Featured Clinical Reviews
- Screening for Atrial Fibrillation: US Preventive Services Task Force Recommendation Statement JAMA Recommendation Statement January 25, 2022
- Evaluating the Patient With a Pulmonary Nodule: A Review JAMA Review January 18, 2022
- Download PDF
- Share X Facebook Email LinkedIn
- Permissions

The FDA and Gene Therapy for Duchenne Muscular Dystrophy
- 1 Institute for Clinical and Economic Review, Harvard Medical School, Boston, Massachusetts
- Research Letter Spending on Targeted Therapies for Duchenne Muscular Dystrophy Liam Bendicksen, BA; Aaron S. Kesselheim, MD, JD, MPH; Benjamin N. Rome, MD, MPH JAMA
- Original Investigation Patient Characteristics in Novel Muscular Dystrophy Drug Trials vs Routine Care Dongzhe Hong, PhD; Jerry Avorn, MD; Richard Wyss, PhD; Aaron S. Kesselheim, MD, JD, MPH JAMA Network Open
The US Food and Drug Administration (FDA) grants accelerated approval that includes the standard of a surrogate outcome that is “reasonably likely” to predict clinical benefit. 1 Gene therapy for Duchenne muscular dystrophy represents a test case for whether the FDA can appropriately apply accelerated approval to a novel gene therapy.
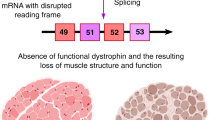
Duchenne muscular dystrophy is a fatal, X-linked neuromuscular disease that results in progressive loss of muscle function. It is caused by alterations in the dystrophin gene ( DMD ) that reduce dystrophin protein production to less than 3% of the normal level. 2 Signs of Duchenne muscular dystrophy usually occur in early childhood. Symptoms include muscle weakness, clumsiness, and difficulty going up and down stairs; untreated children usually progress to a loss of ambulation by 10 years of age. 2 Fatal respiratory or cardiac complications commonly develop in the second or third decade of life. Duchenne muscular dystrophy has a prevalence of 1 in 3500 to 5000 live male births, or about 400 to 600 live male births per year in the US. 2 With treatments such as corticosteroids, assisted ventilation, spinal surgery, and management of cardiomyopathy-related heart failure, some patients are now living into their 30s or 40s.
Read More About
Rind DM. The FDA and Gene Therapy for Duchenne Muscular Dystrophy. JAMA. Published online May 01, 2024. doi:10.1001/jama.2024.5613
Manage citations:
© 2024
Artificial Intelligence Resource Center
Cardiology in JAMA : Read the Latest
Browse and subscribe to JAMA Network podcasts!
Others Also Liked
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 31 August 2023
Therapeutic approaches for Duchenne muscular dystrophy
- Thomas C. Roberts ORCID: orcid.org/0000-0002-3313-7631 1 , 2 , 3 ,
- Matthew J. A. Wood 1 , 2 , 3 &
- Kay E. Davies ORCID: orcid.org/0000-0001-8807-8520 3 , 4
Nature Reviews Drug Discovery volume 22 , pages 917–934 ( 2023 ) Cite this article
6734 Accesses
8 Citations
54 Altmetric
Metrics details
- Drug delivery
- Gene therapy
- Neuroscience
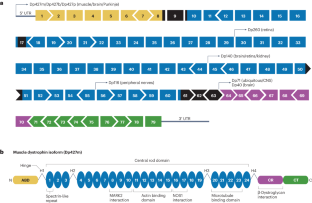
Duchenne muscular dystrophy (DMD) is a monogenic muscle-wasting disorder and a priority candidate for molecular and cellular therapeutics. Although rare, it is the most common inherited myopathy affecting children and so has been the focus of intense research activity. It is caused by mutations that disrupt production of the dystrophin protein, and a plethora of drug development approaches are under way that aim to restore dystrophin function, including exon skipping, stop codon readthrough, gene replacement, cell therapy and gene editing. These efforts have led to the clinical approval of four exon skipping antisense oligonucleotides, one stop codon readthrough drug and one gene therapy product, with other approvals likely soon. Here, we discuss the latest therapeutic strategies that are under development and being deployed to treat DMD. Lessons from these drug development programmes are likely to have a major impact on the DMD field, but also on molecular and cellular medicine more generally. Thus, DMD is a pioneer disease at the forefront of future drug discovery efforts, with these experimental treatments paving the way for therapies using similar mechanisms of action being developed for other genetic diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Therapeutic developments for Duchenne muscular dystrophy
Evaluating the potential of novel genetic approaches for the treatment of Duchenne muscular dystrophy

Genome editing for Duchenne muscular dystrophy: a glimpse of the future?
Hoffman, E. P., Brown, R. H. & Kunkel, L. M. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 51 , 919–928 (1987).
Article CAS PubMed Google Scholar
Moriuchi, T., Kagawa, N., Mukoyama, M. & Hizawa, K. Autopsy analyses of the muscular dystrophies. Tokushima J. Exp. Med. 40 , 83–93 (1993).
CAS PubMed Google Scholar
Chiang, D. Y. et al. Relation of cardiac dysfunction to rhythm abnormalities in patients with Duchenne or Becker muscular dystrophies. Am. J. Cardiol. 117 , 1349–1354 (2016).
Article PubMed Google Scholar
Ishikawa, Y. et al. Duchenne muscular dystrophy: survival by cardio-respiratory interventions. Neuromuscul. Disord. 21 , 47–51 (2011).
Duan, D., Goemans, N., Takeda, S., Mercuri,E. & Aartsma-Rus, A. Duchenne muscular dystrophy Nat. Rev. Dis. Prim. 7 , 14 (2021).
Article Google Scholar
Petrof, B. J., Shrager, J. B., Stedman, H. H., Kelly, A. M. & Sweeney, H. L. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc. Natl Acad. Sci. USA 90 , 3710–3714 (1993).
Article CAS PubMed PubMed Central Google Scholar
Ricotti, V. et al. Long-term benefits and adverse effects of intermittent versus daily glucocorticoids in boys with Duchenne muscular dystrophy. J. Neurol. Neurosurg. Psychiatry 84 , 698–705 (2013).
Birnkrant, D. J. et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: respiratory, cardiac, bone health, and orthopaedic management. Lancet Neurol. 17 , 347–361 (2018).
Article PubMed PubMed Central Google Scholar
Kourakis, S. et al. Standard of care versus new-wave corticosteroids in the treatment of Duchenne muscular dystrophy: can we do better? Orphanet J. Rare Dis. 16 , 117 (2021).
Vestergaard, P. et al. Fracture risk in patients with muscular dystrophy and spinal muscular atrophy. J. Rehabil. Med. 33 , 150–155 (2001).
Hoffman, E. P. et al. Vamorolone trial in Duchenne muscular dystrophy shows dose-related improvement of muscle function. Neurology 93 , e1312–e1323 (2019).
Ervasti, J. M. & Campbell, K. P. Membrane organization of the dystrophin-glycoprotein complex. Cell 66 , 1121–1131 (1991).
Rybakova, I. N., Patel, J. R. & Ervasti, J. M. The dystrophin complex forms a mechanically strong link between the sarcolemma and costameric actin. J. Cell Biol. 150 , 1209–1214 (2000).
Spence, H. J., Dhillon, A. S., James, M. & Winder, S. J. Dystroglycan, a scaffold for the ERK-MAP kinase cascade. EMBO Rep. 5 , 484–489 (2004).
Dumont, N. A. et al. Dystrophin expression in muscle stem cells regulates their polarity and asymmetric division. Nat. Med. 21 , 1455–1463 (2015).
Roberts, T. C. et al. Multi-level omics analysis in a murine model of dystrophin loss and therapeutic restoration. Hum. Mol. Genet. 24 , 6756–6768 (2015).
van Westering, T. L. E. et al. Mutation-independent proteomic signatures of pathological progression in murine models of duchenne muscular dystrophy. Mol. Cell Proteom. 19 , 2047–2067 (2020).
Sandonà, D. & Betto, R. Sarcoglycanopathies: molecular pathogenesis and therapeutic prospects. Expert. Rev. Mol. Med. 11 , e28 (2009).
Consalvi, S. et al. Histone deacetylase inhibitors in the treatment of muscular dystrophies: epigenetic drugs for genetic diseases. Mol. Med. 17 , 457–465 (2011).
Boldrin, L., Zammit, P. S. & Morgan, J. E. Satellite cells from dystrophic muscle retain regenerative capacity. Stem Cell Res. 14 , 20–29 (2015).
Meng, J., Bencze, M., Asfahani, R., Muntoni, F. & Morgan, J. E. The effect of the muscle environment on the regenerative capacity of human skeletal muscle stem cells. Skelet. Muscle 5 , 11 (2015).
Wang, Y. et al. Dystrophin is a tumor suppressor in human cancers with myogenic programs. Nat. Genet. 46 , 601–606 (2014).
Gallia, G. L. et al. Genomic analysis identifies frequent deletions of dystrophin in olfactory neuroblastoma. Nat. Commun. 9 , 5410 (2018).
Bladen, C. L. et al. The TREAT-NMD DMD global database: analysis of more than 7,000 duchenne muscular dystrophy mutations. Hum. Mutat. 36 , 395–402 (2015).
White, S. J. et al. Duplications in the DMD gene. Hum. Mutat. 27 , 938–945 (2006).
Nakamura, A. et al. Deletion of exons 3-9 encompassing a mutational hot spot in the DMD gene presents an asymptomatic phenotype, indicating a target region for multiexon skipping therapy. J. Hum. Genet. 61 , 663–667 (2016).
Nakamura, A. et al. Comparison of the phenotypes of patients harboring in-frame deletions starting at exon 45 in the Duchenne muscular dystrophy gene indicates potential for the development of exon skipping therapy. J. Hum. Genet. 62 , 459–463 (2017).
Muntoni, F., Torelli, S. & Ferlini, A. Dystrophin and mutations: one gene, several proteins, multiple phenotypes. Lancet Neurol. 2 , 731–740 (2003).
England, S. B. et al. Very mild muscular dystrophy associated with the deletion of 46% of dystrophin. Nature 343 , 180–182 (1990).
Matsumura, K. et al. Immunohistochemical analysis of dystrophin-associated proteins in Becker/Duchenne muscular dystrophy with huge in-frame deletions in the NH2-terminal and rod domains of dystrophin. J. Clin. Invest. 93 , 99–105 (1994).
Monaco, A. P., Bertelson, C. J., Liechti-Gallati, S., Moser, H. & Kunkel, L. M. An explanation for the phenotypic differences between patients bearing partial deletions of the DMD locus. Genomics 2 , 90–95 (1988).
Anwar, S., He, M., Lim, K. R. Q., Maruyama, R. & Yokota, T. A genotype-phenotype correlation study of exon skip-equivalent in-frame deletions and exon skip-amenable out-of-frame deletions across the DMD gene to simulate the effects of exon-skipping therapies: a meta-analysis. J. Pers. Med. 11 , 46 (2021).
Koenig, M. et al. The molecular basis for Duchenne versus Becker muscular dystrophy: correlation of severity with type of deletion. Am. J. Hum. Genet. 45 , 498–506 (1989).
CAS PubMed PubMed Central Google Scholar
Malhotra, S. et al. Frame-shift deletions in patients with Duchenne and Becker muscular dystrophy. Science 242 , 755–759 (1988).
Del Rio-Pertuz, G., Morataya, C., Parmar, K., Dubay, S. & Argueta-Sosa, E. Dilated cardiomyopathy as the initial presentation of Becker muscular dystrophy: a systematic review of published cases. Orphanet J. Rare Dis. 17 , 194 (2022).
Aartsma-Rus, A. et al. Theoretic applicability of antisense-mediated exon skipping for Duchenne muscular dystrophy mutations. Hum. Mutat. 30 , 293–299 (2009).
Syed, Y. Y. Eteplirsen: first global approval. Drugs 76 , 1699–1704 (2016).
Heo, Y.-A. Golodirsen: first approval. Drugs 80 , 329–333 (2020).
Shirley, M. Casimersen: first approval. Drugs 81 , 875–879 (2021).
Komaki, H. et al. Systemic administration of the antisense oligonucleotide NS-065/NCNP-01 for skipping of exon 53 in patients with Duchenne muscular dystrophy. Sci. Transl. Med. 10 , eaan0713 (2018).
Clemens, P. R. et al. Safety, tolerability, and efficacy of Viltolarsen in boys with Duchenne muscular dystrophy amenable to exon 53 skipping: a phase 2 randomized clinical trial. JAMA Neurol. 77 , 982–991 (2020).
Dhillon, S. Viltolarsen: first approval. Drugs 80 , 1027–1031 (2020).
Aartsma-Rus, A. & Goemans, N. A sequel to the eteplirsen saga: eteplirsen is approved in the United States but was not approved in Europe. Nucleic Acid. Ther. 29 , 13–15 (2018).
Muntoni, F., Fletcher, S. & Wilton, S. Response to “Railroading at the FDA”. Nat. Biotechnol. 35 , 207–209 (2017).
Aartsma-Rus, A. & Krieg, A. M. FDA approves eteplirsen for Duchenne muscular dystrophy: the next chapter in the eteplirsen saga. Nucleic Acid. Ther. 27 , 1–3 (2017).
Dowling, J. J. Eteplirsen therapy for Duchenne muscular dystrophy: skipping to the front of the line. Nat. Rev. Neurol. 12 , 675–676 (2016).
FDA Briefing Document, Peripheral and Central Nervous System Drugs Advisory Committee Meeting, 22 January 2016, NDA 206488, Eteplirsen (FDA, 2016); https://www.fda.gov/files/advisory%20committees/published/FDA-Briefing-Information-for-the-January-22-2016-Meeting-of-the-Peripheral-and-Central-Nervous-System-Drugs-Advisory-Committee.pdf .
No authors listed. Railroading at the FDA. Nat. Biotechnol. 34 , 1078–1078 (2016).
Charleston, J. S. et al. Eteplirsen treatment for Duchenne muscular dystrophy: exon skipping and dystrophin production. Neurology 90 , e2146–e2154 (2018).
Servais, L. et al. Long-term safety and efficacy data of golodirsen in ambulatory patients with Duchenne muscular dystrophy amenable to exon 53 skipping: a first-in-human, multicenter, two-part, open-label, phase 1/2 trial. Nucleic Acid. Ther. 32 , 29–39 (2022).
Mendell, J. R. et al. Eteplirsen for the treatment of Duchenne muscular dystrophy. Ann. Neurol. 74 , 637–647 (2013).
Mendell, J. R. et al. Longitudinal effect of eteplirsen versus historical control on ambulation in Duchenne muscular dystrophy. Ann. Neurol. 79 , 257–271 (2016).
Wu, B. et al. Dose-dependent restoration of dystrophin expression in cardiac muscle of dystrophic mice by systemically delivered morpholino. Gene Ther. 17 , 132–140 (2010).
Mendell, J. R. et al. Comparison of long-term ambulatory function in patients with Duchenne muscular dystrophy treated with eteplirsen and matched natural history controls. J. Neuromuscul. Dis. 8 , 469–479 (2021).
Roberts, T. C., Langer, R. Wood, M. J. A. Advances in oligonucleotide drug delivery Nat. Rev. Drug Discov. 19 , 673–694 (2020).
Betts, C. et al. Pip6-PMO, a new generation of peptide-oligonucleotide conjugates with improved cardiac exon skipping activity for DMD treatment. Mol. Ther. Nucleic Acids 1 , e38 (2012).
Betts, C. A. et al. Prevention of exercised induced cardiomyopathy following Pip-PMO treatment in dystrophic mdx mice. Sci. Rep. 5 , 8986 (2015).
Sarepta therapeutics reports positive clinical results from phase 2 MOMENTUM study of SRP-5051 in patients with duchenne muscular dystrophy amenable to skipping exon 51. Sarepta Therapeutics (5 May 2021); https://investorrelations.sarepta.com/news-releases/news-release-details/sarepta-therapeutics-reports-positive-clinical-results-phase-2 .
Moulton, H. M. & Moulton, J. D. Morpholinos and their peptide conjugates: therapeutic promise and challenge for Duchenne muscular dystrophy. Biochim. Biophys. Acta 1798 , 2296–2303 (2010).
Amantana, A. et al. Pharmacokinetics, biodistribution, stability and toxicity of a cell-penetrating peptide-morpholino oligomer conjugate. Bioconjug. Chem. 18 , 1325–1331 (2007).
PepGen reports positive data from phase 1 trial of PGN-EDO51 for the treatment of Duchenne muscular dystrophy. PepGen (28 September 2022); https://investors.pepgen.com/news-releases/news-release-details/pepgen-reports-positive-data-phase-1-trial-pgn-edo51-treatment/ .
Kreher, N. et al. P.194 Development of a novel, EEV-conjugated PMO for Duchenne muscular dystrophy. Neuromuscul. Disord. 32 , S126 (2022).
Avidity Biosciences Announces Phase 1/2 EXPLORE44TM Trial of AOC 1044 for Duchenne Muscular Dystrophy Mutations Amenable to Exon 44 Skipping (Avidity Biosciences, 2022); https://aviditybiosciences.investorroom.com/2022-10-11-Avidity-Biosciences-Announces-Phase-1-2-EXPLORE44-TM-Trial-of-AOC-1044-for-Duchenne-Muscular-Dystrophy-Mutations-Amenable-to-Exon-44-Skipping .
Desjardins, C. A. et al. Enhanced exon skipping and prolonged dystrophin restoration achieved by TfR1-targeted delivery of antisense oligonucleotide using FORCE conjugation in mdx mice. Nucleic Acids Res. 50 , 11401–11414 (2022).
Aoki, Y. et al. Bodywide skipping of exons 45–55 in dystrophic mdx52 mice by systemic antisense delivery. Proc. Natl Acad. Sci. USA 109 , 13763–13768 (2012).
Béroud, C. et al. Multiexon skipping leading to an artificial DMD protein lacking amino acids from exons 45 through 55 could rescue up to 63% of patients with Duchenne muscular dystrophy. Hum. Mutat. 28 , 196–202 (2007).
Wave Life Sciences provides positive update on proof-of-concept study for WVE-N531 in Duchenne muscular dystrophy. GlobeNewswire News Room (WAVE Life Science USA, 2022); https://www.globenewswire.com/news-release/2022/12/19/2576214/0/en/Wave-Life-Sciences-Provides-Positive-Update-on-Proof-of-Concept-Study-for-WVE-N531-in-Duchenne-Muscular-Dystrophy.html .
Kandasamy, P. et al. Control of backbone chemistry and chirality boost oligonucleotide splice switching activity. Nucleic Acids Res. 50 , 5443–5466 (2022).
Iwamoto, N. et al. Control of phosphorothioate stereochemistry substantially increases the efficacy of antisense oligonucleotides. Nat. Biotechnol. 35 , 845–851 (2017).
Wan, W. B. et al. Synthesis, biophysical properties and biological activity of second generation antisense oligonucleotides containing chiral phosphorothioate linkages. Nucleic Acids Res. 42 , 13456–13468 (2014).
Wave Life Sciences provides update on phase 1b/2a PRECISION-HD trials - Wave Life Sciences. Wave Life Sciences (2021); https://ir.wavelifesciences.com/news-releases/news-release-details/wave-life-sciences-provides-update-phase-1b2a-precision-hd .
Wave Life Sciences announces discontinuation of suvodirsen development for Duchenne muscular dystrophy. Wave Life Sciences (16 December 2019); https://ir.wavelifesciences.com/news-releases/news-release-details/wave-life-sciences-announces-discontinuation-suvodirsen .
Ito, K. et al. Renadirsen, a novel 2’OMeRNA/ENA® chimera antisense oligonucleotide, induces robust exon 45 skipping for dystrophin in vivo. Curr. Issues Mol. Biol. 43 , 1267–1281 (2021).
Goyenvalle, A. et al. Functional correction in mouse models of muscular dystrophy using exon-skipping tricyclo-DNA oligomers. Nat. Med. 21 , 270–275 (2015).
Zarrouki, F. et al. Partial restoration of brain dystrophin and behavioral deficits by exon skipping in the muscular dystrophy X-linked ( mdx ) mouse. Ann. Neurol. 92 , 213–229 (2022).
De Angelis, F. G. et al. Chimeric snRNA molecules carrying antisense sequences against the splice junctions of exon 51 of the dystrophin pre-mRNA induce exon skipping and restoration of a dystrophin synthesis in Delta 48-50 DMD cells. Proc. Natl Acad. Sci. USA 99 , 9456–9461 (2002).
Goyenvalle, A. et al. Rescue of dystrophic muscle through U7 snRNA-mediated exon skipping. Science 306 , 1796–1799 (2004).
Simmons, T. R. et al. Pre-clinical dose-escalation studies establish a therapeutic range for U7snRNA-mediated DMD exon 2 skipping. Mol. Ther. Methods Clin. Dev. 21 , 325–340 (2021).
Wein, N. et al. Translation from a DMD exon 5 IRES results in a functional dystrophin isoform that attenuates dystrophinopathy in humans and mice. Nat. Med. 20 , 992–1000 (2014).
Nationwide Children’s Hospital Announces Restoration of Full-Length Dystrophin Using dup 2 Gene Therapy Approach (Parent Project Muscular Dystrophy, 2022); https://www.parentprojectmd.org/nationwide-childrens-hospital-announces-restoration-of-full-length-dystrophin-using-duplication-2-gene-therapy-approach/ .
Barton-Davis, E. R., Cordier, L., Shoturma, D. I., Leland, S. E. & Sweeney, H. L. Aminoglycoside antibiotics restore dystrophin function to skeletal muscles of mdx mice. J. Clin. Invest. 104 , 375–381 (1999).
Wagner, K. R. et al. Gentamicin treatment of Duchenne and Becker muscular dystrophy due to nonsense mutations. Ann. Neurol. 49 , 706–711 (2001).
Politano, L. et al. Gentamicin administration in Duchenne patients with premature stop codon. Preliminary results. Acta Myol. 22 , 15–21 (2003).
Malik, V. et al. Gentamicin-induced readthrough of stop codons in Duchenne muscular dystrophy. Ann. Neurol. 67 , 771–780 (2010).
Hayward, R. S. et al. Adverse effects of a single dose of gentamicin in adults: a systematic review. Br. J. Clin. Pharmacol. 84 , 223–238 (2018).
Welch, E. M. et al. PTC124 targets genetic disorders caused by nonsense mutations. Nature 447 , 87–91 (2007).
Ryan, N. J. Ataluren: first global approval. Drugs 74 , 1709–1714 (2014).
Bushby, K. et al. Ataluren treatment of patients with nonsense mutation dystrophinopathy. Muscle Nerve 50 , 477–487 (2014).
McDonald, C. M. et al. Ataluren in patients with nonsense mutation Duchenne muscular dystrophy (ACT DMD): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 390 , 1489–1498 (2017).
No authors listed.Duchenne drug clings on for FDA nod Nat. Biotechnol. 35 , 999 (2017).
Campbell, C. et al. Meta-analyses of ataluren randomized controlled trials in nonsense mutation Duchenne muscular dystrophy. J. Comp. Eff. Res. 9 , 973–984 (2020).
Auld, D. S. et al. Molecular basis for the high-affinity binding and stabilization of firefly luciferase by PTC124. Proc. Natl Acad. Sci. USA 107 , 4878–4883 (2010).
McElroy, S. P. et al. A lack of premature termination codon read-through efficacy of PTC124 (Ataluren) in a diverse array of reporter assays. PLoS Biol. 11 , e1001593 (2013).
Halbert, C. L., Rutledge, E. A., Allen, J. M., Russell, D. W. & Miller, A. D. Repeat transduction in the mouse lung by using adeno-associated virus vectors with different serotypes. J. Virol. 74 , 1524–1532 (2000).
Gruntman, A. M. et al. Gene transfer in skeletal and cardiac muscle using recombinant adeno-associated virus. Curr. Protoc. Microbiol. https://doi.org/10.1002/9780471729259.mc14d03s28 (2013).
Qiao, C., Koo, T., Li, J., Xiao, X. & Dickson, J. G. Gene therapy in skeletal muscle mediated by adeno-associated virus vectors. Methods Mol. Biol. 807 , 119–140 (2011).
Gregorevic, P. et al. Systemic delivery of genes to striated muscles using adeno-associated viral vectors. Nat. Med. 10 , 828–834 (2004).
Duan, D. Systemic AAV micro-dystrophin gene therapy for duchenne muscular dystrophy. Mol. Ther. 26 , 2337–2356 (2018).
Bourdon, A. et al. Evaluation of the dystrophin carboxy-terminal domain for micro-dystrophin gene therapy in cardiac and skeletal muscles in the DMDmdx rat model. Gene Ther. 29 , 520–535 (2022).
Cox, G. A. et al. Overexpression of dystrophin in transgenic mdx mice eliminates dystrophic symptoms without toxicity. Nature 364 , 725–729 (1993).
Potter, R. A. et al. Dose-escalation study of systemically delivered rAAVrh74.MHCK7.micro-dystrophin in the mdx mouse model of duchenne muscular dystrophy. Hum. Gene Ther. 32 , 375–389 (2021).
Yue, Y. et al. Safe and bodywide muscle transduction in young adult Duchenne muscular dystrophy dogs with adeno-associated virus. Hum. Mol. Genet. 24 , 5880–5890 (2015).
Le Guiner, C. et al. Long-term microdystrophin gene therapy is effective in a canine model of Duchenne muscular dystrophy. Nat. Commun. 8 , 16105 (2017).
Salva, M. Z. et al. Design of tissue-specific regulatory cassettes for high-level rAAV-mediated expression in skeletal and cardiac muscle. Mol. Ther. 15 , 320–329 (2007).
Chicoine, L. G. et al. Vascular delivery of rAAVrh74.MCK.GALGT2 to the gastrocnemius muscle of the rhesus macaque stimulates the expression of dystrophin and laminin α2 surrogates. Mol. Ther. 22 , 713–724 (2014).
Zygmunt, D. A., Crowe, K. E., Flanigan, K. M. & Martin, P. T. Comparison of serum rAAV serotype-specific antibodies in patients with duchenne muscular dystrophy, becker muscular dystrophy, inclusion body myositis, or GNE myopathy. Hum. Gene Ther. 28 , 737–746 (2017).
Mendell, J. R. et al. Assessment of systemic delivery of rAAVrh74.MHCK7.micro-dystrophin in children with duchenne muscular dystrophy: a nonrandomized controlled trial. JAMA Neurol. 77 , 1122–1131 (2020).
Willcocks, R. J. et al. Assessment of rAAVrh.74.MHCK7.micro-dystrophin gene therapy using magnetic resonance imaging in children with duchenne muscular dystrophy. JAMA Netw. Open 4 , e2031851 (2021).
Mendell, J. et al. A multicenter randomized, double-blind, placebo-controlled, gene-delivery clinical trial of rAAVrh74.MHCK7.micro-dystrophin for Duchenne muscular dystrophy [Abstr.]. Neurology 96 (Suppl. 15), 4478 (2021).
Google Scholar
Sarepta Therapeutics announces top-line results for part 1 of study 102 evaluating SRP-9001, its investigational gene therapy for the treatment of Duchenne muscular dystrophy. Sarepta Therapeutics (7 Junuary 2021); https://investorrelations.sarepta.com/news-releases/news-release-details/sarepta-therapeutics-announces-top-line-results-part-1-study-102 .
Sarepta Therapeutics’ investigational gene therapy SRP-9001 for Duchenne muscular dystrophy demonstrates significant functional improvements across multiple studies. Sarepta Therapeutics (6 July 2022); https://investorrelations.sarepta.com/news-releases/news-release-details/sarepta-therapeutics-investigational-gene-therapy-srp-9001 .
Sarepta Therapeutics announces that U.S. FDA has accepted for filing and granted priority review for the Biologics License Application for SRP-9001, Sarepta’s gene therapy for the treatment of ambulant individuals with Duchenne muscular dystrophy. Sarepta Therapeutics (28 November 2022); https://investorrelations.sarepta.com/news-releases/news-release-details/sarepta-therapeutics-announces-us-fda-has-accepted-filing-and .
Sarepta Therapeutics announces FDA approval of ELEVIDYS, the first gene therapy to treat Duchenne muscular dystrophy. Sarepta Therapeutics (22 June 2023); https://investorrelations.sarepta.com/news-releases/news-release-details/sarepta-therapeutics-announces-fda-approval-elevidys-first-gene .
Philippidis, A. After patient death, FDA places hold on Pfizer Duchenne muscular dystrophy gene therapy trial. Hum. Gene Ther. 33 , 111–115 (2022).
Article CAS Google Scholar
Philippidis, A. Pfizer eyes resuming phase III enrollment, investigates phase Ib death tied to Duchenne muscular dystrophy candidate. Hum. Gene Ther. 33 , 215–217 (2022).
Pfizer’s new phase 1b results of gene therapy in ambulatory boys with Duchenne muscular dystrophy (DMD) support advancement into pivotal phase 3 study. Pfizer (15 May 2020); https://www.pfizer.com/news/press-release/press-release-detail/pfizers-new-phase-1b-results-gene-therapy-ambulatory-boys .
Philippidis, A. FDA lifts clinical hold on Pfizer DMD gene therapy linked to patient death. GEN - Genetic Engineering and Biotechnology News (28 April 2022); https://www.genengnews.com/topics/genome-editing/gene-therapy/fda-lifts-clinical-hold-on-pfizer-dmd-gene-therapy-linked-to-patient-death/ .
Pfizer announces amendment to ongoing gene therapy phase III trial. Parent Project Muscular Dystrophy (28 September 2021); https://www.parentprojectmd.org/pfizer-announces-amendment-to-ongoing-gene-therapy-phase-iii-trial/ .
Collaborative analysis reveals ‘class effect’ in DMD safety issues. BioSpace (19 May 2022); https://www.biospace.com/article/pfizer-sarepta-genethon-solid-bio-team-up-to-fight-dmd/ .
Solid Biosciences provides SGT-001 program update. Solid Biosciences (19 November 2019); https://www.solidbio.com/about/media/press-releases/solid-biosciences-provides-sgt-001-program-update .
Solid Biosciences announces FDA lifts clinical hold on IGNITE DMD clinical trial. Solid Biosciences (1 October 2020); https://www.solidbio.com/about/media/press-releases/solid-biosciences-announces-fda-lifts-clinical-hold-on-ignite-dmd-clinical-trial .
Solid Biosciences reports fourth quarter and full-year 2021 financial results and 2-year efficacy and safety data from the ongoing phase I/II IGNITE DMD clinical trial of SGT-001. Solid Biosciences (14 March 2022); https://www.solidbio.com/about/media/press-releases/solid-biosciences-reports-fourth-quarter-and-full-year-2021-financial-results-and-2-year-efficacy-and-safety-data-from-the-ongoing-phase-i-ii-ignite-dmd-clinical-trial-of-sgt-001 .
High-dose AAV gene therapy deaths. Nat. Biotechnol. 38 , 910 (2020).
Lysogene confirms child’s death in phase II/III gene therapy trial. GEN (26 October 2020); https://www.genengnews.com/news/lysogene-confirms-childs-death-in-phase-ii-iii-gene-therapy-trial/ .
Reuters. Novartis reports Zolgensma caused two deaths from liver failure. Reuters (11 August 2022); https://www.reuters.com/business/healthcare-pharmaceuticals/novartis-reports-zolgensma-caused-two-deaths-liver-failure-2022-08-11/ .
Hinderer, C. et al. Severe toxicity in nonhuman primates and piglets following high-dose intravenous administration of an adeno-associated virus vector expressing human SMN. Hum. Gene Ther. 29 , 285–298 (2018).
Hale, C. Solid Bio Sees Yet Another Clinical Hold for its DMD Gene Therapy (Fierce Biotech, 2019); https://www.fiercebiotech.com/biotech/solid-bio-sees-yet-another-clinical-hold-for-its-dmd-gene-therapy .
Jeune, V. L., Joergensen, J. A., Hajjar, R. J. & Weber, T. Pre-existing anti–adeno-associated virus antibodies as a challenge in AAV gene therapy. Hum. Gene Ther. Methods 24 , 59–67 (2013).
Article PubMed Central Google Scholar
Mendell, J. R. et al. Dystrophin immunity in Duchenne’s muscular dystrophy. N. Engl. J. Med. 363 , 1429–1437 (2010).
Li, N. et al. The effect of immunomodulatory treatments on anti-Dystrophin immune response after AAV gene therapy in dystrophin deficient mdx mice. J. Neuromuscul. Dis. 8 , S325–S340 (2021).
Rivera, V. M. et al. Long-term pharmacologically regulated expression of erythropoietin in primates following AAV-mediated gene transfer. Blood 105 , 1424–1430 (2005).
Penaud-Budloo, M. et al. Adeno-associated virus vector genomes persist as episomal chromatin in primate muscle. J. Virol. 82 , 7875–7885 (2008).
Le Hir, M. et al. AAV genome loss from dystrophic mouse muscles during AAV-U7 snRNA-mediated exon-skipping therapy. Mol. Ther. 21 , 1551–1558 (2013).
Das, A. et al. Epigenetic silencing of recombinant adeno-associated virus genomes by NP220 and the HUSH complex. J. Virol. 96 , e0203921 (2022).
Mollard, A. et al. Muscle regeneration affects adeno associated virus 1 mediated transgene transcription. Sci. Rep. 12 , 9674 (2022).
Morgan, J. E., Hoffman, E. P. & Partridge, T. A. Normal myogenic cells from newborn mice restore normal histology to degenerating muscles of the mdx mouse. J. Cell Biol. 111 , 2437–2449 (1990).
Partridge, T. A., Morgan, J. E., Coulton, G. R., Hoffman, E. P. & Kunkel, L. M. Conversion of mdx myofibres from dystrophin-negative to -positive by injection of normal myoblasts. Nature 337 , 176–179 (1989).
Garcia, S. M. et al. High-yield purification, preservation, and serial transplantation of human satellite cells. Stem Cell Rep. 10 , 1160–1174 (2018).
Ferrari, G. et al. Muscle regeneration by bone marrow-derived myogenic progenitors. Science 279 , 1528–1530 (1998).
Gussoni, E. et al. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature 401 , 390–394 (1999).
Cossu, G. et al. Intra-arterial transplantation of HLA-matched donor mesoangioblasts in Duchenne muscular dystrophy. EMBO Mol. Med. 7 , 1513–1528 (2015).
Dellavalle, A. et al. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat. Cell Biol. 9 , 255–267 (2007).
Torrente, Y. et al. Autologous transplantation of muscle-derived CD133+ stem cells in Duchenne muscle patients. Cell Transpl. 16 , 563–577 (2007).
Young, C. S. et al. A single CRISPR-Cas9 deletion strategy that targets the majority of DMD patients restores dystrophin function in hiPSC-derived muscle cells. Cell Stem Cell 18 , 533–540 (2016).
Skuk, D. et al. Dystrophin expression in muscles of duchenne muscular dystrophy patients after high-density injections of normal myogenic cells. J. Neuropathol. Exp. Neurol. 65 , 371–386 (2006).
Motohashi, N., Shimizu-Motohashi, Y., Roberts, T. C. & Aoki, Y. Potential therapies using myogenic stem cells combined with bio-engineering approaches for treatment of muscular dystrophies. Cells 8 , 1066 (2019).
Skuk, D. & Tremblay, J. P. Myoblast transplantation: the current status of a potential therapeutic tool for myopathies. J. Muscle Res. Cell Motil. 24 , 285–300 (2003).
Taylor, M. et al. Cardiac and skeletal muscle effects in the randomized HOPE-Duchenne trial. Neurology 92 , e866–e878 (2019).
McDonald, C. M. et al. Repeated intravenous cardiosphere-derived cell therapy in late-stage Duchenne muscular dystrophy (HOPE-2): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 399 , 1049–1058 (2022).
Hanson, B., Wood, M. J. A. & Roberts, T. C. Molecular correction of Duchenne muscular dystrophy by splice modulation and gene editing. RNA Biol. 18 , 1048–1062 (2021).
Cong, L. et al. Multiplex genome engineering using CRISPR/Cas systems. Science 339 , 819–823 (2013).
Tabebordbar, M. et al. In vivo gene editing in dystrophic mouse muscle and muscle stem cells. Science 351 , 407–411 (2016).
Nelson, C. E. et al. In vivo genome editing improves muscle function in a mouse model of Duchenne muscular dystrophy. Science 351 , 403–407 (2016).
Long, C. et al. Postnatal genome editing partially restores dystrophin expression in a mouse model of muscular dystrophy. Science 351 , 400–403 (2016).
Amoasii, L. et al. Single-cut genome editing restores dystrophin expression in a new mouse model of muscular dystrophy. Sci. Transl. Med. 9 , eaan8081 (2017).
Arnett, A. L. et al. Adeno-associated viral (AAV) vectors do not efficiently target muscle satellite cells. Mol. Ther. Methods Clin. Dev. 1 , 14038 (2014).
Nance, M. E. et al. AAV9 edits muscle stem cells in normal and dystrophic adult mice. Mol. Ther. 27 , 1568–1585 (2019).
Kwon, J. B. et al. In vivo gene editing of muscle stem cells with adeno-associated viral vectors in a mouse model of Duchenne muscular dystrophy. Mol. Ther. Methods Clin. Dev. 19 , 320–329 (2020).
Ousterout, D. G. et al. Multiplex CRISPR/Cas9-based genome editing for correction of dystrophin mutations that cause Duchenne muscular dystrophy. Nat. Commun. 6 , 6244 (2015).
Liao, H.-K. et al. In vivo target gene activation via CRISPR/Cas9-mediated trans-epigenetic modulation. Cell 171 , 1495–1507.e15 (2017).
Gilbert, L. A. et al. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell 154 , 442–451 (2013).
To Our Community: An Update On Our CRD-TMH-001 Clinical Trial (Cure Rare Disease, 2022); https://www.cureraredisease.org/blog-posts/to-our-community-an-update-on-our-crd-tmh-001-clinical-trial .
Lek, A. et al. Unexpected death of a Duchenne muscular dystrophy patient in an N-of-1 Trial of rAAV9-delivered CRISPR-transactivator. Preprint at https://doi.org/10.1101/2023.05.16.23289881 (2023).
Pipeline and Progress (Cure Rare Disease, accessed 2023); https://www.cureraredisease.org/our-approach/pipeline-and-progress#section_1 .
Hanson, B. et al. Non-uniform dystrophin re-expression after CRISPR-mediated exon excision in the dystrophin/utrophin double-knockout mouse model of DMD. Mol. Ther. - Nucleic Acids 30 , 379–397 (2022).
Simhadri, V. L. et al. Prevalence of pre-existing antibodies to CRISPR-associated nuclease Cas9 in the USA population. Mol. Ther. Methods Clin. Dev. 10 , 105–112 (2018).
Charlesworth, C. T. et al. Identification of preexisting adaptive immunity to Cas9 proteins in humans. Nat. Med. 25 , 249–254 (2019).
Wagner, D. L. et al. High prevalence of Streptococcus pyogenes Cas9-reactive T cells within the adult human population. Nat. Med. 25 , 242–248 (2019).
Bengtsson, N. E. et al. Muscle-specific CRISPR/Cas9 dystrophin gene editing ameliorates pathophysiology in a mouse model for Duchenne muscular dystrophy. Nat. Commun. 8 , 14454 (2017).
Zhang, Y. et al. CRISPR-Cpf1 correction of muscular dystrophy mutations in human cardiomyocytes and mice. Sci. Adv. 3 , e1602814 (2017).
Porto, E. M., Komor, A. C., Slaymaker, I. M. & Yeo, G. W. Base editing: advances and therapeutic opportunities. Nat. Rev. Drug. Discov. 19 , 839–859 (2020).
Xu, L. et al. Efficient precise in vivo base editing in adult dystrophic mice. Nat. Commun. 12 , 3719 (2021).
Dianov, G. L. & Hübscher, U. Mammalian base excision repair: the forgotten archangel. Nucleic Acids Res. 41 , 3483–3490 (2013).
Ryu, S.-M. et al. Adenine base editing in mouse embryos and an adult mouse model of Duchenne muscular dystrophy. Nat. Biotechnol. 36 , 536–539 (2018).
Chemello, F. et al. Precise correction of Duchenne muscular dystrophy exon deletion mutations by base and prime editing. Sci. Adv. 7 , eabg4910 (2021).
Anzalone, A. V. et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 576 , 149–157 (2019).
Blake, D. J., Tinsley, J. M. & Davies, K. E. Utrophin: a structural and functional comparison to dystrophin. Brain Pathol. 6 , 37–47 (1996).
Tinsley, J. M. et al. Primary structure of dystrophin-related protein. Nature 360 , 591–593 (1992).
Love, D. R. et al. An autosomal transcript in skeletal muscle with homology to dystrophin. Nature 339 , 55–58 (1989).
Anthony, K. et al. Biochemical characterization of patients with in-frame or out-of-frame DMD deletions pertinent to exon 44 or 45 skipping. JAMA Neurol. 71 , 32–40 (2014).
Matsumura, K., Ervasti, J. M., Ohlendieck, K., Kahl, S. D. & Campbell, K. P. Association of dystrophin-related protein with dystrophin-associated proteins in mdx mouse muscle. Nature 360 , 588–591 (1992).
Deconinck, A. E. et al. Utrophin-dystrophin-deficient mice as a model for Duchenne muscular dystrophy. Cell 90 , 717–727 (1997).
Grady, R. M. et al. Skeletal and cardiac myopathies in mice lacking utrophin and dystrophin: a model for Duchenne muscular dystrophy. Cell 90 , 729–738 (1997).
Tinsley, J. et al. Expression of full-length utrophin prevents muscular dystrophy in mdx mice. Nat. Med. 4 , 1441–1444 (1998).
Squire, S. et al. Prevention of pathology in mdx mice by expression of utrophin: analysis using an inducible transgenic expression system. Hum. Mol. Genet. 11 , 3333–3344 (2002).
Fisher, R. et al. Non-toxic ubiquitous over-expression of utrophin in the mdx mouse. Neuromuscul. Disord. 11 , 713–721 (2001).
Song, Y. et al. Non-immunogenic utrophin gene therapy for the treatment of muscular dystrophy animal models. Nat. Med. 25 , 1505–1511 (2019).
Chancellor, D. R. et al. Discovery of 2-arylbenzoxazoles as upregulators of utrophin production for the treatment of Duchenne muscular dystrophy. J. Med. Chem. 54 , 3241–3250 (2011).
Tinsley, J. M. et al. Daily treatment with SMTC1100, a novel small molecule utrophin upregulator, dramatically reduces the dystrophic symptoms in the mdx mouse. PLoS ONE 6 , e19189 (2011).
Muntoni, F. et al. A phase 1b trial to assess the pharmacokinetics of ezutromid in pediatric Duchenne muscular dystrophy patients on a balanced diet. Clin. Pharmacol. Drug Dev. 8 , 922–933 (2019).
Wilkinson, I. V. L. et al. Chemical proteomics and phenotypic profiling identifies the aryl hydrocarbon receptor as a molecular target of the utrophin modulator ezutromid. Angew. Chem. Int. Ed. 59 , 2420–2428 (2020).
Guiraud, S. et al. Second-generation compound for the modulation of utrophin in the therapy of DMD. Hum. Mol. Genet. 24 , 4212–4224 (2015).
Tinsley, J. M. et al. Amelioration of the dystrophic phenotype of mdx mice using a truncated utrophin transgene. Nature 384 , 349–353 (1996).
Deconinck, N. et al. Expression of truncated utrophin leads to major functional improvements in dystrophin-deficient muscles of mice. Nat. Med. 3 , 1216–1221 (1997).
Odom, G. L., Gregorevic, P., Allen, J. M., Finn, E. & Chamberlain, J. S. Microutrophin delivery through rAAV6 increases lifespan and improves muscle function in dystrophic dystrophin/utrophin-deficient mice. Mol. Ther. 16 , 1539–1545 (2008).
Sengupta, K. et al. Genome editing-mediated utrophin upregulation in Duchenne muscular dystrophy stem cells. Mol. Ther. Nucleic Acids 22 , 500–509 (2020).
Pisani, C. et al. Utrophin up-regulation by artificial transcription factors induces muscle rescue and impacts the neuromuscular junction in mdx mice. Biochim. Biophys. Acta Mol. Basis Dis. 1864 , 1172–1182 (2018).
Li, D. et al. Sarcolemmal nNOS anchoring reveals a qualitative difference between dystrophin and utrophin. J. Cell Sci. 123 , 2008–2013 (2010).
Belanto, J. J. et al. Microtubule binding distinguishes dystrophin from utrophin. Proc. Natl Acad. Sci. USA 111 , 5723–5728 (2014).
Markati, T., De Waele, L., Schara-Schmidt, U. & Servais, L. Lessons learned from discontinued clinical developments in Duchenne muscular dystrophy. Front. Pharmacol. 12 , 735912 (2021).
Markati, T. et al. Emerging therapies for Duchenne muscular dystrophy. Lancet Neurol. 21 , 814–829 (2022).
Italfarmaco Group Announces Positive Topline Data From Phase 3 Trial Showing Beneficial Effect Of Givinostat in Patients with Duchenne Muscular Dystrophy (Businesswire, 2022); https://www.businesswire.com/news/home/20220625005001/en/Italfarmaco-Group-Announces-Positive-Topline-Data-from-Phase-3-Trial-Showing-Beneficial-Effect-of-Givinostat-in-Patients-with-Duchenne-Muscular-Dystrophy .
Colussi, C. et al. HDAC2 blockade by nitric oxide and histone deacetylase inhibitors reveals a common target in Duchenne muscular dystrophy treatment. Proc. Natl Acad. Sci. USA 105 , 19183–19187 (2008).
Bettica, P. et al. Histological effects of givinostat in boys with Duchenne muscular dystrophy. Neuromuscul. Disord. 26 , 643–649 (2016).
Webster, C., Silberstein, L., Hays, A. P. & Blau, H. M. Fast muscle fibers are preferentially affected in Duchenne muscular dystrophy. Cell 52 , 503–513 (1988).
Petrof, B. J. et al. Adaptations in myosin heavy chain expression and contractile function in dystrophic mouse diaphragm. Am. J. Physiol. 265 , C834–C841 (1993).
Oldfors, A. Hereditary myosin myopathies. Neuromuscul. Disord. 17 , 355–367 (2007).
Clinical Trials (Edgewise, accessed 2023); https://edgewisetx.com/clinical-trials .
Cordova, G., Negroni, E., Cabello-Verrugio, C., Mouly, V. & Trollet, C. Combined therapies for Duchenne muscular dystrophy to optimize treatment efficacy. Front. Genet. 9 , 114 (2018).
Verhaart, I. E. C. et al. Prednisolone treatment does not interfere with 2’- O -methyl phosphorothioate antisense-mediated exon skipping in Duchenne muscular dystrophy. Hum. Gene Ther. 23 , 262–273 (2012).
Peccate, C. et al. Antisense pre-treatment increases gene therapy efficacy in dystrophic muscles. Hum. Mol. Genet. 25 , 3555–3563 (2016).
Kendall, G. C. et al. Dantrolene enhances antisense-mediated exon skipping in human and mouse models of Duchenne muscular dystrophy. Sci. Transl. Med. 4 , 164ra160 (2012).
Bizot, F. et al. Histone deacetylase inhibitors improve antisense-mediated exon-skipping efficacy in mdx mice. Mol. Ther. Nucleic Acids 30 , 606–620 (2022).
Guiraud, S. et al. The potential of utrophin and dystrophin combination therapies for Duchenne muscular dystrophy. Hum. Mol. Genet. 28 , 2189–2200 (2019).
Hayashita-Kinoh, H. et al. Improved transduction of canine X-linked muscular dystrophy with rAAV9-microdystrophin via multipotent MSC pretreatment. Mol. Ther. Methods Clin. Dev. 20 , 133–141 (2021).
Roberts, T. C. The microRNA machinery. Adv. Exp. Med. Biol. 887 , 15–30 (2015).
Greco, S. et al. Common micro-RNA signature in skeletal muscle damage and regeneration induced by Duchenne muscular dystrophy and acute ischemia. FASEB J. 23 , 3335–3346 (2009).
Roberts, T. C. et al. Expression analysis in multiple muscle groups and serum reveals complexity in the microRNA transcriptome of the mdx mouse with implications for therapy. Mol. Ther. Nucleic Acids 1 , e39 (2012).
Cacchiarelli, D. et al. miR-31 modulates dystrophin expression: new implications for Duchenne muscular dystrophy therapy. EMBO Rep. 12 , 136–141 (2011).
Fiorillo, A. A. et al. TNF-α-induced microRNAs control dystrophin expression in becker muscular dystrophy. Cell Rep. 12 , 1678–1690 (2015).
Basu, U. et al. Translational regulation of utrophin by miRNAs. PLoS ONE 6 , e29376 (2011).
Mishra, M. K., Loro, E., Sengupta, K., Wilton, S. D. & Khurana, T. S. Functional improvement of dystrophic muscle by repression of utrophin: let-7c interaction. PLoS ONE 12 , e0182676 (2017).
Abmayr, S., Gregorevic, P., Allen, J. M. & Chamberlain, J. S. Phenotypic improvement of dystrophic muscles by rAAV/microdystrophin vectors is augmented by Igf1 codelivery. Mol. Ther. 12 , 441–450 (2005).
Dumonceaux, J. et al. Combination of myostatin pathway interference and dystrophin rescue enhances tetanic and specific force in dystrophic mdx mice. Mol. Ther. 18 , 881–887 (2010).
Malerba, A. et al. Dual myostatin and dystrophin exon skipping by morpholino nucleic acid oligomers conjugated to a cell-penetrating peptide is a promising therapeutic strategy for the treatment of Duchenne muscular dystrophy. Mol. Ther. Nucleic Acids 1 , e62 (2012).
Rodino-Klapac, L. R. et al. Micro-dystrophin and follistatin co-delivery restores muscle function in aged DMD model. Hum. Mol. Genet. 22 , 4929–4937 (2013).
Mariot, V. et al. Downregulation of myostatin pathway in neuromuscular diseases may explain challenges of anti-myostatin therapeutic approaches. Nat. Commun. 8 , 1859 (2017).
Godfrey, C. et al. How much dystrophin is enough: the physiological consequences of different levels of dystrophin in the mdx mouse. Hum. Mol. Genet. 24 , 4225–4237 (2015).
van den Bergen, J. C. et al. Dystrophin levels and clinical severity in Becker muscular dystrophy patients. J. Neurol. Neurosurg. Psychiatry 85 , 747–753 (2014).
Hoffman, E. P. et al. Improved diagnosis of Becker muscular dystrophy by dystrophin testing. Neurology 39 , 1011–1017 (1989).
van Westering, T. L. E. et al. Uniform sarcolemmal dystrophin expression is required to prevent extracellular microRNA release and improve dystrophic pathology. J. Cachexia Sarcopenia Muscle 11 , 578–593 (2020).
Chwalenia, K. et al. Exon skipping induces uniform dystrophin rescue with dose-dependent restoration of serum miRNA biomarkers and muscle biophysical properties. Mol. Ther. Nucleic Acids 29 , 955–968 (2022).
Dangouloff, T. & Servais, L. Clinical evidence supporting early treatment of patients with spinal muscular atrophy: current perspectives. Ther. Clin. Risk Manag. 15 , 1153–1161 (2019).
Thomas, S. et al. Time to diagnosis of Duchenne muscular dystrophy remains unchanged: findings from the Muscular Dystrophy Surveillance, Tracking, and Research Network, 2000-2015. Muscle Nerve 66 , 193–197 (2022).
Download references
Author information
Authors and affiliations.
Institute of Developmental and Regenerative Medicine, University of Oxford, Oxford, UK
Thomas C. Roberts & Matthew J. A. Wood
Department of Paediatrics, University of Oxford, Oxford, UK
MDUK Oxford Neuromuscular Centre, Oxford, UK
Thomas C. Roberts, Matthew J. A. Wood & Kay E. Davies
Department of Physiology, Anatomy and Genetics, University of Oxford, Oxford, UK
Kay E. Davies
You can also search for this author in PubMed Google Scholar
Contributions
The manuscript was conceived by K.E.D. and T.C.R. The first draft was written by T.C.R. All authors researched data for the article. All authors contributed substantially to discussion of the content and edited the manuscript before submission.
Corresponding authors
Correspondence to Thomas C. Roberts or Kay E. Davies .
Ethics declarations
Competing interests.
K.E.D. is a member of the scientific advisory board of Sarepta Therapeutics. M.J.A.W. is an adviser and shareholder in PepGen Ltd and Evox Therapeutics. T.C.R. declares no financial competing interests.
Peer review
Peer review information.
Nature Reviews Drug Discovery thanks Alessandra Ferlini, Oxana Ibraghimov-Beskrovnaya and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Roberts, T.C., Wood, M.J.A. & Davies, K.E. Therapeutic approaches for Duchenne muscular dystrophy. Nat Rev Drug Discov 22 , 917–934 (2023). https://doi.org/10.1038/s41573-023-00775-6
Download citation
Accepted : 28 July 2023
Published : 31 August 2023
Issue Date : November 2023
DOI : https://doi.org/10.1038/s41573-023-00775-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Translational Research newsletter — top stories in biotechnology, drug discovery and pharma.
Advertisement
Duchenne muscular dystrophy: pathogenesis and promising therapies
- Published: 01 June 2023
- Volume 270 , pages 3733–3749, ( 2023 )
Cite this article

- Mengyuan Chang 1 na1 ,
- Yong Cai 2 na1 ,
- Zihui Gao 1 na1 ,
- Xin Chen 3 ,
- Boya Liu 1 ,
- Cheng Zhang 1 ,
- Weiran Yu 4 ,
- Qianqian Cao 1 ,
- Yuntian Shen 1 ,
- Xinlei Yao 1 ,
- Xiaoyang Chen 5 &
- Hualin Sun 1 , 6
2788 Accesses
8 Citations
1 Altmetric
Explore all metrics
Duchenne muscular dystrophy (DMD) is a severe, progressive, muscle-wasting disease, characterized by progressive deterioration of skeletal muscle that causes rapid loss of mobility. The failure in respiratory and cardiac muscles is the underlying cause of premature death in most patients with DMD. Mutations in the gene encoding dystrophin result in dystrophin deficiency, which is the underlying pathogenesis of DMD. Dystrophin-deficient myocytes are dysfunctional and vulnerable to injury, triggering a series of subsequent pathological changes. In this review, we detail the molecular mechanism of DMD, dystrophin deficiency-induced muscle cell damage (oxidative stress injury, dysregulated calcium homeostasis, and sarcolemma instability) and other cell damage and dysfunction (neuromuscular junction impairment and abnormal differentiation of muscle satellite). We also describe aberrant function of other cells and impaired muscle regeneration due to deterioration of the muscle microenvironment, and dystrophin deficiency-induced multiple organ dysfunction, while summarizing the recent advances in the treatment of DMD.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others
Skeletal muscle: a brief review of structure and function.

Diagnostic Approach to Proximal Myopathy
The development of skeletal muscle hypertrophy through resistance training: the role of muscle damage and muscle protein synthesis, data availability.
The authors confirm that the data supporting the findings of this study are available within the article.
Huang L, Li M, Deng C, Qiu J, Wang K, Chang M, Zhou S, Gu Y, Shen Y, Wang W et al (2022) Potential therapeutic strategies for skeletal muscle atrophy. Antioxidants (Basel) 12(1):44
Article PubMed Google Scholar
Wang W, Li M, Chen Z, Xu L, Chang M, Wang K, Deng C, Gu Y, Zhou S, Shen Y et al (2022) Biogenesis and function of extracellular vesicles in pathophysiological processes of skeletal muscle atrophy. Biochem Pharmacol 198:114954
Article CAS PubMed Google Scholar
Amenta AR, Yilmaz A, Bogdanovich S, McKechnie BA, Abedi M, Khurana TS, Fallon JR (2011) Biglycan recruits utrophin to the sarcolemma and counters dystrophic pathology in mdx mice. Proc Natl Acad Sci USA 108(2):762–767
Guiraud S, Aartsma-Rus A, Vieira NM, Davies KE, van Ommen GJ, Kunkel LM (2015) The pathogenesis and therapy of muscular dystrophies. Annu Rev Genom Hum Genet 16:281–308
Article CAS Google Scholar
Duan D, Goemans N, Takeda S, Mercuri E, Aartsma-Rus A (2021) Duchenne muscular dystrophy. Nat Rev Dis Primers 7(1):13
Zablocka B, Gorecki DC, Zablocki K (2021) Disrupted calcium homeostasis in duchenne muscular dystrophy: a common mechanism behind diverse consequences. Int J Mol Sci 22(20):11040
Article CAS PubMed PubMed Central Google Scholar
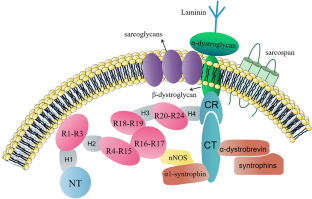
Gao QQ, McNally EM (2015) The dystrophin complex: structure, function, and implications for therapy. Compr Physiol 5(3):1223–1239
Article PubMed PubMed Central Google Scholar
Lai Y, Thomas GD, Yue Y, Yang HT, Li D, Long C, Judge L, Bostick B, Chamberlain JS, Terjung RL et al (2009) Dystrophins carrying spectrin-like repeats 16 and 17 anchor nNOS to the sarcolemma and enhance exercise performance in a mouse model of muscular dystrophy. J Clin Investig 119(3):624–635
Allen DG, Whitehead NP, Froehner SC (2016) Absence of dystrophin disrupts skeletal muscle signaling: roles of Ca 2+ , reactive oxygen species, and nitric oxide in the development of muscular dystrophy. Physiol Rev 96(1):253–305
Patel A, Zhao J, Yue Y, Zhang K, Duan D, Lai Y (2018) Dystrophin R16/17-syntrophin PDZ fusion protein restores sarcolemmal nNOSμ. Skelet Muscle 8(1):36
Brenman JE, Chao DS, Xia H, Aldape K, Bredt DS (1995) Nitric oxide synthase complexed with dystrophin and absent from skeletal muscle sarcolemma in Duchenne muscular dystrophy. Cell 82(5):743–752
Sander M, Chavoshan B, Harris SA, Iannaccone ST, Stull JT, Thomas GD, Victor RG (2000) Functional muscle ischemia in neuronal nitric oxide synthase-deficient skeletal muscle of children with Duchenne muscular dystrophy. Proc Natl Acad Sci USA 97(25):13818–13823
Kodippili K, Hakim CH, Pan X, Yang HT, Yue Y, Zhang Y, Shin JH, Yang NN, Duan D (2018) Dual AAV gene therapy for duchenne muscular dystrophy with a 7-kb Mini-Dystrophin Gene in the canine model. Hum Gene Ther 29(3):299–311
Prosser BL, Ward CW, Lederer WJ (2011) X-ROS signaling: rapid mechano-chemo transduction in heart. Science (New York, NY) 333(6048):1440–1445
Khairallah RJ, Shi G, Sbrana F, Prosser BL, Borroto C, Mazaitis MJ, Hoffman EP, Mahurkar A, Sachs F, Sun Y et al (2012) Microtubules underlie dysfunction in duchenne muscular dystrophy. Sci Signal 5(236):ra56
Pal R, Palmieri M, Loehr JA, Li S, Abo-Zahrah R, Monroe TO, Thakur PB, Sardiello M, Rodney GG (2014) Src-dependent impairment of autophagy by oxidative stress in a mouse model of Duchenne muscular dystrophy. Nat Commun 5:4425
Gissel H (2005) The role of Ca 2+ in muscle cell damage. Ann N Y Acad Sci 1066:166–180
Santulli G, Xie W, Reiken SR, Marks AR (2015) Mitochondrial calcium overload is a key determinant in heart failure. Proc Natl Acad Sci USA 112(36):11389–11394
Rudolf R, Mongillo M, Magalhães PJ, Pozzan T (2004) In vivo monitoring of Ca(2+) uptake into mitochondria of mouse skeletal muscle during contraction. J Cell Biol 166(4):527–536
Zorov DB, Juhaszova M, Sollott SJ (2014) Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev 94(3):909–950
Ng SY, Ljubicic V (2020) Recent insights into neuromuscular junction biology in Duchenne muscular dystrophy: impacts, challenges, and opportunities. EBioMedicine 61:103032
Li L, Xiong WC, Mei L (2018) Neuromuscular junction formation, aging, and disorders. Annu Rev Physiol 80:159–188
Wood SJ, Slater CR (2001) Safety factor at the neuromuscular junction. Prog Neurobiol 64(4):393–429
Suntar I, Sureda A, Belwal T, Sanches Silva A, Vacca RA, Tewari D, Sobarzo-Sánchez E, Nabavi SF, Shirooie S, Dehpour AR et al (2020) Natural products, PGC-1 α, and Duchenne muscular dystrophy. Acta Pharm Sin B 10(5):734–745
Angus LM, Chakkalakal JV, Méjat A, Eibl JK, Bélanger G, Megeney LA, Chin ER, Schaeffer L, Michel RN, Jasmin BJ (2005) Calcineurin-NFAT signaling, together with GABP and peroxisome PGC-1{alpha}, drives utrophin gene expression at the neuromuscular junction. Am J Physiol Cell Physiol 289(4):C908-917
Paredes-Redondo A, Harley P, Maniati E, Ryan D, Louzada S, Meng J, Kowala A, Fu B, Yang F, Liu P et al (2021) Optogenetic modeling of human neuromuscular circuits in Duchenne muscular dystrophy with CRISPR and pharmacological corrections. Sci Adv 7(37):eabi8787
Pratt SJP, Shah SB, Ward CW, Kerr JP, Stains JP, Lovering RM (2015) Recovery of altered neuromuscular junction morphology and muscle function in mdx mice after injury. Cell Mol Life Sci CMLS 72(1):153–164
Hesser BA, Henschel O, Witzemann V (2006) Synapse disassembly and formation of new synapses in postnatal muscle upon conditional inactivation of MuSK. Mol Cell Neurosci 31(3):470–480
Trajanovska S, Ban J, Huang J, Gregorevic P, Morsch M, Allen DG, Phillips WD (2019) Muscle specific kinase protects dystrophic mdx mouse muscles from eccentric contraction-induced loss of force-producing capacity. J Physiol 597(18):4831–4850
Cappellari O, Mantuano P, De Luca A (2020) “The social network” and muscular dystrophies: the lesson learnt about the niche environment as a target for therapeutic strategies. Cells 9(7):1659
Chang NC, Sincennes MC, Chevalier FP, Brun CE, Lacaria M, Segalés J, Muñoz-Cánoves P, Ming H, Rudnicki MA (2018) The dystrophin glycoprotein complex regulates the epigenetic activation of muscle stem cell commitment. Cell Stem Cell 22(5):755-768.e756
Lumeng C, Phelps S, Crawford GE, Walden PD, Barald K, Chamberlain JS (1999) Interactions between beta 2-syntrophin and a family of microtubule-associated serine/threonine kinases. Nat Neurosci 2(7):611–617
Dewey EB, Taylor DT, Johnston CA (2015) Cell Fate decision making through oriented cell division. J Dev Biol 3(4):129–157
Yamashita K, Suzuki A, Satoh Y, Ide M, Amano Y, Masuda-Hirata M, Hayashi YK, Hamada K, Ogata K, Ohno S (2010) The 8th and 9th tandem spectrin-like repeats of utrophin cooperatively form a functional unit to interact with polarity-regulating kinase PAR-1b. Biochem Biophys Res Commun 391(1):812–817
Dumont NA, Wang YX, von Maltzahn J, Pasut A, Bentzinger CF, Brun CE, Rudnicki MA (2015) Dystrophin expression in muscle stem cells regulates their polarity and asymmetric division. Nat Med 21(12):1455–1463
Biressi S, Miyabara EH, Gopinath SD, Carlig PM, Rando TA (2014) A Wnt-TGFβ2 axis induces a fibrogenic program in muscle stem cells from dystrophic mice. Sci Transl Med 6(267):267ra176
Tidball JG, Welc SS, Wehling-Henricks M (2018) Immunobiology of inherited muscular dystrophies. Compr Physiol 8(4):1313–1356
Perandini LA, Chimin P, Lutkemeyer DDS, Câmara NOS (2018) Chronic inflammation in skeletal muscle impairs satellite cells function during regeneration: can physical exercise restore the satellite cell niche? FEBS J 285(11):1973–1984
Deng B, Wehling-Henricks M, Villalta SA, Wang Y, Tidball JG (2012) IL-10 triggers changes in macrophage phenotype that promote muscle growth and regeneration. J Immunology (Baltimore, Md: 1950) 189(7):3669–3680
Pavlidou T, Marinkovic M, Rosina M, Fuoco C, Vumbaca S, Gargioli C, Castagnoli L, Cesareni G (2019) Metformin delays satellite cell activation and maintains quiescence. Stem Cells Int 2019:5980465
Lemos DR, Babaeijandaghi F, Low M, Chang CK, Lee ST, Fiore D, Zhang RH, Natarajan A, Nedospasov SA, Rossi FM (2015) Nilotinib reduces muscle fibrosis in chronic muscle injury by promoting TNF-mediated apoptosis of fibro/adipogenic progenitors. Nat Med 21(7):786–794
Villalta SA, Rinaldi C, Deng B, Liu G, Fedor B, Tidball JG (2011) Interleukin-10 reduces the pathology of mdx muscular dystrophy by deactivating M1 macrophages and modulating macrophage phenotype. Hum Mol Genet 20(4):790–805
Villalta SA, Nguyen HX, Deng B, Gotoh T, Tidball JG (2009) Shifts in macrophage phenotypes and macrophage competition for arginine metabolism affect the severity of muscle pathology in muscular dystrophy. Hum Mol Genet 18(3):482–496
Mercuri E, Muntoni F (2013) Muscular dystrophies. Lancet (London, England) 381(9869):845–860
Tsuda T (2018) Clinical manifestations and overall management strategies for Duchenne muscular dystrophy. Methods Mol Biol (Clifton, NJ) 1687:19–28
Benditt JO, Boitano L (2005) Respiratory support of individuals with Duchenne muscular dystrophy: toward a standard of care. Phys Med Rehabil Clin N Am 16(4):1125–1139, xii
Johnson EK, Zhang L, Adams ME, Phillips A, Freitas MA, Froehner SC, Green-Church KB, Montanaro F (2012) Proteomic analysis reveals new cardiac-specific dystrophin-associated proteins. PLoS ONE 7(8):e43515
Duboc D, Meune C, Lerebours G, Devaux JY, Vaksmann G, Bécane HM (2005) Effect of perindopril on the onset and progression of left ventricular dysfunction in Duchenne muscular dystrophy. J Am Coll Cardiol 45(6):855–857
Silva MC, Magalhães TA, Meira ZM, Rassi CH, Andrade AC, Gutierrez PS, Azevedo CF, Gurgel-Giannetti J, Vainzof M, Zatz M et al (2017) Myocardial fibrosis progression in Duchenne and Becker muscular dystrophy: a randomized clinical trial. JAMA cardiology 2(2):190–199
Pane M, Messina S, Bruno C, D’Amico A, Villanova M, Brancalion B, Sivo S, Bianco F, Striano P, Battaglia D et al (2013) Duchenne muscular dystrophy and epilepsy. Neuromusc Disord NMD 23(4):313–315
Athanasopoulos T, Graham IR, Foster H, Dickson G (2004) Recombinant adeno-associated viral (rAAV) vectors as therapeutic tools for Duchenne muscular dystrophy (DMD). Gene Ther 11(Suppl 1):S109-121
Birch SM, Lawlor MW, Conlon TJ, Guo LJ, Crudele JM, Hawkins EC, Nghiem PP, Ahn M, Meng H, Beatka MJ et al (2023) Assessment of systemic AAV-microdystrophin gene therapy in the GRMD model of Duchenne muscular dystrophy. Sci Transl Med 15(677):eabo1815
Mendell JR, Sahenk Z, Lehman K, Nease C, Lowes LP, Miller NF, Iammarino MA, Alfano LN, Nicholl A, Al-Zaidy S et al (2020) Assessment of systemic delivery of rAAVrh74.MHCK7.micro-dystrophin in children with duchenne muscular dystrophy: a nonrandomized controlled trial. JAMA Neurol 77(9):1122–1131
Bowles DE, McPhee SW, Li C, Gray SJ, Samulski JJ, Camp AS, Li J, Wang B, Monahan PE, Rabinowitz JE et al (2012) Phase 1 gene therapy for Duchenne muscular dystrophy using a translational optimized AAV vector. Mol Ther 20(2):443–455
Duan D (2018) Micro-dystrophin gene therapy goes systemic in Duchenne muscular dystrophy patients. Hum Gene Ther 29(7):733–736
Min YL, Bassel-Duby R, Olson EN (2019) CRISPR correction of Duchenne muscular dystrophy. Annu Rev Med 70:239–255
Li J, Wang K, Zhang Y, Qi T, Yuan J, Zhang L, Qiu H, Wang J, Yang HT, Dai Y et al (2021) Therapeutic exon skipping through a CRISPR-guided cytidine deaminase rescues dystrophic cardiomyopathy in vivo. Circulation 144(22):1760–1776
Moretti A, Fonteyne L, Giesert F, Hoppmann P, Meier AB, Bozoglu T, Baehr A, Schneider CM, Sinnecker D, Klett K et al (2020) Somatic gene editing ameliorates skeletal and cardiac muscle failure in pig and human models of Duchenne muscular dystrophy. Nat Med 26(2):207–214
Xiang X, Zhao X, Pan X, Dong Z, Yu J, Li S, Liang X, Han P, Qu K, Jensen JB et al (2021) Efficient correction of Duchenne muscular dystrophy mutations by SpCas9 and dual gRNAs. Mol Ther Nucleic Acids 24:403–415
Xu L, Park KH, Zhao L, Xu J, El Refaey M, Gao Y, Zhu H, Ma J, Han R (2016) CRISPR-mediated genome editing restores dystrophin expression and function in mdx mice. Mol Ther 24(3):564–569
Kenjo E, Hozumi H, Makita Y, Iwabuchi KA, Fujimoto N, Matsumoto S, Kimura M, Amano Y, Ifuku M, Naoe Y et al (2021) Low immunogenicity of LNP allows repeated administrations of CRISPR-Cas9 mRNA into skeletal muscle in mice. Nat Commun 12(1):7101
Zhang Y, Li H, Min YL, Sanchez-Ortiz E, Huang J, Mireault AA, Shelton JM, Kim J, Mammen PPA, Bassel-Duby R et al (2020) Enhanced CRISPR-Cas9 correction of Duchenne muscular dystrophy in mice by a self-complementary AAV delivery system. Sci Adv 6(8):eaay6812
Majeau N, Fortin-Archambault A, Gérard C, Rousseau J, Yaméogo P, Tremblay JP (2022) Serum extracellular vesicles for delivery of CRISPR-CAS9 ribonucleoproteins to modify the dystrophin gene. Mol Ther 30(7):2429–2442
Pickar-Oliver A, Gough V, Bohning JD, Liu S, Robinson-Hamm JN, Daniels H, Majoros WH, Devlin G, Asokan A, Gersbach CA (2021) Full-length dystrophin restoration via targeted exon integration by AAV-CRISPR in a humanized mouse model of Duchenne muscular dystrophy. Mol Ther 29(11):3243–3257
Koo T, Wood MJ (2013) Clinical trials using antisense oligonucleotides in duchenne muscular dystrophy. Hum Gene Ther 24(5):479–488
Ran N, Lin C, Leng L, Han G, Geng M, Wu Y, Bittner S, Moulton HM, Yin H (2021) MOTS-c promotes phosphorodiamidate morpholino oligomer uptake and efficacy in dystrophic mice. EMBO Mol Med 13(2):e12993
Lin C, Han G, Ning H, Song J, Ran N, Yi X, Seow Y, Yin H (2020) Glycine enhances satellite cell proliferation, cell transplantation, and oligonucleotide efficacy in dystrophic muscle. Mol Ther 28(5):1339–1358
Lim KRQ, Woo S, Melo D, Huang Y, Dzierlega K, Shah MNA, Aslesh T, Roshmi RR, Echigoya Y, Maruyama R et al (2022) Development of DG9 peptide-conjugated single- and multi-exon skipping therapies for the treatment of Duchenne muscular dystrophy. Proc Natl Acad Sci USA 119(9):e2112546119
Gushchina LV, Vetter TA, Frair EC, Bradley AJ, Grounds KM, Lay JW, Huang N, Suhaiba A, Schnell FJ, Hanson G et al (2022) Systemic PPMO-mediated dystrophin expression in the Dup2 mouse model of Duchenne muscular dystrophy. Mol Ther Nucleic Acids 30:479–492
Desjardins CA, Yao M, Hall J, O’Donnell E, Venkatesan R, Spring S, Wen A, Hsia N, Shen P, Russo R et al (2022) Enhanced exon skipping and prolonged dystrophin restoration achieved by TfR1-targeted delivery of antisense oligonucleotide using FORCE conjugation in mdx mice. Nucleic Acids Res 50(20):11401–11414
Gan L, Wu LCL, Wood JA, Yao M, Treleaven CM, Estrella NL, Wentworth BM, Hanson GJ, Passini MA (2022) A cell-penetrating peptide enhances delivery and efficacy of phosphorodiamidate morpholino oligomers in mdx mice. Mol Ther Nucleic Acids 30:17–27
Scaglioni D, Catapano F, Ellis M, Torelli S, Chambers D, Feng L, Beck M, Sewry C, Monforte M, Harriman S et al (2021) The administration of antisense oligonucleotide golodirsen reduces pathological regeneration in patients with Duchenne muscular dystrophy. Acta Neuropathol Commun 9(1):7
McDonald CM, Shieh PB, Abdel-Hamid HZ, Connolly AM, Ciafaloni E, Wagner KR, Goemans N, Mercuri E, Khan N, Koenig E et al (2021) Open-label evaluation of eteplirsen in patients with duchenne muscular dystrophy amenable to exon 51 skipping: PROMOVI Trial. J Neuromusc Dis 8(6):989–1001
Article Google Scholar
Mitelman O, Abdel-Hamid HZ, Byrne BJ, Connolly AM, Heydemann P, Proud C, Shieh PB, Wagner KR, Dugar A, Santra S et al (2022) A combined prospective and retrospective comparison of long-term functional outcomes suggests delayed loss of ambulation and pulmonary decline with long-term eteplirsen treatment. J Neuromusc Dis 9(1):39–52
Iff J, Gerrits C, Zhong Y, Tuttle E, Birk E, Zheng Y, Paul X, Henricson EK, McDonald CM (2022) Delays in pulmonary decline in eteplirsen-treated patients with Duchenne muscular dystrophy. Muscle Nerve 66(3):262–269
Wagner KR, Kuntz NL, Koenig E, East L, Upadhyay S, Han B, Shieh PB (2021) Safety, tolerability, and pharmacokinetics of casimersen in patients with Duchenne muscular dystrophy amenable to exon 45 skipping: a randomized, double-blind, placebo-controlled, dose-titration trial. Muscle Nerve 64(3):285–292
Roshmi RR, Yokota T (2023) Viltolarsen: from preclinical studies to FDA approval. Methods Mol Biol (Clifton, NJ) 2587:31–41
Clemens PR, Rao VK, Connolly AM, Harper AD, Mah JK, McDonald CM, Smith EC, Zaidman CM, Nakagawa T, Hoffman EP (2022) Long-term functional efficacy and safety of viltolarsen in patients with Duchenne muscular dystrophy. J Neuromusc Dis 9(4):493–501
Clemens PR, Rao VK, Connolly AM, Harper AD, Mah JK, Smith EC, McDonald CM, Zaidman CM, Morgenroth LP, Osaki H et al (2020) Safety, tolerability, and efficacy of viltolarsen in boys with Duchenne muscular dystrophy amenable to exon 53 skipping: a phase 2 randomized clinical trial. JAMA Neurol 77(8):982–991
Komaki H, Takeshima Y, Matsumura T, Ozasa S, Funato M, Takeshita E, Iwata Y, Yajima H, Egawa Y, Toramoto T et al (2020) Viltolarsen in Japanese Duchenne muscular dystrophy patients: a phase 1/2 study. Ann Clin Transl Neurol 7(12):2393–2408
Goyenvalle A, Vulin A, Fougerousse F, Leturcq F, Kaplan JC, Garcia L, Danos O (2004) Rescue of dystrophic muscle through U7 snRNA-mediated exon skipping. Science (New York, NY) 306(5702):1796–1799
Forand A, Muchir A, Mougenot N, Sevoz-Couche C, Peccate C, Lemaitre M, Izabelle C, Wood M, Lorain S, Piétri-Rouxel F (2020) Combined treatment with peptide-conjugated phosphorodiamidate morpholino oligomer-PPMO and AAV-U7 rescues the severe DMD phenotype in mice. Mol Ther Methods Clin Dev 17:695–708
Guglieri M, Clemens PR, Perlman SJ, Smith EC, Horrocks I, Finkel RS, Mah JK, Deconinck N, Goemans N, Haberlova J et al (2022) Efficacy and safety of vamorolone vs placebo and prednisone among boys with duchenne muscular dystrophy: a randomized clinical trial. JAMA Neurol 79(10):1005–1014
Smith EC, Conklin LS, Hoffman EP, Clemens PR, Mah JK, Finkel RS, Guglieri M, Tulinius M, Nevo Y, Ryan MM et al (2020) Efficacy and safety of vamorolone in Duchenne muscular dystrophy: an 18-month interim analysis of a non-randomized open-label extension study. PLoS Med 17(9):e1003222
Weiß C, Stoltenburg C, Bayram D, Funk J, Lebek S (2020) Positive effect of the combination of multilevel contracture release and glucocorticoid treatment in Duchenne muscular dystrophy. J Child Orthop 14(4):349–352
Previtali SC, Gidaro T, Díaz-Manera J, Zambon A, Carnesecchi S, Roux-Lombard P, Spitali P, Signorelli M, Szigyarto CA, Johansson C et al (2020) Rimeporide as a first- in-class NHE-1 inhibitor: results of a phase Ib trial in young patients with Duchenne muscular dystrophy. Pharmacol Res 159:104999
Komaki H, Maegaki Y, Matsumura T, Shiraishi K, Awano H, Nakamura A, Kinoshita S, Ogata K, Ishigaki K, Saitoh S et al (2020) Early phase 2 trial of TAS-205 in patients with Duchenne muscular dystrophy. Ann Clin Transl Neurol 7(2):181–190
Finkel RS, McDonald CM, Lee Sweeney H, Finanger E, Neil Knierbein E, Wagner KR, Mathews KD, Marks W, Statland J, Nance J et al (2021) A randomized, double-blind, placebo-controlled, global phase 3 study of edasalonexent in pediatric patients with Duchenne muscular dystrophy: results of the PolarisDMD trial. J Neuromusc Dis 8(5):769–784
Nasomyont N, Keefe C, Tian C, Hornung L, Khoury J, Tilden JC, Hochwalt P, Jackson E, Rybalsky I, Wong BL et al (2020) Safety and efficacy of teriparatide treatment for severe osteoporosis in patients with Duchenne muscular dystrophy. Osteoporos Int 31(12):2449–2459
Tian C, Wong BL, Hornung L, Khoury JC, Rybalsky I, Shellenbarger KC, Rutter MM (2020) Oral bisphosphonate treatment in patients with Duchenne muscular dystrophy on long term glucocorticoid therapy. Neuromusc Disord NMD 30(7):599–610
Segatto M, Szokoll R, Fittipaldi R, Bottino C, Nevi L, Mamchaoui K, Filippakopoulos P, Caretti G (2020) BETs inhibition attenuates oxidative stress and preserves muscle integrity in Duchenne muscular dystrophy. Nat Commun 11(1):6108
Rybalka E, Goodman CA, Campelj DG, Hayes A, Timpani CA (2021) Adenylosuccinic acid: a novel inducer of the cytoprotectant Nrf2 with efficacy in Duchenne muscular dystrophy. Curr Med Res Opin 37(3):465–467
Timpani CA, Goodman CA, Stathis CG, White JD, Mamchaoui K, Butler-Browne G, Gueven N, Hayes A, Rybalka E (2020) Adenylosuccinic acid therapy ameliorates murine Duchenne muscular dystrophy. Sci Rep 10(1):1125
Dort J, Orfi Z, Fabre P, Molina T, Conte TC, Greffard K, Pellerito O, Bilodeau JF, Dumont NA (2021) Resolvin-D2 targets myogenic cells and improves muscle regeneration in Duchenne muscular dystrophy. Nat Commun 12(1):6264
Krishnan SM, Nordlohne J, Dietz L, Vakalopoulos A, Haning P, Hartmann E, Seifert R, Huser J, Mathar I, Sandner P (2021) Assessing the use of the sGC stimulator BAY-747, as a potential treatment for Duchenne muscular dystrophy. Int J Mol Sci 22(15):8016
Dubuisson N, Davis-López de Carrizosa MA, Versele R, Selvais CM, Noel L, Van den Bergh PYD, Brichard SM, Abou-Samra M (2022) Inhibiting the inflammasome with MCC950 counteracts muscle pyroptosis and improves Duchenne muscular dystrophy. Front Immunol 13:1049076
Abou-Samra M, Selvais CM, Boursereau R, Lecompte S, Noel L, Brichard SM (2020) AdipoRon, a new therapeutic prospect for Duchenne muscular dystrophy. J Cachexia Sarcopenia Muscle 11(2):518–533
Hightower RM, Reid AL, Gibbs DE, Wang Y, Widrick JJ, Kunkel LM, Kastenschmidt JM, Villalta SA, van Groen T, Chang H et al (2020) The SINE compound KPT-350 blocks dystrophic pathologies in DMD zebrafish and mice. Mol Ther 28(1):189–201
English KG, Reid AL, Samani A, Coulis GJF, Villalta SA, Walker CJ, Tamir S, Alexander MS (2022) Next-generation SINE compound KPT-8602 ameliorates dystrophic pathology in zebrafish and mouse models of DMD. Biomedicines 10(10):2400
Xu D, Zhao L, Jiang J, Li S, Sun Z, Huang X, Li C, Wang T, Sun L, Li X et al (2020) A potential therapeutic effect of catalpol in Duchenne muscular dystrophy revealed by binding with TAK1. J Cachexia Sarcopenia Muscle 11(5):1306–1320
Stocco A, Smolina N, Sabatelli P, Šileikytė J, Artusi E, Mouly V, Cohen M, Forte M, Schiavone M, Bernardi P (2021) Treatment with a triazole inhibitor of the mitochondrial permeability transition pore fully corrects the pathology of sapje zebrafish lacking dystrophin. Pharmacol Res 165:105421
Lambert MR, Spinazzola JM, Widrick JJ, Pakula A, Conner JR, Chin JE, Owens JM, Kunkel LM (2021) PDE10A inhibition reduces the manifestation of pathology in DMD zebrafish and represses the genetic modifier PITPNA. Mol Ther 29(3):1086–1101
Farr GH 3rd, Morris M, Gomez A, Pham T, Kilroy E, Parker EU, Said S, Henry C, Maves L (2020) A novel chemical-combination screen in zebrafish identifies epigenetic small molecule candidates for the treatment of Duchenne muscular dystrophy. Skelet Muscle 10(1):29
Spreafico M, Cafora M, Bragato C, Capitanio D, Marasca F, Bodega B, De Palma C, Mora M, Gelfi C, Marozzi A et al (2021) Targeting HDAC8 to ameliorate skeletal muscle differentiation in Duchenne muscular dystrophy. Pharmacol Res 170:105750
Ellwood RA, Slade L, Lewis J, Torregrossa R, Sudevan S, Piasecki M, Whiteman M, Etheridge T, Szewczyk NJ (2022) Sulfur amino acid supplementation displays therapeutic potential in a C. elegans model of Duchenne muscular dystrophy. Commun Biol 5(1):1255
Ellwood RA, Hewitt JE, Torregrossa R, Philp AM, Hardee JP, Hughes S, van de Klashorst D, Gharahdaghi N, Anupom T, Slade L et al (2021) Mitochondrial hydrogen sulfide supplementation improves health in the C. elegans Duchenne muscular dystrophy model. Proc Natl Acad Sci USA 118(9):e2018342118
Morroni J, Schirone L, Valenti V, Zwergel C, Riera CS, Valente S, Vecchio D, Schiavon S, Ragno R, Mai A et al (2022) Inhibition of PKCtheta improves dystrophic heart phenotype and function in a novel model of DMD cardiomyopathy. Int J Mol Sci 23(4):2256
Guo Z, Geng M, Huang Y, Han G, Jing R, Lin C, Zhang X, Zhang M, Fan G, Wang F et al (2022) Upregulation of Wilms’ Tumor 1 in epicardial cells increases cardiac fibrosis in dystrophic mice. Cell Death Differ 29(10):1928–1940
Huang D, Yue F, Qiu J, Deng M, Kuang S (2020) Polymeric nanoparticles functionalized with muscle-homing peptides for targeted delivery of phosphatase and tensin homolog inhibitor to skeletal muscle. Acta Biomater 118:196–206
Creisméas A, Gazaille C, Bourdon A, Lallemand MA, François V, Allais M, Ledevin M, Larcher T, Toumaniantz G, Lafoux A et al (2021) TRPC3, but not TRPC1, as a good therapeutic target for standalone or complementary treatment of DMD. J Transl Med 19(1):519
Wasala NB, Yue Y, Lostal W, Wasala LP, Niranjan N, Hajjar RJ, Babu GJ, Duan D (2020) Single SERCA2a therapy ameliorated dilated cardiomyopathy for 18 months in a mouse model of Duchenne muscular dystrophy. Mol Ther 28(3):845–854
Dubinin MV, Starinets VS, Belosludtseva NV, Mikheeva IB, Chelyadnikova YA, Igoshkina AD, Vafina AB, Vedernikov AA, Belosludtsev KN (2022) BK(Ca) activator NS1619 improves the structure and function of skeletal muscle mitochondria in Duchenne dystrophy. Pharmaceutics 14(11):2336
Kamdar F, Das S, Gong W, Klaassen Kamdar A, Meyers TA, Shah P, Ervasti JM, Townsend D, Kamp TJ, Wu JC et al (2020) Stem cell-derived cardiomyocytes and beta-adrenergic receptor blockade in Duchenne muscular dystrophy cardiomyopathy. J Am Coll Cardiol 75(10):1159–1174
Yu L, Zhang X, Yang Y, Li D, Tang K, Zhao Z, He W, Wang C, Sahoo N, Converso-Baran K et al (2020) Small-molecule activation of lysosomal TRP channels ameliorates Duchenne muscular dystrophy in mouse models. Sci Adv 6(6):eaaz2736
Luan P, D’Amico D, Andreux PA, Laurila PP, Wohlwend M, Li H, de Lima TI, Place N, Rinsch C, Zanou N et al (2021) Urolithin A improves muscle function by inducing mitophagy in muscular dystrophy. Sci Transl Med 13(588):eabb0319
Zhang Y, Li Y, Hu Q, Xi Y, Xing Z, Zhang Z, Huang L, Wu J, Liang K, Nguyen TK et al (2020) The lncRNA H19 alleviates muscular dystrophy by stabilizing dystrophin. Nat Cell Biol 22(11):1332–1345
Oliveira-Santos A, Dagda M, Burkin DJ (2022) Sunitinib inhibits STAT3 phosphorylation in cardiac muscle and prevents cardiomyopathy in the mdx mouse model of Duchenne muscular dystrophy. Hum Mol Genet 31(14):2358–2369
Haupenthal D, Possato JC, Zaccaron RP, Mendes C, Rodrigues MS, Nesi RT, Pinho RA, Feuser PE, Machado-de-Ávila RA, Comim CM et al (2020) Effects of chronic treatment with gold nanoparticles on inflammatory responses and oxidative stress in Mdx mice. J Drug Target 28(1):46–54
Li J, Fredericks M, Cannell M, Wang K, Sako D, Maguire MC, Grenha R, Liharska K, Krishnan L, Bloom T et al (2021) ActRIIB:ALK4-Fc alleviates muscle dysfunction and comorbidities in murine models of neuromuscular disorders. J Clin Investig 131(4):e138634
Bella P, Farini A, Banfi S, Parolini D, Tonna N, Meregalli M, Belicchi M, Erratico S, D’Ursi P, Bianco F et al (2020) Blockade of IGF2R improves muscle regeneration and ameliorates Duchenne muscular dystrophy. EMBO Mol Med 12(1):e11019
Sung DK, Kim H, Park SE, Lee J, Kim JA, Park YC, Jeon HB, Chang JW, Lee J (2022) A new method of myostatin inhibition in mice via oral administration of Lactobacillus casei expressing modified myostatin protein, BLS-M22. Int J Mol Sci 23(16):9059
Ran N, Gao X, Dong X, Li J, Lin C, Geng M, Yin H (2020) Effects of exosome-mediated delivery of myostatin propeptide on functional recovery of mdx mice. Biomaterials 236:119826
Xu D, Li S, Wang L, Jiang J, Zhao L, Huang X, Sun Z, Li C, Sun L, Li X et al (2021) TAK1 inhibition improves myoblast differentiation and alleviates fibrosis in a mouse model of Duchenne muscular dystrophy. J Cachexia Sarcopenia Muscle 12(1):192–208
Skuk D, Goulet M, Roy B, Chapdelaine P, Bouchard JP, Roy R, Dugré FJ, Sylvain M, Lachance JG, Deschênes L et al (2006) Dystrophin expression in muscles of duchenne muscular dystrophy patients after high-density injections of normal myogenic cells. J Neuropathol Exp Neurol 65(4):371–386
Nitahara-Kasahara Y, Kuraoka M, Guillermo PH, Hayashita-Kinoh H, Maruoka Y, Nakamura-Takahasi A, Kimura K, Takeda S, Okada T (2021) Dental pulp stem cells can improve muscle dysfunction in animal models of Duchenne muscular dystrophy. Stem Cell Res Ther 12(1):78
Park S, Jeong S, Nam YH, Yum Y, Jung SC (2022) Transplantation of differentiated tonsil-derived mesenchymal stem cells ameliorates murine Duchenne muscular dystrophy via autophagy activation. Tissue Eng Regen Med 19(6):1283–1294
Siemionow M, Langa P, Brodowska S, Kozlowska K, Zalants K, Budzynska K, Heydemann A (2022) Long-term protective effect of human dystrophin expressing chimeric (DEC) cell therapy on amelioration of function of cardiac, respiratory and skeletal muscles in Duchenne muscular dystrophy. Stem Cell Rev Rep 18(8):2872–2892
Siemionow M, Szilagyi E, Cwykiel J, Domaszewska-Szostek A, Heydemann A, Garcia-Martinez J, Siemionow K (2021) Transplantation of dystrophin expressing chimeric human cells of myoblast/mesenchymal stem cell origin improves function in Duchenne muscular dystrophy model. Stem Cells Dev 30(4):190–202
Meng J, Sweeney NP, Doreste B, Muntoni F, McClure M, Morgan J (2020) Restoration of functional full-length dystrophin after intramuscular transplantation of foamy virus-transduced myoblasts. Hum Gene Ther 31(3–4):241–252
Chakkalakal JV, Thompson J, Parks RJ, Jasmin BJ (2005) Molecular, cellular, and pharmacological therapies for Duchenne/Becker muscular dystrophies. FASEB J 19(8):880–891
Tinsley J, Deconinck N, Fisher R, Kahn D, Phelps S, Gillis JM, Davies K (1998) Expression of full-length utrophin prevents muscular dystrophy in mdx mice. Nat Med 4(12):1441–1444
Tinsley JM, Potter AC, Phelps SR, Fisher R, Trickett JI, Davies KE (1996) Amelioration of the dystrophic phenotype of mdx mice using a truncated utrophin transgene. Nature 384(6607):349–353
Babbs A, Berg A, Chatzopoulou M, Davies KE, Davies SG, Edwards B, Elsey DJ, Emer E, Guiraud S, Harriman S et al (2020) 2-Arylbenzo[d]oxazole phosphinate esters as second-generation modulators of utrophin for the treatment of Duchenne muscular dystrophy. J Med Chem 63(14):7880–7891
Chatzopoulou M, Conole D, Emer E, Rowley JA, Willis NJ, Squire SE, Gill B, Brough S, Wilson FX, Wynne GM et al (2022) Structure-activity relationships of 2-pyrimidinecarbohydrazides as utrophin modulators for the potential treatment of Duchenne muscular dystrophy. Bioorg Med Chem 69:116812
Sengupta K, Loro E, Khurana TS (2020) PMO-based let-7c site blocking oligonucleotide (SBO) mediated utrophin upregulation in mdx mice, a therapeutic approach for Duchenne muscular dystrophy (DMD). Sci Rep 10(1):21492
Frank DE, Schnell FJ, Akana C, El-Husayni SH, Desjardins CA, Morgan J, Charleston JS, Sardone V, Domingos J, Dickson G et al (2020) Increased dystrophin production with golodirsen in patients with Duchenne muscular dystrophy. Neurology 94(21):e2270–e2282
Servais L, Mercuri E, Straub V, Guglieri M, Seferian AM, Scoto M, Leone D, Koenig E, Khan N, Dugar A et al (2022) Long-term safety and efficacy data of golodirsen in ambulatory patients with Duchenne muscular dystrophy amenable to exon 53 skipping: a first-in-human, multicenter, two-part, open-label, phase 1/2 trial. Nucleic Acid Ther 32(1):29–39
Chemello F, Chai AC, Li H, Rodriguez-Caycedo C, Sanchez-Ortiz E, Atmanli A, Mireault AA, Liu N, Bassel-Duby R, Olson EN (2021) Precise correction of Duchenne muscular dystrophy exon deletion mutations by base and prime editing. Sci Adv 7(18):eabg4910
Xu L, Zhang C, Li H, Wang P, Gao Y, Mokadam NA, Ma J, Arnold WD, Han R (2021) Efficient precise in vivo base editing in adult dystrophic mice. Nat Commun 12(1):3719
Li G, Jin M, Li Z, Xiao Q, Lin J, Yang D, Liu Y, Wang X, Xie L, Ying W et al (2023) Mini-dCas13X-mediated RNA editing restores dystrophin expression in a humanized mouse model of Duchenne muscular dystrophy. J Clin Investig 133(3):e162809
Download references
Acknowledgements
I am grateful for the contribution to language help and writing assistance from Lai Xu at Nantong University during the research.
This work was supported by the National Natural Science Foundation of China (Nos. 82072160, 32130060, 81901933, 32000725), the Major Natural Science Research Projects in Universities of Jiangsu Province (No. 20KJA310012), the Natural Science Foundation of Jiangsu Province (Nos. BK20202013, BK20201209, BK20200973), the "QingLan Project" in Jiangsu Universities, the Priority Academic Program Development of Jiangsu Higher Education Institutions, and Nantong Science and Technology Program (Nos. JC22022037, MS22022010).
Author information
Mengyuan Chang, Yong Cai, and Zihui Gao have contributed equally to this work.
Authors and Affiliations
Key Laboratory of Neuroregeneration of Jiangsu and Ministry of Education, Co-Innovation Center of Neuroregeneration, NMPA Key Laboratory for Research and Evaluation of Tissue Engineering Technology Products, Nantong University, Nantong, 226001, Jiangsu, People’s Republic of China
Mengyuan Chang, Zihui Gao, Boya Liu, Cheng Zhang, Qianqian Cao, Yuntian Shen, Xinlei Yao & Hualin Sun
Department of Neurology, Binhai County People’s Hospital, Yancheng, 224500, Jiangsu, People’s Republic of China
Department of Neurology, Affiliated Hospital of Nantong University, Nantong, 226001, Jiangsu, People’s Republic of China
Department of Clinical Medicine, Medical College, Nantong University, Nantong, 226001, Jiangsu, People’s Republic of China
Department of Ultrasound, Affiliated Hospital of Nantong University, Nantong, 226001, Jiangsu, People’s Republic of China
Xiaoyang Chen
Research and Development Center for E-Learning, Ministry of Education, Beijing, 100816, People’s Republic of China
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization: HS, XY and XC. Methodology: MC, YC, ZG, XC, BL, CZ, WY, QC, YS. Resources: MC, YC, ZG, XC, BL, CZ, WY, QC, YS. Data curation: MC, YC, ZG, XC, BL, CZ, WY, QC, YS. Writing—original draft preparation: MC, YC, ZG, XY, XC and HS. Review and editing: MC, XY, XC and HS. Visualization: MC, YS, and HS. Supervision: HS. Project administration: HS. Funding acquisition: HS.
Corresponding authors
Correspondence to Xinlei Yao , Xiaoyang Chen or Hualin Sun .
Ethics declarations
Conflicts of interest.
All authors have no conflict of interest to disclose.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Chang, M., Cai, Y., Gao, Z. et al. Duchenne muscular dystrophy: pathogenesis and promising therapies. J Neurol 270 , 3733–3749 (2023). https://doi.org/10.1007/s00415-023-11796-x
Download citation
Received : 27 April 2023
Revised : 24 May 2023
Accepted : 25 May 2023
Published : 01 June 2023
Issue Date : August 2023
DOI : https://doi.org/10.1007/s00415-023-11796-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Duchenne muscular dystrophy
- Muscle atrophy
- Find a journal
- Publish with us
- Track your research
- Open access
- Published: 22 May 2021
The clinical course of Duchenne muscular dystrophy in the corticosteroid treatment era: a systematic literature review
- Shelagh M. Szabo ORCID: orcid.org/0000-0002-9044-3192 1 ,
- Renna M. Salhany 2 ,
- Alison Deighton 1 ,
- Meagan Harwood 1 ,
- Jean Mah 3 &
- Katherine L. Gooch 2
Orphanet Journal of Rare Diseases volume 16 , Article number: 237 ( 2021 ) Cite this article
9856 Accesses
41 Citations
Metrics details
Duchenne muscular dystrophy (DMD) is a severe rare progressive inherited neuromuscular disorder, leading to loss of ambulation (LOA) and premature mortality. The standard of care for patients with DMD has been treatment with corticosteroids for the past decade; however a synthesis of contemporary data describing the clinical course of DMD is lacking. The objective was to summarize age at key clinical milestones (loss of ambulation, scoliosis, ventilation, cardiomyopathy, and mortality) in the corticosteroid-treatment-era.
A systematic review was conducted using MEDLINE and EMBASE. The percentage experiencing key clinical milestones, and the mean or median age at those milestones, was synthesized from studies from North American populations, published between 2007 and 2018.
From 5637 abstracts, 29 studies were included. Estimates of the percentage experiencing key clinical milestones, and age at those milestones, showed heterogeneity. Up to 30% of patients lost ambulation by age 10 years, and up to 90% by 15 years of age. The mean age at scoliosis onset was approximately 14 years. Ventilatory support began from 15 to 18 years, and up to half of patients required ventilation by 20 years of age. Registry-based estimates suggest that 70% had evidence of cardiomyopathy by 15 years and almost all by 20 years of age. Finally, mortality rates up to 16% by age 20 years were reported; among those surviving to adulthood mortality was up to 60% by age 30 years.
Conclusions
Contemporary natural history studies from North America report that LOA on average occurs in the early teens, need for ventilation and cardiomyopathy in the late teens, and death in the third or fourth decade of life. Variability in rates may be due to differences in study design, treatment with corticosteroids or other disease-modifying agents, variations in clinical practices, and dystrophin mutations. Despite challenges in synthesizing estimates, these findings help characterize disease progression among contemporary North American DMD patients.
Duchenne muscular dystrophy (DMD) is a rare, progressive, life-limiting neuromuscular disorder [ 1 ] occurring in 15.9 to 19.5 per 100,000 live male births [ 2 , 3 , 4 ]. It is caused by mutations in the dystrophin gene [ 2 , 5 ]; lack of dystrophin compromises muscle structure and integrity, leading to progressive muscular degeneration [ 6 , 7 ]. Patients with DMD are typically identified in early childhood with symptoms including delays in motor milestones and frequent falls [ 8 ]. Over time, these patients experience progressive functional impairments leading to loss of ambulation (LOA), pulmonary insufficiency, cardiomyopathy, and early mortality [ 2 , 5 , 9 ].
Although there is presently no cure for DMD, advancements to the standard of care, including the introduction of systemic corticosteroids in the 1990s, have helped slow disease progression and improve survival [ 10 , 11 , 12 ]. However, the impact of these changes in standard of care across the full range of clinically-relevant disease progression milestones experienced by those with DMD has not been fully characterized. In 2017, Ryder et al. published a systematic review examining the epidemiology, burden, and treatment of DMD; however this review focused only on studies published between 2011 and 2015 [ 6 ]. Other reviews focused on the prevalence of DMD [ 13 ] or the impact of surgery on pulmonary decline [ 14 ]. While robust outcomes data are available from large cohort studies including the Cooperative International Neuromuscular Research Group (CINRG) [ 15 ], Duchenne Registry [ 16 ], and Centers for Disease Control and Prevention’s Muscular Dystrophy Surveillance, Tracking, and Research Network (MD STARnet) [ 17 ], a synthesis of data from recent studies is lacking [ 18 ]. The objective of this systematic review was to characterize the clinical course of DMD in the era of corticosteroid treatment in North America.
A comprehensive search of the Medline/Medline In-Process and EMBASE databases was performed (see Additional file 1 : Table S1 for search strategy), the design of which was guided by the study-specific PECOS (Population, Exposures, Comparators, Outcomes, Study design) criteria (Table 1 ). Studies published in English between database inception (1946) and November 2018 that reported estimates of the age at occurrence of key clinical milestones occur among males with DMD were selected. To focus on more generalizable outcomes from a more homogeneous set of patients, the review targeted observational studies from North America (or international studies including North America patients) that aimed to estimate the frequency of key clinical events from large (n > 50) samples of DMD patients treated with corticosteroids. Animal studies, or studies that included patients with other muscular dystrophies, were excluded.
Outcomes of interest that describe the clinical course of DMD included LOA, scoliosis, need for ventilatory support (stratified by any ventilation/type unspecified, non-invasive ventilation [NIV] or invasive ventilation [IV]), pulmonary dysfunction, cardiomyopathy, and mortality. Relevant measures included the mean or median age at the outcome of interest, or the percentage experiencing the outcome over time or at a particular time ( t ) . Scores on assessments of ambulatory, pulmonary, or cardiac function over a minimum of one year of follow-up were also included (Table 1 ). Two reviewers independently screened abstracts and potentially eligible full-text articles for inclusion, and any discrepancies were resolved through discussion to achieve consensus.
Data were extracted by two researchers; study characteristics extracted included authors, year, study duration, objective(s) and design, sample size, and inclusion and exclusion criteria. Patient characteristics included details of corticosteroid treatment and baseline demographics. Cohorts were classified as ‘corticosteroid-treated’ if all patients were so treated, ‘mixed corticosteroid use’ if the sample represented a mix of corticosteroid-treated and -untreated patients, and ‘likely corticosteroid-treated’ if the study was published after 2005 and did not state the sample was untreated . Available data on use of cardioprotective medications, such as angiotensin-converting enzyme (ACE) inhibitors, were also extracted where available.
For continuous variables, the mean, median, standard deviation (SD), confidence interval (CI), interquartile ranges (IQR), and range was extracted whenever available. For dichotomous and categorical variables, the number of patients and proportion was extracted. For studies reporting on the mean or median age at the outcome, the range of estimates was tabulated. The percentage of the sample who experienced the outcome at time of reporting was also described (where available). Data on the percentage experiencing the outcome at specific time points or over time were described using Kaplan–Meier (KM) curves, as well as presented as point estimates at time t by the original authors. Where available, scores on functional and clinical measures of interest over time were plotted using line graphs.
The strength of the available evidence was assessed using the STrengthening the Reporting of Observational studies in Epidemiology (STROBE) Statement for observational studies and non-randomized clinical trials [ 19 ].
The search strategy identified 5,637 potentially-relevant records; four (< 1%) were removed after de-duplication and 5,213 (92.5%) were excluded on abstract review (Fig. 1 ). Of the remaining 410 records, 381 were excluded on full-text review, leaving 29 eligible studies. Study designs included single-center or multicenter chart reviews and DMD registries (including 6 publications from CINRG and 4 publications from MD STARnet; Table 2 ). Available details of corticosteroid treatment (including the age at initiation, follow-up protocols, and frequency of reported side effects) are summarized in Additional file 1 : Table S2; however, the level of detail provided varied by study, and few studies examined how variability in parameters such as age at corticosteroid initiation impacted the clinical course of DMD. Available details of treatment with cardioprotective medications are summarized in Additional file 1 : Table S3. A summary of the quality of included studies in Additional file 1 : Table S4.
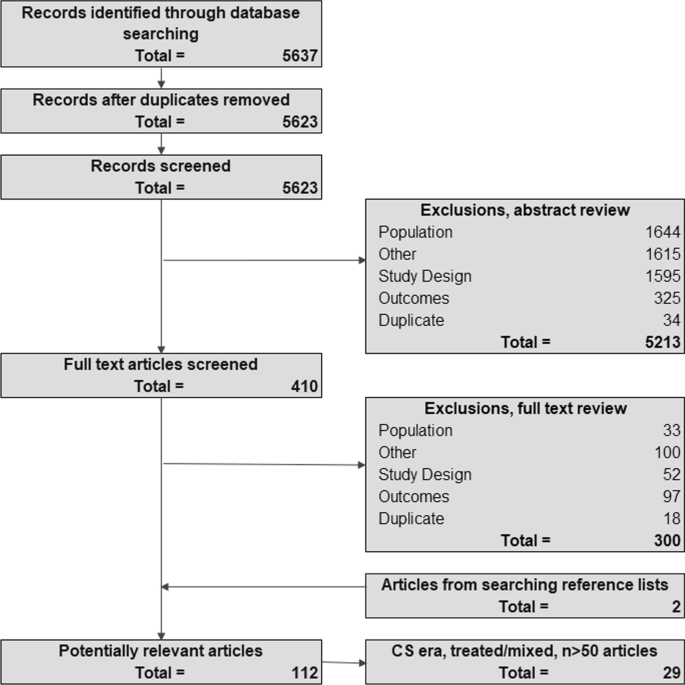
PRISMA diagram outlining study inclusion and exclusion. PRISMA Preferred Reporting Items for Systematic Reviews and Meta-Analyses, CS corticosteroid, RTC randomized controlled trial
- Loss of ambulation
Six studies reported on the mean age at [ 20 , 21 , 22 , 23 , 24 , 25 ], 10 studies on median age at [ 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 ], and 13 studies on the percentage experiencing LOA (Table 2 ) [ 20 , 22 , 23 , 24 , 25 , 28 , 29 , 30 , 31 , 33 , 34 , 36 , 37 ]. Two studies provided subgroup-specific estimates [ 21 , 31 ]. Among studies of corticosteroid-treated patients, the mean (SD) age at LOA ranged from 9.5 (0.2) years (among 112 patients from MD STARnet) [ 21 ] to 12.5 (3.0) years (in 68% of 75 patients from a single-center chart review [ 22 ]; Fig. 2 a). Estimates were similar from the three studies reporting on mixed corticosteroid use patients; the mean ages at LOA ranged from 9.8 (2.2) years (in 26.6% of 432 Mexican DMD patients) [ 24 ] to 10.8 (2.1) years (in 63.2% of 462 patients from MD STARnet) [ 20 ]. The earliest mean age at LOA (9.5 years) was observed among patients with ≤ 3 years of corticosteroid treatment, compared with 12.3 years among those with > 3 year corticosteroid use (MD STARnet) [ 21 ].
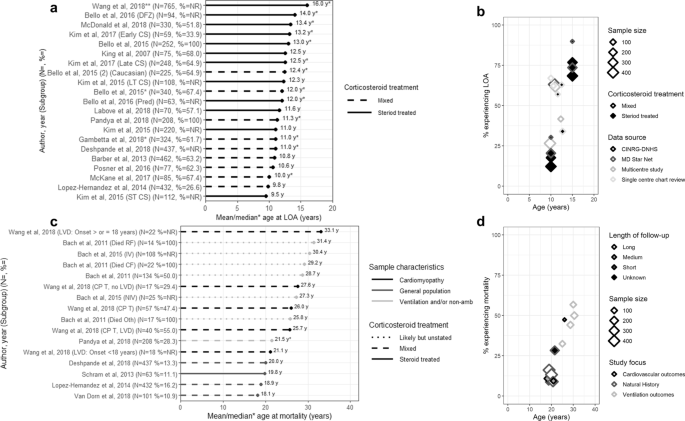
Age at LOA or mortality: a mean/median age at LOA; b LOA over time, c mean/median age at mortality; and d Mortality over time. LOA = loss of ambulation; CS = corticosteroid; LT = long-term; NR = not reported; ST = short term; yrs = years DFZ = deflazacort; NR = not reported; Pred = prednisone; yrs = years; CINRG-DNHS = The Cooperative International Neuromuscular Research Group Duchene Natural History Study; MD STARnet = Muscular Dystrophy Surveillance, Tracking, and Research Network; CM = cardiomyopathy; CPT = cardiopulmonary therapies; Died RF = died from respiratory failure; Died CF = died cardiac failure; Died Oth = died from other causes; IV = invasive ventilation; LVD = left ventricular dysfunction; NIV = non-invasive ventilation; CV = cardiovascular. Notes **Middle value in range of medians. Long follow up = 10–20 years; median follow up = 5.4–7.1 years; short follow up = 1.9–2 years; unknown = not reported
Thirteen estimates from ten studies described median age at LOA (Fig. 2 b) [ 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 ]. Estimates from 7 studies of corticosteroid-treated samples ranged from 12.0 (11.3–14.0) years (in 63 patients from CINRG) [ 29 ] to 16.0 (NR) years (in 765 patients from the Duchenne Registry) [ 26 ]. The latter study reported age at LOA by genotype, from 12 years (patients with exon 51 and 53 skip amenable mutations) to 20 years (patients with exon 44 skip amenable mutations). Six studies reported estimates from mixed corticosteroid use samples, and the range was tighter; from 10.0 (range: 4.0–14.0) years (in 67.4% of 85 patients from a single-center chart review) [ 34 ] to 12.4 years (in 64.9% of 225 patients from CINRG) [ 29 ].
The percentage who experienced LOA increased with time (Fig. 2 c) [ 20 , 22 , 23 , 24 , 25 , 28 , 29 , 30 , 31 , 33 , 34 , 36 , 37 ], from 12.3% at 10 years (from 223 corticosteroid-treated CINRG patients) [ 30 ] to 89.9% at 15 years (from 53 corticosteroid-treated MD STARnet patients) [ 31 ]. Estimates from longitudinal studies report that up to 30% of DMD patients lose ambulation by 10 years (CINRG) [ 28 ], and 90% by 15 years (MD STARnet) [ 31 ]. While these effects were fairly consistent across studies of different sample sizes, mixed corticosteroid use samples tended to have higher rates of LOA at a given age than corticosteroid-treated samples.
One study reported the mean age at scoliosis [ 38 ], 2 studies the median age at scoliosis [ 31 , 35 ], and 5 studies the percentage with scoliosis by age (Table 2 ) [ 22 , 30 , 31 , 35 , 37 ]. How scoliosis was defined varied across studies. In a single-center study of 56 patients, the mean age at spinal surgery was 14.0 years; and 14.5 years in the subset (n = 20) undergoing pulmonary function testing (Fig. 3 a) [ 38 ]. The median (range) age at scoliosis surgery among a mixed corticosteroid use sample from MD STARnet was 14.6 (10.2–20.2) years (with surgery observed in 52.4% of n = 208) [ 35 ]. In the remaining study of 274 corticosteroid-treated patients (also from MD STARnet), the median (range) age (by spinal curvature > 30° or surgery) was 14.2 (12.5–15.6) years among the 107 patients with scoliosis [ 31 ]. The percentage with scoliosis increased with increasing age (Fig. 3 b) [ 22 , 30 , 31 , 35 , 37 ]. Results from a longitudinal study from MD STARnet suggest that up to 59% of patients with DMD will have scoliosis by 15 years of age, and up to 72% by 20 years of age [ 31 ].
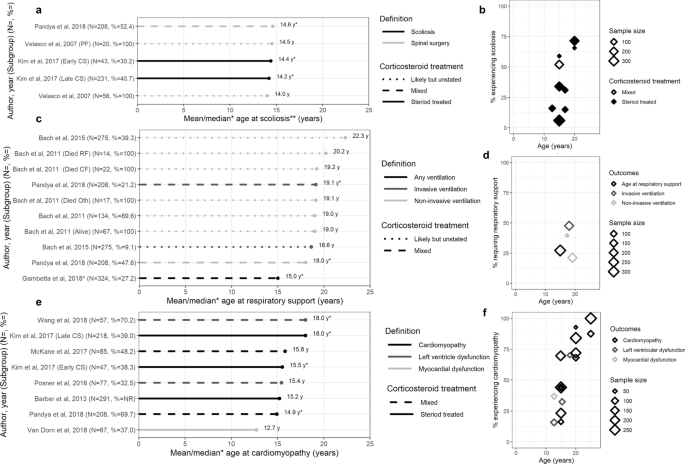
Occurrence of other key clinical milestones: a Mean/median age at scoliosis; b Percentage with scoliosis over time; c Mean/median age at respiratory support; d Percentage on respiratory support over time; e Mean/median age at cardiomyopathy; f Percentage with cardiomyopathy over time. 6MWD = 6 min walk distance; PEF = peak expiratory flow; FVC = forced vital capacity; SF = shortening fraction; LVED = left ventricular end-diastolic dimension; EF = ejection fraction. Notes: **Scoliosis includes both severe scoliosis and spinal surgery
Pulmonary function and need for ventilatory support
Four studies reported the mean or median age at ventilation [ 33 , 35 , 39 , 40 ], 3 studies reported the percentage needing ventilation by age [ 30 , 33 , 35 ], 4 studies reported the age at transitioning to key pulmonary functional milestones [ 30 , 41 , 42 , 43 ], and 2 studies reported pulmonary function over time (Table 2 ) [ 42 , 43 ].
In terms of age at need for ventilation, one multicenter chart review of 324 mixed corticosteroid-treated DMD patients reported a median age at ‘any ventilation’ of 15 years (Fig. 3 c) [ 33 ]. Three studies reported the age at NIV to range from a median (IQR) age of 18.0 (9.4–26.8) years (in 47.6% of 208 mixed corticosteroid-treated patients on nasal NIV from MD STARnet) [ 35 ], to a mean of 22.3 (4.7) years (in 39.3% of 275 likely-corticosteroid-treated patients receiving continuous NIV in a single-center chart review) [ 39 ]. Two studies reported age at IV; a single-center chart review reporting a mean (SD) age of 18.6 (2.3) years (in 9.1% of 275 likely-corticosteroid-treated patients with continuous tracheostomy mechanical ventilation) [ 39 ], and an MD STARnet study reporting a median (IQR) age of 19.1 (13.4–27.0) years (in 21.2% in 208 mixed-corticosteroid-treated patients with tracheostomy) [ 35 ].
The percentage of patients requiring ventilation tended to increase over time, with variability in estimates observed due to type of ventilation (Fig. 3 d) [ 30 , 33 , 35 ]. By 20 years of age, 27.2% (n = 88) of mixed corticosteroid use patients in a multicenter chart review required ‘any ventilation’ [ 33 ]. Two studies describing NIV reported estimates of 21.2% (among 44 corticosteroid-treated patients from MD STARnet) [ 35 ], and 39.6% (among 21 mixed corticosteroid-treated patients from CINRG) [ 30 ] by 20 years. The MD STARnet study also reported that 47.6% of patients with mixed corticosteroid use were on IV by 20 years [ 35 ].
Absolute measures of pulmonary function generally show relatively preserved function until adolescence, which declines with increasing age (Fig. 4 a, c). Two studies reported absolute and percent predicted peak expiratory flow (PEF). A substantial decline in PEF was observed among 330 corticosteroid-treated CINRG patients, from 243.7 L/min (age = 17 years) to 76.1 L/min (age = 29 years). Trends were similar among 60 mixed corticosteroid-treated patients from a single-center chart review (from 269.4 L/min [age = 18 years] to 67.9 L/min [age = 24 years]) [ 42 ]. Estimates of percent predicted PEF show loss of function relative to age-matched healthy controls; the magnitude increases with age (Fig. 4 a), reaching a low of 11.8% by age 29 years in the CINRG study. Those same two studies also reported FVC (L) over time (Fig. 4 d) which demonstrated an initial increase in function followed by progressive decline after approximately 15 years; the percent predicted FVC showed loss of function relative to age-matched controls with increasing age, to 10.4% at 29 years of age (Fig. 4 c) [ 42 , 43 ].
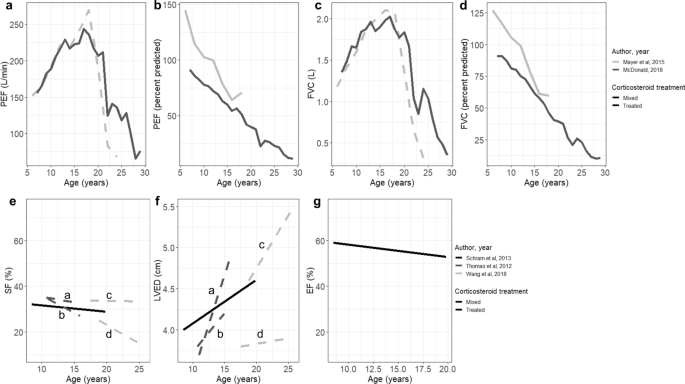
Measures of functional status over time: a – d pulmonary function measures; e – g cardiac function measures. PEF = peak expiratory flow; FVC = forced vital capacity; SF = shortening fraction; LVED = left ventricular end-diastolic dimension; EF = ejection fraction. Notes a = HR in the upper quartile (> 96 BPM), b = HR in the lower quartile (≤ 96 BPM), c = Left ventricular dysfunction, d = No Left ventricular dysfunction
Four studies reported the age at transitioning to key pulmonary milestones; specifically, reaching FVC < 1L, FVC < 30% or PEF < 30% [ 30 , 41 , 42 , 43 ]. FVC < 1L was first reported at 20 years of age in the 60 mixed corticosteroid-treated patients from a single-center chart review [ 42 ] and 23 years of age in a CINRG study of 330 corticosteroid-treated patients [ 30 ]. Mean (SD) ages at FVC < 30% and PEF < 30% were similar from a CINRG study of 223 mixed corticosteroid-treated patients (FVC < 30%: 24.0 (1.5) years, and PEF < 30%: 24.9 (0.8) years); the same CINRG study also reported that 50% progressed to FVC < 30% or PEF < 30% by 25 years of age [ 41 ]. Estimates of the percentage with severe pulmonary dysfunction (FVC < 50%) at 20 years of age ranged from 13.6% [ 43 ] to 29.7% [ 30 ]. Finally, among 330 corticosteroid-treated patients from CINRG, among those with LOA at < 10 years, the median age at FVC < 1L was 18.1 years, vs 20.1 years among those with LOA between 10–13 years of age, and 24.4 years among patients with LOA at ≥ 13 years [ 30 ].
Cardiac function and cardiomyopathy
Seven studies reported the mean or median age at diagnosis [ 20 , 25 , 26 , 31 , 34 , 35 , 44 ] and 9 studies reported the percentage of patients with cardiomyopathy [ 20 , 25 , 31 , 34 , 35 , 37 , 44 , 45 , 46 ]; 3 studies reported changes in cardiac function over time (Table 2 ) [ 45 , 46 , 47 ].
Of the 7 studies reporting the age at cardiomyopathy, 5 described samples not selected using cardiovascular-risk-related criteria (Fig. 3 e) [ 20 , 31 , 34 , 35 , 44 ]. The mean (SD) age at cardiomyopathy ranged from 12.7 (3.0) years (in 37.0% of 67 corticosteroid-treated patients from a multicenter chart review) [ 44 ] to 15.8 (range: 9–29) years (in 48.2% of 85 patients of mixed corticosteroid-treatment status from a single-center chart review) [ 34 ]. Estimates of median (IQR) age at cardiomyopathy ranged from 14.9 (4.9) years (in 69.7% of 208 mixed corticosteroid-treated patients from MD STARnet) [ 35 ] to 18.0 (CI: 6.9–18.5) years (in 39.0% of 218 corticosteroid-treated patients from MD STARnet) [ 31 ]. The reported age at cardiomyopathy was lower among two studies reporting on mixed corticosteroid-treated samples either treated with cardiopulmonary therapy (median 18.0 (7.0–27.3) years, in 70.2% of 57 patients) [ 46 ], or with LV dysfunction (mean, 15.4 (8–27) years, in 32.5% of 77 patients) [ 25 ].
The percentage with cardiomyopathy was higher with increasing age (Fig. 3 f) [ 20 , 25 , 31 , 34 , 35 , 37 , 44 , 45 , 46 ]; this effect was consistent across studies of different sample sizes. At 15 years of age, the percentage with cardiomyopathy ranged from 23.3% (among 218 patients who initiated corticosteroids after 5 years of age) [ 31 ] to 69.7% (among 208 mixed corticosteroid-treated patients) [ 35 ]; both estimates were from MD STARnet. By 20 years of age, the percentage with cardiomyopathy ranged from 68.2% (of 85 mixed corticosteroid-treated patients from a single-center chart review) [ 34 ] to 92.8% (of 47 patients who initiated corticosteroids before 5 years of age from MD STARnet) [ 31 ]. By age 25, the percentage with cardiomyopathy ranged from 87.6% (of 85 mixed corticosteroid-treated patients from a single-center chart review) [ 34 ] to 100% (291 corticosteroid-treated patients from MD STARnet) [ 20 ].
Measures of cardiac function show preserved function until adolescence and then decline with age (Fig. 4 e–g) [ 45 , 46 , 47 ]. In a long-term observational study of 63 DMD patients treated with cardiopulmonary therapies and corticosteroids, the ejection fraction decreased to 53% by 20 years of age [ 45 ]. That study and two other single-center studies also reported worsening of cardiac function by left ventricular end diastolic diameter (LVED) and shortening fraction (SF) among corticosteroid-treated patients with DMD [ 45 , 46 , 47 ].
Eight studies reported the mean age [ 24 , 32 , 35 , 39 , 40 , 44 , 45 , 46 ] and 1 study the median age at mortality [ 35 ]; 9 studies reported case fatality by age (Table 2 ) [ 24 , 30 , 32 , 34 , 35 , 40 , 44 , 46 , 48 ].
Of the 8 studies reporting mean (SD) age at mortality [ 24 , 32 , 35 , 39 , 40 , 44 , 45 , 46 ], 4 were reflective of the overall DMD population [ 24 , 32 , 44 , 45 ] one was from a sample of DMD patients with cardiomyopathy [ 46 ], and 3 were from samples of patients who were non-ambulatory or on ventilation [ 35 , 39 , 40 ]. From studies of the overall population, the mean (SD) age at mortality ranged from 18.1 (3.8) years (in 11% of 101 mixed corticosteroid-treated patients from a multicenter chart review) [ 44 ] to 20.0 (15–31) years (in 13% of 437 mixed corticosteroid-treated patients from an administrative database study; Fig. 2 d) [ 32 ]. In the single study that described outcomes among DMD patients with cardiomyopathy, the mean (SD) age at mortality was 26.0 (6.8) years (in 47.4% of 57 mixed corticosteroid-treated patients from a single-center chart review; Fig. 2 d). The mean (SD) age at mortality among DMD patients who were non-ambulatory or on ventilation ranged from 25.8 (7.8) years (in 17 likely-corticosteroid-treated patients from a single-center chart review, who died from causes other than respiratory or cardiac dysfunction) [ 40 ] to 31.4 (5.7) years (in 14 likely-corticosteroid-treated patients from that single-center chart review, with death due to respiratory complications; Fig. 2 d) [ 40 ]. The median (IQR) age at mortality among DMD patients who were non-ambulatory or on ventilation was 21.5 (3.8) years (in 28.3% of 208 mixed corticosteroid-treated patients from MD STARnet; Fig. 2 d) [ 35 ].
In terms of the proportion surviving over time, up to 16.2% mortality was reported by age 20 years (Fig. 2 e) [ 24 ]. Estimates of survival after 20 years are available only from studies enrolling adult patients with DMD; and these reported rates of 44.2% to 56.8% mortality by age 30 years (Fig. 2 e) [ 40 ].
A comprehensive systematic review was conducted to identify estimates of the age at key clinical milestones, and trajectories on relevant functional measures over time, among studies including North American patients with DMD. Age at LOA was the most widely reported with estimates available from many large studies; these tended to range from 10 to 14 years of age [ 27 , 34 ]. However, robust data on the timing of the onset of scoliosis-, cardiac-, pulmonary- and ventilation-related outcomes were less frequently presented, particularly from large longitudinal studies. While reported estimates of the mean age at diagnosis of scoliosis were fairly consistent across studies (at 14–15 years of age), how scoliosis was classified differed widely [ 31 , 35 , 38 ]. Pulmonary function in DMD patients declines with age from the mid-teens [ 30 , 41 ], and while most have severe pulmonary dysfunction by 25 years [ 30 ], the mean age at initiation of ventilatory support ranged from 15 to 22 years depending on the type of ventilation considered and treatment center [ 33 , 39 ]. Data on age at mortality in DMD were also variable, and estimates were impacted by the inclusion criteria of the individual studies; for example estimates of mortality among those with cardiomyopathy or on ventilation were drawn from populations surviving to adulthood [ 40 , 46 ]. In addition to selection criteria, factors impacting the timing of key clinical milestones include corticosteroid regimen [ 31 ] and disease genotype [ 26 ]. The findings of this review help summarize the likely timing of disease progression milestones for North American patients with DMD, and also highlight potential heterogeneity in timing observed both within and across study populations.
Estimates of time to key clinical milestones in this review included data from studies from the large North American registries (e.g. CINRG and MD STARnet), and findings are consistent with those from large observational studies and registries from outside of North America. The Translational Research in Europe—Assessment and Treatment of Neuromuscular Diseases (TREAT-NMD) network of DMD registries have published studies documenting the clinical course of patients with DMD [ 49 , 50 , 51 , 52 ]. In a large survey of over 1500 DMD patients that characterized the impact of corticosteroid use, mean estimates of age at LOA ranged from 10.1 (non-corticosteroid-treated patients) to 11.4 (corticosteroid-treated) years [ 49 ]. An analysis of over 5000 patients also from TREAT-NMD reported age at LOA of 13 years among corticosteroid-treated patients, and that up to 50% of patients required ventilation by 20 years of age [ 50 ]
That longitudinal data describing survival specifically among North American DMD patients are few, was one of the major gaps identified in this review. However, mortality rates from included studies were consistent with findings of two important studies on mortality in dystrophin gene-related muscular dystrophy, which did not meet the inclusion criteria for the current review as they also included patients with Becker muscular dystrophy (BMD). The first study, which was based on vital statistics, estimated that 71% of mortality among those with BMD/DMD occurred between the ages of 15 and 29 years; the authors assumed it was most likely related to DMD [ 53 ]. The second study, from MD STARnet, estimated mortality in almost 60% of that cohort by age 25 years, with most deaths occurring among those aged 20 to 25 years [ 54 ]. Further follow-up from existing large DMD cohorts will help improve contemporary estimates of the timing of key clinical milestones.
Accurately estimating the time of onset of gradually progressive manifestations of DMD can be difficult, and this along with changes in practice patterns and symptom detection, contribute to observed variability in estimates. For example, many studies reporting on scoliosis classify outcomes based on surgery, however with changing treatment patterns [ 55 ] the utility of surgery as a proxy for clinically-significant scoliosis will decrease. Similarly, recommended strategies for ventilation vary among clinical centers [ 39 , 56 , 57 ], and practice is changing (in particular for how IV is used) [ 58 ], which will impact the comparability of estimates of the timing of respiratory decline across studies from different periods. Finally for cardiomyopathy, with advancements in screening tools [ 59 , 60 ] as well as evidence of benefits to early treatment [ 61 ], it is likely that initial signs will now be detected earlier, which would result in an apparent decrease in the mean age at cardiomyopathy over the coming years.
There are several additional factors impacting the timing of key clinical milestones that require consideration. To capture the impact of corticosteroids in the management of DMD, only studies including patients from the corticosteroid treatment era were included. While details of corticosteroid treatment regimens were extracted and reviewed, there were important limitations that precluded analyzing outcomes according to regimen. First, details on the timing of initiation, duration, type, and dose varied within and between studies. Only a small number of studies reporting on LOA presented results according to agent; but the remainder of the studies for that outcome, and all of the studies for other outcomes of interest, did not stratify by corticosteroid regimen. However, variations in corticosteroid treatment patterns (in terms of duration and dosing) may have affected the timing when patients reached LOA [ 28 , 31 , 62 , 63 ], and other important clinical milestones [ 20 , 31 , 46 , 62 , 63 , 64 , 65 ]. Evidence on the impact of early initiation of corticosteroids (e.g. before age 6 years) remains mixed [ 31 , 66 ]; more work is needed to disentangle the potential confounding effect of disease severity and the potential risk for adverse effects of corticosteroid treatment on outcomes in real-world studies. Treatment with ACE inhibitors has also been shown to impact the clinical course of DMD by delaying the onset of cardiomyopathy; however, the use of ACE inhibitors remains variable [ 20 , 67 ]. While it might be anticipated that studies describing later cohorts would show delayed onset of milestones that define the clinical course of DMD, the interplay between treatment advances and the impact of earlier diagnostics would make these relationships less apparent. Finally, other genetic modifiers may also play a role in the timing of DMD progression [ 26 , 29 , 50 ]; however, outcomes according to genotype are infrequently reported outside of treatment trials [ 68 , 69 ]. The move from biomarkers to precise genetic diagnosis may also impact the apparent clinical course [ 70 ].
Variability in methodology and data sources may also have affected estimates. Data from the CINRG and MD STARnet registries, both large well-documented US cohorts that comprehensively collect longitudinal data on the clinical course of DMD, were used in ten studies within this review. Outside of those, most observational studies and treatment trials do not follow patients for a sufficient time to describe changes across the range of key clinical milestones [ 21 , 30 ]. Other challenges for studying disease progression in rare diseases include small sample sizes which can amplify the impact of heterogeneity in diseases with varied clinical courses; data presented from convenience samples and case series may not be generalizable, and the impact of selection biases on outcomes (particularly for diseases with high early fatality among more severe cases) can be substantial [ 71 , 72 ]. The numerous outcome measures used to assess progression in DMD also make comparisons difficult, a limitation recently acknowledged in a workshop held by the DMD research community [ 73 ]. Finally, there are useful measures for characterizing DMD progression that were infrequently reported in the studies of this review, such as the North Star Ambulatory Assessment or upper arm function, which are important in understanding patient functional status and ability to participate in activities of daily living.
Some limitations to the published data warrant mention. First, while time to event data using KM curves were presented in some studies, many reported the mean age at an occurrence where the entire sample had not experienced the event at the time of study reporting. As such, these values can be interpreted as the lower limit for when key clinical milestones will occur in DMD. Second, some measures may only be administered to individuals who still have some functional capacity (e.g. tests of ambulation), and patients unable to complete the test would have been excluded. This type of survival bias would result in an inflation of apparent functional status for cohorts as a whole. Third, mean scores on functional tests may reflect the inclusion criteria of each study, rather than the underlying distribution of scores on that functional test among the DMD population. Fourth, because of heterogeneity in designs employed, measures selected, and populations included across studies, meta-analysis was judged to be infeasible [ 74 , 75 ]; as a result, overall summary estimates of the time to key clinical milestones were not calculable.
This is the first systematic review of published estimates of the frequency and timing of important milestones that characterize the clinical course of DMD in the corticosteroid era. This review has also leant insight into a number of challenges in the interpretation and comparison of estimates of outcomes to characterize the clinical course of DMD. Additional studies on the ages at occurrence of other important DMD clinical milestones, and the relationships between short-term and long-term outcomes, will be valuable in the continuation of knowledge regarding disease progression in DMD.

Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
Angiotensin-converting enzyme
Becker muscular dystrophy
Confidence interval
Cooperative International Neuromuscular Research Group
- Duchenne muscular dystrophy
Forced vital capacity
Interquartile ranges
Invasive ventilation
Kaplan–Meier
Left ventricle
Left ventricular end diastolic diameter
Muscular Dystrophy Surveillance, Tracking, and Research Network
Non-invasive ventilation
Peak expiratory flow
Standard deviation
Shortening fraction
STrengthening the Reporting of Observational studies in Epidemiology
Translational Research in Europe—Assessment and Treatment of Neuromuscular Diseases
Birnkrant DJ, Bushby K, Bann CM, Apkon SD, Blackwell A, Brumbaugh D, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. Lancet Neurol. 2018;17(3):251–67.
Article PubMed PubMed Central Google Scholar
Wein N, Alfano L, Flanigan KM. Genetics and emerging treatments for Duchenne and Becker muscular dystrophy. Pediatr Clin North Am. 2015;62(3):723–42.
Article PubMed Google Scholar
Mendell JR, Shilling C, Leslie ND, Flanigan KM, al-Dahhak R, Gastier-Foster J, et al. Evidence-based path to newborn screening for Duchenne muscular dystrophy. Ann Neurol. 2012;71(3):304–13.
Article CAS PubMed Google Scholar
Moat SJ, Bradley DM, Salmon R, Clarke A, Hartley L. Newborn bloodspot screening for Duchenne muscular dystrophy: 21 years experience in Wales (UK). Eur J Hum Genet. 2013;21(10):1049.
Article CAS PubMed PubMed Central Google Scholar
Yiu EM, Kornberg AJ. Duchenne muscular dystrophy. J Paediatr Child Health. 2015;51(8):759–64.
Ryder S, Leadley RM, Armstrong N, Westwood M, De Kock S, Butt T, et al. The burden, epidemiology, costs and treatment for Duchenne muscular dystrophy: an evidence review. Orphanet J Rare Dis. 2017;12(1):79.
Medicine; UNLo. Genetics home reference: DMD gene 2017. https://ghr.nlm.nih.gov/gene/DMD .
Ryder S, Leadley R, Armstrong N, Westwood M, de Kock S, Butt T, et al. The burden, epidemiology, costs and treatment for Duchenne muscular dystrophy: an evidence review. Orphanet J Rare Dis. 2017;12(1):79.
Mirski KT, Crawford TO. Motor and cognitive delay in Duchenne muscular dystrophy: implication for early diagnosis. J Pediatr. 2014;165(5):1008–10.
Birnkrant J, Bennett D, Noritz G, Harrington M, Birnkrant D. Prolonged survival and end-of-life care among end-stage patients with Duchenne muscular dystrophy (DMD). Chest. 2011;140(4):1054A.
Article Google Scholar
Birnkrant DJ, Ararat E, Mhanna MJ. Cardiac phenotype determines survival in Duchenne muscular dystrophy. Pediatr Pulmonol. 2016;51(1):70–6.
Andrews JG, Wahl RA. Duchenne and Becker muscular dystrophy in adolescents: current perspectives. Adolesc Health Med Ther. 2018;9:53–63.
Mah JK, Korngut L, Dykeman J, Day L, Pringsheim T, Jette N. A systematic review and meta-analysis on the epidemiology of Duchenne and Becker muscular dystrophy. Neuromuscul Disord. 2014;24(6):482–91.
Roberto R, Fritz A, Hagar Y, Boice B, Skalsky A, Hwang H, et al. The natural history of cardiac and pulmonary function decline in patients with Duchenne muscular dystrophy. Spine. 2011;36(15):E1009–17.
McDonald CM, Henricson EK, Abresch RT, Han JJ, Escolar DM, Florence JM, et al. The cooperative international neuromuscular research group Duchenne natural history study—a longitudinal investigation in the era of glucocorticoid therapy: design of protocol and the methods used. Muscle Nerve. 2013;48(1):32–54.
Parent Project Muscular Dystrophy. The Duchenne registry. 2019. https://www.duchenneregistry.org/ .
Centers for Disease Control and Prevention. Muscular Dystrophy Research and Tracking. 2019. https://www.cdc.gov/ncbddd/musculardystrophy/research.html .
Jewell NP. Natural history of diseases: statistical designs and issues. Clin Pharmacol Ther. 2016;100(4):353–61.
von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–7.
Barber BJ, Andrews JG, Lu Z, West NA, Meaney FJ, Price ET, et al. Oral corticosteroids and onset of cardiomyopathy in Duchenne muscular dystrophy. J Pediatr. 2013;163(4):1080-4.e1.
Kim S, Campbell KA, Fox DJ, Matthews DJ, Valdez R. STARnet MD. Corticosteroid treatments in males with Duchenne muscular dystrophy: treatment duration and time to loss of ambulation. J Child Neurol. 2015;30(10):1275–80.
King WM, Ruttencutter R, Nagaraja HN, Matkovic V, Landoll J, Hoyle C, et al. Orthopedic outcomes of long-term daily corticosteroid treatment in Duchenne muscular dystrophy. Neurology. 2007;68(19):1607–13.
Labove L, Jaworski M, Nguyen CTE. Duchenne muscular dystrophy: do boys with a shorter stature maintain ambulation longer? J Neuromuscul Dis. 2018;5(Supplement 1):S142–3.
Google Scholar
Lopez-Hernandez LB, Gomez-Diaz B, Escobar-Cedillo RE, Gama-Moreno O, Camacho-Molina A, Soto-Valdes DM, et al. Duchenne muscular dystrophy in a developing country: challenges in management and genetic counseling. Genet Couns. 2014;25(2):129–41.
CAS PubMed Google Scholar
Posner AD, Soslow JH, Burnette WB, Bian A, Shintani A, Sawyer DB, et al. The correlation of skeletal and cardiac muscle dysfunction in Duchenne muscular dystrophy. J Neuromuscul Dis. 2016;3(1):91–9.
Wang RT, Barthelemy F, Martin AS, Douine ED, Eskin A, Lucas A, et al. DMD genotype correlations from the Duchenne Registry: endogenous exon skipping is a factor in prolonged ambulation for individuals with a defined mutation subtype. Hum Mutat. 2018;39(9):1193–202.
Bello L, Morgenroth LP, Gordish-Dressman H, Hoffman EP, McDonald CM, Cirak S. DMD genotypes and loss of ambulation in the CINRG Duchenne Natural History Study. Neurology. 2016;87(4):401–9.
Bello L, Gordish-Dressman H, Morgenroth LP, Henricson EK, Duong T, Hoffman EP, et al. Prednisone/prednisolone and deflazacort regimens in the CINRG Duchenne Natural History Study. Neurology. 2015;85(12):1048–55.
Bello L, Kesari A, Gordish-Dressman H, Cnaan A, Morgenroth LP, Punetha J, et al. Genetic modifiers of ambulation in the cooperative international Neuromuscular research group Duchenne natural history study. Ann Neurol. 2015;77(4):684–96.
McDonald CM, Henricson EK, Abresch RT, Duong T, Joyce NC, Hu F, et al. Long-term effects of glucocorticoids on function, quality of life, and survival in patients with Duchenne muscular dystrophy: a prospective cohort study. The Lancet. 2018;391(10119):451–61.
Article CAS Google Scholar
Kim S, Zhu Y, Romitti PA, Fox DJ, Sheehan DW, Valdez R, et al. Associations between timing of corticosteroid treatment initiation and clinical outcomes in Duchenne muscular dystrophy. Neuromuscul Disord. 2017;27(8):730–7.
Deshpande SR, Wittlieb-Weber CA, Gambetta KE, Bock MJ, Lal AK, Conway JL, et al. Duchenne muscular dystrophy-related acute heart failure: multicenter study of hospitalizations and outcomes. J Heart Lung Transpl. 2018;37(4 Supplement 1):S388–9.
Gambetta K, Wittlieb-Weber C, Bock M, Villa C, Johnson J, Lal A, et al. Impact of genotype on boys with duchenne muscular dystrophy. Journal of Heart and Lung Transplantation. 2018;37 (4 Supplement 1):S122.
McKane M, Soslow JH, Xu M, Saville BR, Slaughter JC, Burnette WB, et al. Does body mass index predict premature cardiomyopathy onset for Duchenne muscular dystrophy? J Child Neurol. 2017;32(5):499–504.
Pandya S, James KA, Westfield C, Thomas S, Fox DJ, Ciafaloni E, et al. Health profile of a cohort of adults with Duchenne muscular dystrophy. Muscle Nerve. 2018;58(2):219–23.
Barnard AM, Willcocks RJ, Finanger EL, Daniels MJ, Triplett WT, Rooney WD, et al. Skeletal muscle magnetic resonance biomarkers correlate with function and sentinel events in Duchenne muscular dystrophy. PLoS ONE. 2018;13(3):e0194283.
Article PubMed PubMed Central CAS Google Scholar
Wong BL, Rybalsky I, Shellenbarger KC, Tian C, McMahon MA, Rutter MM, et al. Long-term outcome of interdisciplinary management of patients with Duchenne muscular dystrophy receiving daily glucocorticoid treatment. J Pediatr. 2017;182:296-303.e1.
Velasco MV, Colin AA, Zurakowski D, Darras BT, Shapiro F. Posterior spinal fusion for scoliosis in Duchenne muscular dystrophy diminishes the rate of respiratory decline. Spine. 2007;32(4):459–65.
Bach JR, Tran J, Durante S. Cost and physician effort analysis of invasive vs. noninvasive respiratory management of Duchenne muscular dystrophy. Am J Phys Med Rehabil. 2015;94(6):474–82.
Bach JR, Martinez D. Duchenne muscular dystrophy: continuous noninvasive ventilatory support prolongs survival. Respir Care. 2011;56(6):744–50.
Henricson E, McDonald C, Gordish-Dressman H, Abresch T, Cnaan A. Steroid use delays but does not prevent loss of pulmonary function in patients with Duchene muscular dystrophy (DMD). Dev Med Child Neurol. 2017;59(Supplement 4):30.
Mayer OH, Finkel RS, Rummey C, Benton MJ, Glanzman AM, Flickinger J, et al. Characterization of pulmonary function in Duchenne muscular dystrophy. Pediatr Pulmonol. 2015;50(5):487–94.
McDonald CM, Gordish-Dressman H, Henricson EK, Duong T, Joyce NC, Jhawar S, et al. Longitudinal pulmonary function testing outcome measures in Duchenne muscular dystrophy: long-term natural history with and without glucocorticoids. Neuromuscul Disord. 2018;27:S115–6.
Van Dorn CS, Puchalski MD, Weng HY, Bleyl SB, Butterfield RJ, Williams RV. DMD mutation and LTBP4 haplotype do not predict onset of left ventricular dysfunction in Duchenne muscular dystrophy. Cardiol Young. 2018;28(7):910–5.
Schram G, Fournier A, Leduc H, Dahdah N, Therien J, Vanasse M, et al. All-cause mortality and cardiovascular outcomes with prophylactic steroid therapy in Duchenne muscular dystrophy. J Am Coll Cardiol. 2013;61(9):948–54.
Wang M, Birnkrant DJ, Super DM, Jacobs IB, Bahler RC. Progressive left ventricular dysfunction and long-term outcomes in patients with Duchenne muscular dystrophy receiving cardiopulmonary therapies. Open Heart. 2018;5(1):e000783.
Thomas TO, Morgan TM, Burnette WB, Markham LW. Correlation of heart rate and cardiac dysfunction in Duchenne muscular dystrophy. Pediatr Cardiol. 2012;33(7):1175–9.
Connolly AM, Florence JM, Zaidman CM, Golumbek PT, Mendell JR, Flanigan KM, et al. Clinical trial readiness in non-ambulatory boys and men with Duchenne muscular dystrophy: MDA-DMD network follow-up. Muscle Nerve. 2016;54(4):681–9.
Vry J, Gramsch K, Rodger S, Thompson R, Steffensen BF, Rahbek J, et al. European cross-sectional survey of current care practices for Duchenne muscular dystrophy reveals regional and age-dependent differences. J Neuromuscul Dis. 2016;3(4):517–27.
Koeks Z, Bladen CL, Salgado D, Van Zwet E, Pogoryelova O, McMacken G, et al. Clinical outcomes in Duchenne muscular dystrophy: a study of 5345 patients from the TREAT-NMD DMD global database. J Neuromuscul Dis. 2017;4(4):293–306.
Bladen CL, Rafferty K, Straub V, Monges S, Moresco A, Dawkins H, et al. The TREAT-NMD Duchenne muscular dystrophy registries: conception, design, and utilization by industry and academia. Hum Mutat. 2013;34(11):1449–57.
Thangarajh M, Hendriksen J, McDermott MP, Martens W, Hart KA, Griggs RC, et al. Relationships between DMD mutations and neurodevelopment in dystrophinopathy. Neurology. 2019;93(17):e1597–604.
PubMed PubMed Central Google Scholar
Salzberg DC, Mann JR, McDermott S. Differences in race and ethnicity in muscular dystrophy mortality rates for males under 40 years of age, 2006–2015. Neuroepidemiology. 2018;50(3–4):201–6.
Anonymous. Prevalence of Duchenne/Becker muscular dystrophy among males aged 5–24 years—four states, 2007. Morb Mortal Wkly Rep. 2009; 58(40):1119–22.
Raudenbush BL, Thirukumaran CP, Li Y, Sanders JO, Rubery PT, Mesfin A. Impact of a Comparative Study on the Management of Scoliosis in Duchenne Muscular Dystrophy: Are Corticosteroids Decreasing the Rate of Scoliosis Surgery in the United States? Spine. 2016;41(17):E1030–8.
Jabaley CS, Groff RF, Sharifpour M, Raikhelkar JK, Blum JM. Modes of mechanical ventilation vary between hospitals and intensive care units within a university healthcare system: a retrospective observational study. BMC Res Notes. 2018;11(1):425.
Dellaca RL, Veneroni C, Farre R. Trends in mechanical ventilation: are we ventilating our patients in the best possible way? Breathe (Sheff). 2017;13(2):84–98.
Sheehan DW, Birnkrant DJ, Benditt JO, Eagle M, Finder JD, Kissel J, et al. Respiratory management of the patient with Duchenne muscular dystrophy. Pediatrics. 2018;142(Supplement 2):S62–71.
Brunklaus A, Parish E, Muntoni F, Scuplak S, Tucker SK, Fenton M, et al. The value of cardiac MRI versus echocardiography in the pre-operative assessment of patients with Duchenne muscular dystrophy. Eur J Paediatr Neurol. 2015;19(4):395–401.
Buddhe S, Lewin M, Olson A, Ferguson M, Soriano BD. Comparison of left ventricular function assessment between echocardiography and MRI in Duchenne muscular dystrophy. Pediatr Radiol. 2016;46(10):1399–408.
McNally EM, Kaltman JR, Benson DW, Canter CE, Cripe LH, Duan D, et al. Contemporary cardiac issues in Duchenne muscular dystrophy. Working Group of the National Heart, Lung, and Blood Institute in collaboration with Parent Project Muscular Dystrophy. [Erratum appears in Circulation. 2015 Jun 23;131(25):e539 Note: Groh, William J [added]; PMID: 26099961]. Circulation. 2015;131(18):1590–8.
Moxley RT III, Pandya S, Ciafaloni E, Fox DJ, Campbell K. Change in natural history of Duchenne muscular dystrophy with long-term corticosteroid treatment: implications for management. J Child Neurol. 2010;25(9):1116–29.
Griggs RC, Herr BE, Reha A, Elfring G, Atkinson L, Cwik V, et al. Corticosteroids in Duchenne muscular dystrophy: major variations in practice. Muscle Nerve. 2013;48(1):27–31.
Kim S, Campbell KA, Fox DJ, Matthews DJ, Valdez R. STARnet, MD. Corticosteroid treatments in males with Duchenne muscular dystrophy: treatment duration and time to loss of ambulation. J Child Neurol. 2015;30(10):1275–80.
McDonald CM, Henricson EK, Abresch RT, Duong T, Joyce NC, Hu F, et al. Long-term effects of glucocorticoids on function, quality of life, and survival in patients with Duchenne muscular dystrophy: a prospective cohort study. Lancet. 2018;391(10119):451–61.
Merlini L, Gennari M, Malaspina E, Cecconi I, Armaroli A, Gnudi S, et al. Early corticosteroid treatment in 4 Duchenne muscular dystrophy patients: 14-year follow-up. Muscle Nerve. 2012;45(6):796–802.
Duboc D, Meune C, Lerebours G, Devaux J-Y, Vaksmann G, Bécane H-M. Effect of perindopril on the onset and progression of left ventricular dysfunction in Duchenne muscular dystrophy. J Am Coll Cardiol. 2005;45(6):855–7.
Mendell JR, Goemans N, Lowes LP, Alfano LN, Berry K, Shao J, et al. Longitudinal effect of eteplirsen versus historical control on ambulation in Duchenne muscular dystrophy. Ann Neurol. 2016;79(2):257–71.
McDonald CM, Campbell C, Torricelli RE, Finkel RS, Flanigan KM, Goemans N, et al. Ataluren in patients with nonsense mutation Duchenne muscular dystrophy (ACT DMD): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet. 2017;390(10101):1489–98.
Aartsma-Rus A, Ginjaar IB, Bushby K. The importance of genetic diagnosis for Duchenne muscular dystrophy. J Med Genet. 2016;53(3):145–51.
Kempf L, Goldsmith JC, Temple R. Challenges of developing and conducting clinical trials in rare disorders. Am J Med Genet A. 2018;176(4):773–83.
Administration FaD. Rare diseases: natural history studies for drug development. Guidance for industry. 2019.
Straub V, Mercuri E. Report on the workshop: meaningful outcome measures for Duchenne muscular dystrophy, London, UK, 30–31 January 2017. Neuromuscul Disord. 2018;28(8):690–701.
Egger M, Smith G, Schneider M. Systematic reviews of observational studies. BMJ Publishing Group; 2010.
Colditz GA, Burdick E, Mosteller F. Heterogeneity in meta-analysis of data from epidemiologic studies: a commentary. Am J Epidemiol. 1995;142(4):371–82.
Download references
Sarepta Therapeutics, Inc.
Author information
Authors and affiliations.
Broadstreet HEOR, 201 – 343 Railway St, Vancouver, BC, V6A 1A4, Canada
Shelagh M. Szabo, Alison Deighton & Meagan Harwood
Sarepta Therapeutics, 215 First St, Cambridge, MA, 02142, USA
Renna M. Salhany & Katherine L. Gooch
Cumming School of Medicine, University of Calgary, Calgary, AB, Canada
You can also search for this author in PubMed Google Scholar
Contributions
All authors contributed to study design, interpretation, and analysis. SMS and AD were responsible for data identification, extraction, and synthesis. SMS was responsible for creating the first draft and all authors read, revised, and approved the final manuscript.
Corresponding author
Correspondence to Shelagh M. Szabo .
Ethics declarations
Ethics approval and consent to participate.
Not available.
Consent for publication
Competing interests.
SMS and AMD are employees of Broadstreet HEOR, and MH was at the time of this project; which received funds from Sarepta for this work. RMS and KLG are employees of Sarepta. JM acted as a consultant to Broadstreet HEOR.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: supplementary table 1.
. Search strategy. Supplementary Table 2 . Details of corticosteroid treatment, by study. Supplementary Table 3 . Details of ACE inhibitor treatment, by study. Supplementary Table 4 . STROBE assessments of included studies.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Szabo, S.M., Salhany, R.M., Deighton, A. et al. The clinical course of Duchenne muscular dystrophy in the corticosteroid treatment era: a systematic literature review. Orphanet J Rare Dis 16 , 237 (2021). https://doi.org/10.1186/s13023-021-01862-w
Download citation
Received : 27 May 2020
Accepted : 10 May 2021
Published : 22 May 2021
DOI : https://doi.org/10.1186/s13023-021-01862-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Clinical course
- Systematic review
Orphanet Journal of Rare Diseases
ISSN: 1750-1172
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
- Reference Manager
- Simple TEXT file
People also looked at
Mini review article, treating duchenne muscular dystrophy: the promise of stem cells, artificial intelligence, and multi-omics.

- 1 Stanford Cardiovascular Institute, Stanford University, Stanford, CA, United States
- 2 Division of Cardiovascular Medicine, Department of Medicine, Stanford University, Stanford, CA, United States
Muscular dystrophies are chronic and debilitating disorders caused by progressive muscle wasting. Duchenne muscular dystrophy (DMD) is the most common type. DMD is a well-characterized genetic disorder caused by the absence of dystrophin. Although some therapies exist to treat the symptoms and there are ongoing efforts to correct the underlying molecular defect, patients with muscular dystrophies would greatly benefit from new therapies that target the specific pathways contributing directly to the muscle disorders. Three new advances are poised to change the landscape of therapies for muscular dystrophies such as DMD. First, the advent of human induced pluripotent stem cells (iPSCs) allows researchers to design effective treatment strategies that make up for the gaps missed by conventional “one size fits all” strategies. By characterizing tissue alterations with single-cell resolution and having molecular profiles for therapeutic treatments for a variety of cell types, clinical researchers can design multi-pronged interventions to not just delay degenerative processes, but regenerate healthy tissues. Second, artificial intelligence (AI) will play a significant role in developing future therapies by allowing the aggregation and synthesis of large and disparate datasets to help reveal underlying molecular mechanisms. Third, disease models using a high volume of multi-omics data gathered from diverse sources carry valuable information about converging and diverging pathways. Using these new tools, the results of previous and emerging studies will catalyze precision medicine-based drug development that can tackle devastating disorders such as DMD.
Introduction
Duchenne muscular dystrophy (DMD) is a lethal genetic disorder, primarily characterized by muscle deterioration and wasting. Ultimately, the majority of DMD patients succumb to cardiac and respiratory complications ( 1 ). DMD is caused by mutations in the X-chromosome-linked DMD gene that codes for the dystrophin protein, which is an important component of muscle cells' cytoskeleton. Mutations in DMD generally result in large deletions of the dystrophin protein that reduce structural integrity in muscle cells ( 2 ). Loss of dystrophin heavily disrupts the connection between the inner cytoskeleton and the extracellular matrix, known as the dystrophin associated protein complex, leading to structural and functional abnormalities in these mechanically active muscle cells ( 3 ). As DMD progresses, additional cell functions are impacted (e.g., disrupted calcium regulation, accumulation of reactive oxygen species, poor mitochondrial energetics, etc.). Muscle cells try to adapt to the disrupted processes ( 4 ), but can quickly overcompensate, causing the cell to transition from a stressed to a destabilized state; at this point it begins to secrete inflammatory cytokines, activates fibrosis, and ultimately dies ( 5 ). As more cellular processes are disrupted, more evidence of cellular dysfunction is exhibited that can be detected in the form of higher levels of serum biomarkers like creatine kinase and myoglobin as signs of muscle wasting, and TNF-α, IFN-γ, IL-5 and IL-6 as signs of chronic inflammation ( 6 – 9 ). Therapeutic efforts for DMD are therefore divided between targeting (i) the underlying cause of DMD, loss of dystrophin, or (ii) targeting a secondary pathology ( 10 ).
DMD is a rapidly progressing disease: it presents between the ages 2–5, loss of ambulation can happen by age 12, and premature death can happen by age 25–30 ( 1 ). The guidelines for managing DMD include recommendations on nutrition, physical therapy, and cardiovascular health that can help slow down disease progression ( 11 ), but there is currently no cure for the disease. Emerging therapies are targeting the underlying loss of dystrophin via several ideas like transcriptionally inducing the production of more dystrophin, utilizing oligonucleotides to promote exon-skipping of the mutant region, and genome editing the mutant exon, all with the goal of producing a healthy dystrophin molecule ( 12 – 14 ). All these promising strategies are currently being evaluated, but they are not ready to provide current DMD patients with useful options to delay pathologic onset. Furthermore, fixing the dystrophin issue would not spontaneously fix all the incurred muscle damage, and thus some additional therapy will likely be needed to achieve healthy skeletal muscle and cardiac function. Many of the current interventions target the compensatory processes that contribute to muscle inflammation and oxidative stress, but are able to only mildly decelerate loss of ambulation and cardiomyopathy ( 5 , 13 ). The information gathered through decades of pre-clinical, clinical, and pharmaceutical studies has provided a strong foundation for understanding and treating DMD, and can be used to identify other targets; additionally, the information gathered in clinical trials for any compound can inform on efficacy for these chemicals. Clinical studies have also helped us establish checkpoints for disease progression from early-stage to end-stage, allowing us to predict disease progression and informing treatment.
The advent of combining several new approaches holds promise for better and more specific treatments of DMD in the future ( Figure 1 ). First, stem cell-based studies can investigate cellular processes and the effects of different treatments on DMD-afflicted muscle cells without risk to patients. Second, the evolving AI tools could be used to perform high dimensional drug screening with more efficiently streamlined analysis. Third, multi-omics approaches allow the synthesis of information from diverse sources and enable a more holistic understanding of the mechanisms underlying the disease. In this review, we will discuss all three of these approaches, their recent applications, and their potential.
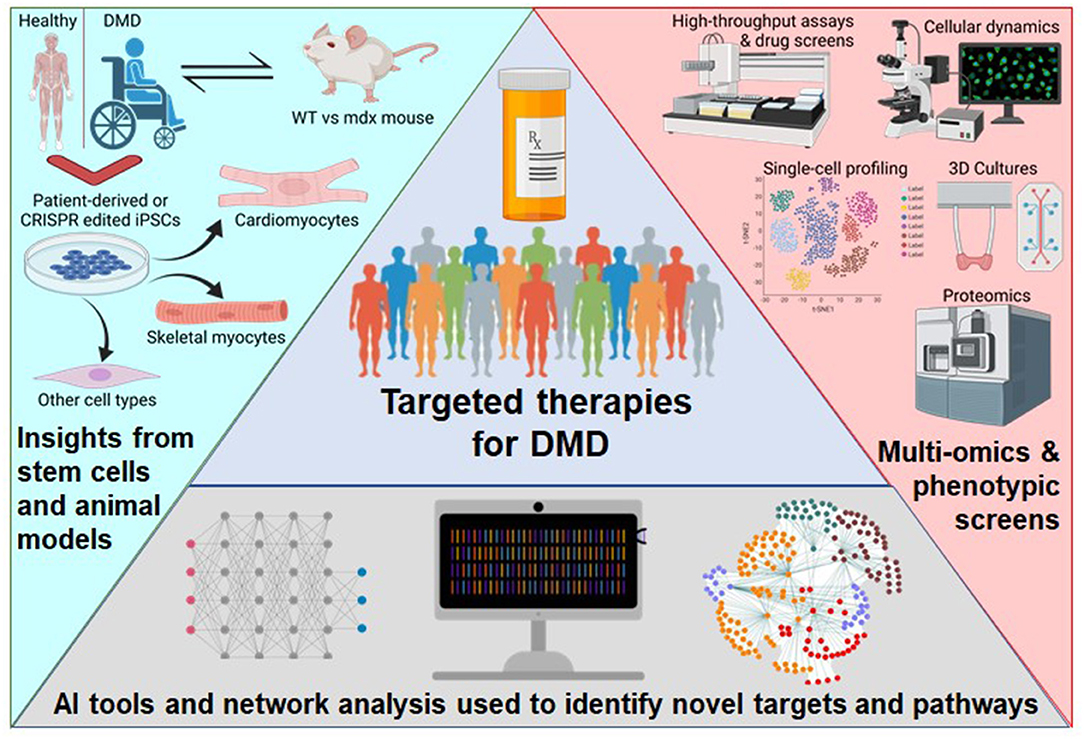
Figure 1 . Schematic of the approaches to broaden the therapeutics available to DMD patients. Patient-derived or genome-edited iPSCs provide a human model for disease specific modeling of various cell types and benefits from previous knowledge using the mdx mouse. Newer experimental technologies are detailing the intricacies of complex phenotypes. Next-generation computational tools are enabling high-dimensional analysis of multi-omics data. This figure was created with BioRender.com .
Human Stem Cells Provide Cell Type- and Disease-Specific Insights
Both DMD-like mouse models and DMD patient samples have yielded mechanistic insights by comparing differential expression of wild-type (WT) and healthy human tissues, pointing to potential pharmaceutical targets ( 16 – 18 ). The mdx mouse has been a standard pre-clinical model for DMD for deciphering how these markers interact and contribute to muscle wasting ( 19 ). It has also been extensively used to test the efficacy of the current interventions for DMD ( 20 ). However, the reliability of animal models in biomedical research has been questioned due to issues concerning physiological context, species specificity, and clinical relevance ( 21 ). An mdx mouse study noted a delayed onset of cardiomyopathy, as opposed to humans which is the main cause of death ( 22 ). Even with their contributions to our understanding of the disease, better models that recapitulate the phenotype are necessary ( 23 ). By utilizing induced pluripotent stem cells (iPSCs), human disease models hold substantial promise for the clinic, because one can study patient-specific pathologies in multiple different tissues and cell types ( 24 , 25 ). The use of human iPSCs, in which somatic cells can be reprogrammed to a pluripotent state by introducing four key transcription factors (Oct4, Sox2, Klf4, and c-Myc) and then be chemically programmed to become a cell type of interest ( 25 , 26 ), has been a ground-breaking technology that promises to treat an entire spectrum of intractable diseases including DMD.
A current effort to directly correct the dystrophin deficiency in DMD is being piloted in the mdx mouse using CRISPR/Cas9 to edit the genetic defect as a therapeutic strategy ( 15 , 27 ). These efforts have generated a single-cell atlas of skeletal muscle from a dystrophic mouse model that covers both its diseased-state and a CRISPR-corrected state ( 28 ). Many known differential expression changes in metabolism, inflammation, and regulatory networks were recapitulated. Moreover, the crosstalk between skeletal myocytes, endothelial cells, macrophages, and various other cell types was explored. In addition, Chemello et al. were able to analyze diverse transcriptional programs stemming from various nuclei, which is important considering that skeletal muscle can house several myonuclei per cell. These spatially defined tissue studies using single-cell RNA-seq can show how different cell types could be targeted for their modified state. Even with the innovation and excitement for spatial transcriptomics studies, there still are trade-offs in utilizing these experiments for mechanistic inference ( 29 ). Depending on the question, if a tissue is too large, certain information could be missed if multiple spaces are not covered or considered for analysis, leading to biases. In addition, processing different samples using different tissue separation or cell isolation protocols can create significant variations in the experimental results, which is why there is much interest in automation. Future advances in spatial transcriptomics may overcome some of the present limitations of these experiments ( 30 ). Studies utilizing iPSCs can be designed for characterizing the disease-state phenotype of various cell types and testing for drugs.
Recently, human stem cell models have been utilized to study DMD. For example, by identifying issues with cell fate during differentiation, studies have highlighted new potential therapeutic targets ( 31 , 32 ). There are other examples of how stem cells were successfully deployed to better understand DMD ( 14 , 33 ). DMD patient-derived iPSCs were used to show accelerated telomere shortening that is seen in DMD patients' heart muscle cells ( 34 ). In yet another example, a recent study combined data from both the mdx mouse and DMD patient-derived iPSC-cardiomyocytes to show the overlap in phenotypes between both species and also how both species' cells react to adrenergic receptor stimulation with an agonist (isoproterenol) and antagonist (beta-blocker) ( 35 ). This is important because it supports the use of iPSC-derived models as a proper tool for characterizing common DMD treatment effects, just as the mdx mouse has been used for decades. Another recent study characterized the functional effects of known Chinese herbal medicine components on DMD iPSC-cardiomyocytes, highlighting the potential of stem cells for drug discovery ( 36 ). This work showed that the anti-oxidant effects of these herbal compounds can be effective at reducing oxidative stress. In a recent review of DMD therapeutics, a meta-analysis of gene expression comparisons of other DMD samples showed that extracellular matrix (ECM) and cell-to-cell interaction molecules are prime targets for reversing late-stage DMD, which makes logical sense because to restore ambulation, the musculature would need to be strengthened ( 10 ). Stem cells give us the ability to generate and characterize the different cell types affected by DMD, as well as the ability to perform high-throughput drug screens, paving the way toward patient-specific treatment programs.
AI Facilitates High-Dimensional Analysis of Large DMD Datasets
Recent advances in omics technologies have led to substantial data generation for disease modeling. However, the wealth of information has resulted in a predictable challenge: how to aggregate and interpret disparate data types, such as transcriptomic, epigenomic, functional, and textual data, which may be distributed across siloed databases ( 37 ). Advances in AI have led to models that perform automated text processing and comprehension. Additional AI models can process high-dimensional, non-linear omics data and learn new features about the data that would not be obvious using traditional analysis ( 38 ). Simultaneously, progress in computational hardware has increased the ability of researchers to develop and train models to aggregate, analyze, and make predictions using very large datasets. An emerging benefit of using “big-data” algorithms on biomedical information is the ability to tailor treatment programs for individual patients. Information obtained from a person's genome is becoming more reliable and pharmaceutically applicable every day ( 39 ). However, designing a specific therapy still requires precisely matching the correct treatment with the unique underlying defect and health state of the patient. The competitive pace of the computational market is driving the current efforts in biotech and pharma to be in position to produce innovative therapies.
Natural language processing (NLP), a machine learning tool with the capability to process text to achieve automated comprehension, translation, and generation, is perhaps one of the most critical AI tools for biomedical research. Initially, NLP primarily relied on recurrent neural networks as the main model of choice. However, recurrent neural networks were limited in the size of the datasets they could train on, and therefore delivered limited performance on clinical and biomedical text comprehension ( 40 ). Transformers and attention-based language models have reshaped the landscape of NLP by bypassing existing limitations of recurrent neural networks ( 41 ). Importantly, transformers are capable of handling larger datasets than seen before and delivering greater text comprehension, both of which are required to discover novel biological mechanisms and therapeutics from existing biomedical literature and databases ( 42 ). When applied to DMD, these recent advances in NLP can query large amounts of DMD-related text and databases to identify key words of interest, annotate DMD with additional clinical phenotypes, molecular pathways, and protein-protein interactions. In drug development, NLP is capable of extracting biological and chemical molecules from existing literature that may interact with DMD pathways and have therapeutics effects.
As an example, Insilico Medicine has been developing an AI-driven platform that combines multiple datasets from various sources to classify therapeutic targets by their disease relevance, “druggability,” and clinical trajectory. One of the tools is called PandaOmics, which uses deep-learning and cloud-stored data to enable researchers to search information on diseases and their drug targets. The OMICs-sourced analysis of disease relevance is derived from Genome Wide Association Studies, Transcriptome Wide Association Studies, Online Mendelian Inheritance in Man, and the Library of Integrated Network-Based Cellular Signatures, to name a few data bases. For example, PandaOmics combs through clinical trial reports, grant applications, and publications data stemming from the labs mostly associated with the study of a specific disease or compound. This can help inform the larger community, including regulatory agencies, investors, and start-ups, on the basic importance of a target. Their proprietary pathway analysis approach uses iPANDA (in silico Pathway Activation Network Decomposition Analysis) to infer pathway alterations and find significant targets ( 43 ). Microarray expression data of skeletal muscles from DMD patients compared to healthy controls are used to quantify common transcriptional changes ( 44 – 47 ). Figures 2A,B illustrate a sample output of a PandaOmics meta-analysis showing some known disease targets and compounds used to treat DMD from the pre-clinical and clinical research stages to full approval for use. Thus, the advent of AI, in particular NLP, allows researchers to aggregate and unearth DMD mechanisms and potential therapeutics that was previously impossible due to the monumental size and siloed nature of existing DMD literature and databases.
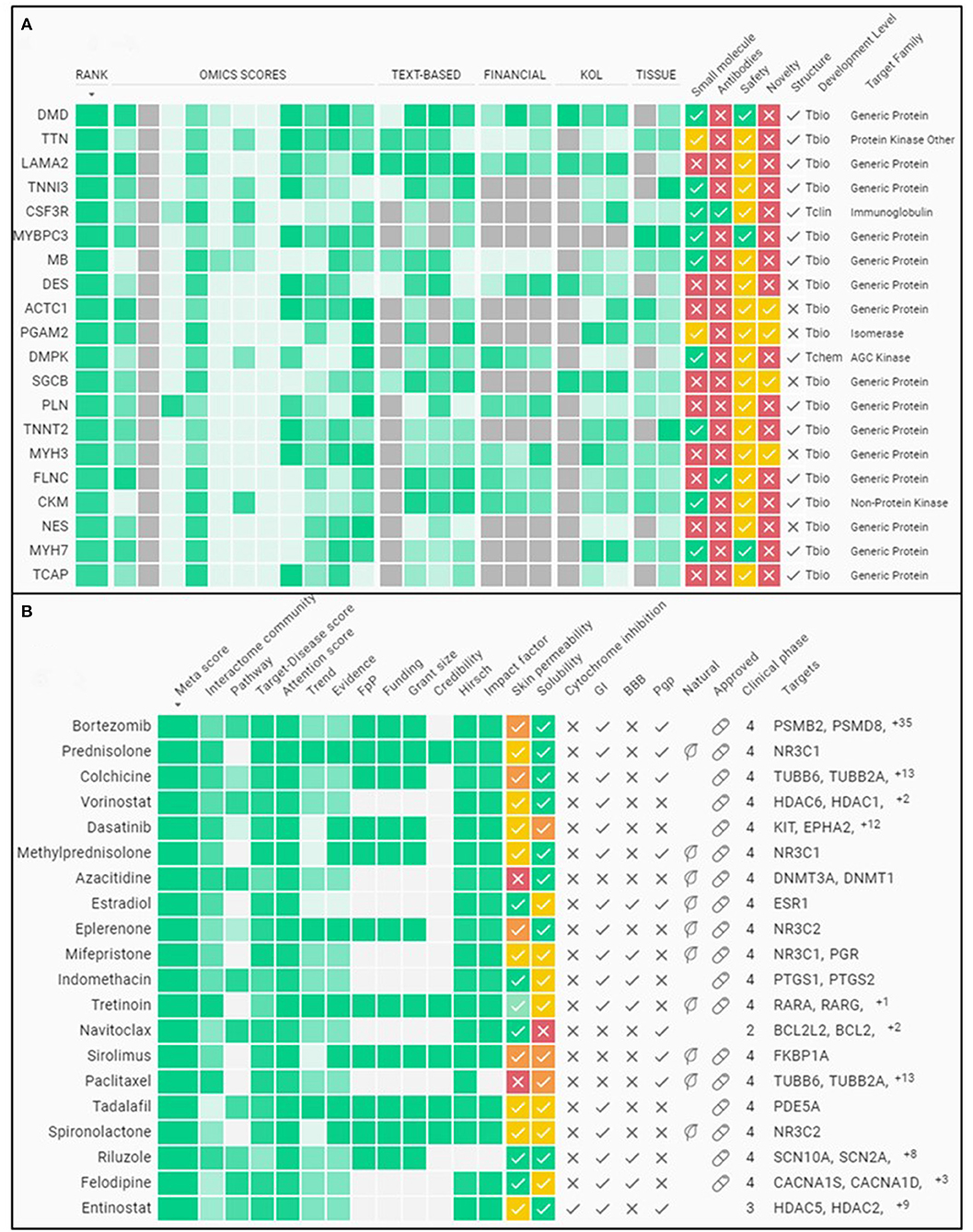
Figure 2 . Sample meta-analysis from an A.I. driven therapeutics platform. (A) Summary of some of the top gene targets associated to DMD. (B) Summary of some of the top compounds associated to DMD for therapy. The evidence for classifying these molecules as the top targets is gleaned from various public databases plus the funding and publications landscape using PandaOmics ( http://pandaomics.com/ ).
Emerging DMD Multi-Omics Analysis Show Concord and Complexity
Multi-omics experiments for mechanistic insights of DMD have been performed on both animal models (e.g., mdx mouse) and human tissues (e.g., muscle biopsies), thus significantly increasing the diversity of datasets available for analysis. One of the earliest studies into the differences between normal and DMD skeletal muscle transcriptomes was performed on the hind limb and diaphragm muscles of mdx mice compared to control mice ( 48 ). This study reported several mechanisms of DMD, including differential expression of IGF-II, NF-kB, SERCA1, RYR1, α-tubulin, and collagens, among many others. A follow-up study comparing mRNA from the gastrocnemius muscle of mdx vs. control mouse used an array analysis of >12,000 genes ( 49 ). This study was in agreement with previous reports confirming differential expression of myogenin, α 2 -tubulin, lysozyme M, and myostatin, among others. It also reported upregulation of both IGF-I and IGF-II in dystrophic muscles, but discovered inhibitory IGF-binding proteins and regulators were also increased, thus counteracting the potential beneficial effects of their upregulation. This demonstrates an example of which analyses of multiple datasets are necessary to provide deeper understanding of mechanistic insights. More importantly, Bakay et al. compared their study with human DMD mRNA analyses ( 49 ). This revealed notable differences between the transcriptomes of the mdx mouse and human samples, including discordant directional changes in mRNA for myogenin, guanidinoacetate methyltransferase, calponin, and mast cell chymase.
Since these early investigations on the transcriptomic differences, subsequent studies further built upon these mechanistic insights using other omics approaches. For example, studies into microRNAs and chromatin changes revealed the importance of nitric oxide and its associated pathway in DMD pathogenesis ( 50 , 51 ). Proteomic analysis revealed that Bromodomain and extra-terminal domain (BET) protein BRD4 is significantly increased in the mdx mouse due to direct association to chromatin regulatory regions of the NADPH oxidase subunits ( 52 ). Epigenomic analysis, including that on histone acetylation of H3K14 and H3K9 and DNA methylations of Notch1, has revealed its role in regulating satellite cell fate during skeletal muscle regeneration and its dysregulation in DMD ( 53 ). Additional chromatin studies also revealed that nuclear pore protein Nup153 associates with chromatin and regulates cardiac gene expression in mdx hearts ( 54 ). Recent studies began looking more comprehensively into multi-omics analysis of mdx mouse and human iPSCs. These studies found discrepancies between different omics, such as only a 53% agreement in fold-change data between the proteome and transcriptome in mdx mice ( 55 ). Nonetheless, multi-omics studies continue to be a valuable approach in elucidating disease mechanisms as demonstrated by their ability to provide insight into early developmental manifestations of DMD in iPSC models of skeletal muscle differentiation ( 32 ). A main challenge remains in integrating these numerous multi-omics datasets into something more precisely meaningful and individually translatable for the patients.
Discovering, developing, and delivering targeted therapeutics requires substantial support and collaboration from both academia and industry. It also requires interdisciplinary studies and combinations of skillsets to enable progress. Much is said about how interdisciplinary research is the proper approach to tackling complex diseases even if it also brings challenges in communication and prioritization ( 56 , 57 ). These challenges can spill over to therapeutics studies where we evaluate how the data we generate correlate with seemingly disconnected sets and how certain factors influence the phenomena we are studying. Machine-learning may not be a new concept, but the influence it has had on biological studies in the past decade is founded on the many novel insights it has generated into how we visualize biological processes and problems ( 58 ). The field of DMD has been enriched by advancements in AI and deep learning that now make it possible to aggregate, interpret, and visualize high volumes of high-dimensional and non-linear datasets.
Theoretically, by combining better models of disease risk and severity with detailed analysis of different compounds, we will significantly improve our ability to treat and reduce cardiomyopathy-related deaths. Furthermore, as we approach the goal of fixing the underlying pathological mechanism of diseases like DMD, we must remember to rehabilitate and regenerate healthy tissues to counter persistent dysfunctions and avoid long-term hauling of these issues. To realize these precise therapies will require a convergence of data acquired from state-of-the-art tools and the prioritization of different research agencies and institutes. This will undoubtedly change business models for pharmaceutical and biotech companies, because this will involve a much greater role for drug repurposing, intellectual property disputes and bargains in the coming years. Moreover, on the scientific front, as the quality of analytical tools increases, we will also need the data to be reproducible and of higher quality. The tools we utilize to study cells from different tissues and lineages like iPSCs are becoming ever more reliable and sustainable with single-cell analysis being the standard. Some advances in AI are providing visually interesting graphics that link disease targets to compounds with clinical context. Multi-omics analysis is highlighting the different pathways a disease can take. These developments hold a great potential for improving healthcare by providing clinical researchers with accurate, high-resolution disease-models that will help illuminate the paths to improving patient outcomes.
Author Contributions
CV wrote the manuscript. AZ, PP, and JW revised the manuscript and provided critical input. All authors contributed to the article and approved the submitted version.
This work is supported by National Institutes of Health grants R01 HL126527, R01 HL130020, R01 HL123968, and R01 HL141371 (JW). Due to space limitation, we apologize in advance for not being able to include all references on this topic.
Conflict of Interest
JW is a co-founder of Greenstone Biosciences.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank Blake Wu and Dr. Adrienne Mueller for critical feedback on the manuscript. The authors would like to acknowledge the platforms BioRender.com and PandaOmics.com for providing the means for creating the figures in this manuscript.
1. Bach JR, O'Brien J, Krotenberg R, Alba AS. Management of end stage respiratory failure in duchenne muscular dystrophy. Muscle & Nerve. (1987) 10:177–82. doi: 10.1002/mus.880100212
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Hoffman EP, Brown RHJ, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. (1987) 51:919–28. doi: 10.1016/0092-8674(87)90579-4
3. Campbell KP. Three muscular dystrophies: Loss of cytoskeleton-extracellular matrix linkage. Cell. (1995) 80:675–9. doi: 10.1016/0092-8674(95)90344-5
4. Klingler W, Jurkat-Rott K, Lehmann-Horn F, Schleip R. The role of fibrosis in Duchenne muscular dystrophy. Acta Myologica. (2012) 31:184–95.
PubMed Abstract | Google Scholar
5. Guiraud S, Aartsma-Rus A, Vieira NM, Davies KE, van Ommen G-JB, Kunkel LM. The pathogenesis and therapy of muscular dystrophies. Annu Rev Genomics Hum Genet. (2015) 16:281–308. doi: 10.1146/annurev-genom-090314-025003
6. Kastenschmidt JM, Coulis G, Farahat PK, Pham P, Rios R, et al. A stromal progenitor and ILC2 niche promotes muscle eosinophilia and fibrosis-associated gene expression. Cell Reports. (2021) 35:108997. doi: 10.1016/j.celrep.2021.108997
7. Kiessling WR, Beckmann R. Serum levels of myoglobin and creatine kinase in Duchenne muscular dystrophy. Klinische Wochenschrift. (1981) 59:347–8. doi: 10.1007/BF01525003
8. Villalta SA, Nguyen HX, Deng B, Gotoh T, Tidball JG. Shifts in macrophage phenotypes and macrophage competition for arginine metabolism affect the severity of muscle pathology in muscular dystrophy. Human Molecular Genetics. (2009) 18:482–96. doi: 10.1093/hmg/ddn376
9. Villalta SA, Rosenberg AS, Bluestone JA. The immune system in Duchenne muscular dystrophy: Friend or foe. Rare Diseases. (2015) 3:e1010966. doi: 10.1080/21675511.2015.1010966
10. Yao S, Chen Z, Yu Y, Zhang N, Jiang H, Zhang G, et al. Current Pharmacological Strategies for Duchenne Muscular Dystrophy. Frontiers in Cell and Developmental Biology. (2021) 9:689533. doi: 10.3389/fcell.2021.689533
11. Bushby K, Bann CM, Apkon SD, Blackwell A, Brumbaugh D, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. Lancet Neurology. (2018) 17:251–67. doi: 10.1016/S1474-4422(18)30024-3
12. Aartsma-Rus A, Corey DR. The 10th Oligonucleotide Therapy Approved : Golodirsen for Duchenne Muscular Dystrophy. Nuclei Acid Ther . (2020) 30: 67–70. doi: 10.1089/nat.2020.0845
13. Sun C Shen L Zhang Z and Xie X. Therapeutic Strategies for Duchenne Muscular. Genes . (2020) 11(837). doi: 10.3390/genes11080837
14. Min Y, Bassel-duby R, Olson EN. CRISPR correction of duchenne muscular dystrophy. Annual Review Medicine . (2019) 70:239–255. doi: 10.1146/annurev-med-081117-010451
15. Min Y-L, Li H, Rodriguez-Caycedo C, Mireault AA, Huang J, et al. CRISPR-Cas9 corrects Duchenne muscular dystrophy exon 44 deletion mutations in mice and human cells Science Advances 5: eaav. (2019) 4324. doi: 10.1126/sciadv.aav4324
16. Camerino GM, Cannone M, Giustino A, Massari AM, Capogrosso RF, Cozzoli A, et al. Gene expression in mdx mouse muscle in relation to age and exercise: aberrant mechanical-metabolic coupling and implications for pre-clinical studies in Duchenne muscular dystrophy. Human Molecular Genetics. (2014) 23:5720–32. doi: 10.1093/hmg/ddu287
17. Chen Y Zhao P Borup R and Hoffman EP. Expression Profiling in the Muscular Dystrophies : Identification of Novel Aspects of Molecular Pathophysiology. (2000) 7:1321–1336. doi: 10.1083/jcb.151.6.1321
18. Hathout Y, Brody E, Clemens PR, Cripe L, DeLisle RK, et al. Large-scale serum protein biomarker discovery in Duchenne muscular dystrophy. Proc. Natl. Acad. Sci. U. S. A. (2015) 112:7153–8. doi: 10.1073/pnas.1507719112
19. Wasala NB, Chen S-J, Duan D. Duchenne muscular dystrophy animal models for high-throughput drug discovery and precision medicine. Expert Opinion on Drug Discovery. (2020) 15:443–56. doi: 10.1080/17460441.2020.1718100
20. Manning J, O'Malley D. What has the mdx mouse model of duchenne muscular dystrophy contributed to our understanding of this disease? J Muscle Res Cell Motil. (2015) 32:155–67. doi: 10.1007/s10974-015-9406-4
21. Chen J, Kang D, Xu J, Lake M, Hogan JO, et al. Species differences and molecular determinant of TRPA1 cold sensitivity. Nature Communications. (2013) 4:2501. doi: 10.1038/ncomms3501
22. Khouzami L, Bourin M-C, Christov C, Damy T, Escoubet B, et al. Delayed cardiomyopathy in dystrophin deficient mdx mice relies on intrinsic glutathione resource. Am J Pathol. (2010) 173:1356–64. doi: 10.2353/ajpath.2010.090479
23. McGreevy JW, Hakim CH, McIntosh MA, Duan D. Animal models of Duchenne muscular dystrophy: from basic mechanisms to gene therapy. Disease Models & Mechanisms. (2015) 8:195–213. doi: 10.1242/dmm.018424
24. Lau E, Paik DT, Wu JC. Systems-wide approaches in induced pluripotent stem cell models. Annu Rev Pathol . (2019) 14:395–19. doi: 10.1146/annurev-pathmechdis-012418-013046
25. Matsa E, Burridge PW, Yu K-H, Ahrens JH, et al. Transcriptome profiling of patient-specific human iPSC- cardiomyocytes predicts individual drug safety and efficacy responses in vitro. Cell Stem Cell. (2016) 19:311–25. doi: 10.1016/j.stem.2016.07.006
26. Shi Y, Inoue H, Wu JC, Yamanaka S. Induced pluripotent stem cell technology: a decade of progress. Nature Reviews Drug Discovery. (2017) 16:115–30. doi: 10.1038/nrd.2016.245
27. Nelson CE, Hakim CH, Ousterout DG, Thakore PI, et al. In vivo genome editing improves muscle function in a mouse model of Duchenne muscular dystrophy. Science (New York, N.Y.) . (2016) 351:403–407. doi: 10.1126/science.aad5143
28. Chemello F Wang Z Li H Mcanally JR and Liu N. Degenerative and regenerative pathways underlying Duchenne muscular dystrophy revealed by single-nucleus RNA sequencing. PNAS . (2020) 117:29691–701. doi: 10.1073/pnas.2018391117
29. Roth R Kim S Kim J Rhee Single-cell S and and spatial transcriptomics approaches of cardiovascular development and disease. BMB Reports. (2020) 53:393–9. doi: 10.5483/BMBRep.2020.53.8.130
CrossRef Full Text | Google Scholar
30. Vickovic S, Eraslan G, Salmén F, et al. High-definition spatial transcriptomics for in situ tissue profiling. Nature Methods. (2019) 16:987–90. doi: 10.1038/s41592-019-0548-y
31. Caron L Kher D Leong Lee K and Robert M. A human pluripotent stem cell model of facioscapulohumeral muscular dystrophy-affected skeletal. Muscles . (2016) 1145–61. doi: 10.5966/sctm.2015-0224
32. Mournetas V, Massouridès E Dupont J.-B., Kornobis E, et al. Myogenesis modelled by human pluripotent stem cells: a multi-omic study of Duchenne myopathy early onset. J Cachexia Sarcopenia Muscle. (2021) 12:209–32. doi: 10.1002/jcsm.12665
33. Caputo L, Granados A, Lenzi J, Rosa A, Ait-Si-Ali S, Puri PL, et al. Acute conversion of patient-derived Duchenne muscular dystrophy iPSC into myotubes reveals constitutive and inducible over-activation of TGFβ-dependent pro-fibrotic signaling. Skeletal Muscle. (2020) 10:13. doi: 10.1186/s13395-020-00224-7
34. Chang ACY, Pardon G, Chang ACH, Wu H, Ong S-G, Eguchi A, et al. Increased tissue stiffness triggers contractile dysfunction and telomere shortening in dystrophic cardiomyocytes. Stem Cell Reports. (2021) 16:2169–81. doi: 10.1016/j.stemcr.2021.04.018
35. Kamdar F, Das S, Gong W, Meyers TA, Shah P, et al. Stem cell – derived cardiomyocytes and beta-adrenergic receptor blockade in duchenne muscular dystrophy cardiomyopathy. J Am Coll Cardiol . (2020) 75 . doi: 10.1016/j.jacc.2019.12.066
36. Li B Xiong W Liang W Chiou J Lin Y and Chang ACY. Targeting of CAT and VCAM1 as novel therapeutic targets for DMD cardiomyopathy. Mol Cell Bio . (2021) 9:1–15. doi: 10.3389/fcell.2021.659177
37. Roszik J, Subbiah V. Mining public databases for precision oncology. Trends in Cancer. (2018) 4:463–5. doi: 10.1016/j.trecan.2018.04.008
38. Sun YV, Hu Y-J. Integrative analysis of multi-omics data for discovery and functional studies of complex human diseases. Advances in Genetics. (2016) 93:147–90. doi: 10.1016/bs.adgen.2015.11.004
39. Goldfeder RL, Wall DP, Khoury MJ, Ioannidis JPA, Ashley EA. Human genome sequencing at the population scale: a primer on high-throughput dna sequencing and analysis. Am J Epidemiol .. (2017) 186:1000–9. doi: 10.1093/aje/kww224
40. Ching T, Himmelstein DS, Beaulieu-Jones BK, Kalinin AA, et al. Opportunities and obstacles for deep learning in biology and medicine. J Royal Society, Interface . (2018) 15. doi: 10.1098/rsif.2017.0387
41. Korngiebel DM, Mooney SD. Considering the possibilities and pitfalls of Generative Pre-trained Transformer 3 (GPT-3) in healthcare delivery. NPJ Digital Medicine. (2021) 4:93. doi: 10.1038/s41746-021-00464-x
42. Yang X, Bian J, Hogan WR, Wu Y. Clinical concept extraction using transformers. J Am Med Inform Assoc. (2020) 27:1935–42. doi: 10.1093/jamia/ocaa189
43. Ozerov IV, Lezhnina KV, Izumchenko E, Artemov AV, et al. In silico pathway activation network decomposition analysis (iPANDA) as a method for biomarker development. Nature Communications. (2016) 7:13427. doi: 10.1038/ncomms13427
44. Bakay M, Wang Z, Melcon G, Schiltz L, Xuan J, Zhao P, et al. Nuclear envelope dystrophies show a transcriptional fingerprint suggesting disruption of Rb-MyoD pathways in muscle regeneration. Brain. (2006) 129:996–1013. doi: 10.1093/brain/awl023
45. Haslett JN, Sanoudou D, Kho AT, Bennett RR, Greenberg SA, et al. Gene expression comparison of biopsies from Duchenne muscular dystrophy (DMD) and normal skeletal muscle. Proc Natl Acad Sci U. S. A. (2002) 99:15000–5. doi: 10.1073/pnas.192571199
46. Kalko SG, Paco S, Jou C, Rodríguez MA, Meznaric M, et al. Transcriptomic profiling of TK2 deficient human skeletal muscle suggests a role for the p53 signalling pathway and identifies growth and differentiation factor-15 as a potential novel biomarker for mitochondrial myopathies. BMC Genomics. (2014) 15:91. doi: 10.1186/1471-2164-15-91
47. Pescatori M, Broccolini A, Minetti C, Bertini E, et al. Gene expression profiling in the early phases of DMD: a constant molecular signature characterizes DMD muscle from early postnatal life throughout disease progression. The FASEB Journal. (2007) 21:1210–26. doi: 10.1096/fj.06-7285com
48. Tkatchenko AV, Le Cam G, Léger JJ, Dechesne CA. Large-scale analysis of differential gene expression in the hindlimb muscles and diaphragm of mdx mouse. Biochimica et Biophysica Acta. (2000) 1500:17–30. doi: 10.1016/S0925-4439(99)00084-8
49. Bakay M, Zhao P, Chen J, Hoffman EP. A web-accessible complete transcriptome of normal human and DMD muscle. Neuromuscular Disorders : NMD. (2002) 12:S125–41. doi: 10.1016/S0960-8966(02)00093-7
50. Cacchiarelli D, Martone J, Girardi E, Cesana M, Incitti T, et al. MicroRNAs involved in molecular circuitries relevant for the Duchenne muscular dystrophy pathogenesis are controlled by the dystrophin/nNOS pathway. Cell Metabolism. (2010) 12:341–51. doi: 10.1016/j.cmet.2010.07.008
51. Colussi C, Gurtner A, Rosati J, Illi B, Ragone G, Piaggio G, et al. Nitric oxide deficiency determines global chromatin changes in Duchenne muscular dystrophy. FASEB Journal. (2009) 23:2131–41. doi: 10.1096/fj.08-115618
52. Segatto M, Szokoll R, Fittipaldi R, Bottino C, Nevi L, Mamchaoui K, et al. BETs inhibition attenuates oxidative stress and preserves muscle integrity in Duchenne muscular dystrophy. Nature Communications. (2020) 11:6108. doi: 10.1038/s41467-020-19839-x
53. Massenet J, Gardner E, Chazaud B, Dilworth FJ. Epigenetic regulation of satellite cell fate during skeletal muscle regeneration. Skeletal Muscle. (2021) 11:4. doi: 10.1186/s13395-020-00259-w
54. Nanni S, Re A, Ripoli C, Gowran A, Nigro P, et al. The nuclear pore protein Nup153 associates with chromatin and regulates cardiac gene expression in dystrophic mdx hearts. Cardiovascular Research. (2016) 112:555–67. doi: 10.1093/cvr/cvw204
55. Van Pelt DW, Kharaz YA, Sarver DC, et al. Multiomics analysis of the mdx/mTR mouse model of Duchenne muscular dystrophy. Connective Tissue Research. (2021) 62:24–39. doi: 10.1080/03008207.2020.1791103
56. Blundell TL. Interdisciplinary research in physics, chemistry and biology is central to understanding biological processes. Prog Biophys Mol . (2020). doi: 10.1016/j.pbiomolbio.2020.09.002
57. Cummins G, Cox BF, Walker JD, Cochran S, Desmulliez MPY. Challenges in developing collaborative interdisciplinary research between gastroenterologists and engineers. J Med Eng Technol. (2018) 42:435–42. doi: 10.1080/03091902.2018.1543466
58. Mirza B, Wang W, Wang J, Choi H, Chung NC, Ping P. (2019). Machine learning and integrative analysis of biomedical big data. Genes. 10 (2). doi: 10.3390/genes10020087
Keywords: Duchenne muscular dystrophy, cardiomyopathy, iPSC disease modeling, drug testing, single-cell technology, artificial intelligence
Citation: Vera CD, Zhang A, Pang PD and Wu JC (2022) Treating Duchenne Muscular Dystrophy: The Promise of Stem Cells, Artificial Intelligence, and Multi-Omics. Front. Cardiovasc. Med. 9:851491. doi: 10.3389/fcvm.2022.851491
Received: 10 January 2022; Accepted: 31 January 2022; Published: 10 March 2022.
Reviewed by:
Copyright © 2022 Vera, Zhang, Pang and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joseph C. Wu, joewu@stanford.edu
This article is part of the Research Topic
Intercellular Communication and Crosstalk in Cardiac Development and Disease
Duchenne Muscular Dystrophy Gene Therapy in 2023: Status, Perspective, and Beyond
Affiliations.
- 1 Department of Molecular Microbiology and Immunology, University of Missouri, Columbia, Missouri, USA.
- 2 Department of Neurology, School of Medicine, University of Missouri, Columbia, Missouri, USA.
- 3 Department of Biomedical Sciences, College of Veterinary Medicine, University of Missouri, Columbia, Missouri, USA.
- 4 Department of Chemical and Biomedical Engineering, College of Engineering, University of Missouri, Columbia, Missouri, USA.
- PMID: 37219994
- PMCID: PMC10325806
- DOI: 10.1089/hum.2023.29242.ddu
Duchenne muscular dystrophy (DMD) was named more than 150 years ago. About four decades ago, the DMD gene was discovered, and the reading frame shift was determined as the genetic underpinning. These pivotal findings significantly changed the landscape of DMD therapy development. Restoration of dystrophin expression with gene therapy became a primary focus. Investment in gene therapy has led to the approval of exon skipping by regulatory agencies, multiple clinical trials of systemic microdystrophin therapy using adeno-associated virus vectors, and revolutionary genome editing therapy using the CRISPR technology. However, many important issues surfaced during the clinical translation of DMD gene therapy (such as low efficiency of exon skipping, immune toxicity-induced serious adverse events, and patient death). In this issue of Human Gene Therapy , several research articles highlighted some of the latest developments in DMD gene therapy. Importantly, a collection of articles from experts in the field reviewed the progress, major challenges, and future directions of DMD gene therapy. These insightful discussions have significant implications for gene therapy of other neuromuscular diseases.
Keywords: CRISPR editing; Duchenne muscular dystrophy (DMD); adeno-associated virus (AAV); exon skipping; gene therapy; microdystrophin.
Publication types
- Research Support, Non-U.S. Gov't
- Research Support, U.S. Gov't, Non-P.H.S.
- Research Support, N.I.H., Extramural
- Clustered Regularly Interspaced Short Palindromic Repeats
- Gene Editing
- Genetic Therapy
- Muscular Dystrophy, Duchenne*
Grants and funding
- R01 AR070517/AR/NIAMS NIH HHS/United States
- R01 NS090634/NS/NINDS NIH HHS/United States
- R21 AR081018/AR/NIAMS NIH HHS/United States
- R56 AR081544/AR/NIAMS NIH HHS/United States
Duchenne Muscular Dystrophy Overview Research Paper
Introduction, background and symptoms, genetic issues, diagnostic process, medical management, and prognosis, concluding remarks.
Even mild muscle weakness can cause children discomfort and reduce their quality of life. Such a severe genetic disorder as Duchenne muscular dystrophy (DMD) leads to tremendous consequences for the muscular system and affects children very early in their development. Understanding the causes and implications of DMD for young children is imperative not only for raising awareness of the problem and recommending solutions for the management of the condition. The purpose of this paper is to provide an in-depth exploration of DMD, including its background and symptomology, genetic significance, diagnostic process and management, as well as prognosis.
Duchenne muscular dystrophy (DMD) is a genetic condition that is characterized by the continuous deterioration and weakness of muscle associated with changes in the composition of a protein called dystrophin, which maintains the functioning of muscle cells. The disorder is rare and affects males predominantly, with women being diagnosed with it only in exceptional cases. DMD causes the muscles in the body to become less resistant to physical impact and get damaged over time. Thus, the purpose of the paper is to explore Duchenne muscular dystrophy in great detail, including its causes, demographic data, signs and symptoms, the overall effect on the body, discuss the diagnostic process and prognosis for the disease.
Duchenne’s dystrophy develops as a result of a genetic mutation that does not allow the body to produce dystrophin, a protein needed to build muscles. Without the availability of enough dystrophin in the body, muscle cells weaken and become damaged. Children diagnosed with the disease at the early stages of life experience significant issues with walking and breathing, which decreases the quality of their lives (Birnkrant et al., 2018). Eventually, the muscles responsible for breathing stop working, which causes death. DMD is an irreversible, progressive disease that currently has no cure that could have alleviated the burden of the disease.
The estimated global prevalence of DMD is 4.78 per 100,000 males (Walter & Reilich, 2017). The implications of the condition also include a yearly disease burden of more than $130 million, without including costs for respiratory management, extra direct costs per one patient, as well as additional medical aids (Walter & Reilich, 2017). According to the official website dedicated to DMD, the average age of diagnosis is 5 years, while it takes 2.5 tears between the initial symptoms and diagnosis (“Duchenne muscular dystrophy: The basics,” 2019).
Being one of the most severe genetic diseases that affect children globally, DMD causes more than 90% of individuals being confined to a wheelchair by age 15 (“Duchenne muscular dystrophy: The basics,” 2019). The burden of the disease is severe, with DMD being diagnosed in 1 among 3,500 to 5,000 males around the world (“Duchenne muscular dystrophy: The basics,” 2019). The statistics on the disease show that it has an adverse effect on the young male population.
The principal symptom of DMD is muscle weakness, which can begin in patients as early as at ages 2-3 (Birnkrant et al., 2018). The proximal muscles get affected first due to the fact that they are the closest to body’s core. The distal limb muscles get affected later because they are close to the body’s extremities (Birnkrant et al., 2018). Furthermore, it is notable that lower external muscles usually show DMD symptoms prior to the upper ones. In affected children, there are noticeable difficulties walking, running, and jumping, which decreases the quality of movement. Other symptoms of the disease include calves’ enlargement, waddle in the gait, as well as lumbar lordosis (Birnkrant et al., 2018).
The latter is a DMD sign implying an inward spine curve. Due to the difficulties associated with muscle strength, the affected children develop issues with the heart and respiratory muscles. The progressive weakness in the body, coupled with scoliosis, can lead to the development of impaired pulmonary function that causes acute respiratory failure.
When exploring the possible symptoms of DMD in their children, parents should understand that muscle deterioration may not be as painful as it is. This is because muscular dystrophy does not have a direct effect on the nerves, with touch and other senses being normal. This is also true for the functions of the bladder and the bowel. Furthermore, it is essential to note the development of learning disabilities among children with DMD.
While serious cognitive disabilities are rare, dystrophin abnormalities in the brain can have minor effects on both cognition and behavior (“Duchenne muscular dystrophy: The basics,” 2019). Learning problems can occur with focusing attention, memory and verbal learning, as well as emotional interactions. Children with learning disabilities who have DMD are usually evaluated by developmental or pediatric neuropsychologists who provide referrals to special education departments. Therefore, the manifestations of DMD can vary from developmental to physical impairments, which points to the need for managing the complications from the point of a multi-dimensional approach.
The underlying genetic defect that causes the condition’s development occurs in the dystrophin gene with an X-linked trait. This means that there is a mutation, an error, in one of the body’s genes that causes the DMD diagnosis. The dystrophin gene contains 79 exons, which are connected to create instructions intended for forming dystrophin protein, which is needed for muscle development (“Genetic testing: A Duchenne fingerprint,” 2019).
Within DMD, there may be three forms of genetic mutations. The first type of mutation is concerned with large deletions, with one and more exons missing from the dystrophin gene. The second type is concerned with large duplications, which means that one or more exons create copies of one another within the dystrophin gene. The third type refers to other changes in the gene, with small alterations taking place and ranging from deletions to changes in a single letter in a gene makeup.
As seen in the diagram below, large deletions of one or more exons in the gene (Figure 1). Similar to a puzzle, the missing pieces in the gene prevent the remaining exons from properly fitting together. This causes errors in the instructions for making dystrophin, with the body not being able to produce the necessary amount of dystrophin protein.
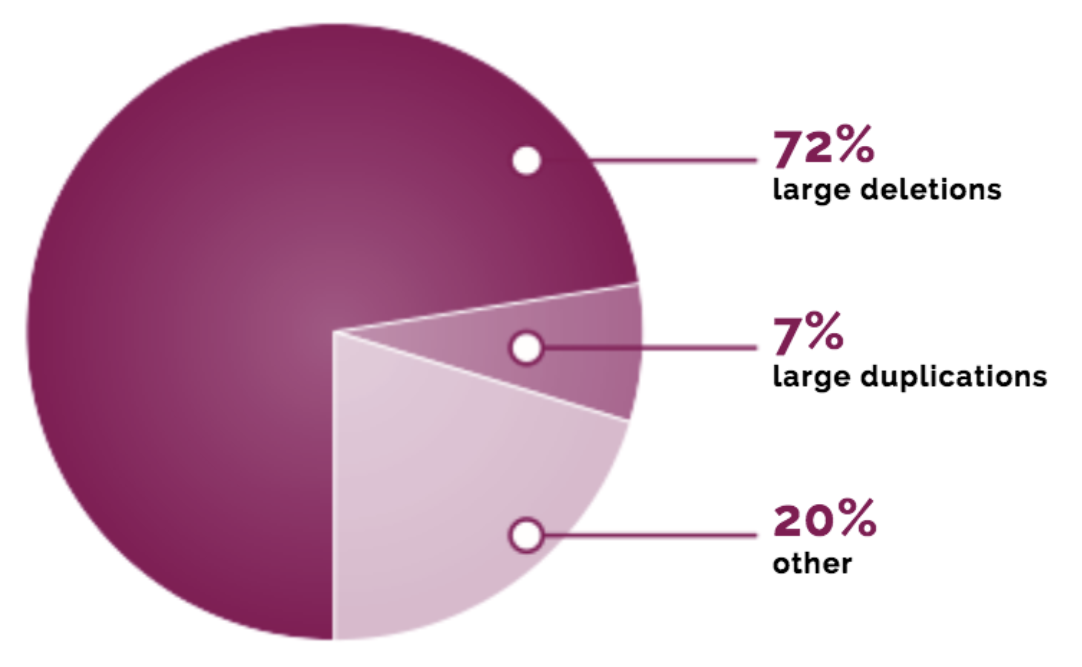
Understanding the importance of genetic mutations as related to DMD is essential because scholars have recorded more than 1,800 different changes in people’s diagnoses with either Duchenne or Becker types of muscular dystrophy. Knowing the kind of mutation that has occurred in a child is a fundamental step for considering the types of management (Bendixen & Houtrow, 2017). In order to determine the kind of genetic change, doctors prescribe a genetic test that provides further information on disease management. Moreover, children can be subjected to clinical trials that are currently being conducted to develop innovative treatments.
Different methods of genetic testing can be used for getting a full picture of a child’s mutation. For instance, complete gene sequencing can help to reveal small modifications in a gene. It is also important to note that since Duchenne is a genetic disorder, it can be inherited from one family member to another. The characteristic of DMD as an X-linked disease means that mutations are only found in the X chromosome (“Genetic testing: A Duchenne fingerprint,” 2019).
Therefore, in cases when women have Duchenne-causing mutations in their chromosomes, they are considered carriers of the disease. Carriers will most likely have no symptoms of DMD but will be capable of passing the gene along to a child; although, there is no certainty that the mutation will be transferred. The chance of having a boy from Duchenne from a carrier mother is 25%, with the same likelihood of 25% applied to having a carrier girl (“Genetic testing: A Duchenne fingerprint,” 2019).
This means that there is a 50% chance that a mother-carrier will have a baby with no mutation at all. In case when Duchenne is under suspicion, genetic testing is recommended, including carrier testing, in order to provide valuable information for making further decisions on treatment. Besides, the expertise of a genetic counselor can be highly helpful for explaining what genetic results on Duchenne can mean for families.
As mentioned in the previous sections, Duchenne muscular dystrophy is a rare inherited disease of the neuromuscular system that has no known cure at this time. DMD is usually suspected in mostly boys who display abnormal gait patterns and complications with physical activity, such as running or climbing stairs. Despite the fact that the current diagnostic process is straightforward, supported with more than 3 decades of research, there is still no solution to overcoming the healthcare challenge (Bendixen & Houtrow, 2017).
Furthermore, the American Academy of Pediatrics (2015) underlines the importance of addressing the concerns of parents about children’s development as soon as possible, especially in cases of more progressive and pronounced motor delays. Getting a formal DMD diagnosis is imperative for understanding a specific genetic mutation and determining an appropriate care path.
When a family pediatrician is concerned with the possibility of a DMD diagnosis in a child, a recommendation and referral for testing are made. An expert in neuromuscular management or a pediatric neurologist will further work to identify the causes of the symptoms and prescribe appropriate genetic and non-genetic testing.
Thus, the typical steps in identifying the disease include observing the signs and symptoms, conducting blood tests for determining enzyme levels (including CK test), genetic testing, and muscle biopsy when needed (Bendixen & Houtrow, 2017). A CK test will measure the blood’s concentration of creatine kinase, which would signify damage in muscle cells (Bendixen & Houtrow, 2017). The high levels of the enzyme in the blood will point to a muscle problem, but not confirm Duchenne as a final diagnosis as the testing is usually the first step.
Genetic testing is prescribed when an elevated level of CK is found, which means that Duchenne is suspected. This test will analyze the genetic makeup of an individual to determine a change in the dystrophin gene (Bendixen & Houtrow, 2017). When such a modification is identified, further decisions on management are made. A muscle biopsy may be prescribed when there is not enough certainty provided by a genetic test. While most patients do not require the biopsy, it is used for gathering more information.
Since there is no treatment for overcoming muscular dystrophy completely, some preventative and management efforts are taken to reduce the burden of disease. Treatment options predominantly range from medications to surgical procedures that help patients live with DMD and manage the condition’s symptoms. Doctors can prescribe heart medications and corticosteroids, as well as the Food and Drug Administration approved a drug called Eteplirsen (Exondys 51) (Bendixen & Houtrow, 2017). Despite being approved and safe for use, the drug has not shown significant evidence of effectiveness. While it may not cure DMD, it has the potential of improving muscle strength through acting on specific gene variants.
Corticosteroids are sometimes necessary for strengthening the muscle mass and delaying the development of certain types of dystrophy in children. The adverse effects of using corticosteroids over prolonged time periods include weight gain and weakened bone integrity, which increases the risk of fracture. Medications for the heart, such as angiotensin-converting enzyme (ACE) inhibitors and beta blockers, are usually prescribed when muscular dystrophy causes damage to the heart (Bourke et al., 2018). Overall, despite being prescribed to patients with DMD, medications cannot guarantee long-term maintenance of relative well-being.
Therapy and assistive devices are used as additional methods for managing life with DMD. Children diagnosed with the condition may do range-of-motion and stretching exercises to maintain high levels of mobility and flexibility of muscles as the disease causes limbs to be drawn forward and maintain in such a position (Magee, Zachazewski, Quillen, & Manske, 2015). Low-impact aerobic exercises that range from swimming to walking can help patients maintain the general health and mobility of children. Although, any activities should be verified with a doctor first since they can also be damaging. Braces and mobility aids are supplementary tools that maintain mobility independence and muscle stretchiness (Magee et at., 2015).
Breathing assistance is implemented when children’s respiratory muscles weaken. Severe cases of muscular dystrophy may call for the use of a ventilator that would force the air to travel to and from the lungs. It is also important to note that Duchenne may call for a surgery that would improve spiral curvature that limits breathing.
At this time, the prognosis for patients diagnosed with DMD does not include recovery. The adverse impact on children’s health causes a significant deterioration in life quality, leading to the need to monitor vitals continuously. For example, parents should always be aware of respiratory infection risks in more severe stages of muscular dystrophy in their children. Proper nutrition is also necessary to help prevent obesity, dehydration, and bowel movement issues. Seeking support from communities to help families cope with DMD contributes to the increased chances of living with the condition (Magee et at., 2015).
Therefore, psychological assistance is as valuable as physical management of the disease when it comes to complex genetic limitations such as Duchenne muscular dystrophy. Since DMD causes individuals with the condition to die the latest in their early twenties, extensive research is necessary to develop treatments that would be effective in overcoming the disease.
Duchenne muscular dystrophy presents a significant challenge for the health care industry because of its severe impact on the well-being and development of children. Since DMD has no cure at the present time, it requires significant control on the part of children’s families and healthcare providers. While the combination of mild exercising and prescription medication can help patients deal with the disorder, the absence of effective interventions and treatment calls for further research and clinical trials.
American Academy of Pediatrics. (2015). Pediatric clinical practice guidelines & policies: A compendium of evidence-based research for pediatric practice . Elk Grove Village, IL: AAP.
Bendixen, R. M., & Houtrow, A. (2017). Parental reflections on the diagnostic process for Duchenne muscular dystrophy: A qualitative study. Journal of Pediatric Health care: Official Publication of National Association of Pediatric Nurse Associates & Practitioners, 31 (3), 285-292.
Birnkrant, D. J., Bushby, K., Bann, C. M., Apkon, S. D., Blackwell, A., Brumbaugh, D., … DMD Care Considerations Working Group (2018). Diagnosis and management of Duchenne muscular dystrophy, part 1: Diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. The Lancet. Neurology, 17 (3), 251-267.
Bourke, J. P., Watson, G., Muntoni, F., Spinty, S., Roper, H., Guglieri, M., … DMD Heart Protection study group (2018). Randomized placebo-controlled trial of combination ACE inhibitor and beta-blocker therapy to prevent cardiomyopathy in children with Duchenne muscular dystrophy? (DMD Heart Protection Study): A protocol study. BMJ Open, 8 (12), e022572.
Duchenne muscular dystrophy: The basics . (2019). Web.
Genetic testing: A Duchenne fingerprint . (2019). Web.
Magee, D., Zachazewski, J., Quillen, W., & Manske, R. (2016 ). Pathology and intervention in musculoskeletal rehabilitation . Maryland Heights, MO: Elsevier.
Walter, M. C., & Reilich, P. (2017). Recent developments in Duchenne muscular dystrophy: facts and numbers. Journal of Cachexia, Sarcopenia and Muscle, 8 (5), 681-685.
- Chicago (A-D)
- Chicago (N-B)
IvyPanda. (2021, July 22). Duchenne Muscular Dystrophy Overview. https://ivypanda.com/essays/duchenne-muscular-dystrophy-overview/
"Duchenne Muscular Dystrophy Overview." IvyPanda , 22 July 2021, ivypanda.com/essays/duchenne-muscular-dystrophy-overview/.
IvyPanda . (2021) 'Duchenne Muscular Dystrophy Overview'. 22 July.
IvyPanda . 2021. "Duchenne Muscular Dystrophy Overview." July 22, 2021. https://ivypanda.com/essays/duchenne-muscular-dystrophy-overview/.
1. IvyPanda . "Duchenne Muscular Dystrophy Overview." July 22, 2021. https://ivypanda.com/essays/duchenne-muscular-dystrophy-overview/.
Bibliography
IvyPanda . "Duchenne Muscular Dystrophy Overview." July 22, 2021. https://ivypanda.com/essays/duchenne-muscular-dystrophy-overview/.
- Muscular Dystrophies in the Children of 3- 12 Years
- Ronald Cohn’s Lab
- The Mental, and Physical Effects Muscular Dystrophy Has On Students
- The Differences between Real and Fake Smiles
- Differences between Real and Fake Smiles
- Human Biology: Human Cells and Chromosomes
- Breast Cancer Susceptibility Gene (BRCA2)
- The Synthesis of a COX II mRNA Ready for Translation in the Cytosol
- The Muscular System of a Human Body
- Articular and Muscular Systems
- Stuttering Management: 9-Year Old Subject
- Bacterial Vaginosis: Watery Fish-Smelling Vaginal Discharge
- Urinary Tract Infections: Diagnosis and Treatment
- The First Aid Knowledge of Youth Soccer Coaches
- Infection Control and Prevention: Analysis
Switch language:

In Association with Novotech
- Partner Content
Duchenne Muscular Dystrophy: the global clinical trials landscape 2024
Affecting muscle and skeletal strength, this rare genetic disorder impacts thousands of men worldwide. Innovative new genetic therapies in development across the globe are improving the quality of life for many.
- Share on Linkedin
- Share on Facebook
Impacting approximately 1 in 5,000 males globally, Duchenne Muscular Dystrophy is a rare genetic disorder characterised by progressive muscle weakness and skeletal degeneration. The prevalence of Duchenne Muscular Dystrophy is higher in certain regions, with Europe, particularly Sweden and Norway, along with the US, Canada, and China reporting elevated rates. This emphasises the considerable health burden associated with this condition, necessitating concerted efforts in research and treatment.
A new report from leading contract research organisation (CRO) Novotech dives deeper into the current clinical trials landscape and the status of experimental treatments for Duchenne Muscular Dystrophy. Treatment typically involves the administration of glucocorticoids, such as deflazacort, to manage symptoms and slow disease progression. These medications have demonstrated effectiveness in improving motor function, strength, and pulmonary function, while also reducing the risk of complications like scoliosis and cardiomyopathy.
Recent advancements in Duchenne Muscular Dystrophy treatment include genetic therapies, such as exon-skipping treatments like eteplirsen, golodirsen, and viltolarsen, as well as premature termination codon read-through therapy with ataluren. These innovative therapies target the underlying cause of Duchenne Muscular Dystrophy by addressing the deficiency of the dystrophin protein, which is central to the disease’s pathology, and could be the key to relief for thousands of patients around the world.
Global trials test new treatments
Novotech’s report finds that the global biotech and biopharmaceutical industry has initiated around 300 clinical trials for Duchenne Muscular Dystrophy since 2019. North America and Europe collectively conducted over 60% of Duchenne Muscular Dystrophy trials, with the UK and US leading in their respective regions. In contrast, the Asia-Pacific region, led by countries like Australia and Japan, contributed around 30% of trials. Europe demonstrated shorter recruitment durations and faster recruitment rates compared to Asia-Pacific and the US, highlighting regional variations in trial efficiency.
Ongoing research initiatives are exploring diverse strategies to comprehend and address the pathogenesis of Duchenne Muscular Dystrophy. Current investigations are actively examining approved gene and RNA therapies, encompassing approaches such as gene replacement, exon skipping, and the suppression of nonsense mutations.
Promising areas of focus include cell therapies utilising muscle precursor cells or stem cells, techniques geared towards membrane stabilisation, and interventions addressing secondary cascades. These secondary cascades include the development of anti-inflammatory and antifibrotic drugs. The dynamic landscape of gene therapies for Duchenne Muscular Dystrophy has witnessed several approved treatments in the past decade, and numerous investigational therapies contribute to ongoing research and development efforts.
Among the marketed drugs for Duchenne Muscular Dystrophy treatments, both Small Molecules and Antisense Oligonucleotides play prominent roles. Drugs like Casimersen, Viltolarsen, Deflazacort, and Delandistrogene Moxeparvovec are available globally, providing tangible options for individuals and families navigating the complexities of managing Duchenne Muscular Dystrophy.
This multifaceted approach to Duchenne Muscular Dystrophy, encompassing genetic therapies, clinical trials, and pharmacologic treatments, offers hope for improved outcomes and enhanced quality of life for those affected by this challenging disorder. Continued research and collaborative efforts worldwide underscore the commitment to addressing the complexities of Duchenne Muscular Dystrophy.
To learn more about the Duchenne Muscular Dystrophy clinical trials landscape in 2024, download the free report below.
Free Report
Duchenne muscular dystrophy - global clinical trial landscape (2024).

By downloading this case study, you acknowledge that GlobalData may share your information with Novotech and that your personal data will be used as described in their Privacy Policy
Sign up for our daily news round-up!
Give your business an edge with our leading industry insights.
- Today's news
- Reviews and deals
- Climate change
- 2024 election
- Fall allergies
- Health news
- Mental health
- Sexual health
- Family health
- So mini ways
- Unapologetically
- Buying guides
Entertainment
- How to Watch
- My Portfolio
- Latest News
- Stock Market
- Premium News
- Biden Economy
- EV Deep Dive
- Stocks: Most Actives
- Stocks: Gainers
- Stocks: Losers
- Trending Tickers
- World Indices
- US Treasury Bonds
- Top Mutual Funds
- Highest Open Interest
- Highest Implied Volatility
- Stock Comparison
- Advanced Charts
- Currency Converter
- Basic Materials
- Communication Services
- Consumer Cyclical
- Consumer Defensive
- Financial Services
- Industrials
- Real Estate
- Mutual Funds
- Credit cards
- Balance Transfer Cards
- Cash-back Cards
- Rewards Cards
- Travel Cards
- Personal Loans
- Student Loans
- Car Insurance
- Morning Brief
- Market Domination
- Market Domination Overtime
- Opening Bid
- Stocks in Translation
- Lead This Way
- Good Buy or Goodbye?
- Fantasy football
- Pro Pick 'Em
- College Pick 'Em
- Fantasy baseball
- Fantasy hockey
- Fantasy basketball
- Download the app
- Daily fantasy
- Scores and schedules
- GameChannel
- World Baseball Classic
- Premier League
- CONCACAF League
- Champions League
- Motorsports
- Horse racing
- Newsletters
New on Yahoo
- Privacy Dashboard
Yahoo Finance
Parent project muscular dystrophy awards $250,000 to support clinical research network for duchenne babies identified by newborn screening.
WASHINGTON , April 29, 2024 /PRNewswire/ -- Parent Project Muscular Dystrophy (PPMD) , a nonprofit organization leading the fight to end Duchenne muscular dystrophy (Duchenne) , is excited to announce a $250,000 award to Bo Hoon Lee , MD, from the University of Rochester to support the development of a clinical research network for Duchenne babies identified through newborn screening efforts in New York State (NYS). The initiative aims to support newborn screening implementation efforts, inform clinical care guidelines for young patients, and lay the foundation for expansion of the network across states.
Duchenne is the most common genetic condition diagnosed in childhood, affecting approximately one in 5,000 live male births. Early diagnosis is critical for implementing timely interventions, establishing strong relationships with care teams, and exploring all available treatment options. Yet, a significant diagnostic delay results in patients not receiving a Duchenne diagnosis until reaching a mean age of 4-5 years.
For over a decade, PPMD has championed newborn screening for Duchenne, including spearheading a pilot study in NYS that took place between 2019 and 2021. The pilot study was a collaborative effort between PPMD, the New York State Newborn Screening (NYS NBS) program, Northwell Health Hospitals, New York-Presbyterian Hospitals, the National Institutes of Health (NIH)-supported Newborn Screening Translational Research Network (NBSTRN) housed at the American College of Medical Genetics and Genomics (ACMG), along with generous funders. In October 2023 , New York Governor Hochul signed bill S6814/A5042, making Duchenne newborn screening mandatory for all babies born in the state.
With the anticipated start of newborn screening in NYS later this year, there is an imminent need and unique opportunity to prospectively collect natural history and clinical outcomes data in these young patients. While outcome measures and rates of progression for children over age 4 are well understood, major gaps persist in trial readiness and evidence-based clinical care guidelines for the 0- to 3-year-old group. Dr. Lee's project, referred to as Baby Duchenne , aims to establish a collaborative clinical network and database to enroll and prospectively follow all babies identified by newborn screening in NYS with genetically confirmed Duchenne. This network will provide the much-needed early natural history of Duchenne, advance understanding of the benefits of early diagnosis to support national newborn screening efforts, and inform clinical trial design for the very young Duchenne patient population.
Dr. Lee's team will leverage the currently established clinical network of pediatric neuromuscular centers initially designated for spinal muscular atrophy newborn screening to build a network infrastructure to capture all Duchenne diagnoses identified via newborn screening in NYS. The network will include two Certified Duchenne Care Centers in NYS, the University of Rochester Medical Center and Stony Brook Children's Hospital, which will serve as the downstate and upstate hubs of the network. Through the development of a REDCap database of minimum core evaluations and assessments to be collected, the team aims to inform guidelines to standardize outcome measures across states implementing newborn screening over time. The ultimate goal is to establish a strong statewide network to allow expansion and inclusion of other early-screening states, such as Ohio and Minnesota , over time into the Baby Duchenne network.
Pat Furlong , PPMD's President and CEO, emphasizes the critical significance of newborn screening and the importance of this project, stating, "Newborn screening is vital for ensuring early diagnosis and intervention for babies with Duchenne. Dr. Lee's Baby Duchenne project holds immense promise in supporting these newborn screening implementation efforts. By establishing a robust clinical research network and database, we aim to not only advance our understanding of Duchenne but also pave the way for more effective treatments and improved outcomes. This initiative further extends PPMD's commitment to break down barriers related to the diagnosis, care, and treatment of every single person living with Duchenne, at all stages of life."
Dr. Lee expresses gratitude for the funding from PPMD, stating:
"This generous support from PPMD is pivotal in our work to advance care for Duchenne babies identified through newborn screening. With this funding, we can establish a collaborative clinical research network and database, enabling us to collect vital data that will inform early care guidelines and improve outcomes for these young patients. PPMD's investment underscores their commitment to advancing research and improving the lives of individuals with Duchenne, and we are deeply grateful for their partnership in this important endeavor."
ABOUT PARENT PROJECT MUSCULAR DYSTROPHY
Duchenne is a genetic disorder that slowly robs people of their muscle strength. Parent Project Muscular Dystrophy (PPMD) fights every single battle necessary to end Duchenne.
We demand optimal care standards and ensure every family has access to expert healthcare providers, cutting edge treatments, and a community of support. We invest deeply in treatments for this generation of Duchenne patients and in research that will benefit future generations. Our advocacy efforts have secured hundreds of millions of dollars in funding and won eight FDA approvals.
Everything we do—and everything we have done since our founding in 1994—helps those with Duchenne live longer, stronger lives. We will not rest until we end Duchenne for every single person affected by the disease. Join our fight against Duchenne at EndDuchenne.org . Follow PPMD on Facebook , Twitter , Instagram , and YouTube .
View original content to download multimedia: https://www.prnewswire.com/news-releases/parent-project-muscular-dystrophy-awards-250-000-to-support-clinical-research-network-for-duchenne-babies-identified-by-newborn-screening-302130082.html
SOURCE Parent Project Muscular Dystrophy (PPMD)
My son's smile gives me hope for Duchenne muscular dystrophy. A new law could help others
Opinion: newborn testing can give families a head start on giving a child with the degenerative muscular disease a shot at the best outcome in life..
Like most mothers, I only saw perfection when my first son was born. That quickly changed when we began to question pediatricians about his physical and cognitive delays.
Don’t worry, they said. Boys develop slower; they all catch up by 3.
However, by that time, Anthony still couldn’t climb stairs, fell often and barely spoke.
Finally, when we were approved for evaluation, we received a devastating diagnosis: Duchenne muscular dystrophy .
Duchenne muscular dystrophy is rare
Duchenne is a rare, progressive disorder in which muscle cells are in a constant state of destruction.
The doctor estimated Anthony would need a wheelchair between ages 8 and 10 , and his heart and lungs would give out between 12 and 20 . There was nothing we could do, they said. We should just go home and love Anthony for the time we had.
I refused to follow those directions, even though zero Duchenne therapies existed.
And today, I’m still challenging passive norms.
With eight FDA-approved tre atments commercially available and 39 more in the pipeline , families shouldn’t have to wait years for symptoms to show when a simple test could deliver a diagnosis and ensure optimal care from birth.
Arizona lawmakers, led by Sen. T.J. Shope, are actively reviewing Senate Bill 1020 to adopt statewide newborn screening for Duchenne. Once the bill passes the budget committee, it will head to the floor for a final vote.
I urge every member to quickly pass this legislation. There is no time to spare when a child’s freedom of mobility is wasting away. In DMD, time is muscle.
Early diagnosis, treatment makes a difference
Our family lived the harrowing diagnostic odyssey firsthand: two years of appointments, three gene-sequencing tests and six doctors to finally get Anthony’s results. Doctors were often unsupportive when I uncovered and attempted to share information they didn’t know.
Several dismissed me as just a “crazy” mom.
My desperation paid off in 2008 when Anthony became Patient No. 1 in the first trial for a Duchenne treatment. We considered ourselves the luckiest of the unlucky.
Most families didn’t (and still don’t) get a diagnosis until after age 5. Although we spent those years putting Anthony through surgeries, traveling back and forth to the clinical site, and missing school and work ... at least we had hope.
Every family that unwillingly joins our Duchenne community deserves the same.
Arizona tests newborns for 35 conditions on the Recommended Uniform Screening Panel from the U.S. Department of Health and Human Services.
Our state lawmakers have life-changing power to add Duchenne to the routine testing every infant already receives.
Universal screening could help other families
By pushing to discover DMD earlier than most, I had time to figure out how to still earn a living despite all the doctor visits and clinical trial travel. I had time to save for a handicap van, home modifications and all the durable medical equipment insurance doesn’t cover.
I had time to get emotional support so I could be healthy and strong enough to care for Anthony and his brother, Oliver.
Arizona must screen: For more disorders
With the benefit of newborn screening, the next generation of families will have even more time to evaluate, pursue and access FDA-approved Duchenne therapies while avoiding extensive surgeries, limitations and suffering.
Even though most new treatments won’t benefit Anthony, his early interventions did.
Today at 24, partly due to staying up on his feet until 14, there has been far less impact on his heart and lungs compared to what was originally projected. He has enjoyed many adventures and been encouraged to pursue his dreams.
And although he now has only limited use of his hands, the muscles DMD don’t impact are the ones he uses to smile.
Even though almost every daily living task — including showering, toileting, eating and even scratching an itch — requires help from family and caregivers, Anthony manages to seek joy every day.
Because of that, when I think about his sacrifices and our legacy efforts to improve Duchenne outcomes for other newborns and families in Arizona, I smile, too.
Jill Castle is an education and behavioral specialist, an adjunct professor at Arizona State University, and a national advocacy leader with Little Hercules Foundation . She's a consultant with Parent Project Muscular Dystrophy . Reach her at [email protected] .

IMAGES
VIDEO
COMMENTS
Introduction. Duchenne muscular dystrophy (DMD) is an atypical inherited musculoskeletal disorder which shows clinical characteristics of progressive muscular weakness at an early stage and pathologic features of fibrosis and fatty replacement, particularly late in the disease course. It is a recessive X-linked disorder occurring 1 in every ...
Duchenne muscular dystrophy (DMD) is a severe X-linked recessive disorder caused by mutations in the dystrophin gene and consequent complete loss of dystrophin protein expression ( Hoffman et al., 1987 ). The incidence of DMD is estimated at 1:5,000 boys worldwide, making it one of the most common recessive disorders in humans.
Abstract. Duchenne muscular dystrophy is a severe, progressive, muscle-wasting disease that leads to difficulties with movement and, eventually, to the need for assisted ventilation and premature ...
Background on Duchenne muscular dystrophy. Duchenne muscular dystrophy (DMD) is a genetic muscle disorder that affects one per 3,500-5,000 live-born males; it is the most common type of muscular dystrophy in childhood. 1, 2 It is caused by mutations of the DMD gene, located on chromosome Xp21, which encodes for dystrophin, a 427 kDa protein that is expressed at the muscle sarcolemma.
Abstract. Duchenne muscular dystrophy (DMD) is a severe, progressive, and ultimately fatal disease of skeletal muscle wasting, respiratory insufficiency, and cardiomyopathy. The identification of the dystrophin gene as central to DMD pathogenesis has led to the understanding of the muscle membrane and the proteins involved in membrane stability ...
Duchenne muscular dystrophy (DMD) is a severe, progressive genetic disorder caused by mutations in the DMD gene that result in the absence of functional dystrophin protein 1.Dystrophin is an ...
Duchenne muscular dystrophy is a fatal, X-linked neuromuscular disease that results in progressive loss of muscle function. It is caused by alterations in the dystrophin gene (DMD) that reduce dystrophin protein production to less than 3% of the normal level. 2 Signs of Duchenne muscular dystrophy usually occur in early childhood.Symptoms include muscle weakness, clumsiness, and difficulty ...
Duchenne muscular dystrophy (DMD) is a genetic muscle-wasting disease and the most common inherited paediatric myopathy, affecting 1 in 3,500-5,000 live male births 1.It is characterized by ...
Introduction. Duchenne muscular dystrophy (DMD) is an X-linked reces-sive disorder characterized by chronic muscle deterioration, weakness, and skeletal deformities [1, 2]. Patients with DMD have mutations in their DMD gene that block the pro-duction of dystrophin. Dystrophin is a cytoskeletal protein.
Duchenne muscular dystrophy (DMD) is a rare, progressive, life-limiting neuromuscular disorder [] occurring in 15.9 to 19.5 per 100,000 live male births [2,3,4].It is caused by mutations in the dystrophin gene [2, 5]; lack of dystrophin compromises muscle structure and integrity, leading to progressive muscular degeneration [6, 7].Patients with DMD are typically identified in early childhood ...
Methods and Results. Duchenne muscular dystrophy subjects prospectively enrolled in observational studies were included. Models using generalized least squares were used to assess the difference of cardiac magnetic resonance measurements between deceased and alive subjects.
Background: Duchenne muscular dystrophy (DMD) is associated with impaired muscle regeneration, progressive muscle weakness, damage, and wasting. While the cause of DMD is an X-linked loss of function mutation in the gene encoding dystrophin, the exact mechanisms that perpetuate the disease progression are unknown.
Muscular dystrophies are chronic and debilitating disorders caused by progressive muscle wasting. Duchenne muscular dystrophy (DMD) is the most common type. DMD is a well-characterized genetic disorder caused by the absence of dystrophin. Although some therapies exist to treat the symptoms and there are ongoing efforts to correct the underlying molecular defect, patients with muscular ...
Abstract. Duchenne muscular dystrophy (DMD) was named more than 150 years ago. About four decades ago, the DMD gene was discovered, and the reading frame shift was determined as the genetic underpinning. These pivotal findings significantly changed the landscape of DMD therapy development. Restoration of dystrophin expression with gene therapy ...
Introduction. Duchenne muscular dystrophy (DMD) is a genetic condition that severely affects the muscles, causing muscle weakness, which begins in early childhood. 1, 2, 3 Although rarely diagnosed in infancy (the diagnosis is usually made between age three and five), children with DMD have marked neck flexor weakness and poor head control from birth. 3
Duchenne muscular dystrophy (DMD) is a disabling and life-threatening, X-linked recessive disorder caused by mutations in dystrophin. Natural history studies can inform the disease characteristics of DMD, and data from these studies can be used to plan and design clinical trials and as external controls for long-term studies.
In Duchenne muscular dystrophy (DMD), muscle is missing a key structural protein called dystrophin, making it more susceptible to injury. Eventually the body cannot keep up with repair and regeneration, which leads to muscle loss (wasting) and weakness. Scientists therefore are working hard on the development of complementary therapies that could replace dystrophin, protect the muscle from ...
In Duchenne muscular dystrophy (DMD) disease progression and the complications associated with muscle weakness influence the overall prognosis. ... (M.G.) and by discussing the relevant literature within the research team. We used the medical subject headings (MeSH) and Embase subject headings (Emtree) terms for Duchenne muscular dystrophy ...
DOI: 10.1016/j.clnesp.2024.04.014 Corpus ID: 269284440; PERSISTENT INFLAMMATION AND NUTRITIONAL STATUS IN DUCHENNE MUSCULAR DYSTROPHY @article{deSouzaCosta2024PERSISTENTIA, title={PERSISTENT INFLAMMATION AND NUTRITIONAL STATUS IN DUCHENNE MUSCULAR DYSTROPHY}, author={{\'A}dila Danielly de Souza Costa and Karina Marques Vermeulen-Serpa and K{\'i}via Maria Batista Marinho and Caroline Addison ...
Introduction. Duchenne muscular dystrophy (DMD) is a rare, X-linked, progressive, degenerative neuromuscular disease caused by mutations in the DMD gene, which result in the absence of functional dystrophin protein. 1, 2 Dystrophin is a large subsarcolemmal protein that plays a key structural role in muscle fibers, protecting them from damage during normal muscle contraction as part of the ...
Duchenne muscular dystrophy (DMD; Online Mendelian Inheritance in Man [OMIM] reference 310200) is an X-linked disease that affects 1 in 3600-6000 live male births.1 -3 Affected individuals can have mildly delayed motor milestones and most are unable to run and jump properly due to proximal muscle weakness, which also results in the use of ...
1 INTRODUCTION. Duchenne muscular dystrophy (DMD) is a rare, degenerative disease with a global prevalence of 1 in 3500−5000 live male births (San Martin & Solis, 2018) and 1 in 50 million live female births (Nozoe et al., 2016).DMD is a multi-faceted disease with key milestones relating to loss of ambulation, loss of the ability to transfer, loss of upper body functions, spinal surgery for ...
References (12) ... Duchenne muscular dystrophy (DMD), the most common of the muscular dystrophies, with an incidence of 1 in 3,500 males, is an X-linked recessive disorder resulting from a ...
To help advance a gene-editing therapy for Duchenne muscular dystrophy, the California Institute for Regenerative Medicine (CIRM) has awarded a $3.4 million "therapeutic translational research projects" grant to MyoGene Bio.
Charlie has Duchenne muscular dystrophy, a degenerative genetic disease that, until recently, has guaranteed death by early adulthood from cardiac or respiratory failure.Like all people with ...
Introduction. Duchenne muscular dystrophy (DMD) is a genetic condition that is characterized by the continuous deterioration and weakness of muscle associated with changes in the composition of a protein called dystrophin, which maintains the functioning of muscle cells. The disorder is rare and affects males predominantly, with women being ...
The prevalence of Duchenne Muscular Dystrophy is higher in certain regions, with Europe, particularly Sweden and Norway, along with the US, Canada, and China reporting elevated rates. This emphasises the considerable health burden associated with this condition, necessitating concerted efforts in research and treatment.
Comprehensive care of individuals with Duchenne muscular dystrophy. ... A few relevant expert-opinion papers and reviews have been ... D Matthews (Children's Hospital Colorado, Aurora, CO, USA); K Bushby (John Walton Muscular Dystrophy Research Centre, Institute of Genetic Medicine, Newcastle University, Newcastle upon Tyne, UK); L E Case ...
Parent Project Muscular Dystrophy (PPMD), a nonprofit organization leading the fight to end Duchenne muscular dystrophy (Duchenne), is excited to announce a $250,000 award to Bo Hoon Lee, MD, from ...
My son's smile gives me hope for Duchenne muscular dystrophy. A new law could help others Opinion: Newborn testing can give families a head start on giving a child with the degenerative muscular ...