Cardiovascular Ultrasound strongly encourages that all datasets on which the conclusions of the paper rely should be available to readers. We encourage authors to ensure that their datasets are either deposited in publicly available repositories (where available and appropriate) or presented in the main manuscript or additional supporting files whenever possible. Please see Springer Nature’s information on recommended repositories .
Authors who need help depositing and curating data may wish to consider contacting our Research Data Support Helpdesk .
Graphical Abstracts
Authors of Research articles are required to submit a graphical abstract (schematic figure) as part of the article, in addition to the text abstract. The graphical abstract should summarize in a visual or conceptual manner the key message of the paper and allow the reader to understand the essence of the study. The graphical abstract may incorporate multiple panels including key figures (numbers, percentages) or graphics, or short text lists summarising key points or variables. The graphical abstract should be submitted for peer review as a separate Additional file. The file should be clearly named, e.g. graphical_abstract.tiff. The graphical abstract should be submitted in a landscape format.

Preparing your manuscript
The information below details the section headings that you should include in your manuscript and what information should be within each section.
Please note that your manuscript must include a 'Declarations' section including all of the subheadings (please see below for more information).
The title page should:
- "A versus B in the treatment of C: a randomized controlled trial", "X is a risk factor for Y: a case control study", "What is the impact of factor X on subject Y: A systematic review"
- or for non-clinical or non-research studies a description of what the article reports
- if a collaboration group should be listed as an author, please list the Group name as an author. If you would like the names of the individual members of the Group to be searchable through their individual PubMed records, please include this information in the “Acknowledgements” section in accordance with the instructions below
- Large Language Models (LLMs), such as ChatGPT , do not currently satisfy our authorship criteria . Notably an attribution of authorship carries with it accountability for the work, which cannot be effectively applied to LLMs. Use of an LLM should be properly documented in the Methods section (and if a Methods section is not available, in a suitable alternative part) of the manuscript.
- indicate the corresponding author
The Abstract should not exceed 350 words. Please minimize the use of abbreviations and do not cite references in the abstract. Reports of randomized controlled trials should follow the CONSORT extension for abstracts. The abstract must include the following separate sections:
- Background: the context and purpose of the study
- Methods: how the study was performed and statistical tests used
- Results: the main findings
- Conclusions: brief summary and potential implications
- Trial registration: If your article reports the results of a health care intervention on human participants, it must be registered in an appropriate registry and the registration number and date of registration should be stated in this section. If it was not registered prospectively (before enrollment of the first participant), you should include the words 'retrospectively registered'. See our editorial policies for more information on trial registration
Three to ten keywords representing the main content of the article.
The Background section should explain the background to the study, its aims, a summary of the existing literature and why this study was necessary or its contribution to the field.
The methods section should include:
- the aim, design and setting of the study
- the characteristics of participants or description of materials
- a clear description of all processes, interventions and comparisons. Generic drug names should generally be used. When proprietary brands are used in research, include the brand names in parentheses
- the type of statistical analysis used, including a power calculation if appropriate
This should include the findings of the study including, if appropriate, results of statistical analysis which must be included either in the text or as tables and figures.
This section should discuss the implications of the findings in context of existing research and highlight limitations of the study.
Conclusions
This should state clearly the main conclusions and provide an explanation of the importance and relevance of the study reported.
List of abbreviations
If abbreviations are used in the text they should be defined in the text at first use, and a list of abbreviations should be provided.
Declarations
All manuscripts must contain the following sections under the heading 'Declarations':
Ethics approval and consent to participate
Consent for publication, availability of data and materials, competing interests, authors' contributions, acknowledgements.
- Authors' information (optional)
Please see below for details on the information to be included in these sections.
If any of the sections are not relevant to your manuscript, please include the heading and write 'Not applicable' for that section.
Manuscripts reporting studies involving human participants, human data or human tissue must:
- include a statement on ethics approval and consent (even where the need for approval was waived)
- include the name of the ethics committee that approved the study and the committee’s reference number if appropriate
Studies involving animals must include a statement on ethics approval and for experimental studies involving client-owned animals, authors must also include a statement on informed consent from the client or owner.
See our editorial policies for more information.
If your manuscript does not report on or involve the use of any animal or human data or tissue, please state “Not applicable” in this section.
If your manuscript contains any individual person’s data in any form (including any individual details, images or videos), consent for publication must be obtained from that person, or in the case of children, their parent or legal guardian. All presentations of case reports must have consent for publication.
You can use your institutional consent form or our consent form if you prefer. You should not send the form to us on submission, but we may request to see a copy at any stage (including after publication).
See our editorial policies for more information on consent for publication.
If your manuscript does not contain data from any individual person, please state “Not applicable” in this section.
All manuscripts must include an ‘Availability of data and materials’ statement. Data availability statements should include information on where data supporting the results reported in the article can be found including, where applicable, hyperlinks to publicly archived datasets analysed or generated during the study. By data we mean the minimal dataset that would be necessary to interpret, replicate and build upon the findings reported in the article. We recognise it is not always possible to share research data publicly, for instance when individual privacy could be compromised, and in such instances data availability should still be stated in the manuscript along with any conditions for access.
Authors are also encouraged to preserve search strings on searchRxiv https://searchrxiv.org/ , an archive to support researchers to report, store and share their searches consistently and to enable them to review and re-use existing searches. searchRxiv enables researchers to obtain a digital object identifier (DOI) for their search, allowing it to be cited.
Data availability statements can take one of the following forms (or a combination of more than one if required for multiple datasets):
- The datasets generated and/or analysed during the current study are available in the [NAME] repository, [PERSISTENT WEB LINK TO DATASETS]
- The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
- All data generated or analysed during this study are included in this published article [and its supplementary information files].
- The datasets generated and/or analysed during the current study are not publicly available due [REASON WHY DATA ARE NOT PUBLIC] but are available from the corresponding author on reasonable request.
- Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
- The data that support the findings of this study are available from [third party name] but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of [third party name].
- Not applicable. If your manuscript does not contain any data, please state 'Not applicable' in this section.
More examples of template data availability statements, which include examples of openly available and restricted access datasets, are available here .
BioMed Central strongly encourages the citation of any publicly available data on which the conclusions of the paper rely in the manuscript. Data citations should include a persistent identifier (such as a DOI) and should ideally be included in the reference list. Citations of datasets, when they appear in the reference list, should include the minimum information recommended by DataCite and follow journal style. Dataset identifiers including DOIs should be expressed as full URLs. For example:
Hao Z, AghaKouchak A, Nakhjiri N, Farahmand A. Global integrated drought monitoring and prediction system (GIDMaPS) data sets. figshare. 2014. http://dx.doi.org/10.6084/m9.figshare.853801
With the corresponding text in the Availability of data and materials statement:
The datasets generated during and/or analysed during the current study are available in the [NAME] repository, [PERSISTENT WEB LINK TO DATASETS]. [Reference number]
If you wish to co-submit a data note describing your data to be published in BMC Research Notes , you can do so by visiting our submission portal . Data notes support open data and help authors to comply with funder policies on data sharing. Co-published data notes will be linked to the research article the data support ( example ).
All financial and non-financial competing interests must be declared in this section.
See our editorial policies for a full explanation of competing interests. If you are unsure whether you or any of your co-authors have a competing interest please contact the editorial office.
Please use the authors initials to refer to each authors' competing interests in this section.
If you do not have any competing interests, please state "The authors declare that they have no competing interests" in this section.
All sources of funding for the research reported should be declared. If the funder has a specific role in the conceptualization, design, data collection, analysis, decision to publish, or preparation of the manuscript, this should be declared.
The individual contributions of authors to the manuscript should be specified in this section. Guidance and criteria for authorship can be found in our editorial policies .
Please use initials to refer to each author's contribution in this section, for example: "FC analyzed and interpreted the patient data regarding the hematological disease and the transplant. RH performed the histological examination of the kidney, and was a major contributor in writing the manuscript. All authors read and approved the final manuscript."
Please acknowledge anyone who contributed towards the article who does not meet the criteria for authorship including anyone who provided professional writing services or materials.
Authors should obtain permission to acknowledge from all those mentioned in the Acknowledgements section.
See our editorial policies for a full explanation of acknowledgements and authorship criteria.
If you do not have anyone to acknowledge, please write "Not applicable" in this section.
Group authorship (for manuscripts involving a collaboration group): if you would like the names of the individual members of a collaboration Group to be searchable through their individual PubMed records, please ensure that the title of the collaboration Group is included on the title page and in the submission system and also include collaborating author names as the last paragraph of the “Acknowledgements” section. Please add authors in the format First Name, Middle initial(s) (optional), Last Name. You can add institution or country information for each author if you wish, but this should be consistent across all authors.
Please note that individual names may not be present in the PubMed record at the time a published article is initially included in PubMed as it takes PubMed additional time to code this information.
Authors' information
This section is optional.
You may choose to use this section to include any relevant information about the author(s) that may aid the reader's interpretation of the article, and understand the standpoint of the author(s). This may include details about the authors' qualifications, current positions they hold at institutions or societies, or any other relevant background information. Please refer to authors using their initials. Note this section should not be used to describe any competing interests.
Footnotes can be used to give additional information, which may include the citation of a reference included in the reference list. They should not consist solely of a reference citation, and they should never include the bibliographic details of a reference. They should also not contain any figures or tables.
Footnotes to the text are numbered consecutively; those to tables should be indicated by superscript lower-case letters (or asterisks for significance values and other statistical data). Footnotes to the title or the authors of the article are not given reference symbols.
Always use footnotes instead of endnotes.
Examples of the Vancouver reference style are shown below.
See our editorial policies for author guidance on good citation practice
Web links and URLs: All web links and URLs, including links to the authors' own websites, should be given a reference number and included in the reference list rather than within the text of the manuscript. They should be provided in full, including both the title of the site and the URL, as well as the date the site was accessed, in the following format: The Mouse Tumor Biology Database. http://tumor.informatics.jax.org/mtbwi/index.do . Accessed 20 May 2013. If an author or group of authors can clearly be associated with a web link, such as for weblogs, then they should be included in the reference.
Example reference style:
Article within a journal
Smith JJ. The world of science. Am J Sci. 1999;36:234-5.
Article within a journal (no page numbers)
Rohrmann S, Overvad K, Bueno-de-Mesquita HB, Jakobsen MU, Egeberg R, Tjønneland A, et al. Meat consumption and mortality - results from the European Prospective Investigation into Cancer and Nutrition. BMC Medicine. 2013;11:63.
Article within a journal by DOI
Slifka MK, Whitton JL. Clinical implications of dysregulated cytokine production. Dig J Mol Med. 2000; doi:10.1007/s801090000086.
Article within a journal supplement
Frumin AM, Nussbaum J, Esposito M. Functional asplenia: demonstration of splenic activity by bone marrow scan. Blood 1979;59 Suppl 1:26-32.
Book chapter, or an article within a book
Wyllie AH, Kerr JFR, Currie AR. Cell death: the significance of apoptosis. In: Bourne GH, Danielli JF, Jeon KW, editors. International review of cytology. London: Academic; 1980. p. 251-306.
OnlineFirst chapter in a series (without a volume designation but with a DOI)
Saito Y, Hyuga H. Rate equation approaches to amplification of enantiomeric excess and chiral symmetry breaking. Top Curr Chem. 2007. doi:10.1007/128_2006_108.
Complete book, authored
Blenkinsopp A, Paxton P. Symptoms in the pharmacy: a guide to the management of common illness. 3rd ed. Oxford: Blackwell Science; 1998.
Online document
Doe J. Title of subordinate document. In: The dictionary of substances and their effects. Royal Society of Chemistry. 1999. http://www.rsc.org/dose/title of subordinate document. Accessed 15 Jan 1999.
Online database
Healthwise Knowledgebase. US Pharmacopeia, Rockville. 1998. http://www.healthwise.org. Accessed 21 Sept 1998.
Supplementary material/private homepage
Doe J. Title of supplementary material. 2000. http://www.privatehomepage.com. Accessed 22 Feb 2000.
University site
Doe, J: Title of preprint. http://www.uni-heidelberg.de/mydata.html (1999). Accessed 25 Dec 1999.
Doe, J: Trivial HTTP, RFC2169. ftp://ftp.isi.edu/in-notes/rfc2169.txt (1999). Accessed 12 Nov 1999.
Organization site
ISSN International Centre: The ISSN register. http://www.issn.org (2006). Accessed 20 Feb 2007.
Dataset with persistent identifier
Zheng L-Y, Guo X-S, He B, Sun L-J, Peng Y, Dong S-S, et al. Genome data from sweet and grain sorghum (Sorghum bicolor). GigaScience Database. 2011. http://dx.doi.org/10.5524/100012 .
Figures, tables and additional files
See General formatting guidelines for information on how to format figures, tables and additional files.
Submit manuscript
Affiliated to the Institute of Clinical Physiology of the Italian National Research Council .
- Editorial Board
- Instructions for Editors
- Sign up for article alerts and news from this journal
Annual Journal Metrics
2022 Citation Impact 1.9 - 2-year Impact Factor 2.4 - 5-year Impact Factor 1.052 - SNIP (Source Normalized Impact per Paper) 0.562 - SJR (SCImago Journal Rank)
2023 Speed 11 days submission to first editorial decision for all manuscripts (Median) 115 days submission to accept (Median)
2023 Usage 560,342 downloads 303 Altmetric mentions
- More about our metrics
Cardiovascular Ultrasound
ISSN: 1476-7120
- General enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 30 January 2023
Single-cell T cell receptor sequencing of paired human atherosclerotic plaques and blood reveals autoimmune-like features of expanded effector T cells
- Marie A. C. Depuydt ORCID: orcid.org/0000-0002-7174-1952 1 na1 ,
- Frank H. Schaftenaar 1 na1 ,
- Koen H. M. Prange ORCID: orcid.org/0000-0002-9835-1735 2 ,
- Arjan Boltjes ORCID: orcid.org/0000-0002-6338-051X 3 ,
- Esmeralda Hemme ORCID: orcid.org/0000-0001-5853-8784 1 ,
- Lucie Delfos 1 ,
- Jill de Mol ORCID: orcid.org/0000-0003-3541-9912 1 ,
- Maaike J. M. de Jong 1 ,
- Mireia N. A. Bernabé Kleijn 1 ,
- Judith A. H. M. Peeters ORCID: orcid.org/0000-0002-0051-9836 4 ,
- Lauren Goncalves 4 ,
- Anouk Wezel 4 ,
- Harm J. Smeets 4 ,
- Gert J. de Borst 5 ,
- Amanda C. Foks ORCID: orcid.org/0000-0002-9747-3458 1 ,
- Gerard Pasterkamp ORCID: orcid.org/0000-0001-5345-1022 3 ,
- Menno P. J. de Winther ORCID: orcid.org/0000-0002-4038-6636 2 ,
- Johan Kuiper 1 ,
- Ilze Bot ORCID: orcid.org/0000-0002-1242-1959 1 na2 &
- Bram Slütter ORCID: orcid.org/0000-0003-3996-0503 1 na2
Nature Cardiovascular Research volume 2 , pages 112–125 ( 2023 ) Cite this article
15k Accesses
23 Citations
48 Altmetric
Metrics details
- Atherosclerosis
- Autoimmunity
Atherosclerosis is a lipid-driven chronic inflammatory disease; however, whether it can be classified as an autoimmune disease remains unclear. In this study, we applied single-cell T cell receptor seqencing (scTCR-seq) on human carotid artery plaques and matched peripheral blood mononuclear cell samples to assess the extent of TCR clonality and antigen-specific activation within the various T cell subsets. We observed the highest degree of plaque-specific clonal expansion in effector CD4 + T cells, and these clonally expanded T cells expressed genes such as CD69 , FOS and FOSB , indicative of recent TCR engagement, suggesting antigen-specific stimulation. CellChat analysis suggested multiple potential interactions of these effector CD4 + T cells with foam cells. Finally, we integrated a published scTCR-seq dataset of the autoimmune disease psoriatic arthritis, and we report various commonalities between the two diseases. In conclusion, our data suggest that atherosclerosis has an autoimmune compondent driven by autoreactive CD4 + T cells.
Similar content being viewed by others

A single-cell atlas enables mapping of homeostatic cellular shifts in the adult human breast
Austin D. Reed, Sara Pensa, … Walid T. Khaled

A lipid atlas of human and mouse immune cells provides insights into ferroptosis susceptibility
Pooranee K. Morgan, Gerard Pernes, … Andrew J. Murphy

An immunophenotype-coupled transcriptomic atlas of human hematopoietic progenitors
Xuan Zhang, Baobao Song, … H. Leighton Grimes
Atherosclerosis is the major underlying pathology of acute cardiovascular events, such as myocardial infarction and stroke. It is characterized by accumulation of lipids and subsequent inflammation of the medium and large arteries. As low-density lipoprotein (LDL) particles are important instigators of atherosclerosis, cardiovascular disease (CVD) has primarily been treated as a lipid-driven disorder, with a treatment focus on lowering LDL cholesterol levels. Nonetheless, inflammation plays a critical role in perpetuating the growth and instability of atherosclerotic lesions, highlighted by the success of recent clinical trials with anti-inflammatory agents 1 , 2 . Elucidating the dominant inflammatory pathways that drive atherosclerosis may, therefore, allow identification of new druggable targets independent of cholesterol lowering.
Single-cell RNA sequencing (scRNA-seq) and mass cytometry have allowed detailed mapping of the leukocyte contents of atherosclerotic plaques 3 , 4 . These studies show that T cells are the largest leukocyte population and that the number of effector T cells within the lesion associates with plaque instability. In combination with previous murine work, this suggests that inflammatory processes inside the plaque are driven by T cells, and atherosclerosis could be considered an autoimmune-like disease. In support of that, autoreactive (LDL-specific) CD4 + T cells have previously been reported in human atherosclerotic lesions and have been identified in elevated levels in the circulation of patients with CVD 5 , 6 , 7 . Moreover, vaccination approaches aimed at the reduction of self-reactive T cells or induction of regulatory T (T reg ) cells have shown promise in murine models of atherosclerosis 8 , 9 . However, when self-reactive CD4 + T cells are indeed the culprit T cells that propagate disease, clonal expansion and accumulation of these cells in the lesions is to be expected. Interestingly, recent work examining the T cell receptor (TCR) distribution in human coronary plaques showed primarily clonal expansion of CD8 + T cells inside the plaque and identified some of these TCRs to be specific for common viral antigens, such as influenza, cytomegalovirus (CMV) and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) 10 . However, this work did not include patient-matched peripheral blood mononuclear cell (PBMC) controls, rendering it impossible to assess whether the virus-specific CD8 + T cells were specifically enriched in the plaque and/or had recently undergone antigen-specific interactions.
Here we present an approach to identify the T cell subsets that are specifically enriched in atherosclerotic lesions and whether these subsets underwent antigen-specific interaction in the plaque. We combine scRNA-seq and single-cell TCR sequencing (scTCR-seq) of human carotid plaques and matched PBMC samples. With this approach, we observed the highest degree of plaque-specific clonal expansion in both effector CD4 + T cells and, to a smaller extent, in the T reg population. By integrating the data from our patients with atherosclerosis with the scTCR-seq data from patients with psoriatic arthritis (PSA), we show that atherosclerosis has major similarities with another prominent autoimmune disease. Thus, our data suggest that atherosclerosis is characterized by an autoimmune component driven by autoreactive CD4 + T cells.
Signature of antigen-specific T cells in atherosclerosis
Recent scRNA-seq studies in human atherosclerosis have shown a prominent accumulation of T cells in the plaque 3 , 4 . However, it remains unclear whether these T cells are bystanders or whether they actively contribute to lesion progression through antigen-specific activation. To examine potential recent antigen encounter and activation, CD69 expression was measured on the surface of both PBMCs and plaque T cells through flow cytometry (cohort 1; Fig. 1a and Supplementary Table 1 ). A significant increase in CD69 + CD4 + (PBMC: 0.82% ± 0.71, plaque: 51.45% ± 16.39; P < 0.0001) and CD8 + T cells (PBMC: 4.95% ± 6.49, plaque: 55.20% ± 19.40; P < 0.0001) was observed in the plaque compared to PBMC (Fig. 1b and Extended Data Fig. 1a,b ). Because CD69 is known to rapidly upregulate after TCR/HLA engagement on T cells 11 , these data suggest that T cells actively engage in TCR-specific interactions within the atherosclerotic plaque.
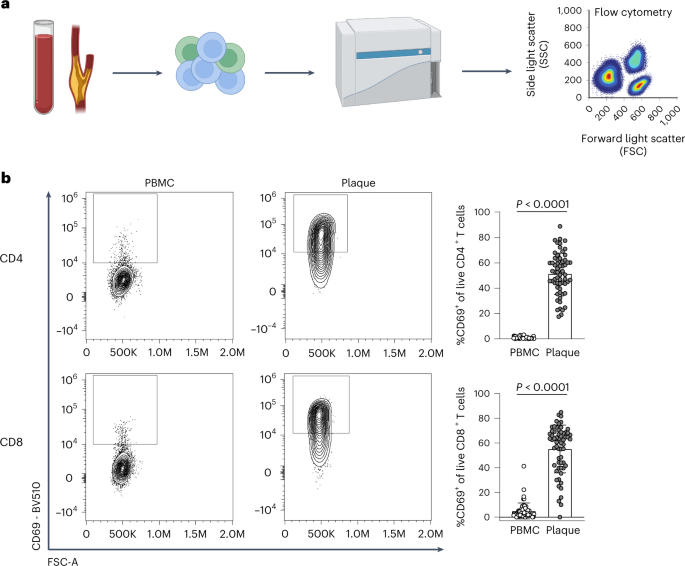
a , Experimental setup: single cells from PBMC and plaque samples were stained with fluorescently labelled antibodies and measured through flow cytometry. b , Flow cytometry analysis of CD69 expression on PBMC and plaque live CD4 + and CD8 + T cells. P values are depicted in the figure panels. Data are presented as mean values ± s.d. PBMC n = 58; plaque n = 61. Statistical analyses were performed using an unpaired Mann–Whitney t -test.
Source data
However, CD69 expression may also indicate the presence of resident memory T cells or may be upregulated by exposure to type I interferon (IFN) 12 , 13 . To determine whether the elevated CD69 expression was due to antigen-specific interactions in the plaque, we aimed to assess whether these T cells were clonally expanded as well. We combined scRNA-seq with scTCR-seq on paired PBMCs and carotid artery plaques from three male patients (cohort 2; Supplementary Table 1 ). The plaques were enzymatically digested, and live CD45 + cells were isolated by fluorescence-activated cell sorting (FACS) (Extended Data Fig. 2a ). Both PBMCs and plaque cells were stained for CD3, CD4, CD8 and CD14 on a protein level with feature barcoding to properly distinguish between myeloid and T cell subsets on both RNA and protein level. All cells were subsequently processed with droplet-based single-cell 5′ RNA sequencing (10x Genomics) and sequenced (Fig. 2a ). Unsupervised clustering revealed clusters consisted of T cells, natural killer (NK) cells, myeloid cells and B cells, originating from both PBMCs and plaque cells and with limited interpatient variability (Extended Data Fig. 2b–e ). We did not further characterize all non-T cells, as we specifically focused on characterizing T cells to assess their clonal expansion in atherosclerosis. Therefore, all T cells were selected based on both RNA and protein expression, and, subsequently, unsupervised clustering was performed independent of the variable TCR genes to prevent clustering based on clonality ( Methods ). Subclustering of both PBMCs and plaque T cells revealed 13 distinct T cell subsets (Fig. 2b,c and Extended Data Fig. 2f ). Within the T cells, we observed one memory (C0) and three naive (C1, C2 and C10) T cell clusters based on different expression levels of TCF7 , LEF1 , SELL and CCR7 (Fig. 2b,d and Supplementary Table 2 ). Furthermore, three effector T cell clusters (C3, C4 and C5) were detected, expressing a multitude of different cytotoxic genes, such as GZMB , GZMK and GZMA (Fig. 2b,d and Supplementary Table 2 ). A T reg cluster was defined based on expression of FOXP3 , IL2RA and TIGIT (C6; Fig. 2b,d and Supplementary Table 2 ) 14 . In addition, an exhausted T cell cluster characterized by expression of HAVCR2 , PDCD1 and TOX 15 , 16 (C7; Fig. 2b,d and Supplementary Table 2 ) and two γδ-T cell clusters expressing TRGC1 , TRGC2 and TRDC (C8 and C9; Fig. 2b,d and Supplementary Table 2 ) were detected. Lastly, we observed two small clusters consisting of mast cells (C11; Fig. 2b and Supplementary Table 2 ) and mucosal-associated invariant T (MAIT) cells (C12; Fig. 2b,d and Supplementary Table 2 ).
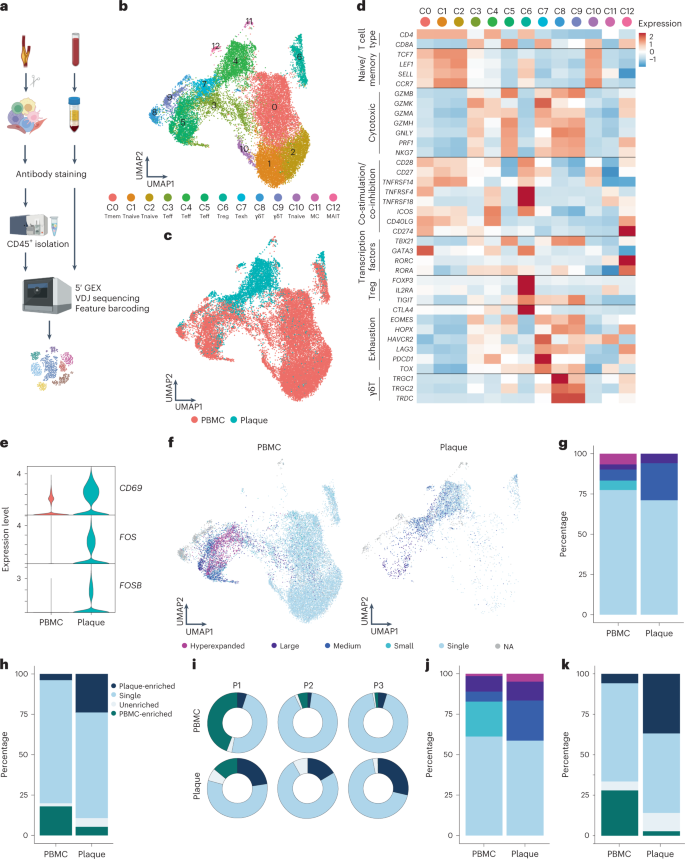
a , Schematic overview of the study design. Human plaques were enzymatically digested, and live CD45 + cells were sorted using FACS. Matched blood samples were processed to isolate PBMCs. Both plaque cells and PBMCs were then further processed using 10x Genomics and sequenced. b , UMAP depicting 13 distinct T cell clusters resulting from unsupervised clustering ( n = 24,443). c , UMAP showing contribution of PBMC or plaque to the T cell clusters. d , Heat map with average expression of T cell function-associated genes. e , Violin plot with expression of CD69 , FOS and FOSB in PBMCs and plaque T cells. f , UMAP visualization of clonotype expansion levels among T cells between PBMC and plaque. g , Bar plot with quantification of clonal expansion levels between plaque and PBMC T cells. h , Bar plot with quantification of tissue enrichment scores of clonotypes. i , Circle plots depicting tissue enrichment scores of all T cells per tissue and per patient. j , Bar plot with quantification of clonal expansion levels between PBMC and plaque T cells of bulk TCR-seq data (cohort 3, n = 10). k , Bar plot with quantification of tissue enrichment scores of bulk TCR-seq data (cohort 3). Clonotype expansion levels: Single (one occurrence), Small (≤0.1%), Medium (>0.1% and ≤1%), Large (>1% and ≤10%) and Hyperexpanded (>10%), percentage of all T cells. Tissue enrichment scores: Plaque-enriched (frequency expanded clone higher in plaque versus PBMC), Single (one occurrence), Unenriched (frequency expanded clone similar in PBMC versus plaque) and PBMC-enriched (frequency expanded clone higher in PBMC versus plaque).
Next, we compared expression of CD69 as well as FOS and FOSB genes, which are also upregulated downstream of TCR signalling 17 , between plaque and blood. In line with the increased CD69 + protein expression measured through flow cytometry, all three genes showed an increased mRNA expression in plaque T cells compared to their PBMC counterparts (Fig. 2e ). Subsequently, we applied VDJ sequencing to map paired α-chains and β-chains of the TCR and to define the clonal composition of the paired PBMCs and plaque T cells. Clonal expansion levels were calculated to indicate the clonotype abundance as percentage of the total measured TCRs per patient, per tissue (Fig. 2f and Methods ). ‘Single’ represents a single clonotype occurrence. Expanded T cells were divided into multiple categories characterized by increasing frequencies of clonotype occurrences, labelled as ‘Small’, ‘Medium’, ‘Large’ and ‘Hyperexpanded’.
Taken together, a small increase in the percentage of total expanded T cells is observed in the plaque compared to PBMCs (PBMC 23% versus plaque 29%; Fig. 2f,g , Extended Data Fig. 3a–c and Supplementary Table 3 ). One clonotype, originating from patient 1, was defined as Hyperexpanded in the PBMC and Large in the plaque. The TCRα sequence of this clonotype matched with a TCRα sequence previously associated with CMV in the VDJdb database ( https://vdjdb.cdr3.net/ ) 18 . The CD8 + T cell-specific clonotype, however, was only expressed in T cells that had little expression of CD69 , FOS and FOSB , suggesting that this was not an active viral infection (Extended Data Fig. 4a–c ). In addition, the tissue enrichment of clonotypes was assessed to investigate whether certain clonotypes specifically accumulated within either of the tissues or whether the clonotype abundance was unaffected by the location. T cells with clonotypes more present in the PBMC were identified as PBMC-enriched and vice versa for plaque-enriched T cells. Indeed, within the plaque, an increased percentage of plaque-enriched T cells was observed in all patients, suggesting a potential plaque-restricted antigen-induced clonal expansion (Fig. 2h,i , Extended Data Fig. 3d,e and Supplementary Table 3 ). To confirm these findings, bulk TCRβ sequencing was performed on matched blood and plaque T cells from ten patients (cohort 3; Supplementary Table 1 ). Both clonal expansion levels and tissue enrichment were similar between TCRβ bulk sequencing and the scTCR-seq data (Fig. 2j,k and Extended Data Fig. 5a ).
Increased percentage of expanded CD8 + T cells in PBMCs
To properly isolate CD4 + and CD8 + T cells for further analysis, a selection was made of CD4 + and CD8 + single-positive T cells based on expression of these proteins as measured by feature barcoding (Extended Data Fig. 6a ). Subclustering of CD8 + T cells resulted in 11 distinct subsets. Most CD8 + T cells had an activated phenotype as indicated by expression of multiple genes with a cytotoxic signature. One naive (C6) and one memory (C2) cluster were mainly detected in the PBMC ( TCF7 , LEF1 , SELL and CCR7 ; Fig. 3a , Extended Data Fig. 6b,c and Supplementary Table 2 ). Four effector clusters were characterized, of which C0 and C10 mostly reside in the PBMC and C3 and C5 predominantly in the plaque. C0, C3 and C10 expressed a multitude of different cytotoxic genes, including GZMK and GZMA , at different levels. C5 was characterized by expression of CD69 , FOS and FOSB (Fig. 3a , Extended Data Fig. 6b,c and Supplementary Table 2 ). Furthermore, three terminally differentiated effector memory T cell (T EMRA ) clusters were defined by expression of, for example, GZMB , PRF1 and NKG7 and lack of CD27 and CD28 (C1, C4 and C7; Fig. 3a , Extended Data Fig. 6b,c and Supplementary Table 2 ). T EMRA clusters were primarily associated with a gradual increase in expression of, among others, KLRD1 , KLRG1 and FCGR3A , indicating various stages of terminal differentiation (Extended Data Fig. 6d ). Using Seurat multimodal reference mapping, which maps your dataset to a large PBMC dataset with feature barcoding data, expression of CD45RA and CD45RO could be predicted. Indeed, T EMRA subsets were predicted to express CD45RA, whereas the effector T cells were predicted to be CD45RO + (Extended Data Fig. 6e ). Finally, a cluster of γδ-T cells (C8) and a cluster of MAIT cells (C9) were detected within the CD8 + T cell subsets (Fig. 3a , Extended Data Fig. 6a,b and Supplementary Table 2 ). Subsequently, clonal expansion levels were examined and quantified within the CD8 + T cells in PBMC and plaque. A large percentage of clonally expanded CD8 + T cells was detected in the plaque; however, a higher percentage of expanded CD8 + T cells was detected in the PBMC (Fig. 3b,c , Extended Data Fig. 6f and Supplementary Table 3 ). Nevertheless, within the plaque, most expanded CD8 + T cells remained plaque enriched (Fig. 3d , Extended Data Fig. 6g and Supplementary Table 3 ). Expanded CD8 + T cells showed upregulation of multiple genes involved in CD8 cytotoxicity—for example, GZMH , KLRD1 , PRF1 and GZMB (Fig. 3e ). Interestingly, when comparing PBMC-enriched versus plaque-enriched CD8 + T cells, PBMC-enriched cells expressed cytotoxic genes, such as GNLY , PRF1 and members of the killer cell lectin-like subfamily ( KLRG1 and KLRD1 ), whereas plaque-enriched CD8 + T cells seemed to have experienced recent antigen-induced TCR activation (Fig. 3f ). To further illustrate the plaque-expanded CD8 + T cell clusters, we selected C1, C3, C5 and C9, which had relatively the most plaque-enriched expansion (Fig. 3g ). C1, C3 and C5 all expressed a multitude of cytotoxic genes. C1 highly expressed NKG7 , GNLY and GZMB , of which the latter was increased in plaque, whereas C3 and C5 had increased expression of GZMA and GZMK in the plaque. C5 plaque T cells had the highest expression of CD69 , FOS and FOSB . Finally, MAIT cells (C9) showed high expression of genes unique for this cell type ( TRAV1-2 , ZBTB16 and IL23R ) 19 and of TCR activation genes. To identify potential dynamics of different CD8 + populations, we applied lineage tracing analyses using Monocle3 and RNA velocity. RNA velocity shows that, within the CD8 + clusters, cells tend to be less prone to switch into another subset. A small trajectory appeared between the memory CD8 + T cells (C2) and the antigen-experienced effector T cells (C5), yet this was not clearly retrieved with pseudotime analysis (Fig. 3i,j ).
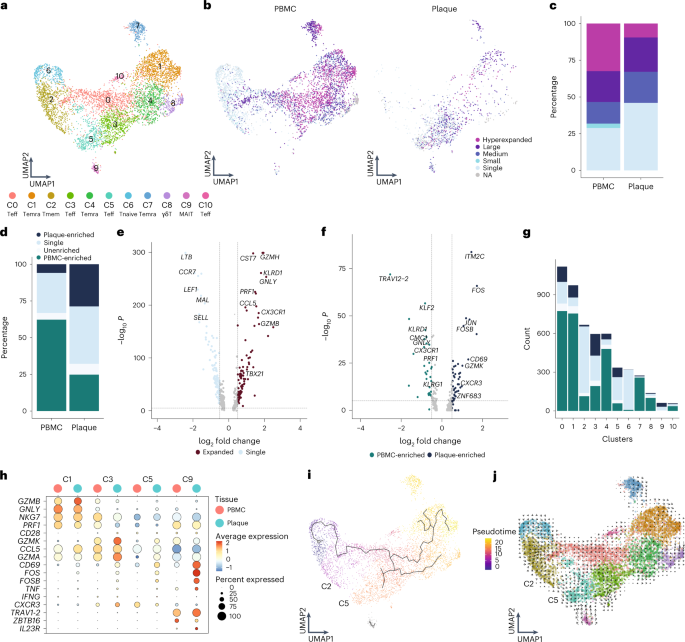
a , UMAP visualization of unsupervised clustering revealed 11 distinct CD8 + T cell populations ( n = 5,730). b , UMAP visualization of different levels of clonotype expansion among CD8 + T cells between PBMC and plaque. c , Quantification of clonal expansion levels between PBMC and plaque CD8 + T cells. d , Quantification of tissue enrichment scores of clonotypes in CD8 + T cells of PBMC and plaque. e , Volcano plot with differentially expressed genes between CD8 + T cells with single clonotypes and all expanded clonotypes (Small–Large). Genes were considered significant with P < 1 × 10 −6 and a fold change of 0.5. For all volcano plots, Bonferroni-corrected P values were calculated based on the total number of genes in the dataset. f , Volcano plot with differentially expressed genes of PBMC-enriched versus plaque-enriched CD8 + T cells. Genes were considered significant with P < 1 × 10 −6 and a fold change of 0.5. g , Bar plot with quantification of tissue enrichment score of individual CD8 + T cell clusters. h , Dot plot of average expression of upregulated genes in clusters 1, 3, 5 and 9. i , UMAP visualization of pseudotime analysis of CD8 + T cells. C2 indicates cluster 2; C5 indicates cluster 5. j , UMAP visualization of RNA velocity analysis of CD8 + T cells. Clonotype expansion levels: Single (one occurrence), Small (≤0.1%), Medium (>0.1% and ≤1%), Large (>1% and ≤10%) and Hyperexpanded (>10%), percentage of all CD8 + T cells. Tissue enrichment scores: Plaque-enriched (frequency expanded clone higher in plaque versus PBMC), Single (one occurrence), Unenriched (frequency expanded clone similar in PBMC versus plaque) and PBMC-enriched (frequency expanded clone higher in PBMC versus plaque).
Increased percentage of expanded CD4 + T cells in plaque
Unsupervised clustering revealed 11 subsets of CD4 + T cells (Fig. 4a ). As previously described, CD4 + T cell clusters are mainly defined by a shift in activation status 3 , 4 . Two naive T cell clusters (C1 and C2) and a memory T cell cluster (C0) were mainly detected within the PBMC (Fig. 4a , Extended Data Fig. 7a,b and Supplementary Table 2 ). Furthermore, a T-helper (T h ) 17-like cluster (C4) expressing RORC , RORA and CCR6 , as well as a T reg cluster (C5; Fig. 4a , Extended Data Fig. 7b and Supplementary Table 2 ), were identified. Whereas T reg cells were found in both PBMC and plaque, T h17 -like cells were mainly detected in PBMC (Extended Data Fig. 7c ). A T cell cluster with genes involved in cell migration (T migr , C6) mainly resided in PBMC (Supplementary Table 2 ). Two different effector subsets were characterized, of which one was more plaque specific with high expression of CD69 , FOS , JUN and GZMA (C3), and one was found in both tissues specifically enriched for GZMK (C8; Fig. 4a , Extended Data Fig. 7a,b and Supplementary Table 2 ). Moreover, a cytotoxic CD4 + T cell cluster, which resembled the previously described CD4 + CD28 null cells 3 , 20 , 21 , was defined by expression of GZMB and PRF1 and lack of CD28 and was found in both PBMC and plaque (Fig. 4a , Extended Data Fig. 6a,b and Supplementary Table 2 ). Finally, a cluster of T cells was observed in the PBMCs that expressed genes involved in IFN I signalling and a small mast cell cluster in the plaque (Fig. 4a and Supplementary Table 2 ). Subsequently, CD4 + T cell clonality was assessed. Clonal expansion levels were projected on the CD4 + T cell uniform manifold approximation and projection (UMAP) and quantified. In line with a recent study by Chowdhury et al. 10 , the percentage of clonal expanded CD8 + T cells in the plaque is larger than those in CD4 + T cells. However, in contrast to CD8 + T cells, a marked increase in the percentage of expanded CD4 + T cells in the plaque was revealed compared to the PBMCs (Fig. 4b,c , Extended Data Fig. 7e and Supplementary Table 3 ). Furthermore, the expanded clonotypes in the plaque CD4 + T cells were mostly plaque enriched (Fig. 4d , Extended Data Fig. 7f and Supplementary Table 3 ). When comparing expanded CD4 + T cells to their single counterparts with a unique clonotype, upregulation of genes involved in T cell activation and cytotoxicity, such as GNLY , GZMH , PRF1 and CX3CR1 , were particularly observed in the expanded T cells, whereas single T cells expressed genes upregulated in naive and memory T cells ( CCR7 , LTB , LEF1 , SELL and CD27 ) (Fig. 4e ). Interestingly, when comparing clonally expanded PBMC-enriched versus the plaque-enriched expanded CD4 + T cells, plaque-enriched CD4 + T cells showed enhanced expression of genes upregulated shortly after antigen-specific TCR interaction ( JUN , CD69 , FOS and FOSB ) (Fig. 4f ), suggesting that there are CD4 + T cells that undergo antigen-specific interactions in the plaque. Next, we quantified the absolute number of plaque-enriched clones per CD4 + T cell cluster (Fig. 4g ), which revealed cluster C3 as the major contributor in absolute number of plaque-specific clonally expanded T cells. Furthermore, C7 and C8 consisted of a relatively large number of plaque-enriched clones compared to the other CD4 + T cell clusters. The C7 cluster, characterized by an increase in cytotoxic genes, including GZMB , NKG7 and PRF1 , has little to no expression of CD69 , FOS and FOSB , indicating that, although these cells have substantial expanded clonotypes, they do not express genes involved in antigen-induced activation (Fig. 4h ). The effector populations C3 and C8 displayed increased expression of TCR proximal genes CD69 , FOS and FOSB . Interestingly, whereas we did not observe increased accumulation of clonally expanded T reg cells (C5) in plaque, we did observe upregulation of FOS , FOSB and JUN in plaque-derived T reg cells compared to PBMC-derived T reg cells, suggesting that T reg cells are encountering antigen in the plaque. Expression of various functional T reg markers ( FOXP3 , IL2RA , TIGIT , CTLA4 and TNFRSF4 (OX40) and TNFRSF18 (GITR)) in the plaque compared to the PBMC indicated increased activity of T reg cells (Fig. 4h,i ).
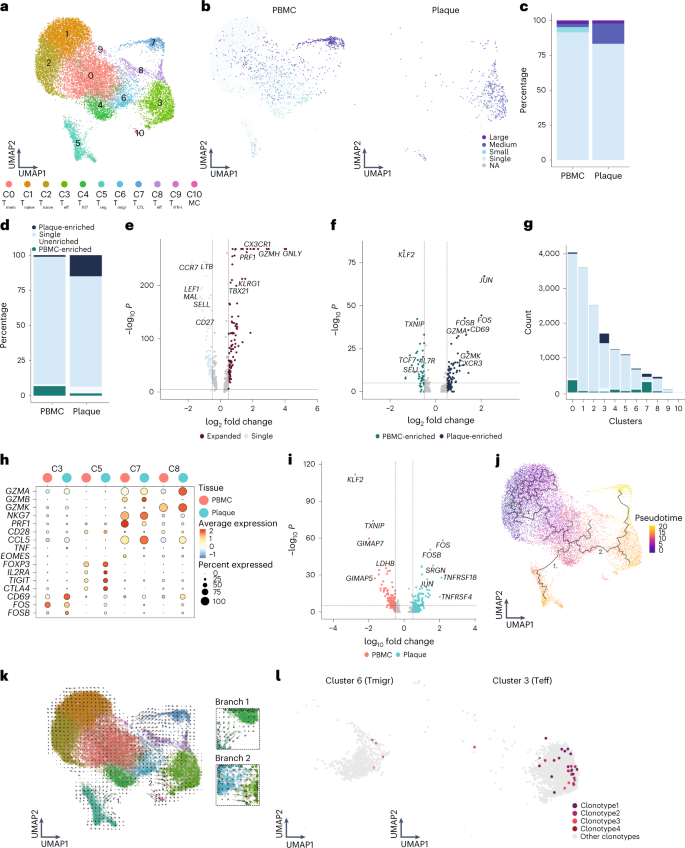
a , UMAP visualization of unsupervised clustering revealed 11 distinct CD4 + T cell populations ( n = 17,073). b , UMAP visualization of different levels of clonotype expansion among CD4 + T cells between PBMC and plaque. c , Bar plot with quantification of clonal expansion levels between PBMC and plaque CD4 + T cells. d , Bar plot with quantification of tissue enrichment scores of clonotypes in CD4 + T cells of PBMC and plaque. e , Volcano plot with differentially expressed genes between CD4 + T cells with single clonotypes and all expanded clonotypes (Small–Large). Genes were considered significant with P <1 × 10 −6 and a fold change of 0.5. For all volcano plots, Bonferroni-corrected P values were calculated based on the total number of genes in the dataset. f , Volcano plot with differentially expressed genes of PBMC-enriched versus plaque-enriched CD4 + T cells. Genes were considered significant with P <1 × 10 −6 and a fold change of 0.5. g , Bar plot with quantification of tissue enrichment score of individual CD4 + T cell clusters. h , Dot plot of average expression of upregulated genes in clusters 3, 5, 7 and 8. i , Volcano plot with differentially expressed genes between T reg cells in PBMC and plaque. Genes were considered significant with P <1 × 10 −6 and a fold change of 0.5. j , UMAP visualization of pseudotime analysis of CD4 + T cells. Two branches of the analysis are indicated with 1 and 2. k , UMAP visualization of RNA velocity analysis of CD4 + T cells with close-up of branches 1 and 2. l , UMAP visualization of four overlapping clonotypes between cluster 6 and cluster 3. Open circles indicate PBMC CD4 + T cells; closed circles indicate plaque CD4 + T cells. Clonotype expansion levels: Single (one occurrence), Small (≤0.1%), Medium (>0.1% and ≤1%), Large (>1% and ≤10%), percentage of all CD4 + T cells. Tissue enrichment scores: Plaque-enriched (frequency expanded clone higher in plaque versus PBMC), Single (one occurrence), Unenriched (frequency expanded clone similar in PBMC versus plaque), PBMC-enriched (frequency expanded clone higher in PBMC versus plaque).
To identify the origin of the antigen-specific effector CD4 + T cell subsets in the plaque, we applied lineage tracing analyses to define the dynamics of the different CD4 + T cell populations. Pseudotime analysis using Monocle3 showed a trajectory ranging from naive T cells toward either the T reg cells (branch 1) or the effector T cell population (branch 2) (Fig. 4j ). The first pseudotime branch directing toward T reg cells is projected through the T h17 -like CD4 + T cell cluster, potentially suggesting a plasticity between both subtypes. However, if the complementary RNA velocity analysis is assessed (time-resolved analysis based on spliced and unspliced mRNA 22 ), the T reg cluster does not seem to be derived from the T h17 -like cells (branch 1; Fig. 4k ). Moreover, T reg cells in tissue also cluster further away from the circulating T h17 -like cells compared to the PBMC T reg cells, indicating that the plaque environment is less likely to induce a phenotype switch from T reg to T h17 . In addition, no overlapping clonotypes were found between both clusters, and FOXP3 and RORC did not co-express (Extended Data Fig. 7b,c ), suggesting that, in our dataset, we were not able to detect the previously described T reg /T h17 plasticity 23 . Looking at the other branch in both pseudotime analysis and RNA velocity (branch 2), a clear path ranging from the T migr cluster (C6) toward the CD69 + T eff cluster (C3) was observed. Their migratory phenotype, highlighted by expression of CCR4 and CCR10 previously described to be expressed on infiltrating T cells in the inflamed skin 24 , suggests that this T migr subset could be the precursor population for the antigen-specific CD4 + T cells in the plaque (Extended Data Fig. 7d ). Indeed, when comparing overlap in TCR sequence between the different CD4 + subpopulations, 37 clonotypes overlapped between both cluster C6 and cluster C3. Within the top five most expanded clonotypes, four plaque-enriched clonotypes were detected and exhibited marked expansion in C3 compared to C6, further confirming our hypothesis that the clonally expanded T eff cells could originate from the circulating migratory T cell subset (Fig. 4l and Extended Data Fig. 7c ).
TREM2 + macrophages can activate antigen-induced CD4 + T cells
Our data suggest that atherosclerotic plaques harbor one major CD4 + T cell subset that regularly undergoes antigen-specific interactions. To understand whether and how these clonally expanded T cells interact with myeloid subsets in the plaque, we selected five plaque myeloid cell populations from the overall dataset: myeloid-derived dendritic cells (DC-M), plasmacytoid dendritic cells (DC-P), proliferating macrophages (M-Prol), inflammatory macrophages (M-Inf) and foamy TREM2 hi macrophages (M-TREM2) (Extended Data Fig. 8a ) 3 . Using CellChat, we examined potential signalling pathways between these myeloid subsets and the CD4 + and CD8 + T cells in the plaque 25 . CellChat can predict incoming (receptor) and outgoing (ligand) activity of cell signalling pathways based on scRNA-seq data, accounting for the multimeric structure of ligand–receptor complexes and the effect of co-factors on the ligand–receptor interactions. Predicted outgoing and incoming pathway signalling was displayed per cluster. Overlap between outgoing and incoming signals of a certain pathway within or between clusters indicates a possible interaction through this pathway. The different CD4 + T cell clusters showed different levels of relative signalling strength in the outgoing signalling patterns (top bar plot heat map, relative to outgoing signals of all pathways in the heat map), whereas CD8 + T cells showed little difference between the clusters (Fig. 5a and Extended Data Fig. 8b ). In general, the most upregulated signalling pathway was MHCII as outgoing signal on all myeloid subsets and incoming signals in multiple CD4 + T cell subsets, including cluster 3 (C3). The plaque-enriched CD69 + C3 displayed elevated outgoing signalling patterns. Interestingly, one of the pathways that was enriched in this cluster was the CD40 pathway, involved in antigen-specific T cell activation 26 . Next, we assessed whether the CD40 pathway was also enriched as an incoming signalling pattern (Fig. 5b ). Specific enrichment was observed in the M-TREM2 (foam cell) subset. Apart from the CD40 pathway, multiple other enriched pathways involved in immune synapse formation and co-stimulation could be defined between C3 and M-TREM2, including the CD99, CD6, CD40, macrophage inhibitory factor (mIF) and annexin A1 pathways (Fig. 5b ) 27 , 28 , 29 , 30 . Together, this suggests that M-TREM2 could be involved in activation of the clonally expanded CD4 + T cells in atherosclerotic lesions.
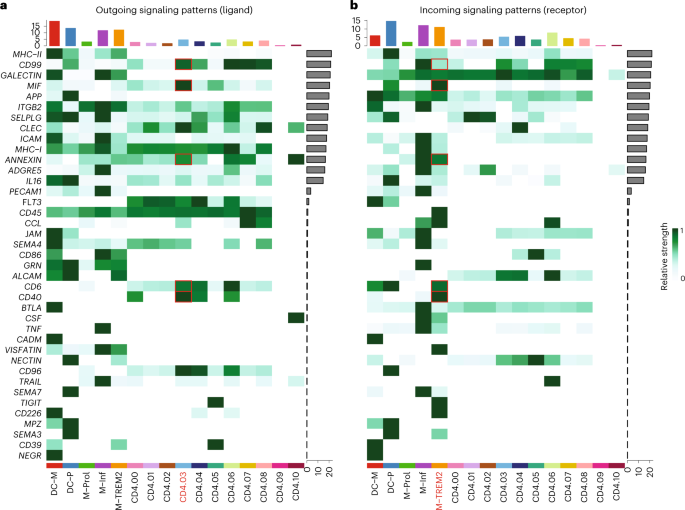
Heat maps displaying outgoing (ligand) ( a ) and incoming (receptor) ( b ) signalling patterns of pathways describing potential ligand–receptor interactions. Scale above the heat map indicates the relative signalling strength of a cell cluster based on all signalling pathways displayed in the heat map. Grey bars to the right of the heat map show the total signalling strength of a pathway in all cell clusters. The relative signalling strength is indicated by ranging colour from white (low) to green (high). All cells included in these graphs originate from the plaque.
Common autoimmune phenotype in expanded plaque T cells
Based on the accumulation of plaque-enriched CD4 + and CD8 + T cell clonotypes, we hypothesized that human atherosclerosis could be characterized as an autoimmune-driven T cell response. To further confirm this hypothesis, we integrated an scTCR-seq dataset of the autoimmune disease PSA, containing data from PBMCs and synovial fluid (SF) 31 . As in this study CD45RA − T cells were isolated, we excluded the naive T cell clusters from our dataset. Moreover, this study did not include feature barcoding. CD4 + and CD8 + T cells were, therefore, selected based on the labels predicted by multimodal reference mapping (Extended Data Fig. 9a–f ). Subsequently, CD4 + and CD8 + T cells of both diseases were integrated (Extended Data Fig. 9g,h ) and projected on the atherosclerosis CD4 + and CD8 + UMAP as reference. Remarkably, a clear overlap between PBMCs from atherosclerosis and PSA was observed in both CD4 + and CD8 + T cells. In addition, this overlap was also seen between plaque and SF for both T cell subsets (Fig. 6a,b ). Next, clonal expansion levels were recalculated for both atherosclerosis and PSA (percentage of all CD4 + or CD8 + TCRs). Indeed, clonally expanded T cells were found in similar CD4 + and CD8 + T cell clusters in both diseases (Fig. 6c,e ). Moreover, quantification of this clonal expansion revealed a similar distribution. An increased percentage of expanded CD8 + T cells versus expanded CD4 + T cells was detected in SF. However, as seen in atherosclerosis, the percentage of expanded CD4 + T cells was increased in SF compared to PBMC, whereas expanded CD8 + T cells did not differ between both tissues (Fig. 6d,f ). Tissue enrichment scores were also determined and again displayed similarities between atherosclerosis and PSA. Tissue-enriched T cells were located in overlapping clusters in both diseases. Quantification resulted in an increase in tissue-enriched T cells in both CD4 + and CD8 + in plaque and SF compared to their matched PBMCs, although this enrichment was more prominent in SF versus plaque T cells (Fig. 6g–j ). Finally, we defined the genes supporting the overlap between the atherosclerosis and PSA subsets in C3 and C5 of both CD4 + and CD8 + T cells. CD4 + T cells from C3 were characterized by high expression of CCL5 , GZMK and GZMA in both plaque and SF (Fig. 6k and Extended Data Fig. 10a ). Atherosclerosis-specific C3 CD4 + T cells had slightly increased GZMA expression compared to PSA PBMCs and SF. In both diseases, FOS and JUN were upregulated in tissue compared to PBMCs, whereas FOSB was specifically upregulated in plaque T cells. Furthermore, regulatory CD4 + T cells in both affected tissues appeared more active by upregulation of activation markers, including IL2RA , TNFRSF4 , TNFRSF18 , TNFSF1B and CTLA4 , compared to the PBMC counterpart (Fig. 6l and Extended Data Fig. 10b ). Nevertheless the T reg subset showed some disparity between SF and plaque-derived cells as plaque T reg also increasingly expressed ICOS and ENTPD1 , compared to PSA SF-derived T regs . Interestingly, atherosclerosis T reg cells in both PBMC and plaque had increased expression of TGFB1 compared to the PSA T reg cells. In both PSA and atherosclerosis CD8 + C3 T cells, expression profiles displayed a similar phenotype with high expression of T cell effector genes—for example, CCL5 , GZMH , GZMA , GZMK and NKG7 (Fig. 6m and Extended Data Fig. 10c ). Lastly, CD8 + T cells from C5 showed upregulation of genes involved in antigen-induced TCR activation in both affected tissues ( FOS and JUN ) (Fig. 6n and Extended Data Fig. 10d ). FOSB was upregulated in plaque only, similarly to CD4 + C3, and JUNB expression was increased in PSA compared to atherosclerosis. Furthermore, increased expression of ZNF683 was observed in both diseased tissues. GZMH was particularly upregulated in plaque CD8 + T cells. To summarize, these data support the hypothesis that atherosclerosis has a considerable autoimmune component, as it has phenotypically similar clonally expanded T cells compared to the autoimmune disease PSA.
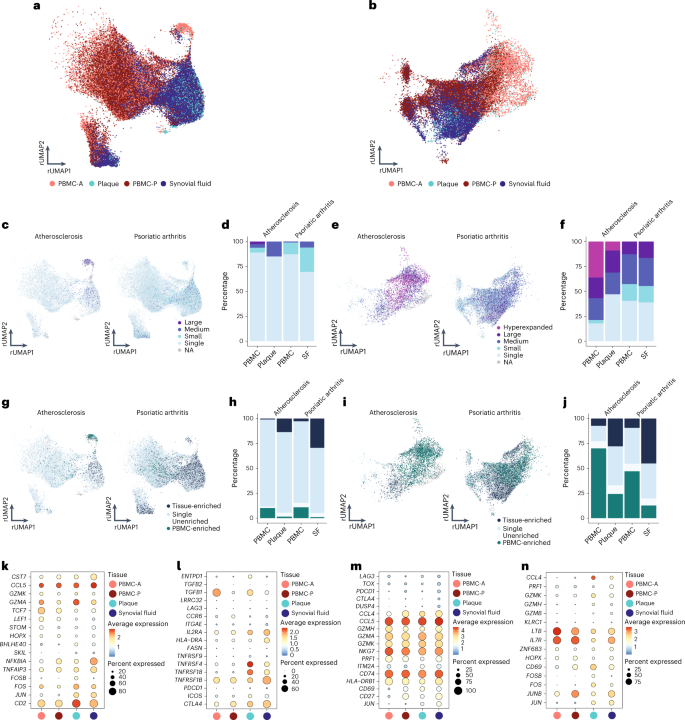
a , Atherosclerosis and PSA CD4 + T cells of PBMC, plaque and SF projected on an atherosclerosis CD4 + T cell reference UMAP (rUMAP). b , Atherosclerosis and PSA CD8 + T cells of PBMC, plaque and SF projected on an atherosclerosis CD8 + T cells rUMAP. c , rUMAP projecting clonal expansion levels of CD4 + T cells in atherosclerosis and PSA. d , Quantification of clonal expansion levels of CD4 + T cells in atherosclerosis, split over PBMC and tissue. e , rUMAP projecting clonal expansion levels of CD8 + T cells in atherosclerosis and PSA. f , Bar plot displaying quantification of clonal expansion levels of CD8 + T cells in atherosclerosis, split over PBMC and tissue. g , rUMAP projecting tissue enrichment scores of clonotypes in CD4 + T cells of atherosclerosis and PSA. h , Bar plot with quantification of tissue enrichment scores of CD4 + T cells in atherosclerosis and PSA, split by PBMC and tissue. i , rUMAP projecting tissue enrichment scores of clonotypes in CD8 + T cells of atherosclerosis and PSA. j , Quantification of tissue enrichment scores of CD8 + T cells in atherosclerosis and PSA, split by PBMC and tissue. k – n , Dot plots with average expression of genes characterizing the genes underlying the overlap between atherosclerosis and PSA in CD4 + T reg cells (C5, k ) and T eff cells (C3, l ) and in CD8 + T eff cells (C3, m ; C5, n ). Clonotype expansion levels: Single (one occurrence), Small (≤0.1%), Medium (>0.1% and ≤1%), Large (>1% and ≤10%) and Hyperexpanded (>10%), percentage of, respectively, CD4 + and CD8 + T cells. Tissue enrichment scores: Tissue-enriched (frequency expanded clone higher in tissue versus PBMC), Single (one occurrence), Unenriched (frequency expanded clone similar in PBMC versus tissue) and PBMC-enriched (frequency expanded clone higher in PBMC versus tissue).
Atherosclerosis has a long history of being treated as metabolic and/or lifestyle disease, with its inflammatory component being overlooked as a potential target of intervention. Groundbreaking work earlier this century has shown that inflammation is an integral part of the disease pathophysiology, and considerable health benefits can be obtained by intervening in inflammatory cascades. Our work here takes these observations a step further and suggests that atherosclerosis is an autoimmune-like disease, with autoreactive T cells driving the inflammation process inside the plaque (Fig. 7 ). Classic autoimmune diseases that involve inflammation of distinct tissue, such as type I diabetes, multiple sclerosis and rheumatoid and psoriatic arthritis, are usually associated with specific HLA class II alleles, suggesting that a pathogenic CD4 + T cell response is a major cause of disease. Moreover, accumulation of antigen-specific T cells at the site of inflammation is a hallmark of autoimmune disease. The absence of clear associations of HLA alleles and atherosclerosis argue against the autoimmune theory in CVD 32 , yet the multifactorial nature of the disease and the large population that it affects make such associations difficult to establish. Accumulation of T cells in atherosclerotic plaques, however, is well established. Moreover, earlier studies investigating TCR diversity using TCRβ sequencing in the plaque indicated an increased clonality in the lesions compared to blood samples from patients with CVD 33 . By taking advantage of scTCR-seq here, we can combine data on distribution of TCRs with their activation state and functionality. Using this approach, we show that a selected number of effector CD4 + T cells and CD8 + T cells accumulate in the lesions and probably undergo antigen-specific activation similarly to autoimmune diseases, such as PSA. Recent work by Chowdhury et al. 10 using a similar approach reached the same conclusion 10 ; however, by using matched PBMC controls, we were able to determine that a large fraction of clonally expanded CD8 + T cells did not specifically accumulate in the plaque and were equally represented, or even overrepresented, in the circulation. One CD8 + T cell clone in particular, whose Vα TCR sequence was identified as specific for CMV, was hyperexpanded and accounted for a substantial percentage of clonally expanded T cells in the plaque while also contributing to the clonally expanded CD8 + T cell pool in the PBMCs of this patient. Moreover, this clone did not show a signature of recent antigen encounter. Apart from classical CD4 + and CD8 + T cells, we also identified a pro-inflammatory MAIT cell population. MAIT cells have been described in multiple autoimmune and inflammatory diseases, including PSA, with contradicting or unknown contributions to disease development. How MAIT cells contribute to atherosclerosis development and whether they are activated through their non-polymorphic MHC class I-like protein MR1 or through TCR-independent activation induced by e.g. IL-12 and IL-18 (refs. 34 , 35 , 36 ) needs further elucidation.
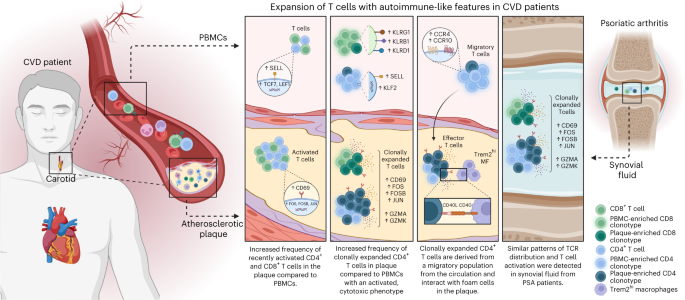
Schematic presentation of the main conclusions.
By instead focusing on the clonally enriched T cells specific for the plaque, we observed that one subset of effector CD4 + T cells was considerably enriched in clonally expanded TCRs and expressed genes indicative of recent antigen engagement. Although we found two such populations in the CD8 + T cells, their clonal enrichment was less pronounced. Interestingly, we also observed an antigen activation signature in the plaque-residing T reg cells, suggesting that these T cells undergo antigen-specific interactions in the plaque. However, these T reg cells did not show substantial clonal expansion, suggesting that these cells do not expand in the plaque. Instead, RNA velocity analysis suggests that T reg cells are not derived from any other T cell population that we detected in PBMC or plaque. Also, we observed minimal overlapping TCR sequences between T reg cells and other T cells in the plaque, in contrast to the effector CD4 + T cell population, which showed considerable TCR overlap with a migratory CD4 + T cell subset in the circulation. Previous work suggests that T reg cells can lose their suppressive capacity and gain expression of pro-inflammatory markers 37 . A shift of autoreactive (ApoB100-specific) T reg cells toward a T h17 phenotype has been associated with severity of CVD. Although the authors show in mice that this shift happens independent of the TCR clonotypes, our data argue against such a shift and suggest that T reg cells and effector CD4 + T cells do not derive from the same ancestor but, rather, develop independent of one another. Alternatively, the number of TCRs detected here may not have been sufficient to find overlapping sequences between T reg cells and effector CD4 + T cells. Also, it is unknown whether ApoB100-specific T cells undergo antigen-specific interaction in the plaque, and, because the antigen specificity of T cells investigated in this study are unknown, it is possible that we did not examine ApoB100-specific CD4 + and CD8 + T cells here.
We attempted to cluster the TCRs in silico using GLIPH2 and GIANA algorithms 38 , 39 , which are based on CDR3β similarity, as this is proposed to be an attractive way to cluster TCRs for a specific antigen together. However, a convincing clustering of plaque-enriched clonotypes was not observed in our dataset. The current clustering algorithms may have some limitations, which, in our data, was illustrated by co-clustering of CD4 + T cell-derived and CD8 + T cell-derived clonotypes, which was resolved only if the CDR3α sequence was included. Moreover, we observed diffuse clustering of clonotypes previously reported as ApoB100 specific 40 , suggesting that the current algorithms are not specific enough to resolve TCR clustering in atherosclerosis. Therefore, we think that a more stringent approach that includes both CDR3α and CDR3β needs to be developed.
As we observe antigen-specific activation in both the effector and T reg subsets, it is currently unclear what the overall effect of TCR engagement in the lesion is. Previous work in mice has shown mixed results with MHCII −/− apoE −/− mice, suggesting that this interaction is protective, whereas various papers suggest a pathogenic role for CD4 + T cells in atherosclerosis 41 , 42 . Interestingly, our work identifies several pathways involved in co-stimulation and immunological synapse formation that potentially drive pathogenic interactions of effector CD4 + T cells with the M-TREM2 (foam cell) population. When limited to effector CD4 + T cell populations, these may be specific and druggable targets. For instance, the expression of CD40LG on the clonally enriched effector population suggests active signalling to foam cells through CD40. This co-stimulatory pathway and that of other TNF superfamily member has been extensively studied in mouse models of atherosclerosis and is the subject of a clinical study 43 , 44 . The observation of antigen-specific T reg interaction also provides a rationale for potential therapeutic possibilities, such as expanding these cells by means of vaccination or development of tolerogenic chimeric antigen receptor (CAR) T cells. Identification of the antigen(s) driving T reg interaction in the plaque will be crucial for this development. Potential antigens, such ApoB100, heat shock proteins and fibronectin, have been suggested as potential self-antigens and have shown therapeutic potential as antigens in mouse models 45 , 46 , 47 and may serve as a potential starting point for vaccine development. Thus, here we highlight an autoimmune component to the pathophysiology of atherosclerosis, and we confirm a rationale for immunotherapeutic interventions in CVD.
Patient cohorts
For flow cytometry (cohort 1) and bulk TCRβ sequencing (cohort 3), whole blood and atherosclerotic plaques were obtained from, respectively, 61 and 10 patients who underwent carotid endarterectomy (CEA) surgery at the Haaglanden Medical Center Westeinde (HMC; The Hague, The Netherlands). The study was approved by the Medical Ethics Committee of the HMC (study approval number, cohort 1: 17-046, protocol number NL57482.098.17; study approval number, cohort 3: Z19.075, protocol number NL71516.058.19). For scTCR-seq, whole blood and atherosclerotic plaques were obtained from three male patients who underwent CEA (cohort 2). Patients were included in the Athero-Express biobank ( www.atheroexpress.nl ), an ongoing biobank study at the University Medical Centre Utrecht (UMCU) 48 . The study was approved by the Medical Ethics Committee of the UMCU (study approval number: TME/C-01.18, protocol number 03/114). All blood samples were collected by venipuncture before surgery. Atherosclerosis specimens were obtained from primary CEAs, and estenotic plaques were excluded due to their different plaque composition as compared to primary atherosclerotic plaques 49 . Informed consent was obtained from all patients involved in this study.
Whole blood processing
Peripheral venous blood was collected in K2-EDTA blood tubes (BD Vacutainer). For scTCR-seq, blood was processed within 10 minutes after withdrawal (cohort 2). For both cohort 1 and cohort 2, blood was diluted 1:2 in PBS containing 2% FCS. A density gradient was created using SepMate PBMC isolation tubes (STEMCELL Technologies) containing Ficoll-Paque Premium (GE Healthcare). Cells were centrifuged at 1,200 g for 10 minutes at room temperature. The intermediate layer containing PBMCs was isolated and washed twice with PBS + 2% FCS (250 g , 10 minutes, room temperature). Cells were taken up in PBS + 1% BSA until further processing. For cohort 3, whole blood samples were lysed twice with ACK lysis buffer in PBS (1:10) for 10 minutes at room temperature and washed with PBS (300 g , 5 minutes). Cells were taken up in RPMI + 1% FCS and cryostored in CryoStor cell cryopreservation medium (Sigma-Aldrich) until further use.
Human atherosclerotic plaque cell isolation
Human carotid plaques were collected during CEA; the culprit segment (5 mm) was used for histology and embedded in paraffin as described elsewhere 48 . In brief, culprit segments were fixed in 4% formaldehyde and decalcified in 10% EDTA, pH 7.5. Afterwards, culprit segments were embedded in paraffin. Time between surgical removal and plaque processing did not exceed 10 minutes. The inclusion of a small medial layer in the dissected tissue could not be excluded during the surgical procedure. The remainder of the plaque was washed in RPMI and minced into small pieces with a razor blade. The tissue was then digested in RPMI 1640 containing 2.5 mg ml −1 of collagenase IV (Thermo Fisher Scientific), 0.25 mg ml −1 of DNAse I (Sigma-Aldrich) and 2.5 mg ml −1 of Human Albumin Fraction V (MP Biomedicals) at 37 °C for 30 minutes. In cohort 2, 1 µM flavopiridol (Selleck Chemicals) was added to the digestion mixture. Subsequently, the plaque cell suspension was filtered through a 70-µm cell strainer and washed with RPMI 1640. Cells were kept in RPMI 1640 with 1% FCS until subsequent staining for flow cytometry (cohort 1), feature barcoding and FACS (cohort 2) or cryostored in CryoStor cell cryopreservation medium (Sigma-Aldrich) until further use.
Flow cytometry
Single-cell suspensions from blood and plaque from cohort 1 were stained with a mixture of extracellular antibodies for 30 minutes at 37 °C (Supplementary Table 4 ). All measurements were performed on a CytoFLEX S (Beckman Coulter) and analysed with FlowJo version 10.7 (Tree Star). A Shapiro log-normality test was performed, and a two-tailed Mann–Whitney test was performed using GraphPad analysis software to determine significance.
Antibody staining for feature barcoding and FACS
PBMCs of cohort 2 were stained with TotalSeq-C antibodies against CD3, CD4, CD8 and CD14 (Supplementary Table 4 ). Antibody pools containing 0.25 µg per antibody were prepared in labeling buffer (PBS + 1% BSA) and spun down at 14,000 g for 10 minutes at room temperature, and supernatant was collected for further staining. First, cells were stained with Human TruStain FcX (BioLegend) for 10 minutes at 4 °C. Next, the antibody pool supernatant was added and incubated for 30 minutes at 4 °C. Cells were washed three times with labeling buffer at 400 g for 5 minutes at 4 °C. Next, cells were taken up in PBS + 0.4% BSA and further processed with 10x Genomics.
Single-cell suspensions of plaques of cohort 2 were stained with TotalSeq-C antibodies against CD3, CD4, CD8 and CD14 (Supplementary Table 4 ). Antibody pools containing 0.25 µg per antibody and plaque (1 µg per antibody) single-cell suspensions were prepared in labeling buffer (PBS + 1% BSA) and spun down at 14,000 g for 10 minutes at room temperature, and supernatant was collected for further staining. First, cells were stained with Human TruStain FcX (BioLegend) for 10 minutes at 4 °C. Next, the antibody pool supernatant was added together with Calcein AM (1:1,000, Thermo Fisher Scientific), Hoechst (1:1,000, Thermo Fisher Scientific) and CD45-PECy7 (1:200, clone HI30, BD Biosciences) and incubated for 30 minutes at 4 °C. Cells were washed three times with labeling buffer at 400 g for 5 minutes at 4 °C. Next, cells were taken up in PBS + 2% FBS. Live CD45 + plaque cells were sorted using the BD FACSAria II (BD Biosciences) in PBS + 0.04% BSA and further processed with 10x Genomics.
scTCR-seq by 10x Genomics
scTCR-seq was performed on PBMCs and live CD45 + plaque cell suspensions from cohort 2 using 10x Genomics 5′ Single Cell Immune Profiling technology. Sequencing libraries were prepared using the 5′ version 1.1 chemistry following standard 10x Genomics protocol. Sequencing was performed using the Illumina NovaSeq 6000 (Novogene).
Bulk TCRβ sequencing
Genomic DNA was extracted from plaque single-cell suspensions and matched PBMC samples (cohort 3) using a DNA extraction kit in accordance with the manufacturer’s instructions (Qiagen). Sequencing of the VDJ locus was performed using the Adaptive Biotechnologies TCRβ sequencing platform.
scTCR-seq data processing, clustering and clonotype quantification
scTCR-seq data analyses were executed in R-4.0.1 and R-4.1.3 environments, primarily using Seurat (version 4.0.0–4.1.1) 50 , 51 . scTCR-seq data were processed as previously described 51 , 52 . In short, reads were filtered for mitochondrial, ribosomal genes and long non-coding RNA genes. To remove apoptotic cells, low-quality cells and doublets, only cells with a gene expression below 2% for KCNQ1OT1, below 2% for UGDH-AS1, below 2% for GHET1 and expressing between 200 and 5,000 genes were used for further analysis. Quality control (QC)-filtered PBMC and plaque Seurat objects were first merged per patient, after which the patient-merged Seurat objects were normalized using the SCT method, integrated using rpca reduction and clustered according to the Seurat ‘scRNA-seq integration’ vignette. VDJ sequencing data were imported into Seurat using the combineExpression function of scRepertoire (version 1.4.0) 53 . The complete integrated dataset was mapped to the pbmc_multimodal.h5seurat dataset ( https://atlas.fredhutch.org/data/nygc/multimodal/pbmc_multimodal.h5seurat ) to transfer cell type labels to the integrated Seurat object.
For subclustering, T cells were selected from the complete integrated dataset, taking the clusters with protein expression of CD3, CD4 and CD8 and without CD14 expression (ADT assay). Before reclustering the T cells, variable TCR genes were removed from the variable genes list, before principal component analysis (PCA) and clustering, to avoid clustering based on TCR, interfering with clustering on T cell phenotypes. However, TCR genes were not removed from the dataset. Separate CD4 + T cell and CD8 + T cell objects were then created by subsetting the T cell object based on, respectively, protein expression of CD4 > 0.75 and CD8 > 1.0 in the ADT assay. Custom clonotype counting functions were used to quantify the clonotype content of the individual samples based on the amino acid sequences of the TCRs. Clonotype frequencies relating to the total TCR repertoire per patient, per tissue are depicted in the atherosclerosis figures. Volcano plots were created using EnhancedVolcano (version 1.8.0) 54 . For all volcano plots, the FindMarkers function of Seurat was used to define differential genes between both groups by using a non-parametric Wilcoxon rank-sum test to determine significance. To assess the differentiation trajectories of the CD4 + T cells and CD8 + T cells, Monocle3 and velocyto.R (version 0.6) were used 22 , 55 . To assess possible interactions of antigen-presenting cells and T cells in the plaque, CellChat (version 1.4.0) was used 25 .
Definition of clonotype expansion levels and tissue enrichment scores
The TCR amino acid sequences were used to define the clonotypes. The clonotype abundance of a clonotype was calculated as the percentage of cells expressing a certain clonotype within a tissue of a patient, divided by the total number of cells in which a TCR was detected in the same tissue of the same patient. Based on the number and percentage of cells expressing the same clonotype, clonotypes were classified as Hyperexpanded, Large, Medium, Small or Single in the tissues of the patients (Supplementary Table 5 ). Furthermore, the tissue enrichment of clonotypes was determined according to the parameters listed in Supplementary Table 6 .
Integration with PSA scTCR-seq data
T cells from our scTCR-seq atherosclerosis dataset were compared with TCR-seq data from donor-matched PBMCs and synovial tissue from patients with PSA (ArrayExpress: E-MTAB-9492; European Genome-phenome Archive: EGAS00001002104 ) 31 . The same QC and processing steps were performed for the PSA dataset as described above for our atherosclerosis dataset. Subsequently, the integrated PSA dataset was mapped to the UMAP reduction of our complete T cell object, using our atherosclerosis dataset as reference. Because CD4 + T cells and CD8 + T cells could not be separated cleanly based on the clustering, and the PSA dataset does not contain protein expression data, the atherosclerosis dataset and the PSA dataset were divided based on the predicted cell type (CD4 T cell or CD8 T cell), derived from the pbmc_multimodal.h5seurat dataset. Subsequently, the atherosclerosis and PSA CD4 + T cell and CD8 + T cell datasets were split by patient and reintegrated as previously described for the atherosclerosis object, to form a CD4 + T cell object and a CD8 + T cell object containing atherosclerosis-derived and PSA-derived T cells. Then, the integrated datasets were mapped to our original CD4 + T cell and CD8 + T cell UMAP reductions. Because the PSA dataset is devoid of naive T cells due to the T cell isolation procedure used by Penkava et al. 31 , naive T cell clusters were removed from the CD4 + T cell dataset (clusters 1 and 2) and the CD8 + T cell dataset (cluster 6) before quantification of the clonotype abundance 31 .
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The raw scTCR-seq data from the Athero-Express cohort are not publicly available due to research participant privacy/consent. These data and the bulk TCRβ sequencing data can be accessed via DataverseNL at this address: https://doi.org/10.34894/DDYKLL . There are restrictions on use by commercial parties and on sharing openly based on (inter)national laws and regulations and written informed consent. Therefore, these data (and additional clinical data) are available only upon discussion and signing a data sharing agreement (see Terms of Access in DataverseNL) and within a specially designed UMCU-provided environment.
Open-source scTCR-seq data from donor-matched PBMCs and synovial tissue from patients with PSA that we used in this study are publicly available (ArrayExpress: E-MTAB-9492; European Genome-phenome Archive: EGAS00001002104 ) 31 .
Code availability
In silico data analysis was performed using custom-made R scripts designed specifically for this study and/or based on the recommended pipelines from pre-existing packages listed above. R scripts are available via Zenodo ( https://doi.org/10.5281/zenodo.7415207 ).
Ridker, P. M. et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 377 , 1119–1132 (2017).
Article CAS PubMed Google Scholar
Nidorf, S. M. et al. Colchicine in patients with chronic coronary disease. N. Engl. J. Med. 383 , 1838–1847 (2020).
Depuydt, M. A. C. et al. Microanatomy of the human atherosclerotic plaque by single-cell transcriptomics. Circ. Res. 127 , 1437–1455 (2020).
Article CAS PubMed PubMed Central Google Scholar
Fernandez, D. M. et al. Single-cell immune landscape of human atherosclerotic plaques. Nat. Med. 25 , 1576–1588 (2019).
Stemme, S. et al. T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Med. Sci. 92 , 3893–3897 (1995).
CAS Google Scholar
Wolf, D. et al. Pathogenic autoimmunity in atherosclerosis evolves from initially protective apolipoprotein B 100 -reactive CD4 + T-regulatory cells. Circulation 142 , 1279–1293 (2020).
Roy, P. et al. Immunodominant MHC-II (major histocompatibility complex II) restricted epitopes in human apolipoprotein B. Circ. Res. 131 , 258–276 (2022).
Benne, N. et al. Anionic 1,2-distearoyl-sn-glycero-3-phosphoglycerol (DSPG) liposomes induce antigen-specific regulatory T cells and prevent atherosclerosis in mice. J. Control. Release 291 , 135–146 (2018).
Gisterå, A. et al. Vaccination against T-cell epitopes of native ApoB100 reduces vascular inflammation and disease in a humanized mouse model of atherosclerosis. J. Intern. Med. 281 , 383–397 (2017).
Article PubMed Google Scholar
Chowdhury, R. R. et al. Human coronary plaque T cells are clonal and cross-react to virus and self. Circ. Res. 130 , 1510–1530 (2022).
Cibrián, D. & Sánchez-Madrid, F. CD69: from activation marker to metabolic gatekeeper. Eur. J. Immunol. 47 , 946–953 (2017).
Article PubMed PubMed Central Google Scholar
Schenkel, J. M. et al. T cell memory. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science 346 , 98–101 (2014).
Kranzer, K. et al. CpG-oligodeoxynucleotides enhance T-cell receptor-triggered interferon-γ production and up-regulation of CD69 via induction of antigen-presenting cell-derived interferon type I and interleukin-12. Immunology 99 , 170–178 (2000).
Mohr, A., Malhotra, R., Mayer, G., Gorochov, G. & Miyara, M. Human FOXP3 + T regulatory cell heterogeneity. Clin. Transl. Immunol. 7 , e1005 (2018).
Article Google Scholar
Wherry, E. J. & Kurachi, M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 15 , 486–499 (2015).
Khan, O. et al. TOX transcriptionally and epigenetically programs CD8 + T cell exhaustion. Nature 571 , 211–218 (2019).
Padhan, K. & Varma, R. Immunological synapse: a multi-protein signalling cellular apparatus for controlling gene expression. Immunology 129 , 322–328 (2010).
Bagaev, D. V. et al. VDJdb in 2019: database extension, new analysis infrastructure and a T-cell receptor motif compendium. Nucleic Acids Res. 48 , D1057–D1062 (2020).
Vorkas, C. K. et al. Single-cell transcriptional profiling reveals signatures of helper, effector, and regulatory MAIT cells during homeostasis and activation. J. Immunol. 208 , 1042–1056 (2022).
Liuzzo, G. et al. Monoclonal T-cell proliferation and plaque instability in acute coronary syndromes. Circulation 102 , 2883–2888 (2000).
Liuzzo, G. et al. Unusual CD4 + CD28 null T lymphocytes and recurrence of acute coronary events. J. Am. Coll. Cardiol. 50 , 1450–1458 (2007).
La Manno, G. et al. RNA velocity of single cells. Nature 560 , 494 (2018).
Ali, A. J., Makings, J. & Ley, K. Regulatory T cell stability and plasticity in atherosclerosis. Cells 9 , 2665 (2020).
Wang, X. et al. Visualizing CD4 T-cell migration into inflamed skin and its inhibition by CCR4/CCR10 blockades using in vivo imaging model. Br. J. Dermatol. 162 , 487–496 (2010).
Jin, S. et al. Inference and analysis of cell–cell communication using CellChat. Nat. Commun. 12 , 1088 (2021).
Foks, A. C. & Kuiper, J. Immune checkpoint proteins: exploring their therapeutic potential to regulate atherosclerosis. Br. J. Pharmacol. 174 , 3940–3955 (2017).
Kwon, I. O. et al. CD99 activates T cells via a costimulatory function that promotes raft association of TCR complex and tyrosine phosphorylation of TCR ζ. Exp. Mol. Med. 39 , 176–184 (2007).
Bixel, G. et al. Mouse CD99 participates in T-cell recruitment into inflamed skin. Blood 104 , 3205–3213 (2004).
Calandra, T. & Roger, T. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat. Rev. Immunol. 3 , 791–800 (2003).
D’Acquisto, F. et al. Annexin-1 modulates T-cell activation and differentiation. Blood 109 , 1095–1102 (2007).
Penkava, F. et al. Single-cell sequencing reveals clonal expansions of pro-inflammatory synovial CD8 T cells expressing tissue-homing receptors in psoriatic arthritis. Nat. Commun. 11 , 4767 (2020).
Björkbacka, H. et al. Weak associations between human leucocyte antigen genotype and acute myocardial infarction. J. Intern. Med. 268 , 50–58 (2010).
PubMed Google Scholar
Lin, Z. et al. Deep sequencing of the T cell receptor β repertoire reveals signature patterns and clonal drift in atherosclerotic plaques and patients. Oncotarget 8 , 99312–99322 (2017).
Treiner, E. et al. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature 422 , 164–169 (2003).
Godfrey, D. I., Koay, H. F., McCluskey, J. & Gherardin, N. A. The biology and functional importance of MAIT cells. Nat. Immunol. 20 , 1110–1128 (2019).
Van Wilgenburg, B. et al. MAIT cells are activated during human viral infections. Nat. Commun. 7 , 11653 (2016).
Kleinewietfeld, M. & Hafler, D. A. The plasticity of human Treg and Th17 cells and its role in autoimmunity. Semin. Immunol. 25 , 305–312 (2013).
Huang, H., Wang, C., Rubelt, F., Scriba, T. J. & Davis, M. M. Analyzing the Mycobacterium tuberculosis immune response by T-cell receptor clustering with GLIPH2 and genome-wide antigen screening. Nat. Biotechnol. 38 , 1194–1202 (2020).
Zhang, H., Zhan, X. & Li, B. GIANA allows computationally-efficient TCR clustering and multi-disease repertoire classification by isometric transformation. Nat. Commun. 12 , 4699 (2021).
Saigusa, R. et al. Single cell transcriptomics and TCR reconstruction reveal CD4 T cell response to MHC-II-restricted APOB epitope in human cardiovascular disease. Nat. Cardiovasc. Res. 1 , 462–475 (2022).
Wigren, M. et al. Lack of ability to present antigens on major histocompatibility complex class II molecules aggravates atherosclerosis in apoE −/− mice. Circulation 139 , 2554–2566 (2019).
Tabas, I. & Lichtman, A. H. Monocyte-macrophages and T cells in atherosclerosis. Immunity 47 , 621–634 (2017).
Smeets, E., Meiler, S. & Lutgens, E. Lymphocytic tumor necrosis factor receptor superfamily co-stimulatory molecules in the pathogenesis of atherosclerosis. Curr. Opin. Lipidol. 24 , 518–524 (2013).
Seijkens, T. T. P. et al. Targeting CD40-induced TRAF6 signaling in macrophages reduces atherosclerosis. J. Am. Coll. Cardiol. 71 , 527–542 (2018).
Chyu, K. Y. et al. Immunization using ApoB-100 peptide-linked nanoparticles reduces atherosclerosis. JCI Insight 7 , e149741 (2022).
Van Puijvelde, G. H. M. et al. Induction of oral tolerance to HSP60 or an HSP60-peptide activates T cell regulation and reduces atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 27 , 2677–2683 (2007).
Dunér, P. et al. Immunization of apoE −/− mice with aldehyde-modified fibronectin inhibits the development of atherosclerosis. Cardiovasc. Res. 91 , 528–536 (2011).
Verhoeven, B. A. N. et al. Athero-Express: differential atherosclerotic plaque expression of mRNA and protein in relation to cardiovascular events and patient characteristics. Rationale and design. Eur. J. Epidemiol. 19 , 1127–1133 (2004).
Hellings, W. E. et al. Histological characterization of restenotic carotid plaques in relation to recurrence interval and clinical presentation: a cohort study. Stroke 39 , 1029–1032 (2008).
R Core Team. R: A Language and Environment for Statistical Computing (R Foundation, 2019); https://www.r-project.org/
Butler, A., Hoffman, P., Smibert, P., Papalexi, E. & Satija, R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 36 , 411–420 (2018).
Stuart, T. et al. Comprehensive integration of single-cell data. Cell 177 , 1888–1902 (2019).
Borcherding, N., Bormann, N. L. & Kraus, G. scRepertoire: an R-based toolkit for single-cell immune receptor analysis. F1000Res. 9 , 47 (2020).
Blighe, K., Rana, S. & Lewis, M. EnhancedVolcano: Publication-Ready Volcano Plots with Enhanced Colouring and Labeling. R Package Version 1.16.0 (GitHub, Inc., 2022); https://github.com/kevinblighe/EnhancedVolcano
Cao, J. et al. The single-cell transcriptional landscape of mammalian organogenesis. Nature 566 , 496–502 (2019).
Download references
Acknowledgements
This work was supported by The Netherlands Heart Foundation (CVON2017-20: GENIUS II, supporting J.K., M.W., G.P., M.A.C.D., I.B. and B.S.); the Dekker Fellowship (2018T051 to A.C.F.); Spark-Holding BV (grant 2015B002 to M.W.); NWO-ZonMW (PTO program grant 95105013, supporting M.A.C.D., I.B. and J.K.); the European Union (ITN grant EPIMAC to M.W.); Fondation Leducq (Transatlantic Network Grants to M.W. and G.P.); EU 755320 Taxinomisis grant (supporting G.J.d.B., A.B. and G.P.); the European Research Area Network on Cardiovascular Diseases (ERA-CVD, 2018T092 supporting M.J.M.J. and B.S. and 2019T107 supporting J.M. and A.C.F.); NWO Veni (VI.Veni.212.196 to K.H.M.P.); NWO-ZonMW (open competition 09120011910025 to M.W.); and established investigator of The Netherlands Heart Foundation (2019T067, supporting E.H., L.D. and I.B.).
We would like to thank Single Cell Discoveries (Utrecht) for processing 10x Genomics samples. Study setup figures and the graphical abstract were created in BioRender.
Author information
These authors contributed equally: Marie A. C. Depuydt, Frank H. Schaftenaar.
These authors jointly supervised this work: Ilze Bot, Bram Slütter.
Authors and Affiliations
Leiden Academic Centre for Drug Research, Division of Biotherapeutics, Leiden University, Leiden, the Netherlands
Marie A. C. Depuydt, Frank H. Schaftenaar, Esmeralda Hemme, Lucie Delfos, Jill de Mol, Maaike J. M. de Jong, Mireia N. A. Bernabé Kleijn, Amanda C. Foks, Johan Kuiper, Ilze Bot & Bram Slütter
Amsterdam University Medical Centers, University of Amsterdam, Experimental Vascular Biology, Department of Medical Biochemistry, Amsterdam Cardiovascular Sciences, Amsterdam Infection and Immunity, Amsterdam, the Netherlands
Koen H. M. Prange & Menno P. J. de Winther
Central Diagnostic Laboratory, University Medical Center, Utrecht University, Utrecht, the Netherlands
Arjan Boltjes & Gerard Pasterkamp
Department of Surgery, Haaglanden Medisch Centrum Westeinde, The Hague, the Netherlands
Judith A. H. M. Peeters, Lauren Goncalves, Anouk Wezel & Harm J. Smeets
Department of Vascular Surgery, University Medical Centre Utrecht, Utrecht, the Netherlands
Gert J. de Borst
You can also search for this author in PubMed Google Scholar
Contributions
M.A.C.D., F.H.S., I.B, J.K., A.C.F. and B.S. drafted the manuscript and designed the figures. J.A.H.M.P., L.G., A.W., H.J.S. and G.J.B. performed carotid endarterectomy procedures and collected patient material. M.A.C.D., A.B., E.H., L.D., J.M., M.N.A.B.K. and M.J.M.J. executed the human plaque processing, FACS and flow cytometry. M.A.C.D., K.H.M.P., F.S., J.K., I.B. and B.S. participated in conceptualization and data interpretation and provided critical feedback on the manuscript. J.K., M.W., G.P., I.B. and B.S. participated in the conceptualization, funding and supervision of the scRNA-seq experiments and analysis and finalization of the manuscript. All authors provided feedback on the research, analyses and manuscript.
Corresponding authors
Correspondence to Ilze Bot or Bram Slütter .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Nature Cardiovascular Research thanks Federica Marelli-Berg, Jong-Eun Park and Jan Nilsson for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended data fig. 1 gating strategy of flow cytometry of cd69 + t cells..
a . Example of gating and gating ancestry of CD69 + T cells in PBMC. b . Example of gating and gating ancestry of CD69 + in the plaque.
Extended Data Fig. 2 Single-cell RNA sequencing of PBMC and live CD45 + plaque cells.
a . Gating strategy used for fluorescent-activated cell sorting (FACS) to isolate plaque live CD45 + cells for 10X Genomics and sequencing. b . UMAP projection of all PBMC and plaque cells, depicting multiple leukocyte types (n = 33249). c . UMAP visualization of tissue distribution of PBMC and plaque cells. d . UMAP projection of protein expression of CD3, CD4, CD8 and CD14 on all PBMC and plaque cells. e . Patient contribution to UMAP of all PBMC and plaque cells. Red dots indicate cells that are retrieved from the abovementioned patient. f . Patient contribution to UMAP of PBMC and plaque T cells. Red dots indicate cells that are retrieved from the abovementioned patient.
Extended Data Fig. 3 Distribution of clonal expansion levels and tissue-enrichment scores in T cell clusters.
a . Circle plots depicting clonal expansion levels of all T cells per tissue and per patient. b . Barplot with relative quantification of clonal expansion levels per cluster. c . Barplot with absolute quantification of clonal expansion levels per cluster. d . Relative quantification of tissue enrichment scores per cluster. e . Barplot with absolute quantification of tissue enrichment scores per cluster. Clonotype expansion levels: Single (one occurrence), Small (≤0.1%), Medium (>0.1 & ≤1%), Large (>1 & ≤10%), Hyperexpanded (>10%), percentage of all T cells. Tissue enrichment scores: Plaque-enriched (Frequency expanded clone higher in Plaque vs. PBMC), Single (one occurrence), Unenriched (Frequency expanded clone similar in PBMC vs. Plaque), PBMC-enriched (Frequency expanded clone higher in PBMC vs Plaque).
Extended Data Fig. 4 Hyperexpanded CMV clonotype does not show signs of recent T cell activation.
a . UMAP projection of clonotype CAVNGGSQGNLIF_CASSPWGGSDTQYF (CMV) on PBMC and plaque T cells. Red dots indicate T cells with clonotype CAVNGGSQGNLIF_CASSPWGGSDTQYF, grey dots indicate T cells with other clonotypes. b . Violin plots projecting gene expression of CD4 , CD8A and protein expression of CD4 and CD8 split by T cells with and without clonotype CAVNGGSQGNLIF_CASSPWGGSDTQYF. c . Violin plots projecting expression of CD69 , FOS and FOSB split by tissue and presence of clonotype CAVNGGSQGNLIF_CASSPWGGSDTQYF.
Extended Data Fig. 5 Distribution of expanded TCRs in scTCRseq and TCRβ bulk data sets.
a . Scatterplot projecting frequencies of clonotypes and their tissue enrichment scores in PBMC and plaque per patient of the single-cell TCR sequencing dataset (Cohort 2) and the TCRβ bulk sequencing data set (Cohort 3). Tissue enrichment scores: Plaque-enriched (Frequency expanded clone higher in Plaque vs. PBMC), Single (1 occurrence), Unenriched (Frequency expanded clone similar in PBMC vs. Plaque), PBMC-enriched (Frequency expanded clone higher in PBMC vs Plaque).
Extended Data Fig. 6 CD8 + T cell marker genes and tissue distribution.
a . CD4 and CD8 protein expression on all T cells colored by cluster ID. Visualization of selection of CD4 + CD8 − , CD4 − CD8 + , double positive (DP) and double negative (DN) cells. CD4 + CD8 − cells were used for subclustering of CD4 + T cells. CD4 − CD8 + cells were used for subclustering of CD8 + T cells. b . UMAP projection of tissue distribution of PBMC and plaque CD8 + T cells. c . Heatmap with expression of T cell function-associated genes in CD8 + T cell clusters. d . Dot plot visualization of a selection of differentially regulated genes, excluding TCR complex genes, between clusters 1, 4 and 7. e . Predicted expression of CD45RA and CD45RO based on mapping the data with Seurat multimodal reference mapping. f . Circle plots depicting clonal expansion levels of CD8 + T cells per tissue and per patient. g . Circle plots depicting tissue-enrichment scores of CD8 + T cells per tissue and per patient. Clonotype expansion levels: Single (one occurrence), Small (≤0.1%), Medium (>0.1 & ≤1%), Large (>1 & ≤10%), Hyperexpanded (>10%), percentage of all CD8 + T cells. Tissue enrichment scores: Plaque-enriched (Frequency expanded clone higher in Plaque vs. PBMC), Single (one occurrence), Unenriched (Frequency expanded clone similar in PBMC vs. Plaque), PBMC-enriched (Frequency expanded clone higher in PBMC vs Plaque).
Extended Data Fig. 7 CD4 + T cell marker genes and tissue distribution.
a . UMAP visualization of tissue distribution of PBMC and plaque CD4 + T cells. b . Heatmap with expression of T cell function-associated genes in CD4 + T cell clusters. c . Circle plot visualizing the overlap of clonotypes between all CD4 + clusters. Each color represents a different cluster. Axis indicates the number of TCRs. Line thickness indicates the number of overlapping clonotypes. d . Violin plots depicting expression of CCR4 and CCR10 in CD4+ T cell clusters. e . Circle plots depicting clonal expansion levels of CD4 + T cells per tissue and per patient. f . Circle plots depicting tissue-enrichment scores of CD4+ T cells per tissue and per patient. Clonotype expansion levels: Single (one occurrence), Small (≤0.1%), Medium (>0.1 & ≤1%), Large (>1 & ≤10%), Hyperexpanded (>10%), percentage of all CD4 + T cells. Tissue enrichment scores: Plaque-enriched (Frequency expanded clone higher in Plaque vs. PBMC), Single (one occurrence), Unenriched (Frequency expanded clone similar in PBMC vs. Plaque), PBMC-enriched (Frequency expanded clone higher in PBMC vs Plaque).

Extended Data Fig. 8 CellChat interaction pathways between CD8 + T cells and myeloid cells.
a . Dotplot displaying average expression of genes describing the different dendritic cell and macrophage clusters. DC-M indicates myeloid-derived dendritic cell (DC); DC-P indicates plasmacytoid DC; M-PROL indicates proliferating macrophages; M-Inf indicates inflammatory macrophage; M-TREM2 indicates TREM2hi macrophages. b . Heatmaps displaying outgoing (Ligand) and incoming (Receptor) signalling patterns of pathways describing potential ligand-receptor interactions. Scale above heatmap indicates the relative signalling strength of a cell cluster based on all signalling pathways displayed in the heatmap. Grey bars right of the heatmap show the total signalling strength of a pathway in all cell clusters. The relative signalling strength indicated by ranging color from white (low) to green (high). All cells included in these graphs originate from the plaque.
Extended Data Fig. 9 Projection of CD4 + and CD8 + T cells of integrated atherosclerosis and psoriatic arthritis single-cell TCR sequencing data on the reference UMAP projection of CD4 + and CD8 + atherosclerosis data.
a UMAP visualization of RNA expression of CD8A and CD4 on atherosclerosis T cells. b . rUMAP visualization of RNA expression of CD8A and CD4 on psoriatic arthritis T cells. c . UMAP visualization of protein expression of CD8 and CD4 on atherosclerosis T cells. d . rUMAP visualization of predicted protein expression of CD8 and CD4 on psoriatic arthritis T cells. e . UMAP visualization of selected CD8 + and CD4 + atherosclerosis T cells. f . UMAP visualization of selected CD8 + and CD4 + psoriatic arthritis T cells. g . UMAP of integrated CD4 + T cells split by diseased and grouped by tissue type. h . UMAP of integrated CD8 + T cells split by diseased and grouped by tissue type.
Extended Data Fig. 10 Extended dot plots with characterizing genes for atherosclerosis and psoriatic arthritis overlapping clonal expanded T cells.
Dotplots with genes used to characterize overlapping clusters of atherosclerosis and psoriatic arthritis per disease and per cluster of respectively CD4 + cluster 3 genes ( a ), CD4 + cluster 5 genes ( b ) CD8 + cluster 3 genes ( c ) and CD8 + cluster 5 genes ( d ).
Supplementary information
Reporting summary, supplementary tables 1 and 4–6..
Table 1: Baseline characteristics of patient cohorts. Table 4: Extracellular and intracellular antibodies used for flow cytometry and feature barcoding. Table 5: Clonal expansion levels. Table 6: Tissue enrichment scores.
Supplementary Table 2.
Differentially expressed genes of T cell, CD4 + and CD8 + T cell clusters.
Supplementary Table 3.
Per-patient display of clonal expansion levels and tissue enrichment scores for the whole dataset.
Source Data Fig. 1
Statistical Source Data
Source Data Fig. 2
Source data extended data fig. 3, source data extended data fig. 6, source data extended data fig. 7, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Depuydt, M.A.C., Schaftenaar, F.H., Prange, K.H.M. et al. Single-cell T cell receptor sequencing of paired human atherosclerotic plaques and blood reveals autoimmune-like features of expanded effector T cells. Nat Cardiovasc Res 2 , 112–125 (2023). https://doi.org/10.1038/s44161-022-00208-4
Download citation
Received : 05 August 2022
Accepted : 20 December 2022
Published : 30 January 2023
Issue Date : February 2023
DOI : https://doi.org/10.1038/s44161-022-00208-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Uncovering atherosclerotic cardiovascular disease by pet imaging.
- Alexander Maier
- Abraham J. P. Teunissen
- Mandy M. T. van Leent
Nature Reviews Cardiology (2024)
Breaking tolerance: the autoimmune aspect of atherosclerosis
Nature Reviews Immunology (2024)
Immunotherapy for atherosclerosis by targeting pro-inflammatory T cells
Cell Research (2024)
Dysregulated cellular metabolism in atherosclerosis: mediators and therapeutic opportunities
- Chad Stroope
- Felix Sebastian Nettersheim
- Arif Yurdagul
Nature Metabolism (2024)
Macrophage profiling in atherosclerosis: understanding the unstable plaque
- Ioanna Gianopoulos
- Stella S. Daskalopoulou
Basic Research in Cardiology (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

Introduction
Research design and methods, conclusions, article information, timing of moderate to vigorous physical activity, mortality, cardiovascular disease, and microvascular disease in adults with obesity.
A.S. and M.N.A. contributed equally to this work.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Cite Icon Cite
- Get Permissions
Angelo Sabag , Matthew N. Ahmadi , Monique E. Francois , Svetlana Postnova , Peter A. Cistulli , Luigi Fontana , Emmanuel Stamatakis; Timing of Moderate to Vigorous Physical Activity, Mortality, Cardiovascular Disease, and Microvascular Disease in Adults With Obesity. Diabetes Care 19 April 2024; 47 (5): 890–897. https://doi.org/10.2337/dc23-2448
Download citation file:
- Ris (Zotero)
- Reference Manager
To assess the association between timing of aerobic moderate to vigorous physical activity (MVPA) and risk of cardiovascular disease (CVD), microvascular disease (MVD), and all-cause mortality in adults with obesity and a subset with obesity and type 2 diabetes (T2D).
Participants included adults with obesity (BMI ≥30 kg/m 2 ) and a subset of those with T2D from the UK Biobank accelerometry substudy. Aerobic MVPA was defined as bouts of MVPA lasting ≥3 continuous minutes. Participants were categorized into morning, afternoon, or evening MVPA based on when they undertook the majority of their aerobic MVPA. The reference group included participants with an average of less than one aerobic MVPA bout per day. Analyses were adjusted for established and potential confounders.
The core sample included 29,836 adults with obesity, with a mean age of 62.2 (SD 7.7) years. Over a mean follow-up period of 7.9 (SD 0.8) years, 1,425 deaths, 3,980 CVD events, and 2,162 MVD events occurred. Compared with activity in the reference group, evening MVPA was associated with the lowest risk of mortality (hazard ratio [HR] 0.39; 95% CI 0.27, 0.55), whereas afternoon (HR 0.60; 95% CI 0.51, 0.71) and morning MVPA (HR 0.67; 95% CI 0.56, 0.79) demonstrated significant but weaker associations. Similar patterns were observed for CVD and MVD incidence, with evening MVPA associated with the lowest risk of CVD (HR 0.64; 95% CI 0.54, 0.75) and MVD (HR 0.76; 95% CI 0.63, 0.92). Findings were similar in the T2D subset ( n = 2,995).
Aerobic MVPA bouts undertaken in the evening were associated with the lowest risk of mortality, CVD, and MVD. Timing of physical activity may play a role in the future of obesity and T2D management.
Graphical Abstract

Obesity is a significant and independent risk factor for the development of type 2 diabetes (T2D) ( 1 ), cardiovascular disease (CVD), microvascular disease (MVD) ( 2 ), and premature mortality ( 3 ). These associations are fueled, in part, by obesity-related imbalances in adipokines, chronic inflammation, insulin resistance, and ensuing impaired glucose tolerance ( 4 , 5 ).
Engaging in moderate to vigorous physical activity (MVPA), particularly aerobic activity ( 6 ), is widely acknowledged as a therapeutic strategy for improving cardiometabolic risk factors ( 7 ). Although historically all MVPA, regardless of bout length, has been considered reflective of aerobic activity, very short MVPA bouts may not truly engage the aerobic energy system. Skeletal muscle predominantly relies on anaerobic energy pathways to meet sudden energy demand up to the first 3 min of MVPA, beyond which aerobic metabolism dominates ( 8 ). Recent evidence suggests that accumulating aerobic MVPA bouts is associated with a lower cardiovascular morbidity and mortality risk compared with accumulating shorter nonaerobic bouts ( 9 ).
Because obesity and T2D are associated with circadian misalignment and impaired metabolic processes ( 10 ), particularly during the evening ( 11 ), modulating the timing of MVPA may offset diurnal variations in glucose tolerance and insulin sensitivity ( 12 ), potentially leading to durable improvements in cardiovascular morbidity. Recent randomized trials have indicated that undertaking late-afternoon or evening aerobic exercise yields superior improvements in glucose control than that generated by morning aerobic exercise ( 13 – 15 ). However, it is unclear whether aerobic MVPA timing is associated with longer-term outcomes, such as morbidity and mortality, among individuals with exacerbated diurnal variations in glucose intolerance. Therefore, this study aimed to determine the association between the timing of MVPA, mortality, and incidence of CVD and MVD among adults with obesity and a subset also diagnosed with T2D.
Study Participants
This study included participants from the UK Biobank study, all of whom were enrolled between 2006 and 2010 and provided written informed consent. Ethical approval was obtained from the National Research Ethics Service of the U.K. National Health Service (NHS; ref. no. 11/NW/0382; London, U.K.). Participants underwent physical examinations conducted by trained staff and completed touchscreen questionnaires. The inclusion criteria were as follows: individuals with prevalent obesity (BMI ≥30 kg/m 2 ; ascertained through health linkage of general practitioner records), including those with T2D (ascertained through health linkage of medication prescription history, general practitioner records, and UK Biobank physical examination) ( Supplementary Table 1 ). Exclusion criteria were as follows: individuals with missing covariate data or those experiencing an event within the initial 24 months of follow-up ( 9 , 16 , 17 ). In analyses considering CVD and MVD as outcomes, participants with prevalent CVD (ascertained through self-report and hospital admission records) or MVD (ascertained through hospital admission records) were excluded where appropriate ( Supplementary Fig. 1 ).
Physical Activity Assessment
Between 2013 and 2015, a total of 103,684 participants wore an Axivity AX3 accelerometer (Axivity, Ltd, Newcastle Upon Tyne, U.K.) on their dominant wrist continuously for 24 h per day over a period of 7 days. Standard procedures were used for device calibration, and nonwear periods were detected using established methods ( 18 ). Participants with a minimum of 3 valid wear days, defined as wearing the accelerometer for at least 16 h per day, were included in the analysis. Physical activity intensity was determined in 10-s intervals using a validated machine-learning accelerometer-based two-level random forest classifier. Physical activity was first classified into one of four activity classes: sedentary, standing utilitarian movements (e.g., ironing a shirt, washing dishes), walking (e.g., active commuting, mopping floors), or running/high-energy activities (active play with children). These activity classes were then assigned to one of four activity intensities: sedentary, light, moderate, or vigorous ( 16 , 17 , 19 ). Walking activities were classified as light (an acceleration value of <100 mg), moderate (≥100 mg), or vigorous (≥400 mg) intensity. As described previously ( 9 ), this two-level physical activity classification scheme minimized the possibility of false-positive MVPA from stationary activities with high wrist movement, such as ironing or cleaning dishes, because an activity had to be classified first by level 1 as an ambulatory activity and then by level 2 as moderate or vigorous. Similar to in a previous study ( 20 ), to assess physical activity timing, participants were categorized into morning (6 a.m. to <12 p.m. ), afternoon (12 p.m. to <6 p.m. ), and evening MVPA (6 p.m. to <12 a.m. ) groups based on when the majority of their MVPA occurred in bouts lasting ≥3 min (e.g., participants undertaking 40%, 30%, and 30% of their total MVPA bouts in the morning, afternoon, and evening, respectively, would be assigned to morning MVPA). Although a previous study categorized morning MVPA as 5 a.m. to 11 a.m. , midday-afternoon MVPA as 11 a.m. to 5 p.m. , and evening MVPA as 5 p.m. to 12 a.m. ( 21 ), we elected to categorize participants into one of three 6-h time windows, as per a previous study ( 20 ), to allow for three even 6-h timing windows. The choice of the ≥3-min bout length aimed to better capture aerobic-based MVPA, known for its established benefits in improving cardiometabolic health in adults with obesity ( 22 ), as well as its association with reduced cardiovascular risk ( 9 ). Participants who did not accumulate at least one MVPA bout lasting ≥3 min in the morning, afternoon, or evening were categorized as having no aerobic physical activity bouts, irrespective of the total minutes of physical activity accumulated. Additionally, we calculated the total time spent undertaking MVPA (regardless of bout length) and MVPA accrued from bouts lasting <3 min.
Mortality, CVD, and MVD Ascertainment
Participants were observed through to 30 November 2022, with deaths obtained through linkage with NHS Digital of England and Wales or the NHS Central Register and National Records of Scotland. Inpatient hospitalization data were sourced from the Hospital Episode Statistics for England, the Patient Episode Database for Wales, or the Scottish Morbidity Record for Scotland. Detailed methods for CVD and MVD assessment are outlined in Supplementary Table 2 . In short, CVD was defined as a disease of the circulatory system, excluding hypertension and diseases of the arteries or lymph nodes ( 23 ). MVD was defined as neuropathy, nephropathy, or retinopathy ( 24 ). Follow-up time was calculated as the time in years from accelerometer wear to the first occurrence of event or censoring.
Covariates considered in the analysis included age, sex, smoking status, alcohol intake, fruit and vegetable consumption, sedentary time, total MVPA, sleep duration, education, medication use (cholesterol, antihypertensive, and/or diabetes medication), waist circumference, and prevalent CVD (all-cause mortality analysis only). Complete definitions for all covariates are provided in Supplementary Table 3 .
Cox proportional hazards regression models were used to estimate hazard ratios (HRs) with 95% CIs for all-cause mortality. For CVD and MVD analyses, participants with prevalent CVD (ascertained through self-report and hospital admission records) or MVD (ascertained through hospital admission records) were excluded where appropriate. Additionally, the Fine-Gray subdistribution method was used, treating deaths resulting from non-CVD or non-MVD causes as competing risks when appropriate. Cox proportionality assumptions were assessed using Schoenfeld residuals, with no observed violations. The association between physical activity timing and risk of all-cause mortality, CVD, and MVD was examined, using the “no aerobic bouts” group as the referent. The analysis also included an examination of adjusted 5-year absolute risk and age- and sex-adjusted incidence rate ratios. A dose-response analysis of activity bout frequency and total duration per day was conducted using restricted cubic splines with knots at the 10th, 50th, and 90th percentiles, with the reference group set to zero bouts and minutes per day. Additionally, the association of physical activity timing with each outcome was explored among participants with T2D, with the no aerobic bouts group as the referent.
Sensitivity Analyses
To assess residual confounding, a negative control outcome of death or hospitalization resulting from an accident (excluding cycling, self-harm, and falls) was used as this outcome does not have an explicit mechanistic link to physical activity ( 25 ). If the negative control had an association pattern similar to that of the primary outcomes, it would be more plausible that the associations were due to bias and confounding than to causality. A sensitivity analysis for total mortality was conducted, excluding participants with prevalent CVD and cancer, recognizing that adjustment for prevalent disease may not fully capture confounding. Additional analyses were performed, categorizing the no aerobic bouts group based on meeting or not meeting physical activity guidelines (150 min of MVPA/week). Additional analyses included assessments for total MVPA and MVPA accrued from bouts lasting <3 min. To assess the influence of more even temporal distributions of aerobic MVPA, sensitivity analyses were conducted for mortality, CVD, and MVD incidence in which participants were only assigned to morning, afternoon, or evening MVPA if >50% of their total daily aerobic MVPA occurred during the same time window; otherwise, they were classified as having mixed MVPA, similar to in previous studies ( 21 , 26 ). Additional analyses were conducted, adjusting for LDL and HDL, blood pressure, ethnicity, Townsend deprivation index, season of accelerometer wear time, ethnicity, and employment status. To assess the influence of diet quality on the primary results, a sensitivity analysis was conducted using the dietary quality index ( 23 ). Finally, sensitivity analyses were also conducted to determine the association of the exposure with all outcomes among nonshift workers. All analyses were conducted using R statistical software, and reporting adhered to the Strengthening the Reporting of Observational Studies in Epidemiology guideline.
Data and Resource Availability
The UK Biobank data that support the findings of this study can be accessed by bona fide researchers when applying to access the UK Biobank research resource to conduct health-related research.
Our sample for all-cause mortality included 29,836 participants, with a mean age of 62.2 years (SD ±7.7) at baseline; 53.2% were female, and 46.8% were either current or previous smokers. A total of 2,995 participants had a prevalent T2D diagnosis at baseline. During an average follow-up time of 7.9 years (SD ±0.8), corresponding to 236,387 person-years, 1,425 deaths occurred. The sample for CVD analyses included 24,660 participants with 3,980 events, and the MVD analysis sample included 28,455 participants with 2,162 events ( Supplementary Fig. 1 ). Throughout the week, participants in the reference group averaged fewer than one MVPA bout per day, whereas the morning MVPA group averaged 4.8 bouts per day in the morning, the afternoon MVPA group averaged 5.0 bouts per day in the afternoon, and the evening MVPA group averaged 3.4 bouts per day in the evening. Participant characteristics by physical activity timing group are listed in Table 1 .
Participant characteristics by physical activity timing group
Data are reported as mean (SD) or median (interquartile range) unless otherwise specified.
Adjusted 5-year absolute risk and incidence rate ratio are presented in Supplementary Table 4 . The 5-year all-cause mortality risk was 25–32% lower for participants in the evening MVPA group (1.79%; 95% CI 2.31%, 1.27%) than for those in the morning (2.64%; 95% CI 3.08%, 2.21%) or afternoon MVPA (2.43%; 95% CI 2.81%, 2.05%) group. Participants in the reference group had a 5-year mortality risk of 4.02% (95% CI 4.48%, 3.57%). This pattern was consistent for the 5-year risk of CVD incidence. For MVD incidence, the 5-year risk was similar between morning, afternoon, and evening MVPA groups and 20–24% lower than that of the reference group (e.g., 5-year risk 5.86%; 95% CI 6.59%, 5.13% vs. 7.78%; 95% CI 8.44%, 7.12% for morning MVPA group vs. reference group). Supplementary Figs. 2 and 3 show the dose-response association for aerobic MVPA (≥3 min) bout duration and frequency. Overall, the magnitude of association was stronger for activity bout frequency (e.g., nadir of the curve HR 0.39 for all-cause mortality) than for activity bout duration (nadir of the curve HR 0.59 for all-cause mortality).
All-Cause Mortality
Compared with the reference group, evening MVPA was associated with the lowest mortality risk (HR 0.39; 95% CI 0.27, 0.55) ( Fig. 1 ). Mortality risk was similar for participants in the afternoon (HR 0.60; 95% CI 0.51, 0.71) and morning MVPA (HR 0.67; 95% CI 0.56, 0.79) groups. Among participants diagnosed with obesity and T2D, evening MVPA was again associated with the lowest mortality risk (HR 0.24; 95% CI 0.08, 0.76), followed by afternoon MVPA (HR 0.44; 95% CI 0.28, 0.72) ( Supplementary Fig. 4 ). Notably, there was no observed association for participants in the morning MVPA (HR 0.86; 95% CI 0.57, 1.29) when compared with those in the reference group.

Association of aerobic MVPA bout (≥3 min) timing with all-cause mortality in adults with obesity. No aerobic bouts group represents participants who did not accumulate an average of one or more aerobic MVPA bout (≥3 min) per day over the week.
CVD Incidence
The findings for CVD incidence among participants with diagnosed obesity mirrored the pattern observed for all-cause mortality ( Fig. 2 ). Evening MVPA was associated with the lowest CVD incidence risk (HR 0.64; 95% CI 0.54, 0.75). Morning MVPA was associated with a CVD incidence risk of 0.83 (95% CI 0.76, 0.91), and afternoon MVPA was associated with an incidence risk of 0.84 (95% CI 0.77, 0.91). Among participants with obesity and T2D, evening MVPA was associated with a CVD incidence risk of 0.54 (95% CI 0.34, 0.86), whereas morning and afternoon MVPA showed smaller or null associations, with HRs of 0.73 (95% CI 0.56, 0.94) and 0.85 (95% CI 0.69, 1.06), respectively ( Supplementary Fig. 5 ).

Association of aerobic MVPA bout (≥3 min) timing with the incidence of CVD in adults with obesity. No aerobic bouts group represents participants who did not accumulate an average of one or more aerobic MVPA bouts (≥3 min) per day over the week.
MVD Incidence
Regarding the incidence of nephropathy, neuropathy, and retinopathy, we observed a similar magnitude of association across each of the three physical activity timing groups. Participants in the morning, afternoon, and evening MVPA groups had respective HRs of 0.79 (95% CI 0.70, 0.89), 0.84 (95% CI 0.75, 0.93), and 0.76 (95% CI 0.63, 0.92) ( Fig. 3 ). Among participants with diagnosed T2D, the strength of association was greatest among those in the evening MVPA group (HR 0.52; 95% CI 0.32, 0.86), with null or smaller associations observed in the morning (HR 0.89; 95% CI 0.69, 1.14) and afternoon MVPA groups (HR 0.75; 95% CI 0.59, 0.95) ( Supplementary Fig. 6 ).

Association of aerobic MVPA bout (≥3 min) timing with the incidence of MVD in adults with obesity. No aerobic bouts group represents participants who did not accumulate an average of one or more aerobic MVPA bouts (≥3 min) per day over the week.
Additional and Sensitivity Analyses
The analyses for negative control outcomes suggested that residual and unmeasured confounding likely had a minimal impact on the findings. Specifically, with the negative control outcome, the HR point estimate pattern was inconsistent relative to the main analyses, with no significant associations for any of the physical activity timing groups ( Supplementary Fig. 7 ). Additional analyses controlling for LDL and HDL, blood pressure, ethnicity, Townsend deprivation index, season of accelerometer wear time, ethnicity, employment status, and diet quality index were consistent with our main analyses ( Supplementary Tables 5 and 6 ). Consistent results for all-cause mortality were observed after excluding participants with prevalent CVD and cancer ( n = 5,258) ( Supplementary Fig. 8 ). The mortality risk ranged from 0.48 (95% CI 0.36, 0.65) for evening MVPA to 0.66 (95% CI 0.56, 0.78) for morning MVPA. Furthermore, separating the no aerobic bouts reference group into those meeting and not meeting physical activity guidelines (i.e., <150 min of MVPA/week) showed that meeting physical activity guidelines did not lower the risk of mortality or CVD or MVD incidence, if no aerobic bouts were undertaken ( Supplementary Figs. 9 , 10 , and 11 ). Physical activity timing from bouts lasting <3 min was not associated with a lower risk of mortality or CVD or MVD incidence ( Supplementary Figs. 12 , 13 , and 14 ). The sensitivity analyses in nonshift workers yielded consistent results ( Supplementary Table 7 ). When undertaking sensitivity analyses to control for more even temporal distributions of aerobic MVPA (i.e., participants not undertaking >50% of total aerobic MVPA in one of the three time windows), the results showed that evening MVPA was associated with the lowest incidence rates in all outcomes relative to afternoon and morning MVPA, although similar to the primary analyses, there was little difference between timing groups for MVD. When compared with activity in the mixed MVPA group, evening MVPA was associated with the lowest risk of mortality, and with a similar incidence of CVD. Mixed MVPA was associated with the lowest incidence of MVD ( Supplementary Figs. 15 , 16 , and 17 ). Finally, there was an inverse dose-response association between total MVPA, including both aerobic and nonaerobic bouts (any bout length), and all-cause mortality and CVD and MVD incidence ( Supplementary Fig. 18 ).
Increasing MVPA is a proven strategy for effectively managing cardiometabolic risk in adults with obesity and related disorders. This study, to our knowledge, is the first to determine the associations between objectively measured aerobic MVPA timing, all-cause mortality, and incidence of CVD and MVD in adults with obesity. These findings demonstrate a compelling connection between MVPA timing and a lower risk of morbidity and mortality in adults with obesity, including those with T2D. Building upon previous clinical studies ( 14 , 15 ), our analyses underscore the consistent association of evening MVPA with the lowest risk in mortality, as well as strong associations with the incidence of CVD and MVD, when compared with not undertaking aerobic MVPA bouts. These findings are robust and extend to the subset of participants with T2D, in whom evening MVPA exhibited even more pronounced associations with mortality and cardiovascular morbidity. Sensitivity analyses demonstrated that when controlling for more even temporal distributions of aerobic MVPA, evening MVPA was associated with the greatest reduction in mortality, whereas more evenly spread MVPA was associated with the greatest reduction in MVD incidence. Finally, the frequency of aerobic bouts seems to be a more important factor in their association with mortality and CVD and MVD incidence than the duration of aerobic MVPA. Although additional well-designed clinical studies are required to confirm these findings, these observational data suggest that MVPA timing may play a significant role in optimizing MVPA-related interventions among adults contending with obesity and T2D.
Insulin resistance, a common feature in both obesity and T2D, denotes impairments in insulin-mediated processes such as glucose uptake, metabolism, and storage across diverse cell types, including adipocytes, hepatocytes, and skeletal muscle ( 27 ). Recognized as a key driver of obesity-related disease and aging ( 27 , 28 ), insulin resistance maintains an inverse association with mortality, independent of body weight ( 29 ). Although MVPA per se exhibits an inverse relationship with insulin resistance ( 30 ) and is linked to a lower mortality risk among individuals with or susceptible to T2D ( 31 , 32 ), the potential impact of undertaking MVPA during specific time windows on morbidity and mortality remains unclear. Previous findings have shown that MVPA performed in the evening is associated with the greatest improvement in insulin sensitivity (+25%) among adults with or without T2D ( 20 ). Our findings add to previous reports by showing that when controlling for total MVPA volume, the timing of MVPA, particularly in the evening, is linked with the lowest risk in all-cause mortality. Additionally, the frequency of aerobic MVPA bouts demonstrated a greater inverse association with mortality risk than the total volume of MVPA, highlighting that accumulating bouts of MVPA within specific timing windows or throughout the day may lead to improved health outcomes.
The robust findings of this study are in line with previously published cohort ( 20 ) and clinical studies ( 14 , 15 ). However, it is important to note that these findings differ from those of other similar studies ( 21 ). For example, a recent study by Feng et al. ( 21 ) showed that afternoon or mixed MVPA but not evening MVPA was associated with a lower mortality risk and CVD incidence than morning MVPA. These seemingly disparate findings may be explained by methodological differences. Firstly, Feng et al. selected morning MVPA as the reference group, whereas in our study, the reference group reflected individuals who did not undertake any aerobic MVPA. Furthermore, where our study focused on individuals with obesity, Feng et al. included individuals from the general population with and without obesity, which likely affected the results, given the association between obesity, circadian misalignment, and metabolic dysfunction ( 10 , 11 ). This may explain why, even when undertaking a sensitivity analysis to account for more even temporal distributions of MVPA, evening MVPA was still associated with a greater reduction in mortality and equal reduction in CVD incidence compared with activity in the mixed group.
Among adults with T2D, more than half of all deaths are related to CVD-related events, including myocardial infarction and ischemic stroke ( 33 ). The results of this study showed that when compared with other MVPA timing windows, evening MVPA was associated with the lowest incidence of CVD among adults with obesity, including those with T2D. Although further research is needed to uncover the precise mechanism behind this association, our findings align with previous studies indicating that moderate- or vigorous-intensity exercise performed in the evening may be linked to lower mean arterial blood pressure, whereas among morning exercisers, it was increased ( 34 ). Similarly, research suggests that evening, but not morning, aerobic exercise can lead to significant reductions in clinic and ambulatory blood pressure, through improvements in systemic vascular resistance and vasomotor sympathetic modulation, as demonstrated in a 10-week randomized trial involving 50 men with hypertension ( 35 ).
An important finding of this study was that aerobic MVPA, regardless of timing, was linked to a reduced risk of MVD. Although the sensitivity analyses revealed that mixed MVPA, followed by evening MVPA, was associated with the greatest reduction in MVD incidence, the differences between the timing windows (morning, afternoon, and evening) were minimal and nonsignificant. This finding highlights the role of MVPA in MVD prevention, and may be explained, in part, by the effects of MVPA on hyperglycemia and oxidative stress, which directly contribute to MVD development and progression ( 36 ). For example, findings from a previous clinical study demonstrated that moderate- to vigorous-intensity exercise directly improved microvascular function through improvements in redox balance via increased nitric oxide production ( 37 ). Furthermore, in addition to the well-established effects of chronic exercise on glycemia ( 38 ), it may be that more frequent episodes of contraction-stimulated glucose uptake into skeletal muscle may reduce hyperglycemic excursions throughout the day. This hypothesis is further supported by the significant association between frequency of MVPA and MVD demonstrated in our results. Thus, it seems plausible that these separate mechanistic pathways may have an additive interaction, thereby reducing the risk of MVD; however, additional studies are required to confirm this hypothesis.
For adults with obesity and T2D, where blood glucose regulation is an ongoing challenge, the results of this study highlight that evening MVPA may yield the greatest benefits in terms of cardiovascular morbidity and mortality. Although the precise mechanisms driving this observation remain unclear, the concept of the dawn phenomenon, suggesting that T2D impairs the circadian rhythm, may offer insights. Individuals with T2D, partly due to desynchronized rhythms, often experience relatively better insulin sensitivity and glycemia in the evening, which progressively worsens overnight to the early morning ( 12 , 39 ). Therefore, engaging in MVPA later in the afternoon or evening, when postprandial glycemia is highest and hepatic insulin sensitivity begins to decline, may elicit the greatest metabolic benefits by directly influencing these pathways and leading to lower morning fasting glucose levels ( 12 ). Additionally, because β-cell function and glucose tolerance are reduced in the circadian evening ( 11 ), particularly in individuals with T2D, MVPA at this time may improve β-cell function when it is needed most. Additional well-designed clinical studies are required to delve deeper into these findings; however, this theory finds support, in part, in a recent prospective study and meta-analysis indicating that MVPA/exercise performed later in the day was associated with the greatest improvements in glucose control ( 13 , 40 ).
Strengths and Limitations
Strengths of our study include the large sample of participants with obesity and a subset concurrently diagnosed with T2D, which allowed for an in-depth exploration of associations with objectively measured physical activity timing using accelerometer-based wearable devices. The extended follow-up duration was instrumental in mitigating the risk of reverse causality by excluding participants with pre-existing CVD or MVD or events within the initial 2 years of follow-up. Despite these robust measures, the potential for reverse causation stemming from prodromal disease and unmeasured or residual confounding cannot be entirely ruled out because of the observational design of the study. However, our use of negative control outcomes suggests minimal impact on our observed associations. There was a median lag of 5.5 years between the UK Biobank baseline, when covariate measurements were taken, and the accelerometry study, although covariates remained generally stable over time, except for medication. The UK Biobank had a low response rate; however, previous work indicates that this factor of poor representativeness does not materially influence associations between physical activity and all-cause or CVD mortality ( 41 ).
In summary, our findings underscore the significant health benefits associated with evening MVPA among adults with or at risk of T2D. The results of this study emphasize that beyond the total volume of MVPA, its timing, particularly in the evening, was consistently associated with the lowest risk of mortality relative to other timing windows. Although future trials and device-based cohort studies are required to further explore MVPA timing as a potential factor in lifestyle interventions targeting cardiometabolic disease management, the available evidence suggests that evening MVPA may be a suitable therapeutic strategy.
This article contains supplementary material online at https://doi.org/10.2337/figshare.25306231 .
This article is featured in podcasts available at diabetesjournals.org/journals/pages/diabetes-core-update-podcasts .
Acknowledgments. The authors thank all the participants and professionals contributing to the UK Biobank.
Funding. This research was conducted using the UK Biobank resource under application 25813. This study was funded by an Australian National Health and Medical Research Council Investigator Grant (APP1194510) and the National Heart Foundation of Australia Postdoctoral Fellowship (APP107158).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. A.S. and M.N.A. wrote the first draft of the manuscript. A.S., M.N.A., and E.S. were involved in the conception and design of the study and the analysis of the results. M.N.A. and E.S. obtained funding. All authors interpreted the results and edited, reviewed, and approved the final version of the manuscript. A.S. and M.N.A. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. The abstract of this work was presented at the 2023 Sydney Cardiovascular Symposium, Sydney, New South Wales, Australia.
Handling Editors. The journal editors responsible for overseeing the review of the manuscript were John B. Buse and Alka M. Kanaya.
Email alerts
- Online ISSN 1935-5548
- Print ISSN 0149-5992
- Diabetes Care
- Clinical Diabetes
- Diabetes Spectrum
- Standards of Medical Care in Diabetes
- Scientific Sessions Abstracts
- BMJ Open Diabetes Research & Care
- ShopDiabetes.org
- ADA Professional Books
Clinical Compendia
- Clinical Compendia Home
- Latest News
- DiabetesPro SmartBrief
- Special Collections
- DiabetesPro®
- Diabetes Food Hub™
- Insulin Affordability
- Know Diabetes By Heart™
- About the ADA
- Journal Policies
- For Reviewers
- Advertising in ADA Journals
- Reprints and Permission for Reuse
- Copyright Notice/Public Access Policy
- ADA Professional Membership
- ADA Member Directory
- Diabetes.org
- X (Twitter)
- Cookie Policy
- Accessibility
- Terms & Conditions
- Get Adobe Acrobat Reader
- © Copyright American Diabetes Association
This Feature Is Available To Subscribers Only
Sign In or Create an Account
[Possibilities of modern echocardiographic technologies in the early diagnosis of the cardiotoxic effect of chemotherapy drugs anthracycline series in cancer patients]
Affiliations.
- 1 Federal State Budgetary Educational Institution of Higher Education, "A. I. Evdokimov Moscow State University for Medicine and Dentistry" of the Ministry of Health of the Russian Federation.
- 2 State Budgetary Institution of Health Care, "Municipal Clinical Hospital #5 Br. Bahrushins of the Health Care Department of Moscow City".
- 3 Federal State Budgetary Educational Institution of Higher Education, "Pirogov Russian National Research Medical University" of the Ministry of Health of the Russian Federation.
- PMID: 29466180
- DOI: 10.18087/cardio.2417
Chronic heart failure following chemotherapy for cancer is a relevant issue of an adverse cardiovascular prognosis and premature death in cancer patients. This category of patients requires thorough and chronic monitoring of the cardiovascular system, prevention and treatment of cardiovascular complications of chemotherapy, such as IHD, systolic or diastolic myocardial dysfunction, arterial or pulmonary hypertension, pulmonary thromboembolism, pericarditis, stroke, and peripheral vascular disease. However, many aspects of this important interdisciplinary issue presently remain understudied. For instance, it is still impossible to predict long-term consequences of chemotherapy for cancer and development of the associated cardiovascular complications listed above. Baseline evaluation of the risk for cardiovascular complications is a major component in management of such patients. High-risk patients need an individual, detailed schedule of cardiovascular treatment throughout and after the course of chemotherapy. Furthermore, early detection of subclinical myocardial dysfunction is critical for prevention of the most threatening cardiovascular complications of chemotherapy, CHF. Detecting impaired LV EF following chemotherapy is, unfortunately, only a late predictor of irreversible changes, such as toxic cardiomyopathy and clinically pronounced, rapidly progressing CHF. Markers of myocardial injury, high-sensitivity troponins and natriuretic peptides, in combination with up-to-date EchoCG technologies have been recently used. Their use, for instance, for evaluation of LV myocardial global longitudinal strain to detect early, reversible changes in structure and mechanics of the myocardium is promising for ultimate improvement of prediction for such patients.
Keywords: chemotherapy, anthracycline cardiotoxicity, speckle tracking, longitudinal global deformation of LV, trimetazidine.
Publication types
- Anthracyclines / adverse effects*
- Anthracyclines / therapeutic use
- Cardiotoxicity / diagnosis*
- Echocardiography / methods*
- Heart Failure / diagnosis
- Heart Failure / etiology*
- Heart Failure / prevention & control
- Middle Aged
- Neoplasms / drug therapy*
- Anthracyclines
Research articles should report on original primary research.
Graphical abstract image
Submission of a Graphical Abstract image is strongly recommended and, together with the article title and the abstract text, should provide the reader with a visual description of the topic covered in the Research article. This is a picture which will appear underneath the Abstract on the Cardiovascular Diabetology website. It should be relevant to the topic covered and serve to attract readers' attention to the article. The graphical abstract image may be one of the images included in the article or any other image the authors feel to be appropriate. It may be a composite of more than one image. It should be approximately 920x300 pixels and should be uploaded as a JPEG, PNG or SVG file. The graphical abstract may have a caption of up to 30 words. This caption must be a part of the image file and must be below the picture. Please note that graphical abstract images must comply with Springer's copyright policy.
Preparing your manuscript
The information below details the section headings that you should include in your manuscript and what information should be within each section.
Please note that your manuscript must include a 'Declarations' section including all of the subheadings (please see below for more information).
The title page should:
- "A versus B in the treatment of C: a randomized controlled trial", "X is a risk factor for Y: a case control study", "What is the impact of factor X on subject Y: A systematic review"
- or for non-clinical or non-research studies a description of what the article reports
- if a collaboration group should be listed as an author, please list the Group name as an author. If you would like the names of the individual members of the Group to be searchable through their individual PubMed records, please include this information in the “Acknowledgements” section in accordance with the instructions below
- Large Language Models (LLMs), such as ChatGPT , do not currently satisfy our authorship criteria . Notably an attribution of authorship carries with it accountability for the work, which cannot be effectively applied to LLMs. Use of an LLM should be properly documented in the Methods section (and if a Methods section is not available, in a suitable alternative part) of the manuscript.
- indicate the corresponding author
The Abstract should not exceed 350 words. Please minimize the use of abbreviations and do not cite references in the abstract. Reports of randomized controlled trials should follow the CONSORT extension for abstracts. The abstract must include the following separate sections:
- Background: the context and purpose of the study
- Methods: how the study was performed and statistical tests used
- Results: the main findings
- Conclusions: brief summary and potential implications
- Trial registration: If your article reports the results of a health care intervention on human participants, it must be registered in an appropriate registry and the registration number and date of registration should be stated in this section. If it was not registered prospectively (before enrollment of the first participant), you should include the words 'retrospectively registered'. See our editorial policies for more information on trial registration
Three to ten keywords representing the main content of the article.
The Background section should explain the background to the study, its aims, a summary of the existing literature and why this study was necessary or its contribution to the field.
The methods section should include:
- the aim, design and setting of the study
- the characteristics of participants or description of materials
- a clear description of all processes, interventions and comparisons. Generic drug names should generally be used. When proprietary brands are used in research, include the brand names in parentheses
- the type of statistical analysis used, including a power calculation if appropriate
This should include the findings of the study including, if appropriate, results of statistical analysis which must be included either in the text or as tables and figures.
This section should discuss the implications of the findings in context of existing research and highlight limitations of the study.
Conclusions
This should state clearly the main conclusions and provide an explanation of the importance and relevance of the study reported.
List of abbreviations
If abbreviations are used in the text they should be defined in the text at first use, and a list of abbreviations should be provided.
Declarations
All manuscripts must contain the following sections under the heading 'Declarations':
Ethics approval and consent to participate
Consent for publication, availability of data and materials, competing interests, authors' contributions, acknowledgements.
- Authors' information (optional)
Please see below for details on the information to be included in these sections.
If any of the sections are not relevant to your manuscript, please include the heading and write 'Not applicable' for that section.
Manuscripts reporting studies involving human participants, human data or human tissue must:
- include a statement on ethics approval and consent (even where the need for approval was waived)
- include the name of the ethics committee that approved the study and the committee’s reference number if appropriate
Studies involving animals must include a statement on ethics approval and for experimental studies involving client-owned animals, authors must also include a statement on informed consent from the client or owner.
See our editorial policies for more information.
If your manuscript does not report on or involve the use of any animal or human data or tissue, please state “Not applicable” in this section.
If your manuscript contains any individual person’s data in any form (including any individual details, images or videos), consent for publication must be obtained from that person, or in the case of children, their parent or legal guardian. All presentations of case reports must have consent for publication.
You can use your institutional consent form or our consent form if you prefer. You should not send the form to us on submission, but we may request to see a copy at any stage (including after publication).
See our editorial policies for more information on consent for publication.
If your manuscript does not contain data from any individual person, please state “Not applicable” in this section.
All manuscripts must include an ‘Availability of data and materials’ statement. Data availability statements should include information on where data supporting the results reported in the article can be found including, where applicable, hyperlinks to publicly archived datasets analysed or generated during the study. By data we mean the minimal dataset that would be necessary to interpret, replicate and build upon the findings reported in the article. We recognise it is not always possible to share research data publicly, for instance when individual privacy could be compromised, and in such instances data availability should still be stated in the manuscript along with any conditions for access.
Authors are also encouraged to preserve search strings on searchRxiv https://searchrxiv.org/ , an archive to support researchers to report, store and share their searches consistently and to enable them to review and re-use existing searches. searchRxiv enables researchers to obtain a digital object identifier (DOI) for their search, allowing it to be cited.
Data availability statements can take one of the following forms (or a combination of more than one if required for multiple datasets):
- The datasets generated and/or analysed during the current study are available in the [NAME] repository, [PERSISTENT WEB LINK TO DATASETS]
- The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
- All data generated or analysed during this study are included in this published article [and its supplementary information files].
- The datasets generated and/or analysed during the current study are not publicly available due [REASON WHY DATA ARE NOT PUBLIC] but are available from the corresponding author on reasonable request.
- Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
- The data that support the findings of this study are available from [third party name] but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of [third party name].
- Not applicable. If your manuscript does not contain any data, please state 'Not applicable' in this section.
More examples of template data availability statements, which include examples of openly available and restricted access datasets, are available here .
BioMed Central strongly encourages the citation of any publicly available data on which the conclusions of the paper rely in the manuscript. Data citations should include a persistent identifier (such as a DOI) and should ideally be included in the reference list. Citations of datasets, when they appear in the reference list, should include the minimum information recommended by DataCite and follow journal style. Dataset identifiers including DOIs should be expressed as full URLs. For example:
Hao Z, AghaKouchak A, Nakhjiri N, Farahmand A. Global integrated drought monitoring and prediction system (GIDMaPS) data sets. figshare. 2014. http://dx.doi.org/10.6084/m9.figshare.853801
With the corresponding text in the Availability of data and materials statement:
The datasets generated during and/or analysed during the current study are available in the [NAME] repository, [PERSISTENT WEB LINK TO DATASETS]. [Reference number]
If you wish to co-submit a data note describing your data to be published in BMC Research Notes , you can do so by visiting our submission portal . Data notes support open data and help authors to comply with funder policies on data sharing. Co-published data notes will be linked to the research article the data support ( example ).
All financial and non-financial competing interests must be declared in this section.
See our editorial policies for a full explanation of competing interests. If you are unsure whether you or any of your co-authors have a competing interest please contact the editorial office.
Please use the authors initials to refer to each authors' competing interests in this section.
If you do not have any competing interests, please state "The authors declare that they have no competing interests" in this section.
All sources of funding for the research reported should be declared. If the funder has a specific role in the conceptualization, design, data collection, analysis, decision to publish, or preparation of the manuscript, this should be declared.
The individual contributions of authors to the manuscript should be specified in this section. Guidance and criteria for authorship can be found in our editorial policies .
Please use initials to refer to each author's contribution in this section, for example: "FC analyzed and interpreted the patient data regarding the hematological disease and the transplant. RH performed the histological examination of the kidney, and was a major contributor in writing the manuscript. All authors read and approved the final manuscript."
Please acknowledge anyone who contributed towards the article who does not meet the criteria for authorship including anyone who provided professional writing services or materials.
Authors should obtain permission to acknowledge from all those mentioned in the Acknowledgements section.
See our editorial policies for a full explanation of acknowledgements and authorship criteria.
If you do not have anyone to acknowledge, please write "Not applicable" in this section.
Group authorship (for manuscripts involving a collaboration group): if you would like the names of the individual members of a collaboration Group to be searchable through their individual PubMed records, please ensure that the title of the collaboration Group is included on the title page and in the submission system and also include collaborating author names as the last paragraph of the “Acknowledgements” section. Please add authors in the format First Name, Middle initial(s) (optional), Last Name. You can add institution or country information for each author if you wish, but this should be consistent across all authors.
Please note that individual names may not be present in the PubMed record at the time a published article is initially included in PubMed as it takes PubMed additional time to code this information.
Authors' information
This section is optional.
You may choose to use this section to include any relevant information about the author(s) that may aid the reader's interpretation of the article, and understand the standpoint of the author(s). This may include details about the authors' qualifications, current positions they hold at institutions or societies, or any other relevant background information. Please refer to authors using their initials. Note this section should not be used to describe any competing interests.
Footnotes can be used to give additional information, which may include the citation of a reference included in the reference list. They should not consist solely of a reference citation, and they should never include the bibliographic details of a reference. They should also not contain any figures or tables.
Footnotes to the text are numbered consecutively; those to tables should be indicated by superscript lower-case letters (or asterisks for significance values and other statistical data). Footnotes to the title or the authors of the article are not given reference symbols.
Always use footnotes instead of endnotes.
Examples of the Vancouver reference style are shown below.
See our editorial policies for author guidance on good citation practice
Web links and URLs: All web links and URLs, including links to the authors' own websites, should be given a reference number and included in the reference list rather than within the text of the manuscript. They should be provided in full, including both the title of the site and the URL, as well as the date the site was accessed, in the following format: The Mouse Tumor Biology Database. http://tumor.informatics.jax.org/mtbwi/index.do . Accessed 20 May 2013. If an author or group of authors can clearly be associated with a web link, such as for weblogs, then they should be included in the reference.
Example reference style:
Article within a journal
Smith JJ. The world of science. Am J Sci. 1999;36:234-5.
Article within a journal (no page numbers)
Rohrmann S, Overvad K, Bueno-de-Mesquita HB, Jakobsen MU, Egeberg R, Tjønneland A, et al. Meat consumption and mortality - results from the European Prospective Investigation into Cancer and Nutrition. BMC Medicine. 2013;11:63.
Article within a journal by DOI
Slifka MK, Whitton JL. Clinical implications of dysregulated cytokine production. Dig J Mol Med. 2000; doi:10.1007/s801090000086.
Article within a journal supplement
Frumin AM, Nussbaum J, Esposito M. Functional asplenia: demonstration of splenic activity by bone marrow scan. Blood 1979;59 Suppl 1:26-32.
Book chapter, or an article within a book
Wyllie AH, Kerr JFR, Currie AR. Cell death: the significance of apoptosis. In: Bourne GH, Danielli JF, Jeon KW, editors. International review of cytology. London: Academic; 1980. p. 251-306.
OnlineFirst chapter in a series (without a volume designation but with a DOI)
Saito Y, Hyuga H. Rate equation approaches to amplification of enantiomeric excess and chiral symmetry breaking. Top Curr Chem. 2007. doi:10.1007/128_2006_108.
Complete book, authored
Blenkinsopp A, Paxton P. Symptoms in the pharmacy: a guide to the management of common illness. 3rd ed. Oxford: Blackwell Science; 1998.
Online document
Doe J. Title of subordinate document. In: The dictionary of substances and their effects. Royal Society of Chemistry. 1999. http://www.rsc.org/dose/title of subordinate document. Accessed 15 Jan 1999.
Online database
Healthwise Knowledgebase. US Pharmacopeia, Rockville. 1998. http://www.healthwise.org. Accessed 21 Sept 1998.
Supplementary material/private homepage
Doe J. Title of supplementary material. 2000. http://www.privatehomepage.com. Accessed 22 Feb 2000.
University site
Doe, J: Title of preprint. http://www.uni-heidelberg.de/mydata.html (1999). Accessed 25 Dec 1999.
Doe, J: Trivial HTTP, RFC2169. ftp://ftp.isi.edu/in-notes/rfc2169.txt (1999). Accessed 12 Nov 1999.
Organization site
ISSN International Centre: The ISSN register. http://www.issn.org (2006). Accessed 20 Feb 2007.
Dataset with persistent identifier
Zheng L-Y, Guo X-S, He B, Sun L-J, Peng Y, Dong S-S, et al. Genome data from sweet and grain sorghum (Sorghum bicolor). GigaScience Database. 2011. http://dx.doi.org/10.5524/100012 .
Figures, tables and additional files
See General formatting guidelines for information on how to format figures, tables and additional files.
Submit manuscript

Cardiovascular Diabetology is affiliated with Sackler Faculty of Medicine, Tel-Aviv University

- Editorial Board
- Manuscript editing services
- Instructions for Editors
- Contact Support for Editors
- Sign up for article alerts and news from this journal
Annual Journal Metrics
2022 Citation Impact 9.3 - 2-year Impact Factor 9.6 - 5-year Impact Factor 2.054 - SNIP (Source Normalized Impact per Paper) 2.361 - SJR (SCImago Journal Rank)
2023 Speed 4 days submission to first editorial decision for all manuscripts (Median) 56 days submission to accept (Median)
2023 Usage 2,523,778 downloads 3,115 Altmetric mentions
- More about our metrics
Cardiovascular Diabetology
ISSN: 1475-2840
- Submission enquiries: [email protected]
- Search Menu
- Advance Articles
- Editor's Choice
- Supplements
- Spotlight Issues
- Image Gallery
- ESC Journals App
- ESC Content Collections
- Author Guidelines
- Submission Site
- Open Access Options
- Read & Publish
- Author Resources
- Self-Archiving Policy
- About Cardiovascular Research
- About the European Society of Cardiology
- ESC Publications
- Journal Career Network
- Editorial Board
- ESC membership
- Advertising and Corporate Services
- Developing Countries Initiative
- Dispatch Dates
- Terms and Conditions
- Journals on Oxford Academic
- Books on Oxford Academic
Article Contents
Mast cells: a novel therapeutic avenue for cardiovascular diseases.
- Article contents
- Figures & tables
- Supplementary Data
Remo Poto, Gianni Marone, Stephen J Galli, Gilda Varricchi, Mast cells: a novel therapeutic avenue for cardiovascular diseases?, Cardiovascular Research , 2024;, cvae066, https://doi.org/10.1093/cvr/cvae066
- Permissions Icon Permissions
Mast cells are tissue-resident immune cells strategically located in different compartments of the normal human heart (the myocardium, pericardium, aortic valve and close to nerves) as well as in atherosclerotic plaques. Cardiac mast cells produce a broad spectrum of vasoactive and proinflammatory mediators, which have potential roles in inflammation, angiogenesis, lymphangiogenesis, tissue remodeling and fibrosis. Mast cells release preformed mediators (e.g., histamine, tryptase, chymase) and de novo synthesized mediators [e.g., cysteinyl leukotriene C 4 (LTC 4 ) and prostaglandin D 2 (PGD 2 )], as well as cytokines and chemokines, which can activate different resident immune cells (e.g., macrophages) and structural cells (e.g., fibroblasts, endothelial cells) in the human heart and aorta. The transcriptional profiles of various mast cell populations highlight their potential heterogeneity and distinct gene and proteome expression. Mast cell plasticity and/or heterogeneity enable these cells the potential for performing different, even opposite, functions in response to changing tissue contexts. Human cardiac mast cells display significant differences compared to mast cells isolated from other organs. These characteristics make cardiac mast cells intriguing, given their dichotomous potential roles of inducing or protecting against cardiovascular diseases. Identification of cardiac mast cell subpopulations represents a prerequisite for understanding their potential multifaceted roles in health and disease. Several new drugs specifically targeting human mast cell activation are under development or in clinical trials. Mast cells and/or their subpopulations can potentially represent novel therapeutic targets for cardiovascular disorders.
Email alerts
Citing articles via.
- Recommend to Your Librarian
- Journals Career Network
Affiliations
- Online ISSN 1755-3245
- Print ISSN 0008-6363
- Copyright © 2024 European Society of Cardiology
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Cardiovasc Diagn Ther
- v.7(Suppl 1); 2017 Apr
Governmental efforts for cardiovascular disease prevention efforts in the Russian Federation
Cardiovascular disease (CVD) is the leading cause of death and disability in Russia as is case with the most other countries of the world, although Russia has unique features and demographic trends. In the late 90’s and early 2000’s Russia has sustained a profound demographical crisis with a period of overmortality but since 2003 the mortality rates are declining. By 2013, the birth rates exceeded mortality. The reversal of the demographic crisis took place on the background of a number of comprehensive governmental efforts with focus on non-communicable diseases prevention. The National Priority Project “Health” implied enhancement of primary care along with improving availability of state-of-art care for CVD patients. The most notable activities in the field of preventive medicine were the launch of Health Centers for universal free-of-charge screening for risk factors and for preventive counseling and the Dispanserization program (a large scale health screening aiming on detection of both people with chronic conditions and of high-risk persons).
Cardiovascular disease (CVD) is the leading cause of death and disability in most countries of the world, including Russia ( 1 - 4 ). At the same time Russia has a number of unique features and demographic trends. First of all, in the late 90’s and early 2000’s Russia has sustained a profound demographical crisis with a period of overmortality primarily due to CVD which has led to the so called “Russian cross” phenomenon when the number of deaths exceeded the number of births in 1992 and the corresponding curves criss-crossed ( 4 ). It was time of turbulent political and economic changes and subsequent decline in population well-being. In recent years the prosperity has increased due to economic growth, but CVD mortality is still much higher in the Russian Federation compared to the average in Europe (55.7% vs. 46% according to the World Health Organization Mortality Database 2013 ) ( 5 , 6 ), but still we have come up with a marked improvement ( 7 ). Surely, these positive trends could also be influenced by migration but in fact the migration rates were higher in the 90’s than nowadays. For instance, the positive migration balance amounted to 877,532 in 1994 and 20 years later in 2014 it was only 299,990 people.
The mortality rates have been declining in Russia since 2003 with an average rate of mortality lowering of 3.2% per year, and in 2013 fertility finally exceeded mortality again (see Figure 1 ). This recent favorable trend coincided with economic growth but improvement of CVD health was also based on huge governmental efforts.

Fertility and mortality in Russia (2000–2014, per 1,000).
The chronological order of state activities in the field of CVD prevention is provided in Table 1 . In January 2006, the National Priority Project called “Health” ( 8 ) was launched in Russia which implied following activities. First of all, enhancement of primary care took place through GPs training, financial support of primary care practitioners and through equipment upgrade in outpatient clinics and ambulance services. The next step was the so called “vascular programme” (implemented from 2008 to 2011) which aimed at establishing of state-of-art care for patients with CVD foremost with acute myocardial infarction and strokes.
CVD, cardiovascular disease.
Since 2008, promoting of healthy lifestyle and prevention of non-communicable diseases and in particular CVD have been in the highlight. That year Russia had joined the WHO Frame Convention on Tobacco Control and after all the necessary arrangements ( 9 ) our country signed the National Antismoking Concept in 2010. Subsequently, the Federal law on health protection from environmental tobacco smoke and consequences of tobacco use ( 10 ) came into effect in 2013 that implied a ban on cigarettes advertising in shops; a 21% increase of tobacco taxes and a ban on smoking in public places.
The 2009 was year of birth for unique preventive care facilities, so called Health Centers for adult population and Health Centers for children ( 11 , 12 ). A total of 695 such institutions have been opened throughout Russia that year. In some regions the Health Centers became the very first institutions dealing with medical prevention. This conceptually new structures in the Russian healthcare system were launched as a part of the National priority project “Health” and their services were free of charge for the citizens of the Russian Federation. The core areas of Health Centers activities are detection and monitoring of risk factors for CVDs and other non-communicable diseases, as well as group and personal counselling on healthy lifestyle and risk factors. The Health Centers are equipped to assess following risk factors and health indicators: the smoking status (by self-report and based on carbon monoxide in the exhaled air and urinary cotinine measurements); the level of physical activity; eating habits; the body mass index (BMI): waist circumference; blood pressure; heart rate; grip strength, stress level; ankle-brachial index; heart rate variability; total cholesterol, blood glucose; adiposity using bioimpedance analysis; oral health and hygiene, vision acuity and intraocular pressure.
As shown in the Figure 2 , the Health Centers have rapidly gained popularity: in 2015 there were a total of 4,739,487 visits versus only 2,364,402 in 2010. To address the growing need for their services additional Health Centers were launched later, so by 2015 we had 806 Health Centers, including 501 Health Centers for adults, 219 Health Centers for children, 23 mixed Health Centers for the whole family and 63 mobile Health Centers for use in rural areas. The latter enhance preventive care in remote and hard to access rural regions. According to the Health Center data up to 81.1% of adults and 66.5% of children visiting these facilities had some risk factors in 2015. Subsequently 86.2% of adult visitors and 82.3% of kids have received individual health counselling.

Health Center visits (absolute numbers and changes relative to the preceding year) of adult and pediatric patients in 2010–2015.
These policies focusing on preventive care were in line with global trends. An important landmark to honor these efforts was the decision to hold the First Global Ministerial Conference on Healthy Lifestyles and NCDs Control in Moscow. The Ministerial Conference took place on 28–29 April, 2011 and resulted in a political declaration ( 13 ), committing world governments to develop a global policy on NCDs prevention as well as a global monitoring framework. The commitment to develop such policy was reflected in the Federal Law #323 “Healthcare of citizens of the Russian Federation” ( 14 ) which was passed later that year and set specific goals for reducing total and disease-specific mortality (see Figure 3 ).

FEDERAL LAW #323 “Healthcare of citizens of the Russian Federation”: main goals.
One of the most striking measures embodied in the new legislation is the dispanserization program, which is a comprehensive health screening program launched in 2013. The word “dispanserization” is another term for screening commonly used in Russia and many other post-Soviet countries. It stems from the French word “dispensaire”, which means a kind of a clinic. All citizens aged 18 and older irrespective of the working status are eligible for this screening program every 3 years. The program is carried out by public outpatient clinics and outpatient departments of hospitals, and people can be referred to such facilities at domiciliary and job or learning locations.
The extent of the work-up in the scope of the dispanserization program depends on age and sex but anyway it consists of two stages. The basic evaluations of the first stage include measurements of height and weight with the calculation of BMI, blood pressure, total cholesterol, blood glucose, ECG, SCORE risk estimates, blood count and urinalysis, chest fluorography, mammography for women aged 39–75 years, pap-smear, PSA for men aged 45 years and older, abdominal ultrasound (every 6 years after 39), intraocular pressure and others. After examination and review of the results primary care physicians can refer patients for additional tests if needed (the second stage of dispanserization). Based on the first stage results patients are categorized into three groups: the 1st health group consists of healthy people with SCORE risk estimates <5%, the 2nd health group implies estimated 10-year fatal CVD risk of ≥5% and the 3rd health group consists of patients with confirmed chronic diseases including CVDs. The identification of the second health group enables follow-up and timely management of high risk patients (approximately 21% of all screened people according to the 2014 dispanserization results). As shown in Figure 4 , the start of the dispanserization program enabled identification of many new CVD patients who could receive appropriate care including high tech procedures and interventions ( 15 ). From the regulatory point of view dispanserization programs are equal in rural and urban areas, but in some scenarios it’s more complicated for rural population to participate in it because of traffic issues. Nevertheless many regions solve this problem with the use of mobile multidisciplinary teams which carry out the screening program at rural primary care facilities. Along with the abovementioned availability issues the dispanserization program is widely criticized for its being not fully evidence based. In fact, many included tests (e.g., complete blood count, urinalysis, abdominal ultrasound and the ECG) really don’t have any proven benefit as screening methods. Their inclusion was primarily based on their wide availability and relatively low cost. Another reason for their utilization was the intent to meet peoples’ conservative expectations because these tests have been long established as a part of dispanserization programs during the Soviet period. It is planned to gradually eliminate them as soon as the population gets used to the program.

Dispanserization results, Moscow 2013. New diagnoses of cardiovascular diseases per 100,000.
Obviously all these efforts would not be possible without most active support from the professional community. From 2009 to 2011 the biggest professional associations in the field, the Russian National Society of Cardiology and Russian National Society of Preventive Cardiology, have carried out the so called Healthy Hearts Project which consisted of public actions, educational and social activities. It involved 27 big Russian cities from Kaliningrad to Vladivostok. Within the Healthy Hearts Project 11,000 GPs and cardiologists were involved in training on contemporary strategies of CVD prevention and more than 57,000 citizens participated in basic health-checks and got a professional advice on CVD prevention. 2011 was also the year of the development of the first Russian National Guidelines on CVD prevention ( 16 ). Since 2013 we also have National Guidelines on non-communicable diseases prevention. By now, after the release of the 2016 European guidelines on CVD prevention the development of a revised Russian National guideline is underway.
Despite prevention being the primary focus of Russian healthcare policy in recent years as noted above the Priority State National Project “Health” included also the so called “Vascular Program”. Since 2008 more than 16,800 million rubles (approx. 525 million USD) were spent in order to increase the availability of the modern technologies of CVD treatment. As part of the Vascular Program 55 regional vascular centers and 146 vascular departments were opened throughout Russia in 2008–2011 providing all essential cardiovascular surgeries and interventions (percutaneous coronary interventions, bypass grafting, valve surgeries, catheter ablation for arrhythmias, carotid artery surgeries, etc.) in particular in emergency settings. The vascular centers network is designed to improve the uptake of emergency interventions throughout the country including but not limited to rural areas. Figure 5 depicts the marked increase of the coronary revascularization rates in Russia from 2004 to 2014 ( 17 ). Current PCI rates have reached 531 PCI per 1,000,000 with placement of 1.37 stents per PCI ( 18 ). Nevertheless, we still need improvement of emergency patient logistics in very many rural areas.

Coronary revascularization procedures in Russian Federation, 2004–2013.
It’s clear that Russian healthcare reform is still at its very beginning and we have a long way to go. But despite the fact that too little time has passed since it started the statistics really began to improve, and it’s very reassuring. In fact, in 2013 the life expectancy of Moscow city inhabitants reached 76.4 years, with only 4 years separating Moscow residents from the European Union population ( Figure 6 ). The life expectancy for the whole country is still much lower than in the capital (70.8 years) but we hope it will improve further.

Life expectancy at birth, years.
Acknowledgements
Conflicts of Interest: The authors have no conflicts of interest to declare.

IMAGES
VIDEO
COMMENTS
The file should be clearly named, e.g. graphical_abstract.tiff. 5. Publisher information 5.1. Language editing pre-submission. Cardiovascular Research publishes in the English (UK) language. Details of pre-submission language editing services can be found on the Language Services page. These services are particularly useful if English is not ...
Cardiovascular Research EP Europace European Heart Journal European Heart Journal - Acute Cardiovascular Care ... Graphical abstract guidance Close. Navbar Search Filter Enter search term Search. Advanced Search. Search Menu. A guide to graphical abstracts Download the PDF. About European Society of Cardiology ...
Atherosclerotic cardiovascular disease (ASCVD) is characterized by a build-up of plaque inside the arteries due to a chronic slowly evolving vascular wall inflammation and lipid accumulation that can lead to several major adverse cardiovascular events including myocardial infarction, stroke, or even death.
Graphic Abstract: All Original Investigations and Review Articles should contain a Graphic Abstract that displays the key points of the manuscript. If requested, Circulation: Cardiovascular Interventions will engage the services of a medical illustrator to work with authors to create final version of the Graphic Abstract. Any title/legend ...
Graphical Abstracts. Authors of Research articles are required to submit a graphical abstract (schematic figure) as part of the article, in addition to the text abstract. The graphical abstract should summarize in a visual or conceptual manner the key message of the paper and allow the reader to understand the essence of the study.
Lastly, we discuss in-depth the significance of precision medicine that is helpful to improve through GWAS analysis in cardiovascular research. Graphical abstract Identification: genome-wide association studies (GWAS) are necessary to identify the disease variants highly linked with an early-onset myocardial infarction (EOMI) and coronary ...
Abstract. Rationale: ... (eg, the median) and dispersion (eg, the minimum, 25th percentile, 75th percentile, and maximum). Graphical representations are helpful in presenting continuous data; chief among these is the box plot ... Most epidemiological and biostatistics mistakes in cardiovascular research derive from 1 flaw—an inadequately ...
Graphical Abstract. Open in a separate window. Mechanisms and clinical phenotypes: the potential impact of acute COVID-19 on the heart and its longer-term sequelae. Reprinted with permission from: Friedrich MG, Cooper LT Jr. ... Cardiovascular mortality was also higher in this group (HR 2.7; 95% CI 1.4-5.1; P = 0.003); however, ...
A graphical abstract is mandatory for this journal. It should summarize the contents of the article in a concise, pictorial form designed to capture the attention of a wide readership online. Authors must provide images that clearly represent the work described in the article. Graphical abstracts should be submitted as a separate file in the ...
Authors must provide an original image that clearly represents the work described in the paper. Graphical abstracts should be submitted as a separate file in the submission system by selecting "graphical abstracts" from the drop-down list when uploading files. Please note that, just as each paper should be unique, so each graphical abstract ...
Study setup figures and the graphical abstract were created in BioRender. Author information. Author notes. ... Nature Cardiovascular Research thanks Federica Marelli-Berg, ...
Graphical abstract summarizing relevant studies published over the last year in the field of percutaneous coronary interventions and transcatheter aortic valve implantation. ... Landshut, Klinikum der Universitaet München, Ludwig-Maximilians-Universitaet and German Centre for Cardiovascular Research (DZHK), Partner Site Munich Heart Alliance, ...
Examples of genome-wide association studies relevant to cardiovascular research. ... on conventional cardiovascular risk factors and lifestyle factors in relation to CVD risk is presented below and in the Graphical Abstract. Some examples of drug target and drug repurposing MR studies are also provided.
HOW to prepare a graphical abstract Your graphical abstract needs to be CLEAR and CONCISE. Content Step 1: Select the key message for your graphical abstract. Step 2: Sketch your idea on paper, or in your design software, keeping the physical size of your graphical abstract in mind (11 cm x 18 cm).
Amended STRONG-HF study design. Eur J Heart Fail. 2021 Nov;23 (11):1981-1982. doi: 10.1002/ejhf.2348. Epub 2021 Oct 4.
Aim To evaluate the prognostic significance of the left ventricular global function index (LV GFI) in patients with acute coronary syndrome (ACS) using echocardiography (EchoCG).Material and methods The LV GFI is an index that integrates LV cavity volumes, stroke volume, and myocardial volume. This …
Data sources. The dataset includes the reference, abstract, address, and citation data for 478 006 cardiovascular publications from 2004 to 2013, including 2014 references to these documents, using an expert informed search strategy and references to core cardiovascular journals, as previously published. 7 The documents span across >5000 journals, and include cardiovascular publications in ...
To assess the association between timing of aerobic moderate to vigorous physical activity (MVPA) and risk of cardiovascular disease (CVD), micr. ... 3 Mackenzie Wearables Research Hub @ Charles Perkins Centre, The University of Sydney, Sydney, New South Wales, ... Graphical Abstract. View large Download slide.
Chronic heart failure following chemotherapy for cancer is a relevant issue of an adverse cardiovascular prognosis and premature death in cancer patients. This category of patients requires thorough and chronic monitoring of the cardiovascular system, prevention and treatment of cardiovascular compl …
The European Heart Journal is an international, peer-reviewed journal, engaged in publishing the highest quality scientific research on all aspects of cardiovascular medicine. It is an official journal of the European Society of Cardiology (ESC) and is published 48 times per year. It publishes articles related to research findings, meta ...
This study aims to explore the association between heat and the incidence of first acute cardiovascular event (CVE) in adults in Madrid between 2015 and 2018, and to assess how social context and other individual characteristics modify the estimated association. We performed a case-crossover study using the individual information collected from ...
Panel A. Primary endpoint of DELIVER [cardiovascular (CV) death or heart failure (HF) hospitalization] in the entire population (left) and in the population with LVEF <60%. Reprinted with permission. 2 Panel B. A pooled analysis of patients enrolled in the DAPA-HF and DELIVER trials reveals a consistent benefit of dapagliflozin on the primary endpoint (CV death or HF hospitalization) across ...
Large-scale genome-wide association studies conducted over the last decade have uncovered numerous genetic variants associated with cardiometabolic traits and risk factors. These discoveries have enabled the Mendelian randomization (MR) design, which uses genetic variation as a natural experiment to improve causal inferences from observational ...
Graphical abstract image. Submission of a Graphical Abstract image is strongly recommended and, together with the article title and the abstract text, should provide the reader with a visual description of the topic covered in the Research article. This is a picture which will appear underneath the Abstract on the Cardiovascular Diabetology ...
As the global population ages, the incidence of Chronic Kidney Disease (CKD) is rising. CKD often remains asymptomatic until advanced stages, which significantly burdens both the healthcare system and patient quality of life. This research developed an explainable machine learning system for predicting CKD in patients with cardiovascular risks, utilizing medical history and laboratory data.
These characteristics make cardiac mast cells intriguing, given their dichotomous potential roles of inducing or protecting against cardiovascular diseases. Identification of cardiac mast cell subpopulations represents a prerequisite for understanding their potential multifaceted roles in health and disease.
Abstract. Cardiovascular disease (CVD) is the leading cause of death and disability in Russia as is case with the most other countries of the world, although Russia has unique features and demographic trends. In the late 90's and early 2000's Russia has sustained a profound demographical crisis with a period of overmortality but since 2003 ...