

Please log in to JScholarship
- Search Menu
- Advance articles
- Editor's Choice
- Special Issues
- Supplements
- Lessons from the field
- Open Access Articles
- Author Guidelines
- Submission Site
- Open Access
- For Reviewers
- About the Journal
- About the Royal Society of Tropical Medicine and Hygiene
- Editorial Board
- Advertising and Corporate Services
- Journals Career Network
- Self-Archiving Policy
- Dispatch Dates
- Journals on Oxford Academic
- Books on Oxford Academic
Article Contents
Introduction, authors’ contributions, acknowledgements, competing interests, ethical approval, data availability, disclaimers, climate change, malaria and neglected tropical diseases: a scoping review.
- Article contents
- Figures & tables
- Supplementary Data
Petra Klepac, Jennifer L Hsieh, Camilla L Ducker, Mohamad Assoum, Mark Booth, Isabel Byrne, Sarity Dodson, Diana L Martin, C Michael R Turner, Kim R van Daalen, Bernadette Abela, Jennifer Akamboe, Fabiana Alves, Simon J Brooker, Karen Ciceri-Reynolds, Jeremy Cole, Aidan Desjardins, Chris Drakeley, Dileepa S Ediriweera, Neil M Ferguson, Albis Francesco Gabrielli, Joshua Gahir, Saurabh Jain, Mbaraka R John, Elizabeth Juma, Priya Kanayson, Kebede Deribe, Jonathan D King, Andrea M Kipingu, Samson Kiware, Jan Kolaczinski, Winnie J Kulei, Tajiri L Laizer, Vivek Lal, Rachel Lowe, Janice S Maige, Sam Mayer, Lachlan McIver, Jonathan F Mosser, Ruben Santiago Nicholls, Cláudio Nunes-Alves, Junaid Panjwani, Nishanth Parameswaran, Karen Polson, Hale-Seda Radoykova, Aditya Ramani, Lisa J Reimer, Zachary M Reynolds, Isabela Ribeiro, Alastair Robb, Kazim Hizbullah Sanikullah, David R M Smith, GloriaSalome G Shirima, Joseph P Shott, Rachel Tidman, Louisa Tribe, Jaspreet Turner, Susana Vaz Nery, Raman Velayudhan, Supriya Warusavithana, Holly S Wheeler, Aya Yajima, Ahmed Robleh Abdilleh, Benjamin Hounkpatin, Dechen Wangmo, Christopher J M Whitty, Diarmid Campbell-Lendrum, T Déirdre Hollingsworth, Anthony W Solomon, Ibrahima Socé Fall, Climate change, malaria and neglected tropical diseases: a scoping review, Transactions of The Royal Society of Tropical Medicine and Hygiene , 2024;, trae026, https://doi.org/10.1093/trstmh/trae026
- Permissions Icon Permissions
To explore the effects of climate change on malaria and 20 neglected tropical diseases (NTDs), and potential effect amelioration through mitigation and adaptation, we searched for papers published from January 2010 to October 2023. We descriptively synthesised extracted data. We analysed numbers of papers meeting our inclusion criteria by country and national disease burden, healthcare access and quality index (HAQI), as well as by climate vulnerability score. From 42 693 retrieved records, 1543 full-text papers were assessed. Of 511 papers meeting the inclusion criteria, 185 studied malaria, 181 dengue and chikungunya and 53 leishmaniasis; other NTDs were relatively understudied. Mitigation was considered in 174 papers (34%) and adaption strategies in 24 (5%). Amplitude and direction of effects of climate change on malaria and NTDs are likely to vary by disease and location, be non-linear and evolve over time. Available analyses do not allow confident prediction of the overall global impact of climate change on these diseases. For dengue and chikungunya and the group of non-vector-borne NTDs, the literature privileged consideration of current low-burden countries with a high HAQI. No leishmaniasis papers considered outcomes in East Africa. Comprehensive, collaborative and standardised modelling efforts are needed to better understand how climate change will directly and indirectly affect malaria and NTDs.
Human activity is driving incremental changes in climate patterns globally. The burning of coal, oil and natural gas; clearing land by cutting down forests; and intensified agriculture all release greenhouse gases, primarily CO 2 , methane and N 2 O. Deforestation also reduces capacity for CO 2 absorption. Increases in atmospheric concentrations of greenhouse gases are driving mean global temperatures upwards, with consequent effects including rising sea levels, changes in rainfall and increases in the frequency and intensity of extreme weather events. 1
Climate change will perturb human health in profound and long-lasting ways, both directly and indirectly. 2 Multiple direct and indirect mechanisms will contribute. Human physiology can be affected directly by changes in air temperature, humidity, stress and diet. Human behaviour can be altered by changing weather, changing economic circumstances, migration, natural disasters and access to or quality of healthcare. Environmental conditions also influence pathogen transmission, reproduction, development and genetics; the reproduction, development, genetics, behaviour, range, longevity and predation of vectors, intermediate hosts and reservoir hosts; and the feasibility and effectiveness of interventions. In fact, there is already empirical evidence of climate change having amplified more than one-half of all known human infectious diseases. 3
The impact of climate change is likely to be disproportionately borne by the poorest people, who are also disproportionately affected by malaria and neglected tropical diseases (NTDs; Table 1 ). In part because of this association with poverty, malaria and NTDs are often co-endemic. Many of these diseases are currently suitable for coordinated control via integrated programmes. 4–7
Diseases and organisms in scope for this review. Included diseases and associated aetiological agents, vectors, reservoir and intermediate hosts and routes of transmission are those listed in the NTD road map 2021–2030, 13 plus malaria. Noma was added to the NTD list in December 2023, 61 and therefore is not included here.
a Ancylostoma ceylanicum is a soil-transmitted helminth species previously believed to only infect dogs but now recognised as a zoonosis that causes symptomatic infections in humans; it was not included in the NTD road map 2021–2030 13 and therefore was not included in this review.
Developing an understanding of the likely effects of climate change on the epidemiology of malaria and NTDs is critical to minimising their health implications. This scoping review explores current predictions of the effects of historical and future climate change on malaria and NTDs, and the potential amelioration of these effects through climate change mitigation and adaptation strategies.
A comprehensive scoping review was conducted. To complete the review efficiently but with high fidelity, we used automation and artificial intelligence-based tools to help define search terms, translate them across different platforms, de-duplicate search results and conduct title-and-abstract screening. 8 We did not otherwise use artificial intelligence assistance. This report adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews guidance 9 ( Supplementary Table 1 ).
Search strategy
Searches were conducted in five electronic databases that index the published peer-reviewed and grey literature: PubMed, Scopus, Embase via Ovid, Global Index Medicus and the WHO's Institutional Repository for Information Sharing.
Databases were searched for papers published from 1 January 2010 to 12 October 2023. No language or other restrictions were applied. Using a combination of Medical Subject Heading (MeSH) and free-text terms, search vocabulary related to ‘climate change’, ‘malaria’ and ‘neglected tropical diseases’ was employed. Searches were designed and conducted in English and included scientific and lay expressions for diseases, pathogens, vectors, reservoir hosts and intermediate hosts; the search strategy was first constructed for PubMed, and the Systematic Review Accelerator Polyglot tool ( https://sr-accelerator.com/#/polyglot ) 10 was then used to translate strings into syntax appropriate for other databases. (Please see the Supplementary material for full search strings.) Using the citationchaser Shiny app ( https://www.eshackathon.org/software/citationchaser.html ), 11 forward and backward screening of relevant seed papers (e.g. reviews, commentaries) was undertaken in December 2023 to identify additional records. The review protocol was not published in advance.
Criteria for paper selection
Inclusion criteria applied to screening are shown in Table 2 . Papers not meeting these criteria were excluded.
Characteristics of included papers.
Papers were eligible for inclusion if they explicitly reported on the observed or modelled effects of historical or future climate change on outcomes framing the distribution, dynamics or transmission of malaria or NTDs; or the range, abundance or transmission potential of their vectors, reservoir hosts or intermediate hosts. Papers were also included if they explicitly reported on the observed or modelled effects of climate change mitigation and adaptation strategies on these outcomes. (‘Climate change mitigation’ was defined as actions intended to reduce the magnitude of climate change, and ‘adaptation’ as actions intended to reduce the vulnerability of human and natural systems to the effects of climate change or to cope with past or future climate change.)
Masters and PhD theses were eligible for inclusion if the analyses were not also presented elsewhere, but we did not specifically search thesis repositories.
Papers were excluded if (i) they did not include original analyses; (ii) they presented methods or protocols without results; (iii) they were clinical case reports; (iv) they were conference proceedings; (v) they were pre-prints (of papers being prepared for, or in the process of being considered by, peer-reviewed journals); or (vi) the full text could not be obtained. In contrast to the exclusion of clinical case reports, we included ecological case reports as potentially important indicators of pathogen or vector range expansion.
Screening and extraction procedures
The Covidence software package ( https://www.covidence.org/ ) was used to support record indexing, removal of duplicate records, title-and-abstract screening, full-text screening and compilation of extracted data.
After de-duplication, titles and abstracts of each record were independently screened by two researchers, with conflicts resolved by a third independent reviewer. Non-English records were translated or reviewed by a fluent speaker of that language.
The Covidence title-and-abstract screening module incorporates a machine-learning algorithm that ranks papers that are yet to be screened according to their acceptance likelihood: based on previous screening decisions, it pushes papers that are more likely to be accepted towards the top of the pile, for all reviewers. This accelerates the screening process by removing the need to screen all search results. The predetermined stopping rule was to discontinue title-and-abstract screening after three criteria were met: (i) more than twice the estimated fraction of relevant records from a previously published systematic review of climate change and NTDs 12 had been screened; (ii) all papers previously determined as relevant (‘seed’ papers) were identified; and (iii) two screeners had each rejected 50 papers in a row. The results of the backward and forward citation searches of seed papers were screened separately with the assistance of the same (trained) machine-learning algorithm, with screening of these records discontinued after a single screener had rejected 100 papers in a row.
Full texts were blind double-screened, with discrepant results arbitrated by an independent third reviewer.
Data from papers selected in the full-text review were independently extracted by one investigator using a pre-piloted standardised form, according to self-identified individual expertise, and checked by a second investigator. Data extraction was limited to the information provided in the published work; authors were not contacted for further clarification or provision of missing information. Extracted information included: first author, year, study title, diseases addressed in the publication, study design (cohort, field, model, laboratory research, ecological case report), whether the paper explicitly discussed adaptation or mitigation strategies, the time frame of the study (cross-sectional, prospective longitudinal, retrospective longitudinal; the length of the time series), location data, and the population, intervention/exposure, comparator and outcome (Table 2 ).
Structured assessment of the methodological limitations and risk of bias of included individual papers was not undertaken.
Data analyses and synthesis
Because of the heterogeneity of included studies in terms of designs, exposures, outcomes and quality, no meta-analyses were attempted. Data were narratively synthesised and thematically categorised to facilitate analysis.
Papers were grouped and synthesised by whether they (i) explored the effect of climate change on malaria and NTDs; or (ii) examined potential amelioration of these effects through climate change mitigation and adaptation. Studies that compared several future climate scenarios of different severities (different representative concentration pathways [RCPs], or different combinations of RCPs and shared socioeconomic pathways, for example; please see Box 1 and Figure 1 ) were defined as projections of the effect of mitigation.
![research paper on malaria pdf Comparison of Intergovernmental Panel on Climate Change (IPCC) scenarios used in papers that met this review's inclusion criteria. The different categories of scenario (special report on emission scenarios [SRES; released in 2000]; representative concentration pathways [RCPs; 2014]; shared socioeconomic pathways [SSP; 2021]; scenarios from the IPCC Sixth Assessment Report Working Group III [AR6 WGIII; 2022]) are laid out in columns against the equivalent scenarios within the full RCP scheme, which are laid out in rows; some of the RCP scenarios (RCP1.9, RCP3.4 and RCP7) were added after the original 2014 publication of the RCP system. (The RCPs were the most commonly applied scenarios used in papers that met this review's inclusion criteria.) Further explanations of all of these scenario categories are included in Box 1.81–83](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/trstmh/PAP/10.1093_trstmh_trae026/3/m_trae026fig1.jpeg?Expires=1718616866&Signature=G3VlrbFu6rVCryZd21YOACnoMq15SJepxjA2hi7KGq8Sb1rNKIjtTYRD~-St996WtCK7usmoQtOrfatybZvhFX~HuyCSVZKA5iN1Gb1xhR2hQw58yM4Y3VRf~CZsmaFhAPacSQCT5miPzkGSadLUhE7-IWENkVek6T-vB2SqBs0Ff3RIMwsHRewZA0jloRllkozycSzOpF0eoDpUFe-WFVbMptYbbhxT85bwgu8hUnIXCNGzWrqAUEP7PEvINHbopZ0bDMMSAjE7E21zWhBihhKd1EZ~kImZtEZcSs1hdZIqQ7tqkQ8rZ-fET6Z5Kot2k3PCFDJnEtmSYVH8jMHRVw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Comparison of Intergovernmental Panel on Climate Change (IPCC) scenarios used in papers that met this review's inclusion criteria. The different categories of scenario (special report on emission scenarios [SRES; released in 2000]; representative concentration pathways [RCPs; 2014]; shared socioeconomic pathways [SSP; 2021]; scenarios from the IPCC Sixth Assessment Report Working Group III [AR6 WGIII; 2022]) are laid out in columns against the equivalent scenarios within the full RCP scheme, which are laid out in rows; some of the RCP scenarios (RCP1.9, RCP3.4 and RCP7) were added after the original 2014 publication of the RCP system. (The RCPs were the most commonly applied scenarios used in papers that met this review's inclusion criteria.) Further explanations of all of these scenario categories are included in Box 1 . 81–83
Climate change scenarios.
The Intergovernmental Panel on Climate Change (IPCC) has periodically published assessment reports on the scientific understanding of climate change, its projected effects and potential response strategies. As a part of these reports, the IPCC outlines future climate scenarios or pathways based on projections from Global Climate Models, for use in modelling. Papers meeting the inclusion criteria for this review used a variety of different future scenarios, so their outcomes and conclusions generally cannot be directly compared. Here, we summarise the history of IPCC climate scenarios, explain their interpretation and note how they relate to each other (Figure 1 ).
IP92 –the first global climate change scenarios, published by IPCC in 1992. These focused on projected future emissions of greenhouse gases (GHGs). 78
Special Report on Emission Scenarios (SRES)—released in 2000 and used in the IPCC's Third Assessment Report (TAR) and Fourth Assessment Report (AR4). The SRES covers a wide range of the main drivers of climate change, from demographic to technological and economic developments, and includes all relevant GHGs and sulphur, and their driving forces. 79 Four main narrative storylines with different contributions of drivers cover a range of possible outcomes:
A1: Rapid economic growth; population peaks mid-century then declines.
A2: Regionalised world; high population growth.
B1: Global sustainability focus; population peaks mid-century then declines.
B2: Local solutions emphasised; slow population growth.
Representative Concentration Pathways (RCPs)—Developed to replace the SRES, for use in climate models as part of the Fifth Assessment Report (AR5) of the IPCC in 2014. Rather than being based on storylines, RCPs describe possible GHG emissions and concentration levels in the atmosphere by 2100, ranging from very low (RCP2.6, radiative forcing peaks at 3 Wm −2 and then declines to 2.6 Wm −2 by 2100, also known as RCP3PD) to intermediate (RCP4.5 and RCP6.0, radiative forcing is stabilised at approximately 4.5 or 6.0 Wm −2 ), to very high (RCP8.5, radiative forcing continues to increase after 2100). RCPs use time series of emissions and concentrations of the full suite of GHGs, aerosols and chemically active gases, as well as projected land use/land cover and population growth. Compared with the SRES, RCP2.6 has no equivalent; RCP4.5 is similar to SRES B1 but median temperatures rise more quickly in RCP4.5 than in B1 in the first half of the twenty-first century, and then more slowly in the second half; RCP6.0 is similar to SRES B2, with median temperatures rising more quickly in RCP6.0 than in B2 from 2060–2090, but otherwise more slowly; and RCP8.5 is similar to SRES A1FI (A1 fossil-fuel intensive scenario), with median temperatures rising more slowly in RCP8.5 than in A1FI during 2035–2080, but more quickly during other periods. 80 , 81
Shared Socioeconomic Pathways (SSPs)—These scenarios were introduced in the IPCC's Sixth Assessment Report (AR6) published in 2021. SSPs are integrated scenarios that combine socioeconomic narratives with emission pathways, describing different plausible futures of societal development based on varying assumptions about demographic, economic, social and technological factors. Briefly, these include:
SSP1 (Sustainability): represents a future in which there is rapid economic growth, low population growth and widespread adoption of environmentally friendly technologies. It is also called ‘Taking the Green Road’ and emphasises sustainable development, international cooperation and a focus on environmental stewardship.
SSP2 (Middle of the Road): represents a future in which trends continue along historic trajectories with moderate economic growth, intermediate population growth and technological progress occurring at a moderate pace. It assumes some improvements in living standards and governance but also incorporates challenges and disparities.
SSP3 (Regional Rivalry): represents a future characterised by high population growth, slow economic development and fragmented governance. Also termed ‘A Rocky Road’, it envisions regions prioritising national interests over global cooperation, resulting in regional rivalries, conflicts and limited efforts to address climate change.
SSP4 (Inequality): represents a future marked by high population growth, slow economic development and high income inequality. It assumes limited international cooperation and emphasises national interests and security concerns over environmental issues. Also termed ‘A Road Divided’, this scenario suggests challenges in achieving sustainable development and addressing climate change due to social disparities.
SSP5 (Fossil-Fuelled Development): represents a future with high population growth, rapid economic development and reliance on fossil fuels. Also termed ‘Taking the Highway’, it envisions limited environmental regulations and technological innovation, leading to high GHG emissions and significant impact from climate change. 82
AR6 WGIII —scenario categories C1-C8 (published in 2022 by the IPCC's Sixth Assessment Report AR6 Working Group III) refer to potential mitigation pathways used to reduce GHG emissions and limit global warming. These scenarios relate to warming levels in the twenty-first century:
C1 (1.5°C, no/limited overshoot). Limits warming to 1.5°C by 2100 (with >50% likelihood). Roughly corresponds to SSP1-1.9 (very low).
C2 (1.5°C, high overshoot). After a high overshoot, warming returns to 1.5°C by 2100 (with >50% likelihood). Roughly corresponds to SSP1-2.6 (low).
C3 (Likely below 2°C). Limits warming to 2°C by 2100 (with >67% likelihood).
C4 (Below 2°C). Limits warming to 2°C by 2100 (with >50% likelihood).
C5 (Below 2.5°C). Limits warming to 2.5°C by 2100 (with >50% likelihood).
C6 (Below 3°C). Limits warming to 3°C by 2100 (with >50% likelihood). Roughly corresponds to SSP2-4.5 (intermediate).
C7 (Below 4°C). Limits warming to 4°C by 2100 (with >50% likelihood). Roughly corresponds to SSP3-7.0 (high).
C8 (Above 4°C). Exceeds warming of 4°C by 2100 (with >50% likelihood). Roughly corresponds to SSP5-8.5 (very high). 83
Based on the volume of literature meeting the inclusion criteria, papers were further grouped by disease into malaria, dengue and chikungunya, other vector-borne NTDs and other non-vector-borne NTDs. Dengue and chikungunya are bracketed as an NTD group in the 2021–2030 NTD road map. 13 We categorised as ‘vector-borne NTDs’ those for which vector control was a recommended control strategy in the 2021–2030 NTD road map. 13
We obtained data on the burden of malaria and NTDs by country, quantified as disability-adjusted life years (DALYs, the cumulative number of years lost by a defined population due to illness, disability and death from a particular cause or group of causes), from the 2019 Global Burden of Disease study. 14 To account for different national levels of health service delivery, we used the healthcare access and quality index (HAQI). This employs data on 32 causes of death from which mortality would be avoidable in the presence of effective healthcare; a high index is a marker of better healthcare. HAQIs were obtained from the 2019 Global Burden of Disease data. 15 To account for countries’ exposure, sensitivity and ability to adapt to the negative impacts of climate change, we used the 2021 vulnerability score from the Notre Dame Global Adaptation Initiative (ND-GAIN; available at: https://gain-new.crc.nd.edu/ranking/vulnerability ). The ND-GAIN measures vulnerability by considering six life-supporting sectors: food, water, health, ecosystem service, human habitat and infrastructure. Countries ranked higher in the index are those that are less vulnerable. After removing papers that examined outcomes at the global level, local polynomial regression was undertaken to predict the number of disease group-specific publications relevant to each country as a function of the country's (i) DALYs for that group of diseases, (ii) HAQI and (iii) climate vulnerability score. As there were <1000 datapoints available for each regression, we used locally estimated scatterplot smoothing (LOESS) for these analyses.
Database searches yielded 31 560 records. Forward and backward citation searching yielded an additional 11 133 (of which 8587 were within the specified publication range), producing a combined total of 42 693 records (40 147 within the specified range). After removal of 14 879 duplicates, 27 814 records were available for title-and-abstract screening. The stopping rules for title-and-abstract screening were met after 9013 records had been examined; of these 9013, 7442 (83%) were excluded on the basis that their titles and abstracts indicated a failure to meet the inclusion criteria. Full-text papers were therefore sought for 1571 records (17% of 9013), of which 1543 (98%) were able to be retrieved. Following full-text review, 511 papers (33% of 1543) were included for data extraction; these are summarised in Supplementary Table 2 . Extracted data are presented in full in Supplementary Table 3 . A flow diagram is included as Figure 2 .

Records included and excluded at each review stage.
Papers that met the inclusion criteria considered outcomes relevant to malaria (185 papers), dengue and chikungunya (181), the leishmaniases (53), schistosomiasis (29), Chagas disease (19), foodborne trematodiases (17), lymphatic filariasis (14), snakebite envenoming (11), rabies (9), human African trypanosomiasis (8), Buruli ulcer (6), echinococcosis (4), onchocerciasis (2), leprosy (1), scabies (1) and soil-transmitted helminthiases (1). No papers meeting the inclusion criteria considered outcomes relevant to dracunculiasis; mycetoma, chromoblastomycosis or other deep mycoses; taeniasis/cysticercosis; trachoma; or yaws (Figure 3A ).

Number of papers by year and total number of papers meeting the inclusion criteria for each (A) disease or disease group, and (B) type of study.
The vast majority of papers used modelling to study the effect of climate change (435 papers). There were 72 field study papers, 54 laboratory research reports, 28 ecological case reports, 10 cohort studies and seven other types of study (Figure 3 B; multiple study types were possible in a single paper). There was little variation in the numbers of papers from year to year (Figure 3 A and B, left plots).
Among papers meeting the inclusion criteria, a total of 174 (35%) considered the possible ameliorating effect of climate change mitigation for any outcome, 24 (5%) considered adaption strategies and two considered both. Sixty-nine papers considered mitigation in relation to malaria outcomes. Nine explicitly considered adaptation strategies for malaria outcomes. Fifty-three papers addressed climate change mitigation and nine considered adaptation strategies in relation to dengue and chikungunya; four of the papers, including mitigation analyses for dengue and chikungunya, also considered malaria outcomes in the light of climate change mitigation. Sixty other papers addressed climate change mitigation or adaptation with respect to NTD outcomes.
There was wide variation in the geographical coverage of papers by disease (Figure 4 ). Apart from malaria, dengue and chikungunya, and schistosomiasis, there were <10 papers per disease per country for every country, with many endemic countries not featured in any papers for several NTDs. We also observed distinct distribution patterns for malaria, dengue and chikungunya, and schistosomiasis. Most papers examining the impact of climate change on malaria focused on countries in Africa, Brazil, China or India. Dengue and chikungunya papers were focused on Australia, China, India, countries in Europe and the USA, many of which are countries where these diseases may spread in future years. Several schistosomiasis papers focused on China. For leishmaniasis, papers meeting our inclusion criteria considered countries that were widely distributed around the globe, with the exception of countries in East Africa.

Geographical coverage of papers by disease. Colours represent the total number of papers that met the inclusion criteria, per disease, across countries.
The observed variation in geographical coverage of papers across diseases prompted us to further investigate the links between study location, number of papers, disease burden and country vulnerability to climate change. Given the discrepancy in the number of papers between diseases, we grouped these into four categories: (i) malaria; (ii) dengue and chikungunya; (iii) other vector-borne NTDs (Chagas disease, dracunculiasis, human African trypanosomiasis, leishmaniasis, lymphatic filariasis, onchocerciasis, schistosomiasis, trachoma); and (iv) non-vector-borne NTDs (Buruli ulcer, echinococcosis, foodborne trematodiases, leprosy, rabies, scabies/tungiasis, soil-transmitted helminthiases, snakebite envenoming, taeniasis and cysticercosis).
Our analysis showed different patterns across groups of diseases. For malaria there were clear trends towards more papers covering countries with a high malaria DALY burden, low HAQI and high vulnerability to climate change (Figure 5 , first column). For dengue and chikungunya, there was a trend towards increasing numbers of papers covering areas with high burden, but at low DALY burden there was an increase in papers due to the relatively large number of studies looking at potential expansion of these diseases into new areas (Figure 5 , second column). This also meant that there was a relative abundance of papers studying these diseases in areas where there is good access to healthcare, and where the climate vulnerability score is low. For the remaining vector-borne NTDs (Figure 5 , third column), there was a suggestion of an increasing numbers of papers addressing countries with increasing burden and decreasing HAQI, but no suggestion that analyses were more commonly focused on areas with high climate vulnerability. For the non-vector-borne NTD group, papers more frequently considered countries with high DALY burden for that disease, high HAQI and low climate vulnerability (Figure 5 , fourth column).

Numbers of papers meeting the inclusion criteria by disease or disease group, compared with country-level (A) DALYs for the disease or disease group; (B) health access and quality index; and (C) climate vulnerability score. Studies with outcomes reported at global level (for all countries) were removed for these analyses. Each circle represents one country; superimposition of multiple circles makes some look darker than others. Lines show locally estimated scatterplot smoothing (LOESS)-generated local polynomial regression.
Detailed analysis of the full text of papers meeting the inclusion criteria revealed additional insights. For malaria, the consensus among papers that met the inclusion criteria was that global warming will extend the area in which transmission is possible to some areas where it was previously too cold for vector or parasite development, while conditions in some currently endemic areas may become too severe to maintain transmission. Zones suitable for transmission may shift both poleward and upwards in altitude. Future expansion could be balanced by more frequent droughts, making environmental conditions unsuitable for transmission in some previously endemic areas, including parts of the Sahel. 16 Other papers concluded that malaria transmission seasons will last longer, as more months of the year will have a suitable climate. 17 While these changes could place a greater proportion of the global population at risk, 18 the potential net impact of climate change on the global burden of malaria remains unclear; 19–22 papers that met the inclusion criteria were vastly different and difficult to compare with each other. Making predictions for regional and global populations was previously difficult because of a general lack of high-resolution monthly incidence data. 23 There is likely to be small-scale heterogeneity in effects on the ground.
One aspect of climate change that is already impacting malaria transmission is the increasing frequency of extreme weather events. Severe flooding in Pakistan in 2022 and cyclones in Mozambique and Madagascar in 2023 were accompanied by local spikes in malaria cases, driven by breeding of Anopheles mosquitos in flood waters. 24
For dengue and chikungunya, a significant proportion of papers that met our inclusion criteria described subnational studies with subdecadal time frames. Models with broader geographic and temporal boundaries predict dramatic expansion in the future range of relevant Aedes vector species, 25–27 in line with trends that are already being observed. 28–30 Some local retreat from geographies currently occupied by Aedes spp. is also predicted. 31 Alongside the overall increase in vector range, considerable increases are projected in the number of future dengue cases under more adverse climate change scenarios (Box 1 ) in some models. 30 , 32 Other models predict a plateauing of dengue in highly endemic regions by 2050. 28 There is undoubtedly uncertainty about the magnitude and direction of future geographic expansion.
For the leishmaniases, 53 papers that met the inclusion criteria considered relevant outcomes. However, there are many different pathogens and vector species contributing to this complex of diseases (Table 1 ), making a reliable, complete picture very difficult to draw. Several large-scale models predict changes in the range of relevant sandfly species around the Mediterranean and in the Americas, with transmission in the latter extending as far north as southern Canada in some future climate scenarios. 33–37 The number of people in North America living in areas in which leishmaniasis is transmitted could double from 2010 to 2080 under SRES B2. 37 The paucity of information on the potential impact of climate change on leishmaniasis in Africa (Figure 4 ) was striking.
For schistosomiasis, one model predicted increased prevalence and intensity of infection in some areas of East Africa over the next few decades, particularly in Rwanda, Burundi, south-west Kenya and eastern Zambia, with concurrent substantial decreases in risk in parts of Kenya, southern South Sudan and eastern Democratic Republic of the Congo. 38 Decreases in transmission are predicted for China, 39 although the possibility was also invoked that disease will re-emerge in parts of mainland China where it was previously eliminated. 40 This concern may account for the relative concentration of publications that focused on China (Figure 4 ).
There were few papers on the remaining NTDs, with a bias towards vector-borne diseases, possibly due to the existence of established methodologies for examining vector suitability and its links to climate. Climate impacts on these other NTDs, whether through environmental changes or societal ones, such as changes in time to diagnosis due to changes in access to health systems, 41 remain largely unexplored.
For thousands of years, societies have been shaped and reshaped by epidemics, with Ebola virus disease, COVID-19 and mpox presenting only the most recent striking examples. A distinct feature of the climate crisis is the pace of change in underlying global ecosystems. This generates uncertainty about the future epidemiology of multiple diseases: not only those that have historically manifested as epidemics, but also those formerly considered as stable and endemic, and those being driven towards elimination or eradication. Climate change will simultaneously reshape the epidemiology of many non-infectious diseases, threaten health infrastructure, affect the health workforce and alter other foundational determinants of human health. These parallel effects will exacerbate the challenge presented by the evolving epidemiology of infectious diseases. 42 , 43
Malaria and many NTDs have relatively complex life cycles, involving overlapping webs of interactions between humans and vertebrate and invertebrate animals. Multiple points of exposure to ecological, biological and social systems increase the probability that climate change will alter disease incidence or prevalence. 43 , 44 This environmental sensitivity makes prediction of future scenarios both difficult and important. The perceived difficulty is borne out in the literature identified here: for most of the outcomes in scope, projections of the effects of future climate change at large scale are scarce. Projections that do exist incorporate considerable uncertainty.
Of all potential outcomes framed for this review, those related to malaria, dengue and chikungunya, and the leishmaniases, were the most studied. Yet even for malaria, long-term projections of future transmission scenarios remain inadequate for robust planning. Beyond the expected short-term effects of extreme weather events on local disease incidence, 45 it is difficult to be definitive 46 –global incidence and attributable deaths may go up, down or stay about the same, depending on multiple factors, including the success or otherwise of nascent vaccination programmes. 6 The arboviral NTDs, dengue and chikungunya, on the other hand, are generally predicted to continue their current surge. 28 , 29 Global expansion in the population at risk of the leishmaniases seems likely. The predicted effect of climate change on these diseases suggests that effects on vectors of other vector-borne NTDs will be similarly important to understand.
For many of the NTDs that we grouped as ‘non-vector-borne’, however, limitations in our capacity for prediction stem in part from gaping deficiencies in our understanding of disease transmission at steady state. Data published only after the searches were conducted for this review pin responsibility for Mycobacterium ulcerans transmission in southeastern Australia on the mosquito Aedes notoscriptus , 47 whereas transmission by mosquitos was previously hypothesised but unproven, and analogous work has not yet been published for Buruli ulcer-endemic areas of the Americas, Japan, Papua New Guinea or West Africa. Postulated mechanisms for the transmission of leprosy are still based on circumstantial evidence. 13 The nematode worm Dracunculus medinensis , the target of a global eradication programme that began in 1980, was only relatively recently discovered to infect paratenic and definitive hosts other than humans. 48 These gaps in our knowledge have lingered because funding for research on NTDs is thin 49 and spread across a very large number of pathogens (Table 1 ), some of which are probably relatively rare. 50 , 51
Multiplying that disease-specific uncertainty are uncertainties surrounding future climate scenarios and their secondary impacts (including, for example, on conflict, migration and demography), which are further clouded by our joint hope that individual and collective behaviours will change sufficiently to allow greenhouse gas concentrations to fall and thereby effectively mitigate climate change. This uncertainty for virtually every parameter of NTD transmission models 52 makes decadal projections of future NTD prevalence or incidence feel ill-advised. Unfortunately, without those projections, NTDs are likely to continue to be given very limited attention in climate-related discussions, including, for example, in the Assessment Reports of the Intergovernmental Panel on Climate Change. 53 Better understanding of NTD transmission dynamics, and estimates—even if heavily caveated—of the potential impacts of climate change, are precisely what is needed now.
The current work builds on previously published reviews, 12 , 43 , 54 but continues to have some limitations. First, although we undertook screening of titles and abstracts by two independent observers, our use of tags to highlight certain characteristics of papers within the Covidence platform may have unmasked some second screeners to the first screener's decision.
Second, screening for mitigation and adaptation strategies was challenging; the process required considerable judgement. It is possible that relevant sources were overlooked in the >42 000 records that our searches identified.
Third, we did not critically quality-appraise the methodology used for each included paper (e.g. number, size and origin of datasets; appropriateness of climate models, such as use of downscaling; and inclusion of all potentially relevant variables). Many studies had low power, did not consider all potential explanatory variables or confounders, or were otherwise methodologically weak. To be useful as a summary of coverage within the published literature, our visualisations imply that all studies contribute equally to the evidence base, whereas they do not.
Fourth, under-ascertainment is an issue for many diseases, but is particularly problematic for malaria and NTDs; their concentration in impoverished populations means that patients with these diseases have HAQIs that are far from ideal. Under-ascertainment is a specifically identified issue for dengue, in which second infections may be considerably more likely to produce disease that leads to clinical presentation and therefore registration. 55
Fifth, to be included in this review, a paper had to explicitly juxtapose climate change and relevant outcomes. Some authors may have made tenuous arguments linking weather-related variables (such as temperature or rainfall) to climate change, resulting in inclusion; others may not have been explicit in framing climate change implications when doing so would have been justifiable.
Sixth, it is likely that source data were used more than once in groups of papers with the same or related outcome measures for overlapping geographies and overlapping timespans.
Seventh, we did not specifically look for the impact of climate change mediated through internal displacement and migration of people; 56 changes in institutional capacity and service provision; 57 , 58 vector microbiome, genetics or gene expression; or pathogen genetics or gene expression. 59 , 60 All of these mechanisms may be important.
Eighth, as for any review, our searches had a fixed date range and were not exhaustive within that range. Not all possible intermediate and reservoir hosts (e.g. for rabies) were specifically included. Insect species with postulated but unproven vectorial capacity were excluded; Culex pipiens is a known vector of lymphatic filariasis in Egypt but papers considering it in other contexts were set aside. Studies in press were excluded by design; we were aware of forthcoming work on the impact of climate change on several diseases that had been submitted for peer review but were not yet published when our searches closed. The December 2023 addition of noma to the WHO's list of NTDs 61 occurred too late for noma to be included in our searches.
Ninth, the high degree of heterogeneity (in questions examined, methods used and so on) precluded quantitative synthesis.
Tenth, projecting disease burdens forward over long timescales means that future changes in treatment and control strategies would ideally also be taken into account. This is difficult to do. Authors of studies from the early part of our 2010–2023 publication window may not have foreseen the scale-up in intervention coverage that has occurred for many diseases in the past 10–15 y.
Despite uncertainty around data, some general conclusions and recommendations are proposed here. It can be inferred from existing data, first, that climate change is likely to have profound direct and indirect implications for malaria, dengue and chikungunya, leishmaniasis and at least several other vector-borne NTDs, even if the amplitude and direction of the effects will probably vary by disease and location, be non-linear 22 , 62–64 and evolve with time. Changes of two kinds will be apparent: diseases will move around, and where endemicity is constant, there will be local increases or decreases in incidence or prevalence. There is a pressing need to safeguard previous global health gains by scaling up proven interventions and achieving impact before future changes render those interventions ineffective. 65 Second, the lack of predictability, even over relatively short timescales, calls for existing surveillance and intervention systems to be reinforced and regularly reviewed. Integrated surveillance and intervention systems, covering multiple diseases 66 and taking a One Health approach, 67 could offer efficiencies. Third, communities should be consulted and involved in these reviews of surveillance and intervention systems, and in research undertaken at the interface of infectious diseases and climate change, to maximise the relevance of such efforts despite changing human populations. Fourth, integrating climate resilience into health systems is critical. This should encompass investing in health infrastructure, fostering cross-sector collaboration, adapting to the needs of displaced populations, improving access to health products and accelerating research and development to fill known gaps. 68 A particular requirement is access to existing and new countermeasures to limit future expansions in disease burden. Fifth, we do not know enough.
Recommendations for future research.
Research to fill current knowledge gaps on the likely impacts of climate change, mitigation and adaptation strategies on malaria and NTDs should:
where projections are modelled, be based on clearly defined climate scenarios, and include multiple scenarios, ideally using the most recent categories defined by the IPCC, in order to facilitate comparisons across studies, diseases and geographies.
where projections are modelled, incorporate not only climate scenarios but also sociodemographic and population density projections. This may require developments in methodology to ensure that demographic transitions underpinning the epidemiological models are in line with those assumed in the projections.
where projections are modelled, ideally incorporate detailed analyses of the likely impact of climate change mitigation and adaptation strategies, which are currently rare, and the modelled effectiveness of existing and new interventions (vector control, vaccines, treatments) under multiple climate scenarios.
explore the potential impacts of climate change on a broader set of NTDs and geographies, prioritising places with the highest disease burdens and the people most vulnerable to the future impacts of climate change.
recognise that, because of the paucity of data, it will remain challenging to estimate the impact of climate change and other secular trends on NTDs, and to anticipate potential interactions between climate change and the impact of interventions. Therefore, new methodologies are needed, based on plausible biological assumptions. This will require distinct study types to prepare for modelling, including prospective population-based investigations, laboratory studies, biological experiments (e.g. mosquito or egg survival) and social science that can be performed in suitable locations to inform projections and provide a data source for future estimates of change. Investigating methods to extrapolate laboratory findings to field settings would be beneficial.
prioritise standardisation and collaboration, including across disciplines. Specifically for modelling, we propose the development and adoption of standardised frameworks for future projections, using, where possible, standardised survey or case data, and an open collaborative model in which source data and contributions to code can be tracked, to speed up and unify research while protecting the rights of countries that generate primary data and acknowledging all collaborators.
facilitate leadership of scientists in affected areas to undertake and communicate research and its implications. An understanding of local context and closer relationships with stakeholders will lead to higher quality analyses with increased uptake in local decision-making processes.
where projections are developed, provide actionable data for policymaking at national and subnational levels. For example, studies focusing on climate-driven dengue expansion to new locations (both in high-income and low- and middle-income countries) should investigate appropriate methods of surveillance, which could be targeted at high-risk areas in a cost-efficient manner. (Risk here could be interpreted in multiple ways: relating to the potential for increases in vector abundance, infection, severe disease or outcomes such as lost gross domestic product (GDP), for example.)
Based on our analysis, we also propose several key recommendations to guide future studies (Box 2 ). The most important of these is for standardisation and collaboration. Our scope included 21 diseases and disease groups, at least 76 distinct pathogens, 373 venomous snakes and humans everywhere; there is insufficient modelling capacity globally for this to be investigated on a competitive basis, particularly when the appropriate modelling methodologies change with the level of endemicity and results are needed at multiple scales. Indeed, our analysis suggests that existing studies may not be sufficiently focused on areas where the need to plan for adaptation may be greatest. We recommend holistic approaches to risk assessment, incorporating more of the available data and recruiting more of the available brainpower to undertake ensemble analyses with agreed best-practice methodology. Modelling efforts should incorporate consideration of humans, pathogens, vectors, intermediate and reservoir hosts and the effect of relevant interventions, as appropriate, to generate predictions over decadal time frames—and not ignore populations where these diseases are currently endemic. Collectively, these measures should reduce potential duplication and hopefully produce more complete and more accurate estimates of future vulnerability, exposure and impact. Open-source collaborative modelling platforms 17 , 23 could facilitate contributions from as many relevant stakeholders as are willing to engage, and allow tailoring of consistently high-quality outputs for specific audiences. Collaboration could include involvement of affected communities through citizen science: in vector surveillance, for example. Accessible global databases on disease and vector occurrence 69–75 should be harnessed and adapted to cover additional diseases. Broader use of remotely sensed climate data should be explored, particularly where locally acquired data are unavailable or microclimates are of relevance. Long-term time-series data should be pooled and re-analysed to tease out the relative contributions of deliberate interventions, secular trends, seasonality and climate change. Production of detailed risk and distribution maps should be facilitated to help plan local control and elimination efforts. The fact that many NTDs are targeted for eradication, interruption of transmission or elimination as a public health problem by 2030 should not dissuade us from taking a long-term view of this work; 43 global health ambitions are not always realised, and the best possible current understanding of counterfactual scenarios should help decision-makers to target resources and chart the most appropriate course.
Conclusions
It is difficult to have immersed ourselves in this literature as we have without acquiring a deepened sense of foreboding over the adverse influence that we as a species are visiting on our planet and its most vulnerable people. Adverse changes have already occurred in the incidence or prevalence of infectious diseases that cause death or profound morbidity. Women, children, older people, indigenous groups and ethnic minorities, migrants and the very poor have contributed least but are likely to experience most of the effects of the climate crisis, 76 notably including through any increase in the burden of malaria or NTDs. An emerging opportunity to correct this inequity arises through financial commitments to NTD control and elimination made at the 28th United Nations Climate Change Conference in December 2023. 77 Allocation of these resources should be guided by informed scenario analyses of current and future disease burden. The work described in this review is a start; convening stakeholders globally to advance the research agenda must be our next collective move.
CLD, TDH, AWS and ISF conceived the overall study design, which was elaborated by PK, JLH, CLD, MA, MB, IB, SD, DLM, CMRT, KRvD, TDH, AWS and ISF, with review by all authors; PK, JLH, CLD, MA, MB, IB, SD, CMRT, KRvD and AWS screened titles and abstracts, located full texts and undertook full-text review; PK and JLH identified seed papers and undertook backward and forward citation searches; PK, JLH, CLD, MA, MB, IB, SD, CMRT, KRvD, JA, AD, DSE, JG, MRJ, AMK, JK, WJK, TLL, JSM, JP, NP, AR, LJR, ZMR, GSGS and AWS extracted data; PK, JLH, KRvD, JFM, CNA, HSR, DRMS and TDH conducted quantitative analyses and constructed figures; PK, KRvD, KCR, CNA and AWS prepared the first draft of the manuscript, which was critically revised by all authors. All authors reviewed and approved the final manuscript. PK and AWS are the paper's guarantors.
We are grateful to Abdisalan M. Noor, lead author of the climate chapter of the World Malaria Report 2023, for his leadership in this space, and to Paul Courtright for assistance with translation from Korean to English.
This work was supported by Reaching the Last Mile; The Fred Hollows Foundation; Bill & Melinda Gates Foundation and the European Commission. PK, SK, CNA and TDH were supported by funding from the Bill & Melinda Gates Foundation [INV-030046], via the NTD Modelling Consortium. TDH is supported by funding from the Li Ka Shing Foundation. JLH acknowledges funding from USAID through an Interagency Agreement with CDC awarded to DLM. KRvD and RL acknowledge funding from the European Union's Horizon Europe research and innovation programme [grant agreement no. 101057554] (Horizon Europe project IDAlert, https://idalertproject.eu ); IDAlert is part of the EU climate change and health cluster ( https://climate-health.eu ). BA, AFG, JK, VL, AR, KHS, RV, SW, AY, DCL, AWS and ISF are staff members of, and CLD and CMRT are consultants to, the WHO. JA was supported by an appointment to the Research Participation Program at the Centers for Disease Control and Prevention, administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and the Centers for Disease Control and Prevention. SK, AMK, JSM, MRJ, TLL and GSGS were supported by funding from the Bill & Melinda Gates Foundation [INV-016807]. NMF was supported by funding from Wellcome, the Jameel Institute and the MRC Centre for Global Infectious Disease Analysis. JFM is supported by grant funding from the Bill & Melinda Gates Foundation and Gavi. RSN and KP are staff members of the Pan American Health Organization.
SD is and JC was an employee of The Fred Hollows Foundation; SJB is an employee of the Bill & Melinda Gates Foundation. Their participation in this work was in their personal capacity. All other authors declare no competing interests.
Not required.
All data relevant to the study are available in Supplementary Table 2 and Supplementary Table 3 . Code available upon request.
The authors alone are responsible for the views expressed in this article and they do not necessarily represent the views, decisions or policies of the institutions with which they are affiliated. The boundaries and names shown and the designations used on the maps in this article do not imply the expression of any opinion whatsoever on the part of the authors, or the institutions with which they are affiliated, concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries.
Intergovernmental Panel on Climate Change . Summary for Policymakers . In: Masson-Delmotte V , Zhai P , Pirani A et al. (eds). Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge and New York : Cambridge University Press , 2021 .
Google Scholar
Google Preview
Romanello M , Napoli CD , Green C et al. The 2023 report of the Lancet Countdown on health and climate change: the imperative for a health-centred response in a world facing irreversible harms . Lancet . 2023 ; 402 ( 10419 ): 2346 – 94 .
Mora C , McKenzie T , Gaw IM et al. Over half of known human pathogenic diseases can be aggravated by climate change . Nat Clim Chang . 2022 ; 12 ( 9 ): 869 – 75 .
Zoleko-Manego R , Okwu DG , Handrich C et al. Effectiveness of antimalarial drug combinations in treating concomitant urogenital schistosomiasis in malaria patients in Lambaréné, Gabon: a non-randomised event-monitoring study . PLoS Negl Trop Dis . 2022 ; 16 ( 10 ): e0010899 .
Afolabi MO , Adebiyi A , Cano J et al. Prevalence and distribution pattern of malaria and soil-transmitted helminth co-endemicity in sub-Saharan Africa, 2000-2018: a geospatial analysis . PLoS Negl Trop Dis . 2022 ; 16 ( 9 ): e0010321 .
World malaria report 2023. Available at https://www.who.int/publications/i/item/9789240086173 (accessed January 26, 2024) . Geneva, Switzerland : World Health Organization , 2023 .
Global report on neglected tropical diseases 2024 . Geneva, Switzerland : World Health Organization , 2024 .
Clark J , Glasziou P , Del Mar C et al. A full systematic review was completed in 2 weeks using automation tools: a case study . J Clin Epidemiol . 2020 ; 121 : 81 – 90 .
Tricco AC , Lillie E , Zarin W et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): checklist and Explanation . Ann Intern Med . 2018 ; 169 ( 7 ): 467 – 73 .
Clark JM , Sanders S , Carter M et al. Improving the translation of search strategies using the Polyglot Search Translator: a randomized controlled trial . J Med Libr Assoc . 2020 ; 108 ( 2 ): 195 – 207 .
Haddaway NR , Grainger MJ , Gray CT. citationchaser: an R package and Shiny app for forward and backward citations chasing in academic searching. Available at https://zenodo.org/records/4543513 (accessed December 12, 2023 ). Zenodo . 2021 .
Tidman R , Abela-Ridder B , de Castañeda RR. The impact of climate change on neglected tropical diseases: a systematic review . Trans R Soc Trop Med Hyg . 2021 ; 115 ( 2 ): 147 – 68 .
Ending the neglect to attain the Sustainable Development Goals: a road map for neglected tropical diseases 2021–2030. Available at https://apps.who.int/iris/handle/10665/338565 (accessed January 28, 2021 ). Geneva, Switzerland : World Health Organization , 2020 .
GBD 2019 Diseases and Injuries Collaborators . Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019 . Lancet . 2020 ; 396 ( 10258 ): 1204 – 22 .
Haakenstad A , Yearwood JA , Fullman N et al. Assessing performance of the Healthcare Access and Quality Index, overall and by select age groups, for 204 countries and territories, 1990-2019: a systematic analysis from the Global Burden of Disease Study . 2019 . Lancet Glob Health . 2022 ; 10 ( 12 ): e1715 – e43 .
Ermert V , Fink AH , Morse AP et al. The impact of regional climate change on malaria risk due to greenhouse forcing and land-use changes in tropical Africa . Environ Health Perspect . 2012 ; 120 ( 1 ): 77 – 84 .
Colón-González FJ , Sewe MO , Tompkins AM et al. Projecting the risk of mosquito-borne diseases in a warmer and more populated world: a multi-model, multi-scenario intercomparison modelling study . Lancet Planet Health . 2021 ; 5 ( 7 ): e404 – e14 .
Caminade C , Kovats S , Rocklov J et al. Impact of climate change on global malaria distribution . Proc Natl Acad Sci USA . 2014 ; 111 ( 9 ): 3286 – 91 .
Feachem RGA , Chen I , Akbari O et al. Malaria eradication within a generation: ambitious, achievable, and necessary . Lancet . 2019 ; 394 ( 10203 ): 1056 – 112 .
Rodó X , Martinez PP , Siraj A et al. Malaria trends in Ethiopian highlands track the 2000 ‘slowdown’ in global warming . Nat Commun . 2021 ; 12 ( 1 ): 1555 .
Stern DI , Gething PW , Kabaria CW et al. Temperature and malaria trends in highland East Africa . PLoS One . 2011 ; 6 ( 9 ): e24524 .
Alonso D , Bouma MJ , Pascual M. Epidemic malaria and warmer temperatures in recent decades in an East African highland . Proc Biol Sci . 2011 ; 278 ( 1712 ): 1661 – 9 .
Edlund S , Davis M , Douglas JV et al. A global model of malaria climate sensitivity: comparing malaria response to historic climate data based on simulation and officially reported malaria incidence . Malar J . 2012 ; 11 : 331 .
Tabassum S , Kalsoom T , Zaheer Z et al. Reflections on the surge in malaria cases after unprecedented flooding in Pakistan-A commentary . Health Sci Rep . 2023 ; 6 ( 10 ): e1620 .
Georgiades P , Proestos Y , Lelieveld J et al. Machine Learning Modeling of Aedes albopictus Habitat Suitability in the 21st Century . Insects . 2023 ; 14 ( 5 ): 447 .
Campbell LP , Luther C , Moo-Llanes D et al. Climate change influences on global distributions of dengue and chikungunya virus vectors . Philos Trans R Soc Lond B Biol Sci . 2015 ; 370 ( 1665 ): 20140135 .
Liu H , Huang X , Guo X et al. Climate change and Aedes albopictus risks in China: current impact and future projection . Infect Dis Poverty . 2023 ; 12 ( 1 ): 26 .
Messina JP , Brady OJ , Golding N et al. The current and future global distribution and population at risk of dengue . Nat Microbiol . 2019 ; 4 ( 9 ): 1508 – 15 .
Schaffner F , Mathis A. Dengue and dengue vectors in the WHO European region: past, present, and scenarios for the future . Lancet Infect Dis . 2014 ; 14 ( 12 ): 1271 – 80 .
Colón-González FJ , Harris I , Osborn TJ et al. Limiting global-mean temperature increase to 1.5-2°C could reduce the incidence and spatial spread of dengue fever in Latin America . Proc Natl Acad Sci USA . 2018 ; 115 ( 24 ): 6243 – 8 .
Escobar LE , Romero-Alvarez D , Leon R et al. Declining Prevalence of Disease Vectors Under Climate Change . Sci Rep . 2016 ; 6 : 39150 .
Bouzid M , Colón-González FJ , Lung T et al. Climate change and the emergence of vector-borne diseases in Europe: case study of dengue fever . BMC Public Health . 2014 ; 14 : 781 .
Chalghaf B , Chemkhi J , Mayala B et al. Ecological niche modeling predicting the potential distribution of Leishmania vectors in the Mediterranean basin: impact of climate change . Parasit Vectors . 2018 ; 11 ( 1 ): 461 .
Moo-Llanes DA , Pech-May A , Ibarra-Cerdeña CN et al. Inferring distributional shifts of epidemiologically important North and Central American sandflies from Pleistocene to future scenarios . Med Vet Entomol . 2019 ; 33 ( 1 ): 31 – 43 .
Moo-Llanes D , Ibarra-Cerdeña CN , Rebollar-Téllez EA et al. Current and future niche of North and Central American sand flies (Diptera: psychodidae) in climate change scenarios . PLoS Negl Trop Dis . 2013 ; 7 ( 9 ): e2421 .
Peterson AT , Campbell LP , Moo-Llanes DA et al. Influences of climate change on the potential distribution of Lutzomyia longipalpis sensu lato (Psychodidae: phlebotominae) . Int J Parasitol . 2017 ; 47 ( 10-11 ): 667 – 74 .
González C , Wang O , Strutz SE et al. Climate change and risk of leishmaniasis in North America: predictions from ecological niche models of vector and reservoir species . PLoS Negl Trop Dis . 2010 ; 4 ( 1 ): e585 .
McCreesh N , Nikulin G , Booth M . Predicting the effects of climate change on Schistosoma mansoni transmission in eastern Africa . Parasit Vectors . 2015 ; 8 : 4 .
Cao B , Bai C , Wu K et al. Tracing the future of epidemics: coincident niche distribution of host animals and disease incidence revealed climate-correlated risk shifts of main zoonotic diseases in China . Glob Chang Biol . 2023 ; 29 ( 13 ): 3723 – 46 .
Zhu G , Fan J , Peterson AT . Schistosoma japonicum transmission risk maps at present and under climate change in mainland China . PLoS Negl Trop Dis . 2017 ; 11 ( 10 ): e0006021 .
Impact of the COVID-19 pandemic on seven neglected tropical diseases: a model-based analysis ( https://www.who.int/publications/i/item/9789240027671 , accessed 10 March 2024 ). Geneva, Switzerland : World Health Organization , 2021 .
Watts N , Amann M , Arnell N et al. The 2020 report of The Lancet Countdown on health and climate change: responding to converging crises . Lancet . 2021 ; 397 ( 10269 ): 129 – 70 .
Booth M. Climate Change and the Neglected Tropical Diseases . Adv Parasitol . 2018 ; 100 : 39 – 126 .
de Souza WM , Weaver SC. Effects of climate change and human activities on vector-borne diseases . Nat Rev Micro . 2024 . https://pubmed.ncbi.nlm.nih.gov/38486116/
Otto FEL , Zachariah M , Saeed F et al. Climate change increased extreme monsoon rainfall, flooding highly vulnerable communities in Pakistan . Environ Res Clim . 2023 ; 2 ( 2 ): 025001 .
Tompkins AM , Thomson MC. Uncertainty in malaria simulations in the highlands of Kenya: relative contributions of model parameter setting, driving climate and initial condition errors . PLoS One . 2018 ; 13 ( 9 ): e0200638 .
Mee PT , Buultjens AH , Oliver J et al. Mosquitoes provide a transmission route between possums and human s for Buruli ulcer in southeastern Australia . Nat Microbiol . 2024 ; 9 ( 2 ): 377–89 .
Callaway E. Dogs thwart effort to eradicate Guinea worm . Nature . 2016 ; 529 ( 7584 ): 10 – 1 .
G-FINDER 2023 . Neglected disease research and development: the higher cost of lower funding . Sydney : Policy Cures Research , 2024 .
Stamm LV . Pinta: Latin America's Forgotten Disease? Am J Trop Med Hyg . 2015 ; 93 ( 5 ): 901 – 3 .
Vighi da Rosa R , Damares Rodrigues de Souza D , Cartell A et al. Mal de Pinta, first autochthonous case from South of Brazil . Int J Dermatol . 2021 ; 60 ( 1 ): e19 – 21 .
Moore JL , Liang S , Akullian A et al. Cautioning the use of degree-day models for climate change projections in the presence of parametric uncertainty . Ecol Appl . 2012 ; 22 ( 8 ): 2237 – 47 .
McIver L , Beavon E , Malm A et al. Impacts of climate change on human health in humanitarian settings: Evidence gaps and future research needs . PLoS Climate . 2024 ; 3 ( 3 ): e0000243
Kulkarni MA , Duguay C , Ost K . Charting the evidence for climate change impacts on the global spread of malaria and dengue and adaptive responses: a scoping review of reviews . Global Health . 2022 ; 18 ( 1 ): 1 .
Clapham HE , Cummings DAT , Johansson MA . Immune status alters the probability of apparent illness due to dengue virus infection: evidence from a pooled analysis across multiple cohort and cluster studies . PLoS Negl Trop Dis . 2017 ; 11 ( 9 ): e0005926 .
Guerra CA , Kang SY , Citron DT et al. Human mobility patterns and malaria importation on Bioko Island . Nat Commun . 2019 ; 10 ( 1 ): 2332 .
Rugai D , Kassenga GR . Climate change impacts and institutional response capacity in Dar es Salaam, Tanzania . In: Macchi S , Ms Tiepolo (eds). Climate Change Vulnerability in Southern African Cities . Cham, Switzerland : Springer , 2014 , 39 – 55 .
Shirley H , Grifferty G , Yates EF et al. The connection between climate change, surgical care and neglected tropical diseases . Ann Glob Health . 2022 ; 88 ( 1 ): 68 .
Greischar MA , Beck-Johnson LM , Mideo N. Partitioning the influence of ecology across scales on parasite evolution . Evolution . 2019 ; 73 ( 11 ): 2175 – 88 .
Wimalasiri-Yapa B , Barrero RA , Stassen L et al. Temperature modulates immune gene expression in mosquitoes during arbovirus infection . Open Biol . 2021 ; 11 ( 1 ): 200246 .
WHO officially recognizes noma as a neglected tropical disease ( https://www.who.int/news/item/15-12-2023-who-officially-recognizes-noma-as-a-neglected-tropical-disease , accessed 25 January 2024 ). Geneva, Switzerland : World Health Organization , 2023 .
Chaves LF , Hashizume M , Satake A et al. Regime shifts and heterogeneous trends in malaria time series from Western Kenya Highlands . Parasitology . 2012 ; 139 ( 1 ): 14 – 25 .
Paaijmans KP , Wandago MO , Githeko AK et al. Unexpected high losses of Anopheles gambiae larvae due to rainfall . PLoS One . 2007 ; 2 ( 11 ): e1146 .
Chemison A , Ramstein G , Tompkins AM et al. Impact of an accelerated melting of Greenland on malaria distribution over Africa . Nat Commun . 2021 ; 12 ( 1 ): 3971 .
Global health community calls for urgent action on climate and health at COP28. Available at https://www.who.int/news/item/27-11-2023-global-health-community-calls-for-urgent-action-on-climate-and-health-at-cop28 (accessed February 21, 2024 ). Geneva, Switzerland : World Health Organization , 2023 .
Cooley GM , Feldstein LR , Bennett SD et al. No serological evidence of trachoma or yaws among residents of registered camps and makeshift settlements in Cox's Bazar, Bangladesh . Am J Trop Med Hyg . 2021 ; 104 ( 6 ): 2031 – 7 .
Ending the neglect to attain the sustainable development goals. One health: approach for action against neglected tropical diseases 2021-2030. Available at https://www.who.int/publications/i/item/9789240042414 (accessed March 21, 2024 ). Geneva, Switzerland : World Health Organization , 2022 .
Mabey D , Agler E , Amuasi JH et al. Towards a comprehensive research and development plan to support the control, elimination and eradication of neglected tropical diseases . Trans R Soc Trop Med Hyg . 2020 ; 115 ( 2 ): 196 – 9 .
Hay SI , Snow RW . The malaria Atlas Project: developing global maps of malaria risk . PLoS Med . 2006 ; 3 ( 12 ): e473 .
International Trachoma Initiative. Global Atlas of Trachoma. Available at www.trachomaatlas.org (accessed February 21, 2024 ). Decatur : Task Force for Global Health , 2024 .
Brooker S , Hotez PJ , Bundy DA. The global atlas of helminth infection: mapping the way forward in neglected tropical disease control . PLoS Negl Trop Dis . 2010 ; 4 ( 7 ): e779 .
Simarro PP , Cecchi G , Paone M et al. The Atlas of human African trypanosomiasis: a contribution to global mapping of neglected tropical diseases . Int J Health Geogr . 2010 ; 9 : 57 .
Pigott DM , Golding N , Messina JP et al. Global database of leishmaniasis occurrence locations, 1960-2012 . Sci Data . 2014 ; 1 : 140036 .
Clarke J , Lim A , Gupte PR et al. OpenDengue: data from the OpenDengue database. Version [1.2]. figshare. Dataset. Available at https://doi.org/10.6084/m9.figshare.24259573.v3 (accessed February 21, 2024 ). London, UK : London School of Hygiene & Tropical Medicine , 2023 .
Malaria threats map . Available at https://apps.who.int/malaria/maps/threats/ (accessed February 29, 2024 ). Geneva, Switzerland : World Health Organization , 2024 .
Lee H , Calvin K , Dasgupta D et al. Climate change 2023: synthesis report. Contribution of working groups I, II and III to the sixth assessment report of the intergovernmental panel on climate change . Available at https://www.ipcc.ch/report/ar6/syr/downloads/report/IPCC_AR6_SYR_LongerReport.pdf (accessed January 23, 2024 ). Geneva, Switzerland : Intergovernmental Panel on Climate Change , 2023 .
Venkatesan P. Millions of dollars pledged at COP28 to fight NTDs . Lancet Infec Dis . 2024 ; 24 ( 2 ): e88 .
Hougton JT , Callander BA , Varney SK. Climate change 1992: the Supplementary Report to the IPCC Scientific Assessment . Cambridge : Cambridge University Press , 1992 .
Intergovernmental Panel on Climate Change . Summary for Policymakers: Emissions Scenarios. A Special Report of IPCC Working Group III . Geneva : IPCC , 2000 .
Intergovernmental Panel on Climate Change . Summary for policymakers . In: Pörtner H-O , Roberts DCV , Masson-Delmotte V et al. (eds). IPCC Special Report on the Ocean and Cryosphere in a Changing Climate Geneva : IPCC , 2019 .
Rogelj J , Meinshausen M , Knutti R. Global warming under old and new scenarios using IPCC climate sensitivity range estimates . Nat Clim Change . 2012 ; 2 ( 4 ): 248 – 53 .
Intergovernmental Panel on Climate Change . Climate Change 2021: the Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [ Masson-Delmotte V. , Zhai P. , Pirani A. , Connors S.L. , Péan C. , Berger S. , Caud N. , Chen Y. , Goldfarb L. , Gomis M.I. , Huang M. , Leitzell K. , Lonnoy E. , Matthews J.B.R. , Maycock T.K. , Waterfield T. , Yelekçi O. , Yu R. , Zhou B. (eds.)]. Cambridge and New York : Cambridge University Press , 2021 .
Intergovernmental Panel on Climate Change . Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [ Core Writing Team , Lee H. , Romero J. (eds.)]. Geneva : IPCC , 2023 .
- chikungunya fever
- dengue fever
- cost of illness
- leishmaniasis
- tropical disease
- climate change
Supplementary data
Email alerts, citing articles via.
- About Transactions of the Royal Society of Tropical Medicine and Hygiene
- Recommend to your Library
Affiliations
- Online ISSN 1878-3503
- Print ISSN 0035-9203
- Copyright © 2024 Royal Society of Tropical Medicine and Hygiene
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
- Open access
- Published: 06 October 2022
Severe malaria
- Nicholas J. White 1 , 2
Malaria Journal volume 21 , Article number: 284 ( 2022 ) Cite this article
22k Accesses
29 Citations
8 Altmetric
Metrics details
Severe malaria is a medical emergency. It is a major cause of preventable childhood death in tropical countries. Severe malaria justifies considerable global investment in malaria control and elimination yet, increasingly, international agencies, funders and policy makers are unfamiliar with it, and so it is overlooked. In sub-Saharan Africa, severe malaria is overdiagnosed in clinical practice. Approximately one third of children diagnosed with severe malaria have another condition, usually sepsis, as the cause of their severe illness. But these children have a high mortality, contributing substantially to the number of deaths attributed to ‘severe malaria’. Simple well-established tests, such as examination of the thin blood smear and the full blood count, improve the specificity of diagnosis and provide prognostic information in severe malaria. They should be performed more widely. Early administration of artesunate and broad-spectrum antibiotics to all children with suspected severe malaria would reduce global malaria mortality.
Severe malaria is important. It is a major cause of preventable childhood death in tropical countries. This large number of avoidable deaths justifies the substantial global investments in malaria control and elimination. But severe malaria is increasingly overlooked by the international agencies, donors and policy makers who determine the direction and support for global malaria initiatives.
Severe malaria, or ague, was recognized long before discovery of the malaria parasite by Laveran in 1880. The Cinchona bark arrived in Europe nearly four hundred years ago providing, for the first time, a potential cure for the pervasive and dangerous illness that then affected most of the inhabited world. But, as today, the specificity of the clinical diagnosis of febrile illnesses was poor. Torti recognized that only some fevers could be cured by the bark [ 1 ]. Even after the malaria parasite was identified first in 1880, severe forms such as algid malaria (shock), haemorrhagic or gastrointestinal malaria bore an uncertain relationship to Plasmodium infection, as did the notorious “blackwater fever”. Until the 1980s, the majority of research on severe malaria was conducted in adults. It derived largely from war-time experiences in the military, or observations from colonial medical services. Specific anti-malarial treatment comprised the parenteral administration of quinine and, from the 1950s, chloroquine. When they became available, renal replacement therapies for adult patients with acute renal failure could also save lives [ 2 ].
Soon after Laveran’s discovery of the causative parasite, the pathological processes underlying severe malaria were elucidated by the great Italian malariologists Marchiafava and Bignami. They considered, correctly, that the sequestration of parasitized erythrocytes in the microvasculature, causing microcirculatory dysfunction, was the key pathological event in “malignant tertian” (severe falciparum) malaria [ 3 , 4 ]. Beginning in the 1960s, coincident with the emergence of immunology as a discipline, and continuing to this day, various novel theories of severe malaria pathogenesis were proposed. These were often derived from observations in a murine “model” of cerebral malaria, which was fundamentally different to the human infection [ 2 , 5 ]. These new theories spawned a long succession of putative adjuvant therapies for severe malaria. Unfortunately, none of these therapies worked, and several were harmful [ 2 , 4 , 5 ].
1985 WHO meeting
Before 1985, there was no standard definition of severe malaria. Cerebral malaria was defined as unrousable coma (no localizing response to a painful stimulus). After publication of the Glasgow Coma Scale (GCS) in 1976 [ 6 ], this level of coma became a GCS less than 11. In 1985 an “informal meeting” was convened by the Malaria Action Programme of the World Health Organization (WHO). It was held in the Institute for Medical Research in Kuala Lumpur where, decades before, Field and colleagues had conducted seminal studies on the diagnosis, pathology and prognosis of severe malaria. The WHO meeting had the objective of reviewing available information on severe falciparum malaria, standardizing the definition, and advising on management [ 7 ]. The resulting document, which derived heavily from studies in Thailand conducted in the previous five years [ 8 ], provided a definition of severe malaria which is broadly similar to that used today, but with the following exceptions.
hyperparasitaemia was defined as > 5% parasitaemia (today this is 10%)
after a convulsion, coma had to persist for 6 h (now 30 min),
severe anaemia was defined as a haematocrit < 20% (now < 15%),
jaundice (total bilirubin > 50 µmol/L) alone was a criterion (today this requires a parasite density > 100,000/uL as well),
‘fluid, electrolyte or acid–base disturbances requiring intravenous therapy’ was a criterion (today more specific criteria have been instituted: either a venous plasma lactate > 5 mmol/L, arterial pH < 7.25, or a plasma bicarbonate < 15 mmol/L is required).
Hyperpyrexia (> 39 °C), vomiting of oral treatment and haemoglobinuria were also included – none of which today are considered defining criteria.
These definitions and descriptions have been generally referred to and referenced as “WHO definitions” although each successive version of the severe malaria review contains a disclaimer that the contents are the opinions of experts, and not those of the WHO itself.
1988 WHO meeting
In 1988 a second informal WHO meeting was held to update the recommendations and to incorporate recent observations in African children with cerebral malaria [ 9 ]. For the definition of severe malaria, hyperparasitaemia, jaundice, and hyperpyrexia were “dropped”, the haematocrit criterion was reduced to 15% and, after some debate, a requirement for a concomitant parasitaemia of > 10,000/µL was added to the severe anaemia criterion. Acidaemia or acidosis were defined as above, and repeated generalized convulsions (more than two observed within 24 h despite cooling) was added as a criterion. In this second meeting, the readily evaluated Blantyre Coma scale [ 10 ] was endorsed as the method to assess the level of consciousness in children.
1995 WHO meeting
The third WHO meeting was held in Geneva in December 1995 to incorporate further experience from clinical research in African children [ 10 – 12 ]. This meeting resulted in a broader, more inclusive and pragmatic, definition of severe malaria in children centred around prostration and respiratory distress (acidotic breathing) [ 13 ].
The hyperparasitaemia threshold was changed to 4% in low transmission settings, and to 20% in high transmission settings. The newly added “prostrate” criterion was very broad. It included many children with acute malaria who had no other signs of severity. This substantially expanded definition of severe malaria therefore encompassed a larger proportion of all children with acute malaria (and so it had a lower case-specific mortality). The new inclusive definition ensured a high proportion of at-risk children would be managed appropriately (i.e. it had high diagnostic sensitivity), but it had low specificity in identifying potentially fatal infections. In clinical research use of the broader inclusion criteria obviously resulted in overall “better outcomes” as more children with a good prognosis were included within the broader definition of severe malaria. Recognizing the disparities with the earlier criteria some investigators continued with the stricter (i.e. more specific) earlier severe malaria criteria [ 9 ] in their clinical research studies (Table 1 ).
2013 WHO meeting
The most recent WHO meeting on severe malaria was convened in 2013, again in Geneva [ 2 ]. By 2013, large prospective series of patients with severe malaria had been studied in Asia and Africa. These studies provided a much larger evidence base than for previous meetings. Many of the data came from randomized controlled trials [ 14 – 21 ]. The key therapeutic advance was the replacement of quinine by artesunate, which had been shown to reduce mortality by between one fifth and one third in very large randomized controlled trials [ 18 , 19 ]. The definitions of severe malaria, and components of the definitions, could now be associated with mortalities [ 22 – 26 ] (which were falling globally as artemisinin combination treatments were rolled out and parenteral artesunate was replacing quinine as first line parenteral treatment) [ 2 , 27 ]. The 2013 “WHO” meeting recognized both the requirements of a definition for practitioners, for whom sensitivity in recognizing potentially severe malaria and thus inclusiveness takes priority, with the contrasting needs of epidemiology and research studies where specificity is more important. The most recent research definition is shown in Table 2 [ 2 ].
Prostration was not included in the ‘research” definition, convulsions were “dropped”, the acidosis criterion was refined, the jaundice criterion was reintroduced with a parasite density > 100,000/µL, and the hyperparasitaemia criterion was changed (again!). In addition, severe Plasmodium vivax and Plasmodium knowlesi infections were reviewed specifically, and slightly modified definitions for severe malaria with these infections were proposed [ 2 ].
The meaning of severe malaria
Strictly speaking, severe malaria is malaria with an increased risk of death at the time of assessment compared to everyone else in that community with malaria illness. How much higher this risk should be (i.e. the lower threshold for the increase in mortality) has not been agreed upon. Mortality varies substantially as it depends on the infection, the host, the circumstances and the treatment. For the same admission severity, outcomes in well-equipped intensive care units (ICUs) with well-trained staff are better than in peripheral health centres. However, tertiary ICUs often receive the very sickest patients, often after long delays in referral – with consequently high mortalities. A frail and debilitated patient may die from a malaria infection that would be regarded as mild in a younger and fitter person. The high mortality of imported malaria (both P. falciparum and P. vivax ) in elderly travellers, and of malariatherapy (all species) in neurosyphilis, testifies to the lethal potential of acute malaria illness, whichever the infecting parasite species, in frail or debilitated persons [ 28 , 29 ]. In contrast, most people with malaria illness in endemic areas are either children or young adults without underlying conditions (although in Southern Africa HIV prevalence is high, and the untreated coinfection predisposes to severe malaria [ 30 ]). Acknowledging that this is an oxymoron, the “uncomplicated falciparum malaria” mortality of orally treated patients ranges from 1 in 10,000 to 1 in 1,000 if effective anti-malarial drugs are being used. Many factors affect this risk. Severe malaria usually has a mortality well over 5%, and therefore represents a > 50 fold increase in the risk of death. In general, as with many infections, mortality in malaria is proportional to the total number of infecting organisms (biomass) in the body. In non-immune adults mortality increases steeply as peripheral blood parasite densities rise over 100,000/µL [ 31 ]. This corresponds approximately to total parasite numbers within the blood of over 10 12 . If severe malaria was defined as clinical and laboratory measures which are associated with > 5% mortality, then the current thresholds would conform, except for the anaemia criterion (see below) which would require a threshold of 3 g/dL rather than 5 g/dL.
Malaria parasite densities
Malaria is traditionally diagnosed by microscopy examination of a peripheral blood smear. Unfortunately, this diagnostic skill is being lost in many places as microscopy is replaced by the more ‘convenient’, but less informative, rapid diagnostic tests. In malaria microscopy, the parasites are speciated and their numbers counted. The result is reported either as the number of parasitized erythrocytes in a stained thin smear or, in a thick film, as the number of parasites seen in a fixed volume or while counting a certain number of white blood cells (usually 200 or 500). The old semi-quantitative ‘cross’ system, in which density is graded from + to + + + + , is no longer recommended. The thin film should be used for high parasite densities (> 0.2% parasitaemia).
In falciparum malaria the parasite count can be misleading. This is because after approximately 12 to 16 h (depending on core temperature) of intraerythrocytic parasite growth (i.e. one quarter to one third of the asexual life cycle) Plasmodium falciparum infected erythrocytes begin to stick (“cytoadhere”) to vascular endothelium. By 20 h the majority have cytoadhered. This “sequestration” is the fundamental pathological process in falciparum malaria [ 2 , 3 ]. It occurs in all P. falciparum infections, although the tissue distribution of sequestration varies between patients. As a result, the parasite densities measured in blood films (reflecting circulating parasites only) variably underestimate the total malaria parasite biomass [ 32 – 34 ]. Nevertheless, the mortality of falciparum malaria is still proportional approximately to the peripheral blood parasite density. Among several factors, the relationship between peripheral blood parasite density and mortality depends on the prevailing intensity of transmission and thus the levels of “immunity” or “premunition”. Field showed in Kuala Lumpur (a generally low transmission area from the 1930s to the 1950s) that the mortality of falciparum malaria in adults with little or no immunity increased markedly when parasite densities rose above 100,000/µL [ 31 ] (Fig. 1 ). There is, therefore, a non-linear relationship between mortality and parasite densities. In a low transmission setting on the Thailand-Myanmar border, where the P. falciparum entomological inoculation rate was approximately 0.5/year, the mortality of children with > 4% P. falciparum parasitaemia (circa 200,000/µL) was 3% [ 35 , 36 ]. In that location a 3% mortality was thirty times higher than the mortality in patients with lower parasite densities, but it was five times lower than in patients who fulfilled the strict WHO definition of severe falciparum malaria [ 9 ]. As the predominant stage of parasite development determines the proportion of the parasite biomass that circulates, some patients with severe falciparum malaria have relatively low parasite densities because most of the malaria parasites are sequestered [ 32 – 34 ]. Others may have low parasite densities because they have already received anti-malarial drugs before assessment. On the other hand, a synchronous infection may have recently undergone schizogony and merozoite release resulting in a high parasite density with a predominance of young ring stage parasites. In this latter case most of the parasites in the body are circulating, and relatively few are still sequestered. Provided the patient receives an artemisinin derivative the prognosis is good. In children in areas of higher transmission, P. falciparum peripheral blood parasite densities over 200,000/uL may be tolerated with relatively few symptoms. Thus, the prognostic value of parasitaemia depends on the epidemiological setting and, overall, it is poor.
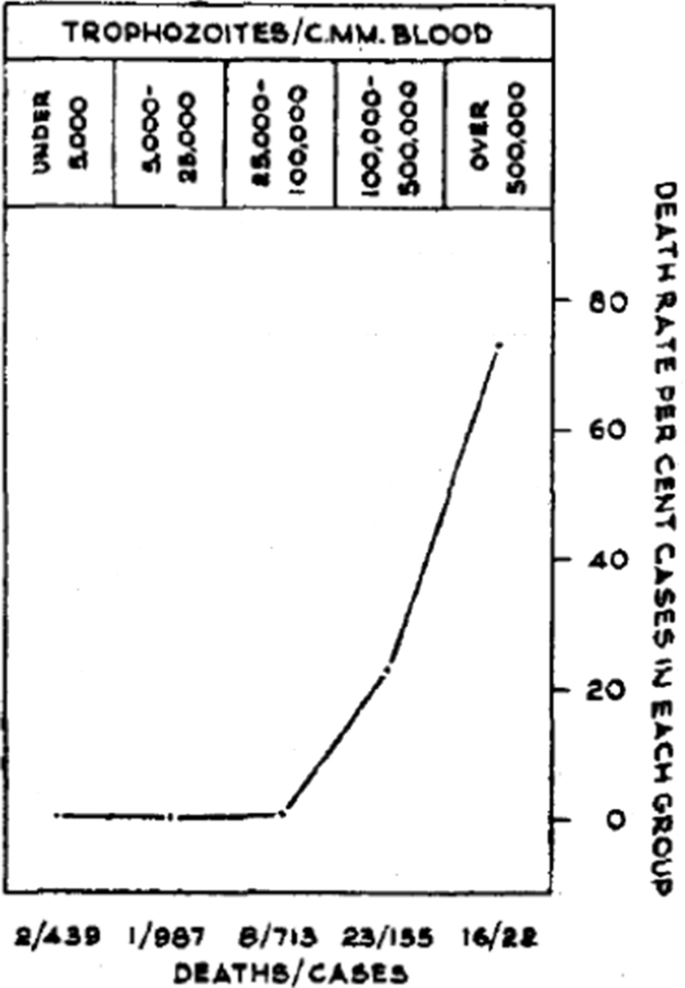
Relationship between peripheral blood parasite density and outcome in patients with acute falciparum malaria studied by Field and colleagues in Kuala Lumpur over 70 years ago [ 31 ]
Factors associated with mortality
The three main clinical presentations of severe malaria in children are coma, metabolic acidosis (usually manifest by an acidotic or “Kussmaul’s” breathing pattern, and commonly termed “respiratory distress”) and anaemia [ 2 , 10 – 13 , 22 – 26 ]. None of these are specific for malaria. These clinical presentations are major manifestations in adults too, although severe anaemia is less common. In contrast many adult patients present with acute kidney injury often accompanied by jaundice [ 37 ]. As noted earlier, there is no agreed threshold mortality threshold to define severe malaria. Among the different syndromes included in the current definition, the lowest case specific mortality is associated with malarial anaemia which can be below 1% [ 38 ]. This is still higher (by a factor of 10–100) than in uncomplicated malaria, but it is substantially lower than the mortalities associated with coma, severe metabolic acidosis, pulmonary oedema or acute renal failure (8–50%) [ 2 , 26 ]. The low mortality of severe anaemia with malaria is explained by the low sequestered parasite biomass and the inclusion, within the definition of severe malaria, of children with chronic anaemia (often as a result of repeated malaria attacks) and either incidental parasitaemia or a concomitant, otherwise uncomplicated, malaria illness. This is a very common presentation in high transmission settings where it is usually the main reason for blood transfusion in young children. The current “WHO” severe anaemia criterion requires an accompanying parasite density of 10,000/µL [ 2 ]. Densities in this range are often found in asymptomatic children, so may be incidental to the anaemia rather than causal. Even if causal the anaemia may result from a chronic process in which the parasite numbers are in a quasi-steady state, controlled by the immune response, and are very unlikely to increase further. If the parasite density requirement in the criterion for “severe anaemia” was raised it would be more specific for acute malaria but, even at higher densities, acute case specific mortalities do not rise above 5% until admission haemoglobin concentrations fall below 3 g/dL. However, it is still very important to recognize children admitted to hospital with severe malaria anaemia as a high risk group. These anaemic children have a high post-discharge mortality [ 39 – 41 ]). Furthermore they may not recover fully from their anaemia for 2–3 months after discharge. Thus, the overall mortality associated with severe malaria anaemia is significantly greater than appreciated from the acute admission [ 39 , 40 ].
The clinical syndromes
Neurological dysfunction.
The most characteristic syndrome of severe falciparum malaria is unrousable coma or cerebral malaria [ 2 , 42 ]. This diffuse, symmetrical, reversible encephalopathy may occur at any age (Fig. 2 ). The main differential diagnoses are bacterial meningoencephalitis, viral encephalitis and, in some areas, toxic encephalopathy. Cerebral malaria occurs typically in people with little or no immunity, so it is seldom seen in residents of areas of high stable malaria transmission where severe anaemia in the first years of life predominates as the manifestation of severe malaria (Fig. 3 ). The outcome of cerebral malaria depends on access to treatment and intensive care, and the degree of associated vital organ dysfunction. ‘Pure’ cerebral malaria (i.e. without other vital organ dysfunction) has approximately half the mortality of patients with coma and other organ dysfunction i.e. renal impairment, pulmonary oedema, jaundice, metabolic acidosis, or hypoglycaemia. Overall, the treated mortality of cerebral malaria in the “quinine era” was approximately 20% in adults and 12–15% in children. These mortalities have been reduced by about one third by parenteral artesunate treatment [ 17 – 20 ]. Falciparum malaria is specifically associated with convulsions, even in otherwise uncomplicated infections. The seizures are usually generalized, and they may herald the onset of coma. Although most children make a full recovery, cerebral malaria in children is associated with significant neurodevelopmental sequelae; stroke, cognitive impairment and an increased risk of epilepsy [ 42 ]. It is very important to distinguish the causal relationship between convulsions in malaria and cerebral malaria and later cognitive impairment and epilepsy, from pre-morbid conditions which may present, sometimes for the first time, as neurological dysfunction in acute malaria (and thus be misdiagnosed as cerebral malaria). Otherwise, the adverse impact of cerebral malaria on long-term neurological outcomes will be overestimated. The specificity of the diagnosis of cerebral malaria is improved by clinical and laboratory examination (see below). For example, demonstration of malaria retinopathy is highly specific for cerebral malaria as the cause of coma [ 44 ]. Severe anaemia has also been associated with neurocognitive deficits [ 45 ]. There is no evidence that severe malaria causes permanent damage to other vital organs.
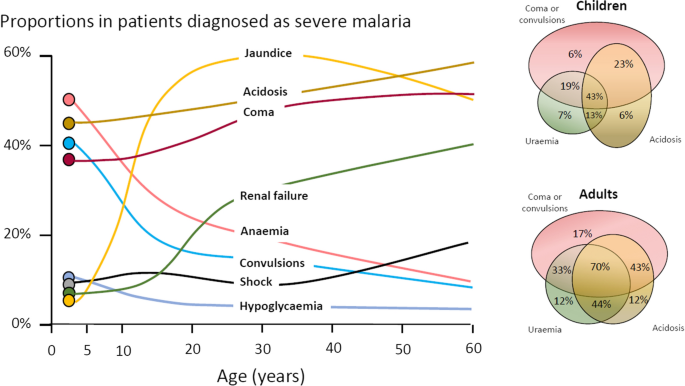
Overlap of clinical syndromes and mortalities in adults and children with severe falciparum malaria. These proportions are derived from prospective studies in SouthEast Asia and Africa of adults and children with severe falciparum malaria conducted or coordinated by the Mahidol Oxford Research Unit over the past 40 years [ 26 ]
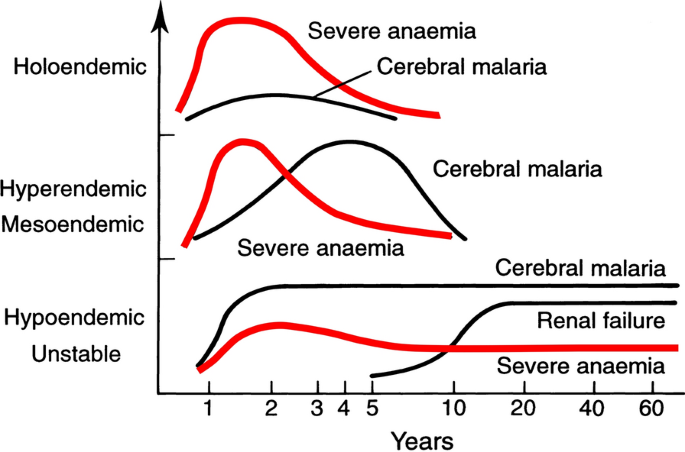
Approximate age relationships for the major clinical manifestations of severe falciparum malaria in relation to the intensity of transmission [ 53 ]. Holoendemic in this illustration approximates to a sustained entomological inoculation rate > 10 per year or a parasite rate (prevalence) in children of 0.5, and hypoendemic refers to an average entomological inoculation rate ≤ 1 year
Acidosis, kidney injury
Metabolic acidosis is a grave sign in both adults and children with severe malaria, [ 2 , 24 , 47 – 49 ] (Fig. 2 ), unless it results from very severe anaemia only, where the prognosis is better [ 38 ]. Lactate (reflecting lactic acid) accumulation is an important component of the malaria acidosis. Other organic acids, mainly of gut origin, are also significant contributors [ 46 , 50 ]. Lactic acidosis is often accompanied by hypoglycaemia reflecting anaerobic glycolysis and impaired hepatic gluconeogenesis [ 47 – 49 ]. Impaired renal function is an important manifestation of severity in younger children, but acute kidney injury (AKI) requiring renal replacement therapies is almost confined to older children and adults [ 2 , 37 , 51 ] (Figs. 2 , 3 ). The fulminant form of AKI, often associated with multiple vital organ dysfunction, is associated with a poor prognosis. In contrast the sub-acute presentation, in which plasma or serum creatinine rises steadily as the patient otherwise recovers, has a good prognosis. A period of renal replacement therapy (preferably haemofiltration or haemodialysis [ 52 ]) may be required, but there is always full recovery of renal function in survivors. The ‘hepatorenal’ combination of jaundice and renal failure became a more common presentation of severe malaria relative to cerebral malaria in Southeast Asia over the past four decades -the prognosis is worse than with AKI alone. Renal dysfunction in malaria can be misattributed in much the same way that neurological dysfunction following malaria can be overdiagnosed. In many tropical regions chronic kidney disease is common, particularly in older adults, and renal impairment may become evident for the first time during hospitalization for malaria. This may be causally attributed to malaria by mistake, and so a diagnosis of malaria nephropathy is made incorrectly. Concomitant anaemia and acidosis may also be ascribed incorrectly to malaria rather than chronic renal disease. In these misattributed cases, renal imaging, if available, often reveals small kidneys, or nephrolithiasis and hydronephrosis, and there may be biochemical or radiological evidence of metabolic bone disease.
Severe anaemia
The definitions of anaemia in malaria vary widely [ 53 ]. The most common classification—used in higher malaria transmission settings- is based on haemoglobin concentrations. In patients with acute malaria haemoglobin (Hb) concentrations between 8 g/dL and 11 g/dL are considered as mild anaemia, Hb between 5 g/dl and 8 g/dL is considered moderate, and Hb < 5 g/dL is defined as severe anaemia [ 53 ]. Unfortunately, despite their simplicity, the point of care measurements of haemoglobin concentrations, which are necessary to ensure appropriate use of blood transfusions, are often unavailable [ 54 ]. In sub-Saharan Africa the Hb ≤ 5 g/dL threshold is used widely as an indication for blood transfusion in children with malaria (whereas Hb ≤ 4 g/dL is often used for other causes of anaemia) (Fig. 4 ). The recent finding, in a large randomized trial, that children with fever (> 37.5 °C) were harmed by higher blood transfusion volumes (30 mL/kg versus 20 mL/kg) whereas children without fever benefited [ 55 – 57 ], has forced a reconsideration of blood transfusion guidelines for African children with severe anaemia [ 58 ] (Fig. 4 ). In low transmission settings an Hb ≤ 7 g/dL has been used as a transfusion indicator [ 2 ]. There is no evidence to support this threshold. Anaemia is the main severe manifestation of malaria in areas of high transmission, where it is largely confined to young children [ 59 ] (Fig. 2 ). Severe anaemia, as a criterion of severe malaria, encompasses a spectrum of aetiologies with several different, but often overlapping, pathological processes which are still not well understood [ 53 ]. At one end of the disease spectrum is an acute illness in patients with high parasite biomass infections and rapid destruction of parasitized and unparasitized red cells. The unparasitized cells comprise the majority of erythrocytes lost. Haemolysis is sometimes sufficient to result in haemoglobinuria (blackwater fever). However, malaria is not the only cause of blackwater fever, which, after over 120 years of investigation, still remains a puzzle [ 60 – 64 ]. Massive haemolysis may occur in any epidemiological setting. At the other end of the disease spectrum, in settings of high transmission or poor access to treatment, are patients (usually young children) with chronic anaemia and incidental parasitaemia. Repeated or untreated malaria infections resulting in shortened erythrocyte survival and protracted dyserythropoeisis are important contributors to this chronic, or acute on chronic, syndrome [ 52 ].
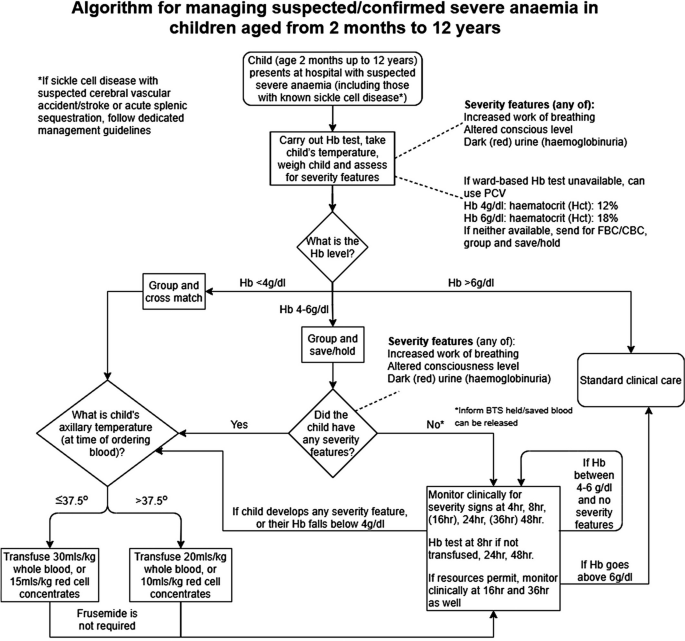
Proposed algorithm for managing suspected/confirmed severe anaemia in African children aged from 2 months to 12 years [ 58 ]
Improved malaria control reduces the frequency of malaria infections and thus the prevalence of severe anaemia [ 59 , 65 ]. As in chronic inflammatory conditions, malaria is associated with iron deficiency [ 66 ]. Other common causes of anaemia in malaria endemic regions are nutritional deficiencies, hookworm, bacterial infections and haemoglobinopathies. Bacterial infections are also associated with acute anaemia presentations [ 67 ]. At presentation to hospital the short-term prognosis of severe anaemia is relatively good as the anaemia is mainly chronic and partially compensated (by the right shifted oxygen dissociation curve). If blood transfusion can be given promptly then the acute mortality is low but, in higher malaria transmission settings, hospitalization for severe anaemia identifies children who are at increased risk of subsequent death. Approximately 5% will die within 6 months. Post-discharge anti-malarial chemoprophylaxis provides temporary protection, which suggests that recurrent malaria is a major contributor to this high mortality [ 40 , 41 ]. The prognosis of children hospitalized with severe anaemia is much better than for the other severe manifestations of falciparum malaria but, because of the longer-term impact, and because it is so common in high transmission settings, the adverse impact at a population level is substantial [ 59 ]. Deaths from malaria overall are positively correlated with transmission intensity [ 59 ], and the direct or indirect consequences of severe anaemia are major contributors to this relationship.
Other complications
Pulmonary oedema (ARDS) carries a very high mortality in falciparum malaria- even with positive pressure ventilation. It often occurs after the other severe manifestations have become evident. Pulmonary oedema results from increased pulmonary capillary permeability. Pulmonary oedema may also occur in vivax malaria, where the prognosis is much better [ 2 ]. Liver dysfunction is usual in severe malaria [ 68 ] although liver failure, as in viral or toxic hepatic injury, never occurs [ 2 ]. Profound thrombocytopenia is associated with an increased mortality in severe malaria, but it is not an independent risk factor and, contrary to some reports, it is not regarded as a criterion of severe malaria [ 69 ]. Although thrombocytopenia is usual in all malarias and coagulation indices are often abnormal in severe illness, significant bleeding (if present, usually from the stomach) and clinically significant coagulopathy are unusual in severe malaria. Overall, the probability of death from severe falciparum malaria depends on the extent and degree of vital organ dysfunction and the access to appropriate treatment [ 2 , 70 ]. Secondary bacterial infection is a potentially lethal complication, particularly in African children. Approximately 6% of children diagnosed with severe malaria have concomitant bacteraemia [ 71 ]. In adults the incidence is much lower (1%) [ 72 ]. Misdiagnosis (see below) is common [ 73 ], as it is difficult to differentiate between severe malaria with concomitant bacteraemia and a primary bacterial infection with incidental parasitaemia [ 74 , 75 ].
Pathophysiology of severe falciparum malaria
Similar to some primate malaria parasites ( P. fragile, P. coatneyi ), but unlike the other human malaria parasites, P. falciparum causes the infected erythrocyte to cytoadhere to vascular endothelium after the first third of the asexual blood cycle [ 2 ]. Severe falciparum malaria results from the extensive sequestration of erythrocytes containing these mature parasite forms in the microvasculature of vital organs [ 2 , 3 , 76 , 77 ] (Fig. 5 ). The microvascular obstruction by highly metabolically active cells, consequent cellular dysfunction, and the liberation of large quantities of bioactive haem are considered the main pathological processes in severe falciparum malaria [ 70 , 76 – 79 ]. There are secondary consequences on vascular function, permeability, tone and on cellular transport. Thus, vital organ dysfunction depends on the extent and the location of parasitized erythrocyte sequestration. The extent of sequestration is heterogeneous, even at a microvascular level [ 80 ]. Magnetic resonance cerebral imaging in paediatric cerebral malaria shows a variety of different patterns. The brain is usually swollen, with restricted diffusion and variable evidence of oedema [ 81 ]. Isolated restricted white matter diffusion is associated with a better prognosis, while oedema is associated with a worse prognosis and an increased risk of sequelae [ 82 , 83 ]. The sequestered static red blood cells occupy space and cause cerebral engorgement [ 3 , 4 ] which contributes to raised intracranial pressure. Cytoadherent parasitised erythrocytes are not the only contributors to disease severity. Very high parasitaemias caused by non-sequestering malaria parasites cause severe malaria across the animal kingdom, and the simian parasite P. knowlesi is potentially lethal in humans – but these parasites do not cause cerebral malaria [ 2 , 84 ]. At very high parasite densities, erythrocyte dysfunction contributes to aggregation and impaired microcirculatory flow and oxygen delivery without cytoadherence. The precise causes of acute kidney injury and acute pulmonary oedema in severe malaria are unclear. Despite extensive research and much speculation over many years, there is little evidence for a primary immunopathological process in severe malaria, or for a final common pathological pathway with bacterial sepsis involving pro-inflammatory cytokine release. As described earlier, the pathobiology of severe malaria has been rich ground for hypothesis and speculation, often fueled by observations in a murine model, which is readily studied in the laboratory but has very little similarity to the human disease [ 5 ]. Observations in the murine ‘model’ have led to a long list of putative adjuvant interventions -all of which have proved either ineffective or harmful. This emphasizes the importance of distinguishing causal pathological processes in malaria from their consequences. From a clinical and operational perspective, it is essential to distinguish causal processes in severe malaria [ 70 ] from those processes in other severe infections with which severe malaria is very often confused (notably bacterial infections). The implications of misdiagnosis on operational disease management and pathobiology understanding are discussed below.
Brain smear from fatal cerebral malaria. The vessels ( A , C and D ) are packed with red cells containing P. falciparum schizonts (many of which are disrupted) and malaria pigment (haemozoin). Vessel segment B , by contrast, contains mainly unparasitized erythrocytes
The diagnosis of severe falciparum malaria
Severe malaria is a medical emergency. Appropriate immediate management is life-saving. An initial brief clinical examination assessing vital signs, peripheral perfusion, respiratory pattern, anaemia, jaundice and level of consciousness, and confirming the absence of rash should be followed rapidly by a blood smear or RDT confirmation [ 2 ]. In a low transmission setting, or with imported malaria, the diagnosis is straightforward. The results of a thin blood film or RDT can be available within minutes of taking a blood sample. Treatment should not be delayed if the blood results take longer than this. Microscopy examination of thin and thick blood smears provides both diagnostic and prognostic information; the parasite count, the parasite stage of development and the presence of neutrophil ingested pigment all have prognostic value and are readily assessed [ 2 , 32 , 33 , 85 – 87 ]. If the parasitaemia is high, the thin film assessment can take less than one minute. The RDT does not provide this quantitative prognostic information. In addition, the Pf HRP2 based RDTs can remain positive for days or weeks following a previous infection [ 88 ]. On the other hand, RDTs are useful in excluding a mixed P. falciparum infection in a patient with a blood slide diagnosis of vivax, malariae or ovale malaria [ 89 ], and they provide a diagnosis in patients who have received treatment with artemisinins several days previously and who are still severely ill (but have cleared their parasitaemia). This is common in adults presenting with acute kidney injury, which may take days or weeks to recover fully. In a low transmission setting, finding malaria parasites in the peripheral blood (by microscopy or RDT) is highly specific for malaria as the cause of illness. PCR diagnosis and speciation has proved very valuable in epidemiological studies, but PCR has no role in the acute diagnosis of severe malaria in endemic areas. It is too slow to be reported and it is too sensitive. PCR detects a higher proportion of people with previously asymptomatic (i.e. incidental) parasitaemia and therefore results in even more misdiagnosis of severe malaria.
At higher levels of transmission, the diagnosis of malaria as the cause of the presenting illness is much more difficult. The prevalence of microscopy or RDT detectable parasitaemia in apparently healthy individuals increases with transmission intensity, so the possibility of ascribing malaria incorrectly as the cause of illness rises too [ 90 ]. In sub-Saharan Africa a high proportion of apparently healthy children have detectable malaria parasitaemia. So how can severe illness caused by malaria parasites be distinguished from severe illness caused by something else with coincident parasitaemia? Good clinical examination is important but diagnostic uncertainty often persists. Other sites and sources of infection should be sought. In unconscious patients a lumbar puncture should be performed to exclude bacterial meningoencephalitis. Sequestration can be seen in-vivo by skilled indirect ophthalmoscopy along with other changes termed “malaria retinopathy” which have high specificity for cerebral malaria [ 44 , 92 – 94 ]. The buccal or rectal microcirculations can be visualized by direct orthogonal polarized light imaging [ 76 , 95 ]. In fatal cases sequestration can be demonstrated in the capillaries and venules of the brain in a post-mortem needle biopsy [ 80 , 96 , 97 ] (Fig. 5 ). But none of these specialist techniques are available in most places where severe malaria is managed. However, most hospitals and many health centres do have microscopes, and many centres now can perform full blood counts. Brief microscopy examination of a stained thin blood film provides valuable diagnostic and prognostic information [ 85 – 87 ]. The blood count is also informative (see below). Point of care blood glucose and lactate measurement is very important, particularly in unconscious or obtunded patients.
The immediate management of severe malaria
The outcomes of severe malaria and of severe sepsis are critically dependent on rapid access to health care and immediate treatment. Delays in giving artesunate and antibiotics are potentially lethal. Sadly, additional delays may still occur after the patient has reached hospital. Any patient suspected of having severe malaria should be treated as such [ 2 ].
Pre-referral
Severe malaria often presents initially far from the health centre or hospital. Referral for medical care can take hours, or sometimes days. At the community level, where giving parenteral drugs is not possible, pre-referral treatment of severe malaria with rectal artesunate reduces mortality by about 25% [ 98 ]. This community-based intervention has been very slow to be deployed, and now the WHO has recommended that it be stopped [ 99 ]. This recent WHO moratorium followed preliminary analysis of a large sequential observational study (“CARAMAL”) in Nigeria, Uganda and the Democratic Republic of the Congo [ 100 ]. Mortality reportedly increased after rectal artesunate was deployed, attributed to delays in the referral of severely ill children. However, there are serious concerns over the design of the study, potential major confounders, the accuracy of the diagnosis, and particularly—the causal interpretation of the results [ 101 ]. The CARAMAL study identified important problems with the referral of severely ill children, but it should not be used to evaluate the effectiveness of pre-referral rectal artesunate. The WHO moratorium appears to be a mistake. Rectal artesunate should be deployed to counter lethal delays in the referral of severe malaria. There are no pre-referral rectal antibiotic formulations unfortunately.
Health centre or hospital
At the level of the health centre or hospital in an area of higher malaria transmission (i.e. most of sub-Saharan Africa), the difficulty in distinguishing malaria from sepsis in children means that both parenteral anti-malarials (i.e. artesunate 3 mg/kg stat for children < 20 kg and 2.4 mg/kg for larger patients) and broad-spectrum antibiotics should be given together as soon as the diagnosis is suspected [ 2 , 73 ]. The most widely used empirical antibiotic treatment of severe sepsis is parenteral ceftriaxone. Administration of antibiotics should not be delayed. The drugs are very safe. Giving anti-malarials initially does no harm if the infection turns out to be bacterial or viral, and giving antibiotics does no harm if the infection is severe malaria only. Immediate administration of parenteral artesunate and broad-spectrum antibiotics to a child suspected of having severe malaria is the single most important life-saving intervention .
In low transmission settings where misdiagnosis is much less likely, it is reasonable in adults to treat only for severe malaria unless there is evidence for concomitant bacterial sepsis. However, antibiotics should be given to all adult patients with a very high parasitaemia (> 20%) [ 72 ], and should be given immediately if there is any unexplained clinical deterioration.
The misdiagnosis of severe malaria
Misdiagnosis of severe malaria is common. Its impact is underestimated. Misdiagnosis can result in incorrect treatment [ 73 ] and it dilutes and distorts genetic, epidemiology, burden of disease, long term impact, pathophysiology and therapeutic studies. In areas of higher transmission (e.g. Sub-Saharan Africa, Oceania), children are often diagnosed as having severe malaria because the blood test is “positive” but, in fact, they have another infection (often bacterial sepsis) causing their severe illness [ 90 ]. As severe bacterial infections have a higher mortality than severe malaria, and require antibiotic treatment, it is essential that both are treated immediately.
The relationship between malaria and bacterial infections is complex [ 71 – 75 , 102 – 109 ]. Severe malaria predisposes to bacterial infections. In a large prospective series of Vietnamese adults with strictly defined severe falciparum malaria (in whom diagnostic specificity for severe malaria is very high), the overall incidence of concomitant septicaemia (identified by positive blood culture) was 1.1% [ 72 ]. Hyperparasitaemia was a risk factor for bacteraemia; in patients with > 20% parasitemia the prevalence of concomitant bacteremia was 5.2%, whereas it was eight times lower (0.65%) in patients with lower parasitaemias. Concomitant bacteraemia is much more frequent in African children diagnosed with severe malaria. Approximately 6% of children hospitalized with a diagnosis of severe falciparum malaria in Africa are also bacteraemic [ 71 ]. As blood cultures are insensitive (but more specific- at least for most organisms) in diagnosis, the true proportion is likely to be much higher. Recent probabilistic assessments based on platelet and white blood cell counts, and also a quantitative parasite biomass indicator (plasma P f HRP2) [ 110 , 111 ] measured in large prospective studies of severe malaria in children, suggest that approximately one third of children diagnosed as having severe malaria in leading research centres actually had another condition (likely mainly sepsis) as the main cause of their illness [ 108 , 109 ] (Fig. 6 ). These probabilistic assessments were validated by comparing the prevalences of sickle cell trait (HbAS), which provides strong protection against severe malaria, between the two groups. The prevalence of HbAS was substantially lower in children with ‘true’ severe malaria than it was in those with a different cause of severe illness. Even for the relatively specific syndrome diagnosed as cerebral malaria, a post-mortem examination study, conducted in a leading research centre in Malawi, revealed a different pathology in one quarter of cases [ 112 ].
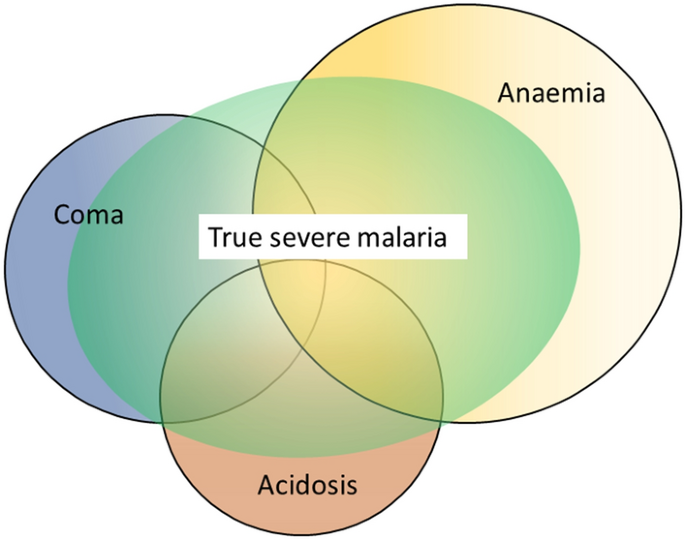
Misdiagnosis of severe falciparum malaria in African children -approximate relationships. [ 108 , 109 ]
The substantial overdiagnosis of severe malaria cannot be ignored in epidemiology, burden of disease, pathophysiology, genetic association and treatment studies. In the large evaluation of African children who had been admitted to leading research centres with a diagnosis of severe malaria (described above), mortality was higher in the likely misdiagnosed group, presumably because most had sepsis [ 108 , 109 ]. This suggests that malaria attributable mortality in African children may have been overestimated. If it has indeed been overestimated then the benefits of the substantial investments in malaria control measures and the provision of effective drugs (i.e. ACTs) have been underestimated [ 113 ]. Progress in reducing the number of deaths from severe malaria may have been better than estimated currently. The high rates of misdiagnosis, even in expert research centres, should be also accomodated by those formulating treatment guidelines and policies for severe malaria. Prompt, or preferably pre-referral, antibiotics must be given together with artesunate. Misdiagnosis also probably explains the difference in mortality reduction with artesunate compared with quinine in adults and children in Asia (where diagnostic specificity is high) compared with children in Africa (where diagnostic specificity is lower) (22.5%) [ 5 , 18 , 19 ]. In Asia the mortality reduction was 35% compared with 22.5% in African children (Fig. 7 ).
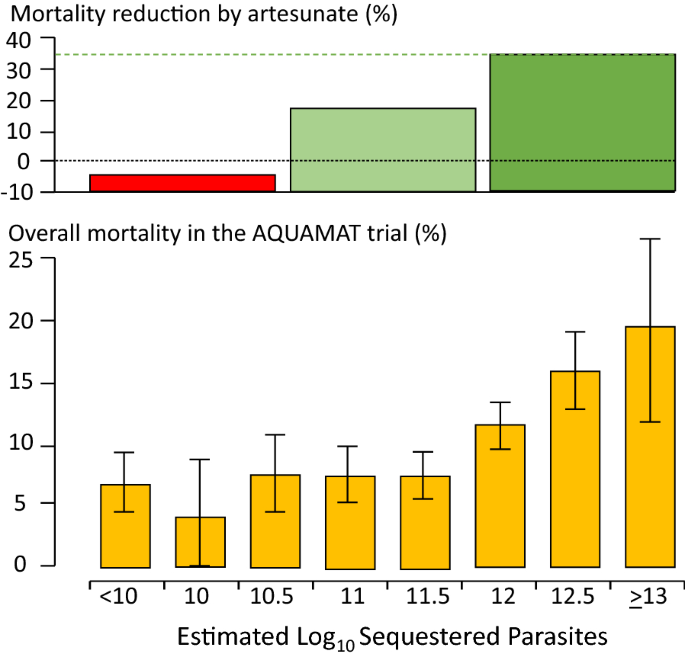
Relationship between estimated parasite biomass and mortality [ 4 , 110 ] in the large randomized controlled trial which compared artesunate and quinine in African children with severe malaria (AQUAMAT) [ 19 ]. The upper panel divides the patients into tertiles by treatment effect (reduction in mortality by artesunate). The mortality reduction in the preceding randomized controlled trial (SEAQUAMAT) which compared artesunate and quinine in Southeast Asia (where the diagnosis of severe malaria is more specific) is shown for comparison [ 18 ] (upper green dashed line). There was no treatment benefit from artesunate in patients in the lowest tertile of parasite biomass (red), likely corresponding to patients with another cause of severe illness (probably sepsis) and incidental parasitaemia [ 108 , 109 ]. The lower panel shows the corresponding relationship between mortality in the AQUAMAT study and the estimated total parasite numbers in the body derived from the admission plasma Pf HRP2 concentration [ 110 ]
Thus, it seems that some of the children with bacteraemia who are diagnosed as having severe malaria may genuinely have a high parasite biomass and extensive sequestration predisposing to bacterial sepsis—but the remainder have a primary bacterial infection and incidental or concomitant malaria. The interaction is complicated further as severe malarial anaemia predisposes to bacterial sepsis, and patients with uncomplicated malaria may have concomitant sepsis. At a population level, as malaria is controlled, the prevalence of sepsis declines (and so does the apparent protective benefit of HbAS against bacterial infections) pointing to the important contribution of malaria to bacterial sepsis, both concomitantly and sequentially [ 104 ]. It is very likely that the same problem of misdiagnosis occurs with Plasmodium vivax. In endemic areas low density chronic P. vivax parasitaemia is common, and so it is not unusual for severely ill patients to have incidental low-density infections, particularly if PCR is used for parasite detection.

The consequences of severe malaria
Children who are admitted with severe malaria anaemia have a high mortality in the months following admission [ 39 – 41 ]. This can be reduced by giving effective antimalarial prophylaxis, which indicates that repeated malaria infection is associated with death. Seizures and coma are associated with neurological deficit in surviving children [ 42 , 114 ]. The deficit is evident immediately following recovery in approximately 10% of children following cerebral malaria [ 115 ]. In two thirds of these cases the clinical picture is of stroke (suggesting a large cerebral vessel territory has been compromised). While many children recover fully, other deficits and behavioural and mental health problems often become apparent -particularly with detailed psychomotor and behavioural evaluation [ 42 , 114 , 116 – 118 ]. Epilepsy is increasingly recognized. These later onset epileptic, psychomotor and behavioural abnormalities may result from cerebral malaria, but they may also be pre-morbid conditions revealed by acute malaria [ 44 ].
Implications for the assessment and treatment of patients diagnosed with severe falciparum malaria
Overall, the consensus definitions of severe malaria described generally as “WHO criteria” have worked well to identify patients at risk and to inform research studies. From a practical case management perspective, specificity in the diagnosis is not as important as recognition that severe malaria could be the cause of the severe illness, and thus starting life-saving treatment with artesunate as soon as possible [ 2 ]. A new simple to administer artesunate formulation is under development. In children with suspected severe malaria in higher transmission settings parenteral broad-spectrum antibiotics should also be given immediately in all cases. As delay in receiving artesunate is a major contributor to death, it is important that referral to a facility capable of managing the sick patient should be as rapid as possible. Pre-referral rectal artesunate should be given to all children with suspected severe malaria [ 2 , 98 ]. The WHO moratorium [ 99 ] on rectal artesunate will hopefully soon be lifted [ 101 ]. Pre-referral antibiotic formulations should be developed.
For patients needing respiratory support, artificial ventilation has improved in recent years as the dangers of high inflation pressures have become evident [ 119 ]. Unfortunately, ventilators and trained staff are often unavailable in the areas where severe malaria is common. Otherwise, apart from the replacement of quinine by artesunate, the overall recommended management of severe malaria has changed relatively little over the past few decades. Aggressive fluid management (as in sepsis) [ 76 , 120 ], high volume (30 mL/kg) blood transfusions (in febrile children)[ 56 ], mannitol to reduce brain swelling [ 121 , 122 ], and unproven adjuvant therapies [ 5 ] have all proved harmful. Studies to optimize blood transfusion and fluid management are ongoing, but the general consensus is returning back to more cautious fluid management in severe malaria [ 7 , 123 ]. Evidence to date does not support red cell concentrates over whole blood in immediate management [ 57 ]. The optimum prevention and treatment of convulsions still remains uncertain. In a large randomized trial, conducted in a centre without access to artificial ventilation, seizure prevention by full dose prophylactic phenobarbitone increased mortality because of respiratory depression [ 16 ]. In a small trial levetiracetam proved safer [ 124 ], and may well become the anticonvulsant of choice, as it is in other settings, although more evidence is needed. Fosphenytoin was ineffective [ 125 ]. Renal replacement should start early in adults, blood glucose should be tested frequently and hypoglycaemia treated promptly [ 2 ]. Studies are ongoing to determine if paracetamol could attenuate renal injury in severe malaria [ 51 ]. If broad spectrum antibiotics have not been started (e.g. in adults in low transmission settings) there should be a low threshold for giving them if the patient deteriorates [ 2 ] -particularly in hyperparasitaemic patients [ 72 ].
From a research or epidemiology perspective, the low specificity of the current definition of severe malaria in African children is a challenge (Fig. 6 ). It has diluted therapeutic evaluations and distorted pathophysiology interpretations and genetic association studies. Most of the techniques to improve the specificity of diagnosis (notably indirect ophthalmoscopy or other methods of visualizing the microcirculation, or measurement of parasite biomass indicators such as plasma Pf HRP2 (Fig. 7 ) or plasma Pf DNA concentrations) are not readily available [ 91 – 95 , 110 , 111 , 126 ]—although simple dilution of a plasma sample and testing (by eye) with a Pf HRP2 RDT is not too difficult [ 127 ]. Importantly, the time-honoured peripheral thin blood smear does contain valuable information. Sadly, it is underused as a diagnostic and as a prognostic tool, and in many centres has been supplanted by the malaria rapid test, which, as currently used, does not provide prognostic information. In blood slides with parasitaemias over 0.5% the stage of parasite development can be easily and rapidly evaluated by microscopy. For any parasite density, finding > 50% tiny rings carries a relatively good prognosis whereas if > 20% parasites contain visible malaria pigment the prognosis is worse [ 85 ]. The proportion of neutrophils containing malaria pigment is also a very useful and readily assessed both for diagnosis and for prognostic assessment [ 86 , 87 ]. Most health facilities have at least one microscope – but sadly it is often old, fungus infested and accompanied by dirty slides, waterlogged methanol and outdated unfiltered stains. Malaria microscopy is well established but it is not well supported, and it is not prioritized in current malaria control funding. Hospital and health centres managing severe malaria should support good microscopy as an essential diagnostic and prognostic measure. Blood counts are valuable too. The haemoglobin concentration or haematocrit guides blood transfusion. The differential white count provides diagnostic information. Although severe malaria may be accompanied by leukocytosis, finding a high neutrophil count (often with toxic granules) together with lymphopenia points to bacterial sepsis. Thrombocytopenia is usual in severe malaria, but not in sepsis. In the recent large probabilistic assessments of severe malaria in African children, the combination of a platelet count of ≤ 150,000/μl and a plasma Pf HRP2 concentration of ≥ 1000 ng/ml had an estimated sensitivity of 74% and specificity of 93% in identifying true severe falciparum malaria [ 109 ] (Table 3 ). Future studies of severe malaria should always include differential blood counts, platelet counts and, preferably, a parasite biomass indicator. The anaemia criterion to define severe malaria should be reviewed.
Severe malaria caused by other malaria species
Plasmodium knowlesi, with its quotidian cycle, can sometimes cause fulminant infections in humans [ 84 , 128 , 129 ]. It does not sequester markedly so the parasite count is a good guide to biomass. P. knowlesi infections do not cause coma (cerebral malaria) but they can cause the other potentially lethal manifestations of severe malaria. Morphologically the younger P. knowlesi parasites resemble P. falciparum , whereas the older forms are often mistaken for Plasmodium malariae . Indeed any P. malariae parasitaemia over 1% should be regarded as P. knowlesi until proved otherwise. Uncomplicated P. vivax infections in a non-immune subject are often worse than uncomplicated P. falciparum malaria infections, causing high fever, weakness, malaise and sometimes rigors and prostration. Some of these vivax malaria illnesses warrant hospital admission. In the past 20 years there has been a marked increase in the number of reports of “severe” vivax malaria, mainly from India [ 130 – 132 ]. In some of the reports, the basis for the classification has been thrombocytopenia, which is not generally regarded as a criterion for severe malaria. Some patients hospitalized with P. vivax malaria die, particularly if they are old or debilitated [ 133 , 134 ]. P. vivax may sometimes cause acute pulmonary oedema-although the prognosis is better than in severe falciparum malaria [ 133 , 135 ]. But severe vivax malaria is overdiagnosed for the same reasons that severe falciparum malaria is overdiagnosed. Incidental parasitaemias are found in patients with severe anaemia or vital organ dysfunction and a causal relationship is inferred. In low transmission settings (i.e. most P. vivax endemic areas) Plasmodium vivax can cause severe illness, but the proportion of symptomatic cases which develop life-threatening illness is substantially less than for P. falciparum infections. However recurrent infections with P. vivax in areas of high transmission, such as the island of New Guinea, are associated with severe anaemia and substantial mortality both in the acute phase and over the longer term [ 136 – 138 ]. Further large and detailed cohort studies of hospitalized P. vivax infections would help clarify the prognostic associations and risk factors. But overall, the mortality of acute P. vivax infections is substantially lower than that of P. falciparum infections.
Conclusions
The apparent lack of progress in reducing the global death toll from malaria despite substantial investment suggests that we should reexamine the evidence, and review the current strategies to prevent and treat severe malaria [ 113 ]. The mortality of this common but frequently misdiagnosed syndrome can and should be reduced. Severe malaria deserves more attention.
Availability of data and materials
Review—individual trial data from trials conducted by MORU can be requested from the MORU data access committee.
Torti F. Therapeutice specialis ad febres quasdam perniciosas, (Venice 1712)
World Health Organization. Severe Malaria. Trop Med Int Health. 2014;19(Suppl 1):1–131.
Google Scholar
Marchiafava E, Bignami A. On summer-autumn malarial fevers. In: Marchiafava E, editor. Two monographs on malaria and the parasites of malarial fevers (translated from the first Italian edition by JH Thompson). London: New Sydenham Society; 1894. p. 1–232.
White NJ, Turner GD, Dondorp AM, Day NP. Lethal malaria: Marchiafava and Bignami were right. J Infect Dis. 2013;208:192–8.
Article CAS PubMed PubMed Central Google Scholar
White NJ, Turner GD, Medana IM, Dondorp AM, Day NP. The murine cerebral malaria phenomenon. Trends Parasitol. 2010;26:11–5.
Article PubMed PubMed Central Google Scholar
Jennett B, Teasdale G, Braakman R, Minderhoud J, Knill-Jones R. Predicting outcome in individual patients after severe head injury. Lancet. 1976;1:1031–4.
Article CAS PubMed Google Scholar
World Health Organization Malaria Action Programme. Severe and complicated malaria. Trans R Soc Trop Med Hyg. 1986;80(suppl):1–50.
Warrell DA, Looareesuwan S, Warrell MJ, Kasemsarn P, Intaraprasert R, Bunnag D, et al. Dexamethasone proves deleterious in cerebral malaria A double-blind trial in 100 comatose patients. N Engl J Med. 1982;306:313–9.
World Health Organization Division of Control of Tropical Diseases. Severe and complicated malaria. 2nd edn. Trans R Soc Trop Med Hyg 1990;2:1‑65.
Molyneux ME, Taylor TE, Wirima JJ, Borgstein A. Clinical features and prognostic indicators in paediatric cerebral malaria: a study of 131 comatose Malawian children. Quart J Med. 1989;71:441–59.
CAS PubMed Google Scholar
Marsh K, Forster D, Waruiru C, Mwangi I, Winstanley M, Marsh V, et al. Indicators of life-threatening malaria in African children. N Engl J Med. 1995;332:1399–404.
Waller D, Krishna S, Crawley J, Miller K, Nosten F, Chapman D, et al. Clinical features and outcome of severe malaria in Gambian children. Clin Infect Dis. 1995;21:577–87.
World Health Organization. Severe falciparum malaria. Trans R Soc Trop Med Hyg. 2000;94(Suppl 1):S1-90.
Article Google Scholar
Tran TH, Day NP, Nguyen HP, Nguyen TH, Tran TH, Pham PL, et al. A controlled trial of artemether or quinine in Vietnamese adults with severe falciparum malaria. N Engl J Med. 1996;335:76–83.
van Hensbroek MB, Palmer A, Onyiorah E, Schneider G, Jaffar S, Dolan G, et al. The effect of a monoclonal antibody to tumor necrosis factor on survival from childhood cerebral malaria. J Infect Dis. 1996;174:1091–7.
Article PubMed Google Scholar
Crawley J, Waruiru C, Mithwani S, Mwangi I, Watkins W, Ouma D, et al. Effect of phenobarbital on seizure frequency and mortality in childhood cerebral malaria: a randomised, controlled intervention study. Lancet. 2000;355:701–6.
The Artemether-Quinine Meta-analysis Study Group. A meta-analysis using individual patient data of trials comparing artemether with quinine in the treatment of severe falciparum malaria. Trans R Soc Trop Med Hyg. 2001;95:637–50.
Dondorp A, Nosten F, Stepniewska K, Day N, White NJ; South East Asian Quinine Artesunate Malaria Trial (SEAQUAMAT) group. Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial. Lancet. 2005; 366: 717–25.
Dondorp AM, Fanello CE, Hendriksen ICE, Gomes E, Seni A, Chhaganlal KD, et al. Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT): an open-label, randomised trial. Lancet. 2010;376:1647–57.
Phu NH, Tuan PQ, Day N, Mai NT, Chau TT, Chuong LV, et al. Randomized controlled trial of artesunate or artemether in Vietnamese adults with severe falciparum malaria. Malar J. 2010;9:97.
Kremsner PG, Taylor T, Issifou S, Kombila M, Chimalizeni Y, Kawaza K, et al. A simplified intravenous artesunate regimen for severe malaria. J Infect Dis. 2012;205:312–9.
Taylor T, Olola C, Valim C, Agbenyega T, Kremsner P, Krishna S, et al. Standardized data collection for multi-center clinical studies of severe malaria in African children: establishing the SMAC network. Trans R Soc Trop Med Hyg. 2006;100:615–22.
Idro R, Aloyo J, Mayende L, Bitarakwate E, John CC, Kivumbi GW. Severe malaria in children in areas with low, moderate and high transmission intensity in Uganda. Trop Med Int Health. 2006;11:115–24.
English M, Waruiru C, Amukoye E, Murphy S, Crawley J, Mwangi I, et al. Deep breathing in children with severe malaria: indicator of metabolic acidosis and poor outcome. Am J Trop Med Hyg. 1996;55:521–4.
Imbert P, Sartelet I, Rogier C, Ka S, Baujat G, Candito D. Severe malaria among children in a low seasonal transmission area, Dakar, Senegal: influence of age on clinical presentation. Trans R Soc Trop Med Hyg. 1997;91:22–4.
von Seidlein L, Olaosebikan R, Hendriksen IC, Lee SJ, Adedoyin OT, Agbenyega T, et al. Predicting the clinical outcome of severe falciparum malaria in African children: findings from a large randomized trial. Clin Infect Dis. 2012;54:1080–90.
WHO. Guidelines for the treatment of malaria. Geneva, World Health Organization, 2015.
Swellengrebel NH, de Buck A. Malaria in The Netherlands: Scheltema & Holkema Ltd. Amsterdam, 1938.
Snounou G, Pérignon JL. Malariotherapy–insanity at the service of malariology. Adv Parasitol. 2013;81:223–55.
Flateau C, Le Loup G, Pialoux G. Consequences of HIV infection on malaria and therapeutic implications: a systematic review. Lancet Infect Dis. 2011;11:541–56.
Field JW. Blood examination and prognosis in acute falciparum malaria. Trans R Soc Trop Med Hyg. 1949;43:33–48.
White NJ, Chapman D, Watt G. The effects of multiplication and synchronicity on the vascular distribution of parasites in falciparum malaria. Trans R Soc Trop Med Hyg. 1992;86:590–7.
Kingston HW, Ghose A, Plewes K, Ishioka H, Leopold SJ, Maude RJ, et al. Disease severity and effective parasite multiplication rate in falciparum malaria. Open Forum Infect Dis. 2017;4:169.
Intharabut B, Kingston HW, Srinamon K, Ashley EA, Imwong M, Dhorda M, et al. Artemisinin resistance and stage dependency of parasite clearance in falciparum malaria. J Infect Dis. 2019;219:1483–9.
Luxemburger C, Thwai KL, White NJ, Webster HK, Kyle DE, Maelankirri L, et al. The epidemiology of malaria in a Karen population on the western border of Thailand. Trans R Soc Trop Med Hyg. 1996;90:105–11.
Luxemburger C, Ricci F, Nosten F, Raimond D, Bathet S, White NJ. The epidemiology of severe malaria in an area of low transmission in Thailand. Trans R Soc Trop Med Hyg. 1997;91:256–62.
Trang TT, Phu NH, Vinh H, Hien TT, Cuong BM, Chau TTH, et al. Acute renal failure in patients with severe falciparum malaria. Clin Infect Dis. 1992;15:874–80.
Brand NR, Opoka RO, Hamre KE, John CC. Differing causes of lactic acidosis and deep breathing in cerebral malaria and severe malarial anemia may explain differences in acidosis-related mortality. PLoS ONE. 2016;11: e0163728.
Phiri KS, Calis JC, Faragher B, Nkhoma E, Ng’oma K, Mangochi B, et al. Long term outcome of severe anaemia in Malawian children. PLoS ONE. 2008;3: e2903.
Kwambai TK, Dhabangi A, Idro R, Opoka R, Watson V, Kariuki S, et al. Malaria chemoprevention in the post-discharge management of severe anemia. N Engl J Med. 2020;383:2242–54.
Kwambai TK, Mori AT, Nevitt S, van Eijk AM, Samuels AM, Robberstad B, et al. Post-discharge morbidity and mortality in children admitted with severe anaemia and other health conditions in malaria-endemic settings in Africa: a systematic review and meta-analysis. Lancet Child Adolesc Health. 2022;6:474–83.
Idro R, Jenkins NE, Newton CR. Pathogenesis, clinical features, and neurological outcome of cerebral malaria. Lancet Neurol. 2005;4:827–40.
Wattanagoon Y, Srivilairit S, Looareesuwan S, White NJ. Convulsions in childhood malaria. Trans R Soc Trop Med Hyg. 1994;88:426–8.
Birbeck GL, Beare N, Lewallen S, Glover SJ, Molyneux ME, Kaplan PW, et al. Identification of malaria retinopathy improves the specificity of the clinical diagnosis of cerebral malaria: findings from a prospective cohort study. Am J Trop Med Hyg. 2010;82:231–4.
Bangirana P, Opoka RO, Boivin MJ, Idro R, Hodges JS, Romero RA, et al. Severe malarial anemia is associated with long-term neurocognitive impairment. Clin Infect Dis. 2014;59:336–44.
Herdman MT, Sriboonvorakul N, Leopold SJ, Douthwaite S, Mohanty S, Hassan MM, et al. The role of previously unmeasured organic acids in the pathogenesis of severe malaria. Crit Care. 2015;19: e317.
White NJ, Warrell DA, Chanthavanich P, Looareesuwan S, Warrell MJ, Krishna S, et al. Severe hypoglycemia and hyperinsulinemia in falciparum malaria. N Engl J Med. 1983;309:61–6.
White NJ, Miller KD, Marsh K, Berry CD, Turner RC, Williamson DH, et al. Hypoglycaemia in African children with severe malaria. Lancet. 1987;11:708–11.
Taylor TE, Molyneux ME, Wirima JJ, Fletcher KA, Morris K. Blood glucose levels in Malawian children before and during the administration of intravenous quinine for severe falciparum malaria. N Engl J Med. 1988;319:1040–7.
Leopold SJ, Ghose A, Allman EL, Kingston HWF, Hossain A, Dutta AK, et al. Identifying the Components of Acidosis in Patients With Severe Plasmodium falciparum Malaria Using Metabolomics. J Infect Dis. 2019;219:1766–76.
Plewes K, Turner GDH, Dondorp AM. Pathophysiology, clinical presentation, and treatment of coma and acute kidney injury complicating falciparum malaria. Curr Opin Infect Dis. 2018;31:69–77.
Phu NH, Hien TT, Mai NT, Chau TT, Chuong LV, Loc PP, et al. Hemofiltration and peritoneal dialysis in infection-associated acute renal failure in Vietnam. N Engl J Med. 2002;347:895–902.
White NJ. Anaemia and malaria. Malar J. 2018;17: e371.
Uyoga S, George EC, Bates I, Olupot-Olupot P, Chimalizeni Y, Molyneux EM, et al. Point-of-care haemoglobin testing in African hospitals: a neglected essential diagnostic test. Br J Haematol. 2021;193:894–901.
Maitland K, Kiguli S, Olupot-Olupot P, Engoru C, Mallewa M, Saramago Goncalves P, et al. Immediate Transfusion in African Children with Uncomplicated Severe Anemia. N Engl J Med. 2019;381:407–19.
Maitland K, Olupot-Olupot P, Kiguli S, Chagaluka G, Alaroker F, Opoka RO, et al. Transfusion volume for children with severe anemia in Africa. N Engl J Med. 2019;381:420–31.
George EC, Uyoga S, M'baya B, Kyeyune Byabazair D, Kiguli S, Olupot-Olupot P et al. Whole blood versus red cell concentrates for children with severe anaemia: a secondary analysis of the transfusion and treatment of African children (TRACT) trial. Lancet Glob Health. 2022;10:e360-e368
Maitland K, Kiguli S, Olupot-Olupot P, Opoka RO, Chimalizeni Y, Alaroker F, et al. Transfusion management of severe anaemia in African children: a consensus algorithm. Br J Haematol. 2021;193:1247–59.
Paton RS, Kamau A, Akech S, Agweyu A, Ogero M, Mwandawiro C, et al. Malaria infection and severe disease risks in Africa. Science. 2021;373:926–31.
Tran TH, Day NP, Ly VC, Nguyen TH, Pham PL, Nguyen HP, et al. Blackwater fever in southern Vietnam: a prospective descriptive study of 50 cases. Clin Infect Dis. 1996;23:1274–81.
Bodi JM, Nsibu CN, Longenge RL, Aloni MN, Akilimali PZ, Tshibassu PM, et al. Blackwater fever in Congolese children: a report of clinical, laboratory features and risk factors. Malar J. 2013;12:205.
Olupot-Olupot P, Engoru C, Uyoga S, Muhindo R, Macharia A, Kiguli S, et al. High frequency of blackwater fever among children presenting to hospital with severe febrile illnesses in Eastern Uganda. Clin Infect Dis. 2017;64:939–46.
Opoka RO, Waiswa A, Harriet N, John CC, Tumwine JK, Karamagi C. Blackwater fever in Ugandan children with severe anemia is associated with poor post-discharge outcomes: a prospective cohort study. Clin Infect Dis. 2020;70:2247–54.
Conroy AL, Hawkes MT, Leligdowicz A, Mufumba I, Starr MC, Zhong K, et al. Blackwater fever and acute kidney injury in children hospitalized with an acute febrile illness: pathophysiology and prognostic significance. BMC Med. 2022;20:221.
Kamau A, Paton RS, Akech S, Mpimbaza A, Khazenzi C, Ogero M, et al. Malaria hospitalisation in East Africa: age, phenotype and transmission intensity. BMC Med. 2022;20: e28.
Muriuki JM, Mentzer AJ, Mitchell R, Webb EL, Etyang AO, Kyobutungi C, et al. Malaria is a cause of iron deficiency in African children. Nat Med. 2021;27:653–8.
Calis JC, Phiri KS, Faragher EB, Brabin BJ, Bates I, Cuevas LE, et al. Severe anemia in Malawian children. N Engl J Med. 2008;358:888–99.
Pukrittayakamee S, Looareesuwan S, Keeratithakul D, Davis TM, Teja-Isavadharm P, Nagachinta B, et al. A study of the factors affecting the metabolic clearance of quinine in malaria. Eur J Clin Pharmacol. 1997;52:487–93.
Hanson J, Phu NH, Hasan MU, Charunwatthana P, Plewes K, Maude RJ, et al. The clinical implications of thrombocytopenia in adults with severe falciparum malaria: a retrospective analysis. BMC Med. 2015;13:97.
Leopold SJ, Watson JA, Jeeyapant A, Simpson JA, Phu NH, Hien TT, et al. Investigating causal pathways in severe falciparum malaria: A pooled retrospective analysis of clinical studies. PLoS Med. 2019;16: e1002858.
Church J, Maitland K. Invasive bacterial co-infection in African children with Plasmodium falciparum malaria: a systematic review. BMC Med. 2014;12:31.
Phu NH, Day NPJ, Tuan PQ, Mai NTH, Chau TTH, Chuong LV, et al. Concomitant bacteremia in adults with severe falciparum malaria. Clin Infect Dis. 2020;71:e465–70.
PubMed PubMed Central Google Scholar
White NJ, Watson JA, Uyoga S, Williams TN, Maitland KM. Substantial misdiagnosis of severe malaria in African children. Lancet. 2022;400:807.
Aung NM, Nyein PP, Kyi MM, Hanson J. Bacterial coinfection in adults with severe malaria. Clin Infect Dis. 2021;72:535–6.
White NJ.Reply to Aung, et al. Clin Infect Dis. 2021;72:536–8.
Hanson J, Lam SW, Mahanta KC, Pattnaik R, Alam S, Mohanty S, et al. Relative contributions of macrovascular and microvascular dysfunction to disease severity in falciparum malaria. J Infect Dis. 2012;206:571–9.
Ishioka H, Ghose A, Charunwatthana P, Maude R, Plewes K, Kingston H, et al. Sequestration and red cell deformability as determinants of hyperlactatemia in falciparum Malaria. J Infect Dis. 2016;213:788–93.
Plewes K, Kingston HWF, Ghose A, Maude RJ, Herdman MT, Leopold SJ, et al. Cell-free hemoglobin mediated oxidative stress is associated with acute kidney injury and renal replacement therapy in severe falciparum malaria: an observational study. BMC Infect Dis. 2017;17:313.
Kingston HWF, Ghose A, Rungpradubvong V, Satitthummanid S, Herdman MT, Plewes K, et al. Cell-free hemoglobin is associated with increased vascular resistance and reduced peripheral perfusion in severe malaria. J Infect Dis. 2020;221:127–37.
Silamut K, Phu NH, Whitty C, Turner GD, Louwrier K, Mai NT, et al. A quantitative analysis of the microvascular sequestration of malaria parasite in the human brain. Am J Pathol. 1999;155:395–410.
Seydel KB, Kampondeni SD, Valim C, Potchen MJ, Milner DA, Muwalo FW, et al. Brain swelling and death in children with cerebral malaria. N Engl J Med. 2015;372:1126–37.
Moghaddam SM, Birbeck GL, Taylor TE, Seydel KB, Kampondeni SD, Potchen MJ. Diffusion-weighted MR imaging in a prospective cohort of children with cerebral malaria offers insights into pathophysiology and prognosis. AJNR Am J Neuroradiol. 2019;40:1575–80.
CAS PubMed PubMed Central Google Scholar
Kampondeni S, Seydel KB, Zhang B, Small DS, Birbeck GL, Hammond CA, et al. Amount of brain edema correlates with neurologic recovery in pediatric cerebral malaria. Pediatr Infect Dis J. 2020;39:277–82.
Anstey NM, Grigg MJ, Rajahram GS, Cooper DJ, William T, Kho S, et al. Knowlesi malaria: Human risk factors, clinical spectrum, and pathophysiology. Adv Parasitol. 2021;113:1–43.
Silamut K, White NJ. Relation of the stage of parasite development in the peripheral blood to prognosis in severe falciparum malaria. Trans R Soc Trop Med Hyg. 1993;87:436–43.
Phu NH, Day NPJ, Diep TS, Ferguson DJP, White NJ. Intraleukocytic malaria pigment and prognosis in severe malaria. Trans R Soc Trop Med Hyg. 1995;89:197–9.
Srinamon K, Watson JA, Silamut K, Intharabut B, Phu NH, Diep PT, et al. The prognostic and diagnostic value of intraleukocytic malaria pigment: an individual patient data pooled meta-analysis of 32,000 patients with severe falciparum malaria in Africa and Asia. Nat Comm, in press.
Poti KE, Sullivan DJ, Dondorp AM, Woodrow CJ. HRP2: transforming malaria diagnosis, but with caveats. Trends Parasitol. 2020;36:112–26.
Mayxay M, Pukritrayakamee S, Chotivanich K, Imwong M, Looareesuwan S, White NJ. Identification of cryptic coinfection with Plasmodium falciparum in patients presenting with vivax malaria. Am J Trop Med Hyg. 2001;65:588–92.
Reyburn H, Mbatia R, Drakeley C, Carneiro I, Mwakasungula E, Mwerinde O, et al. Overdiagnosis of malaria in patients with severe febrile illness in Tanzania: a prospective study. BMJ. 2004;329:1212.
Beare NA, Taylor TE, Harding SP, Lewallen S, Molyneux ME. Malarial retinopathy: a newly established diagnostic sign in severe malaria. Am J Trop Med Hyg. 2006;75:790–7.
Maude RJ, Beare NA, Abu Sayeed A, Chang CC, Charunwatthana P, Faiz MA, et al. The spectrum of retinopathy in adults with Plasmodium falciparum malaria. Trans R Soc Trop Med Hyg. 2009;103:665–71.
Seydel KB, Fox LL, Glover SJ, Reeves MJ, Pensulo P, Muiruri A, et al. Plasma concentrations of parasite histidine-rich protein 2 distinguish between retinopathy-positive and retinopathy-negative cerebral malaria in Malawian children. J Infect Dis. 2012;206:309–18.
Barrera V, MacCormick IJC, Czanner G, Hiscott PS, White VA, Craig AG, et al. Neurovascular sequestration in paediatric P. falciparum malaria is visible clinically in the retina. Elife. 2018;7:e32208.
Dondorp AM, Ince C, Charunwatthana P, Hanson J, van Kuijen A, Faiz MA, et al. Direct in vivo assessment of microcirculatory dysfunction in severe falciparum malaria. J Infect Dis. 2008;197:79–84.
Raja RN. Post-mortem examination in cerebral malaria: a new simple method of demonstrating parasites in the capillaries of the brain. Ind Med Gaz. 1922;57:298–9.
Milner DA Jr, Valim C, Luo R, Playforth KB, Kamiza S, Molyneux ME, et al. Supraorbital postmortem brain sampling for definitive quantitative confirmation of cerebral sequestration of Plasmodium falciparum parasites. J Infect Dis. 2012;205:1601–6.
Gomes MF, Faiz MA, Gyapong JO, Warsame M, Agbenyega T, Babiker A, et al. Pre-referral rectal artesunate to prevent death and disability in severe malaria: a placebo-controlled trial. Lancet. 2009;373:557–66.
WHO. The use of rectal artesunate as a pre- referral treatment for severe P. falciparum malaria. Geneva, World Health Organization, 2022.
Brunner NC, Omoluabi E, Awor P, Okitawutshu J, Tshefu Kitoto A, Signorell A, et al. Prereferral rectal artesunate and referral completion among children with suspected severe malaria in the Democratic Republic of the Congo. Nigeria and Uganda BMJ Glob Health. 2022;7: e008346.
Watson JA, Warsame M, Peto TJ, Onyamboko M, Fanello C, Dondorp AM, et al. Stopping prereferral rectal artesunate - a grave error. BMJ Glob Health. 2022;7: e010006.
Mabey DC, Brown A, Greenwood BM. Plasmodium falciparum malaria and Salmonella infections in Gambian children. J Infect Dis. 1987;155:1319–21.
Berkley J, Mwarumba S, Bramham K, Lowe B, Marsh K. Bacteraemia complicating severe malaria in children. Trans R Soc Trop Med Hyg. 1999;93:283–6.
Scott JA, Berkley JA, Mwangi I, Ochola L, Uyoga S, Ndila C, et al. Relation between falciparum malaria and bacteraemia in Kenyan children: a population-based, case-control study and a longitudinal study. Lancet. 2011;378:1316–23.
Nichols C, Cruz Espinoza LM, von Kalckreuth V, Aaby P, Tayeb M, Ali M, et al. Bloodstream infections and frequency of pretreatment associated with age and hospitalization status in sub-Saharan Africa. Clin Infect Dis. 2015;61(4):S372–9.
Park SE, Pak GD, Aaby P, Adu-Sarkodie Y, Ali M, Aseffa A, et al. The relationship between invasive nontyphoidal Salmonella disease, other bacterial bloodstream infections, and malaria in sub-Saharan Africa. Clin Infect Dis. 2016;62(1):S23-31.
Bruneel F, Tubach F, Mira JP, Houze S, Gibot S, Huisse MG, et al. Imported falciparum malaria in adults: host- and parasite-related factors associated with severity. The French prospective multicenter PALUREA cohort study. Intensive Care Med. 2016;42:1588–96.
Watson JA, Ndila CM, Uyoga S, Macharia A, Nyutu G, Mohammed S, et al. Improving statistical power in severe malaria genetic association studies by augmenting phenotypic precision. Elife. 2021;10: e69698.
Watson JA, Uyoga S, Wanjiku P, Makale J, Nyutu GM, Mturi N, et al. Improving the diagnosis of severe malaria in African children using platelet counts and plasma Pf HRP2 concentrations. Sci Transl Med. 2022;14:5040.
Hendriksen IC, Mwanga-Amumpaire J, von Seidlein L, Mtove G, White LJ, Olaosebikan R, et al. Diagnosing severe falciparum malaria in parasitaemic African children: a prospective evaluation of plasma PfHRP2 measurement. PLoS Med. 2012;9: e1001297.
Hendriksen IC, White LJ, Veenemans J, Mtove G, Woodrow C, Amos B, et al. Defining falciparum-malaria-attributable severe febrile illness in moderate-to-high transmission settings on the basis of plasma PfHRP2 concentration. J Infect Dis. 2013;207:351–61.
Taylor TE, Fu WJ, Carr RA, Whitten RO, Mueller JS, Fosiko NG, et al. Differentiating the pathologies of cerebral malaria by postmortem parasite counts. Nat Med. 2004;10:143–5.
White NJ, Day NPJ, Ashley EA, Smithuis FM, Nosten FH. Have we really failed to roll back malaria? Lancet. 2022;399:799–800.
John CC, Bangirana P, Byarugaba J, Opoka RO, Idro R, Jurek AM, et al. Cerebral malaria in children is associated with long-term cognitive impairment. Pediatrics. 2008;122:e92–9.
Brewster DR, Kwiatkowski D, White NJ. Neurological sequelae of cerebral malaria in children. Lancet. 1990;336:1039–43.
Idro R, Kakooza-Mwesige A, Asea B, Ssebyala K, Bangirana P, Opoka RO, et al. Cerebral malaria is associated with long-term mental health disorders: a cross sectional survey of a long-term cohort. Malar J. 2016;15:184.
Brim R, Mboma S, Semrud-Clikeman M, Kampondeni S, Magen J, Taylor T, et al. Cognitive Outcomes and Psychiatric Symptoms of Retinopathy-Positive Cerebral Malaria: Cohort Description and Baseline Results. Am J Trop Med Hyg. 2017;97:225–31.
Boivin MJ, Mohanty A, Sikorskii A, Vokhiwa M, Magen JG, Gladstone M. Early and middle childhood developmental, cognitive, and psychiatric outcomes of Malawian children affected by retinopathy positive cerebral malaria. Child Neuropsychol. 2019;25:81–102.
Dondorp AM, Hoang MNT, Mer M, Dünser MW, Mohanty S, Nakibuuka J, Management of severe malaria and severe dengue in resource-limited settings., et al. 9. In: Dondorp AM, Dünser MW, Schultz MJ, editors., et al., Sepsis Management in Resource-limited Settings [Internet]. Cham (CH): Springer; 2019. p. 2019.
Chapter Google Scholar
Maitland K, Kiguli S, Opoka RO, Engoru C, Olupot-Olupot P, Akech SO, et al. Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011;364:2483–95.
Namutangula B, Ndeezi G, Byarugaba JS, Tumwine JK. Mannitol as adjunct therapy for childhood cerebral malaria in Uganda: a randomized clinical trial. Malar J. 2007;6:138.
Mohanty S, Mishra SK, Patnaik R, Dutt AK, Pradhan S, Das B, et al. Brain swelling and mannitol therapy in adult cerebral malaria: a randomized trial. Clin Infect Dis. 2011;53:349–55.
Ishioka H, Plewes K, Pattnaik R, Kingston HWF, Leopold SJ, Herdman MT, et al. Associations Between Restrictive Fluid Management and Renal Function and Tissue Perfusion in Adults With Severe Falciparum Malaria: A Prospective Observational Study. J Infect Dis. 2020;221:285–92.
Birbeck GL, Herman ST, Capparelli EV, Dzinjalamala FK, Abdel Baki SG, Mallewa M, et al. A clinical trial of enteral Levetiracetam for acute seizures in pediatric cerebral malaria. BMC Pediatr. 2019;19:399.
Gwer SA, Idro RI, Fegan G, Chengo EM, Mpoya A, Kivaya E, et al. Fosphenytoin for seizure prevention in childhood coma in Africa: a randomized clinical trial. J Crit Care. 2013;28:1086–92.
Imwong M, Woodrow CJ, Hendriksen IC, Veenemans J, Verhoef H, Faiz MA, et al. Plasma concentration of parasite DNA as a measure of disease severity in falciparum malaria. J Infect Dis. 2015;211:1128–33.
Sinha I, Ekapirat N, Dondorp AM, Woodrow CJ. Use of a rapid test to assess plasma Plasmodium falciparum HRP2 and guide management of severe febrile illness. Malar J. 2015;14:362.
Rajahram GS, Cooper DJ, William T, Grigg MJ, Anstey NM, Barber BE. Deaths from Plasmodium knowlesi malaria: case series and systematic review. Clin Infect Dis. 2019;69:1703–11.
Barber BE, Grigg MJ, Cooper DJ, van Schalkwyk DA, William T, Rajahram GS, et al. Clinical management of Plasmodium knowlesi malaria. Adv Parasitol. 2021;113:45–76.
Rahimi BA, Thakkinstian A, White NJ, Sirivichayakul C, Dondorp AM, Chokejindachai W. Severe vivax malaria: a systematic review and meta-analysis of clinical studies since 1900. Malar J. 2014;13:481.
Kojom Foko LP, Arya A, Sharma A, Singh V. Epidemiology and clinical outcomes of severe Plasmodium vivax malaria in India. J Infect. 2021;82:231–46.
Phyo AP, Dahal P, Mayxay M, Ashley EA. Clinical impact of vivax malaria: a collection review. PLoS Med. 2022;19: e1003890.
Anstey NM, Douglas NM, Poespoprodjo JR, Price RN. Plasmodium vivax : clinical spectrum, risk factors and pathogenesis. Adv Parasitol. 2012;80:151–201.
Lacerda MV, Fragoso SC, Alecrim MG, Alexandre MA, Magalhães BM, Siqueira AM, et al. Postmortem characterization of patients with clinical diagnosis of Plasmodium vivax malaria: to what extent does this parasite kill? Clin Infect Dis. 2012;55:e67-74.
Naing C, Whittaker MA, Nyunt Wai V, Mak JW. Is Plasmodium vivax malaria a severe malaria?: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2014;8: e3071.
Douglas NM, Pontororing GJ, Lampah DA, Yeo TW, Kenangalem E, Poespoprodjo JR, et al. Mortality attributable to Plasmodium vivax malaria: a clinical audit from Papua. Indonesia BMC Med. 2014;12:217.
Patriani D, Arguni E, Kenangalem E, Dini S, Sugiarto P, Hasanuddin A, et al. Early and late mortality after malaria in young children in Papua. Indonesia BMC Infect Dis. 2019;19:922.
Dini S, Douglas NM, Poespoprodjo JR, Kenangalem E, Sugiarto P, Plumb ID, et al. The risk of morbidity and mortality following recurrent malaria in Papua, Indonesia: a retrospective cohort study. BMC Med. 2020;18:28.
Download references
Acknowledgements
I am a Wellcome Trust Principal Fellow (093956/Z/10/C). I am very grateful to my colleagues in the Mahidol Oxford Research Unit and associated research programmes for all their advice and help.
NJW is a Wellcome Trust Principal Fellow (093956/Z/10/C).
Author information
Authors and affiliations.
Mahidol Oxford Research Unit, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand
Nicholas J. White
Centre for Tropical Medicine and Global Health, Nuffield Department of Medicine, University of Oxford, Oxford, UK
You can also search for this author in PubMed Google Scholar
Contributions
The author wrote read and approved the final manuscript.
Corresponding author
Correspondence to Nicholas J. White .
Ethics declarations
Ethics approval and consent to participate.
Not relevant.
Consent for publication
Competing interests.
The author declares no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
White, N.J. Severe malaria. Malar J 21 , 284 (2022). https://doi.org/10.1186/s12936-022-04301-8
Download citation
Received : 12 August 2022
Accepted : 23 September 2022
Published : 06 October 2022
DOI : https://doi.org/10.1186/s12936-022-04301-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Malaria Journal
ISSN: 1475-2875
- Submission enquiries: [email protected]
Malaria vaccine: WHO position paper – May 2024
- Loadshedding
- South African News
- African News
- International News
- All Opinions
- Latest Opinions
- Raymond Suttner - Suttner's View
- Politics 360 - Ebrahim Fakir
- Institute for Security Studies
- South African Institute of International Affairs
- Saliem Fakir - Low Carbon Future
- The Conversation
- Real Economy
- Other Opinions
- Author Interviews
- All Legislation
- Constitution
- Audio Articles
- Recommendations
- All Case Law
- Constitutional Court
- Supreme Court of Appeal
- High Courts
- All Legal Briefs
- Company Posts
- Legal Notice

Note: Search is limited to the most recent 250 articles. To access earlier articles, click Advanced Search and set an earlier date range. To search for a term containing the '&' symbol, click Advanced Search and use the 'search headings' and/or 'in first paragraph' options.
And must exclude these words...
Sponsored by
Please enter the email address that you used to register on Polity.org.za. Your password will be sent to this address.
Email this article
separate emails by commas, maximum limit of 4 addresses
Article Enquiry
Malaria vaccine: who position paper – may 2024, embed video.

Embed Video Popup Video Instagram

Download Buy Photos
13th May 2024
ARTICLE ENQUIRY SAVE THIS ARTICLE EMAIL THIS ARTICLE
Font size: - +
- Malaria vaccine: WHO position paper – May 2024 Download 0.44 MB
This position paper supersedes the 2022 WHO position paper on malaria vaccines. It includes the updated WHO recommendations on the use of the RTS,S/AS01 and R21/Matrix-M vaccines for the reduction of malaria morbidity and mortality in children living in endemic areas, prioritizing areas of moderate and high malaria transmission. It also incorporates findings from the evaluation of the WHO-coordinated Malaria Vaccine Implementation Programme (MVIP), through which the RTS,S/AS01 vaccine was introduced in routine immunization programmes through large pilot programmes from 2019 through to 2023 in Ghana, Kenya and Malawi. Recommendations on the use of malaria vaccines were discussed by SAGE and MPAG during a joint session in September 2023 and were subsequently endorsed by WHO.
Report by the World Health Organization
EMAIL THIS ARTICLE SAVE THIS ARTICLE ARTICLE ENQUIRY
To subscribe email [email protected] or click here To advertise email [email protected] or click here
Next article
South africa must create a business-friendly environment to attract foreign investment.
By: Tasneem Bulbulia Business organisation Business Leadership South Africa CEO Busiswe Mavuso writes in her latest weekly newsletter that the withdrawal of foreign investment seems to be “dominating” at present. She avers that while foreign investment in South Africa hinges on many factors, including global strategies and the country’s links to the rest of the world. The more attractive a country is, the more investment it receives. →
Polity.org.za is a product of Creamer Media. www.creamermedia.co.za
Other Creamer Media Products include: Engineering News Mining Weekly Research Channel Africa
Newsletters
Sign up for our FREE daily email newsletter
Subscriptions
We offer a variety of subscriptions to our Magazine, Website, PDF Reports and our photo library.
Subscriptions are available via the Creamer Media Store.
Advertising on Polity.org.za is an effective way to build and consolidate a company's profile among clients and prospective clients. Email [email protected]

An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Emily Buck ; Nancy A. Finnigan .
Affiliations
Last Update: July 31, 2023 .
- Continuing Education Activity
Malaria is a parasitic infection transmitted by the Anopheles mosquito that leads to acute life-threatening disease and poses a significant global health threat. Two billion people risk contracting malaria annually, including those in 90 endemic countries and 125 million travelers. The Plasmodium parasite has a multistage lifecycle, which leads to characteristic cyclical fevers. With timely treatment, most people experience rapid resolution of symptoms; however, significant complications may occur, including cerebral malaria, severe malarial anemia, coma, or death. This activity reviews the epidemiology, presentation, and complications of Plasmodium malaria and the role of the interprofessional team in evaluating and managing patients with this life-threatening infection.
- Review the epidemiology of malaria infection.
- Describe the pathophysiology of malaria infection.
- Summarize the pharmacologic treatment strategies for malaria infection.
- Outline the importance of collaboration amongst an interprofessional team to improve outcomes for patients receiving malaria treatment.
- Introduction
Malaria is a parasitic infection transmitted by the Anopheles mosquito that leads to acute life-threatening disease and poses a significant global health threat. Two billion people risk contracting malaria annually, including those in 90 endemic countries and 125 million travelers, and 1.5 to 2.7 million people die in a year. [1] The Plasmodium parasite has a multistage lifecycle, which leads to characteristic cyclical fevers. With timely treatment, most people experience rapid resolution of symptoms; however, significant complications may occur, including cerebral malaria, severe malarial anemia, coma, or death. Preferred antimalarial therapeutic and chemoprophylactic regimens get dictated by species, geography, susceptibility, and patient demographics. Latent or reactivating infections may be reported years following exposure.
The incubation period, and therefore time to symptom development, varies by species: 8 to 11 days for P. falciparum , 8 to 17 days for P. vivax , 10 to 17 days for P. ovale , 18 to 40 days for P. malariae (though possibly up to several years), and 9 to 12 days for P. knowlesi . [1] The periodicity of the Plasmodium lifecycle creates the classic "malarial paroxysm" of rigors, followed by several hours of fever, followed by diaphoresis, and a drop to normal body temperature ( P. vivax infection establishes a 48-hour cycle), though this is less commonly seen today due to rapid identification and treatment. [1]
- Epidemiology
Forty percent of the global population resides in or visits malaria-endemic regions annually. [1] P. falciparum is present in Western and sub-Saharan Africa and displays the highest morbidity and mortality of the Plasmodia species. [2] P. vivax is present in South Asia, the Western Pacific, and Central America. [2] P. ovale and P. malariae are present in Sub-Saharan Africa. [2] P. knowlesi is present in Southeast Asia. [2] As many as 500 million malaria cases occur annually, with 1.5 to 2.7 million deaths. [1] Ninety percent of fatalities occur in Africa. [1] Those at highest risk include children under age 5, pregnant women, and disease naïve populations, including refugees in Central and Eastern Africa, nonimmune civilian and military travelers, and immigrants returning to their place of origin. [2]
Of the 125 million travelers who visit endemic locations each year, 10000 to 30000 develop malaria, and 1% of these will die from complications of their disease. [2] [3] Rising average global temperatures and changes in weather patterns are projected to expand the burden of malaria; a rise of 3 degrees Celsius is postulated to increase malaria incidence by 50 to 80 million. [1]
- Pathophysiology
Five Plasmodium species possess the ability to infect humans: P. falciparum, P. ovale, P. vivax, P. malariae , and P. knowlesi . [2] The female Anopheles mosquito ingests gametes during a blood meal, which form sporozoites that replicate in the gut. [1] During subsequent bloodmeals, saliva containing sporozoites gets released into a human host's bloodstream. [1] Within 60 minutes, sporozoites reach the liver, invade hepatocytes, and then rapidly divide, forming merozoites. In an active infection, organisms reenter the bloodstream and invade erythrocytes. [1] [4] Within erythrocytes, Plasmodia consume hemoglobin and develop from immature trophozoites (ring stage) to either mature trophozoites or gametocytes (CDC Malaria 2019). Mature trophozoites replicate, forming schizonts, disrupting erythrocyte cell membrane integrity, and leading to capillary endothelial adherence and cell lysis. [1]
Free heme is released into the peripheral blood, which stimulates endothelial activation. [5] [6] Untreated malaria lasts 2 to 24 months. [1] P. vivax and P. ovale infections may display "dormant schizogony," where inactive intrahepatic parasites (hypnozoites) remain until reactivation months to years in the future. [1] Although hypnozoite parasites do not routinely develop in the liver in the setting of P. falciparum and P. malariae infection, there are few reports of resurgent P. falciparum infection years after initial exposure. [7]
Pathogenesis stems from toxin-induced IFN-gamma and TNF-alpha secretion. [8] The innate immune response is dominated by monocyte and macrophage phagocytosis within the splenic red pulp. Adaptive immunity develops by IFN-gamma and TNF-alpha-induced class switching of CD4-positive lymphocytes. [4] TNF also suppresses hematopoiesis, which contributes to anemia. The liver and spleen enlarge, causing massive splenomegaly. [8]
Low arginine, low nitric oxide, and elevated arginase activity have been observed in severe malaria in peripheral blood. [9] Studies have shown that the parasite's arginase enzyme may contribute to low arginine in severely ill patients, thus reducing nitric oxide production. Low nitric may lead to subsequent pulmonary hypertension and myocardial wall stress in children. Therefore, peripheral arginine or inhaled nitric oxide are possible treatment options. [10]
Parasitemia dictates symptom onset and severity: symptoms typically develop with 0.002% parasitemia in naïve patients and 0.2% parasitemia in previously exposed patients. [1] Severe infection usually exhibits parasitemia of 5%. [1] [4]
- Histopathology
Intracellular digestion of hemoglobin by parasites forms hemozoin and makes the membrane less deformable, which results in hemolysis or splenic clearance.
- History and Physical
In taking a history, it is essential to inquire about the location of residence, recent travel and use of chemoprophylaxis, exposures (including sick contacts, fresh water, caves, farm/wild animals, insects/arthropods), HIV status, history of current or recent pregnancy, history of G6PD deficiency, history of sickle cell disease, history of anemia, history of blood or other cancers, and history of prior malarial infections (including successful or failed treatments).
Fever is the dominant symptom of malaria—fever, especially for seven or more days, in a patient residing in or with recent travel to an endemic region is highly suspicious and should prompt evaluation. [3] Adults may exhibit headaches, malaise, weakness, gastrointestinal distress, upper respiratory symptoms, and muscle aches; severe cases may include jaundice, confusion, seizures, and dark urine. [2] [1] Children are more likely to present with non-specific or gastrointestinal symptoms such as fever, lethargy, malaise, nausea, vomiting, abdominal cramps, and somnolence. [2] They are more likely to develop hepatomegaly, splenomegaly, and severe anemia without major organ dysfunction than adults. In the case of severe malaria, they present with more frequent seizures (60 to 80%), hypoglycemia, and concomitant sepsis but are less likely to develop pulmonary edema and renal failure than adults. [11] [2]
Pregnant Women
The clinical features of infection in pregnancy vary from asymptomatic to severe, depending on the degree of (incomplete) immunity that a woman had acquired by the time she got pregnant. In semi-immune pregnant women, only a few infections result in fever or other symptoms. [12] Malaria in pregnancy has a devastating effect on maternal health and has been associated with increased infant mortality due to low birth weight caused by either intrauterine growth restriction or preterm labor, or both. [12] P. falciparum infections are associated with complications such as maternal anemia, low birth weight, miscarriage, stillbirths, and congenital malaria. [13] [12] It is more likely for a pregnant woman in the second or third trimester to develop severe malaria with complications such as hypoglycemia and pulmonary edema compared to non-pregnant adults. [14]
Initial evaluation of undifferentiated fever in stable patients with possible malaria exposure includes a complete blood count, comprehensive metabolic panel, coagulation panel, blood culture, urinalysis, chest radiograph, and thick and thin blood smears. In patients with altered mental status, when cerebral malaria is suspected, a lactate level, arterial blood gas, and lumbar puncture may also be indicated. [2]
In patients with malaria, complete blood count reveals thrombocytopenia in 60-70% of all cases and varying degrees of anemia in 29% of adults and 78% of children. [2] Anemia is more severe in P. falciparum due to invasion of all aged erythrocytes and capillary and splenic erythrocyte sequestration secondary to decreased flexibility and cytoadherence. [1] Anemia is typically moderate with P. vivax and P. malariae due to preferential invasion of reticulocytes and older erythrocytes, respectively. [1] A comprehensive metabolic panel may reveal hepatocellular injury secondary to parasitic invasion, indirect hyperbilirubinemia due to hemolysis, electrolyte abnormalities secondary to the release of intracellular contents, concomitant dehydration, and kidney injury secondary to glomerular damage. [2] The coagulation panel may reveal coagulopathy concerning bleeding risk in patients with severe thrombocytopenia or liver dysfunction. Urinalysis may show proteinuria indicative of nephrotic syndrome. [1]
The gold standard for malaria diagnosis is a microscopic evaluation of Giemsa-stained thick and thin smears of a free-flowing venipuncture blood specimen. [2] [1] Examination with oil immersion must be completed at 100-times and 1000-times magnification to avoid missing low-level parasitemia or "delicate ring forms." [1] The extent of parasitemia is estimated by the number of organisms per high-powered field. [1] Varying microscopic appearance of infected erythrocytes guides speciation:
- The ring stage in P. falciparum appears as a "purple spot with a thin ring;" in P. vivax as a "purple spot with a deformed body;" in P. ovale as a "ring with a large purple spot;" in P. malariae as a "purple spot with a thick body;" and in P. knowlesi as a "purple spot (or spots) with an amorphous thick ring." [15]
- The trophozoite stage in P. falciparum appears as "a bigger spot [growing] around a smaller spot;" in P. vivax as "a misshapen circle which contains an extended spot;" in P. ovale as "an oval circle (sometimes with small corners) which contains a purple spot with undefined shapes;" in P. malariae as "basket or band-shaped [without a] spot;" and in P. knowlesi as a "purple branched spot." [15]
- The schizont stage in P. falciparum is not established; in P. vivax, it appears as "not defined purple spots inside a circle;" in P. ovale as "more than one spot inside an oval circle (sometimes with small corners);" in P. malariae as "diffuse purple spots around a darker spot;" and in P. knowlesi as "defined purple spots [that are] easy to count." [15]
- The gametocyte stage in P. falciparum appears as "banana [or] sausage-shaped;" in P. vivax as an "extended, big spot;" in P. ovale as a "row of accumulated spots;" in P. malariae as a "big stained spot which almost fills[s] the circle;" and in P. knowlesi as a "big spot which contains small spots." [15]
An initial negative smear does not rule out malaria, as infected erythrocytes may become intravascularly sequestered; if clinical suspicion of malaria is high, smears require repetition in 12 and 24 hours. [2] The malarial pigment in monocytes and neutrophils may also manifest on the blood smear, particularly in patients with cerebral malaria. [1]
Other diagnostic modalities include rapid diagnostic testing (RDT), microhematocrit centrifugation, and polymerase chain reaction (PCR). RDTs detecting parasitic antigens histidine-rich-protein-2, lactate dehydrogenase, and aldolase are increasingly being utilized to diagnose P. falciparum infection. [2] [16] Sensitivities approach 100%, though microscopy is still a recommendation at the time of presentation and 12 and 24 hours. Limitations of RDTs include the detection of P. falciparum species only, the inability to quantify parasitic burden, and false-positive results occurring weeks after infection due to persistent blood antigens. [2] Microhematocrit centrifugation isolates infected erythrocytes, then binds to acridine in the collection tube, causing the fluorescence of parasites. [1] PCR is useful in low-level parasitemia detection and speciation.
- Treatment / Management
Treatment for patients diagnosed with malaria includes schizonticidal medications, supportive care, and hospitalization for high-risk patients. Naïve adult and pediatric patients receiving active antimalarial treatment should remain inpatient for at least 24 hours to ensure adequate and correctly timed medication dosing and to trend parasitemia to evaluate treatment response. Higher initial parasitemia and poor downtrend are associated with fluid imbalance, renal dysfunction, and respiratory distress syndrome. [2] Unstable patients, particularly those with cerebral malaria or significant respiratory sequelae, require intensive care. [2]
Treatment involves combination therapy targeting both the hepatic and erythrocytic forms. [17] The chief antimalarials are chloroquine, hydroxychloroquine, primaquine, artemisinin-based combination therapy (ACT), and atovaquone-proguanil. Chloroquine and hydroxychloroquine are synthetic forms of quinine. [18] [19] They disrupt the erythrocytic stage by interfering with parasitic hemoglobin metabolism and increasing intracellular pH. [18] [19] They generally require two days of treatment, allowing for better tolerance and shorter admissions. [2] However, chloroquine may enhance gametogenesis, contributing to resistance, which is a concern, particularly in South Asia. [17] Primaquine is a hypnozointocidal agent added for P. vivax or P. ovale infection for the eradication of liver parasites and the prevention of dormancy and relapse. [2] [20]
Primaquine is contraindicated in pregnant and G6PD deficient patients due to fetal teratogenicity and hemolytic reaction (will see bite cells and Heinz bodies on blood smear), respectively. [3] Artemisinins are active against all parasite lifecycle stages. [2] Atovaquone targets the cellular electron transport chain inhibiting ATP production; proguanil enhances atovaquone’s effect by sensitizing parasitic mitochondria. [21] Atovaquone-proguanil is active against the erythrocytic and extraerythrocytic forms. [17] [21]
Per the 2019 CDC Guidelines below, appropriate treatment depends on the Plasmodium species, clinical stability, age of the patient, and regional antimalarial susceptibility:
- Uncomplicated P. falciparum, P. malariae or P. knowlesi infections in chloroquine-sensitive regions are treated with a chloroquine phosphate 600 mg (pediatric: 10 mg/kg) loading dose, followed by 300 mg (pediatric: 5 mg/kg) at 6, 24, 48 hours; or a hydroxychloroquine 620 mg (pediatric: 10 mg/kg) loading dose, followed by 310 mg (pediatric: 5 mg/kg) at 6, 24, and 48 hours.
- Uncomplicated P. falciparum infections in chloroquine-resistant or unknown regions are treated with atovaquone-proguanil 250 mg/100 mg 4 tabs (pediatric: varied weight-based dosing, 6.5 mg/25 mg tabs) daily for 4 days; or artemether-lumefantrine 20 mg/120 mg 4 tabs (pediatric: varied weight-based tabs) at initial dose, then 8 hours later, then twice daily for 2 days; or quinine sulfate 542 mg (pediatric: 8.3 mg/kg) three times daily for 3 days (7 days if in Southeast Asia) plus either doxycycline 100 mg daily for 7 days (pediatrics 2.2 mg/kg every 12 hours), or tetracycline 250 mg daily for 7 days (pediatric: 25 mg/kg/day divided four times daily for 7 days), or clindamycin 20 mg/kg/day divided three times daily for 7 days (pediatric: same); or mefloquine 684 mg (pediatric: 13.7 mg/kg) loading dose followed by 456 mg (pediatric: 9.1 mg/kg) every 6 to 12 hours for total of 1250 mg (pediatric total: 25 mg/kg).
- Uncomplicated P. vivax or P. ovale infections in chloroquine-sensitive regions receive treatment with chloroquine phosphate or hydroxychloroquine as per above, plus either primaquine phosphate 30 mg (pediatric: 0.5 mg/kg) daily for 14 days or tafenoquine 300 mg once (same in children older than 16 years).
- Uncomplicated P. vivax infections in chloroquine-resistant regions (Indonesia, Papua New Guinea) get treated with quinine sulfate as per above plus either doxycycline, primaquine, or tafenoquine as per above; or atovaquone-proguanil as per above plus either primaquine or tafenoquine; or mefloquine as per above plus either primaquine or tafenoquine as per above.
- Uncomplicated infections with any species in pregnant women in chloroquine-sensitive regions require treatment with chloroquine or hydroxychloroquine as per above.
- Uncomplicated infections with any species in pregnant women in chloroquine-resistant regions are treated with quinine sulfate as per above plus either clindamycin or mefloquine as per above in the first, second, or third trimesters; or artemether-lumefantrine as per above in only the second and third trimesters.
- Severe malaria infection in unstable, non-pregnant patients in all regions includes IV artesunate 2.4 mg/kg (pediatric: children greater than 20 kg receive 2.4 mg/kg, children less than 20 kg receive 3.0 mg/kg) at 0, 12, 24, and 48 hours and either artemether-lumefantrine, atovaquone-proguanil, doxycycline, or mefloquine as per above.
- Differential Diagnosis
The differential for undifferentiated fever is extremely broad and varies based on geographic location and age. In a 2017 review of fever in returning travelers, 77% had protozoal malaria, 18% had a bacterial enteric fever ( Salmonella enterica, typhi, or paratyphi ), and 5% had another infection. In patients presenting with fever and significant somnolence or seizures, viral or bacterial meningitis or meningoencephalitis must remain on the differential and prompt consideration of lumbar puncture. [2] [22] Viral etiologies include avian influenza, Middle East respiratory syndrome coronavirus, hemorrhagic fever (Ebola virus, Lassa fever, Marburg hemorrhagic fever, Crimean-Congo hemorrhagic fever), yellow fever, dengue, Japanese encephalitis, Rift Valley fever, hepatitis virus (A or B), viral gastroenteritis, and rabies. [22] Bacterial etiologies include anthrax, epidemic typhus, ehrlichiosis, leptospirosis, melioidosis, murine (endemic) typhus, spotted fever group rickettsioses, Q fever, and Yersinia pestis. [22] [2]
The differential in children varies by region, with the most likely etiology being a viral or bacterial infection. In a 2014 study of febrile children in a tropical region, 10.5% were diagnosed with malaria, 62% were diagnosed with a respiratory infection, 13.3% with a systemic bacterial infection (usually staphylococcus or streptococcus bacteremia), and 10.3% with gastroenteritis (viral or bacterial). [23] Urinary tract infection and typhoid may also be considerations. Meningitis must be ruled out in somnolent children. [23]
- Treatment Planning
Artemether 20 mg/ lumefantrine 120 mg Artemether 40 mg/ lumefantrine 240 mg
Table 1. Artemisinin combination therapy (ACT) regimens for treatment of uncomplicated Plasmodium falciparum malaria in nonpregnant adults and children
Quinine: 542 mg base (= 650 mg salt) three times daily for a weekClindamycin: 20 mg base/kg/day (up to 1.8 grams) divided three times daily for a week OR Artemisinin combination therapy can be used as an alternate therapy in the first trimester if the above (more...)
Table 2. Oral regimens for treatment of Plasmodium falciparum malaria in pregnant women regions with c hloroquine-resistant P. falciparum infection.
00 hours: 600 mg base (= 1000 mg salt) 06 hours: 300 mg base (= 500 mg salt)
Table 3. Oral regimens for treatment of Plasmodium falciparum malaria in pregnant women in regions with c hloroquine-sensitive P. falciparum infection
The duration of untreated infection and time to relapse vary by location and species. P. falciparum and P. ovale infections last 2 to 3 weeks and may relapse 6 to 18 months later, usually from a new primary infection. [1] P. vivax infection lasts 3 to 8 weeks and may relapse months to up to 5 years later. [1] P. malariae infection lasts 3 to 24 weeks and may relapse up to 20 years later. [1]
Relapse is a case of recurrent symptoms months to years after the resolution of erythrocytic organisms due to reinfection or hypnozoite activation. [2] [1] Recrudescence is defined as recurrent symptoms within days to weeks of acute illness due to remaining parasitemia after ineffective or incomplete treatment or failed host immune response, more commonly in P. falciparum . [2] [1] Appropriate, complete treatment usually results in a full resolution of symptoms.
The two main determinants reflecting the outcome for both adults and children were the level of consciousness assessed by coma scales and the degree of metabolic acidosis, assessed clinically by breathing pattern or, more precisely, with measurement of bicarbonate, base deficit, and plasma lactate. [32] While the general mortality of treated severe malaria is between 10 to 20%, the mortality in pregnant women reaches approximately 50%. [14]
- Complications
The significant complications of malaria are cerebral malaria, severe malarial anemia, and nephrotic syndrome (NS).
Cerebral malaria accounts for 80% of fatal malaria cases, most often occurring with P. falciparum infection. [1] It presents as slow-onset altered mental status, violent behavior, headache, and extremely high fever (up to 42 degrees C), followed by coma, metabolic acidosis, hypoglycemia, and possibly seizures and death. [1] [4] It most commonly affects children under age 5, with a case fatality rate of 18%. [33] Pathogenesis involves malarial rosettes (one infected erythrocyte surrounded by three uninfected erythrocytes), causing cerebral sequestration and vasodilation, as well as excessive oxygen free radicals, IFN-gamma, and TNF-alpha leading to an extreme inflammatory response. [1] [4] [33] This leads to congestion, decreased perfusion, endothelial activation, impairment of the blood-brain barrier, and cerebral edema, which increases brain volume. [33]
Increased brain volume is the major contributor to mortality in cerebral malaria. In a 2015 study of Malawian children with cerebral malaria, 84% of those who died had severely increased brain volume on MRI; children who survived showed lower initial brain volume or a downtrend over time. [33]
Severe malarial anemia stems from TNF-alpha-mediated mechanisms involving both increased destruction and decreased production of erythrocytes, including cell lysis as parasites replicate and exit erythrocytes, splenic removal and autoimmune lysis of immune-marked erythrocytes, poor iron incorporation into new heme molecules, and bone marrow suppression during severe infection leading to decreased production. [1] [4] Blackwater fever is severe anemia with hemoglobinuria and renal failure in the context of "massive intravascular hemolysis" in the setting of repeat P. falciparum infections treated with chronic quinine; it is rare and thought to be associated with G6PD deficiency. [34]
Nephrotic syndrome occurs secondary to glomerular antigen-antibody complex deposition and presents similarly to membranoproliferative glomerulonephritis with proteinuria and decreased renal function, which may lead to renal failure. Nephrotic syndrome is common in P. malariae and P. knowlesi , possible in P. vivax , and rare in P. falciparum and P. ovale infections. [1]
Additional complications include:
- Bilious remittent fever presents with abdominal pain and persistent vomiting that may lead to severe dehydration, jaundice, and dark urine.
- Algid malaria is an adrenal insufficiency due to parasitic congestion and subsequent necrosis of the adrenal glands.
- Acute respiratory distress syndrome, circulatory collapse, disseminated intravascular coagulation, pulmonary edema, coma, and death. [1]
Malaria infection during pregnancy may result in low birth weight or fetal demise. [1]
- Consultations
Recommended consultations for non-infectious disease experts in the management or prevention of malaria include infectious disease and preventive or travel medicine.
- Deterrence and Patient Education
The recommendation is that patients schedule a pre-travel appointment with a preventive medicine or infectious disease physician for education regarding malaria deterrence. Malaria prevention centers around vector control and chemoprophylaxis while exposed to mosquito-ridden environments.
Vector control is the prevention of mosquito bites by way of insecticide-impregnated bed nets, permethrin treatment of clothing, and DEET application to the skin. [3] The three main prophylactic agents for Plasmodium falciparum are atovaquone-proguanil, doxycycline, and mefloquine. Atovaquone-proguanil is taken once daily during and one week after travel to an endemic region; it suppresses the hepatic stage and does not have approval for pregnancy. [2] Doxycycline is taken once daily during and one month after travel; it suppresses the blood stage. [2] Doxycycline has the added benefit of prophylaxis against Rickettsial disease, Q fever, leptospirosis, and travelers’ diarrhea; however, it may cause gastrointestinal distress, photosensitivity, and increased risk of candida infection. Mefloquine is taken once weekly during and one month after travel; it suppresses the blood stage. [2] It has the benefit of safety in the second and third trimester of pregnancy; however, it has a far higher risk of neuropsychiatric side effects. [2] The US military primarily utilizes doxycycline if susceptibilities are equal. [2] For pregnant women in the first trimester or breastfeeding women, chloroquine or mefloquine prophylaxis are preferable; data regarding the safety of atovaquone-proguanil prophylaxis in pregnancy is limited. [35]
- Enhancing Healthcare Team Outcomes
The timely care of patients diagnosed with malaria and clinically relevant research regarding advancing diagnostic techniques and treatment requires interprofessional teamwork and communication between clinicians, infectious disease experts, pharmacists, nurses, and global health professionals.
Any clinician treating malaria will initiate treatment as outlined above. Still, it is good policy to include an infectious disease specialist and involve an infectious disease board-certified pharmacist, who can also examine the regimen and agents chosen, as well as verify dosing and drug interactions. A nurse with infectious disease specialty training can also help by answering patient questions, serving as a bridge to the treating clinician, and monitoring treatment progress and potential adverse drug reactions. All team members must keep accurate and updated records, so everyone involved in treatment has the same information on the patient's case. If there are any concerns, each team member must be free to communicate with other team members so that appropriate interventions can be started or therapeutic modifications can be implemented. This collaborative interprofessional approach can optimize outcomes for malaria patients. [Level 5]
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Life Cycle of the Malaria Parasite Contributed by Wikimedia Commons, National Institutes of Health (NIH) (Public Domain)
Aedes species mosquito Image courtesy of S Bhimji MD
Blood smear malaria Image courtesy S Bhimji MD
Table 1 - Diagnostic criteria for severe P.falciparum malaria. Contributed by Lara Zekar, MD
Disclosure: Emily Buck declares no relevant financial relationships with ineligible companies.
Disclosure: Nancy Finnigan declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Buck E, Finnigan NA. Malaria. [Updated 2023 Jul 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas. [Cochrane Database Syst Rev. 2022] Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas. Crider K, Williams J, Qi YP, Gutman J, Yeung L, Mai C, Finkelstain J, Mehta S, Pons-Duran C, Menéndez C, et al. Cochrane Database Syst Rev. 2022 Feb 1; 2(2022). Epub 2022 Feb 1.
- Malaria Surveillance - United States, 2016. [MMWR Surveill Summ. 2019] Malaria Surveillance - United States, 2016. Mace KE, Arguin PM, Lucchi NW, Tan KR. MMWR Surveill Summ. 2019 May 17; 68(5):1-35. Epub 2019 May 17.
- Malaria surveillance--United States, 2010. [MMWR Surveill Summ. 2012] Malaria surveillance--United States, 2010. Mali S, Kachur SP, Arguin PM, Division of Parasitic Diseases and Malaria, Center for Global Health, Centers for Disease Control and Prevention (CDC). MMWR Surveill Summ. 2012 Mar 2; 61(2):1-17.
- Review [The reemergence of malaria in Israel?]. [Harefuah. 2004] Review [The reemergence of malaria in Israel?]. Anis E, Pener H, Goldmann D, Leventhal A. Harefuah. 2004 Nov; 143(11):815-9, 838, 837.
- Review Diagnosis and Treatment of the Febrile Child. [Reproductive, Maternal, Newbor...] Review Diagnosis and Treatment of the Febrile Child. Herlihy JM, D’Acremont V, Hay Burgess DC, Hamer DH. Reproductive, Maternal, Newborn, and Child Health: Disease Control Priorities, Third Edition (Volume 2). 2016 Apr 5
Recent Activity
- Malaria - StatPearls Malaria - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers

IMAGES
VIDEO
COMMENTS
P a g e | 1. INTRODUCTION. Malaria is a mosquito-borne infectious disease of humans and other animals caused by. protists (a type of microorganism) of the genus Plasmodium. It begins with a bite ...
1. Introduction. Malaria affected an estimated 219 million people causing 435,000 deaths in 2017 globally. This burden of morbidity and mortality is a result of more than a century of global effort and research aimed at improving the prevention, diagnosis, and treatment of malaria [].Malaria is the most common disease in Africa and some countries in Asia with the highest number of indigenous ...
5.6eported malaria cases in R HBHI countries since 2018 and comparisons with estimated cases 48 6.vestments in malaria programmes and research In 52 6.1unding trends for malaria control and elimination 52F 6.2vestments in malaria-related R&D 56In 7.istribution and coverage of malaria prevention, diagnosis and treatment D 58
Malaria vaccine introduction in endemic countries is a game-changing milestone in the fight against the disease. This article examines the inequity in the global pharmaceutical research, development, manufactu... Floriano Amimo. Malaria Journal 2024 23 :136. Perspective Published on: 6 May 2024.
Further research on the use of mAbs against malaria is needed and should be performed in endemic areas and include at risk groups such as under 5s and pregnant women. The future role of mAbs for malaria prevention will depend on accessibility and the ultimate overall cost of implementation. ... •Malaria vaccine: WHO position paper - March ...
The World malaria report 2022 provides comprehensive data and analysis on the global, regional and country-level progress and challenges in the fight against malaria. It covers topics such as antimalarial drug resistance, urban malaria, malaria vaccine and humanitarian emergencies. Download the full report in PDF format from iris.who.int.
of defeat, the neglect of malaria control efforts and abandonment of research into new tools and approaches. Malaria came back with a vengeance; millions of deaths followed. It took decades for the world to be ready to fight back against malaria. Almost 50 years later, the world once again began to consider the feasibility of eradicating malaria.
The GTS 2020 milestone will be missed by an estimated 37% and, at the current rate of progress, the strategy's 2030 target could be missed by 87%. »Mortality rate:In 2020, the estimate for globally projected malaria deaths per 100 000 population at risk was 9.8 against a GTS target of 7.2 deaths (Fig 8.1 b).
Methods: This review considers data from peer‑reviewed articles and information from the National Malaria Control Programme reports, based on field investigations or samples collected from 1992 to 2015. Peer‑reviewed papers were searched throughout online bibliographic databases PubMed, HINARI and Google Scholar using the following terms:
CDC's strategic research helped develop and evaluate each of the effective tools now used throughout the world to prevent and control malaria: Massive scale-up of these proven interventions in the last decade has led to unprecedented gains in the fight against malaria. From 2000 to 2012, 3.3 million lives were saved globally, and malaria ...
the Southern African International Center of Excellence in Malaria Research. This team gave me to ability to research malaria transmission from all angles. I thank Douglas Norris for helping me understand the vectors and ecologies underlying malaria transmission. I thank Frank Curriero for his guidance on spatial dynamics of disease transmission.
This disease is caused disease, each of which is a genetic disorder affecting red by protozoan parasites belonging to the genus Plasmo- blood cells, are commonly found in malaria endemic dium. The strong negative pressure of the disease has areas [1], and people with these two disorders show re-sistance to malaria.
Despite continuous prevention and control strategies in place, malaria remains a major public health problem in sub-Saharan Africa including Ethiopia. Moreover, prevalence of malaria differs in different geographical settings and epidemiological data were inadequate to assure disease status in the study area. This study was aimed to determine the prevalence of malaria and associated risk ...
DOI: 10.1056/NEJMp2216703. In this documentary video from the New England Journal of Medicine, physicians and scientists from across the world discuss the epidemiology of malaria and outline key ...
1. Introduction. Malaria is an important disease of public health problem caused by Plasmodium parasite belonging to the Apicomplexans [].It is spread when an infected female Anopheles mosquito feeds on human blood [].It is majorly infecting people in the world's tropical and subtropical countries, particularly in sub-Saharan Africa [].The four major malaria parasites causing disease in humans ...
The Technical Strategy was developed in close alignment with the Roll Back Malaria Partnership's Action and Investment to defeat Malaria 2016-2030 (AIM) to ensure shared goals and complementarity. Many thanks to the AIM Task force and Vanessa Racloz for the strong coordination and collaboration.
ward" was made in malaria research, particularly in the fields ofimmuneresponse and chemotherapy inasmuch as a great manycompounds were tested. Ananalysis oftheliterature indicated that, during the period 1971-73, at least 800 papers on malaria research were published. The total number of such papers is nodoubtconsiderably greater, as acertain
The vast majority of papers used modelling to study the effect of climate change (435 papers). There were 72 field study papers, 54 laboratory research reports, 28 ecological case reports, 10 cohort studies and seven other types of study (Figure 3B; multiple study types were possible in a
Methods: In this paper, the international scientific publication on malaria and P. vivax has been analyzed using the Scopus database to try to define global trends in this field of study. Results: It has been shown that events such as the emergence of resistance to certain drugs can break a trend.
Malaria is a climate-sensitive, vector-borne disease that caused 619,000 deaths among 247 million cases in 2021 ( 1 ). This burden is acutely focused on Africa, where 95% of global cases are reported ( 1 ). Reductions in cases in Africa have slowed or even reversed in recent years, attributed in part to a stall in investments in global ...
Great progress has been made in recent years to reduce the high level of suffering caused by malaria worldwide. Notably, the use of insecticide-treated mosquito nets for malaria prevention and the use of artemisinin-based combination therapy (ACT) for malaria treatment have made a significant impact. Nevertheless, the development of resistance to the past and present anti-malarial drugs ...
This paper presents a three-country evaluation of 78 version 1 of the eSPT; version 2, which is currently available for free download, ... 509 and non-malaria related research activities with similar calls for the scope to be 510 extended to include other vector borne diseases. Participants had reportedly shared
Severe malaria is a medical emergency. It is a major cause of preventable childhood death in tropical countries. Severe malaria justifies considerable global investment in malaria control and elimination yet, increasingly, international agencies, funders and policy makers are unfamiliar with it, and so it is overlooked. In sub-Saharan Africa, severe malaria is overdiagnosed in clinical practice.
Epidemiology. Malaria is one of the most prevalent human infections worldwide. Over 40% of the world's population live in malaria-endemic areas. 3 Exact numbers are unknown, but an estimated 300 to 500 million cases and 1.5 to 2.7 million deaths occur each year. 3 Ninety percent of deaths occur in sub-Saharan Africa, the majority involving children less than 5 years of age.
This position paper supersedes the 2022 WHO position paper on malaria vaccines. It includes the updated WHO recommendations on the use of the RTS,S/AS01 and R21/Matrix-M vaccines for the reduction of malaria morbidity and mortality in children living in endemic areas, prioritizing areas of moderate and high malaria transmission. It also incorporates findings from the evaluation of the WHO ...
Malaria is a parasitic infection transmitted by the Anopheles mosquito that leads to acute life-threatening disease and poses a significant global health threat. Two billion people risk contracting malaria annually, including those in 90 endemic countries and 125 million travelers, and 1.5 to 2.7 million people die in a year.[1] The Plasmodium parasite has a multistage lifecycle, which leads ...