An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Springer Nature - PMC COVID-19 Collection


Processing strategies to improve the breadmaking potential of whole-grain wheat and non-wheat flours
Tamara dapčević-hadnađev.
University of Novi Sad, Institute of Food Technology, Bulevar cara Lazara 1, 21000 Novi Sad, Serbia
Jelena Tomić
Dubravka Škrobot, bojana Šarić, miroslav hadnađev, associated data.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Not applicable.
Strategies to increase the bio-functionality of staple food, such as bread, by incorporating whole-grain wheat flour or flour from other, non-wheat grains instead of refined wheat flour are often constrained with the lack of their techno-functionality, despite the associated beneficial effect on consumers' health and well-being. Most of the available studies investigating the possibilities to improve technological and sensory quality of bread prepared using whole-grain wheat and non-wheat flours still rely on formulation approaches in which different additives and novel ingredients are used as structuring agents. Less attention has been given to technological approaches which could be applied to induce structural changes on biopolymer level and thus increase the breadmaking potential of whole grains such as: modification of grain and biopolymers structure by germination, flour particle size reduction, dry-heat or hydrothermal treatment, atmospheric cold plasma, high-pressure processing or ultrasound treatment. Strategies to modify processing variables during breadmaking like dough kneading and hydration modification, sourdough fermentation or non-conventional baking techniques application are also poorly exploited for bread preparation from non-wheat grains. In this paper, the challenges and opportunities of abovementioned processing strategies for the development of bread with whole-wheat flours and non-wheat flours from underutilised gluten-containing or gluten-free cereals and pseudocereals will be reviewed throughout the whole breadmaking chain: from grain to bread and from milling to baking. Feasibility of different strategies to increase the technological performance and sensory quality of bread based on whole-grain wheat flours or flours from other, non-wheat grains will be addressed considering both the environmental, safety and nutritive advantages.
Introduction
Bread, regardless of the type, production process and geographical origin, is traditionally produced from refined common wheat ( Triticum aestivum ) flour. However, in recent years, there has been renewed interest in fortifying or replacing refined wheat flour with whole-grain wheat flour, or flour from gluten-free cereals (rice, maize, sorghum, millet), pseudocereals (amaranth, buckwheat, quinoa) and ancient cereals [ 1 , 2 ]. This trend is governed with different reasons: from health-conscious and eco-friendly to economically driven.
Unlike refined wheat flour, whole-grain cereals and pseudocereals possess dense nutritional composition and a range of bioactive compounds. Therefore, their consumption contributes to increased intake of micronutrients, dietary fibres, phenolics, etc. Several studies have shown that regular consumption of whole-grain cereals is associated with health benefits such as a lower risk of chronic-degenerative diseases and improved body weight regulation [ 3 ]. Additionally, gluten-free cereals are finding an increased demand since coeliac disease or other gluten-associated allergies incidence rates are raising over time [ 4 ]. On the other hand, in developing countries, utilization of indigenous grain crops (the case of millet in Africa) is promoted. This contributes to economic development of local agriculture sector through reducing reliance on wheat importation and ensuring food security. Utilization of 'zero km' ingredients and relevance of short food supply chains in increasing the access to healthy and sustainable food has particularly growing attention in crisis situation such as COVID-19 pandemic [ 5 , 6 ].
Despite their contribution to consumers' well-being, sustainability of cereal cultivation and biodiversity protection, whole-grain alternative cereals exploitation in breadmaking is still being diminished due to the lower technological quality compared to refined wheat. The major challenges encountered in whole-grain or non-wheat cereals incorporation in breadmaking are poor gas retention, low loaf volume, hard and/or crumbling crumb texture, altered colour, short shelf-life of bread. This could be related to dilution or absence of gluten complex responsible for viscoelastic properties of dough and/or water competition effect between fibres and gluten [ 1 , 7 ]. The abovementioned quality deficiencies are often coupled with the lower consumers' acceptance of the product sensory properties. The most common sensory attributes of whole-grain and non-wheat cereal-based products are nutty odour, pungent flavour, bitter/astringent/sour taste; associated with the presence of phenolic compounds and in particular the condensed tannins which are located in the outermost bran layers [ 6 ]. In addition, lipid-rich cereals, such as oat, are susceptible to lipid oxidation which leads to development of the undesired sensory attributes evaluated as musty and earthy odour and bitter and rancid flavour [ 8 ]. Generally, altered technological quality (product volume, texture, structure, etc.) and sensory attributes of whole-grain and non-wheat cereal based products represent a limitation in their widespread acceptance.
Different strategies are thus proposed to produce bread from whole-grain and non-wheat cereals with technological and sensory profile comparable to refined wheat bread, while preserving their nutritional value. The most commonly applied strategies are the once involving bread formulation optimization through inclusion of various improvers, such as vital gluten or texturing agents (e.g. hydrocolloids, emulsifiers, enzymes and different food additives) that could act as structure forming agents instead of diluted or absent gluten [ 9 , 10 ]. In order to contribute to 'clean label' products design as well as its cost-effectiveness, some researches have modified abovementioned compositional approach by replacing food additives with fibre rich raw materials or food processing by-products to overcome the gluten deficiency [ 11 , 12 ].
However, relatively little research has been conducted on technological approaches for improving breadmaking potential of whole-grain and non-wheat cereals. As noted by Parenti et al. [ 1 ] instead of modifying process variables to prepare unrefined wheat flour bread, most of the studies are adopting the same methods as for their counterparts prepared with refined flour.
Therefore, the aim of this review is to provide a critical opinion on current and future-looking sustainable technological innovations and strategies utilized to increase the technological performance and sensory quality of bread based on whole-grain and non-wheat cereals. Improvement strategies discussed in this paper encompassed the whole bread production chain (Fig. 1 ): from raw material (cereal, flour, etc.) to process (milling, kneading, leavening, baking, etc.) modification, considering both the environmental, safety and nutritive advantages related to the use of conventional and emerging technologies and approaches.

Summary of technological approaches for increased breadmaking potential of whole-grain wheat and non-wheat flours along the whole breadmaking chain
Strategies to modify raw material for breadmaking
Grain modification approaches, germination.
Modification of grain and biopolymers structure by germination is mostly performed to initiate nutrient compositional changes which are associated to health benefits. During the germination process degradation of macromolecules occurs due to increased enzyme activities: (i) starch is hydrolysed by amylolytic enzymes to maltose, glucose, dextrins and oligosaccharides, resulting in its higher digestibility [ 13 – 15 ]; (ii) storage proteins are degraded by endopeptidases produced from the aleurone layer and scutellum thus releasing peptides and free amino acids [ 15 – 18 ]; (iii) the ratio of soluble to insoluble dietary fibre increases especially when long germination times are applied [ 17 , 20 ]; (iv) a phytate (antinutrient present in cereals) content decreases as a result of increased phytase activity thus releasing chelated cations leading to increased bioavailability of phosphorus and minerals such as Zn 2+ , Fe 2+/3+ , Ca 2+ , Mg 2+ , Mn 2+ and Cu 2+ [ 13 ]. Moreover, germination process results in the increase in free fraction of phenolic acids due to decrease in the bound one contributing to increased antioxidant activity [ 13 , 15 , 18 , 19 ]. Germination is also a strategy to produce important metabolites such as γ-aminobutyric acid (GABA) [ 14 , 18 ], recommended to prevent neurological disorders [ 21 ].
Although increase in enzymatic activity produced by germination has mostly a detrimental effect on the breadmaking potential of cereals, with proper adjustment of the germination parameters it can be a promising tool to improve both the nutritional and technological properties of cereal-based food. In general, germination leads to softer and more fragile grain as a consequence of enzyme action which results in lower damaged starch content upon milling [ 22 ]. This, along with partial protein hydrolysis and decrease in insoluble fibre content, contribute to lower water absorption of flour from germinated wheat [ 17 ]. The germination also affects dough rheological properties in the following directions: (i) weakening of the gluten ability to form viscoelastic network due to decrease in the level of high-molecular-weight glutenin macropolymers which reflects in reduction of the tenacity, an increase of the extensibility of dough, and (ii) reduction of starch gelatinization and retrogradation ability as a result of hydrolysis [ 14 , 23 , 24 ].
However, shorter germination times, low substitution levels or addition of some improvers (vital wheat gluten) to germinated wheat flour could increase technological performance of whole-grain cereals [ 1 , 17 , 25 ]. Activation of slight amount of α-amylase will increase starch transformation to fermentable sugars thus promoting yeast fermentation, carbon dioxide production and increase in dough height during fermentation [ 26 , 27 ], which, along with increased dough extensibility, will contribute to gas cell expansion leading to bread loaves of higher specific volumes as evident from the study of Baranzelli et al. [ 14 ], Johnston et al. [ 28 ], Cardone et al. [ 29 ] and Bhinder et al. [ 18 ] (Table (Table1 1 ).
Impact of germination on the quality of leavened bakery products prepared from whole-grain wheat flour or flour from non-wheat grains
In addition, optimized α -amylase activity can improve the bread shelf-life and sensory attributes [ 17 ]. It was shown that due to restricted starch retrogradation, germination improved crumb softness for 200% after 24 h of storage even when whole-wheat flour was used [ 29 ]. Controlled germination can also yield a product of enhanced starch digestibility [ 15 ] and reduced glycaemic index [ 18 ]. Moreover, germinated whole-wheat breads had improved sensory attributes in comparison to their unsprouted counterparts thanks to their diminished bitterness and graininess, increased sweetness and moistness [ 25 , 28 ]. Breads with germinated wheat flour are also perceived as the ones with dark crust due to the presence of higher contents of reducing sugars that, combined with free amino acids, favoured the occurrence of a Maillard reaction [ 14 ].
Flour modification approaches
Particle size reduction (micronization).
Flour particle size can significantly alter bread functionality and technological quality. If a micronization, such as jet milling, is applied to produce fine wheat flour with extremely low particle size, flour with increased digestible starch content is obtained [ 30 ]. When used in breadmaking, jet milled flour slightly decreased bread glycaemic index.
However, it seems that pulverization of flour is not promising technology concerning bread technological quality since whole-grain wheat jet milled breads (flour volume median diameter = 17–53 μm) were characterized with reduced specific volume and moisture content and increased crumb hardness in comparison to breads with flour having volume median diameter of 84 μm [ 30 ]. The same relationship between flour mean particle size and technological performance was obtained for gluten-free flours. The flours having coarser particle size are the most suitable for making gluten-free maize bread. According to de la Hera et al. [ 31 ], the coarser maize flours (> 150 µm) resulted in breads with higher specific volume and lower crumb firmness than the ones with finer flour (< 106 µm), due to the higher availability of dough to retain the gas produced during fermentation. Concerning rice flour incorporation in breadmaking, de la Hera et al. [ 32 ] concluded that the coarse fraction combined with a high dough hydration was the most suitable combination for developing rice bread when considering the bread volume and crumb texture.
Heat treatment
Different flour heat treatments such as dry-heat treatment or hydrothermal treatments (below or above starch gelatinization temperature) are being increasingly applied to improve the functionality of alternative cereals flour. It was shown that dry-heat treated sorghum flour produced breads with increased specific volume and more cells per slice area. This was ascribed to increased viscosity of sorghum flour dough as a consequence of starch granule swelling due to heat induced partial gelatinization as well as denaturation of both proteins and enzymes [ 33 ]. In addition, protein denaturation and the partial gelatinization of starch granules, led to an increase in gas retention capacity and dough expansion, which all contributed to improvements in structure, strength and volume of dry-heated sorghum containing bread [ 34 ]. Since sorghum-based products are characterized with pungent off-notes, dry heat treatment can also be employed to improve sorghum bread sensory properties [ 35 ]. Dry heating was also promising in upgrading the quality of substandard flour for bread-making applications [ 36 ]. Mann et al. [ 37 ] have shown that heat treatment of flour causes the formation of gluten and starch aggregates and modifies interactions between gluten and starch. The effects were more pronounced in heat-treated flours with increased moisture content where higher mobility of the molecules is enabled.
It was also revealed that gluten-free flours (maize or rice) blanching results in doughs with higher consistency, adhesiveness, springiness and stickiness due to the partial gelatinisation of the starch, which further led to improved bread quality [ 38 , 39 ].
When flour/starch heating is carried out in the presence of water without fostering a complete starch gelatinization, as it is the case with annealing (treatments in excess or at intermediate water contents below the gelatinisation temperature) and heat-moisture treatment (exposure of starch to higher temperatures at very restricted moisture content), increase in the starch gelatinization temperature, water binding capacity and granule susceptibility to enzyme hydrolysis occurs [ 40 , 41 ]. These structural changes improve the volume of breads and their quality, since restricted hydrothermal treatments increase starch emulsifying ability and delay gelatinization which enhance air incorporation in doughs and prolong the period of loaf expansion [ 40 ].
It was shown that application of hydrothermally treated rice and maize flour to manufacture rice and maize semolina-based breads increased the specific volume and decreased the hardness and chewiness of the gluten-free breads, due to higher initial viscosity imparted by treated flours enabling the entrapment of air bubbles in the dough [ 42 ].
When hydrothermal treatments are performed above gelatinization temperature starch granules are irreversibly losing their integrity, a process known as pre-gelatinization [ 40 ]. Parenti et al. [ 43 ] reported an increase in the water absorption capacity, improved alveograph parameters, as well as bread volume, crumb softness and shelf life when pre-gelatinized brown flour (flour having approx. 85% extraction yield, maximum ash content of 0.95 g/100, heated at 1:4 flour to water ratio at 85 °C) was used. Jalali et al. [ 44 ] used microwave-induced pre-gelatinization of maize flour to produce gluten-free pan bread. The authors observed structural expansion and more swelling of the pre-gelatinized maize flour as compared to non-treated one, which consequently resulted in increased firmness of dough, decreased firmness of bread, increased bread crumb moisture, porosity, loaf specific volume and the overall acceptability.
If pre-gelatinization is achieved with the aid of extrusion cooking (flour/starch exposure to high temperatures and mechanical shearing with enough amount of water) besides amylose and amylopectin leaching from disrupter starch granule, breakage of the amylose and amylopectin chains, denaturation of proteins, enzyme (in)activation and Maillard reactions occurred [ 40 ]. Extrusion cooked flour behaves as thickening agent [ 45 ], which is considered as a more 'natural approach' to the use of hydrocolloids as improvers. Substitution of native rice flour by extruded rice flour improved bread volume and crumb structure, decreased initial hardness and delayed bread staling in gluten-free bread [ 46 ].
Atmospheric cold plasma
Atmospheric cold plasma (ACP) is a non-thermal processing technology that so far was applied at different stages of the cereal processing chain for a range of applications including improved germination, microbial decontamination, toxin degradation and biopolymer structural changes for improved functionality [ 47 ]. The mode of action results from plasma generated reactive species (reactive oxygen and nitrogen species), radicals and UV light [ 48 ]. It was revealed that reactive oxygen species generated during wheat flour cold plasma treatment influenced protein oxidation, promoted disulfide bond formation between glutenin proteins, that improved dough strength; led to starch depolymerization and decrease in its crystallinity. These biopolymer structural changes reflected in the increase in bread specific volume, enhancement of its appearance and porosity structure, as well as increase in bread crumb whiteness [ 49 – 51 ].
However, most of the studies investigating plasma-induced changes in grain/flour/dough structure are based on breadmaking potential of refined wheat flour, biopolymer changes in whole grain wheat or the safety aspects of plasma application for alternative grains decontamination. The studies concerning plasma application to enhance breadmaking performance of whole-grain or non-wheat cereals are scarce. Since some preliminary studies have shown that ACP treatment is effective just in increasing breadmaking potential of weak flours [ 52 ], some future studies should be conducted for better exploitation of ACP in whole-grain of gluten-free cereals modification. Moreover, combination of different technologies such as plasma-activated water and heat moisture treatment can also offer novel possibilities in alternative grains utilization in breadmaking [ 53 ].
Dough modification approaches
High-pressure processing.
High-pressure processing (HPP) represents novel processing technology which is mainly used for non-thermal treatment for fruit juices preservation [ 54 ]. Generally, in high-pressure processing, food is subjected to high pressures (usually above 200 MPa, without high temperature treatment) causing structural and textural changes besides microbial inactivation. These changes are mainly influenced by starch gelatinization and polymerization of proteins [ 55 ]. Therefore, this technology can be effectively employed for protein and starch functional properties modification [ 56 ]. Moreover, Kieffer et al. [ 57 ] revealed that high pressure treatment promotes protein network formation. Most of the papers using HPP in cereal technology is mainly focused on gluten-free raw material treatment due to poor technological properties of these materials i.e. the lack of protein network formation, poor gas retention properties, poor volume, acceptability etc. Generally, it was determined that HPP treatment resulted in starch gelatinization and protein polymerization induced by reaction of thiol-disulfide interchange. Consequently, the dough became more viscoelastic, showed better workability, increased water absorption capacity and had better gas retention properties which resulted in increased volume and improved texture of the final product [ 58 , 59 ]. Moreover, the obtained bakery products had improved shelf life [ 60 ] and slower hardening kinetics in comparison to control samples, due to starch gelatinization that occurred in this process. However, according to Vallons et al. [ 61 ] the increase in the addition of pressure treated flour over 10% resulted in lower specific volume and poorer final product quality.
Ultrasound treatment
Ultrasound treatment, as a non-thermal processing tool, has been intensively utilized for microbial and enzyme inactivation, bioactive component extraction and food components modification for increased functionality [ 62 ]. However, application of ultrasound to alter flour functionality and thus improve its breadmaking potential is quite scarce.
While it was shown that ultrasound modulation of flour functionality depends on the treatment time [ 62 , 63 ], there are opposite conclusions concerning the effect of the flour dispersion concentration. According to Vela et al. [ 63 ], effect of ultrasound treatment is independent on the concentration of the treated flour dispersion up to 30%, and in all the treated dispersions (5–30%) particle size of the rice flour was reduced. On the contrary, ultrasound treatment of buckwheat grains caused particles agglomeration in concentrated dispersions (1:5 and 1:2.5 solid:liquid ratio), while higher dilution (1:10) increased smaller particle size fractions [ 64 ].
In general, ultrasound treatment of whole-grain flour significantly increases water solubility, water absorption and swelling power of quinoa, buckwheat and rice flour [ 62 – 64 ]. It also influences starch crystallinity as recorded in the alterations of the flour thermal properties such as reduction of gelatinization enthalpy, increase in pasting temperature and gel strength [ 63 ], as well as in an increase in the in vitro starch digestibility [ 62 ]. However, effects on the flour pasting properties were found to be dependent on treatment time [ 62 ] and dispersion concentration [ 64 ], where lower treatment times [ 62 ] and medium concentrations [ 64 ] led to increase in peak viscosity, breakdown, and setback values.
Jalali et al. [ 44 ] have shown that ultrasound treatment of dough decreased the firmness of maize flour dough and bread, while increasing gluten-free bread specific volume, porosity, and the overall acceptability score. The observed improvement in bread technological, visual, and sensory properties was increased when combination of pre-gelatinization and ultrasound treatment of maize flour was applied [ 44 ].
Strategies to modify processing variables of the breadmaking phases
Dough kneading and hydration modification.
Flour transformation to dough is performed by hydration and mixing operations, where different processing variables can be modified in order to achieve optimum dough and bread quality. Appropriate water content and temperature ensure optimal dough rheology and consistency, avoiding undesired softening or hardening. Proper choice of mixing speed and temperature will avoid dough warming and excessive weakening, while kneading time management prevents both over- and under-mixing and allows dough aeration and its capacity to retain gases [ 5 ].
Water content influences dough quality in the following manner: adding too much water during kneading generates soft and sticky dough, while dough with water content below the optimal water absorption of the flour will be harder to knead [ 5 ]. Increase in total water content in dough from ancient grain flours increases dough extensibility, while it decreases dough tenacity and vice versa [ 65 ]. In the case of gluten-free ingredients, such as rice flour and hydroxypropyl methyl cellulose (HPMC), low hydrated doughs had low ability to retain gas released during proofing, unlike high hydrated doughs which endure longer fermentation time resulting in improved specific volume [ 66 ]. Therefore, different strategies are applied in order to increase water absorption and thus improve gluten-free bread quality. Due to the absence of gluten in gluten-free ingredients, increased water absorption is achieved through fibres/hydrocolloids addition or enzymatic or extrusion treatments to modify amount of water which will be untaken by starch in the early phases of breadmaking [ 67 , 68 ].
Gomez et al. [ 66 ] have also reported that low mixing speed and long mixing time led to gluten-free breads with higher specific volumes and softer texture.
Sourdough fermentation
Although being an ancient biotechnology, sourdough fermentation has gained renewed interest as a tool for better exploitation of non-wheat cereals in breadmaking [ 69 ]. Sourdough can be described as a mixture of flour and water fermented by lactic acid bacteria (LAB) or LAB in combination with yeasts, either spontaneous or inoculated [ 70 ]. The positive effects of sourdough application in breadmaking are associated with the metabolic activities of the LAB and yeasts, such as acidification, production of exopolysaccharides, proteolytic, amylolytic and phytase activity, and production of volatile and antimicrobial substances [ 71 ].
Beside the fact that sourdough fermentation contributes to enhanced nutritional properties of bread (higher free amino acids concentrations, soluble fibre, γ-aminobutyric acid, total phenols and antioxidant activities) and phytic acid reduction, leading to increased mineral, protein and free amino acids bioavailability; it has significant impact on bread techno-functionality [ 6 , 72 ].
Taking advantage of LAB ability to produce certain polymers and modify the main structure-building components of flour such as starch, arabinoxylans and proteins, sourdough fermentation was used to improve dough and bread technological properties such as loaf volume, water absorption of the dough, dough rheology and machinability [ 73 ]. Certain LAB strains produce exopolysaccharides that due to their water-binding ability act as hydrocolloids or gums, and could be considered as gluten mimetics in gluten-free products [ 74 ] in order to improve product texture. In gluten containing flours, organic acids produced by LAB enhance the solubility of the glutenin fraction and improve the swelling power of the gluten, which increase gas retention during fermentation [ 73 ]. Gluten complex structural changes are associated with dough acidification which may also activate some endogenous flour enzymes such as proteases that can hydrolyse gluten under appropriate fermentation conditions and bacteria selection. Gobbetti et al. [ 75 ] suggested that degradation of prolamins of wheat and rye during fermentation by selected sourdough lactic acid bacteria can represent a possibility to use these cereals in the gluten-free diet.
On the contrary, reports on the fate of starch during sourdough fermentation are contradictory. In the case of the wholegrain wheat flour, sourdough fermented bread exhibited higher resistant starch content and lower glycaemic response than the corresponding products leavened with S. cerevisiae [ 76 ]. However, sourdough with a commercial starter added to a gluten-free formulation decreased the glycaemic response in vivo less effective than in wheat sourdough bread. This was explained with lower concentrations of organic acids in gluten-free than in wheat sourdough. In sourdough wheat breads pH decrease upon formation of organic acids led to inhibition of α-amylase and consequently, a decrease in starch hydrolysis. On the contrary, the pH in gluten-free sourdoughs might still be sufficient for α-amylase to proceed with degradation of starch and increase in starch hydrolysis degree [ 77 ].
The effect of sourdough fermentation on techno-functionality of bread prepared with alternative cereals is summarized in Table Table2. 2 . As it can be seen from Table Table2, 2 , the effect of sourdough addition on bread technological performance largely depends on sourdough type, LAB strain and presence of Saccharomyces cerevisiae.
Effect of sourdough fermentation on the quality of bread prepared from whole-grain wheat flour or flour from non-wheat grains
Besides bread technological quality, organic acids together with other LAB metabolites (e.g. CO 2 , ethanol, diacetyl, hydrogen peroxide, fatty acids, reuterin, fungicin, etc.) also contribute to bread preservation thus prolonging its shelf life [ 54 ]. Sourdough was also successfully applied in a sugar reduced bakery product, owning to sourdough bacteria ability to produce polyols [ 87 ]. Because of the synthesis of flavouring amino acids during fermentation, the sourdough efficiently masks salt reduction in bakery products without affecting taste and other quality parameters [ 88 ].
Non-conventional baking techniques
Another interesting approach to improve the breadmaking potential of alternative cereals is to apply a non-conventional baking technique such as vacuum, microwave, infrared, jet-impingement, ohmic or a combination of them (hybrid heating).
In comparison to conventional, partial-vacuum baking of gluten-free bread did not have significant impact on bread volume and texture; however, it resulted in product which became stale more slowly than the control [ 89 ].
Microwave and infrared baking are considered as time- and cost-efficient processes. Although microwave and microwave-assisted hot air baking increase gluten-free bread crumb hardness and result in pale bread crust compared with the hot air baking, it was shown that these techniques can reduce the digestibility of starch and glycaemic index of the bread and increase loaf volume [ 90 ].
Application of single infrared radiation (halogen lamp as NIR source) results mostly in products of inferior quality, due to the high rate of heating which influence sudden and thick crust formation and the prevention of the product expansion thus leading to lower specific volume and higher firmness values than conventional baking [ 91 , 92 ]. However, in the study of Shyu et al. [ 93 ] breads baked by IR had comparable quality in terms volume, water activity, staling rate, or sensory scores with conventionally baked ones.
Another novel baking technique, jet impinging, based on forced convection heating, increases the heat transfer efficiency during the baking process [ 94 ], but results in the formation of a thick crust as compared with infrared radiation and heating in a conventional household oven [ 95 ].
Ohmic heating is an innovative technology in which an alternating electrical current is passed through a material, generating heat by dissipation of the electrical energy due to material's own electrical resistance, allowing rapid and uniform heat distribution [ 54 ].
Bender et al. [ 96 ] have shown that gluten-free breads could benefit from the uniform rapid heating during processing, as these breads exhibit higher loaf volume, finer pore structure, reduced starch digestibility and higher resistant starch content compared to conventionally baked breads. Namely, rapid heating stabilizes the crumb structure at an early stage of baking before CO 2 is released during heating enabling bread expansion.
In order to increase the potential of non-conventional baking techniques while minimizing the disadvantages a combination of them (hybrid heating) can be applied. Combination of infrared lamps and electric heating coils enables 28% reduction in baking time, while resulting in breads comparable with breads baked in conventional electrical heating in terms of crumb firmness, volume, moisture content and colour [ 97 ]. However, there are limited studies applying hybrid heating to produce alternative cereals bread. Demirkesen et al. [ 98 ] compared the quality of the gluten-free breads based on the blends of tigernut flour/rice flour baked in conventional ovens and infrared–microwave combination. They observed higher loaf volume and crumb firmness and less gelatinized starch of IR- microwave baked breads. Moreover, staling of gluten-free breads was not affected by both baking methods [ 99 ].
Impact of abovementioned processing strategies on breadmaking potential of whole-grain wheat and non-wheat flours is summarized in Fig. 2 .

Impact of different technological approaches on breadmaking potential of whole-grain wheat and non-wheat flours
Conclusions and future trends
This review has highlighted that different technological strategies can be used to increase techno-functionality of whole-grain wheat and non-wheat flours and sensory properties of final product—bread. They are mostly performed with the aim to alter biopolymer structure and thus increase its functionality and encompass the ones used to provoke starch pre-gelatinization (high-pressure processing, flour heat treatment), reduce starch retrogradation (germination, extrusion cooking, non-conventional baking techniques), induce gluten strengthening through oxidation (atmospheric cold plasma) or gluten hydrolysis (grain germination, sourdough fermentation). It was elucidated that despite the opportunities offered by different conventional and emerging technologies and approaches, the gaps between technological and nutritional strategies for improving breadmaking potential of whole-grains still exist, especially when other, non-wheat grains are used. Namely, effectiveness of reviewed technological approaches largely depends on initial flour composition and quality. Therefore, further investigations are needed, particularly with respect to the ones including combined technologies (atmospheric pressure plasma/thermal treatment; pre-gelatinization/ultrasound; hybrid heating, etc.) to further increase technological and sensory quality of bread from whole-grain non-wheat cereals while preserving health beneficial properties.
Acknowledgements
This research was financially supported by the Science Fund of the Republic of Serbia, program PROMIS [Grant Number: 6062634], project acronym ReTRA and Ministry of Education, Science and Technological Development of the Republic of Serbia [Grant Number: 451-03-68/2022-14/200222].
Authors' contributions
Idea for the article: TD-H; literature search and data analysis: JT, DŠ, BŠ, MH; drafted the work: TD-H, MH; critically revised the work: JT. All authors read and approved the final manuscript.
Science Fund of the Republic of Serbia [Grant Number: 6062634], Ministry of Education, Science and Technological Development of the Republic of Serbia [Grant Number: 451-03-68/2022-14/200222].
Data availability
Code availability, declarations.
Tamara Dapčević-Hadnađev is Editorial Board Member.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
- Reference Manager
- Simple TEXT file
People also looked at
Review article, wheat proteins: a valuable resources to improve nutritional value of bread.

- 1 Faculty of Agricultural Sciences, Shree Guru Gobind Singh Tricentenary (SGT) University, Gurugram, India
- 2 Department of Bio and Nanotechnology, Guru Jambheshwar University of Science and Technology, Hisar, India
- 3 Centre of Agricultural Biochemistry and Biotechnology, University of Agriculture Faisalabad, Faisalabad, Pakistan
- 4 Mountain Research Centre for Field Crops, Sher-e-Kashmir University of Agricultural Sciences and Technology, Srinagar, India
Triticum aestivum , commonly known as bread wheat, is one of the most cultivated crops globally. Due to its increasing demand, wheat is the source of many nutritious products including bread, pasta, and noodles containing different types of seed storage proteins. Wheat seed storage proteins largely control the type and quality of any wheat product. Among various unique wheat products, bread is the most consumed product around the world due to its fast availability as compared to other traditional food commodities. The production of highly nutritious and superior quality bread is always a matter of concern because of its increasing industrial demand. Therefore, new and more advanced technologies are currently being applied to improve and enrich the bread, having increased fortified nutrients, gluten-free, highly stable with enhanced shelf-life, and long-lasting. This review focused on bread proteins with improving wheat qualities and nutritional properties using modern technologies. We also describe the recent innovations in processing technologies to improve various quality traits of wheat bread. We also highlight some modern forms of bread that are utilized in different industries for various purposes and future directions.
Introduction
Cereals have achieved their well-deserved importance all around the globe owing to their good nutritional profile. People misinterpret cereals as starch-rich foods even though they have proteins, vitamins, antioxidants, and some essential fatty acids, too ( McKevith, 2004 ). Wheat, as bread, contributes maximum nutrients to the global population than any other single food source. The end-product quality of wheat is mainly dependent on wheat proteins and their processing techniques involving harvesting of the grain to the production of flour, which further decrease the bioavailability of some of the nutrients ( Rustgi et al., 2019 ). The product quality is determined by the balanced composition of biochemical components in a seed such as seed storage proteins, starch, minerals, fibers, and phenolic compounds ( Žilić et al., 2011 ). In addition, besides interactions between wheat and companion, additives can also have effects on nutritional quality of end-products. There is a continuous increase in demand for improved wheat products by consumers and baking industries ( Dewettinck et al., 2008 ). Wheat proteins are the responsible agents governing the production of bread and other related end-products. Biotechnological tools are also gaining importance in harnessing cereal proteins for better end-products ( Verni et al., 2019 ; Shewry and Jones, 2020 ). Breeding via cross-hybridization in wheat crop have proved successful for the production of new superior end-use quality products ( Kiszonas and Morris, 2018 ).
Wheat storage proteins are major determinants of wheat flour composed of gluten and non-gluten fractions out of which wheat end-product quality mainly depends on the gluten proteins. Gluten protein mainly provides the elasticity and extensibility of dough, which is unique for wheat, leading to diverse end-products. Gluten protein cysteine residues form disulfide bonds, which are basically the chemical bases for the physical properties of dough ( Islam et al., 2019 ). Gluten was found to be degraded during germination ( Michalcová et al., 2021 ). Worldwide studies are going on to assess various wheat varieties for producing enhanced quality bread ( Lama et al., 2018 ). Various seed storage proteins alleles of wheat have also been explored to dissect their impact on end-product quality of wheat ( Goel et al., 2018a , b ; Altenbach et al., 2019 ). Additional work on enhancement of shelf-life was done to enhance its susceptibility to spoilage ( Nionelli et al., 2020 ). Considering the wide acceptability of wheat bread and other related products, in the present review, we shall be discussing about wheat proteins impact on bread making, also other techniques that are revolutionizing the quality of today's bread, and the effect of interactions with other food components on the nutritional enhancement of bread.
Bread Making
Bread is the product of baking of wheat flour mixed with water, salt, yeast, and flavor ingredients. The characteristic of wheat bread as physical attributes of texture, color, and volume are among the most important parameters taken into account by the consumers ( Tebben et al., 2018 ). The mechanical properties of bread are often associated with the perception of freshness and elasticity that influence the consumption decision ( Fagundes et al., 2018 ). The protein that is responsible for dough elasticity and formation of good bread is gluten produced by mixing gliadin and glutenin, which gives dough its elastic character ( Peña, 2002 ). The gases produced during the rising of the dough and the ability of the dough to hold these gases makes a substantial difference in bread quality as illustrated in Figure 1 ( Janssen et al., 2021 ). The journey of bread making started during Neolithic times; history proves that the mixing of bread with other sources is not a recent tale. In the Second World War, it was called “National Loaf” in which the addition of calcium carbonate was done during that period to counter the expected deficiencies due to shortage of milk and cheese ( Hayden et al., 2016 ).
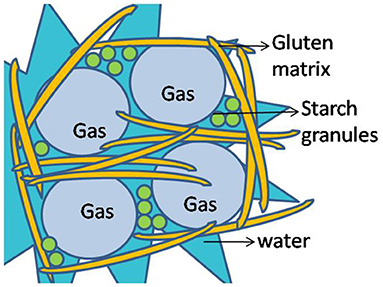
Figure 1 . Illustration of gas molecules entrapped in the gluten matrix of wheat dough.
The basic steps involved in bread making, including mixing, rising, kneading, baking, and cooling, have more or less remained constant since long. Mixing is simply a process causing the uniform distribution of ingredients and allows the formation of a gluten protein mesh-like network to give the product of bread ( Guerrini et al., 2019 ). There is an optimum mixing time, which changes, depending on the flour and mixing method used, because too much mixing produces dough with reduced elastic properties. This results in the development of small unrisen and unmixed patches in the bread, giving the loaf a poor appearance from inside ( Létang et al., 1999 ). Next to mixing, fermentation is done, during which dough slowly moves from a rough non-extensible dense mass into a dough with good gas holding and good extensibility properties. Besides this, breakdown of carbohydrates leads to the formation of alcohol and carbon dioxide that gives the bread its natural flavor and causes rising of the dough ( Rosell, 2011 ). Kneading/molding is done to remove gas from a large hole formed during rising of the dough. The dough is then allowed to rise again and is kneaded if required by the particular production process being used. During the final rising (proving), the dough again fills with more bubbles of gas, and once this has proceeded far enough, the dough is transferred to the oven for baking ( Cauvain, 2012 ).
The baking process transforms initial dough into a flavorful product, which is light and readily digestible. The penetration of intense heat increases the volume and size of the tiny gas cells ( Ishwarya et al., 2018 ). At about 60°C, stabilization of the crumb begins, making the starch granules swell; as they get released in the presence of water, the outer wall of the starch granule cell bursts, making the inside starch form a thick gel-like paste that helps to generate the structure of the dough ( Kumar and Sharma, 2018 ). As baking continues, the internal loaf temperature reaches ~98–100°C. As the moisture is driven off, the crust heats up and eventually reaches the same temperature as the oven. During baking, crust temperature is over 200°C, and the internal temperature of crumb is about 98°C. The loaf is full of saturated steam, which must be evaporated. The whole loaf is cooled to about 35°C before slicing and wrapping can occur without damaging the loaf. In a bakery, special cooling areas are required to ensure efficient cooling before slicing and wrapping of the bread. This completes the process of bread making, which is then consumed by people all over the world ( Pateras, 2007 ).
The rising demand in the food industry emphasized the intervention of research to enhance aspects of improved bread for large production and longer shelf-life. Food additives such as emulsifiers, which belong to a general class of compounds known as surfactants, are used to raise dough strength and as crumb softener in bread quality.
Role of Wheat Proteins in Bread Making
Bread making could be possible due to the viscoelastic properties of wheat doughs. These properties are a result of the structures and interactions of prolamins (a group of seed storage proteins) as observed in previous studies ( Shewry et al., 1999 ). Seed storage proteins of wheat are comprised of the gluten proteins, comprising two prolamin groups, gliadin and glutenin, considered as the main creator of bread. Glutenin is comprised of polymers with subunits linked by disulfide bonds, which is significantly important for bread making ( Shewry and Miflin, 1955 ). Qualitative or quantitative differences in the composition of seed storage proteins account for much of the variation in bread-making quality as observed in diverse wheat cultivars ( Huebner and Wall, 1976 ; Payne, 1987 ; Goel et al., 2018a ). A range of studies has been explored for the probable impact of wheat seed storage proteins and their role in bread making taking different allelic combinations ( Gupta and Shepherd, 1989 ; Goel et al., 2015 ). A minute wheat seed storage protein, Triticin, was also thought to improve quality of bread product ( Goel et al., 2015 , 2018b ). Extensive studies are also available on quantitative trait locus (QTL) analysis, depicting the role of the genetic loci on end-product bread quality and nutritional enhancement ( Charmet et al., 2001 ; Li et al., 2009 ; Goel et al., 2019 ; Suliman et al., 2021 ). Owing to the huge genome size of the wheat, researchers focused on the synteny area of related cereal crops to study the responsible factors of wheat proteins further governing end-product quality ( Quraishi et al., 2017 ). Furthermore, the biotechnological tools have been harnessed to dissect wheat proteins variable actions in improving bread quality ( Goel et al., 2017 , 2020 ).
Recent Advances to Improve Bread Quality
Production of gluten-free bread.
Improvement in the nutritional quality of wheat bread has always been on the priority list of bakers and wheat breeders because of its huge popularity throughout the world. When we look for the additives, one of the best option are legumes, as they are known as rich source of proteins, minerals, and bioactive health-promoting compounds, which may provide texture, structure, and baking quality to the end-products ( Figure 2 ). They also have a low glycemic index, and therefore, their inclusion in bread has enhanced the food menu for people allergic to gluten. Furthermore, various laboratory experiments have proved that along with nutritional value, the viscoelastic properties of gluten-free bread can be improved with the addition of legumes like chickpea, soybean, and lupin ( Melini et al., 2017 ). Industries have started adding barley to the wheat dough to enhance the fiber content without disturbing the glycemic index of traditional wheat bread and without negatively affecting its sensory characteristics ( Cavallero et al., 2002 ). Major emphasis has been given these days toward the reformulation of bread and bakery products by altering the gluten content with the addition of functional compounds such as non-cereal flours, prebiotics, and additives ( Elleuch et al., 2011 ). To improve sensory properties, shelf-life, and quality of gluten-free bread, flour from chestnut seeds, amaranth seeds, and psyllium seeds are added to the dough mix. It has been observed that the addition of prebiotics in dough prevents microbial growth and increases the shelf-life of bread ( Rahaie et al., 2014 ). Production of gluten-free bread is an initiative over rising issues of celiac disorders; sorghum and potato starch were considered as potent options earlier for making bread gluten free. Further addition of hydroxypropyl methylcellulose and whey protein concentrate acts as a technological improver in bread dough, and it was stipulated from the observations that both can be efficiently used to obtain gluten-free protein-rich bread ( Rustagi et al., 2018 ).
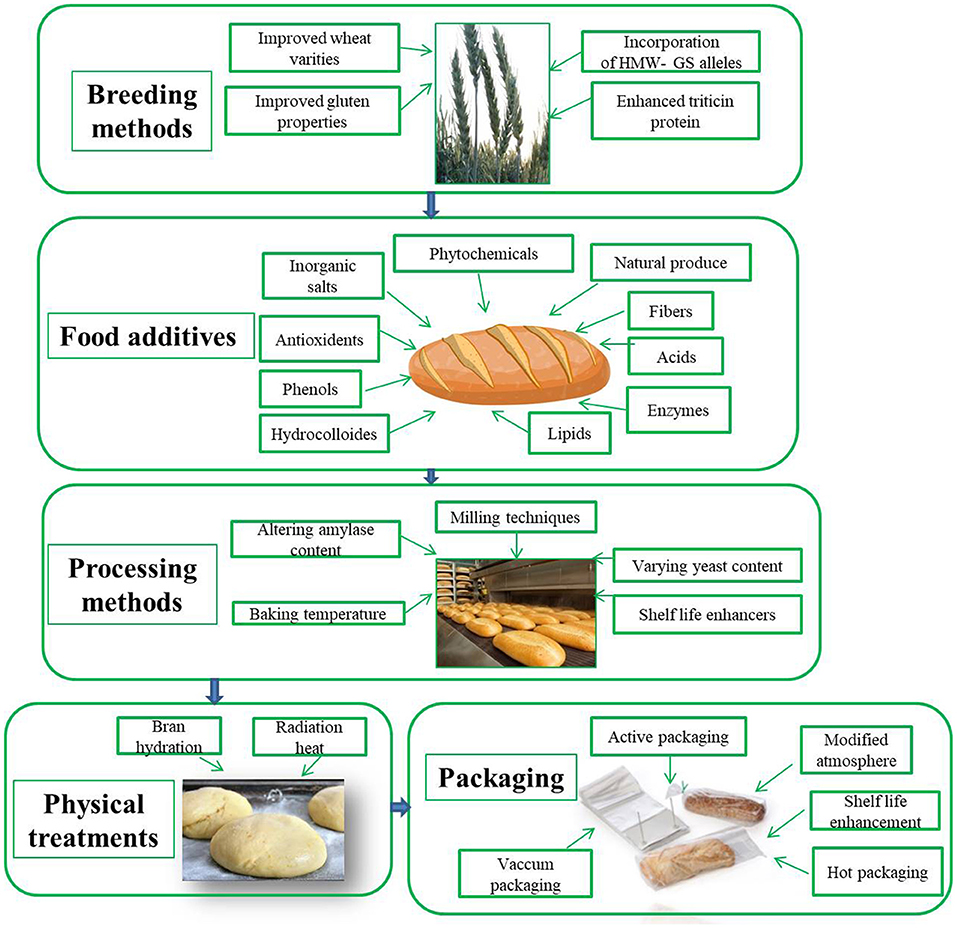
Figure 2 . Advancements in present day improving bread quality from breeding methods to processing techniques.
Improving Texture and Fiber Content
There are different classes of wheat flour that have been gown in different climatic zones around the globe. For example, five classes of wheat are grown in the United States, having their own properties in bread making, like soft white wheat is a special kind that has low moisture content and gives white product such as Asian-style noodles, pastries, and exquisite cakes. Another class is soft red winter wheat, which provides excellent baking and milling properties for making flat breads, cookies, and pretzels ( Nebraska Wheat Board, 2020 ). In certain parts of North America, bread making is practiced by using white wheat flour in which other fibers, germ fractions, phytochemicals, and other important nutrients are generally concentrated. As compared to whole grain bread, white flour products have minimal dietary fibers and non-nutrient phenolics ( Xu et al., 2019 ). On the other hand, hard white wheat is similar to red wheats in its characteristics but has sweet taste and is used in yeast breads, tortillas, and ramen noodles. Hard red winter wheat is mostly used for making pan bread, all-purpose flour, flat breads, and hard rolls, while hard red spring wheat, also called aristocrat of wheat, is used for making pizza crust, bagels, rolls, and hearth breads ( Nebraska Wheat Board, 2020 ).
Therefore, in the direction of quality and texture improvements, several additions have been tried in different proportions such as the addition of course grains, dietary fibers, pectin extracts, and natural coloring and flavoring substances. These fortifications not only improved the nutritional properties of bread but also enhanced its texture and storage. In this light, Angelino et al. investigated the effect of dietary fibers and phenolic compounds on the properties of bread dough and finished bread ( Angelino et al., 2017 ). The phenolic compounds in the form of apple pectin and fruit phenolic extracts showed enhanced antioxidant activity and storability of final bread as compared to the untreated bread ( Sivam et al., 2011 ), although these changes in antioxidant properties entirely depend on the choice of pectin extract (kiwi, apple, and other fruits) ( Rupasinghe et al., 2008 ). Research confirmed that the addition of pectin into bread dough enhances polymeric cross-linking with bread particles of high molecular weight ( Sivam et al., 2012 ).
Increasing Nutritional Value
Providing healthy and safe fresh bakery products and fulfilling expectations of consumers are big challenges before organic farmers, millers, and bakers. The quality of raw organic produce depends upon various factors, viz ., genotype, crop management, soil fertility, and crop rotation practices, which can be modulated as per market requirements, whereas the nutritional quality, taste, and flavor of bakery products varies with changes in the milling and baking process. Among crop management practices, nitrogen application plays an important role in achieving acceptable yield levels of good bread-making qualities. Canadian researchers have established the fact that the quality of bread can be affected by the cultivation practices of wheat to be used in bread making ( Mason et al., 2007 ). The researchers found that organically cultivated wheat produces more nutritive bread (high in protein value indicating excellent grain quality for yeast leavened bread), whereas conventionally grown wheat results in stronger textured bread ( Annett et al., 2007 ). Wheat bran proteins (WBPC) inclusion was observed in bread formulations and studied to determine the impact on nutritional properties without deleterious effects on quality ( Alzuwaid et al., 2021 ). Manganese application through seed treatments (seed priming) is a cost-effective method for improving the productivity of bread wheat particularly in alkaline calcareous soil ( Ullah et al., 2018 ). Additionally, the novel wheat varieties with pigmented grains (black, purple, and blue) with higher amounts of anthocyanins and other phenolics than the traditional wheat varieties can be effectively utilized to bake bread of some medicinal values as well, which may have preventive properties against cancer and chronic diseases ( Sharma et al., 2018 ). The antimicrobial property was reported to improve with the addition of phenolic compounds in many baking products, improving health benefits and extending the shelf-life of bread ( Xu et al., 2019 ).
Innovations in Bread Processing Approaches
Bread milling.
The damage to flour starch, amylase activity, particle size, and ash content of dough largely affects the baking performance of bread. These qualities are modified with milling techniques that in return modifies baking performance and nutritional properties of bread. The mineral bioavailability in bread can be increased using sourdough techniques (acidification process) in the baking method. The responses of bread quality parameters to milling and baking techniques have allowed identifying positive and negative characters of wheat bread, as baking has a multidisciplinary approach ( Abecassis et al., 2008 ).
Addition of Different Yeast Concentrations
Birch et al. (2014) stipulated that the level and strain of yeast along with temperature and duration of fermentation have a significant effect on the aroma of bread crumb. The strain and amount of yeast added to the flour mix control fermentation activities, and modifications in temperature along with time during the fermentation process may alter oxidation of lipids present in flours. The fortification for improving the quality and protein content of homemade bread had also been tried using nutritional yeast. Such fortified bread boosts the nutritional status of poor people and reduces the incidence of protein deficiency diseases. Variable concentrations of yeast (1–15%) is known to increase protein percent in homemade bread; however, fortification with only lower concentrations (1–3.5%) of yeast is acceptable in the market, as higher quantities of yeast alter the taste of bread to an unacceptable level by its consumers ( Harusekwi et al., 2014 ). The impact of addition of organic acids in improving bread quality was studied in China. They analyzed the yeast activity, proteolysis, and amylolysis by adding acetic acid, malic acid, fumaric acid, lactic acid, and citric acid to bread dough ingredients. The organic acids increased specific volume of the bread, whereas they lowered moisture content, pH value, and hardness of bread, which resulted from high yeast activity ( Su et al., 2019 ). Since organic acids improve bread quality, the effects of addition of lactic acid bacteria in dough mix on bread texture and quality have been studied by food technologists. Out of many strains of Lactobacillus plantarum , LAB strains (LB-1, F-3, and F-50) exhibited antifungal activity and found useful for making bread to extend shelf-life. In fact, among these three strains, LB-1 significantly improved water holding capacity, viscosity, elasticity, and extensibility of sourdough ( Sun et al., 2020 ).
The baking interventions include sourdough, which is prepared through natural fermentation using lactobacilli and yeast. The lactic acid produced by the action of lactobacilli adds taste and good keeping qualities to sourdough. It reproduces nutritionally superior, fiber-rich, gluten-free bread with improved mineral bioavailability, making it unsuitable for celiac persons. Poutanen et al. (2009) stated that the inclusion of natural yeast and bacteria in bread dough results in the solubilization of proteins and polysaccharides present in cell wall, which change the texture of baked bread and absorption of nutritional and non-nutritional compounds. The process of natural fermentation may also lead to the synthesis of novel bioactive compounds and metabolites such as prebiotic oligosaccharides.
Loaf Volume and Sensory Qualities
The researchers working on the qualities of loaf stated that the consumers are attracted to higher loaf volume and weight, which is a positive economic character for retail marketing. The buyers often believe that bread with higher loaf volumes offers more substance for similar prices. Shittu et al. (2007) explained that the varying rates of gaseous output and starch gelatinization capacity are responsible for variable loaf volumes, which result from differences in time and temperature of baking. Industries keep these factors in mind to attract the consumers by making little changes in the bread-making process. The crumb moisture content and loaf volume of bread are significantly affected by baking temperatures ( Shittu et al., 2007 ), while dried crumb hardness, bread loaf weight, and density levels can be altered with differential baking durations at variable temperatures. Ghorbani et al. (2019) observed that bread baked at 320°C for 3 min were liked more by the judges of the sensory panel, taking their texture and chewiness, whereas the samples baked at 370°C for 2.5 min did not score well in comparison to other evaluated samples. The research shows that baking at higher temperatures results in hard bread with reduced consumer acceptability. Thus, even a slight degree of change in temperature and time has substantial effect on the overall quality and acceptance of bread.
Reducing Microbial Activity
The shelf-life of bread is a big constraint for the whole bread industry, as the bread is prone to mold contamination due to its moisture content. Within a matter of days, the microbial contamination spoils the product. There have been various studies done focusing on extending the shelf-life, and it was found that replacement or reduction in NaCl in bread making could affect the growth of Penicillium roqueforti and Aspergillus niger . The salt added to the bread mix not only improved the flavor but also increased the water activity (a w ), making the bread more susceptible to mold infections. The infection can be prevented by reducing the amount of NaCl or simply replacing NaCl at least partially with other acceptable salts such as calcium chloride, potassium chloride, magnesium chloride, and magnesium sulfate. The growth of P. roqueforti and A. niger was found to be reduced in the bread dough with 30% less NaCl or with suitable replacers. Mixing wheat flour with other flours (cassava, soybean, etc.) was tried by many bakers to improve the quality of existing bread ( Samapundo et al., 2010 ).
Improving Bread Quality via Value Additions
Non-cereal items.
The addition of non-cereal flours in bread dough is very popular these days. It not only improves the texture of bread but also increases mineral nutrition. According to recent studies, cassava flour (unfermented) is added to bread making due to its high nutritional values. Various combinations of cassava flour with wheat flour have been tried to develop a wide range of food products, viz ., pies, rolls, cakes, biscuits, doughnuts, and breads. Due to low setback viscosity and high peak viscosity, yellow cassava flour is considered good for bread dough, as it imparts low tendency to undergo retro gradation, making it suitable for products that require high elasticity and gel strength ( Ayeh, 2013 ). In addition to the improvement in the quality of bread, the addition of cassava flour reduces the time for dough development as compared to all wheat flour dough. Later, Pasqualone et al. (2010) also reported that cassava-enriched bread is suitable for celiac patients, as it is gluten-free, nutritious, and palatable. The desirable loaf volume and crumb firmness of cassava bread can be achieved by using olive oil (extra virgin) and egg white, even if the hydrocolloids and industrial improvers are not added during dough preparation. The Indian bakeries transformed wheat into an Indian bread, known as chapatti . On an average, chapatti is consumed in every home on a daily basis in India including consumers from weaker economic sections. The recent studies suggested incorporation of 20–50% amaranth seed flour to wheat bread mixture to improve rolling properties, protein content, and mineral availability in the final bread ( Mutahi, 2012 ). The addition of amaranth also increases stickiness, softness, rollability, and elasticity of dough ( Banerji et al., 2019 ). Other non-cereal grains have also been tried to develop multigrain bread, such as buckwheat and quinoa ( Gawlik-Dziki et al., 2009 ), for enhanced protein content, energy, mineral, phytate, and condensed tannin contents; however, when the percentage of wheat was decreased below 70%, the bread quality was found inferior as compared to regular bread ( Ayele et al., 2017 ). The cereal legumes (chickpea, lupin, and soya) are a rich sources of digestible proteins and blend well with wheat dough or other cereals (oat, barley, and rye) for baking purposes. The combination of legume cereals and oats/barley/rye mixed in equal proportions was also found to improve sensory properties and texture of multigrain bread. However, an adverse effect of mixed flours on the technological properties of bread dough was observed that could be corrected by using industrial additives such as ascorbic acid, fungal alpha-amylase, glucose oxidase, xylanase, and vital gluten, alone or in combination ( Yaver and Bilgiçli, 2019 ). Dairy products were also assessed for impact on bread quality ( Graça et al., 2019 ).
Non-plant Ingredients
Monteiro et al. (2018) mixed tilapia-waste flour to bread dough in different proportions (0–20%) and observed that the amount of carbohydrates and total dietary fibers in bread increased with increased levels of tilapia-waste flour, whereas, in sensory evaluation, tilapia-waste flour bread scored low as compared to the traditional bread due to its disagreeable texture, flavor, and aroma. Despite that, the overall acceptance for mixed bread was unaffected as the stickiness in teeth, loaf volume, and cream color of bread did not vary significantly from wheat-based breads. Calcium is another main component that should be adequate in the diet especially for women and children for the health of bones. Recently, to increase the level of calcium in bread, new materials in trend has been used in powder form like skim milk (10%), oyster shell (2%), and eggshell (2%). This also increases set back viscosity, dough stability, percentage of water absorption, the heat of transition, and mixing time. The bread fortified with oyster shell powder showed higher amounts of fiber and ash contents. This bread is also rich in carbohydrates and proteins. It is evident from the latest results that technological properties and nutritional values in bread could be positively increased with the addition of calcium from natural resources. However, the bread fortified with eggshell and oyster shell scored badly in terms of aroma and general acceptability as compared with the bread supplemented with milk powder ( Alsuhaibani, 2018 ). In further continuation with the addition of uncommon substances, seaweed extracts were also tried for improving quality bread for baking purposes. In Indonesia, brown seaweed from coastal areas of Yogyakarta was used to extract alginate, which was proved to be a non-toxic compound with hypo-cholesterolemic effects. Its addition in wheat bread mixture tends to improve proximate values of bread making and is useful for daily consumption ( Supartini and Mushollaeni, 2017 ). However, higher cholesterol levels in alginate added to bread mixture can lead to adverse consequences, which may result in cardiovascular diseases as well. The fortification of wheat bread with plant-based or uncommon additives certainly enhanced the nutritional value, qualities, and texture of the final product. Conte et al. evaluated the effect of bee pollen addition to the flour used for gluten-free bread. They observed that such flour has a higher percentage of total proteins, carotenoids, and minerals, and showed anti-free radical activity ( Conte et al., 2020 ).
Enhanced Aroma Properties
The aroma is among the first few parameters that a consumer is inclined to for bread quality. To date, more than 150 volatile compounds have been characterized in bread loaf, which evolves because of fermentation activities of yeast. Among these, many volatile compounds contribute to the aroma of bread crumb, which is sensed by consumers while eating ( Pico et al., 2016 ). These compounds impart a characteristic odor and flavor to the final baking product. Sensory perception has a major play in the choice of bread by the consumers. The addition of legume flour in gluten-free bread can improve its nutritional value but could harm its sensory properties. Sourdough is often used to improve the sensory properties of bread. Moreover, the addition of sugars and amino acids as precursors of aroma compounds or enzymes that produce them can positively affect bread aroma. In a study, it was established that the addition of pea flour in combination with improvers (fructose, proline, arginine, and protease) helps to enhance sensory properties of bread. The relative amount of pleasant volatiles (key aroma compound 2-acetyl-1-pyrroline) has been found to increase with the addition of quinoa flour (15%) and teff flour (5%) along with wheat and corn starch (40% each). The combination also resulted in lower levels in rancid volatile compounds that originate because of fatty acid oxidation ( Pico et al., 2019 ).
Dough Strength
The starch, which is composed of amylopectin and amylose, is abundantly present in wheat flour and maintains bread stability. However, there are varieties of wheat that are deficient in endosperm amylose. Such wheat is known as “waxy wheat,” which can be utilized in the baking industry to alter amylose levels in wheat-based bakery products. The more waxy wheat is added to the dough mix, the lesser is the amylose content in it, and the better is the quality of bread. The quality of Chinese steamed bread was found to be improved with the addition of waxy flour into the bread mixture, although the addition did not improve the bread quality because the firmness of bread was decreased during storage. In experimental trials, flours of waxy wheat and Canadian spring wheat were mixed in varying amounts (0–20%), where 15% addition of waxy flour improved bread stability without affecting the quality ( Rustagi et al., 2018 ). Wheat varieties with different allelic combinations of seed storage proteins were found to be responsible for a better bread loaf and used for production of better end-product variants of wheat bread ( Goel et al., 2015 , 2017 ).
Increasing Shelf-Life
The fatty acid salts, when used as surface acting agents and food additives, show antibacterial activity. The mold-proofing activity and improved baking property with fatty acid salts have been studied by many food scientists ( Hamaishi et al., 2018 ). The results have proven that the addition of >5% potassium myristate to dough inhibited fungal growth on bread during storage. The length of the carbon chain of fatty acids contributed to the antifungal activity and antimicrobial effects of fatty acids; it was observed that the activity reduces with an increase in the chain length; also, medium-chain fatty acids showed stronger antimicrobial activity than longer chain fatty acids ( Pareyt et al., 2011 ). Efforts have also been made in the direction of increasing the shelf-life of bread, which is largely affected by molds. As the bread is packed and distributed to several destinations in the world, technological interventions are needed to minimize mold infection bread. Liu et al. (2011) studied the impact of radiofrequency energy in addition to the usual hot air treatment for the control of mold in bread packaging. It was found that the radiofrequency treatments decrease moisture content and water activities in bread, which ultimately reduces formation of Penicillium citrinum spores. This method also enhances the storage time by 28 ± 2 days for treated white bread. Some researchers also studied the effect of incorporation of non-plant-based material into bread dough on the final product. Moreover, the addition of marine food products ( Kadam and Prabhasankar, 2010 ), plant extracts such as green tea ( Wang and Zhou, 2004 ), natural antioxidants ( Lim et al., 2011 ), grape seed extracts ( Peng et al., 2010 ), and prebiotics ( Korus et al., 2006 ) to bakery products have been widely proposed to enhance quality and functionality of the bread. Currently, studies are revolutionizing the baking industries and serving in the development of more novel products that are low in calories and cholesterol and suitable for people with celiac disease. There are a lot more opportunities to be tested for ensuring food and nutritional security for the ever-growing population in the world.
Modern Forms of Bread
Bee bread is a specialized fermented product comprised of combination of pollens, bee saliva, and nectar that bees pack in the honeycomb to ferment them with the help of many kinds of yeasts and bacteria ( Khalifa et al., 2020 ). It is very important and considered as a key protein source for bee adults and larvae. Apart from this, bee bread is an excellent source of energy and nutrition for humans due to the higher protein concentration of pollens. The biochemical components of bee bread include vitamins, fatty acids, proteins, enzymes, hormones, antioxidants, carbohydrates, and minerals ( Kieliszek et al., 2018 ). Nowadays, bee bread is very popular in the commercial market due to its high nutritional properties. This bread has high antioxidant activity and phenolic content that contribute to its biological and nutritional properties that can be used as beneficial food supplements ( Mutsaers et al., 2005 ). The bee bread is a product with a long history used mainly in folk medicine due to its therapeutic properties. For example, in recent years, numerous studies have been carried out to study the effectiveness of bee bread to treat different illnesses. Bee bread has been exhibited anti-inflammatory, anticancer, antiradical, and antimicrobial activities ( Khalifa et al., 2020 ).
Steamed Bread
Steaming instead of baking is done in some areas for preparation of bread, which is actually a staple food in China. Bread is consumed after steaming in many countries of the East and Southeast Asian regions ( Peng and Cheng, 2007 ). The People's Republic of China grows large quantities of wheat and is a major wheat importer. The wheats that it produce are both hard red (winter and spring) and soft wheat, which are commonly blended to produce basic flours. Hard red spring wheat is used in northern China to produce steamed breads, which are distinctly different in texture from breads produced in southern China from lower protein hard and soft red winter wheat flours ( Rubenthaler et al., 1990 ).
Multigrain Bread
Multigrain bread is made by mixing wheat flour with flours of some legumes, cereals like oats, and some seeds like flaxseeds and sesame seeds. This bread is more nutritious and flavorful than the normal bread. The study conducted found a positive effect of this multigrain on the dough properties and the quality of bread. Multigrain bread with a 15% multigrain mix proved to be effective in increasing protein, fat, and dietary fiber contents of bread ( Indrani et al., 2010 ). There are enough products in the market that can be claimed as gluten-free and can be safely digested by patients affected by celiac disease. Sourdough is a type of foremost fermentation that is commercially used for baking purposes of gluten-free bread. It has also been proven to be ideal for improving the texture, aroma, palatability, shelf-life, and nutritional enhancement in the case of wheat and rye bread. The concept of sourdough in gluten-free baking industry is a new zone of the experimental area to improve the quality and acceptability of gluten-free bread ( Moroni et al., 2009 ). In addition, the health risk to various celiac diseases has emphasized the focus on gluten-free bread prepared by mixing chestnut, bean flour, and chickpea, with rice flour at different ratios using straight dough bread-making process ( Yildrim and Nadeem, 2019 ). There are challenges even today for optimal formulation when we deal with texture, flavor, and nutrition ( Wang et al., 2017 ). Rye bread is again a variant of ancient bread using rye as a component. A study conducted on rats found that the addition of green tea to rye might help in preventing obesity in rats ( Bajerska et al., 2013 ). High-fiber rye bread was also experimented in menopausal-stage women for insulin secretion and appears to enhance insulin secretion by improving b-cell function ( Juntunen et al., 2003 ). The addition of saffron powder in rye bread showed antidiabetic properties ( Bajerska et al., 2013 ).
Conclusion and Future Perspectives
After rigorous efforts and interventions by researchers and global food industries, we still have a significant proportion of the chronically undernourished populations in developing countries. Surprisingly, even today, around 80% of the world's growing population is devoid of basic balance diet. The research in food sciences should be directed to focus on the quality of food in addition to the quantity of food that is available to humankind. The application of scientific approaches for the improvement of the baking industry provides the potential solution for resolving the challenges of global food and nutritional security. The fortification of bread dough with more nutritive grains and supplements enhances the quality and digestibility of bread. The recent advancement in bread-making process, namely, addition of enzymes, flours of non-plant origin, antimicrobial supplements, improved yeast strains, tools used in baking, and enhancement of dough rheological properties, have helped to bridge the gap between the nutritional demands and fulfillment to some extent. However, the face of ancient bread changed positively with recent research; there are endless possibilities to explore further. Exploration of novel genotypes with varying wheat proteins suitable for bread making, identification of more stable yeast strains, shelf-life enhancement, attractive color, fiber and flavor enhancement in bread, and development of celiac patient-friendly products are the issues that should be considered in future research. It will undoubtedly give rise to new avenues for food and nutrition research, and such advances will allow the development of better end-products from wheat, which can be utilized to reduce global hunger.
Author Contributions
SGr, MS, and SGo: literature survey. SGo, AR, and SW: first draft. SGr, MS, and SW: review and editing. All authors finalized the manuscript before submission.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abecassis, J., David, C., Fontaine, L., Taupier-Létage, B., and Viaux, P. (2008). “A multidisciplinary approach to improve the quality of organic wheat-bread chain,” in Poster at: Cultivating the Future Based on Science: 2nd Conference of the International Society of Organic Agriculture Research ISOFAR, Modena, Italy, June , 18–20.
Google Scholar
Alsuhaibani, A. (2018). Rheological and nutritional properties and sensory evaluation of bread fortified with natural sources of calcium. J. Food Qual . 2018:8308361. doi: 10.1155/2018/8308361
CrossRef Full Text | Google Scholar
Altenbach, S. B., Chang, H. C., Yu, X. B., Seabourn, B. W., Green, P. H., and Alaedini, A. (2019). Elimination of omega-1, 2 gliadins from bread wheat ( Triticum aestivum ) flour: effects on immunogenic potential and end-use quality. Front. Plant. Sci . 10:580. doi: 10.3389/fpls.2019.00580
PubMed Abstract | CrossRef Full Text | Google Scholar
Alzuwaid, N. T., Pleming, D., Fellows, C. M., and Sissons, M. (2021). Fortification of durum wheat spaghetti and common wheat bread with wheat bran protein concentrate-impacts on nutrition and technological properties. Food Chem . 334:127497. doi: 10.1016/j.foodchem.2020.127497
Angelino, D., Cossu, M., Marti, A., Zanoletti, M., Chiavaroli, L., Brighenti, F., et al. (2017). Bio accessibility and bioavailability of phenolic compounds in bread: a review. Food Funct . 8, 2368–2393. doi: 10.1039/C7FO00574A
Annett, L. E., Spaner, D., and Wismer, W. V. (2007). Sensory profiles of bread made from paired samples of organic and conventionally grown wheat grain. J. Food Sci . 7, S254–260. doi: 10.1111/j.1750-3841.2007.00331.x
Ayeh, E. S. (2013). Development and quality characteristics of yam bean (Pachyrhizus erosus) flour and its performance in bread (Doctoral dissertation). Kumasi Ghana: Kwame Nkrumah University of Science and Technology.
Ayele, H. H., Bultosa, G., Abera, T., and Astatkie, T. (2017). Nutritional and sensory quality of wheat bread supplemented with cassava and soybean flours. Cogent Food Agric . 3:1331892. doi: 10.1080/23311932.2017.1331892
Bajerska, J., Mildner-Szkudlarz, S., Podgórski, T., and Oszmatek-Pruszyńska, E. (2013). Saffron ( Crocus sativus L.) powder as an ingredient of rye bread: an anti-diabetic evaluation. J. Med. Food 16, 847–856. doi: 10.1089/jmf.2012.0168
Banerji, A., Ananthanarayan, L., and Lele, S. S. (2019). Dough browning inhibition of multigrain Indian flatbread (chapatti) using a combination of chemical and microwave treatment. J. Food Meas. Charact. 13, 807–820. doi: 10.1007/s11694-018-9993-z
Birch, A. N., Petersen, M. A., and Hansen, Å. S. (2014). Aroma of wheat bread crumb. Cereal Chem . 91, 105–114. doi: 10.1094/CCHEM-06-13-0121-RW
Cauvain, S. (2012). “Breadmaking: an overview,” in Breadmaking , ed. S. P. Cauvain (Woodhead Publishing), 9–31.
Cavallero, A., Empilli, S., Brighenti, F., and Stanca, A. M. (2002). High (1→ 3, 1→ 4)-β-glucan barley fractions in bread making and their effects on human glycemic response. J. Cereal Sci . 36, 59–66. doi: 10.1006/jcrs.2002.0454
Charmet, G., Robert, N., Perretant, M. R., Gay, G., Sourdille, P., Groos, C., et al. (2001). Marker assisted recurrent selection for cumulating QTLs for bread-making related traits. Euphytica 119, 89–93. doi: 10.1023/A:1017577918541
Conte, P., Del, C. A., Urgeghe, P. P., Petretto, G. L., Montanari, L., Piga, A., et al. (2020). Nutritional and aroma improvement of gluten-free bread: is bee pollen effective? LWT 118:108711. doi: 10.1016/j.lwt.2019.108711
Dewettinck, K., Van Bockstaele, F., Kühne, B., Van de Walle, D., Courtens, T. M., and Gellynck, X. (2008). Nutritional value of bread: influence of processing, food interaction and consumer perception. J. Cereal Sci . 4, 243–257. doi: 10.1016/j.jcs.2008.01.003
Elleuch, M., Bedigian, D., Roiseux, O., Besbes, S., Blecker, C., and Attia, H. (2011). Dietary fibre and fibre-rich by-products of food processing: characterisation, technological functionality and commercial applications: a review. Food Chem . 124, 411–421. doi: 10.1016/j.foodchem.2010.06.077
Fagundes, G. A., Rocha, M., and Salas-Mellado, M. M. (2018). Improvement of protein content and effect on technological properties of wheat bread with the addition by cobia ( Rachycentron canadum ). Food Res . 2, 221–227. doi: 10.26656/fr.2017.2(3).275
Gawlik-Dziki, U., Dziki, D., Baraniak, B., and Lin, R. (2009). The effect of simulated digestion in vitro on bioactivity of wheat bread with Tartary buckwheat flavones addition. LWT-Food Sci. Technol . 42, 137–143. doi: 10.1016/j.lwt.2008.06.009
Ghorbani, N. S., Tehrani, M. M., Khodaparast, M. H., and Farhoosh, R. (2019). Effect of temperature, time, and asparaginase on acrylamide formation and physicochemical properties of bread. Acta Alimentaria 48, 160–168. doi: 10.1556/066.2019.48.2.3
Goel, S., Grewal, S., and Singh, N. K. (2017). Evaluation of HMW-GS 20 and 2.2 from near isogenic lines of wheat variety HD2329 for bread quality improvement. J. Sci. Food Agric . 97, 4526–4531. doi: 10.1002/jsfa.8319
Goel, S., Rathore, M., Grewal, S., Jain, N., Singh, B. K., Ahlawat, A. K., et al. (2015). Effect of allelic variation in triticin on bread-and chapati-making qualities of wheat ( Triticum aestivum ). Agric. Res . 4, 139–151. doi: 10.1007/s40003-015-0150-1
Goel, S., Singh, B., Grewal, S., Jaat, R. S., and Singh, N. K. (2018a). Variability in Fe and Zn content among Indian wheat landraces for improved nutritional quality. Indian J. Genet. Plant Breed . 78, 426–432. doi: 10.31742/IJGPB.78.4.4
Goel, S., Singh, K., Grewal, S., and Nath, M. (2020). Impact of “omics” in improving drought tolerance in wheat. Crit. Rev. Plant Sci . 39, 222–235. doi: 10.1080/07352689.2020.1778924
Goel, S., Singh, K., Singh, B., Grewal, S., Dwivedi, N., Alqarawi, A. A., et al. (2019). Analysis of genetic control and QTL mapping of essential wheat grain quality traits in a recombinant inbred population. PLoS ONE 14:e0200669. doi: 10.1371/journal.pone.0200669
Goel, S., Yadav, M., Singh, K., Jaat, R. S., and Singh, N. K. (2018b). Exploring diverse wheat germplasm for novel alleles in HMW-GS for bread quality improvement. J. Food Sci. Technol . 55, 3257–3262. doi: 10.1007/s13197-018-3259-y
Graça, C., Raymundo, A., and Sousa, I. (2019). Wheat bread with dairy products-technology, nutritional, and sensory properties. Appl. Sci . 9:4101. doi: 10.3390/app9194101
Guerrini, L., Parenti, O., Angeloni, G., and Zanoni, B. (2019). The bread making process of ancient wheat: a semi-structured interview to bakers. J. Cereal Sci . 87, 9–17. doi: 10.1016/j.jcs.2019.02.006
Gupta, R. B., and Shepherd, K. W. (1989). “Low molecular weight glutenin subunits in wheat: their variation, inheritance, and association with breadmaking quality,” in Proceedings 7th International Wheat Genetics Symposium , eds. T. E. Miller and R. M. D. Koebner (I.P.S.R.: Cambridge), 943–949.
Hamaishi, T., Morinaga, Y., and Morita, H. (2018). Application of potassium myristate as an antifungal and a dough improving in bread-making. Biocontrol Sci . 23, 223–227. doi: 10.4265/bio.23.223
Harusekwi, S. J., Nyamunda, B. C., and Mutonhodza, B. (2014). Development of high protein content homemade bread by nutritional yeast fortification for disadvantaged communities. Intern. J. Nutrit. Food Sci . 3:194. doi: 10.11648/j.ijnfs.20140303.20
Hayden, B., Nixon-Darcus, L., and Ansell, L. (2016). “Our ‘daily bread’?: The origins of grinding grains and breadmaking,” in Exploring the Materiality of Food'Stuffs' , eds. L. Steel and K. Zinn (London: Routledge), 73–94.
Huebner, F., and Wall, J. S. (1976). Fractionation and quantitative differences of glutenin from wheat varieties varying in baking quality. Cereal Chem ., 53, 258–269.
Indrani, D., Soumya, C., Rajiv, J., and Venkateswara Rao, G. (2010). Multigrain bread-its dough rheology, microstructure, quality and nutritional characteristics. J. Texture Stud . 41, 302–319. doi: 10.1111/j.1745-4603.2010.00230.x
Ishwarya, S. P., Desai, K. M., Srinivasulu, N., and Anandharamakrishnan, C. (2018). Impact of wheat bran addition on the temperature-induced state transitions in dough during bread-baking process. Int. J. Food Sci. Technol . 53, 404–411 doi: 10.1111/ijfs.13598
Islam, S., Yu, Z., She, M., Zhao, Y., and Ma, W. (2019). Wheat gluten protein and its impacts on wheat processing quality. Front. Agric. Sci. Eng . 6, 279–287. doi: 10.15302/J-FASE-2019267
Janssen, F., Wouters, A. G., and Delcour, J. A. (2021). Gas cell stabilization by aqueous-phase constituents during bread production from wheat and rye dough and oat batter: dough or batter liquor as model system. Comp. Rev. Food Sci. Food Saf . 20, 3881–3917. doi: 10.1111/1541-4337.12761
Juntunen, K. S., Laaksonen, D. E., Poutanen, K. S., Niskanen, L. K., and Mykkänen, H. M. (2003). High-fiber rye bread and insulin secretion and sensitivity in healthy postmenopausal women. Am. J. Clinic. Nutrit . 77, 385–391. doi: 10.1093/ajcn/77.2.385
Kadam, S. U., and Prabhasankar, P. (2010). Marine foods as functional ingredients in bakery and pasta products. Food Res. Int . 43, 1975–1980. doi: 10.1016/j.foodres.2010.06.007
Khalifa, S. A., Elashal, M., Kieliszek, M., Ghazala, N. E., Farag, M. A., Saeed, A., et al. (2020). Recent insights into chemical and pharmacological studies of bee bread. Trends Food Sci. Technol . 97, 300–316. doi: 10.1016/j.tifs.2019.08.021
Kieliszek, M., Piwowarek, K., Kot, A. M., Błazejak, S., Chlebowska-Smigiel, A., and Wolska, I. (2018). Pollen and bee bread as new health-oriented products: a review. Trends Food Sci. Technol . 71, 170–180. doi: 10.1016/j.tifs.2017.10.021
Kiszonas, A. M., and Morris, C. F. (2018). Wheat breeding for quality: a historical review. Cereal Chem . 95, 17–34. doi: 10.1002/cche.10033
Korus, J., Grzelak, K., Achremowicz, K., and Sabat, R. (2006). Influence of prebiotic additions on the quality of gluten-free bread and on the content of inulin and fructooligosaccharides. Food Sci. Technol. Int . 12, 489–495. doi: 10.1177/1082013206073072
Kumar, K. A., and Sharma, G. K. (2018). The effect of surfactants on multigrain incorporated short biscuit dough and its baking quality. J. Food Measu. Char. 12, 1360–1368.
Lama, S., Kabir, M. R., and Akhond, M. A. Y. (2018). Biochemical and molecular characterization of Bangladeshi wheat varieties for bread-making quality. Plant Tissue Cult. Biotechnol. 28, 57–68. doi: 10.3329/ptcb.v28i1.37198
Létang, C., Piau, M., and Verdier, C. (1999). Characterization of wheat flour-water doughs. Part I: rheometry and microstructure. J. Food Eng . 41, 121–132. doi: 10.1016/S0260-8774(99)00082-5
Li, Y., Song, Y., Zhou, R., Branlard, G., and Jia, J. (2009). Detection of QTLs for bread-making quality in wheat using a recombinant inbred line population. Plant Breed . 128, 235–243. doi: 10.1111/j.1439-0523.2008.01578.x
Lim, H. S., Park, S. H., Ghafoor, K., Hwang, S. Y., and Park, J. (2011). Quality and antioxidant properties of bread containing turmeric ( Curcuma longa L.) cultivated in South Korea. Food Chem . 124, 1577–1582. doi: 10.1016/j.foodchem.2010.08.016
Liu, Y., Tang, J., Mao, Z., Mah, J. H., Jiao, S., and Wang, S. (2011). Quality and mold control of enriched white bread by combined radio frequency and hot air treatment. J. Food Eng . 104, 492–498. doi: 10.1016/j.jfoodeng.2010.11.019
Mason, H., Navabi, A., Frick, B., O'Donovan, J., Niziol, D., and Spaner, D. (2007). Does growing Canadian Western Hard Red Spring wheat under organic management alter its breadmaking quality? Renew. Agric. Food Syst . 22, 157–167. doi: 10.1017/S1742170507001688
McKevith, B. (2004). Nutritional aspects of cereals. Nutrit. Bullet . 29, 111–142. doi: 10.1111/j.1467-3010.2004.00418.x
Melini, F., Melini, V., Luziatelli, F., and Ruzzi, M. (2017). Current and forward-looking approaches to technological and nutritional improvements of gluten-free bread with legume flours: a critical review. Compr. Rev. Food Sci. Food Saf . 16, 1101–1122. doi: 10.1111/1541-4337.12279
Michalcová, E., Potocká, E., Chmelová, D., and Ondrejovič, M. (2021). Study of wheat protein degradation during germination. J. Microbiol. Biotechnol. Food Sci . 2021, 1437–1449.
Monteiro, M. L. G., Mársico, E. T., Soares Junior, M. S., Deliza, R., de Oliveira, D. C., and Conte-Junior, C. A. (2018). Tilapia-waste flour as a natural nutritional replacer for bread: a consumer perspective. PLoS ONE 13:e0196665. doi: 10.1371/journal.pone.0196665
Moroni, A. V., Dal Bello, F., and Arendt, E. K. (2009). Sourdough in gluten-free bread-making: an ancient technology to solve a novel issue? Food Microbiol . 26, 676–684. doi: 10.1016/j.fm.2009.07.001
Mutahi, A. W. (2012). Effect of sorghum variety on batter rheology and quality of Cassava Sorghum-Amaranth bread. (Doctoral dissertation), University of Nairobi, Kenya.
Mutsaers, M., van Blitterswijk, H., van't Leven, L., and van de Kerkvliet, J. (2005). AD42E Bee Products . Wageningen: Agromisa Foundation.
Nebraska Wheat Board. (2020). 6 Classes of Wheat and Their Uses. Official Nebraska Government Website . Available online at: https://nebraskawheat.com/6-classes-of-wheat-and-their-uses/ (accessed October 8, 2021).
Nionelli, L., Wang, Y., Pontonio, E., Immonen, M., Rizzello, C. G., Maina, H. N., et al. (2020). Antifungal effect of bioprocessed surplus bread as ingredient for bread-making: identification of active compounds and impact on shelf-life. Food Cont . 118:107437. doi: 10.1016/j.foodcont.2020.107437
Pareyt, B., Finnie, S. M., Putseys, J. A., and Delcour, J. A. (2011). Lipids in bread making: sources, interactions, and impact on bread quality. J. Cereal Sci . 54, 266–279. doi: 10.1016/j.jcs.2011.08.011
Pasqualone, A., Caponio, F., Summo, C., Paradiso, V. M., Bottega, G., and Pagani, M. A. (2010). Gluten-free bread making trials from cassava ( Manihot esculenta Crantz) flour and sensory evaluation of the final product. Int. J. Food Prop . 13, 562–573. doi: 10.1080/10942910802713172
Pateras, I. M. (2007). “Bread spoilage and staling,” in Technology of Breadmaking , S. P. Cauvain, L. S. Young (Boston, MA: Springer), 275–298.
Payne, P. I. (1987). Genetics of wheat storage proteins and the effect of allelic variation on breadmaking quality. Ann. Rev. Plant Physiol . 38, 141–153. doi: 10.1146/annurev.pp.38.060187.001041
Peña, R. J. (2002). Wheat for Bread and Other Foods. Bread Wheat Improvement and Production . Food and Agriculture Organization of the United Nations: Rome, 483–542.
Peng, Q.I.N., and Cheng, S.H. (2007). Effect of waxy wheat flour blends on the quality of Chinese steamed bread. Agricultural Sciences in China, 6(10), 1275–1282.
Peng, X., Ma, J., Cheng, K. W., Jiang, Y., Chen, F., and Wang, M. (2010). The effects of grape seed extract fortification on the antioxidant activity and quality attributes of bread. Food Chem . 119, 49–53. doi: 10.1016/j.foodchem.2009.05.083
Pico, J., Gómez, M., Bernal, J., and Bernal, J. L. (2016). Analytical methods for volatile compounds in wheat bread. J. Chromatogr. A . 1428, 55–71. doi: 10.1016/j.chroma.2015.09.045
Pico, J., Reguilón, M. P., Bernal, J., and Gómez, M. (2019). Effect of rice, pea, egg white and whey proteins on crust quality of rice flour-corn starch based gluten-free breads. J. Cereal Sci . 86, 92–101. doi: 10.1016/j.jcs.2019.01.014
Poutanen, K., Flander, L., and Katina, K. (2009). Sourdough and cereal fermentation in a nutritional perspective. Food Microbiol . 26, 693–699. doi: 10.1016/j.fm.2009.07.011
Quraishi, U. M., Pont, C., Ain, Q. U., Flores, R., Burlot, L., Alaux, M., et al. (2017). Combined genomic and genetic data integration of major agronomical traits in bread wheat ( Triticum aestivum L.). Front. Plant Sci . 8:1843. doi: 10.3389/fpls.2017.01843
Rahaie, S., Gharibzahedi, S. M. T., Razavi, S. H., and Jafari, S. M. (2014). Recent developments on new formulations based on nutrient-dense ingredients for the production of healthy-functional bread: a review. J. Food Sci. Technol . 51, 2896–2906. doi: 10.1007/s13197-012-0833-6
Rosell, C. M. (2011). “The science of doughs and bread quality,” in Flour and Breads and Their Fortification in Health and Disease Prevention , eds. V. R. Preedy, R. R. Watson and V. B. Patel (Academic Press), 3–14.
Rubenthaler, G. L., Huang, M. L., and Pomeranz, Y. (1990). Steamed bread. I. Chinese steamed bread formulation and interactions. Cereal Chem . 67, 471–475.
Rupasinghe, H. V., Wang, L., Huber, G. M., and Pitts, N. L. (2008). Effect of baking on dietary fibre and phenolics of muffins incorporated with apple skin powder. Food Chem . 107, 1217–1224. doi: 10.1016/j.foodchem.2007.09.057
Rustagi, S., Khan, S., Choudhary, S., Pandey, A., Khan, M. K., Kumari, A., et al. (2018). Hydroxypropyl methylcellulose and whey protein concentrate as technological improver in formulation of gluten-free protein rich bread. Curr. Res. Nutr. Food Sci . 6, 211–221. doi: 10.12944/CRNFSJ.6.1.24
Rustgi, S., Shewry, P., Brouns, F., Deleu, L. J., and Delcour, J. A. (2019). Wheat seed proteins: factors influencing their content, composition, and technological properties, and strategies to reduce adverse reactions. Compr. Rev. Food Sci. Food Saf . 18, 1751–1769. doi: 10.1111/1541-4337.12493
Samapundo, S., Deschuyffeleer, N., Van Laere, D., De Leyn, I., and Devlieghere, F. (2010). Effect of NaCl reduction and replacement on the growth of fungi important to the spoilage of bread. Food Microbiol . 27, 749–756. doi: 10.1016/j.fm.2010.03.009
Sharma, S., Chunduri, V., Kumar, A., Kumar, R., Khare, P., Kondepudi, K. K., et al. (2018). Anthocyanin bio-fortified colored wheat: nutritional and functional characterization. PLoS ONE 13:e0194367. doi: 10.1371/journal.pone.0194367
Shewry, P. R., and Jones, H. D. (2020). “Improving wheat protein quality for breadmaking: the role of biotechnology,” in Breadmaking , ed. S. P. Cauvain (Woodhead Publishing), 261–288.
Shewry, P. R., and Miflin, B. J. (1955). Seed storage proteins of economically important cereals. Adv. Cereal Sci. Technol . 7, 1–84.
Shewry, P. R., Tatham, A. S., and Halford, N. G. (1999). “The prolamins of the triticeae,” in Seed Proteins (Dordrecht: Springer), 35–78.
Shittu, T. A., Raji, A. O., and Sanni, L. O. (2007). Bread from composite cassava-wheat flour: I. Effect of baking time and temperature on some physical properties of bread loaf. Food Res. Int . 40, 280–290. doi: 10.1016/j.foodres.2006.10.012
Sivam, A. S., Sun-Waterhouse, D., Perera, C. O., and Waterhouse, G. I. N. (2012). Exploring the interactions between blackcurrant polyphenols, pectin and wheat biopolymers in model breads; a FTIR and HPLC investigation. Food Chem . 131, 802–810. doi: 10.1016/j.foodchem.2011.09.047
Sivam, A. S., Sun-Waterhouse, D., Waterhouse, G. I., Quek, S., and Perera, C. O. (2011). Physicochemical properties of bread dough and finished bread with added pectin fiber and phenolic antioxidants. J. Food Sci . 76, H97–H107. doi: 10.1111/j.1750-3841.2011.02086.x
Su, X., Wu, F., Zhang, Y., Yang, N., Chen, F., Jin, Z., et al. (2019). Effect of organic acids on bread quality improvement. Food Chem . 278, 267–275. doi: 10.1016/j.foodchem.2018.11.011
Suliman, S., Alemu, A., Abdelmula, A. A., Badawi, G. H., Al-Abdallat, A., and Tadesse, W. (2021). Genome-wide association analysis uncovers stable QTLs for yield and quality traits of spring bread wheat ( Triticum aestivum ) across contrasting environments. Plant Gene 25:100269. doi: 10.1016/j.plgene.2020.100269
Sun, L., Li, X., Zhang, Y., Yang, W., Ma, G., Ma, N., et al. (2020). A novel lactic acid bacterium for improving the quality and shelf life of whole wheat bread. Food Cont . 109:106914. doi: 10.1016/j.foodcont.2019.106914
Supartini, N., and Mushollaeni, W. (2017). Local alginate as a food additive and nutritional improvement for white bread. Am. J. Res. Commun . 5, 1–22.
Tebben, L., Shen, Y., and Li, Y. (2018). Improvers and functional ingredients in whole wheat bread: a review of their effects on dough properties and bread quality. Trends Food Sci. Technol . 81, 10–24. doi: 10.1016/j.tifs.2018.08.015
Ullah, A., Farooq, M., Rehman, A., Arshad, M. S., Shoukat, H., Nadeem, A., et al. (2018). Manganese nutrition improves the productivity and grain biofortification of bread wheat in alkaline calcareous soil. Exp. Agric . 54, 744–754. doi: 10.1017/S0014479717000369
Verni, M., Rizzello, C. G., and Coda, R. (2019). Fermentation biotechnology applied to cereal industry by-products: nutritional and functional insights. Front. Nutrit . 6:42. doi: 10.3389/fnut.2019.00042
Wang, K., Lu, F., Li, Z., Zhao, L., and Han, C. (2017). Recent developments in gluten-free bread baking approaches: a review. Food Sci. Technol . 37, 1–9. doi: 10.1590/1678-457x.01417
Wang, R., and Zhou, W. (2004). Stability of tea catechins in the breadmaking process. J. Agric. Food Chem . 52, 8224–8229. doi: 10.1021/jf048655x
Xu, J., Wang, W., and Li, Y. (2019). Dough properties, bread quality, and associated interactions with added phenolic compounds: a review. J. Funct. Foods 52, 629–639. doi: 10.1016/j.jff.2018.11.052
Yaver, E., and Bilgiçli, N. (2019). Improvement of physical and sensory properties of bread containing Cereal-Legume composite flour. Selcuk J. Agric. Food Sci . 33, 7–13 doi: 10.15316/SJAFS.2019.149
Yildrim, A., and Nadeem, H. S. (2019). Thermal properties and estimated Glycemic index of different composite flours and their gluten-free bread making performances. Gida 44, 143–152. doi: 10.15237/gida.GD18105
Žilić, S., Barać, M., Pešić, M., Dodig, D., and Ignjatović-Micić, D. (2011). Characterization of proteins from grain of different bread and durum wheat genotypes. Int. J. Mol. Sci . 12, 5878–5894. doi: 10.3390/ijms12095878
Keywords: wheat, seed storage proteins, baking, gluten, nutrition
Citation: Goel S, Singh M, Grewal S, Razzaq A and Wani SH (2021) Wheat Proteins: A Valuable Resources to Improve Nutritional Value of Bread. Front. Sustain. Food Syst. 5:769681. doi: 10.3389/fsufs.2021.769681
Received: 02 September 2021; Accepted: 11 October 2021; Published: 17 November 2021.
Reviewed by:
Copyright © 2021 Goel, Singh, Grewal, Razzaq and Wani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sonia Goel, drsonia.fas@sgtuniversity.org ; Shabir Hussain Wani, shabirhussainwani@gmail.com
This article is part of the Research Topic
Sustaining Protein Nutrition through Plant-Based Foods: A Paradigm Shift
- Review article
- Open access
- Published: 14 November 2022
Evaluation of nutrients in bread: a systematic review
- Zahra Aghalari ORCID: orcid.org/0000-0002-9629-1433 1 ,
- Hans-Uwe Dahms 2 , 3 , 7 &
- Mika Sillanpää 4 , 5 , 6
Journal of Health, Population and Nutrition volume 41 , Article number: 50 ( 2022 ) Cite this article
6646 Accesses
2 Citations
10 Altmetric
Metrics details
A balanced and optimized amount of nutrients in bread, which is the main food in many countries, is necessary to maintain human health. Considering the importance of nutritional values of bread in the food basket of Iranian households, the purpose of this study was to determine the nutrients and their concentrations in breads consumed in Iran.
This systematic review study was performed to determine the types of nutrients in breads consumed in Iran by searching reputable international databases including Scopus and Google scholar, PubMed, Science direct, ISI (Web of Science). Data were collected according to inclusion and exclusion criteria and by searching for relevant keywords, emphasizing the types of nutrients in breads consumed in Iran. Qualitative data were collected using the standard PRISMA checklist (preferential reporting items for systematic reviews and meta-analysis). After verifying the quality of the articles, the information was entered into a checklist such as the name of the first author and year of publication of the research, type of study, number of samples, type of nutrition, type of bread and amount of nutrition measured.
After reviewing the information and quality of articles, 10 articles were qualified for systematic review. The review of the articles showed that different breads were experimented, including: Sangak, Barbari, Taftoon, Lavash, French and local bread. The highest number of experimented bread samples was Sangak. Examination of the articles showed that 6 nutrients were experimented in different breads such as Fe, K, Mg, Ca, Cu and Zn. The highest number of experimented in breads was related to the amount of Zn (13 times) and Cu (10 times), respectively. The results of quality assessment of articles showed that most of the studies were of good quality. The results of articles on the amount of nutrients measured in different breads showed that only in two articles the amount of nutrients was reported to be desirable. In most articles, the amount of nutrients in breads was reported to be lower or higher than standard.
The results of this study showed that the concentration of nutrients in most articles was undesirable. It is suggested that optimal methods of enrichment of breads and flours be done with interdisciplinary cooperation between food hygiene, environmental health, nutrition, farmers and bakers. It is recommended that food hygiene and environmental health researchers investigate other nutrients (including phosphorus, selenium, manganese, boron and molybdenum) in breads and other staple foods used by people to constructive and practical measures to increase public health.
Introduction
Cereals such as wheat, rice, corn and barley are the basis of human nutrition and life and provide 70% of people’s food [ 1 , 2 ]. Bread contains a type of cereals called wheat, which is why bread is a staple food in developing countries, especially in Africa and Asia. Baking bread from farm to bakery has several steps (Fig. 1 ), and each of these steps must comply with the principles of hygiene and food quality because the steps of bread preparation affect the quality of bread. In general, there are three types of bread serial/dough sources worldwide, which are wheat bread (with gluten), bread without gluten and combined bread [ 3 , 4 ].
Industrial and traditional bread preparation steps
Bread provides several nutrients such as sugar, protein, iron, calcium and a variety of vitamins [ 5 ]. An average daily intake of 300 g of bread can provide the nutrients needed by the body and create a desired nutritional status [ 6 ]. Bread can provide 1.2% of protein, 60% of thiamine and niacin, 40% of calcium and 80% of the daily iron needed by an adult [ 7 , 8 ].
Deficiency of nutrients endangers human health. According to the World Health Organization, malnutrition due to nutrient deficiency is a major problem and affects more than two billion people worldwide [ 9 ]. The amount of nutrients (P, K, Se, Mg, Ca, B, MO, Cu, Zn, Mn) varies in food, including different bread samples, and each of the nutrients has a specific structure (Table 1 ).
Nutrients have different biological effects on the body. Iron is one of the nutrients which deficiency causes the most common nutritional problems. For example, iron deficiency anemia is the most common type of anemia in the world [ 20 , 21 ]. Zn is another nutrient that is the second most abundant trace element in the body after iron and participates in the synthesis of brain enzymes [ 22 ]. The recommended amount of Zn for daily absorption is 60 mg [ 23 ]. Zn is contained in several enzymes including carbonic anhydrase, dehydrogenase, proteinase and peptidase, and Zn in wheat reduces the carbohydrate content of leaves and stems [ 24 ]. Zn deficiency causes diseases such as impaired physical development and the immune system, reduced ability to learn, increased risk of infection and cancer [ 25 , 26 ]. Cu is another nutrient that is a vital component of the body. Cu is involved in the structure, function and activity of many enzymes, in the function and activity of catalase, peroxidase and glutathione in the body. Cu deficiency increases the sensitivity of lipoproteins to peroxidation [ 27 , 28 , 29 ]. Se is another nutrient that prevents toxin transmission from mother to fetus. Se is an antioxidant in the body. Scientists have reported that Se is a factor that reduces aging [ 30 ].
Overcoming nutrient deficiencies is a major challenge for humans. There have been many studies on nutrients. A study by Jawad et al. was aiming at the study of copper and iron in wheat, flour and bread grains in Iraq [ 31 ]. In a study by Harmankaya et al., the concentrations of copper, iron, manganese and zinc in bread and wheat produced in Turkey were investigated [ 32 ]. In a study by Kirchmann et al., the amount of copper and iron in wheat grains in Switzerland was investigated [ 33 ].
Bread as the dominant food has a major share in the consumption pattern of households. The per capita consumption of wheat and bread in Iran is about 24 and 300 kg, respectively [ 34 , 35 ]. Considering the nutritional value of bread in the food basket of Iranian households, the purpose of this study was to determine the concentration of nutrients in breads consumed in Iran.
Study protocol
This systematic review study was performed to determine the types of nutrients in breads consumed in Iran by searching reputable international databases including Scopus and Google scholar, PubMed, Science direct, ISI (Web of Science). Search time was from August 1 to September 10, 2020. This search was performed by two authors of this article. To ensure the receipt of all articles related to the objectives of the research, the reference of the articles was reviewed.
Search strategy
Inquired information was collected by searching for keywords on the desired sites. Key words included: ‘Nutrients’ AND ‘Phosphorus’ OR ‘P’ AND ‘Calcium’ OR ‘Ca’ AND ‘Magnesium’ OR ‘Mg’ AND ‘Potassium’ OR ‘K’ AND ‘Selenium’ OR ‘Se’ AND ‘Manganese’ OR ‘Mn’ AND ‘Boron’ OR ‘B’ AND ‘Molybdenum’ OR ‘Mo’ AND ‘Copper’ OR ‘Cu’ AND ‘Zinc’ OR ‘Zn’ AND ‘Iron’ OR ‘Fe’ AND ‘Bread’ AND ‘Iran.’
Inclusion criteria
Inclusion criteria for this study included several items: the year of publication, the type of nutrients, all English and Persian articles with full text that were done in Iran.
Exclusion criteria
Criteria for excluding articles from this study included several items: lack of access to the full article, inconsistency of the subject, lack of methodology, review studies and letter to the editor, case reports, duplicate report results in other articles.
Quality assessment
The quality of the articles was assessed based on the standard checklist PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses). The National Institutes of Health’s Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies was used to assess the quality of the studies [ 36 ]. This checklist examines various aspects in articles such as study objectives, determining the appropriate sample size, type of study, sampling method, research community, data collection method, definition of variables and data collection tools, statistical tests, presentation of findings and results. In this study, the quality of each article was evaluated according to the checklist and scores of each article were given based on Yes, No, cannot determine not applicable and not reported (Table 2 ).
Information extraction
To extract information, all articles were reviewed according to entry and exit criteria by two independent reviewers. Both reviewers eventually summarized the information and used the views of the third author in cases where the information was inconsistent. In case of quality approval of the articles, the information extracted from the articles was entered in the checklist [ 37 , 38 , 39 ]. The checklist included the name of the first author and year of publication of the research, type of study, number of samples, type of nutrition, type of bread and amount of nutrition.
Search results
The steps for selecting articles are shown in Fig. 1 . Using the listed keywords in combination or alone, 537 articles were found. After deleting irrelevant and duplicate articles and deleting articles based on the inclusion and exclusion criteria of this study, 10 articles remained. After reviewing the information and quality of articles, 10 articles qualified for systematic review (Fig. 2 ).
Flowchart showing the process of study selection
Descriptive results of studies
In terms of publication time, 10 articles were published between 2004 and 2017. The largest number of articles (80%) were published between 2012 and 2017. The cities that performed nutrients on bread samples were 9 provinces from the north, south, center and west of Iran, which were shown separately on the map (Fig. 3 ).
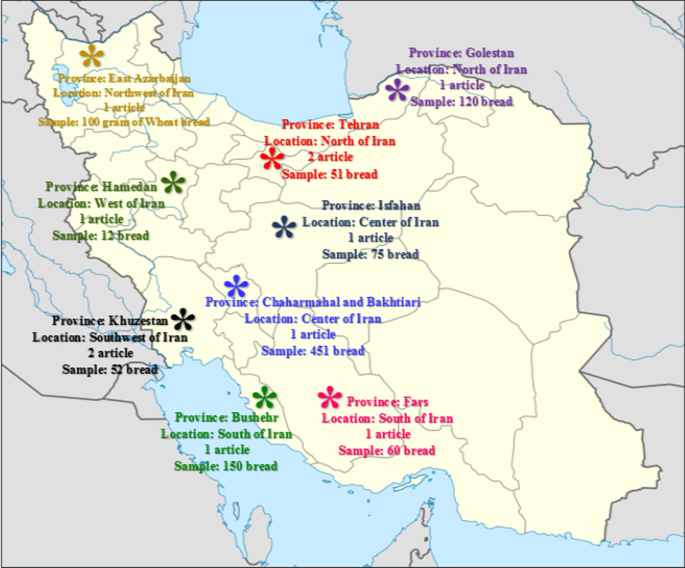
Locations of studies in 10 articles. *The locations of the provinces were shown on the map
The review of the articles showed that different bread samples were taken as samples, including: Sangak, Barbari, Taftoon, Lavash, French and local bread. The highest number of bread samples came from Sangak (5 articles) (Fig. 4 ).
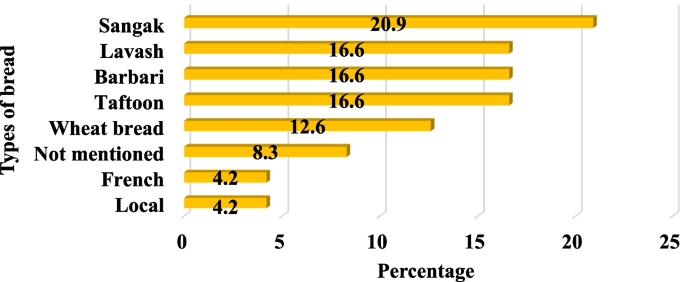
Types of bread sampled in 10 articles
The review of the articles showed that 6 types of nutrients (Fe, K, Mg, Ca, Cu and Zn) were experimented in different breads. The highest number of experimented in breads was related to the amount of Zn (13 times) and Cu (10 times) (Fig. 5 ).

Types of nutrients measured in bread samples in 10 articles
Quality assessment of studies
The results of quality assessment showed that most of the studies were of good quality. In a number of studies such as Torchi et al. [ 45 ] and Kianpoor et al. [ 47 ], the method of determining the sample size was not clear (Q5).
In the articles, participation rate of eligible persons, inclusion and exclusion criteria, the exposure(s) of interest measured prior to the outcome(s), the timeframe sufficient, exposure(s) assessed more than once over time, blinded to the exposure status of participants was not relevant and not applicable (Q3, Q4, Q6, Q7, Q10 and Q12) (Table 3 ).
Article features
All articles reviewed were original articles based on laboratory analysis. The highest sample size in a study by Tabibian et al. [ 48 ] [48 bread samples] and Chaharmahal and Bakhtiari [451 bread samples]. In most articles, Zn nutrients were experimented in breads such as by Falahi et al. [ 40 ], Shockravi et al. [ 42 ], Badii et al. [ 43 ], Hojati et al. [ 44 ], Heidari et al. [ 46 ], Kianpoor et al. [ 47 ] and Shiralipour [ 49 ] (Table 4 ).
In most articles, the type of bread experimented was Sangak such as by Shockravi et al. [ 42 ], Hojati et al. [ 44 ], Torchi et al. [ 45 ], Kianpoor et al. [ 47 ] and Tabibian et al. [ 48 ] (Table 4 ).
A review of article results showed that only in two articles Sadighi et al. [ 41 ] Fe level and in Badii et al. [ 43 ] Zn levels were desirable. In a study by Sadighi et al. [ 41 ], the amount of Fe in different breads was divided into 5 categories, which showed that 77.4% of the bread sampled from Bushehr and 61.7% of the bread sampled from Golestan were acceptable. In 6 articles, the amount of nutrients was reported as lower than standard, and in 2 articles, Sadighi et al. [ 41 ] and Tabibian et al. [ 48 ], the amount of nutrients was reported as higher than standard (Table 4 ).
The findings of this article showed that among the various bread (Sangak, Barbari, Taftoon, Lavash, French and local breads) the highest number of bread samples analyzed was from Sangak, for example, in the studies of Shockravi et al. [ 42 ], Hojati et al. [ 44 ], Torchi et al. [ 45 ], Kianpoor et al. [ 47 ] and Tabibian et al. [ 48 ] (Table 4 ). Most researchers used Sangak bread to study the characteristics of Iranian breads because the use of Sangak is recommended by Iranian nutritionists. The reason is that wholemeal flour is used to bake Sangak and wholemeal flour, and its vitamins and minerals are preserved. Sangak has high levels of vitamins, calcium, protein and iron and is easy to digest due to its high fiber content. Sangak has high levels of vitamins, calcium, protein and iron, and Sangak is easy to digest due to its high fiber content. One of the good features of Sangak is its taste, aroma, nutrition and satiety. The traditional method of baking Sangak, its shape and taste is different from other breads that are made and are highly valued in Iranian culture; especially for breakfast, the presence of Sangak is a priority [ 50 , 51 ].
According to the findings of this article, researchers paid most attention to measuring the amount of Zn in breads. In a study by Falahi et al. [ 40 ] zinc level 1.79 ± 0.01 mg/ 100 g, Shockravi et al. [ 42 ] zinc level 1.66 mg/100 g, Hojati et al. [ 44 ] zinc level 0.591 mg/100 g, Heidari et al. [ 46 ] zinc level 1.3 mg/kg, Kianpoor et al. [ 47 ], the amount of zinc in Lavash, Barbari, Sangak, French breads, respectively, is 5.61 ± 0.32 mg/kg, 8.84 ± 0.30 mg/kg, 4.35 ± 0.16 mg/kg and 3.07 ± 0.09 mg/kg. In the analysis of the results of all studies, it was mentioned that the amount of zinc was less than the standard. Zinc deficiency in bread can cause serious problems for the health of the Iranian people because bread is one of the main sources of zinc in Iranian food. One of the reasons for zinc deficiency in Iran is insufficient intake of zinc from the diet, high consumption of grains, especially unfermented bread [ 52 , 53 ]. Various studies have reported that zinc deficiency in the Middle East is due to a poor diet and zinc deficiency is common in developing countries such as Iran [ 54 , 55 ]. Since deficiency causes problems such as hair loss, imbalance when walking in the elderly, anorexia, taste disturbance, lethargy, fainting, behavioral disorders and growth retardation in children [ 56 , 57 ], attention should be paid to flour fortification and consumer breads in Iran.
Based on the findings of this article, it was reported about K in Tabibian et al. [ 48 ] K level, lower than standard and undesirable. Low levels of potassium in bread can cause problems over time. Potassium disorders are the most common electrolyte abnormality detected in clinical practice [ 58 ]. Potassium deficiency causes fatigue, drowsiness, muscle weakness, constipation, irregular heartbeat and delayed gastric emptying. Many studies have shown that potassium plays an essential role in the normal functioning of cells, and a diet with adequate potassium levels is important for the prevention of cancer and cardiovascular disease [ 59 , 60 ]. Since potassium is one of the basic elements of the body that the body needs the desired amount for a wide range of functions, it is necessary to examine the amount of potassium in people’s basic foods, including bread, and if potassium is undesirable, health and nutritional measures should be taken.
One of the strengths of this study is addressing an issue related to food quality, namely the amount of nutrients in bread consumed by people. Although bread is one of the main foods of Iranian people, researchers did less research on it. Another strength of this study was the review of articles without time limit so that all related articles were included in this study.
Conclusions
According to the findings of this study and by comparing the amount of different nutrients (Fe, K, Mg, Ca, Cu, Zn) in bread, it was determined that the amount of nutrients in breads sampled in an unfavorable condition is lower than standard or higher than standard. It is suggested that optimal methods of enrichment of breads and flours should be done with interdisciplinary cooperation between food hygiene, environmental health, nutrition, farmers and bakers. It is recommended that food hygiene and environmental health researchers investigate other nutrients (including phosphorus, selenium, manganese, boron and molybdenum) in breads and other staple foods used by people as constructive and practical measures to increase public health.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
World Health Organization
Web of Science
Preferred Reporting Items for Systematic Reviews and Meta-analyses
Aghili F, Gamper HA, Eikenberg J, et al. Green manure addition to soil increases grain zinc concentration in bread wheat. PLoS ONE. 2014;9(7):e101487. https://doi.org/10.1371/journal.pone.0101487 .
Article PubMed PubMed Central Google Scholar
Shewry PR, Hey SJ. The contribution of wheat to human diet and health. Food Energy Secur. 2015;4(3):178–202. https://doi.org/10.1002/fes3.64 .
Abdelghafor RF, Mustafa AI, Ibrahim AMH, Krishnan PG. Quality of bread from composite flour of sorghum and hard white winter wheat. Adv J Food Sci Technol. 2011;3(1):9–15.
Google Scholar
Shittu TA, Aminu RA, Abulude EO. Functional effects of xanthan gum oncomposite cassava-wheat dough and bread. Food Hydrocolloids. 2009;23:2254–60.
Article CAS Google Scholar
Alsuhaibani AMA. Rheological and nutritional properties and sensory evaluation of bread fortified with natural sources of calcium. J Food Qual. 2018. https://doi.org/10.1155/2018/8308361 .
Article Google Scholar
Namayandeh SM, Lotfi MH, Jafari V, Dad V, Biabani J, Razi MH, et al. Salt content in traditional and nontraditional breads in Yazd City, Iran, 2015–2016. JNFS. 2018;3(4):185–92. https://doi.org/10.18502/jnfs.v3i4.162 .
Qajarbeygi P, Kazeminia M, Mahmoudi R. Determine the quality of bread samples used in Qazvin, Iran. J Chem Health Risks. 2018. https://doi.org/10.22034/jchr.2018.544195 .
Grafenauer S, Curtain F. An audit of Australian bread with a focus on loaf breads and whole grain. Nutrients. 2018;10(8):1106. https://doi.org/10.3390/nu10081106 .
Article CAS PubMed Central Google Scholar
Hostz C, Brown KH. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr Bull. 2004;25:94–204.
National Center for Biotechnology Information. "PubChem Compound Summary for CID 5462309, Phosphorus" PubChem. https://pubchem.ncbi.nlm.nih.gov/compound/Phosphorus . Accessed 15 Sept 2020.
National Center for Biotechnology Information. "PubChem Compound Summary for CID 5460341, Calcium" PubChem. https://pubchem.ncbi.nlm.nih.gov/compound/Calcium . Accessed 15 Sept 2020.
National Center for Biotechnology Information. "PubChem Compound Summary for CID 5462224, Magnesium" PubChem. https://pubchem.ncbi.nlm.nih.gov/compound/Magnesium . Accessed 15 Sept 2020.
National Center for Biotechnology Information. "PubChem Compound Summary for CID 5462222, Potassium" PubChem. https://pubchem.ncbi.nlm.nih.gov/compound/Potassium . Accessed 15 Sept 2020.
National Center for Biotechnology Information. "PubChem Compound Summary for CID 6326970, Selenium" PubChem. https://pubchem.ncbi.nlm.nih.gov/compound/Selenium . Accessed 15 Sept 2020.
National Center for Biotechnology Information. "PubChem Compound Summary for CID 15551713, CID 15551713" PubChem. https://pubchem.ncbi.nlm.nih.gov/compound/manganese . Accessed 15 Sept 2020.
National Center for Biotechnology Information. "PubChem Compound Summary for CID 5462311, Boron" PubChem. https://pubchem.ncbi.nlm.nih.gov/compound/Boron . Accessed 15 Sept 2020.
National Center for Biotechnology Information. "PubChem Compound Summary for CID 23932, Molybdenum" PubChem. https://pubchem.ncbi.nlm.nih.gov/compound/Molybdenum . Accessed 15 Sept 2020.
National Center for Biotechnology Information. "PubChem Compound Summary for CID 23978, Copper" PubChem. https://pubchem.ncbi.nlm.nih.gov/compound/Copper . Accessed 15 Sept 2020.
National Center for Biotechnology Information. "PubChem Compound Summary for CID 23994, Zinc" PubChem. https://pubchem.ncbi.nlm.nih.gov/compound/Zinc . Accessed 15 Sept 2020.
Black MM. Micronutrient deficiencies and cognitive functioning. J Nutr. 2003;133(11 Suppl 2):3927S-3931S. https://doi.org/10.1093/jn/133.11.3927S .
Article CAS PubMed Google Scholar
Miller JL. Iron deficiency anemia: a common and curable disease. Cold Spring Harb Perspect Med. 2013;3(7):a011866. https://doi.org/10.1101/cshperspect.a011866 .
Article CAS PubMed PubMed Central Google Scholar
Bhattacharya PT, Misra SR, Hussain M. Nutritional aspects of essential trace elements in oral health and disease: an extensive review. Scientifica (Cairo). 2016;2016:5464373. https://doi.org/10.1155/2016/5464373 .
Alam MG, Snow ET, Tanaka A. Arsenic and heavy metal contamination of vegetables grown in Samta village, Bangladesh. Sci Total Environ. 2003;308(1–3):83–96.
Zastrow ML, Pecoraro VL. Designing hydrolytic zinc metalloenzymes. Biochemistry. 2014;53(6):957–78. https://doi.org/10.1021/bi4016617 .
Black RE, Lindsay HA, Bhutta ZA, Caulfield LE, Deonnis M, Ezzati M, Mathers C, Rivera J. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371:243–60.
Article PubMed Google Scholar
Skrajnowska D, Bobrowska-Korczak B. Role of zinc in immune system and anti-cancer defense mechanisms. Nutrients. 2019;11(10):2273. https://doi.org/10.3390/nu11102273 .
Hunter P. A toxic brew we cannot live without. Micronutrients give insights into the interplay between geochemistry and evolutionary biology. EMBO Rep. 2008;9(1):15–8. https://doi.org/10.1038/sj.embor.7401148 .
Matouke MM. FTIR study of the binary effect of titanium dioxide nanoparticles (nTiO2) and copper (Cu2+) on the biochemical constituents of liver tissues of catfish (Clarias gariepinus). Toxicol Rep. 2019;6:1061–70. https://doi.org/10.1016/j.toxrep.2019.10.002 .
Whanger PD. Selenium and its relationship to cancer: an update. Br J Nutr. 2004;91(1):11–38.
Polanska K, Krol A, Sobala W, et al. Selenium status during pregnancy and child psychomotor development-Polish Mother and Child Cohort study. Pediatr Res. 2016;79(6):863–9. https://doi.org/10.1038/pr.2016.32 .
Jawad I, Allafaji SH. The levels of trace metals contaminants in wheat grains, flours and breads in Iraq. Aust J Basic Appl Sci. 2012;6(10):88–92.
CAS Google Scholar
Harmankaya M, Ozcan MM, Gezgin S. Variation of heavy metal and micro and macro element concentrations of bread and durum wheats and their relationship in grain of Turkish wheat cultivars. Environ Monit Assess. 2012;184(9):5511–21.
Kirchmann H, Mattsson L, Eriksson J. Trace element concentration in wheat grain: results from the Swedish long-term soil fertility experiments and national monitoring program. Environ Geochem Health. 2009;31(5):561–71.
Abdollahi M, Mohammadi F, Houshiar-Rad A, HajiFaragi M, Esfarjani F. Shares of energy and nutrients intake from subsidized food items in Iranian households in different socio-economic status. Iran J Nutr Sci Food Technol. 2011;26(1):43–56.
Sakizadeh M, Ghorbani H. Concentration of heavy metals in soil and staple crops and the associated health risk. Arch Hyg Sci. 2017;6(4):303–13.
National Heart, Lung, and Blood Institute. Study quality assessment tools. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools . Accessed 30 July 2019.
Tirgar A, Sajjadi SA, Aghalari Z. The status of international collaborations in compilation of Iranian scientific articles on environmental health engineering. Glob Health. 2019;15(1):17. https://doi.org/10.1186/s12992-019-0460-3 .
Tirgar A, Aghalari Z. Scientific achievements of medical journals in occupational accidents. HDQ. 2018;3(4):179–84. https://doi.org/10.32598/hdq.3.4.179 .
Aghalari Z, Tirgar A. Topics of disasters in scientific outputs of medical sciences: a cross-sectional study. HDQ. 2017;2(2):47–52. https://doi.org/10.18869/nrip.hdq.2.2.47 .
Falahi E, Mohtadinia J, Mahboob SA. Effect of consumption of whole bread baked from cultivated wheat with microfertilizers on blood indices of Iron. Yafte. 2004;5(2):3–11.
Sadighi J, Mohammad K, Sheikholeslam R, Torabi P, Salehi F, Abdolahi Z, et al. Flour fortification with iron and folic acid in Bushehr and Golestan provinces, Iran: program evaluation. Sjsph. 2010;7(4):11–24.
Shockravi S, Mohammad-Shirazi M, Abadi A, Seyedain M, Kimiagar M. Phytase supplementation improves blood zinc in rats fed with high phytate Iranian bread. J Res Med Sci. 2012;17(4):361–7.
PubMed PubMed Central Google Scholar
Badii A, Nekouei N, Fazilati M, Shahedi M, Badiei S. Effect of consuming zinc-fortified bread on serum zinc and iron status of zinc-deficient women: a double blind, randomized clinical trial. Int J Prev Med. 2012;3(Suppl 1):S124–30.
Hojati M, Jahangiri AR, Najafi MA. Evaluation of phytic acid and zinc content in breads produced in Ahwaz. FSCT. 2015;12(47):9–19.
Torchi M, Seyedain Ardabili M, Azizi Nejad R, Nematollahi F. Assessing the effect of baking methods on the levels of heavy metals in Iranian traditional breads. J Food Technol Nutr. 2016;14(1):5–12.
Heidari B, Padash S, Dadkhodaie A. Variations in micronutrients, bread quality and agronomic traits of wheat landrace varieties and commercial cultivars. AJCS. 2016;10(3):377–84. https://doi.org/10.21475/ajcs.2016.10.03.p7231 .
Kianpoor S, Sobhanardakani S. Evaluation of Zn, Pb, Cd and Cu concentrations in wheat and bread consumed in Hamedan city. Food Hyg. 2017;7(4):83–92.
Tabibian M, Torbati M, Afshar Mogaddam MR, Mirlohi M, Sadeghi M, Mohtadinia J. Evaluation of vitamin D3 and D2 stability in fortified flat bread samples during dough fermentation, baking and storage. Adv Pharm Bull. 2017;7(2):323–8. https://doi.org/10.15171/apb.2017.038 .
Shiralipour R, Jahangiri AR, Baaghdezfooli MR. Determination of iron, zinc and copper in fortified wheat flour consumed in Ahvaz. JFST. 2017;65(1):1–7.
Emami SA, Sobhani Z. Characteristics of different ethnic and traditional bread from the perspective of Islamic traditional medicine. J Ethnic Food. 2020;7:13. https://doi.org/10.1186/s42779-020-00051-7 .
Karizaki VM. Ethnic and traditional Iranian breads: different types, and historical and cultural aspects. J Ethnic Foods. 2017;4(1):8–14.
Gargari BP, Mahboob S, Razavieh SV. Content of phytic acid and its mole ratio to zinc in flour and breads in Tabriz, Iran. Food Chem. 2007;100:1115–9.
Houshiar-rad A, Esmaeili M, Abdollahi M, Mazaheri N, Mohammadi M, Kalantari N. Zinc intake pattern in Iranian households and risk of zinc deficiency at national level. Iran J Nutr Sci Food Technol. 2013;7(5):409–20.
Prasad AS, Miale A Jr, Farid Z, Sandstead HH, Schulert AR. Zinc metabolism in patients with the syndrome of iron deficiency anemia, hepatosplenomegaly, dwarfism, and hypognadism. J Lab Clin Med. 1963;61:537–49.
CAS PubMed Google Scholar
Motadi SA, Mbhenyane XG, Mbhatsani HV, Mabapa NS, Mamabolo RL. Prevalence of iron and zinc deficiencies among preschool children ages 3 to 5 y in Vhembe District, Limpopo Province, South Africa. Nutrition. 2015;31:452–8. https://doi.org/10.1016/j.nut.2014.09.016 .
Prasad AS, Bao B. Molecular mechanisms of zinc as a pro-antioxidant mediator: clinical therapeutic implications. Antioxidants (Basel). 2019;8(6):164. https://doi.org/10.3390/antiox8060164 .
Prasad AS, Beck FW, Snell DC, Kucuk O. Zinc in cancer prevention. Nutr Cancer. 2009;61:879–87. https://doi.org/10.1080/01635580903285122 .
Schaefer TJ, Wolford RW. Disorders of potassium. Emerg Med Clin N Am. 2005;23(3):723–ix. https://doi.org/10.1016/j.emc.2005.03.016 .
Kardalas E, Paschou SA, Anagnostis P, Muscogiuri G, Siasos G, Vryonidou A. Hypokalemia: a clinical update. Endocr Connect. 2018;7(4):R135–46. https://doi.org/10.1530/EC-18-0109 .
Tabasum A, Shute C, Datta D, George L. A man with a worrying potassium deficiency. Endocrinol Diabetes Metab Case Rep. 2014;2014:130067. https://doi.org/10.1530/EDM-13-0067 .
Download references
Acknowledgements
Since this research is part of a research project approved at Gonabad University of Medical Sciences, it is hereby sponsored by Gonabad University of Medical Sciences Research and Technology, which supported the research (Project No. T/4/95) and the Code of Ethics. (IR.GMU.REC.1396.110) is appreciated.
This research was funded by the Deputy of Research and Technology of Gonabad University of Medical Sciences. The funders did not have any role in the design of the study and collection, analysis and interpretation of data and in writing the manuscript.
Author information
Authors and affiliations.
Environmental Health Engineer, Faculty of Public Health, Babol University of Medical Sciences, Babol, Islamic Republic of Iran
Zahra Aghalari
Department of Biomedical Science and Environment Biology, College of Life Science, Kaohsiung Medical University, Kaohsiung, Taiwan
Hans-Uwe Dahms
Research Center for Environmental Medicine, KMU - Kaohsiung Medical University, Kaohsiung, 80708, Taiwan
Department of Biological and Chemical Engineering, Aarhus University, Nørrebrogade 44, 8000, Aarhus C, Denmark
Mika Sillanpää
Faculty of Science and Technology, School of Applied Physics, University Kebangsaan Malaysia, 43600, Bangi, Selangor, Malaysia
School of Chemistry, Shoolini University, Solan, Himachal Pradesh, 173229, India
Department of Marine Biotechnology and Resources, National Sun-Yat-Sen University, Kaohsiung, 80424, Taiwan
You can also search for this author in PubMed Google Scholar
Contributions
ZA conceived the study, made final decisions on the inclusion of journal articles and extracted data from them and wrote and revised the manuscript. HUD and MS wrote and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Zahra Aghalari .
Ethics declarations
Ethics approval and consent to participate.
This study was approved and registered by the Code of Ethics (IR.GMU.REC.1396.110).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Aghalari, Z., Dahms, HU. & Sillanpää, M. Evaluation of nutrients in bread: a systematic review. J Health Popul Nutr 41 , 50 (2022). https://doi.org/10.1186/s41043-022-00329-3
Download citation
Received : 08 January 2021
Accepted : 13 October 2022
Published : 14 November 2022
DOI : https://doi.org/10.1186/s41043-022-00329-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Food hygiene
- Environmental health
Journal of Health, Population and Nutrition
ISSN: 2072-1315
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Advertisement
Processing strategies to improve the breadmaking potential of whole-grain wheat and non-wheat flours
- Open access
- Published: 02 March 2022
- Volume 2 , article number 11 , ( 2022 )
Cite this article
You have full access to this open access article
- Tamara Dapčević-Hadnađev ORCID: orcid.org/0000-0001-6222-2889 1 ,
- Jelena Tomić 1 ,
- Dubravka Škrobot 1 ,
- Bojana Šarić 1 &
- Miroslav Hadnađev 1
5460 Accesses
6 Citations
Explore all metrics
Strategies to increase the bio-functionality of staple food, such as bread, by incorporating whole-grain wheat flour or flour from other, non-wheat grains instead of refined wheat flour are often constrained with the lack of their techno-functionality, despite the associated beneficial effect on consumers' health and well-being. Most of the available studies investigating the possibilities to improve technological and sensory quality of bread prepared using whole-grain wheat and non-wheat flours still rely on formulation approaches in which different additives and novel ingredients are used as structuring agents. Less attention has been given to technological approaches which could be applied to induce structural changes on biopolymer level and thus increase the breadmaking potential of whole grains such as: modification of grain and biopolymers structure by germination, flour particle size reduction, dry-heat or hydrothermal treatment, atmospheric cold plasma, high-pressure processing or ultrasound treatment. Strategies to modify processing variables during breadmaking like dough kneading and hydration modification, sourdough fermentation or non-conventional baking techniques application are also poorly exploited for bread preparation from non-wheat grains. In this paper, the challenges and opportunities of abovementioned processing strategies for the development of bread with whole-wheat flours and non-wheat flours from underutilised gluten-containing or gluten-free cereals and pseudocereals will be reviewed throughout the whole breadmaking chain: from grain to bread and from milling to baking. Feasibility of different strategies to increase the technological performance and sensory quality of bread based on whole-grain wheat flours or flours from other, non-wheat grains will be addressed considering both the environmental, safety and nutritive advantages.
Similar content being viewed by others
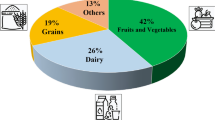
Food preservation techniques and nanotechnology for increased shelf life of fruits, vegetables, beverages and spices: a review
Adithya Sridhar, Muthamilselvi Ponnuchamy, … Ashish Kapoor
Plant-based milk alternatives an emerging segment of functional beverages: a review
Swati Sethi, S. K. Tyagi & Rahul K. Anurag
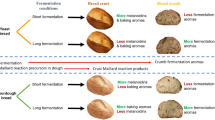
Effect of fermentation conditions of bread dough on the sensory and nutritional properties of French bread
Romane Troadec, Stéphanie Regnault, … Céline Jouquand
Avoid common mistakes on your manuscript.
1 Introduction
Bread, regardless of the type, production process and geographical origin, is traditionally produced from refined common wheat ( Triticum aestivum ) flour. However, in recent years, there has been renewed interest in fortifying or replacing refined wheat flour with whole-grain wheat flour, or flour from gluten-free cereals (rice, maize, sorghum, millet), pseudocereals (amaranth, buckwheat, quinoa) and ancient cereals [ 1 , 2 ]. This trend is governed with different reasons: from health-conscious and eco-friendly to economically driven.
Unlike refined wheat flour, whole-grain cereals and pseudocereals possess dense nutritional composition and a range of bioactive compounds. Therefore, their consumption contributes to increased intake of micronutrients, dietary fibres, phenolics, etc. Several studies have shown that regular consumption of whole-grain cereals is associated with health benefits such as a lower risk of chronic-degenerative diseases and improved body weight regulation [ 3 ]. Additionally, gluten-free cereals are finding an increased demand since coeliac disease or other gluten-associated allergies incidence rates are raising over time [ 4 ]. On the other hand, in developing countries, utilization of indigenous grain crops (the case of millet in Africa) is promoted. This contributes to economic development of local agriculture sector through reducing reliance on wheat importation and ensuring food security. Utilization of 'zero km' ingredients and relevance of short food supply chains in increasing the access to healthy and sustainable food has particularly growing attention in crisis situation such as COVID-19 pandemic [ 5 , 6 ].
Despite their contribution to consumers' well-being, sustainability of cereal cultivation and biodiversity protection, whole-grain alternative cereals exploitation in breadmaking is still being diminished due to the lower technological quality compared to refined wheat. The major challenges encountered in whole-grain or non-wheat cereals incorporation in breadmaking are poor gas retention, low loaf volume, hard and/or crumbling crumb texture, altered colour, short shelf-life of bread. This could be related to dilution or absence of gluten complex responsible for viscoelastic properties of dough and/or water competition effect between fibres and gluten [ 1 , 7 ]. The abovementioned quality deficiencies are often coupled with the lower consumers' acceptance of the product sensory properties. The most common sensory attributes of whole-grain and non-wheat cereal-based products are nutty odour, pungent flavour, bitter/astringent/sour taste; associated with the presence of phenolic compounds and in particular the condensed tannins which are located in the outermost bran layers [ 6 ]. In addition, lipid-rich cereals, such as oat, are susceptible to lipid oxidation which leads to development of the undesired sensory attributes evaluated as musty and earthy odour and bitter and rancid flavour [ 8 ]. Generally, altered technological quality (product volume, texture, structure, etc.) and sensory attributes of whole-grain and non-wheat cereal based products represent a limitation in their widespread acceptance.
Different strategies are thus proposed to produce bread from whole-grain and non-wheat cereals with technological and sensory profile comparable to refined wheat bread, while preserving their nutritional value. The most commonly applied strategies are the once involving bread formulation optimization through inclusion of various improvers, such as vital gluten or texturing agents (e.g. hydrocolloids, emulsifiers, enzymes and different food additives) that could act as structure forming agents instead of diluted or absent gluten [ 9 , 10 ]. In order to contribute to 'clean label' products design as well as its cost-effectiveness, some researches have modified abovementioned compositional approach by replacing food additives with fibre rich raw materials or food processing by-products to overcome the gluten deficiency [ 11 , 12 ].
However, relatively little research has been conducted on technological approaches for improving breadmaking potential of whole-grain and non-wheat cereals. As noted by Parenti et al. [ 1 ] instead of modifying process variables to prepare unrefined wheat flour bread, most of the studies are adopting the same methods as for their counterparts prepared with refined flour.
Therefore, the aim of this review is to provide a critical opinion on current and future-looking sustainable technological innovations and strategies utilized to increase the technological performance and sensory quality of bread based on whole-grain and non-wheat cereals. Improvement strategies discussed in this paper encompassed the whole bread production chain (Fig. 1 ): from raw material (cereal, flour, etc.) to process (milling, kneading, leavening, baking, etc.) modification, considering both the environmental, safety and nutritive advantages related to the use of conventional and emerging technologies and approaches.
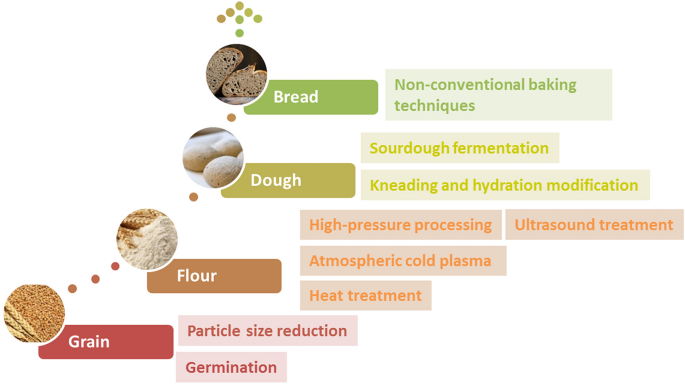
Summary of technological approaches for increased breadmaking potential of whole-grain wheat and non-wheat flours along the whole breadmaking chain
2 Strategies to modify raw material for breadmaking
2.1 grain modification approaches, 2.1.1 germination.
Modification of grain and biopolymers structure by germination is mostly performed to initiate nutrient compositional changes which are associated to health benefits. During the germination process degradation of macromolecules occurs due to increased enzyme activities: (i) starch is hydrolysed by amylolytic enzymes to maltose, glucose, dextrins and oligosaccharides, resulting in its higher digestibility [ 13 , 14 , 15 ]; (ii) storage proteins are degraded by endopeptidases produced from the aleurone layer and scutellum thus releasing peptides and free amino acids [ 15 , 16 , 17 , 18 ]; (iii) the ratio of soluble to insoluble dietary fibre increases especially when long germination times are applied [ 17 , 20 ]; (iv) a phytate (antinutrient present in cereals) content decreases as a result of increased phytase activity thus releasing chelated cations leading to increased bioavailability of phosphorus and minerals such as Zn 2+ , Fe 2+/3+ , Ca 2+ , Mg 2+ , Mn 2+ and Cu 2+ [ 13 ]. Moreover, germination process results in the increase in free fraction of phenolic acids due to decrease in the bound one contributing to increased antioxidant activity [ 13 , 15 , 18 , 19 ]. Germination is also a strategy to produce important metabolites such as γ-aminobutyric acid (GABA) [ 14 , 18 ], recommended to prevent neurological disorders [ 21 ].
Although increase in enzymatic activity produced by germination has mostly a detrimental effect on the breadmaking potential of cereals, with proper adjustment of the germination parameters it can be a promising tool to improve both the nutritional and technological properties of cereal-based food. In general, germination leads to softer and more fragile grain as a consequence of enzyme action which results in lower damaged starch content upon milling [ 22 ]. This, along with partial protein hydrolysis and decrease in insoluble fibre content, contribute to lower water absorption of flour from germinated wheat [ 17 ]. The germination also affects dough rheological properties in the following directions: (i) weakening of the gluten ability to form viscoelastic network due to decrease in the level of high-molecular-weight glutenin macropolymers which reflects in reduction of the tenacity, an increase of the extensibility of dough, and (ii) reduction of starch gelatinization and retrogradation ability as a result of hydrolysis [ 14 , 23 , 24 ].
However, shorter germination times, low substitution levels or addition of some improvers (vital wheat gluten) to germinated wheat flour could increase technological performance of whole-grain cereals [ 1 , 17 , 25 ]. Activation of slight amount of α-amylase will increase starch transformation to fermentable sugars thus promoting yeast fermentation, carbon dioxide production and increase in dough height during fermentation [ 26 , 27 ], which, along with increased dough extensibility, will contribute to gas cell expansion leading to bread loaves of higher specific volumes as evident from the study of Baranzelli et al. [ 14 ], Johnston et al. [ 28 ], Cardone et al. [ 29 ] and Bhinder et al. [ 18 ] (Table 1 ).
In addition, optimized α -amylase activity can improve the bread shelf-life and sensory attributes [ 17 ]. It was shown that due to restricted starch retrogradation, germination improved crumb softness for 200% after 24 h of storage even when whole-wheat flour was used [ 29 ]. Controlled germination can also yield a product of enhanced starch digestibility [ 15 ] and reduced glycaemic index [ 18 ]. Moreover, germinated whole-wheat breads had improved sensory attributes in comparison to their unsprouted counterparts thanks to their diminished bitterness and graininess, increased sweetness and moistness [ 25 , 28 ]. Breads with germinated wheat flour are also perceived as the ones with dark crust due to the presence of higher contents of reducing sugars that, combined with free amino acids, favoured the occurrence of a Maillard reaction [ 14 ].
2.2 Flour modification approaches
2.2.1 particle size reduction (micronization).
Flour particle size can significantly alter bread functionality and technological quality. If a micronization, such as jet milling, is applied to produce fine wheat flour with extremely low particle size, flour with increased digestible starch content is obtained [ 30 ]. When used in breadmaking, jet milled flour slightly decreased bread glycaemic index.
However, it seems that pulverization of flour is not promising technology concerning bread technological quality since whole-grain wheat jet milled breads (flour volume median diameter = 17–53 μm) were characterized with reduced specific volume and moisture content and increased crumb hardness in comparison to breads with flour having volume median diameter of 84 μm [ 30 ]. The same relationship between flour mean particle size and technological performance was obtained for gluten-free flours. The flours having coarser particle size are the most suitable for making gluten-free maize bread. According to de la Hera et al. [ 31 ], the coarser maize flours (> 150 µm) resulted in breads with higher specific volume and lower crumb firmness than the ones with finer flour (< 106 µm), due to the higher availability of dough to retain the gas produced during fermentation. Concerning rice flour incorporation in breadmaking, de la Hera et al. [ 32 ] concluded that the coarse fraction combined with a high dough hydration was the most suitable combination for developing rice bread when considering the bread volume and crumb texture.
2.2.2 Heat treatment
Different flour heat treatments such as dry-heat treatment or hydrothermal treatments (below or above starch gelatinization temperature) are being increasingly applied to improve the functionality of alternative cereals flour. It was shown that dry-heat treated sorghum flour produced breads with increased specific volume and more cells per slice area. This was ascribed to increased viscosity of sorghum flour dough as a consequence of starch granule swelling due to heat induced partial gelatinization as well as denaturation of both proteins and enzymes [ 33 ]. In addition, protein denaturation and the partial gelatinization of starch granules, led to an increase in gas retention capacity and dough expansion, which all contributed to improvements in structure, strength and volume of dry-heated sorghum containing bread [ 34 ]. Since sorghum-based products are characterized with pungent off-notes, dry heat treatment can also be employed to improve sorghum bread sensory properties [ 35 ]. Dry heating was also promising in upgrading the quality of substandard flour for bread-making applications [ 36 ]. Mann et al. [ 37 ] have shown that heat treatment of flour causes the formation of gluten and starch aggregates and modifies interactions between gluten and starch. The effects were more pronounced in heat-treated flours with increased moisture content where higher mobility of the molecules is enabled.
It was also revealed that gluten-free flours (maize or rice) blanching results in doughs with higher consistency, adhesiveness, springiness and stickiness due to the partial gelatinisation of the starch, which further led to improved bread quality [ 38 , 39 ].
When flour/starch heating is carried out in the presence of water without fostering a complete starch gelatinization, as it is the case with annealing (treatments in excess or at intermediate water contents below the gelatinisation temperature) and heat-moisture treatment (exposure of starch to higher temperatures at very restricted moisture content), increase in the starch gelatinization temperature, water binding capacity and granule susceptibility to enzyme hydrolysis occurs [ 40 , 41 ]. These structural changes improve the volume of breads and their quality, since restricted hydrothermal treatments increase starch emulsifying ability and delay gelatinization which enhance air incorporation in doughs and prolong the period of loaf expansion [ 40 ].
It was shown that application of hydrothermally treated rice and maize flour to manufacture rice and maize semolina-based breads increased the specific volume and decreased the hardness and chewiness of the gluten-free breads, due to higher initial viscosity imparted by treated flours enabling the entrapment of air bubbles in the dough [ 42 ].
When hydrothermal treatments are performed above gelatinization temperature starch granules are irreversibly losing their integrity, a process known as pre-gelatinization [ 40 ]. Parenti et al. [ 43 ] reported an increase in the water absorption capacity, improved alveograph parameters, as well as bread volume, crumb softness and shelf life when pre-gelatinized brown flour (flour having approx. 85% extraction yield, maximum ash content of 0.95 g/100, heated at 1:4 flour to water ratio at 85 °C) was used. Jalali et al. [ 44 ] used microwave-induced pre-gelatinization of maize flour to produce gluten-free pan bread. The authors observed structural expansion and more swelling of the pre-gelatinized maize flour as compared to non-treated one, which consequently resulted in increased firmness of dough, decreased firmness of bread, increased bread crumb moisture, porosity, loaf specific volume and the overall acceptability.
If pre-gelatinization is achieved with the aid of extrusion cooking (flour/starch exposure to high temperatures and mechanical shearing with enough amount of water) besides amylose and amylopectin leaching from disrupter starch granule, breakage of the amylose and amylopectin chains, denaturation of proteins, enzyme (in)activation and Maillard reactions occurred [ 40 ]. Extrusion cooked flour behaves as thickening agent [ 45 ], which is considered as a more 'natural approach' to the use of hydrocolloids as improvers. Substitution of native rice flour by extruded rice flour improved bread volume and crumb structure, decreased initial hardness and delayed bread staling in gluten-free bread [ 46 ].
2.2.3 Atmospheric cold plasma
Atmospheric cold plasma (ACP) is a non-thermal processing technology that so far was applied at different stages of the cereal processing chain for a range of applications including improved germination, microbial decontamination, toxin degradation and biopolymer structural changes for improved functionality [ 47 ]. The mode of action results from plasma generated reactive species (reactive oxygen and nitrogen species), radicals and UV light [ 48 ]. It was revealed that reactive oxygen species generated during wheat flour cold plasma treatment influenced protein oxidation, promoted disulfide bond formation between glutenin proteins, that improved dough strength; led to starch depolymerization and decrease in its crystallinity. These biopolymer structural changes reflected in the increase in bread specific volume, enhancement of its appearance and porosity structure, as well as increase in bread crumb whiteness [ 49 , 50 , 51 ].
However, most of the studies investigating plasma-induced changes in grain/flour/dough structure are based on breadmaking potential of refined wheat flour, biopolymer changes in whole grain wheat or the safety aspects of plasma application for alternative grains decontamination. The studies concerning plasma application to enhance breadmaking performance of whole-grain or non-wheat cereals are scarce. Since some preliminary studies have shown that ACP treatment is effective just in increasing breadmaking potential of weak flours [ 52 ], some future studies should be conducted for better exploitation of ACP in whole-grain of gluten-free cereals modification. Moreover, combination of different technologies such as plasma-activated water and heat moisture treatment can also offer novel possibilities in alternative grains utilization in breadmaking [ 53 ].
2.3 Dough modification approaches
2.3.1 high-pressure processing.
High-pressure processing (HPP) represents novel processing technology which is mainly used for non-thermal treatment for fruit juices preservation [ 54 ]. Generally, in high-pressure processing, food is subjected to high pressures (usually above 200 MPa, without high temperature treatment) causing structural and textural changes besides microbial inactivation. These changes are mainly influenced by starch gelatinization and polymerization of proteins [ 55 ]. Therefore, this technology can be effectively employed for protein and starch functional properties modification [ 56 ]. Moreover, Kieffer et al. [ 57 ] revealed that high pressure treatment promotes protein network formation. Most of the papers using HPP in cereal technology is mainly focused on gluten-free raw material treatment due to poor technological properties of these materials i.e. the lack of protein network formation, poor gas retention properties, poor volume, acceptability etc. Generally, it was determined that HPP treatment resulted in starch gelatinization and protein polymerization induced by reaction of thiol-disulfide interchange. Consequently, the dough became more viscoelastic, showed better workability, increased water absorption capacity and had better gas retention properties which resulted in increased volume and improved texture of the final product [ 58 , 59 ]. Moreover, the obtained bakery products had improved shelf life [ 60 ] and slower hardening kinetics in comparison to control samples, due to starch gelatinization that occurred in this process. However, according to Vallons et al. [ 61 ] the increase in the addition of pressure treated flour over 10% resulted in lower specific volume and poorer final product quality.
2.3.2 Ultrasound treatment
Ultrasound treatment, as a non-thermal processing tool, has been intensively utilized for microbial and enzyme inactivation, bioactive component extraction and food components modification for increased functionality [ 62 ]. However, application of ultrasound to alter flour functionality and thus improve its breadmaking potential is quite scarce.
While it was shown that ultrasound modulation of flour functionality depends on the treatment time [ 62 , 63 ], there are opposite conclusions concerning the effect of the flour dispersion concentration. According to Vela et al. [ 63 ], effect of ultrasound treatment is independent on the concentration of the treated flour dispersion up to 30%, and in all the treated dispersions (5–30%) particle size of the rice flour was reduced. On the contrary, ultrasound treatment of buckwheat grains caused particles agglomeration in concentrated dispersions (1:5 and 1:2.5 solid:liquid ratio), while higher dilution (1:10) increased smaller particle size fractions [ 64 ].
In general, ultrasound treatment of whole-grain flour significantly increases water solubility, water absorption and swelling power of quinoa, buckwheat and rice flour [ 62 , 63 , 64 ]. It also influences starch crystallinity as recorded in the alterations of the flour thermal properties such as reduction of gelatinization enthalpy, increase in pasting temperature and gel strength [ 63 ], as well as in an increase in the in vitro starch digestibility [ 62 ]. However, effects on the flour pasting properties were found to be dependent on treatment time [ 62 ] and dispersion concentration [ 64 ], where lower treatment times [ 62 ] and medium concentrations [ 64 ] led to increase in peak viscosity, breakdown, and setback values.
Jalali et al. [ 44 ] have shown that ultrasound treatment of dough decreased the firmness of maize flour dough and bread, while increasing gluten-free bread specific volume, porosity, and the overall acceptability score. The observed improvement in bread technological, visual, and sensory properties was increased when combination of pre-gelatinization and ultrasound treatment of maize flour was applied [ 44 ].
3 Strategies to modify processing variables of the breadmaking phases
3.1 dough kneading and hydration modification.
Flour transformation to dough is performed by hydration and mixing operations, where different processing variables can be modified in order to achieve optimum dough and bread quality. Appropriate water content and temperature ensure optimal dough rheology and consistency, avoiding undesired softening or hardening. Proper choice of mixing speed and temperature will avoid dough warming and excessive weakening, while kneading time management prevents both over- and under-mixing and allows dough aeration and its capacity to retain gases [ 5 ].
Water content influences dough quality in the following manner: adding too much water during kneading generates soft and sticky dough, while dough with water content below the optimal water absorption of the flour will be harder to knead [ 5 ]. Increase in total water content in dough from ancient grain flours increases dough extensibility, while it decreases dough tenacity and vice versa [ 65 ]. In the case of gluten-free ingredients, such as rice flour and hydroxypropyl methyl cellulose (HPMC), low hydrated doughs had low ability to retain gas released during proofing, unlike high hydrated doughs which endure longer fermentation time resulting in improved specific volume [ 66 ]. Therefore, different strategies are applied in order to increase water absorption and thus improve gluten-free bread quality. Due to the absence of gluten in gluten-free ingredients, increased water absorption is achieved through fibres/hydrocolloids addition or enzymatic or extrusion treatments to modify amount of water which will be untaken by starch in the early phases of breadmaking [ 67 , 68 ].
Gomez et al. [ 66 ] have also reported that low mixing speed and long mixing time led to gluten-free breads with higher specific volumes and softer texture.
3.2 Sourdough fermentation
Although being an ancient biotechnology, sourdough fermentation has gained renewed interest as a tool for better exploitation of non-wheat cereals in breadmaking [ 69 ]. Sourdough can be described as a mixture of flour and water fermented by lactic acid bacteria (LAB) or LAB in combination with yeasts, either spontaneous or inoculated [ 70 ]. The positive effects of sourdough application in breadmaking are associated with the metabolic activities of the LAB and yeasts, such as acidification, production of exopolysaccharides, proteolytic, amylolytic and phytase activity, and production of volatile and antimicrobial substances [ 71 ].
Beside the fact that sourdough fermentation contributes to enhanced nutritional properties of bread (higher free amino acids concentrations, soluble fibre, γ-aminobutyric acid, total phenols and antioxidant activities) and phytic acid reduction, leading to increased mineral, protein and free amino acids bioavailability; it has significant impact on bread techno-functionality [ 6 , 72 ].
Taking advantage of LAB ability to produce certain polymers and modify the main structure-building components of flour such as starch, arabinoxylans and proteins, sourdough fermentation was used to improve dough and bread technological properties such as loaf volume, water absorption of the dough, dough rheology and machinability [ 73 ]. Certain LAB strains produce exopolysaccharides that due to their water-binding ability act as hydrocolloids or gums, and could be considered as gluten mimetics in gluten-free products [ 74 ] in order to improve product texture. In gluten containing flours, organic acids produced by LAB enhance the solubility of the glutenin fraction and improve the swelling power of the gluten, which increase gas retention during fermentation [ 73 ]. Gluten complex structural changes are associated with dough acidification which may also activate some endogenous flour enzymes such as proteases that can hydrolyse gluten under appropriate fermentation conditions and bacteria selection. Gobbetti et al. [ 75 ] suggested that degradation of prolamins of wheat and rye during fermentation by selected sourdough lactic acid bacteria can represent a possibility to use these cereals in the gluten-free diet.
On the contrary, reports on the fate of starch during sourdough fermentation are contradictory. In the case of the wholegrain wheat flour, sourdough fermented bread exhibited higher resistant starch content and lower glycaemic response than the corresponding products leavened with S. cerevisiae [ 76 ]. However, sourdough with a commercial starter added to a gluten-free formulation decreased the glycaemic response in vivo less effective than in wheat sourdough bread. This was explained with lower concentrations of organic acids in gluten-free than in wheat sourdough. In sourdough wheat breads pH decrease upon formation of organic acids led to inhibition of α-amylase and consequently, a decrease in starch hydrolysis. On the contrary, the pH in gluten-free sourdoughs might still be sufficient for α-amylase to proceed with degradation of starch and increase in starch hydrolysis degree [ 77 ].
The effect of sourdough fermentation on techno-functionality of bread prepared with alternative cereals is summarized in Table 2 . As it can be seen from Table 2 , the effect of sourdough addition on bread technological performance largely depends on sourdough type, LAB strain and presence of Saccharomyces cerevisiae.
Besides bread technological quality, organic acids together with other LAB metabolites (e.g. CO 2 , ethanol, diacetyl, hydrogen peroxide, fatty acids, reuterin, fungicin, etc.) also contribute to bread preservation thus prolonging its shelf life [ 54 ]. Sourdough was also successfully applied in a sugar reduced bakery product, owning to sourdough bacteria ability to produce polyols [ 87 ]. Because of the synthesis of flavouring amino acids during fermentation, the sourdough efficiently masks salt reduction in bakery products without affecting taste and other quality parameters [ 88 ].
3.3 Non-conventional baking techniques
Another interesting approach to improve the breadmaking potential of alternative cereals is to apply a non-conventional baking technique such as vacuum, microwave, infrared, jet-impingement, ohmic or a combination of them (hybrid heating).
In comparison to conventional, partial-vacuum baking of gluten-free bread did not have significant impact on bread volume and texture; however, it resulted in product which became stale more slowly than the control [ 89 ].
Microwave and infrared baking are considered as time- and cost-efficient processes. Although microwave and microwave-assisted hot air baking increase gluten-free bread crumb hardness and result in pale bread crust compared with the hot air baking, it was shown that these techniques can reduce the digestibility of starch and glycaemic index of the bread and increase loaf volume [ 90 ].
Application of single infrared radiation (halogen lamp as NIR source) results mostly in products of inferior quality, due to the high rate of heating which influence sudden and thick crust formation and the prevention of the product expansion thus leading to lower specific volume and higher firmness values than conventional baking [ 91 , 92 ]. However, in the study of Shyu et al. [ 93 ] breads baked by IR had comparable quality in terms volume, water activity, staling rate, or sensory scores with conventionally baked ones.
Another novel baking technique, jet impinging, based on forced convection heating, increases the heat transfer efficiency during the baking process [ 94 ], but results in the formation of a thick crust as compared with infrared radiation and heating in a conventional household oven [ 95 ].
Ohmic heating is an innovative technology in which an alternating electrical current is passed through a material, generating heat by dissipation of the electrical energy due to material's own electrical resistance, allowing rapid and uniform heat distribution [ 54 ].
Bender et al. [ 96 ] have shown that gluten-free breads could benefit from the uniform rapid heating during processing, as these breads exhibit higher loaf volume, finer pore structure, reduced starch digestibility and higher resistant starch content compared to conventionally baked breads. Namely, rapid heating stabilizes the crumb structure at an early stage of baking before CO 2 is released during heating enabling bread expansion.
In order to increase the potential of non-conventional baking techniques while minimizing the disadvantages a combination of them (hybrid heating) can be applied. Combination of infrared lamps and electric heating coils enables 28% reduction in baking time, while resulting in breads comparable with breads baked in conventional electrical heating in terms of crumb firmness, volume, moisture content and colour [ 97 ]. However, there are limited studies applying hybrid heating to produce alternative cereals bread. Demirkesen et al. [ 98 ] compared the quality of the gluten-free breads based on the blends of tigernut flour/rice flour baked in conventional ovens and infrared–microwave combination. They observed higher loaf volume and crumb firmness and less gelatinized starch of IR- microwave baked breads. Moreover, staling of gluten-free breads was not affected by both baking methods [ 99 ].
Impact of abovementioned processing strategies on breadmaking potential of whole-grain wheat and non-wheat flours is summarized in Fig. 2 .
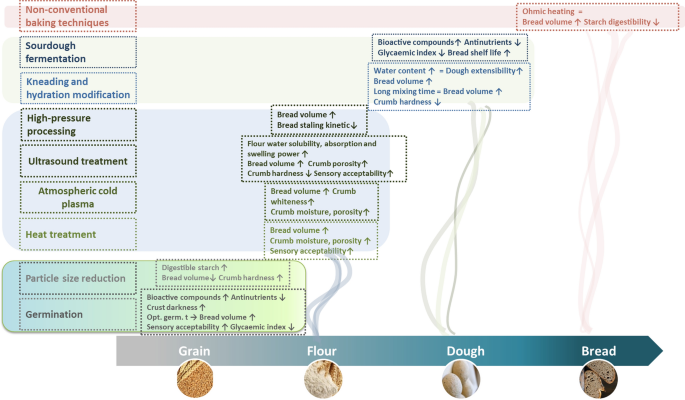
Impact of different technological approaches on breadmaking potential of whole-grain wheat and non-wheat flours
4 Conclusions and future trends
This review has highlighted that different technological strategies can be used to increase techno-functionality of whole-grain wheat and non-wheat flours and sensory properties of final product—bread. They are mostly performed with the aim to alter biopolymer structure and thus increase its functionality and encompass the ones used to provoke starch pre-gelatinization (high-pressure processing, flour heat treatment), reduce starch retrogradation (germination, extrusion cooking, non-conventional baking techniques), induce gluten strengthening through oxidation (atmospheric cold plasma) or gluten hydrolysis (grain germination, sourdough fermentation). It was elucidated that despite the opportunities offered by different conventional and emerging technologies and approaches, the gaps between technological and nutritional strategies for improving breadmaking potential of whole-grains still exist, especially when other, non-wheat grains are used. Namely, effectiveness of reviewed technological approaches largely depends on initial flour composition and quality. Therefore, further investigations are needed, particularly with respect to the ones including combined technologies (atmospheric pressure plasma/thermal treatment; pre-gelatinization/ultrasound; hybrid heating, etc.) to further increase technological and sensory quality of bread from whole-grain non-wheat cereals while preserving health beneficial properties.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
Parenti O, Guerrini L, Zanoni B. Techniques and technologies for the breadmaking process with unrefined wheat flours. Trends Food Sci Technol. 2020;99:152–66.
CAS Google Scholar
Alvarez-Jubete L, Auty M, Arendt EK, Gallagher E. Baking properties and microstructure of pseudocereal flours in gluten-free bread formulations. Eur Food Res Technol. 2010;230(3):437–45.
Aune D, Keum N, Giovannucci E, Fadnes LT, Boffetta P, Greenwood DC, Tonstad S, Vatten LJ, Riboli E, Norat T. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: systematic review and dose-response meta-analysis of prospective studies. BMJ. 2016;353: i2716.
PubMed PubMed Central Google Scholar
King JA, Jeong J, Underwood FE, Quan J, Panaccione N, Windsor JW, Coward S, deBruyn J, Ronksley PE, Shaheen AA, Quan H. Incidence of celiac disease is increasing over time: a systematic review and meta-analysis. Am J Gastroentero. 2020;115(4):507–25.
Google Scholar
Cappelli A, Cini E. Challenges and opportunities in wheat flour, pasta, bread, and bakery product production chains: a systematic review of innovations and improvement strategies to increase sustainability, productivity, and product quality. Sustainability. 2021;13(5):2608.
Wang Y, Maina NH, Coda R, Katina K. Challenges and opportunities for wheat alternative grains in breadmaking: Ex-situ-versus in-situ-produced dextran. Trends Food Sci Technol. 2021;113:232–44.
Naqash F, Gani A, Gani A, Masoodi FA. Gluten-free baking: combating the challenges—a review. Trends Food Sci Technol. 2017;66:98–107.
Heiniö RL, Lehtinen P, Oksman-Caldentey KM, Poutanen K. Differences between sensory profiles and development of rancidity during long-term storage of native and processed oat. Cereal Chem. 2002;79(3):367–75.
Tebben L, Shen Y, Li Y. Improvers and functional ingredients in whole wheat bread: a review of their effects on dough properties and bread quality. Trends Food Sci Technol. 2018;81:10–24.
Pojić M, Hadnađev TD, Hadnađev M, Rakita S, Torbica A. Optimization of additive content and their combination to improve the quality of pure barley bread. J Food Sci Technol. 2017;54(3):579–90.
Torbica A, Hadnađev M, Dapčević T. Rheological, textural and sensory properties of gluten-free bread formulations based on rice and buckwheat flour. Food Hydrocoll. 2010;24(6–7):626–32.
Šarić B, Dapčević-Hadnađev T, Hadnađev M, Sakač M, Mandić A, Mišan A, Škrobot D. Fiber concentrates from raspberry and blueberry pomace in gluten-free cookie formulation: effect on dough rheology and cookie baking properties. J Texture Stud. 2019;50(2):124–30.
PubMed Google Scholar
Benincasa P, Falcinelli B, Lutts S, Stagnari F, Galieni A. Sprouted grains: a comprehensive review. Nutrients. 2019;11(2):421.
CAS PubMed Central Google Scholar
Baranzelli J, Kringel DH, Colussi R, Paiva FF, Aranha BC, de Miranda MZ, da Rosa ZE, Dias AR. Changes in enzymatic activity, technological quality and gamma-aminobutyric acid (GABA) content of wheat flour as affected by germination. LWT. 2018;90:483–90.
Yang Q, Luo Y, Wang H, Li J, Gao X, Gao J, Feng B. Effects of germination on the physicochemical, nutritional and in vitro digestion characteristics of flours from waxy and nonwaxy proso millet, common buckwheat and pea. Innov Food Sci Emerg Technol. 2021;67: 102586.
Hajnal EJ, Tomić J, Torbica A, Rakita S, Pojić M, Živančev D, Hadnađev M, Hadnađev TD. Content of free amino groups during postharvest wheat and flour maturation in relation to gluten quality. Food Chem. 2014;164:158–65.
Lemmens E, Moroni AV, Pagand J, Heirbaut P, Ritala A, Karlen Y, Lê KA, Van den Broeck HC, Brouns FJ, De Brier N, Delcour JA. Impact of cereal seed sprouting on its nutritional and technological properties: a critical review. Compr Rev Food Sci Food Saf. 2019;18(1):305–28.
Bhinder S, Singh N, Kaur A. Impact of germination on nutraceutical, functional and gluten free muffin making properties of Tartary buckwheat ( Fagopyrum tataricum ). Food Hydrocoll. 2022;124: 107268.
Sturza A, Păucean A, Chiș MS, Mureșan V, Vodnar DC, Man SM, Urcan AC, Rusu IE, Fostoc G, Muste S. Influence of buckwheat and buckwheat sprouts flours on the nutritional and textural parameters of wheat buns. Appl Sci. 2020;10(22):7969.
Koehler P, Hartmann G, Wieser H, Rychlik M. Changes of folates, dietary fiber, and proteins in wheat as affected by germination. J Agric Food Chem. 2007;55(12):4678–83.
CAS PubMed Google Scholar
Gajcy K, Lochynski S, Librowski T. A role of GABA analogues in the treatment of neurological diseases. Curr Med Chem. 2010;17(22):2338–47.
Liu T, Hou GG, Cardin M, Marquart L, Dubat A. Quality attributes of whole-wheat flour tortillas with sprouted whole-wheat flour substitution. LWT. 2017;77:1–7.
Žilić S, Janković M, Barać M, Pešić M, Konić-Ristić A, Šukalović VH. Effects of enzyme activities during steeping and sprouting on the solubility and composition of proteins, their bioactivity and relationship with the bread making quality of wheat flour. Food Funct. 2016;7(10):4323–31.
Rimsten L, Haraldsson AK, Andersson R, Alminger M, Sandberg AS, Åman P. Effects of malting on β-glucanase and phytase activity in barley grain. J Sci Food Agric. 2002;82(8):904–12.
Richter K, Christiansen K, Guo G. Wheat sprouting enhances bread baking performance. Cereal Foods World. 2014;59(5):231–3.
Marti A, Cardone G, Nicolodi A, Quaglia L, Pagani MA. Sprouted wheat as an alternative to conventional flour improvers in bread-making. LWT. 2017;80:230–6.
Marti A, Cardone G, Pagani MA, Casiraghi MC. Flour from sprouted wheat as a new ingredient in bread-making. LWT. 2018;89:237–43.
Johnston R, Martin JM, Vetch JM, Byker-Shanks C, Finnie S, Giroux MJ. Controlled sprouting in wheat increases quality and consumer acceptability of whole-wheat bread. Cereal Chem. 2019;96(5):866–77.
Cardone G, D’Incecco P, Pagani MA, Marti A. Sprouting improves the bread-making performance of whole wheat flour ( Triticum aestivum L.). J Sci Food Agric. 2020;100(6):2453–9.
Protonotariou S, Mandala I, Rosell CM. Jet milling effect on functionality, quality and in vitro digestibility of whole wheat flour and bread. Food Bioprocess Technol. 2015;8(6):1319–29.
de la Hera E, Talegón M, Caballero P, Gómez M. Influence of maize flour particle size on gluten-free breadmaking. J Sci Food Agric. 2013;93(4):924–32.
de la Hera E, Rosell CM, Gomez M. Effect of water content and flour particle size on gluten-free bread quality and digestibility. Food Chem. 2014;151:526–31.
Marston K, Khouryieh H, Aramouni F. Effect of heat treatment of sorghum flour on the functional properties of gluten-free bread and cake. LWT Food Sci Technol. 2016;65:637–44.
Collar C. Gluten-free dough-based foods and technologies. In: Taylor JRN, Duodu KG, editors. Sorghum and millets. Cambridge: AACC International Press; 2019. p. 331–54.
Cappelli A, Oliva N, Cini E. A systematic review of gluten-free dough and bread: dough rheology, bread characteristics, and improvement strategies. Appl Sci. 2020;10(18):6559.
Gélinas P, McKinnon CM, Rodrigue N, Montpetit D. Heating conditions and bread-making potential of substandard flour. J Food Sci. 2001;66(4):627–32.
Mann J, Schiedt B, Baumann A, Conde-Petit B, Vilgis TA. Effect of heat treatment on wheat dough rheology and wheat protein solubility. Food Sci Technol Int. 2014;20(5):341–51.
Marco C, Rosell CM. Functional and rheological properties of protein enriched gluten free composite flours. J Food Eng. 2008;88(1):94–103.
Brites C, Trigo MJ, Santos C, Collar C, Rosell CM. Maize-based gluten-free bread: influence of processing parameters on sensory and instrumental quality. Food Bioprocess Technol. 2010;3(5):707–15.
Gómez M, Martínez MM. Changing flour functionality through physical treatments for the production of gluten-free baking goods. J Cereal Sci. 2016;67:68–74.
Tester RF, Debon SJ. Annealing of starch—a review. Int J Biol Macromol. 2000;27(1):1–2.
Bourekoua H, Benatallah L, Zidoune MN, Rosell CM. Developing gluten free bakery improvers by hydrothermal treatment of rice and corn flours. LWT. 2016;73:342–50.
Parenti O, Guerrini L, Canuti V, Angeloni G, Masella P, Zanoni B. The effect of the addition of gelatinized flour on dough rheology and quality of bread made from brown wheat flour. LWT. 2019;106:240–6.
Jalali M, Sheikholeslami Z, Elhamirad AH, Khodaparast MH, Karimi M. The effect of the ultrasound process and pre-gelatinization of the corn flour on the textural, visual, and sensory properties in gluten-free pan bread. J Food Sci Technol. 2020;57(3):993–1002.
Matos ME, Rosell CM. Understanding gluten-free dough for reaching breads with physical quality and nutritional balance. J Sci Food Agric. 2015;95(4):653–61.
Martínez MM, Marcos P, Gómez M. Texture development in gluten-free breads: effect of different enzymes and extruded flour. J Texture Stud. 2013;44(6):480–9.
Ojha S, Fröhling A, Durek J, Ehlbeck J, Tiwari BK, Schlüter OK, Bußler S. Principles and application of cold plasma in food processing. In: Knoerzer K, Muthukumarappan K, editors. Innovative food processing technologies. A comprehensive review. Amsterdam: Elsevier; 2021. p. 519–40.
Feizollahi E, Iqdiam B, Vasanthan T, Thilakarathna MS, Roopesh MS. Effects of atmospheric-pressure cold plasma treatment on deoxynivalenol degradation, quality parameters, and germination of barley grains. Appl Sci. 2020;10(10):3530.
Chaple S, Sarangapani C, Jones J, Carey E, Causeret L, Genson A, Duffy B, Bourke P. Effect of atmospheric cold plasma on the functional properties of whole wheat ( Triticum aestivum L.) grain and wheat flour. Innov Food Sci Emerg Technol. 2020;66: 102529.
Menkovska M, Mangova M, Dimitrov K. Effect of cold plasma on wheat flour and bread making quality. Maced J Anim Sci. 2014;4(1):27–30.
Misra NN, Kaur S, Tiwari BK, Kaur A, Singh N, Cullen PJ. Atmospheric pressure cold plasma (ACP) treatment of wheat flour. Food Hydrocoll. 2015;44:115–21.
Vukić M. Effects of cold atmospheric plasma on the technological quality and safety of wheat flour. Faculty of Technology, University of Novi Sad; 2020.
Shi M, Wang F, Ji X, Yan Y, Liu Y. Effects of plasma-activated water and heat moisture treatment on the properties of wheat flour and dough. Int J Food Sci Technol. 2021. https://doi.org/10.1111/ijfs.15317 .
Article Google Scholar
Bender D, Schönlechner R. Innovative approaches towards improved gluten-free bread properties. J Cereal Sci. 2020;91: 102904.
Vallons KJ, Ryan LA, Arendt EK. Promoting structure formation by high pressure in gluten-free flours. LWT Food Sci Technol. 2011;44(7):1672–80.
Ahmed J, Ramaswamy HS, Ayad A, Alli I, Alvarez P. Effect of high-pressure treatment on rheological, thermal and structural changes in Basmati rice flour slurry. J Cereal Sci. 2007;46(2):148–56.
Kieffer R, Schurer F, Köhler P, Wieser H. Effect of hydrostatic pressure and temperature on the chemical and functional properties of wheat gluten: studies on gluten, gliadin and glutenin. J Cereal Sci. 2007;45(3):285–92.
Stolt M, Oinonen S, Autio K. Effect of high pressure on the physical properties of barley starch. Innov Food Sci Emerg Technol. 2000;1(3):167–75.
Cappa C, Barbosa-Cánovas GV, Lucisano M, Mariotti M. Effect of high pressure processing on the baking aptitude of corn starch and rice flour. LWT. 2016;73:20–7.
Hüttner EK, Dal Bello F, Poutanen K, Arendt EK. Fundamental evaluation of the impact of high hydrostatic pressure on oat batters. J Cereal Sci. 2009;49(3):363–70.
Vallons KJ, Ryan LA, Koehler P, Arendt EK. High pressure–treated sorghum flour as a functional ingredient in the production of sorghum bread. Eur Food Res Technol. 2010;231(5):711–7.
Zhu F, Li H. Modification of quinoa flour functionality using ultrasound. Ultrason Sonochem. 2019;52:305–10.
Vela AJ, Villanueva M, Solaesa ÁG, Ronda F. Impact of high-intensity ultrasound waves on structural, functional, thermal and rheological properties of rice flour and its biopolymers structural features. Food Hydrocoll. 2021;113: 106480.
Harasym J, Satta E, Kaim U. Ultrasound treatment of buckwheat grains impacts important functional properties of resulting flour. Molecules. 2020;25(13):3012.
Cappelli A, Cini E, Guerrini L, Masella P, Angeloni G, Parenti A. Predictive models of the rheological properties and optimal water content in doughs: an application to ancient grain flours with different degrees of refining. J Cereal Sci. 2018;83:229–35.
Gómez M, Talegón M, De La Hera E. Influence of mixing on quality of gluten-free bread. J Food Qual. 2013;36(2):139–45.
Föste M, Verheyen C, Jekle M, Becker T. Fibres of milling and fruit processing by-products in gluten-free bread making: a review of hydration properties, dough formation and quality-improving strategies. Food Chem. 2020;306: 125451.
Morreale F, Garzón R, Rosell CM. Understanding the role of hydrocolloids viscosity and hydration in developing gluten-free bread. A study with hydroxypropylmethylcellulose. Food hydrocoll. 2018;77:629–35.
Ramos L, Alonso-Hernando A, Martínez-Castro M, Morán-Pérez JA, Cabrero-Lobato P, Pascual-Maté A, Téllez-Jiménez E, Mujico JR. Sourdough biotechnology applied to gluten-free baked goods: rescuing the tradition. Foods. 2021;10(7):1498.
CAS PubMed PubMed Central Google Scholar
Hammes WP, Gänzle MG. Sourdough breads and related products. In: Wood BJB, editor. Microbiology of fermented foods. Boston: Springer; 1998. p. 199–216.
Moroni AV, Dal Bello F, Arendt EK. Sourdough in gluten-free bread-making: an ancient technology to solve a novel issue? Food Microbiol. 2009;26(7):676–84.
Gobbetti M, De Angelis M, Di Cagno R, Calasso M, Archetti G, Rizzello CG. Novel insights on the functional/nutritional features of the sourdough fermentation. Int J Food Microbiol. 2019;302:103–13.
Chavan RS, Chavan SR. Sourdough technology—a traditional way for wholesome foods: a review. Compr Rev Food Sci Food Saf. 2011;10(3):169–82.
Lynch KM, Coffey A, Arendt EK. Exopolysaccharide producing lactic acid bacteria: their techno-functional role and potential application in gluten-free bread products. Food Res Int. 2018;110:52–61.
Gobbetti M, Rizzello CG, Di Cagno R, De Angelis M. How the sourdough may affect the functional features of leavened baked goods. Food Microbiol. 2014;37:30–40.
Scazzina F, Del Rio D, Pellegrini N, Brighenti F. Sourdough bread: starch digestibility and postprandial glycemic response. J Cereal Sci. 2009;49(3):419–21.
Wolter A, Hager AS, Zannini E, Arendt EK. Influence of sourdough on in vitro starch digestibility and predicted glycemic indices of gluten-free breads. Food Funct. 2014;5(3):564–72.
Demirkesen-Bicak H, Arici M, Yaman M, Karasu S, Sagdic O. Effect of different fermentation condition on estimated glycemic index, in vitro starch digestibility, and textural and sensory properties of sourdough bread. Foods. 2021;10(3):514.
Nami Y, Gharekhani M, Aalami M, Hejazi MA. Lactobacillus-fermented sourdoughs improve the quality of gluten-free bread made from pearl millet flour. J Food Sci Technol. 2019;56(9):4057–67.
Novotni D, Čukelj N, Smerdel B, Ćurić D. Quality attributes and firming kinetics of partially baked frozen wholewheat bread with sourdough. Int J Food Sci Technol. 2013;48(10):2133–42.
Mariotti M, Garofalo C, Aquilanti L, Osimani A, Fongaro L, Tavoletti S, Hager AS, Clementi F. Barley flour exploitation in sourdough bread-making: a technological, nutritional and sensory evaluation. LWT Food Sci Technol. 2014;59(2):973–80.
Różyło R, Rudy S, Krzykowski A, Dziki D. Novel application of freeze-dried amaranth sourdough in gluten-free bread production. J Food Process Eng. 2015;38(2):135–43.
Axel C, Röcker B, Brosnan B, Zannini E, Furey A, Coffey A, Arendt EK. Application of Lactobacillus amylovorus DSM19280 in gluten-free sourdough bread to improve the microbial shelf life. Food Microbiol. 2015;47:36–44.
Marti A, Marengo M, Bonomi F, Casiraghi MC, Franzetti L, Pagani MA, Iametti S. Molecular features of fermented teff flour relate to its suitability for the production of enriched gluten-free bread. LWT. 2017;78:296–302.
Olojede AO, Sanni AI, Banwo K. Rheological, textural and nutritional properties of gluten-free sourdough made with functionally important lactic acid bacteria and yeast from Nigerian sorghum. LWT. 2020;120: 108875.
Korcari D, Secchiero R, Laureati M, Marti A, Cardone G, Rabitti NS, Ricci G, Fortina MG. Technological properties, shelf life and consumer preference of spelt-based sourdough bread using novel, selected starter cultures. LWT. 2021;151: 112097.
Sahin AW, Zannini E, Coffey A, Arendt EK. Sugar reduction in bakery products: current strategies and sourdough technology as a potential novel approach. Food Res Int. 2019;126: 108583.
Zhao CJ, Kinner M, Wismer W, Gänzle MG. Effect of glutamate accumulation during sourdough fermentation with Lactobacillus reuteri on the taste of bread and sodium-reduced bread. Cereal Chem. 2015;92(2):224–30.
Şimşek ST. Evaluation of partial-vacuum baking for gluten-free bread: effects on quality attributes and storage properties. J Cereal Sci. 2020;91: 102891.
Therdthai N, Tanvarakom T, Ritthiruangdej P, Zhou W. Effect of microwave assisted baking on quality of rice flour bread. J Food Qual. 2016;39(4):245–54.
Keskin SO, Sumnu G, Sahin S. Bread baking in halogen lamp–microwave combination oven. Food Res Int. 2004;37(5):489–95.
De Pilli T, Alessandrino O. Effects of different cooking technologies on biopolymers modifications of cereal-based foods: Impact on nutritional and quality characteristics review. Crit Rev Food Sci Nutr. 2020;60(4):556–65.
Shyu YS, Sung WC, Chang MH, Hwang JY. Effect of far-infrared oven on the qualities of bakery products. J Culin Sci Technol. 2008;6(2–3):105–18.
Li XD, Alamir M, Witrant E, Della-Valle G, Rouaud O, Boillereaux L, Josset C. Further investigations on energy saving by jet impingement in bread baking process. IFAC Proceed Vol. 2013;46(2):701–6.
Olsson EE, Trägårdh AC, Ahrné LM. Effect of near-infrared radiation and jet impingement heat transfer on crust formation of bread. J Food Sci. 2005;70(8):e484–91.
Bender D, Gratz M, Vogt S, Fauster T, Wicki B, Pichler S, Kinner M, Jäger H, Schoenlechner R. Ohmic heating—a novel approach for gluten-free bread baking. Food Bioprocess Technol. 2019;12(9):1603–13.
Chhanwal N, Ezhilarasi PN, Indrani D, Anandharamakrishnan C. Influence of electrical and hybrid heating on bread quality during baking. J Food Sci Technol. 2015;52(7):4467–74.
Demirkesen I, Sumnu G, Sahin S. Quality of gluten-free bread formulations baked in different ovens. Food Bioprocess Technol. 2013;6(3):746–53.
Demirkesen I, Campanella OH, Sumnu G, Sahin S, Hamaker BR. A study on staling characteristics of gluten-free breads prepared with chestnut and rice flours. Food Bioprocess Technol. 2014;7(3):806–20.
Download references
Acknowledgements
This research was financially supported by the Science Fund of the Republic of Serbia, program PROMIS [Grant Number: 6062634], project acronym ReTRA and Ministry of Education, Science and Technological Development of the Republic of Serbia [Grant Number: 451-03-68/2022-14/200222].
Science Fund of the Republic of Serbia [Grant Number: 6062634], Ministry of Education, Science and Technological Development of the Republic of Serbia [Grant Number: 451-03-68/2022-14/200222].
Author information
Authors and affiliations.
University of Novi Sad, Institute of Food Technology, Bulevar cara Lazara 1, 21000, Novi Sad, Serbia
Tamara Dapčević-Hadnađev, Jelena Tomić, Dubravka Škrobot, Bojana Šarić & Miroslav Hadnađev
You can also search for this author in PubMed Google Scholar
Contributions
Idea for the article: TD-H; literature search and data analysis: JT, DŠ, BŠ, MH; drafted the work: TD-H, MH; critically revised the work: JT. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Tamara Dapčević-Hadnađev .
Ethics declarations
Competing interests.
Tamara Dapčević-Hadnađev is Editorial Board Member.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Dapčević-Hadnađev, T., Tomić, J., Škrobot, D. et al. Processing strategies to improve the breadmaking potential of whole-grain wheat and non-wheat flours. Discov Food 2 , 11 (2022). https://doi.org/10.1007/s44187-022-00012-w
Download citation
Received : 28 November 2021
Accepted : 10 February 2022
Published : 02 March 2022
DOI : https://doi.org/10.1007/s44187-022-00012-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Breadmaking
- Whole-grains
- Non-wheat cereals
- Novel technologies
- Cereal processing
- Find a journal
- Publish with us
- Track your research
- Search Menu
- Advance articles
- Darwin Reviews
- Special Issues
- Expert View
- Flowering Newsletter Reviews
- Technical Innovations
- Editor's Choice
- Virtual Issues
- Community Resources
- Reasons to submit
- Author Guidelines
- Peer Reviewers
- Submission Site
- Open Access
- About Journal of Experimental Botany
- About the Society for Experimental Biology
- Editorial Board
- Advertising and Corporate Services
- Journals Career Network
- Permissions
- Self-Archiving Policy
- Dispatch Dates
- Journal metrics
- Journals on Oxford Academic
- Books on Oxford Academic


Article Contents
Introduction, origin and evolution of wheat, cultivated wheats today, why has wheat been so successful, wheat gluten proteins and processing properties, wheat in nutrition and health, adverse reactions to wheat, the future for wheat.
- < Previous
- Article contents
- Figures & tables
- Supplementary Data
P. R. Shewry, Wheat, Journal of Experimental Botany , Volume 60, Issue 6, April 2009, Pages 1537–1553, https://doi.org/10.1093/jxb/erp058
- Permissions Icon Permissions
Wheat is the dominant crop in temperate countries being used for human food and livestock feed. Its success depends partly on its adaptability and high yield potential but also on the gluten protein fraction which confers the viscoelastic properties that allow dough to be processed into bread, pasta, noodles, and other food products. Wheat also contributes essential amino acids, minerals, and vitamins, and beneficial phytochemicals and dietary fibre components to the human diet, and these are particularly enriched in whole-grain products. However, wheat products are also known or suggested to be responsible for a number of adverse reactions in humans, including intolerances (notably coeliac disease) and allergies (respiratory and food). Current and future concerns include sustaining wheat production and quality with reduced inputs of agrochemicals and developing lines with enhanced quality for specific end-uses, notably for biofuels and human nutrition.
Wheat is counted among the ‘big three’ cereal crops, with over 600 million tonnes being harvested annually. For example, in 2007, the total world harvest was about 607 m tonnes compared with 652 m tonnes of rice and 785 m tonnes of maize ( http://faostat.fao.org/ ). However, wheat is unrivalled in its range of cultivation, from 67º N in Scandinavia and Russia to 45º S in Argentina, including elevated regions in the tropics and sub-tropics ( Feldman, 1995 ). It is also unrivalled in its range of diversity and the extent to which it has become embedded in the culture and even the religion of diverse societies.
Most readers will be aware of the significance of bread in the Judaeo-Christian tradition including the use of matzo (hard flat bread) at the Jewish Passover and of bread to represent the ‘host’ at the Christian Eucharist (Holy Communion). The latter may be a thin unleavened wafer, similar to the Jewish matzo, in the Roman Catholic Church and some Protestant denominations, or leavened in other Protestant denominations and the Eastern Orthodox Church. But how many readers are aware that bread is treated as sacred in everyday life in the largely Muslim communities of Central Asia, such as Uzbekistan and Kyrgyzstan? In this culture, the leavened round breads (nan) are stamped before baking and must be treated with respect, including being kept upright and never left on the ground or thrown away in public. These customs almost certainly originate from earlier indigenous religions in the Middle East in which wheat played a similar role and was sometimes equated with the sun and its god.
Although such cultural and religious traditions are fascinating and will certainly reward further study, they are essentially outside the scope of this article which will examine why wheat has developed and continues to be so successful as a crop and food source.
The first cultivation of wheat occurred about 10 000 years ago, as part of the ‘Neolithic Revolution’, which saw a transition from hunting and gathering of food to settled agriculture. These earliest cultivated forms were diploid (genome AA) (einkorn) and tetraploid (genome AABB) (emmer) wheats and their genetic relationships indicate that they originated from the south-eastern part of Turkey ( Heun et al. , 1997 ; Nesbitt, 1998 ; Dubcovsky and Dvorak, 2007 ). Cultivation spread to the Near East by about 9000 years ago when hexaploid bread wheat made its first appearance ( Feldman, 2001 ).
The earliest cultivated forms of wheat were essentially landraces selected by farmers from wild populations, presumably because of their superior yield and other characteristics, an early and clearly non-scientific form of plant breeding! However, domestication was also associated with the selection of genetic traits that separated them from their wild relatives. This domestication syndrome has been discussed in detail by others, but two traits are of sufficient importance to mention here. The first is the loss of shattering of the spike at maturity, which results in seed loss at harvesting. This is clearly an important trait for ensuring seed dispersal in natural populations and the non-shattering trait is determined by mutations at the Br ( brittle rachis ) locus ( Nalam et al. , 2006 ).
The second important trait is the change from hulled forms, in which the glumes adhere tightly to the grain, to free-threshing naked forms. The free forms arose by a dominant mutant at the Q locus which modified the effects of recessive mutations at the Tg ( tenacious glume ) locus ( Jantasuriyarat et al. , 2004 ; Simons et al. , 2006 ; Dubkovsky and Dvorak, 2007 ).
Cultivated forms of diploid, tetraploid, and hexaploid wheat all have a tough rachis apart from the spelt form of bread wheat. Similarly, the early domesticated forms of einkorn, emmer, and spelt are all hulled, whereas modern forms of tetraploid and hexaploid wheat are free-threshing.
Whereas einkorn and emmer clearly developed from the domestication of natural populations, bread wheat has only existed in cultivation, having arisen by hybridization of cultivated emmer with the unrelated wild grass Triticum tauschii (also called Aegilops tauschii and Ae . squarosa ). This hybridization probably occurred several times independently with the novel hexaploid (genome AABBDD) being selected by farmers for its superior properties. The evolution of modern wheats is illustrated in Fig. 1 which also shows examples of spikes and grain.
The evolutionary and genome relationships between cultivated bread and durum wheats and related wild diploid grasses, showing examples of spikes and grain. Modified from Snape and Pánková (2006) , and reproduced by kind permission of Wiley-Blackwell.
The genetic changes during domestication mean that modern wheats are unable to survive wild in competition with better adapted species. This was elegantly demonstrated by John Bennet Lawes in the 1880s when he decided to allow part of the famous long-term Broadbalk experiment at Rothamsted to return to its natural state ( Dyke, 1993 ). He therefore left part of the wheat crop unharvested in 1882 and monitored the growth in successive years. After a good crop in 1883 the weeds dominated and in 1885 the few remaining wheat plants (which were spindly with small ears) were collected and photographed.
The A genomes of tetraploid and hexaploid wheats are clearly related to the A genomes of wild and cultivated einkorn, while the D genome of hexaploid wheat is clearly derived from that of T . tauschii . In fact, the formation of hexaploid wheat occurred so recently that little divergence has occurred between the D genomes present in the hexaploid and diploid species. By contrast, the B genome of tetraploid and hexaploid wheats is probably derived from the S genome present in the Sitopsis section of Aegilops , with Ae . speltoides being the closest extant species. The S genome of Ae . speltoides is also closest to the G genome of T . timopheevi , a tetraploid species with the A and G genomes ( Feldman, 2001 ).
The spread of wheat from its site of origin across the world has been elegantly described by Feldman (2001) and is only summarized here. The main route into Europe was via Anatolia to Greece (8000 BP) and then both northwards through the Balkans to the Danube (7000 BP) and across to Italy, France and Spain (7000 BP), finally reaching the UK and Scandanavia by about 5000 BP. Similarly, wheat spread via Iran into central Asia reaching China by about 3000 BP and to Africa, initially via Egypt. It was introduced by the Spaniards to Mexico in 1529 and to Australia in 1788.
Currently, about 95% of the wheat grown worldwide is hexaploid bread wheat, with most of the remaining 5% being tetraploid durum wheat. The latter is more adapted to the dry Mediterranean climate than bread wheat and is often called pasta wheat to reflect its major end-use. However, it may also be used to bake bread and is used to make regional foods such as couscous and bulgar in North Africa. Small amounts of other wheat species (einkorn, emmer, spelt) are still grown in some regions including Spain, Turkey, the Balkans, and the Indian subcontinent. In Italy, these hulled wheats are together called faro ( Szabó and Hammer, 1996 ) while spelt continues to be grown in Europe, particularly in Alpine areas ( Fossati and Ingold, 2001 ).
The recent interest in spelt and other ancient wheats (including kamut, a tetraploid wheat of uncertain taxonomy, related to durum wheat) as healthy alternatives to bread wheat ( Abdel-Aal et al. , 1998 ) may also lead to wider growth for high value niche markets in the future.
Despite its relatively recent origin, bread wheat shows sufficient genetic diversity to allow the development of over 25 000 types ( Feldman et al. , 1995 ) which are adapted to a wide range of temperate environments. Provided sufficient water and mineral nutrients are available and effective control of pests and pathogens is ensured, yields can exceed 10 tonnes ha −1 , comparing well with other temperate crops. However, deficiencies in water and nutrients and the effects of pests and pathogens cause the global average yield to be low, at about 2.8 tonnes ha −1 . Wheat is also readily harvested using mechanical combine harvesters or traditional methods and can be stored effectively indefinitely before consumption, provided the water content is below about 15% dry weight and pests are controlled.
There is no doubt that the adaptability and high yields of wheat have contributed to its success, but these alone are not sufficient to account for its current dominance over much of the temperate world. The key characteristic which has given it an advantage over other temperate crops is the unique properties of doughs formed from wheat flours, which allow it to be processed into a range of breads and other baked products (including cakes and biscuits), pasta and noodles, and other processed foods. These properties depend on the structures and interactions of the grain storage proteins, which together form the ‘gluten’ protein fraction.
Transcriptomic studies have shown that over 30 000 genes are expressed in the developing wheat grain ( Wan et al. , 2008 ) while proteomic analysis of mature grain has revealed the presence of about 1125 individual components ( Skylas et al. , 2000 ). However, many of these components are present in small amounts and have little or no impact on the utilization of the grain, with one protein fraction being dominant in terms of amount and impact. This fraction is the prolamin storage proteins, which correspond to the gluten proteins. The precise number of individual gluten protein components has not been determined, but 2D gel analyses suggest that about 100 is a reasonable estimate. Together they have been estimated to account for about 80% of the total grain protein in European wheats ( Seilmeier et al. , 1991 ).
Gluten was one of the earliest protein fractions to be described by chemists, being first described by Beccari in 1728 (see translation by Bailey, 1941 ). It is traditionally prepared by gently washing wheat dough in water or dilute salt solution, leaving a cohesive mass which comprises about 80% protein, the remainder being mainly starch granules which are trapped in the protein matrix.
The ability to prepare the gluten proteins in an essentially pure state by such a simple procedure depends on their unusual properties. Firstly, they are insoluble in water or dilute salt solutions but are soluble in alcohol/water mixtures (as discussed below) and were hence defined as ‘prolamins’ by TB Osborne in his classic studies of plant proteins carried out at the end of the 19th century and the start of the 20th century ( Osborne, 1924 ). Secondly, the individual gluten proteins are associated by strong covalent and non-covalent forces which allow the whole fraction to be isolated as a cohesive mass.
What is the origin of gluten?
In common with other seed storage proteins, the gluten proteins are secretory proteins, being synthesized on the rough endoplasmic reticulum and co-translationally transported into the lumen of the ER. Once within the ER lumen, cereal seed storage proteins may follow two routes: a Golgi-dependent route leading to deposition within protein bodies of vacuolar origin or a Golgi-independent route in which protein deposits formed within the ER lumen may ultimately fuse with protein bodies of vacuolar origin (see Kumamaru et al. , 2007 , for a review).
Work carried out by Galili and colleagues ( Levanany et al. , 1992 ; Galili et al. , 1995 ; Galili, 1997 ) indicated that wheat gluten proteins may follow both routes, and this has recently been confirmed using epitope tags and specific antibodies to follow individual proteins and groups of proteins in cells of developing grain ( Tosi et al. , 2009 ). It is also clear that the protein deposits fuse to form a continuous matrix as the cells of the starchy endosperm dry and die during the later stages of grain maturation ( Fig. 2A ). Thus a proteinaceous network is present in each endosperm cell ( Fig. 2B ) and these networks are brought together when flour is mixed with water to form a continuous network in the dough. Washing the dough to remove non-gluten components therefore allows the network to be recovered as the cohesive mass which is called gluten ( Fig. 2C ).
The origin of wheat gluten. (A) Transmission electron microscopy of the developing starchy endosperm cells at 46 d after anthesis shows that the individual protein bodies have fused to form a continuous proteinaceous matrix. Taken from Shewry et al. , 1995 , ( Biotechnology 13, 1185–1190) and provided by Dr M Parker (IFR, Norwich, UK). (B) Digestion of a flour particle with amylases to remove starch reveals a continuous proteinaceous network. Taken from Amend and Beauvais (1995) and reproduced by kind permission of Getreidetechnologie. (C) After kneading, dough can be washed to recover the gluten network as a cohesive mass which is stretched in the photograph to demonstrate its viscoelastic properties.
The biochemical and molecular basis for gluten functionality
Humankind has been aware for many centuries that wheat dough has unusual properties which are shared to a limited extent by doughs made from rye flour but not by those from other cereal flours. These properties, which are usually described as ‘viscoelasticity’, are particularly important in making leavened bread, as they allow the entrapment of carbon dioxide released during leavening. However, they also underpin a range of other uses including making unleavened breads, cakes, and biscuits, pasta (from durum wheat), and noodles (from bread wheat). They are also exploited in the food industry where gluten proteins may be used as a binder in processed foods.
The volume of research carried out on wheat gluten is vast, with a simple search of the Web of Science database showing almost 20 000 papers since 1945. This volume not only reflects the commercial importance of wheat processing, but also the complexity of the system which remains incompletely understood. They include studies at the genetic, biochemical, biophysical, and functional (ie processing) levels.
Genetic studies have exploited the extensive polymorphism which exists between the gluten protein fractions present in different genotypes to establish genetic linkages between either groups of gluten proteins, or allelic forms of these, and aspects of processing quality. Similarly, studies at the biochemical and biophysical levels have demonstrated a relationship between dough strength and the ability of the gluten proteins to form polymeric complexes (called glutenins). Combining results from these two approaches highlighted the importance of a specific group of gluten proteins, called the high molecular weight (HMW) subunits of glutenin.
Cultivars of bread wheat express between three and five HMW subunit genes, with the encoded proteins accounting for up to about 12% of the total grain protein ( Seilmeier et al. , 1991 ; Halford et al. , 1992 ). The HMW subunits are only present in high molecular mass polymers and allelic variation in both the number of expressed genes and the properties of the encoded proteins results in effects on the amount and size of the polymers and hence dough strength (reviewed by Payne, 1987 ; Shewry et al. , 2003 b ). These glutenin polymers are known to be stabilized by inter-chain disulphide bonds, but it is apparent that non-covalent hydrogen bonds are also important in stabilizing the interactions between glutenin polymers and monomeric gluten proteins (called gliadins) ( Belton, 2005 ). Hence, the individual gliadins and glutenin polymers can be separated using solvents which disrupt hydrogen bonding (such as urea) but reducing agents (such as 2-mercaptoethanol or dithiothreitol) are required to break down the glutenin polymers to release the individual subunits.
Although the HMW subunits are the main determinants of glutenin elasticity relationships between other gluten proteins and functional properties have also been reported (reviewed by Shewry et al. , 2003 a ).
The relationship between the HMW subunits and dough strength was first established over 25 years ago ( Payne et al. , 1979 ) and allelic forms associated with good processing quality have been selected by plant breeders for over two decades, using simple SDS-PAGE separations. The established relationships between the number of expressed HMW subunit genes, the total amount of HMW subunit protein and dough strength have also resulted in the HMW subunit genes being an attractive target for genetic transformation, in order to increase their gene copy number and hence dough strength.
The first studies of this type were reported over 10 years ago ( Altpeter et al. , 1996 ; Blechl and Anderson, 1996 ; Barro et al. , 1997 ) and many studies have since been reported (reviewed by Shewry and Jones, 2005 ; Jones et al. , 2009 ). It is perhaps not surprizing that the results have been ‘mixed’, but some conclusions can be drawn. Firstly, expression of an additional HMW subunit gene can lead to increased dough strength, even when a modern good quality wheat cultivar is used as the recipient (see Field et al. , 2008 ; Rakszegi et al. , 2008 , as recent examples, and reviews of earlier work cited above). However, the effect depends on the precise HMW subunit gene which is used and on the expression level, with the transgenes resulting in over-strong (ie too elastic) gluten properties in some studies. Thus, although transgenesis is a realistic strategy to increase dough strength in wheat, it is also necessary to have an understanding of the underlying mechanisms in order to optimize the experimental design.
Wheat is widely consumed by humans, in the countries of primary production (which number over 100 in the FAO production statistics for 2004) and in other countries where wheat cannot be grown. For example, imported wheat is used to meet consumer demands for bread and other food products in the humid tropics, particularly those with a culinary tradition dating back to colonial occupation. Statistics are not available for the total volume of wheat which is consumed directly by humans as opposed to feeding livestock, although figures for the UK indicate about one-third of the total production (approximately 5.7 m tonnes per annum are milled with home production being 15–16 m tonnes). Globally there is no doubt that the number of people who rely on wheat for a substantial part of their diet amounts to several billions.
The high content of starch, about 60–70% of the whole grain and 65–75% of white flour, means that wheat is often considered to be little more than a source of calories, and this is certainly true for animal feed production, with high-yielding, low-protein feed varieties being supplemented by other protein-rich crops (notably soybeans and oilseed residues).
However, despite its relatively low protein content (usually 8–15%) wheat still provides as much protein for human and livestock nutrition as the total soybean crop, estimated at about 60 m tonnes per annum (calculated by Shewry, 2000 ). Therefore, the nutritional importance of wheat proteins should not be underestimated, particularly in less developed countries where bread, noodles and other products (eg bulgar, couscous) may provide a substantial proportion of the diet.
Protein content
Although wheat breeders routinely select for protein content in their breeding programmes (high protein for breadmaking and low protein for feed and other uses), the current range of variation in this parameter in commercial cultivars is limited. For example, Snape et al. (1993) estimated that typical UK breadmaking and feed wheats differed in their protein content by about 2% dry weight (eg from about 12–14% protein) when grown under the same conditions, which is significantly less than the 2-fold differences which can result from high and low levels of nitrogen fertilizer application. This limited variation in conventional wheat lines has led to searches for ‘high protein genes’ in more exotic germplasm.
Early studies of the USDA World Wheat Collection showed approximately 3-fold variation in protein content (from 7–22%), with about one-third of this being under genetic control ( Vogel et al. , 1978 ). However, the strong environmental impact on protein content (accounting for two-thirds of the variation) underpins the difficulty of breeding for this trait. Nevertheless, some success has been achieved by incorporating sources of variation from exotic bread wheat lines or related wild species.
The former include Atlas 50 and Atlas 66, derived from the South American line Frandoso, and Nap Hal from India. These lines appear to have different ‘high protein genes’ and both were extensively used in breeding programmes in Nebraska with the Atlas 66 gene being successfully incorporated into the commercial variety Lancota ( Johnson et al. , 1985 ). Frandoso and related Brazilian lines have also been successfully exploited in other breeding programmes in the USA ( Busch and Rauch, 2001 ). The Kansas variety, Plainsman V, also contained a high protein gene(s) from a related Aegilops species ( Finney, 1978 ).
The most widely studied source of ‘high protein’ is wild emmer (tetraploid Tr . turgidum var. dicoccoides ) wheats from Israel. One accession, FA15-3, accumulates over 40% of protein when grown with sufficient nitrogen ( Avivi, 1978 ). The gene in this line was mapped to a locus on chromosome 6B (called Gpc-B1 ), which accounted for about 70% of the variation in protein content in crosses ( Chee et al. , 2001 ; Distelfeld et al. , 2004, 2006 ). More recent studies have shown that the gene Gpc-B1 encodes a transcription factor which accelerates senescence in the vegetative parts of the plant, resulting in increased mobilization and transfer to the grain of both nitrogen and minerals (notably iron and zinc) ( Uauy et al. , 2006 ). However, it remains to be shown whether this gene can be incorporated into high-yielding and commercially viable lines.
Protein composition
Of the 20 amino acids commonly present in proteins, 10 can be considered to be essential in that they cannot be synthesized by animals and must be provided in the diet. Furthermore, if only one of these is limiting the others will be broken down and excreted. There has been much debate about which amino acids are essential and the amounts that are required, with the most recent values for adult humans being shown in Table 1 . This table includes a combined value for the two aromatic amino acids, tyrosine and phenylalanine, which are biosynthetically related, and both single and combined values for the two sulphur-containing amino acids: methionine, which is truly essential, and cysteine which can be synthesized from methionine. Comparison with the values for whole wheat grain and flour shows that only lysine is deficient, with some essential amino acids being present in considerably higher amounts than the requirements. However, the lysine content of wheat also varies significantly with the values shown in Table 1 being typical of grain of high protein content and the proportion increasing to over 30 mg g −1 protein in low protein grain ( Mossé and Huet, 1990 ). This decrease in the relative lysine content of high protein grain results from proportional increases in the lysine-poor gluten proteins when excess N is available (for example, when fertilizer is applied to increase grain yield and protein content) and also accounts for the lower lysine content of the white flour (the gluten proteins being located in the starchy endosperm tissue).
Recommended levels of essential amino acids for adult humans compared with those in wheat grain and flour (expressed as mg g −1 protein)
FAO/WHO/UNU (2007) .
Calculated from literature values as described in Shewry (2007) .
The amino acid requirements for infants and children vary depending on their growth rate, being particularly high in the first year of life. Similarly, higher levels of essential amino acids are required for rapidly growing livestock such as pigs and poultry.
Wheat as a source of minerals
Iron deficiency is the most widespread nutrient deficiency in the world, estimated to affect over 2 billion people ( Stoltzfus and Dreyfuss, 1998 ). Although many of these people live in less developed countries, it is also a significant problem in the developed world. Zinc deficiency is also widespread, particularly in Sub-Saharan Africa and South Asia, and has been estimated to account for 800 000 child deaths a year (Micronutrient Initiative, 2006 ), in addition to non-lethal effects on children and adults. Wheat and other cereals are significant sources of both of these minerals, contributing 44% of the daily intake of iron (15% in bread) and 25% of the daily intake of zinc (11% in bread) in the UK ( Henderson et al. , 2007 ). There has therefore been considerable concern over the suggestion that the mineral content of modern wheat varieties is lower than that of older varieties.
This was initially suggested by Garvin et al. (2006) who grew 14 red winter wheat cultivars bred between 1873 and 2000 in replicate field experiments and determined their mineral contents. Plants were grown at two locations in Kansas and significant negative correlations were found between grain yield, variety release date, and the concentrations of zinc in material from both of these sites and of iron in materials from only one site. Similar trends were reported by Fan et al. (2008 a , b ) who took a different approach. Rather than carrying out direct comparisons of varieties in field trials, they analysed grain grown on the Rothamsted Broadbalk long-term wheat experiment. This experiment was established in 1843 and uses a single variety which is replaced by a more modern variety at regular intervals. Analysis of archived grain showed significant decreases in the contents of minerals (Zn, Fe, Cu, Mg) since semi-dwarf cultivars were introduced in 1968. A similar difference was observed between the cultivars Brimstone (semi-dwarf) and Squareheads Master (long straw) which were grown side by side in 1988–1990, the concentrations of Zn, Cu, Fe, and Mg being 18–29% lower in Brimstone. A more recent comparison of 25 lines grown also showed a decline in the concentrations of Fe and Zn since semi-dwarf wheats were introduced ( Zhao et al. , 2009 ) ( Fig. 3 ). Although the decrease in the mineral content of modern wheats is partly due to dilution, resulting from increased yield (which was negatively correlated with mineral content), it has been suggested that short-strawed varieties may be intrinsically less efficient at partitioning minerals to the grain compared with the translocation of photosynthate.
The relationship between the iron content of wholemeal flours from 25 wheat cultivars grown on six trial sites/seasons and their release dates. Taken from Zhao et al. (2009) and reproduced by kind permission of Elsevier.
Such genetic differences in mineral content are clearly relevant to international efforts to increase the mineral content of wheat to improve health in less developed countries. Thus, increasing iron, zinc, and vitamin A contents are a major focus of the HarvestPlus initiative of the Consultative Group on International Agricultural Research (CGIAR) which is using conventional plant breeding ( Ortiz-Monasterio et al. , 2007 ) while other laboratories are using genetic engineering approaches (reviewed by Brinch-Pedersen et al. , 2007 ).
These initiatives are focusing not only on contents of minerals but also on their bioavailability. Iron is predominantly located in the aleurone and as complexes with phytate ( myo -inositolphosphate 1,2,3,4,5,6-hexa-kisphosphate). These complexes are largely insoluble, restricting mineral availability to humans and livestock. The use of transgenesis to express phytase in the developing grain can result in increased mineral availability, particularly when a heat-stable form of the enzyme is used to allow hydrolysis to occur during food processing (reviewed by Brinch-Pedersen et al. , 2007 ).
Guttieri et al. (2004) also reported an EMS-induced low phytate mutant of wheat. This mutation resulted in 43% less phytic acid in the aleurone, but has not so far been incorporated into commercial cultivars. However, previous experience with low phytic acid mutants of maize, barley, and soy bean has shown that they may also have significant effects on yield and germination rates (reviewed by Brinch-Pedersen et al. , 2007 ).
Wheat as a source of selenium
Selenium is an essential micronutrient for mammals (but not plants), being present as selenocysteine in a number of enzymes. However, it is also toxic when present in excess (above about 600 μg d −1 ; Yang and Xia, 1995 ). Cereals are major dietary sources of selenium in many parts of the world, including China ( FAO/WHO, 2001 ), Russia ( Golubkina and Alfthan, 1999 ), and the UK (MAFF, 1997). However, the content of selenium in wheat varies widely from about 10 μg kg −1 to over 2000 μg kg −1 ( FAO/ WHO, 2001 ; Combs, 2001 ).
The concentration of selenium in wheat is largely determined by the availability of the element in the soil. Consequently, wheat produced in Western Europe may contain only one-tenth of the selenium that is present in wheat grown in North America. Thus, a survey of 452 grain samples grown in the UK in 1982 and 1992 showed a mean value of 27 μg Se kg −1 fresh weight ( Adams et al. , 2002 ) compared with 370 μg SE kg −1 fresh weight for 290 samples from the USA ( Wolnik et al. , 1983 ).
Because the import of wheat from North America into Europe has declined over the last 25 years, the intake of selenium in the diet has also decreased, which has resulted in concern in some European countries. One response to this is to apply selenium to the crops in fertilizer (called biofortification), which is practised in Finland ( Eurola et al. , 1991 ).
Unlike iron, selenium is not concentrated in the aleurone, being present wherever sulphur is present. The concentration of selenium in grain from the Broadbalk continuous wheat experiment also appeared to be determined principally by the sulphur availability in the soil (which competes to prevent selenium uptake), with no evidence of decreased levels over time ( Fan et al. , 2008 b ). However, sulphur fertilizer is often applied to wheat to improve the grain quality ( Zhao et al. , 1997 ) and this could clearly have negative impacts on selenium in grain.
The reader is referred to a recent review article by Hawkesford and Zhao (2007) for a detailed review of selenium in wheat.
Wholegrain wheat and health
The consumption of white flour and bread have historically been associated with prosperity and the development of sophisticated roller mills in Austro-Hungary during the second part of the 19th century allowed the production of higher volumes of whiter flour than it was possible to produce by traditional milling based on grinding between stones and sieving (see Jones, 2007 , for a fascinating account of the history of roller milling). However, the increased consumption of bread made from highly refined white flour was not accepted universally, leading to what we would today recognize as a movement to increase the consumption of wholegrain products.
In 1880, May Yates founded the Bread Reform League in London to promote a return to wholemeal bread, particularly to improve the nutrition of the children of the poor, and suggested in 1909 that an official minimum standard of 80% flour extraction rate should be adopted. This was called ‘Standard Bread’. Although we now appreciate the nutritional advantages of wholegrain products, this was not supported by the science of the time and clearly conflicted with the tastes of consumers as well as the economics of bread production. Nevertheless, the League continued to campaign and received scientific support in 1911 when Gowland Hopkins agreed that ‘Standard Bread’ may contain ‘unrecognized food substances’ which were vital for health: these were subsequently called vitamins ( Burnett, 2005 ).
By contrast, Thomas Allinson (1858–1918) had a much greater impact by marketing and vigorously promoting his own range of wholemeal products. He can therefore be regarded as the father of the wholegrain movement and remains a household name to this day in the UK ( Pepper, 1992 ).
We now know that wholegrain wheat products contain a range of components with established or proposed health benefits which are concentrated or solely located in the bran. Hence they are either present in lower amounts or absent from white flour which is derived almost exclusively from starchy endosperm cells. They vary widely in their concentrations. For example, lignans, a group of polyphenols with phytoestrogen activity, are present at levels up to about 10 μg g −1 in wholemeal wheat and twice this level in bran ( Nagy-Scholz and Ercsey, 2009 ), while total phenolic acids in wholemeal range up to almost 1200 μg g −1 ( Li et al. , 2008 ).
The most detailed study of wheat phytochemicals which has so far been reported was carried out as part of the EU Framework 6 HEALTHGRAIN programme ( Poutanen et al. , 2008 ; Ward et al. , 2008 ). This study determined a range of phytochemicals in 150 wheat lines grown on a single site in one year, meaning that the levels of the components may have been influenced by environmental as well as genetic effects. The lines were selected to represent a broad range of dates and places of origin. The choice of phytochemicals focused on those which have putative health benefits and for which cereals are recognized dietary sources. For example, cereals are considered to account for about 22% of the daily intake of folate (vitamin B12) in the UK ( Goldberg, 2003 ) and 36% and 43% of the daily intake in Finnish women and men, respectively ( Findiet Study Group, 2003 ). In the HEALTHGRAIN study the contents of folates in wholemeal varied from 364 to 774 ng g −1 dry weight in 130 winter wheats and from 323 to 741 ng g −1 dry weight in 20 spring wheats, with the content in the former being positively correlated with bran yield and negatively correlated with seed weight (indicating concentration in the bran) ( Piironen et al. , 2008 ).
The quantitatively major group of phytochemicals in the wheat grain is phenolic acids, derivatives of either hydroxybenzoic acid or hydroxycinnamic acid. Epidemiological studies indicate that phenolic acids have a number of health benefits which may relate to their antioxidant activity; the total antioxidant activities of grain extracts and their phenolic acid contents being highly correlated ( Drankham et al. , 2003 ; Beta et al. , 2005 ; Wende et al. , 2005 ).
Cereals are also significant sources of tocols (which include vitamin E) (27.6–79.7 μg g −1 in the HEALTHGRAIN study) ( Lampi et al. , 2008 ) and sterols (670–959 μg g −1 ) ( Nurmi et al. , 2008 ).
The HEALTHGRAIN study also determined the levels of dietary fibre. In wheat, this mainly derives from cell wall polymers: arabinoxylans (approximately 70%) with lower amounts of (1-3)(1-4)β- D -glucans (approximately 20%) and other components. The arabinoxylans also occur in soluble and insoluble forms, with the latter being rich in bound phenolic acids which form oxidative cross-links. These bound phenolic acids account, on average, for 77% of the total phenolic acid fraction and are predominantly ferulic acid. Soluble fibre is considered to have health benefits ( Moore et al. , 1998 ; Lewis and Heaton, 1999 ) which are not shared by insoluble fibre and these may therefore be reduced by the phenolic acid cross-linking. However, insoluble fibre may also have benefits in delivering phenolic antioxidants into the colon: these benefits may include reduction in colo-rectal cancer ( Vitaglione et al. , 2008 ).
The HEALTHGRAIN study showed wide variation in the contents of total and water-extractable arabinoxylans in both white flour and bran fractions ( Gebruers et al. , 2008 ) ( Fig. 4 ). Similarly, Ordaz-Ortiz et al. (2005) showed variation from 0.26% to 0.75% dry weight in the content of water-extractable arabinoxylan in 20 French wheat lines and from 1.66% to 2.87% dry weight in total arabinoxylans. A high proportion of the variation in water-extractable arabinoxylans is also heritable ( Martinant et al. , 1999 ).
Contents of arabinoxylan (AX) fibre in flour and bran of 150 wheat cultivars grown on a single site as part of the EU FP6 HEALTHGRAIN project. (A) Total AX in flour (mg g −1 ); (B) water-extractable AX in flour (%); (C) total AX in bran (mg g −1 ), and (D) water-extractable AX in bran. Prepared from data reported by Gebruers et al. (2008) with permission of the authors.
It is clear from these and other studies that there is sufficient genetically determined variation in the phytochemical and fibre contents of wheat to be exploited in breeding for varieties with increased nutritional benefits.
Allergy to wheat
Both respiratory and food allergies to wheat have been reported.
Respiratory allergy (bakers' asthma) has been known since Roman times (when slaves handling flour and dough were required to wear masks) and is currently one of the most important forms of occupational allergy. For example, it is the second most widespread occupational allergy in the UK and has been reported to affect over 8% of apprentice bakers in Poland after only 2 years exposure ( Walusiak et al. , 2004 ). A wide range of wheat grain proteins have been shown to react with immunoglobulin (Ig)E in sera of patients with bakers' asthma, including gliadins, glutenins, serpins (serine proteinase inhibitors), thioredoxin, agglutinin, and a number of enzymes (α- and β-amylases, peroxidase, acyl CoA oxidase, glycerinaldehyde-3-phosphate dehydrogenase and triosephosphate isomerase) (reviewed by Tatham and Shewry, 2008 ). However, it is clear that the predominant wheat proteins responsible for bakers' asthma are a class of α-amylase inhibitors, also known as CM proteins due to their solubility in chloroform:methanol mixtures ( Salcedo et al. , 2004 ). Furthermore, their activity has been demonstrated by a range of approaches including skin pricks and RAST (radioallergosorbent test) as well as immunoblotting, ELISA, and screening expression libraries with IgE fractions.
The CM proteins comprise monomeric, dimeric, and tetrameric forms, with subunit masses ranging between about 10 000 and 16 000. They differ in their spectrum of activity but all inhibit mammalian and insect α-amylases (including those in some pest organisms) rather than endogenous wheat enzymes. Hence, they are considered to be protective rather than regulatory in function. Eleven individual subunits have been shown to play a role in bakers' asthma (using one or more of the assays listed above) but they differ in their activity, with a glycosylated form of CM16 being particularly active.
Wheat is listed among the ‘big eight’ food allergens which together account for about 90% of all allergic responses. However, the incidence of true (ie IgE-mediated) food allergy is, in fact, fairly infrequent in adults, although it may affect up to 1% of children ( Poole et al. , 2006 ). A number of wheat proteins have been reported to be responsible for allergic responses to the ingestion of wheat products but only one syndrome has been studied in detail. Wheat-dependent exercise-induced anaphylaxis (WDEIA) is a well-defined syndrome in which the ingestion of a product containing wheat followed by physical exercise can result in an anaphylactic response. Work carried out by several groups has clearly established that this condition is associated with a group of ω-gliadins (called ω5-gliadins) which are encoded by genes on chromosome 1B ( Palosuo et al. , 2001 ; Morita et al. , 2003 ; Battais et al. , 2005 ). Mutational analysis has also identified immunodominant epitopes in the ω5-gliadins: short glutamine-rich and proline-rich sequences present in the repetitive domains of the proteins ( Matsuo et al. , 2004 , 2005 ; Battais et al. , 2005 ). However, a number of other proteins have also been shown to react with IgE from patients with WDEIA, including gliadins, glutenin subunits, and related proteins from barley and rye (reviewed by Tatham and Shewry, 2008 ).
Other allergic responses to wheat proteins include atopic dermatitis, urticaria, and anaphylaxis. Not surprizingly, these symptoms have been associated with a number of wheat proteins, most notably gluten proteins but also CM proteins, enzymes, and lipid transfer protein (LTP) (reviewed by Tatham and Shewry, 2008 ).
Comparison of the proteins identified as responsible for the respiratory and food allergy shows significant overlap in their functions (most being storage or protective) and identities (notably gluten proteins and CM proteins).
Intolerance to wheat
Dietary intolerance to wheat is almost certainly more widespread than allergy, notably coeliac disease (CD) which is estimated to affect 1% of the population of Western Europe ( Feighery, 1999 ), and dermatitis herpetiformis which has an incidence between about 2-fold and 5-fold lower than CD ( Fry, 1992 ).
CD is a chronic inflammation of the bowel which leads to malabsorption of nutrients. Like bakers' asthma, CD has been known since classical times but it was only defined in detail in 1887 and its relationship to wheat established by Dicke in the late 1940s ( Losowsky, 2008 ).
A series of elegant studies carried out over the past decade, particularly by Sollid, Koning and co-workers, have established that CD results from an autoimmune response which is triggered by the binding of gluten peptides to T cells of the immune system in some (but not all) individuals with the human leucocyte antigens (HLAs) DQ2 or DQ8, expressed by specialized antigen-presenting cells. The presented peptides are then recognized by specific CD4+ T cells which release inflammatory cytokines which lead to the flattening of the intestinal epithelium. It has also been demonstrated that tissue transglutaminase enzyme present in the epithelium of the intestine plays an important role, generating toxic peptides by deamidation of glutamine residues to give glutamate.
The HLA-DQ2 antigen is present in about 95% of coeliac patients ( Karell et al. , 2003 ) and detailed studies have identified the peptide sequences which are recognized by intestinal T cell lines, using either peptide fractions produced from gluten proteins or synthetic peptides. This has led to the definition of two overlapping immunodominant epitopes corresponding to residues 57–68 (α-9) and 62–75 (α-2) of A gliadin (a form of α-gliadin) ( Arentz-Hansen et al. , 2000 , 2002 ; Anderson et al. , 2000 ; Ellis et al. , 2003 ). Related epitopes were similarly defined in γ-gliadins, corresponding to residues 60–79, 102–113, 115–123, and 228–236 ( Sjöström et al. , 1998 ; Arentz-Hansen et al. , 2002 ; Vader et al. , 2002 a ). Furthermore, Vader et al. (2002 b ) showed that the spacing between glutamine and proline residues determined the specificity of glutamine deamidation and hence peptide activation, and developed algorithms to predict the presence of novel T cell stimulatory peptides in gluten proteins and in related proteins from other cereals.
Less work has been carried out on the determinants of the HLA8-DQ8 associated coeliac disease, which affects only about 6% of patients without HLA-DQ2 and 10% of patients with HLA-DQ2 ( Karell et al. , 2003 ). This has again allowed immunodominant epitopes to be identified in gliadins and glutenin subunits ( van der Wal et al. , 1998 , 1999 ; Mazzarella et al. , 2003 ; Tollefsen et al. , 2006 ) although detailed structural studies indicate that the HLA-DQ2 and HLA-DQ8-mediated forms of the disease may differ in their molecular mechanisms ( Henderson et al. , 2007 ).
The possibility of producing wheat which lacks the coeliac toxic peptides has been discussed for many years but interest in the strategy tended to decline as it became clear that most, if not all, gluten proteins are toxic to at least some susceptible individuals, rather than only the α-gliadins as initially thought. However, Spaenij-Dekking et al. (2005) and van Herpen et al. (2006) have shown that it is possible to identify natural forms of gliadin which have few or no coeliac toxic epitopes, raising the possibility of selecting for less toxic lines of wheat by classical plant breeding. RNA interference (RNAi) technology has also been used to silence the α-gliadin ( Becker et al. , 2006 ; Wieser et al. , 2006 ) and γ-gliadin ( Gil-Humanes et al. , 2008 ) gene families, although some effects on grain-processing properties were observed.
The combination of these two approaches may therefore allow the production of less toxic, if not non-toxic, wheat for coeliac patients without significant loss of the processing properties conferred by the gluten proteins.
Dermatitis herpetiformis is a skin eruption resulting from ingestion of gluten, and is associated with the deposition of IgA antibodies in dermal papillae. These include IgA antibodies to epidermal transglutaminase which is considered to be an important autoantigen in disease development ( Hull et al. , 2008 ).
Other medical conditions related to gluten proteins
There are many reports of the association of wheat, and particularly wheat proteins, with medical conditions, ranging from improbable reports in the popular press to scientific studies in the medical literature. Not surprisingly, they include autoimmune diseases such as rheumatoid arthritis which may be more prevalent in coeliac patients and relatives ( Neuhausen et al. , 2008 ). It is perhaps easier to envisage mechanisms for relationships between such diseases which have a common immunological basis ( Hvatum et al. , 2006 ) than to explain a well-established association between wheat, coeliac disease, and schizophrenia ( Singh and Roy, 1975 ; Kalaydiian et al. , 2006 ) Other reported associations include ones with sporadic idiopathic ataxia (gluten ataxia) ( Hadjivassiliou et al. , 2003 ), migraines ( Grant, 1979 ), acute psychoses ( Rix et al. , 1985 ), and a range of neurological illnesses ( Hadjivassiliou et al. , 2002 ). An association with autism has also been reported ( Lucarelli et al. , 1995 ) with some physicians recommending a gluten-free, casein-free diet ( Elder, 2008 ).
Some of these effects may be mediated via the immune system but effects which are not immune-mediated are notoriously difficult to define and diagnose. However, they could result from the release within the body of bioactive peptides, derived particularly from gluten protein. Thus, gluten has been reported to be a source of a range of such peptides including opioid peptides (exorphins) ( Takahashi et al. , 2000 ; Yoshikawa et al. , 2003 ) and an inhibitor of angiotensin I-converting enzyme ( Motoi and Kodama, 2003 ) (see also reviews by Dziuba et al. , 1999 ; Yamamoto et al. , 2003 ). However, these activities were demonstrated in vitro and their in vivo significance has not been established.
There is little doubt that wheat will retain its dominant position in UK and European agriculture due to its adaptability and consumer acceptance. However, it may also need to adapt to face changing requirements from farmers, food processors, governments, and consumers.
Reducing inputs
Currently grown wheat cultivars require high inputs of nitrogen fertilizer and agrochemicals to achieve high yields combined with the protein content required for breadmaking. For example, UK farmers currently apply 250–300 kg N ha −1 in order to achieve the 13% protein content required for the Chorleywood Breadmaking Process, which is the major process used for breadmaking in the UK. Since a 10 tonnes ha −1 crop containing 13% protein equates to about 230 kg N ha −1 , this means that 50–70 kg N ha −1 may be lost. As fertilizer N currently costs about £1 kg −1 this represents a significant financial loss as well as a loss of the energy required for fertilizer production and may also have environmental consequences.
A number of projects worldwide are therefore focusing on understanding the processes that determine the efficiency of uptake, assimilation, and utilization of nitrogen in order to improve the efficiency of nitrogen recovery in the grain (reviewed by Foulkes et al. , 2009 ).
Reducing the nitrogen requirement of wheat does not only relate to the grain protein content, as an adequate supply of nitrogen is also essential for high wheat yields in order to build a canopy and fix carbon dioxide by photosynthesis. Furthermore, a substantial proportion of this nitrogen is remobilized and redistributed to the developing grain during canopy senescence ( Dalling, 1985 ). Hawkesford and colleagues at Rothamsted Research have targeted this process in order to develop a strategy for improving the recovery of N in the grain, using a combination of biochemical analysis and metabolite and transcript profiling to identify differences in metabolites and gene expression which are associated with efficient mobilization and redistribution ( Howarth et al. , 2008 ). Some of the genes identified in these and similar studies are suitable candidates for manipulation to increase the proportion of the total nitrogen recovered in the grain.
Stability of quality
The increases in temperature and carbon dioxide concentration associated with climate change are expected to have effects on crop development and yield, although the magnitude of these is difficult to predict due to interactions with other factors which may also be affected, notably water availability and populations of pests and pathogens ( Coakley et al. , 1999 ; Semenov, 2008 ). Similarly, although it is generally accepted that higher growth temperatures result in greater dough strength, the precise effects are not clearly understood (see review by Dupont and Altenbach, 2003 ) with heat stress (ie above 30–33 °C) actually resulting in dough weakening and reduced quality ( Blumenthal et al. , 1993 ). A recent review of the effects of CO 2 concentration on grain quality also failed to draw clear conclusions ( Högy and Fangmeier, 2008 ).
Of more immediate interest to wheat breeders and grain-utilizing industries are year-to-year fluctuations in growth conditions, and the frequency and magnitude of such fluctuations are also predicted to increase in the future ( Porter and Semenov, 2005 ). Although some cultivars are generally considered to be more consistent in quality than others, this is largely anecdotal with no detailed scientific comparisons.
Given the recent advances in ‘omics’ technologies it should now be possible to dissect the effects of G×E on grain development and quality, and to establish markers suitable for use in plant breeding. However, this will require substantial resources and a multi-disciplinary approach: by growing mapped populations and lines in multi-site/multi-year trials and determining aspects of composition and quality from gene expression profiling to pilot scale breadmaking.
Wan et al. (2009) have reported the application of this approach using a limited set of seven doubled haploid lines to identify a number of transcripts whose expression profile was associated with quality traits independently of environmental conditions. Millar et al. (2008) also reported a larger study in which three doubled haploid populations of wheat were used to map novel QTLs (quantitative trait loci) for breadmaking and pastry making which were stable over two years field trials, but did not relate quality traits to gene expression profiles.
Wheat is an attractive option as a ‘first generation biofuel’ as the high content of starch is readily converted into sugars (saccharification) which can then be fermented into ethanol. Murphy and Power (2008) recently reported that the gross energy recovered in ethanol using wheat was 66 GJ ha −1 a −1 , but that this only corresponds to 50% energy conversion and that the net energy production is as low as 25 GJ ha −1 a −1 . The same authors also calculated that the net energy production could be increased to 72 GJ ha −1 a −1 if the straw was combusted and the residue after distillation, called stillage or distillers grain and solubles (DGS), was converted to biogas (biomethane).
A major concern about using wheat grain for biofuel production is the high energy requirement for crop production, including that required to produce nitrogenous fertilizer. It is therefore necessary to develop new crop management strategies to reduce inputs ( Loyce et al. , 2002 ) as well as exploiting wheats with low N input requirements combined with high starch contents ( Kindred et al. , 2008 ).
The second major concern is, of course, the impact on international grain prices which may exacerbate problems of grain supply to less affluent populations.
New benefits to consumers
The increasing awareness of the important role of wheat-based products in a healthy diet has been discussed above, focusing on the identification and exploitation of natural variation in bioactive components. However, in some cases the natural variation in a trait may be limited in extent or difficult to exploit and, in this case, other approaches may be required. Currently, the most important target of this type of approach is resistant starch.
Most of the starch consumed in the human diet, including wheat starch, is readily digested in the small intestine, resulting in a rapid increase in blood glucose which may contribute to the development of type 2 diabetes and obesity ( Sobal, 2007 ). However, a fraction of the starch may resist digestion and pass through the small intestine to the colon, where it is fermented to short chain fatty acids, notably butyrate, which may have health benefits including reduction of colo-rectal cancer (as discussed by Topping, 2007 ).
Although the proportion of resistant starch (RS) depends on a number of factors including the effects of food processing, the most widely studied form is high amylose starch. In most species, amylose accounts for 20–30% of starch and amylopectin for 70–80%. However, mutant lines have been identified in a number of species in which the proportion of amylose is increased up to about 40% (e.g. Glacier barley; Yoshimoto et al. , 2000 ).
Selection for high amylose mutants is relatively easy in a diploid species such as barley, but more painstaking approaches are required in hexaploid wheat as mutations in homoeologous genes on all three genomes may be required to have a significant effect on the phenotype. This has been demonstrated very elegantly by Yamamori et al. (2000) who combined mutations in the gene encoding the starch synthase II enzyme (also called starch granule protein 1) that catalyses the synthesis of amylopectin. The resulting triple mutant line contained about 37% amylose.
However, the complexity of starch biosynthesis means that similar high amylose phenotypes can result from changes in other enzymes, with a notable example being the use of RNA interference technology to down-regulate the gene encoding starch-branching enzyme IIa ( Regina et al. , 2006 ). The resulting transgenic lines had up to 80% amylose and increased RS as measured in rat feeding trials. This study demonstrates the power of GM technology, although it remains to be shown that lines with such high levels of amylose will have acceptable yields and properties for milling and processing.
It also remains to be shown that consumers will be prepared to eat bread and other foods produced from GM wheat. The wheat grain and its products have been treated with reverence by humans for millennia and GM wheat may just be regarded as a step too far, even in countries in which other GM crops are currently accepted.
I wish to thank all of my colleagues and collaborators who have contributed to the work discussed in this article, Professor John Snape and the John Innes Institute for providing Fig. 1 and Dr Jane Ward (Rothamsted) for preparing Fig. 4 . Rothamsted Research receives grant-aided support from the Biotechnology and Biological Sciences Research Council of the UK.
Google Scholar
Google Preview
Email alerts
Citing articles via.
- Recommend to your Library
Affiliations
- Online ISSN 1460-2431
- Print ISSN 0022-0957
- Copyright © 2024 Society for Experimental Biology
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 25 November 2020
Multiple wheat genomes reveal global variation in modern breeding
- Sean Walkowiak 1 , 2 na1 ,
- Liangliang Gao ORCID: orcid.org/0000-0002-3864-0631 3 na1 ,
- Cecile Monat ORCID: orcid.org/0000-0002-0574-3976 4 na1 ,
- Georg Haberer 5 ,
- Mulualem T. Kassa 6 ,
- Jemima Brinton 7 ,
- Ricardo H. Ramirez-Gonzalez 7 ,
- Markus C. Kolodziej ORCID: orcid.org/0000-0003-3155-2935 8 ,
- Emily Delorean 3 ,
- Dinushika Thambugala 9 ,
- Valentyna Klymiuk ORCID: orcid.org/0000-0001-9308-7886 1 ,
- Brook Byrns 1 ,
- Heidrun Gundlach ORCID: orcid.org/0000-0002-6757-0943 5 ,
- Venkat Bandi 10 ,
- Jorge Nunez Siri 10 ,
- Kirby Nilsen ORCID: orcid.org/0000-0002-9477-5549 1 , 11 ,
- Catharine Aquino 12 ,
- Axel Himmelbach 4 ,
- Dario Copetti ORCID: orcid.org/0000-0002-2680-2568 13 , 14 ,
- Tomohiro Ban 15 ,
- Luca Venturini 16 ,
- Michael Bevan ORCID: orcid.org/0000-0001-8264-2354 7 ,
- Bernardo Clavijo 17 ,
- Dal-Hoe Koo 3 ,
- Jennifer Ens ORCID: orcid.org/0000-0003-2663-1006 1 ,
- Krystalee Wiebe 1 ,
- Amidou N’Diaye ORCID: orcid.org/0000-0001-7769-7801 1 ,
- Allen K. Fritz 3 ,
- Carl Gutwin 10 ,
- Anne Fiebig 4 ,
- Christine Fosker ORCID: orcid.org/0000-0002-3799-9474 17 ,
- Bin Xiao Fu 2 ,
- Gonzalo Garcia Accinelli ORCID: orcid.org/0000-0001-9647-7935 17 ,
- Keith A. Gardner ORCID: orcid.org/0000-0002-4890-301X 18 ,
- Nick Fradgley 18 ,
- Juan Gutierrez-Gonzalez ORCID: orcid.org/0000-0002-6795-6192 19 ,
- Gwyneth Halstead-Nussloch 13 ,
- Masaomi Hatakeyama 12 , 13 ,
- Chu Shin Koh 20 ,
- Jasline Deek ORCID: orcid.org/0000-0002-5584-306X 21 ,
- Alejandro C. Costamagna 22 ,
- Pierre Fobert 6 ,
- Darren Heavens ORCID: orcid.org/0000-0001-5418-7868 17 ,
- Hiroyuki Kanamori 23 ,
- Kanako Kawaura 15 ,
- Fuminori Kobayashi 23 ,
- Ksenia Krasileva 17 ,
- Tony Kuo 24 , 25 ,
- Neil McKenzie 7 ,
- Kazuki Murata 26 ,
- Yusuke Nabeka ORCID: orcid.org/0000-0002-8692-5981 26 ,
- Timothy Paape 13 ,
- Sudharsan Padmarasu ORCID: orcid.org/0000-0003-3125-3695 4 ,
- Lawrence Percival-Alwyn 18 ,
- Sateesh Kagale 6 ,
- Uwe Scholz ORCID: orcid.org/0000-0001-6113-3518 4 ,
- Jun Sese 25 , 27 ,
- Philomin Juliana ORCID: orcid.org/0000-0001-6922-0173 28 ,
- Ravi Singh ORCID: orcid.org/0000-0002-4676-5071 28 ,
- Rie Shimizu-Inatsugi 13 ,
- David Swarbreck 17 ,
- James Cockram ORCID: orcid.org/0000-0002-1014-6463 18 ,
- Hikmet Budak ORCID: orcid.org/0000-0002-2556-2478 29 ,
- Toshiaki Tameshige 15 ,
- Tsuyoshi Tanaka 23 ,
- Hiroyuki Tsuji ORCID: orcid.org/0000-0002-6444-559X 15 ,
- Jonathan Wright ORCID: orcid.org/0000-0001-6471-8749 17 ,
- Jianzhong Wu ORCID: orcid.org/0000-0002-4033-852X 23 ,
- Burkhard Steuernagel 7 ,
- Ian Small ORCID: orcid.org/0000-0001-5300-1216 30 ,
- Sylvie Cloutier 31 ,
- Gabriel Keeble-Gagnère 32 ,
- Gary Muehlbauer 19 ,
- Josquin Tibbets 32 ,
- Shuhei Nasuda 26 ,
- Joanna Melonek ORCID: orcid.org/0000-0003-4471-2520 30 ,
- Pierre J. Hucl 1 ,
- Andrew G. Sharpe ORCID: orcid.org/0000-0002-1832-4009 20 ,
- Matthew Clark ORCID: orcid.org/0000-0002-8049-5423 16 ,
- Erik Legg 33 ,
- Arvind Bharti 33 ,
- Peter Langridge ORCID: orcid.org/0000-0001-9494-400X 34 ,
- Anthony Hall ORCID: orcid.org/0000-0002-1806-020X 17 ,
- Cristobal Uauy ORCID: orcid.org/0000-0002-9814-1770 7 ,
- Martin Mascher ORCID: orcid.org/0000-0001-6373-6013 4 , 35 ,
- Simon G. Krattinger ORCID: orcid.org/0000-0001-6912-7411 8 , 36 ,
- Hirokazu Handa 23 , 37 ,
- Kentaro K. Shimizu ORCID: orcid.org/0000-0002-6483-1781 13 , 15 ,
- Assaf Distelfeld 38 ,
- Ken Chalmers 34 ,
- Beat Keller ORCID: orcid.org/0000-0003-2379-9225 8 ,
- Klaus F. X. Mayer ORCID: orcid.org/0000-0001-6484-1077 5 , 39 ,
- Jesse Poland ORCID: orcid.org/0000-0002-7856-1399 3 ,
- Nils Stein ORCID: orcid.org/0000-0003-3011-8731 4 , 40 ,
- Curt A. McCartney ORCID: orcid.org/0000-0002-9482-3133 9 ,
- Manuel Spannagl ORCID: orcid.org/0000-0003-0701-7035 5 ,
- Thomas Wicker ORCID: orcid.org/0000-0002-6777-7135 8 &
- Curtis J. Pozniak ORCID: orcid.org/0000-0002-7536-3856 1
Nature volume 588 , pages 277–283 ( 2020 ) Cite this article
71k Accesses
427 Citations
497 Altmetric
Metrics details
- Comparative genomics
- Plant breeding
Structural variation
Advances in genomics have expedited the improvement of several agriculturally important crops but similar efforts in wheat ( Triticum spp.) have been more challenging. This is largely owing to the size and complexity of the wheat genome 1 , and the lack of genome-assembly data for multiple wheat lines 2 , 3 . Here we generated ten chromosome pseudomolecule and five scaffold assemblies of hexaploid wheat to explore the genomic diversity among wheat lines from global breeding programs. Comparative analysis revealed extensive structural rearrangements, introgressions from wild relatives and differences in gene content resulting from complex breeding histories aimed at improving adaptation to diverse environments, grain yield and quality, and resistance to stresses 4 , 5 . We provide examples outlining the utility of these genomes, including a detailed multi-genome-derived nucleotide-binding leucine-rich repeat protein repertoire involved in disease resistance and the characterization of Sm1 6 , a gene associated with insect resistance. These genome assemblies will provide a basis for functional gene discovery and breeding to deliver the next generation of modern wheat cultivars.
Similar content being viewed by others
Integration of genetic and genomics resources in einkorn wheat enables precision mapping of important traits
Gautam Saripalli, Laxman Adhikari, … Vijay Tiwari

Long-read genome sequencing of bread wheat facilitates disease resistance gene cloning
Naveenkumar Athiyannan, Michael Abrouk, … Simon G. Krattinger

Improved pearl millet genomes representing the global heterotic pool offer a framework for molecular breeding applications
Punna Ramu, Rakesh K. Srivastava, … Raman Babu
Wheat is a staple food across all parts of the world and is one of the most widely grown and consumed crops 7 . As the human population continues to grow, wheat production must increase by more than 50% over current levels by 2050 to meet demand 7 . Efforts to increase wheat production may be aided by comprehensive genomic resources from global breeding programs to identify within-species allelic diversity and determine the best allele combinations to produce superior cultivars 2 , 8 .
Two species dominate current global wheat production: allotetraploid (AABB) durum wheat ( Triticum turgidum ssp. durum ), which is used to make couscous and pasta 9 , and allohexaploid (AABBDD) bread wheat ( Triticum aestivum ), used for making bread and noodles. A, B and D in these designations correspond to separate subgenomes derived from three ancestral diploid species with similar but distinct genome structure and gene content that diverged between 2.5 and 6 million years ago 10 . The large genome size (16 Gb for bread wheat), high sequence similarity between subgenomes and abundance of repetitive elements (about 85% of the genome) hampered early wheat genome-assembly efforts 3 . However, chromosome-level assemblies have recently become available for both tetraploid 11 , 12 and hexaploid wheat 1 , 13 . Although these genome assemblies are valuable resources, they do not fully capture within-species genomic variation that can be used for crop improvement, and comparative genome data from multiple individuals is still needed to expedite bread wheat research and breeding. Until now, comparative genomics of multiple bread wheat lines have been limited to exome-capture sequencing 4 , 5 , 14 , low-coverage sequencing 2 and whole-genome scaffolded assemblies 13 , 15 , 16 , 17 . Here we report multiple reference-quality genome assemblies and explore genome variation that, owing to past breeder selection, differs greatly between bread wheat lines. These genome assemblies usher a new era for bread wheat and equip researchers and breeders with the tools needed to improve bread wheat and meet future food demands.
Global variation in wheat genomes
To expand on the genome assembly of wheat for Chinese Spring 1 , we generated ten reference-quality pseudomolecule assemblies (RQAs) and five scaffold-level assemblies of hexaploid wheat (Supplementary Note 1 , Supplementary Tables 1 – 3 ). For each RQA, we performed de novo assembly of contigs (contig N50 > 48 kb) that were combined into scaffolds (N50 > 10 Mb) spanning more than 14.2 Gb (Supplementary Note 1 ). The completeness of the genomes was supported by a universal single-copy orthologue (BUSCO) analysis that identified more than 97% of the expected gene content in each genome (Supplementary Note 1 ). More than 94% of the scaffolds were ordered, oriented and curated using 10X Genomics linked reads and three-dimensional chromosome conformation capture sequencing (Hi-C) to generate 21 pseudomolecules, as done previously for wheat 1 , 12 and barley ( Hordeum vulgare ) 18 . The size and structure of the genomes were similar to that of Chinese Spring, and we observed high collinearity between the pseudomolecules (Extended Data Fig. 1 ). We also independently validated the scaffold placement and orientation in the pseudomolecule assembly of CDC Landmark by Oxford Nanopore long-read sequencing (Extended Data Fig. 2a , Supplementary Note 2 ). To complement the RQAs, we generated scaffold-level assemblies of five additional bread wheat lines (Supplementary Note 1 ). To determine the global context of the 15 assemblies, we combined our data with existing datasets 4 , 5 , 19 (Fig. 1a , Supplementary Table 4 ). The genetic relationships were in agreement with those reported in previous studies 4 , 5 and reflected pedigree, geographical location and growth habit (that is, spring versus winter type). There was also a clear separation between the newly assembled genomes and Chinese Spring, supporting that they capture geographical and historical variation not represented in the Chinese Spring assembly.
a , Principal component analysis of polymorphisms from exome-capture sequencing of about 1,200 lines (grey markers), 16 lines from whole-genome shotgun resequencing (orange markers) and our new assemblies (black markers). Text colours reflect different geographical locations and winter or spring growth. b , Dendrogram of pairwise Jaccard similarities for gene PAV between all RQA assemblies. c , Number of unique NLRs at different per cent identity cut-offs as the number of genomes increases. Dashed vertical lines represent 90% of the NLR complement. Markers indicate the mean values of all permutations of the order of adding genomes. Whiskers show maximum and minimum values based on one million random permutations. d , Chromosomal location versus insertion age distribution of unique to (reading downward) increasingly shared syntenic full-length LTR retrotransposons.
Polyploidy and CNV drive gene diversification
Single-nucleotide polymorphisms (SNPs), insertions or deletions (indels), presence/absence variation (PAV) and gene copy number variation (CNV) influence agronomically important traits. This is particularly true for polyploid species such as wheat, in which gene redundancy can buffer the effect of genome variation 17 . To assess gene content, we projected around 107,000 high-confidence gene models from Chinese Spring 1 onto the RQAs (Supplementary Note 3 ). The total number of projected genes exhibited a narrow range, between 118,734 and 120,967 (Supplementary Table 5 ). We identified orthologous groups among projected genes and used the alignment of the orthologous groups to examine SNPs in coding sequences (Supplementary Note 3 ). The peak positions of nucleotide diversity across the three subgenomes were highly similar to those reported in previous studies 20 , supporting a strong representation of breeding diversity within the RQAs (Extended Data Fig. 3a, b ). The correlation of synonymous nucleotide diversity π ( r = 0.11–0.29) and Tajima’s D ( r = 0.02–0.06) between homeologues was low (Supplementary Tables 6 – 8 ). This suggested that polyploidization increased the number of targets of selection and contributed to broad adaptation of bread wheat, as in wild polyploid plant species 20 , 21 , 22 . Further investigation of orthologous groups indicated that 88.1% were unambiguous (clusters containing at most one member in each cultivar) (Extended Data Fig. 3c , Supplementary Table 5 ). Orthologous groups comprising exactly one gene in each line (‘complete’) were the most frequent (approximately 73.5% of genes per cultivar), suggesting strong retention of orthologous genes within the ten RQAs. The residual genes represented either singleton genes with no reciprocal best BLAST hits or genes located in complex clusters in at least one cultivar. Roughly 12% of genes showed PAVs, and their clustering resulted in relationships (Fig. 1b ) that were consistent with SNP-based phylogenetic similarities (Fig. 1a ). In addition, approximately 26% of the projected genes were found in tandem duplications, indicating that CNV is a strong contributor of genetic variation in wheat.
To provide an example of gene expansion on emerging breeding targets, we performed a more detailed analysis of the restorer of fertility ( Rf ) gene families (Supplementary Note 4 ). Rf genes are involved in restoring pollen fertility in hybrid breeding programs 23 , and we identified a previously undescribed clade within the mitochondrial transcription termination factor (mTERF) family (Supplementary Table 9 ), which has recently been implicated in fertility restoration in barley 24 . Of note, this clade shows evolutionary patterns similar to those of Rf- like pentatricopeptide repeat (PPR) proteins, representatives of which are associated with Rf3 , a major locus used in hybrid wheat breeding programs (Extended Data Fig. 4 ). Although wheat is currently not a hybrid crop, there is substantial interest in Rf genes and their potential application in hybrid wheat production systems 25 . To our knowledge, no Rf genes have been cloned in wheat and our analysis of Rf genes in multiple RQAs and identification of an Rf clade in wheat is an important step forward in tackling the challenges of hybrid wheat breeding.
The wheat NLR repertoire
To further exemplify the use of multi-genome comparisons for characterizing agronomically relevant gene families, we examined gene expansion in nucleotide-binding leucine-rich repeat (NLR) proteins, which are major components of the innate immune system and are often causal genes for disease resistance in plants 26 , 27 . We performed de novo annotation of loci that contain conserved NLR motifs (NB-ARC–leucine-rich repeat) and identified around 2,500 loci with NLR signatures in each RQA (Supplementary Tables 10 , 11 ). A redundancy analysis showed that only 31–34% of the NLR signatures are shared across all genomes, and the number of unique signatures ranged from 22 to 192 per wheat cultivar. We estimated the number of unique NLR signatures that can be detected by incrementally adding more wheat genomes to the dataset; this revealed that 90% of the NLR complement is reached at between 8 (considering 95% sequence identity) and 11 wheat lines (considering 100% protein sequence identity) (Fig. 1c ). The total NLR complement of all wheat lines consisted of 5,905 (98% identity) to 7,780 (100% identity) unique NLR signatures, highlighting the size and complexity of the repertoire of receptors involved in disease resistance.
Transposon signatures identify introgressions
Transposable elements make up a large majority of the wheat genome and have a critical role in genome structure and gene regulation. We characterized the overall transposable element content (81.6%) and its composition (69% long terminal-repeat retrotransposons (LTR) and 12.5% DNA transposons) in the RQAs (Supplementary Table 5 ). Across all RQAs, we annotated 1.22 × 10 6 full length (fl)-LTRs, which clustered lines into the same groups we observed from our analysis of PAV and SNPs (Fig. 1a, b , Extended Data Fig. 3d ). Generally, unique fl-LTRs (147,450) were young (median of 0.9 million years) and were enriched in the highly recombining, more distal chromosomal regions (Fig. 1d ). By contrast, shared fl-LTRs were older (median of 1.3 million years) and were more evenly distributed across the pericentric regions (Fig. 1d ). The RLC- Angela fl-LTRs were the most abundant (21,000–27,000 full-length copies per genome) and analysis of variant patterns identified several chromosomal segments that contained numerous unique or rare retrotransposon insertions (Extended Data Fig. 5 ), which, on the basis of breeding history, we hypothesize to represent introgressions. For example, the LongReach Lancer RQA revealed two unique regions, a pericentric region on chromosome 2B and a segment on the end of chromosome 3D (Fig. 2a, b ), both of which affect chromosome length (Extended Data Fig. 5 ). We used pedigree analysis to postulate the source of the introgressions and performed whole-genome sequencing of multiple accessions of putative donors. LongReach Lancer carries the stem rust resistance gene Sr36 , derived from an introgression from Triticum timopheevii , and the resistance genes Lr24 (leaf rust) and Sr24 (stem rust), derived from tall wheatgrass 28 , 29 ( Thinopyrum ponticum ). We generated whole-genome sequence reads from multiple T. ponticum and T. timopheevii accessions (Supplementary Table 12 ) and alignment to the LongReach Lancer RQA confirmed a T. ponticum introgression spanning a region of approximately 60 Mb of chromosome 3D (Fig. 2a ), whereas T. timopheevii aligned to the majority (427 Mb) of chromosome 2B (Fig. 2b ). Overall, we identified 341 chromosomal segments larger than 20 Mb with unique or rare fl-LTR insertion patterns that were present in only 1 to 4 of the RQA genomes, of which 273 insertion patterns were uniquely associated with a single genome (Supplementary Tables 13 – 16 ). The majority of unique regions were in PI190962 (spelt wheat; Triticum aestivum ssp. spelta ), which was expected, given that it diverged from modern bread wheat several thousand years ago.
a – c , T. ponticum introgression on chromosome 3D in LongReach Lancer ( a ), T. timopheevi introgression on chromosome 2B in LongReach Lancer ( b ) and A. ventricosa introgression on chromosome 3D in Jagger ( c ). Track i, map of polymorphic RLC- Angela retrotransposon insertions (legend at bottom); track ii, density of projected gene annotations from Chinese Spring (blue bars, scaled to maximum value); track iii, per cent identity to Chinese Spring based on chromosome alignment (yellow; scale is 0–100%); track iv, read depth of wheat wild relatives (blue–yellow heat map; legend at bottom). d , Dot plot alignment showing chromosome-level collinearity (black) with relative density of CENH3 ChIP–seq mapped to 100-kb bins for Chinese Spring (blue) and Julius (red); the arrow indicates a centromere shift. e , Robertsonian translocation between chromosomes 5B and 7B in Arina LrFor . f , g , Cytology ( f ) and Hi-C ( g ) confirm the 5B/7B translocation in SY Mattis (left) compared with the non-carrier Norin 61 (right). In f , five independent cells were observed; the translocation was confirmed independently ten times. Scale bar, 10 μm.
A similar strategy was used to confirm RLC- Angela variation at the telomeric region of chromosome 2A in Jagger, Mace, SY Mattis and CDC Stanley (Fig. 2c ), which corresponds to the 2NvS introgression from Aegilops ventricosa (Supplementary Note 5 ). This introgression is a well-known source of resistance to wheat blast 30 , and contains the Lr37–Yr17–Sr38 gene cluster, which provides resistance to several rust diseases 31 . Sequencing of A. ventricosa accessions (Supplementary Table 12 ) followed by comparison of chromosomes with the RQAs confirmed that Jagger, Mace, SY Mattis and CDC Stanley carry the 2NvS introgression, which spans about 33 Mb on chromosome 2A (Fig. 2c , Extended Data Fig. 6a ). We annotated the coding genes within this region and identified 535 high-confidence genes; more than 10% were predicted to be associated with disease resistance, including genes that encode putative NB-ARC and NLRs (Extended Data Fig. 6b , Supplementary Tables 17 , 18 ). Furthermore, we used genotyping by sequencing to detect the 2NvS segment in three wheat panels and discovered that its frequency has been increasing in breeding germplasm and its presence is consistently associated with higher grain yield (Extended Data Fig. 6c, d , Supplementary Tables 19 , 20 ). Of note, we identified about 60 genes belonging to the cytochrome P450 superfamily, which have been implicated in abiotic and biotic stress tolerance 32 and have been functionally validated to influence grain yield in wheat 33 . Together, these data indicate that the modern wheat gene pool contains many chromosomal segments of diverse ancestral origins, which can be identified by their transposable-element signatures. We also confirmed the wild-relative origins of three introgressions within the RQA assemblies—a first step towards characterizing causal genes for breeding targets, such as resistance to wheat blast and rust fungi.
Centromere dynamics
Centromeres are vital for cell division and chromosome pairing during meiosis. In plants, functional centromeres are defined by the epigenetic placement of the modified histone CENH3 34 . We therefore used CENH3 chromatin immunoprecipitation and sequencing (ChIP–seq) 35 to determine the positions and sizes (about 7.5–9.6 Mb) of the centromeres for each RQA (Supplementary Tables 21 , 22 ), which were consistent with previous estimates for wheat 1 . Furthermore, all chromosomes showed a single active site, implying that previous reports of multiple active centromeres in Chinese Spring 1 were artefacts of misoriented scaffolds. However, we found examples in which the relative position of the centromere was shifted owing to several pericentric inversions, including inversions on chromosomes 4B and 5B (Extended Data Fig. 7a, b ). We also observed one instance in which the centromeric position changed, but was not associated with a structural event. Specifically, on chromosome 4D in Chinese Spring, the centromere is shifted by around 25 Mb relative to the consensus position (Fig. 2d ). This shift was previously recognized by cytology but was hypothesized to result from a pericentric inversion 36 . However, the high degree of collinearity between genomes supports the hypothesis that Cen4D in Chinese Spring has shifted to a non-homologous position; this shifting of centromeres to non-homologous sites has also been reported in maize 37 . By characterizing the centromere positions for these diverse wheat lines, we provide strong evidence for changes in centromere position caused by structural rearrangements and centromere shifts.
Large-scale structural variation between genomes
Structural variants are common in wheat 38 , and impact genome structure and gene content. We characterized large structural variants using pairwise genome alignments (Extended Data Fig. 1 ), changes in three-dimensional topology of chromosomes revealed by Hi-C conformation capture directionality biases along the genome 39 , 40 (Extended Data Fig. 8 , Supplementary Table 23 ), which were confirmed by Oxford Nanopore long-read sequencing (Extended Data Fig. 2 ) and cytological karyotyping (Extended Data Fig. 7c , Supplementary Table 24 , Supplementary Note 6 ). The most prominent event was a translocation between chromosomes 5B and 7B, observed in Arina LrFor , SY Mattis (Fig. 2e–g ) and Claire. Normally, chromosomes 5B and 7B are approximately 737 and 762 Mb long, respectively, and we estimated that the recombined chromosomes are 488 Mb (5BS/7BS) and 993 Mb (7BL/5BL) long, making 7BL/5BL the largest wheat chromosome (Extended Data Fig. 9a ). In Arina LrFor and SY Mattis, the 7BL/5BL breakpoint resides within an approximately 5-kb GAA microsatellite, which we were able to span using polymerase chain reaction (PCR) (Extended Data Fig. 9b, c ). By contrast, the breakpoint on 5BS/7BS was less syntenic, and we detected polymorphic fluorescence in situ hybridization signals between Arina LrFor and SY Mattis on the 5BS portion of the translocated chromosome segment, suggesting that the regions adjacent to the translocation events differ on 5BS/7BS (Supplementary Note 6 ). To determine the stability of the translocation in breeding, we genotyped for the translocation event in a panel of 538 wheat lines that represent most of the UK wheat gene pool grown since the 1920s 41 . The translocation occurred in 66% of the lines and was selectively neutral (Supplementary Note 7 ). Notably, the Ph1 locus on chromosome 5B, which controls the pairing of homeologous chromosomes during meiosis 42 , is near the translocation breakpoint, but remained highly syntenic between translocation carriers and non-carriers. Genetic mapping and analysis of short-read sequencing data indicated that the 5B/7B translocated chromosomes recombine freely with 5B and 7B chromosomes (Extended Data Fig. 9d ), suggesting that chromosome pairing is not affected by the translocation.
Haplotype-based gene mapping
To develop improved wheat cultivars, breeders shuffle allelic variants by making targeted crosses and exploiting the recombination that occurs during meiosis. These alleles, however, are not inherited independently, but rather as haplotype blocks that often extend across multiple genes that are in genetic linkage 43 , 44 . We quantified haplotype variation along chromosomes across the assemblies, and developed visualization software to support its utility (Supplementary Note 8 ). We used these haplotypes to characterize a locus that provides resistance to the orange wheat blossom midge (OWBM, Sitodiplosis mosellana Géhin), one of the most damaging insect pests of wheat, which is endemic in Europe, North America, west Asia and the Far East. Upon hatching, the first-instar larvae feed on the developing grains and damage the kernels (Fig. 3a ). Sm1 is the only gene in wheat known to provide resistance to OWBM 6 . CDC Landmark, Robigus and Paragon are all resistant to the OWBM, and all three carry the same 7.3-Mb haplotype within the Sm1 locus on chromosome 2B (Fig. 3b ). To identify Sm1 gene candidates, we used high-resolution genetic mapping and refined the locus to a 587-kb interval in the CDC Landmark RQA (Fig. 3c , Extended Data Fig. 10a , Supplementary Table 25 ). Through extensive genotyping of diverse breeding lines, we found an OWBM-susceptible line, Waskada, that displayed a resistant haplotype except near one gene, which we annotated in CDC Landmark to encode a canonical NLR with kinase and major sperm protein (MSP) integrated domains (Fig. 3c ). Oxford Nanopore long-read sequencing further confirmed the structure of the gene in CDC Landmark (Extended Data Fig. 10b ). By contrast, the remaining assemblies (susceptible to OWBM) lacked the NB-ARC domain, but the kinase and MSP domains remained intact (Fig. 3c ). We sequenced the Waskada allele and found it contains the NB-ARC domain, but an alternative haplotype within the kinase domain (Fig. 3c , Extended Data Fig. 10c ). This gene is expressed in wheat kernels and seedlings of Sm1 carrier lines, and the lack of cDNA amplification of the NB-ARC domain for non-carrier lines further supported an alternative gene structure (Extended Data Fig. 10c ). We generated two knockout-mutant lines of this candidate gene in the Sm1 carrier line Unity 45 , and both were consistently rated as susceptible to OWBM (Supplementary Table 26 ). Sequencing of the candidate gene in these two mutants revealed a single point mutation in each line: a G>A mutation resulting in a Gly>Arg (G182R) amino acid substitution in the NB-ARC domain, and a G>A mutation, resulting in a stop codon (W98*) before the NB-ARC domain (Fig. 3c ). The kinase domain encoded by Sm1 belongs to the serine/threonine class 46 , similar to those of Rpg5 , which provides stem rust resistance 47 , and Tsn1 , which encodes sensitivity to the necrotrophic effector ToxA produced by Parastagonospora nodorum and Pyrenophora tritici-repentis 48 ; however, both Rpg5 and Tsn1 lack the MSP domain. To our knowledge, this is the first report of an NB-ARC-LRR-kinase-MSP coding gene associated with insect resistance. Additional research is needed to functionally validate these domains and their putative role in OWBM resistance using tools such as gene editing. Nevertheless, we developed a high-throughput and low-cost competitive allele-specific PCR marker (KASP) that discriminates between OWBM-susceptible and OWBM-resistant lines with perfect accuracy (Extended Data Fig. 10d , Supplementary Table 27 ). Our analyses, along with the haplotype and synteny viewers ( https://kiranbandi.github.io/10wheatgenomes/ , http://10wheatgenomes.plantinformatics.io/ and http://www.crop-haplotypes.com/ ), laid the foundation for identifying haplotypes for Sm1 . Haplotypes can now be genotyped in breeding programs using single-marker or high-throughput-sequencing-based approaches, which can integrate desirable genes into improved cultivars more efficiently.
a , The orange wheat blossom midge oviposits eggs on wheat spikes and the larvae feed on developing wheat grains, resulting in moderate to severe damage to mature kernels. b , Top, sections of chromosome 2B of the same colour in the same position share haplotypes (based on 5-Mb bins), with the exception of those in grey, which indicates a line-specific haplotype. The position of Sm1 is indicated with respect to the CDC Landmark assembly. Bottom, zoomed-in view of haplotype blocks (based on 250-kb bins) from 5 to 25 Mb positions on chromosome 2B, surrounding Sm1 . CDC Landmark, Robigus and Paragon all carry the same haplotype surrounding Sm1 (teal). c , Top, anchoring of the Sm1 fine map to the physical maps of Chinese Spring and CDC Landmark and graphical genotypes of three haplotypes critical to localizing the Sm1 candidate gene. Bottom, annotation of the Sm1 candidate gene, which encodes NB-ARC and LRR motifs in addition to the integrated serine/threonine (S/T) kinase and MSP domains. Two independent ethyl-methanesulfonate-induced mutations (W98* and G182R) result in loss of function and susceptibility to the orange wheat blossom midge (light blue lines). An alternative haplotype was observed in the kinase region of Waskada (black).
We have built on the genome-sequence resources available for wheat and related species to produce ten RQAs and five scaffolded assemblies that represent hexaploid wheat lines from different regions, growth habits and breeding programs 1 , 11 , 12 , 18 , 20 , 49 . We have identified and characterized SNPs, PAV, CNV, centromere shifts, large-scale structural variants and introgressions from wild relatives of wheat that can be used to identify and characterize important breeding targets. This was complemented by a transposable-element-analysis approach to identify candidate introgressions from wild relatives of wheat, for which we provided high-quality assemblies of segments already used in global breeding programs. Together, these RQAs present an opportunity for breeders and researchers to perform high-resolution manipulation of genomic segments and pave the way to identifying genes responsible for in-demand traits, as we demonstrated for resistance to the insect pest OWBM. Functional gene studies will also be facilitated by comparative gene analyses, as exemplified by our analyses of orthologous groups, Rf genes and NLR immune receptors 26 . Finally, we highlight haplotype blocks, which will facilitate marker development for applied breeding 43 , 50 . Equipped with multiple layers of data describing variation in wheat, we now have powerful tools to increase the rate of wheat improvement to meet future food demands.
No statistical methods were used to predetermine sample size. The field experiments were randomized, but the wheat lines sequenced and assembled were not selected at random. The investigators were not blinded to allocation during experiments and outcome assessment.
Assemblies and annotation
Genome assemblies.
We assembled the genomes of 15 diverse wheat lines using two approaches (Supplementary Table 1 ). The RQA approach used the DeNovoMAGIC v.3.0 assembly pipeline, previously used for the wild emmer wheat 11 , durum wheat 12 and Chinese Spring RefSeqv1.0 assemblies. In brief, high-molecular-weight DNA was extracted from wheat seedlings as described previously 51 . Illumina 450-bp paired-end (PE), 800-bp PE and mate-pair (MP) libraries of three different sizes (3 kb, 6 kb and 9 kb) were generated. Sequencing was performed at the University of Illinois Roy J. Carver Biotechnology Center. 10X Genomics Chromium libraries were prepared and sequenced at the Genome Canada Genome Innovation Centre using the manufacturers’ recommendations to achieve a minimum of 30 × coverage. Hi-C libraries were prepared using previously described methods 40 . Using the Illumina PE, MP, 10X Genomics Chromium, and Hi-C, chromosome scale assemblies were prepared as described previously 18 . For cultivars assembled to a scaffold level, we used the W2RAP-contigger using k = 200 (Supplementary Note 1 ). Two MP libraries (10 kb and 13 kb) were produced for each line except Weebill 1, for which two additional MP libraries were used. Mate pairs were processed, filtered and used to scaffold contigs as described in the W2RAP pipeline ( https://github.com/bioinfologics/w2rap ). Scaffolds of less than 500 bp were removed from the final assemblies. Additionally, we performed Oxford Nanopore sequencing of CDC Landmark using R9 flow cells and the GridION sequencing technology (Supplementary Note 2 ).
Nucleotide diversity analysis
The variant call format data files from two wheat exome-capture studies 4 , 5 were retrieved, combined, and filtered to retain hexaploid accessions and polymorphisms detected in both studies. The 10X Genomics Chromium sequencing data for each of the RQA lines were aligned to Chinese Spring RefSeqv1.0 using the LongRanger v.2.1.6 software. Alignment files from the accessions assembled here and 16 Bioplatforms Australia lines 19 with alignments obtained from the DAWN project 52 were then used for variant calling by GATK v.3.8 at the same genomic positions identified by exome-capture sequencing. The variant files from the exome-capture studies, DAWN project and 10+ Wheat Genomes lines were then merged and subjected to principal component analysis (PCA) using the prcomp function in R v.3.6.1.
Gene projections
We used the previously published high-confidence gene models for Chinese Spring to assess the gene content in each assembly. Representative coding sequences of each informant locus were aligned to pseudomolecules of each line separately using BLAT 53 v.3.5 with the ‘fine’ parameter and a maximal intron size of 70 kb. BLAT matches seeded an additional alignment by exonerate 54 in the genomic neighbourhood encompassing 20 kb upstream and downstream of the match position. Exonerate alignments required a minimal and maximal intron sizes of 30 bp and 20 kb, respectively. A linear regression of colocalized matches with complete alignments of the informant were computed for 10,000 such pairs to derive a normalization function and to render comparable scoring schemes for both methods. Subsequently, we selected the top-scoring match for each mapping pair as the locus for the gene projection. Projections were then filtered by alignment coverage (Supplementary Note 3 ), the open reading frame (ORF) contiguity, the observed mapping frequency of the informant, coverage of start and stop codons, and the orthology or potential dislocation of the match scaffold relative to its informant chromosome. Identification of orthologous groups was analogous to the approach used previously 55 . Reciprocal best BLAST hit (RBH) graphs were derived from pairwise all-against-all BLASTn v2.8 transcript searches (minimal e -value ≤ 1 × 10 −30 ). Hits were assigned to homeologous groups on the basis of gene models of Chinese Spring following a previously described homeologue classification 9 . Multiple sequence alignments for the population genetics analysis were performed using MUSCLE v.3.8 with default parameters (Supplementary Note 3 ). Using the gene projections, we quantified average pairwise genetic diversity ( π ), polymorphism (Watterson’s θ W ), and Tajima’s D using compute and polydNdS in the libsequence v.1.0.3-1 package 56 . We retained diversity estimates for genes that were in all of the genomes and had ≤100 segregating sites. PAV was determined from the orthologous groups limited to one-to-one relations where there was no match in at least one genome.
Analysis of the Rf -like gene family
For Rf genes, the genome sequences were scanned for ORFs in six frame translations with the getorf program of the EMBOSS v.6.6.0 package. ORFs longer than 89 codons were searched for the presence of PPR motifs using hmmsearch from the HMMER v.3.2.1 package ( http://hmmer.org ) and the hidden Markov models defined previously. The PF02536 profile from the Pfam v32.0 database ( http://pfam.xfam.org ) was used to screen for ORFs carrying mTERF motifs. Downstream processing of the hmmsearch results followed the pipeline described previously 57 . ORFs with low hmmsearch scores were removed from the analysis as they are unlikely to represent functional PPR proteins. Only genes encoding mTERF proteins longer than 100 amino acids were included in the analysis. RFL -PPR sequences were identified as described 23 . The phylogenetic analyses were performed as described previously 23 . Conserved, non-PPR genes delimiting the borders of analysed RFL clusters were identified in the Chinese Spring RefSeqv1.0 reference genome and used to search for syntenic regions in the remaining wheat accessions with BLAST v.2.8. See Supplementary Note 4 for more details.
NLR repertoire
NLR signatures were annotated using NLR-Annotator 58 , 59 ( https://github.com/steuernb/NLR-Annotator ) with the option -a. We estimated redundancy of NLR signatures between genomes at different thresholds of identity: 95%, 98% and 100%. For the 165 amino acids in the consensus of all NB-ARC motifs, this translates to 8, 3 and 0 mismatches of a concatenated motif sequence. To calculate the overall redundancy in all genomes, we counted the number of LR signatures added to a non-redundant set by adding genomes iteratively. This was done for 1 million random permutations.
Repeat annotation
Transposons were detected and classified by a homology search against the REdat_9.7_Poaceae section of the PGSB transposon library 60 using vmatch ( http://www.vmatch.de ) with the following parameters: identity ≥70%, minimal hit length 75 bp, seedlength 12 bp (exact command line: -d -p -l 75 -identity 70 -seedlength 12 -exdrop 5). To remove overlapping annotations, the output was filtered for redundant hits via a priority-based approach in which higher-scoring matches where assigned first and lower-scoring hits at overlapping positions were either shortened or removed if there was ≥90% overlap with a priority hit or if <50 bp remained. Tandem repeats where identified with TandemRepeatFinder v.4.09 under default parameters 61 and subjected to overlap removal as described above. Full-length LTR retrotransposons were identified with LTRharvest ( http://genometools.org/documents/ltrharvest.pdf ). All candidates were subsequently annotated for PfamA domains using HMMER v.3.0 and filtered to remove false positives, non-canonical hybrids and gene-containing elements. The inner domain order served as a criterion for the LTR retrotransposon superfamily classification, either Gypsy (RLG: RT-RH-INT), Copia (RLC: INT-RT-RH) or undetermined (RLX). The insertion age of fl-LTRs was calculated from the divergence between the 5′ and 3′ long terminal repeats, which are identical upon insertion. The genetic distance was calculated with EMBOSS v.6.6.0 distmat (Kimura2-parameter correction) using a random mutation rate of 1.3 × 10 −8 .
Analysis of centromeric regions
For each line with a RQA, ChIP was performed according to previous methods 62 with slight modification using a wheat-specific CENH3 antibody 36 . An antigen with the peptide sequence RTKHPAVRKTKALPKK, corresponding to the N terminus of wheat CENH3, was used to produce an antibody using the custom-antibody production facility provided by Thermo Fisher Scientific. The customized antibody was purified and obtained as pellets. The antibody pellet (0.396 mg) was dissolved in 2 ml PBS buffer, pH 7.4, resulting in a working concentration of 198 ng μl −1 . Nuclei were isolated from 2-week-old seedlings, digested with micrococcal nuclease and incubated overnight at 4 °C with 3 μg of antibody or rabbit serum (control). Antibodies were captured using Dynabeads Protein G and the chromatin eluted using 100 μl of 1% sodium dodecyl sulfate, 0.1 M NaHCO 3 preheated to 65 °C. DNA isolation was then performed using ChIP DNA Clean & Concentrator Kit, and ChIP–seq libraries were constructed using TruSeq ChIP Library Preparation Kit and sequenced with a NovoSeq S4, which generated 150-bp paired-end reads.
For Chinese Spring, we used two datasets, SRR1686799 63 (dataset 1) and the dataset generated in this study (dataset 2). Sequence reads were de-multiplexed, trimmed and aligned to each of the respective RQAs using HISAT2 v.2.1.0 64 . Alignments were sorted, filtered for minimum alignment quality of 30, counted in 100-kb bins using samtools v.1.10 and BEDtools v.2.29, and visualized in R v.3.6.1. To define the midpoint of each centromere, we identified the highest density of CENH3 ChIP–seq reads using a smoothing spline in R v.3.6.1 with smooth.spline function (number of knots = 1,000) and identified the peak of the smooth spline as the centre of the respective centromere for a given chromosome. To compare centromeric positions of different genomes, the CENH3 ChIP–seq density was plotted along with MUMmer v.4.0 chromosome alignments. To determine the overall size of wheat centromeres, we considered each 100-kb bin with CENH3 ChIP–seq read density that was greater than three times the background (genome average) level of read density to be an active centromeric bin. The number of enriched bins for each genome were counted and averaged to a total of 21 chromosomes. This calculation included counting of unanchored bins.
Analysis of introgressions
Identification of full-length rlc- angela retrotransposons.
Retrotransposon profiles were created for each genome using the RLC- Angela family 65 and consensus sequences obtained from the TREP database ( www.botinst.uzh.ch/en/research/genetics/thomasWicker/trep-db.html ). First, BLASTn was used to compare the ~1,700-bp LTR of RLC- Angela to each genome. Matching elements and 500 bp of flanking sequences were aligned to identify precise LTR borders as well as different sub-families and/or sequences variants. We then used BLASTn to compare the 18 consensus LTR sequences against each genome and then screened for pairs of full-length LTRs that are found in the same orientation within a window of 7.5–9.5 kb (RLC- Angela elements are ~8.7 kb long). These initial candidate full-length elements were screened for the presence of RLC- Angela polyprotein sequences by BLASTx, as well as for the typical 5-bp target-site duplications. We allowed a maximum of two mismatches between the two target-site duplications. All identified full-length RLC- Angela copies were then aligned to a RLC- Angela consensus sequence with the program Water from the EMBOSS v.6.6.0 package ( www.ebi.ac.uk/Tools/emboss/ ). These alignments were used to compile all nucleotide polymorphisms into a single file. The variant call file was then used for PCA using the snpgdsPCA function in the R package SNPrelate v.3.11.
Sequencing of the tertiary gene pool of wheat
Genomic DNA (gDNA) was extracted and purified from young leaf tissue collected from multiple accessions of T. timopheevii , A. ventricosa and T. ponticum (Supplementary Table 12 ) following a standard CTAB–chloroform extraction method. Yield and integrity were evaluated by fluorometry (Qubit 2.0) and agarose gel electrophoresis. Paired-end libraries were prepared following the Nextera DNA Flex protocol. In brief, 500 ng gDNA from each accession was fragmented and amplified with a limited-cycle PCR. Each library was uniquely dual-indexed with a distinct 10-bp index code (IDT for Illumina Nextera DNA UD) for multiplexing, and quantified by qPCR (Kapa Biosystems). Final average library size was estimated on a Tapestation 2200. Libraries were normalized and pooled for sequencing on an Illumina NovaSeq 6000 S4 to generate ~5× coverage per genotype. Sequencing data were de-multiplexed and aligned to appropriate RQAs (Supplementary Table 12 ) in semi-perfect mode using the BBMap v.38 short-read alignment software ( https://sourceforge.net/projects/bbmap/ ).
We karyotyped the lines using mitotic metaphase chromosomes prepared by the conventional acetocarmine-squash method. Non-denaturing fluorescence in situ hybridization (ND-FISH) of three repetitive sequence probes, Oligo-pSc119.2-1, Oligo-pTa535 and Oligo-pTa713, was performed as described 66 , 67 (Supplementary Note 6 ). Chromosomes were counterstained with DAPI. Chromosome images were captured with an Olympus BX61 epifluorescence microscope and a CCD camera DP80. Images were processed and pseudocoloured with ImageJ v.1.51n in the Fiji package. For karyotyping, at least four chromosomes per accession were examined and compared to the karyotype of Chinese Spring as described previously 68 . Hierarchal clustering of karyotype polymorphisms was performed using the Ward method in R v.3.0.2, which was used to estimate distance. Next, we applied Hi-C analysis for inversion calling as described previously 40 . In brief, adapters were removed and reads were mapped to Chinese Spring using minimap2 v.2.10 69 as we have done previously 21 . The raw Hi-C link counts were calculated in 1 Mb non-overlapping sliding windows and then normalized as described in our previous work 40 . Finally, the normalized Hi-C link matrix was subjected to inversion calling using R.
We performed flow cytometry of wheat cultivars Arina and Forno as previously described 70 , except that we used a FACSAria SORP flow cytometer and cell sorter (Becton Dickinson). The 5B/7B translocation breakpoints were identified by comparison of chromosomes 5B and 7B from Arina LrFor and Julius. Sequence collinearity between Arina LrFor and Julius was detected by BLASTn searches of 1,000-bp sequence windows every 100 kb along the chromosomes. Once an interruption of synteny was detected, sequence segments at the positions of synteny loss were extracted and used for local alignments to determine the precise breakpoint positions. PCR amplification of the 5BS/7BS and 7BL/5BL translocation sites was performed using standard PCR cycling conditions.
Characterization of haplotypes
Development of a wheat genome haplotype database.
To identify haplotypes, pairwise chromosome alignments were performed between the RQA using MUMmer v.4.0, which were combined with pairwise nucleotide BLASTn analyses of the genes ± 2,000 bp using custom scripts in R v.3.6.1 ( https://github.com/Uauy-Lab/pangenome-haplotypes ) 71 (Supplementary Note 8). The resultant haplotypes were uploaded to an interactive viewer ( http://www.crop-haplotypes.com/ ). Pairwise BLASTn comparisons of the genes were also used to identify structural variants, and were uploaded into AccuSyn ( https://accusyn.usask.ca/ ) and SynVisio ( https://synvisio.github.io/#/ ) to create a wheat-specific database ( https://kiranbandi.github.io/10wheatgenomes/ ). Pretzel ( https://github.com/plantinformatics/pretzel ) was also used to visualize and compare the RQA and the projected gene annotations ( http://10wheatgenomes.plantinformatics.io/ ).
Characterization of Sm1
Sm1 -linked markers 6 were located in RQAs using BLAST v.2.8.0. Two high-resolution mapping populations were developed, 99B60-EJ2D/Thatcher and 99B60-EJ2G/Infinity. Progeny heterozygous for crossover events near Sm1 were identified in the F 2 generation, and the crossovers were fixed in the F 3 generation. The resulting F 2 -derived F 3 families were analysed with KASP markers within the Sm1 region and tested for resistance to OWBM in field nurseries to identify markers associated with Sm1 . Ethyl methanesulfonate was used to develop knockout mutants in the Sm1 gene. Approximately 3,200 seeds of the Canadian spring wheat variety Unity (an Sm1 carrier) were soaked in a 0.2% (v/v) aqueous ethyl methanesulfonate solution for 22 h at 22 °C. The seed was then rinsed in distilled water and sown in a field nursery. The M 1 seed was grown to maturity and bulk harvested. Approximately 6,000 M 2 seeds were space planted in two field nurseries located in Brandon and Glenlea, Manitoba, Canada. Spikes were collected on a per-plant basis at maturity and were classified as resistant, susceptible or undamaged as done previously 6 , 72 . Putative Sm1 -knockout mutants were re-tested for OWBM resistance in indoor cage tests 73 in the M 3 and M 4 generations. M 4 -derived families were tested for resistance to OWBM in field nurseries (randomized complete block design, six environments, and eight replicates per environment).
Candidate genes were identified between Sm1 flanking markers on the CDC Landmark assembly using the projected gene annotations and FGENESH v.2.6 ( http://www.softberry.com/ ), which were compared to the projected genes of non-carriers. Both 5′ and 3′ rapid amplification of cDNA ends (5′ and 3′ RACE) were used to verify the transcription initiation and termination sites of the gene candidate, whose structure was predicted by FGENESH v.2.6. In brief, RNA was extracted from the leaves of Unity ( Sm1 carrier) seedlings (using the Qiagen RNeasy kit), RACE PCR performed (Invitrogen GeneRacer kit), and the PCR product cloned (Invitrogen TOPO TA Cloning kit for sequencing) and sequenced by Sanger sequencing. Prediction of the conserved domains was done using the NCBI Conserved Domain Search tool ( https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi ) and PROSITE (release 2020_01; https://prosite.expasy.org/ ). The LRR domain was defined on the basis of the presence of 2–42 LRR motif repeats of 20–30 amino acids each. LRR motifs were manually annotated 74 . Prediction of transmembrane regions and orientation was performed using the program TMpred NCBI Conserved Domain Search tool ( https://embnet.vital-it.ch/software/TMPRED_form.html ).
To study the expression of Sm1 , total RNA was extracted from four biological replicates from four wheat genotypes (Unity, CDC Landmark, Waskada and Thatcher) from two different tissues; seedling leaves and developing kernels (five days post anthesis) using NucleoSpin RNA Plant kit (Macherey-Nagel) according to the manufacturer’s instructions. RNA was treated with RNase-free DNase (rDNase) (Macherey-Nagel) and reversed transcribed into cDNA using SuperScript IV Reverse Transcriptase kit (Invitrogen) according to the manufacturer’s instructions and the NB-ARC domain amplified by PCR.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this paper.
Data availability
All sequence reads assemblies have been deposited into the National Center for Biotechnology Information sequence read archive (SRA) (see Supplementary Table 1 for accession numbers). Sequence reads for the RQAs, T. ponticum , A. ventricosa and T. timopheevii have been deposited into the SRA (accession no. PRJNA544491 ) and ChIP–seq short read-data used for centromere characterization is deposited under accession no. PRJNA625537 . All Hi-C data have been deposited in the European Nucleotide Archive (Supplementary Table 1). The RQAs are available for direct user download at https://wheat.ipk-gatersleben.de/ . All assemblies and projected annotations are available for comparative analysis at Ensembl Plants ( https://plants.ensembl.org/index.html ). Comparative analysis viewers are also online for synteny ( https://kiranbandi.github.io/10wheatgenomes/ , http://10wheatgenomes.plantinformatics.io/ ) and haplotypes ( http://www.crop-haplotypes.com/ ). Seed stocks of the assembled lines are available at the UK Germplasm Resources Unit ( https://www.seedstor.ac.uk/ ).
Code availability
Code for custom genome visualizers have been deposited in the public domain for haplotype viewer ( https://github.com/Uauy-Lab/pangenome-haplotypes ), Pretzel ( https://github.com/plantinformatics/pretzel ), AccuSyn ( https://github.com/jorgenunezsiri/accusyn ) and SynVisio ( https://github.com/kiranbandi/synvisio ). Additional scripts used for ChIP–seq analysis of the centromeres are provided at https://github.com/wheatgenetics/centromere .
The International Wheat Genome Sequencing Consortium. Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science 361 , eaar7191 (2018).
Article CAS Google Scholar
Montenegro, J. D. et al. The pangenome of hexaploid bread wheat. Plant J . 90 , 1007–1013 (2017).
Article CAS PubMed Google Scholar
International Wheat Genome Sequencing Consortium (IWGSC). A chromosome-based draft sequence of the hexaploid bread wheat ( Triticum aestivum ) genome. Science 345 , 1251788 (2014).
He, F. et al. Exome sequencing highlights the role of wild-relative introgression in shaping the adaptive landscape of the wheat genome. Nat. Genet . 51 , 896–904 (2019); correction 51 , 1194 (2019).
Pont, C. et al. Tracing the ancestry of modern bread wheats. Nat. Genet. 51 , 905–911 (2019).
Kassa, M. T. et al. A saturated SNP linkage map for the orange wheat blossom midge resistance gene Sm1. Theor. Appl. Genet . 129 , 1507–1517 (2016).
Tadesse, W. et al. Genetic gains in wheat breeding and its role in feeding the world. Crop Breed. Genet. Genom . 1 , e190005 (2019).
Google Scholar
Zhao, Q. et al. Pan-genome analysis highlights the extent of genomic variation in cultivated and wild rice. Nat. Genet . 50 , 278–284 (2018).
Dubcovsky, J. & Dvorak, J. Genome plasticity a key factor in the success of polyploid wheat under domestication. Science 316 , 1862–1866 (2007).
Article CAS PubMed PubMed Central Google Scholar
Marcussen, T. et al. Ancient hybridizations among the ancestral genomes of bread wheat. Science 345 , 1250092 (2014).
Article PubMed CAS Google Scholar
Avni, R. et al. Wild emmer genome architecture and diversity elucidate wheat evolution and domestication. Science 357 , 93–97 (2017).
Article ADS CAS PubMed Google Scholar
Maccaferri, M. et al. Durum wheat genome highlights past domestication signatures and future improvement targets. Nat. Genet . 51 , 885–895 (2019).
Zimin, A. V. et al. The first near-complete assembly of the hexaploid bread wheat genome, Triticum aestivum . Gigascience 6 , 1–7 (2017).
ADS CAS PubMed PubMed Central Google Scholar
Winfield, M. O. et al. Targeted re-sequencing of the allohexaploid wheat exome. Plant Biotechnol. J . 10 , 733–742 (2012).
Arora, D., Gross, T. & Brueggeman, R. Allele characterization of genes required for rpg4-mediated wheat stem rust resistance identifies Rpg5 as the R gene. Phytopathology 103 , 1153–1161 (2013).
Adamski, N. M. et al. A roadmap for gene functional characterisation in crops with large genomes: lessons from polyploid wheat. eLife 9 , e55646 (2020).
Uauy, C. Wheat genomics comes of age. Curr. Opin. Plant Biol . 36 , 142–148 (2017).
Article PubMed Google Scholar
Mascher, M. et al. A chromosome conformation capture ordered sequence of the barley genome. Nature 544 , 427–433 (2017).
Edwards, D. et al. Bread matters: a national initiative to profile the genetic diversity of Australian wheat. Plant Biotechnol. J . 10 , 703–708 (2012).
Jordan, K. W. et al. A haplotype map of allohexaploid wheat reveals distinct patterns of selection on homoeologous genomes. Genome Biol . 16 , 48 (2015).
Article PubMed PubMed Central Google Scholar
Paape, T. et al. Patterns of polymorphism and selection in the subgenomes of the allopolyploid Arabidopsis kamchatica . Nat. Commun . 9 , 3909 (2018).
Article ADS PubMed PubMed Central CAS Google Scholar
Paape, T. et al. Conserved but attenuated parental gene expression in allopolyploids: Constitutive zinc hyperaccumulation in the allotetraploid Arabidopsis kamchatica . Mol. Biol. Evol . 33 , 2781–2800 (2016).
Melonek, J., Stone, J. D. & Small, I. Evolutionary plasticity of restorer-of-fertility-like proteins in rice. Sci. Rep . 6 , 35152 (2016).
Article ADS CAS PubMed PubMed Central Google Scholar
Bernhard, T., Koch, M., Snowdon, R. J., Friedt, W. & Wittkop, B. Undesired fertility restoration in msm1 barley associates with two mTERF genes. Theor. Appl. Genet . 132 , 1335–1350 (2019).
Whitford, R. et al. Hybrid breeding in wheat: technologies to improve hybrid wheat seed production. J. Exp. Bot . 64 , 5411–5428 (2013).
Keller, B., Wicker, T. & Krattinger, S. G. Advances in wheat and pathogen genomics: Implications for disease control. Annu. Rev. Phytopathol . 56 , 67–87 (2018).
Steuernagel, B. et al. Rapid cloning of disease-resistance genes in plants using mutagenesis and sequence capture. Nat. Biotechnol . 34 , 652–655 (2016).
Bariana, H. S. et al. Mapping of durable adult plant and seedling resistances to stripe rust and stem rust diseases in wheat. Aust. J. Agric. Res . 52 , 1247–1255 (2001).
Chemayek, B. et al. Tight repulsion linkage between Sr36 and Sr39 was revealed by genetic, cytogenetic and molecular analyses. Theor. Appl. Genet . 130 , 587–595 (2017).
Cruz, C. D. et al. The 2NS translocation from Aegilops ventricosa confers resistance to the Triticum pathotype of Magnaporthe oryzae . Crop Sci . 56 , 990–1000 (2016).
Helguera, M. et al. PCR assays for the Lr37-Yr17- Sr38 cluster of rust resistance genes and their use to develop isogenic hard red spring wheat lines. Crop Sci . 43 , 1839–1847 (2003).
Li, Y. & Wei, K. Comparative functional genomics analysis of cytochrome P450 gene superfamily in wheat and maize. BMC Plant Biol . 20 , 93 (2020).
Gunupuru, L. R. et al. A wheat cytochrome P450 enhances both resistance to deoxynivalenol and grain yield. PLoS ONE 13 , e0204992 (2018).
Article PubMed PubMed Central CAS Google Scholar
Li, B. et al. Wheat centromeric retrotransposons: the new ones take a major role in centromeric structure. Plant J . 73 , 952–965 (2013).
Gent, J. I., Wang, K., Jiang, J. & Dawe, R. K. Stable patterns of CENH3 occupancy through maize lineages containing genetically similar centromeres. Genetics 200 , 1105–1116 (2015).
Koo, D. H., Sehgal, S. K., Friebe, B. & Gill, B. S. Structure and stability of telocentric chromosomes in wheat. PLoS ONE 10 , e0137747 (2015).
Schneider, K. L., Xie, Z., Wolfgruber, T. K. & Presting, G. G. Inbreeding drives maize centromere evolution. Proc. Natl Acad. Sci. USA 113 , E987–E996 (2016).
Saxena, R. K., Edwards, D. & Varshney, R. K. Structural variations in plant genomes. Brief. Funct. Genomics 13 , 296–307 (2014).
Harewood, L. et al. Hi-C as a tool for precise detection and characterisation of chromosomal rearrangements and copy number variation in human tumours. Genome Biol . 18 , 125 (2017).
Himmelbach, A. et al. Discovery of multi-megabase polymorphic inversions by chromosome conformation capture sequencing in large-genome plant species. Plant J . 96 , 1309–1316 (2018).
Fradgley, N. et al. A large-scale pedigree resource of wheat reveals evidence for adaptation and selection by breeders. PLoS Biol . 17 , e3000071 (2019).
Martín, A. C., Rey, M. D., Shaw, P. & Moore, G. Dual effect of the wheat Ph1 locus on chromosome synapsis and crossover. Chromosoma 126 , 669–680 (2017).
Bevan, M. W. et al. Genomic innovation for crop improvement. Nature 543 , 346–354 (2017).
Luján Basile, S. M. et al. Haplotype block analysis of an Argentinean hexaploid wheat collection and GWAS for yield components and adaptation. BMC Plant Biol . 19 , 553 (2019).
Fox, S. L. et al. Unity hard red spring wheat. Can. J. Plant Sci . 90 , 71–78 (2010).
Article Google Scholar
Hanks, S. K., Quinn, A. M. & Hunter, T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science 241 , 42–52 (1988).
Brueggeman, R. et al. The stem rust resistance gene Rpg5 encodes a protein with nucleotide-binding-site, leucine-rich, and protein kinase domains. Proc. Natl Acad. Sci. USA 105 , 14970–14975 (2008).
Faris, J. D. et al. A unique wheat disease resistance-like gene governs effector-triggered susceptibility to necrotrophic pathogens. Proc. Natl Acad. Sci. USA 107 , 13544–13549 (2010).
Luo, M. C. et al. Genome sequence of the progenitor of the wheat D genome Aegilops tauschii . Nature 551 , 498–502 (2017).
Borrill, P., Harrington, S. A. & Uauy, C. Applying the latest advances in genomics and phenomics for trait discovery in polyploid wheat. Plant J . 97 , 56–72 (2019).
CAS PubMed Google Scholar
Dvorak, J., Mcguire, P. E. & Cassidy, B. Apparent sources of the A genomes of wheats inferred from polymorphism in abundance and restriction fragment length of repeated nucleotide-sequences. Genome 30 , 680–689 (1988).
Watson-Haigh, N. S., Suchecki, R., Kalashyan, E., Garcia, M. & Baumann, U. DAWN: a resource for yielding insights into the diversity among wheat genomes. BMC Genomics 19 , 941 (2018).
Kent, W. J. BLAT—the BLAST-like alignment tool. Genome Res . 12 , 656–664 (2002).
CAS PubMed PubMed Central Google Scholar
Slater, G. S. & Birney, E. Automated generation of heuristics for biological sequence comparison. BMC Bioinformatics 6 , 31 (2005).
Tatusov, R. L., Galperin, M. Y., Natale, D. A. & Koonin, E. V. The COG database: a tool for genome-scale analysis of protein functions and evolution. Nucleic Acids Res . 28 , 33–36 (2000).
Thornton, K. Libsequence: a C++ class library for evolutionary genetic analysis. Bioinformatics 19 , 2325–2327 (2003).
Cheng, S. et al. Redefining the structural motifs that determine RNA binding and RNA editing by pentatricopeptide repeat proteins in land plants. Plant J . 85 , 532–547 (2016).
Steuernagel, B. et al. Physical and transcriptional organisation of the bread wheat intracellular immune receptor repertoire. Preprint at https://doi.org/10.1101/339424 (2018).
Steuernagel, B. et al. The NLR-Annotator tool enables annotation of the intracellular immune receptor repertoire. Plant Physiol . 183 , 468–482 (2020).
Spannagl, M. et al. PGSB PlantsDB: updates to the database framework for comparative plant genome research. Nucleic Acids Res . 44 (D1), D1141–D1147 (2016).
Benson, G. Tandem repeats finder: a program to analyze DNA sequences. Nucleic Acids Res . 27 , 573–580 (1999).
Nagaki, K. et al. Chromatin immunoprecipitation reveals that the 180-bp satellite repeat is the key functional DNA element of Arabidopsis thaliana centromeres. Genetics 163 , 1221–1225 (2003).
Guo, X. et al. De novo centromere formation and centromeric sequence expansion in wheat and its wide hybrids. PLoS Genet . 12 , e1005997 (2016).
Kim, D., Paggi, J. M., Park, C., Bennett, C. & Salzberg, S. L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol . 37 , 907–915 (2019).
Wicker, T. et al. Impact of transposable elements on genome structure and evolution in bread wheat. Genome Biol . 19 , 103 (2018).
Tang, Z., Yang, Z. & Fu, S. Oligonucleotides replacing the roles of repetitive sequences pAs1, pSc119.2, pTa-535, pTa71, CCS1, and pAWRC.1 for FISH analysis. J. Appl. Genet . 55 , 313–318 (2014).
Zhao, L. et al. Cytological identification of an Aegilops variabilis chromosome carrying stripe rust resistance in wheat. Breed. Sci . 66 , 522–529 (2016).
Komuro, S., Endo, R., Shikata, K. & Kato, A. Genomic and chromosomal distribution patterns of various repeated DNA sequences in wheat revealed by a fluorescence in situ hybridization procedure. Genome 56 , 131–137 (2013).
Li, H. Minimap2: pairwise alignment for nucleotide sequences. Bioinformatics 34 , 3094–3100 (2018).
Kubaláková, M., Vrána, J., Cíhalíková, J., Simková, H. & Doležel, J. Flow karyotyping and chromosome sorting in bread wheat ( Triticum aestivum L.). Theor. Appl. Genet . 104 , 1362–1372 (2002).
Brinton, J. et al. A haplotype-led approach to increase the precision of wheat breeding. Commun. Biol. https://doi.org/10.1038/s42003-020-01413-2 (2020).
Thomas, J. et al. Chromosome location and markers of Sm1: a gene of wheat that conditions antibiotic resistance to orange wheat blossom midge. Mol. Breed . 15 , 183–192 (2005).
Lamb, R. J. et al. Resistance to Sitodiplosis mosellana (Diptera: Cecidomyiidae) in spring wheat (Gramineae). Can. Entomol . 132 , 591–605 (2000).
la Cour, T. et al. Analysis and prediction of leucine-rich nuclear export signals. Protein Eng. Des. Sel . 17 , 527–536 (2004).
Download references
Acknowledgements
We are grateful for funding from the Canadian Triticum Applied Genomics research project (CTAG2) funded by Genome Canada, Genome Prairie, the Western Grains Research Foundation, Government of Saskatchewan, Saskatchewan Wheat Development Commission, Alberta Wheat Commission, Viterra and Manitoba Wheat and Barley Growers Association. Funding was also provided by the Biotechnology and Biological Sciences Research Council (BBSRC) via the projects Designing Future Wheat (BB/P016855/1), sLOLA (BB/J003557/1) and MAGIC Pangenome (BB/P010741/1, BB/P010733/1 and BB/P010768/1), by AMED NBRP (JP17km0210142), the German Federal Ministry of Education and Research (FKZ 031B0190, WHEATSeq, 2819103915 and 2819104015), German Network for Bioinformatics and Infrastructure de.NBI (FKZ 031A536A, 031A536B), German Federal Ministry of Food and Agriculture (BMEL FKZ 2819103915 WHEATSEQ), Israel Science Foundation (Grant 1137/17), JST CREST (JPMJCR16O3), US National Science Foundation (1339389), Kansas Wheat Commission and Kansas State University, MEXT KAKENHI, The Birth of New Plant Species (JP16H06469, JP16H06464, JP16H06466 and JP16K21727), National Agriculture and Food Research Organization (NARO) Vice President Fund, Swiss Federal Office of Agriculture (NAP-PGREL), Agroscope, Delley Seeds and Plants, ETH Zurich Institute of Agricultural Sciences, Fenaco Co-operative, IP-SUISSE, swisssem, JOWA, SGPV-FSPC, Swiss National Science Foundation (31003A_182318 and CRSII5_183578), University of Zurich Research Priority Program Evolution in Action, King Abdullah University of Science and Technology, Grains Research and Development Corporation (GRDC), Australian Research Council (CE140100008) and Groupe Limagrain. We are grateful for the computational support of the Functional Genomics Center Zurich, the Molecular Plant Breeding Group—ETH Zurich, and the Global Institute of Food Security (GIFS), Saskatoon. We acknowledge the contribution of the Australian Wheat Pathogens Consortium ( https://data.bioplatforms.com/organization/edit/bpa-wheat-cultivars ) in the generation of data used in this publication. The Initiative is supported by funding from Bioplatforms Australia through the Australian Government National Collaborative Research Infrastructure Strategy (NCRIS). We thank S. Wu for DNA preparations for assembly and ChIP–seq library preparations; O. Francisco-Pabalan and J. Santos, T. Wisk and S. Wolfe for their provision of OWBM images; M. Knauft, I. Walde, S. König, T. Münch, J. Bauernfeind and D. Schüler for their contribution to Hi-C data generation and sequencing, DNA sequencing and IT administration and sequence data management; J. Vrána for karyotyping the wheat cultivars Arina and Forno; and R. Regier for project management, administration and support.
Author information
These authors contributed equally: Sean Walkowiak, Liangliang Gao, Cecile Monat
Authors and Affiliations
Crop Development Centre, University of Saskatchewan, Saskatoon, Saskatchewan, Canada
Sean Walkowiak, Valentyna Klymiuk, Brook Byrns, Kirby Nilsen, Jennifer Ens, Krystalee Wiebe, Amidou N’Diaye, Pierre J. Hucl & Curtis J. Pozniak
Grain Research Laboratory, Canadian Grain Commission, Winnipeg, Manitoba, Canada
Sean Walkowiak & Bin Xiao Fu
Department of Plant Pathology, Kansas State University, Manhattan, KS, USA
Liangliang Gao, Emily Delorean, Dal-Hoe Koo, Allen K. Fritz & Jesse Poland
Leibniz Institute of Plant Genetics and Crop Plant Research (IPK) Gatersleben, Seeland, Germany
Cecile Monat, Axel Himmelbach, Anne Fiebig, Sudharsan Padmarasu, Uwe Scholz, Martin Mascher & Nils Stein
Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany
Georg Haberer, Heidrun Gundlach, Klaus F. X. Mayer & Manuel Spannagl
Aquatic and Crop Resource Development, National Research Council Canada, Saskatoon, Saskatchewan, Canada
Mulualem T. Kassa, Pierre Fobert & Sateesh Kagale
John Innes Centre, Norwich Research Park, Norwich, UK
Jemima Brinton, Ricardo H. Ramirez-Gonzalez, Michael Bevan, Neil McKenzie, Burkhard Steuernagel & Cristobal Uauy
Department of Plant and Microbial Biology, University of Zurich, Zurich, Switzerland
Markus C. Kolodziej, Simon G. Krattinger, Beat Keller & Thomas Wicker
Morden Research and Development Centre, Agriculture and Agri-Food Canada, Morden, Manitoba, Canada
Dinushika Thambugala & Curt A. McCartney
Department of Computer Science, University of Saskatchewan, Saskatoon, Saskatchewan, Canada
Venkat Bandi, Jorge Nunez Siri & Carl Gutwin
Brandon Research and Development Centre, Agriculture and Agri-Food Canada, Brandon, Manitoba, Canada
Kirby Nilsen
Genomics/Transcriptomics group, Functional Genomics Center Zurich, Zurich, Switzerland
Catharine Aquino & Masaomi Hatakeyama
Department of Evolutionary Biology and Environmental Studies, University of Zurich, Zurich, Switzerland
Dario Copetti, Gwyneth Halstead-Nussloch, Masaomi Hatakeyama, Timothy Paape, Rie Shimizu-Inatsugi & Kentaro K. Shimizu
Institute of Agricultural Sciences, ETHZ, Zurich, Switzerland
Dario Copetti
Kihara Institute for Biological Research, Yokohama City University, Yokohama, Japan
Tomohiro Ban, Kanako Kawaura, Toshiaki Tameshige, Hiroyuki Tsuji & Kentaro K. Shimizu
Life Sciences Department, Natural History Museum, London, UK
Luca Venturini & Matthew Clark
Earlham Institute, Norwich Research Park, Norwich, UK
Bernardo Clavijo, Christine Fosker, Gonzalo Garcia Accinelli, Darren Heavens, Ksenia Krasileva, David Swarbreck, Jonathan Wright & Anthony Hall
The John Bingham Laboratory, NIAB, Cambridge, UK
Keith A. Gardner, Nick Fradgley, Lawrence Percival-Alwyn & James Cockram
Department of Agronomy and Plant Genetics, University of Minnesota, Saint Paul, MN, USA
Juan Gutierrez-Gonzalez & Gary Muehlbauer
Global Institute for Food Security, University of Saskatchewan, Saskatoon, Saskatchewan, Canada
Chu Shin Koh & Andrew G. Sharpe
School of Plant Sciences and Food Security, Tel Aviv University, Ramat Aviv, Israel
Jasline Deek
Department of Entomology, University of Manitoba, Winnipeg, Manitoba, Canada
Alejandro C. Costamagna
Institute of Crop Science, NARO, Tsukuba, Japan
Hiroyuki Kanamori, Fuminori Kobayashi, Tsuyoshi Tanaka, Jianzhong Wu & Hirokazu Handa
Centre for Biodiversity Genomics, University of Guelph, Guelph, Ontario, Canada
National Institute of Advanced Industrial Science and Technology (AIST), Tokyo, Japan
Tony Kuo & Jun Sese
Laboratory of Plant Genetics, Graduate School of Agriculture, Kyoto University, Kyoto, Japan
Kazuki Murata, Yusuke Nabeka & Shuhei Nasuda
Humanome Lab, Tokyo, Japan
Global Wheat Program, International Maize and Wheat Improvement Center (CIMMYT), Texcoco, Mexico
Philomin Juliana & Ravi Singh
Montana BioAg, Missoula, MT, USA
Hikmet Budak
Australian Research Council Centre of Excellence in Plant Energy Biology, School of Molecular Sciences, University of Western Australia, Perth, Western Australia, Australia
Ian Small & Joanna Melonek
Ottawa Research and Development Centre, Agriculture and Agri-Food Canada, Ottawa, Ontario, Canada
Sylvie Cloutier
Agriculture Victoria, AgriBio, Centre for AgriBioscience, Bundoora, Victoria, Australia
Gabriel Keeble-Gagnère & Josquin Tibbets
Syngenta, Durham, NC, USA
Erik Legg & Arvind Bharti
School of Agriculture, Food and Wine, University of Adelaide, Adelaide, South Australia, Australia
Peter Langridge & Ken Chalmers
German Centre for Integrative Biodiversity Research (iDiv) Halle-Jena-Leipzig, Leipzig, Germany
- Martin Mascher
Biological and Environmental Science & Engineering Division, King Abdullah University of Science and Technology, Thuwal, Saudi Arabia
Simon G. Krattinger
Graduate School of Life and Environmental Sciences, Kyoto Prefectural University, Kyoto, Japan
Hirokazu Handa
Institute of Evolution and Department of Evolutionary and Environmental Biology, University of Haifa, Haifa, Israel
Assaf Distelfeld
School of Life Sciences Weihenstephan, Technical University of Munich, Freising, Germany
Klaus F. X. Mayer
Center for Integrated Breeding Research (CiBreed), Georg-August-University Göttingen, Göttingen, Germany
You can also search for this author in PubMed Google Scholar
Contributions
Project establishment: K.C., A.D., A. Hall, B.K., S.G.K., E.L., P.L., K.F.X.M., J.P., C.J.P., K.K.S., M.S. and N.S. Project coordination: A. Hall, C.J.P. and N.S. Genome assemblies were contributed as follows: CDC Stanley and CDC Landmark: P.J.H., C.J.P., A.G.S., B.B., C.S.K., A.N., K.N. and S.W.; Julius: K.F.X.M., N.S., M.M., C.M. and U.S.; Jagger: G.M., J.P. and L.G.; Arina LrFor : B.K., S.G.K. and M.C.K.; Mace and LongReach Lancer: K.C., P.L., G.K.-G. and J.T.; Norin 61: K.K.S., H.H., S.N., J.S., K. Kawaura, H.T., T. Tameshige, T.B., D.C., M.H., R.S.-I., C.A., F.K., J.G.-G. and N.S.; SY Mattis: E.L. and A.B.; spelt (PI190962): A.D., C.J.P. and J.D.; Robigus, Claire, Paragon and Cadenza: M.B., M.C., B.C., C.F., N.F. and D.H.; Weebill 1: M.C., B.C., J.C., K.A.G., L.P.-A. and L.V. Sequencing, assembly and analysis were contributed by WRA2P computational assembly: A. Hall, B.C., G.G.A., K. Krasileva, N.M., D.S. and J. Wright; 10X Genomics: H.B., C.J.P., J.E., S.K. and K.W.; Hi-C and structural analysis: M.M., N.S., A. Himmelbach, C.M., S.P. and L.G.; pseudomolecule assemblies: M.M., C.M. and N.S.; gene projections and TE analysis: K.F.X.M., M.S., H.G. and G.H.; diversity and polymorphism analysis: K.K.S., E.D., T.P., G.H.-N., D.C., M.H., G.H., H.H., H.K., M.S., K.M., T. Tameshige, T. Tanaka, J.S. and J. Wu; centromere diversity: J.P. and D.H.K.; 5B/7B translocation: S.G.K., T.W., J.C. and M.C.K; 2N v S introgression: J.P., A.K.F., L.G., P.J., C.J.P., R.S. and S.W.; TE-based introgressions: T.W., B.B., J.E., M.C.K., J.P., C.J.P., J.T. and S.W; cytological karyotyping: S.N., K.M., Y.N., J.S. and T.K.; diversification of Rf genes: J.M. and I.S.; NLR repertoire: S.G.K. and B.S.; Sm1 gene cloning: C.A.M., C.J.P., C.U., J.B., A.C.C., S.C., P.F., M.T.K., V.K., D.T. and K.W.; haplotype database: C.U., J.B. and R.H.R.-G.; visualization software: C.G., V.B., G.K.-G., J.N.S., J.T. and J.M.; BLAST server: M.M., A.F. and U.S.; C.J.P and S.W. drafted the manuscript with input from all authors. All co-authors contributed to and edited the final version.
Corresponding authors
Correspondence to Curt A. McCartney , Manuel Spannagl , Thomas Wicker or Curtis J. Pozniak .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Peer review information Nature thanks Victor Albert, Rudi Appels and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended data fig. 1 chromosome-scale collinearity between the rqa..
Genomes were aligned chromosome by chromosome using MUMmer and are represented as dot plots. The introgression on chromosome 2B of LongReach Lancer (red rectangles) and 5B/7B translocation in SY Mattis and Arina LrFor (purple rectangles) are indicated.
Extended Data Fig. 2 Evaluation of the CDC Landmark RQA using Oxford Nanopore Long Reads.
a , Scaffold-scaffold long read contact map showing shared read IDs between scaffold ends along the ordered scaffolds in the CDC Landmark pseudomolecules. The diagonal pattern indicates that adjacent scaffolds share the same long reads and are therefore properly ordered and oriented by Hi-C in the RQA. b , Characterization of inversion events on chromosomes 2A, 3A, and 3D. The directionality biases estimated from alignments of Hi-C data against Chinese Spring (left, top), and chromosome alignment of the inversion events between CDC Landmark and Chinese Spring RQAs (left, bottom) are shown. Long reads spanning the inversion events and magnified views of the reads aligning to the left and right boundaries of the inversions (right) are provided.
Extended Data Fig. 3 Diversity of genes and TEs.
a , Average pairwise genetic diversity of the homeologues (coding sequences only) of the A, B and D subgenomes. The mode of the A, B and D subgenome is 0.00057, 0.00082, and 0.0002, respectively. b , Tajima’s D estimates of coding sequences for each wheat subgenome. The lower and upper range of the boxplot hinges correspond to the first and third quartiles (the 25th and 75th percentiles). Boxplots show centre line, median; box limits, upper and lower quartiles; whiskers, 1.5 × interquartile range. c , Total gene counts and orthologues for the RQA. Genes in orthologous groups with exactly one gene for each line (Complete; dark brown), genes contained in unambiguous orthologous groups missing an orthologue for at least one line, that is, PAV (2-10 Lines; light brown), and genes with ambiguous orthologues or CNV (Other; pink) are indicated. d , Per cent of pairwise shared syntenic fl-LTRs between wheat lines.
Extended Data Fig. 4 Evolutionary relationships among PPR and mTERF gene sequences.
a , The RFL clade is in blue and all remaining P-class PPRs are in green. b , Clustered mTERF sequences are in blue and the remaining mTERFs are shown in green. The scale bar represents number of substitutions per site. c , Sequence inversions and copy number variation at the Rf3 locus on chromosome 1B. RFL genes are shown as light pink triangles above the chromosome scale. Conserved non-PPR genes used as syntenic anchors are shown on the chromosome scale as coloured triangles. The total number (T) and the number of putatively functional RFL genes with 10 or more PPR motifs (F) are indicated on the right side of each panel.
Extended Data Fig. 5 Identification of alien introgressions from wheat relatives.
A feature of foreign chromosomal introgressions is that they contain unique patterns of TE insertions. Shown are stretches of >20 Mb containing multiple polymorphic RLC- Angela retrotransposons that are found only in one or a few (≤4) of the sequenced lines. One representative chromosome for each wheat subgenome is shown. Individual polymorphic retrotransposons are indicated as coloured vertical lines. Colours correspond to the number of cultivars a foreign segment is found in. Regions of particular interest are indicated by black rectangles. These include the 2N v S alien introgression from A. ventricosa at the end of chromosome 2A in Jagger, Mace, SY Mattis and CDC Stanley, as well as introgression in the central region of chromosome 2B from T. timopheevi in LongReach Lancer, and introgression at the end of chromosome 3D from T. ponticum in LongReach Lancer.
Extended Data Fig. 6 Detailed characterization of the 2N v S introgression from A. ventricosa .
a , Pairwise alignments of the first 50 Mb of chromosome 2A. The black arrow indicates a possible unique haplotype within spelt. b , Orthologous genes between the 2N v S introgression from A. ventricosa in Jagger and the genes on chromosomes 2A, 2B, and 2D in Chinese Spring. c , Frequency of 2N v S introgression carriers in North American datasets from CIMMYT, Kansas State, and the USDA Winter Wheat Regional Performance Nursery (RPN) over time. d , Per cent yield difference in lines that carry the 2N v S introgression. Two sided t -tests were performed to test for the significance of the impact of the 2N v S introgression. ** P < 0.01; *** P < 0.001.
Extended Data Fig. 7 Centromere positions and karyotype variation.
Functional centromere positions in the RQA have undergone structural and positional rearrangement. Chromosome alignments showing collinearity (black scaffolds in same orientation, grey scaffolds in opposite orientation) with relative density of CENH3 ChIP–seq mapped to 100 kb genomic bins for Chinese Spring (blue) and a representative genome of comparison (red) for chromosome 4B of CDC Stanley ( a ), and chromosome 5B of Julius ( b ). c , Detailed list and clustering of cytological features carried by each wheat line (Supplementary Note 6 ). Features that are identical (dark grey) or have a gain (black) or loss (light grey) relative to Chinese Spring are indicated.
Extended Data Fig. 8 Hi-C validates inversions identified from pairwise chromosome alignments.
Pairwise alignments of chromosome 6B from the RQA and Chinese Spring are shown. Above each alignment dot plot, the directionality biases estimated from alignments of Hi-C data against Chinese Spring are shown. Boundaries of diagonal segments are indicative of inversions and coincide with inversion boundaries identified from the chromosome alignments.
Extended Data Fig. 9 Characterization of a translocation involving wheat chromosomes 5B and 7B.
a , Cytogenetic karyotypes of Forno (left) and Arina (right), the parents of Arina LrFor . Note that the large recombinant chromosome 7B is represented by a distinct peak. b , Sequence of the translocation breakpoint on chromosome 7B of Arina LrFor . Note that the exact breakpoint lies in a sequence gap (stretch of Ns). The bp positions are indicated at the left. Forward PCR primers are shown in red and reverse primers in blue. The overlap of the two reverse primers is shown in purple. The outer primer pair was used for PCR, while the inner pair was used for a nested PCR. c , PCR amplification of the fragment spanning the translocation breakpoint. The nested PCR yielded a ~5 kb fragment that spanned the translocation breakpoint and its identity was confirmed by sequencing. Both PCR and nested PCR were performed in duplicate; both replicates of the nested PCR were sequenced using the Sanger method. For gel source data, see Supplementary Fig. 1 . d , Mapping of Illumina reads from the cultivars Arina and Forno on to the pseudomolecules of Arina LrFor . Sequence derived from Forno is shown in blue, while sequenced derived from Arina is in red. Note that chromosomes 5B and 7B are derived from both parents, indicating that these parental chromosomes can recombine freely, despite the presence of a large 5B/7B translocation in Arina.
Extended Data Fig. 10 Confirmation of gene expression and gene structure for Sm1 .
a , Critical recombinants from the 99B60-EJ2G/Infinity and 99B60-EJ2D/Thatcher populations used to fine map Sm1 . The 99B60-EJ2G/Infinity cross had 5,170 F 2 plants, while 99B60-EJ2D/Thatcher cross had 5,264 F 2 plants; only recombinant haplotypes between orange wheat blossom midge resistant (R) and susceptible (S) genotypes are shown. b , Oxford Nanopore long read confirmation of the Sm1 gene candidate in the CDC Landmark RQA (left), and alternative haplotype in Chinese Spring (right). Vertical coloured lines indicate sequence variants. c , Amplification of cDNA for the NB-ARC domain of the Sm1 gene candidate (top) and actin control (bottom) derived from RNA isolated from developing kernels (left) and wheat seedlings (right). Unity and CDC Landmark are carriers of Sm1 . Waskada carries an alternative haplotype and does not carry Sm1 (see main text). Thatcher was used as a susceptible parent for fine mapping of Sm1 and does not contain the associated NB-ARC domain. The experiment was replicated on four independent biological samples for each condition. d , Distribution of an Sm1 allele-specific PCR marker in a diverse panel of >300 wheat lines.
Supplementary information
Supplementary data.
Supplementary Figure 1. Original gel source data used for spanning the breakpoint for the 7B/5B translocation.
Reporting Summary
Supplementary information.
This file contains Supplementary Notes 1-8.
Supplementary Tables
This file contains Supplementary Tables 1-27.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Walkowiak, S., Gao, L., Monat, C. et al. Multiple wheat genomes reveal global variation in modern breeding. Nature 588 , 277–283 (2020). https://doi.org/10.1038/s41586-020-2961-x
Download citation
Received : 03 April 2020
Accepted : 09 September 2020
Published : 25 November 2020
Issue Date : 10 December 2020
DOI : https://doi.org/10.1038/s41586-020-2961-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Introgressions lead to reference bias in wheat rna-seq analysis.
- Benedict Coombes
- Anthony Hall
BMC Biology (2024)
Rapid and cost-effective molecular karyotyping in wheat, barley, and their cross-progeny by chromosome-specific multiplex PCR
- Mohammad Ali
- Dávid Polgári
Plant Methods (2024)
Wheat powdery mildew resistance gene Pm13 encodes a mixed lineage kinase domain-like protein
- Huanhuan Li
- Wenqiang Men
- Wenxuan Liu
Nature Communications (2024)
Plant pangenomes for crop improvement, biodiversity and evolution
- Mona Schreiber
- Murukarthick Jayakodi
Nature Reviews Genetics (2024)
Breeding for water-use efficiency in wheat: progress, challenges and prospects
- Aqsa Hafeez
- Shehzad Ali
Molecular Biology Reports (2024)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Open Access is an initiative that aims to make scientific research freely available to all. To date our community has made over 100 million downloads. It’s based on principles of collaboration, unobstructed discovery, and, most importantly, scientific progression. As PhD students, we found it difficult to access the research we needed, so we decided to create a new Open Access publisher that levels the playing field for scientists across the world. How? By making research easy to access, and puts the academic needs of the researchers before the business interests of publishers.
We are a community of more than 103,000 authors and editors from 3,291 institutions spanning 160 countries, including Nobel Prize winners and some of the world’s most-cited researchers. Publishing on IntechOpen allows authors to earn citations and find new collaborators, meaning more people see your work not only from your own field of study, but from other related fields too.
Brief introduction to this section that descibes Open Access especially from an IntechOpen perspective
Want to get in touch? Contact our London head office or media team here
Our team is growing all the time, so we’re always on the lookout for smart people who want to help us reshape the world of scientific publishing.
Home > Books > Current Trends in Wheat Research
Introductory Chapter: Current Trends in Wheat Research
Published: 11 May 2022
DOI: 10.5772/intechopen.103763
Cite this chapter
There are two ways to cite this chapter:
From the Edited Volume
Current Trends in Wheat Research
Edited by Mahmood-ur-Rahman Ansari
To purchase hard copies of this book, please contact the representative in India: CBS Publishers & Distributors Pvt. Ltd. www.cbspd.com | [email protected]
Chapter metrics overview
230 Chapter Downloads
Impact of this chapter
Total Chapter Downloads on intechopen.com
Total Chapter Views on intechopen.com
Author Information
Nazia nahid.
- Department of Bioinformatics and Biotechnology, GC University–Faisalabad, Pakistan
Parwsha Zaib
Tayyaba shaheen *, kanval shaukat.
- Department of Botany, University of Balochistan, Pakistan
Akmaral U. Issayeva
- M. Auezov South Kazakhstan University, Kazakhstan
Mahmood-ur-Rahman Ansari *
*Address all correspondence to: [email protected] and [email protected]
1. Introduction
Wheat ( Triticum aestivum ) is known as one of the most important cereal crops and is extensively grown worldwide [ 1 ]. Wheat contributes to 50% and 30% of the global grain trade and production respectively [ 2 ]. Wheat is also known as a staple food in more than 40 countries of the world. Wheat provides 82% of basic calories and 85% of proteins to the world population [ 3 , 4 ]. Wheat-based food is rich in fiber contents than meat-based food. Dough produced from bread wheat flour has different viscoelastic properties than other cereals. It is considered a higher fiber food. Therefore, its positive effects on controlling cholesterol, glucose, and intestinal functions in the body were observed [ 5 ]. Primarily, wheat is being used to make Chapatti (Bread) but it also contributes to other bakery products. Wheat utility and high nutritional value made it the staple food for more than 1/3rd population of the world. Wheat grain is separated from the chaff and stalks after the harvesting of wheat. Stalks of wheat are further used in animal bedding and construction material. Globally, the need for wheat production is enhancing even in countries having unfavorable climates for its production. Global climate changes are badly affecting the production of wheat and it raised the concern for food security.
It is estimated that annual cereal production should be increased by 1 billion tons to feed the expected population of 9.1 billion by 2050 [ 1 ]. The current scenario demands an increase in crop productivity to meet the increased requirements of food supply [ 6 ]. Wheat is grown in tropical and subtropical regions which experiences a lot of stress. These stresses result in a reduction of yield [ 7 ]. Major environmental stresses include cold, salinity, heat, and drought which are drastically affecting its yield. However, water and heat are considered as the key environmental stresses which caused in reduction of the wheat yield globally [ 8 , 9 ]. So, genetic improvements related to yield and stress tolerance are mandatory to enhance the production of wheat [ 10 , 11 ].
2. Genetically modified wheat plants
Genetically modified wheat plants have been produced by the use of bacteria. Wheat plants were inoculated with the plant-growth-promoting bacteria (PGPB) which resulted in the higher expression of abiotic stress (mainly drought and salinity) tolerant genes [ 12 ]. PGPB inoculated wheat cultivars also showed the higher expression of genes encoding antioxidant-enzymes, such as catalase (CAT), peroxidase , ascorbate peroxidase (APX), and glutathione peroxidase (GPX). So, it was concluded that PGPB used in wheat plants resulted in increased tolerance to abiotic stresses [ 12 ]. Cold shock proteins increase the survival of bacteria in severe environmental conditions. CspA and CspB genes from bacteria were transformed into wheat. Transgenic wheat plants expressing SeCspA and SeCspB were observed to have decreased water loss rate, increased proline and chlorophyll contents under salinity, and less water-stress conditions [ 13 ]. It was further investigated that SeCsp transgenic wheat plants resulted in enhanced weight and yield of grain than the control plants. SeCspA transgenic wheat plants were observed to have an improved water-stress tolerance than the control plants ( Table 1 , [ 13 ]).
Development of transgenic wheat having various traits/phenotypes.
Gluten is a protein comprised of gliadins found in wheat. Gluten is the main cause of coeliac disease in individuals. Bread-making quality of wheat is determined by the gluten proteins. Wheat varieties with less gliadin contents were produced using gene-editing technologies and RNAi (RNA interference). Wheat lines lacking immunogenic gluten were produced. Low immunogenic gluten and more nutritional values were added in one wheat line named E82. A better microbiota profile (protection microorganisms available in the gut) was observed in the NCWS patients using the bread made with E82 [ 28 ]. Plant cuticle has a positive role in the protection of plant against biotic and abiotic stresses. Wheat plants transformed with TaSHN1 resulted in increased water-stress tolerance by reducing the leaf stomatal density and changing the composition of the cuticle [ 29 ].
3. Biotic stress tolerance in wheat
Wheat is considered an excessive contributor toward the human calorie intake [ 30 ]. Pests and pathogens cause yield losses in wheat up to 21.5% of the total losses and could be reached to 28.1% [ 31 ]. Wheat is affected by the fungal disease, powdery mildew caused by Blumeria graminis f. sp. tritici (Bgt). Powdery mildew is a damaging disease that resulted in greater loss of wheat [ 32 ]. Broad-spectrum resistant genes (BSR) are considered to have the most significant role to control powdery mildew. CMPG1-V gene was cloned from the Hynaldia villosa and it was observed that higher expression of CMPG1-V gene resulted in the Broad-spectrum resistance against powdery mildew [ 33 , 34 ]. Barley chi26 gene could also be used to enhance the resistance against powdery mildew and rust through genetic modification [ 35 ]. Some epigenetic regulators were determined to have a role in wheat powdery mildew resistance. TaHDT701 is a histone deacetylase that was found as a negative regulator of wheat defense against powdery mildew. TaHDT701 was observed to be associated with the one repeat protein (TaHOS15) and RPD3 type histone deacetylase TaHDA6. Knockdown of this histone deacetylase complex ( TaHDT701 , TaHDA6 , TaHOS15 ) in wheat resulted in increased powdery mildew tolerance [ 36 ].
Fusarium graminearum is a plant fungal pathogen that causes a devastating disease called Fusarium head blight in wheat. It results in the reduction of wheat production. Genetic techniques were used to increase the FHB (Fusarium head blight) resistance in wheat. Transgenic wheat plants expressing barley class II chitinase gene 2 were observed to have a higher resistance against Fusarium graminearum [ 37 ]. Lr10 and Lr21 were cloned and transformed into wheat. The transgenic plants were reported to be resistant to leaf rust disease. Evolution and diversification of HIPPs (heavy metal-associated isoprenylated plant proteins) genes were studied in Triticeae [ 38 ]. HIPPs genes of Hynaldia villosa were cloned through homology-based cloning. Transgenic wheat having HIPP1-V was developed and the role of HIPP1-V in cadmium stress was characterized. It was observed that higher expression of this gene resulted in increased tolerance to cadmium stress. Therefore, HIPP1-V could be used to increase the tolerance in wheat against cadmium [ 39 ].
4. Abiotic stress tolerance in wheat
Grain number, weight, and size are greatly reduced under the negative effects of environmental stresses. However, the timing, duration, and intensity of stress determine the severity of the negative effects [ 40 , 41 ]. Wheat is a major source of protein and calories for the human diet. High temperature is badly affecting the yield of wheat which is a main concern worldwide. Drought and heat stresses are the two main abiotic stresses which are playing a greater role in the reduction of wheat yield. Reduction in starch contents, photosynthetic activity, grain number, and chlorophyll contents in the endosperm is caused due to rise in temperature. Heat stress results in the accumulation of reactive oxygen species (ROS) which is the main reason for higher oxidative damage to the plant. Heat stress also results in the variation of wheat biochemistry, morphology, and physiology. Tolerance, avoidance, and escape are known as the three major mechanisms that support the plant to grow in a heat-stress environment. Major heat tolerance mechanisms in wheat are known as stay green, heat shock proteins, and antioxidant defense [ 42 ]. Protein synthesis and folding were observed to be interrupted during heat stress. Heat stress also resulted in the production of several stress agents badly affecting transcription, translation, and DNA replication in plants [ 43 ]. Plants speed up the production of heat shock proteins as a defense mechanism [ 44 ]. Higher activity of antioxidants, such as peroxidases, catalase, and superoxide dismutase, was observed under heat stress. Wheat cultivar showing greater tolerance to heat stress was observed to have higher activity of catalase, ascorbate peroxidase, and S-transferase [ 45 ].
Salt stress greatly affects the growth of wheat plants. Salinity stress has a higher impact on the morphology and physiology of wheat plants. Plants having less tolerance to salinity are not suitable for cropping. Potassium transporter ( HKT ) genes have a greater role in achieving salinity tolerance in wheat. Sodium (Na + ) exclusion through HKT genes is a major mechanism in wheat to have a salinity tolerance. OsMYBSs and AtAB14 are the transcription factors having a role in regulating HKT genes, which are considered as the candidate targets for increasing salinity tolerance in wheat [ 46 ]. Wheat transformed with a mutated transcription factor, HaHB4 showed higher water-use efficiency and was more yielding under drought stress [ 26 ]. Transgenic wheat expressing GmDREB1 gene from soybean was also observed to have higher drought tolerance under water-stress conditions [ 47 ]. DREB1A gene from Arabidopsis thaliana was introduced to bread wheat and increased tolerance against water stress in the transgenic wheat was observed. Bread wheat under drought stress was observed to have a higher level of WRKY proteins [ 48 ]. Higher expression of AtHDG11 gene in transgenic wheat resulted in increased water-stress tolerance during drought-stress conditions. Enhanced TaNAC69 expression in root and leaf of wheat during drought stress was observed [ 49 ]. Researchers are working to develop transgenic wheat having various traits/phenotypes by using advanced approaches of biotechnology for the last several decades ( Table 1 ). Numbers of transgenic wheat cultivars are being grown in the fields and several more are under trial.
5. CRISPR/Cas9 system in wheat
Gliadins and glutenins are known as the gluten proteins and ingestion of these proteins from barley, rye, and wheat could cause the disease called coeliac disease in humans. The only remedy is to develop gluten-free food. Transgenic wheat which retains baking quality and is safe for coeliac could not be produced using conventional methods because of the complexity of the wheat genome. Coeliac disease (CD) is activated by the immunogenic isotopes mainly gliadins. Gliadin families were downregulated by the use of RNA interference. CRISPR/Cas9 is a targeted gene manipulation tool considered to have a potential role in genetic modification ( Table 2 , [ 60 , 61 ]). CRISPR/Cas9 system was recently used for gene editing of gliadins. Offsprings with deleted, edited, or silenced gliadins were produced by CRISPR/Cas9. They helped to decrease the exposure of the patient to the CD epitopes [ 62 ]. This technology has been used to develop wheat cultivars having gluten genes with inactivated CD epitopes [ 62 , 63 ].
Genome edited wheat developed by CRISPR/Cas9 system.
CRISPR/Cas9 system and TALENS (transcription activator-like effector nuclease) were used in the bread wheat to generate the mutations in three homoeoalleles that encode MLO locus proteins against mildew. Mutations in all three TaMLO were generated by using TALENS which resulted in resistance against powdery mildew. The MLO homoeoalleles ( TaMLOA1 , TaMLOB1, and TaMLOD1 ) of bread wheat contributed to the mildew infection. Mutation of MLO alleles resulted in powdery mildew tolerance in wheat [ 50 ]. Genome editing was reported in which pds (phytoene desaturase) and inox (inositol oxygenase) genes in the cell suspension-culture of wheat were targeted. It was demonstrated that the genome-editing technique could also be applied in the cell suspension of wheat [ 64 ]. Very recently, various research groups are involved to develop transgenic wheat by using genome-editing technology. Some of the experiments are listed in Table 2 .
6. Wheat computational analysis
A comprehensive resource for wheat reference genome was developed by International Wheat Genome Sequencing Consortium. The URGI portal ( https://wheat-urgi.versailles.inra.fr/ ) was developed for the breeders and researchers to access the genome sequence data of bread-wheat. InterMine tools, genome browser, and BLAST were established for the exploration of genome sequences together with the additional linked datasets, including gene expression, physical maps, and sequence variation. Portal provided the higher browser and search features that facilitated the use of the latest genomic resources required for the upgradation of wheat [ 65 ].
DNA binding with one finger (Dof) transcription factors is known to have an important role in abiotic stress tolerance as well as the growth of plants. Ninety-six TaDof members of the gene family have been studied using computational approaches. By qPCR analysis, it was revealed that TaDof genes were upregulated under heavy metal and heat stress in wheat. Consequently, it could be concluded that detection of amino acid sites, genome-wide analysis, and identification of the Dof transcription factor family could provide us the new insight into the function, structure, and evolution of the Dof gene family [ 66 ].
Acknowledgments
This work was supported by funds from the Higher Education Commission of Pakistan.
- 1. FAO. World Food and Agriculture-FAO Statistical Pocketbook. Rome, Italy: FAO; 2015
- 2. Akter N, Islam MR. Heat stress effects and management in wheat: A review. Agronomy for Sustainable Development. 2017; 37 (5):1-17
- 3. Chaves MS, Martinelli JA, Wesp-Guterres C, Graichen FAS, Brammer SP, Scagliusi SM, et al. The importance for food security of maintaining rust resistance in wheat. Food Security. 2013; 5 (2):157-176
- 4. Sharma D, Singh R, Tiwari R, Kumar R, Gupta VK. Wheat responses and tolerance to terminal heat stress: A review. In: Hasanuzzaman M, Nahar K, Hossain M, editors. Wheat Production in Changing Environments. Singapore: Springer; 2019. pp. 149-173
- 5. Giraldo P, Benavente E, Manzano-Agugliaro F, Gimenez E. Worldwide research trends on wheat and barley: A bibliometric comparative analysis. Agronomie. 2019; 9 (7):352
- 6. Iqbal M, Raja NI, Yasmeen F, Hussain M, Ejaz M, Shah MA. Impacts of heat stress on wheat: A critical review. Advances in Crop Science and Technology. 2017; 5 (1):01-09
- 7. Rahaie M, Xue GP, Schenk PM. The role of transcription factors in wheat under different abiotic stresses. Abiotic Stress - Plant Responses and Applications in Agriculture. 2013; 2 :367-385
- 8. Lesk C, Rowhani P, Ramankutty N. Influence of extreme weather disasters on global crop production. Nature. 2016; 529 (7584):84-87
- 9. Liu B, Asseng S, Müller C, Ewert F, Elliott J, Lobell DB, et al. Similar estimates of temperature impacts on global wheat yield by three independent methods. Nature Climate Change. 2016; 6 (12):1130-1136
- 10. Tester M, Langridge P. Breeding technologies to increase crop production in a changing world. Science. 2010; 327 (5967):818-822
- 11. He Z, Joshi AK, Zhang W. Climate vulnerabilities and wheat production. In: Pielke RA, editor. Climate Vulnerability: Understanding and Addressing Threats to Essential Resources. Waltham: Academic Press; 2013. pp. 57-67
- 12. Sudan J, Sharma D, Mustafiz A, Kumari S. Signaling peptides: Hidden molecular messengers of abiotic stress perception and response in plants. In: Zargar S, Zargar M, editors. Abiotic Stress-Mediated Sensing and Signaling in Plants: An Omics Perspective. Singapore: Springer; 2018. pp. 95-125
- 13. Yu TF, Xu ZS, Guo JK, Wang YX, Abernathy B, Fu JD, et al. Improved drought tolerance in wheat plants overexpressing a synthetic bacterial cold shock protein gene SeCspA. Scientific Reports. 2017; 7 (1):1-4
- 14. Peña PA, Quach T, Sato S, Ge Z, Nersesian N, Changa T, et al. Expression of the maize Dof1 transcription factor in wheat and sorghum. Frontiers in Plant Science. 2017; 8 :434
- 15. Qu B, He X, Wang J, Zhao Y, Teng W, Shao A, et al. A wheat CCAAT box-binding transcription factor increases the grain yield of wheat with less fertilizer input. Plant Physiology. 2015; 167 (2):411-423
- 16. Yadav D, Shavrukov Y, Bazanova N, Chirkova L, Borisjuk N, Kovalchuk N, et al. Constitutive overexpression of the TaNF-YB4 gene in transgenic wheat significantly improves grain yield. Journal of Experimental Botany. 2015; 66 (21):6635-6650
- 17. He X, Qu B, Li W, Zhao X, Teng W, Ma W, et al. The nitrate-inducible NAC transcription factor TaNAC2-5A controls nitrate response and increases wheat yield. Plant Physiology. 2015; 169 (3):1991-2005
- 18. Tian B, Talukder SK, Fu J, Fritz AK, Trick HN. Expression of a rice soluble starch synthase gene in transgenic wheat improves the grain yield under heat stress conditions. In Vitro Cellular & Developmental Biology. Plant. 2018; 54 (3):216-227
- 19. Hu M, Zhao X, Liu Q , Hong X, Zhang W, Zhang Y, et al. Transgenic expression of plastidic glutamine synthetase increases nitrogen uptake and yield in wheat. Plant Biotechnology Journal. 2018; 16 (11):1858-1867
- 20. Smidansky ED, Meyer FD, Blakeslee B, Weglarz TE, Greene TW, Giroux MJ. Expression of a modified ADP-glucose pyrophosphorylase large subunit in wheat seeds stimulates photosynthesis and carbon metabolism. Planta. 2007; 225 (4):965-976
- 21. Zhao XQ , Nie XL, Xiao XG. Over-expression of a tobacco nitrate reductase gene in wheat (Triticum aestivum L.) increases seed protein content and weight without augmenting nitrogen supplying. PLoS One. 2013; 8 (9):e74678
- 22. Connorton JM, Jones ER, Rodríguez-Ramiro I, Fairweather-Tait S, Uauy C, Balk J. Wheat vacuolar iron transporter TaVIT2 transports Fe and Mn and is effective for biofortification. Plant Physiology. 2017; 174 (4):2434-2444
- 23. Yang Y, Luang S, Harris J, Riboni M, Li Y, Bazanova N, et al. Overexpression of the class I homeodomain transcription factor Ta HDZ ipI-5 increases drought and frost tolerance in transgenic wheat. Plant Biotechnology Journal. 2018; 16 (6):1227-1240
- 24. Saint Pierre C, Crossa JL, Bonnett D, Yamaguchi-Shinozaki K, Reynolds MP. Phenotyping transgenic wheat for drought resistance. Journal of Experimental Botany. 2012; 63 (5):1799-1808
- 25. Castiglioni P, Warner D, Bensen RJ, Anstrom DC, Harrison J, Stoecker M, et al. Bacterial RNA chaperones confer abiotic stress tolerance in plants and improved grain yield in maize under water-limited conditions. Plant Physiology. 2008; 147 (2):446-455
- 26. González FG, Capella M, Ribichich KF, Curín F, Giacomelli JI, Ayala F, et al. Field-grown transgenic wheat expressing the sunflower gene HaHB4 significantly outyields the wild type. Journal of Experimental Botany. 2019; 70 (5):1669-1681
- 27. Shavrukov Y, Baho M, Lopato S, Langridge P. The TaDREB3 transgene transferred by conventional crossings to different genetic backgrounds of bread wheat improves drought tolerance. Plant Biotechnology Journal. 2016; 14 :313-322
- 28. García-Molina MD, Giménez MJ, Sánchez-León S, Barro F. Gluten free wheat: Are we there? Nutrients. 2019; 11 (3):487
- 29. Bi H, Shi J, Kovalchuk N, Luang S, Bazanova N, Chirkova L, et al. Overexpression of the TaSHN1 transcription factor in bread wheat leads to leaf surface modifications, improved drought tolerance, and no yield penalty under controlled growth conditions. Plant, Cell and Environment. 2018; 41 (11):2549-2566
- 30. Godfray HC, Beddington JR, Crute IR, Haddad L, Lawrence D, Muir JF, et al. Food security: The challenge of feeding 9 billion people. Science. 2010; 327 (5967):812-818
- 31. Savary S, Willocquet L, Pethybridge SJ, Esker P, McRoberts N, Nelson A. The global burden of pathogens and pests on major food crops. Nature Ecology & Evolution. 2019; 3 (3):430-439
- 32. Menardo F, Praz CR, Wyder S, Ben-David R, Bourras S, Matsumae H, et al. Hybridization of powdery mildew strains gives rise to pathogens on novel agricultural crop species. Nature Genetics. 2016; 48 (2):201-205
- 33. Zhu Y, Li Y, Fei F, Wang Z, Wang W, Cao A, et al. E3 ubiquitin ligase gene CMPG 1–V from Haynaldia villosa L. contributes to powdery mildew resistance in common wheat (Triticum aestivum L.). The Plant Journal. 2015; 84 (1):154-168
- 34. Liu J, Sun L, Chen Y, Wei L, Hao Y, Yu Z, et al. The regulatory network of CMPG1-V in wheat–Blumeria graminis f. sp. tritici interaction revealed by temporal profiling using RNA-Seq. International Journal of Molecular Sciences. 2020; 21 (17):5967
- 35. Eissa HF, Hassanien SE, Ramadan AM, El-Shamy MM, Saleh OM, Shokry AM, et al. Developing transgenic wheat to encounter rusts and powdery mildew by overexpressing barley chi26 gene for fungal resistance. Plant Methods. 2017; 13 (1):1-3
- 36. Zhi P, Kong L, Liu J, Zhang X, Wang X, Li H, et al. Histone deacetylase TaHDT701 functions in TaHDA6-TaHOS15 complex to regulate wheat defense responses to Blumeria graminis f. sp. tritici. International Journal of Molecular Sciences. 2020; 21 (7):2640
- 37. Shin S, Mackintosh CA, Lewis J, Heinen SJ, Radmer L, Dill-Macky R, et al. Transgenic wheat expressing a barley class II chitinase gene has enhanced resistance against Fusarium graminearum. Journal of Experimental Botany. 2008; 59 (9):2371-2378
- 38. Zhang H, Zhang X, Liu J, Niu Y, Chen Y, Hao Y, et al. Characterization of the heavy-metal-associated Isoprenylated plant protein (HIPP) gene family from Triticeae species. International Journal of Molecular Sciences. 2020; 21 (17):6191
- 39. Hura T. Wheat and barley: Acclimatization to abiotic and biotic stress. International Journal of Molecular Sciences. 2020; 21 (19):7423
- 40. Foulkes MJ, Scott RK, Sylvester-Bradley R. The ability of wheat cultivars to withstand drought in UK conditions: Formation of grain yield. The Journal of Agricultural Science. 2002; 138 (2):153-169
- 41. Weldearegay DF, Yan F, Jiang D, Liu F. Independent and combined effects of soil warming and drought stress during anthesis on seed set and grain yield in two spring wheat varieties. Journal of Agronomy and Crop Science. 2012; 198 (4):245-253
- 42. Poudel PB, Poudel MR. Heat stress effects and tolerance in wheat: A review. Journal of Biology and Today's World. 2020; 9 (3):1-6
- 43. Biamonti G, Caceres JF. Cellular stress and RNA splicing. Trends in Biochemical Sciences. 2009; 34 (3):146-153
- 44. Gupta SC, Sharma A, Mishra M, Mishra RK, Chowdhuri DK. Heat shock proteins in toxicology: How close and how far? Life Sciences. 2010; 86 (11-12):377-384
- 45. Balla K, Bencze S, Janda T, Veisz O. Analysis of heat stress tolerance in winter wheat. Acta Agronomica Hungarica. 2009; 57 (4):437-444
- 46. Kunika BK, Singh PK, Rani V, Pandey GC. Salinity tolerance in wheat: An overview. International Journal of Chemical Studies. 2019; 6 :815-820
- 47. Shiqing GA, Huijun XU, Xianguo C, Ming C, Zhaoshi XU, Liancheng L, et al. Improvement of wheat drought and salt tolerance by expression of a stress-inducible transcription factor GmDREB of soybean (Glycine max). Chinese Science Bulletin. 2005; 50 :2714-2723, 2723
- 48. Okay S, Derelli E, Unver T. Transcriptome-wide identification of bread wheat WRKY transcription factors in response to drought stress. Molecular Genetics and Genomics. 2014; 289 (5):765-781
- 49. Xue GP, Bower NI, McIntyre CL, Riding GA, Kazan K, Shorter R. TaNAC69 from the NAC superfamily of transcription factors is up-regulated by abiotic stresses in wheat and recognises two consensus DNA-binding sequences. Functional Plant Biology. 2006; 33 (1):43-57
- 50. Wang Y, Cheng X, Shan Q , Zhang Y, Liu J, Gao C, et al. Simultaneous editing of three homoeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew. Nature Biotechnology. 2014; 32 (9):947-951
- 51. Ouyang X, Hong X, Zhao X, Zhang W, He X, Ma W, et al. Knock out of the PHOSPHATE 2 gene TaPHO2-A1 improves phosphorus uptake and grain yield under low phosphorus conditions in common wheat. Scientific Reports. 2016; 6 :29850
- 52. Zhang Y, Liang Z, Zong Y, Wang Y, Liu J, Chen K, et al. Efficient and transgene-free genome editing in wheat through transient expression of CRISPR/Cas9 DNA or RNA. Nature Communications. 2016; 7 :12617
- 53. Zhang Y, Bai Y, Wu G, Zou S, Chen Y, Gao C, et al. Simultaneous modification of three homoeologs of TaEDR1 by genome editing enhances powdery mildew resistance in wheat. The Plant Journal. 2017; 91 :714-724
- 54. Wang W, Mauleon R, Hu Z, Chebotarov D, Tai S, Wu Z, et al. Genomic variation in 3,010 diverse accessions of Asian cultivated rice. Nature. 2018; 557 (7703):43-49
- 55. Okada A, Arndell T, Borisjuk N, Sharma N, Watson-Haigh NS, Tucker EJ, et al. CRISPR/Cas9-mediated knockout of Ms1 enables the rapid generation of male-sterile hexaploid wheat lines for use in hybrid seed production. Plant Biotechnology Journal. 2019; 17 :1905-1913
- 56. Li J, Jiao G, Sun Y, Chen J, Zhong Y, Yan L, et al. Modification of starch composition, structure and properties through editing of TaSBEIIa in both winter and spring wheat varieties by CRISPR/Cas9. Plant Biotechnology Journal. 2020; 19 :937-951
- 57. Zong Y, Wang Y, Li C, Zhang R, Chen K, Ran Y, et al. Precise base editing in rice, wheat and maize with a Cas9-cytidine deaminase fusion. Nature Biotechnology. 2017; 35 :438-440
- 58. Zhang R, Liu J, Chai Z, Chen S, Bai Y, Zong Y, et al. Generation of herbicide tolerance traits and a new selectable marker in wheat using base editing. Nature Plants. 2019; 5 :480-485
- 59. Zhang Y, Malzahn AA, Sretenovic S, Qi Y. The emerging and uncultivated potential of CRISPR technology in plant science. Nature Plants. 2019; 5 :778-794
- 60. Schaart JG, van de Wiel CC, Lotz LA, Smulders MJ. Opportunities for products of new plant breeding techniques. Trends in Plant Science. 2016; 21 (5):438-449
- 61. Van de Wiel CC, Schaart JG, Lotz LA, Smulders MJ. New traits in crops produced by genome editing techniques based on deletions. Plant Biotechnology Reports. 2017; 11 (1):1-8
- 62. Jouanin A, Gilissen LJ, Schaart JG, Leigh FJ, Cockram J, Wallington EJ, et al. CRISPR/Cas9 gene editing of gluten in wheat to reduce gluten content and exposure—Reviewing methods to screen for coeliac safety. Frontiers in Nutrition. 2020; 7 :51
- 63. Jouanin A, Schaart JG, Boyd LA, Cockram J, Leigh FJ, Bates R, et al. Outlook for coeliac disease patients: Towards bread wheat with hypoimmunogenic gluten by gene editing of α-and γ-gliadin gene families. BMC Plant Biology. 2019; 19 (1):1-6
- 64. Upadhyay SK, Kumar J, Alok A, Tuli R. RNA-guided genome editing for target gene mutations in wheat. G3: Genes, Genomes, Genetics. 2013; 3 (12):2233-2238
- 65. Alaux M, Rogers J, Letellier T, Flores R, Alfama F, Pommier C, et al. Linking the international wheat genome sequencing consortium bread wheat reference genome sequence to wheat genetic and phenomic data. Genome Biology. 2018; 19 (1):1
- 66. Liu Y, Liu N, Deng X, Liu D, Li M, Cui D, et al. Genome-wide analysis of wheat DNA-binding with one finger (Dof) transcription factor genes: Evolutionary characteristics and diverse abiotic stress responses. BMC Genomics. 2020; 21 (1):1-8
© 2022 The Author(s). Licensee IntechOpen. This chapter is distributed under the terms of the Creative Commons Attribution 3.0 License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Continue reading from the same book
By Harmeet Singh Bakala, Kamalpreet Singh Mandahal, ...
634 downloads
By Mirza Abdul Qayyum, Shafqat Saeed, Unsar Naeem-Ull...
550 downloads
By Juan Manuel Sánchez-Yañez
304 downloads

- Proceedings
Information
International Journal of Food Engineering and Technology

- Processing Charges

Bread Wheat Quality: Physical, Nutritional and Techno-Functional Properties
Cherinet Kasahun
Food Science and Nutrition Research Directorate, Ethiopian Institute of Agricultural Research, Kulumsa Agricultural Research Center, Assela, Ethiopia
Add to Mendeley
The grain physical quality, nutritional composition, milling process and technological characteristics of bread wheat is very important for end-use quality of bread. Many food processing industries utilizing bread wheat as a raw material are being established in Ethiopia. As a result, information on wheat grain physical, chemical and functional characteristics to match end use quality is very important. In line with this, the objective of this review is to characterize the physico-chemical and functional properties in relation to bread making quality and to review more classification of bread wheat cultivars considered as soft and hard wheat based on quality traits characterized. Currently there is an increasing demand among consumers for bread that contains not only traditional nutrients but also provides other compounds that are beneficial to health and well-being. Food systems that feed the world must be changed in ways that will insure that balanced nutrient supplies are available continuously to all people in adequate, accessible and affordable amounts. This paper reviews about the most important wheat grain quality, milling process and their nutritional value including product qualities indices. The opportunities of plant breeding and other technologies to improve the nutritional quality of wheat are also discussed. Different research papers, journals and other national and international resource were used during the review process including agricultural books, thesis, reports and other important scientific resources. According to the present review the bread wheat grain physical qualities, nutritional and technological properties is important quality indices for end-use quality of bread wheat grains. Therefore, working on wheat quality in addition to agronomic and disease resistance is critical issue. Further research work is recommended on wheat quality of landraces, old, newly released and promising bread genotypes for better grain physical qualities, nutritional and product making quality that helps famers, processors, millers and import substitution for the country.
Bread Wheat, Quality, Nutritional
Cherinet Kasahun. (2022). Bread Wheat Quality: Physical, Nutritional and Techno-Functional Properties. International Journal of Food Engineering and Technology , 6 (2), 34-44. https://doi.org/10.11648/j.ijfet.20220602.12
Cherinet Kasahun. Bread Wheat Quality: Physical, Nutritional and Techno-Functional Properties. Int. J. Food Eng. Technol. 2022 , 6 (2), 34-44. doi: 10.11648/j.ijfet.20220602.12
Cherinet Kasahun. Bread Wheat Quality: Physical, Nutritional and Techno-Functional Properties. Int J Food Eng Technol . 2022;6(2):34-44. doi: 10.11648/j.ijfet.20220602.12
Cite This Article
- Author Information
Verification Code/Mostafa Zaman Chowdhury
The verification code is required.
Verification code is not valid.
Science Publishing Group (SciencePG) is an Open Access publisher, with more than 300 online, peer-reviewed journals covering a wide range of academic disciplines.
Learn More About SciencePG

- Special Issues
- AcademicEvents
- ScholarProfiles
- For Authors
- For Reviewers
- For Editors
- For Conference Organizers
- For Librarians
- Article Processing Charges
- Special Issues Guidelines
- Editorial Process
- Peer Review at SciencePG
- Open Access
- Ethical Guidelines
Important Link
- Manuscript Submission
- Propose a Special Issue
- Join the Editorial Board
- Become a Reviewer
- Share full article
Advertisement
Supported by
Can Oats Really Help You Lose Weight?
A viral TikTok trend touts “Oatzempic” as a weight-loss hack. We asked the experts if there’s anything to it.

By Alice Callahan
On TikTok, a woman blends a half cup of rolled oats with a cup of water and the juice of half a lime. She forces a smile and then hesitantly takes a sip. “That,” she says with a colorful flourish, “is nasty.”
The drink isn’t meant to taste good; it’s supposed to be a weight loss hack.
Drink it every day, some influencers on social media claim, and you can lose a staggering 40 pounds in two months. “Oatzempic,” as it’s called, is a reference to the diabetes drug Ozempic , which belongs to a class of medications that have surged in popularity for their remarkable ability to help people lose weight.
It’s riding “on the coattails” of these drugs, said Colleen Tewksbury, an assistant professor in nutrition science at the University of Pennsylvania.
But while oats are certainly nutritious, “there is nothing magical” about them for weight loss, said Emily Haller, a dietitian in the lifestyle medicine program at Trinity Health Ann Arbor in Michigan.
What can oats do for your health?
Oats are a good source of soluble fiber, especially one type called beta-glucan, which has been shown to lower blood cholesterol levels and reduce blood sugar spikes after meals, Ms. Haller said.
In general, consuming enough fiber (which most Americans don’t ) can also reduce your risk of developing heart disease and certain cancers, as well as support a healthy gut and regular bowel habits, she added.
A half cup of rolled oats contains about four grams of fiber; nutrition guidelines advise that adults consume at least 21 to 38 grams per day.
Fiber-rich foods also take longer to digest than low-fiber foods, and they can slow the movement of food through your gut, which can help you feel fuller and more satisfied for longer, Ms. Haller said.
Can a blended oat drink help you lose weight?
Some research suggests that adding oats to your diet may be associated with a small amount of weight loss, maybe because they help us feel sated . But not all studies have found this, Dr. Tewksbury said, adding that she was not aware of any research specifically testing oats blended with water. There is also no evidence that lime juice can help with weight loss.
If people are losing significant amounts of weight on “Oatzempic,” it’s probably because they’re using it to replace a higher-calorie meal, said Dr. Melanie Jay, an obesity researcher at N.Y.U. Langone Health.
One half cup of rolled oats has about 150 calories; if you are consuming that instead of a higher calorie breakfast — an egg and sausage sandwich on a biscuit can have more than 500 calories for instance — you will probably lose weight, she said. It’s similar to replacing a meal with something like a weight-loss shake or a bar, which can be effective for weight loss, at least in the short term, she said.
But oats blended with water and lime juice “is not a balanced meal,” Ms. Haller said. A bowl of oatmeal, perhaps served with milk, nut butter, fruit and seeds, “would be a more well-balanced, satiating breakfast,” she said.
Consuming enough protein is particularly important if you’re losing weight, Dr. Jay said, to help you avoid losing too much muscle. A half cup of oats has about four grams.
Using the “Oatzempic” shake to lose weight is also likely unsustainable, Dr. Jay said. “If you go back to what you were eating before, you’ll gain the weight back,” she said.
Many of Dr. Jay’s patients with obesity “have lost hundreds of pounds in their lifetime” through fad diets and methods similar to “Oatzempic,” she said. But the weight often returns because their bodies respond with a slowed metabolism and more hunger, she said.
The “up and down, up and down” that can come with trying trendy weight loss hacks can be discouraging, she added.
And for some people, fad diets can lead to an “unhealthy obsession” with unrealistic weight loss and a negative relationship with food, Ms. Haller said.
Are oats anything like Ozempic?
“ Oats are not Ozempic,” Ms. Haller said. “Not even close.”
Weight loss drugs are “in such great demand” because they’re effective, Dr. Tewksbury said. “It’s almost Pollyanna to expect the same effect from oatmeal or oats.”
The medications work in part by mimicking a hormone called GLP-1, which your body releases after you eat. The hormone slows the movement of food through the gut and signals fullness to the brain. But, Dr. Jay said, the amount of GLP-1 you release after eating oats or any food is far lower, and not nearly as long-lasting, as that provided by the medications.
In several studies, researchers have measured blood GLP-1 levels after people consumed oat or wheat bread , or a breakfast with or without added oat powder , and found that oats did not increase GLP-1 levels more than the other foods.
The “Oatzempic” craze is “just another trend,” Ms. Haller said. “This is just what the internet does.” And, she added, the drink’s popularity will likely “be very short-lived.”
Alice Callahan is a Times reporter covering nutrition and health. She has a Ph.D. in nutrition from the University of California, Davis. More about Alice Callahan
A Guide to Better Nutrition
How much salt is too much? Should I cut back? We asked experts these and other questions about sodium .
Patients were told for years that cutting calories would ease the symptoms of polycystic ovary syndrome. But research suggests dieting may not help at all .
We asked a nutrition expert how she keeps up healthy habits without stressing about food. Here are seven tips she shared for maintaining that balance.
There are many people who want to lose a few pounds for whom weight loss drugs are not the right choice. Is old-fashioned dieting a good option ?
Salmon is good for you, but choosing the right type to eat isn’t so easy. Here are answers to all your questions about this nutritional powerhouse .
Read these books to shift into a healthier way of thinking about food .

IMAGES
VIDEO
COMMENTS
Bread, traditionally made of wheat flour, is one of the oldest foods and is widely consumed in most cultures [9,10]. ... J.V. Diaz's participation did not affect the authenticity or objectivity of the experimental results in this research paper. The remaining authors declare that the research was conducted in the absence of any commercial or ...
Wheat is an important cereal that is the staple food for more than 2.5 billion people globally 1.Beyond its nutritional and health benefits 2, wheat contributes substantially to food security by ...
Introduction. Bread, regardless of the type, production process and geographical origin, is traditionally produced from refined common wheat (Triticum aestivum) flour.However, in recent years, there has been renewed interest in fortifying or replacing refined wheat flour with whole-grain wheat flour, or flour from gluten-free cereals (rice, maize, sorghum, millet), pseudocereals (amaranth ...
The research is aimed to improve the quality of life of celiac disease or wheat allergy patients. Better bread quality (flavor, texture, and volume), reduced production cost, and wider ...
Triticum aestivum, commonly known as bread wheat, is one of the most cultivated crops globally. Due to its increasing demand, wheat is the source of many nutritious products including bread, pasta, and noodles containing different types of seed storage proteins. Wheat seed storage proteins largely control the type and quality of any wheat product. Among various unique wheat products, bread is ...
An annotated reference sequence representing the hexaploid bread wheat genome in 21 pseudomolecules has been analyzed to identify the distribution and genomic context of ... First release papers; Archive; ... the 21 pseudomolecules position molecular markers for wheat research and breeding [504 single-stranded repeats (SSRs), 3025 diversity ...
Approximately 36% of the world's population depends on wheat as a primary source of food due to the presence of 20% of the food calories, 55% carbohydrates (70-75% starch), 10-12% proteins, 14% ...
Wheat, the third largest cereal crop, is grown globally under spring, winter, and facultative environmental habitats. Modern wheat cultivation started in the Middle East about 9000-11,000 years ago and as a consequence of increased geographical farming, bread wheat became a common staple food from China to England (Heun et al. 1997; Nesbitt 1998; Dubcovsky and Dvorak 2007).
Bread provides several nutrients such as sugar, protein, iron, calcium and a variety of vitamins [].An average daily intake of 300 g of bread can provide the nutrients needed by the body and create a desired nutritional status [].Bread can provide 1.2% of protein, 60% of thiamine and niacin, 40% of calcium and 80% of the daily iron needed by an adult [7, 8].
Introduction. Bread is a staple food across the globe produced from hard wheat. It contains varying levels of nutrients such as starch, complex carbohydrates, and proteins, which have a beneficial effect on human health (Gellynck, Kühne, Van Bockstaele, Van de Walle, & Dewettinck, Citation 2009).Bread composition also varies with the additional ingredients used during the production process ...
Bread, regardless of the type, production process and geographical origin, is traditionally produced from refined common wheat (Triticum aestivum) flour.However, in recent years, there has been renewed interest in fortifying or replacing refined wheat flour with whole-grain wheat flour, or flour from gluten-free cereals (rice, maize, sorghum, millet), pseudocereals (amaranth, buckwheat, quinoa ...
The volume of research carried out on wheat gluten is vast, with a simple search of the Web of Science database showing almost 20 000 papers since 1945. This volume not only reflects the commercial importance of wheat processing, but also the complexity of the system which remains incompletely understood. ... Cultivars of bread wheat express ...
Metrics. Advances in genomics have expedited the improvement of several agriculturally important crops but similar efforts in wheat ( Triticum spp.) have been more challenging. This is largely ...
Wheat contributes to 50% and 30% of the global grain trade and production respectively [ 2 ]. Wheat is also known as a staple food in more than 40 countries of the world. Wheat provides 82% of basic calories and 85% of proteins to the world population [ 3, 4 ]. Wheat-based food is rich in fiber contents than meat-based food.
Trends of innovation in bread and bakery production. January 2021. DOI: 10.1016/B978--12-821048-2.00007-6. In book: Trends in Wheat and Bread Making 1st Edition Editor: Charis Galanakis Published ...
A better understanding of the responsiveness of grain phenotypic indices to terminal water stress (TWS) in wheat might help explain grain weight variations and determine which grain traits are most affected. A two-year field experiment (2020-2021 and 2021-2022) was conducted to identify how TWS and exogenous cytokinin application might affect grain weight and grain dimensions in three ...
Different research papers, journals and other national and international resource were used during the review process including agricultural books, thesis, reports and other important scientific resources. ... According to the present review the bread wheat grain physical qualities, nutritional and technological properties is important quality ...
Wheat has played a fundamental role in human. civilization and improved food security at the global and regional le vels. It provides. about 19% of the calories and 21% of protein needs of daily ...
A half cup of oats has about four grams. Using the "Oatzempic" shake to lose weight is also likely unsustainable, Dr. Jay said. "If you go back to what you were eating before, you'll gain ...
Abstract. Wheat (Triticum aestivum L) is the most extensively grown cereal crop in the world, covering about 237 million hectares annually, accounting for a total of 420 million tonnes (Isitor et ...