- Search by keyword
- Search by citation
Page 1 of 24

Potential role of IGF-1R in the interaction between orbital fibroblasts and B lymphocytes: an implication for B lymphocyte depletion in the active inflammatory phase of thyroid-associated ophthalmopathy
Thyroid eye disease (TED) is an inflammatory process involving lymphocyte-mediated immune response and orbital tissue damage. The anti-insulin-like growth factor-1 receptor (IGF-1R) antibodies produced by B ly...
- View Full Text
Atypical skin conditions of the neck and back as a dermal manifestation of anti-HMGCR antibody-positive myopathy
Immune-mediated necrotizing myopathy (IMNM) is an idiopathic inflammatory myopathy (IIM). Though patients with IMNM were not considered to show skin rash, several reports have showed atypical skin conditions i...
GNUV201, a novel human/mouse cross-reactive and low pH-selective anti-PD-1 monoclonal antibody for cancer immunotherapy
Several PD-1 antibodies approved as anti-cancer therapies work by blocking the interaction of PD-1 with its ligand PD-L1, thus restoring anti-cancer T cell activities. These PD-1 antibodies lack inter-species ...
Effect of immune-modulating metronomic capecitabine as an adjuvant therapy in locoregionally advanced nasopharyngeal carcinoma
Metronomic capecitabine used as an adjuvant therapy improves survival in patients with locoregionally advanced nasopharyngeal carcinoma (LA-NPC). This therapeutic approach may also contribute to improving immu...
A novel chimeric vaccine containing multiple epitopes for simulating robust immune activation against Klebsiella pneumoniae
Due to antibiotic resistance, the Klebsiella genus is linked to morbidity and death, necessitating the development of a universally protective vaccine against Klebsiella pathogens.
The role of the immune system in early-onset schizophrenia: identifying immune characteristic genes and cells from peripheral blood
Early-onset schizophrenia (EOS) is a type of schizophrenia (SCZ) with an age of onset of < 18 years. An abnormal inflammatory immune system may be involved in the occurrence and development of SCZ. We aimed to...
Atypical memory B cells increase in the peripheral blood of patients with breast cancer regardless of lymph node involvement
Breast cancer is the most common cancer in females. The immune system has a crucial role in the fight against cancer. B and T cells, the two main components of the adaptive immunity, are critical players that ...
The influence of neonatal BCG vaccination on in vitro cytokine responses to Plasmodium falciparum
Bacillus Calmette–Guérin (BCG) vaccination has off-target protective effects against infections unrelated to tuberculosis. Among these, murine and human studies suggest that BCG vaccination may protect against...
Basophil activation in insect venom allergy: comparison of an established test using liquid reagents with a test using 5-color tubes with dried antibody reagents
Flow cytometry-based basophil activation tests (BAT) have been performed with various modifications, differing in the use of distinct identification and activation markers. Established tests use liquid reagent...
Advanced in immunological monitoring of HIV infection: profile of immune cells and cytokines in people living with HIV-1 in Benin
Immune cells and cytokines have been linked to viremia dynamic and immune status during HIV infection. They may serve as useful biomarkers in the monitoring of people living with HIV-1 (PLHIV-1). The present w...
Helminth-derived proteins as immune system regulators: a systematic review of their promise in alleviating colitis
Helminth-derived proteins have immunomodulatory properties, influencing the host’s immune response as an adaptive strategy for helminth survival. Helminth-derived proteins modulate the immune response by induc...
Association of interleukin-17A and chemokine/vascular endothelial growth factor-induced angiogenesis in newly diagnosed patients with bladder cancer
The human interleukin-17 (IL-17) family comprises IL-17A to IL-17 F; their receptors are IL-17RA to IL-17RE. Evidence revealed that these cytokines can have a tumor-supportive or anti-tumor impact on human mal...
Causal relationship between immune cells and telomere length: mendelian randomization analysis
The causal relationship between immune cells and telomere length remains controversial.
Subcutaneous immunoglobulin replacement therapy in patients with immunodeficiencies – impact of drug packaging and administration method on patient reported outcomes
Here, the perspective of patients with primary and secondary immunodeficiency receiving subcutaneous immunoglobulin (SCIg) via introductory smaller size pre-filled syringes (PFS) or vials were compared.
Dendritic cells under allergic condition enhance the activation of pruritogen-responsive neurons via inducing itch receptors in a co-culture study
Itch sensitization has been reported in patients with chronic allergic skin diseases and observed in a mouse model of allergic contact dermatitis (ACD). There is evidence suggesting that neuroimmune interactio...
Network pharmacology-based strategy to investigate the mechanisms of artemisinin in treating primary Sjögren’s syndrome
The study aimed to explore the mechanism of artemisinin in treating primary Sjögren’s syndrome (pSS) based on network pharmacology and experimental validation.
Hyperactivation and enhanced cytotoxicity of reduced CD8 + gamma delta T cells in the intestine of patients with Crohn’s disease correlates with disease activity
We aimed to investigate the immune characteristics of intestinal CD8 + gamma delta T (CD8 + γδ T) cells in Crohn’s disease (CD) and their correlation with disease activity.
Immune responses to P falciparum antibodies in symptomatic malaria patients with variant hemoglobin genotypes in Ghana
Haemoglobin (Hb) variants such as sickle cell trait (SCT/HbAS) play a role in protecting against clinical malaria, but little is known about the development of immune responses against malaria parasite ( Plasmodiu...
Systematic evaluation of B-cell clonal family inference approaches
The reconstruction of clonal families (CFs) in B-cell receptor (BCR) repertoire analysis is a crucial step to understand the adaptive immune system and how it responds to antigens. The BCR repertoire of an ind...
Expression of the immune checkpoint molecules CD226 and TIGIT in preeclampsia patients
Imbalanced immune responses are involved in developing preeclampsia (PE). We wish to explore the expression and potential changes of immune checkpoint molecules TIGIT, CD226 and CD155 in PE patients.
Oral administration of DNA alginate nanovaccine induced immune-protection against Helicobacter pylori in Balb/C mice
Helicobacter pylori (H. Pylori), is an established causative factor for the development of gastric cancer and the induction of persistent stomach infections that may lead to peptic ulcers. In recent decades, s...
Profiling of T cell repertoire in peripheral blood of patients from type 2 diabetes with complication
More than 90% of patients with diabetes worldwide are type 2 diabetes (T2D), which is caused by insulin resistance or impaired producing insulin by pancreatic β cells. T2D and its complications, mainly large c...
Tumor microenvironment and immune system preservation in early-stage breast cancer: routes for early recurrence after mastectomy and treatment for lobular and ductal forms of disease
Intra-ductal cancer (IDC) is the most common type of breast cancer, with intra-lobular cancer (ILC) coming in second. Surgery is the primary treatment for early stage breast cancer. There are now irrefutable d...
Predictive biomarkers for immune-related adverse events in cancer patients treated with immune-checkpoint inhibitors
The objective of this study was to identify potential predictors of immune-related adverse events (irAEs) in cancer patients receiving immune checkpoint inhibitor therapy among serum indexes, case data, and li...
Negative prognostic behaviour of PD-L1 expression in tongue and larynx squamous cell carcinoma and its significant predictive power in combination with PD-1 expression on TILs
Biomarkers that can predict outcome will improve the efficacy of treatment for HNSCC patients. In this regard, we retrospectively evaluated the prognostic effect of PD1, PD-L1, and CD45RO in tongue and larynx ...
Whole blood stimulation provides preliminary evidence of altered immune function following SRC
To implement an approach combining whole blood immune stimulation and causal modelling to estimate the impact of sport-related concussion (SRC) on immune function.
IgG antibody response to SARS-CoV-2 infection and its influencing factors in lymphoma patients
The ability of generating effective humoral immune responses to SARS-CoV-2 infection has not been clarified in lymphoma patients. The study aimed to investigate the antibody (Ab) production after SARS-Cov-2 in...
Polarized Th2 cells attenuate high-fat-diet induced obesity through the suppression of lipogenesis
Immune cells, such as macrophages, B cells, neutrophils and T cell subsets, have been implicated in the context of obesity. However, the specific role of Th2 cells in adipose tissue function has remained elusi...
The predictive value of peripheral blood CD4 cells ATP concentration for immune-related adverse events in advanced non-small cell lung cancer patients
Lung cancer with the highest incidence and mortality in the world. Immune checkpoint inhibitors (ICIs), can bring long-term survival benefits to patients, but also can bring immune-related adverse events (irAE...
Evaluation of the TLR3 involvement during Schistosoma japonicum -induced pathology
Despite the functions of TLRs in the parasitic infections have been extensively reported, few studies have addressed the role of TLR3 in the immune response to Schistosoma japonicum infections. The aim of this st...
PRMT2 silencing regulates macrophage polarization through activation of STAT1 or inhibition of STAT6
Macrophages play significant roles in innate immune responses and are heterogeneous cells that can be polarized into M1 or M2 phenotypes. PRMT2 is one of the type I protein arginine methyltransferases involved...
TRPV1 + neurons alter Staphylococcus aureus skin infection outcomes by affecting macrophage polarization and neutrophil recruitment
The interaction between the nervous system and the immune system can affect the outcome of a bacterial infection. Staphylococcus aureus skin infection is a common infectious disease, and elucidating the relations...
Retraction Note: Oral supplementation of diabetic mice with propolis restores the proliferation capacity and chemotaxis of B and T lymphocytes towards CCL21 and CXCL12 by modulating the lipid profile, the pro-inflammatory cytokine levels and oxidative stress
Cd39 identifies a specific cd8 + t cell population in lung adenocarcinoma-related metastatic pleural effusion.
Malignant pleural effusion (MPE), which is a complex microenvironment that contains numerous immune and tumour signals, is common in lung cancer. Gene alterations, such as driver gene mutations, are believed t...
Dissecting cellular states of infiltrating microenvironment cells in melanoma by integrating single-cell and bulk transcriptome analysis
Cellular states of different immune cells can affect the activity of the whole immune microenvironment.
Sec1 regulates intestinal mucosal immunity in a mouse model of inflammatory bowel disease
Inflammatory bowel disease (IBD) is a common immune-mediated condition with its molecular pathogenesis remaining to be fully elucidated. This study aimed to deepen our understanding of the role of FUT2 in human I...
Screening of four lysosome-related genes in sepsis based on RNA sequencing technology
Screening of lysosome-related genes in sepsis patients to provide direction for lysosome-targeted therapy.
Dominant negative biologics normalise the tumour necrosis factor (TNF-α) induced angiogenesis which exploits the Mycobacterium tuberculosis dissemination
Tumor necrosis factor (TNF) is known to promote T cell migration and increase the expression of vascular endothelial growth factor (VEGF) and chemokines. The administration of Xpro-1595, a dominant-negative TN...
Activation dynamics of antigen presenting cells in vivo against Mycobacterium bovis BCG in different immunized route
Control of Tuberculosis (TB) infection is mainly the result of productive teamwork between T-cell populations and antigen presenting cells (APCs). However, APCs activation at the site of initiating cellular im...
Characteristics of circulating immune cells in HBV-related acute-on-chronic liver failure following artificial liver treatment
Liver failure, which is predominantly caused by hepatitis B (HBV) can be improved by an artificial liver support system (ALSS). This study investigated the phenotypic heterogeneity of immunocytes in patients w...
Putative novel outer membrane antigens multi-epitope DNA vaccine candidates identified by Immunoinformatic approaches to control Acinetobacter baumannii
Multi-epitope polypeptide vaccines, a fusion protein, often have a string-of-beads system composed of various specific peptide epitopes, potential adjuvants, and linkers. When choosing the sequence of various ...
Long-term humoral and cellular immunity after primary SARS-CoV-2 infection: a 20-month longitudinal study
SARS-CoV-2 remains a world-wide health issue. SARS-CoV-2-specific immunity is induced upon both infection and vaccination. However, defining the long-term immune trajectory, especially after infection, is limi...
Exploration of biomarkers for systemic lupus erythematosus by machine-learning analysis
In recent years, research on the pathogenesis of systemic lupus erythematosus (SLE) has made great progress. However, the prognosis of the disease remains poor, and high sensitivity and accurate biomarkers are...
Tacrolimus reverses pemphigus vulgaris serum-induced depletion of desmoglein in HaCaT cells via inhibition of heat shock protein 27 phosphorylation
Glucocorticoids are the first-line treatment for Pemphigus vulgaris (PV), but its serious side effects can be life-threatening for PV patients. Tacrolimus (FK506) has been reported to have an adjuvant treatmen...
Increased infiltration of CD4 + T cell in the complement deficient lymphedema model
Lymphedema is an intractable disease that can be caused by injury to lymphatic vessels, such as by surgical treatments for cancer. It can lead to impaired joint mobility in the extremities and reduced quality ...
Vitamin D and biomarkers of inflammation and oxidative stress among pregnant women: a systematic review of observational studies
This systematic review aimed to map the evidence evaluated the relationship between vitamin D and redox and inflammatory status during gestation.
The upregulation of peripheral CD3 - CD56 + CD16 + natural killer cells correlates with Th1/Th2 imbalance in asthma patients during acute upper respiratory viral infections
The aim of this study is to clarify the changes of peripheral CD3 − CD56 + CD16 + NK cells and their correlation with Th1/Th2 immunity profiles in asthma during the phase of acute upper respiratory viral infections (A...
Humoral immune response and changes in peritoneal cell populations in rats immunized against two Leptospira serovars; serovar patoc and serovar pyrogenes
Leptospirosis is a zoonotic disease caused by Leptospira species. Variations in lipopolysaccharide (LPS) structure in Leptospira are known to be associated with the serovar diversity and antigenicity. Development...
Methionine enkephalin(MENK) upregulated memory T cells in anti-influenza response
Novel prophylactic drugs and vaccination strategies for protection against influenza virus should induce specific effector T-cell immune responses in pulmonary airways and peripheral lymphoid organs. Designing...
Transcriptomic analysis identifies CYP27A1 as a diagnostic marker for the prognosis and immunity in lung adenocarcinoma
The association between lipid metabolism disorder and carcinogenesis is well-established, but there is limited research on the connection between lipid metabolism-related genes (LRGs) and lung adenocarcinoma (...
Featured videos
View featured videos from across the BMC-series journals
Important information
Editorial board
For authors
For editorial board members
For reviewers
- Manuscript editing services
Annual Journal Metrics
2022 Citation Impact 3.0 - 2-year Impact Factor 3.6 - 5-year Impact Factor 0.808 - SNIP (Source Normalized Impact per Paper) 0.783 - SJR (SCImago Journal Rank)
2023 Speed 26 days submission to first editorial decision for all manuscripts (Median) 188 days submission to accept (Median)
2023 Usage 671,800 downloads 176 Altmetric mentions
- More about our metrics
- Follow us on Twitter
BMC Immunology
ISSN: 1471-2172
- General enquiries: [email protected]

Immunologic Research
- Offers both traditional publishing route and immediate gold Open Access.
- Maintains a balance between basic and clinical data.
- Provides a platform for presenting historical reviews and landmark discoveries.
- Encourages submissions of editorials, guidelines, and new techniques of innovative importance.
- Rated the overall publishing process as excellent or good by 93% of authors.
- Sakir Ahmed,
- Nicola Bizzaro

Latest issue
Volume 72, Issue 2
Latest articles
Inhibition of ciap1/2 reduces ripk1 phosphorylation in pulmonary endothelial cells and alleviate sepsis-induced lung injury and inflammatory response.
- Guoqiang Zhang

Immunomodulating effects of the single bacterial strain therapy EDP1815 on innate and adaptive immune challenge responses — a randomized, placebo-controlled clinical trial
- Boukje C. Eveleens Maarse
- Micha N. Ronner
- Matthijs Moerland

Inhibition of LSD1 via SP2509 attenuated the progression of rheumatoid arthritis
- Haiping Zhang

Genetic variability of three common NK and γδ T cell receptor genes (FCγ3R, NCR3, and DNAM-1) and their role in Polish patients with rheumatoid arthritis and ankylosing spondylitis
- Sylwia Biały
- Milena Iwaszko
- Katarzyna Bogunia-Kubik

DAP1-2: a synthetic peptide targeting IL-1R1 receptor effectively suppresses IL-1β in vitro
- Ellen De-Pieri
- Rubya Pereira Zaccaron
- Ricardo Andrez Machado-de-Ávila
Journal information
- Biological Abstracts
- CAB Abstracts
- Chemical Abstracts Service (CAS)
- Current Contents/Life Sciences
- Google Scholar
- Japanese Science and Technology Agency (JST)
- OCLC WorldCat Discovery Service
- Pathway Studio
- Science Citation Index Expanded (SCIE)
- TD Net Discovery Service
- UGC-CARE List (India)
Rights and permissions
Editorial policies
© Springer Science+Business Media, LLC, part of Springer Nature
- Find a journal
- Publish with us
- Track your research
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 02 September 2020
Human immunology and immunotherapy: main achievements and challenges
- Jezabel Varadé 1 , 2 na1 ,
- Susana Magadán 1 , 2 na1 &
- África González-Fernández 1 , 2 na1
Cellular & Molecular Immunology volume 18 , pages 805–828 ( 2021 ) Cite this article
47k Accesses
87 Citations
198 Altmetric
Metrics details
- Immunization
- Tumour immunology
The immune system is a fascinating world of cells, soluble factors, interacting cells, and tissues, all of which are interconnected. The highly complex nature of the immune system makes it difficult to view it as a whole, but researchers are now trying to put all the pieces of the puzzle together to obtain a more complete picture. The development of new specialized equipment and immunological techniques, genetic approaches, animal models, and a long list of monoclonal antibodies, among many other factors, are improving our knowledge of this sophisticated system. The different types of cell subsets, soluble factors, membrane molecules, and cell functionalities are some aspects that we are starting to understand, together with their roles in health, aging, and illness. This knowledge is filling many of the gaps, and in some cases, it has led to changes in our previous assumptions; e.g., adaptive immune cells were previously thought to be unique memory cells until trained innate immunity was observed, and several innate immune cells with features similar to those of cytokine-secreting T cells have been discovered. Moreover, we have improved our knowledge not only regarding immune-mediated illnesses and how the immune system works and interacts with other systems and components (such as the microbiome) but also in terms of ways to manipulate this system through immunotherapy. The development of different types of immunotherapies, including vaccines (prophylactic and therapeutic), and the use of pathogens, monoclonal antibodies, recombinant proteins, cytokines, and cellular immunotherapies, are changing the way in which we approach many diseases, especially cancer.
Similar content being viewed by others
Innate lymphoid cells and cancer
Harnessing natural killer cells for cancer immunotherapy: dispatching the first responders
Genetically engineered T cells for cancer immunotherapy
Introduction.
The knowledge of human immunology has improved exponentially in recent years, and more advances in the near future are certainly imminent. The immune system is extremely complex, but we are now developing new tools and skills to study it. Several factors have been involved in these advancements, and the most important ones include the development of thousands of different monoclonal antibodies that allow the identification of a large variety of cell subpopulations and the functional analysis of immune cells. These tools, together with new and sophisticated technologies, such as single-cell analysis, imaging techniques, omics (including massive DNA-RNA sequencing, proteomics, and metabolomics data and new tools for processing these data, such as artificial intelligence and machine learning approaches, mathematical modeling, etc.), newly designed animal models (using conventional transgenic/knockout/knock-in mice or new technologies such as CRISPR-Cas9 (clustered regularly interspaced short palindromic repeats–CRISPR-associated protein 9), are increasing our knowledge about how our immune system functions. The study of the interaction between the immune system and other systems, such as the nervous and endocrine systems or the microbiome, in several illnesses has produced interesting results with important clinical applications.
All of these advances can be applied to several immune-mediated pathologies, but overall, the success achieved with some types of immunotherapies in recent years is revealing new ways to explore and manipulate the immune system for our benefit.
Writing a review about human immunology is a significant challenge, but we have attempted to bring together recent knowledge about the immune system, immune-mediated illnesses and types of immunotherapies.

New findings in fundamental immunology
The last two decades have witnessed a major revolution in the field of immunology. The traditional classification of the immune system into two different arms, namely, innate and adaptive components that collaborate to respond to foreign antigens or to perform self-/nonself-discrimination, has become much more complex. The development and application of new technologies have provided new findings and created a new landscape in which the immune system establishes cross talk, not only between immune components but also with commensal microorganisms 1 , 2 and other important systems, such as the endocrine and nervous systems 3 , 4 , 5 . These developments have forced immunologists to reformulate the immunological architecture that confers protection, which has made the study of the immune system especially attractive. Moreover, these advances have led to an increased interest in better understanding, managing, and manipulating the immune response in both health and disease.
Cell subsets
The characterization of new immune cell subsets has been a constant feature in the immunology field. This evolution is clearly reflected in the discovery of an innate counterpart of T lymphocytes, collectively named innate lymphoid cells (ILCs) 6 , and in the identification of different types of effector CD4 and regulatory T cells 7 .
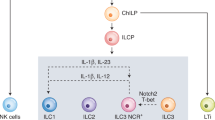
Innate lymphoid cells (ILCs)
ILCs are lymphocytes, but in contrast to adaptive immune cells, they can colonize lymphoid and barrier tissue sites during fetal development, do not undergo somatic recombination and do not express antigen-specific receptors 8 , 9 . In addition to lymphoid organs, ILCs are enriched in barrier tissues, such as the gastrointestinal tract, airways, and skin 10 , 11 . These innate cells have been considered to be tissue-resident cells, but recent studies suggest that ILCs can migrate through the lymphatic system during homeostasis or enter into the circulation upon infection and inflammation 6 , 12 . Currently, five different ILCs are defined on the basis of their transcription factor expression, different cytokine production and/or developmental patterns 6 : natural killer (NK) cells (discussed below), lymphoid tissue inducer cells (LTis) and three subsets of helper-like ILCs (ILC1s, ILC2s, and ILC3s), which are considered to be the innate counterparts of T helper (Th) 1, Th2, and Th17 cells, respectively. The main focus of this review is ILCs.
ILC1s are dependent on the T-box transcription factor T-bet and produce interferon gamma (INF-γ), but they differ in the expression of eomesodermin transcription factor 13 . ILC1s express CD127 in humans and CD200R in mice, but the natural cytotoxicity receptor NKp46 (also known as NCR1) is expressed in both species 14 , 15 .
ILC2s constitute the most homogeneous class of ILCs; they are dependent on GATA3 and RORα, and they produce type 2 cytokines, mainly interleukin 5 (IL-5) and IL-13. ILC2s are involved in immune responses to parasite infection, and in humans, they express chemoattractant receptor-homologous molecule expressed in T H 2 cells (CRTH2) and high levels of CD161, whereas most mouse ILC2s express ST2 (a member of the IL-1 receptor family) 14 , 15 .
The development and function of ILC3s depend on the transcription factor RORγt. Both human and mouse ILC3s can produce granulocyte macrophage colony-stimulating factor (GM-CSF), IL-17, and/or IL-22 16 , 17 . In humans, two major ILC3 subsets can be distinguished on the basis of the expression of the natural cytotoxicity receptor NKp44 (also known as NCR2) 14 , 15 . Both types can produce IL-17, but the production of IL-22 is mainly confined to NKp44 + ILC3s.
Extensive research has focused on deciphering the role of ILCs to ensure the maintenance of tissue homeostasis and immune protection 11 , 18 . ILCs express particular sets of receptors in a tissue-specific manner, and these allow the detection of host-derived signals (including those from alarmins, neuronal mediators, microbia, and the diet) 19 . The integration of these endogenous signals is essential for the maintenance of tissue homeostasis, but dysregulation of ILC responses leads to inflammation and disorder 12 , 20 . ILC are mainly involved in early protection against viruses and bacteria 13 , 21 , but their response to dysregulated local proinflammatory cytokine production in adipose tissues leads to the development of metabolic disorders and obesity 20 . IL-5 and IL-13 produced by ILC2s induce goblet cell differentiation and the recruitment of eosinophils, basophils, and mast cells 22 , which are involved in protection against infection by helminths and viruses, but when uncontrolled, these cells drive allergic responses and metabolic disorders. Moreover, the depletion of ILC2s in animal models suggests a role for these cells in atopic dermatitis and asthma 23 .
ILC3s are abundant in mucosal tissues, and NCR2 + ILC3s have been proven to be essential for regulating the balance between commensal and pathogenic bacteria through the production of IL-22 24 . In contrast, NCR2 − ILC3s can promote colitis in a model of inflammatory bowel disease 25 . The lack of immunodeficiency in ILC-deficient patients led to the proposal that ILCs are dispensable in the presence of functional T cells and B cells 26 . However, recent studies support the idea that ILCs cannot be considered to have functions that only duplicate those of the adaptive immune system.
In addition to those showing the essential role of LTi cells in the formation of secondary lymphoid organs during embryogenesis and the postnatal development of intestinal lymphoid clusters, recent studies also provide evidence that subsets of ILCs express multiple factors that modulate the adaptive immune response in health and disease 27 , 28 . In particular, ILC2s and ILC3s modulate the T-cell response. Studies in mice suggest that in healthy intestine, ILC3s express major histocompatibility complex (MHC) class II molecules but lack the expression of costimulatory molecules; therefore, they inhibit microbiota-specific T-cell responses, thus preventing intestinal inflammation 29 . It seems that the interaction between ILC3s and Tfh cells limits IL-4 secretion and the production of IgA by mucosal B cells 30 .
Studies with murine models have significantly contributed to the classification and understanding of the role of ILCs in the immune system, especially since similarities have been observed between ILCs identified in mice and humans 15 . However, the differences between these two species present real challenges 15 , 31 because human ILCs have unique attributes that are only now being elucidated, with further work required in this exciting field. The roles of ILCs in immunity and their cross talk with other components of the immune response await further analysis. Detailed coverage of this topic is beyond the scope of this review, and we refer the reader to recent reviews that provide more information on the biology of human 32 and mouse 33 , 34 ILCs.
T cells and plasticity
T cells are categorized as Tα/β and Tγ/δ cells, depending on the type of T-cell receptor (TCR) that they express 35 . Human Tγ/δ cells, similar to their murine counterparts, are a minor population (1–10% of nucleated cells) in peripheral blood, but are especially abundant in barrier tissues such as the epidermis 35 , 36 , 37 .
The three main subsets of T cells carrying α/β receptor are the CD4+T helper cells and CD8+cytotoxic and CD4+ CD25+ regulatory T cells 38 .
New effector CD4+ helper T-cell subsets (initially classified as Th1 and Th2) 39 , 40 have been recently described, and at least six human Th cell subsets have been identified to date: Th1, Th2, Th17, Tfh, Th9, and Th22 cells 38 , 41 . All of these cells recognize foreign peptides presented by class II MHC molecules on antigen-presenting cells (dendritic cells, macrophages, and B lymphocytes).
Th1 cells are required to activate macrophages and cell-mediated immunity to kill intracellular pathogens 42 , whereas Th2 cells are important in facilitating eosinophils to fight against parasitic helminths and B cells for antibody production and antibody class-switching to generate IgA or IgE 43 . Th17 cells are required to mobilize neutrophils for the clearance of fungi and extracellular bacteria, and they are also involved in mucosal protection 44 . Th9 and Th22 cells are also involved in mucosal immunity; Th9 cells protect against parasites 45 , 46 , and Th22 cells prevent microbial translocation across epithelial surfaces and promote wound healing 47 , 48 . As mentioned in the introduction to ILCs, studies on human Th cells isolated from lymphoid organs and blood samples, along with recent observations on the developmental mechanism of distinct Th cell subsets, have revealed both similarities and differences of human and mouse Th cells 41 , 49 , 50 .
Tfh cells are very important for germinal center reactions, antibody class switching, affinity maturation, and the development of high affinity antibodies and memory B cells 51 , 52 . At the surface marker level, Tfh cells are generally characterized by the expression of CXCR5, the chemokine receptor for CXCL13, which is highly expressed on B-cell follicles for expressing inducible T-cell costimulator (ICOS) and programmed death protein 1 (PD-1) 53 , 54 , which enable their involvement in the interaction of Tfh cells and B cells 55 .
The definition of a given T cell lineage is based on its ability to sense different inductive cytokines, to produce particular cytokines or to express a lineage-specifying transcription factor. Th1 cells produce IFN-γ and express T-bet 56 ; Th2 cells are characterized by IL-4, IL-5, and IL-13 production and GATA-3 expression 57 , 58 ; pTregs, which are induced in the periphery from naïve precursors, produce TGF-β and express Foxp3 (Tr1 cells are IL-10-secreting Tregs that do not express Foxp3) 59 . Th17 cells produce IL-17A, IL-17F, and IL-22 and express RORγt 60 , 61 , and Tfh cells produce IL-4 and IL-21 and express the BCL6 transcription factor. In addition, Th22 cells, which produce IL-22 and express the aryl hydrocarbon receptor (AHR) 47 , 62 , and Th9 cells, are characterized by the expression of IL-9 and the transcription factor PU.1 63 . Additional levels of regulation, such as the differential expression of microRNAs, long noncoding RNAs (lncRNAs), and protein stability and function, have been found to control various aspects of Th cell differentiation and effector function 64 , 65 .
CD8+ cytotoxic T cells express the dimeric CD8 marker and have specific lytic capacity to target cells through several mechanisms, including the release of cytotoxic granules, secretion of cytokine tumor necrosis factor alpha (TNFa) and interferon gamma, and the induction of cell death through the interactions of Fas and the Fas ligand 38 , 66 . Their TCRs are restricted to interactions with peptides presented by class I MHCs.
Regulatory T cells (Tregs) include thymically derived and peripherally induced regulatory T cells (tTregs and pTregs, respectively), and they produce either IL10, TGF-beta, IL-35 or combinations of these proteins 67 . tTregs express the transcription factor Foxp3 and secrete IL10 and TGF-β; pTregs, which are induced in the periphery from naïve precursors, can also be subdivided into IL-10-induced Tregs [Tr1 cells] (which secrete large amounts of IL-10 and moderate levels of TGFβ), TH3 cells (which produce IL-10 and TGF-β), and TGFβ-induced Tregs [iTregs], which may or may not express Foxp3.
Moreover, new subsets of regulatory T cells have been described. They include follicular regulatory T cells (which express Foxp3 and Bcl-6 and CXCR5), which modulate the function of Tfh cells and fine-tune the germinal center response 68 , 69 , 70 , and a IL-35-dependent regulatory population of cells (referred to as iTr35 cells), which show potent suppressive potential in several mouse disease models 71 . Other regulatory populations have also been described, including Bregs and CD8+ Tregs, which are the analogous counterparts of Tregs 72 , 73 , 74 .
Recent studies have revealed the capacity of differentiated T cells, particularly Th17 cell and pTreg subsets, to change their phenotype in response to changing contexts 75 , 76 , 77 , 78 , 79 . Becattini et al. 78 found that human memory CD4 T cells primed in vivo by pathogens (e.g., Candida albicans and Mycobacterium tuberculosis ) or vaccines (Tetanus toxoid) are highly heterogeneous, both at the population and clonal levels. With respect to studies on human arthritis, Nistala et al. 79 proposed that Th17 cells are recruited to the joint and converted to Th17/1 or Th1 cells in response to local IL-12 levels. This plasticity has also been observed with in vitro assays under conditions that mimic a disease site, namely, low TGF-β and high IL-12 levels 79 . These results are inconsistent with the original idea of Th lineage stability and provide new possibilities for disease treatment aimed at inducing particular Th subsets to modulate the immune response against pathogens or to control detrimental immunity 76 , 77 , 80 .
Trained and adaptive immune memory
Other classical concepts in fundamental immunology, such as immune memory, are also changing. The specificity and the capacity to generate long-lived memory cells are two properties that have been classically used to distinguish innate immunity from adaptive immunity. Adaptive immunity is clearly based on the specific recognition of antigenic determinants by somatically diversified receptors (B cell and T cell receptors (BCR and TCRs, respectively)) and on its capacity to respond more effectively to restimulation with the same antigen. In contrast, innate immune responses have traditionally been considered nonspecific and without the capacity to adapt 81 . However, the discovery of germline-encoded pattern recognition receptors (PRRs) and the “trained innate” immunity (or innate immune memory) have provoked a shift in our understanding of the immune response. In 1997, Medzhitov et al. demonstrated that pattern recognition receptors (PRRs) expressed on innate cells recognize invariant molecular structures expressed by invading pathogens 82 . After the interaction, PRRs trigger the expression of costimulatory molecules and activate important signaling pathways to induce the activation of innate and adaptive immune cells. PRRs mainly belong to four families: Toll-like receptors (TLRs), NOD-like receptors (NLRs), C-type lectin receptors (CLRs), and peptidoglycan recognition proteins (PGRPs) 83 , 84 . The profiles of PRRs expressed by innate cells can lead to partially specific recognition of a type of microorganism; e.g., innate cells can distinguish between gram-negative and gram-positive bacteria and modulate the immune response based on this recognition, although they cannot differentiate between bacterial species 85 .
The idea that only jawed vertebrates developed immunological memory has also been challenged by the observation of resistance to reinfection in organisms that lack an adaptive immune response, such as plants 86 and invertebrates 87 , 88 . Recent studies have shown that monocytes and macrophages exposed to Candida albicans or β-glucans exhibited an enhanced secondary response 89 . In addition, immunization of mice with bacillus Calmette-Guérin (BCG, the tuberculosis vaccine) induces T cell-independent protection against secondary infections by Candida albicans , Schistosoma mansoni or influenza virus 90 , 91 , 92 , 93 . Thus, organisms are protected not only against the original microorganism but also to unrelated pathogens.
The mechanisms underlying the establishment of this innate immune memory differ from those involved in adaptive immune memory 81 . After infection or vaccination, innate immune cells (such as monocytes and macrophages) display long-term functional changes through epigenetic and metabolic reprogramming, including histone acetylation, methylation and modulation of noncoding RNAs 94 , 95 , 96 . In turn, the faster and more pronounced reactivity of adaptive immune cells (T and B lymphocytes) upon reinfection is characterized by permanent changes in the genome of cells, such as mutations, gene rearrangement, clonal expansions, as well as epigenetic modifications, all of which ensure a more persistent effect than is endowed by trained immunity 81 , 94 , 95 .
Other cells for which immunological memory has been described include Tγ/δ cells 97 and innate lymphoid cells 98 . Recently, some authors have proposed that NK cells are also capable of immunological memory 99 , 100 , 101 , 102 . Antigen-specific recall responses by human NK cells were observed by Nikzad et al. 103 in humanized mice and in varicella zoster virus (VZV)-exposed adult human volunteers, in which cytotoxic NK cells were recruited to sites of an VZV test antigen challenge on the skin. Sensitization with haptens using mice lacking T cells and B cells led to the generation of hapten-specific memory NK cells 99 . The recall response persisted for more than four months after priming, and was adoptively transferred to naïve mice 100 . Interestingly, NK cells exhibit memory that is not only specific to a given virus, such as cytomegalovirus 101 , 102 , but that is also induced in the absence of a defined antigen 104 , 105 .
Furthermore, new studies suggest that trained immunity is not a phenomenon that is restricted to immune cells, because epithelial stem cells also retain memory of previous inflammatory challenges by displaying an enhanced wound-healing capacity upon skin damage 106 . Given the data outlined above, immunological memory is now recognized to be highly diverse and not restricted to B cell- or T cell-mediated adaptive immunity. Much remains to be learned in this field, but the different manifestations of immunological memory described above offer an important basis for clinical applications, such as the development of novel vaccination strategies 107 or new therapies for pathological situations in which immunological memory can be detrimental, such as allergies or autoimmune diseases 94 , 108 , 109 .
Interaction of the immune system and the microbiome
The immune system has evolved in the presence of commensal microorganisms that colonize barrier surfaces of vertebrates and invertebrates 1 , 110 . The cross talk between the natural host microbiome and immune system is particularly interesting in the gastrointestinal tract, where the density and diversity of indigenous bacteria, viruses and fungi are greatest compared to those of other anatomical sites 111 . In the literature, reports of observed changes in microbial community composition during diseases are diverse and include those in inflammatory bowel disease (IBD), obesity, metabolic syndrome, and multiple sclerosis 112 , 113 , 114 , 115 , 116 . However, the microbiome can be influenced by different factors, such as the specific niche that it occupies, diet, stress, environmental factors, and host genetics, and a specific correlation does not necessarily infer causation. The presence of these commensals in mucosal tissues has been known since before Metchnikoff, but the current knowledge on the role of the microbiome in shaping the immune system throughout life came mostly from the development of next-generation sequencing (in particular, the reduction in the cost of 16S ribosomal RNA gene sequencing) and the use of germ-free animal models, which can be colonized even with human microbiota 117 .
Germ-free mice are characterized by atrophy of Peyer’s patches with few germinal centers and isolated lymphoid follicles, a lower number of B, T, and dendritic cells and a decreased level of immunoglobulins, particularly IgA and IgG 118 . These effects are observed at the mucosal and systemic levels, and they can be reversed within weeks after the colonization of germ-free mice with commensal bacteria 119 . Moreover, colonization with commensal Bacteroides fragilis revealed the immunomodulatory effect of bacterial polysaccharides in restoring systemic cells and the differentiation of CD4+ T cells into regulatory T cells (Foxp3+ Tregs), which in turn favor mucosal immunomodulation 120 . The induction of Th17 cell maturation by segmented filamentous bacteria has also been reported 121 . These important examples emphasize the major roles of the commensal microbiome in the maturation of mucus-associated lymphoid tissue and the systemic immune system. The development of new technologies to better track the locations and activities of distinct microbial populations is essential to elucidate host-microbe interactions, through which other systems, such as the nervous system, seem to play important roles 2 , 122 , 123 , 124 , 125 .
The better characterization of some immune cell subsets, trained immunity, and host-microbiome interactions provides a few very good examples that prove the maturation of immunology in the last few decades. In this sense, studies with mouse models have significantly contributed to the increase in our fundamental knowledge; however, the differences between murine and human immunology are notable, and conclusions drawn from mouse studies are sometimes not fully translated to humans 31 . If we want to fully exploit the power of the immune system for human health, greater effort is required for understanding human immunology. Immunologists, in cooperation with experts from other fields, have developed a variety of protocols and tools to achieve greater selectivity in the identification and analysis of human cell subsets, types of cytokines and receptors, chemokines, etc. These tools range from biological approaches that rely on next-generation sequencing, mass spectrometry, and bioinformatics to immune monitoring technologies based on multiparameter flow cytometry and single-cell gene expression analysis. Although not without limitations, these techniques provide a much better picture of the whole immune system than individual and independent approaches.
Immune-mediated illnesses
Immune-mediated illnesses comprise a wide variety of diseases characterized by the dysregulation of a normal immune response. Most of these illnesses are complex disorders believed to arise from a combination of genetic and environmental factors 126 .
Infectious diseases
Infectious diseases are caused by pathogens (viruses, bacteria, fungi or parasites that infect the host body), and they remain a leading cause of mortality worldwide. Prominent examples include illnesses produced by Mycobacterium tuberculosis , human immunodeficiency virus (HIV), Plasmodium falciparum or the current coronavirus disease 2019 (COVID-19) outbreak caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which has already infected millions of people and produced thousands of deaths in many countries.
For a number of years, many people believed Koch’s postulates, which implied that virulence traits reside solely in the pathogen. However, recent advances in molecular biology have shown that host genes play major roles in infection, together with a wide range of environmental variables 127 .
To date, six gene products endowing infectious disease susceptibility have been validated in the literature: (1) hemoglobin subunit beta; (2) band 3-anion transport protein; (3) Duffy antigen/receptor, which is associated with Plasmodium spp. infections; (4) the prion protein associated with Creutzfeldt–Jakob disease; (5) fucosyltransferase 2 and 3, which is associated with Norwalk virus infections; and (6) C-C motif chemokine receptor 5 (CCR5) coreceptor, encoded by an immune-related gene and leads to the impairment of the entry of the human immunodeficiency virus (HIV) into helper T cells, thus avoiding/decreasing the progression to acquired immunodeficiency syndrome 128 .
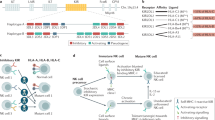
Another gene associated with infectious disease and the immune system is the natural-resistance-associated macrophage protein ( NRAMP1 ), which encodes an integral membrane protein expressed exclusively in the lysosomal compartment of monocytes and macrophages. It is a susceptibility locus for increased ratios of infection with Leishmania spp. parasites and certain strains of Salmonella spp., Mycobacterium bovis and Mycobacterium tuberculosis 129 , 130 . In addition, it has been suggested that functional variants of immunoglobulin Fc gamma RIIa ( CD32 ) are related to the development of invasive encapsulated bacterial infections 131 .
Moreover, because of recently acquired genomic data, new human polymorphisms have been discovered, some of which play roles in changing immunoglobulin levels, seroconversion rates or the intensity of antigen-specific immune responses. In addition, they also contribute to human susceptibility to infection by viruses such as influenza, rhinovirus and respiratory syncytial virus 132 . These polymorphisms are mapped within the MHC ( HLA-DQB1*03 , HLA-DRβ1 , or HLA-DPβ1 ), natural killer cell immunoglobulin-like receptors 1 and 4 ( KIR3DL1 and KIR2DS4 ) and natural killer lectin-like receptor D1 ( KLDR-1 ) 133 .
Several recent studies available as preprints have analyzed certain genes that may explain the differences in the variable expression of and susceptibility to COVID-19 by patients, either by affecting the host receptor for the virus (angiotensin I converting enzyme 2 (ACE-2)) 134 , immune genes (TLR7 and others) or blood groups (group O seems to be the most protective) 135 , and more extensive omics studies are now underway with larger numbers of patients.
Autoimmune diseases
In 1901, the physician Paul Ehrlich first used the term “ Horror autotoxicus ” to describe the way autoimmunity contradicts the natural aversion to self-injury (“Living with the Enemy”, reviewed in 136 ). Currently, according to the American Autoimmune Related Disorders Association, more than 100 autoimmune diseases have been identified. Historically, these diseases were considered to be rare, but current epidemiological data have shown that they affect approximately 3–5% of the population worldwide. Some of the most common autoimmune diseases include type 1 diabetes, rheumatoid arthritis, systemic lupus erythematosus, and inflammatory bowel disease ( https://www.aarda.org/diseaselist/ ). Although significant progress has been made in understanding the mechanisms of autoimmune diseases and the nature of self-tolerance, these disease remain major burdens on health systems around the world.
Autoimmune diseases arise when the immune system attacks normal components of the body 137 . The concept of immune tolerance is defined as the ability of the immune system to prevent the targeting of self-molecules, self-cells or self-tissues. On the other hand, the failure to distinguish self from nonself is often termed a break of tolerance, and it is the basis for an autoimmune disease 138 .
What are the mechanisms that lead to a break in tolerance? Autoimmune diseases are complex disorders that are believed to arise from a combination of genetic (mutations and higher inheritance frequency of some types of major histocompatibility complex alleles), epidemiological (age and sex) and environmental (infections, microbiota, tobacco, chemicals and pharmaceutical drugs). factors These factors trigger a break in self-tolerance with the activation of self-reactive lymphocytes through several mechanisms, such as molecular mimicry, the overexpression and abnormal expression of MHC class II molecules in peripheral tissues, thymic aging, and immunodeficiencies (discussed below) and many others. Some lymphocytes escape control due to polymorphisms in several genes that affect the routes of lymphocyte activation. Other causes may include defective antigen presentation by some MHC variants with specific polymorphisms. Therefore, the self-reactive lymphocytes that have escaped control and react against self-constituents initiate the autoimmune process 139 .
Although a large number of genome-wide association studies (GWAS) have led to the identification of hundreds of polymorphisms associated with the development of different autoimmune diseases, it has proven difficult to define the role of most of these polymorphisms in the breakdown of tolerance to a self-antigen 139 , 140 , 141 , 142 , 143 , 144 , 145 . It is worth highlighting, however, that the MHC remains the main genetic factor associated with human autoimmunity 138 , 139 .
Other gene variants identified are common to many autoimmune diseases, such as rheumatoid arthritis, systemic lupus erythematosus, multiple sclerosis, type I diabetes, ulcerative colitis, autoimmune hepatitis and numerous other autoimmune diseases. For example, the protein tyrosine phosphatase nonreceptor type 22 ( PTPN22 ) gene encodes a protein that inhibits T-cell activation in the adaptive immune system, whereas it promotes myeloid cell activation; interferon regulatory factor 5–transportin 3 (IRF5–TNPO3) is involved in the accumulation of lymphocytes within lymphoid organs and failed elimination of autoreactive naïve T cells; BTB domain and CNC homolog 2 (BACH2) has a critical role in immunoglobulin class-switching recombination, somatic hypermutation of immunoglobulin encoding genes and the activation of tissue macrophages. A more complete list of genes associated with autoimmunity can be found in the review by Wang et al. 138
Researchers are currently looking for the missing heritability in autoimmune diseases by focusing on the study of methylome profiles, genetic cargos in extracellular vesicles, genetic alterations, and ways in which the microbiome may affect these diseases.
Rejection of transplants
Immune-mediated rejection of tissue allografts was first described in 1945 by the British immunologist Peter Medawar 146 , 147 . Only three years later, George Snell described the MHC, which carries the histocompatibility genes, and one decade later, Jean Dausset described the human leukocyte antigen (HLA); each of these scientists was recognized with the Nobel Prize in Physiology and Medicine 148 . Since its discovery, MHC has emerged as the most polymorphic gene locus in eukaryotes with 24093 HLA and related alleles, more than 362709 nucleotide variants reported in the Individual-Participant Data–International ImMunoGeneTics/Human Leukocyte Antigen (IPD–IMGT/HLA) work group database ( https://www.ebi.ac.uk/ipd/imgt/hla/ ), release 3.39.0, 2020/01/20 149 .
Although the main barrier for long-term organ and tissue grafting is driven by HLA incompatibilities, other important players play roles in transplant rejection. In particular, minor histocompatibility antigens, which are peptides derived from allelic variants of normal cellular proteins, presented by class I or II MHC antigens induce cellular immune responses in HLA-matched individuals who lack the same allelic variant 150 .
Natural killer (NK) cells also play important roles in transplantation through their killer cell immunoglobulin-like receptors (KIRs), which are receptors for HLA class I molecules. NK cells expressing an inhibitory KIR-binding self-HLA can be activated when exposed to allografts that lack a ligand for the inhibitory receptor 151 . The locus that codifies these receptors displays a considerable degree of polymorphism, with 1110 alleles reported in the Individual-Participant Data–International/Killer Cell Immunoglobulin-Like Receptors (IPD/KIR) work group database, release 2.9.0, 2019/12/11 149 .
More recently, we have begun to appreciate the importance of non-HLA genetic factors in the development of transplant rejection; examples include polymorphisms in the genes encoding cytokines, such as tumor necrosis factors ( TNF ), interleukins ( IL-1 , IL-6 and IL-10 ), interferon gamma ( IFN-γ ), and transforming growth factor-β3 ( TGF-β3 ). Other genes encode pathogen recognition receptors, with nucleotide-binding oligomerization domain-containing 2 ( NOD2 ( CARD15 )) being the most widely studied, although conclusive data have not been obtained to date 148 .
Immunodeficiencies
Primary immunodeficiencies (PIDs) comprise a heterogeneous group of more than 400 genetic disorders that result in defects in the immune response 152 . PIDs are considered Mendelian disorders because they are mainly autosomal recessive disorders that often display incomplete penetrance, which affects the severity and onset of the disease. With the exception of immunoglobulin A (IgA) deficiency, PIDs are considered to be rare disorders, as their prevalence worldwide ranges from 1 to 9 among 100,000 people 153 . Unsurprisingly, these types of diseases are not uncommon in highly consanguineous populations such as those in the Middle East/Northern Africa (MENA) region. The incidence of consanguinity marriage in these areas ranges between 20 and 56%, which leads to a unique population in which autosomal recessive diseases arise, with the prevalence of PID in these countries as high as 30 in 100,000 people 154 .
Although more than 400 genes have been described for PIDs, approximately 60% of the causal genes remain unknown, and next-generation sequencing studies performed in MENA populations are contributing to the search for currently unknown genes that cause PIDs 155 . A complete and updated list of PID-causing genes and diseases can be found at the European Society for Immunodeficiencies (ESID) webpage ( https://esid.org ) 156 .
Clinical manifestations of PIDs are highly variable; many disorders involve an increased susceptibility to several types of infections, but some patients develop autoimmune diseases. Patients usually present recurrent sinus or ear infections or pneumonia within a one-year period; other indicators are failure to thrive, poor response to prolonged use of antibiotics, and persistent thrush or skin abscesses 153 .
Depending on the affected pathway, PIDs are associated with varying levels of severity, times of onset, and risks of infection by certain groups of microorganisms. According to the International Union of Immunological Societies (IUIS) ( https://iuis.org/committees/iei/ ), 430 inborn errors of immunity can be classified as follows: (a) immunodeficiencies that affect cellular and humoral immunity; (b) combined immunodeficiency (CID) with associated or syndromic features; (c) predominant antibody deficiencies; (d) diseases of immune dysregulation; (e) congenital defects of phagocyte number, function, or both; (f) defects in intrinsic and innate immunity; (g) autoinflammatory disorders; (h) complement deficiencies; and (i) phenocopies of a PID 156 , 157 .
However, PIDs are broadly classified as follows according to the component of the immune system affected:
T-cell immunodeficiency, e.g., defects in the IFN-γ/IL-12 pathway and mutations in the autoimmune regulator (AIRE) gene.
B-cell (antibody-mediated) immunodeficiency: gamma-globulinemia, X-linked common variable immunodeficiency (CVID), selective IgA deficiency, specific antibody deficiency, and IgG subclass deficiency.
Combined immunodeficiency: Wiskott–Aldrich syndrome, ataxia telangiectasia, DiGeorge syndrome and severe combined immunodeficiency (SCID).
Phagocyte defects: chronic granulomatous disease, hyperimmunoglobulin E (IgE) syndrome and leukocyte adhesion deficiency.
Complement defects (deficiency in early, late or regulatory complement components) 158 .
Autoinflammatory diseases
Systemic autoinflammatory diseases (AIDs) are characterized by recurrent acute inflammatory episodes secondary to a dysregulated inflammatory process that typically develops during childhood, with recurrent episodes of fever, rashes, and disease-specific patterns of organ inflammation. Genetically speaking, these are hereditary disorders, andto date, more than 40 genes (Table 1 ) have been identified as causes of AIDs, which can be grouped according to the pathway that is altered 159 .
Inflammasome . The inflammasome is a multiprotein intracellular complex that detects pathogenic microorganisms and stressors and activates the highly pro-inflammatory cytokines IL-1β and IL-18. Genes affected in this group are MEFV (Mediterranean fever pyrin innate immunity regulator), which is related to familial Mediterranean fever (FMF); NLRC4 ( NLR family CARD domain-containing 4); NLRP1 ( NLR family pyrin domain-containing 1) and WDR1 (WD repeat domain 1) 159 .
Type-I interferon (IFN)-mediated disorders . These disorders are characterized by the upregulated expression of genes induced by IFN. The gain of function by variants of TMEM173 (transmembrane protein 173) is the core manifestation of this disorder group, but other genes have been identified, including DDX58 (DExD/H-box helicase 58), DNASE2 (lysosomal deoxyribonuclease 2), POLA1 (DNA polymerase alpha 1 subunit) and USP18 (ubiquitin-specific peptidase 18 ) 159 , 160 .
Ubiquitination disorders . Ubiquitination is a process that marks proteins for degradation via the proteasome, which is required for the processing of intracellular antigens (such as virus proteins or mutated tumor proteins) and their presentation by class I HLA molecules. Ubiquitination involves three main steps: activation, conjugation and ligation, which are performed by ubiquitin-activating enzymes (E1s), ubiquitin-conjugating enzymes (E2s), and ubiquitin ligases (E3s). Ubiquitination disorders are caused by variants of the PSMB8 , PSMB9 , PSMA3 and PSM4 genes (proteasome 20S subunit beta 8, subunit beta 9, subunit alpha 3 and subunit alpha 4, respectively), affecting the proteasome subunits, proteasome maturation protein gene (POMP) and/or proteasome assembly chaperone 2 (PSMG2) , by encoding proteasome assembly molecules 161 . In addition, other genes in this group, such as OTULIN ( OTU deubiquitinase with linear linkage specificity), encode ubiquitin peptidases, i.e., proteins involved in ubiquitination assembly complexes, such as HOIL-1 (heme-oxidized IRP2 ubiquitin ligase 1) and HOIP (NHP2-like protein 1 homolog) . Finally, the loss of function due to variants of the TNFAIP3 ( TNF-alpha-induced protein 3, also known as A20 ) gene, which encodes a protein with ubiquitin ligase and ubiquitinase activity, has also been described 159 .
Inflammatory or innate immune regulators . A large number of genes have been found to affect the pathways/mechanisms involved in macrophage and B-cell differentiation and lymph node development, among many functions. Genes in this group include ADA2 ( adenosine deaminase 2), TNFRSF11A ( TNF receptor superfamily member 11a), ADGRE2 ( adhesion G protein-coupled receptor E2), TRNT1 ( tRNA nucleotidyltransferase 1), LACC1 (laccase domain-containing 1) and AP1S3 ( adaptor related protein complex 1 subunit sigma 3) 159 .
Allergic diseases can be termed complex diseases that involve both genetic and environmental factors, and they influence not only the development of IgE-mediated sensitivity in the case of hypersensitivity type I allergies but also the subsequent development of clinical symptoms in a range of tissues, including skin, nose, and lung tissue 162 .
Since the first report of a link between chromosome 11q12 and atopy in 1989 163 , knowledge about the common risk variants for allergic diseases has increased exponentially, mainly because of GWAS. Most allergic diseases have allergy-related traits such as asthma, with the strongest association mapped to chromosome 17q21. However, the disease-associated gene at this locus remains unclear; one of the candidate genes is ORMDL3 (sphingolipid biosynthesis regulator 3) due to its role in sphingolipid synthesis and the regulation of eosinophils. Other genes associated with asthma are interleukin 33 ( IL33) and its receptor, IL1RL1 (interleukin 1 receptor-like 1), HLA region, SMAD3 ( SMA- and MAD-related protein 3) and IL2RB ( interleukin 2 receptor subunit beta) 164 .
As asthma and other allergic-associated traits could be present in patients without allergies, some researchers performed GWAS analysis on cohorts of patients who had high levels of allergen-specific immunoglobulin E (IgE) or a positive skin prick test. As a result, 18 loci were identified, and the strongest association was on chromosome 11q13. This locus has been associated with two genes: C11orf30 ( EMSY transcriptional repressor, BRCA2 interacting), a potential regulator of interferon-stimulated gene, and LRRC32 ( leucine rich repeat-containing 32), which is involved in Transforming Growth Factor Beta (TGFβ)-signaling in T regulatory cells.
The rest of the associated loci involved in the pathogenesis of allergy highlight the importance of the Th2 responses ( STAT6 (signal transducer and activator of transcription 6), TSLP ( thymic stromal lymphopoietin), BCL6 ( B-cell lymphoma 6 protein), IL1RL1 ( interleukin 1 receptor-like 1), IL33 ( interleukin 33), GATA3 ( trans-acting t-cell-specific transcription factor binding protein 3) ) ; innate immunity ( TLR1/6/10 (Toll-like receptor 1/6/10) ); TGFβ-signaling ( LRRC32 ( leucine rich repeat-containing 32), SMAD3 ( mothers against decapentaplegic homolog 3)); T-cell ( IL2 (interleukin 2), PTGER4 ( Prostaglandin E Receptor 4)) and T regulatory box ( LRRC32 ( leucine rich repeat-containing 32), IL-2 , NFATC2 ( nuclear factor of activated T cells 2), FOXA1 (forkhead box A1)) 164 .
In the last two years, researchers have focused on epigenome-wide association study (EWAS) of allergy processes. The epigenetic landscape is specific for a given cell; thus, EWAS requires careful selection of the relevant cell type for a given biomedical condition. For allergies, EWAS has mainly been performed on nasal mucosal cells and whole blood (although the result was later normalized by the number of circulating eosinophils). Nasal mucosal cells comprise CD8 + T cells, CD4 + T cells, myeloid cells, innate lymphoid cells, B cells, double-negative T cells, granulocytes, CD117 + cells, and plasma cell populations 165 . In all of these studies, 36 CpG-associated regions were identified, from which the SMAD3 gene, coding for an important regulator of T-cell differentiation, was replicated in three independent cohorts 166 . Of all of the genes in whole blood identified using EWAS, only the ACOT7 ( acyl-CoA thioesterase 7), EPX ( eosinophil peroxidase), GJA4 (gap junction protein alpha 4) and METTL1 (methyltransferase-like 1) genes were confirmed in the nasal cell populations 167 .
Cancer immunology
In 1909, Ehrlich proposed the idea that mutant cells arise continuously and that the immune system scans for and eradicates these mutant cells before they manifest clinically 168 . However, immune surveillance remained a controversial topic until its acceptance in the 1990s 169 .
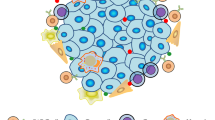
Immune surveillance is the recognition and elimination of cancerous cells by lymphocytes, which act as sentinels that recognize transformed cells. Ultimately, during tumor progression, cancer cells show low immunogenicity and resistance to immune effector cells, thus expanding and escaping immune control. The way in which cancer cells modify the immune system has been called immune editing 169 .
The key of immunosurveillance is cancerous cell expression of tumor antigens that can activate various immune cell phenotypes; for simplicity, any overexpressed, mutated, dysregulated, or rearranged gene product expressed by a cancerous cell may be considered a tumor antigen. It is critical to consider that most of these proteins, except those derived from virus-infected cancer cells, are primarily self-proteins, but they are expressed with mutation(s) or minor changes in their antigenic structure 170 .
One mechanism by which cancer cells escape from immune recognition is antigenic modulation. For example, the loss of MHC class I molecule expression leads to aberrant antigen masking, which is one of the mechanisms described for tumor cells that escape specific antitumor T-cell immune responses 171 . In addition, the MHC-peptide-T cell receptor complex elicited by a tumor antigen shows weak stability, since high-affinity T-cells tend to be rendered tolerant to these antigens 172 .
Another mechanism is the direct inhibition induced by cancer cells due to their interaction with surface regulatory molecules, also called checkpoint molecules. These molecules include programmed cell death-1 (PD1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), which induce the inhibition of host T cells. Although these checkpoints usually help conventional immune responses control immune activation, they can also be used by tumor cells to inhibit antitumoral T-cell responses 173 .
PD1) is a transmembrane protein expressed on T, B, and NK cells, and it binds to PD1 ligands (PD-L1 and PD-L2) on target cells. When it binds to its ligand on tumor cells, PD1 inhibits tumor cell apoptosis, causes peripheral effector T-cell exhaustion, and promotes the conversion of effector T cells into regulatory T cells 172 , 174 .
CTLA4 is also a physiological negative regulator of T-cell activation. The interaction with CD80/CD86 in the tumor leads to the inhibition of T-cell function and suppressed effector activity 175 . Knowledge of these two checkpoint inhibitors has opened the door to new antitumoral therapeutic approaches, such as the use of monoclonal antibodies that block the aforementioned interactions (anti-PD1, anti-PD-L1, or anti-CTLA-4), which are called checkpoint inhibitors 176 .
In addition, tumor cells create an inhibitory microenvironment around them. Malignant cells can recruit other cells, such as immune cells and fibroblasts, which can be corrupted by tumor cells. The interaction between tumor and nontumor cells creates the tumor microenvironment, which is mostly driven by the dynamics of the tumor promoting the proliferation/expansion of cancer cells. For example, tumor and stromal cells release multiple factors, such as the chemokine CCL28 (C-C motif chemokine ligand 28), which inhibits effector T-cell functions and attracts Tregs to the microenvironment 172 .
Tumor cells use different mechanisms to promote cancer progression and further metastasis. The complete immunological eradication of cancer is the goal of antitumoral immunotherapy and is discussed later in this review.
Immunosenescence and inflammaging
Aging is accompanied by the decline and dysregulation of immune efficacy, which results in an increased vulnerability to infectious diseases, diminished responses to vaccination, and reduced tumor clearance. Immune alterations mainly manifest as a reduction in the number of naïve peripheral blood cells and a relative increase in some types of memory cells 177 .
Natural aging causes progressive atrophy of the thymus, which is called thymic involution. The endpoint is a significant decrease in naïve T cells, which reduces the diversity of the T-cell antigen receptor (TCR) repertoire and culminates in disrupted T-cell homeostasis 178 . The cellular and molecular hallmarks of aging have been described as genomic instability, telomere attrition, epigenetic alterations, sarcopenia, changes in intracellular communications, cellular senescence, immunosenescence and mitochondrial dysfunction 179 .
The process of aging alters the innate and adaptive immune systems. In terms of innate immunity, aging results in a decreased number of circulating monocytes and dendritic cells, reduced phagocytic properties of macrophages and neutrophils, and impaired antigen presentation by dendritic cells 179 . As mentioned above, aging also generates a reduction in the T-cell and B-cell receptor repertoire due to the accumulation of senescent or exhausted lymphocytes, together with a decrease in the number of circulating naïve T and B cells 178 , 179 . On the other hand, NK cell cytotoxicity is maintained in centenarians, and an increase in the number of these cells is observed in healthy aging people 177 . Moreover, CD4+ T cells exhibit cytotoxic features in centenarians; this is an acquired characteristic for CD4+ T cells that usually have helper, but not cytotoxic functions under physiological conditions 180 .
In addition to these features, chronic inflammation is considered the key that underlies the phenomenon called ‘inflammaging’, which is related to elevated self-reactivity and results in the typical chronic low-grade, systemic inflammatory phenotype observed in the elderly in the absence of acute infection. Currently, it is believed that self-reactive T cells are the main contributors to this process. It has been proposed that this basal inflammatory state contributes to the development of some diseases, such as Type II diabetes, Alzheimer’s disease and atherosclerosis 178 . Understanding the mechanisms of age-related disorders in immune regulation is important for identifying more efficient strategies of immune rejuvenation and for the effective induction of vaccination-mediated immunity in older individuals 177 .
Immunotherapy
Immunotherapy includes the use of certain components of the immune system (antibodies, cells, cytokines, etc.) for the treatment of various cancers and autoimmune diseases and the manipulation of the immune system through vaccines for the prevention and treatment of infectious and allergic diseases (Fig. 1 ).
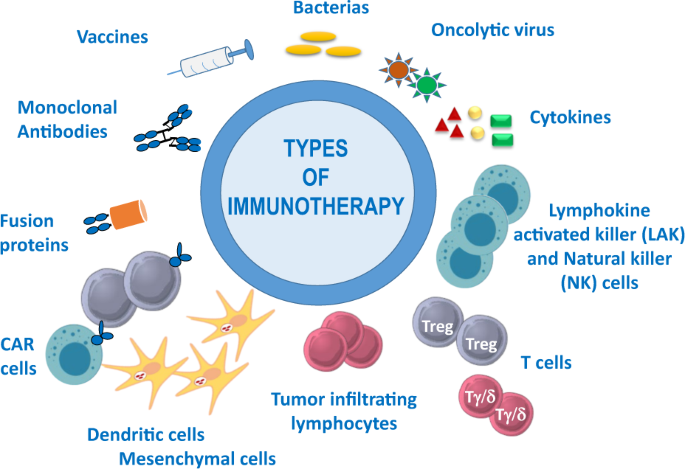
Examples of immunotherapy, including the use of vaccines, monoclonal antibodies, fusion proteins, bacteria, oncolytic viruses, cytokines, and different types of cellular immunotherapy: chimeric antigen receptor (CAR) T cells, dendritic and mesenchymal cells, tumor-infiltrating lymphocytes, regulatory (Treg) and gamma/delta (Tγ/δ) T cells, lymphocyte activated killer (LAK) and natural killer (NK) cells
Immunotherapy using microorganisms or their components in vaccines was first practiced centuries ago; soluble substances such as poly- and monoclonal antibodies, as well as cytokines, have been used for many years, but recently, cellular immunotherapy has emerged in clinical practice. Although immunotherapy can be used for many diseases (infections, autoimmune diseases, macular degeneration, allergic diseases, etc.), it is being used most expansively in the cancer field. The main goal is to destroy the tumor, either directly or indirectly (by enhancing the patient’s immune system), while offering greater specificity and fewer side effects than conferred by conventional therapies.
Pathogens and vaccines for infectious diseases
Immunotherapy associated with pathogens was first linked to the prevention of infectious diseases, starting from variolization (in the X century), followed by Edward Jenner’s vaccination against smallpox (in the XVIII century) and subsequently many other preventive vaccines for infectious diseases. The great advances in the knowledge about infectious diseases took place in the nineteenth century, but the XX and XXI centuries are clearly the vaccination centuries, as many new successful vaccines (with attenuated or dead pathogens, subunits, recombinant proteins, carbohydrates or DNA) introduced against a variety of pathogens. Currently, vaccines are among the factors that, together with hygiene, antibiotics and surgery, save the most lives 181 . Vaccination enabled the eradication of smallpox infection worldwide in 1980, and we are quite close to eradicating polio 182 . However, new and better vaccines are urgently needed; e.g., a vaccine against the new coronavirus 2019, SARS-Cov-2; prevalent pathogens, such as human immunodeficiency virus (HIV); parasites, such as Plasmodium spp., which produce malaria; and bacteria, such as Mycobacterium tuberculosis . However, anti-vaccine groups in more affluent countries are putting society at risk for a return of the serious illnesses that had almost been forgotten, such as diphtheria and tetanus 183 , with an increase in measles in unvaccinated people at epidemic levels, thus negating many of the advances made over many years.
Therapy with microorganisms
Whole pathogens or their products can also be used in human therapy for some types of cancer. At the end of the XIX century, the father of immunotherapy, Dr. Coley, popularized the use of extracts from cultures of Streptococcus pyogenes and Serratia marcescens 184 (called Coley’s toxin) for the treatment of patients with sarcoma, lymphoma, testis cancer, etc., but because of variable results and, indeed, cases of death, these treatments were discontinued. Later, because of the research on cancer performed by Dr. Lloyd J. Old with Mycobacteria , bacillus Calmette-Guérin (BCG) was approved by the American Food and Drug Administration (FDA) in 1976 for use in a therapeutic procedure for bladder cancer —a treatment that is still in use today 185 , 186 .
More recently, and with the increased knowledge of the human microbiome, the use of microorganisms in therapy has seen a resurgence. Some intestinal infections, such as those produced by Clostridium difficile , can be cured with the transfer of intestinal bacteria from healthy people (feces transplantation) 187 . Numerous other attempts to use microorganisms to cure inflammatory illnesses (Crohn’s disease, ulcerative colitis, etc.) have met with limited success 188 , which indicates that this type of therapy is much more complex than initially anticipated. As a consequence, many more studies are required to ensure that this approach can be used for curative immunotherapy. Researchers are also working on genetically modified or artificial bacteria (e.g., based on Salmonella enterica , Listeria monocytogenes or Lactobacillus lactis ), but only limited effects have been observed to date 189 .
Oncolytic viruses (OVs)
Although the use of bacteria in antitumoral therapy has been largely restricted, the use of therapeutic viruses is increasing. Virus-based therapy was introduced in the 1990s with the use of adenovirus, but only in recent years has it been used in practice in the clinic. Oncologic viruses 190 have the capacity to attack tumor cells in a preferential manner and induce immunogenic cell death (ICD) and host antitumor immunity (Fig. 2 ).
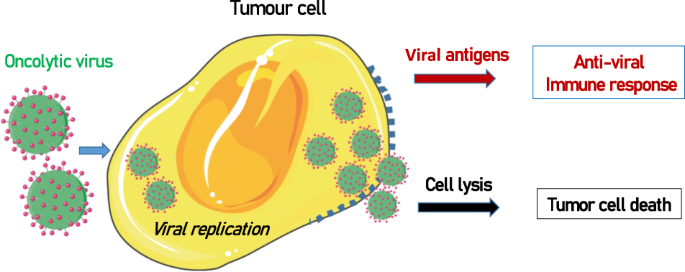
Oncolytic viruses replicate inside tumor cells, which causes cell lysis. In addition, the expression of viral antigens induces an antiviral immune response that helps destroy tumor cells
The first virus approved for use in therapy was a recombinant oncolytic adenovirus named H101, which was licensed in 2005 by the China Food and Drug Administration (CFDA) for treating head and neck carcinoma in combination with chemotherapy 191 . Ten years later, the oncolytic attenuated-modified virus herpes simplex I-talimogene laherparepvec (T-VEC, Imlygic®) was approved by both European (EMEA) and American (FDA) agencies for the treatment of melanoma 192 . The virus is modified by the insertion of human GM-CSF and deletion of the ICP47 gene. Since the approval of T-VEC, a new era has dawned on the use of OVs in cancer therapy 193 , 194 .
Currently, oncolytic viruses from the Adenoviridae , Herpesviridae , Picornaviridae , Reoviridae and Poxviridae families are in different phases of clinical studies for several types of tumors 194 , 195 . For example, reovirus against brain tumors (alone or combined with other therapies) 196 or Maraba virus against triple-negative breast tumors 197 , 198 offer some hope to patients with these types of cancer.
Viral sequences can be modified by genetic engineering techniques, thus making the virus more prone to infect some cells and enhancing viral infiltration and tumor tropism. Combinations with other components (immunomodulators, drugs, and cytokines) are also being explored to suppress antiviral immunity and enhance antitumoral cytotoxicity 199 .
Other vaccines
Vaccines for cancer prevention.
It is clear that certain viruses and bacteria play roles in cancer development. Viruses such as genital herpes, hepatitis B, Epstein Barr or human papilloma and bacteria such as Helicobacter pylori have been associated with cancers of the uterus and liver, in Burkitt’s lymphoma, and oral/genital and stomach cancers, respectively 200 . Therefore, immunization against these pathogens offer protection not only from infection but also from cancer.
Therapeutic vaccines
Once an illness has developed, the intention of a therapeutic vaccine is to eliminate or decrease its pathology. Thus, vaccines are used for cases of allergies, cancers and autoimmune diseases.
Allergy (Type 1)
Allergen-specific immunotherapy (AIT) aims to modulate the immune system against an allergen, thus modifying the natural course of the allergic disease and conferring long-lasting benefits 201 . The basic AIT involves the introduction of repeated doses of allergen (either injectable or sublingual allergen extract tablets) and often in escalating doses in a controlled manner, followed by a maintenance phase. In cases for which long-lasting tolerance is acquired, therapy may be discontinued. Allergen extracts can be obtained from different sources, such as cat hair and pelt, mites, different types of pollen, venom protein, foods, etc. Allergy vaccines are currently the only effective therapy that can stop the progression of the illness because treatment with anti-inflammatory drugs, such as anti-histaminic drugs or corticoids, mitigates the symptoms of the allergic processes but does not modify the natural course of the disease 202 , 203 .
AIT has been shown to induce the activation of antigen-specific Tregs and IL-10-producing Bregs (Br1) subtype cells, which is combined with anergy caused by Th2 cells 201 and the production of allergen-specific IgG antibodies that can compete with IgE for binding to allergens 204 .
In the past, most vaccines were developed using natural allergen extracts. However, significant progress has been made in recent years to correctly characterize the allergen at the molecular level, and some of these allergens are now being produced by recombinant technologies, nucleic acid-based strategies, or synthetic peptide chemistry 205 .
Another therapeutic approach for vaccines is in the field of cancer. Therapeutic cancer vaccines that contain self- or nonself-patient tumor lysates, viral vectors, mutated tumor proteins or peptides, among other types 206 administered in the presence of adjuvants can activate the immune system to induce antitumoral responses 207 . The goal is to activate the Th and Tc cell compartments to expand specific cytotoxic T and NK cells directed against tumor cells.
Some vaccines are more immunogenic than others, and this effect can be related to several factors, such as the types/numbers of genetic mutations in the tumor, expression of neoantigens, production of viral proteins, an immunosuppressive environment, lack of expression of histocompatibility complex molecules, etc., which together may explain the large variability in tumor elimination 208 . Therapeutic cancer vaccines are generally very safe, and major secondary effects have not been observed, although large differences in patient responses are detected. Moreover, this strategy may be used in conjunction with other complementary therapies 209 , such as monoclonal antibodies, chemotherapy or cellular therapy 209 , 210 . Several patients are currently taking part in clinical trials and are receiving therapeutic cancer vaccines against different types of tumors, such as lung (ClinicalTrials.gov Identifier: NCT04397926), prostate (ClinicalTrials.gov Identifier: NCT03525652) or pancreas (ClinicalTrials.gov Identifier: NCT04161755), using individual or combined therapies.
Autoimmunity
In the case of therapeutic vaccines for autoimmune diseases, such as multiple sclerosis, diabetes, Myasthenia gravis or Guillain Barré syndrome, the intention is to induce tolerance to self-antigens through the activation of regulatory cells (Tregs and Bregs) and tolerogenic dendritic cells, thus avoiding the immune response to self-components 211 . Due to the large variety of autoimmune diseases, the different etiologies and extensive variability, even in the same type of disease, designing a vaccine that can be useful for a wide range of patients is very difficult.
However, several researchers are obtaining good results in animal models with nanostructures and peptides that induce specific tolerance, and it is predicted that, in the near future, these types of therapies will be applied to patients suffering from autoimmune diseases (reviewed by Serra and Santamaria 212 ).
Polyclonal antibodies (pAbs)—serotherapy
The discovery of antibodies by Dr. E. von Behring and Kitasato 213 at the end of the XIX century highlighted the potential of antibodies to neutralize tetanus and diphtheria toxins. This discovery opened the way to exploring the potential clinical applications of conventional antiserum-containing polyclonal antibodies from immunized animals/humans 214 . This “serotherapy” was initiated by Dr. Roux and Dr. Yersin, who used anti-diphtheria serum to treat several children 215 . After this initial success, the use of serotherapy was increased for use against diphtheria and other diseases but also led to the identification of problems, such as immunogenicity with the formation of immune complexes (Arthus reaction), the variability and limitation of the antibody batches, the content of a mixture of classes and subclasses of antibodies with different biological activities, and their temporal effects. For all of these reasons, therapy with polyclonal antibodies was very much restricted to special cases, such as the use of gamma-globulins for the prevention of Rhesus (RH) maternal-fetal incompatibility and tetanus or snake venom toxicity 216 .
With the identification of gamma-globulin-deficient patients by Dr. Bruton in 1952 217 , the use of immunoglobulins as therapeutic molecules for the treatment of humoral immunodeficiencies was initiated. However, some problems were encountered in the initial phases, mostly related to the serum preparation and aggregation/fragmentation of antibodies. Since their initial use, several efforts have been made to avoid impurities and to improve the purification process, and several commercial products are now available (as intravenous or subcutaneous preparations). Currently, many patients with humoral immunodeficiencies are successfully being treated to prevent them from catching infectious diseases. More recently, the therapeutic applications of immunoglobulins have expanded to other diseases, such as against COVID-19 caused by SARS-Cov-2 infection (see below), autoimmune disorders and Kawasaki syndrome in children 218 . The beneficial effects seem to be mediated by several immunological mechanisms, including viral neutralization, inhibition of inflammatory cells and activation of immune regulators 214 .
Monoclonal antibodies (mAbs)
The development of monoclonal antibodies (mAbs) by C. Milstein and G. Köhler in 1975 219 (Nobel Prize winners for Physiology/Medicine in 1984) changed medicine and immunology completely, along with many other disciplines. Monoclonal antibodies are produced from the fusion of two cells to generate a hybrid cell or hybridoma with two characteristics, i.e., the production of one specific antibody and immortality. Dr. Milstein is considered to be the father of modern immunology for his crucial contribution 220 . The development of many different mAbs has enabled the identification of new molecules and the development of more accurate diagnostic approaches; specific, fast and inexpensive technologies; processes for the purification/concentration of compounds; and better and more specific therapy. mAbs can now be used against specific targets according to the concept of the “magic bullet”, a term coined by Dr. Paul Ehrlich at the beginning of the XX century (reviewed in ref. 221 ).
Numerous different mouse and rat mAbs were produced against several molecules, but due to their murine origin, patients treated with these mAbs suffered from hypersensitivity and immune responses 222 , 223 . Thus, most mAbs currently used in clinical applications are linked to radioactive elements and used for diagnostic purposes (Table 2 ).
In an effort to avoid immunogenicity, mAbs were subsequently modified by genetic engineering approaches to carry mostly sequences of human origin. Several research groups and companies developed chimeric and humanized mAbs (Table 2 ), and these mAbs included additional modifications, such as changes in the carbohydrates (glycosylation) and/or antibody regions, with the aim of improving their therapeutic action 224 , 225 , 226 , 227 , 228 . Moreover, fragments of recombinant antibodies (Fabs, single-chain Fvs, different V regions, fusion proteins, smaller antibodies, etc.) increased the variability of these potential therapeutic agents.
The generation of fully human mAbs took more time due to technical difficulties and ethical issues; therefore, researchers sought alternative methods to conventional approaches, such as the development of transgenic animals carrying human immunoglobulin genes using minilocus vectors, artificial yeast/human chromosomes or P1 vectors. The generation of knockout mice (in which mice lack Ig genes) and further crosses with transgenic mice carrying human antibody sequences led to the generation of mouse strains that were able to produce fully human mAbs 229 , 230 . Other initiatives, such as the generation of immunodeficient mice in which human bone marrow or libraries of recombinant phages carrying human variable genes were reconstituted, allowed the development of more fully human antibodies (Table 2 ). Sir Greg Winter, Nobel Prize winner in Chemistry in 2018 231 , 232 , became the pioneer of mAb humanization through the genetic engineering of an antibody (Campath 1), later developing a fully human antibody (antitumor necrosis factor alfa, TNF-a) using recombinant phage technology 225 , 233 , 234 . Several companies are currently developing human antibodies using these and new technologies (reviewed in 225 , 227 , 233 , 234 ).
Since 1975, the list of approved mAbs for human therapy has continued to increase (Table 2 ), and many more mAbs are in clinical trials 235 , 236 , 237 . The versatility of mAbs is based on a different mechanism of action 238 :
Neutralization/blocking of soluble elements. For example, the neutralization of cytokines (TNF-α) and growth factors (vascular endothelium growth factor) prevents the exhibition of their effects, i.e., inflammatory and angiogenic effects, respectively 239 , 240 .
Complement activation. IgG/IgM antibodies activate the complement cascade by the classical route, which leads to the death of the target cell 241 , 242 .
Cytotoxicity mediated by NK cells. NK cells can facilitate mAb killing of target cells. The mAb, after binding to a target cell, can attach to Fc receptors on the surface of NK cells to trigger the release of granzymes and perforin, thus inducing cell target death 243 , 244 .
Induction of cell death by apoptosis. Certain mAbs directed against some membrane molecules can directly activate apoptosis 243 .
Blocking activation signals. Antibodies can block some membrane receptors and avoid cell activation/proliferation activation/proliferation 243 , 245 .
Carriers of toxins, pro-drugs, enzymes, and radioactive elements. mAbs are able to concentrate select compounds around target cells, providing a much more selective therapy than conventional chemo- or radiotherapy 244 .
Check point inhibitors. Leading to a recent revolution in cancer therapy, the identification of several inhibitory molecules can be blocked by mAbs, thus leading to the activation and proliferation of antitumoral T cells. Molecules such as CTLA-4 and PD1 and its ligand PDL-1, maintain immune cells under controlled conditions. However, it is possible to reactivate the antitumoral immune responses by blocking some of these molecules with mAbs, either directed to only one of them or by using various antibodies in combination (for example, against CTLA-4 and PD1) 246 .
The results obtained with these therapeutic mAbs against checkpoint inhibitors in some types of cancer have been amazing. For their contribution to the understanding of the roles of CTLA-4 247 and PD-1 248 , the Swedish academy gave the Nobel Prize in 2018 to Dr. J.P. Allison and Dr. T. Honjo, respectively 249 . However, this therapy is not efficacious in all types of cancers for several reasons, such as the expression of these and other checkpoint inhibitors in immune cells, the number of antitumoral cells in each patient, an immunosuppressant microenvironment, the rate of cancer mutations, and the expression of histocompatibility molecules.
Recombinant proteins
There is a large list of recombinant proteins that are currently being used for human therapy, including interleukin 2 (IL-2), interferons (IFNs) and GM-CSF.
IL-2 was identified in 1976 as a growth factor for T lymphocytes, and soon after Dr. Rosenberg started to use it in antitumoral therapy 250 , 251 . Years later, in 1991, IL-2 was approved by the FDA for patients with metastatic renal cancer and in 1998 for the treatment of metastatic melanoma 251 .
Interferon (IFN) was described in 1957 by Isaacs and Lindenmann 252 . The interferon family is the largest family of cytokines and is classified into three different types (I, II, and III). Type I IFNs (including IFN-α and IFN-β) exhibit several molecular actions that may be very relevant for use in therapy for a range of pathologies (such as autoimmune diseases and cancers) 253 . In 1986, the FDA approved human IFN-α2a and IFN-α2b for patients with hairy cell leukemia and later on for patients with multiple sclerosis. Since their initial use, these interferon species have been approved for many other diseases, including chronic hepatitis B and C, lymphoma, advanced melanoma, and as adjuvants together with other therapies for several types of cancers 254 , 255 .
Another cytokine is GM-CSF, which activates the production of granulocytes and monocytes from bone marrow myeloid progenitors and has shown adjuvant antitumoral effects 256 , 257 . Other cytokines, such as IL-5, IL-7, IL-12, IL-15, IL-18, and IL-21 258 , 259 , are being tested in several clinical trials, and it is expected that some of them, either alone or in combination, can be used in future antitumoral therapy.
Other recombinant proteins are already on the market, some of which are derived from antibodies, with some advantages such as small size, low immunogenicity and general ease of production. Examples are etanercept and abatacept (CTLA-4 Ig), which were approved by the European Medicines Agency in 2000 and 2007, respectively. The former is a chimeric protein that carries the external portion of the tumor necrosis factor (TNF) receptor linked to the IgG Fc region, which captures soluble TNF to block its inflammatory effects 260 . The latter example is a fusion protein that combines the extracellular portion of human CTLA-4 and IgG1 Fc. Abatacept is a competitive inhibitor that blocks T-cell activation and can be used in the treatment of inflammatory illnesses such as rheumatoid arthritis 261 .
Cellular immunotherapy
Natural killer (nk) and lymphokine-activated killer (lak) cells.
Natural killer (NK) cells were described in the 1970s based on their capacity to eliminate tumor cells without prior sensitization, with differences observed compared with specific cytotoxic T cells (which are activated based on the recognition of the target cells) 262 , 263 . In 1985, Piontek et al. reported that NK cells have the ability to preferentially kill cells that had lost the expression of the major histocompatibility complex class I molecules 264 , 265 .
Lymphokine-activated killer (LAK) cells are a heterogeneous population that includes not only NK but also NKT and T cells, which can be generated in an in vitro culture of peripheral blood mononuclear cells (PBMCs) in the presence of IL-2 266 . Dr. Rosenberg and collaborators carried out studies using these cells in the presence of IL-2 (reviewed by Rosemberg 251 ). These LAK cells showed good antitumoral responses in 22% of the melanoma patients who received them as therapy 250 . However, secondary effects such as liver toxicity and the expansion of the Treg population limited their therapeutic effect. Researchers started to design new recombinant IL-2 with some mutations to avoid the activation of Tregs 267 , with linking it to polyethylene glycol (PEG) to increase its half-life and efficacy 268 .
Another cytokine described later, IL-15, showed similarities to IL-2 in many respects 269 , and it had some unique advantages, such as the capacity to activate NK and cytotoxic T cells (Tc) but not Tregs. IL-15 is being used in different versions (alone, as a heterodimer with receptor IL-15/IL15Ra or IL15Rα IgFc, or in an agonist complex with ALT-803) 269 and in combination with other therapies in several clinical trials (examples: NCT01021059, NCT03905135, and NCT03759184).
More recently, researchers have focused their attention on other cytokines and combinations (such as IL-15, IL-12, and IL-18) 270 , which are able to activate NK cells in vitro and induce a good responses in animal models. In some human clinical trials, remission has been observed for patients with acute myeloid leukemia 271 , 272 , which broadens the options for the use of NK cells in the treatment of this pathology.
The properties of NK cells reveal their versatility as treatments against tumors. NK cells are able to kill tumors through several mechanisms, including receptor-mediated cytotoxicity, antibody-dependent cell-mediated cytotoxicity (ADCC) and death receptor-mediated apoptosis, but they also secrete cytokines such as interferon gamma that enhance the antitumoral adaptive immune response. NK cell adoptive transfer (either autologous or allogenic NKs) is currently being tested in clinical trials for hematological diseases and solid tumors, and numerous research groups have recognized their potential in other situations, such as transplant rejection and pregnancy. NK cell lines, memory-like NK cells and stem cell-derived NK cells are additional types of cells that can be used for tumor immunotherapy 273 .
Regarding other cellular therapies, NK cells as substitutes for T cells for use upon transformation with an chimeric antibody receptor (CAR) are being explored (see below).
Dendritic cells
Paul Langerhans identified dendritic cells in human skin in 1868 274 , but these cells were not named until 1973 by Dr. Ralph M. Steinman (Nobel Prize in 2011) and Dr. Zanvil A. Cohn, who chose the term because the cell morphology, with long extensions, resembles that of neuronal dendrites 275 . In humans, dendritic cells are obtained from different sources that vary in origin, maturation state and tissue distribution (skin, lymphoid tissue, circulating cells). Among the main types of dendritic cells, plasmocytoids are conventional myeloid DC1 and DC2, pre-DC and monocyte-derived dendritic cells. In the epidermis, there are three types: Langerhans cells (LC), monocyte-derived LC-like cells and inflammatory dendritic epidermal cells (IDECS) 276 . As indicated above, DCs are antigen-presenting cells and are the only cells that are able to activate naïve T lymphocytes. A subpopulation of DCs also carries out a process known as cross-presentation. In this way, they facilitate the activation of both helper and cytotoxic T lymphocytes 277 . In addition to their participation in the immune response, they can be used in antitumoral therapeutic vaccines 277 , 278 .
It is possible to generate a type of blood monocyte-derived dendritic cell in the presence of a mixture of cytokines in culture 279 —a process that induces their subsequent maturation and activation in the presence of tumor antigens (cell lysates, recombinant or purified antigens, peptides, RNA, DNA, and viral vectors 280 ). These cells can also be obtained from bone marrow hematopoietic CD34 + progenitor cells 281 . Other sources, such as circulating or skin dendritic cells, are relatively scarce and are therefore not usually used.
After their differentiation and activation in vitro 278 , 282 , DCs are exposed to tumor antigens and infused back into the patient (either by blood infusion or injected into areas near the lymph nodes or even directly into them) to reach the secondary lymphoid organs as soon as possible, at which point they can present antigens to the T cells. This approach is a type of individualized therapy and is therefore expensive.
The first approved vaccine in which autologous dendritic cells were used was Sipuleucel-T (Provenge) 283 , which was a treatment for prostate cancer refractory to hormonal treatment. Immunotherapy with dendritic cells is currently being tested in more than 200 clinical trials for various tumors: brain, pancreas, mesothelioma, melanoma and many others (ClinicalTrials.gov Identifiers: NCT01204684, NCT02548169, NCT02649829, and NCT03300843, respectively). The data indicate that the therapy is well tolerated and has led to increased patient survival in some trials. Furthermore, complete cure and partial remission outcomes have also been observed. The lack of efficacy on other tests was probably due to the presence of immunosuppressive factors in the tumor environment.
Another therapeutic use of dendritic cells involves their induction of immunosuppression both in transplants and in autoimmune diseases 284 . In an autoimmune pathology such as multiple sclerosis, the intention is to achieve stable tolerogenic dendritic cells that can act against some autoantigens (such as myelin peptides) in the presence of vitamin D3, dexamethasone, or other agents 285 . Phase I clinical trials have generally shown good tolerance to this therapy without serious adverse effects 286 .
However, greater control of this treatment is necessary in several respects to obtain the best therapeutic results 284 ; e.g., the type of dendritic cells and ex vivo differentiation, the antigens used, and the injection route are important considerations.
Gamma/delta T cells (Tγ/δ)
Human T cells expressing γ/δ TCR cells have interesting properties, including the capacity to kill a broad range of tumor cells. The advantages of these cells in cancer therapy are based on their independence from MHC expression on tumor cells and that their relative insensitivity to some inhibitor molecules (such as PD-1). The initial clinical application, with the adoptive transfer of autologous Vδ2+ cells after ex vivo expansion, showed only sporadic responses 287 , and different exploratory studies are currently being carried out to increase their clinical therapeutic use. Allogeneic Vδ2+ cells are also being explored in cancer therapy; e.g., they are being used against refractory hematological malignancies 288 and advanced cholangiocarcinoma 289 .
Regulatory T cells (Tregs)
Although the basis of immune regulation was suggested centuries ago, regulatory T cells were described by Sakaguchi et al. as CD4+ CD25+ natural regulatory T cells 290 that expressed the forkhead box P3 transcription factor (foxp3) 291 . Later, induced or adaptive regulatory T cells were also identified, including different subsets that carry several phenotypic markers and express various cytokine secretion profiles 292 . All of these factors play crucial roles in the maintenance of immunological self-tolerance by suppressing autoreactive T cells.
The manipulation of Tregs to achieve therapeutic outcomes is a field of great interest, because of both their expansion and activation in diseases, such as allergic and autoimmune diseases, and as a potential targets for cancer immunotherapy 293 .
Tumor-infiltrating lymphocytes (TILs)
Lymphocytes that infiltrate solid tumors are called tumor-infiltrating lymphocytes (TILs). In 1957, Thomas and Burnet proposed that the immune system performs tumor immune vigilance, with lymphocytes as sentinel cells leading to the elimination of somatic cells transformed by spontaneous mutations 294 , 295 .
Since the end of the 1980s, Dr. Rosenberg has been trying to prove and improve the effective use of TILs. The process starts with surgery and the isolation of TILs from a tumor, followed by TIL activation in culture in the presence of cytokines, cellular expansion and, finally reinfusion into the patient. Since its inception, this therapy has been improved markedly, with an increase in optimal responses from less than 30% to the current 50–75%, in some cases. These higher success rates are due, in particular, to the prior preparation of the patient, including the depletion of lymphoid tissues, to avoid an expansion of regulatory cells 296 , myeloid suppressor cells and other cells that can compete with the transferred TILs.
Currently, there are more than 200 trials in which TILs are being used alone or in combination with other immunotherapies on several tumors, such as melanoma, metastatic colorectal cancer, glioblastoma, pancreatic cancer, hepatobiliary cancer, ovarian cancer and breast cancer. This individualized therapy has limitations; it can only be used on solid tumors, and the number and specificity of the TILs and the type of tumor and microenvironment make standardizing this therapy difficult.
Chimeric antigen receptor (CAR)
Since TILs include a variety of T lymphocytes with different specificities, the next step was to obtain T cells of a single type (monoclonal cells) carrying a clonal receptor capable of recognizing tumor antigens. This effort was carried out for the first time in mice and subsequently, in 2006, in humans with a transgenic TCR against the MART-1 melanoma antigen 297 , 298 . These types of receptors are known as tTCRs, but their ability to recognize antigens is restricted since they can only identify the peptides presented by antigen-presenting cells on self-histocompatibility molecules.
This situation changed because of one of the latest revolutions in antitumor therapy, the development of T lymphocytes that carry a chimeric antigen receptor (CAR) based on a specific antibody directed to a target surface molecule 299 , 300 . These modified T cells can directly recognize tumor cells without required antigen processing or presentation by professional antigen-presenting cells. Moreover, the CAR includes all of the necessary elements for intracellular signaling and activation of helper and cytotoxic T lymphocytes.
CAR therapy was developed by one of its pioneers, Dr. Carl June at the University of Pennsylvania in the United States 300 , who used modified T lymphocytes that carried a chimeric antigen receptor to target CD19+ leukemic B cells. After interacting with CD19+ cells, these modified CAR T cells were activated and able to proliferate and exert cytotoxic functions against target cells. In this case, both tumor and healthy B cells were affected. Although bone marrow continues to produce B lymphocytes, in cases of severe B lymphopenia, it is possible to provide exogenous immunoglobulins periodically.
The whole process of the current CAR T-cell therapy begins with blood donation, from which lymphocytes are purified and genetically modified in vitro by a viral vector, which carries the genes coding for the chimeric antigen receptor. The cells are expanded in the presence of cytokines in culture and are subsequently reinfused into the patient. This type of cellular immunotherapy is individualized for each patient, with his/her CAR T cells ultimately destroying the tumor.
Since the first generation of CARs appeared, namely, a chimeric receptor composed of an anti-CD19-specific single-chain variable fragment linked to a transmembrane domain and intracellular signaling domain of the T cell receptor (CD3 ζ chain), researchers started to modify the original design. New generations of CARs, including the CD3 ζ subunit together with other signaling domains, such as CD28, CD134, CD137 (4–1BB), CD27, and ICOS, or combinations (CD3 ζ, CD28, and CD134) 301 , have been developed in the second and third generations of CARs to improve several aspects, such as the activation, proliferation and survival of CAR T cells. The fourth generation of CARs show improved the antitumoral effects by carrying additional molecules (such as cytokines or drugs), improvements to the safety of CAR T-cell therapy through the use of suicide genes 301 and many new designs, such as dual CARs or the so-called split universal and programmable (SUPRA) CAR system 302 .
In addition to T cells, other types of cells, such as NK cells, are now being explored for use in antitumoral responses 303 . In an effort to avoid using personalized treatment, researchers are now working on universal CARs that may be used on many different patients without inducing the problem of rejection 304 , 305 , 306 , 307 .
The encouraging results obtained with this therapy have led to interest from companies, and some commercialized examples are available, although many more “in-house” or academia-produced CARs are in clinical trials. CAR T-cell therapy was initially designed for use against hematological cancers (leukemia and lymphomas), but many new opportunities have been opened for its use against solid tumors 308 , infectious diseases (such as HIV) 309 , allotransplantation, autoimmune diseases 310 and severe allergies 311 . China and the USA are the leading countries in producing CAR T-cell therapy, and numerous clinical trials are underway.
Immunotherapy for COVID-19 patients
Coronavirus disease 2019 (COVID-19), which is produced by severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2), affects millions of people in many countries. Most of the infected patients (80–85%) are asymptomatic or have mild symptoms, but the disease in some patients progresses to a moderate or severe illness that requires hospitalization in intensive care units because of respiratory distress, multiorgan failure, and/or other pathologies, and more than one-half million fatal cases have been reported worldwide. The most vulnerable population includes aging patients and those with comorbidities such as hypertension, diabetes and cardiovascular diseases.
There are several aspects of the COVID-19 pathogeny that suggest an overreaction of the immune system in severely ill patients, with increased levels of inflammatory cytokines such as IL-6, IL-1 and others (creating the so-called “cytokine storm”), together with blood lymphopenia and CD8 T cell and NK cell exhaustion. Special therapies have not yet been identified to cure these patients, and preventive vaccines are not yet available, but some immunotherapies have been proposed as adjunct therapies, and some of these are currently in different phases of clinical trials 312 .
The immunotherapeutic strategies include the following:
Targeting inflammatory molecules . To attenuate the cytokine storm (IL-6 receptor, IL-6, IL-1, GM-CSF, VEGF, etc.), monoclonal antibodies against receptors and/or cytokines, receptor antagonists and/or inhibitors are proposed.
Passive immunotherapy . Patients who were infected and recovered, but developed neutralizing antibodies against the SARS-Cov-2 virus, can donate plasma to treat severe/critical patients. Some reports have indicated promising results in a low number of patients who received convalescent plasma 313 , 314 , but conclusions cannot be drawn until several randomized studies and more patients are analyzed. In addition to the use of convalescence plasma, hyperimmune globulin therapy or monoclonal antibodies directed against the virus have also been proposed, and clinical assays are ongoing.
Immunomodulation therapy . Intravenous immunoglobulins are aimed at blocking inflammation and preventing secondary infections 312 . This approach is being used with success in cases of Kawasaki syndrome in children.
Cellular immunotherapy . To date, very little attention has been paid to the cellular immunotherapy approach in treatments of COVID-19, but several attempts may include the use of SARS-Cov-2-specific T and NK cells to trigger antiviral responses and autologous or allogenic Tregs to modulate inflammatory processes.
Future challenges in immunotherapy
Immunotherapy has been used for centuries, but only in recent years has this area expanded rapidly in several respects, mostly by the use of soluble elements (monoclonal antibodies and cytokines) and, more recently, with immune cells (cellular immunotherapy). There are many fields in which immunotherapy faces a range of challenges:
1. Researchers are working on reducing the number of injections by employing a combination of vaccines. Several current vaccines contain components from 3–6 pathogens in a single injection, and these are able to provide adequate protection against all of these pathogens 315 .
2. Researchers are developing more stable and durable vaccines. Improvements in the half-lives of vaccines, for example, by lyophilization, while maintaining immunogenicity is expected to reduce current problems, especially those involved in the transportation of vaccines to remote areas 316 . In this respect, nanotechnology can help in the design of more stable vaccines that lead to slow antigen release and improved immunogenicity 317 .
3. Researchers are working on vaccines that confer protection against all serotypes of a specific pathogen (universal). This outcome is especially important for pathogens with high variability (such as the influenza virus). Researchers are designing vaccines that can protect against several variants by using common regions that can induce protective immune responses to all or most of the variants 318 .
4. Researchers are developing alternative routes of administration (e.g., oral, inhaled, intranasal, skin, rectum, vagina) as substitutes for intramuscular or subcutaneous injections. One of the problems to be solved is the immune tolerance developed to elements delivered by the oral route, but some vaccines are already effectively administered by this route (such as the oral polio, cholera, typhoid fever and rotavirus vaccines). The intranasal route has also proven effective for some vaccines (nasal influenza vaccine), and vaccines administered through other routes are under investigation.
5. Researchers are seeking the early protection of newborns 319 . Newborns are very susceptible to infections due to their immature immune system 320 . Moreover, the protection exerted by maternal antibodies transferred through the placenta during pregnancy against some pathogens interferes with the development of the newborn’s own immune response. Greater knowledge on ways to activate the immature immune system early will enable the development of vaccines for newborns. Moreover, immunization of pregnant women may help to enhance neonatal protection against several pathogens 321 .
6. Researchers are developing new and more effective vaccines. This effort is crucial for very prevalent pathogens such as Mycobacteria tuberculosis , HIV virus or plasmodium falciparum . Although there are treatments against these pathogens, most are not curative—as in the case of HIV; prevention is the best way to stop their spread.
7. Researchers are working to address emerging pandemics. In the case of new pathogens, such as SARS-Cov-2, which has produced a recent global outbreak, effective vaccines are urgently required 322 . New technologies for vaccine formulations and routes of administration, the identification of immune-related factors of protection and modifications to the governmental regulatory and approval process for vaccines for emerging pathogens are challenges that must be faced to achieve a rapid vaccination procedure for outbreaks. Hundreds of vaccines against SARS-Cov-2 (using different strategies such as live attenuated or inactivated pathogens, viral vector-based, viral RNA, DNA, recombinant proteins, peptides, etc.) 323 are now under development, and some are in clinical trials. However, the need to develop a new vaccine in a short period of time should not negate the main principles of vaccination use: safety and immune protection.
8. Researchers are working on genetic (RNA, DNA) vaccines because they have great advantages, including no requirement for growing a pathogen. Genetic vaccines can be obtained in a much shorter time, with much faster and safer production processes, and can be transported much more easily. However, the immunogenicity of these vaccines must be improved, and other problems need be avoided, such as the potential deleterious effects of integrating vaccine sequences into cells 324 .
9. Researchers are developing safer and more powerful adjuvants. Many years ago, the only adjuvant authorized for vaccines was aluminum hydroxide (alum), but currently, several adjuvants are on the market 325 . The use of ligands that activate the innate immune response, such as those linked to TLRs or nanostructures with adjuvant effects, is currently under study.
10. Researchers are boosting trained immunity, a new concept related to the innate immune memory-like described for NK cells (expansion) and macrophages (epigenetic modifications). Knowledge of how to handle trained immunity will enable better vaccine design and more effective secondary responses 326 .
11. Researchers are seeking to eradicate diseases from the earth. The greatest challenge, eradicating disease is possible in the short term for some pathogens, such as poliovirus. Very few cases of polio have been recently reported, and these reports came from only three countries; therefore, it is feasible that this disease can be eradicated in the near future.
12. Advances are challenged by the anti-vaccine movement. Paradoxically, there are people who doubt the beneficial effects of vaccines, and they are putting the health of their own children and society in general at risk 327 . The effectiveness of community protection conferred through vaccinated people is disrupted by decreased numbers of immunized persons. This lesser coverage enables pathogens to infect the most susceptible people, such as small children, elderly patients and those who cannot be vaccinated due to certain pathologies or because they are undergoing immunosuppression treatment. Thus, news about the return of illnesses that were nearly forgotten, such as tetanus in Italy (the first case in 30 years), the death of a child in Catalonia from diphtheria, or the exponential increase of measles cases (already counted in the thousands) worldwide 328 , should make parents think carefully about the risks of not protecting children by vaccines. The World Health Organization ( https://www.who.int/topics/vaccines/en/ ) argues that anti-vaccine movements can roll back all the achievements thus far in this field and have cited this issue as one of the main challenges to be resolved. Addressing the anti-vaccine movement requires a coordinated effort of professionals to inform parents adequately and perhaps other types of coercive measures that some countries are already applying (financial fines, denial of access to public assistance in childcare units, removal of authorization to travel/live in some countries, new laws, and so on).
Antibodies and cytokines
Immunotherapy with monoclonal antibodies has been a true revolution for many pathologies, as has the use of certain cytokines and recombinant fusion proteins. It is therefore predicted that these approaches may have a bright future, and regulatory agencies are expected to authorize many more mAb-based therapies in the coming years, especially given the good results obtained in clinical trials. Complete antibodies or those modified to increase their functionality or decrease their immunogenicity, combinations of antibodies and cytokines, antibody fragments, etc., are only some of the many possibilities for this type of product, which will expand the range of therapeutic options.
One of the main problems regarding the use of antibodies in therapy, especially in cancer, is based on their often unpredictable efficacy. Large variability in terms of remission and durable clinical benefits between patients is observed (for example, in the antitumoral responses by antibodies directed to the checkpoint inhibitors). Thus, the main challenge is to understand the situations in which an antibody will have the desired effect. It is crucial to find validated biomarkers (with predictive and/or prognostic value) that can help to stratify or select patients for the best immunotherapy. A better understanding is also required for tumor heterogeneity, resistance to some drugs and immunosuppressive microenvironments 329 . An in-depth immunological study, together with a personalized approach, is certainly the way to improve the success of these types of therapies.
In combination with conventional therapies (radiotherapy, chemotherapy, and surgery), other immunotherapeutic drugs or cellular immunotherapies can also help to maximize the efficacy of this immunotherapy, but increases in toxicity will be another challenge to face.
The use of oncolytic viruses (OVs), bacteriophages that selectively infect bacteria, modified pathogens for vaccines or for antitumor immuno-activation, and the manipulation/ modification of the microbiota are some of the therapies that are being considered.
OVs are designed to kill tumor cells and to activate the immune system against those cells. However, many of OVs have shown limited therapeutic effects when applied in monotherapy; therefore, much more work is required to improve their systemic antitumor effects and avoid the attenuation of the virus, which limits the viral replication. Several obstacles, such as low viral delivery and spread, resistance to therapy and antiviral immunity, have been observed 330 . Thus, the main challenges with oncolytic viruses are addressed by improving their antitumoral efficacy, including the optimization of viral delivery, the development of OVs engineered to activate the immune system (e.g., by releasing cytokines), and their use as adjuvant therapies or in combination with other immunotherapeutic agents, such as immunomodulators 331 .
Regarding gut microbiota manipulation as a therapeutic approach, fecal microbiota transplantation is an effective therapy for recurrent Clostridium difficile infection 332 and is now being investigated for other indications, such as inflammatory bowel disease and cancer. Some of the challenges facing microbiome transplantation are the lack of precise knowledge about the complete microbiome and the mechanisms of action involved in its therapeutic capacity, the large variability of its effectiveness and the external factors that affect it. More studies are centered on understanding how to manipulate bacterial colonies, the discovery of therapeutic molecules, nutrient competitions, etc., that are required for successful application. The best type of therapy (either individual or the combination of bacteria) is also under debate, along with how to reach the market by translating this individualized therapy into commercial scale products. The safety and potential adverse long-term effects are also being assessed.
Other components (nanomaterials and small molecules)
Nanomaterials. To obtain approval for the use of other elements from incipient fields, such as the use of different types of nanostructures, either alone or in combination with other immunotherapies, it is important to resolve certain issues. In the case of nanoparticle use, a better understanding of the interaction between nanomaterials and biological media; nanoparticle biodistribution, metabolism and biocompatibility; and the reproducibility of the synthesis and scaled up production of nanomaterials are among the issues to address.
Small molecules. A greater knowledge of several molecules involved in the immune system has led to the development of new therapeutic agents, which have been synthesized by traditional chemistry and block or activate intracellular signaling. The low cost of production of these molecules, along with the scaling and reproducibility of small-molecule batches, has attracted the attention of pharmaceutical companies interested in a whole set of new immunomodulatory drugs. A better understanding of the mechanism of action of small-molecule-based drugs and proof that they offer higher efficacy than existing therapies, either in monotherapy or in combination therapy, are challenges that face those seeking to engineer new types of targeting molecules.
To date, cellular immunotherapy has been an individualized therapy with high production costs, and it requires the involvement of multidisciplinary groups in hospitals. A real challenge in the field of cellular immunotherapy is the acquisition of universal off-the-shelf cell therapies to replace those currently made to order in a very personalized manner. The development of universal cells, for example, in the case of CAR T-cell therapy, would increase the number of patients who could benefit from this treatment at thus reduce the costs.
Other challenging aspects of cellular immunotherapy are the life-threatening toxicity of induced and their lack of effect on solid tumors, which is mostly due to the immunosuppressive tumor microenvironment. This approach requires new strategies to overcome these difficulties. In addition to cancer, cellular immunotherapy has a long history of use against autoimmunity, infectious diseases, allergies and transplantation rejection. It is also important to find biomarkers for prognosis/prediction that can help to optimize this method. Other therapies that involve the use of activated NK cells, tumor-infiltrating lymphocytes, vaccination with dendritic cells, etc., are having partial clinical success. Similar to other treatments, these approaches require further study, but it is feasible that they may become reality in the near future.
Conclusions
Greater knowledge of the immune system, especially concerning the variety of cellular and humoral components and the close regulation among them, the interaction with other systems or with elements such as the microbiota, will allow the development of new types of therapies that may be safer, more effective and specific but with much lower toxicity than found in current therapies. This long journey has been possible due to the efforts of numerous researchers (throughout the centuries), who have contributed with their work, creativity, successes and failures to advance our knowledge of the immune system, cellular components, membrane markers, interactions, signaling pathways and many more aspects. This great combined effort has paved the way for the achievements that are currently being realized.
Honda, K. & Littman, D. R. The microbiota in adaptive immune homeostasis and disease. Nature 535 , 75–84 (2016).
Article CAS PubMed Google Scholar
Yoo, B. B. & Mazmanian, S. K. The enteric network: interactions between the immune and nervous systems of the gut. Immunity 46 , 910–926 (2017).
Article CAS PubMed PubMed Central Google Scholar
Mate, I., Madrid, J. & Fuente, M. Chronobiology of the neuroimmunoendocrine system and aging. Curr. Pharm. Des. 20 , 4642–4655 (2014).
Dantzer, R. Neuroimmune interactions: from the brain to the immune system and vice versa. Physiol. Rev. 98 , 477–504 (2018).
Taams, L. S. Neuroimmune interactions: how the nervous and immune systems influence each other. Clin. Exp. Immunol. 197 , 276–277 (2019).
Vivier, E. et al. Innate lymphoid cells: 10 years on. Cell 174 , 1054–1066 (2018).
Sallusto, F. Heterogeneity of human CD4+ T cells against microbes. Annu. Rev. Immunol. 34 , 317–334 (2016).
Artis, D. & Spits, H. The biology of innate lymphoid cells. Nature 517 , 293–301 (2015).
Cherrier, D. E., Serafini, N. & Di Santo, J. P. Innate lymphoid cell development: a T cell perspective. Immunity 48 , 1091–1103 (2018).
Gasteiger, G., Fan, X., Dikiy, S., Lee, S. Y. & Rudensky, A. Y. Tissue residency of innate lymphoid cells in lymphoid and nonlymphoid organs. Science 350 , 981–985 (2015).
Ricardo-Gonzalez, R. R. et al. Tissue signals imprint ILC2 identity with anticipatory function. Nat. Immunol. 19 , 1093–1099 (2018).
Ebbo, M., Crinier, A., Vély, F. & Vivier, E. Innate lymphoid cells: major players in inflammatory diseases. Nat. Rev. Immunol. 17 , 665–678 (2017).
Abt, M. C. et al. Innate immune defenses mediated by two ilc subsets are critical for protection against acute clostridium difficile infection. Cell Host Microbe 18 , 27–37 (2015).
Simoni, Y. et al. Human innate lymphoid cell subsets possess tissue-type based heterogeneity in phenotype and frequency. Immunity 46 , 148–161 (2017).
Guia, S. & Narni-Mancinelli, E. Helper-like innate lymphoid cells in humans and mice. Trends Immunol. 41 , 436–452 (2020).
Melo-Gonzalez, F. & Hepworth, M. R. Functional and phenotypic heterogeneity of group 3 innate lymphoid cells. Immunology 150 , 265–275 (2017).
Shikhagaie, M. M. et al. Neuropilin-1 is expressed on lymphoid tissue residing LTi-like group 3 innate lymphoid cells and associated with ectopic lymphoid aggregates. Cell Rep. 18 , 1761–1773 (2017).
Boulenouar, S. et al. Adipose type one innate lymphoid cells regulate macrophage homeostasis through targeted cytotoxicity. Immunity 46 , 273–286 (2017).
Jacquelot, N., Luong, K. & Seillet, C. Physiological regulation of innate lymphoid cells. Front. Immunol. 10 , 405 (2019).
O’Sullivan, T. E. et al. Adipose-Resident Group 1 innate lymphoid cells promote obesity-associated insulin resistance. Immunity 45 , 428–441 (2016).
Article PubMed PubMed Central CAS Google Scholar
Weizman, O.-E. et al. ILC1 confer early host protection at initial sites of viral infection. Cell 171 , 795–808.e12 (2017).
Nussbaum, J. C. et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature 502 , 245–248 (2013).
Scanlon, S. T. & McKenzie, A. N. J. Type 2 innate lymphoid cells: new players in asthma and allergy. Curr. Opin. Immunol. 24 , 707–712 (2012).
Rankin, L. C. et al. Complementarity and redundancy of IL-22-producing innate lymphoid cells. Nat. Immunol. 17 , 179–186 (2016).
Kirchberger, S. et al. Innate lymphoid cells sustain colon cancer through production of interleukin-22 in a mouse model. J. Exp. Med. 210 , 917–931 (2013).
Vély, F. et al. Evidence of innate lymphoid cell redundancy in humans. Nat. Immunol. 17 , 1291–1299 (2016).
Sonnenberg, G. F. & Hepworth, M. R. Functional interactions between innate lymphoid cells and adaptive immunity. Nat. Rev. Immunol. 19 , 599–613 (2019).
Hepworth, M. R. & Sonnenberg, G. F. Regulation of the adaptive immune system by innate lymphoid cells. Curr. Opin. Immunol. 27 , 75–82 (2014).
Hepworth, M. R. et al. Innate lymphoid cells regulate CD4+ T-cell responses to intestinal commensal bacteria. Nature 498 , 113–117 (2013).
Melo-Gonzalez, F. et al. Antigen-presenting ILC3 regulate T cell-dependent IgA responses to colonic mucosal bacteria. J. Exp. Med. 216 , 728–742 (2019).
Mestas, J. & Hughes, C. C. W. Of mice and not men: differences between mouse and human immunology. J. Immunol. 172 , 2731–2738 (2004).
Hazenberg, M. D. & Spits, H. Human innate lymphoid cells. Blood 124 , 700–709 (2014).
Walker, J. A., Barlow, J. L. & McKenzie, A. N. J. Innate lymphoid cells-how did we miss them? Nat. Rev. Immunol. 13 , 75–87 (2013).
Sonnenberg, G. F. & Artis, D. Innate lymphoid cell interactions with microbiota: implications for intestinal health and disease. Immunity 37 , 601–610 (2012).
Alexandre, D. & Lefranc, M. P. The human γ/δ+ and α/β+ T cells: a branched pathway of differentiation. Mol. Immunol. 29 , 447–451 (1992).
Cruz, M. S., Diamond, A., Russell, A. & Jameson, J. M. Human αβ and γδ T cells in skin immunity and disease. Front. Immunol. 9 , 1 (2018).
Article CAS Google Scholar
Beetz, S., Marischen, L., Kabelitz, D. & Wesch, D. Human γδ T cells: candidates for the development of immunotherapeutic strategies. Immunol. Res. 37 , 97–111 (2007).
Brummelman, J., Pilipow, K. & Lugli, E. The single-cell phenotypic identity of human CD8 + and CD4 + T cells. Int. Rev. Cell Mol. Biol. 341 , 63–124 (2018).
Mosmann, T. R., Cherwinski, H., Bond, M. W., Giedlin, M. A. & Coffman, R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 136 , 2348–2357 (1986).
CAS PubMed Google Scholar
Mosmann, T. R. & Sad, S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol. Today 17 , 138–146 (1996).
Schmitt, N. & Ueno, H. Regulation of human helper T cell subset differentiation by cytokines. Curr. Opin. Immunol. 34 , 130–136 (2015).
Fietta, P. & Delsante, G. The effector T helper cell triade. Riv. Biol. 102 , 61–74 (2009).
PubMed Google Scholar
Romagnani, S. Regulation of the development of type 2 T-helper cells in allergy. Curr. Opin. Immunol. 6 , 838–846 (1994).
Korn, T., Bettelli, E., Oukka, M. & Kuchroo, V. K. IL-17 and Th17 cells. Annu. Rev. Immunol. 27 , 485–517 (2009).
Veldhoen, M. et al. Transforming growth factor-β ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat. Immunol. 9 , 1341–1346 (2008).
Licona-Limón, P., Arias-Rojas, A. & Olguín-Martínez, E. IL-9 and Th9 in parasite immunity. Semin. Immunopathol. 39 , 29–38 (2017).
Article PubMed CAS Google Scholar
Trifari, S., Kaplan, C. D., Tran, E. H., Crellin, N. K. & Spits, H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from TH-17, TH1 and TH2 cells. Nat. Immunol. 10 , 864–871 (2009).
Duhen, T., Geiger, R., Jarrossay, D., Lanzavecchia, A. & Sallusto, F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat. Immunol. 10 , 857–863 (2009).
Ueno, H., Banchereau, J. & Vinuesa, C. G. Pathophysiology of T follicular helper cells in humans and mice. Nat. Immunol. 16 , 142–152 (2015).
De Jong, E., Suddason, T. & Lord, G. M. Translational mini-review series on Th17 cells: development of mouse and human T helper 17 cells. Clin. Exp. Immunol. 159 , 148–158 (2010).
Hams, E. et al. Blockade of B7-H1 (programmed death ligand 1) enhances humoral immunity by positively regulating the generation of T follicular helper cells. J. Immunol. 186 , 5648–5655 (2011).
Good-Jacobson, K. L. et al. PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nat. Immunol. 11 , 535–542 (2010).
Kroenke, M. A. et al. Bcl6 and Maf cooperate to instruct human follicular helper CD4 T cell differentiation. J. Immunol. 188 , 3734–3744 (2012).
Edholm, E.-S. et al. Nonclassical MHC class I-dependent invariant T cells are evolutionarily conserved and prominent from early development in amphibians. Proc. Natl Acad. Sci. U.S.A. 110 , 14342–14347 (2013).
Schaerli, P. et al. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J. Exp. Med. 192 , 1553–1562 (2000).
Szabo, S. J. et al. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 100 , 655–669 (2000).
Lantelme, E., Mantovani, S., Palermo, R ., Campanelli, B., Sallusto, F. & Giachino, C. Kinetics of GATA-3 gene expression in early polarizing and committed human T cells. Immunology 102 , 123–130 (2001).
Zheng, W. P. & Flavell, R. A. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell 89 , 587–596 (1997).
Vieira, P. L. et al. IL-10-secreting regulatory T cells do not express foxp3 but have comparable regulatory function to naturally occurring CD4 + CD25 + regulatory T cells. J. Immunol. 172 , 5986–5993 (2004).
Acosta-Rodriguez, E. V. et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat. Immunol. 8 , 639–646 (2007).
Wilson, N. J. et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat. Immunol. 8 , 950–957 (2007).
Eyerich, S. et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J. Clin. Invest. 119 , 3573–3585 (2009).
CAS PubMed PubMed Central Google Scholar
Jia, L. & Wu, C. Differentiation, regulation and function of Th9 cells. Adv. Exp. Med. Biol. 841 , 181–207 (2014).
Pagani, M. et al. Role of microRNAs and long-non-coding RNAs in CD4+ T-cell differentiation. Immunol. Rev. 253 , 82–96 (2013).
Baumjohann, D. & Ansel, K. M. MicroRNA-mediated regulation of T helper cell differentiation and plasticity. Nat. Rev. Immunol. 13 , 666–678 (2013).
Woodland, D. L. & Dutton, R. W. Heterogeneity of CD4+ and CD8+ T cells. Curr. Opin. Immunol. 15 , 336–342 (2003).
Sawant, D. V., Hamilton, K. & Vignali, D. A. A. Interleukin-35: expanding its job profile. J. Interf. Cytokine Res. 35 , 499–512 (2015).
Linterman, M. A. et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat. Med. 17 , 975–982 (2011).
Maceiras, A. R., Fonseca, V. R., Agua-Doce, A. & Graca, L. T follicular regulatory cells in mice and men. Immunology 152 , 25–35 (2017).
Lim, H. W., Hillsamer, P. & Kim, C. H. Regulatory T cells can migrate to follicles upon T cell activation and suppress GC-Th cells and GC-Th cell–driven B cell responses. J. Clin. Investig. 114 , 1640–1649 (2004).
Collison, L. W. et al. IL-35-mediated induction of a potent regulatory T cell population. Nat. Immunol. 11 , 1093–1101 (2010).
Machicote, A., Belén, S., Baz, P., Billordo, L. A. & Fainboim, L. Human CD8+ HLA-DR+ regulatory T cells, similarly to classical CD4+ Foxp3+ cells, suppress immune responses via PD-1/PD-L1 axis. Front. Immunol. 9 , 2788 (2018).
Nakagawa, H., Wang, L., Cantor, H. & Kim, H. J. New insights into the biology of CD8 regulatory T cells. Adv. Immunol. 140 , 1–20 (2018).
Rosser, E. C. & Mauri, C. Regulatory B cells: origin, phenotype, and function. Immunity 42 , 607–612 (2015).
Mazzoni, A. et al. Demethylation of the RORC2 and IL17A in human CD4 + T lymphocytes defines Th17 origin of nonclassic Th1 cells. J. Immunol. 194 , 3116–3126 (2015).
Maggi, L. et al. Brief report: etanercept inhibits the tumor necrosis factor α-driven shift of Th17 lymphocytes toward a nonclassic Th1 phenotype in juvenile idiopathic arthritis. Arthritis Rheumatol. 66 , 1372–1377 (2014).
Duhen, T. & Campbell, D. J. IL-1β promotes the differentiation of polyfunctional human CCR6 + CXCR3 + Th1/17 cells that are specific for pathogenic and commensal microbes. J. Immunol. 193 , 120–129 (2014).
Becattini, S. et al. Functional heterogeneity of human memory CD4+ T cell clones primed by pathogens or vaccines. Science 347 , 400–406 (2015).
Nistala, K. et al. Th17 plasticity in human autoimmune arthritis is driven by the inflammatory environment. Proc. Natl Acad. Sci. U.S.A. 107 , 14751–14756 (2010).
Dupage, M. & Bluestone, J. A. Harnessing the plasticity of CD4+ T cells to treat immune-mediated disease. Nat. Rev. Immunol. 16 , 149–163 (2016).
Pradeu, T. & Du Pasquier, L. Immunological memory: what’s in a name? Immunol. Rev. 283 , 7–20 (2018).
Medzhitov, R., Preston-Hurlburt, P. & Janeway, C. A. A human homologue of the Drosophila toll protein signals activation of adaptive immunity. Nature 388 , 394–397 (1997).
Gulati, A., Kaur, D., Krishna Prasad, G. V. R. & Mukhopadhaya, A. PRR function of innate immune receptors in recognition of bacteria or bacterial ligands. Adv. Exp. Med. Biol. 1112 , 255–280 (2018).
Dolasia, K., Bisht, M. K., Pradhan, G., Udgata, A. & Mukhopadhyay, S. TLRs/NLRs: shaping the landscape of host immunity. Int. Rev. Immunol. 37 , 3–19 (2018).
Takeuchi, O. & Akira, S. Pattern recognition receptors and inflammation. Cell 140 , 805–820 (2010).
Durrant, W. E. & Dong, X. Systemic acquired resistance. Annu. Rev. Phytopathol. 42 , 185–209 (2004).
Pham, L. N., Dionne, M. S., Shirasu-Hiza, M. & Schneider, D. S. A specific primed immune response in Drosophila is dependent on phagocytes. PLoS Pathog. 3 , e26 (2007).
Kurtz, J. Specific memory within innate immune systems. Trends Immunol. 26 , 186–192 (2005).
Quintin, J. et al. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe 12 , 223–232 (2012).
Wout, J. W., Poell, R. & Furth, R. The rof BCG/PPD-activated macrophages in resistance against systemic candidiasis in mice. Scand. J. Immunol. 36 , 713–720 (1992).
Article Google Scholar
Spencer, J. C., Ganguly, R. & Waldman, R. H. Nonspecific protection of mice against influenza virus infection by local or systemic immunization with Bacille Calmette-Guérin. J. Infect. Dis. 136 , 171–175 (1977).
Angelidou, A. et al. BCG as a case study for precision vaccine development: lessons from vaccine heterogeneity, trained immunity, and immune ontogeny. Front. Microb. 11 , 332 (2020).
Netea, M. G. & Van Crevel, R. BCG-induced protection: effects on innate immune memory. Sem. Immunol. 26 , 512–517 (2014).
Netea, M. G. et al. Trained immunity: a program of innate immune memory in health and disease. Science 352 , 6284 (2016). aaf1098.
Netea, M. G. et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 20 , 375–388 (2020).
Saeed, S. et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 345 , 1251086 (2014).
Ramírez-Valle, F., Gray, E. E. & Cyster, J. G. Inflammation induces dermal Vγ4+ γδT17 memory-like cells that travel to distant skin and accelerate secondary IL-17-driven responses. Proc. Natl Acad. Sci. U.S.A. 112 , 8046–8051 (2015).
Wang, X., Peng, H. & Tian, Z. Innate lymphoid cell memory. Cell Mol. Immunol. 16 , 423–429 (2019).
O’Leary, J. G., Goodarzi, M., Drayton, D. L. & von Andrian, U. H. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat. Immunol. 7 , 507–516 (2006).
Paust, S. et al. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat. Immunol. 11 , 1127–1135 (2010).
Lopez-Vergès, S. et al. Expansion of a unique CD57+ NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc. Natl Acad. Sci. U.S.A. 108 , 14725–14732 (2011).
Article PubMed PubMed Central Google Scholar
Foley, B. et al. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood 119 , 2665–2674 (2012).
Nikzad, R. et al. Human natural killer cells mediate adaptive immunity to viral antigens. Sci. Immunol. 4 , eaat8116 (2019).
Cooper, M. A. et al. Cytokine-induced memory-like natural killer cells. Proc. Natl Acad. Sci. U.S.A. 106 , 1915–1919 (2009).
Cooper, M. A. & Yokoyama, W. M. Memory-like responses of natural killer cells. Immunol. Rev. 235 , 297–305 (2010).
Larsen, S. B., Cowley, C. J. & Fuchs, E. Epithelial cells: liaisons of immunity. Curr. Opin. Immunol. 62 , 45–53 (2020).
de Bree, L. C. J. et al. Non-specific effects of vaccines: current evidence and potential implications. Semin. Immunol. 39 , 35–43 (2018).
Locht, C. & Mielcarek, N. Live attenuated vaccines against pertussis. Exp. Rev. Vaccines 13 , 1147–1158 (2014).
Muramatsu, D. et al. β-Glucan derived from Aureobasidium pullulans is effective for the prevention of influenza in mice. PLoS ONE 7 , e41399 (2012).
Belkaid, Y. & Harrison, O. J. Homeostatic immunity and the microbiota. Immunity 46 , 562–576 (2017).
Costello, E. K. et al. Bacterial community variation in human body habitats across space and time. Science 326 , 1694–1697 (2009).
Abrahamsson, T. R. et al. Low diversity of the gut microbiota in infants with atopic eczema. J. Allergy Clin. Immunol. 129 , 434–440 (2012).
Article PubMed Google Scholar
Abrahamsson, T. R. et al. Low gut microbiota diversity in early infancy precedes asthma at school age. Clin. Exp. Allergy 44 , 842–850 (2014).
Chen, J. et al. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci. Rep. 6 , 28484 (2016).
Scher, J. U. et al. Decreased bacterial diversity characterizes the altered gut microbiota in patients with psoriatic arthritis, resembling dysbiosis in inflammatory bowel disease. Arthritis Rheumatol. 67 , 128–139 (2015).
Round, J. L. & Palm, N. W. Causal effects of the microbiota on immune-mediated diseases. Sci. Immunol. 3 , eaao1603 (2018).
Arrieta, M.-C., Walter, J. & Finlay, B. B. Human microbiota-associated mice: a model with challenges. Cell Host Microbe 19 , 575–578 (2016).
Round, J. L. & Mazmanian, S. K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 9 , 313–323 (2009).
Hapfelmeier, S. et al. Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses. Science 328 , 1705–1709 (2010).
Mazmanian, S. K., Liu, C. H., Tzianabos, A. O. & Kasper, D. L. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 122 , 107–118 (2005).
Tanoue, T., Umesaki, Y. & Honda, K. Immune responses to gut microbiota-commensals and pathogens. Gut Microbes 1 , 224–233 (2010).
Braniste, V. et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 6 , 263ra158 (2014).
Hoban, A. E. et al. Regulation of prefrontal cortex myelination by the microbiota. Transl. Psychiatry 6 , e774 (2016).
Yano, J. M. et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161 , 264–276 (2015).
Hsiao, E. Y. et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 155 , 1451–1463 (2013).
David, T., Ling, S. F. & Barton, A. Genetics of immune-mediated inflammatory diseases. Clin. Exp. Immunol. 193 , 3–12 (2018).
Ferris, M. T. & Hood, D. W. Host genetic regulation of immune-based and infectious diseases: introduction to mammalian genome special issue: genetics of infectious disease. Mamm. Genome 29 , 365–366 (2018).
Klebanov, N. Genetic predisposition to infectious disease. Cureus 10 , e3210 (2018).
PubMed PubMed Central Google Scholar
Bellamy, R. et al. Variations in the NRAMP1 gene and susceptibility to tuberculosis in West Africans. N. Engl. J. Med. 338 , 640–644 (1998).
Vidal, S. M., Malo, D., Vogan, K., Skamene, E. & Gros, P. Natural resistance to infection with intracellular parasites: isolation of a candidate for BCG. Cell 73 , 469–485 (1993).
Platonov, A. E. et al. Association of human Fc gamma RIIa (CD32) polymorphism with susceptibility to and severity of meningococcal disease. Clin. Infect. Dis. 27 , 746–750 (1998).
Bongen, E., Vallania, F., Utz, P. J. & Khatri, P. KLRD1-expressing natural killer cells predict influenza susceptibility. Genome Med. 10 , 45 (2018).
Scepanovic, P. et al. Human genetic variants and age are the strongest predictors of humoral immune responses to common pathogens and vaccines. Genome Med. 10 , 1–13 (2018).
Benetti, E., et al. ACE2 gene variants may underlie interindividual variability and susceptibility to COVID-19 in the Italian population. Eur. J. Hum. Genet . (2020). https://doi.org/10.1038/s41431-020-0691-z .
Zhao, J. et al. Relationship between the ABO Blood Group and the COVID-19 susceptibility. medRxiv (2020). https://doi.org/10.1101/2020.03.11.20031096 .
Silverstein, A. M. Autoimmunity versus horror autotoxicus: the struggle for recognition. Nat. Immunol. 2 , 279–281 (2001).
Goodnow, C. C. Multistep pathogenesis of autoimmune disease. Cell 130 , 25–35 (2007).
Wang, L., Wang, F.-S. & Gershwin, M. E. Human autoimmune diseases: a comprehensive update. J. Intern. Med. 278 , 369–395 (2015).
Rosenblum, M. D., Remedios, K. A. & Abbas, A. K. Mechanisms of human autoimmunity. J. Clin. Investig. 125 , 2228–2233 (2015).
González-Serna, D. et al. Analysis of the genetic component of systemic sclerosis in Iranian and Turkish populations through a genome-wide association study. Rheumatology 58 , 289–298 (2019).
López-Isac, E. et al. GWAS for systemic sclerosis identifies multiple risk loci and highlights fibrotic and vasculopathy pathways. Nat. Commun. 10 , 4955 (2019).
Westra, H.-J. et al. Fine-mapping and functional studies highlight potential causal variants for rheumatoid arthritis and type 1 diabetes. Nat. Genet. 50 , 1366–1374 (2018).
Inshaw, J. R. J., Cutler, A. J., Burren, O. S., Stefana, M. I. & Todd, J. A. Approaches and advances in the genetic causes of autoimmune disease and their implications. Nat. Immunol. 19 , 674–684 (2018).
Márquez, A. et al. Meta-analysis of Immunochip data of four autoimmune diseases reveals novel single-disease and cross-phenotype associations. Genome Med. 10 , 97 (2018).
Catalina, M. D., Owen, K. A., Labonte, A. C., Grammer, A. C. & Lipsky, P. E. The pathogenesis of systemic lupus erythematosus: harnessing big data to understand the molecular basis of lupus. J. Autoimmun. 110 , 102359 (2019).
Medawar, P. B. A second study of the behaviour and fate of skin homografts in rabbits: a report to the War Wounds Committee of the Medical Research Council. J. Anat. 79 , 157–176 (1945).
Medawar, P. B. Immunity to homologous grafted skin; the relationship between the antigens of blood and skin. Br. J. Exp. Pathol. 27 , 15–24 (1946).
Carreras, E., Dufour, C., Mohty, M. & Kröger, N. The EBMT Handbook: Hematopoietic Stem Cell Transplantation and Cellular Therapies. (2019). NBK554031.
Robinson, J. et al. IPD-IMGT/HLA database. Nucleic Acids Res. 48 , 948–955 (2020).
Google Scholar
Spellman, S. et al. Effects of mismatching for minor histocompatibility antigens on clinical outcomes in HLA-matched, unrelated hematopoietic stem cell transplants. Biol. Blood Marrow Transpl. 15 , 856–863 (2009).
Rajalingam, R. The impact of HLA class I-specific killer cell immunoglobulin-like receptors on antibody-dependent natural killer cell-mediated cytotoxicity and organ allograft rejection. Front. Immunol. 7 , 585 (2016).
Gruber, C. & Bogunovic, D. Incomplete penetrance in primary immunodeficiency: a skeleton in the closet. Hum. Genet. 139 , 745–757 (2020).
McCusker, C., Upton, J. & Warrington, R. Primary immunodeficiency. Allergy Asthma Clin. Immunol. 14 , 61 (2018).
Al-Mousa, H. & Al-Saud, B. Primary immunodeficiency diseases in highly consanguineous populations from Middle East and North Africa: epidemiology, diagnosis, and care. Front. Immunol. 8 , 678 (2017).
Abolhassani, H., Hammarström, L. & Cunningham-Rundles, C. Current genetic landscape in common variable immune deficiency. Blood 135 , 656–667 (2020).
Tangye, S. G. et al. Human inborn errors of immunity: 2019 update on the classification from the International Union of Immunological Societies Expert Committee. J. Clin. Immunol. 40 , 24–64 (2020).
Bousfiha, A. et al. The 2017 IUIS phenotypic classification for primary immunodeficiencies. J. Clin. Immunol. 38 , 129–143 (2018).
McCusker, C. & Warrington, R. Primary immunodeficiency. Allergy Asthma Clin. Immunol. 7 , S11 (2011).
Jéru, I. Update on the genetics of autoinflammatory disorders. Curr. Allergy Asthma Rep. 19 , 41 (2019).
Davidson, S., Steiner, A., Harapas, C. R. & Masters, S. L. An update on autoinflammatory diseases: interferonopathies. Curr. Rheumatol. Rep. 20 , 38 (2018).
de Jesus, A. A. et al. Novel proteasome assembly chaperone mutations in PSMG2/PAC2 cause the autoinflammatory interferonopathy CANDLE/PRAAS4. J. Allergy Clin. Immunol. 143 , 1939–1943.e8 (2019).
Holloway, J. W., Yang, I. A. & Holgate, S. T. Genetics of allergic disease. J. Allergy Clin. Immunol. 125 , S81–S94 (2010).
Cookson, W. O., Sharp, P. A., Faux, J. A. & Hopkin, J. M. Linkage between immunoglobulin E responses underlying asthma and rhinitis and chromosome 11q. Lancet 1 , 1292–1295 (1989).
Bønnelykke, K., Sparks, R., Waage, J. & Milner, J. D. Genetics of allergy and allergic sensitization: common variants, rare mutations. Curr. Opin. Immunol. 36 , 115–126 (2015).
Jochems, S. P. et al. Innate and adaptive nasal mucosal immune responses following experimental human pneumococcal colonization. J. Clin. Investig. 130 , 4523–4538 (2019).
DeVries, A. et al. Epigenome-wide analysis links SMAD3 methylation at birth to asthma in children of asthmatic mothers. J. Allergy Clin. Immunol. 140 , 534–542 (2017).
Kabesch, M. & Tost, J. Recent findings in the genetics and epigenetics of asthma and allergy. Semin. Immunopathol. 42 , 43–60 (2020).
Ehrlich, P. Ueber den jetzigen stand der karzinomforschung. Ned. Tijdschr. Geneeskd. 5 , 73–209 (1909).
Kim, R., Emi, M. & Tanabe, K. Cancer immunoediting from immune surveillance to immune escape. Immunology 121 , 1–14 (2007).
Galon, J. & Bruni, D. Tumor immunology and tumor evolution: intertwined histories. Immunity 52 , 55–81 (2020).
Garrido, F. et al. Natural history of HLA expression during tumour development. Immunol. Today 14 , 491–499 (1993).
Sukari, A., Nagasaka, M., Al-Hadidi, A. & Lum, L. G. Cancer immunology and immunotherapy. Anticancer Res. 36 , 5593–5606 (2016).
Seidel, J. A., Otsuka, A. & Kabashima, K. Anti-PD-1 and Anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front. Oncol. 8 , 86 (2018).
Yi, J. S., Cox, M. A. & Zajac, A. J. T-cell exhaustion: characteristics, causes and conversion. Immunology 129 , 474–481 (2010).
Bengsch, F., Knoblock, D. M., Liu, A., McAllister, F. & Beatty, G. L. CTLA-4/CD80 pathway regulates T cell infiltration into pancreatic cancer. Cancer Immunol. Immun. 66 , 1609–1617 (2017).
Lee, H. T., Lee, S. H. & Heo, Y. S. Molecular interactions of antibody drugs targeting PD-1, PD-L1, and CTLA-4 in immuno-oncology. Molecules 24 , 1190 (2019).
Article PubMed Central CAS Google Scholar
Aiello, A. et al. Immunosenescence and its hallmarks: how to oppose aging strategically? A review of potential options for therapeutic intervention. Front. Immunol. 10 , 2247 (2019).
Thomas, R., Wang, W. & Su, D. M. Contributions of age-related thymic involution to immunosenescence and inflammaging. Immun. Ageing 17 , 2 (2020).
Oh, S. J., Lee, J. K. & Shin, O. S. Aging and the immune system: the impact of immunosenescence on viral infection, immunity and vaccine immunogenicity. Immune Netw. 19 , e37 (2019).
Hashimoto, K. et al. Single-cell transcriptomics reveals expansion of cytotoxic CD4 T cells in supercentenarians. Proc. Natl Acad. Sci. USA. 116 , 24242–24251 (2019).
Rappuoli, R., Pizza, M., Del Giudice, G. & De Gregorio, E. Vaccines, new opportunities for a new society. Proc. Natl Acad. Sci. USA. 111 , 12288–12293 (2014).
Meyer, H., Ehmann, R. & Smith, G. L. Smallpox in the post-eradication era. Viruses 12 , 138 (2020).
Article CAS PubMed Central Google Scholar
Greenwood, B. The contribution of vaccination to global health: past, present and future. Philos. Trans. R. Soc. L. B. Biol. Sci. 369 , 20130433 (2014).
Decker, W. K. & Safdar, A. Bioimmunoadjuvants for the treatment of neoplastic and infectious disease: Coley’s legacy revisited. Cytokine Growth Factor Rev. 20 , 271–281 (2009).
Fuge, O., Vasdev, N., Allchorne, P. & Green, J. S. Immunotherapy for bladder cancer. Res. Rep. Urol. 7 , 65–79 (2015).
Old, L. J., Clarke, D. A. & Benacerraf, B. Effect of Bacillus Calmette–Guerin infection on transplanted tumours in the mouse. Nature 184 , 291–292 (1959).
You, J. H. S., Jiang, X., Lee, W. H., Chan, P. K. S. & Ng, S. C. Cost-effectiveness analysis of fecal microbiota transplantation for recurrent Clostridium difficile infection in patients with inflammatory bowel disease. J Gastroenterol. Hepatol. (2020). https://doi.org/10.1111/jgh.15002 .
Tariq, R. et al. Efficacy of fecal microbiota transplantation for recurrent c. difficile infection in inflammatory bowel disease. Inflamm. Bowel Dis. 2019 , izz299 (2019).
Sedighi, M. et al. Therapeutic bacteria to combat cancer; current advances, challenges, and opportunities. Cancer Med. 8 , 3167–3181 (2019).
Davola, M. E. & Mossman, K. L. Oncolytic viruses: how “lytic” must they be for therapeutic efficacy? Oncoimmunology 8 , e1581528 (2019).
Malhotra, A., Sendilnathan, A., Old, M. O. & Wise-Draper, T. M. Oncolytic virotherapy for head and neck cancer: current research and future developments. Oncolytic Virother. 4 , 83–93 (2015).
Greig, S. L. Talimogene laherparepvec: first global approval. Drugs 76 , 147–154 (2016).
Eissa, I. R. et al. The current status and future prospects of oncolytic viruses in clinical trials against melanoma, glioma, pancreatic, and breast cancers. Cancers 10 , 356 (2018).
Kaufman, H. L. & Bommareddy, P. K. Two roads for oncolytic immunotherapy development. J. Immunother. Cancer 7 , 26 (2019).
Miest, T. S. & Cattaneo, R. New viruses for cancer therapy: meeting clinical needs. Nat. Rev. Microbiol. 12 , 23–34 (2014).
Samson, A. et al. Intravenous delivery of oncolytic reovirus to brain tumor patients immunologically primes for subsequent checkpoint blockade. Sci. Transl. Med. 10 , eaam7577 (2018).
Martin, N. T. et al. Pre-surgical neoadjuvant oncolytic virotherapy confers protection against rechallenge in a murine model of breast cancer. Sci. Rep. 9 , 1865 (2019).
Bourgeois-Daigneault, M.-C. et al. Neoadjuvant oncolytic virotherapy before surgery sensitizes triple-negative breast cancer to immune checkpoint therapy. Sci. Transl. Med. 10 , eaao1641 (2018).
Zheng, M., Huang, J., Tong, A. & Yang, H. Oncolytic viruses for cancer therapy: barriers and recent advances. Mol. Ther. - Oncolytics 15 , 234–247 (2019).
De Flora, S. & Bonanni, P. The prevention of infection-associated cancers. Carcinogenesis 32 , 787–795 (2011).
Gutowska-Owsiak, D. & Ogg, G. S. Therapeutic vaccines for allergic disease. npj Vaccines 2 , 12 (2017).
Wu, A. Y. Immunotherapy—vaccines for allergic diseases. J. Thorac. Dis. 4 , 198–202 (2012).
Valenta, R., Campana, R., Focke-Tejkl, M. & Niederberger, V. Vaccine development for allergen-specific immunotherapy based on recombinant allergens and synthetic allergen peptides: lessons from the past and novel mechanisms of action for the future. J. Allergy Clin. Immunol. 137 , 351–357 (2016).
Larché, M., Akdis, C. A. & Valenta, R. Immunological mechanisms of allergen-specific immunotherapy. Nat. Rev. Immunol. 6 , 761–771 (2006).
Zhernov, Y., Curin, M., Khaitov, M., Karaulov, A. & Valenta, R. Recombinant allergens for immunotherapy. Curr. Opin. Allergy Clin. Immunol. 19 , 402–414 (2019).
Schlom, J. et al. Therapeutic cancer vaccines. Adv. Cancer Res. 121 , 67–124 (2014).
Hollingsworth, R. E. & Jansen, K. Turning the corner on therapeutic cancer vaccines. npj Vaccines 4 , 1–10 (2019).
Servín-Blanco, R., Zamora-Alvarado, R., Gevorkian, G. & Manoutcharian, K. Antigenic variability: obstacles on the road to vaccines against traditionally difficult targets. Hum. Vaccin. Immunother. 12 , 2640–2648 (2016).
Hu, Z., Ott, P. A. & Wu, C. J. Towards personalized, tumour-specific, therapeutic vaccines for cancer. Nat. Rev. Immunol. 18 , 168–182 (2018).
Sivori, S. et al. Human NK cells: surface receptors, inhibitory checkpoints and translational applications. Cell. Mol. Immunol. 16 , 430–441 (2019).
Pannemans, K., Hellings, N. & Stinissen, P. Therapeutic vaccines for autoimmune diseases. Drug Discov. Today. Ther. Strateg. 6 , 39–44 (2009).
Serra, P. & Santamaria, P. Nanoparticle-based approaches to immune tolerance for the treatment of autoimmune diseases. Eur. J. Immunol. 48 , 751–756 (2018).
Von Behring, E. & Zustandekommen, K. S. der Diphtherie-Immunitat and der Tetanus-Immunität bei Thieren. Dtsch. Med. Wochenschr. 16 , 1113–1114 (1890).
João, C., Negi, V. S., Kazatchkine, M. D., Bayry, J. & Kaveri, S. V. Passive serum therapy to immunomodulation by IVIG: a fascinating journey of antibodies. J. Immunol. 200 , 1957–1963 (2018).
Roux, E. Y. A. Contribution à l’étude de la diphthérie. 3e mémoire. Ann. Inst. Pasteur 4 , 384–426 (1890).
Galeotti, Caroline, Kaveri, SriniV. & Bayry, J. IVIG-mediated effector functions in autoimmune and inflammatory diseases. Int. Immunol. 29 , 491–498 (2017).
Bruton, O. C. Agamma-globulinemia. Pediatrics 9 , 722–728 (1952).
Perez, E. E. et al. Update on the use of immunoglobulin in human disease: a review of evidence. J. Allergy Clin. Immunol. 139 , S1–S46 (2017).
Köhler, G. & Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256 , 495–497 (1975).
Springer, T. A. César milstein, the father of modern immunology. Nat. Immunol. 3 , 501–503 (2002).
Strebhardt, K. & Ullrich, A. Paul Ehrlich’s magic bullet concept: 100 years of progress. Nat. Rev. Cancer 8 , 473–480 (2008).
Shawler, D. L., Bartholomew, R. M., Smith, L. M. & Dillman, R. O. Human immune response to multiple injections of murine monoclonal IgG. J. Immunol. 135 , 1530–1535 (1985).
Kuus-Reichel, K. et al. Will immunogenicity limit the use, efficacy, and future development of therapeutic monoclonal antibodies? Clin. Diagn. Lab. Immunol. 1 , 365–372 (1994).
Elgundi, Z., Reslan, M., Cruz, E., Sifniotis, V. & Kayser, V. The state-of-play and future of antibody therapeutics. Adv. Drug Deliv. Rev. 122 , 2–19 (2017).
Lonberg, N. Fully human antibodies from transgenic mouse and phage display platforms. Curr. Opin. Immunol. 20 , 450–459 (2008).
Chester, K. A. et al. Phage libraries for generation of clinically useful antibodies. Lancet 343 , 455–456 (1994).
Frenzel, A., Schirrmann, T. & Hust, M. Phage display-derived human antibodies in clinical development and therapy. MAbs 8 , 1177–1194 (2016).
Almagro, J. C., Daniels-Wells, T. R., Perez-Tapia, S. M. & Penichet, M. L. Progress and challenges in the design and clinical development of antibodies for cancer therapy. Front. Immunol. 8 , 1751 (2017).
Magadán, S. et al. Production of antigen-specific human monoclonal antibodies: comparison of mice carrying IgH/κ or IgH/κ/λ transloci. Biotechniques 33, (2002).
Mompó, S. M. & González-Fernández, Á. Antigen-specific human monoclonal antibodies from transgenic mice. Methods Mol. Biol. 1904 , 253–291 (2019).
Barderas, R. & Benito-Peña, E. The 2018 Nobel Prize in chemistry: phage display of peptides and antibodies. Anal. Bioanal. Chem. 411 , 2475–2479 (2019).
Winter, G. Harnessing evolution to make medicines (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 58 , 14438–14445 (2019).
Almagro, J. C., Pedraza-Escalona, M., Arrieta, H. I. & Pérez-Tapia, S. M. Phage display libraries for antibody therapeutic discovery and development. Antibodies (Basel) 8 , 44 (2019).
Winter, G., Griffiths, A. D., Hawkins, R. E. & Hoogenboom, H. R. Making antibodies by phage display technology. Annu. Rev. Immunol. 12 , 433–455 (1994).
Kaplon, H., Muralidharan, M., Schneider, Z. & Reichert, J. M. Antibodies to watch in 2020. MAbs 12 , 1703531 (2020).
Kaplon, H. & Reichert, J. M. Antibodies to watch in 2019. MAbs 11 , 219–238 (2019).
Kaplon, H. & Reichert, J. M. Antibodies to watch in 2018. MAbs 10 , 183–203 (2018).
Jiang, X. R. et al. Advances in the assessment and control of the effector functions of therapeutic antibodies. Nat. Rev. Drug Discov. 10 , 101–110 (2011).
Mitoma, H., Horiuchi, T., Tsukamoto, H. & Ueda, N. Molecular mechanisms of action of anti-TNF-α agents - Comparison among therapeutic TNF-α antagonists. Cytokine 101 , 56–63 (2018).
Buurman, D. J. et al. Quantitative comparison of the neutralizing capacity, immunogenicity and cross-reactivity of anti-TNF-α biologicals and an Infliximab-biosimilar. PLoS ONE 13 , e0208922 (2018).
Taylor, R. P. & Lindorfer, M. A. Cytotoxic mechanisms of immunotherapy: harnessing complement in the action of anti-tumor monoclonal antibodies. Semin. Immunol. 28 , 309–316 (2016).
Rogers, L. M., Veeramani, S. & Weiner, G. J. Complement in monoclonal antibody therapy of cancer. Immunol. Res. 59 , 203–210 (2014).
Singh, S. et al. Monoclonal antibodies: a review. Curr. Clin. Pharmacol. 13 , 85–99 (2018).
Beck, A., Goetsch, L., Dumontet, C. & Corvaïa, N. Strategies and challenges for the next generation of antibody-drug conjugates. Nat. Rev. Drug Discov. 16 , 315–337 (2017).
Carter, P. J. & Lazar, G. A. Next generation antibody drugs: pursuit of the ‘high-hanging fruit’. Nat. Rev. Drug Discov. 17 , 197–223 (2018).
Fritz, J. M. & Lenardo, M. J. Development of immune checkpoint therapy for cancer. J. Exp. Med. 216 , 1244–1254 (2019).
Leach, D. R., Krummel, M. F. & Allison, J. P. Enhancement of antitumor immunity by CTLA-4 blockade. Science 271 , 1734–1736 (1996).
Okazaki, T. & Honjo, T. PD-1 and PD-1 ligands: from discovery to clinical application. Int. Immunol. 19 , 813–824 (2007).
Altmann, D. M. A Nobel Prize-worthy pursuit: cancer immunology and harnessing immunity to tumour neoantigens. Immunology 155 , 283–284 (2018).
Yron, I., Wood, T. A., Spiess, P. J. & Rosenberg, S. A. In vitro growth of murine T cells. V. The isolation and growth of lymphoid cells infiltrating syngeneic solid tumors. J. Immunol. 125 , 238–245 (1980).
Rosenberg, S. A. IL-2: the first effective immunotherapy for human cancer. J. Immunol. 192 , 5451–5458 (2014).
A, I. & J, L. Virus interference. I. interferon Proc. R. Soc. Lond. Ser. B - Biol. Sci. 147 , 258–267 (1957).
Pestka, S. The interferons: 50 years after their discovery, there is much more to learn. J. Biol. Chem. 282 , 20047–20051 (2007).
Borden, E. C. Interferons α and β in cancer: therapeutic opportunities from new insights. Nat. Rev. Drug Discov. 18 , 219–234 (2019).
Parker, B. S., Rautela, J. & Hertzog, P. J. Antitumour actions of interferons: implications for cancer therapy. Nat. Rev. Cancer 16 , 131–144 (2016).
Bhattacharya, P. et al. Dual role of GM-CSF as a pro-inflammatory and a regulatory cytokine: implications for immune therapy. J. Int. Soc. Interface Cytokine Res. 35 , 585–599 (2015).
Chen, P., Chen, F. & Zhou, B. Comparisons of therapeutic efficacy and safety of ipilimumab plus GM-CSF versus ipilimumab alone in patients with cancer: a meta-analysis of outcomes. Drug Des. Devel. Ther. 12 , 2025–2038 (2018).
Conlon, K. C., Miljkovic, M. D. & Waldmann, T. A. Cytokines in the treatment of cancer. J. Interferon Cytokine Res. 39 , 6–21 (2019).
Nicholas, C. & Lesinski, G. B. Immunomodulatory cytokines as therapeutic agents for melanoma. Immunotherapy 3 , 673–690 (2011).
Hassett, B. et al. Manufacturing history of etanercept (Enbrel ® ): consistency of product quality through major process revisions. MAbs 10 , 159–165 (2018).
Ruderman, E. M. & Pope, R. M. The evolving clinical profile of abatacept (CTLA4-lg): a novel co-stimulatory modulator for the treatment of rheumatoid arthritis. Arthritis Res. Ther. 7 , S21 (2005).
Herberman, R. B., Nunn, M. E., Holden, H. T. & Lavrin, D. H. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. II. Characterization of effector cells. Int. J. Cancer 16 , 230–239 (1975).
Kiessling, R., Klein, E., Pross, H. & Wigzell, H. “Natural” killer cells in the mouse. II. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Characteristics of the killer cell. Eur. J. Immunol. 5 , 117–121 (1975).
Piontek, G. E. et al. YAC-1 MHC class I variants reveal an association between decreased NK sensitivity and increased H-2 expression after interferon treatment or in vivo passage. J. Immunol. 135 , 4281–4288 (1985).
Kärre, K., Ljunggren, H. G., Piontek, G. & Kiessling, R. Selective rejection of H–2-deficient lymphoma variants suggests alternative immune defence strategy. Nature 319 , 675–678 (1986).
Grimm, E. A., Mazumder, A., Zhang, H. Z. & Rosenberg, S. A. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J. Exp. Med. 155 , 1823–1841 (1982).
Sun, Z. et al. A next-generation tumor-targeting IL-2 preferentially promotes tumor-infiltrating CD8+ T-cell response and effective tumor control. Nat. Commun. 10 , 1–12 (2019).
Charych, D. H. et al. NKTR-214, an engineered cytokine with biased IL2 receptor binding, increased tumor exposure, and marked efficacy in mouse tumor models. Clin. Cancer Res. 22 , 680–690 (2016).
Waldmann, T. A. The shared and contrasting roles of IL2 and IL15 in the life and death of normal and neoplastic lymphocytes: Implications for cancer therapy. Cancer Immunol. Res. 3 , 219–227 (2015).
Miller, J. S. & Lanier, L. L. Natural killer cells in cancer immunotherapy. Annu. Rev. Cancer Biol. 3 , 77–103 (2019).
Liu, Y., Bewersdorf, J. P., Stahl, M. & Zeidan, A. M. Immunotherapy in acute myeloid leukemia and myelodysplastic syndromes: the dawn of a new era? Blood Rev. 34 , 67–83 (2019).
Mavers, M. & Bertaina, A. High-risk leukemia: past, present, and future role of NK cells. J. Immunol. Res. 2018 , 1586905 (2018).
Davis, Z. B., Felices, M., Verneris, M. R. & Miller, J. S. Natural killer cell adoptive transfer therapy. Cancer J. 21 , 486–491 (2015).
Langerhans, P. Ueber die Nerven der menschlichen Haut. Arch. für Pathol. Anat. und Physiol. und für. Klin. Med. 44 , 325–337 (1868).
Volchenkov, R., Sprater, F., Vogelsang, P. & Appel, S. The 2011 Nobel Prize in physiology or medicine. Scand. J. Immunol. 75 , 1–4 (2012).
Otsuka, M., Egawa, G. & Kabashima, K. Uncovering the mysteries of langerhans cells, inflammatory dendritic epidermal cells, and monocyte-derived Langerhans cell-like cells in the epidermis. Front. immunol. 9 , 1768 (2018).
van Spriel, A. B. & de Jong, E. C. Dendritic cell science: more than 40 years of history. J. Leukoc. Biol. 93 , 33–38 (2013).
Sabado, R. L., Balan, S. & Bhardwaj, N. Dendritic cell-based immunotherapy. Cell Res. 27 , 74–95 (2017).
Posch, W., Lass-Flörl, C. & Wilflingseder, D. Generation of human monocyte-derived dendritic cells from whole blood. J. Vis. Exp. 118 , 54968 (2016).
Fong, L. & Engleman, E. G. Dendritic cells in cancer immunotherapy. Annu. Rev. Immunol. 18 , 245–273 (2000).
Romani, N. et al. Proliferating dendritic cell progenitors in human blood. J. Exp. Med. 180 , 83–93 (1994).
Gardner, A. & Ruffell, B. Dendritic cells and cancer immunity. Trends Immunol. 37 , 855–865 (2016).
Handy, C. E. & Antonarakis, E. S. Sipuleucel-T for the treatment of prostate cancer: novel insights and future directions. Future Oncol. 14 , 907–917 (2018).
Phillips, B. E., Garciafigueroa, Y., Trucco, M. & Giannoukakis, N. Clinical tolerogenic dendritic cells: exploring therapeutic impact on human autoimmune disease. Front. Immunol. 8 , 1279 (2017).
Waisman, A., Lukas, D., Clausen, B. E. & Yogev, N. Dendritic cells as gatekeepers of tolerance. Semin. Immunopathol. 39 , 153–163 (2017).
Prietl, B. et al. High-dose cholecalciferol supplementation significantly increases peripheral CD4+ Tregs in healthy adults without negatively affecting the frequency of other immune cells. Eur. J. Nutr. 53 , 751–759 (2014).
Pauza, C. D. et al. Gamma delta T cell therapy for cancer: it is good to be local. Front. Immunol. 9 , 1305 (2018).
Wilhelm, M. et al. Successful adoptive transfer and in vivo expansion of haploidentical γδ T cells. J. Transl. Med. 12 , 45 (2014).
Alnaggar, M. et al. Allogenic Vγ9Vδ2 T cell as new potential immunotherapy drug for solid tumor: a case study for cholangiocarcinoma. J. Immunother. Cancer 7 , 36 (2019).
Sakaguchi, S., Sakaguchi, N., Asano, M., Itoh, M. & Toda, M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 155 , 1151–1164 (1995).
Hori, S., Nomura, T. & Sakaguchi, S. Control of regulatory T cell development by the transcription factor Foxp3. J. Immunol. 198 , 981–985 (2017).
Holm, T. L., Nielsen, J. & Claesson, M. H. CD4+ CD25+ regulatory T cells: I. Phenotype and physiology. APMIS 112 , 629–641 (2004).
Shitara, K. & Nishikawa, H. Regulatory T cells: a potential target in cancer immunotherapy. Ann. N. Y. Acad. Sci. U.S.A. 1417 , 104–115 (2018).
Dunn, G. P., Bruce, A. T., Ikeda, H., Old, L. J. & Schreiber, R. D. Cancer immunoediting: from immunosurveillance to tumor escape. Nat. Immunol. 3 , 991–998 (2002).
Dunn, G. P., Old, L. J. & Schreiber, R. D. The immunobiology of cancer immunosurveillance and immunoediting. Immunity 21 , 137–148 (2004).
Xu, L. et al. Depletion of CD4+CD25 high regulatory T cells from tumor infiltrating lymphocytes predominantly induces Th1 type immune response in vivo which inhibits tumor growth in adoptive immunotherapy. Cancer Biol. Ther. 8 , 66–72 (2009).
Chodon, T. et al. Adoptive transfer of MART-1 T-cell receptor transgenic lymphocytes and dendritic cell vaccination in patients with metastatic melanoma. Clin. Cancer Res. 20 , 2457–2465 (2014).
Uckert, W. & Schumacher, T. N. M. TCR transgenes and transgene cassettes for TCR gene therapy: status in 2008. Cancer Immunol. Immunother. 58 , 809–822 (2009).
Kalos, M. et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci. Transl. Med. 3 , 95ra73 (2011).
Porter, D. L., Levine, B. L., Kalos, M., Bagg, A. & June, C. H. Chimeric antigen receptor–modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 365 , 725–733 (2011).
Casucci, M. & Bondanza, A. Suicide gene therapy to increase the safety of chimeric antigen receptor-redirected T lymphocytes. J. Cancer 2 , 378–382 (2011).
Cho, J. H., Collins, J. J. & Wong, W. W. Universal chimeric antigen receptors for multiplexed and logical control of T cell responses. Cell 173 , 1426–1438.e11 (2018).
Liu, E. et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N. Engl. J. Med. 382 , 545–553 (2020).
Adachi, K. et al. IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor. Nat. Biotechnol. 36 , 346–351 (2018).
Gomes-Silva, D. & Ramos, C. A. Cancer immunotherapy using CAR-T cells: from the research bench to the assembly line. Biotechnol. J. 13 , 1700097 (2018).
Ruella, M. & Kenderian, S. S. Next-generation chimeric antigen receptor T-cell therapy: going off the Shelf. BioDrugs 31 , 473–481 (2017).
Rezvani, K., Rouce, R., Liu, E. & Shpall, E. Engineering natural killer cells for cancer immunotherapy. Mol. Ther. 25 , 1769–1781 (2017).
Knochelmann, H. M. et al. CAR T cells in solid tumors: blueprints for building effective therapies. Front. immunol. 9 , 1740 (2018).
Kuhlmann, A. S., Peterson, C. W. & Kiem, H. P. Chimeric antigen receptor T-cell approaches to HIV cure. Curr. Opin. HIV AIDS 13 , 446–453 (2018).
Maldini, C. R., Ellis, G. I. & Riley, J. L. CAR T cells for infection, autoimmunity and allotransplantation. Nat. Rev. Immunol. 18 , 605–616 (2018).
Skuljec, J. et al. Chimeric antigen receptor-redirected regulatory T cells suppress experimental allergic airway inflammation, a model of asthma. Front. Immunol. 8 , 1125 (2017).
Bonam, S. R., Kaveri, S. V., Sakuntabhai, A., Gilardin, L. & Bayry, J. Adjunct immunotherapies for the management of severely Ill COVID-19 patients. Cell Rep. Med. 1 , 100016 (2020).
Ahn, J. Y. et al. Use of convalescent plasma therapy in two covid-19 patients with acute respiratory distress syndrome in Korea. J. Korean Med. Sci. 35 , e149 (2020).
Duan, K. et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl Acad. Sci. U.S.A. 117 , 9490–9496 (2020).
Klein, N. P., Abu-Elyazeed, R., Cheuvart, B., Janssens, W. & Mesaros, N. Immunogenicity and safety following primary and booster vaccination with a hexavalent diphtheria, tetanus, acellular pertussis, hepatitis B, inactivated poliovirus and Haemophilus influenzae type b vaccine: a randomized trial in the United States. Hum. Vaccines Immunother. 15 , 809–821 (2019).
Kartoglu, U. & Milstien, J. Tools and approaches to ensure quality of vaccines throughout the cold chain. Exp. Rev. Vaccines 13 , 843–854 (2014).
Peleteiro, M. et al. Polymeric nanocapsules for vaccine delivery: influence of the polymeric shell on the interaction with the immune system. Front. Immunol. 9 , 791 (2018).
Jazayeri, S. D. & Poh, C. L. Development of universal influenza vaccines targeting conserved viral proteins. Vaccines 7 , 169 (2019).
Thompson, K. M., Gellin, B. G., Hinman, A. R. & Orenstein, W. A. The National Vaccine Advisory Committee at 30: impact and opportunity. Vaccine 36 , 1330–1344 (2018).
Saso, A. & Kampmann, B. Vaccine responses in newborns. Semin. Immunopathol. 39 , 627–642 (2017).
Switzer, C., D’Heilly, C. & Macina, D. Immunological and clinical benefits of maternal immunization against pertussis: a systematic review. Infect. Dis. Ther. 8 , 499–541 (2019).
Lurie, N., Saville, M., Hatchett, R. & Halton, J. Developing Covid-19 vaccines at pandemic speed. N. Engl. J. Med. 382 , 1969–1973 (2020).
Amanat, F. & Krammer, F. Perspective SARS-CoV-2 vaccines: status report. Immunity 52 , 583–589 (2020).
Ulmer, J. B., Wahren, B. & Liu, M. A. Gene-based vaccines: recent technical and clinical advances. Trends Mol. Med. 12 , 216–222 (2006).
Petrovsky, N. Comparative safety of vaccine adjuvants: a summary of current evidence and future needs. Drug Saf. 38 , 1059–1074 (2015).
Sánchez-Ramón, S. et al. Trained immunity-based vaccines: a new paradigm for the development of broad-spectrum anti-infectious formulations. Front. Immunol. 9 , 2936 (2018).
Pluviano, S., Watt, C. & Della Sala, S. Misinformation lingers in memory: failure of three pro-vaccination strategies. PLoS ONE 12 , e0181640 (2017).
Sanyaolu, A. et al. Measles outbreak in unvaccinated and partially vaccinated children and adults in the United States and Canada (2018-2019): a narrative review of cases. Inquiry 56 , 004695801989409 (2019).
Lee Ventola, C. Cancer immunotherapy, part 3: challenge and future trends. P T 42 , 514–521 (2017).
Martinez-Quintanilla, J., Seah, I., Chua, M. & Shah, K. Oncolytic viruses: overcoming translational challenges. J. Clin. Investig. 129 , 1407–1418 (2019).
Raja, J., Ludwig, J. M., Gettinger, S. N., Schalper, K. A. & Kim, H. S. Oncolytic virus immunotherapy: future prospects for oncology. J Immunother. Cancer. 6, 140–153 (2018).
Samarkos, M., Mastrogianni, E. & Kampouropoulou, O. The role of gut microbiota in Clostridium difficile infection. Eur. J. Intern. Med. 50 , 28–32 (2018).
Download references
Acknowledgements
This work was financially supported by the Ministerio de Economía y Competitividad (BIO2017-84974-R), Xunta de Galicia “Grupo Referencia Competitiva 2016” (ED431C 2016/041) and the European Union (European Regional Development Fund, Ref. ED431G2019/06). JV and SM acknowledge contracts from Programa INTERREG V-A España-Portugal (POCTEP) 2014-2020 and Retención de Talento Investigador from the University of Vigo, respectively.
Author information
These authors contributed equally: Jezabel Varadé, Susana Magadán, África González-Fernández
Authors and Affiliations
CINBIO, Centro de Investigaciones Biomédicas, Universidade de Vigo, Immunology Group, Campus Universitario Lagoas, Marcosende, 36310, Vigo, Spain
Jezabel Varadé, Susana Magadán & África González-Fernández
Instituto de Investigación Sanitaria Galicia Sur (IIS-Galicia Sur), SERGAS-UVIGO, Vigo, Spain
You can also search for this author in PubMed Google Scholar
Contributions
A.G-F conceptualized the study and conceived the project, and all the authors participated in writing the paper.
Corresponding author
Correspondence to África González-Fernández .
Ethics declarations
Competing interests.
A.G-F is a copromoter of the spin-off Nanoimmunotech. There are no competing financial interests in relation to the work described.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Varadé, J., Magadán, S. & González-Fernández, Á. Human immunology and immunotherapy: main achievements and challenges. Cell Mol Immunol 18 , 805–828 (2021). https://doi.org/10.1038/s41423-020-00530-6
Download citation
Received : 21 April 2020
Revised : 27 July 2020
Accepted : 31 July 2020
Published : 02 September 2020
Issue Date : April 2021
DOI : https://doi.org/10.1038/s41423-020-00530-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- CAR T cells
- Trained immunity
- Monoclonal antibodies
This article is cited by
Aerobic glycolysis enables the effector differentiation potential of stem-like cd4+ t cells to combat cancer.
- Xiaolong Zhang
- Wenhao Chen
Cellular & Molecular Immunology (2024)
A novel multi-variate immunological approach, reveals immune variation associated with environmental conditions, and co-infection in the koala (Phascolarctos cinereus)
- Cristina M. Fernandez
- Mark B. Krockenberger
- Damien P. Higgins
Scientific Reports (2024)
Integrative analysis of homologous recombination repair patterns unveils prognostic signatures and immunotherapeutic insights in breast cancer
- Yan-Shuang Li
- Hong-Chuan Jiang
Journal of Applied Genetics (2024)
Multi-omics characterization of a scoring system to quantify hypoxia patterns in patients with head and neck squamous cell carcinoma
- Zhuguang Yi
Journal of Translational Medicine (2023)
Autoimmune liver disease and multiple sclerosis: state of the art and future perspectives
- Rosanna Villani
- Gaetano Serviddio
- Emanuele D’Amico
Clinical and Experimental Medicine (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts

The science and medicine of human immunology
Bali pulendran.
1 Institute for Immunity, Transplantation and Infection, Stanford University, Stanford, CA 94305, USA.
2 Department of Pathology, Stanford University, Stanford, CA 94305, USA.
3 Department of Microbiology and Immunology, Stanford University, Stanford, CA 94305, USA.
4 Stanford ChEM-H: Chemistry, Engineering and Medicine for Human Health, Stanford University, Stanford, CA 94305, USA.
5 Stanford University School of Medicine, Stanford, CA 94305, USA.
Mark M. Davis
6 Howard Hughes Medical Institute, Stanford University, Stanford, CA 94305, USA.
BACKGROUND:
The mammalian immune system is a remarkable sensory system for the detection and neutralization of pathogens. History is replete with the devastating effects of plagues, and the coronavirus disease 2019 (COVID-19) pandemic is a defining global health crisis of our time. Although the development of effective vaccines has saved many lives, the basic workings of the immune system are complex and require the development of animal models, such as inbred mice. Indeed, research in mice has been enormously productive, and the tremendous insights gleaned have resulted in many Nobel prizes and other accolades. However, past results are not necessarily a reliable guide to the future, and a notable limitation of animal models has been their failure to accurately model some human diseases and their inability to predict human immune responses in many cases. With regard to inbred mice, which have been the principal model of choice for immunology, this is likely due to the compromises that were necessary to create a more tractable and reproducible system for experimentation, such as genetic uniformity and lack of pathogen exposure, as well as the fact that mice are evolutionarily quite distinct. These considerations suggest that direct studies of the human immune system are likely to be extremely rewarding, both from a scientific and a medical perspective.
In the past decade there has been an explosion of new approaches and technologies to explore the human immune system with unprecedented precision. Insights into the human immune response to vaccination, cancers, and viral infections such as COVID-19 have come from high-throughput “omics” technologies that measure the behavior of genes, mRNA (single-cell transcriptomics), proteins (proteomics), metabolites (metabolomics), cells (mass cytometry), and epigenetic modifications (ATAC-seq), coupled with computational approaches.
Sydney Brenner remarked in 2008, “We don’t have to look for a model organism anymore. Because we are the model organisms.” We propose that studying the immune system in humans, who are genetically diverse and afflicted by a multitude of diseases, offers both a direct link to medicine (i.e., “translation”) and the very real prospect of discovering fundamentally new human biology. New approaches and technology are now making this area much more approachable, but profiling immunity in humans is but the first step. Computational mining of the data and biological validation in animal models or human organoids are essential next steps, in an iterative cycle that seeks to bridge fundamental and applied science, as well as mouse and human immunology, in a seamless continuum of scientific discovery and translational medicine. This will represent a new paradigm for accelerating the development of vaccines and therapeutics.
Graphical Abstract

Probing the human immune response to viral infections. Systems biology techniques can be used to probe the human immune response to viral infections and can define molecular signatures that predict disease severity and illuminate the underlying mechanisms of disease.
Although the development of effective vaccines has saved countless lives from infectious diseases, the basic workings of the human immune system are complex and have required the development of animal models, such as inbred mice, to define mechanisms of immunity. More recently, new strategies and technologies have been developed to directly explore the human immune system with unprecedented precision. We discuss how these approaches are advancing our mechanistic understanding of human immunology and are facilitating the development of vaccines and therapeutics for infection, autoimmune diseases, and cancer.
As is the case with other biological disciplines, many of the major paradigms in immunology have emerged from research using animal models. For example, experimental validation of the clonal selection theory, the foundational paradigm on which modern immunology rests ( 1 , 2 ), came from experiments in rats ( 3 ). The role of the thymus in generating T lymphocytes was discovered using mice ( 4 ), and studies in chickens led to the discovery of T and B lymphocytes as the organizing principles of the adaptive immune system ( 5 ). The role of the innate immune system in sensing pathogens through Toll-like receptors (TLRs) was discovered through studies of mice ( 6 ) and fruit flies ( 7 ). Dendritic cells (DCs), the central orchestrators of the immune response, were first discovered in mice ( 8 ). Indeed, the majority of the Nobel prizes for discoveries in immunology have been awarded for research performed in animals ( Table 1 ).
Nobel Prize in Medicine or Physiology award dates are in chronological order.
Yet one must remember that the origin of immunology is rooted deeply in experimentation in humans by Edward Jenner and Louis Pasteur, regarded as the fathers of immunology. Jenner’s first demonstration of the concept of vaccination was performed in 1796, when he inoculated 8-year-old James Phipps with pus from cowpox blisters on the hands of Sarah Nelmes, a milkmaid who had caught cowpox. Phipps developed a fever, but no disease, and was protected from smallpox when subsequently variolated ( 9 ). The findings gradually gained wide acceptance, culminating on 8 May 1980 with the declaration by the World Health Assembly that “the world and all its people have won freedom from smallpox.” Pasteur’s human experimentation began almost a century after Jenner, in 1885, when he treated his first human patient, a 9-year-old boy who had been bitten by rabid dogs. The victim received daily injections of progressively more virulent doses of an attenuated form of the virus ( 10 ). The boy survived and Pasteur became an international hero. Although Jenner and Pasteur would never receive institutional review board approval to perform these studies today, these experiments in humans can be considered to represent the First Golden Age in human immunology.
In the century and a half that followed Pasteur and Jenner, there has been elegant research on the human immune system, particularly in analyzing the cellular composition and functions of immune cell types isolated from the blood of healthy ( 11 – 13 ) and immune-deficient patients ( 14 – 18 ), yet there have been very few studies that have analyzed immune responses in humans in vivo. Instead, it is research in animal models that has been largely responsible for advancing our mechanistic understanding of the immune system. Indeed, a recurring theme in biology is that biologists have adapted unique features of various animal models to address fundamental mechanistic questions. Thus, neurobiologists have used squid with giant axons for studying synaptic action potential ( 19 ), cell biologists have used yeast and sea urchins for studying the cell cycle ( 20 ), developmental biologists have used nematodes for studying development ( 21 ), and immunologists have relied extensively on genetically inbred strains of mice. In such mice, the immune system can be studied without the confounding effects of genetic variability, and genes can be deleted ( 22 , 23 ) or overexpressed ( 24 ) in a given tissue or cell type. However, these mice are genetically homogeneous and are usually housed in abnormally hygienic and specific pathogen-free (SPF) environments. Hence, they are far from ideal models for the immune systems of humans. Some recent studies have used free-living populations of feral mice or pet store mice (which occupy more natural habitats) to study immune responses ( 25 , 26 ), but the extent to which the immune states of such mice reflect the immune states of humans is uncertain. Mice and humans diverged evolutionarily 96 million years ago, and although the immune systems of the two species are broadly similar, they differ in many important details ( Table 2 ).
Adapted from ( 149 ).
Experiments in mice have led to the development of many successful therapies in humans. For example, basic discoveries in mice enabled the development of anti-tumor necrosis factor (TNF) therapy for rheumatoid arthritis ( 27 ) as well as the enormous success of cancer immunotherapy (metastatic melanoma, which was almost incurable previously, can now be in remission in >50% of patients as a result of combined anti-CTLA4 and anti-PD1 therapy) ( 28 ). Yet the number of failures to translate successes from animals to humans has been increasing. One of the most notorious examples in recent years is theralizumab, an anti-CD28 antibody ( 29 ). Its first trial in humans resulted in hospitalization of all six volunteers, at least four of whom suffered multi-organ dysfunction ( 29 ), despite no such adverse effects being observed in preclinical studies in primates. In vaccine development, the world of HIV vaccines suffered a startling setback in 2008 when the first clinical trial of a vaccine designed to elicit cellular immunity against the virus appeared to increase the rate of HIV infection in individuals with prior immunity against the adenovirus 5 vector used in the vaccine ( 30 ). Yet this vaccine had shown protective efficacy against a humanized simian immune deficiency virus (SHIV) in primates, as well as some modest protection against the pathogenic SIV ( 31 ). These failures symbolize the “valley of death” in drug and vaccine development and underscore the imperative to harness the “human model” at a relatively early stage in the discovery-development pipeline. In this context, recent advances have highlighted new approaches to studying immune responses in humans. In particular, advances in systems biology coupled with human phenotypic data have allowed the delineation of molecular networks that drive human immunity. Here, we review these advances and discuss the limitations and critical challenges that need to be overcome to effectively exploit the human model to advance our understanding of the immune system and design novel vaccines and therapeutics.
Humans as model organisms in immunology
In human beings, a widely available resource containing a representation of the immune system is blood, which is routinely taken from almost all patients (as well as from healthy individuals) and provides a kaleidoscope of many lineages and differentiation states within the immune system. Because migration of cells is a key feature of the immune system, blood leukocytes (present at 1 to 3 million per milliliter of blood) represent recent emigrants from tissues, including sites of infection or vaccination. This, coupled with the advent of high-throughput “omics” technologies that measure the behavior of genes, mRNA (transcriptomics), proteins (proteomics), metabolites (metabolomics), cells (mass cytometry), and epigenetic modifications (ATAC-seq), has started to provide unprecedented insight into the human immune response in the context of vaccination, infections, cancers, and autoimmunity ( Fig. 1 ). In particular, the development of next-generation sequencing (NGS) has facilitated the comprehensive evaluation of transcripts including noncoding RNAs, microRNAs, and long noncoding RNAs, and the use of ATAC-seq has further allowed definition of chromatin accessibility loci to identify epigenetic regulators of gene expression ( 32 ). Furthermore, such NGS permits the characterization of T and B cell repertoires ( 33 , 34 ), and high-throughput single-cell sequencing is yielding rich new information about the heterogeneity of cell subsets and identification of new populations of cells ( 35 ). Single-cell mass cytometry techniques allow analysis of the cellular dynamics of immune responses with an exquisite degree of precision ( 36 ) and analysis of the epigenetic landscape of single cells ( 37 ) ( Fig. 1 ). Finally, the development of high-throughput platforms has allowed researchers to analyze the functional properties of antigen-specific antibodies isolated from serum of vaccinated or infected humans ( 38 , 39 ). Specific examples of the application of these technologies are considered below.

The human immune system can be perturbed by vaccination, infection, or allergens, or in autoimmunity. Blood samples or fine needle aspirate samples of lymph nodes or other human tissues (e.g., skin biopsies) can be isolated and the immune response analyzed at multiple levels of the hierarchy of biological organization, using a wide array of technologies including transcriptomics, epigenomics, mass cytometry, and metabolomics.
Vaccines as probes for the human immune system
Vaccination represents the most effective way to prevent infectious diseases. But to immunologists, vaccines represent excellent probes of the human immune system. First, vaccines allow a synchronized perturbation of the immune system, such that the immune response can be assessed from the earliest minutes to several decades after vaccination ( 40 ). Although the immune system is also perturbed in infections, autoimmune diseases, and cancers, in such cases the precise moment of immune perturbation cannot be known. Second, vaccines represent a diverse array of microbial stimuli (e.g., live viruses, carbohydrate vaccines, vaccines containing adjuvants) that can be used to perturb the immune system ( 40 ). Third, vaccines are widely administered to diverse human populations (e.g., neonates versus elderly; undernourished versus obese). For decades, vaccine manufacturers have relied on a single measurement—typically the magnitude of the antigen-specific antibody response (e.g., neutralization antibody titers or binding antibody titers measured by enzyme-linked immunosorbent assay)—to assess the immune response stimulated by vaccination. However, such measurements fail to capture the global architecture of the immune response to vaccination and the multiple immunological mechanisms that can mediate protection against a given pathogen ( 41 ).
Recent advances in omics technologies have enabled scientists to probe the immune response to vaccination in a comprehensive way, by analyzing the cellular and molecular networks driving immune response to vaccination. These omics technologies can measure changes in cellular subsets, transcriptome, metabolome, or epigenome, even at the single-cell level. Coupling these with computational approaches developed to analyze and interpret such data has led to the generation of the new field of “systems vaccinology.” The aim of systems vaccinology is to comprehensively analyze the immune response to vaccination with a view to defining new mechanisms and correlates of protective immunity ( 41 , 42 ).
Initial proof-of-concept studies demonstrating the use of systems biology approaches to identify molecular signatures of vaccination used the yellow fever live-attenuated vaccine YF-17D ( 43 , 44 ). YF-17D is one of the most successful vaccines ever developed, having been administered to more than 600 million people worldwide with an efficacy of 99% ( 45 ); a single immunization results in robust antigen-specific cytotoxic T cell responses and neutralizing antibody responses that can persist for several decades ( 42 ). Transcriptomic analysis of peripheral blood mononuclear cells (PBMCs) isolated 3 to 7 days after vaccination of healthy young adults with YF-17D revealed a gene expression profile pattern consisting of genes encoding proteins involved in antiviral sensing and viral immunity, including the type I interferon (IFN) pathway ( 43 , 44 ). Computational analysis and machine learning approaches revealed signatures of early gene expression, which correlated with and predicted the magnitude of the later antigen-specific CD8 + T cell and neutralizing antibody responses, in an independent study ( 43 ).
Subsequently, several groups applied systems-based analysis to study immune responses to vaccination with seasonal influenza ( 46 – 50 ), meningococcal ( 51 ), shingles ( 52 ), malaria ( 53 , 54 ), smallpox ( 55 ), Ebola ( 56 ) vaccines, as well as a candidate vaccine against HIV ( 57 ). In addition, several groups analyzed the transcriptional profile at “baseline” (i.e., prior to vaccination) to identify signatures of vaccine responsiveness prior to vaccination ( 49 , 58 – 61 ). Furthermore, although until recently most studies have relied on transcriptional profiling to define signatures of vaccine immunity, recent studies have performed integrated analysis of orthogonal datasets to generate an integrated model of vaccine immunity. To understand the mechanisms of immunity induced by the live attenuated shingles vaccine, we constructed a multiscale, multifactorial response network (MMRN) of immunity induced by vaccination in healthy young and older adults. The MMRN revealed striking associations between orthogonal datasets, such as transcriptomic and metabolomics signatures, cell populations, and cytokine levels, and identified immune and metabolic correlates of vaccine immunity ( 52 ).
Defining correlates of vaccine efficacy (i.e., the capacity of vaccination to protect from infection) is more challenging. One approach to defining correlates of efficacy is the controlled human infection model (such as those that involve controlled challenge of humans with pathogenic strains of typhoid, influenza, or malaria), in which subjects can be vaccinated and subsequently challenged with the pathogen to define the mechanisms and correlates of vaccine immunity ( 62 ). Such studies have already been done in the context of the RTS,S malaria vaccine and have yielded important insights about the mechanisms of protection ( 53 , 54 ). In particular, the results from these studies reveal that protection from malaria infection can be mediated by multiple immunological mechanisms involving the magnitude of serum circumsporozoite-specific antibody titers as well as the magnitude of antigen-specific CD4 + T cells with the capacity to produce polyfunctional cytokines ( 53 , 54 ). In the case of phase 2b or phase 3 efficacy trials that involve several thousand subjects, the primary endpoint is efficacy. Typically, the trial design and sample collection schedules do not provide opportunities for retrospective analysis of immune responses. This issue has been brought to the fore recently, as the world races to develop a vaccine against COVID-19. At present, more than 160 vaccine candidates are being developed across the world, and systems vaccinology approaches may be useful to help identify early predictors of efficacy and accelerate the COVID-19 vaccine testing pipeline. Therefore, future efficacy trials should be leveraged to include sample collection schedules that facilitate systems-based immune profiling, with a view to defining mechanisms and correlates of efficacy.
An important question is the extent to which these high-throughput data can yield new insights about the immune system. Several mechanistic insights are emerging from such studies. For example, among the signatures that predicted CD8 + T cell responses to YF-17D was general control nonderepressible 2 kinase (GCN2; also called eukaryotic translation initiation factor 2α kinase 4), a sensor of amino acid starvation and an orchestrator of the integrated stress response ( 43 ). Studies with knockout mice showed a key role for YF-17D– induced GCN2 activation in programming DCs to initiate autophagy and enhance antigen presentation to CD4 + and CD8 + T cells ( 63 ), as well as in the control of intestinal inflammation ( 64 ). These results revealed an unappreciated link between virus-induced integrated stress response in DCs and the adaptive immune response.
Another insight that emerged from the influenza vaccine study was the impact of the gut microbiota on vaccine-induced immunity ( 65 ). Transcriptional profiling of immune responses to vaccination with the seasonal influenza vaccine revealed a striking correlation for the expression of the gene encoding TLR5—a receptor for bacterial flagellin—3 to 7 days after vaccination and the ensuing antibody response a month later ( 47 ). This prompted mechanistic investigation using mice that were genetically deficient in TLR5. Such studies revealed that TLR5-deficient mice were impaired in the vaccine-specific antibody response induced by vaccination with the seasonal influenza vaccine ( 66 ). This effect was shown to depend on sensing of bacterial flagellin by TLR5; consequently, mice administered antibiotics to deplete their microbiota or mice devoid of microbiota from birth (so-called “germ-free mice”) were impaired in their capacity to launch antibody responses to seasonal influenza vaccination ( 66 ). This experiment in mice raised the key question regarding whether the microbiota controlled immune responses after vaccination in humans. A recent clinical trial assessed the impact of administering broad-spectrum antibiotics to healthy adults before and after seasonal influenza vaccination; despite a reduction in gut bacterial load by four orders of magnitude and a long-lasting restriction in gut bacterial diversity, antibody responses were unaffected ( 67 ). However, in a second trial of subjects with low preexisting antibody titers, there was significant impairment in H1N1-specific neutralization and binding immunoglobulin G1 (IgG1) and IgA responses ( 67 ). In addition to these effects on the adaptive immune response, antibiotic administration also enhanced inflammatory signatures (including AP-1/NR4A expression), observed previously in the elderly ( 49 ), as well as perturbation of the serum metabolic profile, with a reduction in serum secondary bile acids by three orders of magnitude. The change in serum bile acids was highly correlated with AP-1/NR4A signaling and inflammasome activation. Integrative analysis of multi-omics datasets revealed significant associations between bacterial species and metabolic phenotypes, highlighting a key role for the microbiome in modulating human immunity ( 67 ). These studies show that systems approaches can help to identify early “signatures” that could predict the later immunogenicity or efficacy of a vaccine and yield new mechanistic insights.
Probing the human immune system in infections
Despite the invention of vaccines and antibiotics, infectious diseases continue to exert a major toll on human health, as evidenced by the recent COVID-19 pandemic ( 68 ). In 2016, lower respiratory infections, diarrheal diseases, tuberculosis (TB), and HIV/AIDS accounted for nearly 7 million deaths per year ( 69 ), and these diseases alone were the leading cause of disability-adjusted life years (DALY), accounting for 12.1% of all DALYs ( 69 ). Animal models have been developed for several infectious diseases and have yielded many valuable insights about mechanisms of immunity, yet differences in pathogenesis between these models and the human model have posed challenges.
In the past decade or so, there has been a growing effort to study immunity to infections in humans. An obvious limitation here, unlike the case with vaccination, is that the precise moment of natural infection cannot be known, thus posing an obstacle to analyzing immune responses during the earliest stages of infection. Nonetheless, there are some notable examples of addressing this issue, such as the FRESH study (Females Rising through Education, Support, and Health), which is a longitudinal study to identify and analyze participants immediately after they are infected with HIV ( 70 ). The FRESH program is located in the Umlazi township of South Africa, where the HIV prevalence rate in young women is as high as 66% at age 23. Investigators are collecting blood samples from 300 noninfected women aged 18 to 23, with a view to studying the earliest events that occur after natural infection with HIV ( 70 ). This FRESH study is providing key mechanistic insights into the biological factors that predispose to HIV infection. For example, the composition and diversity of the cervicovaginal microbiome were found to have a noticeable effect on the local host inflammatory response and the risk of HIV acquisition ( 71 ). In addition, there was profound CD8 + T cell activation and proliferation (>70% of CD8 + T cells in some individuals, the majority being HIV-specific by tetramer staining) in untreated acute infection. These cells rapidly became proapoptotic and were defective at producing antiviral cytokines such as IFN-γ, which suggested that HIV infection caused abnormal activation of CD8 + T cells ( 72 ), different from that observed with live viral vaccines such as YF-17D or smallpox ( 73 ).
Another area that has emerged in recent years is the profiling of transcriptional gene expression signatures in the blood to enhance our understanding of the host immune response to infection ( 74 – 84 ). Mycobacterium tuberculosis has been a scourge of humanity since ancient times, having survived more than 70,000 years, and currently one-third of the world’s population are carriers of the latent pathogen and are at risk for developing active disease ( 85 ). One of its mysteries is that an estimated 10% of infected adults succumb to full-blown TB, but most are able to control the disease throughout their lives ( 85 ). Blood transcriptional analysis of tuberculosis has revealed IFN-inducible genes whose expression diminished after successful treatment ( 76 ). Coupled with sensitive radiography techniques, blood transcriptomics have revealed heterogeneity in patients with active tuberculosis and asymptomatic people with latent tuberculosis, suggesting a continuum of infection and immune states ( 76 ). A recent study using high-dimensional mass cytometry demonstrated that latent TB is associated with enhanced cytotoxic responses and natural killer (NK) cells ( 86 ). Finally, a meta-analysis of 24 datasets containing 3083 transcriptome profiles from whole blood or PBMC samples of healthy controls or patients with active or latent TB from 14 countries yielded a three-gene set in whole blood that was robustly diagnostic for active tuberculosis, suggesting the clinical utility of such a signature ( 87 , 88 ).
An especially fruitful area of research has been the analysis of immune responses in HIV-infected individuals, which has provided new insights about antigen design strategies for vaccination. A small proportion of HIV-infected individuals develop broadly cross-reactive neutralizing antibody responses that can neutralize a broad array of HIV viruses. Monoclonal antibodies have been isolated from such subjects and have been used to define critical antigenic epitopes that represent the targets of the broadly cross-reactive neutralizing antibodies ( 89 – 91 ).
Probing the human immune system in autoimmunity
Autoimmune diseases represent a diverse family of some 80 distinct diseases, such as rheumatoid arthritis, type I diabetes, and systemic lupus erythematosus (SLE), which afflict 5 to 9% of the global population ( 92 ). In healthy individuals, the immune system is considered to be “tolerant” of proteins present within the host’s own body, so-called “self” proteins. However, in humans who develop autoimmune diseases, there is a complex and dynamic interplay between host genes and the environment that results in the loss of immune tolerance to “self antigens,” leading to the development of autoreactive T and B cells that attack the body’s own tissues ( 93 ). Such autoreactivity can be organ-specific (e.g., rheumatoid arthritis) or systemic (e.g., SLE).
Preclinical animal models of human autoimmune diseases have provided important clues to these diseases in the form of possible mechanisms and etiology. Studies using mouse models of spontaneous autoimmune diseases [e.g., the experimental autoimmune encephalomyelitis (EAE) model for multiple sclerosis (MS); NOD mice for diabetes; the MRL/lpr model for lupus] have provided powerful tools to assess mechanisms and test novel therapeutic concepts ( 94 , 95 ). Furthermore, mice genetically deficient in particular genes have established critical regulators of autoimmune processes, and the use of engineered models of autoimmune disease, such as mice transgenic for T and B cell receptors specific for autoantigens, has yielded a wealth of mechanistic insight ( 94 , 95 ). However, the failure to translate promising therapies from these animal models to humans has led to a reassessment of the extent to which such models reflect the complexity and heterogeneity of the clinical presentation of the human disease. For example, of the ~200 treatments that prevent or delay the development of type 1 diabetes in the NOD mouse model, only a few have been shown to have clinical promise, and in only a fraction of patients ( 94 – 96 ). Some of the failures in translation were likely due to differences in the experimental design in the preclinical versus clinical studies, but species differences in the immune systems and human variations have likely been a major impediment.
The discovery of TNF-α as a central regulator in rheumatoid arthritis and the development of therapeutic anti-TNF-α antibodies have established a new paradigm for the treatment of autoimmune diseases ( 97 ). Ironically, it was an experiment with human tissues—the evaluation of cytokine expression in human rheumatoid synovium—that represented the key experiment in defining the cytokines that were overexpressed in the disease site ( 98 ). Many cytokines were overexpressed, so which of those represented the best therapeutic target was unclear. This was analyzed by blocking various cytokines in rheumatoid synovial cultures, which revealed that blockade of TNF-α reduced interleukin-1 (IL-1) synthesis as well as all other pro-inflammatory cytokines found in joints. This was the first demonstration that TNF-α could be an important therapeutic target ( 98 ). Concurrent experiments in mice supported the concept of a TNF-α-dependent cytokine cascade ( 99 ). Subsequent experiments in animal models demonstrated that administration of anti-TNF-α monoclonal antibody ameliorated both inflammation and joint damage in the collagen type II model of arthritis ( 100 ). This provided the rationale for anti-TNF therapy in rheumatoid arthritis, which has heralded a revolution in the introduction of protein therapeutics, termed “biologics.”
Genome-wide association studies (GWASs) have been performed over the past decade, with the goal of analyzing single-nucleotide polymorphisms (SNPs) in genes associated with a disease in large patient populations, and have revealed the polygenetic basis of many autoimmune diseases ( 101 ). Originally it was hoped that genetic studies of autoimmune diseases would discover one or a few defective genes that could explain the pathology and be useful diagnostically. This launched a number of GWASs, but unfortunately these efforts have shown that many loci contribute very small effects. However, some alleles. such as the human leukocyte antigen (HLA) alleles, do show statistically significant associations. Because different HLA alleles bind to distinct antigenic peptides, and because these HLA-peptide complexes are specific targets for T cell recognition, this points to a potential role for T cells in initiating and maintaining the disease ( 102 ).
Transcriptomics of patient PBMCs and whole blood have revealed alterations in the type I IFN network in SLE, Sjögren’s syndrome, inflammatory myopathies, and a subset of systemic sclerosis patients. SLE is an autoimmune disease characterized by loss of tolerance to nucleic acids, including double-stranded DNA and ribonucleoproteins, with diverse clinical manifestation. Recently, Pascual and colleagues analyzed transcriptional changes in the blood of 158 pediatric lupus patients ( 103 ). Using computational approaches, they identified transcriptional signatures of the type I IFN and plasmablast responses as the most robust biomarker of disease activity. They detected increased enrichment of neutrophil transcripts during progression to active nephritis. Strikingly, they were able to stratify the patients into seven groups according to distinct molecular correlates of disease activity. This study reveals the molecular heterogeneity underlying disease manifestation in SLE and suggests that future trials should implement “precision medicine” therapies that target the specific molecular anomalies in genetically and clinically complex autoimmune diseases.
Such approaches have also revealed a key role for IL-1β in systemic-onset juvenile idiopathic arthritis and have led to the development of anti-IL-1-based therapeutics ( 104 ). In the case of MS, IFN-β therapy has been used as a drug therapy, but despite its clinical use, the treatment modality involved a protracted regimen of uncertain clinical benefit ( 105 ). Transcriptomics has revealed transcriptional responses to IFN-β1α and has enabled the stratification of patients according to therapeutic response ( 106 ). Furthermore, systems approaches have been used to probe the immune response in patients with celiac disease, an intestinal autoimmune disease. Administration of gluten to patients with celiac disease not only induced the expansion and mobilization into the circulatory system of gliadin-specific CD4 T cells 6 days after challenge, but also induced CD8 T cells and γδ T cells with oligoclonal T cell receptors with similar kinetics ( 107 ). This same pattern of co-mobilized CD4 + , CD8 + , and γδ T cells was seen in the blood and central nervous system tissue of mice induced to develop EAE, which suggests that this may be a general feature of autoimmune diseases. Surprisingly, upon analysis of the specificities and functional properties of these different T cells, the CD4 + and γδ T cells were found to be pathogenic, whereas the CD8 + cells were found to be suppressive and able to kill the CD4 + cells in vitro ( 108 ). This indicates a complex dynamic in autoimmunity that will be fascinating to investigate further.
Clearly, analysis of the human immune system in the context of autoimmune diseases is yielding novel insights about the mechanisms and potential targets for therapy. Future studies could be aimed at using systems-based approaches to define the immune mechanisms and gene-environment interactions that lead to autoimmunity. However, unlike with vaccination, it has been challenging to assess the earliest stages of the response, and thus the etiology of the disease has been difficult to ascertain. One solution to this problem may be to establish human cohort studies such as the TEDDY study (The Environmental Determinants of Diabetes in the Young), a multi-center prospective cohort study that was designed to follow children with and without a family history of type 1 diabetes to understand the environmental factors that contribute to the disease ( 109 , 110 ). In this study, 424,790 newborn children younger than 4 months were screened for high-risk HLA alleles, of whom 21,589 had the qualifying haplotypes and 8676 were enrolled. Of this group, 672 developed persistent antibodies to insulin, glutamic acid decarboxylase, or islet antigen 2, and these subjects are being followed for 15 years. It is predicted that 390 children will develop diabetes. Blood samples are being collected and banked from the day of birth and every 3 months, and stool samples collected (for microbiome analysis) quarterly, throughout the 15-year period ( 110 ). Clinical metadata (infections, medication, immunizations), exposure to dietary and other environmental factors, negative life events, family history, tap water, and measurements of psychological stress will also be collected. The major goals of the TEDDY study are to discover genes associated with the development of autoantibodies and type 1 diabetes, to identify environmental exposures that modify the risk of autoimmunity, and to use omics technologies to define signatures of the transcriptome, metabolome, epigenome, or microbiome that correlate with and predict autoantibody formation and disease progression ( 110 ). Such system analysis will not only yield novel biomarkers but also reveal mechanistic insights about the sequalae of immunological events, starting at the very earliest stages, that culminate in disease. Studies such as TEDDY also provide unique opportunities for discovering novel autoantigens—for example, by analyzing the B cell repertoire and isolating monoclonal antibodies from plasmablasts, or analyzing the T cell receptor repertoire using the recently discovered GLIPH algorithm (grouping of lymphocyte interactions by paratope hotspots) ( 34 ).
Probing the human immune system in cancer
The development of checkpoint blockade inhibitors has revolutionized cancer immunotherapy ( 28 ) and has led to durable survival outcomes in some patients with metastatic disease ( 111 ). Combination therapy of previously untreated advanced melanoma patients with nivolumab and ipilimumab (monoclonal antibodies that respectively target the PD-1 and CTLA-4 receptors on T cells) resulted in significantly greater objective-response rates and progression-free survival relative to ipilimumab monotherapy alone ( 112 , 113 ). The development of these immune therapy regimens occurred as a result of fundamental discoveries using the mouse model ( 114 , 115 ). However, a major issue with checkpoint inhibitors is that only a fraction of cancer patients experience durable clinical benefit ( 28 ). Thus, the identification of molecular signatures that can be used to predict nonresponsiveness to checkpoint inhibitors would be valuable in identifying patients who would be viable candidates for treatment. In this context, tissue-based immune monitoring is providing important insights into the cellular and molecular mechanisms of immune checkpoint therapy. For example, in a trial of ipilimumab, analysis of the RNA transcriptome in pre- and post-treatment tumor tissues revealed major transcriptional changes related to T cell signaling and activation after CTLA-4 blockade. In particular, ICOS + T cells were increased in tumor tissues from patients with ipilimumab, and the increase in ICOS + T cells was accompanied by similar increases in the blood ( 116 ). To test the hypothesis that ICOS + CD4 T cells may play a role in the therapeutic effect of CTLA-4 blockade, similar studies were conducted in mice; although anti-CTLA-4 treatment was shown to induce tumor rejection in 80 to 90% of mice, the efficacy was less than 50% in ICOS-deficient mice ( 116 ).
In addition to tissue-based immune monitoring, emerging studies demonstrate that analysis of peripheral blood can yield new insights into mechanisms and biomarkers of tumor immune therapy. A recent study used immune profiling of blood from patients with stage IV melanoma before and after treatment with anti-PD-1 antibody (pembro-lizumab); the clinical failure in many patients was not due to a lack of invigoration of exhausted T cells, but rather due to the high tumor burden prior to anti-PD-1 therapy ( 117 ). Thus, the magnitude of reinvigoration of circulating exhausted T cells determined in relation to pretreatment tumor burden correlated with clinical response.
These studies highlight the power of human immune profiling technologies to identify signatures and mechanisms of antitumor immunity. In this context, emerging evidence suggests that patients receiving immunotherapy can be stratified into responders versus nonresponders based on the composition of their gut microbiomes ( 118 – 120 ). Future work should thus be aimed at using multi-omics technologies to delineate the transcriptional, metabolomic, epigenetic, microbiome, and cellular networks that regulate antitumor immunity, with a view to obtaining molecular signatures of responsiveness to cancer immunotherapy, similar to what has been accomplished with analysis of vaccination-induced responses ( 52 , 67 ).
Challenges in the pursuit of human immunology in the 21st century
Human immunology is being rapidly revitalized by spectacular advances in technology and their application to the study of immune responses in humans. Although the preceding discussion has focused on vaccines, infections, autoimmunity, and cancer, systems-based approaches are also beginning to be used to probe the immune response in asthma and allergic diseases in, for example, children before, during, and after placebo-controlled oral challenges to peanuts ( 121 , 122 ) or in the context of transplantation ( 123 , 124 ). Yet the field is just beginning, and major challenges need to be addressed if it is to be a success. These include scientific, practical, and cultural challenges, as discussed below.
Scientific challenges: The obstinately diverse species
One major scientific challenge is dealing with diversity in the human model. Peter Medawar once called us the obstinately diverse species ( 125 ), a reference to our diverse genetic makeup. However, profound demographic, socioeconomic, and environmental changes have resulted in even further diversification with respect to health and age distributions. For example, The State of Food Security and Nutrition in the World 2019 states that maternal and child undernutrition contributes to 45% of deaths in children under 5, yet overweight and obesity are increasing in almost all countries and contribute to 4 million deaths globally ( 126 ). Indeed, paradoxically many populations are afflicted by the dual burdens of undernutrition and overweight. In 2018, Africa and Asia bore the greatest share of all forms of malnutrition, accounting for 90% of all stunted and wasted children but also nearly 75% of all overweight children worldwide ( 126 ). Disparity in life expectancy is also wide; as of 2018, Monaco had an average life expectancy of 89.52 years, whereas Chad was lower at 49.81 years ( 127 ), only moderately better than the 35 years in 17th-century Britain. These wide disparities no doubt impinge on the physiological states of humans and on variations in their immunological states in particular. This in turn is likely to affect responses to vaccination or susceptibility to inflammatory diseases.
The immunological state of an individual can vary longitudinally with time as a result of environmental changes, aging, or developmental changes in early life. In this context, Olin et al . used mass cytometry to analyze the changes in blood leukocyte composition in 100 newborn children during the first 3 months of life. The blood leukocyte composition of preterm and term children differed at birth but converged on a shared trajectory ( 128 ). Alternatively, there can be variations in the immunological states in individuals within a given population (because of genetic, microbiome, and environmental differences) ( 129 , 130 ), or there can be variations in the immunological states between different populations ( Fig. 2 ). This diversity is, however, an excellent source of natural variation or perturbation that enables effective interrogation of the human immune system in a population to draw predictive insights, and is thus a major advantage of the human model ( 131 ). To address this issue, Carr et al . used systems approaches to establish the cellular profiles of 670 healthy individuals and demonstrated high interindividual variation but low longitudinal variation at the level of cellular subset composition. Influenza vaccination induced expansion of antigen-specific B cell clones, but the cellular subset structure was elastic and returned to the individual’s unique baseline state. The largest influence on immune variation between individuals was cohabitation, with 50% less variation between individuals who share an environment than between people in the wider population ( 130 ). Such variations can lead to drastic differences in the responsiveness of the immune systems, such as wide differences in the immunogenicity and efficacy of vaccines. Therefore, a major challenge for human immunology is to embrace this diversity by probing the immune system in diverse human populations, with a view to obtaining mechanistic insights about the factors that lead to this variation. To understand this diversity, it must be accurately captured and recorded. This means aiming to record and standardize as much clinical metadata as possible during human studies. This can be facilitated through close collaboration between immunologists/basic scientists and clinicians. As precision medicine and wearable health technology advance, more and more of these data will become available ( 132 , 133 ).

The immunological state of an individual can vary with time as a result of stochastic changes, environmental perturbations (e.g., vaccination, infection, exposure to allergens), or developmental changes. Alternatively, the immunological state of individuals in a given population, or individuals in different populations, may vary because of differences in host genetics, the environment, or individuals’ microbiomes. Such human variation can lead to different outcomes in responses to vaccination, therapies, or susceptibilities to infections and inflammatory disorders.
The confounding effects of genes can be addressed to some degree by performing studies in monozygotic twins. Immune studies along these lines have shown some strong genetic influences in young children receiving vaccines, but these genetic dependencies dwindle with age. One study reported that 70% of variation had little or no genetic dependency, reinforcing the idea that this is an adaptive system, adapting to microbial exposure similar to the way the nervous system responds to sensory input ( 61 ). An additional way to analyze the effects of genes is to study immune responses in subjects with monogenic lesions that consist of highly penetrant genetic mutations that exhibit large phenotypic effects. Such human knockouts, such as individuals with a common stop codon polymorphism in the ligand-binding domain of TLR5 (TLR5 392STOP ), are unable to mediate flagellin signaling and are susceptible to infection with a flagellated organism, Legionella pneumophila ( 134 ). Given the aforementioned studies demonstrating a role for suboptimal antibody response of TLR5 knockout mice to seasonal influenza vaccination ( 66 ), it would be of interest to use high-throughput systems approaches to profile immune responses in such human mutants. Indeed, associations of SNPs in genes involved in innate and adaptive immunity and with measles and rubella vaccine responses have been described ( 135 ).
In the case of microbial imprinting, one particularly striking example of microbial influence is the case of cytomegalovirus (CMV), a type of herpes virus that has representatives throughout the animal world. Monozygotic twins who were discordant for CMV had many alterations in their immune system; almost 60% of 200+ variables indicated that this virus has an unusual ability to change many aspects of the immune system ( 61 ). Other studies have shown that CMV + young adults have better antibody responses to a flu vaccine than age-matched CMV comparators, and that even more profound effects can be seen in mice infected with mCMV prior to influenza infection, with substantially reduced flu titers, indicating a profound protective effect ( 136 ). This result suggests that CMV is largely beneficial to the immune defense, which may explain its ubiquity around the world and in many organisms.
Scientific challenges: From data to knowledge to understanding
Sydney Brenner has said that systems biology is “low input, high throughput, no output” biology ( 137 ). A major challenge in the field of systems immunology in humans, and indeed for the field of systems biology in general, is learning how to extract knowledge and understanding from the vast sea of data that will be generated using profiling studies. Identification of omics-based signatures that predict susceptibility to autoimmune disease or vaccine response does not provide any mechanistic insight per se. The concept of knowledge-based (“gnostic”) predictors uses groups of biologically related genes that are coordinately regulated in response to infection, autoimmune disease, or vaccination ( 138 ). Although such tools are of great value, it is experimental validation of the signatures that will ultimately yield new biological insight. Data generated in the human model will enable the formulation of novel hypotheses about mechanisms of immune regulation. Mechanistic validation of this hypothesis will require the use of animal models such as gene knockout mice. An example of this approach involves the TLR5-microbiome-influenza vaccination study discussed above ( 47 , 66 , 67 ). The first clue came from systems analysis of immune responses to influenza vaccination in humans, which identified TLR5 as an early correlate of the later antibody titers to vaccination ( 47 ), raising the hypothesis that TLR5 could be functionally relevant in modulating antibody responses to influenza vaccination. This was tested in mice, where flagellin in the gut microbiota was shown to signal through TLR5 and provide adjuvant signals to influenza vaccination ( 66 ). The relevance of the gut microbiota as an adjuvant to antibody responses to influenza vaccination was subsequently shown to be important in humans in a clinical trial ( 67 ). Therefore, this represents an iterative cycle of inquiry that started with correlative observations in a human study, which led to a mechanistic study in mice, which was then validated in a perturbation study in humans.
Such examples illustrate that animal models are key for validating hypotheses generated in human studies. However, this approach of functionally validating genes, one gene knockout mouse at a time, does not have the throughput to rapidly validate the large number of genes that are typically contained in signatures. Therefore, additional approaches for validating hypotheses generated from human studies such as human organoid cultures offer promise in this regard. This, coupled with recent development of CRISPR-based high-throughput screens in primary cells, offers a potential way to rapidly screen and validate immune signatures ( 139 ). In addition, recent advances in genetics and microbiome research are facilitating the design of better mouse models that may more closely mimic humans. For example, mice might better mimic humans if their genomes have been engineered to include modified single bases in coding and noncoding regions or even entire networks ( 140 ), or if they are infection-experienced ( 25 , 26 ), transplanted with human microbiota ( 141 , 142 ), or genetically outbred and collaboratively crossed ( 143 ). Such models would represent an invaluable tool for the mechanistic validation of results observed in humans.
Practical and cultural challenges
A particular challenge to human studies is access to human samples. Although most human studies to date have focused on analyzing blood samples, emerging studies are also analyzing immune responses in other tissues, including the draining lymph nodes (using fine needle aspirates) ( 144 ), bone marrow (to assess long-lived bone marrow plasma cells) ( 145 ), and even liver (using fine needle aspirate) ( 146 ). Thus, establishment of systems for sample banking and management could facilitate human immunology studies tremendously. Another practical challenge is standardization of methods and techniques to profile immune responses in well-characterized human cohorts. An excellent example of this is the NIH-funded Human Immunology Project Consortium (HIPC), a collaborative network of researchers established to characterize the human immune system before and after vaccination or infection ( www.immuneprofiling.org/hipc/page/show ). Through HIPC, well-characterized human cohorts are studied using omics technologies and multiple computational methods. Cross-center and cross-assay analyses enable rigorous standardization of assays and analytical methods.
In addition, there are numerous cultural challenges. The academic ideal has been that of the lone hero/heroine going into a cave and slaying some ferocious dragon of a problem. But science and medicine are actually quite collaborative and team-dependent, and there has been a cultural inertia in academia to reward (or even acknowledge) this. Yet the medical and science worlds are just too complex and not getting any simpler, and to this must be added the computational, statistical, and technical expertise of the team. Ironically, in biotech and pharmaceutical companies, it is a given that tackling important disease areas requires a team approach. Here, the academic approach seems hopelessly outdated and is especially so when faced with the range of skill sets needed to advance human immunology; no one group can do this alone. This also means ensuring that group efforts are rewarded with a fair distribution of the credit in terms of promotions and funding. However, the emphasis on team effort and technology should not detract from the enormous value of individual creativity. To a large extent, scientific discovery has always been, and will continue to be, driven by the creativity and conceptual ideas of individual scientists. Technology, while having a transformative impact on biology, will never be a substitute for biological insight. Therefore, academic institutions and grant-awarding bodies should help to build a culture that stimulates and rewards the creative impulses and aspirations of individual scientists while nurturing a spirit of team science and robust collaboration.
An important cultural challenge is the notion that systems-based approaches to profile human immune response are not hypothesis-driven. This criticism reflects a long-standing philosophical debate on the nature of the scientific method itself. It is unquestionable that hypothesis-driven deductive research has represented the bedrock of the modern scientific method, and Popper has even argued that the so called “hypothetico-deductive” method alone is sufficient for the progress of science ( 147 ). Even so, it is clear that observational science or exploratory research that is not necessarily aimed at testing a specific hypothesis has led to major scientific advances. An example of this concerns Darwin’s observations in his field notebooks, without any apparent focus, during his voyage on the HMS Beagle to the Galapagos, which subsequently led to the theory of evolution by natural selection. Another example involves Galileo’s discovery of the four large moons of Jupiter and the rings of Saturn when he peered through the telescope. A more recent example is the collection of data by the Hubble Telescope about the origins of the universe. In the biological sciences, the omics revolution has stimulated a whole new era of science in which the starting point is data collection. Systems-based approaches represent hypothesis-enabling research rather than hypothesis-driven research.
The way of the future: Human immunology in the 21st century
Sydney Brenner remarked in 2008: “We don’t have to look for a model organism anymore. Because we are the model organisms” ( 148 ). We propose that the immune system, above all other physiological systems, is uniquely amenable to discovering new human biology. Why? Because cells of the immune system are easily accessible in blood samples. Each drop of blood provides a snapshot of many lineages, as well as dozens of differentiation and activation states. Moreover, other immunological tissues such as lymph nodes, skin, rectal mucosae, and even liver can be safely sampled. This is clearly not the case with research on the human brain, heart, or nervous system. Also, cells of the immune system are uniquely sensitive to deliberate and controlled perturbation; vaccination and infection cause profound changes in their transcriptional, epigenetic, and metabolic profiles.
As discussed above, we are firmly convinced that human immunology will form an ever-larger part of the field going forward. This does not mean that the work with mice will disappear. It is too valuable a system built up over 70 years for that; it presents too many excellent opportunities for well-controlled, mechanistic experiments and will remain an absolutely essential part of biology. But exploration of the immune system in humans offers both a much more direct link to medicine (e.g., translation) and the very real prospect of discovering new immunological phenomena, with thousands of diseases and the genetic diversity that come with a more true-to-life immune system. Thus, despite the challenges of human immunology, new approaches and technology are now making this area much more accessible, and the yields— both scientific and translational—are well worth the effort. However, profiling immunity in humans is but the first step. Computational mining of the data and biological validation in animal models are essential next steps, in an iterative cycle that seeks to bridge fundamental and applied science, mouse and human immunology, in a seamless continuum of scientific discovery and translational medicine ( Fig.3 ). Then, humans will occupy their rightful place in the pantheon of model organisms, and the legacy of Jenner and Pasteur may be regained.

Historically, drugs and vaccines have been developed by the “bench-to-bedside” model, which is a linear process that starts with basic research in animal models and culminates in clinical trials. New advances in systems biology have revitalized human immunology and our capacity to profile human immunity with great precision. Thus, the discovery process can now begin with the human model, where systems-based approaches can be used to analyze the immune response (e.g., response to a vaccine or an infection). The ensuing high-throughput data can then be mined computationally to generate novel hypotheses about the underlying biological mechanisms, which can in turn be tested in animal models. The insights gained can then guide the design of new therapeutics and vaccines. This new framework represents an iterative cycle that seeks to bridge basic and applied science, as well as mouse and human immunology, in a continuum of scientific discovery and translational medicine.
ACKNOWLEDGMENTS
We thank G. Nossal and B. Rouse for comments on the manuscript. B.P. thanks members of our lab for excellent feedback.
Funding: Supported by grants from NIH and the Bill & Melinda Gates Foundation to B.P. and M.M.D. M.M.D. acknowledges the generous support of HHMI.
Competing interests: The authors have no competing interests.
REFERENCES AND NOTES

IMAGES
VIDEO
COMMENTS
Immunology is the branch of biomedical sciences concerned with all aspects of the immune system in all multicellular organisms. Featured. ... Latest Research and Reviews.
Current Research in Immunology (CRIMMU) is an international peer-reviewed open access journal publishing original papers, short communications, and reviews that cover all aspects of molecular and cellular immunology. It is a companion to the highly regarded review journal Current Opinion in Immunology and is part of the Current Opinion and Research (CO+RE) suite of journals.
Our research team has focused on cancer signaling, aimed at exploiting emerging information to develop new therapies to prevent and treat cancer. Eight years ago, we moved our lab from the National Institutes of Health to the University of California, San Diego, and this brought many surprises! ... And its allure grows as new discoveries take ...
The immune system has a crucial role in the fight against cancer. B and T cells, the two main components of the adaptive immunity, are critical players that ... Atefeh Azizi, Fereshteh Mehdipour, Morteza Samadi, Reza Rasolmali, Abdol-Rasoul Talei and Abbas Ghaderi. BMC Immunology 2024 25 :25. Research Published on: 3 May 2024.
ABOUT. Science Immunology publishes original, peer-reviewed, science-based research articles that report critical advances in all areas of immunological research, including important new tools and techniques. mission & scope.
About. Immunology & Cell Biology. Immunology & Cell Biology (ICB) is the flagship journal of the ASI, publishing the latest research in immunology, cellular immunology, innate and adaptive immunity, immune responses to pathogens, tumour immunology, immunopathology, immunotherapy, immunogenetics, immunological studies in humans and model ...
Alicante-Winter Immunology Symposium in Health (A-Wish) and the Boulle-SEI awards: A collaboration between the Spanish Society for immunology, the University of Alicante and the Jean Boulle Group to honor the Balmis Expedition. Jordi Ochando, Carmen Camara, Leighton Durham, Jose Miguel Sempere, Marcos Lopez-Hoyos. Pages 136-145.
John D. Lee, Trent M. Woodruff. First published: April 17, 2024. The persistence or recurrence of symptoms after acute SARS-CoV-2 infection, termed 'long COVID', presents a formidable challenge to global healthcare systems. Recent research by Cervia-Hasler and colleagues delves into the intricate immunological landscape in patients with ...
RSS Feeds. Immunology is one of the leading journals in this field with a global representation across authors, editors and reviewers. We publish papers based on original findings in all areas of cellular and molecular immunology, and mechanistic insights into fundamental aspects of the immune system.
Previous vol/issue. Read the latest articles of Current Research in Immunology at ScienceDirect.com, Elsevier's leading platform of peer-reviewed scholarly literature.
Pillars of Immunology. The Journal of Immunology publishes commentaries highlighting scientific reports that were published more than 15 years ago and that have made a major impact on the course of immunological research today. Read published commentaries here . Don't miss the exciting Brief Review Collection, coming in the January 15, 2024 ...
Immunologic Research is a transformative journal that covers the entire spectrum of immunology, from molecular and cellular levels to diseases and clinical applications. Offers both traditional publishing route and immediate gold Open Access. Maintains a balance between basic and clinical data. Provides a platform for presenting historical ...
To find the new drugs, you need to, for the most part, forget the rules and look at the clues and patterns that data and previous research provide. ... The area of application that we have focused on in this paper is AI in immunology. Immunology refers to biomedicine, which deals with the body's response in particular to external contaminants ...
See all (2,782) Learn more about Research Topics. The official journal of the International Union of Immunological Societies (IUIS) and the most cited in its field, leading the way for research across basic, translational and clinical immunology.
However, new and better vaccines are urgently needed; e.g., a vaccine against the new coronavirus 2019, SARS-Cov-2; prevalent pathogens, such as human immunodeficiency virus (HIV); parasites, such ...
Single B cell technologies for monoclonal antibody discovery. Oxenius and colleagues. Published online: November 4, 2021. ISSN: 1471-4981 (online); 1471-4906 (print) Trends in Immunology publishes commissioned, peer-reviewed articles that help link developments in basic and clinical immunology.
The mammalian immune system is a remarkable sensory system for the detection and neutralization of pathogens. History is replete with the devastating effects of plagues, and the coronavirus disease 2019 (COVID-19) pandemic is a defining global health crisis of our time. Although the development of effective vaccines has saved many lives, the ...
1995 ENII (European Network of Immunology Institutes) Conference Sponsored by the Europea. Read the latest articles of Research in Immunology at ScienceDirect.com, Elsevier's leading platform of peer-reviewed scholarly literature.
News-Medical is your trusted source of Immunology news, articles and research for doctors, patients, and families. ... White Papers; Lab Equipment; ... New USC research shows that iron serves as a ...