Appointments at Mayo Clinic
- Pregnancy week by week

Placenta: How it works, what's normal
The placenta plays a crucial role during pregnancy. Find out what the placenta does, issues that might affect it and how it is delivered.
If you're pregnant, you might wonder what exactly the placenta is, what it does and what might affect it. Here's what you need to know about this important organ.
What does the placenta do?
The placenta is an organ that forms in the womb, also called the uterus, during pregnancy. The placenta is connected to a developing baby by a tubelike structure called the umbilical cord. Through the umbilical cord, the placenta provides oxygen and nutrients to a developing baby. It also removes waste from the baby's blood.
The placenta is attached to the wall of the uterus. Most often, it attaches to the top, side, front or back of the uterus. Rarely, it might attach in the lower area of the uterus. When this happens, the placenta may block the passage that connects the uterus to the vagina, called the cervix. If the placenta is near the opening of the cervix, it's known as a low-lying placenta. If it partly or totally covers the opening of the cervix, it causes a condition called placenta previa.
What affects the health of the placenta?
Various factors can affect the health of the placenta, including:
- Age of the pregnant person. Some conditions that affect the placenta are more common in older people, especially after age 40.
- Water breaking before labor. During pregnancy, the developing baby is surrounded and cushioned by a fluid-filled layer of tissue called the amniotic sac. If the sac leaks or breaks before labor starts, it's known as the water breaking. This raises the risk of problems with the placenta.
- High blood pressure. This condition can cause less blood to reach the placenta.
- Being pregnant with twins or other multiples. Being pregnant with more than one baby might raise the risk of some conditions related to the placenta.
- Blood-clotting conditions. Typically, blood hardens into a clump to help control bleeding from cuts. This process is called clotting. Sometimes, blood clots form inside the body and lead to medical problems. Conditions that cause blood to clot too little or too much raise the risk of some conditions related to the placenta.
- Past surgery on the uterus. C-section, surgery to remove tumors called fibroids and other uterine surgeries raise the risk of some conditions that affect the placenta.
- Previous conditions that affected the placenta. The risk of having medical issues with the placenta might be higher if you had problems with the placenta during a past pregnancy.
- Substance use. Some conditions that can affect the placenta are more common in pregnant people who smoke or use cocaine.
- Injury to the stomach area. A blow to the stomach area makes the placenta more likely to separate from the uterus too soon. Risk factors include trauma from a car accident or a serious fall.
What are the most common conditions and concerns?
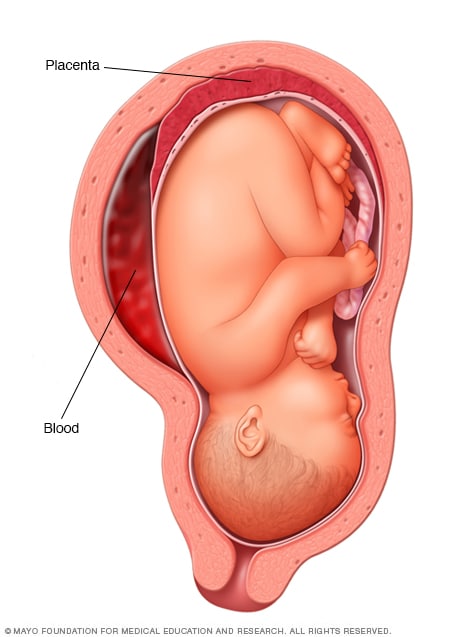
Placental abruption
The placenta is a structure that develops in the uterus during pregnancy. Placental abruption occurs when the placenta separates from the inner wall of the uterus before birth. Placental abruption can deprive the baby of oxygen and nutrients and cause heavy bleeding in the pregnant person. In some people, early delivery is needed.
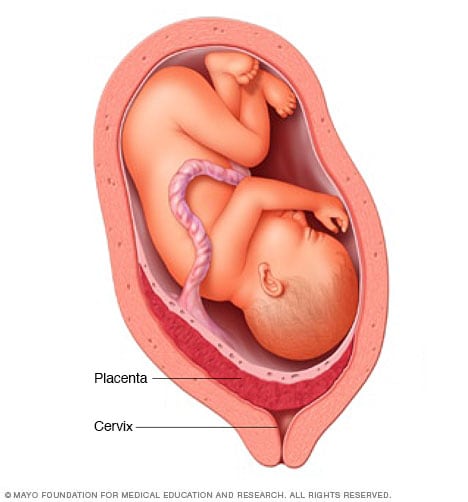
Placenta previa
The placenta is a structure that develops in the uterus during pregnancy. In most pregnancies, the placenta is located at the top or side of the uterus. In placenta previa, the placenta is located low in the uterus. The placenta might partially or completely cover the cervix, as shown here. Placenta previa can cause severe bleeding in a pregnant person before or during delivery. A C-section often is needed.
Conditions that can affect the placenta include:
- Placental abruption. This is when the placenta partly or completely peels away from the inner wall of the uterus before delivery. With placental abruption, the developing baby might not get enough oxygen and nutrients. The pregnant person might have back or stomach pain and bleeding from the vagina. Placental abruption can lead to an emergency in which a baby needs to be delivered early.
Placenta previa. This condition happens when the placenta partly or totally covers the cervix. Placenta previa is more common early in pregnancy. It might get better on its own as the uterus grows.
Placenta previa can cause serious vaginal bleeding during pregnancy or delivery. Treatment depends on various factors. They include the amount of bleeding, whether bleeding stops, how far along the pregnancy is and the placenta's position. If placenta previa continues late into the pregnancy, a healthcare professional likely will recommend a C-section.
Placenta accreta. Most often, the placenta separates from the wall of the uterus after childbirth. With placenta accreta, part or all of the placenta stays firmly attached to the uterus. This condition happens when the blood vessels and other parts of the placenta grow into the uterine wall. This can cause serious blood loss during delivery.
Sometimes, the placenta invades well into the muscles of the uterus or grows through the uterine wall. If this happens, a healthcare professional likely will recommend a C-section followed by surgery to remove the uterus. This is called a C-hysterectomy.
Without treatment, a retained placenta can cause a serious infection or life-threatening blood loss. Treatment may include medicine to help deliver the placenta or a procedure to remove the placenta.
What are symptoms of trouble with the placenta?
Call your healthcare professional if you have any of the following symptoms during pregnancy:
- Bleeding from the vagina, especially if it's heavy.
- Pain in the stomach area, also called the abdomen.
- Tightening and relaxing of the muscles in the uterus, also called uterine contractions.
What can I do to lower my risk of conditions that affect the placenta?
Most medical issues related to the placenta can't be prevented directly. But you can take steps to boost your chances for a healthy pregnancy:
- Go to all of your routine pregnancy checkups.
- Work with your healthcare professional to manage any health conditions, such as high blood pressure.
- Don't smoke or use drugs. If you need help quitting, talk with your health care professional.
- If you're thinking about getting a C-section, ask your healthcare professional about the risks.
If you had a condition that affected the placenta during a past pregnancy and you’re planning another pregnancy, talk with your healthcare professional. Ask about ways to lower the risk of getting that condition again. Also tell your healthcare professional if you've had surgery on your uterus.
How is the placenta delivered?
If you deliver your baby through your vagina, you'll also deliver the placenta that way shortly afterward. This is known as the third stage of labor.
After you give birth, you keep having mild contractions. Your healthcare professional might give you a shot of medicine called oxytocin (Pitocin). This helps you keep having contractions. It also lessens bleeding after you deliver your baby. Your healthcare professional also might massage your lower abdomen. This encourages the uterus to contract and release the placenta through the vagina. You might be asked to push to deliver the placenta.
If you have a C-section, your healthcare professional removes the placenta from your uterus during that procedure.
After it's delivered, your health care professional checks the placenta to make sure it's intact. Any pieces left behind need to be removed from the uterus to prevent bleeding and infection. If you're interested, ask to see the placenta. In some cultures, families bury the placenta in a special place.
If you have questions about the placenta during pregnancy, talk with a member of your healthcare team. Your healthcare professional can help you better understand the placenta's role in pregnancy.
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
From Mayo Clinic to your inbox
Sign up for free and stay up to date on research advancements, health tips, current health topics, and expertise on managing health. Click here for an email preview.
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing!
You'll soon start receiving the latest Mayo Clinic health information you requested in your inbox.
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
- Roberts V, et al. Placental development and physiology. https://www.uptodate.com/contents/search. Accessed Oct. 19, 2023.
- Lockwood CJ, et al. Placenta previa: Epidemiology, clinical features, diagnosis, morbidity and mortality. https://www.uptodate.com/contents/search. Accessed Oct. 19, 2023.
- Baggish MS, et al. Cesarean section. In: Atlas of Pelvic Anatomy and Gynecologic Surgery. 5th ed. Elsevier; 2021. https://www.clinicalkey.com. Accessed Oct. 25, 2023.
- Cunningham FG, et al., eds. Causes of obstetrical hemorrhage. In: Williams Obstetrics. 26th ed. McGraw Hill; 2022. https://accessmedicine.mhmedical.com. Accessed Oct. 19, 2023.
- Lockwood CJ, et al., eds. Placenta previa and accreta, vasa previa, subchorionic hemorrhage, and abruptio placentae. In: Creasy and Resnik's Maternal-Fetal Medicine: Principles and Practice. 9th ed. Elsevier; 2023. https://www.clinicalkey.com. Accessed Oct. 19, 2023.
- Wick MJ, ed. Managing mom's health concerns. In: Mayo Clinic Guide to a Healthy Pregnancy. 2nd ed. Mayo Clinic; 2018.
- Moore KL, et al. Placenta and fetal membranes. In: The Developing Human: Clinically Oriented Embryology. 11th ed. Elsevier; 2020. https://www.clinicalkey.com. Accessed Oct. 19, 2023.
- Martin RJ, et al., eds. Placental pathology. In: Fanaroff and Martin's Neonatal-Perinatal Medicine: Disease of the Fetus and Infant. 11th ed. Elsevier; 2020. https://www.clinicalkey.com. Accessed Oct. 19, 2023.
- Weeks A. Retained placenta after vaginal birth. https://www.uptodate.com/contents/search. Accessed Oct. 19, 2023.
- Landon MB, et al., eds. Placenta accreta spectrum. In: Gabbe's Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021. https://www.clinicalkey.com. Accessed Oct. 19, 2023.
- FAQs: Bleeding during pregnancy. American College of Obstetricians and Gynecologists. https://www.acog.org/womens-health/faqs/bleeding-during-pregnancy. Accessed Oct. 19, 2023.
- What complications can affect the placenta? National Health Service. https://www.nhs.uk/pregnancy/labour-and-birth/what-happens/placenta-complications/. Accessed Oct. 27, 2023.
Products and Services
- Available Solutions for Prenatal Nutrition from Mayo Clinic Store
- A Book: Taking Care of You
- A Book: Obstetricks
- A Book: Mayo Clinic Guide to a Healthy Pregnancy
- Air travel during pregnancy
- Allergy medications during pregnancy
- Ankle swelling during pregnancy
- Antibiotics and pregnancy
- Aspirin during pregnancy
- Pregnancy back pain
- Falling during pregnancy: Reason to worry?
- Fetal ultrasound
- Flu shot in pregnancy
- Headaches during pregnancy: What's the best treatment?
- Iron deficiency anemia during pregnancy: Prevention tips
- Leg cramps during pregnancy
- Pregnancy acne
- Pregnancy and fish
- Pregnancy constipation
- Pregnancy diet: Essential nutrients
- Pregnancy due date calculator
- Pregnancy exercises
- Pregnancy nutrition don'ts
- Pregnancy stretches
- Pregnancy weight gain
- Pregnant. Now What Happens?
- Prenatal testing
- Prenatal vitamins and pregnancy
- Sex during pregnancy
- Twin pregnancy
- Vaccines during pregnancy
- Vaping during pregnancy
- Working during pregnancy
- X-ray during pregnancy
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
- Healthy Lifestyle
- Placenta - How it works whats normal
Make twice the impact
Your gift can go twice as far to advance cancer research and care!
- Type 2 Diabetes
- Heart Disease
- Digestive Health
- Multiple Sclerosis
- COVID-19 Vaccines
- Occupational Therapy
- Healthy Aging
- Health Insurance
- Public Health
- Patient Rights
- Caregivers & Loved Ones
- End of Life Concerns
- Health News
- Thyroid Test Analyzer
- Doctor Discussion Guides
- Hemoglobin A1c Test Analyzer
- Lipid Test Analyzer
- Complete Blood Count (CBC) Analyzer
- What to Buy
- Editorial Process
- Meet Our Medical Expert Board
The Anatomy of the Placenta
The placenta ensures fetuses get necessary food and oxygen during pregnancy.
Associated Conditions
The placenta develops within the uterus during pregnancy, playing a key role in nourishing and providing oxygen to the fetus, as well as removing waste material. This organ is attached to the wall of the uterus, with the baby’s umbilical cord arising from it. Throughout the course of a pregnancy, the placenta grows and changes shape, with its thickness being a reliable measure of how far along the mother-to-be is in gestation. Furthermore, a number of disorders can impact this organ, including placenta previa, in which some or all of the cervix is covered by the placenta, as well as placenta accreta malformations, which involve different degrees of implantation within the uterine wall.
Structure and Location
The largest fetal organ, the placenta undergoes rapid development over the course of pregnancy. By the time the baby is brought to term, it has a flat, round disc-like shape that is about 22 centimeters (cm) in diameter, with walls that are typically between 2 and 2.5 cm.
The placenta typically sits along the back wall of the uterine wall—about 6 cm from the cervix—occasionally accessing the side walls throughout its course of development. Significantly, the umbilical cord (which brings in nutrients and oxygen and takes out waste material) connects the mid-section of the fetus to the placenta; in turn, the fetus is surrounded by the amniotic or gestational sac.
The placenta undergoes consistent change throughout the course of pregnancy; between week 0 and 13 after conception, the fertilized blastocyst (what the embryo becomes once its cells start differentiating at about five days after the egg is fertilized) embeds itself in the mucous membrane (endometrium) of the uterine wall, allowing for the fetus and placenta to start forming. By the fourth or fifth month of pregnancy, the placenta takes up about half of the uterine surface, though this percentage shrinks as the fetus grows. At birth, the placenta is also ejected from the body.
Crucial to placenta (and, by extension, embryonic) development is the formation of small, finger-like structures called chorionic villi, which are composed of two types of cells—cytotrophoblasts and syncytiotrophoblasts. The former of these interact with arteries and veins in the walls of the uterus to ensure the fetus gets the nutrients and oxygen it needs. Throughout pregnancy, this vasculature grows in size and complexity, allowing for the formation of the following two major components.
- Maternal component: Essentially, this is the portion of the placenta that is formed of the mother’s endometrium or the maternal uterine tissue. It forms what is called the decidua basalis, or maternal placenta.
- Fetal component: Also known as the chorion frondosum or villous chorion, this is the portion of the placenta arising from the blastocyte.
These are held together by outgrowths, called anchoring villi, from the maternal component. The placenta is surrounded by a placental membrane or barrier. While it serves to differentiate blood supply for mother and fetus, many substances can still get through.
Anatomical Variations
Not every placenta forms regularly, and this can have serious implications. Several such malformations, including placenta previa, accreta, increta, and percreta, are considered serious medical conditions that can endanger a mother, the fetus, or both. In addition, there are a number of other commonly identified abnormalities.
- Bilobed placenta: Also known as “placenta duplex,” this is a case where the placenta is composed of two roughly equal-sized lobes. The umbilical cord may insert into either lobe, run through both, or sit between them. Though this condition doesn’t increase risk of damage to the fetus, it can cause first-trimester bleeding, excessive amniotic fluid within the gestational sac, abruption (premature separation of the placenta from the womb), or retained placenta (when the placenta remains in the body after birth). This condition is seen in 2% to 8% of women.
- Succenturiate placenta: In these cases, a lobe of placenta forms separately from a main body that is linked via the umbilical cord to the fetus. Essentially, it’s a variation of a bilobed placenta that occurs more commonly in women who are of advanced maternal age or in those who have had in vitro fertilization. Seen about 5% of the time, this condition can also lead to retained placenta as well as placenta previa, among other complications.
- Circumvallate placenta: This is when the membranes of the placenta tuck back around its edges to form a ring-like (annular) shape. In this case, the outer membrane, known as the chorion causes a hematoma (a collection of blood) at the margin of the placenta, and vessels within its ring stop abruptly. This condition can lead to poor outcomes for the pregnancy due to the risk of vaginal bleeding during the first trimester, potential rupture of the membranes, pre-term delivery, insufficient development of the placenta, as well as abruption. This condition isn’t easily diagnosed during pregnancy.
- Circummarginate placenta: This is a much less problematic variant of the above, in which the membranes do not curl back.
- Placenta membranacea: In this rare condition, chorionic villi cover the fetal membrane partially or completely, causing the placenta to develop as a thinner structure at the periphery of the membrane that encloses the chorion. This then leads to vaginal bleeding in the second and/or third trimester of pregnancy and may lead to placenta previa or accreta.
- Ring-shaped placenta: A variation of placenta membranacea, this condition causes the placenta to have either a ring-like or horseshoe-like shape. Occurring in only about 1 in 6,000 pregnancies, this leads to bleeding before or after delivery, as well as reduced growth of the fetus.
- Placenta fenestrata: This condition is characterized by the absence of the central portion of the placenta. Also very rare, the primary concern for doctors is retained placenta at delivery.
- Battledore placenta: Sometimes called “marginal cord insertion,” this is when the umbilical cord runs through the margin of the placenta rather than the center. This occurs in between 7% and 9% of single pregnancies, but is much more common when there are twins, happening between 24% and 33% of the time. This can lead to early (preterm) labor and problems with the fetus, as well as low birth weight.
The placenta plays an absolutely crucial and essential role during the nine months of pregnancy. Via the umbilical cord and the chorionic villi, this organ delivers blood, nutrients, and oxygen to the developing fetus. In addition, it works to remove waste materials and carbon dioxide. As it does so, it creates a differentiation between maternal and fetal blood supply, keeping these separate via its membrane.
Furthermore, the placenta works to protect the fetus from certain diseases and bacterial infections and helps with the development of the baby’s immune system. This organ also secretes hormones—such as human chorionic gonadotropin, human placenta lactogen, and estrogen—necessary to influence the course of pregnancy and fetal growth and metabolism, as well as labor itself.
Aside from the developmental abnormalities listed above, the placenta may also be subject to a number of medical conditions that may be of concern to doctors. Oftentimes, the core of the problem has to do with the position of this organ. Among these are the following.
- Placenta previa : This condition occurs when the placenta forms partially or totally toward the lower end of the uterus, including the cervix, rather than closer to its upper part. In cases of complete previa, the internal os —that is, the opening from the uterus to the vagina —is completely covered by the placenta. Occurring in about 1 in 200 to 250 pregnancies, risk factors for placenta previa include a history of smoking, prior cesarean delivery, abortion, other surgery of the uterus, and older maternal age, among others. Depending on the case, cesarean delivery may be required.
- Placenta accreta : When the placenta develops too deep within the uterine wall without penetrating the uterine muscle (myometrium), the third trimester of the pregnancy can be impacted. A relatively rare occurrence—this is the case in only 1 in every 2,500 pregnancies—this condition is more likely to occur among smokers and those with older maternal age, as well as those with a history of previous surgeries or cesarean deliveries. This also can happen alongside placenta previa. During delivery, this condition can lead to serious complications, including hemorrhage and shock. While hysterectomy —the removal of a woman’s uterus—has been the traditional treatment approach, other, more conservative options are available.
- Placenta increta: Representing 15% to 17% of placenta accreta cases, this form of the condition is when development of the placenta is within the uterine wall and it penetrates the myometrium. Childbirth is severely impacted in these cases, since this can lead to severe hemorrhage due to retention of the placenta within the body. As such, cesarean delivery is required alongside hysterectomy or comparable treatment.
- Placenta percreta: Yet another type of accreta, placenta percreta occurs when this organ develops all the way through the uterine wall. It may even start to grow into surrounding organs, such as the bladder or colon. Occurring in 5% of placenta accreta cases, as with placenta increta, cesarean delivery and hysterectomy is necessary in these cases.
- Placental insufficiency : Arising for a range of reasons, this is when the placenta is unable to provide enough nourishment for the fetus. This can be due to genetic defects, deficiencies of vitamins C and E, chronic infections (such as malaria), high blood pressure, diabetes, anemia, or heart disease, as well as other health issues. Treatment can range from ensuring better diet to taking medications like low-dose aspirin.
Throughout the course of pregnancy, doctors will perform a wide range of tests to ensure the health of the fetus. This can mean everything from blood tests to genetic tests are administered. When it comes to ensuring proper development of the placenta, a number of diagnostic techniques are employed, including the following.
- Ultrasound : A frequently employed approach when it comes to monitoring fetal development as well as the health of the placenta, ultrasound employs high-frequency sound waves to create a real-time video of the uterus and surrounding regions. Especially in the second and third trimesters, this approach can be used for cases of placenta previa, among other disorders. Furthermore, based on ultrasound results, doctors classify placental maturity. This system of placental grading ranges from grade 0 for pregnancy at 18 or less weeks to grade III for when things have progressed beyond week 39. Early onset of grade III, for instance, may be a sign of placental insufficiency.
- Magnetic resonance imaging (MRI): This imaging approach relies on strong magnetic and radio waves to create highly detailed depictions of the fetus and placenta. Though not necessarily the first line of treatment, MRI may be used to diagnose placenta increta and percreta. In addition, this method may be used in cases of placental insufficiency.
National Institutes of Health. Human Placenta Project: How does the placenta form? .
Rathbun K, Hildebrand J. Placenta abnormalities . StatPearls.
Hapugoda S, Jha P. Placenta: radiology reference article . Radiopaedia.
Hill M. Placenta development: embryology . University of New South Wales.
Gude N, Roberts C, Kalionis B, King R. Growth and function of the normal human placenta .
Krishna U, Bhalerao S. Placental insufficiency and fetal growth restriction . J Obstet Gynaecol India . 2011;61(5):505-511.
By Mark Gurarie Gurarie is a freelance writer and editor. He is a writing composition adjunct lecturer at George Washington University.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 29 June 2020
Tracking placental development in health and disease
- John D. Aplin ORCID: orcid.org/0000-0001-8777-9261 1 ,
- Jenny E. Myers ORCID: orcid.org/0000-0003-0913-2096 1 ,
- Kate Timms ORCID: orcid.org/0000-0003-1764-4964 2 &
- Melissa Westwood 1
Nature Reviews Endocrinology volume 16 , pages 479–494 ( 2020 ) Cite this article
8999 Accesses
162 Citations
29 Altmetric
Metrics details
- Endocrine reproductive disorders
Pre-eclampsia and fetal growth restriction arise from disorders of placental development and have some shared mechanistic features. Initiation is often rooted in the maldevelopment of a maternal–placental blood supply capable of providing for the growth requirements of the fetus in later pregnancy, without exerting undue stress on maternal body systems. Here, we review normal development of a placental bed with a safe and adequate blood supply and a villous placenta–blood interface from which nutrients and oxygen can be extracted for the growing fetus. We consider disease mechanisms that are intrinsic to the maternal environment, the placenta or the interaction between the two. Systemic signalling from the endocrine placenta targets the maternal endothelium and multiple organs to adjust metabolism for an optimal pregnancy and later lactation. This signalling capacity is skewed when placental damage occurs and can deliver a dangerous pathogenic stimulus. We discuss the placental secretome including glycoproteins, microRNAs and extracellular vesicles as potential biomarkers of disease. Angiomodulatory mediators, currently the only effective biomarkers, are discussed alongside non-invasive imaging approaches to the prediction of disease risk. Identifying the signs of impending pathology early enough to intervene and ameliorate disease in later pregnancy remains a complex and challenging objective.
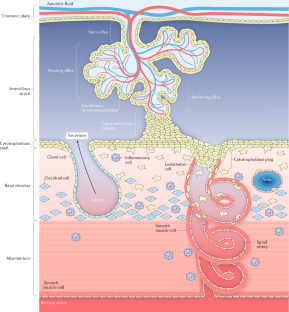
In the first trimester, uterine secretions support embryonic development; remodelling of the maternal vascular supply to the placental site enables increased volume supply of substrates at low pressure as fetal demand increases.
Placental growth and branching of the villous tree yield an increasing surface area for substrate transport, which is coordinated with the elaboration of a fetoplacental vascular network.
Fetal growth restriction arises when the supply of nutrients and oxygen to the fetus is insufficient because of maternal vascular malperfusion and/or inefficient extraction of substrates by the placenta.
Pre-eclampsia is caused by reaction of the placenta to stress, which triggers the release of factors that induce systemic vascular pathology or suppresses factors that stabilize vascular and immune interfaces.
An angiomodulatory imbalance is present in a large proportion of pregnancies with one or more of the clinical features of either pre-eclampsia or fetal growth restriction.
Data on many of the potential biomarkers of disease in pregnancy are conflicting, with reports of unchanged, lower or higher levels in the maternal circulation in complicated versus control pregnancies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
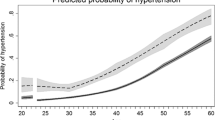
Placental syndromes and long-term risk of hypertension
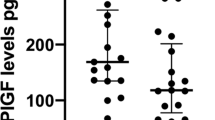
In vivo evidence of significant placental growth factor release by normal pregnancy placentas
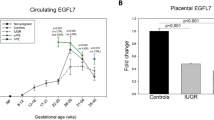
Circulating EGFL7 distinguishes between IUGR and PE: an observational case–control study
Tobi, E. W. et al. Selective survival of embryos can explain DNA methylation signatures of adverse prenatal environments. Cell Rep. 25 , 2660–2667.e4 (2018).
PubMed CAS Google Scholar
Constancia, M. et al. Adaptation of nutrient supply to fetal demand in the mouse involves interaction between the Igf2 gene and placental transporter systems. Proc. Natl Acad. Sci. USA 102 , 19219–19224 (2005).
PubMed CAS PubMed Central Google Scholar
Lane-Cordova, A. D., Khan, S. S., Grobman, W. A., Greenland, P. & Shah, S. J. Long-term cardiovascular risks associated with adverse pregnancy outcomes: JACC review topic of the week. J. Am. Coll. Cardiol. 73 , 2106–2116 (2019).
PubMed Google Scholar
Kesavan, K. & Devaskar, S. U. Intrauterine growth restriction: postnatal monitoring and outcomes. Pediatr. Clin. North. Am. 66 , 403–423 (2019).
Ruane, P. T. et al. Apposition to endometrial epithelial cells activates mouse blastocysts for implantation. Mol. Hum. Reprod. 23 , 617–627 (2017).
Burton, G. J., Watson, A. L., Hempstock, J., Skepper, J. N. & Jauniaux, E. Uterine glands provide histiotrophic nutrition for the human fetus during the first trimester of pregnancy. J. Clin. Endocrinol. Metab. 87 , 2954–2959 (2002).
Jones, C. J., Choudhury, R. H. & Aplin, J. D. Tracking nutrient transfer at the human maternofetal interface from 4 weeks to term. Placenta 36 , 372–380 (2015).
Guo, H. et al. The DNA methylation landscape of human early embryos. Nature 511 , 606–610 (2014).
Aplin, J. D. & Jones, C. J. in The Endometrium Ch. 29 (eds Aplin J. D., Fazleabas A. T., Glasser S. R., & Giudice L. C.) 441–453 (Informa Healthcare, 2008).
Aplin, J. D. & Ruane, P. T. Embryo-epithelium interactions during implantation at a glance. J. Cell Sci. 130 , 15–22 (2017).
Aplin, J. D., Lewis, R. M. & Jones, C. J. in Reference Module in Biomedical Sciences https://doi.org/10.1016/B978-0-12-801238-3.99857-X (Elsevier, 2018).
Cheong, M. L. et al. A positive feedback loop between glial cells missing 1 and human chorionic gonadotropin (hCG) regulates placental hCGbeta expression and cell differentiation. Mol. Cell Biol. 36 , 197–209 (2016).
Gerbaud, P. et al. Mesenchymal activin-A overcomes defective human trisomy 21 trophoblast fusion. Endocrinology 152 , 5017–5028 (2011).
Jones, C. J., Harris, L. K., Whittingham, J., Aplin, J. D. & Mayhew, T. M. A re-appraisal of the morphophenotype and basal lamina coverage of cytotrophoblasts in human term placenta. Placenta 29 , 215–219 (2008).
Kar, M., Ghosh, D. & Sengupta, J. Histochemical and morphological examination of proliferation and apoptosis in human first trimester villous trophoblast. Hum. Reprod. 22 , 2814–2823 (2007).
Mayhew, T. M. Recent applications of the new stereology have thrown fresh light on how the human placenta grows and develops its form. J. Microsc. 186 , 153–163 (1997).
Mayhew, T. M. Villous trophoblast of human placenta: a coherent view of its turnover, repair and contributions to villous development and maturation. Histol. Histopathol. 16 , 1213–1224 (2001).
Lu, X. et al. Fine-tuned and cell-cycle-restricted expression of fusogenic protein syncytin-2 maintains functional placental syncytia. Cell Rep. 21 , 1150–1159 (2017).
Lin, C., Lin, M. & Chen, H. Biochemical characterization of the human placental transcription factor GCMa/1. Biochem. Cell Biol. 83 , 188–195 (2005).
Liang, C. Y. et al. GCM1 regulation of the expression of syncytin 2 and its cognate receptor MFSD2A in human placenta. Biol. Reprod. 83 , 387–395 (2010).
Karolczak-Bayatti, M. et al. IGF signalling and endocytosis in the human villous placenta in early pregnancy as revealed by comparing quantum dot conjugates with a soluble ligand. Nanoscale 11 , 12285–12295 (2019).
Sibley, C. P., Brownbill, P., Glazier, J. D. & Greenwood, S. L. Knowledge needed about the exchange physiology of the placenta. Placenta 64 , S9–S15 (2018).
Aplin, J. D., Haigh, T., Jones, C. J., Church, H. J. & Vicovac, L. Development of cytotrophoblast columns from explanted first-trimester human placental villi: role of fibronectin and integrin alpha5beta1. Biol. Reprod. 60 , 828–838 (1999).
Aplin, J. D., Haigh, T., Vicovac, L., Church, H. J. & Jones, C. J. Anchorage in the developing placenta: an overlooked determinant of pregnancy outcome? Hum. Fertil. 1 , 75–79 (1998).
Google Scholar
Vento-Tormo, R. et al. Single-cell reconstruction of the early maternal-fetal interface in humans. Nature 563 , 347–353 (2018).
Velicky, P. et al. Genome amplification and cellular senescence are hallmarks of human placenta development. PLoS Genet. 14 , e1007698 (2018).
PubMed PubMed Central Google Scholar
al-Lamki, R. S., Skepper, J. N. & Burton, G. J. Are human placental bed giant cells merely aggregates of small mononuclear trophoblast cells? An ultrastructural and immunocytochemical study. Hum. Reprod. 14 , 496–504 (1999).
Aplin, J. D. in Encyclopedia of Reproduction 2nd edn. Vol. 2 (ed. Skinner M. K.) 326–332 (Elsevier, 2018).
Kajihara, T. et al. Differential expression of FOXO1 and FOXO3a confers resistance to oxidative cell death upon endometrial decidualization. Mol. Endocrinol. 20 , 2444–2455 (2006).
Yoshino, O. et al. Endometrial stromal cells undergoing decidualization down-regulate their properties to produce proinflammatory cytokines in response to interleukin-1 beta via reduced p38 mitogen-activated protein kinase phosphorylation. J. Clin. Endocrinol. Metab. 88 , 2236–2241 (2003).
Leitao, B. et al. Silencing of the JNK pathway maintains progesterone receptor activity in decidualizing human endometrial stromal cells exposed to oxidative stress signals. FASEB J. 24 , 1541–1551 (2010).
PubMed PubMed Central CAS Google Scholar
Berkhout, R. P. et al. High-quality human preimplantation embryos actively influence endometrial stromal cell migration. J. Assist. Reprod. Genet. 35 , 659–667 (2018).
Weimar, C. H. et al. Endometrial stromal cells of women with recurrent miscarriage fail to discriminate between high- and low-quality human embryos. PLoS ONE 7 , e41424 (2012).
Tapia-Pizarro, A., Argandona, F., Palomino, W. A. & Devoto, L. Human chorionic gonadotropin (hCG) modulation of TIMP1 secretion by human endometrial stromal cells facilitates extravillous trophoblast invasion in vitro. Hum. Reprod. 28 , 2215–2227 (2013).
Meinhardt, G. et al. Wingless ligand 5a is a critical regulator of placental growth and survival. Sci. Rep. 6 , 28127 (2016).
Murakami, K. et al. Deficiency in clonogenic endometrial mesenchymal stem cells in obese women with reproductive failure — a pilot study. PLoS ONE 8 , e82582 (2013).
Masuda, H., Anwar, S. S., Buhring, H. J., Rao, J. R. & Gargett, C. E. A novel marker of human endometrial mesenchymal stem-like cells. Cell Transpl. 21 , 2201–2214 (2012).
Barragan, F. et al. Human endometrial fibroblasts derived from mesenchymal progenitors inherit progesterone resistance and acquire an inflammatory phenotype in the endometrial niche in endometriosis. Biol. Reprod. 94 , 118 (2016).
Garrido-Gomez, T. et al. Defective decidualization during and after severe preeclampsia reveals a possible maternal contribution to the etiology. Proc. Natl Acad. Sci. USA 114 , E8468–E8477 (2017).
Lucas, E. S. et al. Loss of endometrial plasticity in recurrent pregnancy loss. Stem Cells 34 , 346–356 (2016).
Brighton, P. J. et al. Clearance of senescent decidual cells by uterine natural killer cells in cycling human endometrium. eLife 6 , e31274 (2017).
Brosens, J. J. et al. Uterine selection of human embryos at implantation. Sci. Rep. 4 , 3894 (2014).
Teklenburg, G. et al. Natural selection of human embryos: decidualizing endometrial stromal cells serve as sensors of embryo quality upon implantation. PLoS ONE 5 , e10258 (2010).
Nadkarni, S. et al. Neutrophils induce proangiogenic T cells with a regulatory phenotype in pregnancy. Proc. Natl Acad. Sci. USA 113 , E8415–E8424 (2016).
Amsalem, H. et al. Identification of a novel neutrophil population: proangiogenic granulocytes in second-trimester human decidua. J. Immunol. 193 , 3070–3079 (2014).
Robertson, S. A. et al. Therapeutic potential of regulatory T cells in preeclampsia — opportunities and challenges. Front. Immunol. 10 , 478 (2019).
Kelleher, A. M., DeMayo, F. J. & Spencer, T. E. Uterine glands: developmental biology and functional roles in pregnancy. Endocr. Rev. 40 , 1424–1445 (2019).
Burton, G. J. & Jauniaux, E. The cytotrophoblastic shell and complications of pregnancy. Placenta 60 , 134–139 (2017).
Moser, G., Weiss, G., Gauster, M., Sundl, M. & Huppertz, B. Evidence from the very beginning: endoglandular trophoblasts penetrate and replace uterine glands in situ and in vitro. Hum. Reprod. 30 , 2747–2757 (2015).
Pijnenborg, R., Vercruysse, L. & Hanssens, M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta 27 , 939–958 (2006).
Weiss, G., Sundl, M., Glasner, A., Huppertz, B. & Moser, G. The trophoblast plug during early pregnancy: a deeper insight. Histochem. Cell Biol. 146 , 749–756 (2016).
Rodesch, F., Simon, P., Donner, C. & Jauniaux, E. Oxygen measurements in endometrial and trophoblastic tissues during early pregnancy. Obstet. Gynecol. 80 , 283–285 (1992).
Burton, G. J., Jauniaux, E. & Murray, A. J. Oxygen and placental development; parallels and differences with tumour biology. Placenta 56 , 14–18 (2017).
Foidart, J. M., Hustin, J., Dubois, M. & Schaaps, J. P. The human placenta becomes haemochorial at the 13th week of pregnancy. Int. J. Dev. Biol. 36 , 451–453 (1992).
Okae, H. et al. Derivation of human trophoblast stem cells. Cell Stem Cell 22 , 50–63.e6 (2018).
Haider, S. et al. Self-renewing trophoblast organoids recapitulate the developmental program of the early human placenta. Stem Cell Rep. 11 , 537–551 (2018).
CAS Google Scholar
Turco, M. Y. et al. Trophoblast organoids as a model for maternal-fetal interactions during human placentation. Nature 564 , 263–267 (2018).
Sheridan, M. A. et al. Early onset preeclampsia in a model for human placental trophoblast. Proc. Natl Acad. Sci. USA 116 , 4336–4345 (2019).
Moser, G. et al. Extravillous trophoblasts invade more than uterine arteries: evidence for the invasion of uterine veins. Histochem. Cell Biol. 147 , 353–366 (2017).
Rai, A. & Cross, J. C. Development of the hemochorial maternal vascular spaces in the placenta through endothelial and vasculogenic mimicry. Dev. Biol. 387 , 131–141 (2014).
Pollheimer, J., Vondra, S., Baltayeva, J., Beristain, A. G. & Knofler, M. Regulation of placental extravillous trophoblasts by the maternal uterine environment. Front. Immunol. 9 , 2597 (2018).
Paparini, D. E. et al. Vasoactive intestinal peptide shapes first-trimester placenta trophoblast, vascular, and immune cell cooperation. Br. J. Pharmacol. 176 , 964–980 (2019).
Lacey, H., Haigh, T., Westwood, M. & Aplin, J. D. Mesenchymally-derived insulin-like growth factor 1 provides a paracrine stimulus for trophoblast migration. BMC Dev. Biol. 2 , 5 (2002).
Smith, S. D., Dunk, C. E., Aplin, J. D., Harris, L. K. & Jones, R. L. Evidence for immune cell involvement in decidual spiral arteriole remodeling in early human pregnancy. Am. J. Pathol. 174 , 1959–1971 (2009).
Choudhury, R. H. et al. Extravillous trophoblast and endothelial cell crosstalk mediates leukocyte infiltration to the early remodeling decidual spiral arteriole wall. J. Immunol. 198 , 4115–4128 (2017).
Smith, S. D. et al. Changes in vascular extracellular matrix composition during decidual spiral arteriole remodeling in early human pregnancy. Histol. Histopathol. 31 , 557–571 (2016).
Choudhury, R. H. et al. Decidual leucocytes infiltrating human spiral arterioles are rich source of matrix metalloproteinases and degrade extracellular matrix in vitro and in situ. Am. J. Reprod. Immunol. 81 , e13054 (2019).
Harris, L. K. et al. Trophoblast- and vascular smooth muscle cell-derived MMP-12 mediates elastolysis during uterine spiral artery remodeling. Am. J. Pathol. 177 , 2103–2115 (2010).
Harris, L. K. et al. Invasive trophoblasts stimulate vascular smooth muscle cell apoptosis by a fas ligand-dependent mechanism. Am. J. Pathol. 169 , 1863–1874 (2006).
Keogh, R. J. et al. Fetal-derived trophoblast use the apoptotic cytokine tumor necrosis factor-alpha-related apoptosis-inducing ligand to induce smooth muscle cell death. Circ. Res. 100 , 834–841 (2007).
Dickey, R. P. & Hower, J. F. Ultrasonographic features of uterine blood flow during the first 16 weeks of pregnancy. Hum. Reprod. 10 , 2448–2452 (1995).
Roberts, V. H. J. et al. Early first trimester uteroplacental flow and the progressive disintegration of spiral artery plugs: new insights from contrast-enhanced ultrasound and tissue histopathology. Hum. Reprod. 32 , 2382–2393 (2017).
Hempstock, J. et al. Intralobular differences in antioxidant enzyme expression and activity reflect the pattern of maternal arterial blood flow within the human placenta. Placenta 24 , 517–523 (2003).
Jauniaux, E., Hempstock, J., Greenwold, N. & Burton, G. J. Trophoblastic oxidative stress in relation to temporal and regional differences in maternal placental blood flow in normal and abnormal early pregnancies. Am. J. Pathol. 162 , 115–125 (2003).
Konje, J. C., Kaufmann, P., Bell, S. C. & Taylor, D. J. A longitudinal study of quantitative uterine blood flow with the use of color power angiography in appropriate for gestational age pregnancies. Am. J. Obstet. Gynecol. 185 , 608–613 (2001).
Collins, S. L., Grant, D., Black, R. S., Vellayan, M. & Impey, L. Abdominal pregnancy: a perfusion confusion? Placenta 32 , 793–795 (2011).
Burke, S. D. et al. Spiral arterial remodeling is not essential for normal blood pressure regulation in pregnant mice. Hypertension 55 , 729–737 (2010).
Small, H. Y. et al. Abnormal uterine artery remodelling in the stroke prone spontaneously hypertensive rat. Placenta 37 , 34–44 (2016).
Small, H. Y. et al. Role of tumor necrosis factor-alpha and natural killer cells in uterine artery function and pregnancy outcome in the stroke-prone spontaneously hypertensive rat. Hypertension 68 , 1298–1307 (2016).
Care, A. S. et al. Reduction in regulatory T cells in early pregnancy causes uterine artery dysfunction in mice. Hypertension 72 , 177–187 (2018).
Ellery, P. M., Cindrova-Davies, T., Jauniaux, E., Ferguson-Smith, A. C. & Burton, G. J. Evidence for transcriptional activity in the syncytiotrophoblast of the human placenta. Placenta 30 , 329–334 (2009).
Chan, K. C. et al. Second generation noninvasive fetal genome analysis reveals de novo mutations, single-base parental inheritance, and preferred DNA ends. Proc. Natl Acad. Sci. USA 113 , E8159–E8168 (2016).
Aplin, J. D. et al. Hemangioblastic foci in human first trimester placenta: distribution and gestational profile. Placenta 36 , 1069–1077 (2015).
Burton, G. J. & Jauniaux, E. Development of the human placenta and fetal heart: synergic or independent? Front. Physiol. 9 , 373 (2018).
Burton, G. J., Charnock-Jones, D. S. & Jauniaux, E. Regulation of vascular growth and function in the human placenta. Reproduction 138 , 895–902 (2009).
Brosens, I., Pijnenborg, R., Vercruysse, L. & Romero, R. The “great obstetrical syndromes” are associated with disorders of deep placentation. Am. J. Obstet. Gynecol. 204 , 193–201 (2011).
Burton, G. J. & Jauniaux, E. Pathophysiology of placental-derived fetal growth restriction. Am. J. Obstet. Gynecol. 218 , S745–S761 (2018).
Brosens, J. J., Pijnenborg, R. & Brosens, I. A. The myometrial junctional zone spiral arteries in normal and abnormal pregnancies: a review of the literature. Am. J. Obstet. Gynecol. 187 , 1416–1423 (2002).
Jauniaux, E., Collins, S. & Burton, G. J. Placenta accreta spectrum: pathophysiology and evidence-based anatomy for prenatal ultrasound imaging. Am. J. Obstet. Gynecol. 218 , 75–87 (2018).
Verburg, P. E. et al. Peripheral maternal haemodynamics across pregnancy in hypertensive disorders of pregnancy. Pregnancy Hypertens. 16 , 89–96 (2019).
Chaiworapongsa, T., Chaemsaithong, P., Yeo, L. & Romero, R. Pre-eclampsia part 1: current understanding of its pathophysiology. Nat. Rev. Nephrol. 10 , 466–480 (2014).
Crovetto, F. et al. First-trimester screening for early and late small-for-gestational-age neonates using maternal serum biochemistry, blood pressure and uterine artery Doppler. Ultrasound Obstet. Gynecol. 43 , 34–40 (2014).
Bartsch, E., Medcalf, K. E., Park, A. L., Ray, J. G. & High Risk of Pre-eclampsia Identification Group. Clinical risk factors for pre-eclampsia determined in early pregnancy: systematic review and meta-analysis of large cohort studies. BMJ 353 , i1753 (2016).
Redline, R. W. & Patterson, P. Pre-eclampsia is associated with an excess of proliferative immature intermediate trophoblast. Hum. Pathol. 26 , 594–600 (1995).
Oudejans, C. B. et al. Susceptibility allele-specific loss of miR-1324-mediated silencing of the INO80B chromatin-assembly complex gene in pre-eclampsia. Hum. Mol. Genet. 24 , 118–127 (2015).
Visser, A., Beijer, M., Oudejans, C. B. M. & van Dijk, M. The effect of maternal NODAL on STOX1 expression in extravillous trophoblasts is mediated by IGF1. PLoS ONE 13 , e0202190 (2018).
Rolfo, A. et al. Abnormalities in oxygen sensing define early and late onset preeclampsia as distinct pathologies. PLoS ONE 5 , e13288 (2010).
Lv, S. et al. Impaired decidualization caused by downregulation of circadian clock gene BMAL1 contributes to human recurrent miscarriage. Biol. Reprod. 101 , 138–147 (2019).
Dunk, C. et al. Failure of decidualization and maternal immune tolerance underlies uterovascular resistance in intra uterine growth restriction. Front. Endocrinol. 10 , 160 (2019).
Zhou, Y. et al. Reversal of gene dysregulation in cultured cytotrophoblasts reveals possible causes of preeclampsia. J. Clin. Invest. 123 , 2862–2872 (2013).
Moffett, A., Chazara, O., Colucci, F. & Johnson, M. H. Variation of maternal KIR and fetal HLA-C genes in reproductive failure: too early for clinical intervention. Reprod. Biomed. Online 33 , 763–769 (2016).
Roth, C. J. et al. Dynamic modeling of uteroplacental blood flow in IUGR indicates vortices and elevated pressure in the intervillous space — a pilot study. Sci. Rep. 7 , 40771 (2017).
Burton, G. J., Jauniaux, E. & Charnock-Jones, D. S. The influence of the intrauterine environment on human placental development. Int. J. Dev. Biol. 54 , 303–312 (2010).
Burton, G. J., Yung, H. W. & Murray, A. J. Mitochondrial–endoplasmic reticulum interactions in the trophoblast: stress and senescence. Placenta 52 , 146–155 (2017).
Sultana, Z., Maiti, K., Dedman, L. & Smith, R. Is there a role for placental senescence in the genesis of obstetric complications and fetal growth restriction? Am. J. Obstet. Gynecol. 218 , S762–S773 (2018).
Hutchinson, E. S. et al. Utero-placental haemodynamics in the pathogenesis of pre-eclampsia. Placenta 30 , 634–641 (2009).
Robinson, N. J., Baker, P. N., Jones, C. J. & Aplin, J. D. A role for tissue transglutaminase in stabilization of membrane-cytoskeletal particles shed from the human placenta. Biol. Reprod. 77 , 648–657 (2007).
Kaya, B. et al. Proliferation of trophoblasts and Ki67 expression in preeclampsia. Arch. Gynecol. Obstet. 291 , 1041–1046 (2015).
McGinnis, R. et al. Variants in the fetal genome near FLT1 are associated with risk of preeclampsia. Nat. Genet. 49 , 1255–1260 (2017).
Roberts, J. M. & Escudero, C. The placenta in preeclampsia. Pregnancy Hypertens. 2 , 72–83 (2012).
Redline, R. W. Placental pathology: a systematic approach with clinical correlations. Placenta 29 , S86–91 (2008).
Leavey, K., Cox, B. J., Cargill, Y. & Grynspan, D. Recurrent placental transcriptional profile with a different histological and clinical presentation: a case report. Pediatr. Dev. Pathol. 22 , 584–589 (2019).
Robertson, W. B., Brosens, I. & Dixon, G. Uteroplacental vascular pathology. Eur. J. Obstet. Gynecol. Reprod. Biol. 5 , 47–65 (1975).
Kuzmina, I. Y., Hubina-Vakulik, G. I. & Burton, G. J. Placental morphometry and Doppler flow velocimetry in cases of chronic human fetal hypoxia. Eur. J. Obstet. Gynecol. Reprod. Biol. 120 , 139–145 (2005).
Ong, S. S., Baker, P. N., Mayhew, T. M. & Dunn, W. R. Remodeling of myometrial radial arteries in preeclampsia. Am. J. Obstet. Gynecol. 192 , 572–579 (2005).
Leavey, K. et al. Unsupervised placental gene expression profiling identifies clinically relevant subclasses of human preeclampsia. Hypertension 68 , 137–147 (2016).
Kingdom, J. C., Audette, M. C., Hobson, S. R., Windrim, R. C. & Morgen, E. A placenta clinic approach to the diagnosis and management of fetal growth restriction. Am. J. Obstet. Gynecol. 218 , S803–S817 (2018).
Gibbs, I. et al. Placental transcriptional and histologic subtypes of normotensive fetal growth restriction are comparable to preeclampsia. Am. J. Obstet. Gynecol. 220 , 110.e1–110.e21 (2018).
Myers, J. E., Green, M. & Chappell, L. C. Why is the search for pre-eclampsia prevention so elusive? BMJ 362 , k3536 (2018).
Rolnik, D. L. et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N. Engl. J. Med. 377 , 613–622 (2017).
Sibley, C. P. Treating the dysfunctional placenta. J. Endocrinol. 234 , R81–R97 (2017).
Winterhager, E. & Gellhaus, A. Transplacental nutrient transport mechanisms of intrauterine growth restriction in rodent models and humans. Front. Physiol. 8 , 951 (2017).
Kingdom, J. C. & Kaufmann, P. Oxygen and placental villous development: origins of fetal hypoxia. Placenta 18 , 613–621 (1997).
Junaid, T. O., Bradley, R. S., Lewis, R. M., Aplin, J. D. & Johnstone, E. D. Whole organ vascular casting and microCT examination of the human placental vascular tree reveals novel alterations associated with pregnancy disease. Sci. Rep. 7 , 4144 (2017).
Chen, C. P., Bajoria, R. & Aplin, J. D. Decreased vascularization and cell proliferation in placentas of intrauterine growth-restricted fetuses with abnormal umbilical artery flow velocity waveforms. Am. J. Obstet. Gynecol. 187 , 764–769 (2002).
Junaid, T. O., Brownbill, P., Chalmers, N., Johnstone, E. D. & Aplin, J. D. Fetoplacental vascular alterations associated with fetal growth restriction. Placenta 35 , 808–815 (2014).
Hayward, C. E. et al. Placental adaptation: what can we learn from birthweight:placental weight ratio? Front. Physiol. 7 , 28 (2016).
Glazier, J. D. et al. Association between the activity of the system A amino acid transporter in the microvillous plasma membrane of the human placenta and severity of fetal compromise in intrauterine growth restriction. Pediatr. Res. 42 , 514–519 (1997).
Mayhew, T. M. Fetoplacental angiogenesis during gestation is biphasic, longitudinal and occurs by proliferation and remodelling of vascular endothelial cells. Placenta 23 , 742–750 (2002).
Krebs, C. et al. Intrauterine growth restriction with absent end-diastolic flow velocity in the umbilical artery is associated with maldevelopment of the placental terminal villous tree. Am. J. Obstet. Gynecol. 175 , 1534–1542 (1996).
Mills, T. A., Wareing, M., Bugg, G. J., Greenwood, S. L. & Baker, P. N. Chorionic plate artery function and Doppler indices in normal pregnancy and intrauterine growth restriction. Eur. J. Clin. Invest. 35 , 758–764 (2005).
Lu, L., Kingdom, J., Burton, G. J. & Cindrova-Davies, T. Placental stem villus arterial remodeling associated with reduced hydrogen sulfide synthesis contributes to human fetal growth restriction. Am. J. Pathol. 187 , 908–920 (2017).
Cindrova-Davies, T. et al. Reduced cystathionine gamma-lyase and increased miR-21 expression are associated with increased vascular resistance in growth-restricted pregnancies: hydrogen sulfide as a placental vasodilator. Am. J. Pathol. 182 , 1448–1458 (2013).
Khalil, A. & Thilaganathan, B. Role of uteroplacental and fetal Doppler in identifying fetal growth restriction at term. Best Pract. Res. Clin. Obstet. Gynaecol. 38 , 38–47 (2017).
Gordijn, S. J. et al. Consensus definition of fetal growth restriction: a Delphi procedure. Ultrasound Obstet. Gynecol. 48 , 333–339 (2016).
Simcox, L. E., Myers, J. E., Cole, T. J. & Johnstone, E. D. Fractional fetal thigh volume in the prediction of normal and abnormal fetal growth during the third trimester of pregnancy. Am. J. Obstet. Gynecol. 217 , 453 e451–453.e12 (2017).
Zhu, M. Y. et al. The hemodynamics of late-onset intrauterine growth restriction by MRI. Am. J. Obstet. Gynecol. 214 , 367.e1–367.e17 (2016).
Ingram, E., Morris, D., Naish, J., Myers, J. & Johnstone, E. MR imaging measurements of altered placental oxygenation in pregnancies complicated by fetal growth restriction. Radiology 285 , 953–960 (2017).
Nye, G. A. et al. Human placental oxygenation in late gestation: experimental and theoretical approaches. J. Physiol. 596 , 5523–5534 (2018).
Zamudio, S. et al. Hypoglycemia and the origin of hypoxia-induced reduction in human fetal growth. PLoS ONE 5 , e8551 (2010).
Monkley, S. J., Delaney, S. J., Pennisi, D. J., Christiansen, J. H. & Wainwright, B. J. Targeted disruption of the Wnt2 gene results in placentation defects. Development 122 , 3343–3353 (1996).
Cureton, N. et al. Selective targeting of a novel vasodilator to the uterine vasculature to treat impaired uteroplacental perfusion in pregnancy. Theranostics 7 , 3715–3731 (2017).
Redline, R. W. Classification of placental lesions. Am. J. Obstet. Gynecol. 213 , S21–S28 (2015).
Metzger, R. J., Klein, O. D., Martin, G. R. & Krasnow, M. A. The branching programme of mouse lung development. Nature 453 , 745–750 (2008).
Gaccioli, F., Aye, I., Sovio, U., Charnock-Jones, D. S. & Smith, G. C. S. Screening for fetal growth restriction using fetal biometry combined with maternal biomarkers. Am. J. Obstet. Gynecol. 218 , S725–S737 (2018).
Ilekis, J. V. et al. Placental origins of adverse pregnancy outcomes: potential molecular targets: an executive workshop summary of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Am. J. Obstet. Gynecol. 215 , S1–S46 (2016).
Sahraravand, M., Jarvela, I. Y., Laitinen, P., Tekay, A. H. & Ryynanen, M. The secretion of PAPP-A, ADAM12, and PP13 correlates with the size of the placenta for the first month of pregnancy. Placenta 32 , 999–1003 (2011).
Morris, R. K. et al. Serum screening with Down’s syndrome markers to predict pre-eclampsia and small for gestational age: systematic review and meta-analysis. BMC Pregnancy Childbirth 8 , 33 (2008).
Hui, D. et al. Combinations of maternal serum markers to predict preeclampsia, small for gestational age, and stillbirth: a systematic review. J. Obstet. Gynaecol. Can. 34 , 142–153 (2012).
Townsend, R. et al. Prediction of pre-eclampsia: review of reviews. Ultrasound Obstet. Gynecol. 54 , 16–27 (2018).
Wu, P. et al. Early pregnancy biomarkers in pre-eclampsia: a systematic review and meta-analysis. Int. J. Mol. Sci. 16 , 23035–23056 (2015).
Giguere, Y. et al. Combining biochemical and ultrasonographic markers in predicting preeclampsia: a systematic review. Clin. Chem. 56 , 361–375 (2010).
Zhu, X. L., Wang, J., Jiang, R. Z. & Teng, Y. C. Pulsatility index in combination with biomarkers or mean arterial pressure for the prediction of pre-eclampsia: systematic literature review and meta-analysis. Ann. Med. 47 , 414–422 (2015).
Kuc, S. et al. Evaluation of 7 serum biomarkers and uterine artery Doppler ultrasound for first-trimester prediction of preeclampsia: a systematic review. Obstet. Gynecol. Surv. 66 , 225–239 (2011).
Ray, J. G., Huang, T., Meschino, W. S., Cohen, E. & Park, A. L. Prenatal biochemical screening and long term risk of maternal cardiovascular disease: population based cohort study. BMJ 362 , k2739 (2018).
Ormesher, L. et al. A clinical evaluation of placental growth factor in routine practice in high-risk women presenting with suspected pre-eclampsia and/or fetal growth restriction. Pregnancy Hypertens. 14 , 234–239 (2018).
Birdir, C. et al. Predictive value of sFlt-1, PlGF, sFlt-1/PlGF ratio and PAPP-A for late-onset preeclampsia and IUGR between 32 and 37weeks of pregnancy. Pregnancy Hypertens. 12 , 124–128 (2018).
Zhou, Y. et al. Vascular endothelial growth factor ligands and receptors that regulate human cytotrophoblast survival are dysregulated in severe preeclampsia and hemolysis, elevated liver enzymes, and low platelets syndrome. Am. J. Pathol. 160 , 1405–1423 (2002).
Bushway, M. E. et al. Morphological and phenotypic analyses of the human placenta using whole mount immunofluorescence. Biol. Reprod. 90 , 110 (2014).
De Falco, S. The discovery of placenta growth factor and its biological activity. Exp. Mol. Med. 44 , 1–9 (2012).
Carmeliet, P. et al. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat. Med. 7 , 575–583 (2001).
Luna, R. L. et al. Placental growth factor deficiency is associated with impaired cerebral vascular development in mice. Mol. Hum. Reprod. 22 , 130–142 (2016).
Matsui, M. et al. Placental growth factor as a predictor of cardiovascular events in patients with CKD from the NARA-CKD Study. J. Am. Soc. Nephrol. 26 , 2871–2881 (2015).
Dewerchin, M. & Carmeliet, P. Placental growth factor in cancer. Expert. Opin. Ther. Targets 18 , 1339–1354 (2014).
Maynard, S. E. et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Invest. 111 , 649–658 (2003).
Kendall, R. L., Wang, G. & Thomas, K. A. Identification of a natural soluble form of the vascular endothelial growth factor receptor, FLT-1, and its heterodimerization with KDR. Biochem. Biophys. Res. Commun. 226 , 324–328 (1996).
Sela, S. et al. A novel human-specific soluble vascular endothelial growth factor receptor 1: cell-type-specific splicing and implications to vascular endothelial growth factor homeostasis and preeclampsia. Circ. Res. 102 , 1566–1574 (2008).
Cebe-Suarez, S., Zehnder-Fjallman, A. & Ballmer-Hofer, K. The role of VEGF receptors in angiogenesis; complex partnerships. Cell Mol. Life Sci. 63 , 601–615 (2006).
Rana, S. et al. Angiogenic factors and the risk of adverse outcomes in women with suspected preeclampsia. Circulation 125 , 911–919 (2012).
Levine, R. J. et al. Circulating angiogenic factors and the risk of preeclampsia. N. Engl. J. Med. 350 , 672–683 (2004).
Kumasawa, K. et al. Pravastatin induces placental growth factor (PGF) and ameliorates preeclampsia in a mouse model. Proc. Natl Acad. Sci. USA 108 , 1451–1455 (2011).
Lu, F. et al. The effect of over-expression of sFlt-1 on blood pressure and the occurrence of other manifestations of preeclampsia in unrestrained conscious pregnant mice. Am. J. Obstet. Gynecol. 196 , 396.e1–396.e7 (2007).
Sugimoto, H. et al. Neutralization of circulating vascular endothelial growth factor (VEGF) by anti-VEGF antibodies and soluble VEGF receptor 1 (sFlt-1) induces proteinuria. J. Biol. Chem. 278 , 12605–12608 (2003).
Bergmann, A. et al. Reduction of circulating soluble Flt-1 alleviates preeclampsia-like symptoms in a mouse model. J. Cell Mol. Med. 14 , 1857–1867 (2010).
Vogtmann, R. et al. Human sFLT1 leads to severe changes in placental differentiation and vascularization in a transgenic hsFLT1/rtTA FGR mouse model. Front. Endocrinol. 10 , 165 (2019).
Gilbert, J. S. et al. Recombinant vascular endothelial growth factor 121 infusion lowers blood pressure and improves renal function in rats with placental ischemia-induced hypertension. Hypertension 55 , 380–385 (2010).
Li, Z. et al. Recombinant vascular endothelial growth factor 121 attenuates hypertension and improves kidney damage in a rat model of preeclampsia. Hypertension 50 , 686–692 (2007).
Thadhani, R. et al. Removal of soluble Fms-like tyrosine kinase-1 by dextran sulfate apheresis in preeclampsia. J. Am. Soc. Nephrol. 27 , 903–913 (2016).
Thadhani, R. et al. Pilot study of extracorporeal removal of soluble fms-like tyrosine kinase 1 in preeclampsia. Circulation 124 , 940–950 (2011).
Karumanchi, S. A. Angiogenic factors in preeclampsia: from diagnosis to therapy. Hypertension 67 , 1072–1079 (2016).
Zeisler, H. et al. Predictive value of the sFlt-1:PlGF ratio in women with suspected preeclampsia. N. Engl. J. Med. 374 , 13–22 (2016).
CAS PubMed Google Scholar
Chappell, L. C. et al. Diagnostic accuracy of placental growth factor in women with suspected preeclampsia: a prospective multicenter study. Circulation 128 , 2121–2131 (2013).
National Institute for Health and Care Excellence. PlGF-based testing to help diagnose suspected pre-eclampsia (Triage PlGF test, Elecsys immunoassay sFlt-1/PlGF ratio, DELFIA Xpress PlGF 1-2-3 test, and BRAHMS sFlt-1 Kryptor/BRAHMS PlGF plus Kryptor PE ratio) (NICE, 2016).
Benton, S. J. et al. Can placental growth factor in maternal circulation identify fetuses with placental intrauterine growth restriction? Am. J. Obstet. Gynecol. 206 , 163.e1–163.e7 (2012).
Hoeller, A. et al. Placental expression of sFlt-1 and PlGF in early preeclampsia vs. early IUGR vs. age-matched healthy pregnancies. Hypertens. Pregnancy 36 , 151–160 (2017).
Ehrlich, L. et al. Increased placental sFlt-1 but unchanged PlGF expression in late-onset preeclampsia. Hypertens. Pregnancy 36 , 175–185 (2017).
Powers, R. W. et al. Low placental growth factor across pregnancy identifies a subset of women with preterm preeclampsia: type 1 versus type 2 preeclampsia? Hypertension 60 , 239–246 (2012).
Redman, C. W. & Staff, A. C. Preeclampsia, biomarkers, syncytiotrophoblast stress, and placental capacity. Am. J. Obstet. Gynecol. 213 , S9.e1–S9.e4 (2015).
Griffin, M. et al. Predicting delivery of a small-for-gestational-age infant and adverse perinatal outcome in women with suspected pre-eclampsia. Ultrasound Obstet. Gynecol. 51 , 387–395 (2018).
Rana, S. et al. Circulating angiogenic factors and risk of adverse maternal and perinatal outcomes in twin pregnancies with suspected preeclampsia. Hypertension 60 , 451–458 (2012).
Palomaki, G. E. et al. Modeling risk for severe adverse outcomes using angiogenic factor measurements in women with suspected preterm preeclampsia. Prenat. Diagn. 35 , 386–393 (2015).
Duhig, K. E. et al. Placental growth factor testing to assess women with suspected pre-eclampsia: a multicentre, pragmatic, stepped-wedge cluster-randomised controlled trial. Lancet 393 , 1807–1818 (2019).
Colombo, M., Raposo, G. & Thery, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 30 , 255–289 (2014).
Giacomini, E. et al. Secretome of in vitro cultured human embryos contains extracellular vesicles that are uptaken by the maternal side. Sci. Rep. 7 , 5210 (2017).
Holder, B. et al. Macrophage exosomes induce placental inflammatory cytokines: a novel mode of maternal-placental messaging. Traffic 17 , 168–178 (2016).
Gemmell, C. H., Sefton, M. V. & Yeo, E. L. Platelet-derived microparticle formation involves glycoprotein IIb-IIIa. Inhibition by RGDS and a Glanzmann’s thrombasthenia defect. J. Biol. Chem. 268 , 14586–14589 (1993).
Endzelins, E. et al. Detection of circulating miRNAs: comparative analysis of extracellular vesicle-incorporated miRNAs and cell-free miRNAs in whole plasma of prostate cancer patients. BMC Cancer 17 , 730 (2017).
Théry, C. et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 8 , 1535750 (2019).
Hromadnikova, I., Kotlabova, K., Ivankova, K. & Krofta, L. First trimester screening of circulating C19MC microRNAs and the evaluation of their potential to predict the onset of preeclampsia and IUGR. PLoS ONE 12 , e0171756 (2017).
Ura, B. et al. Potential role of circulating microRNAs as early markers of preeclampsia. Taiwan. J. Obstet. Gynecol. 53 , 232–234 (2014).
Salomon, C. et al. Placental exosomes as early biomarker of preeclampsia: potential role of exosomal microRNAs across gestation. J. Clin. Endocrinol. Metab. 102 , 3182–3194 (2017).
Truong, G. et al. Oxygen tension regulates the miRNA profile and bioactivity of exosomes released from extravillous trophoblast cells — liquid biopsies for monitoring complications of pregnancy. PLoS ONE 12 , e0174514 (2017).
Wu, L. et al. Circulating microRNAs are elevated in plasma from severe preeclamptic pregnancies. Reproduction 143 , 389–397 (2012).
Qin, W., Tang, Y., Yang, N., Wei, X. & Wu, J. Potential role of circulating microRNAs as a biomarker for unexplained recurrent spontaneous abortion. Fertil. Steril. 105 , 1247–1254.e3 (2016).
Pillay, P., Maharaj, N., Moodley, J. & Mackraj, I. Placental exosomes and pre-eclampsia: maternal circulating levels in normal pregnancies and, early and late onset pre-eclamptic pregnancies. Placenta 46 , 18–25 (2016).
Miranda, J. et al. Placental exosomes profile in maternal and fetal circulation in intrauterine growth restriction — liquid biopsies to monitoring fetal growth. Placenta 64 , 34–43 (2018).
Gonzalez-Quintero, V. H. et al. Elevated plasma endothelial microparticles: preeclampsia versus gestational hypertension. Am. J. Obstet. Gynecol. 191 , 1418–1424 (2004).
Tong, M., Chen, Q., James, J. L., Stone, P. R. & Chamley, L. W. Micro- and nano-vesicles from first trimester human placentae carry Flt-1 and levels are increased in severe preeclampsia. Front. Endocrinol. 8 , 174 (2017).
Li, H. et al. Differential proteomic analysis of syncytiotrophoblast extracellular vesicles from early-onset severe preeclampsia, using 8-Plex iTRAQ labeling coupled with 2D nano LC-MS/MS. Cell Physiol. Biochem. 36 , 1116–1130 (2015).
Biro, O. et al. Various levels of circulating exosomal total-miRNA and miR-210 hypoxamiR in different forms of pregnancy hypertension. Pregnancy Hypertens. 10 , 207–212 (2017).
Holder, B. S., Tower, C. L., Jones, C. J., Aplin, J. D. & Abrahams, V. M. Heightened pro-inflammatory effect of preeclamptic placental microvesicles on peripheral blood immune cells in humans. Biol. Reprod. 86 , 103 (2012).
Tannetta, D., Collett, G., Vatish, M., Redman, C. & Sargent, I. Syncytiotrophoblast extracellular vesicles — circulating biopsies reflecting placental health. Placenta 52 , 134–138 (2016).
Shomer, E. et al. Microvesicles of women with gestational hypertension and preeclampsia affect human trophoblast fate and endothelial function. Hypertension 62 , 893–898 (2013).
Chen, Y., Huang, Y., Jiang, R. & Teng, Y. Syncytiotrophoblast-derived microparticle shedding in early-onset and late-onset severe pre-eclampsia. Int. J. Gynaecol. Obstet. 119 , 234–238 (2012).
Tarca, A. L. et al. The prediction of early preeclampsia: results from a longitudinal proteomics study. PLoS ONE 14 , e0217273 (2019).
King, A. et al. Tumor-homing peptides as tools for targeted delivery of payloads to the placenta. Sci. Adv. 2 , e1600349 (2016).
Stephenson, J. et al. Before the beginning: nutrition and lifestyle in the preconception period and its importance for future health. Lancet 391 , 1830–1841 (2018).
Qin, J., Liu, X., Sheng, X., Wang, H. & Gao, S. Assisted reproductive technology and the risk of pregnancy-related complications and adverse pregnancy outcomes in singleton pregnancies: a meta-analysis of cohort studies. Fertil. Steril. 105 , 73–85.e856 (2016).
[No authors listed]. ACOG Practice Bulletin No. 202: gestational hypertension and preeclampsia. Obstet. Gynecol. 133 , e1–e25 (2019).
National Institute for Health and Care Excellence. Hypertension in pregnancy: diagnosis and management (NICE, 2019).
Download references
Acknowledgements
We thank the co-workers in our own groups who have added to our understanding of this field. In summarizing placental development and the evidence for incomplete spiral artery remodelling in disease (especially) we regret being unable to fit in citations to the primary work of many researchers whose contributions were significant.
Author information
Authors and affiliations.
Maternal and Fetal Health Group, Manchester Academic Health Sciences Centre, St Mary’s Hospital, Manchester, UK
John D. Aplin, Jenny E. Myers & Melissa Westwood
Lydia Becker Institute of Inflammation and Immunology, The University of Manchester, Manchester, UK
You can also search for this author in PubMed Google Scholar
Contributions
J.D.A. made substantial contributions to discussion of content. J.D.A., J.E.M., K.T. and M.W. researched data for the article, wrote the article and carried out review/editing of the manuscript before submission.
Corresponding author
Correspondence to John D. Aplin .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Peer review information.
Nature Reviews Endocrinology thanks B. Huppertz, S. Karumanchi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related link
The Centre for Trophoblast Research: https://www.trophoblast.cam.ac.uk/Resources/enders
Supplementary information
Supplementary information.
Covalent epigenetic modifications to the genome that cause genes to be expressed in a parent-of-origin-specific pattern.
Tree-like projections that form the placental exchange surface, and that are the basic functional unit of the placenta, comprising an outer syncytiotrophoblast layer, inner cytotrophoblast layer and a mesenchymal core.
Transformation of endometrial stromal cells that occurs in early pregnancy
The developmental time period between early attachment and gastrulation, or the secondary villous stage in the placenta; it approximates the second week of pregnancy.
The disc-shaped, highly vascularized, fetal aspect of the placenta.
The maternal–fetal interface, where maternal blood passes directly over the outer layer of fetal cells in the placenta.
The tissue layer at the interface between the basal endometrium and the inner myometrium.
The placenta takes over from the corpus luteum as the major source of oestrogens and progesterone at about 8–9 weeks of pregnancy.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Aplin, J.D., Myers, J.E., Timms, K. et al. Tracking placental development in health and disease. Nat Rev Endocrinol 16 , 479–494 (2020). https://doi.org/10.1038/s41574-020-0372-6
Download citation
Accepted : 15 May 2020
Published : 29 June 2020
Issue Date : September 2020
DOI : https://doi.org/10.1038/s41574-020-0372-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
The influence of placenta microbiota of normal term pregnant women on immune regulation during pregnancy.
- Shangrong Fan
BMC Pregnancy and Childbirth (2024)
Single-nucleus multi-omic profiling of human placental syncytiotrophoblasts identifies cellular trajectories during pregnancy
- Meijiao Wang
- Hongmei Wang
Nature Genetics (2024)
Placental transfer and hazards of silver nanoparticles exposure during pregnancy: a review
Environmental Chemistry Letters (2024)
27-Hydroxycholesterol inhibits trophoblast fusion during placenta development by activating PI3K/AKT/mTOR signaling pathway
- Xiaoyan Zhao
- Huanling Yu
Archives of Toxicology (2024)
Proteomic studies of VEGFR2 in human placentas reveal protein associations with preeclampsia, diabetes, gravidity, and labor
- Shannon J. Ho
- Dale Chaput
- John C. M. Tsibris
Cell Communication and Signaling (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Search Menu
- Volume 165, Issue 6, June 2024 (In Progress)
- Volume 165, Issue 5, May 2024
- Advance Articles
- Basic Science Collections
- Thematic Issues
- Clinical Practice Guidelines
- Endocrine Reviews
- Endocrinology
- Journal of the Endocrine Society
- The Journal of Clinical Endocrinology & Metabolism
- JCEM Case Reports
- Molecular Endocrinology
- Endocrine Society Journals
- Author Guidelines
- Submission Site
- Open Access
- Why Publish with the Endocrine Society?
- Advertising & Corporate Services
- Reprints, ePrints, Supplements
- About Endocrinology
- Editorial Board
- Author Resources
- Reviewer Resources
- Rights & Permissions
- Other Society Publications
- Member Access
- Terms and Conditions
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Maternal metabolic adaptations in normal pregnancy, placental hormones in pregnancy, abnormal maternal metabolic adaptations in gestational diabetes, summary and conclusion, acknowledgments, additional information, placental regulation of energy homeostasis during human pregnancy.
- Article contents
- Figures & tables
- Supplementary Data
Brooke Armistead, Eugenia Johnson, Robert VanderKamp, Elzbieta Kula-Eversole, Leena Kadam, Sascha Drewlo, Hamid-Reza Kohan-Ghadr, Placental Regulation of Energy Homeostasis During Human Pregnancy, Endocrinology , Volume 161, Issue 7, July 2020, bqaa076, https://doi.org/10.1210/endocr/bqaa076
- Permissions Icon Permissions
Successful pregnancies rely on sufficient energy and nutrient supply, which require the mother to metabolically adapt to support fetal needs. The placenta has a critical role in this process, as this specialized organ produces hormones and peptides that regulate fetal and maternal metabolism. The ability for the mother to metabolically adapt to support the fetus depends on maternal prepregnancy health. Two-thirds of pregnancies in the United States involve obese or overweight women at the time of conception. This poses significant risks for the infant and mother by disrupting metabolic changes that would normally occur during pregnancy. Despite well characterized functions of placental hormones, there is scarce knowledge surrounding placental endocrine regulation of maternal metabolic trends in pathological pregnancies. In this review, we discuss current efforts to close this gap of knowledge and highlight areas where more research is needed. As the intrauterine environment predetermines the health and wellbeing of the offspring in later life, adequate metabolic control is essential for a successful pregnancy outcome. Understanding how placental hormones contribute to aberrant metabolic adaptations in pathological pregnancies may unveil disease mechanisms and provide methods for better identification and treatment. Studies discussed in this review were identified through PubMed searches between the years of 1966 to the present. We investigated studies of normal pregnancy and metabolic disorders in pregnancy that focused on energy requirements during pregnancy, endocrine regulation of glucose metabolism and insulin resistance, cholesterol and lipid metabolism, and placental hormone regulation.
Human pregnancy is an energetically demanding process and requires synchrony between the mother and fetus. Throughout gestation, maternal basal metabolic rate increases ( 1 ), which causes both the resting and total energy expenditure to increase to support fetal development and growth ( 2 ). Both fetal and placental development cause maternal energy intake and expenditure to increase each day by approximately 375 KJ (89 Kcal) in the first trimester, 1200 KJ (286 Kcal) in the second trimester, and 1950 KJ (466 Kcal) in the third trimester ( 3 ). A mother’s prepregnancy nutritional status, height, and weight determine her ability to energetically and metabolically adapt to fetal needs ( 4 ).
Placental hormones and growth factors regulate maternal metabolism to favor increased fat storage during the first and second trimester ( 5 ), representing an “anabolic” state ( 1 ). This anabolic phase is important because fetal energy demands are not met by only increasing energy intake during the third trimester ( 5 ) and, therefore, rely on fat storage that is accumulated early in the pregnancy. In the third trimester, increased lipolysis and the mobilization of fat stores occur ( 1 ), which is observed by increased blood plasma concentrations of fatty acids and glucose ( 6 ). This shift from anabolic to catabolic lipid metabolism allows lipids to be the main source for maternal energy and preserve glucose for the developing fetus ( 7 ).
Glucose metabolism
The maintenance of glucose metabolism is a key factor in a healthy pregnancy ( 7 ). The fetus is unable to undergo gluconeogenesis and it therefore relies on a supply of glucose from maternal blood plasma and the placenta ( 8 ). During the first trimester of pregnancy, maternal glucose homeostasis is regulated by several hormones, such as estrogen, insulin, and cortisol, which function to increase fat storage, decrease energy expenditure, and delay blood glucose clearance ( 9 ).
Fetal glucose demands increase around week 26 of gestation, which requires maternal basal endogenous glucose production to increase via hepatic gluconeogenesis ( 10 ). At the same time, increases in circulating insulin and decreased insulin sensitivity occur. Estrogen assists in the increased glucose production by enhancing cortisol-binding globulin production by the liver to promote gluconeogenesis ( 11 ). Despite a surge in glucose production, plasma glucose concentrations may simultaneously decrease ( 12 ), suggesting that the circulating glucose is supplied to the fetus and placenta ( 13 ). Riskin-Mashiah et al investigated normal fasting plasma glucose levels in a cohort of 7946 healthy, pregnant, hospitalized women ( 12 ). The team demonstrated that fasting glucose levels are critically maintained in order to remain constant throughout pregnancy ( 12 , 14–19 ).
Insulin resistance
Glucose metabolism is also altered by increasing insulin resistance ( 20 , 21 ), elevated plasma lipid concentrations ( 6 ), and pancreatic β-cell expansion due to maternal pancreatic islet hypertrophy ( 22 , 23 ). Estrogen and progesterone regulate insulin resistance at week 6 of pregnancy ( 22 ). Prolactin and human placental lactogen (hPL) levels peak around week 10 ( 9 ), promoting β-cell proliferation and insulin production and secretion to meet higher insulin demands and further increase insulin resistance ( 20 , 21 ). Insulin resistance continues to develop in the second trimester and peaks in the third trimester of pregnancy ( 24 ). Increased circulating progesterone, prolactin, cortisol, and hPL promote insulin resistance in adipocytes and skeletal muscles ( 24 ). High cortisol assists with the insulin resistance needed for delayed glucose clearance ( 25 ). Insulin initiates glucose uptake by binding to its receptor and through phosphorylation of the β-subunit, followed by phosphorylation of the insulin receptor substrate 1 (IRS-1) at a tyrosine residue, which is then primed for initiating signal transduction pathways ( 26 , 27 ). In normal pregnancies, there is decreased insulin phosphorylation of the insulin receptor ( 28 ), and progesterone causes decreased IRS-1 expression, further decreasing the insulin-induced translocation of glucose transporter 4 (GLUT4) to the cell membrane to dampen glucose cellular uptake ( 26 ).
In addition to hormones, the cytokine tumor necrosis factor-α (TNFα) was identified to be a potential mediator for insulin resistance during later stages in pregnancy ( 29 ). Increases in circulating levels of TNFα have been associated with insulin resistance in obesity, sepsis, muscle damage, and even aging ( 30–32 ). It is also produced by the placenta and increased levels have been reported during pregnancy pathologies, such as preeclampsia and gestational diabetes ( 33 , 34 ). In a prospective study, Kirwan et al showed that insulin resistance during late gestation is significantly correlated with changes in circulating TNFα, irrespective of fat mass ( 29 ).
Lipid metabolism
Pregnancy initiates substantial changes in maternal lipid metabolism that are supportive of fetal growth and development. The first and second trimesters are collectively referred to as the “anabolic phase” of pregnancy whereby increased estrogen, progesterone, and insulin concentrations favor lipid deposition and inhibit lipolysis ( 7 ). Changes in hormones like progesterone, growth hormone (GH), prolactin, and others increase maternal appetite to increase extra body fat ( 6 ). On average, pregnant women with a healthy BMI (body mass index; 18.5–24.9) gain 25–35 lbs of body weight throughout the entirety of pregnancy ( 35 ).
During the first 6 weeks of gestation, plasma lipid levels decrease ( 6 ). Increased insulin sensitivity at this time promotes fatty-acid (FA) synthesis and increases lipoprotein lipase, which facilitates the cellular uptake of circulating triacylglycerides (TAGs) ( 6 ). By week 10, higher levels of FAs, TAGs, cholesterol, and phospholipids are observed in the blood and this continues through the third trimester ( 6 ). At 30 weeks of gestation, a metabolic shift to a catabolic state occurs as lipids are used for maternal energy source, while glucose and amino acids are conserved for the fetus ( 7 , 36 ). These changes are driven by insulin resistance, which promote lipid catabolism and decrease lipoprotein lipase levels during the third trimester ( 6 , 36 ). Increased FAs are released and metabolized into TAGs before being absorbed by the syncytial layer of the placenta ( 6 , 37 ).
Cholesterol is a major component of circulating lipids and is continuously recycled and delivered to sites throughout the body, including the placenta ( 6 ). The placenta utilizes cholesterol to synthesize approximately 400–500 mg of steroid hormones daily ( 6 ). Cholesterol is also important for placental oxidation and placental membrane formation ( 6 ). At week 12 of gestation, high density lipoprotein (HDL) cholesterol increases in response to estrogen and remains elevated throughout the pregnancy ( 6 ). TAGs are elevated by approximately 2-fold, and total and low density lipoprotein (LDL) cholesterol are increased by 30% to 50% in the third trimester ( 6 ).
The human placenta has many functions: it regulates temperature, serves as a protective barrier against the maternal microenvironment and infection, helps to establish immunologic tolerance of the fetus, and provides exchange of gases, nutrients, and waste ( 38 , 39 ). Among the many functions of the human placenta, the numerous hormones produced by this organ have significant influences on establishing and maintaining a healthy pregnancy ( 40 ). Altering energy homeostasis in pregnancy can damage the placenta, leading to inadequate function and subsequent pregnancy complications, which is observed in gestational diabetes mellitus (GDM). In the following sections we provide an overview of hormones and growth factors secreted by the placenta that assist in regulating metabolism throughout pregnancy.
Placental growth hormone
The placental growth hormone (PGH) is a growth hormone variant produced by the placenta, which regulates maternal gluconeogenesis and lipolysis to modulate maternal adaptations during pregnancy ( 41 ). Placental growth hormone replaces pituitary GH in the maternal circulation and its concentrations increase in maternal circulation throughout pregnancy until term ( 42 ). Placental growth hormone functions as an insulin antagonist and mediates insulin resistance by directly modulating insulin-like growth factor 1 (IGF-1) ( 36 , 43 ) and also initates increased growth of maternal tissues ( 24 ). Placental growth hormone may act independently or dependently through IGF-1 to increase nutrient supply for the fetus ( 41 ).
Placental growth hormone is predominantly expressed in and secreted from placental syncytiotrophoblasts and, to a lesser extent, in extravillous trophoblasts ( 43 ). Placental growth hormone has a role in the placenta by acting in both a paracrine and autocrine manner to stimulate trophoblast invasion ( 44 ) and placental growth through its receptor, growth hormone receptor (GHR), on syncytiotrophoblasts ( 41 ). A study by Lacroix et al showed that PGH stimulates trophoblast invasiveness through activation of the Janus kinase-2/signal transducer and activator of the transcription factor-5 (JAK-STAT) signaling pathway ( 44 ) to initiate transcription of invasion-promoting genes ( 45 ). In a transgenic mice study, overexpression of PGH induced hyperinsulinemia, or severe insulin resistance ( 46 ). This is thought to result from maternal pancreatic β-cell expansion and a decrease in body fat, similar to conditions observed in the third trimester of human pregnancy ( 46 ).
Despite the many important functions of PGH during normal pregnancy, studies in pregnant women with diabetes have shown no correlation between changes in PGH levels and insulin levels ( 47 ). Additionally, women with deletions in the PGH gene were also reported to have pregnancies that resulted in children with normal birth weights ( 48 ). This could be explained by other hormones acting in overlapping pathways, which compensate for PGH insufficiency, such as GH or hPL ( 41 ).
Human placental lactogens
Human placental lactogens, also called chorionic somatotrophin hormone (CSH), are types of growth hormones that have several roles, including metabolic regulation by increasing maternal glucose levels, decreasing maternal glucose usage, and promoting lipolysis and insulin resistance ( 21 , 36 ). Human placental lactogen is produced by syncytiotrophoblasts and secreted into maternal–fetal circulations after the sixth week of pregnancy ( 24 ). During early gestation, hPL exhibits anabolic activity by promoting glucose uptake and incorporation of glucose into glycogen, glycerol, and FAs ( 48 ).
Human placental lactogen concentrations rise in the third trimester and become an important contributor to insulin resistance ( 9 , 24 ). During the third trimester, hPL augments lipolysis and fat mobilization, increasing free FA levels in maternal circulation ( 24 ). Human placental lactogen increases to 5000–7000 ng/ml at 32 to 35 weeks, then declines at term to approximately 20–50 ng/ml ( 49 ). Aside from its anabolic/catabolic activities, hPL indirectly controls insulin production and secretion by increasing human pancreatic β-cell replication and cell survival rates ( 50 ). Similar to PGH, women with deletions in the CSH gene experience normal pregnancy outcomes, suggesting that alterations in this hormone may not lead to pregnancy complications such as GDM ( 47 , 48 ).
Ghrelin, also known as growth hormone (GH)-releasing peptide, is a gastric-secreted acylated peptide hormone ( 51 ) that controls feeding behaviors by stimulating GH release through GH secretagogue receptors (GHSR) ( 52 ) and stimulating appetite to increase food intake ( 53 ). At the cellular level, ghrelin regulates energy balance and proliferation ( 52 ). Ghrelin also has a role in activating hepatic gluconeogenesis and inititates glucose uptake through phosphorylation of tyrosine molecules on IRS-1 ( 54 ). Ghrelin is also highly expressed in the first trimester of pregnancy by the human placenta—primarily in cytotrophoblasts and also in placental villi stroma ( 55 ). Ghrelin levels increase midpregnancy and decrease thereafter to undetectable levels in full-term human placenta ( 55 ).
The gestational stage dependent expression of ghrelin in the placenta overlaps with energy intake/expenditure requirement of the fetus. Nakahara et al used a rat model of pregnancy to show that ghrelin has a large effect on fetal growth ( 56 ). Their study shows that maternal treatment with ghrelin increased fetal birth weight, despite a restricted diet ( 56 ). This suggests that ghrelin may have physiological functions in homeostatic control of energy balance in pregnancy as well as in modulating fetal growth and development.
The role of ghrelin in the development of GDM still remains unclear. Women with GDM showed no significant differences in plasma levels of ghrelin compared to healthy pregnant women—although ghrelin mRNA was signficantly higher in the placenta of GDM women compared to healthy pregnancies ( 57 ). This suggests ghrelin may have a role in the placenta during GDM pregnancies, which needs to be further investigated. Interestingly, ghrelin knockout mice show normal fertility with no effect on growth or appetite ( 58 ). However, studies with ghrelin-receptor knockout mice revealed increased levels of IGF-1, suggesting that ghrelin-receptor signaling exerts a physiologic role in energy balance ( 58 ). Similar observations are observed in other rodent models and in humans who have deficiencies in grehlin-receptor function ( 59 ).
Leptin is a hormone characterized by its roles in food intake regulation and energy expenditure in white adipose tissue (WAT), where it is secreted in response to increased energy storage ( 60 ). Leptin is also produced in, and modulates, a wide range of cellular functions in numerous tissues and organs, including the hypothalamus ( 61 ), gastric epithelium ( 62 ), and skeletal muscle ( 63 ). Leptin has recently emerged as an important player in reproductive health, from regulating the menstrual cycle and oocyte maturation ( 64 ) to embryo implantation and development ( 65 , 66 ). Leptin expression in the placenta is regulated by exogenous 17beta-estradiol (E2) via crosstalk between estrogen receptor 1 and MAPK-PI3K signal transduction pathways ( 67 , 68 ).
Leptin suppresses the appetite of healthy, nonpregnant (NP) individuals through its receptors (LRb) ( 69 ) on the hypothalamus located in the brain ( 70 ), where it also influences secretion of thyroid hormones, sex hormones, and growth hormones ( 69 ). Leptin binding to LRb causes transphosphorylation of intracellular LRb and activates the Jak kinase family 2 (Jak2) to intiate further signaling pathways ( 69 ). Despite its role in suppressing appetite, circulating levels of leptin gradually increase throughout gestation. Ladyman et al used a rat model of pregnancy to study the effects of leptin on feeding behavior during pregnancy. In their study, they treated NP and pregnant rats with leptin at gestation days 7 and 14 and measured food intake. They showed NP and gestation day-7 pregnant rats had a reduction in food eaten; however, the leptin did not effect feed behavior for gestation day-14 rats. Their results also revealed that leptin-induced STAT3 phosphorylation was reduced in the hypothalamic nuclei of pregnant rats, which could be the mechanism behind pregnancy-induced leptin resistance ( 71 ). Additionally, decreased mRNA of leptin receptor in the hypothalamus ( 70 ) inidicates that these rats experienced resistance to leptin ( 71 ). This finding is in coordinance with another murine study by Bates et al. They show that STAT3 activation occurs through the tyrosine 1138 residue on LRb ( 72 ). They replaced the tyrosine 1138 residue with a serine residue, and this inhibited STAT3 activation, resulting in hyperphagia and obesity ( 72 ).
A similar trend is observed during human pregnancy, where leptin levels and hyperphagia simultaneously increase throughout gestation. The study by Ladyman et al suggests that a similar mechanism occurs in humans that leads to resistance to the anorexigenic effects of leptin during pregnancy ( 71 , 73 ). Leptin resistance during pregnancy is important to maintain increased energy intake to support fetal growth in the second and third trimester ( 70 ). This also contributes to adipose tissue storage in early and midpregnancy by hyperphagia to prepare for lipid mobilization during the catabolic phase of late pregnancy ( 74 ).
Besides its role in metabolism, studies identified autocrine/paracrine activities for leptin in the placenta due to its expression in placental trophoblasts and amnion cells ( 75 , 76 ). These activities include positive regulation of trophoblast differentiation, promotion of placental angiogenesis and nutrient transport, and local immunomodulation at the maternal–fetal interface ( 77 , 78 ). All such regulatory events are essential for fetal development and adequate placental function ( 77 , 78 ).
Irisin is a newly identified myokine that induces energy expenditure by converting WAT to brown adipose tissue ( 79 ). Irisin is also able to regulate glucose and lipid levels and improve insulin sensitivity ( 80 , 81 ). Irisin consists of 112 amino acids and is produced by FNDC5 (fibronectin domain-containing protein 5) cleavage. Physical activity induces expression irisin through the activation of peroxisome proliferator-activated receptor-γ co-activator 1α (PGC1α), which transcriptionally upregulates FNDC5 ( 82 ). Irisin faciliates glucose uptake in skeletal muscle cells through activation of the AMP-activated protein kinase (AMPK) 2 pathways and translocation of GLUT4 to the plasma membrane ( 82 ). Murine studies have investigated effects of overexpression of irisin and found it decreases fasting insulin levels and improves glucose tolerance during high-fat diets ( 79 ). Irisin also increases expression of uncoupling protein 1, which initiates thermogenesis in adipocytes ( 79 ). It is also hypothesized that irisin can have anti-inflammatory affects through activation of peroxisome proliterator-activated receptor-α (PPARα) ( 82 ).
Garcés et al reported that circulating irisin levels are higher in pregnant compared to NP women; its serum levels increase as gestation progresses, and its levels are significantly lower in preeclampsia ( 83 ). Further studies confirm that irisin is perturbed in other pregnancy complications, such as GDM, spontaneous preterm delivery, and intrauterine growth restriction ( 84–86 ). A study by Chen et al identified that irisin has a role in reducing oxidative stress and improving lipid metabolism in pregnancies complicated by liver dysfunction ( 87 ). Immunohistological evidence shows irisin is localized in cytotrophoblasts in the decidua and in synyctiotrophoblasts ( 82 ). Moreover, Drewlo et al recently showed that irisin may have a role in the placenta by regulating placental trophoblast differentiation in villous and extra villous cell models through activation of AMPK ( 88 ). The detailed physiological role of irisin in human pregnancy remains to be determined, although there are 2 key roles suggested for irisin during normal pregnancy: (1) to contribute to the development of normal gestational insulin resistance, and (2) to regulate body temperature ( 83 ).
Adiponectin
Adiponectin is an antidiabetic adipokine that serves important functions to regulate glucose metabolism and fatty acid oxidation ( 89 ). Adiponectin can promote pancreatic β-cell survival to prevent hepatic production of glucose ( 89 ) through AMPK mechanisms ( 28 ) and increase insulin sensitivity ( 90 ). It is produced in high amounts in lean women compared to overweight or obese NP women ( 91 ).
Adiponectin is produced in and secreted by maternal adipose tissue ( 89 ). Multiple studies described that adiponectin can also be secreted from the human placenta and that it acts in an autocrine/paracrine manner through adiponectin receptors 1 and 2 located on placental trophoblasts ( 91–93 ). However, other studies were unable to detect adiponectin expression in the term placenta ( 94–96 ) and therefore described adiponectin as an “adipose-specific secretory protein” ( 97 ), which is produced by maternal tissues during pregnancy. Depsite this, adiponectin is known to have significant roles in regulating insulin resistance and glucose homeostasis throughout pregnancy ( 98 ); it may function in a paracrine manner to increase adipocyte cell numbers, increase expression of lipid metabolism genes, and regulate local and systemic inflammation ( 97 ). As well, adiponectin decreases glucose production and lipogenesis, and its production is often decreased during unfavorable metabolic situations ( 97 ). Because of this, adiponectin is commonly used as a biomarker to understand metabolic states under certain conditions.
Gestational diabetes mellitus is a type of diabetes mellitus that develops only during pregnancy and usually disappears upon delivery. The American Diabetes Association classifies GDM as “diabetes first diagnosed in the second or third trimester of pregnancy that is not clearly either preexisting type 1 or type 2 diabetes” ( 99 ). It occurs in 15% to 20% of pregnancies and is associated with adverse outcomes, including macrosomia, neonatal hypoglycemia, and an increased rate of cesarean delivery ( 100 ). Gestational diabetes mellitus is generally characterized by maternal hyperglycemia, glucose intolerance, and high insulin resistance ( 101 , 102 ).
In preconception, the median fasting plasma glucose (FPG) for women with normal pregnancies is 81 mg/dL and this slightly decreases to 80 mg/dL, 77 mg/dL, and 76 mg/dL in the first, second, and third trimesters, respectively ( 12 ), as shown in Fig. 1 . Women are screened for GDM through a 1-step or 2-step oral glucose tolerance test (OGTT) at 24 to 28 weeks of gestation, unless they have risk factors for pregestational diabetes or hyperglycemia, in which case they will undergo OGTT at their first visit ( 99 ). The 1-step test involves a 75-gram OGTT that measures FPG, followed by blood glucose levels at 1 hour and 2 hours after glucose consumption ( 99 ). Based on this criteria, women are diagnosed with GDM if their blood glucose levels measure at or above at least 1 of the following: 92 mg/dL FPG, 180 mg/dL at 1 hour, or 153 mg/dL at 2 hours ( 99 ). The 2-step test does not require fasting and is conducted measuring glucose levels 1 hour after a 50-gram OGTT ( 99 ). If the patient measures ≥130, 135, or 140 mg/dL they are required to take a 100-gram OGTT after an 8-hour fast ( 99 ). Gestational diabetes mellitus is then diagnosed if the patient meets at least 2 of the following measurements: 95 mg/dL FPG, 180 mg/dL after 1 hour, 155 mg/dL after 2 hours, or 140 mg/dL after 3 hours ( 99 ).

Fasting plasma glucose levels in normal pregnancy and GDM. In preconception, women who later go on to have normal pregnancies exhibit a mean FPG of 81 mg/dL ( 12 ). Mean FPG levels for women with normal pregnancies slightly decrease to 80 mg/dL, 77 mg/dL, and 76 mg/dL in the first, second, and third trimesters, respectively ( 12 ). A study by Sesmilo et al showed that first trimester FPG may vary, as their cohort of 6845 women had a mean FPG of 83 with a standard deviation of ± 7.3 mg/dL ( 132 ). Of these women, 10.2% developed GDM, which showed that women with a FPG ≥ 88 mg/dL in the first trimester were 1.82 times more likely to be diagnosed with GDM in the second trimester ( 132 ). Gestational diabetes mellitus is diagnosed in the first or second trimester if the patient measures a FPG ≥ 92 mg/dL by the 1-step OGTT ( 99 ). In a study by Seabra et al, they showed that GDM patients had a significantly higher FPG (90 mg/dL) compared to women without pregnancy complications (FPG 77.8 mg/dL, P = 0.016) in the third trimester ( 133 ).
Metabolic disorders in pregnancy, like GDM, often involve aberrant lipid metabolism. Bao et al investigated triglyceride, total cholesterol, LDL cholesterol, and HDL cholesterol levels in women with GDM ( Fig. 2 ) and normal pregnancies ( Fig. 3 ) ( 103 ). High levels of triglycerides and low levels of HDL cholesterol during early pregnancy was shown to increase the risk for developing GDM later on in pregnancy ( 103 ). These data also support the fact that hyperlipidemia is associated with GDM and, in some cases, is a precursor to this condition ( 6 ).

Mean plasma lipid concentrations measured throughout GDM pregnancies. Gestational diabetes mellitus pregnancies often involve abnormal lipid concentrations throughout pregnancy. As well, infants born to mothers with GDM often have increased adipose tissue at birth. Bao et al identified that women with higher triglycerides in early pregnancy have an increased risk of developing GDM in later pregnancy ( 103 ). As well, lower HDL cholesterol in early pregnancy significantly increases the risk for developing GDM in later pregnancy ( 103 ). Changes in LDL cholesterol or total cholesterol throughout pregnancy was not shown to be significantly associated with risk of developing GDM ( 103 ). * P < 0.05 indicates significant differences in HDL, LDL cholesterol, or triglyceride concentration in GDM pregnancies compared to normal pregnancies during that gestation age ( 103 ). Image was adapted from Bao et al. Plasma concentrations of lipids during pregnancy and the risk of gestational diabetes mellitus: a longitudinal study. J Diabetes . 2018;10(6):487–495.

Mean plasma lipid concentrations measured throughout normal pregnancies. Healthy pregnancies typically show HDL cholesterol increases from 60–70 mg/dL in the late first trimester, and these levels decrease to below 60 mg/dL at the end of gestation ( 103 ). Low density lipoprotein cholesterol gradually increases across gestation from around 86–126 mg/dL ( 103 ). Triglycerides increase in the beginning of the second trimester, from around 130 mg/dL, and increase until the end of gestation to approximately 280 mg/dL ( 103 ). Total cholesterol increases from approximately 180 mg/dL at the end of the first trimester to around 230 mg/dL at the end of the third trimester ( 103 ). Image was adapted from Bao et al. Plasma concentrations of lipids during pregnancy and the risk of gestational diabetes mellitus: a longitudinal study. J Diabetes . 2018;10(6):487–495.
Maternal obesity during pregnancy can involve greater lipolysis rates, which may cause lipotoxicity and can lead to maternal endothelial dysfunction ( 37 ). Elevated estrogens and abnormally high insulin resistance may also contribute to high lipid levels during pregnancy ( 6 ). Abnormal lipid metabolism can serve as a precursor to maternal metabolic disease postpartum ( 101 , 104 ) and affect fetal birth weights, as the placenta may accumulate excessive amounts of lipids during obese pregnancies, altering delivery of FAs and TAGs to the fetus ( 37 ).
Gestational diabetes mellitus may also develop due to the dysregulation of pancreatic β-cell function against a background of insufficient insulin action (both insulin sensitivity and secretion defects) and abnormal secretion of pregnancy hormones ( 20 , 23 , 105 ). Leptin, irisin, and adiponectin are among the many placental hormones that are found to be dysregulated in GDM. In this section, we discuss their roles in GDM development.
Leptin involvement during GDM
Leptin and leptin receptor expression are found to be increased in the placenta of women with GDM, and this may result from hyperinsulemia to increase leptin levels ( 106 ). These conditions also promote increases in proinflammatory proteins, which further increase the production of leptin ( 106 ). Besides this, studies have shown that specific single nucleotide polymorphisms in the leptin gene (LEP-2548G/A) predisposes risk for developing GDM ( 106 ). Interestingly, leptin levels measured in early pregnancy have been used as predictive biomarkers for later development of GDM ( 107 ) and in pregnancies complicated by type 1 diabetes ( 108 ). Kautzky-Willer found that pregnant women have higher blood leptin levels than NP women, which peak at around 28 weeks into gestation ( 109 ). In the third trimester, leptin levels start declining and are significantly lowered postpartum in healthy pregnancies ( 109 ). While this trend is similar in women who develop GDM, leptin levels are significantly higher in GDM when compared to normal pregnancies and NP women, as shown in Fig. 4 ( 109 ). It is also reported that leptin levels remain high postpartum in women with GDM, which negatively correlates with placental function and birth weight ( 109 ).

Leptin measurements in the second trimester of pregnancy. Kautzky-Willer et al showed that there is a significant increase in plasma leptin levels in women who have GDM (24.9 ng/mL) compared to normal pregnancies (18.2 ng/mL) ( 109 ). Leptin levels during nonpregnancy are 8.2 ng/mL ( 109 ). *** P < 0.001.
A similar observation was reported by Qiu et al ( 110 ), which investigated a cohort of 823 women and measured plasma leptin in the first trimester of pregnancy. They found that women with higher leptin levels (31.0 ng/ml) had a 4.7% increased risk of developing GDM compared to women with normal or lower leptin levels (≤14.3 ng/ml) ( 110 ). Qiu et al further showed that every 10 ng/ml increase in leptin concentration increased the risk of developing GDM by 20% ( 110 ). A meta-analysis study in 2015 by Bao et al reported similar trends for leptin ( 111 ).
Interestingly, studies by Festa et al and Mosavat et al report significantly lowered leptin levels in women with GDM compared to control ( 112 , 113 ), which is opposite from Qiu et al ( 110 ). Each of these studies compared GDM women with normal pregnant women who have a comparable BMI, maternal age, gestational age, gestational weight gain, and in some cases ethnicity. The opposite results may be attributed to the sampling method, as both Festa et al and Mosavat et al used blood serum to measure leptin levels, while Qiu et al measured leptin from blood plasma. The method and time required for serum and plasma separation from whole blood is shown to have an effect on the levels of certain metabolites such as insulin, peptide C, and other factors such as vascular endothelial growth factor (VEGF) ( 114-116 ). These studies highlight the importance of potential errors introduced in the measurement of clinical samples, and future surveys of blood leptin levels during pregnancy warrant a more critical assessment of specimen collection.
Irisin involvement during GDM
Many reports describe GDM patients as having lower irisin levels compared to healthy, age-matched controls ( 117 , 118 ), as shown in Fig. 5 . A study by Seven et al measured irisin levels in pregnant women with GDM, obese without GDM, and control pregnancies and identified that women with GDM have significantly lower irisin levels, while obese non-GDM women had higher irisin levels (both compared to control pregnancies) ( 119 ). These results point out that irisin likely has different pathogenic effects in GDM and non-GDM obesity during pregnancy ( 119 , 120 ).

Irisin measurements in the first trimester of pregnancy. Erol et al showed that there is a decrease in irisin levels in women who later developed GDM (452 ng/mL) compared to normal pregnancies (752 ng/mL) ( 121 ). *** P < 0.001.
Erol et al also found that GDM patients had significantly lower irisin levels in the first trimester (453 ng/ml in GDM vs. 721 ng/ml in controls) and slightly lower levels during the second trimester (749 ng/ml in GDM vs. 757 ng/ml in controls) ( 121 ). Similarly, Ural et al showed significantly lower irisin levels in blood serum during the second trimester in a cohort of 45 women with GDM and 41 matched controls ( 122 ). However, Ural et al reported values of 1 ug/l (or 1 ng/mL) in comparison to the median value of 749 ng/ml reported by Erol et al. The specificity and sensitivity of ELISA kits used in these studies might contribute to the differences in irisin levels reported by the 2 groups. Both Erol et al and Ural et al report a positive correlation of irisin with fasting insulin levels in these women, which may suggest a role for irisin in glucose and energy metabolism during pregnancy. Moreover, studies have shown that exercise prevents fetal overgrowth ( 123 ), which often occurs during GDM pregnancies. Exercise increases irisin secretion ( 124 ) and insulin sensitivity, decreases plasma glucose concentrations, and increases angiogenic factors in the blood ( 123 ), further showing the importance of irisin during pregnancy.
Current approaches in the field of GDM aim to establish a biomarker panel for diagnosis in the first or second trimester ( 22 ). It is important to indentify women who are at a greater risk for developing GDM in later pregnancy and if their infant is at risk of developing comorbidities as a result ( 22 ). Wang et al suggestes that irisin measurements in the first trimester may serve as a significant risk factor for developing GDM later in the pregnancy ( 125 ). However, a recent study by Jedrychowski et al showed that ELISA-based detection of irisin might not be sufficient and that a more sensitive technology like mass spectrophotometry might be required to correctly assess irisin levels during pregnancy ( 126 ). These results warrant a more thorough investigation into irisin detection and the mechanisms by which irisin contributes to GDM pathology before its development as a biomarker.
Adiponectin involvement in GDM
Adiponectin is known to have major roles regulating energy homeostasis. Multiple reports show decreased levels of adiponectin during GDM pregnancies ( 127–129 ). Adiponectin expression in adipocytes is negatively regulated by proinflammatory factors, like TNFα, which are highly expressed during GDM pregnancies and could explain why adiponectin is decreased in GDM ( 28 ). Using a transgenic knockout mouse model, a recent study revealed that adiponectin-deficient mice developed glucose intolerance and hyperlipidemia in the later stages of pregnancy and their offspring exhibited increased fetal weight ( 130 ). These adiponectin-deficient mice also show increased production of hepatic glucose and triglycerides and decreased β-cell mass compared to the normal pregnant mice, which are characteristic of GDM pregnancies. Interestingly, when these adiponectin knockout mice were then administered adiponectin, it reversed the glucose intolerance and prevented fetal overgrowth. These results show the high impact of adiponectin on maintaining energy homeostasis during pregnancy ( 130 ).
Maternal physiology and overall health prior to pregnancy determine the ability to metabolically adapt to support fetal development. In normal pregnancy, maternal carbohydrate and lipid metabolism and its regulation are altered with advancing gestation. These trends are characterized by a progressive decrease in maternal glucose sensitivity coinciding with increasing lipolysis, indicating a shift from an anabolic state to catabolic state in late pregnancy. Numerous placental hormones—including placental growth hormone, placental lactogens, leptin, ghrelin, irisin, and adiponectin—regulate glucose and lipid metabolism, as well as the insulin sensitivity/resistance that occurs throughout anabolic and catabolic states of pregnancy ( 36 ).
Preconception maternal obesity is a risk factor for placental dysfunction, which drives aberrant metabolic control ( 37 ). Altering the normal trends of maternal energy homeostasis can lead to pregnancy-related metabolic disease, like GDM. This is concerning, as two-thirds of pregnancies in the United States involve overweight or obese women, and conditions like GDM can have lasting effects on maternal–fetal health ( 37 ). Gestational diabetes mellitus leads to insufficient insulin levels and aberrant blood glucose concentrations that impair cognitive, neurological, and endocrine development in the fetus, which negatively impact the offspring in later life. Besides maternal–fetal comorbidities, these pregnancy complications pose a large economic burden accounting for approximately 1.6 billion dollars per year in United States ( 131 ).
It is also important to acknowledge that molecular pathways mediated by placental hormones driving the changes in metabolism during normal pregnancy and GDM are not completely elucidated. This is further complicated by the unavailability of sensitive methods to detect targets like irisin. Understanding the mechanisms that regulate metabolic trends during pregnancy is critical for better identification, treatment, and prevention of metabolic-related pregnancy complications.
The placenta may additionally secrete yet undetected factors that might contribute to this process. Future research should focus on determining accurate levels of known factors and global screening approaches that detect novel factors might be beneficial. It is also crucial to determine the full extent to which aberrant hormone expression may be harmful to the placenta, including the regulation of placental function at the molecular level. Finally, research into means of damping effects of abnormal metabolic trends during metabolic-related pregnancy complications would greatly impact the current means of treatment to alleviate these complications.
We thank Dr. Brian Knight’s team for editoral assistance and Ken Provost from Xavier Studio for the scientific illustrations.
Financial Support: Funding for this article was received from the Department of Obstetrics, Gynecology and Reproductive Biology in the College of Human Medicine at Michigan State University.
Author Contributions: All authors contributed to the article design, literature analysis, and drafting of the article. B.A. served as primary editor of this article.
Disclosure Summary: The authors have nothing to disclose.
Data Availability: Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
King JC , Butte NF , Bronstein MN , Kopp LE , Lindquist SA . Energy metabolism during pregnancy: influence of maternal energy status . Am J Clin Nutr. 1994 ; 59 ( 2 Suppl ): 439S – 445S .
Google Scholar
Abeysekera MV , Morris JA , Davis GK , O’Sullivan AJ . Alterations in energy homeostasis to favour adipose tissue gain: a longitudinal study in healthy pregnant women . Aust N Z J Obstet Gynaecol. 2016 ; 56 ( 1 ): 42 – 48 .
Butte NF , King JC . Energy requirements during pregnancy and lactation . Public Health Nutr. 2005 ; 8 ( 7A ): 1010 – 1027 .
King JC . Physiology of pregnancy and nutrient metabolism . Am J Clin Nutr. 2000 ; 71 ( 5 Suppl ): 1218S – 1225S .
Jebeile H , Mijatovic J , Louie JCY , Prvan T , Brand-Miller JC . A systematic review and metaanalysis of energy intake and weight gain in pregnancy . Am J Obstet Gynecol. 2016 ; 214 ( 4 ): 465 – 483 .
Grimes SB , Wild R . Effect of pregnancy on lipid metabolism and lipoprotein levels. In: Feingold KR , Anawalt B , Boyce A , et al. , eds. Endotext . South Dartmouth, MA: MDText.com, Inc ; 2000 .
Google Preview
Butte NF . Carbohydrate and lipid metabolism in pregnancy: normal compared with gestational diabetes mellitus . Am J Clin Nutr. 2000 ; 71 ( 5 Suppl ): 1256S – 1261S .
Cowett RM , Farrag HM . Selected principles of perinatal-neonatal glucose metabolism . Semin Neonatol. 2004 ; 9 ( 1 ): 37 – 47 .
Kumar P , Magon N . Hormones in pregnancy . Niger Med J. 2012 ; 53 ( 4 ): 179 – 183 .
Bell AW , Bauman DE . Adaptations of glucose metabolism during pregnancy and lactation . J Mammary Gland Biol Neoplasia. 1997 ; 2 ( 3 ): 265 – 278 .
Kumari P , Sharma SN , Kumar S , Kumar M . A comparative study of blood pressure in normal and pregnancy induced hypertensive cases for early diagnosis of hypertensive disorders in a tertiary care hospital . Int J Sci Study. 2014 ; 2 ( 3 ): 33 – 37 .
Riskin-Mashiah S , Damti A , Younes G , Auslander R . Normal fasting plasma glucose levels during pregnancy: a hospital-based study . J Perinat Med. 2011 ; 39 ( 2 ): 209 – 211 .
Baumann MU , Deborde S , Illsley NP . Placental glucose transfer and fetal growth . Endocrine. 2002 ; 19 ( 1 ): 13 – 22 .
Knopp RH , Montes A , Childs M , Li JR , Mabuchi H . Metabolic adjustments in normal and diabetic pregnancy . Clin Obstet Gynecol. 1981 ; 24 ( 1 ): 21 – 49 .
Silverstone FA , Solomons E , Rubricius J . The rapid intravenous glucose tolerance test in pregnancy . J Clin Invest. 1961 ; 40 : 2180 – 2189 .
Lind T , Billewicz WZ , Brown G . A serial study of changes occurring in the oral glucose tolerance test during pregnancy . J Obstet Gynaecol Br Commonw. 1973 ; 80 ( 12 ): 1033 – 1039 .
Victor A . Normal blood sugar variation during pregnancy . Acta Obstet Gynecol Scand. 1974 ; 53 ( 1 ): 37 – 40 .
Hornnes PJ , Kühl C . Cortisol and glucose tolerance in normal pregnancy . Diabetes Metab. 1984 ; 10 ( 1 ): 1 – 6 .
Campbell DM , Sutherland HW , Pearson DW . Maternal glucose response to a standardized test meal throughout pregnancy and postnatally . Am J Obstet Gynecol. 1994 ; 171 ( 1 ): 143 – 146 .
Sorenson RL , Brelje TC . Prolactin receptors are critical to the adaptation of islets to pregnancy . Endocrinology. 2009 ; 150 ( 4 ): 1566 – 1569 .
Goyvaerts L , Lemaire K , Arijs I , et al. Prolactin receptors and placental lactogen drive male mouse pancreatic islets to pregnancy-related mRNA changes . Plos One. 2015 ; 10 ( 3 ): e0121868 .
Lorenzo-Almorós A , Hang T , Peiró C , et al. Predictive and diagnostic biomarkers for gestational diabetes and its associated metabolic and cardiovascular diseases . Cardiovasc Diabetol. 2019 ; 18 ( 1 ): 140 .
Rieck S , Kaestner KH . Expansion of beta-cell mass in response to pregnancy . Trends Endocrinol Metab. 2010 ; 21 ( 3 ): 151 – 158 .
Handwerger S , Freemark M . The roles of placental growth hormone and placental lactogen in the regulation of human fetal growth and development . J Pediatr Endocrinol Metab. 2000 ; 13 ( 4 ): 343 – 356 .
Walejko JM , Antolic A , Koelmel JP , Garrett TJ , Edison AS , Keller-Wood M . Chronic maternal cortisol excess during late gestation leads to metabolic alterations in the newborn heart . Am J Physiol Endocrinol Metab. 2019 ; 316 ( 3 ): E546 – E556 .
Sonagra AD , Biradar SM , K D , Murthy D S J . Normal pregnancy – a state of insulin resistance . J Clin Diagn Res. 2014 ; 8 ( 11 ): CC01 – CC03 .
Kampmann U , Knorr S , Fuglsang J , Ovesen P . Determinants of maternal insulin resistance during pregnancy: an updated overview . J Diabetes Res. 2019 ; 2019 : 5320156 .
Barbour LA , McCurdy CE , Hernandez TL , Kirwan JP , Catalano PM , Friedman JE . Cellular mechanisms for insulin resistance in normal pregnancy and gestational diabetes . Diabetes Care. 2007 ; 30 ( Suppl 2 ): S112 – S119 .
Kirwan JP , Hauguel-De Mouzon S , Lepercq J , et al. TNF-alpha is a predictor of insulin resistance in human pregnancy . Diabetes. 2002 ; 51 ( 7 ): 2207 – 2213 .
Del Aguila LF , Krishnan RK , Ulbrecht JS , et al. Muscle damage impairs insulin stimulation of IRS-1, PI 3-kinase, and Akt-kinase in human skeletal muscle . Am J Physiol Endocrinol Metab. 2000 ; 279 ( 1 ): E206 – E212 .
Kirwan JP , Krishnan RK , Weaver JA , Del Aguila LF , Evans WJ . Human aging is associated with altered TNF-α production during hyperglycemia and hyperinsulinemia . Am J Physiol Endocrinol Metab. 2001 ; 281 ( 6 ): E1137 – E1143 .
Rigato O , Ujvari S , Castelo A , Salomão R . Tumor necrosis factor alpha (TNF-alpha) and sepsis: evidence for a role in host defense . Infection. 1996 ; 24 ( 4 ): 314 – 318 .
Laham N , Brennecke SP , Bendtzen K , Rice GE . Tumour necrosis factor alpha during human pregnancy and labour: maternal plasma and amniotic fluid concentrations and release from intrauterine tissues . Eur J Endocrinol. 1994 ; 131 ( 6 ): 607 – 614 .
Beckmann I , Visser W , Struijk PC , van Dooren M , Glavimans J , Wallenburg HC . Circulating bioactive tumor necrosis factor-alpha, tumor necrosis factor-alpha receptors, fibronectin, and tumor necrosis factor-alpha inducible cell adhesion molecule VCAM-1 in uncomplicated pregnancy . Am J Obstet Gynecol. 1997 ; 177 ( 5 ): 1247 – 1252 .
In: Rasmussen KM , Yaktine AL , eds. Weight Gain During Pregnancy: Reexamining the Guidelines . Washington, DC : National Academy of Sciences ; 2009 .
Freemark M . Placental hormones and the control of fetal growth . J Clin Endocrinol Metab. 2010 ; 95 ( 5 ): 2054 – 2057 .
León-Aguilar LF , Croyal M , Ferchaud-Roucher V , et al. Maternal obesity leads to long-term altered levels of plasma ceramides in the offspring as revealed by a longitudinal lipidomic study in children . Int J Obes (Lond). 2019 ; 43 ( 6 ): 1231 – 1243 .
Hayder H , O’Brien J , Nadeem U , Peng C . MicroRNAs: crucial regulators of placental development . Reproduction. 2018 ; 155 ( 6 ): R259 – R271 .
Armistead B , Kadam L , Drewlo S , Kohan-Ghadr HR . The role of NFκB in healthy and preeclamptic placenta: trophoblasts in the spotlight . Int J Mol Sci. 2020 ; 21 ( 5 ): 1775 .
Burton GJ , Fowden AL . The placenta: a multifaceted, transient organ . Philos Trans R Soc Lond B Biol Sci. 2015 ; 370 ( 1663 ): 20140066 .
Velegrakis A , Sfakiotaki M , Sifakis S . Human placental growth hormone in normal and abnormal fetal growth . Biomed Rep. 2017 ; 7 ( 2 ): 115 – 122 .
Napso T , Yong HEJ , Lopez-Tello J , Sferruzzi-Perri AN . The role of placental hormones in mediating maternal adaptations to support pregnancy and lactation . Front Physiol. 2018 ; 9 : 1091 .
Scippo ML , Frankenne F , Hooghe-Peters EL , Igout A , Velkeniers B , Hennen G . Syncytiotrophoblastic localization of the human growth hormone variant mRNA in the placenta . Mol Cell Endocrinol. 1993 ; 92 ( 2 ): R7 – 13 .
Lacroix MC , Guibourdenche J , Fournier T , et al. Stimulation of human trophoblast invasion by placental growth hormone . Endocrinology. 2005 ; 146 ( 5 ): 2434 – 2444 .
Gupta SK , Malhotra SS , Malik A , Verma S , Chaudhary P . Cell signaling pathways involved during invasion and syncytialization of trophoblast cells . Am J Reprod Immunol. 2016 ; 75 ( 3 ): 361 – 371 .
Barbour LA , Shao J , Qiao L , et al. Human placental growth hormone causes severe insulin resistance in transgenic mice . Am J Obstet Gynecol. 2002 ; 186 ( 3 ): 512 – 517 .
Fuglsang J , Lauszus F , Flyvbjerg A , Ovesen P . Human placental growth hormone, insulin-like growth factor I and -II, and insulin requirements during pregnancy in type 1 diabetes . J Clin Endocrinol Metab. 2003 ; 88 ( 10 ): 4355 – 4361 .
Freemark M . Regulation of maternal metabolism by pituitary and placental hormones: roles in fetal development and metabolic programming . Horm Res. 2006 ; 65 ( Suppl 3 ): 41 – 49 .
Newbern D , Freemark M . Placental hormones and the control of maternal metabolism and fetal growth . Curr Opin Endocrinol Diabetes Obes. 2011 ; 18 ( 6 ): 409 – 416 .
Lombardo MF , De Angelis F , Bova L , et al. Human placental lactogen (hPL-A) activates signaling pathways linked to cell survival and improves insulin secretion in human pancreatic islets . Islets. 2011 ; 3 ( 5 ): 250 – 258 .
Kojima M , Hosoda H , Date Y , Nakazato M , Matsuo H , Kangawa K . Ghrelin is a growth-hormone-releasing acylated peptide from stomach . Nature. 1999 ; 402 ( 6762 ): 656 – 660 .
Harrison JL , Adam CL , Brown YA , et al. An immunohistochemical study of the localization and developmental expression of ghrelin and its functional receptor in the ovine placenta . Reprod Biol Endocrinol. 2007 ; 5 : 25 .
Patterson M , Bloom SR , Gardiner JV . Ghrelin and appetite control in humans–potential application in the treatment of obesity . Peptides. 2011 ; 32 ( 11 ): 2290 – 2294 .
Palik E , Baranyi E , Melczer Z , et al. Elevated serum acylated (biologically active) ghrelin and resistin levels associate with pregnancy-induced weight gain and insulin resistance . Diabetes Res Clin Pract. 2007 ; 76 ( 3 ): 351 – 357 .
Gualillo O , Caminos J , Blanco M , et al. Ghrelin, a novel placental-derived hormone . Endocrinology. 2001 ; 142 ( 2 ): 788 – 794 .
Nakahara K , Nakagawa M , Baba Y , et al. Maternal ghrelin plays an important role in rat fetal development during pregnancy . Endocrinology. 2006 ; 147 ( 3 ): 1333 – 1342 .
Telejko B , Kuzmicki M , Zonenberg A , et al. Ghrelin in gestational diabetes: serum level and mRNA expression in fat and placental tissue . Exp Clin Endocrinol Diabetes. 2010 ; 118 ( 2 ): 87 – 92 .
Sun Y , Ahmed S , Smith RG . Deletion of ghrelin impairs neither growth nor appetite . Mol Cell Biol. 2003 ; 23 ( 22 ): 7973 – 7981 .
Chanoine JP . Ghrelin in growth and development . Horm Res. 2005 ; 63 ( 3 ): 129 – 138 .
Perry RJ . Leptin revisited: the role of leptin in starvation . Mol Cell Oncol. 2018 ; 5 ( 5 ): e1435185 .
Bellefontaine N , Chachlaki K , Parkash J , et al. Leptin-dependent neuronal NO signaling in the preoptic hypothalamus facilitates reproduction . J Clin Invest. 2014 ; 124 ( 6 ): 2550 – 2559 .
Azuma T , Suto H , Ito Y , et al. Gastric leptin and Helicobacter pylori infection . Gut. 2001 ; 49 ( 3 ): 324 – 329 .
Wang J , Liu R , Hawkins M , Barzilai N , Rossetti L . A nutrient-sensing pathway regulates leptin gene expression in muscle and fat . Nature. 1998 ; 393 ( 6686 ): 684 – 688 .
Sylvia KE , Lorenz TK , Heiman JR , Demas GE . Physiological predictors of leptin vary during menses and ovulation in healthy women . Reprod Biol. 2018 ; 18 ( 1 ): 132 – 136 .
Herrid M , Palanisamy SK , Ciller UA , et al. An updated view of leptin on implantation and pregnancy: a review . Physiol Res. 2014 ; 63 ( 5 ): 543 – 557 .
Su RW , Fazleabas AT . Implantation and establishment of pregnancy in human and nonhuman primates . Adv Anat Embryol Cell Biol. 2015 ; 216 : 189 – 213 .
Gambino YP , Maymó JL , Pérez-Pérez A , et al. 17Beta-estradiol enhances leptin expression in human placental cells through genomic and nongenomic actions . Biol Reprod. 2010 ; 83 ( 1 ): 42 – 51 .
Gambino YP , Pérez Pérez A , Dueñas JL , Calvo JC , Sánchez-Margalet V , Varone CL . Regulation of leptin expression by 17beta-estradiol in human placental cells involves membrane associated estrogen receptor alpha . Biochim Biophys Acta. 2012 ; 1823 ( 4 ): 900 – 910 .
Myers MG , Cowley MA , Münzberg H . Mechanisms of leptin action and leptin resistance . Annu Rev Physiol. 2008 ; 70 : 537 – 556 .
Ladyman SR , Augustine RA , Grattan DR . Hormone interactions regulating energy balance during pregnancy . J Neuroendocrinol. 2010 ; 22 ( 7 ): 805 – 817 .
Ladyman SR , Grattan DR . Region-specific reduction in leptin-induced phosphorylation of signal transducer and activator of transcription-3 (STAT3) in the rat hypothalamus is associated with leptin resistance during pregnancy . Endocrinology. 2004 ; 145 ( 8 ): 3704 – 3711 .
Bates SH , Stearns WH , Dundon TA , et al. STAT3 signalling is required for leptin regulation of energy balance but not reproduction . Nature. 2003 ; 421 ( 6925 ): 856 – 859 .
Ladyman SR , Grattan DR . Suppression of leptin receptor messenger ribonucleic acid and leptin responsiveness in the ventromedial nucleus of the hypothalamus during pregnancy in the rat . Endocrinology. 2005 ; 146 ( 9 ): 3868 – 3874 .
Hauguel-de Mouzon S , Lepercq J , Catalano P . The known and unknown of leptin in pregnancy . Am J Obstet Gynecol. 2006 ; 194 ( 6 ): 1537 – 1545 .
Masuzaki H , Ogawa Y , Sagawa N , et al. Nonadipose tissue production of leptin: leptin as a novel placenta-derived hormone in humans . Nat Med. 1997 ; 3 ( 9 ): 1029 – 1033 .
Challier J , Galtier M , Bintein T , Cortez A , Lepercq J , Hauguel-de Mouzon S . Placental leptin receptor isoforms in normal and pathological pregnancies . Placenta. 2003 ; 24 ( 1 ): 92 – 99 .
Jansson T , Wennergren M , Illsley NP . Glucose transporter protein expression in human placenta throughout gestation and in intrauterine growth retardation . J Clin Endocrinol Metab. 1993 ; 77 ( 6 ): 1554 – 1562 .
Zhang Y , Olbort M , Schwarzer K , et al. The leptin receptor mediates apparent autocrine regulation of leptin gene expression . Biochem Biophys Res Commun. 1997 ; 240 ( 2 ): 492 – 495 .
Boström P , Wu J , Jedrychowski MP , et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis . Nature. 2012 ; 481 ( 7382 ): 463 – 468 .
Swick AG , Orena S , O’Connor A . Irisin levels correlate with energy expenditure in a subgroup of humans with energy expenditure greater than predicted by fat free mass . Metabolism. 2013 ; 62 ( 8 ): 1070 – 1073 .
Gizaw M , Anandakumar P , Debela T . A review on the role of irisin in insulin resistance and type 2 diabetes mellitus . J Pharmacopuncture. 2017 ; 20 ( 4 ): 235 – 242 .
Arhire LI , Mihalache L , Covasa M . Irisin: a hope in understanding and managing obesity and metabolic syndrome . Front Endocrinol (Lausanne). 2019 ; 10 : 524 .
Garcés MF , Peralta JJ , Ruiz-Linares CE , et al. Irisin levels during pregnancy and changes associated with the development of preeclampsia . J Clin Endocrinol Metab. 2014 ; 99 ( 6 ): 2113 – 2119 .
Pavlova T , Zlamal F , Tomandl J , Hodicka Z , Gulati S , Bienertova-Vasku J . Irisin maternal plasma and cord blood levels in mothers with spontaneous preterm and term delivery . Dis Markers. 2018 ; 2018 : 7628957 .
Ebert T , Stepan H , Schrey S , et al. Serum levels of irisin in gestational diabetes mellitus during pregnancy and after delivery . Cytokine. 2014 ; 65 ( 2 ): 153 – 158 .
Briana DD , Boutsikou M , Athanasopoulos N , Marmarinos A , Gourgiotis D , Malamitsi-Puchner A . Implication of the myokine irisin in maternal energy homeostasis in pregnancies with abnormal fetal growth . J Matern Fetal Neonatal Med. 2016 ; 29 ( 21 ): 3429 – 3433 .
Chen JQ , Li Q , Ma JR . Maternal serum, placental, and umbilical venous blood irisin levels in intrahepatic cholestasis of pregnancy . J Matern-Fetal Neo M. 2019 : 1 – 8 .
Drewlo S , Johnson E , Kilburn BA , Kadam L , Armistead B , Kohan-Ghadr HR . Irisin induces trophoblast differentiation via AMPK activation in the human placenta . J Cell Physiol. 2020 .
Bozkurt L , Göbl CS , Baumgartner-Parzer S , Luger A , Pacini G , Kautzky-Willer A . Adiponectin and leptin at early pregnancy: association to actual glucose disposal and risk for GDM-A prospective cohort study . Int J Endocrinol. 2018 ; 2018 : 5463762 .
Retnakaran R . Adiponectin and β-cell adaptation in pregnancy . Diabetes. 2017 ; 66 ( 5 ): 1121 – 1122 .
Chen J , Tan B , Karteris E , et al. Secretion of adiponectin by human placenta: differential modulation of adiponectin and its receptors by cytokines . Diabetologia. 2006 ; 49 ( 6 ): 1292 – 1302 .
Caminos JE , Nogueiras R , Gallego R , et al. Expression and regulation of adiponectin and receptor in human and rat placenta . J Clin Endocrinol Metab. 2005 ; 90 ( 7 ): 4276 – 4286 .
Lappas M , Yee K , Permezel M , Rice GE . Release and regulation of leptin, resistin and adiponectin from human placenta, fetal membranes, and maternal adipose tissue and skeletal muscle from normal and gestational diabetes mellitus-complicated pregnancies . J Endocrinol. 2005 ; 186 ( 3 ): 457 – 465 .
McDonald EA , Wolfe MW . Adiponectin attenuation of endocrine function within human term trophoblast cells . Endocrinology. 2009 ; 150 ( 9 ): 4358 – 4365 .
Ichida K , Moriyama T , Morita H , et al. Plasma adiponectin concentrations and placental adiponectin expression in pre-eclamptic women . Gynecol Endocrinol. 2007 ; 23 ( 4 ): 238 – 243 .
Corbetta S , Bulfamante G , Cortelazzi D , et al. Adiponectin expression in human fetal tissues during mid- and late gestation . J Clin Endocrinol Metab. 2005 ; 90 ( 4 ): 2397 – 2402 .
Turer AT , Scherer PE . Adiponectin: mechanistic insights and clinical implications . Diabetologia. 2012 ; 55 ( 9 ): 2319 – 2326 .
Mazaki-Tovi S , Kanety H , Sivan E . Adiponectin and human pregnancy . Curr Diab Rep. 2005 ; 5 ( 4 ): 278 – 281 .
American Diabetes A. 2 . Classification and diagnosis of diabetes: standards of medical care in diabetes-2020 . Diabetes Care. 2020 ; 43 ( Suppl 1 ): S14 – S31 .
Metzger BE , Lowe LP , Dyer AR , et al. Hyperglycemia and adverse pregnancy outcomes . N Engl J Med. 2008 ; 358 ( 19 ): 1991 – 2002 .
Chiefari E , Arcidiacono B , Foti D , Brunetti A . Gestational diabetes mellitus: an updated overview . J Endocrinol Invest. 2017 ; 40 ( 9 ): 899 – 909 .
Dickens LT , Thomas CC . Updates in gestational diabetes prevalence, treatment, and health policy . Curr Diab Rep. 2019 ; 19 ( 6 ): 33 .
Bao W , Dar S , Zhu Y , et al. Plasma concentrations of lipids during pregnancy and the risk of gestational diabetes mellitus: a longitudinal study . J Diabetes. 2018 ; 10 ( 6 ): 487 – 495 .
Wadhwa PD , Buss C , Entringer S , Swanson JM . Developmental origins of health and disease: brief history of the approach and current focus on epigenetic mechanisms . Semin Reprod Med. 2009 ; 27 ( 5 ): 358 – 368 .
Powe CE , Allard C , Battista MC , et al. Heterogeneous contribution of insulin sensitivity and secretion defects to gestational diabetes mellitus . Diabetes Care. 2016 ; 39 ( 6 ): 1052 – 1055 .
Pérez-Pérez A , Toro A , Vilariño-García T , et al. Leptin action in normal and pathological pregnancies . J Cell Mol Med. 2018 ; 22 ( 2 ): 716 – 727 .
Powe CE . Early pregnancy biochemical predictors of gestational diabetes mellitus . Curr Diab Rep. 2017 ; 17 ( 2 ): 12 .
Iciek R , Wender-Ozegowska E , Zawiejska A , et al. Placental leptin and its receptor genes expression in pregnancies complicated by type 1 diabetes . J Physiol Pharmacol. 2013 ; 64 ( 5 ): 579 – 585 .
Kautzky-Willer A , Pacini G , Tura A , et al. Increased plasma leptin in gestational diabetes . Diabetologia. 2001 ; 44 ( 2 ): 164 – 172 .
Qiu C , Williams MA , Vadachkoria S , Frederick IO , Luthy DA . Increased maternal plasma leptin in early pregnancy and risk of gestational diabetes mellitus . Obstet Gynecol. 2004 ; 103 ( 3 ): 519 – 525 .
Bao W , Baecker A , Song Y , Kiely M , Liu S , Zhang C . Adipokine levels during the first or early second trimester of pregnancy and subsequent risk of gestational diabetes mellitus: a systematic review . Metabolism. 2015 ; 64 ( 6 ): 756 – 764 .
Festa A , Shnawa N , Krugluger W , Hopmeier P , Schernthaner G , Haffner SM . Relative hypoleptinaemia in women with mild gestational diabetes mellitus . Diabet Med. 1999 ; 16 ( 8 ): 656 – 662 .
Mosavat M , Omar SZ , Tan PC , Razif MFM , Sthaneshwar P . Leptin and soluble leptin receptor in association with gestational diabetes: a prospective case-control study . Arch Gynecol Obstet. 2018 ; 297 ( 3 ): 797 – 803 .
Liu L , Aa J , Wang G , et al. Differences in metabolite profile between blood plasma and serum . Anal Biochem. 2010 ; 406 ( 2 ): 105 – 112 .
McIlhenny C , George WD , Doughty JC . A comparison of serum and plasma levels of vascular endothelial growth factor during the menstrual cycle in healthy female volunteers . Br J Cancer. 2002 ; 86 ( 11 ): 1786 – 1789 .
Oddoze C , Lombard E , Portugal H . Stability study of 81 analytes in human whole blood, in serum and in plasma . Clin Biochem. 2012 ; 45 ( 6 ): 464 -– 469 .
Du XL , Jiang WX , Lv ZT . Lower circulating irisin level in patients with diabetes mellitus: a systematic review and meta-analysis . Horm Metab Res. 2016 ; 48 ( 10 ): 644 – 652 .
Yuksel MA , Oncul M , Tuten A , et al. Maternal serum and fetal cord blood irisin levels in gestational diabetes mellitus . Diabetes Res Clin Pract. 2014 ; 104 ( 1 ): 171 – 175 .
Seven A , Yalinbas E , Kucur SK , et al. Comprehensive evaluation of irisin levels in fetomaternal circulation of pregnant women with obesity or gestational diabetes mellitus . Ir J Med Sci. 2019 ; 188 ( 4 ): 1213 – 1219 .
Pardo M , Crujeiras AB , Amil M , et al. Association of irisin with fat mass, resting energy expenditure, and daily activity in conditions of extreme body mass index . Int J Endocrinol. 2014 ; 2014 : 857270 .
Erol O , Erkal N , Ellidağ HY , et al. Irisin as an early marker for predicting gestational diabetes mellitus: a prospective study . J Matern Fetal Neonatal Med. 2016 ; 29 ( 22 ): 3590 – 3595 .
Ural UM , Sahin SB , Tekin YB , Cüre MC , Sezgin H . Alteration of maternal serum irisin levels in gestational diabetes mellitus . Ginekol Pol. 2016 ; 87 ( 5 ): 395 – 398 .
Son JS , Liu X , Tian Q , et al. Exercise prevents the adverse effects of maternal obesity on placental vascularization and fetal growth . J Physiol. 2019 ; 597 ( 13 ): 3333 – 3347 .
Daskalopoulou SS , Cooke AB , Gomez YH , et al. Plasma irisin levels progressively increase in response to increasing exercise workloads in young, healthy, active subjects . Eur J Endocrinol. 2014 ; 171 ( 3 ): 343 – 352 .
Wang P , Ma HH , Hou XZ , Song LL , Song XL , Zhang JF . Reduced plasma level of irisin in first trimester as a risk factor for the development of gestational diabetes mellitus . Diabetes Res Clin Pract. 2018 ; 142 : 130 – 138 .
Jedrychowski MP , Wrann CD , Paulo JA , et al. Detection and quantitation of circulating human irisin by tandem mass spectrometry . Cell Metab. 2015 ; 22 ( 4 ): 734 – 740 .
Worda C , Leipold H , Gruber C , Kautzky-Willer A , Knöfler M , Bancher-Todesca D . Decreased plasma adiponectin concentrations in women with gestational diabetes mellitus . Am J Obstet Gynecol. 2004 ; 191 ( 6 ): 2120 – 2124 .
Ranheim T , Haugen F , Staff AC , Braekke K , Harsem NK , Drevon CA . Adiponectin is reduced in gestational diabetes mellitus in normal weight women . Acta Obstet Gynecol Scand. 2004 ; 83 ( 4 ): 341 – 347 .
Retnakaran R , Hanley AJ , Raif N , Connelly PW , Sermer M , Zinman B . Reduced adiponectin concentration in women with gestational diabetes: a potential factor in progression to type 2 diabetes . Diabetes Care. 2004 ; 27 ( 3 ): 799 – 800 .
Qiao L , Wattez JS , Lee S , et al. Adiponectin deficiency impairs maternal metabolic adaptation to pregnancy in mice . Diabetes. 2017 ; 66 ( 5 ): 1126 – 1135 .
Dall TM , Yang W , Gillespie K , et al. The economic burden of elevated blood glucose levels in 2017: diagnosed and undiagnosed diabetes, gestational diabetes, and prediabetes . Diabetes care. 2019 ; 42 ( 9 ): 1661 – 1668 .
Sesmilo G , Prats P , Garcia S , et al. First-trimester fasting glycemia as a predictor of gestational diabetes (GDM) and adverse pregnancy outcomes . Acta Diabetol. 2020 ; 57 ( 6 ): 697 – 703 .
Seabra G , Saunders C , de Carvalho Padilha P , Zajdenverg L , da Silva LB , de Souza Santos MM . Association between maternal glucose levels during pregnancy and gestational diabetes mellitus: an analytical cross-sectional study . Diabetol Metab Syndr. 2015 ; 7 : 17 .
Email alerts
Citing articles via.
- About the Endocrine Society
- Recommend to Your Librarian
- Advertising and Corporate Services
- Journals Career Network
Affiliations
- Online ISSN 1945-7170
- Print ISSN 0013-7227
- Copyright © 2024 Endocrine Society
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Deciphering the Epigenetic Landscape: Placental Development and Its Role in Pregnancy Outcomes
- Published: 08 March 2024
Cite this article

- Yujia Chen 1 , 2 ,
- Zhoujie Ye 1 , 2 ,
- Meijia Lin 4 ,
- Liping Zhu 1 , 2 ,
- Liangpu Xu 3 &
- Xinrui Wang ORCID: orcid.org/0000-0002-7383-9128 1 , 2
405 Accesses
1 Altmetric
Explore all metrics
The placenta stands out as a unique, transitory, and multifaceted organ, essential to the optimal growth and maturation of the fetus. Functioning as a vital nexus between the maternal and fetal circulatory systems, it oversees the critical exchange of nutrients and waste. This exchange is facilitated by placental cells, known as trophoblasts, which adeptly invade and remodel uterine blood vessels. Deviations in placental development underpin a slew of pregnancy complications, notably fetal growth restriction (FGR), preeclampsia (PE), recurrent spontaneous abortions (RSA), and preterm birth. Central to placental function and development is epigenetic regulation. Despite its importance, the intricate mechanisms by which epigenetics influence the placenta are not entirely elucidated. Recently, the scientific community has turned its focus to parsing out the epigenetic alterations during placental development, such as variations in promoter DNA methylation, genomic imprints, and shifts in non-coding RNA expression. By establishing correlations between epigenetic shifts in the placenta and pregnancy complications, researchers are unearthing invaluable insights into the biology and pathophysiology of these conditions. This review seeks to synthesize the latest findings on placental epigenetic regulation, spotlighting its crucial role in shaping fetal growth trajectories and development. Through this lens, we underscore the overarching significance of the placenta in the larger narrative of gestational health.
Graphical Abstract
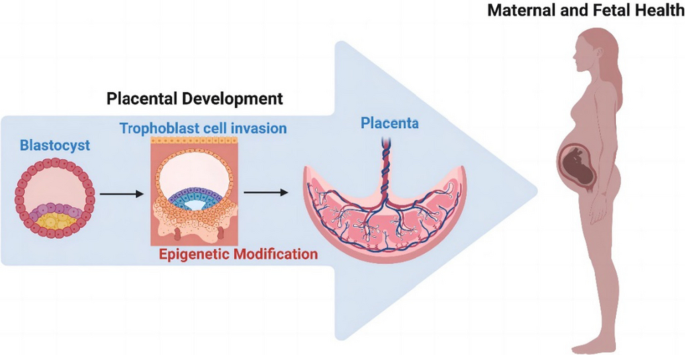
This is a preview of subscription content, log in via an institution to check access.

Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
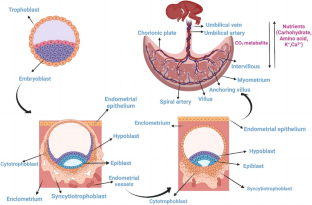
Similar content being viewed by others
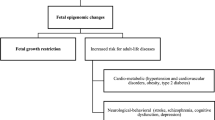
Epigenetics Beyond Fetal Growth Restriction: A Comprehensive Overview
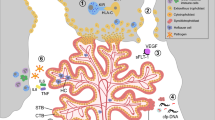
The significance of the placental genome and methylome in fetal and maternal health
Epigenetics of pregnancy: looking beyond the DNA code
Data availability.
Data availability is not applicable to this article as no new data were created or analyzed in this study.
Abbreviations
Angiotensin-converting enzyme
Arteriovenous
Cytotrophoblasts
Antioxidant copper-zinc superoxide dismutase
Chromosome 14 miRNA cluster
Chromosome 19 miRNA cluster
Differentially methylated regions
Decidual natural killer
Extracellular matrix
Endocrine gland-derived vascular endothelial growth factor
Exonic circRNA
BAP treated hESC
Extravillous trophoblasts
Enhancer of Zeste Homolog 2
Fetal growth restriction
Gestational diabetes mellitus
Glucose transporter proteins
G protein γ 7
Human chorionic gonadotropin
Histone deacetylases
Human embryonic stem cells
Human leukocyte antigen
Histone methyltransferases
4-Hydroxynonenal
Heme oxygenase-1
Human pluripotent stem cells
Major histocompatibility complex
Matrix metalloproteinases
Nuclear factor erythroid 2-like protein 2
Preeclampsia
Placental Growth Factor
Recurrent spontaneous abortions
Somatic cell nuclear transfer
Soluble endoglin
Soluble fms-like tyrosine kinase-1
Sodium-coupled neutral amino acid transporter 2
Syncytiotrophoblasts
DNA demethylases
Matrix metalloproteinases tissue inhibitors
Transforming growth factor
Tumor necrosis factor receptor-associated factor 6
Tumor necrosis factor-like weak inducer of apoptosis
Uterine natural killer cells
References
Maltepe, E., Bakardjiev, A. I., & Fisher, S. J. (2010). The placenta: Transcriptional, epigenetic, and physiological integration during development. The Journal of Clinical Investigation, 120 , 1016–1025.
Article CAS PubMed PubMed Central Google Scholar
Reik, W., & Walter, J. (2001). Genomic imprinting: Parental influence on the genome. Nature Reviews Genetics, 2 , 21–32.
Article CAS PubMed Google Scholar
Tobi, E. W., van den Heuvel, J., Zwaan, B. J., Lumey, L. H., Heijmans, B. T., & Uller, T. (2018). Selective Survival of Embryos Can Explain DNA Methylation Signatures of Adverse Prenatal Environments. Cell Reports, 25 (2660–7), e4.
Google Scholar
Nugent, B. M., & Bale, T. L. (2015). The omniscient placenta: Metabolic and epigenetic regulation of fetal programming. Frontiers in Neuroendocrinology, 39 , 28–37.
Hemberger, M., Hanna, C. W., & Dean, W. (2020). Mechanisms of early placental development in mouse and humans. Nature Reviews Genetics, 21 , 27–43.
Gude, N. M., Roberts, C. T., Kalionis, B., & King, R. G. (2004). Growth and function of the normal human placenta. Thrombosis Research, 114 , 397–407.
Gundling, W. E., Jr., & Wildman, D. E. (2015). A review of inter- and intraspecific variation in the eutherian placenta. Philosophical Transactions of the Royal Society of London. Series B, Biological sciences, 370 , 20140072.
Article PubMed PubMed Central Google Scholar
Turco, M., Y., & Moffett, A. (2019) Development of the human placenta. Development . 146 (22), dev163428. https://doi.org/10.1242/dev.163428
Carter, A. M. (1997). When is the maternal placental circulation established in man? 1941. Placenta, 18 , 83–87.
Demir, R., Kaufmann, P., Castellucci, M., Erbengi, T., & Kotowski, A. (1989). Fetal vasculogenesis and angiogenesis in human placental villi. Acta Anatomica (Basel), 136 , 190–203.
Article CAS Google Scholar
Aiko, Y., Askew, D. J., Aramaki, S., Myoga, M., Tomonaga, C., Hachisuga, T., et al. (2014). Differential levels of amino acid transporters System L and ASCT2, and the mTOR protein in placenta of preeclampsia and IUGR. BMC Pregnancy and Childbirth, 14 , 181.
Burton, G. J., Watson, A. L., Hempstock, J., Skepper, J. N., & Jauniaux, E. (2002). Uterine glands provide histiotrophic nutrition for the human fetus during the first trimester of pregnancy. Journal of Clinical Endocrinology and Metabolism, 87 , 2954–2959.
Illsley, N. P. (2000). Glucose transporters in the human placenta. Placenta, 21 , 14–22.
Cariappa, R., Heath-Monnig, E., & Smith, C. H. (2003). Isoforms of amino acid transporters in placental syncytiotrophoblast: Plasma membrane localization and potential role in maternal/fetal transport. Placenta, 24 , 713–726.
Stulc, J. (1997). Placental transfer of inorganic ions and water. Physiological Reviews, 77 , 805–836.
Shennan, D. B., & Boyd, C. A. (1987). Ion transport by the placenta: A review of membrane transport systems. Biochimica et Biophysica Acta, 906 , 437–457.
Costa, M. A. (2016). The endocrine function of human placenta: An overview. Reproductive Biomedicine Online, 32 , 14–43.
Liu, Z., Wang, C., Pei, J., Li, M., & Gu, W. (2022). SIRT1: A Novel Protective Molecule in Pre-eclampsia. International Journal of Medical Sciences, 19 , 993–1002.
Yu, Y., He, J. H., Hu, L. L., Jiang, L. L., Fang, L., Yao, G. D., et al. (2020). Placensin is a glucogenic hormone secreted by human placenta. EMBO Reports, 21 , e49530.
Brady, P. C., Farland, L. V., Racowsky, C., & Ginsburg, E. S. (2020). Hyperglycosylated human chorionic gonadotropin as a predictor of ongoing pregnancy. American Journal of Obstetrics and Gynecology, 222 (68), e1–e12.
Manaster, I., Mizrahi, S., Goldman-Wohl, D., Sela, H. Y., Stern-Ginossar, N., Lankry, D., et al. (2008). Endometrial NK cells are special immature cells that await pregnancy. The Journal of Immunology, 181 , 1869–1876.
Huhn, O., Zhao, X., Esposito, L., Moffett, A., Colucci, F., & Sharkey, A. M. (2021). How Do Uterine Natural Killer and Innate Lymphoid Cells Contribute to Successful Pregnancy? Frontiers in Immunology, 12 , 607669.
Lachapelle, M. H., Miron, P., Hemmings, R., & Roy, D. C. (1996). Endometrial T, B, and NK cells in patients with recurrent spontaneous abortion Altered profile and pregnancy outcome. The Journal of Immunology, 156 , 4027–34.
Rutkowski, K., Sowa, P., Rutkowska-Talipska, J., Kuryliszyn-Moskal, A., & Rutkowski, R. (2014). Dehydroepiandrosterone (DHEA): Hypes and hopes. Drugs, 74 , 1195–1207.
Coulam, C. B., & Roussev, R. G. (2003). Correlation of NK cell activation and inhibition markers with NK cytoxicity among women experiencing immunologic implantation failure after in vitro fertilization and embryo transfer. Journal of Assisted Reproduction and Genetics, 20 , 58–62.
Parham, P. (2004). NK cells and trophoblasts: Partners in pregnancy. Journal of Experimental Medicine, 200 , 951–955.
Kovats, S., Main, E. K., Librach, C., Stubblebine, M., Fisher, S. J., & DeMars, R. (1990). A class I antigen, HLA-G, expressed in human trophoblasts. Science, 248 , 220–223.
Article ADS CAS PubMed Google Scholar
Smith, G. C. (2010). First-trimester determination of complications of late pregnancy. JAMA, 303 , 561–562.
Burton, G. J., Fowden, A. L., & Thornburg, K. L. (2016). Placental Origins of Chronic Disease. Physiological Reviews, 96 , 1509–1565.
Goel, A., Maski, M. R., Bajracharya, S., Wenger, J. B., Zhang, D., Salahuddin, S., et al. (2015). Epidemiology and Mechanisms of De Novo and Persistent Hypertension in the Postpartum Period. Circulation, 132 , 1726–1733.
Rana, S., Lemoine, E., Granger, J. P., & Karumanchi, S. A. (2019). Preeclampsia: Pathophysiology, Challenges, and Perspectives. Circulation Research, 124 , 1094–1112.
Jim, B., & Karumanchi, S. A. (2017). Preeclampsia: Pathogenesis, Prevention, and Long-Term Complications. Seminars in Nephrology, 37 , 386–397.
Phipps, E., Prasanna, D., Brima, W., & Jim, B. (2016). Preeclampsia: Updates in Pathogenesis, Definitions, and Guidelines. Clinical Journal of the American Society of Nephrology, 11 , 1102–1113.
Karumanchi, S. A. (2016). Angiogenic Factors in Preeclampsia: From Diagnosis to Therapy. Hypertension, 67 , 1072–1079.
Young, B. C., Levine, R. J., & Karumanchi, S. A. (2010). Pathogenesis of preeclampsia. Annual Review of Pathology: Mechanisms of Disease, 5 , 173–192.
El-Sayed, A. A. F. (2017). Preeclampsia: A review of the pathogenesis and possible management strategies based on its pathophysiological derangements. Taiwanese Journal of Obstetrics & Gynecology, 56 , 593–598.
Article Google Scholar
Wang, A., Rana, S., & Karumanchi, S. A. (2009). Preeclampsia: The role of angiogenic factors in its pathogenesis. Physiology (Bethesda, Md.), 24 , 147–158.
PubMed Google Scholar
Mustafa, R., Ahmed, S., Gupta, A., & Venuto, R. C. (2012). A comprehensive review of hypertension in pregnancy. Journal of Pregnancy, 2012 , 105918.
Spradley, F. T. (2019). Sympathetic nervous system control of vascular function and blood pressure during pregnancy and preeclampsia. Journal of Hypertension, 37 , 476–487.
Kweider, N., Wruck, C. J., & Rath, W. (2013). New Insights into the Pathogenesis of Preeclampsia - The Role of Nrf2 Activators and their Potential Therapeutic Impact. Geburtshilfe und Frauenheilkunde, 73 , 1236–1240.
Lai, W. S., & Ding, Y. L. (2019). GNG7 silencing promotes the proliferation and differentiation of placental cytotrophoblasts in preeclampsia rats through activation of the mTOR signaling pathway. International Journal of Molecular Medicine, 43 , 1939–1950.
CAS PubMed PubMed Central Google Scholar
Parchem, J. G., Kanasaki, K., Kanasaki, M., Sugimoto, H., Xie, L., Hamano, Y., et al. (2018). Loss of placental growth factor ameliorates maternal hypertension and preeclampsia in mice. The Journal of Clinical Investigation, 128 , 5008–5017.
Resnik, R. (2002). Intrauterine growth restriction. Obstetrics and Gynecology, 99 , 490–496.
Kaufmann, P., Black, S., & Huppertz, B. (2003). Endovascular trophoblast invasion: Implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biology of Reproduction, 69 , 1–7.
Hafner, E., Metzenbauer, M., Hofinger, D., Munkel, M., Gassner, R., Schuchter, K., et al. (2003). Placental growth from the first to the second trimester of pregnancy in SGA-foetuses and pre-eclamptic pregnancies compared to normal foetuses. Placenta, 24 , 336–342.
Proctor, L. K., Toal, M., Keating, S., Chitayat, D., Okun, N., Windrim, R. C., et al. (2009). Placental size and the prediction of severe early-onset intrauterine growth restriction in women with low pregnancy-associated plasma protein-A. Ultrasound in Obstetrics and Gynecology, 34 , 274–282.
Jones, S., Bischof, H., Lang, I., Desoye, G., Greenwood, S. L., Johnstone, E. D., et al. (2015). Dysregulated flow-mediated vasodilatation in the human placenta in fetal growth restriction. Journal of Physiology, 593 , 3077–3092.
Hayward, C. E., Lean, S., Sibley, C. P., Jones, R. L., Wareing, M., Greenwood, S. L., et al. (2016). Placental Adaptation: What Can We Learn from Birthweight: Placental Weight Ratio? Frontiers in Physiology, 7 , 28.
Vaughan, O. R., Maksym, K., Silva, E., Barentsen, K., Anthony, R. V., Brown, T. L., et al. (2021). Placenta-specific Slc38a2/SNAT2 knockdown causes fetal growth restriction in mice. Clinical Science (London, England), 135 , 2049–2066.
Damodaram, M., Story, L., Eixarch, E., Patel, A., McGuinness, A., Allsop, J., et al. (2010). Placental MRI in intrauterine fetal growth restriction. Placenta, 31 , 491–498.
Krebs, C., Macara, L. M., Leiser, R., Bowman, A. W., Greer, I. A., & Kingdom, J. C. (1996). Intrauterine growth restriction with absent end-diastolic flow velocity in the umbilical artery is associated with maldevelopment of the placental terminal villous tree. American Journal of Obstetrics and Gynecology, 175 , 1534–1542.
Chen, C. P., Bajoria, R., & Aplin, J. D. (2002). Decreased vascularization and cell proliferation in placentas of intrauterine growth-restricted fetuses with abnormal umbilical artery flow velocity waveforms. American Journal of Obstetrics and Gynecology, 187 , 764–769.
Article PubMed Google Scholar
Junaid, T. O., Brownbill, P., Chalmers, N., Johnstone, E. D., & Aplin, J. D. (2014). Fetoplacental vascular alterations associated with fetal growth restriction. Placenta, 35 , 808–815.
El Hachem, H., Crepaux, V., May-Panloup, P., Descamps, P., Legendre, G., & Bouet, P. E. (2017). Recurrent pregnancy loss: Current perspectives. Int J Womens Health., 9 , 331–345.
Hustin, J., Jauniaux, E., & Schaaps, J. P. (1990). Histological study of the materno-embryonic interface in spontaneous abortion. Placenta, 11 , 477–486.
Gupta, S. K., Malhotra, S. S., Malik, A., Verma, S., & Chaudhary, P. (2016). Cell Signaling Pathways Involved During Invasion and Syncytialization of Trophoblast Cells. American Journal of Reproductive Immunology, 75 , 361–371.
Abrahams, V. M., Visintin, I., Aldo, P. B., Guller, S., Romero, R., & Mor, G. (2005). A role for TLRs in the regulation of immune cell migration by first trimester trophoblast cells. The Journal of Immunology, 175 , 8096–8104.
Harris, L. K., Smith, S. D., Keogh, R. J., Jones, R. L., Baker, P. N., Knofler, M., et al. (2010). Trophoblast- and vascular smooth muscle cell-derived MMP-12 mediates elastolysis during uterine spiral artery remodeling. American Journal of Pathology, 177 , 2103–2115.
Zhu, J. Y., Pang, Z. J., & Yu, Y. H. (2012). Regulation of trophoblast invasion: The role of matrix metalloproteinases. Reviews in Obstetrics & Gynecology, 5 , e137–e143.
Pollheimer, J., Vondra, S., Baltayeva, J., Beristain, A. G., & Knofler, M. (2018). Regulation of Placental Extravillous Trophoblasts by the Maternal Uterine Environment. Frontiers in Immunology, 9 , 2597.
Deng, W., Cha, J., Yuan, J., Haraguchi, H., Bartos, A., Leishman, E., et al. (2016). p53 coordinates decidual sestrin 2/AMPK/mTORC1 signaling to govern parturition timing. The Journal of Clinical Investigation, 126 , 2941–2954.
Du, L., Deng, W., Zeng, S., Xu, P., Huang, L., Liang, Y., et al. (2021). Single-cell transcriptome analysis reveals defective decidua stromal niche attributes to recurrent spontaneous abortion. Cell Proliferation, 54 , e13125.
Hocher, B., & Hocher, C. F. (2018). Epigenetics of recurrent pregnancy loss. EBioMedicine., 35 , 18–19.
Cross, J. C. (2003). The genetics of pre-eclampsia: A feto-placental or maternal problem? Clinical Genetics, 64 , 96–103.
Menon, R., Taylor, R. N., & Fortunato, S. J. (2010). Chorioamnionitis–a complex pathophysiologic syndrome. Placenta, 31 , 113–120.
Xue, W. C., Chan, K. Y., Feng, H. C., Chiu, P. M., Ngan, H. Y., Tsao, S. W., et al. (2004). Promoter hypermethylation of multiple genes in hydatidiform mole and choriocarcinoma. The Journal of Molecular Diagnostics, 6 , 326–334.
Pliushch, G., Schneider, E., Weise, D., El Hajj, N., Tresch, A., Seidmann, L., et al. (2010). Extreme methylation values of imprinted genes in human abortions and stillbirths. American Journal of Pathology, 176 , 1084–1090.
Vasconcelos, S., Ramalho, C., Marques, C. J., & Doria, S. (2019). Altered expression of epigenetic regulators and imprinted genes in human placenta and fetal tissues from second trimester spontaneous pregnancy losses. Epigenetics, 14 , 1234–1244.
Yuen, R. K., & Robinson, W. P. (2011). Review: A high capacity of the human placenta for genetic and epigenetic variation: Implications for assessing pregnancy outcome. Placenta, 32 (Suppl 2), S136–S141.
Lim, Y. C., Li, J., Ni, Y., Liang, Q., Zhang, J., Yeo, G. S. H., et al. (2017). A complex association between DNA methylation and gene expression in human placenta at first and third trimesters. PLoS ONE, 12 , e0181155.
Ohinata, Y., Payer, B., O’Carroll, D., Ancelin, K., Ono, Y., Sano, M., et al. (2005). Blimp1 is a critical determinant of the germ cell lineage in mice. Nature, 436 , 207–213.
Du, G., Yu, M., Xu, Q., Huang, Z., Huang, X., Han, L., et al. (2020). Hypomethylation of PRDM1 is associated with recurrent pregnancy loss. Journal of Cellular and Molecular Medicine, 24 , 7072–7077.
Wu, A. H., Guo, L. Y., Lu, S., Chen, X. L., Wang, A. A., Wang, X. Y., et al. (2020). Aberrant methylation of IGF2-AS promoter in early pregnancy loss. Taiwanese Journal of Obstetrics & Gynecology, 59 , 109–114.
Yu, M., Du, G., Xu, Q., Huang, Z., Huang, X., Qin, Y., et al. (2018). Integrated analysis of DNA methylome and transcriptome identified CREB5 as a novel risk gene contributing to recurrent pregnancy loss. eBioMedicine, 35 , 334–344.
Hanna, C. W., McFadden, D. E., & Robinson, W. P. (2013). DNA methylation profiling of placental villi from karyotypically normal miscarriage and recurrent miscarriage. American Journal of Pathology, 182 , 2276–2284.
Yin, L. J., Zhang, Y., Lv, P. P., He, W. H., Wu, Y. T., Liu, A. X., et al. (2012). Insufficient maintenance DNA methylation is associated with abnormal embryonic development. BMC Medicine, 10 , 26.
Voss, A. K., & Thomas, T. (2018). Histone Lysine and Genomic Targets of Histone Acetyltransferases in Mammals. BioEssays, 40 , e1800078.
Gebremedhin, K. G., & Rademacher, D. J. (2016). Histone H3 acetylation in the postmortem Parkinson’s disease primary motor cortex. Neuroscience Letters, 627 , 121–125.
Tsaprouni, L. G., Ito, K., Powell, J. J., Adcock, I. M., & Punchard, N. (2011). Differential patterns of histone acetylation in inflammatory bowel diseases. Journal of Inflammation (London), 8 , 1.
Eddy, A. C., Chapman, H., & George, E. M. (2019). Acute Hypoxia and Chronic Ischemia Induce Differential Total Changes in Placental Epigenetic Modifications. Reproductive Sciences, 26 , 766–773.
Seto, E., & Yoshida, M. (2014). Erasers of histone acetylation: The histone deacetylase enzymes. Cold Spring Harbor Perspectives in Biology, 6 , a018713.
Togher, K. L., Kenny, L. C., & O’Keeffe, G. W. (2017). Class-Specific Histone Deacetylase Inhibitors Promote 11-Beta Hydroxysteroid Dehydrogenase Type 2 Expression in JEG-3 Cells. International Journal of Cell Biology, 2017 , 6169310.
Wang, Y., Gu, Y., Alexander, J. S., & Lewis, D. F. (2019). Histone deacetylase inhibition disturbs the balance between ACE and chymase expression in endothelial cells: A potential mechanism of chymase activation in preeclampsia. Hypertension Research, 42 , 155–164.
Cruz-Munoz, W., Sanchez, O. H., Di Grappa, M., English, J. L., Hill, R. P., & Khokha, R. (2006). Enhanced metastatic dissemination to multiple organs by melanoma and lymphoma cells in timp-3-/- mice. Oncogene, 25 , 6489–6496.
Xie, D., Zhu, J., Liu, Q., Li, J., Song, M., Wang, K., et al. (2019). Dysregulation of HDAC9 Represses Trophoblast Cell Migration and Invasion Through TIMP3 Activation in Preeclampsia. American Journal of Hypertension, 32 , 515–523.
Tasta, O., Swiader, A., Grazide, M. H., Rouahi, M., Parant, O., Vayssiere, C., et al. (2021). A role for 4-hydroxy-2-nonenal in premature placental senescence in preeclampsia and intrauterine growth restriction. Free Radical Biology & Medicine, 164 , 303–314.
Tran, T. Q., Lowman, X. H., & Kong, M. (2017). Molecular Pathways: Metabolic Control of Histone Methylation and Gene Expression in Cancer. Clinical Cancer Research, 23 , 4004–4009.
Black, J. C., Van Rechem, C., & Whetstine, J. R. (2012). Histone lysine methylation dynamics: Establishment, regulation, and biological impact. Molecular Cell, 48 , 491–507.
Sheng, W., Gu, Y., Chu, X., Morgan, J. A., Cooper, D. B., Lewis, D. F., et al. (2021). Upregulation of histone H3K9 methylation in fetal endothelial cells from preeclamptic pregnancies. Journal of Cellular Physiology, 236 , 1866–1874.
Padeken, J., Methot, S. P., & Gasser, S. M. (2022). Establishment of H3K9-methylated heterochromatin and its functions in tissue differentiation and maintenance. Nature Reviews Molecular Cell Biology, 23 , 623–640.
Matsui, H., Iriyama, T., Sayama, S., Inaoka, N., Suzuki, K., Yoshikawa, M., et al. (2021). Elevated placental histone H3K4 methylation via upregulated histone methyltransferases SETD1A and SMYD3 in preeclampsia and its possible involvement in hypoxia-induced pathophysiological process. Placenta, 115 , 60–69.
Sirohi, V. K., Medrano, T. I., Kannan, A., Bagchi, I. C., & Cooke, P. S. (2023). Uterine-specific Ezh2 deletion enhances stromal cell senescence and impairs placentation, resulting in pregnancy loss. Science, 26 , 107028.
CAS Google Scholar
Mattick, J. S., & Makunin, I. V. (2006). Non-coding RNA. Human molecular genetics , 15 (suppl_1), R17–R29. https://doi.org/10.1093/hmg/ddl046
Chen, B., & Huang, S. (2018). Circular RNA: An emerging non-coding RNA as a regulator and biomarker in cancer. Cancer Letters, 418 , 41–50.
Bartel, D. P. (2004). MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell, 116 , 281–297.
Bushati, N., & Cohen, S. M. (2007). microRNA functions. Annual Review of Cell and Developmental Biology, 23 , 175–205.
Morales-Prieto, D. M., Ospina-Prieto, S., Chaiwangyen, W., Schoenleben, M., & Markert, U. R. (2013). Pregnancy-associated miRNA-clusters. Journal of Reproductive Immunology, 97 , 51–61.
Liang, Y., Ridzon, D., Wong, L., & Chen, C. (2007). Characterization of microRNA expression profiles in normal human tissues. BMC Genomics, 8 , 166.
Kshitiz, Afzal, J., Maziarz, J. D., Hamidzadeh, A., Liang, C., Erkenbrack, E. M., ... & Wagner, G. P. (2019). Evolution of placental invasion and cancer metastasis are causally linked. Nature Ecology & Evolution, 3(12), 1743–1753. https://doi.org/10.1038/s41559-019-1046-4
Gonzalez, T. L., Eisman, L. E., Joshi, N. V., Flowers, A. E., Wu, D., Wang, Y., et al. (2021). High-throughput miRNA sequencing of the human placenta: Expression throughout gestation. Epigenomics, 13 , 995–1012.
Zhang, H., He, Y., Wang, J. X., Chen, M. H., Xu, J. J., Jiang, M. H., et al. (2020). miR-30-5p-mediated ferroptosis of trophoblasts is implicated in the pathogenesis of preeclampsia. Redox Biology, 29 , 101402.
Ding, J., Zhang, Y., Cai, X., Zhang, Y., Yan, S., Wang, J., et al. (2021). Extracellular vesicles derived from M1 macrophages deliver miR-146a-5p and miR-146b-5p to suppress trophoblast migration and invasion by targeting TRAF6 in recurrent spontaneous abortion. Theranostics., 11 , 5813–5830.
Su, M. T., Tsai, P. Y., Tsai, H. L., Chen, Y. C., & Kuo, P. L. (2017). miR-346 and miR-582-3p-regulated EG-VEGF expression and trophoblast invasion via matrix metalloproteinases 2 and 9. BioFactors, 43 , 210–219.
Herman, A. B., Tsitsipatis, D., & Gorospe, M. (2022). Integrated lncRNA function upon genomic and epigenomic regulation. Molecular Cell, 82 , 2252–2266.
Yu, J., Hong, J. F., Kang, J., Liao, L. H., & Li, C. D. (2017). Promotion of LncRNA HOXA11-AS on the proliferation of hepatocellular carcinoma by regulating the expression of LATS1. European Review for Medical and Pharmacological Sciences, 21 , 3402–3411.
CAS PubMed Google Scholar
Zhang, Q., Wang, Z., Cheng, X., & Wu, H. (2021). lncRNA DANCR promotes the migration an invasion and of trophoblast cells through microRNA-214-5p in preeclampsia. Bioengineered, 12 , 9424–9434.
Ogoyama, M., Ohkuchi, A., Takahashi, H., Zhao, D., Matsubara, S., Takizawa, T. (2021). LncRNA H19-derived miR-675-5p accelerates the invasion of extravillous trophoblast cells by inhibiting GATA2 and Subsequently activating matrix metalloproteinases. International Journal of Molecular Sciences , 22 (3), 1237. https://doi.org/10.3390/ijms22031237
Zhang, L., Deng, X., Shi, X., & Dong, X. (2019). Silencing H19 regulated proliferation, invasion, and autophagy in the placenta by targeting miR-18a-5p. Journal of Cellular Biochemistry, 120 , 9006–9015.
Xu, J., Xia, Y., Zhang, H., Guo, H., Feng, K., & Zhang, C. (2018). Overexpression of long non-coding RNA H19 promotes invasion and autophagy via the PI3K/AKT/mTOR pathways in trophoblast cells. Biomedicine & Pharmacotherapy, 101 , 691–697.
Wu, L., Liu, Q., Fan, C., Yi, X., & Cheng, B. (2021). MALAT1 recruited the E3 ubiquitin ligase FBXW7 to induce CRY2 ubiquitin-mediated degradation and participated in trophoblast migration and invasion. Journal of Cellular Physiology, 236 , 2169–2177.
Chen, H., Meng, T., Liu, X., Sun, M., Tong, C., Liu, J., et al. (2015). Long non-coding RNA MALAT-1 is downregulated in preeclampsia and regulates proliferation, apoptosis, migration and invasion of JEG-3 trophoblast cells. International Journal of Clinical and Experimental Pathology, 8 , 12718–12727.
Wu, H. Y., Wang, X. H., Liu, K., & Zhang, J. L. (2020). LncRNA MALAT1 regulates trophoblast cells migration and invasion via miR-206/IGF-1 axis. Cell Cycle, 19 , 39–52.
Wang, R., & Zou, L. (2020). Downregulation of LncRNA-MEG3 promotes HTR8/SVneo cells apoptosis and attenuates its migration by repressing Notch1 signal in preeclampsia. Reproduction, 160 , 21–29.
Yu, L., Kuang, L. Y., He, F., Du, L. L., Li, Q. L., Sun, W., et al. (2018). The Role and Molecular Mechanism of Long Nocoding RNA-MEG3 in the Pathogenesis of Preeclampsia. Reproductive Sciences, 25 , 1619–1628.
Zhang, J., Liu, X., & Gao, Y. (2021). The long noncoding RNA MEG3 regulates Ras-MAPK pathway through RASA1 in trophoblast and is associated with unexplained recurrent spontaneous abortion. Molecular Medicine, 27 , 70.
Hansen, T. B., Jensen, T. I., Clausen, B. H., Bramsen, J. B., Finsen, B., Damgaard, C. K., et al. (2013). Natural RNA circles function as efficient microRNA sponges. Nature, 495 , 384–388.
Floris, G., Zhang, L., Follesa, P., & Sun, T. (2017). Regulatory Role of Circular RNAs and Neurological Disorders. Molecular Neurobiology, 54 , 5156–5165.
Cheng, J., Huang, J., Yuan, S., Zhou, S., Yan, W., Shen, W., et al. (2017). Circular RNA expression profiling of human granulosa cells during maternal aging reveals novel transcripts associated with assisted reproductive technology outcomes. PLoS ONE, 12 , e0177888.
Qian, Y., Lu, Y., Rui, C., Qian, Y., Cai, M., & Jia, R. (2016). Potential Significance of Circular RNA in Human Placental Tissue for Patients with Preeclampsia. Cellular Physiology and Biochemistry, 39 , 1380–1390.
Zhang, Y. G., Yang, H. L., Long, Y., & Li, W. L. (2016). Circular RNA in blood corpuscles combined with plasma protein factor for early prediction of pre-eclampsia. BJOG, 123 , 2113–2118.
Hu, X., Ao, J., Li, X., Zhang, H., Wu, J., & Cheng, W. (2018). Competing endogenous RNA expression profiling in pre-eclampsia identifies hsa_circ_0036877 as a potential novel blood biomarker for early pre-eclampsia. Clinical Epigenetics, 10 , 48.
Maass, P. G., Glazar, P., Memczak, S., Dittmar, G., Hollfinger, I., Schreyer, L., et al. (2017). A map of human circular RNAs in clinically relevant tissues. Journal of Molecular Medicine (Berlin, Germany), 95 , 1179–1189.
Yan, L., Feng, J., Cheng, F., Cui, X., Gao, L., Chen, Y., et al. (2018). Circular RNA expression profiles in placental villi from women with gestational diabetes mellitus. Biochemical and Biophysical Research Communications, 498 , 743–750.
Wang, H., She, G., Zhou, W., Liu, K., Miao, J., & Yu, B. (2019). Expression profile of circular RNAs in placentas of women with gestational diabetes mellitus. Endocrine Journal, 66 , 431–441.
Zhang, Y., Yang, H., Zhang, Y., Shi, J., Chen, R., & Xiao, X. (2020). CircSFXN1 regulates the behaviour of trophoblasts and likely mediates preeclampsia. Placenta, 101 , 115–123.
Gai, S., Sun, L., Wang, H., & Yang, P. (2020). Circular RNA hsa_circ_0007121 regulates proliferation, migration, invasion, and epithelial-mesenchymal transition of trophoblast cells by miR-182-5p/PGF axis in preeclampsia. Open Med (Wars)., 15 , 1061–1071.
Tang, R., Zhang, Z., & Han, W. (2021). CircLRRK1 targets miR-223-3p to inhibit the proliferation, migration and invasion of trophoblast cells by regulating the PI3K/AKT signaling pathway. Placenta, 104 , 110–118.
Shen, X. Y., Zheng, L. L., Huang, J., Kong, H. F., Chang, Y. J., Wang, F., et al. (2019). CircTRNC18 inhibits trophoblast cell migration and epithelial-mesenchymal transition by regulating miR-762/Grhl2 pathway in pre-eclampsia. RNA Biology, 16 , 1565–1573.
Zhang, S., & Guo, G. (2022). Circ_FURIN promotes trophoblast cell proliferation, migration and invasion in preeclampsia by regulating miR-34a-5p and TFAP2A. Hypertension Research, 45 , 1334–1344.
Jing, M. Y., Xie, L. D., Chen, X., Zhou, Y., Jin, M. M., He, W. H., ... & Liu, A. X. (2022). Circ-CCNB1 modulates trophoblast proliferation and invasion in spontaneous abortion by regulating miR-223/SIAH1 axis. Endocrinology , 163 (8), bqac093. https://doi.org/10.1210/endocr/bqac093
Zhang, Y., Yang, H., Zhang, Y., Shi, J., & Chen, R. (2020). circCRAMP1L is a novel biomarker of preeclampsia risk and may play a role in preeclampsia pathogenesis via regulation of the MSP/RON axis in trophoblasts. BMC Pregnancy and Childbirth, 20 , 652.
Wang, H., Luo, C., Wu, X., Zhang, J., Xu, Z., Liu, Y., et al. (2021). Circular RNA hsa_circ_0081343 promotes trophoblast cell migration and invasion and inhibits trophoblast apoptosis by regulating miR-210-5p/DLX3 axis. Reproductive Biology and Endocrinology, 19 , 123.
Wang, D., Na, Q., Song, G., Wang, Y., & Wang, Y. (2020). The Role of circRNA-SETD2/miR-519a/PTEN Axis in Fetal Birth Weight through Regulating Trophoblast Proliferation. BioMed Research International, 2020 , 9809632.
PubMed PubMed Central Google Scholar
Li, Z., Zhou, G., Tao, F., Cao, Y., Han, W., & Li, Q. (2020). circ-ZUFSP regulates trophoblasts migration and invasion through sponging miR-203 to regulate STOX1 expression. Biochemical and Biophysical Research Communications, 531 , 472–479.
Surani, M. A., & Barton, S. C. (1983). Development of gynogenetic eggs in the mouse: Implications for parthenogenetic embryos. Science, 222 , 1034–1036.
Haycock, P. C., & Ramsay, M. (2009). Exposure of mouse embryos to ethanol during preimplantation development: Effect on DNA methylation in the h19 imprinting control region. Biology of Reproduction, 81 , 618–627.
Constancia, M., Pickard, B., Kelsey, G., & Reik, W. (1998). Imprinting mechanisms. Genome Research, 8 , 881–900.
Wood, A. J., & Oakey, R. J. (2006). Genomic imprinting in mammals: Emerging themes and established theories. PLoS Genetics, 2 , e147.
Constância, M., Hemberger, M., Hughes, J., et al. (2002). Placental-specific IGF-II is a major modulator of placental and fetal growth. Nature, 417 (6892), 945–948. https://doi.org/10.1038/nature00819
Tucci, V., Isles, A. R., Kelsey, G., Ferguson-Smith, A. C., & Erice, I. G. (2019). Genomic Imprinting and Physiological Processes in Mammals. Cell, 176 , 952–965.
Ogata T, Kagami M. Kagami-Ogata syndrome: a clinically recognizable upd(14)pat and related disorder affecting the chromosome 14q32.2 imprinted region. J Hum Genet. 2016; 61: 87–94.
Kagami, M., Sekita, Y., Nishimura, G., Irie, M., Kato, F., Okada, M., ... & Ogata, T. (2008). Deletions and epimutations affecting the human 14q32. 2 imprinted region in individuals with paternal and maternal upd (14)-like phenotypes. Nature Genetics , 40 (2), 237–242. https://doi.org/10.1038/ng.2007.56
Sekita, Y., Wagatsuma, H., Nakamura, K., Ono, R., Kagami, M., Wakisaka, N., et al. (2008). Role of retrotransposon-derived imprinted gene, Rtl1, in the feto-maternal interface of mouse placenta. Nature Genetics, 40 , 243–248.
Li, E., Beard, C., & Jaenisch, R. (1993). Role for DNA methylation in genomic imprinting. Nature, 366 , 362–365.
Inoue, A., Jiang, L., Lu, F., Suzuki, T., & Zhang, Y. (2017). Maternal H3K27me3 controls DNA methylation-independent imprinting. Nature, 547 , 419–424.
Chen, Z., Yin, Q., Inoue, A., Zhang, C., & Zhang, Y. (2019). Allelic H3K27me3 to allelic DNA methylation switch maintains noncanonical imprinting in extraembryonic cells. Science Advances , 5 (12), eaay7246. https://doi.org/10.1126/sciadv.aay7246
Hanna, C. W., Perez-Palacios, R., Gahurova, L., Schubert, M., Krueger, F., Biggins, L., et al. (2019). Endogenous retroviral insertions drive non-canonical imprinting in extra-embryonic tissues. Genome Biology, 20 , 225.
Mira-Bontenbal, H., & Gribnau, J. (2016). New Xist-Interacting Proteins in X-Chromosome Inactivation. Current Biology, 26 , R338–R342.
Inoue, A., Jiang, L., Lu, F., & Zhang, Y. (2017). Genomic imprinting of Xist by maternal H3K27me3. Genes & Development, 31 , 1927–1932.
Okamoto, I., Otte, A. P., Allis, C. D., Reinberg, D., & Heard, E. (2004). Epigenetic dynamics of imprinted X inactivation during early mouse development. Science, 303 , 644–649.
Inoue, A., Chen, Z., Yin, Q., & Zhang, Y. (2018). Maternal Eed knockout causes loss of H3K27me3 imprinting and random X inactivation in the extraembryonic cells. Genes & Development, 32 , 1525–1536.
Wilmut, I., Schnieke, A. E., McWhir, J., Kind, A. J., & Campbell, K. H. (1997). Viable offspring derived from fetal and adult mammalian cells. Nature, 385 , 810–813.
Wang, X., Qu, J., Li, J., He, H., Liu, Z., & Huan, Y. (2020). Epigenetic Reprogramming During Somatic Cell Nuclear Transfer: Recent Progress and Future Directions. Frontiers in Genetics, 11 , 205.
Inoue, K., Ogonuki, N., Kamimura, S., Inoue, H., Matoba, S., Hirose, M., et al. (2020). Loss of H3K27me3 imprinting in the Sfmbt2 miRNA cluster causes enlargement of cloned mouse placentas. Nature Communications, 11 , 2150.
Article ADS CAS PubMed PubMed Central Google Scholar
Romero, R., Kusanovic, J. P., Chaiworapongsa, T., & Hassan, S. S. (2011). Placental bed disorders in preterm labor, preterm PROM, spontaneous abortion and abruptio placentae. Best Practice & Research. Clinical Obstetrics & Gynaecology, 25 , 313–327.
Windsperger, K., Dekan, S., Pils, S., Golletz, C., Kunihs, V., Fiala, C., et al. (2017). Extravillous trophoblast invasion of venous as well as lymphatic vessels is altered in idiopathic, recurrent, spontaneous abortions. Human Reproduction, 32 , 1208–1217.
Zhou, Y., Damsky, C. H., & Fisher, S. J. (1997). Preeclampsia is associated with failure of human cytotrophoblasts to mimic a vascular adhesion phenotype. One cause of defective endovascular invasion in this syndrome?. The Journal of Clinical Investigation , 99 (9), 2152–2164. https://doi.org/10.1172/JCI119388
Schmidt, A., Morales-Prieto, D. M., Pastuschek, J., Frohlich, K., & Markert, U. R. (2015). Only humans have human placentas: Molecular differences between mice and humans. Journal of Reproductive Immunology, 108 , 65–71.
Bentwich, I., Avniel, A., Karov, Y., Aharonov, R., Gilad, S., Barad, O., et al. (2005). Identification of hundreds of conserved and nonconserved human microRNAs. Nature Genetics, 37 , 766–770.
Morales-Prieto, D. M., Ospina-Prieto, S., Schmidt, A., Chaiwangyen, W., & Markert, U. R. (2014). Elsevier Trophoblast Research Award Lecture: Origin, evolution and future of placenta miRNAs. Placenta, 35 (Suppl), S39-45.
Rielland, M., Hue, I., Renard, J. P., & Alice, J. (2008). Trophoblast stem cell derivation, cross-species comparison and use of nuclear transfer: New tools to study trophoblast growth and differentiation. Developmental Biology, 322 , 1–10.
Wilkinson, A. L., Zorzan, I., & Rugg-Gunn, P. J. (2023). Epigenetic regulation of early human embryo development. Cell Stem Cell, 30 , 1569–1584.
Cinkornpumin, J. K., Kwon, S. Y., Guo, Y., Hossain, I., Sirois, J., Russett, C. S., et al. (2020). Naive Human Embryonic Stem Cells Can Give Rise to Cells with a Trophoblast-like Transcriptome and Methylome. Stem Cell Reports., 15 , 198–213.
Pastor, W. A., Chen, D., Liu, W., Kim, R., Sahakyan, A., Lukianchikov, A., et al. (2016). Naive Human Pluripotent Cells Feature a Methylation Landscape Devoid of Blastocyst or Germline Memory. Cell Stem Cell, 18 , 323–329.
Takahashi, S., Okae, H., Kobayashi, N., Kitamura, A., Kumada, K., Yaegashi, N., et al. (2019). Loss of p57(KIP2) expression confers resistance to contact inhibition in human androgenetic trophoblast stem cells. Proc Natl Acad Sci U S A., 116 , 26606–26613.
Sheridan, M. A., Zhao, X., Fernando, R. C., Gardner, L., Perez-Garcia, V., Li, Q., & Turco, M. Y. (2021). Characterization of primary models of human trophoblast. Development , 148 (21), dev199749. https://doi.org/10.1242/dev.199749
Turco, M. Y., Gardner, L., Kay, R. G., Hamilton, R. S., Prater, M., Hollinshead, M. S., et al. (2018). Trophoblast organoids as a model for maternal-fetal interactions during human placentation. Nature, 564 , 263–267.
Goustin, A. S., Betsholtz, C., Pfeifer-Ohlsson, S., Persson, H., Rydnert, J., Bywater, M., et al. (1985). Coexpression of the sis and myc proto-oncogenes in developing human placenta suggests autocrine control of trophoblast growth. Cell, 41 , 301–312.
Lewis, M. P., Clements, M., Takeda, S., Kirby, P. L., Seki, H., Lonsdale, L. B., et al. (1996). Partial characterization of an immortalized human trophoblast cell-line, TCL-1, which possesses a CSF-1 autocrine loop. Placenta, 17 , 137–146.
Rong-Hao, L., Luo, S., & Zhuang, L. Z. (1996). Establishment and characterization of a cytotrophoblast cell line from normal placenta of human origin. Human Reproduction, 11 , 1328–1333.
Msheik, H., El Hayek, S., Bari, M. F., Azar, J., Abou-Kheir, W., Kobeissy, F., et al. (2019). Transcriptomic profiling of trophoblast fusion using BeWo and JEG-3 cell lines. Molecular Human Reproduction, 25 , 811–824.
Burres, N. S., & Cass, C. E. (1986). Density-dependent inhibition of expression of syncytiotrophoblastic markers by cultured human choriocarcinoma (BeWo) cells. Journal of Cellular Physiology, 128 , 375–382.
Burleigh, D. W., Kendziorski, C. M., Choi, Y. J., Grindle, K. M., Grendell, R. L., Magness, R. R., et al. (2007). Microarray analysis of BeWo and JEG3 trophoblast cell lines: Identification of differentially expressed transcripts. Placenta, 28 , 383–389.
Tuan, R. S., Moore, C. J., Brittingham, J. W., Kirwin, J. J., Akins, R. E., & Wong, M. (1991). In vitro study of placental trophoblast calcium uptake using JEG-3 human choriocarcinoma cells. Journal of Cell Science, 98 (Pt 3), 333–342.
Msheik, H., Azar, J., El Sabeh, M., Abou-Kheir, W., & Daoud, G. (2020). HTR-8/SVneo: A model for epithelial to mesenchymal transition in the human placenta. Placenta, 90 , 90–97.
Graham, C. H., Hawley, T. S., Hawley, R. G., MacDougall, J. R., Kerbel, R. S., Khoo, N., et al. (1993). Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Experimental Cell Research, 206 , 204–211.
Abou-Kheir, W., Barrak, J., Hadadeh, O., & Daoud, G. (2017). HTR-8/SVneo cell line contains a mixed population of cells. Placenta, 50 , 1–7.
Harun, R., Ruban, L., Matin, M., Draper, J., Jenkins, N. M., Liew, G. C., et al. (2006). Cytotrophoblast stem cell lines derived from human embryonic stem cells and their capacity to mimic invasive implantation events. Human Reproduction, 21 , 1349–1358.
Genbacev, O., Donne, M., Kapidzic, M., Gormley, M., Lamb, J., Gilmore, J., et al. (2011). Establishment of human trophoblast progenitor cell lines from the chorion. Stem Cells., 29 , 1427–1436.
Xu, R. H., Chen, X., Li, D. S., Li, R., Addicks, G. C., Glennon, C., et al. (2002). BMP4 initiates human embryonic stem cell differentiation to trophoblast. Nature Biotechnology, 20 , 1261–1264.
Amita, M., Adachi, K., Alexenko, A. P., Sinha, S., Schust, D. J., Schulz, L. C., et al. (2013). Complete and unidirectional conversion of human embryonic stem cells to trophoblast by BMP4. Proc Natl Acad Sci U S A., 110 , E1212–E1221.
Zdravkovic, T., Nazor, K. L., Larocque, N., Gormley, M., Donne, M., Hunkapillar, N., et al. (2015). Human stem cells from single blastomeres reveal pathways of embryonic or trophoblast fate specification. Development, 142 , 4010–4025.
Download references
This study was supported by the Key Project on the Integration of Industry, Education and Research Collaborative Innovation of Fujian Province (No. 2021YZ034011), the Key Project on Science and Technology Program of Fujian Health Commission (No. 2021ZD01002),the Joint Funds for the Innovation of Science and Technology, Fujian Province (No: 2021Y9185),the Province-level special subsidy funds for health in Fujian Province (No: Fujian Finance Index [2019] 827),the Fujian Province health scientific research personnel training project(No: Fujian Finance Index [2021] 55),the Fujian Province health scientific research personnel training project(No: Fujian Finance Index [2021] 500).
Author information
Authors and affiliations.
Medical Research Center, Fujian Maternity and Child Health Hospital, College of Clinical Medicine for Obstetrics & Gynecology and Pediatrics, Fujian Medical University, Fuzhou, China
Yujia Chen, Zhoujie Ye, Liping Zhu & Xinrui Wang
National Health Commission (NHC), Key Laboratory of Technical Evaluation of Fertility Regulation for Non-Human Primate, Fujian Maternity and Child Health Hospital, Fuzhou, China
Medical Genetic Diagnosis and Therapy Center of Fujian Maternity and Child Health Hospital, College of Clinical Medicine for Obstetrics and Gynecology and Pediatrics, Fujian Provincial Key Laboratory of Prenatal Diagnosis and Birth Defect, Fuzhou, China
Department of Pathology, Fujian Medical University Cancer Hospital, Fujian Cancer Hospital, Fuzhou, China
You can also search for this author in PubMed Google Scholar
Contributions
YC and ZY write of the manuscript, ML and LZ made diagrams of the manuscript, XW and LX made revisions to the manuscript.
Corresponding authors
Correspondence to Liangpu Xu or Xinrui Wang .
Ethics declarations
Ethical approval.
Ethical Procedure The research meets all applicable standards with regard to the ethics of experimentation and research integrity, and the following is being certified/declared true. As an expert scientist and along with co-authors of concerned field, the paper has been submitted with full responsibility, following due ethical procedure, and there is no duplicate publication, fraud, plagiarism, or concerns about animal or human experimentation.
Consent to Participate
All authors involved in the study obtained informed consent.
Consent to Publish
Paper is containing original research and has not been submitted / published earlier in any journal and is not being considered for publication elsewhere. All authors have seen and approved the manuscript and have contributed significantly for the paper.
Conflict of Interest
None of the authors of this paper has a financial or personal relationship with other people or organizations that could inappropriately influence or bias the content of the paper. It is to specifically state that “No Competing interests are at stake and there is No Conflict of Interest” with other people or organizations that could inappropriately influence or bias the content of the paper.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yujia Chen and Zhoujie Ye are co-first authors.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Chen, Y., Ye, Z., Lin, M. et al. Deciphering the Epigenetic Landscape: Placental Development and Its Role in Pregnancy Outcomes. Stem Cell Rev and Rep (2024). https://doi.org/10.1007/s12015-024-10699-2
Download citation
Accepted : 14 February 2024
Published : 08 March 2024
DOI : https://doi.org/10.1007/s12015-024-10699-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Epigenetics
- Pregnancy Complications
- Fetal Growth
- Clinical Application
- Find a journal
- Publish with us
- Track your research
- How To Get Pregnant
- Infertility
- Pregnancy Week by Week
- Second Pregnancy
- Giving Birth
- Post Pregnancy
- Breastfeeding
- Development
- Browse Names
- Play & Activities
- Coloring Pages
- Food & Nutrition
- Health & Fitness
- Style & Beauty Care
- Collaborations
- New Parents
- Single Parenting
- Relationships
- Baby Eye Color Calculator
- Online Pregnancy Test
- Chinese Gender Predictor
- Implantation Calculator
- hCG Calculator
- Period Calculator
- ovulation calculator
- pregnancy due date calculator
- Child Height Predictor
- Pregnancy Weight Gain Calculator
- Breast Milk Calculator
- Child Growth Percentile Calculator
- Baby Cost Calculator
- BMI Calculator For Kids & Teens
- Contraction Calculator
- Immunization Scheduler and Chart
- C-Section Checklist
- Online Twin Pregnancy Quiz
- Numerology calculator
- Child Blood Type Calculator
- Nakshatra Calculator
- Diaper Bag Checklist
- Baby Name Combiner
Home • Pregnancy • Safety
6 Functions Of Placenta During Pregnancy And Placental Problems
The placenta is responsible for providing the vital oxygen and nutrients the growing fetus needs.
Dr. Subhashis Samajder, a consultant gynecologist-obstetrician with nine years of experience, is currently practicing at Narayana Multispeciality Hospital, Howrah. His area of expertise includes abortion, colposcopy surgery, hysterectomy, h... read full bio
Rebecca is a pregnancy writer and editor with a passion for delivering research-based and engaging content in areas of fertility, pregnancy, birth, and post-pregnancy. She did her graduation in Biotec... read full bio
Swati Patwal is a clinical nutritionist, a Certified Diabetes Educator (CDE) and a toddler mom with more than a decade of experience in diverse fields of nutrition. She started her career as a CSR pro... read full bio
Aneesha holds a Bachelor's degree in Biotechnology from USTM, Meghalaya and Master’s degree in Applied Microbiology from VIT, Vellore. With two years of experience, she has worked on different researc... read full bio
MomJunction believes in providing reliable, research-backed information to you. As per our strong editorial policy requirements, we base our health articles on references (citations) taken from authority sites, international journals, and research studies. However, if you find any incongruencies, feel free to write to us .
Image: Shutterstock
The placenta is an organ that develops in the uterine wall when a woman is pregnant. The critical functions of the placenta during pregnancy include the growth and development of the fetus (1) . It also aids in maternal-fetal exchange by transporting oxygen and nutrients to the fetus and removing the waste from the fetal blood.
Before we begin any further, let us understand what a placenta is. A placenta is a life-supporting organ for the growing fetus; however, sometimes, its functions may be hindered due to several factors, such as blood clots, abdominal trauma, and blood pressure. These factors may lead to pregnancy complications.
This post informs you about the functions of the placenta, the problems related to it, the symptoms of such complications, and ways to reduce the risk.
Why Is The Placenta Important?
The placenta is your unborn baby’s life support system and plays a key role in its development. It connects the mother to the fetus through the umbilical cord during gestation period and carries out the functions your fetus cannot perform by itself (2) .
Functions Of The Placenta During Pregnancy
The placenta serves the functions of organs such as the lungs, kidneys, and liver until your fetus develops them. Some of the main functions that the placenta performs include (1) (3) :
- Respiratory, excretory, nutritive, endocrine i X System of glands that secrete hormones directly into the bloodstream , barrier function, immunological function.
- Supplying oxygen and output of co2 is done via simple diffusion (respiratory) and nutrients to the fetus via the umbilical cord (nutritive).
- Clearing out waste products, such as urea, creatinine, uric acid from the fetus (excretory).
- Metabolizing and releasing food substances and required products into the maternal and fetal blood circulations.
- Protecting the fetus from xenobiotics (compounds including food additives, drugs, and environmental pollutants).
Image: IStock
- Producing steroid and peptide hormones that help in the growth and development of the baby (endocrine).
- Protecting the fetus from infections (bacterial) and maternal diseases.
- Fetal membrane protects the transfer of noxious i X Extremely harmful substances less than 500 dalton except antibody and antigen (barrier).
- Produces different enzymes such as diamine oxidase and oxytocinase (enzymatic).
Factors That Affect The Placental Function
Various factors can affect the placental function during pregnancy and make the mother prone to certain risks. They may include:
- Mother’s age: Mothers who conceive after the age of 35 are likely to experience placental problems (4) .
- Twin or multiple pregnancies: Mothers carrying more than one baby are likely to develop a weak placenta. It may raise the risk of early placental detachment (5) .
- Premature rupture of membranes: Your baby is usually cushioned and protected by the amniotic sac (membrane filled with amniotic fluid). If it breaks or leaks before labor, you may be at risk of placental infections (chorioamnionitis) and placental abruption (premature placental separation from the uterus) (6) .
- Blood-clotting disorders: Blood clotting as a result of genetic susceptibility, obesity, increased maternal age, medical illnesses, prolonged immobility, etc., are likely to form inside the placenta too. It may, sometimes, cut off the blood supply, and pose danger to the baby (7) .
- Abdominal trauma: A fall or any type of blow that causes abdominal trauma increases the risk of placenta abruption (8) .
- Prior placental problems: If you have experienced any placental problems in your previous pregnancy, you might develop it again (9) .
- Prior uterine surgery: Any previous surgery, such as cesarean section or uterine fibroids i X Non-malignant, fibrous tumors that are commonly found on the wall of the uterus removal surgery, may increase your risk of placental conditions (10) .
- Blood pressure: High blood pressure or hypertension levels might affect your placental function (11) .
- Substance abuse: If you smoke or take drugs, you may be at risk of placental conditions (12) .
Problems Related To The Placenta
Some of the possible problems related to the placenta include:
- Placental abruption: It is a condition in which the placenta separates from the uterine wall before delivery. It could deprive the fetus of oxygen and nutrients and may result in premature birth , stillbirth, and growth problems; it can cause severe bleeding (13) . One-third of the cases of abruption are associated with any form of hypertension.
- Placenta previa: It occurs when the placenta lies low in the lower uterine segment of uterus and covers the opening of the cervix partially or totally. It may, therefore, block the baby’s exit from the womb, resulting in preterm labor, placental tear, and antepartum i X The period leading up to the birth of a baby and intrapartum i X The period from the onset of labor to the delivery of the placenta hemorrhage (14) (15) .
- Placenta accreta: This rare complication occurs when the placenta grows into the uterine wall and is unable to be detached properly during delivery. It could lead to vaginal bleeding during and after delivery (16) .
- Retained placenta: A part of the placenta or membranes remain intact in the womb after childbirth. It may occur when the placenta gets stuck behind a uterine muscle. It could be a life-threatening condition and requires manual removal of placenta (MROP) after a few hours of delivery (17) .
- Placental insufficiency: The placenta may not be able to transfer nutrients to the fetus. It may lead to fetal growth restriction (FGR), stillbirth, and low birth weight (18) .
- Anterior placenta: The placenta develops on the front of the uterus with the fetus behind it. It could make it difficult for you to feel the fetal kicks and for sonographers to find the heartbeat. It may lead to placental abruption, intrauterine growth restriction, and fetal death (19) .
Placenta previa may resolve as pregnancy progresses. Liz Miller, a mother from the United States, says, “We had thought for a long time that he might be early- due to the possibility of a c-section because of complications with my placenta. I had what is called placenta previa- where the placenta sits on top of the cervix, getting in the way of a natural birth. However, my placenta began to move out of the way and by 36 weeks, I was cleared to try a vaginal birth. I was ready to go ( i ).”
What Are The Signs And Symptoms Of Placental Problems?
The signs and symptoms that may indicate placental problems include:
- Vaginal bleeding
- Abdominal pain
- Constant uterine contractions
- Decreased fetal movement
You should see your doctor if you begin to experience these symptoms suddenly and often.
Can You Reduce The Risk Of Placental Problems?
You might not be able to prevent several of the placental problems. But you may take a few measures for a healthy pregnancy.
- Go for regular prenatal checkups. According to the CDC National Center for Health Statistics , about 2.1% of mothers received no prenatal care, and 12.5% received insufficient care, potentially leading to undetected placental problems during pregnancy. Therefore, prioritizing proper and timely prenatal care is crucial for supporting a healthy pregnancy.
- Manage health conditions such as blood pressure and gestational diabetes i X A condition characterized by elevated blood sugar levels due to hormonal and physical changes in pregnancy .
- Quit smoking and use of illegal drugs.
- Inform your doctor if you had any placental problem in your previous pregnancy or had any surgery of the uterus.
How Is The Placenta Delivered?
Usually there are mild contractions (sometimes there may not) that could help the placenta to separate from the uterine wall and move through the birth canal.
In a vaginal delivery, the third stage of labor begins with childbirth and ends with placental delivery. Your practitioner may inject Pitocin (oxytocin) into your body to induce uterine contraction and speed up placenta expulsion (20) .
In a C-section, your practitioner physically removes the placenta before closing the incision. The remaining fragments are removed to prevent infection and bleeding (21) .
Does A Doctor Check For Placental Abnormalities Even Without Symptoms?
During the regular ultrasound scans, the healthcare practitioner checks for all possible placental abnormalities. Placental conditions are likely to be associated with vaginal bleeding, and it is important to seek medical attention.
Frequently Asked Questions
1. When does the placenta fully form?
The placenta fully forms by weeks 18 to 20 and continues to grow throughout the pregnancy. It is likely to weigh around one pound at the time of delivery (22) .
2. Is the placenta part of the baby or the mother?
The placenta is a fetomaternal organ comprising two parts—the fetal placenta that develops from the same blastocyst, which forms the fetus (villous chorion), and the maternal placenta that develops from the tissue of the maternal uterus (decidua basalis).
3. Which placenta position is best for normal delivery?
The placental position may not be a cause of concern in several cases. The anterior placenta position—the placenta in front of the stomach—could make it difficult to hear the fetal heart sounds.
4. What is the correlation between the placenta and preterm labor?
Chronic inflammation of the placenta and problems with placental vasculature (blood flow to placenta from maternal and fetal systems) have been associated with preterm birth in scientific studies (24) .
5. What prenatal testing is available to evaluate the placenta?
Placental abnormalities are usually screened using ultrasound scans. Such scans may be performed transabdominally or transvaginally (25) .
6. How does the placenta protect the baby from infections?
The placenta forms a physical barrier between the maternal and fetal circulations that allows only selective substances to pass through and prevents the transfer of pathogens. However, some pathogens may still be transferred from the mother to the fetus and cause fetal infection (26) .
7. Can stress cause placenta problems?
Dr. Laila Kaikavoosi , a UK-based hormone specialist, says, “The placenta can experience a negative impact from stress during pregnancy. The increased transfer of maternal cortisol to the fetus through the placenta, caused by stress or worry is only one explanation. The placenta can typically neutralize cortisol, but this defense system may fail under great stress. In addition, stress can influence the health and function of the placenta by causing disorders like pre-eclampsia, gestational diabetes, pre-diabetes, and maternal microbiota abnormalities.”
The placenta supports a baby’s life inside the womb. It offers the baby nourishment and performs all the necessary functions that a baby can’t do itself. Hence, ensuring proper placental functioning is essential for the viability of the pregnancy. Going for prenatal checkups regularly and quitting smoking, alcohol, and illegal drugs are a few ways to prevent some of the placental problems. Keep your doctor informed about any placental or uterine problems so they can guide you better.
Infographic: What Are The Risks Of Pills Made From Your Own Placenta?
Illustration: Momjunction Design Team
Key Pointers
- The placenta connects the mother to the fetus and provides essential oxygen and nutrients to the fetus.
- It protects the fetus from potential toxins, including food additives, drugs, and pollutants.
- Factors such as the mother’s age, substance abuse, and high blood pressure can affect the functioning of the placenta.
- The placenta produces growth-regulating hormones that aid fetal development.
- Symptoms of placental problems include back and abdominal pain, vaginal bleeding, and decreased fetal movement.
- Regular prenatal care and monitoring can help identify potential placental problems to ensure proper maternal and fetal health.
Personal Experience: Source
MomJunction articles include first-hand experiences to provide you with better insights through real-life narratives. Here are the sources of personal accounts referenced in this article.
i. My birth story; https://apparentinprogress.blogspot.com/2020/11/my-birth-story.html
1. Gude NM, et al.; Growth and function of the normal human placenta ; Thrombosis Research (2004). 2. Wang Y and Zhao S; Chapter 2 – Placental Blood Circulation; Vascular Biology of the Placenta ; Morgan & Claypool Life Sciences; (2010). 3. Graham J. Burton and Abigail L. Fowden; The placenta: a multifaceted, transient organ ; Philosophical Transactions of the Royal Society B: Biological Sciences (2015). 4. Advanced Maternal Age ; Texas Children’s Hospital – Pavilion for Women 5. Complications of Multiple Pregnancy ; University of Rochester Medical Center 6. Premature Rupture of Membranes (PROM)/Preterm Premature Rupture of Membranes (PPROM) ; The Children’s Hospital of Philadelphia 7. Blood Clotting & Pregnancy ; American Society of Hematology 8. Lavin JP and Polsky SS; Abdominal trauma during pregnancy ; Clinics in Perinatology (1983). 9. Kimberly M. Rathbun and Jason P. Hildebrand; Placenta Abnormalities ; Treasure Island (FL): StatPearls Publishing (2020). 10. Tayyaba Majeed, et al.; Frequency of placenta previa in previously scarred and non scarred uterus ; Pakistan Journal of Medical Sciences (2015). 11. Khattak SN, et al.; Association of maternal hypertension with placental abruption ; Journal of Ayub Medical College Abbottabad (2012). 12. Punam Sachdeva, B.G. Patel, and B.K. Patel; Drug Use in Pregnancy; a Point to Ponder ; Indian Journal of Pharmaceutical Sciences (2009). 13. Placental abruption ; Better Health Channel; State Government of Victoria, Australia 14. Placenta previa ; U.S. Department of Health and Human Services National Institutes of Health Abdulrahman Abdulelah Almnabri et al.; Management of Placenta Previa During Pregnancy ; The Egyptian Journal of Hospital Medicine (2017) 15. Placenta Accreta ; USF Health Obstetrics and Gynecology 16. Andrew D Weeks; The Retained Placenta ; African Health Sciences (2001). 17. Usha Krishna and Sarita Bhalerao; Placental Insufficiency and Fetal Growth Restriction ; J Obstet Gynaecol India (2011). 18. Shumaila Zia; Placental location and pregnancy outcome ; Journal of the Turkish-German Gynecological Association (2013). 19. Labour and Delivery Care Module: 6. Active Management of the Third Stage of Labour ; The Open University 20. Cesarean Delivery; Stanford Children’s Health 21. Stages of Development of the Fetus ; The Merck Manual 22. Placenta and Extraembryonic Membranes; Anatomy ; University of Michigan Medical School 23. Placenta ; Cleveland Clinic 24. Sunitha C Suresh et al.; A comprehensive analysis of the association between placental pathology and recurrent preterm birth ; American Journal of Obstetrics and Gynecology (2022) 25. Kimberly M. Rathbun and Jason P. Hildebrand; Placenta Abnormalities ; National Library of Medicine (2022) 26. Regina Hoo et al.; Innate Immune Mechanisms to Protect Against Infection at the Human Decidual-Placental Interface ; Frontiers in Immunology
- Fact-checker
Subhashis Samajder MS, DNB
Dr. laila kaikavoosi mbbs, gp, rebecca malachi bsc, swati patwal m.sc. (food & nutrition), mba, aneesha amonz msc, latest articles, effective ways to break the habit of thumbsucking in babies.
Introducing pacifiers, distractions, and reminders could help deal with a baby's thumb-sucking habits.
What Causes Numbness During Pregnancy And Ways To Deal With It
This condition can be managed by using hot compresses or with massage therapy.
Miralax For Babies And Toddlers: Safety, Uses And Dosage
Little is known about the drug's safety, and it must be administered only as per a doctor's prescription.
Teething Tablets Safe For Babies And Their Alternatives
This is a normal phase of the baby’s development; refrain from using non-prescription drugs.
15+ Early Signs That You’re Pregnant, Before You Miss Period
Understand how PMS signs differ from pregnancy and get possible indications of conception.
Placenta Previa: What It Is, Types, Causes, And Treatment
The cause of placenta previa is unknown, but treatment can alleviate the symptoms.
TT Injection In Pregnancy: Safety, Dosage And Side Effects
TT vaccine during pregnancy isn’t known to cause any harm to the mother or baby.
How To Treat And Prevent Mosquito Bites In Babies?
These itchy bites usually subside in a few hours, but look out for allergic reactions.
Sunburn In Babies: Causes, Treatment And Prevention
Ensure your infant’s delicate skin is adequately protected from harsh sun rays.
Climbing Stairs During Pregnancy: When Is It Safe And When To Avoid?
Whether climbing stair is safe or not depends on the expectant mom’s overall health.
13 Effective Tips To Deal With Your Whiny Baby
Dealing with a whiny baby is any parent’s worst nightmare, but patience is the only key.
Uterine Fibroids During Pregnancy: Symptoms And Treatment
For proper treatment, it is crucial to know the signs of this condition in pregnancy.
REVIEW article
The role of placental hormones in mediating maternal adaptations to support pregnancy and lactation.

- Department of Physiology, Development and Neuroscience, Centre for Trophoblast Research, University of Cambridge, Cambridge, United Kingdom
During pregnancy, the mother must adapt her body systems to support nutrient and oxygen supply for growth of the baby in utero and during the subsequent lactation. These include changes in the cardiovascular, pulmonary, immune and metabolic systems of the mother. Failure to appropriately adjust maternal physiology to the pregnant state may result in pregnancy complications, including gestational diabetes and abnormal birth weight, which can further lead to a range of medically significant complications for the mother and baby. The placenta, which forms the functional interface separating the maternal and fetal circulations, is important for mediating adaptations in maternal physiology. It secretes a plethora of hormones into the maternal circulation which modulate her physiology and transfers the oxygen and nutrients available to the fetus for growth. Among these placental hormones, the prolactin-growth hormone family, steroids and neuropeptides play critical roles in driving maternal physiological adaptations during pregnancy. This review examines the changes that occur in maternal physiology in response to pregnancy and the significance of placental hormone production in mediating such changes.
Introduction
Pregnancy is a dynamic and precisely coordinated process involving systemic and local changes in the mother that support the supply of nutrients and oxygen to the baby for growth in utero and in the subsequent lactation. Inappropriate adaptation of maternal physiology may lead to complications of pregnancy, such as gestational diabetes, preeclampsia, fetal growth restriction, fetal overgrowth and pre-term birth; which can have immediate consequences for fetal and maternal health. Furthermore, these pregnancy complications can also lead to long-term health consequences for the mother and infant. Altered fetal growth is associated with an increased risk of the offspring developing obesity, type-2 diabetes and cardiovascular disease in adulthood ( Hales and Barker, 2001 ; Barker, 2004 ; Fowden et al., 2006 ). Moreover, women who develop gestational diabetes or preeclampsia are more likely to develop type-2 diabetes or cardiovascular disease in later life ( Kim et al., 2002 ; Petry et al., 2007 ). Maternal adaptations to pregnancy are largely mediated by the placenta; the functional interface between the mother and fetus that secretes hormones and growth factors into the mother with physiological effects. This review aims to provide an overview of the physiological changes that occur in the mother in response to pregnancy and to discuss the role of key placental hormones in mediating such adaptations. In particular, this review focuses on the importance of the prolactin-growth hormone family (e.g., prolactin, placental lactogen and growth hormone), steroids (estrogens and progesterone) and neuropeptides (serotonin, melatonin and oxytocin) in adaptations of maternal physiology during pregnancy. Where possible, this review draws upon findings in women and animal models, including rodents and sheep. However, differences exist between species in the specific hormones produced by the placenta, the access of these hormones to the maternal circulation, and the relative proportion of conceptus mass to maternal size (hence constraint on the mother to provide resources for fetal growth; Haig, 2008 ; Carter, 2012 ; Fowden and Moore, 2012 ). Where such differences between species exist, these have been highlighted and discussed as necessary in the relevant sections. Nevertheless, although some effects described may not be applicable to all species, the different animal models of pregnancy still provide novel insight into the fundamental mechanisms of maternal adaptation during gestation.
Adaptations in Maternal Physiology During Pregnancy and Lactation
Most tissues and organs in the mother respond to the pregnant state. Changes include alterations in size, morphology, function and responsiveness of tissues and organs to hormonal and metabolic cues. These changes arise in the cardiovascular, pulmonary, immune, and metabolic systems of the mother (Figure 1 ). Some of these changes are seen from very early in pregnancy, prior to the establishment of a fully functional placenta, highlighting that non-placental factors may also be important ( Paller et al., 1989 ; Drynda et al., 2015 ). The specific nature of changes in maternal physiology depends on the stage of the pregnancy and appears to track with alterations in the metabolic requirements of the mother versus the developing fetus.
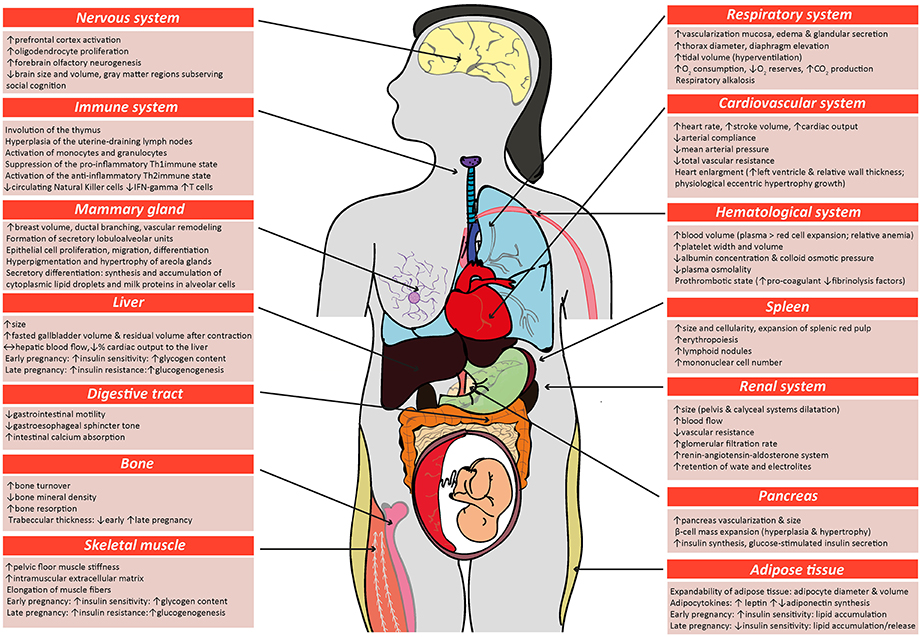
Figure 1 . Schematic diagram highlighting the main physiological modifications in the maternal physiology in response to pregnancy. Many of the changes described in the figure for women during pregnancy also occur in other species, including mice. Respiratory system ( Macrae and Palavradji, 1967 ; Weinberger et al., 1980 ; Contreras et al., 1991 ; Hegewald and Crapo, 2011 ; Frise et al., 2013 ; Lomauro and Aliverti, 2015 ; Soma-Pillay et al., 2016 ); cardiovascular system ( Adamova et al., 2009 ; Li et al., 2012 ; Pieper, 2015 ; Soma-Pillay et al., 2016 ); hematological system ( Shakhmatova et al., 2000 ; Chang and Streitman, 2012 ; Rodger et al., 2015 ; Soma-Pillay et al., 2016 ); spleen ( Maroni and De Sousa, 1973 ; Sasaki et al., 1981 ; Norton et al., 2009 ); renal system ( Davison and Dunlop, 1980 ; Atherton et al., 1982 ; Krutzén et al., 1992 ; Elsheikh et al., 2001 ; Cheung and Lafayette, 2013 ; Lumbers and Pringle, 2014 ; Pieper, 2015 ; Soma-Pillay et al., 2016 ); pancreas ( Ziegler et al., 1985 ; Ernst et al., 2011 ; Ohara-Imaizumi et al., 2013 ; Baeyens et al., 2016 ); adipose tissue ( Catalano et al., 2006 ; Hauguel-De Mouzon et al., 2006 ; Lain and Catalano, 2007 ; Nien et al., 2007 ; Hadden and Mclaughlin, 2009 ; Valsamakis et al., 2010 ; Musial et al., 2016 ); skeletal muscle ( Alperin et al., 2015 , 2016 ; Musial et al., 2016 ); bone ( Shahtaheri et al., 1999 ; Ulrich et al., 2003 ; Hellmeyer et al., 2006 ; Salles, 2016 ); digestive tract ( Everson, 1992 ; Fudge and Kovacs, 2010 ; Pieper, 2015 ); liver ( Munnell and Taylor, 1947 ; Van Bodegraven et al., 1998 ; Lain and Catalano, 2007 ; Bacq, 2013 ); mammary tissue ( Elling and Powell, 1997 ; Neville et al., 2002 ; Sternlicht, 2006 ; Pang and Hartmann, 2007 ); immune system ( Clarke and Kendall, 1994 ; Kendall and Clarke, 2000 ; Veenstra Van Nieuwenhoven et al., 2002 ; Norton et al., 2009 ; Mor and Cardenas, 2010 ; Saito et al., 2010 ; Racicot et al., 2014 ; Groen et al., 2015 ; Zöllner et al., 2017 ; Edey et al., 2018 ); nervous system ( Shingo et al., 2003 ; Gregg, 2009 ; Roos et al., 2011 ; Hoekzema et al., 2017 ).
Alterations in the maternal cardiovascular system begin very early in gestation ( Chapman et al., 1998 ) and ultimately lead to systemic vasodilation and increased blood perfusion of maternal organs, including the gravid uterus. Systemic vascular resistance is reduced by 25–30% and accompanied by a 40% increase in cardiac output during human pregnancy; while in mice, blood pressure decreases by 15% and cardiac output is increased by 48% ( Bader et al., 1955 ; Kulandavelu et al., 2006 ; Soma-Pillay et al., 2016 ). Renal blood flow and glomerular filtration rates are also increased ( Davison and Dunlop, 1980 ; Soma-Pillay et al., 2016 ). The renin-angiotensin-aldosterone system (RAAS) which is a major determinant for sodium balance during gestation, is progressively upregulated toward term with associated plasma volume expansion ( Elsheikh et al., 2001 ; Tkachenko et al., 2014 ). This rise in blood volume, which is required to cope with the oxygen requirements of the maternal organs and the conceptus growth, plateaus by the late gestation, resulting in an increase in total blood volume by approximately 30% at the end of pregnancy ( Chang and Streitman, 2012 ). There is also an increase in the numbers of red blood cells in the mother during pregnancy, due to proliferation of erythroid progenitors in the spleen ( Bustamante et al., 2008 ). Pulmonary function is also altered and encompasses changes in ventilation rates and blood gases. For instance, lung tidal volume and minute ventilation increases by 30–50% ( Hegewald and Crapo, 2011 ). As a result of increased oxygen consumption during hyperventilation, there is greater carbon dioxide production, which leads to chronic respiratory alkalosis that is compensated by an increased renal excretion of bicarbonate ( Weinberger et al., 1980 ). Overall, these adaptations ensure the well-being of the mother, while also providing an adequate blood flow to the placenta for fetal nutrition, oxygenation and maturation.
There are also alterations in maternal metabolic and endocrine state during gestation. In early pregnancy, the maternal pancreatic β-cell mass expands due to both hyperplasia and hypertrophy of islets, which for example in rats, results in a >50% increase ( Ackermann and Gannon, 2007 ; Rieck and Kaestner, 2010 ). The threshold for glucose-stimulated insulin production is also lowered and maternal circulating insulin concentration is greater compared to the non-pregnant state. In early pregnancy, when fetal demands are relatively low, whole body maternal insulin sensitivity is unchanged or increased and there is accumulation of energy reserves in the mother. In particular, early pregnancy is associated with adipocyte hypertrophy, increased lipogenesis and lipid storage and relates to improved insulin sensitivity of white adipose tissue in the mother ( Hadden and Mclaughlin, 2009 ; Mcilvride et al., 2017 ). Interestingly, in pregnant mice, brown adipose stores of the dam also switch to a white adipose tissue-like phenotype in early gestation ( Mcilvride et al., 2017 ). Additionally, glycogen accumulates in the liver, which also increases in size from early gestation ( Bustamante et al., 2010 ). In contrast, late pregnancy is associated with diminished maternal tissue insulin sensitivity and a concomitant increase in lipolysis and hepatic gluconeogenesis ( Freemark et al., 2002 ; Lain and Catalano, 2007 ; Musial et al., 2016 ). Despite the pregnancy-related rise in leptin and insulin concentrations, maternal appetite increases in pregnancy ( Villar et al., 1992 ; Douglas et al., 2007 ; Hadden and Mclaughlin, 2009 ; Díaz et al., 2014 ). Together, these metabolic and endocrine alterations increase lipid and glucose availability for the rapidly growing fetus in late gestation. Intriguingly in rodents, whole body responsiveness to insulin starts to improve near term, which may be important for conserving nutrients for maternal use, as parturition and lactation approach ( Musial et al., 2016 ). There are also notable changes in maternal bone metabolism during pregnancy. In particular, intestinal calcium absorption is enhanced in the mother during pregnancy via upregulation of 1,25-dihydroxyvitamin D levels, improved renal conservation and increased calcium mobilization from the maternal skeleton ( Hellmeyer et al., 2006 ). These processes support the supply of calcium for the formation, growth and mineralization of the fetal skeleton ( King, 2000 ; Kalkwarf and Specker, 2002 ).
The immune system of the mother during pregnancy is tightly regulated to prevent an unwanted immune response against the paternal antigens present in the developing conceptus ( Racicot et al., 2014 ; Groen et al., 2015 ; Zöllner et al., 2017 ). As gestation progresses, there is suppression of the pro-inflammatory Th1 type of immunity and a shift toward a more anti-inflammatory, Th2 immune state in the mother ( Saito et al., 2010 ), which supports fetal growth and maternal well-being ( Mor and Cardenas, 2010 ). In particular, the total abundance of circulating leukocytes, monocytes, granulocytes and T lymphocytes increase in the mother in response to pregnancy ( Groen et al., 2015 ). However, expression of major histocompatibility complex class II by circulating monocytes is reduced in the mother, which would decrease antigen presentation and stimulation of T cells during pregnancy and prevent the maternal immune system from mounting an unwanted response against fetal antigens ( Groen et al., 2015 ). The total number of circulating natural killer cells and secretion of pro-inflammatory cytokines (IFN-gamma) is also reduced in the pregnant state ( Veenstra Van Nieuwenhoven et al., 2002 ). However, close to parturition, the maternal immune system shifts to a pro-inflammatory state, particularly locally within the uterus, to promote labor ( Mor and Cardenas, 2010 ; Edey et al., 2018 ). There are also specific changes in the numbers of different leukocyte populations in the maternal thymus and spleen during pregnancy ( Clarke and Kendall, 1994 ; Kendall and Clarke, 2000 ; Norton et al., 2009 ). The spleen, which also has functions in hematopoiesis, enlarges due to an expansion of the splenic red pulp during pregnancy ( Maroni and De Sousa, 1973 ; Norton et al., 2009 ). Neurological changes must also occur during pregnancy to increase maternal nursing behavior and enable the mother to properly care for her newborn infant ( Bridges et al., 1997 ; Bridges, 2015 ; Kim, 2016 ; Kim et al., 2016 ). For instance, there is increased activation of the prefrontal cortex and neurogenesis of the forebrain olfactory bulb ( Shingo et al., 2003 ), which are important in regulating behavior. In addition, formation of lobulo-alveolar units in the mammary gland commences during pregnancy, in preparation for lactational support of the neonate.
Placental Hormones that Mediate Maternal Adaptations to Pregnancy, Parturition and Lactation
The placenta is a highly active endocrine organ during gestation; secreting a variety of hormones with physiological effects in the mother. Placental hormones include members of the prolactin and growth hormone family, steroid hormones and neuroactive hormones. The function of these hormones in driving physiological changes during pregnancy has been assessed in two main ways. First, the expression and activity of the hormones have been manipulated in vivo by either exogenously administering or genetically manipulating the expression of hormones and hormone receptors to study the physiological consequences for the animal. Secondly, hormones have been manipulated similarly in cultured cells and tissue explants to inform on the cellular and molecular mechanisms by which they modulate function. The effects of hormones in non-pregnant animals have been included as they provide information on the baseline of physiological changes that occur in the absence of hormone expression/activity, which is especially important in the case of some placental-derived hormones, where analyses in the pregnant state have not been conducted.
Prolactin (PRL)-Growth Hormone (GH) Family
The PRL-GH family is one of the main families of hormones secreted by the placenta during gestation. Members of this family consist of prolactin (PRL) ( Handwerger et al., 1992 ), placental lactogens (PLs) ( Wiemers et al., 2003 ), PRL-like hormones ( Wiemers et al., 2003 ), proliferins (PLF) ( Lee et al., 1988 ), proliferin-related proteins (PRP) ( Jackson et al., 1994 ) and growth hormone (GH). Between mammalian species, there are differences in the number and type of family members expressed by the placenta [reviewed elsewhere ( Linzer and Fisher, 1999 ; Soares, 2004 ; Soares et al., 2007 )]. For instance, in the mouse and rat, the placenta expresses all these members except for PRL and GH whereas the human placenta only expresses GH and PL genes. In mice and rats, expression of the individual PRL-GH family members vary spatially and temporally in the placenta ( Dai et al., 2002 ; Simmons et al., 2008 ; Urbanek et al., 2015 ). The anterior pituitary also produces PRL and GH; however this is diminished by mid-pregnancy, when placental hormone production predominates ( Bridges, 2015 ). In several species including rodents and humans, PRL is additionally produced by the decidua during pregnancy. The family members share structural similarity to one another and may bind, with varying affinity to PRL and GH receptors (PRLR and GHR, respectively), which are widely expressed by tissues in the body ( Haig, 2008 ; Trott et al., 2008 ; Ben-Jonathan and Hugo, 2015 ). As the PRL-GH members also exert similar functions, these have been presented in a grouped fashion in the text and tables (Tables 1 , 2 ). However, where possible, the roles of individual family members of the PRL-GH in physiological changes have been described.

Table 1 . Effects of the prolactin-growth hormone family in vivo .

Table 2 . Effects of the prolactin-growth hormone family in vitro .
Studies performed both in vivo and in vitro support a role for the PRL-GH family in mediating maternal metabolic adaptations to pregnancy (Tables 1 , 2 ). PRL, PRL-like proteins and PL, principally via the PRL receptor, induce β-cell mass expansion by both increasing β-cell proliferation and reducing apoptosis of islets in vivo and in vitro (Table 2 ; PRL/PL/GH; Brelje et al., 1993 ; Huang et al., 2009 ). PRL and PL also increase insulin secretion during pregnancy, particularly in response to glucose, by enhancing the expression of glucose sensors (glucokinase, hexokinase and glucose transporter-2) and activating the serotonin biosynthesis pathway in pancreatic islets (Table 2 ; PRL/PL/GH; Nielsen, 1982 ; Brelje et al., 1989 , 1993 ; Weinhaus et al., 1996 ; Sorenson and Brelje, 1997 ; Arumugam et al., 2014 ). Moreover, PL protects β-cells against streptozotocin-induced cell death in mice ( Fujinaka et al., 2004 ). GH may also be important for modulating pancreatic insulin production ( Billestrup and Nielsen, 1991 ; Brelje et al., 1993 ). However, GH from the placenta appears to be primarily important in the acquisition of insulin resistance and shifting metabolic fuel use from glucose to lipid in the mother during pregnancy (Table 1 ; PRL/PL/GH; Horber and Haymond, 1990 ; Goodman et al., 1991 ; Galosy and Talamantes, 1995 ; Barbour et al., 2002 ; Dominici et al., 2005 ; Boparai et al., 2010 ; Liao et al., 2016b ; Sairenji et al., 2017 ). Placental GH reduces insulin receptor expression and signaling, as well as, diminishes the abundance of the insulin-sensitive glucose-transporter, GLUT-4, in the skeletal muscle ( Barbour et al., 2004 ; Kirwan et al., 2004 ). Insulin receptor abundance and signaling in the liver is also reduced in response to increased GH abundance in transgenic mice ( Dominici et al., 1999 ). In white adipose tissue, GH also disrupts the insulin signaling pathway, and inhibits insulin action on glucose uptake and lipid accumulation ( Del Rincon et al., 2007 ). In part, the effects of GH may be mediated through insulin-like growth factor-1 (IGF1), which is primarily secreted from the liver in response to GH and exerts lipolytic effects during pregnancy ( Randle, 1998 ; Sferruzzi-Perri et al., 2006 ; Del Rincon et al., 2007 ). Insulin-like growth factor-2 (IGF2), which is not directly regulated by GH, but is secreted by the placenta is also important for modulating the sensitivity of β cells to glucose (Tables 1 , 2 ; IGF2; Casellas et al., 2015 ; Modi et al., 2015 ) and maternal insulin and glucose concentrations during pregnancy ( Petry et al., 2010 ; Sferruzzi-Perri et al., 2011 ). Polymorphisms/mutations in the PRL-GH family of genes and receptors have been reported in human pregnancies associated with gestational diabetes and fetal growth restriction ( Rygaard et al., 1998 ; Le et al., 2013 ). Moreover, loss of PRLR signaling in β-cells causes gestational diabetes mellitus (GDM) in mice ( Banerjee et al., 2016 ). Taken together, the production of PRL-GH family of hormones by the placenta appears to be important in regulating both insulin production and sensitivity of the mother in response to pregnancy.
The PRL-GH family is also implicated in the regulation of appetite and body weight. For instance, exogenous PRL increases food intake through inhibiting the action of leptin in non-pregnant rats (Table 1 ; PRL/PL/GH; Sorenson et al., 1987 ; Farmer et al., 1991 , 1992 ; Ladyman et al., 2010 ). In contrast, GH appears to decrease food intake in rodents through reducing ghrelin production and hypothalamic expression of appetite-stimulating neuropeptides, AgRP and NPY (Table 1 ; PRL/PL/GH; Farmer et al., 1991 , 1992 ). In non-pregnant animals, GH is important for controlling body weight and composition (such as adiposity; Farmer et al., 1991 , 1992 ; Zhou et al., 1997 ). However, in pregnancy, exogenous GH or GH releasing hormone (GHRH) does not appear to affect maternal weight gain in mice, although increases it in pigs (Table 1 ; PRL/PL/GH; Brown et al., 2012 ). The effect of PRL on weight gain and body adiposity is even less clear; with both no effect and an increase reported for non-pregnant and pregnant rodents.
The PRL-GH family also plays an important role in lactation and maternal behavior. In mice, a deficiency in PRLR or inhibition of PRL secretion in vivo compromises mammary gland development, differentiation and milk production; the latter of which is associated with loss of STAT5 signaling and fewer leaky tight junctions (Table 1 ; PRL/PL/GH; Weinhaus et al., 1996 ; Zhou et al., 1997 ). In contrast, exogenous GHRH in sheep and cows increases mammary gland milk production ( Hart et al., 1985 ; Enright et al., 1988 ). There is also evidence that PRL induces maternal behaviors, such as nurturing, nursing and pup retrieval in non-pregnant rodents (Table 1 ; PRL/PL/GH; Bridges and Millard, 1988 ). Taken together, members of the PRL-GH family appear to promote changes in maternal glucose metabolism, behavior and mammary gland function which are expected to be important for supporting the growth of offspring during pregnancy and lactation.
Steroid Hormones
The placenta is a primary source of steroid hormones during pregnancy. Placental steroid hormones include estrogens and progesterone ( Costa, 2016 ; Edey et al., 2018 ). In species like rodents, the corpus luteum continues to contribute to the circulating pool of steroid hormones during pregnancy, whereas in other species such as humans and ruminants, the placenta serves as the main source ( Costa, 2016 ). Physiological effects of progesterone are mediated predominately by nuclear receptors (PR-A, PR-B) although membrane bound-type receptors (mPR) enable non-genomic actions. Steroid hormones are implicated in pregnancy complications such as gestational diabetes and preeclampsia. High progesterone and estrogen concentrations have been reported for women with gestational diabetes ( Branisteanu and Mathieu, 2003 ; Qi et al., 2017 ). Moreover, placental estrogen and progesterone levels are reduced in preeclamptic patients compared with healthy pregnant women ( Açikgöz et al., 2013 ).
Studies performed in vivo , suggest placental steroid hormones may be important in driving the changes in insulin sensitivity and glucose metabolism of the mother during pregnancy (Table 3 ). Hyperinsulinemic-euglycemic clamp studies in women and rodents highlight a role for progesterone in reducing maternal insulin sensitivity during pregnancy. Progesterone administration decreases the ability of insulin to inhibit glucose production by the liver, and diminishes insulin-stimulated glucose uptake by skeletal muscle and to a lesser extent in the adipose tissue of non-pregnant animals (Table 3 ; Progesterone; Leturque et al., 1984 ; Ryan et al., 1985 ; Kim, 2009 ). In contrast, exogenous estrogen increases whole body insulin sensitivity in non-pregnant state (Table 3 ; Estrogen; Ahmed-Sorour and Bailey, 1980 ). Similarly, genetic deficiency of ERα or aromatase (Cyp19), which is involved in estrogen production, reduces hepatic and whole body insulin sensitivity and impairs glucose tolerance in non-pregnant mice ( Takeda et al., 2003 ; Bryzgalova et al., 2006 ). Loss of the estrogen receptor or estrogen production is also associated with increased body weight, adiposity and hepatic lipogenesis (Table 3 ; Estrogen; Takeda et al., 2003 ; Bryzgalova et al., 2006 ). Progesterone and estrogen also exert opposite effects on food intake in vivo (Table 3 ). In particular, estrogen depresses food intake in part via induction of leptin production by adipose tissue, whereas progesterone increases food intake by enhancing NPY and reducing CART expression by the hypothalamus (Table 3 ; Fungfuang et al., 2013 ; Stelmanska and Sucajtys-Szulc, 2014 ). Estrogen and progesterone however seem to have similar effects on the pancreas; they both appear to induce islet hypertrophy and/or increase pancreatic insulin levels and glucose-stimulated secretion in vivo (Table 3 ; Costrini and Kalkhoff, 1971 ; Bailey and Ahmed-Sorour, 1980 ). Nevertheless, there is some evidence that progesterone may inhibit the PRL-induced proliferation and insulin secretion of β cells in vitro (Table 4 ; Progesterone; Sorenson et al., 1993 ). Furthermore, in rodent models of type 1 and 2 diabetes mellitus, estrogen supplementation protects pancreatic β-cells from oxidative stress, lipotoxicity and apoptosis (Table 3 ; Estrogen; Tiano and Mauvais-Jarvis, 2012 ). Therefore, both estrogen and progesterone play roles in regulating insulin and glucose homeostasis, lipid handling and appetite regulation, which may be important in promoting metabolic changes in the mother during pregnancy.
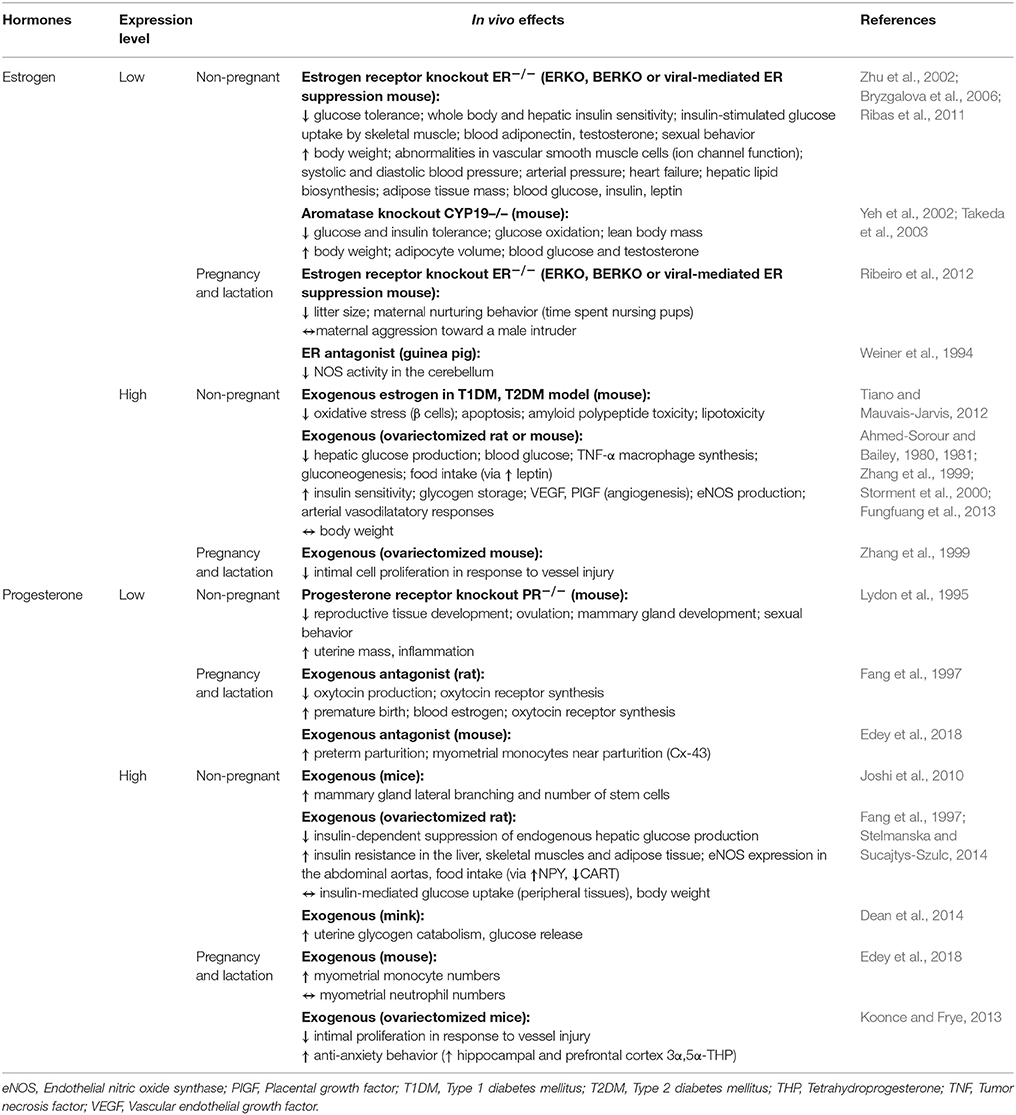
Table 3 . In vivo effects of steroid hormones in vivo .
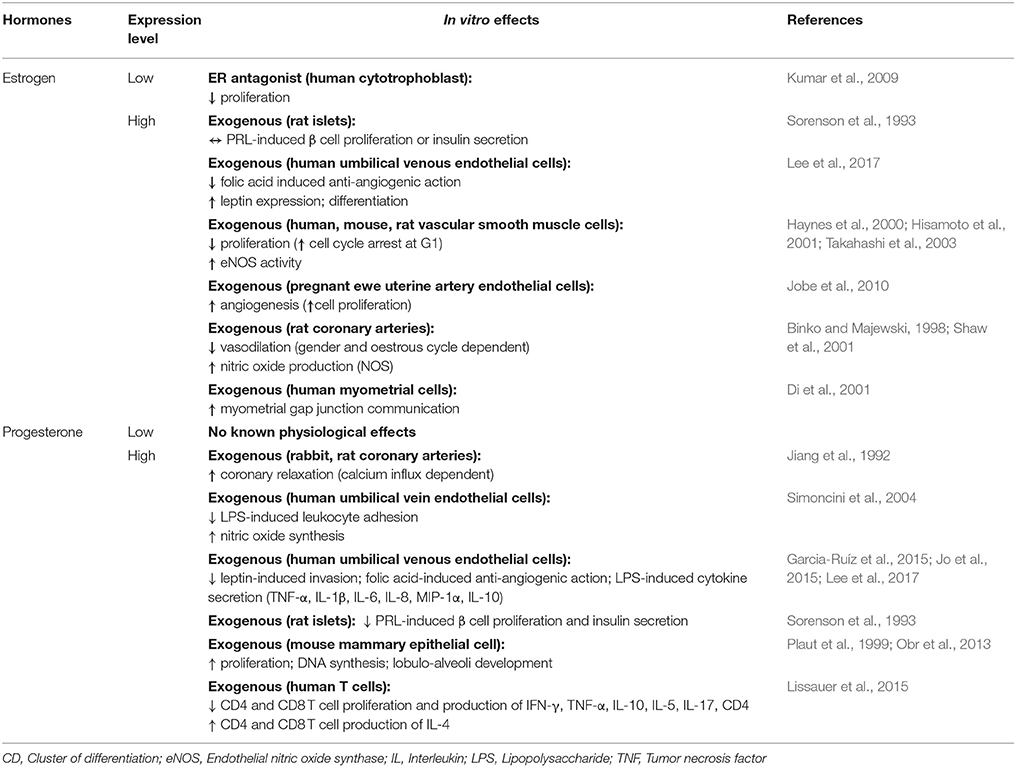
Table 4 . Effects of steroid hormones in vitro .
Work conducted both in vitro and in vivo indicate that estrogen and progesterone may also facilitate some of the cardiovascular changes that accompany pregnancy (Tables 3 , 4 ). Estrogen attenuates the vasoconstrictor responses of blood vessels, impairs vascular smooth muscle cell proliferation and calcium influx, and increases vasodilatory nitric oxide synthase activity in vitro (Table 4 ; Estrogen; Takahashi et al., 2003 ). It also increases uterine artery angiogenesis and amplifies the vasodilatory impact of vascular endothelial growth factor on isolated rat uterine vessels ( Storment et al., 2000 ; Jobe et al., 2010 ). In non-pregnant mice, deficiency of the ERβ gene leads to defects in vascular smooth muscle function, hypertension and signs of heart failure (Table 4 ; Estrogen; Zhu et al., 2002 ; Fliegner et al., 2010 ). Conversely, estrogen supplementation appears to protect the heart and vasculature from pressure overload or vessel injury ( Zhang et al., 1999 ; Zhu et al., 2002 ; Fliegner et al., 2010 ). Progesterone also exerts cardiovascular effects. It stimulates nitric oxide synthesis by human umbilical vein endothelial cells in vitro and by rat abdominal aorta and mesenteric arteries in vivo (Tables 3 , 4 ; Progesterone; Chataigneau et al., 2004 ; Simoncini et al., 2004 ). It also decreases blood pressure, when infused into ovariectomised ewes and protects against vascular injury in non-pregnant mice ( Pecins-Thompson and Keller-Wood, 1997 ; Zhang et al., 1999 ). In culture, progesterone induces hypertrophy and inhibits apoptosis of rodent cardiomyocytes ( Morrissy et al., 2010 ; Chung et al., 2012 ). Thus, via its impacts on cardiomyocytes, progesterone may mediate the pregnancy-induced growth of the mother's heart in vivo . In late pregnancy, the murine heart shifts to use fatty acids, rather than glucose and lactate, as a metabolic fuel. In part, this metabolic shift is proposed to be mediated by progesterone during pregnancy, which inhibits pyruvate dehydrogenase activity in ventricular myocytes ( Liu et al., 2017 ). Thus, placental-derived progesterone and estrogen may mediate part of the changes in the maternal cardiovascular system during pregnancy.
In many mammalian species, progesterone levels decline just before parturition and this is associated with the initiation of labor. Indeed, in rodents, inhibition of progesterone synthesis or administration of a progesterone antagonist results in premature delivery of the neonate (Table 3 ; Progesterone; Fang et al., 1997 ; Kota et al., 2013 ). In humans, circulating progesterone levels continue to be high until birth. Commencement of labor is therefore proposed to be related to a functional withdrawal of progesterone activity in the myometrium of women ( Brown A. G. et al., 2004 ; Norwitz and Caughey, 2011 ). In experimental animals, progesterone reduces the production of prostaglandins and decreases the expression of contraction-associated genes including oxytocin and prostaglandin receptors, gap junction proteins and ion channels in the myometrium (Table 3 ; Progesterone; Fang et al., 1997 ; Soloff et al., 2011 ; Edey et al., 2018 ). Together, these progesterone-mediated actions decrease contractility of uterine smooth muscle cells and maintain uterine quiescence until term. In contrast to progesterone, estrogen levels rise prior to term and estrogen promotes the expression of contraction-associated genes and contraction of the myometrium (Table 4 ; Estrogen; Nathanielsz et al., 1998 ; Di et al., 2001 ; Chandran et al., 2014 ). Therefore, in many species, the high ratio of estrogen to progesterone in the maternal circulation is thought to contribute the onset of labor. Parturition is associated with an influx of inflammatory cells and release of pro-inflammatory cytokines, including interleukin (IL)-1β and tumor necrosis factor (TNF)-α, in the myometrium, cervix and fetal membranes ( Golightly et al., 2011 ). In mice, progesterone reduces the expression of pro-inflammatory cytokines, including IL-1β and IL-6 by the uterus and trophoblast and may modulate the abundance of myometrial monocytes (Table 3 ; Estrogen; Edey et al., 2018 ). Progesterone also decreases the ability of LPS to induce pro-inflammatory cytokine secretion by human myometrium and placental explants ( Youssef et al., 2009 ; Garcia-Ruíz et al., 2015 ). It also diminishes the ability of estrogen to induce the infiltration of macrophages and neutrophils into the uterus, and decreases LPS-induced leukocyte adhesion to human umbilical vein cells ( Simoncini et al., 2004 ). Thus, it is perhaps not surprising that progesterone receptor null mice demonstrate chronic uterine inflammation, particularly in response to estrogen treatment (Table 3 ; Estrogen; Lydon et al., 1995 ). There is also evidence that placental steroids participate in cervical softening, by regulating the expression of matrix remodeling enzymes as well as leukocyte infiltration and function ( Chinnathambi et al., 2014 ; Gopalakrishnan et al., 2016 ; Berkane et al., 2017 ). In addition to regulating the events leading to parturition, recent data suggest that during the course of pregnancy, both estrogen and progesterone contribute to the maternal tolerance of the fetus by modulating proliferation and cytokine expression of CD4 and CD8 T cells and enhancing the suppressive function of T-regulatory cells ( Mao et al., 2010 ; Robinson and Klein, 2012 ; Lissauer et al., 2015 ).
Additionally, both estrogen and progesterone are key stimulators of mammary gland development. For instance, progesterone stimulates proliferation of mammary stem cells and mammary epithelium (Tables 3 , 4 ; Progesterone; Joshi et al., 2010 ; Lee et al., 2013 ). In mice, deficiency of the progesterone receptor restricts mammary gland development, whereas exogenous progesterone induces ductal side branching and lobuloalveolar differentiation and development (Table 3 ; Progesterone; Plaut et al., 1999 ; Joshi et al., 2010 ). In addition, both estrogen and progesterone may have indirect effects on mammary gland development by regulating prolactin secretion from the pituitary gland ( Rezaei et al., 2016 ).
Maternal behavior during and after birth are regulated by the steroid hormones. Estrogen stimulates maternal nurturing behavior in numerous species, including rats, mice, sheep and primates ( Bridges, 2015 ). In particular, maternal care is induced by estrogen treatment, whereas the converse happens when ERα expression is suppressed; deficiency of ERα increases the latency to pup retrieval and reduces the length of time dams spend nursing and licking their pups (Table 3 ; Estrogen; Ribeiro et al., 2012 ). Findings from animal models suggest that progesterone plays a role in regulating anxiety and depression-related behavior. For instance, exogenous progesterone stimulates anti-anxiety and anti-depressive actions in mouse dams (Table 3 ; Progesterone; Koonce and Frye, 2013 ). In contrast, progesterone withdrawal increases these types of behaviors ( Gulinello et al., 2002 ). Thus, placental-derived steroids may modulate several aspects of maternal physiology which are beneficial to both pregnancy and post-partum support of the offspring.
Neuroactive Hormones
One major target of placental hormones is the maternal brain and related neuroendocrine organs such as the hypothalamus and pituitary glands. These neuroendocrine effects enable the mother to respond and adapt accordingly to her environment, so as to mitigate the adverse effects of stress and maintain homeostasis ( Voltolini and Petraglia, 2014 ). Neuroactive hormones also prepare and enable the future mother to adequately care for her young ( Lévy, 2016 ). In addition to their impact on the maternal neuroendocrine system, these hormones have additional functions in vivo and in vitro functions as well, which are detailed in Tables 5 , 6 , respectively.
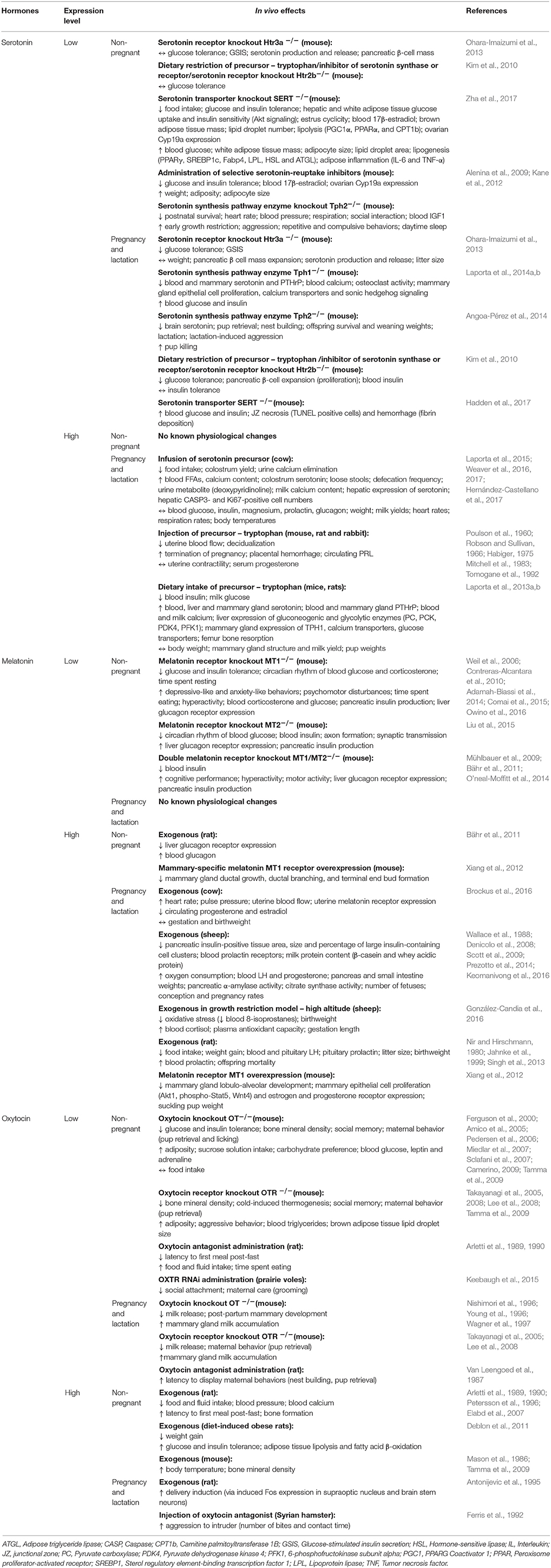
Table 5 . Effects of neuropeptides in vivo .
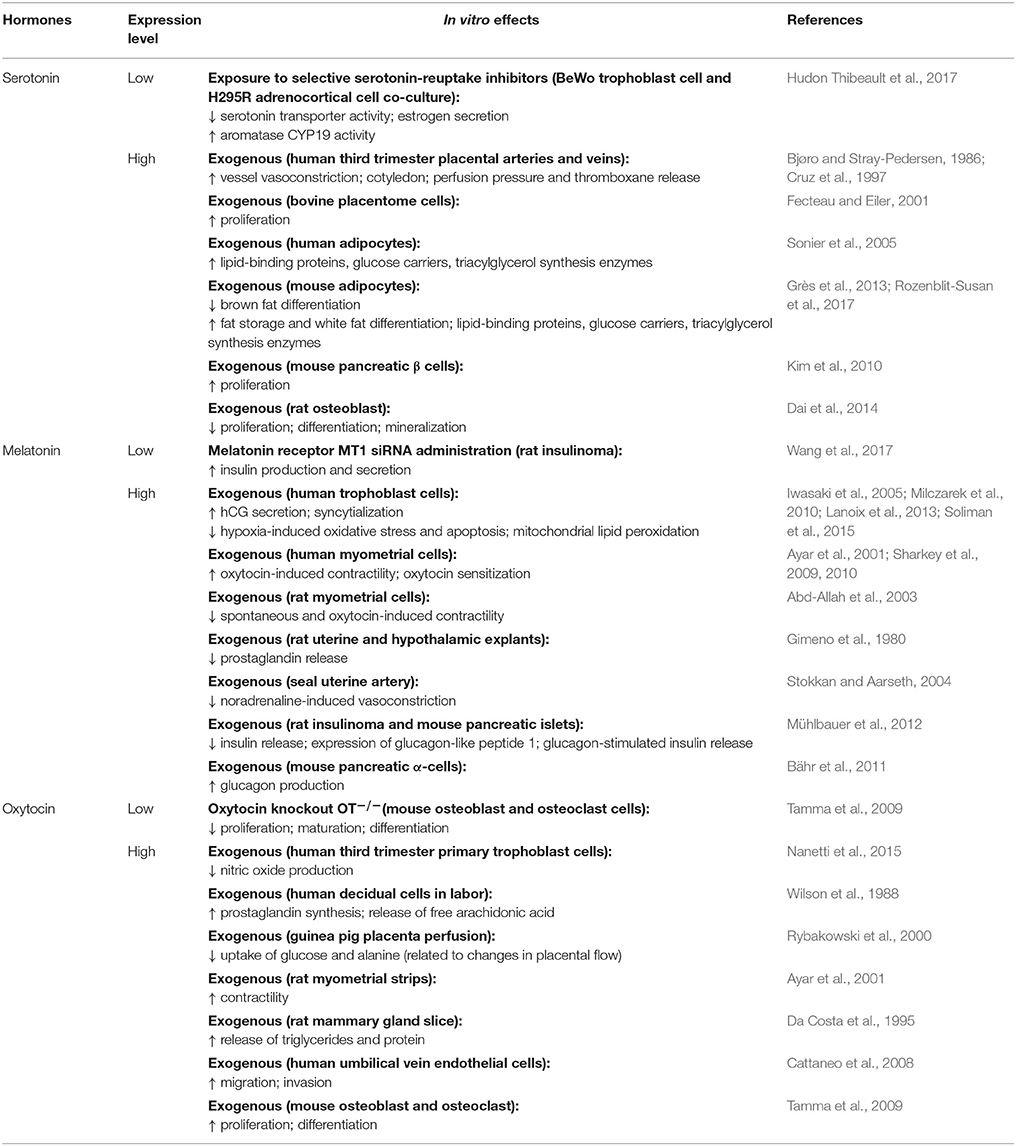
Table 6 . Effects of neuropeptides in vitro .
Melatonin and Serotonin
Melatonin and its precursor, serotonin, are tryptophan-derived hormones with well-known neuroendocrine impacts. In humans, circulating concentrations of melatonin and serotonin increase as pregnancy advances ( Lin et al., 1996 ; Nakamura et al., 2001 ). In the non-pregnant state, melatonin and serotonin are primarily produced by the pineal gland and the brain, respectively. However, the enzymes involved in melatonin and serotonin biosynthesis are also expressed by the human placenta throughout gestation ( Iwasaki et al., 2005 ; Soliman et al., 2015 ; Laurent et al., 2017 ). The mouse placenta similarly expresses the enzymes needed for serotonin synthesis ( Wu et al., 2016 ), although work is required to assess if melatonin synthesizing enzymes are also expressed. The rat placenta does not produce melatonin de novo due to the lack of synthesizing enzymes ( Tamura et al., 2008 ). However, the same study demonstrated that conditioned medium from cultured term rat placentas stimulated melatonin release by the maternal pineal gland ( Tamura et al., 2008 ). These findings suggest that placental-derived factors may indirectly regulate melatonin levels by the mother during pregnancy. Placental expression of melatonin, serotonin and their respective enzymes, also remains to be investigated in other species such as rabbits and sheep, which are commonly used in pregnancy-related studies. Mouse models that result in deficiencies or reduced bioactivity of these hormones demonstrate altered sleep patterns, melancholic behavior, hyperactivity and aggression in the non-pregnant state (Table 5 ; Serotonin and Melatonin; Weil et al., 2006 ; Alenina et al., 2009 ; Kane et al., 2012 ; Adamah-Biassi et al., 2014 ; O'neal-Moffitt et al., 2014 ; Comai et al., 2015 ). Serotonin is thus a major regulator of maternal mood and behavior ( Angoa-Pérez and Kuhn, 2015 ). For instance, genetically-induced serotonin deficiency leads to increased maternal aggression, lower pup retrieval and greater pup cannibalization, which reduces postnatal survival of offspring in mice ( Angoa-Pérez et al., 2014 ). There is some evidence that serotonin and melatonin may also impact maternal feeding behavior. For example, increased serotonin signaling reduces food intake in pregnant cows ( Laporta et al., 2015 ; Weaver et al., 2016 , 2017 ; Hernández-Castellano et al., 2017 ). Similarly, exogenous melatonin lowers food intake in pregnant rats ( Nir and Hirschmann, 1980 ; Jahnke et al., 1999 ; Singh et al., 2013 ). These negative effects on maternal food intake suggest that peak serotonin and melatonin concentrations in late pregnancy may serve to control the maternal appetite and prevent excessive weight gain.
Another key function of melatonin and serotonin is glucose homeostasis and the regulation of steroid synthesis (Table 5 ; Serotonin and Melatonin). In mice, loss of melatonin or serotonin signaling leads to glucose intolerance and insulin resistance, with consequences for blood glucose and insulin concentrations in both the non-pregnant and pregnant state ( Contreras-Alcantara et al., 2010 ; Kim et al., 2010 ; Owino et al., 2016 ). However, these neuroactive hormones appear to have differential effects on the pancreas (Table 6 ; Serotonin and Melatonin). Serotonin promotes pancreatic β-cell proliferation in vitro ( Kim et al., 2010 ), and is thus important for pancreatic β-cell mass expansion during pregnancy in mice ( Goyvaerts et al., 2016 ). In contrast, melatonin reduces insulin release by rodent pancreatic islets in vitro ( Mühlbauer et al., 2012 ). Non-pregnant mice with deficient serotonin signaling have impaired lipid handling and excessive lipid accumulation in association with reduced adipose aromatase expression and circulating estrogen ( Zha et al., 2017 ). Similarly, treating placental-derived trophoblast cells with norfluoxetine, a selective serotonin-reuptake inhibitor, inhibits aromatase activity and estrogen secretion in vitro ( Hudon Thibeault et al., 2017 ). Supplementation of melatonin in non-pregnant humans reduces circulating triglycerides and cholesterol levels, but effects of lipid handling in pregnancy are unknown ( Mohammadi-Sartang et al., 2017 ). Melatonin also modulates steroid production. For instance, melatonin treatment in pregnant cows reduces circulating estrogen and progesterone ( Brockus et al., 2016 ), while lack of melatonin signaling raises blood corticosterone in mice ( Comai et al., 2015 ).
Given melatonin's additional effects on regulating the circadian rhythm ( Mühlbauer et al., 2009 ), there is some weak evidence for its role in the timing of parturition ( Yellon and Longo, 1988 ; González-Candia et al., 2016 ). Melatonin can either enhance or reduce uterine myometrial contractility depending on the species (Table 6 ; Melatonin; Ayar et al., 2001 ; Sharkey et al., 2009 , 2010 ). Both melatonin and serotonin are also important for lactation, specifically for mammary gland development and milk nutrient content ( Okatani et al., 2001 ; Xiang et al., 2012 ; Laporta et al., 2014a , b ). For instance, mammary gland proliferation and calcium transport is impaired in pregnant mice with genetically-induced serotonin deficiency ( Laporta et al., 2014a , b ). Conversely, supplementation of a serotonin precursor increases mammary calcium transporter expression and milk calcium content in lactating mice and cows ( Laporta et al., 2013a , b , 2015 ; Weaver et al., 2016 , 2017 ; Hernández-Castellano et al., 2017 ). In contrast to serotonin, increased melatonin signaling is associated with reduced ductal growth and branching, as well as impaired terminal end bud formation in the non-pregnant state ( Xiang et al., 2012 ). Thus, during lactation, these mice with increased melatonin signaling have impaired mammary gland lobulo-alveolar development and reduced milk protein content, which reduces the weight of suckling pups ( Xiang et al., 2012 ). Indeed, a recent study showed antenatal melatonin supplementation further exacerbated the growth restriction of offspring and raised circulating maternal cortisol in a sheep model of fetal growth restriction ( González-Candia et al., 2016 ). Nevertheless, melatonin supplementation during pregnancy confers significant beneficial neuroprotective effects on the fetus and enhances maternal antioxidant capacity ( Miller et al., 2014 ; González-Candia et al., 2016 ; Castillo-Melendez et al., 2017 ). Therefore, while melatonin supplementation shows promise for use in the clinic, particularly for enhancing the neurodevelopmental outcomes of offspring in growth compromised pregnancies, the potential adverse outcomes for both mother and child must also be considered and should be assessed in further studies.
Another key neuroendocrine factor is oxytocin. Oxytocin is widely known for its role in triggering maternal nursing behavior ( Bosch and Neumann, 2012 ). This is mediated by oxytocin's actions on the maternal brain, as well as, the mammary glands. Indeed, a greater rise in circulating oxytocin concentrations from early to late pregnancy in pregnant women, is associated with a stronger bond between a mother and her infant ( Levine et al., 2007 ). Concurrently, placental expression of oxytocin also peaks at term in humans ( Kim S. C. et al., 2017 ). The rat placenta also produces oxytocin ( Lefebvre et al., 1992 ), while placental expression in other species remains unclear. Reduced oxytocin signaling decreases maternal nurturing behavior such as pup retrieval in rats ( Van Leengoed et al., 1987 ). It also decreases the willingness of female voles to care for, groom and lick unrelated pups ( Keebaugh et al., 2015 ). Low oxytocin signaling can additionally impair social bonding in voles and mice ( Ferguson et al., 2000 ; Takayanagi et al., 2005 ; Lee et al., 2008 ; Keebaugh et al., 2015 ), while high levels builds trust and cooperation in a group setting to facilitate group survival in humans ( Declerck et al., 2010 ; De Dreu et al., 2010 ). Moreover, a lack of oxytocin disrupts mammary gland proliferation and lobuloalveolar development, which impairs milk release from the mammary tissues in mice ( Nishimori et al., 1996 ; Wagner et al., 1997 ). Therefore, high oxytocin levels enable the mother to bond better and protect her newborn, when it is most vulnerable.
Oxytocin is also important in the process of parturition (Table 6 ; Oxytocin); it stimulates the contraction of smooth muscle cells in the myometrium ( Ayar et al., 2001 ; Arrowsmith and Wray, 2014 ), by inducing calcium influx and stimulating prostaglandin release ( Wilson et al., 1988 ; Voltolini and Petraglia, 2014 ; Kim S. H. et al., 2017 ). Cardiovascular effects of oxytocin include its ability to significantly lower blood pressure in non-pregnant rats ( Petersson et al., 1996 ). There is also some evidence that oxytocin induces anti-inflammatory and antioxidant effects in the heart under hypoxic conditions in non-pregnant rats ( Gutkowska and Jankowski, 2012 ). Nevertheless, the specific cardiovascular effects of oxytocin in pregnancy remain to be explored.
Studies performed in non-pregnant rodents show that oxytocin also affects metabolic function in vivo (Table 5 ; Oxytocin). In particular, loss of oxytocin reduces glucose and insulin tolerance and increases adiposity ( Camerino, 2009 ), whereas exogenous oxytocin has the reverse effect ( Deblon et al., 2011 ). Studies are however, required to determine whether the rise in oxytocin in late pregnancy ( Levine et al., 2007 ) may serve to improve insulin sensitivity in the mother in preparation for the metabolic requirements of delivery and lactation. There is some evidence that oxytocin may additionally play a role in controlling energy expenditure and thermoregulation during pregnancy. Even with a similar diet and activity level to control mice, oxytocin-deficient mice become obese due to reduced energy expenditure from poor thermoregulation in the non-pregnant state ( Chaves et al., 2013 ). Furthermore, exogenous oxytocin in non-pregnant mice causes a rise in body temperature ( Mason et al., 1986 ; Tamma et al., 2009 ). Nevertheless, whether oxytocin may play a role in controlling heat dissipation due to the increased maternal energy expenditure during pregnancy requires exploration. Exogenous oxytocin also reduces food intake in non-pregnant rats ( Arletti et al., 1989 , 1990 ). However, the role of oxytocin in appetite regulation during pregnancy remains to be explored. There is also evidence for oxytocin's possible involvement in maternal bone metabolism and calcium homeostasis during pregnancy and lactation. For instance, oxytocin stimulates both bone resorption and bone formation by osteoclasts and osteoblasts respectively in vitro ( Tamma et al., 2009 ). Moreover, oxytocin administration in rats reduces circulating calcium with an overall skew toward bone formation ( Elabd et al., 2007 ). These findings may suggest that the peak in circulating oxytocin toward term promote the restoration of depleted maternal skeletal calcium stores.
Other Neuroactive Hormones
In addition to the aforementioned melatonin, serotonin and oxytocin, the human placenta also produces neuroactive hormones such as kisspeptin and thyrotropin-releasing hormone (TRH), which may function in adapting maternal physiology to support pregnancy ( Bajoria and Babawale, 1998 ; De Pedro et al., 2015 ). In humans, circulating kisspeptin rises throughout pregnancy to concentrations 10,000-fold that of the non-pregnant state, with the placenta speculated as a major source ( Horikoshi et al., 2003 ). In the non-pregnant state, kisspeptin can both stimulate and impede glucose stimulated insulin secretion in mice ( Bowe et al., 2009 ; Song et al., 2014 ). The nature of the effect may partly relate to differences in the actions of kisspeptin isoforms on pancreatic islets ( Bowe et al., 2012 ). Kisspeptin may also have effects on the maternal cardiovascular system, given its reported vasoconstrictive effects on vascular smooth muscle cells and fibrotic effects on the heart in non-pregnant rats ( Mead et al., 2007 ; Zhang et al., 2017 ). Studies in humans highlight the importance of regulating kisspeptin production during gestation; increased placental kisspeptin is associated with pre-eclampsia ( Whitehead et al., 2013 ; Matjila et al., 2016 ) and reduced circulating kisspeptin is observed in women with hypertension and diabetes during pregnancy ( Cetković et al., 2012 ; Matjila et al., 2016 ). Like the human, the murine placenta produces kisspeptin. Although a kisspeptin-deficient mouse has been established, previous work has been focused on feto-placental outcomes, with no examination of maternal physiology ( Herreboudt et al., 2015 ). Studies are required to determine the consequences of abnormal placental kisspeptin on the maternal physiology during pregnancy.
In the non-pregnant state, hypothalamic TRH stimulates release of thyroid-stimulating hormone and PRL from the pituitary ( Hershman et al., 1973 ; Vale et al., 1973 ; Askew and Ramsden, 1984 ). However, during pregnancy, the placenta serves as an additional source of TRH ( Bajoria and Babawale, 1998 ). Excess TRH in pregnancy raises blood concentrations of thyroid-stimulating hormone and PRL in humans, rhesus monkeys, sheep and rats ( Thomas et al., 1975 ; Azukizawa et al., 1976 ; Roti et al., 1981 ; Moya et al., 1986 ; Lu et al., 1998 ). Conversely, a lack of TRH reduces blood PRL in mice ( Rabeler et al., 2004 ; Yamada et al., 2006 ). Thyroid hormones are necessary for optimal brain development as well as thyroid function ( Miranda and Sousa, 2018 ). Impaired TRH signaling is associated with anxiety-like and depressive-like behavior in non-pregnant mice ( Zeng et al., 2007 ; Sun et al., 2009 ) and there is some evidence which suggests a link between thyroid dysfunction and poor maternal mood during pregnancy in humans ( Basraon and Costantine, 2011 ). However, whether any direct causal relationship between placental hormones, like TRH and perinatal depression remains unclear. Additionally, TRH is implicated in glucose homeostasis and appetite regulation. For example, mice with TRH deficiency are hyperglycaemic, due to an impaired insulin response to glucose ( Yamada et al., 1997 ). Reduced TRH signaling also impedes leptin production and ghrelin acylation, which results in less energy conservation during fasting and a lower body mass in the non-pregnant state ( Groba et al., 2013 ; Mayerl et al., 2015 ). Investigations are warranted to identify whether TRH may contribute to the regulation of glucose handling and appetite in the mother during pregnancy.
Additional Hormones
The placenta also produces numerous other hormones with pleiotropic effects. Several key ones, which have been implicated in pregnancy failure or disorders of pregnancy such as hypertension, hyperglycemia and hypercalcemia, are discussed here. The hormones presented here are by no means exhaustive and were selected primarily on their major associations with abnormal maternal physiology during pregnancy. The gonadotropin, chorionic gonadotropin (CG); transforming growth factor β (TGF β) family member, activin; angiogenic factor, relaxin; bone metabolism-associated parathyroid hormone-related protein (PTHrP) and energy homeostasis regulator, leptin are reviewed (Tables 7 , 8 ).
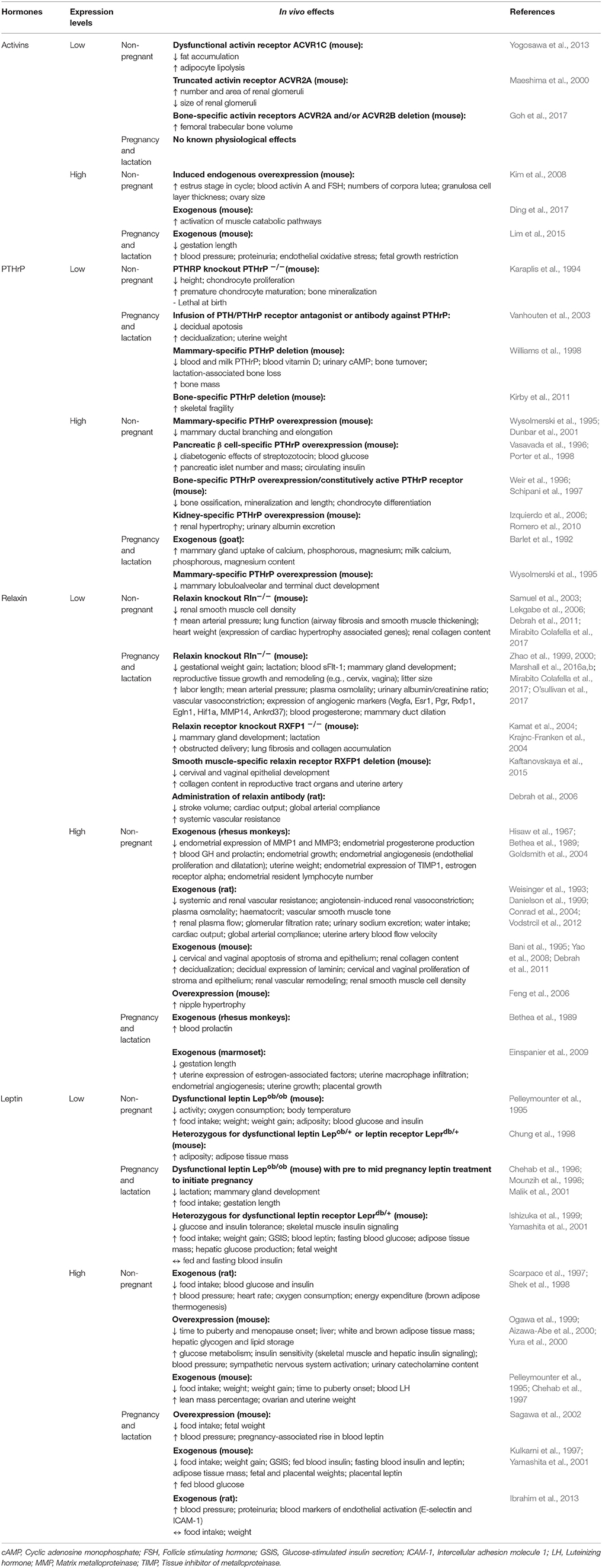
Table 7 . Effects of additional hormones in vivo .

Table 8 . Effects of additional hormones in vitro .
Chorionic Gonadotropin (CG)
CG, is secreted by the human (hCG) and equine (eCG) placenta, although hCG has been more extensively studied. hCG is a large glycoprotein composed of α and β subunits, of which the α subunit identical to luteinizing hormone (LH), follicle stimulating hormone (FSH) and thyroid stimulating hormone (TSH). As a result, hCG can interact with LH, FSH and TSH receptors. In women, hCG is secreted from the trophoblast from very early in gestation and is thought to be the first placental hormone to act on the mother ( Ogueh et al., 2011 ). Indeed, maternal circulating hCG concentrations peak in the first trimester and then decline toward term ( Ogueh et al., 2011 ). In early pregnancy, hCG maintains corpus luteum allowing the continued secretion of ovarian progesterone and estrogens until the steroidogenic activity of the fetal-placental unit can compensate for maternal ovarian function ( Fournier et al., 2015 ). In particular, hCG increases the abundance of low-density lipoprotein receptor and thus uptake of cholesterol for steroidogenesis. It also enhances the expression and/or activity of steroidogenic enzymes including 3β-hydroxysteroid and aromatase. There is also some evidence which suggests hCG may inhibit factors that promote luteal demise, such as the prostaglandins. The high levels of hCG in early pregnancy are also sufficient to bind to the TSH receptor and may act to increase maternal thyroid hormone production, which as mentioned previously, may exert effects in the mother and fetus.
CG may also play important autocrine and paracrine roles at the maternal-fetal interface. Administration of hCG antisera prevents implantation in marmoset in vivo ( Hearn et al., 1988 ). Recent proteomic analysis of estrogen and hCG treated human endometrial epithelial cells demonstrates that hCG targets pathways involved in metabolism, basement membrane and cell connectivity, proliferation and differentiation, cellular adhesion, extracellular-matrix organization, developmental growth, growth factor regulation and cell signaling ( Greening et al., 2016 ). Such pathways are likely to be important for placental development, as attenuating hCG signaling disrupts trophoblast differentiation in vitro ( Shi et al., 1993 ). In contrast, supplementing human trophoblast cells with hCG increases their differentiation, migration, invasion and adhesion to uterine epithelial cells, and decreases their leptin secretion in vitro (Table 8 ; hCG; Shi et al., 1993 ; Prast et al., 2008 ; Lee C. L. et al., 2013 ; Chen et al., 2015 ). hCG also promotes angiogenic vascular endothelial growth factor secretion by both trophoblast and endometrial epithelial cells ( Islami et al., 2003a ; Berndt et al., 2006 ) and enhances endothelial tube formation and migration ( Zygmunt et al., 2002 ). Furthermore, hCG is key in suppressing the maternal immune system from mounting a response against paternal antigens carried by the allogenic conceptus. Administration of hCG in a mouse model of spontaneous abortion significantly reduces the number of fetal resorptions due to improved immune tolerance of the fetus ( Schumacher et al., 2013 ). In vitro , hCG enhances proliferation of immunosuppressive uterine natural killer cells ( Kane et al., 2009 ), and the production of immunosuppressing IL-10 by B cells ( Fettke et al., 2016 ). hCG can also modulate the immune system even in a non-pregnant state, as shown by its efficacy in preventing the development of autoimmune diabetes in a mouse model ( Khil et al., 2007 ). In pregnancy, hCG additionally inhibits the contractile function of smooth muscle cells in the uterus to help sustain myometrial quiescence ( Ambrus and Rao, 1994 ; Eta et al., 1994 ), so as to prevent premature expulsion of the fetus. Glycosylation of hCG affects its biological activity and half-life ( Fournier et al., 2015 ). Given its involvement with multiple systems, it is perhaps unsurprising that abnormal concentrations of hCG and hCG glycoforms have been linked with pregnancy complications such as fetal growth restriction and preeclampsia ( Chen et al., 2012 ). However, whether the abnormal concentrations of hCG are cause or consequence of the disorders remains to be determined.
Activins are members of the TGFβ family and were first discovered for their role in stimulating FSH production and determining estrus cyclicity and fertility in mice ( Ahn et al., 2004 ; Sandoval-Guzmán et al., 2012 ). Activin signaling promotes the decidualization, as well as, apoptosis of endometrial stroma cells (Table 8 ; Activins; Tessier et al., 2003 ; Clementi et al., 2013 ; Yong et al., 2017 ); processes that accommodate implantation and conceptus development ( Peng et al., 2015 ). Additionally, activin A enhances steroid production, invasion and apoptosis of human trophoblast in vitro ( Ni et al., 2000 ; Yu et al., 2012 ; Li et al., 2015 ). However, activins may also be of importance in modulating the physiology of the mother during pregnancy (Table 7 ; Activins). In normal human pregnancy, activin A concentrations gradually rise during gestation and peak at term ( Fowler et al., 1998 ). The placenta is thought to be the main source of activin A in the maternal circulation during pregnancy, given the rapid clearance after delivery of the placenta ( Muttukrishna et al., 1997 ; Fowler et al., 1998 ). A similar rise of activin in the maternal circulation is observed in pregnant ewes ( Jenkin et al., 2001 ), while the circulating profiles in other species remain undetermined. Nevertheless, in mice, impaired activin signaling leads to poor pregnancy outcomes such as fewer viable pups ( Clementi et al., 2013 ; Peng et al., 2015 ). However, there is evidence that an increase in activin may also be pathological and detrimental to pregnancy outcome. For instance in pregnant mice, infusion of activin A or plasmid overexpression of activin A results in the development of a preeclamptic phenotype; dams display hypertension and proteinuria, in addition to growth restriction and greater in utero deaths ( Kim et al., 2008 ; Lim et al., 2015 ). The maternal hypertension observed likely results from pathological concentrations of activin A inducing vascular endothelial dysfunction ( Yong et al., 2015 ). In the non-pregnant state, activins are also important for renal glomeruli development ( Maeshima et al., 2000 ), as well as, for bone, fat and muscle metabolism ( Yogosawa et al., 2013 ; Ding et al., 2017 ; Goh et al., 2017 ). The possible contributions of activin to these latter functions in pregnancy are currently unclear. Therefore, the impact of activin signaling on these other body systems during pregnancy remains to be determined.
Relaxin is a potent vasodilator ( Danielson et al., 1999 ), and regulates hemodynamics in both the non-pregnant and pregnant state (Table 7 ; Relaxin; Conrad et al., 2004 ). In pregnant women, circulating relaxin concentration peaks in the first trimester, declines in the second trimester and is maintained until delivery in the third trimester ( Quagliarello et al., 1979 ; Seki et al., 1985 ). In contrast, circulating relaxin peaks toward term in mice, rats, guinea pigs and hamsters ( O'byrne and Steinetz, 1976 ; O'byrne et al., 1976 ; Renegar and Owens, 2002 ). In pregnant mice, relaxin deficiency leads to proteinuria, suggesting a particular role of relaxin in modulating renal function during pregnancy ( O'sullivan et al., 2017 ). In addition, relaxin-deficient mice remain sensitive to vasoconstrictors such as angiotensin and endothelin, and are hypertensive during pregnancy ( Marshall et al., 2016a ; Mirabito Colafella et al., 2017 ). During pregnancy, relaxin-deficient mice also display stiffer uterine vessels and fetal growth is retarded ( Gooi et al., 2013 ). Relaxin also enhances capillarisation and glucose uptake of skeletal muscles in non-pregnant mice ( Bonner et al., 2013 ). Taken together, these data highlight the importance of relaxin in mediating changes in maternal vascular function that serve to promote blood flow to the gravid uterus during pregnancy.
Relaxin may play additional roles within the uterus that are important for implantation, placentation and pregnancy maintenance (Tables 7 , 8 ; Relaxin). In vitro , relaxin increases decidual cell insulin-like growth factor binding protein-1 expression, a marker of decidualization ( Mazella et al., 2004 ). It also enhances survival and proliferation of cultured human trophoblast cells ( Lodhi et al., 2013 ; Astuti et al., 2015 ). During early mouse pregnancy, relaxin modulates the uterine expression of genes involved in angiogenesis, steroid hormone action and remodeling ( Marshall et al., 2016b ). Indeed in pregnant marmosets, exogenous relaxin improves uterine and placental growth ( Einspanier et al., 2009 ). Relaxin infusion also alters the endometrial lymphocyte number in vivo ( Goldsmith et al., 2004 ), which suggests a possible role of relaxin in achieving immune tolerance of the allogenic conceptus. Relaxin impedes spontaneous contractility of myometrium in humans, rats and pigs ( Maclennan and Grant, 1991 ; Longo et al., 2003 ), and is thus thought to play a role in regulating the onset of parturition ( Vannuccini et al., 2016 ). In mice with a deficiency in relaxin signaling, obstructed deliveries occur at a higher rate due to poor maturation of the cervix ( Zhao et al., 1999 ; Kamat et al., 2004 ; Krajnc-Franken et al., 2004 ; Kaftanovskaya et al., 2015 ). Conversely in hamsters, the rise in circulating relaxin toward term coincides with cervical ripening in preparation for delivery ( O'byrne et al., 1976 ). Insufficient relaxin signaling also impedes mammary development through excessive duct dilation and reduces the nursing of offspring in mice ( Zhao et al., 1999 ; Kamat et al., 2004 ; Krajnc-Franken et al., 2004 ). Conversely, overexpression leads to hypertrophy of the nipples in non-pregnant mice ( Feng et al., 2006 ). Hence, relaxin is important in driving changes at the maternal-fetal interface that establish pregnancy, adapts the cardiovascular system of the mother to support the pregnancy and prepares the mother for lactation post-partum.
Parathyroid Hormone-Related Protein (PTHrP)
During pregnancy, the placenta serves as an additional source of PTHrP ( Bowden et al., 1994 ; Emly et al., 1994 ), a key hormone involved in bone metabolism (Table 7 ; PTHrP). PTHrP concentrations in the maternal blood rise throughout gestation in humans ( Gallacher et al., 1994 ; Ardawi et al., 1997 ; Hirota et al., 1997 ) and correlate with the rise in maternal circulating calcium during pregnancy ( Bertelloni et al., 1994 ). However, excessively high circulating PTHrP can lead to hypercalcaemia during pregnancy ( Winter and Appelman-Dijkstra, 2017 ). PTHrP increases maternal bone resorption, thereby enabling calcium transfer from mother to fetus for bone development ( Salles, 2016 ). Thus, it is perhaps not surprising that complete knockout of PTHrP in mice is lethal at birth in association with abnormal bone development ( Karaplis et al., 1994 ). Carrying one defective PTHrP copy is enough to also impede bone development and reduce snout length in mice ( Amizuka et al., 1996 ). Mammary-specific PTHrP deletion increases maternal bone mass and protects against lactation-associated bone loss by reducing bone turnover in mice ( Williams et al., 1998 ; Vanhouten et al., 2003 ). However, deleting bone-specific PTHrP increases skeletal fragility, both in the non-pregnant and pregnant state ( Kirby et al., 2011 ). PTHrP infusion of lactating goats increases mammary gland uptake calcium, phosphorous and magnesium for transfer in milk to the neonate ( Barlet et al., 1992 ). These findings imply that a fine balance of PTHrP production by gestational and maternal tissues must be achieved for appropriate regulation of maternal bone metabolism and offspring calcium requirements during pregnancy and lactation.
Placental-derived PTHrP may also exert additional effects on the placenta and the mother which are beneficial for offspring development and growth. PTHrP stimulates the proliferation, differentiation, outgrowth and calcium uptake of trophoblast in vitro (Table 8 ; PTHrP; Hershberger and Tuan, 1998 ; El-Hashash and Kimber, 2006 ). In vivo , blocking PTHrP signaling during mouse pregnancy leads to excessive uterine growth and decidualization in association with a decrease in decidual cell apoptosis ( Williams et al., 1998 ; Vanhouten et al., 2003 ). Moreover, over-expression of PTHrP impairs mammary gland branching morphogenesis ( Wysolmerski et al., 1995 ; Dunbar et al., 2001 ). These studies highlight a possible important regulatory role of PTHrP in the control of decidualization and mammary gland development in vivo . In non-pregnant mice, PTHrP enhances pancreatic β-cells proliferation and insulin secretion whilst it inhibits islet cell apoptosis ( Vasavada et al., 1996 ; Porter et al., 1998 ; Cebrian et al., 2002 ; Fujinaka et al., 2004 ). It also increases renal plasma flow and glomerular filtration rate, and exerts proliferative effects on renal glomerular and tubule cells in rodents ( Izquierdo et al., 2006 ; Romero et al., 2010 ). Additionally, in vitro studies show PTHrP can induce relaxation of uterine arteries ( Meziani et al., 2005 ). However, the significance of PTHrP on glucose-insulin dynamics and renal and vascular function of the mother during pregnancy remains to be investigated.
Leptin is an abundant circulating hormone involved in regulating appetite. In the non-pregnant state, the adipose tissue is the exclusive source of circulating leptin. During pregnancy in humans, baboons and mice, concentrations of leptin rapidly rise throughout gestation, peaking toward term ( Highman et al., 1998 ; Henson et al., 1999 ; Malik et al., 2005 ). The rise in leptin positively correlates with increases in maternal body fat ( Highman et al., 1998 ). In humans, blood leptin rapidly falls to non-pregnant concentrations within 24 h of delivery, indicating that the placenta contributes to the main rise of leptin in pregnancy ( Masuzaki et al., 1997 ). In particular, leptin is produced by the human placental trophoblast cells ( Masuzaki et al., 1997 ). A similar post-pregnancy decline and placental trophoblast expression is seen in baboons ( Henson et al., 1999 ). However, this is not the case for mice, as the murine placenta does not produce leptin ( Malik et al., 2005 ). Nevertheless, leptin studies in mice still provide useful knowledge about pregnancy-related effects of leptin (Table 7 ; Leptin). For instance, leptin in pregnancy helps prepare the mother for lactation, as a deficiency results in impaired mammary gland development, which is detrimental for lactation post-delivery ( Mounzih et al., 1998 ; Malik et al., 2001 ). Another significant effect of leptin in pregnancy observed through mouse studies is leptin resistance, whereby the dam increases her food intake in mid-pregnancy to meet increased energy demands despite an increase in circulating leptin, which in the non-pregnant state would lead to satiety ( Mounzih et al., 1998 ). In contrast, excessive leptin significantly decreases maternal food intake and restricts feto-placental growth ( Yamashita et al., 2001 ). Leptin exposure of rat and human islets and cultured insulinoma cells significantly decreases insulin production in vitro , demonstrating that leptin may be directly involved in glucose metabolism (Table 8 ; Leptin; Kulkarni et al., 1997 ). Indeed dysfunctional leptin signaling in pregnancy leads to the spontaneous development of a gestational diabetic phenotype in db/+ mice, who are heterozygous for the leptin receptor (Table 7 ; Leptin; Yamashita et al., 2001 ). Further in vitro studies on placental explants or trophoblast cultures highlight a potential for leptin to be involved in immune modulation and placental hormone production, given its stimulatory effects on HLA-G and hCG expression (Table 8 ; Leptin; Chardonnens et al., 1999 ; Islami et al., 2003a , b ; Barrientos et al., 2015 ). Additional effects of leptin on the placenta are thoroughly reviewed elsewhere ( Schanton et al., 2018 ). Therefore, placental leptin can have systemic effects on the mother in pregnancy.
Pregnancy represents a unique physiological paradigm; there are dynamic and reversible changes in the function of many organ systems in the mother that are designed to support offspring development. In part, these changes are signaled via the placental secretion of hormones, which in turn, alter in abundance, interact with one another and exert wide effects on maternal tissues during pregnancy. For instance, steroid hormones modulate most systems of the mother throughout pregnancy. However, they also alter the production of other hormones, such as prolactin and placental lactogens, which in turn, may contribute to the physiological changes in the mother (Figure 2 ). However, further work is required to better define how placental hormones elicit their actions in the mother, as well as, identify the extent to which they interplay with hormones produced by maternal tissues. As the endocrine and metabolic state of the mother is also influenced by her environment, maternal conditions such as poor nutrition and obesity may modulate placental hormone production and pregnancy adaptations. Indeed, previous work has shown that an obesogenic diet during pregnancy alters the expression of PRL/PL genes in the placenta in association with mal-adaptations of maternal metabolism in mice ( Musial et al., 2017 ). Further studies are nonetheless needed to assess the interaction of the maternal environment with placental endocrine function. Placental hormones are also released into the fetal circulation, where they may have direct impacts on fetal growth and development ( Freemark, 2010 ). Investigations exploring the importance of placental endocrine function on fetal growth, independent of the mother, will require future examination. Collectively, further studies on the nature and role of placental endocrine function in maternal adaptations and fetal growth will undoubtedly provide novel insights into understanding of the potential causes of obstetrical syndromes such as gestational diabetes and preeclampsia that are marked by maternal physiological maladaptation.
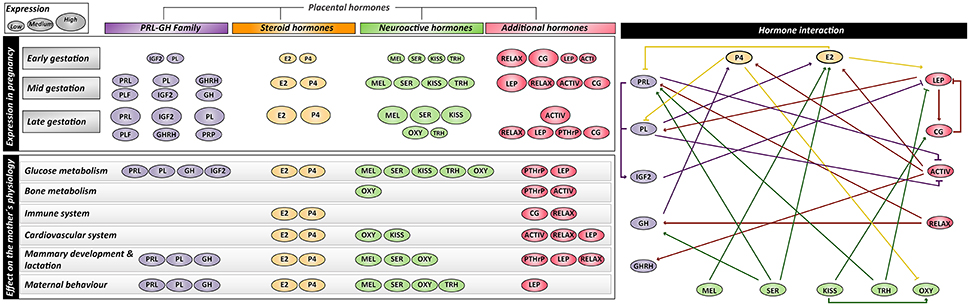
Figure 2 . Summary of expression profiles, interactions and maternal physiological effects of placental-derived hormones. PRL, prolactin; PL, placental lactogen; PLF, proliferins; PRP, proliferin-related proteins; GH, growth hormone; GHRH, growth hormone releasing hormone; IGF1/2, insulin-like growth factor-1/2; E2, estrogen; P4, progesterone; MEL, melatonin; SER, serotonin; KISS, kisspeptin; OXY, Oxytocin; TRH, thyrotropin-releasing hormone; RELAX, relaxin; ACTIV, activin; CG, chorionic gonadotropin; LEP, leptin; PTHrP, parathyroid hormone-related protein.
Author Contributions
TN and HY substantially contributed to the conception of the work, drafting and revision of the manuscript, preparation of the tables and approved of the final version. JL-T substantially contributed to the conception of the work, drafting and revision of the manuscript, preparation of the figures and approved of the final version. AS-P substantially contributed to the conception of the work, critical revision of the manuscript for intellectual content and approved of the final version.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
TN was supported by the Marie Skłodowska-Curie Individual Fellowship from the European Union; HY was supported by an A*STAR International Fellowship from the Agency for Science, Technology and Research; JL-T was supported by the Newton International Fellowship from the Royal Society; AS-P was supported by the Dorothy Hodgkin Research Fellowship from the Royal Society.
Abd-Allah, A. R., El-Sayed El, S. M., Abdel-Wahab, M. H., and Hamada, F. M. (2003). Effect of melatonin on estrogen and progesterone receptors in relation to uterine contraction in rats. Pharmacol. Res. 47, 349–354. doi: 10.1016/S1043-6618(03)00014-8
PubMed Abstract | CrossRef Full Text | Google Scholar
Abribat, T., Lapierre, H., Dubreuil, P., Pelletier, G., Gaudreau, P., Brazeau, P., et al. (1990). Insulin-like growth factor-I concentration in Holstein female cattle: variations with age, stage of lactation and growth hormone-releasing factor administration. Domest. Anim. Endocrinol. 7, 93–102. doi: 10.1016/0739-7240(90)90058-8
Açikgöz, S., Bayar, U. O., Can, M., Güven, B., Mungan, G., Dogan, S., et al. (2013). Levels of oxidized LDL, estrogens, and progesterone in placenta tissues and serum paraoxonase activity in preeclampsia. Mediators Inflamm. 2013:862982. doi: 10.1155/2013/862982
Ackermann, A. M., and Gannon, M. (2007). Molecular regulation of pancreatic beta-cell mass development, maintenance, and expansion. J. Mol. Endocrinol. 38, 193–206. doi: 10.1677/JME-06-0053
Adamah-Biassi, E. B., Hudson, R. L., and Dubocovich, M. L. (2014). Genetic deletion of MT1 melatonin receptors alters spontaneous behavioral rhythms in male and female C57BL/6 mice. Horm. Behav. 66, 619–627. doi: 10.1016/j.yhbeh.2014.08.012
Adamova, Z., Ozkan, S., and Khalil, R. A. (2009). Vascular and cellular calcium in normal and hypertensive pregnancy. Curr. Clin. Pharmacol. 4, 172–190. doi: 10.2174/157488409789375320
Ahmed-Sorour, H., and Bailey, C. J. (1980). Role of ovarian hormones in the long-term control of glucose homeostasis. Interaction with insulin, glucagon and epinephrine. Horm. Res. 13, 396–403. doi: 10.1159/000179307
Ahmed-Sorour, H., and Bailey, C. J. (1981). Role of ovarian hormones in the long-term control of glucose homeostasis, glycogen formation and gluconeogenesis. Ann. Nutr. Metab. 25, 208–212. doi: 10.1159/000176496
Ahn, J. M., Jung, H. K., Cho, C., Choi, D., Mayo, K. E., and Cho, B. N. (2004). Changes in the reproductive functions of mice due to injection of a plasmid expressing an inhibin alpha-subunit into muscle: a transient transgenic model. Mol. Cells 18, 79–86.
PubMed Abstract | Google Scholar
Ahumada-Solórzano, S. M., Martínez-Moreno, C. G., Carranza, M., Ávila-Mendoza, J., Luna-Acosta, J. L., Harvey, S., et al. (2016). Autocrine/paracrine proliferative effect of ovarian GH and IGF-I in chicken granulosa cell cultures. Gen. Comp. Endocrinol. 234, 47–56. doi: 10.1016/j.ygcen.2016.05.008
Aizawa-Abe, M., Ogawa, Y., Masuzaki, H., Ebihara, K., Satoh, N., Iwai, H., et al. (2000). Pathophysiological role of leptin in obesity-related hypertension. J. Clin. Invest. 105, 1243–1252. doi: 10.1172/JCI8341
Alenina, N., Kikic, D., Todiras, M., Mosienko, V., Qadri, F., Plehm, R., et al. (2009). Growth retardation and altered autonomic control in mice lacking brain serotonin. Proc. Natl. Acad. Sci. U.S.A. 106, 10332–10337. doi: 10.1073/pnas.0810793106
Alperin, M., Kaddis, T., Pichika, R., Esparza, M. C., and Lieber, R. L. (2016). Pregnancy-induced adaptations in intramuscular extracellular matrix of rat pelvic floor muscles. Am. J. Obstet. Gynecol. 215, 210 e211–210 e217. doi: 10.1016/j.ajog.2016.02.018
Alperin, M., Lawley, D. M., Esparza, M. C., and Lieber, R. L. (2015). Pregnancy-induced adaptations in the intrinsic structure of rat pelvic floor muscles. Am. J. Obstet. Gynecol. 213, 191 e191–191 e197. doi: 10.1016/j.ajog.2015.05.012
Ambrus, G., and Rao, C. V. (1994). Novel regulation of pregnant human myometrial smooth muscle cell gap junctions by human chorionic gonadotropin. Endocrinology 135, 2772–2779. doi: 10.1210/endo.135.6.7988470
Amico, J. A., Vollmer, R. R., Cai, H. M., Miedlar, J. A., and Rinaman, L. (2005). Enhanced initial and sustained intake of sucrose solution in mice with an oxytocin gene deletion. Am. J. Physiol. Regul. Integr. Comp. Physiol. 289, R1798–R1806. doi: 10.1152/ajpregu.00558.2005
Amizuka, N., Karaplis, A. C., Henderson, J. E., Warshawsky, H., Lipman, M. L., Matsuki, Y., et al. (1996). Haploinsufficiency of parathyroid hormone-related peptide (PTHrP) results in abnormal postnatal bone development. Dev. Biol. 175, 166–176. doi: 10.1006/dbio.1996.0104
Angoa-Pérez, M., Kane, M. J., Sykes, C. E., Perrine, S. A., Church, M. W., and Kuhn, D. M. (2014). Brain serotonin determines maternal behavior and offspring survival. Genes Brain Behav. 13, 579–591. doi: 10.1111/gbb.12159
Angoa-Pérez, M., and Kuhn, D. M. (2015). Neuronal serotonin in the regulation of maternal behavior in rodents. Neurotransmitter (Houst) 2:e615. doi: 10.14800/nt.615
PubMed Abstract
Antonijevic, I. A., Leng, G., Luckman, S. M., Douglas, A. J., Bicknell, R. J., and Russell, J. A. (1995). Induction of uterine activity with oxytocin in late pregnant rats replicates the expression of c-fos in neuroendocrine and brain stem neurons as seen during parturition. Endocrinology 136, 154–163. doi: 10.1210/endo.136.1.7828526
Apa, R., Lanzone, A., Miceli, F., Mastrandrea, M., Macchione, E., Caruso, A., et al. (1995). Growth hormone-releasing factor stimulates meiotic maturation in follicle- and cumulus-enclosed rat oocyte. Mol. Cell. Endocrinol. 112, 195–201. doi: 10.1016/0303-7207(95)03599-3
Ardawi, M. S., Nasrat, H. A., and BA'Aqueel, H. S. (1997). Calcium-regulating hormones and parathyroid hormone-related peptide in normal human pregnancy and postpartum: a longitudinal study. Eur. J. Endocrinol. 137, 402–409. doi: 10.1530/eje.0.1370402
Arletti, R., Benelli, A., and Bertolini, A. (1989). Influence of oxytocin on feeding behavior in the rat. Peptides 10, 89–93. doi: 10.1016/0196-9781(89)90082-X
Arletti, R., Benelli, A., and Bertolini, A. (1990). Oxytocin inhibits food and fluid intake in rats. Physiol. Behav. 48, 825–830. doi: 10.1016/0031-9384(90)90234-U
Arrowsmith, S., and Wray, S. (2014). Oxytocin: its mechanism of action and receptor signalling in the myometrium. J. Neuroendocrinol. 26, 356–369. doi: 10.1111/jne.12154
Arumugam, R., Fleenor, D., and Freemark, M. (2014). Knockdown of prolactin receptors in a pancreatic beta cell line: effects on DNA synthesis, apoptosis, and gene expression. Endocrine 46, 568–576. doi: 10.1007/s12020-013-0073-1
Askew, R. D., and Ramsden, D. B. (1984). Effect of repeated stimulation by thyrotropin-releasing hormone (TRH) on thyrotropin and prolactin secretion in perfused euthyroid and hypothyroid rat pituitary fragments. Horm. Res. 20, 269–276. doi: 10.1159/000180007
Astuti, Y., Nakabayashi, K., Deguchi, M., Ebina, Y., and Yamada, H. (2015). Human recombinant H2 relaxin induces AKT and GSK3beta phosphorylation and HTR-8/SVneo cell proliferation. Kobe J. Med. Sci. 61, E1–8. doi: 10.24546/81008925
Atherton, J. C., Dark, J. M., Garland, H. O., Morgan, M. R., Pidgeon, J., and Soni, S. (1982). Changes in water and electrolyte balance, plasma volume and composition during pregnancy in the rat. J. Physiol. (Lond). 330, 81–93. doi: 10.1113/jphysiol.1982.sp014330
Ayar, A., Kutlu, S., Yilmaz, B., and Kelestimur, H. (2001). Melatonin inhibits spontaneous and oxytocin-induced contractions of rat myometrium in vitro . Neuro Endocrinol. Lett. 22, 199–207.
Azukizawa, M., Murata, Y., Ikenoue, T., Martin, C. B. Jr., and Hershman, J. M. (1976). Effect of thyrotropin-releasing hormone on secretion of thyrotropin, prolactin, thyroxine, and triiodothyronine in pregnant and fetal rhesus monkeys. J. Clin. Endocrinol. Metab. 43, 1020–1028. doi: 10.1210/jcem-43-5-1020
Bacq, Y. (2013). “The liver in normal pregnancy,” in Madame Curie Bioscience Database. (Austin, TX: Landes Bioscience).
Google Scholar
Bader, R. A., Bader, M. E., Rose, D. F., and Braunwald, E. (1955). Hemodynamics at rest and during exercise in normal pregnancy as studies by cardiac catheterization. J. Clin. Invest. 34, 1524–1536. doi: 10.1172/JCI103205
Bae, M. H., Lee, M. J., Bae, S. K., Lee, O. H., Lee, Y. M., Park, B. C., et al. (1998). Insulin-like growth factor II (IGF-II) secreted from HepG2 human hepatocellular carcinoma cells shows angiogenic activity. Cancer Lett. 128, 41–46. doi: 10.1016/S0304-3835(98)00044-5
Baeyens, L., Hindi, S., Sorenson, R. L., and German, M. S. (2016). beta-Cell adaptation in pregnancy. Diabetes Obes. Metab. 18(Suppl. 1), 63–70. doi: 10.1111/dom.12716
Bähr, I., Mühlbauer, E., Schucht, H., and Peschke, E. (2011). Melatonin stimulates glucagon secretion in vitro and in vivo . J. Pineal Res. 50, 336–344. doi: 10.1111/j.1600-079X.2010.00848.x
Bailey, C. J., and Ahmed-Sorour, H. (1980). Role of ovarian hormones in the long-term control of glucose homeostasis. Effects of insulin secretion. Diabetologia 19, 475–481. doi: 10.1007/BF00281829
Bajoria, R., and Babawale, M. (1998). Ontogeny of endogenous secretion of immunoreactive-thyrotropin releasing hormone by the human placenta. J. Clin. Endocrinol. Metab. 83, 4148–4155. doi: 10.1210/jcem.83.11.5216
Banerjee, R. R., Cyphert, H. A., Walker, E. M., Chakravarthy, H., Peiris, H., Gu, X., et al. (2016). Gestational diabetes mellitus from inactivation of prolactin receptor and mafb in islet beta-cells. Diabetes 65, 2331–2341. doi: 10.2337/db15-1527
Bani, G., Maurizi, M., Bigazzi, M., and Bani Sacchi, T. (1995). Effects of relaxin on the endometrial stroma. Studies in mice. Biol. Reprod 53, 253–262. doi: 10.1095/biolreprod53.2.253
Barbour, L. A., Shao, J., Qiao, L., Leitner, W., Anderson, M., Friedman, J. E., et al. (2004). Human placental growth hormone increases expression of the p85 regulatory unit of phosphatidylinositol 3-kinase and triggers severe insulin resistance in skeletal muscle. Endocrinology 145, 1144–1150. doi: 10.1210/en.2003-1297
Barbour, L. A., Shao, J., Qiao, L., Pulawa, L. K., Jensen, D. R., Bartke, A., et al. (2002). Human placental growth hormone causes severe insulin resistance in transgenic mice. Am. J. Obstet. Gynecol. 186, 512–517. doi: 10.1067/mob.2002.121256
Barker, D. J. (2004). The developmental origins of well-being. Philos. Trans. R. Soc. Lond. B Biol. Sci. 359, 1359–1366. doi: 10.1098/rstb.2004.1518
Barlet, J. P., Champredon, C., Coxam, V., Davicco, M. J., and Tressol, J. C. (1992). Parathyroid hormone-related peptide might stimulate calcium secretion into the milk of goats. J. Endocrinol. 132, 353–359. doi: 10.1677/joe.0.1320353
Barrichon, M., Hadi, T., Wendremaire, M., Ptasinski, C., Seigneuric, R., Marcion, G., et al. (2015). Dose-dependent biphasic leptin-induced proliferation is caused by non-specific IL-6/NF-kappaB pathway activation in human myometrial cells. Br. J. Pharmacol. 172, 2974–2990. doi: 10.1111/bph.13100
Barrientos, G., Toro, A., Moschansky, P., Cohen, M., Garcia, M. G., Rose, M., et al. (2015). Leptin promotes HLA-G expression on placental trophoblasts via the MEK/Erk and PI3K signaling pathways. Placenta 36, 419–426. doi: 10.1016/j.placenta.2015.01.006
Basraon, S., and Costantine, M. M. (2011). Mood disorders in pregnant women with thyroid dysfunction. Clin. Obstet. Gynecol. 54, 506–514. doi: 10.1097/GRF.0b013e3182273089
Bearfield, C., Jauniaux, E., Groome, N., Sargent, I. L., and Muttukrishna, S. (2005). The secretion and effect of inhibin A, activin A and follistatin on first-trimester trophoblasts in vitro . Eur. J. Endocrinol. 152, 909–916. doi: 10.1530/eje.1.01928
Ben-Jonathan, N., and Hugo, E. (2015). Prolactin (PRL) in adipose tissue: regulation and functions. Adv. Exp. Med. Biol. 846, 1–35. doi: 10.1007/978-3-319-12114-7_1
Berkane, N., Liere, P., Oudinet, J. P., Hertig, A., Lefèvre, G., Pluchino, N., et al. (2017). From pregnancy to preeclampsia: a key role for estrogens. Endocr. Rev. 38, 123–144. doi: 10.1210/er.2016-1065
Berndt, S., Perrier D'hauterive, S., Blacher, S., Péqueux, C., Lorquet, S., Munaut, C., et al. (2006). Angiogenic activity of human chorionic gonadotropin through LH receptor activation on endothelial and epithelial cells of the endometrium. FASEB J. 20, 2630–2632. doi: 10.1096/fj.06-5885fje
Bertelloni, S., Baroncelli, G. I., Pelletti, A., Battini, R., and Saggese, G. (1994). Parathyroid hormone-related protein in healthy pregnant women. Calcif. Tissue Int. 54, 195–197. doi: 10.1007/BF00301677
Bethea, C. L., Cronin, M. J., Haluska, G. J., and Novy, M. J. (1989). The effect of relaxin infusion on prolactin and growth hormone secretion in monkeys. J. Clin. Endocrinol. Metab. 69, 956–962. doi: 10.1210/jcem-69-5-956
Billestrup, N., and Nielsen, J. H. (1991). The stimulatory effect of growth hormone, prolactin, and placental lactogen on beta-cell proliferation is not mediated by insulin-like growth factor-I. Endocrinology 129, 883–888. doi: 10.1210/endo-129-2-883
CrossRef Full Text | Google Scholar
Binart, N., Helloco, C., Ormandy, C. J., Barra, J., Clément-Lacroix, P., Baran, N., et al. (2000). Rescue of preimplantatory egg development and embryo implantation in prolactin receptor-deficient mice after progesterone administration. Endocrinology 141, 2691–2697. doi: 10.1210/endo.141.7.7568
Binko, J., and Majewski, H. (1998). 17β-Estradiol reduces vasoconstriction in endothelium-denuded rat aortas through inducible NOS. Am. J. Physiol. Heart Circ. Physiol. 274, H853–H859. doi: 10.1152/ajpheart.1998.274.3.H853
Bittorf, T., Jaster, R., Soares, M. J., Seiler, J., Brock, J., Friese, K., et al. (2000). Induction of erythroid proliferation and differentiation by a trophoblast-specific cytokine involves activation of the JAK/STAT pathway. J. Mol. Endocrinol. 25, 253–262. doi: 10.1677/jme.0.0250253
Bjøro, K., and Stray-Pedersen, S. (1986). Effects of vasoactive autacoids on different segments of human umbilicoplacental vessels. Gynecol. Obstet. Invest. 22, 1–6. doi: 10.1159/000298881
Blanchard, M. M., Goodyer, C. G., Charrier, J., Kann, G., Garcia-Villar, R., Bousquet-Melou, A., et al. (1991). GRF treatment of late pregnant ewes alters maternal and fetal somatotropic axis activity. Am. J. Physiol. 260, E575–580. doi: 10.1152/ajpendo.1991.260.4.E575
Bonner, J. S., Lantier, L., Hocking, K. M., Kang, L., Owolabi, M., James, F. D., et al. (2013). Relaxin treatment reverses insulin resistance in mice fed a high-fat diet. Diabetes 62, 3251–3260. doi: 10.2337/db13-0033
Boparai, R. K., Arum, O., Khardori, R., and Bartke, A. (2010). Glucose homeostasis and insulin sensitivity in growth hormone-transgenic mice: a cross-sectional analysis. Biol. Chem. 391, 1149–1155. doi: 10.1515/bc.2010.124
Bosch, O. J., and Neumann, I. D. (2012). Both oxytocin and vasopressin are mediators of maternal care and aggression in rodents: from central release to sites of action. Horm. Behav. 61, 293–303. doi: 10.1016/j.yhbeh.2011.11.002
Bowden, S. J., Emly, J. F., Hughes, S. V., Powell, G., Ahmed, A., Whittle, M. J., et al. (1994). Parathyroid hormone-related protein in human term placenta and membranes. J. Endocrinol. 142, 217–224. doi: 10.1677/joe.0.1420217
Bowe, J. E., Foot, V. L., Amiel, S. A., Huang, G. C., Lamb, M. W., Lakey, J., et al. (2012). GPR54 peptide agonists stimulate insulin secretion from murine, porcine and human islets. Islets 4, 20–23. doi: 10.4161/isl.18261
Bowe, J. E., King, A. J., Kinsey-Jones, J. S., Foot, V. L., Li, X. F., O'byrne, K. T., et al. (2009). Kisspeptin stimulation of insulin secretion: mechanisms of action in mouse islets and rats. Diabetologia 52, 855–862. doi: 10.1007/s00125-009-1283-1
Branisteanu, D. D., and Mathieu, C. (2003). Progesterone in gestational diabetes mellitus: guilty or not guilty? Trends Endocrinol. Metab. 14, 54–56. doi: 10.1016/S1043-2760(03)00003-1
Brelje, T. C., Allaire, P., Hegre, O., and Sorenson, R. L. (1989). Effect of prolactin versus growth hormone on islet function and the importance of using homologous mammosomatotropic hormones. Endocrinology 125, 2392–2399. doi: 10.1210/endo-125-5-2392
Brelje, T. C., Scharp, D. W., Lacy, P. E., Ogren, L., Talamantes, F., Robertson, M., et al. (1993). Effect of homologous placental lactogens, prolactins, and growth hormones on islet B-cell division and insulin secretion in rat, mouse, and human islets: implication for placental lactogen regulation of islet function during pregnancy. Endocrinology 132, 879–887. doi: 10.1210/endo.132.2.8425500
Bridges, R. S. (2015). Neuroendocrine regulation of maternal behavior. Front. Neuroendocrinol. 36, 178–196. doi: 10.1016/j.yfrne.2014.11.007
Bridges, R. S., and Millard, W. J. (1988). Growth hormone is secreted by ectopic pituitary grafts and stimulates maternal behavior in rats. Horm. Behav. 22, 194–206. doi: 10.1016/0018-506X(88)90066-9
Bridges, R. S., Robertson, M. C., Shiu, R. P., Sturgis, J. D., Henriquez, B. M., and Mann, P. E. (1997). Central lactogenic regulation of maternal behavior in rats: steroid dependence, hormone specificity, and behavioral potencies of rat prolactin and rat placental lactogen I. Endocrinology 138, 756–763. doi: 10.1210/endo.138.2.4921
Brockus, K. E., Hart, C. G., Gilfeather, C. L., Fleming, B. O., and Lemley, C. O. (2016). Dietary melatonin alters uterine artery hemodynamics in pregnant Holstein heifers. Domest. Anim. Endocrinol. 55, 1–10. doi: 10.1016/j.domaniend.2015.10.006
Brown, A. G., Leite, R. S., and Strauss, J. F. III. (2004). Mechanisms underlying “functional” progesterone withdrawal at parturition. Ann. N. Y. Acad. Sci. 1034, 36–49. doi: 10.1196/annals.1335.004
Brown, P. A., Davis, W. C., and Draghia-Akli, R. (2004). Immune-enhancing effects of growth hormone-releasing hormone delivered by plasmid injection and electroporation. Mol. Ther. 10, 644–651. doi: 10.1016/j.ymthe.2004.06.1015
Brown, P. A., Khan, A. S., Draghia-Akli, R., Pope, M. A., Bodles-Brakhop, A. M., and Kern, D. R. (2012). Effects of administration of two growth hormone-releasing hormone plasmids to gilts on sow and litter performance for the subsequent three gestations. Am. J. Vet. Res. 73, 1428–1434. doi: 10.2460/ajvr.73.9.1428
Bryant-Greenwood, G. D., Yamamoto, S. Y., Sadowsky, D. W., Gravett, M. G., and Novy, M. J. (2009). Relaxin stimulates interleukin-6 and interleukin-8 secretion from the extraplacental chorionic cytotrophoblast. Placenta 30, 599–606. doi: 10.1016/j.placenta.2009.04.009
Bryzgalova, G., Gao, H., Ahren, B., Zierath, J. R., Galuska, D., Steiler, T. L., et al. (2006). Evidence that oestrogen receptor-alpha plays an important role in the regulation of glucose homeostasis in mice: insulin sensitivity in the liver. Diabetologia 49, 588–597. doi: 10.1007/s00125-005-0105-3
Bustamante, J. J., Copple, B. L., Soares, M. J., and Dai, G. (2010). Gene profiling of maternal hepatic adaptations to pregnancy. Liver Int. 30, 406–415. doi: 10.1111/j.1478-3231.2009.02183.x
Bustamante, J. J., Dai, G., and Soares, M. J. (2008). Pregnancy and lactation modulate maternal splenic growth and development of the erythroid lineage in the rat and mouse. Reprod. Fertil. Dev. 20, 303–310. doi: 10.1071/RD07106
Cameo, P., Bischof, P., and Calvo, J. C. (2003). Effect of leptin on progesterone, human chorionic gonadotropin, and interleukin-6 secretion by human term trophoblast cells in culture. Biol. Reprod. 68, 472–477. doi: 10.1095/biolreprod.102.006122
Camerino, C. (2009). Low sympathetic tone and obese phenotype in oxytocin-deficient mice. Obesity (Silver. Spring). 17, 980–984. doi: 10.1038/oby.2009.12
Carter, A. M. (2012). Evolution of placental function in mammals: the molecular basis of gas and nutrient transfer, hormone secretion, and immune responses. Physiol. Rev. 92, 1543–1576. doi: 10.1152/physrev.00040.2011
Casellas, A., Mallol, C., Salavert, A., Jimenez, V., Garcia, M., Agudo, J., et al. (2015). Insulin-like growth factor 2 overexpression induces beta-cell dysfunction and increases beta-cell susceptibility to damage. J. Biol. Chem. 290, 16772–16785. doi: 10.1074/jbc.M115.642041
Castellucci, M., De Matteis, R., Meisser, A., Cancello, R., Monsurrò, V., Islami, D., et al. (2000). Leptin modulates extracellular matrix molecules and metalloproteinases: possible implications for trophoblast invasion. Mol. Hum. Reprod. 6, 951–958. doi: 10.1093/molehr/6.10.951
Castillo-Melendez, M., Yawno, T., Sutherland, A., Jenkin, G., Wallace, E. M., and Miller, S. L. (2017). effects of antenatal melatonin treatment on the cerebral vasculature in an ovine model of fetal growth restriction. Dev. Neurosci. 39, 323–337. doi: 10.1159/000471797
Catalano, P. M., Hoegh, M., Minium, J., Huston-Presley, L., Bernard, S., Kalhan, S., et al. (2006). Adiponectin in human pregnancy: implications for regulation of glucose and lipid metabolism. Diabetologia 49, 1677–1685. doi: 10.1007/s00125-006-0264-x
Cattaneo, M. G., Chini, B., and Vicentini, L. M. (2008). Oxytocin stimulates migration and invasion in human endothelial cells. Br. J. Pharmacol. 153, 728–736. doi: 10.1038/sj.bjp.0707609
Cebrian, A., García-Ocaña, A., Takane, K. K., Sipula, D., Stewart, A. F., and Vasavada, R. C. (2002). Overexpression of parathyroid hormone-related protein inhibits pancreatic beta-cell death in vivo and in vitro . Diabetes 51, 3003–3013. doi: 10.2337/diabetes.51.10.3003
Cetković, A., Miljic, D., Ljubić, A., Patterson, M., Ghatei, M., Stamenkovic, J., et al. (2012). Plasma kisspeptin levels in pregnancies with diabetes and hypertensive disease as a potential marker of placental dysfunction and adverse perinatal outcome. Endocr. Res. 37, 78–88. doi: 10.3109/07435800.2011.639319
Chandran, S., Cairns, M. T., O'brien, M., and Smith, T. J. (2014). Transcriptomic effects of estradiol treatment on cultured human uterine smooth muscle cells. Mol. Cell. Endocrinol. 393, 16–23. doi: 10.1016/j.mce.2014.05.020
Chang, J., and Streitman, D. (2012). Physiologic adaptations to pregnancy. Neurol. Clin. 30, 781–789. doi: 10.1016/j.ncl.2012.05.001
Chapman, A. B., Abraham, W. T., Zamudio, S., Coffin, C., Merouani, A., Young, D., et al. (1998). Temporal relationships between hormonal and hemodynamic changes in early human pregnancy. Kidney Int. 54, 2056–2063. doi: 10.1046/j.1523-1755.1998.00217.x
Chardonnens, D., Cameo, P., Aubert, M. L., Pralong, F. P., Islami, D., Campana, A., et al. (1999). Modulation of human cytotrophoblastic leptin secretion by interleukin-1α and 17β-oestradiol and its effect on HCG secretion. Mol. Hum. Reprod. 5, 1077–1082. doi: 10.1093/molehr/5.11.1077
Chataigneau, T., Zerr, M., Chataigneau, M., Hudlett, F., Hirn, C., Pernot, F., et al. (2004). Chronic treatment with progesterone but not medroxyprogesterone acetate restores the endothelial control of vascular tone in the mesenteric artery of ovariectomized rats. Menopause 11, 255–263. doi: 10.1097/01.GME.0000097847.95550.E3
Chaves, V. E., Tilelli, C. Q., Brito, N. A., and Brito, M. N. (2013). Role of oxytocin in energy metabolism. Peptides 45, 9–14. doi: 10.1016/j.peptides.2013.04.010
Chehab, F. F., Lim, M. E., and Lu, R. (1996). Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat. Genet. 12, 318–320. doi: 10.1038/ng0396-318
Chehab, F. F., Mounzih, K., Lu, R., and Lim, M. E. (1997). Early onset of reproductive function in normal female mice treated with leptin. Science 275, 88–90. doi: 10.1126/science.275.5296.88
Chen, J. Z., Sheehan, P. M., Brennecke, S. P., and Keogh, R. J. (2012). Vessel remodelling, pregnancy hormones and extravillous trophoblast function. Mol. Cell. Endocrinol. 349, 138–144. doi: 10.1016/j.mce.2011.10.014
Chen, L., Xie, Y., Fan, J., Sui, L., Xu, Y., Zhang, N., et al. (2015). HCG induces beta1,4-GalT I expression and promotes embryo implantation. Int. J. Clin. Exp. Pathol. 8, 4673–4683.
Cheung, K. L., and Lafayette, R. A. (2013). Renal physiology of pregnancy. Adv. Chronic Kidney Dis. 20, 209–214. doi: 10.1053/j.ackd.2013.01.012
Chinnathambi, V., Blesson, C. S., Vincent, K. L., Saade, G. R., Hankins, G. D., Yallampalli, C., et al. (2014). Elevated testosterone levels during rat pregnancy cause hypersensitivity to angiotensin II and attenuation of endothelium-dependent vasodilation in uterine arteries. Hypertension 64, 405–414. doi: 10.1161/HYPERTENSIONAHA.114.03283
Chung, E., Yeung, F., and Leinwand, L. A. (2012). Akt and MAPK signaling mediate pregnancy-induced cardiac adaptation. J Appl. Physiol. (1985) 112, 1564–1575. doi: 10.1152/japplphysiol.00027.2012
Chung, W. K., Belfi, K., Chua, M., Wiley, J., Mackintosh, R., Nicolson, M., et al. (1998). Heterozygosity for Lep(ob) or Lep(rdb) affects body composition and leptin homeostasis in adult mice. Am. J. Physiol. 274, R985–R990.
Clarke, A. G., and Kendall, M. D. (1994). The thymus in pregnancy: the interplay of neural, endocrine and immune influences. Immunol. Today 15, 545–551. doi: 10.1016/0167-5699(94)90212-7
Clementi, C., Tripurani, S. K., Large, M. J., Edson, M. A., Creighton, C. J., Hawkins, S. M., et al. (2013). Activin-like kinase 2 functions in peri-implantation uterine signaling in mice and humans. PLoS Genet. 9:e1003863. doi: 10.1371/journal.pgen.1003863
Comai, S., Ochoa-Sanchez, R., Dominguez-Lopez, S., Bambico, F. R., and Gobbi, G. (2015). Melancholic-Like behaviors and circadian neurobiological abnormalities in melatonin MT1 receptor knockout mice. Int. J. Neuropsychopharmacol. 18. doi: 10.1093/ijnp/pyu075
Conrad, K. P., Debrah, D. O., Novak, J., Danielson, L. A., and Shroff, S. G. (2004). Relaxin modifies systemic arterial resistance and compliance in conscious, nonpregnant rats. Endocrinology 145, 3289–3296. doi: 10.1210/en.2003-1612
Contreras-Alcantara, S., Baba, K., and Tosini, G. (2010). Removal of melatonin receptor type 1 induces insulin resistance in the mouse. Obesity (Silver. Spring). 18, 1861–1863. doi: 10.1038/oby.2010.24
Contreras, G., Gutiérrez, M., Beroíza, T., Fantín, A., Oddó, H., Villarroel, L., et al. (1991). Ventilatory drive and respiratory muscle function in pregnancy. Am. Rev. Respir. Dis. 144, 837–841. doi: 10.1164/ajrccm/144.4.837
Costa, M. A. (2016). The endocrine function of human placenta: an overview. Reprod. Biomed. Online 32, 14–43. doi: 10.1016/j.rbmo.2015.10.005
Costrini, N. V., and Kalkhoff, R. K. (1971). Relative effects of pregnancy, estradiol, and progesterone on plasma insulin and pancreatic islet insulin secretion. J. Clin. Invest. 50, 992–999. doi: 10.1172/JCI106593
Coya, R., Martul, P., Algorta, J., Aniel-Quiroga, M. A., Busturia, M. A., and Señarís, R. (2006). Effect of leptin on the regulation of placental hormone secretion in cultured human placental cells. Gynecol. Endocrinol. 22, 620–626. doi: 10.1080/09513590601012587
Crocker, I., Kaur, M., Hosking, D. J., and Baker, P. N. (2002). Rescue of trophoblast apoptosis by parathyroid hormone-related protein. BJOG 109, 218–220. doi: 10.1111/j.1471-0528.2002.01033.x
Cruz, M. A., Gallardo, V., Miguel, P., Carrasco, G., and Gonzalez, C. (1997). Serotonin-induced vasoconstriction is mediated by thromboxane release and action in the human fetal-placental circulation. Placenta 18, 197–204. doi: 10.1016/S0143-4004(97)90093-X
Díaz, P., Powell, T. L., and Jansson, T. (2014). The role of placental nutrient sensing in maternal-fetal resource allocation. Biol. Reprod. 91:82. doi: 10.1095/biolreprod.114.121798
Da Costa, T. H., Taylor, K., Ilic, V., and Williamson, D. H. (1995). Regulation of milk lipid secretion: effects of oxytocin, prolactin and ionomycin on triacylglycerol release from rat mammary gland slices. Biochem. J. 308(Pt 3), 975–981. doi: 10.1042/bj3080975
Dai, G., Lu, L., Tang, S., Peal, M. J., and Soares, M. J. (2002). Prolactin family miniarray: a tool for evaluating uteroplacental-trophoblast endocrine cell phenotypes. Reproduction 124, 755–765. doi: 10.1530/rep.0.1240755
Dai, S. Q., Yu, L. P., Shi, X., Wu, H., Shao, P., Yin, G. Y., et al. (2014). Serotonin regulates osteoblast proliferation and function in vitro . Braz. J. Med. Biol. Res. 47, 759–765. doi: 10.1590/1414-431X20143565
Danielson, L. A., Sherwood, O. D., and Conrad, K. P. (1999). Relaxin is a potent renal vasodilator in conscious rats. J. Clin. Invest. 103, 525–533. doi: 10.1172/JCI5630
Datta, N. S., Chen, C., Berry, J. E., and Mccauley, L. K. (2005). PTHrP signaling targets cyclin D1 and induces osteoblastic cell growth arrest. J. Bone Miner. Res. 20, 1051–1064. doi: 10.1359/JBMR.050106
Davison, J. M., and Dunlop, W. (1980). Renal hemodynamics and tubular function normal human pregnancy. Kidney Int. 18, 152–161. doi: 10.1038/ki.1980.124
Dean, M., Hunt, J., Mcdougall, L., and Rose, J. (2014). Uterine glycogen metabolism in mink during estrus, embryonic diapause and pregnancy. J. Reprod. Dev. 60, 438–446. doi: 10.1262/jrd.2014-013
Deblon, N., Veyrat-Durebex, C., Bourgoin, L., Caillon, A., Bussier, A. L., Petrosino, S., et al. (2011). Mechanisms of the anti-obesity effects of oxytocin in diet-induced obese rats. PLoS ONE 6:e25565. doi: 10.1371/journal.pone.0025565
Debrah, D. O., Debrah, J. E., Haney, J. L., Mcguane, J. T., Sacks, M. S., Conrad, K. P., et al. (2011). Relaxin regulates vascular wall remodeling and passive mechanical properties in mice. J. Appl. Physiol. (1985) 111, 260–271. doi: 10.1152/japplphysiol.00845.2010
Debrah, D. O., Novak, J., Matthews, J. E., Ramirez, R. J., Shroff, S. G., and Conrad, K. P. (2006). Relaxin is essential for systemic vasodilation and increased global arterial compliance during early pregnancy in conscious rats. Endocrinology 147, 5126–5131. doi: 10.1210/en.2006-0567
Declerck, C. H., Boone, C., and Kiyonari, T. (2010). Oxytocin and cooperation under conditions of uncertainty: the modulating role of incentives and social information. Horm. Behav. 57, 368–374. doi: 10.1016/j.yhbeh.2010.01.006
De Dreu, C. K., Greer, L. L., Handgraaf, M. J., Shalvi, S., Van Kleef, G. A., Baas, M., et al. (2010). The neuropeptide oxytocin regulates parochial altruism in intergroup conflict among humans. Science 328, 1408–1411. doi: 10.1126/science.1189047
Del Rincon, J. P., Iida, K., Gaylinn, B. D., Mccurdy, C. E., Leitner, J. W., Barbour, L. A., et al. (2007). Growth hormone regulation of p85alpha expression and phosphoinositide 3-kinase activity in adipose tissue: mechanism for growth hormone-mediated insulin resistance. Diabetes 56, 1638–1646. doi: 10.2337/db06-0299
Denicolo, G., Morris, S. T., Kenyon, P. R., Morel, P. C., and Parkinson, T. J. (2008). Melatonin-improved reproductive performance in sheep bred out of season. Anim. Reprod. Sci. 109, 124–133. doi: 10.1016/j.anireprosci.2007.10.012
De Pedro, M. A., Morán, J., Díaz, I., Murias, L., Fernández-Plaza, C., Gonzále, C., et al. (2015). Circadian Kisspeptin expression in human term placenta. Placenta 36, 1337–1339. doi: 10.1016/j.placenta.2015.09.009
Dill, R., and Walker, A. M. (2017). Role of prolactin in promotion of immune cell migration into the mammary gland. J. Mammary Gland Biol. Neoplasia 22, 13–26. doi: 10.1007/s10911-016-9369-0
Ding, H., Zhang, G., Sin, K. W., Liu, Z., Lin, R. K., Li, M., et al. (2017). Activin A induces skeletal muscle catabolism via p38beta mitogen-activated protein kinase. J. Cachexia Sarcopenia Muscle 8, 202–212. doi: 10.1002/jcsm.12145
Di, W. L., Lachelin, G. C., Mcgarrigle, H. H., Thomas, N. S., and Becker, D. L. (2001). Oestriol and oestradiol increase cell to cell communication and connexin43 protein expression in human myometrium. Mol. Hum. Reprod. 7, 671–679. doi: 10.1093/molehr/7.7.671
Dominici, F. P., Argentino, D. P., Muñoz, M. C., Miquet, J. G., Sotelo, A. I., and Turyn, D. (2005). Influence of the crosstalk between growth hormone and insulin signalling on the modulation of insulin sensitivity. Growth Horm. IGF Res. 15, 324–336. doi: 10.1016/j.ghir.2005.07.001
Dominici, F. P., Cifone, D., Bartke, A., and Turyn, D. (1999). Loss of sensitivity to insulin at early events of the insulin signaling pathway in the liver of growth hormone-transgenic mice. J. Endocrinol. 161, 383–392. doi: 10.1677/joe.0.1610383
Douglas, A. J., Johnstone, L. E., and Leng, G. (2007). Neuroendocrine mechanisms of change in food intake during pregnancy: a potential role for brain oxytocin. Physiol. Behav. 91, 352–365. doi: 10.1016/j.physbeh.2007.04.012
Drynda, R., Peters, C. J., Jones, P. M., and Bowe, J. E. (2015). The role of non-placental signals in the adaptation of islets to pregnancy. Horm. Metab. Res. 47, 64–71. doi: 10.1055/s-0034-1395691
Dunbar, M. E., Dann, P., Brown, C. W., Van Houton, J., Dreyer, B., Philbrick, W. P., et al. (2001). Temporally regulated overexpression of parathyroid hormone-related protein in the mammary gland reveals distinct fetal and pubertal phenotypes. J. Endocrinol. 171, 403–416. doi: 10.1677/joe.0.1710403
Duval, C., Dilworth, M. R., Tunster, S. J., Kimber, S. J., and Glazier, J. D. (2017). PTHrP is essential for normal morphogenetic and functional development of the murine placenta. Dev. Biol. 430, 325–336. doi: 10.1016/j.ydbio.2017.08.033
Edey, L. F., Georgiou, H., O'dea, K. P., Mesiano, S., Herbert, B. R., Lei, K., et al. (2018). Progesterone, the maternal immune system and the onset of parturition in the mouse. Biol. Reprod. 98, 376–395. doi: 10.1093/biolre/iox146
Einspanier, A., Lieder, K., Husen, B., Ebert, K., Lier, S., Einspanier, R., et al. (2009). Relaxin supports implantation and early pregnancy in the marmoset monkey. Ann. N. Y. Acad. Sci. 1160, 140–146. doi: 10.1111/j.1749-6632.2009.03947.x
Elabd, S. K., Sabry, I., Hassan, W. B., Nour, H., and Zaky, K. (2007). Possible neuroendocrine role for oxytocin in bone remodeling. Endocr. Regul. 41, 131–141.
El-Hashash, A. H., and Kimber, S. J. (2006). PTHrP induces changes in cell cytoskeleton and E-cadherin and regulates Eph/Ephrin kinases and RhoGTPases in murine secondary trophoblast cells. Dev. Biol. 290, 13–31. doi: 10.1016/j.ydbio.2005.10.010
Elling, S. V., and Powell, F. C. (1997). Physiological changes in the skin during pregnancy. Clin. Dermatol. 15, 35–43. doi: 10.1016/S0738-081X(96)00108-3
Elsheikh, A., Creatsas, G., Mastorakos, G., Milingos, S., Loutradis, D., and Michalas, S. (2001). The renin-aldosterone system during normal and hypertensive pregnancy. Arch. Gynecol. Obstet. 264, 182–185. doi: 10.1007/s004040000104
Emly, J. F., Gregory, J., Bowden, S. J., Ahmed, A., Whittle, M. J., Rushton, D. I., et al. (1994). Immunohistochemical localization of parathyroid hormone-related protein (PTHrP) in human term placenta and membranes. Placenta 15, 653–660. doi: 10.1016/S0143-4004(05)80411-4
Enright, W. J., Chapin, L. T., Moseley, W. M., and Tucker, H. A. (1988). Effects of infusions of various doses of bovine growth hormone-releasing factor on growth hormone and lactation in Holstein cows. J. Dairy Sci. 71, 99–108. doi: 10.3168/jds.S0022-0302(88)79530-2
Enright, W. J., Chapin, L. T., Moseley, W. M., Zinn, S. A., Kamdar, M. B., Krabill, L. F., et al. (1989). Effects of infusions of various doses of bovine growth hormone-releasing factor on blood hormones and metabolites in lactating Holstein cows. J. Endocrinol. 122, 671–679. doi: 10.1677/joe.0.1220671
Enright, W. J., Chapin, L. T., Moseley, W. M., Zinn, S. A., and Tucker, H. A. (1986). Growth hormone-releasing factor stimulates milk production and sustains growth hormone release in Holstein cows. J. Dairy Sci. 69, 344–351. doi: 10.3168/jds.S0022-0302(86)80412-X
Ernst, S., Demirci, C., Valle, S., Velazquez-Garcia, S., and Garcia-Ocaña, A. (2011). Mechanisms in the adaptation of maternal beta-cells during pregnancy. Diabetes Manag. (Lond). 1, 239–248. doi: 10.2217/dmt.10.24
Eta, E., Ambrus, G., and Rao, C. V. (1994). Direct regulation of human myometrial contractions by human chorionic gonadotropin. J. Clin. Endocrinol. Metab. 79, 1582–1586.
Etienne, M., Bonneau, M., Kann, G., and Deletang, F. (1992). Effects of administration of growth hormone-releasing factor to sows during late gestation on growth hormone secretion, reproductive traits, and performance of progeny from birth to 100 kilograms live weight. J. Anim. Sci. 70, 2212–2220. doi: 10.2527/1992.7072212x
Everson, G. T. (1992). Gastrointestinal motility in pregnancy. Gastroenterol. Clin. North Am. 21, 751–776.
Fang, X., Wong, S., and Mitchell, B. F. (1997). Effects of RU486 on estrogen, progesterone, oxytocin, and their receptors in the rat uterus during late gestation. Endocrinology 138, 2763–2768. doi: 10.1210/endo.138.7.5247
Farmer, C., Dubreuil, P., Pelletier, G., Petitclerc, D., Gaudreau, P., and Brazeau, P. (1991). Effects of active immunization against somatostatin (SRIF) and/or injections of growth hormone-releasing factor (GRF) during gestation on hormonal and metabolic profiles in sows. Domest. Anim. Endocrinol. 8, 415–422. doi: 10.1016/0739-7240(91)90009-9
Farmer, C., Petitclerc, D., Pelletier, G., and Brazeau, P. (1992). Lactation performance of sows injected with growth hormone-releasing factor during gestation and(or) lactation. J. Anim. Sci. 70, 2636–2642. doi: 10.2527/1992.7092636x
Farmer, C., Robert, S., and Matte, J. J. (1996). Lactation performance of sows fed a bulky diet during gestation and receiving growth hormone-releasing factor during lactation. J. Anim. Sci. 74, 1298–1306. doi: 10.2527/1996.7461298x
Fecteau, K. A., and Eiler, H. (2001). Placenta detachment: unexpected high concentrations of 5-hydroxytryptamine (serotonin) in fetal blood and its mitogenic effect on placental cells in bovine. Placenta 22, 103–110. doi: 10.1053/plac.2000.0596
Feng, S., Bogatcheva, N. V., Kamat, A. A., Truong, A., and Agoulnik, A. I. (2006). Endocrine effects of relaxin overexpression in mice. Endocrinology 147, 407–414. doi: 10.1210/en.2005-0626
Ferguson, J. N., Young, L. J., Hearn, E. F., Matzuk, M. M., Insel, T. R., and Winslow, J. T. (2000). Social amnesia in mice lacking the oxytocin gene. Nat. Genet. 25, 284–288. doi: 10.1038/77040
Ferris, C. F., Foote, K. B., Meltser, H. M., Plenby, M. G., Smith, K. L., and Insel, T. R. (1992). Oxytocin in the amygdala facilitates maternal aggression. Ann. N. Y. Acad. Sci. 652, 456–457. doi: 10.1111/j.1749-6632.1992.tb34382.x
Fettke, F., Schumacher, A., Canellada, A., Toledo, N., Bekeredjian-Ding, I., Bondt, A., et al. (2016). Maternal and fetal mechanisms of B cell regulation during pregnancy: human chorionic gonadotropin stimulates B cells to Produce IL-10 while alpha-fetoprotein drives them into apoptosis. Front. Immunol. 7:495. doi: 10.3389/fimmu.2016.00495
Fliegner, D., Schubert, C., Penkalla, A., Witt, H., Kararigas, G., Dworatzek, E., et al. (2010). Female sex and estrogen receptor-beta attenuate cardiac remodeling and apoptosis in pressure overload. Am. J. Physiol. Regul. Integr. Comp. Physiol. 298, R1597–R1606. doi: 10.1152/ajpregu.00825.2009
Flores-Espinosa, P., Preciado-Martínez, E., Mejía-Salvador, A., Sedano-González, G., Bermejo-Martínez, L., Parra-Covarruvias, A., et al. (2017). Selective immuno-modulatory effect of prolactin upon pro-inflammatory response in human fetal membranes. J. Reprod. Immunol. 123, 58–64. doi: 10.1016/j.jri.2017.09.004
Florio, P., Lombardo, M., Gallo, R., Di Carlo, C., Sutton, S., Genazzani, A. R., et al. (1996). Activin A, corticotropin-releasing factor and prostaglandin F2 alpha increase immunoreactive oxytocin release from cultured human placental cells. Placenta 17, 307–311. doi: 10.1016/S0143-4004(96)90054-5
Fournier, T., Guibourdenche, J., and Evain-Brion, D. (2015). Review: hCGs: different sources of production, different glycoforms and functions. Placenta 36(Suppl. 1), S60–S65. doi: 10.1016/j.placenta.2015.02.002
Fowden, A. L., Giussani, D. A., and Forhead, A. J. (2006). Intrauterine programming of physiological systems: causes and consequences. Physiology (Bethesda). 21, 29–37. doi: 10.1152/physiol.00050.2005
Fowden, A. L., and Moore, T. (2012). Maternal-fetal resource allocation: co-operation and conflict. Placenta 33(Suppl. 2), e11–e15. doi: 10.1016/j.placenta.2012.05.002
Fowler, P. A., Evans, L. W., Groome, N. P., Templeton, A., and Knight, P. G. (1998). A longitudinal study of maternal serum inhibin-A, inhibin-B, activin-A, activin-AB, pro-alphaC and follistatin during pregnancy. Hum. Reprod. 13, 3530–3536. doi: 10.1093/humrep/13.12.3530
Freemark, M. (2010). Placental hormones and the control of fetal growth. J. Clin. Endocrinol. Metab. 95, 2054–2057. doi: 10.1210/jc.2010-0517
Freemark, M., Avril, I., Fleenor, D., Driscoll, P., Petro, A., Opara, E., et al. (2002). Targeted deletion of the PRL receptor: effects on islet development, insulin production, and glucose tolerance. Endocrinology 143, 1378–1385. doi: 10.1210/endo.143.4.8722
Freemark, M., Fleenor, D., Driscoll, P., Binart, N., and Kelly, P. (2001). Body weight and fat deposition in prolactin receptor-deficient mice. Endocrinology 142, 532–537. doi: 10.1210/endo.142.2.7979
Frise, C., Noori, M., and Williamson, C. (2013). Severe metabolic alkalosis in pregnancy. Obstet. Med. 6, 138–140. doi: 10.1258/om.2012.120030
Fudge, N. J., and Kovacs, C. S. (2010). Pregnancy up-regulates intestinal calcium absorption and skeletal mineralization independently of the vitamin D receptor. Endocrinology 151, 886–895. doi: 10.1210/en.2009-1010
Fujinaka, Y., Sipula, D., Garcia-Ocaña, A., and Vasavada, R. C. (2004). Characterization of mice doubly transgenic for parathyroid hormone-related protein and murine placental lactogen: a novel role for placental lactogen in pancreatic beta-cell survival. Diabetes 53, 3120–3130. doi: 10.2337/diabetes.53.12.3120
Fungfuang, W., Terada, M., Komatsu, N., Moon, C., and Saito, T. R. (2013). Effects of estrogen on food intake, serum leptin levels and leptin mRNA expression in adipose tissue of female rats. Lab. Anim. Res. 29, 168–173. doi: 10.5625/lar.2013.29.3.168
Gallacher, S. J., Fraser, W. D., Owens, O. J., Dryburgh, F. J., Logue, F. C., Jenkins, A., et al. (1994). Changes in calciotrophic hormones and biochemical markers of bone turnover in normal human pregnancy. Eur. J. Endocrinol. 131, 369–374. doi: 10.1530/eje.0.1310369
Gallego, M. I., Binart, N., Robinson, G. W., Okagaki, R., Coschigano, K. T., Perry, J., et al. (2001). Prolactin, growth hormone, and epidermal growth factor activate Stat5 in different compartments of mammary tissue and exert different and overlapping developmental effects. Dev. Biol. 229, 163–175. doi: 10.1006/dbio.2000.9961
Galosy, S. S., and Talamantes, F. (1995). Luteotropic actions of placental lactogens at midpregnancy in the mouse. Endocrinology 136, 3993–4003. doi: 10.1210/endo.136.9.7649108
Garcia-Ruíz, G., Flores-Espinosa, P., Preciado-Martínez, E., Bermejo-Martínez, L., Espejel-Nuñez, A., Estrada-Gutierrez, G., et al. (2015). In vitro progesterone modulation on bacterial endotoxin-induced production of IL-1beta, TNFalpha, IL-6, IL-8, IL-10, MIP-1alpha, and MMP-9 in pre-labor human term placenta. Reprod. Biol. Endocrinol. 13:115. doi: 10.1186/s12958-015-0111-3
Gimeno, M. F., Landa, A., Sterin-Speziale, N., Cardinali, D. P., and Gimeno, A. L. (1980). Melatonin blocks in vitro generation of prostaglandin by the uterus and hypothalamus. Eur. J. Pharmacol. 62, 309–317. doi: 10.1016/0014-2999(80)90098-9
Goh, B. C., Singhal, V., Herrera, A. J., Tomlinson, R. E., Kim, S., Faugere, M. C., et al. (2017). Activin receptor type 2A (ACVR2A) functions directly in osteoblasts as a negative regulator of bone mass. J. Biol. Chem. 292, 13809–13822. doi: 10.1074/jbc.M117.782128
Goldsmith, L. T., Weiss, G., Palejwala, S., Plant, T. M., Wojtczuk, A., Lambert, W. C., et al. (2004). Relaxin regulation of endometrial structure and function in the rhesus monkey. Proc. Natl. Acad. Sci. U.S.A. 101, 4685–4689. doi: 10.1073/pnas.0400776101
Golightly, E., Jabbour, H. N., and Norman, J. E. (2011). Endocrine immune interactions in human parturition. Mol. Cell. Endocrinol. 335, 52–59. doi: 10.1016/j.mce.2010.08.005
González-Candia, A., Veliz, M., Araya, C., Quezada, S., Ebensperger, G., Seron-Ferre, M., et al. (2016). Potential adverse effects of antenatal melatonin as a treatment for intrauterine growth restriction: findings in pregnant sheep. Am J Obstet Gynecol 215, 245 e241–245 e247. doi: 10.1016/j.ajog.2016.02.040
Goodman, H. M., Tai, L. R., Ray, J., Cooke, N. E., and Liebhaber, S. A. (1991). Human growth hormone variant produces insulin-like and lipolytic responses in rat adipose tissue. Endocrinology 129, 1779–1783. doi: 10.1210/endo-129-4-1779
Gooi, J. H., Richardson, M. L., Jelinic, M., Girling, J. E., Wlodek, M. E., Tare, M., et al. (2013). Enhanced uterine artery stiffness in aged pregnant relaxin mutant mice is reversed with exogenous relaxin treatment. Biol. Reprod. 89:18. doi: 10.1095/biolreprod.113.108118
Gopalakrishnan, K., Mishra, J. S., Chinnathambi, V., Vincent, K. L., Patrikeev, I., Motamedi, M., et al. (2016). Elevated testosterone reduces uterine blood flow, spiral artery elongation, and placental oxygenation in pregnant rats. Hypertension 67, 630–639. doi: 10.1161/HYPERTENSIONAHA.115.06946
Goyvaerts, L., Schraenen, A., and Schuit, F. (2016). Serotonin competence of mouse beta cells during pregnancy. Diabetologia 59, 1356–1363. doi: 10.1007/s00125-016-3951-2
Greening, D. W., Nguyen, H. P., Evans, J., Simpson, R. J., and Salamonsen, L. A. (2016). Modulating the endometrial epithelial proteome and secretome in preparation for pregnancy: the role of ovarian steroid and pregnancy hormones. J. Proteomics 144, 99–112. doi: 10.1016/j.jprot.2016.05.026
Gregg, C. (2009). Pregnancy, prolactin and white matter regeneration. J. Neurol. Sci. 285, 22–27. doi: 10.1016/j.jns.2009.06.040
Grès, S., Canteiro, S., Mercader, J., and Carpene, C. (2013). Oxidation of high doses of serotonin favors lipid accumulation in mouse and human fat cells. Mol. Nutr. Food Res. 57, 1089–1099. doi: 10.1002/mnfr.201200681
Groba, C., Mayerl, S., Van Mullem, A. A., Visser, T. J., Darras, V. M., Habenicht, A. J., et al. (2013). Hypothyroidism compromises hypothalamic leptin signaling in mice. Mol. Endocrinol. 27, 586–597. doi: 10.1210/me.2012-1311
Groen, B., Van Der Wijk, A. E., Van Den Berg, P. P., Lefrandt, J. D., Van Den Berg, G., Sollie, K. M., et al. (2015). Immunological Adaptations to Pregnancy in Women with Type 1 Diabetes. Sci. Rep. 5:13618. doi: 10.1038/srep13618
Groskopf, J. C., Syu, L. J., Saltiel, A. R., and Linzer, D. I. (1997). Proliferin induces endothelial cell chemotaxis through a G protein-coupled, mitogen-activated protein kinase-dependent pathway. Endocrinology 138, 2835–2840. doi: 10.1210/endo.138.7.5276
Gulinello, M., Gong, Q. H., and Smith, S. S. (2002). Progesterone withdrawal increases the alpha4 subunit of the GABA(A) receptor in male rats in association with anxiety and altered pharmacology-a comparison with female rats. Neuropharmacology 43, 701–714. doi: 10.1016/S0028-3908(02)00171-5
Gutkowska, J., and Jankowski, M. (2012). Oxytocin revisited: its role in cardiovascular regulation. J. Neuroendocrinol. 24, 599–608. doi: 10.1111/j.1365-2826.2011.02235.x
Habiger, V. W. (1975). Serotonin effect on the fetus and the feto-maternal relationship in the rat. Arzneimittelforschung. 25, 626–632.
Hadden, C., Fahmi, T., Cooper, A., Savenka, A. V., Lupashin, V. V., Roberts, D. J., et al. (2017). Serotonin transporter protects the placental cells against apoptosis in caspase 3-independent pathway. J. Cell. Physiol. 232, 3520–3529. doi: 10.1002/jcp.25812
Hadden, D. R., and Mclaughlin, C. (2009). Normal and abnormal maternal metabolism during pregnancy. Semin. Fetal Neonatal Med. 14, 66–71. doi: 10.1016/j.siny.2008.09.004
Haig, D. (2008). Placental growth hormone-related proteins and prolactin-related proteins. Placenta 29(Suppl. A), S36–S41. doi: 10.1016/j.placenta.2007.09.010
Hales, C. N., and Barker, D. J. (2001). The thrifty phenotype hypothesis. Br. Med. Bull. 60, 5–20. doi: 10.1093/bmb/60.1.5
Handwerger, S., Richards, R. G., and Markoff, E. (1992). The physiology of decidual prolactin and other decidual protein hormones. Trends Endocrinol. Metab. 3, 91–95. doi: 10.1016/1043-2760(92)90019-W
Harris, L. K., Crocker, I. P., Baker, P. N., Aplin, J. D., and Westwood, M. (2011). IGF2 actions on trophoblast in human placenta are regulated by the insulin-like growth factor 2 receptor, which can function as both a signaling and clearance receptor. Biol. Reprod. 84, 440–446. doi: 10.1095/biolreprod.110.088195
Hart, I. C., Chadwick, P. M., James, S., and Simmonds, A. D. (1985). Effect of intravenous bovine growth hormone or human pancreatic growth hormone-releasing factor on milk production and plasma hormones and metabolites in sheep. J. Endocrinol. 105, 189–196. doi: 10.1677/joe.0.1050189
Hauguel-De Mouzon, S., Lepercq, J., and Catalano, P. (2006). The known and unknown of leptin in pregnancy. Am. J. Obstet. Gynecol. 194, 1537–1545. doi: 10.1016/j.ajog.2005.06.064
Haynes, M. P., Sinha, D., Russell, K. S., Collinge, M., Fulton, D., Morales-Ruiz, M., et al. (2000). Membrane estrogen receptor engagement activates endothelial nitric oxide synthase via the PI3-kinase-Akt pathway in human endothelial cells. Circ. Res. 87, 677–682. doi: 10.1161/01.RES.87.8.677
Hearn, J. P., Gidley-Baird, A. A., Hodges, J. K., Summers, P. M., and Webley, G. E. (1988). Embryonic signals during the peri-implantation period in primates. J. Reprod. Fertil. Suppl. 36, 49–58.
Hegewald, M. J., and Crapo, R. O. (2011). Respiratory physiology in pregnancy. Clin. Chest Med. 32, 1-13, vii. doi: 10.1016/j.ccm.2010.11.001
Hellmeyer, L., Ziller, V., Anderer, G., Ossendorf, A., Schmidt, S., and Hadji, P. (2006). Biochemical markers of bone turnover during pregnancy: a longitudinal study. Exp. Clin. Endocrinol. Diabetes 114, 506–510. doi: 10.1055/s-2006-951627
Henson, M. C., Castracane, V. D., O'neil, J. S., Gimpel, T., Swan, K. F., Green, A. E., et al. (1999). Serum leptin concentrations and expression of leptin transcripts in placental trophoblast with advancing baboon pregnancy. J. Clin. Endocrinol. Metab. 84, 2543–2549. doi: 10.1210/jc.84.7.2543
Hernández-Castellano, L. E., Hernandez, L. L., Weaver, S., and Bruckmaier, R. M. (2017). Increased serum serotonin improves parturient calcium homeostasis in dairy cows. J. Dairy Sci. 100, 1580–1587. doi: 10.3168/jds.2016-11638
Herreboudt, A. M., Kyle, V. R., Lawrence, J., Doran, J., and Colledge, W. H. (2015). Kiss1 mutant placentas show normal structure and function in the mouse. Placenta 36, 52–58. doi: 10.1016/j.placenta.2014.10.016
Hershberger, M. E., and Tuan, R. S. (1998). Placental 57-kDa Ca(2+)-binding protein: regulation of expression and function in trophoblast calcium transport. Dev. Biol. 199, 80–92. doi: 10.1006/dbio.1998.8926
Hershman, J. M., Kojima, A., and Friesen, H. G. (1973). Effect of thyrotropin-releasing hormone on human pituitary thyrotropin, prolactin, placental lactogen, and chorionic thyrotropin. J. Clin. Endocrinol. Metab. 36, 497–501. doi: 10.1210/jcem-36-3-497
Highman, T. J., Friedman, J. E., Huston, L. P., Wong, W. W., and Catalano, P. M. (1998). Longitudinal changes in maternal serum leptin concentrations, body composition, and resting metabolic rate in pregnancy. Am. J. Obstet. Gynecol. 178, 1010–1015. doi: 10.1016/S0002-9378(98)70540-X
Hirota, Y., Anai, T., and Miyakawa, I. (1997). Parathyroid hormone-related protein levels in maternal and cord blood. Am. J. Obstet. Gynecol. 177, 702–706. doi: 10.1016/S0002-9378(97)70167-4
Hisamoto, K., Ohmichi, M., Kurachi, H., Hayakawa, J., Kanda, Y., Nishio, Y., et al. (2001). Estrogen induces the Akt-dependent activation of endothelial nitric-oxide synthase in vascular endothelial cells. J. Biol. Chem. 276, 3459–3467. doi: 10.1074/jbc.M005036200
Hisaw, F. L., Hisaw, F. L. Jr., and Dawson, A. B. (1967). Effects of relaxin on the endothelium of endometrial blood vessels in monkeys ( Macaca mulatta ). Endocrinology 81, 375–385. doi: 10.1210/endo-81-2-375
Hoekzema, E., Barba-Müller, E., Pozzobon, C., Picado, M., Lucco, F., García-García, D., et al. (2017). Pregnancy leads to long-lasting changes in human brain structure. Nat. Neurosci. 20, 287–296. doi: 10.1038/nn.4458
Horber, F. F., and Haymond, M. W. (1990). Human growth hormone prevents the protein catabolic side effects of prednisone in humans. J. Clin. Invest. 86, 265–272. doi: 10.1172/JCI114694
Horikoshi, Y., Matsumoto, H., Takatsu, Y., Ohtaki, T., Kitada, C., Usuki, S., et al. (2003). Dramatic elevation of plasma metastin concentrations in human pregnancy: metastin as a novel placenta-derived hormone in humans. J. Clin. Endocrinol. Metab. 88, 914–919. doi: 10.1210/jc.2002-021235
Horseman, N. D., Zhao, W., Montecino-Rodriguez, E., Tanaka, M., Nakashima, K., Engle, S. J., et al. (1997). Defective mammopoiesis, but normal hematopoiesis, in mice with a targeted disruption of the prolactin gene. EMBO J. 16, 6926–6935. doi: 10.1093/emboj/16.23.6926
Huang, C., Snider, F., and Cross, J. C. (2009). Prolactin receptor is required for normal glucose homeostasis and modulation of beta-cell mass during pregnancy. Endocrinology 150, 1618–1626. doi: 10.1210/en.2008-1003
Hudon Thibeault, A. A., Laurent, L., Vo Duy, S., Sauve, S., Caron, P., Guillemette, C., et al. (2017). Fluoxetine and its active metabolite norfluoxetine disrupt estrogen synthesis in a co-culture model of the feto-placental unit. Mol. Cell. Endocrinol. 442, 32–39. doi: 10.1016/j.mce.2016.11.021
Hughes, C. K., Xie, M. M., Mccoski, S. R., and Ealy, A. D. (2017). Activities for leptin in bovine trophoblast cells. Domest. Anim. Endocrinol. 58, 84–89. doi: 10.1016/j.domaniend.2016.09.001
Ibrahim, H. S., Omar, E., Froemming, G. R., and Singh, H. J. (2013). Leptin increases blood pressure and markers of endothelial activation during pregnancy in rats. Biomed Res. Int. 2013, 298401. doi: 10.1155/2013/298401
Ishizuka, T., Klepcyk, P., Liu, S., Panko, L., Liu, S., Gibbs, E. M., et al. (1999). Effects of overexpression of human GLUT4 gene on maternal diabetes and fetal growth in spontaneous gestational diabetic C57BLKS/J Lepr(db/+) mice. Diabetes 48, 1061–1069. doi: 10.2337/diabetes.48.5.1061
Islami, D., Bischof, P., and Chardonnens, D. (2003a). Modulation of placental vascular endothelial growth factor by leptin and hCG. Mol. Hum. Reprod. 9, 395–398. doi: 10.1093/molehr/gag053
Islami, D., Bischof, P., and Chardonnens, D. (2003b). Possible interactions between leptin, gonadotrophin-releasing hormone (GnRH-I and II) and human chorionic gonadotrophin (hCG). Eur. J. Obstet. Gynecol. Reprod. Biol. 110, 169–175. doi: 10.1016/S0301-2115(03)00185-4
Islam, M. S., Morton, N. M., Hansson, A., and Emilsson, V. (1997). Rat insulinoma-derived pancreatic beta-cells express a functional leptin receptor that mediates a proliferative response. Biochem. Biophys. Res. Commun. 238, 851–855. doi: 10.1006/bbrc.1997.7399
Iwasaki, S., Nakazawa, K., Sakai, J., Kometani, K., Iwashita, M., Yoshimura, Y., et al. (2005). Melatonin as a local regulator of human placental function. J. Pineal Res. 39, 261–265. doi: 10.1111/j.1600-079X.2005.00244.x
Izquierdo, A., López-Luna, P., Ortega, A., Romero, M., Guitiérrez-Tarrés, M. A., Arribas, I., et al. (2006). The parathyroid hormone-related protein system and diabetic nephropathy outcome in streptozotocin-induced diabetes. Kidney Int. 69, 2171–2177. doi: 10.1038/sj.ki.5000195
Jackson, D., Volpert, O. V., Bouck, N., and Linzer, D. I. (1994). Stimulation and inhibition of angiogenesis by placental proliferin and proliferin-related protein. Science 266, 1581–1584. doi: 10.1126/science.7527157
Jahnke, G., Marr, M., Myers, C., Wilson, R., Travlos, G., and Price, C. (1999). Maternal and developmental toxicity evaluation of melatonin administered orally to pregnant Sprague-Dawley rats. Toxicol. Sci. 50, 271–279. doi: 10.1093/toxsci/50.2.271
Jenkin, G., Ward, J., Loose, J., Schneider-Kolsky, M., Young, R., Canny, B., et al. (2001). Physiological and regulatory roles of activin A in late pregnancy. Mol. Cell. Endocrinol. 180, 131–138. doi: 10.1016/S0303-7207(01)00504-4
Jiang, C. W., Sarrel, P. M., Lindsay, D. C., Poole-Wilson, P. A., and Collins, P. (1992). Progesterone induces endothelium-independent relaxation of rabbit coronary artery in vitro . Eur. J. Pharmacol. 211, 163–167. doi: 10.1016/0014-2999(92)90524-8
Jobe, S. O., Ramadoss, J., Koch, J. M., Jiang, Y., Zheng, J., and Magness, R. R. (2010). Estradiol-17beta and its cytochrome P450- and catechol-O-methyltransferase-derived metabolites stimulate proliferation in uterine artery endothelial cells: role of estrogen receptor-alpha versus estrogen receptor-beta. Hypertension 55, 1005–1011. doi: 10.1161/HYPERTENSIONAHA.109.146399
Jones, R. L., Findlay, J. K., Farnworth, P. G., Robertson, D. M., Wallace, E., and Salamonsen, L. A. (2006). Activin A and inhibin A differentially regulate human uterine matrix metalloproteinases: potential interactions during decidualization and trophoblast invasion. Endocrinology 147, 724–732. doi: 10.1210/en.2005-1183
Joshi, P. A., Jackson, H. W., Beristain, A. G., Di Grappa, M. A., Mote, P. A., Clarke, C. L., et al. (2010). Progesterone induces adult mammary stem cell expansion. Nature 465, 803–807. doi: 10.1038/nature09091
Jo, Y. S., Lee, G. S., Nam, S. Y., and Kim, S. J. (2015). Progesterone inhibits leptin-induced invasiveness of BeWo cells. Int. J. Med. Sci. 12, 773–779. doi: 10.7150/ijms.11610
Kaftanovskaya, E. M., Huang, Z., Lopez, C., Conrad, K., and Agoulnik, A. I. (2015). Conditional deletion of the relaxin receptor gene in cells of smooth muscle lineage affects lower reproductive tract in pregnant mice. Biol. Reprod. 92:91. doi: 10.1095/biolreprod.114.127209
Kalkwarf, H. J., and Specker, B. L. (2002). Bone mineral changes during pregnancy and lactation. Endocrine 17, 49–53. doi: 10.1385/ENDO:17:1:49
Kamat, A. A., Feng, S., Bogatcheva, N. V., Truong, A., Bishop, C. E., and Agoulnik, A. I. (2004). Genetic targeting of relaxin and insulin-like factor 3 receptors in mice. Endocrinology 145, 4712–4720. doi: 10.1210/en.2004-0515
Kane, M. J., Angoa-Peréz, M., Briggs, D. I., Sykes, C. E., Francescutti, D. M., Rosenberg, D. R., et al. (2012). Mice genetically depleted of brain serotonin display social impairments, communication deficits and repetitive behaviors: possible relevance to autism. PLoS ONE 7:e48975. doi: 10.1371/journal.pone.0048975
Kane, N., Kelly, R., Saunders, P. T., and Critchley, H. O. (2009). Proliferation of uterine natural killer cells is induced by human chorionic gonadotropin and mediated via the mannose receptor. Endocrinology 150, 2882–2888. doi: 10.1210/en.2008-1309
Karaplis, A. C., Luz, A., Glowacki, J., Bronson, R. T., Tybulewicz, V. L., Kronenberg, H. M., et al. (1994). Lethal skeletal dysplasia from targeted disruption of the parathyroid hormone-related peptide gene. Genes Dev. 8, 277–289. doi: 10.1101/gad.8.3.277
Keebaugh, A. C., Barrett, C. E., Laprairie, J. L., Jenkins, J. J., and Young, L. J. (2015). RNAi knockdown of oxytocin receptor in the nucleus accumbens inhibits social attachment and parental care in monogamous female prairie voles. Soc. Neurosci. 10, 561–570. doi: 10.1080/17470919.2015.1040893
Kendall, M. D., and Clarke, A. G. (2000). The thymus in the mouse changes its activity during pregnancy: a study of the microenvironment. J. Anat. 197(Pt 3), 393–411. doi: 10.1046/j.1469-7580.2000.19730393.x
Keomanivong, F. E., Lemley, C. O., Camacho, L. E., Yunusova, R., Borowicz, P. P., Caton, J. S., et al. (2016). Influence of nutrient restriction and melatonin supplementation of pregnant ewes on maternal and fetal pancreatic digestive enzymes and insulin-containing clusters. Animal 10, 440–448. doi: 10.1017/S1751731115002219
Khil, L. Y., Jun, H. S., Kwon, H., Yoo, J. K., Kim, S., Notkins, A. L., et al. (2007). Human chorionic gonadotropin is an immune modulator and can prevent autoimmune diabetes in NOD mice. Diabetologia 50, 2147–2155. doi: 10.1007/s00125-007-0769-y
Kim, C., Newton, K. M., and Knopp, R. H. (2002). Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care 25, 1862–1868. doi: 10.2337/diacare.25.10.1862
Kim, H., Toyofuku, Y., Lynn, F. C., Chak, E., Uchida, T., Mizukami, H., et al. (2010). Serotonin regulates pancreatic beta cell mass during pregnancy. Nat. Med. 16, 804–808. doi: 10.1038/nm.2173
Kim, J. K. (2009). Hyperinsulinemic-euglycemic clamp to assess insulin sensitivity in vivo . Methods Mol. Biol. 560, 221–238. doi: 10.1007/978-1-59745-448-3_15
Kim, M. N., Park, M. N., Jung, H. K., Cho, C., Mayo, K. E., and Cho, B. N. (2008). Changes in the reproductive function and developmental phenotypes in mice following intramuscular injection of an activin betaA-expressing plasmid. Reprod. Biol. Endocrinol. 6:63. doi: 10.1186/1477-7827-6-63
Kim, P. (2016). Human maternal brain plasticity: adaptation to parenting. New Dir. Child Adolesc. Dev. 2016, 47–58. doi: 10.1002/cad.20168
Kim, P., Strathearn, L., and Swain, J. E. (2016). The maternal brain and its plasticity in humans. Horm. Behav. 77, 113–123. doi: 10.1016/j.yhbeh.2015.08.001
Kim, S. C., Lee, J. E., Kang, S. S., Yang, H. S., Kim, S. S., and An, B. S. (2017). The regulation of oxytocin and oxytocin receptor in human placenta according to gestational age. J. Mol. Endocrinol. 59, 235–243. doi: 10.1530/JME-16-0223
Kim, S. H., Bennett, P. R., and Terzidou, V. (2017). Advances in the role of oxytocin receptors in human parturition. Mol. Cell. Endocrinol. 449, 56–63. doi: 10.1016/j.mce.2017.01.034
King, J. C. (2000). Physiology of pregnancy and nutrient metabolism. Am. J. Clin. Nutr 71, 1218S–1225S. doi: 10.1093/ajcn/71.5.1218s
Kirby, B. J., Ardeshirpour, L., Woodrow, J. P., Wysolmerski, J. J., Sims, N. A., Karaplis, A. C., et al. (2011). Skeletal recovery after weaning does not require PTHrP. J. Bone Miner. Res. 26, 1242–1251. doi: 10.1002/jbmr.339
Kirwan, J. P., Varastehpour, A., Jing, M., Presley, L., Shao, J., Friedman, J. E., et al. (2004). Reversal of insulin resistance postpartum is linked to enhanced skeletal muscle insulin signaling. J. Clin. Endocrinol. Metab. 89, 4678–4684. doi: 10.1210/jc.2004-0749
Kleiman, A., Keats, E. C., Chan, N. G., and Khan, Z. A. (2013). Elevated IGF2 prevents leptin induction and terminal adipocyte differentiation in hemangioma stem cells. Exp. Mol. Pathol. 94, 126–136. doi: 10.1016/j.yexmp.2012.09.023
Kobayashi, K., Tsugami, Y., Matsunaga, K., Oyama, S., Kuki, C., and Kumura, H. (2016). Prolactin and glucocorticoid signaling induces lactation-specific tight junctions concurrent with beta-casein expression in mammary epithelial cells. Biochim. Biophys. Acta 1863, 2006–2016. doi: 10.1016/j.bbamcr.2016.04.023
Koonce, C. J., and Frye, C. A. (2013). Progesterone facilitates exploration, affective and social behaviors among wildtype, but not 5alpha-reductase Type 1 mutant, mice. Behav. Brain Res. 253, 232–239. doi: 10.1016/j.bbr.2013.07.025
Kota, S. K., Gayatri, K., Jammula, S., Kota, S. K., Krishna, S. V., Meher, L. K., et al. (2013). Endocrinology of parturition. Indian J. Endocrinol. Metab. 17, 50–59. doi: 10.4103/2230-8210.107841
Krajnc-Franken, M. A., Van Disseldorp, A. J., Koenders, J. E., Mosselman, S., Van Duin, M., and Gossen, J. A. (2004). Impaired nipple development and parturition in LGR7 knockout mice. Mol. Cell. Biol. 24, 687–696. doi: 10.1128/MCB.24.2.687-696.2004
Krutzén, E., Olofsson, P., Bäck, S. E., and Nilsson-Ehle, P. (1992). Glomerular filtration rate in pregnancy: a study in normal subjects and in patients with hypertension, preeclampsia and diabetes. Scand. J. Clin. Lab. Invest. 52, 387–392. doi: 10.3109/00365519209088374
Kulandavelu, S., Qu, D., and Adamson, S. L. (2006). Cardiovascular function in mice during normal pregnancy and in the absence of endothelial NO synthase. Hypertension 47, 1175–1182. doi: 10.1161/01.HYP.0000218440.71846.db
Kulkarni, R. N., Wang, Z. L., Wang, R. M., Hurley, J. D., Smith, D. M., Ghatei, M. A., et al. (1997). Leptin rapidly suppresses insulin release from insulinoma cells, rat and human islets and, in vivo , in mice. J. Clin. Invest. 100, 2729–2736. doi: 10.1172/JCI119818
Kumar, P., Kamat, A., and Mendelson, C. R. (2009). Estrogen receptor alpha (ERalpha) mediates stimulatory effects of estrogen on aromatase (CYP19) gene expression in human placenta. Mol. Endocrinol. 23, 784–793. doi: 10.1210/me.2008-0371
Ladyman, S. R., Augustine, R. A., and Grattan, D. R. (2010). Hormone interactions regulating energy balance during pregnancy. J. Neuroendocrinol. 22, 805–817. doi: 10.1111/j.1365-2826.2010.02017.x
Lain, K. Y., and Catalano, P. M. (2007). Metabolic changes in pregnancy. Clin. Obstet. Gynecol. 50, 938–948. doi: 10.1097/GRF.0b013e31815a5494
Lanoix, D., Lacasse, A. A., Reiter, R. J., and Vaillancourt, C. (2013). Melatonin: the watchdog of villous trophoblast homeostasis against hypoxia/reoxygenation-induced oxidative stress and apoptosis. Mol. Cell. Endocrinol. 381, 35–45. doi: 10.1016/j.mce.2013.07.010
Lapensee, C. R., Horseman, N. D., Tso, P., Brandebourg, T. D., Hugo, E. R., and Ben-Jonathan, N. (2006). The prolactin-deficient mouse has an unaltered metabolic phenotype. Endocrinology 147, 4638–4645. doi: 10.1210/en.2006-0487
Lapierre, H., Pelletier, G., Petitclerc, D., Dubreuil, P., Morisset, J., Gaudreau, P., et al. (1988). Effect of human growth hormone-releasing factor (1-29)NH2 on growth hormone release and milk production in dairy cows. J. Dairy Sci. 71, 92–98. doi: 10.3168/jds.S0022-0302(88)79529-6
Laporta, J., Keil, K. P., Vezina, C. M., and Hernandez, L. L. (2014a). Peripheral serotonin regulates maternal calcium trafficking in mammary epithelial cells during lactation in mice. PLoS ONE 9:e110190. doi: 10.1371/journal.pone.0110190
Laporta, J., Keil, K. P., Weaver, S. R., Cronick, C. M., Prichard, A. P., Crenshaw, T. D., et al. (2014b). Serotonin regulates calcium homeostasis in lactation by epigenetic activation of hedgehog signaling. Mol. Endocrinol. 28, 1866–1874. doi: 10.1210/me.2014-1204
Laporta, J., Moore, S. A., Weaver, S. R., Cronick, C. M., Olsen, M., Prichard, A. P., et al. (2015). Increasing serotonin concentrations alter calcium and energy metabolism in dairy cows. J. Endocrinol. 226, 43–55. doi: 10.1530/JOE-14-0693
Laporta, J., Peters, T. L., Merriman, K. E., Vezina, C. M., and Hernandez, L. L. (2013a). Serotonin (5-HT) affects expression of liver metabolic enzymes and mammary gland glucose transporters during the transition from pregnancy to lactation. PLoS ONE 8:e57847. doi: 10.1371/journal.pone.0057847
Laporta, J., Peters, T. L., Weaver, S. R., Merriman, K. E., and Hernandez, L. L. (2013b). Feeding 5-hydroxy-l-tryptophan during the transition from pregnancy to lactation increases calcium mobilization from bone in rats. Domest. Anim. Endocrinol. 44, 176–184. doi: 10.1016/j.domaniend.2013.01.005
Laurent, L., Deroy, K., St-Pierre, J., Côté, F., Sanderson, J. T., and Vaillancourt, C. (2017). Human placenta expresses both peripheral and neuronal isoform of tryptophan hydroxylase. Biochimie 140, 159–165. doi: 10.1016/j.biochi.2017.07.008
Lee, C. L., Chiu, P. C., Hautala, L., Salo, T., Yeung, W. S., Stenman, U. H., et al. (2013). Human chorionic gonadotropin and its free beta-subunit stimulate trophoblast invasion independent of LH/hCG receptor. Mol. Cell. Endocrinol. 375, 43–52. doi: 10.1016/j.mce.2013.05.009
Lee, H. J., Caldwell, H. K., Macbeth, A. H., Tolu, S. G., and Young, W. S. III. (2008). A conditional knockout mouse line of the oxytocin receptor. Endocrinology 149, 3256–3263. doi: 10.1210/en.2007-1710
Lee, H. J., Gallego-Ortega, D., Ledger, A., Schramek, D., Joshi, P., Szwarc, M. M., et al. (2013). Progesterone drives mammary secretory differentiation via RankL-mediated induction of Elf5 in luminal progenitor cells. Development 140, 1397–1401. doi: 10.1242/dev.088948
Lee, O. H., Bae, S. K., Bae, M. H., Lee, Y. M., Moon, E. J., Cha, H. J., et al. (2000). Identification of angiogenic properties of insulin-like growth factor II in in vitro angiogenesis models. Br. J. Cancer 82, 385–391. doi: 10.1054/bjoc.1999.0931
Lee, S. J., Talamantes, F., Wilder, E., Linzer, D. I., and Nathans, D. (1988). Trophoblastic giant cells of the mouse placenta as the site of proliferin synthesis. Endocrinology 122, 1761–1768. doi: 10.1210/endo-122-5-1761
Lee, W. S., Lu, Y. C., Kuo, C. T., Chen, C. T., and Tang, P. H. (2017). Effects of female sex hormones on folic acid-induced anti-angiogenesis. Acta Physiol. (Oxf). 222:e13001. doi: 10.1111/apha.13001
Lefebvre, D. L., Giaid, A., and Zingg, H. H. (1992). Expression of the oxytocin gene in rat placenta. Endocrinology 130, 1185–1192.
Lekgabe, E. D., Royce, S. G., Hewitson, T. D., Tang, M. L., Zhao, C., Moore, X. L., et al. (2006). The effects of relaxin and estrogen deficiency on collagen deposition and hypertrophy of nonreproductive organs. Endocrinology 147, 5575–5583. doi: 10.1210/en.2006-0533
Le, T. N., Elsea, S. H., Romero, R., Chaiworapongsa, T., and Francis, G. L. (2013). Prolactin receptor gene polymorphisms are associated with gestational diabetes. Genet. Test. Mol. Biomarkers 17, 567–571. doi: 10.1089/gtmb.2013.0009
Leturque, A., Burnol, A. F., Ferré, P., and Girard, J. (1984). Pregnancy-induced insulin resistance in the rat: assessment by glucose clamp technique. Am. J. Physiol. 246, E25–31. doi: 10.1152/ajpendo.1984.246.1.E25
Levine, A., Zagoory-Sharon, O., Feldman, R., and Weller, A. (2007). Oxytocin during pregnancy and early postpartum: individual patterns and maternal-fetal attachment. Peptides 28, 1162–1169. doi: 10.1016/j.peptides.2007.04.016
Lévy, F. (2016). Neuroendocrine control of maternal behavior in non-human and human mammals. Ann. Endocrinol. (Paris). 77, 114–125. doi: 10.1016/j.ando.2016.04.002
Liao, S., Vickers, M. H., Evans, A., Stanley, J. L., Baker, P. N., and Perry, J. K. (2016a). Comparison of pulsatile vs. continuous administration of human placental growth hormone in female C57BL/6J mice. Endocrine 54, 169–181. doi: 10.1007/s12020-016-1060-0
Liao, S., Vickers, M. H., Stanley, J. L., Ponnampalam, A. P., Baker, P. N., and Perry, J. K. (2016b). The placental variant of human growth hormone reduces maternal insulin sensitivity in a dose-dependent manner in C57BL/6J Mice. Endocrinology 157, 1175–1186. doi: 10.1210/en.2015-1718
Li, J., Umar, S., Amjedi, M., Iorga, A., Sharma, S., Nadadur, R. D., et al. (2012). New frontiers in heart hypertrophy during pregnancy. Am. J. Cardiovasc. Dis. 2, 192–207.
Lim, R., Acharya, R., Delpachitra, P., Hobson, S., Sobey, C. G., Drummond, G. R., et al. (2015). Activin and NADPH-oxidase in preeclampsia: insights from in vitro and murine studies. Am. J. Obstet. Gynecol. 212, 86 e81–86 e12. doi: 10.1016/j.ajog.2014.07.021
Lin, B., Zhu, S., and Shao, B. (1996). Changes of plasma levels of monoamines in normal pregnancy and pregnancy-induced hypertension women and their significance. Zhonghua Fu Chan Ke Za Zhi 31, 670–672.
Linzer, D. I., and Fisher, S. J. (1999). The placenta and the prolactin family of hormones: regulation of the physiology of pregnancy. Mol. Endocrinol. 13, 837–840. doi: 10.1210/mend.13.6.0286
Lissauer, D., Eldershaw, S. A., Inman, C. F., Coomarasamy, A., Moss, P. A., and Kilby, M. D. (2015). Progesterone promotes maternal-fetal tolerance by reducing human maternal T-cell polyfunctionality and inducing a specific cytokine profile. Eur. J. Immunol. 45, 2858–2872. doi: 10.1002/eji.201445404
Liu, D., Wei, N., Man, H. Y., Lu, Y., Zhu, L. Q., and Wang, J. Z. (2015). The MT2 receptor stimulates axonogenesis and enhances synaptic transmission by activating Akt signaling. Cell Death Differ. 22, 583–596. doi: 10.1038/cdd.2014.195
Liu, H., Wu, Y., Qiao, F., and Gong, X. (2009). Effect of leptin on cytotrophoblast proliferation and invasion. J. Huazhong Univ. Sci. Technol. Med. Sci. 29, 631–636. doi: 10.1007/s11596-009-0519-0
Liu, L. X., Rowe, G. C., Yang, S., Li, J., Damilano, F., Chan, M. C., et al. (2017). PDK4 inhibits cardiac pyruvate oxidation in late pregnancy. Circ. Res. 121, 1370–1378. doi: 10.1161/CIRCRESAHA.117.311456
Li, Y., Klausen, C., Cheng, J. C., Zhu, H., and Leung, P. C. (2014). Activin A, B, and AB increase human trophoblast cell invasion by up-regulating N-cadherin. J. Clin. Endocrinol. Metab. 99, E2216–2225. doi: 10.1210/jc.2014-2118
Li, Y., Klausen, C., Zhu, H., and Leung, P. C. (2015). Activin A Increases human trophoblast invasion by inducing SNAIL-mediated MMP2 up-regulation through ALK4. J. Clin. Endocrinol. Metab. 100, E1415–1427. doi: 10.1210/jc.2015-2134
Lodhi, R. S., Nakabayashi, K., Suzuki, K., Yamada, A. Y., Hazama, R., Ebina, Y., et al. (2013). Relaxin has anti-apoptotic effects on human trophoblast-derived HTR-8/SV neo cells. Gynecol. Endocrinol. 29, 1051–1054. doi: 10.3109/09513590.2013.829444
Lomauro, A., and Aliverti, A. (2015). Respiratory physiology of pregnancy: physiology masterclass. Breathe (Sheff) 11, 297–301. doi: 10.1183/20734735.008615
Longo, M., Jain, V., Vedernikov, Y. P., Garfield, R. E., and Saade, G. R. (2003). Effects of recombinant human relaxin on pregnant rat uterine artery and myometrium in vitro . Am. J. Obstet. Gynecol. 188, 1468–1474; discussion 1474-1466. doi: 10.1067/mob.2003.454
Lucas, B. K., Ormandy, C. J., Binart, N., Bridges, R. S., and Kelly, P. A. (1998). Null mutation of the prolactin receptor gene produces a defect in maternal behavior. Endocrinology 139, 4102–4107. doi: 10.1210/endo.139.10.6243
Lu, C. C., Chen, J. J., Tsai, S. C., Chien, E. J., Chien, C. H., and Wang, P. S. (1998). Increase of thyrotropin response to thyrotropin-releasing hormone (TRH) and TRH release in rats during pregnancy. Chin. J. Physiol. 41, 211–216.
Lumbers, E. R., and Pringle, K. G. (2014). Roles of the circulating renin-angiotensin-aldosterone system in human pregnancy. Am. J. Physiol. Regul. Integr. Comp. Physiol. 306, R91–101. doi: 10.1152/ajpregu.00034.2013
Lydon, J. P., Demayo, F. J., Funk, C. R., Mani, S. K., Hughes, A. R., Montgomery, C. A. Jr., et al. (1995). Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 9, 2266–2278. doi: 10.1101/gad.9.18.2266
Maclennan, A. H., and Grant, P. (1991). Human relaxin. In vitro response of human and pig myometrium. J. Reprod. Med. 36, 630–634.
Macrae, D. J., and Palavradji, D. (1967). Maternal acid-base changes in pregnancy. J. Obstet. Gynaecol. Br. Commonw. 74, 11–16. doi: 10.1111/j.1471-0528.1967.tb03925.x
Maeshima, A., Shiozaki, S., Tajima, T., Nakazato, Y., Naruse, T., and Kojima, I. (2000). Number of glomeruli is increased in the kidney of transgenic mice expressing the truncated type II activin receptor. Biochem. Biophys. Res. Commun. 268, 445–449. doi: 10.1006/bbrc.2000.2171
Magariños, M. P., Sánchez-Margalet, V., Kotler, M., Calvo, J. C., and Varone, C. L. (2007). Leptin promotes cell proliferation and survival of trophoblastic cells. Biol. Reprod. 76, 203–210. doi: 10.1095/biolreprod.106.051391
Malik, N. M., Carter, N. D., Murray, J. F., Scaramuzzi, R. J., Wilson, C. A., and Stock, M. J. (2001). Leptin requirement for conception, implantation, and gestation in the mouse. Endocrinology 142, 5198–5202. doi: 10.1210/endo.142.12.8535
Malik, N. M., Carter, N. D., Wilson, C. A., Scaramuzzi, R. J., Stock, M. J., and Murray, J. F. (2005). Leptin expression in the fetus and placenta during mouse pregnancy. Placenta 26, 47–52. doi: 10.1016/j.placenta.2004.03.009
Mao, G., Wang, J., Kang, Y., Tai, P., Wen, J., Zou, Q., et al. (2010). Progesterone increases systemic and local uterine proportions of CD4+CD25+ Treg cells during midterm pregnancy in mice. Endocrinology 151, 5477–5488. doi: 10.1210/en.2010-0426
Maroni, E. S., and De Sousa, M. A. (1973). The lymphoid organs during pregnancy in the mouse. A comparison between a syngeneic and an allogeneic mating. Clin. Exp. Immunol. 13, 107–124.
Marshall, S. A., Leo, C. H., Senadheera, S. N., Girling, J. E., Tare, M., and Parry, L. J. (2016a). Relaxin deficiency attenuates pregnancy-induced adaptation of the mesenteric artery to angiotensin II in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 310, R847–R857. doi: 10.1152/ajpregu.00506.2015
Marshall, S. A., Ng, L., Unemori, E. N., Girling, J. E., and Parry, L. J. (2016b). Relaxin deficiency results in increased expression of angiogenesis- and remodelling-related genes in the uterus of early pregnant mice but does not affect endometrial angiogenesis prior to implantation. Reprod. Biol. Endocrinol. 14:11. doi: 10.1186/s12958-016-0148-y
Maruo, N., Nakabayashi, K., Wakahashi, S., Yata, A., and Maruo, T. (2007). Effects of recombinant H2 relaxin on the expression of matrix metalloproteinases and tissue inhibitor metalloproteinase in cultured early placental extravillous trophoblasts. Endocrine 32, 303–310. doi: 10.1007/s12020-008-9034-5
Mason, G. A., Caldwell, J. D., Stanley, D. A., Hatley, O. L., Prange, A. J. Jr., and Pedersen, C. A. (1986). Interactive effects of intracisternal oxytocin and other centrally active substances on colonic temperatures of mice. Regul. Pept. 14, 253–260. doi: 10.1016/0167-0115(86)90008-X
Masuzaki, H., Ogawa, Y., Sagawa, N., Hosoda, K., Matsumoto, T., Mise, H., et al. (1997). Nonadipose tissue production of leptin: leptin as a novel placenta-derived hormone in humans. Nat. Med. 3, 1029–1033. doi: 10.1038/nm0997-1029
Matjila, M., Millar, R., Van Der Spuy, Z., and Katz, A. (2016). Elevated placental expression at the maternal-fetal interface but diminished maternal circulatory kisspeptin in preeclamptic pregnancies. Pregnancy Hypertens. 6, 79–87. doi: 10.1016/j.preghy.2015.11.001
Mayerl, S., Liebsch, C., Visser, T. J., and Heuer, H. (2015). Absence of TRH receptor 1 in male mice affects gastric ghrelin production. Endocrinology 156, 755–767. doi: 10.1210/en.2014-1395
Mazella, J., Tang, M., and Tseng, L. (2004). Disparate effects of relaxin and TGFbeta1: relaxin increases, but TGFbeta1 inhibits, the relaxin receptor and the production of IGFBP-1 in human endometrial stromal/decidual cells. Hum. Reprod. 19, 1513–1518. doi: 10.1093/humrep/deh274
Mcilvride, S., Mushtaq, A., Papacleovoulou, G., Hurling, C., Steel, J., Jansen, E., et al. (2017). A progesterone-brown fat axis is involved in regulating fetal growth. Sci. Rep. 7:10671. doi: 10.1038/s41598-017-10979-7
Mead, E. J., Maguire, J. J., Kuc, R. E., and Davenport, A. P. (2007). Kisspeptins: a multifunctional peptide system with a role in reproduction, cancer and the cardiovascular system. Br. J. Pharmacol. 151, 1143–1153. doi: 10.1038/sj.bjp.0707295
Meziani, F., Van Overloop, B., Schneider, F., and Gairard, A. (2005). Parathyroid hormone-related protein-induced relaxation of rat uterine arteries: influence of the endothelium during gestation. J. Soc. Gynecol. Investig. 12, 14–19. doi: 10.1016/j.jsgi.2004.07.005
Miedlar, J. A., Rinaman, L., Vollmer, R. R., and Amico, J. A. (2007). Oxytocin gene deletion mice overconsume palatable sucrose solution but not palatable lipid emulsions. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293, R1063–R1068. doi: 10.1152/ajpregu.00228.2007
Mikaelsson, M. A., Constância, M., Dent, C. L., Wilkinson, L. S., and Humby, T. (2013). Placental programming of anxiety in adulthood revealed by Igf2-null models. Nat. Commun. 4:2311. doi: 10.1038/ncomms3311
Milczarek, R., Hallmann, A., Sokołowska, E., Kaletha, K., and Klimek, J. (2010). Melatonin enhances antioxidant action of alpha-tocopherol and ascorbate against NADPH- and iron-dependent lipid peroxidation in human placental mitochondria. J. Pineal Res. 49, 149–155. doi: 10.1111/j.1600-079X.2010.00779.x
Miller, S. L., Yawno, T., Alers, N. O., Castillo-Melendez, M., Supramaniam, V. G., Vanzyl, N., et al. (2014). Antenatal antioxidant treatment with melatonin to decrease newborn neurodevelopmental deficits and brain injury caused by fetal growth restriction. J. Pineal Res. 56, 283–294. doi: 10.1111/jpi.12121
Mirabito Colafella, K. M., Samuel, C. S., and Denton, K. M. (2017). Relaxin contributes to the regulation of arterial pressure in adult female mice. Clin. Sci. 131, 2795–2805. doi: 10.1042/CS20171225
Miranda, A., and Sousa, N. (2018). Maternal hormonal milieu influence on fetal brain development. Brain Behav. 8:e00920. doi: 10.1002/brb3.920
Mitchell, J. A., Hammer, R. E., and Goldman, H. (1983). Serotonin-induced disruption of implantation in the rat: II. Suppression of decidualization. Biol. Reprod 29, 151–156. doi: 10.1095/biolreprod29.1.151
Modi, H., Jacovetti, C., Tarussio, D., Metref, S., Madsen, O. D., Zhang, F. P., et al. (2015). Autocrine action of IGF2 regulates adult beta-cell mass and function. Diabetes 64, 4148–4157. doi: 10.2337/db14-1735
Mohammadi-Sartang, M., Ghorbani, M., and Mazloom, Z. (2017). Effects of melatonin supplementation on blood lipid concentrations: a systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. doi: 10.1016/j.clnu.2017.11.003. [Epub ahead of print].
Mor, G., and Cardenas, I. (2010). The immune system in pregnancy: a unique complexity. Am J Reprod Immunol 63, 425–433. doi: 10.1111/j.1600-0897.2010.00836.x
Morrissy, S., Xu, B., Aguilar, D., Zhang, J., and Chen, Q. M. (2010). Inhibition of apoptosis by progesterone in cardiomyocytes. Aging Cell 9, 799–809. doi: 10.1111/j.1474-9726.2010.00619.x
Mounzih, K., Qiu, J., Ewart-Toland, A., and Chehab, F. F. (1998). Leptin is not necessary for gestation and parturition but regulates maternal nutrition via a leptin resistance state. Endocrinology 139, 5259–5262. doi: 10.1210/endo.139.12.6523
Moya, F., Mena, P., Heusser, F., Foradori, A., Paiva, E., Yazigi, R., et al. (1986). Response of the maternal, fetal, and neonatal pituitary-thyroid axis to thyrotropin-releasing hormone. Pediatr. Res. 20, 982–986. doi: 10.1203/00006450-198610000-00018
Mühlbauer, E., Albrecht, E., Bazwinsky-Wutschke, I., and Peschke, E. (2012). Melatonin influences insulin secretion primarily via MT(1) receptors in rat insulinoma cells (INS-1) and mouse pancreatic islets. J. Pineal Res. 52, 446–459. doi: 10.1111/j.1600-079X.2012.00959.x
Mühlbauer, E., Gross, E., Labucay, K., Wolgast, S., and Peschke, E. (2009). Loss of melatonin signalling and its impact on circadian rhythms in mouse organs regulating blood glucose. Eur. J. Pharmacol. 606, 61–71. doi: 10.1016/j.ejphar.2009.01.029
Müller, H., Liu, B., Croy, B. A., Head, J. R., Hunt, J. S., Dai, G., et al. (1999). Uterine natural killer cells are targets for a trophoblast cell-specific cytokine, prolactin-like protein A. Endocrinology 140, 2711–2720. doi: 10.1210/endo.140.6.6828
Munnell, E. W., and Taylor, H. C. (1947). Liver Blood Flow in Pregnancy-Hepatic Vein Catheterization. J. Clin. Invest. 26, 952–956. doi: 10.1172/JCI101890
Musial, B., Fernandez-Twinn, D. S., Vaughan, O. R., Ozanne, S. E., Voshol, P., Sferruzzi-Perri, A. N., et al. (2016). Proximity to Delivery Alters Insulin Sensitivity and Glucose Metabolism in Pregnant Mice. Diabetes 65, 851–860. doi: 10.2337/db15-1531
Musial, B., Vaughan, O. R., Fernandez-Twinn, D. S., Voshol, P., Ozanne, S. E., Fowden, A. L., et al. (2017). A Western-style obesogenic diet alters maternal metabolic physiology with consequences for fetal nutrient acquisition in mice. J. Physiol. (Lond). 595, 4875–4892. doi: 10.1113/JP273684
Muttukrishna, S., Child, T. J., Groome, N. P., and Ledger, W. L. (1997). Source of circulating levels of inhibin A, pro alpha C-containing inhibins and activin A in early pregnancy. Hum. Reprod. 12, 1089–1093. doi: 10.1093/humrep/12.5.1089
Nakamura, Y., Tamura, H., Kashida, S., Takayama, H., Yamagata, Y., Karube, A., et al. (2001). Changes of serum melatonin level and its relationship to feto-placental unit during pregnancy. J. Pineal Res. 30, 29–33. doi: 10.1034/j.1600-079X.2001.300104.x
Nanetti, L., Raffaelli, F., Giulietti, A., Sforza, G., Raffaele Giannubilo, S., Ciavattini, A., et al. (2015). Oxytocin, its antagonist Atosiban, and preterm labor: a role for placental nitric oxide. J. Matern. Fetal Neonatal Med. 28, 611–616. doi: 10.3109/14767058.2014.927859
Nathanielsz, P. W., Jenkins, S. L., Tame, J. D., Winter, J. A., Guller, S., and Giussani, D. A. (1998). Local paracrine effects of estradiol are central to parturition in the rhesus monkey. Nat. Med. 4, 456–459. doi: 10.1038/nm0498-456
Neville, M. C., Mcfadden, T. B., and Forsyth, I. (2002). Hormonal regulation of mammary differentiation and milk secretion. J. Mammary Gland Biol. Neoplasia 7, 49–66. doi: 10.1023/A:1015770423167
Nielsen, J. H. (1982). Effects of growth hormone, prolactin, and placental lactogen on insulin content and release, and deoxyribonucleic acid synthesis in cultured pancreatic islets. Endocrinology 110, 600–606. doi: 10.1210/endo-110-2-600
Nien, J. K., Mazaki-Tovi, S., Romero, R., Erez, O., Kusanovic, J. P., Gotsch, F., et al. (2007). Plasma adiponectin concentrations in non-pregnant, normal and overweight pregnant women. J. Perinat. Med. 35, 522–531. doi: 10.1515/JPM.2007.123
Nir, I., and Hirschmann, N. (1980). Melatonin-induced changes in blood and pituitary luteinizing hormone and prolactin levels during the perinatal period in rat dams. J Neural Transm 49, 219–228. doi: 10.1007/BF01252127
Nishimori, K., Young, L. J., Guo, Q., Wang, Z., Insel, T. R., and Matzuk, M. M. (1996). Oxytocin is required for nursing but is not essential for parturition or reproductive behavior. Proc. Natl. Acad. Sci. U.S.A. 93, 11699–11704. doi: 10.1073/pnas.93.21.11699
Ni, X., Luo, S., Minegishi, T., and Peng, C. (2000). Activin A in JEG-3 cells: potential role as an autocrine regulator of steroidogenesis in humans. Biol. Reprod. 62, 1224–1230. doi: 10.1095/biolreprod62.5.1224
Norton, M. T., Fortner, K. A., Bizargity, P., and Bonney, E. A. (2009). Pregnancy alters the proliferation and apoptosis of mouse splenic erythroid lineage cells and leukocytes. Biol. Reprod. 81, 457–464. doi: 10.1095/biolreprod.109.076976
Norwitz, E. R., and Caughey, A. B. (2011). Progesterone supplementation and the prevention of preterm birth. Rev. Obstet. Gynecol. 4, 60–72.
Obr, A. E., Grimm, S. L., Bishop, K. A., Pike, J. W., Lydon, J. P., and Edwards, D. P. (2013). Progesterone receptor and Stat5 signaling cross talk through RANKL in mammary epithelial cells. Mol. Endocrinol. 27, 1808–1824. doi: 10.1210/me.2013-1077
O'byrne, E. M., Sawyer, W. K., Butler, M. C., and Steinetz, B. G. (1976). Serum immunoreactive relaxin and softening of the uterine cervix in pregnant hamsters. Endocrinology 99, 1333–1335. doi: 10.1210/endo-99-5-1333
O'byrne, E. M., and Steinetz, B. G. (1976). Radioimmunoassay (RIA) of relaxin in sera of various species using an antiserum to porcine relaxin. Proc. Soc. Exp. Biol. Med. 152, 272–276. doi: 10.3181/00379727-152-39377
Ogawa, Y., Masuzaki, H., Hosoda, K., Aizawa-Abe, M., Suga, J., Suda, M., et al. (1999). Increased glucose metabolism and insulin sensitivity in transgenic skinny mice overexpressing leptin. Diabetes 48, 1822–1829. doi: 10.2337/diabetes.48.9.1822
Ogueh, O., Clough, A., Hancock, M., and Johnson, M. R. (2011). A longitudinal study of the control of renal and uterine hemodynamic changes of pregnancy. Hypertens. Pregnancy 30, 243–259. doi: 10.3109/10641955.2010.484079
Ohara-Imaizumi, M., Kim, H., Yoshida, M., Fujiwara, T., Aoyagi, K., Toyofuku, Y., et al. (2013). Serotonin regulates glucose-stimulated insulin secretion from pancreatic beta cells during pregnancy. Proc. Natl. Acad. Sci. U.S.A. 110, 19420–19425. doi: 10.1073/pnas.1310953110
Okatani, Y., Wakatsuki, A., Shinohara, K., Taniguchi, K., and Fukaya, T. (2001). Melatonin protects against oxidative mitochondrial damage induced in rat placenta by ischemia and reperfusion. J. Pineal Res. 31, 173–178. doi: 10.1034/j.1600-079x.2001.310212.x
O'neal-Moffitt, G., Pilli, J., Kumar, S. S., and Olcese, J. (2014). Genetic deletion of MT(1)/MT(2) melatonin receptors enhances murine cognitive and motor performance. Neuroscience 277, 506–521. doi: 10.1016/j.neuroscience.2014.07.018
O'sullivan, K. P., Marshall, S. A., Cullen, S., Saunders, T., Hannan, N. J., Senadheera, S. N., et al. (2017). Evidence of proteinuria, but no other characteristics of pre-eclampsia, in relaxin-deficient mice. Reprod. Fertil. Dev. 29, 1477–1485. doi: 10.1071/RD16056
Owino, S., Contreras-Alcantara, S., Baba, K., and Tosini, G. (2016). Melatonin signaling controls the daily rhythm in blood glucose levels independent of peripheral clocks. PLoS ONE 11:e0148214. doi: 10.1371/journal.pone.0148214
Palejwala, S., Stein, D. E., Weiss, G., Monia, B. P., Tortoriello, D., and Goldsmith, L. T. (2001). Relaxin positively regulates matrix metalloproteinase expression in human lower uterine segment fibroblasts using a tyrosine kinase signaling pathway. Endocrinology 142, 3405–3413. doi: 10.1210/endo.142.8.8295
Paller, M. S., Gregorini, G., and Ferris, T. F. (1989). Pressor responsiveness in pseudopregnant and pregnant rats: role of maternal factors. Am. J. Physiol. 257, R866–R871. doi: 10.1152/ajpregu.1989.257.4.R866
Pang, W. W., and Hartmann, P. E. (2007). Initiation of human lactation: secretory differentiation and secretory activation. J. Mammary Gland Biol. Neoplasia 12, 211–221. doi: 10.1007/s10911-007-9054-4
Pecins-Thompson, M., and Keller-Wood, M. (1997). Effects of progesterone on blood pressure, plasma volume, and responses to hypotension. Am. J. Physiol. 272, R377–385. doi: 10.1152/ajpregu.1997.272.1.R377
Pedersen, C. A., Vadlamudi, S. V., Boccia, M. L., and Amico, J. A. (2006). Maternal behavior deficits in nulliparous oxytocin knockout mice. Genes Brain Behav. 5, 274–281. doi: 10.1111/j.1601-183X.2005.00162.x
Pelletier, G., Petitclerc, D., Lapierre, H., Bernier-Cardou, M., Morisset, J., Gaudreau, P., et al. (1987). Injection of synthetic human growth hormone-releasing factors in dairy cows. 1. Effect on feed intake and milk yield and composition. J. Dairy Sci. 70, 2511–2517. doi: 10.3168/jds.S0022-0302(87)80319-3
Pelleymounter, M. A., Cullen, M. J., Baker, M. B., Hecht, R., Winters, D., Boone, T., et al. (1995). Effects of the obese gene product on body weight regulation in ob/ob mice. Science 269, 540–543. doi: 10.1126/science.7624776
Peng, J., Fullerton, P. T. Jr., Monsivais, D., Clementi, C., Su, G. H., and Matzuk, M. M. (2015). Uterine activin-like kinase 4 regulates trophoblast development during mouse placentation. Mol. Endocrinol. 29, 1684–1693. doi: 10.1210/me.2015-1048
Petersson, M., Alster, P., Lundeberg, T., and Uvnäs-Moberg, K. (1996). Oxytocin causes a long-term decrease of blood pressure in female and male rats. Physiol. Behav. 60, 1311–1315. doi: 10.1016/S0031-9384(96)00261-2
Petry, C. J., Evans, M. L., Wingate, D. L., Ong, K. K., Reik, W., Constância, M., et al. (2010). Raised late pregnancy glucose concentrations in mice carrying pups with targeted disruption of H19delta13. Diabetes 59, 282–286. doi: 10.2337/db09-0757
Petry, C. J., Ong, K. K., and Dunger, D. B. (2007). Does the fetal genotype affect maternal physiology during pregnancy? Trends Mol. Med. 13, 414–421. doi: 10.1016/j.molmed.2007.07.007
Pieper, P. G. (2015). Use of medication for cardiovascular disease during pregnancy. Nat. Rev. Cardiol. 12, 718–729. doi: 10.1038/nrcardio.2015.172
Pitera, A. E., Smith, G. C., Wentworth, R. A., and Nathanielsz, P. W. (1998). Parathyroid hormone-related peptide (1 to 34) inhibits in vitro oxytocin-stimulated activity of pregnant baboon myometrium. Am. J. Obstet. Gynecol. 179, 492–496. doi: 10.1016/S0002-9378(98)70385-0
Plaut, K., Maple, R., Ginsburg, E., and Vonderhaar, B. (1999). Progesterone stimulates DNA synthesis and lobulo-alveolar development in mammary glands in ovariectomized mice. J. Cell Physiol. 180, 298–304. doi: 10.1002/(SICI)1097-4652(199908)180:2<298::AID-JCP17>3.0.CO;2-V
Porter, S. E., Sorenson, R. L., Dann, P., Garcia-Ocana, A., Stewart, A. F., and Vasavada, R. C. (1998). Progressive pancreatic islet hyperplasia in the islet-targeted, parathyroid hormone-related protein-overexpressing mouse. Endocrinology 139, 3743–3751. doi: 10.1210/endo.139.9.6212
Poulson, E., Botros, M., and Robson, J. M. (1960). Effect of 5-hydroxytryptamine and iproniazid on pregnancy. Science 131, 1101–1102. doi: 10.1126/science.131.3407.1101
Prast, J., Saleh, L., Husslein, H., Sonderegger, S., Helmer, H., and Knöfler, M. (2008). Human chorionic gonadotropin stimulates trophoblast invasion through extracellularly regulated kinase and AKT signaling. Endocrinology 149, 979–987. doi: 10.1210/en.2007-1282
Prezotto, L. D., Lemley, C. O., Camacho, L. E., Doscher, F. E., Meyer, A. M., Caton, J. S., et al. (2014). Effects of nutrient restriction and melatonin supplementation on maternal and foetal hepatic and small intestinal energy utilization. J. Anim. Physiol. Anim. Nutr. (Berl). 98, 797–807. doi: 10.1111/jpn.12142
Prigent-Tessier, A., Pageaux, J. F., Fayard, J. M., Lagarde, M., Laugier, C., and Cohen, H. (1996). Prolactin up-regulates prostaglandin E2 production through increased expression of pancreatic-type phospholipase A2 (type I) and prostaglandin G/H synthase 2 in uterine cells. Mol. Cell. Endocrinol. 122, 101–108. doi: 10.1016/0303-7207(96)03888-9
Qi, X., Gong, B., Yu, J., Shen, L., Jin, W., Wu, Z., et al. (2017). Decreased cord blood estradiol levels in related to mothers with gestational diabetes. Medicine (Baltimore). 96:e6962. doi: 10.1097/MD.0000000000006962
Quagliarello, J., Szlachter, N., Steinetz, B. G., Goldsmith, L. T., and Weiss, G. (1979). Serial relaxin concentrations in human pregnancy. Am. J. Obstet. Gynecol. 135, 43–44.
Qu, J., and Thomas, K. (1993). Regulation of inhibin secretion in human placental cell culture by epidermal growth factor, transforming growth factors, and activin. J. Clin. Endocrinol. Metab. 77, 925–931.
Rabeler, R., Mittag, J., Geffers, L., Rüther, U., Leitges, M., Parlow, A. F., et al. (2004). Generation of thyrotropin-releasing hormone receptor 1-deficient mice as an animal model of central hypothyroidism. Mol. Endocrinol. 18, 1450–1460. doi: 10.1210/me.2004-0017
Racicot, K., Kwon, J. Y., Aldo, P., Silasi, M., and Mor, G. (2014). Understanding the complexity of the immune system during pregnancy. Am J Reprod Immunol 72, 107–116. doi: 10.1111/aji.12289
Randle, P. J. (1998). Regulatory interactions between lipids and carbohydrates: the glucose fatty acid cycle after 35 years. Diabetes Metab. Rev. 14, 263–283. doi: 10.1002/(sici)1099-0895(199812)14:4<263::aid-dmr233>3.0.co;2-c
Rawn, S. M., Huang, C., Hughes, M., Shaykhutdinov, R., Vogel, H. J., and Cross, J. C. (2015). Pregnancy hyperglycemia in prolactin receptor mutant, but not prolactin mutant, mice and feeding-responsive regulation of placental lactogen genes implies placental control of maternal glucose homeostasis. Biol. Reprod. 93:75. doi: 10.1095/biolreprod.115.132431
Renegar, R. H., and Owens, C. R. III. (2002). Measurement of plasma and tissue relaxin concentrations in the pregnant hamster and fetus using a homologous radioimmunoassay. Biol. Reprod. 67, 500–505. doi: 10.1095/biolreprod67.2.500
Rezaei, R., Wu, Z., Hou, Y., Bazer, F. W., and Wu, G. (2016). Amino acids and mammary gland development: nutritional implications for milk production and neonatal growth. J. Anim. Sci. Biotechnol. 7:20. doi: 10.1186/s40104-016-0078-8
Ribas, V., Drew, B. G., Le, J. A., Soleymani, T., Daraei, P., Sitz, D., et al. (2011). Myeloid-specific estrogen receptor alpha deficiency impairs metabolic homeostasis and accelerates atherosclerotic lesion development. Proc. Natl. Acad. Sci. U.S.A. 108, 16457–16462. doi: 10.1073/pnas.1104533108
Ribeiro, A. C., Musatov, S., Shteyler, A., Simanduyev, S., Arrieta-Cruz, I., Ogawa, S., et al. (2012). siRNA silencing of estrogen receptor-alpha expression specifically in medial preoptic area neurons abolishes maternal care in female mice. Proc. Natl. Acad. Sci. U.S.A. 109, 16324–16329. doi: 10.1073/pnas.1214094109
Rieck, S., and Kaestner, K. H. (2010). Expansion of beta-cell mass in response to pregnancy. Trends Endocrinol. Metab. 21, 151–158. doi: 10.1016/j.tem.2009.11.001
Robinson, D. P., and Klein, S. L. (2012). Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm. Behav. 62, 263–271. doi: 10.1016/j.yhbeh.2012.02.023
Robson, J. M., and Sullivan, F. M. (1966). Analysis of actions of 5-hydroxytryptamine in pregnancy. J. Physiol. (Lond). 184, 717–732. doi: 10.1113/jphysiol.1966.sp007943
Rodger, M., Sheppard, D., Gándara, E., and Tinmouth, A. (2015). Haematological problems in obstetrics. Best Pract. Res. Clin. Obstet. Gynaecol. 29, 671–684. doi: 10.1016/j.bpobgyn.2015.02.004
Romero, M., Ortega, A., Izquierdo, A., López-Luna, P., and Bosch, R. J. (2010). Parathyroid hormone-related protein induces hypertrophy in podocytes via TGF-beta(1) and p27(Kip1): implications for diabetic nephropathy. Nephrol. Dial. Transplant 25, 2447–2457. doi: 10.1093/ndt/gfq104
Roos, A., Robertson, F., Lochner, C., Vythilingum, B., and Stein, D. J. (2011). Altered prefrontal cortical function during processing of fear-relevant stimuli in pregnancy. Behav. Brain Res. 222, 200–205. doi: 10.1016/j.bbr.2011.03.055
Roti, E., Gnudi, A., Braverman, L. E., Robuschi, G., Emanuele, R., Bandini, P., et al. (1981). Human cord blood concentrations of thyrotropin, thyroglobulin, and iodothyronines after maternal administration of thyrotropin-releasing hormone. J. Clin. Endocrinol. Metab. 53, 813–817. doi: 10.1210/jcem-53-4-813
Rozenblit-Susan, S., Chapnik, N., and Froy, O. (2017). Serotonin prevents differentiation into brown adipocytes and induces transdifferentiation into white adipocytes. Int. J. Obes (Lond). 42, 704–710. doi: 10.1038/ijo.2017.261
Ryan, E. A., O'sullivan, M. J., and Skyler, J. S. (1985). Insulin action during pregnancy. Studies with the euglycemic clamp technique. Diabetes 34, 380–389. doi: 10.2337/diab.34.4.380
Rybakowski, C., Niemax, K., Goepel, E., and Schröder, H. J. (2000). The effect of oxytocin, prostaglandin E2 and acetylsalicylic acid on flow distribution and on the transfer of alanine, glucose and water in isolated perfused guinea pig placentae. Placenta 21, 126–131. doi: 10.1053/plac.1999.0459
Rygaard, K., Revol, A., Esquivel-Escobedo, D., Beck, B. L., and Barrera-Saldana, H. A. (1998). Absence of human placental lactogen and placental growth hormone (HGH-V) during pregnancy: PCR analysis of the deletion. Hum. Genet. 102, 87–92. doi: 10.1007/s004390050658
Sagawa, N., Yura, S., Itoh, H., Mise, H., Kakui, K., Korita, D., et al. (2002). Role of leptin in pregnancy–a review. Placenta 23(Suppl. A), S80–S86. doi: 10.1053/plac.2002.0814
Sairenji, T. J., Ikezawa, J., Kaneko, R., Masuda, S., Uchida, K., Takanashi, Y., et al. (2017). Maternal prolactin during late pregnancy is important in generating nurturing behavior in the offspring. Proc. Natl. Acad. Sci. U.S.A. 114, 13042–13047. doi: 10.1073/pnas.1621196114
Saito, S., Nakashima, A., Shima, T., and Ito, M. (2010). Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am. J. Reprod Immunol. 63, 601–610. doi: 10.1111/j.1600-0897.2010.00852.x
Salles, J. P. (2016). Bone metabolism during pregnancy. Ann. Endocrinol. (Paris). 77, 163–168. doi: 10.1016/j.ando.2016.04.004
Samuel, C. S., Zhao, C., Bathgate, R. A., Bond, C. P., Burton, M. D., Parry, L. J., et al. (2003). Relaxin deficiency in mice is associated with an age-related progression of pulmonary fibrosis. FASEB J. 17, 121–123. doi: 10.1096/fj.02-0449fje
Sandoval-Guzmán, T., Gongrich, C., Moliner, A., Guo, T., Wu, H., Broberger, C., et al. (2012). Neuroendocrine control of female reproductive function by the activin receptor ALK7. FASEB J. 26, 4966–4976. doi: 10.1096/fj.11-199059
Sasaki, K., Matsumura, G., and Ito, T. (1981). Effects of pregnancy on erythropoiesis in the splenic red pulp of the mouse: a quantitative electron microscopic study. Arch. Histol. Jpn. 44, 429–438. doi: 10.1679/aohc1950.44.429
Sasaki, Y., Morimoto, T., Saito, H., Suzuki, M., Ichizuka, K., and Yanaihara, T. (2000). The role of parathyroid hormone-related protein in intra-tracheal fluid. Endocr. J. 47, 169–175. doi: 10.1507/endocrj.47.169
Scarpace, P. J., Matheny, M., Pollock, B. H., and Tümer, N. (1997). Leptin increases uncoupling protein expression and energy expenditure. Am. J. Physiol. 273, E226–230. doi: 10.1152/ajpendo.1997.273.1.E226
Schanton, M., Maymó, J. L., Pérez-Pérez, A., Sánchez-Margalet, V., and Varone, C. L. (2018). Involvement of leptin in the molecular physiology of the placenta. Reproduction 155, R1–R12. doi: 10.1530/REP-17-0512
Schipani, E., Lanske, B., Hunzelman, J., Luz, A., Kovacs, C. S., Lee, K., et al. (1997). Targeted expression of constitutively active receptors for parathyroid hormone and parathyroid hormone-related peptide delays endochondral bone formation and rescues mice that lack parathyroid hormone-related peptide. Proc. Natl. Acad. Sci. U.S.A. 94, 13689–13694. doi: 10.1073/pnas.94.25.13689
Schulz, L. C., and Widmaier, E. P. (2004). The effect of leptin on mouse trophoblast cell invasion. Biol. Reprod. 71, 1963–1967. doi: 10.1095/biolreprod.104.032722
Schumacher, A., Heinze, K., Witte, J., Poloski, E., Linzke, N., Woidacki, K., et al. (2013). Human chorionic gonadotropin as a central regulator of pregnancy immune tolerance. J. Immunol. 190, 2650–2658. doi: 10.4049/jimmunol.1202698
Sclafani, A., Rinaman, L., Vollmer, R. R., and Amico, J. A. (2007). Oxytocin knockout mice demonstrate enhanced intake of sweet and nonsweet carbohydrate solutions. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292, R1828–1833. doi: 10.1152/ajpregu.00826.2006
Scott, P. R., Sargison, N. D., Macrae, A. I., and Gough, M. R. (2009). Melatonin treatment prior to the normal breeding season increases fetal number in United Kingdom sheep flocks. Vet. J. 182, 198–202. doi: 10.1016/j.tvjl.2008.07.010
Seki, K., Uesato, T., Tabei, T., and Kato, K. (1985). The secretory patterns of relaxin and human chorionic gonadotropin in human pregnancy. Endocrinol. Jpn. 32, 741–744. doi: 10.1507/endocrj1954.32.741
Seufert, J., Kieffer, T. J., Leech, C. A., Holz, G. G., Moritz, W., Ricordi, C., et al. (1999). Leptin suppression of insulin secretion and gene expression in human pancreatic islets: implications for the development of adipogenic diabetes mellitus. J. Clin. Endocrinol. Metab. 84, 670–676. doi: 10.1210/jc.84.2.670
Sferruzzi-Perri, A. N., Owens, J. A., Pringle, K. G., Robinson, J. S., and Roberts, C. T. (2006). Maternal insulin-like growth factors-I and -II act via different pathways to promote fetal growth. Endocrinology 147, 3344–3355. doi: 10.1210/en.2005-1328
Sferruzzi-Perri, A. N., Owens, J. A., Standen, P., Taylor, R. L., Heinemann, G. K., Robinson, J. S., et al. (2007). Early treatment of the pregnant guinea pig with IGFs promotes placental transport and nutrient partitioning near term. Am. J. Physiol. Endocrinol. Metab. 292, E668–676. doi: 10.1152/ajpendo.00320.2006
Sferruzzi-Perri, A. N., Vaughan, O. R., Coan, P. M., Suciu, M. C., Darbyshire, R., Constancia, M., et al. (2011). Placental-specific Igf2 deficiency alters developmental adaptations to undernutrition in mice. Endocrinology 152, 3202–3212. doi: 10.1210/en.2011-0240
Shahtaheri, S. M., Aaron, J. E., Johnson, D. R., and Purdie, D. W. (1999). Changes in trabecular bone architecture in women during pregnancy. Br. J. Obstet. Gynaecol. 106, 432–438. doi: 10.1111/j.1471-0528.1999.tb08296.x
Shakhmatova, E. I., Osipova, N. A., and Natochin, Y. V. (2000). Changes in osmolality and blood serum ion concentrations in pregnancy. Hum. Physiol. 26, 92–95. doi: 10.1007/BF02760724
Sharkey, J. T., Cable, C., and Olcese, J. (2010). Melatonin sensitizes human myometrial cells to oxytocin in a protein kinase C alpha/extracellular-signal regulated kinase-dependent manner. J. Clin. Endocrinol. Metab. 95, 2902–2908. doi: 10.1210/jc.2009-2137
Sharkey, J. T., Puttaramu, R., Word, R. A., and Olcese, J. (2009). Melatonin synergizes with oxytocin to enhance contractility of human myometrial smooth muscle cells. J. Clin. Endocrinol. Metab. 94, 421–427. doi: 10.1210/jc.2008-1723
Shaw, L., Taggart, M., and Austin, C. (2001). Effects of the oestrous cycle and gender on acute vasodilatory responses of isolated pressurized rat mesenteric arteries to 17 beta-oestradiol. Br. J. Pharmacol. 132, 1055–1062. doi: 10.1038/sj.bjp.0703908
Shek, E. W., Brands, M. W., and Hall, J. E. (1998). Chronic leptin infusion increases arterial pressure. Hypertension 31, 409–414. doi: 10.1161/01.HYP.31.1.409
Shingo, T., Gregg, C., Enwere, E., Fujikawa, H., Hassam, R., Geary, C., et al. (2003). Pregnancy-stimulated neurogenesis in the adult female forebrain mediated by prolactin. Science 299, 117–120. doi: 10.1126/science.1076647
Shi, Q. J., Lei, Z. M., Rao, C. V., and Lin, J. (1993). Novel role of human chorionic gonadotropin in differentiation of human cytotrophoblasts. Endocrinology 132, 1387–1395. doi: 10.1210/endo.132.3.7679981
Sierra-Honigmann, M. R., Nath, A. K., Murakami, C., García-Cardeña G, G., Papapetropoulos, A., Sessa, W. C., et al. (1998). Biological action of leptin as an angiogenic factor. Science 281, 1683–1686. doi: 10.1126/science.281.5383.1683
Simmons, D. G., Rawn, S., Davies, A., Hughes, M., and Cross, J. C. (2008). Spatial and temporal expression of the 23 murine Prolactin/Placental Lactogen-related genes is not associated with their position in the locus. BMC Genomics 9:352. doi: 10.1186/1471-2164-9-352
Simoncini, T., Mannella, P., Fornari, L., Caruso, A., Willis, M. Y., Garibaldi, S., et al. (2004). Differential signal transduction of progesterone and medroxyprogesterone acetate in human endothelial cells. Endocrinology 145, 5745–5756. doi: 10.1210/en.2004-0510
Singh, H. J., Saleh, H. I., Gupalo, S., and Omar, E. (2013). Effect of melatonin supplementation on pregnancy outcome in Wistar-Kyoto and Sprague-Dawley rats. Sheng Li Xue Bao 65, 149–157.
Slattery, M. M., O'leary, M. J., and Morrison, J. J. (2001). Effect of parathyroid hormone-related peptide on human and rat myometrial contractility in vitro . Am. J. Obstet. Gynecol. 184, 625–629. doi: 10.1067/mob.2001.110695
Soares, M. J. (2004). The prolactin and growth hormone families: pregnancy-specific hormones/cytokines at the maternal-fetal interface. Reprod. Biol. Endocrinol. 2:51. doi: 10.1186/1477-7827-2-51
Soares, M. J., Konno, T., and Alam, S. M. (2007). The prolactin family: effectors of pregnancy-dependent adaptations. Trends Endocrinol. Metab. 18, 114–121. doi: 10.1016/j.tem.2007.02.005
Soliman, A., Lacasse, A. A., Lanoix, D., Sagrillo-Fagundes, L., Boulard, V., and Vaillancourt, C. (2015). Placental melatonin system is present throughout pregnancy and regulates villous trophoblast differentiation. J. Pineal Res. 59, 38–46. doi: 10.1111/jpi.12236
Soloff, M. S., Jeng, Y. J., Izban, M. G., Sinha, M., Luxon, B. A., Stamnes, S. J., et al. (2011). Effects of progesterone treatment on expression of genes involved in uterine quiescence. Reprod. Sci. 18, 781–797. doi: 10.1177/1933719111398150
Soma-Pillay, P., Nelson-Piercy, C., Tolppanen, H., and Mebazaa, A. (2016). Physiological changes in pregnancy. Cardiovasc. J. Afr. 27, 89–94. doi: 10.5830/CVJA-2016-021
Song, G. J., Fiaschi-Taesch, N., and Bisello, A. (2009). Endogenous parathyroid hormone-related protein regulates the expression of PTH type 1 receptor and proliferation of vascular smooth muscle cells. Mol. Endocrinol. 23, 1681–1690. doi: 10.1210/me.2009-0098
Song, W. J., Mondal, P., Wolfe, A., Alonso, L. C., Stamateris, R., Ong, B. W., et al. (2014). Glucagon regulates hepatic kisspeptin to impair insulin secretion. Cell Metab. 19, 667–681. doi: 10.1016/j.cmet.2014.03.005
Song, Y., Keelan, J., and France, J. T. (1996). Activin-A stimulates, while transforming growth factor beta 1 inhibits, chorionic gonadotrophin production and aromatase activity in cultured human placental trophoblasts. Placenta 17, 603–610. doi: 10.1016/S0143-4004(96)80078-6
Sonier, B., Lavigne, C., Arseneault, M., Ouellette, R., and Vaillancourt, C. (2005). Expression of the 5-HT2A serotoninergic receptor in human placenta and choriocarcinoma cells: mitogenic implications of serotonin. Placenta 26, 484–490. doi: 10.1016/j.placenta.2004.08.003
Sorenson, R. L., and Brelje, T. C. (1997). Adaptation of islets of Langerhans to pregnancy: beta-cell growth, enhanced insulin secretion and the role of lactogenic hormones. Horm. Metab. Res. 29, 301–307. doi: 10.1055/s-2007-979040
Sorenson, R. L., Brelje, T. C., and Roth, C. (1993). Effects of steroid and lactogenic hormones on islets of Langerhans: a new hypothesis for the role of pregnancy steroids in the adaptation of islets to pregnancy. Endocrinology 133, 2227–2234. doi: 10.1210/endo.133.5.8404674
Sorenson, R. L., Johnson, M. G., Parsons, J. A., and Sheridan, J. D. (1987). Decreased glucose stimulation threshold, enhanced insulin secretion, and increased beta cell coupling in islets of prolactin-treated rats. Pancreas 2, 283–288. doi: 10.1097/00006676-198705000-00006
Spicer, L. J., and Aad, P. Y. (2007). Insulin-like growth factor (IGF) 2 stimulates steroidogenesis and mitosis of bovine granulosa cells through the IGF1 receptor: role of follicle-stimulating hormone and IGF2 receptor. Biol. Reprod. 77, 18–27. doi: 10.1095/biolreprod.106.058230
Steele, G. L., Currie, W. D., Yuen, B. H., Jia, X. C., Perlas, E., and Leung, P. C. (1993). Acute stimulation of human chorionic gonadotropin secretion by recombinant human activin-A in first trimester human trophoblast. Endocrinology 133, 297–303. doi: 10.1210/endo.133.1.8319577
Stelmanska, E., and Sucajtys-Szulc, E. (2014). Enhanced food intake by progesterone-treated female rats is related to changes in neuropeptide genes expression in hypothalamus. Endokrynol. Pol. 65, 46–56. doi: 10.5603/EP.2014.0007
Sternlicht, M. D. (2006). Key stages in mammary gland development: the cues that regulate ductal branching morphogenesis. Breast Cancer Res. 8:201. doi: 10.1186/bcr1368
Stokkan, K. A., and Aarseth, J. J. (2004). Melatonin reduces noradrenaline-induced vasoconstriction in the uterine artery of pregnant hooded seals ( Cystophora cristata ). Pflugers Arch. 447, 405–407. doi: 10.1007/s00424-003-1198-5
Storment, J. M., Meyer, M., and Osol, G. (2000). Estrogen augments the vasodilatory effects of vascular endothelial growth factor in the uterine circulation of the rat. Am. J. Obstet. Gynecol. 183, 449–453. doi: 10.1067/mob.2000.105910
Sun, Y., Zupan, B., Raaka, B. M., Toth, M., and Gershengorn, M. C. (2009). TRH-receptor-type-2-deficient mice are euthyroid and exhibit increased depression and reduced anxiety phenotypes. Neuropsychopharmacology 34, 1601–1608. doi: 10.1038/npp.2008.217
Takahashi, K., Ohmichi, M., Yoshida, M., Hisamoto, K., Mabuchi, S., Arimoto-Ishida, E., et al. (2003). Both estrogen and raloxifene cause G1 arrest of vascular smooth muscle cells. J. Endocrinol. 178, 319–329. doi: 10.1677/joe.0.1780319
Takayanagi, Y., Kasahara, Y., Onaka, T., Takahashi, N., Kawada, T., and Nishimori, K. (2008). Oxytocin receptor-deficient mice developed late-onset obesity. Neuroreport 19, 951–955. doi: 10.1097/WNR.0b013e3283021ca9
Takayanagi, Y., Yoshida, M., Bielsky, I. F., Ross, H. E., Kawamata, M., Onaka, T., et al. (2005). Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 102, 16096–16101. doi: 10.1073/pnas.0505312102
Takeda, K., Toda, K., Saibara, T., Nakagawa, M., Saika, K., Onishi, T., et al. (2003). Progressive development of insulin resistance phenotype in male mice with complete aromatase (CYP19) deficiency. J. Endocrinol. 176, 237–246. doi: 10.1677/joe.0.1760237
Tamma, R., Colaianni, G., Zhu, L. L., Dibenedetto, A., Greco, G., Montemurro, G., et al. (2009). Oxytocin is an anabolic bone hormone. Proc. Natl. Acad. Sci. U.S.A. 106, 7149–7154. doi: 10.1073/pnas.0901890106
Tamura, H., Takayama, H., Nakamura, Y., Reiter, R. J., and Sugino, N. (2008). Fetal/placental regulation of maternal melatonin in rats. J. Pineal Res. 44, 335–340. doi: 10.1111/j.1600-079X.2007.00537.x
Tessier, C., Prigent-Tessier, A., Bao, L., Telleria, C. M., Ferguson-Gottschall, S., Gibori, G. B., et al. (2003). Decidual activin: its role in the apoptotic process and its regulation by prolactin. Biol. Reprod. 68, 1687–1694. doi: 10.1095/biolreprod.102.011684
Thomas, A. L., Jack, P. M., Manns, J. G., and Nathanielsz, P. W. (1975). Effect of synthetic thyrotrophin releasing hormone on thyrotrophin and prolactin concentractions in the peripheral plasma of the pregnant ewe, lamb fetus and neonatal lamb. Biol. Neonate 26, 109–116. doi: 10.1159/000240722
Tiano, J. P., and Mauvais-Jarvis, F. (2012). Importance of oestrogen receptors to preserve functional beta-cell mass in diabetes. Nat. Rev. Endocrinol. 8, 342–351. doi: 10.1038/nrendo.2011.242
Tkachenko, O., Shchekochikhin, D., and Schrier, R. W. (2014). Hormones and hemodynamics in pregnancy. Int J Endocrinol Metab 12:e14098. doi: 10.5812/ijem.14098
Tomogane, H., Mistry, A. M., and Voogt, J. L. (1992). Late pregnancy and rat choriocarcinoma cells inhibit nocturnal prolactin surges and serotonin-induced prolactin release. Endocrinology 130, 23–28. doi: 10.1210/endo.130.1.1727699
Toro, A. R., Maymó, J. L., Ibarbalz, F. M., Pérez-Pérez, A., Maskin, B., Faletti, A. G., et al. (2014). Leptin is an anti-apoptotic effector in placental cells involving p53 downregulation. PLoS ONE 9:e99187. doi: 10.1371/journal.pone.0099187
Trott, J. F., Vonderhaar, B. K., and Hovey, R. C. (2008). Historical perspectives of prolactin and growth hormone as mammogens, lactogens and galactagogues—agog for the future!. J. Mammary Gland Biol. Neoplasia 13, 3–11. doi: 10.1007/s10911-008-9064-x
Ulrich, U., Miller, P. B., Eyre, D. R., Chesnut, C. H. III., Schlebusch, H., and Soules, M. R. (2003). Bone remodeling and bone mineral density during pregnancy. Arch. Gynecol. Obstet. 268, 309–316. doi: 10.1007/s00404-002-0410-8
Unemori, E. N., Erikson, M. E., Rocco, S. E., Sutherland, K. M., Parsell, D. A., Mak, J., et al. (1999). Relaxin stimulates expression of vascular endothelial growth factor in normal human endometrial cells in vitro and is associated with menometrorrhagia in women. Hum. Reprod. 14, 800–806. doi: 10.1093/humrep/14.3.800
Urbanek, M. O., Nawrocka, A. U., and Krzyzosiak, W. J. (2015). Small RNA Detection by in Situ Hybridization Methods. Int. J. Mol. Sci. 16, 13259–13286. doi: 10.3390/ijms160613259
Vaccarello, M. A., Diamond, F. B. Jr., Guevara-Aguirre, J., Rosenbloom, A. L., Fielder, P. J., Gargosky, S., et al. (1993). Hormonal and metabolic effects and pharmacokinetics of recombinant insulin-like growth factor-I in growth hormone receptor deficiency/Laron syndrome. J. Clin. Endocrinol. Metab. 77, 273–280.
Vale, W., Blackwell, R., Grant, G., and Guillemin, R. (1973). TRF and thyroid hormones on prolactin secretion by rat anterior pituitary cells in vitro . Endocrinology 93, 26–33. doi: 10.1210/endo-93-1-26
Valsamakis, G., Kumar, S., Creatsas, G., and Mastorakos, G. (2010). The effects of adipose tissue and adipocytokines in human pregnancy. Ann. N. Y. Acad. Sci. 1205, 76–81. doi: 10.1111/j.1749-6632.2010.05667.x
Van Bodegraven, A. A., Böhmer, C. J., Manoliu, R. A., Paalman, E., Van Der Klis, A. H., Roex, A. J., et al. (1998). Gallbladder contents and fasting gallbladder volumes during and after pregnancy. Scand. J. Gastroenterol. 33, 993–997. doi: 10.1080/003655298750027047
Vanhouten, J. N., Dann, P., Stewart, A. F., Watson, C. J., Pollak, M., Karaplis, A. C., et al. (2003). Mammary-specific deletion of parathyroid hormone-related protein preserves bone mass during lactation. J. Clin. Invest. 112, 1429–1436. doi: 10.1172/JCI200319504
Van Leengoed, E., Kerker, E., and Swanson, H. H. (1987). Inhibition of post-partum maternal behaviour in the rat by injecting an oxytocin antagonist into the cerebral ventricles. J. Endocrinol. 112, 275–282. doi: 10.1677/joe.0.1120275
Vannuccini, S., Bocchi, C., Severi, F. M., Challis, J. R., and Petraglia, F. (2016). Endocrinology of human parturition. Ann. Endocrinol. (Paris). 77, 105–113. doi: 10.1016/j.ando.2016.04.025
Vasavada, R. C., Cavaliere, C., D'ercole, A. J., Dann, P., Burtis, W. J., Madlener, A. L., et al. (1996). Overexpression of parathyroid hormone-related protein in the pancreatic islets of transgenic mice causes islet hyperplasia, hyperinsulinemia, and hypoglycemia. J. Biol. Chem. 271, 1200–1208. doi: 10.1074/jbc.271.2.1200
Vasavada, R. C., Garcia-Ocaña, A., Zawalich, W. S., Sorenson, R. L., Dann, P., Syed, M., et al. (2000). Targeted expression of placental lactogen in the beta cells of transgenic mice results in beta cell proliferation, islet mass augmentation, and hypoglycemia. J. Biol. Chem. 275, 15399–15406. doi: 10.1074/jbc.275.20.15399
Veenstra Van Nieuwenhoven, A. L., Bouman, A., Moes, H., Heineman, M. J., De Leij, L. F., Santema, J., et al. (2002). Cytokine production in natural killer cells and lymphocytes in pregnant women compared with women in the follicular phase of the ovarian cycle. Fertil. Steril. 77, 1032–1037. doi: 10.1016/S0015-0282(02)02976-X
Villar, J., Cogswell, M., Kestler, E., Castillo, P., Menendez, R., and Repke, J. T. (1992). Effect of fat and fat-free mass deposition during pregnancy on birth weight. Am. J. Obstet. Gynecol. 167, 1344–1352. doi: 10.1016/S0002-9378(11)91714-1
Vodstrcil, L. A., Tare, M., Novak, J., Dragomir, N., Ramirez, R. J., Wlodek, M. E., et al. (2012). Relaxin mediates uterine artery compliance during pregnancy and increases uterine blood flow. FASEB J. 26, 4035–4044. doi: 10.1096/fj.12-210567
Voltolini, C., and Petraglia, F. (2014). Neuroendocrinology of pregnancy and parturition. Handb. Clin. Neurol. 124, 17–36. doi: 10.1016/B978-0-444-59602-4.00002-2
Wagner, K. U., Young, W. S. III., Liu, X., Ginns, E. I., Li, M., Furth, P. A., et al. (1997). Oxytocin and milk removal are required for post-partum mammary-gland development. Genes Funct. 1, 233–244. doi: 10.1046/j.1365-4624.1997.00024.x
Wallace, J. M., Robinson, J. J., Wigzell, S., and Aitken, R. P. (1988). Effect of melatonin on the peripheral concentrations of LH and progesterone after oestrus, and on conception rate in ewes. J. Endocrinol. 119, 523–530. doi: 10.1677/joe.0.1190523
Wang, J. W., Jiang, Y. N., Huang, C. Y., Huang, P. Y., Huang, M. C., Cheng, W. T., et al. (2006). Proliferin enhances microvilli formation and cell growth of neuroblastoma cells. Neurosci. Res. 56, 80–90. doi: 10.1016/j.neures.2006.05.011
Wang, S. J., Liu, W. J., Wang, L. K., Pang, X. S., and Yang, L. G. (2017). The role of Melatonin receptor MTNR1A in the action of Melatonin on bovine granulosa cells. Mol. Reprod. Dev. 84, 1140–1154. doi: 10.1002/mrd.22877
Weaver, S. R., Prichard, A. P., Endres, E. L., Newhouse, S. A., Peters, T. L., Crump, P. M., et al. (2016). Elevation of circulating serotonin improves calcium dynamics in the peripartum dairy cow. J. Endocrinol. 230, 105–123. doi: 10.1530/JOE-16-0038
Weaver, S. R., Prichard, A. S., Maerz, N. L., Prichard, A. P., Endres, E. L., Hernández-Castellano, L. E., et al. (2017). Elevating serotonin pre-partum alters the Holstein dairy cow hepatic adaptation to lactation. PLoS ONE 12:e0184939. doi: 10.1371/journal.pone.0184939
Weil, Z. M., Hotchkiss, A. K., Gatien, M. L., Pieke-Dahl, S., and Nelson, R. J. (2006). Melatonin receptor (MT1) knockout mice display depression-like behaviors and deficits in sensorimotor gating. Brain Res. Bull. 68, 425–429. doi: 10.1016/j.brainresbull.2005.09.016
Weinberger, S. E., Weiss, S. T., Cohen, W. R., Weiss, J. W., and Johnson, T. S. (1980). Pregnancy and the lung. Am. Rev. Respir. Dis. 121, 559–581. doi: 10.1164/arrd.1980.121.3.559
Weiner, C. P., Lizasoain, I., Baylis, S. A., Knowles, R. G., Charles, I. G., and Moncada, S. (1994). Induction of calcium-dependent nitric oxide synthases by sex hormones. Proc. Natl. Acad. Sci. USA. 91, 5212–5216. doi: 10.1073/pnas.91.11.5212
Weinhaus, A. J., Stout, L. E., and Sorenson, R. L. (1996). Glucokinase, hexokinase, glucose transporter 2, and glucose metabolism in islets during pregnancy and prolactin-treated islets in vitro : mechanisms for long term up-regulation of islets. Endocrinology 137, 1640–1649. doi: 10.1210/endo.137.5.8612496
Weir, E. C., Philbrick, W. M., Amling, M., Neff, L. A., Baron, R., and Broadus, A. E. (1996). Targeted overexpression of parathyroid hormone-related peptide in chondrocytes causes chondrodysplasia and delayed endochondral bone formation. Proc. Natl. Acad. Sci. U.S.A. 93, 10240–10245. doi: 10.1073/pnas.93.19.10240
Weisinger, R. S., Burns, P., Eddie, L. W., and Wintour, E. M. (1993). Relaxin alters the plasma osmolality-arginine vasopressin relationship in the rat. J. Endocrinol. 137, 505–510. doi: 10.1677/joe.0.1370505
Wennbo, H., Kindblom, J., Isaksson, O. G., and Törnell, J. (1997). Transgenic mice overexpressing the prolactin gene develop dramatic enlargement of the prostate gland. Endocrinology 138, 4410–4415. doi: 10.1210/endo.138.10.5461
Whitehead, C. L., Walker, S. P., Ye, L., Mendis, S., Kaitu'u-Lino, T. J., Lappas, M., et al. (2013). Placental specific mRNA in the maternal circulation are globally dysregulated in pregnancies complicated by fetal growth restriction. J. Clin. Endocrinol. Metab. 98, E429–436. doi: 10.1210/jc.2012-2468
White, V., González, E., Capobianco, E., Pustovrh, C., Martínez, N., Higa, R., et al. (2006). Leptin modulates nitric oxide production and lipid metabolism in human placenta. Reprod. Fertil. Dev. 18, 425–432. doi: 10.1071/RD05105
Wiemers, D. O., Shao, L.-J., Ain, R., Dai, G., and Soares, M. J. (2003). The mouse prolactin gene family locus. Endocrinology 144, 313–325. doi: 10.1210/en.2002-220724
Williams, E. D., Leaver, D. D., Danks, J. A., Moseley, J. M., and Martin, T. J. (1994). Effect of parathyroid hormone-related protein (PTHrP) on the contractility of the myometrium and localization of PTHrP in the uterus of pregnant rats. J. Reprod. Fertil. 102, 209–214. doi: 10.1530/jrf.0.1020209
Williams, E. D., Major, B. J., Martin, T. J., Moseley, J. M., and Leaver, D. D. (1998). Effect of antagonism of the parathyroid hormone (PTH)/PTH-related protein receptor on decidualization in rat uterus. J. Reprod. Fertil. 112, 59–67. doi: 10.1530/jrf.0.1120059
Wilson, T., Liggins, G. C., and Whittaker, D. J. (1988). Oxytocin stimulates the release of arachidonic acid and prostaglandin F2 alpha from human decidual cells. Prostaglandins 35, 771–780. doi: 10.1016/0090-6980(88)90149-9
Winter, E. M., and Appelman-Dijkstra, N. M. (2017). Parathyroid hormone-related protein-induced hypercalcemia of pregnancy successfully reversed by a dopamine agonist. J. Clin. Endocrinol. Metab. 102, 4417–4420. doi: 10.1210/jc.2017-01617
Wu, H. H., Choi, S., and Levitt, P. (2016). Differential patterning of genes involved in serotonin metabolism and transport in extra-embryonic tissues of the mouse. Placenta 42, 74–83. doi: 10.1016/j.placenta.2016.03.013
Wysolmerski, J. J., Mccaughern-Carucci, J. F., Daifotis, A. G., Broadus, A. E., and Philbrick, W. M. (1995). Overexpression of parathyroid hormone-related protein or parathyroid hormone in transgenic mice impairs branching morphogenesis during mammary gland development. Development 121, 3539–3547.
Xiang, S., Mao, L., Yuan, L., Duplessis, T., Jones, F., Hoyle, G. W., et al. (2012). Impaired mouse mammary gland growth and development is mediated by melatonin and its MT1G protein-coupled receptor via repression of ERalpha, Akt1, and Stat5. J. Pineal Res. 53, 307–318. doi: 10.1111/j.1600-079X.2012.01000.x
Yamada, M., Saga, Y., Shibusawa, N., Hirato, J., Murakami, M., Iwasaki, T., et al. (1997). Tertiary hypothyroidism and hyperglycemia in mice with targeted disruption of the thyrotropin-releasing hormone gene. Proc. Natl. Acad. Sci. U.S.A. 94, 10862–10867. doi: 10.1073/pnas.94.20.10862
Yamada, M., Shibusawa, N., Ishii, S., Horiguchi, K., Umezawa, R., Hashimoto, K., et al. (2006). Prolactin secretion in mice with thyrotropin-releasing hormone deficiency. Endocrinology 147, 2591–2596. doi: 10.1210/en.2005-1326
Yamaguchi, M., Endo, H., Tasaka, K., and Miyake, A. (1995). Mouse growth hormone-releasing factor secretion is activated by inhibin and inhibited by activin in placenta. Biol. Reprod. 53, 368–372. doi: 10.1095/biolreprod53.2.368
Yamashita, H., Shao, J., Ishizuka, T., Klepcyk, P. J., Muhlenkamp, P., Qiao, L., et al. (2001). Leptin administration prevents spontaneous gestational diabetes in heterozygous Lepr(db/+) mice: effects on placental leptin and fetal growth. Endocrinology 142, 2888–2897. doi: 10.1210/endo.142.7.8227
Yao, L., Agoulnik, A. I., Cooke, P. S., Meling, D. D., and Sherwood, O. D. (2008). Relaxin acts on stromal cells to promote epithelial and stromal proliferation and inhibit apoptosis in the mouse cervix and vagina. Endocrinology 149, 2072–2079. doi: 10.1210/en.2007-1176
Yeh, S., Tsai, M. Y., Xu, Q., Mu, X. M., Lardy, H., Huang, K. E., et al. (2002). Generation and characterization of androgen receptor knockout (ARKO) mice: an in vivo model for the study of androgen functions in selective tissues. Proc. Natl. Acad. Sci. U.S.A. 99, 13498–13503. doi: 10.1073/pnas.212474399
Yellon, S. M., and Longo, L. D. (1988). Effect of maternal pinealectomy and reverse photoperiod on the circadian melatonin rhythm in the sheep and fetus during the last trimester of pregnancy. Biol. Reprod. 39, 1093–1099. doi: 10.1095/biolreprod39.5.1093
Yogosawa, S., Mizutani, S., Ogawa, Y., and Izumi, T. (2013). Activin receptor-like kinase 7 suppresses lipolysis to accumulate fat in obesity through downregulation of peroxisome proliferator-activated receptor gamma and C/EBPalpha. Diabetes 62, 115–123. doi: 10.2337/db12-0295
Yong, H. E. J., Murthi, P., Kalionis, B., Keogh, R. J., and Brennecke, S. P. (2017). Decidual ACVR2A regulates extravillous trophoblast functions of adhesion, proliferation, migration and invasion i n vitro . Pregnancy Hypertens . 12, 189–193. doi: 10.1016/j.preghy.2017.11.002
Yong, H. E., Murthi, P., Wong, M. H., Kalionis, B., Cartwright, J. E., Brennecke, S. P., et al. (2015). Effects of normal and high circulating concentrations of activin A on vascular endothelial cell functions and vasoactive factor production. Pregnancy Hypertens. 5, 346–353. doi: 10.1016/j.preghy.2015.09.006
Young, W. S. III., Shepard, E., Amico, J., Hennighausen, L., Wagner, K. U., Lamarca, M. E., et al. (1996). Deficiency in mouse oxytocin prevents milk ejection, but not fertility or parturition. J. Neuroendocrinol. 8, 847–853. doi: 10.1046/j.1365-2826.1996.05266.x
Youssef, R. E., Ledingham, M. A., Bollapragada, S. S., O'gorman, N., Jordan, F., Young, A., et al. (2009). The role of toll-like receptors (TLR-2 and−4) and triggering receptor expressed on myeloid cells 1 (TREM-1) in human term and preterm labor. Reprod. Sci. 16, 843–856. doi: 10.1177/1933719109336621
Yu, L., Li, D., Liao, Q. P., Yang, H. X., Cao, B., Fu, G., et al. (2012). High levels of activin A detected in preeclamptic placenta induce trophoblast cell apoptosis by promoting nodal signaling. J. Clin. Endocrinol. Metab. 97, E1370–E1379. doi: 10.1210/jc.2011-2729
Yura, S., Ogawa, Y., Sagawa, N., Masuzaki, H., Itoh, H., Ebihara, K., et al. (2000). Accelerated puberty and late-onset hypothalamic hypogonadism in female transgenic skinny mice overexpressing leptin. J. Clin. Invest. 105, 749–755. doi: 10.1172/JCI8353
Zeng, H., Schimpf, B. A., Rohde, A. D., Pavlova, M. N., Gragerov, A., and Bergmann, J. E. (2007). Thyrotropin-releasing hormone receptor 1-deficient mice display increased depression and anxiety-like behavior. Mol. Endocrinol. 21, 2795–2804. doi: 10.1210/me.2007-0048
Zhang, L., Fishman, M. C., and Huang, P. L. (1999). Estrogen mediates the protective effects of pregnancy and chorionic gonadotropin in a mouse model of vascular injury. Arterioscler. Thromb. Vasc. Biol. 19, 2059–2065. doi: 10.1161/01.ATV.19.9.2059
Zhang, Y., Hou, Y., Wang, X., Ping, J., Ma, Z., Suo, C., et al. (2017). The effects of kisspeptin-10 on serum metabolism and myocardium in rats. PLoS ONE 12:e0179164. doi: 10.1371/journal.pone.0179164
Zhao, L., Roche, P. J., Gunnersen, J. M., Hammond, V. E., Tregear, G. W., Wintour, E. M., et al. (1999). Mice without a functional relaxin gene are unable to deliver milk to their pups. Endocrinology 140, 445–453. doi: 10.1210/endo.140.1.6404
Zhao, L., Samuel, C. S., Tregear, G. W., Beck, F., and Wintour, E. M. (2000). Collagen studies in late pregnant relaxin null mice. Biol. Reprod. 63, 697–703. doi: 10.1095/biolreprod63.3.697
Zha, W., Ho, H. T. B., Hu, T., Hebert, M. F., and Wang, J. (2017). Serotonin transporter deficiency drives estrogen-dependent obesity and glucose intolerance. Sci. Rep. 7:1137. doi: 10.1038/s41598-017-01291-5
Zhou, B., Kong, X., and Linzer, D. I. (2005). Enhanced recovery from thrombocytopenia and neutropenia in mice constitutively expressing a placental hematopoietic cytokine. Endocrinology 146, 64–70. doi: 10.1210/en.2004-1011
Zhou, B., Lum, H. E., Lin, J., and Linzer, D. I. (2002). Two placental hormones are agonists in stimulating megakaryocyte growth and differentiation. Endocrinology 143, 4281–4286. doi: 10.1210/en.2002-220447
Zhou, Y., Xu, B. C., Maheshwari, H. G., He, L., Reed, M., Lozykowski, M., et al. (1997). A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse). Proc. Natl. Acad. Sci. U.S.A. 94, 13215–13220. doi: 10.1073/pnas.94.24.13215
Zhu, Y., Bian, Z., Lu, P., Karas, R. H., Bao, L., Cox, D., et al. (2002). Abnormal vascular function and hypertension in mice deficient in estrogen receptor beta. Science 295, 505–508. doi: 10.1126/science.1065250
Ziegler, B., Lucke, S., Besch, W., and Hahn, H. J. (1985). Pregnancy-associated changes in the endocrine pancreas of normoglycaemic streptozotocin-treated Wistar rats. Diabetologia 28, 172–175.
Zöllner, J., Howe, L. G., Edey, L. F., O'dea, K. P., Takata, M., Gordon, F., et al. (2017). The response of the innate immune and cardiovascular systems to LPS in pregnant and nonpregnant mice. Biol. Reprod. 97, 258–272. doi: 10.1093/biolre/iox076
Zygmunt, M., Herr, F., Keller-Schoenwetter, S., Kunzi-Rapp, K., Münstedt, K., Rao, C. V., et al. (2002). Characterization of human chorionic gonadotropin as a novel angiogenic factor. J. Clin. Endocrinol. Metab. 87, 5290–5296. doi: 10.1210/jc.2002-020642
Keywords: pregnancy, placenta, hormones, maternal adaptations, metabolism, fetal growth, endocrine, cardiovascular
Citation: Napso T, Yong HEJ, Lopez-Tello J and Sferruzzi-Perri AN (2018) The Role of Placental Hormones in Mediating Maternal Adaptations to Support Pregnancy and Lactation. Front. Physiol . 9:1091. doi: 10.3389/fphys.2018.01091
Received: 20 April 2018; Accepted: 23 July 2018; Published: 17 August 2018.
Reviewed by:
Copyright © 2018 Napso, Yong, Lopez-Tello and Sferruzzi-Perri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amanda N. Sferruzzi-Perri, [email protected]
† These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
What is placenta? Mention its role during pregnancy?
The embryo gets nutrition from the mother’s blood with the help of a special tissue which looks like a disc-shaped sac called placenta. the placenta is embedded in the uterine wall. it contains villi on the embryo’s side of the tissue and on the mother’s side are blood spaces, which surround the villi. this provides a large surface area for glucose and oxygen to pass from the mother to the embryo. the developing embryo will also generate metabolic waste which can be removed by transferring them into the mother’s blood through the placenta..

Question 54 What is placenta? Mention its role during pregnancy?
What is placenta? State its two role during pregnancy.


IMAGES
VIDEO
COMMENTS
The placenta is a major endocrine organ, and placental hormones have diverse profound effects on maternal physiology and behaviour [64,65]. During early pregnancy, they drive an increase in food intake and energy storage, whereas towards term, they mobilize these reserves to support fetal growth and preparation for lactation [66,67]. The most ...
The placenta is an organ that forms in the womb, also called the uterus, during pregnancy. The placenta is connected to a developing baby by a tubelike structure called the umbilical cord. Through the umbilical cord, the placenta provides oxygen and nutrients to a developing baby. It also removes waste from the baby's blood.
Discarded at birth, the placenta is a highly complex and fascinating organ. During the course of a pregnancy, it acts as the lungs, gut, kidneys, and liver of the fetus. The placenta also has major endocrine actions that modulate maternal physiology and metabolism and provides a safe and protective milieu in which the fetus can develop. The human placenta undergoes dramatic transformations in ...
The placenta ensures fetuses get necessary food and oxygen during pregnancy. The placenta develops within the uterus during pregnancy, playing a key role in nourishing and providing oxygen to the fetus, as well as removing waste material. This organ is attached to the wall of the uterus, with the baby's umbilical cord arising from it.
The placenta is a temporary organ that connects your baby to your uterus during pregnancy. The placenta develops shortly after conception and attaches to the wall of your uterus. Your baby is connected to the placenta by the umbilical cord. Together, the placenta and umbilical cord act as your baby's lifeline while in the uterus.
Nonetheless, there is much evidence to suggest that the trophoblast, the coelom and the secondary yolk sac combine to act as physiological, rather than a morphological, choriovitelline placenta during the first weeks post-implantation . Figure 3. Schematic of the steps along the histotrophic pathway during early human pregnancy.
The placenta is a central structure in pregnancy and has pleiotropic functions. This organ grows incredibly rapidly during this period, acting as a mastermind behind different fetal and maternal processes. The relevance of the placenta extends far beyond the pregnancy, being crucial for fetal programming before birth. Having integrative knowledge of this maternofetal structure helps ...
Introduction. The placenta delivers oxygen and nutrients to the growing fetus and removes waste products; as such, it is responsible for fetal wellbeing, maintained in the context of maternal ...
Abstract. Successful pregnancies rely on sufficient energy and nutrient supply, which require the mother to metabolically adapt to support fetal needs. The placenta has a critical role in this process, as this specialized organ produces hormones and peptides that regulate fetal and maternal metabolism.
During pregnancy, the human placenta synthesises large amounts of leptin, which is released into maternal and fetal circulation and amniotic fluid (Henson et al, 1998, ... The role of PAPP-A during pregnancy is still poorly understood. Because of its protease activity, PAPP-A decreases IGFBP-4 affinity for IGF-1 and 2. ...
Abstract. The placenta is a transient fetal organ that plays a critical role in the health and wellbeing of both the fetus and its mother. Functionally, the placenta sustains the growth of the fetus as it facilitates delivery of oxygen and nutrients and removal of waste products. Not surprisingly, defective early placental development is the ...
During pregnancy, the mutual interactions between the mother and the fetus in mammals depend on the placenta. Events in early pregnancy can lead to different trajectories of placental development. The establishment of epigenetic patterns during early embryogenesis, coupled with adaptive changes in the placenta, may influence fetal growth, late ...
The placenta acts as a barrier to prevent toxins and pathogens getting into the foetus's blood. Not all toxin molecules or pathogenic organisms (such as viruses e.g. rubella) are stopped from passing through the placenta (this usually depends on the size of the molecule) This is why pregnant women are advised not to smoke during pregnancy as ...
The placenta serves the functions of organs such as the lungs, kidneys, and liver until your fetus develops them. Some of the main functions that the placenta performs include (1) (3): Respiratory, excretory, nutritive, endocrine i. X System of glands that secrete hormones directly into the bloodstream.
Introduction. Pregnancy is a dynamic and precisely coordinated process involving systemic and local changes in the mother that support the supply of nutrients and oxygen to the baby for growth in utero and in the subsequent lactation. Inappropriate adaptation of maternal physiology may lead to complications of pregnancy, such as gestational diabetes, preeclampsia, fetal growth restriction ...
Implications of the new paradigm. pregnancies fail during the first trimester, and an even higher percentage of conceptions are thought to be lost between implantation and clinical detection of a pregnancy [94]. Between 50% and 60% of these early losses are associ-ated with chromosomal abnormalities [95], and although.
Solution. The embryo gets nutrition from the mother's blood with the help of a special tissue which looks like a disc-shaped sac called placenta. The placenta is embedded in the uterine wall. It contains villi on the embryo's side of the tissue and on the mother's side are blood spaces, which surround the villi.
By the numbers: The national C-section delivery rate increased in 2023 to 32.4%, up from 32.1% in 2022, according to provisional CDC numbers. That's the highest rate since 2013, and the fourth annual increase after the rate generally declined 2009 - 2019, the CDC says. The rate of low-risk cesarean deliveries (mothers' first births of full-term ...