- Search by keyword
- Search by citation
Page 1 of 37

Correction: Dietary intake and gastrointestinal symptoms are altered in children with Autism Spectrum Disorder: the relative contribution of autism-linked traits
The original article was published in Nutrition Journal 2024 23 :27
- View Full Text
A late eating midpoint is associated with increased risk of diabetic kidney disease: a cross-sectional study based on NHANES 2013–2020
Modifying diet is crucial for diabetes and complication management. Numerous studies have shown that adjusting eating habits to align with the circadian rhythm may positively affect metabolic health. However, ...
Traditional japanese diet score and the sustainable development goals by a global comparative ecological study
Reducing the environmental impact of the food supply is important for achieving Sustainable Development Goals (SDGs) worldwide. Previously, we developed the Traditional Japanese Diet Score (TJDS) and reported ...
Association between dietary magnesium intake and muscle mass among hypertensive population: evidence from the National Health and Nutrition Examination Survey
Magnesium is critical for musculoskeletal health. Hypertensive patients are at high risk for magnesium deficiency and muscle loss. This study aimed to explore the association between magnesium intake and muscl...
Adult dietary patterns with increased bean consumption are associated with greater overall shortfall nutrient intakes, lower added sugar, improved weight-related outcomes and better diet quality
Limited evidence is available that focuses on beans within American dietary patterns and health. The purpose of this study was to identify commonly consumed adult dietary patterns that included beans and compa...
Validity and reproducibility of the PERSIAN Cohort food frequency questionnaire: assessment of major dietary patterns
Dietary patterns, encompassing an overall view of individuals’ dietary intake, are suggested as a suitable means of assessing nutrition’s role in chronic disease development. The aim of this study was to evalu...
Associations of dietary patterns and longitudinal brain-volume change in Japanese community-dwelling adults: results from the national institute for longevity sciences-longitudinal study of aging
The association of dietary patterns and longitudinal changes in brain volume has rarely been investigated in Japanese individuals. We prospectively investigated this association in middle-aged and older Japane...
Association between serum 25-hydroxyvitamin D and vitamin D dietary supplementation and risk of all-cause and cardiovascular mortality among adults with hypertension
The relationship between vitamin D status and mortality among adults with hypertension remains unclear.
Effect of soy isoflavone supplementation on blood pressure: a meta-analysis of randomized controlled trials
Previous experimental studies have suggested that the consumption of soy isoflavones may have a potential impact on lowering blood pressure. Nevertheless, epidemiological studies have presented conflicting out...
The effects of L-carnitine supplementation on inflammation, oxidative stress, and clinical outcomes in critically Ill patients with sepsis: a randomized, double-blind, controlled trial
Sepsis, a life-threatening organ dysfunction caused by a host’s dysregulated response to infection with an inflammatory process, becomes a real challenge for the healthcare systems. L-carnitine (LC) has antiox...
Metabolic syndrome risk in adult coffee drinkers with the rs301 variant of the LPL gene
Metabolic syndrome (MetS), a cluster of metabolic and cardiovascular risk factors is influenced by environmental, lifestyle, and genetic factors. We explored whether coffee consumption and the rs301 variant of...
Towards objective measurements of habitual dietary intake patterns: comparing NMR metabolomics and food frequency questionnaire data in a population-based cohort
Low-quality, non-diverse diet is a main risk factor for premature death. Accurate measurement of habitual diet is challenging and there is a need for validated objective methods. Blood metabolite patterns refl...
Circulating concentrations of bile acids and prevalent chronic kidney disease among newly diagnosed type 2 diabetes: a cross-sectional study
The relationship between circulating bile acids (BAs) and kidney function among patients with type 2 diabetes is unclear. We aimed to investigate the associations of circulating concentrations of BAs, particul...
Dietary intake and gastrointestinal symptoms are altered in children with Autism Spectrum Disorder: the relative contribution of autism-linked traits
Dietary and gastrointestinal (GI) problems have been frequently reported in autism spectrum disorder (ASD). However, the relative contributions of autism-linked traits to dietary and GI problems in children wi...
The Correction to this article has been published in Nutrition Journal 2024 23 :40
The effect of bovine dairy products and their components on the incidence and natural history of infection: a systematic literature review
Dairy products and their components may impact immune function, although the current evidence base has some research gaps. As part of a larger systematic literature review of dairy products/components (includi...
Food sufficiency status and sleep outcomes in older adults: the National Health and Aging Trends Study (NHATS)
Studies investigating the relationship between food insecurity and sleep among older populations are limited. This study aimed to cross-sectionally examine the associations between food sufficiency status and ...
Effects of vitamin D supplementation on liver fibrogenic factors, vitamin D receptor and liver fibrogenic microRNAs in metabolic dysfunction-associated steatotic liver disease (MASLD) patients: an exploratory randomized clinical trial
Metabolic dysfunction-associated steatotic liver disease (MASLD) is a global metabolic problem which can lead to irreversible liver fibrosis. It has been shown that vitamin D and its receptors contribute to fi...
Validity of food and nutrient intakes assessed by a food frequency questionnaire among Chinese adults
Studies regarding the validity of the food frequency questionnaire (FFQ) and the food composition table (FCT) are limited in Asian countries. We aimed to evaluate the validity of a 64-item FFQ and different me...
Association of sugar intake from different sources with cardiovascular disease incidence in the prospective cohort of UK Biobank participants
The relation between incident cardiovascular disease (CVD) and sugar might not only depend on the quantity consumed but also on its source. This study aims to assess the association between various sources of ...
Association of dietary inflammatory index and the SARS-CoV-2 infection incidence, severity and mortality of COVID-19: a systematic review and dose-response meta-analysis
Several studies have reported the association between dietary inflammatory index (DII) and the SARS-CoV-2 infection risk, severity or mortality of COVID-19, however, the outcomes remain controversial.
Breakfast quality and its sociodemographic and psychosocial correlates among Italian children, adolescents, and adults from the Italian Nutrition & HEalth Survey (INHES) study
Breakfast quality, together with regularity of breakfast, has been suggested to be associated with cardiometabolic health advantages. We aimed to evaluate the quality of breakfast and its socioeconomic and psy...
The association between lifelines diet score (LLDS) with depression and quality of life in Iranian adolescent girls
It has been proposed that a greater degree of adherence to a healthy dietary pattern is associated with a lower risk of depression and a poor quality of life (QoL). The Lifelines diet score (LLDS) is a new, ev...
Diet in secondary prevention: the effect of dietary patterns on cardiovascular risk factors in patients with cardiovascular disease: a systematic review and network meta-analysis
Improving dietary habits is a first-line recommendation for patients with cardiovascular disease (CVD). It is unclear which dietary pattern most effectively lowers cardiovascular risk factors and what the shor...
Prognostic potential of nutritional risk screening and assessment tools in predicting survival of patients with pancreatic neoplasms: a systematic review
The nutritional evaluation of pancreatic cancer (PC) patients lacks a gold standard or scientific consensus, we aimed to summarize and systematically evaluate the prognostic value of nutritional screening and ...
40 years of adding more fructose to high fructose corn syrup than is safe, through the lens of malabsorption and altered gut health–gateways to chronic disease
Labels do not disclose the excess-free-fructose/unpaired-fructose content in foods/beverages. Objective was to estimate excess-free-fructose intake using USDA loss-adjusted-food-availability (LAFA) data (1970–...
Relationship between trajectories of dietary iron intake and risk of type 2 diabetes mellitus: evidence from a prospective cohort study
The association between dietary iron intake and the risk of type 2 diabetes mellitus (T2DM) remains inconsistent. In this study, we aimed to investigate the relationship between trajectories of dietary iron in...
Dietary pattern and precocious puberty risk in Chinese girls: a case-control study
The role of dietary intake on precocious puberty remains unclear. This study aimed to investigate the association between the amount and frequency of dietary intake and the risk of precocious puberty in Chines...
Tracking progress toward a climate-friendly public food service strategy: assessing nutritional quality and carbon footprint changes in childcare centers
Public food procurement and catering are recognized as important leverage points in promoting sustainable and healthy dietary habits. This study aimed to analyze changes in nutritional quality and carbon footp...
Avocado intake and cardiometabolic risk factors in a representative survey of Australians: a secondary analysis of the 2011–2012 national nutrition and physical activity survey
Avocados are a rich source of nutrients including monounsaturated fats, dietary fibre and phytochemicals. Higher dietary quality is reported in studies of consumers with higher avocado intakes. The present stu...
Components in downstream health promotions to reduce sugar intake among adults: a systematic review
Excessive sugar consumption is well documented as a common risk factor for many Non-Communicable Diseases (NCDs). Thus, an adequate intervention description is important to minimise research waste and improve ...
Improving economic access to healthy diets in first nations communities in high-income, colonised countries: a systematic scoping review
Affordability of healthy food is a key determinant of the diet-related health of First Nations Peoples. This systematic scoping review was commissioned by the Ngaanyatjarra Pitjantjatjara Yankunytjatjara Women...
Associations between estimation of salt intake and salt-restriction spoons and hypertension status in patients with poorly controlled hypertension: a community-based study from Huzhou City, Eastern China
As the prevalence of hypertension increases in China, it is advised to use salt-restriction spoons (SRS) as a lifestyle modification. This study aimed to examine the associations between estimated salt consump...
Potassium levels and the risk of all-cause and cardiovascular mortality among patients with cardiovascular diseases: a meta-analysis of cohort studies
Abnormal blood potassium levels are associated with an increased risk of cardiometabolic diseases and mortality in the general population; however, evidence regarding the association between dyskalemia and mor...
Combined versus independent effects of exercise training and intermittent fasting on body composition and cardiometabolic health in adults: a systematic review and meta-analysis
Exercise training (Ex) and intermittent fasting (IF) are effective for improving body composition and cardiometabolic health overweight and obese adults, but whether combining Ex and IF induces additive or syn...
Correction: Associations Between Plant-Based Dietary Patterns and Risks of Type 2 Diabetes, Cardiovascular Disease, Cancer, and Mortality – A Systematic Review and Meta-analysis
The original article was published in Nutrition Journal 2023 22 :46
The association between hyperuricemia and insulin resistance surrogates, dietary- and lifestyle insulin resistance indices in an Iranian population: MASHAD cohort study
Previous studies have reported insulin resistance (IR) to be associated with hyperuricemia. In this study, we aimed to assess the possible associations between the empirical dietary index for IR (EDIR), the em...
Trends and disparities in prevalence of cardiometabolic diseases by food security status in the United States
Previous studies have demonstrated the association between food security and cardiometabolic diseases (CMDs), yet none have investigated trends in prevalence of CMDs by food security status in the United State...
Effect of nutrition education integrating the health belief model and theory of planned behavior on dietary diversity of pregnant women in Southeast Ethiopia: a cluster randomized controlled trial
Maternal anemia, miscarriage, low birth weight (LBW), preterm birth (PTB), intrauterine growth restriction (IUGR), prenatal and infant mortality, morbidity, and the risk of chronic disease later in life are al...
The effect of diet-induced weight loss on circulating homocysteine levels in people with obesity and type 2 diabetes
Having type 2 diabetes (T2D) in combination with being overweight results in an additional increase in cardiovascular disease (CVD) risk. In addition, T2D and obesity are associated with increased levels of to...
Association of early dietary fiber intake and mortality in septic patients with mechanical ventilation based on MIMIC IV 2.1 database: a cohort study
Whether early dietary fiber intake in septic patients is associated with a better clinical prognosis remains unclear, especially the time and the amount. Therefore, we assessed the association between early di...
Comparison of energy expenditure measurements by a new basic respiratory room vs. classical ventilated hood
Nutritional support is often based on predicted resting energy expenditure (REE). In patients, predictions seem invalid. Indirect calorimetry is the gold standard for measuring EE. For assessments over longer ...
Clusters of carbohydrate-rich foods and associations with type 2 diabetes incidence: a prospective cohort study
About one in ten adults are living with diabetes worldwide. Intake of carbohydrates and carbohydrate-rich foods are often identified as modifiable risk factors for incident type 2 diabetes. However, strong cor...
Interaction between CETP Taq1B polymorphism and dietary patterns on lipid profile and severity of coronary arteries stenosis in patients under coronary angiography: a cross-sectional study
Evidence indicates there are still conflicts regarding CETP Taq1B polymorphism and coronary artery disease risk factors. Current findings about whether dietary patterns can change the relationship of the Taq1B...
The effects of curcumin-piperine supplementation on inflammatory, oxidative stress and metabolic indices in patients with ischemic stroke in the rehabilitation phase: a randomized controlled trial
Stroke is a leading cause of death worldwide, which is associated with a heavy economic and social burden. The purpose of this study was to investigate the effects of supplementation with curcumin-piperine com...
Relationship between dietary carotenoid intake and sleep duration in American adults: a population-based study
To investigate the relationship between dietary carotenoid intake and sleep duration.
Different dietary carbohydrate component intakes and long-term outcomes in patients with NAFLD: results of longitudinal analysis from the UK Biobank
This study aimed to investigate the association between the intake of different dietary carbohydrate components and the long-term outcomes of non-alcoholic fatty liver disease (NAFLD).
Association between frequency of breakfast intake before and during pregnancy and developmental delays in children: the Tohoku Medical Megabank Project Birth and Three-Generation Cohort Study
Although an association between maternal nutritional intake and developmental delays in children has been demonstrated, the association of the timing of meal intake and development delays remains unclear. We e...
Development and validation of a novel food exchange system for Chinese pregnant women
The dietary nutritional status of pregnant women is critical for maintaining the health of both mothers and infants. Food exchange systems have been employed in the nutritional guidance of patients in China, a...
Distribution of water turnover by sex and age as estimated by prediction equation in Japanese adolescents and adults: the 2016 National Health and Nutrition Survey, Japan
Although water is essential to the maintenance of health and life, standard values for human water requirements are yet to be determined. This study aimed to evaluate the distribution of water turnover (WT) ac...
Methylmalonic acid, vitamin B12, and mortality risk in patients with preexisting coronary heart disease: a prospective cohort study
The inconsistent relationship between Vitamin B12 (B12), methylmalonic acid (MMA, marker of B12 deficiency) and mortality was poorly understood, especially in patients with coronary heart disease (CHD). This s...
- Editorial Board
- Manuscript editing services
- Instructions for Editors
- Sign up for article alerts and news from this journal
- Follow us on Twitter
Annual Journal Metrics
2022 Citation Impact 5.4 - 2-year Impact Factor 5.3 - 5-year Impact Factor 1.507 - SNIP (Source Normalized Impact per Paper) 1.136 - SJR (SCImago Journal Rank)
2023 Speed 21 days submission to first editorial decision for all manuscripts (Median) 181 days submission to accept (Median)
2023 Usage 2,353,888 downloads 3,953 Altmetric mentions
- More about our metrics
Nutrition Journal
ISSN: 1475-2891
- General enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Open access
- Published: 30 March 2024
Precision nutrition to reset virus-induced human metabolic reprogramming and dysregulation (HMRD) in long-COVID
- A. Satyanarayan Naidu ORCID: orcid.org/0000-0002-6008-0482 1 , 2 ,
- Chin-Kun Wang ORCID: orcid.org/0000-0001-5371-7847 1 , 3 ,
- Pingfan Rao 1 , 4 ,
- Fabrizio Mancini 1 , 5 , 6 ,
- Roger A. Clemens ORCID: orcid.org/0000-0002-5898-9793 1 , 7 ,
- Aman Wirakartakusumah 8 , 9 ,
- Hui-Fang Chiu 10 ,
- Chi-Hua Yen 11 ,
- Sebastiano Porretta 1 , 12 , 13 ,
- Issac Mathai 1 , 14 &
- Sreus A. G. Naidu 1 , 2
npj Science of Food volume 8 , Article number: 19 ( 2024 ) Cite this article
10k Accesses
264 Altmetric
Metrics details
- Mechanisms of disease
SARS‐CoV‐2, the etiological agent of COVID-19, is devoid of any metabolic capacity; therefore, it is critical for the viral pathogen to hijack host cellular metabolic machinery for its replication and propagation. This single-stranded RNA virus with a 29.9 kb genome encodes 14 open reading frames (ORFs) and initiates a plethora of virus–host protein–protein interactions in the human body. These extensive viral protein interactions with host-specific cellular targets could trigger severe human metabolic reprogramming/dysregulation (HMRD), a rewiring of sugar-, amino acid-, lipid-, and nucleotide-metabolism(s), as well as altered or impaired bioenergetics, immune dysfunction, and redox imbalance in the body. In the infectious process, the viral pathogen hijacks two major human receptors, angiotensin-converting enzyme (ACE)-2 and/or neuropilin (NRP)-1, for initial adhesion to cell surface; then utilizes two major host proteases, TMPRSS2 and/or furin, to gain cellular entry; and finally employs an endosomal enzyme, cathepsin L (CTSL) for fusogenic release of its viral genome. The virus-induced HMRD results in 5 possible infectious outcomes: asymptomatic, mild, moderate, severe to fatal episodes; while the symptomatic acute COVID-19 condition could manifest into 3 clinical phases: (i) hypoxia and hypoxemia (Warburg effect), (ii) hyperferritinemia (‘cytokine storm’), and (iii) thrombocytosis (coagulopathy). The mean incubation period for COVID-19 onset was estimated to be 5.1 days, and most cases develop symptoms after 14 days. The mean viral clearance times were 24, 30, and 39 days for acute, severe, and ICU-admitted COVID-19 patients, respectively. However, about 25–70% of virus-free COVID-19 survivors continue to sustain virus-induced HMRD and exhibit a wide range of symptoms that are persistent, exacerbated, or new ‘onset’ clinical incidents, collectively termed as post-acute sequelae of COVID-19 (PASC) or long COVID. PASC patients experience several debilitating clinical condition(s) with >200 different and overlapping symptoms that may last for weeks to months. Chronic PASC is a cumulative outcome of at least 10 different HMRD-related pathophysiological mechanisms involving both virus-derived virulence factors and a multitude of innate host responses. Based on HMRD and virus-free clinical impairments of different human organs/systems, PASC patients can be categorized into 4 different clusters or sub-phenotypes: sub-phenotype-1 (33.8%) with cardiac and renal manifestations; sub-phenotype-2 (32.8%) with respiratory, sleep and anxiety disorders; sub-phenotype-3 (23.4%) with skeleto-muscular and nervous disorders; and sub-phenotype-4 (10.1%) with digestive and pulmonary dysfunctions. This narrative review elucidates the effects of viral hijack on host cellular machinery during SARS-CoV-2 infection, ensuing detrimental effect(s) of virus-induced HMRD on human metabolism, consequential symptomatic clinical implications, and damage to multiple organ systems; as well as chronic pathophysiological sequelae in virus-free PASC patients. We have also provided a few evidence-based, human randomized controlled trial (RCT)-tested, precision nutrients to reset HMRD for health recovery of PASC patients.
Similar content being viewed by others
A ketogenic diet can mitigate SARS-CoV-2 induced systemic reprogramming and inflammation
Amelia Palermo, Shen Li, … Arjun Deb
Metabolic alterations upon SARS-CoV-2 infection and potential therapeutic targets against coronavirus infection
Peiran Chen, Mandi Wu, … Ming-Liang He

Trans cohort metabolic reprogramming towards glutaminolysis in long-term successfully treated HIV-infection
Flora Mikaeloff, Sara Svensson Akusjärvi, … Ujjwal Neogi
Introduction
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), an enveloped, positive-sense, single-stranded RNA virus, is the etiological agent of Coronavirus Disease 2019 (COVID-19) 1 . The World Health Organization (W.H.O.) estimates that after recovery from acute phase of SARS-CoV-2 infection, around a quarter of such population experience persistent or new-onset symptoms in long-term referred to as ‘ post-acute sequalae of COVID ’ (PASC) or long COVID 2 . Accordingly, more than 173 million individuals around the world have PASC, based on a conservative estimated incidence of 25% of infected people and over 692 million documented COVID-19 cases globally 3 , 4 , 5 . The transition of post-COVID patients (after recovery from acute SARS-CoV-2 infection) to a virus-free disease state with lingering/chronic clinical manifestations, has emerged as a new global health crisis—the long-COVID.
PASC could encompass several adverse clinical impairments that may trigger chronic metabolic dysfunctions involving cardiovascular (CV), central/peripheral nervous (CNS/PNS), gastrointestinal (GI), pulmonary, reproductive, skeleto-muscular, and endocrinal systems 6 , 7 . New onset metabolic disorders also include type 2 diabetes mellitus (T2DM), myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and dysautonomia, especially the postural orthostatic tachycardia syndrome (POTS) 8 , 9 , 10 , 11 . PASC could inflict a plethora of long-term symptoms that may linger for years, while clinical manifestations of new onset ME/CFS and POTS may persist throughout lifespan 5 , 12 , 13 .
SARS-CoV-2, the newly emerged RNA (29.9-kb) virus possess a unique genomic ability to insert and ‘reprogram’ a mega-fold larger size human DNA (3.1-Mb) and its cellular metabolic machinery to prime, alter, and redirect host macro-molecules for its own life cycle. The SARS-CoV-2 genome interacts with a few thousands of human metabolites in a specific manner to facilitate its infectious process 14 , 15 , 16 . Furthermore, the virus particle categorically hijacks vital human factors for its cell surface binding, host invasion, and viral RNA integration with human DNA. Accordingly, SARS‐CoV‐2 genomic ‘reprogramming’ of human DNA and its host hijacking of vital cellular factors cumulatively results in metabolic ‘dysregulation’ in the body 17 , 18 , 19 , 20 . Interaction of SARS-CoV-2 proteins with specific host cell targets also rewire metabolic pathways, and alter or impair bioenergetics, immune response, and redox homeostasis in the human body, to favor the virus 21 , 22 . Thus, SARS-CoV-2 proteins could sense the host cellular metabolic status and trigger human metabolic reprogramming/dysregulation (HMRD) in the infected human host. Accordingly, the unique genomic map of SARS-CoV-2 virus and the extent of its mediated virus–host protein–protein interactions that trigger human metabolic reprogramming and dysregulation (HMRD) in favor of the virus life cycle, defines the ultimate severity and fate of COVID-19 23 , 24 , 25 , 26 .
In many COVID-19 cases, the virus-induced HMRD may not reset or revert even after patient discharge as virus-free (RT-PCR negative) survivors. Interestingly, several persistent clinical manifestation(s) of post-COVID symptoms in PASC patients, sustain as an aftermath from earlier SARS-CoV-2-mediated hijack of host cellular factors (i.e., ACE2, NRP1, furin, TMPRSS2, and CTSL); in tandem with other human factors such as the human leukocyte antigen (HLA), epigenetics, preexisting comorbidities (i.e., T2DM, CVD, obesity), age, preceding systemic impairments (i.e., hyperinflammation, micro-thrombosis, fibrosis, dysbiosis, autoimmunity, etc.), and socio-demographic factors (i.e., food security, environment, access to medical care) 22 , 27 , 28 .
Healthcare strategies to combat PASC, the novel virus-induced human metabolic syndrome, requires an in-depth understanding of the following: (i) the genomic and metabolomic (proteomic/lipidomic) signatures of SARS-CoV-2 and their interactions with host cellular metabolic machinery, (ii) the virus-induced HMRD and resulting pathophysiological manifestations in the onset and progression of COVID-19, (iii) the cumulative role of HMRD, symptomatic outcomes (disease spectrum), and comorbidities in systemic/multi-organ dysfunction during COVID-19, (iv) both virus- and host-mediated factors that contribute to transition of acute SARS-CoV-2 infection (COVID-19) into a persistent chronic state of virus-free PASC, (v) protracted effects of HMRD in tandem with patient’s history, in the development of ‘new onset’ metabolic syndromes (i.e., T2DM, CVD, ME/CFS and POTS) among PASC patients, (vi) stratification/categorization of PASC patients based on persistent symptoms, organ/system involvement, and metabolic dysfunction for specific target-delivered health recovery regimens, and (vii) the structure-function activity of specific bio-functional dietary compounds in formulating precision nutrition protocols to reset SARS-CoV-2-induced HMRD in chronic PASC.
This narrative review is an attempt to elaborate and consolidate our current understanding of the molecular mechanisms of SARS-CoV-2 infection, the detrimental effect(s) of this infectious process on human metabolism, consequential symptomatic clinical manifestations, and damage to multiple organ systems; as well as chronic pathophysiological sequelae in virus-free COVID-19 survivors – the long-COVID or PASC patients. We have also provided a few evidence-based, randomized controlled trial (RCT)-tested precision nutrients to reset virus-induced HMRD, a detrimental aftermath resulting from virus-hijacked host cellular metabolic machinery in post-COVID survivors, the affected long-COVID patients worldwide.
SARS-CoV-2 infection: hijack of host cellular metabolic machinery
The SARS‐CoV‐2 obviously lacks metabolic enzymes, a critical requisite for viral genomic replication, protein synthesis, and lipogenesis. Therefore, the virus strategically hijacks host cellular metabolic machinery and re-directs free amino acids (AAs) and fatty acids (FAs), as building blocks for viral progeny and propagation. Accordingly, SARS‐CoV‐2 genome and its products reprogram and dysregulate human metabolism at transcription, translation, and post-translational modification (PTM) levels 17 , 18 , 19 , 20 . Interaction of SARS-CoV-2 proteins with specific host cellular targets could rewire sugar-, AA-, FA-, as well as nucleotide-metabolism(s), and distinctly alter or impair bioenergetics, immune response, and redox homeostasis in the human body, thereby facilitate viral life cycle 21 , 22 . SARS-CoV-2 proteins could sense the host cellular metabolic status and accordingly trigger human metabolic reprogramming/dysregulation (HMRD) in the infected human host.
The viral genome
The SARS-CoV-2 genome is a 29.9-kb RNA that consists of 14 open reading frames (ORFs) encoding two large polyproteins (ORF1a and ORF1b) and 13 small ORFs that encode viral structural proteins and other polypeptides. Polyproteins from the large ORF1a/b are further arranged into 16 non‐structural proteins ( nsp1 to nsp16 ) 1 , 29 . The structural proteins comprise of nucleocapsid (N), membrane (M), envelope (E), and spike (S) proteins. The M and E proteins are located among the S-proteins in the viral envelope 30 . Based on the structural map of SARS-CoV-2, about 6% of the viral proteome mimics human proteins, while nearly 7% has been implicated in cellular hijacking mechanisms, and about 29% of proteome self-assembles into heteromeric components to support viral replication 31 .
Virus–host interactome
Virus-human host protein–protein interactions play a major role in clinical outcomes of acute SARS-CoV-2 infection and its long-term sequelae, the PASC. A ribonucleoprotein (RNP) capture has identified a direct binding of SARS-CoV-2 RNA with 109 human host factors 32 . A comprehensive virus–host interactome of 29 viral (i.e., non-structural/structural) proteins, and 18 host/human cellular proteins (i.e., CSR, proteases, as well as restriction, replication, and trafficking molecules), showed an extensive involvement of >4780 unique high-confidence interactions of SARS-CoV-2 with human metabolome 14 . These diverse virus–host interactions could reprogram/dysregulate host cellular functions such as genomic, mitochondrial, lipidomic, and innate defense activities at various levels in human metabolism.
Viral infection reprograms host genomics
The SARS-CoV-2 protein, nsp1 , binds to human ribosomes and inhibits host cellular translation 33 . The SARS-CoV-2 protein ORF3a interacts with host transcription factor ZNF579 and directly affects human gene transcription 34 . Viral ORF8 acts as a histone mimic and disrupts host cell epigenetic regulation 35 . Viral protein nsp12 (RNA-dependent RNA polymerase) could sense host nucleotide availability and modulate replication efficacy of the viral genome 36 . SARS-CoV-2 infection reprograms host folate and one-carbon metabolism at the PTM level to support de novo purine synthesis for replication of viral genome, through bypassing the viral shutoff of host translation 37 .
Viral infection reprograms cellular mitochondria
Interaction of viral gene nsp6 with mitochondrial proteins (i.e., ATP synthase ) alters cellular ATP synthesis 1 . Thus, SARS-CoV-2 infection dysregulates mitochondrial metabolism and forces the host cell to generate energy (ATP) and other metabolites to support viral life cycle 38 . Viral nsp12 could alter AA metabolism (especially of the branched‐chain amino acids , BCAA), while nsp12, nsp7 , and nsp8 interactions with electron transport chain (ETC) and ribosomal proteins could potentially dysregulate mitochondrial respiration 39 .
Viral infection reprograms host lipid metabolism
Lipids play a major role in viral life cycle, accordingly the SARS-CoV-2 infection affects host lipidome by reprogramming cellular FA metabolism and nucleotide biosynthesis 40 , 41 . Viral protein nsp7 could potentially alter host lipid metabolism, through its avid interaction with host enzymes involved in FA-β‐oxidation and lipogenesis 42 . SARS‐CoV‐2 up-regulates lipid biosynthesis to support the assembly of lipid bilayer‐envelope of virion particle 43 , 44 .
Viral infection impairs innate host defense
Viral ORF3a interacts with heme oxygenase- 1 (HO-1) and reprograms heme metabolism leading to iron (Fe)-redox dysregulation (FeRD) during SARS‐CoV‐2 infection 27 , 45 , 46 . HO-1 is a stress-induced, anti-inflammatory, immune-modulatory, and cyto-protective enzyme that degrades heme into carbon monoxide, free iron, and biliverdin 47 , consequently, the virus-induced HMRD could compromise host innate and adaptive immune responses. Redox imbalance, FeRD in particular, results from virus-induced HMRD and represents a critical state both in the pathogenesis of SARS‐CoV‐2 infection and host inflammatory response 27 , 48 , 49 . Antioxidant enzymes such as superoxide dismutase 1 (SOD1), and glucose‐6‐phosphate dehydrogenase (G6PD) decrease from HMRD-induced oxidative stress (OxS) and protein degradation 50 , 51 . Furthermore, viral protein nsp5 and nsp14 interact with host redox-enzymes: glutathione peroxidase (GPx) and peroxiredoxin (Prx), in both cytoplasm and mitochondria to dysregulate redox balance in different cellular compartments and enhance SARS-CoV-2 infection 21 . The viral protein encoded by ORF6 potently inhibits nuclear trafficking and helps viral evasion of IFN-mediated host defenses 52 . Viral protein, nsp14 , interacts with the catalytic domain of Sirt1, dysregulates Nrf2/HO1 axis, and impairs host antioxidant defense 53 .
Viral binding/attachment to human cell surface receptors (CSRs)
The virulent outcome of a SARS-CoV-2 infection depends on (i) binding/interaction of viral S-protein with human cell surface receptors (CSR) and (ii) priming of S-protein by human cellular proteases 54 , 55 . This infectious process is accomplished by viral hijack of cellular metabolic machinery through sequential steps of viral attachment, invasion, RNA replication, and propagation 56 . The viral S-protein serves as an anchor to interact with host tissue, followed by sequential cleavage of S-protein to facilitate viral entry 57 , 58 . The viral hijack of host cellular metabolic machinery during SARS-CoV-2 infection is depicted in Fig. 1
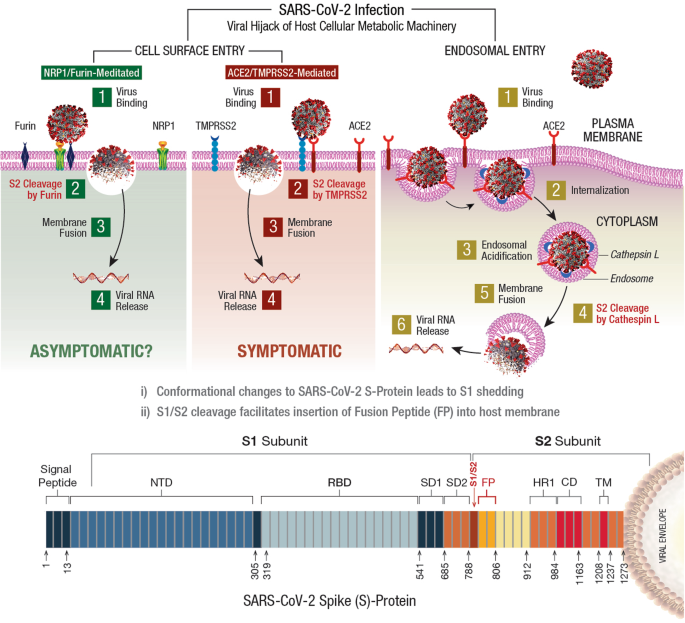
SARS-CoV-2 infection of a susceptible host is achieved through viral spike (S)-protein-mediated hijack of human cell surface receptors (ACE2 and/or NRP1) and cell membrane proteases. The S1-region on viral S-protein contains a receptor-binding domain (RBD) that specifically recognizes host cell surface receptor(s) and exposes the S2 site 55 . For fusion with host cell membrane, the viral S-protein hijacks specific cellular proteases for activation (‘priming’) of viral S-protein at the S1/S2 region. Subsequent conformational changes to viral S-protein lead to S1 shedding by cleavage of S1/S2 fragments. This process facilitates insertion of fusion peptide (FP) into host membrane. Accordingly, proteolytic cleavage by cellular enzymes TMPRSS2 and/or furin accomplish the task of viral FP insertion into host cell membrane. Alternatively, SARS-CoV-2 could also hijack lysosomal protease cathepsin L (CTSL) for direct viral endocytosis, where the viral membrane fuses with luminal face of the endosomal membrane facilitating viral RNA transfer into the cytosol. Thus, SARS-CoV-2 could infect the human by hijacking these 5 major host cellular factors via different routes of entry and elicit a wide range of clinical outcomes. The angiotensin-converting enzyme 2 (ACE2)/TMPRSS2-mediated viral infection and/or the ACE2/CTSL-mediated endosomal route may result in full-spectrum symptomatic COVID-19. The alternative neuropilin 1 ( NRP1)/furin -mediated route 62 , 63 may down-regulate human pain receptors and manifest as asymptomatic to mild disease outcomes.
The SARS-COV-2 S-protein hijacks human angiotensin-converting enzyme 2 (hACE2) to anchor on the host cell surface. The SARS-CoV-2 S/ACE2 complex undergoes conformational change for proteolytic priming/activation. The N-terminal S1 subunit contains receptor-binding domain (RBD) region, which avidly binds to the carboxypeptidase (CPD) domain on the hACE2 receptor and exposes the S2 site 55 . Co-expression of ACE2 with membrane serine proteases is high on ileal absorptive enterocytes in the GI tract, nasal goblet secretory cells and type II pneumocytes in the respiratory tract, as well as on the urogenital epithelia 59 , 60 .
SARS-CoV-2 may also infect host cells independent of the ACE2 receptor binding. The carbohydrate moieties on viral S-protein surface could facilitate viral internalization via innate immune factors, such as neuropilin (NRP)-1, C-lectin type receptors (CLR), and toll-like receptors (TLR), as well as the non-immune receptor glucose-regulated protein 78 (GRP78) for systemic spread of infection 61 . NRP1, a transmembrane glycoprotein involved in cardiovascular (CV), neuronal, and immune regulation, is also hijacked by SARS-CoV-2 for host cell surface binding 62 . NRP1, widely expressed in olfactory and respiratory epithelia, is shown to enhance TMPRSS2-mediated viral cell entry 63 . NRP1 binds to S1 through a multi-basic furin-cleavage site (FCS) and promotes S1 shedding to expose the S2′ site for TMPRSS2 priming 64 .
The S-protein of SARS-CoV-2 has a polybasic insertion (PRRAR) region at the S1/S2 site, which is readily cleaved by furin enzyme 65 . Furin cleavage site (FCS) is an important determinant of SARS-CoV-2 transmission in the human population. After binding to ACE2 and/or NRP1 receptors, the S-protein is proteolytically pre-activated by human proprotein convertase furin 66 . High-affinity interaction of ACE2 and/or NRP1 with the RBD of viral S-protein, followed by cell-mediated furin pre-activation could effectively facilitate host cellular entry of SARS-CoV-2 while evading host immune surveillance 67 .
Viral entry via host cell membrane fusion
The S-protein cleavage site, S1/S2 provides two sequential functions for successful viral entry. The RBD region on the S1 subunit recognizes anchor point(s) on the host cell surface, whereas the S2 subunit facilitates fusion of viral envelope with the host cell membrane after proteolytic cleavage of S1/S2 site to mediate viral entry 68 , 69 . Accordingly, SARS-CoV-2 hijacks several host proteases to enter human target cells and enhance its spread in the body. These proteases include cell surface transmembrane protease/serine (TMPRSS) proteases, cathepsins , furin, elastase, factor Xa, and trypsin 70 .
The S-protein harbors an FCS between the S 1 /S 2 subunits, processed during biogenesis that sets this novel viral pathogen apart from other SARS-related CoVs 71 . Furin cleavage exposes the S2 subunit for further processing by the host serine proteases for subsequent viral entry 72 , 73 . Furin impacts the cellular entry of SARS-CoV-2 in a unique manner by pre-activation of S2 subunit thereby reducing viral dependance on other human proteases for cellular entry 54 . After furin cleavage, the S2′ site requires an additional proteolytic step to facilitate the fusion of viral envelope with host cell membrane. This process involves two major human proteases: the TMPRSS2 in plasma membrane and cathepsin-L (CTSL) in the endo-lysosome 74 .
Human TMPRSS2, an enzyme widely expressed in several human cells, acts on the S2 prime (S2′) region, and cleaves the S-protein 75 . This proteolytic process results in structural rearrangement of S-protein and allows fusion between the viral envelope and host cell membrane 57 , 76 , which cumulatively drives an efficient internalization (infection) of SARS-CoV-2 into target host cells 55 , 77 . CTSL, a pH-dependent endo-lysosomal protease, cleaves the S-protein and facilitates viral fusion with the host endosomal membrane. Also, SARS-CoV-2 could induce cellular transcription, elevate CTSL activity, and increase viral infection 78 .
Distinct variabilities of infection rates, epidemiological transmission, and clinical outcomes during COVID-19 pandemic raises an intriguing question, whether the emergence of SARS-CoV-2 variants of concern (VOCs) with function-specific mutations in ACE2, furin, and TMPRSS2 expression has played any role in disease manifestations and case fatality rates (CFR) 55 , 75 . The estimated reproduction number (R 0 ) of COVID-19 is around 3.28 1 . R 0 represents viral transmissibility, indicating an average number of new infections transmitted by an infected individual in a totally naïve population. For R 0 > 1, the number of infected cases is likely to increase, and for R 0 < 1, viral transmission is likely to die out. From an inanimate transmission standpoint, SARS-CoV-2 has a decay rate of 10 3.5 to 10 2.7 median tissue culture infectious dose (TCID) 50 /L, like the decay rate of SARS-CoV (10 4.3 to 10 3.5 TCID 50 /mL), and the virus could remain infectious in aerosols for several hours and on surfaces for up to one day 79 .
COVID-19: clinical manifestatons
The symptomatic progression of COVID-19 requires that a genetically competent (virulent) SARS-CoV-2 pathogen (i) infects a susceptible host via specific CSR, invades and internalizes into the cell utilizing host membrane proteases, (ii) induces HMRD to ensure ready access to an active host cellular metabolic machinery for an uninterrupted viral replication, (iii) inactivates innate host defense to evade viral elimination, and (iv) exits the infected host cell and repeats the viral propagation cycle for exponential growth and transmission 26 .
The viral load usually reaches its peak at symptomatic onset during the initial weeks of infection and is detectable by reverse transcription polymerase chain reaction (RT-PCR) within the first week of infection. An infected person is estimated to carry about 10 9 to 10 11 virions at the peak of infection 80 . Severe COVID-19 patients might shed viral particles for prolonged periods of up to 4 weeks after symptomatic onset 81 . SARS-CoV-2 RNA (RT-PCR positive) could be detected in the upper respiratory tract (nasopharyngeal for about 7–8 weeks, throat, and sputum for about 4–5 weeks) 82 . Multi-organ viral tropism, mainly localized across lungs, trachea, kidney, heart, or liver, predominantly in cells expressing ACE2, TMPRSS2, or both has been reported. Viral RNA has also been detected in tonsils, salivary glands, oropharynx, thyroid, adrenal gland, testicles, prostate, ovaries, small bowel, lymph nodes, skin and skeletal muscle 83 . SARS-CoV-2 kidney tropism with high viral load in urine sediments from COVID-19 patients (within 2 weeks) correlates with increased incidence of AKI and mortality 84 .
In accordance with its virulence spectrum and host susceptibility pattern, the symptomatic outcomes in COVID-19 patients are manifested in a tri-phasic manner as FeRD-induced hematological syndromes 27 , as shown in Fig. 2 .
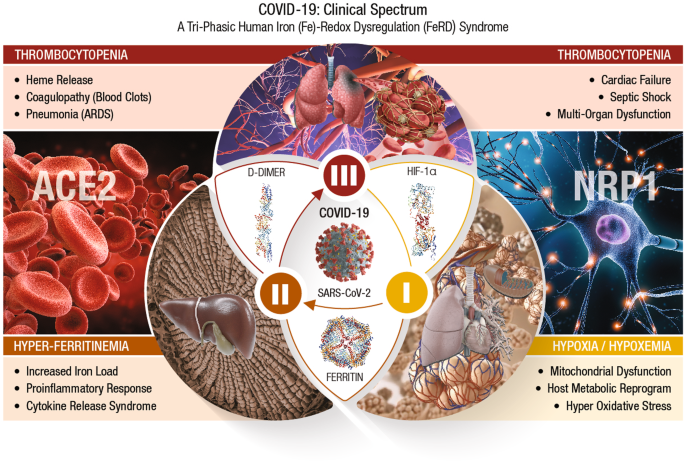
The symptomatic outcomes of SARS-CoV-2 infection manifest in a tri-phasic manner as iron (Fe)-redox disruptive hematological syndromes 27 . Phase-I: Hypoxia/Hypoxemia . Viral binding to ACE2 alters RAAS, subsequently lowers blood pressure, lung function, and reduces O 2 transport (hypoxia) in the infected host. This condition triggers a mitochondrial metabolic shift by alteration of OXPHOS/TCA cycle and activation of anaerobic glycolysis, the ‘Warburg Effect’. This metabolic shift is regulated by HIF-1α that causes impairment of host immune response, exacerbates inflammation, and elicits tissue damage 88 . This clinical phase of COVID-19 is considered a hypoxia-induced blood disease, associated with FeRD and HMRD 27 , 93 . Phase-II: Hyperferritinemia is characterized by a hyper-inflammatory state with elevated proinflammatory cytokines, which stimulates synthesis of both ferritin and hepcidin, the ultimate mediators of FeRD 94 . The altered iron homeostasis is reflected by high iron content in reticuloendothelial cells and elevated serum ferritin levels. Such uncontrolled and dysfunctional immune response associated with macrophage activation leads to hyperferritinemia, and ‘cytokine storm’ or cytokine release syndrome (CRS) 97 . Hyperferritinemia, cellular redox imbalance and FeRD play a critical role in the disease progression of COVID-19, 27 , 98 . Phase-III: Thrombocytopenia . SARS-CoV-2 could invade blood vessels, induce vascular damage, and activate systemic thrombotic events with severe to fatal coagulopathies in COVID-19 patients 100 . This clinical state along with hypoxia, could cast signs of hemolysis with release of heme proteins and accumulation of free heme. Heme from hemolysis could initiate oxidative and inflammatory stress that may cause microvascular thrombosis, organ ischemia and multi-organ failure in severe COVID-19 cases 102 , 105 .
COVID-19/phase-I: hypoxia/hypoxemia
SARS-CoV-2 binding to host CSRs (i.e., ACE2, NRP1) is an initial step in the pathogenesis of COVID-19. Viral binding to ACE2 receptors on alveolar epithelia affects renin-angiotensin-aldosterone system (RAAS), subsequently lowers the blood pressure and lung function of an infected host 85 . The reduced O 2 transport (hypoxia) triggers a mitochondrial metabolic reprogramming/dysregulation via alteration of OXPHOS/TCA cycle and activation of anaerobic glycolysis, known as the ‘Warburg Effect’ 86 , 87 . This shift in mitochondrial energy metabolism (or ATP synthesis) is regulated by different cellular systems, of which the hypoxia-inducible factor (HIF)-1α plays a critical role 88 . HIF-1α induced HMRD affects the available host energy reserves for immune function 89 . Ultimately, HIF-1α could impair host immune response, exacerbate inflammation, and inflict tissue damage. SARS-CoV-2 could evade host innate immunity and sustain intracellular viral replication cycle by altering the mitochondrial dynamics through targeting the mitochondria-associated antiviral signaling (MAVS) pathways 90 HIF-1α could up-regulate vascular endothelial growth factor (VEGF) to cause vascular leakage, damage epithelial barriers of alveoli and vascular endothelia 91 , 92 . Therefore, phase-I of COVID-19 is considered a hypoxia-induced blood disorder, associated with FeRD and HMRD 27 , 93 .
COVID-19/phase-II: hyperferritinemia
Severe COVID-19 is characterized by hyper-inflammation with elevated proinflammatory cytokines that stimulate the synthesis of both ferritin and hepcidin (which ultimately mediate FeRD) 94 . The iron homeostatic imbalance is reflected by high iron content in reticuloendothelial cells and elevated serum ferritin levels. When the iron-binding capacity of transferrin (TF) in the blood exceeds, free iron is released into plasma in a redox-active state known as the labile plasma iron (LPI), which forms tissue-damaging free radicals and cause fibrosis 95 . A ferritin/TF ratio >10 predicts a five-fold higher risk of ICU admission and an eight-fold higher risk for need of mechanical ventilation in COVID-19 patients 96 . A dysfunctional hyperimmune response in tandem with macrophage activation could trigger hyperferritinemia, and ‘cytokine storm’ or cytokine release syndrome (CRS). CRS is characterized by fulminant activation of a large number of lymphocytes that release inflammatory cytokines and result in severe tissue damage with multi-organ dysfunction syndrome (MODS) 97 . Hyperferritinemia, and FeRD collectively play a detrimental role in disease progression of COVID-19 27 , 98 . Phase II of COVID-19 is considered a wide-spectrum hyperinflammatory disease, amplified by CRS from HMRD 27 .
COVID-19/phase-III: thrombocytopenia
Acute COVID-19 due to severe iron toxicity from oxidized iron could modulate several systemic pathways of coagulation cascade and cause thromboembolism 99 . SARS-CoV-2 could invade blood vessels, induce vascular damage, and activate systemic thrombotic events with severe to fatal coagulopathies in COVID-19 patients 100 . Such coagulopathies (or blood clots) are characterized by elevated procoagulant factors such as fibrinogen, along with high levels of D-dimers linked to increased CFR 101 , 102 . Hematological parameters such as anemia of inflammation (AI), reduced numbers of peripheral blood lymphocytes and eosinophils with increased neutrophil-to-lymphocyte ratios are recognized as major risk factors 103 , 104 . This clinical phase along with hypoxia, could exhibit signs of hemolysis with the release of heme proteins and accumulation of free heme. The hemolysis-derived heme could initiate inflammatory OxS that may cause microvascular thrombosis, organ ischemia and MODS in severe COVID-19 102 , 105 .
COVID-19 pathobiological spectrum
The incubation period, defined as the time from infection to the onset of signs and symptoms, is a crucial index of epidemiology in understanding the pathobiological spectrum of acute SARS-CoV-2 infection, and PASC 106 . The median incubation period for COVID-19 was estimated to be 5.1 days, and 99% (101 out of every 10,000 cases) will develop symptoms after 14 days 107 , 108 . The median viral clearance time (VCT, RT-PCR negative) is 24 days. The VCT was 30 days among severe COVID-19 patients and 39 days among ICU-admitted patients 109 , 110 .
About 80% of SARS-CoV-2 infections are asymptomatic to mild, and many COVID-19 patients recover within 2 to 4 weeks. However, the onset of severe pneumonia and critical MODS may occur in 15 and 5% of patients, respectively, which could last for 3 to 6 weeks 111 . COVID‐19 patients may develop a wide range of clinical manifestations, including severe acute pulmonary disease, hepatic dysfunction, kidney injury, heart damage, gastro-intestinal, skeleto-muscular, pancreatic, and sensory (smell and taste) dysfunctions 112 , 113 , 114 , 115 , 116 , 117 . SARS-CoV-2 inflicts severe respiratory symptoms with a substantial pulmonary dysfunction, which may include severe arterial hypoxemia (low blood oxygenation) resulting in acute respiratory distress syndrome (ARDS) 118 . SARS-CoV-2 could also impair cardiovascular (CV) metabolism in COVID-19 patients. The viral S-protein and the ORF9b subunits could alter human cardiomyocyte metabolism and significantly impair the contractile function of the heart 119 . COVID-19 has a major impact on heart health and may lead to myocarditis or cardiac failure.
In COVID patients, the SARS-CoV-2 infection could also reach the brainstem and induce cerebral lesions as long-term sequelae 120 . Several neurological manifestations including cognitive dysfunction are often described in such patients. Thus, SARS-CoV-2 infections impact not only the respiratory organ but also inflict various bodily damage leading to shock and MODS 121 .
Post-acute sequelae of COVID-19 (PASC) or long-COVID
Post-acute sequelae of COVID-19 (PASC) or long-COVID refers to a wide spectrum of symptoms and signs that are persistent, exacerbated, or new clinical incidents during the time period that prolongs after acute SARS-CoV-2 infection 122 , 123 . About 25 to 70% of COVID-19 survivors may experience severe debilitating virus-free disease states with lingering symptoms lasting for weeks to months 2 , 124 . PASC affects asymptomatic, mild symptomatic, or self-quarantined (at home) individuals infected with SARS-CoV-2, as well as moderately to severely inflicted COVID-19 patients that require hospitalization and/or intensive care 4 . The incidence of PASC is estimated at 10–30% of non-hospitalized cases, 50–70% of hospitalized cases, and 10–12% of vaccinated cases 125 , 126 , 127 . PASC is reported in all ages, with the highest percentage of diagnoses observed between the ages 36 and 50 years. PASC is frequently diagnosed in non-hospitalized patients with mild illness, and this population represents most COVID-19 cases 5 .
After two years post-recovery, PASC continues to affect the disability-adjusted life years (DALYs per 1000 persons) of about 25.3% non-hospitalized and 21.3% hospitalized individuals 128 . Accordingly, the substantial cumulative burden of health loss due to persistent long-term PASC is overwhelming.
A prospective cohort study (n = 9764 ) conducted by Researching COVID to Enhance Recovery (RECOVER) consortium of the US National Institutes of Health (NIH) proposed a symptom-based criteria to identify and differentiate PASC cases 129 . The study identified six clinical manifestations, namely: post-exertion malaise (PEM) (87%), fatigue (85%), brain fog (64%), dizziness (62%), GI (59%), and palpitations (57%), as the most prominent PASC symptoms; an additional six common symptoms such as changes in sexual desire or capacity, loss of or change in smell or taste, thirst, chronic cough, chest pain, and abnormal movements were included. Other manifestations associated with selected symptoms such as dry mouth, weakness, headaches, tremor, muscle and abdominal pain, fever/sweats/chills, and sleep disturbance were also recognized.
The long-term sequelae of PASC could manifest with >200 different and overlapping clinical symptoms involving multiple organ/systems such as Pulmonary-PASC (general fatigue, dyspnea, cough, throat pain); Cardiovascular (CV)-PASC (chest pain, tachycardia, palpitations); Gastrointestinal (GI)-PASC (diarrhea, abdominal pain, nausea vomiting); Neuro-cognitive-PASC (brain fog, dizziness, loss of attention, confusion); Renal-PASC (renal failure, electrolyte disorders; Hepato-biliary-PASC; Skeleto-muscular-PASC (myalgias, arthralgias); Psychological-related PASC (post-traumatic stress disorder, anxiety, depression, insomnia); and other PASC manifestations (ageusia, anosmia, parosmia, skin rashes) 130 , 131 .
RECOVER study has proposed the following four multi-symptomatic PASC clusters or subgroups: Cluster-1 —loss of or change in smell or taste ; Cluster-2 —PEM (99%) and fatigue (84%); Cluster-3 —brain fog (100%), PEM (99%), and fatigue (94%); and Cluster-4 with fatigue (94%), PEM (94%), dizziness (94%), brain fog (94%), GI (88%), and palpitations (86%) 129 . Based on relapsing/remitting nature of acute- and post-COVID symptoms, an integrative classification has been proposed 132 . (i) SARS-CoV-2 infection-related acute COVID symptoms (up to 4–5 weeks), (ii) acute post-COVID symptoms (from week 5 to 12), (iii) long post-COVID symptoms (from week 12 to 24), and (iv) persistent post-COVID symptoms (lasting >24 weeks). This classification includes time reference points with predisposing intrinsic/extrinsic factors and hospitalization data in relation to post-COVID symptoms. The clinical transition from acute COVID-19 to symptomatic PASC seems to vary between hospitalized and non-hospitalized patients. In hospitalized COVID-19 patients, about 50–70% cases may continue to PASC symptoms lasting up to 3 months after hospital discharge 133 . In non-hospitalized subjects, about 50–75% may turn PASC-free one month after symptomatic onset 134 . PASC patients may also experience exercise intolerance and impaired daily function and quality of life 135 .
Based on the plethora of symptoms affecting different organs/systems, PASC-affected population could be categorized into four different clusters or sub-phenotypes: Sub-phenotype-1 (33.8%) with cardiac and renal manifestations, Sub-phenotype-2 (32.8%) with respiratory, sleep and anxiety disorders, Sub-phenotype-3 (23.4%) with skeleton-muscular and nervous disorders, and Sub-phenotype-4 (10.1%) with digestive and pulmonary dysfunctions 123 , 136 .
Lon-COVID/PASC: virus-induced human metabolic reprogramming and dysregulation (HMRD)
Several recoverees or survivors of COVID-19 ( RT-PCR negative for SARS-CoV-2) continue to exhibit a plethora of clinical symptoms with impairment(s) of multiple organ systems. Accordingly, PASC or long-COVID is a virus-free, ‘new onset’ disease condition extending from an earlier virus-induced HMRD. The HMRD in PASC pathology is a cumulative clinical outcome of several causative mechanisms comprising both SARS-CoV-2-derived virulence factors, as well as a multitude of host cellular factors and innate responses. A plethora of PASC clinical symptoms and related metabolic impairments indicate involvement of different pathobiological mechanisms such as (i) virus-induced hypoxia/’Warburg’ effect, (ii) iron (Fe)-redox dysregulation (FeRD), (iii) m- Dys and altered bioenergetics, (iv) oxidative stress (OxS) and cellular damage, (v) immuno-pathogenesis and hyperinflammation, (vi) autoimmunity, (vii) dysbiosis, (viii) re-activation of latent pathogens, (ix) persistent viral reservoirs, and (x) viral-hijacked host cellular factors 22 , 27 , 137 , 138 . A wide range of pathophysiological mechanisms involved in the transition of SARS-CoV-2 Infection to virus-free PASC clinical condition is shown in Fig. 3 .
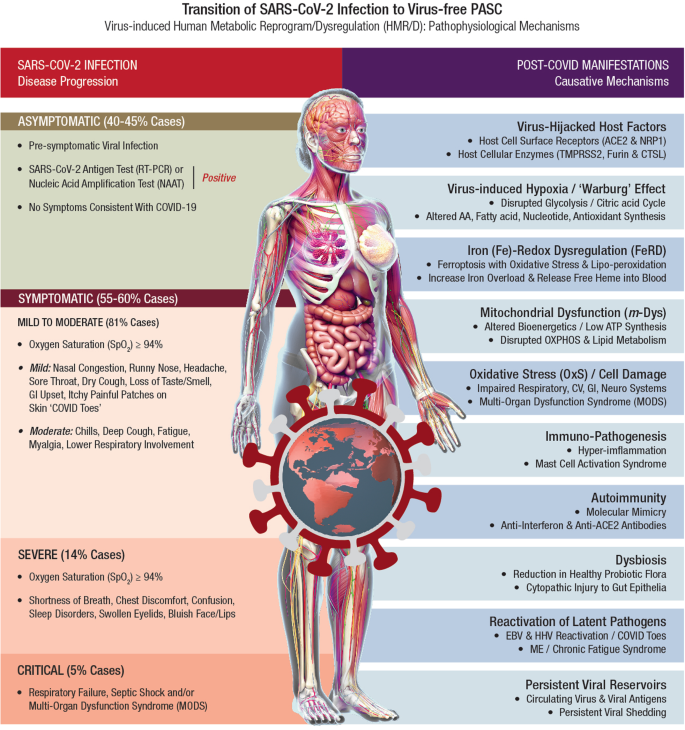
Post-acute sequelae of COVID-19 (PASC) or long-COVID refers to a broad spectrum of symptoms and signs that are persistent, exacerbated, or new clinical incidents in the period that prolongs after acute SARS-CoV-2 infection. In acute COVID-19, the SARS‐CoV‐2 genome and its products critically reprogram and dysregulate human metabolism (HMRD) at transcription, translation, and post-translational modification (PTM) levels. Interaction of SARS-CoV-2 proteins with specific host cellular targets rewires sugar-, amino acid-, lipid-, and nucleotide-metabolism(s), as well as alters or impairs bioenergetics, immune response, and redox homeostasis in the body, to facilitate viral replication and propagation 21 , 22 . However, several recoverees or survivors of COVID-19 ( RT-PCR negative for SARS-CoV-2) continue to exhibit a plethora of clinical symptoms with impairment(s) of multiple organ systems. Accordingly, PASC or long-COVID is a virus-free, ‘new onset’ pathophysiological condition extending from a virus-induced HMRD. The HMRD in PASC pathology is a cumulative clinical outcome of several causative mechanisms comprising both SARS-CoV-2-derived virulence factors, as well as a multitude of host cellular factors and innate responses. A plethora of PASC clinical symptoms and related metabolic impairments indicate an involvement of different pathobiological mechanisms.
Virus-induced hypoxia/’Warburg’ effect
SARS-CoV-2 hijacks host cellular metabolic machinery to extract adequate energy and carbon skeletons to facilitate viral entry and facilitate molecular constructions for viral progeny inside a host cell for replication and propagation. The SARS-CoV-2 infection initiates complex human host-pathogen interactions and alters mitochondrial function with significant disruption of glycolysis/TCA cycle (Warburg effect), affecting several metabolic pathways of amino acid (AA), fatty acid (FA), nucleotide, and antioxidant synthesis 139 , 140 . The virus-induced hypoxia/Warburg effect could potentially compromise endocrinal, cardiovascular, neurocognitive, gastrointestinal, pulmonary, and reproductive functions that demand high levels of mitochondrial O 2 consumption, OXPHOS, and ATP reserve. Failure to reset hypoxia/Warburg effect after viral clearance in COVID-19 survivors, could eventually evoke PASC with metabolic impairments including new onset T2DM, myocardial infarction, chronic fatigue syndrome (CFS), brain fog, and blood clotting issues 141 . Accordingly, PASC could be described as a SARS-CoV-2-induced chronic and self-perpetuating comprised state of m- Dys, where OxS potentially drives inflammation and shifts energy metabolism towards glycolysis while down-regulating OXPHOS 27 , 142 , 143 . Long-term consequences of virus-induced hypoxia/Warburg effect could amplify potential risks of HMRD with chronic multi-organ impairments in PASC (Fig. 4 ).
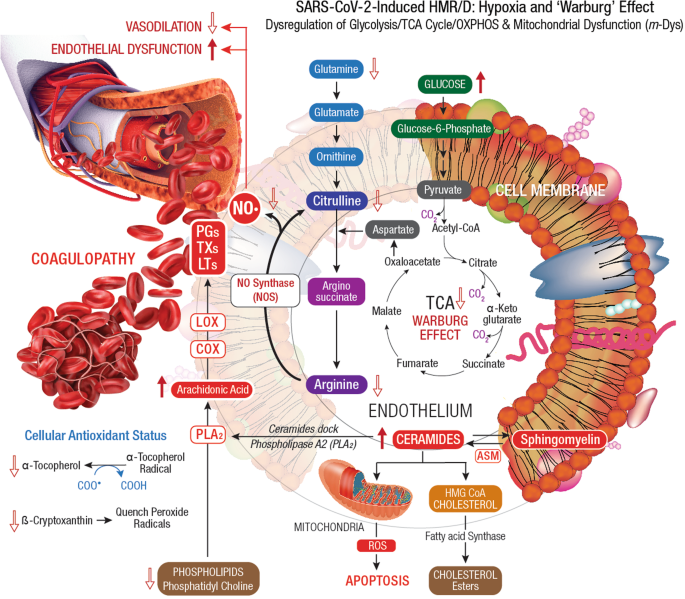
Dysregulation of glycolysis/TCA cycle is a key feature of HMRD. COVID-19 patients exhibit elevated serum glucose levels with an upregulation of glycolytic intermediates. Glutamine deficiency and hyaluronan over synthesis are HMRD-induced metabolic events in SARS-CoV-2 infection 785 . M1 macrophages express nitric oxide synthase (NOS), which oxidizes arginine to nitric oxide (NO•) and citrulline. NO• modulates vascular tone, blood pressure and hemodynamics. Disrupted arginine metabolism further down-regulates NO• synthesis, aggravates endothelial dysfunction and triggers severe coagulopathies in COVID-19 184 . Downstream generation of amino acids ornithine, citrulline, arginine in the circulation also indicates a severe renal dysfunction 51 . Degradation of sphingomyelin by acid sphingomyelinase (ASM) generates stimulatory ceramides, the docking molecules for phospholipase A2 (PLA 2 ). The hydrolysis of phospholipids (i.e., phosphatidyl choline ) by PLA 2 elevates arachidonic acid levels, a precursor for broad spectrum eicosanoids produced by cyclooxygenase (COX) and lipoxygenase (LOX) enzymes. These enzymes further convert arachidonic acid to prostaglandins (PGs), thromboxanes (TXs), and leukotrienes (LTs), which collectively contribute to the development of vascular inflammation and disease severity in COVID-19 786 . Virus-induced HMRD alters host lipid metabolism with major impact on sphingolipid and arachidonic acid pathways 787 . A decline in fat-soluble antioxidants’ vitamin E and carotenoids could compromises ROS quenching capacity in the plasma membrane, causes lipid peroxidation and OxS. Elevated serum lipase levels indicate damaging clinical outcomes in COVID-19 patients 788 . The virus-induced HMRD alternations to glucose, amino acid, and lipid metabolism could aggravate the severity of COVID-19 and may extend to PASC pathology.
Iron (Fe)-redox dysregulation (FeRD)
During SARS-CoV-2 infection, free iron released into the circulation induces inflammation of alveolar macrophages and causes oxidative damage to the lungs 144 . Increased iron load increases blood viscosity with recurrent diffused micro/macro circulatory thrombosis leading to high levels of D-dimers in COVID-19 patients. Altered iron metabolism, iron-restricted erythropoiesis from hyperinflammation causes FeRD 27 , 145 . In COVID-19 patients, FeRD could trigger several clinical manifestations including (i) decrease functional hemoglobin (Hb), (ii) increase cellular iron overload, (iii) release free toxic heme into the circulation, (iv) manifest hypoxemia and systemic hypoxia, (v) reduce nitric oxide (NO•) synthesis, (vi) activate coagulation pathway(s), (vii) trigger ferroptosis with OxS and lipid peroxidation, and (viii) induce mitochondrial degeneration 27 , 146 .
On the other hand, viral protein sequences could form complexes with porphyrin, affect heme on the 1-β chain of Hb, and release free iron 147 . SARS-CoV-2 envelope (E) protein directly binds to heme (from Hb) released from damaged erythrocytes and lysed phagocytes 148 . The viral genomic ORF8 protein could interact with the 1β-chain of Hb, capture the porphyrin and inhibit heme metabolism in the body 149 . Such an array of SARS-CoV-2 interactions with Hb could induce hemolysis and/or form complexes with released heme, generate dysfunctional Hb (hemoglobinopathy) with reduced ability to transport O 2 /CO 2 and lead to O 2 deprived multi-faceted syndromes, including coagulation disorders 146 , 150 . In severe stages of COVID-19, other Hb-associated markers such as bilirubin and ferritin progressively increase and worsen the clinical outcomes.
The FeRD-induced hyperferritinemia strongly correlates with different inflammatory phases of SARS-CoV-2 infection 98 , 101 , 151 . In SARS-CoV-2 infected patients, the plasma levels of ferritin and IL-6 steadily decrease with gradual recovery from COVID-19 152 , 153 . FeRD is highly prevalent among hospitalized COVID-19 patients and this clinical condition may continue for weeks or even months in PASC patients. Biomarkers of iron metabolism (i.e., ferritin, transferrin (TF), lactoferrin (LF), etc.) and Hb could provide risk stratification strategies for COVID-19 management. FeRD determinations are specific and sensitive to predict disease severity in COVID-19 and PASC patients 27 , 154 .
Mitochondrial dysfunction ( m -Dys)/altered bioenergetics
The mitochondrion is the cellular powerhouse involved in oxidative phosphorylation (OXPHOS), ATP synthesis, and regulation of calcium (Ca 2+ ) signaling, redox homeostasis, lipid metabolism, cell differentiation, immune system, apoptosis, and cellular senescence (aging) 155 , 156 . These vital processes are perturbed when the host cellular machinery is hijacked by SARS-CoV-2, which ultimately manifests as mitochondrial dysfunction ( m -Dys). SARS-CoV-2 infection leads to m -Dys including mitochondrial membrane depolarization, mitochondrial permeability transition pore opening, increased release of reactive oxygen species (ROS), and disrupted. mitochondrial redox homeostasis 157 , 158 . SARS-CoV-2 infection also affects fusion/fission kinetics, size, structure, and distribution of mitochondria in the infected host cells. COVID-19 patients with underlying primary mitochondrial disease and secondary m -Dys are prone to increased disease severity and CFR compared to patients with healthy mitochondrial functions 159 . Thus, m -Dys could heavily compromise host bioenergetics with detrimental consequences on COVID-19 and long-term PASC patients 160 , 161 .
After host cell entry, the ORF9b of SARS-CoV-2 RNA could directly manipulate mitochondrial function to evade host cell immunity, facilitate viral replication and trigger the onset of COVID-19. The ORF9b could further manipulate host mitochondria by releasing mitochondrial DNA (mt-DNA) into the cytoplasm to activate mt-DNA-induced inflammasome and suppress innate as well as adaptive immunity 162 . SARS-CoV-2 may also manipulate mitochondrial function via ACE2 regulation. A decline in ACE2 function in aged individuals, coupled with the age-associated deterioration in mitochondrial functions results in chronic metabolic disorders like diabetes or cancer, and predisposes the host for increased susceptibility to infection, vulnerability to health complications, and intensifies the risk of mortality 163 .
SARS-CoV-2 invades mitochondria and evades host defense by the formation of double-membrane vesicles. These virus-induced vesicles could damage mitochondrial membrane integrity, release mt-DNA into circulation, compromise innate immunity, and trigger an exacerbated pro-inflammatory response in COVID-19 patients 164 . SARS-CoV-2 infection could alter mitochondrial function(s), activate TLR9 signaling, induce hyper-inflammation and disrupt endothelial activity 165 . The viral infection could also cause rapid T lymphocytopenia with functional impairment of T cells, which may onset OxS, pro-inflammatory state, cytokine production, and apoptosis 166 , 167 . Hyper-inflammation (with CRS or cytokine storm) due to massive outburst of ROS, is a prominent clinical feature of COVID-19 145 . The mitochondrion is a significant source of ROS in human cellular metabolism that could trigger the onset and development of cytokine storm 168 .
SAR-COV2 could induce m -Dys, activate mitochondrial-dependent intrinsic apoptotic pathways, and cause microglial and neuronal apoptosis leading to neuropathological symptoms in COVID-19 and PASC patients 169 , 170 . In the current pandemic, about 40% of COVID-19 patients demonstrated neurological symptoms, lingering neuro-inflammation, where neuronal damage in PASC patients has emerged as a novel syndrome, the ‘Neuro-COVID’ 169 , 171 . Peripheral blood monocytes of such patients demonstrate altered bioenergetics and reduced basal respiration, reduced spare respiratory capacity, and decreased proton leak 172 . The m -Dys-induced exercise intolerance with elevated arterial blood lactate levels and reduced fatty acid β-oxidation rates is a major health issue in PASC 173 . These patients complain about chronic fatigue during exercise, despite no obvious heart or lung abnormalities 174 .
During the aging process, progressive m -Dys occurs due to the loss of thioretinaco-ozonide-oxygen-ATP complex from mitochondrial membranes through the opening of mitochondrial permeability transition pore 175 . Disruption in mitochondrial OXPHOS could elevate OxS and activate sepsis cascade through HIF-α/Sirtuin pathway. Due to m -Dys, senescent cells fail to meet the hyper-metabolic demands of sepsis in COVID-19 patients. A decline in mitochondrial function in the aging population could be a possible risk factor for increased mortality in COVID-19 and PASC 176 . Furthermore, as a hallmark of the aging population, m -Dys could onset chronic inflammation with massive cytokine release and cause multi-organ failure with fatal outcomes in elderly COVID-19 patients 177 . Age-related comorbidities (metabolic syndromes) such as, obesity, T2DM, asthma, and CVD, could also increase severity and mortality in elderly COVID-19 patients. Preventive therapies to improve mitochondrial turnover, dynamics and activity could prove beneficial in protection against COVID-19 severity 178 . Therefore, nutritional targeting of mitochondrial metabolism could showcase as an effective treatment regimen for PASC management.
Oxidative stress (OxS)/cellular damage
Oxidative stress (OxS) is a nonspecific pathophysiological condition that reflects a redox imbalance between increased production of ROS (free radicals) and the inability of antioxidant defenses to neutralize the reactive intermediates or to repair the ensuing damage 179 , 180 . ROS disrupts cellular metabolism by inflicting DNA strand breaks, protein degradation, lipid peroxidation, and cellular damage 181 . Combined with inflammation, OxS contributes to cardinal patho-mechanisms of both COVID-19 and PASC 182 .
After SARS-CoV-2 infection, the viremia stage could increase OxS, elevated levels of ROS/inflammation markers (i.e., peroxide, NO•, carbonylated proteins, and IL-6) and inflict severe cellular/tissue damage. This clinical condition may compromise mitochondrial functions and trigger apoptosis of leukocytes 183 . Hyper-inflammation, pro-oxidant cytotoxic milieu, and early apoptosis of leukocytes from SARS-CoV-2 infection, could cause severe endothelial-alveolar injury and MODS 184 . PASC patients exhibit a wide range of tissue/organ damage involving pulmonary, cardiovascular, neuro-cognitive, GI, reproductive, and dermatological systems 5 , 127 , 185 .
Pulmonary damage in PASC
SARS-CoV-2 infection of alveolar epithelia could induce cytokine storm and OxS (with ROS release) resulting in severe lung damage 186 . Viral envelope proteins could also trigger abnormal immune response, dysregulate type-1 IFN synthesis, increase NETosis and cause organ injury via microthrombi formation 187 . In COVID-19 survivors, respiratory abnormalities with reduced total lung capacity and airway dysfunction (i.e., dyspnea, chronic cough, and reduced exercise capacity) may persist as chronic manifestations 188 . About 36% of PASC patients complain of shortness of breath and about 26% develop lung impairment. In the long term, virus-induced hyper-inflammation and subsequent disruption of coagulant pathways could increase the risk of thrombosis in PASC patients 138 .
Cardiovascular (CV) damage in PASC
CV complications are prevalent among PASC patients since ACE2 receptor-rich cardiomyocytes provide SARS-CoV-2 direct access to the heart. Disease severity during acute COVID-19 establishes the clinical basis for the onset of CV-PASC. Persistent myocardial inflammation with elevated cardiac troponin levels (2 months after disease onset) is a distinct feature among COVID-19 patients 6 . In acute COVID-19, prominent CV conditions such as myocardial injury, myocarditis, acute heart failure, cardiomyopathy, cardiac dysrhythmias, and venous thromboembolic events may occur 189 . Three months after hospital discharge, about 30% of COVID-19 patients demonstrate adverse ventricular remodeling, which indicates cardiac sequelae 190 , 191 . Causative mechanisms for CV-PASC include chronic inflammation due to viral persistence in heart tissue, molecular mimicry invoking autoimmune responses against cardiac antigens, and ongoing endothelial/microvascular dysfunction 192 . Many PASC patients (89%) report CV symptoms including chest pain (53%), palpitations (68%), and new onset of postural orthostatic tachycardia syndrome (POTS, 31%) 193 .
Neuro-cognitive damage in PASC
SARS-CoV-2 crosses the blood-brain barrier (BBB), invades the brain stem, damages brain parenchyma, and manifests neuro-COVID sequelae 171 . Multiple mechanisms are proposed in the onset and progression of neuro-COVID including hypoxia, hyper-coagulability, endothelial dysfunction, nerve injury, neuro-inflammation, and neurotropism, where all conditions are induced by SARS-CoV-2 infection. Impaired neuron-glial homeostasis, neuron axonal damage, astrogliosis, and microgliosis, are frequent manifestations in neuro-COVID 194 , 195 .
During host cell entry, the viral S-protein disrupts BBB function, damages neurons, and activates brain mast cells 196 . Neuro-invasion of SARS-CoV-2 occurs via transcribrial (nose) route with damage to olfactory mucosa, and olfactory nerves, ultimately manifesting into anosmia (loss of smell) 197 , 198 . COVID-19 patients also display diffused white matter damage, microglial activation, and neuroinflammation at different CNS regions with olfactory neuritis (25%), nodular brainstem encephalitis (31%), and cranial nerve neuritis (6%) 199 . Reactive gliosis, astrocytosis, and microglial activation, along with neuroinflammation gradually advances from COVID-19 to PASC 200 . Fatigue, cognitive dysfunction (brain fog, memory issues, attention disorder) and sleep disturbances are prominent clinical features of PASC. Psychiatric manifestations (sleep disturbances, anxiety, and depression) are also common and significantly increase in due course of neuro-PASC development 201 .
Multi-organ dysfunction syndrome (MODS)
MODS due to virus-induced extensive tissue injury has long-term implications in COVID-19 survivors and in PASC. Patients recovered from COVID-19 show increased risk and about 1-year burden of GI disorders such as irregular bowel movement, acid-related illnesses (i.e., dyspepsia, gastroesophageal reflux condition, peptic ulcers), acute pancreatitis, hepatic and biliary dysfunction 202 . Prolonged GI manifestations in COVID-19 and PASC are attributed to dysbiosis (microbiome imbalance), immune dysregulation and delayed viral clearance from the gut. Bi-directional interactions between respiratory mucosa and gut microbiota (‘Gut-Lung Axis’) plays a major role in the progression of GI-PASC 203 , 204 . Acute kidney injury (AKI) is highly prevalent among discharged COVID-19 patients, and 35% of the recovered patients show reduced kidney function and may require kidney replacement therapy 193 , 205 . Virus-induced hyper-inflammation with complement activation in kidney tissue could inflict focal segmental glomerulo-sclerosis with glomerular involution and lead to AKI 138 .
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)
ME/CFS is defined as persistent or relapsing fatigue for at least six months, which is not resolved by rest, and causes a substantial reduction in the ‘Activity of Daily Living’ (ADL) 206 . It is a hypometabolic state with impairment in multiple metabolic pathways linked to m -Dys with impaired OXPHOS and reduced ATP production 207 . Cognitive dysfunction, depression, and prolonged fatigue are the hallmark of ME/CFS 208 . Aberrant mast cell activation (MCA) could mediate hyper-inflammation in COVID-19 and initiate severe cascades of immune responses that trigger allergic flare-ups in PASC 209 .
Immuno-pathogenesis/hyper-inflammation
SARS-CoV-2 infection could disrupt host immune homeostasis, inflict tissue injury, and may persist during the post-recovery phase of COVID-19 survivors and manifest as PASC 210 . Cell-mediated immune responses with antigen-specific T cells decrease in COVID-19 patients and affect viral clearance from infected host cells 211 . Cytotoxic T cells elevate in peripheral blood and bronchoalveolar lavage of PASC patients with severe airway dysfunction with persistent respiratory symptoms that last for 3 to 6 months 212 . SARS-CoV-2 induced T-cell imbalance resolve over time; however, the markers upregulated from T-cell exhaustion may remain up to 1 year in PASC patients 213 , 214 .
Elevated levels of neutrophils with neutrophil extracellular traps (NETs and NETosis) and associated immune-thrombosis are prominent features of COVID-19 pathology 215 , 216 . Monocytes and macrophages mediate lung fibrotic tissue injury, a drastic consequence in the immunopathogenesis of SARS-CoV-2 infection 217 . Myeloid cells may also incite local fibrosis-mediated tissue injury and sustain proinflammatory cytokine levels contributing to the clinical development of PASC. COVID-19 patients with severe disease exhibit increased monocyte counts with higher frequencies of classical monocytes, lower frequencies of intermediate/non-classical monocytes and elevated plasma levels of C-reactive protein (CRP) and serum TF in comparison to mild disease. This abnormal immune response may persist for >6 months after COVID-19 recovery 218 . Monocyte alterations in acute COVID-19 patients include aberrant expression of leukocyte migration molecules that extend to convalescence and correspond to specific symptoms of PASC. Monocytes from PASC patients with ongoing fatigue show a sustained reduction of prostaglandin-generating enzyme, the cyclooxygenase 2 (COX-2) 219 . Circulating monocytes may remain dysregulated, especially in convalescent subjects for 1 to 3 months of post-COVID.
‘Cytokine storm or CRS’, a clinical state of hyper-inflammation, is a prominent feature of COVID-19 severity, linked to respiratory dysfunction, ARDS with adverse disease outcomes 220 , 221 . About 10% of patients recovered from COVID-19 show persistent symptoms up to 6 months after initial SARS-CoV-2 infection. Cytokine storm, in tandem with lymphopenia, lymphocyte dysfunction, and granulocyte/ monocyte abnormalities, could increase the disease severity of COVID-19 222 . Immune cytokine signatures of PASC patients reflect an ongoing chronic inflammation and angiogenesis with elevated plasma levels of IL-17a, stem cell factor, IL-12p70, IL-1β, macrophage inflammatory protein-1β (MIP-1β), brain-derived neurotrophic factor (BDNF), and VEGF 223 Also, among other immune mediators, reduced levels of cortisol strongly correlate with pulmonary-PASC symptoms 224 . Hyperimmune activation and autoimmunity are considered as potential causative factors in the onset of PASC 225 .
Autoimmunity
In COVID-19 patients, autoantibodies against nuclear bodies ( auto-nuclear antibodies —ANA), phospholipids, type I interferon (IFN), melanoma differentiation-associated protein 5 (MDA5), and ACE2 have been reported 226 . These autoantibodies against immunomodulatory proteins (including cytokines, chemokines, complement components and cell-surface proteins) could attack tissues of patients, thereby impair host cell signals, perturb immune function, damage organ systems, and increase COVID-19 severity 36 . Human leukocyte antigen (HLA) genetic polymorphism has also been observed in COVID-19 patients 227 . HLA polymorphism plays a key role in the onset of several autoimmune diseases 228 . Evidently, SARS-CoV-2 infection could elicit auto-inflammatory and autoimmune disorders such as Guillain-Barré syndrome (GBS), autoimmune hemolytic anemia, immune thrombocytopenic purpura, and Kawasaki disease (KD) 12 , 229 , 230 . Mechanisms of COVID-19-derived autoimmune disorders include (i) viral-mediated host hyper-immune response, (ii) virus-induced excessive NETs formation with neutrophil-associated cytokine responses, and (iii) the molecular mimicry between viral antigenic components and host molecules 231 .
Latent autoimmunity correlates with humoral responses against SARS-CoV-2 infection and autoimmunity has emerged as a prominent feature of PASC pathology 232 . Long-term persistence of immune activation and proinflammation with latent and overt autoimmunity are etiological factors in clinical manifestation(s) of PASC 233 . About 20 distinct autoantibodies that target G-protein-coupled receptors (GPCR) of the CNS and ACE2/RAAS-related molecules were linked to the clinical severity of PASC 234 .
Gut dysbiosis is defined as the reduction in diversity of GI microflora or depletion of autochthonous or host commensal beneficial bacteria with an enrichment of microbial pathogens that may alter host susceptibility to SARS-CoV-2 infection 235 , 236 . Impairment of short-chain fatty acid (SCFA) and l -isoleucine biosynthesis in gut microbiome persists beyond 30 days after recovery from COVID-19, which could contribute to persistent leaky gut and dysbiosis in PASC patients 237 , 238 . Notably, even after viral clearance, more than half of patients suffer from PASC with persistent dysbiosis, deregulated GI metabolism and compromised host immune response 14 , 127 . Gut dysbiosis and disrupted intestinal barrier function could worsen pulmonary symptoms, augment neurological or hepatic inflammation through translocation of endotoxins and bacteria via portal veins 239 , 240 , 241 . Dysbiosis of gut microbiome with ensuing gut barrier dysfunction could severely impact patho-physiologies of both COVID-19 and PASC 242 .
The GI tract is the largest immunological organ in the body and any aberrant immune response to SARS-CoV-2 infection induced by resident microflora could affect recovery from COVID-19. Dysbiosis may lead to GI impairment with persistent symptoms of diarrhea and abdominal pain 243 . Gut dysbiosis could also increase susceptibility to respiratory infections, alter immune responses and affect lung homeostasis (the ‘Gut-Lung Axis’). Persistent gut dysbiosis after resolution of COVID-19 may be linked to PASC, particularly to neurological manifestations 244 . In a prospective follow-up study from Wuhan, China, patients ( n = 187) recovered from COVID-19 demonstrated a strong correlation to gut microbiota dysbiosis and PASC symptoms, 1-year after hospital discharge 245 . In a subset of patients recovered from COVID-19, long-term dysbiosis correlated with PASC symptoms, especially fatigue, joint pain, diarrhea, headache, depression, and anxiety 246 . Dysbiosis in tandem with excess antibiotic use during the pandemic possibly have contributed to an array of PASC manifestations 247 . Since the diversity of gut microbiota erodes during aging, dysbiosis could also be a reason for high susceptibility of older adults to severe COVID-19 248 .
SARS‐CoV‐2 infection inflicts sustained metabolic damage to gut microbiome and GI function; therefore, opportunistic pathogens could selectively enrich in fecal microflora of COVID-19 patients 249 , 250 . Fecal enrichment of bacterial pathogens, such as Coprobacillus spp ., Clostridium ramosum , and Clostridium hathewayi , directly correlates with the severity of SARS‐CoV‐2 infection. Conversely, symbiotic gut microflora ( Bifidobacteria, Roseburia and Faecalibacteria ) with prominent immunomodulatory functions, are extinguished from the gut of PASC patients 251 . Accordingly, SARS-CoV-2 infection could inflict direct cytopathic injury to gut epithelia and elicit indirect immune-mediated damage to endothelial cells 252 . SARS-CoV-2 could also induce GI inflammation, dysregulate intestinal ACE2 activity, and/or infect gut microflora (similar to bacteriophage-type transduction), as three potential inter-connected mechanisms in gut dysbiosis in PASC 253 . Effective clinical management of PASC is contingent upon the following critical evaluation of dysbiosis: (i) the duration of gut dysbiosis after COVID recovery, (ii) the link between gut dysbiosis and long-term persistent symptoms, and (iii) the possible adverse health effects from enriched or depleted specific gut microflora on COVID-19 recovered individuals 254 . Therefore, reversal of dysbiosis and restoration of normal GI function could alleviate PASC symptoms and support patient recovery.
Reactivation of latent viral pathogens
Since the onset of COVID-19 pandemic, a strong correlation between SARS-CoV-2 infection or COVID-19 vaccination and herpesvirus co-infection/reactivation has been reported 255 . To date the reactivation of eight human herpesviruses (HHVs) have been identified, including Herpes Simplex Virus types 1 (HSV‐1) and 2 (HSV‐2), Varicella‐Zoster Virus (VZV or HHV‐3), Epstein-Barr Virus (EBV or HHV‐4), Cytomegalovirus (HCMV or HHV‐5), HHV‐6, HHV‐7, and Kaposi’s Sarcoma‐associated Herpesvirus (KSHV or HHV‐8). Almost 100% of the adult population in the world is infected with at least one HHV during their life 256 . A meta-analysis ( n = 32 studies) has estimated the prevalence of HHV reactivation in hospital-ICU-admitted COVID-19 cases at 38% for HSV, 19% for CMV, 45% for EBV, 18% for HHV-6, 44% for HHV-7, and 19% for HHV-8, respectively 257 .
The incidence of HHV reactivation was found high among patients admitted to the ICU for severe COVID-19 and among individuals administered with COVID-19 vaccine 258 . Simultaneous occurrence of cytokine storm and immune suppression during SARS-CoV-2 infection may lead to reactivation of latent HHV in the body. Lymphopenia with reduced CD8+ levels and elevated CD4 + /CD8+ ratio indicate the severity of COVID-19 215 , 259 . This clinical condition leads to an immune-suppressed state, which could ultimately trigger the reactivation of latent HHV and aggravate SARS-CoV-2 infection 260 . In addition to viral co-infection, anti‐COVID‐19 therapies (i.e., azithromycin, nafamostat mesylate, and remdesivir) could activate various cell signaling pathways and trigger viral lytic reactivation 261 . Remdesivir, a widely administered anti‐COVID‐19 drug, is shown to induce lytic reactivation of KSHV and EBV, from virus‐associated lymphoma cells 262 .
SARS-CoV-2 infected patients demonstrate a wide spectrum of cutaneous manifestations, including maculopapular or perifollicular rash, urticaria, vesicles, petechiae, purpura, livedo racemosa, and pseudo-chilblains, often referred to as the ‘COVID toes’ 263 , 264 . These cutaneous manifestations of COVID-19 are reportedly associated with reactivation of latent HHVs 265 , 266 . Reactivation of HSV-1 coincides with decreased expression of IFN-stimulated genes and concurrent increase in highly activated T-lymphocytes during acute stages of SARS-CoV-2 infection 267 . Reactivation of EBV could enhance the severity of SARS-CoV-2 infection. SARS-CoV-2 infected patients with EBV co-infection are prone to high fever with elevated levels of CRP, and aspartate aminotransferase 268 . A study from China reported higher mortality rates in COVID‐19 cases with EBV reactivation (29.4%) compared to EBV-negative patients (8.1%) 269 .
An exhausted dysfunctional antiviral immune response from SARS-CoV-2 infection could trigger reactivation of human adenovirus with a sequelae effect of ME/CFS in PASC patients 270 . Immuno-compromised individuals susceptible to HHV infection are at higher risk for SARS-CoV-2 infection, PASC, are vulnerable to develop virus‐associated cancers. Several HHVs, such as KSHV, and EBV are oncogenic viruses; therefore, a follow‐up surveillance of COVID‐19 survivors, PASC patients, and vaccinated individuals for possible risk(s) of latent viral reactivation is an important preventive public health strategy 258 .
Persistent viral reservoirs
Infectious viral particle clears out and remain undetectable in the body for most COVID-19 cases; however, among certain patients, SARS-CoV-2 could persist for months after post-recovery 271 . Accordingly, total clearance of SARS-CoV-2 RNA or its protein antigens from host infected tissue may take a longer time, while the virus and its antigenic fragments continue to remain dormant for extended periods of time in the body. The persistence of SARS-CoV-2 or its viral components in the body could trigger a dysregulated immune response and proinflammatory cytokine release, which may cause chronic low-grade inflammation and MODS. These acute sequelae also have a genetic basis that may predispose COVID-19 survivors to a compromised immune status consequently affecting viral clearance 272 .
Multi-organ viral tropism predominantly in cells expressing ACE2, TMPRSS2, or both has been reported 82 , 83 . Viral shedding (as detected by RT-PCR) may be prolonged in certain tissues of post-COVID patients for an extended duration in the lower respiratory tract (59 days), serum (60 days), upper respiratory tract (83 days), and feces (126 days) 273 . Such viral persistence could serve as a chronic trigger for inflammation and cellular activation that may further inflict tissue damage and elicit PASC-related symptoms 274 . In the long-term persistence of COVID-19-associated anosmia (loss of smell), viral transcripts are detected in the inflamed olfactory mucosa. Viral persistence and associated inflammation in olfactory neuro-epithelium may account for prolonged or relapsing symptoms in PASC, such as anosmia 275 . Delayed immune clearance of SARS-CoV-2 antigen(s) or duration of viral antigen burden in the upper respiratory tract and other anatomical sites during acute COVID-19 could be linked to the development of PASC 276 .
Long-term shedding of SARS-CoV-2 is widely reported, even after resolution of symptomatic COVID-19. The continuous replication of live SARS-CoV-2, its viral RNA, or viral protein fragments could play a major role in the clinical onset of PASC. SARS-CoV-2 RNA could persist for several weeks in the respiratory tract of COVID-19 survivors 274 . Viral replication has been reported in multiple respiratory and non-respiratory tissues, including the brain. Persistent shedding of SARS-CoV-2 was detected for months in the feces of patients recovered from COVID-19, regardless of GI symptoms 277 . In gut mucosa of mild to acute cases of COVID-19 (with IBD as comorbidity), the persistence of SARS-CoV-2 RNA was detected in ~70% PASC patients, whereas the viral nucleocapsid (N) protein was found in ~50% of PASC patients after seven months of post-recovery 278 . Such SARS-CoV-2 antigen persistence in infected tissues could possibly trigger immune perturbations that may contribute to the development of PASC.
Also, persistent viral RNA has been detected in multiple tissues of recovered patients even months after the onset of COVID-19. In a cohort of COVID-19 patients with persistent symptoms, about 45% showed detectable plasma SARS-CoV-2 RNA. Viral RNA was also found in blood, stool, and urine of PASC patients 279 . Spike and/or viral RNA fragments could persist in COVID-19 recoverees up to 12 months or longer 280 , 281 . The S1 antigen in peripheral blood monocytes could remain up to 15 months after SARS-CoV-2 infection 282 . The S-protein of SARS-CoV-2 contains structural motifs that affect T-cell receptors and trigger hyperinflammatory responses observed in severe COVID-19 and multi-system inflammatory syndrome in children (MIS-C) 283 . Spike may not activate cytokine storm in PASC patients; however, it could impair endothelial function via down-regulation of ACE2 and disrupt the integrity of BBB 284 , 285 . In summary, viral persistence could play a major role in PASC, considering the ability of the SARS-CoV-2 pathogen to infect and reinfect individuals over a lifetime 274 .
Virus-hijacked host cellular factors
Clinical outcomes of COVID-19 are directly related to the ability of the SARS-CoV-2 pathogen to hijack host metabolic machinery as well as cellular factors of an infected individual, for viral invasion and internalization, followed by intra-cellular replication to assemble and release multiple viral copies for ultimate propagation/transmission. Each of the SARS-CoV-2 hijacked host cellular factor is also a quintessential functional component of several key physiological pathways of human metabolism. In consequence, the virus-hijacked host cellular factors undergo HMRD, with altered or compromised function, which ultimately contributes to a plethora of organ/system impairments with detrimental effects on human metabolism. If not reversed or reset, the virus-induced HMRD condition may persist as PASC for weeks or months, even after viral clearance and recovery from COVID-19. The following section elaborates on five such critical virus-hijacked host cellular factors exploited for initial steps of SARS-CoV-2 infection, the viral host cell surface adhesion, cellular entry, and intracellular invasion (Fig. 5 ).
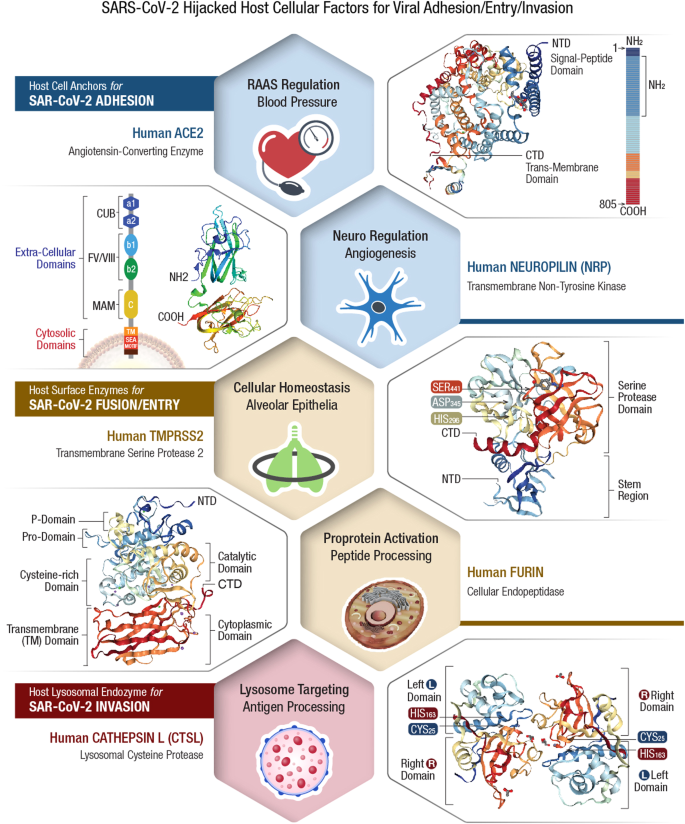
Clinical outcomes of COVID-19 depend on the ability of SARS-CoV-2 pathogen to hijack host metabolic machinery as well as cellular factors of an infected individual for invasion and internalization, followed by intra-cellular replication to assemble and release multiple viral copies for ultimate propagation/transmission. Each of the viral hijacked host cellular factor is also a quintessential functional component of human metabolism. Viral host receptor ACE2 , is a critical regulator of blood pressure, controller of blood volume involved in systemic vascular resistance, and in CV homeostasis 292 . Viral host receptor NRP1 , is vital for several physiological pathways including nervous and vascular development, VEGF-dependent angiogenesis (i.e., new blood vessel formation), immunity and tumorigenesis 321 , 322 . Viral membrane fusion priming enzyme furin , is known for intracellular proteolytic processing of precursor polypeptides, which is an essential step in the maturation of many proteins such as plasma proteins, hormones, neuropeptides, and growth factors 331 . Viral membrane fusion priming enzyme TMPRSS2 plays a key role in digestion, salt-water balance, iron metabolism, tissue remodeling, blood coagulation, auditory nerve development, and fertility 366 . Viral endocytosis-mediator CTSL is involved in functional development of immune system, skeletal physiology including bone collagen degradation/resorption and thyroid hormone release 384 , 385 . Consequential to the viral hijack, these essential host cellular factors could malfunction and lead to a plethora of organ/system impairments with detrimental consequences to the human body. If not corrected or reset, this HMRD condition may persist in a PASC patient for weeks or months even after viral clearance and recovery from COVID-19.
PASC: viral-hijacked host cellular factors and consequences
The initial step of COVID-19 pathogenesis involves that the viral spike (S)-protein interacts and anchors to susceptible host tissue by hijacking of specific CSRs (i.e., ACE2 and NRP1). Subsequent cellular invasion (internalization) of the pathogen takes place as the viral S-protein/host receptor complex is primed by furin cleavage at two sites: S1/S2 and S2’. This proteolytic cleavage induces conformational changes that favors S-protein recognition by host cell membrane proteases (i.e., TMPRSS2, CTSL). The cleavage of S2’ triggers fusion between viral envelope and cell membrane to facilitate SARS-CoV-2 entry into the host cell. The structure-functional properties of these 5 specific viral-hijacked host cellular factors, their ultimate pathophysiological consequence(s) due to virus-induced HMRD in acute COVID-19 and during long-COVID, the persistent virus-free PASC disease state, is comprehensively described below.
Viral-hijacked human angiotensin-converting enzyme-2 (hACE2)
SARS-CoV-2 binds to the human angiotensin-converting enzyme-2 (hACE2) as a potential CSR for anchoring to specific cellular tissue sites in the body 286 , 287 , 288 . Human cells that express ACE2 are potential targets for SARS-CoV-2 infection; however, other cellular factors such as human proteases that prime the viral S-protein are also critical for the next sequential steps of the viral infection process 55 , 289 . The hACE2 levels are highest in the small intestine, testis, kidneys, heart, thyroid, and adipose tissue; moderately present in the lungs, colon, liver, bladder, and adrenal glands; lowest in the blood, spleen, bone marrow, brain, blood vessels and muscle 290 . ACE2 exists either in free soluble form (sACE2) or in bound form immobilized on cell membranes (mACE2) of intestinal, renal, testicular, gall bladder, pulmonary, and cardiovascular (CV) epithelia 291 . Both soluble and membrane-bound ACE2 proteins are critical for the regulation of blood pressure in the body.
ACE2 structure/function
ACE2 is an essential counter-regulatory carboxypeptidase of the hormonal RAAS, a vital regulator of blood volume, systemic vascular resistance, and thus the CV/circulatory homeostasis 292 . ACE2 is expressed in most human tissues and cell types as a type I integral membrane protein solubilized by the action of a disintegrin and metallopeptidase (ADAM)-17 293 . The human ACE gene expresses two distinct isoforms, the somatic ACE (sACE) and testicular ACE (tACE) 294 . The tACE form is exclusive to the male germinal cells with an enzymatic activity critical for male fertility 294 , 295 , 296 . The other isoform, sACE, is widely distributed on the surface of endothelia, neuroepithelia, and immune cell cascade 297 . Besides these two isoforms, humans express an ACE homolog—the ACE2 298 , 299 . ACE2 enzyme degrades ANG II, the major effector of the RAAS that increases hypertension (by lowering baroreceptor sensitivity) to control heart rate, up-regulate vasoconstriction, sodium retention, OxS, inflammation, and fibrosis 300 . Therefore, hijacking hACE2 may shift RAAS homeostasis and compromise CV function 301 . ACE2 was also identified as a potential receptor for the cellular entry of several hCoVs, including HCoV-NL63, SARS-CoV, and SARS-CoV-2 302 , 303 .
Consequences of ACE2 hijack
The viral hijack of hACE2 could compromise patient’s health for extended periods of time, especially among COVID-19 and PASC with comorbidities (i.e., CVD, T2DM, brain and kidney dysfunctions) 300 , 304 . The human ACE2 gene is strongly associated with diabetes; therefore, any loss of hACE2 decreases insulin secretion and impairs the glucose tolerance 305 , 306 . This partly explains the higher morbidity and mortality rates observed in COVID-19 patients with preexisting diabetes 307 . Human ACE2 expression is high in tubular epithelia (from kidney), and the viral hijack of this enzyme could alter sodium transport, affect blood volume/pressure and lead to AKI 308 . Viral hijack of hACE2 in the BBB axis could impair autonomic nervous system (ANS) and dysregulate blood pressure and respiration 76 . Viral hijack of hACE2 in the brain stem may increase sympathetic nerve drive, alter baroreflex, and exacerbate hypertension 309 . Loss of hACE2 in the vasculature may lead to endothelial dysfunction, inflammation and aggravate atherosclerosis and diabetes 284 , 310 . A loss of pulmonary hACE2 may cause hypertension, respiratory distress, and fibrosis post-viral infection 311 . Thus, SARS-CoV-2-mediated cell surface reduction of hACE2 receptors could trigger widespread inflammatory sequelae observed in COVID-19, which may linger through long-COVID for an extended period of time.
Viral-hijacked human neuropilin (hNRP)-1
SARS-CoV-2 anchors to human ACE2 as its primary host CSR; however, the broad-spectrum tissue tropism of COVID-19 raises the possible involvement of other host receptors in the binding of SARS-CoV-2 to other target tissue sites. Neuropilin-1 (NRP1) is another prominent CSR that facilitates entry of SARS-CoV-2 into the CNS through olfactory epithelium in the nasal cavity 63 . Also, NRP1 (but not ACE2) serves as the principal CSR to mediate SARS-CoV-2 infection of astrocytes in the brain tissue. Viral infection of astrocytes resembles reactive astrogliosis with elevated type-I IFN production, increased inflammation, and down-regulation of transporters for water, ions, choline, and neurotransmitters 312 . These events lead to dysfunction and death of uninfected bystander neurons that could inflict severe symptoms of neuro-COVID including anosmia, ageusia, headache, delirium, acute psychosis, seizures, and stroke 171 , 197 .
NRP1 avidly binds to furin-cleaved S1 fragment of viral S-protein and potentiates cellular entry of SARS-CoV-2 63 . NRP1 is abundantly expressed in the respiratory and olfactory epithelia, with the highest localization in endothelial and epithelial cells. Blocking of NRP1/S-protein interaction with small-molecule inhibitors or monoclonal antibodies (mAbs) may reduce SARS-CoV-2 infectivity and provide potential intervention strategies for COVID-19 management 62 .
NRP1 structure/function
NRPs are single-pass transmembrane, non-tyrosine kinase surface glycoproteins, expressed by endothelial, immune, and vascular smooth muscle cells and are regulators of numerous signaling pathways within the vasculature 313 , 314 . Two homologous NRP isoforms are known to exist, namely NRP1 and NRP2, encoded by distinct neuropilin genes ( Nrp1 and Nrp2 ) 315 , 316 , 317 . Both NRPs originally identified as neuronal adhesion molecules, participate in Semaphorin -mediated axonal guidance 318 . They are also expressed in vascular and lymphatic endothelia, affecting proliferation, migration, angiogenesis, as well as the formation of small lymphatic vessels and capillaries 313 . NRP1 plays a key role in VEGF-dependent angiogenesis (i.e., new blood vessel formation) 319 . NRP2 is important for migration, antigen presentation, phagocytosis and cell–cell contact in the immune system 320 . Both NRPs play a multifunctional role in several physiological pathways including nervous and vascular development, as well as in immunity and tumorigenesis 321 , 322 .
Consequences of NRP1 hijack
The SARS-CoV-2 S-protein could hijack NRP1 signaling and directly affect VEGF-A-mediated pain. This may raise the possibility that pain, an early symptom of COVID-19, could be diminished by the SARS-CoV-2 S-protein interaction with NRP1 323 . Such ‘silencing’ of pain through subversion of VEGF-A/NRP-1 signaling could be an underlying factor for disease transmission through SARS-CoV-2 infected asymptomatic or minimally symptomatic individuals 324 . As a key player in VEGF-induced vascular permeability and angiogenesis, the viral hijack of NRP1 could impede capillary formation, tissue repair, and organ function in the body 319 . Furthermore, loss of NRP1 could compromise the integrity of vascular endothelium, a selective barrier that regulates macromolecular exchange between the blood and tissues. The ensuing vascular hyper-permeability of plasma molecules and leukocytes may lead to acute tissue edema and inflammation 325 . SARS-CoV-2 infection of astrocytes via NRP1 hijack could disrupt normal neuron function, induce neuronal cell death leading to abnormal manifestations in the CNS 312 . NRP1 deficiency in visceral smooth muscle cells could negatively impact GI contractility and motility 326 . Also, the viral hijack-mediated suppression of epithelial NRP1 could weaken the gut barrier function 327 . The NRP-1-mediated SARS‐CoV‐2 entry into bone marrow‐derived macrophages (BMM) could impede osteoclast differentiation and affect calcium/phosphorus metabolism in COVID‐19 patients 328 . Accordingly, viral hijacking of NRP1 could exert chronic disorders of bone metabolism such as osteoporosis or osteopetrosis in PASC patients 116 , 117 .
Viral-Hijacked Human Furin
SARS-CoV-2, the etiological agent of COVID-19 pandemic, contains a unique insertion of amino acids (AAs), exclusively proline- arginine -arginine-alanine- arginine (P R RA R 685 ↓ ) at the S1/S2 boundary of its S-protein, which is clearly absent in SARS-CoV and other related hCoVs 72 . Interestingly, this P R RA R insertion generates a ‘furin cleavage site’ on S-protein at the S1/S2 multi-basic region, considered as a potential ‘gain of function’ for the viral pathogen. Furin-mediated priming of viral S protein at S1/S2 (P R RA R 685 ↓ ) [the underlined AAs refer to critical residues needed for the furin recognition] is a key determinant in the pathogenesis of COVID-19 55 , 73 , 329 .
Furin is a member of the proprotein convertase (PC) family of enzymes, known to process latent precursor proteins into their biologically active state 330 . Intracellular proteolytic processing of precursor polypeptides is an essential step in the maturation of many proteins such as plasma proteins, hormones, neuropeptides, and growth factors 331 . Due to their homology with bacterial subtilisin and yeast kexin proteases, PCs are also known as the PC subtilisin/kexin type (PCSK) enzymes 330 . Humans encode nine members of the PCSK family (from 1–9), where the PCSK3 represents ‘furin’ 332 . Most viral envelope glycoproteins, like bacterial exotoxins, also require proteolytic cleavage to mediate entry into host cells. Accordingly, viral pathogens hijack cellular endo-proteases, such as furin/PCSK3 that prime polybasic cleavage sites and provide critical access for tissue tropism and viral spread in an infected host 333 . Accordingly, the canonical polybasic ‘furin cleavage site’ has been reported in several enveloped viruses, including Herpes-, Corona-, Flavi-, Toga-, Borna-, Bunya-, Filo-, Orthomyxo-, Paramyxo-, Pneumo , and Retroviridae 332 .
Furin structure/function
Furin is a 794-AA type-1 transmembrane (TM) protein with large luminal and extracellular regions, a common feature among PC enzymes. Furin is ubiquitously expressed; however, its mRNA and protein levels vary depending on the cell type and tissue. Furin levels are high in salivary glands, liver, and bone marrow, whereas its expression is relatively low in muscle cells 334 . Furin/PCSK3 cleaves basic AA motifs; therefore, also termed as PACE (Paired basic AA Cleaving Enzyme) . It cleaves diverse types of protein precursors in the secretory pathway at downstream of basic-AA target sequence (canonically, R-X(R/K)-R) 335 . Furin most likely cleaves and activates more than 150 mammalian, viral and bacterial substrates. These include viral envelope glycoproteins and bacterial toxins, as well as cellular factors that promote tumorigenesis 336 . Substrates for furin cleavage possess a specific 20-residue recognition sequence motif, which includes: pro- parathyroid hormone (PTH), transforming growth factor (TGF)-β1 precursor, pro - albumin , membrane type-1 matrix metalloproteinase (MMP), β-subunit of pro- nerve growth factor , and von Willebrand factor 337 .
Several bacterial and viral pathogens exploit human furin enzymes for proteolytic activation of their own virulent factors during the infectious process 332 . In bacterial pathogens, furin-activated toxins may promote tissue invasion, increase transmission rates, or suppress cellular immune responses 338 . The presence of ‘furin cleavage site’ in diphtheria toxin (R-V-R-R ↓ ) and Pseudomonas exotoxin A (R-Q-P-R ↓ ), enhances their cytotoxic spectrum 339 , 340 . Similarly, furin could cleave shiga and shiga-like toxins of certain Shigella spp. and Escherichia coli to enhance their ability to inhibit host protein synthesis 341 . Thus, without the proteolytic activation of certain bacterial exotoxins, diseases such as dysentery or diphtheria would not occur. In anthrax toxin, these three sub-unit proteins consist of a receptor-binding protective antigen (PA), an enzyme-active edema factor and a lethal factor 342 . Furin cleavage of PA results in its oligomerization at the cell surface into a pre-pore that facilitates membrane interactions with edema factor and lethal factor. Subsequently, the furin-activated anthrax toxin complex is endocytosed into the cytoplasm and elicits lethal outcomes 341 , 343 .
Consequences of furin hijack
Furin is essential for cardiovascular (CV) function; therefore, viral hijack of this proprotein convertase enzyme could alter lipid metabolism, and affect blood pressure regulation and vascular remodeling in COVID-19 patients 344 , 345 . Furin also regulates the transcription factor NKX2-5, which is essential for both normal heart development and CV function 346 . Viral hijack of furin may increase the susceptibility of tissue epithelia for ferroptosis-like cell injury 347 . Ferroptosis is a type of cell death linked to altered iron metabolism, glutathione (GSH) depletion, GSH peroxidase 4 (GPX4) inactivation, and increased OxS, which is a prominent clinical feature of COVID-19 27 , 348 . Furin is an integral part of specialized cellular machinery that regulates membrane type-1 matrix metalloproteinases (MT1-MMPs) and its deterrence by SARS-CoV-2 could compromise proteolytic events on host cell surface and affect nutrient transport for cellular metabolism 349 . Loss of furin due to viral pre-utilization may result in increased growth, invasiveness and cytokine production in the bone-joint synovium and aggravate rheumatoid arthritis 350 . Dysregulation of furin may lead to neurodegenerative and neuropsychiatric disorders known to persist during PASC 171 , 351 . Co-existence of furin with ACE2/RAAS in the female and male reproductive systems pose a potential risk on human fertility in COVID-19 patients. Viral hijacking of host cellular factors in the female reproductive tract may disrupt ovarian function and thereby the oocyte quality. Higher expression of ACE2 in the endometrium with age and during the secretory phase raises concern about increased susceptibility to SARS-CoV-2 infection 352 . Furin also regulates placenta-specific (PS)-1-mediated oocyte meiosis and fertilization 353 . Furin plays a major role in oocyte development beyond the early secondary follicle stage and any virus-mediated loss of its proteolytic activity could lead to follicular dysplasia and female infertility 354 .
Viral-hijacked human transmembrane protease, serine 2 (TMPRSS2)
Human transmembrane protease, serine 2 (TMPRSS2) has been identified as a key host cell factor that determines the route of viral entry for SARS-CoV-2 infection and the pathogenic spectrum of COVID-19 355 . Specifically, TMPRSS2 processes the SARS-CoV-2 S-protein and enables the viral entry into host cells within <10 min in a pH-independent manner. In TMPRSS2-defecient cellular tissue sites, SARS-CoV-2 is endocytosed into lysosomes, and an alternative route of viral entry into the cytosol is achieved in about 40–60 min of post-infection via acid-activated CTSL protease 356 . TMPRSS2 cleaves the viral S protein at multiple sites, including the canonical S1/S2 cleavage site 355 . TMPRSS2 expression is high in the human prostate gland under androgenic hormone regulation. As an apical surface serine protease, TMPRSS2 regulates epithelial sodium homeostasis in the prostate gland and plays a vital role in male reproduction 357 . In normal prostate, TMPRSS2 is involved with proteolytic cascades to activate prostate-specific antigen (PSA) in the seminal fluid (like fibrinolytic blood coagulation) 358 . TMPRSS2 expression is high in ciliated cells and type I alveolar epithelia (AT1), which is known to upregulate with ageing 359 . This may explain the relative protection of infants and children from severe respiratory illnesses. Recently, both TMPRSS2 and ACE2 were detected in human corneal epithelia, which suggests that the ocular surface is a potential route of cellular entry for SARS-CoV-2 360 .
TMPRSS2 structure/function
TMPRSS2 is a 492 AA polypeptide composed of an intracellular single-pass TM domain, and a bioactive ectodomain with three functional subunits: (i) N-terminal low-density lipoprotein (LDL) receptor type-A (LDLR-A) domain, which is a Ca 2+ binding site; (ii) class-A scavenger receptor cysteine-rich (SRCR) domain, which binds to other cell surface or extracellular molecules; and (iii) C-terminal trypsin-like serine peptidase (SP) domain with a canonical His 296 -Asp 345 -Ser 441 catalytic triad for proteolytic activity to cleave Arg or Lys residues 357 , 360 .
TMPRSS2 belongs to the type 2 transmembrane serine protease (TTSP) family comprising of 19 surface-bound trypsin-like serine proteases. TTSPs initiate several pericellular proteolytic pathways vital for degradative remodeling of extracellular matrix, proteolytic activation of membrane proteins, and putative epithelial homeostasis 361 , 362 , 363 , 364 . Human TMPRSS2 mRNA is expressed in many tissues, including prostate, breast, bile duct, kidney, colon, small intestine, pancreas, ovary, salivary gland, stomach, and lungs 365 . TMPRSS2 plays a vital role in several biological functions such as digestion, salt-water balance, iron metabolism, tissue remodeling, blood coagulation, auditory nerve development, and fertility 366 . It is also required for many pathobiological pathways that involve inflammatory responses, tumor cell invasion, apoptosis, and pain 367 . TMPRSS2 modulates dendritic cells and regulates cytokine release, a major clinical manifestation in COVID-19 pathology 368 .
Consequences of TMPRSS2 hijack
As a membrane-anchored enzyme of the host cellular machinery, TMPRSS2 activates precursor molecules in the pericellular milieu to establish metabolic homeostasis 362 . Viral hijacking of this protease could dysregulate lipid metabolism, adipose tissue phenotype, and thermogenesis via direct growth factor activation or indirect hormonal mechanisms 366 . TMPRSS2 expression is high in human prostate gland and this enzyme regulates sperm function in the seminal prostasome 357 . Increasing evidence suggests that COVID-19 could inflict detrimental effects on spermatogenesis and hormonal regulation in male patients 369 . Abnormal serum follicle-stimulating hormone (FSH), luteinizing hormone (LH), and testosterone (T) levels were also reported, which suggests a dysfunctional hypothalamic-pituitary-gonadal (HPG) axis in COVID-19 patients 370 . These male reproductive health issues may aggravate and continue to linger in PASC patients. Dysregulation of TMPRSS2 expression and/or catalytic activity may cause both tumor formation and metastasis, contributing to the etiology of several cancer types, especially prostate cancer 371 . Interestingly, TMPRSS2 is reportedly associated with tumor cell expression, different complex(es) formation, and pathways, as well as transcriptional mis-regulation in prostate cancer among COVID-19 and PASC patients 372 .
Viral-hijacked human cathepsin L (hCTSL)
In the pathogenesis of COVID-19, the cleavage and priming of S-protein is critical for viral entry into host cells. SARS-CoV-2 uses different routes of host cell entry: i) membrane fusion (with cells that express both ACE2 + serine proteases i.e., TMPRSS2 and furin) and/or ii) receptor-mediated endocytosis (to target cells that express only ACE2 + cysteine proteases i.e., CTSL) 74 , 373 . Interestingly, CTSL alone could activate membrane fusion of viral S-protein and facilitate host cellular entry of the virus 374 . Therefore, viral hijack of CTSL provides an alternative entry mechanism (via endo/lysosomal route) for SARS-CoV-2 invasion of host cells that lack TMPRSS2 enzyme 375 . The activated/primed S protein further mediates fusion of viral envelope with host cell membrane and releases the SARS-CoV genome into the cytoplasm for subsequent viral expression/replication. The CTSL expression is up-regulated during chronic inflammation and is involved in the degradation of the extracellular matrix, an important process for SARS-CoV-2 to enter host cells 376 . Furthermore, the circulating level of CTSL is elevated after SARS-CoV-2 infection and positively correlates with the disease course/severity of COVID-19 78 . The SARS-CoV-2 Omicron variant, which recently dominated the pandemic, prefers the endo/lysosomal cysteine protease CSTL over TMPRSS2 for host cell entry 377 . Inhibition of CTSL is therefore, considered an effective strategy to minimize internalization of the virus.
Cathepsin L (CTSL), a member of the lysosomal cysteine protease family, shares a catalytic mechanism and sequence homology with non-specific plant protease, papain 378 . CTSL contains lysosomal targeting motifs with maximal catalytic activity at acidic pH (3.0–6.5) in the presence of thiol (-SH) compounds. The enzyme activity and stability of CTSL at physiological pH strictly depend on the ionic strength of the milieu 379 . The endopeptidase activity of CTSL generates active enzymes, receptors, transcription factors, and biologically active peptides by limited proteolysis 380 . Limited endosomal proteolytic activity of CTSL is critical for diverse cellular processes such as normal lysosome-mediated protein turnover, antigen/proprotein processing, regulation of signaling molecules, extracellular matrix remodeling, and apoptosis 381 , 382 . CTSL plays a vital role in the functional development of the immune system 383 , in skeletal physiology including bone collagen degradation/resorption and thyroid hormone release 384 , 385 . Human cysteine proteases are involved in pathogenesis of several diseases including rheumatoid arthritis, osteoporosis, tumor metastasis, renal diseases, diabetes, periodontal diseases, and viral infections 376 , 386 .
Consequences of CTSL hijack
In the cytosol and nuclei, CTSL is critical for several biological pathways, including cell division 387 . CTSL regulates oocyte maturation and early embryonic divisions. Any interference with CTSL activity could impair female competence for embryonic development 388 . Accordingly, the viral hijack of CTSL may reduce female competence for embryonic development (also a major cause of infertility) and may account for early miscarriages during COVID-19 pandemic 389 . Furthermore, several lysosomal enzymes are involved in female ovulation, especially CTSL, in ovarian follicle growth and maturation 390 . The CTSL-mediated activation of progesterone receptors in granulosa degrades extracellular matrix in the follicular tissue during female ovulation 391 . Viral hijack of CTSL could severely compromise female reproductive health.
In secretory vesicles, CTSL generates active neuropeptides including enkephalin, β-endorphin, and dynorphin, as well as proopiomelanocortin (POMC)-derived peptide hormones adreno-corticotropin hormone (ACTH), and melanocyte stimulating hormone (MSH), are essential for cell-cell communication in the nervous and endocrine systems 392 . CTSL also converts proenkephalin into the active enkephalin, an opioid peptide neurotransmitter that mediates pain relief 393 . Inhibition of CTSL alleviates microglia-mediated neuroinflammatory responses from caspase-8 and NF-κB pathways 394 . A majority of COVID-19 and PASC patients show neurological symptoms, including headache, impairment of memory, seizures, and encephalopathy, as well as anatomical abnormalities, such as changes in brain morphology 201 , 395 . The viral hijack of CTSL could be a contributing factor for these cognitive dysfunctions in SARS-CoV-2 infection.
In addition to cardiac injury (myocardial infarction, fulminant myocarditis, arrhythmias, venous thromboembolism, and cardiomyopathies), the vasculature is severely affected in COVID-19 and PASC, directly by the SARS-CoV-2 hijack of host factors, and indirectly from the systemic inflammatory cytokine storm 396 . Senescence of vascular endothelium is a hallmark of vascular aging, which leads to the initiation, progression, and advancement of CVD. CTSL plays a key role in vasculo-endothelial senescence via regulation of AKT/ ERK1/2-P21 pathway 397 . Lysosomal CTSL attenuates cardiac hypertrophy and preserves cardiac function by facilitating autophagy and proteasomal protein processing 398 . Hypertension is another independent prognostic factor of poor clinical outcomes in elderly COVID-19 patients 399 . The development of hypertension involves extensive arterial wall remodeling, in which CTSL plays an essential role. CTSL regulates tissue inflammatory responses and extracellular matrix accumulation, thereby preventing arterial remodeling and hypertension, in part by inhibition of smooth muscle cell proliferation in the vessel wall 400 . CTSL is also involved in inflammation and remodeling of vascular as well as extracellular matrix, the cardinal pathological events in systemic sclerosis. Loss in dermal CTSL expression may lead to dermal fibrosis in systemic sclerosis 401 , 402 . Thus, CTSL hijack may have detrimental consequences on CV health in COVID-19 and PASC patients.
Obesity, diabetes, and other related metabolic syndrome pose a higher risk of severe COVID-19 infection with poor prognosis 403 . CTSL is known to degrade fibronectin, insulin receptor (IR), and insulin-like growth factor-1 receptor (IGF-1R), essential molecules for adipogenesis and glucose metabolism. Inhibition of CTSL results in reduced body weight, low serum insulin levels, and increased glucose tolerance 404 . New onset T2DM, arterial hypertension and dyslipidemia are possible sequelae of COVID-19 infection 405 . Viral hijack of CSTL in obese and diabetic COVID-19 patients suggest that this protease is a novel target for new-onset metabolic disorders in PASC patients.
Long-COVID/PASC: precision nutrition to reset virus-induced HMRD
Viral hijacking of host metabolic machinery by SARS-CoV-2 genome and its expressed proteins is critical for viral biogenesis and propagation 21 . During the pathogenesis, SARS-CoV-2 reprograms and dysregulates several host cellular pathways that are involved in metabolism, bioenergetics, iron-redox signaling, and immunity—collectively termed as HMRD 22 , 27 . The pathophysiological onset and persistence HMRD in COVID-19 and PASC patients is a cumulative outcome of metabolic remodeling culminating from both SARS-CoV-2-induced cellular damage as well as host antiviral responses. Extensive clinical data combined with genomic and metabolomic profiles has revealed several acute as well as chronic physio-chemical vulnerabilities both among SARS-CoV-2 infected COVID-19 cases as well as virus-free (RT-PCR negative) PASC patients. SARS-CoV-2-induced HMRD triggers viral pathogenesis by re-directing free AAs and FAs from host cellular metabolism, as building blocks to support viral progeny and propagation. Therefore, precision nutrition to ‘reset’ (or reverse) HMRD is a functional strategy to combat both COVID-19 and PASC. Thus, an ideal nutritional remedy needs to demonstrate human randomized clinical trial (RCT)-proven health benefits with optimal ADME ( Administration, Distribution, Metabolism, Excretion ) profile and functional efficacy to reset HMRD and facilitate total recovery of PASC patients 22 . Accordingly, such precision nutrition protocols consisting of specific bio-functional compounds to reset or resolve SARS-CoV-2-induced HMRD are shown in Table 1 .
Nutritional reset of hypoxia/’Warburg’ effect
Dysregulation of glucose metabolism with elevated serum glucose levels and upregulation of glycolytic intermediates is a prominent feature of COVID‐19. Glucose metabolism supports OXPHOS/TCA cycle (ATP synthesis) in mitochondria and generates malate, an indicator of mitochondrial activity. Interestingly, plasma malate levels dwindle after SARS‐CoV‐2 infection, which suggests m -Dys due to hypoxia/Warburg effect in the pneumonia state 27 , 51 . The SARS-CoV-2-induced hypoxia activates gluconeogenesis and porphyrin (or iron) metabolism, thereby steers the clinical onset/progression of COVID-19. Furthermore, the hypoxia-mediated ‘Warburg’ effect also alters both TCA cycle as well as lipid metabolism through perturbation of tryptophan biosynthesis, aminoacyl-tRNA degradation, phenylalanine, and arachidonic metabolism 406 . Dysregulated arachidonic acid metabolism and fatty acid β‐oxidation in tandem with platelet aggregation and bile acid synthesis help identify SARS-CoV-2 infected asymptomatic individuals, the hidden drivers of COVID-19 pandemic as well as massive victims of PASC 21 , 407 .
l- Arginine metabolism is vital for immune and vascular functions in the body 408 . Its metabolic functions include conversion to nitric oxide (NO•) by NO synthase (NOS) or arginine catabolism to ornithine by arginase 409 . NO• is master regulator of cardiovascular function, metabolism, neurotransmission, and immunity 410 . Upregulation of arginase depletes serum levels of arginine, subsequently inhibits NO• production. Accordingly, low availability of plasma arginine has been implicated in endothelial dysfunction, T cell dysregulation, and thrombocytopenia (coagulopathy) in ARDS patients, the most severe form of COVID-19 408 , 411 . Thus, restoring optimum levels of arginine through oral supplementation could maintain immune homeostasis, particularly to reverse altered T cell activity and reset T cell/macrophage functions in PASC patients 412 , 413 .
Perturbations in l -arginine metabolism is prevalent among COVID-19 and PASC patients, across all disease stages 414 , 415 . Oral supplementation of l -arginine (1.66 g twice a day) for 10 days in COVID-19 patients ( n = 101) show significant reduction both in respiratory/ventilation support (71.1%) and in-hospital stay 416 . Oral supplementation with l -arginine (1.66 g/d), in a human RCT ( n = 110) during COVID-19 pandemic showed patient improvement in cardiac rehabilitation after myocardial infarction and cardiac revascularization 417 . Interestingly, arginine depletion with arginine-metabolizing enzymes has also been suggested as a therapeutic approach to block viral proliferation during acute COVID-19 418 . However, the persistent hyper-inflammatory state and immune dysregulation which is common among virus-free PASC patients, arginine supplementation may prove beneficial to this group. In PASC pathology, the markers of NO• bioavailability are low; accordingly, oral supplementation of these patients with l -arginine + vitamin-C for 4 weeks could significantly elevate serum l -arginine levels and thereby increase NO• bioavailability 419 . In another human RCT ( n = 50), oral supplementation of l -arginine (1.66 g/d) + vit-C (500 mg/d) for 4 weeks restored physical performance, endothelial function, fatigue, and relieved persistent symptoms in PASC patients 415 . Oral supplementation of l -arginine could be a promising nutritional remedial to reset HMRD-associated cardiovascular disorders and immune dysfunction in PASC patients.
l- Tryptophan metabolism is the most prominent pathway affected by HMRD during SARS-CoV-2 infection. The virus-induced re-wiring of l -tryptophan metabolism alters kynurenine pathway, dysregulates host inflammatory and immune responses 420 . The l -tryptophan-kynurenine pathway is a regulatory ‘hub’ for canonical and non-canonical transcription, macrophage release of cytokines, which could trigger hyper-inflammation and cause poor clinical outcomes in COVID-19 and PASC 421 . Major clinical manifestations in PASC such as depression, fatigue, sleep disturbances, attention disorders, anxiety, muscle weakness, and dyspnea are directly linked to ‘kynurenine shunt’, which is known to increase l -tryptophan degradation towards kynurenine and away from serotonin synthesis 422 , 423 , 424 .
l -tryptophan is an essential AA, vital for biosynthesis of neurotransmitter serotonin ( 5-hydroxytryptamine , 5-HT), sleep hormone melatonin and co-factor NAD + through its downstream metabolic pathways 425 . l -tryptophan catabolism by indoleamine-dioxygenase (IDO) through the kynurenine pathway generates several bioactive metabolites collectively referred to as kynurenines 426 . SARS-CoV-2 infection could deplete plasma l -tryptophan levels and increase IDO-stimulated generation of neuroactive tryptophan catabolites, including kynurenine 427 . Kynurenine is precursor for the vital cellular effector NAD + and for several other intermediate metabolites that modulate immune and neuronal functions. SARS-CoV-2 infection deprives the host for NAD + by inhibiting the biosynthetic pathway from quinolinic acid, and simultaneously acquiring NAD + from nicotinamide in a salvage pathway 428 . Thus, l -tryptophan metabolism via kynurenine pathway produces niacin (vit-B3), a building block for NAD + synthesis. In oxidized form, NAD + is a potent inhibitor of pro-inflammatory cytokines and ventilator-induced acute lung injury (ALI) in COVID-19 429 . Accordingly, reduced serum levels of niacin or NAD + reflects l -tryptophan deficiency and increased severity of COVID-19 430 . Activation of kynurenine pathway in elderly COVID-19 and PASC patients is a major cause for cerebrovascular damage 431 . Virus-induced HMRD of tryptophan metabolism and its clinical implication on neuro-cognitive function(s) is shown in Fig. 6 .
The SARS-CoV-2-induced HMR affects tryptophan metabolism by lowering the levels of tryptophan, serotonin, and indole-pyruvate, while elevating the levels of kynurenine, kynurenic acid, picolinic acid, and nicotinic acid 51 , 424 . After conversion to kynurenine, the tryptophan catabolism divides into different branches, leading to the formation of 3-OH-kynurenine, anthranilic acid or kynurenic acid. The 3-OH-kynurenine catabolism further leads to the generation of picolinic acid, quinolinic acid, and nicotinamide. The neuroprotective kynurenic acid is present mainly in astrocytes, neurotoxic 3-OH-kynurenine and excitotoxic quinolinic acid are found in microglial cells. Besides directly targeting neurotransmitter receptors, the tryptophan metabolites, in particular 3-OH-kynurenine and 3-OH-anthranilic acid, are redox active that impact brain physiology 789 . The modulation of the tryptophan-kynurenine pathway is an indicator for a coherent metabolic shift 790 . The tryptophan-nicotinamide pathway is associated with inflammatory signals and coordinator of cell metabolism in SARS-CoV-2 infection 791 . The broader virulence spectrum of SARS-CoV-2 with ability to cross the BBB and inflict a plethora of neuropathological manifestations by HMRD in host brain metabolism has been elucidated as ‘Neuro-COVID-19’ 171 .
Serotonin (5-HT) is a precursor for melatonin, a chrono-biotic pineal hormone, which may elicit potential adjuvant effects to combat COVID-19 and PASC 432 . Melatonin could also reverse aerobic glycolysis in immune cells and inhibit SARS-CoV-2-induced hyper-inflammatory response in COVID-19 patients 433 . l -tryptophan deficiency could deplete melatonin levels and aggravate pathophysiological risks of COVID-19 and PASC. l -tryptophan deficiency could also lower serum levels of 5-HT and augment disease manifestations such as anosmia, ageusia, and dysfunctional chemesthesis in COVID-19 and PASC 434 . Furthermore, 5-HT deficiency could worsen silent hypoxemia, weaken hypoxic pulmonary vasoconstriction, and compromise the recovery of COVID-19 and PASC patients.
The gut-brain’ axis is a bi-directional system that links emotional and cognitive centers of the brain with the peripheral functioning of the GI tract. The serotonergic system forms a diffuse network within the CNS and plays a neuroprotective role while regulating mood and cognition 435 . Based on 11 human RCTs, oral supplementation of l- tryptophan (0.14 to 3 g/d) with regular meal seem to improve the mood of individuals 436 . l- Tryptophan supplementation, especially at ≥1 g is shown to improve sleep quality and resolve insomnia 437 . Nutritional reset of l- tryptophan levels, and optimization of peripheral and central 5-HT levels could help alleviate neuro-cognitive dysfunction in PASC patients.
Nutritional reset of Iron (Fe)-Redox dysregulation (FeRD)
SARS-CoV-2 mediated acute lung injury (ALI) induces death of inflammatory cells with sloughing of alveolar epithelia and damage of pulmonary vasculature with hemolytic consequences 438 . Free heme released during hemolysis could induce pro-inflammatory, pro-oxidative, and pro-thrombotic effects. FeRD is highly prevalent among hospitalized COVID-19 patients. Serum levels of iron and hepcidin are low in COVID-19 patients, whereas erythropoietin (EPO) and haptoglobin levels significantly decline in critical and deceased patients 439 . Other biomarkers of iron metabolism (i.e., ferritin, TF, LF, etc.) and Hb could provide risk stratification strategies for COVID-19 management, as initial anemia is strongly linked to increased CFR. Altered iron-redox homeostasis (Fe-RH) with elevated ferritin/TF ratio predicts subsequent insufficient pulmonary oxygenation (with the need for ICU admission) and mechanical ventilation 96 . Serum TF levels decrease within the 1 st week of hospitalization in many COVID-19 patients; however, a continuous decline is prominent among subjects with fatal outcomes 440 . Therefore, nutritional strategies to reset FeRD with Fe-redox regulators and ferroptosis inhibitors could be an effective strategy for optimal recovery of COVID-19 patients and in post-recovery management of PASC 94 , 441 .
Fe-Redox regulators
In the human body, the total iron content is ~3-g for women and ~4-g for men, distributed in two main forms as heme-iron, mostly found in the Hb, myoglobin, and cytochromes (2 to 2.7-g); and as non-heme-iron, a cofactor for several enzymes 442 . Free iron levels in human body fluids are regulated at <10 –18 M to avert microbial infections as well as to prevent the precipitation of insoluble ferric hydroxides and the formation of toxic free radicals. Innate Fe-Redox regulators such as lactoferrin (LF), heme oxygenase-1 (HO-1), erythropoietin (EPO), and hepcidin (HEP) serve as first innate barriers against free radical damage and hyper-immune responses during COVID-19 and PASC 27 . In COVID-19 recovered virus-free PASC patients, the host Fe-RH is disrupted for an extended period; while the Hb, ferritin, and TF levels slowly restore back to normal after onset of initial FeRD, around a median of 122 days after discharge from the hospital 440 .
Lactoferrin (LF) , an iron-binding glycoprotein present in milk as well as several body fluids including saliva, tears, nasal secretions, gastric/cerebrospinal/synovial fluids, sperm, vaginal secretions, and neutrophils, is a key component of innate host defense 443 . Due to iron-chelation and iron-transport properties, LF is considered an innate iron regulator with a multifunctional role in scavenging iron-catalyzed free radicals (i.e., ROS, RNS) and maintain Fe-RH in the body 171 . Free radical scavenging mechanisms of LF involve stimulated glycolysis, increased ATP generation to sustain ion gradient with membrane potential and morphology of the cell 444 . Oral supplementation of LF could prevent oxidative damage by heme iron and reverse ferritin-bound iron overload during chronic inflammation and aging 445 . Notably, both OxS and related metabolic syndromes are considered as potential risk factors in COVID-19 pathology 446 . As an innate regulator of Fe-RH, LF could combat OxS at the cellular level, modulate inflammatory responses at the tissue level and play a therapeutic role in clinical management of COVID-19 and PASC 27 , 447 .
Several studies have elucidated a broad-spectrum antiviral activity for milk LF against SARS-CoV-2 171 , 448 , 449 , 450 . Breast milk from several positive COVID-19 mothers were found negative for SARS-CoV-2 pathogen 451 , 452 . The antiviral spectrum of milk LF include (i) direct interaction with the viral protein target(s) and blockade of viral attachment to host target cells 453 ; (ii) binding to heparan sulfate proteoglycans (HSPGs) on the host cell surface with subsequent inhibition of viral attachment and cell entry 454 ; and iii) interference with intracellular trafficking of the virus 455 . Also, LF is a potent anti-inflammatory agent that modulates hepcidin and ferroportin (FPN) synthesis through down-regulation of IL-6 456 , 457 ; thereby inhibits intracellular iron overload 442 . Oral administration of 20–30% iron-saturated milk LF (corresponding to 70–84 μg of elemental iron) twice a day could down-regulate IL-6 and restore FPN-mediated iron export from cells to blood in both hepcidin-dependent or independent pathways 456 . LF regulates both pro-inflammatory and anti-inflammatory responses 447 ; thereby could prevent viral insult-induced cytokine storm 458 . LF also activates plasminogen that regulates coagulation cascade and antithrombotic activity, a promising clinical intervention for COVID-19 and PASC 22 , 459 , 460 .
Apo-LF is a normoxic mimetic of hypoxia that effectively stabilizes redox-sensitive transcription factors HIF-1α and HIF-2α 461 . In hypoxia, such as during the early stages of SARS-CoV-2 infection, these transcription factors could provide synergistic protection through activation of the Keap1/Nrf2 signaling pathway 462 . Also, LF could block HIF-1α activity and provide therapeutic benefits to retinal neuronal cells during neuro-COVID 171 , 463 . Taken together, milk LF could reverse iron overload and reduce inflammation, both considered as critical factors in the pathogenesis of COVID-19 and PASC; accordingly, LF could serve as a promising all-natural intervention to resolve FeRD and reset virus-induced HMRD in the ongoing new onset global metabolic syndrome.
Heme Oxygenase-1 (HO-1) also known as the ‘heat shock protein-32’ (hsp32), is an inducible intracellular enzyme upregulated by >100-fold during infections and clinical conditions such as sepsis, renal ischemia-reperfusion injury and acute lung injury (ALI) 464 . At the cellular level, HO-1 exists in the endoplasmic reticulum, mitochondria, nucleus, and plasma membrane 45 . HO-1 mediates catalytic breakdown of heme, a potent pro-oxidant and pro-inflammatory molecule 465 . HO-1 regulates Fe-RH and provides cyto-protection via endogenous mechanisms to sustain body’s antioxidant response against OxS 466 . Human HO-1 binds to the SARS-CoV-2 ORF3a protein and inhibits virus-induced inflammation and tissue damage via the NLRP3 pathway 23 , 467 . The ability of HO-1 to protect against SARS-CoV-2 infection is probably an emergency inducible defense mechanism to ameliorate OxS from heme-released oxidants 27 . Thus, the cyto-protective function of HO-1 is a promising intervention strategy to control SARS-CoV-2 infection and alleviate virus-induced cytokine storm as well as the subsequent lung dysfunction during COVID-19 and PASC 468 , 469 .
Several natural compounds could modulate HO-1 expression. Nimbolide , a limonoid tetranortriterpenoid isolated from neem plant ( Azadirachta indica ) could upregulate the HO-1 enzyme 470 . Phytochemicals such as, the Resveratrol (3,4’,5-trihydroxy stilbene) and the Curcuminoids (with α , β -unsaturated carbonyl groups) are potential inducers of HO-1 expression through Nrf2/ antioxidant-responsive element (ARE) pathway 471 , 472 . Quercetin , a polyphenolic flavonoid found in a variety of fruits and vegetables could induce HO-1 expression via mitogen-activated protein kinase (MAPK)/Nrf2 pathway 473 . In response to inflammation and OxS, the activated HO-1 is a powerful down-regulator of pro-coagulant factors to prevent thrombotic events, endothelial injury from vascular inflammation, resolve FeRD and reset HMRD in COVID-19 and PASC patients 27 , 46 .
Erythropoietin (EPO) is a hypoxia-inducible growth factor expressed in various organs and tissues of the body 474 . Erythropoiesis is the single largest consumer of iron, quintessential for hemoglobin (Hb) synthesis in the body. Critical as well as deceased COVID-19 patients demonstrate significantly lower serum levels of EPO, haptoglobin, and hepcidin compared to survivors or mild cases 439 . EPO treatment could restore Hb levels, increase red blood cell (RBC) count, and improve O 2 delivery to the tissues, thereby help in the recovery of both COVID-19 and PASC patients 475 .
Overexpression of proinflammatory cytokines during COVID-19 results in cytokine storm, which ultimately leads to the clinical development of ALI and ARDS. Such hyper-inflammatory conditions could trigger NO• release and inhibit EPO synthesis causing anemia of inflammation (AI) 476 . Notably, fatal cases of COVID-19 demonstrate 2.5 times lower serum EPO (2.8 vs. 7.1 mU/mL), and 1.24 times lower Hb levels (14.0 vs 17.4 g/dL) compared to survivors 477 . COVID-19 patients also show ground-glass opacities localized to alveoli indicating the presence of ALI 478 , 479 . In a clinical study, recombinant human (rh)-EPO showed effective attenuation of ARDS symptoms and facilitated recovery from COVID-19 via multiple mechanisms including cytokine modulation, anti-apoptotic effects and leukocyte release from the bone marrow 475 .
EPO could potentially benefit neuro-COVID-19 patients with acute and chronic-progressive downstream sequelae of the CNS and peripheral nervous system (PNS) 171 , 480 . Therapeutic benefits of EPO on COVID-19 patients may include (i) respiratory improvement at several levels including lung, brainstem, spinal cord, and respiratory muscles 481 ; (ii) counteract hyperinflammation caused by cytokine storm/inflammasome 482 , 483 ; (iii) neuro-protection and neuro-regeneration in brain and peripheral nervous system 484 . Thus, EPO could be a potential bio-replenishment to reverse FeRD in COVID-19 and help reset HMRD in PASC patients 27 , 484 , 485 .
Hepcidin (HEP) and HEP-modulators
Hepcidin (HEP) , a peptide hormone secreted by the liver, is a master regulator of iron intake and systemic Fe-RH 486 . HEP regulates iron levels by binding to ferroportin (FPN), and the FPN/hepcidin regulatory axis that allows precise control of iron at both systemic as well as cellular levels 487 . HEP synthesis in liver is controlled by four pathways: (i) iron store-related regulation, (ii) erythropoietic activity-driven regulation, (iii) inflammation-related regulation, and (iv) mandatory signaling pathway. These regulatory pathways interact with hepatocytes to initiate or inhibit the production of sufficient HEP to regulate Fe-RH 488 . HEP expression calibrates physiological iron levels, inflammatory cues, and iron requirements for erythropoiesis. Circulating factors (LF, TF, cytokines, erythroid regulators) contribute to HEP modulation in different pathological conditions 456 , 489 .
Several compounds could act as HEP agonists to prevent iron overload from HEP deficiency 490 . Homocysteine up-regulates hepcidin expression through the BMP6/SMAD pathway, which suggests a novel approach to reset Fe-RH 491 . Calcitonin is a potent inducer of HEP expression, which may provide an interventional strategy to reverse FeRD 492 . Genistein , a member of the isoflavone-related estrogen could induce HEP transcription 493 . As potential inducer of HEP expression, phytoestrogens are promising dietary supplements to reduce iron overload and prevent any sequelae of iron-induced toxicities such as hyperferritinemia, coagulopathies and/or thromboembolism, which are prominent clinical manifestations in COVID-19 and PASC 94 , 144 , 494 , 495 . HEP and HEP-modulators are potential candidates to reset FeRD and reverse iron-overload syndromes such as anemia and chronic kidney disease (CKD) in COVID-19 and PASC patients 27 , 496 , 497 .
Ferroptosis inhibitors
Ferroptosis is an iron-catalyzed, non-apoptotic form of regulated necrosis that causes oxidative lipid damage in cell membranes leading to m -Dys 498 . Ferroptosis with characteristic accumulation of oxidized phospholipids (or their breakdown products) in myocardial and renal tissue is responsible for ischemia-reperfusion injury, which is a detrimental factor for cardiac damage and MODS in COVID-19 patients 499 . Ferroptosis is more immunogenic than apoptosis and plays a detrimental role in hyper-inflammation such as the CRS 500 . Accordingly, ferroptosis might serve as a potential treatment target for COVID-19 and PASC management 27 , 112 . A hallmark of ferroptosis is iron-dependent lipid peroxidation, which could be inhibited by the key ferroptosis regulator Gpx4, free radical trapping antioxidants and ferroptosis-specific inhibitors 501 . Nuclear factor (erythroid-derived 2)-like 2 (Nrf2) could suppress ferroptosis and resolve cellular Fe-RH in COVID-19 and PASC 502 .
Vitamin-E (Vit-E) or α-tocopherol is a fat-soluble antioxidant that prevents ROS formation during lipid oxidation. Vit-E could effectively prevent ferroptosis by reducing the Fe 3+ center to inactive Fe 2+ in lipoxygenase (LOX), thereby inhibit enzyme activity of LOX. Notably, LOX oxidizes cell membranes via peroxyl (ROO•) radicals and forms lipid hydroperoxides. Vit-E could neutralize ROO• radicals and prevent formation of lipid hydroperoxides. Afterward, GSH, GPX4, and other antioxidant agents may detoxify oxidized lipids and inhibit ferroptosis 503 . A meta-analysis ( n = 19 studies) of Vit-E supplementation (mean dosage: 384 mg/d) has shown to reduce OxS in hemodialysis patients 504 .
Ferrostatin-1 (FER-1) ( Ethyl 3-amino-4-cyclohexylamino-benzoate) is a potent lipophilic free radical scavenger that could reduce cellular accumulation of lipid peroxides and peroxyl radicals 505 . The anti-ferroptotic activity of FER-1 is associated with its ability to scavenge alkoxyl radicals and remain unconsumed while inhibiting iron-dependent lipid peroxidation 506 . During SARS-CoV-2 infection of bronchial epithelia, the inflammatory IL-6 could induce ferroptosis as well as ROS-dependent lipid peroxidation, leading to severe FeRD 507 . A combination of FER-1 and N-acetylcysteine (NAC) could reverse ferroptotic effects of IL-6 and help resolve FeRD in COVID-19 and PASC patients 27 , 508 . Also, chrono-biotic hormone melatonin could effectively reduce ferroptosis through activation of Nrf2 and HO-1 signaling pathways 509 . The strong ability to inhibit ferroptosis and platelet activation, makes melatonin a potential nutritional intervention to treat hemolytic, thrombotic, and thrombocytopenic conditions 510 , which are widespread among COVID-19 and PASC patients.
Dietary phytochemicals could serve as natural ferroptosis inhibitors to resolve FeRD-related manifestations in COVID-19 and PASC 27 . Glycyrrhizin , the main extract from licorice ( Glycyrrhiza glabra ), is a natural antioxidant, anti-inflammatory, antifibrotic and antiviral agent widely used in the treatment of chronic hepatitis 511 . Glycyrrhizin shows significant reduction in the degree of ferroptosis and inhibits OxS and provides anti-ferroptotic liver protection through up-regulation of Nrf2, HO-1 and Gpx4; and down-regulation of lactate dehydrogenase (LDH), Fe 2+ , malondialdehyde (MDA), and ROS 512 . Quercetin (pentahydroxy flavone), a natural flavonoid, could up-regulate the GSH levels and inhibit ferroptosis by reducing MDA and lipid ROS levels in the renal proximal tubular epithelia 513 . Two tannin hydrolysates, chebulagic acid and chebulinic acid , were identified as potent ferroptosis inhibitors. Their ferroptosis inhibition is mediated by regular antioxidant pathways (ROS scavenging and iron chelation), rather than the redox-based catalytic recycling pathway by FER-1 514 . Curcumin could inhibit myoglobin (Mb)-induced ferroptosis in renal tubular cells. Curcumin could reduce Mb-mediated inflammation and OxS by inhibiting the TLR4/NF-κB axis and activating the cytoprotective enzyme HO-1 515 . Dietary polyphenols such as piceatannol and astringin could strongly inhibit ferroptosis via preferential transfer of hydrogen atoms at the 4’-OH position as conventional antioxidants 154 , 516 .
Nutritional reset of mitochondrial dysfunction ( m -Dys)
Mitochondrial dysfunction ( m -Dys) is characterized by a loss of efficiency in the OXPHOS and reductions in the ATP synthesis, a causative mechanism for several metabolic syndromes including PASC. m -Dys leads to fatigue (with reduced tolerance to exercise), a common persistent symptomatic feature amongst COVID-19 survivors. Nutritional strategies to resolve m -Dys should comprise specific bioactives and cofactors essential for mitochondrial bioenergetic pathways, as well as provide effective free radical (ROS) scavengers to prevent OxS, maintain Fe-RH, and reset virus-induced HMRD.
Nicotinamide adenine dinucleotide (NAD + ) is a vital cofactor in mitochondrial bioenergetic pathways, including glycolysis, fatty acid β-oxidation, and the TCA cycle 517 . It exists in both oxidized (NAD + ) and reduced (NADH) forms, the latter is generated by NAD + accepting high-energy electrons from glycolytic and TCA intermediates and acts as a primary electron donor in ATP synthesis to drive mitochondrial OXPHOS 518 . NAD + also regulates non-redox NAD + -dependent enzymes such as poly-ADP-ribose-polymerases (PARPs) and sirtuins 519 . The macrodomain-containing protein, nsp3 of SARS-CoV-2 could counteract the host antiviral defenses from PARPs, SARM1, sirtuins and CD38 157 . Therefore, NAD + and NAD + -consuming enzymes are critical for immune responses, cellular bioenergetics, and to design antiviral strategies for COVID-19 and PASC.
Furthermore, NAD + plays a key role in several essential cellular processes including DNA repair, immune cell function, senescence, and chromatin remodeling 520 . Cardiac tissue is dense with mitochondria; as one of the most metabolically demanding organs, the heart has the highest NAD + levels. A decline in NAD + metabolism and low tissue levels of NAD + is a common trait among the elderly. Alterations to NAD + metabolism and/or decline in tissue NAD + levels are directly related to m -Dys and pathological conditions such as CVD 521 . SARS-CoV-2 infection dysregulates NAD + metabolism, which could manifest m -Dys and lead to chronic fatigue syndrome (CFS) in PASC patients 522 . Therefore, nutritional reset of m -Dys with NAD + or its natural dietary precursors could be effective in improving myocardial bioenergetics and function.
NAD is synthesized de novo from tryptophan or bio-replenished through NAD + precursors such as nicotinic acid, nicotinamide, or nicotinamide riboside, collectively referred to as niacin/B 3 vitamins. Dietary NAD + could partially resolve SARS-CoV-2-induced dysregulated gene expression and mitochondrial metabolism 523 . NAD supplement could also alleviate intestinal barrier injury by protecting mitochondrial function in gut epithelia 524 . Also, NAD + supplement could directly inhibit PARP-1 and prevent pro-inflammatory cytokines and resolve hyper-activated immune system. Oral administration of NAD + precursors, such as tryptophan, nicotinic acid (niacin), nicotinamide, and nicotinamide riboside (NR), could increase tissue NAD + levels 525 , 526 . Food supplement cocoa flavanol is shown to boost the NAD + and NADH content, stimulate sirtuin metabolism, reduce cellular H 2 O 2 production as well as OxS, and improve mitochondrial function in PASC patients 527 . Increasing NAD + levels could also stabilize telomeres and help recovery of elderly PASC patients 528 .
Alpha-lipoic acid (ALA) is an intracellular redox regulator that reduces OxS, blocks activation of NF-kB and lowers both ADAM17 activity and ACE2 upregulation 529 . ALA upregulates ATP-dependent K + channels in the cell, subsequently raises intracellular pH and thereby inhibits viral entry into host target cells 530 . Furthermore, ALA could increase intracellular GSH levels and reinforce human host antiviral defense 531 . Therefore, ALA could be considered an effective intervention against SARS-CoV-2 as well as a potent redoxeutical to resolve m -Dys-associated clinical manifestations in COVID-19 and PASC 532 , 533 . A combination of ALA (50 µM) and palmitoyl-ethanolamide (5 µM) could reduce OxS (overproduction of ROS and NO•) and modulate the major inflammatory cytokines (IL-β, IL-6,TNFα, and IL-10) involved in COVID-19 infection 534 . A human RCT from Wuhan, China, showed that ALA therapy (1200 mg/d) for 7 days, could reduce a 30-day all-cause mortality rate (37.5%) in critical COVID-19 patients 535 .
Coenzyme Q10 (CoQ10) or ubiquinone is a lipophilic cofactor in the mitochondrial ETC of the OXPHOS system that exerts powerful antioxidant, anti-apoptotic, immuno-modulatory and anti-inflammatory effects in cellular metabolism 536 . CoQ10 is also a potent anti-inflammatory agent that effectively down-regulates cytokines (i.e., TNF-α, IL- 6, CRP) and could optimize viral-disrupted ACE2/RAAS system, by exerting anti-angiotensin II effects and decreasing OxS in COVID-19 patients 537 , 538 . Excess release of cytotoxic ROS during m -Dys leads to OxS, which may hyper-activate platelet function and pose risk of thrombosis in COVID-19 patients. As a potent mitochondrial redox regulator, CoQ10 could prevent thrombotic events in COVID-19 and PASC patients by resolving ROS-induced platelet aggregation 539 . CoQ10 is expressed in all tissues; however, its biosynthesis drops down with ageing and sharply declines during OxS in COVID-19 540 . Thus, CoQ10 as an adjuvant combined with other mitochondrial nutrients could provide potential therapeutic options to resolve hyper-inflammation and reset HMRD in COVID-19 and PASC 541 , 542 .
Creatine could replenish mitochondrial viability and restore cognitive function(s) by down-regulating toll-like receptors (TLRs) involved in neuroinflammation and neurodegeneration. The potent antioxidant activity of creatine could also protect mitochondrial DNA from ROS-mediated oxidative damage 543 , 544 . Orally administered guanidino-acetic acid (GAA) could positively affect creatine metabolism, alleviate several aspects of fatigue and improve both physical as well as work capacity in patients with CFS 545 . Human RCTs have suggested that creatine (monohydrate form) could revitalize cellular bioenergetics, neuro-metabolism, and immune function, thereby may provide a multifunctional benefit in recovery of PASC with MS/CFS complications 546 , 547 .
Vitamin-B12 (Vit-B12) also known as cobalamin, is vital for cardiovascular function, as well as for immune regulation and antiviral defense 548 , 549 . Vit-B12 is also an essential nutrient for ‘skeletomuscular–gut–brain’ axis to maintain skeletal muscle, neuro-cognitive functions, and modulate gut microbiota 550 , 551 . Vit-B 12 has ranked among the top four bioactive nutrients for potential management of COVID-19 and PASC 552 . Thus, vit-B 12 combined with clinical nutrition is a potential adjuvant to reset HMRD in COVID-19 and PASC patients.
Nutritional reset of oxidative stress (OxS)
Virus-induced OxS with excess levels of ROS i.e., superoxide anion (O 2 •- ), hydroxyl radical ( • OH), singlet oxygen ( 1 O 2 ), and hydrogen peroxide (H 2 O 2 ), could trigger severe clinical manifestations including hyper-inflammation, tissue damage, thrombosis, and MODS in COVID-19, which may continue in PASC 553 , 554 . In the body, O 2 •- anions are intended products of redox signaling enzyme cascade and byproducts of several metabolic processes including mitochondrial respiration 555 . Superoxide (O 2 •- ) anions are scavenged by redox enzyme superoxide dismutase (SOD), whereas H 2 O 2 by catalase (CAT), glutathione (GSH), GSH-peroxidase (GPx), thioredoxin peroxidase (Trx), and peroxiredoxins (Prdx) 556 . Any decline in redox enzymes may increase free radical generation with subsequent induction of lipid peroxidation, protein oxidation, and DNA/RNA degradation 557 , 558 , 559 .
Serum levels of SOD, CAT, GSH, and GPx are significantly altered in COVID-19 patients 560 , 561 . The depleted total antioxidant capacity (in blood) of SARS-CoV-2 infected individuals serves as a predictive marker for COVID-19 severity 562 . Both OxS and hyper-inflammatory state during the acute phase of COVID-19, could also predict severity of chronic fatigue, depression, and anxiety symptoms even after 3 to 4 months in the virus-free PASC patients. Based on cluster analysis, a majority of PASC patients show severe abnormalities in SpO 2 , increased OxS and reduced antioxidant indices 563 . Therefore, antioxidant enzymes could be considered an effective nutritional strategy to resolve OxS and reset HMRD in COVID-19 and PASC.
Superoxide dismutases (SODs) are metalloenzymes that trigger endogenous antioxidant machinery, the first-line defense against ROS in the body 564 . SOD catalyzes the conversion of superoxide (O 2 •- ) into O 2 and H 2 O 2 557 . The H 2 O 2 is further hydrolyzed to water via CAT and GPX enzymes 565 . Three isoforms of SOD exist in human body: the cytosolic Cu-, Zn-SOD (SOD1), the mitochondrial Mn-SOD (SOD2) and the extracellular Cu-, Zn-SOD (SOD3) 566 . PC-SOD (recombinant human SOD1 covalently coupled to four molecules of lecithin) is a potent superoxide-radical scavenger with a 100-fold increase in protective effects against endothelial cell injuries, compared to unmodified SOD 567 , 568 . OxS plays a critical role in COVID-19 and PASC; therefore, the therapeutic use of SOD and SOD-mimetics (e.g., Mangafodipir ) may prove beneficial in PASC recovery 565 , 569 .
Catalase (CAT) , a heme enzyme that catalyzes the decomposition of H 2 O 2 to water + molecular O 2 , provides a vital cellular antioxidant defense 570 . Excessive production of H 2 O 2 in mitochondria could damage lipids, proteins, mDNA, resulting in necrosis or apoptosis; where then CAT could protect such cells from H 2 O 2 -induced oxidative injury 571 . Apart from its main substrate H 2 O 2 , the CAT enzyme could also process other oxidative species such as O 2 •- , • OH, 1 O 2 , hypochlorous acid (HOCl), NO•, and peroxynitrite (ONOO - ). A number of these free radicals are formed under oxidative ‘eustress’ (good stress) and ‘distress’ (bad stress), where CAT could help regulate the cellular redox-oxidative status 572 . CAT-mediated decomposition of H 2 O 2 to water minimizes the downstream flow of excessive ROS, which otherwise could trigger OxS and m -Dys in COVID-19 and PASC patients. CAT plays a crucial intermediary role in S-protein binding to hACE2 receptors, thereby affects the host susceptibility to SARS-CoV-2 infection 573 . CAT could also regulate cytokine production in leukocytes, protect alveolar cells from oxidative injury, and block SARS-CoV-2 replication 574 .
Solid lipid nanoparticles based on phosphatidylcholine stabilizers, is a functional CAT supplement designed to resist enteric digestion and deliver potent antioxidant activity 575 . Supplemental CAT is shown to alleviate OxS in the GI tract and improve gut microbiota 576 . Lactic acid bacteria, Lactobacillus casei BL23, L. delbrueckii subsp. bulgaricus CRL 864, and Streptococcus thermophilus CRL 807, produce both antioxidant enzymes CAT as well as SOD, and provide intrinsic immunomodulatory benefits in the GI tract 577 , 578 . Programmable probiotics are promising dietary adjuvants to prevent OxS in the gut, curb intestinal inflammation, and resolve certain persistent GI pathologies (e.g., colitis, inflammatory bowel disease, and dysbiosis) in PASC patients 579 .
Glutathione (GSH ) (γ- l -glutamyl- l -cysteinyl-glycine) is a tripeptide synthesized in the cytosol by two ATP-consuming enzymatic reactions 580 . GSH reaches millimolar levels (1–10 mM) within cells, micromolar levels (10–30 μM) in plasma, and its low redox potential (E ′ 0 = − 240 mV) makes GSH an ideal cellular redox buffer 581 , 582 . GSH is commonly found in reduced GSSG form in cytosol, nucleus, mitochondria, and endoplasmic reticulum 583 . The GSSG/GSH redox couple interacts with other antioxidant enzymes to maintain mitochondrial function and cellular redox homeostasis 584 . The GSSG/GSH redox couple plays a vital role in several enzymatic reactions, including the elimination of peroxides by GSH peroxidases (GPx), in covalent addition of cysteines to proteins by glutaredoxin , and in detoxification of electrophiles by GSH-S-transferase (GST) 585 , 586 , 587 . GSH plays the role of ‘master antioxidant’ in tissues; where the high millimolar levels of GSSG in reduced form emphasizes its regulatory role in processes such as detoxification, protein folding, antiviral defense and immune response 588 . Mitochondria are the main source of ROS, generated from the ETC/OXPHOS and any excess release of toxic free radicals could trigger OxS and m -Dys 589 . GSH is the main cellular antioxidant to reduce H 2 O 2 and lipid hydroperoxides (LOOH) catalyzed by GPXs 589 , 590 . A key enzymatic step in antioxidant clearance with GSH redox cycle is converting H 2 O 2 to water, which is further catalyzed by GPx. This reduction step occurs via oxidation of GSH to GSSG, and the GSSG is subsequently reduced back to GSH in the body via the enzyme glutathione reductase and NADPH 591 .
The SARS-CoV-2-induced FeRD, its ensuing OxS could deplete cellular antioxidant reserves and increase severity of COVID-19 and PASC 27 . Decreased expression of GSH synthesis leads to low free GSH levels, resulting in elevated ROS, immune dysfunction, and increased disease severity in COVID-19 patients 592 . SARS-CoV-2 infection causes GSH deficiency in the body through inhibition of nuclear factor erythroid 2–related factor 2 ( Nrf2 ) pathway, the up-regulator of GSH synthesis 593 . During OxS condition, Nrf2 is transported from cytoplasm into the nucleus by karyopherins , where SARS-CoV-2 interferes with transfer process and reduces GSH synthesis 594 . GSH precursors, particularly NAC, are widely used to revert OxS and replenish low GSH levels in pulmonary episodes such as ARDS, bronchitis, or emphysema in COVID-19 and PASC 595 . Furthermore, comorbidities such as hypertension (56.6%), obesity (41.7%), and diabetes (33.8%) are frequently linked to OxS and chronic inflammation in hospitalized COVID-19 patients 596 . In obese patients, OxS is associated with diminished GSH levels and decreased GSH/GSSG ratio 597 . Low GSH levels could also increase viral replication, pro-inflammatory cytokine release, endothelial damage, and immune-thrombosis, which is a hyper-coagulative clinical condition that could exacerbate morbidity and mortality in COVID-19 and PASC 598 . Since m -Dys and OxS jointly contribute to both COVID-19 and PASC pathology, nutritional to replenish optimal GSH levels could be a promising strategy to reset HMRD and support patient recovery 582 .
Ferroptosis, the programmed cell death due to iron and lipid dependent peroxidation, is associated with ageusia and anosmia, the early clinical manifestations of COVID-19 197 , 599 , 600 . Ferroptosis is regulated by lipid repair enzymes, which also include GSH and GPx4 reducing lipid hydroperoxides (L-OOH) to lipid alcohols (L-OH) 601 . Ferroptosis could cause severe tissue damage and MODS in COVID-19 patients. Administration of liposomal GSH could boost intrinsic GSH levels, enhance GPx4 function and reduce tissue damage from ferroptosis in COVID-19 and PASC 602 .
N-Acetyl-L-Cysteine (NAC) is a sulfur-containing AA that breaks disulfide bonds, increases viscosity of mucoproteins and serves as an antioxidant in pulmonary mucous secretions of the respiratory tract 603 . NAC is widely used as a mucolytic agent to improve airway clearance in chronic respiratory diseases. Glucose 6-phosphate dehydrogenase (G6PD) deficiency predisposes GSH depletion and increases susceptibility to SARS-CoV-2 infection, and such GSH depletion could be reversed with NAC administration 604 . As a precursor for GSH synthesis, adjuvant therapy with NAC could resolve SARS-CoV-2-induced OxS via GSH release and help restore cellular redox homeostasis during COVID-19 and PASC 605 .
SARS-CoV-2 infects type II pneumocytes and disrupts the cellular Fe-RH with increased ROS release causing severe OxS. Treatment of COVID-19 with NAC (dosage: 600 mg) could provide a prophylactic benefit, and at higher dosage (1200 mg) could serve a therapeutic regimen when administered at the first onset of symptoms 606 . Oral administration of NAC (600 mg every 8 h) to COVID-19 patients ( n = 19,208) with high-risk comorbidities (i.e., hypertension, dyslipidemia, diabetes, and COPD) is shown to significantly lower mortality rate 607 . NAC intervention (1200–1800 mg/d) markedly improve oxygenation (SpO 2 /FiO 2 ) parameters in 10 days, reduce inflammatory markers, and shorten the length of hospitalization in COVID-19 patients 608 . In a retrospective study, COVID-19 patients ( n = 1083) receiving NAC (1200 mg/d) had a shorter length of hospital stay 609 . In a cross-sectional study, COVID-19 patients ( n = 164) receiving NAC with standard therapy had an average hospital stay duration of 12 days, a 97% rate of discharge, an average duration of O 2 therapy for 8 days with limited transfer to ICU, and only one case of fatality 610 . NAC, as a precursor for reduced GSH, demonstrates antioxidant, anti-inflammatory and immunomodulatory effects, which may prove beneficial in modulating any excess inflammatory activation during COVID-19 611 , 612 . Therefore, nutritional supplementation with NAC could effectively resolve OxS and target pathophysiological pathways involved in SARS-CoV-2 infection and persistent pulmonary fibrotic sequelae in PASC 613 .
Glutamine is a precursor for several bioactive molecules in plasma and skeletal muscle, largely utilized for gluconeogenesis in the liver. Glutamine increases cellular GSH levels, improves antioxidant capacity, reduces OxS and inflammation in the body 614 . Severe OxS with elevated blood levels of high sensitivity-C-reactive protein (hs-CRP) is a hallmark of hyper-inflammatory state in COVID-19 and PASC 290 , 553 , 615 ; therefore, proper nutrition rich in antioxidants is critical for recovery of these patients 616 . Glutamine is also a widely used nutritional antioxidant in several hospital ICU-admitted patients with respiratory infections 617 . Glutamine supplementation (10 g/3x daily) for 5 days could reduce serum levels of IL-1β, hs-CRP, TNFα and increase appetite in COVID-19 patients with pulmonary complications 614 .
Maillard reaction (MR) and maillard reaction products (MRP)
MR, also known as Maillard conjugation or glycation , is a non-enzymatic process that forms covalent bonds between the NH 2 group of AAs and the carbonyl (C = O) group of reduced sugars 618 . MR generates MRPs that include several protein/peptide-saccharide conjugates. MRPs demonstrate enhanced free radical (ROS) scavenging activity and other bio-functional properties; therefore, widely used in the food industry as emulsifiers, antioxidants, antimicrobials, gelling as well as anti-browning agents. MRPs are also effective delivery systems to enhance stability and bioavailability of several dietary compounds.
Dietary MRPs are powerful antioxidants that chelate metal ions, breakdown radical chains/ hydrogen peroxide (H 2 O 2 ), and scavenge ROS 619 . For example, rutin (a bioflavonoid found in medicinal herbs and plant-derived foods) interacts with α-AAs (i.e., lysine, isoleucine, histidine, or glutamic acid) to generate phenolic-MRPs. A lysine-based thermal MR process (at 120 °C for 30-min) converts rutin to less-polar ‘quercetin’ with increased ROS-scavenging activity in hepatocytes 620 . Quercetin , the rutin-lysine-generated MRP, effectively inhibits free radicals, enhances activity of antioxidant enzymes (i.e., SOD and CAT), initiates Nrf2-dependent pathway, and upregulates phase II detoxifying antioxidant genes (including NQO1, HO-1, GCLG, and GCLM) 621 . Notably, the cellular equilibria between pro-oxidants versus antioxidants (redox homeostasis) regulates the ROS levels and ensuing OxS. The two major transcription factors: i) the NFκB upregulates the pro-oxidant mediators, and ii) the Nrf2 activates the antioxidant responses 622 .
Quercetin is considered a major natural bioactive intervention to combat OxS in the ongoing COVID-19 pandemic (4 RCTs on COVID/PASC; https://ClinicalTrials.gov/ ) 623 . Quercetin affects the expression of 30% of genes that encode viral target proteins in human cells, and potentially interfere with the activities of 85% of SARS-CoV-2 proteins 624 . Quercetin also inhibits protein disulfide isomerase (PDI) enzyme involved in platelet-mediated thrombin formation, thus could ameliorate coagulation abnormalities in PASC 625 .
MRPs could enhance the antioxidant potential of several dietary/food systems and play a supportive role in precision nutrition to help reset virus-induced HMRD. MRPs (i.e., gliadin) in the bread crust are shown to induce NF-kB pathway in macrophages and boost antioxidant defense 626 . Early studies have shown that nutritional reconditioning with MRPs could improve antioxidant status of the heart and provide cardioprotective benefits against severe OxS (as in ischemia reperfusion injury) 627 . MR could significantly enhance the antioxidant and other functional properties of lactoferrin (LF) by forming covalent complexes with beet pectin 628 . Structural modifications to diol type ginsenosides form MRPs that potentiate hydroxyl (OH•) radical-scavenging activity of Panax ginseng 629 . MRPs prepared from fermentation of milk proteins by lactic acid bacteria show higher antioxidant activity than the intact milk protein, low intracellular ROS production and sustain reduced-GSH levels in hepatocytes 630 . Milk-based MRPs could protect against oxidative damage and reduce cardiovascular risks 631 . Milk-based MRP consumption could reduce OxS and resolve dysbiosis from virus-induced HMRD 632 . Taken together, in food systems containing phenolic antioxidants (e.g., quercetin) and proteins (e.g., lactoferrin), MR could enhance antioxidant defense and provide an effective delivery/carrier system to develop nutritional reset strategies to resolve OxS-associated impairments in virus-induced HMRD.
Nutritional reset of virus-hijacked ACE2/RAAS
ACE2 is a key component of the renin-angiotensin-aldosterone system (RAAS) that plays a vital role in regulating blood pressure, vasoconstriction, sodium retention, tissue remodeling, pro-inflammatory and pro-fibrotic functions 633 , 634 . The viral hijack of human ACE2 receptor disrupts RAAS activation, upregulates NF-κB pathway, triggers cytokine storm, hypertension, cell proliferation, inflammation, and fibrosis, where all elicit detrimental effects on every bodily organ during SARS-CoV-2 infection 635 . Nutritional supplementation with l -carnitine could mitigate these pathobiological processes by inhibiting NF-κB and down-regulating NOX1/NOX2, thereby enhance the antioxidant effects of angiotensin II 636 . As an immunomodulator, l -carnitine could decrease proinflammatory cytokines (i.e., TNF-α, IL-6, and IL-1) and help reduce cytokine storm. l -carnitine could also protect against SARS-CoV-2-induced cardiotoxicity resulting from dysregulated ACE2 signaling pathway 637 , 638 .
l-C arnitine , a micronutrient composed of essential AAs (i.e., lysine and methionine), is a cofactor that converts long-chain free fatty acids to acyl-carnitine and transfers these metabolites into the mitochondrial matrix 639 . This hydrophilic AA is widely distributed in CNS, PNS, heart and skeletal muscle, holding >95% of body’s total carnitine 638 , 640 . l -Carnitine plays a vital role in lipid metabolism and its deficiency could induce feeling of tiredness or general fatigue. Therefore, fatigue could possibly be relieved by restoring serum carnitine levels through supplementation 641 . l -carnitine levels in patients with chronic fatigue syndrome (CFS) is 30 to 40% lower than healthy subjects. Oral administration of l -carnitine (3 g/d) with omega-3 fatty acids could increase carnitine palmitoyl-transferase-I activity and relieve clinical symptoms of CFS 642 . SARS-CoV-2 infection requires a high basal energy expenditure for immune activation, hyper-inflammation (‘cytokine storm’), anorexia followed by muscle loss, weakness, and fatigue 643 . Circulating autoantibodies may also have a major role in the manifestation of long-term fatigue in PASC patients 644 . Acetyl- l -carnitine supplementation could generate energy from mitochondrial oxidation of fatty acids and help mitigate fatigue in PASC patients 637 .
Nutritional reset of virus-hijacked NRP1/neuro-cognitive impairment
NRP1 is expressed in olfactory epithelium, astrocytes, and neuronal cells (which lack ACE2 expression) and serve as major CSR for SARS‐CoV‐2 infection of the central nervous system (CNS) 645 , 646 . Viral hijack of NRP1 facilitates CNS invasion of SARS-CoV-2 through the blood brain barrier (BBB), and consequential neuro-cognitive symptoms such as anosmia, ageusia, headaches, confusion, delirium, and strokes in early COVID-19 647 . The ensuing pathophysiology involves virus-induced neuronal damage, neuroinflammation, rupture of the BBB, microvasculitis and hypoxia 648 . Neuroinflammation with hypometabolic lesions cause chronic cognitive impairment in COVID patients 649 . Neurological manifestations of COVID-19 and PASC include damage to CNS and PNS, encephalitis, myelitis, myositis, Guillain Barré syndromes, and cognitive impairments 171 , 650 . These neuro-complications are prevalent among one third of COVID-19 cases, and this clinical condition may persist as chronic symptoms in PASC patients as frequent complaints of brain fog (81%) and fatigue (58%) 651 , 652 . Nutritional reset of neuro-cognitive dysfunction from viral hijack of NRP1 indeed is of high priority in clinical management of PASC.
Melatonin ( N -acetyl-5-methoxytryptamine) is a derivative of tryptophan, synthesized/secreted by the pineal gland and reaches peak levels in plasma during the night hours 653 , 654 . This chrono-biotic hormone serves as a photo-periodic switch, influencing the activity of suprachiasmatic nucleus and facilitates human sleep-wake. Melatonin plays multi-functional roles including the regulation of circadian rhythms, immune modulation, oxidative processes, apoptosis, and mitochondrial homeostasis 655 . Melatonin deficiency may lead to CVD with manifestations of hypertension and myocardial ischemia/reperfusion injury, which are prevalent in COVID-1; as well as neuro-cognitive complications such as brain fog, sleep disorders and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), which are persistent among PASC 656 , 657 .
Melatonin with its antioxidant, anti-inflammatory, immune-modulatory, and anti-apoptotic effects, is considered a potential therapeutic against COVID-19 and PASC. As a powerful antioxidant, melatonin scavenges toxic free radicals (ROS/RNS) and prevents oxidative damage of DNA through activation of DNA repairing pathways 658 . This neuro-hormone could also elevate levels of antioxidant enzymes (i.e., SOD, CAT, GSH and GPx), and inhibit detrimental effects of NLRP3 inflammasome 659 . Melatonin could resolve hyper-inflammatory conditions in COVID-19 and PASC by down-regulating proinflammatory cytokines (i.e., TNF-α, IL-1β, IL-6, and IL-8) and increasing anti-inflammatory cytokines such as IL-10 660 , 661 . The anti-inflammatory activity of melatonin may also involve Sirtuin-1 induction, suppression of NF-κB activation, and stimulation of Nrf2 662 . Furthermore, the anti-apoptotic and cyto-protective effects of melatonin could stabilize mitochondrial membrane and help reset m -Dys 663 .
Based on several human RCTs, melatonin is considered an effective intervention to resolve delirium, ameliorate respiratory stress (i.e., ARDS), and restore circadian balance in COVID and PASC patients 664 , 665 , 666 . Furthermore, as a cyto-protectant, melatonin helps alleviate several COVID-19 comorbidities, including T2DM, metabolic syndromes, and both ischemic as well as non-ischemic CVD 657 . In an open-label RCT ( n = 96), oral administration of melatonin tablets (3 mg/d, 1 h before sleep, for 7 days) with standard treatment, substantially improved sleep quality and blood O 2 saturation parameters in hospitalized COVID-19 patients 667 . In another RCT ( n = 80), prolonged-release melatonin (PRM 2-mg) therapy showed a significant improvement in sleep hours and reduction in delirium episodes among hospitalized insomniac COVID-19 patients 668 . Melatonin activates two of the G-protein-coupled receptors: MT1 that regulates vigilance states of rapid eye movement (REMS), and MT2 that controls non-REMS. In accordance with the circadian rhythm, melatonin release into blood at night could improve sleep quality of insomnia patients through activation of MT1 and MT2 receptors 669 . Finally, melatonin could help alleviate neurological complications such as brain fog, ME/CFS, anxiety, and sleep disorders; resolve ALI/ARDS with related vessel permeability issues. Based on its multi-functionality, high safety profile, melatonin could be considered a promising adjuvant support for nutritional reset of neuro-COVID in PASC patients 171 , 665 , 670 .
Nutritional reset of virus-hijacked serine proteases
In healthy lungs, type II transmembrane serine proteases (i.e., TTSPs: TMPRSS2, CTSL, HAT) play a major role in cellular regeneration, repair, and homeostasis 671 , 672 . Furthermore, anti-proteases (i.e., secretory leukocyte protease inhibitor , SLPI) are important for proteolytic inhibition and host defense 673 . A functional balance between proteases and anti-proteases is vital to ensure respiratory homeostasis. Any imbalance towards increased protease expression and activity may lead to overt inflammation and trigger chronic lung disorders such as COPD and emphysema 674 , 675 . Furthermore, respiratory serine proteases that belong to the TTSP family, increase host susceptibility to SARS-CoV infection-2 55 . Anti-proteases, such as SLPI, inhibit the activity of serine proteases and block viral entry into host target cells 70 . Therefore, the protease/antiprotease balance not only is critical for respiratory homeostasis but also serves as a powerful determinant of SARS-CoV-2 pathogenesis. Nutritional antioxidants could stimulate anti-protease secretion, decrease protease activity and protect epithelial cells against viral infection 676 . Thus, antioxidants serve as regulators of the protease/antiprotease balance that could effectively combat viral infection(s) including COVID-19.
Respiratory epithelium is constantly prone to inhalation insults from excess release of toxic free radicals, ROS/RNS, and peroxides, all cumulatively exert OxS 677 . Nrf2, the innate transcription factor, regulates synthesis and activity of several antioxidant enzymes to help resolve OxS, and prevent tissue damage in lungs 678 . Nrf2 also regulates TMPRSS2, protease/antiprotease balance and protects the respiratory milieu against viral infections 676 , 679 . Thus, bioactive nutrients that activate Nrf2 could reduce persistent OxS as well as help in functional optimization of viral-hijacked serine proteases and reset HMRD in PASC patients 70 .
Flavan-3-ols found in green tea, such as epigallcatechin-3-gallate (EGCG), is shown to induce SLPI secretion, reduce TMPRSS2 secretion, and decrease viral replication 680 . Antioxidant supplementation with flavan-3-ols may serve as a possible nutraceutical therapy to protect against lung disease in the context of a viral infection.
Sulforaphane (SFN), a sulfur-containing isothiocyanate compound naturally found in cruciferous vegetables (i.e., cauliflower, broccoli, brussels sprouts, and cabbage) could enhance Nrf2 activity. SFN could induce cellular antioxidants such as heme oxygenase (HO)-1 and NADPH quinone oxidoreductase 1 (NQO1) activities, thereby inhibit proinflammatory cytokine release 681 . SFN supplementation could also increase SLPI secretion, regulate TMPRSS2 expression and plays an important role in modulation of protease/antiprotease balance 676 , 679 .
Nutritional reset of immune impairment
SARS-CoV-2 infection triggers immune response, a regular host defense strategy to restrain viral entry and constrain disease progression. However, when immune system exhausts and/or compromised, the viral infection become aggressive in vulnerable hosts and evolves into a severe pathophysiological state with hyper-inflammation, extensive tissue damage, and MODS 682 . Such hyper-inflammatory state could lead to loss of appetite, altered intestinal absorption, impaired gut permeability, and malnutrition 683 , 684 . Malnutrition in turn could aggravate inflammatory pathways, compromise the immune system, onset dysbiosis, increase risks of new microbial infection(s) as well as reactivate latent pathogens 685 . Therefore, nutritional resolution of immune dysfunction is an important aspect of recovery from severe inflammation, malnutrition, and sarcopenia during clinical rehabilitation of COVID-19 and PASC.
Vitamin D3 (Vit-D) is a lipid-soluble seco-steroid that exists in two forms as D 2 (ergocalciferol) derived mainly from plant sources and D 3 (cholecalciferol), which is present in higher animals 686 . Both endogenous and exogenous forms of vit-D are inactive and require two successive hydroxylation steps by cytochrome P450 (CYP) enzymes to form fully active vit-D. In humans, vit-D3 is produced by the skin with conversion of 7-dehydro-cholesterol to cholecalciferol via exposure to sunlight 687 . Circulatory vit-D is initially transported to liver by vit-D binding protein 688 . Vit-D is mainly known to regulate calcium/phosphate homeostasis and bone metabolism. However, vit-D is also vital for several biological pathways including modulation of innate and adaptive immune responses 689 . Immuno-modulatory role of vit-D could be categorized into three essential functions: (i) physical barrier, (ii) natural cellular immunity, and (iii) adaptive immunity 690 . Vit-D could activate the release of cathelicidin and defensins to inhibit viral replication; furthermore, could down-regulate release of proinflammatory Th1 cytokines (i.e., TNF-α and IFN-γ) and stimulate macrophages to generate anti-inflammatory cytokines to minimize the risk of ARDS in COVID-19 691 . Vit-D could also inhibit NF-κB activation to down-regulate proinflammatory cytokine synthesis and other key activators of cell-mediated immunity 692 . Vit-D receptor (VDR) is expressed on antigen-presenting cells, T and B lymphocytes that also synthesize active vit-D metabolite. Vit-D can modulate both innate and adaptive immune responses, and vit-D deficiency is linked to increased autoimmunity as well as increased susceptibility to microbial infections. Vit-D deficiency is prevalent among patients with autoimmune disorders 689 . Vit-D is a negative regulator for expression of renin and interacts with the RAAS/ ACE/ACE-2 signaling axis, therefore could affect SARS-CoV-2 infection process as well as host CV/circulatory function 693 .
In COVID-19 patients, vit-D deficiency was reported in 41.7% cases, vit-D insufficiency in 46.0%, and the remaining 12.3% of cases with normal vit-D levels. The odds of severe COVID-19 outcomes increase by 38.1 and 5.6 times for vit-D-deficiency and -insufficiency patients, respectively, for each standard deviation decrease in serum 25(OH)D 694 . Low vit-D levels are associated with elevated inflammatory cytokines with increased risk of pneumonia and viral upper respiratory tract infections. Vit-D deficiency is also associated with an increase in thrombotic episodes, frequently reported in COVID-19 695 . Severe cases of COVID-19 demonstrate 64% more vit-D deficiency than mild cases, while vit-D insufficiency could significantly increase hospitalization and CFR 696 . Vit-D deficiency is a risk factor for unregulated cytokine storm and hyper-inflammation in COVID-19 patients 697 . Also, COVID-19 survivors and PASC patients show lower 25(OH)D levels compared to matched-patients without PASC 698 . Vit-D deficiency refers to serum levels of 25-hydroxyvitamin D , 25(OH)D, <20 ng/mL (50 nmol/L) 699 .
SARS-CoV-2 pneumonitis could rapidly incapacitate the lung and lead to severe ALI/ARDS, including death in some patients. Vit-D deficiency and/or failure to activate the vit-D receptor could trigger a cytotoxic response in stellate cells of the lung and aggravate respiratory complications in COVID-19 and PASC 396 . Vit-D could ameliorate pulmonary inflammation and facilitate repair of epithelial layers, damaged organs with inherent anti-fibrotic properties and help resolve inflammation-induced pathologies, such as fibrosis 690 , 700 , 701 . CVD sequelae such as cardiomyopathy, arrhythmias, thrombotic complications, and cardiogenic shock are prominent features of COVID-19 and PASC pathologies 702 . Vit-D could activate VDR function, regulate calcium flux, reset optimal myocardial contractility, and help reduce risks of myocardial infarction in COVID-19 and PASC patients 703 .
In Spain, a population-based cohort study of 4.6 million people supplemented with cholecalciferol or calcifediol, when achieved serum 25(OH)D levels >30 ng/mL, showed a reduction of about half the risk of SARS-CoV-2 infection, severe COVID-19, or COVID-19 mortality than those not treated 704 . An observational study at the U.S. Veterans Affairs healthcare facilities, patients (n = 4599) when adjusted to 25(OH)D levels from 15 to 60 ng/mL, showed a decrease in probability of COVID-19-related hospitalizations from 24.1 to 18.7%, and mortality rates from 10.4 to 5.7% 704 . There is mounting evidence that optimal 25(OH)D levels are associated with reduced risk of COVID-19.
Reactivation of latent Epstein–Barr virus (EBV) is an emerging risk factor with adverse outcomes for both COVID-19 and PASC 269 , 705 . Vit-D supplementation (20,000 IU/week over 96 weeks) could significantly reduce humoral immune responses against latent EBV antigen in relapsing-remitting multiple sclerosis 706 . Although more evidence is needed on therapeutic benefits of vit-D in COVID-19 and PASC, the multifunctional role of this vitamin on immune system is evident. Vit-D deficiency is cost-effective, safe, and readily available supplement strategy for COVID-19 and PASC management 707 . Therefore, individuals at higher risk of vit-D deficiency during COVID-19 pandemic should consider taking vit-D supplements to reset the circulating 25(OH)D levels to optimum (75–125nmol/L) and avoid and/or recover from COVID-19 and PASC.
Omega-3 Polyunsaturated Fatty Acids (Omega-3 or n-3 PUFAs) , eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA), and docosahexaenoic acid (DHA), are broad-spectrum anti-inflammatory compounds that modulate several pathways of inflammation including leukocyte chemotaxis, adhesion molecule expression, and leukocyte-endothelial adhesive interactions, inflammatory cytokine synthesis and T cell reactivity 708 . As potent antioxidants, omega-3 PUFAs upregulate Nrf2, mitogen-activated protein kinase (MAPK) phosphatases, GSH and HO-1 genes. Metabolites of omega-3 PUFAs are vital for the synthesis of several inflammatory mediators including prostaglandins (PG), leukotrienes (LT), thromboxanes (TX), protectins , and resolvins 709 . Omega-3 PUFAs modulate both innate and acquired immune systems through activation of macrophages, neutrophils, T-cells, B-cells, dendritic cells, NK cells, mast cells, basophils, and eosinophils. As an integral part of the cellular membrane, omega-3 PUFAs regulate membrane fluidity and complex assembly in lipid rafts 710 . PUFAs could help alleviate mitochondrial ROS production in T cells to combat SARS-CoV-2 infection 21 . Virus-induced HMRD of host lipid metabolism may persist as chronic inflammatory condition in PASC patients. Palmitoylethanolamide (PEA), a lipid-derived peroxisome proliferator-activated receptor-α (PPAR-α), is shown to dismantle lipid droplets via β-oxidation and restore innate cellular defenses 711 .
Omega-3 PUFAs may interact at different stages of SARS-CoV-2 infection, particularly during viral entry and replication phases where persistence of viral antigens may lead to sustained inflammatory state in PASC patients 277 , 712 , 713 . Omega-3 PUFAs, particularly EPA, are potential remedials to reduce pro-inflammatory cytokines, alter the HPA axis, modulate neurotransmission via lipid rafts, and alleviate neuro-cognitive complications in PASC patients 714 . Furthermore, omega-3 PUFAs and their metabolites (i.e., specialized pro-resolvin mediators), could effectively ameliorate uncontrolled inflammatory responses, reduce OxS, mitigate coagulopathy, and restore tissue homeostasis 715 . Besides antioxidant and anti-inflammatory activities, omega-3 PUFAs could regulate platelet homeostasis and lower the risk of thrombosis, which indicates its potential use in COVID-19 and PASC management.
COVID-19 patients show significantly low Omega-3 Index (O3I = 4.15%) compared to healthy subjects (O3I = 7.84%). Lower O3I is associated with an increased risk of developing severe COVID-19 with mechanical ventilation and high CFR 716 , 717 . In a retrospective clinical study ( n = 80), oral supplementation with omega-3 PUFAs has significantly lowered proinflammatory procalcitonin and IL-6 levels and reduced prothrombin time in ICU/hospitalized COVID-19 patients 718 . Therefore, the nutritional status of omega-3 PUFAs is particularly important for the overall immune response, tissue inflammation and repair, which may be an effective nutritional strategy for PASC recovery.
Vitamin-C (Vit-C) (ascorbic acid) is a water-soluble, essential nutrient (cannot be synthesized by the human body), important for antioxidant activity and immune-modulation 719 . Vit-C regulates NF-kB release and attenuates pro-inflammatory cytokines production 720 . In a meta-analysis of 7 RCTs and 7 retrospective studies ( n = 751 patients), vit-C supplementation showed a significant alleviation in inflammatory response by increasing ferritin levels and lymphocyte counts in COVID-19 721 . Another meta-analysis of 19 RCTs showed a reduced in-hospital mortality rate in vit-C supplemented COVID-19 patients (24.1%) compared to non-supplemented group (33.9%) 722 . In a retrospective study from UAE ( n = 63), oral supplementation of COVID-19 patients yielded no difference in vit-C levels across BMI categories; however, a significant correlation was noted between vit-C levels and SARS-CoV-2 clearance rate among the obese patient group 723 . Extended fecal viral RNA shedding suggests that SARS-CoV-2 infection in the GI tract could be prolonged in a subset of COVID-19 and PASC patients 724 . The potential viral clearance activity of vit-C supplementation could be a promising adjuvant combo with other ‘reset’ nutritional strategies to treat certain PASC patients.
Nutritional reset of gut dysbiosis
SARS-CoV-2 infection alters gut microflora composition and function, which leads to intestinal barrier dysfunction and immune activation 725 . Epithelial tight junction is a critical intestinal barrier, and its disruption could leak toxic substances from the gut into blood circulation and cause systemic injury. The maintenance of intestinal epithelial tight junctions is closely related to energy homeostasis and mitochondrial function 397 . Such virus-induced dysbiosis triggers cytokine release with NF-κB-mediated hyper-inflammatory response, and the ensuing immune dysregulation could worsen the clinical outcomes of COVID-19 240 . Gut microbiota plays a multi-functional role in the GI tract, including energy extraction from the diet, immune modulation, synthesis of vitamins and short-chain fat acids (SCFAs) 726 . A complex equilibrium exists among prebiotics (i.e., fructo-oligosaccharides), probiotics (i.e., LAB), and postbiotics (i.e., bacteriocins, SCFAs), with the involvement of several networks between gut microflora and other organ systems through different axes (i.e., Gut-Lung, Gut-Liver, Gut-Brain axes) that affect a plethora of pathways in health and disease 244 , 727 .
Gut dysbiosis could persist for at least 6 months in COVID-19 patients after hospital discharge, and this chronic inflammatory condition may onset a wide range of neurological and neuropsychiatric symptoms in PASC patients 244 . Accordingly, several gut metabolic dysfunctions could contribute to long-term neuro-cognitive impairments in PASC, including: (i) perturbed ‘gut-brain’ axis due to loss of SCFA producing intestinal flora, which may cause neuropsychiatric disorders 728 ; (ii) cytokine storm-induced immune-metabolic reprogramming, which could elevate ‘kynurenine:tryptophan’ ratio and trigger chronic depression syndrome 729 ; and iii) ACE2 activation in the gut could alter l -DOPA production and neurotransmitter synthesis, thereby inflict neurological complications including CFS 730 .
Probiotics/lactic acid bacteria (LAB) regulate cytokine secretion and affect both nonspecific as well as specific immune responses. Bacteriocins produced by LAB are antimicrobial compounds known to inhibit adhesion and invasion of microbial pathogens in the GI epithelia 731 . Probiotics could block SARS-CoV-2 proliferation in host cells, via potential immuno-modulation, and inhibit NLRP3 inflammasome activation 732 .
Oral probiotic therapy of hospitalized COVID-19 patients ( n = 70) with Streptococcus thermophilus , L. acidophilus , L. helveticus , L. paracasei , L. plantarum , L. brevis , B. lactis , and B. lactis , experienced remission of diarrhea and other symptoms within 72 h. These probiotics show a significant ameliorating effect and reduce the risk of developing respiratory failure by almost eight times in SARS-CoV-2 infected individuals 733 . Probiotic therapy of COVID-19 patients with a combination of nine different lactobacilli strains (as daily dose), showed significant alterations in gut microbiota, partial restoration of pulmonary dysfunction as well as intestinal dysbiosis. Probiotic therapy also reduced inflammatory markers such as TNF-α, IL-1β, IL-4, and IL-12 734 . Co-administration of L. rhamnosus EH8 with mycelia could potentially inhibit inflammatory cytokine release induced by SARS-CoV-2 membrane (M) glycoprotein 735 . A human RCT with COVID-19 patients ( n = 300) treated with L. plantarum and Pediococcus acidilactici for 30-days, showed total remission of 53.1% in the probiotic-treated compared to 28.1% in the placebo groups, respectively. Probiotic therapy showed significant reduction in pulmonary infiltrates as well as in lowering the nasopharyngeal viral load, shortened the duration of GI and non-GI symptoms, decreased the D-dimer levels, and boosted the synthesis of specific IgM and IgG responses against SARS-CoV-2 736 .
Co-administration of L. plantarum GUANKE (LPG) strain with COVID-vaccine has been suggested to act as an adjuvant, inducing specific and nonspecific immune response(s), thereby extending protection against SARS-CoV-2 737 . A 3-month administration of probiotic strain Loigolactobacillus coryniformis K8 on SARS-CoV-2 (mRNA) vaccine-induced immune responses in elderly population ( n = 200) was evaluated. All participants completed mRNA vaccination, while the intervention started ten days after the first dose. The IgG levels in were significantly higher in the treated group; however, at ages >85, probiotic administration increased IgA antibody levels 738 . Preclinical studies have shown that probiotic therapy confers specific immune responses in inflammatory cytokine expression, prevent cell apoptosis, and induce immunological memory against COVID-19 739 . Thus, re-balancing of healthy gut microbiota through probiotics, prebiotics, and immune nutrients, could help reduce inflammation, promote anti-inflammatory mechanisms, and reset a functional ‘gut-brain’ axis for optimal recovery of COVID-19 and PASC patients.
Nutritional reset of virus-induced metabolic disorders
HMRD in tandem with chronic metabolic syndromes could result in more severe outcomes of COVID-19 and subsequently extends into PASC 740 . A meta-analysis of 120 studies ( n = 125,446 patients) reported that most prevalent comorbidities for SARS-CoV-2 infection include: hypertension (32%), obesity (25%), T2DM (18%), and CVD (16%) 741 . COVID-19 in conjunction with diabetes and obesity (both characterized by severe insulin resistance) has severe clinical consequences 742 , 743 . Therefore, restriction of dietary lipid and sugar intake could potentially benefit COVID-19 and PASC patients with T2DM, obesity or other metabolic syndromes.
Virus-induced metabolic disorder—‘new onset’ diabetes
T2DM is a progressive metabolic disorder due to insulin resistance with underlying chronic inflammation as well as endothelial and β-cell dysfunction 744 . SARS-CoV-2 infection could trigger hyper-inflammation, exacerbate insulin resistance, worsen endothelial dysfunction and lead to new-onset diabetes 745 . COVID-19-induced aberrant glycol-metabolic dysregulation could persist even after COVID-19 recovery. In a cohort of hospitalized COVID-19 patients ( n = 551) about 46% of cases showed long-term hyper-glycemia (who were normo-glycemic prior to infection) 746 . Therefore, not only patients with metabolic and endocrine dysfunction are predisposed to risks of severe COVID-19; but also, SARS-CoV-2 infected normal population could potentially develop ‘new-onset’ diabetes or aggravation of pre-existing metabolic syndromes 747 . The hyperglycemia in non-diabetic COVID‐19 patients could result from impaired pancreatic islet function as well as viral inflammation-induced insulin resistance and abnormal β cell activation 748 , 749 . Oral administration of liposome-embedded SOD (L-SOD) could ameliorate oxidative damage and inflammatory responses via inhibition of myeloperoxidase (MPO) and pro-inflammatory cytokines, as well as protect gut barrier function by promoting the expression of the tight junction proteins occludin and zonula occluden (ZO)-1 in the colon 750 . Furthermore, L-SOD could also reduce OxS to intestinal barrier, thereby ameliorating the vicious circle between hyperglycemia and the oxidative damage 751 . Therefore, targeting the intestinal barrier with dietary bioactive L-SOD could be a promising glucose-lowering approach to reset HMRD-induced new onset diabetes in PASC patients.
Virus-induced metabolic disorder—obesity
Body mass index (BMI) strongly correlates with immune signatures that predict severity of COVID-19 and PASC 752 . Obesity is recognized as a high‐risk factor in COVID‐19 patients, and high-fat diet promotes ACE2 expression on adipocytes 753 . The lipid-rich adipocytes facilitate lipid raft formation on cell membranes to support viral entry, as well as provide building blocks to assemble viral capsules 754 . Viral-mediated adipocyte infection (cellular entry, invasion, and propagation) processes could inflict severe adipose tissue dysfunction and insulin resistance 754 , 755 . Interestingly, SARS‐CoV‐2 has been detected in the adipose tissue of overweight males but not in females. Inhibition of lipase‐mediated breakdown of body fat could effectively block viral propagation in adipocytes 756 . Thus, cellular metabolic state and overall nutrition status are key determinants in the pathobiology of COVID-19 and PASC 21 , 747 .
Nutritional reset of HMRD in complementary and integrative health (CIH) practices
Demographic distribution of SARS-CoV-2 infection and severity of COVID-19 disease spectrum vary around the world. Viral susceptibility and infectious outbreak in a regional population depends on several geo-genomic factors including local dietary habits, public health practices (including traditional/herbal medicines), environmental as well as socio-economic strata, and prevalence of nutrient (vitamin and mineral) deficiencies. Ready access, minimal side effects with low risk of developing drug resistance, makes Complementary and Integrative Health (CIH) practices an ideal adjunct therapeutic strategy to combine with nutrient-based remedial to reset virus-induced HMRD in the global combat of PASC.
Traditional Chinese Medicine (TCM) has played a significant role in combat against COVID-19 and PASC in China. Several herbal formulas have been shown to be efficacious such as Jinhua Qinggan (JHQG) granules, Lianhua Qingwen (LHQW) capsules, Xuanfeibaidu (XFBD) granules, Huashibaidu (HSBD) and Xuebijing (XBJ) 757 . A bedside-to-bench study in Taiwan ( n = 12) reported that a novel TCM formula, Taiwan Chingguan Yihau (NRICM101), could disrupt progression of SARS-CoV-2 infection through antiviral and anti-inflammatory activities, thereby provide both preventive and therapeutic benefits to combat COVID-19 758 . A prospective cohort has reported that TCM could improve pulmonary inflammation and help in early recovery of PASC patients 759 . Bufei Huoxue capsule is shown to resolve hyper-inflammatory response, coagulation abnormalities, and myocardial damage in PASC patients 760 . Acupuncture has been suggested to alleviate many of the clinical symptoms of PASC, including headaches, myalgia, and abdominal pain. A meta-analysis found auricular acupuncture effective in relieving anxiety and depression in COVID-19 patients 761 . Accordingly, stimulation of the Interferon Point (located on tragus/ear helix) is shown to improve innate immune defense and accelerate remission in PASC 762 . A human clinical study in Hubei, China ( n = 84) reported that auricular point pressure involving seed ( Vaccaria segetalis ) placement could relieve insomnia (improve sleep) and reduce situational anxiety in PASC patients 763 . Electro-acupuncture may reduce expression of proinflammatory cytokines and modulate immunity through neuro-regulation 764 . Acupoint stimulation therapy is shown to improve palpitations, dyspnea, cognitive impairment, anxiety, depression, and other symptoms in PASC patients 765 . Nutritional reset of HMRD in combination with TCM could provide a cost-effective remedial strategy to combat PASC, especially in Asia.
Phytotherapy
Several phytochemicals have demonstrated activity against the SARS-CoV-2 through mechanisms such as viral entry inhibition, inhibition of replication enzymes, and virus release blockade 766 . Plant-derived natural non-nucleoside analog inhibitors (NNAIs) effective against SARS-CoV-2 RNA-dependent RNA polymerase complex ( nsp7/nsp8/nsp12 ) were reported 767 . Also, phytochemicals could specifically inhibit in silico, viral protein nsp5 -encoded main protease (M pro ), the autocleavage enzyme critical for COVID-19 pathogenesis 768 . Phytochemicals from medicinal herbs with antiviral activity include hesperidin, apigenin, luteolin, seselin, 6-gingerol, humulene epoxide, quercetin, kaempferol, curcumin, and epigallocatechin-3-gallate (EGCG) have been reported to inhibit multiple molecular targets of SARS-CoV-2 viral replication in silico 769 . For neuro-PASC, the therapeutic potential of 31 phytochemicals (derived from 19 medicinal herbs) to resolve neuro-cognitive impairments such as anxiety, depression , mixed anxiety-depressive (MAD) syndromes, and irreversible dementia has been reported 770 . Polysaccharides, terpenoids, flavonoids, alkaloids, glycosides, and lactones are plant-derived immunomodulators also considered as potential remedials against viral infections 771 .
Ayurvedic Rasayana therapy is traditionally practiced in India for its immunomodulatory and adaptogenic properties, thus used as a therapeutic adjuvant for COVID-19 and PASC recovery. Amongst several others, Withania somnifera (Ashwagandha), Tinospora cordifolia (Guduchi) and Asparagus racemosus (Shatavari) play a major role in Rasayana therapy 772 . Advanced computational technology has provided rapid and cost-effective techniques to screen phytochemicals from AYUSH ( Ayurveda, Yoga, Naturopathy, Unani, Siddha, Sowa-Rigpa , and Homeopathy ) for PASC management. Basti and Rasayana treatments showed potent immunomodulatory effects in regulating pro-inflammatory cytokines, IgG, and T cell function; accordingly, proposed for rejuvenation therapy to combat PASC 773 . Mucormycosis is an opportunistic angio-invasive fungal infection associated with PASC 774 . In a prospective human RCT ( n = 77), Ayurvedic therapy as an adjunct to conventional medical treatments showed significant improvement across the entire spectrum of mucormycosis in PASC patients 775 .
Patients discharged from intensive care pose higher risk of functional loss or undernutrition, even after 6-months post-COVID infection. Malnutrition and loss of muscle strength should be considered in the clinical assessment of these PASC patients 776 . Yoga is a psycho-somatic approach to enhances innate immunity and mental health, so it can be used as complementary therapy with nutritional reset of PASC 777 .
Chiropractic could provide an adjuvant modality to complement the nutritional reset of virus-induced HMRD in PASC. Skeleto-muscular complaints, fatigue, insomnia, and cognitive impairments are prominent clinical manifestations of PASC, which are also common features in fibromyalgia , a disorder of the autonomic nervous system (ANS) 778 . Chiropractic spinal manipulation therapy (SMT) could regulate ANS at peripheral level and reach the CNS. The vagal parasympathetic stimulation by SMT, could then release neurotrophins ( brain-derived neurotrophic factor , BNDF and nerve growth factor , NGF) to help resolve depression and related neuro-cognitive impairments 779 . Other multi-modal chiropractic treatments such as massage and intermittent motorized cervical traction could relieve soft-tissues, inter-vertebral joints, and stretch the core musculatures to facilitate rehabilitation of FM patients 780 . Endogenous paired associative stimulation (ePAS), a neuro-modulatory intervention, could increase muscle power and resolve total neuro-muscular fatigue 781 . Furthermore, chiropractic SMT could also resolve migraine and cervicogenic headaches, which are prevalent among PASC patients. A 17-month RCT in Norwegian patients ( n = 104) showed a significant improvement in migraine duration and headache index with SMT compared to control group 782 . Also, chiropractic modalities with SMT, soft tissue therapy (STT), stretching and mobilizations may also provide safe adjunct treatment for treatment of GI disorders 783 , in combination with nutrient-based reset of HMD/R in PASC. A combination of chiropractic modalities with nutritional reset strategies needs an in-depth evaluation, especially in resolving neuro-cognitive, skeleto-muscular, and GI impairments in PASC patients.
Conclusions/future directives
This narrative review elaborates critical steps involved in the emergence, gradual progression, and chronic manifestation of PASC or long-COVID, the ongoing virus-induced global ‘new-onset’ human metabolic syndrome. The pathophysiology of PASC is described right from the initiation of the ‘novel’ SARS-CoV-2 infection by primordial hijacking of host cellular metabolic machinery, subsequent progression of virus-induced human metabolic reprogramming/dysregulation (HMRD) in a susceptible host, subjecting the patient through a crucial tri-phasic symptomatic clinical onset of COVID-19 (lasting from 3–4 weeks), and ultimate transition of a survivor into PASC or long-COVID, a virus-free state, lingering with earlier and/or new onset disease manifestations resulting from pre-acquired HMRD (lasting for weeks to months).
Besides hijacking of specific host cellular factors (i.e., ACE2, NRP1, furin, TMPRSS2, CTSL), this ‘novel’ RNA (29.9-kb) virus encodes 14 ORFs that could also partake in >4,780 unique high-confidence virus–host protein–protein interactions in the human body. Such extensive viral ORF/protein interactions with host-specific cellular targets could trigger severe HMRD, a rewiring of sugar-, AA-, FA-, and nucleotide-metabolism(s); as well as hypoxia (’Warburg’ effect) with m -Dys (altered ATP synthesis), immune impairment, and FeRD in an infected individual. Accordingly, the SARS‐CoV‐2 genome and its products potentially modulate HMRD at transcription, translation, and post-translational modification (PTM) levels of human metabolism. A plethora of PASC clinical symptoms and related metabolic impairments indicate an involvement of various pathophysiological mechanisms originating from virus-induced HMRD. Thus, PASC is not a simple disease, but a complex disorder of multi-organ systems resulting from virus-induced HMRD; henceforth, should be categorized as a ‘new onset’ human metabolic syndrome.
The virus-free, dysfunctional metabolic state of PASC could manifest with >200 different and overlapping clinical symptoms involving multiple organ/systems. Such dysfunctional metabolic sequelae are cumulative outcomes of virus-induced HMRD involving about 10 divergent pathophysiological mechanisms, comprising of both virus-derived virulence factors as well as a multitude of extreme innate host responses. Each of these underlying etiologies amplified by HMRD would require specific system-targeted remedial(s) to achieve healthy recovery of PASC patients. Therefore, precision nutrition protocols to resolve systemic impairments and ‘reset’ the virus-induced HMRD is the most relevant and effective strategy to combat PASC. An ideal nutritional remedy should demonstrate human RCT proven health benefits with optimal ADME ( Administration, Distribution, Metabolism, Excretion ) profile and deliver functional advantage to reset HMRD and help total recovery of PASC patients. The term ‘RESET’ refers to nutritional or dietary-based remedials or interventions that help resolve dysfunctional cellular pathways from virus-induced HMRD and recalibrate host metabolism to optimal function and homeostasis . We have described a few evidence-based, human RCT tested, bioactive nutritional interventions for resetting of virus-induced HMRD in PASC through precision nutrition ( refer Table 1 ).
FeRD, the cellular iron redox imbalance triggered by HMRD plays a detrimental role during the cytokine storm in COVID-19 and its clinical aftermath 27 , 48 , 49 . Furthermore, hyperferritinemia with oxidized iron levels modulate several pathways of coagulation cascade and cause severe thromboembolism in PASC 99 . Innate Fe-Redox regulators such as lactoferrin (LF), heme oxygenase (HO)-1, erythropoietin (EPO), and hepcidin (HEP) serve as front-line innate barriers against free radical (ROS/RNS) damage and hyper-immune responses during COVID-19 and PASC 27 . Nutrient remedials to reset FeRD with ferroptosis inhibitors (i.e., ferrostatin-1, vit-E) could be effective in post-recovery strategy for PASC 94 , 441 . Virus-induced HMRD in tandem with hypoxia and m- Dys affects several cellular metabolic pathways in COVID-19 survivors (after viral clearance), which could ultimately evoke severe PASC with metabolic impairments including new onset T2DM, cardiovascular disease, chronic fatigue syndrome, brain fog, and blood clotting issues 21 , 143 . l -tryptophan is an essential AA, vital for biosynthesis of serotonin (5-HT), melatonin and co-factor NAD + through its downstream metabolic pathways 425 . l -tryptophan metabolism is the most prominent pathway that undergoes HMRD during SARS-CoV-2 infection. Nutrient availability is the major regulator of life and reproduction, and a complex cell signaling network monitors the cellular energy metabolism, especially the mitochondrial ATP synthesis and NAD + /NADH ratio, are major sensors of metabolic state. Re-activation of TCA cycle, mitochondrial metabolism, OXPHOS, and ATP synthesis could reverse and reset virus-induced HMRD. Nutritional revitalization of m -Dys should include specific bioactives and cofactors (i.e., l -tryptophan, NAD + , CoQ10, α-lipoic acid, creatine, vit-B12), which are essential for mitochondrial-ETC and provide potential benefits to resolve hyper-inflammation, exercise intolerance, CFS and reset HMRD in PASC 541 , 542 .
Administration of antioxidant enzymes could be considered an effective nutritional strategy to resolve OxS and reset Fe-RH in COVID-19 and PASC. Since m -Dys and OxS jointly contribute to both COVID-19 and PASC pathology, nutritional reset of virus-induced HMRD with NAC, glutamine, GSH, and antioxidant enzymes (i.e., SOD, catalase) could potentially resolve OxS and persistent pulmonary fibrotic sequelae in PASC patients 582 . Bioactive nutrients such as flavan-3-ols that activate Nrf2 could also reduce chronic OxS, help restore activity of viral-hijacked serine proteases and reset HMRD in PASC patients. The SARS-CoV-2 infection could reach the brainstem and inflict cerebral lesions as long-term sequelae with several neuro-cognitive dysfunctions in PASC patients. Nutritional reset of HMRD with l- tryptophan and 5-HT could help resolve neuro-psychiatric disorders (resulting from viral hijack of NRP1), is of high priority in clinical management of neuro-PASC. Melatonin could help alleviate neurological complications such as brain fog, ME/CFS, anxiety, and sleep disorders; resolve ALI/ARDS with related vessel permeability issues of neuro-COVID. Considering a high safety profile, melatonin could provide a promising adjuvant support for nutritional reset of HMRD-inflicted neuro-COVID in PASC patients 171 , 665 , 670 . The viral hijack of human ACE2 receptor disrupts RAAS activation, upregulates NF-κB pathway and the ensuing cytokine storm, hypertension, cell proliferation, inflammation, and fibrosis elicits detrimental effects on every bodily organ, the CV system, in particular 635 . Nutritional supplementation with l -carnitine could mitigate these pathophysiological processes by inhibiting NF-κB and down-regulating NOX1/NOX2, thereby enhancing the antioxidant effects of angiotensin II 636 . Also, the vit-D supplementation could activate VDR function, regulate Ca 2+ flux, reset cardiac muscle contractility, and help reduce risks of myocardial infarction in COVID-19 and PASC patients. While the omega-3 PUFAs could regulate platelet homeostasis and lower the risk of thrombosis, oral supplementation of l -arginine is a promising nutritional remedy to reset HMRD-associated CV disorders and immune dysfunction in PASC patients. In PASC, the HMRD-amplified immune disruption could cause tissue damage and aggravate GI disorders such as loss of appetite, leaky gut with severe malnutrition. Such immune exhaustion could onset dysbiosis, increase risk of new microbial infection(s) and reactivate latent viral pathogens with new onset of ME/CFS in PASC patients. Gut dysbiosis may persist for months in COVID-19 patients after hospital discharge, and the virus-induced HMRD could also trigger neuro-cognitive symptoms in PASC patients. Re-balancing the GI tract with effective probiotics, prebiotics, and immune nutrients, could help resolve inflammation, promote anti-inflammatory activity, and reset a functional ‘gut-brain’ axis for recovery of PASC patients.
Epilogue—the final note
Life is a genetically programmed, intricately organized, chemical processing thermo-dynamic system, that self-sustains by breaking chemical bonds (catabolism) to release and capture free energy to form complex macro-molecules (anabolism) for specific structure-function (metabolism) in a lipid-based envelope, known as the cell. Viruses infect almost every species and are probably the most abundant biological entities on the planet Earth. Recently emerged coronavirus, the SARS-CoV-2, is a 29.9-kb RNA virus that possess a unique genomic ability to reprogram a mega-sized 3.1-Mb human DNA and its cellular metabolic machinery to prime, alter, and redirect host macro-molecules for its infection, replication, and propagation. The 14 viral ORFs interact with a few thousands of human metabolites in a specific manner for its intra-cellular invasion, replication, and transmission, which results in HMRD in favor of the viral pathogen. Genomic and meta-genomic data have revealed that co-evolution between viral and cellular genomes involves frequent horizontal gene transfer and the occasional co-option of novel functions over evolutionary time 784 , the virus-induced HMRD with PASC, is one such prime example. Therefore, the nutraceutical/food supplement industry should take a serious note that formulating a RESET composition is not a simple blend of food compounds or nutritional ingredients into a dosage form. A deeper meta-genomic insight to assure 3-D structure/function of nutrient molecules, priming/activation of nutrients with co-factors, compositional stoichiometry, optimal milieu (pH, redox, and ionic strengths), molecular interplay between bioactives (synergism/antagonism), target delivery, ADME/Safety/Toxicity profiles, and most importantly the bio-functional activity, are vital prerequisites in the development of nutrient-based remedials to RESET the virus-induced HMRD in long-COVID, the ‘new onset’ global metabolic syndrome. This precision nutrition-based dietary rehabilitation of PASC patients should be developed as an affordable, interventional regimen for divergent socio-economic populations worldwide for effective public health management of any future emergence of virus-induced ‘new onset’ human metabolic syndromes.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Lu, R. et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 395 , 565–574 (2020).
Article CAS PubMed PubMed Central Google Scholar
Rajan, S. et al. In the wake of the pandemic: Preparing for long covid [internet]. National Center for Biotechnology Information Available at: https://pubmed.ncbi.nlm.nih.gov/33877759/ (Accessed: 27th March 2024).
Coronavirus cases: Worldometer Available at: https://www.worldometers.info/coronavirus/ (Accessed: 27th March 2024).
Hallek, M. et al. Post-COVID syndrome. Dtsch. Arztebl. Int. 120 , 48–55 (2023).
PubMed PubMed Central Google Scholar
Davis, H. E. et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 38 , 101019 (2021).
Article PubMed PubMed Central Google Scholar
Xie, Y., Xu, E., Bowe, B. & Al-Aly, Z. Long-term cardiovascular outcomes of COVID-19. Nat. Med. 28 , 583–590 (2022).
Bornstein, S. R. et al. Long-COVID, metabolic and endocrine disease. Horm. Metab. Res. 54 , 562–566 (2022).
Xie, Y. & Al-Aly, Z. Risks and burdens of incident diabetes in long COVID: a cohort study. Lancet Diabetes Endocrinol. 10 , 311–321 (2022).
Kedor, C. et al. A prospective observational study of post-COVID-19 chronic fatigue syndrome following the first pandemic wave in Germany and biomarkers associated with symptom severity. Nat. Commun. 13 , 5104 (2022).
Larsen, N. W. et al. Characterization of autonomic symptom burden in long COVID: A global survey of 2,314 adults. Front. Neurol. 13 , 1012668 (2022).
Kim, S. H. et al. New-onset diabetes after COVID-19. J. Clin. Endocrinol. Metab. 108 , e1164–e1174 (2023).
Article PubMed Google Scholar
Lopez, C., Kim, J., Pandey, A., Huang, T. & DeLoughery, T. G. Simultaneous onset of COVID-19 and autoimmune haemolytic anaemia. Br. J. Haematol. 190 , 31–32 (2020).
Demko, Z. et al. Post-acute sequelae of SARS-COV-2 (PASC) impact quality of life at 6, 12 and 18 months post-infection. https://doi.org/10.1101/2022.08.08.22278543 (2022).
Liu, X. et al. SARS-CoV-2-host proteome interactions for antiviral drug discovery. Mol. Syst. Biol. 17 , e10396 (2021).
Zhang, Y. et al. In vivo structure and dynamics of the SARS-CoV-2 RNA genome. Nat. Commun. 12 , 5695 (2021).
Warburton, P. E. & Sebra, R. P. Long-read DNA sequencing: recent advances and remaining challenges. Annu. Rev. Genom. Hum. Genet 24 , 109–132 (2023).
Article CAS Google Scholar
Banerjee, A. K. et al. SARS-CoV-2 disrupts splicing, translation, and protein trafficking to suppress host defenses. Cell 183 , 1325–1339.e21 (2020).
Li, J. et al. Virus-host interactome and proteomic survey reveal potential virulence factors influencing SARS-CoV-2 pathogenesis. Med 2 , 99–112.e7 (2021).
Article CAS PubMed Google Scholar
Yang, S. L. et al. Comprehensive mapping of SARS-CoV-2 interactions in vivo reveals functional virus-host interactions. Nat. Commun. 12 , 5113 (2021).
Chen, Z. et al. Interactomes of SARS-CoV-2 and human coronaviruses reveal host factors potentially affecting pathogenesis. EMBO J. 40 , e107776 (2021).
Wang, T. et al. COVID-19 metabolism: mechanisms and therapeutic targets. MedComm 3 , e157 (2022).
Naidu, A. S. et al. SARS-COV-2-induced host metabolic reprogram (HMR): Nutritional Interventions for Global Management of COVID-19 and post-acute sequelae of covid-19 (PASC). J. Food Bioactives 18 , (2022).
Gordon, D. E. et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature 583 , 459–468 (2020).
Gussow, A. B. et al. Genomic determinants of pathogenicity in SARS-CoV-2 and other human coronaviruses. Proc. Natl Acad. Sci. USA 117 , 15193–15199 (2020).
Li, J., Lai, S., Gao, G. F. & Shi, W. The emergence, genomic diversity and global spread of SARS-CoV-2. Nature 600 , 408–418 (2021).
Sicari, D., Chatziioannou, A., Koutsandreas, T., Sitia, R. & Chevet, E. Role of the early secretory pathway in SARS-CoV-2 infection. J. Cell Biol. 219 , e202006005 (2020).
Naidu, S. A. G., Clemens, R. A. & Naidu, A. S. SARS-CoV-2 infection dysregulates host iron (Fe)-redox homeostasis (Fe-R-H): role of Fe-redox regulators, ferroptosis inhibitors, anticoagulants, and iron-chelators in COVID-19 control. J. Diet. Suppl. 20 , 312–371 (2023).
Turner, S. et al. Long COVID: pathophysiological factors and abnormalities of coagulation. Trends Endocrinol. Metab. 34 , 321–344 (2023).
Bai, C., Zhong, Q. & Gao, G. F. Overview of SARS-CoV-2 genome-encoded proteins. Sci. China Life Sci. 65 , 280–294 (2022).
Jin, Y. et al. Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses 12 , 372 (2020).
O’Donoghue, S. I. et al. SARS-CoV-2 structural coverage map reveals viral protein assembly, mimicry, and hijacking mechanisms. Mol. Syst. Biol. 17 , e10079 (2021).
Lee, S. et al. The SARS-CoV-2 RNA interactome. Mol. Cell 81 , 2838–2850.e6 (2021).
Simeoni, M., Cavinato, T., Rodriguez, D. & Gatfield, D. I(nsp1)ecting SARS-CoV-2-ribosome interactions. Commun. Biol. 4 , 715 (2021).
Zhou, Y. et al. A comprehensive SARS-CoV-2-human protein-protein interactome reveals COVID-19 pathobiology and potential host therapeutic targets. Nat. Biotechnol. 41 , 128–139 (2023).
Kee, J. et al. SARS-CoV-2 disrupts host epigenetic regulation via histone mimicry. Nature 610 , 381–388 (2022).
Wang, B. et al. Allosteric activation of SARS-CoV-2 RNA-dependent RNA polymerase by remdesivir triphosphate and other phosphorylated nucleotides. mBio 12 , e0142321 (2021).
Zhang, Y. et al. SARS-CoV-2 hijacks folate and one-carbon metabolism for viral replication. Nat. Commun. 12 , 1676 (2021).
Ripoli, M. et al. Hepatitis C virus-linked mitochondrial dysfunction promotes hypoxia-inducible factor 1 alpha-mediated glycolytic adaptation. J. Virol. 84 , 647–660 (2010).
Bera, S. C. et al. The nucleotide addition cycle of the SARS-CoV-2 polymerase. Cell Rep. 36 , 109650 (2021).
Dias, S. S. G. et al. Lipid droplets fuel SARS-CoV-2 replication and production of inflammatory mediators. PLoS Pathog. 16 , e1009127 (2020).
Casari, I., Manfredi, M., Metharom, P. & Falasca, M. Dissecting lipid metabolism alterations in SARS-CoV-2. Prog. Lipid Res. 82 , 101092 (2021).
Santos-Beneit, F., Raškevičius, V., Skeberdis, V. A. & Bordel, S. A metabolic modeling approach reveals promising therapeutic targets and antiviral drugs to combat COVID-19. Sci. Rep. 11 , 11982 (2021).
Nardacci, R. et al. Evidences for lipid involvement in SARS-CoV-2 cytopathogenesis. Cell Death Dis. 12 , 263 (2021).
Ebrahimi, K. H. & McCullagh, J. S. O. A lipidomic view of SARS-CoV-2. Biosci. Rep. 41 , BSR20210953 (2021).
Hooper, P. L. COVID-19 and heme oxygenase: novel insight into the disease and potential therapies. Cell Stress Chaperones 25 , 707–710 (2020).
Singh, D., Wasan, H. & Reeta, K. H. Heme oxygenase-1 modulation: a potential therapeutic target for COVID-19 and associated complications. Free Radic. Biol. Med. 161 , 263–271 (2020).
Espinoza, J. A., González, P. A. & Kalergis, A. M. Modulation of antiviral immunity by heme oxygenase-1. Am. J. Pathol. 187 , 487–493 (2017).
Paul, B. D., Lemle, M. D., Komaroff, A. L. & Snyder, S. H. Redox imbalance links COVID-19 and myalgic encephalomyelitis/chronic fatigue syndrome. Proc. Natl Acad. Sci. USA 118 , e2024358118 (2021).
Kaundal, R. K., Kalvala, A. K. & Kumar, A. Neurological implications of COVID-19: role of redox imbalance and mitochondrial dysfunction. Mol. Neurobiol. 58 , 4575–4587 (2021).
Wang, Y.-P. et al. Regulation of G6PD acetylation by SIRT2 and KAT9 modulates NADPH homeostasis and cell survival during oxidative stress. EMBO J. 33 , 1304–1320 (2014).
CAS PubMed PubMed Central Google Scholar
Thomas, T. et al. Evidence of structural protein damage and membrane lipid remodeling in red blood cells from COVID-19 patients. J. Proteome Res. 19 , 4455–4469 (2020).
Wong, H. T., Cheung, V. & Salamango, D. J. Decoupling SARS-CoV-2 ORF6 localization and interferon antagonism. J. Cell Sci. 135 , jcs259666 (2022).
Zhang, S., Wang, J., Wang, L., Aliyari, S. & Cheng, G. SARS-CoV-2 virus NSP14 Impairs NRF2/HMOX1 activation by targeting Sirtuin 1. Cell Mol. Immunol. 19 , 872–882 (2022).
Shang, J. et al. Cell entry mechanisms of SARS-CoV-2. Proc. Natl Acad. Sci. USA 117 , 11727–11734 (2020).
Hoffmann, M., Kleine-Weber, H. & Pöhlmann, S. A multibasic cleavage site in the spike protein of SARS-CoV-2 is essential for infection of human lung cells. Mol. Cell 78 , 779–784.e5 (2020).
Evans, J. P. & Liu, S.-L. Role of host factors in SARS-CoV-2 entry. J. Biol. Chem. 297 , 100847 (2021).
Xia, S. et al. Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res. 30 , 343–355 (2020).
Peng, R., Wu, L.-A., Wang, Q., Qi, J. & Gao, G. F. Cell entry by SARS-CoV-2. Trends Biochem Sci. 46 , 848–860 (2021).
Szabo, R. & Bugge, T. H. Membrane-anchored serine proteases in vertebrate cell and developmental biology. Annu. Rev. Cell Dev. Biol. 27 , 213–235 (2011).
Ziegler, C. G. K. et al. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell 181 , 1016–1035.e19 (2020).
Gadanec, L. K. et al. Can SARS-CoV-2 virus use multiple receptors to enter host cells? Int J. Mol. Sci. 22 , 992 (2021).
Daly, J. L. et al. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science 370 , 861–865 (2020).
Cantuti-Castelvetri, L. et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science 370 , 856–860 (2020).
Li, Z.-L. & Buck, M. Neuropilin-1 assists SARS-CoV-2 infection by stimulating the separation of Spike protein S1 and S2. Biophys. J. 120 , 2828–2837 (2021).
Peacock, T. P. et al. The furin cleavage site in the SARS-CoV-2 spike protein is required for transmission in ferrets. Nat. Microbiol 6 , 899–909 (2021).
Zhang, L. et al. Furin cleavage of the SARS-CoV-2 spike is modulated by O-glycosylation. Proc. Natl Acad. Sci. USA 118 , e2109905118 (2021).
Whittaker, G. R., Daniel, S. & Millet, J. K. Coronavirus entry: how we arrived at SARS-CoV-2. Curr. Opin. Virol. 47 , 113–120 (2021).
Ragia, G. & Manolopoulos, V. G. Inhibition of SARS-CoV-2 entry through the ACE2/TMPRSS2 pathway: a promising approach for uncovering early COVID-19 drug therapies. Eur. J. Clin. Pharm. 76 , 1623–1630 (2020).
Benton, D. J. et al. Receptor binding and priming of the spike protein of SARS-CoV-2 for membrane fusion. Nature 588 , 327–330 (2020).
Rahbar Saadat, Y., Hosseiniyan Khatibi, S. M., Zununi Vahed, S. & Ardalan, M. Host serine proteases: a potential targeted therapy for COVID-19 and influenza. Front Mol. Biosci. 8 , 725528 (2021).
Walls, A. C. et al. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 181 , 281–292.e6 (2020).
Andersen, K. G., Rambaut, A., Lipkin, W. I., Holmes, E. C. & Garry, R. F. The proximal origin of SARS-CoV-2. Nat. Med. 26 , 450–452 (2020).
Essalmani, R. et al. Distinctive roles of furin and TMPRSS2 in SARS-CoV-2 infectivity. J. Virol. 96 , e0012822 (2022).
Jackson, C. B., Farzan, M., Chen, B. & Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 23 , 3–20 (2022).
Vankadari, N. Structure of furin protease binding to SARS-CoV-2 spike glycoprotein and implications for potential targets and virulence. J. Phys. Chem. Lett. 11 , 6655–6663 (2020).
Wrapp, D. et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 367 , 1260–1263 (2020).
Limburg, H. et al. TMPRSS2 is the major activating protease of influenza a virus in primary human airway cells and influenza B virus in human type II pneumocytes. J. Virol. 93 , e00649–19 (2019).
Zhao, M.-M. et al. Cathepsin L plays a key role in SARS-CoV-2 infection in humans and humanized mice and is a promising target for new drug development. Signal Transduct. Target Ther. 6 , 134 (2021).
van Doremalen, N. et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 382 , 1564–1567 (2020).
Sender, R. et al. The total number and mass of SARS-CoV-2 virions. Proc. Natl Acad. Sci. USA 118 , e2024815118 (2021).
Folgueira, M. D., Luczkowiak, J., Lasala, F., Pérez-Rivilla, A. & Delgado, R. Prolonged SARS-CoV-2 cell culture replication in respiratory samples from patients with severe COVID-19. Clin. Microbiol. Infect. 27 , 886–891 (2021).
Sun, J. et al. The kinetics of viral load and antibodies to SARS-CoV-2. Clin. Microbiol. Infect. 26 , 1690.e1–1690.e4 (2020).
Wong, D. W. L. et al. Multisystemic cellular tropism of SARS-CoV-2 in autopsies of COVID-19 patients. Cells 10 , 1900 (2021).
Caceres, P. S. et al. High SARS-CoV-2 viral load in urine sediment correlates with acute kidney injury and poor COVID-19 outcome. J. Am. Soc. Nephrol. 32 , 2517–2528 (2021).
Ni, W. et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit. Care 24 , 422 (2020).
Obach, M. et al. 6-Phosphofructo-2-kinase (pfkfb3) gene promoter contains hypoxia-inducible factor-1 binding sites necessary for transactivation in response to hypoxia. J. Biol. Chem. 279 , 53562–53570 (2004).
Palsson-McDermott, E. M. & O’Neill, L. A. J. The Warburg effect then and now: from cancer to inflammatory diseases. Bioessays 35 , 965–973 (2013).
Ferraro, E., Germanò, M., Mollace, R., Mollace, V. & Malara, N. HIF-1, the Warburg effect, and macrophage/microglia polarization potential role in COVID-19 pathogenesis. Oxid. Med. Cell Longev. 2021 , 8841911 (2021).
Ryan, D. G. & O’Neill, L. A. J. Krebs cycle reborn in macrophage immunometabolism. Annu. Rev. Immunol. 38 , 289–313 (2020).
Mehrzadi, S., Karimi, M. Y., Fatemi, A., Reiter, R. J. & Hosseinzadeh, A. SARS-CoV-2 and other coronaviruses negatively influence mitochondrial quality control: beneficial effects of melatonin. Pharm. Ther. 224 , 107825 (2021).
Jahani, M., Dokaneheifard, S. & Mansouri, K. Hypoxia: A key feature of COVID-19 launching activation of HIF-1 and cytokine storm. J. Inflamm. (Lond.) 17 , 33 (2020).
Marchetti, M. COVID-19-driven endothelial damage: complement, HIF-1, and ABL2 are potential pathways of damage and targets for cure. Ann. Hematol. 99 , 1701–1707 (2020).
Debuc, B. & Smadja, D. M. Is COVID-19 a new hematologic disease? Stem Cell Rev. Rep. 17 , 4–8 (2021).
Edeas, M., Saleh, J. & Peyssonnaux, C. Iron: Innocent bystander or vicious culprit in COVID-19 pathogenesis? Int. J. Infect. Dis. 97 , 303–305 (2020).
Le Lan, C. et al. Redox active plasma iron in C282Y/C282Y hemochromatosis. Blood 105 , 4527–4531 (2005).
Bellmann-Weiler, R. et al. Prevalence and predictive value of anemia and dysregulated iron homeostasis in patients with COVID-19 Infection. J. Clin. Med 9 , 2429 (2020).
Ye, Q., Wang, B. & Mao, J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J. Infect. 80 , 607–613 (2020).
Muhoberac, B. B. What can cellular redox, iron, and reactive oxygen species suggest about the mechanisms and potential therapy of COVID-19? Front. Cell Infect. Microbiol. 10 , 569709 (2020).
Tang, N. et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 18 , 1094–1099 (2020).
Jankun, J., Landeta, P., Pretorius, E., Skrzypczak-Jankun, E. & Lipinski, B. Unusual clotting dynamics of plasma supplemented with iron(III). Int J. Mol. Med. 33 , 367–372 (2014).
Zhou, F. et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395 , 1054–1062 (2020).
Varga, Z. et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet 395 , 1417–1418 (2020).
Sun, D.-W. et al. The underlying changes and predicting role of peripheral blood inflammatory cells in severe COVID-19 patients: a sentinel? Clin. Chim. Acta 508 , 122–129 (2020).
Bergamaschi, G. et al. Anemia in patients with Covid-19: pathogenesis and clinical significance. Clin. Exp. Med. 21 , 239–246 (2021).
Wagener, F. A. D. T. G., Pickkers, P., Peterson, S. J., Immenschuh, S. & Abraham, N. G. Targeting the heme-heme oxygenase system to prevent severe complications following COVID-19 infections. Antioxid. (Basel) 9 , 540 (2020).
Cheng, C. et al. The incubation period of COVID-19: a global meta-analysis of 53 studies and a Chinese observation study of 11 545 patients. Infect. Dis. Poverty 10 , 119 (2021).
Lauer, S. A. et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann. Intern. Med . 172 , 577–582 (2020).
Wu, Y. et al. Incubation period of COVID-19 caused by unique SARS-CoV-2 strains: a systematic review and meta-analysis. JAMA Netw. Open 5 , e2228008 (2022).
Samrah, S. M. et al. Viral clearance course of COVID-19 outbreaks. J. Multidiscip. Health 14 , 555–565 (2021).
Article Google Scholar
Hirai, N. et al. Factors associated with viral clearance periods from patients with COVID-19: a retrospective observational cohort study. J. Infect. Chemother. 27 , 864–868 (2021).
Hu, B., Guo, H., Zhou, P. & Shi, Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 19 , 141–154 (2021).
Yang, M. & Lai, C. L. SARS-CoV-2 infection: can ferroptosis be a potential treatment target for multiple organ involvement? Cell Death Discov. 6 , 130 (2020).
Cheung, K. S. et al. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from a hong kong cohort: systematic review and meta-analysis. Gastroenterology 159 , 81–95 (2020).
Mao, L. et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 77 , 683–690 (2020).
Morris, A. Effects of pancreatic SARS-CoV-2 infection identified. Nat. Rev. Endocrinol. 17 , 192 (2021).
di Filippo, L., Doga, M., Frara, S. & Giustina, A. Hypocalcemia in COVID-19: prevalence, clinical significance and therapeutic implications. Rev. Endocr. Metab. Disord. 23 , 299–308 (2022).
Deodatus, J. A. et al. Lower plasma calcium associated with COVID-19, but not with disease severity: a two-centre retrospective cohort study. Infect. Dis. (Lond.) 54 , 90–98 (2022).
Alayash, A. I. The impact of COVID-19 infection on oxygen homeostasis: a molecular perspective. Front. Physiol. 12 , 711976 (2021).
Zhang, P. et al. Ectopic expression of SARS-CoV-2 S and ORF-9B proteins alters metabolic profiles and impairs contractile function in cardiomyocytes. Front. Cell Dev. Biol. 11 , 1110271 (2023).
Hugon, J. Long COVID: does SARS-CoV-2 induce lingering brain lesions? Eur. J. Neurol. 30 , 1165–1166 (2023).
Zaim, S., Chong, J. H., Sankaranarayanan, V. & Harky, A. COVID-19 and multiorgan response. Curr. Probl. Cardiol. 45 , 100618 (2020).
Yelin, D. et al. Long-term consequences of COVID-19: research needs. Lancet Infect. Dis. 20 , 1115–1117 (2020).
Zhang, H. et al. Data-driven identification of post-acute SARS-CoV-2 infection subphenotypes. Nat. Med. 29 , 226–235 (2023).
Chen, C. et al. Global prevalence of post-coronavirus disease 2019 (COVID-19) condition or long COVID: a meta-analysis and systematic review. J. Infect. Dis. 226 , 1593–1607 (2022).
Al-Aly, Z., Bowe, B. & Xie, Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat. Med. 28 , 1461–1467 (2022).
Ayoubkhani, D. et al. Risk of long COVID in people infected with severe acute respiratory syndrome coronavirus 2 after 2 doses of a coronavirus disease 2019 vaccine: community-based, matched cohort study. Open Forum Infect. Dis. 9 , ofac464 (2022).
Nalbandian, A., Desai, A. D. & Wan, E. Y. Post-COVID-19 condition. Annu. Rev. Med. 74 , 55–64 (2023).
Bowe, B., Xie, Y. & Al-Aly, Z. Postacute sequelae of COVID-19 at 2 years. Nat. Med. 29 , 2347–2357 (2023).
Thaweethai, T. et al. Development of a definition of postacute sequelae of SARS-CoV-2 infection. JAMA 329 , 1934 (2023).
Sudre, C. H. et al. Attributes and predictors of long COVID. Nat. Med. 27 , 626–631 (2021).
Yong, S. J. Long COVID or post-COVID-19 syndrome: putative pathophysiology, risk factors, and treatments. Infect. Dis. (Lond.) 53 , 737–754 (2021).
Fernandez, M. et al. Spinal manipulation for the management of cervicogenic headache: a systematic review and meta-analysis. Eur. J. Pain. 24 , 1687–1702 (2020).
Garrigues, E. et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J. Infect. 81 , e4–e6 (2020).
Stavem, K., Ghanima, W., Olsen, M. K., Gilboe, H. M. & Einvik, G. Persistent symptoms 1.5-6 months after COVID-19 in non-hospitalised subjects: a population-based cohort study. Thorax 76 , 405–407 (2021).
Lai, C.-C. et al. Long COVID: An inevitable sequela of SARS-CoV-2 infection. J. Microbiol. Immunol. Infect. 56 , 1–9 (2023).
Mizrahi, B. et al. Long covid outcomes at one year after mild SARS-CoV-2 infection: nationwide cohort study. BMJ 380 , e072529 (2023).
Koc, H. C., Xiao, J., Liu, W., Li, Y. & Chen, G. Long COVID and its management. Int J. Biol. Sci. 18 , 4768–4780 (2022).
Crook, H., Raza, S., Nowell, J., Young, M. & Edison, P. Long covid-mechanisms, risk factors, and management. BMJ 374 , n1648 (2021).
Moolamalla, S. T. R., Balasubramanian, R., Chauhan, R., Priyakumar, U. D. & Vinod, P. K. Host metabolic reprogramming in response to SARS-CoV-2 infection: a systems biology approach. Micro. Pathog. 158 , 105114 (2021).
Shen, T. & Wang, T. Metabolic reprogramming in COVID-19. Int J. Mol. Sci. 22 , 11475 (2021).
Stefano, G. B., Ptacek, R., Ptackova, H., Martin, A. & Kream, R. M. Selective neuronal mitochondrial targeting in SARS-CoV-2 infection affects cognitive processes to induce ‘brain fog’ and results in behavioral changes that favor viral survival. Med Sci. Monit. 27 , e930886 (2021).
Dutta, S., Das, N. & Mukherjee, P. Picking up a fight: fine tuning mitochondrial innate immune defenses against RNA viruses. Front Microbiol 11 , 1990 (2020).
Nunn, A. V. W., Guy, G. W., Brysch, W. & Bell, J. D. Understanding long COVID; mitochondrial health and adaptation-old pathways, new problems. Biomedicines 10 , 3113 (2022).
Sonnweber, T. et al. Persisting alterations of iron homeostasis in COVID-19 are associated with non-resolving lung pathologies and poor patients’ performance: a prospective observational cohort study. Respir. Res. 21 , 276 (2020).
Wessling-Resnick, M. Crossing the iron gate: why and how transferrin receptors mediate viral entry. Annu. Rev. Nutr. 38 , 431–458 (2018).
Cavezzi, A., Troiani, E. & Corrao, S. COVID-19: hemoglobin, iron, and hypoxia beyond inflammation. a narrative review. Clin. Pr. 10 , 1271 (2020).
Wenzhong, L. & Hualan, L. COVID-19: captures iron and generates reactive oxygen species to damage the human immune system. Autoimmunity 54 , 213–224 (2021).
Lechuga, G. C. et al. SARS-CoV-2 proteins bind to hemoglobin and its metabolites. Int J. Mol. Sci. 22 , 9035 (2021).
Radzikowska, U. et al. Distribution of ACE2, CD147, CD26, and other SARS-CoV-2 associated molecules in tissues and immune cells in health and in asthma, COPD, obesity, hypertension, and COVID-19 risk factors. Allergy 75 , 2829–2845 (2020).
Taneri, P. E. et al. Anemia and iron metabolism in COVID-19: a systematic review and meta-analysis. Eur. J. Epidemiol. 35 , 763–773 (2020).
Singh, Y. et al. SARS CoV-2 aggravates cellular metabolism mediated complications in COVID-19 infection. Dermatol. Ther. 33 , e13871 (2020).
Gómez-Pastora, J. et al. Hyperferritinemia in critically ill COVID-19 patients—Is ferritin the product of inflammation or a pathogenic mediator? Clin. Chim. Acta 509 , 249–251 (2020).
Zhu, Z. et al. Clinical value of immune-inflammatory parameters to assess the severity of coronavirus disease 2019. Int J. Infect. Dis. 95 , 332–339 (2020).
Chen, Z., Jiang, J., Fu, N. & Chen, L. Targetting ferroptosis for blood cell-related diseases. J. Drug Target 30 , 244–258 (2022).
Handy, D. E. & Loscalzo, J. Redox regulation of mitochondrial function. Antioxid. Redox Signal 16 , 1323–1367 (2012).
Koklesova, L. et al. Mitochondrial impairments in aetiopathology of multifactorial diseases: common origin but individual outcomes in context of 3P medicine. EPMA J. 12 , 27–40 (2021).
Shang, C. et al. SARS-CoV-2 causes mitochondrial dysfunction and mitophagy impairment. Front Microbiol 12 , 780768 (2021).
Romão, P. R. et al. Viral load is associated with mitochondrial dysfunction and altered monocyte phenotype in acute severe SARS-CoV-2 infection. Int. Immunopharmacol. 108 , 108697 (2022).
Sharma, N. K. & Sarode, S. C. Do compromised mitochondria aggravate severity and fatality by SARS-CoV-2? Curr. Med. Res. Opin. 38 , 911–916 (2022).
Soria-Castro, E. et al. The kidnapping of mitochondrial function associated with the SARS-CoV-2 infection. Histol. Histopathol. 36 , 947–965 (2021).
CAS PubMed Google Scholar
Turton, N., Millichap, L. & Hargreaves, I. P. Potential biomarkers of mitochondrial dysfunction associated with COVID-19 infection. Adv. Exp. Med. Biol. 1412 , 211–224 (2023).
Singh, K. K., Chaubey, G., Chen, J. Y. & Suravajhala, P. Decoding SARS-CoV-2 hijacking of host mitochondria in COVID-19 pathogenesis. Am. J. Physiol. Cell Physiol. 319 , C258–C267 (2020).
Valenzuela, R. et al. An ACE2/Mas-related receptor MrgE axis in dopaminergic neuron mitochondria. Redox Biol. 46 , 102078 (2021).
Valdés-Aguayo, J. J. et al. Mitochondria and mitochondrial DNA: key elements in the pathogenesis and exacerbation of the inflammatory state caused by COVID-19. Med. (Kaunas.) 57 , 928 (2021).
Google Scholar
Costa, T. J. et al. Mitochondrial DNA and TLR9 activation contribute to SARS-CoV-2-induced endothelial cell damage. Vasc. Pharm. 142 , 106946 (2022).
de Las Heras, N., Martín Giménez, V. M., Ferder, L., Manucha, W. & Lahera, V. Implications of oxidative stress and potential role of mitochondrial dysfunction in COVID-19: therapeutic effects of vitamin D. Antioxid. (Basel) 9 , 897 (2020).
Mo, Y. et al. Mitochondrial dysfunction associates with acute T lymphocytopenia and impaired functionality in COVID-19 patients. Front Immunol. 12 , 799896 (2021).
Saleh, J., Peyssonnaux, C., Singh, K. K. & Edeas, M. Mitochondria and microbiota dysfunction in COVID-19 pathogenesis. Mitochondrion 54 , 1–7 (2020).
Clough, E. et al. Mitochondrial dynamics in SARS-COV2 spike protein treated human microglia: implications for neuro-COVID. J. Neuroimmune Pharm. 16 , 770–784 (2021).
Pliss, A., Kuzmin, A. N., Prasad, P. N. & Mahajan, S. D. Mitochondrial dysfunction: a prelude to neuropathogenesis of SARS-CoV-2. ACS Chem. Neurosci. 13 , 308–312 (2022).
Naidu, S. A. G., Wallace, T. C., Davies, K. J. A. & Naidu, A. S. Lactoferrin for mental health: neuro-redox regulation and neuroprotective effects across the blood-brain barrier with special reference to neuro-COVID-19. J. Diet. Suppl. 20 , 218–253 (2023).
Gibellini, L. et al. Altered bioenergetics and mitochondrial dysfunction of monocytes in patients with COVID-19 pneumonia. EMBO Mol. Med. 12 , e13001 (2020).
Guntur, V. P. et al. Signatures of mitochondrial dysfunction and impaired fatty acid metabolism in plasma of patients with post-acute sequelae of COVID-19 (PASC). Metabolites 12 , 1026 (2022).
Chen, T.-H., Chang, C.-J. & Hung, P.-H. Possible pathogenesis and prevention of long COVID: SARS-CoV-2-induced mitochondrial disorder. Int J. Mol. Sci. 24 , 8034 (2023).
McCully, K. S. Review: chemical pathology of homocysteine VI. Aging, cellular senescence, and mitochondrial dysfunction. Ann. Clin. Lab Sci. 48 , 677–687 (2018).
Shenoy, S. Coronavirus (Covid-19) sepsis: revisiting mitochondrial dysfunction in pathogenesis, aging, inflammation, and mortality. Inflamm. Res. 69 , 1077–1085 (2020).
Moreno Fernández-Ayala, D. J., Navas, P. & López-Lluch, G. Age-related mitochondrial dysfunction as a key factor in COVID-19 disease. Exp. Gerontol. 142 , 111147 (2020).
Alfarouk, K. O. et al. Of mitochondrion and COVID-19. J. Enzym. Inhib. Med. Chem. 36 , 1258–1267 (2021).
Betteridge, D. J. What is oxidative stress? Metabolism 49 , 3–8 (2000).
Schieber, M. & Chandel, N. S. ROS function in redox signaling and oxidative stress. Curr. Biol. 24 , R453–R462 (2014).
Galaris, D., Barbouti, A. & Pantopoulos, K. Iron homeostasis and oxidative stress: an intimate relationship. Biochim Biophys. Acta Mol. Cell Res. 1866 , 118535 (2019).
Vollbracht, C. & Kraft, K. Oxidative stress and hyper-inflammation as major drivers of severe COVID-19 and Long COVID: implications for the benefit of high-dose intravenous vitamin C. Front. Pharm. 13 , 899198 (2022).
De la Cruz-Enríquez, J., Rojas-Morales, E., Ruíz-García, M. G., Tobón-Velasco, J. C. & Jiménez-Ortega, J. C. SARS-CoV-2 induces mitochondrial dysfunction and cell death by oxidative stress/inflammation in leukocytes of COVID-19 patients. Free Radic. Res. 55 , 982–995 (2021).
Chang, R., Mamun, A., Dominic, A. & Le, N.-T. SARS-CoV-2 mediated endothelial dysfunction: the potential role of chronic oxidative stress. Front. Physiol. 11 , 605908 (2020).
Lopez-Leon, S. et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci. Rep. 11 , 16144 (2021).
Yuki, K., Fujiogi, M. & Koutsogiannaki, S. COVID-19 pathophysiology: a review. Clin. Immunol. 215 , 108427 (2020).
Gremese, E. & Ferraccioli, G. The pathogenesis of microthrombi in COVID-19 cannot be controlled by DOAC: NETosis should be the target. J. Intern. Med. 289 , 420–421 (2021).
Mo, X. et al. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur. Respir. J. 55 , 2001217 (2020).
Long, B., Brady, W. J., Koyfman, A. & Gottlieb, M. Cardiovascular complications in COVID-19. Am. J. Emerg. Med. 38 , 1504–1507 (2020).
Moody, W. E. et al. Persisting adverse ventricular remodeling in COVID-19 survivors: a longitudinal echocardiographic study. J. Am. Soc. Echocardiogr. 34 , 562–566 (2021).
Carfì, A., Bernabei, R. & Landi, F., Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA 324 , 603–605 (2020).
Raman, B., Bluemke, D. A., Lüscher, T. F. & Neubauer, S. Long COVID: post-acute sequelae of COVID-19 with a cardiovascular focus. Eur. Heart J. 43 , 1157–1172 (2022).
Huang, C. et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet 397 , 220–232 (2021).
Savelieff, M. G., Feldman, E. L. & Stino, A. M. Neurological sequela and disruption of neuron-glia homeostasis in SARS-CoV-2 infection. Neurobiol. Dis. 168 , 105715 (2022).
Hingorani, K. S., Bhadola, S. & Cervantes-Arslanian, A. M. COVID-19 and the brain. Trends Cardiovasc. Med. 32 , 323–330 (2022).
Theoharides, T. C. & Kempuraj, D. Role of SARS-CoV-2 spike-protein-induced activation of microglia and mast cells in the pathogenesis of neuro-COVID. Cells 12 , 688 (2023).
Naidu, A. S. & Clemens, R. A. No smell, no taste—dealing with a “senseless” phase of the pandemic: nutritional management of COVID-19 and postacute sequelae of COVID-19. Nutr. Today 57 , 309–316 (2022).
Fisicaro, F. et al. Neurological sequelae in patients with COVID-19: a histopathological perspective. Int J. Environ. Res. Public Health 18 , 1415 (2021).
Gelpi, E. et al. Multifactorial white matter damage in the acute phase and pre-existing conditions may drive cognitive dysfunction after SARS-CoV-2 infection: neuropathology-based evidence. Viruses 15 , 908 (2023).
Matschke, J. et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 19 , 919–929 (2020).
Premraj, L. et al. Mid and long-term neurological and neuropsychiatric manifestations of post-COVID-19 syndrome: a meta-analysis. J. Neurol. Sci. 434 , 120162 (2022).
Xu, E., Xie, Y. & Al-Aly, Z. Long-term gastrointestinal outcomes of COVID-19. Nat. Commun. 14 , 983 (2023).
de Oliveira, G. L. V., Oliveira, C. N. S., Pinzan, C. F., de Salis, L. V. V. & de B Cardoso, C. R. Microbiota modulation of the gut-lung axis in COVID-19. Front. Immunol. 12 , 635471 (2021).
Ahmadi Badi, S. et al. From the role of microbiota in gut-lung axis to SARS-CoV-2 pathogenesis. Mediators Inflamm. 2021 , 6611222 (2021).
Lumlertgul, N. et al. Acute kidney injury prevalence, progression and long-term outcomes in critically ill patients with COVID-19: a cohort study. Ann. Intensive Care 11 , 123 (2021).
Fukuda, K. et al. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann. Intern. Med. 121 , 953–959 (1994).
Sturm, G. et al. OxPhos defects cause hypermetabolism and reduce lifespan in cells and in patients with mitochondrial diseases. Commun. Biol. 6 , 22 (2023).
Carruthers, B. M. et al. Myalgic encephalomyelitis: International Consensus Criteria. J. Intern. Med. 270 , 327–338 (2011).
Weinstock, L. B. et al. Mast cell activation symptoms are prevalent in Long-COVID. Int J. Infect. Dis. 112 , 217–226 (2021).
Islam, M. S., Wang, Z., Abdel-Mohsen, M., Chen, X. & Montaner, L. J. Tissue injury and leukocyte changes in post-acute sequelae of SARS-CoV-2: review of 2833 post-acute patient outcomes per immune dysregulation and microbial translocation in long COVID. J. Leukoc. Biol. 113 , 236–254 (2023).
Diao, B. et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19). Front. Immunol. 11 , 827 (2020).
Vijayakumar, B. et al. Immuno-proteomic profiling reveals aberrant immune cell regulation in the airways of individuals with ongoing post-COVID-19 respiratory disease. Immunity 55 , 542–556.e5 (2022).
Gaebler, C. et al. Evolution of antibody immunity to SARS-CoV-2. Nature 591 , 639–644 (2021).
Vibholm, L. K. et al. SARS-CoV-2 persistence is associated with antigen-specific CD8 T-cell responses. EBioMedicine 64 , 103230 (2021).
Wang, F. et al. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J. Infect. Dis. 221 , 1762–1769 (2020).
Bautista-Becerril, B. et al. Immunothrombosis in COVID-19: implications of neutrophil extracellular traps. Biomolecules 11 , 694 (2021).
Wendisch, D. et al. SARS-CoV-2 infection triggers profibrotic macrophage responses and lung fibrosis. Cell 184 , 6243–6261.e27 (2021).
Rajamanickam, A. et al. Dynamic alterations in monocyte numbers, subset frequencies and activation markers in acute and convalescent COVID-19 individuals. Sci. Rep. 11 , 20254 (2021).
Scott, N. A. et al. Monocyte migration profiles define disease severity in acute COVID-19 and unique features of long COVID. Eur. Respir. J. 61 , 2202226 (2023).
Fajgenbaum, D. C. & June, C. H. Cytokine storm. N. Engl. J. Med. 383 , 2255–2273 (2020).
Coperchini, F. et al. The cytokine storm in COVID-19: Further advances in our understanding the role of specific chemokines involved. Cytokine Growth Factor Rev. 58 , 82–91 (2021).
Wang, J., Jiang, M., Chen, X. & Montaner, L. J. Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: Review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J. Leukoc. Biol. 108 , 17–41 (2020).
Ong, S. W. X. et al. Persistent symptoms and association with inflammatory cytokine signatures in recovered coronavirus disease 2019 patients. Open Forum Infect. Dis. 8 , ofab156 (2021).
Su, Y. et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell 185 , 881–895.e20 (2022).
Mehandru, S. & Merad, M. Pathological sequelae of long-haul COVID. Nat. Immunol. 23 , 194–202 (2022).
Halpert, G. & Shoenfeld, Y. SARS-CoV-2, the autoimmune virus. Autoimmun. Rev. 19 , 102695 (2020).
Lorente, L. et al. HLA genetic polymorphisms and prognosis of patients with COVID-19. Med Intensiv. (Engl. Ed.) 45 , 96–103 (2021).
Arango, M.-T. et al. HLA-DRB1 the notorious gene in the mosaic of autoimmunity. Immunol. Res. 65 , 82–98 (2017).
Toscano, G. et al. Guillain-Barré syndrome associated with SARS-CoV-2. N. Engl. J. Med. 382 , 2574–2576 (2020).
Verdoni, L. et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet 395 , 1771–1778 (2020).
Dotan, A. et al. The SARS-CoV-2 as an instrumental trigger of autoimmunity. Autoimmun. Rev. 20 , 102792 (2021).
Rojas, M. et al. Autoimmunity is a hallmark of post-COVID syndrome. J. Transl. Med. 20 , 129 (2022).
Acosta-Ampudia, Y. et al. Persistent autoimmune activation and proinflammatory state in post-coronavirus disease 2019 syndrome. J. Infect. Dis. 225 , 2155–2162 (2022).
Dotan, A., David, P., Arnheim, D. & Shoenfeld, Y. The autonomic aspects of the post-COVID19 syndrome. Autoimmun. Rev. 21 , 103071 (2022).
Sarkar, A. et al. The gut microbiome as a biomarker of differential susceptibility to SARS-CoV-2. Trends Mol. Med 27 , 1115–1134 (2021).
Schult, D. et al. Gut bacterial dysbiosis and instability is associated with the onset of complications and mortality in COVID-19. Gut Microbes 14 , 2031840 (2022).
Wang, X. et al. Long-term existence of SARS-CoV-2 in COVID-19 patients: host immunity, viral virulence, and transmissibility. Virol. Sin. 35 , 793–802 (2020).
Zhang, F. et al. Prolonged impairment of short-chain fatty acid and l-isoleucine biosynthesis in gut microbiome in patients with COVID-19. Gastroenterology 162 , 548–561.e4 (2022).
Manna, S., Baindara, P. & Mandal, S. M. Molecular pathogenesis of secondary bacterial infection associated to viral infections including SARS-CoV-2. J. Infect. Public Health 13 , 1397–1404 (2020).
Chen, J., Hall, S. & Vitetta, L. Altered gut microbial metabolites could mediate the effects of risk factors in Covid-19. Rev. Med. Virol. 31 , 1–13 (2021).
Mitrea, L., Nemeş, S.-A., Szabo, K., Teleky, B.-E. & Vodnar, D.-C. Guts imbalance imbalances the brain: a review of gut microbiota association with neurological and psychiatric disorders. Front. Med. (Lausanne) 9 , 813204 (2022).
Sun, Z. et al. Gut microbiome alterations and gut barrier dysfunction are associated with host immune homeostasis in COVID-19 patients. BMC Med. 20 , 24 (2022).
Han, C. et al. Digestive symptoms in COVID-19 patients with mild disease severity: clinical presentation, stool viral RNA testing, and outcomes. Am. J. Gastroenterol. 115 , 916–923 (2020).
Ancona, G. et al. Gut and airway microbiota dysbiosis and their role in COVID-19 and long-COVID. Front. Immunol. 14 , 1080043 (2023).
Zhang, D. et al. Gut microbiota dysbiosis correlates with long COVID-19 at one-year after discharge. J. Korean Med. Sci. 38 , e120 (2023).
Hilpert, K. & Mikut, R. Is there a connection between gut microbiome dysbiosis occurring in COVID-19 patients and post-COVID-19 symptoms? Front. Microbiol. 12 , 732838 (2021).
Ferreira-Junior, A. S. et al. Detection of intestinal dysbiosis in post-COVID-19 patients one to eight months after acute disease resolution. Int J. Environ. Res. Public Health 19 , 10189 (2022).
Roy, K., Agarwal, S., Banerjee, R., Paul, M. K. & Purbey, P. K. COVID-19 and gut immunomodulation. World J. Gastroenterol. 27 , 7925–7942 (2021).
Gu, S. et al. Alterations of the gut microbiota in patients with coronavirus disease 2019 or H1N1 influenza. Clin. Infect. Dis. 71 , 2669–2678 (2020).
Zuo, T. et al. Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology 159 , 944–955.e8 (2020).
Yeoh, Y. K. et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut 70 , 698–706 (2021).
Tsounis, E. P., Triantos, C., Konstantakis, C., Marangos, M. & Assimakopoulos, S. F. Intestinal barrier dysfunction as a key driver of severe COVID-19. World J. Virol. 12 , 68–90 (2023).
Liu, Q. et al. Gut microbiota dynamics in a prospective cohort of patients with post-acute COVID-19 syndrome. Gut 71 , 544–552 (2022).
Clerbaux, L.-A. et al. Mechanisms leading to gut dysbiosis in COVID-19: current evidence and uncertainties based on adverse outcome pathways. J. Clin. Med. 11 , 5400 (2022).
Navarro-Bielsa, A. et al. COVID-19 infection and vaccines: potential triggers of Herpesviridae reactivation. Bras. Dermatol. 98 , 347–354 (2023).
Virgin, H. W., Wherry, E. J. & Ahmed, R. Redefining chronic viral infection. Cell 138 , 30–50 (2009).
Shafiee, A. et al. Reactivation of herpesviruses during COVID-19: A systematic review and meta-analysis. Rev. Med. Virol. 33 , e2437 (2023).
Chen, J., Song, J., Dai, L., Post, S. R. & Qin, Z. SARS-CoV-2 infection and lytic reactivation of herpesviruses: a potential threat in the postpandemic era? J. Med. Virol. 94 , 5103–5111 (2022).
Jiang, M. et al. T-cell subset counts in peripheral blood can be used as discriminatory biomarkers for diagnosis and severity prediction of coronavirus disease 2019. J. Infect. Dis. 222 , 198–202 (2020).
Sugawara-Mikami, M. et al. Skin manifestations of suspected COVID-19: complications of the disease or reactivation of latent viral infections? JAAD Case Rep. 12 , 15–17 (2021).
Chen, J. et al. SARS-CoV-2 proteins and anti-COVID-19 drugs induce lytic reactivation of an oncogenic virus. Commun. Biol. 4 , 682 (2021).
Chen, J., Dai, L., Kendrick, S., Post, S. R. & Qin, Z. The anti-COVID-19 drug remdesivir promotes oncogenic herpesvirus reactivation through regulation of intracellular signaling pathways. Antimicrob. Agents Chemother. 66 , e0239521 (2022).
Galván Casas, C. et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br. J. Dermatol. 183 , 71–77 (2020).
Hubiche, T., Le Duff, F., Chiaverini, C., Giordanengo, V. & Passeron, T. Negative SARS-CoV-2 PCR in patients with chilblain-like lesions. Lancet Infect. Dis. 21 , 315–316 (2021).
Drago, F., Ciccarese, G., Rebora, A. & Parodi, A. Human herpesvirus-6, -7, and Epstein-Barr virus reactivation in pityriasis rosea during COVID-19. J. Med. Virol. 93 , 1850–1851 (2021).
Naendrup, J.-H. et al. Reactivation of EBV and CMV in severe COVID-19-epiphenomena or trigger of hyperinflammation in need of treatment? a large case series of critically ill patients. J. Intensive Care Med. 37 , 1152–1158 (2022).
Seeßle, J. et al. High rate of HSV-1 reactivation in invasively ventilated COVID-19 patients: immunological findings. PLoS ONE 16 , e0254129 (2021).
Chen, T., Song, J., Liu, H., Zheng, H. & Chen, C. Positive Epstein-Barr virus detection in coronavirus disease 2019 (COVID-19) patients. Sci. Rep. 11 , 10902 (2021).
Xie, Y. et al. Clinical characteristics and outcomes of critically ill patients with acute COVID-19 with Epstein-Barr virus reactivation. BMC Infect. Dis. 21 , 955 (2021).
Hannestad, U. et al. Post-COVID sequelae effect in chronic fatigue syndrome: SARS-CoV-2 triggers latent adenovirus in the oral mucosa. Front. Med. (Lausanne) 10 , 1208181 (2023).
Stein, S. R. et al. SARS-CoV-2 infection and persistence in the human body and brain at autopsy. Nature 612 , 758–763 (2022).
Buonsenso, D., Piazza, M., Boner, A. L. & Bellanti, J. A. Long COVID: a proposed hypothesis-driven model of viral persistence for the pathophysiology of the syndrome. Allergy Asthma Proc. 43 , 187–193 (2022).
Cevik, M. et al. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis. Lancet Microbe 2 , e13–e22 (2021).
Chen, B., Julg, B., Mohandas, S. & Bradfute, S. B., RECOVER Mechanistic Pathways Task Force. Viral persistence, reactivation, and mechanisms of long COVID. Elife 12 , e86015 (2023).
de Melo, G. D. et al. COVID-19-related anosmia is associated with viral persistence and inflammation in human olfactory epithelium and brain infection in hamsters. Sci. Transl. Med. 13 , eabf8396 (2021).
Antar, A. A. R. et al. Long COVID brain fog and muscle pain are associated with longer time to clearance of SARS-CoV-2 RNA from the upper respiratory tract during acute infection. Front. Immunol. 14 , 1147549 (2023).
Wu, Y. et al. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol. Hepatol. 5 , 434–435 (2020).
Zollner, A. et al. Postacute COVID-19 is characterized by gut viral antigen persistence in inflammatory bowel diseases. Gastroenterology 163 , 495–506.e8 (2022).
Tejerina, F. et al. Post-COVID-19 syndrome. SARS-CoV-2 RNA detection in plasma, stool, and urine in patients with persistent symptoms after COVID-19. BMC Infect. Dis. 22 , 211 (2022).
Swank, Z. et al. Persistent circulating severe acute respiratory syndrome coronavirus 2 spike is associated with post-acute coronavirus disease 2019 sequelae. Clin. Infect. Dis. 76 , e487–e490 (2023).
Craddock, V. et al. Persistent circulation of soluble and extracellular vesicle-linked Spike protein in individuals with postacute sequelae of COVID-19. J. Med. Virol. 95 , e28568 (2023).
Patterson, B. K. et al. Persistence of SARS CoV-2 S1 protein in CD16+ monocytes in post-acute sequelae of COVID-19 (PASC) up to 15 months post-infection. Front. Immunol. 12 , 746021 (2021).
Cheng, M. H. et al. Superantigenic character of an insert unique to SARS-CoV-2 spike supported by skewed TCR repertoire in patients with hyperinflammation. Proc. Natl Acad. Sci. USA 117 , 25254–25262 (2020).
Lei, Y. et al. SARS-CoV-2 spike protein impairs endothelial function via downregulation of ACE 2. Circ. Res. 128 , 1323–1326 (2021).
DeOre, B. J., Tran, K. A., Andrews, A. M., Ramirez, S. H. & Galie, P. A. SARS-CoV-2 spike protein disrupts blood-brain barrier integrity via RhoA activation. J. Neuroimmune Pharm. 16 , 722–728 (2021).
Lubbe, L., Cozier, G. E., Oosthuizen, D., Acharya, K. R. & Sturrock, E. D. ACE2 and ACE: structure-based insights into mechanism, regulation and receptor recognition by SARS-CoV. Clin. Sci. (Lond.) 134 , 2851–2871 (2020).
Yan, R. et al. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 367 , 1444–1448 (2020).
Zou, X. et al. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med. 14 , 185–192 (2020).
Iwata-Yoshikawa, N. et al. TMPRSS2 contributes to virus spread and immunopathology in the airways of murine models after coronavirus infection. J. Virol. 93 , e01815–e01818 (2019).
Li, Q. et al. Eosinopenia and elevated C-reactive protein facilitate triage of COVID-19 patients in fever clinic: a retrospective case-control study. EClinicalMedicine 23 , 100375 (2020).
Hikmet, F. et al. The protein expression profile of ACE2 in human tissues. Mol. Syst. Biol. 16 , e9610 (2020).
Wang, W. et al. Angiotensin-converting enzyme 2 metabolizes and partially inactivates Pyr-Apelin-13 and Apelin-17: physiological effects in the cardiovascular system. Hypertension 68 , 365–377 (2016).
Lambert, D. W. et al. Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2). J. Biol. Chem. 280 , 30113–30119 (2005).
Kessler, S. P., Rowe, T. M., Gomos, J. B., Kessler, P. M. & Sen, G. C. Physiological non-equivalence of the two isoforms of angiotensin-converting enzyme. J. Biol. Chem. 275 , 26259–26264 (2000).
Hagaman, J. R. et al. Angiotensin-converting enzyme and male fertility. Proc. Natl Acad. Sci. USA 95 , 2552–2557 (1998).
Fuchs, S. et al. Male fertility is dependent on dipeptidase activity of testis ACE. Nat. Med. 11 , 1140–1142 (2005).
Erdös, E. G. Angiotensin I converting enzyme and the changes in our concepts through the years. Lewis K. Dahl memorial lecture. Hypertension 16 , 363–370 (1990).
Tipnis, S. R. et al. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J. Biol. Chem. 275 , 33238–33243 (2000).
Donoghue, M. et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ. Res. 87 , E1–E9 (2000).
South, A. M., Diz, D. I. & Chappell, M. C. COVID-19, ACE2, and the cardiovascular consequences. Am. J. Physiol. Heart Circ. Physiol. 318 , H1084–H1090 (2020).
Patel, V. B., Zhong, J.-C., Grant, M. B. & Oudit, G. Y. Role of the ACE2/angiotensin 1-7 axis of the renin-angiotensin system in heart failure. Circ. Res. 118 , 1313–1326 (2016).
Hamming, I. et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 203 , 631–637 (2004).
Scialo, F. et al. ACE2: the major cell entry receptor for SARS-CoV-2. Lung 198 , 867–877 (2020).
Yousif, M. H. M. et al. Characterization of Angiotensin-(1-7) effects on the cardiovascular system in an experimental model of type-1 diabetes. Pharm. Res. 66 , 269–275 (2012).
Niu, M.-J., Yang, J.-K., Lin, S.-S., Ji, X.-J. & Guo, L.-M. Loss of angiotensin-converting enzyme 2 leads to impaired glucose homeostasis in mice. Endocrine 34 , 56–61 (2008).
Yang, J.-K. et al. Interactions among related genes of renin-angiotensin system associated with type 2 diabetes. Diabetes Care 33 , 2271–2273 (2010).
Hussain, A., Bhowmik, B. & do Vale Moreira, N. C. COVID-19 and diabetes: Knowledge in progress. Diabetes Res. Clin. Pr. 162 , 108142 (2020).
Williams, V. R. & Scholey, J. W. Angiotensin-converting enzyme 2 and renal disease. Curr. Opin. Nephrol. Hypertens. 27 , 35–41 (2018).
Alenina, N. & Bader, M. ACE2 in brain physiology and pathophysiology: evidence from transgenic animal models. Neurochem Res. 44 , 1323–1329 (2019).
Sahara, M. et al. Deletion of angiotensin-converting enzyme 2 promotes the development of atherosclerosis and arterial neointima formation. Cardiovasc. Res. 101 , 236–246 (2014).
Kuba, K. et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 11 , 875–879 (2005).
Kong, W. et al. Neuropilin-1 mediates SARS-CoV-2 infection of astrocytes in brain organoids, inducing inflammation leading to dysfunction and death of neurons. mBio 13 , e0230822 (2022).
Harman, J. L., Sayers, J., Chapman, C. & Pellet-Many, C. Emerging roles for neuropilin-2 in cardiovascular disease. Int J. Mol. Sci. 21 , 5154 (2020).
Chuckran, C. A., Liu, C., Bruno, T. C., Workman, C. J. & Vignali, D. A. Neuropilin-1: a checkpoint target with unique implications for cancer immunology and immunotherapy. J. Immunother. Cancer 8 , e000967 (2020).
Chen, H., Chédotal, A., He, Z., Goodman, C. S. & Tessier-Lavigne, M. Neuropilin-2, a novel member of the neuropilin family, is a high affinity receptor for the semaphorins Sema E and Sema IV but Not Sema III. Neuron 19 , 547–559 (1997).
Soker, S., Takashima, S., Miao, H. Q., Neufeld, G. & Klagsbrun, M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell 92 , 735–745 (1998).
Rossignol, M., Gagnon, M. L. & Klagsbrun, M. Genomic organization of human neuropilin-1 and neuropilin-2 genes: identification and distribution of splice variants and soluble isoforms. Genomics 70 , 211–222 (2000).
Guo, H.-F. & Vander Kooi, C. W. Neuropilin functions as an essential cell surface receptor. J. Biol. Chem. 290 , 29120–29126 (2015).
Lampropoulou, A. & Ruhrberg, C. Neuropilin regulation of angiogenesis. Biochem Soc. Trans. 42 , 1623–1628 (2014).
Schellenburg, S., Schulz, A., Poitz, D. M. & Muders, M. H. Role of neuropilin-2 in the immune system. Mol. Immunol. 90 , 239–244 (2017).
Rizzolio, S. & Tamagnone, L. Multifaceted role of neuropilins in cancer. Curr. Med Chem. 18 , 3563–3575 (2011).
Roy, S. et al. Multifaceted role of neuropilins in the immune system: potential targets for immunotherapy. Front. Immunol. 8 , 1228 (2017).
Perez-Miller, S. et al. Novel compounds targeting neuropilin receptor 1 with potential to interfere with SARS-CoV-2 virus entry. ACS Chem. Neurosci. 12 , 1299–1312 (2021).
Moutal, A. et al. SARS-CoV-2 spike protein co-opts VEGF-A/neuropilin-1 receptor signaling to induce analgesia. Pain 162 , 243–252 (2021).
Domingues, A. & Fantin, A. Neuropilin 1 regulation of vascular permeability signaling. Biomolecules 11 , 666 (2021).
Yamaji, M., Mahmoud, M., Evans, I. M. & Zachary, I. C. Neuropilin 1 is essential for gastrointestinal smooth muscle contractility and motility in aged mice. PLoS ONE 10 , e0115563 (2015).
Pontarollo, G. et al. Commensal bacteria weaken the intestinal barrier by suppressing epithelial neuropilin-1 and Hedgehog signaling. Nat. Metab. 5 , 1174–1187 (2023).
Gao, J. et al. Neuropilin-1-mediated SARS-CoV-2 infection in bone marrow-derived macrophages inhibits osteoclast differentiation. Adv. Biol. (Weinh.) 6 , e2200007 (2022).
Coutard, B. et al. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antivir. Res. 176 , 104742 (2020).
Wise, R. J. et al. Expression of a human proprotein processing enzyme: correct cleavage of the von Willebrand factor precursor at a paired basic amino acid site. Proc. Natl Acad. Sci. USA 87 , 9378–9382 (1990).
Duckert, P., Brunak, S. & Blom, N. Prediction of proprotein convertase cleavage sites. Protein Eng. Des. Sel. 17 , 107–112 (2004).
Braun, E. & Sauter, D. Furin-mediated protein processing in infectious diseases and cancer. Clin. Transl. Immunol. 8 , e1073 (2019).
Klenk, H. D. & Garten, W. Host cell proteases controlling virus pathogenicity. Trends Microbiol. 2 , 39–43 (1994).
Uhlén, M. et al. Proteomics. Tissue-based map of the human proteome. Science 347 , 1260419 (2015).
Tian, S., Huajun, W. & Wu, J. Computational prediction of furin cleavage sites by a hybrid method and understanding mechanism underlying diseases. Sci. Rep. 2 , 261 (2012).
Tian, S., Huang, Q., Fang, Y. & Wu, J. FurinDB: a database of 20-residue furin cleavage site motifs, substrates and their associated drugs. Int J. Mol. Sci. 12 , 1060–1065 (2011).
Thomas, G. Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nat. Rev. Mol. Cell Biol. 3 , 753–766 (2002).
Williams, D. P. et al. Cellular processing of the interleukin-2 fusion toxin DAB486-IL-2 and efficient delivery of diphtheria fragment A to the cytosol of target cells requires Arg194. J. Biol. Chem. 265 , 20673–20677 (1990).
Ogata, M., Fryling, C. M., Pastan, I. & FitzGerald, D. J. Cell-mediated cleavage of Pseudomonas exotoxin between Arg279 and Gly280 generates the enzymatically active fragment which translocates to the cytosol. J. Biol. Chem. 267 , 25396–25401 (1992).
Tsuneoka, M. et al. Evidence for involvement of furin in cleavage and activation of diphtheria toxin. J. Biol. Chem. 268 , 26461–26465 (1993).
Gordon, V. M. & Leppla, S. H. Proteolytic activation of bacterial toxins: role of bacterial and host cell proteases. Infect. Immun. 62 , 333–340 (1994).
Liddington, R. C. Assembly and function of the anthrax toxin protein translocation complex. Subcell. Biochem. 96 , 563–577 (2021).
Molloy, S. S., Bresnahan, P. A., Leppla, S. H., Klimpel, K. R. & Thomas, G. Human furin is a calcium-dependent serine endoprotease that recognizes the sequence Arg-X-X-Arg and efficiently cleaves anthrax toxin protective antigen. J. Biol. Chem. 267 , 16396–16402 (1992).
Liu, Z.-W., Ma, Q., Liu, J., Li, J.-W. & Chen, Y.-D. The association between plasma furin and cardiovascular events after acute myocardial infarction. BMC Cardiovasc. Disord. 21 , 468 (2021).
Patone, M. et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat. Med. 28 , 410–422 (2022).
Dupays, L. et al. Furin, a transcriptional target of NKX2-5, has an essential role in heart development and function. PLoS ONE 14 , e0212992 (2019).
Dong, S. et al. Furin inhibits epithelial cell injury and alleviates experimental colitis by activating the Nrf2-Gpx4 signaling pathway. Dig. Liver Dis. 53 , 1276–1285 (2021).
Li, Q. et al. Ferroptosis and multi-organ complications in COVID-19: mechanisms and potential therapies. Front. Genet. 14 , 1187985 (2023).
Remacle, A. G., Rozanov, D. V., Fugere, M., Day, R. & Strongin, A. Y. Furin regulates the intracellular activation and the uptake rate of cell surface-associated MT1-MMP. Oncogene 25 , 5648–5655 (2006).
Wu, C. et al. Inhibition of furin results in increased growth, invasiveness and cytokine production of synoviocytes from patients with rheumatoid arthritis. Jt. Bone Spine 84 , 433–439 (2017).
Zhang, Y. et al. The emerging role of furin in neurodegenerative and neuropsychiatric diseases. Transl. Neurodegener. 11 , 39 (2022).
Lee, W. Y., Mok, A. & Chung, J. P. W. Potential effects of COVID-19 on reproductive systems and fertility; assisted reproductive technology guidelines and considerations: a review. Hong. Kong Med J. 27 , 118–126 (2021).
Shi, L.-Y. et al. Placenta-specific 1 regulates oocyte meiosis and fertilization through furin. FASEB J. 32 , 5483–5494 (2018).
Meng, T.-G. et al. Oocyte-specific deletion of furin leads to female infertility by causing early secondary follicle arrest in mice. Cell Death Dis. 8 , e2846 (2017).
Fraser, B. J. et al. Structure and activity of human TMPRSS2 protease implicated in SARS-CoV-2 activation. Nat. Chem. Biol. 18 , 963–971 (2022).
Koch, J. et al. TMPRSS2 expression dictates the entry route used by SARS-CoV-2 to infect host cells. EMBO J. 40 , e107821 (2021).
Chen, Y.-W. et al. TMPRSS2, a serine protease expressed in the prostate on the apical surface of luminal epithelial cells and released into semen in prostasomes, is misregulated in prostate cancer cells. Am. J. Pathol. 176 , 2986–2996 (2010).
Lin, B. et al. Prostate-localized and androgen-regulated expression of the membrane-bound serine protease TMPRSS2. Cancer Res. 59 , 4180–4184 (1999).
Schuler, B. A. et al. Age-determined expression of priming protease TMPRSS2 and localization of SARS-CoV-2 in lung epithelium. J. Clin. Invest. 131 , e140766 (2021).
Thunders, M. & Delahunt, B. Gene of the month: TMPRSS2 (transmembrane serine protease 2). J. Clin. Pathol. 73 , 773–776 (2020).
List, K. et al. Epithelial integrity is maintained by a matriptase-dependent proteolytic pathway. Am. J. Pathol. 175 , 1453–1463 (2009).
Antalis, T. M., Bugge, T. H. & Wu, Q. Membrane-anchored serine proteases in health and disease. Prog. Mol. Biol. Transl. Sci. 99 , 1–50 (2011).
Heurich, A. et al. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J. Virol. 88 , 1293–1307 (2014).
Mukai, S. et al. Matriptase and MET are prominently expressed at the site of bone metastasis in renal cell carcinoma: immunohistochemical analysis. Hum. Cell 28 , 44–50 (2015).
Jacquinet, E., Rao, N. V., Rao, G. V. & Hoidal, J. R. Cloning, genomic organization, chromosomal assignment and expression of a novel mosaic serine proteinase: epitheliasin. FEBS Lett. 468 , 93–100 (2000).
Wu, Q., Li, S., Zhang, X. & Dong, N. Type II transmembrane serine proteases as modulators in adipose tissue phenotype and function. Biomedicines 11 , 1794 (2023).
Lam, D. K., Dang, D., Flynn, A. N., Hardt, M. & Schmidt, B. L. TMPRSS2, a novel membrane-anchored mediator in cancer pain. Pain 156 , 923–930 (2015).
Gunne, S. et al. TMPRSS2 impacts cytokine expression in murine dendritic cells. Biomedicines 11 , 419 (2023).
Zeginiadou, T., Symeonidis, E. N., Symeonidis, A. & Vakalopoulos, I. SARS-CoV-2 infection (COVID-19) and male fertility: Something we should be worried about? Urologia 90 , 726–734 (2023).
Li, X. et al. COVID-19 and male reproduction: a thorny problem. Am. J. Mens. Health 16 , 15579883221074816 (2022).
Martin, C. E. & List, K. Cell surface-anchored serine proteases in cancer progression and metastasis. Cancer Metastasis Rev. 38 , 357–387 (2019).
Cheng, J. et al. Prostate adenocarcinoma and COVID-19: The possible impacts of TMPRSS2 expressions in susceptibility to SARS-CoV-2. J. Cell Mol. Med. 25 , 4157–4165 (2021).
Berdowska, I. & Matusiewicz, M. Cathepsin L, transmembrane peptidase/serine subfamily member 2/4, and other host proteases in COVID-19 pathogenesis - with impact on gastrointestinal tract. World J. Gastroenterol. 27 , 6590–6600 (2021).
Simmons, G. et al. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc. Natl Acad. Sci. USA 102 , 11876–11881 (2005).
Bollavaram, K. et al. Multiple sites on SARS-CoV-2 spike protein are susceptible to proteolysis by cathepsins B, K, L, S, and V. Protein Sci. 30 , 1131–1143 (2021).
Gomes, C. P. et al. Cathepsin L in COVID-19: from pharmacological evidences to genetics. Front. Cell Infect. Microbiol. 10 , 589505 (2020).
Metzdorf, K. et al. TMPRSS2 is essential for SARS-CoV-2 beta and omicron infection. Viruses 15 , 271 (2023).
Turk, V., Turk, B. & Turk, D. Lysosomal cysteine proteases: facts and opportunities. EMBO J. 20 , 4629–4633 (2001).
Sosnowski, P. & Turk, D. Caught in the act: the crystal structure of cleaved cathepsin L bound to the active site of Cathepsin L. FEBS Lett. 590 , 1253–1261 (2016).
Dickinson, D. P. Cysteine peptidases of mammals: their biological roles and potential effects in the oral cavity and other tissues in health and disease. Crit. Rev. Oral. Biol. Med. 13 , 238–275 (2002).
Hitzel, C. et al. Thyroglobulin type-I-like domains in invariant chain fusion proteins mediate resistance to cathepsin L digestion. FEBS Lett. 485 , 67–70 (2000).
Turk, B., Turk, D. & Turk, V. Protease signalling: the cutting edge. EMBO J. 31 , 1630–1643 (2012).
Hsing, L. C. & Rudensky, A. Y. The lysosomal cysteine proteases in MHC class II antigen presentation. Immunol. Rev. 207 , 229–241 (2005).
Kakegawa, H. et al. Participation of cathepsin L on bone resorption. FEBS Lett. 321 , 247–250 (1993).
Brix, K., Lemansky, P. & Herzog, V. Evidence for extracellularly acting cathepsins mediating thyroid hormone liberation in thyroid epithelial cells. Endocrinology 137 , 1963–1974 (1996).
Fujishima, A. et al. The crystal structure of human cathepsin L complexed with E-64. FEBS Lett. 407 , 47–50 (1997).
Reinheckel, T. & Tholen, M. Low-level lysosomal membrane permeabilization for limited release and sublethal functions of cathepsin proteases in the cytosol and nucleus. FEBS Open Bio 12 , 694–707 (2022).
Ezz, M. A., Takahashi, M., Rivera, R. M. & Balboula, A. Z. Cathepsin L regulates oocyte meiosis and preimplantation embryo development. Cell Prolif. 57 , e13526 (2024).
Balachandren, N. et al. SARS-CoV-2 infection in the first trimester and the risk of early miscarriage: a UK population-based prospective cohort study of 3041 pregnancies conceived during the pandemic. Hum. Reprod. 37 , 1126–1133 (2022).
Carnevali, O., Cionna, C., Tosti, L., Lubzens, E. & Maradonna, F. Role of cathepsins in ovarian follicle growth and maturation. Gen. Comp. Endocrinol. 146 , 195–203 (2006).
García, V. et al. Transient expression of progesterone receptor and cathepsin-l in human granulosa cells during the periovulatory period. Fertil. Steril. 97 , 707–713.e1 (2012).
Funkelstein, L. & Hook, V. The novel role of cathepsin L for neuropeptide production illustrated by research strategies in chemical biology with protease gene knockout and expression. Methods Mol. Biol. 768 , 107–125 (2011).
Hook, V. Y. H. Unique neuronal functions of cathepsin L and cathepsin B in secretory vesicles: biosynthesis of peptides in neurotransmission and neurodegenerative disease. Biol. Chem. 387 , 1429–1439 (2006).
Xu, S., Zhang, H., Yang, X., Qian, Y. & Xiao, Q. Inhibition of cathepsin L alleviates the microglia-mediated neuroinflammatory responses through caspase-8 and NF-κB pathways. Neurobiol. Aging 62 , 159–167 (2018).
Kettunen, P. et al. SARS-CoV-2 infection of human neurons is TMPRSS2 independent, requires endosomal cell entry, and can be blocked by inhibitors of host phosphoinositol-5 kinase. J. Virol. 97 , e0014423 (2023).
Evans, P. C. et al. Endothelial dysfunction in COVID-19: a position paper of the ESC Working Group for Atherosclerosis and Vascular Biology, and the ESC Council of Basic Cardiovascular Science. Cardiovasc. Res. 116 , 2177–2184 (2020).
Li, C. et al. Critical role of cathepsin L/V in regulating endothelial cell senescence. Biology (Basel) 12 , 42 (2022).
PubMed Google Scholar
Sun, M. et al. Cathepsin-L ameliorates cardiac hypertrophy through activation of the autophagy-lysosomal dependent protein processing pathways. J. Am. Heart Assoc. 2 , e000191 (2013).
Dai, L.-S. et al. Hypertension exacerbates severity and outcomes of COVID-19 in elderly patients: a retrospective observational study. Curr. Med Sci. 42 , 561–568 (2022).
Lu, Y. et al. Angiotensin II-Induced vascular remodeling and hypertension involves cathepsin L/V- MEK/ERK mediated mechanism. Int J. Cardiol. 298 , 98–106 (2020).
Yamashita, T. et al. A potential contribution of altered cathepsin L expression to the development of dermal fibrosis and vasculopathy in systemic sclerosis. Exp. Dermatol. 25 , 287–292 (2016).
Manchanda, M. et al. Cathepsin L and B as potential markers for liver fibrosis: insights from patients and experimental models. Clin. Transl. Gastroenterol. 8 , e99 (2017).
Shah, H., Khan, M. S. H., Dhurandhar, N. V. & Hegde, V. The triumvirate: why hypertension, obesity, and diabetes are risk factors for adverse effects in patients with COVID-19. Acta Diabetol. 58 , 831–843 (2021).
Yang, M. et al. Cathepsin L activity controls adipogenesis and glucose tolerance. Nat. Cell Biol. 9 , 970–977 (2007).
Wrona, M. & Skrypnik, D. New-onset diabetes mellitus, hypertension, dyslipidaemia as sequelae of COVID-19 infection-systematic review. Int J. Environ. Res. Public Health 19 , 13280 (2022).
Barberis, E. et al. Large-scale plasma analysis revealed new mechanisms and molecules associated with the host response to SARS-CoV-2. Int J. Mol. Sci. 21 , 8623 (2020).
Xu, J. et al. Carboxylic submetabolome-driven signature characterization of COVID-19 asymptomatic infection. Talanta 239 , 123086 (2022).
Gambardella, J. et al. Arginine and endothelial function. Biomedicines 8 , 277 (2020).
Durante, W., Johnson, F. K. & Johnson, R. A. Arginase: a critical regulator of nitric oxide synthesis and vascular function. Clin. Exp. Pharm. Physiol. 34 , 906–911 (2007).
Lundberg, J. O. & Weitzberg, E. Nitric oxide signaling in health and disease. Cell 185 , 2853–2878 (2022).
Rees, C. A. et al. Altered amino acid profile in patients with SARS-CoV-2 infection. Proc. Natl Acad. Sci. USA 118 , e2101708118 (2021).
Heyland, D. K. et al. Should immunonutrition become routine in critically ill patients? A systematic review of the evidence. JAMA 286 , 944–953 (2001).
Reizine, F. et al. SARS-CoV-2-induced ARDS associates with MDSC expansion, lymphocyte dysfunction, and arginine shortage. J. Clin. Immunol. 41 , 515–525 (2021).
Adebayo, A. et al. l-Arginine and COVID-19: an update. Nutrients 13 , 3951 (2021).
Tosato, M. et al. Effects of l -arginine plus vitamin c supplementation on physical performance, endothelial function, and persistent fatigue in adults with long COVID: a single-blind randomized controlled trial. Nutrients 14 , 4984 (2022).
Fiorentino, G. et al. Effects of adding L-arginine orally to standard therapy in patients with COVID-19: a randomized, double-blind, placebo-controlled, parallel-group trial. Results of the first interim analysis. EClinicalMedicine 40 , 101125 (2021).
Mone, P. et al. L-Arginine enhances the effects of cardiac rehabilitation on physical performance: new insights for managing cardiovascular patients during the COVID-19 pandemic. J. Pharm. Exp. Ther. 381 , 197–203 (2022).
Grimes, J. M. et al. Arginine depletion as a therapeutic approach for patients with COVID-19. Int J. Infect. Dis. 102 , 566–570 (2021).
Calvani, R. et al. Effects of L-arginine plus vitamin C supplementation on l-arginine metabolism in adults with long COVID: secondary analysis of a randomized clinical trial. Int J. Mol. Sci. 24 , 5078 (2023).
Thomas, T. et al. COVID-19 infection alters kynurenine and fatty acid metabolism, correlating with IL-6 levels and renal status. JCI Insight 5 , e140327 (2020).
Bustamante, S. et al. Tryptophan metabolism ‘hub’ gene expression associates with increased inflammation and severe disease outcomes in COVID-19 infection and inflammatory bowel disease. Int J. Mol. Sci. 23 , 14776 (2022).
Yamamoto, T., Azechi, H. & Board, M. Essential role of excessive tryptophan and its neurometabolites in fatigue. Can. J. Neurol. Sci. 39 , 40–47 (2012).
Yamashita, M. Potential role of neuroactive tryptophan metabolites in central fatigue: establishment of the fatigue circuit. Int. J. Tryptophan Res. 13 , 1178646920936279 (2020).
Eroğlu, İ., Eroğlu, B. Ç. & Güven, G. S. Altered tryptophan absorption and metabolism could underlie long-term symptoms in survivors of coronavirus disease 2019 (COVID-19). Nutrition 90 , 111308 (2021).
Bhat, A., Pires, A. S., Tan, V., Babu Chidambaram, S. & Guillemin, G. J. Effects of sleep deprivation on the tryptophan metabolism. Int. J. Tryptophan Res. 13 , 1178646920970902 (2020).
Sorgdrager, F. J. H., Naudé, P. J. W., Kema, I. P., Nollen, E. A. & Deyn, P. P. D. Tryptophan metabolism in inflammaging: from biomarker to therapeutic target. Front. Immunol. 10 , 2565 (2019).
Al-Hakeim, H. K., Khairi Abed, A., Rouf Moustafa, S., Almulla, A. F. & Maes, M. Tryptophan catabolites, inflammation, and insulin resistance as determinants of chronic fatigue syndrome and affective symptoms in long COVID. Front. Mol. Neurosci. 16 , 1194769 (2023).
Badawy, A. A.-B. The kynurenine pathway of tryptophan metabolism: a neglected therapeutic target of COVID-19 pathophysiology and immunotherapy. Biosci. Rep. 43 , BSR20230595 (2023).
Shakoor, H. et al. Be well: a potential role for vitamin B in COVID-19. Maturitas 144 , 108–111 (2021).
Sen, A. Does serotonin deficiency lead to anosmia, ageusia, dysfunctional chemesthesis and increased severity of illness in COVID-19? Med. Hypotheses 153 , 110627 (2021).
Mahalakshmi, A. M. et al. Alterations in tryptophan metabolism affect vascular functions: connected to ageing population vulnerability to COVID-19 infection? Int. J. Tryptophan Res. 15 , 11786469221083946 (2022).
Vlachou, M., Siamidi, A., Dedeloudi, A., Konstantinidou, S. K. & Papanastasiou, I. P. Pineal hormone melatonin as an adjuvant treatment for COVID‑19 (Review). Int J. Mol. Med. 47 , 47 (2021).
Reiter, R. J. et al. Melatonin inhibits COVID-19-Induced Cytokine Storm by Reversing Aerobic Glycolysis in Immune Cells: A Mechanistic Analysis. Med Drug Discov. 6 , 100044 (2020).
Soria-Castro, R. et al. Severe COVID-19 is marked by dysregulated serum levels of carboxypeptidase A3 and serotonin. J. Leukoc. Biol. 110 , 425–431 (2021).
Jenkins, T. A., Nguyen, J. C. D., Polglaze, K. E. & Bertrand, P. P. Influence of tryptophan and serotonin on mood and cognition with a possible role of the gut-brain axis. Nutrients 8 , 56 (2016).
Kikuchi, A. M., Tanabe, A. & Iwahori, Y. A systematic review of the effect of L-tryptophan supplementation on mood and emotional functioning. J. Diet. Suppl. 18 , 316–333 (2021).
Sutanto, C. N., Loh, W. W. & Kim, J. E. The impact of tryptophan supplementation on sleep quality: a systematic review, meta-analysis, and meta-regression. Nutr. Rev. 80 , 306–316 (2022).
Casey, K., Iteen, A., Nicolini, R. & Auten, J. COVID-19 pneumonia with hemoptysis: acute segmental pulmonary emboli associated with novel coronavirus infection. Am. J. Emerg. Med. 38 , 1544.e1–1544.e3 (2020).
Yağcı, S., Serin, E., Acicbe, Ö., Zeren, M. İ. & Odabaşı, M. S. The relationship between serum erythropoietin, hepcidin, and haptoglobin levels with disease severity and other biochemical values in patients with COVID-19. Int. J. Lab Hematol. 43 , 142–151 (2021).
Lanser, L. et al. Dynamics in anemia development and dysregulation of iron homeostasis in hospitalized patients with COVID-19. Metabolites 11 , 653 (2021).
Soriano, J. B. et al. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 22 , e102–e107 (2022).
Rosa, L., Cutone, A., Lepanto, M. S., Paesano, R. & Valenti, P. Lactoferrin: a natural glycoprotein involved in iron and inflammatory homeostasis. Int J. Mol. Sci. 18 , 1985 (2017).
Naidu, A. S. Natural Food Antimicrobial Systems . (CRC Press, 2000).
Maneva, A., Taleva, B. & Maneva, L. Lactoferrin-protector against oxidative stress and regulator of glycolysis in human erythrocytes. Z. Naturforsch. C. J. Biosci. 58 , 256–262 (2003).
Jegasothy, H., Weerakkody, R., Selby-Pham, S. & Bennett, L. E. In vitro heme and non-heme iron capture from hemoglobin, myoglobin and ferritin by bovine lactoferrin and implications for suppression of reactive oxygen species in vivo. Biometals 27 , 1371–1382 (2014).
Ruan, Q., Yang, K., Wang, W., Jiang, L. & Song, J. Correction to: clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 46 , 1294–1297 (2020).
Bharadwaj, S., Naidu, T. A. G., Betageri, G. V., Prasadarao, N. V. & Naidu, A. S. Inflammatory responses improve with milk ribonuclease-enriched lactoferrin supplementation in postmenopausal women. Inflamm. Res. 59 , 971–978 (2010).
Chang, R., Ng, T. B. & Sun, W.-Z. Lactoferrin as potential preventative and adjunct treatment for COVID-19. Int. J. Antimicrob. Agents 56 , 106118 (2020).
Serrano, G. et al. Liposomal lactoferrin as potential preventative and cure for COVID-19. IJRHS 8 , 08–15 (2020).
Hu, Y., Meng, X., Zhang, F., Xiang, Y. & Wang, J. The in vitro antiviral activity of lactoferrin against common human coronaviruses and SARS-CoV-2 is mediated by targeting the heparan sulfate co-receptor. Emerg. Microbes Infect. 10 , 317–330 (2021).
Naidu, S. A. G. et al. COVID-19 during pregnancy and postpartum. J. Diet. Suppl. 19 , 78–114 (2022).
Lang, G.-J. & Zhao, H. Can SARS-CoV-2-infected women breastfeed after viral clearance? J. Zhejiang Univ. Sci. B 21 , 405–407 (2020).
Pietrantoni, A. et al. Bovine lactoferrin inhibits adenovirus infection by interacting with viral structural polypeptides. Antimicrob. Agents Chemother. 47 , 2688–2691 (2003).
Lang, J. et al. Inhibition of SARS pseudovirus cell entry by lactoferrin binding to heparan sulfate proteoglycans. PLoS ONE 6 , e23710 (2011).
Marr, A. K., Jenssen, H., Moniri, M. R., Hancock, R. E. W. & Panté, N. Bovine lactoferrin and lactoferricin interfere with intracellular trafficking of Herpes simplex virus-1. Biochimie 91 , 160–164 (2009).
Lepanto, M. S. et al. Efficacy of lactoferrin oral administration in the treatment of anemia and anemia of inflammation in pregnant and non-pregnant women: an interventional study. Front. Immunol. 9 , 2123 (2018).
Bonaccorsi di Patti, M. C. et al. The ferroportin-ceruloplasmin system and the mammalian iron homeostasis machine: regulatory pathways and the role of lactoferrin. Biometals 31 , 399–414 (2018).
Zimecki, M., Actor, J. K. & Kruzel, M. L. The potential for Lactoferrin to reduce SARS-CoV-2 induced cytokine storm. Int. Immunopharmacol. 95 , 107571 (2021).
Zwirzitz, A. et al. Lactoferrin is a natural inhibitor of plasminogen activation. J. Biol. Chem. 293 , 8600–8613 (2018).
Marietta, M., Coluccio, V. & Luppi, M. COVID-19, coagulopathy and venous thromboembolism: more questions than answers. Intern. Emerg. Med. 15 , 1375–1387 (2020).
Zakharova, E. T., Kostevich, V. A., Sokolov, A. V. & Vasilyev, V. B. Human apo-lactoferrin as a physiological mimetic of hypoxia stabilizes hypoxia-inducible factor-1 alpha. Biometals 25 , 1247–1259 (2012).
Zakharova, E. T. et al. Erythropoietin and Nrf2: key factors in the neuroprotection provided by apo-lactoferrin. Biometals 31 , 425–443 (2018).
Ibuki, M. et al. Lactoferrin has a therapeutic effect via HIF inhibition in a murine model of choroidal neovascularization. Front. Pharm. 11 , 174 (2020).
Ryter, S. W. & Choi, A. M. K. Targeting heme oxygenase-1 and carbon monoxide for therapeutic modulation of inflammation. Transl. Res. 167 , 7–34 (2016).
Vijayan, V., Wagener, F. A. D. T. G. & Immenschuh, S. The macrophage heme-heme oxygenase-1 system and its role in inflammation. Biochem. Pharm. 153 , 159–167 (2018).
Consoli, V., Sorrenti, V., Grosso, S. & Vanella, L. Heme oxygenase-1 signaling and redox homeostasis in physiopathological conditions. Biomolecules 11 , 589 (2021).
Siu, K.-L. et al. Severe acute respiratory syndrome coronavirus ORF3a protein activates the NLRP3 inflammasome by promoting TRAF3-dependent ubiquitination of ASC. FASEB J. 33 , 8865–8877 (2019).
Batra, N., De Souza, C., Batra, J., Raetz, A. G. & Yu, A.-M. The HMOX1 pathway as a promising target for the treatment and prevention of SARS-CoV-2 of 2019 (COVID-19). IJMS 21 , 6412 (2020).
Rossi, M., Piagnerelli, M., Van Meerhaeghe, A. & Zouaoui Boudjeltia, K. Heme oxygenase-1 (HO-1) cytoprotective pathway: a potential treatment strategy against coronavirus disease 2019 (COVID-19)-induced cytokine storm syndrome. Med. Hypotheses 144 , 110242 (2020).
Mahapatra, S. et al. Antiangiogenic effects and therapeutic targets of azadirachta indica leaf extract in endothelial cells. Evid. Based Complement Altern. Med. 2012 , 303019 (2012).
Chen, C.-Y., Jang, J.-H., Li, M.-H. & Surh, Y.-J. Resveratrol upregulates heme oxygenase-1 expression via activation of NF-E2-related factor 2 in PC12 cells. Biochem. Biophys. Res. Commun. 331 , 993–1000 (2005).
Jeong, S.-O. et al. Dimethoxycurcumin, a synthetic curcumin analogue, induces heme oxygenase-1 expression through Nrf2 activation in RAW264.7 macrophages. J. Clin. Biochem Nutr. 44 , 79–84 (2009).
Yao, P. et al. Quercetin protects human hepatocytes from ethanol-derived oxidative stress by inducing heme oxygenase-1 via the MAPK/Nrf2 pathways. J. Hepatol. 47 , 253–261 (2007).
Krantz, S. B. Erythropoietin. Blood 77 , 419–434 (1991).
Hadadi, A., Mortezazadeh, M., Kolahdouzan, K. & Alavian, G. Does recombinant human erythropoietin administration in critically ill COVID-19 patients have miraculous therapeutic effects? J. Med. Virol. 92 , 915–918 (2020).
Schobersberger, W., Hoffmann, G. & Fandrey, J. Nitric oxide donors suppress erythropoietin production in vitro. Pflug. Arch. 432 , 980–985 (1996).
Viruez-Soto, A. et al. Low serum erythropoietin levels are associated with fatal COVID-19 cases at 4,150 meters above sea level. Respir. Physiol. Neurobiol. 292 , 103709 (2021).
Bhatraju, P. K. et al. Covid-19 in critically Ill patients in the seattle region—case series. N. Engl. J. Med. 382 , 2012–2022 (2020).
Solaimanzadeh, I. Acetazolamide, nifedipine and phosphodiesterase inhibitors: rationale for their utilization as adjunctive countermeasures in the treatment of coronavirus disease 2019 (COVID-19). Cureus 12 , e7343 (2020).
Collantes, M. E. V., Espiritu, A. I., Sy, M. C. C., Anlacan, V. M. M. & Jamora, R. D. G. Neurological manifestations in COVID-19 infection: a systematic review and meta-analysis. Can. J. Neurol. Sci. 48 , 66–76 (2021).
Caravagna, C. & Soliz, J. PI3K and MEK1/2 molecular pathways are involved in the erythropoietin-mediated regulation of the central respiratory command. Respir. Physiol. Neurobiol. 206 , 36–40 (2015).
Peng, T., Du, S.-Y., Son, M. & Diamond, B. HIF-1α is a negative regulator of interferon regulatory factors: Implications for interferon production by hypoxic monocytes. Proc. Natl Acad. Sci. USA 118 , e2106017118 (2021).
Cao, F. et al. Suppression of NLRP3 inflammasome by erythropoietin via the EPOR/JAK2/STAT3 pathway contributes to attenuation of acute lung injury in mice. Front Pharm. 11 , 306 (2020).
Ehrenreich, H. et al. Erythropoietin as candidate for supportive treatment of severe COVID-19. Mol. Med. 26 , 58 (2020).
Soliz, J. et al. Coping with hypoxemia: could erythropoietin (EPO) be an adjuvant treatment of COVID-19? Respir. Physiol. Neurobiol. 279 , 103476 (2020).
Nicolas, G. et al. Lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice. Proc. Natl Acad. Sci. USA 98 , 8780–8785 (2001).
Daher, R. & Karim, Z. Iron metabolism: state of the art. Transfus. Clin. Biol. 24 , 115–119 (2017).
Palaneeswari M, S., Ganesh, M., Karthikeyan, T., Devi, A. J. M. & Mythili, S. V. Hepcidin-minireview. J. Clin. Diagn. Res. 7 , 1767–1771 (2013).
Ravasi, G. et al. Hepcidin expression in iron overload diseases is variably modulated by circulating factors. PLoS ONE 7 , e36425 (2012).
Pietrangelo, A. Hepcidin in human iron disorders: therapeutic implications. J. Hepatol. 54 , 173–181 (2011).
Luo, X. et al. Homocysteine upregulates hepcidin expression through BMP6/SMAD signaling pathway in hepatocytes. Biochem. Biophys. Res. Commun. 471 , 303–308 (2016).
Lei, Y. et al. Calcitonin increases hepatic hepcidin expression through the BMP6 of kidney in mice. J. Trace Elem. Med. Biol. 68 , 126796 (2021).
Zhen, A. W. et al. The small molecule, genistein, increases hepcidin expression in human hepatocytes. Hepatology 58 , 1315–1325 (2013).
Okada, K. et al. Nrf2 inhibits hepatic iron accumulation and counteracts oxidative stress-induced liver injury in nutritional steatohepatitis. J. Gastroenterol. 47 , 924–935 (2012).
Habib, H. M., Ibrahim, S., Zaim, A. & Ibrahim, W. H. The role of iron in the pathogenesis of COVID-19 and possible treatment with lactoferrin and other iron chelators. Biomed. Pharmacother. 136 , 111228 (2021).
Blanchette, N. L., Manz, D. H., Torti, F. M. & Torti, S. V. Modulation of hepcidin to treat iron deregulation: potential clinical applications. Expert Rev. Hematol. 9 , 169–186 (2016).
Banchini, F., Vallisa, D., Maniscalco, P. & Capelli, P. Iron overload and Hepcidin overexpression could play a key role in COVID infection, and may explain vulnerability in elderly, diabetics, and obese patients. Acta Biomed. 91 , e2020013 (2020).
Dixon, S. J. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149 , 1060–1072 (2012).
Jacobs, W. et al. Fatal lymphocytic cardiac damage in coronavirus disease 2019 (COVID-19): autopsy reveals a ferroptosis signature. ESC Heart Fail 7 , 3772–3781 (2020).
Sun, Y. et al. The emerging role of ferroptosis in inflammation. Biomed. Pharmacother. 127 , 110108 (2020).
Stockwell, B. R. & Jiang, X. The chemistry and biology of ferroptosis. Cell Chem. Biol. 27 , 365–375 (2020).
Qiu, Y.-B. et al. Nrf2 protects against seawater drowning-induced acute lung injury via inhibiting ferroptosis. Respir. Res. 21 , 232 (2020).
Tavakol, S. & Seifalian, A. M. Vitamin E at a high dose as an anti-ferroptosis drug and not just a supplement for COVID-19 treatment. Biotechnol. Appl. Biochem. 69 , 1058–1060 (2022).
Bergin, P. et al. The effects of vitamin E supplementation on malondialdehyde as a biomarker of oxidative stress in haemodialysis patients: a systematic review and meta-analysis. BMC Nephrol. 22 , 126 (2021).
Hu, B. et al. Ferrostatin-1 protects auditory hair cells from cisplatin-induced ototoxicity in vitro and in vivo. Biochem. Biophys. Res. Commun. 533 , 1442–1448 (2020).
Miotto, G. et al. Insight into the mechanism of ferroptosis inhibition by ferrostatin-1. Redox Biol. 28 , 101328 (2020).
Shao, Y. et al. Endothelial immunity trained by coronavirus infections, DAMP stimulations and regulated by anti-oxidant NRF2 may contribute to inflammations, myelopoiesis, COVID-19 cytokine storms and thromboembolism. Front. Immunol. 12 , 653110 (2021).
Han, F., Li, S., Yang, Y. & Bai, Z. Interleukin-6 promotes ferroptosis in bronchial epithelial cells by inducing reactive oxygen species-dependent lipid peroxidation and disrupting iron homeostasis. Bioengineered 12 , 5279–5288 (2021).
Ma, H. et al. Melatonin suppresses ferroptosis induced by high glucose via activation of the Nrf2/HO-1 signaling pathway in type 2 diabetic osteoporosis. Oxid. Med. Cell Longev. 2020 , 9067610 (2020).
NaveenKumar, S. K., Hemshekhar, M., Kemparaju, K. & Girish, K. S. Hemin-induced platelet activation and ferroptosis is mediated through ROS-driven proteasomal activity and inflammasome activation: protection by melatonin. Biochim. Biophys. Acta Mol. Basis Dis. 1865 , 2303–2316 (2019).
Lin, C.-C. & Wang, P.-H. Intravenous glycyrrhizin improved serum transaminases rapidly in a chronic hepatitis B patient with acute exacerbation. J. Formos. Med. Assoc. 114 , 188–189 (2015).
Wang, Y., Chen, Q., Shi, C., Jiao, F. & Gong, Z. Mechanism of glycyrrhizin on ferroptosis during acute liver failure by inhibiting oxidative stress. Mol. Med. Rep. 20 , 4081–4090 (2019).
Wang, Y. et al. Quercetin alleviates acute kidney injury by inhibiting ferroptosis. J. Adv. Res. 28 , 231–243 (2021).
Yang, L. et al. Ferroptosis-inhibitory difference between chebulagic acid and chebulinic acid indicates beneficial role of HHDP. Molecules 26 , 4300 (2021).
Guerrero-Hue, M. et al. Curcumin reduces renal damage associated with rhabdomyolysis by decreasing ferroptosis-mediated cell death. FASEB J. 33 , 8961–8975 (2019).
Chen, B. et al. Comparison of ferroptosis-inhibitory mechanisms between ferrostatin-1 and dietary stilbenes (piceatannol and astringin). Molecules 26 , 1092 (2021).
Cantó, C., Menzies, K. J. & Auwerx, J. NAD(+) metabolism and the control of energy homeostasis: a balancing act between mitochondria and the nucleus. Cell Metab. 22 , 31–53 (2015).
Houtkooper, R. H., Cantó, C., Wanders, R. J. & Auwerx, J. The secret life of NAD+: an old metabolite controlling new metabolic signaling pathways. Endocr. Rev. 31 , 194–223 (2010).
Fang, J. et al. NAD+ metabolism-based immunoregulation and therapeutic potential. Cell Biosci. 13 , 81 (2023).
Strømland, Ø., Diab, J., Ferrario, E., Sverkeli, L. J. & Ziegler, M. The balance between NAD+ biosynthesis and consumption in ageing. Mech. Ageing Dev. 199 , 111569 (2021).
Tannous, C. et al. Nicotinamide adenine dinucleotide: biosynthesis, consumption and therapeutic role in cardiac diseases. Acta Physiol. (Oxf.) 231 , e13551 (2021).
Izadpanah, A. et al. SARS-CoV-2 infection dysregulates NAD metabolism. Front. Immunol. 14 , 1158455 (2023).
Jiang, Y. et al. Treatment of SARS-CoV-2-induced pneumonia with NAD+ and NMN in two mouse models. Cell Discov. 8 , 38 (2022).
Li, W. et al. NAD supplement alleviates intestinal barrier injury induced by ethanol via protecting epithelial mitochondrial function. Nutrients 15 , 174 (2022).
de Picciotto, N. E. et al. Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell 15 , 522–530 (2016).
Yoshino, J., Baur, J. A. & Imai, S.-I. NAD+ intermediates: the biology and therapeutic potential of NMN and NR. Cell Metab. 27 , 513–528 (2018).
Daussin, F. N. et al. Dietary cocoa flavanols enhance mitochondrial function in skeletal muscle and modify whole-body metabolism in healthy mice. Nutrients 13 , 3466 (2021).
Omran, H. M. & Almaliki, M. S. Influence of NAD+ as an ageing-related immunomodulator on COVID 19 infection: a hypothesis. J. Infect. Public Health 13 , 1196–1201 (2020).
Ansar, H., Mazloom, Z., Kazemi, F. & Hejazi, N. Effect of alpha-lipoic acid on blood glucose, insulin resistance and glutathione peroxidase of type 2 diabetic patients. Saudi Med. J. 32 , 584–588 (2011).
Hsu, J.-L. et al. Epi-reevesioside F inhibits Na+/K+-ATPase, causing cytosolic acidification, Bak activation and apoptosis in glioblastoma. Oncotarget 6 , 24032–24046 (2015).
Shay, K. P., Moreau, R. F., Smith, E. J., Smith, A. R. & Hagen, T. M. Alpha-lipoic acid as a dietary supplement: molecular mechanisms and therapeutic potential. Biochim. Biophys. Acta 1790 , 1149–1160 (2009).
Zhang, L. & Liu, Y. Potential interventions for novel coronavirus in China: a systematic review. J. Med. Virol. 92 , 479–490 (2020).
Cure, E. & Cumhur Cure, M. Alpha-lipoic acid may protect patients with diabetes against COVID-19 infection. Med Hypotheses 143 , 110185 (2020).
Uberti, F., Ruga, S., Farghali, M., Galla, R. & Molinari, C. A Combination of α-lipoic acid (ALA) and palmitoylethanolamide (PEA) blocks endotoxin-induced oxidative stress and cytokine storm: a possible intervention for COVID-19. J. Diet. Suppl. 20 , 133–155 (2023).
Zhong, M. et al. A randomized, single-blind, group sequential, active-controlled study to evaluate the clinical efficacy and safety of α-lipoic acid for critically ill patients with coronavirus disease 2019 (COVID-19). Front. Med. (Lausanne) 8 , 566609 (2021).
Crane, F. L. Biochemical functions of coenzyme Q10. J. Am. Coll. Nutr. 20 , 591–598 (2001).
Hernández-Camacho, J. D., Bernier, M., López-Lluch, G. & Navas, P. Coenzyme Q10 supplementation in aging and disease. Front. Physiol. 9 , 44 (2018).
Fakhrolmobasheri, M. et al. Coenzyme Q10 and its therapeutic potencies against COVID-19 and other similar infections: a molecular review. Adv. Pharm. Bull. 13 , 233–243 (2023).
Wang, R. et al. Coenzyme Q10 attenuates human platelet aggregation induced by SARS-CoV-2 spike protein via reducing oxidative stress in vitro. Int J. Mol. Sci. 23 , 12345 (2022).
Polymeropoulos, V. M. A potential role of coenzyme Q10 deficiency in severe SARS-cov2 infection. OBM Integrative and Complementary Medicine 5 , (2020).
Pagano, G. et al. Potential roles of mitochondrial cofactors in the adjuvant mitigation of proinflammatory acute infections, as in the case of sepsis and COVID-19 pneumonia. Inflamm. Res. 70 , 159–170 (2021).
Turton, N., Heaton, R. A. & Hargreaves, I. P. COVID-19 and the assessment of coenzyme Q10. Methods Mol. Biol. 2511 , 355–365 (2022).
Sestili, P. et al. Creatine as an antioxidant. Amino Acids 40 , 1385–1396 (2011).
Ostojic, S. M. Diagnostic and pharmacological potency of creatine in post-viral fatigue syndrome. Nutrients 13 , 503 (2021).
Ostojic, S. M. et al. Supplementation with guanidinoacetic acid in women with chronic fatigue syndrome. Nutrients 8 , 72 (2016).
Riesberg, L. A., Weed, S. A., McDonald, T. L., Eckerson, J. M. & Drescher, K. M. Beyond muscles: the untapped potential of creatine. Int. Immunopharmacol. 37 , 31–42 (2016).
Marshall, R. P., Droste, J.-N., Giessing, J. & Kreider, R. B. Role of creatine supplementation in conditions involving mitochondrial dysfunction: a narrative review. Nutrients 14 , 529 (2022).
Yoshii, K., Hosomi, K., Sawane, K. & Kunisawa, J. Metabolism of dietary and microbial vitamin b family in the regulation of host immunity. Front. Nutr. 6 , 48 (2019).
Batista, K. S. et al. The role of vitamin B12 in viral infections: a comprehensive review of its relationship with the muscle-gut-brain axis and implications for SARS-CoV-2 infection. Nutr. Rev. 80 , 561–578 (2022).
Degnan, P. H., Taga, M. E. & Goodman, A. L. Vitamin B12 as a modulator of gut microbial ecology. Cell Metab. 20 , 769–778 (2014).
Wolffenbuttel, B. H. R., Wouters, H. J. C. M., Heiner-Fokkema, M. R. & van der Klauw, M. M. The many faces of cobalamin (Vitamin B12) deficiency. Mayo Clin. Proc. Innov. Qual. Outcomes 3 , 200–214 (2019).
Kandeel, M. & Al-Nazawi, M. Virtual screening and repurposing of FDA approved drugs against COVID-19 main protease. Life Sci. 251 , 117627 (2020).
Laforge, M. et al. Tissue damage from neutrophil-induced oxidative stress in COVID-19. Nat. Rev. Immunol. 20 , 515–516 (2020).
Ahmed, S. A., Alahmadi, Y. M. & Abdou, Y. A. The impact of serum levels of reactive oxygen and nitrogen species on the disease severity of COVID-19. Int J. Mol. Sci. 24 , 8973 (2023).
Wang, Y., Branicky, R., Noë, A. & Hekimi, S. Superoxide dismutases: dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 217 , 1915–1928 (2018).
Roos, G. & Messens, J. Protein sulfenic acid formation: from cellular damage to redox regulation. Free Radic. Biol. Med. 51 , 314–326 (2011).
Weydert, C. J. & Cullen, J. J. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat. Protoc. 5 , 51–66 (2010).
Naidu, A. S. REDOX LIFE . 757 (Bio-Rep Network Media, 2013).
Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: oxidative eustress. Redox Biol. 11 , 613–619 (2017).
Golabi, S. et al. Oxidative stress and inflammatory status in COVID-19 outpatients: a health center-based analytical cross-sectional study. Antioxid. (Basel) 11 , 606 (2022).
Cavalcanti, L. F. et al. Decreased plasma H 2 O 2 levels are associated with the pathogenesis leading to COVID-19 worsening and mortality. Free Radic. Res. 56 , 740–748 (2022).
Yaghoubi, N. et al. Total antioxidant capacity as a marker of severity of COVID-19 infection: possible prognostic and therapeutic clinical application. J. Med. Virol. 94 , 1558–1565 (2022).
Al-Hakeim, H. K., Al-Rubaye, H. T., Al-Hadrawi, D. S., Almulla, A. F. & Maes, M. Long-COVID post-viral chronic fatigue and affective symptoms are associated with oxidative damage, lowered antioxidant defenses and inflammation: a proof of concept and mechanism study. Mol. Psychiatry 28 , 564–578 (2023).
Yasui, K. & Baba, A. Therapeutic potential of superoxide dismutase (SOD) for resolution of inflammation. Inflamm. Res. 55 , 359–363 (2006).
Rosa, A. C., Corsi, D., Cavi, N., Bruni, N. & Dosio, F. Superoxide dismutase administration: a review of proposed human uses. Molecules 26 , 1844 (2021).
Marklund, S. Distribution of CuZn superoxide dismutase and Mn superoxide dismutase in human tissues and extracellular fluids. Acta Physiol. Scand. Suppl. 492 , 19–23 (1980).
Igarashi, R., Hoshino, J., Ochiai, A., Morizawa, Y. & Mizushima, Y. Lecithinized superoxide dismutase enhances its pharmacologic potency by increasing its cell membrane affinity. J. Pharm. Exp. Ther. 271 , 1672–1677 (1994).
CAS Google Scholar
Chen, R. et al. Pharmacokinetics and safety of PC-SOD, a lecithinized recombinant superoxide dismutase, in healthy Chinese subjects: A phase 1, randomized, placebo-controlled, dose-escalation study. Int J. Clin. Pharm. Ther. 57 , 596–602 (2019).
Farella, I., Panza, R., Capozza, M. & Laforgia, N. Lecithinized superoxide dismutase in the past and in the present: any role in the actual pandemia of COVID-19? Biomed. Pharmacother. 141 , 111922 (2021).
Goyal, M. M. & Basak, A. Human catalase: looking for complete identity. Protein Cell 1 , 888–897 (2010).
Bai, J. & Cederbaum, A. I. Mitochondrial catalase and oxidative injury. Biol. Signals Recept. 10 , 189–199 (2001).
Gebicka, L. & Krych-Madej, J. The role of catalases in the prevention/promotion of oxidative stress. J. Inorg. Biochem. 197 , 110699 (2019).
Qian, Y. et al. Evidence for CAT gene being functionally involved in the susceptibility of COVID-19. FASEB J. 35 , e21384 (2021).
Qin, M. et al. An antioxidant enzyme therapeutic for COVID-19. Adv. Mater. 32 , e2004901 (2020).
Qi, C., Chen, Y., Huang, J.-H., Jin, Q.-Z. & Wang, X.-G. Preparation and characterization of catalase-loaded solid lipid nanoparticles based on soybean phosphatidylcholine. J. Sci. Food Agric. 92 , 787–793 (2012).
Wang, W. et al. Dietary catalase supplementation alleviates deoxynivalenol-induced oxidative stress and gut microbiota dysbiosis in broiler chickens. Toxins (Basel) 14 , 830 (2022).
LeBlanc, J. G. et al. Use of superoxide dismutase and catalase producing lactic acid bacteria in TNBS induced Crohn’s disease in mice. J. Biotechnol. 151 , 287–293 (2011).
Del Carmen, S. et al. Genetically engineered immunomodulatory Streptococcus thermophilus strains producing antioxidant enzymes exhibit enhanced anti-inflammatory activities. Appl. Environ. Microbiol. 80 , 869–877 (2014).
Zhou, J. et al. Programmable probiotics modulate inflammation and gut microbiota for inflammatory bowel disease treatment after effective oral delivery. Nat. Commun. 13 , 3432 (2022).
Oestreicher, J. & Morgan, B. Glutathione: subcellular distribution and membrane transport 1. Biochem. Cell Biol. 97 , 270–289 (2019).
Jones, D. P. et al. Redox state of glutathione in human plasma. Free Radic. Biol. Med. 28 , 625–635 (2000).
Marí, M. et al. Mitochondrial glutathione: recent insights and role in disease. Antioxidants (Basel) 9 , 909 (2020).
Hwang, C., Sinskey, A. J. & Lodish, H. F. Oxidized redox state of glutathione in the endoplasmic reticulum. Science 257 , 1496–1502 (1992).
Schafer, F. Q. & Buettner, G. R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 30 , 1191–1212 (2001).
Hayes, J. D., Flanagan, J. U. & Jowsey, I. R. Glutathione transferases. Annu. Rev. Pharm. Toxicol. 45 , 51–88 (2005).
Lillig, C. H., Berndt, C. & Holmgren, A. Glutaredoxin systems. Biochim. Biophys. Acta 1780 , 1304–1317 (2008).
Sies, H. & Reichert, A. S. Selectively addressing mitochondrial glutathione and thioredoxin redox systems. Cell Chem. Biol. 26 , 316–318 (2019).
Forman, H. J., Zhang, H. & Rinna, A. Glutathione: overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 30 , 1–12 (2009).
Marí, M. et al. Mitochondrial glutathione: features, regulation and role in disease. Biochim. Biophys. Acta 1830 , 3317–3328 (2013).
Naik, E. & Dixit, V. M. Mitochondrial reactive oxygen species drive proinflammatory cytokine production. J. Exp. Med. 208 , 417–420 (2011).
Imai, H., Matsuoka, M., Kumagai, T., Sakamoto, T. & Koumura, T. Lipid peroxidation-dependent cell death regulated by GPx4 and ferroptosis. Curr. Top. Microbiol Immunol. 403 , 143–170 (2017).
Polonikov, A. Endogenous deficiency of glutathione as the most likely cause of serious manifestations and death in COVID-19 patients. ACS Infect. Dis. 6 , 1558–1562 (2020).
Theodore, M. et al. Multiple nuclear localization signals function in the nuclear import of the transcription factor Nrf2. J. Biol. Chem. 283 , 8984–8994 (2008).
Sims, A. C. et al. Release of severe acute respiratory syndrome coronavirus nuclear import block enhances host transcription in human lung cells. J. Virol. 87 , 3885–3902 (2013).
Silvagno, F., Vernone, A. & Pescarmona, G. P. The role of glutathione in protecting against the severe inflammatory response triggered by COVID-19. Antioxidants (Basel) 9 , 624 (2020).
Richardson, S. et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 323 , 2052–2059 (2020).
Zamora-Mendoza, R. et al. Dysregulation of mitochondrial function and biogenesis modulators in adipose tissue of obese children. Int J. Obes. (Lond.) 42 , 618–624 (2018).
Glassman, I. et al. The Role of Glutathione in Prevention of COVID-19 Immunothrombosis: A Review. Front Biosci. (Landmark Ed.) 28 , 59 (2023).
Ursini, F. & Maiorino, M. Lipid peroxidation and ferroptosis: the role of GSH and GPx4. Free Radic. Biol. Med. 152 , 175–185 (2020).
Vaira, L. A., Salzano, G., Deiana, G. & De Riu, G. Anosmia and Ageusia: common findings in COVID-19 patients. Laryngoscope 130 , 1787 (2020).
Maiorino, M. et al. Probing the presumed catalytic triad of selenium-containing peroxidases by mutational analysis of phospholipid hydroperoxide glutathione peroxidase (PHGPx). Biol. Chem. Hoppe Seyler 376 , 651–660 (1995).
Guloyan, V. et al. Glutathione supplementation as an adjunctive therapy in COVID-19. Antioxidants (Basel) 9 , 914 (2020).
Tardiolo, G., Bramanti, P. & Mazzon, E. Overview on the effects of N-acetylcysteine in neurodegenerative diseases. Molecules 23 , 3305 (2018).
Ibrahim, H. et al. Therapeutic blockade of inflammation in severe COVID-19 infection with intravenous N-acetylcysteine. Clin. Immunol. 219 , 108544 (2020).
Soto, M. E. et al. N-Acetyl cysteine restores the diminished activity of the antioxidant enzymatic system caused by SARS-CoV-2 infection: preliminary findings. Pharm. (Basel) 16 , 591 (2023).
du Preez, H. N., Aldous, C., Kruger, H. G. & Johnson, L. N-Acetylcysteine and other sulfur-donors as a preventative and adjunct therapy for COVID-19. Adv. Pharm. Pharm. Sci. 2022 , 4555490 (2022).
Izquierdo, J. L. et al. Use of N-Acetylcysteine at high doses as an oral treatment for patients hospitalized with COVID-19. Sci. Prog. 105 , 368504221074574 (2022).
Avdeev, S. N., Gaynitdinova, V. V., Merzhoeva, Z. M. & Berikkhanov, Z. G.-M. N-acetylcysteine for the treatment of COVID-19 among hospitalized patients. J. Infect. 84 , 94–118 (2022).
Faverio, P. et al. Impact of N-acetyl-l-cysteine on SARS-CoV-2 pneumonia and its sequelae: results from a large cohort study. ERJ Open Res. 8 , 00542–02021 (2022).
Bhattacharya, R. et al. The beneficial role of N-acetylcysteine as an adjunctive drug in treatment of COVID-19 patients in a tertiary care hospital in India: an observational study. Int J. Res. Med. Sci. 8 , 3518 (2020).
De Flora, S., Balansky, R. & La Maestra, S. Rationale for the use of N-acetylcysteine in both prevention and adjuvant therapy of COVID-19. FASEB J. 34 , 13185–13193 (2020).
Shi, Z. & Puyo, C. A. N-Acetylcysteine to combat COVID-19: an evidence review. Ther. Clin. Risk Manag. 16 , 1047–1055 (2020).
Micheletto, C., Izquierdo, J. L., Avdeev, S. N., Rada Escobar, R. A. & Pacheco Gallego, M. C. N-acetylcysteine as a therapeutic approach to post-COVID-19 pulmonary fibrosis adjunctive treatment. Eur. Rev. Med. Pharm. Sci. 26 , 4872–4880 (2022).
Mohajeri, M., Horriatkhah, E. & Mohajery, R. The effect of glutamine supplementation on serum levels of some inflammatory factors, oxidative stress, and appetite in COVID-19 patients: a case-control study. Inflammopharmacology 29 , 1769–1776 (2021).
Chernyak, B. V. et al. COVID-19 and oxidative stress. Biochemistry (Mosc.) 85 , 1543–1553 (2020).
Iddir, M. et al. Strengthening the immune system and reducing inflammation and oxidative stress through diet and nutrition: considerations during the COVID-19 crisis. Nutrients 12 , 1562 (2020).
Cruzat, V., Macedo Rogero, M., Noel Keane, K., Curi, R. & Newsholme, P. Glutamine: metabolism and immune function, supplementation and clinical translation. Nutrients 10 , 1564 (2018).
Shen, C.-Y. et al. The development of maillard reaction, and advanced glycation end product (AGE)-receptor for AGE (RAGE) signaling inhibitors as novel therapeutic strategies for patients with age-related diseases. Molecules 25 , 5591 (2020).
Nooshkam, M., Varidi, M. & Bashash, M. The Maillard reaction products as food-born antioxidant and antibrowning agents in model and real food systems. Food Chem. 275 , 644–660 (2019).
Zhang, R. et al. Increase of rutin antioxidant activity by generating Maillard reaction products with lysine. Bioorg. Med. Chem. Lett. 26 , 2680–2684 (2016).
Liu, H. et al. Enhancing the antioxidative effects of foods containing rutin and α-amino acids via the Maillard reaction: A model study focusing on rutin-lysine system. J. Food Biochem. 44 , e13086 (2020).
González, P., Lozano, P., Ros, G. & Solano, F. Hyperglycemia and oxidative stress: an integral, updated and critical overview of their metabolic interconnections. Int J. Mol. Sci. 24 , 9352 (2023).
Matías-Pérez, D. et al. Relationship of quercetin intake and oxidative stress in persistent COVID. Front. Nutr. 10 , 1278039 (2023).
Glinsky, G. V. Tripartite combination of candidate pandemic mitigation agents: vitamin D, quercetin, and estradiol manifest properties of medicinal agents for targeted mitigation of the COVID-19 pandemic defined by genomics-guided tracing of SARS-CoV-2 targets in human cells. Biomedicines 8 , 129 (2020).
Salamanna, F., Maglio, M., Landini, M. P. & Fini, M. Platelet functions and activities as potential hematologic parameters related to Coronavirus Disease 2019 (Covid-19). Platelets 31 , 627–632 (2020).
Wächter, K. et al. AGE-rich bread crust extract boosts oxidative stress interception via stimulation of the NRF2 pathway. Nutrients 13 , 3874 (2021).
Ruhs, S. et al. Preconditioning with Maillard reaction products improves antioxidant defence leading to increased stress tolerance in cardiac cells. Exp. Gerontol. 45 , 752–762 (2010).
Li, Z. et al. Simultaneous ultrasound and heat enhance functional properties of glycosylated lactoferrin. Molecules 25 , 5774 (2020).
Park, C. H., Choi, J. S. & Yokozawa, T. Increase in the hydroxyl radical-scavenging activity of Panax ginseng and ginsenosides by heat-processing. Drug Discov. Ther. 12 , 114–121 (2018).
Oh, N. S. et al. Chemical characteristics and enhanced hepatoprotective activities of Maillard reaction products derived from milk protein-sugar system. J. Dairy Sci. 99 , 947–958 (2016).
Oh, N. S. et al. Preventive effect of fermented Maillard reaction products from milk proteins in cardiovascular health. J. Dairy Sci. 97 , 3300–3313 (2014).
He, S. et al. Potential effects of rapeseed peptide Maillard reaction products on aging-related disorder attenuation and gut microbiota modulation in d-galactose induced aging mice. Food Funct. 10 , 4291–4303 (2019).
Flanagan, J. L., Simmons, P. A., Vehige, J., Willcox, M. D. & Garrett, Q. Role of carnitine in disease. Nutr. Metab. (Lond.) 7 , 30 (2010).
Mirabito Colafella, K. M., Bovée, D. M. & Danser, A. H. J. The renin-angiotensin-aldosterone system and its therapeutic targets. Exp. Eye Res. 186 , 107680 (2019).
Beyerstedt, S., Casaro, E. B. & Rangel, É. B. COVID-19: angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur. J. Clin. Microbiol. Infect. Dis. 40 , 905–919 (2021).
Blanca, A. J. et al. l-Carnitine ameliorates the oxidative stress response to angiotensin II by modulating NADPH oxidase through a reduction in protein kinase c activity and NF-κB translocation to the nucleus. Food Chem. 228 , 356–366 (2017).
Naureen, Z. et al. Proposal of a food supplement for the management of post-COVID syndrome. Eur. Rev. Med Pharm. Sci. 25 , 67–73 (2021).
Vaziri-Harami, R. & Delkash, P. Can l-carnitine reduce post-COVID-19 fatigue? Ann. Med. Surg. (Lond.) 73 , 103145 (2022).
Matsui, H. et al. L-Carnitine supplementation reduces the general fatigue of cancer patients during chemotherapy. Mol. Clin. Oncol. 8 , 413–416 (2018).
Vasiljevski, E. R. et al. L-carnitine supplementation for muscle weakness and fatigue in children with neurofibromatosis type 1: a Phase 2a clinical trial. Am. J. Med. Genet. A 185 , 2976–2985 (2021).
Marx, W. et al. Efficacy and effectiveness of carnitine supplementation for cancer-related fatigue: a systematic literature review and meta-analysis. Nutrients 9 , 1224 (2017).
Reuter, S. E. & Evans, A. M. Long-chain acylcarnitine deficiency in patients with chronic fatigue syndrome. Potential involvement of altered carnitine palmitoyltransferase-I activity. J. Intern. Med. 270 , 76–84 (2011).
Virgens, I. P. A., Santana, N. M., Lima, S. C. V. C. & Fayh, A. P. T. Can COVID-19 be a risk for cachexia for patients during intensive care? Narrative review and nutritional recommendations. Br. J. Nutr. 126 , 552–560 (2021).
Miedema, J. R. et al. Antibodies against angiotensin II receptor type 1 and endothelin A receptor are increased in COVID-19 patients. Front. Immunol. 14 , 1204433 (2023).
Mayi, B. S. et al. The role of Neuropilin-1 in COVID-19. PLoS Pathog. 17 , e1009153 (2021).
Wang, C. et al. ApoE-isoform-dependent SARS-CoV-2 neurotropism and cellular response. Cell Stem Cell 28 , 331–342.e5 (2021).
Chakravarty, N. et al. Neurological pathophysiology of SARS-CoV-2 and pandemic potential RNA viruses: a comparative analysis. FEBS Lett. 595 , 2854–2871 (2021).
Ellul, M. A. et al. Neurological associations of COVID-19. Lancet Neurol. 19 , 767–783 (2020).
Hugon, J., Msika, E.-F., Queneau, M., Farid, K. & Paquet, C. Long COVID: cognitive complaints (brain fog) and dysfunction of the cingulate cortex. J. Neurol. 269 , 44–46 (2022).
Boldrini, M., Canoll, P. D. & Klein, R. S. How COVID-19 affects the brain. JAMA Psychiatry 78 , 682–683 (2021).
Nuzzo, D. et al. Post-acute COVID-19 neurological syndrome: a new medical challenge. J. Clin. Med 10 , 1947 (2021).
Diem, L. et al. Fatigue in post-COVID-19 syndrome: clinical phenomenology, comorbidities and association with initial course of COVID-19. J. Cent. Nerv. Syst. Dis. 14 , 11795735221102727 (2022).
Gunata, M., Parlakpinar, H. & Acet, H. A. Melatonin: a review of its potential functions and effects on neurological diseases. Rev. Neurol. (Paris) 176 , 148–165 (2020).
Gorman, M. R. Temporal organization of pineal melatonin signaling in mammals. Mol. Cell Endocrinol. 503 , 110687 (2020).
Yip, H.-K. et al. Melatonin treatment improves adipose-derived mesenchymal stem cell therapy for acute lung ischemia-reperfusion injury. J. Pineal Res. 54 , 207–221 (2013).
Chitimus, D. M. et al. Melatonin’s impact on antioxidative and anti-inflammatory reprogramming in homeostasis and disease. Biomolecules 10 , 1211 (2020).
Cardinali, D. P., Brown, G. M. & Pandi-Perumal, S. R. Possible application of melatonin in long COVID. Biomolecules 12 , 1646 (2022).
Galano, A., Tan, D.-X. & Reiter, R. J. Melatonin: a versatile protector against oxidative dna damage. Molecules 23 , 530 (2018).
Muñoz-Jurado, A. et al. Melatonin and multiple sclerosis: antioxidant, anti-inflammatory and immunomodulator mechanism of action. Inflammopharmacology 30 , 1569–1596 (2022).
Habtemariam, S. et al. Melatonin and respiratory diseases: a review. Curr. Top. Med. Chem. 17 , 467–488 (2017).
Hardeland, R. Melatonin and inflammation-story of a double-edged blade. J. Pineal Res. 65 , e12525 (2018).
Ahmadi, Z. & Ashrafizadeh, M. Melatonin as a potential modulator of Nrf2. Fundam. Clin. Pharm. 34 , 11–19 (2020).
Reiter, R. J. et al. Melatonin mitigates mitochondrial meltdown: interactions with SIRT3. Int J. Mol. Sci. 19 , 2439 (2018).
Tan, D.-X. & Hardeland, R. Targeting host defense system and rescuing compromised mitochondria to increase tolerance against pathogens by melatonin may impact outcome of deadly virus infection pertinent to COVID-19. Molecules 25 , 4410 (2020).
Wichniak, A., Kania, A., Siemiński, M. & Cubała, W. J. Melatonin as a potential adjuvant treatment for COVID-19 Beyond Sleep Disorders. Int J. Mol. Sci. 22 , 8623 (2021).
Wang, X.-C., Wu, G.-L., Cai, Y.-F. & Zhang, S.-J. The safety and efficacy of melatonin in the treatment of COVID-19: a systematic review and meta-analysis. Med. (Baltim.) 101 , e30874 (2022).
Mousavi, S. A. et al. Melatonin effects on sleep quality and outcomes of COVID-19 patients: an open-label, randomized, controlled trial. J. Med Virol. 94 , 263–271 (2022).
Bologna, C., Madonna, P. & Pone, E. Efficacy of prolonged-release melatonin 2 mg (PRM 2 mg) prescribed for insomnia in hospitalized patients for COVID-19: a retrospective observational study. J. Clin. Med 10 , 5857 (2021).
Comai, S., Ochoa-Sanchez, R. & Gobbi, G. Sleep-wake characterization of double MT 1 /MT 2 receptor knockout mice and comparison with MT 1 and MT 2 receptor knockout mice. Behav. Brain Res. 243 , 231–238 (2013).
Zhang, R. et al. COVID-19: melatonin as a potential adjuvant treatment. Life Sci. 250 , 117583 (2020).
Bertram, S. et al. Cleavage and activation of the severe acute respiratory syndrome coronavirus spike protein by human airway trypsin-like protease. J. Virol. 85 , 13363–13372 (2011).
Hatesuer, B. et al. Tmprss2 is essential for influenza H1N1 virus pathogenesis in mice. PLoS Pathog. 9 , e1003774 (2013).
Yasuoka, S. et al. Purification, characterization, and localization of a novel trypsin-like protease found in the human airway. Am. J. Respir. Cell Mol. Biol. 16 , 300–308 (1997).
Abboud, R. T. & Vimalanathan, S. Pathogenesis of COPD. Part I. The role of protease-antiprotease imbalance in emphysema. Int J. Tuberc. Lung Dis. 12 , 361–367 (2008).
Kersul, A. L. et al. Molecular mechanisms of inflammation during exacerbations of chronic obstructive pulmonary disease. Arch. Bronconeumol. 47 , 176–183 (2011).
Meyer, M. & Jaspers, I. Respiratory protease/antiprotease balance determines susceptibility to viral infection and can be modified by nutritional antioxidants. Am. J. Physiol. Lung Cell Mol. Physiol. 308 , L1189–L1201 (2015).
Benzie, I. F. Evolution of antioxidant defence mechanisms. Eur. J. Nutr. 39 , 53–61 (2000).
Giudice, A., Arra, C. & Turco, M. C. Review of molecular mechanisms involved in the activation of the Nrf2-ARE signaling pathway by chemopreventive agents. Methods Mol. Biol. 647 , 37–74 (2010).
Schultz, M. A. et al. Nrf1 and Nrf2 transcription factors regulate androgen receptor transactivation in prostate cancer cells. PLoS ONE 9 , e87204 (2014).
Kesic, M. J., Simmons, S. O., Bauer, R. & Jaspers, I. Nrf2 expression modifies influenza A entry and replication in nasal epithelial cells. Free Radic. Biol. Med. 51 , 444–453 (2011).
Lee, Y.-J. & Lee, S.-H. Sulforaphane induces antioxidative and antiproliferative responses by generating reactive oxygen species in human bronchial epithelial BEAS-2B cells. J. Korean Med. Sci. 26 , 1474–1482 (2011).
Catanzaro, M. et al. Immune response in COVID-19: addressing a pharmacological challenge by targeting pathways triggered by SARS-CoV-2. Signal Transduct. Target Ther. 5 , 84 (2020).
Holdoway, A. Nutritional management of patients during and after COVID-19 illness. Br. J. Community Nurs. 25 , S6–S10 (2020).
Antwi, J., Appiah, B., Oluwakuse, B. & Abu, B. A. Z. The nutrition-COVID-19 interplay: a review. Curr. Nutr. Rep. 10 , 364–374 (2021).
Junaid, K. et al. Effective immune functions of micronutrients against SARS-CoV-2. Nutrients 12 , 2992 (2020).
Crowe, F. L. et al. Plasma concentrations of 25-hydroxyvitamin D in meat eaters, fish eaters, vegetarians and vegans: results from the EPIC-Oxford study. Public Health Nutr. 14 , 340–346 (2011).
Holick, M. F. Cancer, sunlight and vitamin D. J. Clin. Transl. Endocrinol. 1 , 179–186 (2014).
Kumar, R., Rathi, H., Haq, A., Wimalawansa, S. J. & Sharma, A. Putative roles of vitamin D in modulating immune response and immunopathology associated with COVID-19. Virus Res. 292 , 198235 (2021).
Aranow, C. Vitamin D and the immune system. J. Investig. Med. 59 , 881–886 (2011).
Barrea, L. et al. Vitamin D: a role also in long COVID-19? Nutrients 14 , 1625 (2022).
Grant, W. B. et al. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients 12 , 988 (2020).
Cohen-Lahav, M., Shany, S., Tobvin, D., Chaimovitz, C. & Douvdevani, A. Vitamin D decreases NFkappaB activity by increasing IkappaBalpha levels. Nephrol. Dial. Transpl. 21 , 889–897 (2006).
Shenoy, S. Gut microbiome, vitamin D, ACE2 interactions are critical factors in immune-senescence and inflammaging: key for vaccine response and severity of COVID-19 infection. Inflamm. Res. 71 , 13–26 (2022).
Basaran, N. et al. The relationship between vitamin D and the severity of COVID-19. Bratisl. Lek. Listy 122 , 200–205 (2021).
Weir, E. K., Thenappan, T., Bhargava, M. & Chen, Y. Does vitamin D deficiency increase the severity of COVID-19? Clin. Med. (Lond.) 20 , e107–e108 (2020).
Pereira, M., Dantas Damascena, A., Galvão Azevedo, L. M., de Almeida Oliveira, T. & da Mota Santana, J. Vitamin D deficiency aggravates COVID-19: systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 62 , 1308–1316 (2022).
Daneshkhah, A. et al. Evidence for possible association of vitamin D status with cytokine storm and unregulated inflammation in COVID-19 patients. Aging Clin. Exp. Res. 32 , 2141–2158 (2020).
di Filippo, L. et al. Low vitamin D levels are associated with long COVID syndrome in COVID-19 survivors. J. Clin. Endocrinol. Metab. 108 , e1106–e1116 (2023).
Barrea, L. et al. Vitamin D in obesity and obesity-related diseases: an overview. Minerva Endocrinol. (Torino) 46 , 177–192 (2021).
Ramirez, A. M. et al. Vitamin D inhibition of pro-fibrotic effects of transforming growth factor beta1 in lung fibroblasts and epithelial cells. J. Steroid Biochem. Mol. Biol. 118 , 142–150 (2010).
Ahmad, S. et al. Vitamin D and its therapeutic relevance in pulmonary diseases. J. Nutr. Biochem. 90 , 108571 (2021).
Salabei, J. K. et al. COVID-19 and the cardiovascular system: an update. Am. J. Med. Sci. 364 , 139–147 (2022).
Acharya, P. et al. The effects of vitamin D supplementation and 25-hydroxyvitamin D levels on the risk of myocardial infarction and mortality. J. Endocr. Soc. 5 , bvab124 (2021).
Oristrell, J. et al. Vitamin D supplementation and COVID-19 risk: a population-based, cohort study. J. Endocrinol. Invest. 45 , 167–179 (2022).
Gold, J. E., Okyay, R. A., Licht, W. E. & Hurley, D. J. Investigation of Long COVID Prevalence and Its Relationship to Epstein-Barr Virus Reactivation. Pathogens 10 , 763 (2021).
Røsjø, E. et al. Effect of high-dose vitamin D3 supplementation on antibody responses against Epstein-Barr virus in relapsing-remitting multiple sclerosis. Mult. Scler. 23 , 395–402 (2017).
Zemb, P. et al. Vitamin D deficiency and the COVID-19 pandemic. J. Glob. Antimicrob. Resist 22 , 133–134 (2020).
Calder, P. C. Omega-3 polyunsaturated fatty acids and inflammatory processes: nutrition or pharmacology? Br. J. Clin. Pharm. 75 , 645–662 (2013).
Hathaway, D. et al. Omega 3 fatty acids and COVID-19: a comprehensive review. Infect. Chemother. 52 , 478–495 (2020).
Gutiérrez, S., Svahn, S. L. & Johansson, M. E. Effects of omega-3 fatty acids on immune cells. Int J. Mol. Sci. 20 , 5028 (2019).
Fonnesu, R. et al. Palmitoylethanolamide (PEA) inhibits SARS-CoV-2 entry by interacting with S protein and ACE-2 receptor. Viruses 14 , 1080 (2022).
Carmo, A. et al. Clearance and persistence of SARS-CoV-2 RNA in patients with COVID-19. J. Med. Virol. 92 , 2227–2231 (2020).
Park, S.-K. et al. Detection of SARS-CoV-2 in fecal samples from patients with asymptomatic and mild COVID-19 in Korea. Clin. Gastroenterol. Hepatol. 19 , 1387–1394.e2 (2021).
Yang, C.-P., Chang, C.-M., Yang, C.-C., Pariante, C. M. & Su, K.-P. Long COVID and long chain fatty acids (LCFAs): Psychoneuroimmunity implication of omega-3 LCFAs in delayed consequences of COVID-19. Brain Behav. Immun. 103 , 19–27 (2022).
Calder, P. C., Carr, A. C., Gombart, A. F. & Eggersdorfer, M. Optimal nutritional status for a well-functioning immune system is an important factor to protect against viral infections. Nutrients 12 , 1181 (2020).
Zapata, B. et al. Omega-3 index and clinical outcomes of severe COVID-19: preliminary results of a cross-sectional study. Int J. Environ. Res. Public Health 18 , 7722 (2021).
Ramírez-Santana, M. et al. Inverse association between omega-3 index and severity of COVID-19: a case-control study. Int J. Environ. Res. Public Health 19 , 6445 (2022).
Erdem, D., Segmen, F., Uysal, E. & Kilicarslan, G. Effect of omega-3 fatty acid use on sepsis and mortality in patients with Covıd-19. Niger. J. Clin. Pr. 26 , 102–108 (2023).
Linster, C. L. & Van Schaftingen, E. Vitamin C. Biosynthesis, recycling and degradation in mammals. FEBS J. 274 , 1–22 (2007).
Carr, A. C. & Maggini, S. Vitamin C and immune function. Nutrients 9 , 1211 (2017).
Sun, L. et al. Therapeutic effects of high-dose vitamin C supplementation in patients with covid-19: A meta-analysis. Nutr. Rev. https://doi.org/10.1093/nutrit/nuad105 (2023).
Olczak-Pruc, M. et al. Vitamin C supplementation for the treatment of COVID-19: a systematic review and meta-analysis. Nutrients 14 , 4217 (2022).
Hafez, W. et al. Vitamin C as a potential interplaying factor between obesity and COVID-19 outcome. Healthcare (Basel) 11 , 93 (2022).
Natarajan, A. et al. Gastrointestinal symptoms and fecal shedding of SARS-CoV-2 RNA suggest prolonged gastrointestinal infection. Med 3 , 371–387.e9 (2022).
Marasco, G. et al. Implications of SARS-CoV-2 infection for neurogastroenterology. Neurogastroenterol. Motil. 33 , e14104 (2021).
Adak, A. & Khan, M. R. An insight into gut microbiota and its functionalities. Cell Mol. Life Sci. 76 , 473–493 (2019).
Suez, J. et al. Personalized microbiome-driven effects of non-nutritive sweeteners on human glucose tolerance. Cell 185 , 3307–3328.e19 (2022).
Sajdel-Sulkowska, E. M. Neuropsychiatric ramifications of COVID-19: short-chain fatty acid deficiency and disturbance of microbiota-gut-brain axis signaling. Biomed. Res. Int 2021 , 7880448 (2021).
Xiao, N. et al. Integrated cytokine and metabolite analysis reveals immunometabolic reprogramming in COVID-19 patients with therapeutic implications. Nat. Commun. 12 , 1618 (2021).
Nataf, S. & Pays, L. Molecular insights into SARS-CoV2-induced alterations of the gut/brain axis. Int J. Mol. Sci. 22 , 10440 (2021).
Naidu, A. S., Bidlack, W. R. & Clemens, R. A. Probiotic spectra of lactic acid bacteria (LAB). Crit. Rev. Food Sci. Nutr. 39 , 13–126 (1999).
Kasti, A. N., Synodinou, K. D., Pyrousis, I. A., Nikolaki, M. D. & Triantafyllou, K. D. Probiotics regulating inflammation via NLRP3 inflammasome modulation: a potential therapeutic approach for COVID-19. Microorganisms 9 , 2376 (2021).
d’Ettorre, G. et al. Challenges in the management of SARS-CoV2 infection: the role of oral bacteriotherapy as complementary therapeutic strategy to avoid the progression of COVID-19. Front. Med. (Lausanne) 7 , 389 (2020).
Wu, C. et al. The volatile and heterogeneous gut microbiota shifts of COVID-19 patients over the course of a probiotics-assisted therapy. Clin. Transl. Med. 11 , e643 (2021).
Pham, M. T. et al. Gut probiotic Lactobacillus rhamnosus attenuates PDE4B-mediated interleukin-6 induced by SARS-CoV-2 membrane glycoprotein. J. Nutr. Biochem. 98 , 108821 (2021).
Gutiérrez-Castrellón, P. et al. Probiotic improves symptomatic and viral clearance in Covid19 outpatients: a randomized, quadruple-blinded, placebo-controlled trial. Gut Microbes 14 , 2018899 (2022).
Xu, J. et al. Boosting vaccine-elicited respiratory mucosal and systemic COVID-19 immunity in mice with the oral Lactobacillus plantarum. Front Nutr. 8 , 789242 (2021).
Fernández-Ferreiro, A. et al. Effects of loigolactobacillus coryniformis K8 CECT 5711 on the immune response of elderly subjects to COVID-19 vaccination: a randomized controlled trial. Nutrients 14 , 228 (2022).
Synodinou, K. D., Nikolaki, M. D., Triantafyllou, K. & Kasti, A. N. Immunomodulatory effects of probiotics on COVID-19 infection by targeting the gut-lung axis microbial cross-talk. Microorganisms 10 , 1764 (2022).
Smith, M., Honce, R. & Schultz-Cherry, S. Metabolic syndrome and viral pathogenesis: lessons from influenza and coronaviruses. J. Virol. 94 , e00665–20 (2020).
Thakur, B. et al. A systematic review and meta-analysis of geographic differences in comorbidities and associated severity and mortality among individuals with COVID-19. Sci. Rep. 11 , 8562 (2021).
Apicella, M. et al. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 8 , 782–792 (2020).
Stefan, N., Birkenfeld, A. L. & Schulze, M. B. Global pandemics interconnected - obesity, impaired metabolic health and COVID-19. Nat. Rev. Endocrinol. 17 , 135–149 (2021).
Schwarz, P. E. H. et al. Blood sugar regulation for cardiovascular health promotion and disease prevention: JACC Health Promotion Series. J. Am. Coll. Cardiol. 72 , 1829–1844 (2018).
Santos, A., Magro, D. O., Evangelista-Poderoso, R. & Saad, M. J. A. Diabetes, obesity, and insulin resistance in COVID-19: molecular interrelationship and therapeutic implications. Diabetol. Metab. Syndr. 13 , 23 (2021).
Montefusco, L. et al. Acute and long-term disruption of glycometabolic control after SARS-CoV-2 infection. Nat. Metab. 3 , 774–785 (2021).
Steenblock, C. et al. COVID-19 and metabolic disease: mechanisms and clinical management. Lancet Diabetes Endocrinol. 9 , 786–798 (2021).
Wu, C.-T. et al. SARS-CoV-2 infects human pancreatic β cells and elicits β cell impairment. Cell Metab. 33 , 1565–1576.e5 (2021).
Ji, N. et al. SARS-CoV-2 in the pancreas and the impaired islet function in COVID-19 patients. Emerg. Microbes Infect. 11 , 1115–1125 (2022).
Zhang, C. et al. Liposome-embedded SOD attenuated DSS-induced ulcerative colitis in mice by ameliorating oxidative stress and intestinal barrier dysfunction. Food Funct. 14 , 4392–4405 (2023).
Guo, J. et al. Glucose-lowering effects of orally administered superoxide dismutase in type 2 diabetic model rats. NPJ Sci. Food 6 , 36 (2022).
Kreutmair, S. et al. Distinct immunological signatures discriminate severe COVID-19 from non-SARS-CoV-2-driven critical pneumonia. Immunity 54 , 1578–1593.e5 (2021).
Gupte, M. et al. ACE2 is expressed in mouse adipocytes and regulated by a high-fat diet. Am. J. Physiol. Regul. Integr. Comp. Physiol. 295 , R781–R788 (2008).
Reiterer, M. et al. Hyperglycemia in acute COVID-19 is characterized by insulin resistance and adipose tissue infectivity by SARS-CoV-2. Cell Metab. 33 , 2174–2188.e5 (2021).
Guo, W. et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab. Res. Rev. 36 , e3319 (2020).
Zickler, M. et al. Replication of SARS-CoV-2 in adipose tissue determines organ and systemic lipid metabolism in hamsters and humans. Cell Metab. 34 , 1–2 (2022).
Huang, K. et al. Traditional Chinese Medicine (TCM) in the treatment of COVID-19 and other viral infections: efficacies and mechanisms. Pharm. Ther. 225 , 107843 (2021).
Tsai, K.-C. et al. A traditional Chinese medicine formula NRICM101 to target COVID-19 through multiple pathways: a bedside-to-bench study. Biomed. Pharmacother. 133 , 111037 (2021).
Li, L. et al. Effects of Chinese medicine on symptoms, syndrome evolution, and lung inflammation absorption in COVID-19 convalescent patients during 84-day follow-up after hospital discharge: a prospective cohort and nested case-control study. Chin. J. Integr. Med. 27 , 245–251 (2021).
Jiang, L. et al. The pathological mechanism of the COVID-19 convalescence and its treatment with traditional Chinese medicine. Front. Pharm. 13 , 1054312 (2022).
Wang, X. et al. Treatment of COVID-19 anxiety by auricular points: a protocol for systematic review and meta-analysis. Medicine (Baltimore) 101 , e28984 (2022).
Volf, N., Salques, V. & Lassaux, A. An auricular marker for COVID-19. Med. Acupunct. 32 , 174–175 (2020).
Luo, Y. et al. The beneficial role of auricular point pressure in insomnia and anxiety in isolated COVID-19 patients. Evid. Based Complement Altern. Med. 2021 , 6611942 (2021).
Pan, W.-X., Fan, A. Y., Chen, S. & Alemi, S. F. Acupuncture modulates immunity in sepsis: toward a science-based protocol. Auton. Neurosci. 232 , 102793 (2021).
Feng, B.-W. & Rong, P.-J. Acupoint stimulation for long COVID: A promising intervention. World J. Acupunct. Moxibustion 33 , 191–197 (2023).
Ho, P. et al. Perspective adjunctive therapies for COVID-19: beyond antiviral therapy. Int J. Med. Sci. 18 , 314–324 (2021).
Naidu, S. A. G., Mustafa, G., Clemens, R. A. & Naidu, A. S. Plant-Derived Natural Non-Nucleoside Analog Inhibitors (NNAIs) against RNA-dependent RNA polymerase complex (nsp7/nsp8/nsp12) of SARS-CoV-2. J. Diet. Suppl. 20 , 254–283 (2023).
Naidu, S. A. G., Tripathi, Y. B., Shree, P., Clemens, R. A. & Naidu, A. S. Phytonutrient inhibitors of SARS-CoV-2/NSP5-Encoded Main Protease (Mpro) autocleavage enzyme critical for COVID-19 pathogenesis. J. Diet. Suppl. 20 , 284–311 (2023).
Bachar, S. C., Mazumder, K., Bachar, R., Aktar, A. & Al Mahtab, M. A review of medicinal plants with antiviral activity available in bangladesh and mechanistic insight into their bioactive metabolites on SARS-CoV-2, HIV and HBV. Front. Pharm. 12 , 732891 (2021).
Nawrot, J. et al. Medicinal herbs in the relief of neurological, cardiovascular, and respiratory symptoms after COVID-19 infection a literature review. Cells 11 , 1897 (2022).
Alhazmi, H. A. et al. Medicinal plants and isolated molecules demonstrating immunomodulation activity as potential alternative therapies for viral diseases including COVID-19. Front. Immunol. 12 , 637553 (2021).
Borse, S. et al. Ayurveda botanicals in COVID-19 management: an in silico multi-target approach. PLoS ONE 16 , e0248479 (2021).
Das, K. et al. Inhibition of SARS-CoV2 viral infection with natural antiviral plants constituents: an in-silico approach. J. King Saud. Univ. Sci. 35 , 102534 (2023).
Gupta, I., Baranwal, P., Singh, G. & Gupta, V. Mucormycosis, past and present: a comprehensive review. Future Microbiol. 18 , 217–234 (2023).
Madikonda, P. K., Perugu, S. B. & Ramadevi, C. H. Effect of Ayurvedic Intervention as an adjunct therapy in Post COVID-19 Mucormycosis (PCM): a non-randomized parallel group study. J. Ayurveda Integr. Med. 13 , 100672 (2022).
Gérard, M. et al. Long-term evolution of malnutrition and loss of muscle strength after COVID-19: a major and neglected component of long COVID-19. Nutrients 13 , 3964 (2021).
Capela Santos, D. et al. Yoga for COVID-19: An ancient practice for a new condition—a literature review. Complement Ther. Clin. Pr. 50 , 101717 (2023).
Ursini, F. et al. Fibromyalgia: a new facet of the post-COVID-19 syndrome spectrum? Results from a web-based survey. RMD Open 7 , e001735 (2021).
Kiani, A. K. et al. Neurobiological basis of chiropractic manipulative treatment of the spine in the care of major depression. Acta Biomed. 91 , e2020006 (2020).
Chu, E. C.-P. & Lee, L. Y.-K. Cervical spondylosis as a hidden contributing factor to fibromyalgia: a case report. Int. Med. Case Rep. J. 15 , 639–646 (2022).
Olsen, S. et al. Peripheral electrical stimulation paired with movement-related cortical potentials improves isometric muscle strength and voluntary activation following stroke. Front. Hum. Neurosci. 14 , 156 (2020).
Chaibi, A., Benth, J. Š., Tuchin, P. J. & Russell, M. B. Chiropractic spinal manipulative therapy for migraine: a three-armed, single-blinded, placebo, randomized controlled trial. Eur. J. Neurol. 24 , 143–153 (2017).
Angus, K., Asgharifar, S. & Gleberzon, B. What effect does chiropractic treatment have on gastrointestinal (GI) disorders: a narrative review of the literature. J. Can. Chiropr. Assoc. 59 , 122–133 (2015).
Harris, H. M. B. & Hill, C. A place for viruses on the tree of life. Front. Microbiol 11 , 604048 (2020).
Matsuyama, T., Yoshinaga, S. K., Shibue, K. & Mak, T. W. Comorbidity-associated glutamine deficiency is a predisposition to severe COVID-19. Cell Death Differ. 28 , 3199–3213 (2021).
Törnquist, K., Asghar, M. Y., Srinivasan, V., Korhonen, L. & Lindholm, D. Sphingolipids as modulators of SARS-CoV-2 infection. Front Cell Dev. Biol. 9 , 689854 (2021).
Masoodi, M. et al. Disturbed lipid and amino acid metabolisms in COVID-19 patients. J. Mol. Med (Berl.) 100 , 555–568 (2022).
Barlass, U. et al. Marked elevation of lipase in COVID-19 disease: a cohort study. Clin. Transl. Gastroenterol. 11 , e00215 (2020).
Schwarcz, R., Bruno, J. P., Muchowski, P. J. & Wu, H.-Q. Kynurenines in the mammalian brain: when physiology meets pathology. Nat. Rev. Neurosci. 13 , 465–477 (2012).
Gostner, J. M. et al. Tryptophan metabolism and related pathways in psychoneuroimmunology: the impact of nutrition and lifestyle. Neuropsychobiology 79 , 89–99 (2020).
Blasco, H. et al. The specific metabolome profiling of patients infected by SARS-COV-2 supports the key role of tryptophan-nicotinamide pathway and cytosine metabolism. Sci. Rep. 10 , 16824 (2020).
Download references
Acknowledgements
This work was supported by the Global Nutrition Healthcare Council, Mission COVID ( www.missioncovid.com ). We thank Trevor Amy for digital processing of citations, reference manager, and digital art.
Verdis Lamar Norton (born August 31, 1939) is a visionary who played a pioneering role in the global advancement of iron-transport (lactoferrin) technologies and cellular redox sciences for nutraceutical-based human health applications. As a world-class expert/strategist, Norton’s distinguished career spans over 5 decades, leading several biotech and Fortune 500 food processing companies, including Kraft Foods, N-terminus Labs, and ASEA. Metabolic consequences of iron (Fe)-induced redox dysregulation (FeRD) emerged as a critical pathobiological mechanism in the SARS-CoV2-induced HMRD, the ongoing global health crisis. It is an utmost privilege to dedicate this landmark scientific publication in the honor of Verdis L. Norton .
Author information
Authors and affiliations.
Global Nutrition Healthcare Council (GNHC) Mission-COVID, Yorba Linda, CA, USA
A. Satyanarayan Naidu, Chin-Kun Wang, Pingfan Rao, Fabrizio Mancini, Roger A. Clemens, Sebastiano Porretta, Issac Mathai & Sreus A. G. Naidu
N-terminus Research Laboratory, 232659 Via del Rio, Yorba Linda, CA, 92887, USA
A. Satyanarayan Naidu & Sreus A. G. Naidu
School of Nutrition, Chung Shan Medical University, 110, Section 1, Jianguo North Road, Taichung, 40201, Taiwan
Chin-Kun Wang
College of Food and Bioengineering, Fujian Polytechnic Normal University, No.1, Campus New Village, Longjiang Street, Fuqing City, Fujian, China
Pingfan Rao
World Federation of Chiropractic, Toronto, ON, Canada
Fabrizio Mancini
President-Emeritus, Parker University, 2540 Walnut Hill Lane, Dallas, TX, 75229, USA
University of Southern California, Alfred E. Mann School of Pharmacy/D. K. Kim International Center for Regulatory & Quality Sciences, 1540 Alcazar St., CHP 140, Los Angeles, CA, 90089, USA
Roger A. Clemens
International Union of Food Science and Technology (IUFoST), Guelph, ON, Canada
Aman Wirakartakusumah
IPMI International Business School Jakarta; South East Asian Food and Agriculture Science and Technology, IPB University, Bogor, Indonesia
Department of Chinese Medicine, Taichung Hospital, Ministry of Health & Well-being, Taichung, Taiwan
Hui-Fang Chiu
Department of Family and Community Medicine, Chung Shan Medical University Hospital; School of Medicine, Chung Shan Medical University, Taichung, Taiwan
Chi-Hua Yen
President, Italian Association of Food Technology (AITA), Milan, Italy
Sebastiano Porretta
Experimental Station for the Food Preserving Industry, Department of Consumer Science, Viale Tanara 31/a, I-43121, Parma, Italy
Soukya International Holistic Health Center, Whitefield, Bengaluru, India
Issac Mathai
You can also search for this author in PubMed Google Scholar
Contributions
A.S.N.: Conceptualization, Project administration, Resources, Supervision, Writing—original draft, Writing—review & editing. S.A.G.N.: Formal Analysis, Data Curation, Visualization, Writing—review & editing. C.K.W., P.R., F.M., R.A.C., A.W., H.F.C. and C.H.Y.: Review & editing.
Corresponding author
Correspondence to A. Satyanarayan Naidu .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Naidu, A.S., Wang, CK., Rao, P. et al. Precision nutrition to reset virus-induced human metabolic reprogramming and dysregulation (HMRD) in long-COVID. npj Sci Food 8 , 19 (2024). https://doi.org/10.1038/s41538-024-00261-2
Download citation
Received : 12 October 2023
Accepted : 15 March 2024
Published : 30 March 2024
DOI : https://doi.org/10.1038/s41538-024-00261-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Featured Clinical Reviews
- Screening for Atrial Fibrillation: US Preventive Services Task Force Recommendation Statement JAMA Recommendation Statement January 25, 2022
- Evaluating the Patient With a Pulmonary Nodule: A Review JAMA Review January 18, 2022
- Download PDF
- Share X Facebook Email LinkedIn
- Permissions
Current and Future Landscape of Nutritional Epidemiologic Research
- 1 Departments of Nutrition and Epidemiology, Harvard T. H. Chan School of Public Health, Boston, Massachusetts
- 2 Channing Division of Network Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts
- Viewpoint Improving the Quality of Dietary Research David S. Ludwig, MD, PhD; Cara B. Ebbeling, PhD; Steven B. Heymsfield, MD JAMA
The last half-century of nutrition research has expanded beyond traditional nutrition research based primarily on in vitro biochemistry, animal models, and short-term feeding studies with risk factors as the primary outcomes. Although such studies are still an integral part of nutrition research, they do not directly connect diets with long-term health and disease in humans. Dietary guidelines and recommendations, until recently, were in part based on professional opinions using extrapolations across species and experimental models and limited human evidence from cross-sectional or small feeding studies. Nutritional epidemiology, building on the experience of epidemiology in other fields of public health, has begun to provide important new information and has had substantial effects on diets globally.
Read More About
Hu FB , Willett WC. Current and Future Landscape of Nutritional Epidemiologic Research. JAMA. 2018;320(20):2073–2074. doi:10.1001/jama.2018.16166
Manage citations:
© 2024
Artificial Intelligence Resource Center
Cardiology in JAMA : Read the Latest
Browse and subscribe to JAMA Network podcasts!
Others Also Liked
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
Nutrition Research News
Top headlines, latest headlines.
- Feeding the Lonely Brain
- How Bitter Polyphenols Promote Well-Being
- Crave Snacks After Eating? Food-Seeking Neurons
- Fatty Food Before Surgery May Impair Memory
- Middle-Age Obesity and Shape of Neurons in Brain
- Healthier Diet: Reduced Dementia Risk
- AI-Generated Food Images Look Tastier
- Too Little Sleep: Risk of Type 2 Diabetes
- Pregnant? Are You Getting Enough Omega-3?
- Living Near Fast-Food and Heart Health
Earlier Headlines
Wednesday, march 20, 2024.
- Metformin During Pregnancy Affects the Brain Development in Offspring Mice, Study Finds
Wednesday, March 6, 2024
- Consuming Refined Carbs Might Be Linked to Perceived Facial Attractiveness
Tuesday, February 20, 2024
- Avid Appetite in Childhood Linked to Later Eating Disorder Symptoms
- Could Ultra-Processed Foods Be the New 'silent' Killer?
Friday, February 16, 2024
- Why Do(n't) People Support Being Nudged Towards Healthier Diets?
Thursday, February 15, 2024
- Protein-Rich Breakfast Boosts Satiety and Concentration
- Helping Caregivers Help People With Dementia Eat at Home
Tuesday, February 13, 2024
- A Closer Look at Cannabis Use and Binge Eating
Monday, January 29, 2024
- Study Urges People to Think Twice Before Going on a Diet
Wednesday, January 24, 2024
- 'Furry Fruit' Improves Mental Health -- Fast
Thursday, January 18, 2024
- Removing Largest Serving Sizes of Wine Decreases Alcohol Consumption, Study Finds
- New Gut-Brain Circuits Found for Sugar and Fat Cravings
Saturday, January 13, 2024
- Early Study Shows Health Benefits of Creative Arts Therapies and Nutrition Education for Postmenopausal Women
Thursday, January 11, 2024
- Scientists Identify How Dietary Restriction Slows Brain Aging and Increases Lifespan
Monday, January 8, 2024
- Drugs Used to Treat Type 2 Diabetes Reduce Alcohol Cravings, Use in Individuals With Obesity
Monday, December 18, 2023
- Parents' Top Resolutions: More Patience, Less Time on Phones
Tuesday, December 12, 2023
- Fat Flies Live Longer on a Diet at Any Age
Monday, December 4, 2023
- Survey Finds Americans Struggle to Maintain Healthy Habits During the Holiday Season
Friday, December 1, 2023
- How Pre And Postnatal B-12 Vitamins Improve Breast Milk Vitamin B-12 Levels, Which Supports Infant Brain Development
Tuesday, November 28, 2023
- Fat Cells Help Repair Damaged Nerves
Monday, November 27, 2023
- Scientist Discovers Potential Brain Link Between Stress, Emotional Eating
Wednesday, November 22, 2023
- From the First Bite, Our Sense of Taste Helps Pace Our Eating
Friday, November 17, 2023
- Following a Mediterranean Diet Reduces the Risk of Cognitive Decline in Older People
Thursday, November 16, 2023
- Hunger Hormones Impact Decision-Making Brain Area to Drive Behavior
Monday, November 13, 2023
- Study Finds Melatonin Use Soaring Among Youth
Tuesday, November 7, 2023
- Obesity Linked to Neurodegeneration Through Insulin Resistance
Tuesday, October 24, 2023
- Children as Young as Four Eat More When Bored
Friday, October 20, 2023
- Women With a Heart Healthy Diet in Midlife Are Less Likely to Report Cognitive Decline Later
Wednesday, October 11, 2023
- Jet Lag Disorder Associated With Shift Work Can Lead to Brain Changes Increasing Appetite
Tuesday, October 10, 2023
- How Plant-Derived Nutrients Can Affect the Gut and Brain
Monday, October 9, 2023
- Scientists Says Identifying Some Foods as Addictive Could Shift Attitudes, Stimulate Research
Wednesday, October 4, 2023
- Being a Vegetarian May Be Partly in Your Genes
Monday, October 2, 2023
- Discrimination Alters Brain-Gut 'crosstalk,' Prompting Poor Food Choices and Increased Health Risks
Wednesday, September 27, 2023
- Saturated Fat May Interfere With Creating Memories in Aged Brain
Sunday, September 17, 2023
- Early Treatment of Child Obesity Is Effective
Friday, September 15, 2023
- Living in a Disadvantaged Neighborhood Affects Food Choices, Weight Gain and the Microstructure of the Brain
Thursday, September 14, 2023
- A Quarter of People Are Undoing the Benefits of Healthy Meals by Unhealthy Snacking
Friday, September 1, 2023
- Red Blood Cells Exposed to Oxygen Deficiency Protect Against Myocardial Infarction
Thursday, August 31, 2023
- Adding Complex Component of Milk to Infant Formula Confers Long-Term Cognitive Benefits for Bottle-Fed Babies
- A New Breakthrough in Obesity Research May Allow You to Lose Fat While Eating All You Want
Wednesday, August 30, 2023
- Researchers Identify the Link Between Memory and Appetite in the Human Brain to Explain Obesity
Thursday, August 17, 2023
- A Healthy Diet, Reading, and Doing Sports Promote Reasoning Skills in Children
Wednesday, August 16, 2023
- Adherence to a Mediterranean Lifestyle Associated With Lower Risk of All-Cause and Cancer Mortality
- How Cold Temperatures Trigger the Brain to Boost Appetite
Thursday, August 10, 2023
- Font Size Can 'nudge' Customers Toward Healthier Food Choices
Tuesday, August 8, 2023
- Brain's 'appetite Control Center' Different in People Who Are Overweight or Living With Obesity
- Laboratory Research Finds Gluten Caused Brain Inflammation in Mice
Monday, August 7, 2023
- Out With the Life Coach, in With the Chatbot
Thursday, August 3, 2023
- A Mother's Diet Can Protect Her Grandchildren's Brains: Genetic Model Study
Wednesday, August 2, 2023
- Irregular Sleep Patterns Associated With Harmful Gut Bacteria
Wednesday, July 26, 2023
- Mediterranean Diet and Physical Activity Could Prevent Hospitalization-Associated Disability in Older People
Thursday, June 29, 2023
- Fasting Can Help You Lose Weight, but You Might Gain It Back Quickly
Tuesday, June 27, 2023
- The Worm That Learned: Diet Found to Affect Learning in Older Nematodes
- Molecular Imaging Identifies Brain Changes in Response to Food Cues; Offers Insight Into Obesity Interventions
Monday, June 26, 2023
- Lean Body Mass, Age Linked With Alcohol Elimination Rates in Women

Wednesday, June 21, 2023
- Omega-3 Fatty Acids Linked to Slower Decline in ALS
Monday, June 12, 2023
- Researchers Uncover Why Light-to-Moderate Drinking Is Tied to Better Heart Health
Thursday, June 8, 2023
- Colorful Fresh Foods Improve Athletes' Vision
- How Chronic Stress Drives the Brain to Crave Comfort Food
Tuesday, May 30, 2023
- Junk Food May Impair Our Deep Sleep
Monday, May 29, 2023
- Low-Flavanol Diet Drives Age-Related Memory Loss, Large Study Finds
Wednesday, May 24, 2023
- How Tasty Is the Food?
- Multivitamin Improves Memory in Older Adults, Study Finds
Monday, May 22, 2023
- You Can Satisfy Your Appetite Just by Looking at Pictures of Food on Your Phone
Thursday, May 11, 2023
- The Feeling of Hunger Itself May Slow Aging in Flies
Friday, May 5, 2023
- A Special Omega-3 Fatty Acid Lipid Will Change How We Look at the Developing and Aging Brain
Tuesday, April 25, 2023
- Study Links Nutrients, Brain Structure, Cognition in Healthy Aging
Thursday, April 20, 2023
- Cannabinoids Give Worms the Munchies, Too
Tuesday, April 18, 2023
- How to Get Your Children to Eat More Fruits and Vegetables
Friday, April 14, 2023
- More Structure, Fewer Screens Makes for Healthier Kids in the School Holidays
Thursday, April 13, 2023
- Kombucha to Kimchi: Which Fermented Foods Are Best for Your Brain?
Friday, April 7, 2023
- Researchers Leverage Cell Self-Destruction to Treat Brain Tumors
Wednesday, April 5, 2023
- Exposure Therapy to Feared Foods May Help Kids With Eating Disorders
Tuesday, March 28, 2023
- New Form of Omega-3 Could Prevent Visual Decline With Alzheimer's Disease
Monday, March 27, 2023
- Beneficial Bacteria in the Infant Gut Uses Nitrogen from Breast Milk to Support Baby's Health
Friday, March 24, 2023
- Dieting: Brain Amplifies Signal of Hunger Synapses
Thursday, March 23, 2023
- A Higher Dose of Magnesium Each Day Keeps Dementia at Bay
Wednesday, March 22, 2023
- Sweets Change Our Brain
Monday, March 20, 2023
- Molecular Basis for Alkaline Taste
Tuesday, March 14, 2023
- Meta-Analysis Shows Association Between Autism in Children and Cardiometabolic Diseases
Monday, March 13, 2023
- Mediterranean Diet Associated With Decreased Risk of Dementia, Study Finds
Wednesday, March 8, 2023
- MIND and Mediterranean Diets Associated With Fewer Alzheimer's Plaques and Tangles
Friday, March 3, 2023
- A Good Night's Sleep May Make It Easier to Stick to Exercise and Diet Goals
Wednesday, March 1, 2023
- Taking Vitamin D Could Help Prevent Dementia
Thursday, February 23, 2023
- Leptin Helps Hungry Mice Choose Sex Over Food
Tuesday, February 14, 2023
- Want Healthy Valentine Chocolates? We Can Print Them
Monday, February 13, 2023
- Fructose Could Drive Alzheimer's Disease
Thursday, February 2, 2023
- Sugar Is Processed Differently in the Brains of Obesity-Prone Vs. Obesity-Resistant Rats
Tuesday, January 31, 2023
- Mocktails or Cocktails? Having a Sense of Purpose in Life Can Keep Binge Drinking at Bay
Thursday, January 26, 2023
- Why a High Fat Diet Could Reduce the Brain's Ability to Regulate Food Intake
Wednesday, January 25, 2023
- Supplementation With Amino Acid Serine Eases Neuropathy in Diabetic Mice
Friday, January 20, 2023
- Loneliness Associated With Unhealthful Diets and Physical Inactivity Among US College Students
Wednesday, January 18, 2023
- Body Dissatisfaction Can Lead to Eating Disorders at Any Age
Tuesday, January 17, 2023
- Study Explores Effects of Dietary Choline Deficiency on Neurologic and System-Wide Health
- Vitamin D Benefits and Metabolism May Depend on Body Weight
- Fruit Flies Grow Brainy on a Poor Diet
Wednesday, January 11, 2023
- How Better Planning, Behavior Regulation May Lead to Eating Less Fat
Tuesday, January 10, 2023
- Aware or Not Aware: You Are Affected by Food Cues Either Way
Wednesday, January 4, 2023
- Can Diet Combined With Drugs Reduce Seizures?
Tuesday, January 3, 2023
- Time-Restricted Eating Reshapes Gene Expression Throughout the Body
- LATEST NEWS
- Top Science
- Top Physical/Tech
- Top Environment
- Top Society/Education
- Health & Medicine
- Mind & Brain
- Disorders and Syndromes
- ADD and ADHD
- Alzheimer's
- Bipolar Disorder
- Borderline Personality Disorder
- Brain Injury
- Hearing Impairment
- Huntington's Disease
- Mad Cow Disease
- Multiple Sclerosis
- Obstructive Sleep Apnea
- Parkinson's
- Schizophrenia
- Sleep Disorders
- Education & Learning
- Brain-Computer Interfaces
- Educational Psychology
- Infant and Preschool Learning
- Intelligence
- K-12 Education
- Language Acquisition
- Learning Disorders
- Illegal Drugs
- Crystal Meth
- Psychedelic Drugs
- Living Well
- Anger Management
- Child Development
- Consumer Behavior
- Dieting and Weight Control
- Gender Difference
- Nutrition Research
- Racial Issues
- Relationships
- Spirituality
- Mental Health
- Eating Disorders
- Smoking Addiction
- Neuroscience
- Child Psychology
- Social Psychology
- Space & Time
- Matter & Energy
- Computers & Math
- Plants & Animals
- Earth & Climate
- Fossils & Ruins
- Science & Society
- Business & Industry
Strange & Offbeat
- Drug Development Made Easier
- RNA That Doesn't Age
- 'Rainbow' Detected On an Exoplanet
- Spears and Throwing Sticks 300,000 Years Old
- High Carbon Impact of Tourism at Yellowstone
- Extreme Starburst Galaxy
- Asthma: Disease May Be Stoppable
- Stellar Collisions and Zombie-Like Survivors
- Tiny Robot Swarms Inspired by Herd Mentality
- How the Brain Regulates Emotions
Trending Topics
Embracing complexity: making sense of diet, nutrition, obesity and type 2 diabetes
- Open access
- Published: 14 February 2023
- Volume 66 , pages 786–799, ( 2023 )
Cite this article
You have full access to this open access article
- Nita G. Forouhi 1
13k Accesses
7 Citations
57 Altmetric
Explore all metrics
Nutrition therapy has been emphasised for decades for people with type 2 diabetes, and the vital importance of diet and nutrition is now also recognised for type 2 diabetes prevention. However, the complexity of diet and mixed messages on what is unhealthy, healthy or optimal have led to confusion among people with diabetes and their physicians as well as the general public. What should people eat for the prevention, management and remission of type 2 diabetes? Recently, progress has been made in research evidence that has advanced our understanding in several areas of past uncertainty. This article examines some of these issues, focusing on the role of diet in weight management and in the prevention and management of type 2 diabetes. It considers nutritional strategies including low-energy, low-fat and low-carbohydrate diets, discusses inter-relationships between nutrients, foods and dietary patterns, and examines aspects of quantity and quality together with new developments, challenges and future directions.
Graphical abstract
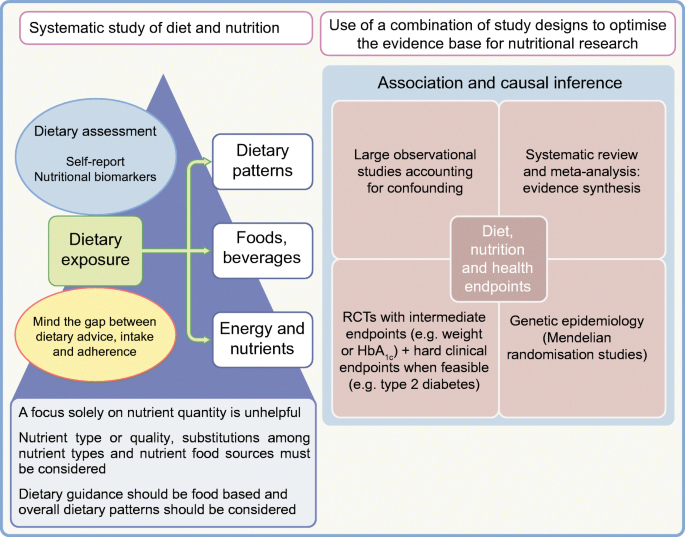
Similar content being viewed by others

Evidence-based European recommendations for the dietary management of diabetes
The Diabetes and Nutrition Study Group (DNSG) of the European Association for the Study of Diabetes (EASD)

Nutrition in Lifestyle Medicine: Overview

Diet: The Balancing Act
Sheel Sharma & Deepika Dhawan
Avoid common mistakes on your manuscript.
Diet, nutrition and type 2 diabetes: what is the evidence?
Diabetes is a metabolic disorder with the potential for multiple adverse health consequences. It is also a public health challenge, with a rising global burden. Estimates indicate that there were approximately 537 million people worldwide with diabetes in 2021, which is projected to rise to 783 million by 2045, with type 2 diabetes constituting the majority (>90%) of this burden [ 1 ]. Diet and nutrition are of indisputable significance in reducing this burden because the development of type 2 diabetes is characterised by obesity and insulin resistance, leading to hyperglycaemia, and both weight and glycaemic control are directly related to food consumption.
Diet and nutrition are thus central as modifiable factors in both the management and the prevention of type 2 diabetes. This is supported by three lines of evidence. First, when adhered to, medical nutrition therapy in those with type 2 diabetes can match or exceed the glycaemic control that can be achieved by glucose-lowering medication in the short term, and can be useful in maintaining control [ 2 ]. Second, the proof of principle was established in the early 2000s that, among people with non-diabetic hyperglycaemia, the onset of type 2 diabetes can be delayed or prevented, with as much as a 58% relative risk reduction, through a supported intensive lifestyle intervention including dietary changes and physical activity [ 3 ]. The real-world impact of lifestyle modification strategies has been demonstrated [ 4 ], outside the highly controlled conditions of clinical trials, and such a strategy has been found to be effective in the UK National Health Service (NHS) [ 5 ]. Third, it has been demonstrated that remission of type 2 diabetes can be achieved through dietary means [ 6 ], resulting in a major shift in scientific understanding of the pathophysiology of type 2 diabetes, from a condition previously thought to be progressive and irreversible to one that can be brought under control to normal functioning.
However, defining the optimal diet for type 2 diabetes is a challenge and dietary strategies used in research have varied between different studies. This is largely because diet is intensely complex, with multiple components and influences on food consumption (Fig. 1 ). Concomitantly, interest in diet, nutrition and health is intense, with a deluge of scientific publications, matched equally by popular media coverage that is saturated with nutrition over-claims and ‘miracle diets’. This is also a field where vested interests are rife [ 7 ]. A search on PubMed (25 November 2022) using the terms ‘diet OR nutrition OR food OR nutrient OR dietary pattern OR diet quality’ and ‘type 2 diabetes OR non-insulin dependent diabetes’ yielded 52,833 hits, with over 3000 articles published each year since 2014; repeating the search using the term ‘obesity’ yielded 165,617 hits. What evidence should we trust?
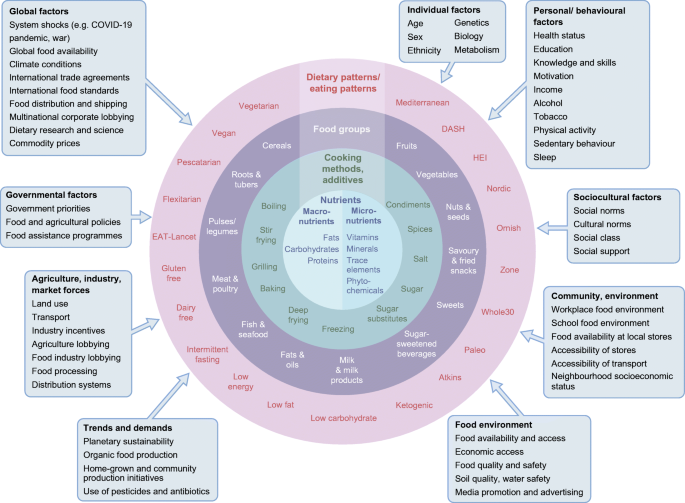
Complexity of diets and multiple influences affecting food intakes. HEI, Healthy Eating Index. Influencing factors (boxes) adapted with permission from Afshin et al [ 83 ] © 2014 John Wiley & Sons. This figure is available as a downloadable slide .
The hierarchy of evidence framework and quality assessment tools have been applied to sift through the vast amount of evidence. Several reviews of the research evidence have been carried out [ 8 , 9 , 10 , 11 , 12 , 13 , 14 ], enabling the incorporation of the best available evidence in dietary guidelines issued by authoritative agencies, including but not limited to the ADA [ 15 ] and Diabetes UK [ 16 ].
In a nutshell, this evidence highlights some key dietary principles. Healthy weight maintenance is critical to both prevent and manage type 2 diabetes; a pattern of food intake that mitigates type 2 diabetes risk includes the habitual consumption of vegetables, fruits, legumes, whole grains and cereal fibre, dairy products such as yoghurt, and nuts, and several overall dietary patterns are effective. In contrast, type 2 diabetes risk is elevated with a pattern of habitual dietary intake that includes processed and unprocessed red meat, refined grains and sugar-sweetened beverages. This evidence provides support that some foods should be emphasised and promoted while the consumption of others should be reduced or avoided, rather than the adage about everything in moderation.
This article does not cover the wide range of topics already discussed in existing reviews and guidelines. It focuses instead on selected hot topics that have been the subject of debate and on new developments in understanding in the field.
Weight management at the core, but how?
Body weight with increased adiposity is mechanistically linked to both the development and the progression of type 2 diabetes, typified by resistance to insulin action (insulin resistance) and an inadequate compensatory insulin secretory response by pancreatic beta cells. The relationship between adiposity, insulin resistance and beta cell function varies between individuals but the benefits of weight loss apply across the different pathophysiologies [ 17 ]. Weight loss is related to improved glycaemic control: the greater the weight loss, the greater the improvement in HbA 1c . A weight loss goal of 5–7% of initial body weight for people with overweight or obesity is recommended for clinical benefit, while weight loss of 15% can be disease modifying with the possibility of remission of type 2 diabetes [ 2 , 18 ].
Of the three options for weight management, bariatric surgery and pharmacotherapy are effective, but dietary strategies offer population-wide benefits without medicalisation. However, the weight loss and weight management diet market is vast and is projected to increase from US$192.2 billion in 2019 to US$295.3 billion by 2027. This promotion of a vast range of dietary products and strategies can be bewildering. An important question is therefore which dietary strategies are effective?
Remission of type 2 diabetes through diet-related weight loss
The proof of principle of the potential for reversibility or remission of type 2 diabetes with weight loss came first from the field of bariatric surgery [ 19 , 20 ]. However, surgery is not suitable for, or acceptable to, all people with type 2 diabetes. Surgery also has the potential for complications, side effects and challenges. One such challenge is the large prevalence of type 2 diabetes, which renders surgery an unrealistic option at the scale required, even if it were financially possible. There is high interest, therefore, in dietary means to achieve diabetes remission.
The nutritional basis for the remission of type 2 diabetes used in the UK-based Diabetes Remission Clinical Trial (DiRECT) was centred on major caloric restriction and weight loss with an associated reduction in hepatic fat and hepatic glucose output and improved beta cell function [ 6 ]. Among people with type 2 diabetes in primary care who were randomised to either a diet very low in energy (very low calorie diet) or usual care, mean body weight fell by 10 kg in the intervention group and 46% remained free of diabetes (i.e. in remission; HbA 1c <48 mmol/mol [<6.5%]) at 1 year and off all glucose-lowering and antihypertensive medications [ 21 ]. The intervention comprised total diet replacement (3452–3569 kJ/day [825–853 kcal/day] liquid formula diet for 12–20 weeks), stepped food reintroduction (2–8 weeks) and then structured support for weight loss maintenance. The greater the weight loss, the greater the likelihood of remission (86% at 1 year for weight loss ≥15kg; 57%, 34% and 7% for weight loss of 10–15 kg, 5–10 kg and <5 kg respectively). In addition, the effects were durable, with 36% of people in sustained remission at 2 years [ 22 ]. Further research is needed to understand the longer term effects of remission on the complications of type 2 diabetes, but current results support the remission of type 2 diabetes as a practical target in primary care.
In an endorsement of this approach, the UK NHS has rolled out a 12 week intervention consisting of a low-energy meal replacement diet for people with type 2 diabetes and a BMI >27 kg/m 2 (or >25 kg/m 2 if from a minority ethnic group in whom risk occurs at a lower BMI) ( https://www.england.nhs.uk/2022/01/nhs-soups-and-shakes-diet-helps-thousands-shed-the-pounds/ ). The goal is to recruit 5000 people from general practice; over 2000 people have already participated, showing the feasibility of this approach.
A focus on nutrients for weight and glycaemic control
Traditionally, dietary guidance has focused on macronutrient composition. Most dietary guidelines recommend intakes of <30–35% of energy from total fat, 45–55% of energy from carbohydrates and the remainder, ~15–20% of energy, from protein, both in the general population and in those with type 2 diabetes. For weight management, low-fat diets were favoured based on the higher energy density of fat, at 38kJ/g (9 kcal/g), compared with that of carbohydrate or protein, at 17kJ/g (4 kcal/g). More recently, low-carbohydrate diets have gained popularity. The optimal macronutrient composition is hotly debated.
Low-fat or low-carbohydrate diets for weight management?
The Look-AHEAD: Action for Health in Diabetes (Look-AHEAD) trial compared an intensive lifestyle intervention with a control condition of support and education in people with type 2 diabetes. The weight loss strategy, comprising energy reduction (5021–7531 kJ/day [1200–1800 kcal/day]) through a low-fat diet, was effective. Greater weight loss was achieved in the intervention group at 1 year, with a net difference in weight of –7.9% (95% CI –8.3% to –7.6%); at year 4, the net difference in weight was –3.9% (95% CI –4.4% to –3.5%) [ 23 ]. Similar low-fat diet approaches have been used in other trials of the primary prevention of type 2 diabetes [ 3 ]. In contrast, in the energy-deficit diet in the type 2 diabetes remission trial (DiRECT), the proportions of macronutrients were inconsequential, with >50% of energy coming from carbohydrates [ 22 ]. A recent umbrella review of the evidence concluded that weight management in type 2 diabetes using hypocaloric diets does not depend on any particular macronutrient profile [ 24 ].
More broadly, among adults with overweight or obesity in the population without consideration of type 2 diabetes, individual studies show differing results favouring one nutrient or another but, when the totality of the evidence is appraised, both low-fat and low-carbohydrate diets of varying protein content are effective for weight loss [ 25 ]. The challenge lies in adherence to the prescribed diets. A systematic review of the effects of low-fat and low-carbohydrate diets on weight loss in RCTs of at least 1 year’s duration and with a similar intervention intensity across groups found that low-fat diets were efficacious compared with usual intake [ 26 ]. But, when low-fat diets were compared with low-carbohydrate diets, there was greater weight loss in the low-carbohydrate diet group. However, the magnitude of the difference in weight loss between low-carbohydrate and low-fat diets was modest at only 1.15 kg, which is statistically significant but may have little clinical meaning. As a limitation, caloric restriction was a component of many of the weight loss interventions included, but not all; for example, some included studies gave dietary advice to eat a low-carbohydrate diet ad libitum [ 26 ]. Future research should seek to address design limitations; however, current research indicates that small effects on weight loss from one macronutrient type or another are unlikely to be of clinical significance. A key challenge is weight maintenance and prevention of weight regain, which is typical following weight loss.
Although overall dietary carbohydrate or fat content has been extensively studied in relation to weight loss and maintenance, protein intake has been less so. Higher protein intake after weight loss has been shown to result in significantly lower weight regain, related to increased satiety and energy efficiency [ 27 ]. For early weight loss maintenance over 6 months, an RCT tested different combinations of protein consumption and glycaemic index (GI) compared with a control diet among those who had lost at least 8% (equivalent to 11 kg) of their initial weight on a 3347 kJ/day (800 kcal/day) diet [ 28 ]. Consuming a low-protein/high GI diet led to subsequent weight regain (mean of 1.7 kg [95% CI 0.5 to 2.9]), while a modest increase in protein content and a modest reduction in GI led to improvements (reductions) in the degree of weight regain over 6 months. Evidence for long-term weight loss maintenance is generally sparse. Observational prospective data from the National Weight Loss Registry indicated that weight loss maintenance over 10 years was related to low-fat-based energy restraint combined with physical activity [ 29 ]. Further research is needed to better understand the dietary strategies and other factors important in weight loss maintenance.
Low-carbohydrate diets for glycaemic control in type 2 diabetes
For glycaemic control in type 2 diabetes, studies from clinical practice or from digital or commercial programmes have promoted low-carbohydrate diets based on significant benefits for HbA 1c , of a mean decrease of 11 mmol/mol (1% unit decrease), together with reductions in glucose-lowering medication use [ 30 , 31 ]. Interpretive challenges include the presence of bias owing to the lack of randomisation, self-selection into groups and unbalanced sample sizes or intensities of interventions in the study arms and lack of a comparator group. However, a number of systematic reviews and meta-analyses of RCTs are available that reduce such limitations [ 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 ].
Evidence from RCTs indicates that lower carbohydrate diets have benefits over higher carbohydrate diets in the short term up to 6 months, but these are not maintained over time [ 34 , 36 ]. In the UK, the Scientific Advisory Committee on Nutrition appraised the available evidence, including 48 individual RCTs from eight systematic reviews. It concluded that lower carbohydrate diets were effective for glycaemic control in type 2 diabetes compared with higher carbohydrate diets, with a greater reduction in HbA 1c (weighted mean difference –4.7 mmol/mol [–0.47%]) in the short term (3–6 months), but this benefit was not maintained at 12 months [ 39 ].
Despite extensive research on low-carbohydrate diets, there are several challenges that limit firm conclusions. First, definitions of what a ‘low-carbohydrate diet’ is range from moderate carbohydrate restriction to very-low-carbohydrate or ketogenic diets (see Text box ‘Definitions of carbohydrate-focused diets’). Across RCTs, prescribed carbohydrate intakes in the lower carbohydrate groups ranged widely, from 14% to 50% of energy intake, and reported carbohydrate intakes were moderate at 26–45% of energy intake in the majority of the primary RCTs [ 39 ]. Second, in the case of isoenergetic diets (maintaining the same overall energy intake), a low-carbohydrate diet is by default higher in fat and vice versa. As many individual studies did not specify isoenergetic study arms, it is difficult to tease out whether the glycaemic change was influenced by differential changes in weight as a result of differing energy intakes. Third, because of differences in or a lack of information in study protocols on adjustment of glucose-lowering medication, it is hard to infer whether criteria for remission of type 2 diabetes were met [ 40 ].
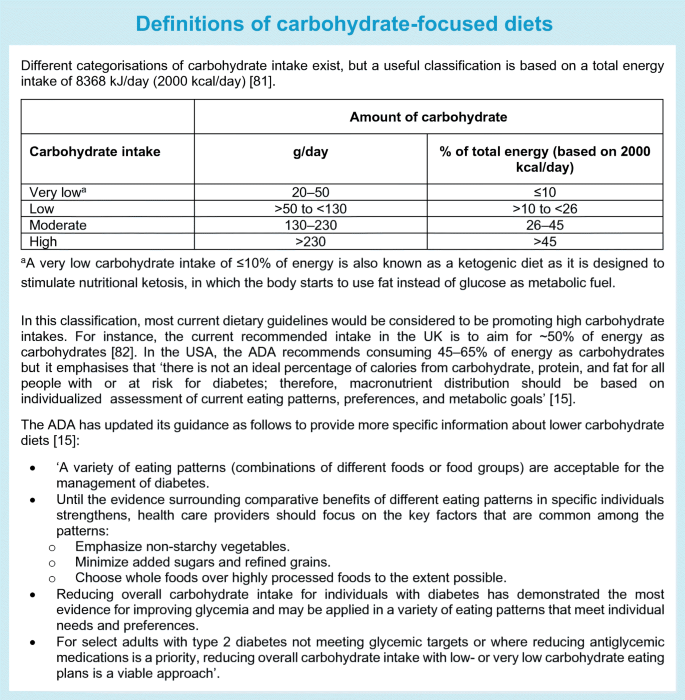
Low-carbohydrate diets seem to be generally safe and well tolerated in the short term; concerns in the longer term relate to the potential atherogenic lipid profile [ 38 , 41 ] or micronutrient deficiency [ 42 ] or their use in people with chronic kidney disease or pregnant women, in whom there is a need for further evaluation. Accumulating evidence from prospective studies with long-term follow-up data indicates that both high and low intakes of carbohydrates may have adverse health impacts on mortality risk, with a U-shaped relationship [ 43 ]. However, such research has been carried out in general populations and needs to be replicated, and further research is needed in those with type 2 diabetes. In the meantime, the ADA dietary guidelines for people with diabetes were updated in 2019, making it explicit that low-carbohydrate diets can be endorsed (see Text box ‘Definitions of carbohydrate-focused diets’).
Nutrition and pathways to obesity and type 2 diabetes
The above focus on energy and macronutrients is rooted in two contesting mechanistic explanations that link dietary intake to obesity and type 2 diabetes. In the energy balance model, energy matters because the law of thermodynamics dictates that when energy intake exceeds energy expenditure weight gain occurs. The link between obesity and the development of type 2 diabetes is strong and, with caloric deficit-induced weight loss, remission of type 2 diabetes is possible. In these scenarios, a calorie is a calorie and excess calories result in adipose tissue accumulation and weight gain.
In contrast, the ‘carbohydrate–insulin model’ proposes that obesity is a cause, not the consequence, of excess caloric intake [ 44 ]. Here, the dysregulation of fat storage and metabolism is the central defect, driven by high-carbohydrate diets that produce spikes of hyperinsulinaemia that promote glucose uptake into tissues, suppress release of fatty acids from adipose tissue and stimulate fat and glycogen storage. Thus, less energy remains available for use by the rest of the body, driving hunger and overeating. In this scenario, not all calories are equal. It has been proposed that energy from refined carbohydrates promote a disturbed hormonal milieu linked with increased hunger, a slower metabolic rate and reduced energy expenditure, leading to adiposity.
The debate between these mechanistic processes continues [ 45 , 46 , 47 ]. However, it is increasingly clear that a focus on energy intake does not account for the impact that diet quality has on long-term weight gain and type 2 diabetes through diverse physiological processes. These include diet-induced thermogenesis, brain reward, appetite, hunger, satiety, digestion, the release and action of hormones, for example insulin, hepatic de novo lipogenesis, interactions with the gut microbiome and energy expenditure [ 48 ]. Moreover, a focus on considering a single macronutrient type has limitations that can lead to unhelpful reductionist messages to avoid a macronutrient without reference to its quality and food sources.
Beyond a focus on nutrient quantity: the relevance of nutrient type, quality and food sources
RCTs of macronutrient manipulation have focused exclusively on quantity. This ignores the fact that health effects will vary substantially by nutrient type or quality. For dietary fats, a vast literature exists on the importance of distinguishing between saturated, polyunsaturated, monounsaturated and trans fats. Health effects also vary by carbohydrate type (starch, sugar or fibre), degree of processing (whole grain vs refined grain), glycaemic response after consumption (GI and load) and food structure (solid or liquid form).
There is substantial evidence from meta-analyses for inverse (beneficial) associations between the consumption of fibre [ 49 ], particularly cereal fibre [ 50 ] and wholegrains [ 11 ], and the incidence of type 2 diabetes. However, evidence is more mixed for the dietary GI, which reflects the differential blood glucose-raising potential of foods with similar carbohydrate content, and a related measure, the glycaemic load (GL), which accounts for the amount of available carbohydrate. For example, the meta-analysis by Reynolds et al found inverse associations between fibre intake and several disease endpoints, including type 2 diabetes and mortality, but associations with GI and GL were non-significant [ 49 ]. Mixed and inconclusive results were also reported in reviews of a link between GI, GL and HbA 1c or fasting glucose [ 15 ]. The OmniCarb RCT compared four diets with varying GI and carbohydrate content in overweight or obese individuals with hypertension or pre-hypertension. This was a crossover feeding study with each diet based on a Dietary Approaches to Stop Hypertension (DASH)-type diet pattern [ 51 ]. Compared with a high GI (65% on the glucose scale), high-carbohydrate (58% energy) diet, a low GI (40% on the glucose scale), low-carbohydrate (40% energy) diet did not significantly improve insulin sensitivity, lipid levels or blood pressure. This type of evidence indicates that GI values have a low utility, but further research contradicts this. Other reviews with a more nuanced approach have reported a positive association between GI or GL and type 2 diabetes [ 52 ]. Similarly, some reviews and individual large cohorts have also reported a positive (adverse) association of high GI or GL with CHD or CVD [ 53 ], as well as a likely benefit of low GI or GL dietary patterns for glycaemic control and cardiometabolic risk factors in people with type 1 diabetes or type 2 diabetes [ 54 ]. A take-home message is that multiple aspects of carbohydrate quality are relevant and should be considered where possible because intakes of fibre, wholegrain and the GI and GL values of foods are likely to be highly correlated and may have confounding effects if not accounted for in diet–disease associations.
A point to note is that, when consumption of one nutrient type is manipulated (to eat less or more of it), this impacts the consumption of other nutrient types—the so-called ‘nutrient substitution’, in which one nutrient substitutes for another within isoenergetic consumption. Moreover, there are both ‘healthy’ and ‘unhealthy’ low-fat or low-carbohydrate diets.
The Diet Intervention Examining The Factors Interacting with Treatment Success (DIETFITS) RCT tested diet quality, comparing ‘healthy’ low-carbohydrate and low-fat regimens [ 55 ]. Both diet groups were instructed to maximise their non-starchy vegetable intake, minimise added sugars, refined flours and trans fats and focus on whole foods. Both diet types were effective, with a mean weight loss of 5.3 kg and 6 kg for the healthy low-fat and healthy low-carbohydrate diets, respectively, at 12 months, but there was no significant between-group difference [ 55 ]. In both diet groups there were also improvements at 12 months in secondary outcomes, including fasting glucose and insulin levels, body fat percentage, waist circumference, blood pressure and lipid profiles, except for LDL-cholesterol level, which was reduced in the low-fat group but increased in the low-carbohydrate group.
A crossover trial compared different levels of carbohydrate restriction and food sources in people with prediabetes or type 2 diabetes over two 12 week periods. Carbohydrates comprised <20% of energy in the very-low-carbohydrate ketogenic diet and <40% in the low-carbohydrate Mediterranean-style diet [ 56 ]. Both diets incorporated non-starchy vegetables and avoided added sugars and refined grains; the ketogenic diet avoided legumes, most fruits (except a few berries in small amounts) and whole grains whereas the Mediterranean-style diet incorporated these foods. Both diets resulted in improvements that were not significantly different. Specifically, mean HbA 1c levels decreased by 9% and 7% in the ketogenic and Mediterranean-style diet groups, respectively, and weight decreased by 8% and 7%, respectively. The ketogenic diet group achieved greater improvements in triglyceride and HDL-cholesterol levels than the Mediterranean-style diet group but had higher LDL-cholesterol levels (percentage change +10% vs –5%, respectively). The diets were ad libitum but participants in both groups reported consuming on average 1046–1255 kJ/day (250–300 kcal/day) less compared with baseline. The ketogenic diet group had a lower fibre intake and consumed lower levels of micronutrients (folate, vitamin C and magnesium). This study was of short duration and longer term research is needed, but its findings do not justify achieving a low-carbohydrate status by avoiding fruits, legumes and whole grains, which are considered part of a healthy diet in other longstanding research.
In sum, the consideration of nutrients in isolation has led to unhelpful polarised debates on whether low-fat or low-carbohydrate diets are superior. Macronutrients are not homogeneous entities: individual nutrients are derived from foods and people eat food in overall dietary patterns.
Beyond nutrients: foods and dietary patterns
Foods are complex mixtures of thousands of components—the food matrix—that have different physicochemical properties and health effects. This is illustrated by the opposite directions of association with the incidence of CHD seen for different foods rich in saturated fats. Consumption of dairy products such as yoghurt and cheese is inversely related to CHD incidence whereas consumption of red and processed meat is positively associated with CHD incidence [ 57 ]. This was corroborated by research showing that people who ate more saturated fats from red meat and butter were more likely to develop CHD than those who ate more saturated fats from cheese, yoghurt and fish [ 58 ]. This highlights the need to consider food sources together with the macronutrients they contain rather than the nutrients in isolation.
A consensus on dietary factors for the prevention of type 2 diabetes has been established from the comprehensive evidence base and incorporated into dietary guidelines. Broadly this suggests the benefits of the consumption of fruit, vegetables, nuts, seeds, wholegrains and yoghurt and the potential harms associated with sugar-sweetened beverages and red and processed meat. For some foods, such as fruit juice, artificially sweetened beverages, lean and fatty fish, milk and eggs, uncertainty remains with regard to their benefits for type 2 diabetes prevention [ 14 ].
Highly processed or ultra-processed foods of both plant and animal origin are increasingly consumed globally and have been related to a number of adverse health impacts. They include foods that have undergone industrial processing and that contain added ingredients such as salt, sugar, fat and artificial preservatives, stabilisers or colours, prolonging shelf life and reducing cost. An RCT compared the ad libitum consumption of ultra-processed foods with consumption of unprocessed foods. A total of 20 participants received all meals, matched for energy and macronutrient content, in a controlled setting for 28 days [ 59 ]. Ultra-processed food consumption led to substantially greater energy intake (+2090 kJ/day [+500 kcal/day] on average over 14 days) and weight gain (+0.9 kg over 14 days vs weight loss of equal magnitude during the 14 days of the unprocessed diet phase). Longer term prospective studies have provided evidence for an association of ultra-processed food consumption with the development of type 2 diabetes [ 60 ].
A number of food-based dietary patterns have a place in the prevention of type 2 diabetes based on observational evidence, including the Mediterranean, DASH and plant-based diets, but only the Mediterranean diet has been investigated in an RCT, both for the prevention and for the management of type 2 diabetes [ 61 ]. For many named popular diets such as the paleo, Atkins, Ornish and Zone diets, there is RCT evidence for short-term weight management but without any meaningful differences between them [ 25 ], while no evidence for their role in the prevention of type 2 diabetes is available.
For dietary patterns, quality matters too. For instance, plant-based diets are generally considered healthy, but not all such diets are alike. In one study, plant-based diets that were high in refined carbohydrates or were ultra-processed were associated positively with the incidence of type 2 diabetes [ 62 ].
Embracing complexity: key messages
Diet is a complex risk exposure.
Diet is non-binary, unlike, for example, tobacco, for which zero is best. Diet is multidimensional and hierarchical in nature. Foods belong within food groups and may be consumed unprocessed (e.g. beef or pork) or processed (e.g. ham or bacon). Foods contain nutrients (e.g. meat fat or protein as macronutrients; haem iron as a micronutrient) or additives and preservatives if processed, and are part of overall dietary patterns (e.g. the Mediterranean diet with relatively low intakes of red meat or a low-carbohydrate diet regimen with relatively high intakes of meat).
The continuum of dietary exposures should be considered, as well as ‘food substitution effects’, because when more or less of one food type is consumed it impacts the consumption of other foods as part of the overall energy intake.
Diet is hard to measure
Tools such as food frequency questionnaires or 24 h dietary recall instruments are commonly used to assess habitual dietary intakes. Despite efforts towards validating these tools and their ability to produce credible estimates of diet–disease associations, critics have called for them to be abandoned, considering them flawed because of their reliance on memory and cognition and issues of bias and measurement error [ 63 , 64 ]. Suggestions for suitable alternatives are sparse, however. Emerging digital technologies—smartphone apps, cameras for food imaging and wearable devices—hold promise but are not yet of ‘research grade’, with demonstrable validity and reliability [ 65 ]. They are also not free from measurement error, nor gaming, consciously or subconsciously. A promising complementary approach is the use of objective biomarkers of dietary intakes, for instance plasma vitamin C and carotenoids as markers for fruit and vegetable intake, or plasma omega-3 fatty acids as a marker for seafood consumption [ 66 ]. However, these too have sources of random and systematic errors as well as interpretive challenges, that is, the extent to which circulating levels reflect intake compared with metabolism.
No method is perfect, but the use of validated dietary instruments with repeat measures can approximate habitual diet. Moreover, there are benefits in using a combination of methods to harness their complementary strengths and deal with relative weaknesses.
The study design of nutritional research is challenging
The RCT design is considered the gold standard in the hierarchy of evidence-based medicine framework, but for complex behavioural exposures such as diet, unlike for pharmaceuticals, RCTs are more challenging. The bulk of the evidence base for nutrition and health has come from long-term observational prospective cohort studies. Both observational and interventional studies have relative strengths and weaknesses. Observational studies are typically limited by confounding and bias but when rigorously conducted they can yield reliable and valid results, from which causal inference can be made [ 14 ]. Dietary RCTs have several challenges. They have a specific set of limitations including a lack of blinding, lack of an appropriate control group, issues with feasibility and cost and challenges of adherence and attrition. The inability to pinpoint the specific nutritional component(s) is another challenge, such as in some of the above-cited RCTs, which could not separate out the effects of macronutrient type and energy intake. Moreover, dietary trials can vary greatly in quality, and consistency of findings and comparability are limited by the populations and endpoints included, for example healthy or diseased participants, free-living or tightly controlled conditions, and a variety of intermediate endpoints or clinical outcomes. In practice, RCTs also suffer from poor methodology and unreliable findings, as evidenced by an appraisal of nearly 21,000 RCTs [ 67 ].
Causal inference is strengthened when there is consistent evidence from different study designs. Inferring causality from observational evidence is possible by applying the Bradford Hill criteria, and Mendelian randomisation is a tool that can be applied in some situations to evaluate causal relationships [ 68 ].
No design is perfect and the evolution of improvements in all study designs—RCTs and observational studies—must continue. New concepts are emerging, such as ‘ n -of-1’ trials and adaptive trial design, which need robust testing in the nutrition field. There is strong concordance in findings from prospective observational studies and RCTs and the two study designs should complement each other [ 7 ]. The best evidence base is that which evaluates all the relevant diverse types of evidence.
Uncertainty remains for some dietary factors
Consensus on the potential benefits and harms of many foods and dietary patterns has been established. However, for some dietary factors controversy remains, for example in the case of non-nutritive or artificial sweeteners such as aspartame, saccharin and sucralose. These sugar substitutes can help decrease daily energy and carbohydrate intakes but whether they are helpful for obesity and type 2 diabetes in the long term is debated [ 69 ]. The use of such sweeteners is predicted to rise in line with the public health policy on sugar reduction, which in the UK includes a soft drinks industry levy applied to soft drinks containing high amounts of added sugar; manufacturers have responded to this with reformulations using sugar substitutes. To resolve this uncertainty, future research will ideally use a combination of research designs including well-conducted short-term RCTs and long-term prospective studies and employ nutritional biomarkers of artificial sweeteners.
Noise and confusion are commonplace in the nutritional field
Everyone is interested in food. From news media to social media, books and blogs, information and misinformation on nutritional topics is everywhere. Conflicts of interest cannot always be avoided. Trusted resources are needed, including high-quality research evidence, improved dietary guidelines [ 70 ] and greater involvement of academic institutions and health agencies.
There are many influences on what we eat beyond individual lifestyle choice (Fig. 1 )
There is a gap between dietary advice and dietary intakes. Consider the public health message to eat five portions a day of fruit and vegetables. Despite strong health promotion efforts, ~12% of the population aged over 15 years in Europe meet this goal [ 71 ]. In a global context, compliance with eating five portions a day of fruit and vegetables is affected disproportionately by income, such that achieving this goal costs an estimated 52%, 18%, 16% and 2% of household income in low-, low- to middle-, middle- to upper- and high-income countries, respectively [ 72 ]. Further, sobering current examples of wider determinants of food choice include the effects of Brexit, the COVID-19 pandemic and the Russian invasion of Ukraine on availability, access and food security.
To improve and maintain dietary adherence, there is a need to operate both at the individual level and in the policy space across the entire food system (see Text box ‘Strategies to promote dietary adherence to healthy eating’). Education, dietary guidelines and strategies that enable people to make healthy food choices are necessary but not yet universally available.

Interest has recently risen in ‘food is medicine’ interventions in healthcare systems, such that a healthy diet can be prescribed in a manner equivalent to the prescription of medication, particularly for those with food insecurity. Such interventions include food prescriptions or the provision of medically tailored groceries or meals, which in those with diabetes can achieve improvements in diet quality and in HbA 1c of a comparable magnitude to those seen with glucose-lowering medication [ 73 ]. Pilot data in people with uncontrolled type 2 diabetes and food insecurity are impressive, with substantial reductions in HbA 1c in those enrolled to receive fresh food on prescription [ 74 ]. Similarly, a meta-analysis of healthy food prescription programmes reported that an increase in consumption of fruit and vegetables by a mean of 0.8 daily servings was associated with significant reductions in BMI and HbA 1c [ 75 ]. Although there were methodological limitations, these studies highlight the potential effectiveness of such dietary interventions and the case for investment in further research.
There are exciting new developments on the horizon
This is illustrated by two examples. First, greater understanding of the relationships between eating and circadian biology is emerging to shed light on so-called chrononutrition [ 76 ]. In addition to considerations of quantity and quality appraised above, chrononutrition considers the impact of the timing of food intake on metabolic health. As an example, the benefits of intermittent fasting and time-restricted feeding are becoming apparent for weight loss [ 77 ] and health more broadly [ 78 ], but research specifically targeted at type 2 diabetes is needed. Second, to improve on current dietary guidance, which is based on population averages, promising research on ‘precision nutrition’ aims to combine information from personal, biological, social and environmental factors to target individuals or population subgroups sharing similar characteristics [ 79 ]. Although still in its infancy, the use of technologies that enable information from genetics, metabolomics, proteomics and the gut microbiome to be integrated with clinical and biochemical data together with machine learning has the potential to enable the development of personalised nutrition interventions [ 80 ].
Conclusions
Diet and nutrition play a central role in both the prevention and the management of type 2 diabetes but the complexity of diet and some key controversies have posed challenges in the field. The latest research evidence has advanced our understanding of the importance of shifting away from the decades-long focus on the quantity of isolated nutrients to nutrient quality, nutrient food sources and overall dietary patterns. New advances in research hold promise for helping to resolve current ongoing uncertainties, and exciting future directions are anticipated (see Text box ‘Future directions: food for thought’).
Abbreviations
Dietary Approaches to Stop Hypertension
Glycaemic index
Glycaemic load
National Health Service
Sun H, Saeedi P, Karuranga S et al (2022) IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract 183:109119. https://doi.org/10.1016/j.diabres.2021.109119
Article PubMed Google Scholar
Franz MJ, MacLeod J, Evert A et al (2017) Academy of nutrition and dietetics nutrition practice guideline for type 1 and type 2 diabetes in adults: systematic review of evidence for medical nutrition therapy effectiveness and recommendations for integration into the nutrition care process. J Acad Nutr Diet 117:1659–1679. https://doi.org/10.1016/j.jand.2017.03.022
Tuomilehto J, Lindstrom J, Eriksson JG et al (2001) Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 344:1343–1350. https://doi.org/10.1056/NEJM200105033441801
Article CAS PubMed Google Scholar
Galaviz KI, Weber MB, Straus A, Haw JS, Narayan KMV, Ali MK (2018) Global diabetes prevention interventions: a systematic review and network meta-analysis of the real-world impact on incidence, weight, and glucose. Diabetes Care 41:1526–1534. https://doi.org/10.2337/dc17-2222
Article PubMed PubMed Central Google Scholar
Valabhji J, Barron E, Bradley D et al (2020) Early outcomes from the english national health service diabetes prevention programme. Diabetes Care 43:152–160. https://doi.org/10.2337/dc19-1425
Taylor R, Ramachandran A, Yancy WS Jr, Forouhi NG (2021) Nutritional basis of type 2 diabetes remission. BMJ 374:n1449. https://doi.org/10.1136/bmj.n1449
Mozaffarian D, Forouhi NG (2018) Dietary guidelines and health-is nutrition science up to the task? Bmj 360:k822. https://doi.org/10.1136/bmj.k822
Forouhi NG, Misra A, Mohan V, Taylor R, Yancy W (2018) Dietary and nutritional approaches for prevention and management of type 2 diabetes. BMJ 361:k2234. https://doi.org/10.1136/bmj.k2234
Mozaffarian D (2016) Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: a comprehensive review. Circulation 133:187–225. https://doi.org/10.1161/CIRCULATIONAHA.115.018585
Article CAS PubMed PubMed Central Google Scholar
Ley SH, Hamdy O, Mohan V, Hu FB (2014) Prevention and management of type 2 diabetes: dietary components and nutritional strategies. Lancet 383:1999–2007. https://doi.org/10.1016/S0140-6736(14)60613-9
Schwingshackl L, Hoffmann G, Lampousi AM et al (2017) Food groups and risk of type 2 diabetes mellitus: a systematic review and meta-analysis of prospective studies. Eur J Epidemiol 32:363–375. https://doi.org/10.1007/s10654-017-0246-y
Micha R, Shulkin ML, Penalvo JL et al (2017) Etiologic effects and optimal intakes of foods and nutrients for risk of cardiovascular diseases and diabetes: Systematic reviews and meta-analyses from the Nutrition and Chronic Diseases Expert Group (NutriCoDE). PLoS One 12:e0175149. https://doi.org/10.1371/journal.pone.0175149
Neuenschwander M, Ballon A, Weber KS et al (2019) Role of diet in type 2 diabetes incidence: umbrella review of meta-analyses of prospective observational studies. BMJ 366:l2368. https://doi.org/10.1136/bmj.l2368
Miller V, Micha R, Choi E, Karageorgou D, Webb P, Mozaffarian D (2022) Evaluation of the quality of evidence of the association of foods and nutrients with cardiovascular disease and diabetes: a systematic review. JAMA Netw Open 5:e2146705. https://doi.org/10.1001/jamanetworkopen.2021.46705
Evert AB, Dennison M, Gardner CD et al (2019) Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care 42:731–754. https://doi.org/10.2337/dci19-0014
Diabetes UK (2018) Evidence-based nutrition guidelines for the prevention and management of diabetes. Available from https://diabetes-resources-production.s3.eu-west-1.amazonaws.com/resources-s3/2018-03/1373_Nutrition%20guidelines_0.pdf . Accessed 25 Nov 2022.
Lingvay I, Sumithran P, Cohen RV, le Roux CW (2022) Obesity management as a primary treatment goal for type 2 diabetes: time to reframe the conversation. Lancet 399:394–405. https://doi.org/10.1016/S0140-6736(21)01919-X
American Diabetes Association Professional Practice Committee, Draznin B, Aroda VR et al (2022) 8. Obesity and Weight Management for the Prevention and Treatment of Type 2 Diabetes: Standards of Medical Care in Diabetes-2022. Diabetes Care 45:S113–S124. https://doi.org/10.2337/dc22-S008
Article Google Scholar
Sjöström L, Lindroos A-K, Peltonen M et al (2004) Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 351:2683–2693. https://doi.org/10.1056/NEJMoa035622
Panunzi S, Carlsson L, De Gaetano A et al (2016) Determinants of diabetes remission and glycemic control after bariatric surgery. Diabetes Care 39:166–174. https://doi.org/10.2337/dc15-0575
Lean ME, Leslie WS, Barnes AC et al (2018) Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet 391:541–551. https://doi.org/10.1016/S0140-6736(17)33102-1
Lean MEJ, Leslie WS, Barnes AC et al (2019) Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol 7:344–355. https://doi.org/10.1016/S2213-8587(19)30068-3
Gregg EW, Chen H, Wagenknecht LE et al (2012) Association of an intensive lifestyle intervention with remission of type 2 diabetes. JAMA 308:2489–2496. https://doi.org/10.1001/jama.2012.67929
Churuangsuk C, Hall J, Reynolds A, Griffin SJ, Combet E, Lean MEJ (2022) Diets for weight management in adults with type 2 diabetes: an umbrella review of published meta-analyses and systematic review of trials of diets for diabetes remission. Diabetologia 65:14–36. https://doi.org/10.1007/s00125-021-05577-2
Ge L, Sadeghirad B, Ball GDC et al (2020) Comparison of dietary macronutrient patterns of 14 popular named dietary programmes for weight and cardiovascular risk factor reduction in adults: systematic review and network meta-analysis of randomised trials. BMJ 369:m696. https://doi.org/10.1136/bmj.m696
Tobias DK, Chen M, Manson JE, Ludwig DS, Willett W, Hu FB (2015) Effect of low-fat diet interventions versus other diet interventions on long-term weight change in adults: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 3:968–979. https://doi.org/10.1016/S2213-8587(15)00367-8
Westerterp-Plantenga MS, Lejeune MPGM, Nijs I, van Ooijen M, Kovacs EMR (2003) High protein intake sustains weight maintenance after body weight loss in humans. Int J Obes 28:57–64. https://doi.org/10.1038/sj.ijo.0802461
Article CAS Google Scholar
Larsen TM, Dalskov S-M, van Baak M et al (2010) Diets with high or low protein content and glycemic index for weight-loss maintenance. N Engl J Med 363:2102–2113. https://doi.org/10.1056/NEJMoa1007137
Thomas JG, Bond DS, Phelan S, Hill JO, Wing RR (2014) Weight-loss maintenance for 10 years in the national weight control registry. Am J Prev Med 46:17–23. https://doi.org/10.1016/j.amepre.2013.08.019
Athinarayanan SJ, Adams RN, Hallberg SJ et al (2019) Long-term effects of a novel continuous remote care intervention including nutritional ketosis for the management of type 2 diabetes: a 2-year non-randomized clinical trial. Front Endocrinol (Lausanne) 10:348. https://doi.org/10.3389/fendo.2019.00348
Saslow LR, Summers C, Aikens JE, Unwin DJ (2018) Outcomes of a digitally delivered low-carbohydrate type 2 diabetes self-management program: 1-year results of a single-arm longitudinal study. JMIR Diabetes 3:e12. https://doi.org/10.2196/diabetes.9333
Huntriss R, Campbell M, Bedwell C (2018) The interpretation and effect of a low-carbohydrate diet in the management of type 2 diabetes: a systematic review and meta-analysis of randomised controlled trials. Eur J Clin Nutr 72:311–325. https://doi.org/10.1038/s41430-017-0019-4
Korsmo-Haugen HK, Brurberg KG, Mann J, Aas AM (2019) Carbohydrate quantity in the dietary management of type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab 21:15–27. https://doi.org/10.1111/dom.13499
Sainsbury E, Kizirian NV, Partridge SR, Gill T, Colagiuri S, Gibson AA (2018) Effect of dietary carbohydrate restriction on glycemic control in adults with diabetes: a systematic review and meta-analysis. Diabetes Res Clin Pract 139:239–252. https://doi.org/10.1016/j.diabres.2018.02.026
van Zuuren EJ, Fedorowicz Z, Kuijpers T, Pijl H (2018) Effects of low-carbohydrate- compared with low-fat-diet interventions on metabolic control in people with type 2 diabetes: a systematic review including GRADE assessments. Am J Clin Nutr 108:300–331. https://doi.org/10.1093/ajcn/nqy096
Snorgaard O, Poulsen GM, Andersen HK, Astrup A (2017) Systematic review and meta-analysis of dietary carbohydrate restriction in patients with type 2 diabetes. BMJ Open Diabetes Res Care 5:e000354. https://doi.org/10.1136/bmjdrc-2016-000354
McArdle PD, Greenfield SM, Rilstone SK, Narendran P, Haque MS, Gill PS (2019) Carbohydrate restriction for glycaemic control in Type 2 diabetes: a systematic review and meta-analysis. Diabet Med 36:335–348. https://doi.org/10.1111/dme.13862
Goldenberg JZ, Day A, Brinkworth GD et al (2021) Efficacy and safety of low and very low carbohydrate diets for type 2 diabetes remission: systematic review and meta-analysis of published and unpublished randomized trial data. BMJ 372:m4743. https://doi.org/10.1136/bmj.m4743
Scientific Advisory Committee on Nutrition (2021) Lower carbohydrate diets for adults with type 2 diabetes. Available from https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1014673/SACN_report_on_lower_carbohydrate_diets_for_type_2_diabetes.pdf . Accessed 25 Nov 2022
Riddle MC, Cefalu WT, Evans PH et al (2021) Consensus report: definition and interpretation of remission in type 2 diabetes. Diabetes Care 44:2438–2444. https://doi.org/10.2337/dci21-0034
Nordmann AJ, Nordmann A, Briel M et al (2006) Effects of low-carbohydrate vs low-fat diets on weight loss and cardiovascular risk factors: a meta-analysis of randomized controlled trials. Arch Intern Med 166:285–293. https://doi.org/10.1001/archinte.166.3.285
Churuangsuk C, Griffiths D, Lean MEJ, Combet E (2019) Impacts of carbohydrate-restricted diets on micronutrient intakes and status: a systematic review. Obes Rev 20:1132–1147. https://doi.org/10.1111/obr.12857
Seidelmann SB, Claggett B, Cheng S et al (2018) Dietary carbohydrate intake and mortality: a prospective cohort study and meta-analysis. Lancet Public Health 3:e419–e428. https://doi.org/10.1016/S2468-2667(18)30135-X
Ludwig DS, Ebbeling CB (2018) The carbohydrate-insulin model of obesity: beyond “Calories In, Calories Out”. JAMA Intern Med 178:1098–1103. https://doi.org/10.1001/jamainternmed.2018.2933
Ludwig DS, Aronne LJ, Astrup A et al (2021) The carbohydrate-insulin model: a physiological perspective on the obesity pandemic. Am J Clin Nutr 114:1873–1885. https://doi.org/10.1093/ajcn/nqab270
Hall KD, Farooqi IS, Friedman JM et al (2022) The energy balance model of obesity: beyond calories in, calories out. Am J Clin Nutr 115:1243–1254. https://doi.org/10.1093/ajcn/nqac031
Ludwig DS, Apovian CM, Aronne LJ et al (2022) Competing paradigms of obesity pathogenesis: energy balance versus carbohydrate-insulin models. Eur J Clin Nutr 76:1209–1221. https://doi.org/10.1038/s41430-022-01179-2
Mozaffarian D (2017) Foods, obesity, and diabetes-are all calories created equal? Nutr Rev 75:19–31. https://doi.org/10.1093/nutrit/nuw024
Reynolds A, Mann J, Cummings J, Winter N, Mete E, Te Morenga L (2019) Carbohydrate quality and human health: a series of systematic reviews and meta-analyses. Lancet 393:434–445. https://doi.org/10.1016/S0140-6736(18)31809-9
Kuijsten A, Aune D, Schulze MB et al (2015) Dietary fibre and incidence of type 2 diabetes in eight European countries: the EPIC-InterAct Study and a meta-analysis of prospective studies. Diabetologia 58:1394–1408. https://doi.org/10.1007/s00125-015-3585-9
Sacks FM, Carey VJ, Anderson CA et al (2014) Effects of high vs low glycemic index of dietary carbohydrate on cardiovascular disease risk factors and insulin sensitivity: the OmniCarb randomized clinical trial. JAMA 312:2531–2541. https://doi.org/10.1001/jama.2014.16658
Livesey G, Livesey H (2019) Coronary heart disease and dietary carbohydrate, glycemic index, and glycemic load: dose-response meta-analyses of prospective cohort studies. Mayo Clin Proc Innov Qual Outcomes 3:52–69. https://doi.org/10.1016/j.mayocpiqo.2018.12.007
Jenkins DJA, Dehghan M, Mente A et al (2021) Glycemic index, glycemic load, and cardiovascular disease and mortality. N Engl J Med 384:1312–1322. https://doi.org/10.1056/NEJMoa2007123
Chiavaroli L, Lee D, Ahmed A et al (2021) Effect of low glycaemic index or load dietary patterns on glycaemic control and cardiometabolic risk factors in diabetes: systematic review and meta-analysis of randomised controlled trials. Bmj 374:n1651. https://doi.org/10.1136/bmj.n1651
Gardner CD, Trepanowski JF, Del Gobbo LC et al (2018) Effect of low-fat vs low-carbohydrate diet on 12-month weight loss in overweight adults and the association with genotype pattern or insulin secretion: the DIETFITS randomized clinical trial. Jama 319:667–679. https://doi.org/10.1001/jama.2018.0245
Gardner CD, Landry MJ, Perelman D et al (2022) Effect of a ketogenic diet versus mediterranean diet on HbA1c in individuals with prediabetes and type 2 diabetes mellitus: the interventional keto-med randomized crossover trial. Am J Clin Nutr 116:640–652. https://doi.org/10.1093/ajcn/nqac154
Key TJ, Appleby PN, Bradbury KE et al (2019) Consumption of meat, fish, dairy products, and eggs and risk of ischemic heart disease. Circulation 139:2835–2845. https://doi.org/10.1161/CIRCULATIONAHA.118.038813
Steur M, Johnson L, Sharp SJ et al (2021) Dietary fatty acids, macronutrient substitutions, food sources and incidence of coronary heart disease: findings from the EPIC-CVD case-cohort study across nine european countries. J Am Heart Assoc 10:e019814. https://doi.org/10.1161/JAHA.120.019814
Hall KD, Ayuketah A, Brychta R et al (2019) Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake. Cell Metab 30:67–77 e63. https://doi.org/10.1016/j.cmet.2019.05.008
Delpino FM, Figueiredo LM, Bielemann RM et al (2021) Ultra-processed food and risk of type 2 diabetes: a systematic review and meta-analysis of longitudinal studies. Int J Epidemiol 51:1120–1141. https://doi.org/10.1093/ije/dyab247
Schulze MB, Martinez-Gonzalez MA, Fung TT, Lichtenstein AH, Forouhi NG (2018) Food based dietary patterns and chronic disease prevention. BMJ 361:k2396. https://doi.org/10.1136/bmj.k2396
Satija A, Bhupathiraju SN, Rimm EB et al (2016) Plant-based dietary patterns and incidence of type 2 diabetes in US men and women: results from three prospective cohort studies. PLoS Med 13:e1002039. https://doi.org/10.1371/journal.pmed.1002039
Archer E, Marlow ML, Lavie CJ (2018) Controversy and debate: memory-based methods paper 1: the fatal flaws of food frequency questionnaires and other memory-based dietary assessment methods. J Clin Epidemiol 104:113–124. https://doi.org/10.1016/j.jclinepi.2018.08.003
Archer E, Lavie CJ, Hill JO (2018) The failure to measure dietary intake engendered a fictional discourse on diet-disease relations. Front Nutr 5:105. https://doi.org/10.3389/fnut.2018.00105
Eldridge A, Piernas C, Illner A-K et al (2019) Evaluation of new technology-based tools for dietary intake assessment—an ILSI europe dietary intake and exposure task force evaluation. Nutrients 11:55. https://doi.org/10.3390/nu11010055
Zheng JS, Sharp SJ, Imamura F et al (2020) Association of plasma biomarkers of fruit and vegetable intake with incident type 2 diabetes: EPIC-InterAct case-cohort study in eight European countries. BMJ 370:m2194. https://doi.org/10.1136/bmj.m2194
Dechartres A, Trinquart L, Atal I et al (2017) Evolution of poor reporting and inadequate methods over time in 20 920 randomised controlled trials included in Cochrane reviews: research on research study. Bmj 357:j2490. https://doi.org/10.1136/bmj.j2490
Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey SG (2008) Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med 27:1133–1163. https://doi.org/10.1002/sim.3034
Lohner S, Kuellenberg de Gaudry D, Toews I, Ferenci T, Meerpohl JJ (2020) Non-nutritive sweeteners for diabetes mellitus. Cochrane Database Syst Rev 5:CD012885. https://doi.org/10.1002/14651858.CD012885.pub2
Bero LA, Norris SL, Lawrence MA (2019) Making nutrition guidelines fit for purpose. Bmj 365:l1579. https://doi.org/10.1136/bmj.l1579
Eurostat (2022) How much fruit and vegetables do you eat daily? Available from https://ec.europa.eu/eurostat/web/products-eurostat-news/-/ddn-20220104-1 . Accessed 25 Nov 2022
Miller V, Yusuf S, Chow CK et al (2016) Availability, affordability, and consumption of fruits and vegetables in 18 countries across income levels: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet Global Health 4:e695–e703. https://doi.org/10.1016/S2214-109X(16)30186-3
Sherifali D, Nerenberg K, Pullenayegum E, Cheng JE, Gerstein HC (2010) The effect of oral antidiabetic agents on A1C levels: a systematic review and meta-analysis. Diabetes Care 33:1859–1864. https://doi.org/10.2337/dc09-1727
Hess A, Passaretti M, Coolbaugh S (2019) Fresh food farmacy. Am J Health Promotion 33:830–832. https://doi.org/10.1177/0890117119845711d
Bhat S, Coyle DH, Trieu K et al (2021) Healthy food prescription programs and their impact on dietary behavior and cardiometabolic risk factors: a systematic review and meta-analysis. Adv Nutr (Bethesda, Md) 12:1944–1956. https://doi.org/10.1093/advances/nmab039
Hawley JA, Sassone-Corsi P, Zierath JR (2020) Chrono-nutrition for the prevention and treatment of obesity and type 2 diabetes: from mice to men. Diabetologia 63:2253–2259. https://doi.org/10.1007/s00125-020-05238-w
Welton S, Minty R, O'Driscoll T et al (2020) Intermittent fasting and weight loss: systematic review. Can Fam Physician 66:117–125
PubMed PubMed Central Google Scholar
de Cabo R, Mattson MP (2019) Effects of intermittent fasting on health, aging, and disease. N Engl J Med 381:2541–2551. https://doi.org/10.1056/NEJMra1905136
Merino J (2022) Precision nutrition in diabetes: when population-based dietary advice gets personal. Diabetologia 65:1839–1848. https://doi.org/10.1007/s00125-022-05721-6
National Institutes of Health (2022) NIH awards $170 million for precision nutrition study 2022. Available from https://www.nih.gov/news-events/news-releases/nih-awards-170-million-precision-nutrition-study . Accessed 25 Nov 2022
Feinman RD, Pogozelski WK, Astrup A et al (2015) Dietary carbohydrate restriction as the first approach in diabetes management: critical review and evidence base. Nutrition 31:1–13. https://doi.org/10.1016/j.nut.2014.06.011
Scientific Advisory Committee on Nutrition (2015) Carbohydrates and health. TSO, London. Available from https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/445503/SACN_Carbohydrates_and_Health.pdf . Accessed 6 Feb 2023
Afshin A, Micha R, Khatibzadeh S et al (2014) Dietary policies to reduce non-communicable diseases. In: Brown GW, Yamey G, Wamala S (eds) The handbook of global health policy, 1st edn. Wiley, Chichester, UK, pp 175–193
Chapter Google Scholar
Download references
Acknowledgements
I acknowledge D. Bhagtani’s help with Fig. 1 (MRC Epidemiology Unit, University of Cambridge School of Clinical Medicine).
Author’s relationships and activities
NGF was a member of the Joint Scientific Advisory Committee on Nutrition/NHS England/Diabetes UK Working Group, which was initiated to review the evidence on lower carbohydrate diets compared with current government advice for adults with type 2 diabetes. The views expressed are her own and not those of the Group.
Contribution statement
The author was the sole contributor to this article.
NGF is supported by the Medical Research Council Epidemiology Unit (MC_UU_00006/3) and the NIHR Biomedical Research Centre Cambridge: Nutrition, Diet, and Lifestyle Research Theme (IS-BRC-1215-20014). She is an NIHR Senior Investigator. The views expressed are those of the author and not necessarily those of the NIHR or the Department of Health and Social Care.
Author information
Authors and affiliations.
MRC Epidemiology Unit, University of Cambridge School of Clinical Medicine, Institute of Metabolic Science, Cambridge Biomedical Campus, Cambridge, UK
Nita G. Forouhi
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Nita G. Forouhi .
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Figure slide.
(PPTX 232 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Forouhi, N.G. Embracing complexity: making sense of diet, nutrition, obesity and type 2 diabetes. Diabetologia 66 , 786–799 (2023). https://doi.org/10.1007/s00125-023-05873-z
Download citation
Received : 27 July 2022
Accepted : 13 December 2022
Published : 14 February 2023
Issue Date : May 2023
DOI : https://doi.org/10.1007/s00125-023-05873-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Epidemiology
- Study design
- Type 2 diabetes
- Find a journal
- Publish with us
- Track your research

Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- ASCEND for Better Health

At USDA we take a “food first” approach to improving human health and well-being and reducing the burden of chronic diet-related diseases. When it comes to using food and nutrition to improve health-related outcomes, one size doesn’t fit all. This requires a more precise understanding of nutritional needs, and broader consideration of the social, economic, and cultural factors that influence food selection. Our research efforts focus on the entire food and ag value chain to better understand how farming practices, different crops and animals, food selection, food preparation, and nutritional content and composition contribute to health-related outcomes.
Featured Stories
Usda invests more than $59m to improve dietary health and nutrition security.

Keep Cool and Stay Fresh

Research and Extension Supports Local Food Systems

Selected Resources
Agricultural research service.
Arkansas Children's Nutrition Center
Beltsville Human Nutrition Research Center
Children's Nutrition Research Center at Baylor College of Medicine
Grand Forks Human Nutrition Research Center
Jean Mayer Human Nutrition Research Center on Aging at Tufts University
Western Human Nutrition Research Center
U.S. Plant, Soil, and Nutrition Laboratory
Food Systems Research Unit, Burlington, VT
Responsive Agricultural Food Systems Research, College Station, TX
Economic Research Service
Diet Quality and Nutrition
Food Choices & Health
Food Insecurity, Chronic Disease, and Health Among Working-Age Adults
Racial and Ethnic Diversification Will Likely Shape U.S. Food Demand and Diet Quality
Use of Nutrition Information and the Food Healthfulness Gap
National Institute of Food and Agriculture (NIFA)
Food and Nutrition Security
Nutrition and Food Systems
NIFA Grants
Foundational and Applied Science Program
Sustainable Agricultural Systems
Gus Schumacher Nutrition Incentive Program
Expanded Food and Nutrition Education Program (EFNEP)
Other Key Federal Partners and Federal Initiatives
Interagency Committee on Human Nutrition Research
National Institutes of Health – Office of Nutrition Research
Food and Nutrition Service – Office of Policy Support
White House Conference on Hunger, Nutrition, and Health
White House Cancer Moonshot Initiative
USDA Cancer Moonshot Initiative
- Open access
- Published: 27 January 2023
Toward nutrition improving outcome of critically ill patients: How to interpret recent feeding RCTs?
- Jan Gunst ORCID: orcid.org/0000-0003-2470-6393 1 ,
- Michael P. Casaer ORCID: orcid.org/0000-0002-7087-0795 1 ,
- Jean-Charles Preiser ORCID: orcid.org/0000-0003-3163-0390 2 ,
- Jean Reignier ORCID: orcid.org/0000-0002-3768-3496 3 &
- Greet Van den Berghe ORCID: orcid.org/0000-0002-5320-1362 1
Critical Care volume 27 , Article number: 43 ( 2023 ) Cite this article
8877 Accesses
16 Citations
18 Altmetric
Metrics details
Although numerous observational studies associated underfeeding with poor outcome, recent randomized controlled trials (RCTs) have shown that early full nutritional support does not benefit critically ill patients and may induce dose-dependent harm. Some researchers have suggested that the absence of benefit in RCTs may be attributed to overrepresentation of patients deemed at low nutritional risk, or to a too low amino acid versus non-protein energy dose in the nutritional formula. However, these hypotheses have not been confirmed by strong evidence. RCTs have not revealed any subgroup benefiting from early full nutritional support, nor benefit from increased amino acid doses or from indirect calorimetry-based energy dosing targeted at 100% of energy expenditure. Mechanistic studies attributed the absence of benefit of early feeding to anabolic resistance and futile catabolism of extra provided amino acids, and to feeding-induced suppression of recovery-enhancing pathways such as autophagy and ketogenesis, which opened perspectives for fasting-mimicking diets and ketone supplementation. Yet, the presence or absence of an anabolic response to feeding cannot be predicted or monitored and likely differs over time and among patients. In the absence of such monitor, the value of indirect calorimetry seems obscure, especially in the acute phase of illness. Until now, large feeding RCTs have focused on interventions that were initiated in the first week of critical illness. There are no large RCTs that investigated the impact of different feeding strategies initiated after the acute phase and continued after discharge from the intensive care unit in patients recovering from critical illness.
In critically ill patients, severe physical stress induces a catabolic response, leading to muscle wasting and weakness [ 1 ]. The longer the stay in the intensive care unit (ICU), the higher the risk of weakness, and the poorer the outcome [ 1 , 2 ]. Indeed, severe weakness may preclude weaning from mechanical ventilation and may cause life-threatening complications by difficulties to cough up secretions and swallowing dysfunction, among others [ 1 ]. Also in patients surviving critical illness, persistent weakness is considered part of the post-intensive care syndrome [ 3 , 4 , 5 ]. Apart from weakness, also increased bone resorption may occur, with increased fracture risk after intensive care [ 5 , 6 , 7 ].
To counteract catabolism, nutrition has been advocated, since prolonged underfeeding could contribute to catabolism [ 8 ]. Moreover, some patients already have sarcopenia and prolonged low nutrition intake before ICU admission. Numerous observational studies have associated increased nutrition intake with improved outcome of critically ill patients [ 9 , 10 ]. Yet, a causal relationship cannot be derived from such associations, since feeding tolerance closely associates with illness severity, with in general a better feeding tolerance in patients who are less ill. Hence, in observational studies, there is an inherent risk of residual confounding. Until a decade ago, in view of the absence of large randomized controlled trials (RCTs), European experts advocated to avoid any caloric or protein deficit in critically ill patients, and to start early artificial feeding, especially in patients considered to be at high nutritional risk [ 8 ]. Since then, however, several large RCTs have shown that early full feeding did not benefit adult and pediatric critically ill patients, and some even showed harm [ 11 , 12 , 13 , 14 , 15 ]. These at first sight counterintuitive results indicate that critical illness-associated catabolism is much more complex than merely a consequence of underfeeding, and that anorexia and temporary starvation may to some extent be an adaptive component of the stress response to severe illness. We here summarize the RCT evidence and review potential mechanisms explaining the negative results of recent feeding RCTs, which will hopefully guide future research and which may ultimately lead to individualization of feeding.
The impact of early full feeding in critical illness: evidence from recent feeding RCTs
As shown in a recent meta-analysis, no large-scale RCT in critically ill patients found benefit by early full feeding, as compared to more restrictive feeding regimens [ 16 ]. Two RCTs—one in adults and one in children—even found significant harm by early supplementation of insufficient or contraindicated enteral nutrition with parenteral nutrition. Indeed, in both the adult EPaNIC ( N = 4640) and pediatric PEPaNIC RCTs ( N = 1440), providing early supplemental parenteral nutrition prolonged ICU dependency, with increased dependency on vital organ support and incidence of new infections as compared to withholding supplemental parenteral nutrition until one week after ICU admission [ 11 , 12 ]. In adults, early supplemental parenteral nutrition further increased the incidence of ICU-acquired weakness, and hampered recovery hereof [ 17 ]. In theory, harm by early supplemental parenteral nutrition could be explained by an increased nutritional dose, or by an inferior feeding route. However, 2 large RCTs in adults—the CALORIES ( N = 2400) and Nutrirea-2 ( N = 2410) RCT—showed no harm by parenteral nutrition when provided at isocaloric doses as enteral nutrition [ 18 , 19 ], suggesting that harm by early supplemental parenteral nutrition in the EPaNIC and PEPaNIC RCTs is explained by the higher nutritional dose, rather than by the intravenous route. Moreover, the large-scale EDEN ( N = 1000), PermiT ( N = 894) and TARGET ( N = 3957) RCTs, which compared early full enteral nutrition with lower-dose enteral nutrition for 6, respectively 14 or 28 days in ICU in critically ill adults, did not find benefit with higher nutritional doses [ 13 , 14 , 15 ].Two of these RCTs found more gastrointestinal intolerance with early full enteral nutrition [ 13 , 15 ]. Also in long-term follow-up, providing early enhanced nutrition was not beneficial with regard to functional outcome [ 20 , 21 , 22 , 23 , 24 ]. Of note, the EPaNIC and PEPaNIC RCTs showing harm by early enhanced feeding had the highest relative difference in caloric intake between the 2 study groups, which enhances the statistical power to detect a treatment effect [ 25 ].
Based on this recent high level evidence, the most recent European feeding guidelines for adult critically ill patients shifted from promoting early full feeding to less aggressive artificial feeding in the first week of critical illness [ 26 ]. However, it is important to note that the shift toward providing less feeding in the acute phase should not increase the risk of refeeding syndrome, which is caused by a deficiency in micronutrients and electrolytes, including vitamin B1, potassium and phosphate [ 27 ]. Indeed, when artificial feeding is restarted after a prolonged period of starvation, the metabolic need and intracellular transport of several micronutrients and electrolytes increases, which may unmask preexisting deficiencies and lead to life-threatening symptoms [ 27 ]. A biochemical hallmark of this condition is refeeding hypophosphatemia, which has been defined as a drop in phosphate levels below 0.65 mmol/l within 72 h after institution of artificial feeding [ 28 , 29 ], explained by intracellular uptake and incorporation in energy-rich phosphate bonds. Once refeeding hypophosphatemia occurs early in critical illness, temporarily limiting nutrition intake while correcting existing vitamin and electrolyte deficiencies is likely beneficial, as shown in the Refeeding RCT ( N = 339) [ 28 ]. To prevent refeeding syndrome, it seems prudent to ensure sufficient micronutrient intake in all patients, which may, especially in the acute phase of illness, require parenteral administration of micronutrients and electrolytes [ 11 , 26 , 30 ].
Critiques on recent feeding RCTs
The neutral or negative effect of early enhanced feeding in recent RCTs has been suggested to be explained by over-representation of patients presumed to carry low risk of malnutrition, by administration of too low amino acid doses, and by the use of calculated energy targets [ 31 , 32 , 33 ]. However, these critiques have not been supported by high level evidence and several lines of evidence have contradicted them, as outlined below. The Nutrirea-3 RCT, which randomized 3044 adult patients with shock requiring mechanical ventilation and vasopressor support to early full feeding versus one week of calorie-protein restriction irrespective of the feeding route, recently finalized recruitment and will provide more insight (NCT03573739) [ 34 ].
Inclusion of patients presumed to be at low risk of malnutrition
Researchers have suggested that the absence of benefit in recent RCTs may be explained by including too many patients considered to be at low risk of malnutrition, whereby any potential benefit in perceived high-risk patients may have been obscured by no impact or even harm in hypothesized low-risk patients [ 32 ]. However, this hypothesis is not confirmed by subgroup analyses of RCTs. Indeed, in large RCTs, there was no subgroup of patients identifiable who benefited from early enhanced feeding, as defined by age, the nutritional risk screening (NRS) score, the modified Nutrition Risk in Critically Ill (NUTRIC) score, or body mass index (BMI) upon admission [ 11 , 12 , 13 , 15 , 35 ]. In a secondary analysis of the PermiT RCT, studied biomarkers did not discriminate adult patients who would benefit from early full enteral nutrition as compared to lower-dose enteral nutrition [ 35 ]. If anything, there was a signal in the opposite direction. Indeed, patients with low prealbumin levels, who would be considered at highest nutritional risk, had an increased risk of mortality associated with early full enteral nutrition [ 35 ]. Also in the large EPaNIC subgroup of patients for whom enteral nutrition was contraindicated ( N = 517), early total parenteral nutrition was harmful as compared to virtual starvation for one week in ICU [ 11 ].
Low amino acid doses
It has been suggested that several feeding RCTs did not show benefit because of imbalanced feeding solutions, whereby the doses of amino acids would have been too low [ 31 ]. However, the largest RCT on amino acid supplements in adult critically ill patients, the Nephroprotective RCT ( N = 474), did not find benefit from early amino acid supplements provided at doses of approximately 1.75 g/kg per day throughout ICU stay, while significantly increasing ureagenesis [ 36 ]. Also, in other RCTs in both adults and children, early full feeding significantly increased ureagenesis [ 37 , 38 , 39 ]. In a secondary analysis of the EPaNIC RCT, it was estimated that approximately two third of the extra amino acids provided through early parenteral nutrition were net wasted in ureagenesis, even with amino acid doses that are considered relatively low (approximately 0.8 g/kg per day) [ 37 ]. Concomitantly, both microscopic and macroscopic muscle loss were not prevented by providing early full feeding [ 17 , 40 ]. In contrast, early supplementation of insufficient enteral nutrition by parenteral nutrition increased muscular fat content, aggravated muscle weakness, and hampered recovery from weakness [ 17 ]. In secondary analyses of both EPaNIC and PEPaNIC RCTs, harm by early parenteral nutrition was statistically explained by the amino acid doses, and not by the glucose or lipid doses [ 38 , 41 ]. Evidently, these findings are observational and require confirmation in RCTs. Of note, in the absence of solid evidence supporting a clear protein target, the most recent European ESPEN guidelines for adult critically ill patients do not make a strong recommendation [ 26 ]. Instead, there is a grade 0 recommendation suggesting that 1.3 g/kg protein can be delivered progressively [ 26 ]. The EFFORT RCT ( N = 4000; study completed December 3, 2021, with 1329 patients included according to clinicaltrials.gov) will fill this evidence gap, as it investigates whether or not a higher dose of proteins improves outcome of adult critically ill patients [ 42 ].
Calculated energy targets
The absence of benefit of early full feeding has also been attributed to the absence of indirect calorimetry to guide the energy target [ 43 ]. In acute illness, indirect calorimetry is the gold standard to measure energy expenditure, which is derived from measurement of VO2 and VCO2, and the obtained value has been proposed as energy target after the first days in ICU [ 44 ]. In most recent large feeding RCTs, indirect calorimetry was not routinely used, reflecting daily practice in most centers [ 11 , 12 , 13 , 14 , 15 ]. Instead, the energy target was determined by predictive equations that only provide an estimation of energy expenditure that may considerably deviate from the measured energy expenditure [ 11 , 12 , 13 , 14 , 15 , 44 ]. However, there is no solid evidence that the feeding target should equal energy expenditure at all times, since the largest RCTs comparing indirect calorimetry-based feeding versus predictive equation-based feeding in adult critically ill patients did not show clear benefit [ 45 , 46 ]. The EAT-ICU RCT ( N = 199) even found harm, with an increased ICU stay in patients randomized to the intervention group in which early full feeding was guided by indirect calorimetry and by nitrogen balances as compared with the control group in which early enteral nutrition was delivered up to a fixed energy target [ 39 ]. Interestingly, the EAT-ICU intervention resulted in higher protein and energy intake in the first week than the control group, further supporting harm by a higher nutritional dose early during critical illness [ 39 ].
Despite the absence of benefit from using indirect calorimetry to target 100% of energy expenditure by feeding in large RCTs, proponents of its use in the first week of critical illness have referred to the results of a recent meta-analysis that suggested potential mortality benefit by indirect calorimetry-based feeding initiated in the first week in critically ill adults as compared with calculated energy target-based feeding [ 47 , 48 ]. The potential mechanisms of mortality benefit remain unclear, however, since morbidity outcomes did not differ [ 47 ]. Although this meta-analysis may seem encouraging, the results should be interpreted with great caution, for several reasons. First, none of the included studies had a low risk of bias, and the mortality difference was barely significant [ 47 ]. It is highly likely that the statistical difference would be lost if a small number of patients (close to 1)—the fragility index of the study—would have had a different outcome. Moreover, there are concerns with regard to the reported mortality data in the largest RCT, the TICACOS-International RCT ( N = 417) [ 46 , 49 ]. Indeed, the reported mortality at consecutive time points decreased over time in this RCT, which is obviously impossible, and reported numbers in abstract and full text do not match [ 46 ], as reported in a letter to the editor [ 49 ]. Moreover, according to the reported numbers in TICACOS-International, there was only a statistically insignificant, but numerical difference in mortality at 90 days, which was the mortality rate used in the meta-analysis [ 46 , 47 ]. In contrast, reported ICU mortality and mortality at 6 months, not used in the meta-analysis, were virtually identical [ 46 , 47 ]. In view of these important concerns and unresolved issues, the level of evidence put forward by the meta-analysis remains low. Moreover, the TICACOS-International RCT may indirectly question the feasibility of widespread implementation of indirect calorimetry, since the authors, who are experts in the field, only included 417 patients over 6 years in 7 centers, whereby slow recruitment led to premature stopping of the RCT [ 46 ].
Apart from the absence of benefit from full feeding guided by indirect calorimetry in the largest RCTs, there are also pathophysiological concerns with regard to its early use to guide nutritional energy dosing. Indeed, if the ideal energy target would equal energy expenditure at all times, one intrinsically assumes that all endogenous energy production can be suppressed by providing calories by feeding, which is not the case (Fig. 1 ). Indeed, acute critical illness is characterized by feeding-resistant catabolism and severe insulin resistance, especially in the liver, whereby endogenous glucose production cannot be suppressed by providing nutrients and insulin [ 50 ]. Hence, providing extra calories on top of not suppressible gluconeogenesis may aggravate hyperglycemia and hypertriglyceridemia, and may only pose an additional burden on the liver [ 51 ]. Unfortunately, there is no monitor of endogenous glucose production available at the bedside, so the duration and extent of unsuppressible endogenous substrate production of individual patients remain unclear. There are no RCTs that investigated the impact of reducing feeding intake to a fixed percentage of the measured energy expenditure, to compensate for endogenous glucose production [ 52 ]. Also, no large RCTs have investigated the impact of indirect calorimetry-based feeding that is initiated in prolonged critically ill patients and continued after ICU discharge. Of note, the current ESPEN guidelines and recent experts’ opinion do not recommend to match the energy expenditure measured by indirect calorimetry with the feeding target at all times in adult critically ill patients [ 26 , 53 ].
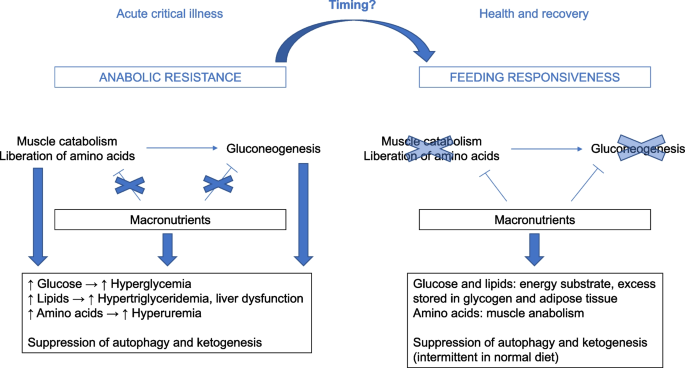
Selected mechanisms explaining the lack of benefit by early full feeding in critical illness. Evoked by the stress response to severe illness, anabolic resistance occurs, whereby muscle catabolism and hepatic gluconeogenesis cannot be counteracted by providing macronutrients, unlike in normal health. Providing extra macronutrients in such condition increases the risk of overfeeding, manifested as hyperglycemia, hypertriglyceridemia, liver dysfunction and hyperuremia by catabolism of extra provided amino acids. In addition, continuous artificial nutrition continuously suppresses autophagy and ketogenesis as potentially important repair pathways. The time when anabolic resistance ceases and the condition reverses into metabolic feeding responsiveness cannot be predicted or monitored at the bedside. Theoretically, feeding responsiveness may undergo dynamic changes over time, and the timing of such changes likely differs between patients
Mechanisms explaining lack of benefit of early full feeding
Suppression of fasting-induced recovery pathways.
The lack of benefit from early full nutrition in RCTs may be explained by a continuous suppression of the fasting response. Although fasting has traditionally been considered a negative process in critical illness [ 8 ], a normal diet involves alteration of feeding periods with fasting intervals, and fasting has been attributed health-promoting effects. Indeed, diets that induce a prolonged fasting response such as fasting-mimicking diets or caloric restriction diets protected against age-related disease and improved longevity in animal models, and ameliorated risk factors of age-related disease in humans [ 54 , 55 ]. This suggests that fasting-activated pathways are important to maintain normal cellular integrity and function. In large feeding RCTs in critically ill patients, however, artificial feeding has always been provided in a continuous manner [ 11 , 12 , 13 , 14 , 15 ], hereby continuously suppressing any fasting response.
Part of the beneficial effects of fasting in normal health are mediated by activation of macroautophagy [ 54 ]. Macroautophagy, hereafter referred to as autophagy, is a cellular process whereby cytoplasmic content is digested in the lysosome after its delivery to the lysosome in an intermediate vesicle that is called an autophagosome [ 56 ]. Autophagy is activated by fasting and by a variety of stress signals [ 56 ]. Particularly deprivation of amino acids is a strong stimulus of autophagy [ 56 ]. Autophagy is the only process able to remove macromolecular damage, including damaged organelles, potentially toxic protein aggregates and intracellular microorganisms and as such, it is a crucial process that is necessary to maintain homeostasis [ 57 ]. Aging is accompanied by a gradual decline in autophagic activity, and activation of autophagy has been shown to protect against age-related disease and to improve life span in animals [ 57 , 58 ]. Increasing evidence also implicates autophagy as crucial repair process to recover from critical illness [ 59 , 60 , 61 ]. Moreover, mechanistic studies have implicated autophagy suppression as potential mechanism explaining harm by early full nutrition in critical illness [ 62 ]. In a critically ill animal model, early parenteral nutrition, especially with higher amino acid doses, increased liver damage and signs of muscle degeneration as compared to relative fasting, while it suppressed autophagy [ 63 ]. In this model, administration of the autophagy activator rapamycin protected against kidney injury in fed critically ill animals [ 64 ]. Likewise, in critically ill patients, early parenteral nutrition suppressed autophagy in muscle, which associated with more weakness [ 17 ]. Altogether, this evidence puts forward autophagy as potential therapeutic target in critical illness. However, pharmacological autophagy activation is complicated, since there are no specific pharmacological autophagy inducers available [ 65 ], and excessive autophagy stimulation may also be detrimental [ 66 ].
A second process that may explain the negative impact of early full feeding in critically ill patients is suppression of ketogenesis. Apart from being an alternative energy substrate during fasting, ketones serve signaling roles, may stimulate autophagy and enhance muscle regeneration [ 67 , 68 ]. In a mouse model of sepsis-induced critical illness, administration of ketones improved muscle force, which appeared not related to its use as energy substrate, but by activating muscle regeneration pathways [ 68 ]. A recent study showed that ketones increase resilience of muscle stem cells to cellular stress via signaling effects [ 69 ]. Secondary analyses of the EPaNIC and PEPaNIC RCTs showed that withholding early parenteral nutrition activated ketogenesis, most robustly in critically ill children, in whom it statistically mediated part of the outcome benefit of the intervention [ 70 , 71 ].
Anabolic resistance
One of the main aims of providing nutrients to critically ill patients is to inhibit or limit critical illness-associated catabolism, which would attenuate muscle wasting and weakness, and improve long-term functional outcome. However, recent nutritional RCTs have shown that early full feeding is unable to counteract catabolism. Indeed, both muscle wasting and weakness were not prevented, and long-term functional outcome was not improved [ 17 , 20 , 21 , 22 , 40 ]. Instead, providing higher doses of amino acids in the acute phase significantly increased ureagenesis in several RCTs [ 36 , 37 , 38 , 39 ]. Currently, there are no bedside monitors or biomarkers that predict or document feeding responsiveness. The failure of artificial feeding to suppress catabolism may to some extent be explained by the so-called muscle-full effect [ 72 , 73 ]. Indeed, in healthy adults, muscle protein synthesis only rises temporarily in response to continuous amino acid infusion [ 72 ]. However, whether bolus feeding or intermittent feeding would indeed lead to more anabolism in critically ill patients remains to be studied. A relatively small RCT in critically ill adults ( N = 121) found lower rise in the urea over creatinine ratio as marker of catabolism by intermittent feeding as compared to continuous feeding, while there was no impact on ultrasound-assessed muscle wasting [ 74 , 75 ]. Moreover, there was already a baseline difference in urea over creatinine ratio, precluding a strong conclusion [ 75 ]. Regardless of the mode of delivering nutrition, the degree of anabolic resistance likely varies over time and among patients, since critical illness-associated catabolism has been related to the stress response and the accompanying inflammatory and endocrine alterations (Fig. 1 ) [ 1 ]. Apart from the muscle-full effect, another potential mechanism contributing to anabolic resistance is the relative immobilization of the patient. Outside critical illness, protein supplementation is most effective in achieving anabolism when combined with exercise [ 76 ]. There are no data regarding early mobilization in large feeding RCTs in critical illness, and large RCTs investigating the interaction between early mobilization and feeding in critically ill patients are lacking [ 77 ]. However, as for early feeding, early enhanced, active mobilization is not beneficial for critically ill patients and increases the risk of adverse events [ 78 ].
Perspectives for future research
These mechanistic insights provide a base for novel feeding regimens, to be developed and to be tested ultimately in RCTs powered for clinical endpoints. Although fasting may activate beneficial cellular pathways that are also essential in normal health, prolonged starvation will likely come at a price. Novel feeding strategies that may exploit these fasting-associated benefits while avoiding prolonged starvation include intermittent feeding, ketogenic diets and ketone supplementation.
Intermittent feeding diets, which alternate feeding with fasting intervals, would theoretically allow to provide feeding while intermittently activating the fasting response and its associated benefits [ 79 ]. In animal models of aging, so-called fasting-mimicking diets could replicate the benefits observed with caloric restriction [ 80 ]. Apart from activating fasting responses, intermittent feeding strategies could theoretically be beneficial through preventing the muscle-full effect and better preservation of circadian rhythm [ 79 ]. However, it remains unclear how long critically ill patients should fast before a metabolic fasting response that includes autophagy stimulation develops [ 81 ]. In a pilot crossover RCT, 12 h fasting activated ketogenesis and other components of the fasting response, while it had no impact on autophagy assessed in peripheral blood cells [ 82 ]. Yet, it remains unclear whether 12 h fasting was able to activate autophagy in vital tissues, or whether 12 h fasting was merely insufficient to initiate autophagy stimulation at all [ 82 ]. RCTs investigating the impact of intermittent versus continuous feeding strategies in critical illness did not show consistent benefit of intermittent feeding [ 74 , 83 ]. Yet, RCTs were relatively small and likely underpowered to detect or exclude a meaningful clinical benefit, and the fasting interval was relatively short (in general 4–6 h), which may have been too short to induce a fasting response and its associated benefits [ 79 ]. Nevertheless, intermittent feeding may also be challenging, since the daily nutritional intake has to be given over a shorter time, which may increase the risk of complications due to enteral feeding intolerance and large glucose variability, among others [ 84 ]. Hence, efficacy and safety remain to be studied. Apart from intermittent feeding strategies, ketogenic diets or ketone supplementation could be beneficial [ 85 ]. Although ketogenic diets have been used in selected patients including patients with refractory status epilepticus, the efficacy and safety of ketogenic diets or ketone supplements for general critically ill patients remain to be studied [ 85 ].
Apart from the ideal feeding regimen or the ideal feeding mode, there is a need for validated markers of feeding tolerance and responsiveness [ 86 ]. Indeed, although enteral nutrition is usually favored over parenteral nutrition, patients on enteral nutrition may suffer feeding intolerance and, in severe cases, non-occlusive mesenteric ischemia, especially when delivered at higher doses in patients with shock [ 19 , 86 ]. Currently, there are no validated biomarkers or bedside monitoring devices that can predict enteral feeding tolerance, which could help avoid complications of too early enteral feeding, such as aspiration pneumonia [ 87 , 88 ]. At current, gastric residual volumes are still widely used and recommended by guidelines [ 89 ], although a RCT ( N = 449) did not show benefit of measuring gastric residual volumes in adult patients receiving mechanical ventilation [ 90 ]. Also, metabolic responsiveness to feeding cannot be predicted or monitored at the bedside, which requires further investigation (Fig. 1 ) [ 87 ]. In the past, experts have recommended to use nutritional risk scores to inform which patients would benefit most from early enhanced nutrition [ 91 , 92 ]. However, RCT data have shown that no biomarker was able to discern subpopulations of patients benefiting from early full nutrition [ 35 ]. Future research in metabolomics could help to identify which patients may benefit from enhanced or more restricted feeding and at what time [ 93 , 94 ]. Currently used signs of energy or protein overload are nonspecific and frequently occur outside the context of overfeeding, including hyperglycemia, hypertriglyceridemia, elevated liver enzymes, hyperbilirubinemia, hyperuremia and hyperammonemia [ 95 ]. A potential sign that may assist in determining readiness for feeding may be the degree of insulin resistance, as can be derived from the amount of insulin required to maintain blood glucose at a predefined level [ 95 , 96 ]. One important biomarker is, however, phosphate, to detect and early treat refeeding syndrome [ 95 ].
The target population and outcomes studied in RCTs on ICU nutrition also need to be considered. As responsiveness to feeding likely changes over time, there is a need for RCTs that investigate the impact of optimized nutrition started after the acute phase and continued throughout the recovery phase [ 97 ], since anabolic resistance is expected to cease at a particular time. In this regard, a large RCT in hospitalized non-critically ill adults at risk of malnutrition ( N = 2088) showed that intensified nutritional support, achieved predominantly through increased oral intake, improved short-term mortality [ 98 ]. Nevertheless, the mortality difference was only transient [ 99 ], there was no impact on functional outcome after 6 months [ 99 ], and less than 2% of patients in the intervention group received enteral or parenteral nutrition [ 98 ]. Hence, it is not clear to what extent these findings can be extrapolated to patients who are still in need of artificial nutrition while recovering from critical illness. Theoretically, indirect calorimetry could be a useful adjunct in patients who are responsive to feeding, to prevent over- and underfeeding. Yet, feeding responsiveness cannot be monitored at the bedside at this time.
In future nutritional RCTs, the use of uniform endpoints would facilitate comparisons and meta-analyses, although there is only limited agreement on essential outcomes [ 100 ]. There has been considerable variability in the primary and secondary outcomes of RCTs [ 101 , 102 ]. For large efficacy RCTs, the primary endpoint should be a patient-centered outcome that is likely affected by feeding [ 101 ]. The anticipated effect size should be realistic with regard to the nature and the duration of the intervention, avoiding an underpowered study. Evidently, potential confounders should be taken into account, including competing risks [ 101 , 103 ].
Recent RCTs have not confirmed the hypothesized benefit of early full feeding, and several RCTs even showed harm of early parenteral nutrition supplementing insufficient enteral nutrition. Harm by early parenteral nutrition appeared explained by a higher nutritional dose in the acute phase, and not by the parenteral route per se, since a short period of parenteral nutrition did not cause harm as compared to an isocaloric dose of enteral nutrition. There are no large RCTs favoring indirect calorimetry-guided full feeding as compared to calculation-based feeding. The absence of benefit of early full feeding has been attributed to suppression of autophagy and ketogenesis, and to feeding-resistant muscle catabolism. Hence, intermittent feeding, ketone supplementation and ketogenic diets emerge as potential novel feeding strategies that may allow continuation of nutrition while avoiding prolonged suppression of beneficial fasting responses, which needs further study. Despite many large-scale RCTs in the last decade, it remains unclear how to optimally administer feeding, since the ideal timing and dose remain unclear. Since recent feeding practices have shifted toward lower-dose artificial nutrition and avoiding early PN, sufficient micronutrient intake should be ensured to prevent deficiencies. To allow individualization of feeding, novel biomarkers, predictive models or monitoring devices that predict and indicate the response to feeding are needed, since the presence or absence of feeding resistance and unsuppressible gluconeogenesis is likely dynamic and time-dependent, depending on the stress response to severe illness and the recovery hereof. Until that time, it will remain unclear for whom, when and how to optimally use indirect calorimetry. Evidently, any feeding strategy, even in case of a solid pathophysiological rationale, requires confirmation of efficacy and safety in a large-scale RCT powered for clinical endpoints before it can be strongly recommended in clinical practice.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Abbreviations
Body mass index
European Society for Clinical Nutrition and Metabolism
Intensive care unit
Nutritional risk screening
Nutrition risk in critically ill
Randomized controlled trial
Vanhorebeek I, Latronico N, Van den Berghe G. ICU-acquired weakness. Intensive Care Med. 2020;46(4):637–53.
Article Google Scholar
Hermans G, Van Mechelen H, Clerckx B, Vanhullebusch T, Mesotten D, Wilmer A, et al. Acute outcomes and 1-year mortality of intensive care unit-acquired weakness: a cohort study and propensity-matched analysis. Am J Respir Crit Care Med. 2014;190(4):410–20.
Herridge MS, Tansey CM, Matte A, Tomlinson G, Diaz-Granados N, Cooper A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364(14):1293–304.
Article CAS Google Scholar
Van Aerde N, Meersseman P, Debaveye Y, Wilmer A, Gunst J, Casaer MP, et al. Five-year impact of ICU-acquired neuromuscular complications: a prospective, observational study. Intensive Care Med. 2020;46(6):1184–93.
Rousseau AF, Prescott HC, Brett SJ, Weiss B, Azoulay E, Creteur J, et al. Long-term outcomes after critical illness: recent insights. Crit Care. 2021;25(1):108.
Orford NR, Saunders K, Merriman E, Henry M, Pasco J, Stow P, et al. Skeletal morbidity among survivors of critical illness. Crit Care Med. 2011;39(6):1295–300.
Orford NR, Lane SE, Bailey M, Pasco JA, Cattigan C, Elderkin T, et al. Changes in bone mineral density in the year after critical illness. Am J Respir Crit Care Med. 2016;193(7):736–44.
Singer P, Berger MM, Van den Berghe G, Biolo G, Calder P, Forbes A, et al. ESPEN guidelines on parenteral nutrition: intensive care. Clin Nutr. 2009;28(4):387–400.
Alberda C, Gramlich L, Jones N, Jeejeebhoy K, Day AG, Dhaliwal R, et al. The relationship between nutritional intake and clinical outcomes in critically ill patients: results of an international multicenter observational study. Intensive Care Med. 2009;35(10):1728–37.
Dvir D, Cohen J, Singer P. Computerized energy balance and complications in critically ill patients: an observational study. Clin Nutr. 2006;25(1):37–44.
Casaer MP, Mesotten D, Hermans G, Wouters PJ, Schetz M, Meyfroidt G, et al. Early versus late parenteral nutrition in critically ill adults. N Engl J Med. 2011;365(6):506–17.
Fivez T, Kerklaan D, Mesotten D, Verbruggen S, Wouters PJ, Vanhorebeek I, et al. Early versus late parenteral nutrition in critically ill children. N Engl J Med. 2016;374(12):1111–22.
Rice TW, Wheeler AP, Thompson BT, Steingrub J, Hite RD, Moss M, et al. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA. 2012;307(8):795–803.
Arabi YM, Aldawood AS, Haddad SH, Al-Dorzi HM, Tamim HM, Jones G, et al. Permissive underfeeding or standard enteral feeding in critically ill adults. N Engl J Med. 2015;372(25):2398–408.
Chapman M, Peake SL, Bellomo R, Davies A, Deane A, Horowitz M, et al. Energy-dense versus routine enteral nutrition in the critically ill. N Engl J Med. 2018;379(19):1823–34.
Compher C, Bingham AL, McCall M, Patel J, Rice TW, Braunschweig C, et al. Guidelines for the provision of nutrition support therapy in the adult critically ill patient: The American Society for Parenteral and Enteral Nutrition. JPEN J Parenter Enteral Nutr. 2022;46(1):12–41.
Hermans G, Casaer MP, Clerckx B, Guiza F, Vanhullebusch T, Derde S, et al. Effect of tolerating macronutrient deficit on the development of intensive-care unit acquired weakness: a subanalysis of the EPaNIC trial. Lancet Respir Med. 2013;1(8):621–9.
Harvey SE, Parrott F, Harrison DA, Bear DE, Segaran E, Beale R, et al. Trial of the route of early nutritional support in critically ill adults. N Engl J Med. 2014;371(18):1673–84.
Reignier J, Boisramé-Helms J, Brisard L, Lascarrou JB, Ait Hssain A, Anguel N, et al. Enteral versus parenteral early nutrition in ventilated adults with shock: a randomised, controlled, multicentre, open-label, parallel-group study (NUTRIREA-2). Lancet. 2018;391(10116):133–43.
Needham DM, Dinglas VD, Bienvenu OJ, Colantuoni E, Wozniak AW, Rice TW, et al. One year outcomes in patients with acute lung injury randomised to initial trophic or full enteral feeding: prospective follow-up of EDEN randomised trial. BMJ. 2013;346:f1532.
Needham DM, Dinglas VD, Morris PE, Jackson JC, Hough CL, Mendez-Tellez PA, et al. Physical and cognitive performance of patients with acute lung injury 1 year after initial trophic versus full enteral feeding: EDEN trial follow-up. Am J Respir Crit Care Med. 2013;188(5):567–76.
Deane AM, Little L, Bellomo R, Chapman MJ, Davies AR, Ferrie S, et al. Outcomes six months after delivering 100% or 70% of enteral calorie requirements during critical illness (TARGET): a randomized controlled trial. Am J Respir Crit Care Med. 2020;201(7):814–22.
Verstraete S, Verbruggen SC, Hordijk JA, Vanhorebeek I, Dulfer K, Güiza F, et al. Long-term developmental effects of withholding parenteral nutrition for 1 week in the paediatric intensive care unit: a 2-year follow-up of the PEPaNIC international, randomised, controlled trial. Lancet Respir Med. 2019;7(2):141–53.
Jacobs A, Dulfer K, Eveleens RD, Hordijk J, Van Cleemput H, Verlinden I, et al. Long-term developmental effect of withholding parenteral nutrition in paediatric intensive care units: a 4-year follow-up of the PEPaNIC randomised controlled trial. Lancet Child Adolesc Health. 2020;4(7):503–14.
Casaer MP, Van den Berghe G. Nutrition in the acute phase of critical illness. N Engl J Med. 2014;370(13):1227–36.
Singer P, Blaser AR, Berger MM, Alhazzani W, Calder PC, Casaer MP, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38(1):48–79.
Vankrunkelsven W, Gunst J, Amrein K, Bear DE, Berger MM, Christopher KB, et al. Monitoring and parenteral administration of micronutrients, phosphate and magnesium in critically ill patients: the VITA-TRACE survey. Clin Nutr. 2021;40(2):509–99.
Doig GS, Simpson F, Heighes PT, Bellomo R, Chesher D, Caterson ID, et al. Restricted versus continued standard caloric intake during the management of refeeding syndrome in critically ill adults: a randomised, parallel-group, multicentre, single-blind controlled trial. Lancet Respir Med. 2015;3(12):943–52.
Olthof LE, Koekkoek WACK, van Setten C, Kars JCN, van Blokland D, van Zanten ARH. Impact of caloric intake in critically ill patients with, and without, refeeding syndrome: a retrospective study. Clin Nutr. 2018;37(5):1609–17.
Eveleens RD, Witjes BCM, Casaer MP, Vanhorebeek I, Guerra GG, Veldscholte K, et al. Supplementation of vitamins, trace elements and electrolytes in the PEPaNIC randomised controlled trial: composition and preparation of the prescription. Clin Nutr ESPEN. 2021;42:244–51.
Hoffer LJ, Bistrian BR. Nutrition in critical illness: a current conundrum. F1000Res. 2016;5:2531.
Felbinger TW, Weigand MA, Mayer K. Early or late parenteral nutrition in critically ill adults. N Engl J Med. 2011;365(19):1839.
O’Leary MJ, Ferrie S. Early or late parenteral nutrition in critically ill adults. N Engl J Med. 2011;365(19):1839–40.
Reignier J, Le Gouge A, Lascarrou JB, Annane D, Argaud L, Hourmant Y, et al. Impact of early low-calorie low-protein versus standard-calorie standard-protein feeding on outcomes of ventilated adults with shock: design and conduct of a randomised, controlled, multicentre, open-label, parallel-group trial (NUTRIREA-3). BMJ Open. 2021;11(5):e045041.
Arabi YM, Aldawood AS, Al-Dorzi HM, Tamim HM, Haddad SH, Jones G, et al. Permissive underfeeding or standard enteral feeding in high and low nutritional risk critically ill adults: post-hoc analysis of the PermiT trial. Am J Respir Crit Care Med. 2017;195(5):652–62.
Doig GS, Simpson F, Bellomo R, Heighes PT, Sweetman EA, Chesher D, et al. Intravenous amino acid therapy for kidney function in critically ill patients: a randomized controlled trial. Intensive Care Med. 2015;41(7):1197–208.
Gunst J, Vanhorebeek I, Casaer MP, Hermans G, Wouters PJ, Dubois J, et al. Impact of early parenteral nutrition on metabolism and kidney injury. J Am Soc Nephrol. 2013;24(6):995–1005.
Vanhorebeek I, Verbruggen S, Casaer MP, Gunst J, Wouters PJ, Hanot J, et al. Effect of early supplemental parenteral nutrition in the paediatric ICU: a preplanned observational study of post-randomisation treatments in the PEPaNIC trial. Lancet Respir Med. 2017;5(6):475–83.
Allingstrup MJ, Kondrup J, Wiis J, Claudius C, Pedersen UG, Hein-Rasmussen R, et al. Early goal-directed nutrition versus standard of care in adult intensive care patients: the single-centre, randomised, outcome assessor-blinded EAT-ICU trial. Intensive Care Med. 2017;43(11):1637–47.
Casaer MP, Langouche L, Coudyzer W, Vanbeckevoort D, De Dobbelaer B, Guiza FG, et al. Impact of early parenteral nutrition on muscle and adipose tissue compartments during critical illness. Crit Care Med. 2013;41(10):2298–309.
Casaer MP, Wilmer A, Hermans G, Wouters PJ, Mesotten D, Van den Berghe G. Role of disease and macronutrient dose in the randomized controlled EPaNIC trial: a post hoc analysis. Am J Respir Crit Care Med. 2013;187(3):247–55.
Heyland DK, Patel J, Bear D, Sacks G, Nixdorf H, Dolan J, et al. The effect of higher protein dosing in critically ill patients: a multicenter registry-based randomized trial: the EFFORT Trial. JPEN J Parenter Enteral Nutr. 2019;43(3):326–34.
Oshima T, Berger MM, De Waele E, Guttormsen AB, Heidegger CP, Hiesmayr M, et al. Indirect calorimetry in nutritional therapy: a position paper by the ICALIC study group. Clin Nutr. 2017;36(3):651–62.
De Waele E, van Zanten ARH. Routine use of indirect calorimetry in critically ill patients: pros and cons. Crit Care. 2022;26(1):123.
Singer P, Anbar R, Cohen J, Shapiro H, Shalita-Chesner M, Lev S, et al. The tight calorie control study (TICACOS): a prospective, randomized, controlled pilot study of nutritional support in critically ill patients. Intensive Care Med. 2011;37(4):601–9.
Singer P, De Waele E, Sanchez C, Ruiz Santana S, Montejo JC, Laterre PF, et al. TICACOS international: a multi-center, randomized, prospective controlled study comparing tight calorie control versus Liberal calorie administration study. Clin Nutr. 2021;40(2):380–7.
Duan JY, Zheng WH, Zhou H, Xu Y, Huang HB. Energy delivery guided by indirect calorimetry in critically ill patients: a systematic review and meta-analysis. Crit Care. 2021;25(1):88.
De Waele E, Jonckheer J, Wischmeyer PE. Indirect calorimetry in critical illness: a new standard of care? Curr Opin Crit Care. 2021;27(4):334–43.
Casaer MP, Van den Berghe G, Gunst J. Indirect calorimetry: a faithful guide for nutrition therapy, or a fascinating research tool? Clin Nutr. 2021;40(2):651.
Langouche L, Vander Perre S, Wouters PJ, D’Hoore A, Hansen TK, Van den Berghe G. Effect of intensive insulin therapy on insulin sensitivity in the critically ill. J Clin Endocrinol Metab. 2007;92(10):3890–7.
Berger MM, Pichard C. Feeding should be individualized in the critically ill patients. Curr Opin Crit Care. 2019;25(4):307–13.
Fraipont V, Preiser JC. Energy estimation and measurement in critically ill patients. JPEN J Parenter Enteral Nutr. 2013;37(6):705–13.
Preiser JC, Arabi YM, Berger MM, Casaer M, McClave S, Montejo-González JC, et al. A guide to enteral nutrition in intensive care units: 10 expert tips for the daily practice. Crit Care. 2021;25(1):424.
de Cabo R, Mattson MP. Effects of intermittent fasting on health, aging, and disease. N Engl J Med. 2019;381(26):2541–51.
Flanagan EW, Most J, Mey JT, Redman LM. Calorie restriction and aging in humans. Annu Rev Nutr. 2020;40:105–33.
Kroemer G, Marino G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40(2):280–93.
Mizushima N, Levine B. Autophagy in human diseases. N Engl J Med. 2020;383(16):1564–76.
Eisenberg T, Knauer H, Schauer A, Büttner S, Ruckenstuhl C, Carmona-Gutierrez D, et al. Induction of autophagy by spermidine promotes longevity. Nat Cell Biol. 2009;11(11):1305–14.
Gunst J. Recovery from critical illness-induced organ failure: the role of autophagy. Crit Care. 2017;21(1):209.
Thiessen SE, Derese I, Derde S, Dufour T, Pauwels L, Bekhuis Y, et al. The role of autophagy in critical illness-induced liver damage. Sci Rep. 2017;7(1):14150.
Vanhorebeek I, Gunst J, Derde S, Derese I, Boussemaere M, Guiza F, et al. Insufficient activation of autophagy allows cellular damage to accumulate in critically ill patients. J Clin Endocrinol Metab. 2011;96(4):E633–45.
Van Dyck L, Casaer MP, Gunst J. Autophagy and its implications against early full nutrition support in critical illness. Nutr Clin Pract. 2018;33(3):339–47.
Derde S, Vanhorebeek I, Guiza F, Derese I, Gunst J, Fahrenkrog B, et al. Early parenteral nutrition evokes a phenotype of autophagy deficiency in liver and skeletal muscle of critically ill rabbits. Endocrinology. 2012;153(5):2267–76.
Gunst J, Derese I, Aertgeerts A, Ververs E-J, Wauters A, Van den Berghe G, et al. Insufficient autophagy contributes to mitochondrial dysfunction, organ failure, and adverse outcome in an animal model of critical illness. Crit Care Med. 2013;41(1):182–94.
Levine B, Packer M, Codogno P. Development of autophagy inducers in clinical medicine. J Clin Invest. 2015;125(1):14–24.
Nah J, Zhai P, Huang CY, Fernández Á, Mareedu S, Levine B, et al. Upregulation of Rubicon promotes autosis during myocardial ischemia/reperfusion injury. J Clin Invest. 2020;130(6):2978–91.
Newman JC, Verdin E. β-hydroxybutyrate: a signaling metabolite. Annu Rev Nutr. 2017;37:51–76.
Goossens C, Weckx R, Derde S, Dufour T, Vander Perre S, Pauwels L, et al. Adipose tissue protects against sepsis-induced muscle weakness in mice: from lipolysis to ketones. Crit Care. 2019;23(1):236.
Benjamin DI, Both P, Benjamin JS, Nutter CW, Tan JH, Kang J, et al. Fasting induces a highly resilient deep quiescent state in muscle stem cells via ketone body signaling. Cell Metab. 2022;34(6):902-918.e906.
De Bruyn A, Gunst J, Goossens C, Vander Perre S, Guerra GG, Verbruggen S, et al. Effect of withholding early parenteral nutrition in PICU on ketogenesis as potential mediator of its outcome benefit. Crit Care. 2020;24(1):536.
De Bruyn A, Langouche L, Vander Perre S, Gunst J, Van den Berghe G. Impact of withholding early parenteral nutrition in adult critically ill patients on ketogenesis in relation to outcome. Crit Care. 2021;25(1):102.
Bohé J, Low JF, Wolfe RR, Rennie MJ. Latency and duration of stimulation of human muscle protein synthesis during continuous infusion of amino acids. J Physiol. 2001;532(Pt 2):575–9.
Atherton PJ, Etheridge T, Watt PW, Wilkinson D, Selby A, Rankin D, et al. Muscle full effect after oral protein: time-dependent concordance and discordance between human muscle protein synthesis and mTORC1 signaling. Am J Clin Nutr. 2010;92(5):1080–8.
McNelly AS, Bear DE, Connolly BA, Arbane G, Allum L, Tarbhai A, et al. Effect of intermittent or continuous feed on muscle wasting in critical illness: a phase 2 clinical trial. Chest. 2020;158(1):183–94.
Flower L, Haines RW, McNelly A, Bear DE, Koelfat K, Damink SO, et al. Effect of intermittent or continuous feeding and amino acid concentration on urea-to-creatinine ratio in critical illness. JPEN J Parenter Enteral Nutr. 2022;46(4):789–97.
Morton RW, Traylor DA, Weijs PJM, Phillips SM. Defining anabolic resistance: implications for delivery of clinical care nutrition. Curr Opin Crit Care. 2018;24(2):124–30.
Kagan I, Cohen J, Bendavid I, Kramer S, Mesilati-Stahy R, Glass Y, et al. Effect of combined protein-enriched enteral nutrition and early cycle ergometry in mechanically ventilated critically ill patients-a pilot study. Nutrients. 2022;14(8):1.
Hodgson CL, Bailey M, Bellomo R, Brickell K, Broadley T, Buhr H, et al. Early active mobilization during mechanical ventilation in the ICU. N Engl J Med. 2022;387(19):1747–58.
Puthucheary Z, Gunst J. Are periods of feeding and fasting protective during critical illness? Curr Opin Clin Nutr Metab Care. 2021;24(2):183–8.
Di Francesco A, Di Germanio C, Bernier M, de Cabo R. A time to fast. Science. 2018;362(6416):770–5.
Pletschette Z, Preiser JC. Continuous versus intermittent feeding of the critically ill: have we made progress? Curr Opin Crit Care. 2020;26(4):341–5.
Google Scholar
Van Dyck L, Vanhorebeek I, Wilmer A, Schrijvers A, Derese I, Mebis L, et al. Towards a fasting-mimicking diet for critically ill patients: the pilot randomized crossover ICU-FM-1 study. Crit Care. 2020;24(1):249.
Van Dyck L, Casaer MP. Intermittent or continuous feeding: any difference during the first week? Curr Opin Crit Care. 2019;25(4):356–62.
Bear DE, Hart N, Puthucheary Z. Continuous or intermittent feeding: pros and cons. Curr Opin Crit Care. 2018;24(4):256–61.
Gunst J, Casaer MP, Langouche L, Van den Berghe G. Role of ketones, ketogenic diets and intermittent fasting in ICU. Curr Opin Crit Care. 2021;27(4):385–9.
Reintam Blaser A, Preiser JC, Fruhwald S, Wilmer A, Wernerman J, Benstoem C, et al. Gastrointestinal dysfunction in the critically ill: a systematic scoping review and research agenda proposed by the section of metabolism, endocrinology and nutrition of the European Society of Intensive Care Medicine. Crit Care. 2020;24(1):224.
Van Dyck L, Gunst J, Casaer MP, Peeters B, Derese I, Wouters PJ, et al. The clinical potential of GDF15 as a “ready-to-feed indicator” for critically ill adults. Crit Care. 2020;24(1):557.
Deane AM, Chapman MJ. Technology to inform the delivery of enteral nutrition in the intensive care unit. JPEN J Parenter Enteral Nutr. 2022;46(4):754–6.
Reintam Blaser A, Starkopf J, Alhazzani W, Berger MM, Casaer MP, Deane AM, et al. Early enteral nutrition in critically ill patients: ESICM clinical practice guidelines. Intensive Care Med. 2017;43(3):380–98.
Reignier J, Mercier E, Le Gouge A, Boulain T, Desachy A, Bellec F, et al. Effect of not monitoring residual gastric volume on risk of ventilator-associated pneumonia in adults receiving mechanical ventilation and early enteral feeding: a randomized controlled trial. JAMA. 2013;309(3):249–56.
Kondrup J, Allison SP, Elia M, Vellas B, Plauth M. Educational and Clinical Practice Committee ErSoPaENE: ESPEN guidelines for nutrition screening 2002. Clin Nutr. 2003;22(4):415–21.
Heyland DK, Dhaliwal R, Jiang X, Day AG. Identifying critically ill patients who benefit the most from nutrition therapy: the development and initial validation of a novel risk assessment tool. Crit Care. 2011;15(6):R268.
Wernerman J, Christopher KB, Annane D, Casaer MP, Coopersmith CM, Deane AM, et al. Metabolic support in the critically ill: a consensus of 19. Crit Care. 2019;23(1):318.
Christopher KB. Nutritional metabolomics in critical illness. Curr Opin Clin Nutr Metab Care. 2018;21(2):121–5.
Berger MM, Reintam-Blaser A, Calder PC, Casaer M, Hiesmayr MJ, Mayer K, et al. Monitoring nutrition in the ICU. Clin Nutr. 2019;38(2):584–93.
Uyttendaele V, Dickson JL, Shaw GM, Desaive T, Chase JG. Untangling glycaemia and mortality in critical care. Crit Care. 2017;21(1):152.
Ridley EJ, Bailey M, Chapman M, Chapple LS, Deane AM, Hodgson C, et al. Protocol summary and statistical analysis plan for Intensive nutrition therapy comparEd to usual care iN criTically ill adults (INTENT): a phase II randomised controlled trial. BMJ Open. 2022;12(3):e050153.
Schuetz P, Fehr R, Baechli V, Geiser M, Deiss M, Gomes F, et al. Individualised nutritional support in medical inpatients at nutritional risk: a randomised clinical trial. Lancet. 2019;393(10188):2312–21.
Kaegi-Braun N, Tribolet P, Gomes F, Fehr R, Baechli V, Geiser M, et al. Six-month outcomes after individualized nutritional support during the hospital stay in medical patients at nutritional risk: secondary analysis of a prospective randomized trial. Clin Nutr. 2021;40(3):812–9.
Davies TW, van Gassel RJJ, van de Poll M, Gunst J, Casaer MP, Christopher KB, et al. Core outcome measures for clinical effectiveness trials of nutritional and metabolic interventions in critical illness: an international modified Delphi consensus study evaluation (CONCISE). Crit Care. 2022;26(1):240.
Fetterplace K, Ridley EJ, Beach L, Abdelhamid YA, Presneill JJ, MacIsaac CM, et al. Quantifying response to nutrition therapy during critical illness: implications for clinical practice and research? A narrative review. JPEN J Parenter Enteral Nutr. 2021;45(2):251–66.
Chapple LS, Summers MJ, Weinel LM, Deane AM. Outcome measures in critical care nutrition interventional trials: a systematic review. Nutr Clin Pract. 2020;35(3):506–13.
Schetz M, Casaer MP, Van den Berghe G. Does artificial nutrition improve outcome of critical illness? Crit Care. 2013;17(1):302.
Download references
Acknowledgements
Not applicable.
JG holds a postdoctoral research fellowship granted by the University Hospitals Leuven. MPC holds a postdoctoral research fellowship granted by the Research Foundation—Flanders. JG and MPC receive C2 project funding by KU Leuven. JR received grants from the French Ministry of Health (Programme Hospitalier de Recherche Clinique National 2012 and 2017: #PHRC-12-0184; #PHRC-17-0213). GVdB receives structural research financing by the Methusalem programme of the Flemish Government through the University of Leuven (METH14/06), and a European Research Council (ERC) Advanced Grant from the Horizon 2020 Program of the EU (AdvG-2017-785809).
Author information
Authors and affiliations.
Clinical Division and Laboratory of Intensive Care Medicine, Department of Cellular and Molecular Medicine, KU Leuven, Herestraat 49, 3000, Leuven, Belgium
Jan Gunst, Michael P. Casaer & Greet Van den Berghe
Erasme University Hospital, Université Libre de Bruxelles, Brussels, Belgium
Jean-Charles Preiser
Service de Médecine Intensive Réanimation, Centre Hospitalier Universitaire de Nantes, Université de Nantes, Nantes, France
Jean Reignier
You can also search for this author in PubMed Google Scholar
Contributions
JG wrote the first draft, which was subsequently reviewed by all authors. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Jan Gunst .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Gunst, J., Casaer, M.P., Preiser, JC. et al. Toward nutrition improving outcome of critically ill patients: How to interpret recent feeding RCTs?. Crit Care 27 , 43 (2023). https://doi.org/10.1186/s13054-023-04317-9
Download citation
Received : 25 November 2022
Accepted : 11 January 2023
Published : 27 January 2023
DOI : https://doi.org/10.1186/s13054-023-04317-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Critical illness
- Enteral nutrition
- Parenteral nutrition
- Indirect calorimetry
- Energy target
- Intermittent feeding
Critical Care
ISSN: 1364-8535
- Submission enquiries: [email protected]
- Open access
- Published: 09 September 2021
International Society of Sports Nutrition position stand: sodium bicarbonate and exercise performance
- Jozo Grgic ORCID: orcid.org/0000-0003-2349-8078 1 ,
- Zeljko Pedisic ORCID: orcid.org/0000-0003-2886-3556 1 ,
- Bryan Saunders ORCID: orcid.org/0000-0003-0995-9077 2 , 3 ,
- Guilherme G. Artioli ORCID: orcid.org/0000-0001-8463-2213 4 ,
- Brad J. Schoenfeld ORCID: orcid.org/0000-0003-4979-5783 5 ,
- Michael J. McKenna ORCID: orcid.org/0000-0001-9998-0093 1 ,
- David J. Bishop ORCID: orcid.org/0000-0002-6956-9188 1 ,
- Richard B. Kreider ORCID: orcid.org/0000-0002-3906-1658 6 ,
- Jeffrey R. Stout ORCID: orcid.org/0000-0001-6114-1649 7 ,
- Douglas S. Kalman ORCID: orcid.org/0000-0003-0882-9748 8 , 9 ,
- Shawn M. Arent ORCID: orcid.org/0000-0003-0647-0591 10 ,
- Trisha A. VanDusseldorp ORCID: orcid.org/0000-0001-9057-2720 11 ,
- Hector L. Lopez ORCID: orcid.org/0000-0003-0666-4222 12 , 13 ,
- Tim N. Ziegenfuss ORCID: orcid.org/0000-0001-5644-3263 12 ,
- Louise M. Burke ORCID: orcid.org/0000-0001-8866-5637 14 ,
- Jose Antonio ORCID: orcid.org/0000-0002-8930-1058 15 &
- Bill I. Campbell ORCID: orcid.org/0000-0002-4106-3871 16
Journal of the International Society of Sports Nutrition volume 18 , Article number: 61 ( 2021 ) Cite this article
59k Accesses
34 Citations
184 Altmetric
Metrics details
Based on a comprehensive review and critical analysis of the literature regarding the effects of sodium bicarbonate supplementation on exercise performance, conducted by experts in the field and selected members of the International Society of Sports Nutrition (ISSN), the following conclusions represent the official Position of the Society:
Supplementation with sodium bicarbonate (doses from 0.2 to 0.5 g/kg) improves performance in muscular endurance activities, various combat sports, including boxing, judo, karate, taekwondo, and wrestling, and in high-intensity cycling, running, swimming, and rowing. The ergogenic effects of sodium bicarbonate are mostly established for exercise tasks of high-intensity that last between 30 s and 12 min.
Sodium bicarbonate improves performance in single- and multiple-bout exercise.
Sodium bicarbonate improves exercise performance in both men and women.
For single-dose supplementation protocols, 0.2 g/kg of sodium bicarbonate seems to be the minimum dose required to experience improvements in exercise performance. The optimal dose of sodium bicarbonate dose for ergogenic effects seems to be 0.3 g/kg. Higher doses (e.g., 0.4 or 0.5 g/kg) may not be required in single-dose supplementation protocols, because they do not provide additional benefits (compared with 0.3 g/kg) and are associated with a higher incidence and severity of adverse side-effects.
For single-dose supplementation protocols, the recommended timing of sodium bicarbonate ingestion is between 60 and 180 min before exercise or competition.
Multiple-day protocols of sodium bicarbonate supplementation can be effective in improving exercise performance. The duration of these protocols is generally between 3 and 7 days before the exercise test, and a total sodium bicarbonate dose of 0.4 or 0.5 g/kg per day produces ergogenic effects. The total daily dose is commonly divided into smaller doses, ingested at multiple points throughout the day (e.g., 0.1 to 0.2 g/kg of sodium bicarbonate consumed at breakfast, lunch, and dinner). The benefit of multiple-day protocols is that they could help reduce the risk of sodium bicarbonate-induced side-effects on the day of competition.
Long-term use of sodium bicarbonate (e.g., before every exercise training session) may enhance training adaptations, such as increased time to fatigue and power output.
The most common side-effects of sodium bicarbonate supplementation are bloating, nausea, vomiting, and abdominal pain. The incidence and severity of side-effects vary between and within individuals, but it is generally low. Nonetheless, these side-effects following sodium bicarbonate supplementation may negatively impact exercise performance. Ingesting sodium bicarbonate (i) in smaller doses (e.g., 0.2 g/kg or 0.3 g/kg), (ii) around 180 min before exercise or adjusting the timing according to individual responses to side-effects, (iii) alongside a high-carbohydrate meal, and (iv) in enteric-coated capsules are possible strategies to minimize the likelihood and severity of these side-effects.
Combining sodium bicarbonate with creatine or beta-alanine may produce additive effects on exercise performance. It is unclear whether combining sodium bicarbonate with caffeine or nitrates produces additive benefits.
Sodium bicarbonate improves exercise performance primarily due to a range of its physiological effects. Still, a portion of the ergogenic effect of sodium bicarbonate seems to be placebo-driven.
Introduction
Sodium bicarbonate is used as an ergogenic aid and as an ingredient in prescription and over-the-counter medications [ 1 ]. Many studies have explored the effects of sodium bicarbonate on performance in various modes of exercise, including combat sport tasks, resistance exercise, and single and repeated high-intensity cycling, running, swimming, and rowing (Table 1 ) [ 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 ]. The effects of different sodium bicarbonate ingestion protocols to maximize the ergogenic effects while minimizing the incidence and severity of side-effects have also been examined [ 38 , 46 , 47 , 55 , 75 , 77 , 99 , 121 ]. Studies have also investigated the interaction of sodium bicarbonate with other ergogenic aids, such as beta-alanine, caffeine, and creatine (Table 2 ) [ 126 , 127 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 , 144 , 145 , 146 , 147 ]. The purpose of this position stand was to: (1) critically evaluate and summarize the scientific literature on the ergogenic effects of sodium bicarbonate; (2) provide recommendations for the use of sodium bicarbonate as an ergogenic aid; and (3) suggest key topics for future research on sodium bicarbonate supplementation.
International Society of Sports Nutrition (ISSN) position stands are invited papers on topics the ISSN editors and Research Committee identify as being of interest to our readers. Here, we briefly outline the process utilized in preparing ISSN position stands. Editors and/or the Research Committee first identify a lead author or team of authors to perform a comprehensive literature review. After the authors develop the content of the position stand, the draft is sent to leading scholars in the field for a detailed review. Following a critical review by the scholars, the paper is revised by the team of authors, approved by the Research Committee and Editors, and published as a consensus statement and the official position of the ISSN on the topic.
History of research on sodium bicarbonate and exercise
The effects of sodium bicarbonate on exercise performance have been researched since the 1930s [ 148 ]. The first published study on this topic was carried out in the Fatigue Laboratory at Harvard University by Dennig and colleagues [ 148 ]. In this single-participant study, 10 g of sodium bicarbonate was provided before treadmill running. The authors concluded that performance was improved by establishing a pre-exercise state of alkalosis. While several studies on this topic were published in the 1950s and early 1970s [ 149 , 150 , 151 ], the beginning of modern-day research on sodium bicarbonate is generally linked to the study by Jones et al. [ 58 ] published in the Journal of Applied Physiology in 1977. In this study, 5 participants undertook 40 min of submaximal cycling prior to a cycling task to exhaustion at 95% of their maximum power on three occasions, following the ingestion of calcium carbonate (placebo condition), ammonium chloride (acidosis condition), or sodium bicarbonate (alkalosis condition). On average, participants cycled for 438 ± 120 s after ingesting sodium bicarbonate, which was significantly longer than in the acidosis (160 ± 22 s) and control (270 ± 13 s) conditions. Interestingly, sodium bicarbonate was ingested 3 h before exercise in a dose of 0.3 g/kg; this dose is used in most studies today and therefore considered a precursor to later studies. Many additional studies on sodium bicarbonate and exercise were published by this group, collectively demonstrating their major impact on the development of this field of research [ 61 , 74 , 111 , 152 , 153 ].
In the 1980s, the effects of sodium bicarbonate on exercise performance began to receive international attention, with studies published by independent research groups from Australia, Canada, France, Israel, Japan, the Netherlands, Sweden, the UK, and the USA [ 10 , 11 , 19 , 42 , 43 , 44 , 55 , 56 , 57 , 60 , 61 , 65 , 73 , 74 , 75 , 89 , 97 , 111 , 119 ]. Most of these studies were conducted as randomized, double-blinded, cross-over trials using small sample sizes (e.g., 6 to 7 participants per study). During this period, ergogenic effects of sodium bicarbonate were established for single and repeated high-intensity cycling, running, swimming, and muscular endurance [ 10 , 19 , 42 , 44 , 73 , 75 , 119 ]. Since then, there has been a large increase in the number of studies on the effects of sodium bicarbonate on exercise performance (Table 1 and Table 2 ), making it one of the most studied ergogenic aids.
Mechanisms for bicarbonate absorption
Sodium bicarbonate is highly soluble in water, promptly dissociating into its constituent ions, namely sodium (Na + ) and bicarbonate (HCO 3 - ), on contact with aqueous solutions, including the stomach acid, as described by the following reaction:
NaHCO 3 ➔ HCO 3 - + Na +
HCl + HCO 3 - + Na + ➔ H 2 CO 3 + Cl - + Na +
H 2 CO 3 ➔ CO 2 + H 2 O
Part of the ingested HCO 3 - is removed via carbon dioxide (CO 2 ) formation in the stomach acid. Since CO 2 is a gas, it is released from the gastric juice on formation and then expelled. However, the rate of CO 2 release is rather slow and increases in a concentration-dependent fashion [ 154 ]. Sodium bicarbonate ingestion increases CO 2 formation, thus increasing the requirement for and rate of CO 2 release, a mechanism that explains commonly reported side-effects of sodium bicarbonate such as belching and bloating. Despite the removal of some HCO 3 - due to acid neutralization in the stomach, the alkalization of the gastric juice following sodium bicarbonate ingestion will likely stimulate the basolateral Cl - /HCO 3 - antiporter in parietal cells mediated by apical gastric H + /K + -ATPase, leading to increased HCO 3 - transport into the blood [ 155 , 156 ] (Fig. 1 ).
Schematic representation of the mechanism of HCO 3 - absorption in the stomach and the impact of sodium bicarbonate ingestion on muscle metabolism and selected blood parameters. Sodium bicarbonate ingestion increases the concentration of HCO 3 - in the stomach lumen, some of which neutralizes HCl to form CO 2 and increases luminal pH. The rise in pH stimulates the Cl - /HCO 3 - antiporter in the parietal cells, which transports HCO 3 - into the extracellular fluid. This transport is coupled with the H-K-ATPase pump that secretes H + into the stomach lumen to restore the pH. This results in increased pH and HCO 3 - concentration, which increases the activity of monocarboxylate transporters (MCT1 and MCT4, represented in light blue), thereby enhancing the transport of H + out of muscle cells and improving intramuscular acid-base balance. Improved pH control in the muscle cells allows higher glycolytic rates, resulting in higher rates of ATP production and higher muscle and blood lactate concentrations. Solid lines indicate reactions. Dashed lines indicate transport across membranes or movement within the cell compartment. Created using BioRender.com
In addition to HCO 3 - absorption in the stomach, other absorption mechanisms exist in the human intestine [ 157 ]. Plausibly, the high doses of sodium bicarbonate commonly ingested (see section “Sodium bicarbonate dose”) do not entirely react with the stomach acid since the bicarbonate load exceeds the amount of acid in the stomach. Thus, some HCO 3 - will enter the intestine and reach the jejunum, where it can be absorbed. This mechanism has been shown to be concentration-dependent and to involve either coupled Na + absorption or active H + secretion [ 157 ]. These multiple mechanisms seem to account for the rapid increase in plasma HCO 3 - concentration that occurs following acute sodium bicarbonate ingestion [ 19 ].
Mechanisms for the ergogenic effect of sodium bicarbonate
During high-intensity short-term exercise, the rate of intramuscular ATP hydrolysis exceeds the maximum rate of ATP re-synthesis by mitochondria. Therefore, ATP production heavily relies on anaerobic systems, namely phosphoryl-creatine (ATP-PCr) hydrolysis and glycolysis. The contribution of each of these systems to ATP production varies according to different factors, such as age [ 158 ] and training [ 159 ]. However, exercise intensity is a major determinant of the energy system contribution [ 160 ]. Illustrated in Fig. 2 , the higher the intensity, the higher the predominance of the ATP-PCr system. As intensity reduces, the contribution of ATP-PCr reduces accordingly and the ATP demands are increasingly met by glycolysis [ 160 ]. Thus, the metabolic perturbations elicited by maximal all-out exercises lasting less than 30 s are distinct from those that occur during intense exercise lasting from ~30 s to ~5 min. While the former is characterized by rapid phosphoryl-creatine depletion, the latter is characterized by substantive accumulation of lactate and H + in both intra and extracellular fluids. The differences in the metabolic perturbations induced by exercise of different intensities and durations seem to be related to the ergogenic potential of sodium bicarbonate supplementation. Specifically, a large body of evidence indicates that exercise that is more reliant on glycolysis and thus results in greater H + accumulation is more likely to benefit from sodium bicarbonate supplementation in comparison to exercise that is too short or too long to result in marked acidosis [ 1 ].
Illustration of the contribution of different energy systems to the production of ATP to sustain maximal or near-maximal exercise over a given amount of time. The horizontal gradient-filled bars indicate exercise intensity/duration zones that are more (filled) or less (shaded) likely to result in acidosis and thus benefit from sodium bicarbonate supplementation
In response to intense contractile activity, muscle temporarily loses much of its ability to generate force and power in a process defined as fatigue. The associated accumulation of metabolites and ions is thought to play a causative role in the fatigue process, although the exact causes of fatigue continue to be debated [ 161 , 162 , 163 ]. Accumulation of H + (i.e., muscle acidosis or a decrease in muscle pH) has been shown to be an important contributing factor to fatigue due to its inhibitory effects on key glycolytic enzymes [ 164 ], depressing effects on Ca 2+ sensitivity, and direct effects on cross-bridge cycling [ 165 ].
High-intensity exercise is also associated with marked changes in the intracellular and extracellular concentrations of various ions [ 163 , 166 ]. One important alteration is the release of K + from the muscle cells to the interstitium, leading to increased interstitial K + concentration. Whilst moderate elevations in extracellular K + may enhance muscle function [ 167 ], very high interstitial K + can depolarize sarcolemma and T-tubule membranes, thus affecting muscle fiber conduction [ 168 ] and excitability [ 169 ], ultimately leading to muscle fatigue. Exercise also imposes important disturbances to Na + , Cl - and Ca 2+ transmembrane gradients, which also contribute to the loss of excitability [ 162 ]. Reduced sarcolemmal and T-tubule excitability are linked with decreased Ca 2+ release from the sarcoplasmic reticulum, which is another important contributor to fatigue [ 152 , 166 , 170 ]. During fatigue, the rise in Mg 2+ and inorganic phosphate concentrations, as well as the fall in ATP concentrations, in skeletal muscle have also been linked with reduced force production and, therefore, appear to contribute to fatigue.
The ergogenic mechanisms of sodium bicarbonate are not yet fully understood. Nevertheless, an increase in extracellular buffering capacity is a widely accepted mechanism for the ergogenic effect of sodium bicarbonate. Since the sarcolemma is not permeable to HCO 3 - , sodium bicarbonate ingestion leads to an increase in plasma HCO 3 - concentration in a dose-dependent manner [ 19 , 33 , 77 ]. This is associated with changes in blood acid-base balance, including an increased pH and base excess, which characterize a state of metabolic alkalosis.
Increased extracellular pH leads to a greater transmembrane H + concentration gradient [ 153 ] that stimulates H + and lactate co-transport out of exercising muscle cells, most likely via monocarboxylate transporters MCT4 and MCT1. Evidence from in vitro studies also suggests that increased extracellular HCO 3 - concentration may contribute to lactate efflux from skeletal muscle [ 171 ]. Direct evidence of increased lactate efflux following sodium bicarbonate ingestion has been provided by Hollidge-Horvat et al. [ 153 ]. Given that lactate transport is stoichiometrically coupled with H + , increased lactate efflux also indicates increased H + efflux during exercise, therefore reducing the intramuscular H + accumulation. Following sodium bicarbonate ingestion, some studies reported higher intramuscular pH during exercise [ 19 , 110 ], although conflicting data exist [ 35 ]. A study measuring pH with phosphorous magnetic resonance spectroscopy during exercise confirmed a delayed onset of intramuscular acidosis with sodium bicarbonate ingestion [ 96 ], providing support for the notion of increased H + efflux and improved intramuscular acid-base balance as a primary ergogenic mechanism following sodium bicarbonate supplementation.
Improved control of intramuscular pH during exercise results in increased glycolytic rates and higher rates of ATP re-synthesis to sustain higher exercise demands. Indeed, studies with muscle biopsies have shown increases in post-exercise muscle lactate content following sodium bicarbonate ingestion, as well as increased glycolytic activity and glycogen utilization [ 7 , 10 , 111 , 161 ]. More direct evidence of increased glycolytic rates has been provided by studies showing increased rates of glycogen utilization and lactate production during exercise after sodium bicarbonate ingestion [ 153 ]. Recent studies assessing the whole-body contribution of the energy systems during exercise after sodium bicarbonate ingestion [ 68 , 131 ] have also reported an increased glycolytic contribution to explain improvements in high-intensity performance via longer periods in which higher rates of ATP re-synthesis are sustained.
In addition to the improved glycolytic metabolism in the exercising muscle cells, improved pH regulation stemming from sodium bicarbonate supplementation may also have a direct effect on the cross-bridge cycle, potentially attenuating the suppressive effects of acidosis on muscle contractility [ 172 ]. Moreover, because pH alters Ca 2+ sensitivity [ 173 ], improved intramuscular pH regulation might also result in increased force production in response to similar cytosolic Ca 2+ concentration during muscle contraction. Whether sodium bicarbonate ingestion can indeed affect the cross-bridge cycle and Ca 2+ sensitivity in human muscle remains to be experimentally determined.
The traditional Henderson’s interpretation of acid-base regulation has been expanded by the physicochemical approach proposed by Stewart in the 1980s [ 174 , 175 ]. This interpretation based acid-base as disturbances, having either respiratory or metabolic origins, to describe three independent variables that collectively drive H + and HCO 3 - ion concentrations as dependent variables. The independent variables are: the Strong Ion Difference ([SID]), defined as the excess of strong cations in relation to strong anions; PCO 2 ; and the total concentration of weak acids and bases ([A TOT ]). It is assumed that any changes in plasma Na + , Cl - , K + or Lac - concentrations, as well as in PCO 2 with sodium bicarbonate ingestion may directly affect plasma pH and plasma HCO 3 - concentrations [ 109 , 152 ]. Changes in muscle intracellular and extracellular strong ion concentrations can therefore affect not only acid-base status but can also directly modulate sarcolemmal and transverse membrane excitability. Alkalosis induced by sodium citrate ingestion has been shown to attenuate the increase in interstitial K + concentration during exercise [ 176 ], thereby minimizing an important factor contributing to fatigue. A study with sodium bicarbonate ingestion [ 109 ] showed lower K + concentration and an increased K + efflux from the exercising muscle, along with an increase in post-exercise K + reuptake, indicating enhanced Na + ,K + -ATPase activity. Thus, sodium bicarbonate helps to minimize K + disturbances in muscle and potentially also preserve sarcolemmal excitability. Intense exercise also induces disturbances in Na + , Cl - , and Ca 2+ transmembrane gradients; altogether, these changes lead to transsarcolemmal and T-tubular depolarization and loss of excitability [ 162 ], a condition that results in impaired force production and can be mitigated by sodium bicarbonate ingestion [ 109 ].
Effects of sodium bicarbonate on high-intensity exercise performance
A number of studies have investigated the effects of sodium bicarbonate on high-intensity exercise performance. Many, but not all, studies have reported an ergogenic effect of sodium bicarbonate. However, the duration of the exercise task is an important component to consider when interpreting the evidence.
Single-bout running and cycling
The effects of sodium bicarbonate on high-intensity, single-bout running or cycling have been thoroughly explored [ 6 , 10 , 27 , 28 , 38 , 40 , 44 , 45 , 46 , 47 , 49 , 50 , 52 , 55 , 56 , 57 , 70 , 76 , 77 , 78 , 79 , 90 , 98 , 111 , 114 , 115 , 119 ]. A recent meta-analysis pooled results from studies exploring the effects of sodium bicarbonate on performance in the Wingate test, which in its original form involves 30-s all-out cycling [ 177 ]. In this meta-analysis, no significant difference was found between the effects of a placebo and sodium bicarbonate on mean or peak power [ 177 ]. The pooled effect size and corresponding 95% confidence intervals (CI) for mean power were in the range of trivial/very small effects (Cohen’s d : 0.02; 95% CI: –0.07, 0.11), suggesting any actual effect of sodium bicarbonate on this outcome would likely be negligible from a practical perspective, at least for most individuals. Footnote 1 Therefore, it seems that a single-bout, high-intensity task lasting 30 s may not be of sufficient duration to benefit from sodium bicarbonate.
The minimum duration of a high-intensity task to experience an ergogenic effect of sodium bicarbonate is yet to be established. However, McNaughton [ 78 ] examined the effects of sodium bicarbonate supplementation on performance in cycling tasks lasting 10, 30, 120, and 240 s. Whereas no significant differences in total work and peak power were found between sodium bicarbonate and placebo trials for cycling tests lasting 10 or 30 s, significant performance improvements were shown for the two cycling tests of longer duration (120 and 240 s). Running-based investigations have focused on distances ranging from 400 m to 1500 m, lasting from 57 to 254 s. For example, Wilkes et al. [ 119 ] included 6 male university runners who performed an 800-m run following the ingestion of a placebo or 0.3 g/kg of sodium bicarbonate. In this study, running time following placebo ingestion was 125.1 ± 4.9 s and consumption of sodium bicarbonate reduced the time needed to complete the test on average by 2.9 s (1.8%). Other studies reported an average reduction in the time required to complete 400-m and 1500-m runs of 1.5 s and 3 s (1.1% to 2.9%) [ 6 , 44 ]. In addition to reducing the time to finish a run, sodium bicarbonate supplementation has also been shown to delay fatigue during high-intensity running. Van Montfoort et al. [ 114 ] evaluated running on a treadmill using an incline of 2% and speeds between 19 and 23 km/h, set to elicit maximum effort in 1 to 2 min. The ingestion of sodium bicarbonate was ergogenic, as the average running time was 77.4 s and 82.3 s (6%) following placebo and sodium bicarbonate ingestion, respectively. Overall, it seems that the minimum duration of a single-bout, high-intensity task to experience an ergogenic benefit with sodium bicarbonate needs to be longer than 30 s.
In terms of the upper limits of the task duration, several studies [ 45 , 46 , 47 , 49 , 50 , 52 , 53 , 86 ] have used high-intensity cycling or running tests lasting between 4 and 12 min and reported ergogenic effects of sodium bicarbonate ingestion. For example, Gough et al. [ 47 , 48 ] used 4-km cycling time trials (lasting around 6 min) and observed that sodium bicarbonate reduced the time to complete this test by 5 – 8 s (~2%). Mueller et al. [ 86 ] used a test involving cycling to exhaustion at critical power that lasted on average 12 min and documented an ergogenic effect of sodium bicarbonate ingestion. Overall, the evidence indicates that sodium bicarbonate may improve single-bout high-intensity cycling and running if the exercise test is of sufficient intensity and lasts approximately between 30 s and 12 min. Single-bout exercise tasks of longer duration, and thus lower intensity, are less likely to be influenced by sodium bicarbonate ingestion.
Stephens and colleagues examined the effects of sodium bicarbonate on 60-min cycling performance [ 110 ]. Even though the ingestion of 0.3 g/kg of sodium bicarbonate significantly increased plasma bicarbonate concentration and decreased plasma and muscle H + , exercise performance was not affected [ 110 ]. Three other studies also did not find a significant difference between the effects of placebo and sodium bicarbonate in different exercise tasks lasting from 20 to 68 min (i.e., running to exhaustion, cycling to exhaustion, and 40-km cycling time trial) [ 39 , 61 , 87 ]. However, three studies reported a positive effect of sodium bicarbonate on performance in exercise tasks of similar duration [ 36 , 43 , 80 ]. McNaughton et al. [ 80 ] found that sodium bicarbonate ingestion increased mean power during a 1-h cycling exercise, while others reported that time to fatigue in cycling or running to exhaustion (lasting from 26 to 50 min) was delayed following sodium bicarbonate ingestion [ 36 , 43 ]. While this area requires further research, it should be mentioned that sodium bicarbonate may be beneficial during prolonged exercise, when such exercise involves periods of increased intensity. For example, athletes competing in road cycling or track running commonly finish the race with an all-out sprint, which may be enhanced with sodium bicarbonate supplementation [ 178 ]. A recent study provided sodium bicarbonate in the dose of 0.3 g/kg, supplemented before and during a 3-h simulated cycling race that involved a 90-s all-out sprint at the end of the race [ 21 ]. Sodium bicarbonate ingestion enhanced mean power in the 90-s sprint by ~3% [ 21 ]. Based on this recent evidence, it seems that sodium bicarbonate may enhance performance during endurance events if they include sprints during or at the end of the competition.
Repeated-bout running and cycling
While sodium bicarbonate ingestion appears ergogenic for single-bout running and cycling tasks, its effects are proposed to be more pronounced in multiple bouts of maximal exercise since the performance in the latter bouts might be more affected by acidosis [ 19 ]. Costill et al. [ 19 ] used a protocol consisting of 4 × 1-min cycling (including 1-min rest periods), with the fifth sprint performed to exhaustion. This final sprint was extended by an average of 47 s following sodium bicarbonate ingestion (placebo: 113 ± 41 s; sodium bicarbonate: 160 ± 63 s), demonstrating an increase in exercise performance during high-intensity, repeated-bout exercise.
While sodium bicarbonate ingestion does not seem to be ergogenic for a single 30-s all-out exercise bout, several studies have investigated the effects of sodium bicarbonate ingestion on repeated maximal 30-s efforts [ 4 , 85 , 88 , 89 , 122 , 123 , 125 ]. For example, Artioli et al. [ 4 ] used a 4 × 30-s arm cranking test with intervening 3-min rest and observed that sodium bicarbonate ingestion increased peak and mean power only in bouts 3 and 4. These results were confirmed by another study from the same research group [ 88 ]. However, other studies have used similar protocols and did not show ergogenic effects of sodium bicarbonate [ 122 , 123 ]. The lack of an effect in these studies might be due to the longer duration of rest intervals between cycling tasks (15 to 30 min) [ 122 , 123 ]. The benefits of sodium bicarbonate may be greatest when employing rest intervals of shorter duration [ 60 ]. Indeed, this is supported by a recent meta-analysis [ 177 ] that included 10 studies and found an ergogenic effect of sodium bicarbonate on mean power in bout 2 of maximal 30-s cycling (pooled Cohen’s d = 0.09) and bout 4 (pooled Cohen’s d = 0.62). In bout 3, a significant effect of sodium bicarbonate was found on mean power (pooled Cohen’s d = 0.40), albeit only when considering studies that used shorter rest intervals between repeated tests (i.e., 3 to 6 min) [ 177 ]. Similarly, Siegler et al. [ 101 ] reported that sodium bicarbonate supplementation increased average speed in a protocol involving three bouts of running at maximum speed for 30 s followed by a 3-min rest, but only in the third bout [ 101 ]. As a result, total distance covered was also greater in the sodium bicarbonate trial.
Subsequent research has observed an ergogenic effect of sodium bicarbonate on multiple bouts of cycling and running of even shorter duration. Bishop et al. [ 7 ] used a repeated-sprint protocol where the participants were required to perform 5 × 6-s cycle sprints with intervening 30-s rest periods, with the analyzed outcomes being total work and peak power. Total work across all sprints was higher following the ingestion of sodium bicarbonate (0.4 g/kg), and this was largely attributed to the increase in peak power in sprints 3, 4, and 5. In other words, sodium bicarbonate enhanced performance by attenuating the fatigue-induced decline in power during repeated-sprints. Lavender and Bird [ 65 ] evaluated performance following sodium bicarbonate ingestion in a protocol involving 10 × 10-s cycle sprints, interspersed with 50-s rest periods. Sodium bicarbonate ingestion improved mean power in 8 sprints (sprints 2 and 3, and 5 to 10) and peak power in 2 out of the 10 performed sprints (sprints 2 and 10). In both of these studies, performance in the first sprint was not affected by sodium bicarbonate ingestion, further demonstrating no benefit of this supplement on shorter duration exercise tasks [ 7 , 65 ].
The Yo-Yo intermittent recovery test [ 179 ] is widely used to assess performance in interval running, which is particularly relevant for many team sports. It determines an individual’s capacity to undertake, repeatedly perform, and recover from high-intensity running. In this test, the individual must run 2 × 20-m distances at progressively increasing speeds, interspersed with a 10-s rest interval [ 179 ]. This test has two types, “level 1” and “level 2”, with the latter starting at a higher speed and requiring a greater contribution from anaerobic energy systems [ 179 ]. Due to its popularity in practice, studies have also explored the effects of sodium bicarbonate on performance in this test [ 25 , 63 , 69 , 113 ]. For example, Marriot et al. [ 69 ] included 12 male team-sport athletes who ingested 0.4 g/kg of sodium bicarbonate or placebo 60 to 90 min before exercise. Sodium bicarbonate increased the distance covered in the level 2 Yo-Yo intermittent recovery test by 23% [ 69 ]. A recent meta-analysis also reported an ergogenic effect of sodium bicarbonate on performance in this test (pooled Cohen’s d = 0.36; 16%) [ 180 ]. As the Yo-Yo test has been reported to correlate with some sport-specific outcomes (e.g., amount of high-intensity running performed at the end of each half of a game) [ 179 ], athletes competing in intermittent sports, such as basketball, football, hockey, and rugby, may consider supplementation with sodium bicarbonate to acutely improve their performance. At least theoretically, the effects of sodium bicarbonate are likely to be greater in the level 2 versions of the Yo-Yo test, which require a larger contribution from the anaerobic energy system [ 179 ]. Still, future research is needed to directly compare the effects of sodium bicarbonate on level 1 and level 2 Yo-Yo test performance.
Single-bout rowing
At the Olympic Games and world championships, all rowing races are performed over a 2000-m distance. It is estimated that approximately 30% of the energy necessary to complete a 2000-m row is derived from anaerobic sources [ 181 ], meaning that sodium bicarbonate may be beneficial in such exercise tasks.
Several studies have investigated the effects of sodium bicarbonate on rowing using a 2000-m rowing ergometer time trial as a measure of performance [ 15 , 54 , 129 , 138 ]. Carr et al. [ 15 , 129 ] conducted two studies to explore the effects of sodium bicarbonate on performance in the 2000-m rowing ergometer test among well-trained rowers. They did not find an ergogenic effect of sodium bicarbonate on rowing performance using either a single-dose (0.3 g/kg consumed 120 to 90 min before the test) or multiple-day (0.5 g/kg per day consumed for 3 days before the test) supplementation protocol. In their study that used the single-dose protocol, average rowing time following placebo and sodium bicarbonate ingestion was 403.8 ± 23.4 s and 404.4 ± 23.4 s, respectively. However, limitations of these studies are a small sample including only 7 to 8 participants and high intra-individual variability in performance, blood alkalosis, and gastrointestinal symptoms [ 15 , 129 ]. In a much larger sample ( n = 20 club-level rowers), sodium bicarbonate ingestion in the dose of 0.3 g/kg consumed from 240 to 120 min before exercise improved 2000-m rowing performance [ 54 ]. In this study, the time needed to complete 2000 m of rowing was 410.7 ± 14.9 s following sodium bicarbonate ingestion and 412.0 ± 15.1 s following placebo ingestion. This study also analyzed the 500-m split times and reported a reduction in rowing time in the second half of the race (i.e., from 1000 m to 2000 m), supporting previous findings that sodium bicarbonate is effective towards the latter stages of exercise.
The positive effects of sodium bicarbonate on rowing performance were also established in a recent meta-analysis [ 182 ] that pooled the results of all these previous studies. This analysis reported an improvement in rowing performance by 1.4% (90% CI: 0.1%, 2.6%) following the ingestion of sodium bicarbonate compared to placebo. While such an improvement may seem small, it is likely to be practically important for competitive rowing, where small differences often determine placings. For example, at the 2016 Rio Olympic Games, the first and second places in the men’s single scull event were determined by a photo-finish. It is estimated that improvements in performance as small as 0.3% may have meaningful effects on performance outcomes in rowing competitions, highlighting that rowers may consider supplementation with sodium bicarbonate [ 183 ]. Overall, we conclude that sodium bicarbonate is likely to have a small but positive effect on rowing performance.
Single-bout swimming
Competitive swimming is a single-bout event (e.g., 100-m swimming, 200-m swimming). Therefore, studies that examined the effects of sodium bicarbonate on single-bout swimming tasks likely offer the most practically important findings for competition [ 59 , 64 , 67 , 92 , 121 , 142 ]. Lindh et al. [ 67 ] conducted a study that involved nine elite male swimmers who performed 200-m freestyle swimming after the ingestion of placebo or 0.3 g/kg of sodium bicarbonate. In this study, swimming time following placebo ingestion was 114 ± 3.6 s. Ingestion of sodium bicarbonate reduced the time needed to complete 200-m of swimming on average by 1.8 s (1.6%). Despite this finding, other studies investigating the effects of sodium bicarbonate on 200-m swimming performance and did not report significant ergogenic effects of sodium bicarbonate among highly trained swimmers [ 59 , 142 ]. Reasons for this discrepancy in the findings are currently unclear, but they may be associated with the individual variation in responses to sodium bicarbonate supplementation and/or due to the small samples ( n = 6 to 7) included in these studies [ 59 , 142 ].
To address the limitation of small sample sizes commonly observed in the literature, one meta-analysis explored the effects of sodium bicarbonate on swimming performance [ 184 ]. Sodium bicarbonate was not found to be ergogenic for 100-yard (91.4-m) and 100-m swimming tests lasting between 50 and 60 s. Nevertheless, an ergogenic effect was shown in a subgroup analysis that included only 200-m and 400-m swimming tests, lasting between 112 s and 270 s [ 184 ]. Based on this analysis and consistent with the results for running and cycling, it seems that sodium bicarbonate may improve swimming performance in longer-distance events. Even though the magnitude of improvement was small (pooled Cohen’s d = 0.22; 1.3%), it may be of practical importance as placings in swimming competitions are often determined by narrow margins. For example, at the 2016 Olympic Games finales in the 200-m butterfly stroke, the difference between first and second place was only 0.04 s (i.e., 1:53.36 vs. 1:53.40-min). Data suggest that improvements in swimming performance of ~0.4% may represent a practically relevant effect in regard to event outcomes [ 185 ]; therefore, sodium bicarbonate supplementation seems to be beneficial in swimming competitions.
Repeated-bout swimming
Several studies have also explored the effects of sodium bicarbonate on interval swimming performance, which is relevant for the interval-based training practices of swimmers [ 42 , 102 , 124 , 186 ]. In the first study on this topic, Gao et al. [ 42 ] examined the effects of sodium bicarbonate on swimming velocity using a 5 × 100-yard swimming protocol, with a 2-min rest between intervals. Results showed that sodium bicarbonate increased swimming velocity in swimming intervals 4 and 5 (~2%). A similar ergogenic effect (1.1% to 2%) was also reported in studies using 8 × 25-m (5-s rest) and 4 × 50-m swimming (1-min rest) protocols [ 102 , 124 ].
Two studies that used interval swimming protocols did not report an ergogenic effect of sodium bicarbonate [ 14 , 112 ]. One of them [ 14 ] used a protocol involving 6 × 100-m swimming, but the rest interval between bouts lasted for 6 min, much longer than in the studies that reported ergogenic effects (with rest periods between 5-s and 2-min). The other study [ 112 ] employed a 56 × 10-m sprint swimming protocol (~7 s per sprint), where the rest interval ranged between 17 s and 5 min. The study’s lack of a significant ergogenic effect may be because the outcome variable was the average time across all 56 sprints. This is a limitation, given that the effects of sodium bicarbonate may not have been uniform in all the sprints. In the early work by Gao et al. [ 42 ], the ergogenic effects of sodium bicarbonate on 100-yard swimming performance were only recorded in bouts 4 and 5. Therefore, future studies that use interval swimming should consider analyzing the effects of sodium bicarbonate on each sprint separately. Overall, it seems that sodium bicarbonate may enhance interval swimming performance and that these effects are more pronounced when using shorter rest intervals between swimming bouts and longer swimming distances.
Effects of sodium bicarbonate on performance in combat sports
Competitive events in many combat sports consist of high-intensity actions of short duration that are interspaced with brief intervals of lower exertion [ 187 , 188 ]. For example, an analysis of movements in judo showed that most actions were of high intensity, lasted between 20 and 30 s, and were separated by short periods of lower exertion lasting from 5 to 10 s [ 187 ]. Due to their structure, many combat sports (e.g., judo, wrestling) rely heavily on glycolysis [ 187 ], which explains the interest of researchers to explore the effects of sodium bicarbonate supplementation on performance exercise tasks relevant to these sports.
Three studies involving judo athletes [ 4 , 135 , 147 ] have examined the effects of sodium bicarbonate on the number of throws in three bouts of the “Special Judo Fitness Test”. The “Special Judo Fitness Test” involves three periods lasting between 15 s and 30 s during which the participant attempts to complete as many throws as possible on two other individuals. Supplementation with sodium bicarbonate increased the number of throws by ~2 (6%) in the “Special Judo Fitness Test” bouts 2 and 3 [ 4 , 135 ].
Similar studies have also been conducted with athletes competing in boxing, karate, taekwondo, and wrestling [ 32 , 34 , 68 , 100 , 143 ]. In a study including amateur boxers [ 100 ], 0.3 g/kg of sodium bicarbonate ingested 60 min before exercise increased the number of punches performed during four rounds of sparring, where each round lasted 3 min with a 1-min rest interval. Sodium bicarbonate ingestion also prolonged the time to fatigue during a Karate-specific aerobic test in 8 karate athletes, and it increased attack time during simulated taekwondo combat [ 68 , 143 ]. Durkalec-Michalski et al. [ 32 ] examined the effects of sodium bicarbonate ingestion on the number of throws during a dummy throw test in a cohort of elite competitive wrestlers. Results did not show an ergogenic effect of sodium bicarbonate ( p = 0.07), using a multiple-day, progressive protocol of sodium bicarbonate supplementation (from 0.025 g/kg per day to 0.100 g/kg per day over 10 days). However, a follow-up study by the same research group [ 34 ] reported an increase in the number of throws (~2) in the same dummy throw test using the same supplementation protocol, even though the improvement was found only in male participants. Overall, most studies suggest that sodium bicarbonate is an effective supplement for enhancing performance in combat sports, such as boxing, judo, karate, taekwondo, and wrestling. Therefore, athletes competing in combat sports may consider using supplementation with sodium bicarbonate to improve their performance. However, future research is needed to explore the effects of sodium bicarbonate supplementation specifically in combat sport competitions.
Effects of sodium bicarbonate on resistance exercise performance
A considerable amount of evidence exists on the effects of sodium bicarbonate supplementation on resistance exercise performance, particularly for two outcomes, muscular endurance and muscular strength [ 3 , 16 , 18 , 30 , 37 , 71 , 73 , 104 , 105 , 106 , 107 , 108 , 118 ]. Muscular endurance is commonly assessed as the maximum number of completed repetitions of a movement with a given load or as the maximum duration of maintaining isometric force production. A recent meta-analysis [ 189 ] of 12 studies showed sodium bicarbonate supplementation to be ergogenic for muscular endurance (Cohen’s d = 0.37; 95% CI: 0.15, 0.59). While an acute improvement in muscular endurance might be expected following sodium bicarbonate intake, its magnitude will likely depend on a number of factors.
Two particularly important factors to consider are the external load and duration of the task. Maughan et al. [ 73 ] evaluated the effects of consuming 0.3 g/kg of sodium bicarbonate on time to maintain an isometric contraction at 80%, 50%, and 20% of maximum isometric strength. In this study, an ergogenic effect was observed when utilizing the lowest load, increasing the time of the isometric contraction from 210 ± 77 s in the placebo trial to 227 ± 65 s after sodium bicarbonate ingestion (8%). With the two higher loads, the participants could only maintain the contraction from 20 to 58 s. The duration of these tasks might have been too short to observe a benefit from sodium bicarbonate supplementation. Alternatively, the restricted blood flow to the contracting muscles with these more intense isometric contractions may have minimized lactate, H + , and K + release from muscles and thus diminished benefits of sodium bicarbonate supplementation. Another study [ 18 ] used isokinetic knee flexion and extension test that lasted 85 s, where total work was used as a proxy of muscular endurance and found an ergogenic effect of sodium bicarbonate. Similar to the findings observed for high-intensity exercise, the evidence is suggesting that the duration of the muscular endurance task may need to exceed ~1 min for sodium bicarbonate to be ergogenic, at least when a single set is performed.
Another factor that needs to be considered is the set protocol. Webster et al. [ 118 ] used a protocol where the participants first performed 4 sets of 12 repetitions of the leg press, followed by one set performed to muscular failure at 70% of one-repetition maximum (1RM) following sodium bicarbonate or placebo ingestion. The outcome variable in this study was the number of completed repetitions in the fifth set. The study did not find significant differences in the number of repetitions between the two conditions (average of 18 and 19 repetitions for placebo and sodium bicarbonate, respectively). Assuming that this study adopted a tempo of 1 s for the eccentric and concentric phase of the movement, the duration of the exercise task would only have been around 40 s, which might explain the absence of an ergogenic effect. However, when multiple set protocols performed to muscular failure were used, an ergogenic effect of sodium bicarbonate on muscular endurance was reported [ 16 , 30 ]. For example, Carr et al. [ 16 ] assessed the number of repetitions performed in 13 sets across three lower-body exercises. An ergogenic effect was observed, as the total number of performed repetitions was increased from 157 ± 15 to 164 ± 15 (4.5%) in the placebo and sodium bicarbonate trials, respectively. This study only analyzed the total number of repetitions across all exercises and did not evaluate performance in each set separately. It is conceivable that the effects of sodium bicarbonate increase with increasing the number of sets. This hypothesis is somewhat supported by the work of Duncan et al. [ 30 ]. After the ingestion of sodium bicarbonate and placebo, the participants in their study were able to complete, on average, 12 repetitions in the first set of squats at 80% of 1RM. In the second set that took place after a 3-min rest interval, the participants performed 11 repetitions in the sodium bicarbonate trial and 7 repetitions in the placebo trial. Favorable effects of sodium bicarbonate were also observed in the third set (9 vs. 6 repetitions). Therefore, it seems that the effects of sodium bicarbonate on muscular performance are more pronounced when using multiple-set protocols.
Muscular strength, commonly evaluated using isometric, isokinetic, or isotonic tests, is another important muscular quality in resistance exercise [ 190 ]. Given that muscular strength tests are characterized by very brief duration and maximal exertion, it appears less likely that sodium bicarbonate would be ergogenic for this outcome. Indeed, a recent meta-analysis [ 189 ] did not find a significant difference between the effects of sodium bicarbonate and placebo on muscular strength (Cohen’s d = –0.03; 95% CI: –0.18, 0.12). Furthermore, the upper and lower limits of the 95% CI were in the range of trivial/very small effects, suggesting that even if there is a true effect of sodium bicarbonate on muscular strength in the population, it is likely practically negligible. While sodium bicarbonate may not increase strength per se , it might prevent the fatigue-induced decline in strength [ 3 ]. Ansdell et al. [ 3 ] evaluated the effect of sodium bicarbonate supplementation on isometric strength of the knee extensors assessed before a basketball game and after each quarter. The authors observed that the decline in strength was on average 9% when sodium bicarbonate was ingested, compared to a 15% decline in the placebo condition [ 3 ]. Although sodium bicarbonate may not enhance muscle strength directly, this supplement seems to enhance the capacity to train maximally, thus leading to increased strength gains with resistance training. Indeed, attenuating the decline in strength during multiple sets has been found to contribute to greater gains in strength [ 191 ].
In animal models, studies commonly report that increases in bicarbonate concentration and/or intracellular accumulation of H + do not impact maximum force production, even though these effects may be temperature-dependent [ 192 , 193 ]. This lack of an effect on force production might also explain why sodium bicarbonate supplementation in humans generally does not enhance maximum strength. However, data using animal models also show that a decline in pH negatively affects muscle conduction velocity, a metric that is positively related to the rate of force development (RFD) [ 194 , 195 ]. Given that one of the mechanisms of sodium bicarbonate is buffering of H + , the ingestion of this supplement may positively impact RFD. A series of studies explored the effects of sodium bicarbonate on RFD following either 30 s of cycling, maximum strength testing, or five sets of knee extensions [ 104 , 106 , 107 , 108 ]. RFD was higher following sodium bicarbonate ingestion in three of these studies. These findings might be of practical importance as RFD is associated with several aspects of athletic performance, such as sprinting and jumping [ 196 , 197 ]. This might partially explain some of the positive results shown for the effect of sodium bicarbonate supplementation on repeated-sprint activities. While these initial findings are promising, future work is still needed, as only a few studies have focused on this outcome.
Effects of sodium bicarbonate on training adaptations
Most studies in the field have explored the acute effects of sodium bicarbonate supplementation. However, a handful of studies have also explored the effect of long-term supplementation with sodium bicarbonate. Edge et al. [ 35 ] used a volume- and intensity-equated cycling interval training program in which female student participants ( n = 16) were randomized to consume 0.4 g/kg of sodium bicarbonate one hour before every training session for 8 weeks (overall 24 sessions) or to ingest the same amount of placebo. After 8 weeks of training, greater improvements in the lactate threshold (26% vs. 15%) and time to fatigue while cycling at 100% of peak oxygen uptake (164% vs. 123%) were shown in the group ingesting sodium bicarbonate. It is important to emphasize that the capacity tests were performed without prior acute supplementation, meaning these differences can be attributed to greater adaptations throughout the training period with sodium bicarbonate supplementation. The authors suggested that less disturbance to metabolic homeostasis associated with the use of sodium bicarbonate around exercise sessions might have promoted muscle protein balance, contributing to greater increases in intracellular muscle proteins involved in mitochondrial respiration or ion balance [ 35 ]. As suggested in a subsequent study conducted with rats, the effects might indeed be attributed to greater mitochondrial adaptations in the group ingesting sodium bicarbonate [ 198 ].
Another study [ 117 ] explored the effect of high-intensity interval training coupled with sodium bicarbonate supplementation in a group of 20 recreationally active men. Following six weeks of high-intensity interval training, incorporating 18 training sessions, the group ingesting sodium bicarbonate prior to every training session experienced greater improvements in relative peak power during 30-s all-out cycling than the group ingesting a placebo (21% vs. 10%). However, these findings were not confirmed in two other studies [ 29 , 108 ]. Sodium bicarbonate supplementation during 4 weeks of high-intensity interval training (8 sessions) did not positively impact outcomes such as rowing time, peak power, and mean power [ 29 ]. Another study explored the effect of sodium bicarbonate supplementation during 10 weeks of resistance training and did not find an ergogenic effect on 1RM strength [ 108 ]. However, in both studies that did not find an ergogenic effect, the sample sizes were very small (i.e., 4 to 6 participants per group), which resulted in a low statistical power [ 29 , 108 ]. Overall, there is some evidence that the repeated use of sodium bicarbonate supplementation (0.2 to 0.4 g/kg ingested 90 to 60 min before every exercise session for 6 to 8 weeks) may impact long-term adaptations to exercise (i.e., delaying time to fatigue and increasing peak power), but much more work in the area is needed.
Sex-specific effects of sodium bicarbonate on exercise performance
A recent systematic review [ 199 ] showed that only around 20% of studies examining the effects of sodium bicarbonate involved women as participants. Of these studies, several reported an ergogenic effect of sodium bicarbonate. For example, two studies by Bishop et al. [ 7 , 8 ] reported that sodium bicarbonate improved repeated-sprint and intermittent sprint performance. While others reported similar findings [ 24 , 99 ], some studies [ 62 ] did not find an ergogenic effect of sodium bicarbonate. According to the meta-analytical data of Saunders et al. [ 199 ], only 11 studies provided group analyses exclusively for women. When the results of these studies were pooled in a meta-analysis, an average increase in plasma bicarbonate following sodium bicarbonate ingestion was found to be around 7 mmol/L. The review also indicated that sodium bicarbonate is ergogenic in females with small-to-moderate exercise performance improvements (pooled effect size = 0.37). Nonetheless, the vast majority of studies that have explored (and established) the ergogenic effects of sodium bicarbonate on exercise performance included men only as participants. It currently seems that sodium bicarbonate is ergogenic for both sexes; however, the evidence base for a performance-enhancing effect in women is not as large. Although menstrual cycle phase has been shown to have very little influence on exercise outcomes [ 200 ], the size of some differences between phases represents >30% of the ergogenic effects shown in this meta-analysis (effect size 0.14 vs. 0.37), meaning it might be an important factor. Further work with female participants is required to determine the influence of the menstrual cycle phase on exercise outcomes following sodium bicarbonate supplementation.
Training status and the effects of sodium bicarbonate on exercise performance
Studies that examined the ergogenic effects of sodium bicarbonate supplementation on exercise performance included different populations, ranging from elite athletes to untrained individuals (Table 1 ). Accordingly, training status could be a variable moderating the effects of sodium bicarbonate on exercise performance. Studies included well-trained basketball players [ 2 ], judo competitors [ 4 ], distance runners [ 6 ], team-sport athletes [ 7 , 8 ], varsity track athletes [ 10 ], resistance-trained individuals [ 16 ], well-trained cyclists [ 27 ], hockey players [ 33 ], professional boxers [ 49 ], elite swimmers [ 67 ], taekwondo black belt athletes [ 68 ], and triathletes [ 86 ], and all reported an ergogenic effect of sodium bicarbonate on exercise outcomes. Studies have also included untrained participants and reported an ergogenic effect of this supplement [ 18 , 19 , 57 , 73 , 96 , 97 , 109 ]. Therefore, the absence of an ergogenic effect in some studies does not seem to be related to the training status of included participants. However, the studies analyzed herein also differed in a range of methodological characteristics that may have affected the effect sizes independent of the training status (e.g., exercise test, sodium bicarbonate dose, and timing of ingestion). Therefore, future studies should consider including participants with different training levels (e.g., untrained, trained, competitive, and elite athletes) to determine directly whether the effects of sodium bicarbonate vary according to training status.
Optimal protocols of sodium bicarbonate supplementation
Powder and capsule form of sodium bicarbonate.
In recent years, studies generally provided sodium bicarbonate supplementation to participants in capsule form. This form of supplementation is used to maintain a double-blind study design. Still, some of the early studies [ 6 , 18 , 19 , 41 , 42 , 44 , 77 ] mixed sodium bicarbonate with water (with or without additional substances such as orange juice or low-calorie sweetener), and this solution was ingested before exercise. There are several limitation with the latter approach. The taste of sodium bicarbonate is bitter and salty, which many participants may find unpleasant. In addition, due to its taste, it is difficult to disguise the sodium bicarbonate condition with this form of ingestion, which may compromise blinding. Still, a limitation with capsules is that a large number of them need to be ingested to get to ergogenic doses of sodium bicarbonate. Therefore, readers are also advised to consider the form of sodium bicarbonate supplementation used in a given study when interpreting its findings.
Sodium bicarbonate dose
Because the dose of sodium bicarbonate most commonly used in research trials is 0.3 g/kg, the evidence base for the ergogenic effects of sodium bicarbonate is most established for this dose. The origin for this protocol likely stems from the work of Jones et al. [ 58 ]. Additional support for the use of this dose can be found in one of the most cited dose-response studies on the topic, published by McNaughton in 1992 [ 77 ]. This study explored the effects of consuming sodium bicarbonate in incremental doses of 0.1 g/kg (from 0.1 to 0.5 g/kg) on peak power and total work produced during 60 s of high-intensity cycling. Increases in total work were observed with doses from 0.2 to 0.5 g/kg, and for peak power, doses from 0.3 to 0.5 g/kg were required to record an improvement. No significant differences in ergogenic effects were found between doses from 0.3 to 0.5 g/kg. However, side-effects were higher with 0.4 and 0.5 g/kg; thus, it was concluded that the dose of 0.3 g/kg provides an optimal cost/benefit balance [ 77 ].
Other studies have also investigated the effects of consuming different doses of sodium bicarbonate on exercise performance [ 38 , 46 , 47 , 55 , 75 , 99 , 121 ]. The smallest researched dose was 0.1 g/kg. A study by Ferreira et al. [ 38 ] compared the effects of consuming 0.1 g/kg vs. 0.3 g/kg of sodium bicarbonate on performance in a cycling test to exhaustion. Time to fatigue was improved only following the ingestion of 0.3 g/kg (76 ± 4 s cycling). The dose of 0.1 g/kg was not ergogenic, and the average performance value with this dose was very similar to the placebo condition (65 ± 8 s and 68 ± 5 s, respectively). Similar findings were reported in another study [ 99 ] that compared the effects of consuming 0.1 vs. 0.2 g/kg of sodium bicarbonate on repeated cycling in 12 moderately trained females. Time to exhaustion increased following the ingestion of 0.2 g/kg (162.4 ± 107.3 s), compared with 0.1 g/kg (133.9 ± 83.3 s) and a placebo (129.4 ± 104.0 s). There were no significant differences between the lower dose of sodium bicarbonate and placebo. Another study used doses of 0.1, 0.15, and 0.2 g/kg [ 55 ]. While the dose of 0.1 g/kg was not found to be ergogenic, there was also a general absence of improvements in performance (4 × 2-min cycling sprints) following sodium bicarbonate ingestion at any dose. Still, it is unclear whether the doses or some other element of the study protocol led to the absence of significant effects. It currently seems that a sodium bicarbonate dose of 0.1 g/kg is not ergogenic for exercise performance. The reason for this lack of an ergogenic effect might be that increases in plasma bicarbonate concentration following the ingestion of 0.1 g/kg of sodium bicarbonate are very small (1 to 2 mmol/L) [ 55 , 201 ].
A dose of 0.15 g/kg has also been used, albeit only in two studies [ 55 , 75 ]. One study [ 75 ] reported ergogenic effects with 0.15 g/kg and 0.3 g/kg of sodium bicarbonate on repeated-cycling performance (5 × 1-min cycling with the sixth sprint to exhaustion), but no significant difference between the doses. The other study [ 55 ] used 2-min cycling sprints and evaluated total work as the performance outcome following the ingestion of 0.1 g/kg, 0.15 g/kg, and 0.2 g/kg, although none of the doses were shown to be ergogenic. Thus, the efficacy of a 0.15 g/kg dose remains unclear.
Several studies have reported an ergogenic effect with a dose of 0.2 g/kg [ 19 , 43 , 57 , 117 ]. Costill et al. [ 19 ] used this dose in their study that reported an improvement in time to exhaustion in a repeated-cycling test, coupled with substantial increases in plasma bicarbonate concentration of 5 mmol/L. In addition to McNaughton’s previously discussed work [ 77 ], several other studies compared the effects of 0.2 g/kg and 0.3 g/kg of sodium bicarbonate [ 46 , 47 , 48 , 50 , 51 , 121 ]. Most studies reported an ergogenic effect for both doses, with no significant differences in the effects between the doses [ 46 , 48 , 50 , 51 , 121 ]. However, one study [ 47 ] reported that performance in a 4-km cycling time trial was improved only following the ingestion of 0.3 g/kg. Examination of individual responses in one study indicated that a dose of 0.2 g/kg was ergogenic in 8 out of 12 participants, while the dose of 0.3 g/kg was ergogenic for 11 out of 12 participants [ 51 ]. The difference between the percentages of participants whose performance improved following the ingestion of 0.2 g/kg and 0.3 g/kg of sodium bicarbonate was not statistically significant ( p = 0.132). Future research is needed to clarify any potential differences between ergogenic effects of consuming 0.2 g/kg and 0.3 g/kg of sodium bicarbonate.
In summary, the following is currently known regarding the dose of sodium bicarbonate: (1) a dose of 0.1 g/kg does not appear to be ergogenic for exercise performance; (2) a dose of 0.15 g/kg may be the minimal ergogenic dose, albeit this was found only in one out of two studies; (3) in several studies, a dose of 0.2 g/kg was ergogenic, with some showing similar effects to those with higher doses; (4) a dose of 0.3 g/kg has been the most thoroughly studied dose of sodium bicarbonate, it has consistently produced ergogenic effects and is considered as the optimal dose; (5) doses of 0.4 g/kg and 0.5 g/kg are also ergogenic, but they do not seem to be a requirement to elicit improvements in exercise performance and are associated with a higher incidence and severity of side-effects.
Of note, this section reviewed the evidence and provided recommendations only for single-dose protocols of sodium bicarbonate ingestion. However, the optimal dose for multiple-day sodium bicarbonate supplementation protocols might be higher (see the section “Multiple-day protocols of sodium bicarbonate supplementation”).
Timing of sodium bicarbonate supplementation
In most studies, sodium bicarbonate was consumed at a standardized time point 60 to 180 min before exercise for all participants, making the evidence of its effectiveness around this timing of ingestion well established (Table 1 ). One of the determinants of the ergogenic effect of sodium bicarbonate may be the increase in plasma bicarbonate concentration [ 202 ]. It has been suggested that to increase the likelihood of an ergogenic effect, the start of the exercise session should coincide with the peak concentration of plasma bicarbonate concentration or with an increase in plasma bicarbonate concentration of 5 mmol/L [ 202 ]. However, there is a considerable variation between individuals in time from ingestion of sodium bicarbonate to the peak plasma bicarbonate concentration. For example, in one study [ 201 ], time to peak plasma bicarbonate concentration varied highly between individuals, as it occurred from 30 to 150 min, 40 to 165 min, and 75 to 180 min after the ingestion of sodium bicarbonate in the doses of 0.1, 0.2, and 0.3 g/kg, respectively.
The large individual variability in time from sodium bicarbonate ingestion to the peak plasma bicarbonate concentration may have presented a methodological issue in previous studies on ergogenic effects of this supplement using a standardized ingestion time. An alternative approach, incorporated into several recent studies involves sodium bicarbonate ingestion according to individualized considerations of time to peak plasma bicarbonate concentration [ 9 , 22 , 23 , 46 , 47 , 50 , 84 ]. In such studies, time to peak plasma bicarbonate concentration following sodium bicarbonate supplementation was first evaluated under resting conditions. Then, sodium bicarbonate intake is adjusted for the exercise trials according to individual timing at which peak plasma bicarbonate occurred. While the time to peak value is typically 60 to 70 min across individuals, this can vary from 10 min to 240 min after ingestion [ 22 , 23 , 46 , 47 , 50 , 53 , 84 , 201 , 203 ]. When using such an ingestion protocol, most studies showed ergogenic effects of sodium bicarbonate. For example, one study [ 84 ] used a repeated-sprint ability protocol (10 × 6-s sprints with 60-s rest) and reported that ingesting 0.3 g/kg according to individualized time to peak plasma bicarbonate concentration increased total work across all 10 sprints. The study by Boegman et al. [ 9 ] is the only one to date that compared the effects of individualized vs. non-individualized ingestion timing and reported that the former protocol produced greater ergogenic effects, although this study was limited by a lack of a placebo trial. It showed that individualized timing produces larger ergogenic effects than supplementation at a standardized pre-exercise time point (60 min), although this time point might be on the lower end of the optimal standardized timeframe [ 203 ]. Supplementation protocols that utilize individualized ingestion timing provide substantial practical difficulties because most athletes do not have access to a blood gas analyzer that would be required to determine peak plasma bicarbonate concentration and individualize supplementation timing.
Despite the generally favorable effects of individualizing ingestion timing, some have questioned the reliability of time to peak plasma bicarbonate concentration following sodium bicarbonate supplementation [ 203 ]. Oliveira and colleagues used three repeated administrations of sodium bicarbonate and reported that the time to peak plasma bicarbonate concentration was inconsistent and non-reproducible [ 203 ]. This study also suggested that ingesting a dose of 0.3 g/kg produces a long-lasting ergogenic potential window that lasts around 3 h, starting around 60 min after ingestion, when considering increases of plasma bicarbonate concentration >5 mmol/L [ 203 ]. Indeed, studies providing sodium bicarbonate supplementation at 60 min, 90 min, 120 min, 150 min, or 180 min before exercise have reported ergogenic effects [ 4 , 7 , 19 , 25 , 58 , 102 ]. Based on these results, we conclude that sodium bicarbonate can be ergogenic when ingested from 60 to 180 min before exercise, but individual experimentation around timing is recommended, particularly concerning the incidence of side-effects.
When deciding about the timing of sodium bicarbonate ingestion, possible side-effects and their interference with the activity should also be considered (see section “Side-effects associated with sodium bicarbonate supplementation”). The decision to use or not to use individualized ingestion timing may also depend on the dose of sodium bicarbonate. Specifically, lower doses of sodium bicarbonate (e.g., 0.2 g/kg) result in shorter increases in plasma bicarbonate concentration [ 77 ]. However, when using higher doses of sodium bicarbonate, such as 0.3 g/kg, the increases in plasma bicarbonate concentration are longer-lasting [ 203 ]. Therefore, individualized ingestion timing may be more important when using lower doses of sodium bicarbonate. More dose-response studies are needed to confirm this hypothesis.
Finally, when deciding about the timing of sodium bicarbonate ingestion, the size of the capsules may also need to be considered. A recent study reported that peak plasma bicarbonate concentration following the ingestion of 0.3 g/kg of sodium bicarbonate occurred at 94 ± 24 min, 141 ± 27 min, and 121 ± 29 min when using small, medium and large size capsules, respectively [ 204 ]. For those aiming to increase extracellular buffering capacity more quickly, these recent results suggest that supplementation with sodium bicarbonate in smaller capsules should be considered [ 204 ].
Multiple-day protocols of sodium bicarbonate supplementation
Most studies have used protocols in which sodium bicarbonate is ingested acutely, from 60 to 180 min before exercise. However, several studies [ 15 , 24 , 26 , 27 , 31 , 32 , 33 , 34 , 59 , 66 , 81 , 82 , 83 , 88 , 147 ] have provided supplementation for several consecutive days before testing exercise performance, ceasing on the evening before the trial. This approach is interesting because it could help avoid potential sodium bicarbonate-induced side-effects on the day of competition. Specifically, in these studies, sodium bicarbonate is provided daily for several days before the exercise test, with the last dose commonly ingested on the night before evaluating exercise performance. In most of these studies, there was no sodium bicarbonate ingestion on the day of the exercise test. For example, in one study [ 27 ], sodium bicarbonate was provided for three consecutive days before testing, with the last dose ingested at 19:00 h on the night before testing. The multiple-day protocol was initially used by McNaughton et al. [ 81 ], who provided an overall dose of 0.5 g/kg per day (split into four smaller doses to be ingested throughout the day) to their participants for 5 days before evaluating performance in a 60-s cycling test on the 6 th day (i.e., the day after the final ingested dose). This supplementation protocol was effective for improving total work (~14% increase) and peak power (~10% increase) during the exercise task.
Whilst there are no likely benefits of ingesting doses higher than 0.3 g/kg for single-dose supplementation protocols, multiple-day supplementation protocols may require a larger daily sodium bicarbonate dose to produce an ergogenic effect. One dose-response study [ 26 ] evaluated performance in the Wingate test following 5 days of supplementation with either 0.3 or 0.5 g/kg per day of sodium bicarbonate and found increases in mean power only with the higher dose. Other studies [ 59 , 66 ] that used multiple-day supplementation (lasting from 3 to 7 days) also did not show significant ergogenic effects with a 0.3 g/kg per day dose. In contrast, most other studies [ 24 , 26 , 27 , 81 , 82 , 83 , 88 , 147 ] that provided supplementation for 3 to 7 days with doses of 0.4 or 0.5 g/kg per day reported ergogenic effects of sodium bicarbonate. Therefore, for protocols that last between 3 and 7 days, it seems that a dose of 0.4 or 0.5 g/kg per day is needed to elicit an ergogenic effect. Still, it should be mentioned that a research group recently conducted a series of studies [ 31 , 32 , 33 , 34 ] in which much smaller doses of sodium bicarbonate were ingested over 10 days, with progressive increases in the doses (i.e., daily doses ranged from 0.025 g/kg per day to 0.100 g/kg per day). These studies reported ergogenic effects on mean power, muscular endurance, and the number of throws in a dummy throw test. This would suggest that a lower dose (i.e., up to 0.100 g/kg per day) of sodium bicarbonate ingested in a multiple-day protocol may also be ergogenic, at least when ingested for 10 days. However, future studies replicating this protocol of ingestion are needed to confirm the findings.
While it is clear that multiple-day sodium bicarbonate supplementation protocols can be effective, it is unclear how their effects compare to those observed following single-dose ingestion of sodium bicarbonate. A meta-analysis [ 205 ] included studies that provided sodium bicarbonate supplementation for 5 to 7 days before a Wingate test [ 26 , 88 , 147 ]. In this analysis, an ergogenic effect was found for mean power. This same meta-analysis, when focusing on studies that provided single-dose supplementation, did not find a significant ergogenic effect of sodium bicarbonate on performance in single and repeated Wingate tests [ 205 ]. Still, none of the studies included in this meta-analysis directly compared these two protocols of supplementation, so no inferences can be made on which (if any) of the protocols are more effective.
In two studies [ 15 , 59 ] that compared the effects of single-dose vs. multiple-day protocols of sodium bicarbonate ingestion, no significant ergogenic effect was found for any of the two protocols. In another study, both protocols were comparably ergogenic [ 27 ]. One study [ 33 ] reported that only a multiple-day ingestion protocol was ergogenic for mean and peak power in the Wingate test. In the same study, both protocols elicited an ergogenic effect on performance in a hockey-specific field performance test [ 33 ]. Finally, McNaughton and Thompson [ 83 ] evaluated exercise performance during 90 s cycling after ingesting 0.5 g/kg 90 min before exercise and after ingesting 0.5 g/kg for 5 days before exercise. Exercise performance was evaluated on three consecutive days. On the second and third testing day, there was no sodium bicarbonate supplementation. Both ingestion protocols increased the total work in the cycling task on the first testing day. However, only the multiple-day protocol was ergogenic on the following two days. This suggests that the improvement in performance with this supplementation protocol may be longer-lasting. These results suggest that sodium bicarbonate loading may benefit athletes who have competition events scheduled on consecutive days. However, given that the mechanism underpinning this ergogenic effect is unclear, these findings should be taken with caution and they require further confirmation.
A final point to consider is that multiple-day sodium bicarbonate supplementation protocols require ingestion of a high number of capsules for several days, which may result in a continuous sensation of satiety [ 206 ]. This may, at least hypothetically, prevent athletes from meeting their daily energy demands and thus has the potential to negatively affect their performance. To circumvent this, athletes may consider using powder form of sodium bicarbonate mixed with liquid, even though there are limitations with this approach as well (see section “Powder and capsule form of sodium bicarbonate”).
Influence of warm-up on the ergogenic effects of sodium bicarbonate
The influence of different warm-up strategies on the ergogenic effects of sodium bicarbonate has been explored in two studies. Gurton et al. [ 207 ] explored whether an intermittent, sprint-based warm-up relative to lactate threshold (5 min at 50%; 2 min at 60%; 2 min at 80%; 1 min at 100%; 2 min at 50%; and 3 × 10-s maximal sprints with 90-s recovery) impacted the ergogenic effects of individualized sodium bicarbonate ingestion on 4-km cycling time-trial performance compared to a control warm-up (16.5 min cycling at 150 W). Their results showed that the high-intensity warm-up mitigated the ergogenic effects of sodium bicarbonate in club-level cyclists, likely due to the use of the increased buffering capacity during this intense warm-up. More recent data [ 208 ] reported that a high-intensity warm-up relative to maximal power output (5 min at 60% maximal power output, 5 min at 70% maximal power output, 5 min at 80% maximal power output, and 30 s at maximal power output) did not modulate the ergogenic effect of sodium bicarbonate on exercise capacity during a cycle to exhaustion tasks compared to a low-intensity warm-up (15-min cycling at 60% maximal power output). The difference between these studies may be related to the time between the warm-up and the exercise bout. Specifically, Gurton et al. [ 207 ] allowed 10 min between the end of the warm-up and the exercise bout, while Jones et al. [ 208 ] used 30 min; both studies cited personal experience in elite sport for these periods. The 30 min recovery period allowed for a substantial rebound effect in blood variables, leading to higher pre-exercise blood values with sodium bicarbonate regardless of exercise intensity, whereas the 10-min recovery period led to lower blood values following the high-intensity warm-up compared to the low-intensity warm-up. Thus, athletes may wish to leave sufficient recovery time between the end of the warm-up and the start of the main exercise event to ensure blood variables have returned to levels from which they will benefit. Further work should determine how much different recovery periods influence the ergogenic effects of sodium bicarbonate and whether these effects are seen across different modalities (e.g., running, swimming).
Sodium bicarbonate supplementation for multiple events
In many sporting events that would theoretically benefit from sodium bicarbonate ingestion, competition outcomes are decided through a series of heats and finals (e.g., 4000-m track cycling pursuit, rowing events) or via multiple events (e.g., events within the decathlon and modern pentathlon). Besides, in some sports, athletes may compete in more than one event in the same competition program (e.g., a swimmer may compete in several individual events and relays). In specific scenarios, including those identified above, the interval between bouts/events is often between 1 and 24 h, and the subsequent event may fall within the half-life of the changes in plasma bicarbonate concentrations achieved via sodium bicarbonate supplementation or before the body’s return to normal physiological status following the preceding event. One study [ 59 ] explored the effects of sodium bicarbonate on 200-m swimming time (measured on two consecutive days) in eight highly trained male swimmers. This study compared the effectiveness of single-dose and multiple-day supplementation protocols, neither of which was continued on the day of the second performance test. Such intervention design mimicked a real-life swimming competition timetable, with repeated races held 24 h apart. This study did not find a significant ergogenic effect, despite attempting to simulate an event known to cause acidosis [ 59 ]. This study did not provide a detailed time course of changes in plasma bicarbonate concentration over the period from the last dose to the second trial, which would be essential information for assessing their protocol and informing future similar studies. Therefore, future research is needed to explore the effects of sodium bicarbonate for multiple events.
Side-effects associated with sodium bicarbonate supplementation
It is important to consider potential side-effects associated with sodium bicarbonate supplementation, including gastrointestinal discomfort. The buildup of CO 2 in the gut, resulting from supplementation with sodium bicarbonate (Figure 1 ), may cause bloating, nausea, vomiting, and abdominal pain [ 209 ]. Furthermore, the incidence and severity of these side-effects increase linearly with the dose of sodium bicarbonate ingested and should be considered in terms of their overall effect on performance [ 77 ]. Additionally, it should be mentioned that long-term sodium bicarbonate supplementation at commonly employed doses (0.3 g/kg) will lead to an increased habitual sodium load for the body, which may contribute to exceeding the Tolerable Upper Intake Level for sodium specified in dietary guidelines. However, the effects of sodium bicarbonate supplementation on kidney function are not yet known and are deserving of investigation.
A 1995 study by Bird et al. [ 6 ] reported an ergogenic effect of sodium bicarbonate on 1500-m running. However, scrutiny of the individual responses revealed that two participants did not improve their running performance following sodium bicarbonate ingestion. Both of these participants experienced side-effects, such as stomachache and diarrhea, suggesting that these adverse reactions may have influenced their race times. Accordingly, the absence of an ergogenic effect in some studies might be due to the side-effects associated with sodium bicarbonate. Indeed, adverse gastrointestinal effects negated the performance benefits from sodium bicarbonate supplementation in the study by Saunders et al. [ 98 ]. They found a significant overall performance improvement only after excluding four participants who experienced gastrointestinal discomfort (from the total sample of 21 participants). The potential for side-effects to diminish the ergogenic effect of sodium bicarbonate was confirmed in a subsequent study from the same research group [ 40 ]. However, not all studies had shown a significant impact of side-effects on ergogenic effects of sodium bicarbonate, at least when the side-effects tended to be less severe (e.g., minor gastrointestinal distress) [ 95 ]. Unfortunately, many studies, especially the older ones, did not provide a detailed and comprehensive record of side-effects, making it difficult to determine how much this may have contributed to the lack of ergogenic effects of sodium bicarbonate in some studies. Therefore, future studies that examine the ergogenic effects of sodium bicarbonate should aim to also record and report side-effects associated with this supplement.
Reducing or eliminating the side-effects of sodium bicarbonate ingestion will likely increase its ergogenic effects. To accomplish this, several strategies can be considered. One is related to the timing of ingestion. Siegler et al. [ 103 ] reported lower gastrointestinal discomfort 180 min after supplementation, compared to 60 and 120 min after supplementation, even though the increase in plasma bicarbonate concentration was similar between the conditions. Carr et al. [ 210 ] reported that the greatest incidence of side-effects occurred 90 min after ingestion, suggesting that conducting performance testing at this time might not be optimal. Therefore, merely adjusting the timing of sodium bicarbonate ingestion relative to exercise may reduce the incidence and severity of side-effects that occur during the exercise.
Another option to minimize side-effects is to ingest sodium bicarbonate alongside a meal. Compared to protocols that involved isolated sodium bicarbonate ingestion, combining sodium bicarbonate (0.3 g/kg) with a high-carbohydrate meal (bread with fruit spread and cereal bars, including 1.5 g of carbohydrate per kilogram of body weight) resulted in alkalosis coupled with the lowest incidence of gastrointestinal symptoms [ 210 ]. This may be an optimal strategy for athletes who consume a high-carbohydrate meal within a few hours of training or competition. Additionally, these results highlight the potential importance of controlling pre-trial diet when exploring the effects of sodium bicarbonate supplementation. As mentioned previously, using lower doses of sodium bicarbonate (e.g., 0.2 g/kg) may be another strategy to reduce the incidence and severity of side-effects (see section “Sodium bicarbonate dose”).
More recent evidence suggests that the use of enteric-coated capsules may minimize side-effects by avoiding the interaction with stomach acid [ 53 ]. Such capsules can be used for acid-sensitive ingredients, such as sodium bicarbonate, to bypass the stomach [ 53 ]. As shown in a proof-of-principle case study [ 211 ] that included a post-bariatric surgery participant, bypassing the stomach led to some of the greatest increases of plasma bicarbonate concentration ever reported, of 12 to 20 mmol/L, while minimizing the associated side-effects. Hilton et al. [ 53 ] compared the effects of sodium bicarbonate ingestion in a dose of 0.3 g/kg in enteric-coated or gelatin capsules on performance in 4-km cycling time trials. The time required to complete 4 km of cycling was reduced following the ingestion of sodium bicarbonate in both enteric-coated and gelatin capsules (8.5 s and 9.6 s, respectively). Out of 11 participants, only three experienced gastrointestinal discomfort after ingesting enteric-coated capsules, compared to seven after ingesting gelatin capsules. Given the relatively small sample size in this study, more research is needed to draw solid conclusions about the possible reduction in the incidence and severity of side-effects following the ingestion of sodium bicarbonate in enteric-coated capsules. Finally, it should be considered that enteric-coated capsules can potentially prevent gastric symptoms, but they are unlikely to prevent intestinal side-effects such as osmotic diarrhea.
In summary, the most common side-effects associated with sodium bicarbonate ingestion include bloating, nausea, and abdominal pain, and their incidence and severity increase with increasing dose. Importantly, experiencing these side-effects may negatively affect exercise performance and diminish the ergogenic effect of sodium bicarbonate. Ingesting sodium bicarbonate (i) in smaller doses (e.g., 0.2 g/kg or 0.3 g/kg), (ii) around 180 min before exercise or adjusting the timing according to individual responses to side-effects, (iii) alongside a high-carbohydrate meal, and (iv) in enteric-coated capsules, are possible strategies to minimize the likelihood and severity of side-effects. Finally, a prudent recommendation for athletes is to experiment with different sodium bicarbonate supplementation protocols during training to find their own “optimal” protocol with minimal side-effects, which they can later also use in competitions.
Placebo effects associated with sodium bicarbonate supplementation
It is well-established that a favorable outcome can arise purely from believing that one has received a beneficial treatment [ 212 ]. Indeed, there is considerable evidence to support placebo effects in sport and exercise nutrition, including sodium bicarbonate supplementation. In a recent meta-analysis, Marticorena et al. [ 213 ] examined the effects of placebo ingestion compared to a non-placebo control condition (no substance ingestion) on exercise performance in studies involving supplementation of caffeine and buffering agents (including sodium bicarbonate). In this meta-analysis, placebo ingestion was found to provide a small but ergogenic effect (Cohen’s d = 0.09). Additional analyses from this review showed that around 30% of the performance-enhancing benefits of buffering agents could be attributed to the placebo effect [ 213 ]. A double-blind crossover design, in which all participants receive both the active treatment and placebo prior to performing an exercise task, is the study design most used when investigating the effect of sodium bicarbonate supplementation on exercise outcomes. Thus, the studies that do not include a non-placebo control session, in which no inert supplement is provided, may underestimate the total effect of the intervention. This is important because in the practical context a given individual will either ingest or not ingest the supplement (i.e., the deliberate use of a placebo is unlikely) [ 214 ].
To minimize the possibility of a placebo effect, studies generally attempt participant blinding (i.e., keeping participants unaware of the assigned treatment). While this is a recommended methodological approach, it is important to consider that some participants may discern between the sodium bicarbonate and placebo trials. Specifically, sodium bicarbonate supplementation may cause side-effects such as stomach cramps and nausea, which may enable some, but usually not all, participants to correctly identify the substance ingested. This observation was placed in context by the study of Higgins and Shabir [ 215 ], in which participants ingested a standard placebo (sodium chloride) or a sham placebo that aimed to mimic gut fullness and abdominal discomfort associated with sodium bicarbonate ingestion. The authors found that cycling at 100% of peak power output to exhaustion was improved by 9.5% (Cohen’s d = 0.27) following sham placebo ingestion. While significant, this improvement was smaller than the one observed in a study using the same exercise test, where sodium bicarbonate was actually ingested (17.5%; Cohen’s d = 0.50), likely because placebo ingestion does not alter physiological buffering capacity [ 52 ].
McClung et al. [ 216 ] conducted a similar study on the placebo effect of sodium bicarbonate supplementation. When giving the placebo pill to the participants, the researchers told them that the pill contains an ergogenic substance—sodium bicarbonate. The ingestion of placebo (alongside the misleading instruction) enhanced performance in 1000-m running time trials similar to the actual effect of sodium bicarbonate (1.5% and 1.7%, respectively). Therefore, while sodium bicarbonate may enhance performance due to physiological effects, some portion of the ergogenic effect may be purely due to outcome expectancy. Another possibility is that the placebo effect is similar to the effect of sodium bicarbonate on some exercise performance outcomes and for some supplementation protocols. Due to the possibility of a placebo effect, in studies that attempt participant blinding, researchers should evaluate the effectiveness of blinding and consider it when interpreting their findings [ 217 ]. Most of the available studies did not evaluate the effectiveness of blinding, which is a limitation that needs to be addressed in future research.
Interaction of sodium bicarbonate with other ergogenic aids
Many athletes compete in events in which the use of several supplements may be justified to maximize performance gains [ 218 ]. This has important implications for how research findings are interpreted because most studies have investigated the isolated effects of ergogenic supplements on performance. Nonetheless, evidence is growing regarding combinations of supplements and whether they provide synergistic ergogenic effects or counteract each other. Studies have explored supplement combinations of sodium bicarbonate with other ergogenic aids including, beta-alanine, caffeine, creatine, and nitrates [ 1 ].
Sodium bicarbonate and beta-alanine
Concurrent use of sodium bicarbonate and beta-alanine is one of the most well-researched supplement combinations. Beta-alanine is an effective ergogenic aid that must be ingested over a longer-term (e.g., over several weeks) to increase muscle carnosine content, thereby increasing the buffering capacity of the muscle [ 219 , 220 , 221 , 222 ]. Given that beta-alanine increases intracellular pH-buffering and sodium bicarbonate increases extracellular pH-buffering, it appears reasonable to consider that combining the two would produce an additive ergogenic effect. The first study to investigate this showed a 12% improvement in high-intensity cycling capacity with beta-alanine, a 7% improvement with sodium bicarbonate, and a 16% improvement when concurrently using sodium bicarbonate and beta-alanine [ 144 ]. Other studies have also suggested potentially greater improvements when combining sodium bicarbonate and beta-alanine compared to sodium bicarbonate alone during a repeated 30-s arm cranking test [ 147 ], 2000-m rowing [ 138 ], and 100-m and 200-m swimming [ 133 ]. However, several studies have not shown significant additive benefits of co-supplementing these ergogenic aids [ 127 , 132 , 134 , 146 ]. A meta-analysis showed that the addition of sodium bicarbonate to beta-alanine supplementation produced greater ergogenic effects than beta-alanine alone (pooled Cohen’s d : 0.43 vs. 0.18) [ 221 ]. Overall, the findings suggest that beta-alanine and sodium bicarbonate co-supplementation may generate potentially meaningful increases in exercise performance over supplementation with either alone.
Sodium bicarbonate and caffeine
Caffeine is a well-established ergogenic aid, with performance benefits reported for aerobic and muscular endurance, power, jump height, and muscular strength [ 223 , 224 , 225 , 226 , 227 , 228 , 229 ]. Caffeine’s ergogenic effects are generally explained by its ability to act as an adenosine receptor antagonist, which acts to reduce fatigue, pain, or perception of effort [ 228 ]. Given that sodium bicarbonate and caffeine could improve performance through different mechanisms, their concurrent use could produce additive effects. A recent review [ 230 ] reported that only one [ 135 ] out of eight studies on this topic showed additive effects of combining sodium bicarbonate and caffeine. Despite this, it should be considered that sodium bicarbonate supplementation protocols in some of the studies [ 137 , 142 ] resulted in high incidence and severity of side-effects and were also not found to be effective. Therefore, it was concluded that further work is required to truly determine whether co-supplementation of caffeine and sodium bicarbonate produces larger ergogenic effects compared with supplementation with either of the supplements alone.
Sodium bicarbonate and creatine
Creatine is another well-researched ergogenic supplement [ 231 ]. Several studies explored the effects of using sodium bicarbonate and creatine concurrently and reported an additive effect. Co-supplementation with sodium bicarbonate (0.5 g/kg per day for 5 days) and creatine (20 g for 5 days) enhanced exercise performance (“Taekwondo Anaerobic Intermittent Kick Test”) in taekwondo athletes [ 143 ]. Another study involving competitive female and male swimmers provided 20 g of creatine for 6 days, followed by an acute dose of sodium bicarbonate in the dose of 0.3 g/kg on the morning of the seventh day. On the same day, repeated 100-m swim performance was evaluated [ 140 ]. Concurrent use of these supplements reduced the time needed to complete the second 100-m swim by 1 s (1.5%). Similarly, the combination of creatine and sodium bicarbonate increased peak and mean power and attenuated the decline in peak power to the greatest extent over repeated-sprints [ 126 ]. While only a handful of studies have been published on this topic thus far, the combination of sodium bicarbonate and creatine may provide superior benefits compared to ingesting sodium bicarbonate or creatine alone.
Sodium bicarbonate and nitrates
Due to their mechanisms of action, co-supplementation of sodium bicarbonate and nitrates may be counterproductive. Specifically, the alkalosis achieved by bicarbonate supplementation may decrease the effect of nitrate supplementation because the conversion of nitrate to nitric oxide is facilitated by an acidic environment [ 128 ]. Only one study to date has investigated the combined effect of these two supplements, with no significant effects on 4-km cycling time-trial performance found for nitrates (consumed through beetroot crystals) or sodium bicarbonate either in isolation or combined [ 128 ]. Given the paucity of studies investigating the effects of combining these supplements on exercise performance, no conclusions can be drawn at present. More research is needed on this topic.
Position of the International Society of Sports Nutrition (ISSN)
Availability of data and materials.
Not applicable.
Cohen's d effect sizes are commonly interpreted as: “trivial/very small” (<0.20), “small” (0.20–0.49), “medium” (0.50–0.79), and “large” (≥0.80)
Maughan RJ, Burke LM, Dvorak J, Larson-Meyer DE, Peeling P, Phillips SM, et al. IOC consensus statement: dietary supplements and the high-performance athlete. Int J Sport Nutr Exerc Metab. 2018;28(2):104–25.
Article CAS PubMed Google Scholar
Afman G, Garside RM, Dinan N, Gant N, Betts JA, Williams C. Effect of carbohydrate or sodium bicarbonate ingestion on performance during a validated basketball simulation test. Int J Sport Nutr Exerc Metab. 2014;24(6):632–44.
Ansdell P, Dekerle J. Sodium bicarbonate supplementation delays neuromuscular fatigue without changes in performance outcomes during a basketball match simulation protocol. J Strength Cond Res. 2020;34(5):1369–75.
Article PubMed Google Scholar
Artioli GG, Gualano B, Coelho DF, Benatti FB, Gailey AW, Lancha AH Jr. Does sodium-bicarbonate ingestion improve simulated judo performance? Int J Sport Nutr Exerc Metab. 2007;17(2):206–17.
Aschenbach W, Ocel J, Craft L, Ward C, Spangenburg E, Williams J. Effect of oral sodium loading on high-intensity arm ergometry in college wrestlers. Med Sci Sports Exerc. 2000;32(3):669–75.
Bird SR, Wiles J, Robbins J. The effect of sodium bicarbonate ingestion on 1500-m racing time. J Sports Sci. 1995;13(5):399–403.
Bishop D, Edge J, Davis C, Goodman C. Induced metabolic alkalosis affects muscle metabolism and repeated-sprint ability. Med Sci Sports Exerc. 2004;36(5):807–13.
Bishop D, Claudius B. Effects of induced metabolic alkalosis on prolonged intermittent-sprint performance. Med Sci Sports Exerc. 2005;37(5):759–67.
Boegman S, Stellingwerff T, Shaw G, Clarke N, Graham K, Cross R, et al. The impact of individualizing sodium bicarbonate supplementation strategies on world-class rowing performance. Front Nutr. 2020;7:138.
Article PubMed PubMed Central CAS Google Scholar
Bouissou P, Defer G, Guezennec CY, Estrade PY, Serrurier B. Metabolic and blood catecholamine responses to exercise during alkalosis. Med Sci Sports Exerc. 1988;20(3):228–32.
Brien DM, McKenzie DC. The effect of induced alkalosis and acidosis on plasma lactate and work output in elite oarsmen. Eur J Appl Physiol Occup Physiol. 1989;58(8):797–802.
Brisola GM, Miyagi WE, da Silva HS, Zagatto AM. Sodium bicarbonate supplementation improved MAOD but is not correlated with 200-and 400-m running performances: a double-blind, crossover, and placebo-controlled study. Appl Physiol Nutr Metab. 2015;40(9):931–7.
Cameron SL, McLay-Cooke RT, Brown RC, Gray AR, Fairbairn KA. Increased blood pH but not performance with sodium bicarbonate supplementation in elite rugby union players. Int J Sport Nutr Exerc Metab. 2010;20(4):307–21.
Campos EZ, Sangali EB, Neto JG, Gobbi RB, Freitas Junior IF, Papoti M. Effects of sodium bicarbonate ingestion during an intermittent exercise on blood lactate, stroke parameters, and performance of swimmers. J Exerc Physiol Online. 2012;15(6):84–92.
Google Scholar
Carr AJ, Slater GJ, Gore CJ, Dawson B, Burke LM. Reliability and effect of sodium bicarbonate: buffering and 2000-m rowing performance. Int J Sports Physiol Perform. 2012;7(2):152–60.
Carr BM, Webster MJ, Boyd JC, Hudson GM, Scheett TP. Sodium bicarbonate supplementation improves hypertrophy-type resistance exercise performance. Eur J Appl Physiol. 2013;113(3):743–52.
Cholewa JM, Grannis DJ, Jaffe DA, Guimarães-Ferreira L, Matthews TD, Paolone VJ. The effects of sodium bicarbonate supplementation on a soccer specific conditioning test in division III soccer players. J Trainol. 2015;4:19–24.
Article Google Scholar
Coombes J, McNaughton LR. Effects of bicarbonate ingestion on leg strength and power during isokinetic knee flexion and extension. J Strength Cond Res. 1993;7(4):241–9.
Costill DL, Verstappen F, Kuipers H, Janssen E, Fink W. Acid-base balance during repeated bouts of exercise: influence of HCO3. Int J Sports Med. 1984;5(5):228–31.
Dalle S, De Smet S, Geuns W, Rompaye BV, Hespel P, Koppo K. Effect of stacked sodium bicarbonate loading on repeated all-out exercise. Int J Sports Med. 2019;40(11):711–6.
Dalle S, Koppo K, Hespel P. Sodium bicarbonate improves sprint performance in endurance cycling. J Sci Med Sport. 2021;24(3):301–6.
Deb SK, Gough LA, Sparks SA, McNaughton LR. Determinants of curvature constant (W') of the power duration relationship under normoxia and hypoxia: the effect of pre-exercise alkalosis. Eur J Appl Physiol. 2017;117(5):901–12.
Article CAS PubMed PubMed Central Google Scholar
Deb SK, Gough LA, Sparks SA, McNaughton LR. Sodium bicarbonate supplementation improves severe-intensity intermittent exercise under moderate acute hypoxic conditions. Eur J Appl Physiol. 2018;118(3):607–15.
Delextrat A, Mackessy S, Arceo-Rendon L, Scanlan A, Ramsbottom R, Calleja-Gonzalez J. Effects of three-day serial sodium bicarbonate loading on performance and physiological parameters during a simulated basketball test in female university players. Int J Sport Nutr Exerc Metab. 2018;28(5):547–52.
Dixon H, Baker CE, Baker JS, Dewhurst S, Hayes LD. Sodium bicarbonate ingestion improves Yo-Yo intermittent recovery test 1 performance: a randomized crossover trial. Nutr Diet Suppl. 2017;9:23–7.
Douroudos II, Fatouros IG, Gourgoulis V, Jamurtas AZ, Tsitsios T, Hatzinikolaou A, et al. Dose-related effects of prolonged NaHCO3 ingestion during high-intensity exercise. Med Sci Sports Exerc. 2006;38(10):1746–53.
Driller MW, Gregory JR, Williams AD, Fell JW. The effects of serial and acute NaHCO3 loading in well-trained cyclists. J Strength Cond Res. 2012;26(10):2791–7.
Driller M, Williams A, Bellinger P, Howe S, Fell J. The effects of NaHCO3 and NaCl loading on hematocrit and high-intensity cycling performance. J Exerc Physiol Online. 2012;15(1):47–56.
Driller MW, Gregory JR, Williams AD, Fell JW. The effects of chronic sodium bicarbonate ingestion and interval training in highly trained rowers. Int J Sport Nutr Exerc Metab. 2013;23(1):40–7.
Duncan MJ, Weldon A, Price MJ. The effect of sodium bicarbonate ingestion on back squat and bench press exercise to failure. J Strength Cond Res. 2014;28(5):1358–66.
Durkalec-Michalski K, Zawieja EE, Podgórski T, Łoniewski I, Zawieja BE, Warzybok M, et al. The effect of chronic progressive-dose sodium bicarbonate ingestion on CrossFit-like performance: a double-blind, randomized cross-over trial. PLoS One. 2018;13(5):e0197480.
Durkalec-Michalski K, Zawieja EE, Podgórski T, Zawieja BE, Michałowska P, Łoniewski I, et al. The effect of a new sodium bicarbonate loading regimen on anaerobic capacity and wrestling performance. Nutrients. 2018;10(6):697.
Article PubMed Central CAS Google Scholar
Durkalec-Michalski K, Nowaczyk PM, Adrian J, Kamińska J, Podgórski T. The influence of progressive-chronic and acute sodium bicarbonate supplementation on anaerobic power and specific performance in team sports: a randomized, double-blind, placebo-controlled crossover study. Nutr Metab (Lond). 2020;17:38.
Article CAS Google Scholar
Durkalec-Michalski K, Zawieja EE, Zawieja BE, Michałowska P, Podgórski T. The gender dependent influence of sodium bicarbonate supplementation on anaerobic power and specific performance in female and male wrestlers. Sci Rep. 2020;10(1):1878.
Edge J, Bishop D, Goodman C. Effects of chronic NaHCO3 ingestion during interval training on changes to muscle buffer capacity, metabolism, and short-term endurance performance. J Appl Physiol. 2006;101(3):918–25.
Egger F, Meyer T, Such U, Hecksteden A. Effects of sodium bicarbonate on high-intensity endurance performance in cyclists: a double-blind, randomized cross-over trial. PLoS One. 2014;9(12):e114729.
Farney TM, MacLellan MJ, Hearon CM, Johannsen NM, Nelson AG. The effect of aspartate and sodium bicarbonate supplementation on muscle contractile properties among trained men. J Strength Cond Res. 2020;34(3):763–70.
Ferreira LHB, Smolarek AC, Chilibeck PD, Barros MP, McAnulty SR, Schoenfeld BJ, et al. High doses of sodium bicarbonate increase lactate levels and delay exhaustion in a cycling performance test. Nutrition. 2019;60:94–9.
Freis T, Hecksteden A, Such U, Meyer T. Effect of sodium bicarbonate on prolonged running performance: A randomized, double-blind, cross-over study. PLoS One. 2017;12(8):e0182158.
Froio de Araujo Dias G, da Eira Silva V, de Salles Painelli V, Sale C, Giannini Artioli G, Gualano B, et al. (In)consistencies in responses to sodium bicarbonate supplementation: a randomised, repeated measures, counterbalanced and double-blind study. PLoS One. 2015;10(11):e0143086.
Gaitanos GC, Nevill ME, Brooks S, Williams C. Repeated bouts of sprint running after induced alkalosis. J Sports Sci. 1991;9(4):355–70.
Gao JP, Costill DL, Horswill CA, Park SH. Sodium bicarbonate ingestion improves performance in interval swimming. Eur J Appl Physiol Occup Physiol. 1988;58(1-2):171–4.
George KP, MacLaren DP. The effect of induced alkalosis and acidosis on endurance running at an intensity corresponding to 4 mM blood lactate. Ergonomics. 1988;31(11):1639–45.
Goldfinch J, Mc Naughton L, Davies P. Induced metabolic alkalosis and its effects on 400-m racing time. Eur J Appl Physiol Occup Physiol. 1988;57(1):45–8.
Gough LA, Rimmer S, Osler CJ, Higgins MF. Ingestion of sodium bicarbonate (NaHCO3) following a fatiguing bout of exercise accelerates postexercise acid-base balance recovery and improves subsequent high-intensity cycling time to exhaustion. Int J Sport Nutr Exerc Metab. 2017;27(5):429–38.
Gough LA, Deb SK, Sparks AS, McNaughton LR. The reproducibility of 4-km time trial (TT) performance following individualised sodium bicarbonate supplementation: a randomised controlled trial in trained cyclists. Sports Med Open. 2017;3(1):34.
Article PubMed PubMed Central Google Scholar
Gough LA, Brown D, Deb SK, Sparks SA, McNaughton LR. The influence of alkalosis on repeated high-intensity exercise performance and acid-base balance recovery in acute moderate hypoxic conditions. Eur J Appl Physiol. 2018;118(12):2489–98.
Gough LA, Deb SK, Sparks SA, McNaughton LR. Sodium bicarbonate improves 4 km time trial cycling performance when individualised to time to peak blood bicarbonate in trained male cyclists. J Sports Sci. 2018;36(15):1705–12.
Gough LA, Rimmer S, Sparks SA, McNaughton LR, Higgins MF. Post-exercise supplementation of sodium bicarbonate improves acid base balance recovery and subsequent high-intensity boxing specific performance. Front Nutr. 2019;6:155.
Gough LA, Deb SK, Brown D, Sparks SA, McNaughton LR. The effects of sodium bicarbonate ingestion on cycling performance and acid base balance recovery in acute normobaric hypoxia. J Sports Sci. 2019;37(13):1464–71.
Gurton WH, Gough LA, Sparks SA, Faghy MA, Reed KE. Sodium bicarbonate ingestion improves time-to-exhaustion cycling performance and alters estimated energy system contribution: a dose-response investigation. Front Nutr. 2020;7:154.
Higgins MF, James RS, Price MJ. The effects of sodium bicarbonate (NaHCO3) ingestion on high intensity cycling capacity. J Sports Sci. 2013;31(9):972–81.
Hilton NP, Leach NK, Hilton MM, Sparks SA, McNaughton LR. Enteric-coated sodium bicarbonate supplementation improves high-intensity cycling performance in trained cyclists. Eur J Appl Physiol. 2020;120(7):1563–73.
Hobson RM, Harris RC, Martin D, Smith P, Macklin B, Elliott-Sale KJ, et al. Effect of sodium bicarbonate supplementation on 2000-m rowing performance. Int J Sports Physiol Perform. 2014;9(1):139–44.
Horswill CA, Costill DL, Fink WJ, Flynn MG, Kirwan JP, Mitchell JB, et al. Influence of sodium bicarbonate on sprint performance: relationship to dosage. Med Sci Sports Exerc. 1988;20(6):566–9.
Inbar O, Rotstein A, Jacobs I, Kaiser P, Dlin R, Dotan R. The effects of alkaline treatment on short-term maximal exercise. J Sports Sci. 1983;1(2):95–104.
Iwaoka K, Okagawa S, Mutoh Y, Miyashita M. Effects of bicarbonate ingestion on the respiratory compensation threshold and maximal exercise performance. Jpn J Physiol. 1989;39(2):255–65.
Jones NL, Sutton JR, Taylor R, Toews CJ. Effect of pH on cardiorespiratory and metabolic responses to exercise. J Appl Physiol Respir Environ Exerc Physiol. 1977;43(6):959–64.
CAS PubMed Google Scholar
Joyce S, Minahan C, Anderson M, Osborne M. Acute and chronic loading of sodium bicarbonate in highly trained swimmers. Eur J Appl Physiol. 2012;112(2):461–9.
Katz A, Costill DL, King DS, Hargreaves M, Fink WJ. Maximal exercise tolerance after induced alkalosis. Int J Sports Med. 1984;5(2):107–10.
Kowalchuk JM, Heigenhauser GJ, Jones NL. Effect of pH on metabolic and cardiorespiratory responses during progressive exercise. J Appl Physiol Respir Environ Exerc Physiol. 1984;57(5):1558–63.
Kozak-Collins K, Burke ER, Schoene RB. Sodium bicarbonate ingestion does not improve performance in women cyclists. Med Sci Sports Exerc. 1994;26(12):1510–5.
Krustrup P, Ermidis G, Mohr M. Sodium bicarbonate intake improves high-intensity intermittent exercise performance in trained young men. J Int Soc Sports Nutr. 2015;12:25.
Kumstát M, Hlinský T, Struhár I, Thomas A. Does sodium citrate cause the same ergogenic effect as sodium bicarbonate on swimming performance? J Hum Kinet. 2018;65:89–98.
Lavender G, Bird SR. Effect of sodium bicarbonate ingestion upon repeated sprints. Br J Sports Med. 1989;23(1):41–5.
Limmer M, de Marées M, Platen P. Effects of daily ingestion of sodium bicarbonate on acid-base status and anaerobic performance during an altitude sojourn at high altitude: a randomized controlled trial. J Int Soc Sports Nutr. 2020;17(1):22.
Lindh AM, Peyrebrune MC, Ingham SA, Bailey DM, Folland JP. Sodium bicarbonate improves swimming performance. Int J Sports Med. 2008;29(6):519–23.
Lopes-Silva JP, Da Silva Santos JF, Artioli GG, Loturco I, Abbiss C, Franchini E. Sodium bicarbonate ingestion increases glycolytic contribution and improves performance during simulated taekwondo combat. Eur J Sport Sci. 2018;18(3):431–40.
Marriott M, Krustrup P, Mohr M. Ergogenic effects of caffeine and sodium bicarbonate supplementation on intermittent exercise performance preceded by intense arm cranking exercise. J Int Soc Sports Nutr. 2015;12:13.
Marx JO, Gordon SE, Vos NH, Nindl BC, Gómez AL, Volek JS, et al. Effect of alkalosis on plasma epinephrine responses to high intensity cycle exercise in humans. Eur J Appl Physiol. 2002;87(1):72–7.
Materko W, Santos EL, Novaes JDS. Effect of bicarbonate supplementation on muscular strength. J Exerc Physiol Online. 2008;11(6):25–33.
Matsuura R, Arimitsu T, Kimura T, Yunoki T, Yano T. Effect of oral administration of sodium bicarbonate on surface EMG activity during repeated cycling sprints. Eur J Appl Physiol. 2007;101(4):409–17.
Maughan RJ, Leiper JB, Litchfield PE. The effects of induced acidosis and alkalosis on isometric endurance capacity in man. In: Dotson CO, Humphrey JH, editors. Exercise physiology, current selected research, vol. 2. New York: AMS Press; 1986. p. 73–82.
McCartney N, Heigenhauser GJ, Jones NL. Effects of pH on maximal power output and fatigue during short-term dynamic exercise. J Appl Physiol Respir Environ Exerc Physiol. 1983;55(1 Pt 1):225–9.
McKenzie DC, Coutts KD, Stirling DR, Hoeben HH, Kuzara G. Maximal work production following two levels of artificially induced metabolic alkalosis. J Sports Sci. 1986;4(1):35–8.
McNaughton L, Curtin R, Goodman G, Perry D, Turner B, Showell C. Anaerobic work and power output during cycle ergometer exercise: effects of bicarbonate loading. J Sports Sci. 1991;9(2):151–60.
McNaughton LR. Bicarbonate ingestion: effects of dosage on 60 s cycle ergometry. J Sports Sci. 1992;10(5):415–23.
McNaughton LR. Sodium bicarbonate ingestion and its effects on anaerobic exercise of various durations. J Sports Sci. 1992;10(5):425–35.
McNaughton LR, Ford S, Newbold C. Effect of sodium bicarbonate ingestion on high intensity exercise in moderately trained women. J Strength Cond Res. 1997;11(2):98–102.
McNaughton L, Dalton B, Palmer G. Sodium bicarbonate can be used as an ergogenic aid in high-intensity, competitive cycle ergometry of 1 h duration. Eur J Appl Physiol Occup Physiol. 1999;80(1):64–9.
McNaughton L, Backx K, Palmer G, Strange N. Effects of chronic bicarbonate ingestion on the performance of high-intensity work. Eur J Appl Physiol Occup Physiol. 1999;80(4):333–6.
McNaughton L, Strange N, Backx K. The effects of chronic sodium bicarbonate ingestion on multiple bouts of anaerobic work and power output. J Hum Mov Stud. 2000;38:307–22.
McNaughton L, Thompson D. Acute versus chronic sodium bicarbonate ingestion and anaerobic work and power output. J Sports Med Phys Fitness. 2001;41(4):456–62.
CAS Google Scholar
Miller P, Robinson AL, Sparks SA, Bridge CA, Bentley DJ, McNaughton LR. The effects of novel ingestion of sodium bicarbonate on repeated sprint ability. J Strength Cond Res. 2016;30(2):561–8.
Mündel T. Sodium bicarbonate ingestion improves repeated high-intensity cycling performance in the heat. Temperature. 2018;5(4):343–7.
Mueller SM, Gehrig SM, Frese S, Wagner CA, Boutellier U, Toigo M. Multiday acute sodium bicarbonate intake improves endurance capacity and reduces acidosis in men. J Int Soc Sports Nutr. 2013;10(1):16.
Northgraves MJ, Peart DJ, Jordan CA, Vince RV. Effect of lactate supplementation and sodium bicarbonate on 40-km cycling time trial performance. J Strength Cond Res. 2014;28(1):273–80.
Oliveira LF, de Salles PV, Nemezio K, Gonçalves LS, Yamaguchi G, Saunders B, et al. Chronic lactate supplementation does not improve blood buffering capacity and repeated high-intensity exercise. Scand J Med Sci Sports. 2017;27(11):1231–9.
Parry-Billings M, MacLaren DP. The effect of sodium bicarbonate and sodium citrate ingestion on anaerobic power during intermittent exercise. Eur J Appl Physiol Occup Physiol. 1986;55(5):524–9.
Peart DJ, McNaughton LR, Midgley AW, Taylor L, Towlson C, Madden LA, et al. Pre-exercise alkalosis attenuates the heat shock protein 72 response to a single-bout of anaerobic exercise. J Sci Med Sport. 2011;14(5):435–40.
Peinado AB, Holgado D, Luque-Casado A, Rojo-Tirado MA, Sanabria D, González C, et al. Effect of induced alkalosis on performance during a field-simulated BMX cycling competition. J Sci Med Sport. 2019;22(3):335–41.
Pierce EF, Eastman NW, Hammer WH, Lynn TD. Effect of induced alkalosis on swimming time trials. J Sports Sci. 1992;10(3):255–9.
Potteiger JA, Webster MJ, Nickel GL, Haub MD, Palmer RJ. The effects of buffer ingestion on metabolic factors related to distance running performance. Eur J Appl Physiol Occup Physiol. 1996;72(4):365–71.
Price M, Moss P, Rance S. Effects of sodium bicarbonate ingestion on prolonged intermittent exercise. Med Sci Sports Exerc. 2003;35(8):1303–8.
Price MJ, Simons C. The effect of sodium bicarbonate ingestion on high-intensity intermittent running and subsequent performance. J Strength Cond Res. 2010;24(7):1834–42.
Raymer GH, Marsh GD, Kowalchuk JM, Thompson RT. Metabolic effects of induced alkalosis during progressive forearm exercise to fatigue. J Appl Physiol. 2004;96(6):2050–6.
Robertson RJ, Falkel JE, Drash AL, Swank AM, Metz KF, Spungen SA, et al. Effect of induced alkalosis on physical work capacity during arm and leg exercise. Ergonomics. 1987;30(1):19–31.
Saunders B, Sale C, Harris RC, Sunderland C. Sodium bicarbonate and high-intensity-cycling capacity: variability in responses. Int J Sports Physiol Perform. 2014;9(4):627–32.
Schauf M, Ball TE. Effects of sodium bicarbonate ingestion on anaerobic performance of women: dosage effect. J Hum Mov Stud. 1996;31(4):179–87.
Siegler JC, Hirscher K. Sodium bicarbonate ingestion and boxing performance. J Strength Cond Res. 2010;24(1):103–8.
Siegler JC, McNaughton LR, Midgley AW, Keatley S, Hillman A. Metabolic alkalosis, recovery and sprint performance. Int J Sports Med. 2010;31(11):797–802.
Siegler JC, Gleadall-Siddall D. Sodium bicarbonate ingestion and repeated swim sprint performance. J Strength Cond Res. 2010;24(11):3105–11.
Siegler JC, Marshall PW, Bray J, Towlson C. Sodium bicarbonate supplementation and ingestion timing: does it matter? J Strength Cond Res. 2012;26(7):1953–8.
Siegler JC, Marshall PW, Raftry S, Brooks C, Dowswell B, Romero R, et al. The differential effect of metabolic alkalosis on maximum force and rate of force development during repeated, high-intensity cycling. J Appl Physiol. 2013;115(11):1634–40.
Siegler JC, Marshall P, Pouslen MK, Nielsen NP, Kennedy D, Green S. The effect of pH on fatigue during submaximal isometric contractions of the human calf muscle. Eur J Appl Physiol. 2015;115(3):565–77.
Siegler JC, Marshall P. The effect of metabolic alkalosis on central and peripheral mechanisms associated with exercise-induced muscle fatigue in humans. Exp Physiol. 2015;100(5):519–30.
Siegler JC, Mudie K, Marshall P. The influence of sodium bicarbonate on maximal force and rates of force development in the triceps surae and brachii during fatiguing exercise. Exp Physiol. 2016;101(11):1383–91.
Siegler JC, Marshall PWM, Finn H, Cross R, Mudie K. Acute attenuation of fatigue after sodium bicarbonate supplementation does not manifest into greater training adaptations after 10-weeks of resistance training exercise. PLoS One. 2018;13(5):e0196677.
Sostaric SM, Skinner SL, Brown MJ, Sangkabutra T, Medved I, Medley T, et al. Alkalosis increases muscle K+ release, but lowers plasma [K+] and delays fatigue during dynamic forearm exercise. J Physiol. 2006;570(Pt 1):185–205.
Stephens TJ, McKenna MJ, Canny BJ, Snow RJ, McConell GK. Effect of sodium bicarbonate on muscle metabolism during intense endurance cycling. Med Sci Sports Exerc. 2002;34(4):614–21.
Sutton JR, Jones NL, Toews CJ. Effect of pH on muscle glycolysis during exercise. Clin Sci. 1981;61(3):331–8.
Tan F, Polglaze T, Cox G, Dawson B, Mujika I, Clark S. Effects of induced alkalosis on simulated match performance in elite female water polo players. Int J Sport Nutr Exerc Metab. 2010;20(3):198–205.
Tanaka S, Yamaguchi D, Igawa S. Effect of low-dose sodium bicarbonate sup-plementation on intermittent endurance performance. Food Nutr Sci. 2018;9:1316–26.
Van Montfoort MC, Van Dieren L, Hopkins WG, Shearman JP. Effects of ingestion of bicarbonate, citrate, lactate, and chloride on sprint running. Med Sci Sports Exerc. 2004;36(7):1239–43.
Article PubMed CAS Google Scholar
Vanhatalo A, McNaughton LR, Siegler J, Jones AM. Effect of induced alkalosis on the power-duration relationship of "all-out" exercise. Med Sci Sports Exerc. 2010;42(3):563–70.
Verbitsky O, Mizrahi J, Levin M, Isakov E. Effect of ingested sodium bicarbonate on muscle force, fatigue, and recovery. J Appl Physiol. 1997;83(2):333–7.
Wang J, Qiu J, Yi L, Hou Z, Benardot D, Cao W. Effect of sodium bicarbonate ingestion during 6 weeks of HIIT on anaerobic performance of college students. J Int Soc Sports Nutr. 2019;16(1):18.
Webster MJ, Webster MN, Crawford RE, Gladden LB. Effect of sodium bicarbonate ingestion on exhaustive resistance exercise performance. Med Sci Sports Exerc. 1993;25(8):960–5.
Wilkes D, Gledhill N, Smyth R. Effect of acute induced metabolic alkalosis on 800-m racing time. Med Sci Sports Exerc. 1983;15(4):277–80.
Wu CL, Shih MC, Yang CC, Huang MH, Chang CK. Sodium bicarbonate supplementation prevents skilled tennis performance decline after a simulated match. J Int Soc Sports Nutr. 2010;7:33.
Yong GY, Yin LH, Hoe WE. Effects of different dosage of sodium bicarbonate on 200 m freestyle swimming performance in competitive swimmers. Int J Life Sci Pharma Res. 2018;1:66–71.
Zabala M, Requena B, Sánchez-Muñoz C, González-Badillo JJ, García I, Oöpik V, et al. Effects of sodium bicarbonate ingestion on performance and perceptual responses in a laboratory-simulated BMX cycling qualification series. J Strength Cond Res. 2008;22(5):1645–53.
Zabala M, Peinado AB, Calderón FJ, Sampedro J, Castillo MJ, Benito PJ. Bicarbonate ingestion has no ergogenic effect on consecutive all out sprint tests in BMX elite cyclists. Eur J Appl Physiol. 2011;111(12):3127–34.
Zajac A, Cholewa J, Poprzecki S, Waskiewicz Z, Langfort J. Effects of sodium bicarbonate ingestion on swim performance in youth athletes. J Sports Sci Med. 2009;8(1):45–50.
PubMed PubMed Central Google Scholar
Zinner C, Wahl P, Achtzehn S, Sperlich B, Mester J. Effects of bicarbonate ingestion and high intensity exercise on lactate and H+-ion distribution in different blood compartments. Eur J Appl Physiol. 2011;111(8):1641–8.
Barber JJ, McDermott AY, McGaughey KJ, Olmstead JD, Hagobian TA. Effects of combined creatine and sodium bicarbonate supplementation on repeated sprint performance in trained men. J Strength Cond Res. 2013;27(1):252–8.
Bellinger PM, Howe ST, Shing CM, Fell JW. Effect of combined β-alanine and sodium bicarbonate supplementation on cycling performance. Med Sci Sports Exerc. 2012;44(8):1545–51.
Callahan MJ, Parr EB, Hawley JA, Burke LM. Single and combined effects of beetroot crystals and sodium bicarbonate on 4-km cycling time trial performance. Int J Sport Nutr Exerc Metab. 2017;27(3):271–8.
Carr AJ, Gore CJ, Dawson B. Induced alkalosis and caffeine supplementation: effects on 2,000-m rowing performance. Int J Sport Nutr Exerc Metab. 2011;21(5):357–64.
Christensen PM, Petersen MH, Friis SN, Bangsbo J. Caffeine, but not bicarbonate, improves 6 min maximal performance in elite rowers. Appl Physiol Nutr Metab. 2014;39(9):1058–63.
da Silva RP, de Oliveira LF, Saunders B, de Andrade KC, de Salles PV, da Eira SV, et al. Effects of β-alanine and sodium bicarbonate supplementation on the estimated energy system contribution during high-intensity intermittent exercise. Amino Acids. 2019;51(1):83–96.
Danaher J, Gerber T, Wellard RM, Stathis CG. The effect of β-alanine and NaHCO3 co-ingestion on buffering capacity and exercise performance with high-intensity exercise in healthy males. Eur J Appl Physiol. 2014;114(8):1715–24.
de Salles Painelli Vde S, Roschel H, Fd J, Sale C, Harris RC, Solis MY, et al. The ergogenic effect of beta-alanine combined with sodium bicarbonate on high-intensity swimming performance. Appl Physiol Nutr Metab. 2013;38(5):525–32.
Ducker KJ, Dawson B, Wallman KE. Effect of beta alanine and sodium bicarbonate supplementation on repeated-sprint performance. J Strength Cond Res. 2013;27(12):3450–60.
Felippe LC, Lopes-Silva JP, Bertuzzi R, McGinley C, Lima-Silva AE. Separate and combined effects of caffeine and sodium-bicarbonate intake on judo performance. Int J Sports Physiol Perform. 2016;11(2):221–6.
Griffen C, Rogerson D, Ranchordas M, Ruddock A. Effects of creatine and sodium bicarbonate coingestion on multiple indices of mechanical power output during repeated Wingate tests in trained men. Int J Sport Nutr Exerc Metab. 2015;25(3):298–306.
Higgins MF, Wilson S, Hill C, Price MJ, Duncan M, Tallis J. Evaluating the effects of caffeine and sodium bicarbonate, ingested individually or in combination, and a taste-matched placebo on high-intensity cycling capacity in healthy males. Appl Physiol Nutr Metab. 2016;41(4):354–61.
Hobson RM, Harris RC, Martin D, Smith P, Macklin B, Gualano B, et al. Effect of beta-alanine, with and without sodium bicarbonate, on 2000-m rowing performance. Int J Sport Nutr Exerc Metab. 2013;23(5):480–7.
Kilding AE, Overton C, Gleave J. Effects of caffeine, sodium bicarbonate, and their combined ingestion on high-intensity cycling performance. Int J Sport Nutr Exerc Metab. 2012;22(3):175–83.
Mero AA, Keskinen KL, Malvela MT, Sallinen JM. Combined creatine and sodium bicarbonate supplementation enhances interval swimming. J Strength Cond Res. 2004;18(2):306–10.
PubMed Google Scholar
Mero AA, Hirvonen P, Saarela J, Hulmi JJ, Hoffman JR, Stout JR. Effect of sodium bicarbonate and beta-alanine supplementation on maximal sprint swimming. J Int Soc Sports Nutr. 2013;10(1):52.
Pruscino CL, Ross ML, Gregory JR, Savage B, Flanagan TR. Effects of sodium bicarbonate, caffeine, and their combination on repeated 200-m freestyle performance. Int J Sport Nutr Exerc Metab. 2008;18(2):116–30.
Rezaei S, Akbari K, Gahreman DE, Sarshin A, Tabben M, Kaviani M, et al. Caffeine and sodium bicarbonate supplementation alone or together improve karate performance. J Int Soc Sports Nutr. 2019;16(1):44.
Sale C, Saunders B, Hudson S, Wise JA, Harris RC, Sunderland CD. Effect of β-alanine plus sodium bicarbonate on high-intensity cycling capacity. Med Sci Sports Exerc. 2011;43(10):1972–8.
Sarshin A, Fallahi V, Forbes SC, Rahimi A, Koozehchian MS, Candow DG, et al. Short-term co-ingestion of creatine and sodium bicarbonate improves anaerobic performance in trained taekwondo athletes. J Int Soc Sports Nutr. 2021;18(1):10.
Saunders B, Sale C, Harris RC, Sunderland C. Effect of sodium bicarbonate and beta-alanine on repeated sprints during intermittent exercise performed in hypoxia. Int J Sport Nutr Exerc Metab. 2014;24(2):196–205.
Tobias G, Benatti FB, de Salles PV, Roschel H, Gualano B, Sale C, et al. Additive effects of beta-alanine and sodium bicarbonate on upper-body intermittent performance. Amino Acids. 2013;45(2):309–17.
Dennig H, Talbott JH, Edwards HT, Dill DB. Effect of acidosis and alkalosis upon capacity for work. J Clin Invest. 1931;9(4):601–13.
Johnson WR, Black DH. Comparison of effects of certain blood alkalinizers and glucose upon competitive endurance performance. J Appl Physiol. 1953;5(10):577–8.
Margaria R, Aghemo P, Sassi G. Effect of alkalosis on performance and lactate formation in supramaximal exercise. Int Z Angew Physiol. 1971;29(3):215–23.
Simmons RW, Hardt AB. The effect of alkali ingestion on the performance of trained swimmers. J Sports Med Phys Fitness. 1973;13(3):159–63.
Lindinger MI, Franklin TW, Lands LC, Pedersen PK, Welsh DG, Heigenhauser GJ. Role of skeletal muscle in plasma ion and acid-base regulation after NaHCO3 and KHCO3 loading in humans. Am J Physiol. 1999;276(1):R32–43.
Hollidge-Horvat MG, Parolin ML, Wong D, Jones NL, Heigenhauser GJ. Effect of induced metabolic alkalosis on human skeletal muscle metabolism during exercise. Am J Physiol Endocrinol Metab. 2000;278(2):E316–29.
Fordtran JS, Morawski SG, Santa Ana CA, Rector FC Jr. Gas production after reaction of sodium bicarbonate and hydrochloric acid. Gastroenterology. 1984;87(5):1014–21.
Petrovic S, Ju X, Barone S, Seidler U, Alper SL, Lohi H, et al. Identification of a basolateral Cl-/HCO3- exchanger specific to gastric parietal cells. Am J Physiol Gastrointest Liver Physiol. 2003;284(6):G1093–103.
Tosco M, Orsenigo MN, Gastaldi G, Faelli A. pH dependence of Cl/HCO3 exchanger in the rat jejunal enterocyte. Biochim Biophys Acta. 1998;1372(2):323–30.
Turnberg LA, Fordtran JS, Carter NW, Rector FC Jr. Mechanism of bicarbonate absorption and its relationship to sodium transport in the human jejunum. J Clin Invest. 1970;49(3):548–56.
Vilmi N, Äyrämö S, Nummela A, Pullinen T, Linnamo V, Häkkinen K, et al. Oxygen uptake, acid-base balance and anaerobic energy system contribution in maximal 300-400 m running in child, adolescent and adult athletes. Athl Enhanc. 2016;5:3.
La Monica MB, Fukuda DH, Starling-Smith TM, Clark NW, Panissa VLG. Alterations in energy system contribution following upper body sprint interval training. Eur J Appl Physiol. 2020;120(3):643–51.
Gastin PB. Energy system interaction and relative contribution during maximal exercise. Sports Med. 2001;31(10):725–41.
Dawson MJ, Gadian DG, Wilkie DR. Muscular fatigue investigated by phosphorus nuclear magnetic resonance. Nature. 1978;274(5674):861–6.
Cairns SP, Lindinger MI. Do multiple ionic interactions contribute to skeletal muscle fatigue? J Physiol. 2008;586(17):4039–54.
Lindinger MI, Cairns SP. Regulation of muscle potassium: exercise performance, fatigue and health implications. Eur J Appl Physiol. 2021;121(3):721–48.
Fitts RH. The role of acidosis in fatigue: pro perspective. Med Sci Sports Exerc. 2016;48(11):2335–8.
Debold EP, Fitts RH, Sundberg CW, Nosek TM. Muscle fatigue from the perspective of a single crossbridge. Med Sci Sports Exerc. 2016;48(11):2270–80.
McKenna MJ, Bangsbo J, Renaud JM. Muscle K+, Na+, and Cl disturbances and Na+-K+ pump inactivation: implications for fatigue. J Appl Physiol. 2008;104(1):288–95.
Pedersen KK, Cheng AJ, Westerblad H, Olesen JH, Overgaard K. Moderately elevated extracellular [K+] potentiates submaximal force and power in skeletal muscle via increased [Ca2+]i during contractions. Am J Physiol Cell Physiol. 2019;317(5):C900–9.
Fortune E, Lowery MM. Effect of extracellular potassium accumulation on muscle fiber conduction velocity: a simulation study. Ann Biomed Eng. 2009;37(10):2105–17.
Juel C, Pilegaard H, Nielsen JJ, Bangsbo J. Interstitial K(+) in human skeletal muscle during and after dynamic graded exercise determined by microdialysis. Am J Physiol Regul Integr Comp Physiol. 2000;278(2):R400–6.
Allen DG, Lamb GD, Westerblad H. Impaired calcium release during fatigue. J Appl Physiol. 2008;104(1):296–305.
Mainwood GW, Worsley-Brown P. The effects of extracellular pH and buffer concentration on the efflux of lactate from frog sartorius muscle. J Physiol. 1975;250(1):1–22.
Debold EP. Recent insights into muscle fatigue at the cross-bridge level. Front Physiol. 2012;3:151.
MacIntosh BR, Holash RJ, Renaud JM. Skeletal muscle fatigue--regulation of excitation-contraction coupling to avoid metabolic catastrophe. J Cell Sci. 2012;125(Pt 9):2105–14.
Stewart PA. Modern quantitative acid-base chemistry. Can J Physiol Pharmacol. 1983;61(12):1444–61.
Jensen WB. The Lewis Acid-Base Concepts: An Overview. Krieger Pub Co., 1979. 1st edition.
Street D, Nielsen JJ, Bangsbo J, Juel C. Metabolic alkalosis reduces exercise-induced acidosis and potassium accumulation in human skeletal muscle interstitium. J Physiol. 2005;566(Pt 2):481–9.
Grgic J. Effects of sodium bicarbonate ingestion on measures of Wingate test performance: a meta-analysis. J Am Coll Nutr. 2020. https://doi.org/10.1080/07315724.2020.1850370 .
Faria EW, Parker DL, Faria IE. The science of cycling: physiology and training - part 1. Sports Med. 2005;35(4):285–312.
Bangsbo J, Iaia FM, Krustrup P. The Yo-Yo intermittent recovery test: a useful tool for evaluation of physical performance in intermittent sports. Sports Med. 2008;38(1):37–51.
Grgic J, Garofolini A, Pickering C, Duncan MJ, Tinsley GM, Del Coso J. Isolated effects of caffeine and sodium bicarbonate ingestion on performance in the Yo-Yo test: A systematic review and meta-analysis. J Sci Med Sport. 2020;23(1):41–7.
Hagerman FC. Applied physiology of rowing. Sports Med. 1984;1(4):303–26.
Turnes T, Cruz RSO, Caputo F, De Aguiar RA. The impact of preconditioning strategies designed to improve 2000-m rowing ergometer performance in trained rowers: a systematic review and meta-analysis. Int J Sports Physiol Perform. 2019;14(7):871–9.
Smith TB, Hopkins WG. Variability and predictability of finals times of elite rowers. Med Sci Sports Exerc. 2011;43(11):2155–60.
Grgic J, Mikulic P. Ergogenic effects of sodium bicarbonate supplementation on middle, but not short-distance swimming tests: a meta-analysis. J Diet Suppl. 2021. https://doi.org/10.1080/19390211.2021.1942381 .
Trewin CB, Hopkins WG, Pyne DB. Relationship between world-ranking and Olympic performance of swimmers. J Sports Sci. 2004;22(4):339–45.
Sperlich B, Zinner C, Heilemann I, Kjendlie PL, Holmberg HC, Mester J. High-intensity interval training improves VO(2peak), maximal lactate accumulation, time trial and competition performance in 9-11-year-old swimmers. Eur J Appl Physiol. 2010;110(5):1029–36.
Franchini E, Artioli GG, Brito CJ. Judo combat: time-motion analysis and physiology. Int J Perf Anal Spor. 2013;13(3):624–41.
Amtmann JA, Amtmann KA, Spath WK. Lactate and rate of perceived exertion responses of athletes training for and competing in a mixed martial arts event. J Strength Cond Res. 2008;22(2):645–7.
Grgic J, Rodriguez RF, Garofolini A, Saunders B, Bishop DJ, Schoenfeld BJ, et al. Effects of sodium bicarbonate supplementation on muscular strength and endurance: a systematic review and meta-analysis. Sports Med. 2020;50(7):1361–75.
Suchomel TJ, Nimphius S, Stone MH. The importance of muscular strength in athletic performance. Sports Med. 2016;46(10):1419–49.
Hill-Haas S, Bishop D, Dawson B, Goodman C, Edge J. Effects of rest interval during high-repetition resistance training on strength, aerobic fitness, and repeated-sprint ability. J Sports Sci. 2007;25(6):619–28.
Spriet LL, Lindinger MI, Heigenhauser GJ, Jones NL. Effects of alkalosis on skeletal muscle metabolism and performance during exercise. Am J Physiol. 1986;251(5 Pt 2):R833–9.
Pate E, Bhimani M, Franks-Skiba K, Cooke R. Reduced effect of pH on skinned rabbit psoas muscle mechanics at high temperatures: implications for fatigue. J Physiol. 1995;486(3):689–94.
Brody L, Pollock M, Roy S, De Luca C, Celli B. pH-induced effects on median frequency and conduction velocity of the myoelectric signal. J Appl Physiol. 1991;71(5):1878–85.
Methenitis S, Terzis G, Zaras N, Stasinaki AN, Karandreas N. Intramuscular fiber conduction velocity, isometric force and explosive performance. J Hum Kinet. 2016;51:93–101.
Tillin NA, Pain MT, Folland J. Explosive force production during isometric squats correlates with athletic performance in rugby union players. J Sports Sci. 2013;31(1):66–76.
Maffiuletti NA, Aagaard P, Blazevich AJ, Folland J, Tillin N, Duchateau J. Rate of force development: physiological and methodological considerations. Eur J Appl Physiol. 2016;116(6):1091–116.
Bishop DJ, Thomas C, Moore-Morris T, Tonkonogi M, Sahlin K, Mercier J. Sodium bicarbonate ingestion prior to training improves mitochondrial adaptations in rats. Am J Physiol Endocrinol Metab. 2010;299(2):E225–33.
Saunders B, Oliveira LF, Dolan E, Durkalec-Michalski K, McNaughton L, Artioli GG, et al. Sodium bicarbonate supplementation and the female athlete: A brief commentary with small scale systematic review and meta-analysis. Eur J Sport Sci. 2021. https://doi.org/10.1080/17461391.2021.1880649 .
McNulty KL, Elliott-Sale KJ, Dolan E, Swinton PA, Ansdell P, Goodall S, et al. The effects of menstrual cycle phase on exercise performance in eumenorrheic women: a systematic review and meta-analysis. Sports Med. 2020;50(10):1813–27.
Jones RL, Stellingwerff T, Artioli GG, Saunders B, Cooper S, Sale C. Dose-response of sodium bicarbonate ingestion highlights individuality in time course of blood analyte responses. Int J Sport Nutr Exerc Metab. 2016;26(5):445–53.
Heibel AB, Perim PHL, Oliveira LF, McNaughton LR, Saunders B. Time to optimize supplementation: modifying factors influencing the individual responses to extracellular buffering agents. Front Nutr. 2018;5:35.
Oliveira LF, Saunders B, Yamaguchi G, Swinton P, Giannini AG. Is individualization of sodium bicarbonate ingestion based on time to peak necessary? Med Sci Sports Exerc. 2020;52(8):1801–8.
Middlebrook I, Peacock J, Tinnion DJ, Leach NK, Hilton NP, Saunders B, et al. Capsule size alters the timing of metabolic alkalosis following sodium bicarbonate supplementation. Front Nutr. 2021;8:634465.
Lopes-Silva JP, Reale R, Franchini E. Acute and chronic effect of sodium bicarbonate ingestion on Wingate test performance: a systematic review and meta-analysis. J Sports Sci. 2019;37(7):762–71.
Lancha Junior AH, Painelli Vde S, Saunders B, Artioli GG. Nutritional strategies to modulate intracellular and extracellular buffering capacity during high-intensity exercise. Sports Med. 2015;45(Suppl 1):S71–81.
Gurton WH, Faulkner SH, James RM. Effect of warm-up and sodium bicarbonate ingestion on 4-km cycling time-trial performance. Int J Sports Physiol Perform. 2021. https://doi.org/10.1123/ijspp.2020-0743 .
Jones RL, Stellingwerff T, Swinton P, Artioli GG, Saunders B, Sale C. Warm-up intensity does not affect the ergogenic effect of sodium bicarbonate in adult men. Int J Sport Nutr Exerc Metab. 2021. In press.
Kahle LE, Kelly PV, Eliot KA, Weiss EP. Acute sodium bicarbonate loading has negligible effects on resting and exercise blood pressure but causes gastrointestinal distress. Nutr Res. 2013;33(6):479–86.
Carr AJ, Slater GJ, Gore CJ, Dawson B, Burke LM. Effect of sodium bicarbonate on [HCO3-], pH, and gastrointestinal symptoms. Int J Sport Nutr Exerc Metab. 2011;21(3):189–94.
de Oliveira LF, Saunders B, Artioli GG. Is bypassing the stomach a means to optimize sodium bicarbonate supplementation? A case study with a postbariatric surgery individual. Int J Sport Nutr Exerc Metab. 2018;28(6):660–3.
Clark VR, Hopkins WG, Hawley JA, Burke LM. Placebo effect of carbohydrate feedings during a 40-km cycling time trial. Med Sci Sports Exerc. 2000;32(9):1642–7.
Marticorena FM, Carvalho A, de Oliveira LF, Dolan E, Gualano B, Swinton S, et al. Nonplacebo controls to determine the magnitude of ergogenic interventions: a systematic review and meta-analysis. Med Sci Sports Exerc. 2021. https://doi.org/10.1249/MSS.0000000000002635 .
Grgic J, Venier S, Mikulic P. Both caffeine and placebo improve vertical jump performance compared with a nonsupplemented control condition. Int J Sports Physiol Perform. 2021;16(3):448–51.
Higgins MF, Shabir A. Expectancy of ergogenicity from sodium bicarbonate ingestion increases high-intensity cycling capacity. Appl Physiol Nutr Metab. 2016;41(4):405–10.
McClung M, Collins D. "Because I know it will!": placebo effects of an ergogenic aid on athletic performance. J Sport Exerc Psychol. 2007;29(3):382–94.
Saunders B, de Oliveira LF, da Silva RP, de Salles PV, Gonçalves LS, Yamaguchi G, et al. Placebo in sports nutrition: a proof-of-principle study involving caffeine supplementation. Scand J Med Sci Sports. 2017;27(11):1240–7.
Burke LM. Practical issues in evidence-based use of performance supplements: supplement interactions, repeated use and individual responses. Sports Med. 2017;47(Suppl 1):79–100.
Harris RC, Tallon MJ, Dunnett M, Boobis L, Coakley J, Kim HJ, et al. The absorption of orally supplied beta-alanine and its effect on muscle carnosine synthesis in human vastus lateralis. Amino Acids. 2006;30(3):279–89.
Sale C, Saunders B, Harris RC. Effect of beta-alanine supplementation on muscle carnosine concentrations and exercise performance. Amino Acids. 2010;39(2):321–33.
Saunders B, Elliott-Sale K, Artioli GG, Swinton PA, Dolan E, Roschel H, et al. β-alanine supplementation to improve exercise capacity and performance: a systematic review and meta-analysis. Br J Sports Med. 2017;51(8):658–69.
Grgic J. Effects of beta-alanine supplementation on Yo-Yo test performance: A meta-analysis. Clin Nutr ESPEN. 2021;43:158–62.
Guest NS, VanDusseldorp TA, Nelson MT, Grgic J, Schoenfeld BJ, Jenkins NDM, et al. International society of sports nutrition position stand: caffeine and exercise performance. J Int Soc Sports Nutr. 2021;18(1):1.
Grgic J, Trexler ET, Lazinica B, Pedisic Z. Effects of caffeine intake on muscle strength and power: a systematic review and meta-analysis. J Int Soc Sports Nutr. 2018;15:11.
Grgic J. Caffeine ingestion enhances Wingate performance: a meta-analysis. Eur J Sport Sci. 2018;18(2):219–25.
Grgic J, Mikulic P. Caffeine ingestion acutely enhances muscular strength and power but not muscular endurance in resistance-trained men. Eur J Sport Sci. 2017;17(8):1029–36.
Sabol F, Grgic J, Mikulic P. The effects of 3 different doses of caffeine on jumping and throwing performance: a randomized, double-blind, crossover study. Int J Sports Physiol Perform. 2019;14(9):1170–7.
McLellan TM, Caldwell JA, Lieberman HR. A review of caffeine's effects on cognitive, physical and occupational performance. Neurosci Biobehav Rev. 2016;71:294–312.
Grgic J. Effects of caffeine on resistance exercise: a review of recent research. Sports Med. 2021. https://doi.org/10.1007/s40279-021-01521-x .
Grgic J. Effects of combining caffeine and sodium bicarbonate on exercise performance: a review with suggestions for future research. J Diet Suppl. 2021;18(4):444–60.
Kreider RB, Kalman DS, Antonio J, Ziegenfuss TN, Wildman R, Collins R, et al. International Society of Sports Nutrition position stand: safety and efficacy of creatine supplementation in exercise, sport, and medicine. J Int Soc Sports Nutr. 2017;14:18.
Download references
No funding was received for the research, writing or publication of this manuscript. B.S. (2016/50438-0) has been financially supported by Fundação de Amparo à Pesquisa do Estado de São Paulo and has also received a grant from Faculdade de Medicina da Universidade de São Paulo (2020.1.362.5.2).
Author information
Authors and affiliations.
Institute for Health and Sport, Victoria University, Melbourne, Australia
Jozo Grgic, Zeljko Pedisic, Michael J. McKenna & David J. Bishop
Applied Physiology and Nutrition Research Group, School of Physical Education and Sport; Rheumatology Division; Faculdade de Medicina FMUSP, Universidade de Sao Paulo, Sao Paulo, SP, BR, University of São Paulo, Sao Paulo, Brazil
Bryan Saunders
Institute of Orthopaedics and Traumatology, Faculty of Medicine FMUSP, University of São Paulo, Sao Paulo, Brazil
Centre for Bioscience, Manchester Metropolitan University, Manchester, M1 5GD, UK
Guilherme G. Artioli
Department of Health Sciences, Lehman College, Bronx, NY, USA
Brad J. Schoenfeld
Exercise & Sport Nutrition Lab, Human Clinical Research Facility, Department of Health & Kinesiology, Texas A&M University, College Station, TX, USA
Richard B. Kreider
Physiology of Work and Exercise Response (POWER) Laboratory, Institute of Exercise Physiology and Rehabilitation Science, School of Kinesiology and Physical Therapy, University of Central Florida, Orlando, FL, USA
Jeffrey R. Stout
Nutrion Department, College of Osteopathic Medicine, Nova Southeastern University, Fort Lauderdale, FL, 33314, USA
Douglas S. Kalman
Scientific Affairs. Nutrasource, Guelph, ON, Canada
Department of Exercise Science, Arnold School of Public Health, University of South Carolina, Columbia, SC, USA
Shawn M. Arent
Department of Exercise Science and Sport Management, Kennesaw State University, Kennesaw, GA, USA
Trisha A. VanDusseldorp
The Center for Applied Health Sciences, Stow, OH, USA
Hector L. Lopez & Tim N. Ziegenfuss
Supplement Safety Solutions, Bedford, MA, 01730, USA
Hector L. Lopez
Exercise and Nutrition Research Program, Mary MacKillop Institute for Health Research, Australian Catholic University, Melbourne, Australia
Louise M. Burke
Exercise and Sport Science, Nova Southeastern University, Davie, FL, 33314, USA
Jose Antonio
Performance & Physique Enhancement Laboratory, University of South Florida, Tampa, FL, 33612, USA
Bill I. Campbell
You can also search for this author in PubMed Google Scholar
Contributions
JG conceptualized the idea for the review and the content of the manuscript, drafted the initial version, created the tables, and oversaw all edits and revisions. BS and GGA drafted two sections of the manuscript, and critically reviewed all other sections. GGA created the figures. ZP, BJS, MJM, DJB, RBK, JRS, DSK, SMA, TAV, HLL, TNZ, LMB, JA, and BIC critically reviewed and contributed to all sections of the draft manuscript and their revisions.
Corresponding author
Correspondence to Jozo Grgic .
Ethics declarations
Ethics approval and consent to participate.
This paper was reviewed by the International Society of Sports Nutrition Research Committee and represents the official position of the Society.
Consent for publication
Competing interests.
J.G, Z.P, B.S, G.G.A, B.J.S, and M.J.M have no competing interests.
R.B.K. has conducted industry sponsored studies at the universities he has been affiliated with and occasionally serves as a scientific and legal consultant related to exercise and nutrition intervention studies.
J.R.S. has conducted industry-sponsored research on nutraceuticals over the past 25 years. Further, J.R.S. has also received financial support for presenting on the science of various nutraceuticals at industry-sponsored scientific conferences.
D.S.K. declares that in part, he works for a contract research company that conducts research and human clinical trials for industries including dietary supplements, medical foods, beverages, foods, pharmaceuticals and medical devices. He also sits on the Scientific Advisory Board for Dymatize Nutrition (BellRing Brands). At the time this review was developed and published, Dymatize Nutrition was not producing any supplements containing sodium bicarbonate.
S.M.A. has received grants to evaluate the effects of dietary supplements, and serves or has served on scientific advisory boards for sport nutrition companies.
D.J.B. and T.A.V. have received research grants related to dietary supplements.
H.L.L. has no conflict in terms of financial or business interests related to this manuscript. He has received grants to conduct research on dietary supplements; has served as a paid consultant for industry on Nutravigilance, post-market safety and regulatory compliance; receives royalties from the licensing of several patents in the dietary supplements industry (but not on sodium bicarbonate supplements).
T.N.Z. has no conflict in terms of financial or business interests related to this manuscript. T.N.Z. has received grants and contracts to conduct research on dietary supplements; has served as a paid consultant for industry; has received honoraria for speaking at conferences and writing lay articles about sports nutrition ingredients; receives royalties from the sale of several sports nutrition products (but not on sodium bicarbonate supplements); and has served as an expert witness on behalf of the plaintiff and defense in cases involving dietary supplements.
L.M.B. is a Director of the International Olympic Committee Diploma in Sports Nutrition.
J.A. is the CEO of the ISSN.
B.I.C. is on the scientific advisory board for Dymatize Nutrition (BellRing Brands), a manufacturer of sports supplements. At the time this review was developed and published, Dymatize Nutrition was not producing any supplements containing sodium bicarbonate
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Grgic, J., Pedisic, Z., Saunders, B. et al. International Society of Sports Nutrition position stand: sodium bicarbonate and exercise performance. J Int Soc Sports Nutr 18 , 61 (2021). https://doi.org/10.1186/s12970-021-00458-w
Download citation
Received : 13 August 2021
Accepted : 17 August 2021
Published : 09 September 2021
DOI : https://doi.org/10.1186/s12970-021-00458-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Journal of the International Society of Sports Nutrition
ISSN: 1550-2783
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
We are a non-profit organization that funds nutritional research
Nutrition research saves lives
Be the first to know about food news, events and more, the mission.
Let’s spread the message about the vital role excellent nutrition plays in our health and its ability to reverse most chronic disease. If we can change the way health professionals and physicians regard nutrition, then we can save millions from endless suffering and premature death.
The Challenge
There are millions of people with serious diseases who can be helped if only they become informed about these remarkable nutritional protocols.
Did you know: the NRF has heard the stories of thousand of individuals who successfully reversed their chronic diseases when they made the right dietary choices?
Our Solution
The Nutrition Research Foundation is committed now more than ever to funding research that demonstrates the power of nutrition. With your support, we can accelerate our efforts to fund clinical trials to increase professional and public confidence to use nutritional therapies as a potent weapon in the fight against chronic disease.
Research that changes the way we think about health
We raise money to fund nutritional research that utilizes nutrition to prevent and reverse disease.
Using our research, the healthcare system can implement new nutritional protocols.
Publications
We publish our research in key medical journals to document findings in the field of nutrition.
Our Success
Thanks to generous donors and our partners we were able to conduct research and publish several studies in the field of nutrition. Check out our publications to learn more.
Donate Today!
CALL US
1-888-511-4443
Join our mailing list
Nutritional Research Foundation © All Rights Reserved
501(c)3 Information Privacy Policy
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.4(5); 2013 Sep
Nutrition research to affect food and a healthy lifespan 1, 2
Sarah d. ohlhorst.
3 American Society for Nutrition, Bethesda, MD
Robert Russell
4 NIH Office of Dietary Supplements, Bethesda, MD, and Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University, Boston, MA
Dennis Bier
5 USDA/Agricultural Research Service Children’s Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine, Houston, TX
David M. Klurfeld
6 Human Nutrition Program, USDA/Agricultural Research Service, Beltsville, MD
Zhaoping Li
7 Center for Human Nutrition, University of California Los Angeles, and David Geffen School of Medicine at UCLA, Los Angeles, CA
Jonathan R. Mein
8 Monsanto Center for Food and Nutrition Research, Monsanto Vegetable Seed, Kannapolis, NC
John Milner
9 NIH National Cancer Institute, Bethesda, MD
A. Catharine Ross
10 Department of Nutritional Sciences, Pennsylvania State University, University Park, PA; and
Patrick Stover
11 Division of Nutritional Sciences, Cornell University, Ithaca, NY.
Emily Konopka
Proper nutrition offers one of the most effective and least costly ways to decrease the burden of many diseases and their associated risk factors, including obesity. Nutrition research holds the key to increasing our understanding of the causes of obesity and its related comorbidities and thus holds promise to markedly influence global health and economies. After outreach to 75 thought leaders, the American Society for Nutrition (ASN) convened a Working Group to identify the nutrition research needs whose advancement will have the greatest projected impact on the future health and well-being of global populations. ASN’s Nutrition Research Needs focus on the following high priority areas: 1 ) variability in individual responses to diet and foods; 2 ) healthy growth, development, and reproduction; 3 ) health maintenance; 4 ) medical management; 5 ) nutrition-related behaviors; and 6 ) food supply/environment. ASN hopes the Nutrition Research Needs will prompt collaboration among scientists across all disciplines to advance this challenging research agenda given the high potential for translation and impact on public health. Furthermore, ASN hopes the findings from the Nutrition Research Needs will stimulate the development and adoption of new and innovative strategies that can be applied toward the prevention and treatment of nutrition-related diseases. The multidisciplinary nature of nutrition research requires stakeholders with differing areas of expertise to collaborate on multifaceted approaches to establish the evidence-based nutrition guidance and policies that will lead to better health for the global population. In addition to the identified research needs, ASN also identified 5 tools that are critical to the advancement of the Nutrition Research Needs: 1 ) omics, 2 ) bioinformatics, 3 ) databases, 4 ) biomarkers, and 5 ) cost-effectiveness analysis.
INTRODUCTION
The attainment of good nutrition depends on and encompasses the entire food supply. Plant and animal foods and their various components are the primary vehicles that provide nourishment to human beings. Nutrition is vital, not only in the growth and development of humans and animals but also in the prevention and treatment of disease. Nutrition is also fundamental to the maintenance of good health and functionality. Basic and applied research on the interrelations between nutrition and noncommunicable diseases, nutrient composition, and nutrition monitoring represents the underpinnings for healthy populations and robust economies. Thus, innovative nutrition research and education provide the basis for solutions to larger health-related issues, allowing individuals to live healthier, more productive lives.
The importance of nutrition, as an integral part of the solution to many societal, environmental, and economic challenges facing the world, has just started to be fully appreciated. The American Society for Nutrition (ASN) has identified the “grand” challenges facing nutrition research and science in the 21st century, termed “Nutrition Research Needs.” Findings from these Nutrition Research Needs will elucidate strategies that can be applied toward the prevention and treatment of both infectious and noncommunicable diseases, including cardiovascular disease, diabetes, and cancer. Nutrition research holds the key to increasing our understanding of the underlying causes of obesity and its related comorbidities and thus holds promise to markedly influence global economies. Knowledge about adequate nutrition also has an important role in reducing or ending global and domestic food insecurity through direct and purposeful agricultural practices. Population growth will undeniably lead to increased global demand for a safe, available, sustainable, and affordable food supply, while continuing to demand nutritional adequacy.
The ASN Nutrition Research Needs project was originally conceptualized by ASN’s Public Policy Committee to identify worldwide nutrition research needs. This effort will be used to educate and communicate to policy makers and other stakeholders the need and value of increased nutrition research funding to meet societal needs. ASN’s Public Policy Committee reached out to nearly 75 thought leaders in September 2011 to develop a draft list of nutrition research needs.
In February 2012, ASN convened a Working Group of nutrition scientists and researchers representing a cross-section of the Society’s membership to determine the nutrition research needs that will have the greatest impact on the health and well-being of global populations. The names of the Working Group members are listed in the Acknowledgments. Starting with the draft list, the Working Group narrowed down and pulled together 6 nutrition research needs for which advancement would have the greatest projected impact on future health and well-being.
The ASN then informed its membership of the 6 priority research needs and sought further member input. A workshop was held during ASN’s 2012 Scientific Sessions and Annual Meeting in San Diego, CA, with nearly 250 attendees. The research needs were also shared via ASN’s member newsletter, which reaches the entire membership base of nearly 5000 individuals, to inform and seek input from members who did not attend the annual meeting or the workshop. Member feedback on the Nutrition Research Needs was incorporated during development of the final document.
THE TOP NUTRITION RESEARCH NEEDS
The top 6 nutrition research needs cut across the entire research spectrum from basic science to health policy, from discovery to application. Specific research areas are listed under each research need. These 6 nutrition research needs are highlighted in the hope that they will prompt scientists from all disciplines to collaborate to advance these challenging research needs that have high potential for translation and public health impact. Although the topics presented focus principally on human nutrition research, the Working Group recognized that nutrition research using animal models is an essential foundation for making new discoveries that can be translated to advances in human nutrition. Further, the importance of animal nutrition research is emphasized within these research needs in particular: “Understanding the role of nutrition in health maintenance” and “Understanding the food supply/environment.” The research community will benefit from clearly articulated nutrition research priorities that will lead to science-based information, help to shape policy and enhance future funding for nutrition research, and thereby further promote the field of nutrition science.
1) Understanding variability in individual responses to diet and foods
A top priority for future nutrition research is the need to better understand variability in metabolic responses to diet and food. Enormous variability exists in individual responses to diet and food components that affect overall health. Discoveries underpinning this variability will lead to advances in personalized nutrition interventions and will better inform health and food policies, including Dietary Reference Intakes (DRIs) for nutrient needs and, ideally, future recommendations for known bioactive food components. Research in the following areas is necessary to determine the origins and architecture of variability and to explain similar or dissimilar responses to diet and food components by subpopulations, as influenced by genetic, epigenetic, and ethnic and/or racial differences.
Omics research, such as nutrigenetics and nutrigenomics (e.g., epigenetics, transcriptomics, proteomics, and metabolomics), will help to determine how specific nutrients interact with genes, proteins, and metabolites to predict an individual’s health. Omics provide information on individualized nutrient requirements, including how nutrients are digested, absorbed, and metabolized, and their functions in the body. Omics will help to determine and reflect an individual’s nutritional status and will aid in the creation of new nutritional and disease biomarkers.
Microbiome.
Diverse microbes, such as bacteria and viruses, live in and on the body and contribute to the microbiome, which is estimated to have 10 times as many cells as the body itself ( 1 ). Microbes can vary in type and quantity, making each organism’s microbiome unique—although subpopulations may have similar microbiome characteristics. The microbiota needs to be better defined, and changes due to diet, age, physiologic state, and disease need to be determined. Research is needed to determine the microbiome’s role in varying biological responses to diet and food components and its importance in disease prevention and progression. Conversely, research is also needed to determine how the microbiome is influenced by diet and other environmental factors.
Biological networks.
Basic research is needed to provide a better understanding of biological networks, such as an individuals’ genome (DNA/RNA protein profiles), and how these networks affect metabolic responses to diet and food. Environmental interactions, including nutrients and other dietary components, bacteria, viruses, and chemical contaminants, all may affect the responsiveness of biological networks to specific foods and the entire diet.
Tissue specificity and temporality.
Research is needed to describe the mechanisms by which dietary factors affect variability in development and functioning, including which tissues are most influenced by dietary factors and when during the most critical stages in life this influence occurs.
2) Understanding the impact of nutrition on healthy growth, development, and reproduction
Epigenetics/imprinting..
Epigenetics and imprinting research examines how exposures to dietary components during critical periods of development may “program” long-term health and well-being. Research is needed to determine how early nutritional events contribute to disease later in life and alter normal developmental progression.
Early nutrition.
Research is necessary to better understand the role of diet and individual food components on normal growth and development. This includes the role of parent’s preconception diets, the maternal diet during pregnancy, and early nutritional events. Studies indicate that the timing of an infant’s introduction to solid foods may increase the likelihood of becoming obese later in life ( 2 ). These findings are important given that the number of overweight children in the United States has increased dramatically in recent years ( 3 ). Research is now needed to determine the best approaches to influence these factors during early life. The important role of nutrition throughout early life on growth and development, as well as on health and well-being, needs to be continually assessed.
Nutrition and reproductive health.
The impact of nutrition on reproductive health, including before and after conception, requires further research. Nutrition has a direct impact on both maternal and paternal fertility and the ability to conceive and also plays a key role in preventing diseases related to reproductive organs, including prostate and ovarian cancers. Although numerous studies have investigated how fruit and vegetable consumption may affect risk of breast, prostate, and other cancers, there is no clear consensus in the scientific literature. Thus, well-designed controlled intervention studies are needed to determine whether effects are limited to subpopulations, what factors influence a response and what mechanisms may account for changes in health.
3) Understanding the role of nutrition in health maintenance
Health maintenance includes noncommunicable disease prevention and treatment as well as weight management. The role that food components, particularly novel ingredients, contribute to health maintenance requires continuing research. Researchers and the public rely on dietary guidance, including the DRIs, to guide nutrition recommendations and health policy. Research is needed to better define the nutrient needs that best support health maintenance in all populations and their subgroups, from infancy throughout life. Nutrition across life is a fundamental issue that requires investigation so that recommendations will “match” with true biological needs.
Optimal bodily function.
Research is needed to determine the roles that nutrition and fitness, both singularly and together, have in maintaining bodily functions, including cognitive, immune, skeletal, muscular, and other functions. Evolving research areas include prevention of disease-related processes, such as inflammation, and definition of mechanisms that have an important role in health maintenance, such as immunocompetence. Animal models are used to understand the requirements for optimal health in humans and production animals.
Energy balance.
Research is also needed to examine the use of a systems approach to achieve energy balance including and integrating environmental, biological, psychosocial, and food system factors. A systems approach is preferable because the standard experimental approach of varying one factor at a time has accomplished little to address the populationwide problem of energy imbalance. A solution-oriented approach that is comprehensive in nature and takes into account the complexities of achieving energy balance must be created. Although far more research is needed to identify systemwide changes that maximize energy balance, intriguing examples exist. “Shape Up Somerville, MA,” effectively reduced weight gain in high-risk children through a multifaceted community-based environmental change campaign ( 4 ). Shape Up Somerville increased the community’s physical activity and healthful eating through physical infrastructure improvements and citywide policy and programming changes.
4) Understanding the role of nutrition in medical management
The rapid translation of nutrition research advances into evidence-based practice and policy is a priority for ensuring optimal patient care and effective disease management. Nutrition researchers have a key role in bridging the gap between disease prevention and disease treatment by fostering clinical research, providing innovative education for caregivers and patients, and delineating best practices for medical nutrition in primary care settings.
Disease progression.
To improve the medical management of disease, research is needed to determine how nutritional factors influence both disease initiation and progression, as well as how nutrition affects a patient’s response to therapy. Genetic and epigenetic variations among individuals can result in both positive and negative responses to diets, to specific foods, and to novel food components. The issue of individual variability is of considerable importance in refining medical management, including nutrition support, and requires continuing research.
Expanded research will allow us to better understand and minimize unfavorable impacts of both reduced and elevated nutrient intakes on disease progression and overall health. Disease/mortality response curves are U-shaped for many nutrients (that is, there is an increased risk of adverse outcomes if the nutrient is ingested in either too low or too high amounts). The importance of achieving a proper nutrient balance is seen in the example of chronic inflammation. Chronic inflammation contributes to many noncommunicable diseases and can result from high intakes of proinflammatory omega-6 fatty acids in the face of low intakes of anti-inflammatory omega-3 fatty acids ( 5 ). Research will help to determine the desired intake for essential and nonessential nutrients alone and when combined with other nutrients in the diet.
Nutrition support for special subgroups.
Nutrition research is needed to establish the required nutritional needs that best support survival, growth, and development in subpopulations, such as in chronically diseased patients, in children, and in aging adults. With the success of medical advances, as have been seen with in vitro fertilization and neonatal care, caring for preterm infants presents a new challenge in early nutritional management. Preterm infants have special nutrition needs that will greatly affect their future growth and development, as well as their eventual health status as adults.
5) Understanding nutrition-related behaviors
Drivers of food choice..
Understanding the link between behavior and food choices can help tackle obesity and other nutrition-related issues that are a public health priority. Individual food choices can be influenced by a number of different drivers including the following:
- Government policy
- Environmental cues
- Cultural differences
- Communication tools, such as social networking and food marketing
Research is needed to identify the impact of these various drivers and understand how they work alone or together to influence nutrition-related behavior. Research will show how these drivers should be altered to have the highest positive influence on individual behavior and therefore public health. For example, the state of Mississippi recorded a 13% decline in obesity among elementary school students from 2005 to 2011 ( 6 ). Multiple changes in the environment occurred, such as the setting of standards for foods sold in school vending machines, setting a requirement for more school exercise time, mandating healthier environments in childcare settings, and establishing programs that encouraged fruit and vegetable consumption. The challenge now is to determine what effect these combined actions will have on obesity-related behaviors in the long run.
Nutrition and brain functioning.
Further explorations of the biochemical and behavioral bases for food choices and intake over time are essential. Brain function as it relates to food desire and choice needs to be clarified through research, and the multiple hormones that affect eating require further study as well. Factors such as meal frequency and size, speed of meal consumption, and how these factors are influenced by social cues require objective data, which can only be provided by research. Understanding how the marketing of healthy behaviors could help consumers achieve dietary guidance goals should be a priority. As part of this approach, innovative and practical methods for accurately measuring and evaluating food purchases and eating occasions must be developed.
Imprinting.
Because of the high propensity of obese children remaining obese as adults ( 7 ), additional research is needed to determine how eating and satiety behaviors are imprinted during critical periods of development and to show how food components affect neural biochemistry and brain functioning—and therefore shape behavior. This research will provide us with a better understanding of how and why an individual makes particular food choices. Although scientists recently validated the concept that food availability during pregnancy has permanent effects on gene expression in children ( 8 ), human studies are needed to confirm or refute the hypothesis that fetal programming, resulting from maternal obesity, leads to excess weight in children and into adulthood.
6) Understanding the food supply/environment
Food environment and food choice..
Simply knowing or understanding what constitutes a healthy diet is not enough to change an individual’s diet or lifestyle. Understanding how the food environment affects dietary and lifestyle choices is necessary before effective policies can be instituted that will change a population’s diet in a meaningful way. Examples of key questions that should be addressed include the following:
- Is current dietary guidance an effective way of communicating dietary change?
- Do food assistance programs promote positive dietary patterns or have negative dietary and health consequences?
- What role does food advertising play in food decision-making among different age groups and educational levels?
- How do farm-to-fork food systems, with an increased emphasis on local agricultural production and consumption, influence dietary patterns and behaviors?
- How can farm-to-fork food systems ultimately be used to promote healthy behaviors and improve public health?
- How can we most effectively measure, monitor, and evaluate dietary change?
Food composition and novel foods and food ingredients.
Having an affordable, available, sustainable, safe, and nutritious food supply is also an important underpinning for making significant changes to a population’s diet and lifestyle. Examples of key research areas to address include the following:
- Enhancing our knowledge of the nutrient and phytonutrient content and bioavailability of foods produced, processed, and consumed
- Studying how to better align and foster collaboration between nutrition and agricultural production
- Can shifting agricultural focus from principally agronomic to include quality factors (such as taste, flavor, and nutritional value) have positive effects on fruit and vegetable consumption?
- Can we leverage technologies, such as biotechnology and nanotechnology, to develop novel foods and food ingredients that will improve health, both domestically and abroad, and provide credible, tangible functional health benefits?
Public/private partnerships.
To tackle these enormous challenges requires the coordinated efforts of public and private partners. The development of public/private partnerships between food and agricultural industries, government, academia, and nongovernmental organizations has the potential to advance nutrition research, enabling meaningful changes to be made to American and global diets (e.g., increased fruit and vegetable consumption to match government recommendations). We need to examine successful examples of public/private partnerships that have resulted in improved nutritional status and food security in specific populations ( 9 ).
CROSS-CUTTING TOOLS TO ADVANCE NUTRITION RESEARCH
Nutrition research is truly a cross-cutting discipline, and the Working Group identified several tools that are also necessary to advance the priority needs in nutrition research. Adequately powered intervention trials continue to be essential for validating research theories arising from experimental and epidemiologic studies. However, the development of new, impactful tools will help us to more effectively quantify dietary intake and food waste and to determine the effectiveness of nutrition standards, such as DRI values and the Dietary Guidelines for Americans . Although not a traditional tool, multidisciplinary partnerships among scientific societies, government, industry, academia, and others are fundamental to advance the nutrition research agenda. ASN and its membership must be proactive not only in efforts to advance nutrition research (including initiating and leading partnerships) but also in developing the tools needed to enhance the field. ASN recognizes the need to facilitate effective communication among academia, industry, government agencies, consumers, and other stakeholders to advance nutrition.
Omics (especially genomics, proteomics, and metabolomics) will enable us to determine how specific nutrients interact with genes, proteins, and metabolites to predict the future health of an individual. A field of study that encompasses technological advances as well as omics-based research, it is sometimes referred to as personalized nutrition. Omics hold the keys to major nutrition breakthroughs in noncommunicable disease and obesity prevention. Omics provide information on how well nutrients are digested, absorbed, metabolized, and used by an individual. Moreover, omics will lead to new biomarkers that reveal a person’s nutritional status and health status all at one time.
2) Bioinformatics
Bioinformatics is an interdisciplinary field that uses computer science and information technology to develop and enhance techniques to make it easier to acquire, store, organize, retrieve, and use biological data. Bioinformatics will enable nutrition researchers to manage, analyze, and understand nutrition data and to make connections between diet and health that were not previously possible. Databases are necessary to gain the full benefits of bioinformatics, because they make nutrition data easily accessible in a machine-readable format.
3) Databases
Accurate, up-to-date food and nutrient databases are essential to track and observe trends related to the nutrition and health of individuals. Databases link food and supplement composition and intake data to health outcomes. Nutrient databases should be expanded to cover more foods and their bioactive components, including nonessential nutrients. Nutrition data must be incorporated into databases related to novel research areas, such as nutrigenomics and the microbiome, to adequately link these areas with nutrition. Data collection must also be improved with enhancements such as photographic food intake documentation, direct upload of food composition and sensory characteristics (if not proprietary) from food manufacturers, and biological sample collection.
4) Biomarkers
Intake, effect, and exposure biomarkers allow us to determine and monitor the health and nutritional status of individuals and subpopulations, including ethnic and racial minorities. Biomarkers that are responsive to diet and nutrition will help assess disease progression and variability in response to treatment, while improving early diagnosis and prevention. Biomarkers must continue to be developed and validated to accurately track food and nutrient intake given our rapidly changing food supply.
5) Cost-effectiveness analysis
Cost-effectiveness analysis is a tool used to calculate and compare the relative costs and benefits of nutrition research interventions. Cost effectiveness analysis helps to determine the most cost-effective option that will have the greatest benefit to public health.
CONCLUSIONS
The multidisciplinary nature of nutrition research requires collaboration among research scientists with differing areas of expertise, many different stakeholders, and multifaceted approaches to develop the knowledge base required for establishing the evidence-based nutrition guidance and policies that will lead to better health and well-being of world populations. Proper nutrition offers one of the most effective and least costly ways to decrease the burden of chronic and noncommunicable diseases and their risk factors, including obesity. Although there is skepticism about the ability to complete large, well-controlled dietary interventions at a reasonable cost in the United States, the success of the Lyon Diet Heart study in France ( 10 , 11 ) and the PREvención con DIeta MEDiterránea (PREDIMED) study in Spain ( 12 ), both of which used variations of the Mediterranean diet, show this approach can be successful, even in the presence of drug treatment of cardiovascular risks in the latter study. Both of these studies showed significant reductions in cardiovascular disease (and cancer in the Lyon study) after relatively modest dietary changes.
Perhaps the greatest barrier to advancing the connections between food and health is the variability in individual responses to diet; it is also the origin of public skepticism to acceptance of dietary advice and the opportunity for entrepreneurship in the private sector. Imagine being able to identify, with certainty, those most likely to benefit from prescriptive nutrition advice through the various omic technologies and then providing these groups of people with customized nutrition advice based on their metabolic risk profiles. This is the new frontier of the nutritional sciences that offers the opportunity to predictably engineer our physiologic networks for health through diet. The confidence this approach would bring to the skeptical consumer would improve adherence to weight management and disease treatment techniques and improve the chances of success for disease prevention. To realize the full positive impact of achieving good nutrition on disease prevention and the health of populations, we must have the will to invest in and support the 6 key areas of nutrition research that have been outlined above.
Acknowledgments
The Nutrition Research Needs Working Group consisted of Dennis Bier, David M Klurfeld, Zhaoping Li, Jonathan R Mein, John Milner, A Catharine Ross, Robert Russell (Chair), and Patrick Stover. They were supported by ASN staff members Sarah D. Ohlhorst and Emily Konopka.

IMAGES
VIDEO
COMMENTS
Since 2010 Nutrition Research has sponsored the David Kritchevsky Graduate Student Award with support provided by Elsevier. This prestigious award recognizes outstanding articles published in Nutrition Research by graduate and professional students. Annually, two awards of $1000 (USD) each are presented to these early-career scientists.
Water insecurity: A barrier to healthy eating. In a national survey of lower-income United States adults, indicators of water insecurity were found to be associated with lower. Dedicated to bringing together the world's top researchers, clinical nutritionists, and industry to advance our knowledge and application of nutrition.
2. Nutrition for Healthy Ageing. The science of nutrition or the "nutritional science" is a highly advanced field of study, and numerous excellent books, journals and other resources are available for fundamental information about all nutritional components [].Briefly, the three essential macronutrients which provide the basic materials for building biological structures and for producing ...
Nutrition is the organic process of nourishing or being nourished, including the processes by which an organism assimilates food and uses it for growth and maintenance. Latest Research and Reviews
About. ISSN: 0022-3166. The Journal of Nutrition (JN/J Nutr) the official publication of the American Society for Nutrition (ASN), publishes high impact peer-reviewed original research papers covering all aspects of experimental nutrition in humans and other animal species. More.
Low-quality, non-diverse diet is a main risk factor for premature death. Accurate measurement of habitual diet is challenging and there is a need for validated objective methods. Blood metabolite patterns refl... Anna Winkvist, Ingegerd Johansson, Lars Ellegård and Helen M Lindqvist. Nutrition Journal 2024 23 :29.
2005 — Volume 25. Page 1 of 3. Read the latest articles of Nutrition Research at ScienceDirect.com, Elsevier's leading platform of peer-reviewed scholarly literature.
Nutrition and Health is an online international peer-reviewed journal that focusses on the relationship between nutrition and health. The journal welcomes original investigations, short communications, reviews, systematic reviews and meta-analyses, protocols, commentaries, hypotheses and case studies on current topics relating to the full spectrum of the effects of diet and nutrition on health ...
The World Cancer Research Fund/American Institute for Cancer Research Third Expert Report on Diet, Nutrition, Physical Activity, and Cancer: Impact and future directions. J Nutr. 2020;150:663-71.
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), an enveloped, positive-sense, single-stranded RNA virus, is the etiological agent of Coronavirus Disease 2019 (COVID-19) 1. The World ...
The last half-century of nutrition research has expanded beyond traditional nutrition research based primarily on in vitro biochemistry, animal models, and short-term feeding studies with risk factors as the primary outcomes. Although such studies are still an integral part of nutrition research, they do not directly connect diets with long ...
What factors influence sustainable and healthy diet consumption? A review and synthesis of literature within the university setting and beyond. Patrick S. Elliott, Lauren D. Devine, Eileen R. Gibney, Aifric M. O'Sullivan. In Press, Journal Pre-proof, Available online 16 March 2024.
Nutrition research holds the key to increasing our understanding of the causes of obesity and its related comorbidities and thus holds promise to markedly influence global health and economies. After outreach to 75 thought leaders, the American Society for Nutrition (ASN) convened a Working Group to identify the nutrition research needs whose ...
Additionally, NIH is committed to modernizing nutrition research through precision nutrition initiatives. The NIH Nutrition Research Report summarizes nutrition research activities supported and conducted by NIH Institutes, Centers, and Offices (ICOs) in Fiscal Years 2020 (FY20) and 2021 (FY21). This report was compiled and produced by the NIH ...
Nutritional Information. Answers to questions about nutrition, obesity, herbal and nutritional supplements, and the role of diet in improving and maintaining your health.
The NIH Nutrition Research Report 2020-2021 summarizes nutrition research activities supported and conducted by NIH ICOs in Fiscal Years 2020 and 2021. This report and an accompanying Executive Summary are now available. These documents detail FY19-FY21 funding levels for NIH-supported nutrition research and training, as well as providing highlights of selected accomplishments, gaps and ...
The NIH nutrition research portfolio includes extramural and intramural research as well as research training. In F Y 21, 81 percent of the nutrition research portfolio was extramural research, conducted by hundreds of institutions in the United States and in . several countries across the world.
The authors concluded that one avocado a day in a heart-healthy diet decreased oxLDL in adults with overweight and obesity, and the effect was associated with the reduction in sdLDL. This research was featured on ASN's nutrition research blog in February 2020, and in over 70 other news stories, including this one by Business Insider.
Food & Nutrition Research is a peer-reviewed journal that presents the latest scientific research in various fields focusing on human nutrition. The journal publishes both quantitative and qualitative research papers. Through an Open Access publishing model, Food & Nutrition Research opens an important forum for researchers from academic and ...
January 5, 2022 by ASN Staff. 2021 has come to a close, take a look back at some trending nutrition research articles from ASN's four journals: The Journal of Nutrition, The American Journal of Clinical Nutrition, Advances in Nutrition, and Current Developments in Nutrition. Here are 15 articles that were mentioned the most in news and social ...
Nutrition therapy has been emphasised for decades for people with type 2 diabetes, and the vital importance of diet and nutrition is now also recognised fo. ... (MC_UU_00006/3) and the NIHR Biomedical Research Centre Cambridge: Nutrition, Diet, and Lifestyle Research Theme (IS-BRC-1215-20014). She is an NIHR Senior Investigator.
Dietary n-3 and n-6 polyunsaturated fatty acids differentially modulate the adiponectin and leptinmediated major signaling pathways in visceral and subcutaneous white adipose tissue in high fat diet induced obesity in Wistar rats. Prerna Sharma, Chetna Bhandari, Navneet Agnihotri. Pages 74-86.
Research. At USDA we take a "food first" approach to improving human health and well-being and reducing the burden of chronic diet-related diseases. When it comes to using food and nutrition to improve health-related outcomes, one size doesn't fit all. This requires a more precise understanding of nutritional needs, and broader ...
Although numerous observational studies associated underfeeding with poor outcome, recent randomized controlled trials (RCTs) have shown that early full nutritional support does not benefit critically ill patients and may induce dose-dependent harm. Some researchers have suggested that the absence of benefit in RCTs may be attributed to overrepresentation of patients deemed at low nutritional ...
Based on a comprehensive review and critical analysis of the literature regarding the effects of sodium bicarbonate supplementation on exercise performance, conducted by experts in the field and selected members of the International Society of Sports Nutrition (ISSN), the following conclusions represent the official Position of the Society: 1. Supplementation with sodium bicarbonate (doses ...
The Nutrition Research Foundation is committed now more than ever to funding research that demonstrates the power of nutrition. With your support, we can accelerate our efforts to fund clinical trials to increase professional and public confidence to use nutritional therapies as a potent weapon in the fight against chronic disease.
Nutrition research holds the key to increasing our understanding of the causes of obesity and its related comorbidities and thus holds promise to markedly influence global health and economies. After outreach to 75 thought leaders, the American Society for Nutrition (ASN) convened a Working Group to identify the nutrition research needs whose ...
Critical Reviews in Food Science and Nutrition Latest Articles. Submit an article Journal homepage. 110 ... supported by National Natural Science Foundation of China (No. 32372475, No. 32101990, No. 32130084), National Key Research and Development Program of China (No. 2022YFF1100103), the Interdisciplinary Integration Innovation Project ...
CEP Final Rule Briefing, Child Nutrition Programs: Community Eligibility Provision--Increasing Options for Schools (September 2023) CEP 101, The Basics (February 2024) The CEP Estimator Tool. The CEP monthly federal reimbursement estimator tool may be used to estimate the level of federal reimbursement a CEP school, group of schools, or school ...