Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, generate accurate citations for free.
- Knowledge Base
Methodology
- How to Write a Literature Review | Guide, Examples, & Templates

How to Write a Literature Review | Guide, Examples, & Templates
Published on January 2, 2023 by Shona McCombes . Revised on September 11, 2023.
What is a literature review? A literature review is a survey of scholarly sources on a specific topic. It provides an overview of current knowledge, allowing you to identify relevant theories, methods, and gaps in the existing research that you can later apply to your paper, thesis, or dissertation topic .
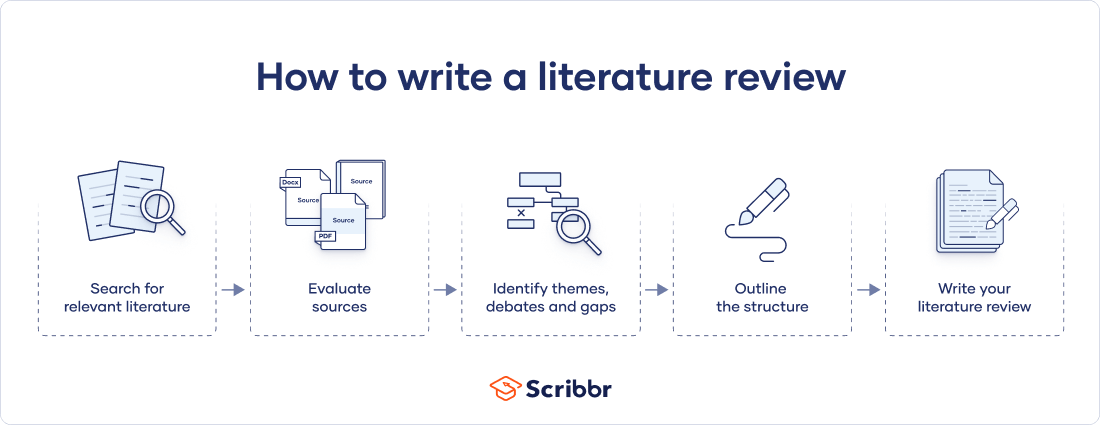
There are five key steps to writing a literature review:
- Search for relevant literature
- Evaluate sources
- Identify themes, debates, and gaps
- Outline the structure
- Write your literature review
A good literature review doesn’t just summarize sources—it analyzes, synthesizes , and critically evaluates to give a clear picture of the state of knowledge on the subject.
Instantly correct all language mistakes in your text
Upload your document to correct all your mistakes in minutes

Table of contents
What is the purpose of a literature review, examples of literature reviews, step 1 – search for relevant literature, step 2 – evaluate and select sources, step 3 – identify themes, debates, and gaps, step 4 – outline your literature review’s structure, step 5 – write your literature review, free lecture slides, other interesting articles, frequently asked questions, introduction.
- Quick Run-through
- Step 1 & 2
When you write a thesis , dissertation , or research paper , you will likely have to conduct a literature review to situate your research within existing knowledge. The literature review gives you a chance to:
- Demonstrate your familiarity with the topic and its scholarly context
- Develop a theoretical framework and methodology for your research
- Position your work in relation to other researchers and theorists
- Show how your research addresses a gap or contributes to a debate
- Evaluate the current state of research and demonstrate your knowledge of the scholarly debates around your topic.
Writing literature reviews is a particularly important skill if you want to apply for graduate school or pursue a career in research. We’ve written a step-by-step guide that you can follow below.
Receive feedback on language, structure, and formatting
Professional editors proofread and edit your paper by focusing on:
- Academic style
- Vague sentences
- Style consistency
See an example

Writing literature reviews can be quite challenging! A good starting point could be to look at some examples, depending on what kind of literature review you’d like to write.
- Example literature review #1: “Why Do People Migrate? A Review of the Theoretical Literature” ( Theoretical literature review about the development of economic migration theory from the 1950s to today.)
- Example literature review #2: “Literature review as a research methodology: An overview and guidelines” ( Methodological literature review about interdisciplinary knowledge acquisition and production.)
- Example literature review #3: “The Use of Technology in English Language Learning: A Literature Review” ( Thematic literature review about the effects of technology on language acquisition.)
- Example literature review #4: “Learners’ Listening Comprehension Difficulties in English Language Learning: A Literature Review” ( Chronological literature review about how the concept of listening skills has changed over time.)
You can also check out our templates with literature review examples and sample outlines at the links below.
Download Word doc Download Google doc
Before you begin searching for literature, you need a clearly defined topic .
If you are writing the literature review section of a dissertation or research paper, you will search for literature related to your research problem and questions .
Make a list of keywords
Start by creating a list of keywords related to your research question. Include each of the key concepts or variables you’re interested in, and list any synonyms and related terms. You can add to this list as you discover new keywords in the process of your literature search.
- Social media, Facebook, Instagram, Twitter, Snapchat, TikTok
- Body image, self-perception, self-esteem, mental health
- Generation Z, teenagers, adolescents, youth
Search for relevant sources
Use your keywords to begin searching for sources. Some useful databases to search for journals and articles include:
- Your university’s library catalogue
- Google Scholar
- Project Muse (humanities and social sciences)
- Medline (life sciences and biomedicine)
- EconLit (economics)
- Inspec (physics, engineering and computer science)
You can also use boolean operators to help narrow down your search.
Make sure to read the abstract to find out whether an article is relevant to your question. When you find a useful book or article, you can check the bibliography to find other relevant sources.
You likely won’t be able to read absolutely everything that has been written on your topic, so it will be necessary to evaluate which sources are most relevant to your research question.
For each publication, ask yourself:
- What question or problem is the author addressing?
- What are the key concepts and how are they defined?
- What are the key theories, models, and methods?
- Does the research use established frameworks or take an innovative approach?
- What are the results and conclusions of the study?
- How does the publication relate to other literature in the field? Does it confirm, add to, or challenge established knowledge?
- What are the strengths and weaknesses of the research?
Make sure the sources you use are credible , and make sure you read any landmark studies and major theories in your field of research.
You can use our template to summarize and evaluate sources you’re thinking about using. Click on either button below to download.
Take notes and cite your sources
As you read, you should also begin the writing process. Take notes that you can later incorporate into the text of your literature review.
It is important to keep track of your sources with citations to avoid plagiarism . It can be helpful to make an annotated bibliography , where you compile full citation information and write a paragraph of summary and analysis for each source. This helps you remember what you read and saves time later in the process.
The only proofreading tool specialized in correcting academic writing - try for free!
The academic proofreading tool has been trained on 1000s of academic texts and by native English editors. Making it the most accurate and reliable proofreading tool for students.
Try for free
To begin organizing your literature review’s argument and structure, be sure you understand the connections and relationships between the sources you’ve read. Based on your reading and notes, you can look for:
- Trends and patterns (in theory, method or results): do certain approaches become more or less popular over time?
- Themes: what questions or concepts recur across the literature?
- Debates, conflicts and contradictions: where do sources disagree?
- Pivotal publications: are there any influential theories or studies that changed the direction of the field?
- Gaps: what is missing from the literature? Are there weaknesses that need to be addressed?
This step will help you work out the structure of your literature review and (if applicable) show how your own research will contribute to existing knowledge.
- Most research has focused on young women.
- There is an increasing interest in the visual aspects of social media.
- But there is still a lack of robust research on highly visual platforms like Instagram and Snapchat—this is a gap that you could address in your own research.
There are various approaches to organizing the body of a literature review. Depending on the length of your literature review, you can combine several of these strategies (for example, your overall structure might be thematic, but each theme is discussed chronologically).
Chronological
The simplest approach is to trace the development of the topic over time. However, if you choose this strategy, be careful to avoid simply listing and summarizing sources in order.
Try to analyze patterns, turning points and key debates that have shaped the direction of the field. Give your interpretation of how and why certain developments occurred.
If you have found some recurring central themes, you can organize your literature review into subsections that address different aspects of the topic.
For example, if you are reviewing literature about inequalities in migrant health outcomes, key themes might include healthcare policy, language barriers, cultural attitudes, legal status, and economic access.
Methodological
If you draw your sources from different disciplines or fields that use a variety of research methods , you might want to compare the results and conclusions that emerge from different approaches. For example:
- Look at what results have emerged in qualitative versus quantitative research
- Discuss how the topic has been approached by empirical versus theoretical scholarship
- Divide the literature into sociological, historical, and cultural sources
Theoretical
A literature review is often the foundation for a theoretical framework . You can use it to discuss various theories, models, and definitions of key concepts.
You might argue for the relevance of a specific theoretical approach, or combine various theoretical concepts to create a framework for your research.
Like any other academic text , your literature review should have an introduction , a main body, and a conclusion . What you include in each depends on the objective of your literature review.
The introduction should clearly establish the focus and purpose of the literature review.
Depending on the length of your literature review, you might want to divide the body into subsections. You can use a subheading for each theme, time period, or methodological approach.
As you write, you can follow these tips:
- Summarize and synthesize: give an overview of the main points of each source and combine them into a coherent whole
- Analyze and interpret: don’t just paraphrase other researchers — add your own interpretations where possible, discussing the significance of findings in relation to the literature as a whole
- Critically evaluate: mention the strengths and weaknesses of your sources
- Write in well-structured paragraphs: use transition words and topic sentences to draw connections, comparisons and contrasts
In the conclusion, you should summarize the key findings you have taken from the literature and emphasize their significance.
When you’ve finished writing and revising your literature review, don’t forget to proofread thoroughly before submitting. Not a language expert? Check out Scribbr’s professional proofreading services !
This article has been adapted into lecture slides that you can use to teach your students about writing a literature review.
Scribbr slides are free to use, customize, and distribute for educational purposes.
Open Google Slides Download PowerPoint
If you want to know more about the research process , methodology , research bias , or statistics , make sure to check out some of our other articles with explanations and examples.
- Sampling methods
- Simple random sampling
- Stratified sampling
- Cluster sampling
- Likert scales
- Reproducibility
Statistics
- Null hypothesis
- Statistical power
- Probability distribution
- Effect size
- Poisson distribution
Research bias
- Optimism bias
- Cognitive bias
- Implicit bias
- Hawthorne effect
- Anchoring bias
- Explicit bias
A literature review is a survey of scholarly sources (such as books, journal articles, and theses) related to a specific topic or research question .
It is often written as part of a thesis, dissertation , or research paper , in order to situate your work in relation to existing knowledge.
There are several reasons to conduct a literature review at the beginning of a research project:
- To familiarize yourself with the current state of knowledge on your topic
- To ensure that you’re not just repeating what others have already done
- To identify gaps in knowledge and unresolved problems that your research can address
- To develop your theoretical framework and methodology
- To provide an overview of the key findings and debates on the topic
Writing the literature review shows your reader how your work relates to existing research and what new insights it will contribute.
The literature review usually comes near the beginning of your thesis or dissertation . After the introduction , it grounds your research in a scholarly field and leads directly to your theoretical framework or methodology .
A literature review is a survey of credible sources on a topic, often used in dissertations , theses, and research papers . Literature reviews give an overview of knowledge on a subject, helping you identify relevant theories and methods, as well as gaps in existing research. Literature reviews are set up similarly to other academic texts , with an introduction , a main body, and a conclusion .
An annotated bibliography is a list of source references that has a short description (called an annotation ) for each of the sources. It is often assigned as part of the research process for a paper .
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the “Cite this Scribbr article” button to automatically add the citation to our free Citation Generator.
McCombes, S. (2023, September 11). How to Write a Literature Review | Guide, Examples, & Templates. Scribbr. Retrieved April 15, 2024, from https://www.scribbr.com/dissertation/literature-review/
Is this article helpful?
Shona McCombes
Other students also liked, what is a theoretical framework | guide to organizing, what is a research methodology | steps & tips, how to write a research proposal | examples & templates, what is your plagiarism score.
- USC Libraries
- Research Guides
Organizing Your Social Sciences Research Paper
- 5. The Literature Review
- Purpose of Guide
- Design Flaws to Avoid
- Independent and Dependent Variables
- Glossary of Research Terms
- Reading Research Effectively
- Narrowing a Topic Idea
- Broadening a Topic Idea
- Extending the Timeliness of a Topic Idea
- Academic Writing Style
- Applying Critical Thinking
- Choosing a Title
- Making an Outline
- Paragraph Development
- Research Process Video Series
- Executive Summary
- The C.A.R.S. Model
- Background Information
- The Research Problem/Question
- Theoretical Framework
- Citation Tracking
- Content Alert Services
- Evaluating Sources
- Primary Sources
- Secondary Sources
- Tiertiary Sources
- Scholarly vs. Popular Publications
- Qualitative Methods
- Quantitative Methods
- Insiderness
- Using Non-Textual Elements
- Limitations of the Study
- Common Grammar Mistakes
- Writing Concisely
- Avoiding Plagiarism
- Footnotes or Endnotes?
- Further Readings
- Generative AI and Writing
- USC Libraries Tutorials and Other Guides
- Bibliography
A literature review surveys prior research published in books, scholarly articles, and any other sources relevant to a particular issue, area of research, or theory, and by so doing, provides a description, summary, and critical evaluation of these works in relation to the research problem being investigated. Literature reviews are designed to provide an overview of sources you have used in researching a particular topic and to demonstrate to your readers how your research fits within existing scholarship about the topic.
Fink, Arlene. Conducting Research Literature Reviews: From the Internet to Paper . Fourth edition. Thousand Oaks, CA: SAGE, 2014.
Importance of a Good Literature Review
A literature review may consist of simply a summary of key sources, but in the social sciences, a literature review usually has an organizational pattern and combines both summary and synthesis, often within specific conceptual categories . A summary is a recap of the important information of the source, but a synthesis is a re-organization, or a reshuffling, of that information in a way that informs how you are planning to investigate a research problem. The analytical features of a literature review might:
- Give a new interpretation of old material or combine new with old interpretations,
- Trace the intellectual progression of the field, including major debates,
- Depending on the situation, evaluate the sources and advise the reader on the most pertinent or relevant research, or
- Usually in the conclusion of a literature review, identify where gaps exist in how a problem has been researched to date.
Given this, the purpose of a literature review is to:
- Place each work in the context of its contribution to understanding the research problem being studied.
- Describe the relationship of each work to the others under consideration.
- Identify new ways to interpret prior research.
- Reveal any gaps that exist in the literature.
- Resolve conflicts amongst seemingly contradictory previous studies.
- Identify areas of prior scholarship to prevent duplication of effort.
- Point the way in fulfilling a need for additional research.
- Locate your own research within the context of existing literature [very important].
Fink, Arlene. Conducting Research Literature Reviews: From the Internet to Paper. 2nd ed. Thousand Oaks, CA: Sage, 2005; Hart, Chris. Doing a Literature Review: Releasing the Social Science Research Imagination . Thousand Oaks, CA: Sage Publications, 1998; Jesson, Jill. Doing Your Literature Review: Traditional and Systematic Techniques . Los Angeles, CA: SAGE, 2011; Knopf, Jeffrey W. "Doing a Literature Review." PS: Political Science and Politics 39 (January 2006): 127-132; Ridley, Diana. The Literature Review: A Step-by-Step Guide for Students . 2nd ed. Los Angeles, CA: SAGE, 2012.
Types of Literature Reviews
It is important to think of knowledge in a given field as consisting of three layers. First, there are the primary studies that researchers conduct and publish. Second are the reviews of those studies that summarize and offer new interpretations built from and often extending beyond the primary studies. Third, there are the perceptions, conclusions, opinion, and interpretations that are shared informally among scholars that become part of the body of epistemological traditions within the field.
In composing a literature review, it is important to note that it is often this third layer of knowledge that is cited as "true" even though it often has only a loose relationship to the primary studies and secondary literature reviews. Given this, while literature reviews are designed to provide an overview and synthesis of pertinent sources you have explored, there are a number of approaches you could adopt depending upon the type of analysis underpinning your study.
Argumentative Review This form examines literature selectively in order to support or refute an argument, deeply embedded assumption, or philosophical problem already established in the literature. The purpose is to develop a body of literature that establishes a contrarian viewpoint. Given the value-laden nature of some social science research [e.g., educational reform; immigration control], argumentative approaches to analyzing the literature can be a legitimate and important form of discourse. However, note that they can also introduce problems of bias when they are used to make summary claims of the sort found in systematic reviews [see below].
Integrative Review Considered a form of research that reviews, critiques, and synthesizes representative literature on a topic in an integrated way such that new frameworks and perspectives on the topic are generated. The body of literature includes all studies that address related or identical hypotheses or research problems. A well-done integrative review meets the same standards as primary research in regard to clarity, rigor, and replication. This is the most common form of review in the social sciences.
Historical Review Few things rest in isolation from historical precedent. Historical literature reviews focus on examining research throughout a period of time, often starting with the first time an issue, concept, theory, phenomena emerged in the literature, then tracing its evolution within the scholarship of a discipline. The purpose is to place research in a historical context to show familiarity with state-of-the-art developments and to identify the likely directions for future research.
Methodological Review A review does not always focus on what someone said [findings], but how they came about saying what they say [method of analysis]. Reviewing methods of analysis provides a framework of understanding at different levels [i.e. those of theory, substantive fields, research approaches, and data collection and analysis techniques], how researchers draw upon a wide variety of knowledge ranging from the conceptual level to practical documents for use in fieldwork in the areas of ontological and epistemological consideration, quantitative and qualitative integration, sampling, interviewing, data collection, and data analysis. This approach helps highlight ethical issues which you should be aware of and consider as you go through your own study.
Systematic Review This form consists of an overview of existing evidence pertinent to a clearly formulated research question, which uses pre-specified and standardized methods to identify and critically appraise relevant research, and to collect, report, and analyze data from the studies that are included in the review. The goal is to deliberately document, critically evaluate, and summarize scientifically all of the research about a clearly defined research problem . Typically it focuses on a very specific empirical question, often posed in a cause-and-effect form, such as "To what extent does A contribute to B?" This type of literature review is primarily applied to examining prior research studies in clinical medicine and allied health fields, but it is increasingly being used in the social sciences.
Theoretical Review The purpose of this form is to examine the corpus of theory that has accumulated in regard to an issue, concept, theory, phenomena. The theoretical literature review helps to establish what theories already exist, the relationships between them, to what degree the existing theories have been investigated, and to develop new hypotheses to be tested. Often this form is used to help establish a lack of appropriate theories or reveal that current theories are inadequate for explaining new or emerging research problems. The unit of analysis can focus on a theoretical concept or a whole theory or framework.
NOTE : Most often the literature review will incorporate some combination of types. For example, a review that examines literature supporting or refuting an argument, assumption, or philosophical problem related to the research problem will also need to include writing supported by sources that establish the history of these arguments in the literature.
Baumeister, Roy F. and Mark R. Leary. "Writing Narrative Literature Reviews." Review of General Psychology 1 (September 1997): 311-320; Mark R. Fink, Arlene. Conducting Research Literature Reviews: From the Internet to Paper . 2nd ed. Thousand Oaks, CA: Sage, 2005; Hart, Chris. Doing a Literature Review: Releasing the Social Science Research Imagination . Thousand Oaks, CA: Sage Publications, 1998; Kennedy, Mary M. "Defining a Literature." Educational Researcher 36 (April 2007): 139-147; Petticrew, Mark and Helen Roberts. Systematic Reviews in the Social Sciences: A Practical Guide . Malden, MA: Blackwell Publishers, 2006; Torracro, Richard. "Writing Integrative Literature Reviews: Guidelines and Examples." Human Resource Development Review 4 (September 2005): 356-367; Rocco, Tonette S. and Maria S. Plakhotnik. "Literature Reviews, Conceptual Frameworks, and Theoretical Frameworks: Terms, Functions, and Distinctions." Human Ressource Development Review 8 (March 2008): 120-130; Sutton, Anthea. Systematic Approaches to a Successful Literature Review . Los Angeles, CA: Sage Publications, 2016.
Structure and Writing Style
I. Thinking About Your Literature Review
The structure of a literature review should include the following in support of understanding the research problem :
- An overview of the subject, issue, or theory under consideration, along with the objectives of the literature review,
- Division of works under review into themes or categories [e.g. works that support a particular position, those against, and those offering alternative approaches entirely],
- An explanation of how each work is similar to and how it varies from the others,
- Conclusions as to which pieces are best considered in their argument, are most convincing of their opinions, and make the greatest contribution to the understanding and development of their area of research.
The critical evaluation of each work should consider :
- Provenance -- what are the author's credentials? Are the author's arguments supported by evidence [e.g. primary historical material, case studies, narratives, statistics, recent scientific findings]?
- Methodology -- were the techniques used to identify, gather, and analyze the data appropriate to addressing the research problem? Was the sample size appropriate? Were the results effectively interpreted and reported?
- Objectivity -- is the author's perspective even-handed or prejudicial? Is contrary data considered or is certain pertinent information ignored to prove the author's point?
- Persuasiveness -- which of the author's theses are most convincing or least convincing?
- Validity -- are the author's arguments and conclusions convincing? Does the work ultimately contribute in any significant way to an understanding of the subject?
II. Development of the Literature Review
Four Basic Stages of Writing 1. Problem formulation -- which topic or field is being examined and what are its component issues? 2. Literature search -- finding materials relevant to the subject being explored. 3. Data evaluation -- determining which literature makes a significant contribution to the understanding of the topic. 4. Analysis and interpretation -- discussing the findings and conclusions of pertinent literature.
Consider the following issues before writing the literature review: Clarify If your assignment is not specific about what form your literature review should take, seek clarification from your professor by asking these questions: 1. Roughly how many sources would be appropriate to include? 2. What types of sources should I review (books, journal articles, websites; scholarly versus popular sources)? 3. Should I summarize, synthesize, or critique sources by discussing a common theme or issue? 4. Should I evaluate the sources in any way beyond evaluating how they relate to understanding the research problem? 5. Should I provide subheadings and other background information, such as definitions and/or a history? Find Models Use the exercise of reviewing the literature to examine how authors in your discipline or area of interest have composed their literature review sections. Read them to get a sense of the types of themes you might want to look for in your own research or to identify ways to organize your final review. The bibliography or reference section of sources you've already read, such as required readings in the course syllabus, are also excellent entry points into your own research. Narrow the Topic The narrower your topic, the easier it will be to limit the number of sources you need to read in order to obtain a good survey of relevant resources. Your professor will probably not expect you to read everything that's available about the topic, but you'll make the act of reviewing easier if you first limit scope of the research problem. A good strategy is to begin by searching the USC Libraries Catalog for recent books about the topic and review the table of contents for chapters that focuses on specific issues. You can also review the indexes of books to find references to specific issues that can serve as the focus of your research. For example, a book surveying the history of the Israeli-Palestinian conflict may include a chapter on the role Egypt has played in mediating the conflict, or look in the index for the pages where Egypt is mentioned in the text. Consider Whether Your Sources are Current Some disciplines require that you use information that is as current as possible. This is particularly true in disciplines in medicine and the sciences where research conducted becomes obsolete very quickly as new discoveries are made. However, when writing a review in the social sciences, a survey of the history of the literature may be required. In other words, a complete understanding the research problem requires you to deliberately examine how knowledge and perspectives have changed over time. Sort through other current bibliographies or literature reviews in the field to get a sense of what your discipline expects. You can also use this method to explore what is considered by scholars to be a "hot topic" and what is not.
III. Ways to Organize Your Literature Review
Chronology of Events If your review follows the chronological method, you could write about the materials according to when they were published. This approach should only be followed if a clear path of research building on previous research can be identified and that these trends follow a clear chronological order of development. For example, a literature review that focuses on continuing research about the emergence of German economic power after the fall of the Soviet Union. By Publication Order your sources by publication chronology, then, only if the order demonstrates a more important trend. For instance, you could order a review of literature on environmental studies of brown fields if the progression revealed, for example, a change in the soil collection practices of the researchers who wrote and/or conducted the studies. Thematic [“conceptual categories”] A thematic literature review is the most common approach to summarizing prior research in the social and behavioral sciences. Thematic reviews are organized around a topic or issue, rather than the progression of time, although the progression of time may still be incorporated into a thematic review. For example, a review of the Internet’s impact on American presidential politics could focus on the development of online political satire. While the study focuses on one topic, the Internet’s impact on American presidential politics, it would still be organized chronologically reflecting technological developments in media. The difference in this example between a "chronological" and a "thematic" approach is what is emphasized the most: themes related to the role of the Internet in presidential politics. Note that more authentic thematic reviews tend to break away from chronological order. A review organized in this manner would shift between time periods within each section according to the point being made. Methodological A methodological approach focuses on the methods utilized by the researcher. For the Internet in American presidential politics project, one methodological approach would be to look at cultural differences between the portrayal of American presidents on American, British, and French websites. Or the review might focus on the fundraising impact of the Internet on a particular political party. A methodological scope will influence either the types of documents in the review or the way in which these documents are discussed.
Other Sections of Your Literature Review Once you've decided on the organizational method for your literature review, the sections you need to include in the paper should be easy to figure out because they arise from your organizational strategy. In other words, a chronological review would have subsections for each vital time period; a thematic review would have subtopics based upon factors that relate to the theme or issue. However, sometimes you may need to add additional sections that are necessary for your study, but do not fit in the organizational strategy of the body. What other sections you include in the body is up to you. However, only include what is necessary for the reader to locate your study within the larger scholarship about the research problem.
Here are examples of other sections, usually in the form of a single paragraph, you may need to include depending on the type of review you write:
- Current Situation : Information necessary to understand the current topic or focus of the literature review.
- Sources Used : Describes the methods and resources [e.g., databases] you used to identify the literature you reviewed.
- History : The chronological progression of the field, the research literature, or an idea that is necessary to understand the literature review, if the body of the literature review is not already a chronology.
- Selection Methods : Criteria you used to select (and perhaps exclude) sources in your literature review. For instance, you might explain that your review includes only peer-reviewed [i.e., scholarly] sources.
- Standards : Description of the way in which you present your information.
- Questions for Further Research : What questions about the field has the review sparked? How will you further your research as a result of the review?
IV. Writing Your Literature Review
Once you've settled on how to organize your literature review, you're ready to write each section. When writing your review, keep in mind these issues.
Use Evidence A literature review section is, in this sense, just like any other academic research paper. Your interpretation of the available sources must be backed up with evidence [citations] that demonstrates that what you are saying is valid. Be Selective Select only the most important points in each source to highlight in the review. The type of information you choose to mention should relate directly to the research problem, whether it is thematic, methodological, or chronological. Related items that provide additional information, but that are not key to understanding the research problem, can be included in a list of further readings . Use Quotes Sparingly Some short quotes are appropriate if you want to emphasize a point, or if what an author stated cannot be easily paraphrased. Sometimes you may need to quote certain terminology that was coined by the author, is not common knowledge, or taken directly from the study. Do not use extensive quotes as a substitute for using your own words in reviewing the literature. Summarize and Synthesize Remember to summarize and synthesize your sources within each thematic paragraph as well as throughout the review. Recapitulate important features of a research study, but then synthesize it by rephrasing the study's significance and relating it to your own work and the work of others. Keep Your Own Voice While the literature review presents others' ideas, your voice [the writer's] should remain front and center. For example, weave references to other sources into what you are writing but maintain your own voice by starting and ending the paragraph with your own ideas and wording. Use Caution When Paraphrasing When paraphrasing a source that is not your own, be sure to represent the author's information or opinions accurately and in your own words. Even when paraphrasing an author’s work, you still must provide a citation to that work.
V. Common Mistakes to Avoid
These are the most common mistakes made in reviewing social science research literature.
- Sources in your literature review do not clearly relate to the research problem;
- You do not take sufficient time to define and identify the most relevant sources to use in the literature review related to the research problem;
- Relies exclusively on secondary analytical sources rather than including relevant primary research studies or data;
- Uncritically accepts another researcher's findings and interpretations as valid, rather than examining critically all aspects of the research design and analysis;
- Does not describe the search procedures that were used in identifying the literature to review;
- Reports isolated statistical results rather than synthesizing them in chi-squared or meta-analytic methods; and,
- Only includes research that validates assumptions and does not consider contrary findings and alternative interpretations found in the literature.
Cook, Kathleen E. and Elise Murowchick. “Do Literature Review Skills Transfer from One Course to Another?” Psychology Learning and Teaching 13 (March 2014): 3-11; Fink, Arlene. Conducting Research Literature Reviews: From the Internet to Paper . 2nd ed. Thousand Oaks, CA: Sage, 2005; Hart, Chris. Doing a Literature Review: Releasing the Social Science Research Imagination . Thousand Oaks, CA: Sage Publications, 1998; Jesson, Jill. Doing Your Literature Review: Traditional and Systematic Techniques . London: SAGE, 2011; Literature Review Handout. Online Writing Center. Liberty University; Literature Reviews. The Writing Center. University of North Carolina; Onwuegbuzie, Anthony J. and Rebecca Frels. Seven Steps to a Comprehensive Literature Review: A Multimodal and Cultural Approach . Los Angeles, CA: SAGE, 2016; Ridley, Diana. The Literature Review: A Step-by-Step Guide for Students . 2nd ed. Los Angeles, CA: SAGE, 2012; Randolph, Justus J. “A Guide to Writing the Dissertation Literature Review." Practical Assessment, Research, and Evaluation. vol. 14, June 2009; Sutton, Anthea. Systematic Approaches to a Successful Literature Review . Los Angeles, CA: Sage Publications, 2016; Taylor, Dena. The Literature Review: A Few Tips On Conducting It. University College Writing Centre. University of Toronto; Writing a Literature Review. Academic Skills Centre. University of Canberra.
Writing Tip
Break Out of Your Disciplinary Box!
Thinking interdisciplinarily about a research problem can be a rewarding exercise in applying new ideas, theories, or concepts to an old problem. For example, what might cultural anthropologists say about the continuing conflict in the Middle East? In what ways might geographers view the need for better distribution of social service agencies in large cities than how social workers might study the issue? You don’t want to substitute a thorough review of core research literature in your discipline for studies conducted in other fields of study. However, particularly in the social sciences, thinking about research problems from multiple vectors is a key strategy for finding new solutions to a problem or gaining a new perspective. Consult with a librarian about identifying research databases in other disciplines; almost every field of study has at least one comprehensive database devoted to indexing its research literature.
Frodeman, Robert. The Oxford Handbook of Interdisciplinarity . New York: Oxford University Press, 2010.
Another Writing Tip
Don't Just Review for Content!
While conducting a review of the literature, maximize the time you devote to writing this part of your paper by thinking broadly about what you should be looking for and evaluating. Review not just what scholars are saying, but how are they saying it. Some questions to ask:
- How are they organizing their ideas?
- What methods have they used to study the problem?
- What theories have been used to explain, predict, or understand their research problem?
- What sources have they cited to support their conclusions?
- How have they used non-textual elements [e.g., charts, graphs, figures, etc.] to illustrate key points?
When you begin to write your literature review section, you'll be glad you dug deeper into how the research was designed and constructed because it establishes a means for developing more substantial analysis and interpretation of the research problem.
Hart, Chris. Doing a Literature Review: Releasing the Social Science Research Imagination . Thousand Oaks, CA: Sage Publications, 1 998.
Yet Another Writing Tip
When Do I Know I Can Stop Looking and Move On?
Here are several strategies you can utilize to assess whether you've thoroughly reviewed the literature:
- Look for repeating patterns in the research findings . If the same thing is being said, just by different people, then this likely demonstrates that the research problem has hit a conceptual dead end. At this point consider: Does your study extend current research? Does it forge a new path? Or, does is merely add more of the same thing being said?
- Look at sources the authors cite to in their work . If you begin to see the same researchers cited again and again, then this is often an indication that no new ideas have been generated to address the research problem.
- Search Google Scholar to identify who has subsequently cited leading scholars already identified in your literature review [see next sub-tab]. This is called citation tracking and there are a number of sources that can help you identify who has cited whom, particularly scholars from outside of your discipline. Here again, if the same authors are being cited again and again, this may indicate no new literature has been written on the topic.
Onwuegbuzie, Anthony J. and Rebecca Frels. Seven Steps to a Comprehensive Literature Review: A Multimodal and Cultural Approach . Los Angeles, CA: Sage, 2016; Sutton, Anthea. Systematic Approaches to a Successful Literature Review . Los Angeles, CA: Sage Publications, 2016.
- << Previous: Theoretical Framework
- Next: Citation Tracking >>
- Last Updated: Apr 16, 2024 10:20 AM
- URL: https://libguides.usc.edu/writingguide

Literature review sources
Sources for literature review can be divided into three categories as illustrated in table below. In your dissertation you will need to use all three categories of literature review sources:
Sources for literature review and examples
Generally, your literature review should integrate a wide range of sources such as:
- Books . Textbooks remain as the most important source to find models and theories related to the research area. Research the most respected authorities in your selected research area and find the latest editions of books authored by them. For example, in the area of marketing the most notable authors include Philip Kotler, Seth Godin, Malcolm Gladwell, Emanuel Rosen and others.
- Magazines . Industry-specific magazines are usually rich in scholarly articles and they can be effective source to learn about the latest trends and developments in the research area. Reading industry magazines can be the most enjoyable part of the literature review, assuming that your selected research area represents an area of your personal and professional interests, which should be the case anyways.
- Newspapers can be referred to as the main source of up-to-date news about the latest events related to the research area. However, the proportion of the use of newspapers in literature review is recommended to be less compared to alternative sources of secondary data such as books and magazines. This is due to the fact that newspaper articles mainly lack depth of analyses and discussions.
- Online articles . You can find online versions of all of the above sources. However, note that the levels of reliability of online articles can be highly compromised depending on the source due to the high levels of ease with which articles can be published online. Opinions offered in a wide range of online discussion blogs cannot be usually used in literature review. Similarly, dissertation assessors are not keen to appreciate references to a wide range of blogs, unless articles in these blogs are authored by respected authorities in the research area.
Your secondary data sources may comprise certain amount of grey literature as well. The term grey literature refers to type of literature produced by government, academics, business and industry in print and electronic formats, which is not controlled by commercial publishers. It is called ‘grey’ because the status of the information in grey literature is not certain. In other words, any publication that has not been peer reviewed for publication is grey literature.
The necessity to use grey literature arises when there is no enough peer reviewed publications are available for the subject of your study.

John Dudovskiy
- Privacy Policy
Buy Me a Coffee

Home » Literature Review – Types Writing Guide and Examples
Literature Review – Types Writing Guide and Examples
Table of Contents

Literature Review
Definition:
A literature review is a comprehensive and critical analysis of the existing literature on a particular topic or research question. It involves identifying, evaluating, and synthesizing relevant literature, including scholarly articles, books, and other sources, to provide a summary and critical assessment of what is known about the topic.
Types of Literature Review
Types of Literature Review are as follows:
- Narrative literature review : This type of review involves a comprehensive summary and critical analysis of the available literature on a particular topic or research question. It is often used as an introductory section of a research paper.
- Systematic literature review: This is a rigorous and structured review that follows a pre-defined protocol to identify, evaluate, and synthesize all relevant studies on a specific research question. It is often used in evidence-based practice and systematic reviews.
- Meta-analysis: This is a quantitative review that uses statistical methods to combine data from multiple studies to derive a summary effect size. It provides a more precise estimate of the overall effect than any individual study.
- Scoping review: This is a preliminary review that aims to map the existing literature on a broad topic area to identify research gaps and areas for further investigation.
- Critical literature review : This type of review evaluates the strengths and weaknesses of the existing literature on a particular topic or research question. It aims to provide a critical analysis of the literature and identify areas where further research is needed.
- Conceptual literature review: This review synthesizes and integrates theories and concepts from multiple sources to provide a new perspective on a particular topic. It aims to provide a theoretical framework for understanding a particular research question.
- Rapid literature review: This is a quick review that provides a snapshot of the current state of knowledge on a specific research question or topic. It is often used when time and resources are limited.
- Thematic literature review : This review identifies and analyzes common themes and patterns across a body of literature on a particular topic. It aims to provide a comprehensive overview of the literature and identify key themes and concepts.
- Realist literature review: This review is often used in social science research and aims to identify how and why certain interventions work in certain contexts. It takes into account the context and complexities of real-world situations.
- State-of-the-art literature review : This type of review provides an overview of the current state of knowledge in a particular field, highlighting the most recent and relevant research. It is often used in fields where knowledge is rapidly evolving, such as technology or medicine.
- Integrative literature review: This type of review synthesizes and integrates findings from multiple studies on a particular topic to identify patterns, themes, and gaps in the literature. It aims to provide a comprehensive understanding of the current state of knowledge on a particular topic.
- Umbrella literature review : This review is used to provide a broad overview of a large and diverse body of literature on a particular topic. It aims to identify common themes and patterns across different areas of research.
- Historical literature review: This type of review examines the historical development of research on a particular topic or research question. It aims to provide a historical context for understanding the current state of knowledge on a particular topic.
- Problem-oriented literature review : This review focuses on a specific problem or issue and examines the literature to identify potential solutions or interventions. It aims to provide practical recommendations for addressing a particular problem or issue.
- Mixed-methods literature review : This type of review combines quantitative and qualitative methods to synthesize and analyze the available literature on a particular topic. It aims to provide a more comprehensive understanding of the research question by combining different types of evidence.
Parts of Literature Review
Parts of a literature review are as follows:
Introduction
The introduction of a literature review typically provides background information on the research topic and why it is important. It outlines the objectives of the review, the research question or hypothesis, and the scope of the review.
Literature Search
This section outlines the search strategy and databases used to identify relevant literature. The search terms used, inclusion and exclusion criteria, and any limitations of the search are described.
Literature Analysis
The literature analysis is the main body of the literature review. This section summarizes and synthesizes the literature that is relevant to the research question or hypothesis. The review should be organized thematically, chronologically, or by methodology, depending on the research objectives.
Critical Evaluation
Critical evaluation involves assessing the quality and validity of the literature. This includes evaluating the reliability and validity of the studies reviewed, the methodology used, and the strength of the evidence.
The conclusion of the literature review should summarize the main findings, identify any gaps in the literature, and suggest areas for future research. It should also reiterate the importance of the research question or hypothesis and the contribution of the literature review to the overall research project.
The references list includes all the sources cited in the literature review, and follows a specific referencing style (e.g., APA, MLA, Harvard).
How to write Literature Review
Here are some steps to follow when writing a literature review:
- Define your research question or topic : Before starting your literature review, it is essential to define your research question or topic. This will help you identify relevant literature and determine the scope of your review.
- Conduct a comprehensive search: Use databases and search engines to find relevant literature. Look for peer-reviewed articles, books, and other academic sources that are relevant to your research question or topic.
- Evaluate the sources: Once you have found potential sources, evaluate them critically to determine their relevance, credibility, and quality. Look for recent publications, reputable authors, and reliable sources of data and evidence.
- Organize your sources: Group the sources by theme, method, or research question. This will help you identify similarities and differences among the literature, and provide a structure for your literature review.
- Analyze and synthesize the literature : Analyze each source in depth, identifying the key findings, methodologies, and conclusions. Then, synthesize the information from the sources, identifying patterns and themes in the literature.
- Write the literature review : Start with an introduction that provides an overview of the topic and the purpose of the literature review. Then, organize the literature according to your chosen structure, and analyze and synthesize the sources. Finally, provide a conclusion that summarizes the key findings of the literature review, identifies gaps in knowledge, and suggests areas for future research.
- Edit and proofread: Once you have written your literature review, edit and proofread it carefully to ensure that it is well-organized, clear, and concise.
Examples of Literature Review
Here’s an example of how a literature review can be conducted for a thesis on the topic of “ The Impact of Social Media on Teenagers’ Mental Health”:
- Start by identifying the key terms related to your research topic. In this case, the key terms are “social media,” “teenagers,” and “mental health.”
- Use academic databases like Google Scholar, JSTOR, or PubMed to search for relevant articles, books, and other publications. Use these keywords in your search to narrow down your results.
- Evaluate the sources you find to determine if they are relevant to your research question. You may want to consider the publication date, author’s credentials, and the journal or book publisher.
- Begin reading and taking notes on each source, paying attention to key findings, methodologies used, and any gaps in the research.
- Organize your findings into themes or categories. For example, you might categorize your sources into those that examine the impact of social media on self-esteem, those that explore the effects of cyberbullying, and those that investigate the relationship between social media use and depression.
- Synthesize your findings by summarizing the key themes and highlighting any gaps or inconsistencies in the research. Identify areas where further research is needed.
- Use your literature review to inform your research questions and hypotheses for your thesis.
For example, after conducting a literature review on the impact of social media on teenagers’ mental health, a thesis might look like this:
“Using a mixed-methods approach, this study aims to investigate the relationship between social media use and mental health outcomes in teenagers. Specifically, the study will examine the effects of cyberbullying, social comparison, and excessive social media use on self-esteem, anxiety, and depression. Through an analysis of survey data and qualitative interviews with teenagers, the study will provide insight into the complex relationship between social media use and mental health outcomes, and identify strategies for promoting positive mental health outcomes in young people.”
Reference: Smith, J., Jones, M., & Lee, S. (2019). The effects of social media use on adolescent mental health: A systematic review. Journal of Adolescent Health, 65(2), 154-165. doi:10.1016/j.jadohealth.2019.03.024
Reference Example: Author, A. A., Author, B. B., & Author, C. C. (Year). Title of article. Title of Journal, volume number(issue number), page range. doi:0000000/000000000000 or URL
Applications of Literature Review
some applications of literature review in different fields:
- Social Sciences: In social sciences, literature reviews are used to identify gaps in existing research, to develop research questions, and to provide a theoretical framework for research. Literature reviews are commonly used in fields such as sociology, psychology, anthropology, and political science.
- Natural Sciences: In natural sciences, literature reviews are used to summarize and evaluate the current state of knowledge in a particular field or subfield. Literature reviews can help researchers identify areas where more research is needed and provide insights into the latest developments in a particular field. Fields such as biology, chemistry, and physics commonly use literature reviews.
- Health Sciences: In health sciences, literature reviews are used to evaluate the effectiveness of treatments, identify best practices, and determine areas where more research is needed. Literature reviews are commonly used in fields such as medicine, nursing, and public health.
- Humanities: In humanities, literature reviews are used to identify gaps in existing knowledge, develop new interpretations of texts or cultural artifacts, and provide a theoretical framework for research. Literature reviews are commonly used in fields such as history, literary studies, and philosophy.
Role of Literature Review in Research
Here are some applications of literature review in research:
- Identifying Research Gaps : Literature review helps researchers identify gaps in existing research and literature related to their research question. This allows them to develop new research questions and hypotheses to fill those gaps.
- Developing Theoretical Framework: Literature review helps researchers develop a theoretical framework for their research. By analyzing and synthesizing existing literature, researchers can identify the key concepts, theories, and models that are relevant to their research.
- Selecting Research Methods : Literature review helps researchers select appropriate research methods and techniques based on previous research. It also helps researchers to identify potential biases or limitations of certain methods and techniques.
- Data Collection and Analysis: Literature review helps researchers in data collection and analysis by providing a foundation for the development of data collection instruments and methods. It also helps researchers to identify relevant data sources and identify potential data analysis techniques.
- Communicating Results: Literature review helps researchers to communicate their results effectively by providing a context for their research. It also helps to justify the significance of their findings in relation to existing research and literature.
Purpose of Literature Review
Some of the specific purposes of a literature review are as follows:
- To provide context: A literature review helps to provide context for your research by situating it within the broader body of literature on the topic.
- To identify gaps and inconsistencies: A literature review helps to identify areas where further research is needed or where there are inconsistencies in the existing literature.
- To synthesize information: A literature review helps to synthesize the information from multiple sources and present a coherent and comprehensive picture of the current state of knowledge on the topic.
- To identify key concepts and theories : A literature review helps to identify key concepts and theories that are relevant to your research question and provide a theoretical framework for your study.
- To inform research design: A literature review can inform the design of your research study by identifying appropriate research methods, data sources, and research questions.
Characteristics of Literature Review
Some Characteristics of Literature Review are as follows:
- Identifying gaps in knowledge: A literature review helps to identify gaps in the existing knowledge and research on a specific topic or research question. By analyzing and synthesizing the literature, you can identify areas where further research is needed and where new insights can be gained.
- Establishing the significance of your research: A literature review helps to establish the significance of your own research by placing it in the context of existing research. By demonstrating the relevance of your research to the existing literature, you can establish its importance and value.
- Informing research design and methodology : A literature review helps to inform research design and methodology by identifying the most appropriate research methods, techniques, and instruments. By reviewing the literature, you can identify the strengths and limitations of different research methods and techniques, and select the most appropriate ones for your own research.
- Supporting arguments and claims: A literature review provides evidence to support arguments and claims made in academic writing. By citing and analyzing the literature, you can provide a solid foundation for your own arguments and claims.
- I dentifying potential collaborators and mentors: A literature review can help identify potential collaborators and mentors by identifying researchers and practitioners who are working on related topics or using similar methods. By building relationships with these individuals, you can gain valuable insights and support for your own research and practice.
- Keeping up-to-date with the latest research : A literature review helps to keep you up-to-date with the latest research on a specific topic or research question. By regularly reviewing the literature, you can stay informed about the latest findings and developments in your field.
Advantages of Literature Review
There are several advantages to conducting a literature review as part of a research project, including:
- Establishing the significance of the research : A literature review helps to establish the significance of the research by demonstrating the gap or problem in the existing literature that the study aims to address.
- Identifying key concepts and theories: A literature review can help to identify key concepts and theories that are relevant to the research question, and provide a theoretical framework for the study.
- Supporting the research methodology : A literature review can inform the research methodology by identifying appropriate research methods, data sources, and research questions.
- Providing a comprehensive overview of the literature : A literature review provides a comprehensive overview of the current state of knowledge on a topic, allowing the researcher to identify key themes, debates, and areas of agreement or disagreement.
- Identifying potential research questions: A literature review can help to identify potential research questions and areas for further investigation.
- Avoiding duplication of research: A literature review can help to avoid duplication of research by identifying what has already been done on a topic, and what remains to be done.
- Enhancing the credibility of the research : A literature review helps to enhance the credibility of the research by demonstrating the researcher’s knowledge of the existing literature and their ability to situate their research within a broader context.
Limitations of Literature Review
Limitations of Literature Review are as follows:
- Limited scope : Literature reviews can only cover the existing literature on a particular topic, which may be limited in scope or depth.
- Publication bias : Literature reviews may be influenced by publication bias, which occurs when researchers are more likely to publish positive results than negative ones. This can lead to an incomplete or biased picture of the literature.
- Quality of sources : The quality of the literature reviewed can vary widely, and not all sources may be reliable or valid.
- Time-limited: Literature reviews can become quickly outdated as new research is published, making it difficult to keep up with the latest developments in a field.
- Subjective interpretation : Literature reviews can be subjective, and the interpretation of the findings can vary depending on the researcher’s perspective or bias.
- Lack of original data : Literature reviews do not generate new data, but rather rely on the analysis of existing studies.
- Risk of plagiarism: It is important to ensure that literature reviews do not inadvertently contain plagiarism, which can occur when researchers use the work of others without proper attribution.
About the author
Muhammad Hassan
Researcher, Academic Writer, Web developer
You may also like

Data Collection – Methods Types and Examples

Delimitations in Research – Types, Examples and...

Research Process – Steps, Examples and Tips

Research Design – Types, Methods and Examples

Institutional Review Board – Application Sample...

Evaluating Research – Process, Examples and...
How To Structure Your Literature Review
3 options to help structure your chapter.
By: Amy Rommelspacher (PhD) | Reviewer: Dr Eunice Rautenbach | November 2020 (Updated May 2023)
Writing the literature review chapter can seem pretty daunting when you’re piecing together your dissertation or thesis. As we’ve discussed before , a good literature review needs to achieve a few very important objectives – it should:
- Demonstrate your knowledge of the research topic
- Identify the gaps in the literature and show how your research links to these
- Provide the foundation for your conceptual framework (if you have one)
- Inform your own methodology and research design
To achieve this, your literature review needs a well-thought-out structure . Get the structure of your literature review chapter wrong and you’ll struggle to achieve these objectives. Don’t worry though – in this post, we’ll look at how to structure your literature review for maximum impact (and marks!).

But wait – is this the right time?
Deciding on the structure of your literature review should come towards the end of the literature review process – after you have collected and digested the literature, but before you start writing the chapter.
In other words, you need to first develop a rich understanding of the literature before you even attempt to map out a structure. There’s no use trying to develop a structure before you’ve fully wrapped your head around the existing research.
Equally importantly, you need to have a structure in place before you start writing , or your literature review will most likely end up a rambling, disjointed mess.
Importantly, don’t feel that once you’ve defined a structure you can’t iterate on it. It’s perfectly natural to adjust as you engage in the writing process. As we’ve discussed before , writing is a way of developing your thinking, so it’s quite common for your thinking to change – and therefore, for your chapter structure to change – as you write.
Need a helping hand?
Like any other chapter in your thesis or dissertation, your literature review needs to have a clear, logical structure. At a minimum, it should have three essential components – an introduction , a body and a conclusion .
Let’s take a closer look at each of these.
1: The Introduction Section
Just like any good introduction, the introduction section of your literature review should introduce the purpose and layout (organisation) of the chapter. In other words, your introduction needs to give the reader a taste of what’s to come, and how you’re going to lay that out. Essentially, you should provide the reader with a high-level roadmap of your chapter to give them a taste of the journey that lies ahead.
Here’s an example of the layout visualised in a literature review introduction:
Your introduction should also outline your topic (including any tricky terminology or jargon) and provide an explanation of the scope of your literature review – in other words, what you will and won’t be covering (the delimitations ). This helps ringfence your review and achieve a clear focus . The clearer and narrower your focus, the deeper you can dive into the topic (which is typically where the magic lies).
Depending on the nature of your project, you could also present your stance or point of view at this stage. In other words, after grappling with the literature you’ll have an opinion about what the trends and concerns are in the field as well as what’s lacking. The introduction section can then present these ideas so that it is clear to examiners that you’re aware of how your research connects with existing knowledge .

2: The Body Section
The body of your literature review is the centre of your work. This is where you’ll present, analyse, evaluate and synthesise the existing research. In other words, this is where you’re going to earn (or lose) the most marks. Therefore, it’s important to carefully think about how you will organise your discussion to present it in a clear way.
The body of your literature review should do just as the description of this chapter suggests. It should “review” the literature – in other words, identify, analyse, and synthesise it. So, when thinking about structuring your literature review, you need to think about which structural approach will provide the best “review” for your specific type of research and objectives (we’ll get to this shortly).
There are (broadly speaking) three options for organising your literature review.

Option 1: Chronological (according to date)
Organising the literature chronologically is one of the simplest ways to structure your literature review. You start with what was published first and work your way through the literature until you reach the work published most recently. Pretty straightforward.
The benefit of this option is that it makes it easy to discuss the developments and debates in the field as they emerged over time. Organising your literature chronologically also allows you to highlight how specific articles or pieces of work might have changed the course of the field – in other words, which research has had the most impact . Therefore, this approach is very useful when your research is aimed at understanding how the topic has unfolded over time and is often used by scholars in the field of history. That said, this approach can be utilised by anyone that wants to explore change over time .

For example , if a student of politics is investigating how the understanding of democracy has evolved over time, they could use the chronological approach to provide a narrative that demonstrates how this understanding has changed through the ages.
Here are some questions you can ask yourself to help you structure your literature review chronologically.
- What is the earliest literature published relating to this topic?
- How has the field changed over time? Why?
- What are the most recent discoveries/theories?
In some ways, chronology plays a part whichever way you decide to structure your literature review, because you will always, to a certain extent, be analysing how the literature has developed. However, with the chronological approach, the emphasis is very firmly on how the discussion has evolved over time , as opposed to how all the literature links together (which we’ll discuss next ).
Option 2: Thematic (grouped by theme)
The thematic approach to structuring a literature review means organising your literature by theme or category – for example, by independent variables (i.e. factors that have an impact on a specific outcome).
As you’ve been collecting and synthesising literature , you’ll likely have started seeing some themes or patterns emerging. You can then use these themes or patterns as a structure for your body discussion. The thematic approach is the most common approach and is useful for structuring literature reviews in most fields.
For example, if you were researching which factors contributed towards people trusting an organisation, you might find themes such as consumers’ perceptions of an organisation’s competence, benevolence and integrity. Structuring your literature review thematically would mean structuring your literature review’s body section to discuss each of these themes, one section at a time.

Here are some questions to ask yourself when structuring your literature review by themes:
- Are there any patterns that have come to light in the literature?
- What are the central themes and categories used by the researchers?
- Do I have enough evidence of these themes?
PS – you can see an example of a thematically structured literature review in our literature review sample walkthrough video here.
Option 3: Methodological
The methodological option is a way of structuring your literature review by the research methodologies used . In other words, organising your discussion based on the angle from which each piece of research was approached – for example, qualitative , quantitative or mixed methodologies.
Structuring your literature review by methodology can be useful if you are drawing research from a variety of disciplines and are critiquing different methodologies. The point of this approach is to question how existing research has been conducted, as opposed to what the conclusions and/or findings the research were.

For example, a sociologist might centre their research around critiquing specific fieldwork practices. Their literature review will then be a summary of the fieldwork methodologies used by different studies.
Here are some questions you can ask yourself when structuring your literature review according to methodology:
- Which methodologies have been utilised in this field?
- Which methodology is the most popular (and why)?
- What are the strengths and weaknesses of the various methodologies?
- How can the existing methodologies inform my own methodology?
3: The Conclusion Section
Once you’ve completed the body section of your literature review using one of the structural approaches we discussed above, you’ll need to “wrap up” your literature review and pull all the pieces together to set the direction for the rest of your dissertation or thesis.
The conclusion is where you’ll present the key findings of your literature review. In this section, you should emphasise the research that is especially important to your research questions and highlight the gaps that exist in the literature. Based on this, you need to make it clear what you will add to the literature – in other words, justify your own research by showing how it will help fill one or more of the gaps you just identified.
Last but not least, if it’s your intention to develop a conceptual framework for your dissertation or thesis, the conclusion section is a good place to present this.

Example: Thematically Structured Review
In the video below, we unpack a literature review chapter so that you can see an example of a thematically structure review in practice.
Let’s Recap
In this article, we’ve discussed how to structure your literature review for maximum impact. Here’s a quick recap of what you need to keep in mind when deciding on your literature review structure:
- Just like other chapters, your literature review needs a clear introduction , body and conclusion .
- The introduction section should provide an overview of what you will discuss in your literature review.
- The body section of your literature review can be organised by chronology , theme or methodology . The right structural approach depends on what you’re trying to achieve with your research.
- The conclusion section should draw together the key findings of your literature review and link them to your research questions.
If you’re ready to get started, be sure to download our free literature review template to fast-track your chapter outline.

Psst… there’s more!
This post is an extract from our bestselling Udemy Course, Literature Review Bootcamp . If you want to work smart, you don't want to miss this .
You Might Also Like:

27 Comments
Great work. This is exactly what I was looking for and helps a lot together with your previous post on literature review. One last thing is missing: a link to a great literature chapter of an journal article (maybe with comments of the different sections in this review chapter). Do you know any great literature review chapters?
I agree with you Marin… A great piece
I agree with Marin. This would be quite helpful if you annotate a nicely structured literature from previously published research articles.
Awesome article for my research.
I thank you immensely for this wonderful guide
It is indeed thought and supportive work for the futurist researcher and students
Very educative and good time to get guide. Thank you
Great work, very insightful. Thank you.
Thanks for this wonderful presentation. My question is that do I put all the variables into a single conceptual framework or each hypothesis will have it own conceptual framework?
Thank you very much, very helpful
This is very educative and precise . Thank you very much for dropping this kind of write up .
Pheeww, so damn helpful, thank you for this informative piece.
I’m doing a research project topic ; stool analysis for parasitic worm (enteric) worm, how do I structure it, thanks.
comprehensive explanation. Help us by pasting the URL of some good “literature review” for better understanding.
great piece. thanks for the awesome explanation. it is really worth sharing. I have a little question, if anyone can help me out, which of the options in the body of literature can be best fit if you are writing an architectural thesis that deals with design?
I am doing a research on nanofluids how can l structure it?
Beautifully clear.nThank you!
Lucid! Thankyou!
Brilliant work, well understood, many thanks
I like how this was so clear with simple language 😊😊 thank you so much 😊 for these information 😊
Insightful. I was struggling to come up with a sensible literature review but this has been really helpful. Thank you!
You have given thought-provoking information about the review of the literature.
Thank you. It has made my own research better and to impart your work to students I teach
I learnt a lot from this teaching. It’s a great piece.
I am doing research on EFL teacher motivation for his/her job. How Can I structure it? Is there any detailed template, additional to this?
You are so cool! I do not think I’ve read through something like this before. So nice to find somebody with some genuine thoughts on this issue. Seriously.. thank you for starting this up. This site is one thing that is required on the internet, someone with a little originality!
I’m asked to do conceptual, theoretical and empirical literature, and i just don’t know how to structure it
Submit a Comment Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
- Print Friendly

Research Process :: Step by Step
- Introduction
- Select Topic
- Identify Keywords
- Background Information
- Develop Research Questions
- Refine Topic
- Search Strategy
- Popular Databases
- Evaluate Sources
- Types of Periodicals
- Reading Scholarly Articles
- Primary & Secondary Sources
- Organize / Take Notes
- Writing & Grammar Resources
- Annotated Bibliography
- Literature Review
- Citation Styles
- Paraphrasing
- Privacy / Confidentiality
- Research Process
- Selecting Your Topic
- Identifying Keywords
- Gathering Background Info
- Evaluating Sources

Organize the literature review into sections that present themes or identify trends, including relevant theory. You are not trying to list all the material published, but to synthesize and evaluate it according to the guiding concept of your thesis or research question.
What is a literature review?
A literature review is an account of what has been published on a topic by accredited scholars and researchers. Occasionally you will be asked to write one as a separate assignment, but more often it is part of the introduction to an essay, research report, or thesis. In writing the literature review, your purpose is to convey to your reader what knowledge and ideas have been established on a topic, and what their strengths and weaknesses are. As a piece of writing, the literature review must be defined by a guiding concept (e.g., your research objective, the problem or issue you are discussing, or your argumentative thesis). It is not just a descriptive list of the material available, or a set of summaries
A literature review must do these things:
- be organized around and related directly to the thesis or research question you are developing
- synthesize results into a summary of what is and is not known
- identify areas of controversy in the literature
- formulate questions that need further research
Ask yourself questions like these:
- What is the specific thesis, problem, or research question that my literature review helps to define?
- What type of literature review am I conducting? Am I looking at issues of theory? methodology? policy? quantitative research (e.g. on the effectiveness of a new procedure)? qualitative research (e.g., studies of loneliness among migrant workers)?
- What is the scope of my literature review? What types of publications am I using (e.g., journals, books, government documents, popular media)? What discipline am I working in (e.g., nursing psychology, sociology, medicine)?
- How good was my information seeking? Has my search been wide enough to ensure I've found all the relevant material? Has it been narrow enough to exclude irrelevant material? Is the number of sources I've used appropriate for the length of my paper?
- Have I critically analyzed the literature I use? Do I follow through a set of concepts and questions, comparing items to each other in the ways they deal with them? Instead of just listing and summarizing items, do I assess them, discussing strengths and weaknesses?
- Have I cited and discussed studies contrary to my perspective?
- Will the reader find my literature review relevant, appropriate, and useful?
Ask yourself questions like these about each book or article you include:
- Has the author formulated a problem/issue?
- Is it clearly defined? Is its significance (scope, severity, relevance) clearly established?
- Could the problem have been approached more effectively from another perspective?
- What is the author's research orientation (e.g., interpretive, critical science, combination)?
- What is the author's theoretical framework (e.g., psychological, developmental, feminist)?
- What is the relationship between the theoretical and research perspectives?
- Has the author evaluated the literature relevant to the problem/issue? Does the author include literature taking positions she or he does not agree with?
- In a research study, how good are the basic components of the study design (e.g., population, intervention, outcome)? How accurate and valid are the measurements? Is the analysis of the data accurate and relevant to the research question? Are the conclusions validly based upon the data and analysis?
- In material written for a popular readership, does the author use appeals to emotion, one-sided examples, or rhetorically-charged language and tone? Is there an objective basis to the reasoning, or is the author merely "proving" what he or she already believes?
- How does the author structure the argument? Can you "deconstruct" the flow of the argument to see whether or where it breaks down logically (e.g., in establishing cause-effect relationships)?
- In what ways does this book or article contribute to our understanding of the problem under study, and in what ways is it useful for practice? What are the strengths and limitations?
- How does this book or article relate to the specific thesis or question I am developing?
Text written by Dena Taylor, Health Sciences Writing Centre, University of Toronto
http://www.writing.utoronto.ca/advice/specific-types-of-writing/literature-review
- << Previous: Annotated Bibliography
- Next: Step 5: Cite Sources >>
- Last Updated: Apr 16, 2024 4:42 PM
- URL: https://libguides.uta.edu/researchprocess
University of Texas Arlington Libraries 702 Planetarium Place · Arlington, TX 76019 · 817-272-3000
- Internet Privacy
- Accessibility
- Problems with a guide? Contact Us.
Research Methods
- Getting Started
- Literature Review Research
- Research Design
- Research Design By Discipline
- SAGE Research Methods
- Teaching with SAGE Research Methods
Literature Review
- What is a Literature Review?
- What is NOT a Literature Review?
- Purposes of a Literature Review
- Types of Literature Reviews
- Literature Reviews vs. Systematic Reviews
- Systematic vs. Meta-Analysis
Literature Review is a comprehensive survey of the works published in a particular field of study or line of research, usually over a specific period of time, in the form of an in-depth, critical bibliographic essay or annotated list in which attention is drawn to the most significant works.
Also, we can define a literature review as the collected body of scholarly works related to a topic:
- Summarizes and analyzes previous research relevant to a topic
- Includes scholarly books and articles published in academic journals
- Can be an specific scholarly paper or a section in a research paper
The objective of a Literature Review is to find previous published scholarly works relevant to an specific topic
- Help gather ideas or information
- Keep up to date in current trends and findings
- Help develop new questions
A literature review is important because it:
- Explains the background of research on a topic.
- Demonstrates why a topic is significant to a subject area.
- Helps focus your own research questions or problems
- Discovers relationships between research studies/ideas.
- Suggests unexplored ideas or populations
- Identifies major themes, concepts, and researchers on a topic.
- Tests assumptions; may help counter preconceived ideas and remove unconscious bias.
- Identifies critical gaps, points of disagreement, or potentially flawed methodology or theoretical approaches.
- Indicates potential directions for future research.
All content in this section is from Literature Review Research from Old Dominion University
Keep in mind the following, a literature review is NOT:
Not an essay
Not an annotated bibliography in which you summarize each article that you have reviewed. A literature review goes beyond basic summarizing to focus on the critical analysis of the reviewed works and their relationship to your research question.
Not a research paper where you select resources to support one side of an issue versus another. A lit review should explain and consider all sides of an argument in order to avoid bias, and areas of agreement and disagreement should be highlighted.
A literature review serves several purposes. For example, it
- provides thorough knowledge of previous studies; introduces seminal works.
- helps focus one’s own research topic.
- identifies a conceptual framework for one’s own research questions or problems; indicates potential directions for future research.
- suggests previously unused or underused methodologies, designs, quantitative and qualitative strategies.
- identifies gaps in previous studies; identifies flawed methodologies and/or theoretical approaches; avoids replication of mistakes.
- helps the researcher avoid repetition of earlier research.
- suggests unexplored populations.
- determines whether past studies agree or disagree; identifies controversy in the literature.
- tests assumptions; may help counter preconceived ideas and remove unconscious bias.
As Kennedy (2007) notes*, it is important to think of knowledge in a given field as consisting of three layers. First, there are the primary studies that researchers conduct and publish. Second are the reviews of those studies that summarize and offer new interpretations built from and often extending beyond the original studies. Third, there are the perceptions, conclusions, opinion, and interpretations that are shared informally that become part of the lore of field. In composing a literature review, it is important to note that it is often this third layer of knowledge that is cited as "true" even though it often has only a loose relationship to the primary studies and secondary literature reviews.
Given this, while literature reviews are designed to provide an overview and synthesis of pertinent sources you have explored, there are several approaches to how they can be done, depending upon the type of analysis underpinning your study. Listed below are definitions of types of literature reviews:
Argumentative Review This form examines literature selectively in order to support or refute an argument, deeply imbedded assumption, or philosophical problem already established in the literature. The purpose is to develop a body of literature that establishes a contrarian viewpoint. Given the value-laden nature of some social science research [e.g., educational reform; immigration control], argumentative approaches to analyzing the literature can be a legitimate and important form of discourse. However, note that they can also introduce problems of bias when they are used to to make summary claims of the sort found in systematic reviews.
Integrative Review Considered a form of research that reviews, critiques, and synthesizes representative literature on a topic in an integrated way such that new frameworks and perspectives on the topic are generated. The body of literature includes all studies that address related or identical hypotheses. A well-done integrative review meets the same standards as primary research in regard to clarity, rigor, and replication.
Historical Review Few things rest in isolation from historical precedent. Historical reviews are focused on examining research throughout a period of time, often starting with the first time an issue, concept, theory, phenomena emerged in the literature, then tracing its evolution within the scholarship of a discipline. The purpose is to place research in a historical context to show familiarity with state-of-the-art developments and to identify the likely directions for future research.
Methodological Review A review does not always focus on what someone said [content], but how they said it [method of analysis]. This approach provides a framework of understanding at different levels (i.e. those of theory, substantive fields, research approaches and data collection and analysis techniques), enables researchers to draw on a wide variety of knowledge ranging from the conceptual level to practical documents for use in fieldwork in the areas of ontological and epistemological consideration, quantitative and qualitative integration, sampling, interviewing, data collection and data analysis, and helps highlight many ethical issues which we should be aware of and consider as we go through our study.
Systematic Review This form consists of an overview of existing evidence pertinent to a clearly formulated research question, which uses pre-specified and standardized methods to identify and critically appraise relevant research, and to collect, report, and analyse data from the studies that are included in the review. Typically it focuses on a very specific empirical question, often posed in a cause-and-effect form, such as "To what extent does A contribute to B?"
Theoretical Review The purpose of this form is to concretely examine the corpus of theory that has accumulated in regard to an issue, concept, theory, phenomena. The theoretical literature review help establish what theories already exist, the relationships between them, to what degree the existing theories have been investigated, and to develop new hypotheses to be tested. Often this form is used to help establish a lack of appropriate theories or reveal that current theories are inadequate for explaining new or emerging research problems. The unit of analysis can focus on a theoretical concept or a whole theory or framework.
* Kennedy, Mary M. "Defining a Literature." Educational Researcher 36 (April 2007): 139-147.
All content in this section is from The Literature Review created by Dr. Robert Larabee USC
Robinson, P. and Lowe, J. (2015), Literature reviews vs systematic reviews. Australian and New Zealand Journal of Public Health, 39: 103-103. doi: 10.1111/1753-6405.12393

What's in the name? The difference between a Systematic Review and a Literature Review, and why it matters . By Lynn Kysh from University of Southern California

Systematic review or meta-analysis?
A systematic review answers a defined research question by collecting and summarizing all empirical evidence that fits pre-specified eligibility criteria.
A meta-analysis is the use of statistical methods to summarize the results of these studies.
Systematic reviews, just like other research articles, can be of varying quality. They are a significant piece of work (the Centre for Reviews and Dissemination at York estimates that a team will take 9-24 months), and to be useful to other researchers and practitioners they should have:
- clearly stated objectives with pre-defined eligibility criteria for studies
- explicit, reproducible methodology
- a systematic search that attempts to identify all studies
- assessment of the validity of the findings of the included studies (e.g. risk of bias)
- systematic presentation, and synthesis, of the characteristics and findings of the included studies
Not all systematic reviews contain meta-analysis.
Meta-analysis is the use of statistical methods to summarize the results of independent studies. By combining information from all relevant studies, meta-analysis can provide more precise estimates of the effects of health care than those derived from the individual studies included within a review. More information on meta-analyses can be found in Cochrane Handbook, Chapter 9 .
A meta-analysis goes beyond critique and integration and conducts secondary statistical analysis on the outcomes of similar studies. It is a systematic review that uses quantitative methods to synthesize and summarize the results.
An advantage of a meta-analysis is the ability to be completely objective in evaluating research findings. Not all topics, however, have sufficient research evidence to allow a meta-analysis to be conducted. In that case, an integrative review is an appropriate strategy.
Some of the content in this section is from Systematic reviews and meta-analyses: step by step guide created by Kate McAllister.
- << Previous: Getting Started
- Next: Research Design >>
- Last Updated: Aug 21, 2023 4:07 PM
- URL: https://guides.lib.udel.edu/researchmethods
- UConn Library
- Literature Review: The What, Why and How-to Guide
- Strategies to Find Sources
Literature Review: The What, Why and How-to Guide — Strategies to Find Sources
- Getting Started
- Introduction
- How to Pick a Topic
- Evaluating Sources & Lit. Reviews
- Tips for Writing Literature Reviews
- Writing Literature Review: Useful Sites
- Citation Resources
- Other Academic Writings
The Research Process
![highlight four sources of literature review in research Interative Litearture Review Research Process image (Planning, Searching, Organizing, Analyzing and Writing [repeat at necessary]](https://libapps.s3.amazonaws.com/accounts/64450/images/ResearchProcess.jpg)
Planning : Before searching for articles or books, brainstorm to develop keywords that better describe your research question.
Searching : While searching, take note of what other keywords are used to describe your topic, and use them to conduct additional searches
♠ Most articles include a keyword section
♠ Key concepts may change names throughout time so make sure to check for variations
Organizing : Start organizing your results by categories/key concepts or any organizing principle that make sense for you . This will help you later when you are ready to analyze your findings
Analyzing : While reading, start making notes of key concepts and commonalities and disagreement among the research articles you find.
♠ Create a spreadsheet to record what articles you are finding useful and why.
♠ Create fields to write summaries of articles or quotes for future citing and paraphrasing .
Writing : Synthesize your findings. Use your own voice to explain to your readers what you learned about the literature on your topic. What are its weaknesses and strengths? What is missing or ignored?
Repeat : At any given time of the process, you can go back to a previous step as necessary.
Advanced Searching
All databases have Help pages that explain the best way to search their product. When doing literature reviews, you will want to take advantage of these features since they can facilitate not only finding the articles that you really need but also controlling the number of results and how relevant they are for your search. The most common features available in the advanced search option of databases and library online catalogs are:
- Boolean Searching (AND, OR, NOT): Allows you to connect search terms in a way that can either limit or expand your search results
- Proximity Searching (N/# or W/#): Allows you to search for two or more words that occur within a specified number of words (or fewer) of each other in the database
- Limiters/Filters : These are options that let you control what type of document you want to search: article type, date, language, publication, etc.
- Question mark (?) or a pound sign (#) for wildcard: Used for retrieving alternate spellings of a word: colo?r will retrieve both the American spelling "color" as well as the British spelling "colour."
- Asterisk (*) for truncation: Used for retrieving multiple forms of a word: comput* retrieves computer, computers, computing, etc.
Want to keep track of updates to your searches? Create an account in the database to receive an alert when a new article is published that meets your search parameters!
- EBSCOhost Advanced Search Tutorial Tips for searching a platform that hosts many library databases
- Library's General Search Tips Check the Search tips to better used our library catalog and articles search system
- ProQuest Database Search Tips Tips for searching another platform that hosts library databases
There is no magic number regarding how many sources you are going to need for your literature review; it all depends on the topic and what type of the literature review you are doing:
► Are you working on an emerging topic? You are not likely to find many sources, which is good because you are trying to prove that this is a topic that needs more research. But, it is not enough to say that you found few or no articles on your topic in your field. You need to look broadly to other disciplines (also known as triangulation ) to see if your research topic has been studied from other perspectives as a way to validate the uniqueness of your research question.
► Are you working on something that has been studied extensively? Then you are going to find many sources and you will want to limit how far back you want to look. Use limiters to eliminate research that may be dated and opt to search for resources published within the last 5-10 years.
- << Previous: How to Pick a Topic
- Next: Evaluating Sources & Lit. Reviews >>
- Last Updated: Sep 21, 2022 2:16 PM
- URL: https://guides.lib.uconn.edu/literaturereview

Want to create or adapt books like this? Learn more about how Pressbooks supports open publishing practices.
The Research Proposal
83 Components of the Literature Review
Krathwohl (2005) suggests and describes a variety of components to include in a research proposal. The following sections present these components in a suggested template for you to follow in the preparation of your research proposal.
Introduction
The introduction sets the tone for what follows in your research proposal – treat it as the initial pitch of your idea. After reading the introduction your reader should:
- Understand what it is you want to do;
- Have a sense of your passion for the topic;
- Be excited about the study´s possible outcomes.
As you begin writing your research proposal it is helpful to think of the introduction as a narrative of what it is you want to do, written in one to three paragraphs. Within those one to three paragraphs, it is important to briefly answer the following questions:
- What is the central research problem?
- How is the topic of your research proposal related to the problem?
- What methods will you utilize to analyze the research problem?
- Why is it important to undertake this research? What is the significance of your proposed research? Why are the outcomes of your proposed research important, and to whom or to what are they important?
Note : You may be asked by your instructor to include an abstract with your research proposal. In such cases, an abstract should provide an overview of what it is you plan to study, your main research question, a brief explanation of your methods to answer the research question, and your expected findings. All of this information must be carefully crafted in 150 to 250 words. A word of advice is to save the writing of your abstract until the very end of your research proposal preparation. If you are asked to provide an abstract, you should include 5-7 key words that are of most relevance to your study. List these in order of relevance.
Background and significance
The purpose of this section is to explain the context of your proposal and to describe, in detail, why it is important to undertake this research. Assume that the person or people who will read your research proposal know nothing or very little about the research problem. While you do not need to include all knowledge you have learned about your topic in this section, it is important to ensure that you include the most relevant material that will help to explain the goals of your research.
While there are no hard and fast rules, you should attempt to address some or all of the following key points:
- State the research problem and provide a more thorough explanation about the purpose of the study than what you stated in the introduction.
- Present the rationale for the proposed research study. Clearly indicate why this research is worth doing. Answer the “so what?” question.
- Describe the major issues or problems to be addressed by your research. Do not forget to explain how and in what ways your proposed research builds upon previous related research.
- Explain how you plan to go about conducting your research.
- Clearly identify the key or most relevant sources of research you intend to use and explain how they will contribute to your analysis of the topic.
- Set the boundaries of your proposed research, in order to provide a clear focus. Where appropriate, state not only what you will study, but what will be excluded from your study.
- Provide clear definitions of key concepts and terms. As key concepts and terms often have numerous definitions, make sure you state which definition you will be utilizing in your research.
Literature Review
This is the most time-consuming aspect in the preparation of your research proposal and it is a key component of the research proposal. As described in Chapter 5 , the literature review provides the background to your study and demonstrates the significance of the proposed research. Specifically, it is a review and synthesis of prior research that is related to the problem you are setting forth to investigate. Essentially, your goal in the literature review is to place your research study within the larger whole of what has been studied in the past, while demonstrating to your reader that your work is original, innovative, and adds to the larger whole.
As the literature review is information dense, it is essential that this section be intelligently structured to enable your reader to grasp the key arguments underpinning your study. However, this can be easier to state and harder to do, simply due to the fact there is usually a plethora of related research to sift through. Consequently, a good strategy for writing the literature review is to break the literature into conceptual categories or themes, rather than attempting to describe various groups of literature you reviewed. Chapter V, “ The Literature Review ,” describes a variety of methods to help you organize the themes.
Here are some suggestions on how to approach the writing of your literature review:
- Think about what questions other researchers have asked, what methods they used, what they found, and what they recommended based upon their findings.
- Do not be afraid to challenge previous related research findings and/or conclusions.
- Assess what you believe to be missing from previous research and explain how your research fills in this gap and/or extends previous research
It is important to note that a significant challenge related to undertaking a literature review is knowing when to stop. As such, it is important to know how to know when you have uncovered the key conceptual categories underlying your research topic. Generally, when you start to see repetition in the conclusions or recommendations, you can have confidence that you have covered all of the significant conceptual categories in your literature review. However, it is also important to acknowledge that researchers often find themselves returning to the literature as they collect and analyze their data. For example, an unexpected finding may develop as one collects and/or analyzes the data and it is important to take the time to step back and review the literature again, to ensure that no other researchers have found a similar finding. This may include looking to research outside your field.
This situation occurred with one of the authors of this textbook´s research related to community resilience. During the interviews, the researchers heard many participants discuss individual resilience factors and how they believed these individual factors helped make the community more resilient, overall. Sheppard and Williams (2016) had not discovered these individual factors in their original literature review on community and environmental resilience. However, when they returned to the literature to search for individual resilience factors, they discovered a small body of literature in the child and youth psychology field. Consequently, Sheppard and Williams had to go back and add a new section to their literature review on individual resilience factors. Interestingly, their research appeared to be the first research to link individual resilience factors with community resilience factors.
Research design and methods
The objective of this section of the research proposal is to convince the reader that your overall research design and methods of analysis will enable you to solve the research problem you have identified and also enable you to accurately and effectively interpret the results of your research. Consequently, it is critical that the research design and methods section is well-written, clear, and logically organized. This demonstrates to your reader that you know what you are going to do and how you are going to do it. Overall, you want to leave your reader feeling confident that you have what it takes to get this research study completed in a timely fashion.
Essentially, this section of the research proposal should be clearly tied to the specific objectives of your study; however, it is also important to draw upon and include examples from the literature review that relate to your design and intended methods. In other words, you must clearly demonstrate how your study utilizes and builds upon past studies, as it relates to the research design and intended methods. For example, what methods have been used by other researchers in similar studies?
While it is important to consider the methods that other researchers have employed, it is equally important, if not more so, to consider what methods have not been employed but could be. Remember, the methods section is not simply a list of tasks to be undertaken. It is also an argument as to why and how the tasks you have outlined will help you investigate the research problem and answer your research question(s).
Tips for writing the research design and methods section:
- Specify the methodological approaches you intend to employ to obtain information and the techniques you will use to analyze the data.
- Specify the research operations you will undertake and he way you will interpret the results of those operations in relation to the research problem.
- Go beyond stating what you hope to achieve through the methods you have chosen. State how you will actually do the methods (i.e. coding interview text, running regression analysis, etc.).
- Anticipate and acknowledge any potential barriers you may encounter when undertaking your research and describe how you will address these barriers.
- Explain where you believe you will find challenges related to data collection, including access to participants and information.
Preliminary suppositions and implications
The purpose of this section is to argue how and in what ways you anticipate that your research will refine, revise, or extend existing knowledge in the area of your study. Depending upon the aims and objectives of your study, you should also discuss how your anticipated findings may impact future research. For example, is it possible that your research may lead to a new policy, new theoretical understanding, or a new method for analyzing data? How might your study influence future studies? What might your study mean for future practitioners working in the field? Who or what may benefit from your study? How might your study contribute to social, economic, environmental issues? While it is important to think about and discuss possibilities such as these, it is equally important to be realistic in stating your anticipated findings. In other words, you do not want to delve into idle speculation. Rather, the purpose here is to reflect upon gaps in the current body of literature and to describe how and in what ways you anticipate your research will begin to fill in some or all of those gaps.
The conclusion reiterates the importance and significance of your research proposal and it provides a brief summary of the entire proposed study. Essentially, this section should only be one or two paragraphs in length. Here is a potential outline for your conclusion:
- Discuss why the study should be done. Specifically discuss how you expect your study will advance existing knowledge and how your study is unique.
- Explain the specific purpose of the study and the research questions that the study will answer.
- Explain why the research design and methods chosen for this study are appropriate, and why other design and methods were not chosen.
- State the potential implications you expect to emerge from your proposed study,
- Provide a sense of how your study fits within the broader scholarship currently in existence related to the research problem.
As with any scholarly research paper, you must cite the sources you used in composing your research proposal. In a research proposal, this can take two forms: a reference list or a bibliography. A reference list does what the name suggests, it lists the literature you referenced in the body of your research proposal. All references in the reference list, must appear in the body of the research proposal. Remember, it is not acceptable to say “as cited in …” As a researcher you must always go to the original source and check it for yourself. Many errors are made in referencing, even by top researchers, and so it is important not to perpetuate an error made by someone else. While this can be time consuming, it is the proper way to undertake a literature review.
In contrast, a bibliography , is a list of everything you used or cited in your research proposal, with additional citations to any key sources relevant to understanding the research problem. In other words, sources cited in your bibliography may not necessarily appear in the body of your research proposal. Make sure you check with your instructor to see which of the two you are expected to produce.
Overall, your list of citations should be a testament to the fact that you have done a sufficient level of preliminary research to ensure that your project will complement, but not duplicate, previous research efforts. For social sciences, the reference list or bibliography should be prepared in American Psychological Association (APA) referencing format. Usually, the reference list (or bibliography) is not included in the word count of the research proposal. Again, make sure you check with your instructor to confirm.
An Introduction to Research Methods in Sociology Copyright © 2019 by Valerie A. Sheppard is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License , except where otherwise noted.
Share This Book
Get science-backed answers as you write with Paperpal's Research feature
What is a Literature Review? How to Write It (with Examples)

A literature review is a critical analysis and synthesis of existing research on a particular topic. It provides an overview of the current state of knowledge, identifies gaps, and highlights key findings in the literature. 1 The purpose of a literature review is to situate your own research within the context of existing scholarship, demonstrating your understanding of the topic and showing how your work contributes to the ongoing conversation in the field. Learning how to write a literature review is a critical tool for successful research. Your ability to summarize and synthesize prior research pertaining to a certain topic demonstrates your grasp on the topic of study, and assists in the learning process.
Table of Contents
- What is the purpose of literature review?
- a. Habitat Loss and Species Extinction:
- b. Range Shifts and Phenological Changes:
- c. Ocean Acidification and Coral Reefs:
- d. Adaptive Strategies and Conservation Efforts:
- How to write a good literature review
- Choose a Topic and Define the Research Question:
- Decide on the Scope of Your Review:
- Select Databases for Searches:
- Conduct Searches and Keep Track:
- Review the Literature:
- Organize and Write Your Literature Review:
- Frequently asked questions
What is a literature review?
A well-conducted literature review demonstrates the researcher’s familiarity with the existing literature, establishes the context for their own research, and contributes to scholarly conversations on the topic. One of the purposes of a literature review is also to help researchers avoid duplicating previous work and ensure that their research is informed by and builds upon the existing body of knowledge.

What is the purpose of literature review?
A literature review serves several important purposes within academic and research contexts. Here are some key objectives and functions of a literature review: 2
- Contextualizing the Research Problem: The literature review provides a background and context for the research problem under investigation. It helps to situate the study within the existing body of knowledge.
- Identifying Gaps in Knowledge: By identifying gaps, contradictions, or areas requiring further research, the researcher can shape the research question and justify the significance of the study. This is crucial for ensuring that the new research contributes something novel to the field.
- Understanding Theoretical and Conceptual Frameworks: Literature reviews help researchers gain an understanding of the theoretical and conceptual frameworks used in previous studies. This aids in the development of a theoretical framework for the current research.
- Providing Methodological Insights: Another purpose of literature reviews is that it allows researchers to learn about the methodologies employed in previous studies. This can help in choosing appropriate research methods for the current study and avoiding pitfalls that others may have encountered.
- Establishing Credibility: A well-conducted literature review demonstrates the researcher’s familiarity with existing scholarship, establishing their credibility and expertise in the field. It also helps in building a solid foundation for the new research.
- Informing Hypotheses or Research Questions: The literature review guides the formulation of hypotheses or research questions by highlighting relevant findings and areas of uncertainty in existing literature.
Literature review example
Let’s delve deeper with a literature review example: Let’s say your literature review is about the impact of climate change on biodiversity. You might format your literature review into sections such as the effects of climate change on habitat loss and species extinction, phenological changes, and marine biodiversity. Each section would then summarize and analyze relevant studies in those areas, highlighting key findings and identifying gaps in the research. The review would conclude by emphasizing the need for further research on specific aspects of the relationship between climate change and biodiversity. The following literature review template provides a glimpse into the recommended literature review structure and content, demonstrating how research findings are organized around specific themes within a broader topic.
Literature Review on Climate Change Impacts on Biodiversity:
Climate change is a global phenomenon with far-reaching consequences, including significant impacts on biodiversity. This literature review synthesizes key findings from various studies:
a. Habitat Loss and Species Extinction:
Climate change-induced alterations in temperature and precipitation patterns contribute to habitat loss, affecting numerous species (Thomas et al., 2004). The review discusses how these changes increase the risk of extinction, particularly for species with specific habitat requirements.
b. Range Shifts and Phenological Changes:
Observations of range shifts and changes in the timing of biological events (phenology) are documented in response to changing climatic conditions (Parmesan & Yohe, 2003). These shifts affect ecosystems and may lead to mismatches between species and their resources.
c. Ocean Acidification and Coral Reefs:
The review explores the impact of climate change on marine biodiversity, emphasizing ocean acidification’s threat to coral reefs (Hoegh-Guldberg et al., 2007). Changes in pH levels negatively affect coral calcification, disrupting the delicate balance of marine ecosystems.
d. Adaptive Strategies and Conservation Efforts:
Recognizing the urgency of the situation, the literature review discusses various adaptive strategies adopted by species and conservation efforts aimed at mitigating the impacts of climate change on biodiversity (Hannah et al., 2007). It emphasizes the importance of interdisciplinary approaches for effective conservation planning.

How to write a good literature review
Writing a literature review involves summarizing and synthesizing existing research on a particular topic. A good literature review format should include the following elements.
Introduction: The introduction sets the stage for your literature review, providing context and introducing the main focus of your review.
- Opening Statement: Begin with a general statement about the broader topic and its significance in the field.
- Scope and Purpose: Clearly define the scope of your literature review. Explain the specific research question or objective you aim to address.
- Organizational Framework: Briefly outline the structure of your literature review, indicating how you will categorize and discuss the existing research.
- Significance of the Study: Highlight why your literature review is important and how it contributes to the understanding of the chosen topic.
- Thesis Statement: Conclude the introduction with a concise thesis statement that outlines the main argument or perspective you will develop in the body of the literature review.
Body: The body of the literature review is where you provide a comprehensive analysis of existing literature, grouping studies based on themes, methodologies, or other relevant criteria.
- Organize by Theme or Concept: Group studies that share common themes, concepts, or methodologies. Discuss each theme or concept in detail, summarizing key findings and identifying gaps or areas of disagreement.
- Critical Analysis: Evaluate the strengths and weaknesses of each study. Discuss the methodologies used, the quality of evidence, and the overall contribution of each work to the understanding of the topic.
- Synthesis of Findings: Synthesize the information from different studies to highlight trends, patterns, or areas of consensus in the literature.
- Identification of Gaps: Discuss any gaps or limitations in the existing research and explain how your review contributes to filling these gaps.
- Transition between Sections: Provide smooth transitions between different themes or concepts to maintain the flow of your literature review.
Conclusion: The conclusion of your literature review should summarize the main findings, highlight the contributions of the review, and suggest avenues for future research.
- Summary of Key Findings: Recap the main findings from the literature and restate how they contribute to your research question or objective.
- Contributions to the Field: Discuss the overall contribution of your literature review to the existing knowledge in the field.
- Implications and Applications: Explore the practical implications of the findings and suggest how they might impact future research or practice.
- Recommendations for Future Research: Identify areas that require further investigation and propose potential directions for future research in the field.
- Final Thoughts: Conclude with a final reflection on the importance of your literature review and its relevance to the broader academic community.

Conducting a literature review
Conducting a literature review is an essential step in research that involves reviewing and analyzing existing literature on a specific topic. It’s important to know how to do a literature review effectively, so here are the steps to follow: 1
Choose a Topic and Define the Research Question:
- Select a topic that is relevant to your field of study.
- Clearly define your research question or objective. Determine what specific aspect of the topic do you want to explore?
Decide on the Scope of Your Review:
- Determine the timeframe for your literature review. Are you focusing on recent developments, or do you want a historical overview?
- Consider the geographical scope. Is your review global, or are you focusing on a specific region?
- Define the inclusion and exclusion criteria. What types of sources will you include? Are there specific types of studies or publications you will exclude?
Select Databases for Searches:
- Identify relevant databases for your field. Examples include PubMed, IEEE Xplore, Scopus, Web of Science, and Google Scholar.
- Consider searching in library catalogs, institutional repositories, and specialized databases related to your topic.
Conduct Searches and Keep Track:
- Develop a systematic search strategy using keywords, Boolean operators (AND, OR, NOT), and other search techniques.
- Record and document your search strategy for transparency and replicability.
- Keep track of the articles, including publication details, abstracts, and links. Use citation management tools like EndNote, Zotero, or Mendeley to organize your references.
Review the Literature:
- Evaluate the relevance and quality of each source. Consider the methodology, sample size, and results of studies.
- Organize the literature by themes or key concepts. Identify patterns, trends, and gaps in the existing research.
- Summarize key findings and arguments from each source. Compare and contrast different perspectives.
- Identify areas where there is a consensus in the literature and where there are conflicting opinions.
- Provide critical analysis and synthesis of the literature. What are the strengths and weaknesses of existing research?
Organize and Write Your Literature Review:
- Literature review outline should be based on themes, chronological order, or methodological approaches.
- Write a clear and coherent narrative that synthesizes the information gathered.
- Use proper citations for each source and ensure consistency in your citation style (APA, MLA, Chicago, etc.).
- Conclude your literature review by summarizing key findings, identifying gaps, and suggesting areas for future research.
The literature review sample and detailed advice on writing and conducting a review will help you produce a well-structured report. But remember that a literature review is an ongoing process, and it may be necessary to revisit and update it as your research progresses.
Frequently asked questions
A literature review is a critical and comprehensive analysis of existing literature (published and unpublished works) on a specific topic or research question and provides a synthesis of the current state of knowledge in a particular field. A well-conducted literature review is crucial for researchers to build upon existing knowledge, avoid duplication of efforts, and contribute to the advancement of their field. It also helps researchers situate their work within a broader context and facilitates the development of a sound theoretical and conceptual framework for their studies.
Literature review is a crucial component of research writing, providing a solid background for a research paper’s investigation. The aim is to keep professionals up to date by providing an understanding of ongoing developments within a specific field, including research methods, and experimental techniques used in that field, and present that knowledge in the form of a written report. Also, the depth and breadth of the literature review emphasizes the credibility of the scholar in his or her field.
Before writing a literature review, it’s essential to undertake several preparatory steps to ensure that your review is well-researched, organized, and focused. This includes choosing a topic of general interest to you and doing exploratory research on that topic, writing an annotated bibliography, and noting major points, especially those that relate to the position you have taken on the topic.
Literature reviews and academic research papers are essential components of scholarly work but serve different purposes within the academic realm. 3 A literature review aims to provide a foundation for understanding the current state of research on a particular topic, identify gaps or controversies, and lay the groundwork for future research. Therefore, it draws heavily from existing academic sources, including books, journal articles, and other scholarly publications. In contrast, an academic research paper aims to present new knowledge, contribute to the academic discourse, and advance the understanding of a specific research question. Therefore, it involves a mix of existing literature (in the introduction and literature review sections) and original data or findings obtained through research methods.
Literature reviews are essential components of academic and research papers, and various strategies can be employed to conduct them effectively. If you want to know how to write a literature review for a research paper, here are four common approaches that are often used by researchers. Chronological Review: This strategy involves organizing the literature based on the chronological order of publication. It helps to trace the development of a topic over time, showing how ideas, theories, and research have evolved. Thematic Review: Thematic reviews focus on identifying and analyzing themes or topics that cut across different studies. Instead of organizing the literature chronologically, it is grouped by key themes or concepts, allowing for a comprehensive exploration of various aspects of the topic. Methodological Review: This strategy involves organizing the literature based on the research methods employed in different studies. It helps to highlight the strengths and weaknesses of various methodologies and allows the reader to evaluate the reliability and validity of the research findings. Theoretical Review: A theoretical review examines the literature based on the theoretical frameworks used in different studies. This approach helps to identify the key theories that have been applied to the topic and assess their contributions to the understanding of the subject. It’s important to note that these strategies are not mutually exclusive, and a literature review may combine elements of more than one approach. The choice of strategy depends on the research question, the nature of the literature available, and the goals of the review. Additionally, other strategies, such as integrative reviews or systematic reviews, may be employed depending on the specific requirements of the research.
The literature review format can vary depending on the specific publication guidelines. However, there are some common elements and structures that are often followed. Here is a general guideline for the format of a literature review: Introduction: Provide an overview of the topic. Define the scope and purpose of the literature review. State the research question or objective. Body: Organize the literature by themes, concepts, or chronology. Critically analyze and evaluate each source. Discuss the strengths and weaknesses of the studies. Highlight any methodological limitations or biases. Identify patterns, connections, or contradictions in the existing research. Conclusion: Summarize the key points discussed in the literature review. Highlight the research gap. Address the research question or objective stated in the introduction. Highlight the contributions of the review and suggest directions for future research.
Both annotated bibliographies and literature reviews involve the examination of scholarly sources. While annotated bibliographies focus on individual sources with brief annotations, literature reviews provide a more in-depth, integrated, and comprehensive analysis of existing literature on a specific topic. The key differences are as follows:
References
- Denney, A. S., & Tewksbury, R. (2013). How to write a literature review. Journal of criminal justice education , 24 (2), 218-234.
- Pan, M. L. (2016). Preparing literature reviews: Qualitative and quantitative approaches . Taylor & Francis.
- Cantero, C. (2019). How to write a literature review. San José State University Writing Center .
Paperpal is an AI writing assistant that help academics write better, faster with real-time suggestions for in-depth language and grammar correction. Trained on millions of research manuscripts enhanced by professional academic editors, Paperpal delivers human precision at machine speed.
Try it for free or upgrade to Paperpal Prime , which unlocks unlimited access to premium features like academic translation, paraphrasing, contextual synonyms, consistency checks and more. It’s like always having a professional academic editor by your side! Go beyond limitations and experience the future of academic writing. Get Paperpal Prime now at just US$19 a month!
Related Reads:
- Empirical Research: A Comprehensive Guide for Academics
- How to Write a Scientific Paper in 10 Steps
- Life Sciences Papers: 9 Tips for Authors Writing in Biological Sciences
- What is an Argumentative Essay? How to Write It (With Examples)
6 Tips for Post-Doc Researchers to Take Their Career to the Next Level
Self-plagiarism in research: what it is and how to avoid it, you may also like, paperpal’s new ai research finder empowers authors to..., why traditional editorial process needs an upgrade, what is hedging in academic writing , how to use ai to enhance your college..., ai + human expertise – a paradigm shift..., how to use paperpal to generate emails &..., ai in education: it’s time to change the..., is it ethical to use ai-generated abstracts without..., do plagiarism checkers detect ai content, word choice problems: how to use the right....

Graduate Research: Guide to the Literature Review
- "Literature review" defined
- Research Communication Graphic
- Literature Review Steps
- Search techniques
- Finding Additional "Items
- Evaluating information
- Citing Styles
- Ethical Use of Information
- Research Databases This link opens in a new window
- Get Full Text
- Reading a Scholarly Article
- Author Rights
- Selecting a publisher
Introduction to Research Process: Literature Review Steps
When seeking information for a literature review or for any purpose, it helps to understand information-seeking as a process that you can follow. 5 Each of the six (6) steps has its own section in this web page with more detail. Do (and re-do) the following six steps:
1. Define your topic. The first step is defining your task -- choosing a topic and noting the questions you have about the topic. This will provide a focus that guides your strategy in step II and will provide potential words to use in searches in step III.
2. Develop a strategy. Strategy involves figuring out where the information might be and identifying the best tools for finding those types of sources. The strategy section identifies specific types of research databases to use for specific purposes.
3. Locate the information . In this step, you implement the strategy developed in II in order to actually locate specific articles, books, technical reports, etc.
4. Use and Evaluate the information. Having located relevant and useful material, in step IV you read and analyze the items to determine whether they have value for your project and credibility as sources.
5. Synthesize. In step V, you will make sense of what you've learned and demonstrate your knowledge. You will thoroughly understand, organize and integrate the information --become knowledgeable-- so that you are able to use your own words to support and explain your research project and its relationship to existing research by others.
6. Evaluate your work. At every step along the way, you should evaluate your work. However, this final step is a last check to make sure your work is complete and of high quality.
Continue below to begin working through the process.
5. Eisenberg, M. B., & Berkowitz, R. E. (1990). Information Problem-Solving: the Big Six Skills Approach to Library & Information Skills Instruction . Norwood, NJ: Ablex Publishing.
1. Define your topic.
I. Define your topic
A. Many students have difficulty selecting a topic. You want to find a topic you find interesting and will enjoy learning more about.
B. Students often select a topic that is too broad. You may have a broad topic in mind initially and will need to narrow it.
1. To help narrow a broad topic :
a. Brainstorm.
1). Try this technique for brainstorming to narrow your focus.
a) Step 1. Write down your broad topic.
b) Step 2. Write down a "specific kind" or "specific aspect" of the topic you identified in step 1.
c) Step 3. Write down an aspect --such as an attribute or behavior-- of the "specific kind" you identified in step 2.
d) Step 4. Continue to add levels of specificity as needed to get to a focus that is manageable. However, you may want to begin researching the literature before narrowing further to give yourself the opportunity to explore what others are doing and how that might impact the direction that you take for your own research.
2) Three examples of using the narrowing technique. These examples start with very, very broad topics, so the topic at step 3 or 4 in these examples would be used for a preliminary search in the literature in order to identify a more specific focus. Greater specificity than level 3 or 4 will ultimately be necessary for developing a specific research question. And we may discover in our preliminary research that we need to alter the direction that we originally were taking.
a) Example 1.
Step 1. information security
Step 2. protocols
Step 3. handshake protocol
Brainstorming has brought us to focus on the handshake protocol.
b) Example 2.
Step 1. information security
Step 2. single sign-on authentication
Step 3. analyzing
Step 4. methods
Brainstorming has brought us to focus on methods for analyzing the security of single sign-on authentication
c) Example 3. The diagram below is an example using the broad topic of "software" to show two potential ways to begin to narrow the topic.
C. Once you have completed the brainstorming process and your topic is more focused, you can do preliminary research to help you identify a specific research question .
1) Examine overview sources such as subject-specific encyclopedias and textbooks that are likely to break down your specific topic into sub-topics and to highlight core issues that could serve as possible research questions. [See section II. below on developing a strategy to learn how to find these encyclopedias]
2). Search the broad topic in a research database that includes scholarly journals and professional magazines (to find technical and scholarly articles) and scan recent article titles for ideas. [See section II. below on developing a strategy to learn how to find trade and scholarly journal articles]
D. Once you have identified a research question or questions, ask yourself what you need to know to answer the questions. For example,
1. What new knowledge do I need to gain?
2. What has already been answered by prior research of other scholars?
E. Use the answers to the questions in C. to identify what words to use to describe the topic when you are doing searches.
1. Identify key words
a. For example , if you are investigating "security audits in banking", key terms to combine in your searches would be: security, audits, banking.
2. Create a list of alternative ways of referring to a key word or phrase
a.For example , "information assurance" may be referred to in various ways such as: "information assurance," "information security," and "computer security."
b. Use these alternatives when doing searches.
3. As you are searching, pay attention to how others are writing about the topic and add new words or phrases to your searches if appropriate.
2. Develop a strategy.
II. Develop a strategy for finding the information.
A. Start by considering what types of source might contain the information you need . Do you need a dictionary for definitions? a directory for an address? the history of a concept or technique that might be in a book or specialized encyclopedia? today's tech news in an online tech magazine or newspaper? current research in a journal article? background information that might be in a specialized encyclopedia? data or statistics from a specific organization or website? Note that you will typically have online access to these source types.
B. This section provides a description of some of the common types of information needed for research.
1. For technical and business analysis , look for articles in technical and trade magazines . These articles are written by information technology professionals to help other IT professionals do their jobs better. Content might include news on new developments in hardware or software, techniques, tools, and practical advice. Technical journals are also likely to have product ads relevant to information technology workers and to have job ads. Examples iof technical magazines include Network Computing and IEEE Spectrum .
2. To read original research studies , look for articles in scholarly journals and conference proceedings . They will provide articles written by information technology professionals who are reporting original research; that is, research that has been done by the authors and is being reported for the first time. The audience for original research articles is other information technology scholars and professionals. Examples of scholarly journals include Journal of Applied Security Research , Journal of Management Information Systems , IEEE Transactions on Computers , and ACM Transactions on Information and System Security .
3. For original research being reported to funding agencies , look for technical reports on agency websites. Technical reports are researcher reports to funding agencies about progress on or completion of research funded by the agency.
4. For in-depth, comprehensive information on a topic , look for book-length volumes . All chapters in the book might be written by the same author(s) or might be a collection of separate papers written by different authors.
5. To learn about an unfamiliar topic , use textbooks , specialized encyclopedias and handbooks to get get overviews of topics, history/background, and key issues explained.
6. For instructions for hardware, software, networking, etc., look for manuals that provide step-by-step instructions.
7. For technical details about inventions (devices, instruments, machines), look for patent documents .
C. NOTE - In order to search for and find original research studies, it will help if you understand how information is produced, packaged and communicated within your profession. This is explained in the tab "Research Communication: Graphic."
3. Locate the information.
III. Locate the information
A. Use search tools designed to find the sources you want. Types of sources were described in section II. above.
Always feel free to Ask a librarian for assistance when you have questions about where and how locate the information you need.
B. Evaluate the search results (no matter where you find the information)
1. Evaluate the items you find using at least these 5 criteria:
a. accuracy -- is the information reliable and error free?
1) Is there an editor or someone who verifies/checks the information?
2) Is there adequate documentation: bibliography, footnotes, credits?
3) Are the conclusions justified by the information presented?
b. authority -- is the source of the information reputable?
1) How did you find the source of information: an index to edited/peer-reviewed material, in a bibliography from a published article, etc.?
2) What type of source is it: sensationalistic, popular, scholarly?
c. objectivity -- does the information show bias?
1) What is the purpose of the information: to inform, persuade, explain, sway opinion, advertise?
2) Does the source show political or cultural biases?
d. currency -- is the information current? does it cover the time period you need?
e. coverage -- does it provide the evidence or information you need?
2. Is the search producing the material you need? -- the right content? the right quality? right time period? right geographical location? etc. If not, are you using
a. the right sources?
b. the right tools to get to the sources?
c. are you using the right words to describe the topic?
3. Have you discovered additional terms that should be searched? If so, search those terms.
4. Have you discovered additional questions you need to answer? If so, return to section A above to begin to answer new questions.
4. Use and evaluate the information.
IV. Use the information.
A. Read, hear or view the source
1. Evaluate: Does the material answer your question(s)? -- right content? If not, return to B.
2. Evaluate: Is the material appropriate? -- right quality? If not, return to B.
B. Extract the information from the source : copy/download information, take notes, record citation, keep track of items using a citation manager.
1. Note taking (these steps will help you when you begin to write your thesis and/or document your project.):
a. Write the keywords you use in your searches to avoid duplicating previous searches if you return to search a research database again. Keeping track of keywords used will also save you time if your search is interrupted or you need return and do the search again for some other reason. It will help you remember which search terms worked successfully in which databases
b. Write the citations or record the information needed to cite each article/document you plan to read and use, or make sure that any saved a copy of the article includes all the information needed to cite it. Some article pdf files may not include all of the information needed to cite, and it's a waste of your valuable time to have to go back to search and find the items again in order to be able to cite them. Using citation management software such as EndNote will help keep track of citations and help create bibliographies for your research papers.
c. Write a summary of each article you read and/or why you want to use it.
5. Synthesize.
V. Synthesize.
A. Organize and integrate information from multiple sources
B. Present the information (create report, speech, etc. that communicates)
C. Cite material using the style required by your professor or by the venue (conference, publication, etc.). For help with citation styles, see Guide to Citing Sources . A link to the citing guide is also available in the "Get Help" section on the left side of the Library home page
6. Evaluate your work.
VI. Evaluate the paper, speech, or whatever you are using to communicate your research.
A. Is it effective?
B. Does it meet the requirements?
C. Ask another student or colleague to provide constructive criticism of your paper/project.
- << Previous: Research Communication Graphic
- Next: Search techniques >>
- Last Updated: Apr 15, 2024 3:27 PM
- URL: https://library.dsu.edu/graduate-research
We use cookies on this site to enhance your experience
By clicking any link on this page you are giving your consent for us to set cookies.
A link to reset your password has been sent to your email.
Back to login
We need additional information from you. Please complete your profile first before placing your order.
Thank you. payment completed., you will receive an email from us to confirm your registration, please click the link in the email to activate your account., there was error during payment, orcid profile found in public registry, download history, deciding what to include and exclude as you begin to write your literature review.
- Charlesworth Author Services
- 06 October, 2021
Once you have completed your literature search , you can start thinking about creating a structure to best explain the literature and to link existing studies to your paper. Having a firm structure provides the foundation for laying out your discussions of the literature and/or the development of your research question(s) or hypothesis. Before that, though, you may still need to make some key decisions regarding what literature or texts should be included and excluded in your paper. This article highlights four essential considerations as you start drafting a structure for your literature review .
Comprehensiveness
There are two types of literature review : one is the literature review section within a paper and the other is a literature review synthesis paper that aims to provide a framework for future studies. For the first, a more focused review of only relevant studies would be more appropriate and useful. For the second type, however, you would usually be expected to provide a much more comprehensive review.
For a literature review section within a paper, a focused review that is more tightly related to your study will help you to build arguments more succinctly, and enable you to link existing studies to your own research more easily. If you find that the literature that is most relevant to your study still falls in large, broad categories, then breaking this section down into different, smaller subsections can be helpful for making sure the various ideas and themes are presented clearly and are easy to follow.
Level of detail
In the literature review, you should be aiming to clearly explain prior and current studies so you can better contextualise your own research within the field. However, the level of detail that you include in this section needs to be carefully considered.
If several studies are key to your paper or sound similar to your study, you may need to compare and contrast them more closely in order to differentiate them from prior studies, create connections between them or to build on existing literature.
In addition, if you need to draw from specific papers for your methodology or your theoretical framework , it is a good idea to go into slightly more detail and provide as much information as is reasonably possible, rather than assuming that the reader already knows about these studies.
However, too many detailed descriptions can be distracting and it is important to try to strike a balance between providing enough information for your reader to follow your argument without overwhelming them with too much detail. In some instances therefore, offering a summarised key message can work more effectively.
In order to better gauge the level of detail needed, go through your writing several times to sharpen your focus, ask your colleagues for feedback or engage professional editing services to check that your structure and overall narrative are clear.
Online sources and extended quotations
Sometimes, you may want to include online sources in your discussion of the literature. For example, government reports or reputable reports released by major organisations can be quite useful for helping you develop your narrative and arguments. These reports may also provide some initial evidence. However, if you do choose to use such studies, they should be engaged alongside other studies from different sources to make them more plausible.
In addition, unless really necessary, try to avoid very long or extended quotations. A better practice is to paraphrase and/or summarise the key points that you are trying to make. Drawing from your notes can be useful here and will also help to avoid potential concerns about plagiarism . Using your own words to explain complex issues or to summarise long quotations can also make reading easier for the reader.
The literature review is supposed to comprise a summary of thoughts and findings in prior or existing studies related to the topic that you are addressing in your study. Accordingly, the discussion of these studies should be as objective as possible and should not include your personal opinions, comments or even article preferences. This will help you to describe what has already been done in the field more clearly and use this review as a basis for developing your own research.
Use the above four points to help you stay on track as you write. By being very clear about what your literature review will include or exclude, you will be able to provide an effective, focused overview of existing research, upon which you can build and structure your own study .
Read next (fourth) in series: How to refer to other studies or literature in the different sections of a research paper
Read previous (second) in series: How to structure and write your literature review
Maximise your publication success with Charlesworth Author Services .
Charlesworth Author Services, a trusted brand supporting the world’s leading academic publishers, institutions and authors since 1928.
To know more about our services, visit: Our Services

Share with your colleagues
Related articles.

Important factors to consider as you Start to Plan your Literature Review
Charlesworth Author Services 06/10/2021 00:00:00

Conducting a Literature Review
Charlesworth Author Services 10/03/2021 00:00:00

How to Structure and Write your Literature Review
Charlesworth Author Services 07/10/2021 00:00:00
Related webinars

Bitesize Webinar: How to write and structure your academic article for publication - Module 1: Know when are you ready to write
Charlesworth Author Services 04/03/2021 00:00:00

Bitesize Webinar: How to write and structure your academic article for publication- Module 3: Understand the structure of an academic paper

Bitesize Webinar: How to write and structure your academic article for publication: Module 5: Conduct a Literature Review

Bitesize Webinar: How to write and structure your academic article for publication: Module 11: Know when your article is ready for submission
Charlesworth Author Services 05/03/2021 00:00:00
Literature search

Why and How to do a literature search
Charlesworth Author Services 17/08/2020 00:00:00

Best tips to do a PubMed search
Charlesworth Author Services 26/08/2021 00:00:00

Best tips to do a Scopus search
How to write a literature review introduction (+ examples)
The introduction to a literature review serves as your reader’s guide through your academic work and thought process. Explore the significance of literature review introductions in review papers, academic papers, essays, theses, and dissertations. We delve into the purpose and necessity of these introductions, explore the essential components of literature review introductions, and provide step-by-step guidance on how to craft your own, along with examples.
Why you need an introduction for a literature review
When you need an introduction for a literature review, what to include in a literature review introduction, examples of literature review introductions, steps to write your own literature review introduction.
A literature review is a comprehensive examination of the international academic literature concerning a particular topic. It involves summarizing published works, theories, and concepts while also highlighting gaps and offering critical reflections.
In academic writing , the introduction for a literature review is an indispensable component. Effective academic writing requires proper paragraph structuring to guide your reader through your argumentation. This includes providing an introduction to your literature review.
It is imperative to remember that you should never start sharing your findings abruptly. Even if there isn’t a dedicated introduction section .
Instead, you should always offer some form of introduction to orient the reader and clarify what they can expect.
There are three main scenarios in which you need an introduction for a literature review:
- Academic literature review papers: When your literature review constitutes the entirety of an academic review paper, a more substantial introduction is necessary. This introduction should resemble the standard introduction found in regular academic papers.
- Literature review section in an academic paper or essay: While this section tends to be brief, it’s important to precede the detailed literature review with a few introductory sentences. This helps orient the reader before delving into the literature itself.
- Literature review chapter or section in your thesis/dissertation: Every thesis and dissertation includes a literature review component, which also requires a concise introduction to set the stage for the subsequent review.
You may also like: How to write a fantastic thesis introduction (+15 examples)
It is crucial to customize the content and depth of your literature review introduction according to the specific format of your academic work.
In practical terms, this implies, for instance, that the introduction in an academic literature review paper, especially one derived from a systematic literature review , is quite comprehensive. Particularly compared to the rather brief one or two introductory sentences that are often found at the beginning of a literature review section in a standard academic paper. The introduction to the literature review chapter in a thesis or dissertation again adheres to different standards.
Here’s a structured breakdown based on length and the necessary information:
Academic literature review paper
The introduction of an academic literature review paper, which does not rely on empirical data, often necessitates a more extensive introduction than the brief literature review introductions typically found in empirical papers. It should encompass:
- The research problem: Clearly articulate the problem or question that your literature review aims to address.
- The research gap: Highlight the existing gaps, limitations, or unresolved aspects within the current body of literature related to the research problem.
- The research relevance: Explain why the chosen research problem and its subsequent investigation through a literature review are significant and relevant in your academic field.
- The literature review method: If applicable, describe the methodology employed in your literature review, especially if it is a systematic review or follows a specific research framework.
- The main findings or insights of the literature review: Summarize the key discoveries, insights, or trends that have emerged from your comprehensive review of the literature.
- The main argument of the literature review: Conclude the introduction by outlining the primary argument or statement that your literature review will substantiate, linking it to the research problem and relevance you’ve established.
- Preview of the literature review’s structure: Offer a glimpse into the organization of the literature review paper, acting as a guide for the reader. This overview outlines the subsequent sections of the paper and provides an understanding of what to anticipate.
By addressing these elements, your introduction will provide a clear and structured overview of what readers can expect in your literature review paper.
Regular literature review section in an academic article or essay
Most academic articles or essays incorporate regular literature review sections, often placed after the introduction. These sections serve to establish a scholarly basis for the research or discussion within the paper.
In a standard 8000-word journal article, the literature review section typically spans between 750 and 1250 words. The first few sentences or the first paragraph within this section often serve as an introduction. It should encompass:
- An introduction to the topic: When delving into the academic literature on a specific topic, it’s important to provide a smooth transition that aids the reader in comprehending why certain aspects will be discussed within your literature review.
- The core argument: While literature review sections primarily synthesize the work of other scholars, they should consistently connect to your central argument. This central argument serves as the crux of your message or the key takeaway you want your readers to retain. By positioning it at the outset of the literature review section and systematically substantiating it with evidence, you not only enhance reader comprehension but also elevate overall readability. This primary argument can typically be distilled into 1-2 succinct sentences.
In some cases, you might include:
- Methodology: Details about the methodology used, but only if your literature review employed a specialized method. If your approach involved a broader overview without a systematic methodology, you can omit this section, thereby conserving word count.
By addressing these elements, your introduction will effectively integrate your literature review into the broader context of your academic paper or essay. This will, in turn, assist your reader in seamlessly following your overarching line of argumentation.
Introduction to a literature review chapter in thesis or dissertation
The literature review typically constitutes a distinct chapter within a thesis or dissertation. Often, it is Chapter 2 of a thesis or dissertation.
Some students choose to incorporate a brief introductory section at the beginning of each chapter, including the literature review chapter. Alternatively, others opt to seamlessly integrate the introduction into the initial sentences of the literature review itself. Both approaches are acceptable, provided that you incorporate the following elements:
- Purpose of the literature review and its relevance to the thesis/dissertation research: Explain the broader objectives of the literature review within the context of your research and how it contributes to your thesis or dissertation. Essentially, you’re telling the reader why this literature review is important and how it fits into the larger scope of your academic work.
- Primary argument: Succinctly communicate what you aim to prove, explain, or explore through the review of existing literature. This statement helps guide the reader’s understanding of the review’s purpose and what to expect from it.
- Preview of the literature review’s content: Provide a brief overview of the topics or themes that your literature review will cover. It’s like a roadmap for the reader, outlining the main areas of focus within the review. This preview can help the reader anticipate the structure and organization of your literature review.
- Methodology: If your literature review involved a specific research method, such as a systematic review or meta-analysis, you should briefly describe that methodology. However, this is not always necessary, especially if your literature review is more of a narrative synthesis without a distinct research method.
By addressing these elements, your introduction will empower your literature review to play a pivotal role in your thesis or dissertation research. It will accomplish this by integrating your research into the broader academic literature and providing a solid theoretical foundation for your work.
Comprehending the art of crafting your own literature review introduction becomes significantly more accessible when you have concrete examples to examine. Here, you will find several examples that meet, or in most cases, adhere to the criteria described earlier.
Example 1: An effective introduction for an academic literature review paper
To begin, let’s delve into the introduction of an academic literature review paper. We will examine the paper “How does culture influence innovation? A systematic literature review”, which was published in 2018 in the journal Management Decision.

The entire introduction spans 611 words and is divided into five paragraphs. In this introduction, the authors accomplish the following:
- In the first paragraph, the authors introduce the broader topic of the literature review, which focuses on innovation and its significance in the context of economic competition. They underscore the importance of this topic, highlighting its relevance for both researchers and policymakers.
- In the second paragraph, the authors narrow down their focus to emphasize the specific role of culture in relation to innovation.
- In the third paragraph, the authors identify research gaps, noting that existing studies are often fragmented and disconnected. They then emphasize the value of conducting a systematic literature review to enhance our understanding of the topic.
- In the fourth paragraph, the authors introduce their specific objectives and explain how their insights can benefit other researchers and business practitioners.
- In the fifth and final paragraph, the authors provide an overview of the paper’s organization and structure.
In summary, this introduction stands as a solid example. While the authors deviate from previewing their key findings (which is a common practice at least in the social sciences), they do effectively cover all the other previously mentioned points.
Example 2: An effective introduction to a literature review section in an academic paper
The second example represents a typical academic paper, encompassing not only a literature review section but also empirical data, a case study, and other elements. We will closely examine the introduction to the literature review section in the paper “The environmentalism of the subalterns: a case study of environmental activism in Eastern Kurdistan/Rojhelat”, which was published in 2021 in the journal Local Environment.

The paper begins with a general introduction and then proceeds to the literature review, designated by the authors as their conceptual framework. Of particular interest is the first paragraph of this conceptual framework, comprising 142 words across five sentences:
“ A peripheral and marginalised nationality within a multinational though-Persian dominated Iranian society, the Kurdish people of Iranian Kurdistan (a region referred by the Kurds as Rojhelat/Eastern Kurdi-stan) have since the early twentieth century been subject to multifaceted and systematic discriminatory and exclusionary state policy in Iran. This condition has left a population of 12–15 million Kurds in Iran suffering from structural inequalities, disenfranchisement and deprivation. Mismanagement of Kurdistan’s natural resources and the degradation of its natural environmental are among examples of this disenfranchisement. As asserted by Julian Agyeman (2005), structural inequalities that sustain the domination of political and economic elites often simultaneously result in environmental degradation, injustice and discrimination against subaltern communities. This study argues that the environmental struggle in Eastern Kurdistan can be asserted as a (sub)element of the Kurdish liberation movement in Iran. Conceptually this research is inspired by and has been conducted through the lens of ‘subalternity’ ” ( Hassaniyan, 2021, p. 931 ).
In this first paragraph, the author is doing the following:
- The author contextualises the research
- The author links the research focus to the international literature on structural inequalities
- The author clearly presents the argument of the research
- The author clarifies how the research is inspired by and uses the concept of ‘subalternity’.
Thus, the author successfully introduces the literature review, from which point onward it dives into the main concept (‘subalternity’) of the research, and reviews the literature on socio-economic justice and environmental degradation.
While introductions to a literature review section aren’t always required to offer the same level of study context detail as demonstrated here, this introduction serves as a commendable model for orienting the reader within the literature review. It effectively underscores the literature review’s significance within the context of the study being conducted.
Examples 3-5: Effective introductions to literature review chapters
The introduction to a literature review chapter can vary in length, depending largely on the overall length of the literature review chapter itself. For example, a master’s thesis typically features a more concise literature review, thus necessitating a shorter introduction. In contrast, a Ph.D. thesis, with its more extensive literature review, often includes a more detailed introduction.
Numerous universities offer online repositories where you can access theses and dissertations from previous years, serving as valuable sources of reference. Many of these repositories, however, may require you to log in through your university account. Nevertheless, a few open-access repositories are accessible to anyone, such as the one by the University of Manchester . It’s important to note though that copyright restrictions apply to these resources, just as they would with published papers.
Master’s thesis literature review introduction
The first example is “Benchmarking Asymmetrical Heating Models of Spider Pulsar Companions” by P. Sun, a master’s thesis completed at the University of Manchester on January 9, 2024. The author, P. Sun, introduces the literature review chapter very briefly but effectively:
PhD thesis literature review chapter introduction
The second example is Deep Learning on Semi-Structured Data and its Applications to Video-Game AI, Woof, W. (Author). 31 Dec 2020, a PhD thesis completed at the University of Manchester . In Chapter 2, the author offers a comprehensive introduction to the topic in four paragraphs, with the final paragraph serving as an overview of the chapter’s structure:
PhD thesis literature review introduction
The last example is the doctoral thesis Metacognitive strategies and beliefs: Child correlates and early experiences Chan, K. Y. M. (Author). 31 Dec 2020 . The author clearly conducted a systematic literature review, commencing the review section with a discussion of the methodology and approach employed in locating and analyzing the selected records.

Having absorbed all of this information, let’s recap the essential steps and offer a succinct guide on how to proceed with creating your literature review introduction:
- Contextualize your review : Begin by clearly identifying the academic context in which your literature review resides and determining the necessary information to include.
- Outline your structure : Develop a structured outline for your literature review, highlighting the essential information you plan to incorporate in your introduction.
- Literature review process : Conduct a rigorous literature review, reviewing and analyzing relevant sources.
- Summarize and abstract : After completing the review, synthesize the findings and abstract key insights, trends, and knowledge gaps from the literature.
- Craft the introduction : Write your literature review introduction with meticulous attention to the seamless integration of your review into the larger context of your work. Ensure that your introduction effectively elucidates your rationale for the chosen review topics and the underlying reasons guiding your selection.
Get new content delivered directly to your inbox!
Subscribe and receive Master Academia's quarterly newsletter.
The best answers to "What are your plans for the future?"
10 tips for engaging your audience in academic writing, related articles.

How to peer review an academic paper

PhD thesis types: Monograph and collection of articles

How to disagree with reviewers (with examples!)

How to introduce yourself in a conference presentation (in six simple steps)
This paper is in the following e-collection/theme issue:
Published on 11.4.2024 in Vol 26 (2024)
This is a member publication of Imperial College London (Jisc)
Regulatory Standards and Guidance for the Use of Health Apps for Self-Management in Sub-Saharan Africa: Scoping Review
Authors of this article:

- Benard Ayaka Bene 1, 2 , MBBS, MPH ;
- Sunny Ibeneme 3 , MD, PhD ;
- Kayode Philip Fadahunsi 1 , MBBS, MPH ;
- Bala Isa Harri 4 , MBBS, MPH, MSc ;
- Nkiruka Ukor 5 , MSc ;
- Nikolaos Mastellos 1 , BSc, PhD ;
- Azeem Majeed 1 , MD ;
- Josip Car 1, 6 , MSc, MD, PhD
1 Department of Primary Care and Public Health, School of Public Health, Imperial College London, London, United Kingdom
2 Department of Public Health, Federal Ministry of Health, Abuja, Nigeria
3 Digital Health Specialist, UNICEF East Asia Pacific Regional Office, Bangkok, Thailand
4 Department of Health Planning, Research and Statistics, Federal Ministry of Health, Abuja, Nigeria
5 Strategic Health Information Cluster, World Health Organization, Abuja, Nigeria
6 School of Life Course & Population Sciences, King’s College London, London, United Kingdom
Corresponding Author:
Benard Ayaka Bene, MBBS, MPH
Department of Primary Care and Public Health
School of Public Health
Imperial College London
The Reynolds Building
St Dunstan’s Road
London, W6 8RP
United Kingdom
Phone: 44 7598439185
Email: [email protected]
Background: Health apps are increasingly recognized as crucial tools for enhancing health care delivery. Many countries, particularly those in sub-Saharan Africa, can substantially benefit from using health apps to support self-management and thus help to achieve universal health coverage and the third sustainable development goal. However, most health apps published in app stores are of unknown or poor quality, which poses a risk to patient safety. Regulatory standards and guidance can help address this risk and promote patient safety.
Objective: This review aims to assess the regulatory standards and guidance for health apps supporting evidence-based best practices in sub-Saharan Africa with a focus on self-management.
Methods: A methodological framework for scoping reviews was applied. A search strategy was built and applied across the following databases, gray literature sources, and institutional websites: PubMed, Scopus, World Health Organization (WHO) African Index Medicus, OpenGrey, WHO Regional Office for Africa Library, ICTworks, WHO Directory of eHealth policies, HIS Strengthening Resource Center, International Telecommunication Union, Ministry of Health websites, and Google. The search covered the period between January 2005 and January 2024. The findings were analyzed using a deductive descriptive content analysis. The policy analysis framework was adapted and used to organize the findings. The Reporting Items for Stakeholder Analysis tool guided the identification and mapping of key stakeholders based on their roles in regulating health apps for self-management.
Results: The study included 49 documents from 31 sub-Saharan African countries. While all the documents were relevant for stakeholder identification and mapping, only 3 regulatory standards and guidance contained relevant information on regulation of health apps. These standards and guidance primarily aimed to build mutual trust; promote integration, inclusion, and equitable access to services; and address implementation issues and poor coordination. They provided guidance on systems quality, software acquisition and maintenance, security measures, data exchange, interoperability and integration, involvement of relevant stakeholders, and equitable access to services. To enhance implementation, the standards highlight that legal authority, coordination of activities, building capacity, and monitoring and evaluation are required. A number of stakeholders, including governments, regulatory bodies, funders, intergovernmental and nongovernmental organizations, academia, and the health care community, were identified to play key roles in regulating health apps.
Conclusions: Health apps have huge potential to support self-management in sub-Saharan Africa, but the lack of regulatory standards and guidance constitutes a major barrier. Hence, for these apps to be safely and effectively integrated into health care, more attention should be given to regulation. Learning from countries with effective regulations can help sub-Saharan Africa build a more robust and responsive regulatory system, ensuring the safe and beneficial use of health apps across the region.
International Registered Report Identifier (IRRID): RR2-10.1136/bmjopen-2018-025714
Introduction
Health apps are the most widely used digital health products globally [ 1 , 2 ]. Harnessing the potential of health apps creates a huge opportunity in providing support for health care delivery, including patient communication, patient education, and decision support for self-management [ 3 - 8 ]. Health apps can be an effective tool to strengthen health systems worldwide, especially in low- and middle-income countries including those in sub-Saharan Africa [ 4 , 5 , 9 ]. As a result, the attainment of universal health coverage (UHC) and sustainable development goal (SDG) 3, good health and well-being, can be accelerated [ 8 , 10 ].
Many health apps fall below the expected quality threshold [ 11 ]. Several studies have found that widely used health apps are often technically unreliable and clinically unsafe [ 12 - 14 ] and do not comply with ethical standards and the principles of confidentiality of information and data privacy [ 15 , 16 ]. In addition, many commercially available health apps were not developed using interoperability standards that are widely accepted in sub-Saharan Africa (eg, Fast Healthcare Interoperability Resources [FHIR]) [ 17 - 20 ]. Consequently, it becomes difficult to integrate these apps into a clinical workflow.
Hence, regulation through robust mechanisms is crucial to enhance the development, implementation, and adoption of health apps. Regulatory standards and guidance are essential for the safety of patients as they ensure quality assurance of any new technology in health care and contribute to building mutual trust while promoting the optimal use of the technology [ 21 - 23 ]. Therefore, to ensure that health apps that are used to support the self-management of patients are technically reliable and clinically safe, interoperable across systems, and compliant with the principles of confidentiality of information and data privacy, there is a need for effective regulatory standards. Furthermore, effective regulation can help ensure that health apps for self-management are culturally functional and competent and are accessible to those who need them regardless of gender, ethnicity, geographical location, or financial status [ 24 - 31 ].
Since 2005, there have been ongoing efforts to strengthen digital health governance at both the national and international levels [ 32 , 33 ]. In 2018, the World Health Organization (WHO) member states renewed their commitment to using digital health technologies (DHTs) to advance UHC and SDG 3 [ 33 ]. However, to date, the extent to which the use of health apps for self-management is regulated across countries within the WHO African Region (also known as sub-Saharan Africa) remains unclear. Therefore, this review was conducted to identify available regulatory standards and guidance and assess the extent to which they regulate health apps for self-management in sub-Saharan Africa. The review also mapped out the key stakeholders and their roles in regulating health apps for self-management across sub-Saharan Africa.
Review Questions
The review attempted to answer the following questions: (1) What regulatory standards and guidance are available for regulating health apps for self-management across sub-Saharan Africa? (2) To what extent do regulatory standards and guidance regulate health apps for self-management in terms of what aspects are regulated; why, how, and for whom; and what aspects are not regulated? (3) Who are the key stakeholders and what are their roles in regulating health apps for self-management?
Study Design
The process of this scoping review followed the methodological framework for conducting a scoping study originally described by Arksey and O’Malley [ 34 ] and the updated methodological guidance for conducting a Joanna Briggs Institute scoping review [ 34 - 37 ]. The reporting of the review was guided by the PRISMA-ScR (Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews) checklist [ 38 ]. A completed PRISMA-ScR checklist is provided in Multimedia Appendix 1 . The protocol of this scoping review was published in BMJ Open [ 30 ].
Identifying Relevant Documents
Two reviewers (BAB and SI) developed the search strategy with the assistance of a librarian and in consultation with other research team members (KPF, BIH, NU, NM, AM, and JC). The following key terms were included: policy, legislation, strategy, regulation, standard, criterion, framework, guidance, guideline, digital health, eHealth, app, WHO African Region, and sub-Saharan Africa, and the names of all sub-Saharan African countries.
Owing to the absence of regulatory standards and guidance in scientific databases, the search focus was narrowed down to gray literature sources and institutional websites, including OpenGrey, WHO Regional Office for Africa (AFRO) Library, repositories for digital health policies (ICTworks, WHO’s Directory of eHealth Policies, and Health Information System Strengthening Resource Center), as well as the websites of WHO, International Telecommunication Union (ITU), and Ministries of Health (MOHs). The only scientific databases searched were PubMed, Scopus, and WHO AIM. PubMed was not included in the protocol. We also conducted a systematic search on Google. We used truncation to increase the yield of the results. The search strategy was then applied across PubMed, Scopus, and WHO AIM databases using Boolean terms (mainly OR and AND ) to combine search results. Gray literature sources and institutional websites were searched using phrases containing ≥2 keywords such as “eHealth regulation,” “digital health regulatory standard,” “eHealth regulatory standard,” “digital health regulation,” “digital health policy,” “eHealth policy,” “digital health strategy,” and “eHealth strategy.” For Google search, we added the names of the country to the phrases (eg, “digital health regulation Nigeria”). The reference lists of the included documents were also searched, and key individuals at the MOHs, WHO Country Offices, and the WHO AFRO were contacted for related documents. When our search was conducted, the WHO Directory of eHealth policies website was unavailable, and the WHO AFRO Library was undergoing reconstruction. The search strategies for PubMed, Scopus, and WHO AIM are provided in Multimedia Appendix 2 . The search was conducted between 2005 and January 2024.
Study Selection
The search results obtained from PubMed, Scopus, and WHO AIM were imported into Mendeley (Elsevier) [ 39 ] to remove duplicates. The search conducted on OpenGrey did not yield any results, whereas relevant records obtained from institutional websites, repositories, and Google were downloaded as PDF copies and uploaded to Mendeley. After removing duplicates, the remaining results were imported into Covidence (Veritas Health Innovation) [ 40 ] for screening. Two reviewers (BAB and SI) applied the predefined eligibility criteria ( Textbox 1 ) to screen the documents in 2 stages (title and abstract or executive summary). All discrepancies were discussed until the reviewers reached agreement.
Inclusion criteria
- Type of document: Regulatory standards, guidance, policies, strategies, and committee or government reports that address regulatory issues related to the use of health apps for self-management
- Location: Documents developed and implemented in countries within sub-Saharan Africa
- Date of publication: Documents developed since 2005; the global efforts toward promoting standards to minimize variability and potential harms that could arise from poorly regulated use of digital health began in 2005 [ 33 ]
- Language: Documents written in English language and other official languages of sub-Saharan African countries (Portuguese and French)
Exclusion criteria
- Type of document: Standards, guidance, policies, strategies, and reports not related to regulation of health apps
- Location: Documents from countries outside sub-Saharan Africa
- Date of publication: Documents developed before 2005
- Language: None
Data Charting (Extraction)
Two reviewers (BAB and SI), in consultation with the other members of the research team, developed the data extraction forms using an iterative process that included piloting data extraction and refinement until a consensus was reached.
We proposed in the study protocol [ 30 ] that data extraction would be conducted by the 2 reviewers independently. However, owing to the approach adopted for data extraction (deductive qualitative content analysis), 1 reviewer, rather than 2, initially extracted data from the included documents, and any concerns were discussed with a second reviewer [ 41 ]. Unresolved issues were then discussed and resolved with a third reviewer in a steering group meeting.
Collating, Summarizing, and Reporting Results
To address the research questions (particularly question 2), we adopted a deductive descriptive qualitative content analysis method to analyze and report the key findings. The policy analysis framework by Walt and Gilson [ 42 ] was adapted and applied to ensure that there was a consistent way of organizing the key findings: (1) Content (which aspects are regulated and which aspects are not?)—these are the components that directly or indirectly address regulatory issues related to the use of health apps for self-management, including areas that have not been addressed. (2) Context (why are those aspects regulated?)—this characterizes the rationale indicated for addressing regulatory issues related to the use of health apps for self-management. (3) Process (how are the regulatory standards developed and implemented?)—this describes the methods or approaches used to develop and implement regulatory standards. (4) Actors (who are the regulatory standards targeted toward?)—these are the key actors targeted by the standards.
Using a deductive descriptive qualitative content analysis approach, we examined each included document to systematically identify texts for concepts, patterns, and other relevant information. We then categorized them under content, context, process, or actors in relation to regulating health apps for self-management. The findings under content and context were further organized based on 4 predefined regulatory categories or themes as documented in the literature, namely (1) technical and clinical safety [ 12 - 14 ], (2) data protection and security [ 15 , 16 ], (3) standards and interoperability [ 28 , 31 ], and (4) inclusion and equitable access [ 24 - 29 ].
To address the third research question, the Reporting Items for Stakeholder Analysis (RISA) tool [ 41 ] was used as a guide to group key stakeholders based on role categorization as recognized globally by the WHO, the ITU, and UNESCO [ 32 , 33 , 43 ].
Ethical Considerations
Primary data were not collected in this study. Therefore, no ethics approval was required.
Search Results
A total of 2900 records were obtained after removing duplicates. Although the literature search was conducted in English, the search also yielded documents written in French and Portuguese from the ICTworks repository [ 44 ]. Following the initial screening of the title and abstract (or executive summaries), 73 documents were retrieved for full-text assessment. After applying the inclusion criteria for the full-text assessment, 49 documents were found eligible for inclusion in the review.
The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram [ 45 ] showing the study selection process is presented in Figure 1 .

Types of Documents
On the basis of the inclusion criteria, 3 categories of documents were considered for this review, namely “stand-alone regulatory standards and guidance that potentially regulate health apps for self-management,” “national policies and strategies on digital health,” and “other national documents that relate to the regulation of health apps for self-management.” Table 1 presents the types of documents obtained for each country within sub-Saharan Africa.
Characteristics of the Included Documents
Stand-alone regulatory standards and guidance.
We identified and included 6 stand-alone regulatory standards [ 18 , 19 , 46 - 49 ] from 3 countries (Ethiopia, Kenya, and Nigeria). All 6 documents were written in English. The years of development ranged between 2013 and 2021, as indicated in Multimedia Appendix 3 . The years of implementation were not specifically stated.
Although none of the included regulatory standards were exclusively developed to regulate health apps for self-management, 3 of them (Kenya Standards and Guidelines for mHealth Systems [ 18 ], Kenya Standards and Guidelines for E-Health Systems Interoperability [ 47 ], and Health Sector Information and Communications Technology Standards and Guidelines [ 48 ]) provided concept and information relevant to the regulation of health apps and were included in the qualitative content analysis. The Kenya Standards and Guidelines for mHealth Systems [ 18 ] provides standards and guidelines on the design, development, and implementation of mobile health (mHealth) solutions to ensure they are interoperable, scalable, and sustainable. The Kenya Standards and Guidelines for E-Health Systems Interoperability [ 47 ] outlines the principles, requirements, and standards for eHealth systems interoperability in Kenya. The Health Sector Information and Communications Technology Standards and Guidelines [ 48 ] provide guidance and a consistent approach across the health sector in Kenya for establishing, acquiring, and maintaining current and future information systems and information and communications technology (ICT) infrastructure that foster interoperability across systems. These 3 documents are a good combination of regulatory standards and guidance that provide content and context relevant to the regulation of health apps in sub-Saharan Africa.
The remaining 3 standards (standard for electronic health record [EHR] system in Ethiopia [ 19 ], standards and guidelines for electronic medical record systems in Kenya [ 46 ], and the health information exchange standard operating procedure and guideline [ 49 ]) were exclusively developed for EHRs or electronic medical records. However, they contain information relevant for mapping stakeholders with potential roles in regulating health apps for supporting self-management.
National Policies and Strategies on Digital Health
This review includes 35 national policies and strategies that are related to digital health (potentially covering health apps) [ 50 - 84 ] from 31 countries written in English, French, and Portuguese (Benin, Botswana, Burkina Faso, Burundi, Cameroon, Comoros, Côte d’Ivoire [Ivory Coast], Democratic Republic of the Congo, Eswatini, Ethiopia, Gabon, Ghana, Kenya, Liberia, Madagascar, Malawi, Mali, Mauritius, Mozambique, Namibia, Niger, Nigeria, Rwanda, Senegal, Sierra Leone, South Africa, Tanzania, Togo, Uganda, Zambia, and Zimbabwe). Although the literature search was conducted in English, it also yielded documents written in French and Portuguese from the ICTworks repository. The years of development and implementation range between 2005 and 2030. Policies and strategies written in French and Portuguese were translated into English using Google Translate. Documents labeled as national development plans, strategic plans, and strategic development plans were considered as national strategies.
National policies and strategies do not offer specific standards or guidance, but rather outline the country’s vision, policy directions, and strategies for using digital technologies in health care. They provide useful information for identifying digital health stakeholders who can play a role in regulating health apps for self-management. For example, Nigeria has a separate National Digital Health Policy [ 72 ] and a National Digital Health Strategy [ 71 ]. Both documents were developed by building on the lessons learned from the end-term evaluation of the previous National Health ICT Strategic Framework [ 85 ]. They describe Nigeria’s renewed vision, mission, goals, objectives, and strategies for the development and implementation of digital health with the aim to improve the quality, efficiency, and effectiveness of health service delivery and health outcomes.
It is worth noting that for countries with >1 policy or strategy, we included only the most recent versions. For instance, as mentioned earlier, Nigeria now has both a national digital health policy and a national digital health strategy. These 2 documents supersede and thus replace the old National Health ICT Strategic Framework [ 86 ]. Details of included documents are presented in Multimedia Appendix 3 .
Other Related National Documents
We included 8 other documents [ 20 , 85 , 87 - 92 ] from 6 countries (Ethiopia, Kenya, Liberia, Nigeria, South Africa, and Tanzania) that did not fall under either stand-alone regulatory standards and guidance or national policies and strategies. These were mostly frameworks, road maps, and reports that potentially provide information relevant to the use of health apps. The years of development and implementation range from 2016 to 2025. These documents do not provide standards or guidance, but they contain information that can help map the digital health stakeholders that potentially play a role in regulating health apps for self-management. When multiple versions of a document exist, only the latest version was taken into consideration. Multimedia Appendix 3 provides details of the included documents.
Content: Aspects That Are Regulated and Aspects That Are Not
Technical and clinical safety.
Technical and clinical safety standards are required to prevent or minimize the harm that may arise from the use of the health ICT systems (including mHealth systems) as well as to improve the health outcomes and user satisfaction. As shown in Figure 2 , two subthemes were generated from included standards [ 18 , 47 , 48 ] as content under technical and clinical safety: v(1) guidance on system quality and (2) guidance on software or app development, acquisition, support, and maintenance.

Notably, 2 of the included standards [ 18 , 47 ] provide guidance on system quality to ensure the quality, security, reliability, performance, and maintenance of eHealth and mHealth systems. The Kenya Standards and Guidelines for E-Health Systems Interoperability [ 47 ] recommend the implementation of a data quality protocol to ensure that the data collection, collation, analysis, interpretation, dissemination, and use are managed in accordance with the quality standards. Similarly, the Kenya Standards and Guidelines for mHealth Systems [ 18 ] recommends the inclusion of the following requirements in the technical manual: (1) minimum hardware requirements that should incorporate the preferred hardware architecture, (2) minimum software requirements that should include the minimum version of the underlying operating system as well as acceptable versions of related software, and (3) a detailed list of software dependencies (external libraries) necessary for the system to function properly.
The included standards [ 18 , 48 ] cover guidance on software or app development, acquisition, support, and maintenance, which aim to ensure the efficiency and effectiveness of eHealth and mHealth systems. The Kenya Standards and Guidelines for mHealth Systems [ 18 ] recommends a technical manual to provide a detailed description of the system’s installation and maintenance processes for system administrators and implementers; a developer’s guide for software developers and programmers to provide them with an overview of the system, description of the software design methodologies, description of the system architecture, and technical design diagrams; and a user manual to aid users in understanding how the system works and how each feature operates; in addition, the technical manual contains instructions for operating the software; entering and updating data; and generating, saving, and printing reports.
Although the contents generated here provide guidance that is relevant to health apps, they are not specific to health apps. Moreover, there are no clear measures to enable individuals or organizations that use health apps to manage clinical risk appropriately.
Data Protection and Security
Data protection and security are crucial aspects of managing patient information, thus ensuring the confidentiality, integrity, and availability of data as well as the rights and interests of the patient. Two subthemes related to data protection and security are (1) security measures for adequate protection of patients’ digital records and (2) guidance on data exchange.
The included standards [ 18 , 48 ] provide security measures for eHealth or mHealth systems to ensure the adequate protection of digitally accessible patient records. These measures include authentication, accountability, identification, authorization, integrity, confidentiality, availability, security, administration, and audit. This will help to achieve confidentiality, integrity, availability, and nonrepudiation of patient data or health records. Additional levels of security such as data encryption are required when there is a need to store sensitive information on removable devices or media or outside the MOH premises.
The Kenya Standards and Guidelines for mHealth Systems [ 18 ] provide the following guidance on data exchange to ensure privacy: (1) anonymize client data as much as possible before they can be shared; (2) where possible, use pseudonyms for the client data before they can be shared; (3) aggregate client data before they can be shared to reduce possibilities of tracing the data back to the client; and (4) minimize data so that access is available only to the data set required for that particular use. With regard to privacy rules, the Kenya Standards and Guidelines for E-Health Systems Interoperability [ 47 ] propose that a notice of privacy practices should be given to patients describing how their information may be used or shared while also specifying their legal rights.
Standards and Interoperability
Standards and interoperability are essential concepts in the field of IT, especially for systems that need to communicate and exchange data, as seen in the use of health apps for self-management. Two subthemes related to standards and interoperability are (1) interoperability as a basic requirement and (2) minimum standards to enable integration.
All the regulatory standards [ 18 , 47 , 48 ] highlight the importance of having interoperability as a basic requirement when selecting software products or services for use within the health system. This facilitates interaction across systems. For instance, to facilitate seamless interaction between mHealth systems and primary information systems for data capture, reporting, and decision support in various domains of the health system, the Kenya Standards and Guidelines for mHealth Systems [ 18 ] recommends the incorporation of at least 3 types of interoperability, namely, technical interoperability, semantic interoperability, and process interoperability.
Furthermore, 2 regulatory standards [ 18 , 47 ] proposed minimum interoperability standards to enable the integration of services and data exchange between various systems in health care. For instance, the Kenya Standards and Guidelines for mHealth Systems [ 18 ] suggests standards (for interoperability) for mHealth systems that are consistent with the recommendations in internationally accepted standards. They include the following: (1) clinical messaging—ensuring mHealth systems conform to Health Level 7 (HL7) version 3 standards and corresponding implementation guideline; (2) clinical terminology—ensuring terminologies and classifications for clinical concepts (eg, International Classification of Diseases, tenth revision—for diseases; Systemized Nomenclature of Medicine—for clinical data coding; Logical Observation Identifiers Names and Codes—for laboratories; and RxNorm—for Pharmacies); (3) the mHealth system must use the latest versions of international standards, such as HL7 Clinical Document Architecture for electronic sharing of clinical documents; (4) concepts—mHealth systems will use the idea of “concepts” so that information can be transmitted between systems without losing meaning or context, and HL7 Reference Implementation Model or other appropriate standards are recommended for implementing concepts; (5) architecture—to develop mHealth systems, developers should define the system architecture that should include data elements and business logic. Furthermore, to define how mHealth systems interact with other systems, developers of mHealth solutions must provide application programming interfaces. FHIR is the preferred application programming interface interoperability standard.
Inclusion and Equitable Access
Inclusion and equitable access are essential principles to ensure that health apps are culturally appropriate and relevant and accessible to everyone, regardless of gender, ethnicity, location, or economic status.
All the included regulatory standards [ 18 , 47 , 48 ] indicate that they were developed based on a combination of participatory and consultative approaches involving multiple actors or stakeholders, thus promoting inclusion. However, there are no specific measures or guidance to ensure adequate engagement and representation of all the relevant stakeholders and to sustain that engagement.
The Kenya Standards and Guidelines for mHealth Systems [ 18 ] proposes the following systems attributes to ensure equitable access to mHealth services at all times and from anywhere: (1) allocation of adequate storage and bandwidth capacity; (2) fast response time; (3) fast recovery capabilities; (4) performance monitoring; (5) business continuity processes, for example, backups; and (6) redundant sites and links. Furthermore, the Kenya Standards and Guidelines for mHealth Systems [ 18 ] prescribes the following metrics for measuring system availability: (1) downtime per year, (2) mean time between failure, (3) mean time to repair, and (4) failure in time.
Although the abovementioned systems attributes and metrics for measuring system availability are important, the included standards do not offer any concrete guidance or model for achieving a sustainable funding mechanism for health apps to ensure that they are readily available and accessible to those who need them.
Context: Reasons Why Those Aspects Are Regulated
The 3 standards [ 18 , 47 , 48 ] were developed to address unsafe, isolated, and inconsistent implementation. The Health Sector ICT Standards and Guidelines [ 48 ] suggest that although there has been a lot of ICT investment in the health sector leading to improvement in service delivery and information exchange, there remains the challenge of inconsistency in ICT implementation and harmonization of the health sector system requirements. Hence, there is a need to adopt global best practices for software development, acquisition, support, and maintenance by the MOH. In addition, the Kenya Standards and Guidelines for mHealth Systems [ 18 ] indicates that standards and guidelines are necessary to ensure a consistent approach to the development of ICT systems. Similarly, the Kenya Standards and Guidelines for E-Health Systems Interoperability [ 47 ] recognize the need to ensure that the processes of collecting, collating, analyzing, interpreting, disseminating, and using data are consistent with data quality standards.
To build mutual trust and maximize the benefits of eHealth information exchange, the Kenya Standards and Guidelines for E-Health Systems Interoperability [ 47 ] reiterate that as health data are constantly being exchanged across health information systems, robust security standards are required to maintain their integrity and confidentiality. This will build the trust of service users and consequently help to maximize the benefits of eHealth information exchange such as in self-management.
Two of the included regulatory standards [ 47 , 48 ] indicate that the context for standards and interoperability was (1) to address poor coordination, duplication of efforts, and inefficient use of resources and (2) to promote the integration of ICT systems.
The Kenya Standards and Guidelines for E-Health Systems Interoperability [ 47 ] acknowledge that the absence of interoperability standards over the years has led to the duplication of efforts and the inefficient use of ICT resources in health care. Now that ICT has become increasingly relevant in improving efficiency in health service delivery, the Kenya MOH recognizes the need to adopt a standardized approach, hence the development of interoperability standards for eHealth systems. In addition, the Health Sector ICT Standards and Guidelines [ 48 ] emphasize the relevance of interoperability as a requirement for addressing the inconsistency in implementing ICT in the health sector.
The Health Sector ICT Standards and Guidelines [ 48 ] consider “integration of ICT systems” as one of its key guiding principles, acknowledging the lack of information systems integration as a challenge experienced by ICT services across Kenya.
The contexts for inclusion and equitable access as generated from included standards [ 18 , 47 , 48 ] were (1) to promote inclusion and (2) to promote equitable access to services.
To promote inclusion, the standards [ 18 , 47 , 48 ] highlight the importance of involving and engaging multiple actors and stakeholders during the development process. However, no emphasis was placed on the need to sustain stakeholder engagement during the implementation process.
Pertaining to equitable access, the Kenya Standards and Guidelines for mHealth Systems [ 18 ] acknowledges that the public health care system is largely unavailable to most of the population in many developing countries because of geographical location, resource constraints, inefficiencies, and lack of awareness. Hence, it recognizes the importance of ensuring that mHealth services are always accessible by users and from anywhere as well as the need to put in place mechanisms to make this happen.
Process: How the Regulations Are Developed and Implemented
Two themes were generated from the included standards: development and implementation processes [ 18 , 47 , 48 ].
Development Process
All the included standards [ 18 , 47 , 48 ] indicate that they were developed through a participatory process and in consultation with a range of subject experts and interest groups. In addition, the standards [ 18 , 47 , 48 ] adopted a multisectoral approach to engage health-related stakeholders from government ministries or agencies and development partners and a range of subject experts and interest groups. It has also been reported that these standards [ 18 , 47 , 48 ] were developed based on international best practices and with reference to international standards. However, there is no indication that a stakeholder engagement strategy was adopted to sustain the engagement of stakeholders through the entire development and implementation process.
Implementation Process
The 3 regulatory standards [ 18 , 47 , 48 ] identify the key requirements to ensure effective implementation of IT services in the health sector. These are (1) legal authority, (2) coordination, (3) building capacity, and (4) monitoring and evaluation.
The included standards [ 18 , 47 , 48 ] were established based on the legal provisions enshrined in the health and other related acts and laws of Kenya as well as the relevant policies and strategies. Hence, it is expected that their implementation will comply with and be backed by those legal provisions. For example, the Health Sector ICT Standards and Guidelines [ 48 ] indicate that its implementation will be supported by the authority from the Kenya Communications Act 2009, E-Government Strategy, and National ICT Policy. Similarly, the Kenya Standards and Guidelines for mHealth Systems [ 18 ] asserts that it will be implemented by complying with existing and relevant national policies, legal frameworks, strategies, and standards, including the Health Information Policy, ICT Standards, and System Interoperability Principles.
The included standards [ 18 , 47 , 48 ] report that the implementation of regulations will require robust coordination mechanisms. For instance, the Health Sector ICT Standards and Guidelines [ 48 ] indicate that, as the Ministry’s ICT resource manager, the principal secretary (also the head of ICT), in collaboration with the ICT Governance Committee, is responsible for coordinating the implementation of the standard. The ICT Governance Committee comprises representatives from the heads of departments and ICT development partners in the health sector. The committee’s responsibilities include overseeing, enforcing, and reviewing standards as well as initiating ICT projects.
The Health Sector ICT Standards and Guidelines [ 48 ] highlight the need for capacity building or training of the MOH staff and stakeholders who are the primary users of the Ministry’s ICT services. This will enhance their capacity to implement the guidelines provided in the document in line with the ministry’s human resource development policies, regulations, and rules. However, it is acknowledged that building capacity for health ICT is a challenge given that there is low adoption of ICT among health providers, and ICT is not routinely included in the course content of most training programs. The Kenya Standards and Guidelines for mHealth Systems [ 18 ] listed the “number of mHealth practitioners trained on the standards and guidelines” as one of the indicators for monitoring and evaluating mHealth interventions.
The Health Sector ICT Standards and Guidelines [ 48 ] assert that monitoring and evaluation is an essential role of the MOH to ensure efficiency, accountability, and transparency throughout the implementation period. It further stresses that all those who use the Ministry’s ICT services are required to adhere to the provisions in the standard as the MOH will carry out quarterly monitoring exercises on the use of the standard to ensure compliance based on clear indicators. Furthermore, the ICT Governance Committee will periodically review and amend the standard to keep it relevant and effective. Similarly, the Kenya Standards and Guidelines for mHealth Systems [ 18 ] establishes the following key indicators for effectively monitoring and evaluating the implementation of the standards and guidelines: (1) the number of counties in which the MOH has disseminated the standards and guidelines, (2) the number of counties successfully implementing the standards and guidelines, (3) the number of mHealth practitioners trained on the standards and guidelines, (4) the number of mHealth practitioners accessing the standards and guidelines, (5) the number of mHealth practitioners who correctly understand the standards and guidelines, (6) the number of stakeholders who adhere to the standards and guidelines, (7) the number of mHealth systems that follow the required development steps, and (8) the number of mHealth practitioners who have implemented their systems by using the standards and guidelines. In addition, the Kenya Standards and Guidelines for mHealth Systems [ 18 ] indicates that the outlined standards will be reviewed every 3 years to ensure they are up to date with new changes including the changes in policies and systems upgrades.
Although all the abovementioned indicators are relevant, the implementation process is not explicit on the approach for regulating health apps and ensuring compliance with regulatory standards and guidance.
Actors: Those the Regulations Are Targeted at
The included standards [ 18 , 47 , 48 ] identified 2 main groups of actors for whom the regulations and guidance were targeted. They included (1) those who provide digital health services and (2) those who use the ICT infrastructure of the MOH.
Two of the standards [ 47 , 48 ] indicated that the regulations should be implemented by all individuals and organizations that provide ICT-related health care services to the public. Similarly, the Health Sector ICT Standards and Guidelines [ 48 ] state that all those who access or use the MOH ICT infrastructure are expected to adhere to the guidelines outlined in the document.
Mapping of Stakeholders
To address the third research question, we conducted a stakeholder mapping guided by the RISA tool [ 41 ].
A total of 11 categories of key stakeholders were identified from all 49 included documents (6 stand-alone regulatory standards and guidance, 35 national policies or strategies, and 8 other related documents). These categories are consistent with the digital health stakeholders recognized by the WHO, ITU, and UNESCO [ 32 , 33 , 43 ]. Table 2 presents the mapping of stakeholders according to their role categorization. A more detailed table with a potential role description with regard to regulating health apps for self-management is presented in Multimedia Appendix 4 .
a WHO: World Health Organization.
This paper presents the findings of a scoping review of regulatory standards and guidance for the use of health apps for self-management in sub-Saharan Africa. To the best of our knowledge, this is the first study that attempted to identify and assess the extent to which regulatory standards and guidance regulate and guide the use of health apps for self-management in sub-Saharan Africa as well as map out the key stakeholders and their potential roles.
Our findings reveal that only 1 country (Kenya) in sub-Saharan Africa currently has national regulatory standards that could potentially regulate the use of health apps for self-management. The included standards failed to adequately address adequate attention to inclusion and equitable access. This is concerning given the growing need to promote the adoption of culturally appropriate and relevant health apps and to ensure that they are available to those who need them regardless of gender, ethnicity, geographical location, or financial status [ 24 - 29 ]. Consequently, this review provides insights into the regulation of health apps for self-management in sub-Saharan Africa, which needs to be given more attention if the potential of these apps is to be harnessed in the region.
Principal Findings
We identified 49 documents from 31 countries in sub-Saharan Africa. Although none of the included standards provided a specific set of regulations on health apps for self-management, we identified 3 standards [ 18 , 47 , 48 ] that provided relevant information regarding the regulation of health apps. The included national policies and strategies, in contrast, only outline the goals and commitments made by national governments to promote the adoption of digital technologies in the health sector and the plans and paths set forth to achieve these goals. However, the information they provided was relevant for identifying and mapping digital health stakeholders who potentially have vital roles in regulating the use of health apps for self-management.
The policy analysis framework (content, context, process, and actors) [ 42 ] was adapted and applied to organize the key findings. The content covered the following areas: guidance on systems quality; guidance on software and app development, acquisition, support, and maintenance; security measures for adequate protection of patients’ digital records; guidance on data exchange; interoperability as a basic requirement; minimum standards to enable integration; involvement and engagement of relevant stakeholders; and system attributes for equitable access to services. Meanwhile, the context was to address unsafe, isolated, and inconsistent implementation; to build mutual trust and maximize the benefits of eHealth information exchange; to address poor coordination, duplication of efforts, and inefficient use of resources; to promote the integration of ICT systems; and to promote inclusion and equitable access to services. The process involved the development process (which covers participatory and consultative processes and multisectoral approach, with reference to international standards and best practices) and the implementation process (which covers legal authority, coordination, capacity building, and monitoring and evaluation). The targeted actors were those who provided digital health services and those who used the ICT infrastructure of the MOH.
Furthermore, key stakeholders with potential roles in regulating health apps for self-management were identified. They include the government, regulatory bodies, funders, intergovernmental and nongovernmental organizations, academia, and the health care community.
Implications of the Study Findings for Practice
Regulatory standards and guidance act as a bridge between technological innovation and its safe and effective use in health care. They ensure that while technology continues to advance, the safety and trust of patients are never compromised. Among the plethora of health apps on the market, the over-the-counter, nonregulated apps such as wellness and fitness apps are the most mainstream [ 93 - 95 ]. On the other side of the spectrum, there are regulated health apps that are classified under medical devices or software as medical device products [ 94 , 95 ]. Some of these are prescription-only apps, such as digital therapeutics (DTx) apps for managing substance dependence [ 95 , 96 ].
Although some high-income countries have made significant strides in ensuring the safety, effectiveness, and accessibility of health apps, the journey has indeed not been without challenges and hurdles. Sub-Saharan Africa, although dealing with its own unique set of challenges, has the opportunity to learn from the experiences of these high-income countries. This could potentially allow the region to bypass some of the hurdles encountered by high-income countries in their journeys.
Technical and clinical safety are essential requirements that health apps must meet before they can be considered for use for self-management to minimize the risk of harm to patients. It is well documented that health apps that function poorly pose a serious threat to the safety of patients. An example illustrating how health apps used for self-management can threaten patient safety is evident in a study [ 12 ]. This study [ 12 ] revealed that widely used health apps designed to calculate and estimate insulin doses could endanger patients by providing incorrect or inappropriate dose recommendations. Similarly, 2 successive studies that assessed the contents and tools of apps for asthma discovered that none of the apps in the first study offered comprehensive information or adequate tools for asthma self-management, whereas the follow-up study, which was conducted 2 years later, showed a 2-fold increase in the number of asthma apps, yet there was no improvement in the content and tools offered by the newer apps. In fact, many apps recommended self-management procedures that were not supported by evidence [ 13 , 14 ]. Accordingly, some health apps that support the self-management of long-term conditions do not adhere to evidence-based guidelines and are unresponsive to the evolving health needs of patients.
Although the context of included regulatory standards with regard to technical and clinical safety was to address unsafe, isolated, and inconsistent implementation, the guidance provided by these regulatory standards is not specific to health apps, and they do not provide appropriate guidance and standards for health organizations and other key stakeholders to establish a framework for managing the clinical risks associated with deploying and implementing self-management health apps. Considering the rapid advancements in digital health (including artificial intelligence [AI] or machine learning and big data), health apps will increasingly play a crucial role in supporting self-management through digitally enabled care pathways that will improve personalized care and health outcomes [ 97 , 98 ]. Therefore, it is imperative to ensure the technical reliability and clinical safety of health apps for self-management through robust regulatory standards and guidance. For instance, a guide on the criteria for health app assessment, developed by the UK government, includes technical stability and clinical safety as criteria for deciding whether health apps should be considered for use in the National Health Service (NHS) [ 99 ]. In addition, medical device apps are required to conform to the NHS clinical risk management standards as part of the clinical safety requirements [ 99 , 100 ]. In the event of any concerns regarding the safety of a medical device app, the Yellow Card reporting system can be used by a responsible clinical safety officer or any other individual to notify the Medicines and Healthcare products Regulatory Agency (MHRA) [ 101 , 102 ].
To adequately manage patient information when health apps are used for self-management, data protection and security standards and guidance are required. They guarantee that data are kept and handled safely and responsibly within the provisions of the law and that patients’ rights and interests are respected.
There have been ongoing concerns about compliance with ethical standards, the principles of confidentiality of information, and data privacy. For example, an assessment of apps that had previously been endorsed by the former UK NHS Apps Library revealed substantial gaps in compliance with data protection principles regarding the collection, storage, and transmission of personal information. This has raised a fundamental concern about the credibility of developer disclosures and whether these disclosures can be trusted by certification programs [ 15 ]. A study assessed the privacy practices of the 36 most popular apps for depression and smoking cessation for Android and iOS in the United States and Australia [ 16 ]. The findings revealed that although only 69% (25/36) of the apps included a privacy policy, 92% (33/36) of the apps shared data with a third party, and only 92% (23/25 with privacy policy) of the apps disclosed sharing data with a third party in their policy. Although 81% (29/36) of the apps shared data with Google and Facebook for the purposes of advertising, marketing, or analytics, only 43% (12/28) of the apps that shared data with Google and 50% (6/12) of the apps that shared data with Facebook disclosed this in their policy [ 16 ].
In this regard, health app developers and providers in the United Kingdom are required to conduct a data protection risk assessment before they launch or update their apps to ensure compliance with the United Kingdom General Data Protection Regulation (GDPR) and other relevant regulations, including the Data Protection Act 2018 [ 103 ]. By conducting a data protection risk assessment, health app developers and providers can demonstrate that they are accountable; they respect the privacy and dignity of their users; and that they deliver safe, effective, and ethical solutions [ 104 ].
Health apps are expected to play an increasingly important role in supporting self-management. However, this ambition can only be achieved if citizens trust that these apps are collecting and analyzing data safely and in accordance with robust regulatory standards and guidance. It is also crucial that these apps provide reliable information that clinicians can act on [ 98 ]. The context of the standards included in this study regarding data protection and security was to build mutual trust and maximize the benefits of eHealth information exchange. Trust is a key factor in the successful adoption and use of health apps, and transparency in data handling and clinical decision-making is essential to build and maintain that trust. This is also paramount for the widespread acceptance and impact of health apps on health care outcomes in sub-Saharan Africa.
We acknowledge the existence of numerous national laws related to data protection and security outside the health sector. Hence, guidelines that link these legislations together must be provided to ensure compliance with all relevant laws and guidance when using patient data. An example of how to achieve this is the United Kingdome’s guide to good practice for digital and data-driven health technologies that provides guidelines on how to abide by the laws and principles that govern data security and protection in the United Kingdom, including the GDPR, Data Protection Act 2018, and Caldicott Principles [ 105 ].
Standards and interoperability are essential for effectively developing, deploying, and implementing health apps to support self-management in sub-Saharan Africa. Interoperability is the ability of different systems, devices, or applications to communicate and exchange data with each other in a coordinated manner, thus providing timely and seamless portable information across organizational, regional, and national boundaries and optimizing both individual and population health [ 106 ]. In the same vein, standards enable interoperability between systems or devices through a common language and a common set of expectations [ 106 ].
Interoperability is crucial in improving the quality, safety, and efficiency of care delivery as well as empowering patients and providers with access to relevant and timely information [ 99 ]. One of the most widely used and accepted interoperability standards for health care data exchange is FHIR [ 106 , 107 ]. FHIR is a global industry standard developed by HL7 International. FHIR is designed to be quick to learn and implement and to support a variety of use cases, including self-management [ 108 ]. By using apps that are based on an FHIR standard, patients can benefit from data analytics that show how their health data relate to their chronic conditions or wellness goals [ 109 ]. They could also access all their health information from one place, even if they visit different health professionals who use different electronic medical records or EHR, thus promoting integrated care [ 28 , 31 , 33 , 109 - 115 ]. As a result, patient care can easily be coordinated.
The context of the included regulatory standards with regard to standards and interoperability was to address poor coordination, duplication of efforts, and inefficient use of resources and to promote the integration of ICT systems. However, in sub-Saharan Africa, there are many challenges and barriers to the adoption and implementation of interoperability standards, such as the lack of awareness or knowledge of the benefits and requirements of interoperability standards among stakeholders; lack of incentives or regulations to encourage or enforce the adoption of interoperability standards by app developers and vendors; lack of resources or capacity to implement interoperability standards, including technical expertise, infrastructure, funding, or governance; and lack of alignment or coordination among the different actors and initiatives involved in developing, deploying, and implementing the digital health interventions [ 30 , 116 - 119 ]. To address these challenges, some possible solutions may include raising awareness and education on the importance and value of interoperability standards for health apps among all relevant actors; developing and implementing policies and guidelines that promote or mandate the use of interoperability standards by app developers and vendors; providing technical assistance and support for app developers and vendors to adopt and implement interoperability standards, such as tools, frameworks, testing, certification, or accreditation; and establishing and strengthening collaboration and coordination among the different stakeholders and initiatives involved in health app development, deployment, and implementation in sub-Saharan Africa. In addition, the Digital Health Platform Handbook, a toolkit developed by the collaborative efforts of the WHO and ITU [ 120 ], can help countries in sub-Saharan Africa to develop and implement digital health platforms as the underlying infrastructure for interoperable and integrated national digital health systems. The digital health platform is a system-wide approach to developing digital health solutions with the aim to overcome the problems of siloed, vertical, and isolated applications and systems that hamper data management, innovation, efficiency, and impact in the health sector.
Inclusion and equitable access are crucial to ensuring that health apps and related services are culturally appropriate and relevant as well as accessible to all who need them, regardless of gender, ethnicity, geographical location, ability, or financial status [ 24 - 29 ]. This is the key to promoting a “sense of belonging” and “ownership” and thus underscoring the importance of stakeholder mapping and involvement or engagement through the development and implementation process [ 22 ].
In this study, the included regulatory standards demonstrate the importance of inclusion by adopting both a participatory and consultative approach involving multiple stakeholders from different sectors. However, the standards do not provide clear guidance to ensure the adequate participation and sustained engagement of all relevant stakeholders. The lack of concise guidance to ensure the adequate participation and engagement of all relevant stakeholders, especially the susceptible and disadvantaged groups, can increase the risk of tokenistic tendencies, which can undermine the cultural appropriateness of health apps [ 25 , 121 ]. Some susceptible groups, such as women and people with low socioeconomic status, may face additional barriers to accessing and using health apps, such as lack of digital literacy, privacy concerns, cultural norms, or stigma [ 25 ]. Similarly, the cost of developing, maintaining, and updating health apps may not be covered by public or private health insurance schemes, which could limit their affordability and availability for low-income or uninsured populations [ 95 ]. However, there is no specific guidance or model for an effective funding mechanism for health apps in the included regulatory standards.
To address these challenges and ensure equitable access to health apps for self-management in sub-Saharan Africa, possible measures may include developing policies and regulations that support integrating health app interventions into existing health systems and financing mechanisms and engaging with stakeholders from different sectors and backgrounds (including health professionals, patients, communities, governments, civil society, academia, and industry) to co-develop and co-implement frameworks or models that promote the use of health apps for self-management in ways that are responsive to the local context and needs. Moreover, establishing regulations that provide appropriate financing or reimbursement options will reduce the risk of developers of good quality health apps turning to data mining for revenue, thus increasing privacy concerns [ 95 ]. For instance, in Germany, the reimbursement of health apps classified as medical devices (Digitale Gesundheitsanwendungen) was introduced in 2021 under the statutory health insurance [ 122 , 123 ]. When a medical device is prescribed by a physician or a physiotherapist, the manufacturer must submit an application to the German Federal Institute for Drugs and Medical Devices (Bundesinstitut für Arzneimittel und Medizinprodukte) for approval [ 123 ]. The Federal Association of the Statutory Health Insurance Funds (Spitzenverband Bund der Krankenkassen) determines and negotiates the reimbursement thresholds following approval. However, the manufacturer must demonstrate that the app is safe, functional, and of good quality; complies with data protection requirements; and benefits patient care [ 123 ].
The process of regulating health apps essentially involves the development and implementation of regulatory standards and guidance. According to our study, the development process comprises a participatory and consultative process, a multisectoral approach, and a reference to international standards and best practices. In contrast, the implementation process is ongoing and requires appropriate legal authority, coordination, capacity building, and monitoring and evaluation.
We recognize that health apps can be accessed and used by patients from different parts of the world, and this means that countries need to carefully consider whether health apps that are accessed and used by their citizens meet the national or regional legal and ethical requirements, including their cultural and linguistic needs [ 23 ]. For countries in sub-Saharan Africa, a cross-border or regional collaboration between national legal authorities through the coordination of agencies such as the African Medicines Regulatory Harmonization (AMRH) may help to ensure that health apps built for the region are safe, effective, and user-friendly for everyone, considering the contextual differences of the countries [ 23 ]. For instance, all medical device companies that want to sell their products in the European market must obtain a Conformité Européenne (CE) mark for their devices, which indicates that they meet the legal requirements and can be freely circulated within the European Union [ 124 ]. Although the European Union member states regulate medical devices, the European Medicines Agency is involved in the regulatory process.
The regulation of health apps is extremely complex and involves a wide range of stakeholders. Therefore, a robust coordination mechanism is essential to reduce the risk of fragmentation and duplication of efforts and to promote the efficient use of resources. Most countries in sub-Saharan Africa have units in health ministries that coordinate and oversee the regulation of medical products. These units should be autonomous, full-fledged departments with legal authority (boards or commissions) to ensure independent, transparent, and accountable decision-making, but this is often not the case [ 125 ]. These units are recognized by the national authorities as regulators (eg, the National Medicines Regulatory Authority [NMRA]) [ 126 ]. Such organizational structures hinder the effectiveness of the national regulatory authorities in fulfilling their mandate and prevent the establishment of quality management systems to ensure transparent and accountable decision-making [ 125 ].
Furthermore, Essén et al [ 23 ] analyzed health app policy or regulation in 9 high-income countries (Sweden, Norway, Denmark, Netherlands, Belgium, Germany, England, the United States, and Singapore) and found that most of these countries adopted centralized approaches to app evaluation. Although centralized approaches might have advantages over self-evaluation, they may create bottlenecks and limit the availability of high-quality health apps for users. As suggested by Essén et al [ 23 ], a decentralized approach, such as the accreditation of evaluation agencies, maybe a worthwhile solution. However, this will require adequate coordination to ensure the consistency and reliability of the evaluation criteria and methods across different agencies as well as the transparency and accountability of the accreditation process. A possible way to achieve this is to adopt a common framework that can guide the evaluation and accreditation of health apps.
Similarly, the postmarket surveillance (PMS) system, which is a new regulation for medical devices in Europe, is a process of collecting and analyzing data on medical devices after they have been launched into the market to ensure their safety and performance and to identify any problems or need for improvements [ 127 , 128 ]. The PMS system is important because premarket data, which are obtained from testing a medical device before it is launched, have limitations in capturing the long-term performance and risks of the device [ 128 ]. Currently, the PMS system does not cover fitness and wellness apps, which are commonly used in self-management. Hence, Yu [ 93 ] proposed that the PMS system should also be applied to DHTs, such as fitness and wellness apps. They argue that the postmarket data would help regulators periodically review and adjust the regulatory standards for these groups of health apps based on their risks and benefits.
Drawing on the experience of the United Kingdom, it can be clearly demonstrated that the regulation of health apps is a complex, a multifaceted, and an evolving process that involves different regulators and criteria depending on the nature and function of the app. For instance, a centralized NHS Apps Library was launched as a beta site in April 2017 to provide patients with a collection of trusted and easy-to-use digital health tools [ 129 ]. The library provided access to a range of health apps that were reviewed and approved by the NHS, including apps that could help patients manage conditions such as diabetes, mental health, and chronic obstructive pulmonary disease [ 130 ]. However, the library was closed in December 2021 [ 131 ]. Although no reason for the closure was provided on the website, it is likely because of persistent concerns regarding the safety of patients and data privacy involving multiple apps including those listed in the library [ 12 , 14 - 16 , 131 , 132 ]. The NHS App was introduced in January 2019 before the closure of the NHS Apps Library to serve as the gateway for accessing NHS services including ordering repeat prescriptions and booking or managing appointments [ 133 ].
Furthermore, the United Kingdom Health Security Agency, formerly known as Public Health England, issued a guidance on criteria for health app assessment in October 2017 [ 99 ]. The purpose of this guidance was to ensure that all health apps built for the UK population work well and provide clear information about their functions, benefits, and intended outcomes for patients and health care professionals. On the basis of this guidance, those intending to build an app are required to conform to certain regulations before being considered for the app assessment process. The 2 main regulations are the medical device regulation and the Care Quality Commission (CQC) registration. Apps that are considered as medical devices must register with the MHRA and have a CE mark. Apps providing health or social care that fit into 1 of 14 regulated activities are required to register with the CQC before they can be assessed [ 134 ]. CQC is an independent regulator of health and social care services in England.
Similarly, the Organisation for the Review of Care and Health Apps (ORCHA) is a UK-based organization that independently evaluates and distributes health apps. It provides services such as app review, accreditation, curation, and recommendation within the United Kingdom and across the world [ 135 ]. ORCHA also enables organizations (including the NHS) to build a decentralized web-based digital health library of consumer-friendly over-the-counter apps [ 135 - 137 ]. These apps are continuously assessed by ORCHA against the standards and regulations in clinical and professional assurance, data quality and privacy, and usability and accessibility [ 137 ].
In addition, the Digital Technology Assessment Criteria (DTAC) were introduced in beta in October 2020, and its first official version was subsequently launched in February 2021 [ 138 ]. The DTAC plays a crucial role in ensuring that digital health tools meet the necessary standards in areas such as clinical safety, data protection, technical security, interoperability, usability, and accessibility. By serving as the national baseline criteria for DHTs in the NHS and social care, it provides a valuable framework for health care organizations during procurement. It also offers guidance for developers on the expectations for their digital technologies within the NHS and social care. This is an example of how a harmonized framework can help ensure the quality and safety of DHTs, including health apps.
In addition, the National Institute for Health and Care Excellence Evidence Standards Framework is a set of evidence standards for a wide range of DHTs designed to help evaluators and decision makers in the health care system to consistently identify DHTs that are likely to offer benefits to the users and the health care system [ 139 ]. The Evidence Standards Framework was first published in March 2019 and is ideally used before DHTs (including health apps) are considered for commissioning or procurement by the NHS [ 140 ]. It is a crucial tool for ensuring that DHTs are clinically effective and offer value to the health and care system in the United Kingdom. In August 2022, the framework was updated to include AI and data-driven technologies with adaptive algorithms [ 140 ].
Furthermore, DTx apps, which are a type of medical device, are not allowed into the UK market unless they comply with the UK GDPR and meet the requirements of DTAC. In addition, they must bear the CE or UK Conformity Assessed marks [ 141 ]. This means that DTx apps must demonstrate their safety and efficacy through clinical trials and comply with the relevant regulations for data protection and quality standards as regulated by the MHRA. DTx products are also recognized as DHTs under the National Institute for Health and Care Excellence Evidence Standards Framework [ 142 ]. DTx incorporates software to treat, prevent, or manage specific diseases or conditions [ 143 , 144 ]. The fact that DTx products typically focus on a narrow clinical indication and generate evidence of clinical efficacy underscores their potential to make a substantial contribution to self-management and health care delivery in general. The increasing recognition of the role of DTx in patient care by regulators is also noteworthy, and the creation of regulatory and reimbursement pathways for approved apps further enables DTx products to continue to play an important role in impacting health care delivery [ 1 , 143 ]. This is a testament to the potential of regulated health apps to revolutionize health care and improve patient outcomes.
Among the many lessons to learn from the experience of the United Kingdom is that the regulation of health apps must evolve to keep pace with advances in DHTs and adapt to the changing needs and demands of digital health. Moreover, efforts are being made to streamline the multifaceted approaches to simplify app regulation and access in the United Kingdom [ 23 ]. Therefore, a robust and dynamic coordination mechanism, along with political will, skilled personnel, reliable funding, and a robust framework for monitoring and evaluating progress and aligning key performance indicators, is essential for countries in sub-Saharan Africa to keep pace with the advancement in the regulation of health apps. There is also a need to strengthen collaboration and ensure regulatory harmonization among national regulatory authorities and continental bodies such as the regional economic communities, AMRH, and the WHO AFRO [ 126 ].
Capacity building and monitoring and evaluation are important factors for ensuring effective regulation of health apps given the complex nature of the process. The regulation of medical products (including health apps) in sub-Saharan Africa generally includes licensing and accreditation, evaluation, inspection, quality control, information dissemination and promotion, and monitoring of adverse events [ 125 ]. Therefore, high-level skills as well as effective monitoring and evaluation will be required to ensure the success of the process. For most countries in sub-Saharan Africa, the NMRA is responsible for coordinating and overseeing the regulatory system of medical products [ 125 , 126 ]. However, in most cases, NMRAs are unable to perform the core regulatory functions expected of them [ 145 ]. More than 90% of African countries have limited or no capacity to regulate medical products, with only 7% having moderately developed capabilities [ 145 ]. The lack of effective NMRAs in Africa exposes the citizens to potential harm by allowing unsafe, low-quality, and fake medical products to circulate and be used [ 145 ].
Although it is the responsibility of governments to establish functional regulatory systems and ensure effective monitoring and evaluation of the regulatory process, the involvement of international and continental organizations to support sub-Saharan African countries improve the regulatory capacity of their national regulatory agencies would be extremely beneficial. For instance, the African Medicines Agency (AMA) was established in November 2019 as a treaty adopted by the African Union Member States to help address the concerns arising from weak regulatory systems on the continent. At present, 37 countries have signed the AMA treaty, including 26 countries that have ratified it [ 146 ]. The main objective of the AMA is to enhance the capacity of States Parties and regional economic communities to regulate medical products to improve the quality, safety, and efficacy of medical products on the continent [ 147 ]. The AMA, in collaboration with other existing capacity building initiatives or organizations, such as the WHO Global Initiative on Digital Health, ITU, AMRH, WHO AFRO, and United Nations Children’s Fund, can assist sub-Saharan African countries in aligning their regulatory requirements with available resources and support them to acquire the necessary tools and skills to build effective and sustainable regulatory systems for health apps. This can be achieved by adopting a decentralized approach to engage a network of technical experts across the African Union similar to the model of the European Medicines Agency [ 148 ].
Actors or Stakeholders
The regulation of health apps often requires working with a wide range of actors or stakeholders. However, in this review, we identified only 2 main actor groups (those who provide digital health services and those who use the ICT infrastructure of the health ministry). These are the groups that are targeted by the included regulatory standards.
From a broader perspective, 12 categories of stakeholders according to their potential role in regulating health apps for the self-management were mapped in this study. The potential contribution of these stakeholders to the regulation of health apps for self-management in sub-Saharan Africa not only depends on their roles and responsibilities but also on their interests, needs, expectations, and influence [ 41 , 149 - 151 ]. Thus, a robust stakeholder analysis is paramount as it can help define the scope of the regulatory process, prioritize the requirements, manage the expectations, and ensure the engagement and participation of stakeholders throughout the regulatory process [ 41 , 152 - 156 ]. Our stakeholder mapping, as presented in Table 2 (refer to Multimedia Appendix 4 for more details), lays the foundation for national governments to conduct a robust stakeholder analysis and to adopt an all-inclusive stakeholder engagement strategy to manage and sustain the engagement and participation of all relevant stakeholders [ 157 , 158 ].
Recommendations
Our review found that the regulation of health apps in sub-Saharan Africa is especially poor and almost nonexistent, as only Kenya has national standards that could address some of the regulatory issues related to health apps. Therefore, we recommend the following actions to help sub-Saharan African countries improve the regulation of health apps to support self-management:
- Establish a clear and consistent definition of what constitutes a health app (considering AI or machine learning) and what level of regulation is required for different types of apps.
- Develop and implement criteria and guidelines that ensure the quality, safety, and usability of health apps.
- Engage with independent app evaluators, such as ORCHA, to adopt a common framework that can guide the evaluation and accreditation of health apps and use the framework to create and maintain decentralized and transparent platforms that showcase and evaluate health apps for users and health care professionals.
- Develop and implement policies and regulations that enable sustainable funding for health apps such as integrating the use of health apps for self-management into existing health systems and financing pathways or mechanisms.
- Support and facilitate innovation and collaboration across the sub-Saharan Africa region, especially in areas including but not limited to data security and privacy, interoperability standards, usability, accessibility, funding, capacity building, and monitoring and evaluation of the regulatory process.
- Manage and sustain the engagement, involvement, and participation of all relevant stakeholders in the regulatory process by conducting a robust stakeholder analysis and adopting an all-inclusive stakeholder engagement strategy.
Strengths and Limitations of the Study
This study has several strengths, which include an extensive search of gray literature and repositories, contact with key individuals, and the use of a systematic approach. Given that regulatory standards and guidance are unavailable in scientific databases, a wide range of gray literature and repositories were searched. In addition, contact was made with key staff members to obtain relevant documents, including those at the MOHs, the WHO country offices, and the WHO AFRO. Second, to enhance the strength of the study, a policy analysis framework was adapted and used to systematically organize the key study findings, whereas a deductive descriptive qualitative content analysis approach was used to identify and analyze texts that contained relevant concepts and other related information based on the 4 predefined themes. Third, the RISA tool was used to guide the mapping of key stakeholders. This has further increased the robustness of the study findings.
The limitations of this study include the fact that our literature search was conducted in English. Although the literature search was conducted in English, it yielded documents written in French and Portuguese from the ICTworks repository. Second, regulatory standards and guidance are not readily available on scientific databases; hence, it is possible that some relevant documents might have been missed. However, efforts were made to obtain these documents by contacting key stakeholders including key contact persons at the WHO AFRO, WHO country offices, and MOHs. In addition, contacting key individuals only for the purposes of requesting documents rather than conducting direct interviews was one of the limitations of this study. Interviewing key contact persons and stakeholders to obtain additional information could have strengthened the review; however, we did not interview any key individuals or stakeholders because it was beyond the scope of this review. Nonetheless, we recommend that future studies consider incorporating interviews to explore the perspectives of key stakeholders.
Conclusions
Health apps are increasingly being used by patients to manage their health, and sub-Saharan African countries can leverage these apps to advance their progress toward achieving SDG 3 (good health and well-being) and UHC, especially given the rapid advancement of AI and big data. However, our study has established that the regulation of health apps in sub-Saharan Africa is inadequate to ensure that health apps are technically reliable and clinically safe; interoperable across systems; compliant with the principles of confidentiality of information and data privacy; culturally appropriate and relevant; and accessible to everyone regardless of gender, ethnicity, location, or income. Therefore, the region can learn from the experiences of some high-income countries such as the United Kingdom and Germany to develop and implement a robust and responsive regulatory system that supports the widespread adoption of safe, effective, and beneficial health apps for its population.
Following the publication of this review, a summary of the findings will be disseminated to the relevant organizations. In addition, the key findings will be summarized and presented at national, regional, and international conferences.
Acknowledgments
The authors would like to thank Rebecca Jones, the Library Manager and Liaison Librarian at Charing Cross Library, who advised and assisted with the search strategy for this study. This work is part of the PhD research of BAB, which is sponsored by the government of Nigeria. AM and JC were supported by the National Institute for Health and Care Research (NIHR) Applied Research Collaboration Northwest London (NIHR200180). The views expressed in this publication are those of the authors and not necessarily those of the government of Nigeria or the NIHR or the Department of Health and Social Care. In the Results and Discussion sections, Microsoft Copilot in Bing [ 159 ] was used to help summarize and modify a few texts as well as suggest some citations.
Data Availability
The search strategy for PubMed, Scopus, and the World Health Organization AIM is presented in Multimedia Appendix 1 . All data generated or analyzed during this study are included in this published article (and its supplementary information files). The documents analyzed are available directly from the relevant institutional websites, ICTworks repository [ 44 ] or upon request from the relevant government departments in each country. Additionally, documents in the list of references that are not accessible on the web can be solicited from the corresponding author on reasonable request.
Authors' Contributions
BAB and JC conceived the study. BAB designed the study with contributions from JC and NM. BAB drafted the manuscript, and JC, NM, AM, SI, KPF, BIH, and NU read and contributed to it. AM was the clinical lead, and JC acted as a guarantor for this study. The final manuscript was read and approved by all the authors.
Conflicts of Interest
None declared.
PRISMA-ScR (Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews) checklist.
Database search strategy.
Details of included documents.
Mapping of the stakeholders according to their potential role in regulating health apps for self-management.
- Aitken M, Nass D. Digital health trends 2021: innovation, evidence, regulation, and adoption. IQVIA Institute for Human Data Science. 2021. URL: https://www.iqvia.com/-/media/iqvia/pdfs/institute-reports/digital-health-trends-2021/iqvia-institute-digital-health-trends-2021.pdf?&_=1669449368070 [accessed 2022-11-26]
- Mobile app threat landscape report. RiskIQ. 2020. URL: https://www.riskiq.com/2020-mobile-threat-landscape-report-thank-you/ [accessed 2021-07-19]
- El-Sappagh S, Ali F, Hendawi A, Jang JH, Kwak KS. A mobile health monitoring-and-treatment system based on integration of the SSN sensor ontology and the HL7 FHIR standard. BMC Med Inform Decis Mak. May 10, 2019;19(1):97. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Labrique AB, Vasudevan L, Kochi E, Fabricant R, Mehl G. mHealth innovations as health system strengthening tools: 12 common applications and a visual framework. Glob Health Sci Pract. Aug 06, 2013;1(2):160-171. [ FREE Full text ] [ CrossRef ]
- Adepoju IOO, Albersen BJA, De Brouwere V, van Roosmalen J, Zweekhorst M. mHealth for clinical decision-making in sub-Saharan Africa: a scoping review. JMIR Mhealth Uhealth. Mar 23, 2017;5(3):e38. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Vegesna A, Tran M, Angelaccio M, Arcona S. Remote patient monitoring via non-invasive digital technologies: a systematic review. Telemed J E Health. Jan 2017;23(1):3-17. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Use of appropriate digital technologies for public health: Report by the Director-General. World Health Organization. 2016. URL: https://iris.who.int/handle/10665/274134 [accessed 2023-05-06]
- El-Osta A, Rowe C, Majeed A. Developing a shared definition of self-driven healthcare to enhance the current healthcare delivery paradigm. J R Soc Med. Nov 2022;115(11):424-428. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Hussein R. A review of realizing the universal health coverage (UHC) goals by 2030: part 2- what is the role of eHealth and technology? J Med Syst. Jul 2015;39(7):72. [ CrossRef ] [ Medline ]
- Sustainable development goal 3: Ensure healthy lives and promote well-being for all at all ages. United Nations. URL: https://sdgs.un.org/goals/goal3 [accessed 2023-05-07]
- Coronavirus: apps to help the elderly. Organisation for the Review of Care and Health Apps. 2020. URL: https://orchahealth.com/coronavirus-apps-to-help-the-elderly/ [accessed 2021-07-19]
- Huckvale K, Adomaviciute S, Prieto JT, Leow MKS, Car J. Smartphone apps for calculating insulin dose: a systematic assessment. BMC Med. May 06, 2015;13:106. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Huckvale K, Car M, Morrison C, Car J. Apps for asthma self-management: a systematic assessment of content and tools. BMC Med. Nov 22, 2012;10:144. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Huckvale K, Morrison C, Ouyang J, Ghaghda A, Car J. The evolution of mobile apps for asthma: an updated systematic assessment of content and tools. BMC Med. Mar 23, 2015;13:58. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Huckvale K, Prieto JT, Tilney M, Benghozi PJ, Car J. Unaddressed privacy risks in accredited health and wellness apps: a cross-sectional systematic assessment. BMC Med. Sep 07, 2015;13:214. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Huckvale K, Torous J, Larsen ME. Assessment of the data sharing and privacy practices of smartphone apps for depression and smoking cessation. JAMA Netw Open. Apr 05, 2019;2(4):e192542. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Ndlovu K, Mars M, Scott RE. Interoperability frameworks linking mHealth applications to electronic record systems. BMC Health Serv Res. May 13, 2021;21(1):459. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Kenya standards and guidelines for mHealth systems. Kenya Ministry of Health. 2017. URL: https://www.health.go.ke/wp-content/uploads/2020/02/Revised-Guidelines-For-Mhealth-Systems-May-Version.pdf [accessed 2023-03-21]
- Standard for electronic health record system (EHRs) in Ethiopia. Ethiopia Minister of Health. 2021. URL: https://registry.betterehealth.eu/ehealth-policies/standard-electronic-health-record-system-ehrs-ethiopia [accessed 2023-04-21]
- National health normative standards framework for digital health interoperability in South Africa. South Africa Department of Health. 2021. URL: https://www.health.gov.za/wp-content/uploads/2022/10/HNSF_Gazette_21_October_2022.pdf [accessed 2023-05-15]
- Ferretti A, Ronchi E, Vayena E. From principles to practice: benchmarking government guidance on health apps. Lancet Digit Health. Jun 2019;1(2):e55-e57. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Diao JA, Venkatesh KP, Raza MM, Kvedar JC. Multinational landscape of health app policy: toward regulatory consensus on digital health. NPJ Digit Med. May 11, 2022;5(1):61. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Essén A, Stern AD, Haase CB, Car J, Greaves F, Paparova D, et al. Health app policy: international comparison of nine countries' approaches. NPJ Digit Med. Mar 18, 2022;5(1):31. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Brown SA, Garcia AA, Kouzekanani K, Hanis CL. Culturally competent diabetes self-management education for Mexican Americans: the Starr County border health initiative. Diabetes Care. Feb 2002;25(2):259-268. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Chaney SC, Mechael P. Self-Care Trailblazer Group. 2020. URL: https://media.psi.org/wp-content/uploads/2020/09/31000510/Digital-Self-Care-Final.pdf [accessed 2021-05-20]
- Kanzaveli T. Healthcare: shiftingfrom “one size fits all” to “one size fits one”. Medium. 2017. URL: https://tkanzaveli.medium.com/healthcare-shifting-from-one-size-fits-all-to-one-size-fits-one-d56136ded705 [accessed 2022-03-04]
- Myth 1 – one app will fit all!. Organisation for the Review of Care and Health Apps. URL: https://orchahealth.com/myth-1-one-app-will-fit-all/ [accessed 2022-03-04]
- Aitken M, Lyle J. Patient adoption of mHealth: use, evidence and remaining barriers to mainstream acceptance. IQVIA Institute for Human Data Science. Sep 2015. URL: https://www.iqvia.com/-/media/iqvia/pdfs/institute-reports/patient-adoption-of-mhealth.pdf [accessed 2021-05-21]
- Mechael P, Batavia H, Kaonga N. Barriers and gaps affecting mhealth in low and middle income countries: policy white paper. Center for Global Health and Economic Development Earth Institute, Columbia University. 2010. URL: http://www.globalproblems-globalsolutions-files.org/pdfs/mHealth_Barriers_White_Paper.pdf [accessed 2021-03-24]
- Bene BA, Ibeneme S, Fadahunsi KP, Harri BI, Ukor N, Mastellos N, et al. Regulatory standards and guidance for the use of health applications for self-management in Africa: scoping review protocol. BMJ Open. Feb 11, 2022;12(2):e058067. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Aitken M, Gauntlett C. Patient apps for improved healthcare: from novelty to mainstream. IMS Institute for Healthcare Informatics. 2013. URL: https://ignacioriesgo.es/wp-content/uploads/2014/03/iihi_patient_apps_report_editora_39_2_1.pdf [accessed 2024-03-10]
- National eHealth Strategy Toolkit. World Health Organization, International Telecommunication Union. 2012. URL: https://www.itu.int/pub/D-STR-E_HEALTH.05-2012 [accessed 2021-06-28]
- Global strategy on digital health 2020-2025. World Health Organization. 2021. URL: https://www.who.int/docs/default-source/documents/gs4dhdaa2a9f352b0445bafbc79ca799dce4d.pdf [accessed 2021-06-23]
- Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. Feb 2005;8(1):19-32. [ FREE Full text ] [ CrossRef ]
- Anderson S, Allen P, Peckham S, Goodwin N. Asking the right questions: scoping studies in the commissioning of research on the organisation and delivery of health services. Health Res Policy Syst. Jul 09, 2008;6:7. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Levac D, Colquhoun H, O'Brien KK. Scoping studies: advancing the methodology. Implement Sci. Sep 20, 2010;5:69. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Peters MDJ, Marnie C, Tricco AC, Pollock D, Munn Z, Alexander L, et al. Updated methodological guidance for the conduct of scoping reviews. JBI Evid Synth. Oct 2020;18(10):2119-2126. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. Oct 02, 2018;169(7):467-473. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Leitner C, Potenziani D. Mendeley reference manager. Mendeley. 2022. URL: https://www.mendeley.com/reference-management/reference-manager [accessed 2022-08-03]
- Better systematic review management. Covidence. URL: https://www.covidence.org/ [accessed 2023-02-13]
- Franco-Trigo L, Fernandez-Llimos F, Martínez-Martínez F, Benrimoj SI, Sabater-Hernández D. Stakeholder analysis in health innovation planning processes: A systematic scoping review. Health Policy. Oct 2020;124(10):1083-1099. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Walt G, Gilson L. Reforming the health sector in developing countries: the central role of policy analysis. Health Policy Plan. Dec 1994;9(4):353-370. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Digital health: a call for government leadership and cooperation between ICT and health. Broadband Commission. 2017. URL: https://broadbandcommission.org/wp-content/uploads/2021/09/WGHealth_Report2017-.pdf [accessed 2021-06-28]
- Vota W. Every African country’s national eHealth strategy or digital health policy. ICT works. 2019. URL: https://www.ictworks.org/african-national-ehealth-strategy-policy/ [accessed 2023-12-10]
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. Jul 21, 2009;6(7):e1000097. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Standards and guidelines for electronic medical record systems in Kenya. Kenya Ministry of Medical Services, Kenya Ministry of Public Health and Sanitation. 2010. URL: http://guidelines.health.go.ke:8000/media/Standards_and_Guidelines_for_EMR_Systems.pdf [accessed 2023-04-21]
- Kenya standards and guidelines for E-health systems interoperability. Kenya Ministry of Health, AfyaInfo Project. 2014. URL: https://pdf.usaid.gov/pdf_docs/PA00TB2K.pdf [accessed 2023-03-21]
- Health sector ICT standards and guidelines. Kenya Ministry of Health. 2013. URL: https://www.medbox.org/pdf/5e148832db60a2044c2d2895 [accessed 2023-03-21]
- Health information exchange standard operating procedure (SOP) and guideline. Nigeria Federal Ministry of Health. Jul 2020.
- National eHealth strategy 2018-2022. Benin Ministry of Health. 2017.
- The eHealth strategy of botswana 2020-2024. Botswana Ministry of Health. URL: https://ehealth.ub.bw/bhdc/Docs/MOH%20ehealth%20Strategy%20Book%20A4.pdf [accessed 2023-04-22]
- Health sector digital strategy 2016-2020. Burkina Faso Ministry of Health.
- National health informatics development plan of Burundi. Burundi Ministry of Public Health. 2015.
- The 2020-2024 national digital health strategic plan. Cameroon Ministry of Public Health. 2020.
- National eHealth strategy 2017-2021. Comoros Ministry of Health. 2016.
- eHealth strategic plan. Cote d’Ivoire Minister of Health and Public Hygiene. 2011.
- National development plan for health informatics. Democratic Republic of Congo Ministry of Public Health. 2014.
- Kingdom of Swaziland eHealth strategy 2016 - 2020. Kingdom of Swaziland Ministry Of Health. 2016.
- Information revolution strategic plan (2018-2025). Ethiopia Ministry of Health. 2018.
- Strategic master plan of the health information system of the Gabon. Gabon Ministry of Public Health and Population. 2017.
- National e-Health strategy. Ghana Ministry of Health. 2010.
- Kenya national e-Health strategy. Kenya Ministry of Medical Services, Kenya Ministry of Public Health & Sanitation. 2011.
- Kenya national eHealth policy 2016-2030. Kenya Ministry of Health. 2016.
- National strategy - Liberia - 2016-2021. Liberia Ministry of Health. 2016.
- Strategic plan for strengthening the health information system of Madagascar 2018–2022. Madagascar Ministry of Public Health. 2017.
- National digital health strategy 2020-2025. Malawi Ministry of Health. 2020.
- National eHealth policy in Mali. Mali Ministry of Health and Public Hygiene. 2013.
- Health 2015: seamless continuity of care. Mauritius Ministry of Health and Quality of Life. 2015.
- Strategic plan of information system for health 2009-2014. Mozambique Ministry of Health. 2009.
- National eHealth strategy 2019-2023. Niger Ministry of Public Health. 2018.
- National digital health strategy 2021-2025. Nigeria Federal Ministry of Health. 2021.
- National digital health policy. Nigeria Federal Ministry of Health. 2021.
- National digital health strategic plan 2018-2023. Rwanda Ministry of Health. 2018. URL: https://elearning.helinanet.org/mod/resource/view.php?id=890 [accessed 2023-05-09]
- Strategic plan for digital health 2018-2023. Senegal Ministry of Health and Social Action. 2018.
- National digital health strategy 2018-2023. Sierra Leone Ministry of Health and Sanitation, Sierra Leone Ministry of Information and Communication. 2018.
- The national digital health strategy 2019 – 2024. Tanzania Ministry of Health, Community Development, Gender, Elderly and Children. 2019.
- National digital health strategy for South Africa 2019 - 2024. South Africa Department of Health. 2019.
- Strategic plan for the development of eHealth in Togo 2013-2015. Togo Ministry of Health. 2012.
- Uganda national eHealth policy. Uganda Ministry of Health. 2016.
- Uganda national eHealth strategy 2017 - 2021. Uganda Ministry of Health. URL: https://health.go.ug/sites/default/files/National%20e_Health%20Strategy_0.pdf [accessed 2023-05-16]
- National eHealth strategy 2017-2021. Zambia Ministry of Health. 2017.
- Zimbabwe’s E-Health strategy 2012-2017. Ministry of Health and Child Welfare. 2012.
- National eHealth strategy 2021-2025. Namibia Ministry of Health & Social Services. 2021. URL: https://www.scribd.com/document/639371316/eHealth-Strategy-Namibia-2021# [accessed 2023-05-13]
- Health sector ICT policy and strategy. Ghana Ministry of Health. 2005. URL: https://www.moh.gov.gh/wp-content/uploads/2016/02/Health-Sector-ICT-Policy-and-Strategy.pdf [accessed 2023-05-08]
- Adebola OJ. Beyond national digital health strategy: final report of end term evaluation for the National Health ICT Strategic Framework 2015-2020. Nigeria Federal Ministry of Health. May 2021.
- National Health ICT Strategic Framework 2015 - 2020. Nigeria Federal Ministry of Health. 2016. URL: https://www.health.gov.ng/doc/HealthICTStrategicFramework.pdf [accessed 2023-05-16]
- Digital health blueprint. Ethiopia Ministry of Health. 2021. URL: http://repository.iifphc.org/bitstream/handle/123456789/1658/Ethiopian-Digital-Health-Blueprint.pdf?sequence=1&isAllowed=y [accessed 2023-05-16]
- Kenya health information systems interoperability framework. Kenya Ministry of Health. 2020. URL: https://www.data4sdgs.org/sites/default/files/services_files/Kenya%20Health%20Information%20Systems%20Interoperability%20Framework.pdf [accessed 2023-05-16]
- National community health digitization strategy 2020-2025. Kenya Ministry of Health, Division of Community Health Services. 2021. URL: https://www.eahealth.org/sites/www.eahealth.org/files/content/attachments/2021-08-02/eCHIS-Strategy-2020-2025.pdf [accessed 2023-05-16]
- Leitner C, Potenziani D. Health information systems interoperability in Liberia. IntraHealth International. 2016. URL: https://elearning.helinanet.org/mod/resource/view.php?id=938 [accessed 2023-05-16]
- Narrative for 2022 national digital health annual operational plan (AOP). Nigeria Federal Ministry of Health. 2022.
- Tanzania digital health investment road map 2017-2023. Tanzania Ministry of Health, Community Development, Gender, Elderly and Children, President’s Office Regional Administration and Local Government. 2017.
- Yu H. Regulation of digital health technologies in the European Union: intended versus actual use. In: Cohen GI, Minssen T, Price II NW, Robertson C, Shachar C, editors. The Future of Medical Device Regulation: Innovation and Protection. Cambridge. Cambridge University Press; Mar 31, 2022;103-114.
- Policy for device software functions and mobile medical applications: guidance for industry and Food and Drug Administration staff. U.S. Food and Drug Administration. 2022. URL: https://www.fda.gov/media/80958/download [accessed 2023-10-10]
- Gordon WJ, Landman A, Zhang H, Bates DW. Beyond validation: getting health apps into clinical practice. NPJ Digit Med. 2020;3:14. [ FREE Full text ] [ CrossRef ] [ Medline ]
- FDA clears mobile medical app to help those with opioid use disorder stay in recovery programs. U.S. Food and Drug Administration. 2018. URL: https://www.fda.gov/news-events/press-announcements/fda-clears-mobile-medical-app-help-those-opioid-use-disorder-stay-recovery-programs [accessed 2021-01-27]
- Digital maturity model: achieving digital maturity to drive growth. Deloitte. 2018. URL: https://www2.deloitte.com/content/dam/Deloitte/global/Documents/Technology-Media-Telecommunications/deloitte-digital-maturity-model.pdf [accessed 2021-10-20]
- May E. How digital health apps are empowering patients. Deloitte. 2021. URL: https://www2.deloitte.com/us/en/blog/health-care-blog/2021/how-digital-health-apps-are-empowering-patients.html [accessed 2023-10-06]
- Guidance: criteria for health app assessment. Public Health England. 2017. URL: https://www.gov.uk/government/publications/health-app-assessment-criteria/criteria-for-health-app-assessment [accessed 2023-10-16]
- Clinical risk management standards. National Health Service Digital. 2020. URL: https://digital.nhs.uk/services/clinical-safety/clinical-risk-management-standards [accessed 2023-10-28]
- Report a problem with a medicine or medical device. Gov.uk. URL: https://www.gov.uk/report-problem-medicine-medical-device [accessed 2023-11-07]
- Digital technology assessment criteria for health and social care (DTAC) - Version 1.0. National Health Service X. 2021. URL: https://view.officeapps.live.com/op/view.aspx?src=https%3A%2F%2Ftransform.england.nhs.uk%2Fmedia%2Fdocuments%2FDTAC_version_1.0_FINAL_updated_16.04.odt&wdOrigin=BROWSELINK [accessed 2023-11-07]
- Data protection impact assessment: NHS login - formerly Citizen Identity. National Health Service Digital. 2022. URL: https://digital.nhs.uk/services/nhs-login/data-protection-impact-assessment [accessed 2023-11-07]
- Risks and data protection impact assessments (DPIAs). Information Commissioner’s Office. URL: https://ico.org.uk/for-organisations/uk-gdpr-guidance-and-resources/accountability-and-governance/accountability-framework/risks-and-data-protection-impact-assessments-dpias/ [accessed 2023-11-07]
- A guide to good practice for digital and data-driven health technologies. Department of Health and Social Care. 2021. URL: https://www.gov.uk/government/publications/code-of-conduct-for-data-driven-health-and-care-technology/initial-code-of-conduct-for-data-driven-health-and-care-technology [accessed 2023-10-30]
- Interoperability in healthcare. Healthcare Information and Management Systems Society (HIMSS). 2023. URL: https://www.himss.org/resources/interoperability-healthcare [accessed 2023-10-17]
- DAPB4020: UK core Fast Healthcare Interoperability Resources (FHIR) release 4 (R4) governance. National Health Service Digital. 2022. URL: https://digital.nhs.uk/data-and-information/information-standards/information-standards-and-data-collections-including-extractions/publications-and-notifications/standards-and-collections/dapb4020-uk-core-fhir-r4-governance [accessed 2023-10-17]
- Fast Healthcare Interoperability Resources (FHIR). National Health Service Digital. 2022. URL: https://digital.nhs.uk/services/fhir-apis [accessed 2023-10-17]
- FHIR Interoperability Basics: 4 things to know. Health IT Analytics. 2022. URL: https://healthitanalytics.com/news/4-basics-to-know-about-the-role-of-fhir-in-interoperability [accessed 2023-11-07]
- Giordanengo A, Bradway M, Pedersen R, Grøttland A, Hartvigsen G, Årsand E. Integrating data from apps, wearables and personal electronic health record (pEHR) systems with clinicians’ electronic health records (EHR) systems. Int J Integr Care. Nov 09, 2016;16(5):16. [ FREE Full text ] [ CrossRef ]
- A plan for digital health and social care. Department of Health & Social Care. 2022. URL: https://www.gov.uk/government/publications/a-plan-for-digital-health-and-social-care/a-plan-for-digital-health-and-social-care [accessed 2022-12-01]
- Ryu B, Kim N, Heo E, Yoo S, Lee K, Hwang H, et al. Impact of an electronic health record-integrated personal health record on patient participation in health care: development and randomized controlled trial of MyHealthKeeper. J Med Internet Res. Dec 07, 2017;19(12):e401. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Winter A, Takabayashi K, Jahn F, Kimura E, Engelbrecht R, Haux R, et al. Quality requirements for electronic health record systems*. A Japanese-German information management perspective. Methods Inf Med. Aug 07, 2017;56(7):e92-e104. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Wachter RM. Making IT work: harnessing the power of health information technology to improve care in England. National Advisory Group on Health Information Technology. 2016. URL: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/550866/Wachter_Review_Accessible.pdf [accessed 2021-07-22]
- Framework on integrated people-centred health services (IPCHS). World Health Organisation. 2023. URL: https://www.who.int/teams/integrated-health-services/clinical-services-and-systems/service-organizations-and-integration [accessed 2023-06-05]
- Ibeneme S, Karamagi H, Muneene D, Goswami K, Chisaka N, Okeibunor J. Strengthening health systems using innovative digital health technologies in Africa. Front Digit Health. 2022;4:854339. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Ibeneme S, Ukor N, Ongom M, Dasa T, Muneene D, Okeibunor J. Strengthening capacities among digital health leaders for the development and implementation of national digital health programs in Nigeria. BMC Proc. 2020;14(Suppl 10):9. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Delivering safe digital health. Organisation for the Review of Care and Health Apps. URL: https://orchahealth.com/ [accessed 2023-10-22]
- Mamuye AL, Yilma TM, Abdulwahab A, Broomhead S, Zondo P, Kyeng M, et al. Health information exchange policy and standards for digital health systems in Africa: a systematic review. PLOS Digit Health. Oct 2022;1(10):e0000118. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Digital health platform handbook: Building a digital information infrastructure (infostructure) for health. World Health Organization, International Telecommunication Union. 2022. URL: https://www.itu.int/dms_pub/itu-d/opb/str/D-STR-E_HEALTH.10-2020-PDF-E.pdf [accessed 2021-05-22]
- Framework for involving patients in patient safety 2021. National Health Service England. 2021. URL: https://www.england.nhs.uk/patient-safety/framework-for-involving-patients-in-patient-safety/ [accessed 2023-03-23]
- Olesch A. Towards harmonised EU landscape for digital health: summary of the roundtable discussions in selected EIT Health InnoStars countries. EIT Health InnoStars. Jan 2023. URL: https://eithealth.eu/wp-content/uploads/2023/02/EIT_Health_DiGA_report_Jan2023.pdf [accessed 2023-10-10]
- Grieb J, Tschammler D, Färber C, Woitz S. Digital health laws and regulations germany. Global Legal Group. 2023. URL: https://iclg.com/practice-areas/digital-health-laws-and-regulations/germany [accessed 2023-11-03]
- Human regulatory: medical devices. European Medicines Agency. URL: https://www.ema.europa.eu/en/human-regulatory/overview/medical-devices [accessed 2023-10-12]
- Strengthening the capacity for regulation of medical products in the African region. World Health Organization Regional Office for Africa. 2013. URL: https://iris.who.int/bitstream/handle/10665/94308/AFR_RC63_7.pdf?sequence=1 [accessed 2023-10-17]
- Ncube BM, Dube A, Ward K. Establishment of the African Medicines Agency: progress, challenges and regulatory readiness. J Pharm Policy Pract. Mar 08, 2021;14(1):29. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Post market surveillance system. European Union Medical Device Regulation. 2023. URL: https://eumdr.com/post-market-surveillance-system/ [accessed 2023-10-31]
- Dayal R. Effective post-market surveillance for medical devices: An essential part of medical devices regulation (MDR). Capgemini. 2020. URL: https://www.capgemini.com/insights/expert-perspectives/effective-post-market-surveillance-for-medical-devices-an-essential-part-of-mdr/ [accessed 2023-10-31]
- NHS app library reaches 70 apps in honour of the NHS birthday. Northampton General Hospital NHS Trust. 2018. URL: https://www.northamptongeneral.nhs.uk/News/News-Archive/2018/NHS-App-Library-reaches-70-apps-in-honour-of-the-NHS-birthday.aspx [accessed 2023-09-21]
- Developers invited to add to NHS apps library. National Health Service Digital. 2018. URL: https://digital.nhs.uk/news/2018/developers-invited-to-add-to-nhs-apps-library [accessed 2023-09-22]
- The NHS apps library has closed. National Health Service Digital. 2021. URL: https://digital.nhs.uk/services/nhs-apps-library#:~:text=The%20NHS%20Apps%20Library%20was%20decommissioned%20in%20December%202021.&text=Further%20information%20can%20be%20found%20on%20the%20NHS.UK%20website [accessed 2023-09-22]
- Larsen ME, Huckvale K, Nicholas J, Torous J, Birrell L, Li E, et al. Using science to sell apps: evaluation of mental health app store quality claims. NPJ Digit Med. 2019;2:18. [ FREE Full text ] [ CrossRef ] [ Medline ]
- About the NHS app. National Health Service. Dec 4, 2023. URL: https://www.nhs.uk/nhs-app/about-the-nhs-app/ [accessed 2023-09-22]
- Scope of registration: regulated activities. Care Quality Commission. 2022. URL: https://www.cqc.org.uk/guidance-providers/scope-registration-regulated-activities [accessed 2023-11-05]
- Distributing great apps into health and care services across the world. Organisation for the Review of Care and Health Apps. 2020. URL: https://orchahealth.com/wp-content/uploads/2020/12/Health-and-Care-1.pdf [accessed 2023-10-09]
- Our founder, our story and our values: we exist to make digital health healthy. Organisation for the Review of Care and Health Apps. URL: https://orchahealth.com/about-us/ [accessed 2023-10-09]
- Health app library: empower your community with safe access to health apps and digital health products. Organisation for the Review of Care and Health Apps. URL: https://orchahealth.com/our-products/health-app-library/#:~:text=A%20Health%20App%20Library%20is,on%20the%20Health%20App%20Library [accessed 2023-10-09]
- Digital technology assessment criteria (DTAC). National Health Service X. URL: https://www.nhsx.nhs.uk/key-tools-and-info/digital-technology-assessment-criteria-dtac/ [accessed 2023-10-09]
- Evidence standards framework (ESF) for digital health technologies. National Institute for Health and Care Excellence. 2023. URL: https://www.nice.org.uk/about/what-we-do/our-programmes/evidence-standards-framework-for-digital-health-technologies [accessed 2023-10-08]
- Tsang L, Kerr-Peterson H. UK NICE updates its evidence standards framework for data-driven digital health technologies. Ropes & Gray. 2022. URL: https://www.ropesgray.com/en/insights/alerts/2022/10/uk-nice-updates-its-evidence-standards-framework-for-data-driven-digital-health-technologies [accessed 2023-10-09]
- Guidance: medical device stand-alone software including apps (including IVDMDs). Medicines and healthcare products regulatory agency. 2023. URL: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1168485/Medical_device_stand-alone_software_including_apps__including_IVDMDs_.pdf [accessed 2023-10-09]
- Digital therapeutics in the United Kingdom. Digital Therapeutics Alliance. 2021. URL: https://dtxalliance.org/wp-content/uploads/2021/06/DTA_DTx-Overview_UK.pdf [accessed 2023-10-09]
- Transforming global healthcare by advancing digital therapeutics. Digital Therapeutics Alliance. 2023. URL: https://dtxalliance.org/ [accessed 2023-10-10]
- International Organization for Standardization (ISO) digital therapeutic definition. Digital Therapeutic Alliance. Jun 2023. URL: https://dtxalliance.org/wp-content/uploads/2023/06/DTA_FS_ISO-Definition.pdf [accessed 2023-10-09]
- Ndomondo-Sigonda M, Miot J, Naidoo S, Dodoo A, Kaale E. Medicines regulation in Africa: current state and opportunities. Pharmaceut Med. 2017;31(6):383-397. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Chinele J. East Africa shows solid support for African Medicines Agency treaty. Health Policy Watch. Aug 16, 2023. URL: https://healthpolicy-watch.news/east-africa-shows-solid-support-for-african-medicines-agency-treaty/ [accessed 2023-10-09]
- Treaty for the establishment of the African Medicines Agency 2019. African Union. 2019. URL: https://au.int/sites/default/files/treaties/36892-treaty-0069_-_ama_treaty_e.pdf [accessed 2023-10-17]
- European Medicines Agency: about us. European Medicines Agency. Mar 1, 2023. URL: https://www.ema.europa.eu/en/documents/other/about-us-european-medicines-agency-ema_en.pdf [accessed 2023-10-18]
- Bryson JM. What to do when stakeholders matter. Public Adm Rev. Mar 2004;6(1):21-53. [ FREE Full text ] [ CrossRef ]
- Iyawa G, Herselman M, Botha A. Potential stakeholders and perceived benefits of a digital health innovation ecosystem for the Namibian context. Procedia Computer Science. 2017;121:431-438. [ CrossRef ]
- Ferretti V. From stakeholders analysis to cognitive mapping and multi-attribute value theory: an integrated approach for policy support. European Journal of Operational Research. Sep 2016;253(2):524-541. [ CrossRef ]
- Brugha R, Varvasovszky Z. Stakeholder analysis: a review. Health Policy Plan. Sep 2000;15(3):239-246. [ CrossRef ] [ Medline ]
- Schmeer K. Guidelines for conducting a stakeholder analysis 1999. Partnerships for Health Reform, Abt Associates. 1999. URL: https://www.ktecop.ca/wordpress/wp-content/uploads/guidelines-stakeholder-analysis-PHR-1999.pdf [accessed 2023-10-17]
- Gilmour J, Beilin R. Stakeholder mapping for effective risk assessment and communication. Australian Centre of Excellence for Risk Analysis, University of Melbourne. Apr 2007. URL: https://cebra.unimelb.edu.au/__data/assets/pdf_file/0006/2220990/gilmour0609.pdf [accessed 2023-10-17]
- Quality, service improvement and redesign tools: stakeholder analysis. National Health Service England, National Health Service Improvement. 2022. URL: https://www.england.nhs.uk/wp-content/uploads/2022/02/qsir-stakeholder-analysis.pdf [accessed 2023-10-20]
- Craven MP, Lang AR, Martin JL. Developing mHealth apps with researchers: multi-stakeholder design considerations. Springer; 2014. Presented at: Third International Conference, DUXU 2014, held as a part of HCI International; June 22-27, 2014;15-24; Heraklion, Greece. URL: https://doi.org/10.1007/978-3-319-07635-5_2 [ CrossRef ]
- How to encourage stakeholder participation. SustaiNet Software International. URL: https://sustainet.com/how-to-encourage-stakeholder-participation/ [accessed 2023-10-20]
- Stakeholder engagement. Organisation for Economic Cooperation and Development. URL: https://www.oecd.org/governance/better-international-rulemaking/compendium/keyprinciples/stakeholderengagement.htm [accessed 2023-10-20]
- Microsoft Copilot in Bing. Microsoft. URL: https://www.bing.com/chat?form=NTPCHB [accessed 2023-03-15]
Abbreviations
Edited by A Mavragani; submitted 19.05.23; peer-reviewed by N O'Brien, A Essén; comments to author 07.09.23; revised version received 08.12.23; accepted 23.02.24; published 11.04.24.
©Benard Ayaka Bene, Sunny Ibeneme, Kayode Philip Fadahunsi, Bala Isa Harri, Nkiruka Ukor, Nikolaos Mastellos, Azeem Majeed, Josip Car. Originally published in the Journal of Medical Internet Research (https://www.jmir.org), 11.04.2024.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work, first published in the Journal of Medical Internet Research, is properly cited. The complete bibliographic information, a link to the original publication on https://www.jmir.org/, as well as this copyright and license information must be included.
- Open access
- Published: 14 October 2023
A scoping review of ‘Pacing’ for management of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): lessons learned for the long COVID pandemic
- Nilihan E. M. Sanal-Hayes 1 , 7 ,
- Marie Mclaughlin 1 , 8 ,
- Lawrence D. Hayes 1 ,
- Jacqueline L. Mair ORCID: orcid.org/0000-0002-1466-8680 2 , 3 ,
- Jane Ormerod 4 ,
- David Carless 1 ,
- Natalie Hilliard 5 ,
- Rachel Meach 1 ,
- Joanne Ingram 6 &
- Nicholas F. Sculthorpe 1
Journal of Translational Medicine volume 21 , Article number: 720 ( 2023 ) Cite this article
3333 Accesses
5 Citations
21 Altmetric
Metrics details
Controversy over treatment for people with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a barrier to appropriate treatment. Energy management or pacing is a prominent coping strategy for people with ME/CFS. Whilst a definitive definition of pacing is not unanimous within the literature or healthcare providers, it typically comprises regulating activity to avoid post exertional malaise (PEM), the worsening of symptoms after an activity. Until now, characteristics of pacing, and the effects on patients’ symptoms had not been systematically reviewed. This is problematic as the most common approach to pacing, pacing prescription, and the pooled efficacy of pacing was unknown. Collating evidence may help advise those suffering with similar symptoms, including long COVID, as practitioners would be better informed on methodological approaches to adopt, pacing implementation, and expected outcomes.
In this scoping review of the literature, we aggregated type of, and outcomes of, pacing in people with ME/CFS.
Eligibility criteria
Original investigations concerning pacing were considered in participants with ME/CFS.
Sources of evidence
Six electronic databases (PubMed, Scholar, ScienceDirect, Scopus, Web of Science and the Cochrane Central Register of Controlled Trials [CENTRAL]) were searched; and websites MEPedia, Action for ME, and ME Action were also searched for grey literature, to fully capture patient surveys not published in academic journals.
A scoping review was conducted. Review selection and characterisation was performed by two independent reviewers using pretested forms.
Authors reviewed 177 titles and abstracts, resulting in 17 included studies: three randomised control trials (RCTs); one uncontrolled trial; one interventional case series; one retrospective observational study; two prospective observational studies; four cross-sectional observational studies; and five cross-sectional analytical studies. Studies included variable designs, durations, and outcome measures. In terms of pacing administration, studies used educational sessions and diaries for activity monitoring. Eleven studies reported benefits of pacing, four studies reported no effect, and two studies reported a detrimental effect in comparison to the control group.
Conclusions
Highly variable study designs and outcome measures, allied to poor to fair methodological quality resulted in heterogenous findings and highlights the requirement for more research examining pacing. Looking to the long COVID pandemic, our results suggest future studies should be RCTs utilising objectively quantified digitised pacing, over a longer duration of examination (i.e. longitudinal studies), using the core outcome set for patient reported outcome measures. Until these are completed, the literature base is insufficient to inform treatment practises for people with ME/CFS and long COVID.
Introduction
Post-viral illness occurs when individuals experience an extended period of feeling unwell after a viral infection [ 1 , 2 , 3 , 4 , 5 , 6 ]. While post-viral illness is generally a non-specific condition with a constellation of symptoms that may be experienced, fatigue is amongst the most commonly reported [ 7 , 8 , 9 ]. For example, our recent systematic review found there was up to 94% prevalence of fatigue in people following acute COVID-19 infection [ 3 ]. The increasing prevalence of long COVID has generated renewed interest in symptomology and time-course of post-viral fatigue, with PubMed reporting 72 articles related to “post-viral fatigue” between 2020 and 2022, but less than five for every year since 1990.
As the coronavirus pandemic developed, it became clear that a significant proportion of the population experienced symptoms which persisted beyond the initial viral infection, meeting the definition of a post-viral illness. Current estimates suggest one in eight people develop long COVID [ 10 ] and its symptomatology has repeatedly been suggested to overlap with clinical demonstrations of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). In a study by Wong and Weitzer [ 11 ], long COVID symptoms from 21 studies were compared to a list of ME/CFS symptoms. Of the 29 known ME/CFS symptoms the authors reported that 25 (86%) were reported in at least one long COVID study suggesting significant similarities. Sukocheva et al. [ 12 ] reported that long COVID included changes in immune, cardiovascular, metabolic, gastrointestinal, nervous and autonomic systems. When observed from a pathological stance, this list of symptoms is shared with, or is similar to, the symptoms patients with ME/CFS describe [ 13 ]. In fact, a recent article reported 43% of people with long COVID are diagnosed with ME/CFS [ 13 ], evidencing the analogous symptom loads.
A striking commonality between long COVID and similar conditions such as ME/CFS is the worsening of symptoms including fatigue, pain, cognitive difficulties, sore throat, and/or swollen lymph nodes following exertion. Termed post exertional malaise (PEM) [ 14 , 15 , 16 , 17 ], lasting from hours to several days, it is arguably one of the most debilitating side effects experienced by those with ME/CFS [ 16 , 17 , 18 ]. PEM is associated with considerably reduced quality of life amongst those with ME/CFS, with reduced ability to perform activities of daily living, leading to restraints on social and family life, mental health comorbidities such as depression and anxiety, and devastating employment and financial consequences [ 19 , 20 , 21 , 22 ]. At present, there is no cure or pharmacological treatments for PEM, and therefore, effective symptom management strategies are required. This may be in part because the triggers of PEM are poorly understood, and there is little evidence for what causes PEM, beyond anecdotal evidence. The most common approach to manage PEM is to incorporate activity pacing into the day-to-day lives of those with ME/CFS with the intention of reducing the frequency of severity of bouts of PEM [ 23 ]. Pacing is defined as an approach where patients are encouraged to be as active as possible within the limits imposed by the illness [ 23 , 24 , 25 ]. In practice, pacing requires individuals to determine a level at which they can function, but which does not lead to a marked increase in fatigue and other symptoms [ 26 , 27 ].
Although long COVID is a new condition [ 3 , 14 ], the available evidence suggests substantial overlap with the symptoms of conditions such as ME/CFS and it is therefore pragmatic to consider the utility of management strategies (such as pacing) used in ME/CFS for people with long COVID. In fact, a recent Delphi study recommended that management of long COVID should incorporate careful pacing to avoid PEM relapse [ 28 ]. This position was enforced by a multidisciplinary consensus statement considering treatment of fatigue in long COVID, recommending energy conservation strategies (including pacing) for people with long COVID [ 29 ]. Given the estimated > 2 million individuals who have experienced long COVID in the UK alone [ 30 , 31 , 32 ], there is an urgent need for evidence-based public health strategies. In this context, it seems pragmatic to borrow from the ME/CFS literature.
From a historical perspective, the 2007 NICE guidelines for people with ME/CFS advised both cognitive behavioural therapy (CBT) and graded exercise therapy (GET) should be offered to people with ME/CFS [ 33 ]. As of the 2021 update, NICE guidelines for people with ME/CFS do not advise CBT or GET, and the only recommended management strategy is pacing [ 34 ]. In the years between changes to these guidelines, the landmark PACE trial [ 35 ] was published in 2011. This large, randomised control trial (RCT; n = 639) compared pacing with CBT and reported GET and CBT were more effective than pacing for improving symptoms. Yet, this study has come under considerable criticism from patient groups and clinicians alike [ 36 , 37 , 38 , 39 ]. This may partly explain why NICE do not advise CBT or GET as of 2021, and only recommend pacing for symptom management people with ME/CFS [ 34 ]. There has been some controversy over best treatment for people with ME/CFS in the literature and support groups, potentially amplified by the ambiguity of evidence for pacing efficacy and how pacing should be implemented. As such, before pacing can be advised for people with long COVID, it is imperative previous literature concerning pacing is systematically reviewed. This is because a consensus is needed within the literature for implementing pacing so practitioners treating people with ME/CFS or long COVID can do so effectively. A lack of agreement in pacing implementation is a barrier to adoption for both practitioners and patients. Despite several systematic reviews concerning pharmacological interventions or cognitive behavioural therapy in people with ME/CFS [ 36 , 40 , 41 ], to date, there are no systematic reviews concerning pacing.
Despite the widespread use of pacing, the literature base is limited and includes clinical commentaries, case studies, case series, and few randomised control trials. Consequently, while a comprehensive review of the effects of pacing in ME/CFS is an essential tool to guide symptom management advice, the available literature means that effective pooling of data is not feasible [ 42 ] and therefore, a traditional systematic review and meta-analysis, with a tightly focussed research question would be premature [ 43 ]. Consequently, we elected to undertake a scoping review. This approach retains the systematic approach to literature searching but aims to map out the current state of the research [ 43 ]. Using the framework of Arksey and O'Malley [ 44 ], a scoping review aims to use a broad set of search terms and include a wide range of study designs and methods (in contrast to a systematic review [ 44 ]). This approach, has the benefit of clarifying key concepts, surveying current data collection approaches, and identifying critical knowledge gaps.
We aimed to provide an overview of existing literature concerning pacing in ME/CFS. Our three specific objectives of this scoping review were to (1) conduct a systematic search of the published literature concerning ME/CFS and pacing, (2) map characteristics and methodologies used, and (3) provide recommendations for the advancement of the research area.
Protocol and registration
The review was conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for scoping reviews (PRISMA-ScR) guidelines [ 45 ] and the five-stage framework outlined in Arksey and O’Malley [ 44 ]. Registration is not recommended for scoping reviews.
Studies that met the following criteria were included in this review: (1) published as a full-text manuscript; (2) not a review; (3) participants with ME/CFS; (4) studies employed a pacing intervention or retrospective analysis of pacing or a case study of pacing. Studies utilising sub-analysis of the pacing, graded activity, and cognitive behaviour therapy: a randomised evaluation (PACE) trial were included as these have different outcome measures and, as this is not a meta-analysis, this will not influence effect size estimates. Additionally, due to the paucity of evidence, grey literature has also been included in this review.
Search strategy
The search strategy consisted of a combination of free-text and MeSH terms relating to ME/CFS and pacing, which were developed through an examination of published original literature and review articles. Example search terms for PubMed included: ‘ME/CFS’ OR ‘ME’ OR ‘CFS’ OR ‘chronic fatigue syndrome’ OR ‘PEM’ OR ‘post exertional malaise’ OR ‘pene’ OR ‘post-exertion neurogenic exhaust’ AND ‘pacing’ OR ‘adaptive pacing’. The search was performed within title/abstract. Full search terms can be found in Additional file 1 .
Information sources
Six electronic databases [PubMed, Scholar, ScienceDirect, Scopus, Web of Science, and the Cochrane Central Register of Controlled Trials (CENTRAL)] were searched to identify original research articles published from the earliest available date up until 02/02/2022. Additional records were identified through reference lists of included studies. ‘Grey literature’ repositories including MEPedia, Action for ME, and ME Action were also searched with the same terms.
Study selection and data items
Once each database search was completed and manuscripts were sourced, all studies were downloaded into a single reference list (Zotero, version 6.0.23) and duplicates were removed. Titles and abstracts were screened for eligibility by two reviewers independently and discrepancies were resolved through discussion between reviewers. Subsequently, full text papers of potentially relevant studies were retrieved and assessed for eligibility by the same two reviewers independently. Any uncertainty by reviewers was discussed in consensus meetings and resolved by agreement. Data extracted from each study included sample size, participant characteristics, study design, trial registration details, study location, pacing description (type), intervention duration, intervention adherence, outcome variables, and main outcome data. Descriptions were extracted with as much detail as was provided by the authors. Study quality was assessed using the Physiotherapy Evidence Database (PEDro) scale [ 46 , 47 ].
Role of the funding source
The study sponsors had no role in study design, data collection, analysis, or interpretation, nor writing the report, nor submitting the paper for publication.
Study selection
After the initial database search, 281 records were identified (see Fig. 1 ). Once duplicates were removed, 177 titles and abstracts were screened for inclusion resulting in 22 studies being retrieved as full text and assessed for eligibility. Of those, five were excluded, and 17 articles remained and were used in the final qualitative synthesis.
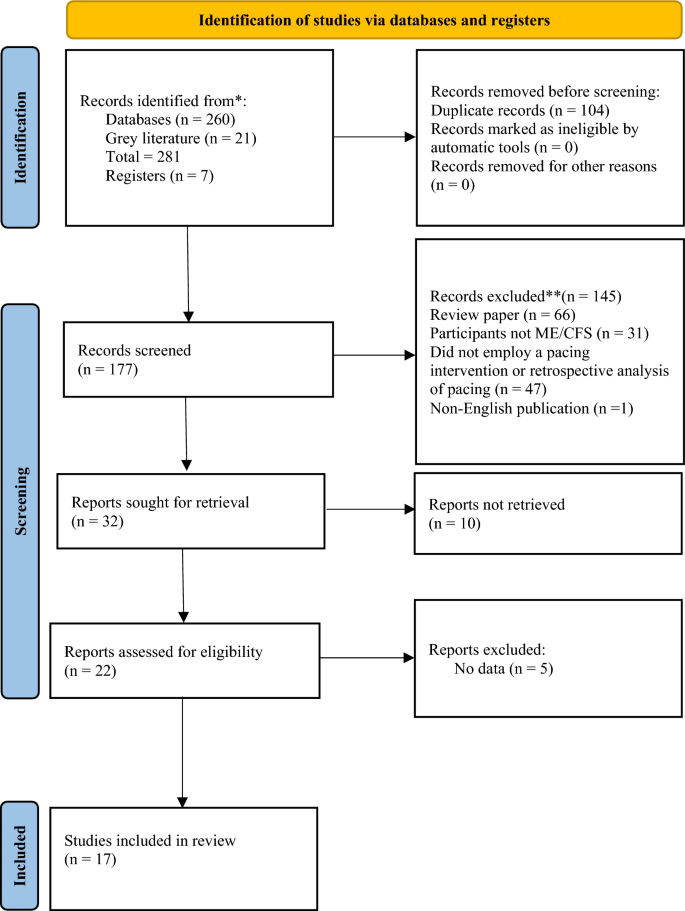
Schematic flow diagram describing exclusions of potential studies and final number of studies. RCT = randomized control trial. CT = controlled trial. UCT = uncontrolled trial
Study characteristics
Study characteristics are summarised in Table 1 . Of the 17 studies included, three were randomised control trials (RCTs [ 35 , 48 , 49 ]); one was an uncontrolled trial [ 50 ]; one was a case series [ 51 ]; one was a retrospective observational study [ 52 ], two were prospective observational studies [ 53 , 54 ]; four were cross-sectional observational studies [ 25 , 55 , 56 ]; and five were cross-sectional analytical studies [ 57 , 58 , 59 , 60 , 61 ] including sub-analysis of the PACE trial [ 35 , 56 , 59 , 61 ]. Seven of the studies were registered trials [ 35 , 48 , 49 , 50 , 56 , 57 , 58 ]. Diagnostic criteria for ME/CFS are summarised in Table 2 .
Types of pacing
Pacing interventions.
Of the 17 studies included, five implemented their own pacing interventions and will be discussed in this section. Sample sizes ranged from n = 7 in an interventional case series [ 51 ] to n = 641 participants in the largest RCT [ 35 ]. The first of these five studies considered an education session on pacing and self-management as the ‘pacing’ group, and a ‘pain physiology education’ group as the control group [ 49 ]. Two studies included educational sessions provided by a therapist plus activity monitoring via ActiGraph accelerometers [ 51 ] and diaries [ 48 ] at baseline and follow-up. In the first of these two studies, Nijs and colleagues [ 51 ] implemented a ‘self-management program’ which asked patients to estimate their current physical capabilities prior to commencing an activity and then complete 25–50% less than their perceived energy envelope. They[ 51 ] did not include a control group and had a sample size of only n = 7. Six years later, the same research group [ 48 ] conducted another pacing study which utilised relaxation as a comparator group (n = 12 and n = 14 in the pacing and relaxation groups, respectively). The pacing group underwent a pacing phase whereby participants again aimed to complete 25–50% less than their perceived energy envelope, followed by a gradual increase in exercise after the pacing phase (the total intervention spanned three weeks, and it is unclear how much was allocated to pacing, and how much to activity increase). Therefore, it could be argued that Kos et al. [ 48 ] really assessed pacing followed by a gradual exercise increase as outcome measures were assessed following the graded activity phase. Another pacing intervention delivered weekly educational sessions for six weeks and utilised a standardised rehabilitation programme using the ‘activity pacing framework’ [ 50 ] in a single-arm, no comparator group feasibility study. Finally, the PACE trial adopted an adaptive pacing therapy intervention consisting of occupational therapists helping patients to plan and pace activities utilising activity diaries to identify activities associated with fatigue and staying within their energy envelope [ 35 ]. This study incorporated standard medical care, cognitive behavioural therapy (CBT) and graded exercise therapy (GET) as comparator groups [ 35 ]. It is worth noting that the pacing group and the CBT group were both ‘encouraged’ to increase physical activity levels as long as participants did not exceed their energy envelope. Although not all five intervention studies explicitly mentioned the “Energy Envelope Theory”, which dictates that people with ME/CFS should not necessarily increase or decrease their activity levels, but moderate activity and practice energy conservation [ 62 ], all intervention studies used language analogous to this theory, such as participants staying within limits, within capacity, or similar.
The interventions included in this review were of varying durations, from a single 30-min education session [ 49 ], a 3-week (one session a week) educational programme [ 51 ], a 3-week (3 × 60–90 min sessions/week) educational programme [ 48 ], a 6-week rehabilitation programme [ 50 ], to a 24-week programme [ 35 ]. Intervention follow-up durations also varied across studies from immediately after [ 49 ], 1-week [ 51 ], 3-weeks [ 48 ], 3-months [ 50 ], and 1-year post-intervention [ 35 ].
Observational studies of pacing
Eight studies were observational and, therefore, included no intervention. Observational study sample sizes ranged from 16 in a cross-sectional interview study [ 25 ] to 1428 in a cross-sectional survey [ 52 ]. One study involved a retrospective analysis of participants’ own pacing strategies varying from self-guided pacing or pacing administered by a therapist compared with implementation of CBT and GET [ 52 ]. Five involved a cross-sectional analysis of participants own pacing strategies which varied from activity adjustment, planning and acceptance [ 50 , 55 ], and the Energy Envelope method [ 58 , 60 ]. Two studies were prospective observational studies investigating the Energy Envelope theory [ 53 , 54 ]. Four studies [ 56 , 57 , 59 , 61 ] included in this review involved sub-analysis of results of the PACE trial [ 35 ].
Outcome measures
Quantitative health outcomes.
ME/CFS severity and general health status were the most common outcome measures across studies (16/17) [ 35 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 63 ]. Studies utilised different instruments, including the Short-Form 36 (SF-36; 8/16) [ 35 , 51 , 53 , 54 , 56 , 57 , 58 , 60 ], SF-12 (2/16) [ 50 , 63 ], ME symptom and illness severity (2/16) [ 52 , 55 ], Patient health (PHQ-15; 1/16) [ 59 ], DePaul symptom questionnaire (DSQ; 1/16) [ 58 ], and the Patient health questionnaire-9 (1/16) [ 50 ]. Additionally, some studies used diagnostic criteria for ME/CFS as an outcome measure to determine recovery [ 57 , 59 , 61 ].
Pain was assessed by most included studies (11/17) [ 35 , 49 , 50 , 51 , 53 , 54 , 55 , 57 , 59 , 60 , 61 , 63 ]. Two studies [ 59 , 61 ] included the international CDC criteria for CFS which contain five painful symptoms central to a diagnosis of CFS: muscle pain and joint pain. Other methods of assessment included Brief Pain Inventory (1/11) [ 53 ], Chronic Pain Coping Inventory (CPCI; 1/11) [ 49 ], Pain Self Efficacy Questionnaire (PSEQ; 1/11) [ 50 ], Tampa Scale for Kinesiophobia–version CFS (1/11) [ 49 ], algometry (1/11) [ 49 ], Knowledge of Neurophysiology of Pain Test (1/12) [ 49 ], Pain Catastrophizing Scale (1/11) [ 49 ], Pain Anxiety Symptoms Scale short version (PASS-20; 1/11) [ 50 ], Pain Numerical Rating Scale (NRS; 1/11) [ 63 ].
Fatigue or post-exertional malaise was assessed by 11 of the 17 studies [ 35 , 48 , 50 , 51 , 53 , 54 , 56 , 57 , 60 , 61 , 63 ]. Again, measurement instruments were divergent between studies and included the Chalder Fatigue Questionnaire (CFQ; 4/11) [ 35 , 50 , 57 , 63 ], Fatigue Severity Scale (2/11) [ 53 , 60 ], the Chronic Fatigue Syndrome Medical Questionnaire (1/11) [ 60 ], and Checklist Individual Strength (CIS; 2/11) [ 48 , 51 ].
Anxiety and depression were also common outcome measures, utilised by four studies (4/17) [ 50 , 53 , 59 , 63 ]. These were also assessed using different instruments including Hospital Anxiety and Depression Scale (HADS; 2/4) [ 59 , 63 ], Generalised Anxiety Disorder Assessment (1/4 [ 50 ]), Beck Depression Inventory (BDI-II; 1/4) [ 53 ], Beck Anxiety Inventory (BAI; 1/4) [ 53 ], and Perceived Stress Scale (PSS; 1/4) [ 53 ].
Outcome measures also included sleep (2/17) [ 53 , 59 ], assessed by The Pittsburgh Sleep Quality Index (1/2) [ 53 ] and Jenkins sleep scale (1/2) [ 59 ]; and quality of life (2/17) [ 50 , 53 ] as assessed by the EuroQol five-dimensions, five-levels (EQ-5D-5L; 1/2) [ 50 ] and The Quality-of-Life Scale (1/2) [ 53 ]. Self-Efficacy was measured in four studies [ 50 , 53 , 59 , 60 ], assessed by the Brief Coping Orientation to Problems Experienced Scale (bCOPE; 1/4) [ 60 ] and the Chronic Disease Self-Efficacy measure (3/4) [ 50 , 53 , 59 ].
Quantitative evaluation of pacing
Some studies (4/17) [ 25 , 50 , 52 , 63 ] included assessments of the participants’ experiences of pacing, using the Activity Pacing Questionnaire (APQ-28; 1/4 [ 50 ], APQ-38 (2/4) [ 25 , 63 ]), a re-analysis of the 228 question survey regarding treatment (1/4) [ 52 ] originally produced by the ME Association [ 55 ], and qualitative semi-structured telephone interviews regarding appropriateness of courses in relation to individual patient needs (1/4) [ 25 ]. The APQ-28 and -38 have been previously validated, but the 228-question survey has not. When outcome measures included physical activity levels (4/17), the Canadian Occupational Performance Measure (COPM) was used in two studies [ 48 , 51 ], and two studies used accelerometers to record physical activity [ 51 , 54 ]. Of these two studies, Nijs [ 51 ] examined accelerometery after a 3-week intervention based on the Energy Envelope Theory and Brown et al. [ 54 ] evaluated the Energy Envelope Theory of pacing over 12 months.
Other outcomes
Two [ 53 , 59 ] of the 17 studies included structured clinical interviews for the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) to assess psychiatric comorbidity and psychiatric exclusions. One study included a disability benefits questionnaire [ 55 ], and one study included employment and education questionnaire [ 55 ]. Additionally, satisfaction of primary care was also used as an outcome measure (2/17) [ 25 , 55 ] assessed using the Chronic Pain Coping Inventory (CPCI).
Efficacy of pacing interventions
The majority of studies (12/17) [ 25 , 48 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 58 , 60 , 63 ] highlighted improvements in at least one outcome following pacing (Fig. 2 ). When the effect of pacing was assessed by ME symptomology and general health outcomes, studies reported pacing to be beneficial [ 25 , 50 , 51 , 53 , 54 , 55 , 56 , 58 ]. It is worth noting however that pacing reportedly worsened ME symptoms in 14% of survey respondents, whilst improving symptoms in 44% of respondents [ 52 ]. Most studies using fatigue as an outcome measure reported pacing to be efficacious (7/10) [ 50 , 51 , 53 , 54 , 56 , 60 , 63 ]. However, one study reported no change in fatigue with a pacing intervention (1/10) [ 35 ], and 2/10 studies [ 53 , 63 ] reported a worsening of fatigue with pacing. Physical function was used to determine the efficacy of pacing in 11 studies [ 35 , 48 , 50 , 51 , 53 , 54 , 56 , 58 , 59 , 60 , 63 ]. Of these, the majority found pacing improved physical functioning (8/10) [ 48 , 50 , 51 , 53 , 54 , 56 , 58 , 60 ], with 1/10 [ 35 ] studies reporting no change in physical functioning, and 1/10 [ 59 ] reporting a worsening of physical functioning from pre- to post-pacing. Of the seven studies [ 35 , 49 , 50 , 51 , 53 , 54 , 60 ] which used pain to assess pacing efficacy, 4/7 [ 50 , 51 , 53 , 60 ] reported improvements in pain and 3/7 [ 35 , 51 , 53 ] reported no change in pain scores with pacing. All studies reporting quality of life (1/1) [ 53 ], self-efficacy (3/3) [ 50 , 53 , 59 ], sleep (2/2) [ 53 , 59 ], and depression and anxiety (4/4) [ 50 , 53 , 59 , 63 ], found pacing to be efficacious for ME/CFS participants.
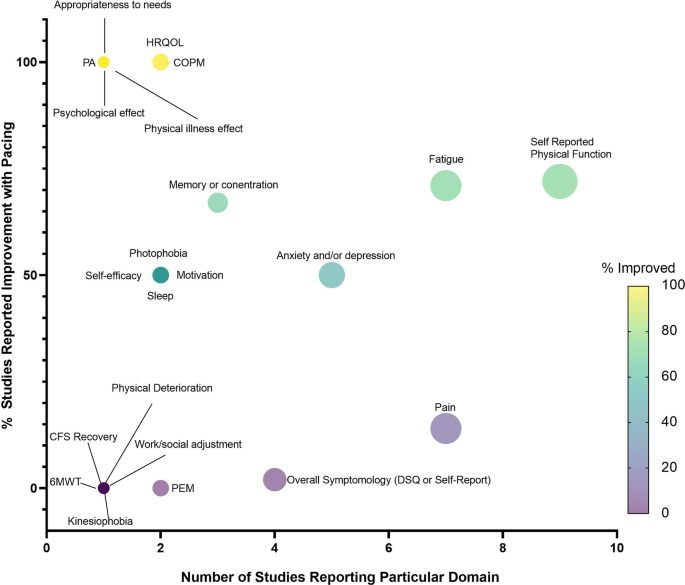
Bubble plot displaying number of studies reporting each domain (x-axis) and the percentage of studies reporting improvement with pacing (y-axis), including a coloured scale of improvement from 0–100%. PEM = post-exertional malaise, 6MWT = 6-min walk time, CFS = chronic fatigue syndrome, DSQ = DePaul Symptom Questionnaire, PA = Physical Activity, HRQOL = Health-related quality of life, COPM = The Canadian Occupational Performance Measure
Participant characteristics
The majority of studies (10/17) [ 25 , 50 , 52 , 53 , 54 , 58 , 59 , 60 , 61 , 63 ] did not report age of the participants. For those which did report age, this ranged from 32 ± 14 to 43 ± 13 years. Where studies reported sex (11/17) [ 35 , 48 , 49 , 50 , 51 , 54 , 55 , 56 , 57 , 58 , 60 ], this was predominantly female, ranging from 75 to 100% female. Only six studies [ 35 , 54 , 56 , 57 , 58 , 60 ] reported ethnicity, with cohorts predominantly Caucasian (94–98%). Time since diagnosis was mostly unreported (12/17) [ 25 , 48 , 49 , 50 , 52 , 53 , 54 , 58 , 59 , 60 , 61 , 63 ] but ranged from 32 to 96 months, with a cross-sectional survey reporting 2% of the participants were diagnosed 1–2 years previously; 6% 3–4 years since diagnosis; 13% 3–4 years since diagnosis; 12% 5–6 years since diagnosis; 20% 7–10 years since diagnosis; 29% 11–21 years since diagnosis; 13% 21–30 years since diagnosis; and 5% > 30 years since diagnosis. Of the studies which reported comorbidities of the participants (6/17) [ 25 , 35 , 50 , 56 , 57 , 63 ], the comorbidities were chronic pain, depressive disorder, psychiatric disorder.
Study location
Of the 17 studies, 14 were from Europe [ 25 , 35 , 48 , 49 , 50 , 51 , 52 , 55 , 56 , 57 , 58 , 59 , 61 , 63 ], and three from North America [ 53 , 54 , 60 ]. Of the 14 studies[ 25 , 35 , 48 , 49 , 50 , 51 , 52 , 55 , 56 , 57 , 58 , 59 , 61 , 63 ] from Europe, ten [ 25 , 35 , 50 , 52 , 55 , 56 , 57 , 58 , 59 , 61 , 63 ] were conducted in the United Kingdom, three in Belgium [ 48 , 49 , 51 ], and one was a multicentred study between the United Kingdom and Norway [ 58 ].
Recruitment strategy
Of the 17 studies, three [ 53 , 54 , 60 ] used announcements in a newspaper and physician referrals to recruit participants, two [ 50 , 63 ] recruited patients referred by a consultant from a National Health Service (NHS) Trust following a pain diagnosis, two [ 52 , 55 ] concerned online platforms on the web, two [ 59 , 61 ] recruited from secondary care clinics, and two used the PACE trial databases [ 56 , 57 ]. Moreover, one study recruited from the hospital [ 58 ], one from physiotherapist referrals [ 25 ], two from specialist clinic centres [ 35 , 64 ], one from waiting list of rehabilitation centre [ 48 ], and one from medical files [ 49 ].
Study settings
Ten studies were carried out in hospital and clinic setting [ 25 , 35 , 48 , 49 , 50 , 51 , 58 , 59 , 61 , 63 ]. Two studies were performed on online platforms [ 52 , 55 ]. Three studies did not report study setting [ 53 , 54 , 60 ]. Two studies generated output from PACE trial databases [ 56 , 57 ]
Adherence and feasibility
All five intervention studies reported adherence rates (which they defined as number of sessions attended), which ranged from 4–44% (4% [ 49 ], 8% [ 35 ], 25% [ 48 ], 29% [ 51 ], and 44% [ 50 ]). One study reported the median number of rehabilitation programme sessions attended was five out of six possible sessions, with 58.9% [ 50 ] participants attending ≥ 5 sessions; 83.2% participants attending at least one educational session on activity pacing and 56.1% attending both activity pacing sessions.
This scoping review summarises the existing literature, with a view to aid physicians and healthcare practitioners better summarise evidence for pacing in ME/CFS and use this knowledge for other post-viral fatiguing conditions. Overall, studies generally reported pacing to be beneficial for people with ME/CFS. The exception to this trend is the controversial PACE trial [ 36 , 37 , 38 , 39 ], which we will expand on in subsequent sections. We believe information generated within this review can facilitate discussion of research opportunities and issues that need to be addressed in future studies concerning pacing, particularly given the immediate public health issue of the long COVID pandemic. As mentioned, we found some preliminary evidence for improved symptoms following pacing interventions or strategies. However, we wish to caution the reader that the current evidence base is extremely limited and hampered by several limitations which preclude clear conclusions on the efficacy of pacing. Firstly, studies were of poor to fair methodological quality (indicated by the PEDro scores), often with small sample sizes, and therefore unknown power to detect change. Moreover, very few studies implemented pacing, with most studies merely consulting on people’s views on pacing. This may of course lead to multiple biases such as reporting, recruitment, survivorship, confirmation, availability heuristic, to name but a few. Thus, there is a pressing need for more high-quality intervention studies. Secondly, the reporting of pacing strategies used was inconsistent and lacked detail, making it difficult to describe current approaches, or implement them in future research or symptom management strategies. Furthermore, outcome evaluations varied greatly between studies. This prevents any appropriate synthesis of research findings.
The lack of evidence concerning pacing is concerning given pacing is the only NICE recommended management strategy for ME/CFS following the 2021 update [ 34 ]. Given the analogous nature of long COVID with ME/CFS, patients and practitioners will be looking to the ME/CFS literature for guidance for symptom management. There is an urgent need for high quality studies (such as RCTs) investigating the effectiveness of pacing and better reporting of pacing intervention strategies so that clear recommendations can be made to patients. If this does not happen soon, there will be serious healthcare and economic implications for years to come [ 65 , 66 ].
Efficacy of pacing
Most studies (12/17) highlighted improvements in at least one outcome measure following pacing. Pacing was self-reported to be the most efficacious, safe, acceptable, and preferred form of activity management for people with ME/CFS [ 55 ]. Pacing was reported to improve symptoms and improve general health outcomes [ 25 , 50 , 52 , 58 , 63 ], fatigue and PEM [ 48 , 50 , 51 , 53 , 54 , 55 , 56 , 60 , 63 ], physical functioning [ 48 , 50 , 51 , 53 , 56 , 58 , 60 , 63 ], pain [ 25 , 50 , 55 , 63 ], quality of life [ 50 ], self-efficacy [ 50 , 53 ], sleep [ 53 , 55 ], and depression and anxiety [ 50 , 53 , 63 ]. These positive findings provide hope for those with ME/CFS, and other chronic fatiguing conditions such as long COVID, to improve quality of life through symptom management.
Conversely, some studies reported no effects of pacing on ME/CFS symptoms [ 52 ], fatigue, physical functioning [ 35 ], or pain scores [ 49 , 61 ]. Some studies even found pacing to have detrimental effects in those with ME/CFS, including a worsening of symptoms in 14% of survey participants recalling previous pacing experiences [ 52 ]. Furthermore, a worsening of fatigue [ 35 , 59 ], and physical functioning from pre- to post-pacing [ 35 , 57 , 59 , 61 ] was reported by the PACE trial and sub-analysis of the PACE trial [ 56 , 57 , 61 ]. The PACE trial [ 35 ], a large RCT (n = 639) comparing pacing with CBT and GET, reported GET and CBT were more effective for reducing ME/CFS-related fatigue and improving physical functioning than pacing. However, the methodology and conclusions from the PACE trial have been heavily criticised, mainly due to the authors lowering the thresholds they used to determine improvement [ 36 , 37 , 38 , 67 ]. With this in mind, Sharpe et al. [ 56 ] surveyed 75% of the participants from the PACE trial 1-year post-intervention and reported pacing improved fatigue and physical functioning, with effects similar to CBT and GET.
Lessons for pacing implementation
All pacing intervention studies (5/5) implemented educational or coaching sessions. These educational components were poorly reported in terms of the specific content and how and where they had been developed, with unclear pedagogical approaches. Consequently, even where interventions reported reduction in PEM or improved symptoms, it would be impossible to transfer that research into practice, future studies, or clinical guidance, given the ambiguity of reporting. Sessions typically contained themes of pacing such as activity adjustment (decrease, break-up, and reschedule activities based on energy levels), activity consistency (maintaining a consistently low level of activity to prevent PEM), activity planning (planning activities and rest around available energy levels), and activity progression (slowly progressing activity once maintaining a steady baseline) [ 35 , 48 , 49 , 50 , 51 ]. We feel it is pertinent to note here that although activity progression has been incorporated as a pacing strategy in these included studies, some view activity progression as a form of GET. The NICE definition of GET is “first establishing an individual's baseline of achievable exercise or physical activity, then making fixed incremental increases in the time spent being physically active” [ 34 ]. Thus, this form of pacing can also be considered a type of ‘long-term GET’ in which physical activity progression is performed over weeks or months with fixed incremental increases in time spent being physically.
Intervention studies attempted to create behaviour change, through educational programmes to modify physical activity, and plan behaviours. However, none of these studies detailed integrating any evidence-based theories of behaviour change [ 68 ] or reported using any frameworks to support behaviour change objectives. This is unfortunate since there is good evidence that theory-driven behaviour change interventions result in greater intervention effects [ 69 ]. Indeed, there is a large body of work regarding methods of behaviour change covering public health messaging, education, and intervention design, which has largely been ignored by the pacing literature. Interventions relied on subjective pacing (5/5 studies), with strategies including keeping an activity diary (3/5 studies) to identify links between activity and fatigue [ 35 , 48 , 50 ]. Given the high prevalence of ‘brain fog’ within ME/CFS [ 70 , 71 , 72 , 73 ], recall may be extremely difficult and there is significant potential for under-reporting. Other strategies included simply asking participants to estimate energy levels available for daily activities (2/5 studies [ 48 , 51 ]). Again, this is subjective and relies on participants’ ability to recall previous consequences of the activity. Other methods of activity tracking and measuring energy availability, such as wearable technology [ 74 , 75 , 76 , 77 , 78 ] could provide a more objective measure of adherence and pacing strategy fidelity in future studies. Despite technology such as accelerometers being widely accessible since well-before the earliest interventional study included in this review (which was published in 2009), none of the interventional studies utilised objective activity tracking to track pacing and provide feedback to participants. One study considered accelerometery alongside an activity diary [ 51 ]. However, accelerometery was considered the outcome variable, to assess change in activity levels from pre- to post-intervention and was not part of the intervention itself (which was one pacing coaching sessions per week for 3 weeks). Moreover, most research-grade accelerometers cannot be used as part of the intervention since they have no ability to provide continuous feedback and must be retrieved by the research team in order to access any data. Consequently, their use is mostly limited to outcome assessments only. As pacing comprises a limit to physical activity to prevent push-crash cycles, it is an astonishing observation from this scoping review that only two studies objectively measured physical activity to quantify changes to activity as a result of pacing [ 51 , 54 ]. If the aim of pacing is to reduce physical activity, or reduce variations in physical activity (i.e., push-crash cycles), only two studies have objectively quantified the effect pacing had on physical activity, so it is unclear whether pacing was successfully implemented in any of the other studies.
By exploring the pacing strategies previously used, in both intervention studies and more exploratory studies, we can identify and recommend approaches to improve symptoms of ME/CFS. These approaches can be categorised as follows: activity planning, activity consistency, activity progression, activity adjustment and staying within the Energy Envelope [ 50 , 53 , 60 , 63 ]. Activity planning was identified as a particularly effective therapeutic strategy, resulting in improvement of mean scores of all symptoms included in the APQ-28, reducing current pain, improvement of physical fatigue, mental fatigue, self-efficacy, quality of life, and mental and physical functioning [ 50 ]. Activity planning aligns with the self-regulatory behaviour change technique ‘Action Planning’ [ 79 ] which is commonly used to increase physical activity behaviour. In the case of ME/CFS, activity planning is successfully used to minimise rather than increase physical activity bouts to prevent expending too much energy and avoid PEM. Activity consistency, meaning undertaking similar amounts of activity each day, was also associated with reduced levels of depression, exercise avoidance, and higher levels of physical function [ 63 ]. Activity progression was associated with higher levels of current pain. Activity adjustment associated with depression and avoidance, and lower levels of physical function [ 63 ]. Staying within the Energy Envelope was reported to reduce PEM severity [ 53 , 60 ], improve physical functioning [ 53 , 60 ] and ME/CFS symptom scores [ 53 ], and more hours engaged in activity than individuals with lower available energy [ 53 ]. These results suggest that effective pacing strategies would include activity planning, consistency, and energy management techniques while avoiding progression. This data is, of course, limited by the small number of mostly low-quality studies and should be interpreted with some caution. Nevertheless, these are considerations that repeatedly appear in the literature and, as such, warrant deeper investigation. In addition, and as outlined earlier, most studies are relatively old, and we urgently need better insight into how modern technologies, particularly longitudinal activity tracking and contemporaneous heart-rate feedback, might improve (or otherwise) adaptive pacing. Such longitudinal tracking would also enable activities and other behaviours (sleep, diet, stress) to be linked to bouts of PEM. Linking would enable a deeper insight into potential PEM triggers and mitigations that might be possible.
The PACE trial
We feel it would be remiss of us to not specifically address the PACE trial within this manuscript, as five of the 17 included studies resulted from the PACE trial [ 35 , 56 , 57 , 59 , 61 ]. There has been considerable discussion around the PACE trial, which has been particularly divisive and controversial [ 37 , 38 , 39 , 59 , 67 , 80 , 81 ]. In the PACE trial, GET and CBT were deemed superior to pacing by the authors. Despite its size and funding, the PACE trial has received several published criticisms and rebuttals. Notably, NICE's most recent ME/CFS guideline update removed GET and CBT as suggested treatment options, which hitherto had been underpinned by the PACE findings. While we will not restate the criticisms and rebuttals here, what is not in doubt, is that the PACE trial has dominated discussions of pacing, representing almost a third of all the studies in this review. However, the trial results were published over a decade ago, with the study protocol devised almost two decades ago [ 82 ]. The intervening time has seen a revolution in the development of mobile and wearable technology and an ability to remotely track activity and provide real-time feedback in a way which was not available at that time. Furthermore, there has been no substantive research since the PACE trial that has attempted such work. Indeed, possibly driven by the reported lack of effect of pacing in the PACE trial, this review has demonstrated the dearth of progress and innovation in pacing research since its publication. Therefore, regardless of its findings or criticisms, the pacing implementation in the PACE trial is dated, and there is an urgent need for more technologically informed approaches to pacing research.
Limitations of the current evidence
The first limitation to the literature included in this scoping review is that not all studies followed the minimum data set (MDS) of patient-reported outcome measures (PROMs) agreed upon by the British Association of CFS/ME Professionals (BACME) (fatigue, sleep quality, self-efficacy, pain/discomfort, anxiety/depression, mobility, activities of daily living, self-care, and illness severity) [ 83 , 84 ]. All but one study included in this review measured illness severity, most studies included fatigue and pain/discomfort, and some studies included assessments of anxiety/depression. There was a lack of quantitative assessment of sleep quality, self-efficacy, mobility, activities of daily living, and self-care. Therefore, studies did not consistently capture the diverse nature of the symptoms experienced, with crucial domains missing from the analyses. The MDS of PROMs were established in 2012 [ 83 , 84 ] and therefore, for studies published out prior to 2012, these are not applicable [ 35 , 49 , 51 , 53 , 54 ]. However, for the 12 studies carried out after this time, the MDS should have been considered elucidate the effects of pacing on ME/CFS. Importantly, despite PEM being a central characteristic of ME/CFS, only two studies included PEM as an outcome measure [ 55 , 60 ]. This may be because of the difficulty of accurately measuring fluctuating symptoms, as PEM occurs multiple times over a period of months, and therefore pre- to post- studies and cross-sectional designs cannot adequately capture PEM incidence. Therefore, it is likely studies opted for measuring general fatigue instead. More appropriate longitudinal study designs are required to track PEM over time to capture a more representative picture of PEM patterns. Secondly, reporting of participant characteristics was inadequate, but in the studies that did describe participants, characteristics were congruent with the epidemiological literature and reporting of ME/CFS populations (i.e., 60–65% female) [ 85 ]. Therefore, in this respect, studies included herein were representative samples. However, the lack of reporting of participant characteristics limits inferences we can draw concerning any population-related effects (i.e. whether older, or male, or European, or people referred by a national health service would be more or less likely to respond positively to pacing). Thirdly, comparison groups (where included) were not ideal, with CBT or GET sometimes used as comparators to pacing [ 35 ], and often no true control group included. Penultimately, there is a distinct lack of high-quality RCTs (as mentioned throughout this manuscript). Finally, in reference to the previous section, inferences from the literature are dated and do not reflect the technological capabilities of 2023.
Recommendations for advancement of the investigative area
It is clear from the studies included in this scoping review for the last decade or more, progress and innovation in pacing research have been limited. This is unfortunate for several reasons. People with ME/CFS or long COVID are, of course, invested in their recovery. From our patient and public involvement (PPI) group engagement, it is clear many are ahead of the research and are using wearable technology to track steps, heart rate, and, in some cases, heart rate variability to improve their own pacing practice. While the lack of progress in the research means this is an understandable response by patients, it is also problematic. Without underpinning research, patients may make decisions based on an individual report of trial-and-error approaches given the lack of evidence-based guidance.
A more technologically-informed pacing approach could be implemented by integrating wearable trackers [ 77 , 78 , 86 , 87 ] to provide participants with live updates on their activity and could be integrated with research-informed messaging aimed at supporting behaviour change, as has been trialled in other research areas [ 88 , 89 , 90 , 91 ]. However, more work is needed to evaluate how to incorporate wearable activity trackers and which metrics are most helpful.
A more technologically-informed approach could also be beneficial for longitudinal symptom tracking, particularly useful given the highly variable symptom loads of ME/CFS and episodic nature of PEM. This would overcome reliance on assessments at a single point in time (as the studies within this review conducted). Similarly, mobile health (mHealth) approaches also allow questionnaires to be digitised to make it easier for participants to complete if they find holding a pen or reading small font problematic [ 92 ]. Reminders and notifications can also be helpful for patients completing tasks [ 77 , 93 , 94 , 95 ]. This approach has the added advantage of allowing contemporaneous data collection rather than relying on pre- to post-intervention designs limited by recall bias. Future work must try to leverage these approaches, as unless we collect large data sets on symptoms and behaviours (i.e. activity, diet, sleep, and pharmacology) in people with conditions like ME/CFS we will not be able to leverage emerging technologies such as AI and machine learning to improve the support and care for people with these debilitating conditions. The key areas for research outline in the NICE guidelines (2021 update) speaks to this, with specific mention of improved self-monitoring strategies, sleep strategies, and dietary strategies, all of which can be measured using mHealth approaches, in a scalable and labour-inexpensive way.
The potential for existing pacing research to address the long COVID pandemic
There is now an urgent public health need to address long COVID, with over 200 million sufferers worldwide [ 30 ]. Given the analogous symptomology between ME/CFS and long COVID, and the lack of promising treatment and management strategies in ME/CFS, pacing remains the only strategy for managing long COVID symptoms. This is concerning as the quality of evidence to support pacing is lacking. Given long COVID has reached pandemic proportions, scalable solutions will be required. In this context, we propose that technology should be harnessed to a) deliver, but also b) evaluate, pacing. We recently reported on a just-in-time adaptive intervention to increase physical activity during the pandemic [ 78 ]. However, this method could be adapted to decrease or maintain physical activity levels (i.e., pacing) in long COVID. This method has the advantage of scalability and remote data collection, reducing resource commitments and participant burden, essential for addressing a condition with so many sufferers.
This review highlights the need for more studies concerning pacing in chronic fatiguing conditions. Future studies would benefit from examining pacing’s effect on symptomology and PEM with objectively quantified pacing, over a longer duration of examination, using the MDS. It is essential this is conducted as an RCT, given that in the case of long COVID, participants may improve their health over time, and it is necessary to determine whether pacing exerts an additional effect over time elapsing. Future studies would benefit from digitising pacing to support individuals with varying symptom severity and personalise support. This would improve accessibility and reduce selection bias, in addition to improving scalability of interventions. Finally, clinicians and practitioners should be cognisant of the strength of evidence reported in this review and should exert caution when promoting pacing in their patients, given the varying methods utilised herein.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
Activity Pacing Questionnaire
Beck Anxiety Inventory
Beck Depression Inventory
Brief Coping Orientation to Problems Experienced Scale
Canadian Occupational Performance Measure
Centers for disease control and prevention
Chalder Fatigue Questionnaire
Checklist Individual Strength
Chronic Pain Coping Inventory
Cognitive behavioural therapy
Cochrane Central Register of Controlled Trials
DePaul symptom questionnaire
EuroQol five-dimensions, five-levels questionnaire
Graded exercise therapy
Hospital Anxiety and Depression Scale
Myalgic encephalomyelitis/chronic fatigue syndrome
Pain Self Efficacy Questionnaire
Pain Anxiety Symptoms Scale short version
Pain Numerical Rating Scale
Patient health questionnaire
Patient reported outcome measures
Physiotherapy Evidence Database
Perceived Stress Scale
Post exertional malaise
Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for scoping reviews
Randomised control trial
McMurray JC, May JW, Cunningham MW, Jones OY. Multisystem Inflammatory Syndrome in Children (MIS-C), a post-viral myocarditis and systemic vasculitis-a critical review of its pathogenesis and treatment. Front Pediatr. 2020;8: 626182.
Article PubMed PubMed Central Google Scholar
Perrin R, Riste L, Hann M, Walther A, Mukherjee A, Heald A. Into the looking glass: post-viral syndrome post COVID-19. Med Hypotheses. 2020;144: 110055.
Article PubMed PubMed Central CAS Google Scholar
Hayes LD, Ingram J, Sculthorpe NF. More than 100 persistent symptoms of SARS-CoV-2 (Long COVID): A scoping review. Front Med. 2021. https://doi.org/10.3389/fmed.2021.750378 .
Article Google Scholar
McLaughlin M, Cerexhe C, Macdonald E, Ingram J, Sanal-Hayes NEM, Hayes LD, et al. A Cross-sectional study of symptom prevalence, frequency, severity, and impact of long-COVID in Scotland: part I. Am J Med. 2023. https://doi.org/10.1016/j.amjmed.2023.07.009 .
Article PubMed Google Scholar
McLaughlin M, Cerexhe C, Macdonald E, Ingram J, Sanal-Hayes NEM, Hayes LD, et al. A cross-sectional study of symptom prevalence, frequency, severity, and impact of long-COVID in Scotland: part II. Am J Med. 2023. https://doi.org/10.1016/j.amjmed.2023.07.009 .
Hayes LD, Sanal-Hayes NEM, Mclaughlin M, Berry ECJ, Sculthorpe NF. People with long covid and ME/CFS exhibit similarly impaired balance and physical capacity: a case-case-control study. Am J Med. 2023;S0002–9343(23):00465–75.
Google Scholar
Jenkins R. Post-viral fatigue syndrome. Epidemiology: lessons from the past. Br Med Bull. 1991;47:952–65.
Article PubMed CAS Google Scholar
Sandler CX, Wyller VBB, Moss-Morris R, Buchwald D, Crawley E, Hautvast J, et al. Long COVID and post-infective fatigue syndrome: a review. Open Forum Infect Dis. 2021;8:440.
Carod-Artal FJ. Post-COVID-19 syndrome: epidemiology, diagnostic criteria and pathogenic mechanisms involved. Rev Neurol. 2021;72:384–96.
PubMed CAS Google Scholar
Ballering AV, van Zon SKR, Olde Hartman TC, Rosmalen JGM. Lifelines corona research initiative. Persistence of somatic symptoms after COVID-19 in the Netherlands: an observational cohort study. Lancet. 2022;400:452–61.
Wong TL, Weitzer DJ. Long COVID and Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS)-a systemic review and comparison of clinical presentation and symptomatology. Medicina (Kaunas). 2021;57:418.
Sukocheva OA, Maksoud R, Beeraka NM, Madhunapantula SV, Sinelnikov M, Nikolenko VN, et al. Analysis of post COVID-19 condition and its overlap with myalgic encephalomyelitis/chronic fatigue syndrome. J Adv Res. 2021. https://doi.org/10.1016/j.jare.2021.11.013 .
Bonilla H, Quach TC, Tiwari A, Bonilla AE, Miglis M, Yang P, et al. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) is common in post-acute sequelae of SARS-CoV-2 infection (PASC): results from a post-COVID-19 multidisciplinary clinic. medrxiv. 2022. https://doi.org/10.1101/2022.08.03.22278363v1 .
Twomey R, DeMars J, Franklin K, Culos-Reed SN, Weatherald J, Wrightson JG. Chronic fatigue and postexertional malaise in people living with long COVID: an observational study. Phys Ther. 2022;102:005.
Barhorst EE, Boruch AE, Cook DB, Lindheimer JB. Pain-related post-exertional malaise in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and fibromyalgia: a systematic review and three-level meta-analysis. Pain Med. 2022;23:1144–57.
Goudsmit EM. The psychological aspects and management of chronic fatigue syndrome [Internet] [Thesis]. Brunel University, School of Social Sciences; 1996 [cited 2022 Jan 20]. https://scholar.google.co.uk/scholar_url?url=https://bura.brunel.ac.uk/bitstream/2438/4283/1/FulltextThesis.pdf&hl=en&sa=X&ei=kNYjZdeuA4-8ywTAmKmADQ&scisig=AFWwaeZvdxcuHmzGL08L3jp-QwNn&oi=scholarr . Accessed 2 Aug 2022
Stussman B, Williams A, Snow J, Gavin A, Scott R, Nath A, et al. Characterization of post-exertional malaise in patients with myalgic encephalomyelitis/chronic fatigue syndrome. Front Neurol. 2020;11:1025.
Holtzman CS, Bhatia KP, Cotler J, La J. Assessment of Post-Exertional Malaise (PEM) in Patients with Myalgic Encephalomyelitis (ME) and Chronic Fatigue Syndrome (CFS): a patient-driven survey. Diagnostics. 2019. https://doi.org/10.3390/diagnostics9010026 .
Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann Intern Med. 1994;121:953–9.
Carruthers BM, van de Sande MI, De Meirleir KL, Klimas NG, Broderick G, Mitchell T, et al. Myalgic encephalomyelitis: international consensus criteria. J Intern Med. 2011;270:327–38.
Carruthers JD, Lowe NJ, Menter MA, Gibson J, Eadie N, Botox Glabellar Lines II Study Group. Double-blind, placebo-controlled study of the safety and efficacy of botulinum toxin type A for patients with glabellar lines. Plast Reconstr Surg. 2003;112:1089–98.
Jason LA, Jordan K, Miike T, Bell DS, Lapp C, Torres-Harding S, et al. A pediatric case definition for myalgic encephalomyelitis and chronic fatigue syndrome. J Chronic Fatigue Syndrome. 2006;13:1–44.
Goudsmit EM, Nijs J, Jason LA, Wallman KE. Pacing as a strategy to improve energy management in myalgic encephalomyelitis/chronic fatigue syndrome: a consensus document. Disabil Rehabil. 2012;34:1140–7.
Antcliff D, Keenan A-M, Keeley P, Woby S, McGowan L. Engaging stakeholders to refine an activity pacing framework for chronic pain/fatigue: a nominal group technique. Musculoskeletal Care. 2019;17:354–62.
Antcliff D, Keeley P, Campbell M, Woby S, McGowan L. Exploring patients’ opinions of activity pacing and a new activity pacing questionnaire for chronic pain and/or fatigue: a qualitative study. Physiotherapy. 2016;102:300–7.
Yoshiuchi K, Cook DB, Ohashi K, Kumano H, Kuboki T, Yamamoto Y, et al. A real-time assessment of the effect of exercise in chronic fatigue syndrome. Physiol Behav. 2007;92:963–8.
Davenport TE, Stevens SR, Baroni K, Van Ness M, Snell CR. Diagnostic accuracy of symptoms characterising chronic fatigue syndrome. Disabil Rehabil. 2011;33:1768–75.
Nurek M, Rayner C, Freyer A, Taylor S, Järte L, MacDermott N, Delaney BC, Panellists D, et al. Recommendations for the recognition, diagnosis, and management of long COVID: a Delphi study. Br J Gen Pract. 2021. https://doi.org/10.3399/BJGP.2021.0265 .
Herrera JE, Niehaus WN, Whiteson J, Azola A, Baratta JM, Fleming TK, Kim SY, Naqvi H, Sampsel S, Silver JK, Gutierrez MV, Maley J, Herman E, Abramoff Benjamin, et al. Multidisciplinary collaborative consensus guidance statement on the assessment and treatment of fatigue in postacute sequelae of SARS-CoV-2 infection (PASC) patients. PM & R. 2021. https://doi.org/10.1002/pmrj.12684 .
Chen C, Haupert SR, Zimmermann L, Shi X, Fritsche LG, Mukherjee B. Global prevalence of post COVID-19 condition or long COVID: a meta-analysis and systematic review. J Infect Dis. 2022. https://doi.org/10.1093/infdis/jiac136 .
Office for National Statistics. Prevalence of ongoing symptoms following coronavirus (COVID-19) infection in the UK [Internet]. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk/7july2022 . Accessed 2 Aug 2022
Office for National Statistics. Prevalence of ongoing symptoms following coronavirus (COVID-19) infection in the UK [Internet]. [cited 2022 Apr 1]. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk/3march2022
Baker R, Shaw EJ. Diagnosis and management of chronic fatigue syndrome or myalgic encephalomyelitis (or encephalopathy): summary of NICE guidance. BMJ. 2007;335:446–8.
NICE. Overview | Myalgic encephalomyelitis (or encephalopathy)/chronic fatigue syndrome: diagnosis and management | Guidance | NICE [Internet]. NICE; [cited 2022 Aug 22]. https://www.nice.org.uk/guidance/ng206 . Accessed 2 Aug 2022
White P, Goldsmith K, Johnson A, Potts L, Walwyn R, DeCesare J, et al. Comparison of adaptive pacing therapy, cognitive behaviour therapy, graded exercise therapy, and specialist medical care for chronic fatigue syndrome (PACE): a randomised trial. The Lancet. 2011;377:823–36.
Article CAS Google Scholar
Vink M. PACE trial authors continue to ignore their own null effect. J Health Psychol. 2017;22:1134–40.
Petrie K, Weinman J. The PACE trial: it’s time to broaden perceptions and move on. J Health Psychol. 2017;22:1198–200.
Stouten B. PACE-GATE: an alternative view on a study with a poor trial protocol. J Health Psychol. 2017;22:1192–7.
Agardy S. Chronic fatigue syndrome patients have no reason to accept the PACE trial results: response to Keith J Petrie and John Weinman. J Health Psychol. 2017;22:1206–8.
Kim D-Y, Lee J-S, Park S-Y, Kim S-J, Son C-G. Systematic review of randomized controlled trials for chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME). J Transl Med. 2020;18:7.
Twisk FNM, Maes M. A review on cognitive behavorial therapy (CBT) and graded exercise therapy (GET) in myalgic encephalomyelitis (ME) / chronic fatigue syndrome (CFS): CBT/GET is not only ineffective and not evidence-based, but also potentially harmful for many patients with ME/CFS. Neuro Endocrinol Lett. 2009;30:284–99.
PubMed Google Scholar
Mays N, Roberts E, Popay J. Synthesising research evidence. In: Fulop N, Allen P, Clarke A, Black N, editors. Studying the organisation and delivery of health services: research methods. London: Routledge; 2001. p. 188–220.
Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. 2018;18:143.
Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8:19–32.
Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169:467–73.
de Morton NA. The PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic study. Aust J Physiother. 2009;55:129–33.
Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83:713–21.
Kos D, van Eupen I, Meirte J, Van Cauwenbergh D, Moorkens G, Meeus M, et al. Activity pacing self-management in chronic fatigue syndrome: a randomized controlled trial. Am J Occup Ther. 2015;69:6905290020.
Meeus M, Nijs J, Van Oosterwijck J, Van Alsenoy V, Truijen S. Pain physiology education improves pain beliefs in patients with chronic fatigue syndrome compared with pacing and self-management education: a double-blind randomized controlled trial. Arch Phys Med Rehabil. 2010;91:1153–9.
Antcliff D, Keenan A-M, Keeley P, Woby S, McGowan L. Testing a newly developed activity pacing framework for chronic pain/fatigue: a feasibility study. BMJ Open. 2021;11: e045398.
Nijs J, van Eupen I, Vandecauter J, Augustinus E, Bleyen G, Moorkens G, et al. Can pacing self-management alter physical behavior and symptom severity in chronic fatigue syndrome? A case series. J Rehabil Res Dev. 2009;46:985–96.
Geraghty K, Hann M, Kurtev S. Myalgic encephalomyelitis/chronic fatigue syndrome patients’ reports of symptom changes following cognitive behavioural therapy, graded exercise therapy and pacing treatments: Analysis of a primary survey compared with secondary surveys. J Health Psychol. 2019;24:1318–33.
Jason L, Muldowney K, Torres-Harding S. The energy envelope theory and myalgic encephalomyelitis/chronic fatigue syndrome. AAOHN J. 2008;56:189–95.
Brown M, Khorana N, Jason LA. The role of changes in activity as a function of perceived available and expended energy in non-pharmacological treatment outcomes for ME/CFS. J Clin Psychol. 2011;67:253.
Association ME. ME/CFS illness management survey results:‘“No decisions about me without me.” Part 1: Results and in-depth analysis of the 2012 ME association patient survey examining the acceptability, efficacy and safety of cognitive behavioural therapy, graded exercise therapy and pacing, as interventions used as management strategies for ME/CFS. 2015. https://www.meassociation.org.uk/wp-content/uploads/NO-DECISIONS-WITHOUT-ME-report.docx . Accessed 2 Feb 2022
Sharpe M, Goldsmith KA, Johnson AL, Chalder T, Walker J, White PD. Rehabilitative treatments for chronic fatigue syndrome: long-term follow-up from the PACE trial. The Lancet Psychiatry. 2015;2:1067–74.
White PD, Goldsmith K, Johnson AL, Chalder T, Sharpe M. Recovery from chronic fatigue syndrome after treatments given in the PACE trial. Psychol Med. 2013;43:2227–35.
O’connor K, Sunnquist M, Nicholson L, Jason LA, Newton JL, Strand EB. Energy envelope maintenance among patients with myalgic encephalomyelitis and chronic fatigue syndrome: Implications of limited energy reserves. Chronic Illn. 2019;15:51–60.
Dougall D, Johnson A, Goldsmith K, Sharpe M, Angus B, Chalder T, et al. Adverse events and deterioration reported by participants in the PACE trial of therapies for chronic fatigue syndrome. J Psychosom Res. 2014;77:20–6.
Brown AA, Evans MA, Jason LA. Examining the energy envelope and associated symptom patterns in chronic fatigue syndrome: does coping matter? Chronic Illn. 2013;9:302–11.
Bourke JH, Johnson AL, Sharpe M, Chalder T, White PD. Pain in chronic fatigue syndrome: response to rehabilitative treatments in the PACE trial. Psychol Med. 2014;44:1545–52.
Jason LA, Brown M, Brown A, Evans M, Flores S, Grant-Holler E, et al. Energy conservation/envelope theory interventions to help patients with myalgic encephalomyelitis/chronic fatigue syndrome. Fatigue. 2013;1:27–42.
Antcliff D, Campbell M, Woby S, Keeley P. Activity pacing is associated with better and worse symptoms for patients with long-term conditions. Clin J Pain. 2017;33:205–14.
Nijs T, Klein Y, Mousavi S, Ahsan A, Nowakowska S, Constable E, et al. The different faces of 4’-Pyrimidinyl-Functionalized 4,2’:6’,4"-Terpyridin es: metal-organic assemblies from solution and on Au(111) and Cu(111) surface platforms. J Am Chem Soc. 2018;140:2933–9.
Cutler DM, Summers LH. The COVID-19 pandemic and the $16 Trillion Virus. JAMA. 2020;324:1495–6.
Cutler DM. The costs of long COVID. JAMA Health Forum. 2022;3:e221809–e221809.
Geraghty K. ‘PACE-Gate’: when clinical trial evidence meets open data access. J Health Psychol. 2017;22:1106–12.
Davis R, Campbell R, Hildon Z, Hobbs L, Michie S. Theories of behaviour and behaviour change across the social and behavioural sciences: a scoping review. Health Psychol Rev. 2015;9:323–44.
Prestwich A, Sniehotta FF, Whittington C, Dombrowski SU, Rogers L, Michie S. Does theory influence the effectiveness of health behavior interventions? Meta-analysis Health Psychol. 2014;33:465–74.
Balinas C, Eaton-Fitch N, Maksoud R, Staines D, Marshall-Gradisnik S. Impact of life stressors on Myalgic encephalomyelitis/chronic fatigue syndrome symptoms: an Australian longitudinal study. Int J Environ Res Public Health. 2021;18:10614.
McGregor NR, Armstrong CW, Lewis DP, Gooley PR. Post-exertional malaise is associated with hypermetabolism, hypoacetylation and purine metabolism deregulation in ME/CFS cases. Diagnostics. 2019;9:70.
Nacul LC, Lacerda EM, Campion P, Pheby D, de Drachler M, Leite JC, et al. The functional status and well being of people with myalgic encephalomyelitis/chronic fatigue syndrome and their carers. BMC Public Health. 2011;11:402.
Deumer U-S, Varesi A, Floris V, Savioli G, Mantovani E, López-Carrasco P, et al. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): an overview. J Clin Med. 2021. https://doi.org/10.3390/jcm10204786 .
Düking P, Giessing L, Frenkel MO, Koehler K, Holmberg H-C, Sperlich B. Wrist-worn wearables for monitoring heart rate and energy expenditure while sitting or performing light-to-vigorous physical activity: validation study. JMIR Mhealth Uhealth. 2020;8: e16716.
Falter M, Budts W, Goetschalckx K, Cornelissen V, Buys R. Accuracy of apple watch measurements for heart rate and energy expenditure in patients with cardiovascular disease: cross-sectional study. JMIR Mhealth Uhealth. 2019;7: e11889.
Fuller D, Colwell E, Low J, Orychock K, Tobin MA, Simango B, et al. Reliability and validity of commercially available wearable devices for measuring steps, energy expenditure, and heart rate: systematic review. JMIR Mhealth Uhealth. 2020;8: e18694.
Mair JL, Hayes LD, Campbell AK, Sculthorpe N. Should we use activity tracker data from smartphones and wearables to understand population physical activity patterns? J Measur Phys Behav. 2022;1:1–5.
Mair JL, Hayes LD, Campbell AK, Buchan DS, Easton C, Sculthorpe N. A personalized smartphone-delivered just-in-time adaptive intervention (JitaBug) to increase physical activity in older adults: mixed methods feasibility study. JMIR Formative Res. 2022;6: e34662.
Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W, et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med. 2013;46:81–95.
Feehan SM. The PACE trial in chronic fatigue syndrome. The Lancet. 2011;377:1831–2.
Giakoumakis J. The PACE trial in chronic fatigue syndrome. The Lancet. 2011;377:1831.
White PD, Sharpe MC, Chalder T, DeCesare JC, Walwyn R, PACE trial group. Protocol for the PACE trial: a randomised controlled trial of adaptive pacing, cognitive behaviour therapy, and graded exercise, as supplements to standardised specialist medical care versus standardised specialist medical care alone for patients with the chronic fatigue syndrome/myalgic encephalomyelitis or encephalopathy. BMC Neurol. 2007;7:6.
Reuben DB, Tinetti ME. Goal-oriented patient care–an alternative health outcomes paradigm. N Engl J Med. 2012;366:777–9.
Roberts D. Chronic fatigue syndrome and quality of life. PROM. 2018;9:253–62.
Valdez AR, Hancock EE, Adebayo S, Kiernicki DJ, Proskauer D, Attewell JR, et al. Estimating prevalence, demographics, and costs of ME/CFS using large scale medical claims data and machine learning. Front Pediatr. 2019. https://doi.org/10.3389/fped.2018.00412 .
Greiwe J, Nyenhuis SM. Wearable technology and how this can be implemented into clinical practice. Curr Allergy Asthma Rep. 2020;20:36.
Sun S, Folarin AA, Ranjan Y, Rashid Z, Conde P, Stewart C, et al. Using smartphones and wearable devices to monitor behavioral changes during COVID-19. J Med Internet Res. 2020;22: e19992.
Hardeman W, Houghton J, Lane K, Jones A, Naughton F. A systematic review of just-in-time adaptive interventions (JITAIs) to promote physical activity. Int J Behav Nutr Phys Act. 2019;16:31.
Perski O, Hébert ET, Naughton F, Hekler EB, Brown J, Businelle MS. Technology-mediated just-in-time adaptive interventions (JITAIs) to reduce harmful substance use: a systematic review. Addiction. 2022;117:1220–41.
AhmedS A, van Luenen S, Aslam S, van Bodegom D, Chavannes NH. A systematic review on the use of mHealth to increase physical activity in older people. Clinical eHealth. 2020;3:31–9.
Valenzuela T, Okubo Y, Woodbury A, Lord SR, Delbaere K. Adherence to technology-based exercise programs in older adults: a systematic review. J Geriatric Phys Ther. 2018;41:49–61.
Bowling A. Mode of questionnaire administration can have serious effects on data quality. J Public Health. 2005;27:281–91.
Burns SP, Terblanche M, Perea J, Lillard H, DeLaPena C, Grinage N, et al. mHealth intervention applications for adults living with the effects of stroke: a scoping review. Arch Rehabil Res Clin Transl. 2021;3: 100095.
Vandelanotte C, Müller AM, Short CE, Hingle M, Nathan N, Williams SL, et al. Past, present, and future of eHealth and mHealth research to improve physical activity and dietary behaviors. J Nutr Educ Behav. 2016;48:219-228.e1.
Ludwig K, Arthur R, Sculthorpe N, Fountain H, Buchan DS. Text messaging interventions for improvement in physical activity and sedentary behavior in youth: systematic review. JMIR Mhealth Uhealth. 2018;6:e10799.
Download references
Acknowledgements
We have no acknowledgements to make.
Open access funding provided by Swiss Federal Institute of Technology Zurich. This work was supported by grants from the National Institute for Health and Care Research (COV-LT2-0010) and the funder had no role in the conceptualisation, design, data collection, analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and affiliations.
Sport and Physical Activity Research Institute, School of Health and Life Sciences, University of the West of Scotland, Glasgow, UK
Nilihan E. M. Sanal-Hayes, Marie Mclaughlin, Lawrence D. Hayes, David Carless, Rachel Meach & Nicholas F. Sculthorpe
Future Health Technologies, Singapore-ETH Centre, Campus for Research Excellence and Technological Enterprise (CREATE), Singapore, Singapore
Jacqueline L. Mair
Saw Swee Hock School of Public Health, National University of Singapore, Singapore, Singapore
Long COVID Scotland, 12 Kemnay Place, Aberdeen, UK
Jane Ormerod
Physios for ME, London, UK
Natalie Hilliard
School of Education and Social Sciences, University of the West of Scotland, Glasgow, UK
Joanne Ingram
School of Health and Society, University of Salford, Salford, UK
Nilihan E. M. Sanal-Hayes
School of Sport, Exercise & Rehabilitation Sciences, University of Hull, Hull, UK
Marie Mclaughlin
You can also search for this author in PubMed Google Scholar
Contributions
Authors’ contributions are given according to the CRediT taxonomy as follows: Conceptualization, N.E.M.S–H., M.M., L.D.H, and N.F.S.; methodology, N.E.M.S–H., M.M., L.D.H., and N.F.S.; software, N.E.M.S–H., M.M., L.D.H., and N.F.S.B.; validation, N.E.M.S–H., M.M., L.D.H, and N.F.S.; formal analysis, N.E.M.S–H., M.M., L.D.H., and N.F.S.; investigation, N.E.M.S–H., M.M., L.D.H., and N.F.S.; resources, L.D.H., J.O., D.C., N.H., J.L.M., and N.F.S.; data curation, N.E.M.S.-H., M.M., L.D.H., and N.F.S.; writing—original draft preparation, N.E.M.S.-H., M.M., L.D.H., and N.F.S.; writing—review and editing, N.E.M.S–H., M.M., L.D.H., J.O., D.C., N.H., R.M., J.L.M., J.I., and N.F.S.; visualisation, N.E.M.S–H. and M.M., supervision, N.F.S; project administration, N.E.M.S–H., M.M., L.D.H., and N.F.S.; funding acquisition, L.D.H., J.O., D.C., N.H., J.L.M., J.I., and N.F.S. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Correspondence to Jacqueline L. Mair .
Ethics declarations
Ethical approval and content to participate.
This manuscript did not involve human participants, data, or tissues, so did not require ethical approval.
Consent for publication
This paper does not contain any individual person’s data in any form.
Competing interests
We report no financial and non-financial competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1..
Supplementary file 1. Full search string for databse searching.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Sanal-Hayes, N.E.M., Mclaughlin, M., Hayes, L.D. et al. A scoping review of ‘Pacing’ for management of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): lessons learned for the long COVID pandemic. J Transl Med 21 , 720 (2023). https://doi.org/10.1186/s12967-023-04587-5
Download citation
Received : 30 June 2023
Accepted : 03 October 2023
Published : 14 October 2023
DOI : https://doi.org/10.1186/s12967-023-04587-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Myalgic encephalomyelitis
- Chronic fatigue syndrome
- Post-exertional malaise
Journal of Translational Medicine
ISSN: 1479-5876
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
Examples of literature reviews. Step 1 - Search for relevant literature. Step 2 - Evaluate and select sources. Step 3 - Identify themes, debates, and gaps. Step 4 - Outline your literature review's structure. Step 5 - Write your literature review.
A literature review may consist of simply a summary of key sources, but in the social sciences, a literature review usually has an organizational pattern and combines both summary and synthesis, often within specific conceptual categories.A summary is a recap of the important information of the source, but a synthesis is a re-organization, or a reshuffling, of that information in a way that ...
Sources for literature review and examples. Generally, your literature review should integrate a wide range of sources such as: Books. Textbooks remain as the most important source to find models and theories related to the research area. Research the most respected authorities in your selected research area and find the latest editions of ...
Writing a literature review requires a range of skills to gather, sort, evaluate and summarise peer-reviewed published data into a relevant and informative unbiased narrative. Digital access to research papers, academic texts, review articles, reference databases and public data sets are all sources of information that are available to enrich ...
Types of Literature Review are as follows: Narrative literature review: This type of review involves a comprehensive summary and critical analysis of the available literature on a particular topic or research question. It is often used as an introductory section of a research paper. Systematic literature review: This is a rigorous and ...
Okay - with the why out the way, let's move on to the how. As mentioned above, writing your literature review is a process, which I'll break down into three steps: Finding the most suitable literature. Understanding, distilling and organising the literature. Planning and writing up your literature review chapter.
Demonstrate your knowledge of the research topic. Identify the gaps in the literature and show how your research links to these. Provide the foundation for your conceptual framework (if you have one) Inform your own methodology and research design. To achieve this, your literature review needs a well-thought-out structure.
In writing the literature review, your purpose is to convey to your reader what knowledge and ideas have been established on a topic, and what their strengths and weaknesses are. As a piece of writing, the literature review must be defined by a guiding concept (e.g., your research objective, the problem or issue you are discussing, or your ...
literature Review and Focusing the Research 93 The Search Process No matter what the reason for the literature review or the paradigm within which the researcher is working, many aspects of the literature review process are the same. A general outline for conducting a literature review is provided in Box 3.1.
Critically reviewing the literature is an indispensible skill which is used throughout a research career. This article demystifies the processes involved in systematically and critically reviewing the literature to demonstrate knowledge, identify research ideas, position research and develop theory. Although aimed primarily at research students ...
Literature Review is a comprehensive survey of the works published in a particular field of study or line of research, usually over a specific period of time, in the form of an in-depth, critical bibliographic essay or annotated list in which attention is drawn to the most significant works. Also, we can define a literature review as the ...
Compares management and healthcare research • Highlights the challenges of conducting a systematic review in management research ... the basics steps and important choices involved in conducting a literature review will be suggested and discussed using four phases; (1) designing the review, (2) conducting the review, (3) analysis and (4 ...
Finding sources (scholarly articles, research books, dissertations, etc.) for your literature review is part of the research process. This process is iterative, meaning you repeat and modify searches until you have gathered enough sources for your project. The main steps in this research process are:
A literature review determines where your research question falls within the body of research within a discipline. Things to keep in mind: Cast a wide search at first to find all the related material to your topic. Then select the most relevant source material as it pertains to your topic and purpose. Consider: Time periods
Literature Review. This is the most time-consuming aspect in the preparation of your research proposal and it is a key component of the research proposal. As described in Chapter 5, the literature review provides the background to your study and demonstrates the significance of the proposed research. Specifically, it is a review and synthesis ...
A literature review is a critical analysis and synthesis of existing research on a particular topic. It provides an overview of the current state of knowledge, identifies gaps, and highlights key findings in the literature. 1 The purpose of a literature review is to situate your own research within the context of existing scholarship, demonstrating your understanding of the topic and showing ...
A literature review surveys books, scholarly articles, and any other sources relevant to a particular. issue, area of research, or theory, and by so doing, provides a description, summary, and ...
The main purpose of the review is to introduce the readers to the need for conducting the said research. A literature review should begin with a thorough literature search using the main keywords in relevant online databases such as Google Scholar, PubMed, etc. Once all the relevant literature has been gathered, it should be organized as ...
C. Once you have completed the brainstorming process and your topic is more focused, you can do preliminary research to help you identify a specific research question.. 1) Examine overview sources such as subject-specific encyclopedias and textbooks that are likely to break down your specific topic into sub-topics and to highlight core issues that could serve as possible research questions.
Literature reviews are essential in moving our evidence-base forward. "A literature review makes a significant contribution when the authors add to the body of knowledge through providing new insights" (Bearman, 2016, p. 383).Although there are many methods for conducting a literature review (e.g., systematic review, scoping review, qualitative synthesis), some commonalities in ...
This article highlights four essential considerations as you start drafting a structure for your literature review. Comprehensiveness. There are two types of literature review: one is the literature review section within a paper and the other is a literature review synthesis paper that aims to provide a framework for future studies. For the ...
These sections serve to establish a scholarly basis for the research or discussion within the paper. In a standard 8000-word journal article, the literature review section typically spans between 750 and 1250 words. The first few sentences or the first paragraph within this section often serve as an introduction.
Some Issues in Liter ature R eview. 1. A continuous and time consuming process runs. through out r esearch work (more whil e selecting. a resear ch problem and writing 'r eview of. liter ature ...
Many countries around the world are rapidly advancing sustainable development (SD) through the exploitation of clean energy sources such as solar and wind energy, which are becoming the core of the sustainable energy transition. In recent years, the continuous advancement of Earth system models (ESMs) has facilitated numerous studies utilizing them to predict long-term and large-scale ...
Background: Health apps are increasingly recognized as crucial tools for enhancing health care delivery. Many countries, particularly those in sub-Saharan Africa, can substantially benefit from using health apps to support self-management and thus help to achieve universal health coverage and the third sustainable development goal. However, most health apps published in app stores are of ...
Consequently, while a comprehensive review of the effects of pacing in ME/CFS is an essential tool to guide symptom management advice, the available literature means that effective pooling of data is not feasible and therefore, a traditional systematic review and meta-analysis, with a tightly focussed research question would be premature ...