
Want to create or adapt books like this? Learn more about how Pressbooks supports open publishing practices.

6.3 IR Spectrum and Characteristic Absorption Bands
With a basic understanding of IR theory, we will now take a look at the actual output from IR spectroscopy experiments and learn how to get structural information from the IR spectrum. Below is the IR spectrum for 2-hexanone.
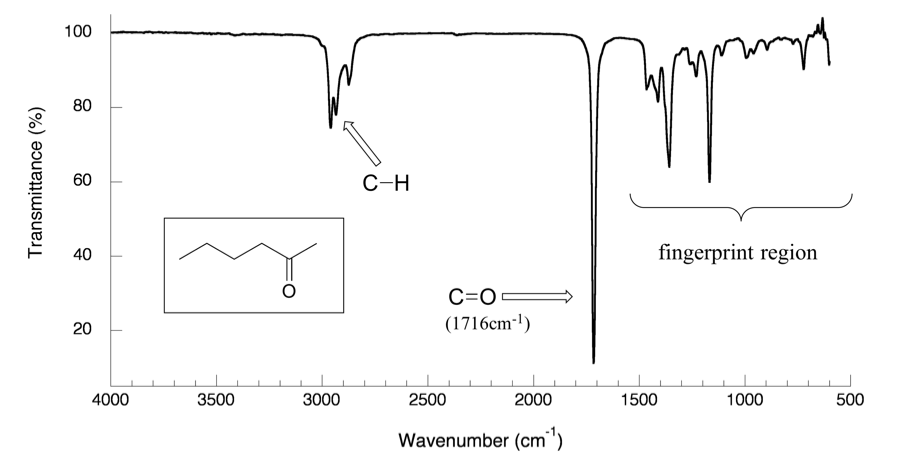
Notes for interpreting IR spectra:
- The vertical axis is ‘% transmittance’, which indicates how strongly light was absorbed at each frequency. The solid line traces the values of % transmittance for every wavelength passed through the sample. At the high end of the axis, 100% transmittance means no absorption occurred at that frequency. Lower values of % transmittance mean that some of the energy is absorbed by the compound and gives downward spikes. The spikes are called absorption bands in the IR spectrum. A molecule has a variety of covalent bonds, and each bond has different vibration modes, so the IR spectrum of a compound usually shows multiple absorption bands.
Please note that the direction of the horizontal axis (wavenumber) in IR spectra decreases from left to right. The larger wavenumbers (shorter wavelengths) are associated with higher frequencies and higher energy.
Stretching Vibrations
Generally, stretching vibrations require more energy and show absorption bands in the higher wavenumber/frequency region. The characteristics of stretching vibration bands associated with the bonds in some common functional groups are summarized in Table 6.1 .
Table 6.1 Characteristic IR Frequencies of Stretching Vibrations
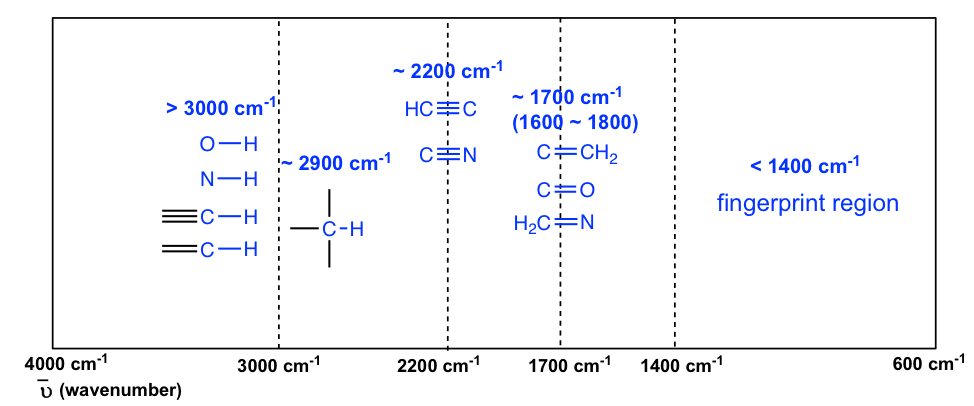
The information in Table 6.1 can be summarized in the diagram for easier identification (Figure 6.3b) , in which the IR spectrum is divided into several regions, with the characteristic band of certain groups labelled.
The absorption bands in IR spectra have different intensities that can usually be referred to as strong (s), medium (m), weak (w), broad and sharp. The intensity of an absorption band depends on the polarity of the bond, and a bond with higher polarity will show a more intense absorption band. The intensity also depends on the number of bonds responsible for the absorption, and an absorption band with more bonds involved has a higher intensity.
The polar O-H bond (in alcohol and carboxylic acid) usually shows strong and broad absorption bands that are easy to identify. The broad shape of the absorption band results from the hydrogen bonding of the OH groups between molecules. The OH bond of an alcohol group usually has absorption in the range of 3200–3600 cm -1 , while the OH bond of the carboxylic acid group occurs at about 2500–3300 cm -1 (Figure 6.4a and Figure 6.4c).
The polarity of the N-H bond (in amine and amide) is weaker than the OH bond, so the absorption band of N-H is not as intense or as broad as O-H, and the position is in the 3300–3500 cm -1 region.
The C-H bond stretching of all hydrocarbons occurs in the range of 2800–3300 cm -1 , and the exact location can be used to distinguish between alkane, alkene and alkyne. Specifically:
- ≡C-H (sp C-H) bond of terminal alkyne gives absorption at about 3300 cm -1
- =C-H (sp 2 C-H) bond of alkene gives absorption at about 3000-3100 cm -1
- -C-H (sp 3 C-H) bond of alkane gives absorption at about ~2900 cm -1 (see the example of the IR spectrum of 2-hexanone in Figure 6.3a; the C-H absorption band at about 2900 cm -1 )
A special note should be made for the C-H bond stretching of an aldehyde group that shows two absorption bands: one at ~2800 cm -1 and the other at ~ 2700 cm -1 . It is therefore relatively easy to identify the aldehyde group (together with the C=O stretching at about 1700 cm -1 ) since essentially no other absorptions occur at these wavenumbers (see the example of the IR spectrum of butanal in Figure 6.4d ).
The stretching vibration of triple bonds C≡C and C≡N have absorption bands of about 2100–2200 cm -1 . The band intensity is in a medium to weak level. The alkynes can generally be identified with the characteristic weak but sharp IR absorbance bands in the range of 2100–2250 cm -1 due to stretching of the C≡C triple bond, and terminal alkynes can be identified by their absorbance at about 3300 cm -1 due to stretching of sp C-H.
As mentioned earlier, the C=O stretching has a strong absorption band in the 1650–1750 cm -1 region. Other double bonds like C=C and C=N have absorptions in lower frequency regions of about 1550–1650 cm -1 . The C=C stretching of an alkene only shows one band at ~1600 cm -1 (Figure 6.4b) , while a benzene ring is indicated by two sharp absorption bands: one at ~1600 cm -1 and one at 1500–1430 cm -1 (see the example of the IR spectrum of ethyl benzene in Figure 6.4e ) .
You will notice in Figure 6.3a and 6.3b that a region with the lower frequency 400–1400 cm -1 in the IR spectrum is called the fi ngerprint region . Similar to a human fingerprint, the pattern of absorbance bands in the fingerprint region is characteristic of the compound as a whole. Even if two different molecules have the same functional groups, their IR spectra will not be identical, and such a difference will be reflected in the bands in the fingerprint region. Therefore, the IR from an unknown sample can be compared to a database of IR spectra of known standards in order to confirm the identification of the unknown sample.
Organic Chemistry I Copyright © 2021 by Xin Liu is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License , except where otherwise noted.
Share This Book
- Metal Nanoparticles
- Metal Oxide Nanoparticles
- Nanocomposites
- Carbon Nanomaterials
- XRD Analysis
- FTIR Analysis
- SEM Analysis
- TEM Analysis
- XPS Analysis
- BET Analysis
- TGA Analysis
- DSC Analysis
- Raman Analysis
- PL Analysis
- NMR Analysis
- SAXS Analysis
- UV-Vis Analysis
- Scientific Graph Plotter
- Reaction Image Builder
- DOI to Reference: Scientific Writing
FTIR Functional Group Database Table with Search
Search by Group
Search by Class
Ask Your Question Cancel reply
You must be logged in to post a comment.
Top Paid Services

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

Lab 3: Fourier Transform Infrared Spectroscopy (FTIR)
- Last updated
- Save as PDF
- Page ID 204119
- Students should develop an understanding of molecular symmetry and vibrational modes.
- Students should be able to use symmetry elements to predict which vibrational modes are IR active.
- Student's should be able to use a solve the pollution mystery by determining the air pollutant and the source of the air pollutant.
Introduction
The procedure of this lab does not follow the normal format. In this lab, you have been hired by an environmental testing company to monitor air pollutants. By working through a series of exercises, you will gain important information to determine the air pollutant in an air sample and therefore determine the source of the pollution. The air sample was taken in a location 20 m away from an open farm field where strawberries were growing. In the field on one side, there were cows grazing; on the other side, there was a natural gas pumping station. Across the street, there was a gas station with an auto repair shop specializing in air conditioner repair and a dry cleaner. This information narrows the list of suspected chemicals to six. Test your air sample using an infrared spectrometer and determine the culprit.
The FTIR / Vibrational spectroscopy experiment is an adaptation of "Pollution Police" by Profs. Jodye Selco and Janet Beery at the University of Redlands, which was presented at the Division of Chemical Education Regional ACS meeting in Ontario, CA 1999. Many students currently in CHE 115 have already taken CHE 124A which focuses on symmetry, molecular vibrations, and point groups. For students that have taken CHE 124A, use this lab as an opportunity to use your knowledge and assist your lab partners that have not taken the class yet.
There are resources available to further an understanding of FTIR. The theory of FTIR spectrometer operation is discussed in SHN Chapters 16 and 17. The group theory and vibrational quantum mechanics are discussed in McQuarrie and Simon (Chem 110B text) Chapters 12 and 13. Principles of Infrared Spectrometry and its application can be found in Skoog, Holler, and Crouch (the textbook for CHE 115) in chapters 16 and 17.
The FTIR exercise follows the format of a detective story involving solving a series of problems rather than the normal lab format. The experimental portion of the exercises are problems #9 and #10. The computer in room 3475 will be used to complete problems #2, 7, and 8. The computers are set up to run HyperChem, Gaussian, and Spartan.
Setting the Scene
You have been hired by an environmental testing company to monitor air pollutants. Air pollutants can be released from many types of sources. A few examples of sources are: factories, cars, or cattle. Sometimes, volatile chemicals can evaporate from agricultural fields. Since most air pollutant chemicals can absorb infrared light, we are able to detect them with an infrared spectrometer. Some of the molecules commonly found in the atmosphere include those in Table 3.1. Water and small amounts of carbon dioxide, methane, and sulfur trioxide are found in “clean” air samples. Large amounts of any of the chemicals other than water usually indicate atmospheric pollution.
When testing, a test sample is first obtained by taking an evacuated cell to the target location, opening a valve, and allowing the ambient air to fill the cell. An FTIR – Fourier Transform Infrared Spectrometer will be used to perform Infrared Spectrometry. The final output from the spectrometer called an infrared spectrum ( Figure 3.1 ), is a plot of the intensity of light reaching the detector divided by the initial intensity of light, as a function of frequency (%Transmittance= I/I o vs. frequency). The goal of this project is to gain a better understanding of group theory and to identify atmospheric pollutants from their infrared spectra.
For the molecules listen in Table 4.1, examine the three-dimensional ball-and-stick models for these molecule s and compare them with the two-dimensional representations of the molecules in Figure 3.2. In the drawings in Figure 3.2, straight lines represent bonds that lie in the plane of the paper; two lines between a pair of atoms represent a double bond; a filled arrowhead indicates a single bond that is angled out of the plane of the paper toward you; and the dashed arrowhead indicates a bond angled into the plane of the paper away from you. Carbon dioxide is an example of a linear molecule; water, ethylene, sulfur trioxide, and benzene are planar mol ecules.
Figure 3.2: Molecules of Study originally listed in Table 4.1.
Problem #1: Center-of-mass and Coordinate Axes
In order to accomplish our goal of identifying the chemical pollutant you are investigating from its infrared spectrum, we must know which infrared frequencies, if any, the molecule absorbs. To decide which molecules absorb infrared radiation and at which frequencies, we will need to examine various properties of the molecules themselves, including their centers of mass, their Cartesian coordinate axes, their vibrations, and their symmetries. Specifically, we will compare the actions of the symmetry operations of a molecule on its Cartesian coordinate axes with the actions of the symmetry operations on its vibrations. When a molecule absorbs infrared radiation of a given frequency, this energy causes the molecule to vibrate in a specific way; the atoms bounce against each other much like balls connected by a spring. The vibrational motions of a molecule that absorb infrared radiation are the ones that exhibit the same behavior as do the Cartesian coordinate axes of the molecule when the atoms of the molecule are permuted in certain ways. This is a result of the orthogonal interaction between the electromagnetic field of the light and the electric field of the molecule itself. Therefore, you will begin by drawing in a three-dimensional coordinate system for each of the molecules in the set assigned (see Table 3.2). The convention for molecules is that the origin of the axis system is placed at the center of mass of the molecule. (This is the weighted average of the positions of the atoms.) First, determine approximately where this should be. (You might want to reexamine the ball-and-stick models.) Remember, the masses of the different atoms are different. To find out how much each atom weighs; consult a periodic table of the elements. The mass number for each type of atom appears at the bottom of the square in which the atomic symbol appears. By convention, the z-axis is the unique axis, if there is one. This axis is also called the molecular axis, or axis of highest symmetry. In a linear molecule, it corresponds to the line formed by the molecule; this is true for carbon dioxide. For benzene, the z-axis is the out-of-plane axis since it is the unique axis. If there doesn’t seem to be a unique axis, then place the heaviest atoms in the molecule along the z-axis (often, there is more than one way to do this). Once the z-axis is assigned, the in-plane axis usually is the y-axis and the out-of-plane axis is the x-axis. In addition, axes should be placed along the molecular bonds whenever possible.
Example 3.1
The y- and z-axes for water and carbon dioxide are shown in Figure 3.3. The x-axis is out-of-plane from the origin (just below the O atom and pointing straight out at you for water, but centered in the C atom and pointing directly away from you for carbon dioxide).
Figure 3.3: Cartesian coordinate axes for water and carbon dioxide
Draw in the three Cartesian coordinate axes in a picture of each molecule assigned to you in Table 3.2 as outlined in the steps below. (If you do not assign them correctly now, you will have a chance later to re-label them.)
- Estimate, by eye, the center of mass for each molecule, keeping in mind the atomic masses for each type of atom.
- Draw in the z-axis using the rules above.
- Draw in the two remaining axes using the rules above.
Table 3.2: Assigned molecules for each group
Problem #2: Molecular Vibrations
The types of molecular vibrations a molecule has determine whether or not it absorbs infrared light. Hence, you need to determine the types of vibrations your molecules make. To ensure that all of them have been identified, we need to know how many are possible. Consider a molecule that is a collection of N atoms connected together in a specific way by chemical bonds. In order to describe the motions of the molecule, we need to consider the motions of each individual atom. This means that we need 3 degrees of freedom for every atom within the molecule for a total of 3N degrees of freedom. However, the atoms within the molecule have a specific geometric relationship to the other atoms in the molecule; this results in a redistribution of the number of independent degrees of freedom. The motion through space of the molecule uses three degrees of freedom, reducing the 3N degrees of freedom to 3N - 3. Since the molecule also can rotate (like a spinning baton or Frisbee), we require two degrees of freedom to describe the coordinates about which a linear molecule can spin and three degrees of freedom for a non-linear molecule (for a linear molecule there is no concerted rotation about the molecular axis (z-axis). This leaves 3N - 5 degrees of freedom for the linear molecule and 3N - 6 for the non-linear molecule still unaccounted for; each of the remaining degrees of freedom describes a distinct coordinated internal motion, or vibration, of the atoms within the molecule.
For example, when there are only two atoms in the molecule (e.g. O 2 , N 2 , or CO), there is only one vibrational motion: 3(2) - 5 = 1. In the case of benzene (C 6 H 6 ) there are 12 atoms and 3(12) - 6 = 30 vibrational motions possible! As it turns out, not all of these vibrations are capable of absorbing infrared radiation. For the simplest molecules, such as water, it is easy to draw pictures representing the vibrational motions.
Example 3.2
Water has 3(3)-6=3 vibrations and carbon dioxide has 3(3)-5=4 vibrations as shown in Figures 3.4 and 3.5.
Figure 3.4: Vibrations of water
Consider the water molecule as it undergoes the asymmetric stretch; as it reaches its most extreme position, it has one “arm” extended and the other “arm” contracted. The bend is a change largely in angle and not inter-atom distances.
Figure 3.5 : Vibrations of carbon dioxide
The four vibrations for CO 2 consist of two stretches (one symmetric and one asymmetric) and two bends, that are degenerate (with the same energy) but involve perpendicular motions.
For each molecule, calculate the number of vibrational motions using the formula given above. Then, use a computer program to view the vibrational motions for water, carbon dioxide and the other molecules assigned. Record the motions of the atoms in the molecule using the symbols from the examples above, and record the frequency calculated for each vibration. Note that some of these motions are out-of-plane motions. Be sure to rotate the molecules on the computer screen so that you examine the motions from many different angles. (Many of the programs calculate the frequencies in cm -1 ; this is actually the frequency, in s -1 , divided by the speed of light (3.00 x 10 10 cm/s). In this exercise, make note of the frequencies in cm -1 given by the HyperChem program.) Again, you are to complete the following steps for the molecules assigned to you in Table 3.2 . This assignment will require a lot of space in your lab notebook due to all of the vibrations you will be drawing.
- Determine the number of vibrational motions, 3N – 6 or 3N – 5, to make sure that you know how many vibrational motions to record.
- Record the vibrational motions for your molecules, following the notation used in the examples above.
- Record the frequency for each of these vibrations.
Spartan '16 Operation
- Double-click “PC Spartan '16” on desktop. The Spartan computer is located in the TA area of 3475 and has a bright orange name tag.
- Click the new page icon in the top left corner. Atoms with various bond choices will appear. If you prefer a more advanced setting, click the Expert tab.
- Draw your molecule by clicking on the atom with the correct number of bonds needed. To join two atoms, repeat this process by touching the mouse arrow to the open bond.
- Once you are done drawing your molecule, click the Glasses ("View") icon located just below the Geometry scroll-down. This will finalize your drawing.
- Calculate: Equilibrium Geometry with Hartree-Fock 3-21G(*)
- Compute: IR
- Print: Vibrational Modes
- Go to Setup and scroll down to Submit. Make a new folder with your group's letter in the Chem 115 folder. Label within your folder as you see fit.
- After you save the file you will be prompted twice that Spartan has started and completed. Press OK both times.
- Go to Display and scroll to Spectra. Here you will find the frequencies associated with the different vibrations of your molecule. Click on any of the checkboxes to view the animation for the vibration associated with that particular frequency.
Problem #3: Symmetry Elements and Symmetry Operations
Because the molecules we are examining are very small, the rules of quantum mechanics govern the processes in which we are interested in. According to quantum mechanics, not all light absorption processes are allowed; many are “forbidden” by symmetry. If we want to determine which molecular vibrations absorb infrared light, we need to examine the actions of the symmetries of the molecules on the coordinate axes and on the molecular vibrations. We begin by determining the symmetry elements that each molecule possesses.
A symmetry operation on a molecule is an action that moves the molecule into a position that is indistinguishable from the starting position. A symmetry element of a molecule is a geometric feature of the molecule about which a symmetry operation is performed. Symmetry elements include planes and axes; symmetry operations include reflections across planes and rotations about axes. In the case of water, 180° rotation about the z-axis is a symmetry operation, denoted C 2 ; while the z-axis itself is a symmetry element, a C 2 axis. The symbol C 3 indicates a three-fold axis of symmetry, a symmetry element; while C 3 indicates a 120°rotation about a C 3 axis, a symmetry operation. Rotation by 240° about a C 3 axis is denoted C 3 2 . Rotation by 360° about a C 3 axis is equivalent to doing nothing; that is, C 3 3 =Ê, where Ê is the identity operation.
The plane of symmetry, σ, is also referred to as a reflection plane or mirror plane. The symbol σ v is used to denote a “vertical” plane of symmetry that is parallel to an axis of highest symmetry (z-axis, or C n with largest n), while the symbol σ h is used to denote a “horizontal” plane of symmetry that is perpendicular to the axis of highest symmetry (taken as z-axis). The symbol σ d denotes a “dihedral” plane of symmetry that bisects an angle between atoms.
Example 3.3
Figures 3.6 and 3.7 illustrate several of the symmetry elements listed in Table 3.3.
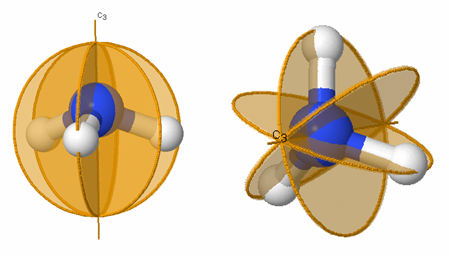
Figure 3.7: Symmetry elements for allene (C 3 H 2 ). The C 2 and S 4 axis is labeled and the two mirror planes, σ d , σ d ' are labeled as gold circles.
Example 3.4
Figures 3.8 and 3.9 show the symmetry elements and operations of water and carbon dioxide, respectively.
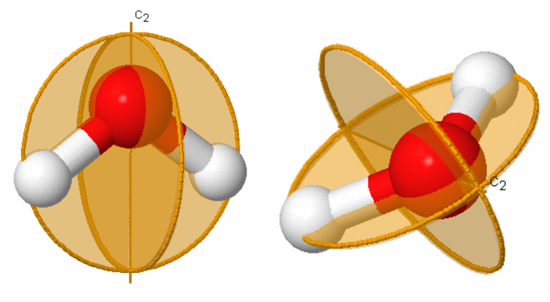
In the case of water the symmetry elements are E, C 2 (shown), σ v (xz-plane, perpendicular to the plane of the molecule), and σ v’ (yz-plane, the plane of the molecule). Note that it does not matter whether σ v represents the xz- or yz-plane. The corresponding symmetry operations for water are Ê, C 2 , σ v , and σ v’ .
The symmetry elements not shown above are E, σ v (yz-plane, the plane of the paper), σ h (xy-plane, perpendicular to the C 2 axis), and i (center of inversion at the coordinate origin). The symmetry operations for carbon dioxide are Ê, infinitely many C 2 , C ∞ , infinitely many σv, S ∞ , σ h , and i . A subscript of ∞ means that rotation through any angle about that axis results in a valid symmetry operation. Note that σ h is identical to S 0 and is often omitted.
Reexamine the ball-and-stick models for molecules assigned to you in Table 3.2 . Determine all of the symmetry elements and corresponding symmetry operations for each molecule. (Hint: At least one molecule on the list contains the symmetry element σ h and one contains an S 3 symmetry element.)
There are a few things to keep in mind while trying to determine the symmetry elements for chemical compounds. The first is that molecules are three-dimensional objects. This means that we can tell the difference between the “front” and “back” or the “top” and “bottom” of planar molecules. For instance, the σ v’ reflection of the water molecule across the yz-plane is not the same as the identity operation Ê. Second, since atoms of the same kind (or color) are indistinguishable, you may want to number the atoms in the models in order to keep track of the results of the symmetry operations. Finally, when molecules have hexagonal rings with alternating double bonds, all of these bonds---both single and double---are equivalent (e.g. benzene and toluene). It is only the orientation of the atoms themselves that can be “seen” spectroscopically and hence needs to be considered here.
After you have determined the symmetries of the molecules, you can double-check your axis assignments. The z-axis should be the axis of highest C n symmetry. In H 2 O, there is only one C n axis, C 2 , so it is the z-axis. In CO 2 , there is a C 2 axis and a C ∞ axis, so the C ∞ axis is the z-axis. Check the other molecules to make sure that the z-axis you assigned is the one of highest symmetry. Remember that the axis of highest symmetry may not be unique.
Problem #4: Orders of Symmetry Operations
The order of a symmetry operation is the number of times the operation must be applied to obtain the identity operation, Ê. More specifically, the order of a symmetry operation  is n, if n is the smallest positive integer such that  n = Ê. For instance, for H 2 O, the non-identity symmetry operations each have order 2. Note that an inversion always has order 2. The symmetry operation S 4 has order 4 because it must be applied four times in succession to return the molecule to its original orientation when the outside atoms are labeled. (Try it for methane!) Therefore, S 4 generates 4 symmetry operations: S 4 , S 4 2 = C 2 , S 4 3 , and S 4 4 = Ê o f orders 4, 2, 4, and 1, respectively.
For each of the molecules assigned to you in Table 3.2 , find the order of each symmetry operation. Use the description of order to aid you.
Problem #5: Symmetry Groups
You may have noticed that the set of symmetry operations forms a group under composition of operations, called the symmetry group of the molecule. The symmetry elements of a molecule can be used to determine the group to which the molecule belongs. Using Figure 3.10 and the molecule's symmetry elements, the group can be identified.

Figure 3.10: Flow Map for identifying the symmetry group to which any molecule belongs.
Identify the symmetry group for each of the molecules assigned to you in Table 3.2 . The order of a symmetry group is the number of operations which comprise the group. What is the order of each group? Verify by determining the group for your molecules from HyperChem and by examining the character tables in Chapter 12 of McQuarrie and Simon (Chem 110B text) or Chemical Applications of Group Theory , by F. A. Cotton. There are also tutorials available on to help identify the group and practice finding groups of other molecules.
Problem #6: Action of Symmetries on Coordinate Axes
Your overall goal is to identify the molecular origins of the infrared peaks observed in a spectrum of contaminated air. Since a molecule’s infrared light absorption depends on how the Cartesian axes transform under the symmetry operations, the next step is to determine what happens to each of the Cartesian axes as the different symmetry operations are performed upon the molecule.
Example 3.5
In Figure 3.11 and Table 3.4, we illustrate this process for water. Note that under the identity operation, Ê, none of the axes are inverted or reversed. When the molecule is rotated about the C 2 axis, the orientation of the z-axis remains the same but the x- and y-axes are oriented in the opposite direction; each point (x,y,z) is moved to the point (-x,-y,z). In this case, the rotation is equivalent to multiplying the x and y values by -1. For any operation, -1 indicates that there is a reversal in the orientation of the axis relative to the original orientation, whereas +1 indicates that the orientation remains the same.
Construct similar tables for the FIRST THREE molecules assigned to you in Table 3.2 . First, check with your TA to make sure you have drawn the Cartesian coordinate axes in the standard way for each of these molecules.
Problem #7: Action of Symmetries on Vibrational Motions
The vibrational motions of the molecule that absorb infrared radiation are the ones that transform under the symmetry operations of the molecule in the same way as do the Cartesian coordinate axes of the molecule. Therefore, we need to determine how the molecular vibrations behave under each of the symmetry operations, so that we can compare them to the transformations of the Cartesian coordinate axes.
Example 3.6
Let us examine the vibrations of water again ( Figure 3.12 ).
If we ask how each of the vibrations of water behaves under each of the symmetry operations, we can add more entries to Table 3.5. When we examine what happens to the vibrating molecules as the symmetry operations are performed, we are interested only in whether or not the geometrical orientation of the molecule has changed. For instance, consider the water molecule as it undergoes the asymmetric stretch. Imagine the molecule (or stop the computer program) when it reaches its most extreme position, with one “arm” extended and the other “arm” contracted. Now perform the C 2 operation (rotation by 180°) on this “distorted” molecule. Its orientation after the C 2 operation is different from its orientation before. Note that the configuration has been reversed. The fact that its new position is distinguishable from its original position is represented in Table 3 by -1.
Now let’s examine the water molecule as it undergoes the bend or symmetric stretch. If we perform the C 2 operation (rotation by 180 ° ) on this vibrating molecule, its geometric orientation is unchanged. Its new position is indistinguishable from its original position. The fact that it appears unchanged is represented in Table 3.5 by +1.
Construct tables as in example 3.6 for the FIRST THREE molecules assigned to you in Table 3.2 . Examine the motions of the atoms for each different vibration. If you were to imagine the molecule (or stop the computer program) when it reaches its most extreme position, consider how that “version” of the molecular shape would behave under each of the different symmetry operations. That the molecule is indistinguishable after the symmetry operation is indicated by a 1, while a distinguishable molecule is represented by a -1. Fill in a line in your table for each vibrational motion. Note this procedure works for most, but not all simple molecules. For example, this procedure will not work for your third molecule.

Problem #8: Comparing Symmetry Operations on Axes and Vibrations
When a symmetry operation and an axis transform in the same way, the frequency associated with that symmetry operation will absorb light. By comparing the tables you generated in
Example 3.7
In the case of water, as illustrated in Table 3.5, the bending vibration transforms under the symmetry operations in the same way as does the z-axis. This is also true for the symmetric stretching motion. On the other hand, the asymmetric stretch transforms in the same way as does the y-axis. In this case, all three of the vibrational motions of water would absorb infrared light since each one of them transforms under the symmetry operations as does one of the Cartesian coordinate axes. All of the vibrations also have frequencies that are in the appropriate frequency range. We would expect the infrared spectrum of water to have three peaks corresponding to frequencies of 1840, 3587, and 3652 cm -1 .
For the FIRST THREE molecules assigned to you in Table 3.2 , use the tables you constructed in Problem #7 to determine how the vibrational motions transform under the symmetry operations. List the frequency calculated of the ones that transform as do the x-, y-, or z-axes. (You listed the frequencies you need for this problem in Problem #2.) Given that infrared spectrometers operate in the range of about 600 cm -1 to 4000 cm -1 (wavenumbers), which vibrational frequencies should you observe in the infrared spectra for your molecules? Make a rough sketch of the infrared spectrum you would expect to see for each of your molecules, labeling peaks with their frequencies. Assume that if peaks are separated by less than 25 cm -1 , they will not be resolved and will appear as a single peak.
Problem #9: Obtaining Infrared Spectra
You have now reached the experimental portion of this exercise. Before you can measure your contaminated air sample. You will need to measure some common air pollutants. The IR spectra that you measure for these molecules will help you decide which compound is in your air sample.
Breathing into the sample compartment of the infrared spectrometer can generate the spectrum of water and carbon dioxide. You will then take spectra of methyl iodide, ethylene, acetylene, trans-DCE, and a sample of contaminated air , which have already been prepared in infrared cells. Do not be overly concerned if the windows on the cells appear to be hazy; they will work fine. Do you observe the predicted absorptions? If not, try multiplying all of your vibrational frequencies by 0.89 (a factor theoretical chemists recommend to compensate for over calculations). Do they come closer to what you observe now?
Operating the Bruker FTIR Spectrometer (room 3475)
- Start the OPUS 6.0 software on the computer next to the instrument.
- Scans: 16 for sample and 16 for background
- Resolution: 2.0
- Signal Gain: 1
- IR Data Type: Transmittance
- Name your sample in the Filename: field
- Close the sample compartment and wait 2-3 minutes for the sample compartment to be purged of air.
- In the Measure Menu , go to the Basic tab . Click the “Background Single Channel” button, the instrument will start scanning the background. The progress status will be shown at the bottom of the screen.
- Open the chamber, and load your sample in. Close the chamber.
- To acquire your sample scan. Give your sample a description under the Basic tab . Run the sample spectrum by clicking on the “Sample Single Channel” button. The progress of the scan can be seen at the bottom of the screen.
Operating the FTIR Spectrometer (room 3480)
- Start the OPUS 7.2 software on the computer next to the instrument.

- Signal Gain: auto
2017-An Agilent 630 FTIR might be available, ask Paul
Collect a spectrum of water and carbon dioxide:
- Open the sample compartment.
- Exhale into the sample compartment.
- Collect the spectrum by clicking on the “ Sample Single Channel ” button on the Basic Tab .
Collect spectra of the four reference samples and your unknown:
- Put the cell holder with the cell into the sample compartment. Align the holes in the bottom of the holder with the holes in the plate in the bottom of the compartment.
- Wait 2-3 minutes for the compartment to purge.
- Name the file.
- Acquire the spectrum by pressing Sample Single Channel button.
To pick peaks: (This procedure may not work depending on the instrument you are using.)
Click on the peak picking icon, or select Peak Picking from the Evaluate menu.
- The peak picking screen will show up. Choose the Interactive mode . Sliding the Threshold square up or down so that all the peaks are below it.
- Each peak above the threshold will be labeled with its frequency and the peak list will be written to a report file that you must save by selecting Save Report from the File pulldown menu.
- You can annotate the plot by selecting the annotator options from the Tools pulldown menu.
- The file path and name are the default title for your plot. You can change this by selecting Title under the Display pulldown menu.
- Once you have picked your peaks and annotated your plot, save the window as before by selecting Save Sample from the File pulldown menu.
- Select Plot from the File pulldown menu. In the Plot menu select the window you wish to print. Check that the size of the plot is correct and then click on the “plot”. This will take 1-2 minutes. Be patient. You can then select another window to print or click on the “done” button.
Problem #10: Identifying the Pollutant
As a reminder: The sample was taken in a location 20 m away from an open farm field where strawberries were growing. In the field on one side, there were cows grazing; on the other side, there was a natural gas pumping station. Across the street, there was a gas station with an auto repair shop specializing in air conditioner repair and a dry cleaner. This information narrows the list of suspected chemicals to six. Test your air sample and determine the culprit. The spectrometer automatically purges the cell cavity with N 2 , which is transparent in infrared. This removes water and carbon dioxide, which exhibit strong IR absorptions, so you should be able to see the pollutant's spectrum relatively easily.
Examine the spectrum from your sample of the unknown, contaminated air. Note that it is a very different spectrum from that of carbon dioxide and water, which is shown in Figure 3.1. Which of the chemicals that you studied is responsible for this spectrum? To which potential polluter described above would you attribute this pollution?
Post-Lab Questions
1. Include all of the information recorded during the assignments.
2. Include all of the FTIR spectra
3. Identify the pollutant and predict the source of the pollutant.
4. Explain the process used to identify the pollutant.
Outside Links
- http://symmetry.otterbein.edu/index.html
- Point group symmetry character tables
- McQuarrie, D. A.; Simon, J. D. Physical Chemistry: A Molecular Approach ; University Science Books: Sausalito, CA, 1997.
- Cotton, F. A. Chemical Applications of Group Theory ; Wiley: New York, NY, 1990.
- Drago, R. S. Physical Methods in Chemistry ; W. B. Saunders: Philadelphia, PA, 1977.
- Skoog, D. A.; Holler, F. J.; Crouch, S. R. Principles of Instrumental Analysis, Seventh Edition; Cengage Learning: Boston, MA, 2016.
Contributors
- Dr. Christopher Brazier, Dr. Steven Morics, Dr. Teresa Longin, Dr. Dara Gilbert, Dr. David Goodin, and Brooke McMahon
Photometry & Reflectometry

Photometry is the measurement of light absorbed in the ultraviolet (UV) to visible (VIS) to infra-red (IR) range. This measurement is used to determine the amount of an analyte in a solution or liquid. Photometers utilize a specific light source and detectors that convert light passed through a sample solution into a proportional electric signal. These detectors may be for example photodiodes, photoresistors, or photomultipliers. Photometry uses Beer–Lambert’s law to calculate coefficients obtained from the transmittance measurement. A correlation between absorbance and analyte concentration is then established by a test-specific calibration function to achieve highly accurate measurements.
Photometry is a widely used quantitative analysis in research laboratories to determine the amounts of inorganic and organic compounds in solutions and other liquids. Photometry also has broad industrial applications in the determination of contaminants in drinking and wastewater, analysis of nutrients in soil, food and beverage samples, building material composition, and many other areas.
Construction and Working of Photometer
The components of a typical photometer include a light source, monochromator, sample, and detector. Light sources can be tungsten-halogen lamps (generally the source of light used for analyses in the visible light range) or LEDs. For measurements in the UV–Visible range a xenon flash lamp may be used. The monochromator filters the light radiated by the light source to allow only a very narrow spectrum to pass. The light then passes through the cuvette or the sample holding cell. Based on the amount of analyte (or a dye derived from it) that is present in the sample solution, some part of the light is absorbed by the solution and the remaining is transmitted. The transmitted light is directed towards the detectors, which produce an electric current proportional to the light intensity.
Beer–Lambert Law
Beer–Lambert law, also known as Beer’s law, states that the quantity of light absorbed by a sample is directly proportional to the concentration of the analyte present and path length of the light through the sample. “Sample” in this context means either the analyte itself (direct measurement) or a dye derived from the analyte (when using reagents or kits). The relationship is described by the formula:
Reflectometry
Reflectometry (also known as remission photometry) is a non-destructive analytical technique that uses the reflection of light by surfaces and interfaces to measure characteristics such as color intensity, film thickness and refractive index.
As with other photometers, the main elements of reflectometers include a light source, usually long-life LEDs of specific wavelengths that are focused onto a sample surface via a lens system and the reflected light is measured by detectors.
Reflectometers are often designed to measure physical characteristics of surfaces like color changes on a test strip. In this approach, a sample can be placed on a test strip and its remission (REM) value can be compared against appropriate controls and standards. As in photometry, the difference in intensity between emitted and reflected light allows a quantitative determination of the concentration of specific analytes.
Reflectometry is commonly used in industrial applications as a rapid, sensitive method for quantitating a wide variety of organic and inorganic parameters in water, food, beverages, and environmental samples as well as other diverse uses such as surface analysis of building materials and skin color quantification.
Related Technical Articles
- Transmittance to Absorbance Table A transmittance to absorbance table enables fast conversion from transmittance values to absorbance in the lab or in the field.
- Measuring Chemical Oxygen Demand in Water Treatment Facilities We are a global leader in the life science industry and have produced test kits to measure numerous analytes. Water Online spoke with us about advances in measuring chemical oxygen demand.
- Ammonium in Wastewater Step-by-step accurate reflectometric determination of ammonium in wastewater with Nessler’s reagent or Indophenol blue using Reflectroquant® system and test strips.
- Analytical Method: Analytical Quality Assurance, Standard for COD/Chloride Preparation of a standard solution for COD/chloride
- K268 Olive Oil Commission Regulation (EEC) No 2568/91 Annex IX Learn about the UV-spectrometric determination of the K268 nm value of olive oil, assessing the UV absorbance at 268 nm, to ensure quality and compliance with regulatory standards.
- See All (24)
Related Protocols
- Analytical Method: Analytical Quality Assurance, Standard for Hydrogen peroxide Preparation of a standard solution for Hydrogen peroxide
- Analytical Method: Aluminium (total) in effluents Photometric determination with Chromazurole S subsequent to acid mineralisation; DIN decomposition
- Analytical Method: Aluminium (total) in effluents, high organic content Photometric determination with Chromazurole S subsequent to acid mineralisation
- Analytical Method: Aluminium (total) in lime stone Photometric determination with Chromazurole S subsequent to fusion melting
- Ammonium in Effluents Step-by-step method describing the photometric determination of ammonium ions in effluents using Spectroquant® test kits and spectrophotometer.
- See All (33)
Find More Articles and Protocols

Join our webinar on June 15th to find out how to quickly analyze chemical disinfectant parameters to ensure the safety of your production line after disinfection & the different methods available.
To continue reading please sign in or create an account.
Research. Development. Production.
We are a leading supplier to the global Life Science industry with solutions and services for research, biotechnology development and production, and pharmaceutical drug therapy development and production.
© 2024 Merck KGaA, Darmstadt, Germany and/or its affiliates. All Rights Reserved.
Reproduction of any materials from the site is strictly forbidden without permission.
- English - EN
- Español - ES

IMAGES
VIDEO
COMMENTS
Contributors and Attributions. OChemOnline. Infrared Spectroscopy Absorption Table is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. \ (^1H\) NNR Solvent Shifts.
An infrared spectroscopy correlation table (or table of infrared absorption frequencies) is a list of absorption peaks and frequencies, typically reported in wavenumber, for common types of molecular bonds and functional groups. In physical and analytical chemistry, infrared spectroscopy (IR spectroscopy) is a technique used to identify chemical compounds based on the way infrared radiation is ...
Table of Characteristic IR Absorptions. Table of Characteristic IR Absorptions. m=medium, w=weak, s=strong, n=narrow, b=broad, sh=sharp. frequency, cm. -1. bond functional group. 3640-3610 (s, sh) O-H stretch, free hydroxyl alcohols, phenols 3500-3200 (s,b) O-H stretch, H-bonded alcohols, phenols 3400-3250 (m) N-H stretch 1˚, 2 ...
The values given in the tables that follow are typical values. Specific bands may fall over a range of wavenumbers, cm-1. Specific substituents may cause variations in absorption frequencies. Absorption intensities may be stronger or weaker than expected, often depending on dipole moments. Additional bands may confuse the interpretation.
%PDF-1.6 %âãÏÓ 157 0 obj > endobj 176 0 obj >/Filter/FlateDecode/ID[29E5AF2A7F9D0F4196F4DB70B6DBCA78>89F80795035FDF4A95A9FB23652E2839>]/Index[157 33]/Info 156 0 R ...
For example, the most characteristics absorption band in the spectrum of 2-hexanone ( Figure 6.3a) is that from the stretching vibration of carbonyl double bond C=O, at 1716 cm -1. It is a very strong band comparing to the others on the spectrum. A strong absorbance band in the 1650-1750 cm-1 region indicate that a carbonyl group (C=O) is present.
Table 6.1 Characteristic IR Frequencies of Stretching Vibrations. The information in Table 6.1 can be summarized in the diagram for easier identification (Figure 6.3b), in which the IR spectrum is divided into several regions, with the characteristic band of certain groups labelled.. Figure 6.3b Approximate IR Absorption Range. The absorption bands in IR spectra have different intensities that ...
IR Tables, UCSC Table 1. Characteristic IR Absorption Peaks of Functional Groups* Vibration Position (cm-1) Intensity* Notes Alkanes C-H stretch 2990 - 2850 m to s ... 3250 br, m Primary (two bands) Secondary (one band) Nitriles C≡N stretch 2280 -2200 s Aldehydes C-H stretch 2900 - 2800 & 2800 - 2700 s H-C=O Fermi doublet C=O stretch ...
Infrared Spectroscopy. 1. Introduction. As noted in a previous chapter, the light our eyes see is but a small part of a broad spectrum of electromagnetic radiation. On the immediate high energy side of the visible spectrum lies the ultraviolet, and on the low energy side is the infrared. The portion of the infrared region most useful for ...
Table 1. Secondary structure band assignments for protein in water. 2 Figure 1. Transmission-FTIR spectra for cytochrome C in phosphate buffer (cytc_12) at 12 mg/mL and 6 mg/mL (cytc_6), and phosphate buffer blank. Secondary structure Band assignment in water α-Helix 1,648-1,657 cm-1 β-Sheet (high-frequency component) 1,623-1,641 cm-1
Infrared Spectrum of Ethyl benzoate. The carbonyl stretch C=O of a carboxylic acid appears as an intense band from 1760-1690 cm -1. The exact position of this broad band depends on whether the carboxylic acid is saturated or unsaturated, dimerized, or has internal hydrogen bonding. O-H stretch from 3300-2500 cm -1.
Table S1.Major IR absorption bands and possible assignmentin the typicalFT- IR spectrumof saffron Band (cm-1)* Assignment 3365-3333 (broad) Stretching vibration of bonded and non-bonded -O-H groups 2924 (m) and 2857 (m) ... Table S2. The results of PCA application on the transmittance FT-IR spectral data of Saffron samples ...
Download Table | List of band assignments for FTIR spectra from publication: Monitoring of marine mucilage formation in Italian seas investigated by infrared spectroscopy and independent component ...
You can search for FTIR functional groups by peak position, group, or class quickly from the table. You can search for FTIR functional groups by peak position, group, or class quickly from the table. I n s t a. Chat With Us [email protected] Donate Us. ... Band gap Calculation from Tauc Plot. Sale! Instrument Raw Data File Conversion to Excel File.
Download Table | List of FTIR band assignment from publication: Effects of the environmental stress on two fish populations revealed by statistical and spectral analysis | The aquatic species are ...
According to literature band assignments (Table 6), the bands observed in FT-NIR (Fig. 2C) and NIR portable (Fig. 2D) are mainly related to carbohydrate (e. g C-H, C-O, O-H and CH 2 ) and protein ...
MilliporeSigma
Table 4 as already observed by FTIR analysis ... [36] 38,39].avenumber (cm −1 ) Band Assignment B 3399 O-H stretching vibration of carbohydrate C-OH 2934 C-H asymmetric stretching in alkanes ...
Again, you are to complete the following steps for the molecules assigned to you in Table 3.2. This assignment will require a lot of space in your lab notebook due to all of the vibrations you will be drawing. Determine the number of vibrational motions, 3N - 6 or 3N - 5, to make sure that you know how many vibrational motions to record.
Reflectometry. Reflectometry (also known as remission photometry) is a non-destructive analytical technique that uses the reflection of light by surfaces and interfaces to measure characteristics such as color intensity, film thickness and refractive index. As with other photometers, the main elements of reflectometers include a light source ...
The spectra of all individual components, including NEPA, PVA, poloxamer 407, and HPβCD and the spectra of electrospun fibrous samples are presented in Fig. 3. The spectra of nepafenac powder ...
functional groups present in FTIR spectra. By limiting spectral preprocessing, we explore a minimalistic approach to allow the network to learn spectral patterns for successful recognition of the fifteen most common organic functional groups (Table 1). Table 1. Functional groups for which successful models were trained.
AD-0178. The resulting protein difference spectra were smoothened Table 2: Secondary structure band wavenumber and using a seven-point Savizky-Golay function to remove any assignments for protein in water possible white noise. Amide band I (1600 - 1700 cm-1) was baseline-corrected. LabSolutionsTM IR Curve-Fitting Program was used for band ...
The assignment of absorption bands and their frequencies is summarized in the Table 4. The FTIR sector for the PbO 2 matrix is characteristic of the amorphous structure of lead dioxide, and the progressive incorporation of SiO 2 into the vitreous matrix results in notable alterations in both the intensity and position of these bands, along with ...