Research Paper Writing Checklist
- Writing Research Papers
- Writing Essays
- English Grammar
- M.Ed., Education Administration, University of Georgia
- B.A., History, Armstrong State University
A research paper checklist is an essential tool because the task of putting together a quality paper involves many steps. Nobody writes a perfect report in one sitting!
Before you get started on your project, you should review the checklist on research ethics .
Later, once you have finished the final draft of your research paper, you can use this checklist to make sure that you have remembered all the details.

Research Paper Checklist
- How to Develop a Research Paper Timeline
- What Is a Research Paper?
- How to Organize Research Notes
- Make Your Paragraphs Flow to Improve Writing
- What Is a Senior Thesis?
- Explore and Evaluate Your Writing Process
- How to Outline and Organize an Essay
- German Grammar Checklist
- Brainstorming Techniques for Students
- Revising a Paper
- 14 Ways to Write Better in High School
- An Introduction to Academic Writing
- Documentation in Reports and Research Papers
- Abstract Writing for Sociology
- Writing an Annotated Bibliography for a Paper
- How to Write a Research Paper That Earns an A
Home / Guides / Writing Guides / Writing Tips / Research Paper Checklist
Research Paper Checklist
Research papers are hard. As tempting as it may be to just hand in your paper the second you finish that last citation, it is super important to review everything to make sure you don’t have any silly mistakes!
Use this 10-step checklist to make sure your paper is in top-notch form:
- Credit and cite all information from other sources.
- Place direct quotes from other sources between quotation marks.
- Add all appropriate in-text citations or footnotes.
- All in-text citations/footnotes have a matching citation in the bibliography.
- Alphabetize bibliography and check formatting/ capitalization of titles .
- The thesis statement (purpose of the paper) is clearly stated.
- The paper has a clear conclusion/closing statement.
- Check for spelling and grammatical errors.
- Check for slang words or contractions in writing.
- The title page is properly formatted (when required by your instructor).
How useful was this post?
Click on a star to rate it!
We are sorry that this post was not useful for you!
Let us improve this post!
Tell us how we can improve this post?
Grammar and Plagiarism Checkers
Grammar Basics
Plagiarism Basics
Writing Basics
Plagiarism Checker
Upload a paper to check for plagiarism against billions of sources and get advanced writing suggestions for clarity and style.
Get Started
- Writing Worksheets and Other Writing Resources
- Research Paper
Checklist for Research Papers
About the slc.
- Our Mission and Core Values

- Do I have a sufficient number of sources?
- Are a significant number of my sources critical sources (e.g., from academic journals)?
- Are my sources integrated smoothly into the paper?
- Is there a dialogue between my own analysis of the text and the research I'm including?
- Is my own scholarly opinion strongly present in the research paper, rather than the paper reading like a review of the opinions of other scholars?
- Have I cited all sources I draw from?
- If my paper is a revision, have I considered the changes I want to make, the comments of my peers, and the instructor's comments towards the end of significantly improving on the previous version of this paper?
María Villaseñor
Student Learning Center, University of California, Berkeley
© 2002 UC Regents
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License.
Get science-backed answers as you write with Paperpal's Research feature
Research Paper Writing: A 15-Point Academic Writing Checklist

Research paper writing can be a challenging task for PhD students, early career researchers, and even some more experienced academics. When writing a research paper, authors need to be able to present their work in a compelling, easy to understand way, and in doing so demonstrate a deep understanding of their subject. This requires a structured process, a proper approach, careful attention to detail, meticulous planning, excellent writing skills and a significant amount of effort. In this article, we will outline the key elements of a good research paper and provide researchers with a 15-point research paper writing checklist to streamline the process.
Key elements of research paper writing
The research paper writing process requires you to include some essential elements to deliver a well-written, complete manuscript.
- An engaging title: Ensuring that the title of your research paper is concise, impactful, and engaging is important as it is the first thing that readers will see. It should be able to convey the subject of your research paper clearly and simply.
- Well-articulated research questions : Research paper writing is incomplete without a well-defined thesis statement or research question that clearly conveys the purpose and scope of the research.
- Thorough literature reviews: This key element in research paper writing covers a comprehensive overview of existing research in the field, which helps researchers identify and address potential gaps in knowledge.
- Structured methodology: A good research paper must present a detailed description of the methodology used to collect and analyze data as part of their study. This allows readers to assess the validity and reliability of the research.
- Accurate results : Communicating the results accurately and logically is an important element of research paper writing. Use graphs, tables and other visual aids as needed to organize and present your findings in an easy-to-understand way.
- Analysis and discussion: In research paper writing, this isthe section that helps readers evaluate, discuss, and understand the relevance, significance, and implications of the research being done.
- Strong conclusion : A well-rounded conclusion summarizes the key research findings and provides a clear, concise answer to the research question or thesis statement.
- Citations and references: A good research paper writing practice is to ensure you have got the citations and references right; this establishes your expertise and adds credibility to your work.
THE academic writing checklist for researchers
Now that you know the key elements to include in your research paper writing process, it’s time to get started. If it seems like a lot, don’t worry. Follow this 15-point academic writing checklist to ensure that all the key research paper writing elements have been included and carefully checked before you submit.
- Assess the research paper title to see if it clearly conveys the focus of the research paper, has relevant keywords, and is engaging enough to attract reader attention.
- Read the abstract to make sure that it contains the aims and objectives of the study, the research design and methodology, the main findings, and final conclusions.
- Ensure the introduction is well structured and addresses key issues like the aim, research questions, and arguments made in the research paper.
- Present a clear statement of the rationale for the study in the introduction and ensure that relevant literature is cited as it relates to the study.
- Ensure that the research paper is well structured and organized with sub-sections and paragraphs and presented in a logical flow; this will aid reader comprehension.
- Craft short, engaging paragraphs that communicate often complex ideas clearly; use appropriate transitional phrases or words to connect paragraphs and sub-sections smoothly.
- Provide relevant headings for sub-sections to improve overall readability and convey important points quickly.
- Ensure that the conclusion summarizes and clearly communicates the findings and analysis of the research problem. Remember not to introduce any new ideas or findings that are nor reflected in the main body of the text.
- Avoid repetition – differentiate the results from the discussion section; the former should present the findings, while the latter evaluates how the study adds to existing knowledge, practice and/or policy formulation.
- Check the citations follows the target journal’s style guidelines. Make sure that in-text citations match the references and bibliography.
- Check that the guidelines for formatting provided by the target journal have been strictly followed. Page numbers must be provided in the proper format, line spacing must be even, and tables and figures should be given titles and numbers that match with the references.
- Proofread several times to ensure that your final manuscript duly addresses and resolves all the feedback comments and recommendations shared by the supervisor/reviewer/editor.
- Check for possible plagiarism by checking and properly citing all the sources used for research paper writing; use the appropriate citation style (APA, MLA, etc.) preferred by your journal.
- Make sure that the research paper adheres to the recommended word count and length specified by the journal. Check abbreviations have been provided in full at least once and a glossary has been provided, where necessary. Any non-English language should be accurately translated.
- Include an acknowledgement section mentioning and thanking individuals or teams who have helped and supported you with the research study.
The research paper writing process requires careful attention to detail and adherence to academic standards. By following this comprehensive 15-point academic writing checklist, PhD students and researchers can optimize their research paper writing and ensure they consistently deliver coherent, well-structured manuscripts that meet the high standards expected by journal editors.
Paperpal is an AI writing assistant that help academics write better, faster with real-time suggestions for in-depth language and grammar correction. Trained on millions of research manuscripts enhanced by professional academic editors, Paperpal delivers human precision at machine speed. Try it for free or upgrade to Paperpal Prime , which unlocks unlimited access to premium features like academic translation, paraphrasing, contextual synonyms, consistency checks, submission readiness and more. It’s like always having a professional academic editor by your side! Go beyond limitations and experience the future of academic writing. Get Paperpal Prime now at just US$12 a month !
Related Reads:
- Top 5 Ethical Considerations in Research
- Good Writing Habits: 7 Ways to Improve Your Academic Writing
- Self-Plagiarism in Research: What it is and How to Avoid It
- How to Write a Conclusion for Research Papers (with Examples)
How to Ask a Journal Editor About Manuscript Status (Email Template Included)
Are you using the right verbs in your research paper, you may also like, phd qualifying exam: tips for success , ai in education: it’s time to change the..., is it ethical to use ai-generated abstracts without..., what are journal guidelines on using generative ai..., should you use ai tools like chatgpt for..., publish research papers: 9 steps for successful publications , how to make translating academic papers less challenging, self-plagiarism in research: what it is and how..., 6 tips for post-doc researchers to take their..., presenting research data effectively through tables and figures.

The Plagiarism Checker Online For Your Academic Work
Start Plagiarism Check
Editing & Proofreading for Your Research Paper
Get it proofread now
Online Printing & Binding with Free Express Delivery
Configure binding now
- Academic essay overview
- The writing process
- Structuring academic essays
- Types of academic essays
- Academic writing overview
- Sentence structure
- Academic writing process
- Improving your academic writing
- Titles and headings
- APA style overview
- APA citation & referencing
- APA structure & sections
- Citation & referencing
- Structure and sections
- APA examples overview
- Commonly used citations
- Other examples
- British English vs. American English
- Chicago style overview
- Chicago citation & referencing
- Chicago structure & sections
- Chicago style examples
- Citing sources overview
- Citation format
- Citation examples
- College essay overview
- Application
- How to write a college essay
- Types of college essays
- Commonly confused words
- Definitions
- Dissertation overview
- Dissertation structure & sections
- Dissertation writing process
- Graduate school overview
- Application & admission
- Study abroad
- Master degree
- Harvard referencing overview
- Language rules overview
- Grammatical rules & structures
- Parts of speech
- Punctuation
- Methodology overview
- Analyzing data
- Experiments
- Observations
- Inductive vs. Deductive
- Qualitative vs. Quantitative
- Types of validity
- Types of reliability
- Sampling methods
- Theories & Concepts
- Types of research studies
- Types of variables
- MLA style overview
- MLA examples
- MLA citation & referencing
- MLA structure & sections
- Plagiarism overview
- Plagiarism checker
- Types of plagiarism
- Printing production overview
- Research bias overview
- Types of research bias
- Example sections
- Types of research papers
- Research process overview
- Problem statement
- Research proposal
- Research topic
- Statistics overview
- Levels of measurment
- Frequency distribution
- Measures of central tendency
- Measures of variability
- Hypothesis testing
- Parameters & test statistics
- Types of distributions
- Correlation
- Effect size
- Hypothesis testing assumptions
- Types of ANOVAs
- Types of chi-square
- Statistical data
- Statistical models
- Spelling mistakes
- Tips overview
- Academic writing tips
- Dissertation tips
- Sources tips
- Working with sources overview
- Evaluating sources
- Finding sources
- Including sources
- Types of sources
Your Step to Success
Plagiarism Check within 10min
Printing & Binding with 3D Live Preview
Research Paper Checklist For Academic Success
How do you like this article cancel reply.
Save my name, email, and website in this browser for the next time I comment.

Embarking on the journey of writing a research paper can be a daunting task. To aid in this process and ensure thoroughness, we’ve curated an article titled ‘Research Paper Checklist.’ This guide is designed to provide step-by-step essentials to guarantee the quality and rigor of your research paper. In this article, we’ll look at how you can formulate a workable research paper checklist to help you with your dissertations.
Inhaltsverzeichnis
- 1 Research Paper Checklist – In a Nutshell
- 2 Definition: Research paper checklist
- 3 The importance of a research paper checklist
- 4 Research paper checklist
- 5 Research paper checklist: The benefits
Research Paper Checklist – In a Nutshell
- A research paper checklist is a document that lists the required criteria for writing a thesis.
- It is a guiding tool that allows you to express your research ideas logically and systematically.
- A research paper checklist provides an overview of the writing process, enabling proper planning.
Definition: Research paper checklist
A research paper checklist is a document that briefly highlights the scope and structure of a research report in point form. It provides step-by-step instructions that you can use to complete a given task, which in this case is drafting a research thesis.
Ideally, the checklist enables you to ascertain that you meet all the requirements when writing and submitting a dissertation. It also allows you to achieve a coherent flow of opinions when discussing a given topic.
The importance of a research paper checklist
All these elements can be overwhelming to remember, so it is advisable to use a checklist.
A research paper checklist is a general guideline on the most crucial aspects of writing a report. It clarifies what to prioritize in your thesis; where, when, and why. It also allows you to break down the writing process into bite-sized chunks that you can tackle easily without feeling overwhelmed by the overall workload. Additionally, the research paper checklist assists you in comprehensively adhering to report writing criteria before presenting your work.
A typical research report provides in-depth information that accounts for the flow of events when studying a particular subject. It features organized documents containing defined elements, including the:
- introduction
- literature review
- methodology
- recommendations
- conclusions
A research paper checklist is a general guideline on the most crucial aspects of writing a report. It clarifies what to prioritize in your thesis; where, when, and why.
It also allows you to break down the writing process into bite-sized chunks that you can tackle easily without feeling overwhelmed by the overall workload.
Additionally, the research paper checklist assists you in comprehensively adhering to report writing criteria before presenting your work.
Research paper checklist
As discussed, a research paper checklist lets you write an adequate research report.
Here are some pointers you can use to guide you when drafting your thesis:
- What is the required writing style for the research paper?
- Does the report have a title?
- Is the title clear and specific?
- Are my transitions smooth and coherent?
- Does the research paper have adequate source citations?
- Are all the sources listed in the bibliography?
- Are most, if not all, of the source citations derived from critical sources?
- Do the source citations listed adhere to the prevailing writing format?
- Are there spelling issues and grammatical errors in the report?
- Does the research report risk having stylistic problems that can obscure meaning?
- Does the text contain overused words or phrases?
- Do paragraphs adhere to effective paragraphing criteria?
- Does the overall report depict a coherent flow of ideas?
- Is the content thoroughly addressing the subject matter?
- Do the summary and recommendations relate to the topic in question?
- Are the examples used relevant to the research paper?
- Does the summary restate the thesis briefly and conclusively?
- Do the number of pages of the report compare appropriately with the subject matter?

Research paper checklist: The benefits
A research report checklist is essential in helping you write a well-organized thesis.
It can be beneficial in the following ways:
- A research paper checklist allows you to express your thoughts and ideas in a concise flow that readers can comprehend effortlessly.
- It helps you limit your ideas and discussion to the scope of the subject matter.
- You can use it to strategically approach the writing process without feeling overwhelmed.
- A research paper checklist lets you put together and submit a high-quality report that sufficiently addresses the topic in question.
- It also acts as an evaluation document to help you ascertain that you considered all the essential aspects of your report, like the writing style.
- Additionally, a report-writing checklist minimizes the time needed to write a research paper.
What is a research paper checklist?
A research paper checklist is a document that provides a detailed overview of essential factors to consider and prioritize when writing a research report.
Why should you use a research paper checklist?
A research paper checklist helps you define your workload. In doing so, you can strategize how to approach the task accordingly and achieve timely delivery.
How do you formulate a research paper checklist?
Start by considering the scope of the topic in question and the style guidelines required to write the report, and then create a list that prominently highlights the major focus areas .
We use cookies on our website. Some of them are essential, while others help us to improve this website and your experience.
- External Media
Individual Privacy Preferences
Cookie Details Privacy Policy Imprint
Here you will find an overview of all cookies used. You can give your consent to whole categories or display further information and select certain cookies.
Accept all Save
Essential cookies enable basic functions and are necessary for the proper function of the website.
Show Cookie Information Hide Cookie Information
Statistics cookies collect information anonymously. This information helps us to understand how our visitors use our website.
Content from video platforms and social media platforms is blocked by default. If External Media cookies are accepted, access to those contents no longer requires manual consent.
Privacy Policy Imprint
Check List for Research Papers
Statement of purpose, review of literature, transitions..
We have discussed the topic of code-switching in Swahili from what might be termed the point of view of the mechanics of code-switching; i.e. how it operates in Swahili; let us now examine its function in Swahili linguistic culture, i.e. why Swahili speakers choose to code-switch, and when. We will then show how code-switching is used in popular media, print advertising, and other genres.
No discussion of print-medium advertising in Swahili would be complete without a discussion of another, related phenomenon, which is the use of language(s) and varieties in comic books. Particularly instructive are Swahili renditions of Tarzan comics, which depict Tarzan as fluent in Swahili and English, while other characters are depicted only as speaking English inadequately.
"The work of Ferguson (Ferguson 1959:32) is crucial for our understanding of the concept of diglossia."
"No discussion of language maintenance would be complete without the work of Fishman (1959, 1960, 1963, 1966, 1972, [...])"
Most researchers on the subject of bilingualism accept the notion that one language or code will be dominant, i.e. they assume that individual speakers have more facility, or higher proficiency, in one language than in another; that is, the so-called balanced bilingual is a rare phenomenon. I will follow this practice, but will also point out examples where this has been shown to be problematical.
Which sounds better, `English's worldwide spread' or `the worldwide spread of English'? `The Queen of England's unruly children' vs. the unruly children of the Q of England'? Spelling problems are marked in margins etc. with "sp" Stylistic problems are given alternative suggestions. (sometimes marked in margin "awk" for awkward.) If some words or phrases are repeated or overused, the mark repet for `repetitious' may appear in the margin. If the symbol PP appears, it means "start a new paragraph". Content I stated at the beginning of the course ("Helpful Hints...") that I would like your paper to reflect issues and problems we have discussed in various ways during the class. I will not try to summarize these here, but I would like to see evidence that these ideas have been considered, and brought to bear on the material you are discussing. I do not expect you to parrot what others say, or what I have said; I expect you to contrast different ideas and weigh them; or bring two disparate opinions or approaches together and show how the conjunction of these ideas throws new light on the subject. Example: Much has been written about the status of French in France, and much has also been written about French attempts to control the corpus of Standard French. What has not often been made clear is how the French themselves do not usually distinguish between corpus and status issues, and that is what I wish to focus on in this paper. As such I introduce no new facts into the situation, but I do introduce a new interpretation of existing facts.
I have discussed in this paper how such issues as code-switching, code-shifting, and bilingualism in Swahili linguistic culture are manifested in print-medium advertising in East Africa. I have attempted to describe in a general way both the mechanics of these phenomena, as well as the social motivation for them, and how advertisers use these techniques for their own means in advertising.
In the process I have attempted to demonstrate that these phenomena are intricately interwoven with social forces identified by various writers as modernization, power-relations, and gender relations in East African society. They do not constitute in any way a failure of the linguistic code, but are in fact a manifestation of shifting identities in the culture.
My own contribution, if any, has been to bring in the work of A, B, and C, and relate their research to the ideas presented by X, Y, and Z, who are the acknowledged primary researchers in this field.
Example: In the process of this review, an attempt to define the role of language in the definition of ethnicity, I have to conclude that many researchers seem to define ethnicity in a circular or tautological way, i.e., as a constellation of factors involving language, race, descent, culture, history, etc. but often with one or more of these factors missing. Some researchers act as if ethnicity were a given, something that must be present in society, rather than a construct they themselves have invented. And, they often act as if all languages are equal in their impact on ethnicity, or as if any `language' at all would do for their definition of ethnicity , with no sense of the complexity of any one language. My conclusion, therefore, is that ethnicity is a problematical construct, and that in the society I examined, Eastern Rumelia, ethnicity indeed seems to involve a language factor, but having said this, I am unable to state what ethnicity actually means to the Rumelians. Perhaps this is a factor of the recent political shift in eastern Europe, but in any event, the concept of ethnicity seems to be in a very fluid state. Much more work, beyond the scope of this paper, is obviously required.
Entertainment Value
Research Paper Checklist
- By Nicole Nichols-West on Jan 2, 2012

- Choose a Topic
- Pick a topic which interests and challenges you.
- Focus on a limited aspect.
- Obtain teacher approval for your topic.
- Re-read your assignment sheet carefully to be certain you know what is expected.
- Select a subject you can manage.
- Avoid subjects that are too technical, learned, or specialized.
- Avoid topics that have only a very narrow range of source materials.
- Find Information
- Use search engines and other search tools as a starting point.
- Pay attention to domain name extensions (if it ends with edu or org)
- Be selective of com (commercial) sites.
- Be wary of personal home pages - their quality vary greatly.
- Check out public and university libraries, businesses, government agencies.
- Contact knowledgeable people in your community.
- Write down full bibliographical information.
- Your Thesis
- Write your thesis statement down in one sentence.
- Find arguments to support and defend this belief.
- Think through your topic carefully and organize it logically
- Include in your outline an introduction, a body, and a conclusion.
- Make the first outline tentative.
- In the introdutions, state your thesis and the purpose of your research.
- State how you plan to approach your topic.
- Explain briefly the major points you plan to cover.
- In the body, present your arguments to support your thesis statement.
- In the conclusion, restate or reword your thesis and summarize your arguments.
- Thesis statement is concise and clear.
- Arguments are presented in a logical sequence.
- All sources are properly cited.
- Your intentions and points are clear in the essay.
- Read your paper for grammatical errors.
- Correct all errors that you can spot.
- Get someone else to read it over.
- Share via: Twitter , LinkedIn , Facebook , Whatsapp
- 55 views today
- 7 uses today
Related Checklists

By Lawless French

By Lawless Spanish

More Related Checklist Templates

More Checklist Templates
"research paper checklist" not suitable, search the world's largest free library of checklist templates.
38+ SAMPLE Research Checklist in PDF | MS Word
Research checklist | ms word, 38+ sample research checklist, what is a research checklist, importance of research, advantages of checklist in research, how to write a research checklist, what are the five checklist components of a research paper, what are the different types of a checklist, can a checklist be used for the qualitative study.

Research Checklist Template
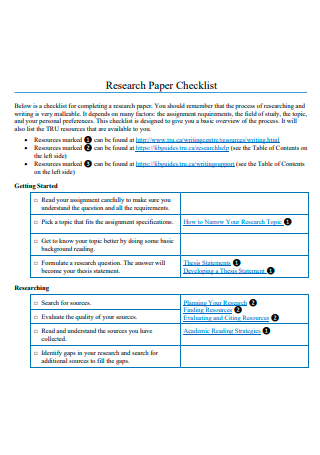
Research Paper Checklist

Basic Research Checklist

Research Proposal Checklist
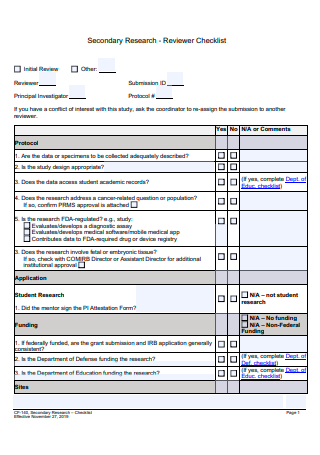
Secondary Research Reviewer Checklist

Research Request Checklist
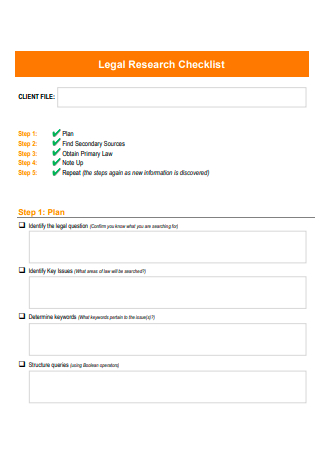
Legal Research Checklist

Work Based Learning Pre-Experience Research Checklist

Research Statement Checklist

Research Faculty Position Checklist

Undergraduate Research Experience Checklist

Research Start up Checklist
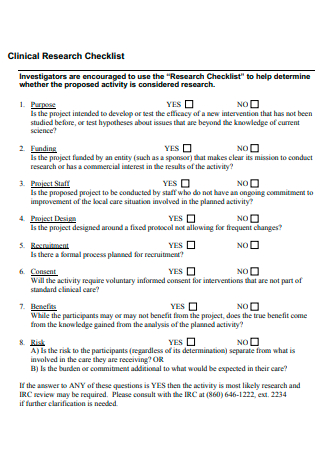
Clinical Research Checklist
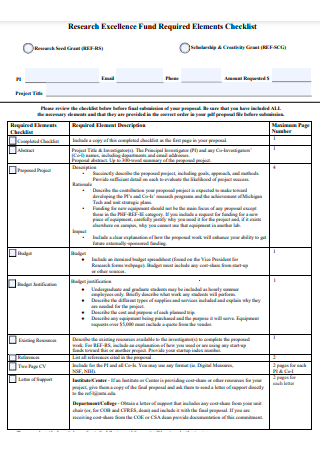
Research Excellence Fund Required Elements Checklist
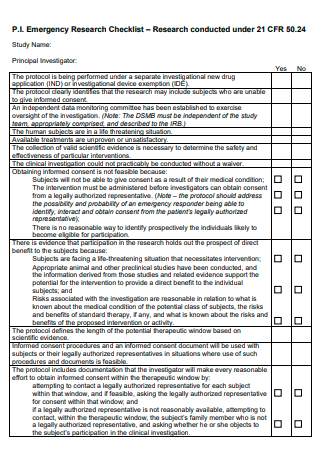
Emergency Research Checklist

Product Research Checklist
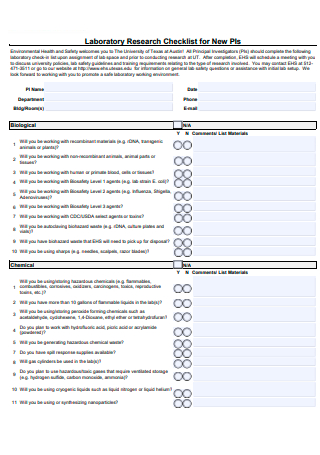
Laboratory Research Checklist

Research Presentation Checklist

Research Assignment Checklist


Research Training Checklist

Research Checklist Example

Covid-19 Research Checklist

Return to Research Checklist
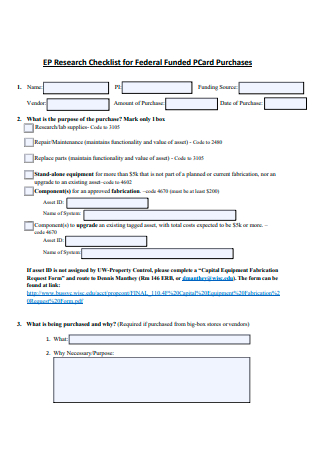
Federal Funded Purchases Research Checklist

Research Process Checklist
Human Research Checklist

Research Restart Plan Review Checklist

Domestic Research Administrative Assistant Checklist

Research Investigator Project Checklist

Formal Research Checklist
Research Ethics Checklist

Forest Field Research Checklist

Research Onboarding Checklist

Research Collaboration Checklist

Research Checklist in PDF
Classroom Research Project Checklist
Department of Defense Research Checklist

Research Project Ethics Checklist

Research Checklist in DOC
1. define the topic, 2. list down your sources, 3. synthesize your research, 4. add boxes, share this post on your network, file formats, word templates, google docs templates, excel templates, powerpoint templates, google sheets templates, google slides templates, pdf templates, publisher templates, psd templates, indesign templates, illustrator templates, pages templates, keynote templates, numbers templates, outlook templates, you may also like these articles, 3+ sample vendor audit checklist in pdf.

Kathy Burlison once said, “Given the risks of an expensive audit, paying strict attention to the rules is the only smart decision you can make.” Since the term audit…
18+ SAMPLE Food Safety Inspection Checklist in PDF

The food industry is one of the most dominant and flourishing industries present in the status quo. With that, food companies are doing their best to keep their products…
browse by categories
- Questionnaire
- Description
- Reconciliation
- Certificate
- Spreadsheet
Information
- privacy policy
- Terms & Conditions
This paper is in the following e-collection/theme issue:
Published on 19.4.2024 in Vol 26 (2024)
Investigating the Cost-Effectiveness of Telemonitoring Patients With Cardiac Implantable Electronic Devices: Systematic Review
Authors of this article:

- Sarah Raes 1 , MSc ;
- Andrea Prezzi 1 , MSc ;
- Rik Willems 2 , PhD ;
- Hein Heidbuchel 3 , PhD ;
- Lieven Annemans 1 , PhD
1 Department of Public Health and Primary Care, Ghent University, Gent, Belgium
2 Department of Cardiovascular Sciences, Universiteit Leuven, Leuven, Belgium
3 Department of Genetics, Pharmacology and Physiopathology of Heart, Blood Vessels and Skeleton (GENCOR), Antwerp University, Antwerp, Belgium
Corresponding Author:
Sarah Raes, MSc
Department of Public Health and Primary Care
Ghent University
Corneel Heymanslaan 10
Phone: 32 9 332 83 59
Email: [email protected]
Background: Telemonitoring patients with cardiac implantable electronic devices (CIEDs) can improve their care management. However, the results of cost-effectiveness studies are heterogeneous. Therefore, it is still a matter of debate whether telemonitoring is worth the investment.
Objective: This systematic review aims to investigate the cost-effectiveness of telemonitoring patients with CIEDs, focusing on its key drivers, and the impact of the varying perspectives.
Methods: A systematic review was performed in PubMed, Web of Science, Embase, and EconLit. The search was completed on July 7, 2022. Studies were included if they fulfilled the following criteria: patients had a CIED, comparison with standard care, and inclusion of health economic evaluations (eg, cost-effectiveness analyses and cost-utility analyses). Only complete and peer-reviewed studies were included, and no year limits were applied. The exclusion criteria included studies with partial economic evaluations, systematic reviews or reports, and studies without standard care as a control group. Besides general study characteristics, the following outcome measures were extracted: impact on total cost or income, cost or income drivers, cost or income drivers per patient, cost or income drivers as a percentage of the total cost impact, incremental cost-effectiveness ratios, or cost-utility ratios. Quality was assessed using the Consensus Health Economic Criteria checklist.
Results: Overall, 15 cost-effectiveness analyses were included. All studies were performed in Western countries, mainly Europe, and had primarily a male participant population. Of the 15 studies, 3 (20%) calculated the incremental cost-effectiveness ratio, 1 (7%) the cost-utility ratio, and 11 (73%) the health and cost impact of telemonitoring. In total, 73% (11/15) of the studies indicated that telemonitoring of patients with implantable cardioverter-defibrillators (ICDs) and cardiac resynchronization therapy ICDs was cost-effective and cost-saving, both from a health care and patient perspective. Cost-effectiveness results for telemonitoring of patients with pacemakers were inconclusive. The key drivers for cost reduction from a health care perspective were hospitalizations and scheduled in-office visits. Hospitalization costs were reduced by up to US $912 per patient per year. Scheduled in-office visits included up to 61% of the total cost reduction. Key drivers for cost reduction from a patient perspective were loss of income, cost for scheduled in-office visits and transport. Finally, of the 15 studies, 8 (52%) reported improved quality of life, with statistically significance in only 1 (13%) study ( P =.03).
Conclusions: From a health care and patient perspective, telemonitoring of patients with an ICD or a cardiac resynchronization therapy ICD is a cost-effective and cost-saving alternative to standard care. Inconclusive results were found for patients with pacemakers. However, telemonitoring can lead to a decrease in providers’ income, mainly due to a lack of reimbursement. Introducing appropriate reimbursement could make telemonitoring sustainable for providers while still being cost-effective from a health care payer perspective.
Trial Registration: PROSPERO CRD42022322334; https://tinyurl.com/puunapdr
Introduction
The implantation rates of cardiac implantable electronic devices (CIEDs), including pacemakers and implantable cardioverter-defibrillators (ICDs), have increased over the last decades due to expanded indications and a progressively aging population [ 1 ]. To evaluate the clinical status of the patient and device functioning, current guidelines recommend that older patients with pacemakers should be evaluated every 3 to 12 months and patients with ICDs should be evaluated every 3 to 6 months [ 2 ]. This regimen imposes a considerable burden on patients and physicians if the patient is required to be seen in person.
Telemonitoring, referring to the process of using telecommunication and information technology to monitor the health status of a patient and device function from a distance, can reduce this burden by replacing some in-office visits with transmissions from the patients’ home [ 3 ]. Existing research indicated that telemonitoring is safe (eg, experiencing equal major adverse events to standard care) [ 4 , 5 ]. The advantages of telemonitoring include fewer inappropriate shocks for patients with ICDs [ 4 , 6 ] and fewer hospitalizations for patients with atrial arrhythmias and strokes [ 4 , 6 , 7 ]. Moreover, there is a rapid detection of cardiovascular events and device malfunction [ 5 , 7 ], leading to a time reduction between clinical decision and intervention [ 8 ].
Besides the effectiveness of telemonitoring, patient experience is essential in high-quality health care services. Overall, patients with pacemakers on telemonitoring reported positive experiences comparable to the experience of patients with in-hospital monitoring [ 9 ]. Telemonitored patients with pacemakers tended to receive less information about their diagnosis but no significant differences were found in other items, such as confidence in clinicians, treatment decision involvement, treatment satisfaction, and waiting time before admission [ 9 ]. Another study indicated that telemonitoring of patients with a cardiac resynchronization therapy defibrillator (CRT-D) was time-saving for both patients and physicians [ 10 ].
Cost-effectiveness analyses are important to quantify the value of new interventions, informing both medical decision-making and public policy [ 11 ]. However, cost-effectiveness analyses depend on the perspective considered. The different perspectives are the health care payer perspective (eg, Medicare or Medicaid and British National Health Service), the patient perspective, the provider perspective (eg, physician), and the society perspective. The health care payer and societal perspectives differ from each other as the societal perspective includes indirect nonmedical costs (eg, transport) [ 12 ].
As cost-effectiveness analyses have shown heterogeneous results, it is still debatable whether telemonitoring is worth the investment relative to standard care. However, data on cost-effectiveness are important for health care payers to make decisions on the reimbursement of telemonitoring. Lack of reimbursement can be an important adoption barrier for new technology [ 13 , 14 ]. For these 2 reasons, this paper reviews the cost-effectiveness of telemonitoring, reviews how the results differ from different perspectives, and describes the key drivers of the cost-effectiveness of telemonitoring.
The review protocol was published by PROSPERO (International Prospective Register of Systematic Reviews; CRD42022322334). This systematic review was carried out in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) reporting guideline of 2020 [ 15 ], and the PRISMA-ScR (Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews) [ 16 ], which can be found in the Multimedia Appendix 1 . Guidelines for preparing a systematic review of health economic evaluations were followed [ 17 ].
Literature Search
For this review, PubMed, Embase, EconLit, and Web of Science Core Collection were systematically searched. The last search was performed on July 7, 2022. No filters (eg, publication date or type of study) were applied. Search strategies for all electronic databases can be found in Multimedia Appendix 2 .
Search strings were developed based on explorations of databases and previous reviews. The following key concepts were translated into strings: (1) CIEDs, (2) telemonitoring, and (3) economic evaluations (eg, cost-effectiveness analyses and cost-utility analyses). The latter was based on a validated search filter, designed to identify economic evaluations, and was broadened for this study to maximize sensitivity [ 18 ]. The search terms for CIEDs and telemonitoring were based on existing reviews [ 19 - 21 ].
Study Selection
Studies were included if their primary focus was on the cost-effectiveness of telemonitoring patients with a CIED. The eligibility criteria were defined a priori for study selection ( Textbox 1 ). The population, intervention, comparator, and outcome strategy was applied to describe the criteria. Only complete and peer-reviewed studies were included. Specific exclusion criteria included partial economic evaluations, systematic reviews or reports, and studies without standard care as a control group. Only studies published in English, Dutch, French, or German were eligible for inclusion. The reference lists of the included studies were searched manually to identify relevant studies. Two reviewers (SR and AP) independently screened the titles and abstracts of all records using Rayyan (Rayyan Systems Inc) [ 22 ]. After the initial screening, full texts were retrieved and screened for a second time. The second screening round was independently performed by 2 reviewers (SR and AP). Reasons for exclusion were documented ( Figure 1 ). For both screening rounds, reviewers were blinded from each other’s decision, and disagreements were resolved through discussion.
Inclusion criteria
- Cardiac implantable electronic devices: pacemaker, implantable cardioverter-defibrillator, cardiac resynchronization therapy defibrillator, cardiac resynchronization therapy pacemaker, and loop recorder
- Standard care
- Complete health economic evaluations (within-trial and model-based)
- All settings
- English, French, German, or Dutch
Exclusion criteria
- Implantable pulmonary artery pressure monitor
- Partial health economic evaluations (outcomes related to costs or effectiveness only)
- Systematic reviews, reports, commentaries, congress abstracts, protocols, and animal studies

Quality Assessment
Two researchers (SR and AP) independently evaluated the original papers using the Consensus Health Economic Criteria (CHEC) checklist to assess the risk of bias [ 23 ]. The CHEC checklist included 19 items. Any disagreement was resolved by discussion and consensus. Interpretation of the CHEC list can be found in Multimedia Appendix 3 . The included studies were classified into 4 quality categories: excellent (score of 100%), good quality (score between 75% and 100%), moderate quality (score between 50% and 75%), and low quality (score <50%) [ 24 ].
Synthesis of Results
The study characteristics and main outcomes of the original papers are presented in the Results section. SR extracted all data. A data extraction sheet was developed using an existing template [ 17 ]. The following information was extracted from the included studies: study identification, general study characteristics, results, and authors’ conclusion. The principal outcome measures were health outcomes, cost or income outcomes (eg, the impact on total cost or income, cost or income drivers, cost or income drivers per patient, and cost or income drivers as a percentage of the total cost impact), and incremental cost-effectiveness ratios (ICERs) or cost-utility ratios.
To facilitate comparison across studies, the following adjustments and interpretations were made. First, the cost or income outcomes were presented per patient per year, and different currencies were converted to US Dollar (reference year: 2019 and reference country: United States) [ 25 ]. Second, perspectives were categorized into the health care payer perspective, patient perspective, provider perspective, and societal perspective. For the purpose of our study, the provider includes physicians who are directly involved in the care of patients with CIED.
The selection process is shown in Figure 1 . From a total of 3305 publications, 15 (0.45%) unique publications were reviewed. Studies were excluded because one of the following reasons: (1) intervention: the paper did not describe telemonitoring patients with a CIED; (2) outcome: the paper contained only a cost analysis and not a cost-effectiveness analysis; and (3) study design or publication: the paper was a partial health economic evaluation, congress abstract, protocol, systematic review, animal study, or with no peer review.
Characteristics of the included studies can be found in Table 1 . All 15 (100%) studies had a primarily male population, except for the Nordland study, which had an almost equal sex distribution ( Table 1 ) [ 26 ]. The mean age of the population with pacemakers was between 75 (SD 24.64) and 81 (SD 6.47) years. The mean age of patients with an ICD or CRT-D was between 61 (SD 12.6) and 69 (SD not calculated) years, except for the PREDICT RM study, where >50% of the population was aged >75 years [ 27 ]. Furthermore, of the 15 studies, 1 (7%) included only older patients (with a mean age of 81 years) with pacemakers [ 28 ], and 2 (13%) ICD or CRT-D studies only included patients with heart failure [ 11 , 29 ].
a CIED: cardiac implantable electronic device.
b N/A: not applicable.
c Age was a discrete variable in this study (higher of lower than 75 years old).
d ICD: implantable cardioverter-defibrillator.
e TARIFF: Health Economics Evaluation Registry for Remote Follow-Up.
f CRT-D: cardiac resynchronization therapy defibrillator.
g EVOLVO: Evolution of Management Strategies of Heart Failure Patients With Implantable Defibrillators.
h MORE-CARE: Monitoring Resynchronization Devices and Cardiac Patients.
i CONNECT: Clinical Evaluation of Remote Notification to Reduce Time to Clinical Decision.
j ECOST: Effectiveness and Cost of ICD Follow-Up Schedule With Telecardiology.
k EuroEco: European Health Economic Trial on Home Monitoring in ICD Patients.
l VVI-ICD: single-chamber ICD.
m DDD-ICD: dual-chamber ICD.
n SAVE-HM: Socio-Economic Effects and Cost Saving Potential of Remote Patient Monitoring.
Study Designs
Tables 2 and 3 show the summary table of results. Of 15 studies, 11 (73%) were conducted in Europe [ 11 , 26 , 28 - 32 , 34 , 35 , 37 ], 3 (20%) in the United States [ 27 , 33 , 38 ], and 1 (7%) in Canada [ 36 ]. Of the 15 studies, 3 (20%) calculated the ICER [ 26 - 28 ], 1 (7%) calculated the cost-utility ratio [ 11 ], and 11 (73%) calculated the cost impact of telemonitoring. All studies analyzed the health care payer perspective, with 33% (5/15) analyzing the patient perspective [ 11 , 28 , 30 , 32 , 34 ], 13% (2/15) analyzing the societal perspective [ 33 , 35 ], and 13% (2/15) analyzing the provider perspective [ 13 , 30 ].
b RCT: randomized controlled trial.
c QALY: quality-adjusted life year.
d ICER: incremental cost-effectiveness ratio.
e SAVE-HM: Socio-Economic Effects and Cost Saving Potential of Remote Patient Monitoring.
f N/A: not applicable.
g ICD: implantable cardioverter-defibrillator.
h CRT-D: cardiac resynchronization therapy defibrillator.
i TARIFF: Health Economics Evaluation Registry for Remote Follow-Up.
j EVOLVO: Evolution of Management Strategies of Heart Failure Patients With Implantable Defibrillators.
k The values are statistically significant.
l MORE-CARE: Monitoring Resynchronization Devices and Cardiac Patients.
m QOL: quality of life.
n CONNECT: Clinical Evaluation of Remote Notification to Reduce Time to Clinical Decision.
o ECOST: Effectiveness and Cost of ICD Follow-Up Schedule With Telecardiology.
p SF-36: The 36-Item Short Form Survey.
q EuroEco: European Health Economic Trial on Home Monitoring in ICD Patients.
a If the perspective is health care system or patient, then cost and if the perspective is provider, then income .
b pp: per patient (in health care and patient perspectives) or per physician (in provider perspective).
c The values are statistically significant.
d SAVE-HM: Socio-Economic Effects and Cost Saving Potential of Remote Patient Monitoring.
e ED: emergency department.
f ICD: implantable cardioverter-defibrillator.
g CRT-D: cardiac resynchronization therapy defibrillator.
h TARIFF: Health Economics Evaluation Registry for Remote Follow-Up.
i EVOLVO: Evolution of Management Strategies of Heart Failure Patients With Implantable Defibrillators.
j MORE-CARE: Monitoring Resynchronization Devices and Cardiac Patients.
k Costs were recalculated per patient.
l CONNECT: Clinical Evaluation of Remote Notification to Reduce Time to Clinical Decision.
m ECOST: Effectiveness and Cost of ICD Follow-Up Schedule With Telecardiology.
n EuroEco: European Health Economic Trial on Home Monitoring in ICD Patients.
Intervention and Comparator
Telemonitoring entailed data transmission and data review. Table 4 shows the frequencies of data transmission, review, and in-office visits of the included studies. In 47% (7/15) of the studies, data were transmitted continuously or daily [ 26 , 28 , 30 , 31 , 34 , 35 ]; in 20% (3/15) studies, data were transmitted after a device alert [ 8 , 11 , 29 ]; and in 13% (2/15) studies, data were transmitted every 3 months [ 32 , 33 ]. In 20% (3/15) of the studies, data review was performed daily [ 28 , 34 , 35 ]; however, in 40% (6/15) of the studies, it was performed after a device alert was received [ 8 , 11 , 26 , 29 - 31 ]. Besides data transmission and review, telemonitoring included scheduled in-office visits. In 33% (5/15) of the studies, all scheduled in-office visits were based on the protocol [ 11 , 13 , 29 , 30 , 35 ]. In 7% (1/15) of the studies, at least 1 scheduled in-office visit was protocol based [ 37 ]. In 3 (20%) of the 15 studies, only 1 scheduled in-office visit was protocol based [ 32 - 34 ]. Protocol-based in-office visits are described in Table 4 .
b pp: per patient.
c TARIFF: Health Economics Evaluation Registry for Remote Follow-Up.
d EVOLVO: Evolution of Management Strategies of Heart Failure Patients With Implantable Defibrillators.
e MORE-CARE: Monitoring Resynchronization Devices and Cardiac Patients.
g ECOST: Effectiveness and Cost of ICD Follow-Up Schedule With Telecardiology.
h EuroEco: European Health Economic Trial on Home Monitoring in ICD Patients.
i Save-HM: Socio-Economic Effects and Cost Saving Potential of Remote Patient Monitoring.
j ICD: implantable-cardioverter defibrillator.
k N/A: not applicable.
Effectiveness
Effectiveness results of telemonitoring can be found in Table 2 . Of the 15 studies, 9 (60%) investigated a quality-adjusted life year (QALY) or quality of life (QOL) difference [ 11 , 26 - 30 , 33 , 34 ]. A total of 53% (8/15) of studies reported an increase in QALY or QOL [ 11 , 26 - 28 , 30 , 33 , 34 , 39 ], but the QALY or QOL increase was only statistically significant in 1 (13%; P =.03) of the 8 studies [ 26 - 28 , 30 , 33 , 34 ]. In contrast, only 1 (11%) of the 9 studies investigating QOL or QALY reported a significant decrease in QOL [ 29 ]. Comparing all studies, QALY differences ranged from 0.03 to 0.27 in patients with pacemakers and ranged from −1 to 0.64 in patients with ICD or CRT-D.
Besides QALY or QOL, several studies reported other health outcomes. Chew et al [ 36 ] indicated that the risk of death was lower with telemonitoring. Al-Khatib et al [ 33 ] reported that mortality and general patient satisfaction with telemonitoring were equal to those of standard care. Crossley et al [ 8 ] reported that the time between the clinical event and the clinical decision was 17.4 days shorter in patients with an ICD or CRT-D on telemonitoring than in those on standard care ( P <.001). Burri et al [ 31 ] indicated that telemonitoring patients with ICD or CRT-D led to fewer inappropriate shocks (−51%) and a reduction in battery exhaustion (−7%). Raatikainen et al [ 32 ] indicated that telemonitoring patients with an ICD reduced the average total time spent on device follow-up, with 17 minutes per patient per follow-up for physicians and 175 minutes per patient per follow-up for patients. Similarly, Dario et al [ 37 ] indicated that the time spent by physicians to treat the patient reduced by an average of 4.1 minutes per follow-up in patients with pacemakers and an average of 13.7 minutes per follow-up in patients with an ICD (SD was not reported).
Economic Impact
The results of the economic impact of telemonitoring are presented in Table 2 . Of the 15 studies, 4 (27%) investigated the cost impact of telemonitoring in patients with pacemakers [ 26 , 28 , 35 , 37 ]. From a health care payer perspective, 1 (25%) of the 4 pacemaker studies indicated that telemonitoring increased costs with US $2183 per patient per year (not statistically significant) mainly because of increased hospitalization costs [ 26 ]. A total of 2 (50%) of the 4 pacemaker studies indicated that telemonitoring reduced costs by US $8.9 and US $1054 per patient per year mainly because of a reduction in hospitalization and staff costs, respectively [ 28 , 37 ]. Therefore, hospitalizations reduced costs in the study by Dario et al [ 37 ] but increased costs in the study by Lopez-Villegas et al [ 26 ]. From a patient and societal perspective, the results indicated that telemonitoring reduced costs by US $11 and US $1113 per patient per year, respectively, mainly because of lower transport costs [ 28 , 35 ].
Of the 15 studies, 13 (87%) investigated the cost or income impact of telemonitoring in patients with an ICD or CRT-D [ 8 , 11 , 13 , 27 , 29 - 37 ]. A total of 11 (85%) of the 13 ICD or CRT-D studies investigated the cost impact of telemonitoring from a health care payer perspective, all indicating that telemonitoring reduced costs for patients with an ICD or CRT-D [ 8 , 11 , 13 , 27 , 29 - 32 , 34 , 36 , 37 ]. A total of 9 (82%) of the 11 health care payer perspective studies indicated that hospitalization was the largest driver for cost reduction for patients with an ICD or CRT-D [ 8 , 11 , 13 , 27 , 29 , 30 , 34 , 36 , 37 ]. The hospitalization cost reduced by up to US $912.3 per patient per year [ 34 ]. In addition, scheduled in-office visits were reported as a driver for cost reduction in 5 (45%) of the 11 health care payer perspective studies, as up to 61% of the total cost reduction was due to a decrease in the number of scheduled in-office visits [ 11 , 29 , 30 , 32 , 34 ]. Besides cost drivers that reduced costs, there were also drivers that increased costs. In 3 (27%) of the 11 health care payer perspective studies, unscheduled visits increased the total cost impact of telemonitoring [ 11 , 13 , 29 , 30 , 33 ]. A total of 3 (20%) of the 15 studies indicated that the cost reduction for scheduled in-office visits outweighed the cost increase for unscheduled in-office visits (−US $81.4 vs US $15.6, −US $45.4 vs US $7.8, and −US $44.1 vs US $14/patient/year) [ 11 , 29 , 30 ].
The results of 4 (31%) of the 13 ICD or CRT-D studies that investigated the cost impact of telemonitoring from the patients’ perspective [ 11 , 30 , 32 , 34 ] indicated that patient and caregiver loss of work or activity [ 30 ], scheduled in-office visits [ 11 ], and transport [ 34 ] were the largest drivers for cost reduction. The results of 2 (15%) of the 13 ICD or CRT-D studies that investigated the income impact of telemonitoring from a provider perspective indicated that the loss of reimbursed (scheduled) in-office visits was the most important factor for income loss due to telemonitoring [ 13 , 30 ], reducing income by up to €72.7 (US $77.21) per patient per year [ 30 ].
ICER and Cost-Utility Ratio
Results on ICER and the cost-utility ratio are presented in Table 2 . Of the 15 studies, 3 (20%) calculated the ICER from a health care payer perspective [ 26 - 28 ] and 1 (7%) calculated the cost-utility ratio from a health care payer perspective [ 11 ]. Of the 15 studies, 2 (13%) calculating ICER were conducted with patients with pacemakers [ 26 , 28 ]. Notably, of the 2 studies, 1 (50%) indicated that telemonitoring was cost-effective (ICER: US $270.09/QALY) [ 28 ], and 1 (50%) indicated that telemonitoring was not cost-effective (ICER: US $64,410/QALY) [ 26 ]. For patients with an ICD or CRT-D, of the 2 studies, 1 (50%) indicated that telemonitoring was cost-effective (ICER: US $12,069/QALY) [ 27 ] and 1 (50%) indicated that telemonitoring was dominant [ 11 ].
Critical Appraisal
The critical appraisal of the individual studies is provided in Tables 5 and 6 . Of the 15 studies, 1 (7%) was classified as excellent (score of 100%) [ 13 ], 8 (53%) had a good quality score (100%<score>75%) [ 26 , 28 , 30 , 31 , 33 , 34 , 36 , 37 ], and 6 (40%) had a moderate quality score (75%<score>50%) [ 8 , 11 , 27 , 29 , 32 , 35 ]. A total of 3 (20%) of the 15 studies scored the lowest, with 59% each [ 8 , 29 , 32 ]. More than 50% (>8/15) of the studies scored low for the items cost valuation (item 9) [ 11 , 27 - 32 , 34 , 35 , 37 ], discounting (item 14) [ 8 , 11 , 29 , 30 , 32 , 34 , 35 , 37 ], and no conflict of interest (item 18) [ 8 , 11 , 27 , 29 , 30 , 32 , 34 , 35 ]. All studies scored high on the items study population (item 1), study design (item 4), time horizon (item 10), outcome identification (item 11), outcome measurement (item 12), and ethics (item 19).
a EuroEco: European Health Economic Trial on Home Monitoring in ICD patients.
b TARIFF: Health Economics Evaluation Registry for Remote Follow-Up.
c ECOST: Effectiveness and Cost of ICD Follow-Up Schedule With Telecardiology.
d Sufficient attention was given to this aspect.
e Insufficient attention was given to this aspect.
a Save-HM: Socio-Economic Effects and Cost Saving Potential of Remote Patient Monitoring.
b EVOLVO: Evolution of Management Strategies of Heart Failure Patients With Implantable Defibrillators.
c MORE-CARE: Monitoring Resynchronization Devices and Cardiac Patients.
d CONNECT: Clinical Evaluation of Remote Notification to Reduce Time to Clinical Decision.
e Sufficient attention is given to this aspect.
f Insufficient attention is given to this aspect.
g N/A: not applicable.
Principal Findings and Comparison With Prior Work
The primary aim of this study was to investigate the cost-effectiveness of telemonitoring patients with an ICD or CRT-D and a pacemaker from different perspectives.
From a health care payer perspective, most studies indicated that telemonitoring was a cost-saving and effective alternative to standard care. The most important driver for cost reduction was hospitalizations, both in patients with a pacemaker and those with an ICD or CRT-D. The cost of hospitalizations was reduced by up to US $912.3 per patient per year [ 34 ]. Moreover, the reduction of scheduled in-office visits was the second most important cost-saving factor in most ICD or CRT-D studies, with up to 61% of the total cost reduction. Previous research indicated that up to 55% of the device follow-ups were routine checks with no actionable events or device programming [ 35 , 40 , 41 ]. Several researchers pointed out that most scheduled in-office visits could be replaced by telemonitoring without affecting the quality of care [ 7 , 34 ] and with potentially diagnosing >99.5% of arrhythmia and device problems [ 41 ]. Although scheduled in-office visits decreased, our results show that unscheduled in-office visits increased because of telemonitoring patients with an ICD or CRT-D, probably because of the possible faster detection of arrhythmia and device malfunction by telemonitoring [ 8 ]. However, in all studies analyzing both scheduled and unscheduled in-office visits, the cost reduction for scheduled in-office visits outweighed the cost increase for unscheduled in-office visits [ 11 , 29 , 30 ].
From a patient perspective, our results indicated that the reduction of professional activity, transport time, and costs due to scheduled in-office visits are the most important factors for cost reduction.
The provider perspective was investigated less frequently in the included studies, although it is very relevant. Owing to the reduction of scheduled in-office visits, providers will lose income with telemonitoring if no reimbursement exists for telemonitoring but only for in-office visits. As a result, providers will be stimulated to maintain the classic follow-up instead of telemonitoring. Of the 15 studies, 1 (7%) observed that the total cost for insurance payers does not increase in countries where telemonitoring is reimbursed [ 13 ]. As telemonitoring decreases the overall costs from a health care payer perspective, there is room for proper compensation for providers to transition from in-office care to remote care. Hence, correct compensation (which is possible while still saving on the overall health care cost) will stimulate providers to switch to telemonitoring as the desired care path for patients with a CIED.
All studies reported the effectiveness of telemonitoring. Of the 15 studies, 9 (60%) indicated a QALY or QOL difference. Furthermore, 89% (8/9) of these studies indicated an increase in QALYs or QOL for telemonitoring patients with pacemakers or ICD or CRT-D, ranging from −1 to 0.64. Some studies (3/9, 33%) indicated this QALY or QOL increase was the result of the reduced routine in-office visits [ 7 , 34 ]. However, the QALY or QOL increase was only statistically significant (and positive) in 1 (11%) of the 9 studies [ 11 ]. Nevertheless, patient questionnaires have demonstrated a high acceptance of telemonitoring among patients with pacemakers and those with ICDs [ 39 ]. Moreover, telemonitoring is reported to lead to an increased sense of security [ 39 ]. Furthermore, the results indicated that telemonitoring leads to fewer inappropriate shocks, an important determinant of QALY, in patients with an ICD or CRT-D [ 31 ].
The cost-effectiveness analyses may be sensitive to the heterogeneity among the organization of telemonitoring in different hospitals. This may include different devices, the number of transmissions, the configuration of alerts, and hospital visit scheduling [ 26 ]. It seems reasonable to expect that the efficiency of telemonitoring not only depends on the technology but also on the organization of the service. If hospitals see telemonitoring as an additional service, on top of standard care, less cost-savings may be seen than if hospitals see telemonitoring as a substitute for standard care. A radical organizational change could lead to larger cost-savings, as suggested by an observational study by Facchin et al [ 42 ]. Moreover, such radical change may include a strategy involving other physicians, such as general practitioners, and referring cardiologists, that is, an integrated health care delivery [ 37 ].
Furthermore, the comparison between studies is challenged by differences in study design. The Poniente study by Bautista-Mesa et al [ 28 ] followed up patients with pacemakers for 12 months and indicated a QALY increase of 0.09 for telemonitoring. However, after 5 years of follow-up, the results indicated a QALY decrease of 0.20 for telemonitoring. Bautista-Mesa et al [ 28 ] indicated that some of the telemonitoring benefits (eg, reduction of in-office visits) may not be appreciated in the long term. Therefore, the evolution of utilities may be different depending on the follow-up time. In addition, the results indicated that hospitalizations reduced costs in the study by Dario et al [ 37 ] but increased costs in the study by Lopez-Villegas et al [ 26 ]. This discrepancy might be explained because significantly fewer patients were included in the study by Lopez-Villegas et al (50 vs 2101 patients). None of the 25 patients in the conventional follow-up group were hospitalized, whereas 12% (3/25) of the patients were hospitalized in the remotely monitored group (all for pacemaker problems) [ 26 ]. Furthermore, the included studies relied disproportionally on male participants, except for the Nordland study [ 26 ]. This may be explained by the significant sex disparity in ICD implantation rates, pointed out by Ingelaere et al [ 43 ]. Ingelaere et al [ 43 ] could not completely explain these differences by prevalence differences of cardiomyopathies and imply a possible undertreatment of women. Another study [ 44 ] observed an undertreatment of women with coronary heart disease, as they are less likely to undergo coronary angiography. Therefore, men may undergo more expensive treatments than women. This can explain why the included cost-effectiveness studies may present an overly positive result. In addition, time differences may impact the quality and cost-effectiveness of telemonitoring, as telemonitoring may evolve over time. However, our results did not provide meaningful insights in this respect.
The cost-effectiveness analyses may be sensitive to the heterogeneity among health care systems. From a provider perspective, our results indicated that telemonitoring generates lesser profit than standard care in the absence of reimbursement. Therefore, the lack of reimbursement is generally perceived as a major implementation barrier to telemonitoring, affecting 80% of the centers [ 45 ]. Consequently, providers tend to continue with standard care instead of telemonitoring. However, from a health care payer perspective, our results indicated that telemonitoring was still cost-saving even with reimbursement [ 13 , 34 ]. To stimulate providers to use telemonitoring, provider compensation should be provided based on overall health care cost-savings, making telemonitoring possible if it is preferred as the way to deliver CIED follow-up care.
Limitations
Because of the large discrepancies between health care systems’ organization, costs, access, delivery, quality, and reimbursement of cardiac care, any generalization may be perceived as inaccurate [ 37 , 46 ]. For instance, the included studies were mainly performed in Western countries. The results may not be generalizable to non-Western countries. Therefore, the cost-effectiveness results are contingent on the context in which they were analyzed [ 46 ]. Another limitation of this research is that 40% (6/15) of the included studies are not randomized controlled trials. These studies may have unobserved confounding factors that cannot be controlled for. Finally, cost analyses were excluded in this study because of our research objective. However, future cost analyses could draw a lot of information from analyzing these excluded studies.
Conclusions
Telemonitoring patients with CIED may be a cost-effective alternative to standard follow-up. Moreover, telemonitoring may lead to a cost reduction from a health care and patient perspective, mainly by the reduction of hospitalizations and scheduled in-office visits. Owing to the reduction in scheduled in-office visits, providers’ income tends to decrease when implementing telemonitoring without proper reimbursement. Introducing appropriate reimbursement could make telemonitoring sustainable for providers, while still being cost-effective from a health care payer perspective.
Acknowledgments
The authors would like to thank Dr Ingrid Kremer of Maastricht University for her help in the manuscript review. This work was supported by the Fund for Scientific Research Flanders (Fonds Wetenschappelijk Onderzoek Vlaanderen, grant 1SC9322N, 2021). RW is supported as a postdoctoral clinical researcher by the Fund for Scientific Research Flanders (Fonds Wetenschappelijk Onderzoek Vlaanderen).
Data Availability
All data generated or analyzed during this study are included in this published article and its multimedia appendices.
Authors' Contributions
SR and LA were responsible for the conceptualization of the manuscript. SR, LA, RW, and HH acquired the financial support necessary for this paper and developed the methodology. SR analyzed and investigated the data. AP, LA, RW, and HH validated the results. SR was responsible for the first and final drafts. LA, RW, and HH were involved in editing the drafts. All authors approved the final manuscript.
Conflicts of Interest
RW reports research funding from Abbott, Biotronik, Boston Scientific, and Medtronic and speakers and consultancy fees from Medtronic, Boston Scientific, Biotronik, and Abbott. None of these payments were personal; all were handled through the University of Leuven. HH received personal lecture and consultancy fees from Abbott, Biotronik, Daiichi-Sankyo, Pfizer-BMS, Medscape, and Springer Healthcare Limited. He received unconditional research grants through the University of Antwerp and the University of Hasselt from Abbott, Bayer, Biotronik, Biosense Webster, Boston Scientific, Boehringer Ingelheim, Daicchi-Sankyo, Fibricheck or Qompium, Medtronic, and Pfizer-BMS, all outside the scope of this work. All other authors declare no other conflicts of interest.
PRISMA-ScR (Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews) checklist.
Search strategy.
Interpretation Consensus Health Economic Criteria list.
- Blomström-Lundqvist C, Traykov V, Erba PA, Burri H, Nielsen JC, Bongiorni MG, et al. European Heart Rhythm Association (EHRA) international consensus document on how to prevent, diagnose, and treat cardiac implantable electronic device infections-endorsed by the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), the Latin American Heart Rhythm Society (LAHRS), International Society for Cardiovascular Infectious Diseases (ISCVID) and the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Europace. Apr 01, 2020;22(4):515-549. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Wilkoff BL, Auricchio A, Brugada J, Cowie M, Ellenbogen KA, Gillis AM, et al. HRS/EHRA expert consensus on the monitoring of cardiovascular implantable electronic devices (CIEDs): description of techniques, indications, personnel, frequency and ethical considerations. Heart Rhythm. Jun 2008;5(6):907-925. [ CrossRef ] [ Medline ]
- de la Torre Díez I, Garcia-Zapirain B, Méndez-Zorrilla A, López-Coronado M. Monitoring and follow-up of chronic heart failure: a literature review of eHealth applications and systems. J Med Syst. Jul 2016;40(7):179. [ CrossRef ] [ Medline ]
- Guédon-Moreau L, Lacroix D, Sadoul N, Clémenty J, Kouakam C, Hermida J, et al. A randomized study of remote follow-up of implantable cardioverter defibrillators: safety and efficacy report of the ECOST trial. Eur Heart J. Feb 2013;34(8):605-614. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Ricci RP, Morichelli L, Santini M. Remote control of implanted devices through Home Monitoring technology improves detection and clinical management of atrial fibrillation. Europace. Jan 2009;11(1):54-61. [ FREE Full text ] [ CrossRef ] [ Medline ]
- García-Fernández FJ, Osca Asensi J, Romero R, Fernández Lozano I, Larrazabal JM, Martínez Ferrer J, et al. Safety and efficiency of a common and simplified protocol for pacemaker and defibrillator surveillance based on remote monitoring only: a long-term randomized trial (RM-ALONE). Eur Heart J. Jun 14, 2019;40(23):1837-1846. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Varma N, Epstein AE, Irimpen A, Schweikert R, Love C. Efficacy and safety of automatic remote monitoring for implantable cardioverter-defibrillator follow-up: the Lumos-T Safely Reduces Routine Office Device Follow-up (TRUST) trial. Circulation. Jul 27, 2010;122(4):325-332. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Crossley GH, Boyle A, Vitense H, Chang Y, Mead RH. The CONNECT (Clinical Evaluation of Remote Notification to Reduce Time to Clinical Decision) trial: the value of wireless remote monitoring with automatic clinician alerts. J Am Coll Cardiol. Mar 8, 2011;57(10):1181-1189. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Catalan-Matamoros D, Lopez-Villegas A, Tore-Lappegard K, Lopez-Liria R. Patients' experiences of remote communication after pacemaker implant: The NORDLAND study. PLoS One. 2019;14(6):e0218521. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Masella C, Zanaboni P, Di Stasi F, Gilardi S, Ponzi P, Valsecchi S. Assessment of a remote monitoring system for implantable cardioverter defibrillators. J Telemed Telecare. 2008;14(6):290-294. [ CrossRef ] [ Medline ]
- Zanaboni P, Landolina M, Marzegalli M, Lunati M, Perego GB, Guenzati G, et al. Cost-utility analysis of the EVOLVO study on remote monitoring for heart failure patients with implantable defibrillators: randomized controlled trial. J Med Internet Res. 2013;15(5):e106. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Annemans L. Health Economics for Non-economists: An Introduction to the Concepts, Methods and Pitfalls of Health Economic Evaluations. Kalmthout, Belgium. Pelckmans Pro; 2018.
- Heidbuchel H, Hindricks G, Broadhurst P, Van Erven L, Fernandez-Lozano I, Rivero-Ayerza M, et al. EuroEco (European Health Economic Trial on Home Monitoring in ICD Patients): a provider perspective in five European countries on costs and net financial impact of follow-up with or without remote monitoring. Eur Heart J. Jan 14, 2015;36(3):158-169. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Klersy C, Boriani G, De Silvestri A, Mairesse GH, Braunschweig F, Scotti V, et al. Effect of telemonitoring of cardiac implantable electronic devices on healthcare utilization: a meta-analysis of randomized controlled trials in patients with heart failure. Eur J Heart Fail. Feb 2016;18(2):195-204. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. Mar 29, 2021;372:n71. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. Oct 02, 2018;169(7):467-473. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Wijnen B, Van Mastrigt G, Redekop WK, Majoie H, De Kinderen R, Evers S. How to prepare a systematic review of economic evaluations for informing evidence-based healthcare decisions: data extraction, risk of bias, and transferability (part 3/3). Expert Rev Pharmacoecon Outcomes Res. Dec 2016;16(6):723-732. [ CrossRef ] [ Medline ]
- Search strategies: the centre for reviews and dissemination. University of York. 2018. URL: http://www.crd.york.ac.uk/crdweb/searchstrategies.asp [accessed 2024-07-01]
- Auener SL, Remers TE, van Dulmen SA, Westert GP, Kool RB, Jeurissen PP. The effect of noninvasive telemonitoring for chronic heart failure on health care utilization: systematic review. J Med Internet Res. Sep 29, 2021;23(9):e26744. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Lopez-Villegas A, Leal-Costa C, Perez-Heredia M, Villegas-Tripiana I, Catalán-Matamoros D. Knowledge update on the economic evaluation of pacemaker telemonitoring systems. Int J Environ Res Public Health. Nov 18, 2021;18(22):12120. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Vehmeijer JT, Brouwer TF, Limpens J, Knops RE, Bouma BJ, Mulder BJ, et al. Implantable cardioverter-defibrillators in adults with congenital heart disease: a systematic review and meta-analysis. Eur Heart J. May 07, 2016;37(18):1439-1448. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Scott AM, Forbes C, Clark J, Carter M, Glasziou P, Munn Z. Systematic review automation tools improve efficiency but lack of knowledge impedes their adoption: a survey. J Clin Epidemiol. Oct 2021;138:80-94. [ CrossRef ] [ Medline ]
- Evers S, Goossens M, de VH, van TM, Ament A. Criteria list for assessment of methodological quality of economic evaluations: consensus on Health Economic Criteria. Int J Technol Assess Health Care. 2005;21(2):240-245. [ Medline ]
- Jiang X, Ming W, You JH. The cost-effectiveness of digital health interventions on the management of cardiovascular diseases: systematic review. J Med Internet Res. Jun 17, 2019;21(6):e13166. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Consumptieprijsindex. Statbel. URL: https://statbel.fgov.be/nl/themas/consumptieprijsindex/consumptieprijsindex#figures [accessed 2022-07-08]
- Lopez-Villegas A, Catalan-Matamoros D, Peiro S, Lappegard KT, Lopez-Liria R. Cost-utility analysis of telemonitoring versus conventional hospital-based follow-up of patients with pacemakers. The NORDLAND randomized clinical trial. PLoS One. 2020;15(1):e0226188. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Hummel JP, Leipold RJ, Amorosi SL, Bao H, Deger KA, Jones PW, et al. Outcomes and costs of remote patient monitoring among patients with implanted cardiac defibrillators: an economic model based on the PREDICT RM database. J Cardiovasc Electrophysiol. Jul 2019;30(7):1066-1077. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Bautista-Mesa RJ, Lopez-Villegas A, Peiro S, Catalan-Matamoros D, Robles-Musso E, Lopez-Liria R, et al. Long-term cost-utility analysis of remote monitoring of older patients with pacemakers: the PONIENTE study. BMC Geriatr. Nov 16, 2020;20(1):474. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Boriani G, Da Costa A, Quesada A, Ricci RP, Favale S, Boscolo G, et al. Effects of remote monitoring on clinical outcomes and use of healthcare resources in heart failure patients with biventricular defibrillators: results of the MORE-CARE multicentre randomized controlled trial. Eur J Heart Fail. Mar 2017;19(3):416-425. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Ricci RP, Vicentini A, D'Onofrio A, Sagone A, Rovaris G, Padeletti L, et al. Economic analysis of remote monitoring of cardiac implantable electronic devices: results of the health economics evaluation registry for remote follow-up (TARIFF) study. Heart Rhythm. Jan 2017;14(1):50-57. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Burri H, Sticherling C, Wright D, Makino K, Smala A, Tilden D. Cost-consequence analysis of daily continuous remote monitoring of implantable cardiac defibrillator and resynchronization devices in the UK. Europace. Nov 2013;15(11):1601-1608. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Raatikainen MJ, Uusimaa P, van Ginneken MM, Janssen JP, Linnaluoto M. Remote monitoring of implantable cardioverter defibrillator patients: a safe, time-saving, and cost-effective means for follow-up. Europace. Oct 2008;10(10):1145-1151. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Al-Khatib SM, Piccini JP, Knight D, Stewart M, Clapp-Channing N, Sanders GD. Remote monitoring of implantable cardioverter defibrillators versus quarterly device interrogations in clinic: results from a randomized pilot clinical trial. J Cardiovasc Electrophysiol. May 2010;21(5):545-550. [ CrossRef ] [ Medline ]
- Guédon-Moreau L, Lacroix D, Sadoul N, Clémenty J, Kouakam C, Hermida J, et al. Costs of remote monitoring vs. ambulatory follow-ups of implanted cardioverter defibrillators in the randomized ECOST study. Europace. Aug 2014;16(8):1181-1188. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Perl S, Stiegler P, Rotman B, Prenner G, Lercher P, Anelli-Monti M, et al. Socio-economic effects and cost saving potential of remote patient monitoring (SAVE-HM trial). Int J Cardiol. Nov 30, 2013;169(6):402-407. [ CrossRef ] [ Medline ]
- Chew DS, Zarrabi M, You I, Morton J, Low A, Reyes L, et al. Clinical and economic outcomes associated with remote monitoring for cardiac implantable electronic devices: a population-based analysis. Can J Cardiol. Jun 2022;38(6):736-744. [ CrossRef ] [ Medline ]
- Dario C, Delise P, Gubian L, Saccavini C, Brandolino G, Mancin S. Large controlled observational study on remote monitoring of pacemakers and implantable cardiac defibrillators: a clinical, economic, and organizational evaluation. Interact J Med Res. Jan 13, 2016;5(1):e4. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Crossley G, Boyle A, Vitense H, Sherfesee L, Mead RH. Trial design of the clinical evaluation of remote notification to reduce time to clinical decision: the clinical evaluation Of remote NotificatioN to rEduCe Time to clinical decision (CONNECT) study. Am Heart J. Nov 2008;156(5):840-846. [ CrossRef ] [ Medline ]
- Ricci RP, Morichelli L, Quarta L, Sassi A, Porfili A, Laudadio MT, et al. Long-term patient acceptance of and satisfaction with implanted device remote monitoring. Europace. May 2010;12(5):674-679. [ CrossRef ] [ Medline ]
- Boriani G, Auricchio A, Klersy C, Kirchhof P, Brugada J, Morgan J, et al. Healthcare personnel resource burden related to in-clinic follow-up of cardiovascular implantable electronic devices: a European Heart Rhythm Association and Eucomed joint survey. Europace. Aug 2011;13(8):1166-1173. [ CrossRef ] [ Medline ]
- Heidbüchel H, Lioen P, Foulon S, Huybrechts W, Ector J, Willems R, et al. Potential role of remote monitoring for scheduled and unscheduled evaluations of patients with an implantable defibrillator. Europace. Mar 2008;10(3):351-357. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Facchin D, Baccillieri MS, Gasparini G, Zoppo F, Allocca G, Brieda M, et al. Findings of an observational investigation of pure remote follow-up of pacemaker patients: is the in-clinic device check still needed? Int J Cardiol. Oct 01, 2016;220:781-786. [ CrossRef ] [ Medline ]
- Ingelaere S, Hoffmann R, Guler I, Vijgen J, Mairesse GH, Blankoff I, et al. Inequality between women and men in ICD implantation. Int J Cardiol Heart Vasc. Aug 2022;41:101075. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Steingart RM, Packer M, Hamm P, Coglianese ME, Gersh B, Geltman EM, et al. Sex differences in the management of coronary artery disease. Survival and Ventricular Enlargement Investigators. N Engl J Med. Jul 25, 1991;325(4):226-230. [ CrossRef ] [ Medline ]
- Mairesse GH, Braunschweig F, Klersy K, Cowie MR, Leyva F. Implementation and reimbursement of remote monitoring for cardiac implantable electronic devices in Europe: a survey from the health economics committee of the European Heart Rhythm Association. Europace. May 2015;17(5):814-818. [ CrossRef ] [ Medline ]
- Lucà F, Cipolletta L, Di Fusco SA, Iorio A, Pozzi A, Rao CM, et al. Remote monitoring: doomed to let down or an attractive promise? IJC Heart Vasculature. Sep 2019;24:100380. [ CrossRef ]
Abbreviations
Edited by T Leung; submitted 28.03.23; peer-reviewed by P Jeurissen, B Dechert; comments to author 07.09.23; revised version received 13.09.23; accepted 13.02.24; published 19.04.24.
©Sarah Raes, Andrea Prezzi, Rik Willems, Hein Heidbuchel, Lieven Annemans. Originally published in the Journal of Medical Internet Research (https://www.jmir.org), 19.04.2024.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work, first published in the Journal of Medical Internet Research, is properly cited. The complete bibliographic information, a link to the original publication on https://www.jmir.org/, as well as this copyright and license information must be included.

IMAGES
VIDEO
COMMENTS
Checklist: Research paper 0 / 14. I have followed all instructions in the assignment sheet. My introduction presents my topic in an engaging way and provides necessary background information.. My introduction presents a clear, focused research problem and/or thesis statement.. My paper is logically organized using paragraphs and (if relevant) section headings.
This checklist corresponds to the writing and formatting guidelines described in full in the Publication Manual of the American Psychological Association (7th ed.). Refer to the following chapters for specific information: paper elements and format in Chapter 2. writing style and grammar in Chapter 4. bias-free language in Chapter 5.
A research paper checklist is an essential tool because the task of putting together a quality paper involves many steps. Nobody writes a perfect report in one sitting! Before you get started on your project, you should review the checklist on research ethics . Later, once you have finished the final draft of your research paper, you can use ...
Use this checklist to help you write a beginner-friendly student paper in seventh edition APA Style, consisting of a title page, text, and reference list. If your paper has more elements, such as tables and figures, use the Publication Manual checklist or the Concise Guide checklist. Links in this checklist lead to free resources on the APA ...
This checklist is to help you along your research paper writing process. Make sure you read and understand the specific requirements and instructions that are specific to your paper. It is a good idea to have professional paper editors review your work, to make sure you submit original content and that your work is formatted correctly and free ...
Research papers are hard. As tempting as it may be to just hand in your paper the second you finish that last citation, it is super important to review everything to make sure you don't have any silly mistakes! Use this 10-step checklist to make sure your paper is in top-notch form: Credit and cite all information from other sources.
Checklist for Research Papers. Do I have a sufficient number of sources? Are a significant number of my sources critical sources (e.g., from academic journals)? Are my sources integrated smoothly into the paper? Is there a dialogue between my own analysis of the text and the research I'm including?
By following this comprehensive 15-point academic writing checklist, PhD students and researchers can optimize their research paper writing and ensure they consistently deliver coherent, well-structured manuscripts that meet the high standards expected by journal editors. Paperpal is an AI writing assistant that help academics write better ...
Checklist: Writing a Research Article. The title emphasizes what is most important about the paper (often the main conclusion but sometimes also the methods used) The results are described in a logical order that fits into a scientific narrative (and not necessarily the order with which you performed the experiments) The abstract reads like a ...
Research Paper Checklist Below is a checklist for completing a research paper. You should remember that the process of researching and writing is very malleable. It depends on many factors: the assignment requirements, the field of study, the topic, and your personal preferences. This checklist is designed to give you a basic overview of the ...
Research Paper Checklist - In a Nutshell. A research paper checklist is a document that lists the required criteria for writing a thesis.; It is a guiding tool that allows you to express your research ideas logically and systematically.; A research paper checklist provides an overview of the writing process, enabling proper planning.
Use this article submission checklist to find out. By the time you're ready to submit your paper to a journal, there are a lot of things you'll need to have checked, understood, and incorporated into your article. We've created this checklist to help you make sure that you don't miss anything important, both in writing and preparing ...
Do not use a period after your title or after any heading in the paper (e.g., Works Cited). Begin your text on a new, double-spaced line after the title, indenting the first line of the paragraph half an inch from the left margin. Fig. 1. The top of the first page of a research paper.
Many research papers suffer from rough transitions; they shift from one topic to another abruptly, without adequately warning the reader that a transition is about to take place. Or, a ... This abbreviated checklist incorporates the detailed items in the above list, and is what I will hand back to you with your various writing samples, to give ...
Education. 36 tasks. By Nicole Nichols-West on Jan 2, 2012. Choose a Topic. Pick a topic which interests and challenges you. Focus on a limited aspect. Obtain teacher approval for your topic. Re-read your assignment sheet carefully to be certain you know what is expected. Select a subject you can manage.
Table of contents. Step 1: Introduce your topic. Step 2: Describe the background. Step 3: Establish your research problem. Step 4: Specify your objective (s) Step 5: Map out your paper. Research paper introduction examples. Frequently asked questions about the research paper introduction.
eligibility, confirmed eligible, included in the study, completing follow-up, and analyzed. (b) Give reasons for nonparticipation at each stage. (c) Consider use of a flow diagram. Descriptive ...
What are the five checklist components of a research paper? A full research paper in APA format reporting on experimental research will typically include a title page, abstract, introduction, methods, results, discussion, and references sections. These parts of a research paper are crucial to include because the formulated thesis study is ...
Background: Telemonitoring patients with cardiac implantable electronic devices (CIEDs) can improve their care management. However, the results of cost-effectiveness studies are heterogeneous. Therefore, it is still a matter of debate whether telemonitoring is worth the investment. Objective: This systematic review aims to investigate the cost-effectiveness of telemonitoring patients with ...