Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, generate accurate citations for free.
- Knowledge Base

The Beginner's Guide to Statistical Analysis | 5 Steps & Examples
Statistical analysis means investigating trends, patterns, and relationships using quantitative data . It is an important research tool used by scientists, governments, businesses, and other organizations.
To draw valid conclusions, statistical analysis requires careful planning from the very start of the research process . You need to specify your hypotheses and make decisions about your research design, sample size, and sampling procedure.
After collecting data from your sample, you can organize and summarize the data using descriptive statistics . Then, you can use inferential statistics to formally test hypotheses and make estimates about the population. Finally, you can interpret and generalize your findings.
This article is a practical introduction to statistical analysis for students and researchers. We’ll walk you through the steps using two research examples. The first investigates a potential cause-and-effect relationship, while the second investigates a potential correlation between variables.
Table of contents
Step 1: write your hypotheses and plan your research design, step 2: collect data from a sample, step 3: summarize your data with descriptive statistics, step 4: test hypotheses or make estimates with inferential statistics, step 5: interpret your results, other interesting articles.
To collect valid data for statistical analysis, you first need to specify your hypotheses and plan out your research design.
Writing statistical hypotheses
The goal of research is often to investigate a relationship between variables within a population . You start with a prediction, and use statistical analysis to test that prediction.
A statistical hypothesis is a formal way of writing a prediction about a population. Every research prediction is rephrased into null and alternative hypotheses that can be tested using sample data.
While the null hypothesis always predicts no effect or no relationship between variables, the alternative hypothesis states your research prediction of an effect or relationship.
- Null hypothesis: A 5-minute meditation exercise will have no effect on math test scores in teenagers.
- Alternative hypothesis: A 5-minute meditation exercise will improve math test scores in teenagers.
- Null hypothesis: Parental income and GPA have no relationship with each other in college students.
- Alternative hypothesis: Parental income and GPA are positively correlated in college students.
Planning your research design
A research design is your overall strategy for data collection and analysis. It determines the statistical tests you can use to test your hypothesis later on.
First, decide whether your research will use a descriptive, correlational, or experimental design. Experiments directly influence variables, whereas descriptive and correlational studies only measure variables.
- In an experimental design , you can assess a cause-and-effect relationship (e.g., the effect of meditation on test scores) using statistical tests of comparison or regression.
- In a correlational design , you can explore relationships between variables (e.g., parental income and GPA) without any assumption of causality using correlation coefficients and significance tests.
- In a descriptive design , you can study the characteristics of a population or phenomenon (e.g., the prevalence of anxiety in U.S. college students) using statistical tests to draw inferences from sample data.
Your research design also concerns whether you’ll compare participants at the group level or individual level, or both.
- In a between-subjects design , you compare the group-level outcomes of participants who have been exposed to different treatments (e.g., those who performed a meditation exercise vs those who didn’t).
- In a within-subjects design , you compare repeated measures from participants who have participated in all treatments of a study (e.g., scores from before and after performing a meditation exercise).
- In a mixed (factorial) design , one variable is altered between subjects and another is altered within subjects (e.g., pretest and posttest scores from participants who either did or didn’t do a meditation exercise).
- Experimental
- Correlational
First, you’ll take baseline test scores from participants. Then, your participants will undergo a 5-minute meditation exercise. Finally, you’ll record participants’ scores from a second math test.
In this experiment, the independent variable is the 5-minute meditation exercise, and the dependent variable is the math test score from before and after the intervention. Example: Correlational research design In a correlational study, you test whether there is a relationship between parental income and GPA in graduating college students. To collect your data, you will ask participants to fill in a survey and self-report their parents’ incomes and their own GPA.
Measuring variables
When planning a research design, you should operationalize your variables and decide exactly how you will measure them.
For statistical analysis, it’s important to consider the level of measurement of your variables, which tells you what kind of data they contain:
- Categorical data represents groupings. These may be nominal (e.g., gender) or ordinal (e.g. level of language ability).
- Quantitative data represents amounts. These may be on an interval scale (e.g. test score) or a ratio scale (e.g. age).
Many variables can be measured at different levels of precision. For example, age data can be quantitative (8 years old) or categorical (young). If a variable is coded numerically (e.g., level of agreement from 1–5), it doesn’t automatically mean that it’s quantitative instead of categorical.
Identifying the measurement level is important for choosing appropriate statistics and hypothesis tests. For example, you can calculate a mean score with quantitative data, but not with categorical data.
In a research study, along with measures of your variables of interest, you’ll often collect data on relevant participant characteristics.
Prevent plagiarism. Run a free check.
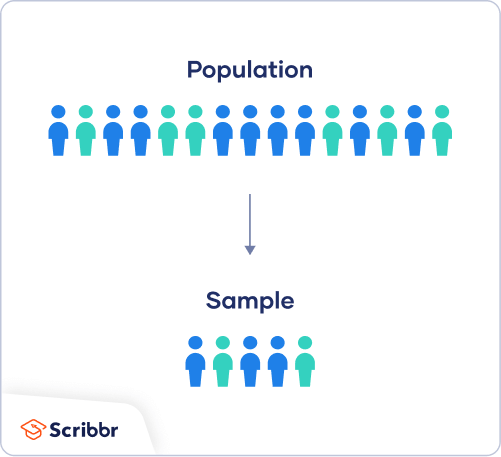
In most cases, it’s too difficult or expensive to collect data from every member of the population you’re interested in studying. Instead, you’ll collect data from a sample.
Statistical analysis allows you to apply your findings beyond your own sample as long as you use appropriate sampling procedures . You should aim for a sample that is representative of the population.
Sampling for statistical analysis
There are two main approaches to selecting a sample.
- Probability sampling: every member of the population has a chance of being selected for the study through random selection.
- Non-probability sampling: some members of the population are more likely than others to be selected for the study because of criteria such as convenience or voluntary self-selection.
In theory, for highly generalizable findings, you should use a probability sampling method. Random selection reduces several types of research bias , like sampling bias , and ensures that data from your sample is actually typical of the population. Parametric tests can be used to make strong statistical inferences when data are collected using probability sampling.
But in practice, it’s rarely possible to gather the ideal sample. While non-probability samples are more likely to at risk for biases like self-selection bias , they are much easier to recruit and collect data from. Non-parametric tests are more appropriate for non-probability samples, but they result in weaker inferences about the population.
If you want to use parametric tests for non-probability samples, you have to make the case that:
- your sample is representative of the population you’re generalizing your findings to.
- your sample lacks systematic bias.
Keep in mind that external validity means that you can only generalize your conclusions to others who share the characteristics of your sample. For instance, results from Western, Educated, Industrialized, Rich and Democratic samples (e.g., college students in the US) aren’t automatically applicable to all non-WEIRD populations.
If you apply parametric tests to data from non-probability samples, be sure to elaborate on the limitations of how far your results can be generalized in your discussion section .
Create an appropriate sampling procedure
Based on the resources available for your research, decide on how you’ll recruit participants.
- Will you have resources to advertise your study widely, including outside of your university setting?
- Will you have the means to recruit a diverse sample that represents a broad population?
- Do you have time to contact and follow up with members of hard-to-reach groups?
Your participants are self-selected by their schools. Although you’re using a non-probability sample, you aim for a diverse and representative sample. Example: Sampling (correlational study) Your main population of interest is male college students in the US. Using social media advertising, you recruit senior-year male college students from a smaller subpopulation: seven universities in the Boston area.
Calculate sufficient sample size
Before recruiting participants, decide on your sample size either by looking at other studies in your field or using statistics. A sample that’s too small may be unrepresentative of the sample, while a sample that’s too large will be more costly than necessary.
There are many sample size calculators online. Different formulas are used depending on whether you have subgroups or how rigorous your study should be (e.g., in clinical research). As a rule of thumb, a minimum of 30 units or more per subgroup is necessary.
To use these calculators, you have to understand and input these key components:
- Significance level (alpha): the risk of rejecting a true null hypothesis that you are willing to take, usually set at 5%.
- Statistical power : the probability of your study detecting an effect of a certain size if there is one, usually 80% or higher.
- Expected effect size : a standardized indication of how large the expected result of your study will be, usually based on other similar studies.
- Population standard deviation: an estimate of the population parameter based on a previous study or a pilot study of your own.
Once you’ve collected all of your data, you can inspect them and calculate descriptive statistics that summarize them.
Inspect your data
There are various ways to inspect your data, including the following:
- Organizing data from each variable in frequency distribution tables .
- Displaying data from a key variable in a bar chart to view the distribution of responses.
- Visualizing the relationship between two variables using a scatter plot .
By visualizing your data in tables and graphs, you can assess whether your data follow a skewed or normal distribution and whether there are any outliers or missing data.
A normal distribution means that your data are symmetrically distributed around a center where most values lie, with the values tapering off at the tail ends.
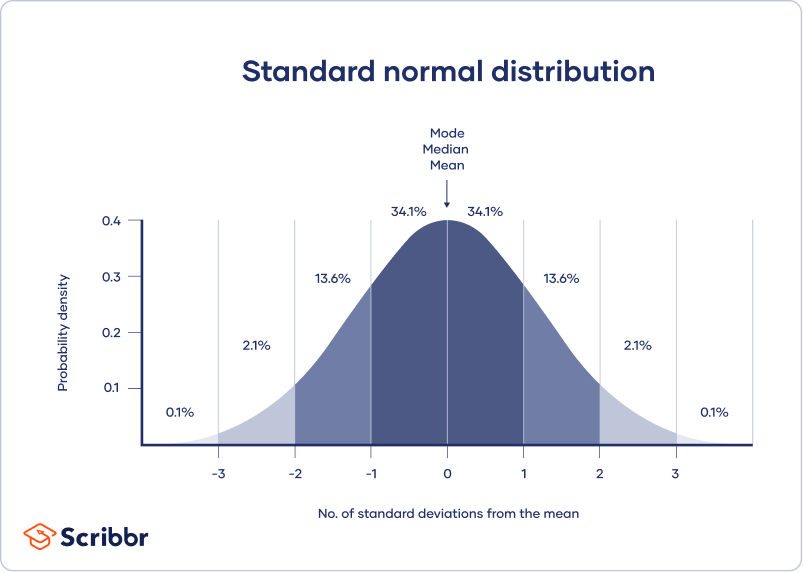
In contrast, a skewed distribution is asymmetric and has more values on one end than the other. The shape of the distribution is important to keep in mind because only some descriptive statistics should be used with skewed distributions.
Extreme outliers can also produce misleading statistics, so you may need a systematic approach to dealing with these values.
Calculate measures of central tendency
Measures of central tendency describe where most of the values in a data set lie. Three main measures of central tendency are often reported:
- Mode : the most popular response or value in the data set.
- Median : the value in the exact middle of the data set when ordered from low to high.
- Mean : the sum of all values divided by the number of values.
However, depending on the shape of the distribution and level of measurement, only one or two of these measures may be appropriate. For example, many demographic characteristics can only be described using the mode or proportions, while a variable like reaction time may not have a mode at all.
Calculate measures of variability
Measures of variability tell you how spread out the values in a data set are. Four main measures of variability are often reported:
- Range : the highest value minus the lowest value of the data set.
- Interquartile range : the range of the middle half of the data set.
- Standard deviation : the average distance between each value in your data set and the mean.
- Variance : the square of the standard deviation.
Once again, the shape of the distribution and level of measurement should guide your choice of variability statistics. The interquartile range is the best measure for skewed distributions, while standard deviation and variance provide the best information for normal distributions.
Using your table, you should check whether the units of the descriptive statistics are comparable for pretest and posttest scores. For example, are the variance levels similar across the groups? Are there any extreme values? If there are, you may need to identify and remove extreme outliers in your data set or transform your data before performing a statistical test.
From this table, we can see that the mean score increased after the meditation exercise, and the variances of the two scores are comparable. Next, we can perform a statistical test to find out if this improvement in test scores is statistically significant in the population. Example: Descriptive statistics (correlational study) After collecting data from 653 students, you tabulate descriptive statistics for annual parental income and GPA.
It’s important to check whether you have a broad range of data points. If you don’t, your data may be skewed towards some groups more than others (e.g., high academic achievers), and only limited inferences can be made about a relationship.
A number that describes a sample is called a statistic , while a number describing a population is called a parameter . Using inferential statistics , you can make conclusions about population parameters based on sample statistics.
Researchers often use two main methods (simultaneously) to make inferences in statistics.
- Estimation: calculating population parameters based on sample statistics.
- Hypothesis testing: a formal process for testing research predictions about the population using samples.
You can make two types of estimates of population parameters from sample statistics:
- A point estimate : a value that represents your best guess of the exact parameter.
- An interval estimate : a range of values that represent your best guess of where the parameter lies.
If your aim is to infer and report population characteristics from sample data, it’s best to use both point and interval estimates in your paper.
You can consider a sample statistic a point estimate for the population parameter when you have a representative sample (e.g., in a wide public opinion poll, the proportion of a sample that supports the current government is taken as the population proportion of government supporters).
There’s always error involved in estimation, so you should also provide a confidence interval as an interval estimate to show the variability around a point estimate.
A confidence interval uses the standard error and the z score from the standard normal distribution to convey where you’d generally expect to find the population parameter most of the time.
Hypothesis testing
Using data from a sample, you can test hypotheses about relationships between variables in the population. Hypothesis testing starts with the assumption that the null hypothesis is true in the population, and you use statistical tests to assess whether the null hypothesis can be rejected or not.
Statistical tests determine where your sample data would lie on an expected distribution of sample data if the null hypothesis were true. These tests give two main outputs:
- A test statistic tells you how much your data differs from the null hypothesis of the test.
- A p value tells you the likelihood of obtaining your results if the null hypothesis is actually true in the population.
Statistical tests come in three main varieties:
- Comparison tests assess group differences in outcomes.
- Regression tests assess cause-and-effect relationships between variables.
- Correlation tests assess relationships between variables without assuming causation.
Your choice of statistical test depends on your research questions, research design, sampling method, and data characteristics.
Parametric tests
Parametric tests make powerful inferences about the population based on sample data. But to use them, some assumptions must be met, and only some types of variables can be used. If your data violate these assumptions, you can perform appropriate data transformations or use alternative non-parametric tests instead.
A regression models the extent to which changes in a predictor variable results in changes in outcome variable(s).
- A simple linear regression includes one predictor variable and one outcome variable.
- A multiple linear regression includes two or more predictor variables and one outcome variable.
Comparison tests usually compare the means of groups. These may be the means of different groups within a sample (e.g., a treatment and control group), the means of one sample group taken at different times (e.g., pretest and posttest scores), or a sample mean and a population mean.
- A t test is for exactly 1 or 2 groups when the sample is small (30 or less).
- A z test is for exactly 1 or 2 groups when the sample is large.
- An ANOVA is for 3 or more groups.
The z and t tests have subtypes based on the number and types of samples and the hypotheses:
- If you have only one sample that you want to compare to a population mean, use a one-sample test .
- If you have paired measurements (within-subjects design), use a dependent (paired) samples test .
- If you have completely separate measurements from two unmatched groups (between-subjects design), use an independent (unpaired) samples test .
- If you expect a difference between groups in a specific direction, use a one-tailed test .
- If you don’t have any expectations for the direction of a difference between groups, use a two-tailed test .
The only parametric correlation test is Pearson’s r . The correlation coefficient ( r ) tells you the strength of a linear relationship between two quantitative variables.
However, to test whether the correlation in the sample is strong enough to be important in the population, you also need to perform a significance test of the correlation coefficient, usually a t test, to obtain a p value. This test uses your sample size to calculate how much the correlation coefficient differs from zero in the population.
You use a dependent-samples, one-tailed t test to assess whether the meditation exercise significantly improved math test scores. The test gives you:
- a t value (test statistic) of 3.00
- a p value of 0.0028
Although Pearson’s r is a test statistic, it doesn’t tell you anything about how significant the correlation is in the population. You also need to test whether this sample correlation coefficient is large enough to demonstrate a correlation in the population.
A t test can also determine how significantly a correlation coefficient differs from zero based on sample size. Since you expect a positive correlation between parental income and GPA, you use a one-sample, one-tailed t test. The t test gives you:
- a t value of 3.08
- a p value of 0.001
The final step of statistical analysis is interpreting your results.
Statistical significance
In hypothesis testing, statistical significance is the main criterion for forming conclusions. You compare your p value to a set significance level (usually 0.05) to decide whether your results are statistically significant or non-significant.
Statistically significant results are considered unlikely to have arisen solely due to chance. There is only a very low chance of such a result occurring if the null hypothesis is true in the population.
This means that you believe the meditation intervention, rather than random factors, directly caused the increase in test scores. Example: Interpret your results (correlational study) You compare your p value of 0.001 to your significance threshold of 0.05. With a p value under this threshold, you can reject the null hypothesis. This indicates a statistically significant correlation between parental income and GPA in male college students.
Note that correlation doesn’t always mean causation, because there are often many underlying factors contributing to a complex variable like GPA. Even if one variable is related to another, this may be because of a third variable influencing both of them, or indirect links between the two variables.
Effect size
A statistically significant result doesn’t necessarily mean that there are important real life applications or clinical outcomes for a finding.
In contrast, the effect size indicates the practical significance of your results. It’s important to report effect sizes along with your inferential statistics for a complete picture of your results. You should also report interval estimates of effect sizes if you’re writing an APA style paper .
With a Cohen’s d of 0.72, there’s medium to high practical significance to your finding that the meditation exercise improved test scores. Example: Effect size (correlational study) To determine the effect size of the correlation coefficient, you compare your Pearson’s r value to Cohen’s effect size criteria.
Decision errors
Type I and Type II errors are mistakes made in research conclusions. A Type I error means rejecting the null hypothesis when it’s actually true, while a Type II error means failing to reject the null hypothesis when it’s false.
You can aim to minimize the risk of these errors by selecting an optimal significance level and ensuring high power . However, there’s a trade-off between the two errors, so a fine balance is necessary.
Frequentist versus Bayesian statistics
Traditionally, frequentist statistics emphasizes null hypothesis significance testing and always starts with the assumption of a true null hypothesis.
However, Bayesian statistics has grown in popularity as an alternative approach in the last few decades. In this approach, you use previous research to continually update your hypotheses based on your expectations and observations.
Bayes factor compares the relative strength of evidence for the null versus the alternative hypothesis rather than making a conclusion about rejecting the null hypothesis or not.
If you want to know more about statistics , methodology , or research bias , make sure to check out some of our other articles with explanations and examples.
- Student’s t -distribution
- Normal distribution
- Null and Alternative Hypotheses
- Chi square tests
- Confidence interval
Methodology
- Cluster sampling
- Stratified sampling
- Data cleansing
- Reproducibility vs Replicability
- Peer review
- Likert scale
Research bias
- Implicit bias
- Framing effect
- Cognitive bias
- Placebo effect
- Hawthorne effect
- Hostile attribution bias
- Affect heuristic
Is this article helpful?
Other students also liked.
- Descriptive Statistics | Definitions, Types, Examples
- Inferential Statistics | An Easy Introduction & Examples
- Choosing the Right Statistical Test | Types & Examples
More interesting articles
- Akaike Information Criterion | When & How to Use It (Example)
- An Easy Introduction to Statistical Significance (With Examples)
- An Introduction to t Tests | Definitions, Formula and Examples
- ANOVA in R | A Complete Step-by-Step Guide with Examples
- Central Limit Theorem | Formula, Definition & Examples
- Central Tendency | Understanding the Mean, Median & Mode
- Chi-Square (Χ²) Distributions | Definition & Examples
- Chi-Square (Χ²) Table | Examples & Downloadable Table
- Chi-Square (Χ²) Tests | Types, Formula & Examples
- Chi-Square Goodness of Fit Test | Formula, Guide & Examples
- Chi-Square Test of Independence | Formula, Guide & Examples
- Coefficient of Determination (R²) | Calculation & Interpretation
- Correlation Coefficient | Types, Formulas & Examples
- Frequency Distribution | Tables, Types & Examples
- How to Calculate Standard Deviation (Guide) | Calculator & Examples
- How to Calculate Variance | Calculator, Analysis & Examples
- How to Find Degrees of Freedom | Definition & Formula
- How to Find Interquartile Range (IQR) | Calculator & Examples
- How to Find Outliers | 4 Ways with Examples & Explanation
- How to Find the Geometric Mean | Calculator & Formula
- How to Find the Mean | Definition, Examples & Calculator
- How to Find the Median | Definition, Examples & Calculator
- How to Find the Mode | Definition, Examples & Calculator
- How to Find the Range of a Data Set | Calculator & Formula
- Hypothesis Testing | A Step-by-Step Guide with Easy Examples
- Interval Data and How to Analyze It | Definitions & Examples
- Levels of Measurement | Nominal, Ordinal, Interval and Ratio
- Linear Regression in R | A Step-by-Step Guide & Examples
- Missing Data | Types, Explanation, & Imputation
- Multiple Linear Regression | A Quick Guide (Examples)
- Nominal Data | Definition, Examples, Data Collection & Analysis
- Normal Distribution | Examples, Formulas, & Uses
- Null and Alternative Hypotheses | Definitions & Examples
- One-way ANOVA | When and How to Use It (With Examples)
- Ordinal Data | Definition, Examples, Data Collection & Analysis
- Parameter vs Statistic | Definitions, Differences & Examples
- Pearson Correlation Coefficient (r) | Guide & Examples
- Poisson Distributions | Definition, Formula & Examples
- Probability Distribution | Formula, Types, & Examples
- Quartiles & Quantiles | Calculation, Definition & Interpretation
- Ratio Scales | Definition, Examples, & Data Analysis
- Simple Linear Regression | An Easy Introduction & Examples
- Skewness | Definition, Examples & Formula
- Statistical Power and Why It Matters | A Simple Introduction
- Student's t Table (Free Download) | Guide & Examples
- T-distribution: What it is and how to use it
- Test statistics | Definition, Interpretation, and Examples
- The Standard Normal Distribution | Calculator, Examples & Uses
- Two-Way ANOVA | Examples & When To Use It
- Type I & Type II Errors | Differences, Examples, Visualizations
- Understanding Confidence Intervals | Easy Examples & Formulas
- Understanding P values | Definition and Examples
- Variability | Calculating Range, IQR, Variance, Standard Deviation
- What is Effect Size and Why Does It Matter? (Examples)
- What Is Kurtosis? | Definition, Examples & Formula
- What Is Standard Error? | How to Calculate (Guide with Examples)
What is your plagiarism score?
The Data Deep Dive: Statistical Analysis Guide
Table of contents
- 1 What Is Statistical Analysis and Its Role?
- 2 Preparing for Statistical Analysis
- 3 Data Collection and Management
- 4 Performing Descriptive Statistical Analysis
- 5 Performing Inferential Statistics Analysis
- 6 Writing the Statistical Research Paper
- 7 Common Mistakes in Statistical Analysis
- 8 Ethical Considerations
- 9 Concluding the Research Paper
- 10 Examples of Good and Poor Statistical Analysis in Research Paper
- 11 Key Insights: Navigating Statistical Analysis
Statistical analysis is fundamental if you need to reveal patterns or identify trends in datasets, employing numerical data analysis to eliminate bias and extract meaningful vision. Accordingly, it is crucial in research explanation, model evolution, and survey planning.
Statistical analysts make valuable results from the raw data, facilitating informed decision-making and predictive statistical analytics based on historical information.
Do you need to make a statistical analysis for your university studies? In this statistical study article, you will find instructions on how to write statistical analysis, as well as types of statistical analysis, statistical tools, and common mistakes students face.
What Is Statistical Analysis and Its Role?
Statistical analysis is the systematic process of collecting, organizing, and interpreting numbers to reveal patterns and identify trends and relationships. It plays a crucial role in research by providing tools to analyze data objectively, remove bias, and draw conclusions. Moreover, statistical analysis aids in identifying correlations, testing hypotheses, and making predictions, thereby informing decision-making in various fields such as computer science, medicine, economics, and social sciences. Thus, it enables quantitative data and statistical analytics researchers to assess results.
Struggling with how to analyze data in research? Feel free to address our specialists to get skilled and qualified help with research paper data analysis.
Preparing for Statistical Analysis
Preparing for statistical analysis requires some essential steps to ensure the validity and reliability of results.
- Firstly, formulating understandable and measurable questions is critical for valid statistics in a research paper. Questions lead the entire data analysis process and help define the scope of the study. Accordingly, scientists should develop specific, relevant, and capable issues that can be answered through statistical methods.
- Secondly, identifying appropriate data is vital. Picking an accordant data set that aligns with the investigations guarantees the analysis and business intelligence are focused and meaningful. For this purpose, researchers should consider data origin, quality, and responsibility when selecting data for analysis.
By scrupulously formulating problems and selecting appropriate statistical analytics data, researchers can lay a solid foundation for statistical analysis, guiding to strong and prudent results.
Data Collection and Management
Information collection and management are integral components of the statistical analysis process, ensuring the accuracy and reliability of results. Firstly, considering the techniques of data collection is essential. Here, researchers may employ primary approaches, such as examinations, interviews, or experiments, to gather direct information. Secondary methods involve utilizing existing data sources like databases, statistical analysis software, literature reviews, or archival records.
To collect data, specialists need to analyze:
- dependent variable;
- categorical variables;
- outcome variable;
- patterns and trends;
- alternative hypothesis states.
Once data is collected, organizing it is crucial for efficient analysis. As a rule, researchers utilize statistical analysis software tools or spreadsheets to manage data systematically, ensuring clarity and accessibility. Besides, proper organization includes labeling variables, formatting data consistently, and documenting any transformations or cleaning statistical procedures undertaken.
Effective data management also facilitates coherent analysis, empowering scientists to get meaningful insights and draw valid conclusions. By using suitable data collection approaches and organizing data systematically, researchers can unlock the full potential of statistical analysis, advancing knowledge and driving proof-based replies.
Performing Descriptive Statistical Analysis
Performing descriptive statistics is essential in knowing and summarizing data sets for statistics in research. Usually, it involves exploring the crucial characteristics of the data to gain insights into its normal allotting and changeability.
The basics of descriptive statistics encompass measures of central tendency, dispersion, and graphical representations.
- Measures of main bias , such as mean, median, and mode, summarize a dataset’s typical or main value.
- Dispersion repeated measures , including range, variance, and standard deviation, quantify the spread or variability of the data points.
- Graphical representations , such as histograms, box plots, and scatter plots, offer visual insights into the distribution and patterns within the data based on statistical observations.
Explaining descriptive statistical analysis results involves understanding and presenting the findings effectively. Accordingly, researchers should show understandable explanations of the descriptive statistics in the research paper calculated, highlighting key insights and trends within the data. Indeed, visual representations can enhance understanding by illustrating the distribution and relationships in the data. Hence, it’s essential to consider the context of the analysis and the questions when interpreting the results, ensuring that the conclusions drawn are suggestive and relevant.
Overall, performing descriptive statistical data analysis enables researchers to summarize and derive the crucial characteristics after collecting data. It is vital to provide a foundation for further research study and interpretation. By mastering the basics of different types of statistical analysis and correctly explaining the results, experimentals can uncover valuable insights and communicate their findings clearly and precisely.
If you struggle on this step, ask for help from our writers. They can’t write an essay or paper for you but are eager to assist you with each step.
Performing Inferential Statistics Analysis
When students perform statistical analysis, it involves making statistical inference and drawing conclusions about a population based on sample data. For this reason, the inferential statistical tool in research revolves around hypothesis testing, confidence intervals, and significance levels.
On the one side, hypothesis testing allows researchers to assess the validity of assumptions underlying entire population parameters by comparing one sample data to theoretical expectations. On the other side, sample data, null hypothesis, and confidence intervals provide a range of normal and extreme values within which the proper population parameter will likely fall. Lastly, significance levels indicate the probability of obtaining the observed results by chance alone, helping researchers determine the reliability of their findings.
Choosing the proper approach is crucial for conducting meaningful inferential statistics analysis. Accordingly, researchers must select appropriate parametric tests based on the research design, collect data type, null hypothesis, and hypothesis being tested. For example, standard parametric tests and non parametric tests include:
- T-tests: a parametric statistical test used to determine if there is a significant difference between the means of two groups. It is commonly used when the sample size is small, and the population standard deviation is unknown.
- Z test: similar to the t-test, the z-test is a parametric test used to compare means, but it is typically employed when the sample size is large and/or the population standard deviation is known.
- ANOVA: this parametric statistical test compares the means of three or more groups simultaneously. It assesses whether there are any statistically significant differences between the means of the groups.
- Regression: a statistical method used to examine the relationship between one dependent variable (often denoted as Y) and one or more independent variables (often denoted as X) within one case study analysis . Thus, it helps in understanding how the value of the dependent variable changes when one or more independent variables are varied. Here, case study analysis refers to applying regression analysis in specific scenarios or case studies to explore relationships between quantitative variables.
Importantly to note, interpreting results from inferential studies requires a nuanced understanding of statistical concepts and diligent consideration of the context. Here, investigators should assess the strength of evidence supporting their conclusions, considering factors such as effect size, statistical power, and potential biases. Besides, communicating inferential statistics results involves presenting findings and standard deviation to highlight the implications for the research question or troublesome under investigation.
Writing the Statistical Research Paper
Writing a research paper involves integrating and presenting your findings coherently. You need to know the answers to the questions: “What is statistical analysis?” and “How do you do a statistical analysis?”. As a rule, the typical structure includes several essential sections:
- Introduction : This section provides backdrop information on the research theme, states the research questions or null hypothesis, patterns, and trends, and outlines the study’s objectives and statistical attention.
- Methodology : Here, researchers detail the methods and procedures for analyzing and collecting data. This section should be thorough enough for other researchers to replicate the study.
- Results : This section presents the study’s findings, often through descriptive and inferential statistical data analysis. It’s essential to present results objectively and accurately, using appropriate statistical study measures and techniques.
- Discussion : In this segment, investigators interpret statistics and the results, discuss their implications, and compare them to existing literature. It’s an opportunity to critically evaluate the findings and address any limitations or potential biases.
- Conclusion : The conclusion summarizes the study’s key findings, discusses their significance, and suggests avenues for future research.
When you present or write a statistical report in each section, it’s crucial to clearly and concisely explain the methods, results, and research design. Therefore, students usually need to test it in the sample group. In the methodology section, describe the statistical techniques used and justify their appropriateness for the research question. Otherwise, use descriptive statistics to summarize data and inferential statistics to test hypotheses or explore relationships between variables.
Whereas, graphics and tables are potent statistical instruments for presenting data effectively. Choose the most appropriate format for your data, whether it’s a bar graph, scatter plot, or table of descriptive statistics for research.
As a result, writing your research essay must involve such steps:
- Arranging your decisions analytically;
- Integrating statistical analysis throughout;
- Using visuals and tables to enhance clarity and understanding.
Common Mistakes in Statistical Analysis
Common mistakes in statistical analysis can undermine the validity and reliability of research findings. Here are some key pitfalls to avoid:
- Confusing terms like ‘mean’ and ‘median’ or misinterpreting p value and confidence intervals can lead to incorrect conclusions.
- Selecting the wrong test for the research question or ignoring test assumptions can compromise the accuracy of the results.
- Ignoring missing data and outliers or failing to preprocess data properly can introduce bias and skew results.
- Focusing solely on statistical significance without considering practical significance or engaging in p-hacking practices can lead to misleading conclusions.
- Failing to share facts or selectively report results can hinder research reproducibility and transparency.
- Both small and large sample sizes can impact the reliability and generalizability of findings.
- Repeatedly testing hypotheses on the same data set or creating overly complicated models can result in spurious decisions.
- Failing to interpret statistical results within the broader context or generalize findings appropriately can limit the practical relevance of research.
- Misrepresenting graphics or neglecting to label and interval scale graphs correctly can distort the statistical analysis of data.
- Managing redundant analyses or ignoring existing knowledge in the field can hinder the promotion of research.
Avoiding common mistakes in statistical analysis requires diligence and attention to detail. Consequently, researchers should prioritize understanding statistical concepts systematically and using appropriate methods for exploratory data analysis. Thus, it’s essential to double-check calculations, verify assumptions, and seek guidance from statistical analysts if needed.
Furthermore, maintaining transparency and reproducibility in research practices is leading. It includes sharing data, code, and methodology details to facilitate equivalent surveys and replication of findings.
Continuous data learning and staying updated on best practices in statistical analysis are also vital for avoiding pitfalls and improving the quality of research. By addressing these common mistakes and adopting robust practices, researchers can ensure the morality and reliability of their findings, contributing to advancing knowledge in their respective fields.
Ethical Considerations
Ethical considerations in statistical analysis encompass safeguarding data privacy and integrity. That being said, researchers must uphold ethical practices in handling data, ensuring confidentiality, and respecting participants’ rights. Indeed, transparent reporting of results is vital, as is disclosing potential conflicts of passion and holding to moral guidelines data dedicated to relevant institutions and controlling bodies. By prioritizing ethical principles, researchers can maintain trust and integrity in their work, fostering a culture of responsible data analysis in research and decision-making.
Concluding the Research Paper
Concluding a research paper involves summarizing key findings and suggesting future research directions. Here, reiterating the paper’s main points and highlighting the significance of the results is essential. Statistical analysts can also discuss limitations and areas for further investigation, providing context for future studies. By showing insightful outcomes and figuring out avenues for future research, scientists can contribute to the ongoing discourse in their field and inspire further inquiry and exploration.
Examples of Good and Poor Statistical Analysis in Research Paper
Good statistical analysis examples in research:
- A study on the effectiveness of a new drug uses appropriate parametric tests, presents results clearly with confidence intervals, and discusses both statistical and practical significance.
- A survey-based research project employs stratified random sampling, ensuring a representative sample, and utilizes advanced regression analysis to explore complex relationships between variables.
- An experiment investigating the impact of a teaching method on student performance controls for potential confounding variables and conducts power statistical analysis basics to determine sample size, ensuring adequate statistical power.
Examples of poor stat analysis in research:
- A study fails to report key details about information collection and statistical methods, making it impossible to evaluate the validity of the findings.
- A research paper relies solely on p value to conclude without considering effect sizes or practical significance, leading to misleading interpretations.
- An analysis uses an inappropriate statistical test for the research question, resulting in flawed conclusions and misinterpretation of the data.
Here are two good examples.
Example 1: The Effect of Regular Exercise on Anxiety Levels among College Students
Introduction: In recent years, mental health issues among college students have become a growing concern. Anxiety, in particular, is prevalent among this demographic. This study aims to investigate the potential impact of regular exercise on anxiety levels among college students. Understanding this relationship could inform interventions aimed at improving mental well-being in this population.
Methodology: Participants (N = 100) were recruited from a local university and randomly assigned to either an exercise or control group. The exercise group engaged in a supervised 30-minute aerobic exercise session three times a week for eight weeks, while the control group maintained regular activities. Anxiety levels were assessed using the State-Trait Anxiety Inventory (STAI) before and after the intervention period.
Results: The results revealed a significant decrease in anxiety levels among participants in the exercise group compared to the control group (t(98) = -2.45, p < 0.05). Specifically, the mean anxiety score decreased from 45.2 (SD = 7.8) to 38.6 (SD = 6.4) in the exercise group, while it remained relatively stable in the control group (mean = 44.5, SD = 8.2).
Discussion: These findings suggest that regular aerobic exercise may have a beneficial effect on reducing anxiety levels among college students. Engaging in physical activity could serve as a potential non-pharmacological intervention for managing anxiety symptoms in this population. Further research is warranted to explore this relationship’s underlying mechanisms and determine optimal exercise duration and intensity for maximum mental health benefits.
Example 2: The Relationship between Service Quality, Customer Satisfaction, and Loyalty in Retail Settings
Introduction: Maintaining high levels of customer satisfaction and loyalty is essential for the success of retail businesses. This study investigates the relationship between service quality, customer satisfaction, and loyalty in a local retail chain context. Understanding these dynamics can help businesses identify areas for improvement and develop strategies to enhance customer retention.
Methodology: A survey was conducted among the retail chain’s customers (N = 300) to assess their perceptions of service quality, satisfaction with their shopping experience, and intention to repurchase from the store. Service quality was measured using the SERVQUAL scale, while customer satisfaction and loyalty were assessed using Likert-type scales.
Results: The results indicated a strong positive correlation between service quality, customer satisfaction, and loyalty (r = 0.75, p < 0.001). Furthermore, regression analysis revealed that service quality significantly predicted both customer satisfaction (β = 0.60, p < 0.001) and loyalty (β = 0.45, p < 0.001). Additionally, customer satisfaction emerged as a significant predictor of loyalty (β = 0.50, p < 0.001), indicating its mediating role in the relationship between service quality and loyalty.
Discussion: These findings underscore the importance of high-quality service in enhancing customer satisfaction and fostering loyalty in retail settings. Businesses should prioritize investments in service training, infrastructure, and customer relationship management to ensure positive shopping experiences and promote repeat patronage. Future research could explore additional factors influencing customer loyalty and examine the effectiveness of specific loyalty programs and incentives in driving repeat business.
Key Insights: Navigating Statistical Analysis
To sum up, mastering a statistical analysis system is essential for researchers to derive meaningful insights from data. Understanding statistical concepts, choosing appropriate methods, and adhering to ethical guidelines are paramount.
Additionally, transparent reporting, rigorous methodology, and careful interpretation ensure the integrity and reliability of research findings. By avoiding common pitfalls and embracing best practices, researchers can contribute to advancing knowledge and making informed decisions across various fields.
Ultimately, statistical analysis is a powerful tool for unlocking the mysteries hidden within data, guiding us toward more profound understanding and innovation.
Readers also enjoyed

WHY WAIT? PLACE AN ORDER RIGHT NOW!
Just fill out the form, press the button, and have no worries!
We use cookies to give you the best experience possible. By continuing we’ll assume you board with our cookie policy.

Statistical Analysis in Research: Meaning, Methods and Types
Home » Videos » Statistical Analysis in Research: Meaning, Methods and Types
The scientific method is an empirical approach to acquiring new knowledge by making skeptical observations and analyses to develop a meaningful interpretation. It is the basis of research and the primary pillar of modern science. Researchers seek to understand the relationships between factors associated with the phenomena of interest. In some cases, research works with vast chunks of data, making it difficult to observe or manipulate each data point. As a result, statistical analysis in research becomes a means of evaluating relationships and interconnections between variables with tools and analytical techniques for working with large data. Since researchers use statistical power analysis to assess the probability of finding an effect in such an investigation, the method is relatively accurate. Hence, statistical analysis in research eases analytical methods by focusing on the quantifiable aspects of phenomena.
What is Statistical Analysis in Research? A Simplified Definition
Statistical analysis uses quantitative data to investigate patterns, relationships, and patterns to understand real-life and simulated phenomena. The approach is a key analytical tool in various fields, including academia, business, government, and science in general. This statistical analysis in research definition implies that the primary focus of the scientific method is quantitative research. Notably, the investigator targets the constructs developed from general concepts as the researchers can quantify their hypotheses and present their findings in simple statistics.
When a business needs to learn how to improve its product, they collect statistical data about the production line and customer satisfaction. Qualitative data is valuable and often identifies the most common themes in the stakeholders’ responses. On the other hand, the quantitative data creates a level of importance, comparing the themes based on their criticality to the affected persons. For instance, descriptive statistics highlight tendency, frequency, variation, and position information. While the mean shows the average number of respondents who value a certain aspect, the variance indicates the accuracy of the data. In any case, statistical analysis creates simplified concepts used to understand the phenomenon under investigation. It is also a key component in academia as the primary approach to data representation, especially in research projects, term papers and dissertations.
Most Useful Statistical Analysis Methods in Research
Using statistical analysis methods in research is inevitable, especially in academic assignments, projects, and term papers. It’s always advisable to seek assistance from your professor or you can try research paper writing by CustomWritings before you start your academic project or write statistical analysis in research paper. Consulting an expert when developing a topic for your thesis or short mid-term assignment increases your chances of getting a better grade. Most importantly, it improves your understanding of research methods with insights on how to enhance the originality and quality of personalized essays. Professional writers can also help select the most suitable statistical analysis method for your thesis, influencing the choice of data and type of study.
Descriptive Statistics
Descriptive statistics is a statistical method summarizing quantitative figures to understand critical details about the sample and population. A description statistic is a figure that quantifies a specific aspect of the data. For instance, instead of analyzing the behavior of a thousand students, research can identify the most common actions among them. By doing this, the person utilizes statistical analysis in research, particularly descriptive statistics.
- Measures of central tendency . Central tendency measures are the mean, mode, and media or the averages denoting specific data points. They assess the centrality of the probability distribution, hence the name. These measures describe the data in relation to the center.
- Measures of frequency . These statistics document the number of times an event happens. They include frequency, count, ratios, rates, and proportions. Measures of frequency can also show how often a score occurs.
- Measures of dispersion/variation . These descriptive statistics assess the intervals between the data points. The objective is to view the spread or disparity between the specific inputs. Measures of variation include the standard deviation, variance, and range. They indicate how the spread may affect other statistics, such as the mean.
- Measures of position . Sometimes researchers can investigate relationships between scores. Measures of position, such as percentiles, quartiles, and ranks, demonstrate this association. They are often useful when comparing the data to normalized information.
Inferential Statistics
Inferential statistics is critical in statistical analysis in quantitative research. This approach uses statistical tests to draw conclusions about the population. Examples of inferential statistics include t-tests, F-tests, ANOVA, p-value, Mann-Whitney U test, and Wilcoxon W test. This
Common Statistical Analysis in Research Types
Although inferential and descriptive statistics can be classified as types of statistical analysis in research, they are mostly considered analytical methods. Types of research are distinguishable by the differences in the methodology employed in analyzing, assembling, classifying, manipulating, and interpreting data. The categories may also depend on the type of data used.
Predictive Analysis
Predictive research analyzes past and present data to assess trends and predict future events. An excellent example of predictive analysis is a market survey that seeks to understand customers’ spending habits to weigh the possibility of a repeat or future purchase. Such studies assess the likelihood of an action based on trends.
Prescriptive Analysis
On the other hand, a prescriptive analysis targets likely courses of action. It’s decision-making research designed to identify optimal solutions to a problem. Its primary objective is to test or assess alternative measures.
Causal Analysis
Causal research investigates the explanation behind the events. It explores the relationship between factors for causation. Thus, researchers use causal analyses to analyze root causes, possible problems, and unknown outcomes.
Mechanistic Analysis
This type of research investigates the mechanism of action. Instead of focusing only on the causes or possible outcomes, researchers may seek an understanding of the processes involved. In such cases, they use mechanistic analyses to document, observe, or learn the mechanisms involved.
Exploratory Data Analysis
Similarly, an exploratory study is extensive with a wider scope and minimal limitations. This type of research seeks insight into the topic of interest. An exploratory researcher does not try to generalize or predict relationships. Instead, they look for information about the subject before conducting an in-depth analysis.
The Importance of Statistical Analysis in Research
As a matter of fact, statistical analysis provides critical information for decision-making. Decision-makers require past trends and predictive assumptions to inform their actions. In most cases, the data is too complex or lacks meaningful inferences. Statistical tools for analyzing such details help save time and money, deriving only valuable information for assessment. An excellent statistical analysis in research example is a randomized control trial (RCT) for the Covid-19 vaccine. You can download a sample of such a document online to understand the significance such analyses have to the stakeholders. A vaccine RCT assesses the effectiveness, side effects, duration of protection, and other benefits. Hence, statistical analysis in research is a helpful tool for understanding data.
Sources and links For the articles and videos I use different databases, such as Eurostat, OECD World Bank Open Data, Data Gov and others. You are free to use the video I have made on your site using the link or the embed code. If you have any questions, don’t hesitate to write to me!
Support statistics and data, if you have reached the end and like this project, you can donate a coffee to “statistics and data”..
Copyright © 2022 Statistics and Data
When you choose to publish with PLOS, your research makes an impact. Make your work accessible to all, without restrictions, and accelerate scientific discovery with options like preprints and published peer review that make your work more Open.
- PLOS Biology
- PLOS Climate
- PLOS Complex Systems
- PLOS Computational Biology
- PLOS Digital Health
- PLOS Genetics
- PLOS Global Public Health
- PLOS Medicine
- PLOS Mental Health
- PLOS Neglected Tropical Diseases
- PLOS Pathogens
- PLOS Sustainability and Transformation
- PLOS Collections
- How to Report Statistics

Ensure appropriateness and rigor, avoid flexibility and above all never manipulate results
In many fields, a statistical analysis forms the heart of both the methods and results sections of a manuscript. Learn how to report statistical analyses, and what other context is important for publication success and future reproducibility.
A matter of principle
First and foremost, the statistical methods employed in research must always be:
Appropriate for the study design
Rigorously reported in sufficient detail for others to reproduce the analysis
Free of manipulation, selective reporting, or other forms of “spin”
Just as importantly, statistical practices must never be manipulated or misused . Misrepresenting data, selectively reporting results or searching for patterns that can be presented as statistically significant, in an attempt to yield a conclusion that is believed to be more worthy of attention or publication is a serious ethical violation. Although it may seem harmless, using statistics to “spin” results can prevent publication, undermine a published study, or lead to investigation and retraction.
Supporting public trust in science through transparency and consistency
Along with clear methods and transparent study design, the appropriate use of statistical methods and analyses impacts editorial evaluation and readers’ understanding and trust in science.
In 2011 False-Positive Psychology: Undisclosed Flexibility in Data Collection and Analysis Allows Presenting Anything as Significant exposed that “flexibility in data collection, analysis, and reporting dramatically increases actual false-positive rates” and demonstrated “how unacceptably easy it is to accumulate (and report) statistically significant evidence for a false hypothesis”.
Arguably, such problems with flexible analysis lead to the “ reproducibility crisis ” that we read about today.
A constant principle of rigorous science The appropriate, rigorous, and transparent use of statistics is a constant principle of rigorous, transparent, and Open Science. Aim to be thorough, even if a particular journal doesn’t require the same level of detail. Trust in science is all of our responsibility. You cannot create any problems by exceeding a minimum standard of information and reporting.

Sound statistical practices
While it is hard to provide statistical guidelines that are relevant for all disciplines, types of research, and all analytical techniques, adherence to rigorous and appropriate principles remains key. Here are some ways to ensure your statistics are sound.
Define your analytical methodology before you begin Take the time to consider and develop a thorough study design that defines your line of inquiry, what you plan to do, what data you will collect, and how you will analyze it. (If you applied for research grants or ethical approval, you probably already have a plan in hand!) Refer back to your study design at key moments in the research process, and above all, stick to it.
To avoid flexibility and improve the odds of acceptance, preregister your study design with a journal Many journals offer the option to submit a study design for peer review before research begins through a practice known as preregistration. If the editors approve your study design, you’ll receive a provisional acceptance for a future research article reporting the results. Preregistering is a great way to head off any intentional or unintentional flexibility in analysis. By declaring your analytical approach in advance you’ll increase the credibility and reproducibility of your results and help address publication bias, too. Getting peer review feedback on your study design and analysis plan before it has begun (when you can still make changes!) makes your research even stronger AND increases your chances of publication—even if the results are negative or null. Never underestimate how much you can help increase the public’s trust in science by planning your research in this way.
Imagine replicating or extending your own work, years in the future Imagine that you are describing your approach to statistical analysis for your future self, in exactly the same way as we have described for writing your methods section . What would you need to know to replicate or extend your own work? When you consider that you might be at a different institution, working with different colleagues, using different programs, applications, resources — or maybe even adopting new statistical techniques that have emerged — you can help yourself imagine the level of reporting specificity that you yourself would require to redo or extend your work. Consider:
- Which details would you need to be reminded of?
- What did you do to the raw data before analysis?
- Did the purpose of the analysis change before or during the experiments?
- What participants did you decide to exclude?
- What process did you adjust, during your work?
Even if a necessary adjustment you made was not ideal, transparency is the key to ensuring this is not regarded as an issue in the future. It is far better to transparently convey any non-optimal techniques or constraints than to conceal them, which could result in reproducibility or ethical issues downstream.
Existing standards, checklists, guidelines for specific disciplines
You can apply the Open Science practices outlined above no matter what your area of expertise—but in many cases, you may still need more detailed guidance specific to your own field. Many disciplines, fields, and projects have worked hard to develop guidelines and resources to help with statistics, and to identify and avoid bad statistical practices. Below, you’ll find some of the key materials.
TIP: Do you have a specific journal in mind?
Be sure to read the submission guidelines for the specific journal you are submitting to, in order to discover any journal- or field-specific policies, initiatives or tools to utilize.
Articles on statistical methods and reporting
Makin, T.R., Orban de Xivry, J. Science Forum: Ten common statistical mistakes to watch out for when writing or reviewing a manuscript . eLife 2019;8:e48175 (2019). https://doi.org/10.7554/eLife.48175
Munafò, M., Nosek, B., Bishop, D. et al. A manifesto for reproducible science . Nat Hum Behav 1, 0021 (2017). https://doi.org/10.1038/s41562-016-0021
Writing tips
Your use of statistics should be rigorous, appropriate, and uncompromising in avoidance of analytical flexibility. While this is difficult, do not compromise on rigorous standards for credibility!

- Remember that trust in science is everyone’s responsibility.
- Keep in mind future replicability.
- Consider preregistering your analysis plan to have it (i) reviewed before results are collected to check problems before they occur and (ii) to avoid any analytical flexibility.
- Follow principles, but also checklists and field- and journal-specific guidelines.
- Consider a commitment to rigorous and transparent science a personal responsibility, and not simple adhering to journal guidelines.
- Be specific about all decisions made during the experiments that someone reproducing your work would need to know.
- Consider a course in advanced and new statistics, if you feel you have not focused on it enough during your research training.

Don’t
- Misuse statistics to influence significance or other interpretations of results
- Conduct your statistical analyses if you are unsure of what you are doing—seek feedback (e.g. via preregistration) from a statistical specialist first.
- How to Write a Great Title
- How to Write an Abstract
- How to Write Your Methods
- How to Write Discussions and Conclusions
- How to Edit Your Work
The contents of the Peer Review Center are also available as a live, interactive training session, complete with slides, talking points, and activities. …
The contents of the Writing Center are also available as a live, interactive training session, complete with slides, talking points, and activities. …
There’s a lot to consider when deciding where to submit your work. Learn how to choose a journal that will help your study reach its audience, while reflecting your values as a researcher…
Have a thesis expert improve your writing
Check your thesis for plagiarism in 10 minutes, generate your apa citations for free.
- Knowledge Base
The Beginner's Guide to Statistical Analysis | 5 Steps & Examples
Statistical analysis means investigating trends, patterns, and relationships using quantitative data . It is an important research tool used by scientists, governments, businesses, and other organisations.
To draw valid conclusions, statistical analysis requires careful planning from the very start of the research process . You need to specify your hypotheses and make decisions about your research design, sample size, and sampling procedure.
After collecting data from your sample, you can organise and summarise the data using descriptive statistics . Then, you can use inferential statistics to formally test hypotheses and make estimates about the population. Finally, you can interpret and generalise your findings.
This article is a practical introduction to statistical analysis for students and researchers. We’ll walk you through the steps using two research examples. The first investigates a potential cause-and-effect relationship, while the second investigates a potential correlation between variables.
Table of contents
Step 1: write your hypotheses and plan your research design, step 2: collect data from a sample, step 3: summarise your data with descriptive statistics, step 4: test hypotheses or make estimates with inferential statistics, step 5: interpret your results, frequently asked questions about statistics.
To collect valid data for statistical analysis, you first need to specify your hypotheses and plan out your research design.
Writing statistical hypotheses
The goal of research is often to investigate a relationship between variables within a population . You start with a prediction, and use statistical analysis to test that prediction.
A statistical hypothesis is a formal way of writing a prediction about a population. Every research prediction is rephrased into null and alternative hypotheses that can be tested using sample data.
While the null hypothesis always predicts no effect or no relationship between variables, the alternative hypothesis states your research prediction of an effect or relationship.
- Null hypothesis: A 5-minute meditation exercise will have no effect on math test scores in teenagers.
- Alternative hypothesis: A 5-minute meditation exercise will improve math test scores in teenagers.
- Null hypothesis: Parental income and GPA have no relationship with each other in college students.
- Alternative hypothesis: Parental income and GPA are positively correlated in college students.
Planning your research design
A research design is your overall strategy for data collection and analysis. It determines the statistical tests you can use to test your hypothesis later on.
First, decide whether your research will use a descriptive, correlational, or experimental design. Experiments directly influence variables, whereas descriptive and correlational studies only measure variables.
- In an experimental design , you can assess a cause-and-effect relationship (e.g., the effect of meditation on test scores) using statistical tests of comparison or regression.
- In a correlational design , you can explore relationships between variables (e.g., parental income and GPA) without any assumption of causality using correlation coefficients and significance tests.
- In a descriptive design , you can study the characteristics of a population or phenomenon (e.g., the prevalence of anxiety in U.S. college students) using statistical tests to draw inferences from sample data.
Your research design also concerns whether you’ll compare participants at the group level or individual level, or both.
- In a between-subjects design , you compare the group-level outcomes of participants who have been exposed to different treatments (e.g., those who performed a meditation exercise vs those who didn’t).
- In a within-subjects design , you compare repeated measures from participants who have participated in all treatments of a study (e.g., scores from before and after performing a meditation exercise).
- In a mixed (factorial) design , one variable is altered between subjects and another is altered within subjects (e.g., pretest and posttest scores from participants who either did or didn’t do a meditation exercise).
- Experimental
- Correlational
First, you’ll take baseline test scores from participants. Then, your participants will undergo a 5-minute meditation exercise. Finally, you’ll record participants’ scores from a second math test.
In this experiment, the independent variable is the 5-minute meditation exercise, and the dependent variable is the math test score from before and after the intervention. Example: Correlational research design In a correlational study, you test whether there is a relationship between parental income and GPA in graduating college students. To collect your data, you will ask participants to fill in a survey and self-report their parents’ incomes and their own GPA.
Measuring variables
When planning a research design, you should operationalise your variables and decide exactly how you will measure them.
For statistical analysis, it’s important to consider the level of measurement of your variables, which tells you what kind of data they contain:
- Categorical data represents groupings. These may be nominal (e.g., gender) or ordinal (e.g. level of language ability).
- Quantitative data represents amounts. These may be on an interval scale (e.g. test score) or a ratio scale (e.g. age).
Many variables can be measured at different levels of precision. For example, age data can be quantitative (8 years old) or categorical (young). If a variable is coded numerically (e.g., level of agreement from 1–5), it doesn’t automatically mean that it’s quantitative instead of categorical.
Identifying the measurement level is important for choosing appropriate statistics and hypothesis tests. For example, you can calculate a mean score with quantitative data, but not with categorical data.
In a research study, along with measures of your variables of interest, you’ll often collect data on relevant participant characteristics.

In most cases, it’s too difficult or expensive to collect data from every member of the population you’re interested in studying. Instead, you’ll collect data from a sample.
Statistical analysis allows you to apply your findings beyond your own sample as long as you use appropriate sampling procedures . You should aim for a sample that is representative of the population.
Sampling for statistical analysis
There are two main approaches to selecting a sample.
- Probability sampling: every member of the population has a chance of being selected for the study through random selection.
- Non-probability sampling: some members of the population are more likely than others to be selected for the study because of criteria such as convenience or voluntary self-selection.
In theory, for highly generalisable findings, you should use a probability sampling method. Random selection reduces sampling bias and ensures that data from your sample is actually typical of the population. Parametric tests can be used to make strong statistical inferences when data are collected using probability sampling.
But in practice, it’s rarely possible to gather the ideal sample. While non-probability samples are more likely to be biased, they are much easier to recruit and collect data from. Non-parametric tests are more appropriate for non-probability samples, but they result in weaker inferences about the population.
If you want to use parametric tests for non-probability samples, you have to make the case that:
- your sample is representative of the population you’re generalising your findings to.
- your sample lacks systematic bias.
Keep in mind that external validity means that you can only generalise your conclusions to others who share the characteristics of your sample. For instance, results from Western, Educated, Industrialised, Rich and Democratic samples (e.g., college students in the US) aren’t automatically applicable to all non-WEIRD populations.
If you apply parametric tests to data from non-probability samples, be sure to elaborate on the limitations of how far your results can be generalised in your discussion section .
Create an appropriate sampling procedure
Based on the resources available for your research, decide on how you’ll recruit participants.
- Will you have resources to advertise your study widely, including outside of your university setting?
- Will you have the means to recruit a diverse sample that represents a broad population?
- Do you have time to contact and follow up with members of hard-to-reach groups?
Your participants are self-selected by their schools. Although you’re using a non-probability sample, you aim for a diverse and representative sample. Example: Sampling (correlational study) Your main population of interest is male college students in the US. Using social media advertising, you recruit senior-year male college students from a smaller subpopulation: seven universities in the Boston area.
Calculate sufficient sample size
Before recruiting participants, decide on your sample size either by looking at other studies in your field or using statistics. A sample that’s too small may be unrepresentative of the sample, while a sample that’s too large will be more costly than necessary.
There are many sample size calculators online. Different formulas are used depending on whether you have subgroups or how rigorous your study should be (e.g., in clinical research). As a rule of thumb, a minimum of 30 units or more per subgroup is necessary.
To use these calculators, you have to understand and input these key components:
- Significance level (alpha): the risk of rejecting a true null hypothesis that you are willing to take, usually set at 5%.
- Statistical power : the probability of your study detecting an effect of a certain size if there is one, usually 80% or higher.
- Expected effect size : a standardised indication of how large the expected result of your study will be, usually based on other similar studies.
- Population standard deviation: an estimate of the population parameter based on a previous study or a pilot study of your own.
Once you’ve collected all of your data, you can inspect them and calculate descriptive statistics that summarise them.
Inspect your data
There are various ways to inspect your data, including the following:
- Organising data from each variable in frequency distribution tables .
- Displaying data from a key variable in a bar chart to view the distribution of responses.
- Visualising the relationship between two variables using a scatter plot .
By visualising your data in tables and graphs, you can assess whether your data follow a skewed or normal distribution and whether there are any outliers or missing data.
A normal distribution means that your data are symmetrically distributed around a center where most values lie, with the values tapering off at the tail ends.

In contrast, a skewed distribution is asymmetric and has more values on one end than the other. The shape of the distribution is important to keep in mind because only some descriptive statistics should be used with skewed distributions.
Extreme outliers can also produce misleading statistics, so you may need a systematic approach to dealing with these values.
Calculate measures of central tendency
Measures of central tendency describe where most of the values in a data set lie. Three main measures of central tendency are often reported:
- Mode : the most popular response or value in the data set.
- Median : the value in the exact middle of the data set when ordered from low to high.
- Mean : the sum of all values divided by the number of values.
However, depending on the shape of the distribution and level of measurement, only one or two of these measures may be appropriate. For example, many demographic characteristics can only be described using the mode or proportions, while a variable like reaction time may not have a mode at all.
Calculate measures of variability
Measures of variability tell you how spread out the values in a data set are. Four main measures of variability are often reported:
- Range : the highest value minus the lowest value of the data set.
- Interquartile range : the range of the middle half of the data set.
- Standard deviation : the average distance between each value in your data set and the mean.
- Variance : the square of the standard deviation.
Once again, the shape of the distribution and level of measurement should guide your choice of variability statistics. The interquartile range is the best measure for skewed distributions, while standard deviation and variance provide the best information for normal distributions.
Using your table, you should check whether the units of the descriptive statistics are comparable for pretest and posttest scores. For example, are the variance levels similar across the groups? Are there any extreme values? If there are, you may need to identify and remove extreme outliers in your data set or transform your data before performing a statistical test.
From this table, we can see that the mean score increased after the meditation exercise, and the variances of the two scores are comparable. Next, we can perform a statistical test to find out if this improvement in test scores is statistically significant in the population. Example: Descriptive statistics (correlational study) After collecting data from 653 students, you tabulate descriptive statistics for annual parental income and GPA.
It’s important to check whether you have a broad range of data points. If you don’t, your data may be skewed towards some groups more than others (e.g., high academic achievers), and only limited inferences can be made about a relationship.
A number that describes a sample is called a statistic , while a number describing a population is called a parameter . Using inferential statistics , you can make conclusions about population parameters based on sample statistics.
Researchers often use two main methods (simultaneously) to make inferences in statistics.
- Estimation: calculating population parameters based on sample statistics.
- Hypothesis testing: a formal process for testing research predictions about the population using samples.
You can make two types of estimates of population parameters from sample statistics:
- A point estimate : a value that represents your best guess of the exact parameter.
- An interval estimate : a range of values that represent your best guess of where the parameter lies.
If your aim is to infer and report population characteristics from sample data, it’s best to use both point and interval estimates in your paper.
You can consider a sample statistic a point estimate for the population parameter when you have a representative sample (e.g., in a wide public opinion poll, the proportion of a sample that supports the current government is taken as the population proportion of government supporters).
There’s always error involved in estimation, so you should also provide a confidence interval as an interval estimate to show the variability around a point estimate.
A confidence interval uses the standard error and the z score from the standard normal distribution to convey where you’d generally expect to find the population parameter most of the time.
Hypothesis testing
Using data from a sample, you can test hypotheses about relationships between variables in the population. Hypothesis testing starts with the assumption that the null hypothesis is true in the population, and you use statistical tests to assess whether the null hypothesis can be rejected or not.
Statistical tests determine where your sample data would lie on an expected distribution of sample data if the null hypothesis were true. These tests give two main outputs:
- A test statistic tells you how much your data differs from the null hypothesis of the test.
- A p value tells you the likelihood of obtaining your results if the null hypothesis is actually true in the population.
Statistical tests come in three main varieties:
- Comparison tests assess group differences in outcomes.
- Regression tests assess cause-and-effect relationships between variables.
- Correlation tests assess relationships between variables without assuming causation.
Your choice of statistical test depends on your research questions, research design, sampling method, and data characteristics.
Parametric tests
Parametric tests make powerful inferences about the population based on sample data. But to use them, some assumptions must be met, and only some types of variables can be used. If your data violate these assumptions, you can perform appropriate data transformations or use alternative non-parametric tests instead.
A regression models the extent to which changes in a predictor variable results in changes in outcome variable(s).
- A simple linear regression includes one predictor variable and one outcome variable.
- A multiple linear regression includes two or more predictor variables and one outcome variable.
Comparison tests usually compare the means of groups. These may be the means of different groups within a sample (e.g., a treatment and control group), the means of one sample group taken at different times (e.g., pretest and posttest scores), or a sample mean and a population mean.
- A t test is for exactly 1 or 2 groups when the sample is small (30 or less).
- A z test is for exactly 1 or 2 groups when the sample is large.
- An ANOVA is for 3 or more groups.
The z and t tests have subtypes based on the number and types of samples and the hypotheses:
- If you have only one sample that you want to compare to a population mean, use a one-sample test .
- If you have paired measurements (within-subjects design), use a dependent (paired) samples test .
- If you have completely separate measurements from two unmatched groups (between-subjects design), use an independent (unpaired) samples test .
- If you expect a difference between groups in a specific direction, use a one-tailed test .
- If you don’t have any expectations for the direction of a difference between groups, use a two-tailed test .
The only parametric correlation test is Pearson’s r . The correlation coefficient ( r ) tells you the strength of a linear relationship between two quantitative variables.
However, to test whether the correlation in the sample is strong enough to be important in the population, you also need to perform a significance test of the correlation coefficient, usually a t test, to obtain a p value. This test uses your sample size to calculate how much the correlation coefficient differs from zero in the population.
You use a dependent-samples, one-tailed t test to assess whether the meditation exercise significantly improved math test scores. The test gives you:
- a t value (test statistic) of 3.00
- a p value of 0.0028
Although Pearson’s r is a test statistic, it doesn’t tell you anything about how significant the correlation is in the population. You also need to test whether this sample correlation coefficient is large enough to demonstrate a correlation in the population.
A t test can also determine how significantly a correlation coefficient differs from zero based on sample size. Since you expect a positive correlation between parental income and GPA, you use a one-sample, one-tailed t test. The t test gives you:
- a t value of 3.08
- a p value of 0.001
The final step of statistical analysis is interpreting your results.
Statistical significance
In hypothesis testing, statistical significance is the main criterion for forming conclusions. You compare your p value to a set significance level (usually 0.05) to decide whether your results are statistically significant or non-significant.
Statistically significant results are considered unlikely to have arisen solely due to chance. There is only a very low chance of such a result occurring if the null hypothesis is true in the population.
This means that you believe the meditation intervention, rather than random factors, directly caused the increase in test scores. Example: Interpret your results (correlational study) You compare your p value of 0.001 to your significance threshold of 0.05. With a p value under this threshold, you can reject the null hypothesis. This indicates a statistically significant correlation between parental income and GPA in male college students.
Note that correlation doesn’t always mean causation, because there are often many underlying factors contributing to a complex variable like GPA. Even if one variable is related to another, this may be because of a third variable influencing both of them, or indirect links between the two variables.
Effect size
A statistically significant result doesn’t necessarily mean that there are important real life applications or clinical outcomes for a finding.
In contrast, the effect size indicates the practical significance of your results. It’s important to report effect sizes along with your inferential statistics for a complete picture of your results. You should also report interval estimates of effect sizes if you’re writing an APA style paper .
With a Cohen’s d of 0.72, there’s medium to high practical significance to your finding that the meditation exercise improved test scores. Example: Effect size (correlational study) To determine the effect size of the correlation coefficient, you compare your Pearson’s r value to Cohen’s effect size criteria.
Decision errors
Type I and Type II errors are mistakes made in research conclusions. A Type I error means rejecting the null hypothesis when it’s actually true, while a Type II error means failing to reject the null hypothesis when it’s false.
You can aim to minimise the risk of these errors by selecting an optimal significance level and ensuring high power . However, there’s a trade-off between the two errors, so a fine balance is necessary.
Frequentist versus Bayesian statistics
Traditionally, frequentist statistics emphasises null hypothesis significance testing and always starts with the assumption of a true null hypothesis.
However, Bayesian statistics has grown in popularity as an alternative approach in the last few decades. In this approach, you use previous research to continually update your hypotheses based on your expectations and observations.
Bayes factor compares the relative strength of evidence for the null versus the alternative hypothesis rather than making a conclusion about rejecting the null hypothesis or not.
Hypothesis testing is a formal procedure for investigating our ideas about the world using statistics. It is used by scientists to test specific predictions, called hypotheses , by calculating how likely it is that a pattern or relationship between variables could have arisen by chance.
The research methods you use depend on the type of data you need to answer your research question .
- If you want to measure something or test a hypothesis , use quantitative methods . If you want to explore ideas, thoughts, and meanings, use qualitative methods .
- If you want to analyse a large amount of readily available data, use secondary data. If you want data specific to your purposes with control over how they are generated, collect primary data.
- If you want to establish cause-and-effect relationships between variables , use experimental methods. If you want to understand the characteristics of a research subject, use descriptive methods.
Statistical analysis is the main method for analyzing quantitative research data . It uses probabilities and models to test predictions about a population from sample data.
Is this article helpful?
Other students also liked, a quick guide to experimental design | 5 steps & examples, controlled experiments | methods & examples of control, between-subjects design | examples, pros & cons, more interesting articles.
- Central Limit Theorem | Formula, Definition & Examples
- Central Tendency | Understanding the Mean, Median & Mode
- Correlation Coefficient | Types, Formulas & Examples
- Descriptive Statistics | Definitions, Types, Examples
- How to Calculate Standard Deviation (Guide) | Calculator & Examples
- How to Calculate Variance | Calculator, Analysis & Examples
- How to Find Degrees of Freedom | Definition & Formula
- How to Find Interquartile Range (IQR) | Calculator & Examples
- How to Find Outliers | Meaning, Formula & Examples
- How to Find the Geometric Mean | Calculator & Formula
- How to Find the Mean | Definition, Examples & Calculator
- How to Find the Median | Definition, Examples & Calculator
- How to Find the Range of a Data Set | Calculator & Formula
- Inferential Statistics | An Easy Introduction & Examples
- Levels of measurement: Nominal, ordinal, interval, ratio
- Missing Data | Types, Explanation, & Imputation
- Normal Distribution | Examples, Formulas, & Uses
- Null and Alternative Hypotheses | Definitions & Examples
- Poisson Distributions | Definition, Formula & Examples
- Skewness | Definition, Examples & Formula
- T-Distribution | What It Is and How To Use It (With Examples)
- The Standard Normal Distribution | Calculator, Examples & Uses
- Type I & Type II Errors | Differences, Examples, Visualizations
- Understanding Confidence Intervals | Easy Examples & Formulas
- Variability | Calculating Range, IQR, Variance, Standard Deviation
- What is Effect Size and Why Does It Matter? (Examples)
- What Is Interval Data? | Examples & Definition
- What Is Nominal Data? | Examples & Definition
- What Is Ordinal Data? | Examples & Definition
- What Is Ratio Data? | Examples & Definition
- What Is the Mode in Statistics? | Definition, Examples & Calculator
Table of Contents
Types of statistical analysis, importance of statistical analysis, benefits of statistical analysis, statistical analysis process, statistical analysis methods, statistical analysis software, statistical analysis examples, career in statistical analysis, choose the right program, become proficient in statistics today, what is statistical analysis types, methods and examples.

Statistical analysis is the process of collecting and analyzing data in order to discern patterns and trends. It is a method for removing bias from evaluating data by employing numerical analysis. This technique is useful for collecting the interpretations of research, developing statistical models, and planning surveys and studies.
Statistical analysis is a scientific tool in AI and ML that helps collect and analyze large amounts of data to identify common patterns and trends to convert them into meaningful information. In simple words, statistical analysis is a data analysis tool that helps draw meaningful conclusions from raw and unstructured data.
The conclusions are drawn using statistical analysis facilitating decision-making and helping businesses make future predictions on the basis of past trends. It can be defined as a science of collecting and analyzing data to identify trends and patterns and presenting them. Statistical analysis involves working with numbers and is used by businesses and other institutions to make use of data to derive meaningful information.
Given below are the 6 types of statistical analysis:
Descriptive Analysis
Descriptive statistical analysis involves collecting, interpreting, analyzing, and summarizing data to present them in the form of charts, graphs, and tables. Rather than drawing conclusions, it simply makes the complex data easy to read and understand.
Inferential Analysis
The inferential statistical analysis focuses on drawing meaningful conclusions on the basis of the data analyzed. It studies the relationship between different variables or makes predictions for the whole population.
Predictive Analysis
Predictive statistical analysis is a type of statistical analysis that analyzes data to derive past trends and predict future events on the basis of them. It uses machine learning algorithms, data mining , data modelling , and artificial intelligence to conduct the statistical analysis of data.
Prescriptive Analysis
The prescriptive analysis conducts the analysis of data and prescribes the best course of action based on the results. It is a type of statistical analysis that helps you make an informed decision.
Exploratory Data Analysis
Exploratory analysis is similar to inferential analysis, but the difference is that it involves exploring the unknown data associations. It analyzes the potential relationships within the data.
Causal Analysis
The causal statistical analysis focuses on determining the cause and effect relationship between different variables within the raw data. In simple words, it determines why something happens and its effect on other variables. This methodology can be used by businesses to determine the reason for failure.
Statistical analysis eliminates unnecessary information and catalogs important data in an uncomplicated manner, making the monumental work of organizing inputs appear so serene. Once the data has been collected, statistical analysis may be utilized for a variety of purposes. Some of them are listed below:
- The statistical analysis aids in summarizing enormous amounts of data into clearly digestible chunks.
- The statistical analysis aids in the effective design of laboratory, field, and survey investigations.
- Statistical analysis may help with solid and efficient planning in any subject of study.
- Statistical analysis aid in establishing broad generalizations and forecasting how much of something will occur under particular conditions.
- Statistical methods, which are effective tools for interpreting numerical data, are applied in practically every field of study. Statistical approaches have been created and are increasingly applied in physical and biological sciences, such as genetics.
- Statistical approaches are used in the job of a businessman, a manufacturer, and a researcher. Statistics departments can be found in banks, insurance businesses, and government agencies.
- A modern administrator, whether in the public or commercial sector, relies on statistical data to make correct decisions.
- Politicians can utilize statistics to support and validate their claims while also explaining the issues they address.
Become a Data Science & Business Analytics Professional
- 28% Annual Job Growth By 2026
- 11.5 M Expected New Jobs For Data Science By 2026
Data Analyst
- Industry-recognized Data Analyst Master’s certificate from Simplilearn
- Dedicated live sessions by faculty of industry experts
Data Scientist
- Add the IBM Advantage to your Learning
- 25 Industry-relevant Projects and Integrated labs
Here's what learners are saying regarding our programs:
Gayathri Ramesh
Associate data engineer , publicis sapient.
The course was well structured and curated. The live classes were extremely helpful. They made learning more productive and interactive. The program helped me change my domain from a data analyst to an Associate Data Engineer.

A.Anthony Davis
Simplilearn has one of the best programs available online to earn real-world skills that are in demand worldwide. I just completed the Machine Learning Advanced course, and the LMS was excellent.
Statistical analysis can be called a boon to mankind and has many benefits for both individuals and organizations. Given below are some of the reasons why you should consider investing in statistical analysis:
- It can help you determine the monthly, quarterly, yearly figures of sales profits, and costs making it easier to make your decisions.
- It can help you make informed and correct decisions.
- It can help you identify the problem or cause of the failure and make corrections. For example, it can identify the reason for an increase in total costs and help you cut the wasteful expenses.
- It can help you conduct market analysis and make an effective marketing and sales strategy.
- It helps improve the efficiency of different processes.
Given below are the 5 steps to conduct a statistical analysis that you should follow:
- Step 1: Identify and describe the nature of the data that you are supposed to analyze.
- Step 2: The next step is to establish a relation between the data analyzed and the sample population to which the data belongs.
- Step 3: The third step is to create a model that clearly presents and summarizes the relationship between the population and the data.
- Step 4: Prove if the model is valid or not.
- Step 5: Use predictive analysis to predict future trends and events likely to happen.
Although there are various methods used to perform data analysis, given below are the 5 most used and popular methods of statistical analysis:
Mean or average mean is one of the most popular methods of statistical analysis. Mean determines the overall trend of the data and is very simple to calculate. Mean is calculated by summing the numbers in the data set together and then dividing it by the number of data points. Despite the ease of calculation and its benefits, it is not advisable to resort to mean as the only statistical indicator as it can result in inaccurate decision making.
Standard Deviation
Standard deviation is another very widely used statistical tool or method. It analyzes the deviation of different data points from the mean of the entire data set. It determines how data of the data set is spread around the mean. You can use it to decide whether the research outcomes can be generalized or not.
Regression is a statistical tool that helps determine the cause and effect relationship between the variables. It determines the relationship between a dependent and an independent variable. It is generally used to predict future trends and events.
Hypothesis Testing
Hypothesis testing can be used to test the validity or trueness of a conclusion or argument against a data set. The hypothesis is an assumption made at the beginning of the research and can hold or be false based on the analysis results.
Sample Size Determination
Sample size determination or data sampling is a technique used to derive a sample from the entire population, which is representative of the population. This method is used when the size of the population is very large. You can choose from among the various data sampling techniques such as snowball sampling, convenience sampling, and random sampling.
Everyone can't perform very complex statistical calculations with accuracy making statistical analysis a time-consuming and costly process. Statistical software has become a very important tool for companies to perform their data analysis. The software uses Artificial Intelligence and Machine Learning to perform complex calculations, identify trends and patterns, and create charts, graphs, and tables accurately within minutes.
Look at the standard deviation sample calculation given below to understand more about statistical analysis.
The weights of 5 pizza bases in cms are as follows:
Calculation of Mean = (9+2+5+4+12)/5 = 32/5 = 6.4
Calculation of mean of squared mean deviation = (6.76+19.36+1.96+5.76+31.36)/5 = 13.04
Sample Variance = 13.04
Standard deviation = √13.04 = 3.611
A Statistical Analyst's career path is determined by the industry in which they work. Anyone interested in becoming a Data Analyst may usually enter the profession and qualify for entry-level Data Analyst positions right out of high school or a certificate program — potentially with a Bachelor's degree in statistics, computer science, or mathematics. Some people go into data analysis from a similar sector such as business, economics, or even the social sciences, usually by updating their skills mid-career with a statistical analytics course.
Statistical Analyst is also a great way to get started in the normally more complex area of data science. A Data Scientist is generally a more senior role than a Data Analyst since it is more strategic in nature and necessitates a more highly developed set of technical abilities, such as knowledge of multiple statistical tools, programming languages, and predictive analytics models.
Aspiring Data Scientists and Statistical Analysts generally begin their careers by learning a programming language such as R or SQL. Following that, they must learn how to create databases, do basic analysis, and make visuals using applications such as Tableau. However, not every Statistical Analyst will need to know how to do all of these things, but if you want to advance in your profession, you should be able to do them all.
Based on your industry and the sort of work you do, you may opt to study Python or R, become an expert at data cleaning, or focus on developing complicated statistical models.
You could also learn a little bit of everything, which might help you take on a leadership role and advance to the position of Senior Data Analyst. A Senior Statistical Analyst with vast and deep knowledge might take on a leadership role leading a team of other Statistical Analysts. Statistical Analysts with extra skill training may be able to advance to Data Scientists or other more senior data analytics positions.
Supercharge your career in AI and ML with Simplilearn's comprehensive courses. Gain the skills and knowledge to transform industries and unleash your true potential. Enroll now and unlock limitless possibilities!
Program Name AI Engineer Post Graduate Program In Artificial Intelligence Post Graduate Program In Artificial Intelligence Geo All Geos All Geos IN/ROW University Simplilearn Purdue Caltech Course Duration 11 Months 11 Months 11 Months Coding Experience Required Basic Basic No Skills You Will Learn 10+ skills including data structure, data manipulation, NumPy, Scikit-Learn, Tableau and more. 16+ skills including chatbots, NLP, Python, Keras and more. 8+ skills including Supervised & Unsupervised Learning Deep Learning Data Visualization, and more. Additional Benefits Get access to exclusive Hackathons, Masterclasses and Ask-Me-Anything sessions by IBM Applied learning via 3 Capstone and 12 Industry-relevant Projects Purdue Alumni Association Membership Free IIMJobs Pro-Membership of 6 months Resume Building Assistance Upto 14 CEU Credits Caltech CTME Circle Membership Cost $$ $$$$ $$$$ Explore Program Explore Program Explore Program
Hope this article assisted you in understanding the importance of statistical analysis in every sphere of life. Artificial Intelligence (AI) can help you perform statistical analysis and data analysis very effectively and efficiently.
If you are a science wizard and fascinated by the role of AI in statistical analysis, check out this amazing Caltech Post Graduate Program in AI & ML course in collaboration with Caltech. With a comprehensive syllabus and real-life projects, this course is one of the most popular courses and will help you with all that you need to know about Artificial Intelligence.
Our AI & Machine Learning Courses Duration And Fees
AI & Machine Learning Courses typically range from a few weeks to several months, with fees varying based on program and institution.
Get Free Certifications with free video courses
Data Science & Business Analytics
Introduction to Data Analytics Course
Introduction to Data Science
Learn from Industry Experts with free Masterclasses
Ai & machine learning.
Gain Gen AI expertise in Purdue's Applied Gen AI Specialization
Unlock Your Career Potential: Land Your Dream Job with Gen AI Tools
Make Your Gen AI & ML Career Shift in 2024 a Success with iHUB DivyaSampark, IIT Roorkee
Recommended Reads
Free eBook: Guide To The CCBA And CBAP Certifications
Understanding Statistical Process Control (SPC) and Top Applications
A Complete Guide on the Types of Statistical Studies
Digital Marketing Salary Guide 2021
What Is Data Analysis: A Comprehensive Guide
A Complete Guide to Get a Grasp of Time Series Analysis
Get Affiliated Certifications with Live Class programs
- PMP, PMI, PMBOK, CAPM, PgMP, PfMP, ACP, PBA, RMP, SP, and OPM3 are registered marks of the Project Management Institute, Inc.

Statistical Papers
Statistical Papers is a forum for presentation and critical assessment of statistical methods encouraging the discussion of methodological foundations and potential applications.
- The Journal stresses statistical methods that have broad applications, giving special attention to those relevant to the economic and social sciences.
- Covers all topics of modern data science, such as frequentist and Bayesian design and inference as well as statistical learning.
- Contains original research papers (regular articles), survey articles, short communications, reports on statistical software, and book reviews.
- High author satisfaction with 90% likely to publish in the journal again.
- Werner G. Müller,
- Carsten Jentsch,
- Shuangzhe Liu,
- Ulrike Schneider

Latest issue
Volume 65, Issue 2
Latest articles
A high-dimensional single-index regression for interactions between treatment and covariates.
- Thaddeus Tarpey
- R. Todd Ogden

Flexible-dimensional L-statistic for mean estimation of symmetric distributions
- Diego García-Zamora
- Luis Martínez

Matrix-variate generalized linear model with measurement error

Some practical and theoretical issues related to the quantile estimators
- Dagmara Dudek
- Anna Kuczmaszewska
A sequential feature selection approach to change point detection in mean-shift change point models
- Baolong Ying

Journal updates
Write & submit: overleaf latex template.
Overleaf LaTeX Template
Journal information
- Australian Business Deans Council (ABDC) Journal Quality List
- Current Index to Statistics
- Google Scholar
- Japanese Science and Technology Agency (JST)
- Mathematical Reviews
- Norwegian Register for Scientific Journals and Series
- OCLC WorldCat Discovery Service
- Research Papers in Economics (RePEc)
- Science Citation Index Expanded (SCIE)
- TD Net Discovery Service
- UGC-CARE List (India)
Rights and permissions
Springer policies
© Springer-Verlag GmbH Germany, part of Springer Nature
- Find a journal
- Publish with us
- Track your research

Why is Statistical Analysis an Important Part of Research?

You’ve probably heard the old joke about statistics: “Torture the numbers long enough, and they’ll say whatever you want them to say.” Statistics are quoted daily in the news media, yet often these quotes are poorly explained or fail to provide context or analysis. Misrepresentation of statistics is no reason to avoid using statistical analysis in research.
On the contrary, it makes an even stronger argument for including statistical analysis in research and academic studies. High-quality statistical analysis in research is vital to making it clear what the importance of the research is and helping future researchers build on your work. It can also make it easier for laypersons to understand the significance of complex academic research. Let’s talk about what statistical analysis in research is, and explore why it is such a critical tool to help us understand our world better, from science and medicine to humanities and social sciences.
What is Statistical Analysis in Research?
The word statistics, simply put, describes a range of methods for collecting, arranging, analyzing, and reporting quantitative data. Because statistics focuses on quantitative data, data in this case is usually in the form of numbers. So, we can understand statistical analysis in research as a systematic, proven approach to analyzing numerical data so that we can maximize our understanding of what the numbers are telling us. It’s a numerical form of analysis in a research paper.
We use statistical analysis in research for several reasons. The main reason is that while it is often impossible to study all of a target set, it is usually possible to study a small sample of that set. Whether you want to examine humans, animals, plants, rocks, stars, or atoms, a small subset is often all that is available and possible to study within the time and budgetary constraints that researchers must work under. However, the chances that a random sample will be representative of the whole are not high. There is always going to be some variation. How do researchers handle this type of variation when trying to understand data collected from samples? You guessed it -- through statistical analysis. Statistical analysis methods account for variation and differences, and that is why they are so critical to performing high quality research analysis in your paper.
Collecting and analyzing data through statistics is a really important part of furthering our understanding of everything from human behavior to the universe itself. One reason for this is that our assumptions about the world around us are often based on personal experience and anecdotes, but we as individuals lack a broad perspective. It takes time, dedication, and careful work to really understand what is happening in the world around us. Much of what we know and understand about the world today is thanks to hardworking researchers performing statistical analysis in research. Without this research analysis, it would be a lot harder to know what’s going on around us.
Are Statistics Always Useful?
Statistics can be, but are not always, useful. We can determine whether statistics are helpful by looking at the quality of the data gathered and the quality and rigor of the statistical analysis performed. Great statistical analysis is worthless if the data itself is poorly collected or not representative. And even the best data can mislead us when the statistical analysis performed is poor and leads to erroneous results.
It is a sad fact that some writers and researchers who use obscure or inappropriate statistical methodology as a way to get the results they are aiming for, or who fail to provide clear statistical analysis and therefore mislead their readers about the significance of their data. Poor use of statistical methods have led to a replication crisis in science, where the results of many studies cannot be reproduced. Some have argued that this replication crisis is because while collecting data has become much easier in recent decades, scientists and researchers are not adequately trained in statistical analysis in research. The replication crisis has led to falling public trust in science, the consequences of which became sharply clear during the COVID-19 pandemic.
How Can You Improve Statistical Analysis in Your Research?
So how can you avoid misusing data and producing poor quality statistical analysis in your research paper? The first way is to make sure you have a solid understanding of the scientific method. Create a hypothesis, test that hypothesis, and then analyze what happened. Be familiar with the ways that people use and misuse statistical analysis in research to present false conclusions. This will help you improve the quality of your own work and increase your ability to identify and call out poorly performed research analysis in academic papers.
Finally, don’t be afraid to reach out for assistance with your work. You don’t need to spend hours in front of your computer trying to perform statistical analysis with software and statistical analysis methods you don’t fully understand. There are services available to help you turn your data and methods into easy-to-understand results - and they are legitimate services that help researchers perform technical work without interfering their research. These services can do everything from recommending what statistical model you should use to verifying your data. They can also perform full statistical analysis in your research paper for you. Don’t worry about becoming a casualty of poor quality statistical analysis ever again - use the statistical analysis services available to you!
Our English Editing Services

Top Impact Scientific Editing New
Top Impact Scientific Editing Service offers in-depth scientific and developmental

Substantive Editing Popular
Substantive Editing, our popular flagship service, is specifically tailored for quality.

Copy Editing
Our professional Copy Editing service is designed for quality-conscious authors.
Our Experts

Similar Articles

7 Proven Strategies to Proofread Effectively

How are Editing, Copy Editing & Proofreading Different?

Editing vs. Copyediting: What's the Difference?

What Are The Different Types of Book Editing?

What Are the Steps in Editing a Document?

Substantive Editing vs. Copyediting: What's the Difference?

Why Copy Editing is Important

A Step-by-Step Guide to Journal Editing

We use cookies to offer you a personalized experience. By continuing to use this website, you consent to the use of cookies in accordance with our Cookie Policy.
- Correspondence
- Open access
- Published: 25 April 2012
An evaluation of the quality of statistical design and analysis of published medical research: results from a systematic survey of general orthopaedic journals
- Nick R Parsons 1 ,
- Charlotte L Price 2 ,
- Richard Hiskens 3 ,
- Juul Achten 1 &
- Matthew L Costa 1
BMC Medical Research Methodology volume 12 , Article number: 60 ( 2012 ) Cite this article
12k Accesses
30 Citations
2 Altmetric
Metrics details
The application of statistics in reported research in trauma and orthopaedic surgery has become ever more important and complex. Despite the extensive use of statistical analysis, it is still a subject which is often not conceptually well understood, resulting in clear methodological flaws and inadequate reporting in many papers.
A detailed statistical survey sampled 100 representative orthopaedic papers using a validated questionnaire that assessed the quality of the trial design and statistical analysis methods.
The survey found evidence of failings in study design, statistical methodology and presentation of the results. Overall, in 17% (95% confidence interval; 10–26%) of the studies investigated the conclusions were not clearly justified by the results, in 39% (30–49%) of studies a different analysis should have been undertaken and in 17% (10–26%) a different analysis could have made a difference to the overall conclusions.
It is only by an improved dialogue between statistician, clinician, reviewer and journal editor that the failings in design methodology and analysis highlighted by this survey can be addressed.
Peer Review reports
Statistics is an essential component of medical research from design initiation to project reporting, and it influences all aspects of the research process from data collection and management to analysis and interpretation. The application of statistics to medical sciences, and particularly in our area of interest, trauma and orthopaedic surgery, has become more widespread and complex. However, there is considerable evidence, both anecdotal and in the literature [ 1 ], of poor reporting and use of statistical methods in orthopaedics papers. Although our experience providing statistical support more widely in medicine leads us to suspect that similar opinions, about the quality of both design and statistical analysis, exists within many other medical disciplines. So our selection of general orthopaedic journals is not solely to highlight particularly bad practice in this discipline, as we suspect much of what we report here is generally applicable to research across all disciplines, and as such orthopaedic publications simply provide an exemplar of this larger population. In an attempt to quantify the extent of poor reporting and use of statistical methods, Parsons et al. [ 2 ] undertook a large survey of the orthopaedic literature to assess both the quality of reporting and the appropriate and correct use of statistical methods. The first part of this study found major deficiencies in reporting, with 59% (95% confidence interval; 56–62%) and 58% (56–60%) compliance with CONSORT [ 3 ] and STROBE [ 4 ] guidelines, and commented on differences between journals and paper types [ 2 ]. In the second part of the study, the quality of statistical analysis methods was assessed using a detailed questionnaire which was completed for a random sample of orthopaedics papers by two experienced statisticians. The results of this survey are discussed in detail here.
A random sample of 100 papers from the general orthopaedic literature was obtained and included 27 randomized controlled trials (RCTs), 30 case–control (CC) studies, 16 longitudinal (L) studies and 27 cross-sectional (CS) studies. The sample was stratified by study type to ensure accurate representation of each of the four types of study and additional inclusion criteria were as follows:
Published research papers from seven general orthopaedic journals [ 5 ] covering a range of impact factors [ 6 ]; Journal of Bone and Joint Surgery (American), Clinical Orthopaedics and Related Research, Journal of Bone and Joint Surgery (British), Acta Orthopaedica, Archives of Orthopaedic and Trauma Surgery, International Orthopaedics and BMC Musculoskeletal Disorders
Original research only – excluding trial protocols, reviews, meta-analyses, short reports, communications and letters
Published between 1 st January 2005 and 1 st March 2010 (study start date)
No more than one paper from any single research group
Papers published by research groups based at our own institutes were excluded to avoid assessment bias
Full details of the search strategy and methods used to collect the sample are provided by Parsons et al. [ 2 ].
The statistical quality of each paper was assessed using a validated questionnaire [ 7 ], which was adapted to reflect the specific application to orthopaedic research [ 2 ]. After randomly numbering the papers from 1 to 100, each paper was read and independently assessed using the questionnaire by two experiences statisticians (NP and CP). Even numbered papers were read by NP and odd numbered papers were read by CP. The questionnaire was divided into two parts. Part one captured data describing the type of study, population under study, design, outcome measures and the methods of statistical analysis and the results of this were reported in Parsons et al. [ 2 ]. A random sample of 16 papers from the original 100, stratified by study type to ensure balance, was selected and read by both statisticians to assess the level of agreement between the two reviewers for individual items on part one of the questionnaire. Parsons et al. [ 2 ] reported kappa statistics in the range 0.76 to 1.00 with a mean of 0.96 suggesting good agreement between the reviewers for this more objective part of the survey. The second part of the questionnaire required generally more subjective assessments concerning the presentation of data and the quality and appropriateness of the statistical methods used (see Additional file 1 for questionnaire details). The results of this part are reported in detail here. The survey allowed a detailed investigation of issues such as the description of the sample size calculation, missing data, the use of blinding in trials, the experimental unit, multiple testing and presentation of results.
The correctness, robustness, efficiency and relevance [ 7 ] of the statistical methods reported in the sample papers were assessed using a yes or no assignment for each characteristic. Correctness refers to whether the statistical method was appropriate. For instance, it is not correct to use an unpaired t -test to compare an outcome from baseline to the trial endpoint for a single group of patients. Many statistical methods rely on a number of assumptions (e.g. normality, independence etc.); if those assumptions are incorrect, the selected method can produce misleading results. In this context we would describe the selected methods as lacking robustness . A statistical method was rated as inefficient if, for example, a nonparametric rather than a parametric method was used for an analysis where data conformed to a known distribution (e.g. using a Mann–Whitney test, rather than a t -test). Finally, an analysis was regarded as relevant if it answered the question posed in the study. For instance, a principal components analysis may be correct and efficient for summarising a multivariate dataset, but may have no bearing on the stated aim of a paper.
The majority of the survey items were objective assessments of quality, e.g. an incorrect method of analysis was used, with a small number of more subjective items, e.g. could a different analysis make a difference to the conclusions?
The outcomes of part two of the statistical questionnaire are summarized in the following three subsections covering study design, statistical methods and the presentation of results.
Study design
A number of key themes emerged from the analysis of the questionnaire data. Foremost amongst these were the description of the study design, identification of the experimental unit, details of the sample size calculation, the handling of missing data and blinding for subjective measures. These topics are discussed individually below.
Experimental unit
The experimental unit is a physical object which can be assigned to a treatment or intervention. In orthopaedics research, it is often an individual patient. However, other possibilities include things such as a surgeon, a hip or a knee. The experimental unit is the unit of statistical analysis and, for simple study designs, it is synonymous with the data values, i.e. there is a single outcome measure for each experimental unit. For more complex designs, such as repeated measures, there may be many data values for each experimental unit. Failure to correctly identify the experimental unit is a common error in medical research, and often leads to incorrect inferences from a study [ 1 , 8 ].
The experimental unit was not identified correctly in 23% (15–33%; 95% confidence interval based on normal approximation to binomial) of the sampled studies. Of the 77 papers that correctly identified the experimental unit, 86% (75–92%) correctly summarised the data by patient. By far the most common reason for incorrect identification of the experimental unit was confusion between limbs and individual patients when analysing and reporting results. For example, one paper reported data for 100 patients but summarised outcomes for 120 feet, whereas another reported patient pain scores after surgery for both left and right ankles on some patients and single ankles for other patients. Failure to identify the correct experimental unit can lead to ‘dependencies’ in data. For example, outcome measures made on left and right hips for the same patient will be correlated, but outcome measures between individual hips from two different patients will be uncorrelated. Only one paper, where data were available from one or both legs for patients, identified this as an important issue and the authors decided to use what we would regard as an inappropriate strategy by taking the mean of the two values as the outcome for bilateral patients. Almost all of the statistical analyses reported in these studies (e.g. t-tests, ANOVA, regression) are based on an assumption that outcome data (formally the residuals) are uncorrelated; if this is not the case then the reported inferences are unlikely to be valid.
Sample size
The size of the sample used in a study, i.e. the number of experimental units (usually patients), largely determines the precision of estimates of study population characteristics such as means and variances. That is, the number of patients in the study determines how confidently we can draw inferences from the results of that study and use them to inform decisions about the broader population of patients with that particular condition or problem. In clinical trials, a pre-study power analysis is usually used to estimate the sample size [ 9 ], although methods are available for many other study types [ 10 ]. It is particularly important for RCTs, where specific null hypotheses are tested, that a clear description of the methodology and rationale for choosing a sample size is given. For example, the outcome is assumed to be normally distributed, treatment group differences will be assessed using a t -test, the power to detect a defined clinically meaningful difference is set to at least 80% and the type I error rate, or significance level, is set to 5%.
The sample size was not justified in the Methods section for 19% (7–39%) of the 27 papers describing RCTs. A specific calculation, with sufficient details to allow the reader to judge the validity, was not given for 30% (14–50%) of RCTs. These studies often simply described the sample size in vague terms, for instance "…based on a priori sample size estimation, a total of 26 patients were recruited…" . For 3 papers reporting RCTs, the validity of the sample size calculation was questionable, for 3 papers there was a lack of clearly stated assumptions and in 2 papers the calculation was simply not credible. For example, one paper gave sparse details about the population variance, minimum clinically important difference and required power which resulted in a recruitment target of 27 patients for a two arm trial. For purely practical reasons one would always want an even number of patients in a two arm trial. In another paper, 400 patients were recruited to a study, based on a vague description about how this number was arrived at, and exactly 200 patients were randomly allocated to each of two treatment groups. A cynical reader might question the likelihood of such an exact split of patients between treatment groups; there is only a 1 in 25 chance of an exact split for a simple 1 to 1 randomization. However, this might simply be a case of poor reporting, where in reality blocking or minimization were used to equalise numbers in the treatment arms, thus giving more credence to the description of the design. For the 73 observational studies, only 34% (24–46%) justified the sample size, that is there was some discussion in the paper on how the sample size was arrived at; this was often minimal, for instance a simple statement that the number of patients required to answer the research question was the number of patients who were available at the time of study, or those who accepted an invitation to participate (e.g. "…all patients were invited to join the study…" ).
Missing data
Missing data are observations that were intended to be made but were not made [ 11 ]; the data may be missing for unexpected reasons (e.g. patient withdrawal from a study), or intentionally omitted or not collected. It is important to carefully document why data are missing in the study design when reporting. If data can be considered to be missing at random, then valid inferences can still be made. However, if values are missing systematically, then it is more dangerous to draw conclusions from that study. For example, if in a clinical trial comparing different types of hip replacement all of the missing data occurs in one particular arm of the trial, the remaining data is unlikely to be representative of the overall result in that group of patients; the missing data may be because those patients went to another hospital for their revision surgery.
Data were missing, either for a complete unit or a single observation, in 34% (25–44%) of the papers, of these 34 papers only 62% (44–77%) documented and explained the reasons for this. An audit of the data reported in each paper allowed the statistical assessors to identify 13 papers (13% of the total sample) where data were missing with no explanation. Data missingness was generally inferred from the numbers reported in the results being less than those reported in the methods, with no explanation or reason offered by the authors of the study. In the 34 papers reporting missing data, 28 based the analysis on complete cases, 2 imputed missing data and for the remaining 4 papers it was unclear as to what methodology was used.
Subjective assessments and blinding
Many orthopaedic studies report subjective assessments, such as a pain or a functional score after surgery or a radiological assessment of the quality of a scan. To reduce the risk of bias for these kinds of assessments it is desirable, where possible, to ‘blind’ the assessor to the treatment groups to which the patient was allocated.
Subjective assessments were undertaken in 16 of the 27 RCTs (59%; 95% CI 39–77%) and in 6 of these studies (38%; 95% CI 16–64%), the assessments were not done blind and no explanation was given as to why this was not possible.
Statistical methods
Statistical methods should always be fully described in the methods section of a paper and only the statistics described in the methods should be reported in the results section. In 20% (13–29%) of the papers in our survey, statistical methods not previously stated in the methods section were reported in the results section [ 2 ]. In addition to the poor reporting of the methods used, a number of specific issues were identified.
Analysis methods
The most commonly reported statistical methods were chi-squared (χ 2 ) and Fisher’s exact tests (47%; 95% CI 37–57%), t-tests (45%; 95% CI 35–55%), regression analysis (33%; 95% CI 24–43%) and Mann–Whitney tests (28%; 95% CI 20–38%). The selection of an appropriate method of analysis is crucial to making correct inferences from study data.
In 52% (32–71%) of papers where a Mann–Whitney, Wilcoxon rank sum or Wilcoxon signed rank test was used, the analysis was considered to be inefficient and the reported analysis was only considered to be correct 70% (50–86%) of the time. The t -test was used inappropriately, with a lack of robustness, in 26% (14–41%) of papers and in an equivalent proportion of papers (26%; 95% CI 14–41%) it was reported in such a way as to be irrelevant to the stated aims of the paper. This lack of relevance was, on occasion, due to method selection such as the choice between a parametric and a nonparametric test, but more often was simply a result of poor reporting and lack of clarity in the description. Many papers reported a list of the statistical tools used in the analysis, but in the results gave only short statements such as “A was better than B (p = 0.03)” with no details as to which test was used to obtain the p-value; so-called ‘orphan’ p-values [ 12 ]. It was therefore impossible to assess whether the correct test was used for the relevant comparison.
Seven papers (7%; 95% CI 3–14%) reported clear methodological errors in the analysis. Two papers wrongly used the Wilcoxon signed-rank test to compare independent samples and another paper used an independent samples t -test where a paired test should have been used. One paper committed the reverse error of using a paired t -test to compare cases and controls in an unpaired case–control study and another paper used a t -test to compare differences in proportions rather than, for instance, a χ 2 test. Another study calculated the arithmetic mean of a number of percentages, all based on different denominator populations. And finally, one study outlined reasons for conducting a non-parametric analysis in the methods only to later report an analysis of covariance, a parametric method of analysis based on assumptions of normality.
Parametric versus non-parametric tests
Parametric statistical tests assume that data come from a probability distribution with a known form. That is, the data from the study can be described by a known mathematical model; the most widely used being the normal distribution. Such tests make inferences about the parameters of the distribution based on estimates obtained from the data. For example, the arithmetic mean and variance are parameters of the normal distribution measuring location and spread respectively. Non-parametric tests are often used in place of parametric tests when the assumptions necessary for the parametric method do not hold; for instance the data might be more variable or more skewed than expected. However, if the assumptions are (approximately) correct, parametric methods should be used in preference to non-parametric methods as they provide more accurate and precise estimates, and greater statistical power [ 13 ].
Many of the papers in this survey showed no clear understanding of the distinction between these types of tests, evidenced by reporting that made no statistical sense: e.g. "…continuous variables were determined to be parametric using Kolmogorov-Smirnov tests…" , "…the t-test was used for parametric variances…" , "…non-parametric statistics were used to compare outcome measures between groups (one way ANOVA)…" and "…Student's t-test and the Mann–Whitney test were used to analyse continuous data with and without normal distribution…" . Continuous variables may be assumed to be approximately normal in an analysis, but it makes no sense to describe variables or variances as parametric. It is also incorrect to label an analysis of variance (ANOVA) as non-parametric. In at least 5 papers (5%; 95% CI 2–12%), the authors opted to use non-parametric statistical methods, but then summarised data in tables and figures using means and standard deviations, the parameters of the normal distribution, rather than correctly using medians and ranges or inter-quartile ranges.
The survey showed that 52% (42–62%) of papers used non-parametric tests inefficiently; that is they reported the results of non-parametric tests for outcomes that evidence from the paper suggested were approximately normal. Three papers (3%; 95% CI 0–9%) compared the lengths of time to an outcome event between groups by using the non-parametric Mann–Whitney (M-W) test based on converting the times to ranks. By doing this, much of the information about real differences between individual records is lost; for example outcomes of 1 day, 2 days and 100 days become 1, 2 and 3 when converted to ranks. Although times are often positively skewed, they are usually approximately normally distributed after logarithmic transformation [ 14 ]. A more efficient analysis can therefore usually be achieved by using a t -test on log-transformed times rather than applying a M-W test to untransformed data. This is not to say that non-parametric tests should never be used, but that for many variable types (e.g. times, areas, volumes, ratios or percentages) there are simple and well-known transformations that can be used to force the data to conform more closely to the assumptions required for parametric analysis, such as normality or equality of variances between treatment groups.
Multiple comparisons
Problems of multiple comparisons, or multiple testing, occur when considering the outcomes of more than one statistical inference simultaneously. In the context of this survey, it is best illustrated by considering a number of reported statistical tests for one study all reporting evidence for significance at the 5% level. By definition, if one undertakes 20 hypothesis tests on data where we know that there is no true difference, we will expect to see one significant result at the 5% level by chance alone. Therefore, if we undertake multiple tests, we require a stronger level of evidence to compensate for this. For example, the Bonferroni correction preserves the ‘familywise error rate’ (α), or the probability of making one or more false discoveries, by requiring that each of n tests should be conducted at the α/n level of significance, i.e. it adjusts the significance level to account for multiple comparisons [ 15 ].
The questionnaire recorded the number of hypotheses tested in each paper, based on an approximate count of the number of p-values reported. Three papers did not report p-values, 31 papers (31%; 95% CI 22–41%) reported less than 5 p-values, 36 papers (36%; 95% CI 27–46%) reported between 5 and 20 p-values and 30 papers (30%; 95% CI 21–40%) reported more than 20 p-values. Issues of the relevance and the need for formal adjustment for multiple comparisons will clearly be very problem specific [ 16 ]. Whilst most statisticians would concede that the formal adjustment of p-values to account for multiple comparisons may not necessarily be required when reporting a small number of hypothesis tests, if reporting more than 20 p-values from separate analyses, some discussion of the rationale and need for so many statistical tests should be provided and formal adjustment for multiple-comparison considered. In an extreme case, one paper reported a total of 156 p-values without considering the effect of this on inferences from the study. A Bonferroni correction to the significance level would have resulted in at least 21 of the 35 reported significant p-values in this study to be regarded as no longer significant. Where some adjustment was made for multiple comparisons (7 papers), the Bonferroni correction was the most common method (5 papers). One other paper used Tukey’s Honestly Significant Difference (HSD) test and another set the significance level to 1% (rather than 5%) in an ad-hoc manner to account for undertaking 10 tests.
Presentation of results
The clear and concise presentation of results, be it the labelling of tables and graphs or the terminology used to describe a method of analysis or a p-value, is an important component of all research papers. The statistical assessment of the study papers identified two important presentational issues.
Graphs and tables
The statistical assessors were asked to comment on the quality of the data presentation in the papers which included tables and graphs. Graphs and tables were clearly titled in only 29% (21–39%) of papers. For instance, typical examples of uninformative labels included “Table I: Details of Study” and “Table II: Surgical Information” . Furthermore, only 43% of graphs and tables were considered to be clearly labelled. In particular, a number of the papers included tables with data in parentheses without further explanation. The reader was then left to decide whether the numbers indicated, for example, 95% confidence intervals, inter-quartile ranges (IQRs) or ranges. Some tables also included p-values with no indication of the statistical test used. The description of graphical displays was occasionally confusing. One paper stated that the bars of a box-and-whisker plot represented the maximum and minimum values in a dataset, when there were clearly points outside the bars. By convention, the bars represent 1.5 times the inter-quartile range, with points outside the bars identified as ‘outliers’. Interestingly, another paper claimed that the boxes showed standard deviations, rather than the correct IQR, so there is clearly a wider misunderstanding of these figures.
Raw data for individual patients (or experimental units) were displayed graphically or in tables in only 9% (4–17%) of papers. Raw data, as opposed to means, medians or other statistics, always provide the simplest and clearest summary of a study, and direct access to the data for the interested reader. Although we accept that there may be practical reasons why authors would not want to present such data, it is disappointing that such a small proportion of investigators decided to do so.
Terminology
The lack of appropriate statistical review, either prior to submission or at the review stage, was apparent in the catalogue of simple statistical reporting errors found in these papers. For instance, methods were reported that, to our knowledge, do not exist: e.g. "multiple variance analysis" or the “least squares difference" post-hoc test after ANOVA. Presumably the latter refers to a least significant difference test, but the former is ambiguous. Another class of reporting error were those that simply made no statistical sense in the context they were reported: e.g. "…there was no difference in the incidence among the corresponding groups (chi-squared test, p = 0.05)…" , and "…there were no significant differences in the mean T-score or Z-score between the patients and controls…" . The former remark was made in the context of rejecting the null hypothesis at the 5% level for significance and the latter presumably implied that mean t-statistics and z-scores were compared between groups, which makes no statistical sense. The inadequate or poor reporting of p-values was also widespread, and typical errors included "p < 0.000009" , “p < 0.134” and, more generally, the use of “p = NS” or “p < 0.05” . P-values should generally be quoted to no more than 3 decimal places, and be exact (as opposed to an inequality e.g. p < 0.05), unless very small when p < 0.001 is acceptable.
A number of key issues have emerged from our survey of 100 papers investigating the quality of statistical analysis and design in trauma and orthopaedic research. These points are summarised below with recommendations for improvement.
It is important that authors clearly identify the experimental unit when reporting. This was a source of confusion for 23% (95% CI 15–33%) of the papers in our survey and reflects a fundamental lack of understanding. If no attempt is made to modify the analysis to account for data dependencies, researchers should at least state that they are making an assumption of (approximate) independence between multiple observations for the same unit (e.g. functional outcomes from the left and right side for the same individual after a bilateral procedure). This then at least allows the reader to decide whether the assumption is reasonable in the context of the study.
Where specific hypotheses are being tested, for instance in an RCT, the sample size should be discussed, and usually a power calculation reported with sufficient details to allow one to verify the reported sample size. In this survey, 30% (14–50%) of the RCTs gave no such calculation and 19% (7–39%) of them provided no justification for the sample size in the methods section. A clear description of the methodology used for sample size determination (e.g. power level, significance) and the design used (e.g. randomization) is critical for judging the quality of research. However, in studies where a sample size calculation may not be relevant, for example if the research is exploratory or researchers have access to a limited number of participants, authors should provide an open discussion of this in the methods section.
The lack of a clear explanation offered by a number of the papers in this survey for missing data goes hand-in-hand with the poor reporting of patient numbers. Parsons et al. [ 2 ] showed that only 57% of these papers stated exact numbers of patients in both the methods and results sections. RCTs should report a (CONSORT-style [ 3 ]) flowchart documenting exactly what happened to all of the participants in the trial, and all studies should state how and why any patient data was missing or excluded from the analysis. Furthermore, all studies should state the size of sample used to estimate parameters, as a way of explicitly stating whether all or only partial information was available for inference.
It is important for the credibility of reported research to take all practical steps to remove potential sources of bias from a study. Blinding an assessor to the treatment allocation in RCTs is a simple method to achieve this. We expect that when subjective scores are used then blinding is necessary, or if blinding is not possible some explanation should be offered as to why it was not possible or practical.
This survey has highlighted the common use of inefficient or irrelevant statistical methods, with 7 papers reporting clear methodological errors. Not only does this suggest that many of the studies reported in these papers have had little or no expert statistical input, it is clear that many of the papers have not undergone adequate statistical review prior to publication. The lack of clear association between the description of the statistical methods and the reporting of the outcome (e.g. p-value) was widespread. However, this kind of issue could be easily corrected by obtaining appropriate expert statistical review prior to submission. If a reviewer notes a statistical error, or does not understand a statistical analysis plan, they should recommend that an expert opinion is sought during the review process after submission to the journal.
Non-parametric tests were used widely in the studies in this survey in a manner that suggested a lack of understanding as to when they are appropriate. For instance when selecting between a (parametric) t -test or a (non-parametric) Mann–Whitney test, the latter test should only be used for outcomes that are not approximately normally distributed and should be reported with medians and ranges (or interquartile ranges), not means and standard deviations [ 13 ]. However, where a natural transformation is available to make an outcome ‘more normal’, undertaking the analysis on the transformed scale using normal test statistics is usually a more efficient and powerful option than the non-parametric alternative. The widespread misuse of non-parametric tests in this survey suggests that this issue is not widely appreciated.
Carrying out multiple hypothesis tests was a common practice in many of the papers reviewed, with 30% (21–40%) of the papers reporting over 20 p-values and one study reporting a massive 156. Whilst we accept that the details of statistical methods to correct for multiple comparisons may not be common knowledge in the orthopaedic research community, and the circumstances when it is appropriate to adjust for multiple testing remain contentious amongst both statistical and clinical communities [ 16 ], we would expect most researchers to have some appreciation that carrying out large numbers of hypothesis tests and reporting significance at the standard 5% level is bad practice. We would advise that often the best way of dealing with this issue is to insist that a clear description and justification of all tests of significance that have been performed be included; this process of questioning will generally lead to a marked reduction in the number of tests reported. Graphs and tables: The presentation of results was a particular weak point in the papers included in this survey. All graphs and tables should be clearly labelled and sufficiently detailed to allow at least some inference to be made in isolation from the rest of the paper. Authors should include a clear title so that readers can quickly understand the information on display without reference to the main body of the text. With the increasing availability of software for producing sophisticated plots, it is tempting for authors to indulge in novel ways to present results. However, this is often one area where clear and simple tends to be best.
Although not formally one of the items in the questionnaire, we noted that 5 of the 27 RCTs (19%; 95% CI 7–39%) tested for differences between baseline characteristics (e.g. age, gender ratio, BMI etc.) after recruitment and randomization of patients to treatment arms of a trial. Since the randomization process produces treatment groups that are random samples from the same population , a null hypothesis of no difference between two populations must, by definition, be true. As such, any significant difference observed between groups must have arisen by chance; i.e. it is a type I error. Despite the widespread appreciation of this argument within the statistics community, this is still a widely reported error in many medical disciplines that, with adequate statistical input during the course of a study and at review, could be avoided.
The opinions expressed here are the result of independent assessments made by two statisticians using a sample of 100 representative orthopaedic papers and, as such, are limited by the experience and prejudices of the assessors and the size and nature of the survey. However, the carefully designed sampling strategy and the random selection methods ensured that the papers surveyed were indeed representative of the target literature [ 2 ]. Furthermore, the fact that many of the issues highlighted in this paper are familiar topics to those providing statistical reviews of medical research [ 17 ], suggests that the views expressed here are likely to be widely held within this community. For those who are unfamiliar with good practice in research, others have provided guidance in the use of statistics in the orthopaedic setting [ 1 , 18 ] and also specifically in the design of RCTs [ 19 , 20 ]. More generally, the series of short articles on the use of statistics for medical researchers published in the British Medical Journal [ 21 ] provide a rich resource of information on good statistical practice. Although our focus here has been on research published in general orthopaedic journals, the nature and extent of the issues raised here are clearly not exclusive to this discipline and as such we expect that the issues raised in the discussion and our recommendations for improvement to be applicable across all medical disciplines. To the non-statistically trained reader, many of the criticisms reported here may seem niggling and unimportant relative to the clinical details of a study. However, it is troubling to report that the statistical assessors in this survey thought that in 17% (10–26%) of the studies, the conclusions were not clearly justified by the results. For 39% (30–49%) of studies a different analysis should have been undertaken and for 17% (10–26%) of them, a different analysis could have made a difference to the conclusions. The results of this survey present challenges for us all, whether statistician, clinician, reviewer or journal editor, and it is only by greater dialogue between us all that these important issues can be addressed.
Petrie A: Statistics in orthopaedic papers. J Bone Joint Surg Br. 2006, 88: 1121-1136. 10.1302/0301-620X.88B9.17896.
Article CAS PubMed Google Scholar
Parsons N, Hiskens R, Price CL, Costa ML: A systematic survey of the quality of research reporting in general orthopaedic journals. J Bone Joint Surg Br. 2011, 93: 1154-1159. 10.1302/0301-620X.93B9.27193.
Moher D: CONSORT: an evolving tool to help improve the quality of reports of randomized controlled trials. Consolidated Standards of Reporting Trials. JAMA. 1998, 279: 1489-1491.
CAS PubMed Google Scholar
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC: The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet. 2007, 370: 1453-1457. 10.1016/S0140-6736(07)61602-X.
Article PubMed Google Scholar
Siebelt M, Siebelt T, Pilot P, Bloem RM, Bhandari M, Poolman RW: Citation analysis of orthopaedic literature: 18 major orthopaedic journals compared for Impact Factor and SCImago. BMC Musculoskelet Disord. 2010, 11: 4-10.1186/1471-2474-11-4.
Article PubMed PubMed Central Google Scholar
Web of Knowledge. [ http://wok.mimas.ac.uk/ ]
Kilkenny C, Parsons N, Kadyszewski E, Festing MFW, Cuthill IC, Fry D, Hutton J, Altman D: Survey of the quality of experimental design, statistical analysis and reporting of research using animals. PLoS One. 2009, 4: e7824-10.1371/journal.pone.0007824.
Altman DG, Bland JM: Units of analysis. BMJ. 1874, 1997: 314-
Google Scholar
Chow S-C, Shao J, Wang H: Sample size calculations in clinical research. 2008, New York: Chapman and Hall
Schlesselman JJ: Sample size requirements in cohort and case–control studies of disease. American J Epidemiol. 1974, 99: 381-384.
CAS Google Scholar
Missing data analysis. [ http://missingdata.lshtm.ac.uk/ ]
Oliver D, Hall JC: Usage of statistics in the surgical literature and the 'orphan P' phenomenon. Aust N Z J Surg. 1989, 59: 449-451. 10.1111/j.1445-2197.1989.tb01609.x.
Altman DG, Bland JM: Parametric v non-parametric methods for data analysis. BMJ. 2009, 338: a3167-10.1136/bmj.a3167.
Bland M: An introduction to medical statistics. 2003, Oxford: OUP
Bland JM, Altman DG: Multiple significance tests: the Bonferroni method. BMJ. 1995, 310: 170-10.1136/bmj.310.6973.170.
Article CAS PubMed PubMed Central Google Scholar
Perneger TV: What's wrong with Bonferroni adjustments. BMJ. 1998, 316: 1236-10.1136/bmj.316.7139.1236.
Bland M: How to upset the Statistical Referee. [ http://www-users.york.ac.uk/~mb55/talks/upset.htm ]
Petrie A: Statistical power in testing a hypothesis. J Bone Joint Surg Br. 2010, 92: 1192-1194. 10.1302/0301-620X.92B9.25069.
Simunovic N, Devereaux PJ, Bhandari M: Design considerations for randomised trials in orthopaedic fracture surgery. Injury. 2008, 39: 696-704. 10.1016/j.injury.2008.02.012.
Soucacos PN, Johnson EO, Babis G: Randomised controlled trials in orthopaedic surgery and traumatology: overview of parameters and pitfalls. Injury. 2008, 39: 636-642. 10.1016/j.injury.2008.02.011.
BMJ Statistics Notes Series. [ http://openwetware.org/wiki/BMJ_Statistics_Notes_series ]
Pre-publication history
The pre-publication history for this paper can be accessed here: http://www.biomedcentral.com/1471-2288/12/60/prepub
Download references
Author information
Authors and affiliations.
Warwick Medical School, University of Warwick, Coventry, CV2 2DX, UK
Nick R Parsons, Juul Achten & Matthew L Costa
Public Health, Epidemiology and Biostatistics Group, University of Birmingham, Birmingham, B15 2TT, UK
Charlotte L Price
University Hospitals Coventry and Warwickshire NHS Trust, Coventry, UK
Richard Hiskens
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Nick R Parsons .
Additional information
Competing interests.
The authors declare that they have no competing interests.
Authors’ contributions
NP, JA, MC, RH and CP designed the study and extracted the data. RH identified the papers for inclusion in the study. NP and CP, reviewed the papers, extracted the data, conducted the analyses and created the first draft of the manuscript. All authors participated in editing the manuscript and approved final manuscript for publication.
Electronic supplementary material
Additional file 1: statistical questionnaire.(doc ), rights and permissions.
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License ( http://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Reprints and permissions
About this article
Cite this article.
Parsons, N.R., Price, C.L., Hiskens, R. et al. An evaluation of the quality of statistical design and analysis of published medical research: results from a systematic survey of general orthopaedic journals. BMC Med Res Methodol 12 , 60 (2012). https://doi.org/10.1186/1471-2288-12-60
Download citation
Received : 01 November 2011
Accepted : 16 April 2012
Published : 25 April 2012
DOI : https://doi.org/10.1186/1471-2288-12-60
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Experimental Unit
- Statistical Review
- Medical Discipline
- Orthopaedic Research
- Orthopaedic Literature
BMC Medical Research Methodology
ISSN: 1471-2288
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- BMJ Open Access

How to write statistical analysis section in medical research
Alok kumar dwivedi.
Department of Molecular and Translational Medicine, Division of Biostatistics and Epidemiology, Texas Tech University Health Sciences Center El Paso, El Paso, Texas, USA
Associated Data
jim-2022-002479supp001.pdf
Data sharing not applicable as no datasets generated and/or analyzed for this study.
Reporting of statistical analysis is essential in any clinical and translational research study. However, medical research studies sometimes report statistical analysis that is either inappropriate or insufficient to attest to the accuracy and validity of findings and conclusions. Published works involving inaccurate statistical analyses and insufficient reporting influence the conduct of future scientific studies, including meta-analyses and medical decisions. Although the biostatistical practice has been improved over the years due to the involvement of statistical reviewers and collaborators in research studies, there remain areas of improvement for transparent reporting of the statistical analysis section in a study. Evidence-based biostatistics practice throughout the research is useful for generating reliable data and translating meaningful data to meaningful interpretation and decisions in medical research. Most existing research reporting guidelines do not provide guidance for reporting methods in the statistical analysis section that helps in evaluating the quality of findings and data interpretation. In this report, we highlight the global and critical steps to be reported in the statistical analysis of grants and research articles. We provide clarity and the importance of understanding study objective types, data generation process, effect size use, evidence-based biostatistical methods use, and development of statistical models through several thematic frameworks. We also provide published examples of adherence or non-adherence to methodological standards related to each step in the statistical analysis and their implications. We believe the suggestions provided in this report can have far-reaching implications for education and strengthening the quality of statistical reporting and biostatistical practice in medical research.
Introduction
Biostatistics is the overall approach to how we realistically and feasibly execute a research idea to produce meaningful data and translate data to meaningful interpretation and decisions. In this era of evidence-based medicine and practice, basic biostatistical knowledge becomes essential for critically appraising research articles and implementing findings for better patient management, improving healthcare, and research planning. 1 However, it may not be sufficient for the proper execution and reporting of statistical analyses in studies. 2 3 Three things are required for statistical analyses, namely knowledge of the conceptual framework of variables, research design, and evidence-based applications of statistical analysis with statistical software. 4 5 The conceptual framework provides possible biological and clinical pathways between independent variables and outcomes with role specification of variables. The research design provides a protocol of study design and data generation process (DGP), whereas the evidence-based statistical analysis approach provides guidance for selecting and implementing approaches after evaluating data with the research design. 2 5 Ocaña-Riola 6 reported a substantial percentage of articles from high-impact medical journals contained errors in statistical analysis or data interpretation. These errors in statistical analyses and interpretation of results do not only impact the reliability of research findings but also influence the medical decision-making and planning and execution of other related studies. A survey of consulting biostatisticians in the USA reported that researchers frequently request biostatisticians for performing inappropriate statistical analyses and inappropriate reporting of data. 7 This implies that there is a need to enforce standardized reporting of the statistical analysis section in medical research which can also help rreviewers and investigators to improve the methodological standards of the study.
Biostatistical practice in medicine has been improving over the years due to continuous efforts in promoting awareness and involving expert services on biostatistics, epidemiology, and research design in clinical and translational research. 8–11 Despite these efforts, the quality of reporting of statistical analysis in research studies has often been suboptimal. 12 13 We noticed that none of the methods reporting documents were developed using evidence-based biostatistics (EBB) theory and practice. The EBB practice implies that the selection of statistical analysis methods for statistical analyses and the steps of results reporting and interpretation should be grounded based on the evidence generated in the scientific literature and according to the study objective type and design. 5 Previous works have not properly elucidated the importance of understanding EBB concepts and related reporting in the write-up of statistical analyses. As a result, reviewers sometimes ask to present data or execute analyses that do not match the study objective type. 14 We summarize the statistical analysis steps to be reported in the statistical analysis section based on review and thematic frameworks.
We identified articles describing statistical reporting problems in medicine using different search terms ( online supplemental table 1 ). Based on these studies, we prioritized commonly reported statistical errors in analytical strategies and developed essential components to be reported in the statistical analysis section of research grants and studies. We also clarified the purpose and the overall implication of reporting each step in statistical analyses through various examples.
Supplementary data
Although biostatistical inputs are critical for the entire research study ( online supplemental table 2 ), biostatistical consultations were mostly used for statistical analyses only 15 . Even though the conduct of statistical analysis mismatched with the study objective and DGP was identified as the major problem in articles submitted to high-impact medical journals. 16 In addition, multivariable analyses were often inappropriately conducted and reported in published studies. 17 18 In light of these statistical errors, we describe the reporting of the following components in the statistical analysis section of the study.
Step 1: specify study objective type and outcomes (overall approach)
The study objective type provides the role of important variables for a specified outcome in statistical analyses and the overall approach of the model building and model reporting steps in a study. In the statistical framework, the problems are classified into descriptive and inferential/analytical/confirmatory objectives. In the epidemiological framework, the analytical and prognostic problems are broadly classified into association, explanatory, and predictive objectives. 19 These study objectives ( figure 1 ) may be classified into six categories: (1) exploratory, (2) association, (3) causal, (4) intervention, (5) prediction and (6) clinical decision models in medical research. 20

Comparative assessments of developing and reporting of study objective types and models. Association measures include odds ratio, risk ratio, or hazard ratio. AUC, area under the curve; C, confounder; CI, confidence interval; E, exposure; HbA1C: hemoglobin A1c; M, mediator; MFT, model fit test; MST, model specification test; PI, predictive interval; R 2 , coefficient of determinant; X, independent variable; Y, outcome.
The exploratory objective type is a specific type of determinant study and is commonly known as risk factors or correlates study in medical research. In an exploratory study, all covariates are considered equally important for the outcome of interest in the study. The goal of the exploratory study is to present the results of a model which gives higher accuracy after satisfying all model-related assumptions. In the association study, the investigator identifies predefined exposures of interest for the outcome, and variables other than exposures are also important for the interpretation and considered as covariates. The goal of an association study is to present the adjusted association of exposure with outcome. 20 In the causal objective study, the investigator is interested in determining the impact of exposure(s) on outcome using the conceptual framework. In this study objective, all variables should have a predefined role (exposures, confounders, mediators, covariates, and predictors) in a conceptual framework. A study with a causal objective is known as an explanatory or a confirmatory study in medical research. The goal is to present the direct or indirect effects of exposure(s) on an outcome after assessing the model’s fitness in the conceptual framework. 19 21 The objective of an interventional study is to determine the effect of an intervention on outcomes and is often known as randomized or non-randomized clinical trials in medical research. In the intervention objective model, all variables other than the intervention are treated as nuisance variables for primary analyses. The goal is to present the direct effect of the intervention on the outcomes by eliminating biases. 22–24 In the predictive study, the goal is to determine an optimum set of variables that can predict the outcome, particularly in external settings. The clinical decision models are a special case of prognostic models in which high dimensional data at various levels are used for risk stratification, classification, and prediction. In this model, all variables are considered input features. The goal is to present a decision tool that has high accuracy in training, testing, and validation data sets. 20 25 Biostatisticians or applied researchers should properly discuss the intention of the study objective type before proceeding with statistical analyses. In addition, it would be a good idea to prepare a conceptual model framework regardless of study objective type to understand study concepts.
A study 26 showed a favorable effect of the beta-blocker intervention on survival outcome in patients with advanced human epidermal growth factor receptor (HER2)-negative breast cancer without adjusting for all the potential confounding effects (age or menopausal status and Eastern Cooperative Oncology Performance Status) in primary analyses or validation analyses or using a propensity score-adjusted analysis, which is an EBB preferred method for analyzing non-randomized studies. 27 Similarly, another study had the goal of developing a predictive model for prediction of Alzheimer’s disease progression. 28 However, this study did not internally or externally validate the performance of the model as per the requirement of a predictive objective study. In another study, 29 investigators were interested in determining an association between metabolic syndrome and hepatitis C virus. However, the authors did not clearly specify the outcome in the analysis and produced conflicting associations with different analyses. 30 Thus, the outcome should be clearly specified as per the study objective type.
Step 2: specify effect size measure according to study design (interpretation and practical value)
The study design provides information on the selection of study participants and the process of data collection conditioned on either exposure or outcome ( figure 2 ). The appropriate use of effect size measure, tabular presentation of results, and the level of evidence are mostly determined by the study design. 31 32 In cohort or clinical trial study designs, the participants are selected based on exposure status and are followed up for the development of the outcome. These study designs can provide multiple outcomes, produce incidence or incidence density, and are preferred to be analyzed with risk ratio (RR) or hazards models. In a case–control study, the selection of participants is conditioned on outcome status. This type of study can have only one outcome and is preferred to be analyzed with an odds ratio (OR) model. In a cross-sectional study design, there is no selection restriction on outcomes or exposures. All data are collected simultaneously and can be analyzed with a prevalence ratio model, which is mathematically equivalent to the RR model. 33 The reporting of effect size measure also depends on the study objective type. For example, predictive models typically require reporting of regression coefficients or weight of variables in the model instead of association measures, which are required in other objective types. There are agreements and disagreements between OR and RR measures. Due to the constancy and symmetricity properties of OR, some researchers prefer to use OR in studies with common events. Similarly, the collapsibility and interpretability properties of RR make it more appealing to use in studies with common events. 34 To avoid variable practice and interpretation issues with OR, it is recommended to use RR models in all studies except for case–control and nested case–control studies, where OR approximates RR and thus OR models should be used. Otherwise, investigators may report sufficient data to compute any ratio measure. Biostatisticians should educate investigators on the proper interpretation of ratio measures in the light of study design and their reporting. 34 35

Effect size according to study design.
Investigators sometimes either inappropriately label their study design 36 37 or report effect size measures not aligned with the study design, 38 39 leading to difficulty in results interpretation and evaluation of the level of evidence. The proper labeling of study design and the appropriate use of effect size measure have substantial implications for results interpretation, including the conduct of systematic review and meta-analysis. 40 A study 31 reviewed the frequency of reporting OR instead of RR in cohort studies and randomized clinical trials (RCTs) and found that one-third of the cohort studies used an OR model, whereas 5% of RCTs used an OR model. The majority of estimated ORs from these studies had a 20% or higher deviation from the corresponding RR.
Step 3: specify study hypothesis, reporting of p values, and interval estimates (interpretation and decision)
The clinical hypothesis provides information for evaluating formal claims specified in the study objectives, while the statistical hypothesis provides information about the population parameters/statistics being used to test the formal claims. The inference about the study hypothesis is typically measured by p value and confidence interval (CI). A smaller p value indicates that the data support against the null hypothesis. Since the p value is a conditional probability, it can never tell about the acceptance or rejection of the null hypothesis. Therefore, multiple alternative strategies of p values have been proposed to strengthen the credibility of conclusions. 41 42 Adaption of these alternative strategies is only needed in the explanatory objective studies. Although exact p values are recommended to be reported in research studies, p values do not provide any information about the effect size. Compared with p values, the CI provides a confidence range of the effect size that contains the true effect size if the study were repeated and can be used to determine whether the results are statistically significant or not. 43 Both p value and 95% CI provide complementary information and thus need to be specified in the statistical analysis section. 24 44
Researchers often test one or more comparisons or hypotheses. Accordingly, the side and the level of significance for considering results to be statistically significant may change. Furthermore, studies may include more than one primary outcome that requires an adjustment in the level of significance for multiplicity. All studies should provide the interval estimate of the effect size/regression coefficient in the primary analyses. Since the interpretation of data analysis depends on the study hypothesis, researchers are required to specify the level of significance along with the side (one-sided or two-sided) of the p value in the test for considering statistically significant results, adjustment of the level of significance due to multiple comparisons or multiplicity, and reporting of interval estimates of the effect size in the statistical analysis section. 45
A study 46 showed a significant effect of fluoxetine on relapse rates in obsessive-compulsive disorder based on a one-sided p value of 0.04. Clearly, there was no reason for using a one-sided p value as opposed to a two-sided p value. A review of the appropriate use of multiple test correction methods in multiarm clinical trials published in major medical journals in 2012 identified over 50% of the articles did not perform multiple-testing correction. 47 Similar to controlling a familywise error rate due to multiple comparisons, adjustment of the false discovery rate is also critical in studies involving multiple related outcomes. A review of RCTs for depression between 2007 and 2008 from six journals reported that only limited studies (5.8%) accounted for multiplicity in the analyses due to multiple outcomes. 48
Step 4: account for DGP in the statistical analysis (accuracy)
The study design also requires the specification of the selection of participants and outcome measurement processes in different design settings. We referred to this specific design feature as DGP. Understanding DGP helps in determining appropriate modeling of outcome distribution in statistical analyses and setting up model premises and units of analysis. 4 DGP ( figure 3 ) involves information on data generation and data measures, including the number of measurements after random selection, complex selection, consecutive selection, pragmatic selection, or systematic selection. Specifically, DGP depends on a sampling setting (participants are selected using survey sampling methods and one subject may represent multiple participants in the population), clustered setting (participants are clustered through a recruitment setting or hierarchical setting or multiple hospitals), pragmatic setting (participants are selected through mixed approaches), or systematic review setting (participants are selected from published studies). DGP also depends on the measurements of outcomes in an unpaired setting (measured on one occasion only in independent groups), paired setting (measured on more than one occasion or participants are matched on certain subject characteristics), or mixed setting (measured on more than one occasion but interested in comparing independent groups). It also involves information regarding outcomes or exposure generation processes using quantitative or categorical variables, quantitative values using labs or validated instruments, and self-reported or administered tests yielding a variety of data distributions, including individual distribution, mixed-type distribution, mixed distributions, and latent distributions. Due to different DGPs, study data may include messy or missing data, incomplete/partial measurements, time-varying measurements, surrogate measures, latent measures, imbalances, unknown confounders, instrument variables, correlated responses, various levels of clustering, qualitative data, or mixed data outcomes, competing events, individual and higher-level variables, etc. The performance of statistical analysis, appropriate estimation of standard errors of estimates and subsequently computation of p values, the generalizability of findings, and the graphical display of data rely on DGP. Accounting for DGP in the analyses requires proper communication between investigators and biostatisticians about each aspect of participant selection and data collection, including measurements, occasions of measurements, and instruments used in the research study.

Common features of the data generation process.
A study 49 compared the intake of fresh fruit and komatsuna juice with the intake of commercial vegetable juice on metabolic parameters in middle-aged men using an RCT. The study was criticized for many reasons, but primarily for incorrect statistical methods not aligned with the study DGP. 50 Similarly, another study 51 highlighted that 80% of published studies using the Korean National Health and Nutrition Examination Survey did not incorporate survey sampling structure in statistical analyses, producing biased estimates and inappropriate findings. Likewise, another study 52 highlighted the need for maintaining methodological standards while analyzing data from the National Inpatient Sample. A systematic review 53 identified that over 50% of studies did not specify whether a paired t-test or an unpaired t-test was performed in statistical analysis in the top 25% of physiology journals, indicating poor transparency in reporting of statistical analysis as per the data type. Another study 54 also highlighted the data displaying errors not aligned with DGP. As per DGP, delay in treatment initiation of patients with cancer defined from the onset of symptom to treatment initiation should be analyzed into three components: patient/primary delay, secondary delay, and tertiary delay. 55 Similarly, the number of cancerous nodes should be analyzed with count data models. 56 However, several studies did not analyze such data according to DGP. 57 58
Step 5: apply EBB methods specific to study design features and DGP (efficiency and robustness)
The continuous growth in the development of robust statistical methods for dealing with a specific problem produced various methods to analyze specific data types. Since multiple methods are available for handling a specific problem yet with varying performances, heterogeneous practices among applied researchers have been noticed. Variable practices could also be due to a lack of consensus on statistical methods in literature, unawareness, and the unavailability of standardized statistical guidelines. 2 5 59 However, it becomes sometimes difficult to differentiate whether a specific method was used due to its robustness, lack of awareness, lack of accessibility of statistical software to apply an alternative appropriate method, intention to produce expected results, or ignorance of model diagnostics. To avoid heterogeneous practices, the selection of statistical methodology and their reporting at each stage of data analysis should be conducted using methods according to EBB practice. 5 Since it is hard for applied researchers to optimally select statistical methodology at each step, we encourage investigators to involve biostatisticians at the very early stage in basic, clinical, population, translational, and database research. We also appeal to biostatisticians to develop guidelines, checklists, and educational tools to promote the concept of EBB. As an effort, we developed the statistical analysis and methods in biomedical research (SAMBR) guidelines for applied researchers to use EBB methods for data analysis. 5 The EBB practice is essential for applying recent cutting-edge robust methodologies to yield accurate and unbiased results. The efficiency of statistical methodologies depends on the assumptions and DGP. Therefore, investigators may attempt to specify the choice of specific models in the primary analysis as per the EBB.
Although details of evidence-based preferred methods are provided in the SAMBR checklists for each study design/objective, 5 we have presented a simplified version of evidence-based preferred methods for common statistical analysis ( online supplemental table 3 ). Several examples are available in the literature where inefficient methods not according to EBB practice have been used. 31 57 60
Step 6: report variable selection method in the multivariable analysis according to study objective type (unbiased)
Multivariable analysis can be used for association, prediction or classification or risk stratification, adjustment, propensity score development, and effect size estimation. 61 Some biological, clinical, behavioral, and environmental factors may directly associate or influence the relationship between exposure and outcome. Therefore, almost all health studies require multivariable analyses for accurate and unbiased interpretations of findings ( figure 1 ). Analysts should develop an adjusted model if the sample size permits. It is a misconception that the analysis of RCT does not require adjusted analysis. Analysis of RCT may require adjustment for prognostic variables. 23 The foremost step in model building is the entry of variables after finalizing the appropriate parametric or non-parametric regression model. In the exploratory model building process due to no preference of exposures, a backward automated approach after including any variables that are significant at 25% in the unadjusted analysis can be used for variable selection. 62 63 In the association model, a manual selection of covariates based on the relevance of the variables should be included in a fully adjusted model. 63 In a causal model, clinically guided methods should be used for variable selection and their adjustments. 20 In a non-randomized interventional model, efforts should be made to eliminate confounding effects through propensity score methods and the final propensity score-adjusted multivariable model may adjust any prognostic variables, while a randomized study simply should adjust any prognostic variables. 27 Maintaining the event per variable (EVR) is important to avoid overfitting in any type of modeling; therefore, screening of variables may be required in some association and explanatory studies, which may be accomplished using a backward stepwise method that needs to be clarified in the statistical analyses. 10 In a predictive study, a model with an optimum set of variables producing the highest accuracy should be used. The optimum set of variables may be screened with the random forest method or bootstrap or machine learning methods. 64 65 Different methods of variable selection and adjustments may lead to different results. The screening process of variables and their adjustments in the final multivariable model should be clearly mentioned in the statistical analysis section.
A study 66 evaluating the effect of hydroxychloroquine (HDQ) showed unfavorable events (intubation or death) in patients who received HDQ compared with those who did not (hazard ratio (HR): 2.37, 95% CI 1.84 to 3.02) in an unadjusted analysis. However, the propensity score-adjusted analyses as appropriate with the interventional objective model showed no significant association between HDQ use and unfavorable events (HR: 1.04, 95% CI 0.82 to 1.32), which was also confirmed in multivariable and other propensity score-adjusted analyses. This study clearly suggests that results interpretation should be based on a multivariable analysis only in observational studies if feasible. A recent study 10 noted that approximately 6% of multivariable analyses based on either logistic or Cox regression used an inappropriate selection method of variables in medical research. This practice was more commonly noted in studies that did not involve an expert biostatistician. Another review 61 of 316 articles from high-impact Chinese medical journals revealed that 30.7% of articles did not report the selection of variables in multivariable models. Indeed, this inappropriate practice could have been identified more commonly if classified according to the study objective type. 18 In RCTs, it is uncommon to report an adjusted analysis based on prognostic variables, even though an adjusted analysis may produce an efficient estimate compared with an unadjusted analysis. A study assessing the effect of preemptive intervention on development outcomes showed a significant effect of an intervention on reducing autism spectrum disorder symptoms. 67 However, this study was criticized by Ware 68 for not reporting non-significant results in unadjusted analyses. If possible, unadjusted estimates should also be reported in any study, particularly in RCTs. 23 68
Step 7: provide evidence for exploring effect modifiers (applicability)
Any variable that modifies the effect of exposure on the outcome is called an effect modifier or modifier or an interacting variable. Exploring the effect modifiers in multivariable analyses helps in (1) determining the applicability/generalizability of findings in the overall or specific subpopulation, (2) generating ideas for new hypotheses, (3) explaining uninterpretable findings between unadjusted and adjusted analyses, (4) guiding to present combined or separate models for each specific subpopulation, and (5) explaining heterogeneity in treatment effect. Often, investigators present adjusted stratified results according to the presence or absence of an effect modifier. If the exposure interacts with multiple variables statistically or conceptually in the model, then the stratified findings (subgroup) according to each effect modifier may be presented. Otherwise, stratified analysis substantially reduces the power of the study due to the lower sample size in each stratum and may produce significant results by inflating type I error. 69 Therefore, a multivariable analysis involving an interaction term as opposed to a stratified analysis may be presented in the presence of an effect modifier. 70 Sometimes, a quantitative variable may emerge as a potential effect modifier for exposure and an outcome relationship. In such a situation, the quantitative variable should not be categorized unless a clinically meaningful threshold is not available in the study. In fact, the practice of categorizing quantitative variables should be avoided in the analysis unless a clinically meaningful cut-off is available or a hypothesis requires for it. 71 In an exploratory objective type, any possible interaction may be obtained in a study; however, the interpretation should be guided based on clinical implications. Similarly, some objective models may have more than one exposure or intervention and the association of each exposure according to the level of other exposure should be presented through adjusted analyses as suggested in the presence of interaction effects. 70
A review of 428 articles from MEDLINE on the quality of reporting from statistical analyses of three (linear, logistic, and Cox) commonly used regression models reported that only 18.5% of the published articles provided interaction analyses, 17 even though interaction analyses can provide a lot of useful information.
Step 8: assessment of assumptions, specifically the distribution of outcome, linearity, multicollinearity, sparsity, and overfitting (reliability)
The assessment and reporting of model diagnostics are important in assessing the efficiency, validity, and usefulness of the model. Model diagnostics include satisfying model-specific assumptions and the assessment of sparsity, linearity, distribution of outcome, multicollinearity, and overfitting. 61 72 Model-specific assumptions such as normal residuals, heteroscedasticity and independence of errors in linear regression, proportionality in Cox regression, proportionality odds assumption in ordinal logistic regression, and distribution fit in other types of continuous and count models are required. In addition, sparsity should also be examined prior to selecting an appropriate model. Sparsity indicates many zero observations in the data set. 73 In the presence of sparsity, the effect size is difficult to interpret. Except for machine learning models, most of the parametric and semiparametric models require a linear relationship between independent variables and a functional form of an outcome. Linearity should be assessed using a multivariable polynomial in all model objectives. 62 Similarly, the appropriate choice of the distribution of outcome is required for model building in all study objective models. Multicollinearity assessment is also useful in all objective models. Assessment of EVR in multivariable analysis can be used to avoid the overfitting issue of a multivariable model. 18
Some review studies highlighted that 73.8%–92% of the articles published in MEDLINE had not assessed the model diagnostics of the multivariable regression models. 17 61 72 Contrary to the monotonically, linearly increasing relationship between systolic blood pressure (SBP) and mortality established using the Framingham’s study, 74 Port et al 75 reported a non-linear relationship between SBP and all-cause mortality or cardiovascular deaths by reanalysis of the Framingham’s study data set. This study identified a different threshold for treating hypertension, indicating the role of linearity assessment in multivariable models. Although a non-Gaussian distribution model may be required for modeling patient delay outcome data in cancer, 55 a study analyzed patient delay data using an ordinary linear regression model. 57 An investigation of the development of predictive models and their reporting in medical journals identified that 53% of the articles had fewer EVR than the recommended EVR, indicating over half of the published articles may have an overfitting model. 18 Another study 76 attempted to identify the anthropometric variables associated with non-insulin-dependent diabetes and found that none of the anthropometric variables were significant after adjusting for waist circumference, age, and sex, indicating the presence of collinearity. A study reported detailed sparse data problems in published studies and potential solutions. 73
Step 9: report type of primary and sensitivity analyses (consistency)
Numerous considerations and assumptions are made throughout the research processes that require assessment, evaluation, and validation. Some assumptions, executions, and errors made at the beginning of the study data collection may not be fixable 13 ; however, additional information collected during the study and data processing, including data distribution obtained at the end of the study, may facilitate additional considerations that need to be verified in the statistical analyses. Consistencies in the research findings via modifications in the outcome or exposure definition, study population, accounting for missing data, model-related assumptions, variables and their forms, and accounting for adherence to protocol in the models can be evaluated and reported in research studies using sensitivity analyses. 77 The purpose and type of supporting analyses need to be specified clearly in the statistical analyses to differentiate the main findings from the supporting findings. Sensitivity analyses are different from secondary or interim or subgroup analyses. 78 Data analyses for secondary outcomes are often referred to as secondary analyses, while data analyses of an ongoing study are called interim analyses and data analyses according to groups based on patient characteristics are known as subgroup analyses.
Almost all studies require some form of sensitivity analysis to validate the findings under different conditions. However, it is often underutilized in medical journals. Only 18%–20.3% of studies reported some forms of sensitivity analyses. 77 78 A review of nutritional trials from high-quality journals reflected that 17% of the conclusions were reported inappropriately using findings from sensitivity analyses not based on the primary/main analyses. 77
Step 10: provide methods for summarizing, displaying, and interpreting data (transparency and usability)
Data presentation includes data summary, data display, and data from statistical model analyses. The primary purpose of the data summary is to understand the distribution of outcome status and other characteristics in the total sample and by primary exposure status or outcome status. Column-wise data presentation should be preferred according to exposure status in all study designs, while row-wise data presentation for the outcome should be preferred in all study designs except for a case–control study. 24 32 Summary statistics should be used to provide maximum information on data distribution aligned with DGP and variable type. The purpose of results presentation primarily from regression analyses or statistical models is to convey results interpretation and implications of findings. The results should be presented according to the study objective type. Accordingly, the reporting of unadjusted and adjusted associations of each factor with the outcome may be preferred in the determinant objective model, while unadjusted and adjusted effects of primary exposure on the outcome may be preferred in the explanatory objective model. In prognostic models, the final predictive models may be presented in such a way that users can use models to predict an outcome. In the exploratory objective model, a final multivariable model should be reported with R 2 or area under the curve (AUC). In the association and interventional models, the assessment of internal validation is critically important through various sensitivity and validation analyses. A model with better fit indices (in terms of R 2 or AUC, Akaike information criterion, Bayesian information criterion, fit index, root mean square error) should be finalized and reported in the causal model objective study. In the predictive objective type, the model performance in terms of R 2 or AUC in training and validation data sets needs to be reported ( figure 1 ). 20 21 There are multiple purposes of data display, including data distribution using bar diagram or histogram or frequency polygons or box plots, comparisons using cluster bar diagram or scatter dot plot or stacked bar diagram or Kaplan-Meier plot, correlation or model assessment using scatter plot or scatter matrix, clustering or pattern using heatmap or line plots, the effect of predictors with fitted models using marginsplot, and comparative evaluation of effect sizes from regression models using forest plot. Although the key purpose of data display is to highlight critical issues or findings in the study, data display should essentially follow DGP and variable types and should be user-friendly. 54 79 Data interpretation heavily relies on the effect size measure along with study design and specified hypotheses. Sometimes, variables require standardization for descriptive comparison of effect sizes among exposures or interpreting small effect size, or centralization for interpreting intercept or avoiding collinearity due to interaction terms, or transformation for achieving model-related assumptions. 80 Appropriate methods of data reporting and interpretation aligned with study design, study hypothesis, and effect size measure should be specified in the statistical analysis section of research studies.
Published articles from reputed journals inappropriately summarized a categorized variable with mean and range, 81 summarized a highly skewed variable with mean and standard deviation, 57 and treated a categorized variable as a continuous variable in regression analyses. 82 Similarly, numerous examples from published studies reporting inappropriate graphical display or inappropriate interpretation of data not aligned with DGP or variable types are illustrated in a book published by Bland and Peacock. 83 84 A study used qualitative data on MRI but inappropriately presented with a Box-Whisker plot. 81 Another study reported unusually high OR for an association between high breast parenchymal enhancement and breast cancer in both premenopausal and postmenopausal women. 85 This reporting makes suspicious findings and may include sparse data bias. 86 A poor tabular presentation without proper scaling or standardization of a variable, missing CI for some variables, missing unit and sample size, and inconsistent reporting of decimal places could be easily noticed in table 4 of a published study. 29 Some published predictive models 87 do not report intercept or baseline survival estimates to use their predictive models in clinical use. Although a direct comparison of effect sizes obtained from the same model may be avoided if the units are different among variables, 35 a study had an objective to compare effect sizes across variables but the authors performed comparisons without standardization of variables or using statistical tests. 88
A sample for writing statistical analysis section in medical journals/research studies
Our primary study objective type was to develop a (select from figure 1 ) model to assess the relationship of risk factors (list critical variables or exposures) with outcomes (specify type from continuous/discrete/count/binary/polytomous/time-to-event). To address this objective, we conducted a (select from figure 2 or any other) study design to test the hypotheses of (equality or superiority or non-inferiority or equivalence or futility) or develop prediction. Accordingly, the other variables were adjusted or considered as (specify role of variables from confounders, covariates, or predictors or independent variables) as reflected in the conceptual framework. In the unadjusted or preliminary analyses as per the (select from figure 3 or any other design features) DGP, (specify EBB preferred tests from online supplemental table 3 or any other appropriate tests) were used for (specify variables and types) in unadjusted analyses. According to the EBB practice for the outcome (specify type) and DGP of (select from figure 3 or any other), we used (select from online supplemental table 1 or specify a multivariable approach) as the primary model in the multivariable analysis. We used (select from figure 1 ) variable selection method in the multivariable analysis and explored the interaction effects between (specify variables). The model diagnostics including (list all applicable, including model-related assumptions, linearity, or multicollinearity or overfitting or distribution of outcome or sparsity) were also assessed using (specify appropriate methods) respectively. In such exploration, we identified (specify diagnostic issues if any) and therefore the multivariable models were developed using (specify potential methods used to handle diagnostic issues). The other outcomes were analyzed with (list names of multivariable approaches with respective outcomes). All the models used the same procedure (or specify from figure 1 ) for variable selection, exploration of interaction effects, and model diagnostics using (specify statistical approaches) depending on the statistical models. As per the study design, hypothesis, and multivariable analysis, the results were summarized with effect size (select as appropriate or from figure 2 ) along with (specify 95% CI or other interval estimates) and considered statistically significant using (specify the side of p value or alternatives) at (specify the level of significance) due to (provide reasons for choosing a significance level). We presented unadjusted and/or adjusted estimates of primary outcome according to (list primary exposures or variables). Additional analyses were conducted for (specific reasons from step 9) using (specify methods) to validate findings obtained in the primary analyses. The data were summarized with (list summary measures and appropriate graphs from step 10), whereas the final multivariable model performance was summarized with (fit indices if applicable from step 10). We also used (list graphs) as appropriate with DGP (specify from figure 3 ) to present the critical findings or highlight (specify data issues) using (list graphs/methods) in the study. The exposures or variables were used in (specify the form of the variables) and therefore the effect or association of (list exposures or variables) on outcome should be interpreted in terms of changes in (specify interpretation unit) exposures/variables. List all other additional analyses if performed (with full details of all models in a supplementary file along with statistical codes if possible).
Concluding remarks
We highlighted 10 essential steps to be reported in the statistical analysis section of any analytical study ( figure 4 ). Adherence to minimum reporting of the steps specified in this report may enforce investigators to understand concepts and approach biostatisticians timely to apply these concepts in their study to improve the overall quality of methodological standards in grant proposals and research studies. The order of reporting information in statistical analyses specified in this report is not mandatory; however, clear reporting of analytical steps applicable to the specific study type should be mentioned somewhere in the manuscript. Since the entire approach of statistical analyses is dependent on the study objective type and EBB practice, proper execution and reporting of statistical models can be taught to the next generation of statisticians by the study objective type in statistical education courses. In fact, some disciplines ( figure 5 ) are strictly aligned with specific study objective types. Bioinformaticians are oriented in studying determinant and prognostic models toward precision medicine, while epidemiologists are oriented in studying association and causal models, particularly in population-based observational and pragmatic settings. Data scientists are heavily involved in prediction and classification models in personalized medicine. A common thing across disciplines is using biostatistical principles and computation tools to address any research question. Sometimes, one discipline expert does the part of others. 89 We strongly recommend using a team science approach that includes an epidemiologist, biostatistician, data scientist, and bioinformatician depending on the study objectives and needs. Clear reporting of data analyses as per the study objective type should be encouraged among all researchers to minimize heterogeneous practices and improve scientific quality and outcomes. In addition, we also encourage investigators to strictly follow transparent reporting and quality assessment guidelines according to the study design ( https://www.equator-network.org/ ) to improve the overall quality of the study, accordingly STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) for observational studies, CONSORT (Consolidated Standards of Reporting Trials) for clinical trials, STARD (Standards for Reporting Diagnostic Accuracy Studies) for diagnostic studies, TRIPOD (Transparent Reporting of a multivariable prediction model for Individual Prognosis OR Diagnosis) for prediction modeling, and ARRIVE (Animal Research: Reporting of In Vivo Experiments) for preclinical studies. The steps provided in this document for writing the statistical analysis section is essentially different from other guidance documents, including SAMBR. 5 SAMBR provides a guidance document for selecting evidence-based preferred methods of statistical analysis according to different study designs, while this report suggests the global reporting of essential information in the statistical analysis section according to study objective type. In this guidance report, our suggestion strictly pertains to the reporting of methods in the statistical analysis section and their implications on the interpretation of results. Our document does not provide guidance on the reporting of sample size or results or statistical analysis section for meta-analysis. The examples and reviews reported in this study may be used to emphasize the concepts and related implications in medical research.

Summary of reporting steps, purpose, and evaluation measures in the statistical analysis section.

Role of interrelated disciplines according to study objective type.
Acknowledgments
The author would like to thank the reviewers for their careful review and insightful suggestions.
Contributors: AKD developed the concept and design and wrote the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: AKD is a Journal of Investigative Medicine Editorial Board member. No other competing interests declared.
Provenance and peer review: Commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Ethics statements, patient consent for publication.
Not required.
AI Index Report
The AI Index Report tracks, collates, distills, and visualizes data related to artificial intelligence. Our mission is to provide unbiased, rigorously vetted, broadly sourced data in order for policymakers, researchers, executives, journalists, and the general public to develop a more thorough and nuanced understanding of the complex field of AI. The report aims to be the world’s most credible and authoritative source for data and insights about AI.
Subscribe to receive the 2024 report in your inbox!
Coming Soon: 2024 AI Index Report!
The 2024 AI Index Report will be out April 15! Sign up for our mailing list to receive it in your inbox.
Steering Committee Co-Directors

Ray Perrault
Steering committee members.

Erik Brynjolfsson

John Etchemendy

Katrina Ligett

Terah Lyons

James Manyika

Juan Carlos Niebles

Vanessa Parli

Yoav Shoham

Russell Wald
Staff members.

Loredana Fattorini

Nestor Maslej
Letter from the co-directors.
AI has moved into its era of deployment; throughout 2022 and the beginning of 2023, new large-scale AI models have been released every month. These models, such as ChatGPT, Stable Diffusion, Whisper, and DALL-E 2, are capable of an increasingly broad range of tasks, from text manipulation and analysis, to image generation, to unprecedentedly good speech recognition. These systems demonstrate capabilities in question answering, and the generation of text, image, and code unimagined a decade ago, and they outperform the state of the art on many benchmarks, old and new. However, they are prone to hallucination, routinely biased, and can be tricked into serving nefarious aims, highlighting the complicated ethical challenges associated with their deployment.
Although 2022 was the first year in a decade where private AI investment decreased, AI is still a topic of great interest to policymakers, industry leaders, researchers, and the public. Policymakers are talking about AI more than ever before. Industry leaders that have integrated AI into their businesses are seeing tangible cost and revenue benefits. The number of AI publications and collaborations continues to increase. And the public is forming sharper opinions about AI and which elements they like or dislike.
AI will continue to improve and, as such, become a greater part of all our lives. Given the increased presence of this technology and its potential for massive disruption, we should all begin thinking more critically about how exactly we want AI to be developed and deployed. We should also ask questions about who is deploying it—as our analysis shows, AI is increasingly defined by the actions of a small set of private sector actors, rather than a broader range of societal actors. This year’s AI Index paints a picture of where we are so far with AI, in order to highlight what might await us in the future.
- Jack Clark and Ray Perrault
Our Supporting Partners

Analytics & Research Partners

Stay up to date on the AI Index by subscribing to the Stanford HAI newsletter.

IMAGES
VIDEO
COMMENTS
Table of contents. Step 1: Write your hypotheses and plan your research design. Step 2: Collect data from a sample. Step 3: Summarize your data with descriptive statistics. Step 4: Test hypotheses or make estimates with inferential statistics.
Introduction. Statistical analysis is necessary for any research project seeking to make quantitative conclusions. The following is a primer for research-based statistical analysis. It is intended to be a high-level overview of appropriate statistical testing, while not diving too deep into any specific methodology.
1 Introduction. Statistics is a set of methods used to analyze data. The statistic is present in all areas of science involving the. collection, handling and sorting of data, given the insight of ...
Preparing for statistical analysis requires some essential steps to ensure the validity and reliability of results. Firstly, formulating understandable and measurable questions is critical for valid statistics in a research paper. Questions lead the entire data analysis process and help define the scope of the study.
Statistical methods involved in carrying out a study include planning, designing, collecting data, analysing, drawing meaningful interpretation and reporting of the research findings. The statistical analysis gives meaning to the meaningless numbers, thereby breathing life into a lifeless data. The results and inferences are precise only if ...
This article is a practical guide to conducting data analysis in general literature reviews. The general literature review is a synthesis and analysis of published research on a relevant clinical issue, and is a common format for academic theses at the bachelor's and master's levels in nursing, physiotherapy, occupational therapy, public health and other related fields.
Using statistical analysis methods in research is inevitable, especially in academic assignments, projects, and term papers. It's always advisable to seek assistance from your professor or you can try research paper writing by CustomWritings before you start your academic project or write statistical analysis in research paper. Consulting an ...
The descriptive statistical analysis allows organizing and summarizing the large data into graphs and tables. Descriptive analysis involves various processes such as tabulation, measure of central tendency, measure of dispersion or variance, skewness measurements etc. 2. Inferential Analysis.
In many fields, a statistical analysis forms the heart of both the methods and results sections of a manuscript. Learn how to report statistical analyses, and what other context is important for publication success and future reproducibility. A matter of principle. First and foremost, the statistical methods employed in research must always be:
Type and distribution of the data used. For the same objective, selection of the statistical test is varying as per data types. For the nominal, ordinal, discrete data, we use nonparametric methods while for continuous data, parametric methods as well as nonparametric methods are used.[] For example, in the regression analysis, when our outcome variable is categorical, logistic regression ...
Provides well-organized coverage of statistical analysis and applications in biology, kinesiology, and physical anthropology with comprehensive insights into the techniques and interpretations of R, SPSS®, Excel®, and Numbers® output An Introduction to Statistical Analysis in Research: With Applications in the Biological and Life Sciences develops a conceptual foundation in statistical ...
Statistical analysis means investigating trends, patterns, and relationships using quantitative data. It is an important research tool used by scientists, governments, businesses, and other organisations. To draw valid conclusions, statistical analysis requires careful planning from the very start of the research process. You need to specify ...
As you read papers also notice the construction of the papers (learn from the good and bad examples). Abstract and Introduction { keys for getting readers engaged. Be gentle with your audience. Tell them your story. Writing is work { but ultimately rewarding! 13
Statistical analysis is the process of collecting and analyzing data in order to discern patterns and trends. It is a method for removing bias from evaluating data by employing numerical analysis. This technique is useful for collecting the interpretations of research, developing statistical models, and planning surveys and studies.
Overview. Statistical Papers is a forum for presentation and critical assessment of statistical methods encouraging the discussion of methodological foundations and potential applications. The Journal stresses statistical methods that have broad applications, giving special attention to those relevant to the economic and social sciences.
Statistical analysis methods account for variation and differences, and that is why they are so critical to performing high quality research analysis in your paper. Collecting and analyzing data through statistics is a really important part of furthering our understanding of everything from human behavior to the universe itself.
The choice of statistical test used for analysis of data from a research study is crucial in interpreting the results of the study. This article gives an overview of the various factors that determine the selection of a statistical test and lists some statistical testsused in common practice. How to cite this article: Ranganathan P. An ...
The application of statistics in reported research in trauma and orthopaedic surgery has become ever more important and complex. Despite the extensive use of statistical analysis, it is still a subject which is often not conceptually well understood, resulting in clear methodological flaws and inadequate reporting in many papers. A detailed statistical survey sampled 100 representative ...
The Statistical Research Report Series (RR) covers research in statistical methodology and estimation. Page Last Revised - October 8, 2021. View Statistical Research reports by their topics.
Results. Although biostatistical inputs are critical for the entire research study (online supplemental table 2), biostatistical consultations were mostly used for statistical analyses only 15.Even though the conduct of statistical analysis mismatched with the study objective and DGP was identified as the major problem in articles submitted to high-impact medical journals. 16 In addition ...
AI Index Report. The AI Index Report tracks, collates, distills, and visualizes data related to artificial intelligence. Our mission is to provide unbiased, rigorously vetted, broadly sourced data in order for policymakers, researchers, executives, journalists, and the general public to develop a more thorough and nuanced understanding of the ...