
Permission Letter To Conduct Research: How To Draft It Right!
In this article, I’ll share my insights and provide you with a step-by-step guide, including customizable templates , to craft your own effective permission letter for research.
Key Takeaways Understand the purpose and importance of a permission letter for research. Learn the essential components to include in your letter. Get a step-by-step guide to writing a compelling permission letter. Benefit from a customizable template to streamline your writing process. Discover practical tips from my personal experience to enhance your letter.
Understanding the Importance of a Permission Letter for Research
A permission letter for research is a crucial document that formally requests authorization to conduct a study in specific locations or collect data from a particular group.

It serves as a formal agreement between the researcher and the authority or individuals involved, ensuring that the research is conducted ethically and legally.
Step-by-Step Guide to Writing Your Permission Letter
Step 1: start with contact information and date.
Always begin your letter by stating your contact information at the top, followed by the date. This should include your name, address, phone number, and email address.
Step 2: Address the Recipient Properly
Address the recipient by their proper title and name. If you’re unsure, a general “To Whom It May Concern” can suffice, but personalized greetings are always more impactful.
Step 3: Introduce Yourself and Your Affiliation
Introduce yourself, your position, and your affiliation. This sets the context and establishes your credibility.
Step 4: Clearly State the Purpose of Your Letter
Be clear and concise about your intent to seek permission for research. Mention the research topic and why the specific site or group is essential for your study.
Step 5: Provide Details of Your Research
Explain the scope of your research, the methodology you’ll use, and the expected duration. Transparency is key to gaining trust and approval.
Step 6: Assure Ethical Compliance
Highlight your commitment to ethical standards, including how you’ll ensure participant confidentiality and data protection.
Step 7: Request for Approval
Politely request permission to proceed with your research, expressing your willingness to comply with any required protocols or guidelines.
Step 8: Include Contact Information for Follow-up
Offer your contact information again, encouraging the recipient to reach out with any questions or requests for further details.
Step 9: Close with a Professional Salutation
End your letter with a professional closing, such as “Sincerely,” followed by your name and signature.
Template for a Permission Letter To Conduct Research
[Your Name] [Your Address] [City, State, Zip Code] [Phone Number] [Email Address] [Date]
[Recipient’s Name or Title] [Organization’s Name] [Address] [City, State, Zip Code]
Dear [Recipient’s Name or Title],
I am writing to request permission to conduct research at [location/site/group], as part of my [research project/study] on [topic]. My name is [Your Name], and I am a [Your Position] at [Your Institution or Organization].
The purpose of my research is to [briefly state the objective]. I believe that [location/site/group] is essential for my study because [reason]. The research will involve [describe the methodology], and I anticipate it will take approximately [duration] to complete.
I assure you that all research activities will adhere to the highest ethical standards. Participant confidentiality and data protection will be strictly maintained throughout the research process.
Your approval to conduct this research would be greatly appreciated. I am more than willing to adhere to any specific protocols or requirements you may have. Please feel free to contact me at [Your Phone Number] or [Your Email Address] if you have any questions or need further information.
Thank you for considering my request. I look forward to your positive response.
[Your Name] [Your Signature, if sending a hard copy]
Personal Tips from My Experience
- Personalize Your Letter: Tailoring the letter to the recipient shows respect and attention to detail.
- Be Concise but Thorough: Provide enough detail to inform but not so much that it overwhelms the reader.
- Follow-Up: Don’t hesitate to follow up if you haven’t received a response within a reasonable time frame.
- Show Appreciation: Always express gratitude for the recipient’s time and consideration.
I hope this guide helps you craft an effective permission letter for your research. I’d love to hear about your experiences or any additional tips you might have. Please share your thoughts and questions in the comments below!
Related Posts
- Free Templates for Research Permission Letters
- 3 Must-Have Templates for Requesting Permission Easily
- Sample Letter To Request To Attend A Conference: Free & Effective
Frequently Asked Questions (FAQs)

Q: What is a permission letter to conduct research?
Answer : A permission letter to conduct research is a formal request to obtain permission from an organization or individual to conduct research on a particular topic. This type of letter is commonly used by students, researchers, and scholars who require permission to carry out their research.
Q: Why is a permission letter to conduct research important?
Answer : A permission letter to conduct research is important because it shows that the researcher has obtained the necessary permissions to conduct their research. It also provides a clear understanding of the scope and nature of the research and how it will be conducted, which can help to prevent misunderstandings or legal issues.
Q: Who should I address my permission letter to?
Answer : You should address your permission letter to the individual or organization that has the authority to grant permission for your research. This could be the head of the organization, a department manager, or an individual who is responsible for the area that you wish to conduct research in.
Q: What should I include in my permission letter to conduct research?
Answer : Your permission letter to conduct research should include an introduction that outlines your research topic and objectives, an explanation of why you need permission, an overview of your research methodology, details on the timeline and logistics of your research, and a formal closing that thanks the recipient for their time and consideration.
Q: How do I ensure that my permission letter to conduct research is effective?
Answer : To ensure that your permission letter to conduct research is effective, make sure that it is clear, concise, and polite. Provide detailed information about your research and the nature of your request, and address any potential concerns or objections that the recipient may have. Finally, proofread your letter carefully to ensure that it is free from errors and typos.
Related Articles
Sample request letter for air conditioner replacement: free & effective, goodbye email to coworkers after resignation: the simple way, sample absence excuse letter for work: free & effective, salary negotiation counter offer letter sample: free & effective, formal complaint letter sample against a person: free & effective, medical reimbursement letter to employer sample: free & effective, leave a comment cancel reply.
Your email address will not be published. Required fields are marked *
Home » Letters » Approval Letters » Approval Letter for Research – Sample Letter for Approval of Research
Approval Letter for Research – Sample Letter for Approval of Research
(Sender’s details) ____________ ____________ ____________
Date: __/__/____ (Date)
(Receiver’s details) ____________ ____________ ____________
Subject: Approval of research
Dear Sir/madam
This is with reference to your letter dated __/__/____ (Date) regarding approval for research. I am glad to announce that you have been granted permission to research the topic of ________ (mention topic).
As a ________ (graduate/masters/scholar/any other) student, we have high hopes from you. We noticed that your dream of studying ________ (mention topic with details). This research will provide you with interesting facts and we hope you continue doing research using ____________ (surveys/questionnaire/any other) method.
Your efforts are appreciated. We are happy to cooperate with you and we wish you luck and success in the future. Kindly contact me on _______________ for any further questions.
Sincerely, ___________
By lettersdadmin
Related post, request letter for issuance of approval letter for delivery – sample letter for delivery approval.
Request Letter for Extension of Time to Deliver Goods – Sample Letter Requesting for Extension of Timeto Deliver Goods
Request letter for annual leave approval – sample letter requesting annual leave approval.
Leave a Reply Cancel reply
You must be logged in to post a comment.
Internship Request Letter – How to Write an Application for Internship | Sample Letter
Salary increment request letter – sample request letter for salary increment, application for half day leave – sample leave application to principal for half day leave, simple leave application in office – sample request letter for leave of absence.
As the nation’s largest public research university, the Office of the Vice President for Research (OVPR) aims to catalyze, support and safeguard U-M research and scholarship activity.
The Office of the Vice President for Research oversees a variety of interdisciplinary units that collaborate with faculty, staff, students and external partners to catalyze, support and safeguard research and scholarship activity.
ORSP manages pre-award and some post-award research activity for U-M. We review contracts for sponsored projects applying regulatory, statutory and organizational knowledge to balance the university's mission, the sponsor's objectives, and the investigator's intellectual pursuits.
Ethics and compliance in research covers a broad range of activity from general guidelines about conducting research responsibly to specific regulations governing a type of research (e.g., human subjects research, export controls, conflict of interest).
eResearch is U-M's site for electronic research administration. Access: Regulatory Management (for IRB or IBC rDNA applications); Proposal Management (eRPM) for the e-routing, approval, and submission of proposals (PAFs) and Unfunded Agreements (UFAs) to external entities); and Animal Management (for IACUC protocols and ULAM).
Sponsored Programs manages the post-award financial activities of U-M's research enterprise and other sponsored activities to ensure compliance with applicable federal, state, and local laws as well as sponsor regulations. The Office of Contract Administration (OCA) is also part of the Office of Finance - Sponsored Programs.
Ethics & Compliance
You are here, site approval/letter of cooperation.
(Word) Two sample letters for site approval cooperation between U-M and other institutions, organizations, etc. Letters of cooperation must be on U-M letterhead and signed by an appropriate official. These letters are uploaded into the Performance Site section of the eResearch IRB application.
Letter Samples & Templates
Letter of authorization to conduct research.

It is not uncommon for students and researchers to require use of, or access to, a facility in order to complete the research project. Prior to a project being started, a letter of authorization to conduct research will likely be required. This letter will demonstrate that the project is understood, and the facilities in use are willing and able to support the research. Depending on the nature of the research, the letter range from quite simple to very specific .
Providing authorization to conduct research within your facility should be considered a serious manner . Any risks associated with the research may affect the facility. You may also carry some risk regarding the safety of the persons conducting or participating in the research.
Before giving authorization, review the materials regarding the nature and duration of the research. Make sure you understand who is conducting the research, and who you should contact with any questions. You should also have a thorough understanding of how the research is to be conducted. Research can range from simple observation to medical experimentation. It can involve the use of already existing devices and materials, or the attempted creation of something new. The term “ research ” covers a large range of categories and activities. If you have any questions regarding the processes that will take place, get satisfactory answers prior to allowing them to begin.
Once you are comfortable with giving authorization , you should begin your letter by stating to whom you are giving permission . This could be an individual or group. You should also include with whom they are associated. If the research is sponsored by a university or business, include that information. This ensures you have properly specified how your authorization may be applied.
You will also need to include information on yourself. This includes your name, as well as an explanation of why you have the authority to provide the authorization. You may want to clarify that you are the facility owner, business manager, or another authority figure with the capacity to make such a commitment. This allows the proposed researches the assurance that they have obtained permission from a proper point of contact.
If there is limited risk within the research, that may be all you require. For more complex situation, you may need to authorize specific actions or situations that will present as the research goes forward . Maybe you are willing to allow one phase of a project to occur on your property, while another stage will need to take place elsewhere. List any limitations you would like in place, as well as any rights you would like the option to exercise. This can include the duration your authorization remains valid, or if you must be contacted as certain tasks progress.
Once complete, sign and date your letter of authorization to conduct research . You will also want to make sure your contact information is included. Make sure to keep a copy of the letter for your own records.
- Administration
- Toggle Search
- Find People

Sample Documents

540 Devall Drive, Suite 200
Auburn, AL 36832
Phone: 334.844.5966
New Page Bar
TC IRB Submission Document Templates & Samples

Researchers working with human subjects must submit their research plans and any research-related documents to Teachers College (TC) Institutional Review Board (IRB) for review and approval. Researchers may not begin recruitment or research until IRB has issued a final approval letter on the protocol.
Submission Document Templates
TC IRB has designed templates for most documents required for IRB submission. The templates are located in MyTC/Resources/TC Mentor IRB (right hand side), or accessible through the Submitting a New Protocol page.
- IRB Application Template : required for all submissions, including exempt existing data .
- Informed Consent Form Template : for adults competent to consent.
- Parental Permission Form Template : required if the research includes youth under the age of 18.
- Assent Form Template - for participants under the age of 18.
Note : The Informed Consent, Parent Permission, and Assent Form templates are designed using a three-color-code-scheme:
- BLUE text includes suggestions from TC IRB where researchers can freely edit
- RED text is tailored for your study
- BLACK text is standard and should be kept in the final consent form copy
The templates are not designed as one-size-fits-all documents. Instead, they are intended to help guide researchers through the most common submission contexts. Researchers should read through the templates carefully and consider their population of interest when creating final versions of documents.
Researchers are responsible for informing individuals of the research study activities, expectations, risks, and potential benefits of study participation.
Sample Application Documents
When writing human subject research materials, investigators often use an academic writing style for both the IRB submission and participant materials. For information on distinguishing your writing style between a general and academic audience, please visit our Writing for an IRB Review page.
In addition to writing style, new researchers may struggle with knowing how to frame their research study within a TC IRB application. To assist researchers in the writing process, TC IRB has identified common types of studies frequently used among TC researchers and created sample applications with relevant explanations. Following these sample applications does not guarantee a protocol will be approved or that a researcher will have a flawless review process. It does, however, offer some suggestions on how to frame materials for a formal IRB review.
- Exempt Category 4 - Existing Data Sample Application : This research does not involve new recruitment of human participants, and falls under Exempt Review . Researchers hoping to conduct this type of research must still submit an IRB protocol through TC Mentor IRB, however, many questions on the application related to participant recruitment and study activities should be marked “Not Applicable.” For more information on existing data studies, please visit our Exempt Existing Data Guide .
- Review of Public Data (Exempt Category 4)
- Review of Existing Data (Exempt Category 4)
- Anonymous Online Surveys
- Audio-Recorded In-Person Interviews and Focus Groups
Sample Consent Documents
Language used on consent, parent permission, and assent forms should be tailored to the population of interest, taking into account the participants’ reading level and familiarity with research procedures. In all of these documents, researchers should aim to clearly state the following:
- The study activities
- The duration of time the activities will take
- Explanation of risks and benefits
- Compensation and any limits to receiving it
- Protections and limits of confidentiality
The consent samples outline different types of research studies and the information researchers should provide participants. Following these samples does not guarantee a protocol will be approved or that a researcher will have a flawless review process. It does, however, offer some suggestions on how to write consent materials for participants.
- Online Survey Consent Sample Form : This consent form is for an online survey conducted with adults competent to consent. The following survey was part of an exempt study which posed minimal risk to participants. The sample demonstrates how to explain an online study to participants, as well as possible ways to obtain consent through digital platforms.
- Audio Recorded Individual Interview and Focus Group Sample Form : This study pertains to in-person data collection with recorded elements. Researchers hoping to conduct interviews or focus groups over digital platforms (e.g., Zoom) may also use this sample as a guide.
For information specifically related to working with youth, please review The Assent Process with Minors Guide and the Obtaining Parent Permission Guide. TC IRB reviewers also provide their insights into the consenting process on TC IRB’s Blog , and in the TC Reviewer Questions .
Sample Memo Documents
Throughout the IRB process, circumstances may call for a Primary Investigator (PI) to follow-up with an IRB reviewer. These follow up actions may be required through modification memos or responses to IRB revision feedback. When planning to submit a new modification or respond to request for revisions, PIs should submit a memo style document describing their specific scenario, revisions to their protocol and documents, and a list of files associated with the change. To assist in the writing process and timeliness, the IRB has provided sample memos for PIs to follow. These samples do not guarantee a protocol approval or take place of the PIs specific responses, but can be used as a model for writing a memo.
- Modification Memo Sample : This Modification Memo Sample can be used as a model for PIs when requesting modifications to an active protocol. This sample provides a format as well as examples of the adequate amount of information necessary for IRB administrators to review.
- Revision Memo Sample : This Revision Memo Sample can be used as a model for PIs to respond to specific IRB administrator requested revisions. Following this format allows for both PIs and IRB administrators to effectively communicate protocol revisions and feedback.
Institutional Review Board
Address: Russell Hall, Room 13
* Phone: 212-678-4105 * Email: [email protected]
Appointments are available by request . Make sure to have your IRB protocol number (e.g., 19-011) available. If you are unable to access any of the downloadable resources, please contact OASID via email [email protected] .
How to Write A Letter of Approval Examples and Samples Included

A letter of approval can take two forms or can be written from two positions. The first is as the requesting personnel. The second is as the approving personnel. This article looks at the fundamental principles of handling a letter of approval from an approving personnel point of view and offers samples to help you handle approvals properly.
The need to write a letter of approval is bound to arise as a project leader, manager or business owner. When tasked with oversight functions in an organization, you will be called on to review and approve initiatives, expenses and even absences from work.
Writing a proper letter of approval requires good email writing skills coupled with sound organizational behavior and management skill. We will look at some approval request handling best practice and lay out how to write a letter of approval with examples to help you succeed in your oversight role.
How To Write An Email Requesting For The Approval Of The Boss

How to Write an Approval Letter
Writing an approval letter involves conveying consent or authorization to a recipient for a specific request or action. Here are the key points to include in a condensed bullet-point format:
Opening: Use a professional salutation (e.g., “Dear [Recipient’s Name],”). Clearly state the purpose of the letter (e.g., “Subject: Approval Letter”).
Express Approval: Clearly and directly state that approval is granted. Mention the specific request or action being approved.
Provide Details: Include relevant details, such as dates, amounts, or any specific conditions.
If necessary, Justify the Approval: Briefly explain the reasons for granting approval.
Encourage Action: If the approval requires any subsequent actions from the recipient, provide necessary instructions or deadlines.
Closing: Express goodwill and appreciation (e.g., “Thank you for your cooperation”). Use a polite closing (e.g., “Sincerely,” “Best regards,” etc.).
Signature: Sign the letter if it is a physical document, or use a digital signature if it’s an email.
Letter of Approval Best Practice
Anyone that has ever waited several weeks to receive feedback on a leave request or budget approval can relate with the fact that the manner in which these requests and subsequent approvals are handled is critical to organizational culture and employee morale.
Be Timely with Approval Letters
Approval requests are time sensitive as they are prerequisite factors for other actions and operations. Aim to respond to approval requests within 24 to 48 hours to avoid delaying other operations dependent on your approval. Prompt replies to letters of approval are also a relief to the sender as their anxiety about the matter can be alleviated.
In cases of sensitive approvals, you may need some time to review information, documentation and conduct your own analysis. Don’t keep the sender in the dark rather respond with a short email stating that you need time to review the request.
Email Requesting Approval for Payment: Templates Included
Email Task Approval Example Email
Letter of approval example, sample i: email acknowledging an approval request.
Here is a sample letter of approval acknowledgment letter:
Re: Approval Needed – End of Year Budget
Use Objective Criteria for Approvals
The presence of diverse personalities in the workplace is bound to lead to interpersonal conflict which is often referred to as “office politics”. Business leaders and managers must be careful to manage approval requests in a manner that clearly shows the absence of sentiment.
Let all approval decisions be backed by company policy and objectives. Provide explanations for all decisions in your approval letters to reinforce your objectivity and impartiality.
Here’s a sample letter of approval when a team leader has to decline a leave request because the requesting officer has not met the requirements to be entitled to leave according to company policy.
Sample II: Email Declining a Leave Request
Re: Leave Request – Adam Kain
Keep the Tone Warm & Professional
Similar to other business emails and letters, keep the tone of your approval letter warm and professional . Emails written in warm professional tones breed an organizational culture of open communication, collaboration, and team orientation. Receiving strongly worded cold emails are not just a stressor but slowly erode healthy working relationships within an organization.
Displaying empathy is essential for healthy organizational relationships. Show understanding and care for the requesting personnel by using consolatory tones in cases when approval is denied. Use an appropriate congratulatory tone when sharing positive news. Use the closing paragraph of your approval letters to congratulate or offer alternatives to requesting parties as shown in the various samples.
Provide Information Relevant to the Approval
Ensure that your letter of approval contains all essential information relevant to the approval request. Confirm all necessary information to ensure that there is no misunderstanding about timelines, amounts or requirements. Granting an approval then canceling is unprofessional and stressful for all involved.
Take advantage of technology to stay abreast of pending approvals and how each approval correlates with other organizational functions. Leave rosters can be used to ensure no conflicts exist and your teams are adequately staffed at every point. You can also use the calendar app of email management platforms to ensure you do not miss approval deadlines.
Close the Letter Appropriately
Instead of closing your approval letters with generic endings, use an appropriate closing for every approval letter . For leave requests, you may wish the recipient a restful holiday. For projects, share your excitement for the project and warm wishes for the success of the implementing team. As shown in the samples above structure all communication to be consistent with the overall message of the approval letter.
Sample III: Typical Letter of Approval for a Leave Request
A typical letter of approval most businesses will encounter is for Leave requests. Here’s a sample letter of approval for a leave request.
Letters of Approval are instrumental to the operational efficiency of every business, therefore an understanding of how to write a letter of approval is prerequisite to leadership and managerial roles. Combine the principles discussed in this article with sound email writing skills and you are on your way to running an efficient organization.
About The Author

Chinazom Elizabeth Izuora
Related posts.

9 Tips You Need to Write and Respond to Emails Professionally

12 Rules of Writing Emails Professionally and Effectively

How to Write Professional Emails: 7 Critical Ingredients

8 Simple Lessons for Writing Irresistible Business to Business Emails
Leave a comment cancel reply.
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
- Privacy Policy
Buy Me a Coffee

Home » Institutional Review Board – Application Sample and Approval Guide
Institutional Review Board – Application Sample and Approval Guide
Table of Contents

Institutional Review Board (IRB)
Institutional Review Board (IRB) is a committee responsible for reviewing and approving research proposals involving human subjects. An IRB ensures that the proposed research meets ethical and legal standards to protect the rights and welfare of the participants.
Purpose of Institutional Review Board
Some specific purposes of an IRB include:
- Ensuring that the rights and welfare of human subjects are protected. This includes ensuring that participants are fully informed about the study and have given their informed consent to participate.
- Assessing the risks and benefits of the research study. An IRB must evaluate the potential risks to participants and ensure that the benefits of the research outweigh any potential harms.
- Reviewing the study design and methods. The IRB must ensure that the study is designed in a way that minimizes risks to participants and that the methods used to collect data are ethical.
- Monitoring ongoing studies . An IRB must ensure that studies are conducted in accordance with the approved protocol and that any changes to the study are reviewed and approved by the IRB.
- Ensuring compliance with regulatory requirements . An IRB must ensure that studies are conducted in compliance with applicable laws and regulations, such as the Common Rule and the Health Insurance Portability and Accountability Act (HIPAA).
Institutional Review Board Guidelines
Here are some general guidelines that IRBs follow:
- Informed Consent : Researchers must obtain informed consent from all participants. This means that the participants must be fully informed about the study, including its purpose, procedures, risks, and benefits, and they must be given the opportunity to ask questions and decide whether or not they want to participate.
- Risk Assessment: The IRB must assess the risks associated with the research and determine whether they are reasonable in relation to the potential benefits. The IRB must also consider ways to minimize risks and protect participants.
- Confidentiality : The IRB must ensure that the confidentiality of the participants is protected. This may involve using pseudonyms, removing identifying information, or limiting access to data.
- Privacy : The IRB must ensure that participants’ privacy is protected. This may involve conducting the research in a private setting or using measures to prevent unauthorized access to participants’ personal information.
- Conflict of Interest : The IRB must ensure that researchers do not have a conflict of interest that could bias the study results. Researchers must disclose any potential conflicts of interest to the IRB.
- Review and Monitoring : The IRB must review and monitor the study to ensure that it continues to meet ethical standards. The IRB has the authority to suspend or terminate a study if it determines that it is no longer ethical or safe.
How to Get IRB Approval
Here are the general steps to obtain IRB approval:
- Determine if your study requires IRB approval : IRB approval is required for any research study involving human subjects, which includes collecting data through interviews, surveys, or experiments.
- Complete required training: Many IRBs require that researchers complete training in the responsible conduct of research and human subjects protection.
- Develop the study protocol : The study protocol should describe the research question, methodology, recruitment and consent procedures, and data collection and analysis plans.
- Submit the IRB application : Once the study protocol is developed, submit the IRB application to your institution’s IRB. The application will include the study protocol, consent forms, and any other required documents.
- Respond to any requests for revisions : The IRB may request revisions to the study protocol or consent forms before granting approval.
- Receive IRB approval: Once the study protocol is approved, you may begin your research study.
Institutional Review Board Example
Here is an example of an IRB:
Let’s say that a researcher wants to conduct a study on the effects of a new medication on patients with depression. The study would involve giving the medication to a group of patients and monitoring their symptoms over a period of several months. Before the study can begin, the researcher must submit a detailed research protocol to the IRB.
The IRB will then review the protocol to ensure that the study meets certain ethical standards. For example, the IRB will want to make sure that:
- The risks to the participants are minimized and are reasonable in relation to the potential benefits of the study.
- The participants are fully informed about the nature of the study and have given their informed consent to participate.
- The confidentiality of the participants is protected.
- The study design is scientifically sound and will yield reliable results.
If the IRB determines that the study meets these and other ethical standards, it will issue a formal approval letter. The researcher can then begin the study, but must continue to follow the protocol and report any adverse events or other issues to the IRB. The IRB may also conduct periodic reviews of the study to ensure that it continues to meet ethical standards.
Institutional Review Board Proposal Sample
Here is a sample proposal for an Institutional Review Board (IRB) submission:
Title : Effects of Meditation on Stress Reduction in College Students
Introduction:
Stress is a common issue among college students, and it can have negative effects on academic performance and overall well-being. Meditation has been shown to be an effective tool for reducing stress and improving mental health. However, the effectiveness of meditation for stress reduction in college students has not been well-studied. This study aims to investigate the effects of a meditation intervention on stress reduction in college students.
Objectives:
The main objective of this study is to determine if a meditation intervention is effective in reducing stress levels in college students. Secondary objectives include assessing the feasibility and acceptability of the intervention and exploring potential moderators of the intervention’s effectiveness.
Participants will be recruited from a large university campus and randomly assigned to either a meditation intervention or a waitlist control group. The meditation intervention will consist of eight weekly 60-minute group meditation sessions led by a certified meditation instructor. The waitlist control group will receive the intervention after the study is completed.
Participants will complete self-report measures of stress, anxiety, and depression at baseline, post-intervention, and 1-month follow-up. Demographic information and previous experience with meditation will also be collected. Intervention participants will also complete measures of intervention satisfaction and adherence.
Data Analysis:
Descriptive statistics will be used to summarize demographic and baseline variables. Independent samples t-tests and chi-squared tests will be used to compare groups at baseline. Repeated-measures analysis of variance (ANOVA) will be used to compare changes in stress, anxiety, and depression between groups over time. Moderation analyses will also be conducted to examine potential moderators of intervention effectiveness.
Ethical Considerations:
Participants will provide informed consent prior to participating in the study. All data will be kept confidential and stored securely. The study has been approved by the university’s Institutional Review Board.
Conclusion:
This study will provide valuable information about the effectiveness of meditation for stress reduction in college students. If the intervention is found to be effective, it may have important implications for promoting mental health among this population.
Advantages of Institutional Review Board
Here are some advantages of IRBs:
- Ethical Review : IRBs ensure that research studies are conducted in an ethical manner and that the rights and welfare of human subjects are protected. This includes ensuring that informed consent is obtained, risks are minimized, and benefits outweigh potential harms.
- Compliance : IRBs help ensure that researchers comply with relevant laws and regulations governing human subjects research, such as the Common Rule, HIPAA, and FDA regulations.
- Expertise : IRBs are typically composed of individuals with diverse expertise, including scientists, ethicists, and community members. This expertise allows IRBs to provide valuable feedback to researchers and ensure that research studies are well-designed and scientifically sound.
- Transparency : IRBs provide transparency in the research process by reviewing study protocols, monitoring ongoing research activities, and ensuring that study results are reported accurately.
- Trust : IRBs help build trust between researchers and the public by ensuring that research studies are conducted in a responsible and ethical manner. This can enhance the credibility and reputation of researchers and institutions conducting the research.
About the author
Muhammad Hassan
Researcher, Academic Writer, Web developer
You may also like

Data Collection – Methods Types and Examples

Delimitations in Research – Types, Examples and...

Research Process – Steps, Examples and Tips

Research Design – Types, Methods and Examples

Evaluating Research – Process, Examples and...

Research Questions – Types, Examples and Writing...
Compliance protocols MUST be approved and linked in SeRA to a SPO project record prior to award acceptance.
Pre-Proposals and Letters of Intent (LOIs)
Main navigation.
Sponsors sometimes require applicants to submit a pre-proposal or letter of intent in response to a funding opportunity announcement. Sponsors then review the pre-proposal or letter of intent submissions and select and invite a subset of applicants to submit full applications for the relevant funding opportunity announcement.
NOTE: Pre-proposals or letters of intent (LOIs) that REQUIRE and/or include any of the following require institutional (OSR or RMG) review, ARE subject to the Stanford Internal 5- Day Proposal Deadline Policy and REQUIRE a SeRA Pre-Proposal .
- Institutional signature (written or electronic)
- Submission through a portal or platform where OSR or RMG as institutional officials/Authorized Organizational Representatives (AORs) must complete the submission i.e., "push the button"
- ANY commitment of institutional resources including, but not limited to: PI time/effort, staff time, space, facilities use, project completion, students, and/ or post-doc participation/time, etc. Most often, but not always, these resources are expressed in a budget and/or matching and/or cost sharing information.
For example: National Science Foundation (NSF) letters of intent (LOIs) and pre-proposals, like NSF proposals, are prepared under an investigator's NSF account in Research.gov and are submitted to the NSF by an Authorized Organizational Representative (AOR) i.e., by an OSR Contract and Grant Officer (CGO) or a School of Medicine Research Management Group (RMG) Research Process Manager (RPM). Thus, because NSF LOIs and pre-proposals REQUIRE institutional level review and submission, they ARE subject to the Stanford Internal 5- Day Proposal Deadline Policy and REQUIRE a SeRA Pre-Proposal .
<< Back
Read our research on: Gun Policy | International Conflict | Election 2024
Regions & Countries
9 facts about americans and marijuana.

The use and possession of marijuana is illegal under U.S. federal law, but about three-quarters of states have legalized the drug for medical or recreational purposes. The changing legal landscape has coincided with a decades-long rise in public support for legalization, which a majority of Americans now favor.
Here are nine facts about Americans’ views of and experiences with marijuana, based on Pew Research Center surveys and other sources.
As more states legalize marijuana, Pew Research Center looked at Americans’ opinions on legalization and how these views have changed over time.
Data comes from surveys by the Center, Gallup , and the 2022 National Survey on Drug Use and Health from the U.S. Substance Abuse and Mental Health Services Administration. Information about the jurisdictions where marijuana is legal at the state level comes from the National Organization for the Reform of Marijuana Laws .
More information about the Center surveys cited in the analysis, including the questions asked and their methodologies, can be found at the links in the text.
Around nine-in-ten Americans say marijuana should be legal for medical or recreational use, according to a January 2024 Pew Research Center survey . An overwhelming majority of U.S. adults (88%) say either that marijuana should be legal for medical use only (32%) or that it should be legal for medical and recreational use (57%). Just 11% say the drug should not be legal in any form. These views have held relatively steady over the past five years.
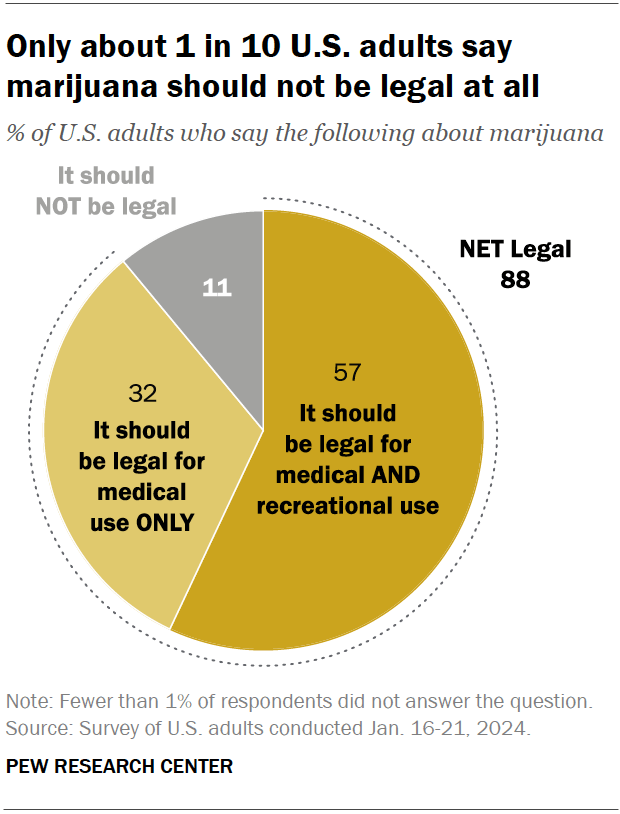
Views on marijuana legalization differ widely by age, political party, and race and ethnicity, the January survey shows.

While small shares across demographic groups say marijuana should not be legal at all, those least likely to favor it for both medical and recreational use include:
- Older adults: 31% of adults ages 75 and older support marijuana legalization for medical and recreational purposes, compared with half of those ages 65 to 74, the next youngest age category. By contrast, 71% of adults under 30 support legalization for both uses.
- Republicans and GOP-leaning independents: 42% of Republicans favor legalizing marijuana for both uses, compared with 72% of Democrats and Democratic leaners. Ideological differences exist as well: Within both parties, those who are more conservative are less likely to support legalization.
- Hispanic and Asian Americans: 45% in each group support legalizing the drug for medical and recreational use. Larger shares of Black (65%) and White (59%) adults hold this view.
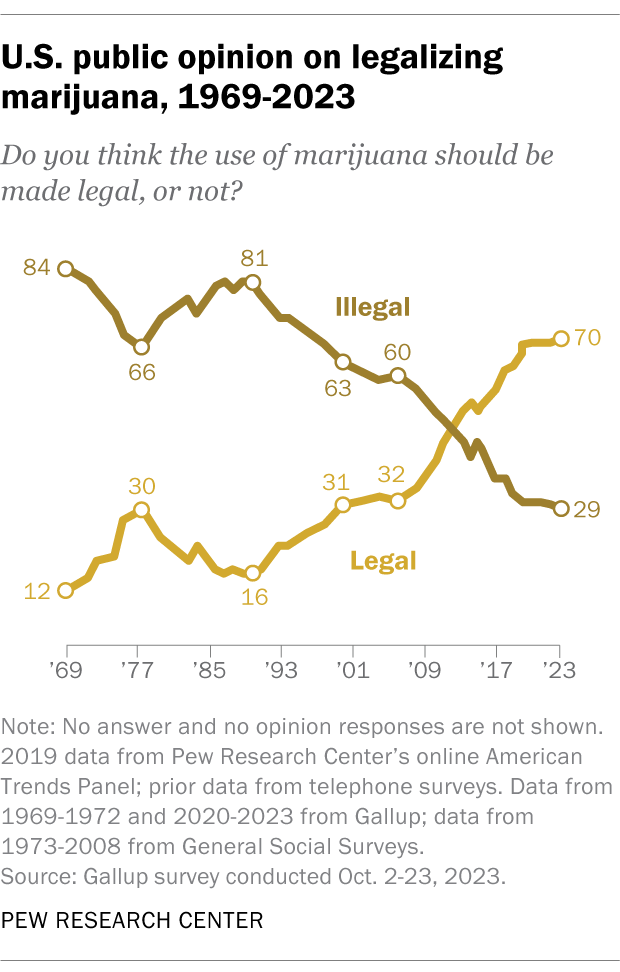
Support for marijuana legalization has increased dramatically over the last two decades. In addition to asking specifically about medical and recreational use of the drug, both the Center and Gallup have asked Americans about legalizing marijuana use in a general way. Gallup asked this question most recently, in 2023. That year, 70% of adults expressed support for legalization, more than double the share who said they favored it in 2000.
Half of U.S. adults (50.3%) say they have ever used marijuana, according to the 2022 National Survey on Drug Use and Health . That is a smaller share than the 84.1% who say they have ever consumed alcohol and the 64.8% who have ever used tobacco products or vaped nicotine.
While many Americans say they have used marijuana in their lifetime, far fewer are current users, according to the same survey. In 2022, 23.0% of adults said they had used the drug in the past year, while 15.9% said they had used it in the past month.
While many Americans say legalizing recreational marijuana has economic and criminal justice benefits, views on these and other impacts vary, the Center’s January survey shows.
- Economic benefits: About half of adults (52%) say that legalizing recreational marijuana is good for local economies, while 17% say it is bad. Another 29% say it has no impact.
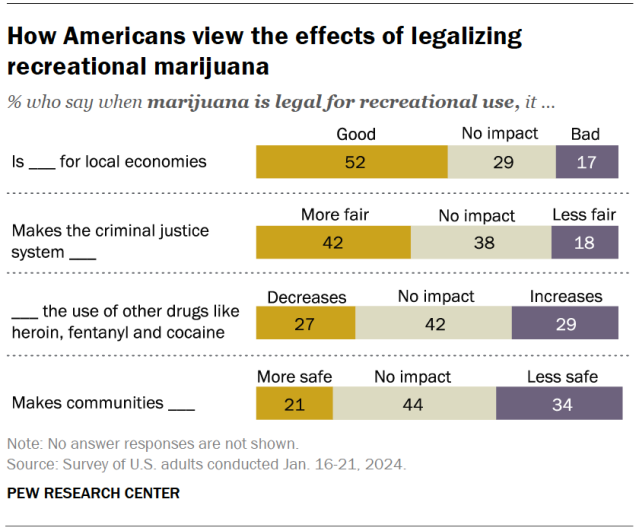
- Criminal justice system fairness: 42% of Americans say legalizing marijuana for recreational use makes the criminal justice system fairer, compared with 18% who say it makes the system less fair. About four-in-ten (38%) say it has no impact.
- Use of other drugs: 27% say this policy decreases the use of other drugs like heroin, fentanyl and cocaine, and 29% say it increases it. But the largest share (42%) say it has no effect on other drug use.
- Community safety: 21% say recreational legalization makes communities safer and 34% say it makes them less safe. Another 44% say it doesn’t impact safety.
Democrats and adults under 50 are more likely than Republicans and those in older age groups to say legalizing marijuana has positive impacts in each of these areas.
Most Americans support easing penalties for people with marijuana convictions, an October 2021 Center survey found . Two-thirds of adults say they favor releasing people from prison who are being held for marijuana-related offenses only, including 41% who strongly favor this. And 61% support removing or expunging marijuana-related offenses from people’s criminal records.
Younger adults, Democrats and Black Americans are especially likely to support these changes. For instance, 74% of Black adults favor releasing people from prison who are being held only for marijuana-related offenses, and just as many favor removing or expunging marijuana-related offenses from criminal records.
Twenty-four states and the District of Columbia have legalized small amounts of marijuana for both medical and recreational use as of March 2024, according to the National Organization for the Reform of Marijuana Laws (NORML), an advocacy group that tracks state-level legislation on the issue. Another 14 states have legalized the drug for medical use only.
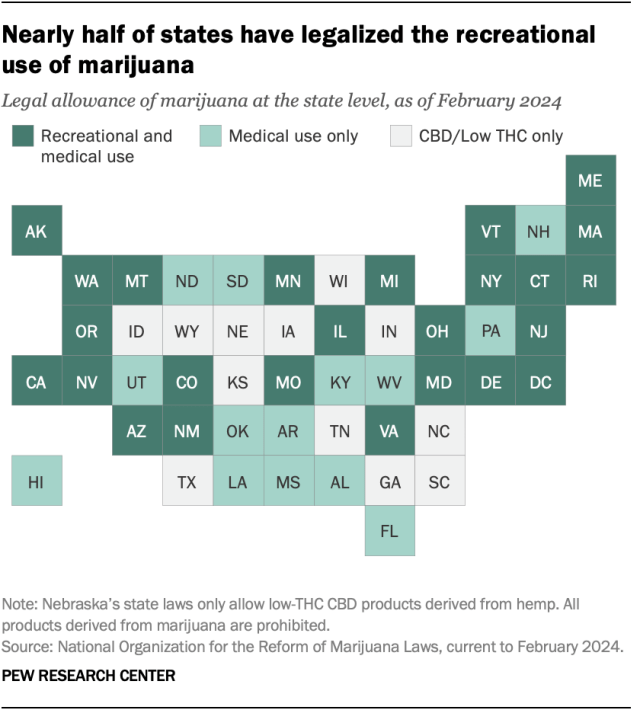
Of the remaining 12 states, all allow limited access to products such as CBD oil that contain little to no THC – the main psychoactive substance in cannabis. And 26 states overall have at least partially decriminalized recreational marijuana use , as has the District of Columbia.
In addition to 24 states and D.C., the U.S. Virgin Islands , Guam and the Northern Mariana Islands have legalized marijuana for medical and recreational use.
More than half of Americans (54%) live in a state where both recreational and medical marijuana are legal, and 74% live in a state where it’s legal either for both purposes or medical use only, according to a February Center analysis of data from the Census Bureau and other outside sources. This analysis looked at state-level legislation in all 50 states and the District of Columbia.
In 2012, Colorado and Washington became the first states to pass legislation legalizing recreational marijuana.
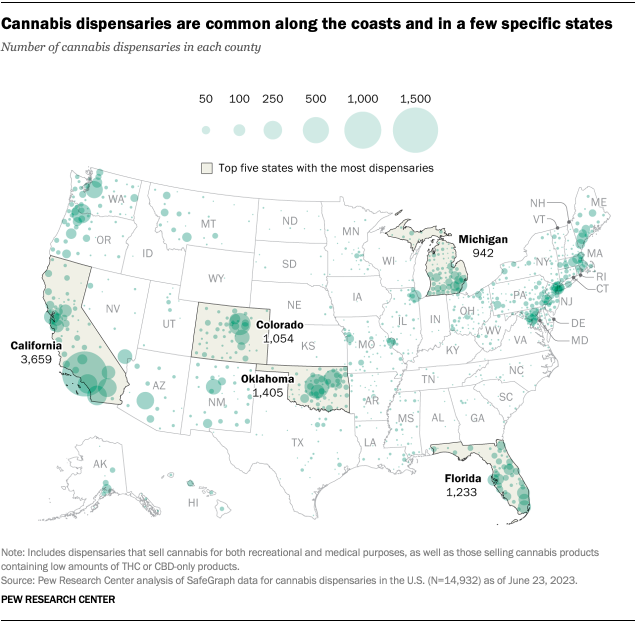
About eight-in-ten Americans (79%) live in a county with at least one cannabis dispensary, according to the February analysis. There are nearly 15,000 marijuana dispensaries nationwide, and 76% are in states (including D.C.) where recreational use is legal. Another 23% are in medical marijuana-only states, and 1% are in states that have made legal allowances for low-percentage THC or CBD-only products.
The states with the largest number of dispensaries include California, Oklahoma, Florida, Colorado and Michigan.
Note: This is an update of a post originally published April 26, 2021, and updated April 13, 2023.

Sign up for our weekly newsletter
Fresh data delivered Saturday mornings
Americans overwhelmingly say marijuana should be legal for medical or recreational use
Religious americans are less likely to endorse legal marijuana for recreational use, four-in-ten u.s. drug arrests in 2018 were for marijuana offenses – mostly possession, two-thirds of americans support marijuana legalization, most popular.
About Pew Research Center Pew Research Center is a nonpartisan fact tank that informs the public about the issues, attitudes and trends shaping the world. It conducts public opinion polling, demographic research, media content analysis and other empirical social science research. Pew Research Center does not take policy positions. It is a subsidiary of The Pew Charitable Trusts .

IMAGES
VIDEO
COMMENTS
A permission letter for research is a crucial document that formally requests authorization to conduct a study in specific locations or collect data from a particular group. It serves as a formal agreement between the researcher and the authority or individuals involved, ensuring that the research is conducted ethically and legally.
Approval Letter for Research - Sample Letter for Approval of Research. Date: __/__/____ (Date) Subject: Approval of research. Dear Sir/madam. This is with reference to your letter dated __/__/____ (Date) regarding approval for research. I am glad to announce that you have been granted permission to research the topic of ________ (mention topic).
Description: (Word) Two sample letters for site approval cooperation between U-M and other institutions, organizations, etc. Letters of cooperation must be on U-M letterhead and signed by an appropriate official. These letters are uploaded into the Performance Site section of the eResearch IRB application.
This letter will demonstrate that the project is understood, and the facilities in use are willing and able to support the research. Depending on the nature of the research, the letter range from quite simple to very specific. Providing authorization to conduct research within your facility should be considered a serious manner.
Essential Elements for Documented Approval to Conduct Study at an Off-Campus Site On agency letterhead, the letter from the Off-Campus site administrator should include the following: Investigator's first and last name. A statement that the site will receive a copy of the IRB approval letter.
Informed Consent for Adults This is a sample document that can be used as a guide to develop an Informed Consent specific to a research study. (Requires AU IRB approval stamp on all pages.) **IMPORTANT: Consent forms must have a concise summary table. The table should be placed at the beginning of the document.**.
Researchers working with human subjects must submit their research plans and any research-related documents to Teachers College (TC) Institutional Review Board (IRB) for review and approval. Researchers may not begin recruitment or research until IRB has issued a final approval letter on the protocol. Submission Document Templates
New Study Application: 1. Resources and Responsibilities Section, Question 11.3: This response should describe any location(s) where research is being conducted. 2. Attachment: Letters of Support should be submitted with the new study application. Please contact the OPRS Office at (217) 333-2670 or [email protected] for additional guidance.
As research supervisor of Mr. John Smith, I confirm that I approve and support the research proposal submitted by the candidate. c) Before the commencement of the award, Mr. Smith will have completed all the required courses for the master's/PhD degree and the comprehensive /oral examination. e) John Smith will be traveling to xxx, where ...
A letter of approval sample for research is a formal document that grants consent and authorization for conducting a specific research project. This letter typically serves as an agreement between the researchers and the institution or individuals responsible for overseeing the research. The content of a letter of approval sample for research ...
ÐÏ à¡± á> þÿ + - þÿÿÿ ...
Approval letter format. Your address. Date. Recipients address. Subject: Dear Mr. / MS. /Mrs. [Name of the individual], I write this letter to inform you the board of directors has approved your application for [reason] for [amount]. This loan will be repaid at an interest rate of [percentage] for [duration]. In this respect, you are requested ...
Letter of Approval to Conduct Research - Free download as PDF File (.pdf), Text File (.txt) or read online for free. Sample letter
An approval letter sample for research paper is a formal document issued by a reviewing committee or an institutional body to acknowledge and endorse a research paper project for further development, publication, or funding. Such letters play a crucial role in the academic and research community to ensure the quality, relevance, and ethical ...
The research study "<dissertation title>" submitted to Flagler Schools by <applicant name> was approved by the Flagler Schools Research Oversight Committee on August 20, 2021. Please contact individual school principals to set your on-site data collection timeline. You have a maximum of eighteen (18) months from the approval date above to ...
Here are the key points to include in a condensed bullet-point format: Opening: Use a professional salutation (e.g., "Dear [Recipient's Name],"). Clearly state the purpose of the letter (e.g., "Subject: Approval Letter"). Express Approval: Clearly and directly state that approval is granted.
Institutional Review Board Proposal Sample. Here is a sample proposal for an Institutional Review Board (IRB) submission: Title: Effects of Meditation on Stress Reduction in College Students. Introduction: Stress is a common issue among college students, and it can have negative effects on academic performance and overall well-being.
Use kind and respectful terms like 'Sir,' 'Madam,' 'Mr.,' and so on. That is to show some respect and to establish a reasonable connection between you and the reader. It also increases your chances of receiving your wish. State what you need. Now get to the core of the letter.
2. Obtain written approval from the Human Subjects Committee of any changes from the originally approved protocol BEFORE implementing those changes. 3. Retain signed consent forms in a secure location separate from the data for at least three years after the completion of the research. 4.
Research Approval Letter to respondents - Free download as Word Doc (.doc / .docx), PDF File (.pdf), Text File (.txt) or read online for free. sample of approval letter for the respondents
6753 Groove Street. Huntington, NY 56474. Dear Ms Arthur. I am pleased to let you know that the fifteen days of absence you had previously requested have been granted and approved by the company. Starting July 15, 2018, you will be able to take fifteen consecutive days off while continuing to collect 30% of your pay.
National Science Foundation (NSF) letters of intent (LOIs) and pre-proposals, like NSF proposals, are prepared under an investigator's NSF account in Research.gov and are submitted to the NSF by an Authorized Organizational Representative (AOR) i.e., by an OSR Contract and Grant Officer (CGO) or a School of Medicine Research Management Group ...
Around nine-in-ten Americans say marijuana should be legal for medical or recreational use, according to a January 2024 Pew Research Center survey.An overwhelming majority of U.S. adults (88%) say either that marijuana should be legal for medical use only (32%) or that it should be legal for medical and recreational use (57%).Just 11% say the drug should not be legal in any form.