
Clinical Trials Market Size & Share Analysis - Growth Trends & Forecasts (2024 - 2029)
The Report Covers Global Clinical Trials Market Growth Analysis and is Segmented by Phase (Phase I, Phase II, Phase III, and Phase IV), Design (Treatment Studies and Observational Studies), and Geography (North America, Europe, Asia-Pacific, Middle East and Africa, and South America).
Clinical Trial Market Size

Need a report that reflects how COVID-19 has impacted this market and its growth?
Clinical Trial Market Analysis
The Clinical Trials Market size is estimated at USD 50.66 billion in 2024, and is expected to reach USD 67.5 billion by 2029, growing at a CAGR of 5.91% during the forecast period (2024-2029).
The COVID-19 pandemic tremendously impacted the market for clinical trials, as there has been a rising focus on developing new therapeutics or vaccines to treat the disease. Also, COVID-19 brought a shift in terms of the way clinical trials are performed. There has been an increased interest in virtual/decentralized trials in the clinical trial space, and those have been featured on conference agendas and in articles for a long time. Moreover, COVID-19 forced some of the trials to move to a virtual model to keep the trials on track during the pandemic. Additionally, as of November 8, 2022, a total of 8,397 studies have been registered for COVID-19 on the ClinicalTrials.gov website, among which 2,932 studies were registered for Europe alone, followed by 2,290 studies in North America. Thus, such an increase in the number of clinical trials registered to find an effective treatment for the disease is anticipated to drive market growth. Therefore, COVID-19 is predicted to have a significant impact on the market studied.
The major factors propelling the market's growth include the high demand for clinical trials in emerging markets, increased research and development (R&D) spending in the pharmaceutical industry, an increasing prevalence of diseases; and the focus on rare diseases and multiple orphan drugs in the pipeline. For instance, Novartis AG, one of the major players in the studied market, invested USD 9,540 million in the year 2021, which increased from USD 8,980 million in 2020. In addition, another market player, Pfizer Inc., invested USD 13,829 million in 2021 on R&D as compared to USD 9,393 in FY 2020. Thus, the increased research and development expenses by the major players in the market are expected to drive the growth of the market.
Factors such as growing burden of diseases need more advanced and effective medicines for treatment and thus contributes to the growth of the market. For instance, as per IDF Atlas 2021 edition, in 2021, there were 536.6 million people aged 20-79 years living with diabetes around the world and this number is expected to reach 783.7 million by 2045. Such high burden of diseases also propel the growth of the market.
Additionally, the initiatives taken by the government in different regions also conbtribute to the growth of the market. For instance, in January 2022, the European Commission (EC), the Heads of Medicines Agencies (HMA), and the European Medicines Agency (EMA) launched an initiative to transform how clinical trials are initiated, designed, and ran, referred to as Accelerating Clinical Trials in the EU (ACT EU). The aim of ACT EU is to further develop Europe as a focal point for clinical research, to further promote the development of high-quality, safe, and effective medicines, and to better integrate clinical research into the European health system. Such, initiatives by governments across the globe are contributing to market growth.
Therefore, owing to the factors mentioned above, the studied market is anticipated to grow over the forecast period. However, the lack of a skilled workforce in clinical research and stringent regulations for patient enrolment are factors that are expected to hinder the market growth during the analysis period.
Clinical Trial Market Trends
Phase iii by phase segment is expected to grow over the forecast period.
Phase III clinical trials evaluate the comparative effect of the new medication over the previous medications available or conducted to confirm and expand on safety and effectiveness results from Phase 1 and 2 trials. This usually involves up to 3,000 participants with the condition that the new medication is meant to treat and may last for many years. Also, the number of Phase III clinical trials remains comparatively higher than Phase II and Phase I trials, owing to their greater complexity and need for a larger patient pool. Factors such as increasing research activities, the growing burden of diseases, and many investigative drugs in Phase III are propelling the growth of the market segment.
The high number of clinical trials in Phase III is driving the growth of the market segment. For instance, according to the data from clinicaltrials.gov, as of November 8, 2022, 9,137 clinical trials were in Phase III for cancer, 5,069 for cardiology, and 5,217 for respiratory studies. Thus, such a high number of clinical trials registered under phase III of clinical trials is expected to contribute to the segment's growth.
Additionally, the Phase III trials conducted by market players are also contributing to the growth of the market segment. For instance, in May 2022, Lipidor AB reported that half of the patients have been enrolled in the Phase III study of AKP02 skin spray for mild to moderate psoriasis. Also, in August 2022, Wockhardt Ltd initiated a global Phase III clinical study of its new antibiotic candidate WCK 5222. It is entirely a new class of antibiotic known as "β-lactam ENHANCER', and is targeted for the treatment of hospitalized adults with complicated urinary tract infections, including acute pyelonephritis. Such a high number of studies in Phase III depicts the growth of the segment.
Thus, the factors mentioned above are expected to propel the segment's growth over the forecast period.

North America is Expected to Dominate the Market Over The Forecast Period
The North American region is expected to contribute significantly to the market growth during the study period owing to factors such as high R&D expenditure of the pharmaceutical industry, presence of well-established players, robust regulatory framework, and rising prevalence of diseases, coupled with the significant contribution of the United States.
The American Cancer Society estimated that in the United States, around 1,918,0303 new cases are estimated to register in 2022. Thus, the high burden of cancer is expected to boost the demand for the development of drugs and devices for disease diagnosis and treatment, thereby driving the market growth.
Additionally, the support from the government of the countries in the region is also contributing to the growth of the market. For instance, in June 2022, the Government of Canada launched the Clinical Trials Fund (CTF), supported by a Budget 2021 investment of USD 250 million over three years for the Canadian Institutes of Health Research (CIHR). With this funding, the government aims to improve health outcomes for Canadians while ensuring Canada is well-positioned to respond to future pandemics and other health priorities. The CTF will strengthen the clinical trials infrastructure in Canada and support the training of new clinical researchers.
Furthermore, the major market players in the region are active in the innovation of new drugs and devices, which is another factor predicted to contribute to the market growth in the region. For instance, in September 2021, Janssen started the Phase III trial for the investigational respiratory syncytial virus (RSV) vaccine among older adults. The study will evaluate the efficacy, safety and immunogenicity of Janssen's investigational adult vaccine against lower respiratory tract disease (LRTD) throughout North America and some other countries of different regions. Such trials are expected to propel the growth of the market in the region.
Such continuous developments are expected to fuel the clinical trials market in the North American region.

Clinical Trial Industry Overview
The clinical trials market is moderately competitive. Strategic partnerships between pharmaceutical companies and CROs are expected to impact the market's growth significantly. Also, the quick adoption of advanced technology for improved healthcare contributes to the growth of the market. Some of the key players are Clinipace, Eli Lilly and Company, Laboratory Corporation of America, ICON PLC, and Novo Nordisk AS.
Clinical Trial Market Leaders
Laboratory Corporation of America
Eli Lilly and Company
Novo Nordisk AS
*Disclaimer: Major Players sorted in no particular order

Clinical Trial Market News
- July 2022: An early-stage clinical trial investigating an investigational vaccine to stave off Nipah virus infection was started by the National Institute of Allergy and Infectious Diseases (NIAID), a division of the National Institutes of Health (NIH) of the United States.
- May 2022: The International AIDS Vaccine Initiative (IAVI) and Moderna Inc. started a Phase I clinical trial of an mRNA vaccine antigen in Rwanda and South Africa.
Clinical Trial Market Report - Table of Contents
1. INTRODUCTION
1.1 Study Assumptions and Market Definition
1.2 Scope of the Study
2. RESEARCH METHODOLOGY
3. EXECUTIVE SUMMARY
4. MARKET DYNAMICS
4.1 Market Overview
4.2 Market Drivers
4.2.1 Demand for Clinical Trials in the Emerging Markets
4.2.2 High R&D Expenditure of the Pharmaceutical Industry
4.2.3 Rising Prevalence of Diseases
4.3 Market Restraints
4.3.1 Lack of Skilled Workforce in Clinical Research
4.3.2 Stringent Regulations for Patient Enrollment
4.4 Porter's Five Forces Analysis
4.4.1 Threat of New Entrants
4.4.2 Bargaining Power of Buyers/Consumers
4.4.3 Bargaining Power of Suppliers
4.4.4 Threat of Substitute Products
4.4.5 Intensity of Competitive Rivalry
5. MARKET SEGMENTATION (Market Size by Value - USD million)
5.1 By Phase
5.1.1 Phase I
5.1.2 Phase II
5.1.3 Phase III
5.1.4 Phase IV
5.2 By Design
5.2.1 Treatment Studies
5.2.1.1 Randomized Control Trial
5.2.1.2 Adaptive Clinical Trial
5.2.1.3 Non-randomized Control Trial
5.2.2 Observational Studies
5.2.2.1 Cohort Study
5.2.2.2 Case Control Study
5.2.2.3 Cross Sectional Study
5.2.2.4 Ecological Study
5.3 Geography
5.3.1 North America
5.3.1.1 United States
5.3.1.2 Canada
5.3.1.3 Mexico
5.3.2 Europe
5.3.2.1 Germany
5.3.2.2 United Kingdom
5.3.2.3 France
5.3.2.4 Italy
5.3.2.5 Spain
5.3.2.6 Rest of Europe
5.3.3 Asia-Pacific
5.3.3.1 China
5.3.3.2 Japan
5.3.3.3 India
5.3.3.4 Australia
5.3.3.5 South Korea
5.3.3.6 Rest of Asia-Pacific
5.3.4 Middle East and Africa
5.3.4.1 GCC
5.3.4.2 South Africa
5.3.4.3 Rest of Middle East and Africa
5.3.5 South America
5.3.5.1 Brazil
5.3.5.2 Argentina
5.3.5.3 Rest of South America
6. COMPETITIVE LANDSCAPE
6.1 Company Profiles
6.1.1 Clinipace
6.1.2 Laboratory Corporation of America
6.1.3 Eli Lilly and Company
6.1.4 ICON PLC
6.1.5 Novo Nordisk AS
6.1.6 PAREXEL International Corporation
6.1.7 Pfizer Inc.
6.1.8 Pharmaceutical Product Development LLC
6.1.9 IQVIA
6.1.10 F. Hoffmann-La Roche Ltd
6.1.11 Sanofi SA
6.1.12 Syneos Health
6.1.13 ClinDatrix Inc
6.1.14 Charles River Laboratory
- *List Not Exhaustive
7. MARKET OPPORTUNITIES AND FUTURE TRENDS
Clinical Trial Industry Segmentation
As per the scope of the report, clinical trials are experiments that are conducted under clinical research and follow a regulated protocol. These experiments are primarily performed to obtain data regarding the safety and efficacy of newly developed drugs. Clinical trial data is mandatory for drug approval and for it to be introduced in the market. This process is expensive and time-consuming and requires expertise at all stages. The Clinical Trials Market is Segmented by Phase (Phase I, Phase II, Phase III, and Phase IV), Design (Treatment Studies and Observational Studies), and Geography (North America, Europe, Asia-Pacific, Middle East and Africa, and South America). The market report also covers the estimated market sizes and trends for 17 different countries across major regions globally. The report offers values (in USD million) for the above segments.
Clinical Trial Market Research FAQs
How big is the clinical trials market.
The Clinical Trials Market size is expected to reach USD 50.66 billion in 2024 and grow at a CAGR of 5.91% to reach USD 67.50 billion by 2029.
What is the current Clinical Trials Market size?
In 2024, the Clinical Trials Market size is expected to reach USD 50.66 billion.
Who are the key players in Clinical Trials Market?
Clinipace, Laboratory Corporation of America, ICON PLC, Eli Lilly and Company and Novo Nordisk AS are the major companies operating in the Clinical Trials Market.
Which is the fastest growing region in Clinical Trials Market?
North America is estimated to grow at the highest CAGR over the forecast period (2024-2029).
Which region has the biggest share in Clinical Trials Market?
In 2024, the Asia Pacific accounts for the largest market share in Clinical Trials Market.
What years does this Clinical Trials Market cover, and what was the market size in 2023?
In 2023, the Clinical Trials Market size was estimated at USD 47.83 billion. The report covers the Clinical Trials Market historical market size for years: 2019, 2020, 2021, 2022 and 2023. The report also forecasts the Clinical Trials Market size for years: 2024, 2025, 2026, 2027, 2028 and 2029.
Why is North America anticipated to witness a rapid growth rate in the Clinical Trials Market?
North America is anticipated to witness a rapid growth rate in the Clinical Trials Market due to a) High R&D expenditure of the pharmaceutical industry b) Presence of well-established players c) Robust regulatory framework d) Rising prevalence of diseases.
Which is the major segment in the Clinical Trials Market by phase?
The major segment in the Clinical Trials Market by phase is Phase III. This is mainly due to the high number of clinical trials conducted in this phase compared to others.
Our Best Selling Reports
- Sports Apparel Market
- EV Charging Station Market
- Automotive Suspension Systems Market
- Electric 3 Wheeler Market
- Pharmaceutical Plastic Packaging Market
- Security Paper Market
- Japan Used Car Market
- Lens Market
- Footwear Market
- Automotive Transmission Market
Clinical Research Industry Report
This comprehensive report offers a deep dive into the clinical trials industry, providing a detailed analysis of key market drivers and market segments. Mordor Intelligence offers customization based on your specific interests, including: 1. Service - Laboratory, Analytical Testing, Bioanalytical Testing 2. Therapeutic Area - Oncology, Neurology, CNS, Autoimmune/Inflammation 3. Application - MABs, CGT 4. End-User - Hospitals, Laboratories, and Clinics
Clinical Research Market Report Snapshots
- Clinical Research Market Size
- Clinical Research Market Share
- Clinical Research Market Trends
- Clinical Research Companies
Please enter a valid email id!
Please enter a valid message!

Clinical Trials Market Get a free sample of this report
Please enter your name
Business Email
Please enter a valid email
Please enter your phone number
Get this Data in a Free Sample of the Clinical Trials Market Report
Please enter your requirement

Thank you for choosing us for your research needs! A confirmation has been sent to your email. Rest assured, your report will be delivered to your inbox within the next 72 hours. A member of our dedicated Client Success Team will proactively reach out to guide and assist you. We appreciate your trust and are committed to delivering precise and valuable research insights.
Please be sure to check your spam folder too.
Sorry! Payment Failed. Please check with your bank for further details.
Add Citation APA MLA Chicago
➜ Embed Code X
Get Embed Code
Want to use this image? X
Please copy & paste this embed code onto your site:
Images must be attributed to Mordor Intelligence. Learn more
About The Embed Code X
Mordor Intelligence's images may only be used with attribution back to Mordor Intelligence. Using the Mordor Intelligence's embed code renders the image with an attribution line that satisfies this requirement.
In addition, by using the embed code, you reduce the load on your web server, because the image will be hosted on the same worldwide content delivery network Mordor Intelligence uses instead of your web server.
- Health, Pharma & Medtech ›
Pharmaceutical Products & Market
Clinical trials – statistics & facts
How much do pharma companies spend on r&d, the complexities of clinical studies, drug developers seek approval, key insights.
Detailed statistics
Pharmaceuticals: cost of drug development in the U.S. since 1975
Total global pharmaceutical R&D spending 2014-2028
Number of drugs in the R&D pipeline worldwide 2001-2023
Editor’s Picks Current statistics on this topic
Current statistics on this topic.
Pharmaceuticals
Total number of registered clinical studies worldwide 2000-2024
CRO market size worldwide forecast 2028
Biotechnology
Top companies by COVID-19 treatment vaccines in development June 2022
Related topics
Recommended.
- Global pharmaceutical industry
- Pharmaceutical industry in the U.S.
- Top pharmaceutical drugs
- Coronavirus (COVID-19) vaccines and treatments
- Pharmaceutical research and development (R&D)
Recommended statistics
R&d overview.
- Premium Statistic Total global pharmaceutical R&D spending 2014-2028
- Premium Statistic Industry sectors - expenditure on research and development 2021
- Premium Statistic Top pharmaceutical R&D projects based on net present value August 2023
- Basic Statistic Research and development expenditure: U.S. pharmaceutical industry 1995-2022
- Premium Statistic Research and development in European pharmaceutical industry by country 2021
- Premium Statistic Total clinical research funding by National Institutes for Health 2013-2024
Total global pharmaceutical R&D spending 2014-2028
Total global spending on pharmaceutical research and development from 2014 to 2028 (in billion U.S. dollars)
Industry sectors - expenditure on research and development 2021
Percentage of spending on research and development of total revenue in 2021, by industrial sector
Top pharmaceutical R&D projects based on net present value August 2023
Selected top pharmaceutical R&D projects based on net present value (NPV) as of August 2023 (in billion U.S. dollars)
Research and development expenditure: U.S. pharmaceutical industry 1995-2022
Research and development expenditure of total U.S. pharmaceutical industry from 1995 to 2022 (in billion U.S. dollars)
Research and development in European pharmaceutical industry by country 2021
Pharmaceutical research and development spending in selected European countries in 2021 (in million euros)
Total clinical research funding by National Institutes for Health 2013-2024
Total clinical research funding by the National Institutes for Health (NIH) from FY 2013 to FY 2024 (in million U.S. dollars)
Top R&D companies
- Basic Statistic Global top pharmaceutical companies based on R&D spending 2026
- Basic Statistic Top 50 global pharmaceutical and biotech companies by R&D intensity in 2022
- Basic Statistic Top pharmaceutical companies in R&D spending growth 2022
- Premium Statistic Leading global contract research organizations based on revenue 2022
- Basic Statistic Roche: participation of patients in clinical trials 2009-2017
Global top pharmaceutical companies based on R&D spending 2026
Global top 10 pharmaceutical companies based on projected R&D spending in 2026 (in billion U.S. dollars)
Top 50 global pharmaceutical and biotech companies by R&D intensity in 2022
World's top 50 pharmaceutical and biotechnology companies based on R&D intensity in 2022
Top pharmaceutical companies in R&D spending growth 2022
World's top 50 pharmaceutical and biotechnology companies based on R&D spending growth in 2022
Leading global contract research organizations based on revenue 2022
Leading global contract research organizations (CROs) based on 2022 revenue (in million U.S. dollars)
Roche: participation of patients in clinical trials 2009-2017
Number of patients who took part in clinical trials for pharmaceutical company Roche from 2009 to 2017*
Clinical studies
- Basic Statistic Increase in clinical trials' complexity 2001-2015
- Premium Statistic Clinical trial success rates by therapeutic area 2020
- Basic Statistic Number of registered clinical studies by location worldwide 2024
- Basic Statistic Percent of registered clinical studies worldwide by location 2024
- Basic Statistic Share of recruiting clinical studies worldwide by location 2024
- Premium Statistic Total number of registered clinical studies worldwide 2000-2024
- Basic Statistic Total number of registered clinical studies with posted results worldwide 2008-2024
Increase in clinical trials' complexity 2001-2015
Increase in clinical trials' complexity between 2001-2005 and 2011-2015
Clinical trial success rates by therapeutic area 2020
Clinical trial success rates by therapeutic area as of 2020*
Number of registered clinical studies by location worldwide 2024
Number of registered clinical studies worldwide by location as of April 2024
Percent of registered clinical studies worldwide by location 2024
Percentage of registered clinical studies worldwide by location as of April 2024
Share of recruiting clinical studies worldwide by location 2024
Percentage of registered recruiting clinical studies worldwide by location as of April 2024
Total number of registered clinical studies worldwide since 2000 (as of April 2024)
Total number of registered clinical studies with posted results worldwide 2008-2024
Total number of registered clinical studies with posted results worldwide since 2008 (as of April 2024)
Participation
- Basic Statistic Top clinical trial participant countries worldwide 2015-19, by share
- Basic Statistic Clinical trial participants U.S. vs. rest of world by therapy area 2015-2019
- Basic Statistic Clinical trial participants gender share worldwide 2015-19, by geographic location
- Basic Statistic Clinical trial participants ethnicity share worldwide 2015-19, by geographic location
Top clinical trial participant countries worldwide 2015-19, by share
Top 20 clinical trial participant countries worldwide in 2015-2019, by share of participants
Clinical trial participants U.S. vs. rest of world by therapy area 2015-2019
Number of clinical trial participants in the U.S. and rest of the world in 2015-2019, by therapeutic area (in 1,000s)
Clinical trial participants gender share worldwide 2015-19, by geographic location
Gender share of clinical trial participants worldwide in 2015-2019, by geographic location
Clinical trial participants ethnicity share worldwide 2015-19, by geographic location
Ethnicity share of clinical trial participants worldwide in 2015-2019, by geographic location
Costs and market
- Basic Statistic Pharmaceuticals: cost of drug development in the U.S. since 1975
- Premium Statistic R&D expenditure of new therapeutic drugs 2009-2018
- Basic Statistic Estimated clinical trial cost per patient by therapeutic class 2015-2017
- Basic Statistic Estimated clinical trial cost per drug by therapeutic class 2015-2017
- Premium Statistic CRO market size worldwide forecast 2028
- Premium Statistic Global pharma CRO market size 2015-2024, by pre-clinical, clinical and discovery
- Premium Statistic Size of total U.S. clinical trial supplies market 2016-2025
Cost of developing a drug in the U.S. from the 1970s until today (in million U.S. dollars)*
R&D expenditure of new therapeutic drugs 2009-2018
Mean and median R&D expenditure on new drugs by therapeutic area between 2009 and 2018* (in million U.S. dollars)
Estimated clinical trial cost per patient by therapeutic class 2015-2017
Estimated clinical trial cost per patient by therapeutic area in 2015-2017* (in U.S. dollars)
Estimated clinical trial cost per drug by therapeutic class 2015-2017
Estimated clinical trial cost per drug by therapeutic area in 2015-2017* (in million U.S. dollars)
Global contract research organization (CRO) market in 2023 and 2028 (in billion U.S. dollars)
Global pharma CRO market size 2015-2024, by pre-clinical, clinical and discovery
Global pharmaceutical CRO market size from 2015 to 2024, by pre-clinical, clinical and discovery (in billion U.S. dollars)
Size of total U.S. clinical trial supplies market 2016-2025
Size of the total U.S. clinical trial supplies market from 2016 to 2025 (in million U.S. dollars)
Approvals, launches, setbacks
- Premium Statistic Pharmaceutical industry - number of new substances 1998-2022
- Premium Statistic Number of novel drugs approved annually by CDER 2008-2023
- Basic Statistic Key measurements of U.S. CDER drug approvals in 2023
- Basic Statistic FDA first premarket approvals for medtech products granted 2005-2022
- Premium Statistic Projection of top 2024 pharma and biotech launches by revenue 2028
- Basic Statistic Number of unsuccessful Alzheimer’s drugs in development in the U.S. 1998-2017
Pharmaceutical industry - number of new substances 1998-2022
Number of new chemical or biological entities developed between 1998 and 2022, by region of origin
Number of novel drugs approved annually by CDER 2008-2023
Total number of novel drugs approved by CDER from 2008 to 2023
Key measurements of U.S. CDER drug approvals in 2023
Percentage of drugs approved by the U.S. Center for Drug Evaluation and Research (CDER) in 2023 that met select key measurements
FDA first premarket approvals for medtech products granted 2005-2022
Number of first premarket approvals (PMA/HDE) granted by the FDA for medtech products from 2005 to 2022
Projection of top 2024 pharma and biotech launches by revenue 2028
Leading biotech and pharma product launches in 2024 and revenue forecasts for 2028 (in billion U.S. dollars)
Number of unsuccessful Alzheimer’s drugs in development in the U.S. 1998-2017
Number of unsuccessful Alzheimer’s drugs in development in the U.S. from 1998 to 2017
- Premium Statistic Top companies by COVID-19 treatment vaccines in development June 2022
- Basic Statistic Number of COVID-19 drugs in development worldwide by phase June 2022
- Basic Statistic Number of COVID-19 treatment vaccine trials worldwide by phase June 2022
- Basic Statistic Number of COVID-19 treatment vaccine trials worldwide by type June 2022
Leading companies by number of COVID-19 drugs and vaccines in development as of June 3, 2022
Number of COVID-19 drugs in development worldwide by phase June 2022
Number of coronavirus (COVID-19) drugs and vaccines in development worldwide as of June 3, 2022, by phase
Number of COVID-19 treatment vaccine trials worldwide by phase June 2022
Number of coronavirus (COVID-19) clinical trials for drugs and vaccines worldwide as of June 3, 2022, by phase
Number of COVID-19 treatment vaccine trials worldwide by type June 2022
Number of coronavirus (COVID-19) clinical trials for drugs and vaccines worldwide as of June 3, 2022, by type*
Further reports Get the best reports to understand your industry
Get the best reports to understand your industry.
- Pharmaceutical trends in Europe
- Generics and biosimilars
Mon - Fri, 9am - 6pm (EST)
Mon - Fri, 9am - 5pm (SGT)
Mon - Fri, 10:00am - 6:00pm (JST)
Mon - Fri, 9:30am - 5pm (GMT)
- Healthcare IT
- Clinical Trials Market
"Designing Growth Strategies is in our DNA"
Clinical Trials Market Size, Share & Industry Analysis, By Phase (Phase I, Phase II, Phase III, and Phase IV), By Application (Oncology, CNS Disorder, Cardiology, Infectious Disease, Metabolic Disorder, Renal/Nephrology, and Others), and Regional Forecast, 2024-2032
Last Updated: April 30, 2024 | Format: PDF | Report ID: FBI106930
- Segmentation
- Methodology
- Infographics
- Request Sample PDF
KEY MARKET INSIGHTS
The global clinical trials market size was valued at USD 57.76 billion in 2023 and is projected to grow from USD 61.58 billion in 2024 to USD 106.78 billion by 2032, exhibiting a CAGR of 7.1% during the forecast period (2024-2032). Clinical trials are an important process in developing new therapeutics or medical devices. These studies are performed to analyze novel pharmaceuticals, medical devices, or any other therapeutics. These studies provide a scientific foundation for guiding and treating patients and evaluating novel medications and equipment. Increasing R&D by pharmaceutical and biotechnological companies globally has fueled the number of clinical trials being conducted globally.
Furthermore, increased companies' focus on the development of novel treatments for chronic diseases and rising demand for outsourcing R&D activities have also been fueling the market’s growth. For instance, in July 2021, the Beijing Illness Challenge Foundation (ICF) in China formed a strategic relationship with Parexel. This ground-breaking collaboration aimed to obtain direct feedback from individuals with rare diseases to improve their access to and participation in these studies.
The impact of COVID-19 pandemic resulted in the slow growth of the market during the pandemic. Many clinical studies were put on hold after the sudden outbreak of COVID-19 due to lockdown restrictions and the low presence of resources.
However, many pharmaceutical and biotechnological companies increased their focus on the development of drugs, test kits, and vaccines against the SARS-CoV-2 virus. These companies increased their focus on collaboration and partnership with CRO service providers for R&D and clinical studies.
- In January 2021, ICON plc, BioNTech, and Pfizer announced their partnership to develop an experimental COVID-19 vaccine program to provide clinical trial services.
However, the market experienced a significant recovery in 2021 compared to the prior year, due to the release of lockdown restrictions, mass vaccination, and increased demand for clinical trials to develop novel treatments.
Clinical Trials Market Trends
Pharmaceutical and Biotechnological Companies Increased their Investments in R&D
Many medical device, pharmaceutical, and biopharmaceutical companies continue to put significant resources into the development of technologies and new medications. The pharmaceutical sector, in particular, has been majorly making investments in R&D initiatives for the development of novel therapeutics. Pharmaceutical and biotechnology companies have increased their focus on expanding their R&D efficiencies by investing in R&D.
- For instance, according to the research article published by NCBI, in 2021, overall pharmaceutical expenditures in the U.S. increased by 7.7% as compared to 2020, for a total of USD 576.90 billion.
Moreover, over the past two decades, both R&D spending and the launch of new drugs have witnessed notable increases. This is due to the rapidly growing demand for innovative medicines to treat a wide range of diseases.
- For instance, as per the data published by the Congressional Budget Office in April 2021, the pharmaceutical industry’s research and development expenditure reached USD 83.00 billion in 2019, marking a tenfold increase compared to its yearly spending in the 1980s when adjusted for inflation.
Furthermore, these companies also initiated outsourcing of their R&D activities to CRO companies for time efficient and smooth conduction of these trials.
Request a Free sample to learn more about this report.
Clinical Trials Market Growth Factors
Increasing Prevalence of Chronic Diseases to Increase the Demand for the Development of Efficient Therapeutics
The burden of chronic diseases, such as diabetes, several types of cancers, neurological disorders, and arthritis, has been increasing at a significant pace globally. This is expected to fuel the demand for the development of more effective therapeutics.
- For instance, as per the data provided by the University of Washington (UW) education, in June 2023, the global population of individuals living with diabetes currently exceeds half a billion, and projections indicate it will surpass 1.30 billion within the next three decades. This growth is anticipated across all countries, marking a significant global increase.
- Similarly, according to the data published by Globocan in 2020, Europe accounted for 22.8% of all cancer cases and 19.6% of cancer deaths, representing 9.7% of the global population.
People worldwide are affected by chronic diseases. Compared to developing countries, emerging countries are more prone to chronic disease-related public health problems. According to the World Health Organization (WHO), in five out of six regions, chronic disease is the major reason for mortality. Infectious diseases, such as malaria, TB, HIV/AIDS, and other conditions, are still predominant in Sub-Saharan Africa and are expected to prevail in the coming years.
The global market is anticipated to grow significantly due to the high prevalence of chronic diseases during the forecast period.
Rising Number of Clinical Trials Globally has been Fueling the Market Growth
Registration of trials has been growing at a significant rate annually to meet the increasing demand to treat chronic diseases.
- For instance, as per the data published by WHO in 2022, the total number of these trials conducted in 2021 experienced an increase of 11.7% from the prior year.
These study trials conducted in the U.S. are comparatively fewer than in other countries worldwide. Due to its cost-effectiveness and easy process, many of the trials are conducted outside of the U.S. and the European Union. Clinical trial success rates are majorly dependent on the stage of the study and the treatments or items being developed.
In recent years, the number of registered trials has increased significantly.
- For instance, as per the International Clinical Trials Registry Platform (ICTRP), the annual number of these registered trials by high-income countries increased from 21,028 in 2010 to 29,538 in 2020.
In addition, clinical trials have addressed chronic diseases, emerging infectious diseases, and other global health problems through research and development of novel medicines. Clinical trial research has recognized several necessary interventions for various diseases. Therefore, increase in development of new drugs & medical devices is expected to drive the clinical trials market growth during the forecast period.
RESTRAINING FACTORS
Limited Availability of Skilled Workforce and High Costs of Study Trials Limit the Market Growth
Increasing globalization has been fueling the adoption of advanced technology. New opportunities are emerging in terms of occupation. Furthermore, rising industrialization and the requirement for new amenities have fueled the need for new skills. This factor has also enhanced competency in job opportunities.
Contract Research Organization (CRO) services face issues in drawing and maintaining vastly proficient experts as they require qualified as well as experienced scientists from the field of pharmaceutical, biotechnology, academic & research institutes, and medical device businesses. Companies must give high rewards and other such recognitions to compete efficiently, impacting other players’ capitals and operational outcomes, majorly small-scale analytical testing providers. This limited availability of experienced specialists could limit the adoption of advanced technologies and processes, limiting the market’s growth in the coming years.
Furthermore, adherence to regulatory requirements significantly impacts clinical trial costs. From research to the final drug approval, the drug development process is quite costly. According to the Tufts Center for the Study of Drug Development, on average, the complete drug development process costs USD 2.60 billion to develop a new medicine, including the cost of failures. Moreover, only 12.0% of new drug candidates that enter study trials get the U.S. FDA approval.
An additional important factor affecting the costs of a trial is the complexity of the study design and protocol. Apart from financial costs, there are several obstructions to conducting clinical trials, including difficulties in recruitment, lengthy time frames, retention of participants, insufficiencies in the clinical research workforce, and drug sponsor-imposed barriers.
These factors are expected to restrict the market growth during the forecast period.
Clinical Trials Market Segmentation Analysis
By phase analysis.
Increased Registration of Clinical Trials along with the Emergence of CRO Services has been Fueling the Segment’s Growth
Based on phase, the market is segregated into phase I, phase II, phase III, and phase IV.
Phase III segment generated the highest revenue in 2023. The market players’ increased initiative to outsource their R&D activities is responsible for the segment’s dominance in the market.
Furthermore, the phase II segment is expected to grow at the fastest CAGR over the forecast period. The segment's growth is attributed to the increasing prevalence of chronic diseases and increasing investment by pharmaceutical companies in the R&D of novel treatments.
To know how our report can help streamline your business, Speak to Analyst
By Application Analysis
Limited Presence for Effective Treatment of Cancer has been Fueling the Segment’s Growth
Based on application, the market is segmented into renal/nephrology, cardiology, metabolic disorder, infectious disease, CNS disorder, oncology, and others.
The oncology segment dominated the market by generating the highest revenue in 2023. The segment's growth is due to the growing demand for effective cancer treatment therapies and the rising number of drug approvals for cancer treatment.
- For instance, in 2020, Pralsetinib (Gavreto) received FDA approval. It is indicated for adult and pediatric patients aged 12 or older suffering from advanced or metastatic RET-mutant medullary thyroid cancer, which requires systemic therapy or RET fusion.
Furthermore, CNS disorder is expected to grow at a significant CAGR during the forecast period. The increase in the prevalence of CNS disorders is expected to accelerate the segment's growth. For instance, in 2019, neurological conditions accounted for 47.39 per 100,000 among the American population.
Moreover, the metabolic disorder segment is expected to grow substantially during the forecast period. This is due to the rise in prevalence of chronic diseases, such as diabetes, globally. For instance, according to a published article in 2020 by OECD-iLibrary, in Asia Pacific, around 227 million people are living with type 2 diabetes; half of them are undiagnosed, and thus could develop long-term complications. These factors are propelling CRO services for the metabolic disorder segment.
REGIONAL INSIGHTS
North America Clinical Trials Market Size, 2023 (USD Billion)
To get more information on the regional analysis of this market, Request a Free sample
North America accounted for a major clinical trials market share, generating a revenue of USD 27.65 billion in 2023. Pharmaceutical companies have increased their spending on R&D to improve their drug development. This has been fueling the market growth during the forecast period in the region.
- For instance, as per the Pharmaceutical Research and Manufacturers of America (PhRMA) trade group, in 2021, PhRMA's member companies' Research & Development (R&D) expenditure reached around USD 102.3 billion worldwide.
The market in Europe accounted for a substantial market share in 2021 and is expected to witness stagnant growth during the forecast period. The market growth in the region is attributed to increased R&D expenditure by leading pharmaceutical, biotechnology, and MedTech companies.
- For instance, in 2020, Roche Diagnostics spent around USD 11.30 billion on R&D.
Moreover, the market across the Asia Pacific region is expected to expand at the fastest CAGR during the forecast. The increasing prevalence of infectious and chronic diseases across Asia Pacific is expected to propel the demand for new drugs, thereby increasing the overall market in Asia Pacific.
- For instance, as per the data published in December 2023, around 35.0% of the Indian population is suffering from chronic illnesses, including diabetes and cardiac-related disorders.
List of Key Companies in Clinical Trials Market
Pharmaceutical Companies with a Strong Focus on the Expansion of Product Portfolios to Hold Key Market Share
IQVIA, Laboratory Corporation of America Holdings, and Pfizer, Inc. are among the prominent players in the market and captured a considerable global market share in 2023.
IQVIA and Laboratory Corporation of America Holdings accounted for significant market share in 2023. This is due to their strong emphasis on R&D to introduce solutions and to upskill their offerings.
- For instance, in November 2021, IQVIA announced its data aggregation strategy as a foundation to improve market insights. This helped the company to connect the right data and services to help patients. This increased the efficacy of the company’s services.
Similarly, Pfizer, Inc. held a considerable share of the market in 2023. This was due to the company’s strong brand presence with a strong pipeline of products. Moreover, the company strongly focuses on developing advanced and highly efficient therapeutics for chronic disease treatment.
Other significant players operating in the market, such as Icon PLC, Syneos Health, and Pharmaceutical Product Development, LLC (Thermo Fisher Scientific), emphasize various strategic developments such as service expansion, partnerships, and collaborations.
LIST OF KEY COMPANIES PROFILED:
- IQVIA Inc. (U.S.)
- Laboratory Corporation of America Holdings (U.S.)
- Thermo Fisher Scientific Inc . (U.S.)
- Parexel International Corporation (U.S.)
- Medpace Holdings, Inc. (U.S.)
- Icon plc (Ireland)
- Syneos Health (U.S.)
- WuXi AppTec (China)
- Charles River Laboratories (U.S.)
- Pfizer Inc . (U.S.)
- Lilly (U.S.)
KEY INDUSTRY DEVELOPMENTS:
- December 2023 – Thermo Fisher Scientific Inc. introduced CorEvidence, a cloud-based optimizing pharmacovigilance case processing and safety data management processes.
- December 2021 – Thermo Fisher Scientific Inc. announced the completion of the acquisition of Pharmaceutical Product Development, LLC. This acquisition expanded the key services and offerings provided by the company.
- December 2021 – Laboratory Corporation of America Holdings acquired Toxikon Corporation. This acquisition fueled the company’s strong non-clinical development portfolio.
- November 2021 – Icon plc announced the expansion of its Accellacare Site Network in reach and capabilities through new partnerships with six research sites across four countries.
- October 2021 - Parexel International Corporation and Kyoto University Hospital formed a strategic partnership to expand clinical research opportunities.
- July 2021 – Icon plc announced the acquisition of PRA Health Sciences, creating the world’s most advanced healthcare intelligence and clinical research organization.
REPORT COVERAGE
The research report provides a detailed competitive landscape. It includes the number of clinical trials and key industry developments such as partnerships, mergers, and acquisitions. Additionally, it focuses on key points such as new product launches in the market. Furthermore, the report covers regional analysis of different segments, company profiles of key players, and market trends. The report consists of quantitative and qualitative insights that contribute to the market growth.
To gain extensive insights into the market, Request for Customization
Report Scope & Segmentation
Frequently asked questions.
Fortune Business Insights says that the global market stood at USD 57.76 billion in 2023 and is projected to reach USD 106.78 billion by 2032.
The market is expected to exhibit a CAGR of 7.1% during the forecast period (2024-2032).
The phase III segment is set to lead the market by phase.
The key factors driving the market are increasing prevalence of chronic diseases and rising number of clinical trials.
IQVIA, Laboratory Corporation of America Holdings, and Pfizer, Inc. are the top players in the market.
Seeking Comprehensive Intelligence on Different Markets? Get in Touch with Our Experts
- STUDY PERIOD: 2019-2032
- BASE YEAR: 2023
- HISTORICAL DATA: 2019-2022
- NO OF PAGES: 151
Personalize this Research
- Granular Research on Specified Regions or Segments
- Companies Profiled based on User Requirement
- Broader Insights Pertaining to a Specific Segment or Region
- Breaking Down Competitive Landscape as per Your Requirement
- Other Specific Requirement on Customization

Healthcare Clients

Related Reports
- Contract Research Organization (CRO) Services Market
- Europe CRO Services Market
- ASEAN Contract Development and Manufacturing Organization (CDMO) Market
- Preclinical CRO Market
Client Testimonials
“We are quite happy with the methodology you outlined. We really appreciate the time your team has spent on this project, and the efforts of your team to answer our questions.”
“Thanks a million. The report looks great!”
“Thanks for the excellent report and the insights regarding the lactose market.”
“I liked the report; would it be possible to send me the PPT version as I want to use a few slides in an internal presentation that I am preparing.”
“This report is really well done and we really appreciate it! Again, I may have questions as we dig in deeper. Thanks again for some really good work.”
“Kudos to your team. Thank you very much for your support and agility to answer our questions.”
“We appreciate you and your team taking out time to share the report and data file with us, and we are grateful for the flexibility provided to modify the document as per request. This does help us in our business decision making. We would be pleased to work with you again, and hope to continue our business relationship long into the future.”
“I want to first congratulate you on the great work done on the Medical Platforms project. Thank you so much for all your efforts.”
“Thank you very much. I really appreciate the work your team has done. I feel very comfortable recommending your services to some of the other startups that I’m working with, and will likely establish a good long partnership with you.”
“We received the below report on the U.S. market from you. We were very satisfied with the report.”
“I just finished my first pass-through of the report. Great work! Thank you!”
“Thanks again for the great work on our last partnership. We are ramping up a new project to understand the imaging and imaging service and distribution market in the U.S.”
“We feel positive about the results. Based on the presented results, we will do strategic review of this new information and might commission a detailed study on some of the modules included in the report after end of the year. Overall we are very satisfied and please pass on the praise to the team. Thank you for the co-operation!”
“Thank you very much for the very good report. I have another requirement on cutting tools, paper crafts and decorative items.”
“We are happy with the professionalism of your in-house research team as well as the quality of your research reports. Looking forward to work together on similar projects”
“We appreciate the teamwork and efficiency for such an exhaustive and comprehensive report. The data offered to us was exactly what we were looking for. Thank you!”
“I recommend Fortune Business Insights for their honesty and flexibility. Not only that they were very responsive and dealt with all my questions very quickly but they also responded honestly and flexibly to the detailed requests from us in preparing the research report. We value them as a research company worthy of building long-term relationships.”
“Well done Fortune Business Insights! The report covered all the points and was very detailed. Looking forward to work together in the future”
“It has been a delightful experience working with you guys. Thank you Fortune Business Insights for your efforts and prompt response”
“I had a great experience working with Fortune Business Insights. The report was very accurate and as per my requirements. Very satisfied with the overall report as it has helped me to build strategies for my business”
“This is regarding the recent report I bought from Fortune Business insights. Remarkable job and great efforts by your research team. I would also like to thank the back end team for offering a continuous support and stitching together a report that is so comprehensive and exhaustive”
“Please pass on our sincere thanks to the whole team at Fortune Business Insights. This is a very good piece of work and will be very helpful to us going forward. We know where we will be getting business intelligence from in the future.”
“Thank you for sending the market report and data. It looks quite comprehensive and the data is exactly what I was looking for. I appreciate the timeliness and responsiveness of you and your team.”
Get in Touch with Us
+1 424 253 0390 (US)
+44 2071 939123 (UK)
+91 744 740 1245 (APAC)
[email protected]
- Request Sample
Sharing this report over the email

The global clinical trials market size is projected to grow from $61.58 billion in 2024 to $106.78 billion by 2032, at a CAGR of 7.1% during the forecast period
Read More at:-
- Healthcare and Pharmaceuticals
- Healthcare Equipment and Services
Clinical Trials Market
Global Clinical Trials Market to Grow at a CAGR of 5.4% in the Forecast Period, Aided by the Increasing Focus on Therapeutic Drugs Generation

Global Clinical Trials Market Size, Share, Trends, Forecast: By Design: Treatment Studies, Observational Studies; By Phase; By Service Type: Site Identification, Patient Recruitment, Laboratory Services, Analytical Testing Services, Others; By Therapy Area; By Application; Regional Analysis; Patent Analysis; Supplier Landscape; 2024-2032
- Report Summary
- Table of Contents
- Pricing Detail
- Request Sample
Global Clinical Trials Market Outlook
The clinical trials market size attained a value of USD 49.22 billion in 2023. The market is anticipated to grow at a CAGR of 5.4% during the forecast period of 2024-2032 to attain a value of nearly USD 79.02 billion by 2032.

Read more about this report - REQUEST FREE SAMPLE COPY IN PDF
Increasing Demand for Oncology to Augment the Clinical Trials Industry Growth
Oncology clinical trials are more complex than other therapeutic disciplines since they require advanced treatments for specific malignancies. An oncology trial looks not only into the efficacy and safety of an antibiotic against infection, but also how it can improve the subject's quality of life. Because cancer research is growing at a quick rate, immunotherapy has shifted away from chemotherapy procedures and toward molecularly targeted medicines. The evolution of oncology clinical trials has seen rapid development in available clinical data, as well as a strong and rising pipeline of therapeutic candidates.
North America to Provide Significant Growth Opportunities to the Clinical Trial Industry
Geographically, North America accounts for a dominant share in the industry owing to large investment for outsourcing the big R&D companies. This is mainly due to the major demand for investments in drug development. Due to the rising COVID-19 cases, many big R&D firms have collaborated with government and non-government organisations to manufacture therapeutic drug injections. The United States is one of the largest global manufacturers of clinical trials as they are investing more in R&D development. Meanwhile, the Asia Pacific is expected to witness a robust growth in the forecast period due to the increasing focus of manufacturers to produce vaccines to decrease death risk due to the COVID-19 pandemic.
Clinical Trials: Market Segmentation
Clinical trials are research studies that are used to assess the effectiveness of a medicinal, surgical, or behavioural intervention. They are the primary method by which scientists determine whether a new treatment, such as a new medicine, diet, or medical instrument, is safe and effective in humans. A clinical trial is frequently performed to determine whether a new medication is more effective and has fewer negative side effects.

By design, the market is divided into:
- Randomised Control Trial
- Adaptive Clinical Trail
- Non- Randomised Control Trial
- Cohort Studies
- Case Control Study
- Cross Section Study
- Ecological Study
The regional markets for clinical trials include North America, Europe, the Asia Pacific, Latin America, and the Middle East and Africa.

Increasing Focus on Therapeutic Drugs Generation to Bolster the Growth of Clinical Trials Industry
The global clinical trial industry is being driven by the increasing demand for the production of therapeutic drugs across the globe. Due to the COVID-19 pandemic, significant changes in the clinical trials industry has shifted the central point of many research and commercial companies to give attention on the development of new therapeutic drugs and vaccines for COVID-19. The industry is also predicted to develop as a result of government initiatives in emerging nations to promote medication discovery and constant technical advances. Over the forecast period, it can be expected that Phase III will grow and become significant as it is one of the most important stages in determining whether or not a new intervention is effective and useful for clinical purposes.
Key Industry Players in the Global Clinical Trials Market
The report presents a detailed analysis of the following key players in the global clinical trials market, looking into their capacity, market shares, and latest developments like capacity expansions, plant turnarounds, and mergers and acquisitions:
- Parexel International Corporation
- Charles River laboratory
- Syneos Health
The comprehensive report looks into the macro and micro aspects of the industry. The EMR report gives an in-depth insight into the market by providing a SWOT analysis as well as an analysis of Porter’s Five Forces model.
Key Highlights of the Report
*At Expert Market Research, we strive to always give you current and accurate information. The numbers depicted in the description are indicative and may differ from the actual numbers in the final EMR report.
1 Preface 1.1 Objectives of the Study 1.2 Key Assumptions 1.3 Report Coverage – Key Segmentation and Scope 1.4 Research Methodology 2 Executive Summary 3 Global Clinical Trial Market Overview 3.1 Global Clinical Trial Market Historical Value (2017-2023) 3.2 Global Clinical Trial Market Forecast Value (2024-2032) 4 Global Clinical Trial Market Landscape 4.1 Global Clinical Trial Developers Landscape 4.1.1 Analysis by Year of Establishment 4.1.2 Analysis by Company Size 4.1.3 Analysis by Region 4.2 Global Clinical Trial Product Landscape 4.2.1 Analysis by Service Type 4.2.2 Analysis by Design 4.2.3 Analysis by Therapy Area 5 Global Clinical Trial Market Dynamics 5.1 Market Drivers and Constraints 5.2 SWOT Analysis 5.3 Porter’s Five Forces Model 5.4 Key Demand Indicators 5.5 Key Price Indicators 5.6 Industry Events, Initiatives, and Trends 5.7 Value Chain Analysis 6 Global Clinical Trial Market Segmentation 6.1 Global Clinical Trial Market by Design 6.1.1 Market Overview 6.1.2 Treatment Studies 6.1.2.1 Randomised Control Trial 6.1.2.2 Adaptive Clinical Trail 6.1.2.3 Non- Randomised Control Trial 6.1.3 Observational Studies 6.1.3.1 Cohort Studies 6.1.3.2 Case Control Studies 6.1.3.3 Cross Section Study 6.1.3.4 Ecological Study 6.1.3.5 Others 6.2 Global Clinical Trial Market by Phase 6.2.1 Market Overview 6.2.2 Phase I 6.2.3 Phase II 6.2.4 Phase III 6.2.5 Phase IV 6.3 Global Clinical Trial Market by Service Type 6.3.1 Market Overview 6.3.2 Site Identification 6.3.3 Patient Recruitment 6.3.4 Laboratory Services 6.3.5 Analytical Testing Services 6.3.6 Bio-Analytical Testing Services 6.3.6.1 Cell Based Assay 6.3.6.2 Virology Testing 6.3.6.3 PK/PD Testing Services 6.3.6.4 Method Development, Optimization, Validation 6.3.6.5 Serology, Immunogenicity & Neutralizing Antibodies 6.3.6.6 Bio Marker Testing Service 6.3.6.7 Other Bioanalytical Testing Services 6.3.7 Clinical Trial Data Management Services 6.3.8 Clinical Trial Supply and Logistic Services 6.3.9 Medical Device Testing Services 6.3.10 Other Clinical Trial Services 6.4 Global Clinical Trial Market by Therapy Area 6.4.1 Market Overview 6.4.2 Oncology 6.4.3 Infectious Disease 6.4.4 Neurology 6.4.5 Immunology 6.4.6 Cardiology 6.4.7 Genetic Disease 6.4.8 Women’s Health 6.4.9 Other Therapy Areas 6.5 Global Clinical Trial Market by Application 6.5.1 Market Overview 6.5.2 Small Molecule 6.5.3 Vaccine 6.5.4 Cell & Gene Therapy 6.5.5 Other Applications 6.6 Global Clinical Trial Market by Region 6.6.1 Market Overview 6.6.2 North America 6.6.3 Europe 6.6.4 Asia Pacific 6.6.5 Latin America 6.6.6 Middle East and Africa 7 North America Clinical Trial Market 7.1 Market Share by Country 7.2 United States of America 7.3 Canada 8 Europe Clinical Trial Market 8.1 Market Share by Country 8.2 United Kingdom 8.3 Germany 8.4 France 8.5 Italy 8.6 Others 9 Asia Pacific Clinical Trial Market 9.1 Market Share by Country 9.2 China 9.3 Japan 9.4 India 9.5 ASEAN 9.6 Australia 9.7 Others 10 Latin America Clinical Trial Market 10.1 Market Share by Country 10.2 Brazil 10.3 Argentina 10.4 Mexico 10.5 Others 11 Middle East and Africa Clinical Trial Market 11.1 Market Share by Country 11.2 Saudi Arabia 11.3 United Arab Emirates 11.4 Nigeria 11.5 South Africa 11.6 Others 12 Patent Analysis 12.1 Analysis by Type of Patent 12.2 Analysis by Publication year 12.3 Analysis by Issuing Authority 12.4 Analysis by Patent Age 12.5 Analysis by CPC Analysis 12.6 Analysis by Patent Valuation 12.7 Analysis by Key Players 13 Grants Analysis 13.1 Analysis by year 13.2 Analysis by Amount Awarded 13.3 Analysis by Issuing Authority 13.4 Analysis by Grant Application 13.5 Analysis by Funding Institute 13.6 Analysis by NIH Departments 13.7 Analysis by Recipient Organization 14 Funding Analysis 14.1 Analysis by Funding Instances 14.2 Analysis by Type of Funding 14.3 Analysis by Funding Amount 14.4 Analysis by Leading Players 14.5 Analysis by Leading Investors 14.6 Analysis by Geography 15 Partnership and Collaborations Analysis 15.1 Analysis by Partnership Instances 15.2 Analysis by Type of Partnership 15.3 Analysis by Leading Players 15.4 Analysis by Geography 16 Regulatory Framework 16.1 Regulatory Overview 16.1.1 US FDA 16.1.2 EU EMA 16.1.3 INDIA CDSCO 16.1.4 JAPAN PMDA 16.1.5 Others 17 Supplier Landscape 17.1 IQVIA 17.1.1 Financial Analysis 17.1.2 Service Portfolio 17.1.3 Demographic Reach and Achievements 17.1.4 Mergers and Acquisitions 17.1.5 Certifications 17.2 PAREXEL International Corporation 17.2.1 Financial Analysis 17.2.2 Service Portfolio 17.2.3 Demographic Reach and Achievements 17.2.4 Mergers and Acquisitions 17.2.5 Certifications 17.3 Charles River Laboratory 17.3.1 Financial Analysis 17.3.2 Service Portfolio 17.3.3 Demographic Reach and Achievements 17.3.4 Mergers and Acquisitions 17.3.5 Certifications 17.4 ICON Plc 17.4.1 Financial Analysis 17.4.2 Service Portfolio 17.4.3 Demographic Reach and Achievements 17.4.4 Mergers and Acquisitions 17.4.5 Certifications 17.5 Syneos Health 17.5.1 Financial Analysis 17.5.2 Service Portfolio 17.5.3 Demographic Reach and Achievements 17.5.4 Mergers and Acquisitions 17.5.5 Certifications 17.6 Labcorp Drug Development (COVANCE) 17.6.1 Financial Analysis 17.6.2 Service Portfolio 17.6.3 Demographic Reach and Achievements 17.6.4 Mergers and Acquisitions 17.6.5 Certifications 17.7 Wuxi Apptec 17.7.1 Financial Analysis 17.7.2 Service Portfolio 17.7.3 Demographic Reach and Achievements 17.7.4 Mergers and Acquisitions 17.7.5 Certifications 17.8 Charles River Laboratories 17.8.1 Financial Analysis 17.8.2 Service Portfolio 17.8.3 Demographic Reach and Achievements 17.8.4 Mergers and Acquisitions 17.8.5 Certifications 17.9 PPD Inc 17.9.1 Financial Analysis 17.9.2 Service Portfolio 17.9.3 Demographic Reach and Achievements 17.9.4 Mergers and Acquisitions 17.9.5 Certifications 17.10 ICON Plc 17.10.1 Financial Analysis 17.10.2 Service Portfolio 17.10.3 Demographic Reach and Achievements 17.10.4 Mergers and Acquisitions 17.10.5 Certifications 17.11 Medpace Holdings Inc 17.11.1 Financial Analysis 17.11.2 Service Portfolio 17.11.3 Demographic Reach and Achievements 17.11.4 Mergers and Acquisitions 17.11.5 Certifications 17.12 Acm Global Laboratories 17.12.1 Financial Analysis 17.12.2 Service Portfolio 17.12.3 Demographic Reach and Achievements 17.12.4 Mergers and Acquisitions 17.12.5 Certifications 17.13 Advanced Clinical 17.13.1 Financial Analysis 17.13.2 Service Portfolio 17.13.3 Demographic Reach and Achievements 17.13.4 Mergers and Acquisitions 17.13.5 Certifications 17.14 SGS 17.14.1 Financial Analysis 17.14.2 Service Portfolio 17.14.3 Demographic Reach and Achievements 17.14.4 Mergers and Acquisitions 17.14.5 Certifications 17.15 PSI CRO AG 17.15.1 Financial Analysis 17.15.2 Service Portfolio 17.15.3 Demographic Reach and Achievements 17.15.4 Mergers and Acquisitions 17.15.5 Certifications 17.16 Bio Agile Therapeutics 17.16.1 Financial Analysis 17.16.2 Service Portfolio 17.16.3 Demographic Reach and Achievements 17.16.4 Mergers and Acquisitions 17.16.5 Certifications 18 Global Clinical Trial Market- Distribution Model (Additional Insight) 18.1 Overview 18.2 Potential Distributors 18.3 Key Parameters for Distribution Partner Assessment 19 Key Opinion Leaders (KOL) Insights (Additional Insight) 20 Company Competitiveness Analysis (Additional Insight) 20.1 Very Small Companies 20.2 Small Companies 20.3 Mid-Sized Companies 20.4 Large Companies 20.5 Very Large Companies 21 Payment Methods (Additional Insight) 21.1 Government Funded 21.2 Private Insurance 21.3 Out-of-Pocket
*Additional insights provided are customisable as per client requirements.
What was the clinical trial market size in 2023?
The global clinical trial Market was valued at USD 49.22 billion in 2023.
What is the forecast outlook for the clinical trial market?
The market is expected to grow at a CAGR of 5.4% from 2024 to 2032 to reach a value of USD 79.02 billion by 2032.
What are the major industry drivers?
The industry is primarily being driven by the growing rates of R&D projects for the development of therapeutic drugs, development in technologies, increasing focus of manufacturers to produce vaccines to decrease death risk due to the COVID-19 pandemic, and increasing cancer research.
What are the key industry trends of the global clinical trials market?
The key trends driving the market’s expansion are the increasing demand for the production of therapeutic drugs and increasing government initiatives.
What are the major regional markets of the global clinical trials market, according to the EMR report?
The major regions in the industry are North America, Latin America, Europe, Middle East and Africa, and the Asia Pacific with North America accounting for the largest share in the market.
What are the segments based on design in the market?
By design, the market is divided into treatment studies and observational studies. Treatment studies are further divided into randomised control trial, adaptive clinical trail and non- andomized control trial while observational studies are segmented into cohort studies, case control study, cross section study, and ecological study, among others.
Who are the key industry players, according to the report?
The major players in the industry are IQVIA, PAREXEL International Corporation, Charles River Laboratory, ICON Plc, and Syneos Health, among others.
Purchase Options 10% off
Methodology
Request Customisation
Report Sample
Request Brochure
Ask an Analyst
( USA & Canada ) +1-415-325-5166 [email protected]
( United Kingdom ) +44-702-402-5790 [email protected]
Mini Report
- Selected Sections, One User
- Printing Not Allowed
- Email Delivery in PDF
- Free Limited Customisation
- Post Sales Analyst Support
- 50% Discount on Next Update
Single User License
- All Sections, One User
- One Print Allowed

Five User License
- All Sections, Five Users
- Five Prints Allowed
Corporate License
- All Sections, Unlimited Users
- Unlimited Prints Allowed
- Email Delivery in PDF + Excel
Any Question? Speak With An Analyst
View A Sample
Did You Miss Anything, Ask Now
Right People
We are technically excellent, strategic, practical, experienced and efficient; our analysts are hand-picked based on having the right attributes to work successfully and execute projects based on your expectations.
Right Methodology
We leverage our cutting-edge technology, our access to trusted databases, and our knowledge of the current models used in the market to deliver you research solutions that are tailored to your needs and put you ahead of the curve.
Right Price
We deliver in-depth and superior quality research in prices that are reasonable, unmatchable, and shows our understanding of your resource structure. We, additionally, offer attractive discounts on our upcoming reports.
Right Support
Our team of expert analysts are at your beck and call to deliver you optimum results that are customised to meet your precise needs within the specified timeframe and help you form a better understanding of the industry.

Stay informed with our free industry updates.
We use cookies, just to track visits to our website, we store no personal details. Privacy Policy X
Press Release
Industry Statistics

- Our Solution
- Food & Beverage
- Chemical & Materials
- Semiconductors and Electronics
- Materials & packaging
- Agriculture & Animal Feed
- Industrial Automation
- OIL, GAS & ENERGY
- Cloud Solutions
- Procurement Consulting
- Company Profile Analysis
- Primary Research
- DBMR Market Position Grid
- Bulk Report
- Industry Subscription
- Annual Update
- Quarterly Update
- Pharma Insights
- Market Insights
- Press Release
- Infographics
- White Paper
- Case Studies
- Business Case Studies
- COVID-19 Resources
- Our Company
- Company News Room
- Investor Relations

- US: +1 614 591 3140 UK: +44 845 154 9652 APAC : +653 1251 975
- Database Cloud Login
Global Clinical Trials Market – Industry Trends and Forecast to 2030
- Medical Devices

- Upcoming Report
- No of Tables: 60
- No of Figures: 220
- Report Description
- Table of Content
- List of Table
- List of Figure
- Request for TOC
- Speak to Analyst
- Inquire Before Buying
- Free Sample Report
Market Size in USD Billion
CAGR : 5.15 %
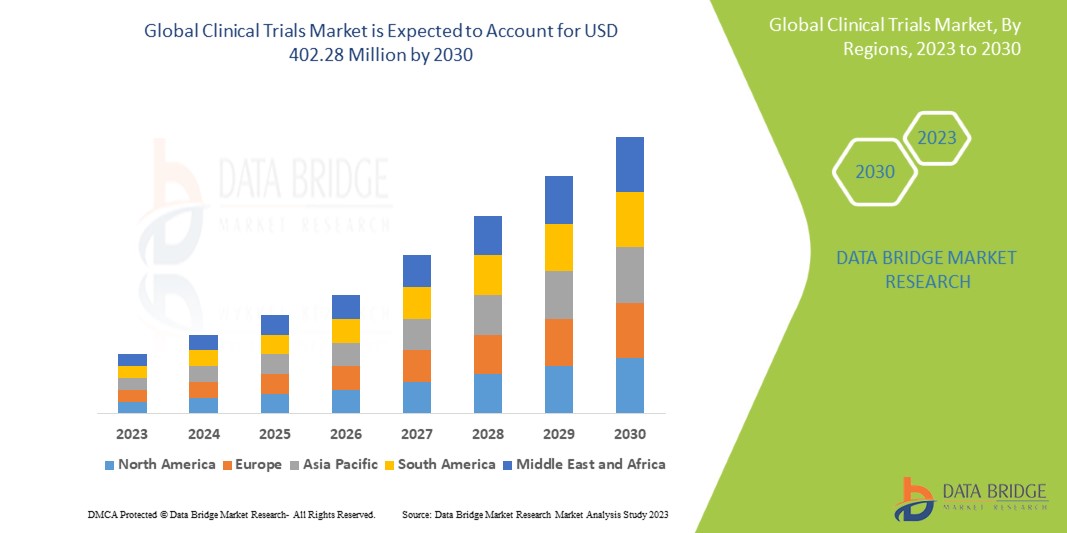
Global Clinical Trials Market, By Phase (Phase I, Phase II, Phase III, Phase IV), Indication (Autoimmune/Inflammation, Pain Management, Oncology, CNS Condition, Diabetes, Obesity, Cardiovascular, Others), Design (Interventional, Treatment Studies, Observational Studies, Expanded Access), End User (Hospital, Laboratories, Clinics) – Industry Trends and Forecast to 2030.
Clinical Trials Market Analysis and Size
The growing demand for clinical trial in developing countries, growing geriatric population, globalization of clinical trials, technological evolution are the significant factors responsible for driving the growth of the clinical trials market. The leveraging online resources to increase patient recruitment rates in clinical trials is also projected to boost the market’s growth. In addition, the increasing occurrences of chronic diseases coupled with globalizing drug development activities also heighten the overall growth of the market.
Data Bridge Market Research analyzes that the global clinical trials market which was USD 269.18 million in 2022, is likely to reach USD 402.28 million by 2030, and is expected to undergo a CAGR of 5.15% during the forecast period. “Laboratories" dominates the end segment of the clinical trials market due to increase in the number of research and development activities. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Report Scope and Market Segmentation
Market Definition
Clinical trials are basically the research studies performed by researchers in people that are aimed to find out a new treatment, like a new drug or diet or medical device . It is also used to find out whether it is safe and effective in people.
Clinical Trials Market Dynamics
- Advancements in medical research
Increasing focus on developing novel therapies and treatments for various diseases drives the demand for clinical trials. Advances in genomics and personalized medicine also contribute to the growth.
- Rising prevalence of diseases
The growing incidence of chronic diseases like cancer, diabetes, and cardiovascular disorders necessitates extensive clinical trials for innovative drugs and therapies.
- Technological advancements
Integration of technologies like big data analytics, AI, and IoT in clinical trials streamlines processes, enhances data accuracy, and reduces costs, thereby driving market growth.
Opportunities
- Real-world evidence (RWE) studies
The integration of real-world data into clinical trials provides valuable insights into drug effectiveness and safety, opening avenues for more pragmatic and efficient trials
- Patient-centric trials
Emphasizing patient experience and convenience through methods like virtual trials and home healthcare services can enhance participation rates and data accuracy.
Restraints/Challenges
- Stringent regulatory procedures
Complex regulatory processes and the need for compliance with diverse guidelines in different countries pose challenges for sponsors, leading to delays and increased costs.
- Data security and privacy concerns
With the rise in digitalization, ensuring the security and privacy of patient data in clinical trials is a significant challenge. Adhering to data protection regulations adds complexity.
- Patient recruitment and retention
Identifying suitable participants and retaining them throughout the trial period is challenging. This affects the timeline and success of the trials.
This clinical trials market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the clinical trials market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Global Clinical Trials Market Scope
Clinical trails market is segmented on the basis of phase, indication, design, and end user. The growth amongst these segments will help you analyze meagre growth segments in the industries, and provide the users with valuable market overview and market insights to help them in making strategic decisions for identification of core market applications.
- Autoimmune/inflammation
- Pain management
- Cns condition
- Cardiovascular
- Interventional
- Treatment studies
- Observational studies
- Expanded access
- Laboratories
Clinical Trials Market Regional Analysis/Insights
Clinical trials market is analyzed and market size insights and trends are provided by country, phase, indication, design and end user as referenced above.
The countries covered in the clinical trials market report is U.S., Canada, Mexico, Germany, Italy, U.K., France, Spain, Netherland, Belgium, Switzerland, Turkey, Russia, rest of Europe, Japan, China, India, South Korea, Australia, Singapore, Malaysia, Thailand, Indonesia, Philippines, rest of Asia-Pacific, Brazil, Argentina, rest of South America, South Africa, Saudi Arabia, U.A.E, Egypt, Israel, rest of Middle East & Africa.
North America is expected to dominate the market due to increasing research and development and increasing adoption of new technologies in clinical research.
Asia-Pacific is expected to show fastest growth during the forecast period of 2023 to 2030 due to expected to show a rapid and lucrative growth rate in the forecast period owing to the increasing availability of large patient pool facilitating easy recruitment of candidates.
The country section of the report also provides individual market impacting factors and domestic regulation changes that impact the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, and case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, the impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Healthcare Infrastructure growth Installed base and New Technology Penetration
The clinical trials market also provides you with detailed market analysis for every country growth in healthcare expenditure for capital equipment, installed base of different kind of products for clinical trials market, impact of technology using life line curves and changes in healthcare regulatory scenarios and their impact on clinical trials market. The data is available for historic period 2010-2020.
Competitive Landscape and Clinical Trials Market Share Analysis
The clinical trials market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to clinical trials market.
Some of the major players operating in the global clinical trials market are:
- Clinipace (U.S.)
- Laboratory Corporation of America Holdings (LabCorp) (U.S.)
- Eli Lilly and Company (U.S.)
- ICON Plc. (Ireland)
- Novo Nordisk A/S (Denmark)
- Parexel International Corporation (U.S.)
- Pfizer Inc. (U.S.)
- PPD, Inc. (U.S.)
- IQVIA (U.S.)
- Sanofi (France)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Alcami Corporation, Inc. (U.S.)
- Accell Clinical Research LLC (U.S.)
- Congenix LLP (U.S.)
- Labcorp Drug Development (U.S.)
- Ecron Acunova (India)
- Medpace (U.S.)
- LUMITOS AG (Germany)
- ICON plc (Ireland)
- SIRO Clapham Private Limited (India)
Please fill in the below form for detailed Table of Content
By clicking the "Submit" button, you are agreeing to the Data Bridge Market Research Privacy Policy and Terms and Conditions
Please fill in the below form for detailed List of Table
Please fill in the below form for detailed list of figure, please fill in the below form for infographics, research methodology:.
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Please fill in the below form for Research Methodology
Customization available:.
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.
Please fill in the below form for Available Customization
Frequently ask questions, browse related reports, browse related blogs.
- View Infographics
- Research Methodology
- Available Customization
CHOOSE LICENCE TYPE
- Enterprise User 7000.00
- Single User 4800.00
- DBMR Factbook 3000.00
- DBMR Prime 8000.00
- DBMR Supreme 12000.00
Can be used by entire organization across the globe + Downloadable and Printable PDF + 30 + Countries
Why Choose Us
Industry coverage.
DBMR works across the globe in multiple industries which equip us with knowledge across verticals and provide our clients with insights not only from their industry but how other industries will impact their ecosystem.
Regional Coverage
Coverage of Data Bridge is not restricted to developed or emerging economies. We work across the globe covering the largest array of countries where no other market research or business consulting firm has ever conducted research; creating growth opportunities for our clients in areas which are still unknown.
Technology Coverage
In today’s world, technology drives the market sentiment, so our vision is to provide our clients insights not only for developed technologies but upcoming and disrupting technological changes throughout the product lifecycle by enabling them with unforeseen opportunities in the market which will create disruption in their industry. This leads to innovation and our clients to come out as winners.
Goal Oriented Solutions
DBMR goal is to help our clients achieve their goals through our solutions; hence we formatively create the most appropriate solutions for our client needs, saving time and efforts for them to drive their grand strategies.
Unparallel Analyst Support
Our analysts take pride in our clients’ success. Unlike others, we believe in working along our clients to achieve their goals with 24 hours analyst support determining the correct needs and inspire innovation through service.

Client Testimonials
David manning - thermo fisher scientific.
Dear Ricky, I want to thank you for the excellent market analysis (LIMS INSTALLED BASE DATA) that you and your team delivered, especially end of year on short notice. Sachin and Shraddha captured the requirements, determined their path forward and executed quickly. You, Sachin and Shraddha have been a pleasure to work with – very responsive, professional and thorough. Your work is much appreciated.

Manager - Market Analytics,
Thank you for all the assistance and the level of detail in the market report. We are very pleased with the results and the customization. We would like to continue to do business.
Business Development Manager,
DBMR was attentive and engaged while discussing the Global Nasal Spray Market. They understood what we were looking for and was able to provide some examples from the report as requested. DBMR Service team has been responsive as needed. Depending on what my colleagues were looking for, I will recommend your services and would be happy to stay connected in case we can utilize your research in the future.
Business Intelligence and Analytics,
We are impressed by the CENTRAL PRECOCIOUS PUBERTY (CPP) TREATMENT report - so a BIG thanks to you colleagues.

Competition Analyst,
I just wanted to share a quick note and let you know that you guys did a really good job. I’m glad I decided to work with you. I shall continue being associated with your company as long as we have market intelligence needs.

Marketing Director,
It was indeed a good experience, would definitely recommend and come back for future prospects.
DBMR did an outstanding job on the Global Drug Delivery project, We were extremely impressed by the simple but comprehensive presentation of the study and the quality of work done. This report really helped us to access untapped opportunities across the globe.

The study was customized to our targets and needs with well-defined milestones. We were impressed by the in-depth customization and inclusion of not only major but also minor players across the globe. The DBMR Market position grid helped us to analyze the market in different dimension which was very helpful for the team to get into the minute details.

Product manager,
Thankful to the team for the amazing coordination, and helping me at the last moment with my presentation. It was indeed a comprehensive report that gave us revenue impacting solution enabling us to plan the right move.

Investor relations,
Thank you for the report, and addressing our needs in such short time. DBMR has outdone themselves in this project with such short timeframe.

Market Analyst,
We found the results of this study compelling and will help our organization validate a market we are considering to enter. Thank you for a job well done.

Andrew - Senior Global Marketing Manager,
I want to thank you for your help with this report – It’s been very helpful in our business planning and it well organized.

Amarildo - Manager, Global Strategic Alignment
We believe the work done by Data Bridge Team for our requirements in the North America Loyalty Management Market was fantastic and would love to continue working with your team moving forward.
Thank you for your quick response to this unfortunate circumstance. Please extend my thanks to your reach team. I will be contacting you in the future with further projects
Tommaso Finocchiaro
I acknowledge the difficulty given by the very short warning for this report, and I think that its quality and your delivering time have been very satisfying. Obviously, as a provider Data Bridge Market Research will be considered as a plus for future needs of Nippon Gases.
Yuki Kopyl (Asian Business Development Department)
Xylose report was very useful for our team. Thank you very much & hope to work with you again in the future

Share Your Experience With Us
Avail pdf sample reports.

By clicking the "Submit" button, you are agreeing to the Data Bridge Market Research Privacy Policy. and Terms and Conditions
Corporate Email ID Only (Avoid Using Generic mail ID such as Gmail)
Industry Verticals »
- Chemicals And Materials
- Consumer Goods
- Electronics and Semiconductors
- Energy and Natural Resources
- Factory Automation
- Food and Beverages
- Heavy Engineering Equipment
- IT and Telecom
- Pharmaceutical
- Latest Reports
- Forthcoming Reports
- Top Industry Reports
Press Releases »
About tmr ».
Clinical Trials Market
Clinical Trials Market (Phase: Phase I, Phase II, Phase III, and Phase IV; Study Design: Interventional Trials, Observational Trials, and Expanded Access Trials; and Indication: Autoimmune/Inflammation, Pain Management, Cardiovascular, CNS Condition, Oncology, Diabetes, Obesity, and Others) - Global Industry Analysis, Size, Share, Growth, Trends, and Forecast, 2020-2030
- Report Preview
- Table of Content
- Request Brochure
Success of Clinical Trials for COVID-19 Vaccines Boosts Market Growth
The coronavirus pandemic has brought research labs and healthcare institutions under great scrutiny for accelerating the clinical trials for COVID-19 vaccines. As such, the success in these clinical trials has led to global recognition of India for supplying several lack of free doses to Brazil, Bangladesh, Algeria, and South Africa. Countries such as Sri Lanka are following suit whilst creating incremental opportunities for stakeholders in the clinical trials market.
The Food & Drug Administration (FDA) is creating awareness about Coronavirus Treatment Acceleration Program (CTAP) in order to bring economies to normal. Companies in the clinical trials market are taking advantage of this program to make new treatments available to patients as quickly as possible.

To know the scope of our report Get a Sample on Clinical Trials Market
Clinical Trial Recruitment Companies Essential for Matching Patients with Right Trial
The clinical trials market is estimated to cross US$ 83.5 Bn by the end of 2030 . However, finding the right patients is one of the most important pieces of the puzzle with respect to conducting clinical trials. Hence, stakeholders are working with experts at clinical trial recruitment companies to match patients with the right trials.
Slow recruitment is another challenge faced by companies in the market. Hence, companies are becoming aware about scrutinizing existing patient data to anticipate potential recruitment challenges in the long run. Clinical trial innovations are anticipated to bolster participation from volunteers.
Get a glimpse of the in-depth analysis through our Report Brochure
FDA Guidelines Pave Way for Innovations for At-home, At-clinic Clinical Trials
Companies in the market are following guidelines of the FDA to advance in processes. They are referring to FDA guidance about Severely Debilitating or Life-Threatening Hematologic Disorders (SDLTHDs) in patients. Companies are increasing efforts to fill in the gap between scientific and technical complexities of clinical trials. Thus, companies are investing in improving the academia and are developing new incentives that reward collaboration from volunteers.
Companies in the market are taking additional efforts to develop networks that enable procedures at a patient’s home or at their private doctor’s clinic. This has led to innovations in sensor devices, patient reported outcomes on their computers, and flash pictures of their lesions from their cellphones that contribute toward processes.
Coronavirus Pandemic Pushes Sponsors, Patients to Adopt Virtual Clinical Trials
Compliance with protocols is one of the key trends followed by stakeholders in the clinical trials market. Clinical trials supervised by Principal Investigators are gaining prominence in the market landscape. Continued positive cases for coronavirus have increased relevancy and role of stakeholders in the market. This shift has pushed clinical trials to go virtual.
Stakeholders in the market are teaming up with virtual clinical trial experts to capitalize on business opportunities during the ongoing pandemic. Even regulatory authorities have released guidelines to assist sponsors and patients via telemedicine and virtual trial tools to create viable solutions for current operational problems.
Digital Health Innovations Give Impetus to IoMT for Enhancing Clinical Development Programs
The proliferation of digital innovations with the help of wearables and sensors is translating into value grab opportunities for companies in the clinical trials market. ICON plc— a clinical research organization company is researching how digital endpoints, including digital biomarkers can improve trial outcomes. This has led to the adoption of digital health technologies that enable appropriate device selection and data strategies.
Digital health innovations hold promising potentials to better manage chronic diseases and improve patient access to healthcare services. Stakeholders and sponsors are taking efforts to improve adherence to medications and prevent its complications in patients. The Internet of Medical Things (IoMT) has the potential to enhance clinical development programs involving med-tech and pharmaceutical companies.
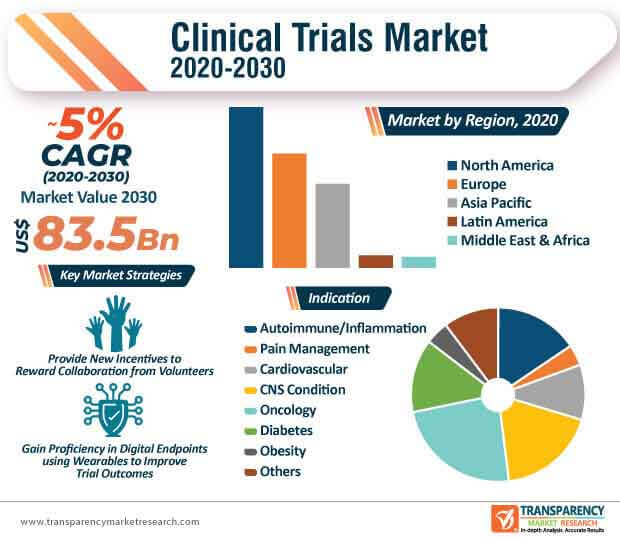
Expanding operations in future? To get the perfect launch ask for a custom report
Analysts’ Viewpoint There is a need for trial innovations since recently several volunteers were given wrong dose of the late stage clinical trial of the Oxford/AstraZeneca Covid-19 vaccine. The clinical trials market is predicted to advance at a modest CAGR of 5.4% during the forecast period. This is evident since stakeholders need to address challenges pertaining to site management and compliance in order to improve trial outcomes. Hence, companies are developing clinical trial timelines to avoid delays, and are increasing transparency with their sponsors to offer them a 360 degree view of the processes happening at development centers. IoMT is acting as a key driver for the market.
Clinical Trials Market: Overview
- According to Transparency Market Research’s latest report on the global market for the historical period 2018–2019 and forecast period 2020–2030 , high prevalence and increase in incidence rate of chronic diseases and rise in R&D activities in biotechnology & pharmaceuticals industries are projected to drive the global market during the forecast period
- According to the report, the global market was valued over US$ 46.7 Bn in 2019 and is anticipated to expand at a CAGR of 5.4% from 2020 to 2030
High Prevalence and Increase in Incidence Rate of Chronic Diseases to Drive Demand for Clinical Trials: Key Drivers
- Chronic diseases such as chronic respiratory diseases (CRD), diabetes, chronic kidney diseases (CKD), cancer, cardiac stroke, and neurological disorders are the leading causes of disability and mortality across the globe
- The emergence and outbreak of various infectious and chronic diseases have created challenges and new opportunities for researchers to develop new diagnostic tools, tests, drugs, and vaccines for early diagnosis, prevention, and cure of such diseases
- Hepatitis and HIV are the other major infectious diseases. According to the World Health Organization (WHO), as of October 2017, around 71 million people across the world were estimated to have hepatitis C infection. The WHO also stated that 36.7 million people were affected with HIV across the globe.
- An article published by Columbia, Mailman School of Public Health on infectious disease epidemiology stated that infectious diseases continue to have a substantial impact on the health of communities across the world. Various seasonal outbreaks of diseases in certain parts of the world, global epidemics, the threat of resistant bacteria, and the challenge of emerging and newly identified pathogens increase the need of new methods to detect such pathogens, to understand their pathogenesis, and to devise effective interventions for their prevention and control.
- These factors have led to an increase in demand for clinical trials, and are expected to drive the market for clinical trials from 2020 to 2030
Rise in R&D Activities in Biotechnology and Pharmaceuticals Industries Boosts Market Growth
- Clinical research organizations, diagnostic laboratories, and biotechnology players are engaged in the development of newer diagnostic tests to address the unmet needs in the healthcare industry. The life science industry’s R&D spending is driven primarily by the mass and research intensity of the biopharmaceutical sector, which accounts for nearly 85% of all expenditure.
- Additionally, laboratories strive to improve equipment cost and performance. UHPLC and ultra-fast mass spectrometers are more reliable, faster, and more sensitive as a result of research initiatives by major players.
- Acceleration in biopharmaceutical R&D innovation, buoyed by several contributing factors such as precision medicine getting into gear in rare diseases, cancer, and autoimmune diseases; immunotherapy, expansion of therapeutic modalities exploiting natural and synthetic biology innovation, and expedited regulatory pathways boost the growth of the clinical trials market
Patient Recruitment and Retention to Hamper Global Market
- Shift in focus toward genetic and rare diseases has made recruitment of relevant patient population a challenging task. Moreover, lack of awareness among patients of clinical trials presents a challenging environment for CROs.
- Lack of appropriate patient recruitment could have an impact on the scientific and financial viability. According to the Tufts Center for the Study of Drug Development, in 2013, 11% of the sites failed to recruit even a single patient for clinical trial.
- Other factors responsible for poor patient recruitment are patient’s fear of side effects, illiteracy, language barrier for region-specific clinical trials, and recording of the consent process
- Factors affecting the retention of patients for clinical trials include serious adverse events, fear of complex medical procedures, poor compliance of protocol, lack of dedication toward patient safety, and lack of support from family. This significantly affects the advancement of clinical trial process and product development.
Clinical Trials Market: Competition Landscape
- This report profiles major players in the global clinical trials industry based on various attributes such as company overview, financial overview, product portfolio, business strategies, and recent developments
- Laboratory Corporation of America Holdings
- IQVIA, Inc.
- Syneos Health
- Parexel International Corporation
- PRA Health Sciences, Inc.
- Charles River Laboratories, Inc.
- WuXi AppTec
- Medpace Holdings, Inc.
Key Developments
- In February 2020, Charles River Laboratories International, Inc. signed an agreement to acquire Citoxlab for US$ 510 Mn. Citoxlab is a non-clinical CRO specialized in regulated safety assessment services, non-regulated discovery services, and medical device testing.
- In November 2019, PPD, Inc. launched site coach where doctors and healthcare centers will receive clinical research at new sites, which will provide sufficient training for physicians and other academic institutions through online for the new clinical researchers
- In October 2019, Icon plc acquired Symphony Clinical Research to enhance the ability in offering customers hybrid trial solutions and site support services. This acquisition affected the clinical trials directly and improved the services in the research centers.
- In June 2019, Laboratory Corporation’s Covance Drug Development segment completed the acquisition of Envigo’s nonclinical contract research services business. This acquisition enabled the company to increase its global nonclinical drug development capabilities. As part of this move, Envigo acquired Covance’s research models & services business, leading to the establishment of an organization dedicated to offering a complete range of research models.
- In October 2018, Parexel International Corporation announced that it would launch new wearable, mobile tech for clinical trials, which will expand the services in clinical research. Microsoft's Azure App Services is one of the mobile tech services, which alerts a healthcare team when there is a patient safety threat.
- In February 2018, PPD, Inc. and Acurian introduced an innovative clinical trial patient concierge service that provides personalized patient support and improves customer retention. PPD’s Site Coach Service addresses this by providing specialized clinical research education, coaching, and support to new clinical research physicians, hospitals, and academic institutions.
- In April 2018, ICON plc, a global biopharmaceutical company, announced that it recently agreed with the Intel Pharma Analytics platform in clinical trials, which will help to reduce costs in clinical trials and delivers an excellent patient experience
- In August 2017, LabCorp, a leading company in life sciences, acquired Chiltren for around US$ 1.2 Bn. The company enhanced end-to-end drug development services and engaged patient care more effectively in the next few years.
- In May 2017, WuXi AppTec Co., Ltd. announced completion of the acquisition of HD Biosciences (HDB), a leading biology-focused preclinical drug discovery contract research organization (CRO). The acquisition would further strengthen WuXi’s R&D capability from target validation to lead discovery and optimization, improving and expanding WuXi’s open-access enabling service platform.
- In June 2016, IQVIA, the most significant research organization, launched a continuous glucose monitoring device, which is helpful to record a person's glucose levels throughout the day. Medical device CGM is combined with the consumer wearable devices to record the readings.
- In June 2015, IQVIA and PRA Health Sciences, two leading companies in pharmaceuticals, announced that with new predictive and advanced analytics technology they plan to improve the performance in clinical trials and improve safety issues
- In October 2014, Laboratory Corporation of America Holdings acquired Covance, Inc. for US$ 5.6 Bn and became world’s leading health care diagnostic company and leading end-to-end solutions provider for drug development and commercialization
- The report on the global clinical trials market discussed individual strategies, followed by company profiles of service providers of clinical trials. The competition landscape section has been included in the report to provide readers with a dashboard view and a company market share analysis of key players operating in the global market.
Clinical Trials Market – Segmentation
Frequently Asked Questions
What is the total market worth of clinical trials market.
It is projected to reach a value of US$ 83.5 Bn by the end of 2030
What will be the CAGR during the forecast period?
The CAGR is anticipated to be 5% from 2020 to 2030.
Which region will account for major share?
North America is expected to account for leading share during the forecast period.
What are the key driving factors for the growth of the clinical trials market?
High prevalence and increase in incidence rate of chronic diseases, and rise in R&D activities in biotechnology & pharmaceuticals industries are anticipated to drive the global market.
Who are the prominent players in the sector?
Laboratory Corporation of America Holdings, IQVIA, Inc., Syneos Health, Parexel International Corporation, PRA Health Sciences, Inc., PPD, Inc., Icon plc, Charles River Laboratories, Inc., WuXi AppTec, and Medpace Holdings, Inc.
1.1. Market Definition and Scope
1.2. Market Segmentation
1.3. Key Research Objectives
1.4. Research Highlights
2. Assumptions and Research Methodology
3. Executive Summary: Global Clinical Trials Market
4. Market Overview
4.1. Introduction
4.2. Overview
4.3. Market Dynamics
4.3.1. Drivers
4.3.2. Restraints
4.3.3. Opportunities
4.4. Global Clinical Trials Market Analysis and Forecast, 2018–2030
4.4.1. Market Revenue Projections (US$ Mn)
5. Key Insights
5.1. Number of Clinical Trials
5.2. Key Industry Events
5.3. COVID-19 Pandemic Impact on Industry
6. Global Clinical Trials Market Analysis and Forecast, by Phase
6.1. Introduction & Definition
6.2. Key Findings / Developments
6.3. Market Value Forecast, by Phase, 2018–2030
6.3.1. Phase I
6.3.2. Phase II
6.3.3. Phase III
6.3.4. Phase IV
6.4. Market Attractiveness Analysis, by Phase
7. Global Clinical Trials Market Analysis and Forecast, by Study Design
7.1. Introduction & Definition
7.2. Key Findings / Developments
7.3. Market Value Forecast, by Study Design, 2018–2030
7.3.1. Interventional Trials
7.3.2. Observational Trials
7.3.3. Expanded Access Trials
7.4. Market Attractiveness Analysis, by Study Design
8. Global Clinical Trials Market Analysis and Forecast, by Indication
8.1. Introduction & Definition
8.2. Key Findings / Developments
8.3. Market Value Forecast, by Indication, 2018–2030
8.3.1. Autoimmune/Inflammation
8.3.2. Pain Management
8.3.3. Cardiovascular
8.3.4. CNS Condition
8.3.5. Oncology
8.3.6. Diabetes
8.3.7. Obesity
8.3.8. Others
8.4. Market Attractiveness Analysis, by Indication
9. Global Clinical Trials Market Analysis and Forecast, by Region
9.1. Key Findings
9.2. Market Value Forecast, by Region
9.2.1. North America
9.2.2. Europe
9.2.3. Asia Pacific
9.2.4. Latin America
9.2.5. Middle East & Africa
9.3. Market Attractiveness Analysis, by Country/Region
10. North America Clinical Trials Market Analysis and Forecast
10.1. Introduction
10.1.1. Key Findings
10.2. Market Value Forecast, by Phase, 2018–2030
10.2.1. Phase I
10.2.2. Phase II
10.2.3. Phase III
10.2.4. Phase IV
10.3. Market Value Forecast, by Study Design, 2018–2030
10.3.1. Interventional Trials
10.3.2. Observational Trials
10.3.3. Expanded Access Trials
10.4. Market Value Forecast, by Indication, 2018–2030
10.4.1. Autoimmune/Inflammation
10.4.2. Pain Management
10.4.3. Cardiovascular
10.4.4. CNS Condition
10.4.5. Oncology
10.4.6. Diabetes
10.4.7. Obesity
10.4.8. Others
10.5. Market Value Forecast, by Country, 2018–2030
10.5.1. U.S.
10.5.2. Canada
10.6. Market Attractiveness Analysis
10.6.1. By Phase
10.6.2. By Study Design
10.6.3. By Indication
10.6.4. By Country
11. Europe Clinical Trials Market Analysis and Forecast
11.1.Introduction
11.1.1. Key Findings
11.2.Market Value Forecast, by Phase, 2018–2030
11.2.1. Phase I
11.2.2. Phase II
11.2.3. Phase III
11.2.4. Phase IV
11.3.Market Value Forecast, by Study Design, 2018–2030
11.3.1. Interventional Trials
11.3.2. Observational Trials
11.3.3. Expanded Access Trials
11.4.Market Value Forecast, by Indication, 2018–2030
11.4.1. Autoimmune/Inflammation
11.4.2. Pain Management
11.4.3. Cardiovascular
11.4.4. CNS Condition
11.4.5. Oncology
11.4.6. Diabetes
11.4.7. Obesity
11.4.8. Others
11.5.Market Value Forecast, by Country/Sub-region, 2018–2030
11.5.1. Germany
11.5.2. U.K.
11.5.3. France
11.5.4. Spain
11.5.5. Italy
11.5.6. Rest of Europe
11.6.Market Attractiveness Analysis
11.6.1. By Phase
11.6.2. By Study Design
11.6.3. By Indication
11.6.4. By Country/Sub-region
12. Asia Pacific Clinical Trials Market Analysis and Forecast
12.1.Introduction
12.1.1. Key Findings
12.2.Market Value Forecast, by Phase, 2018–2030
12.2.1. Phase I
12.2.2. Phase II
12.2.3. Phase III
12.2.4. Phase IV
12.3.Market Value Forecast, by Study Design, 2018–2030
12.3.1. Interventional Trials
12.3.2. Observational Trials
12.3.3. Expanded Access Trials
12.4.Market Value Forecast, by Indication, 2018–2030
12.4.1. Autoimmune/Inflammation
12.4.2. Pain Management
12.4.3. Cardiovascular
12.4.4. CNS Condition
12.4.5. Oncology
12.4.6. Diabetes
12.4.7. Obesity
12.4.8. Others
12.5.Market Value Forecast, by Country/Sub-region, 2018–2030
12.5.1. China
12.5.2. Japan
12.5.3. India
12.5.4. Australia & New Zealand
12.5.5. Rest of Asia Pacific
12.6.Market Attractiveness Analysis
12.6.1. By Phase
12.6.2. By Study Design
12.6.3. By Indication
12.6.4. By Country/Sub-region
13. Latin America Clinical Trials Market Analysis and Forecast
13.1.Introduction
13.1.1. Key Findings
13.2.Market Value Forecast, by Phase, 2018–2030
13.2.1. Phase I
13.2.2. Phase II
13.2.3. Phase III
13.2.4. Phase IV
13.3.Market Value Forecast, by Study Design, 2018–2030
13.3.1. Interventional Trials
13.3.2. Observational Trials
13.3.3. Expanded Access Trials
13.4.Market Value Forecast, by Indication, 2018–2030
13.4.1. Autoimmune/Inflammation
13.4.2. Pain Management
13.4.3. Cardiovascular
13.4.4. CNS Condition
13.4.5. Oncology
13.4.6. Diabetes
13.4.7. Obesity
13.4.8. Others
13.5.Market Value Forecast, by Country/Sub-region, 2018–2030
13.5.1. Brazil
13.5.2. Mexico
13.5.3. Rest of Latin America
13.6.Market Attractiveness Analysis
13.6.1. By Phase
13.6.2. By Study Design
13.6.3. By Indication
13.6.4. By Country/Sub-region
14. Middle East & Africa Clinical Trials Market Analysis and Forecast
14.1.Introduction
14.1.1. Key Findings
14.2.Market Value Forecast, by Phase, 2018–2030
14.2.1. Phase I
14.2.2. Phase II
14.2.3. Phase III
14.2.4. Phase IV
14.3.Market Value Forecast, by Study Design, 2018–2030
14.3.1. Interventional Trials
14.3.2. Observational Trials
14.3.3. Expanded Access Trials
14.4.Market Value Forecast, by Indication, 2018–2030
14.4.1. Autoimmune/Inflammation
14.4.2. Pain Management
14.4.3. Cardiovascular
14.4.4. CNS Condition
14.4.5. Oncology
14.4.6. Diabetes
14.4.7. Obesity
14.4.8. Others
14.5.Market Value Forecast, by Country/Sub-region, 2018–2030
14.5.1. GCC Countries
14.5.2. South Africa
14.5.3. Rest of Middle East & Africa
14.6.Market Attractiveness Analysis
14.6.1. By Phase
14.6.2. By Study Design
14.6.3. By Indication
14.6.4. By Country/Sub-region
15. Competitive Landscape
15.1. Market Player - Competition Matrix (by tier and size of companies)
15.2. Market Share Analysis, by Company, 2019
15.3. Company Profiles
15.3.1. Laboratory Corporation of America Holdings
15.3.1.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.1.2. Financial Overview
15.3.1.3. Product Portfolio
15.3.1.4. Strategic Overview
15.3.1.5. SWOT Analysis
15.3.2. IQVIA Inc.
15.3.2.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.2.2. Financial Overview
15.3.2.3. Product Portfolio
15.3.2.4. Strategic Overview
15.3.2.5. SWOT Analysis
15.3.3. Syneos Health
15.3.3.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.3.2. Financial Overview
15.3.3.3. Product Portfolio
15.3.3.4. Strategic Overview
15.3.3.5. SWOT Analysis
15.3.4. Parexel International Corporation
15.3.4.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.4.2. Financial Overview
15.3.4.3. Product Portfolio
15.3.4.4. Strategic Overview
15.3.4.5. SWOT Analysis
15.3.5. PPD Inc.
15.3.5.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.5.2. Financial Overview
15.3.5.3. Product Portfolio
15.3.5.4. Strategic Overview
15.3.5.5. SWOT Analysis
15.3.6. Icon plc
15.3.6.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.6.2. Financial Overview
15.3.6.3. Product Portfolio
15.3.6.4. Strategic Overview
15.3.6.5. SWOT Analysis
15.3.7. Charles River Laboratories, Inc.
15.3.7.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.7.2. Financial Overview
15.3.7.3. Product Portfolio
15.3.7.4. Strategic Overview
15.3.8. WuXi AppTec
15.3.8.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.8.2. Financial Overview
15.3.8.3. Product Portfolio
15.3.8.4. Strategic Overview
15.3.8.5. SWOT Analysis
15.3.9. Medpace Holdings, Inc.
15.3.9.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.9.2. Financial Overview
15.3.9.3. Product Portfolio
15.3.9.4. Strategic Overview
15.3.9.5. SWOT Analysis
List of Tables
Table 01: Global Clinical Trials Market Value (US$ Mn) Forecast, by Phase, 2018–2030
Table 02: Global Clinical Trials Market Value (US$ Mn) Forecast, by Study Design, 2018–2030
Table 03: Global Clinical Trials Market Value (US$ Mn) Forecast, by Indication, 2018–2030
Table 04: Global Clinical Trials Market Value (US$ Mn) Forecast, by Region, 2018–2030
Table 05: North America Clinical Trials Market Value (US$ Mn) Forecast, by Country, 2018–2030
Table 06: North America Clinical Trials Market Value (US$ Mn) Forecast, by Phase, 2018–2030
Table 07: North America Clinical Trials Market Value (US$ Mn) Forecast, by Phase, 2018–2030
Table 08: North America Clinical Trials Market Value (US$ Mn) Forecast, by Indication, 2018–2030
Table 09: Europe Clinical Trials Market Value (US$ Mn) Forecast, by Country/Sub-region, 2018–2030
Table 10: Europe Clinical Trials Market Value (US$ Mn) Forecast, by Phase, 2018–2030
Table 11: Europe Clinical Trials Market Value (US$ Mn) Forecast, by Study Design, 2018–2030
Table 12: Europe Clinical Trials Market Value (US$ Mn) Forecast, by Indication, 2018–2030
Table 13: Asia Pacific Clinical Trials Market Value (US$ Mn) Forecast, by Country/Sub-region, 2018–2030
Table 14: Asia Pacific Clinical Trials Market Value (US$ Mn) Forecast, by Phase, 2018–2030
Table 15: Asia Pacific Clinical Trials Market Value (US$ Mn) Forecast, by Study Design, 2018–2030
Table 16: Asia Pacific Clinical Trials Market Value (US$ Mn) Forecast, by Indication, 2018–2030
Table 17: Latin America Clinical Trials Market Value (US$ Mn) Forecast, by Country/Sub-region, 2018–2030
Table 18: Latin America Clinical Trials Market Value (US$ Mn) Forecast, by Phase, 2018–2030
Table 19: Latin America Clinical Trials Market Value (US$ Mn) Forecast, by Study Design, 2018–2030
Table 20: Latin America Clinical Trials Market Value (US$ Mn) Forecast, by Indication, 2018–2030
Table 21: Middle East & Africa Clinical Trials Market Value (US$ Mn) Forecast, by Country/Sub-region, 2018–2030
Table 22: Middle East & Africa Clinical Trials Market Value (US$ Mn) Forecast, by Phase, 2018–2030
Table 23: Middle East & Africa Clinical Trials Market Value (US$ Mn) Forecast, by Study Design, 2018–2030
Table 24: Middle East & Africa Clinical Trials Market Value (US$ Mn) Forecast, by Indication, 2018–2030
List of Figures
Figure 01: Global Clinical Trials Market Value (US$ Mn) Forecast, 2018–2030
Figure 02: Global Clinical Trials Market Value Share, by Phase, 2019
Figure 03: Global Clinical Trials Market Value Share, by Indication, 2019
Figure 04: Global Clinical Trials Market Value Share, by Study Design, 2019
Figure 05: Global Clinical Trials Market Value Share, by Region, 2019
Figure 06: Global Clinical Trials Market Value Share Analysis, by Phase, 2019 and 2030
Figure 07: Global Clinical Trials Market Value (US$ Mn), by Phase I, 2018–2030
Figure 08: Global Clinical Trials Market Value (US$ Mn), by Phase II, 2018–2030
Figure 09: Global Clinical Trials Market Value (US$ Mn), by Phase III, 2018–2030
Figure 10: Global Clinical Trials Market Value (US$ Mn), by Phase IV, 2018–2030
Figure 11: Global Clinical Trials Market Attractiveness Analysis, by Phase, 2020–2030
Figure 12: Global Clinical Trials Market Value Share Analysis, by Study Design, 2019 and 2030
Figure 13: Global Clinical Trials Market Value (US$ Mn), Interventional Trials, 2018–2030
Figure 14: Global Clinical Trials Market Value (US$ Mn), Observational Trials, 2018–2030
Figure 15: Global Clinical Trials Market Value (US$ Mn), Expanded Access Trials, 2018–2030
Figure 16: Global Clinical Trials Market Attractiveness Analysis, by Study Design, 2020–2030
Figure 17: Global Clinical Trials Market Value Share Analysis, by Indication, 2019 and 2030
Figure 18: Global Clinical Trials Market Value (US$ Mn), Autoimmune /Inflammation, 2018–2030
Figure 19: Global Clinical Trials Market Value (US$ Mn), Pain Management, 2018–2030
Figure 20: Global Clinical Trials Market Value (US$ Mn), Cardiovascular, 2018–2030
Figure 21: Global Clinical Trials Market Value (US$ Mn), CNS Condition, 2018–2030
Figure 22: Global Clinical Trials Market Value (US$ Mn), Oncology, 2018–2030
Figure 23: Global Clinical Trials Market Value (US$ Mn), Diabetes, 2018–2030
Figure 24: Global Clinical Trials Market Value (US$ Mn), Obesity, 2018–2030
Figure 25: Global Clinical Trials Market Value (US$ Mn), Others, 2018–2030
Figure 26: Global Clinical Trials Market Attractiveness Analysis, by Indication, 2020–2030
Figure 27: Global Clinical Trials Market Value Share Analysis, by Region, 2019 and 2030
Figure 28: Global Clinical Trials Market Attractiveness Analysis, by Region, 2020–2030
Figure 29: North America Clinical Trials Market Value (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, 2018–2030
Figure 30: North America Clinical Trials Market Value Share (%), by Country, 2019 and 2030
Figure 31: North America Clinical Trials Market Attractiveness, by Country, 2020–2030
Figure 32: North America Clinical Trials Market Value Share Analysis, by Phase, 2019 and 2030
Figure 33: North America Clinical Trials Market Attractiveness Analysis, by Phase, 2020–2030
Figure 34: North America Clinical Trials Market Value Share Analysis, by Study Design, 2019 and 2030
Figure 35: North America Clinical Trials Market Attractiveness Analysis, by Study Design, 2020–2030
Figure 36: North America Clinical Trials Market Value Share Analysis, by Indication, 2019 and 2030
Figure 37: North America Clinical Trials Market Attractiveness Analysis, by Indication, 2020–2030
Figure 38: Europe Clinical Trials Market Value (US$ Mn) Forecast, 2018–2030
Figure 39: Europe Clinical Trials Market Value Share Analysis, by Country/Sub-region, 2019 and 2030
Figure 40: Europe Clinical Trials Market Attractiveness Analysis, by Country/Sub-region, 2020–2030
Figure 41: Europe Clinical Trials Market Value Share Analysis, by Phase, 2019 and 2030
Figure 42: Europe Clinical Trials Market Attractiveness Analysis, by Phase, 2020–2030
Figure 43: Europe Clinical Trials Market Value Share Analysis, by Study Design, 2019 and 2030
Figure 44: Europe Clinical Trials Market Attractiveness Analysis, by Study Design, 2020–2030
Figure 45: Europe Clinical Trials Market Value Share Analysis, by Indication, 2019 and 2030
Figure 46: Europe Clinical Trials Market Attractiveness Analysis, by Indication, 2020–2030
Figure 47: Asia Pacific Clinical Trials Market Value (US$ Mn) Forecast, 2018–2030
Figure 48: Asia Pacific Clinical Trials Market Value Share Analysis, by Country/Sub-region, 2019 and 2030
Figure 49: Asia Pacific Clinical Trials Market Attractiveness Analysis, by Country/Sub-region, 2020–2030
Figure 50: Asia Pacific Clinical Trials Market Value Share Analysis, by Phase, 2019 and 2030
Figure 51: Asia Pacific Clinical Trials Market Attractiveness Analysis, by Phase, 2020–2030
Figure 52: Asia Pacific Clinical Trials Market Value Share Analysis, by Study Design, 2019 and 2030
Figure 53: Asia Pacific Clinical Trials Market Attractiveness Analysis, by Study Design, 2020–2030
Figure 54: Asia Pacific Clinical Trials Market Value Share Analysis, by Indication, 2019 and 2030
Figure 55: Asia Pacific Clinical Trials Market Attractiveness Analysis, by Indication, 2020–2030
Figure 56: Latin America Clinical Trials Market Value (US$ Mn) Forecast, 2018–2030
Figure 57: Latin America Clinical Trials Market Value Share Analysis, by Country/Sub-region, 2019 and 2030
Figure 58: Latin America Clinical Trials Market Attractiveness Analysis, by Country/Sub-region, 2020–2030
Figure 59: Latin America Clinical Trials Market Value Share Analysis, by Phase, 2019 and 2030
Figure 60: Latin America Clinical Trials Market Attractiveness Analysis, by Phase, 2020–2030
Figure 61: Latin America Clinical Trials Market Value Share Analysis, by Study Design, 2019 and 2030
Figure 62: Latin America Clinical Trials Market Attractiveness Analysis, by Study Design, 2020–2030
Figure 63: Latin America Clinical Trials Market Value Share Analysis, by Indication, 2019 and 2030
Figure 64: Latin America Clinical Trials Market Attractiveness Analysis, by Indication, 2020–2030
Figure 65: Middle East & Africa Clinical Trials Market Value (US$ Mn) Forecast, 2018–2030
Figure 66: Middle East & Africa Clinical Trials Market Value Share Analysis, by Country/Sub-region, 2019 and 2030
Figure 67: Middle East & Africa Clinical Trials Market Attractiveness Analysis, by Country/Sub-region, 2020–2030
Figure 68: Middle East & Africa Clinical Trials Market Value Share Analysis, by Phase, 2019 and 2030
Figure 69: Middle East & Africa Clinical Trials Market Attractiveness Analysis, by Phase, 2020–2030
Figure 70: Middle East & Africa Clinical Trials Market Value Share Analysis, by Study Design, 2019 and 2030
Figure 71: Middle East & Africa Clinical Trials Market Attractiveness Analysis, by Study Design, 2020–2030
Figure 72: Middle East & Africa Clinical Trials Market Value Share Analysis, by Indication, 2019 and 2030
Figure 73: Middle East & Africa Clinical Trials Market Attractiveness Analysis, by Indication, 2020–2030
Figure 74: Global Clinical Trials Market Share Analysis, by Company, 2019
Download FREE Sample!
Get a sample copy of this report
Your personal details are safe with us. Privacy Policy*
Get a copy of Brochure!
Copyright © Transparency Market Research, Inc. All Rights reserved
Trust Online

Clinical Trials Market
Clinical trials market size, share & trends analysis report by phase (phase i, phase ii, phase iii, phase iv), by study design (interventional, observational, expanded access), by indication (autoimmune/inflammation, pain management, oncology, cns condition, diabetes, obesity, cardiovascular, others ) and by region(north america, europe, apac, middle east and africa, latam) forecasts, 2023-2031.
Market Dynamics
Regional analysis, report scope, segmental analysis.
- Recent Development
Top Key Players
- Report Overview
- Table of Content
- Segmentation
Download Free Sample
Market overview.
The global clinical trials market size was valued at USD 49.49 billion in 2022. It is estimated to reach USD 16.77 billion by 2031, growing at a CAGR of 5.2% during the forecast period (2023–2031). Globalization of clinical trials has led to an increase in investment in new product development in emerging nations, thereby positively impacting the overall clinical trials market.
Clinical trials are investigation studies conducted with human participants to evaluate the safety and success of new medical interventions. These interventions can include drugs, vaccines, medical devices, diagnostic tests, or treatment methods. The primary motive of clinical trials is to gather scientifically rigorous evidence regarding the intervention under investigation. The trials aim to determine whether the new intervention is safe, how well it works, and how it compares to existing treatments or standard care. A clinical trial follows a well-defined protocol or plan that outlines various aspects of the study. This protocol includes the trial's objectives, the study design, participant eligibility criteria, treatment procedures, data collection methods, and statistical analysis. By adhering to this protocol, researchers ensure that the trial is conducted consistently and reliably.
Clinical trials are typically conducted in various phases. In Phase 1, a few healthy participants are enrolled to assess the intervention's safety, dosage, and potential side effects. Phase 2 trials concern a larger group of participants, including patients with the target condition or disease. The main objective of Phase 2 is to gather preliminary data on the intervention's effectiveness and further evaluate its safety. Phase 3 trials involve a larger population and are usually randomized and controlled. They compare the new intervention against the current standard treatment or placebo to determine its overall efficacy, safety, and side effects. Phase 4 trials are conducted after the intervention is approved and available to the public. They aim to monitor its long-term safety, effectiveness in different populations, and any rare or long-term side effects that may emerge over time.
Global Market Drivers
Globalization of clinical trials.
The number of clinical studies in each area is slowly changing from growing nations to emerging countries. The rising cost of clinical trials and difficulty in patient recruitment have led biopharmaceutical companies to shift towards regions like central and Eastern Europe, Asia pacific, Latin America, and the Middle East for cost savings and quick patient recruitment. Emerging countries also possess greater disease variation than the West, where traditional diseases are growing. The greater disease variation among developing countries helps biopharmaceutical companies to perform clinical trials for rare diseases.
Additionally, regions like Asia-Pacific provide greater economic benefits to biopharmaceutical companies as governments in countries like Singapore and China allocate funds to promote biomedical research. Regions like Latin America, where the population speaks only Portuguese and Spanish, also reduce barriers to informed consent, which helps in easy patient recruitment and faster clinical trials. The Middle East is expected to be a potential region for clinical research owing to its increasing population, improved infrastructure, and greater disease variation. Globalization of clinical trials has led to an increase in investment in new product development in emerging nations, thereby positively impacting the overall Clinical trials market.
Shift Toward Personalized Medicine
The paradigm shift towards personalized medicine will positively impact the clinical trial market. The classic clinical trial process is carried out on thousands of people, while personalized medicine focuses only on the effect of drugs on individual patients for a specific period. Due to the traditional clinical trial approach, few medicines in the development phase pass all clinical trial phases. The mentality of “one size will fit all” is a barrier for drugs currently in the pipeline, but we will never see the day.
The use of pharmacogenetics in the clinical trial process is expected to increase the number of drugs passing all phases of the clinical trial process. The shift towards personalized medicine is expected to increase the use of pharmacogenetics in the clinical trial phase, thereby increasing the pipeline of drugs. This trend is expected to instigate biopharmaceutical companies to invest more in the clinical trial phase.
Global Market Restraints
Regulations in new drug development.
The complex regulation in the clinical trial process, from the dossier application to the approval of new drugs, is a major barrier for biopharmaceuticals to invest in the recent drug development phase. Biopharmaceutical companies file applications in different countries for approval of a specific drug. In regions like the EU, companies file separate applications in different countries, and this process is very tedious and discourages companies from investing in R&D. Majority of the drugs in preclinical studies are not passed through the final stages of a clinical trial, and this discourages biopharmaceutical companies from investing heavily on the clinical trial process. The regulations in the EU are expected to streamline the drug development and approval process, which is a positive sign for the global clinical trials market.
Global Market Opportunities
Expansion of precision medicine.
The expansion of precision medicine presents a significant opportunity in the global clinical trials market. Precision medicine involves tailoring treatments to individual patients based on their genetic and molecular characteristics. This approach represents a shift from a generalized treatment approach to a more personalized and targeted approach to patient care. Clinical trials plays an important role in advancing precision medicine by identifying biomarkers, validating diagnostic tests, and assessing the effectiveness of targeted therapies.
One key contribution of clinical trials is identifying and validating biomarkers associated with specific diseases or treatment responses. Through careful analysis of patient samples and monitoring of biomarkers throughout the trial, researchers gain insights into disease mechanisms, treatment effectiveness, and patient stratification.
North America Dominates the Global Market
Based on region, the global clinical trials market is bifurcated into North America, Europe, Asia-Pacific, Latin America, and the Middle East and Africa.
North America is the most significant global clinical trials market shareholder and is estimated to grow at a CAGR of 4.9% over the forecast period. North America dominates global clinical trials, accounting for the largest market share in 2020. This is primarily attributed to several factors, including significant investments in research and development, the presence of global players, and efforts to develop newer patents.
In addition, the region has witnessed substantial R&D investments, contributing to its market leadership. One notable example is the precision medicine initiative announced by President Obama in January 2015. The initiative involved a substantial investment of USD 215 million in the National Institutes of Health (NIH), the National Cancer Institute, and the Food and Drug Administration. The funding was allocated to various areas, such as encouraging volunteer participation, genomic driver identification in cancer, and evaluating Next Generation Sequencing for enhanced patient care.
Europe is anticipated to exhibit a CAGR of 5.7% over the forecast period. Countries like Germany and the U.K. have a large patient pool and advanced medical expertise. The doctors advocate for these trials, thus increasing patient recruitment and benefiting research and patients. The legal aspects are developing in Germany but fully evolved in the U.K. and France. The regulations are formulated to ensure volunteers' safety, rights, and proper reimbursement. With clearer protocols and increased funding, Europe is expected to be a promising region for clinical trials. Factors such as greater access to the treatment-naïve patient pool, a higher concentration of patients near sites, cheap labor costs, and close relationships between doctors and patients drive the clinical research market in Europe.
Asia-Pacific is the fastest-growing market as many developed nations invest in Asia-Pacific regions. In October 2014, the Australian CRO Novotech started a new office in China with its ninth office in Asia. Novotech as a company has expanded in many developing nations owing to the requests of its U.S. and EU clients. In the case of China, Novotech is not the only company. Even Charles River Laboratories, in February 2014, declared China as having disproportionate growth and expressed interest in merging with potential regions.
In Latin America, 80% of the residents live in metropolitan cities. Three megacities, Sao Paulo, Buenos Aires, and Mexico City, have a huge patient pool. Though the regulatory timelines in Latin America are a drawback, the time saved in patient recruitment compensates for it. In addition to patient recruitment, patient retention is very high due to the healthy patient-physician relationship. Lower cost, highly qualified investigators, medical personnel, and study coordinators with GCP-compliant knowledge contribute to a higher clinical trial success rate. Moreover, Mexico and Brazil are expected to witness lucrative growth in the CRO market due to their cost advantage over developed economies such as the U.S. and European nations such as Germany.
Conducting clinical trials in the Middle East and Africa offers advantages such as patient diversity, cost advantage, infrastructure, and world-class medical facilities. The only drawback is discrepancies between the laws of different regions. Many regions are initiating to adapt to the global standards and collaborating with other global CROs to mark their presence. For instance, Hassan Labs of Egypt and Saham Group of North Africa joined hands to provide international medical expertise across Africa. MCT participated in the Partnership in Clinical Trials in April 2014 to reach out to clients and expand in developed countries. Through this, even MCT met sponsors from North America and presented the advantages of conducting trials in MEA.
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports
The global clinical trials market is bifurcated into phase, study design, and indication.
Based on phase, the global market is bifurcated into phase I, phase II, phase III, and phase IV.
The phase I segment dominates the global market and is projected to exhibit a CAGR of 6.8% over the forecast period. Phase I studies evaluate the safety of a device or drug and the tolerability and pharmacokinetics of molecules. It determines the result of a device or drug on the person, including how it is absorbed, metabolized, and excreted. It also examines the side effects of the drug in case of increased dosage levels. The stage includes 20 to 100 healthy volunteers or people with the disease.
In addition, conducting other studies to assess drug-drug interaction and the effects of food is also necessary in this phase. For patients with clear tolerability studies, oncology studies must be conducted as a primary endpoint. The main aim of phase I is to evaluate doses and their adverse effects, which includes testing about 70% of experimental drugs.
The global market is bifurcated based on study design into interventional, observational, and expanded access.
The interventional segment dominates the global market and is projected to exhibit a CAGR of 5.5% over the forecast period. The growth can be attributed to many interventional studies being conducted on autoimmune/inflammation across the globe. Over 7,000 interventional studies listed on clinicaltrails.gov related to autoimmune/inflammation. For instance, an interventional study titled Immunoadsorption or Plasma Exchange - What is the Best Treatment Option of Steroid Refractory Neurological Autoimmune Diseases was completed on December 1, 2019.
Based on the study design, the global market is bifurcated into Autoimmune/inflammation, pain management , oncology, CNS conditions, diabetes, obesity, cardiovascular, and others.
The autoimmune/inflammation segment dominates the global market and is projected to exhibit a CAGR of 5.4% over the forecast period. Autoimmune diseases have increased considerably in the past decade and are expected to rise further owing to the lifestyle and aging population. Due to significant growth in the prevalence of autoimmune diseases, there has been a significant increase in R&D spend on autoimmune diseases. The autoimmune diseases affect one in 15 Americans, including 18 million women and 5 million men. They are among the top 10 causes of death in women and female children in all age groups up to 64 years of age. According to the NIH’s estimate, autoimmune medical care costs approximately USD 100 billion yearly.
Market Size By Phase
Recent developments.
- January 2023- the FDA announced that it would streamline the clinical trial process for certain drugs and biologics. This is expected to make it easier and faster for companies to bring new therapies to market.
- February 2023- the European Medicines Agency (EMA) announced that it would be launching a new initiative to improve the efficiency of clinical trials. This initiative is expected to reduce the time and cost of bringing new therapies to market in Europe.
Frequently Asked Questions (FAQs)
Sample details.

- You will receive a link to download the sample pages on your email immediately after filling out a form
- A sample report will help you understand the structure of our full report
- The sample shared will be for our off-the-shelf market report
- The off-the-shelf report is curated considering a broader audience for this market, we have insights extending well beyond the scope of this report. Please reach out to discuss
- Should the sample pages not contain the specific information you seek, we extend the option to request additional details, which our dedicated team will incorporate.
- The sample is free of cost, while the full report is available for a fee.
Custom Scope
Connect with our analyst, we are featured on :.

- Terms & Conditions
- Privacy Policy
- Return Policy

Clinical Trials Market (By Phase: Phase 1, Phase 2, Phase 3, and Phase 4; By Study Design: Observational, Interventional, and Expanded Access; By Indication: Oncology, Autoimmune/Inflammation, Diabetes, Central Nervous System, Cardiovascular, Pain Management, and Others) - Global Market Size, Share, Trends Analysis, Segment Forecasts, Regional Outlook 2023 - 2032
- Report Description
- Table of Content
The global clinical trials market size was valued at USD 48.68 billion in 2022 and is predicted to hit USD 83.55 bn by 2032 with a registered CAGR of 5.6% during the forecast period 2023 to 2032. U.S. clinical trials market was valued at USD 24.61 billion in 2022.
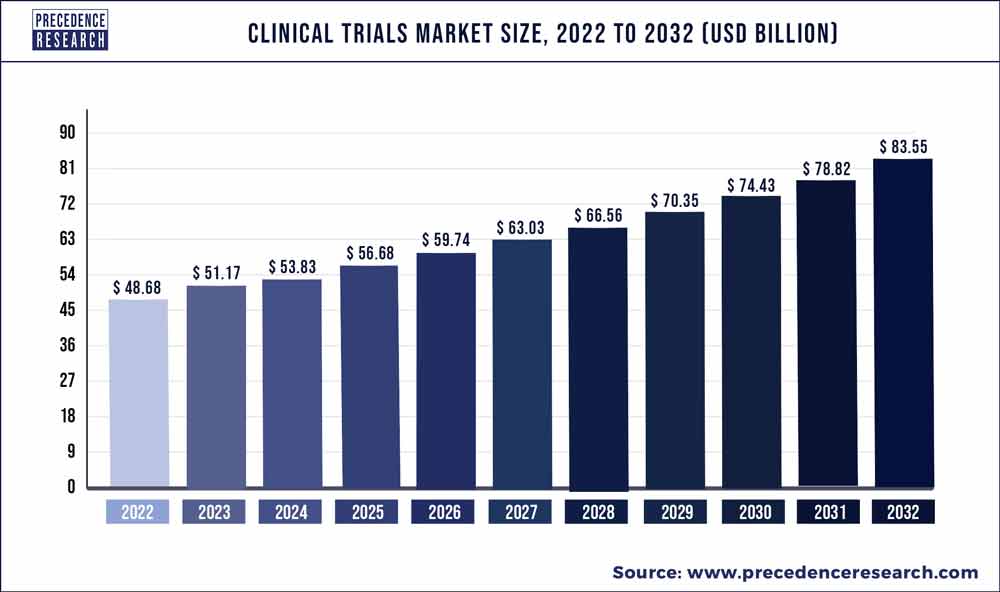
Access our Healthcare Data Intelligence Tool with 10000+ Database, Visit: Towards Healthcare
Key Takeaway:
- North America has held 51.7% of the total market share in 2022.
- Asia Pacific region is growing at a CAGR of 6.8% during the forecast period.
- By indication, the oncology segment has held a market share of around 24.7% in 2022.
- The cardiovascular condition segment is growing at a CAGR of 6.4% during the forecast period.
- By sponsor, The pharmaceutical & biopharmaceutical companies has generated the largest revenue share of around 72% in 2022.
- By Indication by study design, the interventional trials market for autoimmune/inflammation has captured 83% market share in 2022.
- By study design, the interventional design segment has accounted revenue share of 46% in 2022.
- The expanded access trials segment is projected to register at a CAGR of 6.4% over the forecast period.
Key market Insights:
Clinical trials are a process of clinical research that is governed by a defined protocol which is carefully established to answer a precise patient care query. Clinical trials can be divided into five phases, with every phase playing a distinct purpose within the clinical trial. Every trial adheres to a procedure that designates what types of individuals may participate in the study.
The trials also outline exact plan of procedures, tests, medications, and doses within the trial apart from specifying the span of the study. In recent years, the costs associated with drug development have increased significantly, driving pharma and biotech companies to look for modernizations and smarter ways of conducting business.
One important trend is the outsourcing of clinical research activities by manufacturers. By subcontracting their R&D activities, pharma and biotech companies are reforming the drug development facilities business. The R&D service providers have risen from just a few establishments providing restricted clinical trial facilities to big conglomerates offering a extensive range of facilities like study design, preclinical evaluations, clinical trial management and planning, autonomous safety data audit, bio-statistical analysis and several more. CROs (Contract Research Organizations) started off by providing preclinical & clinical trial services, however they are now venturing into project administration.
Crucial factors accountable for market growth are:
- Growing prevalence of chronic disorders
- Increasing number of clinical trials in developing regions
- Growing number of biologics
- Increasing demand for advanced treatments such as personalized medicines
Production, manufacturing, investment data and market statistics Clinical Trials Market
- According to the U.S. National Library of Medicine, as of June 2023, approximately 55,483 interventional clinical studies were posted with results on the Clinicatrial.gov. website.
- According to the World Health Organization (WHO), as of 2022, approximately 39% clinical trials were done for non-communicable diseases across the globe. Whereas the clinical trials for communicable diseases reached 60%.
- Europe registered almost 13,254 clinical trials in 2022. Whereas South-East Asia enrolled 11,052 number of clinical trials in the same year.
- In 2022, approximately 54% clinical trial initiations reached at the Phase-III level in India. Whereas 28% of them made it to the Phase IV level.
- Charles River Laboratory, a globally leading clinical trial company witnessed a significant growth in drug research fundings in 2022, the company’s annual generated revenue for 2022 was $3.98 billion. The revenue represented the growth of 12.3% over the previous year.
- The Cancer Research UK funded 193 million Euros on clinical research projects that focus on specific cancers in the financial year 2021-2022
- Japan accounted for 4.7% share in the worldwide clinical trial activities in 2022. The industry sponsored clinical trials held 54% share of the total Japanese industry in 2022
- For year 2022, in Japan, the oncology sector led the clinical trial activities with 25.1% share of the overall industry for clinical trials in the country. Whereas clinicals trials on infectious diseases held 7% share.
- The United Kingdom held total 178 Advanced Therapy Medicinal Product (ATMP) clinical trials in 2022.
- MedPace, a globally leading contract research organization’s annual revenue increased by 27.7% in 2022 and reached $394.1 million.
Report Scope of the Clinical Trials Market
Market drivers.
ADOPTION OF NEW TECHNOLOGY IN CLINICAL RESEARCH
Digitization in biomedical research is also paving the way for the market growth. Digitization also helps in meeting stringent regulations by maintaining patient data records that reduces trial process errors through adoption of software such as electronic clinical outcome assessment (e-COA). Digitization helps in streamlining the clinical trial process thereby probing sponsors to invest more in the clinical trial process as chances of successful clinical trials are more through the adoption of newer technologies.
SHIFT TOWARDS PERSONALIZED MEDICINE
The paradigm shift towards personalized medicine is expected to have a positive impact on the clinical trial market. The classic clinical trial process is carried out on thousands of people while personalized medicine will focus only on the effect of drugs on individual patients for a specific period. Very few medicines in the development phase pass all phases of a clinical trial due to the traditional clinical trial approach. The mentality of “one size will fit all” is acting as a barrier for drugs that are currently in pipeline but would never see the day. The use of pharmacogenetics in the clinical trial process is expected to increase the number of drugs passing all phases of the clinical trial process. The shift towards personalized medicine is expected to increase the use of pharmacogenetics in the clinical trial phase thereby increasing the pipeline of drugs. This trend is expected to instigate biopharmaceutical companies to invest more in the clinical trial phase.
GROWING DISEASE VARIATION AND PREVALENCE
Growing prevalence of disease and incidence of new disease is expected to give further boost to the clinical trial market. Worldwide population has varied disease profile with emerging countries having the most diverse disease profile. This is expected to boost the clinical trial of new or rare disease which otherwise would not have found any sponsors. More number of patients having a specific disease would act as a stimulus for biopharmaceutical companies to invest more in clinical trials for a disease segment. Diverse population would also mean easy recruitment of patients and faster clinical trial process. Rare diseases are given a status of “Orphan disease” in U.S and biopharmaceutical companies who sponsor clinical trials for Orphan drugs would get incentives for the process. This trend is likely to have a positive impact on clinical trials for rare disease thereby increasing the global clinical trials market.
INCREASING COLLABORATION IN BIOMEDICAL RESEARCH
The trend of combination trials and collaborations in clinical trial is expected to rise which would further boost the global clinical trials market. Due to high drug development cost biopharmaceutical companies are now forming an alliance with each other to increase the resources and share the risk of high cost. Immuno-oncology collaborations in 2015 is a prime example of increase collaboration to combat risk associated with clinical trials. Recent example of immuno-oncology collaboration is of MSD and Lily for clinical trial phase for treatment of advanced soft tissue sarcoma.
The race to launch the molecule in the market in a feasible timeline and cost will propel the need for CROs service provides. The pharmaceutical companies, along with the increased research cost, also witness global regulation complexity. All of these variables create a need for expertise in different portfolios, driving the need for outsourcing the market. Drug companies are not only outsourcing the production of medicines but also clinical trials. With the increasing clinical trial privatization, there is a surge in outsourcing to developing countries such as India, China, and Latin America.
Phase Insights
Phase 3 Segment Reported Foremost Market Stake in 2022
Phase 3 segment recorded the major market stake in the worldwide clinical trials market in 2022 and has garnered revenue share 54.6%. Implementation on a large scale is the major reason for high market share of phase 3. Other factors such as high cost and increasing trend of outsourcing are expected to boost the demand over the estimate period.
The Phase 2 clinical trials are anticipated to advance at the maximum CAGR through the forecast period. The Phase II segment held market share for 19.5% in 2021.
Study Design
Interventional Study is Projected to Dominate the Study Design Segment of Clinical Trials Market Revenue
The requirement of clinical trials in developing diagnostic tests and vaccines for viral diseases such as the SARS CoV-2 has augmented the demand for clinical trials exponentially. Thus, high incidence of novel viral diseases and ongoing technological improvements in clinical trials are major reason for the high revenue share of interventional study. Interventional study segment is expected to grow at a CAGR of 5.4% from 2022 to 2030. The interventional design segment accounted largest market share 46% in 2022.
Indication Insights
Oncology is Projected to Dominate the Indication Segment of Clinical Trials Market Revenue
Constant research on cancer treatment and increasing demand for precision medicine are the major reasons responsible for high market share of oncology. The oncology segment is expected to witness growth at a CAGR of 6.4% over the forecast period 2022 to 2030.
Regional Insights
North America is Estimated to be the Largest Market for Clinical Trials
The research study covers key prospects and trends of clinical trials products throughout different regions including Europe, North America, Asia-Pacific, Middle East and Africa, and Latin America. Regionally, clinical trials market is dominated by North America due to high incidence of chronic disorders and presence of latest healthcare infrastructure. North America accounted largest revenue share 51.7% in 2022.
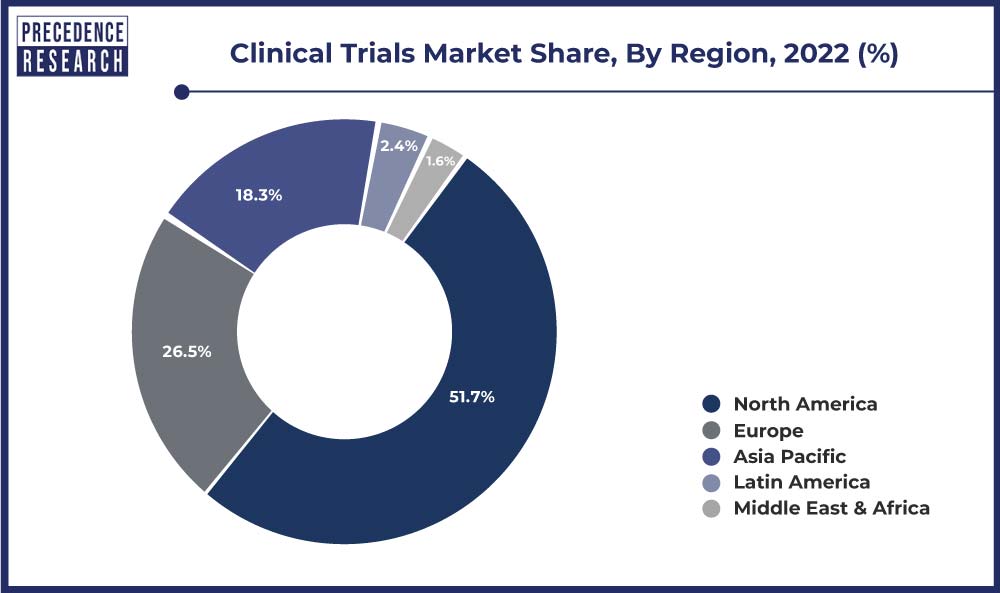
On the other hand, Asia-Pacific is anticipated to witness the rapid growth rate 6.8%, on account of increasing investment by governments in research and growing awareness regarding precision medicine.
- The North America clinical trials market was valued at USD 27.5 billion in 2022 and it is expected to grow at a CAGR of 5.6% over the forecast period.
- The Europe clinical trials market was valued at USD 14.8 billion in 2022 and it is expected to grow at a CAGR of 5.8% over the forecast period.
- The Asia Pacific clinical trials market was valued at USD 10.7 billion in 2022 and it is expected to grow at a CAGR of 6.8% over the forecast period.
Clinical Trials Market Companies
- Charles River Laboratory
- Pharmaceutical Product Development, LLC
In order to better recognize the current status of clinical trials, and policies adopted by the foremost countries, Precedence Research predicted the future evolution of the clinical trials market. This research study bids qualitative and quantitative insights on clinical trials market and assessment of market size and growth trend for potential market segments.
Major Market Segments Covered:
By Study Design
- Observational
- Interventional
- Expanded Access
By Indication
- Rheumatoid arthritis
- Multiple Sclerosis
- Osteoarthritis
- Irritable Bowel Syndrome (IBS)
- Chronic Pain
- Blood Cancer
- Solid Tumors
- Parkinson's Disease (PD)
- Huntington's Disease
- Traumatic Brain Injury (TBI)
- Amyotrophic Lateral Sclerosis (ALS)
- Muscle Regeneration
- Cardiovascular
By Service Type
- Protocol Designing
- Patient Recruitment
- Laboratory Services
- Site Identification
- Cell-based Assays
- Virology Testing
- Method Development, Optimization, & Validation
- Serology, Immunogenicity, & Neutralizing Antibodies
- Biomarker Testing Services
- PK/PD (Pharmacokinetics/Pharmacodynamics) Testing Services
- Other Bioanalytical Testing Services
- Analytical Testing Services
- Clinical Trial Supply & Logistic Services
- Clinical Trial Data Management Services
- Decentralized Clinical Services
- Medical Device Testing Services
- Pharmaceutical & Biopharmaceutical Companies
- Medical Device Companies
By End User
- Laboratories
By Application
- Cell & Gene Therapy
- Small Molecules
- Other Applications
By Geography
- North America
- United Kingdom
- Rest of Europe
- Asia Pacific
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Rest of Latin America
- Middle East & Africa (MEA)
- North Africa
- South Africa
- Rest of Middle East & Africa
Frequently Asked Questions
- Report Code: 1185
- Category: Healthcare
- No. of Pages: 150+
- Format: PDF/PPT/Excel
- Published: December 2022
- Historical Year: 2021-2022
- Base Year: 2023
- Estimated Years: 2024-2033
PROCEED TO BUY :
ASK FOR SAMPLE
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client

- Privacy Policy
- Return Policy
- Terms and Conditions
- About Us
- Contact Us

- Pharmaceuticals /
- Clinical Trials

Clinical Trials Market Size, Share & Trends Analysis Report by Phase (Phase I, Phase II, Phase III, Phase IV), by Study Design, by Indication (Pain Management, Oncology, CNS Condition, Diabetes, Obesity), by Region, and Segment Forecasts, 2022-2030
- Region: Global
- Grand View Research
- ID: 4396385
- Description
Table of Contents
Companies mentioned, methodology, related topics, related reports.
- Purchase Options
- Ask a Question
- Recently Viewed Products
Clinical Trials Market Report Highlights
- The phase III clinical trials segment dominated the market with a 53.4% share in 2021. This can be attributed to the complexity of this phase.
- The interventional design segment accounted for the largest share of 45.7% in 2021 in the study design segment owing to the increasing demand for the intervention for clinical trials by researchers.
- North America held 50.7% of the market share in 2021. Favorable government initiatives and the presence of a large number of players in the U.S. that offer advanced services are responsible for market growth.
- The Asia Pacific region is expected to grow at the fastest CAGR of 6.8% over the forecast period owing to the increasing patient pool and cost-efficient services.
- PAREXEL International Corporation
- Pharmaceutical Product Development, LLC
- Charles River Laboratory
- Wuxi AppTec Inc
- PRA Health Sciences
- Syneos Health
- Chiltern International Ltd
- Eli Lilly and Company
- Novo Nordisk A/S
Table Information

Virtual Clinical Trials Market Size, Share & Trends Analysis Report By Study Design (Interventional, Observational, Expanded Access), By Indication (Oncology, Cardiovascular Disease), By Phase, By Region, And Segment Forecasts, 2023 - 2030
- Report

Global Clinical Trial Support Services Market by Service (Administrative Staff, Clinical Trial Site Management, Data Management), Phase (Phase I, Phase II, Phase III), Study Design, Indication, Sponsor - Forecast 2024-2030

Mental Health Clinical Trials Market Size, Share & Trends Analysis Report By Phase, By Study Design (Interventional, Observational), By Sponsor, By Disorder (Anxiety Disorders, Depression, Schizophrenia), By Region, And Segment Forecasts, 2023 - 2030

Global Clinical Trials Market by Design (Interventional Study, Observational Study), Phase (Phase I, Phase II, Phase III), Indication - Forecast 2024-2030

Clinical Trials Market, Size, Global Forecast 2024-2030, Industry Trends, Share, Growth, Insight, Impact of Inflation, Company Analysis
- February 2024
ASK A QUESTION
We request your telephone number so we can contact you in the event we have difficulty reaching you via email. We aim to respond to all questions on the same business day.
Request a Quote
YOUR ADDRESS
YOUR DETAILS
PRODUCT FORMAT
DOWNLOAD SAMPLE
Please fill in the information below to download the requested sample.
- Today's news
- Reviews and deals
- Climate change
- 2024 election
- Fall allergies
- Health news
- Mental health
- Sexual health
- Family health
- So mini ways
- Unapologetically
- Buying guides
Entertainment
- How to Watch
- My Portfolio
- Latest News
- Stock Market
- Premium News
- Biden Economy
- EV Deep Dive
- Stocks: Most Actives
- Stocks: Gainers
- Stocks: Losers
- Trending Tickers
- World Indices
- US Treasury Bonds
- Top Mutual Funds
- Highest Open Interest
- Highest Implied Volatility
- Stock Comparison
- Advanced Charts
- Currency Converter
- Basic Materials
- Communication Services
- Consumer Cyclical
- Consumer Defensive
- Financial Services
- Industrials
- Real Estate
- Mutual Funds
- Credit cards
- Balance Transfer Cards
- Cash-back Cards
- Rewards Cards
- Travel Cards
- Personal Loans
- Student Loans
- Car Insurance
- Morning Brief
- Market Domination
- Market Domination Overtime
- Opening Bid
- Stocks in Translation
- Lead This Way
- Good Buy or Goodbye?
- Fantasy football
- Pro Pick 'Em
- College Pick 'Em
- Fantasy baseball
- Fantasy hockey
- Fantasy basketball
- Download the app
- Daily fantasy
- Scores and schedules
- GameChannel
- World Baseball Classic
- Premier League
- CONCACAF League
- Champions League
- Motorsports
- Horse racing
- Newsletters
New on Yahoo
- Privacy Dashboard
Analysis and Review: Clinical Research Organization Market by Service (Drug Discovery Services, Pre-Clinical Services, Clinical Services, Post-Approval Services), by Production (In-House, Outsourced), by Indication (Oncology, CNS, Cardiovascular Diseases, Metabolic Disorders, Immunology, Respiratory, Musculoskeletal Disorders, Hematological Disorders), by End User (Pharmaceutical & Biotechnology Companies, Medical Device Companies, Governments & Private Firms, Academic Institutions, Others) and By Region- Forecast for 2023 – 2033
Market Insights on Clinical Research Organization Market covering sales outlook, demand forecast and up-to-date key trends
- Report Preview
- Request Methodology
Clinical Research Organization Market Snapshot (2023 to 2033)
According to Future Market Insights (FMI) analysis in a recent market survey, the global clinical research organization market was valued at US$ 58.0 Billion in 2022 and is expected to reach US$ 139.6 Billion by 2033.
Market Outlook:
The Clinical Research Organization (CRO) market refers to the industry segment companies and organizations providing clinical research services to pharmaceutical, biotechnology, and medical device companies. It encompasses the commercial activities and financial transactions associated with outsourcing clinical trials and research studies.
The market is driven by the increasing demand for efficient and cost-effective drug development processes. Pharmaceutical and biotech companies often outsource clinical research activities to CROs to leverage their specialized expertise, infrastructure, and resources. This allows the companies to focus on their core competencies, such as drug discovery and marketing, while relying on CROs for the execution of clinical trials.
Don't pay for what you don't need
Customize your report by selecting specific countries or regions and save 30%!
Sales Analysis of Clinical Research Organization Market from 2018 to 2022 Vs Market Outlook for 2023 to 2033
Sales of the market grew at a CAGR of 6.4% between 2018 to 2022.
With a historical forecast of stable growth, the clinical research organization (CRO) industry has seen significant growth in recent years. The rise in demand for outsourced clinical trials from pharmaceutical, biotechnology, and medical device businesses is what is causing this surge.
The clinical research organization (CRO) market is anticipated to maintain its growth trajectory in the upcoming years, according to the projection. The expansion of the CRO market is anticipated to be fueled by an increase in clinical trials, a rise in the need for personalized medication, and the prevalence of chronic diseases. Various clinical trial processes, including patient recruiting and retention, data analysis, and medication discovery, are using these technologies.
Considering this, FMI expects the global clinical research organization market to grow at a CAGR of 8.4% through the forecasted years.
What are the Key Opportunities in the Clinical Research Organization Market?
CROs can provide specialized services for carrying out clinical studies in this field as personalized medicine and targeted medicines gain popularity. This covers the identification of biomarkers, patient screening, and customized trial planning. CROs now have more potential due to the use of real-world data and virtual trials.
Companies with expertise in these fields can provide pharma/biotech firms with specialized services and assistance for real-world research as well as for virtual studies, which can shorten the trial duration and expense. It can also include telemedicine and digital health technology in clinical trials to raise study compliance, increase patient participation, and offer ongoing monitoring. CROs can be assisted in adopting these technologies and incorporating them into clinical trial designs by businesses offering specialised services.
They are now also able to provide specialized services in data analytics and machine learning due to the increased availability of real-time data in clinical trials. To do this, one can employ predictive analytics to foresee dangers, spot trends, and improve study design, which thereby helps in the growth of the global market.

Principal Consultant
Talk to Analyst
Find your sweet spots for generating winning opportunities in this market.
Which Factors Could Possibly Restrain the Growth of the Clinical Research Organization Market?
Ensuring compliance with regulatory regulations is one of the biggest issues for CRO businesses. Navigating through each nation's unique legislation can be time-consuming and expensive. To make sure that all applicable regulations are followed, CROs must have a strong regulatory staff. Companies in the pharma and biotech industries are increasingly looking for CROs that can offer services at a low cost without sacrificing quality or timeliness. To remain competitive, CROs may need to modify their pricing strategies and business models in response to margin concerns.
Large-scale multicenter trials might be difficult to finance since there is sometimes a lack of funding for clinical trials. To maximize the cost-effectiveness of clinical studies, sponsors, researchers, and CROs must collaborate due to the high cost of drug development and growing cost constraints. The capabilities of CROs may also be constrained by a lack of highly skilled workers, including researchers, data administrators, and statisticians. Lack of qualified personnel may cause inefficiencies, delays in hiring and retaining staff, and a general decline in the quality of project outputs.
Country-wise Insights
What makes the usa the dominating country in the clinical research organization market.
The USA clinical research organization market is expected to register 32.0% in the global market in 2022.
There are several life science businesses in the United States, and these businesses are embracing innovations in clinical research. CROs that offer these services are in demand as technological innovations like telemedicine and virtual trials are used more frequently. Drug development efforts by biotech and pharmaceutical firms are increasing in the USA These businesses are turning more and more to CROs for assistance, including access to facilities and programs for current patients, enrollment and recruiting information, personalized protocol design, higher trial completion rates, and lower costs.
Why is China considered a Lucrative Market for Clinical Research Organization Services?
China accounted for around 9.3% market share in 2022 globally.
The clinical research organization (CRO) market in China is expanding due to several factors. The rapidly ageing and expanding Chinese population is a significant contributing element, which has raised demand for cutting-edge medical cures and treatments. Furthermore, recent reforms and laws adopted by the Chinese government have made it simpler to conduct clinical trials and research there.
The government has put in place many steps to hasten the regulatory approval procedure for novel pharmaceuticals and therapies, luring multinational companies to locate their research and development operations in the nation. Additionally, the development of healthcare and rising income levels in China have raised the demand for advanced healthcare services, such as clinical research and triage.
What Makes India an Emerging Market for Clinical Research Organization?
India holds a 7.4% value share in the global market in 2022.
The clinical research organization (CRO) market is expanding in India for a variety of reasons. Large and diversified patient populations are a significant contributing element, which makes India a desirable location for carrying out clinical studies. Due to the country's accessible healthcare services and cheaper cost of living, clinical trials in India are also more economical than in many other nations.
The supportive regulatory environment created by the Indian government, which promotes clinical research activities by streamlining the approval procedure for clinical trials and cutting the time needed to secure regulatory approvals, is another important aspect. More foreign money has also been invested in India's clinical research sector as a result of the rise in medical tourism there.
Get the data you need at a Fraction of the cost
Personalize your report by choosing insights you need and save 40%!
Category-wise Insights
Which service is largely adopted for clinical research organizations.
The post-approval services segment held the major chunk of about 45.7% of the global market by the end of 2022.
A key factor in the expansion of the clinical research organization (CRO) market is post-approval services. Post-approval services are the actions taken after regulatory bodies or authorities have given their clearance for the use of a product or therapy. These services are essential for maintaining the product's safety and effectiveness as well as for carrying out additional studies that could increase the product or therapy's effectiveness.
In order to diversify their service portfolio and give their clients value-added services, several CROs offer post-approval services. Pharmacovigilance, medical monitoring, safety monitoring, data management, statistical analysis, quality assurance, and regulatory compliance are a few examples of the services that may be provided.
Which production type is largely preferred for the clinical research organization market?
The In-house production segment contribute 58.3% share of the global market in 2022.
The term in-house describes a method of conducting all phases of clinical research within a single organization, often comprising study design, data management, monitoring, and statistical analysis. Clinical research organizations (CRO) market development is still mostly fueled by internal clinical research conducted by pharmaceutical and biotech corporations. The in-house methodology has several benefits, including more control over studies, quicker decision-making, and reduced reliance on outside suppliers.
A pharmaceutical or biotech company's ability to quickly and efficiently react to any noteworthy events or changes in the study protocol can be facilitated by in-house clinical research, which provides better control over study data and technological competence.
Which Indication Dominates the Global Market in 2022?
The oncology by indication segment accounted for a revenue share of 23.4% in the global market at the end of 2022.
One of the markets for clinical research organizations (CROs) that is expanding the quickest is oncology, which has had a big impact on the development of the sector. Cancer research is an appealing subject for CROs to concentrate their attention on because it is a complex and fast-developing field of medicine that calls for a high degree of knowledge and resources.
Many variables, such as the rising cancer burden globally, developments in molecular biology and genomics, and the creation of more advanced and novel immuno-oncology medicines, all contribute to the expansion of oncology research and the CRO business. The need for more advanced oncology therapies that can address patients' complicated requirements is growing as cancer rates are expected to keep rising.
Which End User Segment Propels the Market Growth?
The pharmaceutical and biotechnology companies hold a share of 45.6% of the global market in 2022.
The market for clinical research organizations (CROs) is expanding mostly due to the efforts of the pharmaceutical and biotechnology industries. These businesses invest a lot of money in the discovery of novel treatments and medications, and they frequently contract out some or all of the clinical research work to CROs to increase productivity and access to funding.
A great deal of financing for clinical research studies-particularly those involving new medicinal compounds-comes from pharmaceutical and biotech corporations. These businesses frequently look to collaborate with CROs who can offer specialized knowledge in research design, data administration, and general project management. Pharmaceutical and biotech companies can shorten the time it takes to develop new drugs by outsourcing clinical trial efforts to CROs and concentrating internal resources on core research and development tasks.
Competitive Landscape
The market's key vendors are concentrating on diversifying their product offerings to strengthen their market share in clinical research organizations and to broaden their presence in developing nations. Pricing strategies, market strategies, technology improvements, regulatory compliance, and acquisition and distribution agreements with other companies to extend their business are the main tactics used by manufacturers to acquire a competitive edge in the market.
For instance:
- In January 2020, Charles River announced a scientific collaboration with Takeda Pharmaceutical Company Limited in order to focus on drug discovery in the four core therapeutic areas that Takeda works on. These four therapeutic areas include oncology, gastroenterology, neuroscience, and rare disease. This collaboration focuses on delivering preclinical candidates.
- In July 2022, Labcorp launched a new test called the neurofilament light chain (NfL) blood test, which will help in the identification and confirmation of neurodegenerative diseases like multiple sclerosis, Alzheimer’s disease, Parkinson’s disease, and others.
Similarly, recent developments have been tracked by the team at Future Market Insights related to companies in the clinical research organization market space, which are available in the full report
Scope of the Clinical Research Organization Report
Key segments covered in clinical research organization industry research, by service:.
- Drug discovery Services
- Pre-clinical Services
- Clinical Services
- Post Approval Services
By Production:
By indication:.
- Cardiovascular Diseases
- Metabolic Disorders
- Respiratory
- Musculoskeletal Disorders
- Hematological Disorders
By End User:
- Pharmaceutical & Biotechnology Companies
- Medical Device Companies
- Governments & Private Firms
- Academic Institutions
- North America
- Latin America
- Middle East and Africa (MEA)
Frequently Asked Questions
How big is the clinical research organization market.
The clinical research organization market is slated to attain US$ 62.43 billion in 2023.
What Might be the Market’s Size by 2033?
The market is expected to attain a value of US$ 139.56 billion by 2033.
Which Opportunities are Emerging in the Market?
Increasing popularity of targeted medicines and personalized medicines are creating opportunities for key players.
Which Factors Pull Back the Market Growth?
Strict regulatory regulations and lack of funding for clinical trials are pulling back the market’s growth.
Who are the Key Players in the CRO Industry?
IQVIA Inc, Parexel International Corporation, and ICON plc. are the key players in the CRO industry.
Table of Content
List of tables, list of charts.
Explore Healthcare Insights
Talk To Analyst
Your personal details are safe with us. Privacy Policy*
- Talk To Analyst -
This report can be customized as per your unique requirement
- Get Free Brochure -
Request a free brochure packed with everything you need to know.
- Customize Now -
I need Country Specific Scope ( -30% )
I am searching for Specific Info.
- Download Report Brochure -

You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Clinical Research Trends & Insights for 2023
In our 2023 preview, 14 experts from WCG share the important shifts, trends, regulations, and priorities that will inform clinical trial development this year and beyond.
After the disruptions to the clinical research field in 2020 and 2021, many looked forward to 2022 as a year of “getting back to normal.” Instead, we found that many pandemic-associated changes have forced us to look at a lot of issues through a new lens.
Certainly, “decentralized trials” dominated our conversations, and considerations of the importance of proactive efforts to facilitate the inclusion of historically underrepresented populations in research is finally being recognized. Both biopharma sponsors and clinical sites are finding their footing again, allowing them to renegotiate some old practices from new perspectives (e.g., investigator oversight responsibilities in decentralized trials, how much of site monitoring really needs to be on-site).
Our clinical trials continued to evolve in response to new therapeutic advances, with more protocols incorporating complex design elements, earlier evaluations of efficacy, and an increasing number of platform studies to move potential drug candidates to decisions more quickly.
2023 looks like it will be just as exciting. In this report, WCG experts from a variety of areas talk about what they’re watching, expecting, and looking forward to in 2023. We’re exploring study design, study conduct, new proposed regulations and guidance, and even at the types of therapeutic agents moving into clinical investigation.
Thank you for joining us as we look ahead to the next exciting year!
Explore Insights:
Perspectives on clinical research sites in 2023, redesigning the clinical research landscape, diversity strategies and empowering sites to support them, the fda’s recent notice of proposed rulemaking (nprm) and guidance, diversifying clinical research by providing research participants payments, clinical trial technology integration now and into the future, interoperability required for the future of clinical trials, quality by design and slowing down to speed up, the emerging use of adjudication in oncology for determining patient eligibility, mrna: not just for vaccines.
Current Trends in Neurodevelopmental and Rare Pediatric Disease
Neuroscience Research Trends to Pay Attention to in 2023
Epilepsy treatments and research: where we started and where we are heading, watch our 2023 trends & insights webinar.
Hear our online panel of clinical trial experts discuss the future of clinical trials, and answer questions from your peers.
This engaging discussion is facilitated by Jill Johnston, Chief Innovation Officer of WCG.

Sandra Smith, RN, MSN, AOCN
Senior Vice President, Clinical Solutions & Strategic Partnerships, WCG
To combat the tumultuous conditions of the industry, research organizations have been forced to look internally to identify operational efficiencies over the last two years, and institutional reconfiguration will continue to evolve in 2023. Centralization of research services, embedding enabling technologies, and establishing research partnerships – both with service providers and with sponsors – will remain key themes in addressing site capacity challenges. Many non-institutional research sites will aggregate into networks to achieve higher operational performance.
Human capital remains critical, and the skillset for research expertise will continue to be in high demand in 2023. Sites are reporting fewer vacant positions, but turnover will persist, and training the newly hired, less experienced research personnel will remain critical. Sponsor-supported staffing augmentation solutions will be key to trial conduct for many organizations.
Oncology will endure as a dominant therapeutic area in clinical research in 2023. Approximately 50 percent of all clinical trials started in 2022 1 were in oncology, with more than 1M patients (about the population of Delaware) needed to complete those open trials 2 . Increasing trial complexity will challenge sites with narrow inclusion/exclusion criteria, multiple study arms (i.e., 19-20 percent increase in four to five arm trials in oncology) 3 , and an increasing number of amendments per clinical trial (i.e., 57 percent increase since 2017) 4 .
Declining average rates of enrollment per site 5 and the need for increased enrollment diversity will accelerate sponsor interest in opening trials at community-based sites and in supporting new investigators/sites. Rapid trial activation, bringing trial access to new participant populations, and utilization of decentralized trial components, will be critical components for trial success.
References:
1, 2, 3, 4, 5 WCG Knowledge Base TM , 2022

Jamie Harper, MHA
Director of Site Engagement and Relations, WCG
In 2023, clinical trial access and improving participant diversity will remain key concerns within the clinical research and healthcare community. Compounded by the number of physician investigators continuing to decrease, the industry will be tasked with developing solutions to ensure scientific medical advancements continue while supporting these diverse populations. Identifying and supporting new physician investigators at research sites, especially those ingrained into the community setting, is critical to offsetting these concerns and accelerating advancements.
Redesigning the clinical trial landscape to mitigate these challenges and create an environment that allows engaged community physicians to provide clinical trial access to the communities they serve is a must. By engaging community sites and physicians who may not have previously considered clinical research as an option, it presents a path toward addressing the concerns of clinical trial access and participant diversity. However, this pathway will require the clinical research industry to provide support for the tools and mentorship needed to successfully conduct compliant clinical trials.
The new environment will require evolution of the current collaborations between sponsors, clinical research organizations (CROs), third-party clinical research vendors, and experienced physician investigators. This collaboration’s success will depend on a shared focus to cultivating a redesigned clinical research landscape through inclusion.
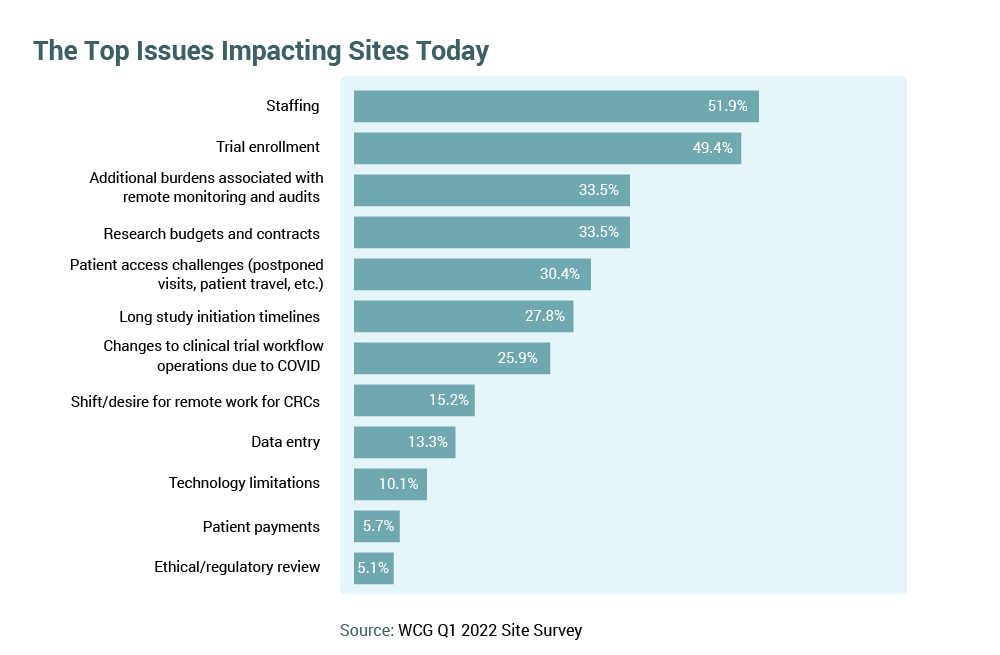
Katherine Cornish, PhD, PMP
Former Associate Director, Patient Advocacy & Clinical Trial Diversity, WCG
As the FDA (U.S. Food and Drug Administration) moves closer to finalizing the current draft guidance on establishing diversity plans 1 to improve clinical trial participation of underrepresented racial and ethnic populations, it becomes imperative to ensure that the strategies outlined in these plans are translated to the site level in intentional and actionable ways.
Sponsors must empower their sites by providing pragmatic strategies that site staff can use to effectively identify, engage, enroll, and retain underrepresented populations. This should be done through the creation of site-focused diversity plans that tie into the overarching diversity plan. Key partnerships with advocacy groups, community-based organizations, and other similar entities outlined in diversity plans at a national level should be translated to individual sites locally. Sponsors must also provide the necessary support to ensure these strategies can be enacted by their sites.
Additionally, these strategies must be identified, documented, and mobilized early in the trial lifecycle; they must be thought about proactively rather than as rescue methods. Sponsors should reach out to the FDA to discuss their diversity plans as early as possible – certainly prior to study start. Thinking about these approaches early on can also ensure that the necessary support is preemptively budgeted at the site level, allowing sites to initiate local advocacy and community engagement efforts from the start. This ensures that sites are prepared and ready when recruitment begins and can enact their action plans from first patient to last visit.
- https://www.fda.gov/media/157635/download

Dave Borasky, MPH, CIP
Vice President, IRB Compliance WCG IRB
The year 2022 ended with two interesting developments for Institutional Review Boards (IRBs) and industry on the FDA front. First, the FDA published two Notices of Proposed Rulemaking (NPRMs) 1,2 that are intended to bring greater harmony between the FDA regulations for human subjects research, and the Common Rule regulations that apply to U.S. federally funded research that is not subject to FDA regulation. While this harmonization is expected – it was mandated by the 21st Century Cures Act – it was not clear when the NPRMs would be issued. Harmonization on issues of informed consent requirements and the use of a single IRB for multisite trials will improve the review and implementation of clinical research in 2023 and beyond.
The second late-year offering from the FDA was the draft guidance Ethical Considerations for Clinical Investigations of Medical Products Involving Children . Through this guidance, the FDA has finally provided clear direction on the expectation that IRBs use component analysis when reviewing research with children that includes multiple research-related interventions or procedures. Component analysis, which has the IRB assess each study procedure (and each study arm), considering the risks and benefits, is often confusing to sponsors. The guidance also provides direction on the FDA’s thinking on what constitutes a “minor increase over minimal risk” (21 CFR 56.102(i)), which is not defined in the regulation, but represents an important threshold for reviewing research involving children, particularly in the context of component analysis.
- https://www.federalregister.gov/documents/2022/09/28/2022-21088/protection-of-human-subjects-and-institutional-review-boards
- https://www.federalregister.gov/documents/2022/09/28/2022-21089/institutional-review-boards-cooperative-research

Kelly Fitzgerald, PhD
IRB Executive Chair and Vice President, IBC Affairs WCG IRB
Payment to research participants is a critical aspect of diversifying clinical research participation. There will be a continued increase in awareness of this issue in 2023. IRBs are often seen as a barrier to paying participants, and that may have been true in the past, but the thinking on this topic has evolved significantly in the last few years, informed in part by the recognition of the importance of research participants as partners rather than as research “subjects. ”1
IRBs are tasked with ensuring that research-recruiting and consenting processes do not exert undue influence on participants, and high payments may be seen by IRB members as unduly influential 2 . Some people believe that payments could incentivize someone to participate in activities they would otherwise choose not to do, particularly people with low incomes. However, when an IRB insists on lower payments, participants who have less free time, less available income, or more burdensome lives are less likely to participate, and the study ends up with a participant population that does not match society because those people who can bear the financial burdens of research participation will participate.
The clinical research world now recognizes that payments can incentivize without being unduly influential. While there is a perception that IRBs will not approve high payments for participants, this is changing. During the last 24 months, out of approximately 10,000 IRB reviews, only 13 reviews resulted in the IRB specifically requiring modifications to the payment plan (see table), and no records included a request to decrease the proposed payments to participants.
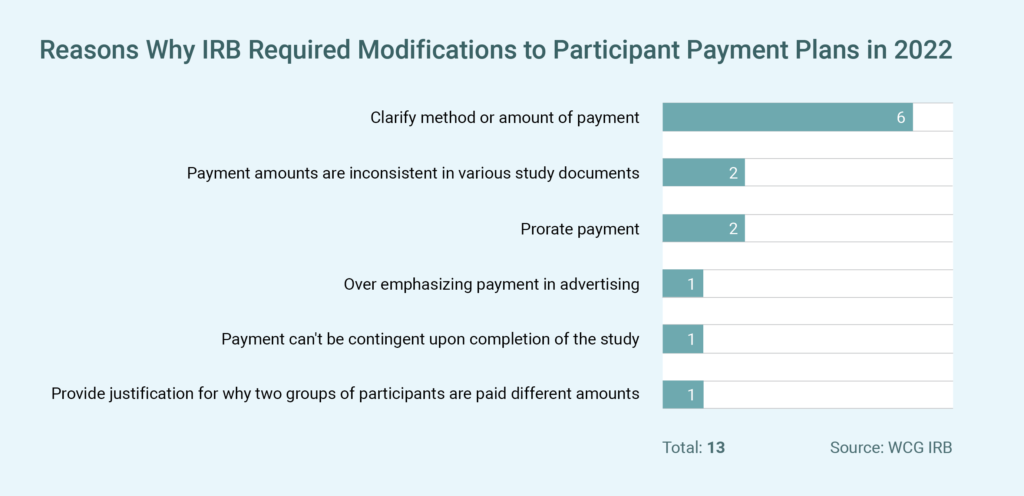
IRB members reflect the values and norms of their communities, and the events of the last few years have led to the awareness of the importance, both scientifically and ethically, of diverse representation in clinical trials. To improve in this area, there must be support for higher payments for research participation and a reliance on mechanisms other than limiting payment to ensure participants are recruited ethically. In 2023, there needs to be more discussions around accurately assessing participant costs for research participation and providing just compensation for their service 3 .
- National Academies of Sciences, Engineering, and Medicine 2022. Improving Representation in Clinical Trials and Research: Building Research Equity for Women and Underrepresented Groups. Washington, DC: The National Academies Press. https://doi.org/10.17226/26479.
- Payment and Reimbursement to Research Subjects, FDA, Jan 2018.
- Largent EA, Lynch HF. Paying Research Participants: The Outsized Influence of “Undue Influence”. IRB. 2017 Jul-Aug;39(4):1-9. PMID: 29038611; PMCID: PMC5640154.

Sonia Abrol
Senior Vice President, Product and Strategy, WCG Velos
The COVID-19 pandemic made many stakeholders in clinical research reassess their technology infrastructure. To sustain and accelerate research, they prioritized taking various research management workflows online to vendor managed systems, ensuring centralized, easy, and secure access to data. As sites and sponsors expanded their technology portfolio, sites are now carrying a bigger technology burden. They are supporting the costs of multiple site technologies and then putting additional time and effort into entering data in both site and sponsor systems. Approximately 90 percent of data needed by sponsors now lives in one or more site systems 1 .
Additionally, the uptick in adoption of innovative technologies for helping with decentralized trials, patient recruitment, patient retention, and engagement has produced new data silos for sites and sponsors. As a result, clinical research stakeholders are recognizing the need for more interoperability and workflow-driven integrations.
In 2023, sites and sponsors will continue to make technology integration one of their top priorities as they select the right solutions, enhance their technology infrastructure, and budget for such investments. Sites starting their journeys toward integrated ecosystems may begin with key multi-level integrations between their e-regulatory/e-Binder, EHR/EMR, CTMS, IRB and Financial systems. Those that are already ahead of the curve will continue to expand and develop new integration workflows by connecting one silo at a time.
In parallel, technology vendors will continue to work toward offering more open systems and actively defining their own integration roadmaps to help sites and sponsors gain operational efficiencies and reduce the technology burden on users.
Also, it will be interesting to see what the gained momentum in collaboration among sites, sponsors, and vendors will bring in 2023. As these consortia and partnerships come together to help define data exchange standards, map best workflows among site systems and site-sponsor systems and prioritize implementation of cost-effective and standard technology integrations, we will see remarkable growth and acceleration in conducting clinical research and drug development.
- WCG Knowledge Base TM , 2022

Rahul Bafna
Former Chief Product Officer, WCG
The use of healthcare technology at the consumer level has exploded over the past decade. This started with the broad availability and adoption of wearable devices, taking advantage of smaller sensors, better batteries, and always-on connectivity. Over this same time period, there has been a ton of investment in healthcare technology for clinicians – the HITECH Act of 2009 was designed to accelerate medical product development. Many of these advancements in technology have the potential to be transformative in the realm of clinical trials, which for decades have suffered from low patient participation, unrepresentative study populations, heavy operational burden, and long timelines. The key to making these advancements work in real-world settings is the seamless interoperability of patient data.
As an example, patient-trial matching is one of the most time-consuming operations of running a trial. Multiple software platforms exist that can match eligible patients – based on the clinical record stored in one or more EHRs and lab systems – to the various inclusion and exclusion criteria of trials. But for this matching to happen, these solutions need access to those clinical records in a standardized format. Once found, matches need to be pushed back into the physician’s daily workflow – often the EHR – to drive patient conversations, consent and enrollment. Once enrolled, technology can simplify or even automate the data collection necessary for some types of studies. This will be critical for enabling decentralized trials (DCT).
DCTs will allow more patients to participate, potentially from underserved communities who would benefit from trial designs that require fewer or no site visits. The use of technology can also enable more frequent collection of study data, and any data collected in novel systems will need to be pushed back to a unified source, such as an EHR or EDC.
Making sense of clinical data from multiple sources – whether for clinical trials or routine care – requires that data to be collected, retained, transformed, transmitted, standardized, and made available to researchers and clinicians at the right time and in the correct format. Interoperability between all these systems is critical to driving innovation and bringing new treatments to patients. For several years now, the Office of the National Coordinator for Health Information Technology (ONC) has been leading the charge to improve the interoperability of patient clinical data through regulation – mandating specific data standards that enable data to be exported in standardized formats. The 21st Century Cures Act, which includes interoperability standards not only for accessing clinical data but also for enabling new workflows and within platforms like EHRs, will continue to drive advancement in 2023.
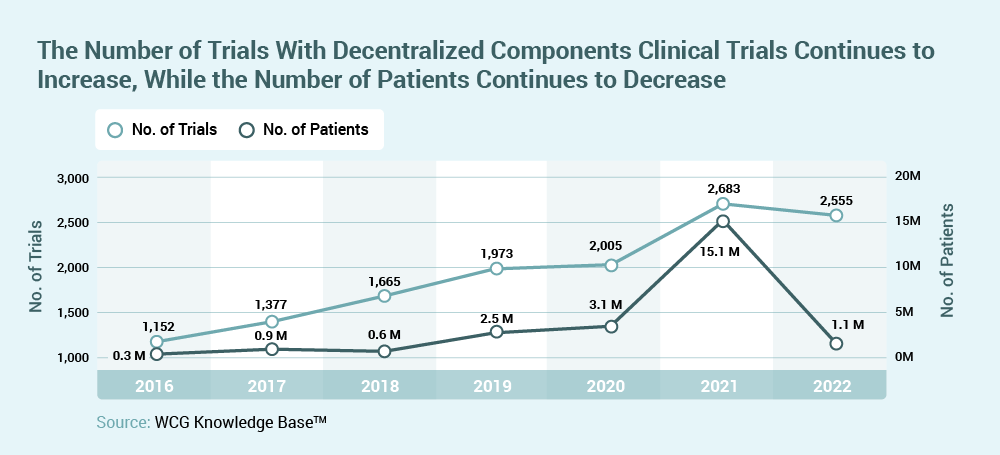
Cristin MacDonald, PhD
VP Client Delivery, WCG Avoca
The year 2023 should be seen as an opportunity for everyone in the clinical research industry to embrace the concept of “slowing down to speed up.” While the concept of Quality by Design is not new, the newly finalized ICH E8 guidance, EU CTR, along with the upcoming ICH E6 R3 and FDA’s Diversity guidance are all examples of the regulators prioritizing efficiency in clinical trial execution by forcing quality into the forefront of study design.
Whether it is by requiring that sponsors show their diversity strategies in advance of launching a pivotal trial, or by subjecting sponsors to regulatory holds for failed submissions, it is time to spend more resources early in study planning. It is time to slow down, think ahead, and ensure quality is built in study design.
Gone are the days of rushing to a first subject in timeframe and reactive protocol amendments that have negative time and budget impacts. Entirely new benchmarks need to be established that encourage study teams to invest the appropriate time and effort in study planning and reward those who demonstrate rapid study completion with minimal amendments by leveraging the proper resourcing up front.

Shaena Kauffman
Executive Director, Operations EAC, WCG
One of the most interesting trends over the past year has been the increased use of Endpoint Adjudication Committees (EACs) to determine patient eligibility, especially in oncology clinical trials. This trend will likely continue in 2023. Adjudication of inclusion criteria, or eligibility adjudication, has been used in clinical trials where inclusion criteria are subjective. The independent assessment by an EAC gives regulators and scientists confidence in the comparability of clinical trial subjects. Previously, this type of adjudication has been applied to areas such as progressive neurological disease or heart failure, where medical expertise is required, or eligibility is subjective. However, there is a rising trend in using EACs to confirm oncology disease progression to demonstrate a consistent evaluation for patient eligibility and provide real-time feedback to the Investigator study sites.
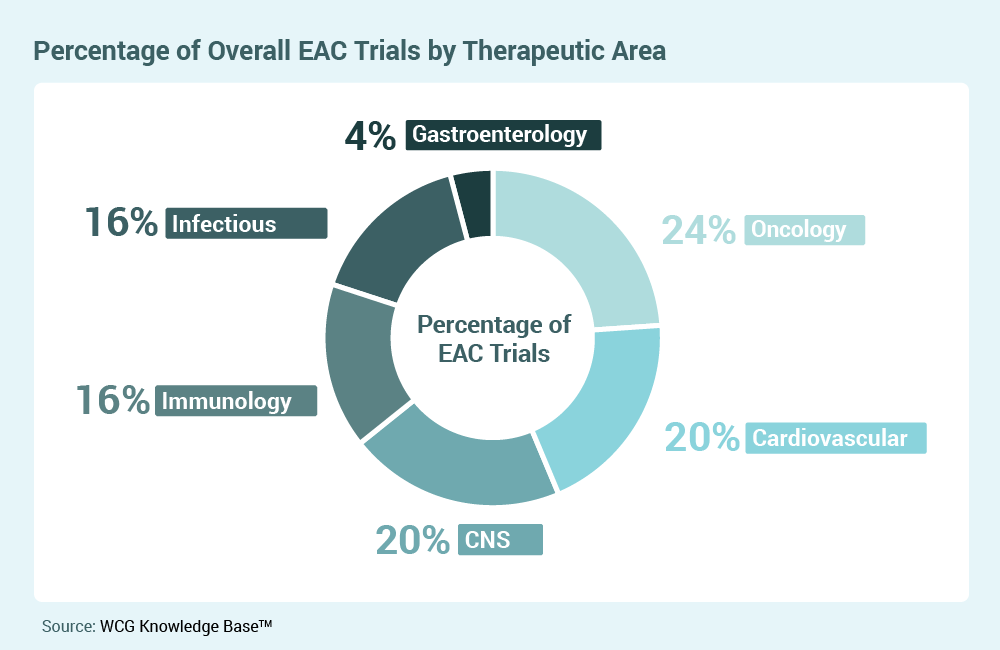
In the area of disease progression, adjudication has the potential to mitigate variability and increase the strength of the clinical trial results. In addition to bolstering the clinical trial data against the regulatory and scientific rigors, EAC members can serve as a resource to investigator study sites that may need access to experts to assess some of the more complex patient eligibility criteria. Of course, the most significant benefits are that adjudication of inclusion criteria reduces protocol deviations and enforces scientific rigor through a pre-randomization, standardized, objective assessment. The use of eligibility EACs will continue to expand in 2023 as sponsors and sites report the benefits.

Daniel Kavanagh , PhD
Senior Scientific Advisor, Gene Therapy, WCG
Reflecting a new emphasis on cell and gene therapy (CGT) for 2023, the FDA recently announced a new “Super Office,” the Office of Therapeutic Products (OTP), to replace the former Office of Tissues and Advanced Therapies. Included in the announcement are plans to increase hiring and issue more CGT guidance documents in the coming year.
The year 2022 saw many new advances in cell and gene therapy, including FDA approvals for new products and supplemental indications for genetically modified cellular therapies and vectored gene therapies. In 2023, one area to watch closely is that of “vectorless” mRNA therapeutics. Synthetic mRNA is a powerful tool that can program cells for new therapeutic functions. When combined with rapidly developing nanoparticle technologies, mRNA therapeutics may be targeted to a wide array of cell types and tissues. One potential application of gene transfer technology is to bypass the complex in-vitro manufacturing process currently required for biologics and turn the body’s own cells into temporary factories to produce antibodies, enzymes, or vaccine antigens. Many such approaches in development rely on mRNA.
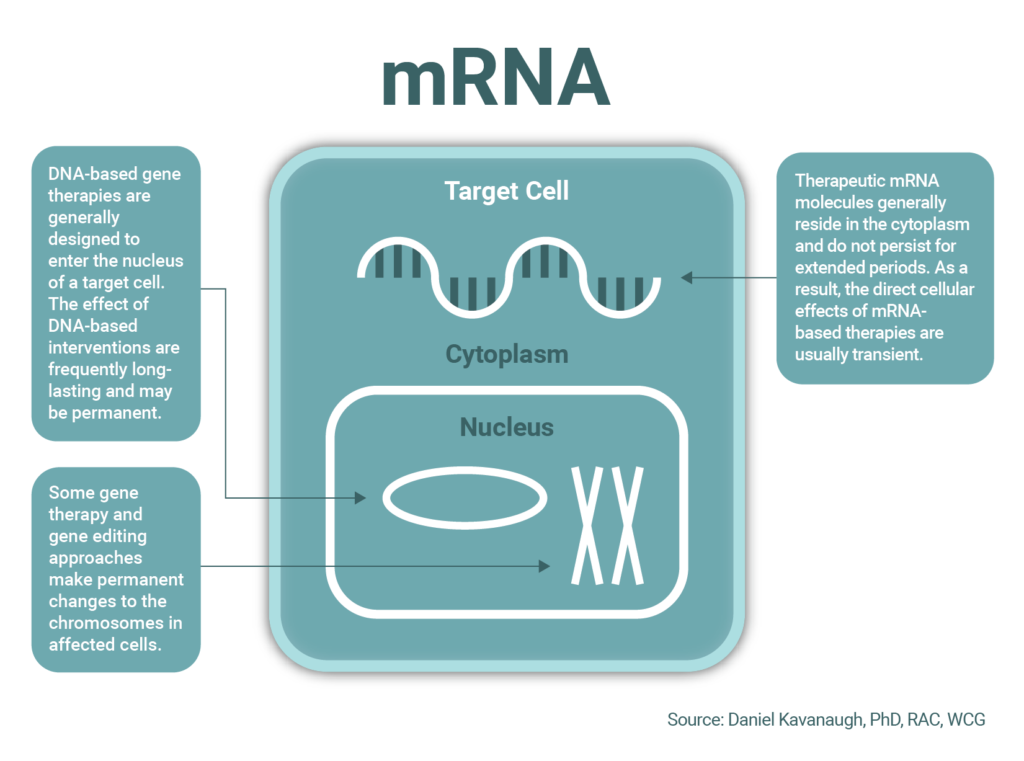
Currently approved gene therapies tend to produce long-lasting or permanent changes in cellular programming and may induce immune responses that prevent re-dosing. For these reasons, many gene therapies are designed for “one-and-done” curative administration. This offers new hope to patients and their families, but also presents challenges for safety, commercialization, and reimbursement. In contrast, mRNA molecules are normally short-lived and exert transient effects on cell biology. Thus, for some applications, mRNA is an important alternative to DNA-based therapies, with potential advantages for safety, re-dosing, and commercialization.
Current Trends in Neuro-developmental and Rare Pediatric Disease

Scott Hunter, PhD
Senior Scientific Expert, Neurodevelopment Disorders, WCG
In 2023, there will continue to be an emphasis on developing new outcome measures that can be used across rare pediatric and Central Nervous System (CNS) diseases. This has been an area of shared concern among caregivers regarding challenges observed with their impacted children that affect both individual and family engagement and quality of life. Moving from broader cognitive measures that are often experienced as less sensitive, focus has moved more to communication (specifically receptive and expressive language skills in areas including Fragile-X, Angelman, Rett, Autism Spectrum Disorders) and motor control (e.g., Rett, Angelman, Fragile-X).
Seeking greater sensitivity with scales developed, foundation and patient advocacy partners are developing mechanisms to build disorder-specific scales to address key areas of caregiver and patient concern, while also collaborating across indications to support better measurement design. The goal of this is to amplify acceptance by the FDA of caregiver and clinician report measures (ClinROs and patient reported outcomes, or PROs) in the rare disease space.
We will move toward using wearables and engaging more directly with samples collected from these technologies that can be expert reviewed and rated. Collecting simultaneous biological and behavioral outcomes through direct movement assessment, recording of communication efforts, and video monitoring of responses, provides significant multipoint data that can be aggregated in tandem with use of performance measurements in rare and neurodevelopmental disease.
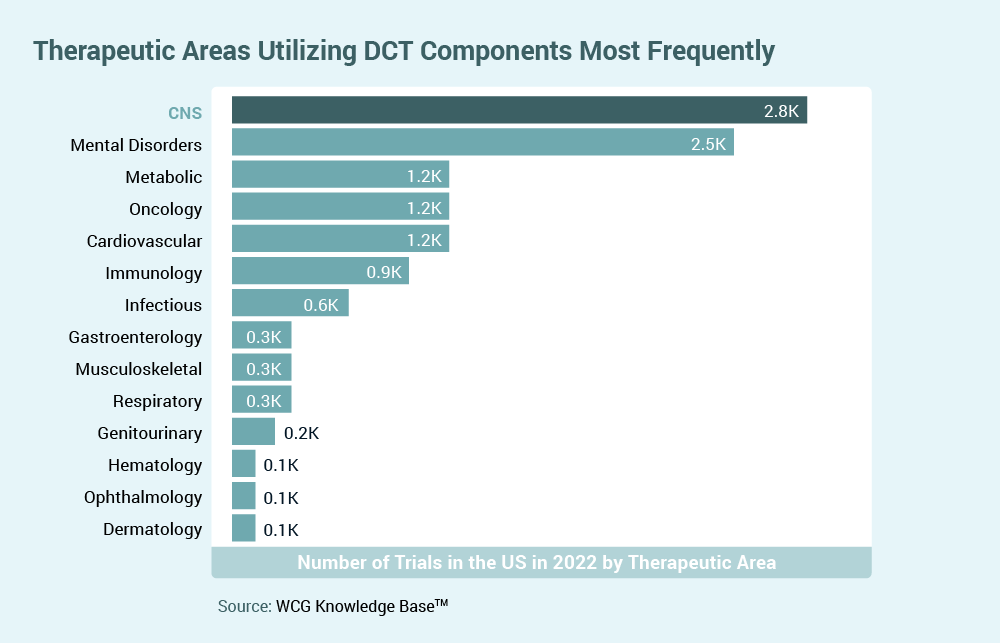
Mark G. A. Opler, PhD, MPH
Chief Research Officer, WCG Endpoint Solutions
The last five years have brought a remarkable burst of innovation in neuroscience drug development. From the approval of the first, rapid-acting agent to treat depression, to the past year’s massive surge of activity in the study of psychedelic compounds, to the ongoing, exploratory use of wearables and other technology-oriented methods for data collection, there has been a tremendous degree of change. In the coming year, we’ll see some of these exciting yet unproven innovations begin to demonstrate their promise in a more substantive way with enduring, real-world impacts.
A few of the most important trends to watch include:
- Late-Stage Studies of Psychedelics and Cannabinoids: The first large-scale, Phase III industry-sponsored studies of psilocybin by Compass Pathways should help prove the viability of the psychedelics movement in neuroscience. The published results of the Compass Phase II program, if confirmed in Phase III, would solidify this while also clarifying the direction that this exciting new class of treatments is likely to take in years to come.
- Development of Wearables and “Digital Biomarkers ”: Although regulatory agencies have held off on explicitly approving the use of novel endpoints related to wearable devices or similar device-rated digital biomarkers, the proliferation of these tools as exploratory endpoints is creating a growing wealth of data. As the evidence base for them continues to grow, standardization and adoption will almost certainly follow.
Mike Cioffi
Senior Vice President, Clinical Solutions and Strategic Partnerships, WCG
Currently, there are more than 30 antiseizure medications (ASMs) available to clinicians to treat patients. But even with such a significant amount of treatment options, there are still over 30 percent of individuals who do not respond to common ASMs and are addressed as “drug-resistant.” 1 This will drive innovation in both pharmacological and non-pharmacological interventions aimed at improving symptoms and quality of life for patients along with their caregivers.
Research is ongoing in many areas, and we will see important advances in the field in the coming years. An increased application of technologies to aid in epilepsy management is likely. Smartwatches that can detect imminent seizures and alert caregivers to the location of the patient, and apps that can inform clinicians with real-time information on their patients’ status and seizure counts are all on the horizon.
Precision medicine has begun to take a more prominent role as we understand more about the pathogenesis of epilepsies. Significant advancement has been made in identifying the genetics of epilepsies, and the discovery of gene mutations responsible for a large portion of patients with developmental and epileptic encephalopathies (DEEs) 2 .
Additionally, the search for biomarkers to guide drug development is moving at a fast pace and will potentially allow clinicians to improve diagnostic accuracy, predict response to ASMs, and improve outcomes for patients.
While there are certainly limitations to current technologies and biomarker identification, and despite precision medicine being further researched, this multidisciplinary approach will redefine our ability to improve the treatment and management of epilepsy in the future.
- Kalilani L, Sun X, Pelgrims B, Noack-Rink M, Villanueva V. The epidemiology of drug-resistant epilepsy: a systematic review and meta-analysis. Epilepsia. (2018) 59:2179–93. doi: 10.1111/epi.14596
- Perucca P, Bahlo M, Berkovic SF. The genetics of epilepsy. Annu Rev Genomics Hum Genet. 2020;21(1):205–30
Request a Meeting with a WCG Expert
Interested to learn how the insights above will affect your studies? Submit this form to request a meeting with one of WCG’s consultants featured in this report.
2024 Avoca Quality Consortium Summit
Event Details
- Arcus Biosciences-stock
- News for Arcus Biosciences
Promising Outlook for Arcus Biosciences Anchored by Strong Clinical Trials and Strategic Market Positioning
Analyst Kaveri Pohlman of BTIG maintained a Buy rating on Arcus Biosciences ( RCUS – Research Report ), retaining the price target of $70.00.
Kaveri Pohlman’s rating is based on the promising clinical trial data and strategic positioning of Arcus Biosciences in the competitive landscape of cancer treatments. The upcoming presentations at ASCO 2024 for both the Edge-Gastric and ARC-9 studies are pivotal, with the Edge-Gastric study showing encouraging six-month progression-free survival (PFS) rates that align with historical benchmarks. Additionally, Arcus’ TIGIT program, despite expected efficacy similar to competitors, stands out with a cleaner safety profile, which may offer a competitive advantage over treatments associated with gastrointestinal toxicities.
Furthermore, the ARC-9 study targets a market with limited competition for the third-line treatment of metastatic colorectal cancer (mCRC), and Arcus’s inclusive approach to patients with liver metastases, who represent a substantial portion of the mCRC patient population, presents a significant opportunity. The breadth of the TIGIT franchise, with multiple ongoing Phase 3 trials and expected data readouts, including from partner Roche’s Skyscraper-01 study, underscores the potential for Arcus’s therapies in areas of high unmet need. Pohlman’s Buy rating reflects confidence in Arcus’s ability to deliver value through its robust pipeline and strategic clinical development.
According to TipRanks , Pohlman is a 4-star analyst with an average return of 5.9% and a 31.54% success rate. Pohlman covers the Healthcare sector, focusing on stocks such as Gritstone Oncology, Arcus Biosciences, and Nuvation Bio.
In another report released on April 28, Citi also maintained a Buy rating on the stock with a $37.00 price target.
TipRanks tracks over 100,000 company insiders, identifying the select few who excel in timing their transactions. By upgrading to TipRanks Premium, you will gain access to this exclusive data and discover crucial insights to guide your investment decisions. Begin your TipRanks Premium journey today.
Arcus Biosciences (RCUS) Company Description:
Arcus Biosciences, Inc. engages in the development and commercialization of immunotherapies. The firm compete in the segments of the pharmaceutical, biotechnology and other related markets that develop immunotherapies for the treatment of cancer. The company was founded by Terry J. Rosen and Juan Carlos Jaen in 2015 and is headquartered in Hayward, CA.
Read More on RCUS:
- Arcus Biosciences price target lowered to $37 from $44 at Citi
Arcus Biosciences News MORE
Related stocks.
Stop COVID Cohort: An Observational Study of 3480 Patients Admitted to the Sechenov University Hospital Network in Moscow City for Suspected Coronavirus Disease 2019 (COVID-19) Infection
Collaborators.
- Sechenov StopCOVID Research Team : Anna Berbenyuk , Polina Bobkova , Semyon Bordyugov , Aleksandra Borisenko , Ekaterina Bugaiskaya , Olesya Druzhkova , Dmitry Eliseev , Yasmin El-Taravi , Natalia Gorbova , Elizaveta Gribaleva , Rina Grigoryan , Shabnam Ibragimova , Khadizhat Kabieva , Alena Khrapkova , Natalia Kogut , Karina Kovygina , Margaret Kvaratskheliya , Maria Lobova , Anna Lunicheva , Anastasia Maystrenko , Daria Nikolaeva , Anna Pavlenko , Olga Perekosova , Olga Romanova , Olga Sokova , Veronika Solovieva , Olga Spasskaya , Ekaterina Spiridonova , Olga Sukhodolskaya , Shakir Suleimanov , Nailya Urmantaeva , Olga Usalka , Margarita Zaikina , Anastasia Zorina , Nadezhda Khitrina
Affiliations
- 1 Department of Pediatrics and Pediatric Infectious Diseases, Institute of Child's Health, Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russia.
- 2 Inflammation, Repair, and Development Section, National Heart and Lung Institute, Faculty of Medicine, Imperial College London, London, United Kingdom.
- 3 Soloviev Research and Clinical Center for Neuropsychiatry, Moscow, Russia.
- 4 School of Physics, Astronomy, and Mathematics, University of Hertfordshire, Hatfield, United Kingdom.
- 5 Biobank, Institute for Regenerative Medicine, Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russia.
- 6 Institute for Regenerative Medicine, Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russia.
- 7 Chemistry Department, Lomonosov Moscow State University, Moscow, Russia.
- 8 Department of Polymers and Composites, N. N. Semenov Institute of Chemical Physics, Moscow, Russia.
- 9 Department of Clinical and Experimental Medicine, Section of Pediatrics, University of Pisa, Pisa, Italy.
- 10 Institute of Social Medicine and Health Systems Research, Faculty of Medicine, Otto von Guericke University Magdeburg, Magdeburg, Germany.
- 11 Institute for Urology and Reproductive Health, Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russia.
- 12 Department of Intensive Care, Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russia.
- 13 Clinic of Pulmonology, Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russia.
- 14 Department of Internal Medicine No. 1, Institute of Clinical Medicine, Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russia.
- 15 Department of Forensic Medicine, Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russia.
- 16 Department of Statistics, University of Oxford, Oxford, United Kingdom.
- 17 Medical Research Council Population Health Research Unit, Nuffield Department of Population Health, University of Oxford, Oxford, United Kingdom.
- 18 Centre for Tropical Medicine and Global Health, Nuffield Department of Medicine, University of Oxford, Oxford, United Kingdom.
- 19 Oxford University Hospitals NHS Foundation Trust, John Radcliffe Hospital, Oxford, United Kingdom.
- 20 Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russia.
- PMID: 33035307
- PMCID: PMC7665333
- DOI: 10.1093/cid/ciaa1535
Background: The epidemiology, clinical course, and outcomes of patients with coronavirus disease 2019 (COVID-19) in the Russian population are unknown. Information on the differences between laboratory-confirmed and clinically diagnosed COVID-19 in real-life settings is lacking.
Methods: We extracted data from the medical records of adult patients who were consecutively admitted for suspected COVID-19 infection in Moscow between 8 April and 28 May 2020.
Results: Of the 4261 patients hospitalized for suspected COVID-19, outcomes were available for 3480 patients (median age, 56 years; interquartile range, 45-66). The most common comorbidities were hypertension, obesity, chronic cardiovascular disease, and diabetes. Half of the patients (n = 1728) had a positive reverse transcriptase-polymerase chain reaction (RT-PCR), while 1748 had a negative RT-PCR but had clinical symptoms and characteristic computed tomography signs suggestive of COVID-19. No significant differences in frequency of symptoms, laboratory test results, and risk factors for in-hospital mortality were found between those exclusively clinically diagnosed or with positive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RT-PCR. In a multivariable logistic regression model the following were associated with in-hospital mortality: older age (per 1-year increase; odds ratio, 1.05; 95% confidence interval, 1.03-1.06), male sex (1.71; 1.24-2.37), chronic kidney disease (2.99; 1.89-4.64), diabetes (2.1; 1.46-2.99), chronic cardiovascular disease (1.78; 1.24-2.57), and dementia (2.73; 1.34-5.47).
Conclusions: Age, male sex, and chronic comorbidities were risk factors for in-hospital mortality. The combination of clinical features was sufficient to diagnose COVID-19 infection, indicating that laboratory testing is not critical in real-life clinical practice.
Keywords: COVID-19; Russia; SARS-CoV-2; cohort; mortality risk factors.
© The Author(s) 2020. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: [email protected].
Publication types
- Observational Study
- Research Support, Non-U.S. Gov't
- Hospitalization
- Middle Aged
Grants and funding
- 20-04-60063/Russian Foundation for Basic Research

IMAGES
VIDEO
COMMENTS
The global clinical trials market size was valued at USD 80.7 billion in 2023 and is projected to grow at a compound annual growth rate (CAGR) of 6.49% from 2024 to 2030. The market growth spiked in 2020 owing to the COVID-19 pandemic. This growth pattern was witnessed by both virtual clinical trials and traditional ones.
The Clinical Trials Market size is estimated at USD 50.66 billion in 2024, and is expected to reach USD 67.5 billion by 2029, growing at a CAGR of 5.91% during the forecast period (2024-2029). The COVID-19 pandemic tremendously impacted the market for clinical trials, as there has been a rising focus on developing new therapeutics or vaccines ...
The clinical trials market recorded more than USD 55.5 billion in 2022 and is anticipated to exhibit 5.9% CAGR from 2023-2032 due to growing demand for outsourcing clinical trials to CROs. ... The clinical trials market research report includes in-depth coverage of the industry with estimates & forecast in terms of revenue in USD Million from ...
Global pharmaceutical CRO market size from 2015 to 2024, by pre-clinical, clinical and discovery (in billion U.S. dollars) Premium Statistic Size of total U.S. clinical trial supplies market 2016-2025
KEY MARKET INSIGHTS. The clinical trials market size was valued at USD 54.24 billion in 2022 and is projected to grow from USD 57.76 billion in 2023 to USD 92.45 billion by 2030, exhibiting a CAGR of 6.9% during the forecast period (2023-2030). North America accounted for a market value of USD 26.13 billion in 2022.
Global Clinical Trials Market Outlook. The clinical trials market size attained a value of USD 49.22 billion in 2023. The market is anticipated to grow at a CAGR of 5.4% during the forecast period of 2024-2032 to attain a value of nearly USD 79.02 billion by 2032. Read more about this report - REQUEST FREE SAMPLE COPY IN PDF.
The global clinical trials market in terms of revenue was estimated to be worth $48.2 billion in 2023 and is poised to reach $73.2 billion by 2028, growing at a CAGR of 8.7% from 2023 to 2028. ... Clinical research entities partner with their clients (such as pharmaceutical and biotechnology companies) to design strategies, implement them, and ...
Clinical Trials: Essential Pillars of Evidence-Based Medicine, Guiding Informed Healthcare Decisions for Optimal Patient Outcomes. share : The clinical trials market value was USD 269.18 million in 2022 & it crosses USD 402.28 million by 2030, following a CAGR of 5.15% by the forecasted period.
The clinical trials market is estimated to cross US$ 83.5 Bn by the end of 2030. However, finding the right patients is one of the most important pieces of the puzzle with respect to conducting clinical trials. Hence, stakeholders are working with experts at clinical trial recruitment companies to match patients with the right trials.
The global clinical trials market size was valued at USD 49.49 billion in 2022. It is estimated to reach USD 16.77 billion by 2031, growing at a CAGR of 5.2% during the forecast period (2023-2031). Globalization of clinical trials has led to an increase in investment in new product development in emerging nations, thereby positively impacting ...
The clinical trial market is estimated to be US$ 120.97 billion in 2024. From 2024 to 2034, the market is expected to progress at a solid clip, registering a CAGR of 4.3%. By 2034, the clinical trial market is anticipated to have reached a value of US$ 184.61 billion. Clinical Trial Market Growth Report. Governments are making efforts to step ...
Market Size & Trends. The North America clinical trials market size was estimated at USD 40.9 billion in 2023 and is projected to grow at a compound annual growth rate (CAGR) of 5.99% from 2024 to 2030. This is attributed to increasing clinical trial cycles, the need for novel therapies, a surge in chronic diseases such as cancer, diabetes, and ...
Report Description. Table of Content. Request Customization. The global clinical trials market size was valued at USD 48.68 billion in 2022 and is predicted to hit USD 83.55 bn by 2032 with a registered CAGR of 5.6% during the forecast period 2023 to 2032. U.S. clinical trials market was valued at USD 24.61 billion in 2022.
The U.S. clinical trials market was valued at USD 22.6 billion in 2021 and is expected to expand at a compound annual growth rate (CAGR) of 5.4% from 2022 to 2030. The growth is majorly driven by a rise in R&D activities, increasing vaccine trials, the need for personalized medicine, and the growing use of new technologies across clinical research.
The global clinical trials market size is expected to reach USD 78.3 billion by 2030, registering a CAGR of 5.8% during the forecast period. An increase in the volume and complexity of clinical trials has been witnessed lately, which plays an important role in the R&D of new drugs and products.
Clinical Research market's key players are ICON Plc, Wuxi AppTec, PRA Health Sciences, SGS SA, Syneos Health, Eli Lilly and Company, Novo Nordisk A/S, Pfizer, Clinipace, Other key players. To ...
Clinical Research Organization Market Snapshot (2023 to 2033) According to Future Market Insights (FMI) analysis in a recent market survey, the global clinical research organization market was valued at US$ 58.0 Billion in 2022 and is expected to reach US$ 139.6 Billion by 2033. Market Outlook: The key players in the market are Charles River ...
In our 2023 preview, 14 experts from WCG share the important shifts, trends, regulations, and priorities that will inform clinical trial development this year and beyond. After the disruptions to the clinical research field in 2020 and 2021, many looked forward to 2022 as a year of "getting back to normal.".
2. Integrating Technology. This pandemic has accelerated remote clinical trial conduct and management. With the integration of technology, it has been possible to collect clinical data of subjects ...
Market by Research Stage: Clinical Trials Stage Dominates the Market Different research stages, encompassing Pre-clinical Research, Clinical Trials, Public Health Research, Cross-disciplinary ...
Analyst Kaveri Pohlman of BTIG maintained a Buy rating on Arcus Biosciences (RCUS - Research Report), retaining the price target of $70.00. Kaveri Pohlman's rating is based on the promising ...
Company expands structural heart portfolio and begins the limited market release of a bipolar temporary pacing guidewire intended to introduce and position catheters and other interventional devices within the chambers of the heart while transmitting an electrical signal from an external pulse generator to the heart WAYNE, Pa., April 30, 2024 (GLOBE NEWSWIRE) - Teleflex Incorporated (NYSE: TFX ...
Today's top 236 Research jobs in Moscow, Moscow City, Russia. Leverage your professional network, and get hired. New Research jobs added daily.
Vladyslav Logos will present his clinical research at the eighth annual International Conference on Education and Psychology (ICEAP) July 18-20 in Fukuoka, Japan. Logos, originally from Kyiv, Ukraine, is in his second year of master's studies in clinical mental health counseling at Tarleton State's Fort Worth campus.
(SACRAMENTO) Neuroblastoma is the leading cause of cancer death in children under the age of five. Thanks to an award from The Hartwell Foundation, UC Davis pediatric surgeon Erin Brown will develop a stem cell treatment to target this difficult-to-treat disease.. Brown is one of only ten scientists selected to receive the 2024 Individual Biomedical Research Award.
1 Clinical Research Department, ... The rate of clinical improvement on Day 7 in the favipiravir group was 1.5-fold higher than in SOC group: 52.7% vs. 35.8% (RR 1.50; 95% CI 1.02-2.22; P=0.020). Favipiravir was well-tolerated and the most common adverse reactions were asymptomatic hyperuricemia, transient elevation of ALT & AST, and mild ...
Introduction. Emerging data suggest that a substantial proportion of people experience ongoing symptoms including fatigue and muscle weakness, breathlessness, and neurological problems more than 6 months after the acute phase of coronavirus disease 2019 (COVID-19) [1, 2].This phenomenon is commonly referred to as "long COVID", a term defined by patient groups, and also known as post-COVID ...
The combination of clinical features was sufficient to diagnose COVID-19 infection, indicating that laboratory testing is not critical in real-life clinical practice. Age, male sex, and chronic comorbidities were risk factors for in-hospital mortality. ... 3 Soloviev Research and Clinical Center for Neuropsychiatry, Moscow, Russia. 4 School of ...