Get the Most Legit Information and Guide on the Latest Jobs in Nigeria, Facebook and Education Here

WAEC Chemistry Questions and Answers for 2023/2024 (Theory and Objectives)
2023 WAEC Chemistry Answers, WAEC Chemistry questions and answers, WAEC Chemistry questions and everything you need to know about 2023 WAEC Chemistry Examination will be provided in this article. You will find Chemistry Paper 1 (Objective) and Chemistry 2 (Essay) here
Scroll to the bottom for today’s Chemistry Answers
Below are the WAEC Chemistry questions. Read them and get ready to score high in your WAEC Chemistry exam.
The West Africa Examination Council (WAEC) is an examination body in Nigeria that conducts the Senior Secondary Certificate Examination and the General Certificate in Education in May/June and November/December respectively.
Table of Contents
WAEC Chemistry Questions and Answers 2023
WAEC Chemistry Questions and Answers 2023 Loading…
The 2023 answers will be posted here on 24th May during the exam
WAEC Chemistry OBJ Answers Loading…
1-10: ACADABBDAC
11-20: BCAABBCCBB
21-30: ACBABDACBB
31-40: ABBDAADCDD
41-50: BDCDDCDDCD
A transition element, also known as a transition metal, is an element that belongs to the d-block of the periodic table.
(i) Element B (2:8:2)
(ii) Element C (2:8:1)
The increase in the first ionization energies across a period is mainly due to the increasing effective nuclear charge and decreasing atomic radius. The stronger pull from the increasing nuclear charge and the closer proximity of the electrons to the nucleus make it harder to remove an electron, leading to higher ionization energies.
Number 1di)
Two examples of aliphatic compounds are:
A. Ethane (C2H6): Ethane is a saturated hydrocarbon belonging to the alkane family. It consists of a straight chain of two carbon atoms bonded to each other, with six hydrogen atoms attached to the carbons.
B. Butanol (C4H10O): Butanol is an alcohol with a four-carbon straight chain. It has the chemical formula C4H10O and exists in four isomeric forms: n-butanol (normal butanol), sec-butanol (secondary butanol), isobutanol (isopropyl alcohol), and tert-butanol (tert-butyl alcohol).

*Reaction between Iron (Fe) and Dilute H2SO4:*
Iron reacts with dilute sulfuric acid to form iron sulfate (FeSO4) and hydrogen gas (H2). The balanced chemical equation for this reaction is:
3Fe(s) + 4H2SO4(aq) -> 3FeSO4(aq) + 2H2(g) + 2H2O(l)
In this reaction, iron displaces hydrogen from the acid, resulting in the production of iron sulfate and hydrogen gas. The iron sulfate is soluble in water and remains in solution, while the hydrogen gas is released as a gas.
*Reaction between Aluminum (Al) and Dilute H2SO4:*
Aluminum reacts differently with dilute sulfuric acid due to the protective oxide layer that forms on its surface. The oxide layer prevents further reaction by acting as a barrier between the aluminum metal and the acid. However, the reaction can occur if the protective layer is disrupted or removed
2Al(s) + 3H2SO4(aq) -> Al2(SO4)3(aq) + 3H2(g)
In this reaction, aluminum sulfate (Al2(SO4)3) and hydrogen gas are produced. The aluminum sulfate formed is soluble in water and remains in solution, while the hydrogen gas is liberated as a gas.

More Answers Loading…
—————————————————————-
—————————————————–
The questions below are the WAEC Chemistry Questions. Go through them and be ready to score high in your WAEC 2023 Chemistry Examination.
1. What condition favours the formation of the product for the endothermic reaction, N2O4(g) —><—– 2NO2(g)
A. Decrease in pressure
B. A decrease in volume
C. An increase in pressure
D. A constant volume
ANSWER: A ( Decrease in pressure)
2. How many alkoxy alkanes can be obtained from the molecular formula C 4 H 4 O 4
ANSWER: C (3)
3. Element Y has two isotopes Y and Y present in the ratio 1:3. The relative atomic mass of Y would be
ANSWER: C (21.5)
4. 100.0g of KClO 3 was added to 40.0 cm^3 of water to give a saturated solution at 298K. If the solubility of the salt is 20.0 moldm^-3 at 298K, what percentage of the salt is left undissolved? {K= 39, Cl = 35.5, O = 16}
5. Elements X and Y have electronic configuration 1S 2 2S 2 2P 4 and 1S 2 2S 2 2P 6 3S 2 3P 1 respectively. When they combine the formula of the compound formed is
ANSWER: B (Y 2 X 3 )
Recommended: WAEC Timetable
6. A solution of 0.20 mole of NaBr and 0.20 mole of MgBr 2 in 2.0 dm 3 of water is to be analysed. How many moles of Pb(NO 3 ) 2 must be added to precipitate all the bromide as insoluble PbBr 2
A. 0.30 mol
B. 0.10 mol
C. 0.20 mol
D. 0.40 mol
ANSWER: A (0.30 mol)
7. Na 2 CO 3 + HCl —-> NaHCO 3 + NaCl. The indicator most suitable for this reaction should have pH equal to.
ANSWER: D (9)
8. A satuarted solution of silver trioxocarbonate (IV), was found to have concentration of 1.30 x 10 -5 moldm -3 . The solubility product of the trioxocarbonate (IV) is
A. 8.79 x 10 -15
B. 1.69 x 10 -10
C. 1.82 x 10 -11
D. 9.84 x 10 -10
ANSWER: A (8.79 x 10 -15 )
9. When platinum electodes are used during the electrolysis of copper (II) tetraoxosulphate (IV) solution, the solution gets progressive
D. Atmospheric
ANSWER: A (Acidic)
10. Tetraoxosulphate (VI) ions are final test using
A. acidified silver nitrate
B. acidic barium chloride
C. lime water
D. dilute hydrochloric acid
ANSWER: D (dilute hydrochloric acid)
- WAEC Physics Questions and Answers
- WAEC Biology Questions and Answers
- WAEC English Questions and Answers
WAEC 2020 Chemistry Theory Questions
Paper 2 (essay).
1. Identify the solid remaining when each of the following is heated. (a) lithium trioxonitrate (V) (b) potassium trioxonitrate (V) (c) calcium trioxonitrate (V)
2. (a) When calcium oxide and coke are heated in an electric furnace, the products are carbon (ii) oxide and calcium carbide (CaC2), write the equation for this reaction.
(b) The addition of water to calcium carbide leads to the formation of calcium hydroxide and ethyne. Write the equation for the production of ethyne.
5. The first ionization energy of chlorine is +1260KJmol-1. (a) Define the term first ionization energy. (b) State and explain the general trend in the values of the first ionization energy for the elements across the period, sodium to argon in the periodic table.
7. State two factors other than a change in temperature or the use of a catalyst that influence the rate of a chemical reaction.
1. (a) Two elements represented by the letters Q and R have atomic numbers 9 and 12 respectively. (i) Write the election configuration of R. (ii) To what group does Q belong in the periodic table. (iii) Write the formula of the compound formed when Q combines with R. (iv) Explain briefly, why Q is a good oxidizing agent. (v) State whether R would be expected to form acidic or basic oxide. (b) (i) State two assumptions of the kinetic theory of gases. (ii) When some solids are heated, they change directly into the gaseous state. What name is given to this phenomenon? (iii) List two substances which exhibit the phenomenon mentioned in (ii). (iv) Write an expression to show the mathematical relationship between the rate of diffusion of a gas and its vapour.
2. An aqueous solution has a pH of 4.0. (a) (i) What is the hydrogen ion concentration of the solution? (ii) What effect will it have on litmus paper? (iii) Which of the following salt solutions would have the same effect on litmus? Give a reason for your answer. NH4Cl(aq); NaCl(aq) ; CH3OON(aq). (b) (i) Differentiate between a fine chemical and a heavy chemical. (ii) Name two sources of air pollution. (iii) Suggest one way of reducing air pollution in cities
3. (a) (i) Explain briefly the fermentation process. (ii) Write a balanced equation for the fermentation of glucose. (iii) What substance must be added to glucose solution to ferment it? (iv) Explain briefly why tightly corked glass filled to the brim with palm wine shatters on standing. (b) State one industrial application of each of the following methods of separation: (i) Crystallization; (ii) Fractional distillation. (c) Explain the following terms: (i) Saponification; (ii) Esterification. (d) Write a balanced equation to illustrate each of the terms in (c). (e) i) What is hydrocarbon compound? (ii) Name two principal sources of hydrocarbons.
WAEC Chemistry Essay and Objective 2023 (EXPO)
The above questions are not exactly 2023 WAEC Chemistry questions and answers but likely WAEC Chemistry repeated questions and answers.
These questions are for practice. The 2023 WAEC Chemistry expo will be posted on this page during the WAEC Chemistry examination. Keep checking and refreshing this page for the answers.
Keep reloading this page for more answers
Tips to Pass WAEC Chemistry Questions
1. study hard.
It is known to everyone that hard work brings success. Ensure to read the chemistry textbooks recommended by The West Africa Examination Council (WAEC).
This will help you to take a look at most of the questions you will see on the day of WAEC Chemistry examination.
Let me clearly state that questions are not imported from heaven but from those textbooks recommended by WAEC.
2. Read WAEC Chemistry Past Questions
WAEC usually picks questions from the previous years to form fresh questions. Studying WAEC Chemistry past questions would give you an idea of how WAEC Chemistry questions are set.
3. Start Preparing for WAEC 2023 :
Stop wasting your time. Now that you have gotten textbooks and past questions, the next thing is to begin your reading. Early reading and practice is good for you; you will pass well on WAEC 2023 Chemistry
4. Solve and try to understand every example and exercise you see in textbooks
It is very unfortunate that Secondary school students are fond of skipping exercises and even examples while studying textbooks.
They loved notebooks so much that they could ask, “can I read my Chemistry notebook and pass WAEC 2023?”. Don’t be scared of attempting exercises; they are there to help you. Face it and overcome it!
5. Practise Regularly
Don’t get discouraged when certain topics are annoying, keep on practicing until you master everything. Never give up and never say never. Keep pushing….
If you have any questions about WAEC Chemistry Questions and Answers for 2023, kindly drop your question in the comment box and please, remember to share this information by clicking the Facebook share icon or any of the social media share icons below.
Last Updated on May 24, 2023 by Admin
Related posts:

144 thoughts on “WAEC Chemistry Questions and Answers for 2023/2024 (Theory and Objectives)”
Can we get waec questions for 2024 pls?
Sir pls send me the past question and answer i beg Sir pls send me the past question and answer i beg sir
I need number four question please
Pls upload the waec chemistry questions
Pls this this are correct very correct this is what came out in chemistry
Pls sir, i need the questions on civic education tomorrow
Pls sir send me waec question and answer for this year
Please sir i will like You to send waec 2023 chemistry theory and essay
Thanks u alot sir But I need waec chemistry around 9am Please update me soon
Leave a Comment Cancel reply
Save my name, email, and website in this browser for the next time I comment.
Notify me of follow-up comments by email.
Notify me of new posts by email.

WAEC GCE Chemistry Questions and Answers 2023 – Objectives and Essay
The West African Examination Council (WAEC) conducts the GCE Chemistry exam for Senior School Certificate candidates. If you are preparing for the 2023 WAEC GCE Chemistry exam, you might be wondering about the types of questions you can expect and how to effectively prepare for them. In this comprehensive guide, we will provide you with valuable insights and tips to help you excel in the exam. We will cover both the objective and essay sections, providing you with a complete understanding of the topics to focus on.

Objectives Section
The objectives section of the WAEC GCE Chemistry exam tests your knowledge and understanding of various concepts. It consists of multiple-choice questions that require you to select the correct answer from the options provided. Here are some key points to keep in mind for this section:
1. Familiarize Yourself with Past Questions
To excel in the objectives section, it is essential to familiarize yourself with past questions. These questions often follow a similar pattern, allowing you to identify recurring topics and concepts. By practicing with past questions, you can develop a deeper understanding of the exam format and improve your speed and accuracy in answering.
2. Understand the Marking Scheme
Understanding the marking scheme is crucial for maximizing your score in the objectives section. Each question carries equal marks, and there is no negative marking for incorrect answers. Therefore, it is advisable to attempt all questions, even if you are unsure of the correct answer. Eliminate options that you know are incorrect and make an educated guess if necessary.
3. Review Key Concepts and Formulas
The objectives section covers a wide range of topics, including atomic structure, chemical reactions, organic chemistry, and more. Reviewing key concepts and formulas related to these topics is essential for answering questions accurately. Create concise notes or flashcards to help you memorize important information and revise regularly to reinforce your understanding.
4. Practice Time Management
Time management is crucial during the exam. The objectives section is time-sensitive, and you need to allocate enough time to answer all the questions. Practice solving past papers within the allotted time frame to improve your speed and efficiency. Additionally, learn to prioritize questions based on your strengths and weaknesses to maximize your score.
Essay Section
The essay section of the WAEC GCE Chemistry exam tests your ability to express your understanding of the subject matter in a coherent and structured manner. Here are some tips to help you excel in the essay section:
1. Understand the Question
Before you start writing your essay, take the time to carefully read and understand the question. Identify the key points and concepts the question is asking you to address. This will help you structure your essay effectively and ensure that you stay on topic throughout.
2. Plan Your Essay
Planning your essay is essential for organizing your thoughts and presenting a well-structured argument. Create an outline that includes an introduction, body paragraphs, and a conclusion. Map out the main points you want to discuss in each section, ensuring a logical flow of ideas.
3. Provide Relevant Examples
To support your arguments and demonstrate a deep understanding of the subject matter, provide relevant examples in your essay. These examples can be drawn from real-life situations, experiments, or case studies. Use specific details and data to strengthen your points and make your essay more persuasive.
4. Use Proper Scientific Terminology
The essay section of the WAEC GCE Chemistry exam requires you to use proper scientific terminology to communicate your ideas effectively. Make sure to use the appropriate terms and definitions related to the topic you are discussing. Avoid using vague or ambiguous language that may confuse the examiner.
Additional Tips for Success
Here are some additional tips to help you succeed in the WAEC GCE Chemistry exam:
- Practice Regularly : Consistent practice is key to mastering the concepts and improving your problem-solving skills. Dedicate regular study sessions to revise the topics and solve practice questions.
- Seek Clarification : If you come across any concepts or questions that you find difficult to understand, don’t hesitate to seek clarification from your teacher or classmates. Understanding the fundamentals will help you tackle more complex problems.
- Manage Your Time : Develop a study schedule that allows you to cover all the necessary topics before the exam. Allocate sufficient time to practice past questions and identify areas where you need to improve.
- Stay Calm and Confident : On the day of the exam, stay calm and confident in your preparation. Trust in your abilities and approach each question with a clear mind. Avoid rushing and carefully read each question before answering.
Preparing for the WAEC GCE Chemistry exam requires diligent study and practice. By familiarizing yourself with past questions, understanding the marking scheme, reviewing key concepts, and practicing time management, you can excel in both the objectives and essay sections. Remember to plan your essay effectively, provide relevant examples, and use proper scientific terminology. With consistent effort and a strategic approach, you can achieve excellent results in the 2023 WAEC GCE Chemistry exam.
Remember, success in the exam is not just about memorizing answers but also demonstrating a deep understanding of the subject matter. Use this comprehensive guide as a roadmap to guide your preparation and ace the WAEC GCE Chemistry exam. Best of luck!
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Related Articles

School Of Health Technology Kano Tafara Jarrabawar Tantancewa

Covenant University Post UTME Screening Admission Form 2023/2024 Session – How ToApply

How To Access WAEC Digital Certificate Platform In 2023

ABU ZARIA TA BUDE PORTAL 2020/2021

WAEC Literature Questions and Answers 2022

JAMB: ePIN PAYMENT PROCESS BY CANDIDATES 2022

YADDA AKE SAMUN ADMISSION TA HANYAR JAMB UPLOADING RESULT

VACANCY AT THE COMMONWEALTH SECRETARIAT LONDON UNITED KINGDOM

How to Retrieve your Lost Jamb profile Code in Few Minutes

Sule Lamido University IJMB Admission Form 2022/2023

JIGAWA STATE COLLEGE OF EDUCATION SCREENING/INTERVIEW 2021/ 2022

JAMB TA SANARDA RANAR FARA UTME

- Username Password Remember me Sign in New here ? Join Us

Chemistry 2023 WAEC Past Questions

The vapour density of an organic compound with the molecular formula \(C_2H_4O_2\), is [H=1.0, C=12.0, O= 16.0]
The following statements are correct except
- A. energy is released when liquids change to solids.
- B. carbon atoms in gaseous methane are further apart than those in solid diamond.
- C. there is large decrease in the volume of a solid metal when pressure is applied to it.
- D. particles move faster in the gaseous state than in the liquid state.
Which of the following reactions represents the hydrolysis of an alkanoate?
- A. \(CH_3COOH + OH^- ⇌H^+⇌ CH_3COO^- + H_2O\)
- B. \(CH_3COOH + C_2H_5OH ⇌H^+⇌ CH_3COOC_2H_5 + H_2O\)
- C. \(CH_3COOC_2H_5 + H_2O ⇌H^+⇌ CH_3COO^- + CH_3CH_2OH\)
- D. \(CH_3COOCH_2CH_3 + H_2O ⇌H^+⇌ CH_3COOH + CH_3CH_2OH\)
The following steps are scientific methods except
- A. experimentation
- B. problem identification
- C. Analysis
- D. Open-mindedness
Alkenes can be manufactured by
- A. the combustion of alkanes.
- B. the cracking of hydrocarbons.
- C. polymerization reactions.
- D. addition of hydrogen to unsaturated vegetable oils.
- Mathematics
- English Language
- Animal Husbandry
- Literature in English
- Accounts - Principles of Accounts
- Christian Religious Knowledge (CRK)
- Agricultural Science
- Islamic Religious Knowledge (IRK)
- Civic Education
- Further Mathematics
- Home Economics
- Book Keeping
- Data Processing
- Catering Craft Practice
- Computer Studies
- Physical Education
- Office Practice
St Charles Edu Services
Genuine Exam Past Questions and Answers Online Bookshop – PDF and MS Word Download
WAEC Chemistry Past Questions and Answers in 2023 PDF Download Objective & Theory
Are you writing the West Africa Examination Council WAEC Internal or External examination, if yes you need the WAEC Past Questions on Chemistry
we at stcharlesedu.com has compiled a good number of Chemistry WAEC Past Questions and Answers in Pdf Chemistry 2 – Theory/Essay Questions. Chemistry 1 – Objective Test Questions.
Our research has confirm that candidate that uses WASSCE Chemistry past questions to prepare is ten times better than those who do not.
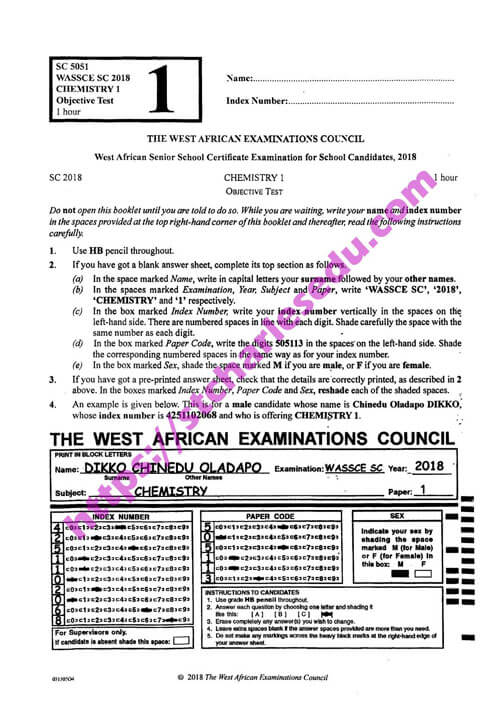
Table of Contents
- 1.1 Chemistry WAEC Objective Questions
- 2 SSCE WAEC Chemistry Theory Questions
- 3 Chemistry WAEC Essay Questions
- 4 Free WAEC Chemistry Exam Past Questions Download
- 5 How to Get WASSCE Chemistry Exam Past Questions and Answers
SSCE WAEC Chemistry Objective Questions and Answers
CHEMISTRY Paper 1 (Objective Test Questions) Paper 1 will last for 1 hours Use HB pencil throughout.
Answer All Questions Each question is followed by four options lettered A to D. Find out the correct options for each question and shade in pencil on your answer sheet, the answer space which bears the same letter as the option you Chosen. Give only one answer to each question. An example is given below
What others are downloading WAEC Past Questions for all Subjects
Chemistry WAEC Objective Questions
Which of the following elements reacts with water? A. Carbon B. Iodine C. Sodium D. Sulphur
The correct answer is Sodium, which is lettered C and therefore answer space C would be shaded. [A] [ B ] [C] [ D ]
Think carefully before you shade the answer spaces; erase completely any answer you wish to change.
Which of the following raw materials is used in the plastic industry? A. Ethene B. Methane C. Sulphur D. Hydrogen
Which of the following organic compounds can undergo both addition and substitution reactions? A. Petane B. Benzene C. Propane D. Hexane
Which of the following equations represents a redox reaction? A. AgNO 3 (aq) + KCl(ag)->AgCl(s)+ KNO 3 (aq) B. HNO 3 (aq)+ NaOH(aq) -> NaNO 3 (aq) + H 2 O(l) C. CaCO 3 (s) -> CaO(s) + CO 2 (g) D. 2H 2 S(g) + SO 2 (g) -> 2H 2 O(I) + 3S(g)
T he process of extraction of iron from its ore is A. decomposition. B. oxidation. C. reduction. D. sublimation.
What is the solubility of a salt if 0.4 g of it is obtained on evaporating 200 cm3 of its saturated solution to dryness? A. 0.08 gdm -3 B. 2.00 gdm -3 C. 8.00 gdm -3 D. 80.00 gdm -3
An acidic salt has A. double anions in its aqueous solution. B. a single cation in its aqueous solution. C. hydrogen ions in its aqueous solution. D. hydrogen atoms in its aqueous solution.
A reaction is endothermic if the A. reaction vessel feels cool during the reaction. B. enthalpy change is negative. C. bond forming energy exceeds bond breaking energy. D. heat of formation of reactants exceeds heat of formation of products.
In which of the following compounds does hydrogen form ionic compounds? A. CH 4 B. HCl C. NH 3 D. NaH
Consider the following reaction equation: Br 2 + 2KI -> 2KBr + I 2 . Bromine is acting as A. an oxidizing agent. B. a reducing agent. C. an acid. D. a base.
An organic compound has the empirical formula CH 2 . If its molar mass is 42 gmol-1 what is its molecular formula? [H = 1.0, C = 12.0] A. C 2 H 4 B. C 3 H 4 C. C 3 H 6 D. C 4 H 8
Ethene is produced from ethanol by A. decomposition. B. hydrolysis. C. ozonolysis. D. dehydration.
Consider the following equilibrium reaction: 2 AB(g) + B 2 (g) -><- 2AB 3 (g) AH = -XkJmol -1 The backward reaction will be favored by A. a decrease in pressure. B. an increase in pressure. C. a decrease in temperature. D. an introduction of a positive catalyst.
What is the mass of solute in 500 cm 3 of 0.005 moldm -3 H 2 SO 4 ? [H =1.0, O = 16.0, S = 32.0] A. 0.490 g B. 0.049 g C. 0.245 g D. 0.0245 g
Pure water can be made to boil at a temperature lower than 100 °C by A. reducing its quantity. B. decreasing the external pressure. C. distilling it. D. increasing the external pressure.
Consider the following sketch of the solubility curve of some substances. Note: scroll down to download the free chemistry waec questions in pdf copy to view the sketch
At what temperature does the solubility of KNO 3, equal that of NaNO 3 ? A. 0°C B. 20 °C C. 30 °C D. 40 °C
When a salt is added to its saturated solution, the salt A. dissolves and the solution becomes super saturated. B. dissolves and the solution becomes unsaturated. C. precipitates and the solution remains unchanged. D. dissolves and crystals are formed.
When substance X was added to a solution of bromine water, the solution became colorless. X is likely to be A. propane. B. propanoic acid. C. propyne. D. propanol.
The preferential discharge of ions during electrolysis is influenced by the A. mechanism of electrolysis. B. electrolytic reactions. C. nature of the electrode. D. type of electrolytic cell.
The valence electrons of 12 Mg are in the A. 3s orbital. B. 2px orbital. C. 2s orbital. D. 1s orbital.
Stainless Steel is an alloy comprising of A. Fe and C. B. Fe and Ni. C. Fe, C and Ni. D. Fe, C and Al.
The number of hydrogen ions in 1.0 dm 3 of 0.02 moldm -3 tetraoxosulphate(VI) acid is [NA = 6.02 x 1023] A. 1.2 x 10 22 B. 1.2 x 10 23 . C. 2.4 x 10 22 . D. 2.4 x 10 23 .
The most suitable substance for putting out petrol fire is A. water. B. carbon(IV)oxide. C. fire blanket. D. sand.
The following factors would contribute to environmental pollution except A. production of ammonia. B. manufacture of cement. C. photosynthesis. D. combustion.
The position of equilibrium in a reversible reaction is affected by A. particle size of the reactants. B. vigorous stirring of the reaction mixture. C. presence of a catalyst. D. change in concentration of the reactants.
The diagram below illustrates a conical flask containing water and ice.
NOTE: scroll down and download the free chemistry pdf past questions to see the diagram
Which of the following statements about the diagram is correct? A. The water is at a lower temperature than the ice B. Energy is absorbed when the ice changes to water C. Energy is released when the ice changes to water D. The water molecules vibrate about a fixed point
Which of the following statements best explains the differences between a gas and a vapor? A. Unlike gases, vapors are liquids at room temperature B. Unlike gases, vapor can easily be condensed into liquids C. Unlike gases, vapour is readily converted into solids D. Vapours are generally denser than gases
Consider the following reaction equation: 2HCl + Ca(OH) 2 –> CaCl 2 + H 2 O. What is the volume of 0.1 moldrn -3 HCl that would completely neutralize 25cm 3 of 0.3 moldm -3 Ca(OH) 2 ? A. 150 cm 3 B. 75 cm 3 C. 30 cm 3 D. 25 cm 3
Cu and HNO 3 are not suitable for preparing hydrogen gas because of their A. reactivity and oxidation respectively. B. conductivity and corrosiveness respectively. C. melting point and reduction respectively. D. electro negativity and solubility respectively.
Which of the following formulae cannot be an empirical formula? A. CH B. CH2 C. P2O5 D. N204
One of the criteria for confirming the purity of benzene is to determine its A. heat capacity. B. boiling point. C. mass. D. colour.
Want more Chemistry Objective Test Questions like this? Get the Complete WAEC Chemistry Exam Past Questions and Answers (Obj and Essay) in PDF Format from us.
SSCE WAEC Chemistry Theory Questions
Chemistry Paper 2 Paper 2 will last for 2 hours This paper consists of two sections A and B. Answer one questions from Section A and three questions from Section B.
Credit will be given for clarity of expression and orderly presentation of material.
SECTION A (1ai) Define the term fermentation. (1aii) Name the catalyst that can be used for this process.
(b) Name two factors which determines the choice of an indicator for an acid-base titration. (c) Consider the following reaction equation: [Fe + H2S04 ] FeS04 + H2. Calculate the mass of unreacted iron when 5.0g of iron reacts with 10cm3 of 1.0 moldrrv3 H SO [Fe = 56.0] (d) Name one: (di) Heavy chemical used in electrolytic cells; (dii) Fine chemical used in textile industries.
(e) Explain briefly how a catalyst increases the rate of a chemical reaction. (f) (i) Write the chemical formula for the product formed when ethanoic acid reacts with ammonia. (ii) Give the name of the product formed in 1 (f) (i)..
(g) List three properties of aluminum that makes it suitable for the manufacture of drink can. (h) State two industrial uses of alkylalkanoates. (i) List two effects of global warming. (j) Name two steps involved in the crystallization of a salt from its solution.
Chemistry WAEC Essay Questions
SECTION B. 2ai. State the collision theory of reaction rates. 2aii.Using the collision theory, explain briefly how temperature can affect the rate of a chemical reaction.
bi. Sketch a graphical representation of Charles’s law. bii. Calculate the volume of oxygen that would be required for the complete combustion of 2.5moles of ethanol at s.t.p. [ molar volume at s.t.p = 22.4dm3]
ci. Define esterification. cii. Give two uses of alkanoates. ciii. Give the products of the alkaline hydrolysis of ethyl ethanoate.
d. A tin coated plate and a galvanized plate were exposed for the same length of time. di. Which of the two plates corrodes faster? dii. Explain briefly your answer in 2 (d) (i).
Want more Chemistry Theory Questions like this? Get the Complete WAEC Chemistry Exam Past Questions and Answer (Obj and Essay) in PDF Format from us.
Free WAEC Chemistry Exam Past Questions Download
Click to Download your free NECO Past Question on Painting and Decorating Paper 2 and 3
Link 1: WASSCE Chemistry Questions Booklet Link 2: WASSCE Chemistry Questions Booklet
How to Get WASSCE Chemistry Exam Past Questions and Answers
To get the complete and more recent copy of the West Africa Examination Council WAEC Past Questions and answer
Take Note of the following step
Make a Call Call or whatsapp us on 08051311885 for the account number to make payment and how to received your complete copy of the past questions to be sent directly to your email address or whatsapp number.
Mode of Payment. Mobile Transfer or Direct Bank Deposit.
After Payment send us the following Depositor Name: Name of Product Paid for: Valid email address.
DELIVERY ASSURANCE We will deliver the past question to you 10 mins after confirmation of payment to the email you will send to us.
Related Posts:
- WAEC Technical Drawing Past Questions PDF Download – Objective, Essay, Building Plan/Practical Drawing
- WAEC Government Past Questions and Answers in 2023 PDF Download Objective & Theory
- WAEC Financial Accounting Past Questions and Answer 2023 – Objective & Essay
- WAEC Visual Art Past Questions and Answers – Objective, Theory in 2023
- WASSCE/WAEC Electrical Installation & Maintenance Past Questions PDF – Objective/Essay
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Education News and Academic Guides
- WAEC Questions and Answers
WAEC Chemistry Questions And Answers For 2023 | Objectives And Theories

Join one of the lucky candidates who will have access to the May/June WAEC Chemistry Questions and Answers for 2023 | Objectives and Theories . It is not everyone who is writing the 2023 WAEC examination will be privileged to see the information that I am going to reveal to you here.
Are you a candidate who is sitting for the May/June WAEC examination for 2023? This article is very important for you especially if you are a science student.
In this article, I am going to be revealing to you those questions that you are going to see in chemistry on the examination date. If you are interested in becoming one of the early birds who will have access to the exact questions and the answers, kindly read this article to the end.
First of all, let me start by answering some of the frequently asked questions about WAEC chemistry questions every year. These questions are going to give you more guides about the 2023 WAEC questions and how you would be able to answer them.
How Many Questions are in WAEC Chemistry
How to answer waec chemistry questions, reasons for poor performance in chemistry, what you should read for waec chemistry 2023, waec chemistry objective questions for 2023, waec chemistry objective answers for 2023, waec chemistry essay questions for 2023, related links, related posts.
The WAEC chemistry examination is usually made up of not less than three subsections with each subsection containing a specified number of questions.
This section presents to you the number of questions that you are going to see in the WAEC Chemistry Examination for 2023.
This part is usually the objective part of the Chemistry examination. It is usually made up of 50 questions in all and candidates are expected to answer all the questions contained therein.
These are usually compulsory questions that you are expected to finish. They carry 1 mark respectively, making a total of 50 marks.
In this section, you will be given about 7 questions and would be required to answer just 5 questions in all. While entering this part, it is necessary to check for the question(s) that have been made compulsory by WAEC.
The paper 3 is made up of 3 questions which are usually practical questions. You would be expected to answer all the questions compulsorily.
They are quite good number of students who have concluded that chemistry is one of the hardest subjects to write in WAEC examination. This could be as a result of how they were thought in their respective secondary schools. Some of them do not even have a good knowledge of chemistry.
For one to pass chemistry examination at one sitting in WAEC , they are some principles that must be put in practice. These are the rules that are applicable during the time of writing the examination.
If you follow the guidelines that I am going to give you in this section, then you will make an excellent result in chemistry
The principles for answering WAEC chemistry examination include the following:
- Read Instructions
Once you have settled to take your examination in the examination hall, the next thing that you must do first is to read through the instructions on top of the page before you can proceed with reading the questions.
Most times, some instructions are specific to some questions, maybe one or two questions. Here, you still have to take those instructions very serious.
2. Read Carefully
Before you can proceed to answering WAEC chemistry questions, it is highly recommended that you read carefully to understand any question before you can start to answer the questions.
It is possible to see questions that would be similar to what you have been seeing before, probably in past questions, but they are not the same.
This is the point where it is very necessary that you meticulously go through the question before you answer, to avoid choosing the wrong option for objectives and giving wrong explanations for theory questions.
3. Take Note of Compulsory Questions
In every WAEC examination, there is/are usually some questions that are specially made compulsory by the West Africa Examination Council. These questions are always seen in the paper 2 which is theory aspect of it.
You must ensure that you are able to answer those compulsory questions before you can think of answering any other ones. The compulsory questions do carry special mark.
4. Start with the Simplest
The complexity of every question in WAEC chemistry varies. After you have gone through the questions, you would be able to tell which of the questions are simple and the ones that are difficult.
The best approach in such case requires that you answer the questions from the simplest to the most complex ones. This is important because it will help to cushion you against examination tension.
Not only that, it also helps in the management of your time. If you are finding any question difficult to answer, you have to leave it and go to the next. Thereafter, you can re-visit those questions that you have left unanswered.
5. Attempt all the Question
Though it is advisable that you start answering your question from the simplest, leaving the more difficult ones at the first attempts, it does not imply that you should submit you examination without answering those ones you skipped.
It quite understandable that you may not know all the asked questions, you are still required choose your answers even the ones that you do not really know very well.
Sometime, your guess can fall in place and become the right answer. So always ensure that you attempt all you question before you submit.
6. Review your Answers
After you have attempted the entire given questions, you still have to go through all the answers to check for any errors and possible corrections.
By going through the questions, you would be able to see any skipped question(s), if any and the ones you mistakenly clicked the wrong options.
It is an error to assume that WAEC chemistry examination is hard. Failure to perform excellently in WAEC chemistry examination is a question of whether the candidate knows the right things to do and if he/she is doing it right.
The poor performance that have been seen in some candidates’ results over the years are as a result of poor application of the basic principles for writing WAEC chemistry examination and some other factors that I am going to show you here.
1. Inadequate Preparation
This is the most important aspect of the reasons for poor performance in WAEC Chemistry that should be considered.
It is obtainable in all areas that when there is poor preparation for any examination they will be poor result. As a good student, you are required to prepare for the WAEC chemistry examination to the point that you would be sure of scoring high.
2. Poor Time Management
Time management is another important factor that can lead to poor performance in every examination. You should learn how to manage your time especially when you are in the examination hall.
The practice of time management starts from the period of the candidate’s preparation for the examination. Once you can answer your past questions comfortably with the limited time that will be provided, you will still manage your time very well on the examination date.
3. Lack of Adherence to Given Instructions
There are some students who are fond of ignoring instructions when come for examination. Jumping into answering of questions without reading the given instructions is very wrong. This contributes in a big way to poor performance in the examination.
If you want to have good performance in WAEC chemistry, you have to strictly pay close attention to any given instructions.
4. Submitting Incomplete Answers
When a candidate submits the examination without attempting all the questions, it will automatically affect his/her score. It is important you answer all the questions before your submission.
In WAEC chemistry examination, they are some topics that are considered very important. These topics are where most of the questions that are repeatedly asked by the WAEC for every year.
As you continue to read this section, I am going to reveal those topics to you. If you are able to read the chemistry topics that I am going to show you here very well before the examination, it means that you can attempt up to 90% of WAEC chemistry questions correctly.
These topics include the following:
- Nature of Matter
- Separation Techniques
- Atomic Structure
- Chemical Combination
- Kinetic Theory of Matter
- Acids, Bases and Salts
- Periodic Table
- Non-metals and their Compounds
- Metals and their Compounds
- Electrolysis
- Energy and Chemical Reactions
- Chemical Equilibrium
- Rate of Reaction
- Air and Air Pollution
- Water, Solution and Solubility
- Shapes of Molecules and Solids
- Radioactivity
- Hydrocarbon
- Organic chemistry
However, you should note that reading with the WAEC syllables is the best practice. The topics listed above are just suggestion of areas that you can concentrate so as to get a good result in the WAEC chemistry examination.
The following are what you are likely going to see in the 2023 WAEC chemistry examination for objective section.
1. A mixture of sugar and sulphur can be separated by
A. dissolution in water, evaporation and filtration
B. filtration, evaporation and dissolution in water
C. dissolution in water, filtration and evaporation
D evaporation dissolution in water and filtration
2. Which of the following is a physical change?
A. Freezing ice-cream
B. Dissolving calcium in water
C. Burning kerosene
D. Exposing white phosphorus to air.
3. The percentage of water of crystallization in ZnSO.7H 2 O is
[Zn = 65, S = 32, O = 16 , H=1]
4. 0.0075 mole of calcium trioxocarbonate(IV) is added to 0.015 mole of a solution of hydrochloric acid. The volume of gas evolved at s.t p. is
[Molar volume of a gas at s t.p = 22.4dm 3 ]
5. A gas exerts pressure on its container because
A. the molecules of a gas collide with the walls of the container.
B some of the molecules are moving faster than others.
C. of the collisions of the molecules with each other
D. of the mass of the molecules of the gas
6. The basic assumption in the kinetic theory of gases that the collisions of the gaseous molecules are perfectly elastic implies that the
A. forces of attraction and repulsion are in equilibrium
B. gaseous molecules can occupy any available space
C. gaseous molecules will continue their motion indefinitely
D gases can be compressed
7. If an atom is represented as 11 23 X, which of the following deductions is correct?
A. It contains 12 protons.
B. It forms a covalent chloride.
C. Its atomic number is 23
D. It is an alkali metal.
8. If the relative molecular mass of an element is not a whole number, it can be deduced that the element is
A naturally radioactive
B. abundant in nature
C. a transition metal
D. an isotopic mixture
9. Cathode rays cause an object placed behind a perforated anode to cast a shadow on the screen. This observation shows that the rays
A. are positively charged
B. are negatively charged
C. have mass
D. travel in straight lines
10. Which quantum number divides shells into orbitals?
A. Principal
B. Azimuthal
C. Magnetic
11. The type of bonding in [Cu(NH 3 ) 4 ] 2+ is
A. coordinate B. electrovalent C. metallic D. covalent.
12. The mixture of gases used in a photographer’s flash tube is
A. Argon and krypton
B. Krypton and Xenon
C helium and argon
D. argon and xenon
13. When sodium trioxocarbonate(IV) decahydrate loses its water of crystallization to the atmosphere, the process is
A deliquescence
B. efflorescence
C. hygroscopic
D. effervescence
14. Water can be obtained as the only product during the
A. combustion of hydrocarbons
B. neutralization of an acid by a base
C. combustion of hydrogen
D. Electrolysis of brine.
15. If 10.5g of lead (II) trioxonitrate (V) is dissolved in 20cm³ of distilled water at 18°C, the solubility of the solute in moldm -3 is
[Pb = 207 N = 14, O = 16]
16. For a given solute, the concentrations of its saturated solution in different solvents are
A. the same at the same temperature
B. different at the same temperature
C. the same at different temperatures
D. constant
17. The major source of oxides of nitrogen is from the burning of
D. chlorofluorocarbons
18. The acid used in electrolysis of water is dilute
C. H 2 SO 4
19. What volume of 1.5 M solution of KOH would contain 0 045 moles? A 67.50cm³
B 30.00cm 3
C. 6.75cm 3
20. The salt formed from a reaction between citric acid and sodium hydroxide in solution will be
21. The colour change observed when testing for reducing agents using acidified potassium heptaoxodichromate(VI) solution is
A. yellow to purple
B. orange to green
C. green to orange
D. purple to yellow
22. The oxidation state of Cr in K 2 Cr 2 O 7 is
23. Which of the following metals is purified commercially by electrolysis?
24. What current will deposit 3.25g of zinc in 2 hrs?
See: Updated May/June WAEC Timetable for the 2023
[Zn = 65, F = 96500C mol – ¹]
25. C (s) +H 2 O (q) → H 2(q) +CO (q) ∆G for the reaction above at 1300K is – 43kJ At this temperature, the reaction is
A not feasible
B. at equilibrium
C. feasible
D. exothermic.
26. Two equal bulbs, one containing ammonia and the other nitrogen, are opened mouth-to-mouth to each other at room temperature. The entropy in the mixture of gases is likely to
A. remain unchanged
C. decrease

27. In the diagram above, which of the curves represents the evolution of oxygen with time in the equation 2KCIO 3(s) → 2KCl (s) + 3O 2(g) ?
28. A catalyst increases the rate of a chemical reaction by providing a path that
A. raises the activation energy
B. increases the temperature
C. lowers the activation energy
D. increases the concentration.
29. CuO (s) + H 2(g) ↔ Cu (s) + H 2 O (l) .
What is the effect of increasing the pressure on the equilibrium reaction above?
A. The equilibrium is shifted to the left.
B. The equilibrium is shifted to the right.
C. There is no effect.
D. More H 2(g) is produced.
30. The equilibrium of an endothermic reaction which proceeds with an increase in volume can be shifted in the reverse direction by
A. increasing the temperature and decreasing the pressure
B. increasing the pressure and decreasing the temperature
C. decreasing the temperature and increasing the pressure
D. decreasing the pressure and decreasing the temperature
31. The oxidation of ammonia in excess air produces
32. Hydrogen sulphide gas can act as
A. an oxidizing agent
B. a dehydrating agent
C. a bleaching agent
D. a precipitating agent
33. The gasification of coke is used for the manufacture of
A. producer gas
B. natural gas
C. synthetic gas
D. industrial gas
34. The gas that is most useful in protecting humans against solar radiations is
A. chlorine
C. carbon (IV) oxide
D. hydrogen sulphide.
35. Which of the allotrope of carbon is a constituent of a lead pencil?
A. Graphite
C. Lampblack
36. Which of the following metals will show the highest metallic character?
37. Copper metal dissolves in concentrated trioxonitrate(V) acid with the resultant evolution of
38. The type of iron that is best suited for welding, making nails, chains and iron rods is
A. pig iron
B. wrought iron
C. cast iron
D. iron pyrites.
39. In the electrolytic extraction of aluminium, the function of the molten cryolite is to
A. precipitate aluminium hydroxide
B. lower the melting point of aluminium oxide
C. act as a raw material
D. act as a solvent
40. One of the products of the thermal decomposition of sodium trioxonitrate(V) is
B. nitrogen
C. sodium dioxonitrate (III)
D. sodium oxide.
41. A cracking process in petroleum refining can be represented by
A. heptane to heptene
B. heptane to 3-methylhexame
C. heptane to propene and butane
D. heptane to 2,3,3-trimethybutane.
42. Which of the following molecule has two other positional isomers?
A. CH 3 CH₂Br
B. CH 3 CHBr
C. CH 3 CBr₂F 2
D. CH₂CHBrCH₂Br
43. Which of the following class of compounds can exist as dipolar ions in solution?
A. Alkanoic acids.
B. Fatty acids.
C. Dialkanoic acids.
D. Amino acids.
44 Lucas reagent is used to test for
B. alkanoic acids.
C. alkanols
45. A compound commonly used for sterilization and preservation of specimens and food is
D. ammonia.
46. An organic compound reacted with bromine water to give a colourless solution. The compound is probably an
D. alkanone.
47. Which of the following hydrocarbons is mainly used as fuel?
A. Methylene.
B. Ethylene.
48. The molecular formula of a common organic laboratory anaesthetic is
49. The simplest branched-chain hydrocarbon is
50. Organic molecules that have the suffix-ene are unsaturated hydrocarbons that have
A. a single bond
B. a double bond
C. a triple bond
D. an ionic bond
See also: How to Check WAEC Result Online – 2023
ZnSO 4 .7H 2 O = 65 + 64 + 7(18) = 287
% water of crystallization = 126/287 x 100/1 = 44% (A)
4. CaCO 3 + 2HCl → CaCl 2 + CO 2 + H 2 O
1 mole of CaCO 3 produce 1 mole CO 2
∴ 0.0075 mole CaCO 3 will produce 0.0075 mole CO 2
1 mole of CO 2 occupy 22.4dm 3 = 22400cm 3
∴0.0075 CO 2 will occupy 0.0075 x 22400cm 3
5. A 6. B 7. D 8. D 9. A 10. B 11. A 12. B 13. B 14. C
15. Pb(NO 3 ) 2 = 331
20 cm 3 of distilled water is saturated by 10.5g Pb(NO 3 ) 2
1000cm 3 will be saturated by
= 10.5/20 x 1000/1 = 525gdm -3
In moldmol -3 = 525/331 = 1.59 = 1.60moldm -3 (A)
16. A 17. C 18. C
19. 1.0M of 1000cm 3 contains 56g of KOH
1.5M of 1000cm 3 will contain 84g of KOH
∴1.5M contain 84/56 = 1.5M
i.e 1.5M = 1000cm 3
∴0.045 moles 1000/1 x 0.045 = 30.00cm 3 Ans = B
20. D 21. B
More answers loading…>>
See also: 2023 WAEC Biology Practical Specimen, Questions and Answers
These are the kinds of theory questions that you are likely going to see in WAEC Chemistry for 2023.
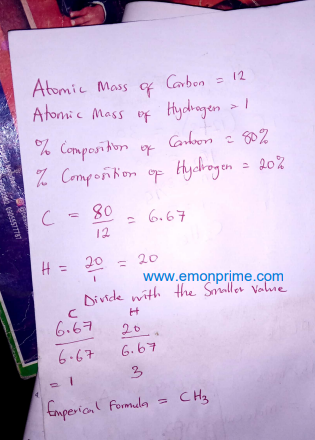
1. Compound A consisting of carbon and hydrogen only. The compound was found to contain 80% carbon by mass. (a) Calculate the empirical formula of compound A using the data above. (b) The relative molecular mass of compound A was found to be 30. Use this information to deduce the molecular formula of compound A. [H = 1.00 C = 12.00]
2. (a) When calcium oxide and coke are heated in an electric furnace, the products are carbon (ii) oxide and calcium carbide (CaC2), write the equation for this reaction.
(b) The addition of water to calcium carbide leads to the formation of calcium hydroxide and ethyne. Write the equation for the production of ethyne.
3. Calculate the percentage by mass of silicon tetrachloride. [2 marks] 4. Ammonia, NH3, and phosphine, Ph3, are the hydrides of the first two elements in group 5. (a) Draw a dot and cross diagram for the ammonia molecule. [2 marks] (b) Sketch and explain the shape of the ammonia molecule. [3 marks]
5. The first ionization energy of chlorine is +1260KJmol-1. (a) Define the term first ionization energy. (b) State and explain the general trend in the values of the first ionization energy for the elements across the period, sodium to argon in the periodic table.
6. An aqueous solution has a pH of 4.0. (a) (i) What is the hydrogen ion concentration of the solution? (ii) What effect will it have on litmus paper? (iii) Which of the following salt solutions would have the same effect on litmus? Give a reason for your answer. NH4Cl(aq); NaCl(aq) ; CH3OON(aq). (b) (i) Differentiate between a fine chemical and a heavy chemical. (ii) Name two sources of air pollution. (iii) Suggest one way of reducing air pollution in cities
WAEC Chemistry Theory Answers for 2023
Keep refreshing this page if you wish to get all solutions to the chemistry theory question for 2023.
WAEC English Questions And Answers 2023 | Objectives, Test Of Orals and Essay
WAEC Economics Questions And Answers 2023 | Theories And Objectives
WAEC French Questions And Answers 2023 | Theories And Objectives
WAEC Mathematics Questions And Answers 2023 | Theory And Objectives
WAEC Physics Practical For 2023 | Specimens/Apparatus, Questions And Answers
2023 WAEC Biology Practical Specimen | Questions And Answers
Complete WAEC Physics Questions And Answers For 2023 (Objectives & Theory)
I believe that you have found this article very useful. For any other questions about the WAEC Chemistry Questions and Answers for 2023 , kindly make use of the comment section.
Please do well to share this information with others
WAEC Literature Questions and Answers 2024/2025 | Novels, Essay & Objective
Waec civic education questions and answers 2024 | essay & objective, leave a reply cancel reply.
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Notify me of follow-up comments by email.
Notify me of new posts by email.

JOIN OUR WAEC & NECO WHATSAPP SAPP GROUP

CHEMISTRY-ANSWERS!
Chemistry-Obj Use this more Accurate 1ACADABBDAC 11BCACBBCCBA 21ACBABDCCCB 31CBBDAABDAD 41ACCDABDDCD
============================== (3)

==============================

(1a) Transition element can be defined as an element in which at least one of its simple ions or atoms has an incomplete inner shell of d-electrons. They are known as d-block elements. They are in three rows with ten elements in each row
(1b) i – B ii – C
(1c) There is an increase in the first ionization energy accross the periodic table due to increase in the number of volume electrons leading to an increase in the screening effect and nuclear attraction between the nucleus and the outermost electrons.
(1d) – Alkane eg Butane – Alkene eg Ethene
(1e) Alkanols are stronger base than water due to the presence of hydroxyl ions (OH-) in them.
(1f) – Salt (Nacl) – Limestone (CaCO3) – Ammonia (NH3) – Coke (C)
(1g) This is also known as Cis-trans isomerism. It is a term which involves the spatial arrangement of atoms within the molecules
(1h) This is because it has a high calorific value due to the combustible nature of the component gases
(1i) Heat of combustion can be define as the amount of heat involved when 1 mole of substance burns in air or oxygen under standard conditions
(1j) (i) Faraday’s second law of electrolysis states that when the same quantity of electricity is allowed to pass through different electrolytes, the number of moles of the ions deposited is inversely proportional to the charge.
(ii) Q=10920c If = 96500C mol-¹ N= Q/nf N= 10920/1×96500 N= 0.113 moles :. mole= 0.113 moles

I’m a blogger living in Nigeria. I like to share education guides and information from various sources. I created Jambclass to serve as a platform to disseminate quality, credible and dependable information regarding various ways to excel academically. I strive to keep Nigerian youths and students informed. I started this Blog with the Vision To Inspire and Empower Young Persons; helping them Realize and Maximize their Potential.
Leave a Reply
Name (required)
Email (required, but never shared)
NOTE:- Your comment will appear after it has been approved by an admin .
Home | Recent Posts | Pages
About Jambclass
Copyright © 2022 Jambclass
- Now Trending:
- WAEC Literature 2024 Pro...
- WAEC Financial Accountin...
- WAEC Mathematics Questio...
- 2024 WAEC Catering Craft...
WAEC Chemistry Expo 2023 Essay and Objective for 24th May
Table of Contents
The West African Examination Council (WAEC) Has Scheduled The Waec Chemistry Expo 2023 Paper To Kick Of On The 24th of May, 2023.
This brings the attention of candidates writing the exam in to searching for 2023 WAEC Chemistry Question And Answer, WAEC Chemistry Expo 2023, waec Chemistry questions and answers 2023, Waec 2023 Chemistry Questions and Answers and etc.
How to Get WAEC Chemistry Expo 2023 Essay and Objective Questions and Answers for 24th May
In this section, you will read the steps and requirements needed for you to get WAEC Chemistry Expo 2023 Essay and Objective Question And Answer 4 hours before exam.
WAEC Chemistry Expo 2023 Essay and Objective Paper is Categorized in to 2 parts:
- WAEC Chemistry Theory 2023
- WAEC Chemistry Objective 2023
Here on zamgist, we have solved all the questions. That is 2023 Waec Chemistry Theory and Objective 2023.

CLICK HERE TO CHAT ME UP ON WHATSAPP & GET IT NOW
WAEC Chemistry Expo 2023
In this section, you will get waec chemistry expo 2023, check the subscription details below on how get it:
==> Direct SMS: N1000 MTN CARD Direct SMS MEANS all answers(theory & obj) will come direct to ur phone as sMs.
==> Online PIN: N500 MTN CARD Online PIN MEANS The Pin to access our answers online via https://examdubs.com.ng/ will be sent to u at least 4 hours before the exam to access our answers.
==> WhatsApp: N500 MTN CARD Whatsapp MEANS The answer will be sent to you on WhatsApp after we confirm your subscription. Add Us In WhatsApp With 08023429251.
Send The Following details:-
(i) MTN CARD Pin(s) (ii) Your Name (iii) Subject (iv) Phone number ===> 08023429251 via sms
- 2023 WAEC Physics Question And Answer Expo
- WAEC Financial Accounting 2023 Questions And Answers
- WAEC Literature 2023 Prose and Objective Questions And Answers
- WAEC Chemistry Practical 2023 Expo Questions And Answers
- 2023 WAEC Agricultural Science Questions And Answers Expo
- WAEC Biology Practical 2023 Expo Questions And Answers
- WAEC Further Mathematics Objective Answers 2023
- 2023 WAEC Computer Studies Questions and Answers
- Biology Specimen for WAEC 2023 and Answer
NOTE:- All sms messages sent to the above number on whatsapp are attended to. always send us whatsapp messages of your complaint, your message(s) will get to us and we will reply immediately.
Notice To Our WAEC 2023 Daily Subscribers
Candidates who are planning to take the 2023 West African Examination Council (WAEC) are advised to subscribe at least two days before the scheduled examination date.
This will allow them to avoid any delay and receive the password/code immediately, which will enable them to study the answers before the exam time.
We highly recommend that candidates make their payment via WhatsApp only to avoid delay, as we reply to Whatsapp messages faster than SMS messages.
At our platform, we understand the importance of providing candidates with authentic and accurate answers to their WAEC questions. That’s why we offer a 100% assurance and guarantee that authentic answers will be delivered to you.
If you do not receive the 2023 WAEC questions and answers before the examination time, we will provide a full refund of your money.
Candidates who wish to receive their questions and answers on website/Online PIN, are advised to subscribe early.
We will contact them on WhatsApp to ensure they receive their materials on time.
It is essential to note that subscribing early will give candidates a significant advantage as they will have more time to study and prepare for the exam.
Our materials are carefully prepared by a team of experienced tutors who are knowledgeable about the exam requirements and will provide candidates with an in-depth understanding of the subject matter.
We also understand that candidates may have reservations about paying for such services online. Rest assured that our payment platform is secure, and we take the privacy of our clients very seriously.
We have put in place robust security measures to ensure that our clients’ personal information is protected at all times.
At our platform, we believe in providing quality services to our clients, and we are committed to ensuring that candidates who subscribe to our service receive the best possible assistance to excel in their WAEC exams.
In conclusion, subscribing to our platform two days before the scheduled examination date will enable candidates to avoid any delay and receive their password/code immediately.
About ZAMGIST
Welcome to Zamgist, the most reliable and valuable examination portal in Nigeria. We are dedicated to providing useful and substantial educational resources to Secondary School students, university aspirants seeking admission into various higher institutions, and undergraduates.
Our portal is designed to help students achieve excellent results in their examinations by providing them with the necessary materials and support.
At Zamgist, we understand that education is the key to success, and we are committed to ensuring that our candidates have access to the best resources and support available.
Our team of experienced teachers and tutors work tirelessly to provide our students with the guidance and assistance they need to excel academically.
Whether you are preparing for your O’level and A’Level exams, WAEC, NECO, GCE, JUPEB, IJMB, UTME, Post-UTME, or any other examination, Zamgist is here to help.
We offer a wide range of examination assistance, including expo, past questions, study materials, exam tips, and more. Our aim is to help our candidates prepare effectively for their exams and achieve their academic goals.
Our commitment to excellence is evident in the quality of our materials and the success of our candidates. Many of our students have gone on to excel in their academic pursuits, and we are proud to have played a part in their success.
Why Choose Zamgist?
At Zamgist, we are committed to providing our students with the best possible support and resources. Here are just a few reasons why you should choose Zamgist for your examination assistance:
1.Comprehensive Study Materials
We offer a wide range of study materials, including past questions, textbooks, and exam tips, to help our candidates prepare effectively for their exams.
2. Experienced Teachers and Tutors
Our team of experienced teachers and tutors are dedicated to helping our candidates achieve academic excellence. They work tirelessly to provide guidance and support to our students.

3. Convenient Online Portal
Our online portal is easy to use and accessible from anywhere, making it convenient for our candidates to access the resources they need.
4. Affordable
We believe that education should be accessible to everyone, which is why we offer our services at an affordable price.
5. Reliable and Trusted
Zamgist is a reliable and trusted examination portal that has helped countless students achieve academic success.
Get in Touch with Zamgist Today!
If you are a student looking for examination assistance, look no further than Zamgist. Contact us today on WhatsApp: 08023429251 to learn more about our services and how we can help you achieve your academic goals.
TAGS: waec chemistry questions and answers 2023, waec chemistry obj and theory 2023, waec chemistry objective and essay 2023, lasu waec chemistry, joberplanet waec chemistry, waec chemistry question for 2023, waec chemistry expo for 2023, waec chemistry expo 2023, waec chemistry area of concentration, 2023 waec chemistry question and answer
Related Posts
Home » EXAM NEWS » NOV/DEC 2023 WAEC GCE Chemistry (Essay & OBJ) Answers For All GCE Candidates
NOV/DEC 2023 WAEC GCE Chemistry (Essay & OBJ) Answers For All GCE Candidates

TODAY ANSWERS ON 2023 WAEC GCE CHEMISTRY OBJ AND THEORY ANSWERS WE BE UPLOADED HERE….
The Time Of the WAEC GCE Chemistry Essay | Chemistry Objective | Chemistry Practical Exam is 11:30pm – 3:30pm for all the 2023 WAEC GCE Chemistry exams.
A. 2023 WAEC GCE CHEMISTRY ESSAY | (THEORY) ANSWERS
Kindly bookmark the website for the answers that will be released here, or better still reload the site to check if the answers for the 2023 Waec GCE Chemistry Questions and Answers have dropped.
2023 WAEC GCE CHEMISTRY OBJ AND THEORY ANSWERS
Loading……………………………………………
B. 2023 WAEC GCE CHEMISTRY OBJECTIVES (OBJ) ANSWERS
WAEC GCE Chemistry Questions and Answers Answers Loading……………………………………………
C. 2023 WAEC GCE CHEMISTRY PRACTICAL ANSWERS
Feel free to see the 2023 Waec Gce questions and answers we’ve shared already on Waec Gce Igbo, Waec Gce Hausa, Waec Gce Yoruba for free below. Many students/candidates who regularly visit our site find helpful answers for their subjects.
Related Content For You:
- 2023 Free WAEC GCE Answers For All WAEC GCE Subjects – Obj and Theory
- JOIN THE GCE WHATSAPP GROUP LINK FOR 2023-2024 FOR FREE
If you have any doubts about whether the questions you’ve come across are indeed the 2023 WAEC GCE questions and answers, you can easily confirm by asking your friends who took the exam to share their questions. Compare their questions with our provided answers to ensure accuracy.
You can Join our Whatsapp here .
WAEC GCE IGBO | WAEC GCE YOURUBA | WAEC GCE LANGUAGE QUESTIONS AND ANSWERS – 16th November 2023
2023 WAEC GCE YORUBA OBJ === You can confirm it yourself.
1-10: BABCDBBDAC 11-20: BBDDABCDAD 21-30: BAACDDAAAA 31-40: DBACCACDAC 41-50: CADBBCDACC 51-60: DBABACDDBB
WAEC GCE YORUBA THEORY ANSWERS ======= You can confirm it yourself.
(12) Dandogo jẹ ẹwu nla kan, ti o de awọn eekun ti awọn ọlọrọ ati awọn agbalagba wọ, ti o wọpọ laarin awọn olori ilu, lati jade. Aṣọ yii le jẹ kedere ni gbangba nipa sisọpọ awọn ohun elo pẹlẹbẹ pẹlu ohun elo akọkọ tabi o kan lati dara aṣọ naa ni iṣẹ-ọnà le ṣee ṣe lori rẹ. eyi yoo fun ni irisi ọlọrọ. Dandogo jẹ aṣa Yoruba ati pe o gba akoko ati ifarada lati ran.” Òwe Yorùbá gbajúmọ ‘Dandogo kọjá aso a binu da’ ( A kì í kánjú ra aṣọ gígùn ) fi èyí hàn, bẹẹ ni ọrọ náà ṣe ń lọ, tí ẹ bá fẹ ran Dandogo a nílò 36yards, ó sì gba àkókò. lati ran.
A Le wo Dandogo pẹlu awọn oriṣiriṣi sokoto…..
WAEC GCE HAUSA OBJ ANSWERS === You can confirm it yourself.

WAEC GCE IGBO OBJECTIVE ANSWERS === You can confirm it yourself.
1-10: BCDCDCBBDB 11-20: BCADCDBCCC 21-30: ABDCADCDDB 31-40: CACDBDDABC 41-50: DDBAACBCCD 51-60: BACCBDAAAC
WAEC GCE IGBO THEORY ANSWERS========== You can confirm it yourself.
(1a) Izunna: Mmaduka enyim! Anyi aga ekwugodi okwu obere oge
Mmaduka: Izuu nwannem, ọfuma! ka anyi kpaa. Kedu ihe di gi n’obi
Izunna: E nwere kepukepu m na-anụ gbasara gi.
Mmaduka: Hahaha, kedu ihe i nụrụ maka m?
Izunna: A nụrụm na i na-añuzi anwụrụ ike.
Mmaduka: Kedu ebe inukwara asiri nke a? Nke a bụ asiri, ọ bughi eziokwu.
Izunna: Ya burukwa asiri. Mana achọputaram na agwa gi gbanweziri na nke ikpeazu a. Ujọ na-atụm maka na-amaram ọghọm di na anwụrụ ike.
Mmaduka: Izunna nwannem, m ga-eletawu onwe m anya ọfuma. Echena oke uche maka m. A maram ebe ihe osise m jedebere.
Izunna: Enweghi kwa njede na-abia na ebe anwụrụ ike nọ. O nwere ike ighasa gi aru, ma ghasakwaa gi ụbụlụ. O nwekwara ike ibutere gi ọgba-aghara n’etiti gi na ndi be ụnụ.
Mmaduka: Echegbuna onwe gi, Izuu. Ihe m ji wee na-añuchalu obere bu ka m wee wepụ echiche.
Izunna: Mmadu, aghọtaram na ụwa nke anyi nọ n’ime ya siri ike mana obughi anwụrụ ike ka e ji eso ije uwa. Kama, anwụrụ ike ka enyere gi aka, otisaara gi ihe. Tinyezie gi nsogbu kariri nke inobụ na ya. O nwere ike ime ka igaa nga, maobu gbaa gi ara.
Mmaduka: Echebeyikwam ya otu a isi wee kwuo
Izunna: Nwanne, lee anya, obim di ebe inọ oge ọbụla. Aghaghim achọ ka ihe ọjọọ dakwasa gi.
Mmaduka: O bụ eziokwu ka ikwụrụ. Dalu na ihe nkea igwagasirim n’ehihie a. Chukwu gozie gi nwannem.
Izunna: Chukwu gozikwaa gi ezi oyim. oge ọbụla odi ka i ga-aba n;echiche, kpoturum ka mụna gi nọrọ, ka anyi tụkọọ aro ọnu, na anyụkọọ mamiri ọnụ ọ gbọọ ụfụfụ.
(5a) Nnochiaha bu mkpuruokwu na-anochite anya aha n’ahiriokwu maobu ebe obula o no. O bukwa umu irighiri mkpuruokwu e ji edochi anya aha ihe n’ahiriokwu ka a ghara ide ya ugboro ugboro.
NNOCHIONYE: (i) Nochionwe na-anochite anya onye(mmadu) (ii) O na-anochite anya ihe a kporo aha ya (iii) Omumatu bu ndi a: m, mụ anyi, gi, ụnụ, ọ, o, ya na ha
NNOCHIMPESIN: (i) O na-anaghi aru aka kpomkwem onye mere ihe (ii) O na-anaghi aru aka kpomkwem onye a na-ekwu maka ya (iii) Omumatụ bụ ndi a: A kụrụ aka, A sara efere, E riri nri ahụ.
(5bii) NNOCHIONYE: (i) Emeka bu di ya. (ii) O na-esi nri. (iii) Nnewi bu obodo m.
NNOCHIMPESIN: (i) A kụrụ aka (ii) A sara efere (iii) E riri nri ahụ
(8) (i) Nne na nna ga ekuziri umu ha otu esi aza ụlo (ii) Ha ga-ekuziri ha otu esi asu asusu Igbo (iii) Ha ga-ekuziri umu ha otu esi ekene aha (iv) Ha ga-azukwa ha n’uzo eziokwu (v) Ha ga-apiakwa ha ihe oge ha mere ihee ọjọọ (vi) Ha ga-ekuzikwara ihe gbasara mmekọ nwoke na nwanyi na ha tolite (vii) Ha ga-ekuzikwara ha ọghọm di na ibi ajọ ndu (viii) Ha ha ekuzikwara ha otu esi ekpe ekpere (ix) Ha ga-akuzikwara umu ha otu esi asopuru ndi tọrọ ha ato (x) Ha ga-ekuzikwara maka ihu mmadu ibe ha n’anya.
Kindly bookmark this website for quick access to the released WAEC GCE answers. Alternatively, you can reload the site periodically to check if the answers for the 2023 WAEC GCE Chemistry questions have been posted. Stay tuned for the latest updates.
You may also like

Approved Jamb Cut-off Mark For Law Into Nigeria Law...

How Many Candidates Registered For 2024 UTME JAMB Exam...

Check UTME MOCK JAMB RESULTS – MOCK JAMB RESULT...

JAMB Expo WhatsApp Group Link For 2024/2025 –...
How Do I Get My NECO Token Online and How Much Is NECO...
Today News On Jamb Registration Complains and Jamb...
About the author.
Leave a Comment X
Save my name, email, and website in this browser for the next time I comment.

- Forums New posts Search Forums
- Members Registered members Current visitors Recent Activity
- Free Assessment
Free 2023 WAEC Chemistry Expo Questions (Complete Obj&Essay) Ditto/Runz
- Thread starter Ackley8
- Start date May 23, 2023
- Fundamental Concepts: Familiarize yourself with the basic principles, laws, and theories of Chemistry. Understand topics such as atomic structure, chemical bonding, and the periodic table.
- Organic Chemistry: Pay special attention to organic compounds, their nomenclature, properties, and reactions. Focus on topics like hydrocarbons, alcohols, carboxylic acids, and their derivatives.
- Inorganic Chemistry: Study the properties and reactions of various inorganic compounds, including acids, bases, salts, and transition metals. Understand concepts related to stoichiometry, redox reactions, and equilibrium.
- Physical Chemistry: Gain a strong foundation in physical chemistry by mastering topics such as thermodynamics, chemical kinetics, electrochemistry, and atomic structure.
- Practicing Past Questions: Solve previous years' WAEC Chemistry questions to become familiar with the exam pattern and identify areas where you need more practice.
- Objective (OBJ) Section: The OBJ section consists of multiple-choice questions. Each question carries equal marks, and there is no negative marking for incorrect answers. The total marks for the OBJ section may vary each year.
- Theory Section: In the theory section, marks are allocated to specific questions. Each question is assigned a specific number of marks based on its complexity and depth of explanation required. It is crucial to answer the theory questions clearly and concisely, providing relevant explanations and examples where necessary.
Good
- Privacy Policy
- ADMISSION UPDATES
- SCHOOL GISTS

WAEC Chemistry Questions and Answers 2023 Obj & Essay Free Expo

The WAEC Chemistry will be written on Wednesday 8th June 2023, Chemistry – Essay & OBJ.
For more WAEC subjects questions and answers you can check this website, you’ll also find the available tutorial for WAEC, NECO and JAMB.
- Recommended: NECO Result 2023 is out: Check Now Free Online | NECO SSCE Result Checker
Also find: Waec Chemistry question and answers, waec 2023 Chemistry questions and answers, questions and answers for WAEC 2023 Chemistry.
Chemistry Area of Concentration
(a) Define the following terms:
(i) Saturated solution;
(ii) Solubility. 100
(b)In an experiment to determine the solubility of a given salt Y, the following data were provided: Mass of dry empty dish = 7.16g = 17.85g Mass of dish + salt Y = 9.30g Temperature of solution = 20oC Molar mass of salt Y = 100 Density of solution Y = 1.00gcm-3
Calculate the solubility of salt Y in (i) g dm-30f solution, (ii) mol dm-3 of solution. [6 marks]
(c) State the type of bond broken on melting each of the following substances:
NaCl(s); CO2(s); SiO2(s); Al(s)
WAEC Chemistry Questions and Answers 2023 Objective Answers and Essay
(1a) A molecular formula consists of the chemical symbols for the constituent followed by numeric subscripts showing the number of atoms of each element present while structural formula consists of symbols for the atoms connected by short lines that represent chemical bonds.
(1b) (i) Screening effect (ii) Size of atom (iii) Nuclear change
(1c) (i) The reaction must proceed both forward and backward i.e reversible (ii) DG = O
(1d) (i) B (ii) It has the least ionization energy among the three
(1e) Graham’s law of diffusion states that the rate of diffusion of a gas is inversely proportional to the square root of their densities provided other conditions remained constant
(1f) (i) Na2SO4. Mg(NO3)2 (ii) CaCO3
It is available now HERE to get
https://paystack.com/pay/waecchem
Thanks for reading, good luck.
Source: Edustuff

RELATED ARTICLES MORE FROM AUTHOR
Jamb activates portal for reprinting 2024 exam slip, jamb past questions and answers pdf free download1983 – 2024 now, jamb 2024 reprint portal slipsprinting.jamb.gov.ng, leave a reply cancel reply.
Save my name, email, and website in this browser for the next time I comment.
WAEC Chemistry Questions and Answers 2023 | Theory and OBJ- Download PDF
WAEC Chemistry Questions and Answers 2023 | Theory and OBJ- Download PDF. WAEC Chemistry Questions and Answers 2023… If you are about to write the West African Senior Certificate Exams (WASSCE), you will realize that you need the WAEC Past Questions and Answers.
This article provides you with WAEC Chemistry Questions and Answers for the WASSCE 2023. It contains information regarding the WAEC Chemistry Theory and Objective Questions and Answers.
WAEC Chemistry Objective Questions
1. Which of the statements below is correct about Isotopes of the same element?
- WAEC CASS 2023 | Offline Download, Portal and Registration Software.
- WAEC GCE Result 2023/2024 is Out- (August/September 2nd Series Exam)
- WAEC Result 2023/2024 is Out- WAEC Result Checker 2023
- Have the Same number of Protons, neutrons, and electrons.
- Same Protons and neutrons but a different number of electrons.
- Same number Protons and electrons but a different number of neutrons.
- The number of Neutrons and electrons is the same but the number of protons differs.
2. The chemical bond that is formed by the transfer of electrons is known as?
- Covalent Bond
- Dative Bond
- Metallic bond
3. Two electrons can fill the same orbital if only?
- They have different Angular momentum quantum numbers.
- Magnetic quantum numbers differ
- Principal quantum numbers are different.
- Spin quantum numbers not the same
4. Which of the substances listed below is not a hydrocarbon?
Related Posts:
- WAEC Chemistry Practical Questions and Answers…
- NECO Chemistry Past Questions and Answers | Theory…
- WAEC Biology Questions and Answers 2023 | Theory and…
- WAEC Commerce Questions and Answers 2023 | Theory…
- WAEC Economics Questions and Answers 2023 | Theory…
- WAEC English Questions and Answers 2023 | Theory and…
5. The complete ionization of A substance into hydroxonium ions indicates:
- Strong acid.
- Strong base.
6. One of the solutions below that can resist changes in pH when a small quantity of a base or acid is added is?
- Buffer solution
- Neutral solution
- Saturated solution
- Supersaturated solution
7. Write a balanced chemical equation for a 2.47g dry pure copper (II) oxide which is completely reduced copper using a laboratory gas. If the mass of the residue left is found to be 1.97g.
- Cu + O → CuO.
- 2Cu+O 2 → 2CuO
The correct answer is B. 2Cu+O 2 → 2CuO. See how this question is solved in the number one (No.1) question in the theory section.
Use this question to answer question 2 & 3
8. Calculate the mass of anhydrous sodium-trioxocarbonate (IV) present in a 300cm 3 of 0.1M; (Na =23, C=12, O=16).
The correct answer is A. 3.18g. See solving in No.2 ques. in the theory section.
9. The number of Na 2 CO 3 particles present in the solution
- 6.02 X 10 23
- 0.1 X 6.02 10 23
- 1.81 X 10 22
- 1.81 X 10 23
- 6.02 X 10 22
The correct answer is C. 1.81 X 10 22.
WAEC Chemistry Theory Questions
These are possible WAEC Chemistry theory questions and answers 2023 that may appear in this year’s exam.
There are not actual questions and answers but, sample questions for practices.
2.47g of dry pure copper (II) oxide was completely reduced to a copper using laboratory gas. The mass of the residue left was found to be 1.97g. Write a chemical equation for this reaction.
Molar mass of copper atoms =63.5
No. of moles of copper atoms =1.97
=63.5 = 0.03 mole
Molar mass of oxygen atoms = 16
No. of moles Oxygen atom = (2.47 – 1.97) = 0.5
Mole ratio of Cu to O = 0.03 : 0.03
= 1 : 1
Therefore, the equation is Cu + O → CuO.
But Oxygen is diatomic, so we have 2Cu+O 2 → 2CuO
2. Calculate the;
- mass of anhydrous sodiumtrioxocarbonate (IV) present in a 300cm 3 of 0.1M;
- Number of Na 2 CO 3 particles present in the solution (Na =23, C=12, O=16)
a. Molar concentration of Na 2 CO 3 =0.1M
Molar mass of Na 2 CO 3 = 106gmol -1
Mass concentration = Molar concentration X Molar mass
=10.6gdm -3
i.e 1000cm3 of 0.1M solution contain 10.6g of Na 2 CO 3
Therfore,300cm 3 of 0.1M solution will contain
= 300 X 10.6/1000
=3.18g of Na 2 CO 3
b. Number of Na 2 CO 3 particles
=Molar concentration X 6.02 X 10 23
=0.1 X 6.02 10 23
=6.02 X 10 22
Now, 100cm 3 of 0.1M solution contains 6.02X10 22 Na 2 CO 3 particles
Therefore, 300cm 3 of 0.1M solution will contain 300X6.02 X10 22 /1000
= 1.81 X 10 22 particular of Na 2 CO 3 .
How to Download WAEC Past Questions and Answers
WAEC Past Questions and Answers are available for download for all subjects in PDF. To download WAEC Past Questions and Answers for Chemistry, you only need a phone, a whatsapp account and a Gmail address.
Then call 07063986527 and drop and WhatsApp message.
Click the Link Below to download WAEC Past Questions and Answers for Chemistry.
Download WAEC Past Questions and Answers for all Subjects Including practical.
Disclaimer: Please note that we are not in any way affiliated to any of the organization’s mention here. Some of the organizations here do not release their past questions, so we can only compile likely questions and answers very related to the organization.
If You Want More Information, please subscribe to our email list and leave a comment with your number below, also Follow us on Facebook , Twitter , and Linkedin
Disclaimer: Please note that we are not in any way affiliated with any of the organizations or institutions here. All articles here are for the sole purpose of providing information. All Past questions are gotten from previous years’ examinations and likely questions from the Internet and related exams. We are not in any way promising that what you find in the past questions is what you will find in your examination. We are not in any way responsible for how the reader uses the information here. Please do consult an expert or professional in your field should the need arise. Copyrights Infringement: No article from this website should be copied without a proper reference and link to the page picked from. Anything otherwise will lead to legal action of copyright infringement. All articles on this website are products of painstaking research from our writers and journalist. Should you find any material bearing semblance here to any material on your page, please quickly notify us by sending a mail to [email protected] and we will immediately commence the process of taking it down.
WAEC Building Construction Questions and Answers 2023 | Theory and OBJ- Download PDF
Waec gce timetable 2023/2024: january/february first series, related articles, waec applied electricity/ basic electricity questions and answers 2023 | theory and obj- download pdf, waec christian religious studies (crs/crk) questions and answers 2023 | theory and obj- download pdf, waec biology practical questions and answers 2023/2024 pdf download, waec physics practical questions and answers 2023/2024 download pdf, waec forestry and answers 2023 | theory and obj- download pdf.
- WAEC Biology Questions and Answers 2023 | Theory and OBJ- Download PDF
WAEC Chemistry Questions and Answers 2024 Objectives and Essay
- Post author: Study Admin
- Post published: November 19, 2023
- Post category: School News
- Post comments: 0 Comments
WAEC chemistry 2024 answers are now available. WAEC chemistry questions and answers 2024/2025 objective and essay and other exam details for WASSCE 2024 are on this page. See the 2024 WAEC chemistry answers for both objective and theory below. Get the WAEC chemistry objective and essay answers here.
The 2024 chemistry WAEC OBJ and theory questions and answers are provided here for free. All you have to do is to go through the questions and take note of the WAEC chemistry answers 2024. Read on to find out.
WAEC Chemistry Questions and Answers 2024 Objective and Essay
Have you been searching on Google in order to get the WASSCE chemistry questions and answers 2024? If so, we have got you covered!
We have the 2024 WAEC chemistry questions and our team of experts will soon upload the WAEC chemistry questions and their accurate answers to help you pass the 2024 WAEC chemistry examination.
The 2024 WAEC chemistry theory questions and OBJ will be uploaded any moment from now. So if you are searching for the WAEC chemistry answers 2024 for objective and theory, then you are on the right page. See WAEC chemistry objective and essay questions and answers below.
WAEC Chemistry Answers 2024 Objective and Theory
The West Africa Examinations Council (WAEC) is an examination body in Nigeria that conducts the Senior Secondary Certificate Examination and the General Certificate in Education in May/June and November/December respectively.
The 2024 WAEC chemistry questions are set from the SS1 to SS3 chemistry syllabus. So all the questions you will encounter in this year’s examination are in the syllabus, and nearly 90% of the questions are repeated.
You don’t have to worry about the 2024 WAEC chemistry questions and answers PDF (essay and objective). The WAEC chemistry answers 2024 will be uploaded any moment from now. All you need to do is to keep refreshing this page so as not to miss out.
Once again, keep refreshing this page because we will upload the original WAEC chemistry questions and answers for this year’s exams on this page at any moment from now. Also, to download the past questions and answers, click on this link WAEC chemistry past questions .
If you have any questions about the WAEC chemistry questions 2024 and answers, feel free to use the comment box below or use the Chat With Us button and we will respond immediately.
The 2024 WAEC chemistry answers will be posted here. Be patient. Keep checking and reloading this page for the correct answers. WAEC 2024 chemistry answers loading…….
There is nothing like WAEC chemistry expo 2024 online. All students are advised to avoid all patronizing online fraudsters/vendors who claim to provide such services.
You Might Also Like
Federal poly nekede post utme form 2024/2025 academic session, kasu post utme form 2024/2025 academic session, summit university post utme form 2023/2024 academic session, leave a reply cancel reply.
Save my name, email, and website in this browser for the next time I comment.
- 98494519845
- [email protected]
- Tyagal, Patan, Lalitpur

CHEMISTRY THEORY And OBJECTIVE: QUESTIONS & ANSWERS For 2022/2023 WAEC EXAM.
Chemistry Theory And Objective: Questions & Answers For 2022/2023 WAEC Exam. Are you a student who wants to make a good grade in Chemistry? Here are the WAEC Chemistry questions and everything you need to know about 2021 WAEC Chemistry. This post provides provided for you with all you need to know in Chemistry Paper 1 (Objective) and Chemistry 2 (Essay).
Table of Contents
Welcome to the Chemistry Theory and Objective: Questions & Answers for the 2022/2023 WAEC Exam. This comprehensive guide has been designed to assist students in their preparation for the upcoming WAEC Chemistry examination. It presents a collection of theory and objective questions along with their corresponding answers, covering the essential topics and concepts outlined in the WAEC syllabus. By studying and practicing these questions, you will gain a solid foundation in chemistry and improve your chances of achieving excellent results in the exam. So let’s delve into the world of chemistry and embark on a journey of learning and success.
The Question & Answers: CHEMISTRY THEORY And OBJECTIVE: QUESTIONS & ANSWERS For 2022/2023 WAEC EXAM.
Furthermore, below are the WAEC Chemistry questions. Read them properly. In fact, they will make you ready to score high in your WAEC Chemistry exam.
The West Africa Examination Council (WAEC) is an examination body in Nigeria. It has the statutory power to conduct the Senior Secondary Certificate Examination in May/June and the General Certificate in Education in November/December.
THE OBJECTIVE OF POST: CHEMISTRY THEORY And OBJECTIVE: QUESTIONS & ANSWERS For 2022/2023 WAEC EXAM.
Because people are asking about WAEC Chemistry and objective questions and answer 2022/2023. In fact, they are also asking for JAMB 2022 CHEMISTRY questions and answers pdf, The solutions are the objectives of this post. This post, therefore, takes care of those students who plan to do well in the WAEC exams this year.
The best way to read this post is to read it by clicking the highlighted topics for referencing. So check out these related topics that follow.
- Canada Visa Status
- Netherlands International Students guide
- 3 most demanded undergraduate courses in the Netherlands
- Most Popular Degree courses in Australia
- UK-approved graduate schools
- Most popular degree courses in the UK
- Approved Undergraduate – in UK
CHEMISTRY THEORY And OBJECTIVE: QUESTIONS & ANSWERS For 2022/2023 WAEC EXAM.
General tips and advice to help you prepare for your exams effectively..
- Study the syllabus: Familiarize yourself with the syllabus for your respective exams. Understand the topics and concepts that are likely to be covered and focus your preparation accordingly.
- Review past papers: Practice solving past exam papers to get an idea of the types of questions that may be asked. This will help you become familiar with the exam format and identify any areas where you need further improvement.
- Understand the concepts: Chemistry involves understanding fundamental concepts and principles. Ensure you have a strong foundation by studying the theory thoroughly. Take notes, create summaries, and use diagrams or visual aids to help you grasp complex concepts.
- Practice problem-solving: Chemistry often requires application and problem-solving skills. Practice solving numerical problems and chemical equations regularly to strengthen your problem-solving abilities. This will also help you become more familiar with the calculations and techniques needed for the exam.
- Seek clarification: If you come across any challenging topics or concepts, don’t hesitate to seek clarification from your teachers, classmates, or online resources. Understanding the material thoroughly will boost your confidence during the exam. CHEMISTRY THEORY And OBJECTIVE: QUESTIONS & ANSWERS For 2022/2023 WAEC EXAM..
- Create a study schedule: Plan your study sessions and allocate sufficient time to each topic based on its importance and your level of understanding. Break down your study material into manageable chunks and set realistic goals to stay motivated.
- Collaborate with peers: Consider forming study groups with classmates or friends who are also preparing for the exams. Discussing and explaining concepts to each other can enhance your understanding and retention of the material.
- Utilize available resources: Take advantage of textbooks, reference materials, online resources, and educational platforms that provide study materials, practice questions, and tutorials specific to your exam. These resources can provide valuable insights and additional practice opportunities. CHEMISTRY THEORY And OBJECTIVE: QUESTIONS & ANSWERS For 2022/2023 WAEC EXAM.
- Practice time management: During the exam, time management is crucial. Practice solving questions within the allocated time limits to improve your speed and accuracy. This will ensure that you can complete the exam within the given time frame.
- Stay focused and maintain a healthy lifestyle: Avoid distractions and create a conducive study environment. Take regular breaks, exercise, eat nutritious meals, and get enough sleep to keep your mind and body in optimal condition for studying.
Remember, success in any exam is a result of consistent effort, effective preparation, and a positive mindset. Good luck with your exams!
WAEC Chemistry Questions and Answers 2022:
Furthermore, the questions below are the WAEC Chemistry Questions. Going through them will make you ready to score high in your WAEC 2021 Chemistry Examination. Congratulations.
- How many alkoxy alkanes can be obtained from the molecular formula C4 H4O4
ANSWER: C (3)
- Element Y has two isotopes Y and Y present in the ratio 1:3. The relative atomic mass of Y would be
ANSWER: C (21.5)
- What condition favors the formation of the product for the endothermic reaction, N2O4(g) —><—– 2NO2(g)
A. Decrease in pressure
B. A decrease in volume
C. An increase in pressure
D. A constant volume
ANSWER: A ( Decrease in pressure)
- Elements X and Y have electronic configurations 1S22S22P4 and 1S22S22P63S23P1 respectively. When they combine the formula of the compound formed is
ANSWER: B (Y2X3)
CHEMISTRY THEORY And OBJECTIVE: QUESTIONS & ANSWERS For 2022/2023 WAEC EXAM
- A solution of 0.20 mole of NaBr and 0.20 mole of MgBr2 in 2.0 dm3 of water is to be analyzed. How many moles of Pb(NO3 )2 must be added to precipitate all the bromide as insoluble PbBr2
A. 0.30 mol
B. 0.10 mol
C. 0.20 mol
D. 0.40 mol
ANSWER: A (0.30 mol)
- Na2CO3 + HCl —-> NaHCO3 + NaCl. The indicator most suitable for this reaction should have pH equal to.
ANSWER: D (9)
- A saturated solution of silver trioxocarbonate (IV), was found to have a concentration of 1.30 x 10-5 moldm-3 . The solubility product of the trioxocarbonate (IV) is
A. 8.79 x 10-15
B. 1.69 x 10-10
C. 1.82 x 10-11
D. 9.84 x 10-10
ANSWER: A (8.79 x 10-15)
QUESTIONS & ANSWERS FOR THE 2022/2023 WAEC EXAM
- 100.0g of KClO3 was added to 40.0 cm^3 of water to give a saturated solution at 298K. If the solubility of the salt is 20.0 moldm^-3 at 298K, what percentage of the salt is left undissolved? {K= 39, Cl = 35.5, O = 16}
- Tetraoxosulphate (VI) ions are the final test using
A. acidified silver nitrate
B. acidic barium chloride
C. lime water
D. dilute hydrochloric acid
ANSWER: D (dilute hydrochloric acid)
- When platinum electrodes are used during the electrolysis of copper (II) tetraoxosulphate (IV) solution, the solution gets progressive
D. Atmospheric
ANSWER: A (Acidic)
WAEC 2022/2023 Chemistry Theory Questions
PAPER 2 (ESSAY) SECTION A
- (a) When calcium oxide and coke are heated in an electric furnace, the products are carbon (ii) oxide and calcium carbide (CaC2), write the equation for this reaction.
(b) The addition of water to calcium carbide leads to the formation of calcium hydroxide and ethyne. Write the equation for the production of ethyne.
- Calculate the percentage by mass of silicon tetrachloride. [2 marks]
- Ammonia, NH3, and phosphine, Ph3, are the hydrides of the first two elements in group 5. (a) Draw a dot and cross diagram for the ammonia molecule. [2 marks] (b) Sketch and explain the shape of the ammonia molecule. [3 marks]
- The first ionization energy of chlorine is +1260KJmol-1. (a) Define the term first ionization energy. (b) State and explain the general trend in the values of the first ionization energy for the elements across the period, sodium to argon in the periodic table.
- Compound A consists of carbon and hydrogen only. The compound was found to contain 80% carbon by mass. (a) Calculate the empirical formula of compound A using the data above. (b) The relative molecular mass of compound A was found to be 30. Use this information to deduce the molecular formula of compound A. [H = 1.00 C = 12.00]
- State two factors other than a change in temperature or the use of a catalyst that influence the rate of a chemical reaction.
- Identify the solid remaining when each of the following is heated. (a) lithium trioxonitrate (V) (b) potassium trioxonitrate (V) (c) calcium trioxonitrate (V)
CHEMISTRY THEORY And OBJECTIVE: QUESTIONS & ANSWERS For the 2022/2023 WAEC EXAM
- An aqueous solution has a pH of 4.0. (a) (i) What is the hydrogen ion concentration of the solution? (ii) What effect will it have on litmus paper? (iii) Which of the following salt solutions would have the same effect on litmus? Give a reason for your answer. NH4Cl(aq); NaCl(aq) ; CH3OON(aq). (b) (i) Differentiate between a fine chemical and a heavy chemical. (ii) Name two sources of air pollution. (iii) Suggest one way of reducing air pollution in cities
- (a) (i) Explain briefly the fermentation process. (ii) Write a balanced equation for the fermentation of glucose. (iii) What substance must be added to glucose solution to ferment it? (iv) Explain briefly why tightly corked glass filled to the brim with palm wine shatters on standing. (b) State one industrial application of each of the following methods of separation: (i) Crystallization; (ii) Fractional distillation. (c) Explain the following terms: (i) Saponification; (ii) Esterification. (d) Write a balanced equation to illustrate each of the terms in (c). (e) i) What is hydrocarbon compound? (ii) Name two principal sources of hydrocarbons.

QUESTIONS & ANSWERS FOR 2022/2023 WAEC EXAM
- (a) Two elements represented by the letters Q and R have atomic numbers 9 and 12 respectively. (i) Write the electron configuration of R. (ii) To what group does Q belong in the periodic table? (iii) Write the formula of the compound formed when Q combines with R. (iv) Explain briefly, why Q is a good oxidizing agent. (v) State whether R would be expected to form acidic or basic oxide. (b) (i) State two assumptions of the kinetic theory of gases. (ii) When some solids are heated, they change directly into the gaseous state. What name is given to this phenomenon? (iii) List two substances that exhibit the phenomenon mentioned in (ii). (iv) Write an expression to show the mathematical relationship between the rate of diffusion of a gas and its vapor.
READ ALSO FOR CHEMISTRY THEORY And OBJECTIVE: QUESTIONS & ANSWERS For 2022/2023 WAEC EXAM.
- Latest Secondary Education updates
- Physics 2021 WAEC Questions & Answers
- English Language for WAEC May/ June 2021
- How to Become a professional
- Secondary Education List of Subjects – WAEC approved
- Tips for Successful Professionals
- Tertiary Education updates
- Latest professional tips
- How the basic primary education works
- Professionals and Recruitment
HOW TO PARTNER WITH US: CHEMISTRY THEORY And OBJECTIVE: QUESTIONS & ANSWERS For 2022/2023 WAEC EXAM.
In conclusion, mastering the Chemistry theory and objectives is crucial for success in the upcoming 2022/2023 WAEC exam. By diligently studying and understanding the key concepts, practicing with a wide range of questions, and seeking clarification when needed, students can enhance their understanding of this fascinating subject. Remember, a strong foundation in Chemistry will not only boost your performance in the exam but also equip you with valuable knowledge for future academic pursuits. Stay focused, stay determined, and embark on this journey with confidence. Best of luck in your preparations and may you excel in the upcoming WAEC exam! CHEMISTRY THEORY And OBJECTIVE: QUESTIONS & ANSWERS For 2022/2023 WAEC EXAM.
Finally, for those who would want to win a scholarship and at times part-time studies, this website is for you. For career professional tutorials for ICAN, CITN, NBA exams, etc. this site is available for your current information. Therefore, send us your questions through the comment box. And keep in touch with us by filling in the email list box below for further updates. Our social media buttons will also help if you like us on then or follow us. Thanks Get ready with CHEMISTRY THEORY And OBJECTIVE: QUESTIONS & ANSWERS For 2022/2023 WAEC EXAM.
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
- Career & Training
- Career Guide
- Loans & Grants
- Professional Tips
- Scholarship
- Travel Visas

WAEC 2023 Chemistry Practical Questions and Answers
Table of Contents
Thursday, 18th May, 2023
- Chemistry 3 (Practical) ( Alternative A) – 09:30am – 11:30am (1st Set)
- Chemistry 3 (Practical) (Alternative A) – 12:00pm – 2:00pm (2nd Set)
This page contains the questions and answers to the 2023 WAEC Chemistry Practical. We have carefully solved the problem and here’s are the answers to the questions.
Continually refresh this page as we will keep updating the answers.
QUESTION PAPER
Free download now.

PRACTICAL ANSWERS

Answers will be posted here as soon as possible, so be patient and keep refreshing this page to see wonders.
Join FREE Junior WAEC Expo WhatsApp Group Link 2023/2024
Join FREE GCE Expo WhatsApp Group Link 2023/2024
Join FREE NECO Expo WhatsApp Group Link 2023/2024
Join FREE WAEC Expo WhatsApp Group Link 2023/2024
Leave a Comment Cancel reply
Save my name, email, and website in this browser for the next time I comment.
- All Profiles
- Login/Register
- Privacy Policy
- Terms and Conditions

Best Online Education Platform for waec ,neco,Jamb, Nabteb and Jupeb
2023 WAEC GCE 2ND SERIES Chemistry Practical (Alternative A & B) Questions and Answers
2023 WAEC chemistry (Alternative A & B ) Questions and Answers is out. Get free live 2023 WAEC May/June Chemistry Practical (Alternative A) and WAEC Chemistry 3 (Practical) (Alternative B) questions and answers for school candidates free of charge. Visit this free WAEC Expo Room (18th May, 2023) to get all your Free 2023 WAEC May/June chemistry Practical Questions and Answers .
There will be a Free Chemistry Practical Answer Room for School Candidates for the (Alternative A & B ) WAEC May/June 2023 Examination. This paper covers all of the questions and answers from WAEC 2023 for chemistry
Table of Contents
COMPLETE 2023 CHEMISTRY PRACTICAL (ALTERNATIVE A) ANSWERS.

2023 WAEC CHEMISTRY PRACTICAL ANSWERS is Now Available
2023 waec chemistry practical answer..

WAEC 2023 CHEMISTRY PRACTICAL QUESTIONS (ALT. A & B) ANSWERS

WAEC CHEMISTRY PRACTICAL 2023 SPECIMEN
Waec 2023 chemistry practical answers , how to subscribe for 2023 waec chemistry practical answer room (alt. a), how to pass 2023 waec chemistry practical alternative question and answers.
- Study the syllabus : Review the WAEC Chemistry syllabus to understand the topics and practical exercises that may be covered in the exam. Pay attention to the required laboratory techniques and procedures.
- Practice experiments : Set up a makeshift laboratory at home or utilize your school’s laboratory facilities to practice the practical experiments outlined in the syllabus. Perform the experiments following the instructions and record your observations and results accurately.
- Understand concepts and theories : Study the theoretical aspects of Chemistry to have a solid understanding of the principles behind the practical experiments. This will help you explain the purpose, expected outcomes, and underlying theories during the exam.
- Safety precautions : Familiarize yourself with safety measures and precautions in the laboratory. Be aware of proper handling and disposal of chemicals, use safety equipment such as goggles and lab coats, and follow all guidelines to ensure a safe environment.
- Time management : During the exam, manage your time effectively. Read the instructions carefully, plan your approach for each experiment, and allocate time accordingly. Be mindful of the time available and try to complete all tasks within the given duration.
- Review past papers : Solve previous years’ WAEC Chemistry Practical papers to get acquainted with the format and types of questions asked. This will give you an idea of the level of difficulty and help you identify areas where you need more practice.
- Seek guidance : If you’re having difficulty with specific concepts or experiments, don’t hesitate to reach out to your teacher or instructor for clarification. They can provide additional guidance and support to help you better understand the material.
Related Post
Waec gce economics 2023 2nd series question and answer, waec gce agric question and answer 2023 november/decembr, waec gce literature in english answer 2023 nov/december, leave a reply cancel reply.
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Pinterest Affiliate Marketing Guide – How to Make $2,000 in 30 Days
Teslar ai review – unlimited free automated traffic (teslar ai app by glynn kosky), 7 internet-based home businesses to start in 2024, 9 freelance business ideas perfect for side hustlers.
Typically replies within minutes
Any questions related to 2023 WAEC GCE 2ND SERIES Chemistry Practical (Alternative A & B) Questions and Answers?
WhatsApp Us
🟢 Online | Privacy policy
WhatsApp us

IMAGES
VIDEO
COMMENTS
The 2023 answers will be posted here on 24th May during the exam. WAEC Chemistry OBJ Answers Loading…. 1-10: ACADABBDAC. 11-20: BCAABBCCBB. 21-30: ACBABDACBB. 31-40: ABBDAADCDD. 41-50: BDCDDCDDCD. (1a) A transition element, also known as a transition metal, is an element that belongs to the d-block of the periodic table.
In conclusion, preparing for the Objectives and Essay sections of the WAEC Chemistry examination requires dedication, persistence, and a strong understanding of the subject matter. By following the tips provided in this guide and practicing with the sample questions and answers, you will be well on your way to achieving success in the 2023 WAEC ...
Objectives Section. The objectives section of the WAEC GCE Chemistry exam tests your knowledge and understanding of various concepts. It consists of multiple-choice questions that require you to select the correct answer from the options provided. Here are some key points to keep in mind for this section: 1.
Chemistry 2023 WAEC Past Questions. A. energy is released when liquids change to solids. B. carbon atoms in gaseous methane are further apart than those in solid diamond. C. there is large decrease in the volume of a solid metal when pressure is applied to it. D. particles move faster in the gaseous state than in the liquid state.
The 2023 Waec Chemistry Theory and objective questions and answers are loading! 2023 Chemistry Essay answers Loading!! 2023 Waec Chemistry objective Answers Loading!!! Kindly bookmark the website for the answers that will be released. or better still reload the site to check if the answers for the 2023 Waec Chemistry questions and answers have ...
SSCE WAEC Chemistry Objective Questions and Answers. CHEMISTRY Paper 1 (Objective Test Questions) Paper 1 will last for 1 hours Use HB pencil throughout. Answer All Questions Each question is followed by four options lettered A to D. Find out the correct options for each question and shade in pencil on your answer sheet, the answer space which bears the same letter as the option you Chosen.
A. 2023 WAEC GCE CHEMISTRY ESSAY (THEORY) ANSWERS | 12TH DECEMBER, 2023. (1d) (i) Ensure that both copper (II) oxide and dilute trioxonitrate (V) acid used in the reaction are of high purity. (ii) Ensure that the beakers and stirring rods used are clean and dry. OR.
WAEC Chemistry Objective Questions for 2023. The following are what you are likely going to see in the 2023 WAEC chemistry examination for objective section. 1. A mixture of sugar and sulphur can be separated by. A. dissolution in water, evaporation and filtration. B. filtration, evaporation and dissolution in water.
ii - C. (1c) There is an increase in the first ionization energy accross the periodic table due to increase in the number of volume electrons leading to an increase in the screening effect and nuclear attraction between the nucleus and the outermost electrons. (1d) - Alkane eg Butane. - Alkene eg Ethene. (1e)
In this section, you will read the steps and requirements needed for you to get WAEC Chemistry Expo 2023 Essay and Objective Question And Answer 4 hours before exam. WAEC Chemistry Expo 2023 Essay and Objective Paper is Categorized in to 2 parts: Here on zamgist, we have solved all the questions. That is 2023 Waec Chemistry Theory and Objective ...
This video is the second part of the correction to the 2023 WAEC Chemistry Theory. It covers the answers to Questions 2 and 3.You can find the answers to Sec...
PAPER 2: Will be a 2-hour essay paper covering the entire syllabus and carrying 100 marks. The paper will be in two sections; Sections A and B. Section A: Will consist of ten short structured questions drawn from the common portion of the syllabus. (i.e. Section A of the syllabus). Candidates will be required to answer all the questions for 25 ...
The Time Of the WAEC GCE Chemistry Essay | Chemistry Objective | Chemistry Practical Exam is 11:30pm - 3:30pm for all the 2023 WAEC GCE Chemistry exams. A. 2023 WAEC GCE CHEMISTRY ESSAY | (THEORY) ANSWERS. Kindly bookmark the website for the answers that will be released here, or better still reload the site to check if the answers for the ...
Understanding the marking scheme for the WAEC Chemistry examination is essential for maximizing your score. The examination is divided into two sections: the Objective (OBJ) section and the Theory section. CLICK HERE TO GET FREE WAEC 2023 CHEMISTRY (ESSAY AND OBJECTIVE) QUESTIONS AND ANSWERS DIRECTLY ON WHATSAPP MAY/JUNE EXAM RIGHT NOW
2023 waec chemistry (chem) objectives (obj) answers: 1-10: acadabbdac 11-20: bcaabbccbb 21-30: acbabdacbb 31-40: ... to subscribe for 2023 waec chemistry essay & obj answers via link. just go out and buy mtn cards of n600 (400 + 400 = 800) go to your message, type the card pins correctly and send to 08107431933.
WAEC Chemistry 2023 question and answer: This page contains both the WAEC Chemistry Objective and Essay Questions and Answers for the 2023 West African Senior School Certificate Examination (WASSCE). The WAEC Chemistry will be written on Wednesday 8th June 2023, Chemistry - Essay & OBJ.
These are possible WAEC Chemistry theory questions and answers 2023 that may appear in this year's exam. There are not actual questions and answers but, sample questions for practices. 2.47g of dry pure copper (II) oxide was completely reduced to a copper using laboratory gas. The mass of the residue left was found to be 1.97g.
The 2024 WAEC chemistry questions are set from the SS1 to SS3 chemistry syllabus. So all the questions you will encounter in this year's examination are in the syllabus, and nearly 90% of the questions are repeated. You don't have to worry about the 2024 WAEC chemistry questions and answers PDF (essay and objective).
WAEC 2022/2023 Chemistry Theory Questions. PAPER 2 (ESSAY) SECTION A. (a) When calcium oxide and coke are heated in an electric furnace, the products are carbon (ii) oxide and calcium carbide (CaC2), write the equation for this reaction. (b) The addition of water to calcium carbide leads to the formation of calcium hydroxide and ethyne.
Thursday, 18th May, 2023. Chemistry 3 (Practical) ( Alternative A) - 09:30am - 11:30am (1st Set) Chemistry 3 (Practical) (Alternative A) - 12:00pm - 2:00pm (2nd Set) This page contains the questions and answers to the 2023 WAEC Chemistry Practical. We have carefully solved the problem and here's are the answers to the questions.
For question 1. Use your school's endpoint and use it wherever you see our endpoint (15.15cm³) …and calculate with it. Replace our 15.15 in 1b and do the calculation. If your school wants to use mine, then the chemistry teacher must submit endpoint similar to ours without much difference…eg 15.15, 15.20, 15.30, etc.
2023 WAEC GCE Chemistry Questions & Answers for Essay and Objective Released. The Waec GCE chemistry answers 2023 essay and objective questions for the West African Examination Council (WAEC) Chemistry SSCE exam paper scheduled to be written on Tuesday, 12th December, 2023 can now be studied here.
Feb 25, 2023 - Free WAEC Chemistry Objective Questions and Answers - Free download as PDF File (.pdf), Text File (.txt) or read online for free. Download Free SSCE WAEC Chemistry Past Questions in PDF, Objective, theory, Practical radioactivity, periodic table, electrolysis, radioactivity, gas laws, e.t.c with Answers.