Learn how UpToDate can help you.
Select the option that best describes you
- Medical Professional
- Resident, Fellow, or Student
- Hospital or Institution
- Group Practice
- Patient or Caregiver
- Find in topic

RELATED PATHWAYS
Related topics.
INTRODUCTION
This topic will review the clinical presentation, diagnosis, and initial evaluation of diabetes in nonpregnant adults. Screening for and prevention of diabetes, the etiologic classification of diabetes mellitus, the treatment of diabetes, as well as diabetes during pregnancy are discussed separately.
● (See "Screening for type 2 diabetes mellitus" .)
● (See "Prevention of type 2 diabetes mellitus" and "Type 1 diabetes mellitus: Prevention and disease-modifying therapy" .)
● (See "Classification of diabetes mellitus and genetic diabetic syndromes" .)

- Previous Article
- Next Article
Classification
Diagnostic tests for diabetes, type 1 diabetes, prediabetes and type 2 diabetes, cystic fibrosis–related diabetes, posttransplantation diabetes mellitus, monogenic diabetes syndromes, pancreatic diabetes or diabetes in the context of disease of the exocrine pancreas, gestational diabetes mellitus, 2. classification and diagnosis of diabetes: standards of medical care in diabetes—2021.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Cite Icon Cite
- Get Permissions
American Diabetes Association; 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021 . Diabetes Care 1 January 2021; 44 (Supplement_1): S15–S33. https://doi.org/10.2337/dc21-S002
Download citation file:
- Ris (Zotero)
- Reference Manager
The American Diabetes Association (ADA) “Standards of Medical Care in Diabetes” includes the ADA's current clinical practice recommendations and is intended to provide the components of diabetes care, general treatment goals and guidelines, and tools to evaluate quality of care. Members of the ADA Professional Practice Committee, a multidisciplinary expert committee ( https://doi.org/10.2337/dc21-SPPC ), are responsible for updating the Standards of Care annually, or more frequently as warranted. For a detailed description of ADA standards, statements, and reports, as well as the evidence-grading system for ADA's clinical practice recommendations, please refer to the Standards of Care Introduction ( https://doi.org/10.2337/dc21-SINT ). Readers who wish to comment on the Standards of Care are invited to do so at professional.diabetes.org/SOC .
Diabetes can be classified into the following general categories:
Type 1 diabetes (due to autoimmune β-cell destruction, usually leading to absolute insulin deficiency, including latent autoimmune diabetes of adulthood)
Type 2 diabetes (due to a progressive loss of adequate β-cell insulin secretion frequently on the background of insulin resistance)
Specific types of diabetes due to other causes, e.g., monogenic diabetes syndromes (such as neonatal diabetes and maturity-onset diabetes of the young), diseases of the exocrine pancreas (such as cystic fibrosis and pancreatitis), and drug- or chemical-induced diabetes (such as with glucocorticoid use, in the treatment of HIV/AIDS, or after organ transplantation)
Gestational diabetes mellitus (diabetes diagnosed in the second or third trimester of pregnancy that was not clearly overt diabetes prior to gestation)
This section reviews most common forms of diabetes but is not comprehensive. For additional information, see the American Diabetes Association (ADA) position statement “Diagnosis and Classification of Diabetes Mellitus” ( 1 ).
Type 1 diabetes and type 2 diabetes are heterogeneous diseases in which clinical presentation and disease progression may vary considerably. Classification is important for determining therapy, but some individuals cannot be clearly classified as having type 1 or type 2 diabetes at the time of diagnosis. The traditional paradigms of type 2 diabetes occurring only in adults and type 1 diabetes only in children are no longer accurate, as both diseases occur in both age-groups. Children with type 1 diabetes typically present with the hallmark symptoms of polyuria/polydipsia, and approximately one-third present with diabetic ketoacidosis (DKA) ( 2 ). The onset of type 1 diabetes may be more variable in adults; they may not present with the classic symptoms seen in children and may experience temporary remission from the need for insulin ( 3 – 5 ). Occasionally, patients with type 2 diabetes may present with DKA ( 6 ), particularly ethnic and racial minorities ( 7 ). It is important for the provider to realize that classification of diabetes type is not always straightforward at presentation and that misdiagnosis is common (e.g., adults with type 1 diabetes misdiagnosed as having type 2 diabetes; individuals with maturity-onset diabetes of the young [MODY] misdiagnosed as having type 1 diabetes, etc.). Although difficulties in distinguishing diabetes type may occur in all age-groups at onset, the diagnosis becomes more obvious over time in people with β-cell deficiency.
In both type 1 and type 2 diabetes, various genetic and environmental factors can result in the progressive loss of β-cell mass and/or function that manifests clinically as hyperglycemia. Once hyperglycemia occurs, patients with all forms of diabetes are at risk for developing the same chronic complications, although rates of progression may differ. The identification of individualized therapies for diabetes in the future will require better characterization of the many paths to β-cell demise or dysfunction ( 8 ). Across the globe many groups are working on combining clinical, pathophysiological, and genetic characteristics to more precisely define the subsets of diabetes currently clustered into the type 1 diabetes versus type 2 diabetes nomenclature with the goal of optimizing treatment approaches. Many of these studies show great promise and may soon be incorporated into the diabetes classification system ( 9 ).
Characterization of the underlying pathophysiology is more precisely developed in type 1 diabetes than in type 2 diabetes. It is now clear from studies of first-degree relatives of patients with type 1 diabetes that the persistent presence of two or more islet autoantibodies is a near certain predictor of clinical hyperglycemia and diabetes. The rate of progression is dependent on the age at first detection of autoantibody, number of autoantibodies, autoantibody specificity, and autoantibody titer. Glucose and A1C levels rise well before the clinical onset of diabetes, making diagnosis feasible well before the onset of DKA. Three distinct stages of type 1 diabetes can be identified ( Table 2.1 ) and serve as a framework for future research and regulatory decision-making ( 8 , 10 ). There is debate as to whether slowly progressive autoimmune diabetes with an adult onset should be termed latent autoimmune diabetes in adults (LADA) or type 1 diabetes. The clinical priority is awareness that slow autoimmune β-cell destruction can occur in adults leading to a long duration of marginal insulin secretory capacity. For the purpose of this classification, all forms of diabetes mediated by autoimmune β-cell destruction are included under the rubric of type 1 diabetes. Use of the term LADA is common and acceptable in clinical practice and has the practical impact of heightening awareness of a population of adults likely to develop overt autoimmune β-cell destruction ( 11 ), thus accelerating insulin initiation prior to deterioration of glucose control or development of DKA ( 4 , 12 ).
Staging of type 1 diabetes ( 8 , 10 )
FPG, fasting plasma glucose; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; 2-h PG, 2-h plasma glucose.
The paths to β-cell demise and dysfunction are less well defined in type 2 diabetes, but deficient β-cell insulin secretion, frequently in the setting of insulin resistance, appears to be the common denominator. Type 2 diabetes is associated with insulin secretory defects related to inflammation and metabolic stress among other contributors, including genetic factors. Future classification schemes for diabetes will likely focus on the pathophysiology of the underlying β-cell dysfunction ( 8 , 9 , 13 – 15 ).
Diabetes may be diagnosed based on plasma glucose criteria, either the fasting plasma glucose (FPG) value or the 2-h plasma glucose (2-h PG) value during a 75-g oral glucose tolerance test (OGTT), or A1C criteria ( 16 ) ( Table 2.2 ).
Criteria for the diagnosis of diabetes
DCCT, Diabetes Control and Complications Trial; FPG, fasting plasma glucose; OGTT, oral glucose tolerance test; WHO, World Health Organization; 2-h PG, 2-h plasma glucose.
In the absence of unequivocal hyperglycemia, diagnosis requires two abnormal test results from the same sample or in two separate test samples.
Generally, FPG, 2-h PG during 75-g OGTT, and A1C are equally appropriate for diagnostic screening. It should be noted that the tests do not necessarily detect diabetes in the same individuals. The efficacy of interventions for primary prevention of type 2 diabetes ( 17 , 18 ) has mainly been demonstrated among individuals who have impaired glucose tolerance (IGT) with or without elevated fasting glucose, not for individuals with isolated impaired fasting glucose (IFG) or for those with prediabetes defined by A1C criteria.
The same tests may be used to screen for and diagnose diabetes and to detect individuals with prediabetes ( Table 2.2 and Table 2.5 ) ( 19 ). Diabetes may be identified anywhere along the spectrum of clinical scenarios—in seemingly low-risk individuals who happen to have glucose testing, in individuals tested based on diabetes risk assessment, and in symptomatic patients.
Fasting and 2-Hour Plasma Glucose
The FPG and 2-h PG may be used to diagnose diabetes ( Table 2.2 ). The concordance between the FPG and 2-h PG tests is imperfect, as is the concordance between A1C and either glucose-based test. Compared with FPG and A1C cut points, the 2-h PG value diagnoses more people with prediabetes and diabetes ( 20 ). In people in whom there is discordance between A1C values and glucose values, FPG and 2-h PG are more accurate ( 21 ).
Recommendations
2.1 To avoid misdiagnosis or missed diagnosis, the A1C test should be performed using a method that is certified by the NGSP and standardized to the Diabetes Control and Complications Trial (DCCT) assay. B
2.2 Marked discordance between measured A1C and plasma glucose levels should raise the possibility of A1C assay interference and consideration of using an assay without interference or plasma blood glucose criteria to diagnose diabetes. B
2.3 In conditions associated with an altered relationship between A1C and glycemia, such as hemoglobinopathies including sickle cell disease, pregnancy (second and third trimesters and the postpartum period), glucose-6-phosphate dehydrogenase deficiency, HIV, hemodialysis, recent blood loss or transfusion, or erythropoietin therapy, only plasma blood glucose criteria should be used to diagnose diabetes. (See other conditions altering the relationship of a1c and glycemia below for more information.) B
The A1C test should be performed using a method that is certified by the NGSP ( www.ngsp.org ) and standardized or traceable to the Diabetes Control and Complications Trial (DCCT) reference assay. Although point-of-care A1C assays may be NGSP certified and cleared by the U.S. Food and Drug Administration (FDA) for use in monitoring glycemic control in people with diabetes in both Clinical Laboratory Improvement Amendments (CLIA)-regulated and CLIA-waived settings, only those point-of-care A1C assays that are also cleared by the FDA for use in the diagnosis of diabetes should be used for this purpose, and only in the clinical settings for which they are cleared. As discussed in Section 6 “Glycemic Targets” ( https://doi.org/10.2337/dc21-S006 ), point-of-care A1C assays may be more generally applied for assessment of glycemic control in the clinic.
A1C has several advantages compared with FPG and OGTT, including greater convenience (fasting not required), greater preanalytical stability, and less day-to-day perturbations during stress, changes in diet, or illness. However, these advantages may be offset by the lower sensitivity of A1C at the designated cut point, greater cost, limited availability of A1C testing in certain regions of the developing world, and the imperfect correlation between A1C and average glucose in certain individuals. The A1C test, with a diagnostic threshold of ≥6.5% (48 mmol/mol), diagnoses only 30% of the diabetes cases identified collectively using A1C, FPG, or 2-h PG, according to National Health and Nutrition Examination Survey (NHANES) data ( 22 ).
When using A1C to diagnose diabetes, it is important to recognize that A1C is an indirect measure of average blood glucose levels and to take other factors into consideration that may impact hemoglobin glycation independently of glycemia, such as hemodialysis, pregnancy, HIV treatment ( 23 , 24 ), age, race/ethnicity, pregnancy status, genetic background, and anemia/hemoglobinopathies. (See other conditions altering the relationship of a1c and glycemia below for more information.)
The epidemiologic studies that formed the basis for recommending A1C to diagnose diabetes included only adult populations ( 22 ). However, recent ADA clinical guidance concluded that A1C, FPG, or 2-h PG can be used to test for prediabetes or type 2 diabetes in children and adolescents (see screening and testing for prediabetes and type 2 diabetes in children and adolescents below for additional information) ( 25 ).
Race/Ethnicity/Hemoglobinopathies
Hemoglobin variants can interfere with the measurement of A1C, although most assays in use in the U.S. are unaffected by the most common variants. Marked discrepancies between measured A1C and plasma glucose levels should prompt consideration that the A1C assay may not be reliable for that individual. For patients with a hemoglobin variant but normal red blood cell turnover, such as those with the sickle cell trait, an A1C assay without interference from hemoglobin variants should be used. An updated list of A1C assays with interferences is available at www.ngsp.org/interf.asp .
African Americans heterozygous for the common hemoglobin variant HbS may have, for any given level of mean glycemia, lower A1C by about 0.3% compared with those without the trait ( 26 ). Another genetic variant, X-linked glucose-6-phosphate dehydrogenase G202A, carried by 11% of African Americans, was associated with a decrease in A1C of about 0.8% in homozygous men and 0.7% in homozygous women compared with those without the variant ( 27 ).
Even in the absence of hemoglobin variants, A1C levels may vary with race/ethnicity independently of glycemia ( 28 – 30 ). For example, African Americans may have higher A1C levels than non-Hispanic Whites with similar fasting and postglucose load glucose levels ( 31 ). Though conflicting data exists, African Americans may also have higher levels of fructosamine and glycated albumin and lower levels of 1,5-anhydroglucitol, suggesting that their glycemic burden (particularly postprandially) may be higher ( 32 , 33 ). Similarly, A1C levels may be higher for a given mean glucose concentration when measured with continuous glucose monitoring ( 34 ). Despite these and other reported differences, the association of A1C with risk for complications appears to be similar in African Americans and non-Hispanic Whites ( 35 , 36 ).
Other Conditions Altering the Relationship of A1C and Glycemia
In conditions associated with increased red blood cell turnover, such as sickle cell disease, pregnancy (second and third trimesters), glucose-6-phosphate dehydrogenase deficiency ( 37 , 38 ), hemodialysis, recent blood loss or transfusion, or erythropoietin therapy, only plasma blood glucose criteria should be used to diagnose diabetes ( 39 ). A1C is less reliable than blood glucose measurement in other conditions such as the postpartum state ( 40 – 42 ), HIV treated with certain protease inhibitors (PIs) and nucleoside reverse transcriptase inhibitors (NRTIs) ( 23 ), and iron-deficient anemia ( 43 ).
Confirming the Diagnosis
Unless there is a clear clinical diagnosis (e.g., patient in a hyperglycemic crisis or with classic symptoms of hyperglycemia and a random plasma glucose ≥200 mg/dL [11.1 mmol/L]), diagnosis requires two abnormal test results, either from the same sample ( 44 ) or in two separate test samples. If using two separate test samples, it is recommended that the second test, which may either be a repeat of the initial test or a different test, be performed without delay. For example, if the A1C is 7.0% (53 mmol/mol) and a repeat result is 6.8% (51 mmol/mol), the diagnosis of diabetes is confirmed. If two different tests (such as A1C and FPG) are both above the diagnostic threshold when analyzed from the same sample or in two different test samples, this also confirms the diagnosis. On the other hand, if a patient has discordant results from two different tests, then the test result that is above the diagnostic cut point should be repeated, with careful consideration of the possibility of A1C assay interference. The diagnosis is made on the basis of the confirmed test. For example, if a patient meets the diabetes criterion of the A1C (two results ≥6.5% [48 mmol/mol]) but not FPG (<126 mg/dL [7.0 mmol/L]), that person should nevertheless be considered to have diabetes.
Each of the tests has preanalytic and analytic variability, so it is possible that a test yielding an abnormal result (i.e., above the diagnostic threshold), when repeated, will produce a value below the diagnostic cut point. This scenario is likely for FPG and 2-h PG if the glucose samples remain at room temperature and are not centrifuged promptly. Because of the potential for preanalytic variability, it is critical that samples for plasma glucose be spun and separated immediately after they are drawn. If patients have test results near the margins of the diagnostic threshold, the health care professional should discuss signs and symptoms with the patient and repeat the test in 3 – 6 months.
In a patient with classic symptoms, measurement of plasma glucose is sufficient to diagnose diabetes (symptoms of hyperglycemia or hyperglycemic crisis plus a random plasma glucose ≥200 mg/dL [11.1 mmol/L]). In these cases, knowing the plasma glucose level is critical because, in addition to confirming that symptoms are due to diabetes, it will inform management decisions. Some providers may also want to know the A1C to determine the chronicity of the hyperglycemia. The criteria to diagnose diabetes are listed in Table 2.2 .
2.4 Screening for type 1 diabetes risk with a panel of islet autoantibodies is currently recommended in the setting of a research trial or can be offered as an option for first-degree family members of a proband with type 1 diabetes. B
2.5 Persistence of autoantibodies is a risk factor for clinical diabetes and may serve as an indication for intervention in the setting of a clinical trial. B
Immune-Mediated Diabetes
This form, previously called “insulin-dependent diabetes” or “juvenile-onset diabetes,” accounts for 5 – 10% of diabetes and is due to cellular-mediated autoimmune destruction of the pancreatic β-cells. Autoimmune markers include islet cell autoantibodies and autoantibodies to GAD (GAD65), insulin, the tyrosine phosphatases IA-2 and IA-2β, and zinc transporter 8 (ZnT8). Numerous clinical studies are being conducted to test various methods of preventing type 1 diabetes in those with evidence of islet autoimmunity ( www.clinicaltrials.gov and www.trialnet.org/our-research/prevention-studies ) ( 12 , 45 – 49 ). Stage 1 of type 1 diabetes is defined by the presence of two or more of these autoimmune markers. The disease has strong HLA associations, with linkage to the DQA and DQB genes. These HLA-DR/DQ alleles can be either predisposing or protective ( Table 2.1 ). There are important genetic considerations, as most of the mutations that cause diabetes are dominantly inherited. The importance of genetic testing is in the genetic counseling that follows. Some mutations are associated with other conditions, which then may prompt additional screenings.
The rate of β-cell destruction is quite variable, being rapid in some individuals (mainly infants and children) and slow in others (mainly adults) ( 50 ). Children and adolescents may present with DKA as the first manifestation of the disease. Others have modest fasting hyperglycemia that can rapidly change to severe hyperglycemia and/or DKA with infection or other stress. Adults may retain sufficient β-cell function to prevent DKA for many years; such individuals may have remission or decreased insulin needs for months or years and eventually become dependent on insulin for survival and are at risk for DKA ( 3 – 5 , 51 , 52 ). At this latter stage of the disease, there is little or no insulin secretion, as manifested by low or undetectable levels of plasma C-peptide. Immune-mediated diabetes is the most common form of diabetes in childhood and adolescence, but it can occur at any age, even in the 8th and 9th decades of life.
Autoimmune destruction of β-cells has multiple genetic predispositions and is also related to environmental factors that are still poorly defined. Although patients are not typically obese when they present with type 1 diabetes, obesity is increasingly common in the general population, and there is evidence that it may also be a risk factor for type 1 diabetes. As such, obesity should not preclude the diagnosis. People with type 1 diabetes are also prone to other autoimmune disorders such as Hashimoto thyroiditis, Graves disease, celiac disease, Addison disease, vitiligo, autoimmune hepatitis, myasthenia gravis, and pernicious anemia (see Section 4 “Comprehensive Medical Evaluation and Assessment of Comorbidities,” https://doi.org/10.2337/dc21-S004 ).
Idiopathic Type 1 Diabetes
Some forms of type 1 diabetes have no known etiologies. These patients have permanent insulinopenia and are prone to DKA but have no evidence of β-cell autoimmunity. However, only a minority of patients with type 1 diabetes fall into this category. Individuals with autoantibody-negative type 1 diabetes of African or Asian ancestry may suffer from episodic DKA and exhibit varying degrees of insulin deficiency between episodes (possibly ketosis-prone diabetes). This form of diabetes is strongly inherited and is not HLA associated. An absolute requirement for insulin replacement therapy in affected patients may be intermittent. Future research is needed to determine the cause of β-cell destruction in this rare clinical scenario.
Screening for Type 1 Diabetes Risk
The incidence and prevalence of type 1 diabetes is increasing ( 53 ). Patients with type 1 diabetes often present with acute symptoms of diabetes and markedly elevated blood glucose levels, and approximately one-third are diagnosed with life-threatening DKA ( 2 ). Multiple studies indicate that measuring islet autoantibodies in individuals genetically at risk for type 1 diabetes (e.g., relatives of those with type 1 diabetes or individuals from the general population with type 1 diabetes–associated genetic factors) identifies individuals who may develop type 1 diabetes ( 10 ). Such testing, coupled with education about diabetes symptoms and close follow-up, may enable earlier identification of type 1 diabetes onset. A study reported the risk of progression to type 1 diabetes from the time of seroconversion to autoantibody positivity in three pediatric cohorts from Finland, Germany, and the U.S. Of the 585 children who developed more than two autoantibodies, nearly 70% developed type 1 diabetes within 10 years and 84% within 15 years ( 45 ). These findings are highly significant because while the German group was recruited from offspring of parents with type 1 diabetes, the Finnish and American groups were recruited from the general population. Remarkably, the findings in all three groups were the same, suggesting that the same sequence of events led to clinical disease in both “sporadic” and familial cases of type 1 diabetes. Indeed, the risk of type 1 diabetes increases as the number of relevant autoantibodies detected increases ( 48 , 54 , 55 ). In The Environmental Determinants of Diabetes in the Young (TEDDY) study, type 1 diabetes developed in 21% of 363 subjects with at least one autoantibody at 3 years of age ( 56 ).
There is currently a lack of accepted and clinically validated screening programs outside of the research setting; thus, widespread clinical testing of asymptomatic low-risk individuals is not currently recommended due to lack of approved therapeutic interventions. However, one should consider referring relatives of those with type 1 diabetes for islet autoantibody testing for risk assessment in the setting of a clinical research study (see www.trialnet.org ). Individuals who test positive should be counseled about the risk of developing diabetes, diabetes symptoms, and DKA prevention. Numerous clinical studies are being conducted to test various methods of preventing and treating stage 2 type 1 diabetes in those with evidence of autoimmunity with promising results (see www.clinicaltrials.gov and www.trialnet.org ).
2.6 Screening for prediabetes and type 2 diabetes with an informal assessment of risk factors or validated tools should be considered in asymptomatic adults. B
2.7 Testing for prediabetes and/or type 2 diabetes in asymptomatic people should be considered in adults of any age with overweight or obesity (BMI ≥25 kg/m 2 or ≥23 kg/m 2 in Asian Americans) and who have one or more additional risk factors for diabetes ( Table 2.3 ). B
2.8 Testing for prediabetes and/or type 2 diabetes should be considered in women with overweight or obesity planning pregnancy and/or who have one or more additional risk factor for diabetes ( Table 2.3 ). C
2.9 For all people, testing should begin at age 45 years. B
2.10 If tests are normal, repeat testing carried out at a minimum of 3-year intervals is reasonable, sooner with symptoms. C
2.11 To test for prediabetes and type 2 diabetes, fasting plasma glucose, 2-h plasma glucose during 75-g oral glucose tolerance test, and A1C are equally appropriate ( Table 2.2 and Table 2.5 ). B
2.12 In patients with prediabetes and type 2 diabetes, identify and treat other cardiovascular disease risk factors. A
2.13 Risk-based screening for prediabetes and/or type 2 diabetes should be considered after the onset of puberty or after 10 years of age, whichever occurs earlier, in children and adolescents with overweight (BMI ≥85th percentile) or obesity (BMI ≥95th percentile) and who have one or more risk factor for diabetes. (See Table 2.4 for evidence grading of risk factors.) B
2.14 Patients with HIV should be screened for diabetes and prediabetes with a fasting glucose test before starting antiretroviral therapy, at the time of switching antiretroviral therapy, and 3−6 months after starting or switching antiretroviral therapy. If initial screening results are normal, fasting glucose should be checked annually. E
Criteria for testing for diabetes or prediabetes in asymptomatic adults
CVD, cardiovascular disease; GDM, gestational diabetes mellitus; IFG, impaired fasting glucose; IGT, impaired glucose tolerance.
Risk-based screening for type 2 diabetes or prediabetes in asymptomatic children and adolescents in a clinical setting ( 202 )
GDM, gestational diabetes mellitus.
After the onset of puberty or after 10 years of age, whichever occurs earlier. If tests are normal, repeat testing at a minimum of 3-year intervals (or more frequently if BMI is increasing or risk factor profile deteriorating) is recommended. Reports of type 2 diabetes before age 10 years exist, and this can be considered with numerous risk factors.
Prediabetes
“Prediabetes” is the term used for individuals whose glucose levels do not meet the criteria for diabetes but are too high to be considered normal ( 35 , 36 ). Patients with prediabetes are defined by the presence of IFG and/or IGT and/or A1C 5.7 – 6.4% (39 – 47 mmol/mol) ( Table 2.5 ). Prediabetes should not be viewed as a clinical entity in its own right but rather as an increased risk for diabetes and cardiovascular disease (CVD). Criteria for testing for diabetes or prediabetes in asymptomatic adults is outlined in Table 2.3 . Prediabetes is associated with obesity (especially abdominal or visceral obesity), dyslipidemia with high triglycerides and/or low HDL cholesterol, and hypertension.
Criteria defining prediabetes *
FPG, fasting plasma glucose; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; OGTT, oral glucose tolerance test; 2-h PG, 2-h plasma glucose.
For all three tests, risk is continuous, extending below the lower limit of the range and becoming disproportionately greater at the higher end of the range.
IFG is defined as FPG levels from 100 to 125 mg/dL (from 5.6 to 6.9 mmol/L) ( 57 , 58 ) and IGT as 2-h PG during 75-g OGTT levels from 140 to 199 mg/dL (from 7.8 to 11.0 mmol/L) ( 59 ). It should be noted that the World Health Organization (WHO) and numerous other diabetes organizations define the IFG cutoff at 110 mg/dL (6.1 mmol/L).
As with the glucose measures, several prospective studies that used A1C to predict the progression to diabetes as defined by A1C criteria demonstrated a strong, continuous association between A1C and subsequent diabetes. In a systematic review of 44,203 individuals from 16 cohort studies with a follow-up interval averaging 5.6 years (range 2.8 – 12 years), those with A1C between 5.5% and 6.0% (between 37 and 42 mmol/mol) had a substantially increased risk of diabetes (5-year incidence from 9% to 25%). Those with an A1C range of 6.0–6.5% (42 – 48 mmol/mol) had a 5-year risk of developing diabetes between 25% and 50% and a relative risk 20 times higher compared with A1C of 5.0% (31 mmol/mol) ( 60 ). In a community-based study of African American and non-Hispanic White adults without diabetes, baseline A1C was a stronger predictor of subsequent diabetes and cardiovascular events than fasting glucose ( 61 ). Other analyses suggest that A1C of 5.7% (39 mmol/mol) or higher is associated with a diabetes risk similar to that of the high-risk participants in the Diabetes Prevention Program (DPP) ( 62 ), and A1C at baseline was a strong predictor of the development of glucose-defined diabetes during the DPP and its follow-up ( 63 ). Hence, it is reasonable to consider an A1C range of 5.7 – 6.4% (39 – 47 mmol/mol) as identifying individuals with prediabetes. Similar to those with IFG and/or IGT, individuals with A1C of 5.7 – 6.4% (39 – 47 mmol/mol) should be informed of their increased risk for diabetes and CVD and counseled about effective strategies to lower their risks (see Section 3 “Prevention or Delay of Type 2 Diabetes,” https://doi.org/10.2337/dc21-S003 ). Similar to glucose measurements, the continuum of risk is curvilinear, so as A1C rises, the diabetes risk rises disproportionately ( 60 ). Aggressive interventions and vigilant follow-up should be pursued for those considered at very high risk (e.g., those with A1C >6.0% [42 mmol/mol]).
Table 2.5 summarizes the categories of prediabetes and Table 2.3 the criteria for prediabetes testing. The ADA diabetes risk test is an additional option for assessment to determine the appropriateness of testing for diabetes or prediabetes in asymptomatic adults ( Fig. 2.1 ) ( diabetes.org/socrisktest ). For additional background regarding risk factors and screening for prediabetes, see screening and testing for prediabetes and type 2 diabetes in asymptomatic adults and also screening and testing for prediabetes and type 2 diabetes in children and adolescents below.

ADA risk test ( diabetes.org/socrisktest ).
Type 2 Diabetes
Type 2 diabetes, previously referred to as “noninsulin-dependent diabetes” or “adult-onset diabetes,” accounts for 90 – 95% of all diabetes. This form encompasses individuals who have relative (rather than absolute) insulin deficiency and have peripheral insulin resistance. At least initially, and often throughout their lifetime, these individuals may not need insulin treatment to survive.
There are various causes of type 2 diabetes. Although the specific etiologies are not known, autoimmune destruction of β-cells does not occur, and patients do not have any of the other known causes of diabetes. Most, but not all, patients with type 2 diabetes have overweight or obesity. Excess weight itself causes some degree of insulin resistance. Patients who do not have obesity or overweight by traditional weight criteria may have an increased percentage of body fat distributed predominantly in the abdominal region.
DKA seldom occurs spontaneously in type 2 diabetes; when seen, it usually arises in association with the stress of another illness such as infection, myocardial infarction, or with the use of certain drugs (e.g., corticosteroids, atypical antipsychotics, and sodium–glucose cotransporter 2 inhibitors) ( 64 , 65 ). Type 2 diabetes frequently goes undiagnosed for many years because hyperglycemia develops gradually and, at earlier stages, is often not severe enough for the patient to notice the classic diabetes symptoms caused by hyperglycemia. Nevertheless, even undiagnosed patients are at increased risk of developing macrovascular and microvascular complications.
Patients with type 2 diabetes may have insulin levels that appear normal or elevated, yet the failure to normalize blood glucose reflects a relative defect in glucose-stimulated insulin secretion. Thus, insulin secretion is defective in these patients and insufficient to compensate for insulin resistance. Insulin resistance may improve with weight reduction, exercise, and/or pharmacologic treatment of hyperglycemia but is seldom restored to normal. Recent interventions with intensive diet and exercise or surgical weight loss have led to diabetes remission ( 66 – 72 ) (see Section 8 “Obesity Management for the Treatment of Type 2 Diabetes,” https://doi.org/10.2337/dc21-S008 ).
The risk of developing type 2 diabetes increases with age, obesity, and lack of physical activity. It occurs more frequently in women with prior gestational diabetes mellitus (GDM), with hypertension or dyslipidemia, with polycystic ovary syndrome, and in certain racial/ethnic subgroups (African American, American Indian, Hispanic/Latino, and Asian American). It is often associated with a strong genetic predisposition or family history in first-degree relatives (more so than type 1 diabetes). However, the genetics of type 2 diabetes is poorly understood and under intense investigation in this era of precision medicine ( 13 ). In adults without traditional risk factors for type 2 diabetes and/or younger age, consider islet autoantibody testing (e.g., GAD65 autoantibodies) to exclude the diagnosis of type 1 diabetes.
Screening and Testing for Prediabetes and Type 2 Diabetes in Asymptomatic Adults
Screening for prediabetes and type 2 diabetes risk through an informal assessment of risk factors ( Table 2.3 ) or with an assessment tool, such as the ADA risk test ( Fig. 2.1 ) (online at diabetes.org/socrisktest ), is recommended to guide providers on whether performing a diagnostic test ( Table 2.2 ) is appropriate. Prediabetes and type 2 diabetes meet criteria for conditions in which early detection via screening is appropriate. Both conditions are common and impose significant clinical and public health burdens. There is often a long presymptomatic phase before the diagnosis of type 2 diabetes. Simple tests to detect preclinical disease are readily available. The duration of glycemic burden is a strong predictor of adverse outcomes. There are effective interventions that prevent progression from prediabetes to diabetes (see Section 3 “Prevention or Delay of Type 2 Diabetes,” https://doi.org/10.2337/dc21-S003 ) and reduce the risk of diabetes complications ( 73 ) (see Section 10 “Cardiovascular Disease and Risk Management,” https://doi.org/10.2337/dc21-S010 , and Section 11 “Microvascular Complications and Foot Care,” https://doi.org/10.2337/dc21-S011 ). In the most recent National Institutes of Health (NIH) Diabetes Prevention Program Outcomes Study (DPPOS) report, prevention of progression from prediabetes to diabetes ( 74 ) resulted in lower rates of developing retinopathy and nephropathy ( 75 ). Similar impact on diabetes complications was reported with screening, diagnosis, and comprehensive risk factor management in the U.K. Clinical Practice Research Datalink database ( 73 ). In that report, progression from prediabetes to diabetes augmented risk of complications.
Approximately one-quarter of people with diabetes in the U.S. and nearly half of Asian and Hispanic Americans with diabetes are undiagnosed ( 57 , 58 ). Although screening of asymptomatic individuals to identify those with prediabetes or diabetes might seem reasonable, rigorous clinical trials to prove the effectiveness of such screening have not been conducted and are unlikely to occur. Based on a population estimate, diabetes in women of childbearing age is underdiagnosed ( 76 ). Employing a probabilistic model, Peterson et al. ( 77 ) demonstrated cost and health benefits of preconception screening.
A large European randomized controlled trial compared the impact of screening for diabetes and intensive multifactorial intervention with that of screening and routine care ( 78 ). General practice patients between the ages of 40 and 69 years were screened for diabetes and randomly assigned by practice to intensive treatment of multiple risk factors or routine diabetes care. After 5.3 years of follow-up, CVD risk factors were modestly but significantly improved with intensive treatment compared with routine care, but the incidence of first CVD events or mortality was not significantly different between the groups ( 59 ). The excellent care provided to patients in the routine care group and the lack of an unscreened control arm limited the authors' ability to determine whether screening and early treatment improved outcomes compared with no screening and later treatment after clinical diagnoses. Computer simulation modeling studies suggest that major benefits are likely to accrue from the early diagnosis and treatment of hyperglycemia and cardiovascular risk factors in type 2 diabetes ( 79 ); moreover, screening, beginning at age 30 or 45 years and independent of risk factors, may be cost-effective (<$11,000 per quality-adjusted life year gained—2010 modeling data) ( 80 ). Cost-effectiveness of screening has been reinforced in cohort studies ( 81 , 82 ).
Additional considerations regarding testing for type 2 diabetes and prediabetes in asymptomatic patients include the following.
Age is a major risk factor for diabetes. Testing should begin at no later than age 45 years for all patients. Screening should be considered in adults of any age with overweight or obesity and one or more risk factors for diabetes.
BMI and Ethnicity
In general, BMI ≥25 kg/m 2 is a risk factor for diabetes. However, data suggest that the BMI cut point should be lower for the Asian American population ( 83 , 84 ). The BMI cut points fall consistently between 23 and 24 kg/m 2 (sensitivity of 80%) for nearly all Asian American subgroups (with levels slightly lower for Japanese Americans). This makes a rounded cut point of 23 kg/m 2 practical. An argument can be made to push the BMI cut point to lower than 23 kg/m 2 in favor of increased sensitivity; however, this would lead to an unacceptably low specificity (13.1%). Data from WHO also suggests that a BMI of ≥23 kg/m 2 should be used to define increased risk in Asian Americans ( 85 ). The finding that one-third to one-half of diabetes in Asian Americans is undiagnosed suggests that testing is not occurring at lower BMI thresholds ( 86 , 87 ).
Evidence also suggests that other populations may benefit from lower BMI cut points. For example, in a large multiethnic cohort study, for an equivalent incidence rate of diabetes, a BMI of 30 kg/m 2 in non-Hispanic Whites was equivalent to a BMI of 26 kg/m 2 in African Americans ( 88 ).
Medications
Certain medications, such as glucocorticoids, thiazide diuretics, some HIV medications ( 23 ), and atypical antipsychotics ( 66 ), are known to increase the risk of diabetes and should be considered when deciding whether to screen.
Individuals with HIV are at higher risk for developing prediabetes and diabetes on antiretroviral (ARV) therapies, so a screening protocol is recommended ( 89 ). The A1C test may underestimate glycemia in people with HIV; it is not recommended for diagnosis and may present challenges for monitoring ( 24 ). In those with prediabetes, weight loss through healthy nutrition and physical activity may reduce the progression toward diabetes. Among patients with HIV and diabetes, preventive health care using an approach used in patients without HIV is critical to reduce the risks of microvascular and macrovascular complications. Diabetes risk is increased with certain PIs and NRTIs. New-onset diabetes is estimated to occur in more than 5% of patients infected with HIV on PIs, whereas more than 15% may have prediabetes ( 90 ). PIs are associated with insulin resistance and may also lead to apoptosis of pancreatic β-cells. NRTIs also affect fat distribution (both lipohypertrophy and lipoatrophy), which is associated with insulin resistance. For patients with HIV and ARV-associated hyperglycemia, it may be appropriate to consider discontinuing the problematic ARV agents if safe and effective alternatives are available ( 91 ). Before making ARV substitutions, carefully consider the possible effect on HIV virological control and the potential adverse effects of new ARV agents. In some cases, antihyperglycemic agents may still be necessary.
Testing Interval
The appropriate interval between screening tests is not known ( 92 ). The rationale for the 3-year interval is that with this interval, the number of false-positive tests that require confirmatory testing will be reduced and individuals with false-negative tests will be retested before substantial time elapses and complications develop ( 92 ). In especially high-risk individuals, particularly with weight gain, shorter intervals between screening may be useful.
Community Screening
Ideally, testing should be carried out within a health care setting because of the need for follow-up and treatment. Community screening outside a health care setting is generally not recommended because people with positive tests may not seek, or have access to, appropriate follow-up testing and care. However, in specific situations where an adequate referral system is established beforehand for positive tests, community screening may be considered. Community testing may also be poorly targeted; i.e., it may fail to reach the groups most at risk and inappropriately test those at very low risk or even those who have already been diagnosed ( 93 ).
Screening in Dental Practices
Because periodontal disease is associated with diabetes, the utility of screening in a dental setting and referral to primary care as a means to improve the diagnosis of prediabetes and diabetes has been explored ( 94 – 96 ), with one study estimating that 30% of patients ≥30 years of age seen in general dental practices had dysglycemia ( 96 , 97 ). A similar study in 1,150 dental patients >40 years old in India reported 20.69% and 14.60% meeting criteria for prediabetes and diabetes using random blood glucose. Further research is needed to demonstrate the feasibility, effectiveness, and cost-effectiveness of screening in this setting.
Screening and Testing for Prediabetes and Type 2 Diabetes in Children and Adolescents
In the last decade, the incidence and prevalence of type 2 diabetes in children and adolescents has increased dramatically, especially in racial and ethnic minority populations ( 53 ). See Table 2.4 for recommendations on risk-based screening for type 2 diabetes or prediabetes in asymptomatic children and adolescents in a clinical setting ( 25 ). See Table 2.2 and Table 2.5 for the criteria for the diagnosis of diabetes and prediabetes, respectively, which apply to children, adolescents, and adults. See Section 13 “Children and Adolescents” ( https://doi.org/10.2337/dc21-S013 ) for additional information on type 2 diabetes in children and adolescents.
Some studies question the validity of A1C in the pediatric population, especially among certain ethnicities, and suggest OGTT or FPG as more suitable diagnostic tests ( 98 ). However, many of these studies do not recognize that diabetes diagnostic criteria are based on long-term health outcomes, and validations are not currently available in the pediatric population ( 99 ). The ADA acknowledges the limited data supporting A1C for diagnosing type 2 diabetes in children and adolescents. Although A1C is not recommended for diagnosis of diabetes in children with cystic fibrosis or symptoms suggestive of acute onset of type 1 diabetes and only A1C assays without interference are appropriate for children with hemoglobinopathies, the ADA continues to recommend A1C for diagnosis of type 2 diabetes in this cohort to decrease barriers to screening ( 100 , 101 ).
2.15 Annual screening for cystic fibrosis–related diabetes (CFRD) with an oral glucose tolerance test should begin by age 10 years in all patients with cystic fibrosis not previously diagnosed with CFRD. B
2.16 A1C is not recommended as a screening test for cystic fibrosis–related diabetes. B
2.17 Patients with cystic fibrosis–related diabetes should be treated with insulin to attain individualized glycemic goals. A
2.18 Beginning 5 years after the diagnosis of cystic fibrosis–related diabetes, annual monitoring for complications of diabetes is recommended. E
Cystic fibrosis–related diabetes (CFRD) is the most common comorbidity in people with cystic fibrosis, occurring in about 20% of adolescents and 40 – 50% of adults ( 102 ). Diabetes in this population, compared with individuals with type 1 or type 2 diabetes, is associated with worse nutritional status, more severe inflammatory lung disease, and greater mortality. Insulin insufficiency is the primary defect in CFRD. Genetically determined β-cell function and insulin resistance associated with infection and inflammation may also contribute to the development of CFRD. Milder abnormalities of glucose tolerance are even more common and occur at earlier ages than CFRD. Whether individuals with IGT should be treated with insulin replacement has not currently been determined. Although screening for diabetes before the age of 10 years can identify risk for progression to CFRD in those with abnormal glucose tolerance, no benefit has been established with respect to weight, height, BMI, or lung function. OGTT is the recommended screening test; however, recent publications suggest that an A1C cut point threshold of 5.5% (5.8% in a second study) would detect more than 90% of cases and reduce patient screening burden ( 103 , 104 ). Ongoing studies are underway to validate this approach. Regardless of age, weight loss or failure of expected weight gain is a risk for CFRD and should prompt screening ( 103 , 104 ). The Cystic Fibrosis Foundation Patient Registry ( 105 ) evaluated 3,553 cystic fibrosis patients and diagnosed 445 (13%) with CFRD. Early diagnosis and treatment of CFRD was associated with preservation of lung function. The European Cystic Fibrosis Society Patient Registry reported an increase in CFRD with age (increased 10% per decade), genotype, decreased lung function, and female sex ( 106 , 107 ). Continuous glucose monitoring or HOMA of β-cell function ( 108 ) may be more sensitive than OGTT to detect risk for progression to CFRD; however, evidence linking these results to long-term outcomes is lacking, and these tests are not recommended for screening outside of the research setting ( 109 ).
CFRD mortality has significantly decreased over time, and the gap in mortality between cystic fibrosis patients with and without diabetes has considerably narrowed ( 110 ). There are limited clinical trial data on therapy for CFRD. The largest study compared three regimens: premeal insulin aspart, repaglinide, or oral placebo in cystic fibrosis patients with diabetes or abnormal glucose tolerance. Participants all had weight loss in the year preceding treatment; however, in the insulin-treated group, this pattern was reversed, and patients gained 0.39 (± 0.21) BMI units (P = 0.02). The repaglinide-treated group had initial weight gain, but this was not sustained by 6 months. The placebo group continued to lose weight ( 110 ). Insulin remains the most widely used therapy for CFRD ( 111 ). The primary rationale for the use of insulin in patients with CFRD is to induce an anabolic state while promoting macronutrient retention and weight gain.
Additional resources for the clinical management of CFRD can be found in the position statement “Clinical Care Guidelines for Cystic Fibrosis–Related Diabetes: A Position Statement of the American Diabetes Association and a Clinical Practice Guideline of the Cystic Fibrosis Foundation, Endorsed by the Pediatric Endocrine Society” ( 112 ) and in the International Society for Pediatric and Adolescent Diabetes's 2014 clinical practice consensus guidelines ( 102 ).
2.19 Patients should be screened after organ transplantation for hyperglycemia, with a formal diagnosis of posttransplantation diabetes mellitus being best made once a patient is stable on an immunosuppressive regimen and in the absence of an acute infection. B
2.20 The oral glucose tolerance test is the preferred test to make a diagnosis of posttransplantation diabetes mellitus. B
2.21 Immunosuppressive regimens shown to provide the best outcomes for patient and graft survival should be used, irrespective of posttransplantation diabetes mellitus risk. E
Several terms are used in the literature to describe the presence of diabetes following organ transplantation ( 113 ). “New-onset diabetes after transplantation” (NODAT) is one such designation that describes individuals who develop new-onset diabetes following transplant. NODAT excludes patients with pretransplant diabetes that was undiagnosed as well as posttransplant hyperglycemia that resolves by the time of discharge ( 114 ). Another term, “posttransplantation diabetes mellitus” (PTDM) ( 114 , 115 ), describes the presence of diabetes in the posttransplant setting irrespective of the timing of diabetes onset.
Hyperglycemia is very common during the early posttransplant period, with ∼90% of kidney allograft recipients exhibiting hyperglycemia in the first few weeks following transplant ( 114 – 117 ). In most cases, such stress- or steroid-induced hyperglycemia resolves by the time of discharge ( 117 , 118 ). Although the use of immunosuppressive therapies is a major contributor to the development of PTDM, the risks of transplant rejection outweigh the risks of PTDM and the role of the diabetes care provider is to treat hyperglycemia appropriately regardless of the type of immunosuppression ( 114 ). Risk factors for PTDM include both general diabetes risks (such as age, family history of diabetes, etc.) as well as transplant-specific factors, such as use of immunosuppressant agents ( 119 ). Whereas posttransplantation hyperglycemia is an important risk factor for subsequent PTDM, a formal diagnosis of PTDM is optimally made once the patient is stable on maintenance immunosuppression and in the absence of acute infection ( 117 – 120 ). In a recent study of 152 heart transplant recipients, 38% had PTDM at 1 year. Risk factors for PTDM included elevated BMI, discharge from the hospital on insulin, and glucose values in the 24 h prior to hospital discharge ( 121 ). In an Iranian cohort, 19% had PTDM after heart and lung transplant ( 122 ). The OGTT is considered the gold standard test for the diagnosis of PTDM (1 year posttransplant) ( 114 , 115 , 123 , 124 ). However, screening patients using fasting glucose and/or A1C can identify high-risk patients requiring further assessment and may reduce the number of overall OGTTs required.
Few randomized controlled studies have reported on the short- and long-term use of antihyperglycemic agents in the setting of PTDM ( 119 , 125 , 126 ). Most studies have reported that transplant patients with hyperglycemia and PTDM after transplantation have higher rates of rejection, infection, and rehospitalization ( 117 , 119 , 127 ). Insulin therapy is the agent of choice for the management of hyperglycemia, PTDM, and preexisting diabetes and diabetes in the hospital setting. After discharge, patients with preexisting diabetes could go back on their pretransplant regimen if they were in good control before transplantation. Those with previously poor control or with persistent hyperglycemia should continue insulin with frequent home self-monitoring of blood glucose to determine when insulin dose reductions may be needed and when it may be appropriate to switch to noninsulin agents.
No studies to date have established which noninsulin agents are safest or most efficacious in PTDM. The choice of agent is usually made based on the side effect profile of the medication and possible interactions with the patient's immunosuppression regimen ( 119 ). Drug dose adjustments may be required because of decreases in the glomerular filtration rate, a relatively common complication in transplant patients. A small short-term pilot study reported that metformin was safe to use in renal transplant recipients ( 128 ), but its safety has not been determined in other types of organ transplant. Thiazolidinediones have been used successfully in patients with liver and kidney transplants, but side effects include fluid retention, heart failure, and osteopenia ( 129 , 130 ). Dipeptidyl peptidase 4 inhibitors do not interact with immunosuppressant drugs and have demonstrated safety in small clinical trials ( 131 , 132 ). Well-designed intervention trials examining the efficacy and safety of these and other antihyperglycemic agents in patients with PTDM are needed.
2.22 All children diagnosed with diabetes in the first 6 months of life should have immediate genetic testing for neonatal diabetes. A
2.23 Children and those diagnosed in early adulthood who have diabetes not characteristic of type 1 or type 2 diabetes that occurs in successive generations (suggestive of an autosomal dominant pattern of inheritance) should have genetic testing for maturity-onset diabetes of the young. A
2.24 In both instances, consultation with a center specializing in diabetes genetics is recommended to understand the significance of these mutations and how best to approach further evaluation, treatment, and genetic counseling. E
Monogenic defects that cause β-cell dysfunction, such as neonatal diabetes and MODY, represent a small fraction of patients with diabetes (<5%). Table 2.6 describes the most common causes of monogenic diabetes. For a comprehensive list of causes, see Genetic Diagnosis of Endocrine Disorders ( 133 ).
Most common causes of monogenic diabetes ( 133 )
AD, autosomal dominant; AR, autosomal recessive; IUGR, intrauterine growth restriction; OGTT, oral glucose tolerance test; UPD6, uniparental disomy of chromosome 6; 2-h PG, 2-h plasma glucose.
Neonatal Diabetes
Diabetes occurring under 6 months of age is termed “neonatal” or “congenital” diabetes, and about 80 – 85% of cases can be found to have an underlying monogenic cause ( 134 – 137 ). Neonatal diabetes occurs much less often after 6 months of age, whereas autoimmune type 1 diabetes rarely occurs before 6 months of age. Neonatal diabetes can either be transient or permanent. Transient diabetes is most often due to overexpression of genes on chromosome 6q24, is recurrent in about half of cases, and may be treatable with medications other than insulin. Permanent neonatal diabetes is most commonly due to autosomal dominant mutations in the genes encoding the Kir6.2 subunit ( KCNJ11 ) and SUR1 subunit ( ABCC8 ) of the β-cell K ATP channel. A recent report details a de novo mutation in EIF2B1 affecting eIF2 signaling associated with permanent neonatal diabetes and hepatic dysfunction, similar to Wolcott-Rallison syndrome but with few severe comorbidities ( 138 ). Correct diagnosis has critical implications because most patients with K ATP -related neonatal diabetes will exhibit improved glycemic control when treated with high-dose oral sulfonylureas instead of insulin. Insulin gene ( INS ) mutations are the second most common cause of permanent neonatal diabetes, and, while intensive insulin management is currently the preferred treatment strategy, there are important genetic counseling considerations, as most of the mutations that cause diabetes are dominantly inherited.
Maturity-Onset Diabetes of the Young
MODY is frequently characterized by onset of hyperglycemia at an early age (classically before age 25 years, although diagnosis may occur at older ages). MODY is characterized by impaired insulin secretion with minimal or no defects in insulin action (in the absence of coexistent obesity). It is inherited in an autosomal dominant pattern with abnormalities in at least 13 genes on different chromosomes identified to date. The most commonly reported forms are GCK-MODY (MODY2), HNF1A-MODY (MODY3), and HNF4A-MODY (MODY1).
For individuals with MODY, the treatment implications are considerable and warrant genetic testing ( 139 , 140 ). Clinically, patients with GCK-MODY exhibit mild, stable fasting hyperglycemia and do not require antihyperglycemic therapy except sometimes during pregnancy. Patients with HNF1A- or HNF4A-MODY usually respond well to low doses of sulfonylureas, which are considered first-line therapy. Mutations or deletions in HNF1B are associated with renal cysts and uterine malformations (renal cysts and diabetes [RCAD] syndrome). Other extremely rare forms of MODY have been reported to involve other transcription factor genes including PDX1 ( IPF1 ) and NEUROD1 .
Diagnosis of Monogenic Diabetes
A diagnosis of one of the three most common forms of MODY, including GCK-MODY, HNF1A-MODY, and HNF4A-MODY, allows for more cost-effective therapy (no therapy for GCK-MODY; sulfonylureas as first-line therapy for HNF1A-MODY and HNF4A-MODY). Additionally, diagnosis can lead to identification of other affected family members. Genetic screening is increasingly available and cost-effective ( 138 , 140 ).
A diagnosis of MODY should be considered in individuals who have atypical diabetes and multiple family members with diabetes not characteristic of type 1 or type 2 diabetes, although admittedly “atypical diabetes” is becoming increasingly difficult to precisely define in the absence of a definitive set of tests for either type of diabetes ( 135 – 137 , 139 – 145 ). In most cases, the presence of autoantibodies for type 1 diabetes precludes further testing for monogenic diabetes, but the presence of autoantibodies in patients with monogenic diabetes has been reported ( 146 ). Individuals in whom monogenic diabetes is suspected should be referred to a specialist for further evaluation if available, and consultation is available from several centers. Readily available commercial genetic testing following the criteria listed below now enables a cost-effective ( 147 ), often cost-saving, genetic diagnosis that is increasingly supported by health insurance. A biomarker screening pathway such as the combination of urinary C-peptide/creatinine ratio and antibody screening may aid in determining who should get genetic testing for MODY ( 148 ). It is critical to correctly diagnose one of the monogenic forms of diabetes because these patients may be incorrectly diagnosed with type 1 or type 2 diabetes, leading to suboptimal, even potentially harmful, treatment regimens and delays in diagnosing other family members ( 149 ). The correct diagnosis is especially critical for those with GCK-MODY mutations where multiple studies have shown that no complications ensue in the absence of glucose-lowering therapy ( 150 ). Genetic counseling is recommended to ensure that affected individuals understand the patterns of inheritance and the importance of a correct diagnosis.
The diagnosis of monogenic diabetes should be considered in children and adults diagnosed with diabetes in early adulthood with the following findings:
Diabetes diagnosed within the first 6 months of life (with occasional cases presenting later, mostly INS and ABCC8 mutations) ( 134 , 151 )
Diabetes without typical features of type 1 or type 2 diabetes (negative diabetes-associated autoantibodies, nonobese, lacking other metabolic features, especially with strong family history of diabetes)
Stable, mild fasting hyperglycemia (100 – 150 mg/dL [5.5 – 8.5 mmol/L]), stable A1C between 5.6% and 7.6% (between 38 and 60 mmol/mol), especially if nonobese
Pancreatic diabetes includes both structural and functional loss of glucose-normalizing insulin secretion in the context of exocrine pancreatic dysfunction and is commonly misdiagnosed as type 2 diabetes. Hyperglycemia due to general pancreatic dysfunction has been called “type 3c diabetes” and, more recently, diabetes in the context of disease of the exocrine pancreas has been termed pancreoprivic diabetes ( 1 ). The diverse set of etiologies includes pancreatitis (acute and chronic), trauma or pancreatectomy, neoplasia, cystic fibrosis (addressed elsewhere in this chapter), hemochromatosis, fibrocalculous pancreatopathy, rare genetic disorders ( 152 ), and idiopathic forms ( 1 ), which is the preferred terminology. A distinguishing feature is concurrent pancreatic exocrine insufficiency (according to the monoclonal fecal elastase 1 test or direct function tests), pathological pancreatic imaging (endoscopic ultrasound, MRI, computed tomography), and absence of type 1 diabetes–associated autoimmunity ( 153 – 157 ). There is loss of both insulin and glucagon secretion and often higher-than-expected insulin requirements. Risk for microvascular complications is similar to other forms of diabetes. In the context of pancreatectomy, islet autotransplantation can be done to retain insulin secretion ( 158 , 159 ). In some cases, autotransplant can lead to insulin independence. In others, it may decrease insulin requirements ( 160 ).
2.25 Test for undiagnosed prediabetes and diabetes at the first prenatal visit in those with risk factors using standard diagnostic criteria. B
2.26 Test for gestational diabetes mellitus at 24 – 28 weeks of gestation in pregnant women not previously found to have diabetes. A
2.27 Test women with gestational diabetes mellitus for prediabetes or diabetes at 4 – 12 weeks postpartum, using the 75-g oral glucose tolerance test and clinically appropriate nonpregnancy diagnostic criteria. B
2.28 Women with a history of gestational diabetes mellitus should have lifelong screening for the development of diabetes or prediabetes at least every 3 years. B
2.29 Women with a history of gestational diabetes mellitus found to have prediabetes should receive intensive lifestyle interventions and/or metformin to prevent diabetes. A
For many years, GDM was defined as any degree of glucose intolerance that was first recognized during pregnancy ( 60 ), regardless of the degree of hyperglycemia. This definition facilitated a uniform strategy for detection and classification of GDM, but this definition has serious limitations ( 161 ). First, the best available evidence reveals that many, perhaps most, cases of GDM represent preexisting hyperglycemia that is detected by routine screening in pregnancy, as routine screening is not widely performed in nonpregnant women of reproductive age. It is the severity of hyperglycemia that is clinically important with regard to both short- and long-term maternal and fetal risks. Universal preconception and/or first trimester screening is hampered by lack of data and consensus regarding appropriate diagnostic thresholds and outcomes and cost-effectiveness ( 162 , 163 ). A compelling argument for further work in this area is the fact that hyperglycemia that would be diagnostic of diabetes outside of pregnancy and is present at the time of conception is associated with an increased risk of congenital malformations that is not seen with lower glucose levels ( 164 , 165 ).
The ongoing epidemic of obesity and diabetes has led to more type 2 diabetes in women of reproductive age, with an increase in the number of pregnant women with undiagnosed type 2 diabetes in early pregnancy ( 166 – 169 ). Because of the number of pregnant women with undiagnosed type 2 diabetes, it is reasonable to test women with risk factors for type 2 diabetes ( 170 ) ( Table 2.3 ) at their initial prenatal visit, using standard diagnostic criteria ( Table 2.2 ). Women found to have diabetes by the standard diagnostic criteria used outside of pregnancy should be classified as having diabetes complicating pregnancy (most often type 2 diabetes, rarely type 1 diabetes or monogenic diabetes) and managed accordingly. Women who meet the lower glycemic criteria for GDM should be diagnosed with that condition and managed accordingly. Other women should be rescreened for GDM between 24 and 28 weeks of gestation (see Section 14 “Management of Diabetes in Pregnancy,” https://doi.org/10.2337/dc21-S014 ). The International Association of the Diabetes and Pregnancy Study Groups (IADPSG) GDM diagnostic criteria for the 75-g OGTT as well as the GDM screening and diagnostic criteria used in the two-step approach were not derived from data in the first half of pregnancy, so the diagnosis of GDM in early pregnancy by either FPG or OGTT values is not evidence based ( 171 ) and further work is needed.
GDM is often indicative of underlying β-cell dysfunction ( 172 ), which confers marked increased risk for later development of diabetes, generally but not always type 2 diabetes, in the mother after delivery ( 173 , 174 ). As effective prevention interventions are available ( 175 , 176 ), women diagnosed with GDM should receive lifelong screening for prediabetes to allow interventions to reduce diabetes risk and for type 2 diabetes to allow treatment at the earliest possible time ( 177 ).
GDM carries risks for the mother, fetus, and neonate. The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study ( 178 ), a large-scale multinational cohort study completed by more than 23,000 pregnant women, demonstrated that risk of adverse maternal, fetal, and neonatal outcomes continuously increased as a function of maternal glycemia at 24 – 28 weeks of gestation, even within ranges previously considered normal for pregnancy. For most complications, there was no threshold for risk. These results have led to careful reconsideration of the diagnostic criteria for GDM.
GDM diagnosis ( Table 2.7 ) can be accomplished with either of two strategies:
The “one-step” 75-g OGTT derived from the IADPSG criteria, or
The older “two-step” approach with a 50-g (nonfasting) screen followed by a 100-g OGTT for those who screen positive, based on the work of Carpenter and Coustan's interpretation of the older OʼSullivan ( 179 ) criteria.
Screening for and diagnosis of GDM
GDM, gestational diabetes mellitus; GLT, glucose load test; OGTT, oral glucose tolerance test.
American College of Obstetricians and Gynecologists notes that one elevated value can be used for diagnosis ( 189 ).
Different diagnostic criteria will identify different degrees of maternal hyperglycemia and maternal/fetal risk, leading some experts to debate, and disagree on, optimal strategies for the diagnosis of GDM.
One-Step Strategy
The IADPSG defined diagnostic cut points for GDM as the average fasting, 1-h, and 2-h PG values during a 75-g OGTT in women at 24 – 28 weeks of gestation who participated in the HAPO study at which odds for adverse outcomes reached 1.75 times the estimated odds of these outcomes at the mean fasting, 1-h, and 2-h PG levels of the study population. This one-step strategy was anticipated to significantly increase the incidence of GDM (from 5 – 6% to 15–20%), primarily because only one abnormal value, not two, became sufficient to make the diagnosis ( 180 ). Many regional studies have investigated the impact of adopting the IADPSG criteria on prevalence and have seen a roughly one- to threefold increase ( 181 ). The anticipated increase in the incidence of GDM could have a substantial impact on costs and medical infrastructure needs and has the potential to “medicalize” pregnancies previously categorized as normal. A recent follow-up study of women participating in a blinded study of pregnancy OGTTs found that 11 years after their pregnancies, women who would have been diagnosed with GDM by the one-step approach, as compared with those without, were at 3.4-fold higher risk of developing prediabetes and type 2 diabetes and had children with a higher risk of obesity and increased body fat, suggesting that the larger group of women identified by the one-step approach would benefit from increased screening for diabetes and prediabetes that would accompany a history of GDM ( 182 , 183 ). The ADA recommends the IADPSG diagnostic criteria with the intent of optimizing gestational outcomes because these criteria are the only ones based on pregnancy outcomes rather than end points such as prediction of subsequent maternal diabetes.
The expected benefits of using IADPSG to the offspring are inferred from intervention trials that focused on women with lower levels of hyperglycemia than identified using older GDM diagnostic criteria. Those trials found modest benefits including reduced rates of large-for-gestational-age births and preeclampsia ( 184 , 185 ). It is important to note that 80 – 90% of women being treated for mild GDM in these two randomized controlled trials could be managed with lifestyle therapy alone. The OGTT glucose cutoffs in these two trials overlapped with the thresholds recommended by the IADPSG, and in one trial ( 185 ), the 2-h PG threshold (140 mg/dL [7.8 mmol/L]) was lower than the cutoff recommended by the IADPSG (153 mg/dL [8.5 mmol/L]). No randomized controlled trials of treating versus not treating GDM diagnosed by the IADPSG criteria but not the Carpenter-Coustan criteria have been published to date. Data are also lacking on how the treatment of lower levels of hyperglycemia affects a mother's future risk for the development of type 2 diabetes and her offspring's risk for obesity, diabetes, and other metabolic disorders. Additional well-designed clinical studies are needed to determine the optimal intensity of monitoring and treatment of women with GDM diagnosed by the one-step strategy ( 186 , 187 ).
Two-Step Strategy
In 2013, the NIH convened a consensus development conference to consider diagnostic criteria for diagnosing GDM ( 188 ). The 15-member panel had representatives from obstetrics and gynecology, maternal-fetal medicine, pediatrics, diabetes research, biostatistics, and other related fields. The panel recommended a two-step approach to screening that used a 1-h 50-g glucose load test (GLT) followed by a 3-h 100-g OGTT for those who screened positive. The American College of Obstetricians and Gynecologists (ACOG) recommends any of the commonly used thresholds of 130, 135, or 140 mg/dL for the 1-h 50-g GLT ( 189 ). A systematic review for the U.S. Preventive Services Task Force compared GLT cutoffs of 130 mg/dL (7.2 mmol/L) and 140 mg/dL (7.8 mmol/L) ( 190 ). The higher cutoff yielded sensitivity of 70–88% and specificity of 69 – 89%, while the lower cutoff was 88 – 99% sensitive and 66 – 77% specific. Data regarding a cutoff of 135 mg/dL are limited. As for other screening tests, choice of a cutoff is based upon the trade-off between sensitivity and specificity. The use of A1C at 24–28 weeks of gestation as a screening test for GDM does not function as well as the GLT ( 191 ).
Key factors cited by the NIH panel in their decision-making process were the lack of clinical trial data demonstrating the benefits of the one-step strategy and the potential negative consequences of identifying a large group of women with GDM, including medicalization of pregnancy with increased health care utilization and costs. Moreover, screening with a 50-g GLT does not require fasting and is therefore easier to accomplish for many women. Treatment of higher-threshold maternal hyperglycemia, as identified by the two-step approach, reduces rates of neonatal macrosomia, large-for-gestational-age births ( 192 ), and shoulder dystocia, without increasing small-for-gestational-age births. ACOG currently supports the two-step approach but notes that one elevated value, as opposed to two, may be used for the diagnosis of GDM ( 189 ). If this approach is implemented, the incidence of GDM by the two-step strategy will likely increase markedly. ACOG recommends either of two sets of diagnostic thresholds for the 3-h 100-g OGTT—Carpenter-Coustan or National Diabetes Data Group ( 193 , 194 ). Each is based on different mathematical conversions of the original recommended thresholds by O'Sullivan ( 179 ), which used whole blood and nonenzymatic methods for glucose determination. A secondary analysis of data from a randomized clinical trial of identification and treatment of mild GDM ( 195 ) demonstrated that treatment was similarly beneficial in patients meeting only the lower thresholds per Carpenter-Coustan ( 193 ) and in those meeting only the higher thresholds per National Diabetes Data Group ( 194 ). If the two-step approach is used, it would appear advantageous to use the Carpenter-Coustan lower diagnostic thresholds as shown in step 2 in Table 2.7 .
Future Considerations
The conflicting recommendations from expert groups underscore the fact that there are data to support each strategy. A cost-benefit estimation comparing the two strategies concluded that the one-step approach is cost-effective only if patients with GDM receive postdelivery counseling and care to prevent type 2 diabetes ( 196 ). The decision of which strategy to implement must therefore be made based on the relative values placed on factors that have yet to be measured (e.g., willingness to change practice based on correlation studies rather than intervention trial results, available infrastructure, and importance of cost considerations).
As the IADPSG criteria (“one-step strategy”) have been adopted internationally, further evidence has emerged to support improved pregnancy outcomes with cost savings ( 197 ), and IADPSG may be the preferred approach. Data comparing population-wide outcomes with one-step versus two-step approaches have been inconsistent to date ( 198 , 199 ). In addition, pregnancies complicated by GDM per the IADPSG criteria, but not recognized as such, have outcomes comparable to pregnancies with diagnosed GDM by the more stringent two-step criteria ( 200 , 201 ). There remains strong consensus that establishing a uniform approach to diagnosing GDM will benefit patients, caregivers, and policy makers. Longer-term outcome studies are currently underway.
Suggested citation: American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2021 . Diabetes Care 2021;44(Suppl. 1):S15−S33
Email alerts
- Addendum. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021 . Diabetes Care 2021;44(Suppl. 1):S15–S33
- Online ISSN 1935-5548
- Print ISSN 0149-5992
- Diabetes Care
- Clinical Diabetes
- Diabetes Spectrum
- Standards of Medical Care in Diabetes
- Scientific Sessions Abstracts
- BMJ Open Diabetes Research & Care
- ShopDiabetes.org
- ADA Professional Books
Clinical Compendia
- Clinical Compendia Home
- Latest News
- DiabetesPro SmartBrief
- Special Collections
- DiabetesPro®
- Diabetes Food Hub™
- Insulin Affordability
- Know Diabetes By Heart™
- About the ADA
- Journal Policies
- For Reviewers
- Advertising in ADA Journals
- Reprints and Permission for Reuse
- Copyright Notice/Public Access Policy
- ADA Professional Membership
- ADA Member Directory
- Diabetes.org
- X (Twitter)
- Cookie Policy
- Accessibility
- Terms & Conditions
- Get Adobe Acrobat Reader
- © Copyright American Diabetes Association
This Feature Is Available To Subscribers Only
Sign In or Create an Account
An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Entire Site
- Research & Funding
- Health Information
- About NIDDK
- Diabetes Overview
What Is Diabetes?
- Español
Diabetes is a disease that occurs when your blood glucose, also called blood sugar, is too high. Glucose is your body’s main source of energy. Your body can make glucose, but glucose also comes from the food you eat.
Insulin is a hormone made by the pancreas that helps glucose get into your cells to be used for energy. If you have diabetes, your body doesn’t make enough—or any—insulin, or doesn’t use insulin properly. Glucose then stays in your blood and doesn’t reach your cells.
Diabetes raises the risk for damage to the eyes, kidneys, nerves, and heart. Diabetes is also linked to some types of cancer. Taking steps to prevent or manage diabetes may lower your risk of developing diabetes health problems.

What are the different types of diabetes?
The most common types of diabetes are type 1, type 2, and gestational diabetes.
Type 1 diabetes
If you have type 1 diabetes , your body makes little or no insulin. Your immune system attacks and destroys the cells in your pancreas that make insulin. Type 1 diabetes is usually diagnosed in children and young adults, although it can appear at any age. People with type 1 diabetes need to take insulin every day to stay alive.
Type 2 diabetes
If you have type 2 diabetes , the cells in your body don’t use insulin properly. The pancreas may be making insulin but is not making enough insulin to keep your blood glucose level in the normal range. Type 2 diabetes is the most common type of diabetes. You are more likely to develop type 2 diabetes if you have risk factors , such as overweight or obesity , and a family history of the disease. You can develop type 2 diabetes at any age, even during childhood.
You can help delay or prevent type 2 diabetes by knowing the risk factors and taking steps toward a healthier lifestyle, such as losing weight or preventing weight gain.
Gestational diabetes
Gestational diabetes is a type of diabetes that develops during pregnancy. Most of the time, this type of diabetes goes away after the baby is born. However, if you’ve had gestational diabetes, you have a higher chance of developing type 2 diabetes later in life. Sometimes diabetes diagnosed during pregnancy is type 2 diabetes.
Prediabetes
People with prediabetes have blood glucose levels that are higher than normal but not high enough to be diagnosed with type 2 diabetes. If you have prediabetes, you have a higher risk of developing type 2 diabetes in the future. You also have a higher risk for heart disease than people with normal glucose levels.
Other types of diabetes
A less common type of diabetes, called monogenic diabetes , is caused by a change in a single gene . Diabetes can also come from having surgery to remove the pancreas, or from damage to the pancreas due to conditions such as cystic fibrosis or pancreatitis .
How common are diabetes and prediabetes?
More than 133 million Americans have diabetes or prediabetes. 1
As of 2019, 37.3 million people—or 11.3% of the U.S. population—had diabetes. 1 More than 1 in 4 people over the age of 65 had diabetes. Nearly 1 in 4 adults with diabetes didn’t know they had the disease. 2
About 90% to 95% of diabetes cases are type 2 diabetes. 3
In 2019, 96 million adults—38% of U.S. adults—had prediabetes. 4
What other health problems can people with diabetes develop?
Over time, high blood glucose can damage your heart , kidneys , feet , and eyes . If you have diabetes, you can take steps to lower your chances of developing diabetes health problems by taking steps to improve your health and learning how to manage the disease . Managing your blood glucose, blood pressure, and cholesterol levels can help prevent future health problems.

This content is provided as a service of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), part of the National Institutes of Health. NIDDK translates and disseminates research findings to increase knowledge and understanding about health and disease among patients, health professionals, and the public. Content produced by NIDDK is carefully reviewed by NIDDK scientists and other experts.
NIDDK would like to thank: Daniel Bessesen, M.D., University of Colorado; Domenico Accili, M.D., Columbia University
Diabetes Basics

An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Dean L, McEntyre J. The Genetic Landscape of Diabetes [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004.

The Genetic Landscape of Diabetes [Internet].
Chapter 1 introduction to diabetes.
Created: July 7, 2004 .
Diabetes mellitus is characterized by abnormally high levels of sugar (glucose) in the blood.
When the amount of glucose in the blood increases, e.g., after a meal, it triggers the release of the hormone insulin from the pancreas. Insulin stimulates muscle and fat cells to remove glucose from the blood and stimulates the liver to metabolize glucose, causing the blood sugar level to decrease to normal levels .
In people with diabetes, blood sugar levels remain high. This may be because insulin is not being produced at all, is not made at sufficient levels, or is not as effective as it should be. The most common forms of diabetes are type 1 diabetes (5%), which is an autoimmune disorder, and type 2 diabetes (95%), which is associated with obesity. Gestational diabetes is a form of diabetes that occurs in pregnancy, and other forms of diabetes are very rare and are caused by a single gene mutation.
For many years, scientists have been searching for clues in our genetic makeup that may explain why some people are more likely to get diabetes than others are. "The Genetic Landscape of Diabetes" introduces some of the genes that have been suggested to play a role in the development of diabetes.
- Classification
Diabetes is classified by underlying cause. The categories are: type 1 diabetes—an autoimmune disease in which the body's own immune system attacks the pancreas , rendering it unable to produce insulin; type 2 diabetes—in which a resistance to the effects of insulin or a defect in insulin secretion may be seen; gestational diabetes; and “other types”. Table 1 compares the presentation (phenotype) of type 1 and type 2 diabetes.
Comparison of Type 1 and Type 2 Diabetes.
Type 2 diabetes commonly occurs in adults who are obese. There are many underlying factors that contribute to the high blood glucose levels in these individuals. An important factor is the body's resistance to insulin in the body, essentially ignoring its insulin secretions. A second factor is the falling production of insulin by the beta cells of the pancreas. Therefore, an individual with type 2 diabetes may have a combination of deficient secretion and deficient action of insulin.
In contrast to type 2, type 1 diabetes most commonly occurs in children and is a result of the body's immune system attacking and destroying the beta cells. The trigger for this autoimmune attack is not clear, but the result is the end of insulin production.
- History of Diabetes
Physicians have observed the effects of diabetes for thousands of years. For much of this time, little was known about this fatal disease that caused wasting away of the body, extreme thirst, and frequent urination. It wasn't until 1922 that the first patient was successfully treated with insulin.
One of the effects of diabetes is the presence of glucose in the urine (glucosuria). Ancient Hindu writings, many thousands of years old, document how black ants and flies were attracted to the urine of diabetics. The Indian physician Sushruta in 400 B.C. described the sweet taste of urine from affected individuals, and for many centuries to come, the sweet taste of urine was key to diagnosis.
Around 250 B.C., the name “diabetes” was first used. It is a Greek word that means “to syphon”, reflecting how diabetes seemed to rapidly drain fluid from the affected individual. The Greek physician Aretaeus noted that as affected individuals wasted away, they passed increasing amounts of urine as if there was “liquefaction of flesh and bones into urine”. The complete term “diabetes mellitus” was coined in 1674 by Thomas Willis, personal physician to King Charles II. Mellitus is Latin for honey, which is how Willis described the urine of diabetics (“as if imbued with honey and sugar”).
Up until the mid-1800s, the treatments offered for diabetes varied tremendously. Various “fad” diets were prescribed, and the use of opium was suggested, as were bleeding and other therapies. The most successful treatments were starvation diets in which calorie intake was severely restricted. Naturally, this was intolerable for the patient and at best extended life expectancy for a few years.
A breakthrough in the puzzle of diabetes came in 1889. German physicians Joseph von Mering and Oskar Minkowski surgically removed the pancreas from dogs. The dogs immediately developed diabetes. Now that a link was established between the pancreas gland and diabetes, research focused on isolating the pancreatic extract that could treat diabetes.
When Dr. Frederick Banting took up the challenge of isolating a pancreatic extract, he was met with much skepticism. Many great physiologists had tried and failed to isolate an internal secretion from the pancreas. But Banting, a surgeon, persisted and in May 1921, he began work in the laboratory of Professor John Macloed in Toronto, Canada. Charles Best, a medical student at the time, worked as his assistant.
To concentrate what we now know as insulin, Banting tied the pancreatic ducts of dogs. The pancreatic cells that released digestive enzymes (and could also destroy insulin) degenerated, but the cells that secreted insulin were spared. Over several weeks the pancreas degenerated into a residue from which insulin could be extracted. In July 1921, a dog that had had its pancreas surgically removed was injected with an extract collected from a duct-tied dog. In the two hours that followed the injection, the blood sugar level of the dog fell, and its condition improved. Another de-pancreatized (diabetic-like) dog was kept alive for eight days by regular injections until supplies of the extract, at that time called "isletin", were exhausted.
Further experiments on dogs showed that extracts from the pancreas caused a drop in blood sugar, caused glucose in the urine to disappear, and produced a marked improvement in clinical condition. So long as the extract was being given, the dogs were kept alive. The supply of the extract was improved: the pancreas of different animals were used until that of the cow was settled upon. This extract kept a de-pancreatized dog alive for 70 days. Dr. J. Collip, a biochemist, was drafted to continue improving the purity of the pancreas extract, and later, Best carried on this work.
A young boy, Leonard Thompson, was the first patient to receive insulin treatment. On January 11, 1922, aged 14 and weighing only 64 pounds, he was extremely ill. The first injections of insulin only produced a slight lowering of blood sugar level. The extract still was not pure enough, and abscesses developed at the injection site. Collip continued to refine the extract. Several weeks later, Leonard was treated again and showed a remarkable recovery. His blood sugar levels fell, he gained weight and lived for another 13 years. He died from pneumonia at the age of 27.
During the spring of 1922, Best increased the production of insulin to enable the treatment of diabetic patients coming to the Toronto clinic. Over the next 60 years, insulin was further refined and purified, and long-acting and intermediate types were developed to provide more flexibility. A revolution came with the production of recombinant human DNA insulin in 1978. Instead of collecting insulin from animals, new human insulin could be synthesized.
In 1923, Banting and Macloed were awarded the Nobel Prize for the discovery of insulin. Banting split his prize with Best, and Macloed split his prize with Collip. In his Nobel Lecture, Banting concluded the following about their discovery:
“Insulin is not a cure for diabetes; it is a treatment. It enables the diabetic to burn sufficient carbohydrates, so that proteins and fats may be added to the diet in sufficient quantities to provide energy for the economic burdens of life.”
- Epidemiology
Box 1 Increase of diabetes in adults in the United States
Click on the arrow to start the animation.
Download video file. (1.8M, mov)
- Mokdad A H , Ford E S , Bowman B A , Dietz W H , Vinicor F , Bales V S , Marks J S . Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2001; 289(1) :76–79. [ PubMed : 12503980 ]
Box 2 Rise of obesity in the United States
Download video file. (2.6M, mov)
Over 18 million Americans have diabetes; of these, about 5 million do not know they have the disease ( 1 ).
Type 1 diabetes accounts for 5-10% of cases, affecting 1 of 400 children and adolescents.
Type 2 diabetes is extremely common, accounting for 90-95% of all cases of diabetes. This form of diabetes can go undiagnosed for many years, but the number of cases that are being diagnosed is rising rapidly, leading to reports of a diabetes epidemic.
The Type 2 Diabetes Epidemic
When people think of epidemics, they often think of infectious diseases such as SARS, HIV, or the flu. However, the prevalence of type 2 diabetes is now at epidemic proportions. In the United States, diabetes accounts for over 130 billion dollars of health care costs and is the fifth leading cause of death ( 2 ). The number of new cases being diagnosed continues to rise. It has been estimated that of the children born in the year 2000, 1 of 3 will suffer from diabetes at some point in their lifetime ( 3 ). Diabetes is predicted to become one of the most common diseases in the world within a couple of decades, affecting at least half a billion people ( 4 ).
Estimate your risk of developing Type 2 Diabetes
In the past, type 2 was rarely seen in the young, hence its original name of “adult-onset diabetes”. But now type 2 diabetes is increasingly being diagnosed in young adults and even in children. In Japan, more children suffer from type 2 than type 1 (“juvenile onset”) diabetes. This young generation of diabetics will have many decades in which to develop the complications of diabetes.
In 1990, 4.9% of the American population were diagnosed with diabetes (see Flash Animation 1 ). This increased to 7.9% by the year 2001 ( 5 ).
The driving force behind the high prevalence of diabetes is the rise of obesity in the population. In today's society, it can be difficult to maintain a healthy weight. We have the combination of ample food and a sedentary lifestyle. This is in stark contrast to only a couple of hundred years ago, when people were more active and food supplies were not as abundant. As a result, many of us are heavier than we should be.
Calculate your ideal weight
Being overweight or obese is defined by a calculation called the Body Mass Index (BMI). It is a calculation that takes your height and weight into consideration and gives you a score. A score of 18–24.9 is a healthy weight. If you are overweight, your score lies within the range to 25–29.9; a score of 30 and above indicates obesity.
Calculate your BMI
In 1991, it was estimated that 12% of the population were obese ( 5 ). By the year 2001, this had increased to an estimated 20.9% of the population; this represents over 44 million obese adult Americans. A more recent study estimated that a record 30% of the American population are now obese ( 6 ) (see Flash Animation 2 ).
Obesity is a major problem for the United states. Every year, an estimated 300,000 US adults die of causes related to obesity ( 7 ). Obesity is also a huge economic burden, accounting for up to 4% of healthcare costs in the United States ( 8 ).
Thrifty Genes
Epidemics of infectious diseases increase when there is increased spread of the infectious agent and decrease when the number of victims who are susceptible falls (they either become immune or they die). An epidemic of a genetic disease such as type 2 diabetes is similar. The number of cases rises when there is a rise in environmental risk (abundant food supplies, lack of activity) and decreases when the number of susceptible individuals falls (by deaths from the complications of diabetes).
The classic example of an epidemic of diabetes is found on an remote island in the Pacific Ocean, the island of Nauru. Before the turn of the 20th century, the lifestyle of Nauruans was harsh. The soil was poor, agriculture was difficult, and frequent episodes of starvation were common. Despite these adverse conditions, the islanders were noted to be “heavy”. In 1922, it was discovered that Nauru contained phosphate rock, which was then mined for use in fertilizer, and for which the islanders received royalties. Over several decades, the Nauruans became extremely wealthy, and with their new-found riches came major lifestyle changes. Food was now abundant and could be bought from stores. Instead of fishing and farming, Nauruans now led sedentary lives. By the 1950s, type 2 diabetes exploded from being non-existent in this population to affecting 2 of 3 adults over the age of 55 and becoming a common cause of death.
The case of the Nauruans is an extreme case of how type 2 diabetes can rapidly reach epidemic proportions, and “thrifty genes” may be involved. It has been postulated by Neel ( 9 ) that genes that are metabolically thrifty give a survival advantage in times when there is a constant threat of famine and starvation. When food is abundant, these genes aid the efficient metabolism of the food, enabling rapid build up of fat stores. This enabled people like the Nauruans to survive food shortages later on. But when food is always abundant, a thrifty genetic makeup turns into a survival disadvantage. Thrifty genes cause obesity, which in turn predisposes to diabetes. The epidemic that took hold of the island of Nauru is now emerging in developing countries and already has a firm hold on the developed world.
- Physiology and Biochemistry of Sugar Regulation
Overview of Glucose Metabolism
Glucose is an essential fuel for the body ( Figure 1 ).The amount of glucose in the bloodstream is regulated by many hormones, the most important being insulin.
Overview of glucose metabolism. Glucose is used for many purposes in the body. It can be converted into energy via pyruvate and the tricarboxylic acid (TCA) cycle, as well as being converted to fat (long-term storage) and glycogen (short-term storage). Some (more...)
- muscle and fat cells to remove glucose from the blood
- cells to breakdown glucose, releasing its energy in the form of ATP (via glycolysis and the citric acid cycle)
- the liver and muscle to store glucose as glycogen (short-term energy reserve)
- adipose tissue to store glucose as fat (long-term energy reserve)
- cells to use glucose in protein synthesis
Glucagon is the main hormone opposing the action of insulin and is released when food is scarce. Whereas insulin triggers the formation of glycogen (an energy-requiring process, or an anabolic effect), glucagon triggers glycogen breakdown, which releases energy (a catabolic effect). Glucagon also helps the body to switch to using resources other than glucose, such as fat and protein ( Figure 2 ).
Anabolism and catabolism of glucose. Glucose metabolism involves both energy-producing (catabolic, shown in orange) and energy-consuming (anabolic, shown in green) processes.
Regulation of Blood Glucose
Blood glucose levels are not constant—they rise and fall depending on the body's needs, regulated by hormones. This results in glucose levels normally ranging from 70 to 110 mg/dl.
The blood glucose level can rise for three reasons: diet, breakdown of glycogen, or through hepatic synthesis of glucose.
Eating produces a rise in blood glucose, the extent of which depends on a number of factors such as the amount and the type of carbohydrate eaten (i.e., the glycemic index), the rate of digestion, and the rate of absorption. Because glucose is a polar molecule, its absorption across the hydrophobic gut wall requires specialized glucose transporters (GLUTS) of which there are five types. In the gut, GLUT2 and GLUT5 are the most common.
The liver is a major producer of glucose—it releases glucose from the breakdown of glycogen and also makes glucose from intermediates of carbohydrate, protein, and fat metabolism. The liver is also a major consumer of glucose and can buffer glucose levels (see Box 1 ). It receives glucose-rich blood directly from the digestive tract via the portal vein ( Figure 3 ). The liver quickly removes large amounts of glucose from the circulation so that even after a meal, the blood glucose levels rarely rise above 110 mg/dl in a non-diabetic.
The liver buffers glucose levels.
The portal circulation. The portal vein drains almost all of the blood from the digestive tract and empties directly into the liver. This circulation of nutrient-rich blood between the gut and liver is called the portal circulation. It enables the liver (more...)
After a Meal—the Role of Insulin
The rise in blood glucose following a meal is detected by the pancreatic beta cells, which respond by releasing insulin. Insulin increases the uptake and use of glucose by tissues such as skeletal muscle and fat cells. This rise in glucose also inhibits the release of glucagon, inhibiting the production of glucose from other sources, e.g., glycogen break down ( Figure 4 ).
Changes in key hormones after a meal. Changes in blood levels of glucose, insulin, and glucagon after a carbohyrate-rich meal (ingested at time 0 minutes).
1. Use Glucose
Once inside the cell, some of the glucose is used immediately via glycolysis. This is a central pathway of carbohydrate metabolism because it occurs in all cells in the body, and because all sugars can be converted into glucose and enter this pathway. During the well-fed state, the high levels of insulin and low levels of glucagon stimulate glycolysis, which releases energy and produces carbohydrate intermediates that can be used in other metabolic pathways.
Glycolysis in Stryer's Biochemistry
2. Make Glycogen
- stimulating hepatic glycogen synthetase (the enzyme that catalyzes glycogen synthesis in the liver)
- inhibiting hepatic glycogen phosphorylase (the enzyme that catalyzes glycogen breakdown in the liver)
- inhibiting glucose synthesis from other sources (inhibits gluconeogenesis)
Glycogen metabolism in Stryer's Biochemistry
3. Make Fat
- increasing the number of glucose transporters (GLUT4) expressed on the surface of the fat cell, causing a rapid uptake of glucose
- increasing lipoprotein lipase activity, which frees up more fatty acids for triglyceride synthesis
Fatty acid metabolism in Stryer's Biochemistry
Insulin also has an anabolic effect on protein metabolism. It stimulates the entry of amino acids into cells and stimulates protein production from amino acids.
Fasting—the Role of Glucagon
Fasting is defined as more than eight hours without food. The resulting fall in blood sugar levels inhibits insulin secretion and stimulates glucagon release. Glucagon opposes many actions of insulin. Most importantly, glucagon raises blood sugar levels by stimulating the mobilization of glycogen stores in the liver, providing a rapid burst of glucose. In 10–18 hours, the glycogen stores are depleted, and if fasting continues, glucagon continues to stimulate glucose production by favoring the hepatic uptake of amino acids, the carbon skeletons of which are used to make glucose.
In addition to low blood glucose levels, many other stimuli stimulate glucagon release including eating a protein-rich meal (the presence of amino acids in the stomach stimulates the release of both insulin and glucagon, glucagon prevents hypoglycemia that could result from unopposed insulin) and stress (the body anticipates an increased glucose demand in times of stress).
The metabolic state of starvation in the USA is more commonly found in people trying to lose weight rapidly or in those who are too unwell to eat. After a couple of days without food, the liver will have exhausted its stores of glycogen but continues to make glucose from protein (amino acids) and fat (glycerol).
The metabolism of fatty acids (from adipose tissue) is a major source of energy for organs such as the liver. Fatty acids are broken down to acetyl-CoA, which is channeled into the citric acid cycle and generates ATP. As starvation continues, the levels of acetyl-CoA increase until the oxidative capacity of the citric acid cycle is exceeded. The liver processes these excess fatty acids into ketone bodies (3-hydroxybutyrate) to be used by many tissues as an energy source.
The most important organ that relies on ketone production is the brain because it is unable to metabolize fatty acids. During the first few days of starvation, the brain uses glucose as a fuel. If starvation continues for more than two weeks, the level of circulating ketone bodies is high enough to be used by the brain. This slows down the need for glucose production from amino acid skeletons, thus slowing down the loss of essential proteins.
“Starvation in the Midst of Plenty”
Diabetes is often referred to as “starvation in the midst of plenty” because the intracellular levels of glucose are low, although the extracellular levels may be extremely high.
As in starvation, type 1 diabetics use non-glucose sources of energy, such as fatty acids and ketone bodies, in their peripheral tissues. But in contrast to the starvation state, the production of ketone bodies can spiral out of control. Because the ketones are weak acids, they acidify the blood. The result is the metabolic state of diabetic ketoacidosis (DKA). Hyperglycemia and ketoacidosis are the hallmark of type 1 diabetes (Figure 5) .
Metabolic changes in diabetic ketoacidosis. Hyperglycemia is caused by the increased production of glucose by the liver (driven by glucagon) and the decreased use of glucose of insulin by peripheral tissues (because of the lack of insulin).
Hypertriglyceridemia is also seen in DKA. The liver combines triglycerol with protein to form very low density lipoprotein (VLDL). It then releases VLDL into the blood. In diabetics, the enzyme that normally degrades lipoproteins (lipoprotein lipase) is inhibited by the low level of insulin and the high level of glucagon. As a result, the levels of VLDL and chylomicrons (made from lipid from the diet) are high in DKA.
Low-Carbohydrate Diet
Low-carbohydrate diets, such as the “Atkins” and “South Beach” diets, are currently popular ways to lose weight. Such diet plans involve restricting the type and amount of carbohydrate eaten.
One of the earliest descriptions of a low-carbohydrate diet was by William Banting in the 1860s in England. At the age of 66, Banting found success in following a carbohydrate-restricted diet: in the course of one year, he lost 46 pounds of his initial weight of 202 pounds. His impression was that “any starchy or saccharine matter tends to the disease of corpulence in advanced life”. He claimed he was never hungry and that “the great charms and comfort of the system are that its effects are palpable within a week of trial and creates a natural stimulus to persevere for a few weeks more”.
In a recent small trial, 63 obese men and women were assigned to either a low-carbohydrate diet or a low-fat diet ( 1 ). People on both diets lost weight. The carbohydrate-restricted group initially lost weight at a faster rate, but when reviewed at the end of the year there was no significant difference in weight loss between the two groups ( 1 ). It was found that low-carbohydrate dieters (who were allowed unrestricted amounts of protein and fat) actually had a lower energy intake than the low-fat diets (who were limited in their calorie intake). It may be that when carbohydrates are restricted, weight loss is due to a lower calorie intake due to the monotony of the diet. It is also possible that the lower calorie intake may be because of a change in peripheral or central saiety signals, leaving people feeling more full after a meal.
A second study compared the effects of a carbohydrate-restricted diet on the risk of developing atherosclerosis ( 2 ). 132 severely obese men and women were assigned to either a low-carbohydrate or low-fat diet. Again, after a 6-month period both groups lost weight. They became more sensitive to insulin, and their triglyceride (TG) levels, a type of fat that is a risk factor for atherosclerosis, improved. However, the carbohydrate-restricted group lost more weight and showed a greater improvement in insulin sensitivity and TG levels. After one year, the weight loss between the two groups was similar, but the cardiogenic risk factors were improved in the low-carbohydrate dieters, TG levels were lower, and levels of HDL cholesterol, a type of fat that protects against atherosclerosis, were higher ( 3 ). Also, long-term sugar control, which can be measured by checking for the amount of glycosylated hemoglobin (HbA1c), was better in people on the low-carbohydrate diet. However, it remains unclear whether these beneficial effects would continue after 1 year.
At present, the risks of obesity are well known, and the benefits of weight loss by traditional low-calorie, low-fat, and high-complex carbohydrate diets are also well documented. Future research will clarify the long-term outcomes of a low-carbohydrate diet for achieving and maintaing a healthy weight together with the effects on the heart and other systems of the body.
- The Story of Insulin
Insulin Synthesis
The insulin-making cells of the body are called beta cells, and they are found in the pancreas gland. These cells clump together to form the "islets of Langerhans", named for the German medical student who described them.
The synthesis of insulin begins at the translation of the insulin gene, which resides on chromosome 11. During translation, two introns are spliced out of the mRNA product, which encodes a protein of 110 amino acids in length. This primary translation product is called preproinsulin and is inactive. It contains a signal peptide of 24 amino acids in length, which is required for the protein to cross the cell membrane.
Once the preproinsulin reaches the endoplasmic reticulum, a protease cleaves off the signal peptide to create proinsulin. Proinsulin consists of three domains: an amino-terminal B chain, a carboxyl-terminal A chain, and a connecting peptide in the middle known as the C-peptide.
Within the endoplasmic reticulum, proinsulin is exposed to several specific peptidases that remove the C-peptide and generate the mature and active form of insulin. In the Golgi apparatus, insulin and free C-peptide are packaged into secretory granules, which accumulate in the cytoplasm of the beta cells. Exocytosis of the granules is triggered by the entry of glucose into the beta cells. The secretion of insulin has a broad impact on metabolism.
Insulin Structure
In 1958, Frederick Sanger was awarded his first Nobel Prize for determining the sequence of the amino acids that make up insulin. This marked the first time that a protein had had the order of its amino acids (the primary sequence) determined.
Insulin is composed of two chains of amino acids named chain A (21 amino acids) and chain B (30 amino acids) that are linked together by two disulfide bridges. There is a 3rd disulfide bridge within the A chain that links the 6th and 11th residues of the A chain together.
In most species, the length and amino acid compositions of chains A and B are similar, and the positions of the three disulfide bonds are highly conserved. For this reason, pig insulin can be used to replace deficient human insulin levels in diabetes patients. Today, porcine insulin has largely been replaced by the mass production of human proinsulin by bacteria (recombinant insulin).
Insulin molecules have a tendency to form dimers in solution, and in the presence of zinc ions, insulin dimers associate into hexamers. Whereas monomers of insulin readily diffuse through the blood and have a rapid effect, hexamers diffuse slowly and have a delayed onset of action. In the design of recombinant insulin, the structure of insulin can be modified in a way that reduces the tendency of the insulin molecule to form dimers and hexamers but that does not interrupt binding to the insulin receptor. In this way, a range of preparations of insulin is made, varying from short acting to long acting.
Insulin secretion
Rising levels of glucose inside the pancreatic beta cells trigger the release of insulin:
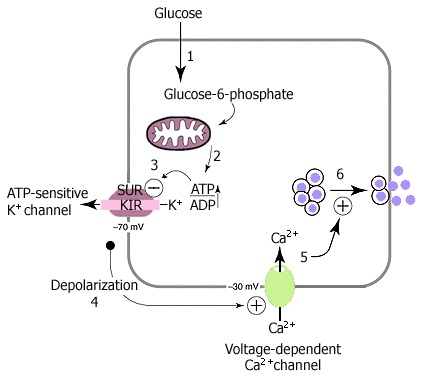
Glucose is transported into the beta cell by type 2 glucose transporters (GLUT2). Once inside, the first step in glucose metabolism is the phosphorylation of glucose to produce glucose-6-phosphate. This step is catalyzed by glucokinase—it is the rate-limiting step in glycolysis, and it effectively traps glucose inside the cell.
As glucose metabolism proceeds, ATP is produced in the mitochondria.
The increase in the ATP:ADP ratio closes ATP-gated potassium channels in the beta cell membrane. Positively charged potassium ions (K + ) are now prevented from leaving the beta cell.
The rise in positive charge inside the beta cell causes depolarization.
Voltage-gated calcium channels open, allowing calcium ions (Ca 2+ ) to flood into the cell.
The increase in intracellular calcium concentration triggers the secretion of insulin via exocytosis.
There are two phases of insulin release in response to a rise in glucose. The first is an immediate release of insulin. This is attributable to the release of preformed insulin, which is stored in secretory granules. After a short delay, there is a second, more prolonged release of newly synthesized insulin.
Once released, insulin is active for a only a brief time before it is degraded by enzymes. Insulinase found in the liver and kidneys breaks down insulin circulating in the plasma, and as a result, insulin has a half-life of only about 6 minutes. This short duration of action allows rapid changes in the circulating levels of insulin.
Insulin Receptor
The net effect of insulin binding is to trigger a cascade of phosphorylation and dephosphorylation reactions. These actions are terminated by dephosphorylation of the insulin receptor.
Similar to the receptors for other polypeptide hormones, the receptor for insulin is embedded in the plasma membrane and is composed of a pair of alpha subunits and a pair of beta subunits ( Figure 1 ). The alpha subunits are extracellular and contain the insulin-binding site. The beta subunits span the membrane and contain the enzyme tyrosine kinase. Kinases are a group of enzymes that phosphorylate proteins (the reverse reaction is catalyzed by a group of enzymes called phosphatases).
The insulin receptor. The insulin receptor is a tyrosine kinase receptor and is composed of a pair of alpha subunits and a pair of beta subunits. Insulin binds to the alpha subunits and induces a conformational change that is transmitted to the beta (more...)
Insulin binding to the alpha subunits induces a conformational change that is transmitted to the beta subunits and causes them to phosphorylate themselves (autophosphorylation). A specific tyrosine of each beta subunit is phosphorylated along with other target proteins, such as insulin receptor substrate (IRS). As these and other proteins inside the cell are phosphorylated, this in turn alters their activity, bringing about the wide biological effects of insulin.
Insulin Action
The binding of insulin results in a wide range of actions that take place over different periods of time.
Almost immediately, insulin promotes the uptake of glucose into many tissues that express GLUT4 glucose transporters, such as skeletal muscle and fat. Insulin increases the the activity of these transporters and increases their numbers by stimulating their recruitment from an intracellular pool to the cell surface. Not all tissues require insulin for glucose uptake. Tissues such as liver cells, red blood cells, the gut mucosa, the kidneys, and cells of the nervous system use a glucose transporter that is not insulin dependent.
Over minutes to hours, insulin alters the activity of various enzymes as a result of changes in their phosphorylation status.
Over a period of days, insulin increases the amounts of many metabolic enzymes. These reflect an increase in gene transcription, mRNA, and enzyme synthesis.
- Cite this Page Dean L, McEntyre J. The Genetic Landscape of Diabetes [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004. Chapter 1, Introduction to Diabetes. 2004 Jul 7.
- PDF version of this page (213K)
- PDF version of this title (2.2M)
In this Page
Discovery of insulin.
- Nobel Prize for the discovery of insulin
- Development of Insulin , University of Toronto
- Banting Digital library , New Tecumseth Public Library, Canada
Calculators
- Diabetes Type 2 Risk Calculator , University of Maryland Medical Center.
- Calculate your ideal weight , according to Dr. Koop.
- Calculate your BMI at the National Heart, Lung, and Blood Institute.
Healthy Living
- The DASH diet , National Heart, Lung, and Blood Institute.
- Advice from the Obesity Education Initiative , National Heart, Lung, and Blood Institute.
- The President's challenge .
- Advice from the Surgeon General .
- The glycemic index , Joslin Diabetes Center, Boston.
Read more about Insulin on the Bookshelf
- In Stryer's Biochemistry , read about the receptors that contain tyrosine kinase domains | See the release of insulin , insulin crystals , and the synthesis of proinsulin by bacteria .
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Recent Activity
- Introduction to Diabetes - The Genetic Landscape of Diabetes Introduction to Diabetes - The Genetic Landscape of Diabetes
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
Diabetes Mellitus Clinical Presentation
During prediabetes and the early stages of diabetes, the majority of patients are asymptomatic. As a result, obtaining a diagnosis may be delayed for many years if routine screening measures for diabetes, such as laboratory work, are not performed during regular healthcare visits. Typically, patients with type 1 diabetes mellitus (T1DM) present with symptomatic hyperglycemia and sometimes with diabetic ketoacidosis (DKA). DKA is defined as an acute metabolic complication of diabetes distinguished by hyperglycemia, hyperketonemia, and metabolic acidosis. DKA occurs primarily in T1DM and is less common in T2DM; it can present with nausea, vomiting, and abdominal pain and cause cerebral edema, coma, and death. DKA may be the initial presentation in an estimated 25% of adults with T1DM. The most common symptoms associated with T1DM are polyuria, polydipsia, and polyphagia, along with lethargy, nausea, and blurred vision, all of which result from the hyperglycemia. The onset of symptoms may be abrupt. Polyuria is caused by osmotic diuresis secondary to hyperglycemia. In young children, severe nocturnal enuresis secondary to polyuria could be a warning sign of diabetes onset. In T1DM, thirst is a response to the hyperosmolar state and dehydration. The American Diabetes Association notes that adults with new-onset T1DM may present with a short duration of illness of 1 to 4 weeks or more, a gradually progressing process that can be misinterpreted as type 2 diabetes mellitus (T2DM). Patients with T1DM may also present with fatigue, weakness, and weight loss despite normal appetite. Patients with T2DM may present with symptomatic hyperglycemia but are frequently asymptomatic for diabetes, and T2DM is often discovered during routine checkups and laboratory testing. Classic symptoms of T2DM include polyuria, polydipsia, polyphagia, and weight loss. Other symptoms that may suggest hyperglycemia include blurred vision, lower-extremity paresthesia, and delayed wound healing. In some patients, initial symptoms may actually be indicative of diabetic complications (such as neuropathy, retinopathy, skin acanthosis, and recurring Candida infections), signifying that the T2DM has been present for some time. This highlights the need for routine screening, especially in high-risk patient populations. In some patients, a hyperosmolar hyperglycemic state occurs initially, particularly during a period of extreme stress or when glucose metabolism is further impaired by use of certain pharmacologic agents, such as corticosteroids. Since early diagnosis and clinical intervention are essential for preventing and/or reducing the complications associated with poorly controlled diabetes, patients should be reminded to maintain routine healthcare and seek medical care if they are experiencing any symptoms associated with hyperglycemia. The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
« Click here to return to Diabetes Awareness.

- Advertising Contacts
- Editorial Staff
- Professional Organizations
- Submitting a Manuscript
- Privacy Policy
- Classifieds
- Diabetes & Primary Care
- Vol:11 | No:06

Clinical presentations, diagnosis and prevention of diabetes
- 14 Dec 2009
Researchers, public health physicians and frontline clinicians, including GPs, are increasingly convinced that we are entering an epidemic (if not a pandemic) of diabetes mellitus. Rates of diabetes prevalence are increasing across the world, particularly in developing countries, and an increasing number of people are being diagnosed in primary care. This article explores the classification and diagnosis of diabetes, focusing on risk factors, pre-diabetes, and management and prevention strategies for type 2 diabetes in primary care.
Share this article + Add to reading list – Remove from reading list ↓ Download pdf
In 2007 it was estimated that 4.82% of the UK population have diabetes (2.45 million people) (Yorkshire and Humber Public Health Observatory, 2007). Data from the author’s own practice alone show a trebling in the prevalence of type 2 diabetes in the past 20 years, with a relentless year on year increase (Evans et al, 2008). With the diagnosis of diabetes comes an increased risk of cardiovascular disease (CVD) and three-quarters of people with diabetes will die from cardiovascular causes (Garber, 2003).
Along with this rise in the prevalence of diabetes there is also a growing number of people in the UK with intermediate or borderline hyperglycaemia (often known as “pre-diabetes”). The challenge to primary care is therefore to encourage early diagnosis, intervention and, if possible, prevention of both of these disorders.
The questions, therefore, are: how do we define diabetes and pre-diabetes, and how can we prevent people developing these potentially life-threatening conditions?
Type 1 and type 2 diabetes Raised blood glucose (hyperglycaemia) has numerous health implications. Diabetes mellitus is “a group of metabolic diseases characterised by hyperglycaemia resulting from defects in insulin secretion, action or both”. This definition by the American Diabetes Association (ADA, 2009) illustrates the fact that diabetes is a syndrome with multiple causes.
The vast majority of people with diabetes fall into two main groups: type 1 and type 2 (ADA, 2009). As described in an earlier module in the series, type 1 diabetes is caused by an absolute deficiency of insulin thought to be due to autoimmune destruction of pancreatic islet cells. Type 1 accounts for between 5% and 10% of all cases and is often seen in younger people, usually before the age of 40 (Diabetes UK, 2009). Type 2 diabetes, however, is far more common (90% of all cases) and is usually diagnosed in people over 45 years of age who are often obese or physically inactive (Diabetes UK, 2009). It is rapidly increasing in prevalence and is the driver for the current diabetes epidemic.
Type 2 diabetes is strongly dependent on ethnicity and is more common in south Asian or Afro-Caribbean populations. In these populations in the UK, people may develop type 2 diabetes at a younger age and at lower BMI levels than their Caucasian counterparts. Unlike type 1 diabetes, type 2 is characterised by a relative insulin deficiency and is often associated with insulin resistance and features of the so-called metabolic syndrome – an increase in waist circumference and raised blood pressure, low HDL-cholesterol, raised plasma triglycerides or a raised blood glucose (Alberti et al, 2005).
Type 2 diabetes usually develops after a long prodromal period of several years of gradually increasing blood glucose levels (Harris et al, 1992), and most people pass through a period of pre-diabetes before their hyperglycaemia reaches the diabetes threshold. Recent data from the Whitehall II study (Tabák et al, 2009) showed that before diagnosis with type 2 diabetes, study participants had a slow increase in their blood glucose levels over the 13 years of the study, but that blood glucose levels then rose rapidly in the 2–3 years preceding diagnosis.
People with type 2 diabetes often do not need insulin for a period of time after diagnosis (hence the previous term “non-insulin dependent”). In addition, type 2 diabetes is often asymptomatic until blood glucose levels rise (Evans et al, 2003).
Whatever the cause of the hyperglycaemia, however, be it type 1 or 2 diabetes, the symptoms include polyuria, polydipsia, weight loss, tiredness, blurred vision and susceptibility to infections. Long-term complications can be disabling, even fatal, and include neuropathy, retinopathy, CVD, sexual dysfunction and a significant impact on the individual’s quality of life and social functioning. However, even at diagnosis of type 2 diabetes, around 25% of people may already have complications (UK Prospective Diabetes Study Group, 1998).
Rarer causes of diabetes Type 2 diabetes is generally considered to be a polygenic disorder. Monogenic causes of diabetes are seen less frequently (1–2% of all cases) (Murphy et al, 2008), but nevertheless can present to GPs. For example, it is thought that each GP practice has at least one person whose diabetes is due to maturity-onset diabetes of the young (MODY), although this is unlikely to have been recognised as such.
MODY is a monogenic autosomal dominant condition often causing hyperglycaemia in people under the age of 20, and hence is likely to be diagnosed as either type 1 or early type 2 diabetes. The chromosomal defects and functional deficiencies have now been determined, and the most common form involves a mutation in one of the liver transcription factors known as hepatocyte nuclear factor (HNF-1 α ). People with MODY usually present with early-onset diabetes aged 15–30 years, are not insulin-dependent and usually not obese. There is usually a strong family history of diabetes, often with family members developing the condition before the age of 25.
MODY is important to the primary care team for several reasons, including the need to screen other family members and offer genetic counselling, the need to define the precise sub-type of MODY by genetic testing, and the need for specialist referral to ensure the right diagnosis is made. Treatment options are often dependent on the individual’s genetic sub-type (e.g. the use of low-dose sulphonylureas in people with the HNF-1 α subtype) (Murphy et al, 2008).
Another monogenic cause of diabetes in middle-aged adults is maternally inherited diabetes and deafness (MIDD). People with the condition have hyperglycaemia and a maternal history of diabetes as well as young-onset bilateral sensori-neural hearing loss. A mitochondrial mutation has been identified (m.3243A>G) (Fischel-Ghodsian, 2001).
When a more unusual form of diabetes is suspected, e.g. younger onset, a strong family history or a lack of the usual insulin resistance features, then discussion with your local specialist about the possibility of monogenic diabetes, the need for genetic testing and possible referral may be helpful. A very practical and educational website is www.diabetesgenes.org.
Diagnosing diabetes Diabetes can and should be diagnosed in primary care without specialist referral unless the individual’s condition is potentially life-threatening, such as diabetic ketoacidosis, or hyperglycaemia is severe and requiring immediate insulin treatment.
Currently, both the World Health Organization and International Diabetes Federation (WHO and IDF, 2006) and the ADA (2009) recommend that the diagnosis of diabetes (and pre-diabetes states) is based on a blood glucose measurement ( Table 1 ). Unless people have hyperglycaemic symptoms then this blood glucose estimation should be repeated; either repeated fasting plasma measures (after at least an 8-hour fast) or an oral glucose tolerance test (OGTT) (75 g of anhydrous glucose which equates to 410 ml of Lucozade Energy Original) are commonly used in primary care.
Traditionally, the OGTT has been promoted as the gold standard for the diagnosis of diabetes and has been used extensively in epidemiological studies. However, the recommended use of repeated fasting plasma glucose (FPG) estimations, which are cheap and more convenient for both doctor and patient, may well have moved UK primary care teams away from the OGTT. The use of OGTT is therefore debatable as it is intensive in terms of patient time, nurse time, and has surprisingly poor repeatability. A proportion of general practices do not therefore use it as a diagnostic tool. However, OGTT should be considered in people with impaired fasting glucose (IFG), 30% of whom will have diabetes if challenged with a glucose load (WHO and IDF, 2006).
Currently, there is also debate regarding the introduction of HbA 1c as the diagnostic test for diabetes. HbA 1c is the predominant form of glycated haemoglobin, present in red blood cells, which reflects the average plasma glucose concentration over the preceding 2–3 months, and is expressed as a percentage of HbA (International Expert Committee [IEC], 2009), and hence would give a better overall glycaemic picture. The new NHS Health Check Programme (2009) advocates the use of HbA 1c with a cut-off of >6.5% (>48 mmol/mol) as diagnostic of diabetes. The use of HbA 1c may therefore rapidly gain in popularity. It is more convenient (as it does not require a fasting specimen), is reliable and correlates well with long-term complications, hence its use in people once they are diagnosed with diabetes. International recommendations promoting the use of HbA 1c in diagnosis were recently published (IEC, 2009), and national bodies across the world are currently considering whether to implement HbA 1c as the diagnostic test for diabetes.
It should be noted that the diagnostic cut-offs for the development of diabetes specified in Table 1 are derived from plasma glucose levels associated with increased risk of retinopathy, as well as the population distribution of plasma glucose (WHO and IDF, 2006).
Risk factors for diabetes The most important risk factor for type 2 diabetes is obesity. There are, however, other modifiable and non-modifiable risk factors ( Table 2 ). These are used as risk indicators to identify those at higher risk of type 2 diabetes in several clinical settings, for example in risk-screening questionnaires such as FINDRISC (Finnish Type 2 Diabetes Risk Score; Lindström and Tuomilehto, 2003); in opportunistic screening in GP surgeries (Evans et al, 2008); in risk calculations using routinely collected data held in GP databases such as the QDScore (Hippisley-Cox et al, 2009); and in the new NHS Health Check Programme (2009) to identify those who should have a glucose test.
Pre-diabetes Another area of debate is the diagnosis of the intermediate hyperglycaemic states collectively known as pre-diabetes. All these conditions have in common the fact that blood glucose levels are raised yet are not above the threshold that is diagnostic of type 2 diabetes. The two most important features of pre-diabetes in primary care are the increased risk of CVD, which is two to three times that of normoglycaemic individuals (Coutinho et al, 1999), and the increased risk of progression to type 2 diabetes. Hence the potential for prevention of both diabetes and CVD in this high-risk group.
The term “pre-diabetes” has been considered by some as being potentially misleading, as a large proportion of people with pre-diabetes do not progress to diabetes. Other terms such as non-diabetic hyperglycaemia, intermediate hyperglycaemia and impaired glucose regulation are therefore gaining popularity. Risk factors for pre-diabetes are generally considered to be the same as those for type 2 diabetes as both conditions share the common pathology of insulin resistance.
The terminology is complicated, but currently two states are recognised: IFG diagnosed on repeated fasting blood glucose (FBG) measurements and impaired glucose tolerance (IGT) diagnosed on an OGTT ( Table 1 ). There is some debate, however, about the level of FPG in IFG. The ADA (2009) recommend that IFG includes an FPG of 5.6–6.9 mmol/L rather than the stricter criterion of 6.1–6.9 mmol/L in the WHO and IDF (2006) recommendations. A person may have either IFG or IGT (in isolation) or both (i.e. an FPG of 6.1–6.9 mmol/L and a 2-hour glucose ≥7.8 mmol/L and
People with pre-diabetes are asymptomatic. Nevertheless, some features of the metabolic syndrome may often be present. Also, a number of associated conditions, such as peripheral neuropathy (Singleton et al, 2005) and carpal tunnel syndrome (Gulliford et al, 2006), are increasingly being recognised. Despite these associations, people with pre-diabetes are usually diagnosed by screening.
Both IFG and IGT are increasingly prevalent. For example, it is estimated that 5.1% of the UK population aged 20–79 may have IGT (IDF, 2003). Pre-diabetes carries an increased risk of progression to type 2 diabetes, although this can vary dependent on ethnicity and other factors such as initial level of glycaemia (Unwin et al, 2002). On average, around 5% of people with IGT progress to type 2 diabetes annually (Santaguida et al, 2005). It is widely accepted that people with these conditions are at greater risk of both type 2 diabetes and CVD (Coutinho et al, 1999), and interventions designed to prevent diabetes have, in the main, been targeted at this population.
Education of people with pre-diabetes Previous work in developing a pragmatic screening programme using the GP database identified a large proportion of people with pre-diabetes (Greaves et al, 2004).
Studies had previously shown that individuals and healthcare professionals alike were confused about the implications of the diagnosis of pre-diabetes (Wylie et al, 2002; Whitford et al, 2003; Williams et al, 2004). The author and colleagues therefore developed an educational package for people with pre-diabetes and their healthcare professionals. This package, known as WAKEUP (Ways of Addressing Knowledge Education and Understanding in Prediabetes), was found to be acceptable both to people with pre-diabetes and healthcare professionals (Evans et al, 2006).
Managing pre-diabetes Although generic guidance was given to GPs and practice nurses, the qualitative data from healthcare professionals in the WAKEUP study revealed a need for robust practice systems to facilitate effective management and follow-up of individuals with pre-diabetes (Evans et al, 2006). Key messages in the WAKEUP study that should be conveyed to people with pre-diabetes were identified ( Table 3 ). Similar qualitative work undertaken by Troughton et al (2008) has also shown that this population expected structured follow-up after their diagnosis.
It should not be forgotten that people with pre-diabetes need appropriate lifestyle advice regarding smoking, alcohol, and possible prescription of lipid-lowering drugs, such as statins, and also blood pressure medication if appropriate. For these reasons an annual review in primary care would seem reasonable with these cardiovascular risk factors being addressed, and also an FBG test (or even OGTT) undertaken to assess any progression towards diabetes.
Primary prevention of type 2 diabetes As the transition from normoglycaemia through impaired glucose regulation to type 2 diabetes takes several years, it is logical to intervene and aim to prevent or delay the onset of diabetes. This can be at individual or population level. The best evidence regarding prevention exists in high-risk individuals, although several countries such as Finland have a national population programme to prevent diabetes that involves all stakeholders.
There is now substantial evidence from large-scale randomised trials in various populations across the world that progression to diabetes can be prevented or delayed in high-risk groups both by behavioural (Tuomilehto et al, 2001; Knowler et al, 2002; Ramachandran et al, 2006) and pharmacological interventions (Chiasson et al, 2002; Knowler et al, 2002; Lindström and Tuomilehto, 2003; Torgerson et al, 2004; Gerstein et al, 2006).
Lifestyle A meta-analysis has shown that lifestyle interventions can produce a 50% relative risk reduction in the incidence of type 2 diabetes at 1 year (Yamaoka and Tango, 2005). Typically these interventions are in high-risk individuals, such as those with pre-diabetes (usually IGT), and interventions are targeted at halting or slowing beta-cell dysfunction.
The majority of behavioural interventions are relatively intensive and designed to increase an individual’s physical activity levels and encourage weight loss and dietary change. Relatively modest changes in lifestyle, such as a 5% reduction in weight or an increase in moderate physical activity to 4 hours a week, can have important benefits in reducing the risk of diabetes.
In the Finnish Diabetes Prevention Study (DPS; Tuomilehto et al, 2001) a clear “dose–response” curve was observed, such that the greater the number of behavioural changes (the success score), the lower the risk of diabetes in an individual ( Figure 1 ). It was also noted that the beneficial effects observed in the Finnish DPS persisted when the participants were followed-up a median of 3 years after the intervention had finished (Lindström et al, 2006).
Lifestyle interventions of course have other general benefits for the individual. However, the majority of these interventions are not feasible or affordable in a resource-limited NHS, and there is therefore a need to develop, pilot and evaluate a pragmatic intervention that could be delivered in primary care or in the community. It is possible that this could be based on motivational interviewing (MI), and early results with MI in promoting weight loss in obese people through lay facilitators are encouraging (Greaves et al, 2008).
The need for a pragmatic intervention is now more urgent as the NHS Health Check Programme begins. A large number of people with pre-diabetes will undoubtedly be identified and will need intervention. These interventions will also need to be culturally sensitive in the light of the large number of people from ethnic communities in the UK with pre-diabetes.
Pharmacological interventions As well as lifestyle interventions, drugs have also been shown to reduce progression to type 2 diabetes, including metformin (Knowler et al, 2002; Ramachandran et al, 2006), acarbose (Chiasson et al, 2002), orlistat (Torgerson et al, 2004) as well as troglitazone – although later withdrawn (Azen et al, 1998) – and rosiglitazone (Gerstein et al, 2006).
A meta-analysis by Gillies et al (2008) showed that drug interventions were both less effective and less cost-effective than lifestyle. The IDF (Alberti et al, 2007) recommends drug therapy as second-line after lifestyle intervention for diabetes prevention, yet, unfortunately, no pharmaceutical agent is licensed for diabetes prevention in the UK.
There is also debate about whether these drugs simply mask progression to diabetes by lowering blood glucose, which then rises in the subsequent wash-out period once treatment has finished. On balance, however, it is generally thought that diabetes prevention through lifestyle or drugs is cost-effective and should be actively promoted in clinical practice (Gillies et al, 2008).
Practitioner behaviour In UK primary care there is a considerable gap between the theory of diabetes prevention and its active implementation. Several qualitative and questionnaire studies have shown that GPs and primary care staff are confused by the whole area of pre-diabetes and its diagnosis and wanted more information and guidance (Wylie et al, 2002; Whitford et al, 2003; Williams et al, 2004). GPs also expressed a variety of attitudes towards pre-diabetes, ranging from enthusiastically embracing its management to diagnostic nihilism (Fearn-Smith et al, 2007).
In the biggest database study to date (Holt et al, 2008), it was demonstrated that GPs were missing opportunities to diagnose both pre-diabetes and diabetes in their registered patients. For example, borderline blood glucose results were not being followed-up with either a repeat test or OGTT. Better education of healthcare professionals is therefore needed. Box 1 gives a case study highlighting some common problems encountered in primary care.
Screening for diabetes and pre-diabetes Although population screening is not thought to be appropriate (Wareham and Griffin, 2001), targeted or selective screening for both diabetes and pre-diabetes is now considered to be both effective and cost-effective (Waugh et al, 2008). Most authorities advise two-stage screening. First, individuals at higher risk of diabetes are identified using GP data or a questionnaire, such as FINDRISC (Lindström and Tuomilehto, 2003), and then a blood glucose test such as an FBG, an OGTT or an HbA 1c test is used.
NICE guidance on preventing type 2 diabetes will not be available until June 2011, although European guidance from the IMAGE (Development and Implementation of a European Guideline and Training Standards for Diabetes Prevention) project will be available in early 2010 ( http://www.image-project.eu/ ).
In the new NHS Health Check Programme, all people aged 40–74 years who are not on a disease register will be called in for a face-to-face check and assessment of their vascular risk. Those who are overweight or obese or have a raised blood pressure will also be screened for diabetes. Managing this exercise and its implications will be a major challenge to all practitioners in primary care who wish to prevent type 2 diabetes and its complications.
Conclusion The prevalence of type 2 diabetes is rapidly increasing in the UK, although primary care teams should be aware of the rarer types of diabetes (e.g. MODY or MIDD) as well as type 1 diabetes.
The risk factors for type 2 diabetes and pre-diabetes are well recognised and primary care teams are in an ideal position to screen for both conditions (either opportunistically or systematically). Finally, it is now clear that type 2 diabetes can be prevented or delayed by lifestyle or pharmacological interventions in those at highest risk.
To complete the CPD module, click here.
Alberti KG, Zimmet P, Shaw J; IDF Epidemiology Task Force Consensus Group (2005) The metabolic syndrome – a new worldwide definition. Lancet 366 : 1059–62 Alberti KG, Zimmet P, Shaw J (2007) International Diabetes Federation: a consensus on Type 2 diabetes prevention. Diabet Med 24 : 451–63 American Diabetes Association (2009) Diagnosis and classification of diabetes mellitus. Diabetes Care 32 : S62–7 Azen SP, Peters RK, Berkowitz K et al (1998) TRIPOD (TRoglitazone In the Prevention Of Diabetes): a randomized, placebo-controlled trial of troglitazone in women with prior gestational diabetes mellitus. Control Clin Trials 19 : 217–31 Chiasson JL, Josse RG, Gomis R et al (2002) Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet 359 : 2072–7 Coutinho M, Gerstein HC, Wang Y, Yusuf S (1999) The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care 22 : 233–40 Diabetes UK (2009) Diabetes in the UK 2009: Key Statistics on Diabetes. Diabetes UK, London Evans PH, Luthra M, Powell R et al (2003) Diagnosis of type 2 diabetes in primary care. British Journal of Diabetes and Vascular Disease 3 : 342–4 Evans PH, Winder R, Greaves C et al (2006) Ways of addressing knowledge, education and understanding in pre-diabetes: the WAKEUP study. Abstract. Diabet Med 23 (Suppl2): 132–3 Evans P, Langley P, Gray DP (2008) Diagnosing type 2 diabetes before patients complain of diabetic symptoms – clinical opportunistic screening in a single general practice. Fam Pract 25 : 376–81 Fearn-Smith JDG, Evans PH, Harding G, Campbell JL (2007) Attitudes of GPs to the diagnosis and management of impaired glucose tolerance: The practitioners’ attitudes to hyperglycaemia (PAtH) questionnaire. Primary Care Diabetes 1 : 35–41 Fischel-Ghodsian N (2001) Mitochondrial DNA mutations and diabetes: another step toward individualized medicine. Ann Intern Med 134 : 777–9 Garber AJ (2003) Cardiovascular complications of diabetes: prevention and management. Clin Cornerstone 5 : 22–37 Gerstein HC, Yusuf S, Bosch J et al (2006) Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet 368 : 1096–105 Gillies CL, Lambert PC, Abrams KR et al (2008) Different strategies for screening and prevention of type 2 diabetes in adults: cost effectiveness analysis. Br Med J 336 : 1180–5 Greaves CJ, Stead JW, Hattersley A et al (2004) A simple pragmatic system for detecting new cases of type 2 diabetes and impaired fasting glycaemia in primary care. Fam Pract 21 : 57–62 Greaves CJ, Middlebrooke A, O’Loughlin L et al (2008) Motivational interviewing for modifying diabetes risk: a randomised controlled trial. Br J Gen Pract 58 : 535–40 Gulliford MC, Latinovic R, Charlton J, Hughes RA (2006) Increased incidence of carpal tunnel syndrome up to 10 years before diagnosis of diabetes. Diabetes Care 29 : 1929–30 Harris MI, Klein R, Wellborn TA, Knuiman MW (1992) Onset of NIDDM occurs at least 4–7 years before clinical diagnosis. Diabetes Care 15 : 815–19 Hippisley-Cox J, Coupland C, Robson J et al (2009) Predicting risk of type 2 diabetes in England and Wales: prospective derivation and validation of QDScore. Br Med J 338 : b880 Holt TA, Stables D, Hippisley-Cox J et al (2008) Identifying undiagnosed diabetes: cross-sectional survey of 3.6 million patients’ electronic records. Br J Gen Pract 58 : 192–6 International Diabetes Federation (2003) Diabetes Atlas. 2nd ed. IDF, Brussels International Expert Committee (2009) Report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 32 : 1327–34 Knowler WC, Barrett-Connor E, Fowler SE et al (2002) Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346 : 393–403 Lindström J, Tuomilehto J (2003) The diabetes risk score: a practical tool to predict type 2 diabetes risk. Diabetes Care 26 : 725–31 Lindström J, Ilanne-Parikka P, Peltonen M et al (2006) Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet 368 : 1673–9 Murphy R, Ellard S, Hattersley AT (2008) Clinical implications of a molecular genetic classification of monogenic beta-cell diabetes. Nat Clin Pract Endocrinol Metab 4 : 200–13 NHS Health Check Programme (2009) NHS Health Check: Vascular Risk Assessment and Management Best Practice Guidance. Department of Health, London Ramachandran A, Snehalatha C, Mary S et al (2006) The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 49 : 289–97 Santaguida PL, Balion C, Hunt D et al (2005) Diagnosis, prognosis, and treatment of impaired glucose tolerance and impaired fasting glucose. Evid Rep Technol Assess (Summ) Aug: 1–11 Singleton JR, Smith AG, Russell J, Feldman EL (2005) Polyneuropathy with impaired glucose tolerance: implications for diagnosis and therapy. Curr Treat Options Neurol 7 : 33–42 Tabák AG, Jokela M, Akbaraly TN et al (2009) Trajectories of glycaemia, insulin sensitivity, and insulin secretion before diagnosis of type 2 diabetes: an analysis from the Whitehall II study. Lancet 373 : 2215–21 Torgerson JS, Hauptman J, Boldrin MN, Sjöström L (2004) XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care 27 : 155–61 Troughton J, Jarvis J, Skinner C et al (2008) Waiting for diabetes: perceptions of people with pre-diabetes: a qualitative study. Patient Educ Couns 72 : 88–93 Tuomilehto J, Lindström J, Eriksson JG et al (2001) Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 344 : 1343–50 UK Prospective Diabetes Study (UKPDS) Group (1998) Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352 : 837–53 Unwin N, Shaw J, Zimmet P, Alberti KG (2002) Impaired glucose tolerance and impaired fasting glycaemia: the current status on definition and intervention. Diabet Med 19 : 708–23 Wareham NJ, Griffin SJ (2001) Should we screen for type 2 diabetes? Evaluation against National Screening Committee criteria. BMJ 322 : 986–8 Waugh N, Scotland G, McNamee P et al (2008) Screening for type 2 diabetes: literature review and economic modelling. Health Technol Assess 11 : iii–iv, ix–xi, 1–125 Whitford DL, Lamont SS, Crosland A (2003) Screening for type 2 diabetes: is it worthwhile? Views of general practitioners and practice nurses. Diabet Med 20 : 155–8 Williams R, Rapport F, Elwyn G et al (2004) The prevention of type 2 diabetes: general practitioner and practice nurse opinions. Br J Gen Pract 54 : 531–5 World Health Organization, International Diabetes Federation (2006) Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycaemia. Report of a WHO/IDF Consultation. WHO, Geneva Wylie G, Hungin AP, Neely J (2002) Impaired glucose tolerance: qualitative and quantitative study of general practitioners’ knowledge and perceptions. BMJ 324 : 1190 Yamaoka K, Tango T (2005) Efficacy of lifestyle education to prevent type 2 diabetes: a meta-analysis of randomized controlled trials. Diabetes Care 28 : 2780–6 Yorkshire and Humber Public Health Observatory (2007) Diabetes Key Facts Supplement 2007. YHPHO, York
Diabetes Distilled: Smoking cessation cuts excess mortality rates after as little as 3 years
Impact of freestyle libre 2 on diabetes distress and glycaemic control in people on twice-daily pre-mixed insulin, updated guidance from the pcds and abcd: managing the national glp-1 ra shortage, diabetes distilled: fib-4 – a diagnostic and prognostic marker for liver and cardiovascular events and mortality, at a glance factsheet: tirzepatide for management of type 2 diabetes, editorial: lipid management, tirzepatide and hybrid closed-loop: what does new nice guidance recommend, case report: pancreatic cancer – assessing diabetes in a thin elderly person.

The mortality benefits of smoking cessation may be greater and accrue more rapidly than previously understood.

Expanding CGM eligibility criteria to include this patient group may be beneficial.
27 Mar 2024

Advice on selecting alternative glucose-lowering therapies when GLP-1 RAs used in the management of type 2 diabetes in adults are unavailable.
22 Mar 2024

Should sequential Fib-4 testing now be made part of ongoing care in people with obesity and/or type 2 diabetes?
18 Mar 2024
Sign up to all DiabetesontheNet journals
- CPD Learning
- Journal of Diabetes Nursing
- Diabetes Care for Children & Young People
- The Diabetic Foot Journal
- Diabetes Digest
Useful information
- Terms and conditions
- Privacy policy
- Editorial policies and ethics

By clicking ‘Subscribe’, you are agreeing that DiabetesontheNet.com are able to email you periodic newsletters. You may unsubscribe from these at any time. Your info is safe with us and we will never sell or trade your details. For information please review our Privacy Policy .
Are you a healthcare professional? This website is for healthcare professionals only. To continue, please confirm that you are a healthcare professional below.
We use cookies responsibly to ensure that we give you the best experience on our website. If you continue without changing your browser settings, we’ll assume that you are happy to receive all cookies on this website. Read about how we use cookies .

Diabetes Presentations
This list of presentations is designed to be a resource for people with diabetes, health care professionals, diabetes educators, and students. These presentations can be downloaded but the original authors should be referenced if used elsewhere. To download, right-click the link and choose “Save Link As” to download the PDFs. Take a look at presentations by:
John Walsh PA, CDE – Physician assistant and diabetes clinical specialist at Advanced Metabolic Care and Research in Escondido, CA. The webmaster of Diabetesnet.com, co-author of Pumping Insulin , Using Insulin , and Stop the Rollercoaster.
Gary Scheiner MS, CDE – Certified diabetes educator and owner and operator of Integrated Diabetes Services , which specializes in intensive blood glucose control and lifestyle intervention for people with diabetes.
Andrea Gasper, MS, PA-C – Physician assistant who provides clinical care at the VA San Diego Department of Endocrinology and Metabolism.
Presentations by John Walsh
Full list of presentations, past and present
Novel and Basic Pump Ideas
A presentation John gave to 60 industry personnel on novel and basic ideas to improve glucose outcomes for insulin pump wearers.
New devices and research regarding Type 1 diabetes from the 2018 ADA Conference in Orlando
Presented to the San Diego Pump Club on July 9, 2018
Tried and True Tips and New Advances in Diabetes
These slides review pump tips and pump advances with new information on artificial pancreas projects, implanted CGMs, and faster insulins. This presentation was given at the San Diego Take Control of Your Diabetes (TCOYD) meeting and at the San Diego Pump Club meeting in late 2016.
Clinical Benefits of CGM
Scripps Whittier Diabetes Institute on May 3, 2016 and Canadian endocrinologists visiting Dexcom on April 29, 2016 This talk covers benefits of pumps and CGMs, the 5 paths to better readings, identification of glucose patterns, and lots of tips on how to use real time and downloaded CGM data.
Infusion Sets—The Weakest Link?
San Diego Pump Club in September 2015 Little attention has been paid over the years to the effectiveness of the infusion set in the push to improve insulin pumps and CGMs and connect them with an algorithm into an artificial pancreas. Not all infusion sets are delivering insulin well. Too often Unexplained High Glucose or UHG happens from a malfunctioning infusion set and the wearer misdiagnoses it as “I ate the wrong thing.” Is the problem coming from a mismatch between poor infusion set design, the user’s technique, or other physiology? See what the latest research and thinking has to say about the state of infusion sets, including a discussion of a reduction in silent occlusions with BD’s new FlowSmart infusion set.
Glucose Control With Today’s Insulin Pumps and CGMs
3rd Annual Diabetes Type 1 Conference at ANA Intercontinental Tokyo in May 2015 Great info on all the latest insulin pumps available. Also has information on CGMs, optimal TDDs and more.
Insulin Pumps Secrets and Settings and for Great Glucose Control
Type One Nation Presentation at the Sansum Clinic Support Group in March 2015 Tips on state-of-the-art pumping with a CGM, steps to optimize pump settings, plus things you never knew about pumping. Why your TDD and DIA are so important. Common causes for unwanted readings. How CGM downloads and trend lines can help you trouble-shoot frequent highs, frequent lows, roller-coaster readings, post-meal spikes, and more. Tips on when your bolus calculator or infusion set may be part of the problem.
Insulin Pump Settings and Secrets for Great Glucose Control (PDF 9.7 mb)
Presentation by John Walsh PA, CDTC at Type One Nation in March 2015 Learn the nuts and bolts of pumping and get an overview of today’s state-of-the-art insulin pumps and how to set them up for best results. Get ready to learn the things you never knew about pumps and pumping.
Troubleshooting Common Control Issues (PDF 12.3 mb)
Presentation by John Walsh PA, CDTC at Type One Nation in March 2015 Review common sources for failure on an insulin pump and how to solve them. Learn how to trouble shoot unexpected high or low patterns and learn clever tricks to get the most out of your pump and CGM.
Insulin Pump Tune Up (PDF 2.7 mb)
Presentation by John Walsh PA, CDTC and Chris Sadler MA, PA-C, CDE, CDTC for TCOYD
Latest on Pumps, CGMs, and Connectivity (PDF 9.9 mb)
A presentation given in Ft Murray in November 2014
The Latest on Insulin Pumps and Glycemia (PDF 6.3 mb)
This Grand Rounds presentation, entitled “The Latest on Insulin Pumps and Glycemia” was given at the UCSD/VA Hospital in La Jolla, CA, on Feb. 12, 2014. Lots of tools, tips, and insights.
Advanced Pumping Techniques – TCOYD 2013 (PDF 6.8 mb)
A presentation on the latest in pumps and CGM, tips to achieve better glucose levels, and the latest breakthrough technologies in diabetes.
Exercise Tech – insulinINdependance Conference 2013 (PDF 7.2 mb)
John’s presentation at the Insulindependence Conference 2013 on the latest diabetes technology with a focus on insulin pumps and exercise.
The Latest on Insulin Pumps (PDF – 3.2 mb)
How to get the most out of an insulin pump for pump wearers. Includes lots of tips and tricks and how to remedy common mistakes.
Advanced Pump Workshop (PDF – 5.9 mb)
This half-day clinical workshop covers insulin pump therapy in great detail using results of the Actual Pump Practices Study. Topics include glucose management, how to tune the bolus calculator, BOB, DIA, insulin stacking, infusion set issues and solutions, and CGMs for better control.
Insulin Therapy – Taking it to the Next Level (PDF – 1.9 mb)
This presentation discusses insulin therapy and includes numerous tips on how to achieve the best results.
Glucose Management and the Actual Pump Practices Study
Presentation for St. Michael’s Hospital Staff for Medtronic of Canada, June 22, 2012
Slideshow (in parts) – #1-Glucose Management and Actual Pump Practices Study – #2-Bolus Calculator Settings – # 3 -DIA, BOB, and Insulin Stacking – # 4- Infusion Set Issues – # 5-CGMs for Better Control – or A PDF of all 5 Workshops
Advanced Pump Strategies – The Latest on Pumps and CGMs
Presentation at Toronto Congress Center for Animas Canada, June 23, 2012
2 Presentations- The Latests on Pumps & CGMs – or a PDF of Both Talks (4.6 mb)
Patch or Line Pump – Which Works Best?
Presentation at TCOYD Conference with Karmeen Kulkarni, RD, in San Diego, CA – November 12, 2011
Presentation on Patch and Line Pumps (PDF – 9.6 mb)
Evolution of Insulin Pumps & CGMs Toward An Artificial Pancreas
Presentation at Diabetes Technology Meeting in Burlingame, CA – Oct 27, 2011
Evolution of Insulin Pumps & CGMs – Toward An Artificial Pancreas (PDF – 9.4 mb)
Emerging Technologies: Bolus Calculators, Pumps And CGMs
Presentation to Children With Diabetes in Orlando, FL – July 2011
Web Presentation – Emerging Technologies (PDF – 12 mb)
Advanced Pumping Strategies That Work
Presentation to Children With Diabetes in Orlando, FL -July 2011
Web Presentation – PDF or Advanced Pumping Strategies (PDF – 9.8 mb)
Pumps and Sensors Practical Problem Solving
Presentation to Children With Diabetes in Charlotte, NC – September 2010
Web Presentation – PPT Presentation (8.5 mb ) – PDF (9.6 mb)
Advanced Pump Features And Their Use
Presentation to Children With Diabetes in La Jolla, CA- Oct 2009
Advanced Pump Features and Their Use provides information on special and future pump features, CGMs, pump settings, pumps for Type 2s, DIA and BOB. Please view the slideshow .
Pumping Basics: Start for Success
Pumping Basics provides information on reasons to use a pump, who’s a candidate, brands and features, CGMs, infusion set choices, pump start and the future of pumping. Please view the slideshow .
Management Tips For Insulin Use
Presentation to Diabetes Educators in Calgary, Alberta
Management Tips For Insulin Use covers why insulin is needed, what it does, and how to replace it when production is lost in Type 1a and Type 2 diabetes. Discusses long and rapid acting insulins, mixed insulin, insulin pens, use in Type 2 diabetes, causes for lows and highs, and new treatments in using insulin. Please view the slideshow or download a PDF version (2.6 MB) for easy viewing.
Future Insulin Pump Features
We have been developing new ideas for several years to make insulin pumps more helpful to wearers. You can see the full list of proposed insulin pump features as a slideshow or Powerpoint . Previous Versions: 12/18/08 – Slideshow , PDF
Proposed Insulin Pump Standards
This slideshow presents a preliminary set of Manufacturing Standards For Insulin Pumps designed to improve insulin pump use and medical outcomes. Comments and suggestions on these guidelines are welcomed. Please view the slideshow . Be patient — there are 29 proposed features presented over 157 slides. Previous versions: 12/03/08 ( Slideshow or Powerpoint ), 11/10/08 ( Slideshow or Powerpoint ) and 10/10/08 ( Slideshow or Powerpoint ).
Current & Emerging Technologies In Insulin Pumps & Continuous Monitors – May 2008
Learn about new trends in insulin pumps and continuous monitors in this web slideshow or downloadable Powerpoint presentation .
Introduction to Pumping – Starting and Success
August 2007 at the CWD Conference
Learn how to start pumping the right way with this helpful presentation. View the slideshow or download the Powerpoint Presentation .
Exercise, Pumps, & Continuous Monitors – June 2007 at the DESA Exercise Conference
Get the latest tips and tricks for combining insulin pumps and continuous monitors with your fitness plan from this slideshow or the downloadable Powerpoint presentation .
Insulin Pumps Give Different Bolus Recommendations When BOB Is Large – February 2007
Find out why differences in Bolus on Board calculations are important and when they will occur by viewing the BOB slideshow . You can also download the Powerpoint presentation .
Comparison Of The Two Currently Available Continuous Monitors – January 2007
The Dexcom STS and the Paradigm RT continuous monitors are currently available in the U.S. with a prescription. In this study, they are compared head to head while being worn by one person with Type 1 diabetes. Is one monitor better than the other? How close are the monitor readings to the Ultra fingerstick readings? Find out by viewing the comparison slideshow or downloading the Powerpoint presentation .
Smart Pumps & Tomorrow’s Intelligent Devices – July 2005
See how classic diabetes devices have improved and will continue to improve in this presentation. View the Slideshow or download the Powerpoint Presentation
Changes In Diabetes Care – September 2004
Learn about the history of insulin pumps in this slideshow
The Super Bolus and the Intelligent BG Alert – September 2004
This slideshow details new insulin pump ideas to improve glucose levels, avoid hypoglycemia, and speed correction of hyperglycemia.
Intelligent Devices – September 2004
This slideshow details the idea of a “Smart Pen” that demonstrates the possibilities for intelligent diabetes devices.
Simultaneous Device Wear May Disclose Disparate Continuous Glucose Monitoring Performance (PDF – 739 kb) by John Walsh PA CDE and Timothy Bailey MD FACE CPI Disparate Bolus Recommendations In Insulin Pump Therapy (PDF – 363 kb) by John Walsh PA CDE and Timothy Bailey MD FACE CPI Insulin Pump Settings A Major Source For Insulin Dose Errors (PDF – 507 kb) by John Walsh PA CDE, Dariusz Wroblewski PhD, and Timothy S. Bailey MD FACE CPI
Presentations by Gary Scheiner
Improving diabetes control with accurate carb counting.
One major advantage of carbohydrate counting is that it can improve your blood glucose levels. This slideshow details the best tips, tricks, and methods to accurately count carb. You can also download the Powerpoint Presentation .
Strategies for Combating After-Meal Highs
Learn how to measure and control after meal blood sugar levels in this helpful slideshow . You can also download the Powerpoint Presentation .
Hypoglycemia: Prevention and Treatment
This slideshow details a number of ways to prevent and treat hypoglycemia. You can also download the Powerpoint Presentation .
Presentations by Andrea Gasper
Pumps and dosing software: the latest advances.
Presented in 2007 at the ADA Diabetes Technology Clinical Focus Meeting
Read about the latest advances in insulin pump and dosing software in this helpful PDF presentation.
Got any suggestions?
We want to hear from you! Send us a message and help improve Slidesgo
Top searches
Trending searches

solar eclipse
25 templates

education technology
180 templates

32 templates

28 templates

thanksgiving
38 templates

Diabetes Presentation templates
Here at slidesgo, we would like to help you raise awareness about diabetes, a metabolic disorder related to insulin and the levels of glucose in our blood. with these google slides & powerpoint templates, you can make sure that the audience will pay attention to your speech, as these slides will keep them focused on the screen and your explanations..

Premium template
Unlock this template and gain unlimited access
Diabetes Education Center
Running a medical center specialized on diabetes involves lots of organization and management. What is the admission procedure? How are patients going to be allocated? What fun/free time activities do you offer? That’s an important question to think about, because offering your patients activities to disconnect can improve their mental...
Diabetes Infographics
Information on health issues requires pedagogical resources to make it understandable to others. At Slidesgo we have created this diabetes infographic template so that you can easily and entertainingly report on this disease. It contains numerous icons and illustrations to make the data more visual and easier to remember. With...

Gestational Diabetes
Gestational diabetes is diabetes that first appears during pregnancy. It usually occurs in the middle of the gestation period and it is necessary to take care of it so that it does not cause problems. For this, information is key, so we have created this beautiful pink template, with creative...

Patient with Gestational Diabetes Case Report
Download the Patient with Gestational Diabetes Case Report presentation for PowerPoint or Google Slides. A clinical case is more than just a set of symptoms and a diagnosis. It is a unique story of a patient, their experiences, and their journey towards healing. Each case is an opportunity for healthcare...

World Diabetes Day
Diabetes is a serious illness that affects millions of people all around the world, and making proper treatment, care and information accessible to everyone who needs it is an unresolved issue that needs more awareness. For reasons like these, the United Nations decided to establish November 14th as the World...

Diabetes Mellitus Breakthrough
Diabetes mellitus refers to a group of diseases that affects the way the body uses blood glucose. Many people in the world suffer from this disease, and we are sure that your breakthrough will give a new perspective to its diagnosis and treatment. Present it with this complete and impressive...

Type 2 Diabetes Breakthrough
Type 2 diabetes is a metabolic disorder with a high prevalence in the world. Hundreds of millions of people suffer from it every year. Basically, a person cannot produce enough insulin, which can cause very severe consequences. Are there news on the treatment of type 2 diabetes? Waste no time...

Diabetes Breakthrough: Insulin Increase
This is a modern template full of illustrations and resources that gives medical presentations a visual and creative touch. This design focuses on diabetes: a medical issue that affects the lives of millions of people. If you have investigated on this and have come up with a new treatment, these...

World Diabetes Day Infographics
Slidesgo is aware of how healthcare professionals love to have extra help when sharing information about diseases, treatments or breakthroughs. Today, we're releasing a series of infographic designs for presentations about diabetes, a serious illness that affects millions of people. Check them out! The main color used is blue, a...

Treatment of Hypoglycemia Breakthrough
When the glucose levels in our blood drop below normal, our body can experience symptoms such as shakiness, confusion, sweating, and fatigue. A more correct name for this condition is hypoglycemia, but there's treatment (usually, ingesting sugar and carbohydrates), but perhaps there's more to it. Any new breakthrough in this...

Diabetes Devices and Technology Breakthrough
This modern and cool template is the perfect way to showcase the amazing breakthroughs in diabetes technologies and devices. With this template, you can easily present the latest developments to help treat and control diabetes. It features modern visuals and graphics that provide insight into the technology, as well as...

Type 2 Diabetes Disease
Type 2 diabetes is a chronic condition that affects the way the body processes glucose. With this disease, the patient's body does not produce enough insulin or is resistant to it. Share your research on this important disease using this modern and minimalist template designed in pastel colors, with which...

Happy World Diabetes Day!
Celebrate World Diabetes Day in style with our fully editable Google Slides and PowerPoint template. Complete with illuminating light green design and vivid photographs, it provides a perfect platform to give an informative presentation about diabetes, its varied types, and summarize significant research. It also offers a fantastic opportunity to...

Diabetes Mellitus Disease
Diabetes mellitus refers to a group of diseases that affects the way the body uses blood glucose. Glucose is vital for health, as it is an important source of energy for the cells that make up muscles and tissues. Understanding a disease thoroughly is the first step to being able...

Download the Gestational Diabetes presentation for PowerPoint or Google Slides. Taking care of yourself and of those around you is key! By learning about various illnesses and how they are spread, people can get a better understanding of them and make informed decisions about eating, exercise, and seeking medical attention....

Atypical Diabetic Ketoacidosis Case Report
Are you tired of scribbling notes on scratch paper, or worse - forgetting vital details about your patients' cases? Well, don’t worry about it anymore! We've got a template that will make organizing your atypical diabetic ketoacidosis case reports a breeze! This watercolor beauty features a gentle pastel palette with...

Diabetic Ketoacidosis Disease
When it comes to educating others about diseases, having a clear and organized presentation is key. With tools like this Google Slides and PowerPoint template, creating a comprehensive and professional-looking presentation has never been easier. For those looking to inform others about diabetic ketoacidosis disease, this is a perfect design....

Diabetes Breakthrough
Medical breakthroughs are always good news, especially when it comes to diseases that affect millions of people in the world, such as diabetes. With this template you can share these achievements simply and clearly. It contains graphs, lists, infographics and tables to explain the research, methods used, clinical trial, phases,...
- Page 1 of 2
New! Make quick presentations with AI
Slidesgo AI presentation maker puts the power of design and creativity in your hands, so you can effortlessly craft stunning slideshows in minutes.

Register for free and start editing online

IMAGES
VIDEO
COMMENTS
This topic will review the clinical presentation, diagnosis, and initial evaluation of diabetes in nonpregnant adults. Screening for and prevention of diabetes, the etiologic classification of diabetes mellitus, the treatment of diabetes, as well as diabetes during pregnancy are discussed separately. (See "Screening for type 2 diabetes mellitus" .)
Type 1 diabetes and type 2 diabetes are heterogeneous diseases in which clinical presentation and disease progression may vary considerably. Classification is important for determining therapy, but some individuals cannot be clearly classified as having type 1 or type 2 diabetes at the time of diagnosis.
Type 2 diabetes mellitus consists of an array of dysfunctions characterized by hyperglycemia and resulting from the combination of resistance to insulin action, inadequate insulin secretion, and excessive or inappropriate glucagon secretion. Poorly controlled type 2 diabetes is associated with an array of microvascular, macrovascular, and neu...
Diabetes is a condition that happens when your blood sugar (glucose) is too high. It develops when your pancreas doesn't make enough insulin or any at all, or when your body isn't responding to the effects of insulin properly. Diabetes affects people of all ages. Most forms of diabetes are chronic (lifelong), and all forms are manageable ...
Diabetes mellitus is a chronic heterogeneous metabolic disorder with complex pathogenesis. It is characterized by elevated blood glucose levels or hyperglycemia, which results from abnormalities in either insulin secretion or insulin action or both. Hyperglycemia manifests in various forms with a varied presentation and results in carbohydrate ...
Diabetes mellitus (DM) is a chronic metabolic disorder characterized by persistent hyperglycemia. It may be due to impaired insulin secretion, resistance to peripheral actions of insulin, or both. ... DM is broadly classified into three types by etiology and clinical presentation, type 1 diabetes, type 2 diabetes, and gestational diabetes (GDM ...
Diabetes is a disease that occurs when your blood glucose, also called blood sugar, is too high. Glucose is your body's main source of energy. Your body can make glucose, but glucose also comes from the food you eat. Insulin is a hormone made by the pancreas that helps glucose get into your cells to be used for energy.
Diabetes mellitus is taken from the Greek word diabetes, meaning siphon - to pass through and the Latin word mellitus meaning sweet. A review of the history shows that the term "diabetes" was first used by Apollonius of Memphis around 250 to 300 BC. Ancient Greek, Indian, and Egyptian civilizations discovered the sweet nature of urine in this condition, and hence the propagation of the word ...
People with type 2 diabetes mellitus (T2DM) may be asymptomatic or present with classic symptoms. In the context of thirst, polydipsia and polyuria, the presence or absence of weight loss is an important diagnostic feature. The mode of presentation of slow-onset type 1, monogenic and pancreatic diabetes is helpful in diagnosis.
Type 1 Diabetes. • About 5 -10% of people with diabetes have Type 1 • Autoimmune disorder - Body attacks itself and destroys cells in pancreas which produce insulin • Pancreas is unable to produce insulin and cannot use glucose • Must inject themselves with insulin through shots or insulin pump • This is a blood glucose monitor used ...
Diabetes is a chronic (long-lasting) health condition that affects how your body turns food into energy. Your body breaks down most of the food you eat into sugar (glucose) and releases it into your bloodstream. When your blood sugar goes up, it signals your pancreas to release insulin. Insulin acts like a key to let the blood sugar into your ...
Diabetes Basics. Diabetes is a chronic (long-lasting) disease that affects how your body turns food into energy. There are three main types of diabetes: type 1, type 2, and gestational diabetes (diabetes while pregnant). More than 133 million Americans are living with diabetes (37.3 million) or prediabetes (96 million).
Slide Deck. This comprehensive slide deck of ADA's 2023 Standards of Care contains content created, reviewed, and approved by the American Diabetes Association. You are free to use the slides in presentations without further permission as long as the slide content is not altered in any way and appropriate attribution is made to the American ...
The designations employed and the presentation of the material in this publication do not imply the expression of ... Diabetes is a serious, chronic disease that occurs either when the pancreas does not produce enough insulin (a hormone that regulates blood sugar, or glucose), or when the body cannot effectively use the insulin it ...
6 /25. Some health habits and medical conditions related to your lifestyle can raise your odds of having type 2 diabetes, including: Being overweight, especially at the waist. A couch potato ...
General presentation. Diabetes mellitus (DM) is an important endocrine disorder that presents commonly in children and adolescents. There are two types of diabetes mellitus: type 1 and type 2. Type 1 DM is one of the most common chronic diseases in children and is characterized by insulin deficiency as a result of autoimmune destruction of ...
Type 1 diabetes is a chronic illness characterized by the body's inability to produce insulin due to the autoimmune destruction of the beta cells in the pancreas. Onset most often occurs in childhood, but the disease can also develop in adults in their late 30s and early 40s. ... Symptoms at the time of the first clinical presentation can ...
Diabetes mellitus is characterized by abnormally high levels of sugar (glucose) in the blood. When the amount of glucose in the blood increases, e.g., after a meal, it triggers the release of the hormone insulin from the pancreas. Insulin stimulates muscle and fat cells to remove glucose from the blood and stimulates the liver to metabolize ...
DKA is defined as an acute metabolic complication of diabetes distinguished by hyperglycemia, hyperketonemia, and metabolic acidosis. DKA occurs primarily in T1DM and is less common in T2DM; it can present with nausea, vomiting, and abdominal pain and cause cerebral edema, coma, and death. DKA may be the initial presentation in an estimated 25% ...
This article explores the classification and diagnosis of diabetes, focusing on risk factors, pre-diabetes, and management and prevention strategies for type 2 diabetes in primary care. In 2007 it was estimated that 4.82% of the UK population have diabetes (2.45 million people) (Yorkshire and Humber Public Health Observatory, 2007).
One year before the current presentation, the weight was 48 kg, and the BMI was 19.4. Medications for diabetes included subcutaneous insulin glargine (34 units daily), subcutaneous insulin lispro ...
This list of presentations is designed to be a resource for people with diabetes, health care professionals, diabetes educators, and students. These presentations can be downloaded but the original authors should be referenced if used elsewhere. To download, right-click the link and choose "Save Link As" to download the PDFs.
Diabetes Presentation templates Here at Slidesgo, we would like to help you raise awareness about diabetes, a metabolic disorder related to insulin and the levels of glucose in our blood. With these Google Slides & PowerPoint templates, you can make sure that the audience will pay attention to your speech, as these slides will keep them focused ...
Emilia Gómez Hoyos, Patricia Cabrera García, Marcelino Gómez Balaguer, en nombre del Grupo de Trabajo de Gónada, Identidad y Diferenciación Sexual de la Sociedad Española de Endocrinología y Nutrición (GT-GIDSEEN)
Presentation Title: Dermal Fibroblast Spheroid-based Treatment of Chronic Wounds in a Diabetes Mouse Model. Presenter: Subhiksha Raghuram, MS, MD, Associate Scientist, FibroBiologics. Poster ...