research into new mechanistic approaches and offered hope for the development of novel, fast-acting antidepressants. While ketamine’s underlying mechanism of action remains the subject of active investigation, several theories have been propsed 144 . These include N-methyl- d -aspartate receptor (NMDAR)-dependent mechanisms, such as the inhibition of NMDARs on gamma aminobutyric acid (GABA)-ergic interneurons, the inhibition of spontaneous NMDAR-mediated transmission, the inhibition of extrasynaptic NMDARs, the inhibition of lateral habenula neurons, and GABA B receptor expression/function 144 . Substantial evidence also supports additional NMDAR-independent mechanisms, including the stabilization of glutamate release/excitatory transmission, active metabolites such as hydroxynorketamine, regulation of the dopaminergic system, G-alpha subunit translocation, and activation of cyclic adenosine monophosphate, as well as potential sigma-1 and mu-opioid receptor activation 145 . Among those theories, a leading hypothesis remains that NMDAR antagonism increases BDNF synthesis, a process mediated by decreased phosphorylation of eukaryotic elongation factor-2 and the subsequent activation of the mammalian target of rapamycin pathway by BDNF activation of the TrkB receptor 146 , 147 . These putative mechanisms of action are not mutually exclusive and may complement each other to induce potentiation of excitatory synapses in affective-regulating brain circuits, resulting in improved depressive symptoms.
The initial serendipitous discovery that a single, subanesthetic-dose ketamine infusion has rapid-acting antidepressant effects in MDD 148 , a finding subsequently confirmed by numerous randomized trials, has been hailed as one of the most important discoveries in psychiatry in the last decades 149 . The initial proof-of-concept studies demonstrated that a single dose of ketamine (0.5 mg/kg, IV) administered over 40 min led to rapid, robust, and relatively sustained antidepressant effects in TRD—both MDD 150 , 151 , 152 , 153 and bipolar depression 154 , 155 . In research settings, studies of TRD patients found response rates of >70% within 24 h post-infusion 153 , with about 50–70% of participants exhibiting a variable duration of response 156 , 157 . Ketamine has also been shown to be superior to any blinding counterpart 158 . Off-label ketamine use has also been associated with significant and rapid (one to four hours) antisuicidal effects 150 , 159 , 160 , a finding supported by a large, recent metanalysis showing that ketamine exerted rapid (within hours) and sustained (up to 7 days) improvements in suicidal thoughts compared to placebo 161 .
Esketamine hydrochloride
The ketamine enantiomer esketamine received approval by the FDA for TRD and is currently undergoing further Phase III clinical trials. A Phase II, 10-week, clinical trial of flexibly dosed intranasal esketamine (28 mg/56 mg or 84 mg) found that, in TRD patients, this agent demonstrated rapid and clinically relevant improvements in depressive symptoms compared to placebo 162 . Strikingly, 65% of TRD patients met response criteria through Day 57. In another Phase II proof-of-concept, multi-site, 4-week, double-blind study, standard treatment plus intranasal esketamine (84 mg) was compared to standard treatment plus placebo in individuals with MDD at imminent risk of suicide 163 . The authors found a rapid antisuicidal effect, as assessed via the Montgomery-Åsberg Depression Rating Scale Suicide Item score at 4 h.
Other rapid acting and novel antidepressants
Based on the success of ketamine, other rapid-acting or novel antidepressant substances within the glutamatergic/GABA neurotransmitter systems are being developed, several of which are in Phase III clinical trials. A prototype novel substance is AV-101 (L-4-cholorkynurenine). This is a potent selective antagonist at the glycine-binding site of the NMDAR NR1 subunit and has demonstrated antidepressant-like effects in animal models, while human Phase II studies are currently ongoing 164 . Brexanolone is a formulation of the endogenous neurosteroid allopregnanolone, which modulates neuronal activation of GABA A receptors and has met positive endpoints in Phase III, leading to FDA approval for postpartum depression. A comparable substance is under development for MDD 165 . In addition, serotonergic agonists have been studied as our understanding of their mechanism of action (e.g., their effects on glutamate release or plasticity) has increased 166 . Encouraging results have been seen for the serotonin 2A receptor agonist psilocybin 167 , but these findings need to be replicated in larger systematic clinical trials. Initial positive trials of add-on agents—such as buprenorphine 168 , 169 , rapastinel 170 , or scopolamine 145 —have also been conducted. However, it is beyond the scope of this manuscript to review all of these findings, and we refer the interested reader to recent comprehensive reviews of this subject 144 , 145 , 165 , 171 .
Transcranial stimulation paradigms
In contrast to pharmaceutical treatments that exert their efficacy at the molecular level, electrical stimulation techniques target entire neuronal circuits. TMS of the (left) dorsolateral prefrontal cortex has been FDA-approved since 2008 to treat depression in patients who failed to respond to one standard antidepressant treatment. Apart from transient local skin and muscle irritation at the stimulation site and headaches, it is a very safe technique with few side effects. Studies have repeatedly demonstrated the superiority of rTMS over sham procedures, though effect sizes have been moderate 172 , 173 , 174 . Initial studies suggest that rTMS is also effective in TRD but the data are too few to draw definitive conclusions 175 , 176 . Improvements in rTMS techniques known as theta-burst stimulation (TBS) provide significantly shortened treatment times (3 min for TBS versus 37 min for rTMS) and hence allow more patients to be treated per day. A large non-inferiority trial of 414 moderately resistant MDD patients found that TBS was at least as effective as rTMS in reducing depressive symptoms 177 .
Electroconvulsive therapy (ECT)
Regarded as the ‘gold standard’, ECT has been successfully used for many years to treat severe TRD and exhibits both relatively rapid and sustained onset of efficacy; approximately 50% of all patients reach response criteria at the third treatment, typically within 1 week. It is also one of the most effective antidepressant therapies 178 , yielding response rates of ~80%, remission rates of ~75% 179 , and antisuicidal effects 180 . Remission is achieved by about 30% of patients within six ECT sessions 179 . ECT also reduces the risk of readmission 181 and is likewise safe to use in depressed elderly subjects 182 . The side effects of ECT include intermediate disorientation, impaired learning, and retrograde amnesia, all of which usually resolve 183 . The optimal anatomic location of the stimulus electrodes is a topic of current debate 184 , 185 . Recent evidence suggests that all three methods for electrode placement (bifrontal, bitemporal, and unilateral) show clinically significant effects 186 . While no difference in cognitive side effects was observed, bitemporal placement should be considered the first-line choice for urgent clinical situations. Despite its clinical efficacy, ECT remains underutilized. Its use is declining 187 because it needs to be administered in hospital settings under anesthesia, and partly because of misleading portrayals of the procedure itself. Adjusting the dose of electrical stimuli (e.g., through refined electrode placement or individually adjusted pulse amplitudes) may improve ECT’s side effect profile.
Magnetic seizure therapy (MST)
MST uses high doses of rTMS to induce seizures 188 . The electromagnetically induced electrical field generated by MST is unifocal and variable, as there are individual differences in the degree to which the skull provides electrical resistance 189 . As an advantage over ECT, MST is associated with a more superficial stimulation, which exerts less impact on the medial-temporal lobe where cognitive side effects are thought to be elicited. To date, few research sites across the world have used MST, with a concomitant dearth of open-label trials. Nevertheless, the preliminary treatment data suggest that results obtained with MST are similar to those obtained with ECT but with a more favorable side effect profile 190 , 191 .
Vagus nerve stimulation (VNS)
VNS is a surgically implanted pacemaker-like device attached to a stimulating wire threaded along the left vagus nerve. Since 2005, the FDA has approved VNS use for the adjunctive long-term treatment of long-lasting recurrent depression in patients 18 years and older who are experiencing a major depressive episode and have failed to respond to four or more previous adequate standard antidepressant treatment trials. In such cases, it has been shown to have superior long-term effects over conventional psychopharmacological treatment 192 . A recent, large, observational, adjunctive, open-label, naturalistic study followed TRD patients over 5 years 193 . In this group, adjunctive VNS led to significantly better clinical outcomes and higher remission rates than treatment as usual (67.6% vs. 40.9%, respectively).
Deep-brain stimulation (DBS)
DBS involves the neurosurgical implantation of electrodes and has become clinically routine in the treatment of Parkinson’s disease and Dystonia. The technique is safe, removable, and does not cause lasting neuronal lesions. In TRD, anatomical targets include the subgenual cingulate, nucleus accumbens, habenula, and medial forebrain bundle. Clinical trials typically only enroll severely ill TRD patients whose current episode has lasted >12 months, whose age of onset is <45 years, and who have failed to respond to at least four adequate prior treatment trials of standard antidepressants, ECT, and/or psychotherapy. Initial open-label or single-blind trials found that DBS had both rapid and sustained antidepressant effects 194 , 195 , 196 . In contrast, one large and one smaller sham-controlled clinical study both failed to achieve their primary endpoints of symptom reduction 197 , 198 . To date, the number of MDD patients treated with DBS has been very small compared to other treatment options, including ECT and TMS. Nevertheless, brain-electrode interfaces are evolving quickly and it is possible that next generation brain-responsive stimulation devices will be able to adjust stimulation on-demand only when abnormal biological marker impulses (e.g., pulse amplitude) are detected 199 .
Conclusions
Although enormous progress has been made in measuring, predicting, and improving outcomes, depression remains a relentless disease that places a heavy burden on both individuals and society. The research reviewed above indicates that early recognition and early adequate treatment at illness onset are preferable to watch-and-wait strategies. The studies reviewed above also underscore the manner in which SLEs, as well as physical and psychiatric comorbidities, contribute to impaired outcomes. Together, these factors contribute toward treatment resistance, which has gained a substantial amount of importance as a patient-stratifying variable.
This paper also reviewed biological markers, where research has grown exponentially to encompass enormous projects with potentially tens of thousands of subjects enrolled in real world studies. In parallel, studies exploring the underlying genetics of depression have evolved from early candidate gene studies of neurotransmitters, stress, or gene-regulatory systems to large GWAS that help reveal potential new pathways and treatment targets. Moreover, the burgeoning field of proteomics has found promising target molecules. Nevertheless, despite the wealth of recent work in this area, no single biomarker has yet been used in clinical applications. A substantial need exists for replication and, because many biomarker studies are currently open-label, for controlled studies. In combination with neuroimaging techniques such as fMRI, genes or blood-based markers have a high potential of future implementation in stratification of MDD or serve as prognostic marker on treatment outcome.
Above, we also outlined efforts to optimize outcomes. We argue that disease-inherent heterogeneity, in concert with inaccurate group stratification tools, might have contributed to the lack of clinically applicable stratification and response prediction markers. Successful subgroup identification, and the ability to use this information in clinical settings, is crucial to improving future treatment paradigms. While recent research has increasingly focused on TRD, we wish to reiterate that no standard definition of TRD presently exists. Thus, based on currently available guidelines, we have outlined a sequential treatment optimization scheme that includes options for TRD; such work highlights the substantial need to develop and improve “third-line-and-beyond” therapeutics. In this context, this manuscript also reviews novel treatments and brain stimulation techniques that have demonstrated rapid antidepressant effects in TRD, including ketamine, esketamine, ECT, MST, TMS/TBS, VNS, DBS, and others. When treating TRD patients, physicians should consider illness severity, the chronicity of past and recent depressive episodes, the side effect profile of available treatment options, as well as previous refractoriness to particular treatment approaches. If acuity supersedes chronicity, one could consider fast-acting interventions such as ketamine or ECT/MST.
This review, though comprehensive, was not able to consider several lines of evidence on outcome prediction and treatment improvement. In particular, we focused on clinical outcomes in humans and were, thus, unable to fully explore the highly valuable advances made in translational science. Similarly, it was beyond the scope of this manuscript to review the richness of results from animal research and their relevance to MDD. Moreover, given the amount of literature, we were not able to incorporate many proteomic, genetic, or psychopharmacological findings.
Taken together, this review outlines important clinical, psychosocial, and biological factors associated with response and remission to antidepressant treatment (see Table 3 ). Recent studies have led to important insights into neurobiological disease markers that could result in improved disease stratification and response prediction in the near future. Key discoveries into novel rapid-acting substances, in concert with improvements in brain stimulation techniques, may also result in significantly improved treatment outcomes in formerly hard-to-treat patients.
Wittchen, H. U. et al. The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur. Neuropsychopharmacol.: J. Eur. Coll. Neuropsychopharmacol. 21 , 655–679 (2011).
Article CAS Google Scholar
Lim, S. S. et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380 , 2224–2260 (2012).
Article PubMed PubMed Central Google Scholar
Lecrubier, Y. Widespread underrecognition and undertreatment of anxiety and mood disorders: results from 3 European studies. J . Clin . Psychiatry 68 Suppl 2 , 36–41 (2007).
Riedel, M. et al. Clinical predictors of response and remission in inpatients with depressive syndromes. J. Affect. Disord. 133 , 137–149 (2011).
Article PubMed Google Scholar
Rost, K. et al. Persistently poor outcomes of undetected major depression in primary care. Gen. Hosp. Psychiatry 20 , 12–20 (1998).
Article CAS PubMed Google Scholar
Ghio, L., Gotelli, S., Marcenaro, M., Amore, M. & Natta, W. Duration of untreated illness and outcomes in unipolar depression: a systematic review and meta-analysis. J. Affect. Disord. 152–154 , 45–51 (2014).
Hung, C. I., Liu, C. Y. & Yang, C. H. Untreated duration predicted the severity of depression at the two-year follow-up point. PLoS ONE 12 , e0185119 (2017).
Article PubMed PubMed Central CAS Google Scholar
Bukh, J. D., Bock, C., Vinberg, M. & Kessing, L. V. The effect of prolonged duration of untreated depression on antidepressant treatment outcome. J. Affect. Disord. 145 , 42–48 (2013).
Habert, J. et al. Functional recovery in major depressive disorder: Focus on early optimized treatment. Prim Care Companion CNS Disord. 18 (2016).
Kautzky, A. et al. Clinical factors predicting treatment resistant depression: affirmative results from the European multicenter study. Acta Psychiatr. Scand. 139 , 78–88 (2018).
Furukawa, T. A., Kitamura, T. & Takahashi, K. Time to recovery of an inception cohort with hitherto untreated unipolar major depressive episodes. Br. J. Psychiatry.: J. Ment. Sci. 177 , 331–335 (2000).
Feffer, K. et al. Early symptom improvement at 10 sessions as a predictor of rTMS treatment outcome in major depression. Brain Stimul. 11 , 181–189 (2018).
Martinez-Amoros, E. et al. Early improvement as a predictor of final remission in major depressive disorder: New insights in electroconvulsive therapy. J. Affect. Disord. 235 , 169–175 (2018).
Soares, C. N., Endicott, J., Boucher, M., Fayyad, R. S. & Guico-Pabia, C. J. Predictors of functional response and remission with desvenlafaxine 50 mg/d in patients with major depressive disorder. CNS Spectr. 19 , 519–527 (2014).
Lam, R. W. et al. Predictors of functional improvement in employed adults with major depressive disorder treated with desvenlafaxine. Int Clin. Psychopharmacol. 29 , 239–251 (2014).
Jha, M. K. et al. Early improvement in work productivity predicts future clinical course in depressed outpatients: Findings from the CO-MED trial. Am. J. Psychiatry 173 , 1196–1204 (2016).
Spies, M. et al. Default mode network deactivation during emotion processing predicts early antidepressant response. Transl. Psychiatry 7 , e1008 (2017).
Article CAS PubMed PubMed Central Google Scholar
Bartlett, E. A. et al. Pretreatment and early-treatment cortical thickness is associated with SSRI treatment response in major depressive disorder. Neuropsychopharmacol.: Off. Publ. Am. Coll. Neuropsychopharmacol. 43 , 2221–2230 (2018).
Olgiati, P. et al. Early improvement and response to antidepressant medications in adults with major depressive disorder. Meta-analysis and study of a sample with treatment-resistant depression. J. Affect Disord. 227 , 777–786 (2018).
de Vries, Y. A. et al. Predicting antidepressant response by monitoring early improvement of individual symptoms of depression: individual patient data meta-analysis. Br. J. Psychiatry.: J. Ment. Sci. 214 , 1–7 (2018).
Google Scholar
Trivedi, M. H. et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am. J. Psychiatry 163 , 28–40 (2006).
Blom, M. B. et al. Severity and duration of depression, not personality factors, predict short term outcome in the treatment of major depression. J. Affect. Disord. 104 , 119–126 (2007).
Kautzky, A. et al. Refining prediction in treatment-resistant depression: results of machine learning analyses in the TRD III sample. J. Clin. Psychiatry 79 , 16m11385 (2018).
Katon, W., Unutzer, J. & Russo, J. Major depression: the importance of clinical characteristics and treatment response to prognosis. Depress Anxiety 27 , 19–26 (2010).
Papakostas, G. I. Surrogate markers of treatment outcome in major depressive disorder. Int. J. Neuropsychopharmacol./Off. Sci. J. Coll. Int. Neuropsychopharmacol. 15 , 841–854 (2012).
Friedman, E. S. et al. Baseline depression severity as a predictor of single and combination antidepressant treatment outcome: results from the CO-MED trial. Eur. Neuropsychopharmacol.: J. Eur. Coll. Neuropsychopharmacol. 22 , 183–199 (2012).
Balestri, M. et al. Socio-demographic and clinical predictors of treatment resistant depression: A prospective European multicenter study. J. Affect Disord. 189 , 224–232 (2016).
Souery, D. et al. Clinical factors associated with treatment resistance in major depressive disorder: results from a European multicenter study. J. Clin. Psychiatry 68 , 1062–1070 (2007).
Mandelli, L. et al. Opinion paper: poor response to treatment of depression in people in high occupational levels. Psychol. Med. 49 , 49–54 (2019).
Kautzky, A. et al. A new prediction model for evaluating treatment-resistant depression. J. Clin. Psychiatry 78 , 215–222 (2017).
Paksarian, D. et al. Stability and change in reported age of onset of depression, back pain, and smoking over 29 years in a prospective cohort study. J. Psychiatr. Res. 88 , 105–112 (2017).
Wells, K. et al. Five-year impact of quality improvement for depression: results of a group-level randomized controlled trial. Arch. Gen. Psychiatry 61 , 378–386 (2004).
Vinkers, C. H. et al. Stress exposure across the life span cumulatively increases depression risk and is moderated by neuroticism. Depress Anxiety 31 , 737–745 (2014).
Kendler, K. S., Kuhn, J. & Prescott, C. A. The interrelationship of neuroticism, sex, and stressful life events in the prediction of episodes of major depression. Am. J. Psychiatry 161 , 631–636 (2004).
Nanni, V., Uher, R. & Danese, A. Childhood maltreatment predicts unfavorable course of illness and treatment outcome in depression: a meta-analysis. Am. J. Psychiatry 169 , 141–151 (2012).
Thompson, A. E. & Kaplan, C. A. Childhood emotional abuse. Br. J. Psychiatry.: J. Ment. Sci. 168 , 143–148 (1996).
Nelson, J., Klumparendt, A., Doebler, P. & Ehring, T. Childhood maltreatment and characteristics of adult depression: meta-analysis. Br. J. Psychiatry.: J. Ment. Sci. 210 , 96–104 (2017).
Article Google Scholar
Kendler, K. S. & Gardner, C. O. Sex differences in the pathways to major depression: a study of opposite-sex twin pairs. Am. J. Psychiatry 171 , 426–435 (2014).
Keers, R. et al. Stressful life events, cognitive symptoms of depression and response to antidepressants in GENDEP. J. Affect. Disord. 127 , 337–342 (2010).
Henriksen, C. A. et al. Identifying factors that predict longitudinal outcomes of untreated common mental disorders. Psychiatr. Serv. 66 , 163–170 (2015).
Dennehy, E. B., Marangell, L. B., Martinez, J., Balasubramani, G. K. & Wisniewski, S. R. Clinical and functional outcomes of patients who experience partial response to citalopram: secondary analysis of STAR*D. J. Psychiatr. Pract. 20 , 178–187 (2014).
Dold, M. et al. Clinical characteristics and treatment outcomes of patients with major depressive disorder and comorbid anxiety disorders—results from a European multicenter study. J. Psychiatr. Res. 91 , 1–13 (2017).
Fava, M. et al. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am. J. Psychiatry 165 , 342–351 (2008).
Angstman, K. B. et al. Personality disorders in primary care: impact on depression outcomes within collaborative care. J. Prim. Care Community Health 8 , 233–238 (2017).
Zeeck, A. et al. Prognostic and prescriptive predictors of improvement in a naturalistic study on inpatient and day hospital treatment of depression. J. Affect. Disord. 197 , 205–214 (2016).
Newton-Howes, G., Tyrer, P. & Johnson, T. Personality disorder and the outcome of depression: meta-analysis of published studies. Br. J. Psychiatry.: J. Ment. Sci. 188 , 13–20 (2006).
Whooley, M. A. et al. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA 300 , 2379–2388 (2008).
Ducat, L., Philipson, L. H. & Anderson, B. J. The mental health comorbidities of diabetes. JAMA 312 , 691–692 (2014).
Fugger, G. et al. Comorbid thyroid disease in patients with major depressive disorder—results from the European Group for the Study of Resistant Depression (GSRD). Eur. Neuropsychopharmacol. 28 , 752–760 (2018).
Iosifescu, D. V. et al. The impact of medical comorbidity on acute treatment in major depressive disorder. Am. J. Psychiatry 160 , 2122–2127 (2003).
Oslin, D. W. et al. Association between medical comorbidity and treatment outcomes in late-life depression. J. Am. Geriatr. Soc. 50 , 823–828 (2002).
Amital, D. et al. Physical co-morbidity among treatment resistant vs. treatment responsive patients with major depressive disorder. Eur. Neuropsychopharmacol. 23 , 895–901 (2013).
Karp, J. F. et al. Pain predicts longer time to remission during treatment of recurrent depression. J. Clin. Psychiatry 66 , 591–597 (2005).
Ohayon, M. M. & Schatzberg, A. F. Using chronic pain to predict depressive morbidity in the general population. Arch. Gen. Psychiatry 60 , 39–47 (2003).
Racine, M. Chronic pain and suicide risk: A comprehensive review. Prog. Neuropsychopharmacol. Biol. Psychiatry 87 , 269–280 (2018).
Bogner, H. R. et al. The role of medical comorbidity in outcome of major depression in primary care: the PROSPECT study. Am. J. Geriatr. Psychiatry 13 , 861–868 (2005).
MacQueen, G. M., Yucel, K., Taylor, V. H., Macdonald, K. & Joffe, R. Posterior hippocampal volumes are associated with remission rates in patients with major depressive disorder. Biol. Psychiatry 64 , 880–883 (2008).
Phillips, J. L., Batten, L. A., Tremblay, P., Aldosary, F. & Blier, P. A prospective, longitudinal study of the effect of remission on cortical thickness and hippocampal volume in patients with treatment-resistant depression. Int. J. Neuropsychopharmacol. / Off. Sci. J. Coll. Int. Neuropsychopharmacol. 18 , pyv037 (2015).
Frodl, T. et al. Effect of hippocampal and amygdala volumes on clinical outcomes in major depression: a 3-year prospective magnetic resonance imaging study. J. Psychiatry Neurosci. 33 , 423–430 (2008).
PubMed PubMed Central Google Scholar
Fu, C. H., Steiner, H. & Costafreda, S. G. Predictive neural biomarkers of clinical response in depression: a meta-analysis of functional and structural neuroimaging studies of pharmacological and psychological therapies. Neurobiol. Dis. 52 , 75–83 (2013).
Frodl, T. et al. Reduced hippocampal volumes associated with the long variant of the serotonin transporter polymorphism in major depression. Arch. Gen. Psychiatry 61 , 177–183 (2004).
Vythilingam, M. et al. Hippocampal volume, memory, and cortisol status in major depressive disorder: effects of treatment. Biol. Psychiatry 56 , 101–112 (2004).
Schmaal, L. et al. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol. Psychiatry 22 , 900–909 (2017).
Wilkinson, S. T., Sanacora, G. & Bloch, M. H. Hippocampal volume changes following electroconvulsive therapy: a systematic review and meta-analysis. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2 , 327–335 (2017).
Nordanskog, P. et al. Increase in hippocampal volume after electroconvulsive therapy in patients with depression: a volumetric magnetic resonance imaging study. J. ECT 26 , 62–67 (2010).
Dukart, J. et al. Electroconvulsive therapy-induced brain plasticity determines therapeutic outcome in mood disorders. Proc. Natl Acad. Sci. USA 111 , 1156–1161 (2014).
Gryglewski, G. et al. Structural changes in amygdala nuclei, hippocampal subfields and cortical thickness following electroconvulsive therapy in treatment-resistant depression: longitudinal analysis. Br. J. Psychiatr 214 , 159–167 (2019).
Oltedal, L. et al. Volume of the human hippocampus and clinical response following electroconvulsive therapy. Biol. Psychiatry 84 , 574–581 (2018).
Drysdale, A. T. et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat. Med. 23 , 28–38 (2017).
Dunlop, B. W. et al. Functional connectivity of the subcallosal cingulate cortex and differential outcomes to treatment with cognitive-behavioral therapy or antidepressant medication for major depressive disorder. Am. J. Psychiatry 174 , 533–545 (2017).
Pizzagalli, D. A. et al. Pretreatment rostral anterior cingulate cortex theta activity in relation to symptom improvement in depression: A randomized clinical trial. JAMA Psychiatry 75 , 547–554 (2018).
Gryglewski, G., Lanzenberger, R., Kranz, G. S. & Cumming, P. Meta-analysis of molecular imaging of serotonin transporters in major depression. J. Cereb. Blood Flow. Metab. 34 , 1096–1103 (2014).
Spies, M., Knudsen, G. M., Lanzenberger, R. & Kasper, S. The serotonin transporter in psychiatric disorders: insights from PET imaging. Lancet Psychiatry 2 , 743–755 (2015).
Wang, L. et al. Serotonin-1A receptor alterations in depression: a meta-analysis of molecular imaging studies. BMC Psychiatry 16 , 319 (2016).
Miller, J. M. et al. Brain serotonin 1A receptor binding as a predictor of treatment outcome in major depressive disorder. Biol. Psychiatry 74 , 760–767 (2013).
Miller, J. M., Oquendo, M. A., Ogden, R. T., Mann, J. J. & Parsey, R. V. Serotonin transporter binding as a possible predictor of one-year remission in major depressive disorder. J. Psychiatr. Res. 42 , 1137–1144 (2008).
Lanzenberger, R. et al. Prediction of SSRI treatment response in major depression based on serotonin transporter interplay between median raphe nucleus and projection areas. NeuroImage 63 , 874–881 (2012).
Drevets, W. C. Neuroimaging studies of mood disorders. Biol. Psychiatry 48 , 813–829 (2000).
Kennedy, S. H. et al. Differences in brain glucose metabolism between responders to CBT and venlafaxine in a 16-week randomized controlled trial. Am. J. Psychiatry 164 , 778–788 (2007).
Setiawan, E. et al. Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry 72 , 268–275 (2015).
Richards, E. M. et al. PET radioligand binding to translocator protein (TSPO) is increased in unmedicated depressed subjects. EJNMMI Res. 8 , 57 (2018).
Holmes, S. E. et al. Elevated translocator protein in anterior cingulate in major depression and a role for inflammation in suicidal thinking: a positron emission tomography study. Biol. Psychiatry 83 , 61–69 (2018).
Wium-Andersen, M. K., Orsted, D. D. & Nordestgaard, B. G. Elevated plasma fibrinogen, psychological distress, antidepressant use, and hospitalization with depression: two large population-based studies. Psychoneuroendocrinology 38 , 638–647 (2013).
Jha, M. K. et al. Can C-reactive protein inform antidepressant medication selection in depressed outpatients? Findings from the CO-MED trial. Psychoneuroendocrinology 78 , 105–113 (2017).
Kohler-Forsberg, O. et al. Association between C-reactive protein (CRP) with depression symptom severity and specific depressive symptoms in major depression. Brain Behav. Immun. 62 , 344–350 (2017).
Strawbridge, R. et al. Inflammation and clinical response to treatment in depression: A meta-analysis. Eur. Neuropsychopharmacol.: J. Eur. Coll. Neuropsychopharmacol. 25 , 1532–1543 (2015).
Lamers, F. et al. Serum proteomic profiles of depressive subtypes. Transl. Psychiatry 6 , e851 (2016).
Kadriu, B. et al. Acute ketamine administration corrects abnormal inflammatory bone markers in major depressive disorder. Mol. Psychiatry 23 , 1626–1631 (2017).
Machado-Vieira, R. et al. The role of adipokines in the rapid antidepressant effects of ketamine. Mol. Psychiatry 22 , 127–133 (2017).
Schmidt, H. D., Shelton, R. C. & Duman, R. S. Functional biomarkers of depression: diagnosis, treatment, and pathophysiology. Neuropsychopharmacology 36 , 2375–2394 (2011).
Molendijk, M. L. et al. Serum BDNF concentrations as peripheral manifestations of depression: evidence from a systematic review and meta-analyses on 179 associations (N = 9484). Mol. Psychiatry 19 , 791–800 (2014).
Brunoni, A. R., Baeken, C., Machado-Vieira, R., Gattaz, W. F. & Vanderhasselt, M. A. BDNF blood levels after electroconvulsive therapy in patients with mood disorders: a systematic review and meta-analysis. World J. Biol. Psychiatry 15 , 411–418 (2014).
Polyakova, M. et al. BDNF as a biomarker for successful treatment of mood disorders: a systematic & quantitative meta-analysis. J. Affect. Disord. 174 , 432–440 (2015).
Wray, N. R. et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat. Genet. 50 , 668–681 (2018).
Fabbri, C. et al. Consensus paper of the WFSBP Task Force on Genetics: Genetics, epigenetics and gene expression markers of major depressive disorder and antidepressant response. World J. Biol. Psychiatry 18 , 5–28 (2017).
Kautzky, A. et al. The combined effect of genetic polymorphisms and clinical parameters on treatment outcome in treatment-resistant depression. Eur. Neuropsychopharmacol. 25 , 441–453 (2015).
Stamm, T. J. et al. The FKBP5 polymorphism rs1360780 influences the effect of an algorithm-based antidepressant treatment and is associated with remission in patients with major depression. J. Psychopharmacol. 30 , 40–47 (2016).
Binder, E. B. et al. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat. Genet. 36 , 1319–1325 (2004).
Fabbri, C. et al. Pleiotropic genes in psychiatry: Calcium channels and the stress-related FKBP5 gene in antidepressant resistance. Prog. Neuropsychopharmacol. Biol. Psychiatry 81 , 203–210 (2018).
Klengel, T. & Binder, E. B. Gene x environment interactions in the prediction of response to antidepressant treatment. Int. J. Neuropsychopharmacol./Off. Sci. J. Coll. Int. Neuropsychopharmacol. 16 , 701–711 (2013).
CAS Google Scholar
Schosser, A. & Kasper, S. The role of pharmacogenetics in the treatment of depression and anxiety disorders. Int. Clin. Psychopharmacol. 24 , 277–288 (2009).
Zeier, Z. et al. Clinical implementation of pharmacogenetic decision support tools for antidepressant drug prescribing. Am. J. Psychiatry 175 , 873–886 (2018).
Jukic, M. M., Haslemo, T., Molden, E. & Ingelman-Sundberg, M. Impact of CYP2C19 genotype on escitalopram exposure and therapeutic failure: A retrospective study based on 2,087 patients. Am. J. Psychiatry 175 , 463–470 (2018).
Bauer, M. et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders, part 1: update 2013 on the acute and continuation treatment of unipolar depressive disorders. World J. Biol. Psychiatry 14 , 334–385 (2013).
Uher, R. et al. Melancholic, atypical and anxious depression subtypes and outcome of treatment with escitalopram and nortriptyline. J. Affect. Disord. 132 , 112–120 (2011).
Arnow, B. A. et al. Depression subtypes in predicting antidepressant response: A report from the iSPOT-D trial. Am. J. Psychiatry 172 , 743–750 (2015).
Kasper, S. & Montgomery, S. A. Ohio Library and Information Network, Wiley Online Library (Online service). Treatment-resistant Depression , 1 online resource (2013).
Schosser, A. et al. European Group for the Study of Resistant Depression (GSRD)–where have we gone so far: review of clinical and genetic findings. Eur. Neuropsychopharmacol. 22 , 453–468 (2012).
Bergfeld, I. O. et al. Treatment-resistant depression and suicidality. J. Affect. Disord. 235 , 362–367 (2018).
Mann, J. J. et al. Suicide prevention strategies: a systematic review. JAMA 294 , 2064–2074 (2005).
Nimalasuriya, K., Compton, M. T. & Guillory, V. J., Prevention Practice Committee of the American College of Preventive M. Screening adults for depression in primary care: A position statement of the American College of Preventive Medicine. J. Fam. Pract. 58 , 535–538 (2009).
PubMed Google Scholar
Kroenke, K., Spitzer, R. L. & Williams, J. B. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med. Care 41 , 1284–1292 (2003).
Horowitz, L. M. et al. Ask suicide-screening questions to everyone in medical settings: the asQ’em Quality Improvement Project. Psychosomatics 54 , 239–247 (2013).
King, M. et al. Predicting onset of major depression in general practice attendees in Europe: extending the application of the predictD risk algorithm from 12 to 24 months. Psychol. Med. 43 , 1929–1939 (2013).
Kupfer, D. J., Frank, E. & Perel, J. M. The advantage of early treatment intervention in recurrent depression. Arch. Gen. Psychiatry 46 , 771–775 (1989).
Lopizzo, N. et al. Gene-environment interaction in major depression: focus on experience-dependent biological systems. Front. Psychiatry 6 , 68 (2015).
Gutierrez, B. et al. The risk for major depression conferred by childhood maltreatment is multiplied by BDNF and SERT genetic vulnerability: a replication study. J. Psychiatry Neurosci.: JPN 40 , 187–196 (2015).
Serretti, A. et al. The impact of adverse life events on clinical features and interaction with gene variants in mood disorder patients. Psychopathology 46 , 384–389 (2013).
Herbison, C. E., Allen, K., Robinson, M., Newnham, J. & Pennell, C. The impact of life stress on adult depression and anxiety is dependent on gender and timing of exposure. Dev. Psychopathol. 29 , 1443–1454 (2017).
Keers, R. & Uher, R. Gene-environment interaction in major depression and antidepressant treatment response. Curr. Psychiatry Rep. 14 , 129–137 (2012).
Gold, P. W. & Chrousos, G. P. Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol. Psychiatry 7 , 254–275 (2002).
Carroll, B. J. et al. A specific laboratory test for the diagnosis of melancholia. Standardization, validation, and clinical utility. Arch. Gen. Psychiatry 38 , 15–22 (1981).
Musil, R. et al. Subtypes of depression and their overlap in a naturalistic inpatient sample of major depressive disorder. Int. J. Methods Psychiatr. Res . 27 (2018).
Angst J., Gamma A., Benazzi F., Ajdacic V., Rossler W. Melancholia and atypical depression in the Zurich study: epidemiology, clinical characteristics, course, comorbidity and personality. Acta Psychiatr. Scand. Suppl. 72–84 (2007).
Ionescu, D. F., Niciu, M. J., Henter, I. D. & Zarate, C. A. Defining anxious depression: a review of the literature. CNS Spectr. 18 , 252–260 (2013).
Lamers, F. et al. Evidence for a differential role of HPA-axis function, inflammation and metabolic syndrome in melancholic versus atypical depression. Mol. Psychiatry 18 , 692–699 (2013).
Simmons, W. K. et al. Appetite changes reveal depression subgroups with distinct endocrine, metabolic, and immune states. Mol. Psychiatry , https://doi.org/10.1038/s41380-018-0093-6 (2018).
Woody, M. L. & Gibb, B. E. Integrating NIMH research domain criteria (RDoC) into depression. Res. Curr. Opin. Psychol. 4 , 6–12 (2015).
Ballard, E. D. et al. Parsing the heterogeneity of depression: An exploratory factor analysis across commonly used depression rating scales. J. Affect. Disord. 231 , 51–57 (2018).
Ruhe, H. G., van Rooijen, G., Spijker, J., Peeters, F. P. & Schene, A. H. Staging methods for treatment resistant depression. A systematic review. J. Affect. Disord. 137 , 35–45 (2012).
Nugent, A. C. et al. Safety of research into severe and treatment-resistant mood disorders: analysis of outcome data from 12 years of clinical trials at the US National Institute of Mental Health. Lancet Psychiatry 3 , 436–442 (2016).
Herzog, D. P. et al. Guideline adherence of antidepressant treatment in outpatients with major depressive disorder: a naturalistic study. Eur. Arch. Psychiatry Clin. Neurosci. 267 , 711–721 (2017).
Trivedi, M. H. et al. Clinical results for patients with major depressive disorder in the Texas Medication Algorithm Project. Arch. Gen. Psychiatry 61 , 669–680 (2004).
Adli, M. et al. How effective is algorithm-guided treatment for depressed inpatients? results from the randomized controlled multicenter german algorithm project 3 trial. Int J. Neuropsychopharmacol. 20 , 721–730 (2017).
Bauer, M. et al. Efficacy of an algorithm-guided treatment compared with treatment as usual: a randomized, controlled study of inpatients with depression. J. Clin. Psychopharmacol. 29 , 327–333 (2009).
Ricken, R. et al. Algorithm-guided treatment of depression reduces treatment costs–results from the randomized controlled German Algorithm Project (GAPII). J. Affect. Disord. 134 , 249–256 (2011).
Khan, A. & Brown, W. A. Antidepressants versus placebo in major depression: an overview. World Psychiatry 14 , 294–300 (2015).
Fava, M., Evins, A. E., Dorer, D. J. & Schoenfeld, D. A. The problem of the placebo response in clinical trials for psychiatric disorders: culprits, possible remedies, and a novel study design approach. Psychother. Psychosom. 72 , 115–127 (2003).
Enck, P., Bingel, U., Schedlowski, M. & Rief, W. The placebo response in medicine: minimize, maximize or personalize? Nat. Rev. Drug Discov. 12 , 191–204 (2013).
Desseilles, M. et al. Massachusetts general hospital SAFER criteria for clinical trials and research. Harv. Rev. Psychiatry 21 , 269–274 (2013).
Finniss, D. G., Kaptchuk, T. J., Miller, F. & Benedetti, F. Biological, clinical, and ethical advances of placebo effects. Lancet 375 , 686–695 (2010).
Henter, I. D., de Sousa, R. T. & Zarate, C. A. Jr. Glutamatergic modulators in depression. Harv. Rev. Psychiatry 26 , 307–319 (2018).
Ionescu, D. F. & Papakostas, G. I. Current trends in identifying rapidly acting treatments for depression. Curr. Behav. Neurosci. Rep. 3 , 185–191 (2016).
Kadriu, B. et al. Glutamatergic neurotransmission: pathway to developing novel rapid-acting antidepressant treatments. Int. J. Neuropsychopharmacol. / Off. Sci. J. Coll. Int. Neuropsychopharmacol. 22 , 119–135 (2018).
Zanos, P. et al. Convergent mechanisms underlying rapid antidepressant action. CNS Drugs 32 , 197–227 (2018).
Li, N. et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329 , 959–964 (2010).
Autry, A. E. et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475 , 91–95 (2011).
Berman, R. M. et al. Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry 47 , 351–354 (2000).
Duman, R. S. & Aghajanian, G. K. Synaptic dysfunction in depression: potential therapeutic targets. Science 338 , 68–72 (2012).
Diazgranados, N. et al. Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J. Clin. Psychiatry 71 , 1605–1611 (2010).
Iadarola, N. D. et al. Ketamine and other N-methyl-D-aspartate receptor antagonists in the treatment of depression: a perspective review. Ther. Adv. Chronic Dis. 6 , 97–114 (2015).
Ibrahim, L. et al. Rapid decrease in depressive symptoms with an N-methyl-d-aspartate antagonist in ECT-resistant major depression. Progress. neuro-Psychopharmacol. Biol. Psychiatry 35 , 1155–1159 (2011).
Zarate, C. A. et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry 63 , 856–864 (2006).
Diazgranados, N. et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch. Gen. Psychiatry 67 , 793–802 (2010).
Zarate, C. A. et al. Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol. Psychiatry 71 , 939–946 (2012).
aan het Rot, M. et al. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol. Psychiatry 67 , 139–145 (2010).
Wan, L. B. et al. Ketamine safety and tolerability in clinical trials for treatment-resistant depression. J. Clin. Psychiatry 76 , 247–252 (2015).
Murrough, J. W. et al. Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol. Psychiatry 74 , 250–256 (2013).
Murrough, J. W. et al. Ketamine for rapid reduction of suicidal ideation: a randomized controlled trial. Psychol. Med. 45 , 3571–3580 (2015).
Price, R. B., Nock, M. K., Charney, D. S. & Mathew, S. J. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol. Psychiatry 66 , 522–526 (2009).
Wilkinson, S. T. et al. The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am. J. Psychiatry 175 , 150–158 (2018).
Daly, E. J. et al. Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: A randomized clinical trial. JAMA Psychiatry 75 , 139–148 (2018).
Canuso, C. M. et al. Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: results of a double-blind, randomized, placebo-controlled study. Am. J. Psychiatry 175 , 620–630 (2018).
Zanos, P. et al. The prodrug 4-chlorokynurenine causes ketamine-like antidepressant effects, but not side effects, by NMDA/glycineB-site inhibition. J. Pharmacol. Exp. Ther. 355 , 76–85 (2015).
Wilkinson, S. T. & Sanacora, G. A new generation of antidepressants: an update on the pharmaceutical pipeline for novel and rapid-acting therapeutics in mood disorders based on glutamate/GABA neurotransmitter systems. Drug Discov. Today 24 , 606–615 (2018).
Article PubMed CAS PubMed Central Google Scholar
Vollenweider, F. X. & Kometer, M. The neurobiology of psychedelic drugs: implications for the treatment of mood disorders. Nat. Rev. Neurosci. 11 , 642–651 (2010).
Carhart-Harris, R. L. et al. Psilocybin with psychological support for treatment-resistant depression: an open-label feasibility study. Lancet Psychiatry 3 , 619–627 (2016).
Karp, J. F. et al. Safety, tolerability, and clinical effect of low-dose buprenorphine for treatment-resistant depression in midlife and older adults. J. Clin. Psychiatry 75 , e785–e793 (2014).
Fava, M. et al. Opioid modulation with buprenorphine/samidorphan as adjunctive treatment for inadequate response to antidepressants: A randomized double-blind placebo-controlled trial. Am. J. Psychiatry 173 , 499–508 (2016).
Preskorn, S. et al. Randomized proof of concept trial of GLYX-13, an N-methyl-D-aspartate receptor glycine site partial agonist, in major depressive disorder nonresponsive to a previous antidepressant agent. J. Psychiatr. Pract. 21 , 140–149 (2015).
Garay, R. P. et al. Investigational drugs in recent clinical trials for treatment-resistant depression. Expert Rev. Neurother. 17 , 593–609 (2017).
Kolbinger, H. M., Hoflich, G., Hufnagel, A., Moller, H. J. & Kasper, S. Transcranial magnetic stimulation (TMS) in the treatment of major depression—a pilot study. Human. Psychopharmacol. 10 , 305–310 (1995).
Lisanby, S. H. et al. Daily left prefrontal repetitive transcranial magnetic stimulation in the acute treatment of major depression: clinical predictors of outcome in a multisite, randomized controlled clinical trial. Neuropsychopharmacol.: Off. Publ. Am. Coll. Neuropsychopharmacol. 34 , 522–534 (2009).
Brunoni, A. R. et al. Repetitive transcranial magnetic stimulation for the acute treatment of major depressive episodes: A systematic review with network meta-analysis. JAMA Psychiatry 74 , 143–152 (2017).
Benadhira, R. et al. A randomized, sham-controlled study of maintenance rTMS for treatment-resistant depression (TRD). Psychiatry Res 258 , 226–233 (2017).
Cusin, C. & Dougherty, D. D. Somatic therapies for treatment-resistant depression: ECT, TMS, VNS, DBS. Biol. Mood Anxiety Disord. 2 , 14 (2012).
Blumberger, D. M. et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. Lancet 391 , 1683–1692 (2018).
Fava, M. Diagnosis and definition of treatment-resistant depression. Biol. Psychiatry 53 , 649–659 (2003).
Husain, M. M. et al. Speed of response and remission in major depressive disorder with acute electroconvulsive therapy (ECT): a Consortium for Research in ECT (CORE) report. J. Clin. Psychiatry 65 , 485–491 (2004).
Kellner, C. H. et al. Relief of expressed suicidal intent by ECT: a consortium for research in ECT study. Am. J. Psychiatry 162 , 977–982 (2005).
Slade, E. P., Jahn, D. R., Regenold, W. T. & Case, B. G. Association of electroconvulsive therapy with psychiatric readmissions in US hospitals. JAMA Psychiatry 74 , 798–804 (2017).
Kellner, C. H. et al. Right unilateral ultrabrief pulse ECT in geriatric depression: phase 1 of the PRIDE study. Am. J. Psychiatry 173 , 1101–1109 (2016).
McClintock, S. M. et al. Multifactorial determinants of the neurocognitive effects of electroconvulsive therapy. J. ECT 30 , 165–176 (2014).
Fink, M. & Taylor, M. A. Electroconvulsive therapy: evidence and challenges. JAmA 298 , 330–332 (2007).
American Psychiatric Association. Task Force on Electroconvulsive Therapy. The practice of ECT: recommendations for treatment, training and privileging. Convuls. Ther. 6 , 85–120 (1990).
Kellner, C. H. et al. Bifrontal, bitemporal and right unilateral electrode placement in ECT: randomised trial. Br. J. Psychiatry.: J. Ment. Sci. 196 , 226–234 (2010).
Wilkinson, S. T., Agbese, E., Leslie, D. L. & Rosenheck, R. A. Identifying recipients of electroconvulsive therapy: data from privately insured Americans. Psychiatr. Serv. 69 , 542–548 (2018).
Lisanby, S. H., Schlaepfer, T. E., Fisch, H. U. & Sackeim, H. A. Magnetic seizure therapy of major depression. Arch. Gen. Psychiatry 58 , 303–305 (2001).
Deng, Z. -D., Lisanby, S. H. & Peterchev, A. V. Electric field strength and focality in electroconvulsive therapy and magnetic seizure therapy: a finite element simulation study. J. Neural Eng. 8 , 016007 (2011).
Fitzgerald, P. B. et al. Pilot study of the clinical and cognitive effects of high-frequency magnetic seizure therapy in major depressive disorder. Depress Anxiety 30 , 129–136 (2013).
Kayser, S. et al. Magnetic seizure therapy in treatment-resistant depression: clinical, neuropsychological and metabolic effects. Psychol. Med. 45 , 1073–1092 (2015).
Nahas, Z. et al. Two-year outcome of vagus nerve stimulation (VNS) for treatment of major depressive episodes. J. Clin. Psychiatry 66 , 1097–1104 (2005).
Aaronson, S. T. et al. A 5-Year observational study of patients with treatment-resistant depression treated with vagus nerve stimulation or treatment as usual: comparison of response, remission, and suicidality. Am. J. Psychiatry 174 , 640–648 (2017).
Schlaepfer, T. E., Bewernick, B. H., Kayser, S., Madler, B. & Coenen, V. A. Rapid effects of deep brain stimulation for treatment-resistant major depression. Biol. Psychiatry 73 , 1204–1212 (2013).
Bewernick, B. H. et al. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol. Psychiatry 67 , 110–116 (2010).
Bergfeld, I. O. et al. Deep brain stimulation of the ventral anterior limb of the internal capsule for treatment-resistant depression: A randomized clinical trial. JAMA Psychiatry 73 , 456–464 (2016).
Dougherty, D. D. et al. A randomized sham-controlled trial of deep brain stimulation of the ventral capsule/ventral striatum for chronic treatment-resistant depression. Biol. Psychiatry 78 , 240–248 (2015).
Holtzheimer, P. E. et al. Subcallosal cingulate deep brain stimulation for treatment-resistant depression: a multisite, randomised, sham-controlled trial. Lancet Psychiatry 4 , 839–849 (2017).
Widge, A. S., Malone, D. A. Jr. & Dougherty, D. D. Closing the loop on deep brain stimulation for treatment-resistant depression. Front Neurosci. 12 , 175 (2018).
Jain, K. K. in The Handbook of Biomarkers . 1–26 (Springer New York, New York, NY, 2017).
Zohar, J. et al. A review of the current nomenclature for psychotropic agents and an introduction to the Neuroscience-based Nomenclature. Eur. Neuropsychopharmacol.: J. Eur. Coll. Neuropsychopharmacol. 25 , 2318–2325 (2015).
Dold, M. & Kasper, S. Evidence-based pharmacotherapy of treatment-resistant unipolar depression. Int J. Psychiatry Clin. Pract. 21 , 13–23 (2017).
Colle, R. et al. BDNF/TRKB/P75NTR polymorphisms and their consequences on antidepressant efficacy in depressed patients. Pharmacogenomics 16 , 997–1013 (2015).
Porcelli, S., Fabbri, C. & Serretti, A. Meta-analysis of serotonin transporter gene promoter polymorphism (5-HTTLPR) association with antidepressant efficacy. Eur. Neuropsychopharmacol.: J. Eur. Coll. Neuropsychopharmacol. 22 , 239–258 (2012).
Download references
Acknowledgements
We thank the 7SE research unit and staff for their support. Ioline Henter (NIMH) provided invaluable editorial assistance. We also thank E. Acevedo-Diaz, Z.D. Deng, and J.W. Evans for scientific input.
Author information
Authors and affiliations.
Department of Psychiatry and Psychotherapy, Medical University of Vienna, Vienna, Austria
Christoph Kraus, Rupert Lanzenberger & Siegfried Kasper
Section on Neurobiology and Treatment of Mood Disorders, Intramural Research Program, National Institute of Mental Health, National Institutes of Health, Bethesda, MD, USA
Christoph Kraus, Bashkim Kadriu & Carlos A. Zarate Jr.
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Siegfried Kasper .
Ethics declarations
Conflict of interest.
Funding for this work was supported in part by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH; ZIA MH002927). All support given to authors was not related to the design of the manuscript or the ideas stated in this review. Dr. Kasper received grants/research support, consulting fees, and/or honoraria within the last 3 years from Angelini, AOP Orphan Pharmaceuticals AG, AstraZeneca, Eli Lilly, Janssen, KRKA-Pharma, Lundbeck, Neuraxpharm, Pfizer, Pierre Fabre, Schwabe, and Servier. Dr. Lanzenberger received travel grants and/or conference speaker honoraria from AstraZeneca, Lundbeck A/S, Dr. Willmar Schwabe GmbH, Orphan Pharmaceuticals AG, Janssen-Cilag Pharma GmbH, and Roche Austria GmbH. Dr. Kraus has received travel grants from Roche Austria GmbH and AOP Orphan. Dr. Zarate is a full-time U.S government employee. He is listed as a co-inventor on a patent for the use of ketamine in major depression and suicidal ideation; as a co-inventor on a patent for the use of (2 R ,6 R )-hydroxynorketamine, ( S )-dehydronorketamine, and other stereoisomeric dehydro and hydroxylated metabolites of ( R,S )-ketamine metabolites in the treatment of depression and neuropathic pain; and as a co-inventor on a patent application for the use of (2 R ,6 R )-hydroxynorketamine and (2 S ,6 S )-hydroxynorketamine in the treatment of depression, anxiety, anhedonia, suicidal ideation, and post-traumatic stress disorders. He has assigned his patent rights to the U.S. government but will share a percentage of any royalties that may be received by the government.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Kraus, C., Kadriu, B., Lanzenberger, R. et al. Prognosis and improved outcomes in major depression: a review. Transl Psychiatry 9 , 127 (2019). https://doi.org/10.1038/s41398-019-0460-3
Download citation
Received : 07 November 2018
Revised : 10 January 2019
Accepted : 11 February 2019
Published : 03 April 2019
DOI : https://doi.org/10.1038/s41398-019-0460-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Cannabinoid type 2 receptor inhibition enhances the antidepressant and proneurogenic effects of physical exercise after chronic stress.
- R. S. Rodrigues
- J. B. Moreira
Translational Psychiatry (2024)
Developing an individualized treatment rule for Veterans with major depressive disorder using electronic health records
- Nur Hani Zainal
- Robert M. Bossarte
- Ronald C. Kessler
Molecular Psychiatry (2024)
Multiomics and blood-based biomarkers of electroconvulsive therapy in severe and treatment-resistant depression: study protocol of the DetECT study
- Iven-Alex von Mücke-Heim
- Julius C. Pape
- Elisabeth B. Binder
European Archives of Psychiatry and Clinical Neuroscience (2024)
Functional and structural alterations in different durations of untreated illness in the frontal and parietal lobe in major depressive disorder
- Xiaowei Jiang
- Yanqing Tang
Multi-site benchmark classification of major depressive disorder using machine learning on cortical and subcortical measures
- Vladimir Belov
- Tracy Erwin-Grabner
- Roberto Goya-Maldonado
Scientific Reports (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Ohio State nav bar
The Ohio State University
- BuckeyeLink
- Find People
- Search Ohio State
Patient Case Presentation

Figure 1. Blue and silver stethoscope (Pixabay, N.D.)
Ms. S.W. is a 48-year-old white female who presented to an outpatient community mental health agency for evaluation of depressive symptoms. Over the past eight weeks she has experienced sad mood every day, which she describes as a feeling of hopelessness and emptiness. She also noticed other changes about herself, including decreased appetite, insomnia, fatigue, and poor ability to concentrate. The things that used to bring Ms. S.W. joy, such as gardening and listening to podcasts, are no longer bringing her the same happiness they used to. She became especially concerned as within the past two weeks she also started experiencing feelings of worthlessness, the perception that she is a burden to others, and fleeting thoughts of death/suicide.
Ms. S.W. acknowledges that she has numerous stressors in her life. She reports that her daughter’s grades have been steadily declining over the past two semesters and she is unsure if her daughter will be attending college anymore. Her relationship with her son is somewhat strained as she and his father are not on good terms and her son feels Ms. S.W. is at fault for this. She feels her career has been unfulfilling and though she’d like to go back to school, this isn’t possible given the family’s tight finances/the patient raising a family on a single income.
Ms. S.W. has experienced symptoms of depression previously, but she does not think the symptoms have ever been as severe as they are currently. She has taken antidepressants in the past and was generally adherent to them, but she believes that therapy was more helpful than the medications. She denies ever having history of manic or hypomanic episodes. She has been unable to connect to a mental health agency in several years due to lack of time and feeling that she could manage the symptoms on her own. She now feels that this is her last option and is looking for ongoing outpatient mental health treatment.
Past Medical History
- Hypertension, diagnosed at age 41
Past Surgical History
- Wisdom teeth extraction, age 22
Pertinent Family History
- Mother with history of Major Depressive Disorder, treated with antidepressants
- Maternal grandmother with history of Major Depressive Disorder, Generalized Anxiety Disorder
- Brother with history of suicide attempt and subsequent inpatient psychiatric hospitalization,
- Brother with history of Alcohol Use Disorder
- Father died from lung cancer (2012)
Pertinent Social History
- Works full-time as an enrollment specialist for Columbus City Schools since 2006
- Has two children, a daughter age 17 and a son age 14
- Divorced in 2015, currently single
- History of some emotional abuse and neglect from mother during childhood, otherwise denies history of trauma, including physical and sexual abuse
- Smoking 1/2 PPD of cigarettes
- Occasional alcohol use (approximately 1-2 glasses of wine 1-2 times weekly; patient had not had any alcohol consumption for the past year until two weeks ago)
- Research article
- Open access
- Published: 30 March 2020
Mental and somatic comorbidity of depression: a comprehensive cross-sectional analysis of 202 diagnosis groups using German nationwide ambulatory claims data
- Annika Steffen ORCID: orcid.org/0000-0003-4072-9245 1 ,
- Julia Nübel 2 ,
- Frank Jacobi 3 ,
- Jörg Bätzing 1 &
- Jakob Holstiege 1
BMC Psychiatry volume 20 , Article number: 142 ( 2020 ) Cite this article
20k Accesses
104 Citations
25 Altmetric
Metrics details
Depression is frequently accompanied by other mental disorders and various somatic diseases; however, previous comorbidity studies often relied on self-reported data and have not simultaneously assessed the entire spectrum of mental and somatic diagnoses. The aim is to provide a complete picture of mental and somatic comorbidity of depression in routine outpatient care in a high income country with a relatively well equipped health care system.
Using ambulatory claims data covering 87% of the German population (age 15+), we designed a cross-sectional study by identifying persons diagnosed with mild, moderate and severe depression in 2017 ( N = 6.3 million) and a control group matched 4:1 on sex, 5-year age group and region of residence ( N = 25.2 million). Stratified by severity, we calculated the prevalence of 202 diagnosis groups included in the ICD-10 in persons with depression as compared to matched controls using prevalence ratios (PR).
Nearly all mental disorders were at least twice as prevalent in persons with depression relative to controls, showing a dose-response relationship with depression severity. Irrespective of severity, the three most prevalent somatic comorbid diagnosis groups were ‘other dorsopathies’ (M50-M54), ‘hypertensive diseases’ (I10-I15) and ‘metabolic disorders’ (E70-E90), exhibiting PRs in moderate depression of 1.56, 1.23 and 1.33, respectively. Strong associations were revealed with diseases of the central nervous system (i.e. multiple sclerosis) and several neurological diseases, among them sleep disorders, migraine and epilepsy, most of them exhibiting at least 2- to 3-fold higher prevalences in depression relative to controls. Utilization of health care was higher among depression cases compared to controls.
Conclusions
The present study based on data from nearly the complete adolescent and adult population in Germany comprehensively illustrates the comorbidity status of persons diagnosed with depression as coded in routine health care. Our study should contribute to increasing the awareness of the strong interconnection of depression with all other mental and the vast majority of somatic diseases. Our findings underscore clinical and health-economic relevance and the necessity of systematically addressing the high comorbidity of depression and somatic as well as other mental diseases through prevention, early identification and adequate management of depressive symptoms.
Peer Review reports
Major depressive disorder (MDD) is one of the most common mental disorders worldwide with a lifetime risk of 15–18% [ 1 , 2 ]. Roughly 7% of the general population experiences a depressive episode within a 12-month period [ 1 , 3 , 4 ]. Depression severely limits psychosocial functioning and quality of life and ranks as a leading cause of disease burden worldwide [ 5 , 6 ]. With an increased mortality risk of 50%, depression is a risk factor comparable in strength to smoking [ 7 , 8 , 9 ].
A large body of evidence from epidemiological surveys has documented a strong interconnection of depression with other mental disorders, most notably with anxiety disorders and substance use disorders [ 10 , 11 ]. Approximately, 50–60% of individuals with a lifetime history of depression also report a lifetime history of at least one anxiety disorder [ 11 , 12 ]. Results from a large US survey showed that 14% of respondents with MDD in the prior 12 months also had an alcohol use disorder and 4.6% had a drug use disorder [ 13 ]. Among those with lifetime MDD, 40% had an alcohol use disorder and 17% had a drug use disorder [ 13 ]. The presence of psychiatric comorbidity in depression is associated with greater severity (e. g. suicidality [ 14 ]), as well as slower recovery, higher risk of chronicity, recurrence, treatment resistance [ 15 , 16 ], and increased utilization of medical services compared to “pure” depression [ 11 , 12 , 17 ].
Depression has been further identified as independent risk factor and negative prognostic factor for many chronic somatic disorders, including diabetes, cardiovascular disease, hypertension, chronic respiratory disorders, and arthritis [ 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 ]. Comorbid depression in physical disease is related to poor quality of life, worse course of the physical disorder, higher functional impairment and disability, increased service utilization and higher medical costs, and increased mortality compared to the presence of either depression or the physical disease alone [ 18 , 20 , 24 , 25 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 ].
Data from the US demonstrate that comorbidities account for the largest portion of the growing economic burden of depression and highlight the importance of considering comorbidities in the treatment of depression [ 30 ]. This, however, requires empirical evidence on the relative importance of individual comorbidities in depression. Previous studies investigating the co-occurrence of depression with other mental or somatic disorders have usually focused on single or a few, mainly common, diseases and assessed these comorbidities via self-report [ 1 , 28 , 29 , 38 , 39 , 40 , 41 ]. In addition, a comprehensive quantification of the excess risk for a broad range of comorbid diseases in individuals with depression relative to persons without depression is currently lacking. On a large-scale basis, the present comparison allows identifying comorbidities based on actually coded medical diagnoses that a) are of high prevalence among individuals with depression and/or b) exhibiting a high excess risk in comparison to persons without depression and, thereby, may ultimately contribute to an improved medical care of persons with depression. Finally, the associations with specific comorbidities may differ according to severity of depression, though a differentiated view on the population level is currently lacking.
Given their size and nearly full coverage, administrative data offer the unprecedented opportunity to simultaneously investigate the association between unipolar depression (MDD or Dysthymic Disorder [DD]) and a large number of mental and somatic disorders. Using claims data from 87% of the German population, the present study set out to simultaneously quantify associations of depression with 202 diagnosis groups included in the ICD-10 as coded by physicians and other mental health professionals. This allows identifying a) the most prevalent mental and somatic comorbidities among individuals with depression as well as b) identifying those comorbidities exhibiting the highest excess risk in depression relative to controls without depression. Such a comprehensive evaluation of the comorbidity in depression at the population level within one study is fundamental to understanding the size and nature of the health challenges posed by depression.
Data source
The present analysis was based on nationwide statutory health insurance (SHI) physicians’ claims data from the years 2009 to 2017, with 2017 being the reporting year. The data cover all SHI insurants in Germany amounting to roughly 70 million individuals and reflecting approximately 87% of the total German population. No data were available for residents with private health insurance (~ 13% of the German population). Among others, the present data source includes information on the insurant’s age, sex, residential area and on all diagnoses documented in the ambulatory care setting according to the ICD-10.
Study design and study population
We designed a case-control study focusing on patients with a diagnosis of unipolar depression (F32x, F33x or F34.1) in the year 2017 aged ≥15 years. Cases of depression were categorized into mild, moderate and severe based on the documented diagnostic code according to the ICD-10 (Table 1 ).
As illustrated in Fig. 1 , we used a hierarchical classification and considered only the most severe diagnosis. In brief, from all persons with a least one diagnosis of depression in 2017 ( n = 9,827,889), cases of severe depression were defined as patients with at least one diagnosis of F32.2, F32.3, F33.2 or F33.3 ( n = 1,404,250). From the remaining patients, those who had at least one diagnosis of F32.1 or F33.1 were classified as cases with moderate depression ( n = 3,213,925). Finally, patients with a diagnosis of F32.0, F33.0 or F34.1 were classified to have mild depression (1,685,108). Patients who did not have at least one specific diagnostic code of depression to differentiate the severity of the disease were not included in the present analysis ( n = 3,524,606). Thus, cases exclusively coded with F32.8, F32.9, F33.8 and F33.9 were not considered as cases in the present study. Depressive syndromes within other ICD-10 categories beyond F3.x (e.g., in organic mental disorders, adjustment disorder with depressive features, mixed anxiety and depression, depressive syndromes in Parkinson’s disease, dementias, or stroke) were not considered as depression cases but as comorbidities.
A control group of persons without depression matched by age (5-year categories), sex and residential area (17 regions representing the different Associations of Statutory Health Insurance Physicians in Germany) was drawn separately for each subgroup of cases (mild, moderate and severe) with a case-control ratio of 1:4. To be included as a control, insurants had to be a) free of any diagnosis of moderate and severe depression during the whole observation period (2009–2017) and b) free of any diagnosis of mild and unspecified depression during the preceding 4 years (2014–2017). This relaxed criterion with respect to previous diagnoses of mild and unspecified depression was used to account for our observation that within specific age and sex strata it appears rather uncommon to not have received such a diagnosis at least once during a period of 9 years.
Selection of study participants. A hierarchical classification, considering only the most severe diagnosis, was used to define cases of mild, moderate and severe depression based on diagnostic codes documented in ambulatory care. From the group of all persons with a least one diagnosis of depression in 2017 ( n = 9,827,889), cases of severe depression were defined as patients with at least one diagnosis of F32.2, F32.3, F33.2 or F33.3 ( n = 1,404,250). From the remaining patients, those who had at least one diagnosis of F32.1 or F33.1 were classified as cases with moderate depression ( n = 3,213,925). Finally, patients with a diagnosis of F32.0, F33.0 or F34.1 were classified to have mild depression (1,685,108). Patients who did not have at least one specific diagnostic code of depression to differentiate the severity of the disease were not included in the present analysis ( n = 3,524,606). Thus, cases exclusively coded with F32.8, F32.9, F33.8 and F33.9 were not considered as cases in the present study
Selection of comorbid mental and somatic diseases
The present analysis was based on 202 diagnosis groups stemming from 18 chapters of the ICD-10, excluding diagnostic groups from chapters XVI (‘Certain conditions originating in the perinatal period’; P00-P96), XVIII (‘Symptoms, signs and abnormal clinical laboratory findings, not elsewhere classified’; R00-R99), XXI (‘Factors influencing health status and contact with health services’; Z00-Z99) and XXII (‘Codes for special purposes’; U00-U85).
Statistical analysis
Characteristics of the study population with regard to sex, age and service utilization in 2017 are reported by depression severity. To describe outpatient service utilization, we used the frequency of contact to ambulatory health care in 2017 as measured by a) the number of different doctors consulted, b) the number of diagnosis groups reflecting the number of different diagnoses a person has received, and c) the number of treatment cases that were generated. In German SHI data, a treatment case is defined on the patient-level as the sum of all medical services delivered by one physician during one quarter. Lastly, we computed the total cost of ambulatory care as the sum of costs related to all medical services that were utilized in ambulatory care in 2017.
Associations of depression with other mental and somatic diseases (202 diagnosis groups) were quantified by computing prevalence ratios (PR), defined as the ratio of the prevalence among depression cases to the prevalence among controls. Results of all investigated comorbidities were presented separately for mental and somatic diseases. Given the high number of somatic diseases included and in order to depict their clinical and public health relevance, PRs for comorbid somatic conditions are displayed in scatterplots across the range of the comorbidity prevalence among controls. Further, a ranking of the 20 most prevalent comorbidities (mental and somatic combined) is presented to illustrate the global picture of relevant comorbidities based on their occurrence. For the sake of completeness, we additionally provide a supplemental table presenting information on all included disease groups, their description according to ICD, the respective ICD chapter they belong to and the prevalence among cases as well as the corresponding prevalence ratio stratified by depression severity (Appendix table A 1 ).
Comorbidity associations were examined by severity of depression in the total study population and for men and women separately. All analyses were conducted using SAS®, version 9.4.
Sociodemographic characteristics and service utilization of the study population
We identified 6,303,283 patients with a specific diagnosis of depression in 2017. According to severity, 27% ( N = 1,685,108) had mild depression, 51% ( N = 3,213,925) moderate and 22% ( N = 1,404,250) severe depression. Characteristics of the study population are presented in Table 2 . Mean age of the study population was 55 years and two thirds were women. Depression cases showed a higher utilization of medical services than controls, as assessed by several parameters. In brief, patients with depression consulted a higher number of different doctors than controls, generated more treatment cases and were more likely to have received diagnoses from a larger number of diagnosis groups relative to control persons (e.g. more than threefold multimorbid cases with > 20 diagnostic codes). Ultimately, the higher health care utilization of depression cases was reflected in more than two-fold higher treatment costs compared to controls in the ambulatory setting.
Mental comorbidity
Overall, 64% of mild depression cases (72% of moderate, 78% of severe) had a comorbid mental disorder. Figure 2 presents the prevalence of diagnosed mental disorders in depression cases according to severity of depression and the prevalence ratio of the respective comorbidity relative to controls. In general, the prevalence of comorbid disorders increased with depression severity, amounting to a 30 to 40% higher prevalence for most disorders in severe compared to mild depression cases. Larger differences were only observed for schizophrenia (3-times and 2-times higher in severe and moderate compared to mild depression cases, respectively). Neurotic, stress-related and somatoform disorders (F4) were by far the most prevalent comorbidity in depression, irrespective of depression severity; 65% of severe depression cases (52% of mild and 61% of moderate cases) had additionally received an F4-diagnosis. The second most frequent psychiatric comorbidity was the group of substance use disorders (F1) which amounted to a diagnostic prevalence of 12, 16 and 20% in mild, moderate and severe cases. Personality disorders (F6) and behavioral syndromes (F5) ranked third and fourth with prevalence values ranging between 6 and 14% (Fig. 2 ).
Overall, depression cases were at least twice as likely to have a mental comorbidity compared with age- and sex-matched controls, with mental retardation (F7) being the only exception (PR between 1.4 and 2.0). While most mental comorbidities exhibited prevalence ratios between 2 and 6, personality disorders showed strikingly higher PRs (PR = 11 in moderate and PR = 17 in severe depression). In addition, severe depression cases had a 10-fold higher risk of being diagnosed with schizophrenia compared to controls, while the risk of mild and moderate depression cases was substantially lower (PRs of 3.5 and 4.6, respectively, Fig. 2 ).
Prevalence of mental disorders among cases with unipolar depression and prevalence ratios relative to controls. Diagnosis groups according to ICD-10: F00-F09, Organic mental disorders; F10-F19, Substance use disorders; F20-F29, Schizophrenia; F40-F48, Neurotic, stress-related and somatoform disorders; F50-F59, Behavioral syndromes associated with physiological disturbances and physical factors; F60-F69, Disorders of adult personality and behavior; F70-F79, Mental retardation; F80-F89, Disorders of psychological development; F90-F98, Behavioral and emotional disorders with onset in childhood and adolescence; F99-F99, Unspecified mental disorders. The prevalence ratio is defined as the ratio of the prevalence of the respective diagnosis group among depression cases to the prevalence among age-, sex- and region-matched controls
Somatic comorbidity
The associations between 192 somatic disease groups and depression severity are illustrated in Fig. 3 . Almost all somatic diseases were more prevalent among depression cases compared to controls. Exceptions were the group of diagnoses concerning pregnancy, childbirth and puerperium (Chapter XV) which showed an inverse association with moderate and severe depression and no association with mild depression. As already observed for mental disorders, the strength of association gradually increased with severity of depression diagnosis.
Irrespective of severity, the three most prevalent somatic comorbid diagnosis groups were ‘other dorsopathies’ (M50-M54), ‘hypertensive diseases’ (I10-I15) and ‘metabolic disorders’ (E70-E90) (Fig. 3 and Table 3 ). A total of 54% of moderate depression cases had also received a diagnosis of ‘other dorsopathies’, relating to a 56% higher risk compared to controls (PR = 1.56). In terms of hypertensive diseases and metabolic diseases, moderate depression was related to a 23 and 33% higher risk, respectively (45 and 38% affected moderate depression cases).
Overall, three major groups of somatic comorbidities can be identified in Fig. 3 :
Prevalence ratios of somatic comorbidities according to prevalence among controls by depression severity. Prevalence ratios were estimated for 191 somatic diagnosis groups from the ICD-10 based on administrative data from outpatient care including 6.3 million patients with a specific diagnosis of depression in 2017 and 25.2 million age-, sex- and region-matched controls. The prevalence ratio is defined as the ratio of the prevalence of the respective diagnosis group among depression cases to the prevalence among controls. Diagnosis groups are colored according to the respective chapter of the ICD
First, there is the group of diseases that are relatively common in the general population (prevalence > 10% among controls). This group comprises diseases of the musculoskeletal system (Chapter XIII), endocrine, nutritional and metabolic disorders (Chapter IV) and diseases of the circulatory (Chapter IX) and respiratory system (Chapter X). These prevalent diseases were mainly associated with low to moderate increased risks in depression (PRs between 1.2 and 2.0). In the group of circulatory diseases, ‘Essential (primary) hypertension’ (I10.9) was the single most frequent comorbid diagnosis in moderate depression (38% vs. 31% in controls, PR = 1.2%). Among musculoskeletal diseases, ‘low back pain’ (M54.5) and ‘Cervicalgia’ (M54.2) both affecting 15% of moderate depression cases constituted the most frequent comorbid diagnoses (PR = 1.7 and PR = 2.0, respectively). With regard to endocrine, nutritional and metabolic disorders (Chapter IV), pure hyperchlesterolaemia (E78.0) was the single most frequent diagnosis, affecting 17% of moderate depression cases (PR = 1.3), followed by type 2 diabetes mellitus (E11.9) with a prevalence of 13% (PR = 1.4) and hypothyroidism (E03.9) with a prevalence of 12% (PR = 1.6). Within the group of respiratory diseases, apart from acute upper respiratory infections (J00-J06; P = 28%; PR = 1.3), diagnoses of asthma and chronic obstructive pulmonary disease belonged to the group of common diseases (J40-J47), generally displaying a 1.8- to 2.2-fold higher prevalence in depression cases compared to controls.
Second, we observed a cluster of somatic comorbidities that were rather infrequent in the general population (prevalence < 2%, except ‘G40-G47’) and strongly related to the diagnosis of depression, exhibiting prevalence ratios > 2.5. Among mild, moderate and severe cases a total of 4, 9 and 16 disease groups displayed a PR beyond this threshold, predominantly comprising diseases of the nervous system (Chapter VI, G.x). In mild depression, the highest PR was observed for demyelinating diseases of the central nervous system (G35-G37; PR = 3.02), mainly reflecting the diagnosis of multiple sclerosis (G35.9 and G35.1). In moderate and severe depression, extrapyramidal and movement disorders (G20-G26; PR of 3.33 and 4.02, respectively) were related to the highest excess risks. The main contributor to this disease group were ‘restless legs syndrome’ (G25.81; prevalence of 2.8% in moderate and of 3.3% in severe depression cases) followed by ‘primary Parkinson disease’ (G20.9) with a prevalence of 1.2% (PR = 3.6) in moderate and 1.5% (PR = 4.3) in severe depression. Additionally, in moderate depression, other degenerative diseases of the nervous system (G30-G32) and demyelinating diseases of the central nervous system (G35-G37) ranked second and third in terms of increased risk (PR of 2.95 and 2.93, respectively). As indicated above, the group of ‘Episodic and paroxysmal disorders’ (G40-G47) stood out in that it was much more frequent among controls (12%) than the other diseases of the nervous system and strongly associated with depression (PR = 2.7), indicating that 31% of moderate depression cases received a diagnosis from this group. The main contributors to this group in terms of prevalence in moderate depression were unspecified sleep disorders (G47.9; P = 7.4%; PR = 4.3), migraine (G43.9; P = 7.1%; PR = 2.1) and disorders of initiating and maintaining sleep (G47.0; P = 5.8%; PR = 5.5). Diagnoses of epilepsy exhibited prevalence ratios between 2.3 and 4.1, with unspecified epilepsy ranking 9th in terms of prevalence in the total group of episodic and paroxysmal disorders (G40.9; P = 2.0%; PR = 2.3).
A third cluster of disease groups can be defined as having low to moderate prevalence in the general population (< 10%) and low to moderate increased risk in depression (PRs > 1.0 and < 2.0). The majority of disease groups were comprised in this cluster, amounting to 145 (72%), 134 (66%), and 124 (61%) among mild, moderate and severe depression cases, respectively.
Ranking of mental and somatic comorbidity
Table 3 provides an integrated view of the 20 most frequent mental and somatic comorbidities in cases with depression - ranked by their prevalence - and their excess risk in comparison to controls. Irrespective of depression severity, neurotic, stress-related and somatoform disorders (F4) emerged as a highly relevant comorbidity (rank 2 in mild depression, rank 1 in moderate and severe depression). With the exception of substance use disorders that ranked 19th in the group of severe depression cases, all remaining top 20 comorbidities were of somatic nature. While the set of identified comorbidities was largely the same for mild, moderate and severe depression, the ranking slightly differed for some of the comorbidities. For instance, while episodic and paroxysmal disorders (G40-G47) ranked 10th in mild depression (prevalence of 28%), they ranked 6th in moderate (prevalence of 34%) and 5th in severe depression (prevalence of 34%). As a result, the prevalence ratio increased with severity from 2.4 in mild depression to 2.9 in severe depression.
Within control persons, the group of neurotic, stress-related and somatoform disorders (F4) was the only mental comorbidity among the 20 most prevalent comorbidities, ranking 13th (controls of moderate depression cases) or 14th place (controls of mild and severe depression) with a prevalence of nearly 16% (see Table 2 ). The three most prevalent disorders among controls were hypertension (I10-I15) followed by other dorsopathies (M50-M54) and metabolic disorders (E70-E90), affecting roughly 38, 35 and 30% among control patients, respectively.
Comparison of men and women
For the most prevalent comorbidities in the total population, we compared the prevalence among depression cases and the corresponding prevalence ratio between men and women. For this comparison, we included all diagnosis groups from Table 3 , irrespective of depression severity, excluding N80-N98 as a women-specific diagnosis group. Figure 4 displays the results for moderate depression, while the findings for mild and severe depression can be found in the appendix (Table A 2 ). For the majority of the displayed disease groups, age-adjusted prevalence was higher among female depression cases compared to male cases, except for metabolic disorders (E70-E90), substance use disorders (F10-F19), hypertensive diseases (I10-I15) and other forms of heart disease (I30-I52). Further, the excess risk relative to controls was mainly similar for males and females with depression, with a tendency towards slightly higher risks among women though. A striking exception was observed for the group of neurotic, stress-related and somatoform disorders (F4). Despite the age-adjusted prevalence of this mental comorbidity being substantially lower in men compared to women (55% vs. 65%), the excess risk compared to control persons was 47% higher in men than in women (PR of 5.13 vs. 3.48), implying that male cases of depression are much more likely to additionally have a diagnosis of an F4-disorder compared to controls than female depression cases. In other words, F4-disorders are generally more prevalent among women than men (18% vs. 11% in female and male control persons), but prevalence in men increases more drastically when they are diagnosed with depression.
Age-adjusted prevalence and prevalence ratios for the most prevalent comorbidities by sex . Prevalence ratios were estimated for 201 diagnosis groups from the ICD-10 reflecting mental and somatic diseases using administrative data from outpatient care including 6.3 million patients with a specific diagnosis of depression in 2017 and 25.2 million age- and sex-matched controls. Sex-specific prevalence was age-adjusted using the joint age distribution of depression cases as reference, stratified by severity. The prevalence ratio is defined as the ratio of the prevalence of the respective diagnosis group among depression cases to the prevalence among controls
Ranking of the comorbidities according to their prevalence among depression cases was largely similar between men and women. A few striking differences should be noted though. First, substance use disorders ranked 13th in moderate depression and 10th in severe depression among men, while they ranked 29th and 26th among women, respectively. Despite a comparable prevalence ratio, they were 50 to 60% more likely to occur in men with depression compared to women. Second, diabetes mellitus (E10-E14) which did not reach the top 20 most prevalent comorbidities in the total population (Table 3 ), came up to rank 16 among men (prevalence: 20.5%), while it ranked only 25th in women (prevalence: 15.4%). Third, other forms of heart disease (I30-I52) ranked substantially higher among men compared to women (i.e. 11th and 22nd in severe depression), despite a comparable prevalence among depression cases (23.5% in men, 20.8% in women).
To our knowledge, the present study is the first to comprehensively illustrate the comorbid status of persons diagnosed with depression with regard to the entire spectrum of diseases diagnosed by medical professionals in outpatient care (i.e. not assessed via self-report such as in the World Mental health surveys [ 29 ]). With our unique approach of a) simultaneously investigating about 200 diagnosis groups, while b) differentiating analyses according to depression severity and c) quantifying the excess risk of comorbidity in depression relative to controls based on data from nearly the complete adolescent and adult population in a high-income country with a well-equipped health care system, we were able to generate an overarching picture of the distribution and implications of depression comorbidity for the individual and public health in high-income countries.
The present study confirms the strong association of depression with anxiety and substance use disorders, as described previously [ 10 , 40 , 41 ]. It further illustrates that nearly all mental disorders are at least twice as likely in persons with depression relative to controls and that excess risk increases with depression severity. Overall, about two thirds of depression cases had at least one other concomitant mental disorder which corresponds well with findings from population surveys documenting that 60–65% of persons with 12-month MDD have at least one other mental comorbid disorder [ 10 , 42 , 43 , 44 ].
In line with results from international epidemiological surveys in high-income countries [ 10 , 42 , 44 , 45 ], we identified neurotic, stress-related and somatoform disorders, which mainly comprise anxiety disorders, as the most frequent mental comorbidity, affecting between 60 and 65% of moderate and severe depression cases. This highly frequent comorbidity has been explained based on a close relation in terms of genetics [ 46 ], shared environmental risk factors (i.e. childhood adversities and negative life events [ 47 ]) and overlap in symptoms. A recent study further underscored the close relation of both disorders by documenting that MDD, as a narrowly defined episodic disorder, may lead to an underestimation of both the prognosis of the majority of patients and the appropriate type of care [ 48 ]. In that large prospective study, 32% of MDD patients appeared to be fully recovered after a follow-up of 6 years when considering only MDD, while this proportion reduced to 17% when taking into account symptoms of anxiety, suggesting that the majority of patients suffer from chronic disorders and that full recovery is the exception. In concordance with European and US population surveys in the general population [ 49 ] and also with regard to comorbid anxiety [ 45 ], we observed a female preponderance of anxiety disorders in depression, though in men we observed a substantially higher risk for anxiety disorders when relative to their healthy counterparts than in women. This strikingly higher comorbidity risk observed in men needs further investigation; though we hypothesize it might be artefactual. It is well documented that men are less likely 1) to seek help for mental health problems than women, i.e. related to conformity to traditional gender roles, 2) to have symptoms fitting standard measurement tools and 3) to have their mental health problem identified by primary care physicians, all promoting an underestimation of the prevalence, particularly with regard to depression and anxiety disorders [ 50 , 51 ]. Taking these observations into account, it seems likely that the prevalence of anxiety disorders in the male control group may be underestimated in the present study and/or that depression without anxiety was underdiagnosed in men, both artificially increasing the prevalence ratio. In relation to this, we recently observed that the prevalence of diagnosed depression increased more strongly in (young) men compared to women between 2009 and 2017, proposing a continuous reduction of the gender difference in prevalence of diagnosed depression [ 52 ].
Substance use disorders emerged as the second most prevalent mental comorbidity in depression, affecting between 12 and 20% of depression cases, depending on severity of depression. Previous population studies not differentiating between severity status yielded estimates ranging between 9% in the US [ 10 ], 13% in New Zealand [ 44 ], 17% in the Netherlands [ 43 ], and 18% in Australia [ 45 ].
With regard to personality disorders, our findings deviate from previous studies that were mainly based on structured interviews using the DSM-system [ 53 , 54 ]. First, the prevalence of diagnosed comorbid personality disorders observed in our study is strikingly lower than determined in a recent meta-analysis where 30–50% of MDD patients had a comorbid DSM-diagnosed personality disorder [ 53 ]. Second, the prevalence of personality disorders in the general population is estimated to be about 9% [ 54 ], suggesting a population prevalence as high as observed in our depression cases and much higher than in our control group. Thus, our study supports the general notion of differences in the identification of personality disorders between the DSM-system and ICD-system, with a substantial underdiagnosis in the ICD [ 53 , 55 ]. The artificial dichotomy that requires clinicians to decide on whether a person does or does not have a personality disorder has been made responsible for the observation that in most cases, the diagnosis is avoided [ 56 ]. The 11th version of the ICD has now addressed the need for a dimensional diagnostic approach and allows for classifying different degrees of severity which may ultimately improve identification of personality disturbances in clinical practice. It will be interesting to evaluate this aspect in future studies using administrative health data.
By taking into account the whole range of somatic disease groups, we were able to provide an overall picture of somatic comorbidity in depression, including a ranking of the comorbidities according to their prevalence as well as to their excess risk relative to controls. Thereby, our study adds to the existing literature that has already generated compelling evidence on comorbid associations with depression for a large number of somatic diseases, but usually focused on single or a few common diseases at the same time. In general, our findings on the cross-sectional associations of diagnosed depression and comorbid somatic disorders in the ambulatory setting are in large agreement with previous evidence on associations with various somatic diseases, including cardiovascular diseases [ 29 , 57 ], metabolic diseases [ 22 , 32 , 58 ], neurological diseases [ 31 , 59 , 60 ], cancer [ 61 ], immune-mediated inflammatory diseases [ 62 , 63 , 64 , 65 ], chronic lower respiratory diseases [ 34 , 66 ] and musculoskeletal diseases [ 67 ].
In the following, we discuss some of our findings in the context of previous research by focusing on the very common diseases and high-risk disease groups as described in relation to Fig. 3 .

Depression and common diseases
We found depression to be associated with several common somatic illnesses, including diseases of the circular system (i.e. hypertension, heart disease and diseases of veins), endocrine, nutrition and metabolic diseases (i.e. diabetes mellitus, obesity) as well as muscoloskeletal diseases (i.e. low back pain, cervicalgia). In general, individuals with depression were 20–60% more likely to additionally have a diagnosis of one of these diseases compared to individuals without depression.
Within this set of relatively common diseases, the group of ‘other dorsopathies’, reflecting low back pain and cervicalgia, was the most frequent diagnosis group in depression cases and, at the same time, was related to the highest risk compared to controls. A significant comorbidity of depression and (chronic) back pain has been observed previously in cross-sectional population surveys in Germany [ 33 ] and Canada [ 68 ] and has been linked to significant increases in sick days and doctor visits [ 33 ] as well as substantially higher direct health care costs [ 69 ]. Depression has been shown to increase risk of future low back pain [ 70 ], to have a negative impact on pain severity and perception in patients with low back pain [ 71 ], and to relate to an impaired quality of life, increased disability and higher risk of chronicity [ 29 , 72 ].
Hypertensive diseases, as a leading risk factor for morbidity and mortality in the general population, ranked second among depression cases in terms of prevalence and related to a 20% higher risk compared to individuals without depression. In addition, further diseases of the cardiovascular system are found within the top 20 of the most prevalent diseases in depression. Our findings are supported by a large body of evidence documenting associations of depression with incident hypertension [ 73 , 74 , 75 ] as well as the incidence and poor prognosis of other cardiovascular diseases, including stroke [ 18 , 76 ], coronary heart disease [ 18 , 77 ], and heart failure [ 57 ]. Similarly, for various endocrine and metabolic disorders such as hypercholesterolaemia [ 78 ], obesity [ 79 ] and diabetes mellitus [ 22 , 32 ], all of which risk factors for cardiovascular diseases, some cancers and overall mortality, associations with depressions have been documented.
Depression and high-risk comorbidities
In line with previous literature [ 31 , 59 , 63 , 80 , 81 ], the present study revealed strong associations of depression with diseases of the central nervous system (i.e. multiple sclerosis) and with several neurological diseases, among them sleep disorders, migraine and epilepsy, most of them exhibiting at least 2- to 3-fold higher prevalence in depression relative to controls. Results of a meta-analysis on depression and epilepsy indicate that 23% of persons with epilepsy also have a depression [ 80 ], though studies suggest depression to be underrecognized in individuals with epilepsy [ 82 ]. With regard to multiple sclerosis, a Canadian study based on administrative health data has recently estimated the incidence of depression to be 2.4-fold higher in individuals with multiple sclerosis compared to their healthy counterparts [ 81 ]. Further, the authors demonstrated a greater than additive interaction of multiple sclerosis with depression on mortality risk, implying that 13% of the increased mortality in multiple sclerosis being due to the joint effects of having multiple sclerosis and depression [ 63 ].
Epidemiological studies on the association of migraine and depression have shown a 50% higher risk of depression in persons with migraine and, among persons with depression, a 1.6–3.4-times higher risk for developing migraine [ 59 ].
Depression and pregnancy
In contrast to all other diagnosis groups, pregnancy-related diagnosis groups were inversely related to moderate and severe, but not mild, depression in our study. A range of cross-sectional studies has previously suggested that depression may be associated with decreased fecundability, though the temporal sequence of events remained unclear [ 83 ]. A recent prospective internet-based preconception cohort study of women attempting to conceive recently suggests that moderate to severe depressive symptoms at baseline, independent of psychotropic medication use, were related to decreased fecundability compared to no or low depressive symptoms [ 84 ].
Biological mechanisms linking depression to comorbid diseases
The high comorbidity of depression with a broad range of somatic diseases may reflect various mechanisms. First, depression is associated with unhealthy behavior, i.e. smoking, alcohol consumption, lack of physical activity, poor diet, and impaired sleep, all well-established risk factors for common chronic diseases such as diabetes and cardiovascular diseases [ 25 ]. In addition, depression has been associated with non-adherence to treatment regimens, which may explain the worse prognosis among patients with somatic disease and comorbid depression [ 85 ]. Second, depression has been shown to have neuroendocrine effects, i.e. the activation of the sympathetic nervous system and dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis which among others promotes endothelial dysfunction, hypertension, abdominal obesity, hypercholesterolemia and hypertriglyceridemia, conferring higher risks of diabetes and cardiovascular diseases [ 18 , 85 ]. Also, elevated HPA activity may impact menstrual cycle characteristics, subsequently affecting the ability to conceive [ 86 ]. Third, accumulating evidence indicates that depression is related to a state of chronic low-grade inflammation, with significantly increased levels of interleukin (IL)-1, IL-6, tumor-necrosis factor (TNF)-alpha and C-reactive protein (CRP). The role of immune-mediated inflammation is increasingly recognized as the universal pathophysiological process that underlies numerous somatic diseases (diabetes, stroke, heart disease, many cancers, autoimmune diseases such as multiple sclerosis and rheumatoid arthritis) as well as mental disorders, including depression [ 18 , 65 ]. Based on these emerging observations on shared biological mechanisms involved, the association of depression with various somatic diseases (i.e. coronary heart disease, stroke, migraine, chronic obstructive pulmonary disease, rheumatoid arthritis) is likely bidirectional, with abnormalities present in depression increasing the risk of the somatic disease and the presence of the somatic disease or its determinants contributing to the development of depression [ 18 , 25 , 59 , 65 , 66 , 87 , 88 ]. It is increasingly understood that the inflammatory processes in individuals with depression and a comorbid somatic disease together with neuroendocrine effects and behavioral factors associated with depression all feed off each other in a self-perpetuating feedback loop, affecting development, severity, prognosis and outcome of both disorders [ 18 , 87 ]. A recent study using Mendelian randomization to elucidate shared mechanisms underlying the association of depression and coronary heart disease provided evidence that triglycerides, IL-6 and CRP are causally related to depression [ 89 ]. Using a similar approach with regard to obesity, studies indicate that obesity is a causal risk factor for depression, but not vice versa, with a recent study specifying body fat mass to be the driver of this causal relationship [ 58 ].
Implications for clinical practice and public health
As key finding, our study underscores the importance of closely surveying symptoms associated with depression in chronic somatic diseases in primary care and, conversely, considering the increased risk of somatic diseases, i.e. cardiometabolic disturbances, related to depression. The results of our study support the development of interdisciplinary and multidisciplinary treatment strategies, integrating mental health services into primary care, which have been shown to improve treatment adherence, outcomes and quality of life [ 90 ]. This may eventually require changes in the organization of the health care system, i.e. including the establishment of a regular and standardized screening for depression in primary care, adjustment of training of health workers in the primary care with regard to the frequent comorbidity of depression and somatic disorders and inclusion of mental health workers, improved referral to qualified health care provider, harmonization of care between healthcare providers, as well as education of the general population and individuals with somatic diseases [ 25 , 32 ]. As important step towards early detection and improved treatment, some of the latest treatment guidelines for common somatic diseases have now included recommendations on screening for depression, i.e. the European guidelines on cardiovascular disease prevention [ 91 ] or the American clinical practice recommendations on the management of diabetes [ 92 ]. In Germany, the Federal Join Committee (G-BA) has recently issued the implementation of a structured treatment plan for depression (disease management program), defining the adequate treatment of relevant comorbidities as one major treatment target [ 93 , 94 ].
Limitations
Limitations of our study refer to the commonly recognized constraints of administrative data for epidemiological research. Results are based on the presence of specific diagnostic codes that were routinely collected for the purpose of physician billing claims. Hence, the validity depends on the accuracy of those codes and studies have shown validity to vary by disease [ 95 ].
Given the cross-sectional design, it is not possible to draw conclusions about causes and effects and interactions of the observed associations. Potential pathways include a) the contact to the health care system is more pronounced among persons with depression compared to persons without depression, which increases the likelihood of early detection and diagnosis of further existing diseases in depression cases; b) the presence of (somatic) diseases or its medical treatment may lead to the development of depressive symptoms and similarly increase the likelihood of the diagnosis; c) for some diseases, the typical symptoms overlap with or mimic the specific symptoms of depression (i.e. hypothyroidism); d) further, enhanced comorbidity may be due to shared risk factors and pathological pathways between depression and other conditions.
Finally, the present analysis did not evaluate excess mortality in comorbid cases (as was documented in particular in severe mental disorders, i.e. [ 96 , 97 ]).
The present study provides a comprehensive overview of depression comorbidity and should stimulate awareness of the strong interconnection of depression with all other mental and the vast majority of somatic diseases. Our findings underscore the necessity of systematically addressing the high comorbidity of depression and somatic diseases in primary care through prevention, early identification of vulnerable persons and management of depressive symptoms, i.e. within the framework of disease management programs. Given the extensive association with several of the most burdensome somatic diseases and other mental disorders, as well as excess utilization of health care services (which may reflect excess needs), depression has evolved to a central health care problem that requires the integration of interdisciplinary and multidisciplinary treatment strategies.
Availability of data and materials
The datasets analyzed during the current study are not publicly available due to ethical and privacy reasons.
Abbreviations
C-reactive protein
Dysthymic Disorder
Diagnostic and Statistical Manual of Mental Disorders
Hypothalamic-pituitary-adrenal axis
International Classification of Diseases
Interleukin-1
Major Depressive Disorder
Prevalence Ratio
Statistical Analysis Software
Statutory Health Insurance
Tumor-necrosis factor alpha
Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS. National Comorbidity Survey R: the epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA. 2003;289(23):3095–105.
Article PubMed Google Scholar
Malhi GS, Mann JJ. Depression. Lancet. 2018;392(10161):2299–312.
Jacobi F, Hofler M, Siegert J, Mack S, Gerschler A, Scholl L, Busch MA, Hapke U, Maske U, Seiffert I, et al. Twelve-month prevalence, comorbidity and correlates of mental disorders in Germany: the mental health module of the German health interview and examination survey for adults (DEGS1-MH). Int J Methods Psychiatr Res. 2014;23(3):304–19.
Article PubMed PubMed Central Google Scholar
Jacobi F, Hofler M, Strehle J, Mack S, Gerschler A, Scholl L, Busch MA, Hapke U, Maske U, Seiffert I, et al. Twelve-months prevalence of mental disorders in the German health interview and examination survey for adults - mental health module (DEGS1-MH): a methodological addendum and correction. Int J Methods Psychiatr Res. 2015;24(4):305–13.
Wittchen HU, Jacobi F, Rehm J, Gustavsson A, Svensson M, Jönsson B, Olesen J, Allgulander C, Alonso J, Faravelli C, et al. The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur Neuropsychopharmacol. 2011;21(9):655–79.
Article CAS PubMed Google Scholar
Disease GBD, Injury I, Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the global burden of Disease study 2017. Lancet. 2018;392(10159):1789–858.
Article Google Scholar
Mykletun A, Bjerkeset O, Overland S, Prince M, Dewey M, Stewart R. Levels of anxiety and depression as predictors of mortality: the HUNT study. Br J Psychiatry. 2009;195(2):118–25.
Gilman SE, Sucha E, Kingsbury M, Horton NJ, Murphy JM, Colman I. Depression and mortality in a longitudinal study: 1952-2011. CMAJ. 2017;189(42):E1304–10.
Pequignot R, Dufouil C, Peres K, Artero S, Tzourio C, Empana JP. Depression increases the risk of death independently from vascular events in elderly individuals: the Three-City study. J Am Geriatr Soc. 2019;67(3):546–52.
Kessler RC, Merikangas KR, Wang PS. Prevalence, comorbidity, and service utilization for mood disorders in the United States at the beginning of the twenty-first century. Annu Rev Clin Psychol. 2007;3:137–58.
Kaufman J, Charney D. Comorbidity of mood and anxiety disorders. Depress Anxiety. 2000;12(Suppl 1):69–76.
Hirschfeld RM. The comorbidity of major depression and anxiety disorders: recognition and Management in Primary Care. Prim Care Companion J Clin Psychiatry. 2001;3(6):244–54.
Hasin DS, Goodwin RD, Stinson FS, Grant BF. Epidemiology of major depressive disorder: results from the National Epidemiologic Survey on alcoholism and related conditions. Arch Gen Psychiatry. 2005;62(10):1097–106.
Nock MK, Hwang I, Sampson NA, Kessler RC. Mental disorders, comorbidity and suicidal behavior: results from the National Comorbidity Survey Replication. Mol Psychiatry. 2010;15(8):868–76.
Wiethoff K, Bauer M, Baghai TC, Moller HJ, Fisher R, Hollinde D, Kiermeir J, Hauth I, Laux G, Cordes J, et al. Prevalence and treatment outcome in anxious versus nonanxious depression: results from the German algorithm project. J Clin Psychiatry. 2010;71(8):1047–54.
Richards D. Prevalence and clinical course of depression: a review. Clin Psychol Rev. 2011;31(7):1117–25.
Mack S, Jacobi F, Gerschler A, Strehle J, Hofler M, Busch MA, Maske UE, Hapke U, Seiffert I, Gaebel W, et al. Self-reported utilization of mental health services in the adult German population--evidence for unmet needs? Results of the DEGS1-mental health module (DEGS1-MH). Int J Methods Psychiatr Res. 2014;23(3):289–303.
Voinov B, Richie WD, Bailey RK. Depression and chronic diseases: it is time for a synergistic mental health and primary care approach. Prim Care Companion CNS Disord. 2013;15:2.
Google Scholar
Kessler RC, Bromet EJ. The epidemiology of depression across cultures. Annu Rev Public Health. 2013;34:119–38.
Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the world health surveys. Lancet. 2007;370(9590):851–8.
Lotfaliany M, Bowe SJ, Kowal P, Orellana L, Berk M, Mohebbi M. Depression and chronic diseases: co-occurrence and communality of risk factors. J Affect Disord. 2018;241:461–8.
Maske UE, Scheidt-Nave C, Busch MA, Jacobi F, Weikert B, Riedel-Heller SG. Hapke U: [comorbidity of diabetes mellitus and depression in the general population in Germany]. Psychiatr Prax. 2015;42(4):202–7.
PubMed Google Scholar
Maske UE, Busch MA, Jacobi F, Riedel-Heller SG, Scheidt-Nave C, Hapke U. Chronic somatic conditions and mental health problems in the general population in Germany. Results of the national telephone health interview survey “German health update (GEDA)” 2010. Psychiatr Prax. 2013;40(4):207–13.
Evans DL, Charney DS, Lewis L, Golden RN, Gorman JM, Krishnan KR, Nemeroff CB, Bremner JD, Carney RM, Coyne JC, et al. Mood disorders in the medically ill: scientific review and recommendations. Biol Psychiatry. 2005;58(3):175–89.
Vaccarino V, Badimon L, Bremner JD, Cenko E, Cubedo J, Dorobantu M, Duncker DJ, Koller A, Manfrini O, Milicic D, et al. Depression and coronary heart disease: 2018 ESC position paper of the working group of coronary pathophysiology and microcirculation developed under the auspices of the ESC Committee for practice guidelines. Eur Heart J. 2019;1:28.
Maske UE, Buttery AK, Beesdo-Baum K, Riedel-Heller S, Hapke U, Busch MA. Prevalence and correlates of DSM-IV-TR major depressive disorder, self-reported diagnosed depression and current depressive symptoms among adults in Germany. J Affect Disord. 2016;190:167–77.
Welch CA, Czerwinski D, Ghimire B, Bertsimas D. Depression and costs of health care. Psychosomatics. 2009;50(4):392–401.
Kang HJ, Kim SY, Bae KY, Kim SW, Shin IS, Yoon JS, Kim JM. Comorbidity of depression with physical disorders: research and clinical implications. Chonnam Med J. 2015;51(1):8–18.
Article CAS PubMed PubMed Central Google Scholar
Scott KM, Von Korff M, Alonso J, Angermeyer MC, Bromet E, Fayyad J, de Girolamo G, Demyttenaere K, Gasquet I, Gureje O, et al. Mental-physical co-morbidity and its relationship with disability: results from the world mental health surveys. Psychol Med. 2009;39(1):33–43.
Greenberg PE, Fournier AA, Sisitsky T, Pike CT, Kessler RC. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry. 2015;76(2):155–62.
Josephson CB, Jette N. Psychiatric comorbidities in epilepsy. Int Rev Psychiatry. 2017;29(5):409–24.
Sartorius N. Depression and diabetes. Dialogues Clin Neurosci. 2018;20(1):47–52.
PubMed PubMed Central Google Scholar
Martini L, Hoffmann F. Comorbidity of chronic back pain and depression in Germany: results from the GEDA study, 2009 and 2010. Z Evid Fortbild Qual Gesundhwes. 2018;137-138:62–8.
Gerald JK, Moreno FA. Asthma and depression: It's complicated. J Allergy Clin Immunol Pract. 2016;4(1):74–5.
Long-term conditions and mental health. The cost of comorbidities [ https://www.kingsfund.org.uk/sites/default/files/field/field_publication_file/long-term-conditions-mental-health-cost-comorbidities-naylor-feb12.pdf ].
Deschenes SS, Burns RJ, Schmitz N. Associations between depression, chronic physical health conditions, and disability in a community sample: a focus on the persistence of depression. J Affect Disord. 2015;179:6–13.
van Melle JP, de Jonge P, Spijkerman TA, Tijssen JG, Ormel J, van Veldhuisen DJ, van den Brink RH, van den Berg MP. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis. Psychosom Med. 2004;66(6):814–22.
Buist-Bouwman MA, de Graaf R, Vollebergh WA, Ormel J. Comorbidity of physical and mental disorders and the effect on work-loss days. Acta Psychiatr Scand. 2005;111(6):436–43.
Thaipisuttikul P, Ittasakul P, Waleeprakhon P, Wisajun P, Jullagate S. Psychiatric comorbidities in patients with major depressive disorder. Neuropsychiatr Dis Treat. 2014;10:2097–103.
Lamers F, van Oppen P, Comijs HC, Smit JH, Spinhoven P, van Balkom AJ, Nolen WA, Zitman FG, Beekman AT, Penninx BW. Comorbidity patterns of anxiety and depressive disorders in a large cohort study: the Netherlands study of depression and anxiety (NESDA). J Clin Psychiatry. 2011;72(3):341–8.
Lai HM, Cleary M, Sitharthan T, Hunt GE. Prevalence of comorbid substance use, anxiety and mood disorders in epidemiological surveys, 1990-2014: A systematic review and meta-analysis. Drug Alcohol Depend. 2015;154:1–13.
Kessler RC, Nelson CB, McGonagle KA, Liu J, Swartz M, Blazer DG. Comorbidity of DSM-III-R major depressive disorder in the general population: results from the US National Comorbidity Survey. Br J Psychiatry Suppl. 1996;30:17–30.
de Graaf R, Bijl RV, Smit F, Vollebergh WA, Spijker J. Risk factors for 12-month comorbidity of mood, anxiety, and substance use disorders: findings from the Netherlands mental health survey and incidence study. Am J Psychiatry. 2002;159(4):620–9.
Scott KM, McGee MA, Oakley Browne MA, Wells JE. New Zealand mental health survey research T: mental disorder comorbidity in Te Rau Hinengaro: the New Zealand mental health survey. Aust N Z J Psychiatry. 2006;40(10):875–81.
Teesson M, Slade T, Mills K. Comorbidity in Australia: findings of the 2007 National Survey of mental health and wellbeing. Aust N Z J Psychiatry. 2009;43(7):606–14.
Smoller JW. The genetics of stress-related disorders: PTSD, depression, and anxiety disorders. Neuropsychopharmacology. 2015;41:297.
Article PubMed PubMed Central CAS Google Scholar
Spinhoven P, Elzinga BM, Hovens JG, Roelofs K, Zitman FG, van Oppen P, Penninx BW. The specificity of childhood adversities and negative life events across the life span to anxiety and depressive disorders. J Affect Disord. 2010;126(1–2):103–12.
Verduijn J, Verhoeven JE, Milaneschi Y, Schoevers RA, van Hemert AM, Beekman ATF, Penninx B. Reconsidering the prognosis of major depressive disorder across diagnostic boundaries: full recovery is the exception rather than the rule. BMC Med. 2017;15(1):215.
Bandelow B, Michaelis S. Epidemiology of anxiety disorders in the 21st century. Dialogues Clin Neurosci. 2015;17(3):327–35.
Seidler ZE, Dawes AJ, Rice SM, Oliffe JL, Dhillon HM. The role of masculinity in men’s help-seeking for depression: a systematic review. Clin Psychol Rev. 2016;49:106–18.
Smith DT, Mouzon DM, Elliott M. Reviewing the assumptions about Men's mental health: an exploration of the gender binary. Am J Mens Health. 2018;12(1):78–89.
Steffen A, Thom J, Jacobi F, Holstiege J, Bätzing J. Trends in prevalence of depression in Germany between 2009 and 2017 based on nationwide ambulatory claims data (in revision with journal of affective disorders); 2019.
Friborg O, Martinsen EW, Martinussen M, Kaiser S, Overgard KT, Rosenvinge JH. Comorbidity of personality disorders in mood disorders: a meta-analytic review of 122 studies from 1988 to 2010. J Affect Disord. 2014;152-154:1–11.
Lenzenweger MF, Lane MC, Loranger AW, Kessler RC. DSM-IV personality disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;62(6):553–64.
Reed GM. Progress in developing a classification of personality disorders for ICD-11. World Psychiatry. 2018;17(2):227–9.
Tyrer P, Crawford M, Mulder R, Blashfield R, Farnam A, Fossati A, Kim Y-R, Koldobsky N, Lecic-Tosevski D, Ndetei D, et al. The rationale for the reclassification of personality disorder in the 11th revision of the international classification of diseases (ICD-11). Personal Ment Health. 2011;5(4):246–59.
Celano CM, Villegas AC, Albanese AM, Gaggin HK, Huffman JC. Depression and anxiety in heart failure: a review. Harv Rev Psychiatry. 2018;26(4):175–84.
Speed MS, Jefsen OH, Børglum AD, Speed D, Østergaard SD. Investigating the association between body fat and depression via Mendelian randomization. Transl Psychiatry. 2019;9(1):184.
Yang Y, Ligthart L, Terwindt GM, Boomsma DI, Rodriguez-Acevedo AJ, Nyholt DR. Genetic epidemiology of migraine and depression. Cephalalgia. 2016;36(7):679–91.
Hsu Y-T, Liao C-C, Chang S-N, Yang Y-W, Tsai C-H, Chen T-L, Sung F-C. Increased risk of depression in patients with Parkinson Disease: a Nationwide cohort study. Am J Geriatr Psychiatry. 2015;23(9):934–40.
Sotelo JL, Musselman D, Nemeroff C. The biology of depression in cancer and the relationship between depression and cancer progression. Int Rev Psychiatry. 2014;26(1):16–30.
Marrie RA, Hitchon CA, Walld R, Patten SB, Bolton JM, Sareen J, Walker JR, Singer A, Lix LM, El-Gabalawy R, et al. Increased burden of psychiatric disorders in rheumatoid arthritis. Arthritis Care Res. 2018;70(7):970–8.
Marrie RA, Walld R, Bolton JM, Sareen J, Patten SB, Singer A, Lix LM, Hitchon CA, El-Gabalawy R, Katz A, et al. Psychiatric comorbidity increases mortality in immune-mediated inflammatory diseases. Gen Hosp Psychiatry. 2018;53:65–72.
Bernstein CN, Hitchon CA, Walld R, Bolton JM, Sareen J, Walker JR, Graff LA, Patten SB, Singer A, Lix LM, et al. Increased burden of psychiatric disorders in inflammatory bowel Disease. Inflamm Bowel Dis. 2018;25(2):360–8.
Article PubMed Central Google Scholar
Nerurkar L, Siebert S, McInnes IB, Cavanagh J. Rheumatoid arthritis and depression: an inflammatory perspective. Lancet Psychiatry. 2019;6(2):164–73.
Yohannes AM, Alexopoulos GS. Depression and anxiety in patients with COPD. Eur Respir Rev. 2014;23(133):345–9.
Bletzer J, Gantz S, Voigt T, Neubauer E, Schiltenwolf M. Chronische untere Rückenschmerzen und psychische Komorbidität. Schmerz. 2017;31(2):93–101.
Currie SR, Wang J. Chronic back pain and major depression in the general Canadian population. Pain. 2004;107(1–2):54–60.
Baumeister H, Knecht A, Hutter N. Direct and indirect costs in persons with chronic back pain and comorbid mental disorders--a systematic review. J Psychosom Res. 2012;73(2):79–85.
Pinheiro MB, Ferreira ML, Refshauge K, Ordonana JR, Machado GC, Prado LR, Maher CG, Ferreira PH. Symptoms of depression and risk of new episodes of low Back pain: a systematic review and meta-analysis. Arthritis Care Res. 2015;67(11):1591–603.
Bletzer J, Gantz S, Voigt T, Neubauer E, Schiltenwolf M. Chronic low back pain and psychological comorbidity: a review. Schmerz. 2017;31(2):93–101.
Pincus T, Burton AK, Vogel S, Field AP. A systematic review of psychological factors as predictors of chronicity/disability in prospective cohorts of low back pain. Spine (Phila Pa 1976). 2002;27(5):E109–20.
Meng L, Chen D, Yang Y, Zheng Y, Hui R. Depression increases the risk of hypertension incidence: a meta-analysis of prospective cohort studies. J Hypertens. 2012;30(5):842–51.
Ginty AT, Carroll D, Roseboom TJ, Phillips AC, de Rooij SR. Depression and anxiety are associated with a diagnosis of hypertension 5 years later in a cohort of late middle-aged men and women. J Hum Hypertens. 2013;27(3):187–90.
Carroll D, Phillips AC, Gale CR, Batty GD. Generalized anxiety and major depressive disorders, their comorbidity and hypertension in middle-aged men. Psychosom Med. 2010;72(1):16–9.
Pan A, Sun Q, Okereke OI, Rexrode KM, Hu FB. Depression and risk of stroke morbidity and mortality: a meta-analysis and systematic review. JAMA. 2011;306(11):1241–9.
Davidson KW. Depression and coronary heart disease. ISRN Cardiol. 2012;2012:743813.
Tyrovolas S, Lionis C, Zeimbekis A, Bountziouka V, Micheli M, Katsarou A, Papairakleous N, Metallinos G, Makri K, Polychronopoulos E, et al. Increased body mass and depressive symptomatology are associated with hypercholesterolemia, among elderly individuals; results from the MEDIS study. Lipids Health Dis. 2009;8(1):10.
Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BW, Zitman FG. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry. 2010;67(3):220–9.
Fiest KM, Dykeman J, Patten SB, Wiebe S, Kaplan GG, Maxwell CJ, Bulloch AG, Jette N. Depression in epilepsy: a systematic review and meta-analysis. Neurology. 2013;80(6):590–9.
Marrie RA, Walld R, Bolton JM, Sareen J, Walker JR, Patten SB, Singer A, Lix LM, Hitchon CA, El-Gabalawy R, et al. Increased incidence of psychiatric disorders in immune-mediated inflammatory disease. J Psychosom Res. 2017;101:17–23.
Barry JJ. The recognition and Management of Mood Disorders as a comorbidity of epilepsy. Epilepsia. 2003;44(s4):30–40.
Williams KE, Marsh WK, Rasgon NL. Mood disorders and fertility in women: a critical review of the literature and implications for future research. Hum Reprod Update. 2007;13(6):607–16.
Nillni YI, Wesselink AK, Gradus JL, Hatch EE, Rothman KJ, Mikkelsen EM, Wise LA. Depression, anxiety, and psychotropic medication use and fecundability. Am J Obstet Gynecol. 2016;215(4):453 e451–8.
Dhar AK, Barton DA. Depression and the link with cardiovascular Disease. Front Psychiatry. 2016;7:33.
Joseph DN, Whirledge S. Stress and the HPA Axis: Balancing Homeostasis and Fertility. Int J Mol Sci. 2017;18:10.
Lippi G, Montagnana M, Favaloro EJ, Franchini M. Mental depression and cardiovascular disease: a multifaceted, bidirectional association. Semin Thromb Hemost. 2009;35(3):325–36.
Wium-Andersen MK, Wium-Andersen IK, Prescott EIB, Overvad K, Jørgensen MB, Osler M. An attempt to explain the bidirectional association between ischaemic heart disease, stroke and depression: a cohort and meta-analytic approach. Br J Psychiatry. 2019;1:1–8.
Khandaker GM, Zuber V, Rees JMB, Carvalho L, Mason AM, Foley CN, Gkatzionis A, Jones PB, Burgess S. Shared mechanisms between coronary heart disease and depression: findings from a large UK general population-based cohort. Mol Psychiatry. 2019. https://www.ncbi.nlm.nih.gov/pubmed/30886334 .
Ivbijaro GO, Enum Y, Khan AA, Lam SS, Gabzdyl A. Collaborative care: models for treatment of patients with complex medical-psychiatric conditions. Curr Psychiatry Rep. 2014;16(11):506.
Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corra U, Cosyns B, Deaton C, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37(29):2315–81.
American Diabetes Association. 4. Comprehensive medical evaluation and assessment of comorbidities: standards of medical Care in Diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S34–45.
What are disease management programs (DMPs)? [ https://www.ncbi.nlm.nih.gov/books/NBK279412/ ].
Beschluss des Gemeinsamen Bundesausschusses über die 17. Änderung der DMP-Anforderungen-Richtlinie (DMP-A-RL): Änderung der Anlage 2, Ergänzung der Anlage 17 (DMP Depression) und der Anlage 18 (Depression – Dokumentation) [ https://www.g-ba.de/downloads/39-261-3930/2019-08-15_DMP-A-RL_Depression.pdf ].
Johnson EK, Nelson CP. Values and pitfalls of the use of administrative databases for outcomes assessment. J Urol. 2013;190(1):17–8.
Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry. 2015;72(4):334–41.
Schneider F, Erhart M, Hewer W, Loeffler LA, Jacobi F. Mortality and medical comorbidity in the severely mentally ill. Dtsch Arztebl Int. 2019;116(23–24):405–11.
Download references
Acknowledgements
Not applicable.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and affiliations.
Central Research Institute of Ambulatory Health Care in Germany (Zi), Berlin, Germany
Annika Steffen, Jörg Bätzing & Jakob Holstiege
Department of Epidemiology and Health Monitoring, Unit 26 Mental Health, Robert Koch Institute, Berlin, Germany
Julia Nübel
Psychologische Hochschule Berlin, Berlin, Germany
Frank Jacobi
You can also search for this author in PubMed Google Scholar
Contributions
Study concept and design: AS, JN, FJ, JB, and JH. Analysis and interpretation of data: AS, JN, FJ, JB, and JH. Drafting of the manuscript: AS and JN. Critical revision of the manuscript for important intellectual content: AS, JN, FJ, JB, and JN. Statistical analysis: AS and JH. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Annika Steffen .
Ethics declarations
Ethics approval and consent to participate.
In Germany, the use of claims data for scientific research is regulated by the Code of Social Law (SGB X). An ethical approval and informed consent are not required as this study used routinely collected anonymized data.
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1:.
Table A1. Prevalence (%) and prevalence ratio (PR) for all 202 ICD diagnosis groups included in the present study. This supplemental table provides the prevalence and prevalence ratios stratified by depression severity for all 202 included diagnosis groups.
Additional file 2:
Table A2. Age-adjusted prevalence (%) and prevalence ratios (PR) for the 20 most prevalent comorbidities by sex among individuals with mild depressive disorder. This supplemental table provides age-adjusted prevalence and prevalence ratios for the 20 most prevalent comorbidities by sex among individuals with mild depressive disorder.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Steffen, A., Nübel, J., Jacobi, F. et al. Mental and somatic comorbidity of depression: a comprehensive cross-sectional analysis of 202 diagnosis groups using German nationwide ambulatory claims data. BMC Psychiatry 20 , 142 (2020). https://doi.org/10.1186/s12888-020-02546-8
Download citation
Received : 04 December 2019
Accepted : 13 March 2020
Published : 30 March 2020
DOI : https://doi.org/10.1186/s12888-020-02546-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Ambulatory claims data
- Comorbidity
- Mental disorders
- Somatic diseases

Mental Health Case Study: Understanding Depression through a Real-life Example
Imagine feeling an unrelenting heaviness weighing down on your chest. Every breath becomes a struggle as a cloud of sadness engulfs your every thought. Your energy levels plummet, leaving you physically and emotionally drained. This is the reality for millions of people worldwide who suffer from depression, a complex and debilitating mental health condition.
Understanding depression is crucial in order to provide effective support and treatment for those affected. While textbooks and research papers provide valuable insights, sometimes the best way to truly comprehend the depths of this condition is through real-life case studies. These stories bring depression to life, shedding light on its impact on individuals and society as a whole.
In this article, we will delve into the world of mental health case studies, using a real-life example to explore the intricacies of depression. We will examine the symptoms, prevalence, and consequences of this all-encompassing condition. Furthermore, we will discuss the significance of case studies in mental health research, including their ability to provide detailed information about individual experiences and contribute to the development of treatment strategies.
Through an in-depth analysis of a selected case study, we will gain insight into the journey of an individual facing depression. We will explore their background, symptoms, and initial diagnosis. Additionally, we will examine the various treatment options available and assess the effectiveness of the chosen approach.
By delving into this real-life example, we will not only gain a better understanding of depression as a mental health condition, but we will also uncover valuable lessons that can aid in the treatment and support of those who are affected. So, let us embark on this enlightening journey, using the power of case studies to bring understanding and empathy to those who need it most.
Understanding Depression
Depression is a complex and multifaceted mental health condition that affects millions of people worldwide. To comprehend the impact of depression, it is essential to explore its defining characteristics, prevalence, and consequences on individuals and society as a whole.
Defining depression and its symptoms
Depression is more than just feeling sad or experiencing a low mood. It is a serious mental health disorder characterized by persistent feelings of sadness, hopelessness, and a loss of interest in activities that were once enjoyable. Individuals with depression often experience a range of symptoms that can significantly impact their daily lives. These symptoms include:
1. Persistent feelings of sadness or emptiness. 2. Fatigue and decreased energy levels. 3. Significant changes in appetite and weight. 4. Difficulty concentrating or making decisions. 5. Insomnia or excessive sleep. 6. feelings of guilt, worthlessness, or hopelessness. 7. Loss of interest or pleasure in activities.
Exploring the prevalence of depression worldwide
Depression knows no boundaries and affects individuals from all walks of life. According to the World Health Organization (WHO), an estimated 264 million people globally suffer from depression. This makes depression one of the most common mental health conditions worldwide. Additionally, the WHO highlights that depression is more prevalent among females than males.
The impact of depression is not limited to individuals alone. It also has significant social and economic consequences. Depression can lead to impaired productivity, increased healthcare costs, and strain on relationships, contributing to a significant burden on families, communities, and society at large.
The impact of depression on individuals and society
Depression can have a profound and debilitating impact on individuals’ lives, affecting their physical, emotional, and social well-being. The persistent sadness and loss of interest can lead to difficulties in maintaining relationships, pursuing education or careers, and engaging in daily activities. Furthermore, depression increases the risk of developing other mental health conditions, such as anxiety disorders or substance abuse.
On a societal level, depression poses numerous challenges. The economic burden of depression is significant, with costs associated with treatment, reduced productivity, and premature death. Moreover, the social stigma surrounding mental health can impede individuals from seeking help and accessing appropriate support systems.
Understanding the prevalence and consequences of depression is crucial for policymakers, healthcare professionals, and individuals alike. By recognizing the significant impact depression has on individuals and society, appropriate resources and interventions can be developed to mitigate its effects and improve the overall well-being of those affected.
The Significance of Case Studies in Mental Health Research
Case studies play a vital role in mental health research, providing valuable insights into individual experiences and contributing to the development of effective treatment strategies. Let us explore why case studies are considered invaluable in understanding and addressing mental health conditions.
Why case studies are valuable in mental health research
Case studies offer a unique opportunity to examine mental health conditions within the real-life context of individuals. Unlike large-scale studies that focus on statistical data, case studies provide a detailed examination of specific cases, allowing researchers to delve into the complexities of a particular condition or treatment approach. This micro-level analysis helps researchers gain a deeper understanding of the nuances and intricacies involved.
The role of case studies in providing detailed information about individual experiences
Through case studies, researchers can capture rich narratives and delve into the lived experiences of individuals facing mental health challenges. These stories help to humanize the condition and provide valuable insights that go beyond a list of symptoms or diagnostic criteria. By understanding the unique experiences, thoughts, and emotions of individuals, researchers can develop a more comprehensive understanding of mental health conditions and tailor interventions accordingly.
How case studies contribute to the development of treatment strategies
Case studies form a vital foundation for the development of effective treatment strategies. By examining a specific case in detail, researchers can identify patterns, factors influencing treatment outcomes, and areas where intervention may be particularly effective. Moreover, case studies foster an iterative approach to treatment development—an ongoing cycle of using data and experience to refine and improve interventions.
By examining multiple case studies, researchers can identify common themes and trends, leading to the development of evidence-based guidelines and best practices. This allows healthcare professionals to provide more targeted and personalized support to individuals facing mental health conditions.
Furthermore, case studies can shed light on potential limitations or challenges in existing treatment approaches. By thoroughly analyzing different cases, researchers can identify gaps in current treatments and focus on areas that require further exploration and innovation.
In summary, case studies are a vital component of mental health research, offering detailed insights into the lived experiences of individuals with mental health conditions. They provide a rich understanding of the complexities of these conditions and contribute to the development of effective treatment strategies. By leveraging the power of case studies, researchers can move closer to improving the lives of individuals facing mental health challenges.
Examining a Real-life Case Study of Depression
In order to gain a deeper understanding of depression, let us now turn our attention to a real-life case study. By exploring the journey of an individual navigating through depression, we can gain valuable insights into the complexities and challenges associated with this mental health condition.
Introduction to the selected case study
In this case study, we will focus on Jane, a 32-year-old woman who has been struggling with depression for the past two years. Jane’s case offers a compelling narrative that highlights the various aspects of depression, including its onset, symptoms, and the treatment journey.
Background information on the individual facing depression
Before the onset of depression, Jane led a fulfilling and successful life. She had a promising career, a supportive network of friends and family, and engaged in hobbies that brought her joy. However, a series of life stressors, including a demanding job, a breakup, and the loss of a loved one, began to take a toll on her mental well-being.
Jane’s background highlights a common phenomenon – depression can affect individuals from all walks of life, irrespective of their socio-economic status, age, or external circumstances. It serves as a reminder that no one is immune to mental health challenges.
Presentation of symptoms and initial diagnosis
Jane began noticing a shift in her mood, characterized by persistent feelings of sadness and a lack of interest in activities she once enjoyed. She experienced disruptions in her sleep patterns, appetite changes, and a general sense of hopelessness. Recognizing the severity of her symptoms, Jane sought help from a mental health professional who diagnosed her with major depressive disorder.
Jane’s case exemplifies the varied and complex symptoms associated with depression. While individuals may exhibit overlapping symptoms, the intensity and manifestation of those symptoms can vary greatly, underscoring the importance of personalized and tailored treatment approaches.
By examining this real-life case study of depression, we can gain an empathetic understanding of the challenges faced by individuals experiencing this mental health condition. Through Jane’s journey, we will uncover the treatment options available for depression and analyze the effectiveness of the chosen approach. The case study will allow us to explore the nuances of depression and provide valuable insights into the treatment landscape for this prevalent mental health condition.
The Treatment Journey
When it comes to treating depression, there are various options available, ranging from therapy to medication. In this section, we will provide an overview of the treatment options for depression and analyze the treatment plan implemented in the real-life case study.
Overview of the treatment options available for depression
Treatment for depression typically involves a combination of approaches tailored to the individual’s needs. The two primary treatment modalities for depression are psychotherapy (talk therapy) and medication. Psychotherapy aims to help individuals explore their thoughts, emotions, and behaviors, while medication can help alleviate symptoms by restoring chemical imbalances in the brain.
Common forms of psychotherapy used in the treatment of depression include cognitive-behavioral therapy (CBT), interpersonal therapy (IPT), and psychodynamic therapy. These therapeutic approaches focus on addressing negative thought patterns, improving relationship dynamics, and gaining insight into underlying psychological factors contributing to depression.
In cases where medication is utilized, selective serotonin reuptake inhibitors (SSRIs) are commonly prescribed. These medications help rebalance serotonin levels in the brain, which are often disrupted in individuals with depression. Other classes of antidepressant medications, such as serotonin-norepinephrine reuptake inhibitors (SNRIs) or tricyclic antidepressants (TCAs), may be considered in specific cases.
Exploring the treatment plan implemented in the case study
In Jane’s case, a comprehensive treatment plan was developed with the intention of addressing her specific needs and symptoms. Recognizing the severity of her depression, Jane’s healthcare team recommended a combination of talk therapy and medication.
Jane began attending weekly sessions of cognitive-behavioral therapy (CBT) with a licensed therapist. This form of therapy aimed to help Jane identify and challenge negative thought patterns, develop coping strategies, and cultivate more adaptive behaviors. The therapeutic relationship provided Jane with a safe space to explore and process her emotions, ultimately helping her regain a sense of control over her life.
In conjunction with therapy, Jane’s healthcare provider prescribed an SSRI medication to assist in managing her symptoms. The medication was carefully selected based on Jane’s specific symptoms and medical history, and regular follow-up appointments were scheduled to monitor her response to the medication and adjust the dosage if necessary.
Analyzing the effectiveness of the treatment approach
The effectiveness of treatment for depression varies from person to person, and it often requires a period of trial and adjustment to find the most suitable intervention. In Jane’s case, the combination of cognitive-behavioral therapy and medication proved to be beneficial. Over time, she reported a reduction in her depressive symptoms, an improvement in her overall mood, and increased ability to engage in activities she once enjoyed.
It is important to note that the treatment journey for depression is not always linear, and setbacks and challenges may occur along the way. Each individual responds differently to treatment, and adjustments might be necessary to optimize outcomes. Continuous communication between the individual and their healthcare team is crucial to addressing any concerns, monitoring progress, and adapting the treatment plan as needed.
By analyzing the treatment approach in the real-life case study, we gain insights into the various treatment options available for depression and how they can be tailored to meet individual needs. The combination of psychotherapy and medication offers a holistic approach, addressing both psychological and biological aspects of depression.
The Outcome and Lessons Learned
After undergoing treatment for depression, it is essential to assess the outcome and draw valuable lessons from the case study. In this section, we will discuss the progress made by the individual in the case study, examine the challenges faced during the treatment process, and identify key lessons learned.
Discussing the progress made by the individual in the case study
Throughout the treatment process, Jane experienced significant progress in managing her depression. She reported a reduction in depressive symptoms, improved mood, and a renewed sense of hope and purpose in her life. Jane’s active participation in therapy, combined with the appropriate use of medication, played a crucial role in her progress.
Furthermore, Jane’s support network of family and friends played a significant role in her recovery. Their understanding, empathy, and support provided a solid foundation for her journey towards improved mental well-being. This highlights the importance of social support in the treatment and management of depression.
Examining the challenges faced during the treatment process
Despite the progress made, Jane faced several challenges during her treatment journey. Adhering to the treatment plan consistently proved to be difficult at times, as she encountered setbacks and moments of self-doubt. Additionally, managing the side effects of the medication required careful monitoring and adjustments to find the right balance.
Moreover, the stigma associated with mental health continued to be a challenge for Jane. Overcoming societal misconceptions and seeking help required courage and resilience. The case study underscores the need for increased awareness, education, and advocacy to address the stigma surrounding mental health conditions.
Identifying the key lessons learned from the case study
The case study offers valuable lessons that can inform the treatment and support of individuals with depression:
1. Holistic Approach: The combination of psychotherapy and medication proved to be effective in addressing the psychological and biological aspects of depression. This highlights the need for a holistic and personalized treatment approach.
2. Importance of Support: Having a strong support system can significantly impact an individual’s ability to navigate through depression. Family, friends, and healthcare professionals play a vital role in providing empathy, understanding, and encouragement.
3. Individualized Treatment: Depression manifests differently in each individual, emphasizing the importance of tailoring treatment plans to meet individual needs. Personalized interventions are more likely to lead to positive outcomes.
4. Overcoming Stigma: Addressing the stigma associated with mental health conditions is crucial for individuals to seek timely help and access the support they need. Educating society about mental health is essential to create a more supportive and inclusive environment.
By drawing lessons from this real-life case study, we gain insights that can improve the understanding and treatment of depression. Recognizing the progress made, understanding the challenges faced, and implementing the lessons learned can contribute to more effective interventions and support systems for individuals facing depression.In conclusion, this article has explored the significance of mental health case studies in understanding and addressing depression, focusing on a real-life example. By delving into case studies, we gain a deeper appreciation for the complexities of depression and the profound impact it has on individuals and society.
Through our examination of the selected case study, we have learned valuable lessons about the nature of depression and its treatment. We have seen how the combination of psychotherapy and medication can provide a holistic approach, addressing both psychological and biological factors. Furthermore, the importance of social support and the role of a strong network in an individual’s recovery journey cannot be overstated.
Additionally, we have identified challenges faced during the treatment process, such as adherence to the treatment plan and managing medication side effects. These challenges highlight the need for ongoing monitoring, adjustments, and open communication between individuals and their healthcare providers.
The case study has also emphasized the impact of stigma on individuals seeking help for depression. Addressing societal misconceptions and promoting mental health awareness is essential to create a more supportive environment for those affected by depression and other mental health conditions.
Overall, this article reinforces the significance of case studies in advancing our understanding of mental health conditions and developing effective treatment strategies. Through real-life examples, we gain a more comprehensive and empathetic perspective on depression, enabling us to provide better support and care for individuals facing this mental health challenge.
As we conclude, it is crucial to emphasize the importance of continued research and exploration of mental health case studies. The more we learn from individual experiences, the better equipped we become to address the diverse needs of those affected by mental health conditions. By fostering a culture of understanding, support, and advocacy, we can strive towards a future where individuals with depression receive the care and compassion they deserve.
Similar Posts

Exploring the World of Bipolar Bingo: Understanding the Game and Its Connection to Bipolar Disorder
Imagine a world where a game of chance holds the power to bring solace and support to individuals affected by bipolar disorder. A seemingly unlikely connection, but one that exists nonetheless. Welcome to the world of…

Can Anxiety Disorders be Genetic? Exploring the Hereditary Aspects of Anxiety Disorders
Introduction to Genetic Factors in Anxiety Disorders Understanding anxiety disorders Anxiety disorders are a common mental health condition that affect millions of individuals worldwide. People with anxiety disorders experience overwhelming feelings of fear, worry, and unease…

Understanding the Abbreviations and Acronyms for Bipolar Disorder
Living with bipolar disorder can be a challenging journey. The constant rollercoaster of emotions, the highs and lows, can take a toll on a person’s mental and emotional well-being. But what if there was a way…

The Connection Between Ice Cream and Depression: Can Ice Cream Really Help?
Ice cream has long been hailed as a delightful treat, bringing joy to people of all ages. It is often associated with carefree summer days, laughter, and moments of pure bliss. But what if I told…

Understanding the Relationship Between Bipolar Disorder and PTSD
Imagine living with extreme mood swings that can range from feeling on top of the world one day to plunging into the depths of despair the next. Now add the haunting memories of a traumatic event…

The Connection Between Meth and Bipolar: Understanding the Link and Seeking Treatment
It’s a tangled web of interconnectedness – the world of mental health and substance abuse. One doesn’t often think of them as intertwined, but for those battling bipolar disorder, the link becomes all too clear. And…
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.15(3); 2023 Mar
- PMC10097604

A Case Study of Depression in High Achieving Students Associated With Moral Incongruence, Spiritual Distress, and Feelings of Guilt
Bahjat najeeb.
1 Institute of Psychiatry, Rawalpindi Medical University, Rawalpindi, PAK
Muhammad Faisal Amir Malik
Asad t nizami, sadia yasir.
Psychosocial and cultural factors play an important, but often neglected, role in depression in young individuals. In this article, we present two cases of young, educated males with major depressive disorder and prominent themes of guilt and spiritual distress. We explore the relationship between moral incongruence, spiritual distress, and feelings of guilt with major depressive episodes by presenting two cases of depression in young individuals who were high-achieving students. Both cases presented with low mood, psychomotor slowing, and selective mutism. Upon detailed history, spiritual distress and feelings of guilt due to internet pornographic use (IPU) and the resulting self-perceived addiction and moral incongruence were linked to the initiation and progression of major depressive episodes. The severity of the depressive episode was measured using the Hamilton Depression Scale (HAM-D). Themes of guilt and shame were measured using the State of Guilt and Shame Scale (SSGS). High expectations from the family were also a source of stress. Hence, it is important to keep these factors in mind while managing mental health problems in young individuals. Late adolescence and early adulthood are periods of high stress and vulnerabe to mental illness. Psychosocial determinants of depression in this age group generally go unexplored and unaddressed leading to suboptimal treatment, particularly in developing countries. Further research is needed to assess the importance of these factors and to determine ways to mitigate them.
Introduction
More attention needs to be paid to the psychological and societal factors which precipitate, prolong, and cause a relapse of depression in high-achieving young individuals. A young, bright individual has to contend with the pressures of -- often quite strenuous -- moral and financial expectations from the family, moral incongruence, spiritual distress, and feelings of guilt.
Moral incongruence is the distress that develops when a person continues to behave in a manner that is at odds with their beliefs. It may be associated with self-perceptions of addictions, including, for example, to pornographic viewing, social networking, and online gaming [ 1 ]. Perceived addiction to pornographic use rather than use is related to the high incidence of feelings of guilt and shame and predicts religious and spiritual struggle [ 2 - 3 ]. Guilt is a negative emotional and cognitive experience that occurs when a person believes that they have negated a standard of conduct or morals. It is a part of the diagnostic criteria for depression and various rating scales for depressive disorders [ 4 ]. Generalized guilt has a direct relationship with major depressive episodes. Guilt can be a possible target for preventive as well as therapeutic interventions in patients who experience major depressive episodes [ 5 ].
We explored the relationship between moral incongruence, spiritual distress, and feelings of guilt with major depressive episodes in high-achieving students. Both patients presented with symptoms of low mood, extreme psychomotor slowing, decreased oral intake, decreased sleep, and mutism. The medical evaluation and lab results were unremarkable. The severity of depressive episodes was measured using the Hamilton Depression Scale (HAM-D). Themes of guilt and shame were measured by using the State of Guilt and Shame Scale (SSGS). This case study was presented as a poster abstract at the ‘RCPsych Faculty of General Adult Psychiatry Annual Conference 2021.’
Case presentation
A 25-year-old Sunni Muslim, Punjabi male educated till Bachelors presented with a one-month history of fearfulness, weeping spells during prolonged prostration, social withdrawal, complaints of progressively decreasing verbal communication to the extent of giving nods and one-word answers, and decreased oral intake. His family believed that the patient's symptoms were the result of ‘Djinn’ possession. This was the patient’s second episode. The first episode was a year ago with similar symptoms of lesser severity that resolved on its own. Before being brought to us, he had been taken to multiple faith healers. No history of substance use was reported. Psychosexual history could not be explored at the time of admission. His pre-morbid personality was significant for anxious and avoidant traits.
On mental state examination (MSE), the patient had psychomotor retardation. He responded non-verbally, and that too slowly. Once, he wept excessively and said that he feels guilt over some grave sin. He refused to explain further, saying only that ‘I am afraid of myself.’ All baseline investigations returned normal. His score on the Hamilton Depression Rating Scale (HAM-D) was 28 (Very Severe). A diagnosis of major depressive disorder was made. The patient was started on tab sertraline 50 mg per day and tab olanzapine 5 mg per day. After the second electroconvulsive therapy (ECT), his psychomotor retardation improved and he began to open up about his stressors. His HAM-D score at this time was 17 (moderate). He revealed distress due to feelings of excessive guilt and shame due to moral incongruence secondary to internet pornography use (IPU). The frequency and duration of IPU increased during the last six months preceding current illness. That, according to him, led him to withdraw socially and be fearful. He felt the burden of the high financial and moral expectations of the family. He complained that his parents were overbearing and overinvolved in his life. His family lacked insight into the cause of his illness and had difficulty accepting his current state. All these factors, particularly spiritual distress, were important in precipitating his illness. He scored high on both the shame and guilt domains (14/25, and 20/25, respectively) of the State of Shame and Guilt Scale (SSGS).
He was discharged after three weeks following a cycle of four ECTs, psychotherapy, and psychoeducation of the patient and family. At the time of discharge, his HAM-D score was 10 (mild) and he reported no distress due to guilt or feeling of shame. He has been doing well for the past 5 months on outpatient follow-up.
A 21-year-old Sunni Muslim, Punjabi male, high-achieving student of high school presented with low mood, low energy, anhedonia, weeping spells, decreased oral intake, decreased talk, and impaired biological functions. His illness was insidious in onset and progressively worsened over the last 4 months. This was his first episode. He was brought to a psychiatric facility after being taken to multiple faith healers. Positive findings on the MSE included psychomotor slowing, and while he followed commands, he remained mute throughout the interview. Neurological examination and laboratory findings were normal. His score on HAM-D was 24 (very severe). He was diagnosed with major depressive disorder and started on tab lorazepam 1 mg twice daily with tab mirtazapine 15 mg which was built up to 30 mg once daily. He steadily improved, and two weeks later his score on HAM-D was 17 (moderate). His scores on SSGS signified excessive shame and guilt (16/25, and 21/25; respectively). He communicated his stressors which pertained to the psychosexual domain: he started masturbating at the age of 15, and he felt guilt following that but continued to do so putting him in a state of moral incongruence. He perceived his IPU as ‘an addiction’ and considered it a ‘gunahe kabira’ (major sin) and reported increased IPU in the months leading to the current depressive episode leading to him feeling guilt and anguish. Initially, during his illness, he was taken to multiple faith healers as the family struggled to recognize the true nature of the disease. Their understanding of the illness was of him being under the influence of ‘Kala Jadu’ (black magic). His parents had high expectations of him due to him being their only male child. After 3 weeks of treatment and psychotherapy, his condition improved and his HAM-D score came out to be 08 (mild). He was discharged on 30 mg mirtazapine HS and seen on fortnightly visits. His guilt and shame resolved with time after the resolution of depressive symptoms and counseling. We lost the follow-up after 6 months.
Late adolescence and young adulthood can be considered a unique and distinct period in the development of an individual [ 6 ]. It is a period of transition marked by new opportunities for development, growth, and evolution, as well as bringing new freedom and responsibilities. At the same time, this period brings interpersonal conflicts and an increased vulnerability to mental health disorders such as depression and suicidality. Biological, social, and psychological factors should all be explored in the etiology of mental health problems presenting at this age [ 6 ].
Socio-cultural factors played a significant role in the development and course of disease in our patients, and these included the authoritarian parenting style, initial lack of awareness about psychiatric illnesses causing a delay in seeking treatment, high expressed emotions in the family, and the burden of expectations from the family and the peer group. The strict and often quite unreasonable societal and family expectations in terms of what to achieve and how to behave and the resultant onus on a high-scoring, bright young individual make for a highly stressful mental state.
We used the ICD-10 criteria to diagnose depression clinically in our patients and the HAMD-17 to measure the severity of symptoms [ 7 ]. Both our patients had scores signifying severe depression initially. Psychomotor retardation was a prominent and shared clinical feature. Psychomotor retardation is the slowing of cognitive and motor functioning, as seen in slowed speech, thought processes, and motor movements [ 8 - 9 ]. The prevalence of psychomotor retardation in major depressive disorder ranges from 60% to 70% [ 10 ]. While psychomotor retardation often responds poorly to selective serotonin reuptake inhibitors (SSRI), both tricyclic antidepressants (TCAs) and noradrenergic and specific serotonergic antidepressants (NaSSA) produce a better response [ 9 , 11 ]. In addition, ECT shows a high treatment response in cases with significant psychomotor retardation [ 11 - 12 ].
A growing body of evidence shows that shame and guilt are features of numerous mental health problems. Guilt is the negative emotional and cognitive experience that follows the perception of negating or repudiating a set of deeply held morals [ 4 ]. Guilt can be generalized as well as contextual and is distinct from shame [ 13 ]. The distinction between guilt and shame allows for an independent assessment of the association of both guilt and shame with depressive disorder. As an example, a meta-analysis of 108 studies including 22,411 individuals measuring the association of shame and guilt in patients with depressive disorder found both shame and guilt to have a positive association with depressive symptoms. This association was stronger for shame (r=0.43) than for guilt (r=0.28) [ 14 ]. In our study, we used the State of Shame and Guilt Scale (SSGS), to measure the feelings of guilt and shame [ 15 ]. The SSGS is a self-reported measure and consists of 5 items each for subsets of guilt and shame. SSGS scores showed high levels of guilt and shame in both of our patients.
During the course of treatment, we paid special attention to the psychological, cultural, and social factors that likely contributed to the genesis of the illness, delayed presentation to seek professional help, and could explain the recurrence of the depressive episodes. In particular, we observe the importance, particularly in this age group, of family and societal pressure, spiritual distress, moral incongruence, and feelings of guilt and shame. Moral incongruence is when a person feels that his behavior and his values or judgments about that behavior are not aligned. It can cause a person to more negatively perceive a behavior. As an example, the presence of moral congruence in an individual is a stronger contributor to perceiving internet pornographic use (IPU) as addictive than the actual use itself [ 16 ]. Therefore, moral congruence has a significant association with increased distress about IPU, enhanced psychological distress in general, and a greater incidence of perceived addiction to IPU [ 16 ].
Self-perceived addiction is an individual’s self-judgment that he or she belongs to the group of addicts. The pornography problems due to moral incongruence (PPMI) model is one framework that predicts the factors linking problematic pornographic use with self-perceived addiction. This model associates moral incongruence with self-perceived addiction to problematic pornographic use [ 17 ]. A recent study on the US adult population also showed a high association of guilt and shame with moral incongruence regarding IPU [ 18 ]. Another factor of importance in our patients was spiritual distress, which is the internal strain, tension, and conflict with what people hold sacred [ 19 ]. Spiritual distress can be intrapersonal, interpersonal, or supernatural [ 20 ]. Research indicates that IPU causes people to develop spiritual distress that can ultimately lead to depression [ 16 - 17 ].
Conclusions
In both our cases the initial presentation was that of psychomotor slowing, selective mutism, and affective symptoms of low mood, therefore, a diagnosis of depressive illness was made. One week into treatment, improvement was noted both clinically as well as on the psychometric scales. Upon engaging the patients to give an elaborate psychosexual history, moral incongruence, spiritual distress, and feelings of guilt, linked particularly to self-perceived addiction to IPU were found. Sensitivity to the expectations of the parents, the cognizance of failing them because of illness, and their own and family’s lack of understanding of the situation were additional sources of stress. Hence, it is imperative to note how these factors play an important role in the initiation, progression, and relapse of mental health problems in young individuals.
Acknowledgments
We are thankful to the participants of this study for their cooperation.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study

IMAGES
VIDEO
COMMENTS
Case Study 142 Depressed Patient with Suicidal Thoughts. ... signs of depression in the older adult include hopelessness, changes in sleeping and or eating patterns, worthlessness, lonliness, diminished pleasure in activities, and indeciciveness. CASE STUDY PROGRESS.
There should not be a loss of desire or interest in activities or even thoughts of death. In order to tell the difference between old age and depression, the characteristics and risk factors of the illness should be assessed to fully determine. 6. List five of the most common signs of depression in the older adult. 1.
Case Study 142. Scenario You are working the day shift on a medical inpatient unit. You are discussing discharge instructions with J., an 86-year-old man who was admitted for mitral valve repair. His serum blood glucose had been averaging 250 mg/dL or higher for the past several months.
Data from the Sequential Treatment Alternatives to Relieve Depression (STAR*D) study, ... novel and effective antidepressants 142 ... Case, B. G. Association of electroconvulsive therapy with ...
View Case Study 142 Depressed Patient with Suicidal Thoughts.docx from NUR MISC at Florida State College at Jacksonville. Case Study 142 Depressed Patient with Suicidal Thoughts 1. ... View Depression case study.docx from PSY 2012 at Palm Beach State College. Depression C... Case Study2.pdf. Texas A&M University, Kingsville. PHARMACY PHARM 810.
Sara, a 35-year-old married female. Sara was referred to treatment after having a stillbirth. Sara showed symptoms of grief, or complicated bereavement, and was diagnosed with major depression, recurrent. The clinician recommended interpersonal psychotherapy (IPT) for a duration of 12 weeks. Bleiberg, K.L., & Markowitz, J.C. (2008).
Case Study 142 Depressed Patient with Suicidal Thoughts 1. If he has a plan to commit suicide and if he has the means to carry out his plan 2. Lack of social connectedness, age, depression, and stress 3. Mood disorders, personality disorders, schizophrenia 4. Assess for depressed mood; diminished interest or pleasure in activities; poor appetite or overeating; insomnia or hypersomnia; fatigue ...
Since the turn of the century, numerous studies have demonstrated the efficacy of VNS in resistant depression[140-142]. However, only one randomized, double-blind, controlled trial comparing VNS with usual medical treatment has been conducted over a short period of 10 wk[ 141 ].
When a patient with depression is feeling sleepy, be aware of sleep apnoea. A 67-year-old man was referred to an outpatient clinic of geriatric psychiatry because of persistent symptoms of depression and anxiety, accompanied by sleepiness. The latter had been evaluated multiple times in the general practice over several years; each time it was ...
Studies on depression have increased significantly over the past few decades. However, the literature remains fragmented and the interpretation of heterogeneous findings across studies and between fields is difficult. The cross-pollination of ideas between disciplines, such as genetics, neurology, immunology, and psychology, is limited.
Case Study 142 Depressed Patient with Suicidal Thoughts Difficulty: Beginning Setting: Inpatient medicine unit Index Words: suicidal ideation, suicide methods, depression, electroconvulsive therapy (ECT), selective serotonin reuptake inhibitors (SSRIs) Giddens Concepts: Clinical Judgment, Coping, Mood and Affect HESI Concepts: Assessment, Clinical Decision Making—Clinical Judgment, Mood ...
Patient Case Presentation. Figure 1. Blue and silver stethoscope (Pixabay, N.D.) Ms. S.W. is a 48-year-old white female who presented to an outpatient community mental health agency for evaluation of depressive symptoms. Over the past eight weeks she has experienced sad mood every day, which she describes as a feeling of hopelessness and emptiness.
A 37-year-old woman was admitted to the hospital because of suicidal ideation during the Covid-19 pandemic. She had presented with symptoms consistent with Covid-19 on two previous occasions and ha...
Study design and study population. We designed a case-control study focusing on patients with a diagnosis of unipolar depression (F32x, F33x or F34.1) in the year 2017 aged ≥15 years. Cases of depression were categorized into mild, moderate and severe based on the documented diagnostic code according to the ICD-10 (Table 1).
the case study had a therapist who was a doctoral level graduate student in clinical psychology trained in CBT who received weekly supervision from a licensed clinical psychologist with a Ph.D. Qualitative data for this case study were analyzed by reviewing progress notes and video recordings of therapy sessions. SESSIONS 1-4
Case Studies for Midterm. 17 terms. celeste122567. Preview. Study with Quizlet and memorize flashcards containing terms like Characteristics that make patient at high risk for suicide, Psychiatric disorders that can result in suicidal ideation or gestures:, Depression vs Illness: depression and more.
In a study of 239 outpatients diagnosed with major depressive disorder in a NIMH. 16-week multi-center clinical trial, participants were assigned to interpersonal therapy, CBT, imipramine with clinical management, or placebo with clinical management. One. hundred sixty-two patients completed the trial.
Enhanced Document Preview: Case Study 142: Depressed Patient with Suicidal Thoughts SCENARIO: You are working the day shift on a medical inpatient unit. You are discussing discharge instructions with J.B., an 86-year-old man who was admitted for mitral valve repair.
Introduction to the selected case study. In this case study, we will focus on Jane, a 32-year-old woman who has been struggling with depression for the past two years. Jane's case offers a compelling narrative that highlights the various aspects of depression, including its onset, symptoms, and the treatment journey.
Study with Quizlet and memorize flashcards containing terms like Pt is 52 AAF, widowed accompanied to ED with daughter. Pt's grooming/fair, but overall disheveled. Pt's motor activity is slow, she rarely makes eye contact, and her responses to questions are slow and barely audible. When asked what she prefers to be called, "I don't care.", The nurse completes a physical assessment. When asked ...
current issue. current issue; browse recently published; browse full issue index; learning/cme
Individuals with depression often face problems in activities of daily living, work functioning and interpersonal relationships. Aim and Objectives: The present case study aimed to assess psychosocial problems and to provide psychiatric social work intervention based on cognitive behaviour therapy (CBT) to the client. Methods and materials: The ...
The severity of depressive episodes was measured using the Hamilton Depression Scale (HAM-D). Themes of guilt and shame were measured by using the State of Guilt and Shame Scale (SSGS). This case study was presented as a poster abstract at the 'RCPsych Faculty of General Adult Psychiatry Annual Conference 2021.'
They analysed data from 14,056 participants in the German Ageing Survey, which includes people born between 1911 and 1974. Study participants were surveyed up to eight times over 25 years and ...
When treated by a female doctor, female patients had a mortality rate of 8.15 per cent compared to an 8.38 per cent rate when a male doctor treated them. While a "large and clinically meaningful ...