Editor's Choice: JAMA Neurology —The Year in Review, 2023

Josefin E. Kaufmann, MMed; Eric L. Harshfield, PhD; Henrik Gensicke, MD; et al
Original Investigation | May 13, 2024

Just Published
- Staged Bilateral MRI-Guided Ultrasound Subthalamotomy for Parkinson Disease Raúl Martínez-Fernández, MD, PhD; et al. Original Investigation online first has multimedia Raúl Martínez-Fernández, MD, PhD; et al.
- Antithrombotic Treatment for Cervical Artery Dissection Josefin E. Kaufmann, MMed; et al. Original Investigation online first Josefin E. Kaufmann, MMed; et al.
- Ghrelin for Neuroprotection in Post–Cardiac Arrest Coma Sjoukje Nutma, MD; et al. Original Investigation online first Sjoukje Nutma, MD; et al.
- Fluid Biomarker Changes After Amyloid-β–Targeting Drugs Rik Ossenkoppele, PhD; et al. Editorial online first Rik Ossenkoppele, PhD; et al.
- Personalized Management of Device-Detected Atrial Fibrillation James E. Siegler, MD; et al. Viewpoint online first James E. Siegler, MD; et al.
- Improving Trends in Brain Health Explain Declining Dementia Risk? Prashanthi Vemuri, PhD Editorial Prashanthi Vemuri, PhD
- Clinical Spectrum of Anti-GQ1b Antibody Syndrome Sun-Uk Lee, MD, PhD; et al. Review online first has active quiz has multimedia Sun-Uk Lee, MD, PhD; et al.
- A 24-Year-Old Man With Spastic Ataxia and Hypodontia Lissa Marien, MD; et al. JAMA Neurology Clinical Challenge online first has active quiz Lissa Marien, MD; et al.
- Progress in Pharmacologic Management of Neuropsychiatric Syndromes in Neurodegenerative Disorders Jeffrey Cummings, MD, ScD(HC); et al. Review online first has active quiz Jeffrey Cummings, MD, ScD(HC); et al.
- 7,845 Views Diagnostic Accuracy of a Plasma Phosphorylated Tau 217 Immunoassay for Alzheimer Disease Pathology
- 6,583 Views Clinicopathologic Heterogeneity and Glial Activation Patterns in Alzheimer Disease
- 5,542 Views Intracranial and Cerebral Volumes in Framingham Heart Study Participants
- 4,950 Views Downstream Biomarker Effects of Gantenerumab or Solanezumab in Dominantly Inherited Alzheimer Disease
- 3,948 Views Effects of Tirofiban on Neurological Deterioration in Patients With Acute Ischemic Stroke
- 3,888 Views Brexpiprazole for the Treatment of Agitation in Alzheimer Dementia
- 3,489 Views Emerging Cerebrospinal Fluid Biomarkers of Disease Activity and Progression in Multiple Sclerosis
- 3,477 Views Progress in Pharmacologic Management of Neuropsychiatric Syndromes in Neurodegenerative Disorders
- 3,314 Views Association of New-Onset Seizures With SARS-CoV-2 Vaccines
- 3,082 Views Ghrelin for Neuroprotection in Post–Cardiac Arrest Coma
- 226 Citations Amyloid-Related Imaging Abnormalities in 2 Phase 3 Studies Evaluating Aducanumab in Patients With Early Alzheimer Disease
- 192 Citations SARS-CoV-2 Infection in a North American Registry of Patients With Multiple Sclerosis
- 186 Citations Differences Between Plasma and CSF GFAP Levels Across the Alzheimer Disease Continuum
- 175 Citations Head-to-Head Comparison of 8 Plasma Amyloid-β 42/40 Assays in Alzheimer Disease
- 128 Citations Noncontrast CT vs CT Perfusion or MRI Selection in Late Presentation of Large-Vessel–Occlusion Stroke
- 125 Citations Humoral and T-Cell Response to SARS-CoV-2 Vaccination in Patients With Multiple Sclerosis Treated With Ocrelizumab
- 124 Citations Global Prevalence of Young-Onset Dementia
- 124 Citations Accuracy of Tau PET as a Prognostic Marker
- 121 Citations Five-Year Extension Results of the Phase 1 START Trial
- 112 Citations Effect of High-frequency (10-kHz) Spinal Cord Stimulation in Patients With Painful Diabetic Neuropathy
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
Molecular Neurobiology
- Provides thorough and constructive peer review managed by our Editor-in-Chief and Associate editors.
- Offers attractive turnaround times for research publication.
- Committed to high levels of author satisfaction, with strong publication return rates.
- Serves as essential reading for neuroscientists at the cutting edge of their field.
- Benedict C. Albensi
Latest issue
Volume 61, Issue 6
Latest articles
Circulating levels of t-cell traits and the risk of amyotrophic lateral sclerosis: a mendelian randomization study.

Disease-Associated Q159X Mutant Prion Protein Is Sufficient to Cause Fatal Degenerative Disease in Mice
- Runchuan Yan
Dysregulation of the Gut Microbiota Contributes to Sevoflurane-Induced Cognitive Dysfunction in Aged Mice by Activating the NLRP3 Inflammasome
- Shanshan Han
- Dengxin Zhang

MicroRNA-451 Regulates Angiogenesis in Intracerebral Hemorrhage by Targeting Macrophage Migration Inhibitory Factor
- Zhouping Tang

MSC Promotes the Secretion of Exosomal lncRNA KLF3-AS1 to Regulate Sphk1 Through YY1-Musashi-1 Axis and Improve Cerebral Ischemia–Reperfusion Injury
- Daodao Wang
- Dingzhou Zhou
Journal updates
Special issue: molecular neurobiology of mitochondria: focus on mitochondrial therapeutics.
The purpose of this Collection , Guest Edited by Dr. Hemachandra Reddy , is to assess the recent developments in mitochondrial research in health and disease. The major themes are:
1) biochemistry and molecular basis of mitochondria 2) current status mitochondrial dynamics, mitophagy, mitophagy enhancers 3) impact of oxidative stress, inflammation on mitochondrial function/dysfunction 4) mitochondrial therapeutics
The special issue will cover articles on aging, diabetes/obesity, age-related neurodegenerative diseases, and inherited mitochondrial diseases.
Articles will be consider for publication in the special issue from January 1, 2024 until July 1, 2024. We invite original research, high quality mini and comprehensive reviews and commentaries.
Microbes & Alzheimer’s: Bridging Silos to Accelerate Innovation
Molecular Neurobiology Guest Editors Brian Balin and Nikki Schultek are hosting an in-person and virtual educational symposium on July 27th on Microbes & Alzheimer's . There will be over 20 global experts presenting.
Special Issue Exploring the molecular mechanisms of infectious microbes and microbiota in chronic neurologic and psychiatric diseases
In this special issue, Guest Edited by Brian J. Balin and Nikki M. Schultek , we invite the scientific community to submit original research papers, reviews, meta-analyses, etc pertaining to the molecular mechanisms of neurologic and psychiatric diseases with emphasis on infectious microbes (the pathobiota) and/or the alterations to the microbiota that may lead to these disease states
Journal information
- Biological Abstracts
- Chemical Abstracts Service (CAS)
- Google Scholar
- Japanese Science and Technology Agency (JST)
- OCLC WorldCat Discovery Service
- Pathway Studio
- Science Citation Index Expanded (SCIE)
- TD Net Discovery Service
- UGC-CARE List (India)
Rights and permissions
Editorial policies
© Springer Science+Business Media, LLC, part of Springer Nature
- Find a journal
- Publish with us
- Track your research
- Research article
- Open access
- Published: 27 November 2020
Routinely collected patient data in neurology research: a systematic mapping review
- Fran Biggin 1 ,
- Hedley C. A. Emsley ORCID: orcid.org/0000-0003-0129-4488 1 , 2 &
- Jo Knight 1
BMC Neurology volume 20 , Article number: 431 ( 2020 ) Cite this article
3251 Accesses
5 Citations
3 Altmetric
Metrics details
This review focuses on neurology research which uses routinely collected data. The number of such studies is growing alongside the expansion of data collection. We aim to gain a broad picture of the scope of how routine healthcare data have been utilised.
This study follows a systematic mapping review approach which does not make a judgement on the quality of the papers included in the review, thereby enabling a complete overview of the field.
Of 4481 publications retrieved, 386 met the eligibility criteria for this study. These publications covered a wide range of conditions, but the majority were based on one or only a small number of neurological conditions. In particular, publications concerned with three discrete areas of neurological practice - multiple sclerosis (MS), epilepsy/seizure and Parkinson’s disease - accounted for 60% of the total. MS was the focus of the highest proportion of eligible studies (35%), yet in the recent Global Burden of Neurological Disease study it ranks only 14th out of 15 neurological disorders for DALY rates. In contrast, migraine is the neurological disorder with the highest ranking of DALYs globally (after stroke) and yet it was represented by only 4% of eligible studies.
This review shows that there is a disproportionately large body of literature pertaining to relatively rare disorders, and a correspondingly small body of literature describing more common conditions. Therefore, there is potential for future research to redress this balance.
Peer Review reports
The global burden of neurological disorders is increasing [ 1 ]. The Global Burden of Disease neurology collaborators reported that there has been a 39% increase in deaths due to neurological disorders between 1990 and 2016 [ 2 ]. Alongside this increase in the burden of disease, there is a predicted future shortfall in the US neurology workforce [ 3 ], and in the UK there is considerable concern surrounding services for people with neurological disorders [ 4 , 5 , 6 ]. A 2011 report by the UK National Audit Office (NAO) highlighted issues including delays in diagnosis, geographical inequalities in access to care; and a lack of good quality data [ 6 ].
Neurology is a large and diverse area of medicine with a correspondingly wide and varied body of research literature. Current neurology practice is heavily informed by the evidence provided by research, and the development of a focus on evidence based practice has been widely reported [ 7 , 8 , 9 ]. The use of data that have not been specifically collected for research is growing but we do not currently know how these data are being used in neurology research.
Routinely collected health data are collected from many different sources. For example, data may be collected at a patient’s face-to-face appointment with a healthcare professional, from administrative processes pertaining to the booking of the appointment, from laboratory results arising from tests requested at the appointment, for insurance information, or diagnostic coding for costing purposes [ 10 ]. Increasingly, health data are being recorded in an electronic manner, making it easier to store and access for research purposes.
Whilst the traditional hierarchy of evidence holds the randomised controlled trial (RCT) in highest regard, the use of routinely collected data to both supplement RCTs and conduct research outside of clinical trials is growing [ 8 ]. The 2018 scoping review for an extension to the Consolidated Standards of Reporting Trials (CONSORT) guidelines acknowledges the difficulties and limitations of RCTs and proposes that routinely collected data can be used to help address challenges such as cost, ‘limited real-world generalisability’ and recruiting representative samples to trials [ 11 ]. In addition, the use of routinely collected data to conduct stand-alone research is also being advocated. For example in their 2017 article Casey et al. explore in depth the advantages and disadvantages of using data obtained from the Electronic Health Record (EHR), a key source of routinely collected data, in population health research [ 12 ]. They conclude that research using EHRs has many advantages such as low cost, large sample sizes and the ability to link to other records, enabling, for example, the incorporation of social, behavioural and environmental data.
This review aims to explore how routinely collected patient data are currently being used in neurology research outside of clinical trials. We will take a broad view of the field in order to understand themes relating to study purpose, statistical methodology, and geographical location of the research. By understanding how routinely collected data are currently being used in neurology research this study intends to identify areas in which these data can be used to enhance future research.
Search strategy and selection criteria
Searches were carried out in eight online databases which span the topics of health, statistics, computing and general science. No restrictions were placed on the language of the research. All eight databases were searched between the 13th and 18th December 2018. No restriction was placed on the date of the publications to be retrieved; thus, the search was designed to retrieve all available studies published before December 2018. However, it is worth noting that the searches make use of the term Electronic Health Record (EHR) and as EHRs did not come into widespread use until the twenty-first century, the majority of studies retrieved were published after the year 2000. Searches were not restricted to full journal articles, allowing abstracts to be retrieved. Details of the search strategy and the databases searched can be found in the supplementary materials [Additional file 1 ].
In order to gain a large enough number of studies for analysis the searches were not limited by geographical location. However, this study concerned itself particularly with neurology research in the UK and so the search terms for the ‘neurology’ concept were developed using previous research carried out in the UK [ 13 ]. Specifically, neither stroke nor dementia were used as individual search terms as neither of these conditions are routinely seen in general neurology clinics in the UK, but rather, for the most part, in their own speciality settings [ 14 ].
Data collection
Once the searches had been completed, 10 % of the retrieved papers were screened against draft eligibility criteria. This subset of the papers was then examined to refine the criteria. Following this initial screen the following eligibility criteria were defined.
Papers were included in this review if:
Neurology or a neurological condition was the main focus of the study (excluding stroke and dementia).
The study used only routinely collected data. This includes hospital records, primary care records, health insurance databases, and dispensary data.
Papers were excluded if:
The primary focus was stroke or dementia.
Any extra data were collected for the study. For example, patient questionnaires, focus groups or tests ordered specifically for the research.
They were a systematic review or qualitative study.
The population included individuals under 16 years of age.
These eligibility criteria were then applied to the whole set of retrieved papers. To reduce the impact of human error, 20% of the papers were audited by Emsley and Knight, ensuring consistent application of the criteria.
A data extraction form was used to extract relevant data from all eligible studies. See the supplementary materials [Additional file 1 ] for a table showing the data items extracted and used in the analysis.
The information required for the data extraction was taken from the study titles and abstracts. The full text of a paper was only retrieved if the necessary information could not be found in the abstract. Where possible, the variables were recorded verbatim as found in the paper. However, the information in the papers regarding study objective was not always explicitly clear so this was categorised whilst extracting the data. If the geographical location of the study was not explicitly mentioned in the paper then the country of the lead author’s first listed institution was taken as a proxy.
Data analysis
Variables relating to neurological condition and statistical methodology were categorised, allowing for coherent analysis. The nine diagnosis categories used to analyse the data regarding the neurological condition(s) that formed the focus of the papers were defined using previous research and clinical expertise [ 13 ]. The statistical methodologies were categorised based on descriptive information contained within the individual articles combined with formal definitions of various statistical methodologies. Definitions for both the diagnosis and statistical categories can be found in the supplementary materials [Additional file 1 ].
We retrieved 4481 papers from our database searches and five further papers by searching citations by hand. Once duplicates had been removed, 3075 papers remained for screening. The eligibility criteria were applied to these 3075 papers and 386 papers were deemed eligible for this study. Of these 386 papers, 207 were full research articles and 179 were abstracts only. This selection process can be seen in Fig. 1 .
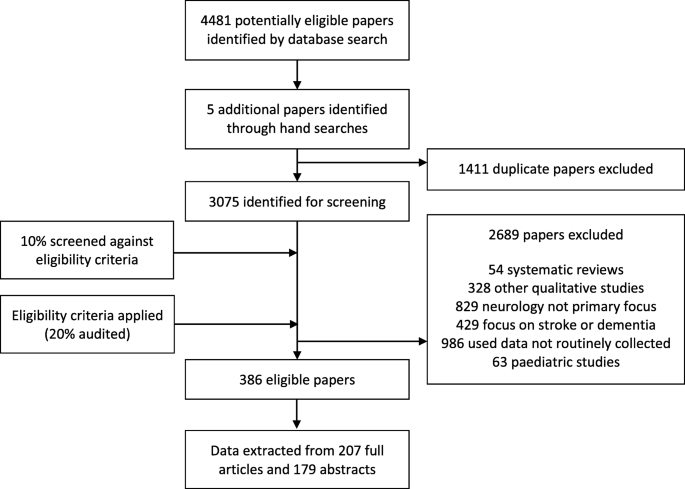
Flow chart showing study selection procedure
We compared the number of papers retrieved by our search in PubMed to an equivalent search on all medical papers. Overall, there are relatively few papers using EHRs and routinely collected data until around the year 2000, since when the number of papers has increased steadily. The earliest neurology specific paper was published in 1991, and the numbers follow the same general upward trend (see supplementary materials [Additional file 1 ] Table vi ). Figure 2 shows that as a percentage of all medical papers referencing EHRs and routine data, neurology accounted for between 0 to 3.3% until 2012, apart from in 1991 when the single neurology paper published accounts for 4.8% of all papers. Since 2012 this percentage has been steadily increasing to reach 8.1% in 2017 and 2018.
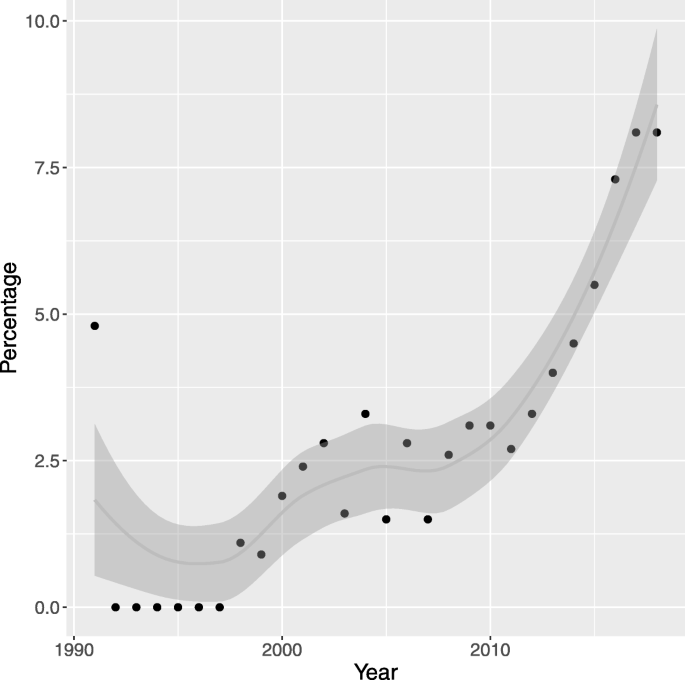
Neurology studies as a percentage of all medical studies. A graph of neurology papers as a percentage of all papers relating to the use of EHRs and Routinely Collected Data each year retrieved from a PubMed search
An overview of the characteristics of the included papers can be seen in Table 1 . They have been split into two separate columns – one for full articles and one abstracts only. This distinction has been made as many abstracts become, or contribute in a large part, to future full articles and it is not always possible to identify when this has occurred.
Most of the papers, both full articles and abstracts, focus on a single type of neurological condition, with only four articles and seven abstracts referring to studies analysing data from multiple conditions. The most frequently studied condition in this analysis is multiple sclerosis (MS), followed by epilepsy/seizure and Parkinson’s disease (PD), which can be clearly seen in Fig. 3 a. When comparing this to the global burden of neurological disorders we see that the frequency of the conditions studied does not reflect the burden of those conditions in the population [ 2 ]. Setting aside stroke and dementia (as they were specifically excluded from this study for reasons previously explained in the methods section) the top three neurological conditions ranked by age-standardised DALY (Disability-adjusted Life Years) rates in both Western Europe and North America are: migraine, spinal cord injury, and brain and central nervous system cancer.
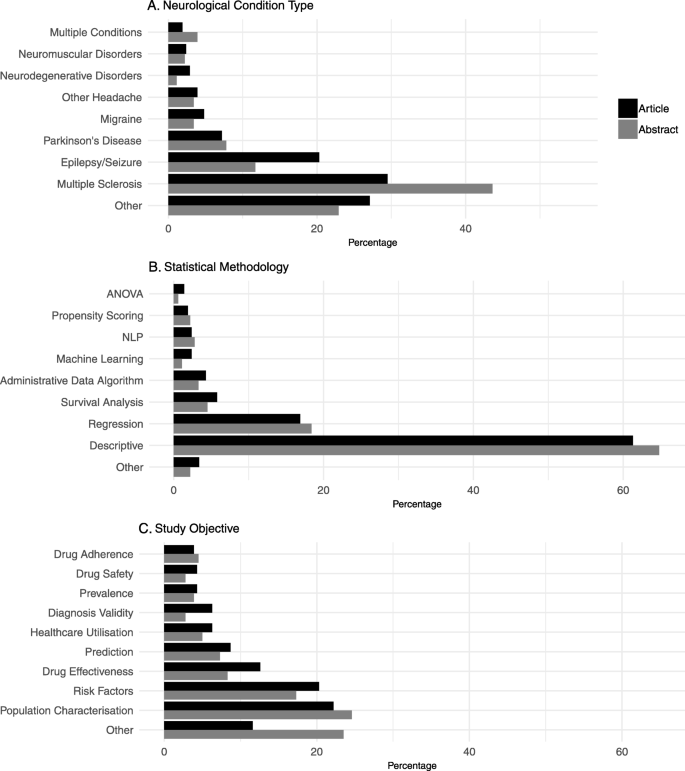
Visualisation of study characteristics. A breakdown of 3 of the variables extracted from each study. Panel a shows the percentage of studies focused on each neurological condition, b shows the percentage of studies using different statistical methodologies and c shows study objectives
In this review MS is the most frequently studied condition, yet globally it ranks only 14th out of 15 neurological disorders for DALY rates. In contrast, migraine is the neurological disorder with the highest ranking of DALYs globally (after stroke) and yet in this study we see only 4·8% of the full articles and 3·4% of the abstracts focus on this condition. This may reflect a number of issues such as the perception of the validity of research into a condition within the research community, the ease with which the condition can be studied, the availability of data and the availability of funding.
There are 11 papers which analyse many different neurological conditions together and are categorised as ‘multiple conditions’, four full articles and seven abstracts. Six of these papers cover a wide range of neurological conditions, however five of them focus on subsets of neurological conditions such as those treated with immunoglobulins [ 15 , 16 ], neurologic emergencies [ 17 , 18 ], and neuro-ophthalmology [ 19 ].
Figure 3 b shows that the majority of the papers (61·3% of the full articles and 64·8% of the abstracts) exclusively used descriptive statistics in their analysis. This includes means, proportions and statistical tests such as t-tests used to test hypotheses on single variables. Of those papers that moved beyond descriptive statistics the most common type of statistical modelling used is regression modelling (16·9% of full articles and 18·4% of the abstracts). The benefit of using these forms of modelling over hypothesis testing on descriptive statistics is that the effect of many variables can be taken into account at once.
A small number of papers used methods which build on similar foundations to regression modelling including survival analysis (12 articles and eight abstracts) and propensity scoring (four articles and four abstracts). Other papers used a completely different approach to analysis using algorithmic methods. A small number of papers (nine articles and six abstracts) were dedicated to developing administrative data algorithms. Typical of these papers is Ho C et al. who used a set of rules applied to data stored in a discharge database to identify patients with non-traumatic spinal cord dysfunction [ 20 ].
There were relatively few papers using computationally intensive methods such as Natural Language Processing (NLP) (five articles and five abstracts) and machine learning (ML) (five articles and two abstracts). However, those few papers which have taken advantage of the large body of ‘Big Data’ available in routinely collected health records have used some innovative techniques. For example Chase et al. used NLP with a naïve bayes classifier to identify patients with MS from the EHR, demonstrating how analysing large amounts of routinely collected data could lead to early diagnosis of a neurological illness [ 21 ].
The majority of the studies used hospital data in their research (44% of full papers and 37% of abstracts), with the use of claims data second most common but more prevalent in abstracts (25%) than full papers (11%). Data from specialist clinics accounts for 14.5% of the full papers and 15.6% of abstracts. In addition, Tables vii and viii in the supplementary materials [Additional file 1 ] give a more detailed breakdown of the data type used for each condition (Table vii ), and the types statistical analysis used for each data type (Table viii ). From Table vii we see that for studies focusing on Multiple Sclerosis claims data was most commonly used (36% of studies), however conclusions are hard to draw regarding other conditions due to sample sizes. Table viii shows that descriptive analyses are the most common across all data types, however the distribution of statistical analyses used does vary across different data types.
The studies included in this review had a number of different study objectives, as can be seen in Fig. 3 c. The most common objectives for both full articles and abstracts are ‘Characterisation of a clinical population’ and ‘Risk factors’. Characterising a clinical population refers to those types of study which seek to describe a group, or groups, of patients. For example in their 2016 paper Kestenbaum M et al. describe the characteristics of patients with either Parkinson’s disease or essential tremor who underwent deep brain stimulation [ 22 ]. In contrast, the studies regarding risk factors focus more on the factors leading to a disease or outcome, for example Modi SY et.al. published a paper examining the predictors of long hospital stays in status migrainosus [ 23 ].
Other common study objectives include research on drug effectiveness, safety and adherence. Taken together these types of study account for 20·8% of the full articles and 15·6% of the abstracts. The most common condition investigated by these types of study is MS, with two thirds of all the drug studies dedicated to this condition.
The vast majority of included studies were based in the USA (54·1% of full articles and 70·9% of the abstracts), 26·1% of the full articles were from Europe and 19·8% from the rest of the world. Of all the European papers eligible for inclusion in this study 29 were UK based, 14 of which were abstracts and 15 full articles. All of the UK based research focused on single types of neurological condition with epilepsy/seizure being the most commonly researched (seven papers), followed by Parkinson’s disease (four papers) and MS (four papers) showing a broadly similar trend to that seen in the whole body of eligible studies. However, we did not find a large enough number of UK based studies to do a full mapping review on this subset of research.
This study synthesises and summarises neurological research that has been carried out using routinely collected data, that is, data which were not initially collected for research purposes, but for reasons such as diagnosis, treatment or administration.
The results show that routinely collected patient data has been used for a number of different purposes in neurology research. Primarily, the data has been used to study single neurological conditions in isolation. Within these papers we found a variety of study objectives, the most common of which relate to the characterisation of a population, risk factors for an outcome and drug safety, adherence and effectiveness outside of clinical trials. Whilst these conditions are well researched, this study highlights the fact that there are potentially areas of neurology which remain under-researched in comparison.
There is an imbalance between the numbers of papers found for particular types of conditions, and the impact of those conditions (measured in DALYs) according to the global burden of neurological disease [ 3 ]. This indicates that there may be an opportunity for high impact research to take place into conditions that have a very real effect on healthcare systems, on society, and on individual patient’s lives. Previous research has highlighted the fact that there is an imbalance between the amount of research conducted and the rarity of a condition, with rare neurological conditions receiving disproportionately more attention than common ones [ 24 , 25 ]. Bishop proposes that the reason that rare conditions receive more research focus is because of their severity [ 24 ], and Al-Shahi et al. propose that the amount of research conducted should be proportional to the burden of the disease in society [ 25 ]. A high rate of DALYs indicates the potential economic and social cost of common conditions such as migraine. Research into these less well-studied areas using routinely collected data could contribute to reducing the burden of disease and consequently the economic and social cost.
The statistical methodologies used in the papers included in this study range from descriptive statistics to more complex analyses based on Machine Learning techniques. Machine Learning techniques generally require large amounts of data from which to ‘learn’ a mathematical model which can then be applied to an unseen set of data to predict or classify future results. As the amount of routinely collected data grows, this is an area in which future neurology research could have an impact – for example by using Machine Learning to find previously unknown associations, or for phenotyping diseases [ 26 ]. However, the use of complex algorithms and computationally intensive methods relies on having the right kind of question as well as suitable data. This study shows that there are differences in statistical analyses used on different types of data (see table viii in Supplementary Materials [Additional file 1 ]). For example, the relatively high number of regression analyses undertaken on claims data may occur because claims data is often highly numerical and abundant, and therefore lends itself to this type of analysis. In addition, data from hospital records can be highly complex and include pages of written notes, and so we see that analyses using Machine Learning and NLP are used in these types of data. It is worth noting that not all types of data lend themselves to complex analyses, and statistical analyses should only be as complex as is required to answer the question at hand.
As expected, this review did not identify many studies using routinely collected data to investigate neurology services managing multiple conditions, such as outpatient clinics. Rather, this review clearly shows that the majority of research relates to single conditions or condition types such as epilepsy and MS. We found only 11 studies which included multiple conditions, and of those, only four were studies into the provision of services. Many neurology clinics provide treatment and care to patients with a wide range of conditions and as such, research relating to these services should incorporate all of those conditions [ 14 ]. There is a real opportunity here for research to be conducted using routinely collected data which could be used in many different ways to support the efficient delivery of services. For example, in other disciplines, routinely collected data have been used to examine waiting times for appointment and explore patient visit patterns [ 27 , 28 ].
Limitations
Systematic mapping reviews, like all systematic reviews have some underlying limitations, which include reporting and selection biases and inaccuracies in data extraction. Particular to mapping reviews is the issue of oversimplification – because a mapping review is designed to give a broad overview of an area, it can mask underlying variations in the included studies [ 29 ]. In this study we have sought to limit the impact of reporting bias (the tendency for research with positive studies more likely to be published) by searching for and including papers that have been published as abstracts. This ensures that research in emerging areas is included, as well as studies that have perhaps not yet merited full publication.
Selection bias was limited by defining strict eligibility criteria before the papers were screened for inclusion. The application of the eligibility criteria to the list of potential papers was also quality assured in 20% of the papers to ensure that the criteria were applied consistently.
Other limitations include inaccuracy in data extraction and classification, which is inevitable when using categories to define study characteristics, however we have been consistent throughout the study and the definitions used for the categories can be found in the supplementary materials.
Applying the results of this review across different geographical areas should be done with caution. The majority of the studies in this review were conducted in the USA and Western Europe where neurology services and policies may differ significantly from other areas with different healthcare structures and populations. Even within Western Europe there are many differences in the way in which services are delivered and the data recorded [ 30 ]. Future studies should endeavour to relate the findings of this review to their own context, and as more neurology research emerges in different countries and contexts, the gaps in research in individual areas will become clearer. In addition, applying conclusions drawn from the location of the studies should take into account the fact that study location was not always explicit. In these cases, study location was taken to be the location of the lead author’s main institution.
The main strength of this review is that research on neurological conditions using routinely collected data has not been reviewed in this way before. This study allows us to see what work is already being done, and where future research could have an impact. As with all systematic reviews the methodology of this study has been well documented such that it could be repeated and the results replicated in the future.
There is a large body of research within neurology that exclusively uses routinely collected data, including data from electronic health records, public health records, and primary care data as well as administrative data such as medical insurance claims. This research covers a wide range of conditions, outcomes and study objectives. We have discovered an underrepresentation of studies into common conditions. It is also clear from this study that there are few studies which include multiple conditions in the same research, or which study neurology services as a whole. Future research using routinely collected data could make a large impact by considering the more common but less well-researched conditions or by considering how services could be improved by utilising data from many conditions.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
Consolidated Standards of Reporting Trials
Disability Adjusted Life Years
Electronic Health Record
Machine Learning
Multiple Sclerosis
National Audit Office
Natural Language Processing
Parkinson’s Disease
World Health Organisation. Neurological disorders: public health challenges. Switzerland: World Health Organisation; 2006.
Google Scholar
Feigin VL, Nichols E, Alam T, Bannick MS, Beghi E, Blake N, et al. Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2019;18(5):459–80.
Article Google Scholar
Freeman WD, Vatz KA, Griggs RC, Pedley T. Clinical implications for neurology. Neurology. 2013;81:479–86.
Morrish PK. Inadequate neurology services undermine patient care in the UK. BMJ. 2015;350:1–2.
Morrish P. What is happening to English neurology: an update. Clin Med. 2013;11(1):101–2.
National Audit Office. Services for people with neurological conditions. 2011.
Smith R, Rennie D. Evidence-based medicine - an oral history. JAMA. 2014;311(4):365–7.
Article CAS Google Scholar
Zuiderent-Jerak T, Forland F, Macbeth F. Guidelines should reflect all knowledge, not just clinical trials. BMJ. 2012;345(oct054):e6702.
Sackett DL, Rosenberg WMC, Gray JAM, Haynes RB, Richardson WS. Evidence based medicine: what it is and what it isnt. BMJ. 1996;312(7023):71–2.
Nie X, Peng X. The reporting of studies conducted using observational routinely-collected health data (RECORD) statement. Chin J Evid Based Med. 2017;17(4):475–87.
Kwakkenbos L, Imran M, McCord KA, Sampson M, Fröbert O, Gale C, et al. Protocol for a scoping review to support development of a CONSORT extension for randomised controlled trials using cohorts and routinely collected health data. BMJ Open. 2018;8(8):1–5.
Casey JA, Schwartz B, Stewart W, Adler NE. Using electronic health Records for Population Health Research: a review of methods and applications. Annu Rev Public Health. 2016;37:61–81.
Stone J, Carson A, Duncan R, Roberts R, Warlow C, Hibberd C, et al. Who is referred to neurology clinics? - the diagnoses made in 3781 new patients. Clin Neurol Neurosurg. 2010;112(9):747–51.
Bateman D. The future of neurology services in the UK. Pract Neurol. 2011;11(3):134–5.
Tankersley A. Tolerability of intravenously administered immune globulin in the home setting. Value Health. 2009;12(3):A132.
Sommer C, Brunnert M, Fricke S, Wallenstein L, Baehr M, Langebrake C. Evaluation of pharmacoeconomic interventions in neurological patients treated with immunoglobulins. Eur J Hosp Pharm Sci Pract. 2014;21(Suppl 1):A55 LP–A56.
Singh V, Darger B, Madhok D, Diaz M, Wybourn C. Prevalence of neurologic emergencies in patients presenting as trauma activations to an urban level I trauma center. Neurocrit Care. 2017;27(2 Supplement 1):S292.
Diaz M, Darger B, Wybourn C, Singh V, Madhok D. Neurologic emergencies presenting as trauma activations to a level i trauma center. Acad Emerg Med. 2018;25(Supplement 1):S159.
Pula J, Shiloach M, Heunisch C. Medication Reconciliation in a Neuro-Ophthalmology Clinic (P1.360). Neurology. 2016;86(16 Supplement):P1.360.
Ho C, Guilcher SJT, McKenzie N, Mouneimne M, Williams A, Voth J, et al. Validation of algorithm to identify persons with non-traumatic spinal cord dysfunction in Canada using administrative health data. Top Spinal Cord Inj Rehabil. 2017;23(4):333–42.
Chase HS, Mitrani LR, Lu GG, Fulgieri DJ. Early recognition of multiple sclerosis using natural language processing of the electronic health record. BMC Med Inform Decis Mak. 2017;17(1):24.
Kestenbaum M, Robakis D, Ford B, Alcalay RN, Louis ED. Clinical characteristics of patients with parkinson’s disease and essential tremor undergoing deep brain stimulation surgery at Columbia university medical center, 2009–2014. Isr Med Assoc J. 2016;18(7):386–90.
PubMed Google Scholar
Modi SY, Dharaiya D, Katramados AM, Mitsias P. Predictors of prolonged hospital stay in status Migrainosus. Neurohospitalist. 2016;6(4):141–6.
Bishop DVM. Which neurodevelopmental disorders get researched and why? PLoS One. 2010;5(11):e15112.
Al-Shahi R, Will RG, Warlow CP. Amount of research interest in rare and common neurological conditions: Bibliometric study. Br Med J. 2001;323(7327):1461–2.
Obermeyer Z, Emanuel EJ. Predicting the future-big data, machine learning, and clinical medicine HHS public access. N Engl J Med. 2016;375(13):1216–9.
Siciliani L, Moran V, Borowitz M. Measuring and comparing health care waiting times in OECD countries. Health Policy. 2014;118(3):292–303.
Ou-Yang C, Wulandari CP, Hariadi RAR, Wang H-C, Chen C. Applying sequential pattern mining to investigate cerebrovascular health outpatients’ re-visit patterns. PeerJ. 2018;6:e5183.
Grant MJ, Booth A. A typology of reviews: an analysis of 14 review types and associated methodologies. Health Inf Libr J. 2009;26(2):91–108.
Grisold W, Galvin R, Lisnic V, Lopes Lima J, Mueller E, Oberndorfer S, et al. One Europe, one neurologist? Eur J Neurol. 2007;14(3):241–7.
Download references
Acknowledgements
Not applicable.
Fran Biggin is funded by an EPSRC doctoral training partnership grant number EP/R513076/1. The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Author information
Authors and affiliations.
Lancaster University Faculty of Health and Medicine, Furness College, Lancaster University, Bailrigg, Lancaster, LA1 4YG, England
Fran Biggin, Hedley C. A. Emsley & Jo Knight
Lancashire Hospitals NHS Foundation Trust, Department of Neurology, Royal Preston Hospital, Sharoe Green Lane, Fulwood, Preston, PR2 9HT, England
Hedley C. A. Emsley
You can also search for this author in PubMed Google Scholar
Contributions
FB, HE and JK conceived and designed the study. FB carried out the literature searches, extracted and analysed the data, JK and HE audited data extraction. All authors interpreted the data. FB wrote the manuscript and HE and JK revised it. All authors approved the final manuscript.
Corresponding author
Correspondence to Hedley C. A. Emsley .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:.
Detailed Search Strategy; Data Items Extracted; Definitions; and Supplementary Results Tables.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions

About this article
Cite this article.
Biggin, F., Emsley, H.C.A. & Knight, J. Routinely collected patient data in neurology research: a systematic mapping review. BMC Neurol 20 , 431 (2020). https://doi.org/10.1186/s12883-020-01993-w
Download citation
Received : 24 January 2020
Accepted : 09 November 2020
Published : 27 November 2020
DOI : https://doi.org/10.1186/s12883-020-01993-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Routinely collected data
- Electronic health record
BMC Neurology
ISSN: 1471-2377
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts

The Neuroscience of Goals and Behavior Change
Elliot t. berkman.
Department of Psychology, Center for Translational Neuroscience, University of Oregon, and Berkman Consultants, LLC
The ways that people set, pursue, and eventually succeed or fail in accomplishing their goals are central issues for consulting psychology. Goals and behavior change have long been the subject of empirical investigation in psychology, and have been adopted with enthusiasm by the cognitive and social neurosciences in the last few decades. Though relatively new, neuroscientific discoveries have substantially furthered the scientific understanding of goals and behavior change. This article reviews the emerging brain science on goals and behavior change, with particular emphasis on its relevance to consulting psychology. I begin by articulating a framework that parses behavior change into two dimensions, one motivational (the will ) and the other cognitive (the way ). A notable feature of complex behaviors is that they typically require both. Accordingly, I review neuroscience studies on cognitive factors, such as executive function, and motivational factors, such as reward learning and self-relevance, that contribute to goal attainment. Each section concludes with a summary of the practical lessons learned from neuroscience that are relevant to consulting psychology.
Setting goals is easy; achieving them is hard. Why? This question has long stumped humanity and will certainly not be answered in this article. A full explanation of why it is hard to accomplish a goal or change old habits may never be possible. However, all hope is not lost. Research at the interface of neuroscience and psychology has made significant strides in uncovering the machinery behind goal pursuit. This knowledge, in turn, provides clues about the various ways that behavior change can go wrong and how to improve it. In this article, I present a brain-based framework for understanding how goal pursuit works and how to facilitate behavior change. Along the way, I highlight specific and practical lessons learned that are relevant to the science and practice of consulting psychology.
Goals and the Four Types of Behavior
What do I mean by goals? Colloquially, a goal is any desired outcome that wouldn’t otherwise happen without some kind of intervention. In other words, a goal is a detour from the path of least resistance. Formally, a goal is a desired future state (an end) coupled with a set of antecedent acts that promote the attainment of that end state (means; see Kruglanski, Shah, Fishbach, Friedman, Chun, & Sleeth-Keppler, 2002 for a summary). I present the informal definition first because it captures something that is missing from the formal one: a sense of what people actually mean by the word “goals” and how we use them. Technically, according to the formal definition, going out with friends to celebrate someone’s birthday is goal; it is an imagined end state and one must deploy various means to make it happen. But most people wouldn’t think of planning to go to a party later tonight as a goal. In practice, we set goals in cases where we need to do something that hasn’t happened yet and isn’t likely to happen on its own.
The difference between the two definitions of goals highlights an important aspect of goals and the way it is often overlooked. Goals are usually things we want but have difficulty achieving even when we know they are achievable. Otherwise, we wouldn’t need a goal in the first place. That sense of struggle is also captured in the term behavior change , which I use interchangeably with goal pursuit here. It’s not engaging in behavior, per se, but rather new behavior that is hard. To pursue what most people call a goal involves doing something different than what has been done before. For example, a primary incentive underlying achievement motivation (i.e., the need for achievement) is to demonstrate one’s capability to perform well on a new or challenging task ( McClelland, 1985 ).
To understand why new behavior is so hard, it’s useful to think about two dimensions that give rise to behaviors. The first dimension captures the skills, capacities, and knowledge required to engage in a behavior. This includes mapping out the steps to take and having the skill to execute an action, as well as related cognitive processes such as attentional focus, inhibitory control, and working memory capacity. Because it reflects the means used to achieve a goal, I refer to the first dimension as the way . The second dimension captures the desire for and importance of a behavior. This includes wanting to achieve a goal and prioritizing it over other goals, as well as related motivational processes such as volition, intention, and the nature and strength of the drive for achievement. Because it relates to the motivation to engage in a behavior, I refer to the second dimension as the will .
As shown in Figure 1 , these two dimensions give rise to four broad types of action. Complex-Routine behavior, in the top-left quadrant, requires some level of skill or knowledge but little motivation. Habitual behaviors reside in this quadrant: they can be quite complex yet are often triggered by external cues without motivation. For example, many drivers have piloted their car somewhere familiar, such as a child’s school, without thinking and despite an intention to go elsewhere. Indeed, a hallmark of habitual behavior is engaging in it even (or especially) in the absence of a conscious goal to do so ( Wood & Neal, 2007 ). Simple-Routine behavior, in the bottom-left quadrant, requires little skill and motivation. For example, walking, eating, and other behaviors related to primary rewards reside in this quadrant. These behaviors are so easy and effortless that we hardly think of them as goals at all. Because they are located in the same place on the horizontal axis and on different places on the vertical axis, the key difference between the first two types of behaviors is the level of skill they require. Simple-Novel behavior, in the bottom-right quadrant, requires high motivation but low skill to accomplish. Simple but new (and at times unpleasant) tasks such as changing a diaper belong in this quadrant. The most interesting kind of behavior is in the fourth quadrant: Complex-Novel behavior that requires high skill and high motivation. The goals that people care about most reside there.

Behavior can be divided into four broad categories defined by the level of motivation they demand (horizontal axis) and the level of skill or ability they require (vertical axis). Behavior change typically involves moving from left-to-right, from bottom-to-top, or both. Moving from left-to-right increases the motivational demand ( why ) of an action, whereas moving from bottom-to-top increases the skill level ( how ). It is useful to identify the vector of change required during goal pursuit and to target motivational (horizontal) and cognitive (vertical) processes as necessary.
Differences between adjacent quadrants within this space are instructive. The key distinction between a rote, unpleasant task (bottom-right) and a complex, hard one (top-right) is skill- and knowledge-oriented. Changing one diaper doesn’t take much ability, but building a machine to do the task for you would require decades of schooling. Both require high levels of motivation. The lesson is that moving up and down in this space is a matter of skill-building. In contrast, the distinction between a complex task that happens easily (top-left) and one that requires effort (top-right) is motivational. Driving to your child’s school is easy because you’ve done it so many times that it has become a matter of habit. In contrast, driving for the first time in a new country relies on the same skillset but feels much harder because it forces you to focus and apply the driving and navigation skills you already have. As you do it more it becomes easier, of course, but you can still do it on the first attempt as long as you try hard enough. Moving from left to right in this space, therefore, is a matter of effort more than one of skill or knowledge. Once a person possesses the capacity and knowledge to accomplish a difficult task, the missing piece is motivation.
Lessons learned for consulting psychology
In light of this framework, the first step to facilitating behavior change is to diagnose the source of the difficulty. Consultants and coaches can do foundational work with their clients early in the behavior change process to pinpoint the nature of the behavior change and identify how the new behavior is different from old patterns. The first step to helping a client with behavior change can involve answering these questions:
- Does the client already have the skills required for the new task?
- Is the barrier to change a lack of a way or a lack of a will?
- Is the person trying to move up, to the right, or both on the axes in Figure 1 ?
Once the most relevant dimension of change is identified, the second step is to drill down to learn more about the specific nature of the motivation or skills/capacities that will be the target. For example, consider the questions:
- If motivation, is the client lacking motivation to approach a desirable outcome or to avoid an undesirable one (e.g., Berkman & Lieberman, 2010 )?
- If motivation, is the client generally unmotivated, or highly motivated to a different goal besides than the behavior change goal?
- If skills, are they related to interpersonal abilities (e.g., empathy and perspective taking) or executive functioning (e.g., inhibition and attentional control)?
- If skills, is it possible that the client already possesses the skills but is stuck in a closed mindset and overly focused on one aspect of the behavior, such that a broadening of perspective might open new avenues for progress using other skills?
The relevant neuroscience will be quite different depending on the answer to these questions. In the following sections, I summarize the neuroscientific literatures on the will and the way with an emphasis on practical lessons for consulting psychology.
The neuroscience of the “way”: Executive function and cognitive control
Research on “the way” of goals and behavior change has mostly focused on constructs such as attention, working memory, inhibitory control, and planning – collectively known as executive function. A great deal of knowledge has been gained from neuroscientific studies about executive function, mostly about the neural systems and circuits that implement executive function (sometimes referred to as the task-positive network; Fox et al., 2005 ), and also about how disruptions to those circuits can cause alternately specific or broad impairment depending on the precise location and nature of the damage ( Alvarez & Emory, 2006 ; Stuss & Knight, 2012 ). Recent work has even begun to explore the bidirectional relationship between central and peripheral nervous system functioning in the context of goals, such as how activation of the sympathetic nervous system and hypothalamic-pituitary-adrenal axis during stress can influence executive function ( Roos et al., 2017 ). Together, imaging and lesion studies have illuminated many of the mechanistic elements and processes involved in complex goal pursuit ( Stuss, 2011 ). This information, in turn, contains some important lessons for consulting psychology about the capabilities and limits of executive function that are directly relevant to goals.
Despite substantial progress in knowledge of how executive function operate at the level of the brain, there is only sparse neuroscience research about how executive function might be improved. What little research there is suggests that executive function is more fixed than malleable by intervention, but there are some hints that targeted improvement might be possible. In this section, I review recent neuroscientific studies on executive function with respect to three questions that are pertinent to goals and behavior change: What is the nature of executive function? Is executive function a limited resource? And can executive function be improved with practice?
What is the nature of executive function?
Executive function refers to a suite of higher-level cognitive skills and capacities that generally promote successful human functioning. Attention, task switching, working memory, and inhibitory control are usually described as executive functions, though there is debate about the precise definition of the term ( Banich, 2009 ). Executive function involves some degree of updating information, shifting focus between targets or mental sets, and inhibiting irrelevant or distracting information ( Miyake, Friedman, Emerson, Witzki, Howerter, & Wager, 2000 ). Rather than enter that debate, I will describe broad features of executive function that are shared across most definitions. These features are useful for providing clarity and context for the subsequent questions regarding the limits and improvability of executive functions.
Executive function has three characteristic features: it is effortful , operates consciously , and engaged in service of novel goals as opposed to rote or overlearned ones (e.g., Miyake & Friedman, 2012). Effortful means that they feel hard and must be completed serially. In fact, emerging evidence suggests that one function of the dorsal anterior cingulate cortex (dACC; Figure 2 ), among several others, is to efficiently allocate cognitive resources by tracking the amount of mental work a task will require ( Shenhav, Cohen, & Botvinick, 2016 ). For example, activity in the dACC scales with the upcoming demand for control and also the potential payoff of that control ( Kouneiher, Charron, & Koechlin, 2009 ). It appears that the brain has dedicated regions not only to executing control but also allocating that control to various tasks.

Regions implicated in the will and the way. Left: Lateral view featuring the lateral prefrontal cortex (LPFC) and the ventrolateral prefrontal cortex (VLPFC), premotor cortex (pMC) and motor cortex (MC), and the temporalparietal junction (TPJ) and supramarginal gyrus (SMG). Top Right: Medial view featuring the dorsal anterior cingulate cortex (dACC) and ventral striatum (vS), and the dorsomedial (dmPFC), medial (mPFC) and ventromedial (vmPFC) aspects of the prefrontal cortex. Bottom Right: Coronal view featuring the ventral (vS) and dorsolateral (dlS) aspects of the striatum.
Executive function is conscious, which means that it occurs within awareness and requires conscious attention. People know when they are engaging in executive function because it becomes the center of attention in a given moment. A classic example of executive function is mental math, such as multiplying 13 by 17. In contrast to things such as breathing or adding 1+1, you know when it happens because it occupies all of your attention, and it is generally voluntary. The steps involved in solving that problem recruit a host of executive functions surrounding attention: focusing attention on the appropriate column, swapping information in and out of attention, and restricting attention to the desired part of the operation to the exclusion of others. These short-term memory and attentional processes are supported by complex interactions among lateral prefrontal and parietal cortices including aspects of all three frontal gyri, the superior frontal sulcus and precentral gyrus, and the supramarginal gyrus and temporalparietal junction ( Figure 2 ; Nee, Brown, Askren, Berman, Demiralp, Krawitz, & Jonides, 2012 ). The role of these regions is not just to maintain information, but also to disengage attention from irrelevant or previously-relevant information as appropriate to the task ( Shipstead, Harrison, & Engle, 2016 ). The importance of redirecting attention underscores the limited-capacity nature of working memory and executive function more generally. Extensive cognitive processes and neural resources are dedicated to gating which information enjoys the focus of attention and which must be ignored. In this way, executive function generally, and attention specifically, play a key role in how open or closed we are to new ideas and perspectives during goal setting and goal striving.
In addition to feeling effortful and occupying conscious attention, a third characteristic property of executive function is that it specializes in novel tasks. It enables humans to do things that we’ve never done before. In fact, the basic role of the entire prefrontal cortex has been described broadly as coordinating behavior to achieve novel goals ( Miller & Cohen, 2001 ). The ability of our prefrontal cortex to plan and execute novel behaviors is one of the defining characteristics of humans and one that sets us apart from nearly all other animals. However, this ability is not unlimited. In light of the limited capacity of attention and working memory, the prefrontal cortex has a second function that is nearly as critical: to learn to automate novel behaviors to the point that they no longer take up precious space in consciousness. Research on this process of habit formation shows that as a particular behavior in a particular behavior is repeatedly rewarded, the systems that control it shift from the dorsomedial to the ventral and dorsolateral aspects of the striatum ( Figure 2 ; Yin, Mulcare, Kilario, Clouse, Holloway, Davis, et al., 2009 ). This shift is in part supported by the differential connectivity in these parts of the striatum, with the dorsomedial more strongly connected to the prefrontal and parietal cortices (involved in attention and working memory) and the other two parts of the striatum more strongly connected to the sensory and motor cortices ( Liljeholm & O’Doherty, 2012 ). That the process of routinizing behavior has a robust pathway embedded within some of the oldest structures in the brain speaks to the evolutionary importance of offloading effortful mental activities from the cortex as early and efficiently as possible. Thus, these regions are key for habit formation.
Is executive function a limited resource?
The answer to this question is both yes and no. Many readers will be familiar with the concept of ego depletion, or the idea that the “active self” that implements executive functions draws upon a finite resource that exhausts over time with repeated use, not unlike a fuel tank ( Baumeister, Bratlavsky, Muraven, & Tice, 1998 ). Though there are literally hundreds of published studies showing the effect ( Hagger, Wood, Stiff, & Chatzisarants, 2010 ), it is likely that many of those studies are false positives or unreliable ( Hagger, Chatzisarantis, Alberts, Anggono, Batailler, Birt, et al., 2016 ). A large, highly powered, preregistered study recently failed to replicate the ego depletion effect ( Lurquin, Michaelson, Barker, Gustavson, von Bastian, Carruth, et al., 2016 ), and a meta-analysis uncovered evidence of publication bias in the ego depletion field such that studies finding the effect are much more likely to appear in print than those that do not ( Carter & McCullough, 2014 ).
On a deeper level, there is strong counter-evidence to the basic ego depletion effect, for example that taking a short break, watching a fun film clip, or even smoking a cigarette can reverse the effect (see Inzlicht & Berkman, 2015 for a summary). Active-self processes such as executive function are unlikely to draw upon a limited physiological resource if simple psychological manipulations can replenish it. Even more suggestive, there is strong physiological evidence that the neuronal processes involved in executive function demand no more energy than simpler functions or even than the brain at rest (see Kurzban, 2010 , for a review). There is simply no special physiological resource for executive function to deplete. The bottom line is that people get tired when they work hard – which is nothing new – but that, contrary to popular belief about ego depletion, that sense of fatigue is mostly psychological and can be short circuited by a short rest and a variety of positive experiences.
But what about the experience of depletion? Everyone has the intuition that some mental activities – certainly including executive function – feel hard and seem to drain our energy. The answer may be found by adjusting our understanding what exactly the limited resource is. The original formulation of ego depletion hypothesized a physiological resource, likely centered in the brain. That prediction is no longer tenable given the data. Newer models focus on the contributions of psychological and motivational factors to depletion instead beyond strictly physiological ones. For example, a shift in priorities from effortful, obligation-based, and prevention-focused “have-to” goals to enjoyable, desire-based, promotion-focused “want-to” goals could explain the decline in performance on tough cognitive tasks ( Inzlicht, Schmeichel, & Macrae, 2014 ); perhaps the “resource” is prioritization. Another possibility is that depletion results from an interaction between psychological processes, such as perceptions of upcoming task demands and available resources, and physiological factors including the peripheral nervous system, hormones, and afferent inputs ( Evans, Boggero, & Segerstrom, 2016 ).
A psychological model that fits particularly well with the characterization of executive function above focuses on its opportunity cost ( Kurzban, Duckworth, Kable, & Myers, 2013 ). Because we can only focus our executive function capacity on one task at a time, then any time we engage in one executive function task we are likely forgoing others. The cost of what we’re giving up is reflected in the sense of effort that comes along with executive function. The feeling of depletion, therefore, reflects the tipping point when the cost of putting off alternative tasks begins to outweigh the benefit of continuing on the current course of action ( Berkman, Kahn, & Livingston, 2016 ).
The evidence at this point indicates that executive function is limited in terms of bandwidth – how much can be done or stored or attended to in a given moment – but not in terms of duration in the ego depletion sense. That limit stems directly from the properties of the executive function system: the facts that only a small amount of information can be consciously accessible and operated upon in a given moment ( Unsworth, Fukuda, Awh, & Vogel, 2015 ), and that we actively track the processing costs of potential cognitive operations with respect to ongoing goals ( Westbrook & Braver, 2015 ). For precisely this reason, executive function was likened by the mathematician and philosopher Alfred North Whitehead to cavalry in an army, “Operations of thought are like cavalry charges in a battle – they are strictly limited in number, they require fresh horses, and must only be made at decisive moments.” (pp. 61; Whitehead, 1911 ).
Can executive function be improved with practice?
There is naturally great interest in the question of whether executive function can be improved, expanded, or strengthened with practice given its bandwidth limitations. Study of this kind of “brain training” is an active research area and a controversial one. Some researchers make claims about the ability to improve executive function with training ( Jaeggi, Buschkuehl, Jonides, & Shah, 2011 ), though these claims have been tempered by compelling counter-evidence ( Redick, Shipstead, Harrison, Hicks, Fried, Hambrick, et al., 2013 ). A fair characterization of the research to date is that people can certainly improve on a given executive function task with practice, but there is no evidence that practice generalizes to other, even closely related tasks, and task-specific improvements are unlikely to endure over time ( Berkman, 2016 ).
The core issue in executive function training is transfer , or whether the improvements on a training task generalize to other tasks. In some theories such as the Strength Model, on which the ego depletion hypothesis is based, executive function is a common resource that is shared across many discrete capacities (e.g., working memory and self-control), so expanding that common resource should improve a range of executive abilities ( Muraven, 2010 ). However, counter-evidence to ego depletion specifically and the Strength Model generally have raised the question about whether a common underlying resource even exists ( Inzlicht et al., 2014 ). A recent meta-analysis of studies attempting to train one form of executive function, self-control, revealed a negligible transfer effect ( Inzlicht & Berkman, 2015 ). Additionally, at least two highly-powered studies have failed to find generalizable training effects on executive function despite showing practice effects on the training task ( Miles, Sheeran, Baird, Macdonald, Webb, & Harris, in press ; Redick et al., 2013 ).
What is happening? Neuroscientific investigations provide some clues. A series of training studies on inhibitory control, an executive function involving the prevention of ongoing or prepotent behavior, found that performance on an inhibitory control task improves with practice and does not transfer to other tasks. Interestingly, to the degree that performance on the training task improved, activity in the lateral prefrontal regions and dACC that is associated with successful inhibitory control shifted earlier in time, peaking in anticipation of the need for control ( Beauchamp, Kahn, & Berkman, 2016 ; Berkman, Kahn, & Merchant, 2014 ). This effect can be characterized as a reactive-to-proactive shift in the neural activation involved in inhibitory control, and is akin to gently applying a car’s brakes when a light turns yellow instead of slamming on the brakes only upon a red light.
The observed shift in brain activity from later to earlier in time fits well with the general characteristics of executive function described earlier. Inhibitory control feels hard and occupies attention, so it is beneficial to the individual to automate the operation when possible. With enough practice and exposure, the habit learning system discovers regularities in the environment that allow the need for inhibitory control to be anticipated using contextual cues. Just as the frequent association of a yellow light with a red light teaches experienced drivers to automatically move their foot to the brake when seeing a yellow, so too do participants in inhibitory control training studies learn the specific task cues that anticipate the need for control. This cue-learning effect in training occurs automatically ( Lenartowicz, Verbruggen, Logan, & Poldrack, 2011 ), suggesting that performance improvements during inhibitory control training studies are a result of the transfer of at least some effortful behavior to the habit system. Habits increase efficiency during goal striving.
This habit learning process also explains the lack of transfer to new tasks. The advantages of executive function are mirrored in the limitations of the habit learning system. Specifically, while executive function evolved to deal with novel challenges, habit learning evolved for routine ones. Habits create efficiency by shrinking the range of responses in a situation down to one behavior. By function, they forestall new and creative behaviors in that situation. Habitual behaviors are triggered by specific contextual cues, which is why habits do not require vigilant and costly monitoring; that work is offloaded to more efficient stimulus-response mappings. The tradeoff is that habitual behaviors are necessarily tied to a particular context. If the cues that had been associated with a response change, then the habitual response will no longer emerge. For example, the ease of slowing on a yellow would be lost if the cue that preceded a red light suddenly became blue instead. In the case of executive function, training doesn’t transfer to new contexts (or tasks) because the cues are different. The brain treats the tests of transfer as novel tasks, which is exactly what executive function evolved to deal with in the first place.
Lessons learned from neuroscience about “the way”
The neuroscience literature on executive function offers some practical if not entirely hopeful advice about the “way” of behavior change. The first lesson is that executive function feels hard for a reason. It is a serial process, so the sense of effort that accompanies executive function is a signal that working on a difficult task necessarily means losing out on other opportunities. In other words, effort reflects an opportunity cost. In this view, effort also signals one’s internal priorities; the more important the alternatives are, the harder a focal task will feel. The inverse is also true: a given task will feel relatively easy when it is more important to a person than the alternative choices. Consultants and coaches can work with clients to reflect on their priorities and make them explicit, which can explain why some goals feel harder than others.
The mental processes related to the “way” operate sequentially, not in parallel. Executive functions can only be performed one at a time, so the most important ones should come first even if executive processing will not exhaust over time with use. Based on the portrait of executive function drawn here, the factors that influence the capacity for executive function most directly are other concurrent cognitive operations and the relative importance of the task compared to other possibilities. Together, this suggests that it is optimal to carve out dedicated, distraction-free time to work on important novel tasks and challenges ( Berkman & Rock, 2014 ). Our cognitive bandwidth is precious and operates most efficiently in (mental) solitude. Licensing clients to reserve work time specifically for new tasks can help.
Our executive function abilities evolved to help us deal with novel challenges. So, the precious resource of executive function should be brought to bear on any and all aspects of behavior change, such as goal setting, that benefit from openness to new ideas, broadened attention, and a wide survey of possibilities. In contrast, habit formation evolved to create efficiency by rigidly attaching one behavior to one cue. Habits can be formed to aid in other aspects of behavior change, such as goal striving, that benefit from a narrower focus and relatively consistent, fixed behaviors in a given situation.
Finally, there is not much evidence that executive function can be improved broadly by focused interventions (e.g., Lumosity; Redick et al., 2013 ; Shute, Ventura, & Ke, 2015 ), and some compelling counter-evidence. However, complex mental operations can become routinized by leveraging the habit learning system ( Foerde, Knowlton, & Poldrack, 2006 ). Habit learning is facilitated when the new behavior is consistently preceded by specific cues and then rewarded. This procedure can be particularly useful for behavior change if the new behavior will occur repeatedly in similar contexts. Research is underway to test whether a highly variable set of cues used in training can broaden the range of contexts to which training effects generalize. Nonetheless, some executive functions such as working memory may simply be fixed capacities for neuroarchitectural reasons ( Zhange & Luck, 2008 ). Rather than attempting to improve executive function generally, consultants and coaches should help their clients focus on improving specifically the skillsets relevant to the goal or new behavior. These will improve with practice and, with some proper motivation, become habitual in time.
The neuroscience of the “will”: Motivation, Reward, and Subjective Value
The question of what motivates behavior, in a general sense, runs at least back to the Greeks, with Plato’s famous analogy of the charioteer and his horses, through William James and Abraham Maslow, and continues to this day. In contrast, the question of what motivates behavior change has received considerably less attention. Psychologists have developed taxonomies of different “stages of change” to capture individual variability in readiness to engage in sustained behavior change (Transtheoretical Model; Prochaska, DiClemente, & Norcross, 1992 ), and of different types of behaviors within a person to capture relatively self-motivated, “intrinsic” versus more externally-motivated, “extrinsic” types of goals (Self-Determination Theory; Deci & Ryan, 2000 ). Much of this work is descriptive rather than prescriptive – it says what motivation is but does not indicate how to increase it. A person can be confidently described as in the precontemplation stage, but there is not much evidence-backed knowledge about moving him or her to the contemplation stage; likewise, some behaviors are clearly extrinsically motivated, though there is a lack of prescriptive advice about how one can transform them into intrinsically motivated ones.
As it did with studies on the “way,” neuroimaging research provides some clues about how to increase motivation to change a specific behavior. In this section, I review neuroscientific insights into the “way” of behavior change surrounding three questions that are relevant to consulting psychology. Which brain systems are involved in motivational processes? How do those systems interact with other networks in the brain? And what does neuroscience indicate about motivating behavior change?
How and where is motivation represented in the brain?
Motivation is conceptualized here as the strength of the desire to attain a particular outcome, irrespective of how pleasant or unpleasant the experience of actually attaining it is. This distinction between the motivational component of a reward – “wanting” – and the hedonic component of consuming it – “liking” – is maintained with remarkable evolutionary consistency in the brains of both humans and animals ( Berridge & Robinson, 2003 ). I focus here on the “wanting” side because of its direct bearing on behavior and behavior change. Wanting a reward is closely tied with activity of mesolimbic dopaminergic neurons, particularly within the ventral striatum and ventromedial prefrontal cortex ( Berridge, 2006 ; Figure 2 ), which is sometimes also called the orbitofrontal cortex ( Wallis, 2007 ). Of course, there are many other regions and interactions involved in reward learning, but I focus on these because they are the best characterized in terms of human functional neuroanatomy to date.
The dopaminergic reward system has been conserved evolutionarily because it plays a critical role in the reinforcement learning cycle. When a particular behavior in a given context it is rewarded, that behavior and context are paired and tagged with reward value for later repetition ( Rescorla & Wagner, 1972 ). Reinforcement learning is why behaviors that are rewarded are likely to be repeated in the future. (This is also why the dopamine system is implicated in addictive behavior.) The amount of cumulative, learned reward value of a behavior is its expected value, sometimes referred to as subjective value ( Rangel & Hare, 2010 ). In short, subjective value represents the amount of reward that an actor expects to receive for a given action, largely based on past learning. This learning cycle is one of the key impediments to behavior change: old behavior has been rewarded and new behavior has not. A protein called brain-derived neurotrophic factor (BDNF) is important for maintaining new behaviors after engaging in them initially because of its critical role in memory consolidation ( Bekinschtein et al., 2008 ). As described in the following sections, the key to launching this reward learning and consolidation cycle is finding ways to increase the subjective value of new behavior.
A notable feature of activity in the ventromedial prefrontal cortex is that it represents the subjective values of diverse types of actions, presumably to facilitate “apples to oranges” decisions between qualitatively different behaviors ( Levy & Glimcher, 2011 ). For example, activity in the ventromedial prefrontal cortex tracks the value of approach appetitive and avoiding aversive stimuli ( Tom, Fox, Trepel, & Poldrack, 2007 ), and also the subjective value of a range of stimulus types, including food, money, gains for the self and others, charitable decisions, and emotional and utilitarian benefits of moral actions ( Hare, Camerer, Knoepfle, O’Doherty, & Rangel, 2010 ; Hutcherson, Montaser-Kouhsari, Woodward, & Rangel, 2015 ; Lebreton, Jorge, Michel, Thirion, & Pessiglione, 2009 ; Zaki, Lopez, & Mitchell, 2014 ). These findings converge on the idea that the ventromedial prefrontal cortex plays a central role in tracking the subjective value of different kinds of actions during choice, which strongly implicates that region in motivational processing during behavior change.
How do motivation regions interact with other brain systems?
One way to approach the deeper issue of where motivation originates is to examine the connectivity of its neural systems. In the same way that it is adaptive to humans and informative to scientists that sensory and motor regions in the brain are adjacent and highly interconnected, the regions involved in motivation are themselves intertwined with several other brain networks. Those interrelations contain insights about how motivation operates and how it might be increased in the service of behavior change.
As Self-Determination Theory suggests, autonomously choosing to engage in a behavior (relative to being forced) increases performance on that behavior because autonomy is an intrinsic motive. At the neural level, autonomy also prevents a reduction in reward system activity in the face of negative feedback, particularly in the ventromedial prefrontal cortex ( Murayama, Matsumoto, Izuma, Sugiura, Ryan, Deci, et al., 2013 ). Interestingly, the ventromedial prefrontal cortex has also been found to be active in studies of self-processing and particularly of self-affirmation , such as considering one’s core personal values ( Cascio, O’Donnell, Tinney, Lieberman, Taylor, Strecher, et al., 2016 ). Brain activation related to self-affirmation during health messaging has even been shown to predict the eventual degree of health behavior change that would follow ( Falk, O’Donnell, Cascio, Tinney, Kang, Lieberman, et al., 2015 ). Finally, a meta-analysis using the Neurosynth study database ( Yarkoni, Poldrack, Nichols, Van Essen, & Wager, 2011 ) found that the ventromedial prefrontal cortex was one of the largest regions of overlap between 812 studies on identity (“self” and “self-referential” terms in the database) and 324 subjective value and reward (“value” term in the database). The meta-analysis contained several regions along the medial cortical wall including the ventromedial prefrontal cortex, the posterior cingulate cortex, and the mid-cingulate. The ventromedial prefrontal cortex was the single largest cluster to be consistently associated with both identity and value.
The overlap between intrinsic goals, core values, and subjective value has several implications for consulting psychology. First, identity (e.g., self-concept) and subjective value are closely functionally connected to one another. This is not a surprise given the extensive evidence from social psychology and other fields that people have disproportionate positive regard for themselves (and behaviors related to the self) compared to others ( Greenwald, 1980 ; Pelham & Swann, 1989 ). We want, and perhaps need, to see our selves as good ( Rosenberg, 1979 ). Second, the value derived from identity and other self-related processes may have a special status compared to other sources of value (e.g., monetary) because of the high degree of overlap in the neural systems and conceptual representation of identity and value. It may even be that identity and value are inseparable, leading one researcher to hypothesize that the defining function of the self is to organize and prioritize the world by assigning it motivational significance ( Northoff & Hayes, 2011 ). By this definition, the self-concept is exactly the set of places, things, and actions in the world that hold value.
It is important to note that the valuation process subserved by the vmPFC reflects not only positive value, but negative value as well. For example, just as social affiliation holds positive value, the threat of social rejection can be highly negative in value. The experience of social rejection invokes similar brain networks as physical pain ( Lieberman & Eisenberger, 2015 ). Beyond its unpleasantness, this experience can enhance defensiveness and facilitate a stress response that detracts from other ongoing goals because it narrows attentional focus on the social threat ( Muscatell et al., 2016 ).
The ventromedial prefrontal cortex and related dopaminergic motivational structures also interact with cognitive networks, including those related to executive function ( Botvinick & Braver, 2015 ). The ventromedial prefrontal cortex appears to be a point of convergence where the motivational value of various options in a choice are integrated, notably including both effortful actions that require cognitive control and also easier, more hedonic ones ( Bartra, McGuire, & Kable, 2013 ). For example, the dorsolateral prefrontal cortex is functionally connected with the ventromedial prefrontal cortex when higher-order goals such as health concerns or social factors are made salient ( Hare et al., 2010 ; Hutcherson, Plassman, Gross, & Rangel, 2012 ). There is also evidence that the value of potential actions are reflected in the ventromedial prefrontal cortex before any specific action plans is selected ( Wunderlich, Rangel, & O’Doherty, 2010 ), but that value signals provide input to downstream brain regions that are responsible for selecting and implementing behavior ( Hare, Schultz, Camerer, O’Doherty, & Rangel, 2011 ). Taken together, then, the emergent view from the neuroscience literature is that the ventromedial prefrontal cortex receives a variety of value signals relevant to decisions about behavior, and its activation reflects a dynamic value integration process that subsequently biases behavior toward higher-valued actions. A promising route to increasing motivation, then, is identifying the value inputs to a new behavior (i.e., the reasons why the behavior is or is not valued) and learning ways to modulate them. I address this possibility in the next section.
How can motivation be increased?
The neurally-informed model described above suggests that motivation is guided by an integration of the value of features of the behavioral options. Behavior change can be accomplished by amplifying the value of the new (goal-related) behavior, reducing the value of old (goal-counter or goal-unrelated) behaviors, or some combination of the two. A clear example of the effectiveness of the first approach is contingency management treatment for substance use disorders ( Bigelow & Silverman, 1999 ), in which the value of drug abstinence is increased with monetary incentives. A meta-analysis found this approach to have an effect size d = 0.42 on treatment for alcohol, tobacco, and illicit drugs, which was larger than therapy (d = 0.25) and outpatient treatment (d = 0.37), and comparable to methadone treatment for opiate use ( Prendergast, Podus, Finney, Greenwell, & Roll, 2006 ). Similarly, “precommitting” to buy more healthy foods at the risk of losing financial incentives is more effective than having the incentives alone ( Schwartz, Mochon, Wyper, Maroba, Patel, & Ariely, 2014 ). Monetary incentives also increase persistence at exercise ( Cabanac, 1986 ), endurance on a cold-pressor task ( Baker & Kirsch, 1991 ), and performance on a difficult cognitive task ( Boksem, Meijman, & Lorist, 2006 ). Simple monetary payments are an effective way to motivate behavior change.
“Money walks,” as the saying goes, but its scarcity makes it a less than ideal option for many goal pursuit contexts. Above, I noted the deep connections between identity and motivation. Other researchers have, too, and are now beginning to deploy identity interventions to increase motivation. For example, one study leveraged the fact that most people consider willpower to be a desirable trait ( Magen & Gross, 2007 ). The participants in that study completed an executive function task twice, and in between were randomly assigned to reconstrue the task itself as a measure of their own willpower or not. Performance improved from the first to the second run only among participants whose perceptions of the task were changed from non-diagnostic to diagnostic of willpower. Similarly, noting that identity is somewhat susceptible to cognitive shifts such as framing, construal, or priming effects, other researchers used a simple “noun-verb” manipulation to increase motivation for behavior change, presumably through a subtle shift in the extent to which the new behavior is construed as identity-relevant. For example, phrasing questions about voting intentions in terms of identity (noun: “being a voter”) instead of an action (verb: “voting”) increased voting intentions and actual turnout in statewide elections ( Bryan, Walton, Rogers, & Dweck, 2011 ). In another study, participants were less likely to cheat by claiming money they were not entitled to if that behavior was described as a (negative) identity (noun: “being a cheater”) instead of an action (verb: “cheating”; Bryan, Adams, & Monin, 2013 ). Each of these results is consistent with the idea that identity can influence motivation, presumably by highlighting the subjective value of desired (e.g., “voter”, “willpower”) or undesired (e.g., “cheater”) identity. This path is a promising future direction for motivation interventions because it is low-cost, modest in scope, and easily scalable to a broad range of populations and types of desired identities.
Finally, merely highlighting certain attributes of a behavior can alter the value placed on that behavior. After all, our attentional bandwidth is fairly narrow, so not all relevant properties will be equally salient at all times. For example, people’s motivation to act on a choice option increases as attention is allocated to it ( Krajbich, Armel, & Rangel, 2010 ). In another study ( Hare et al., 2011 ), participants were presented with health-versus-taste decisions with or without reminders about health. As expected, health reminders increased the likelihood of healthy choices. Tellingly, the healthiness rating of the foods (assessed earlier, and separate from the tastiness) was strongly correlated with activity in the ventromedial prefrontal cortex at the moment of decision, which in turn predicted the food choice. In contrast, when unhealthy foods were selected, the earlier tastiness ratings were correlated with ventromedial prefrontal cortex activity during choice. The results of these studies are broadly consistent with psychological framing effects (e.g., gain vs. loss frame; Kahneman & Tversky, 1984 ), whereby altering the relative salience of the features of a decision can dramatically change it. Though they are most often applied to decision-making, the neuroscientific evidence presented here suggests that motivation may also be susceptible to framing effects.
In light of the present framework, I focused on ways to increase motivation that are grounded in valuation. But there are other ways to increase motivation from complementary lines of research that nonetheless may be connected to subjective value. For example, Higgins has argued that people experience “value from fit” when their regulatory style (promotion versus prevention focus) matches the particular means through which goals are pursued ( Higgins, Idson, Freitas, Spiegel, & Molden, 2003 ). A similar “matching” effect on motivation has been observed with achievement motivation and performance goals: people high in achievement motivation experience greater intrinsic motivation when provided with performance (vs. mastery) goals, whereas people low in achievement motivation experience greater intrinsic motivation with mastery (vs. performance) goals ( Elliot & Harackiewicz, 1994 ). A plausible cause of these kinds of “matching” effects, which can be tested in future research, is that there is subjective value in experiencing fit between one’s dispositional tendencies and the nature of the goal at hand.
Lessons learned from neuroscience about “the will”
Neuroscientific investigations of motivation have established the major brain systems for motivation and identified ways that those systems interact with other parts of the brain. This knowledge, in turn, contains clues about how motivation works and how to increase it on the psychological level. Two are particularly relevant to consulting psychology.
The first lesson surrounds the extent to which motivation is tied to the past. The neural mechanisms of reinforcement learning are some of the most basic and ancient parts of our brains. For good reason, we evolved to be highly sensitive to learn where we receive rewards and to work hard to recreate the situations that brought them about. Attempting to change behavior in a systematic way by engaging in new behaviors, which have never been reinforced, often means working against this powerful system. Thus, wise advice for clients that is grounded in the neuroscience of motivation and reinforcement learning is to start behavior change with modest goals and reward even the smallest steps toward them. New behaviors emerge slowly because they are usually working against the power of prior reinforcement. Consultants and coaches can help clients anticipate and understand the difficulty of behavior change by explaining the neuroscience of reinforcement learning. Being cognizant of the challenges of behavior change can prevent frustration on both sides.
The second lesson is to leverage the intrinsic connections between the motivation system and other parts of the brain, particularly self and identity. The elaborated web of memories, beliefs, values, objects, and relationships that comprise our sense of self is paralleled perhaps only by executive function in its distinctiveness to humans. And it may offer a pathway to behavior change and goal achievement that is just as potent. A behavior will hold greater subjective value to the degree that it is related to one’s core values and sense of self. Identity-linked goals are more likely to be successful than identity-irrelevant or identity-counter ones. Consultants and coaches can be particularly helpful to clients in this arena by helping them discover core aspects of their self-concepts and the ways those aspects are linked to the behavior change at hand. And remember that identity is not a fixed construct, but rather is susceptible to framing, reconstrual, and other kinds of subtle influences. To some extent, motivation can be gained by finding ways to think about goals that makes their connection to important parts of one’s identity salient. Sometimes it is easier for other people to make these connections than for us because they have more distance from them ( Berkman & Rock, 2014 ); coaches can be particularly helpful in this regard. Paying people works, too, but connecting goals to the self-concept in various ways may be a more sustainable and accessible approach to increasing motivation.
Pursing goals and changing behavior is hard. Neuroscience will never change that fact, but it can provide some brain-level explanations for the difficulty as well as some new insights about how to mitigate it. This article reviewed the neuroscientific literatures on the “way” of goal pursuit – the set of cognitive skills, capacities, and abilities collectively known as executive function – and the “will” – the motivational factors that propel behavior. Although parts of the “way” are limited by constraints that may be difficult to change, the “will” can be influenced by incentives both within the person and without. Though neuroscientific investigations into long-term behavior change are only just starting to emerge they have already begun to contribute to the body of practical scientific knowledge about goals. The science and practice of consulting psychology will benefit directly from this research in the coming years.
Functional neuroanatomy of key networks
Acknowledgments
This work was supported by grants AG048840, CA175241, and DA035763 from the National Institutes of Health to ETB, as well as support from the Bezos Family Foundation and the Center for the Developing Child at Harvard University.
- Alvarez JA, Emory E. Executive function and the frontal lobes: A meta-analytic review. Neuropsychology Review. 2006; 16 (1):17–42. [ PubMed ] [ Google Scholar ]
- Baker SL, Kirsch I. Cognitive mediators of pain perception and tolerance. Journal of Personality and Social Psychology. 1991; 61 (3):504–510. [ PubMed ] [ Google Scholar ]
- Banich MT. Executive function: The search for an integrated account. Current Directions in Psychological Science. 2009; 18 (2):89–94. [ Google Scholar ]
- Bartra O, McGuire JT, Kable JW. The valuation system: A coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. NeuroImage. 2013; 76 :412–427. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Baumeister RF, Bratslavsky E, Muraven M, Tice DM. Ego depletion: Is the active self a limited resource? Journal of Personality and Social Psychology. 1998; 74 (5):1252–1265. [ PubMed ] [ Google Scholar ]
- Beauchamp KG, Kahn LE, Berkman ET. Does inhibitory control training transfer?: behavioral and neural effects on an untrained emotion regulation task. Social Cognitive and Affective Neuroscience. 2016; 11 (9):1374–1382. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bekinschtein P, Cammarota M, Katche C, Slipczuk L, Rossato JI, Goldin A, et al. BDNF is essential to promote persistence of long-term memory storage. Proceedings of the National Academy of Sciences. 2008; 105 (7):2711–2716. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Berkman ET. Self-regulation training. In: Vohs KD, Baumeister RF, editors. Handbook of Self-Regulation. 3. New York: Guilford Press; 2016. pp. 440–457. [ Google Scholar ]
- Berkman ET, Rock D. AIM: An integrative model of goal pursuit. NeuroLeadership Journal. 2014; 5 :1–11. [ Google Scholar ]
- Berkman ET, Kahn LE, Livingston JL. Self-Regulation and Ego Control. New York: Elsevier; 2016. Valuation as a mechanism of self-control and ego depletion; pp. 255–279. [ Google Scholar ]
- Berkman ET, Kahn LE, Merchant JS. Training-induced changes in inhibitory control network activity. The Journal of Neuroscience. 2014; 34 (1):149–157. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Berkman ET, Lieberman MD. Using neuroscience to broaden emotion regulation: Theoretical and methodological considerations. Social and Personality Psychology Compass. 2009; 3 (4):475–493. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Berkman ET, Lieberman MD. Approaching the bad and avoiding the good: Lateral prefrontal cortical asymmetry distinguishes between action and valence. Journal of Cognitive Neuroscience. 2010; 22 (9):1970–1979. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Berridge KC. The debate over dopamine’s role in reward: The case for incentive salience. Psychopharmacology. 2006; 191 (3):391–431. [ PubMed ] [ Google Scholar ]
- Berridge KC, Robinson TE. Parsing reward. Trends in Neurosciences. 2003; 26 (9):507–513. [ PubMed ] [ Google Scholar ]
- Bigelow GE, Silverman K. Theoretical and empirical foundations of contingency management treatments for drug abuse. In: Higgins ST, Silverman K, editors. Motivating Behavior Change Among Illicit-Drug Abusers: Research on Contingency Management Interventions. Washington, DC: American Psychological Association; 1999. pp. 15–31. [ Google Scholar ]
- Boksem MAS, Meijman TF, Lorist MM. Mental fatigue, motivation and action monitoring. Biological Psychology. 2006; 72 (2):123–132. [ PubMed ] [ Google Scholar ]
- Botvinick M, Braver T. Motivation and cognitive control: From behavior to neural mechanism. Annual Review of Psychology. 2015; 66 (1):83–113. [ PubMed ] [ Google Scholar ]
- Bryan CJ, Adams GS, Monin B. When cheating would make you a cheater: Implicating the self prevents unethical behavior. Journal of Experimental Psychology: General. 2013; 142 (4):1001–1005. [ PubMed ] [ Google Scholar ]
- Bryan CJ, Walton GM, Rogers T, Dweck CS. Motivating voter turnout by invoking the self. Proceedings of the National Academy of Sciences. 2011; 108 (31):12653–12656. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Cabanac M. Money versus pain: Experimental study of a conflict in humans. Journal of the Experimental Analysis of Behavior. 1986; 46 (1):37–44. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Carter EC, McCullough ME. Publication bias and the limited strength model of self-control: Has the evidence for ego depletion been overestimated? Frontiers in Psychology. 2014; 5 (1):1–11. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Cascio CN, O’Donnell MB, Tinney FJ, Lieberman MD, Taylor SE, Strecher VJ, Falk EB. Self-affirmation activates brain systems associated with self-related processing and reward and is reinforced by future orientation. Social Cognitive and Affective Neuroscience. 2016; 11 (4):621–629. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Deci EL, Ryan RM. The “what” and “why” of goal pursuits: Human needs and the self-determination of behavior. Psychological Inquiry. 2000; 11 (4):227–268. [ Google Scholar ]
- Elliot AJ, Harackiewicz JM. Goal setting, achievement orientation, and intrinsic motivation: A mediational analysis. Journal of Personality and Social Psychology. 1994; 66 (5):968–980. [ PubMed ] [ Google Scholar ]
- Evans DR, Boggero IA, Segerstrom SC. The nature of self-regulatory fatigue and “ego depletion”: Lessons from physical fatigue. Personality and Social Psychology Review. 2016; 20 (4):291–310. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Falk EB, O’Donnell MB, Cascio CN, Tinney F, Kang Y, Lieberman MD, et al. Self-affirmation alters the brain’s response to health messages and subsequent behavior change. Proceedings of the National Academy of Sciences. 2015; 112 (7):201500247–7. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Foerde K, Knowlton BJ, Poldrack RA. Modulation of competing memory systems by distraction. Proceedings of the National Academy of Sciences. 2006; 103 (31):11778–11783. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences. 2005; 102 (27):9673–9678. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Greenwald AG. The totalitarian ego: Fabrication and revision of personal history. American Psychologist. 1980; 35 (7):603–618. [ Google Scholar ]
- Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-State Functional Connectivity Reflects Structural Connectivity in the Default Mode Network. Cerebral Cortex. 2008; 19 (1):72–78. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hagger MS, Chatzisarantis N. A multi-lab pre-registered replication of the ego-depletion effect. Perspectives on Psychological Science. 2016; 11 (4):546–573. [ PubMed ] [ Google Scholar ]
- Hagger MS, Wood C, Stiff C, Chatzisarantis NLD. Ego depletion and the strength model of self-control: A meta-analysis. Psychological Bulletin. 2010; 136 (4):495–525. [ PubMed ] [ Google Scholar ]
- Hare TA, Camerer CF, Knoepfle DT, O’Doherty JP, Rangel A. Value computations in ventral medial prefrontal cortex during charitable decision making incorporate input from regions involved in social cognition. The Journal of Neuroscience. 2010; 30 (2):583–590. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hare TA, Schultz W, Camerer CF, O’Doherty JP, Rangel A. Transformation of stimulus value signals into motor commands during simple choice. Proceedings of the National Academy of Sciences. 2011; 108 (44):18120–18125. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Higgins ET, Chen Idson L, Freitas AL, Spiegel S, Molden DC. Transfer of value from fit. Journal of Personality and Social Psychology. 2003; 84 (6):1140–1153. [ PubMed ] [ Google Scholar ]
- Hutcherson CA, Montaser-Kouhsari L, Woodward J, Rangel A. Emotional and utilitarian appraisals of moral dilemmas are encoded in separate areas and integrated in ventromedial prefrontal cortex. The Journal of Neuroscience. 2015; 35 (36):12593–12605. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hutcherson CA, Plassmann H, Gross JJ, Rangel A. Cognitive regulation during decision making shifts behavioral control between ventromedial and dorsolateral prefrontal value systems. The Journal of Neuroscience. 2012; 32 (39):13543–13554. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Inzlicht M, Berkman E. Six questions for the resource model of control (and some answers) Social and Personality Psychology Compass. 2015; 9 (10):511–524. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Inzlicht M, Schmeichel BJ, Macrae CN. Why self-control seems (but may not be) limited. Trends in Cognitive Sciences. 2014; 18 (3):127–133. [ PubMed ] [ Google Scholar ]
- Jaeggi SM, Buschkuehl M, Jonides J, Shah P. Short- and long-term benefits of cognitive training. Proceedings of the National Academy of Sciences. 2011; 108 (5):10081–10086. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kahneman D, Tversky A. Values, choices and frames. American Psychologist. 1984; 39 (4):341–350. [ Google Scholar ]
- Kouneiher F, Charron S, Koechlin E. Motivation and cognitive control in the human prefrontal cortex. Nature Neuroscience 2009 [ PubMed ] [ Google Scholar ]
- Krajbich I, Armel C, Rangel A. Visual fixations and the computation and comparison of value in simple choice. Nature Neuroscience. 2010; 13 (10):1292–1298. [ PubMed ] [ Google Scholar ]
- Kruglanski AW, Shah JY, Fishbach A, Friedman R, Chun WY, Sleeth-Keppler D. A theory of goal systems. Advances in Experimental Social Psychology. 2002; 34 (1):331–378. [ Google Scholar ]
- Kurzban R. Does the brain consume additional glucose during self-control tasks? Evolutionary Psychology. 2010; 8 (2):244–259. [ PubMed ] [ Google Scholar ]
- Kurzban R, Duckworth A, Kable JW, Myers J. An opportunity cost model of subjective effort and task performance. The Behavioral and Brain Sciences. 2013; 36 (06):661–679. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lebreton M, Jorge S, Michel V, Thirion B, Pessiglione M. An automatic valuation system in the human brain: Evidence from functional neuroimaging. Neuron. 2009; 64 (3):431–439. [ PubMed ] [ Google Scholar ]
- Lenartowicz A, Verbruggen F, Logan GD, Poldrack RA. Inhibition- related activation in the right inferior frontal gyrus in the absence of inhibitory cues. Journal of Cognitive Neuroscience. 2011; 23 (11):3388–3399. [ PubMed ] [ Google Scholar ]
- Levy DJ, Glimcher PW. Comparing apples and oranges: Using reward-specific and reward-general subjective value representation in the brain. The Journal of Neuroscience. 2011; 31 (41):14693–14707. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lieberman MD, Eisenberger NI. The dorsal anterior cingulate cortex is selective for pain: Results from large-scale reverse inference. Proceedings of the National Academy of Sciences. 2015; 112 (49):15250–15255. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Liljeholm M, O’Doherty JP. Contributions of the striatum to learning, motivation, and performance: an associative account. Trends in Cognitive Sciences. 2012; 16 (9):467–475. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lurquin JH, Michaelson LE, Barker JE, Gustavson DE, von Bastian CC, Carruth NP, Miyake A. No evidence of the ego-depletion effect across task characteristics and individual differences: A pre-registered study. PLoS ONE. 2016; 11 (2):e0147770–20. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Magen E, Gross JJ. Harnessing the need for immediate gratification: Cognitive reconstrual modulates the reward value of temptations. Emotion. 2007; 7 (2):415–428. [ PubMed ] [ Google Scholar ]
- McClelland DC. Human Motivation. Glenview, IL: Scott, Foresman and Company; 1985. [ Google Scholar ]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Structure and Function. 2010; 214 (5–6):655–667. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Miles E, Sheeran P, Baird H, Macdonald I, Webb TL, Harris PR. Does self-control improve with practice? Evidence from a six-week training program. Journal of Experimental Psychology: General. :1–18. in press. [ PubMed ] [ Google Scholar ]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001; 24 :167–202. [ PubMed ] [ Google Scholar ]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology. 2000; 41 (1):49–100. [ PubMed ] [ Google Scholar ]
- Muraven M. Building self-control strength: Practicing self-control leads to improved self-control performance. Journal of Experimental Social Psychology. 2010; 46 (2):465–468. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Murayama K, Matsumoto M, Izuma K, Sugiura A, Ryan RM, Deci EL, Matsumoto K. How self-determined choice facilitates performance: A key role of the ventromedial prefrontal cortex. Cerebral Cortex. 2013; 25 (5):1241–1251. [ PubMed ] [ Google Scholar ]
- Muscatell KA, Dedovic K, Slavich GM, Jarcho MR, Breen EC, Bower JE, et al. Neural mechanisms linking social status and inflammatory responses to social stress. Social Cognitive and Affective Neuroscience. 2016; 11 (6):915–922. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Nee DE, Brown JW, Askren MK, Berman MG, Demiralp E, Krawitz A, Jonides J. A meta-analysis of executive components of working memory. Cerebral Cortex 2012 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, Carter CS. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cognitive, Affective, and Behavioral Neuroscience. 2012; 12 (2):241–268. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Northoff G, Hayes DJ. Is our self nothing but reward? Biological Psychiatry. 2011; 69 (11):1019–1025. [ PubMed ] [ Google Scholar ]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain—A meta-analysis of imaging studies on the self. NeuroImage. 2006; 31 (1):440–457. [ PubMed ] [ Google Scholar ]
- Pelham BW, Swann WB. From self-conceptions to self-worth: On the sources and structure of global self-esteem. Journal of Personality and Social Psychology. 1989; 57 (4):672–680. [ PubMed ] [ Google Scholar ]
- Prendergast M, Podus D, Finney J, Greenwell L, Roll J. Contingency management for treatment of substance use disorders: a meta-analysis. Addiction. 2006; 101 (11):1546–1560. [ PubMed ] [ Google Scholar ]
- Prochaska JO, DiClemente CC, Norcross JC. In search of how people change: Applications to addictive behaviors. The American Psychologist. 1992; 47 (9):1102–1114. [ PubMed ] [ Google Scholar ]
- Rangel A, Hare T. Neural computations associated with goal-directed choice. Current Opinion in Neurobiology. 2010; 20 (2):262–270. [ PubMed ] [ Google Scholar ]
- Redick TS, Shipstead Z, Harrison TL, Hicks KL, Fried DE, Hambrick DZ, et al. No evidence of intelligence improvement after working memory training: A randomized, placebo-controlled study. Journal of Experimental Psychology: General. 2013; 142 (2):359–379. [ PubMed ] [ Google Scholar ]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. Classical Conditioning II Current Research and Theory. 1972; 2 :64–99. [ Google Scholar ]
- Roos LE, Knight EL, Beauchamp KG, Berkman ET, Faraday K, Hyslop K, Fisher PA. Acute stress impairs inhibitory control based on individual differences in parasympathetic nervous system activity. Biological Psychology. 2017; 125 :58–63. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rosenberg M. Conceiving the Self. New York: Basic Books; 1979. [ Google Scholar ]
- Schwartz J, Mochon D, Wyper L, Maroba J, Patel D, Ariely D. Healthier by precommitment. Psychological Science. 2014; 25 (2):538–546. [ PubMed ] [ Google Scholar ]
- Shenhav A, Cohen JD, Botvinick MM. Dorsal anterior cingulate cortex and the value of control. Nature Neuroscience. 2016; 19 (10):1286–1291. [ PubMed ] [ Google Scholar ]
- Shipstead Z, Harrison TL, Engle RW. Working memory capacity and fluid intelligence: Maintenance and disengagement. Perspectives on Psychological Science. 2016; 11 (6):771–799. [ PubMed ] [ Google Scholar ]
- Shute VJ, Ventura M, Ke F. The power of play: The effects of Portal 2 and Lumosity on cognitive and noncognitive skills. Computers & Education. 2015; 80 :58–67. [ Google Scholar ]
- Stuss DT. Functions of the frontal lobes: Relation to executive functions. Journal of the International Neuropsychological Society. 2011; 17 (05):759–765. [ PubMed ] [ Google Scholar ]
- Stuss DT, Knight RT. Principles of Frontal Lobe Function. 2. New York: Oxford University Press; 2012. [ Google Scholar ]
- Tom SM, Fox CR, Trepel C, Poldrack RA. The neural basis of loss aversion in decision-making under risk. Science. 2007; 315 (5811):515–518. [ PubMed ] [ Google Scholar ]
- Unsworth N, Fukuda K, Awh E, Vogel EK. Working memory delay activity predicts individual differences in cognitive abilities. Journal of Cognitive Neuroscience. 2015; 27 (5):853–865. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wallis JD. Orbitofrontal cortex and its contribution to decision-making. Annual Review of Neuroscience. 2007; 30 (1):31–56. [ PubMed ] [ Google Scholar ]
- Westbrook A, Braver TS. Cognitive effort: A neuroeconomic approach. Cognitive, Affective, and Behavioral Neuroscience. 2015; 15 (2):395–415. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Whitehead AN. An Introduction to Mathematics. New York: Holt; 1911. [ Google Scholar ]
- Wood W, Neal DT. A new look at habits and the habit-goal interface. Psychological Review. 2007; 114 (4):843–863. [ PubMed ] [ Google Scholar ]
- Wunderlich K, Rangel A, O’Doherty JP. Economic choices can be made using only stimulus values. Proceedings of the National Academy of Sciences. 2010; 107 (34):15005–15010. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nature Methods. 2011; 8 (8):665–670. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Yin HH, Mulcare SP, Hilário MRF, Clouse E, Holloway T, Davis MI, et al. Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nature Neuroscience. 2009; 12 (3):333–341. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Zaki J, Lopez G, Mitchell JP. Activity in ventromedial prefrontal cortex covaries with revealed social preferences: Evidence for person-invariant value. Social Cognitive and Affective Neuroscience. 2014; 9 (4):464–469. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Zhang W, Luck SJ. Discrete fixed-resolution representations in visual working memory. Nature. 2008; 453 (7192):233–235. [ PMC free article ] [ PubMed ] [ Google Scholar ]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- 09 May 2024
Cubic millimetre of brain mapped in spectacular detail
- Carissa Wong
You can also search for this author in PubMed Google Scholar
Rendering based on electron-microscope data, showing the positions of neurons in a fragment of the brain cortex. Neurons are coloured according to size. Credit: Google Research & Lichtman Lab (Harvard University). Renderings by D. Berger (Harvard University)
Researchers have mapped a tiny piece of the human brain in astonishing detail. The resulting cell atlas, which was described today in Science 1 and is available online , reveals new patterns of connections between brain cells called neurons, as well as cells that wrap around themselves to form knots, and pairs of neurons that are almost mirror images of each other.
The 3D map covers a volume of about one cubic millimetre, one-millionth of a whole brain, and contains roughly 57,000 cells and 150 million synapses — the connections between neurons. It incorporates a colossal 1.4 petabytes of data. “It’s a little bit humbling,” says Viren Jain, a neuroscientist at Google in Mountain View, California, and a co-author of the paper. “How are we ever going to really come to terms with all this complexity?”
Slivers of brain
The brain fragment was taken from a 45-year-old woman when she underwent surgery to treat her epilepsy. It came from the cortex, a part of the brain involved in learning, problem-solving and processing sensory signals. The sample was immersed in preservatives and stained with heavy metals to make the cells easier to see. Neuroscientist Jeff Lichtman at Harvard University in Cambridge, Massachusetts, and his colleagues then cut the sample into around 5,000 slices — each just 34 nanometres thick — that could be imaged using electron microscopes.
Jain’s team then built artificial-intelligence models that were able to stitch the microscope images together to reconstruct the whole sample in 3D. “I remember this moment, going into the map and looking at one individual synapse from this woman’s brain, and then zooming out into these other millions of pixels,” says Jain. “It felt sort of spiritual.”
A single neuron (white) shown with 5,600 of the axons (blue) that connect to it. The synapses that make these connections are shown in green. Credit: Google Research & Lichtman Lab (Harvard University). Renderings by D. Berger (Harvard University)
When examining the model in detail, the researchers discovered unconventional neurons, including some that made up to 50 connections with each other. “In general, you would find a couple of connections at most between two neurons,” says Jain. Elsewhere, the model showed neurons with tendrils that formed knots around themselves. “Nobody had seen anything like this before,” Jain adds.
The team also found pairs of neurons that were near-perfect mirror images of each other. “We found two groups that would send their dendrites in two different directions, and sometimes there was a kind of mirror symmetry,” Jain says. It is unclear what role these features have in the brain.
Proofreaders needed
The map is so large that most of it has yet to be manually checked, and it could still contain errors created by the process of stitching so many images together. “Hundreds of cells have been ‘proofread’, but that’s obviously a few per cent of the 50,000 cells in there,” says Jain. He hopes that others will help to proofread parts of the map they are interested in. The team plans to produce similar maps of brain samples from other people — but a map of the entire brain is unlikely in the next few decades, he says.
“This paper is really the tour de force creation of a human cortex data set,” says Hongkui Zeng, director of the Allen Institute for Brain Science in Seattle. The vast amount of data that has been made freely accessible will “allow the community to look deeper into the micro-circuitry in the human cortex”, she adds.
Gaining a deeper understanding of how the cortex works could offer clues about how to treat some psychiatric and neurodegenerative diseases. “This map provides unprecedented details that can unveil new rules of neural connections and help to decipher the inner working of the human brain,” says Yongsoo Kim, a neuroscientist at Pennsylvania State University in Hershey.
doi: https://doi.org/10.1038/d41586-024-01387-9
Shapson-Coe, A. et al. Science 384 , eadk4858 (2024).
Article Google Scholar
Download references
Reprints and permissions
Related Articles
- Neuroscience

How does ChatGPT ‘think’? Psychology and neuroscience crack open AI large language models
News Feature 14 MAY 24
Brain-reading device is best yet at decoding ‘internal speech’
News 13 MAY 24
Retuning of hippocampal representations during sleep
Article 08 MAY 24
Found: the dial in the brain that controls the immune system
News 01 MAY 24
Global Talent Recruitment of Xinjiang University in 2024
Recruitment involves disciplines that can contact the person in charge by phone.
Wulumuqi city, Ürümqi, Xinjiang Province, China
Xinjiang University
Tenure-Track Assistant Professor, Associate Professor, and Professor
Westlake Center for Genome Editing seeks exceptional scholars in the many areas.
Westlake Center for Genome Editing, Westlake University
Faculty Positions at SUSTech School of Medicine
SUSTech School of Medicine offers equal opportunities and welcome applicants from the world with all ethnic backgrounds.
Shenzhen, Guangdong, China
Southern University of Science and Technology, School of Medicine
Assistant/Associate Professor in Sustainable Biobased Products Manufacturing
Lubbock, Texas
Texas Tech University
Professor and Center for Vector-borne and Zoonotic Diseases Director
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

IMAGES
VIDEO
COMMENTS
Brain clearance is reduced during sleep and anesthesia. It has been widely believed that a key function of sleep is to actively clear metabolites and toxins from the brain. Miao, Luo et al. show ...
We are proud to introduce Current Research in Neurobiology. (CRNEUR), a new primary research journal from Elsevier. CRNEUR publishes timely original papers, short communications, resources and news & views that cover any aspect of the field of neural science from molecules to mind. We ambitiously aim to be the neuroscientific community's ...
Nature Neuroscience is a ... This paper shows that compulsive-like grooming in Sapap3-knockout mice ... We seek exceptional candidates to lead vigorous independent research programs working in any ...
Research Articles, Neurobiology of Disease. TRPM2 and CaMKII Signaling Drives Excessive GABAergic Synaptic Inhibition Following Ischemia. May 08, 2024. Early Release. Research Articles, Systems/Circuits. Inhibitory subpopulations in preBötzinger Complex play distinct roles in modulating inspiratory rhythm and pattern.
Top 100 in Neuroscience. This collection highlights our most downloaded* neuroscience papers published in 2021. Featuring authors from around the world, these papers showcase valuable research ...
Research Articles, Neurobiology of Disease. TRPM2 and CaMKII Signaling Drives Excessive GABAergic Synaptic Inhibition Following Ischemia. Amelia M. Burch, Joshua D. Garcia, Heather O'Leary, Ami Haas, James E. Orfila, Erika Tiemeier, Nicholas Chalmers, Katharine R. Smith, Nidia Quillinan and Paco S. Herson.
The Journal of Neuroscience Research ( JNR) publishes pioneering research relevant to the development, function, and pathophysiology of the nervous system. Molecular, cellular, systems, and translational approaches are all considered. Papers explore basic research and clinical aspects of neurology, neuropathology, psychiatry, or psychology.
Publications. Macrophages protect against sensory axon degeneration in diabetic neuropathy. Nerve injury disrupts temporal processing in the spinal cord dorsal horn through alterations in PV+ interneurons. The secondary somatosensory cortex gates mechanical and heat sensitivity.
Formerly Archives of Neurology. Explore the latest in brain science including dementia, traumatic brain injury, cerebellar stroke, restless arms, herpes ... Economics, and Policy Promoting EDI in Genetics Research PTSD and Cardiovascular Disease Red Blood Cell Transfusion: 2023 AABB International Guidelines Reimagining Children's Rights in ...
Neuroscience Research is an international journal for high quality articles in all branches of neuroscience, from the molecular to the behavioral levels. The journal is published in collaboration with the Japan Neuroscience Society and is open to all contributors in the world. Neuroscience Research publishes original full-length research ...
1. Introduction. Fear and anxiety play a central role in the lives of humans and other mammals, and there is an abiding interest among scientists, clinicians, philosophers, artists, and the public at large in understanding their nature, identifying their biological underpinnings, and determining their contribution to other psychological processes, from cognition and decision-making, to health ...
Overview. Molecular Neurobiology is a journal focusing on contemporary developments in molecular brain research. Provides thorough and constructive peer review managed by our Editor-in-Chief and Associate editors. Offers attractive turnaround times for research publication. Committed to high levels of author satisfaction, with strong ...
Abstract. Schizophrenia is a debilitating disease that presents with both positive and negative symptoms affecting cognition and emotions. Extensive studies have analyzed the different factors that contribute to the disorder. There is evidence of significant genetic etiology involving multiple genes such as dystrobrevin binding protein 1 ...
Analysis of Published Papers. In the past decade, the total number of papers on depression published worldwide has increased year by year as shown in Fig. Fig.1A. 1 A. Searching the Web of Science database, we found a total of 43,863 papers published in the field of depression from 2009 to 2019 (search strategy: TI = (depression$) or ts = ("major depressive disorder$")) and py = (2009-2019 ...
Atom. RSS Feed. Neuroscience is a multidisciplinary science that is concerned with the study of the structure and function of the nervous system. It encompasses the evolution, development ...
Two—not necessarily opposing—classification systems are now at the forefront of clinical research: (1) Diagnostic and Statistical Manual, Fifth Edition (DSM-5 1) and (2) the National Institutes of Mental Health (NIMH) Research Domain Criteria (RDoC 2-4).RDoC provides a fresh perspective on new ways to approach anxiety research (e.g., through the construct of "potential threat"), but ...
7. Future Directions. The principal intent of this paper is to highlight a potential educational neuroscience research in areas of growth mindset and intrinsic motivation. Although there are some empirical studies on mindset and motivation, the neuroscience of intrinsic motivation is still unclear and at its infancy.
Alexander Kaltenboeck reports receiving research funding from the Medical Research Council and the Department of Psychiatry, University of Oxford. Catherine Harmer reports receiving grants from Johnson & Johnson, UCB, and Sunovion; and personal fees from P1vital and Lundbeck (outside this work).
Background This review focuses on neurology research which uses routinely collected data. The number of such studies is growing alongside the expansion of data collection. We aim to gain a broad picture of the scope of how routine healthcare data have been utilised. Methods This study follows a systematic mapping review approach which does not make a judgement on the quality of the papers ...
This collection highlights our most downloaded* neuroscience papers published in 2022. Featuring authors from around the world, these papers showcase valuable research from an international community.
The paper addresses the main problems of discrepancy between neurobiological research and philosophical perspective. Current opinions concerning neural correlates and models of consciousness are ...
Our large nationwide population-based case-control study used the Taiwanese National Health Insurance Research Database. A total of 7,843 adults with newly diagnosed dementia without a history of dementia or neurodegenerative disease between 2006 and 2013 were identified and included in our study.
Research at the interface of neuroscience and psychology has made significant strides in uncovering the machinery behind goal pursuit. This knowledge, in turn, provides clues about the various ways that behavior change can go wrong and how to improve it. In this article, I present a brain-based framework for understanding how goal pursuit works ...
Top 100 in Neuroscience. This collection highlights our most downloaded* neuroscience papers published in 2019. Featuring authors from around the world, these papers feature valuable research from ...
@article{Hong2024TheUO, title={The University of Toronto Scarborough Psychology and Neuroscience Departmental Students' Association (PNDA) 2024 Academic Research Panel}, author={Lauren Hong and Jerlene Pandian and Isabelle Pastula and Kayla Relleve and Solomon Tse}, journal={Undergraduate Research in Natural and Clinical Science and Technology ...
Credit: Google Research & Lichtman Lab (Harvard University). Renderings by D. Berger (Harvard University) Researchers have mapped a tiny piece of the human brain in astonishing detail.