
- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.


Experiment_726_Paper Chromatography_1_2_1
- Last updated
- Save as PDF
- Page ID 305609
Experiment 726: Paper Chromatography: A Technique of Separation and Identification
Section 1: Purpose and Summary
One of the problems encountered most frequently in chemistry is that of separating a mixture into the pure substances which compose it. Most natural materials, such as seawater, air, oil, coal, and so on are mixtures. In order to study these materials chemically, we must first treat the mixture in some way to separate it into single, pure substances. (A "pure substance" consists entirely of just one thing; meaning one kind of molecule.) Because it does consist of just one thing, it always behaves in the same way when tested. For example, pure water always boils at 100°C (212°F), provided that the test is done at atmospheric pressure.
Many different methods have been devised for separating mixtures into their components. In the present experiment, we will use a method called chromatography. It is used quite widely for making small-scale separations and identifications. The method works because of the differences in the ways various components of a dissolved mixture interact with a fixed solid. The fixed solid can be made of different materials and in different shapes, depending on the version of chromatography that is being done. One version is called paper chromatography.
To perform paper chromatography to separate components of a mixture, there are several steps. First, the mixture that is to be separated is dissolved (if it is not already in liquid form.) Then, a small drop of the solution is applied to a piece of special chromatography paper, which is a porous paper similar to filter paper. This drop makes a tiny spot on the paper. Many such spots (of different materials) may be placed side by side on the same piece of paper. Next, the spotted paper is made to stand in a small amount of some special solvent (liquid), in such a way that only the bottom edge of the paper is submerged in the liquid, not the spots. The paper acts like a wick, drawing the liquid up the paper by capillary action. The solvent then slowly rises up the paper, reaches the spots, and begins to dissolve them and carry the substances up the paper with it.
The key to this separation is that the different components of the mixture in each spot interact with the paper differently, and so will be found (after a few minutes) to have reached different heights on the paper. This happens because some components are more strongly attracted to the paper (and so move more slowly), while others are more strongly attracted to the solvent. Any particular substance always moves at the same rate, no matter what else it may have been mixed with originally. For that reason, it can always be recognized and identified, because it will always rise to the same height, relative to the heights that the other components rise.
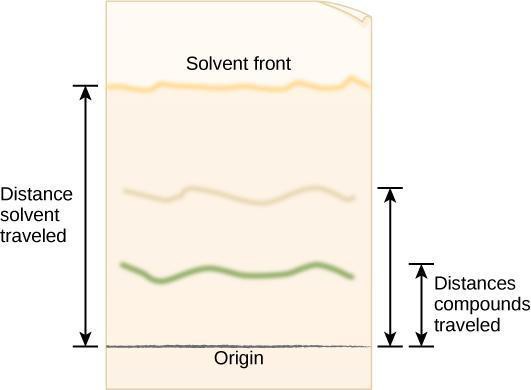
The movement of any spot on the paper can be quantified by calculating its R f ( retention factor ) value after you have stopped the experiment and the paper has dried.
R f = distance traveled by the solute
distance traveled by the solvent front
The distances used in calculating R f values are measured as shown in the following figure. To determine the distance traveled by the solute , measure from the point at which you originally applied the spot to the center or densest part of the spot. The distance traveled by the solvent front is measured from the original point of application of the spot to the limit of movement of the solvent front (which must be marked immediately after the paper is removed from the beaker, because it may be nearly invisible after the solvent evaporates).
If all conditions could be maintained constant, R f values would be constant. However, either variations in temperature or in the composition of the solvent phase or changes in the paper can alter the R f value. The R f value is useful mainly for expressing the relative mobility of two or more solutes in a particular chromatographic system. The absolute R f values may change from day to day, but their values in relation to each other remain nearly constant.
Figure 1: Completed paper chromatography containing only 1 dye.
In this experiment, students will measure the values of several dyes in 3 different solvent systems. Students will also analyze an unknown mixture of dyes in order to identify the dyes present in the mixture. The three different solvent systems are 1) laboratory water, 2) an aqueous solution of 0.10% sodium chloride (table salt), and 3) rubbing alcohol (70% isopropyl alcohol and 30% water). By using different solvents, the dyes will travel differently in paper chromatography, and the R f values will be different. The goal is to find a solvent that gives a different value for every dye as this will separate all the dyes from each other (scientists say that the dyes are resolved when the solvent system creates distinctly different values for each dye.) When every dye is separated from all the other dyes, then you can match up R f values and identify each dye in the unknown mixture.
Section 2: Safety Precautions and Waste Disposal
Safety Precautions:
Use of eye protection is recommended for all experimental procedures.
The rubbing alcohol used in this lab gives off fumes, so keep the beaker containing this solvent covered with plastic wrap when it is not in the hood.
Waste Disposal:
The rubbing alcohol should be disposed of in the organic waste container in the hood. The aqueous solvents may be poured down the drain. The chromatography paper can be disposed in the trash.
Section 3: Procedure
Preparing and developing the chromatogram
Section 4 : Analysis of the Chromatogra m s
Section 5: Identifying the components in the Unknown Mixture
Post Lab Questions:
Why use a pencil and not a pen to mark your chromatograms?
2) Why is it important that the chromatography paper not touch the sides of the beaker?
3) Which solvent system worked the best for you? Explain.
4) Suppose a student did today’s experiment and obtained the following results:
4a) Which solvent is best for separating Dyes #1-3?
4b) Assume that the student receives an Unknown mixture that contains Component Dye #2 and Component Dye #3. Draw the chromatogram the student would expect to obtain when analyzing Dye #1, Dye #2, Dye #3 and her “Component” mixture when using the best solvent, the same solvent you picked in question 4a. Include the points of application and the solvent front in your drawing.
Notes:
Sciencing_Icons_Science SCIENCE
Sciencing_icons_biology biology, sciencing_icons_cells cells, sciencing_icons_molecular molecular, sciencing_icons_microorganisms microorganisms, sciencing_icons_genetics genetics, sciencing_icons_human body human body, sciencing_icons_ecology ecology, sciencing_icons_chemistry chemistry, sciencing_icons_atomic & molecular structure atomic & molecular structure, sciencing_icons_bonds bonds, sciencing_icons_reactions reactions, sciencing_icons_stoichiometry stoichiometry, sciencing_icons_solutions solutions, sciencing_icons_acids & bases acids & bases, sciencing_icons_thermodynamics thermodynamics, sciencing_icons_organic chemistry organic chemistry, sciencing_icons_physics physics, sciencing_icons_fundamentals-physics fundamentals, sciencing_icons_electronics electronics, sciencing_icons_waves waves, sciencing_icons_energy energy, sciencing_icons_fluid fluid, sciencing_icons_astronomy astronomy, sciencing_icons_geology geology, sciencing_icons_fundamentals-geology fundamentals, sciencing_icons_minerals & rocks minerals & rocks, sciencing_icons_earth scructure earth structure, sciencing_icons_fossils fossils, sciencing_icons_natural disasters natural disasters, sciencing_icons_nature nature, sciencing_icons_ecosystems ecosystems, sciencing_icons_environment environment, sciencing_icons_insects insects, sciencing_icons_plants & mushrooms plants & mushrooms, sciencing_icons_animals animals, sciencing_icons_math math, sciencing_icons_arithmetic arithmetic, sciencing_icons_addition & subtraction addition & subtraction, sciencing_icons_multiplication & division multiplication & division, sciencing_icons_decimals decimals, sciencing_icons_fractions fractions, sciencing_icons_conversions conversions, sciencing_icons_algebra algebra, sciencing_icons_working with units working with units, sciencing_icons_equations & expressions equations & expressions, sciencing_icons_ratios & proportions ratios & proportions, sciencing_icons_inequalities inequalities, sciencing_icons_exponents & logarithms exponents & logarithms, sciencing_icons_factorization factorization, sciencing_icons_functions functions, sciencing_icons_linear equations linear equations, sciencing_icons_graphs graphs, sciencing_icons_quadratics quadratics, sciencing_icons_polynomials polynomials, sciencing_icons_geometry geometry, sciencing_icons_fundamentals-geometry fundamentals, sciencing_icons_cartesian cartesian, sciencing_icons_circles circles, sciencing_icons_solids solids, sciencing_icons_trigonometry trigonometry, sciencing_icons_probability-statistics probability & statistics, sciencing_icons_mean-median-mode mean/median/mode, sciencing_icons_independent-dependent variables independent/dependent variables, sciencing_icons_deviation deviation, sciencing_icons_correlation correlation, sciencing_icons_sampling sampling, sciencing_icons_distributions distributions, sciencing_icons_probability probability, sciencing_icons_calculus calculus, sciencing_icons_differentiation-integration differentiation/integration, sciencing_icons_application application, sciencing_icons_projects projects, sciencing_icons_news news.
- Share Tweet Email Print
- Home ⋅
- Science Fair Project Ideas for Kids, Middle & High School Students ⋅
Paper Chromatography Science Projects With a Hypothesis

Chemicals in Dry-Erase Markers
Paper chromatography analyzes mixtures by separating the chemical contents onto paper. For instance, chromatography is used in forensic science to separate chemical substances such as drugs in urine and blood samples. Students can perform paper chromatography projects using ink to understand how scientists are able to determine the presence of different chemicals.
Separate Ink Colors
Form an experiment to separate ink colors using paper chromatography. Hypothesize that regular black ink will show colors on the paper chromatography more noticeably than permanent ink. Set up the experiment using coffee filters and washable and permanent markers. Cut the coffee filters into long strips for each pen. Form a loop by stapling the ends of the strips together. Place a dot of ink on the bottoms of the coffee filter strips. Label each strip using a pencil, specifying the type of pen. Place the strips into a glass, then add water until it touches the bottom of the paper. Observe the strip. Compare your results between permanent marker and washable marker ink. The washable marker colors should spread out onto the paper, while the permanent marker does not because of its permanent ink.
Water vs. Rubbing Alcohol
Create an experiment to separate permanent marker ink colors using paper chromatography in water and rubbing alcohol. Hypothesize that rubbing alcohol will separate the ink colors in permanent markers, while water will not. Set up the experiment using coffee filters and permanent markers. Cut the coffee filters into long strips for each pen. Form a loop by stapling the ends of each strip together. Place a dot of ink on the bottom of the coffee filter strips. Place one strip into a glass of water and place another strip into a glass of rubbing alcohol until the fluid touches the bottom of the paper. Observe the strips. Compare your results between the water and rubbing alcohol solution. The colors should separate on the strip dipped in the rubbing alcohol, but won’t separate when using water.
Different Solvents
Conduct a paper chromatography project to find out if different types of solvents separate ink differently. Set up the experiment using coffee filters and permanent markers. Cut the coffee filters into long strips. Form a loop by stapling the ends of each strip together. Place a dot of ink on the bottom of the coffee filter strips. Place a strip each into a glass of water, rubbing alcohol, vinegar and nail polish remover. Make sure to only add liquid to touch the bottom of the strip. Observe the strips and compare results. Indicate which solvent separated the ink colors the best.
Use a Black Light
Perform an ink paper chromatography test and use a black light to determine if there are any more components visible on the paper than in regular light. Hypothesize that more components will be seen under black light, because some chemicals are invisible under white light. Make sure to look at the paper the same day the paper chromatography test was conducted in order to assure there is no fading on the paper.
Related Articles
How to separate the components of ink, how can parts of a solution be separated by chromatography, why does chromatography work, methods on how to determine ph in ph paper, cool science experiments for teens, what is contained in a permanent marker, how does paper chromatography work & why do pigments..., 5th grade solubility experiment, how-to science experiments for kids with iodine and..., what color would a tester ph paper turn if is dipped..., chalk and vinegar science projects, how to make disappearing ink reappear, how to use litmus papers, what turns ph paper green, food coloring & science projects, food coloring experiments, science fair projects & ideas on art, what is the difference between a solution and a suspension, how to test for hydrochloric acid.
- Science Buddies; Paper Chromatography: Basic Version; Amber Hess; April 2008
About the Author
Based in Huntington Beach, Calif., Dana Schafer has been writing environmental articles and grant proposals since 2006. Schafer has written for Grace Unlimited Corporation and Youth Have Vision. Schafer is in the process of receiving a Master of Science in biology from California State University, Long Beach.
Photo Credits
Comstock/Comstock/Getty Images
Find Your Next Great Science Fair Project! GO

BIOLOGY JUNCTION
Test And Quizzes for Biology, Pre-AP, Or AP Biology For Teachers And Students
Paper Chromatography Report
Introduction The purpose of this experiment is to observe how chromatography can be used to separate mixtures of chemical substances. Chromatography serves mainly as a tool for the examination and separation of mixtures of chemical substances. Chromatography is using a flow of solvent or gas to cause the components of a mixture to migrate differently from a narrow starting point in a specific medium, in the case of this experiment, filter paper. It is used for the purification and isolation of various substances. A chromatographically pure substance is the result of the separation. Because purification of substances is required to determine their properties, chromatography is an indispensable tool in the sciences concerned with chemical substances and their reactions.
Chromatography is also used to compare and describe chemical substances. The chromatographic sequence of sorbed substances is related to their atomic and molecular structures. A change in a chemical substance produced by a chemical or biological reaction often alters the solubility and migration rate. With this knowledge, alterations or changes can be detected in the substance.
In all chromatographic separations, there is an important relationship between the solvent, the chromatography paper, and the mixture. For a particular mixture, the solvent and the paper must be chosen so the solubility is reversible and be selective for the components of the mixture. The main requirement, though, of the solvent is to dissolve the mixture needing to be separated. The porous paper used must also absorb the components of the mixtures selectively and reversibly. For the separation of a mixture, the substances making up the mixture must be evenly dispersed in a solution, a vapor, or a gas. Once all of the above criteria have been met, chromatography can be a simple tool for separating and comparing chemical mixtures.
Hypothesis Paper can be used to separate mixed chemicals.
Materials The materials used for this lab are paper, pencil, eraser, filter paper, test tube, rubber stopper, paper clip, metric ruler, black felt-tip pen, and a computer.
Methods The first step of the method is to bend a paper clip so that it is straight with a hook at one end. Push the straight end of the paper clip into the bottom of the rubber stopper. Next, you hang a thin strip of filter paper on the hooked end of the paper clip. Insert the paper strip into the test tube. The paper should not touch the sides of the test tube and should almost touch the bottom of the test tube. Now you will remove the paper strip from the test tube. Draw a solid 5-mm-wide band about 25 mm from the bottom of the paper, using the black felt-tip pen. Use a pencil to draw a line across the paper strip 10 cm above the black band.
Pour about 2 mL of water into the test tube. The water will act as a solvent. Put the filter paper back into the test tube with the bottom of the paper in the water and the black band above the water. Observe what happens as the liquid travels up the paper. Record the changes you see. When the solvent has reached the pencil line, remove the paper from the test tube. Measure how far the solvent traveled before the strip dries. Finally, let the strip dry on the desk. With the metric ruler, measure the distance from the starting point to the top edge of each color. Record this data in a data table. Calculate a ratio for each color by dividing the distance the color traveled by the distance the solvent traveled.
Results The results of the experiment are shown in a chart and a graph.
1. How many colors separated from the black ink? Five colors separated from the black ink: yellow, pink, red, purple, and blue.
2. What served as the solvent for the ink? Water served as the solvent for the ink. As the solvent traveled up the paper, which color of ink appeared first? The color orange first appeared as the solvent traveled up the paper.
3. List the colors in order, from top to bottom, which separated from the black ink. The colors separated in this order, from top to bottom: blue, purple, red, pink, and then yellow.
4. In millimeters, how far did the solvent travel? The solvent traveled 111 mm.
5. From your results, what can you conclude is true about black ink? Black ink is a mixture of several different colors.
6 . Why did the inks separate? The inks separated because the black ink was a mixture of different pigments with different molecular characteristics. These differences allow for different rates of absorption by the filter paper.
7. Why did some inks move a greater distance? The ink least readily absorbed by the paper would then travel the farthest from the starting mark. You can conclude from this information that the different pigments were absorbed at different rates.
Error Analysis Possible errors could include inaccurate measurements of the distances traveled by the inks and mistakes when calculating the ratio traveled by the water and colors. If a longer test tube was used, a longer strip of filter paper could have been used. This may have changed the ratios. Another color may have been present, but not detected because of the filter paper length.
Conclusion The proposed hypothesis was correct. The paper chromatography did show that black ink could be separated into various colors. The black ink gets its color from a mixture of various colored inks blended together. The first color of ink to appear on the filter paper was yellow followed by pink, red, purple then blue. The colors separated the way they did because of the differences in their molecular characteristics, specifically, their solubility in water and their rate of absorption by the paper. The most soluble and readily absorbed ink color was the yellow. The least soluble and least absorbable ink color was the blue.

Jove Lab Bio
Lab 7: photosynthesis — procedure.
- NOTE: In this experiment you will separate pigments from spinach leaves using chromatography paper. Individual pigments travel along the paper at different rates and may have different colors. By calculating the relative distance the pigments travel, their resolution factor, and comparing them with literature values, you can identify different pigments. HYPOTHESES: In this exercise the experimental hypothesis is that there will be multiple pigments within the spinach leaves that absorb different wavelengths of sunlight. The null hypothesis is that there is only one type of pigment within the spinach leaf.
- Use a pencil to make a line two centimeters from one end of the chromatography paper.
- Then, lay a pipe cleaner horizontally across the top of a clean 400 mL beaker.
- Place the pencil-marked side of the chromatography strip at the bottom of the beaker.
- Next, wrap the paper around the pipe cleaner so that the bottom edge is barely touching the bottom of the beaker and then secure it with a paperclip.
- When the paper is secured around the pipe cleaner, remove it from the beaker, and then place a patted-dry spinach leaf over the marked line on the chromatography paper.
- Roll a coin over the spinach leaf along the pencil line going back and forth multiple times and applying steady pressure. When the leaf is removed, a green line should be clearly present.
- Next, place 8 mL of chromatography solvent in the beaker.
- Lower the chromatography strip into the beaker so that the edge of the paper touches the solvent but the green line does not. Adjust the pipe cleaner if needed.
- Without disturbing the beaker, observe the solvent as it moves up the paper and the individual pigments separate.
- When the solvent has traveled half way up the chromatography paper, which will take approximately 10 minutes, and the pigments have separated into well-defined bands, remove the paper from the beaker.
- Mark how far the solvent traveled with a pencil and then allow the paper to dry. NOTE: The solvent evaporates quickly.
- Next, record the number of visible bands and describe their color and relative size.
- Measure how far the solvent and pigments traveled, and record this information for each pigment in Table 1. Click Here to download Table 1
- Dispose of the chromatography solvent in a waste container under a fume hood. Throw the chromatography strips into the regular trash, and then clean the beakers with soap and water.
- NOTE: In this experiment you will indirectly observe photosynthesis and cellular respiration using a floating leaf disc in a solution. During photosynthesis, air bubbles will cause the leaves to float, and during respiration, the discs will sink. HYPOTHESES: In this exercise, the experimental hypothesis is that the leaf discs will have a greater rate of photosynthesis in the bicarbonate solution, because bicarbonate provides added CO 2 to fuel photosynthesis, causing more leaf discs to float. Additionally, all of the discs will sink in dark conditions as they perform cellular respiration. The null hypothesis is that there will be no difference in the rate of photosynthesis, and therefore the number of floating discs, between the bicarbonate and water, or light and dark treatments.
- To place leaf discs under vacuum, first remove the plungers from two 20 mL syringes, and then place 10 leaf discs inside each syringe tube. Label one syringe “bicarbonate”, and label the other syringe “water”.
- Replace the plungers and push the plunger until only a small amount of air remains in the syringe. Take care not to damage the leaf discs.
- Pull 5 mL of the bicarbonate solution into one of the syringes. Invert and swirl the syringe to suspend the leaf discs in solution.
- Push as much air out as possible without expelling the solution or damaging the leaf discs.
- Then pull 5 mL of the water solution into the other syringe and swirl it as previously described (step 3).
- To create a vacuum, hold one finger over the tip of the syringe while pulling back on the plunger. Hold this for 10 seconds while swirling the syringe to keep the leaf discs in suspension.
- Then, release the vacuum. NOTE: The discs should have absorbed the solution into the air spaces in their tissues and you should see them sink. If the discs don't sink, you can repeat the vacuum creation up to three times.
- Next, add 50 mL of bicarbonate solution to a plastic cup or a glass beaker, and then gently add the discs from the bicarbonate vacuum syringe.
- For the control, add the same amount of water to an identical cup, and then add the leaf discs from the water vacuum syringe. Label the containers appropriately.
- Place both cups under a light source.
- Every five minutes record the number of discs floating on the surface of the cup in Table 3 until 20 minutes have passed. Click Here to download Table 3
- Next, remove the cups from the light source and then swirl them so that the discs at the surface intermix with any gases also at the surface.
- Move the cups to a dark place. Every five minutes record the number of leaf discs floating at the surface until 20 minutes have passed. Swirl the cup each time before placing it back in the dark.
- To clean up, dispose of the leaf discs in the trash, and pour the bicarbonate solution down the drain. Wash the syringes and cups thoroughly.
- NOTE: In the first experiment, you observed how far pigments from spinach leaves traveled on chromatography paper. Different pigments absorb light at different wavelengths.
- Using colored pens or pencils, draw the positions of the pigment bands and the solvent on Figure 3.
- Calculate the retention factor, or Rf values for the pigments, which is done by dividing the distance the pigment in question moved up the paper from the line by the distance the solvent moved up the paper from the line.
- Compare your calculated Rf values to those in Table 2 to determine the identity of the pigment. Click Here to download Table 2
- Record these data in Table 1. NOTE: In the second experiment, you observed floating and sinking leaf discs as an indirect measurement of photosynthesis and respiration.
- Graph the results with time and minutes on the x-axis and number of floating discs on the y-axis. Use two different lines to represent the water control and the bicarbonate treatment.
- Add a line to the graph to indicate the point where the discs were removed from the light condition and placed into the dark.
- Next, starting with the bicarbonate condition, use the graph to determine the point at which 50% of the leaf discs were floating. This is referred to as the effective time, or ET50. NOTE: You will notice that the discs likely hit the 50% floating mark once in the light condition and then again in the dark condition.
- Your water samples may or may not have reached the ET50 mark. If they did, add the line for this sample also.
- Finally, compare your ET50 values and graphs with the rest of the class.

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

13.3: Lab Report
- Last updated
- Save as PDF
- Page ID 105849
Exercise 1 Data:
Make a sketch of your chromatogram using the template that follows. Using colored pencils, note the color of the bands. Use Table 1 to help you identify each pigment. On your sketch, label each band with the name of the pigment. Mark the distance (in cm) from the initial pigment band to each colored band as well as the total distance from the initial pigment band to the solvent front.
Sketch of Resulting Chromatogram
- Use the data from your sketch to complete the data table below.
- Calculate the value and include that in your data table.
Exercise 1 Review Questions:
- Which plant pigments are most polar?
- Which are least polar?
- How do these differences in polarity affect the movement of the pigments up the chromatography paper? Explain why this is observed.
4. What does the abbreviation Rf stand for? How is it calculated? What does it tell us?
- If Solution A moves 4 cm and Solution B moves 4.5 cm on a piece of chromatography paper, when the solvent moves 10 cm, which is the most polar solution? Explain your answer.
Exercise 2 Data and Review Questions:
*peaks may vary and are pH-dependent.
- How do you think the knowledge obtained from your chromatogram and the spectrograms relate to our understanding of plant pigments?
- Why do leaves change color in the fall?
Exercise 3 Employing Steps in the Scientific Method:
- Record the Question that is being investigated in this experiment. ________________________________________________________________
- Record a Hypothesis for the question stated above. ________________________________________________________________
- Predict the results of the experiment based on your hypothesis (if/then). ________________________________________________________________
Exercise 3 Review Questions:
- Complete the following sentence.
Photosynthesis is a set of ____________________________ in which ____________energy is converted to __________________energy.
- Why do some trees appear green in the summer but change colors in the fall?
3. What optimal wavelengths (or peaks) did you observe for both chlorophyll a and b ?
4. Do your leaf disks float? Use the information in this diagram of a cross-section of a leaf to explain why a leaf disk would float.
- Where does photosynthesis occur in a leaf? State which organelles carry out photosynthesis and which type or types of leaf cells have this organelle.
- Explain why it is useful to the plant to have air spaces around the spongy mesophyll cells in the leaves. (Hint: Recall the chemical equation for photosynthesis.)
- What was the purpose of the sodium bicarbonate in this experiment?
- Leaf disks normally float. What caused the leaf disks to sink?
- To measure the rate of photosynthesis, you replaced the air in the spongy mesophyll in your leaf disks with a liquid. This caused the leaf disks to sink. Then you put these leaf disks in water with dissolved CO2 and measured the amount of time it took for the leaf disks to float. Which product of photosynthesis accumulated in the spongy mesophyll and caused the leaf disks to float?
- Suppose that a leaf disk that has had the air sucked out is placed in a bicarbonate solution under a dim light that results in a low rate of photosynthesis that just equals the rate of cellular respiration. Would you expect this leaf disk to float? Explain why or why not.
Exercise 4: Observing and Quantifying Stomata (Optional)
- Develop a hypothesis about the number of open stomata found on the upper side of a leaf as compared to the lower side of the leaf. ___________________________________________________________
- Develop a hypothesis about the number of open stomata found on the upper side of a leaf as compared to the lower side of the leaf. Write your hypothesis in the space below. ____________________________________________________________
Extension Activity: (Optional)
The results of this experiment can be presented graphically. The presentation of your data in a graph will assist you in interpreting your results. Based on your results, you can complete the final step of scientific investigation, in which you must be able to propose a logical argument that either allows you to support or reject your initial hypothesis.
- Graph your results using the data from Table 3.
- What is the dependent variable? Which axis is used to graph this data? ______________________________________________________________________
- What is your independent variable? Which axis is used to graph this data? _____________________________________________________________________
Exercise 4 Review Questions:
- What are stomata?
2. What is the importance of stomata in photosynthesis?
3. What is the function of the guard cells?
4. During the lab activity, were more stomata observed on the upper surface of the leaf or the lower surface of the leaf? Explain why you think this distribution exists.
- Earth Science
- Physics & Engineering
- Science Kits
- Microscopes
- Science Curriculum and Kits
- About Home Science Tools
Science Projects > Chemistry Projects > Candy Chromatography Project + Video
Candy Chromatography Project + Video
Discover the colors behind the colors of popular candies with a Candy Chromatography Science Project. First you’ll learn what chromatography means. Then you’ll use a candy chromatography science experiment to test two types of candy. Find out if there are other colors hidden behind the colors you see!
Chromatography Concepts
The word chromatography comes from the two Greek words for color and writing , and this project will teach you why. Chromatography is a simple technique for separating a mixture’s individual components.
In chemistry, a mixture is a combination of substances that can be separated because they are not chemically bonded. Conversely, a compound cannot be separated since its elements are chemically bonded.
In this paper chromatography project, you’ll dissolve a mixture and then watch as it pulls across a piece of paper. The mixture separates and its components travel across the paper at different rates. The result is what’s known as a chromatogram , or the pattern of separated substances revealed through chromatography.

Candy Chromatography Science Project
The three primary colors for mixing dyes or paints are red, yellow, and blue. Other colors are often a mixture of these three colors. Do you think M&Ms and Skittles will have the same chromatograms? Find out with this easy and fun 7-step candy chromatography science project!
What You Need:
- M&Ms and Skittles, or other candy with colored coating
- Petri dish or a clean plate
- Pipet or dropper
- Filter paper (or coffee filters cut into strips)
- Ruler or pencil
- Clips or tape

What You Do:
1. Prepare a salt water solution by mixing 1/8 teaspoon of salt into 3 cups of water, shaking or stirring until completely dissolved. This will be your chromatography solvent. Pour about 100 ml of salt water into the beaker.
2. Get two pieces of chromatography paper, or cut out two 4×8 cm rectangles from the coffee filter. Mark a line in pencil 1 cm from the bottom of each. Use the pencil to label one for Skittles and one for M&Ms.
3. Sort the candies to find several matching colors: both packs should contain some red, orange, green, etc.
4. Use the pipet to put a single drop of water for each M&M color in the bottom of the petri dish. (Make sure the drops are evenly spaced.) Place an M&M on each water drop and set aside. The water will dissolve the candy coloring. Remove the candy after 1-2 minutes.
5. Repeat step 3 for the Skittles, this time using the lid of the petri dish.
6. Dab the end of a toothpick in one of the colored water droplets and apply the pigment to the filter paper. Apply 2-3 coats, letting the spots dry in between. Use a clean toothpick and repeat for each color.
7. Tape or clip the papers side-by-side (but not touching) to your pencil or ruler. Place the pencil or ruler over the mouth of the beaker. You want your papers barely touching the water. The paper will soak up the water and move up the paper. When the water nears the top, take the papers out, transfer them to a clean, dry, flat surface, and let them dry.
Examine your results. What colors do you see on your chromatogram? Are the two chromatograms similar? Where do you see differences? Look at the ingredient list on the packaging and see if some of the same dyes are listed. If the dyes overlap, what do you think might be the reason for different chromatograms?
Candy Chromatography Science Project + Video
What happened:
The water travels up the paper strip by capillary action .
Capillary action occurs because the water is attracted to the surface of the paper, and as the first water molecules stick to the paper, they pull others along with them.
(Capillary action is one way water moves up through the roots of plants.)
As the candy coating dissolves in the water, it is pulled up the paper too.
With this candy chromatography science experiment, you probably found that the candy coating is actually a mixture of several pigments. Certain pigments dissolve in water more easily and are pulled with the water farther up the paper. Others are more attracted to the paper and move more slowly. Usually smaller molecules move farther than larger ones.
For further study, instead of a candy chromatography science project, experiment with colored markers, flavored gelatin, powdered drink mix, or food coloring. Try to predict your results.
Learn more color chromatography science projects + watch another video.

Welcome! Read other Chemistry articles or explore the rest of the Resource Center, which consists of hundreds of free science articles!
Shop for Chemistry Supplies!
Home Science tools offers a wide variety of Chemistry products and kits. Find affordable beakers, test tubes, chemicals, kits, and everything else you need for lab experiments.
Related Articles

Science Fair Projects for 7th Graders
Science Fair Projects for 7th Graders Science fair projects for 7th graders are a step up in complexity. Because 7th graders have a better grasp of science concepts, they’re expected to practice the scientific method in the way they approach their experiments–which...

Home Science Experiments for Preschoolers
Home Science Experiments for Preschoolers Home science experiments for preschoolers are a great way to pique your child’s curiosity, teach them valuable knowledge, and allow them to have some fun in the comfort of their own home. There are plenty of activities your...

Easy Science Fair Projects for Kids
Easy Science Fair Projects for Kids Science fairs are a long-standing tradition that provide kids with the opportunity to better understand practical concepts in fun and innovative ways. The great thing about the experiments presented at these events is that they...

How to Make a Pollinator Hotel
Have you ever wondered how you can help provide habitat for pollinators like honey bees and butterflies in your back yard? Learn how to make a pollinator hotel with this step-by-step guide and lesson. Pollinators are animals that help move pollen. Most pollinators are...

Valentine’s Day Science Projects
Valentine’s Day is a great opportunity to inspire your student’s LOVE for science! Engage your kids with science concepts such as diffusion, density, and surfactants. These three, hands-on science projects include the Dancing Conversational Hearts, Rainbow Heart, and...
JOIN OUR COMMUNITY
Get project ideas and special offers delivered to your inbox.

Paper Chromatography of Plant Pigments
Learning Objectives
After completing the lab, the student will be able to:
- Extract pigments from plant material.
- Separate pigments by paper chromatography.
- Measure R f (retention factor) values for pigments.
Activity 2: Pre-Assessment
- The leaves of some plants change color in fall. Green foliage appears to turn to hues of yellow and brown. Does the yellow color appear because carotenoids replace the green chlorophylls? Explain your reasoning.
- Examine the molecular structures of photosynthetic pigments in Figure 10.1. Photosynthetic pigments are hydrophobic molecules located in thylakoid membranes. Will these pigments dissolve in water?
Activity 2: Paper Chromatography of Plant Pigments
Paper chromatography is an analytical method that separates compounds based on their solubility in a solvent.
The solvent is used to separate a mixture of molecules that have been applied to filter paper. The paper, made of cellulose, represents the stationary or immobile phase. The separation mixture moves up the paper by capillary action. It is called the mobile phase. The results of chromatography are recorded in a chromatogram. Here, the chromatogram is the piece of filter paper with the separated pigment that you will examine at the end of your experiment (see Figure 10.4).
We separate the compounds based on how quickly they move across the paper. Compounds that are soluble in the solvent mixture will be more concentrated in the mobile phase and move faster up the paper. Polar compounds will bind to the cellulose in the paper and trail behind the solvent front. As a result, the different compounds will separate according to their solubility in the mixture of organic solvents we use for chromatography.
This video demonstrates the principles and examples of chromatography. You will experiment with only paper chromatography in this lab; however, you will see that you are already familiar with some uses of thin layer chromatography.
Safety Precautions
- Work under a hood or in a well-ventilated space and avoid breathing solvents.
- Do not have any open flames when working with flammable solvents.
- Wear aprons and eye protection.
- Do not pour any organic solvent down the drain.
- Dispose of solvents per local regulations.
- Use forceps to handle chromatography paper that has been immersed in solvent and wash your hands after completing this activity.
For this activity, you will need the following:
- Plant material: intact leaves of spinach and Coleus (one leaf of each plant per pair of students)
- Filter or chromatography paper
- Ruler (one per group)
- Colored pencils
- Beakers (400 mL) (Mason jars are an acceptable substitute)
- Aluminum foil
- Petroleum ether: acetone: water in a 3:1:1 proportion
- If no hood or well-ventilated place is available, the mixture can be substituted with 95 percent isopropyl alcohol. Note that, if isopropyl alcohol is used, the pigment bands will smear. You may not be able to separate and identify the chlorophylls or carotene from xanthophyll.
For this activity, you will work in pairs .
Structured Inquiry
Step 1: Hypothesize/Predict: Discuss with your lab partner what color pigments will likely be present in the spinach leaves. Write your predictions in your lab notebook and draw a diagram of how you think the pigments will separate out on the chromatography paper.
Step 2: Student-led Planning: Read step 3 below. Discuss with your lab partner the setup of the experiment. Then agree upon the dimensions of the filter/chromatography paper that you will use. To allow good separation, the paper should not touch the walls of the container. The paper must fit inside the container while being long enough for maximum separation. Write all your calculations in your lab notebook.
Step 3: Follow the steps below to set up your filter paper and perform the chromatography experiment.
- Prepare the chromatogram by cutting a piece of filter paper. Transfer pigments from spinach leaves as in Activity 1. A heavy application line will yield stronger colors when the pigments separate, making it easier to read results. Allow the pigments to dry between applications. Wet extracts diffuse on the paper and yield blurry lines.
- Form a cylinder with the filter paper without overlapping the edges (to avoid edge effects). The sample should face the outside of the cylinder. Secure the top and bottom of the cylinder with staples.
- Pour enough separation mixture to provide a mobile phase while staying below the origin line on the chromatogram. The exact volume is not critical if the origin, the start line where you applied the solvent, is above the solvent. See Figure 10.4.
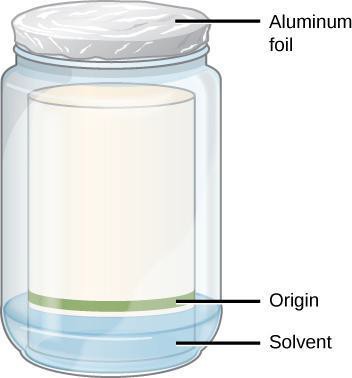
- Label the beaker with a piece of tape with your initials and your partner’s initials.
- Lower the paper into the container with the band from the extraction in the lower section. The paper must touch the solvent, but not reach the band of pigment you applied. Why must the band be above the solvent line? Write your answer in your notebook.
- Cover the container tightly with a piece of aluminum foil.
- Track the rising of the solvent front. Can you see a separation of colors on the paper?
- When the solvent front is within 1 cm of the upper edge of the paper, remove the cylinder from the beaker using forceps. Trace the solvent front with a pencil before it evaporates and disappears! Draw the colored bands seen on your chromatography paper in your lab notebook immediately. The colors will fade upon drying. If no colored pencils are available, record the colors of the lines.
- Let the paper dry in a well-ventilated area before making measurements because the wet paper is fragile and may break when handled. This is also a precaution to avoid breathing fumes from the chromatogram.
- Discard solvent mixture per your instructor’s directions. Do not pour down the drain.
Step 4: Critical Analysis: Open the dried cylinder by removing the staples. Measure the distance from the first pencil line to the solvent front, as shown in Figure 10.5. This is the distance traveled by the solvent front. Measure the distance from the pencil line to the middle point of each color band and the original pencil line. Record your results in your notebook in a table modeled after Table 10.1. The retention factor (R f ) is the ratio of the distance traveled by a colored band to the distance traveled by the solvent front. Calculate R f values for each pigment using the following equation:
R f=Distance traveled by colored band/Distance traveled by solvent front
Step 5: After determining the color of the band, tentatively identify each band. Did your results support your hypothesis about the color of each band? Discuss which aspects of the experiments may have yielded inconclusive results. How could you improve the experiment?
Guided Inquiry
Step 1: Hypothesize/Predict: What type of pigments are present in Coleus leaves and where are the different colors located? Can you make a hypothesis based on the coloration of the variegated leaves? Write your hypothesis down in your lab notebook. Would there be a difference if you performed chromatography on pigment composition from different colored regions of the leaves?
Step 2: Student-led Planning: Cut the chromatography/filter paper to the dimensions needed. Apply pigments from different parts of the Coleus leaves following the procedure described under Activity 1, keeping in mind that a darker line will yield stronger colors when the pigments are separated, which will make it easier to read the results. Allow the pigments to dry between applications. Wet extracts diffuse on the paper and yield blurry lines.
Step 3: When the solvent front reaches 1 cm from the top of the filter paper, stop the procedure. Draw the pigment bands you see on the filter paper in your lab notebook. Clearly indicate the color you observed for each band.
Step 4: Let the cylinder dry and measure the distance the front traveled from the origin and the distances traveled by each of the pigments. If the bands broadened during separation, take measurements to the middle of each band.
Step 5: Critical Analysis: Calculate R f for each of the bands and record them in a table in your notebook. Compare the R f you obtained with those of other groups. Are the R f values similar? What may have altered R f values?
Assessments
- Carotenoids and chlorophylls are hydrophobic molecules that dissolve in organic solvents. Where would you find these molecules in the cell? What would happen if you ran the chromatography in this lab with water as the solvent?
- All chlorophyll molecules contain a complexed magnesium ion. Your houseplant is developing yellow leaves. What may cause this, and how can you restore your plant’s health?
- Seeds that grow under dim light are said to be etiolated, which describes their pale and spindly appearance. They soon waste away after exhausting their food reserves. Can you explain this observation?
Lab Manual for Biology Part I Copyright © 2022 by LOUIS: The Louisiana Library Network is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License , except where otherwise noted.
Share This Book

- Science Notes Posts
- Contact Science Notes
- Todd Helmenstine Biography
- Anne Helmenstine Biography
- Free Printable Periodic Tables (PDF and PNG)
- Periodic Table Wallpapers
- Interactive Periodic Table
- Periodic Table Posters
- How to Grow Crystals
- Chemistry Projects
- Fire and Flames Projects
- Holiday Science
- Chemistry Problems With Answers
- Physics Problems
- Unit Conversion Example Problems
- Chemistry Worksheets
- Biology Worksheets
- Periodic Table Worksheets
- Physical Science Worksheets
- Science Lab Worksheets
- My Amazon Books
Leaf Chromatography Experiment – Easy Paper Chromatography

Leaf chromatography is paper chromatography using leaves. Paper chromatography is a separation technique. When applied to leaves, it separates the pigment molecules mostly according to their size. The main pigment molecule in green leaves is chlorophyll, which performs photosynthesis in the plant. Other pigments also occur, such as carotenoids and anthocyanins. When leaves change color in the fall , the amount and type of pigment molecules changes. Leaf chromatography is a fun science project that lets you see these different pigments.
Leaf Chromatography Materials
You only need a few simple materials for the leaf chromatography project:
- Rubbing alcohol (isopropyl alcohol)
- Coffee filters or thick paper towels
- Small clear jars or glasses with lids (or plastic wrap to cover the jars)
- Shallow pan
- Kitchen utensils
You can use any leaves for this project. A single plant leaf contains several pigment molecules, but for the most colors, use a variety of leaves. Or, collect several of each kind of leaf and compare them to each other. Good choices are colorful autumn leaves or chopped spinach.
Perform Paper Chromatography on Leaves
The key steps are breaking open the cells in leaves and extracting the pigment molecule and then separating the pigment using the alcohol and paper.
- Finely chop 2-3 leaves or several small leaves. If available, use a blender to break open the plant cells. The pigment molecules are in the chloroplasts of the cells, which are organelles encased within the plant cell walls. The more you break up the leave, the more pigment you’ll collect.
- Add enough alcohol to just cover the leaves.
- If you have more samples of leaves, repeat this process.
- Cover the container of leaves and alcohol and set it in a shallow pan filled with enough hot tap water to surround and heat the container. You don’t want water getting into your container of leaves.
- Replace the hot water with fresh water as it cools. Swirl the container of leaves around from time to time to aid the pigment extraction into the alcohol. The extraction is ready when the alcohol is deeply colored. The darker its color, the brighter the resulting chromatogram.
- Cut a long strip of coffee filter or sturdy paper towel for each chromatography jar. Paper with an open mesh (like a paper towel) works quickly, but paper with a denser mesh (like a coffee filter) is slower but gives a better pigment separation.
- Place a strip of paper into jar, with one end in the leaf and alcohol mixture and the other end extending upward and out of the jar.
- The alcohol moves via capillary action and evaporation, pulling the pigment molecules along with it. Ultimately, you get bands of color, each containing different pigments. After 30 to 90 minutes (or whenever you achieve pigment separation), remove the paper strips and let them dry.
How Leaf Chromatography Works
Paper chromatography separates pigments in leaf cells on the basis of three criteria:
- Molecule size
Solubility is a measure of how well a pigment molecule dissolves in the sol vent. In this project, the solvent is alcohol . Crushing the leaves breaks open cells so pigments interact with alcohol. Only molecules that are soluble in alcohol migrate with it up the paper.
Assuming a pigment is soluble, the biggest factor in how far it travels up the paper is particle size. Smaller molecules travel further up the paper than larger molecules. Small molecules fit between fibers in the paper more easily than big ones. So, they take a more direct path through the paper and get further in less time. Large molecules slowly work their way through the paper. In the beginning, not much space separates large and small molecules. The paper needs to be long enough that the different-sized molecules have enough time to separate enough to tell them apart.
Paper consists of cellulose, a polysaccharide found in wood, cotton, and other plants. Cellulose is a polar molecule . Polar molecules stick to cellulose and don’t travel very far in paper chromatography. Nonpolar molecules aren’t attracted to cellulose, so they travel further.
Of course, none of this matters if the solvent doesn’t move through the paper. Alcohol moves through paper via capillary action . The adhesive force between the liquid and the paper is greater than the cohesive force of the solvent molecules. So, the alcohol moves, carrying more alcohol and the pigment molecules along with it.
Interpreting the Chromatogram
- The smallest pigment molecules are the ones that traveled the greatest distance. The largest molecules are the ones that traveled the least distance.
- If you compare chromatograms from different jars, you can identify common pigments in their leaves. All things being equal, the lines made by the pigments should be the same distance from the origin as each other. But, usually conditions are not exactly the same, so you compare colors of lines and whether they traveled a short or long distance.
- Try identifying the pigments responsible for the colors.
There are three broad classes of plant pigments: porphyrins, carotenoids, and flavonoids. The main porphyrins are chlorophyll molecules. There are actually multiple forms of chlorophyll, but you can recognize them because they are green. Carotenoids include carotene (yellow or orange), lycopene (orange or red), and xanthophyll (yellow). Flavonoids include flavone and flavonol (both yellow) and anthocyanin (red, purple, or even blue).
Experiment Ideas
- Collect leaves from a single tree or species of tree as they change color in the fall. Compare chromatograms from different colors of leaves. Are the same pigments always present in the leaves? Some plants produce the same pigments, just in differing amounts. Other plants start producing different pigments as the seasons change.
- Compare the pigments in leaves of different kinds of trees.
- Separate leaves according to color and perform leaf chromatography on the different sets. See if you can tell the color of leaves just by looking at the relative amount of different pigments.
- The solvent you use affects the pigments you see. Repeat the experiment using acetone (nail polish remover) instead of alcohol.
- Block, Richard J.; Durrum, Emmett L.; Zweig, Gunter (1955). A Manual of Paper Chromatography and Paper Electrophoresis . Elsevier. ISBN 978-1-4832-7680-9.
- Ettre, L.S.; Zlatkis, A. (eds.) (2011). 75 Years of Chromatography: A Historical Dialogue . Elsevier. ISBN 978-0-08-085817-3.
- Gross, J. (1991). Pigments in Vegetables: Chlorophylls and Carotenoids . Van Nostrand Reinhold. ISBN 978-0442006570.
- Haslam, Edwin (2007). “Vegetable tannins – Lessons of a phytochemical lifetime.” Phytochemistry . 68 (22–24): 2713–21. doi: 10.1016/j.phytochem.2007.09.009
- McMurry, J. (2011). Organic chemistry With Biological Applications (2nd ed.). Belmont, CA: Brooks/Cole. ISBN 9780495391470.
Related Posts

IMAGES
VIDEO
COMMENTS
The 3 different solvent systems are 1) laboratory water, 2) an aqueous solution of 0.10% sodium chloride (table salt), and 3) rubbing alcohol (70% isopropyl alcohol and 30% water). 8. To the empty beaker, add a few milliliters of the appropriate solvent to each beaker. Use just enough solvent so that it will wet the bottom edge of the cylinder ...
Chromatography is defined to be a chemical method of component separation where two phases - the mobile phase and the stationary phase - are used to dissolve and divide parts of a ... For this lab, I developed a hypothesis regarding the solutions that would best identify my unknown ink samples: "Based on observations of the base paper ...
Conduct a paper chromatography project to find out if different types of solvents separate ink differently. Set up the experiment using coffee filters and permanent markers. Cut the coffee filters into long strips. Form a loop by stapling the ends of each strip together. Place a dot of ink on the bottom of the coffee filter strips.
Thin Layer Chromatography Ryan Huckaby CHEM 2123-3-5-Ryan Huckaby Dr. Srinivasan 2423- 3-5-Thin Layer Chromatography ABSTRACT The process of thin layer chromatography allows compounds to rise a TLC plate through capillary action as the chromatogram develops.
Hypothesis Paper can be used to separate mixed chemicals. Materials The materials used for this lab are paper, pencil, eraser, filter paper, test tube, rubber stopper, paper clip, metric ruler, black felt-tip pen, and a computer. Methods The first step of the method is to bend a paper clip so that it is straight with a hook at one end. Push the ...
Chromatography. Chromatography is an analytical technique used to separate the components of a mixture. All forms of chromatography work on the same principle. liquid supported on a solid) and a mobile phase (a liquid or a gas). The mobile phase flows through the stationary phase and carries the components of the mixture with it.
Experiment #7: Chromatography Simran Sharda CHM 1004 ETX[41725] Dr. Olga Lavinda October 27, 2019. Abstract— Chromatography is an analytical technique commonly used for separating a mixture of chemical substances into its individual components, so that the individual components can be thoroughly analyzed, and chromatography is thus a form of purification.
The null hypothesis is that there is only one type of pigment within the spinach leaf. Use a pencil to make a line two centimeters from one end of the chromatography paper. Then, lay a pipe cleaner horizontally across the top of a clean 400 mL beaker.
Make a sketch of your chromatogram using the template that follows. Using colored pencils, note the color of the bands. Use Table 1 to help you identify each pigment. On your sketch, label each band with the name of the pigment. Mark the distance (in cm) from the initial pigment band to each colored band as well as the total distance from the ...
Lab report for a chromatography lab. biol chromatography hypothesis: the dye molecules that have more ionic bonds will stay stationary on the chromatography ... This molecule is derived from Gentian violet; two ionic bonds were added. B. My hypothesis was that the dye molecules with more ionic bonds will stay stationary and not move up the ...
A lab report starts with a title page covering the name, student number, and experiment name. In this case, the title will be paper chromatography. Next in line is the abstract that summarizes the significant points. It would help determine why you are experimenting and state the materials you need to perform the task.
Get two pieces of chromatography paper, or cut out two 4×8 cm rectangles from the coffee filter. Mark a line in pencil 1 cm from the bottom of each. Use the pencil to label one for Skittles and one for M&Ms. 3. Sort the candies to find several matching colors: both packs should contain some red, orange, green, etc. 4.
Fill a capillary tube by placing it in the leaf extract (it will fill by capillary action). Keep your finger off the end of the capillary tube. Apply the extract to the center of the dot (e) on the paper by quickly touching the end of the TLC applicator to the plate. Allow to dry (you can gently blow on the strip).
Step 1: Hypothesize/Predict: Discuss with your lab partner what color pigments will likely be present in the spinach leaves. Write your predictions in your lab notebook and draw a diagram of how you think the pigments will separate out on the chromatography paper. Step 2: Student-led Planning: Read step 3 below.
Ap Bio Lab Report. Chromatography comes from the Greek words chroma and graph for Color Writing. The technique was developed by Mikhail Tsvet who used it for separating pigments that made up plant dyes. Chromatography is a very valuable technique used for separating mixtures.
Perform Paper Chromatography on Leaves. The key steps are breaking open the cells in leaves and extracting the pigment molecule and then separating the pigment using the alcohol and paper. Finely chop 2-3 leaves or several small leaves. If available, use a blender to break open the plant cells.
Chromatography Lab 1. Hypothesis- In the Chromatography lab, my hypothesis is that the structure with the most charges will rise higher than the dye with less charges. The dye with the less charges would be more stable than the dye with more charges because it makes less ionic bonds than the dye that can make more ionic bonds.
Cup (preparably clear, plastic cup but not necessary) 7. Tape. Procedure: 1. Preparing chromatography paper: Cut the paper towel: 2 cm by 15 cm. Then, measure 4 cm from one end and draw a line using a pencil and also a midline dividing the 4 cm line you just drew (as shown in the picture below). This is the baseline.
Chromatography lab report.docx. School. University of Botswana-Gaborone * *We aren't endorsed by this school. Course. BIOLOGY 111. Subject. Biology. Date. Nov 14, 2023. ... Alternative hypothesis: leaves of the same plant contain different pigments and thin-layer chromatography is an efficient method for the separation and identification of ...
Lab—plant pigment chromatography and photosynthesis Introduction: Background information Photosynthesis is the process that the plants use sunlight, water, and carbon dioxide to create oxygen and energy in the form of sugar (National Geographic, 2023). ... Take decent photo of your labeled chromatograph(s) for your lab report. (5 pts) Our own ...
Biology_ Chromatography Lab Report - Free download as PDF File (.pdf), Text File (.txt) or read online for free. The students conducted a chromatography experiment to separate and identify the pigments in leaves, specifically chlorophyll. They used a mortar and pestle to grind leaves with ethanol, then applied the mixture to a filter paper strip and placed it in a test tube with ethanol.