An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Neuropsychiatr Dis Treat
- v.3(5); 2007 Oct

Sleep deprivation: Impact on cognitive performance
Paula alhola.
1 Department of Psychology
Päivi Polo-Kantola
2 Sleep Research Unit (Department of Physiology), University of Turku, Turku, Finland
Today, prolonged wakefulness is a widespread phenomenon. Nevertheless, in the field of sleep and wakefulness, several unanswered questions remain. Prolonged wakefulness can be due to acute total sleep deprivation (SD) or to chronic partial sleep restriction. Although the latter is more common in everyday life, the effects of total SD have been examined more thoroughly. Both total and partial SD induce adverse changes in cognitive performance. First and foremost, total SD impairs attention and working memory, but it also affects other functions, such as long-term memory and decision-making. Partial SD is found to influence attention, especially vigilance. Studies on its effects on more demanding cognitive functions are lacking. Coping with SD depends on several factors, especially aging and gender. Also interindividual differences in responses are substantial. In addition to coping with SD, recovering from it also deserves attention. Cognitive recovery processes, although insufficiently studied, seem to be more demanding in partial sleep restriction than in total SD.
Introduction
A person’s quality of life can be disrupted due to many different reasons. One important yet underestimated cause for that is sleep loss ( National Sleep Foundation 2007 ). Working hours are constantly increasing along with an emphasis on active leisure. In certain jobs, people face sleep restriction. Some professions such as health care, security and transportation require working at night. In such fields, the effect of acute total sleep deprivation (SD) on performance is crucial. Furthermore, people tend to stretch their capacity and compromise their nightly sleep, thus becoming chronically sleep deprived.
When considering the effects of sleep loss, the distinction between total and partial SD is important. Although both conditions induce several negative effects including impairments in cognitive performance, the underlying mechanisms seem to be somewhat different. Particularly, results on the recovery from SD have suggested different physiological processes. In this review, we separately consider the effects of acute total and chronic partial SD and describe the effects on cognitive performance. The emphasis on acute total SD reflects the quantity of studies carried out compared with partial SD. The effects of aging and gender, as well as interindividual differences are discussed. We concentrate on the studies that have been published since 1990.
Sleep and sleep loss
The need for sleep varies considerably between individuals ( Shneerson 2000 ). The average sleep length is between 7 and 8.5 h per day ( Kripke et al 2002 ; Carskadon and Dement 2005 ; Kronholm et al 2006 ). Sleep is regulated by two processes: a homeostatic process S and circadian process C (eg, Achermann 2004 ). The homeostatic process S depends on sleep and wakefulness; the need for sleep increases as wakefulness continues. The theory for circadian process C suggests a control of an endogenous circadian pacemaker, which affects thresholds for the onset and offset of a sleep episode. The interaction of these two processes determines the sleep/wake cycle and can be used to describe fluctuations in alertness and vigilance. Although revised “three-process models” (eg, Akerstedt and Folkard 1995 ; Van Dongen et al 2003b ; Achermann 2004 ) have been suggested, this classical model is the principal one used for study designs in SD research.
There are many unanswered questions regarding both the functions of sleep and the effects of sleep loss. Sleep is considered to be important to body restitution, like energy conservation, thermoregulation, and tissue recovery ( Maquet 2001 ). In addition, sleep is essential for cognitive performance, especially memory consolidation ( Maquet 2001 ; Stickgold 2005 ). Sleep loss, instead, seems to activate the sympathetic nervous system, which can lead to a rise of blood pressure ( Ogawa et al 2003 ) and an increase in cortisol secretion ( Spiegel et al 1999 ; Lac and Chamoux 2003 ). Immune response may be impaired and metabolic changes such as insulin resistance may occur (for review, see Spiegel et al 2005 ). People who are exposed to sleep loss usually experience a decline in cognitive performance and changes in mood (for meta-analyses, see Pilcher and Huffcutt 1996 ; Philibert 2005 ).
Sleep deprivation is a study design to assess the effects of sleep loss. In acute total SD protocols, the subjects are kept awake continuously, generally for 24–72 hours. In chronic partial SD, subjects are allowed restricted sleep time during several consecutive nights. Although chronic sleep restriction is more common in the normal population and thus offers a more accurate depiction of real life conditions, total SD has been more thoroughly explored.
Cognitive performances measured in SD studies have included several domains. The most thoroughly evaluated performances include different attentional functions, working memory, and long-term memory. Visuomotor and verbal functions as well as decision-making have also been assessed. Sleep deprivation effects on cognitive performance depend on the type of task or the modality it occupies (eg, verbal, visual, or auditory). In addition, task demands and time on task may play a role. The task characteristics are discussed in more detail in following sections where the existing literature on the cognitive effects of SD is reviewed.
Mechanisms behind sleep loss effects
Some hypotheses are proposed to explain why cognitive performance is vulnerable to prolonged wakefulness. The theories can be divided roughly in two main approaches, in which SD is assumed to have (1) general effects on alertness and attention, or (2) selective effects on certain brain structures and functions. In addition, individual differences in the effects have been reported.
The general explanation relies on the two-process model of sleep regulation. Cognitive impairments would be mediated through decreased alertness and attention through lapses, slowed responses, and wake-state instability. Attentional lapses, brief moments of inattentiveness, have been considered the main reason for the decrease in cognitive performance during sleep deprivation (on lapse hypothesis, eg, Williams et al 1959, see Dorrian et al 2005 ; Kjellberg 1977 ). The lapses are caused by microsleeps characterized by very short periods of sleep-like electro-encephalography (EEG) activity ( Priest et al 2001 ). Originally, it was thought that in between the lapses, cognitive performance almost remained intact, but the slowing of cognitive processing has also been observed independent of lapsing ( Kjellberg 1977 ; Dorrian et al 2005 ). According to these hypotheses, performance during SD would most likely deteriorate in long, simple, and monotonous tasks requiring reaction speed or vigilance. In addition to the lapses and response slowing, considerable fluctuations in alertness and effort have been observed during SD. According to the wake-state instability hypothesis, those fluctuations lead to variation in performance ( Doran et al 2001 ).
According to explanations on selective impact, SD interferes with the functioning of certain brain areas and thus impairs cognitive performance. This approach is also referred to as the ‘sleep-based neuropsychological perspective’ ( Babkoff et al 2005 ). Perhaps the most famous theory in this category is the prefrontal vulnerability hypothesis, first proposed by Horne (1993) . It suggests that SD especially impairs cognitive performances that depend on the prefrontal cortex. These include higher functions, such as language, executive functions, divergent thinking, and creativity. In order to show the SD effect, the tests should be complex, new, and interesting. A good performance would require cognitive flexibility and spontaneity. This theory also assumes that the deterioration of subjects’ performance in simple and long tasks is merely due to boredom ( Harrison and Horne 1998 ; Harrison and Horne 1999 ; Harrison and Horne 2000 ). The specific brain areas that are vulnerable to sleep loss have been explored using functional magnetic resonance imaging (fMRI) and positron emission tomography (PET). Those studies, however, have mainly measured working memory or other attentional functions with the type of tasks that are not traditionally emphasized in the prefrontal vulnerability hypothesis (for summary, see Chee et al 2006 ).
Individuals differ in terms of the length, timing, and structure of sleep. Therefore, it is logical to hypothesize that interindividual differences are also important in reaction to SD. Studies have consistently found that some people are more vulnerable to sleep loss than others (for review, see Van Dongen et al 2005 ). In reference to trait differential vulnerability to SD, Van Dongen et al (2005) have proposed the concept of the “trototype”, as compared to the terms “chronotype” and “somnotype”, which define interindividual differences in the timing of circadian rhythmicity and sleep duration. Since a comprehensive review of the interindividual differences in sleep and performance has been published recently ( Van Dongen et al 2005 ), we will focus here on the studies with group comparisons and just briefly address the trait-like vulnerability.
Acute total sleep deprivation
Attention and working memory.
The two most widely studied cognitive domains in SD research are attention and working memory, which in fact are interrelated. Working memory can be divided into four subsystems: phonological loop, visuospatial sketchpad, episodic buffer and central executive ( Baddeley and Hitch 1974 ; Baddeley 2000 ). The phonological loop is assumed to temporarily store verbal and acoustic information (echo memory); the sketchpad, to hold visuospatial information (iconic memory), and the episodic buffer to integrate information from several different sources. The central executive controls them all. Executive processes of working memory play a role in certain attentional functions, such as sustained attention ( Baddeley et al 1999 ), which is referred to here as vigilance. Both attention and working memory are linked to the functioning of frontal lobes (for a review, see Naghavi and Nyberg 2005 ). Since the frontal brain areas are vulnerable to SD ( Harrison et al 2000 ; Thomas et al 2000 ), it can be hypothesized that both attention and working memory are impaired during prolonged wakefulness.
The decrease in attention and working memory due to SD is well established. Vigilance is especially impaired, but a decline is also observed in several other attentional tasks ( Table 1 ). These include measures of auditory and visuo-spatial attention, serial addition and subtraction tasks, and different reaction time tasks ( Table 1 ). The most frequently used task is the psychomotor vigilance test (PVT, lasts usually 10 min) ( Dinges and Powell 1985 ), which is sensitive to sleep loss effects and provides information about both reaction speed and lapses. In working memory, the tests have varied from n-back style tasks with different demand levels to choice-reaction time tasks with a working memory component ( Table 1 ). However, some studies have also failed to find any effect. After one night of SD, no difference was observed between deprived and non-deprived subjects in simple reaction time, vigilance, or selective attention tasks in one study ( Forest and Godbout 2000 ). Performance on the Wisconsin Card Sorting Test, a measure of frontal lobe function, also remained even ( Binks et al 1999 ; Forest and Godbout 2000 ). These results may be partly biased because of small sample sizes, inadequate control of the subjects’ sleep history or the use of stimulants before the study.
Cognitive tests in which deterioration of performance has been reported during acute total sleep deprivation
Abbreviations: SD, sleep deprivation; WAIS, Wechsler Adult Intelligence Scale; WAIS-R, Wechsler Adult Intelligence Scale-Revised.
Outcomes are inconsistent in various dual tasks used for measuring divided attention. Sleep deprivation of 24 h impaired performance in one study ( Wright and Badia 1999 ), whereas in two others, performance was maintained after 25–35 h of SD ( Drummond et al 2001 ; Alhola et al 2005 ). The divergent findings in these studies may be explained by the uneven loads between different subtests as well as by uncontrolled practice effect. Although dividing attention between different tasks puts high demands on cognitive capacity, subjects often attempt to reduce the load by automating some easier procedures of a dual or multitask. In the study by Wright and Badia (1999) , the test was not described; in the study by Alhola et al (2005) , subjects had to count backwards and carry out a visual search task simultaneously, and in the study by Drummond et al (2001) subjects had to memorize words and complete a serial subtraction task sequentially. In addition, differences in essential study elements, like the age and gender of participants, as well as the duration of SD, further complicate comparison of the results.
In the tasks measuring attention or working memory, two aspects of performance are important: speed and accuracy. In practice, people can switch their emphasis between the two with attentional focusing ( Rinkenauer et al 2004 ). Oftentimes, concentrating on improving one aspect leads to the deterioration of the other. This is called the speed/accuracy trade-off phenomenon. Some SD studies have found impairment only in performance speed, whereas accuracy has remained intact ( De Gennaro et al 2001 ; Chee and Choo 2004 ). In others, the results are the opposite ( Kim et al 2001 ; Gosselin et al 2005 ). De Gennaro et al (2001) proposed that in self-paced tasks, there is likely to be a stronger negative impact on speed, while accuracy remains intact. In experimenter-paced tasks, the effect would be the opposite. However, many studies show detrimental effect on both speed and accuracy (eg, Smith et al 2002 ; Jennings et al 2003 ; Chee and Choo 2004 ; Habeck et al 2004 ; Choo et al 2005 ). The speed/accuracy trade-off phenomenon is moderately affected by gender, age, and individual differences in response style ( Blatter et al 2006 ; Karakorpi et al 2006 ), which could be a reason for inconsistencies in the SD results. It has been argued that low signal rates increase fatigue during performance in SD studies and that subjects may even fall asleep during the test ( Dorrian et al 2005 ). Therefore, tasks with different signal loads may produce different results in terms of performance speed and accuracy.
Long-term memory
Long-term memory can be divided between declarative and non-declarative (procedural) memory. Declarative memory is explicit and limited, whereas non-declarative memory is implicit and has a practically unlimited capacity. Declarative memory includes semantic memory, which consists of knowledge about the world, and episodic memory, which holds autobiographical information. The contents of declarative memory can be stored in visual or verbal forms and they can be voluntarily recalled. Non-declarative or procedural memory includes the information needed in everyday functioning and behavior, eg, motor and perceptual skills, conditioned functions and priming. In previous studies, long-term memory has been measured with a variety of tasks, and the results are somewhat inconsistent.
In verbal episodic memory, SD of 35 h impaired free recall, but not recognition ( Drummond et al 2000 ). The opposite results were obtained with one night of SD ( Forest and Godbout 2000 ). The groups in both studies were quite small (in Drummond’s study, N = 13; in Forest and Godbout’s study, experimental group = 9, control group = 9), which offers a possible explanation for the variation in results. In addition, Drummond et al (2000) used a within-subject design, whereas Forest and Godbout (2000) had a between-subject design. In visual memory, recognition was similar in the experimental and control groups when the measurement was taken once after 36 h SD ( Harrison and Horne 2000 ), whereas the practice effect in visual recall was postponed by SD in a study with three measurements (baseline, 25 h SD, recovery; Alhola et al 2005 ). Performance was impaired in probed forced memory recall ( Wright and Badia 1999 ), and memory search ( McCarthy and Waters 1997 ), but no effect was found in episodic memory ( Nilsson et al 2005 ), implicit memory, prose recall, crystallized semantic memory, procedural memory, or face memory ( Quigley et al 2000 ). In the studies failing to find an effect, however, the subjects spent only the SD night under controlled conditions ( Quigley et al 2000 ; Nilsson et al 2005 ).
Free recall and recognition are both episodic memory functions which seem to be affected differently by SD. Temporal memory for faces (recall) deteriorated during 36 h of SD, although in the same study, face recognition remained intact ( Harrison and Horne 2000 ). In verbal memory, the same pattern was observed ( Drummond et al 2000 ). One explanation may be different neural bases, which supports the prefrontal vulnerability hypothesis. Episodic memory is strongly associated with the functioning of the medial temporal lobes ( Scoville and Milner 2000 ), but during free recall in a rested state, even stronger brain activation is found in the prefrontal cortex ( Hwang and Golby 2006 ). It is unclear whether this prefrontal activation reflects episodic memory function, the organization of information in working memory, or the executive control of attention and memory. Recognition, instead, presumably relies on the thalamus in addition to medial temporal lobes ( Hwang and Golby 2006 ). Since SD especially disturbs the functioning of frontal brain areas ( Drummond et al 1999 ; Thomas et al 2000 ), it is not surprising that free recall is more affected than recognition.
Although the prefrontal cortex vulnerability hypothesis has received wide support in the field of SD research, other brain areas are also involved. For instance, the exact role of the thalamus remains unknown. Some studies measuring attention or working memory have noted an increase in thalamic activation during SD (eg, Portas et al 1998 ; Chee and Choo 2004 ; Habeck et al 2004 ; Choo et al 2005 ). This may reflect an increase in phasic arousal or an attempt to compensate attentional performance during a demanding condition of low arousal caused by SD ( Coull et al 2004 ). In other cognitive tasks such as verbal memory ( Drummond and Brown 2001 ) or logical reasoning ( Drummond et al 2004 ), no increase in thalamic activation was found despite the fact that behavioral deterioration occurred. This implies that thalamic activation during SD is mainly related to some attentional function or compensation, providing further support for the hypothesis that “prefrontal dependent” recall is more affected by SD than “thalamus dependent” recognition. However, it is possible that the brain activation patterns during SD reflect something more than merely different cognitive domains. Harrison and Horne (2000) stated that their results may also reflect the difficulty of the task assigned to subjects.
Other cognitive functions
Sleep deprivation impairs visuomotor performance, which is measured with tasks of digit symbol substitution, letter cancellation, trail-making or maze tracing ( Table 1 ). It is believed that visual tasks would be especially vulnerable to sleep loss because iconic memory has short duration and limited capacity ( Raidy and Scharff 2005 ). Another suggestion is that SD impedes engagement of spatial attention, which can be observed as impairments in saccadic eye movements ( Bocca and Denise 2006 ). Decreased oculomotor functioning is associated with impaired visual performance ( De Gennaro et al 2001 ) and sleepiness (eg, De Gennaro et al 2001 ; Zils et al 2005 ). However, further research is needed to confirm this explanation, since not all studies have found oculomotor impairment with cognitive performance decrements ( Quigley et al 2000 ).
Reasoning ability during SD has for the most part been measured with Baddeley’s logical reasoning task or its modified versions. Again the results are inconsistent (deteriorated performance was reported by Blagrove et al 1995 ; McCarthy and Waters 1997 ; Monk and Carrier 1997 , and Harrison and Horne 1999 ; no effects were noted by Linde and Bergstrom 1992 ; Quigley et al 2000 , or Drummond et al 2004 ). The studies reporting no effect have mainly used SD of ca. 24 h ( Linde and Bergström 1992 ; Quigley et al 2000 ), whereas in the studies showing an adverse effect, the SD period has been longer (36 h). Thus reasoning ability seems to be maintained during short-term SD. However, choosing divergent study designs may result in different outcomes. Monk and Carrier (1997) repeated the cognitive test every 2 h and found deterioration after as little as 16 h of SD. In the studies with zero-results, cognitive tests were carried out in the morning ( Linde and Bergström 1992 ; Quigley et al 2000 ) or the practice effect was not adequately controlled ( Drummond et al 2004 ). In the studies with longer SD, the tests have been conducted either in the late afternoon ( McCarthy and Waters 1997 ; Harrison and Horne 1999 ) or have been repeated several times ( Blagrove et al 1995 ; Monk and Carrier 1997 ). Therefore, the different results may reflect the effect of circadian rhythm on alertness and cognitive performance. In the morning or before noon, the circadian process reaches its peak, inducing greater alertness, whereas the timing of the circadian nadir coincides with the late afternoon testing (see Achermann 2004 ).
In addition to the cognitive domains already introduced, total SD affects several other cognitive processes as well. It increases rigid thinking, perseveration errors, and difficulties in utilizing new information in complex tasks requiring innovative decision-making ( Harrison and Horne 1999 ). Deterioration in decision-making also appears as more variable performance and applied strategies ( Linde et al 1999 ), as well as more risky behavior ( Killgore et al 2006 ). Several other tasks have been used in the sleep deprivation studies ( Table 1 ). For example, motor function, rhythm, receptive and expressive speech, and memory measured with the Luria-Nebraska Neuropsychological Battery deteriorated after one night of SD, whereas tactile function, reading, writing, arithmetic and intellectual processes remain intact ( Kim et al 2001 ).
The adverse effects of total SD shown in experimental designs have also been confirmed in real-life settings, mainly among health care workers, professional drivers and military personnel ( Samkoff and Jacques 1991 ; Otmani et al 2005 ; Philibert 2005 ; Russo et al 2005 ). Performance of residents in routine practice and repetitive tasks requiring vigilance becomes more error-prone when wakefulness is prolonged (for a review, see Samkoff and Jacques 1991 ). However, in new situations or emergencies, the residents seem to be able to mobilize additional energy sources to compensate for the effects of tiredness. More recent meta-analysis shows that SD of less than 30 h causes a significant decrease in both the clinical and overall performance of both residents and non-physicians ( Philibert 2005 ).
What role does motivation play in cognitive performance? Can high motivation reverse the adverse effect of SD? Does poor motivation further deteriorate performance? According to a commonly held opinion, high motivation compensates for a decrease in performance, but only a few attempts have been made to confirm this theory. Estimating the compensatory effect of motivation in performance during SD is generally difficult, because persons participating in research protocols, especially in SD studies, usually have high initial motivation. The concept of motivation is closely linked to the “attentional effort” that is considered a cognitive incentive (for a review, see Sarter et al 2006 ). According to Sarter et al (2006) , “increases in attentional effort do not represent primarily a function of task demands but of subjects’ motivation to perform.” Furthermore, attentional effort is a function of explicit and implicit motivational forces and may be increased especially when the subjects are motivated or when they detect signals of performance decrements ( Sarter et al 2006 ).
Harrison and Horne (1998 , 1999) suggest that the deterioration of cognitive performance during SD could be due to boredom and lack of motivation caused by repeated tasks, especially if the tests are simple and monotonous. They used short, novel, and interesting tasks to abolish this motivational gap, yet still noted that SD impaired performance. In contrast, other researchers suggest that sleep-deprived subjects could maintain performance in short tasks by being able to temporarily increase their attentional effort. When a task is longer, performance deteriorates as a function of time. A meta-analysis by Pilcher and Huffcutt (1996) provides support for that: total SD of less than 45 h deteriorated performance more severely in complex tasks with a long duration than in simple and short tasks. Based on this, it is probably necessary to make a distinction between mere attentional effort and more general motivation. Although attentional effort reflects motivational aspects in performance, motivation in a broader sense can be considered a long-term process such as achieving a previously set goal, eg, completing a study protocol. If one has already invested a great deal of time and effort in the participation, motivation to follow through may be increased.
Different aspects of motivation were investigated in a study with 72 h SD, where the subjects evaluated both motivation to perform the tasks and motivation to carry out leisure activities ( Mikulincer et al 1989 ). Cognitive tasks were repeated every two hours. Performance motivation decreased only during the second night of SD, whereas leisure motivation decreased from the second day until the end of the study on the third day. The authors concluded that the subjects were more motivated to complete experimental testing than to enjoy leisure activities because by performing the tasks, they could advance the completion of the study. The researchers suggested that the increased motivation towards the tasks on the third day reflected the “end spurt effect” caused by the anticipation of sleep.
Providing the subjects with feedback on their performance or rewarding them for effort or good performance is shown to help maintain performance both in normal, non-deprived conditions ( Tomporowski and Tinsley 1996 ) and during SD ( Horne and Pettitt 1985 ; Steyvers 1987 ; Steyvers and Gaillard 1993 ). In a large study with 61 subjects (experimental group = 29), with SD of 34–36 h, and with a comprehensive test battery, the subjects were continuously encouraged and provided with 2–3 minute breaks between the tests ( Binks et al 1999 ). Furthermore, they were told they would receive a monetary award for completing all tests with “honest effort”. As result, no deteriorating effect on cognitive performance was found. Unfortunately, a non-motivated control group was not included and thus the effect of motivation remained uncertain. In general, since this issue has not been addressed sufficiently, it is difficult to specify the role of motivation in performance. It seems that motivation affects performance, but it also appears that SD can lead to a loss of motivation.
Self-evaluation of cognitive performance
It has been suggested that the self-evaluation of cognitive performance is impaired by SD. During 36 h SD, the subjects became more confident that their answers were correct as the wakefulness continued ( Harrison and Horne 2000 ). Confidence was even stronger when the answer was actually wrong. In another study, performance was similar between sleep-deprived and control groups in several attentional assessments, but the deprived subjects evaluated their performance as moderately impaired ( Binks et al 1999 ). The controls considered that their performance was high.
The ability to evaluate one’s own cognitive performance depends on age and on the study design. Young people seem to underestimate the effect of SD, whereas older people seem to overestimate it. In a simple reaction time task, both young (aged 20–25 years) and aging (aged 52–63 years) subjects considered that their performance had deteriorated after 24 h SD, although performance was actually impaired only in young subjects ( Philip et al 2004 ). When it comes to the study design and methodology, the way in which the self-evaluation is done may affect the outcome. The answers possibly reflect presuppositions of the subjects or their desire to please the researcher. The repetition of tasks is also essential. Evaluation ability is poor in studies with one measurement only ( Binks et al 1999 ; Harrison and Horne 2000 ; Philip et al 2004 ), whereas in repeated measures, the subjects are shown to be able to assess their performance quite reliably during 60–64 h SD and recovery ( Baranski et al 1994 ; Baranski and Pigeau 1997 ). Thus, self-evaluation is likely to be more accurate when subjects can compare their performance with baseline.
Chronic partial sleep restriction
Although chronic partial sleep restriction is common in everyday life and even more prevalent than total SD, surprisingly few studies have evaluated its effects on cognitive performance. Even fewer studies have compared the effects of acute total sleep deprivation and chronic partial sleep restriction. Belenky and co-workers (2003) evaluated the effect of partial sleep restriction in a laboratory setting in groups which were allowed to spend 3, 5 or 7 h in bed daily for seven consecutive days. The control group spent 9 h in bed. In the 3 h group, both speed and accuracy in the PVT deteriorated almost linearly as the sleep restriction continued. In this group, performance was clearly the worst. In the 5- and 7 h groups, performance speed deteriorated after the first two restriction nights, but then remained stable (though impaired) during the rest of the sleep restriction from the third night onwards. Impairment was greater in the 5- than 7 h group. Accuracy followed the same pattern in the 7 h group, but further declined in the 5 h group as the study went on. The control group’s performance did not change during the study. Intriguingly, a highly similar pattern was observed in another study with the same task when sleep was restricted by 33% of the subject’s habitual nightly sleep, which resulted in 5 h of sleep per night on average ( Dinges et al 1997 ). Both speed and accuracy were impaired at the beginning of the sleep restriction period followed by a plateau and finally, another drop after the seventh night of deprivation. However, no change was found in probed recall memory or serial addition tests, probably because of the practice effect and short duration of the tests (serial addition test: 1 min).
It is difficult to compare the effects of total and partial SD based on existing literature due to large variation in methodologies, including the length of SD or the type of cognitive measures. The only study that has compared total and partial SD found that after controlling learning effects, cognitive performance declined almost linearly in the course of the study in all four experimental groups ( Van Dongen et al 2003a ): one group was exposed to 3 nights total SD, and in other experimental groups, time in bed was restricted to 4 or 6 h for 14 consecutive days. The control group was allowed 8 h in bed for 14 days. Impairment in psychomotor vigilance test and digit symbol substitution task for the 4 h group after 14 days was equal to that of the total SD group after 2 nights. Deterioration in the serial addition/subtraction task for the 4 h group was similar to that of the total SD group after 1 night. The effect of 6 h restricted sleep corresponded to 1 night of total SD in psychomotor vigilance and digit symbol. Performance remained unaffected in the control group.
According to the well-controlled studies ( Dinges et al 1997 ; Belenky et al 2003 ; Van Dongen et al 2003a ), the less sleep obtained due to sleep restriction, the more cognitive performance is impaired. Otherwise, it is difficult to draw conclusions about the effects of chronic sleep restriction because of methodological problems in the previous studies. Blagrove et al (1995) compared subjects that slept at home either 5 h or 8 h per night for 4 weeks and found no effect in a short task of logical reasoning (duration 5 min). The statistical analyses were compromised by the small sample size (6 subjects in the experimental group and only 4 subjects in the control group). In another protocol, they also carried out auditory vigilance test, two column addition, finding embedded figures, and logical reasoning (10 min) tasks, and again no effect was observed with groups of 6–8 subjects having 4, 5 or 8 h sleep per night for 7, 19 or 40 weeks respectively ( Blagrove et al 1995 ). Casement et al (2006) reported no change in working memory and motor speed in the group whose sleep was restricted to 4 h per night for 9 nights. In the control group, performance improved. The study was carried out in a controlled clinical environment, but only one short test session per day was included, which means that subjects may have been able to temporarily increase their effort and thus maintain their performance. Furthermore, the results were confounded by the practice effect. In other sleep restriction studies, SD cannot be considered chronic, since the length of the restriction has been 1–3 nights ( Stenuit and Kerkhofs 2005 ; Swann et al 2006 ; Versace et al 2006 ).
Since chronic partial SD mimics every day life situations more than acute total SD, additional studies on how it affects cognitive performance are warranted. In addition, the tasks used in previous studies have been quite short and simple, and trials with more demanding cognitive tasks are required. The effects of sleep restriction have also been addressed by drive simulation studies, which are interesting and practical designs. Just one night of restricted sleep (4 h) increased right edge-line crossings in a motorway drive simulation of 90 minutes ( Otmani et al 2005 ). However, neither the drivers’ position in the lane nor the amplitude and frequency of steering wheel movements were affected. One sleep-restricted night did not increase the probability of a crash, but after five nights of partial SD, the quantity of accidents increased ( Thorne et al 1999 ).
Cognitive recovering from sleep deprivation
The recovery processes of cognitive performance after sleep loss are still obscure. In many SD studies, the recovery period has either not been included in the protocol or was not reported. Recovery sleep is distinct from normal sleep. Sleep latency is shorter, sleep efficiency is higher, the amounts of SWS and REM-sleep are increased and percentages of stage 1 sleep and awake are decreased ( Armitage et al 2001 ; Kilduff et al 2005 ). The characteristics of recovery sleep may also depend on circumstances and some differences seem to come with eg, aging ( Kalleinen et al 2006 ). Evidence suggests that one sleep period (at least eight hours) can reverse the adverse effects of total SD on cognition ( Brendel et al 1990 ; Corsi-Cabrera et al 2003 ; Adam et al 2006 ; Drummond et al 2006 ; Kendall et al 2006 ). The tasks have been mainly simple attentional tasks; for example, the PVT used by Adam et al (2006) has been proven to have practically no learning curve and little if any correlation with aptitude ( Durmer and Dinges 2005 ). Thus, it is likely that the improvement was mostly caused by the recovery process and not just the practice effect.
After chronic partial sleep restriction, the recovery process of cognitive functioning seems to take longer than after acute total SD. Performance in the PVT was not restored after one 10 h recovery night, but approached the baseline level after two 10 h nights in a study with seven consecutive sleep restriction nights with 5 h sleep/night ( Dinges et al 1997 ). Using the same test, three 8 h recovery nights were not enough to restore performance after one week of sleep restriction even in the group that spent 7 h time in bed (the study is explained in greater detail in paragraph 1 of “Partial sleep restriction”, Belenky et al 2003 ). The group that spent 3 h in bed showed the greatest decline as well as the greatest recovery, although it did not reach baseline level again. In the 5 h group, a similar deterioration-recovery curve was observed, although it was not as steep. Those authors concluded that during mild and moderate chronic partial SD, the brain adapted to a stressful condition to maintain performance, yet at a reduced level. This adaptation process was obviously so demanding that it postponed the restoration of normal functioning. According to their results, it could be further interpreted that when sleep restriction was severe, no such adaptation occurred, which in turn allowed for greater recovery. However, these results may be biased because of poor statistical sensitivity in multiple comparisons. They have also been criticized by eg, Van Dongen et al (2004) , who pointed out that another confounding factor may have been considerable interindividual differences in recovery rates. Since interindividual differences have been observed in response to SD, it is likely – although not yet adequately verified – that those individual traits also affect the recuperation.
Sleep deprivation in different populations
Sleep structure changes with aging. Slow wave sleep and sleep efficiency decrease, and alterations in the circadian rhythm occur (for reviews, see Dzaja et al 2005 ; Gaudreau et al 2005 ). Sleep complaints also become more frequent ( Leger et al 2000 ). Yet, during prolonged wakefulness, cognitive performance seems to be maintained better in aging people than in younger ones ( Bonnet and Rosa 1987 ; Smulders et al 1997 ; Philip et al 2004 ; Stenuit and Kerkhofs 2005 ). Total SD of 24 h deteriorated vigilance in young subjects (20–25 years), whereas performance in aging subjects (52–63 years) remained unaffected ( Philip et al 2004 ). Similarly, during three consecutive nights of partial SD (4 h in bed) performance in psychomotor vigilance task declined more in young subjects (20–30 years) than in aging ones (55–65 years, Stenuit and Kerkhofs 2005 ). In visual episodic memory, visuomotor performance and divided attention, aging subjects (58–72 years) were able to maintain their performance after 25 h of SD and showed improvement only after a recovery night ( Alhola et al 2005 ). However, no comparison with young subjects was made in that study.
Sleep deprivation deteriorates accuracy of performance, especially in young subjects ( Brendel et al 1990 ; Smulders et al 1997 ; Adam et al 2006 ; Karakorpi et al 2006 ). Regarding performance speed, however, results have been inconsistent and the performance of aging subjects has declined more, less, or equally compared to that of younger people. In simple and two-choice reaction time tasks as well as in a vigilance task, reaction speed was impaired in aging subjects (59–72 years) during 40 h SD, whereas young subjects (20–26 years) kept up their speed ( Karakorpi et al 2006 ). These results followed the speed/accuracy trade-off phenomenon so that aging subjects maintained accuracy at the expense of speed and the younger ones did the opposite. In contrast, two other studies found that young subjects were slower than aging subjects ( Brendel et al 1990 ; Adam et al 2006 ). During 24 h wakefulness, performance speed in a vigilance task was impaired in both 20- and 80-year-olds, but more so in the young subjects ( Brendel et al 1990 ). This was confirmed in another study with 40 h SD ( Adam et al 2006 ). When measuring reaction speed in three different choice-reaction time tasks, performance deteriorated similarly in young (18–24 years) and aging (62–73 years) subjects after 28 h total SD ( Smulders et al 1997 ).
Even though there is some evidence that older subjects tolerate SD better than young subjects, it is difficult to determine the age effect during SD with precision. However, because of age-related changes in many aspects of sleep and wakefulness, it is plausible that aging influences reactions to SD. As suggested previously, the weaker SD effect in aging may be due to attenuation of the circadian amplitude, which is reflected in the performance curve in vigilance tasks ( Blatter et al 2006 ). Also, changes in the homeostatic process may play a role. During wakefulness, the accumulation of sleep pressure seems to be reduced in aging ( Murillo-Rodriguez et al 2004 ), which could leave older subjects more alert. There is also evidence that aging subjects recover faster from SD than young subjects in terms of physiological sleep ( Bonnet and Rosa 1987 ; Brendel et al 1990 ). This faster recovery in sleep state may also mean better restoration of cognitive performance ( Bonnet and Rosa 1987 ; Brendel et al 1990 ). However, more research is necessary to confirm these hypotheses.
The age effect found in previous studies could also be explained by methodological factors, such as inadequate control of the baseline conditions. Younger subjects are usually more chronically sleep deprived ( National Sleep Foundation 2002 ) due to several reasons, such as studying, career building or raising children. Chronic sleep restriction may cause long-term changes in brain functions that are not reversible during short adaptation and baseline periods in sleep laboratory studies. Even though subjects of certain studies were instructed to maintain a regular 8 h sleep schedule for 3–5 days, this may not be enough to erase the previous “sleep debt” ( Brendel et al 1990 ; Philip et al 2004 ; Adam et al 2006 ). Furthermore, in the long run, people tend to get used to experiencing sleepiness ( Van Dongen et al 2003a ) and thus may not even recognize being chronically sleep deprived. Perhaps aging people also have more experience that helps them to cope with the challenges posed by SD. Nevertheless, based on the available studies, it is impossible to distinguish the factors behind the age effect.
There are dissimilarities between genders in sleep structure measured with polysomnography (for a review, see Manber and Armitage 1999 ). Furthermore, women of all ages report more sleeping problems than men ( Leger et al 2000 ). Sex hormones affect sleep through several mechanisms, both genomic and nongenomic, including neurochemical and vascular mechanisms (for a review, see Dzaja et al 2005 ). This ensures instant and short-term effects as well as long-term ones.
It is possible that physiological responses to SD are not equal among men and women. During SD of 38 h, EEG showed more sleep activity in men than in women during waking rest and cognitive performance ( Corsi-Cabrera et al 2003 ). Presumably, therefore, one recovery night of nine hours would be enough to restore waking EEG activity in men, but not in women. Only a few studies have examined gender differences in cognitive performance during SD. In a vigilance task, performance was more impaired in men but returned to the baseline level in both men and women after recovery sleep ( Corsi-Cabrera et al 2003 ). In another study, women performed better than men in verbal and in visuo-constructive tasks during 35 h SD ( Binks et al 1999 ). No gender differences were observed in word fluency, maintenance or suppression of attention, auditory attention or cognitive flexibility. In that study, however, only one point of measurement was included, and so the difference in performance could be caused by SD or initial distinctions between the gender groups.
Few attempts have been made to evaluate the effect of sex hormones on coping with SD. It has been suggested that hormone therapy, which is widely used for women during their menopausal transition to help alleviate climacteric symptoms, attenuates physiological stress response ( Lindheim et al 1992 ). However, after 25 h of total SD, no difference was observed between hormone therapy users and nonusers in visual episodic memory, visuomotor performance, verbal attention and shared attention ( Alhola et al 2005 ). In addition, during 40 h of SD, hormone therapy did not produce any advantage in reaction time or vigilance tasks ( Karakorpi et al 2006 ).
The previous studies suggest that women cope with continuous wakefulness better than men. According to evolution, the demands of child nurturing and rearing in women would support this hypothesis ( Corsi-Cabrera et al 2003 ), but that certainly does not constitute a comprehensive explanation today. Gender differences during SD could be due to either physiological or social factors. There are differences in the brain structure and functioning of men and women ( Ragland et al 2000 ; Cowell et al 2007 ). These can be seen in cognitive performance in normal, non-deprived conditions: men typically have better spatial abilities and mental rotation, and higher visuo-constructive performance, whereas women perform better in visuomotor speed and some verbal functions, especially verbal fluency (for a review, see Kimura 1996 ). Men and women also exhibit behavioral and lifestyle differences, which are mainly due to socialization and gender roles ( Eagly and Wood 1999 ). Current literature, however, provides only minimal evidence of differential effects during SD, and does not resolve the issue of sexual dimorphism in coping with SD.
Interindividual differences
Several studies provide evidence that during total SD, performance becomes more variable as assessed from the within-subject point of view (eg, Smith et al 2002 ; Habeck et al 2004 ; Choo et al 2005 ). This is considered to reflect the wake-state instability caused by prolonged wakefulness. However, Doran et al (2001) were probably the first to also examine between-subjects variability, which they found to increase in PVT as wakefulness was extended to 88 hours. They suggested that some people are more vulnerable to the effects of sleep loss than others, which could probably explain the lack of significant results in some group comparisons. These differences between subjects could have arguably been caused by differences in sleep history, but the sleep patterns for the preceding week were controlled with sleep diaries, actigraph, and calls to the time-stamped voice recorder.
The interindividual variability has been further examined with a thorough protocol where a three night study (baseline, 36 h SD and recovery) was carried out three times ( Van Dongen et al 2004 ). Sleep history was manipulated by instructing subjects to stay in bed for either 6 or 12 h per night for one week before the study. The 12 h procedure was repeated and the order of the conditions was counterbalanced. The cognitive test selection included serial addition/subtraction task, digit symbol, critical tracking, word detection, repeated acquisition of response sequences, and PVT. The authors concluded that interindividual differences were systematic and independent from sleep history. The trait-like differential vulnerability to sleep loss has received support from an fMRI study attempting to reveal the neural basis for the interindividual differences ( Chuah et al 2006 ). They used a go/no-go task to measure response inhibition after 24 h of sleep deprivation. The results indicated that the subjects less vulnerable to SD had lower prefrontal cortex activation at the rested wakefulness than the more vulnerable subjects. During SD, activation increased temporarily in the prefrontal cortex and in some other areas only in the less vulnerable subjects. Since interindividual differences have also been found in other sleep-related variables, such as duration, timing, and quality of sleep, sleepiness, and circadian phase ( Van Dongen 1998 ; Van Dongen et al 2005 ), it is plausible that the tolerance to SD may also vary. Nevertheless, more studies are needed for further support.
Methodological issues and common biases
Although the adverse effects of SD on cognitive performance are quite well established, some studies have failed to detect any deterioration. Inadequate descriptions of study protocols or subject characteristics in some studies make it difficult to interpret the neutral results. However, it is likely that such results are due to methodological shortcomings, such as insensitive cognitive measures, failure to control the practice effect or other confounding factors, like individual sleep history or napping during the study. Also, if the task is carried out only once during the SD period, the results may be influenced by circadian rhythm.
Sleep deprivation studies are laborious and expensive to carry out, which may lead to compromises in the study design: for example, a small sample size can reduce the statistical power of the study, but a larger population may come at the expense of other methodological issues, such as a reduction in the cognitive test selection or in the number of nights spent in the sleep laboratory. Comparison of the results is also complicated because the length of sleep restriction varies and the studies are designed either within- or between-subjects.
Sleeping in unfamiliar surroundings may impair sleep quality. An adaptation night at the sleep laboratory is used to minimize this first night effect. However, in several studies, this has been neglected and the SD period has been preceded by a “normal” night at home (eg, Harrison and Horne 2000 ; Jennings et al 2003 ; Choo et al 2005 ). Although sleeping at home certainly reflects a subject’s reality more accurately, it does not allow for precise control and information of sleeping conditions. Adding a portable recording, such as an actigraph, provides objective information about eg, bedtime and resting periods. In some studies, the first night in the sleep laboratory has been the baseline (eg, Drummond et al 2000 ; Forest and Godbout 2000 ; De Gennaro et al 2001 ; Drummond et al 2001 ), whereas others have included one adaptation night (eg, Casagrande et al 1997 ; Alhola et al 2005 ). Yet, it may be questionable to use data from the second night as the baseline because sleep quality can be better than normal due to the rebound from the first night. Accordingly, only data from the third night should be accepted, which has been the case in a few studies ( Thomas et al 2000 ; Van Dongen et al 2003a ). This, however, makes the procedure very hard. Furthermore, study protocols can be improved by adding an ambulatory EEG recording to confirm the wakefulness of the subjects during the study.
In sleep studies, a common pitfall is recruitment methods. Enrolment via advertisements or from sleep clinics favors the selection of subjects with sleeping problems or concerns about their cognitive performance. Thus, strict exclusion criteria regarding physical or mental diseases or sleeping problems are essential. Further, sleeping habits should be controlled to make sure that the subjects are not initially sleep deprived. For this, use of a sleep diary for eg, 1–3 weeks before the experiment (eg, De Gennaro et al 2001 ; Habeck et al 2004 ; Alhola et al 2005 ) or an actigraph is applicable ( Harrison and Horne 1999 ; Thomas et al 2000 ).
The use of medication or stimulants, such as caffeine, alcohol or tobacco, is often prohibited before the experiment (eg, Thomas et al 2000 ; Van Dongen et al 2003a ; Habeck et al 2004 ; Alhola et al 2005 ; Choo et al 2005 ). In some studies, the subjects have been required to refrain from these substances only 24 h before the study ( Habeck et al 2004 ; Choo et al 2005 ), which may increase withdrawal symptoms and dropping out of the study. Thus a longer abstinence, eg, 1–2 weeks, is more appropriate ( Van Dongen et al 2003a ; Alhola et al 2005 ).
A variety of cognitive tests, from simple reaction time measures to complex decision-making tasks requiring creativity and reasoning, have been used to evaluate the effect of SD on cognition. The greatest problem in repeated cognitive testing is the practice effect, which easily conceals any adverse effects of SD. Therefore, careful control over learning is essential. Cognitive processes are also intertwined in several ways, which makes it difficult to specify exactly which cognitive functions are utilized in certain performances. Because attention is involved in performing any cognitive task, a decrease in other cognitive domains during SD may be mediated through impaired attention. In complex tasks, however, applying previous knowledge and use of strategies or creativity may be more essential. Some studies have concentrated on neural correlates of cognitive functioning during continuous wakefulness. Both fMRI ( Portas et al 1998 ; Drummond et al 2000 ; Drummond et al 2001 ; Chee and Choo 2004 ; Habeck et al 2004 ; Choo et al 2005 ) and PET have been used ( Thomas et al 2000 ). Although these trials yield interesting information about brain functioning, the use of imaging techniques limits the selection of cognitive tests that could be carried out at the same time.
Dorrian et al (2005) have compiled a list of criteria for neurocognitive tests that would be suitable for investigating sleep deprivation effects. The criteria include psychometric quality, ie, reliability and validity, but the tests should also reflect a fundamental aspect of waking neurocognitive functions and it should be possible to interpret them in a meaningful way. The tasks should be repeatable, independent of aptitude, and they should be short with a high signal load. These criteria are not met in some studies. Dorrian et al (2005) also argued that vigilance is the underlying factor through which the sleep deprivation effects are mediated in all other tasks. However, although attention is needed to perform any task to some extent, the hypothesis that sleep deprivation can have an independent effect on other cognitive functions such as memory cannot be ruled out. Nevertheless, when measuring other cognitive functions, the characteristics of the task should be considered carefully and, eg, for repeated measures of memory, parallel test versions should be used.
The negative effect of both acute total and chronic partial SD on attention and working memory is supported by existing literature. Total SD impairs a range of other cognitive functions as well. In partial SD, a more thorough evaluation of higher cognitive functions is needed. Furthermore, the effects of SD have not been thoroughly compared among some essential subpopulations.
Aging influences a person’s ability to cope with SD. Although in general the cognitive performance of aging people is often poorer than that of younger individuals, during SD performance in older subjects seems to deteriorate less. Based on the scarce evidence, it seems that in terms of cognitive performance, women may endure prolonged wakefulness better than men, whereas physiologically they recover slower. Tolerating SD can also depend on individual traits. However, mechanisms inducing differences between the young and aging and between men and women or different individuals are mostly unclear. Several reasons such as physiological mechanisms as well as social or environmental factors may be involved. In conclusion, there is great variation in SD studies in terms of both subject selections and methods, and this makes it difficult to compare the different studies. In the future, methodological issues should be considered more thoroughly.
- Achermann P. The two-process model of sleep regulation revisited. Aviat Space Environ Med. 2004; 75 :A37–43. [ PubMed ] [ Google Scholar ]
- Adam M, Retey JV, Khatami R, et al. Age-related changes in the time course of vigilant attention during 40 hours without sleep in men. Sleep. 2006; 29 :55–7. [ PubMed ] [ Google Scholar ]
- Akerstedt T, Folkard S. Validation of the S and C components of the three-process model of alertness regulation. Sleep. 1995; 18 :1–6. [ PubMed ] [ Google Scholar ]
- lhola P, Tallus M, Kylmälä M, et al. Sleep deprivation, cognitive performance, and hormone therapy in postmenopausal women. Menopause. 2005; 12 :149–55. [ PubMed ] [ Google Scholar ]
- Armitage R, Smith C, Thompson S, et al. Sex differences in slow-wave activity in response to sleep deprivation. Sleep Research Online. 2001; 4 (1):33–41. [ Google Scholar ]
- Babkoff H, Zukerman G, Fostick L, et al. Effect of the diurnal rhythm and 24 h of sleep deprivation on dichotic temporal order judgment. J Sleep Res. 2005; 14 :7–15. [ PubMed ] [ Google Scholar ]
- Baddeley AD, Hitch GJ. Working memory . Academic Press; 1974. pp. 47–89. [ Google Scholar ]
- Baddeley A. The episodic buffer: A new component of working memory? Trends Cogn Sci. 2000; 4 :417–23. [ PubMed ] [ Google Scholar ]
- Baddeley A, Cocchini G, Della Sala S, et al. Working memory and vigilance: Evidence from normal aging and alzheimer’s disease. Brain Cogn. 1999; 41 :87–108. [ PubMed ] [ Google Scholar ]
- Baranski JV, Pigeau RA. Self-monitoring cognitive performance during sleep deprivation: Effects of modafinil, d-amphetamine and placebo. J Sleep Res. 1997; 6 :84–91. [ PubMed ] [ Google Scholar ]
- Baranski JV, Pigeau RA, Angus RG. On the ability to self-monitor cognitive performance during sleep deprivation: A calibration study. J Sleep Res. 1994; 3 :36–44. [ PubMed ] [ Google Scholar ]
- Belenky G, Wesensten NJ, Thorne DR, et al. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: A sleep dose-response study. J Sleep Res. 2003; 12 :1–12. [ PubMed ] [ Google Scholar ]
- Binks PG, Waters WF, Hurry M. Short-term total sleep deprivations does not selectively impair higher cortical functioning. Sleep. 1999; 22 :328–34. [ PubMed ] [ Google Scholar ]
- Blagrove M, Alexander C, Horne JA. The effects of chronic sleep reduction on the performance of cognitive tasks sensitive to sleep deprivation. App Cogn Psych. 1995; 9 :21–40. [ Google Scholar ]
- Blatter K, Graw P, Munch M, et al. Gender and age differences in psychomotor vigilance performance under differential sleep pressure conditions. Behav Brain Res. 2006; 168 :312–7. [ PubMed ] [ Google Scholar ]
- Bocca ML, Denise P. Total sleep deprivation effect on disengagement of spatial attention as assessed by saccadic eye movements. Clin Neurophysiol. 2006; 117 :894–9. [ PubMed ] [ Google Scholar ]
- Bonnet MH, Rosa RR. Sleep and performance in young adults and older normals and insomniacs during acute sleep loss and recovery. Biol Psychol. 1987; 25 :153–72. [ PubMed ] [ Google Scholar ]
- Brendel DH, Reynolds CF, 3rd, Jennings JR, et al. Sleep stage physiology, mood, and vigilance responses to total sleep deprivation in healthy 80-year-olds and 20-year-olds. Psychophysiology. 1990; 27 :677–85. [ PubMed ] [ Google Scholar ]
- Carskadon MA, Dement WC. Normal human sleep: An overview. Philadelphia: Elsevier Saunders; 2005. pp. 13–23. [ Google Scholar ]
- Casagrande M, Violani C, Curcio G, et al. Assessing vigilance through a brief pencil and paper letter cancellation task (LCT): Effects of one night of sleep deprivation and of the time of day. Ergonomics. 1997; 40 :613–30. [ PubMed ] [ Google Scholar ]
- Casement MD, Broussard JL, Mullington JM, et al. The contribution of sleep to improvements in working memory scanning speed: A study of prolonged sleep restriction. Biol Psychol. 2006; 72 :208–12. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chee MW, Choo WC. Functional imaging of working memory after 24 hr of total sleep deprivation. J Neurosci. 2004; 24 :4560–7. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chee MW, Chuah LY, Venkatraman V, et al. Functional imaging of working memory following normal sleep and after 24 and 35 h of sleep deprivation: Correlations of fronto-parietal activation with performance. Neuroimage. 2006; 31 :419–28. [ PubMed ] [ Google Scholar ]
- Choo WC, Lee WW, Venkatraman V, et al. Dissociation of cortical regions modulated by both working memory load and sleep deprivation and by sleep deprivation alone. Neuroimage. 2005; 25 :579–87. [ PubMed ] [ Google Scholar ]
- Chuah YM, Venkatraman V, Dinges DF, et al. The neural basis of interindividual variability in inhibitory efficiency after sleep deprivation. J Neurosci. 2006; 26 :7156–62. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Corsi-Cabrera M, Sanchez AI, del-Rio-Portilla Y, et al. Effect of 38 h of total sleep deprivation on the waking EEG in women: Sex differences. Int J Psychophysiol. 2003; 50 :213–24. [ PubMed ] [ Google Scholar ]
- Coull JT, Jones MEP, Egan TD, et al. Attentional effects of nor-adrenaline vary with arousal level: Selective activation of thalamic pulvinar in humans. NeuroImage. 2004/5; 22 :315–22. [ PubMed ] [ Google Scholar ]
- Cowell PE, Sluming VA, Wilkinson ID, et al. Effects of sex and age on regional prefrontal brain volume in two human cohorts. Eur J Neurosci. 2007; 25 :307–18. [ PubMed ] [ Google Scholar ]
- De Gennaro L, Ferrara M, Curcio G, et al. Visual search performance across 40 h of continuous wakefulness: Measures of speed and accuracy and relation with oculomotor performance. Physiol Behav. 2001; 74 :197–204. [ PubMed ] [ Google Scholar ]
- Dinges DF, Douglas SD, Zaugg L, et al. Leukocytosis and natural killer cell function parallel neurobehavioral fatigue induced by 64 hours of sleep deprivation. J Clin Invest. 1994; 93 :1930–9. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Dinges DF, Pack F, Williams K, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4–5 hours per night. Sleep. 1997; 20 :267–77. [ PubMed ] [ Google Scholar ]
- Dinges DF, Powell JW. Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behav Res Meth Instr Comp. 1985; 17 :652–5. [ Google Scholar ]
- Doran SM, Van Dongen HP, Dinges DF. Sustained attention performance during sleep deprivation: Evidence of state instability. Arch Ital Biol. 2001; 139 :253–67. [ PubMed ] [ Google Scholar ]
- Dorrian J, Rogers NL, Dinges DF. Psychomotor vigilance performance: Neurocognitive assay sensitive to sleep loss. New York: Marcel Dekker; 2005. pp. 39–70. [ Google Scholar ]
- Drummond SP, Brown GG. The effects of total sleep deprivation on cerebral responses to cognitive performance. Neuropsychopharmacology. 2001; 25 :S68–73. [ PubMed ] [ Google Scholar ]
- Drummond SP, Brown GG, Gillin JC, et al. Altered brain response to verbal learning following sleep deprivation. Nature. 2000; 403 :655–7. [ PubMed ] [ Google Scholar ]
- Drummond SP, Brown GG, Salamat JS, et al. Increasing task difficulty facilitates the cerebral compensatory response to total sleep deprivation. Sleep. 2004; 27 :445–51. [ PubMed ] [ Google Scholar ]
- Drummond SP, Brown GG, Stricker JL, et al. Sleep deprivation-induced reduction in cortical functional response to serial subtraction. Neuroreport. 1999; 10 :3745–8. [ PubMed ] [ Google Scholar ]
- Drummond SP, Gillin JC, Brown GG. Increased cerebral response during a divided attention task following sleep deprivation. J Sleep Res. 2001; 10 :85–92. [ PubMed ] [ Google Scholar ]
- Drummond SP, Paulus MP, Tapert SF. Effects of two nights sleep deprivation and two nights recovery sleep on response inhibition. J Sleep Res. 2006; 15 :261–5. [ PubMed ] [ Google Scholar ]
- Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2005; 25 :117–29. [ PubMed ] [ Google Scholar ]
- Dzaja A, Arber S, Hislop J, et al. Women’s sleep in health and disease. J Psychiatr Res. 2005; 39 :55–76. [ PubMed ] [ Google Scholar ]
- Eagly AH, Wood W. The Origins of Sex Differences in Human Behavior: Evolved Dispositions Versus Social Roles. Am Psychol. 1999; 54 :408–23. [ Google Scholar ]
- Forest G, Godbout R. Effects of sleep deprivation on performance and EEG spectral analysis in young adults. Brain Cogn. 2000; 43 :195–200. [ PubMed ] [ Google Scholar ]
- Frey DJ, Badia P, Wright KP., Jr Inter- and intra-individual variability in performance near the circadian nadir during sleep deprivation. J Sleep Res. 2004; 13 :305–15. [ PubMed ] [ Google Scholar ]
- Gaudreau H, Carrier J, Tchiteya B. Age and individual determinants of sleep loss effects. New York: Marcel Dekker; 2005. pp. 441–479. [ Google Scholar ]
- Gosselin A, De Koninck J, Campbell KB. Total sleep deprivation and novelty processing: Implications for frontal lobe functioning. Clin Neurophysiol. 2005; 116 :211–22. [ PubMed ] [ Google Scholar ]
- Graw P, Krauchi K, Knoblauch V, et al. Circadian and wake-dependent modulation of fastest and slowest reaction times during the psychomotor vigilance task. Physiol Behav. 2004; 80 :695–701. [ PubMed ] [ Google Scholar ]
- Habeck C, Rakitin BC, Moeller J, et al. An event-related fMRI study of the neurobehavioral impact of sleep deprivation on performance of a delayed-match-to-sample task. Brain Res Cogn Brain Res. 2004; 18 :306–21. [ PubMed ] [ Google Scholar ]
- Harrison Y, Espelid E. Loss of negative priming following sleep deprivation. Q J Exp Psychol A. 2004; 57 :437–46. [ PubMed ] [ Google Scholar ]
- Harrison Y, Horne JA. Sleep loss and temporal memory. Q J Exp Psychol A. 2000; 53 :271–9. [ PubMed ] [ Google Scholar ]
- Harrison Y, Horne JA. One night of sleep loss impairs innovative thinking and flexible decision making. Organ Behav Hum Decis Process. 1999; 78 :128–45. [ PubMed ] [ Google Scholar ]
- Harrison Y, Horne JA. Sleep loss impairs short and novel language tasks having a prefrontal focus. J Sleep Res. 1998; 7 :95–100. [ PubMed ] [ Google Scholar ]
- Harrison Y, Horne JA, Rothwell A. Prefrontal neuropsychological effects of sleep deprivation in young adults–a model for healthy aging? Sleep. 2000; 23 :1067–73. [ PubMed ] [ Google Scholar ]
- Heuer H, Klein W. One night of total sleep deprivation impairs implicit learning in the serial reaction task, but not the behavioral expression of knowledge. Neuropsychology. 2003; 17 :507–16. [ PubMed ] [ Google Scholar ]
- Heuer H, Kleinsorge T, Klein W, et al. Total sleep deprivation increases the costs of shifting between simple cognitive tasks. Acta Psychol (Amst) 2004; 117 :29–64. [ PubMed ] [ Google Scholar ]
- Heuer H, Kohlisch O, Klein W. The effects of total sleep deprivation on the generation of random sequences of key-presses, numbers and nouns. Q J Exp Psychol A. 2005; 58 :275–307. [ PubMed ] [ Google Scholar ]
- Heuer H, Spijkers W, Kiesswetter E, et al. Effects of sleep loss, time of day, and extended mental work on implicit and explicit learning of sequences. J Exp Psychol Appl. 1998; 4 :139–62. [ PubMed ] [ Google Scholar ]
- Horne JA. Human sleep, sleep loss and behaviour. implications for the prefrontal cortex and psychiatric disorder. Br J Psychiatry. 1993; 162 :413–9. [ PubMed ] [ Google Scholar ]
- Horne JA, Pettitt AN. High incentive effects on vigilance performance during 72 hours of total sleep deprivation. Acta Psychol (Amst) 1985; 58 :123–39. [ PubMed ] [ Google Scholar ]
- Hwang DY, Golby AJ. The brain basis for episodic memory: Insights from functional MRI, intracranial EEG, and patients with epilepsy. Epilepsy Behav. 2006; 8 :115–26. [ PubMed ] [ Google Scholar ]
- Jennings JR, Monk TH, van der Molen MW. Sleep deprivation influences some but not all processes of supervisory attention. Psychol Sci. 2003; 14 :473–9. [ PubMed ] [ Google Scholar ]
- Johnsen BH, Laberg JC, Eid J, et al. Dichotic listening and sleep deprivation: Vigilance effects. Scand J Psychol. 2002; 43 :413–17. [ PubMed ] [ Google Scholar ]
- Kalleinen N, Polo O, Himanen SL, et al. Sleep deprivation and hormone therapy in postmenopausal women. Sleep Med. 2006; 7 :436–47. [ PubMed ] [ Google Scholar ]
- Karakorpi M, Alhola P, Urrila AS, et al. Hormone treatment gives no benefit against cognitive changes caused by acute sleep deprivation in postmenopausal women. Neuropsychopharmacology. 2006; 31 :2079–88. [ PubMed ] [ Google Scholar ]
- Kendall AP, Kautz MA, Russo MB, et al. Effects of sleep deprivation on lateral visual attention. Int J Neurosci. 2006; 116 :1125–38. [ PubMed ] [ Google Scholar ]
- Kilduff TS, Kushida CA, Terao A. Recovery from sleep deprivation. New York: Marcel Dekker; 2005. pp. 485–502. [ Google Scholar ]
- Killgore WD, Balkin TJ, Wesensten NJ. Impaired decision making following 49 h of sleep deprivation. J Sleep Res. 2006; 15 :7–13. [ PubMed ] [ Google Scholar ]
- Kim DJ, Lee HP, Kim MS, et al. The effect of total sleep deprivation on cognitive functions in normal adult male subjects. Int J Neurosci. 2001; 109 :127–37. [ PubMed ] [ Google Scholar ]
- Kimura D. Sex, sexual orientation and sex hormones influence human cognitive function. Curr Opin Neurobiol. 1996; 6 :259–63. [ PubMed ] [ Google Scholar ]
- Kjellberg A. Sleep deprivation and some aspects of performance. II. lapses and other attentional effects. Waking Sleeping. 1977; 1 :145–8. [ Google Scholar ]
- Kripke DF, Garfinkel L, Wingard DL, et al. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002; 59 :131–6. [ PubMed ] [ Google Scholar ]
- Kronholm E, Harma M, Hublin C, et al. Self-reported sleep duration in finnish general population. J Sleep Res. 2006; 15 :276–90. [ PubMed ] [ Google Scholar ]
- Lac G, Chamoux A. Elevated salivary cortisol levels as a result of sleep deprivation in a shift worker. Occup Med (Lond) 2003; 53 :143–5. [ PubMed ] [ Google Scholar ]
- Lee HJ, Kim L, Suh KY. Cognitive deterioration and changes of P300 during total sleep deprivation. Psychiatry Clin Neurosci. 2003; 57 :490–6. [ PubMed ] [ Google Scholar ]
- Leger D, Guilleminault C, Dreyfus JP, et al. Prevalence of insomnia in a survey of 12,778 adults in france. J Sleep Res. 2000; 9 :35–42. [ PubMed ] [ Google Scholar ]
- Linde L, Bergstrom M. The effect of one night without sleep on problem-solving and immediate recall. Psychol Res. 1992; 54 :127–36. [ PubMed ] [ Google Scholar ]
- Linde L, Edland A, Bergström M. Auditory attention and multi-attribute decision-making during a 33 h sleep-deprivation period: Mean performance and between-subject dispersions. Ergonomics. 1999; 42 :696–713. [ PubMed ] [ Google Scholar ]
- Lindheim SR, Legro RS, Bernstein L, et al. Behavioral stress responses in premenopausal and postmenopausal women and the effects of estrogen. Am J Obstet Gynecol. 1992; 167 :1831–6. [ PubMed ] [ Google Scholar ]
- Manber R, Armitage R. Sex, steroids, and sleep: A review. Sleep. 1999; 22 :540–55. [ PubMed ] [ Google Scholar ]
- Maquet P. The role of sleep in learning and memory. Science. 2001; 294 :1048–52. [ PubMed ] [ Google Scholar ]
- McCarthy ME, Waters WF. Decreased attentional responsivity during sleep deprivation: Orienting response latency, amplitude, and habituation. Sleep. 1997; 20 :115–23. [ PubMed ] [ Google Scholar ]
- Mikulincer M, Babkoff H, Caspy T, et al. The effects of 72 hours of sleep loss on psychological variables. Br J Psychol. 1989; 80 (Pt 2):145–62. [ PubMed ] [ Google Scholar ]
- Monk TH, Carrier J. Speed of mental processing in the middle of the night. Sleep. 1997; 20 :399–401. [ PubMed ] [ Google Scholar ]
- Mu Q, Nahas Z, Johnson KA, et al. Decreased cortical response to verbal working memory following sleep deprivation. Sleep. 2005; 28 :55–67. [ PubMed ] [ Google Scholar ]
- Murillo-Rodriguez E, Blanco-Centurion C, Gerashchenko D, et al. The diurnal rhythm of adenosine levels in the basal forebrain of young and old rats. Neuroscience. 2004; 123 :361–70. [ PubMed ] [ Google Scholar ]
- Naghavi HR, Nyberg L. Common fronto-parietal activity in attention, memory, and consciousness: Shared demands on integration? Conscious Cogn. 2005; 14 :390–425. [ PubMed ] [ Google Scholar ]
- National Sleep Foundation. “Sleep in America” poll. 2007. [Accessed 8 March 2007]. pp. 1–52. URL: http://www.sleepfoundation.org .
- National Sleep Foundation. “Sleep in America” poll. 2002. [Accessed 6 September 2006]. pp. 1–44. URL: http://www.sleepfoundation.org .
- Nilsson JP, Soderstrom M, Karlsson AU, et al. Less effective executive functioning after one night’s sleep deprivation. J Sleep Res. 2005; 14 :1–6. [ PubMed ] [ Google Scholar ]
- Ogawa Y, Kanbayashi T, Saito Y, et al. Total sleep deprivation elevates blood pressure through arterial baroreflex resetting: A study with microneurographic technique. Sleep. 2003; 26 :986–9. [ PubMed ] [ Google Scholar ]
- Otmani S, Pebayle T, Roge J, et al. Effect of driving duration and partial sleep deprivation on subsequent alertness and performance of car drivers. Physiol Behav. 2005; 84 :715–24. [ PubMed ] [ Google Scholar ]
- Philibert I. Sleep loss and performance in residents and nonphysicians; a meta-analytic examination. Sleep. 2005; 28 :1393–402. [ PubMed ] [ Google Scholar ]
- Philip P, Taillard J, Sagaspe P, et al. Age, performance and sleep deprivation. J Sleep Res. 2004; 13 :105–10. [ PubMed ] [ Google Scholar ]
- Pilcher JJ, Huffcutt AI. Effects of sleep deprivation on performance: A meta-analysis. Sleep. 1996; 19 :318–26. [ PubMed ] [ Google Scholar ]
- Portas CM, Rees G, Howseman AM, et al. A specific role for the thalamus in mediating the interaction of attention and arousal in humans. J Neurosci. 1998; 18 :8979–89. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Priest B, Brichard C, Aubert G, et al. Microsleep during a simplified maintenance of wakefulness test. A validation study of the OSLER test. Am J Respir Crit Care Med. 2001; 163 :1619–25. [ PubMed ] [ Google Scholar ]
- Quigley N, Green JF, Morgan D, et al. The effect of sleep deprivation on memory and psychomotor function in healthy volunteers. Hum Psychopharmacol. 2000; 15 :171–7. [ PubMed ] [ Google Scholar ]
- Ragland JD, Coleman AR, Gur RC, et al. Sex differences in brain-behavior relationships between verbal episodic memory and resting regional cerebral blood flow. Neuropsychologia. 2000; 38 :451–61. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Raidy DJ, Scharff LF. Effects of sleep deprivation on auditory and visual memory tasks. Percept Mot Skills. 2005; 101 :451–67. [ PubMed ] [ Google Scholar ]
- Rinkenauer G, Osman A, Ulrich R, et al. On the locus of speed-accuracy trade-off in reaction time: Inferences from the lateralized readiness potential. J Exp Psychol Gen. 2004; 133 :261–82. [ PubMed ] [ Google Scholar ]
- Russo MB, Kendall AP, Johnson DE, et al. Visual perception, psychomotor performance, and complex motor performance during an overnight air refueling simulated flight. Aviat Space Environ Med. 2005; 76 :C92–103. [ PubMed ] [ Google Scholar ]
- Sagaspe P, Sanchez-Ortuno M, Charles A, et al. Effects of sleep deprivation on color-word, emotional, and specific stroop interference and on self-reported anxiety. Brain Cogn. 2006; 60 :76–87. [ PubMed ] [ Google Scholar ]
- Samkoff JS, Jacques CH. A review of studies concerning effects of sleep deprivation and fatigue on residents’ performance. Acad Med. 1991; 66 :687–93. [ PubMed ] [ Google Scholar ]
- Sarter M, Gehring WJ, Kozak R. More attention must be paid: The neurobiology of attentional effort. Brain Res Brain Res Rev. 2006; 51 :145–60. [ PubMed ] [ Google Scholar ]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. 1957. J Neuropsychiatry Clin Neurosci. 2000; 12 :103–13. [ PubMed ] [ Google Scholar ]
- Shneerson JM. Handbook of sleep medicine. Cambridge: Blackwell Science; 2000. [ Google Scholar ]
- Smith ME, McEvoy LK, Gevins A. The impact of moderate sleep loss on neurophysiologic signals during working-memory task performance. Sleep. 2002; 25 :784–94. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Smulders FT, Kenemans JL, Jonkman LM, et al. The effects of sleep loss on task performance and the electroencephalogram in young and elderly subjects. Biol Psychol. 1997; 45 :217–39. [ PubMed ] [ Google Scholar ]
- Spiegel K, Knutson K, Leproult R, et al. Sleep loss: A novel risk factor for insulin resistance and type 2 diabetes. J Appl Physiol. 2005; 99 :2008–19. [ PubMed ] [ Google Scholar ]
- Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999; 354 :1435–9. [ PubMed ] [ Google Scholar ]
- Stenuit P, Kerkhofs M. Age modulates the effects of sleep restriction in women. Sleep. 2005; 28 :1284–8. [ PubMed ] [ Google Scholar ]
- Steyvers FJ. The influence of sleep deprivation and knowledge of results on perceptual encoding. Acta Psychol (Amst) 1987; 66 :173–87. [ PubMed ] [ Google Scholar ]
- Steyvers FJ, Gaillard AW. The effects of sleep deprivation and incentives on human performance. Psychol Res. 1993; 55 :64–70. [ PubMed ] [ Google Scholar ]
- Stickgold R. Sleep-dependent memory consolidation. Nature. 2005; 437 :1272–8. [ PubMed ] [ Google Scholar ]
- Strangman G, Thompson JH, Strauss MM, et al. Functional brain imaging of a complex navigation task following one night of total sleep deprivation: A preliminary study. J Sleep Res. 2005; 14 :369–75. [ PubMed ] [ Google Scholar ]
- Swann CE, Yelland GW, Redman JR, et al. Chronic partial sleep loss increases the facilitatory role of a masked prime in a word recognition task. J Sleep Res. 2006; 15 :23–9. [ PubMed ] [ Google Scholar ]
- Taillard J, Moore N, Claustrat B, et al. Nocturnal sustained attention during sleep deprivation can be predicted by specific periods of subjective daytime alertness in normal young humans. J Sleep Res. 2006; 15 :41–5. [ PubMed ] [ Google Scholar ]
- Thomas M, Sing H, Belenky G, et al. Neural basis of alertness and cognitive performance impairments during sleepiness. I. effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. 2000; 9 :335–52. [ PubMed ] [ Google Scholar ]
- Thorne DR, Thomas ML, Sing HC, et al. Driving-simulator accident rates before, during and after one week of restricted nightly sleep. Sleep. 1999; 21 :S135. [ Google Scholar ]
- Tomporowski PD, Tinsley VF. Effects of memory demand and motivation on sustained attention in young and older adults. Am J Psychol. 1996; 109 :187–204. [ PubMed ] [ Google Scholar ]
- Tsai LL, Young HY, Hsieh S, et al. Impairment of error monitoring following sleep deprivation. Sleep. 2005; 28 :707–13. [ PubMed ] [ Google Scholar ]
- Van Dongen HP, Baynard MD, Maislin G, et al. Systematic interin-dividual differences in neurobehavioral impairment from sleep loss: Evidence of trait-like differential vulnerability. Sleep. 2004; 27 :423–33. [ PubMed ] [ Google Scholar ]
- Van Dongen HP, Maislin G, Mullington JM, et al. The cumulative cost of additional wakefulness: Dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003a; 26 :117–26. [ PubMed ] [ Google Scholar ]
- Van Dongen HP, Rogers NL, Dinges DF. Sleep debt: Theoretical and empirical issues. Sleep Biol Rhythms. 2003b; 1 :5–13. [ Google Scholar ]
- Van Dongen HP, Vitellaro KM, Dinges DF. Individual differences in adult human sleep and wakefulness: Leitmotif for a research agenda. Sleep. 2005; 28 :479–96. [ PubMed ] [ Google Scholar ]
- Van Dongen HPA. Inter- and intra-individual differences in circadian phase 1998 [ Google Scholar ]
- Versace F, Cavallero C, De Min Tona G, et al. Effects of sleep reduction on spatial attention. Biol Psychol. 2006; 71 :248–55. [ PubMed ] [ Google Scholar ]
- Wilkinson RT. Response-stimulus interval in choice serial reaction time: Interaction with sleep deprivation, choice, and practice. Q J Exp Psychol A. 1990; 42 :401–23. [ PubMed ] [ Google Scholar ]
- Wimmer F, Hoffmann RF, Bonato RA, et al. The effects of sleep deprivation on divergent thinking and attention processes. J Sleep Res. 1992; 1 :223–30. [ PubMed ] [ Google Scholar ]
- Wright KP, Jr, Badia P. Effects of menstrual cycle phase and oral contraceptives on alertness, cognitive performance, and circadian rhythms during sleep deprivation. Behav Brain Res. 1999; 103 :185–94. [ PubMed ] [ Google Scholar ]
- Wu JC, Gillin JC, Buchsbaum MS, et al. The effect of sleep deprivation on cerebral glucose metabolic rate in normal humans assessed with positron emission tomography. Sleep. 1991; 14 :155–62. [ PubMed ] [ Google Scholar ]
- Zils E, Sprenger A, Heide W, et al. Differential effects of sleep deprivation on saccadic eye movements. Sleep. 2005; 28 :1109–15. [ PubMed ] [ Google Scholar ]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 17 March 2020
The relationship between subjective sleep quality and cognitive performance in healthy young adults: Evidence from three empirical studies
- Zsófia Zavecz ORCID: orcid.org/0000-0003-2532-7491 1 , 2 , 3 ,
- Tamás Nagy ORCID: orcid.org/0000-0001-5244-0356 2 ,
- Adrienn Galkó 2 ,
- Dezso Nemeth ORCID: orcid.org/0000-0002-9629-5856 2 , 3 , 4 na1 &
- Karolina Janacsek ORCID: orcid.org/0000-0001-7829-8220 2 , 3 , 5 na1
Scientific Reports volume 10 , Article number: 4855 ( 2020 ) Cite this article
31k Accesses
43 Citations
260 Altmetric
Metrics details
- Human behaviour
- Working memory
The role of subjective sleep quality in cognitive performance has gained increasing attention in recent decades. In this paper, our aim was to test the relationship between subjective sleep quality and a wide range of cognitive functions in a healthy young adult sample combined across three studies. Sleep quality was assessed by the Pittsburgh Sleep Quality Index, the Athens Insomnia Scale, and a sleep diary to capture general subjective sleep quality, and the Groningen Sleep Quality Scale to capture prior night’s sleep quality. Within cognitive functions, we tested working memory, executive functions, and several sub-processes of procedural learning. To provide more reliable results, we included robust frequentist as well as Bayesian statistical analyses. Unequivocally across all analyses, we showed that there is no association between subjective sleep quality and cognitive performance in the domains of working memory, executive functions and procedural learning in healthy young adults. Our paper can contribute to a deeper understanding of subjective sleep quality and its measures, and we discuss various factors that may affect whether associations can be observed between subjective sleep quality and cognitive performance.
Similar content being viewed by others
Optimizing the methodology of human sleep and memory research
Dezső Németh, Emilie Gerbier, … Karolina Janacsek

Macro and micro sleep architecture and cognitive performance in older adults
Ina Djonlagic, Sara Mariani, … Shaun M. Purcell
Impact of sleep duration on executive function and brain structure
Xin You Tai, Cheng Chen, … Masud Husain
Introduction
There is a widely accepted belief that experiencing poor sleep quality, including subjective experiences (e.g., reporting difficulties falling asleep, waking up frequently during the night, or feeling tired during the day), indisputably decreases cognitive performance. We can often hear people complaining about weaker memory and/or attentional performance in relation to their experienced sleep insufficiency. This phenomenon can be particularly prevalent amongst university students since the pressure for academic performance in this population is exceptionally high. The possible overestimation of the importance of one’s subjective sleep quality can even lead to placebo or nocebo effects on cognitive performance 1 , 2 . However, scientific evidence on the relationship between experienced subjective sleep quality and cognition is still inconclusive 3 , 4 , 5 , 6 , 7 . Therefore, our aim in the current study was to test whether subjective sleep quality is associated with cognitive performance in healthy young adults.
The role of sleep in cognitive performance has gained increasing attention in neuroscience and sleep research in recent decades 8 , 9 . Numerous experimental methods exist that can be employed for examining the association between sleep and cognitive performance. Sleep parameters can be evaluated based on actigraph or electroencephalograph measurements (i.e., objective measures), which are time-consuming and require expensive equipment. Hence, researchers and clinicians often tend to rely on questionnaires (i.e., subjective measures) to assess sleep parameters (e.g., sleep latency, sleep quality, sleep disturbances, or sleep duration). This inclination has also motivated the current study to explore the relationship between sleep questionnaires and cognitive functions.
Previous studies have shown that subjective and objective sleep parameters, such as sleep latency, sleep duration, or sleep efficiency could differ 10 , 11 , 12 ; the strength of correlation between the subjective and objective measures of the same parameters varied between 0.21 and 0.62 for sleep latency and duration, while it was close to 0 for sleep efficiency. Subjective sleep quality can vary from objective sleep quality as it is typically estimated from a combination of parameters, such as sleep initiation, sleep continuity (number of awakenings), and/or depth of sleep. For instance, extreme deviations can occur between subjective and objective measures in sleep disorders, such as insomnia or sleep-state misperception. According to Zhang and Zhao 13 , the subjective and objective measures together should determine the type of treatment and medication in sleep disorders. Stepanski et al . 7 showed that, within insomniac patients, the decisive factor of whether a patient seeks medication is their subjective evaluation of their sleep quality and daytime functioning. Furthermore, Gavriloff et al . 1 found that providing sham feedback about their sleep to patients with insomnia influenced their daytime symptoms and performance in attention and vigilance tasks. Similarly, in a placebo sleep study, young adults were randomly told they had below or above average sleep quality based on their brainwaves and other psychophysiological measures 2 . This constructed belief about their sleep quality affected their performance in attentional and executive function tasks. Thus, beyond therapeutic importance, it appears that subjective sleep quality can have further explanatory value for cognitive performance compared to objective measures.
One of the most widely-used sleep questionnaires is the Pittsburgh Sleep Quality Index (PSQI) 14 , a self-administered questionnaire, in which participants rate their subjective sleep quality based on several questions. These questions deal with various aspects of sleep that range from the average amount of sleep during the night, the difficulty experienced in falling asleep, and other sleep disturbances. Nevertheless, there are other popular measurements, such as the Athens Insomnia Scale (AIS) 15 , which measures difficulties in falling asleep or maintaining sleep, as well as sleep diaries, which capture the sleeping habits of the participants from day to day, spanning a few days or weeks. Sleep questionnaires and sleep diaries are two different types of self-reported measures: while sleep questionnaires are administered at a single point in time, and ask about various aspects of sleep experience in a longer time period retrospectively, sleep diaries are ongoing, daily self-monitoring tools. Libman et al . 16 showed that the two measurement types are tapping the same domains but lead to somewhat different results due to methodological differences: questionnaires can be susceptible to memory distortion while sleep diaries may be distorted by atypical sleep experiences during the monitored period.
Previous research on subjective sleep quality and cognitive performance has led to mixed findings. While some studies focusing on healthy participants have shown that poorer sleep quality as measured by the PSQI score was associated with weaker working memory 4 , executive functions 5 , and decision-making performance 17 , others have failed to find an association between subjective sleep quality and cognitive performance 6 , 7 . Bastien et al . 3 showed different associations between subjective sleep quality as measured by a sleep diary and cognitive performance in patients with insomnia who received or did not receive treatment and in elderly participants who reported good sleep quality. Interestingly, in good sleepers, greater subjective depth, quality, and efficiency of sleep were associated with better performance on attention and concentration tasks but poorer memory performance. These findings suggest that further studies are needed to clarify the complex relationship between subjective sleep quality and aspects of cognitive functioning.
Notably, these previous studies focused on diverse populations, including adolescents, elderly and clinical groups, and relied on sample sizes ranging from around 20 to 100, with smaller sample sizes potentially limiting the robustness of the observed results. In these studies, subjective sleep quality was assessed by a combination of self-reported measures, such as difficulty in sleep initiation, sleep continuity, and/or depth of sleep. In contrast to subjective sleep quality captured by a combination of such measures, self-reported sleep duration has been studied more thoroughly. In a large study with more than 100,000 participants, Sternberg et al . 18 reported a quadratic relationship between self-reported sleep duration and performance in cognitive tasks assessing working memory and arithmetics. Furthermore, a recent powerful meta-analysis focusing on elderly participants also showed that both short and long sleep increased the odds of poor cognitive performance 19 . A similar association was shown in another study investigating insomnia symptoms and cognitive performance in a large sample of participants 20 : self-reported sleep duration extremes were associated with impaired performance. Systematic investigations on the relationship between subjective sleep quality as captured by a combination of parameters (such as sleep latency, subjective sleep quality, sleep disturbances) and cognitive performance using larger sample sizes are, however, still lacking.
Moreover, in previous investigations focusing on the association between subjective sleep quality and various aspects of cognitive performance, the potential relationship with procedural learning/memory has largely been neglected. The procedural memory system underlies the learning, storage, and use of cognitive and perceptual-motor skills and habits 21 . Evidence suggests that the system is multifaceted in that it supports numerous functions that are performed automatically, including sequences, probabilistic categorization, and grammar, and perhaps aspects of social skills 22 , 23 , 24 , 25 , 26 . Considering the importance of this memory system, the clarification of its relationship with subjective sleep quality would be indispensable.
Here we aimed to fill the gaps identified in previous research by providing an extensive investigation on the relationship between subjective sleep quality and cognitive performance in healthy young adults. Within cognitive functions, we focused on working memory, executive functions, and procedural learning. We chose these domains because 1) the relationship between working memory, executive functions and subjective sleep quality has remained inconclusive, and 2) the relationship between procedural learning/memory and subjective sleep quality has largely been neglected in previous studies. Therefore, in the latter case, we explored several measures of procedural learning in order to obtain a more detailed picture of the potential associations with subjective sleep quality. To increase the robustness of our analyses, we created a database of 235 participants’ data by pooling three separate datasets from our lab. We assessed subjective sleep quality by PSQI and AIS (Study 1–3), Groningen Sleep Quality Scale (GSQS, Study 2), and a sleep diary (Study 2). These separate measures capture somewhat different aspects of self-reported sleep quality and thus provide a detailed picture. We tested working memory, executive functions and several sub-processes of procedural learning in all three studies. To control for possible confounding effects, we included age, gender and chronotype as covariates in our analyses. To test the amount of evidence either for associations or no associations between subjective sleep quality and cognitive performance, we calculated Bayes Factors that offer a way of evaluating the evidence against or in favor of the null hypothesis, respectively.
Participants
Participants were selected from a large pool of undergraduate students from Eötvös Loránd University. The selection procedure was based on the completion of an online questionnaire assessing mental and physical health status. Respondents reporting current or prior chronic somatic, psychiatric or neurological disorders, or the regular consumption of drugs other than contraceptives were excluded. In addition, individuals reporting the occurrence of any kind of extreme life event (e.g., accident) during the last three months that might have had an impact on their mood or daily rhythms were also excluded from the study.
The data was obtained from three different studies, each with a slightly different focus. Importantly, the analyses presented in the current paper are completely novel, none of the separate studies focused on the relationship between subjective sleep quality and cognitive performance. Forty-seven participants took part in Study 1 27 , 103 participants took part in Study 2 28 , and 85 participants took part in Study 3 29 . The descriptive characteristics of participants in the three studies are listed in Table 1 . All participants were white/Caucasian. All participants provided written informed consent and received course credits for taking part. The studies were approved by the Research Ethics Committee of Eötvös Loránd University (201410, 2016/209). The study was conducted in accordance with the Declaration of Helsinki.
We conducted three separate studies on the association of subjective sleep quality and procedural learning, working memory, and executive functions in healthy young adults. The sleep questionnaires included in the studies and the timing of the procedural learning task slightly differed. While we assessed subjective sleep quality by PSQI and AIS in all three studies, in Study 2, we included further measures of subjective sleep quality as well: (1) a sleep diary to assess day-to-day general sleep quality and (2) Groningen Sleep Quality Scale (GSQS) to assess prior night’s sleep quality. To control for the potential confounding effect of chronotype, we also administered the Morningness-Eveningness Questionnaire (MEQ) 30 , 31 , henceforth referred to as morningness score because a larger score on this questionnaire indicates greater morningness.
In all three studies, PSQI and AIS sleep quality questionnaires and the MEQ were administered online, while the GSQS in Study 2 and the tasks assessing cognitive performance in all studies were administered in a single session in the lab. Due to technical problems, the data of six participants on executive functions are missing. To ensure that participants do the tests in their preferred time of the day, the timing of the session was chosen by the participants themselves (between 7 am and 7 pm). The timing of the sessions was normally distributed in all three studies, with most participants performing the tasks during the daytime between 11 am and 3 pm. The sleep diary in Study 2 was filled by the participants for at least one week, and to a maximum of two weeks, prior to the cognitive assessment that was scheduled based on the participants’ availability.
Questionnaires and tasks
All cognitive performance tasks and subjective sleep questionnaires are well-known and widely used in the field of psychology and neuroscience (for details about each task and questionnaire, see Supplementary methods).
Subjective sleep quality questionnaires
To capture the general sleep quality of the last month, we administered the Pittsburgh Sleep Quality Index (PSQI) 14 , 32 and the Athens Insomnia Scale (AIS) 15 , 33 . Additionally, in Study 2, we administered a Sleep diary 34 to assess the sleep quality of the last one-two weeks, and the Groningen Sleep Quality Scale (GSQS) 35 , 36 to capture the sleep quality of the night prior testing.
Cognitive performance tasks
Working memory was measured by the Counting Span task 37 , 38 , 39 , 40 . Executive functions were assessed by the Wisconsin Card Sorting Test (WCST) 41 , 42 , 43 . The outcome measure of the WCST task was the number of perseverative errors, which shows the inability/difficulty to change the behavior despite feedback. Procedural learning was measured by the explicit version of the Alternating Serial Reaction Time (ASRT) task (Figure S1 , see also 44 ). There are several learning indices that can be acquired from this task. Higher-order sequence learning refers to the acquisition of the sequence order of the stimuli. Statistical learning refers to the acquisition of frequency information embedded in the task. However, previous ASRT studies often assessed Triplet learning, which is a mixed measure of acquiring frequency and sequential information (for details, see Supplementary methods). In addition to these learning indices, we measured the average reaction times (RTs) and accuracy (ACC), which reflect the average general performance of the participants across the task, and the changes in RT and ACC from the beginning to the end of the task, which indicate general skill learning that occurs due to more efficient visuomotor and motor-motor coordination as the task progresses 45 .
Data analysis
Statistical analyses were conducted in R 3.6.1 46 using the lme4 package 47 . Bootstrapped confidence intervals and p-values were calculated using the boot package 48 , 49 . The data and analysis code can be found on the following link: https://github.com/nthun/performance_sleep_quality/
Analysis of the relationship between subjective sleep quality and cognitive performance
Subjective sleep quality scales (PSQI and AIS) were combined into a single metric, using principal component analysis. Then separate linear mixed-effect models were created for each outcome measure (i.e., performance metric), where the aggregated sleep quality metric (hereinafter referred to as sleep disturbance) was used as a predictor, and ‘Study’ (1, 2 or 3) was added as a random intercept. This way we could estimate an aggregated effect while accounting for the potential differences across studies. To control for possible confounding effects, we included age, gender and morningness score as covariates in our analyses. Thus, the estimates reported in the Results section are controlled for these factors.
As the residuals did not show normal distribution, we used bootstrapped estimates and confidence intervals, using 1000 bootstrap samples, from which we calculated the p-values 48 , 49 . Bayes Factors (BF 01 ) were calculated by using the exponential of the Bayesian Information Criterion (BIC) of the fitted models minus the BIC of the null models – that contained the confounders only, and a random intercept by study 50 . The BF is a statistical technique that helps conclude whether the collected data favors the null-hypothesis ( H 0) or the alternative hypothesis ( H 1); thus, the BF could be considered as a weight of evidence provided by the data 51 . It is an effective mathematical approach to show if there is no association between two measures. In Bayesian correlation analyses, H 0 is the lack of associations between the two measures, and H 1 states that association exists between the two measures. Here we report BF 01 values. According to Wagenmakers et al . 51 , BF 01 values between 1 and 3 indicate anecdotal evidence for H 0, while values between 3 and 10 indicate substantial evidence for H 0. Conversely, while values between 1/3 and 1 indicate anecdotal evidence for H 1, values between 1/10 and 1/3 indicate substantial evidence for H 1. If the BF is below 1/10, 1/30, or 1/100, it indicates strong, very strong, or extreme evidence for H 1, respectively. Values around 1 do not support either H 0 or H 1. Thus, Bayes Factor is a valuable tool to provide evidence for no associations between constructs as opposed to frequentists analyses, where no such evidence can be obtained based on non-significant results.
To test the association between the additional subjective sleep quality measures and cognitive performance in Study 2, we used robust linear regression, this time without random effects. We included the same potential confounders (age, gender, morningness score), and Bayes factors were calculated in the previously described way.
Analysis of the ASRT data
Performance in the ASRT task was analyzed by repeated-measures analyses of variance (ANOVA) in each study (for details of these analyses, see Supplementary methods). Based on these ANOVAs, Triplet learning, Higher-order sequence learning, and Statistical learning occurred in all three studies, both in ACC and RT (all p s < 0.001; for details, see Supplementary results and Figure S2 ).
Cognitive performance in the three studies
The working memory capacity (measured by the counting span) and executive functions (measured by the number of perseverative errors in the WCST task) of the participants were in the standard range for their age 52 , 53 . The mean counting span for the entire sample was 3.59 ( SD = 0.85) in the three studies. This average score represents a mid-range cognitive performance, as obtainable scores range from 1 to 6. The mean number of perseverative errors was 14.76 ( SD = 5.27) in the three studies (no maximum score can be defined in this case). For procedural learning, mean scores were 26.48 ( SD = 26.37) for RT Triplet learning, 16.63 ( SD = 40.34) for RT Higher-order sequence learning, 16.74 ( SD = 9.94) for RT Statistical learning, 359.88 ( SD = 40.94) for average RT, and 31.13 ( SD = 30.15) for RT general skill learning. Accuracy scores were as follows: 0.04 ( SD = 0.03) for ACC Triplet learning, 0.02 ( SD = 0.03) for ACC Higher-order sequence learning, 0.03 (SD = 0.03) for ACC Statistical learning, 0.90 ( SD = 0.10) for average ACC, −0.02 ( SD = 0.09) for ACC general skill learning, in all three studies. Note that for accuracy, these values represent proportions (e.g., the average ACC was 90%, hence 0.90), and the learning scores are difference scores (e.g., the ACC Triplet learning score shows that participants were on average 4% more accurate on high-frequency triplets compared to the low-frequency ones). All presented RT and ACC scores represent typical values in ASRT studies with healthy young adults.
We also provide descriptive data for Study 2 separately, as additional analyses were run on cognitive performance from this dataset and GSQS and sleep diary scores. In Study 2, the mean counting span was 3.65 ( SD = 1.01), and the mean number of perseverative errors was 14.46 ( SD = 6.37). For procedural learning in Study 2, mean scores were 33.04 ( SD = 27.96) for RT Triplet learning, 28.53 ( SD = 51.44) for RT Higher-order sequence learning, 18.77 ( SD = 9.78) for RT Statistical learning, 348.29 ( SD = 42.26) for average RT, and 39.30 ( SD = 34.74) for RT general skill learning. Accuracy scores were as follows: 0.03 ( SD = 0.02) for ACC Triplet learning, 0.01 ( SD = 0.02) for ACC Higher-order sequence learning, 0.02 ( SD = 0.02) for ACC Statistical learning, 0.94 ( SD = 0.03) for average ACC, 0.02 ( SD = 0.03) for ACC general skill learning.
Overall, these values represent a mid-range cognitive performance with a sufficient level of variability in the sample to conduct the planned analyses.
Subjective sleep questionnaire scores in the three studies
The obtainable scores, means, standard deviations, and proportions of good, moderate and poor sleepers for each questionnaire are presented in Table 2 . The mean scores of PSQI in the current sample were higher than the score of 1.91 for the same components in Buysse et al . 14 , and in the range or even higher than the global PSQI score (which aggregates seven components; M = 2.67) for the control participants, whose age was between 24 and 83 years. In the same study 14 , the participants with sleep disorders had a mean score of 4.78 for the three components of PSQI, suggesting that ~18% of the current sample had a score higher than the average score of sleep-disordered patients. The mean scores of AIS were somewhat higher than the mean score of 3 reported for a representative Hungarian adult sample in Novak et al . 33 . According to the cut-off score of 10 suggested in that paper, ~5% of our sample would fall into the diagnostic category of insomnia. However, according to a stricter cut-off score of 6 suggested by Soldatos, Dikeos & Paparrigopoulos 54 , up to 23% of the participants would have complaints comparable to those of insomniac patients. The mean of the GSQS score was lower than the mean score reported for a Hungarian sample of young adults ( M = 4.70, SD = 1.78) in Simor et al . 35 . The mean of the Sleep diary score in Study 2 was comparable to the mean PSQI score of 1.3 for the same components for the control participants and lower than the score of 6.36 for the participants with sleep disorders in Buysse et al . 14 .
Although with some differences across questionnaires, these sleep scores suggest a moderate to poor sleep quality of the current sample, with about 15% of participants experiencing very poor sleep quality, comparable to those of patients with sleep disorders. Overall, all sleep measures used in the current study appear to have a sufficient level of variability to conduct the planned analyses.
Combining sleep quality metrics
Principal component analysis was used to combine PSQI and AIS into a single ‘sleep disturbance’ metric. The Bartlett’s test of sphericity indicated that the correlation between the scales was adequately large for a PCA, χ 2 (235) = 84.88, p < 0.0001. One principal factor with an eigenvalue of 1.55 was extracted to represent sleep disturbance. The component explained 83.7% of the variance, and it was named ‘sleep disturbance’ as higher values of this metric show more disturbed sleep. The aggregated sleep disturbance index across the three studies ranged from -1.9 to 3.86.
Associations between subjective sleep quality and cognitive performance
As described above, to study the associations between subjective sleep quality and cognitive performance, separate linear mixed-effect models were created for each outcome measure (i.e., cognitive performance metric), where sleep disturbance was used as a fixed predictor, and ‘Study’ was added as a random intercept. Sleep disturbance did not show an association with any of the cognitive performance metrics (see Table 3 and Fig. 1 ). Bayes Factors ranged from 5.01 to 14.35, indicating substantial evidence for no association between subjective sleep quality and the measured cognitive processes 51 .
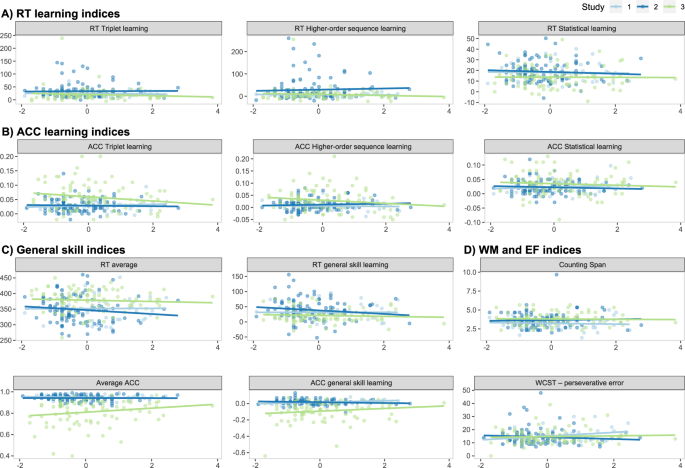
Association between sleep disturbance and cognitive performance metrics by study. Horizontal axes represent the sleep disturbance index, while vertical axes represent the outcome variables, with their names shown in the panel titles. The scatterplots and the linear regression trendlines show no association between subjective sleep quality and procedural learning indices in terms of reaction time (RT, A ), or accuracy (ACC, B ), general skill indices in terms of RT or ACC ( C ), and working memory and executive function indices ( D ).
To test whether AIS or PSQI scores separately are associated with cognitive performance, we performed similar analyses as for the sleep disturbance metric. Additionally, we also tested whether cognitive performance differed between “good” and “poor” sleepers as defined by the extremes in the overall PSQI score. For this analysis, we considered those with a score of 0 or 1 as good sleepers (N = 36), while those with a score of 5 to 8 as poor sleepers (N = 43), corresponding to approximately the upper and lower 15% of the data (see Table 2 ). These additional analyses (reported in the Supplementary results) are consistent with the above findings for the sleep disturbance metric, suggesting no relationship between subjective sleep quality and cognitive performance using these measures.
In Study 2, to investigate the associations between further subjective sleep quality questionnaires and cognitive performance, we created a separate linear mixed-effect model for each outcome measure (i.e., cognitive performance metric), and each additional sleep questionnaire (i.e., sleep diary and GSQS). Sleep diary scores did not show association with any of the cognitive performance metrics (all p s > 0.05, see Table 4 and Fig. 2 ). Bayes Factors ranged from 2.51 to 12.58, indicating, in all but one cases, substantial evidence for no association between subjective sleep quality and measures of cognitive performance 51 . The lowest value of 2.51 for ACC general skill learning also pointed to the same direction, indicating slightly weaker evidence for no association with subjective sleep quality.
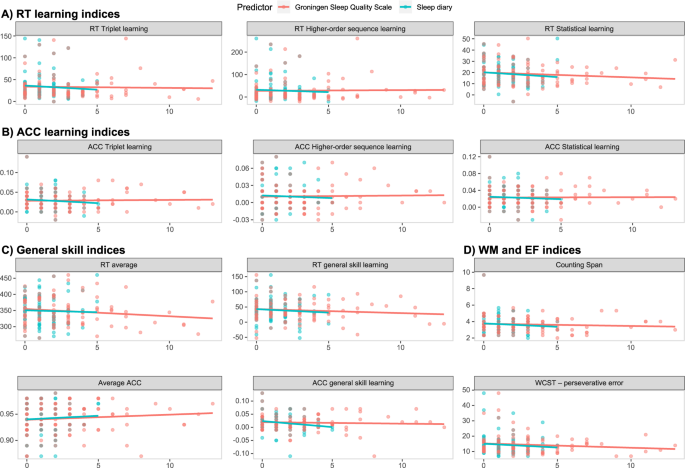
Association between sleep diary and GSQS scores and cognitive performance metrics. Horizontal axes represent the sleep disturbance index, while vertical axes represent the outcome variables, with their names shown in the panel titles. The scatterplots and the linear regression trendlines show no association between subjective sleep quality (measured with a sleep diary (blue) or the GSQS (red)) and procedural learning indices in terms of reaction time (RT, A ), or accuracy (ACC, B ), general skill indices in terms of RT or ACC ( C ), and working memory and executive function indices ( D ).
Similarly, GSQS scores did not show association with any of the cognitive performance metrics (all p s > 0.11, see Table 5 and Fig. 2 ). Bayes Factors ranged from 3.46 to 16.46, indicating substantial evidence for no association between subjective sleep quality and the measured cognitive processes 51 .
Our aim was to investigate the relationship between subjective sleep quality and cognitive performance in healthy young adults. Cognitive performance was tested in the domains of working memory, executive functions, and procedural learning. To provide more reliable results, we pooled data from three different studies, controlled for possible confounders, such as age, gender, and chronotype, and performed robust frequentists as well as Bayesian statistical analyses. We did not find associations between subjective sleep quality and cognitive performance measures using the robust frequentist statistical analyses. Moreover, the Bayes factors provided substantial evidence for no association between subjective sleep quality and measures of working memory, executive functions, and procedural learning. This pattern held when subjective sleep quality was reported retrospectively for a longer period (i.e., a month; with PSQI and AIS), as well as when monitored daily (for one to two weeks; with the sleep diary) or reported for the night prior to testing (with GSQS). These results suggest that neither moderately persistent nor transient subjective sleep quality is associated with cognitive performance in healthy young adults.
There are several factors to consider why subjective sleep quality showed no associations with cognitive performance in our sample of healthy young adults. First, it is possible that methodological issues contributed to the null effects. For example, having a lower range of obtainable scores on the selected subjective sleep quality and cognitive performance measures can limit the possibility of finding a relationship between these measures. Importantly, all measures that we used in the current study have been well-established in previous research and have a reasonable range of obtainable values. Although the sample choice of healthy young adults has naturally limited the range of scores on the used measures, our analyses showed a sufficient level of variability in all measures. Therefore, the obtained null results seem unlikely to be explained by such methodological issues.
Second, as we studied healthy university students, there may be a ceiling effect in subjective sleep quality. Sleep disturbance can be more prevalent in elderly populations and clinical disorders 14 , 33 . Consequently, variance and extremities in subjective sleep quality could be greater in these populations, while it can remain relatively low in healthy young adults. Nevertheless, previous research has found that university students are also prone to sleep disturbances, and in particular to chronic sleep deprivation 55 . Although with some variation across sleep questionnaires, most participants’ subjective sleep quality ranged from moderate to poor in our sample, with about 15% of participants experiencing very poor sleep quality similar to those of patients with sleep disorders. Thus, it seems unlikely that the obtained results are due to a ceiling effect in subjective sleep quality.
Third, it is possible that because young adults typically show a peak cognitive performance, poor subjective sleep quality may not have a substantial impact on it. In line with this explanation, the studies that reported associations between subjective sleep quality and cognitive performance 4 , 5 , 17 focused primarily on adolescents, older adults, or clinical populations, where cognitive performance has not yet peaked or have declined. Further supporting this explanation, Saksvik et al . 56 found in their meta-analysis that young adults are not as prone to the negative consequences of shift work as the elderly. Moreover, Gao et al . 57 in a recent study showed that above-average cognitive abilities buffer against insufficient sleep durations. However, not all cognitive functions peak in adulthood: while previous studies have reported the best performance in working memory and executive functions in young adulthood 58 , 59 , 60 , 61 , some aspects of procedural learning (as measured by the ASRT task) has been shown to peak in childhood and to decline already around adolescents 44 , 62 , 63 . Consequently, a cognitive peak may explain finding no relationship between subjective sleep quality and aspects of working memory and executive functions, while this explanation for the measures of procedural learning seems unlikely.
Fourth, the conditions under which the data collection took place could have also contributed to the null results. We conducted our experiments during the term-time when the workload in the university is typically moderate. Moreover, students could choose the time of day for cognitive testing, and they may have chosen a time when they typically felt well-rested. There is evidence that performing in a preferred circadian time period can attenuate the effect of sleep disturbances 64 . Consistently, previous studies showed that participants exhibit better performance on working memory and executive functions tasks in their preferred time of day 65 , 66 . However, a recent study found that participants, in fact, exhibit weaker performance in procedural learning in their preferred time of day, and better performance in their non-preferred time of day, suggesting variability in the relationship between circadian effects and cognitive functions 67 . Additionally, independent of the time of day, participants may have perceived the session with the cognitive tasks as a testing situation and may have been motivated to show their best performance, compensating for any possible effect of poor subjective sleep quality. Indeed, there is evidence that highly motivated participants are less prone to the effect of sleep deprivation 68 . Thus, the time of testing and participants’ motivation may have contributed to our findings by potentially compensating for any negative effects of poor subjective sleep quality on cognitive performance.
Fifth, the relationship between sleep and cognitive performance can vary depending on what parameters of sleep are assessed. Associations between objective sleep quality (measured by actigraphy or electroencephalography) and various aspects of working memory, executive functions, and procedural learning have been frequently reported in previous studies (for a review, see 8 , 9 ). Here we showed that subjective sleep quality is not associated with these cognitive functions, at least under the circumstances described above. As outlined in the Introduction, this dissociation suggests that objective and subjective sleep quality, although measure the same domains, do not necessarily capture the same aspects of sleep quality and sleep disturbances 11 . Subjective sleep quality may be estimated based on a combination of objective sleep parameters. Moreover, some objective parameters of sleep that contribute to cognitive performance may not be captured with self-reported instruments. For example, it is often reported that spindle activity or time spent in slow-wave sleep (SWS) or in rapid eye movement (REM) sleep is essential for memory consolidation 69 , 70 , 71 . Also, in laboratory sleep examinations, sleep quality is usually carefully controlled for several days prior to the examination. Potentially, the objective sleep parameters showing associations with cognitive performance may only be measured in these carefully controlled conditions (i.e., when sleep quality on the night of testing as well as in the preceding days are good). Hence, it is possible that while results with objective sleep quality may show how healthy sleep is related to cognitive functioning, results with subjective sleep quality may reflect how aspects of sleep disturbances are related to cognitive functioning.
Sixth, and relatedly, there could be differences in the association with cognitive performance within self-reported measures of sleep as well. In our study, we captured the perceived disturbances in initiating and maintaining sleep rather than the self-reported duration of sleep. While we found no associations between these measures of subjective sleep quality and cognitive performance, there is solid evidence that self-reported extreme sleep durations (both long and short sleep times) are associated with worse cognitive performance 18 , 19 , 20 . These findings suggest a dissociation between sleep quality as measured by extreme self-reported sleep durations and other types of sleep quality disturbances.
Seventh, it is possible that while interindividual differences in subjective sleep quality do not contribute to at least some aspects of cognitive performance, intraindividual fluctuations do. The possible importance of intraindividual rather than interindividual differences was also suggested by Ackerman et al . 72 in a large study, in which contrary to previous studies they showed no associations between declarative memory consolidation and objective sleep parameters. Further studies are warranted to test whether day-to-day variations in subjective sleep quality predict day-to-day changes in cognitive performance.
Finally, our paper has some limitations. As mentioned above, it is possible that investigating populations more susceptible to sleep disturbances or cognitive performance problems could yield different results and the lack of associations could be specific to healthy young adults. Furthermore, it would be interesting to test whether individual differences in other factors (for example, interoceptive ability, i.e., how accurately one perceives their own body sensations) influence the relationship between subjective sleep quality and cognitive performance.
Conclusions
In conclusion, we showed that self-reported, subjective sleep quality is not associated with working memory, executive functions, and various aspects of procedural learning in a relatively large sample of healthy young adults. These findings were supported not only by frequentist statistical analyses but also by Bayes factors that provided substantial evidence for no associations between these functions. Importantly, however, our findings do not imply that sleep per se has no relationship with these cognitive functions; instead, it emphasizes the dissociation between subjective and objective sleep quality. We believe that our approach of systematically testing the relationship between self-reported sleep questionnaires and a relatively wide range of cognitive functions can inspire future systematic studies on the relationship between subjective/objective sleep parameters and cognition. Within healthy young adults, future studies are warranted to probe the relationship between subjective sleep quality and cognitive performance assessed in the non-preferred time of day, include other aspects of cognitive functions, and test intraindividual, day-to-day variations in the relationship between sleep and cognitive performance.
Data availability
The dataset and analysis code of the current study are available in the Open Science Framework repository, https://osf.io/hcnsx/ .
Gavriloff, D. et al . Sham sleep feedback delivered via actigraphy biases daytime symptom reports in people with insomnia: Implications for insomnia disorder and wearable devices. J. Sleep Res ., e12726 (2018).
Article PubMed Google Scholar
Draganich, C. & Erdal, K. Placebo sleep affects cognitive functioning. J. Exp. Psychol. Learn. Mem. Cogn. 40 , 857 (2014).
Bastien, C. H. et al . Cognitive performance and sleep quality in the elderly suffering from chronic insomnia: relationship between objective and subjective measures. J. Psychosom. Res. 54 , 39–49 (2003).
van den Noort, M. et al . Schizophrenia and depression: The relation between sleep quality and working memory. Asian J. Psychiatr. 24 , 73–78 (2016).
Nebes, R. D., Buysse, D. J., Halligan, E. M., Houck, P. R. & Monk, T. H. Self-reported sleep quality predicts poor cognitive performance in healthy older adults. J. Gerontol. B 64 , 180–187 (2009).
Article Google Scholar
Miyata, S. et al . Poor sleep quality impairs cognitive performance in older adults. J. Sleep Res. 22 , 535–541 (2013).
Stepanski, E. et al . Characteristics of individuals who do or do not seek treatment for chronic insomnia. Psychosomatics 30 , 421–427 (1989).
Article CAS PubMed Google Scholar
Diekelmann, S. & Born, J. The memory function of sleep. Nat. Rev. Neurosci. 11 , 114–126 (2010).
Jones, K. & Harrison, Y. Frontal lobe function, sleep loss and fragmented sleep. Sleep Med. Rev. 5 , 463–475 (2001).
Guedes, L. G. et al . Comparison between self-reported sleep duration and actigraphy among adolescents: gender differences. Revista Brasileira de Epidemiologia 19 , 339–347 (2016).
Armitage, R., Trivedi, M., Hoffmann, R. & Rush, A. J. Relationship between objective and subjective sleep measures in depressed patients and healthy controls. Depression and anxiety 5 , 97–102 (1997).
Landry, G. J., Best, J. R. & Liu-Ambrose, T. Measuring sleep quality in older adults: a comparison using subjective and objective methods. Front. Aging Neurosci. 7 , 166 (2015).
PubMed PubMed Central Google Scholar
Zhang, L. & Zhao, Z.-X. Objective and subjective measures for sleep disorders. Neurosci. Bull. 23 , 236–240 (2007).
Buysse, D. J., Reynolds, C. F. III, Monk, T. H., Berman, S. R. & Kupfer, D. J. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 28 , 193–213 (1989).
Soldatos, C. R., Dikeos, D. G. & Paparrigopoulos, T. J. Athens Insomnia Scale: validation of an instrument based on ICD-10 criteria. J. Psychosom. Res. 48 , 555–560 (2000).
Libman, E., Fichten, C. S., Bailes, S. & Amsel, R. Sleep questionnaire versus sleep diary: which measure is better? International Journal of Rehabilitation and Health 5 , 205–209 (2000).
Telzer, E. H., Fuligni, A. J., Lieberman, M. D. & Galván, A. The effects of poor quality sleep on brain function and risk taking in adolescence. Neuroimage 71 , 275–283 (2013).
Sternberg, D. A. et al . The largest human cognitive performance dataset reveals insights into the effects of lifestyle factors and aging. Front. Hum. Neurosci. 7 , 292 (2013).
Article PubMed PubMed Central Google Scholar
Lo, J. C., Groeger, J. A., Cheng, G. H., Dijk, D.-J. & Chee, M. W. Self-reported sleep duration and cognitive performance in older adults: a systematic review and meta-analysis. Sleep Med. 17 , 87–98 (2016).
Kyle, S. D. et al . Sleep and cognitive performance: cross-sectional associations in the UK Biobank. Sleep Med. 38 , 85–91 (2017).
Poldrack, R. A. et al . Interactive memory systems in the human brain. Nature 414 , 546–550 (2001).
Fiser, J. & Aslin, R. N. Unsupervised statistical learning of higher-order spatial structures from visual scenes. Psychol. Sci. 12 , 499–504 (2001).
Howard, J. H. Jr. & Howard, D. V. Age differences in implicit learning of higher-order dependencies in serial patterns. Psychol. Aging 12 , 634–656 (1997).
Lieberman, M. D. Intuition: a social cognitive neuroscience approach. Psychol. Bull. 126 , 109–137 (2000).
Poldrack, R. A. & Foerde, K. Category learning and the memory systems debate. Neurosci. Biobehav. Rev. 32 , 197–205 (2008).
Pothos, E. M. Theories of artificial grammar learning. Psychol. Bull. 133 , 227–244 (2007).
Török, C., Janacsek, K. & Nemeth, D. In Internetional Conference On Memory (Budapest, Hungary, 2016).
Simor, P. et al . Deconstructing procedural memory: Different learning trajectories and consolidation of sequence and statistical learning. Front. Psychol. 9 , 2708 (2019).
Takács, Á. et al . In 3rd Conference of the European Society for Cognitive and Affective Neuroscience (Porto, Portugal, 2016).
Horne, J. A. & Östberg, O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int. J. Chronobiol (1976).
Zavecz, Z., Török, C., Köteles, F., Pálosi, V. & Simor, P. The psychometric properties of the Hungarian version of the Morningness-Eveningness Questionnaire (MEQ-H): The separate factors of morning freshness and circadian rhythmicity. Psychiatria Hungarica: A Magyar Pszichiátriai Társaság Tudományos Folyóirata 30 , 318–331 (2015).
Google Scholar
Takács, J. et al . Reliability and validity of the Hungarian version of the Pittsburgh Sleep Quality Index (PSQI-HUN): comparing psychiatric patients with control subjects. Sleep. Breath. 20 , 1045–1051 (2016).
Novak, M., Mucsi, I., Shapiro, C. M., Rethelyi, J. & Kopp, M. S. Increased utilization of health services by insomniacs—an epidemiological perspective. J. Psychosom. Res. 56 , 527–536 (2004).
Gilson, M. et al . REM-enriched naps are associated with memory consolidation for sad stories and enhance mood-related reactivity. Brain Sci. 6 , 1 (2015).
Article MathSciNet PubMed Central Google Scholar
Simor, P., Köteles, F., Bódizs, R. & Bárdos, G. A questionnaire based study of subjective sleep quality: the psychometric evaluation of the Hungarian version of the Groningen Sleep Quality Scale. Mentálhigiéné és Pszichoszomatika 10 , 249–261 (2009).
Meijman, T., de Vries-Griever, A., De Vries, G. & Kampman, R. The evaluation of the Groningen sleep quality scale. Groningen: Heymans Bulletin (HB 88-13-EX) 2006 (1988).
Case, R., Kurland, D. M. & Goldberg, J. Operational efficiency and the growth of short-term memory span. J. Exp. Child Psychol. 33 , 386–404 (1982).
Engle, R. W., Tuholski, S. W., Laughlin, J. E. & Conway, A. R. A. Working memory, short-term memory, and general fluid intelligence: A latent-variable approach. J. Exp. Psychol. 128 , 309–331 (1999).
Conway, A. R. et al . Working memory span tasks: A methodological review and user’s guide. Psychon. Bull. Rev. 12 , 769–786 (2005).
Virag, M. et al . Competition between frontal lobe functions and implicit sequence learning: evidence from the long-term effects of alcohol. Exp. Brain Res. 233 , 2081–2089 (2015).
Berg, E. A. A simple objective treatment for measuring flexibility in thinking. J. Gen. Psychol. 39 , 15–22 (1948).
Piper, B. J. et al . Reliability and validity of neurobehavioral function on the Psychology Experimental Building Language test battery in young adults. PeerJ 3 , e1460 (2015).
Nemeth, D., Janacsek, K., Polner, B. & Kovacs, Z. A. Boosting Human Learning by Hypnosis. Cereb. Cortex 23 , 801–805, https://doi.org/10.1093/cercor/bhs068 (2013).
Nemeth, D., Janacsek, K. & Fiser, J. Age-dependent and coordinated shift in performance between implicit and explicit skill learning. Front. Comput. Neurosci . 7 , https://doi.org/10.3389/fncom.2013.00147 (2013).
Hallgato, E., Győri-Dani, D., Pekár, J., Janacsek, K. & Nemeth, D. The differential consolidation of perceptual and motor learning in skill acquisition. Cortex 49 , 1073–1081 (2013).
R Core Team. (ISBN 3-900051-07-0: URL http://www. R-project. org 2018).
Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting Linear Mixed-Effects Models Using lme4. Journal of Statistical Software 67 , 1–48 (2015).
Canty, A. & Ripley, B. boot: Bootstrap R (S-Plus) Functions. (2019).
Davison, A. C. & Hinkley, D. V. Bootstrap methods and their application. Vol. 1 (Cambridge university press, 1997).
Wagenmakers, E. J. A practical solution to the pervasive problems of p values. Psychon Bull Rev 14 , 779–804, https://doi.org/10.3758/BF03194105 (2007).
Wagenmakers, E. J., Wetzels, R., Borsboom, D. & van der Maas, H. L. Why psychologists must change the way they analyze their data: the case of psi: comment on Bem (2011). J. Pers. Soc. Psychol. 100 , 426–432, https://doi.org/10.1037/a0022790 (2011).
Heaton, R. K. A manual for the Wisconsin card sorting test. (Western Psycological Services, 1981).
Racsmány, M., Lukács, Á., Németh, D. & Pléh, C. A verbális munkamemória magyar nyelvű vizsgálóeljárásai (Hungarian Diagnostic Tools of Verbal Working Memory Functions). Magyar Pszichológiai Szemle (Hungarian Review of Psychology) 60 , 479–506 (2005).
Soldatos, C. R., Dikeos, D. G. & Paparrigopoulos, T. J. The diagnostic validity of the Athens Insomnia Scale. J. Psychosom. Res. 55 , 263–267 (2003).
Gaultney, J. F. The prevalence of sleep disorders in college students: impact on academic performance. J. Am. Coll. Health 59 , 91–97 (2010).
Saksvik, I. B., Bjorvatn, B., Hetland, H., Sandal, G. M. & Pallesen, S. Individual differences in tolerance to shift work–a systematic review. Sleep Med. Rev. 15 , 221–235 (2011).
Gao, C., Terlizzese, T. & Scullin, M. K. Short sleep and late bedtimes are detrimental to educational learning and knowledge transfer: An investigation of individual differences in susceptibility. Chronobiol. Int. 36 , 307–318 (2019).
Tanczos, T., Janacsek, K. & Nemeth, D. Verbal fluency tasks I. Investigation of the Hungarian version of the letter fluency task between 5 and 89 years of age. Psychiatria Hungarica: A Magyar Pszichiatriai Tarsasag tudomanyos folyoirata 29 , 158–180 (2013).
Tanczos, T., Janacsek, K. & Nemeth, D. Verbal fluency tasks II. Investigation of the Hungarian version of the semantic fluency task between 5 and 89 years of age. Psychiatria Hungarica: A Magyar Pszichiatriai Tarsasag tudomanyos folyoirata 29 , 181–207 (2013).
Craik, F. I. & Bialystok, E. Cognition through the lifespan: mechanisms of change. TRENDS in Cognitive Sciences 10 , 131–138 (2006).
Zelazo, P. D., Craik, F. I. & Booth, L. Executive function across the life span. Acta Psychol. (Amst.) 115 , 167–183 (2004).
Janacsek, K., Fiser, J. & Nemeth, D. The best time to acquire new skills: age-related differences in implicit sequence learning across the human lifespan. Dev. Sci. 15 , 496–505 (2012).
Juhasz, D., Nemeth, D. & Janacsek, K. Is there more room to improve? The lifespan trajectory of procedural learning and its relationship to the between-and within-group differences in average response times. PLoS One , https://doi.org/10.1371/journal.pone.0215116 (2019).
Article CAS PubMed PubMed Central Google Scholar
Goel, N., Basner, M., Rao, H. & Dinges, D. F. Circadian rhythms, sleep deprivation, and human performance. Prog. Mol. Biol. Transl. Sci. 119 , 155–190 (2013).
Rowe, G., Hasher, L. & Turcotte, J. Short article: Age and synchrony effects in visuospatial working memory. Q. J. Exp. Psychol. 62 , 1873–1880 (2009).
Matchock, R. L. & Mordkoff, J. T. Chronotype and time-of-day influences on the alerting, orienting, and executive components of attention. Exp. Brain Res. 192 , 189–198 (2009).
Delpouve, J., Schmitz, R. & Peigneux, P. Implicit learning is better at subjectively defined non-optimal time of day. Cortex 58 , 18–22 (2014).
Hull, J. T., Wright, K. P. Jr & Czeisler, C. A. The influence of subjective alertness and motivation on human performance independent of circadian and homeostatic regulation. J. Biol. Rhythms 18 , 329–338 (2003).
Walker, M. P. The Role of Slow Wave Sleep in Memory Processing. J. Clin. Sleep Med. 5 , S20–S26 (2009).
Siegel, D. J. Memory: an overview, with emphasis on developmental, interpersonal, and neurobiological aspects. J. Am. Acad. Child Adolesc. Psychiatry 40 , 997–1011 (2001).
Clemens, Z., Fabo, D. & Halasz, P. Overnight verbal memory retention correlates with the number of sleep spindles. Neuroscience 132 , 529–535 (2005).
Ackermann, S., Hartmann, F., Papassotiropoulos, A., de Quervain, D. J. & Rasch, B. No associations between interindividual differences in sleep parameters and episodic memory consolidation. Sleep 38 , 951–959 (2015).
Download references
Acknowledgements
This research was supported by the Research and Technology Innovation Fund, Hungarian Brain Research Program (National Brain Research Program, project 2017-1.2.1-NKP-2017-00002); IDEXLYON Fellowship of the University of Lyon as part of the Programme Investissements d’Avenir (ANR-16-IDEX-0005); Hungarian Scientific Research Fund (NKFIH-OTKA PD 124148, PI: KJ; NKFIH-OTKA K 128016, to DN); and Janos Bolyai Research Fellowship of the Hungarian Academy of Sciences (to KJ). The authors are thankful to Csenge Török, Kata Horváth, Eszter Tóth-Fáber, Orsolya Pesthy, Noémi Éltető, Andrea Kóbor, and Ádám Takács for their help in data collection, to Kate Schipper for proofreading the manuscript, and to the reviewers for their helpful comments and suggestions to improve the paper.

Author information
These authors contributed equally: Dezso Nemeth and Karolina Janacsek
Authors and Affiliations
Doctoral School of Psychology, ELTE Eötvös Loránd University, Budapest, Hungary
Zsófia Zavecz
Institute of Psychology, ELTE Eötvös Loránd University, Budapest, Hungary
Zsófia Zavecz, Tamás Nagy, Adrienn Galkó, Dezso Nemeth & Karolina Janacsek
Institute of Cognitive Neuroscience and Psychology, Hungarian Academy of Sciences, Budapest, Hungary
Zsófia Zavecz, Dezso Nemeth & Karolina Janacsek
Lyon Neuroscience Research Center (CRNL), INSERM, CNRS, Université Claude Bernard Lyon 1, Lyon, France
- Dezso Nemeth
School of Human Sciences, Faculty of Education, Health and Human Sciences, University of Greenwich, London, United Kingdom
Karolina Janacsek
You can also search for this author in PubMed Google Scholar
Contributions
Z.Z., K.J. and D.N. designed the present study and wrote the manuscript. A.G. and Z.Z. collected the data. A.G., Z.Z., K.J. and T.N. analyzed the data. Z.Z., K.J., T.N. and D.N. contributed to the interpretation of the results and critically revised the previous versions of the manuscript. All authors read and approved the final version of the manuscript.
Corresponding authors
Correspondence to Dezso Nemeth or Karolina Janacsek .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information ., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Zavecz, Z., Nagy, T., Galkó, A. et al. The relationship between subjective sleep quality and cognitive performance in healthy young adults: Evidence from three empirical studies. Sci Rep 10 , 4855 (2020). https://doi.org/10.1038/s41598-020-61627-6
Download citation
Received : 19 August 2019
Accepted : 17 February 2020
Published : 17 March 2020
DOI : https://doi.org/10.1038/s41598-020-61627-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Deterministic and probabilistic regularities underlying risky choices are acquired in a changing decision context.
- Andrea Kóbor
- Eszter Tóth-Fáber
Scientific Reports (2023)
Sleep Duration and Executive Function in Adults
- Aayushi Sen
- Xin You Tai
Current Neurology and Neuroscience Reports (2023)
Association Between Internet Use, Sleep, Cognition and Physical Activity Levels During COVID-19 Lockdown
- Deepika Singla
- Ona P. Desai
- Sohrab A. Khan
Sleep and Vigilance (2023)
Too Sour to be True? Tart Cherries (Prunus cerasus) and Sleep: a Systematic Review and Meta-analysis
- Brandon Stretton
- Aditya Eranki
Current Sleep Medicine Reports (2023)
Bidirectional associations between physical activity and sleep in older adults: a multilevel analysis using polysomnography
- Jaehoon Seol
- Tomohiro Okura
Scientific Reports (2022)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
Neurophysiological Effects of Sleep Deprivation in Healthy Adults, a Pilot Study
* E-mail: [email protected]
Affiliations Department of Psychiatry, VU University Medical Center, Amsterdam, The Netherlands, Neuroscience Campus Amsterdam, VU University Medical Center, Amsterdam, The Netherlands
Affiliation Neuroimaging Center University Medical Center, Groningen, The Netherlands
Affiliations Department of Nuclear Medicine & PET Research, VU University Medical Center, Amsterdam, The Netherlands, Neuroscience Campus Amsterdam, VU University Medical Center, Amsterdam, The Netherlands
Current address: Department of Psychiatry, Erasmus Medical Center, Rotterdam, The Netherlands
- Ursula M. H. Klumpers,
- Dick J. Veltman,
- Marie-Jose van Tol,
- Reina W. Kloet,
- Ronald Boellaard,
- Adriaan A. Lammertsma,
- Witte J. G. Hoogendijk

- Published: January 21, 2015
- https://doi.org/10.1371/journal.pone.0116906
- Reader Comments
Total sleep deprivation (TSD) may induce fatigue, neurocognitive slowing and mood changes, which are partly compensated by stress regulating brain systems, resulting in altered dopamine and cortisol levels in order to stay awake if needed. These systems, however, have never been studied in concert. At baseline, after a regular night of sleep, and the next morning after TSD, 12 healthy subjects performed a semantic affective classification functional magnetic resonance imaging (fMRI) task, followed by a [ 11 C]raclopride positron emission tomography (PET) scan. Saliva cortisol levels were acquired at 7 time points during both days. Affective symptoms were measured using Beck Depression Inventory (BDI), Spielberger State Trait Anxiety Index (STAI) and visual analogue scales. After TSD, perceived energy levels, concentration, and speed of thought decreased significantly, whereas mood did not. During fMRI, response speed decreased for neutral words and positive targets, and accuracy decreased trendwise for neutral words and for positive targets with a negative distracter. Following TSD, processing of positive words was associated with increased left dorsolateral prefrontal activation. Processing of emotional words in general was associated with increased insular activity, whereas contrasting positive vs. negative words showed subthreshold increased activation in the (para)hippocampal area. Cortisol secretion was significantly lower after TSD. Decreased voxel-by-voxel [ 11 C]raclopride binding potential (BP ND ) was observed in left caudate. TSD induces widespread cognitive, neurophysiologic and endocrine changes in healthy adults, characterized by reduced cognitive functioning, despite increased regional brain activity. The blunted HPA-axis response together with altered [ 11 C]raclopride binding in the basal ganglia indicate that sustained wakefulness requires involvement of additional adaptive biological systems.
Citation: Klumpers UMH, Veltman DJ, van Tol M-J, Kloet RW, Boellaard R, Lammertsma AA, et al. (2015) Neurophysiological Effects of Sleep Deprivation in Healthy Adults, a Pilot Study. PLoS ONE 10(1): e0116906. https://doi.org/10.1371/journal.pone.0116906
Academic Editor: Hengyi Rao, University of Pennsylvania, UNITED STATES
Received: August 17, 2013; Accepted: December 16, 2014; Published: January 21, 2015
Copyright: © 2015 Klumpers et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Funding: This study was supported in part by ZONMW (Dutch Organization for Health Research and Development), The Netherlands, grant no. 016.066.309, to Dr. Ronald Boellaard. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Introduction
Lack of sleep is a common condition in everyday life, either related to psychosocial demands or related to working shift hours. In healthy individuals, this may induce decreased alertness and vigilance, together with a general decline in mood. Total sleep deprivation (TSD) has been associated with general psychomotor slowing and diminished cognitive performance [ 1 , 2 ]. In affective disorders, only one night of sleep deprivation may improve mood in 40–60% of subjects with major depressive disorder [ 3 ], whereas bipolar patients may even turn into (hypo)mania [ 4 ]. Thus, in humans, sleep deprivation is clearly related to altered emotional and affective functioning.
From an evolutionary perspective, staying awake has served to guard against outside threats, requiring increased alertness. Motivational control over the waking state is necessary and presumed to be modulated by top-down cortical control systems, involving prefrontal executive regions [ 5 ]. Using [ 18 F]-2-fluoro-2-deoxy-D-glucose ([ 18 F]FDG) as a ligand in positron emission tomography (PET) studies, sleep deprivation has been associated with reduced metabolic activity in a network of brain regions, including prefrontal and limbic regions, the thalamo-basal ganglia circuit, and cerebellum [ 6 , 7 ]. Neurophysiologically, dopamine (DA) release is supposed to increase wakefulness, partly through the D2 receptor [ 8 , 9 , 10 ] and partly by acting as a stimulator of corticotropin releasing hormone (CRH) [ 11 ]. Ultimately, CRH releases cortisol from the adrenal cortex via the hypothalamic pituitary adrenal (HPA) axis, a key endocrine response mechanism to a stressful situation. These effects are superimposed upon the circadian rhythm of the HPA axis, and largely controlled by the central body clock, the suprachiasmatic nucleus (SCN). HPA axis functioning can be assessed by the cortisol awakening response (CAR), reflecting the natural HPA response to stress of sleep-wake transitions [ 12 ]. It is unknown, however, how cortical, dopaminergic and HPA axis activities interact to maintain wakefulness. Studying their interaction may also provide insight into the pathophysiology of depressive disorder, with its frequently occurring sleeping problems and HPA-axis hyperactivity [ 13 , 14 ].
The purpose of this pilot study was to assess how the healthy brain responds to TSD and how compensatory and regulatory stress mechanisms may interact as opposed to future clinical studies in mood disorder. It was hypothesized that wakefulness would be associated with an increase in dopamine release and CRH activation, in the presence of altered emotional functioning.
Materials and Methods
Participants.
Twelve healthy adults (6 female, mean age 29.2 ± 10.2 years; 6 male, mean age 28.5 ± 4.8 years) were recruited through newspaper advertisements. Exclusion criteria included a lifetime history of psychiatric disorders, as assessed by Mini international neuropsychiatric interview [ 15 ] and reported contacts with mental health counselors, previous use of psychotropic medication known to interfere with the dopaminergic system, 1 st degree relatives with psychiatric disorder, somatic disorders, pregnancy, use of sleep medication and past or current abuse of psychoactive drugs. All subjects were good sleepers, defined as feeling rested after a night’s sleep, and in good physical health as assessed by medical history, physical examination and routine laboratory tests. On the night preceding TSD, subjects slept 6.6 ± 1.1 hours. Mean body mass index was 21.0 ± 1.4 kg·m −2 , 2 were cigarette smokers (10 per day), and 10 consumed alcohol (1.5 ± 1.1 units day).
Ethics Statement
Written informed consent was obtained from all participants. The study protocol was approved by the Medical Ethics Review Committee of the VU University Medical Center in Amsterdam.
Design and Procedure
Cortisol saliva was collected on both days. At baseline (day 1), after a regular night of sleep at home, all subjects underwent functional magnetic resonance imaging (fMRI) scanning in the morning, followed by a 60min [ 11 C]raclopride PET scan. After this scanning session, participants returned to their daily activities, including study and/or work. They returned to the hospital at 22.00h for effectuation of total sleep deprivation. During the night, subjects were monitored by a trained observer and engaged in reading, conversation, short walks on the ward, and board games in a well-lit room. At arrival, urine toxicology was screened and found negative for a subset of dopaminergic and wake enhancing drugs, including cocaine, tetrahydrocannabinol (THC) and amphetamines. Use of alcohol, caffeinated beverages and smoking was prohibited during the night, as on both days in-between scan experiments. At day 2, a light meal was served at 6.00h. After having been awake for about 25 hours, fMRI scanning was repeated, followed by a second [ 11 C]raclopride PET scan for all participants. After finishing the scan sessions, subjects were asked to stay awake during the remainder of the day, and to postpone sleep until the evening.
Psychometric Data
Depressive symptoms over the prior week were assessed using the Beck Depression Inventory [ 16 ]. At baseline and before scanning, trait and state anxiety were measured using the Spielberger State-Trait Anxiety Inventory (STAI) [ 17 ]. During sleep deprivation, self and observer based visual analogue scales (VAS) were registered every 3 hours, starting at 24.00h and finishing at 12.00h, documenting mood, interest, motor inhibition, speed of thought, self appreciation, energy level and concentration on a scale from 0–100. Psychometric data were analyzed using Statistical Package for the Social Sciences (SPSS) version 15.0 for Windows (SPPS Inc, Chicago, Illinois, USA), using Repeated Measures ANOVA.
Cortisol Measurements
Data acquisition..
At the baseline interview, participants were instructed to collect saliva samples using Salivettes (Starstedt, Germany)[ 18 , 19 ], at 7 time points per day. One hour cortisol awakening response (CAR) measurements included three sampling points, immediately after awakening (T1), at +30min (T2) and at +60min (T3). Additional saliva samples were taken at +90min (T4) after awakening, at 14.00h (T5), 17.00h (T6) and 23.00h (T7). Subjects were instructed to write down the exact sampling time. On the following day, samples were collected at identical time points (T8–T14). Eating, smoking, drinking tea or coffee or brushing their teeth was prohibited within 15min before sampling. No dental work was allowed within 24 hours prior to sampling. Samples were stored in a refrigerator and returned by the participant or by regular mail. Salivettes were centrifuged at 2000g for 10min, aliquoted and stored at −80°C. Free cortisol analysis was performed by competitive electrochemiluminescence immunoassay (Architect, Abbott Laboratories, Illinois, USA) [ 20 ]. The lower limit of quantification was 2.0nmol·L −1 , the intra- and inter-assay variability coefficients were less than 9 and 11%.
Data analysis.
The CAR area under the curve (AUC), with respect to increase (AUC I ) and to ground (AUC G ), was calculated. AUC I is calculated with reference to the baseline measurement at T1, ignoring the distance from zero for all measurements, and emphasizing change over time. AUC G is the total area under the curve of all measurements [ 21 ]. The mean increase in the 1 st hour (MnInc) was calculated by subtracting the baseline value at T1 from the mean of the subsequent values at T2 and T3. Using the real sampling time at T2, T3, T9 and T10, cortisol levels were interpolated using piecewise linear spline to +30 and +60min, in order to derive the individual CAR AUC for identical time points on both days [ 22 ]. For AUC G T1-T7 and T8-T14, mixed model analysis was used to include time points available, with missing values being interpolated [ 23 ].
Task design.
We used a semantic emotional classification task adapted from Murphy [ 24 ] and Elliot [ 25 ], where subjects had to respond as quickly as possible to affective target stimuli and ignore distracter stimuli. The fMRI study consisted of two task sessions (runs), one to be executed at baseline and one after sleep deprivation. Each participant therefore performed two versions of the task, their order randomized across subjects. Each task comprised a blocked design with 16 blocks, programmed in E-prime software (Psychology Software Tools, Inc., Pittsburgh, PA, USA). The first two blocks were practice blocks while being in the magnet, to become acquainted with the task and to reduce anticipation anxiety. Within each session, eight different task conditions were presented twice in a pseudo-randomized order, to generate 16 blocks ( Table 1 ). In each block, 22 trials were presented in a randomized order, half of these being targets, and the other half consisting of distracters. Targets and distracters were defined on the basis of emotional valence, with happy (positive (P)), sad (negative (N)), or neutral (O) words as targets, presented with one of the other categories as distracters (e.g. positive targets with negative distracters). All the words were selected from the Centre for Lexical Information (Celex) Database [ 26 ], and matched for frequency of written use and word length. Affective words were selected on high emotional impact (positive words 6.0 ± 1.6 letters, intensity 2.2 ± 0.5; negative words 5.7 ± 0.4 letters, intensity 5.9 ± 1). A baseline neutral condition was included, where targets and distracters were defined on the basis of physical properties (italic (I) vs. regular (R) font), providing similar visual input. Each of the 16 blocks started with a written instruction for a fixed 5s, followed by a 1s rest, in which subjects were instructed to respond as fast as possible to the appropriate task condition by pressing a button with the preferred index finger. Following a fixation cross for 800ms, a word was shown for 500ms to which subjects were allowed to respond within an additional fixed inter-stimulus interval of 900ms. After pressing, the word was no longer visible. At the end of a block, a 1s rest was included prior to the next block.
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0116906.t001
T1-weighted MRI scans were acquired using a 1.5T Sonata MR system (Siemens Medical Solutions, Erlangen, Germany) to exclude anatomical abnormalities and for PET and fMRI co-registration purposes. A sagittal 3D gradient-echo T1-weighted image was acquired using the following sequence: repetition time (TR) = 2.7ms, echo time (TE) = 3.97ms, matrix 256×160, voxel size 1×1×1.5mm 3 . Echo-planar images (EPI) were obtained using a T2*-weighted gradient echo sequence TR = 2.18s, TE = 45ms, 35 axial slices; voxel size 3×3×3mm 3 , flip angle 90°, matrix 64×64). For the fMRI task, stimuli were projected onto a screen at the end of the scanner table, visible through a mirror mounted above the subject’s head. Two magnetic field compatible response boxes were used to record the subject’s responses.
Data processing.
Functional imaging data were preprocessed and analyzed using Statistical Parametric Mapping (SPM) software (SPM8, Wellcome Trust Neuroimaging Centre, London, UK), implemented in Matlab 7.1.0 (The MathWorks Inc., Natick, MA, USA). Preprocessing included reorientation of the functional images to the anterior commissure, slice time correction, image realignment, co-registration of the T1 scan to the mean image, warping of the co-registered T1 image to Montreal Neurological Institute (MNI) space as defined by SPM’s T1 template, applying the transformations to the slice-timed and realigned images, reslicing to voxels of 3×3×3mm 3 and applying spatial smoothing using an 8mm full width at half maximum (FWHM) Gaussian kernel. Subject movements of more than 3mm in more than one direction resulted in exclusion of data.
In the first level analysis, scanner drifts were modeled using a high pass filter with a cut off of 128s. For each regressor, the onset of the block and the duration of the total block were modeled as a block design, consisting of 22 trial words × [800msec (fixation cross) + 500msec (word presentation) + 900msec (maximum time to press the button)] per word, plus 21 intervals × 32 msec (refresh rate word in scanner), totaling 49.072 ms. Task instructions were modeled separately as a regressor of no interest ( Table 1 ).
The following contrast images were computed:
- 1). [−2 1 0 1 0 0 0 0] positive classification vs. baseline, in which the positive-neutral (P-O) and neutral-positive (O-P) word pairs were grouped and contrasted to the baseline (italic-regular font pairs and vice versa).
- 2). [−2 0 1 0 1 0 0 0] negative classification vs. baseline, in which the negative-neutral (N-O) and neutral-negative (O-N) word pairs were grouped and contrasted to the baseline (italic-regular font pairs and vice versa).
- 3). [−2 0 0 0 0 1 1 0] both emotional valences vs. baseline, in which exclusively emotional valence pairs (P-N and N-P) were grouped and contrasted to the baseline (italic-regular font pairs and vice versa).
- 4). [−6 1 1 1 1 1 1 0] any emotional valence vs. baseline, in which all emotional valences (P-O, N-O, O-P, O-N, P-N and N-P) were grouped and contrasted to the baseline (italic-regular font pairs and vice versa).
These contrasts were defined for both pre-deprivation and post-deprivation sessions.
Next, on a second level, the contrast images for positive vs. baseline for the pre-deprivation session and the post-deprivation session were entered in a two-sample t -test, with session as dependent variable. Additionally, separate models were set up for negative vs. baseline, exclusively emotional valence pairs and any emotional valence vs. baseline. Due to the relative low number of subjects, no additional covariates were entered to these models.
The main effect of time (day 1 vs. day 2) was explored at a threshold of p uncorrected <0.005, with an extent threshold of 10 contiguous voxels. Additionally, correction for multiple comparisons was performed by applying Small Volume Correction (SVC) for regions of interest (ROIs) with known involvement in depression, sleep abnormalities and emotional attention. As described in the introduction, the following regions were selected: dorsolateral prefrontal cortex, subgenual cingulate, hippocampal gyrus/ amgydala and insula, defined using the Automated Anatomical Labeling (AAL) system as implemented in the WFU-pickatlas toolbox [ 27 ]. Effects occurring in these regions were thus followed up using SVC-correction and results are reported at a Family Wise error (FWE) corrected p-value <.05. Psychometric and performance data (correct responses, false alarms, misses and mean response time for events (RT)) for both days were likewise analysed using paired sample t -testing.
[ 11 C]Raclopride PET
[ 11 C]Raclopride scans were performed on an ECAT EXACT HR+ scanner (Siemens/CTI, Knoxville, TN, USA). Participants were studied at rest, in supine position, with a nurse nearby and ice cubes in both hands to prevent them from falling asleep. Head movement was restricted by a head immobilization device and Velcro tape. A venous catheter was placed in the forearm for [ 11 C]raclopride infusion. A 10min 2D transmission scan using three rotating 68 Ge/ 68 Ga sources was acquired for photon attenuation correction. 370MBq [ 11 C]raclopride was dissolved in 5mL saline and administered by an infusion pump (Med-Rad, Beek, The Netherlands), at a rate of 0.8mL·s −1 , followed by a 35mL saline flush at a rate of 2.0mL·s −1 . Meanwhile, a 60min dynamic 3D raclopride scan was acquired, consisting of 20 frames with progressively increasing frame lengths (1×15, 3×5, 3×10, 2×30, 3×60, 2×150, 2×300, 4×600s). All PET sinograms were normalized and corrections were applied for decay, dead time, attenuation scatter and randoms. Emission data were reconstructed using FORE+2D filtered back projection [ 28 , 29 ] applying a 5.0mm Hanning filter with a Y-offset of 4cm and a 2.123 zoom. Frames 12–20 were summed (i.e. 5–60min after injection) to create a single frame emission sinogram with high count statistics. Reconstruction of this emission sinogram was performed using ordered-subset expectation maximization (OSEM) with 4 iterations and 16 subsets. OSEM images underwent a 5mm FWHM Gaussian post smoothing, to obtain a transaxial spatial resolution of 7mm FWHM, equal to that of filtered back projected (FBP) images. Final images consisted of 63 planes of 128×128 voxels, each 2.4×2.4×2.4 mm 3 .
All structural MRI scans were rotated to the axial (horizontal) plane, parallel to the anterior and posterior commissure (AC–PC) line. To correct for possible motion, each frame (1–20) was coregistered to the summed image over frames 12–20. These motion corrected PET images were subsequently coregistered to the realigned MRI scan using Volume Imaging in Neurological Research (VINCI) software [ 30 ].
Kinetic analysis.
Mean non-displaceable binding potential (BP ND ) was used as a measure of dopamine D2/D3 receptor availability. Using the in-house developed software package PPET [ 31 ], parametric BP ND images were generated using receptor parametric imaging (RPM2), a basis function implementation of the simplified reference tissue model (SRTM) [ 32 ]. Cerebellum grey matter was used as reference tissue, for which automated cerebellar volumes of interest (VOIs) were defined using partial volume effect (PVE) lab [ 33 ]. This analysis also provided parametric R 1 images, representing local tracer delivery relative to that to the cerebellar reference region. Basis function settings used were: start exponential = 0.05min −1 , end = 0.5min −1 , number of basis functions 32.
Statistical parametric mapping.
Parametric BP ND images were analyzed using SPM8. After spatial preprocessing, including reorientation and normalization to MNI space, images were analyzed on a voxel by voxel basis, using a basal ganglia mask created with WFU Pickatlas software [ 27 ]. No proportional scaling was applied. SPM RPM2 and R 1 BP ND images were entered in paired sample t -tests. The threshold was set at p uncorrected ≤0.005 with an extent threshold of 10 voxels.
At baseline, depressive symptoms were low to absent (BDI score 1.8 ± 2.0). Using the Spielberger State-Trait Inventory (STAI), containing 20 items to be scored on a four-point Likert scale (range 20–80), mean trait anxiety score was 29.4 ± 4.8 and state anxiety at baseline scanning 30.4 ± 3.9. During the TSD night, VAS energy levels declined significantly (F(1,11) 20.2, p = 0.001), in line with decreased concentration (F(1,11) 10.6, p = 0.01), speed of thought (F(1,11) 12.0, p = 0.007), and increased perceived motor retardation (F(1,11) 12.0, p = 0.007), but not significantly for mood (F(1,11) 2.9, p = 0.122). STAI scores indicated a trendwise increased anxiousness after TSD, 36.3 ± 10.7 ( p = 0.068).
Cortisol Data
After TSD, CAR AUC I and AUC G showed significant blunting ( p = 0.029 and p = 0.022, respectively) ( Table 2 , Fig. 1 ). On day 1, nine subjects showed a rise in cortisol during the first hour after awakening, compared with a much smaller increase in five subjects after TSD, signified by a decreasing MnInc CAR. Similarly, cortisol AUC G T1-7 vs. T8-14 showed a robust decline after TSD. Cortisol levels were normally distributed on both days and showed no significant gender differences. Evening cortisol was not discriminating.
Individual saliva cortisol curves (grey line) and cortisol mean value (nmol/L) per Tx sampling point (solid line). Day 1 shows baseline cortisol sampling at T1-T7, day 2 shows effects of one night of total sleep deprivation on cortisol levels at T8-T14. T1, 2 and 3 comprise the cortisol awakening response (CAR). T8, 9 and 10 are sampled at identical time points the following day. T5 and T12 are sampled at 14.00hr, T6 and T13 at 17.00hr and T7 and T14 at 23.00hr. p values show effects of TSD, # p = 0.016.
https://doi.org/10.1371/journal.pone.0116906.g001
https://doi.org/10.1371/journal.pone.0116906.t002
Twelve data sets were available on day 1, and 11 on day 2 due to scanner logistic problems. After TSD, subjects were significantly slower in reacting during the neutral condition ( p = 0.043), but also to positive targets with a neutral distracter ( p = 0.008). The proportion of correct versus false answers decreased trendwise for neutral words ( p = 0.082) and for positive targets with a negative distracter ( p = 0.079) ( Table 3 ). Post hoc , results were additionally analyzed using general linear model statistics (GLM). When performing multivariate testing, the effect of sleep deprivation on reaction time for emotional words was significant at F(1,20) = 34.14, p <0.001; the effect of time for sleep deprivation was significant at F(1,20) = 5.78, p = 0.037, indicating that participants were slower at day 2, due to sleep deprivation. The interaction effect of sleep deprivation on emotion* time was not significant (F(1,20) = 0.81, p = 0.475), indicating that the general slowing following deprivation was common for all emotions presented.
https://doi.org/10.1371/journal.pone.0116906.t003
After 25 hours of wakefulness, the neutral condition showed no significant activation differences at a group level ( Table 4 ). Evaluation and processing of positive words was associated with increased bilateral prefrontal activation in addition to increased activation of left medial prefrontal working memory areas ( Fig. 2A ). Left DLPFC activation remained significant after Small Volume Correction (SVC; AAL p FWE 0.02). Processing of negative words was associated with increased activity in left insular area, but this effect did not survive SVC. During conditions containing emotional words only, viz. positive targets and negative distracters (P-N), or vice versa (N-P), left insular, limbic and parahippocampal lobes were activated, as well as right parietal lobe ( Fig. 2B ), showing SVC subthreshold increased activation in the hippocampal/parahippocampal region. All emotional conditions (i.e. target and/or distracter) resulted in increased activation in the anterior part of the left insula (AAL p FWE 0.043), mainly driven by the response to words with a negative valence, in addition to activation of the parietal lobe.
p <0.005, extent threshold 10 voxels. A and B are task related fMRI results, showing increased prefrontal and limbic activation respectively, in the conditions (A) positive valence versus baseline and (B) both emotional valences. C is a [ 11 C]raclopride PET image, showing decreased voxel-by-voxel RPM2 binding potential (BP ND ) in nucleus caudatus in n = 8. At the bottom right is the Z-score scale depicted.
https://doi.org/10.1371/journal.pone.0116906.g002
https://doi.org/10.1371/journal.pone.0116906.t004
[ 11 C]Raclopride
A subset of 8 paired data sets was available due to a failed synthesis (1 TSD scan) and technical problems with 1 baseline and 2 TSD scans. For n = 8, injected masses of raclopride were 2.36 ± 1.08 and 1.45 ± 0.55μg, on days 1 and 2 respectively ( p = 0.06) and injected doses of [ 11 C]raclopride were 378 ± 12 and 390 ± 19MBq on days 1 and 2, respectively ( p = 0.230). TSD resulted in a significantly decreased voxel-by-voxel based BP ND in left caudate nucleus, as shown in Table 4 and Fig. 2C . In addition, there was a TSD induced decrease in R 1 in right caudate nucleus.
Clinical Interactions
Post hoc we tested for correlations for TSD related changes in cortisol AUC, regions of interest (ROI) based BOLD response to emotional words, and altered [ 11 C]raclopride binding, using anatomical automatic labeling (AAL) defined striatal regions, according to WFU Pick atlas [ 27 ]. No statistically significant correlations were observed.
In the present study the effects of total sleep deprivation on stress regulating brain systems in healthy subjects were investigated as preliminary work for a TSD study in mood disorder. During a sleep deprived night, VAS scores on energy, concentration and speed of thought, but not mood, declined significantly. Although at baseline participants were not clinically depressed or overly anxious, as witnessed by BDI and STAI-scores, validated instruments like the Positive and Negative Affect Schedule (PANAS)[ 34 , 35 ] and the Profile of Mood State (POMS)[ 36 ] could have been used to score a broad range of mood states, both at baseline and during the night of sleep deprivation and the subsequent day.
After 24 hours of prolonged wakefulness, significant blunting of the cortisol awakening response (CAR) and secretion over the day (AUC G ) were found. Normally, under the influence of the SCN, HPA activity increases during the night, resulting in a cortisol rise two to three hours after sleep onset, which continues to rise into the early waking hours [ 12 , 14 ]. The present results indicate robust attenuation of the HPA-mediated stress response after TSD, congruous with decreasing VAS scores and lowered arousal, which may be due to the absence of the initial physiological awakening response [ 12 , 37 ]. These findings are in line with Vzontgas and colleagues, finding lowered, albeit not significantly, 24 hour plasma cortisol levels in blood in a laboratory setting in a group of 10 men [ 38 ].
In the present study, no significantly altered cortisol levels were found after 14.00h (T5-T12). Evening cortisol, indicating return to baseline levels, was slightly lower than those reported by Vreeburg [ 19 ] in healthy subjects.
In order to investigate effects of TSD on processing of both positive and negative stimuli, as well as on cognitive inhibition, we chose to adapt the Murphy and Elliott fMRI paradigm [ 24 , 25 , 39 , 40 ], as this task was originally developed to investigate emotional bias in mood disorders in the context of cognitive processing. After TSD, in healthy adults, task performance during fMRI was slower, indicating that TSD overruled any learning or practice effects. Slowing of task performance after TSD is in line with previous reports and likely due to loss of sustained attention and vigilance [ 41 ]. Slowing was particularly evident for positive targets with a neutral distracter. Accuracy was trendwise decreased for the neutral (italic vs. regular font) condition and for positive targets with negative distracters, suggesting decreased sensitivity to detect positive valence. On processing emotionally salient versus neutral words, TSD was associated with increased left dorsolateral prefrontal activity, suggesting increased mental effort to perform semantic judgements and to maintain control, in a setting of less efficient functional circuitry. Although we do not intend to overstate the relevance of these findings in this emotionally healthy group, cognitive biases in depressive disorder are thought to reflect maladaptive bottom-up processes, which are generally perpetuated by weakened cognitive control [ 42 ]. Processing of solely affective stimuli (target and distracter) showed subthreshold increased activation in the left parahippocampal /hippocampal region. Activation of the subgenual gyrus and amygdala was remarkably absent, though for amygdala this is line with findings by Elliott et al., [ 25 ], fostering [presumably reflecting] a lower affective salience for words compared to pictures. Processing of any affective stimulus (target and/or distracter) showed increased activation of the anterior part of the insula in a context of performance anxiety as indicated by trendwise increased STAI scores in these healthy, but weary adults [ 43 ]. Activation of the insula was mainly driven by the response to words with a negative valence, suggestive of an increased effort to handle negative affect [ 1 ] and in line with the insular function of emotional interference resolution in working memory [ 44 ]. With due caution, we propose that these neural responses reflect modulation of cognitive performance by emotional tone. Therefore, these regions likely represent an interface between cognition and emotion processing [ 25 ].
After TSD, voxel-by-voxel based BP ND of [ 11 C]raclopride was significantly decreased in left caudate, which is partly in accordance with a report by Volkow [ 9 ]. This was not explained by regional altered delivery (R 1 ) of the tracer, although metabolic activity in the cerebellar reference tissue may be altered after sleep deprivation [ 6 , 7 ]. A reduction in [ 11 C]raclopride specific binding is consistent with either an increase in dopamine release, or a decreased affinity of the synaptic D2/D3 receptor in these regions [ 45 ], which may be due to internalization of receptors [ 46 ]. This could not be determined on the basis of our design, and may have resulted from a combination of these factors.
Using both [ 11 C]raclopride and a dopamine transporter blocking radioligand, [ 11 C]methylphenidate, Volkow [ 10 ] argued TSD induced decreased [ 11 C]raclopride binding not to be due to increased dopamine availability, but to decreased affinity of the D2/D3 receptor, resulting in dopamine receptor downregulation in the synaptic cleft. As dopamine D2 receptors are thought to be involved in wakefulness, and partially responsible for maintaining arousal and alertness [ 8 , 47 ], the present reduced VAS on energy and concentration and efficiency in fMRI task performance, are in line with D2 down-regulation. This would further be exemplified by the blunted cortisol response, since dopaminergic stimulation of the HPA axis is mediated through D1 and D2 receptors [ 11 ]. Decreased affinity in the head of the left caudate could be in line with increased difficulty in controlling word interference from task unrelated processing [ 48 ], explaining both the general slowing and increased prefrontal activity. However, we were not able to corroborate this explanation in a correlational analysis, which may be primarily due to insufficient power, but may also indicate that regional brain activation as measured with fMRI is not tightly coupled to either striatal dopaminergic transmission or HPA axis activity. Excluding two smokers did not change results significantly, although smoking may influence dopamine release and therefore raclopride binding [ 49 ].
Clinical Relevance
Individual vulnerability to sleep deprivation is known to be variable [ 3 ]. From the present study, it cannot be ruled out that decreased D2 receptor affinity is the brain’s response to initially increased dopamine levels, induced by TSD. Blunting of the HPA axis response may reflect the absence of awakening stress and possibly explain some of the beneficial effects of sleep deprivation in depressive mood disorder.
Limitations
This pilot study in healthy adults contains several potential limitations. In view of our modest sample size and fixed-order design, the current results are in clear need of replication.
Regarding baseline characteristics, the participants’ number of hours of sleep was adequate at the start of the experiment, but we did not control objectively for sleep quality and duration. Baseline CAR may have been affected by waking up earlier, or by the excitement of taking part in a research study, which may have released additional ACTH [ 50 ]. A higher CAR has been associated with shorter sleep duration [ 51 ]. However, excluding three subjects who slept 6 hours or less, did not have a major effect on the CAR ( p = 0.022). During the night, participants were kept in a well-lit room. Melatonin suppression may have dampened the SCN-mediated CRH response.
For our fMRI runs, we have chosen to adapt the original Murphy and Elliott task [ 24 , 25 ], who described their paradigm to investigate emotional bias in depressive disorder as a go/no-go task. However, go/no-go paradigms do not typically feature an even split of valid and invalid targets, and therefore we have renamed the task as a semantic affective classification task. The task was modeled as a block design, and because the inter-stimulus interval (ISI) was fixed, could not be analyzed as an event-related design. Evidently, a block design is preferable when sample sizes are modest, as it is generally more robust [ 52 ], although it lacks the flexibility of event-related designs. Therefore, for assessing individual cognitive and emotional responses in e.g. a patient population, an event-related design would be more appropriate [ 53 ]. Finally, for our voxel-based analyses we set an a priori threshold of p = 0.005 and 10 voxels to obtain a reasonable balance between Type I and Type II error [ 54 ], again highlighting the need for a replication in a larger sample.
With respect to mood enhancers, other drugs of abuse were not tested for. At baseline, we did not control for caffeine use at home before the start of the experiments. Caffeine evokes its stimulating effects through blockade of the adenosine receptor [ 55 ], which in turn is involved in the control of dopamine release [ 56 ]. As raclopride is a dopamine receptor antagonist, in theory, TSD induced changes in raclopride binding may therefore have been underestimated.
Although changes in [ 11 C]raclopride BP ND clearly show a dose dependent relationship with extracellular DA levels, the nature of this relationship is complex [ 57 ]. [ 11 C]Raclopride BP ND does not differentiate between binding to receptors in high or low affinity states, whereas endogenous dopamine is mainly conveyed by high affinity state receptors [ 58 ], acting on pre- and postsynaptic (extra)-striatal dopaminergic D1 receptors to bring about its effect [ 59 ]. Therefore, dopaminergic effects due to TSD may have been underestimated and future research should resolve this issue, for example by comparing [ 11 C]raclopride to the purported high-affinity ligand [ 11 C]PHNO [ 60 ]. As [ 11 C]raclopride scans were performed in the second half of the morning, and the time sequence of dopamine release is not known, effects may have been either over- or underestimated. A variable response to TSD is in line with observations in depressed patients, where the therapeutic response to TSD may vanish within hours to a day [ 3 ].
Sleep deprivation in healthy adults induces widespread neurophysiological and endocrine changes, characterized by impaired cognitive functioning, despite increased regional brain activity. Our pilot findings indicate that activation of the dopaminergic system occurs together with a blunted cortisol response, suggesting augmented motivational top down control and requiring increased involvement of prefrontal and limbic cortical areas. Sustained wakefulness requires the involvement of compensatory brain systems, and may help to understand the therapeutic effects of sleep deprivation in affective disorders.
Acknowledgments
The authors thank Ms Marieke Mink for accompanying the participants to the PET scanning sessions, Dr. Marjan Nielen for help in designing the fMRI task, Dr. Sophie Vreeburg for help in interpreting cortisol data, Dr. Adriaan Hoogendoorn for statistical support, Neuroradiology staff for interpretation of MRI scans, staff of the department of Nuclear Medicine & PET Research for tracer production, technical assistance and data acquisition and staff of the VU Medical Center Neuroendocrine lab for cortisol saliva analysis.
Author Contributions
Conceived and designed the experiments: UK DV MJT RK RB AAL WH. Performed the experiments: UK DV MJT RK. Analyzed the data: UK DV MJT RK RB AAL WH. Contributed reagents/materials/analysis tools: UK DV MJT RK RB AAL WH. Wrote the paper: UK DV MJT RK RB AAL WH.
- View Article
- PubMed/NCBI
- Google Scholar
- 36. McNair DM, Lorr M, Droppleman LF (1971) Manual for the Profile of Mood States.
- 53. Huettel SA, Song AW, McCarthy G (2009) Functional Magnetic Resonance Imaging. Sunderland MA: Sinauer Associates.

IMAGES
VIDEO
COMMENTS
The Influence of Sleep Deprivation on Human Body. It contradicts living in harmony with God, as when the person is irritated and moody, it is more difficult to be virtuous and to be a source of joy for others. We will write. a custom essay specifically for you by our professional experts. 809 writers online.
Insufficient sleep is a pervasive and prominent problem in the modern 24-h society. A considerable body of evidence suggests that insufficient sleep causes hosts of adverse medical and mental dysfunctions. An extensive literature search was done in all the major databases for “insufficient sleep” and “public health implications” in this ...
A summary of the details of the 19 RCTs included in this systematic review is presented in Table 2. A total of 766 young adults with mean ages ranging from 20.2 to 27.5 years (mean age, 23.7 ± 3.09 years; 55.2% male) were included in these RCTs. BMI was only reported in one trial, and the mean was 20.0 ± 1.9 kg/m 2.
People who are exposed to sleep loss usually experience a decline in cognitive performance and changes in mood (for meta-analyses, see Pilcher and Huffcutt 1996; Philibert 2005 ). Sleep deprivation is a study design to assess the effects of sleep loss. In acute total SD protocols, the subjects are kept awake continuously, generally for 24–72 ...
Sleep deprivation is a state that arises when an organism has less sleep than is optimal, and is followed by a 'rebound' in slow-wave sleep when the opportunity arises. This can be induced ...
In a study, sleep deprivation was defined as a sleep duration of 6 h or less (Roberts and Duong, 2014). Sleep disorder overarches disorders related to sleep. Sleep disorder overarches disorders ...
The role of subjective sleep quality in cognitive performance has gained increasing attention in recent decades. In this paper, our aim was to test the relationship between subjective sleep ...
Total sleep deprivation (TSD) may induce fatigue, neurocognitive slowing and mood changes, which are partly compensated by stress regulating brain systems, resulting in altered dopamine and cortisol levels in order to stay awake if needed. These systems, however, have never been studied in concert. At baseline, after a regular night of sleep, and the next morning after TSD, 12 healthy subjects ...
INTRODUCTION. Sleep is vital for health and well-being in children, adolescents, and adults. 1–3 Healthy sleep is important for cognitive functioning, mood, mental health, and cardiovascular, cerebrovascular, and metabolic health. 4 Adequate quantity and quality of sleep also play a role in reducing the risk of accidents and injuries caused by sleepiness and fatigue, including workplace ...