Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- RESEARCH BRIEFINGS
- 20 December 2023

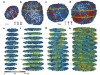
Atomic electron tomography reveals chemical order in medium- and high-entropy alloys
This is a summary of: Moniri, S. et al . Three-dimensional atomic structure and local chemical order of medium- and high-entropy nanoalloys. Nature 624 , 564–569 (2023) .
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
185,98 € per year
only 3,65 € per issue
Rent or buy this article
Prices vary by article type
Prices may be subject to local taxes which are calculated during checkout
doi: https://doi.org/10.1038/d41586-023-03656-5
‘Expert opinion’ is published under a CC BY 4.0 licence.
George, E. P., Raabe, D. & Ritchie, R. O. Nature Rev. Mater. 4 , 515–534 (2019).
Article Google Scholar
Yao, Y. et al. Science 376 , eabn3103 (2022).
Article PubMed Google Scholar
Nature Mater. 22 , 925 (2023).
Miao, J., Ercius, P. & Billinge, S. J. L. Science 353 , aaf2157 (2016).
Zhou, J. et al. Nature 570 , 500–503 (2019).
Download references
Reprints and permissions
Related Articles
- Nanoparticles
- Materials science

Landmark study links microplastics to serious health problems
News 06 MAR 24

Directive giant upconversion by supercritical bound states in the continuum
Article 21 FEB 24

Three-dimensional atomic structure and local chemical order of medium- and high-entropy nanoalloys
Article 20 DEC 23
Selenium alloyed tellurium oxide for amorphous p-channel transistors
Article 10 APR 24

Phononic switching of magnetization by the ultrafast Barnett effect

A hybrid topological quantum state in an elemental solid
Force-controlled release of small molecules with a rotaxane actuator
Nanoscale scythe cuts molecular tethers using mechanical forces
News & Views 10 APR 24
Trio of radicals choreographed for versatile chemical reaction
News & Views 03 APR 24
Junior Group Leader Position at IMBA - Institute of Molecular Biotechnology
The Institute of Molecular Biotechnology (IMBA) is one of Europe’s leading institutes for basic research in the life sciences. IMBA is located on t...
Austria (AT)
IMBA - Institute of Molecular Biotechnology
Open Rank Faculty, Center for Public Health Genomics
Center for Public Health Genomics & UVA Comprehensive Cancer Center seek 2 tenure-track faculty members in Cancer Precision Medicine/Precision Health.
Charlottesville, Virginia
Center for Public Health Genomics at the University of Virginia
Husbandry Technician I
Memphis, Tennessee
St. Jude Children's Research Hospital (St. Jude)
Lead Researcher – Department of Bone Marrow Transplantation & Cellular Therapy
Researcher in the center for in vivo imaging and therapy.
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

Students' representations of the atomic structure – the effect of some individual differences in particular task contexts
George Papageorgiou *, Angelos Markos and Nikolaos Zarkadis Democritus University of Thrace, Greece. E-mail: [email protected]
First published on 9th December 2015
The current study aims to investigate students' representations of the atomic structure in a number of student cohorts with specific characteristics concerning age, grade, class curriculum and some individual differences, such as formal reasoning and field dependence/independence. Two specific task contexts, which were designed in accordance with corresponding teaching contexts for the atomic structure, one based on Bohr's model and the other on the quantum mechanical model, were examined as for their potential to differentiate initial students' representations of the atomic structure (when no specific context was provided). Participants ( n = 421) were students of 8th, 10th and 12th grades of secondary schools from Northern Greece. Results showed that, although developmental factors, like formal reasoning, were associated with a better representation of the atomic structure, the task context appeared to have the dominant role, since positive associations were found between student cohort characteristics and representation of the atomic structure in context dependent tasks, even after accounting for the effects of individual differences.
Introduction
Students' representations of the atomic structure.
However, more interesting are the cases where student models comprise paths of electrons, either with or without references to certain levels of orbits and/or to energy quantization. These cases are reported mostly as the ‘solar system model’ ( Harrison and Treagust, 1996 ; Nicoll, 2001 ; Nakiboglu, 2003 ; Cokelez and Dumon, 2005 ; Cokelez, 2012 ), the ‘planetary model’ ( Petri and Niedderer, 1998 ; Papaphotis and Tsaparlis, 2008 ; Adbo and Taber, 2009 ; Tsaparlis and Papaphotis, 2009 ) or ‘Bohr's model’ ( Fischler and Lichtfield, 1992 ; Nicoll, 2001 ; McKagan et al. , 2008 ; Papaphotis and Tsaparlis, 2008 ; Park and Light, 2009 ; Tsaparlis and Papaphotis, 2009 ; Wang and Barrow, 2013 ), and are considered to be the most typical ones in students' descriptions of the atomic structure. Even though these cases refer to scientifically and historically different models ( Justi and Gilbert, 2000 ), in the majority of students' mental model categorizations as articulated by science education researchers, they have been treated as one and the same kind of deterministic model. For instance, Park and Light (2009) studied students' representations of the atomic structure. Although from a scientific point of view Bohr's model has been clearly based on quantum theory, the researchers incorporated all the above cases – even those without any references to quantum theory – into the same student model, i.e. ‘Bohr's model’. Possible student references to certain levels of orbits and/or energy quantization were taken into account only in the formation of the corresponding sub-categories inside this model. Other researchers, when they similarly blend scientifically different models in order to form a particular student mental model, they use appropriate representative terms, such as ‘planetary Bohr's model’ ( Tsaparlis and Papaphotis, 2009 ) or ‘Bohr/solar system model’ ( Stevens et al. , 2010 ). In any case, this kind of mental model – let us refer to it as ‘Bohr's model’ – appears to be the most dominant in students' thinking, even in the upper grades of secondary education, where the conditions could probably facilitate the development of a more sophisticated approach to the atomic structure ( Fischler and Lichtfield, 1992 ; Petri and Niedderer, 1998 ; Papaphotis and Tsaparlis, 2008 ; Tsaparlis and Papaphotis, 2009 ).
Towards a quantum description of the atomic structure, the literature shows more sophisticated students' representations that are reported as ‘orbital model’ ( Harrison and Treagust, 1996 ; Kalkanis et al. , 2003 ; Taber 2005 ), ‘electron cloud model’ ( Petri and Niedderer, 1998 ; Cokelez and Dumon, 2005 ; Tsaparlis and Papaphotis, 2009 ; Stevens et al. , 2010 ; Cokelez, 2012 ), ‘quantum model’ ( Taber, 2002a, 2005 ; Park and Light, 2009 ) or ‘Schrödinger model’ ( McKagan et al. , 2008 ). In these student models, concepts such as energy quantization, wave function or/and probability are frequently present. Similar to those reported above for ‘Bohr's model’, an incorporation of scientifically different relevant models into a particular student mental model has also been noticed – let us refer to it as the ‘quantum mechanical model’. However, research has demonstrated that students generally have difficulties and hold misconceptions in adopting this model, since its understanding also requires an understanding of many other abstract concepts such as ‘orbital’ ( Tsaparlis, 1997 ; Taber, 2002a, 2002b, 2005 ; Tsaparlis and Papaphotis, 2002, 2009 ; Nakiboglu, 2003 ; Papaphotis and Tsaparlis, 2008 ; Stefani and Tsaparlis, 2009 ), ‘electron cloud’ ( Harrison and Treagust, 1996, 2000 ; Tsaparlis and Papaphotis, 2002, 2009 ), ‘quantization of energy’ and ‘angular momentum’ ( Taber, 2002a ; Didiş et al. , 2014 ), ‘probability’ ( Park and Light, 2009 ), ‘Heisenberg's uncertainty principle’ ( Tsaparlis and Papaphotis, 2009 ) and other characteristics of a quantum probabilistic approach.
The effect of the context
However, apart from the implications for the teaching/learning procedure, the idea that these two models ( i.e. ‘Bohr's model’ and the ‘quantum mechanical model’) define two different contexts has also implications for researchers trying to explore relevant students' representations. In the process of developing an appropriate research instrument and creating certain tasks, a researcher has the option to define a ‘task context’ with particular contextual features/settings, on which students could base their representations of the atomic structure. In other words, ‘task context’ refers to a set of situational settings in a specific area, in which cueing and prompting are given to students ( Sağlam, 2010 ). The question here is: Could such a task context have a significant effect on the corresponding student's representation?
According to a number of studies, a ‘task context’ can generally affect student responses ( e.g. Palmer, 1997 ; Petri and Niedderer, 1998 ; Bao et al. , 2002 ; Itza-Ortiz et al. , 2004 ; Bao and Redish, 2006 ; Redish and Smith, 2008 ; Teichert et al. , 2008 ; Hrepic et al. , 2010 ; Sağlam, 2010 ; Didiş et al. , 2014 ). This is known as ‘context dependence’ ( e.g. Bao and Redish, 2006 ; Redish and Smith, 2008 ) and it is also connected to the theory of ‘knowledge in pieces’ ( diSessa, 1993 ). According to the latter, student responses to particular questions are based on the ‘ in situ ’ combination of small pieces of knowledge that could produce inconsistent answers, since they are influenced by the contextual features of the questions. On this basis, when students are trying to work within two different task contexts towards a representation of the atomic structure, they could activate different knowledge resources, which would significantly differentiate their thinking. For example, in the study of Tsaparlis and Papaphotis (2009) on students' atomic representations, it appears that the context of the tasks during the interviews and the relevant discussions affected the high-school students' (12th grade) representations, leading those who initially provided simple model representations ( e.g. Bohr model) to adopt a more sophisticated model (electron cloud model). Such context dependence could be affected by a number of factors. Wang and Barrow (2013) , for instance, when investigated the undergraduate students' general chemistry conceptual frameworks about the atomic structure, found that ‘high conceptual knowledge’ (HCK) students were much more context dependent than ‘low conceptual knowledge’ (LCK) students. HCK students had the ability to adapt their representations of the atomic structure to the context of the task and thus, could switch from the Bohr model to the electron-cloud model using quantum mechanics descriptions in order to explain, for instance, the electron distribution of a polar bond. In contrast, LCK students could only work on simple models and had difficulties in using quantum mechanics descriptions in the context of the electron-cloud model.
Individual differences
Formal Reasoning (FR), also reported as Logical Thinking , is in fact a Piagetian concept and it refers to the ability of an individual to use concrete and formal operational reasoning ( Lawson, 1978, 1985, 1993 ). In the general context of science education many studies have reported a correlation between FR and student performance ( e.g. Lawson, 1982 ; Chandran et al. , 1987 ; Niaz, 1996 ). On the other hand, Field Dependence/Independence (FDI) is associated with the ability of an individual to disembed relevant information from a complex context or, in other words, the ability to efficiently separate the ‘signal’ from the ‘noise’ ( Witkin et al. , 1971 ). In the literature, FDI appears to be very important for the conceptual understanding of science concepts ( Bahar and Hansell, 2000 ; Kang et al. , 2005 ; Tsaparlis, 2005 ; Danili and Reid, 2006 ).
The atomic structure in Greek secondary education
Particularly relevant to the context of chemistry, students in the 8th grade receive a one-hour lesson per week about (among others) the concept of the atom, the subatomic particles and their characteristics, as well as an introduction to Bohr's atomic model. In the 10th grade, during two one-hour lessons per week, students are taught the electronic configuration of the atom based on Bohr's atomic model. In the 12th grade, students in the ‘science and math’ direction also receive two one-hour lessons per week, where they are taught (among others) the quantum mechanical model and relevant concepts, such as the atomic orbital, the uncertainty principle, the electron cloud and its density.
In the context of physics, all students of the 12th grade receive a one-hour lesson per week, where they are taught (among others) more in-depth concepts related to the Bohr atomic model, such as the electron stimulation or the ionization.
Rationale of the study and research questions
With regard to individual differences, choices were theory driven considering also previous research findings. According to those, a number of Neo-Piagetian cognitive variables are identified as predictors of student achievement in science ( Johnstone and Al-Naeme, 1995 ; Niaz, 1996 ; Tsitsipis et al. , 2010 ). Among them, formal reasoning and field dependence/independence were sought as closely associated with the tasks usually involved in the learning process related to science topics ( Tsitsipis et al. , 2010 ; Stamovlasis and Papageorgiou, 2012 ), especially those for which ‘context’ is an issue.
Thus, the present study aims to investigate:
1. What are the students' representations of the atomic structure, in both the presence and absence of task context?
2. To what extent do cognitive factors ( i.e. individual differences concerning formal reasoning and field dependence/independence) and student cohort characteristics (grade, age and curriculum) explain a possible variability in student competence for representing the atomic structure in the presence and absence of a task context?
Taking into account recent relevant research evidence, a number of hypotheses could be articulated:
• Hypothesis 1 : It is expected that a task context will have a significant impact on students' representations of the atomic structure and consequently, representations will differ when the context changes.
• Hypothesis 2 : It is expected that dependent on or independent of a task context, an increase in the odds of possessing a scientifically sufficient representation will be associated with an increase in formal reasoning, field dependence/independence and student cohort characteristics (age and grade).
• Hypothesis 3 : In line with the literature ( e.g. Wang and Barrow, 2013 ), it is expected that student cohort characteristics will be associated with representations, even after accounting for the effects of cognitive factors.
Methodology
Subjects and procedure, instruments.
The construct validity of the two cognitive constructs was examined in the context of Confirmatory Factor Analysis (CFA). Due to the dichotomous/categorical nature of the test items (the correct and incorrect dichotomy was obtained by collapsing the options representing the wrong alternatives) the analysis was performed on the tetrachoric correlation matrix using the WLSMV (Weighted Least Squares with Mean and Variance Adjustment) estimator implemented in Mplus software Version 7.31 ( Muthén and Muthén, 2012 ). The model fit was evaluated using the following indices: comparative fit index (CFI), Tucker–Lewis index (TLI), root mean square error of approximation (RMSEA), and 90% confidence interval (CI) of RMSEA. According to previous research, CFI and TLI values ≥0.95 and RMSEA values ≤0.08 were considered as good indicators of the data-model fit ( Hu and Bentler, 1999 ). Internal consistency of all measures was assessed using Cronbach's alpha coefficient. Scores for all scales used in the study were computed by summing the items that constitute the scale.
Table 1 summarizes the descriptive statistics of each one of the cognitive scales.
Students' representations were assessed in all tasks, taking into account both drawings and relevant feature descriptions, according to their correctness and completeness in comparison to the scientific view. Student scoring categorization took into account other similar categorizations already presented in relevant research ( e.g. , Harrison and Treagust, 1996 ; Park and Light, 2009 ; Cokelez, 2012 ). A summary of the categories for all tasks is presented in Table 3 together with the corresponding sub-categories. Category E refers to the most scientifically sufficient, whereas A refers to the most naïve.
In fact, the two contexts of tasks 2a and 2b could potentially lead students towards categories D and E, respectively. So, a shift from categories A, B and C to category D was expected in task 2a and a shift from categories A, B, C and D to category E in task 2b. Of course, this does not mean that all students shifting to D or E could fully understand the corresponding model, i.e. Bohr's model in task 2a and the quantum mechanical model in task 2b; what was assessed in that case was the ability of students to adapt their representations of the atomic structure within the given characteristics of a specific context. A possible shift from E to D in task 2a could also not be excluded.
Statistical analysis
In order to investigate the influence of student cohort characteristics on students' representations, after accounting for the effects of cognitive variables (hypothesis 3), a Categorical Regression Analysis model (CATREG in SPSS) was examined with student cohort characteristics as the outcome and the two cognitive factors as the independent variables. The model residuals were subsequently correlated with students' representations using Kendall's tau-b correlation coefficient.
Preliminary analyses
With regard to the context dependent tasks (2a and 2b), shifts towards categories D and E were observed ( Table 4 ). In task 2a, as expected, the majority of students appeared to be affected by the characteristics of the corresponding context and they represented the atomic structure according to Bohr's model (65.8%), although the nuclear model still held a significant percentage (26.6%). A closer look at the distribution per student cohort ( Table 5 ) revealed that the effect of a task context was greater in the 3rd and 4th cohorts, where 92.7% and 91.1% of students of each one of these cohorts, respectively, responded within the Bohr's model.
Interestingly, the effect of context on students' representations was even more obvious in task 2b, where the quantum mechanical model reached unexpectedly a percentage of 43.5% of the sample. The effect was even larger in the 4th cohort, reaching 93.3% of the students of this particular cohort. Taking into account the difficulty in perceiving the characteristics of this model, which also justifies the high percentage of missing values, these figures seem to be higher than expected. However, one should also take into account that this cohort included students who had been taught this model. In addition, as already mentioned, this does not mean that all these students have developed a good knowledge of the quantum mechanical model.
Results of ordinal logistic regression
Student cohort characteristics had also a statistically significant effect ( χ 2 (3) = 15.820, p = 0.001). Specifically, the odds for the 4th cohort (12th grade students – science and math direction) of possessing a sufficient representation of the atomic structure in task 1 were (1/0.270) = 3.70 times greater than the odds for the 1st cohort (8th grade students), (1/0.214) = 4.67 times higher than the odds for the 2nd cohort (10th grade students) and (1/0.289) = 3.46 times higher than the odds for the 3rd cohort (12th grade students – technological direction). In contrast, the odds of the 2nd cohort and the 3rd cohort were similar to those of the 1st cohort. Finally, the odds for the 3rd cohort were similar to those of the 2nd cohort. All the effects were found to be statistically significant.
However, student cohort characteristics had a statistically significant effect ( χ 2 (3) = 27.230, p < 0.001). The odds for the 4th cohort of possessing a sufficient representation of the atomic structure in task 2a were (1/0.034) = 29.41 times greater than the odds for the 1st cohort, (1/0.062) = 16.12 times higher than the odds for the 2nd cohort and (1/0.220) = 4.54 times higher than the odds for the 3rd cohort. The odds for the 2nd cohort were 1.82 times greater than the odds for the 1st cohort. Finally, the odds for the 3rd cohort were 4.54 and 3.56 times greater than the odds for the 1st and 2nd cohorts. All the effects were statistically significant.
Student cohort characteristics had also a statistically significant effect ( χ 2 (3) = 12.899, p = 0.005). The odds for the 4th cohort of possessing a sufficient representation of the atomic structure in task 2b were (1/0.322) = 3.10 times greater than the odds for the 1st cohort, (1/0.184) = 5.43 times higher than the odds for the 2nd cohort and (1/0.223) = 4.48 times higher than the odds for the 3rd cohort. Moreover, the odds of the 2nd cohort were 1.95 times greater than that for the 1st cohort. Lastly, the odds for the 3rd cohort are 5.44 and 7.12 times greater than the odds for the 1st and the 2nd cohorts, respectively. All the effects were statistically significant.
Finally, the unique influence of student cohort characteristics on the representations of the atomic structure, after accounting for the effects of formal reasoning and field dependence/independence was investigated. For this purpose, the residuals of a categorical regression analysis model with cohort characteristics as the dependent variable and the two cognitive factors (FR and FDI) as independent were correlated with student performance in each of the three tasks, using Kendall's tau-b correlation coefficient. A non-significant correlation was detected for context independent task 1 (tau-b = 0.061, p > 0.05), whereas for context dependent tasks 2a and 2b the correlations were found to be positive and statistically significant (tau-b = 0.177 and tau-b = 0.192, p < 0.01). These results indicate, in the case of context dependent tasks, a positive association between student cohort characteristics and possessing a sufficient representation of the atomic structure, even after controlling for the effects of formal reasoning and field dependence/independence.
Discussion and educational implications
The effect of a task context.
With regard to hypothesis 1, it was expected that students' representations would change when students had to work within specific characteristics of particular task contexts. Indeed, the effect of such contexts was found to be significant for both tasks 2a and 2b. In the Bohr's context (task 2a) students reached very high percentages, which were higher than 90% in 3rd and 4th cohorts. Rather unexpectedly, it was observed that in task 2b (quantum mechanical context), students of the 4th cohort were not the only ones affected by the context (very high percentage, 93.3%), but significant percentages of students in the other cohorts were affected as well. Although possible implications for the corresponding teaching context is a big step ahead, one could make speculations for the possibility to have analogous effects on the adoption of more sophisticated models by students through an appropriately designed teaching/learning process. Relevant research provides evidence to this direction. Petri and Niedderrer (1998), for instance, when differentiated the teaching context by setting different teaching inputs during a series of 80 appropriately designed lessons for the atomic structure, they found that, despite the domination of the planetary model in students' representations, different inputs had as a result the development of different conceptions for the atom, moving to a significant degree, from the planetary model to the electron cloud model.
The effect of individual differences
Consequently, the findings concerning individual differences address to anyone who can configure the ‘teaching context’ and especially, to teachers and science curriculum designers. As has been suggested elsewhere ( e.g. , Stamovlasis and Papageorgiou, 2012 ), both of them can improve teaching outcomes, by adapting teaching context and relevant subject matter to the understanding level of the students of each grade and by using appropriately designed means, such as illustrations, diagrams, and representations, in order to stimulate student attention on critical attributes of the atomic structure, especially when this is studied through more sophisticated models.
The effect of student cohorts
Conclusions.
As for the implications for the teaching and learning process, although the effect of a ‘task context’ does not necessarily imply direct analogous effects of ‘teaching context’, some general considerations could be possibly made. In that context, it appears that, although developmental factors, like formal reasoning, will always play an important role in the learning process, having a significant effect on students' representations and conceptions in general, the success of this process is actually defined by the role of the teacher and the curricula designers. A science curriculum that takes into account student characteristics in each grade and an appropriate teaching methodology on the basis of a compatible context could potentially overcome the challenges that arise by other factors, like FDI, and drive students through learning paths.
- Adbo K. and Taber K. S., (2009), Learners' mental models of the particle nature of matter: a study of 16-yearold Swedish science students, Int. J. Sci. Educ. , 31 (6), 757–786.
- Bahar M. and Hansell M., (2000), The relationship between some psychological factors and their effects on the performance of grid questions and word association tests, Educ. Psychol.: Int. J. Exp. Educ. Psychol. , 20 , 349–363.
- Bao L. and Redish E. F., (2006), Model analysis: representing and assessing the dynamics of student learning, Phys. Rev. Spec. Top. Phys. Educ. Res. , 2 (1), 010103.
- Bao L., Hogg K. and Zollman D., (2002), Model analysis of fine structures of student models: an example with Newton's third law, Am. J. Phys. , 70 (7), 766–778.
- Beaton D. E., Bombardier C., Guillemin F. and Ferraz M. B., (2000), Guidelines for the process of cross-cultural adaptation of self-report measures, Spine , 25 , 3186–3191.
- Chandran S., Treagust D. F. and Tobin K., (1987), The role of cognitive factors in chemistry achievement, J. Res. Sci. Teach. , 24 (2), 145–160.
- Chi M. T. H., (2005), Common sense conceptions of emergent processes: why some misconceptions are robust, J. Learn. Sci. , 14 (2), 161–199.
- Cokelez A., (2012), Junior High School Students' Ideas about the Shape and Size of the Atom, Res. Sci. Educ. , 42 , 673–686.
- Cokelez A. and Dumon A., (2005), Atom and molecule: upper secondary school French students' representations in long-term memory, Chem. Educ. Res. Pract. , 6 (3), 119–135.
- Danili E. and Reid N., (2006), Cognitive factors that can potentially affect pupils' test performance, Chem. Educ. Res. Pract. , 7 (2), 64–83.
- Didiş N., Eryılmaz A. and Erkoç Ş., (2014), Investigating students' mental models about the quantization of light, energy, and angular momentum, Phys. Rev. Spec. Top. Phys. Educ. Res. , 10 (2), 020127.
- diSessa A. A., (1993), Toward an epistemology of physics, Cognition Instruct. , 10 (2 & 3), 105–225.
- Fischler, H and Lichtfield M., (1992), Modern physics and students; conceptions, Int. J. Sci. Educ. , 14 (2), 181–190.
- Greek Pedagogical Institute, (2003), National Program of Study for Primary and Secondary Education: Science , Athens (Greece): Greek Pedagogical Institute Publications.
- Griffiths K. A. and Preston R. K., (1992), Grade-12 students' misconceptions relating to fundamental characteristics of atoms and molecules, J. Res. Sci. Teach. , 29 (6), 611–628.
- Harrison A. G. and Treagust D. F., (1996), Secondary students' mental models of atoms and molecules: implications for teaching chemistry, Sci. Educ. , 80 (5), 509–534.
- Harrison A. G. and Treagust D. F., (2000), Learning about atoms, molecules, and chemical bonds: a case study of multiple-model use in grade 11 chemistry, Sci. Educ. , 84 (3), 352–381.
- Hrepic Z., Zollman D. A. and Rebello N. S., (2010), Identifying students' mental models of sound propagation: the role of conceptual blending in understanding conceptual change, Phys. Rev. Spec. Top. Phys. Educ. Res. , 6 (2), 020114.
- Hu L. T. and Bentler P. M., (1999), Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives, Struct. Equat. Model. , 6 , 1–55.
- Itza-Ortiz S. F., Rebello S. and Zollman D., (2004), Students' models of Newton's second law in mechanics and electromagnetism, Eur. J. Phys. , 25 (1), 81.
- Johnstone A. H. and Al-Naeme F. F., (1995), Filling a curriculum gap in chemistry, Int. J. Sci. Educ. , 17 (2), 219–232.
- Justi R. and Gilbert J. K., (2000), History and philosophy of science through models: some challenges in the case of ‘the atom’, Int. J. Sci. Educ. , 22 (9), 993–1009.
- Kalkanis G., Hadzidaki P. and Stavrou D., (2003), An instructional model for a radical conceptual change towards quantum mechanics concepts, Sci. Educ. , 87 , 257–280.
- Kang S., Scharmann L. C., Noh T. and Koh H., (2005), The influence of students' cognitive and motivational variables in respect of cognitive conflict and conceptual change, Int. J. Sci. Educ. , 27 (9), 1037–1058.
- Kypraios N., Papageorgiou G. and Stamovlasis D., (2014), The Role of Some Individual Differences in Understanding Chemical Changes: a study in Secondary Education, Int. J. Envir. Sci. Educ. , 9 (4), 413–427.
- Lawson A. E., (1978), Development and validation of the classroom test of formal reasoning, J. Res. Sci. Teach. , 15 , 11–24.
- Lawson A. E., (1982), Formal reasoning, achievement, and intelligence: an issue of importance, Sci. Educ. , 66 (1), 77–83.
- Lawson A. E., (1985), A review of research on formal reasoning and science instruction, J. Res. Sci. Teach. , 22 , 569–617.
- Lawson A. E., (1993), Classroom test of scientific reasoning: revised paper-pencil edition , Tempe, AZ: Arizona State University.
- McKagan S. B., Perkins K. K. and Wieman C. E., (2008), Why we should teach the Bohr model and how to teach it effectively, Phys. Rev. Spec. Top. Phys. Educ. Res. , 4 , 010103.
- Muthén B. and Muthén L., (2012), Mplus User ' s Guide , 7th edn, Los Angeles, CA: Muthén and Muthén.
- Nakiboglu C., (2003), Instructional misconceptions of Turkish prospective chemistry teachers about orbitals and hybridization, Chem. Educ. Res. Pract. , 4 (2), 171–188.
- Niaz M., (1996), Reasoning strategies of students in solving chemistry problems as a function of developmental level, functional M-capacity and disembedding ability, Int. J. Sci. Educ. , 18 (5), 525–541.
- Nicoll G., (2001), A report of undergraduates' bonding misconceptions, Int. J. Sci. Educ. , 23 (7), 707–730.
- Palmer D., (1997), The effect of context on students' reasoning about forces, Int. J. Sci. Educ. , 19 (6), 681–696.
- Papaphotis G. and Tsaparlis G., (2008), Conceptual versus algorithmic learning in high school chemistry: the case of basic quantum chemical concepts. Part 2. Students' common errors, misconceptions and difficulties in understanding, Chem. Educ. Res. Pract. , 9 (4), 332–340.
- Park E. J. and Light G., (2009), Identifying Atomic Structure as a Threshold Concept: Student Mental Models and Troublesomeness, Int. J. Sci. Educ. , 31 (2), 233–258.
- Petri J. and Niedderer H., (1998), A learning pathway in high-school level quantum atomic physics, Int. J. Sci. Educ. , 20 (9), 1075–1088.
- Redish E. F. and Smith K. A., (2008), Looking beyond content: skill development for engineers, J. Engin. Educ. , 97 (3), 295–307.
- Sağlam M., (2010), University students' explanatory models of the interactions between electric charges and magnetic fields, Educ. Res. Rev. , 5 (9), 538–544.
- Stamovlasis D. and Papageorgiou G., (2012), Understanding Chemical Change in Primary Education: The Effect of two Cognitive Variables, J. Sci. Teach. Educ. , 23 (2), 177–197.
- Stefani C. and Tsaparlis G., (2009), Students' levels of explanations, models, and misconceptions in basic quantum chemistry: a phenomenographic study, J. Res. Sci. Teach. , 46 (5), 520–536.
- Stevens S. Y., Delgato C. and Krajcik J. S., (2010), Developing a hypothetical multi-dimensional learning progression for the nature of matter, J. Res. Sci. Teach. , 47 (6), 687–715.
- Taber K. S., (2002a), Conceptualizing quanta—illuminating the ground state of student understanding of atomic orbitals, Chem. Educ. Res. Pract. , 3 (2), 145–158.
- Taber K. S., (2002b), Compounding quanta: probing the frontiers of student understanding of molecular orbitals, Chem. Educ. Res. Pract. , 3 (2), 159–173.
- Taber K. S., (2003), The atom in the chemistry curriculum: fundamental concept, teaching model or epistemological obstacle? Foundat. Chem. , 5 (1), 43–84.
- Taber K. S., (2005), Learning quanta: barriers to stimulating transitions in student understanding of orbital ideas, Sci. Educ. , 89 (1), 94–116.
- Taber K. S., (2008), Conceptual resources for learning science: issues of transcience and grain-size in cognition and cognitive structure, Int. J. Sci. Educ. , 30 (8), 1027–1053.
- Teichert M. A., Tien L. T., Anthony S. and Rickey D., (2008), Effects of context on students' molecular-level ideas, Int. J. Sci. Educ. , 30 (8), 1095–1114.
- Tsaparlis G., (1997), Atomic and molecular structure in chemical education, J. Chem. Educ. , 74 (8), 922–925.
- Tsaparlis G., (2005), Non-algorithmic quantitative problem solving in university physical chemistry: a correlation study of the role of selective cognitive factors, Res. Sci. Technol. Educ. , 23 , 125–148.
- Tsaparlis G. and Papaphotis G., (2002), Quantum-chemical concepts: are they suitable for secondary students? Chem. Educ. Res. Pract. , 3 (2), 129–144.
- Tsaparlis G. and Papaphotis G., (2009), High-school students' conceptual difficulties and attempts at conceptual change: the case of basic quantum chemical concepts, Int. J. Sci. Educ. , 31 (7), 895–930.
- Tsitsipis G., Stamovlasis D. and Papageorgiou G., (2010), The effect of three cognitive variables on students' understanding of the particulate nature of matter and its changes of state, Int. J. Sci. Educ. , 32 (8), 987–1016.
- Tsitsipis G., Stamovlasis D. and Papageorgiou G., (2012), A probabilistic model for students' errors and misconceptions in relation to three cognitive variables, Int. J. Sci. Math. Educ. , 10 (4), 777–802.
- Vosniadou S. and Brewer W. F., (1992), Mental models of the earth, a study of conceptual change in childhood, Cognitive Psychol. , 24 , 535–585.
- Vosniadou S. and Brewer W. F., (1994), Mental models of the day/night cycle, Cognitive Sci. , 18 , 123–183.
- Wang C. Y. and Barrow L. H., (2013), Exploring conceptual frameworks of models of atomic structures and periodic variations, chemical bonding, and molecular shape and polarity: a comparison of undergraduate general chemistry students with high and low levels of content knowledge, Chem. Educ. Res. Pract. , 14 (1), 130–146.
- Witkin H. A., Oltman P. K., Raskin E. and Karp S. A., (1971), Embedded figures test, children ' s embedded figures test, group embedded figures test: manual , Palo Alto, CA: Consulting Psychologists Press.
- Reference Manager
- Simple TEXT file
People also looked at
Editorial article, editorial: the future of nuclear structure: challenges and opportunities in the microscopic description of nuclei.

- 1 Istituto Nazionale di Fisica Nucleare, Sezione di Napoli, Napoli, Italy
- 2 Physics Department and McDonnell Center for the Space Sciences at Washington University in St. Louis, St. Louis, MO, United States
- 3 Department of Physics, University of Surrey, Guildford, United Kingdom
- 4 Dipartimento di Fisica, Universita Degli Studi di Milano, Milano, Italy
- 5 INFN, Sezione di Milano, Milano, Italy
Editorial on the Research Article The Future of Nuclear Structure: Challenges and Opportunities in the Microscopic Description of Nuclei
The past two decades have witnessed tremendous progress in the microscopic description of atomic nuclei. Within this approach, nuclei are described in terms of nucleons interacting via realistic two- and three-body forces, constrained to accurately reproduce a large body of data for few nucleons systems. The goal of the nuclear theory community is to gain an accurate and predictable understanding of how the properties of many-body systems, along with their dynamics and structure, emerge from internucleon correlations induced by the strong interaction.
Progress in the microscopic (or, ab initio ) theory has been quite notable and it has been supported by two major pillars: First, thanks to the advent of Effective Field Theories (EFTs), we can now systematically develop nuclear Hamiltonians that are rooted in the fundamental properties and symmetries of the underlying theory of QCD. Second, advances in computational resources and novel powerful algorithms allow us to solve 1) the many-nucleon problem efficiently, and 2) quantify the degree of reliability of theoretical calculations and predictions. In many cases, microscopic computations achieve an accuracy that is comparable or superior to the precision delivered by current EFT interactions. This sparked a renewed interest to further broaden the focus of ab initio theory and address open problems in nuclear physics.
While the status of the first pillar has been recently discussed by “The Long-Lasting Quest for Nuclear Interactions: The Past, the Present and the Future” Topical Review on this Journal, here we focus on the exciting new developments in microscopic theory. At present, ab initio computations of nuclear structure include up to medium-mass isotopes. The heaviest systems currently reached—with different degrees of accuracy—have mass number A ≈ 140 . These computational limits are constantly being pushed forward. At the same time, the community is expanding into new directions, in particular toward the study of electroweak observables and nuclear reactions, that nowadays require predictions with an accuracy never reached before for similar mass ranges.
In collecting the contributions for this Research Topic, we sought to gather contributions from authors who could summarize the current state-of-the-art microscopic calculations in Nuclear Theory, favoring a selected but broad view over an attempt to cover every application. All presented contributions stem from well-established methods in computational nuclear structure, and indicate recent theoretical advances and prospective outlooks, challenges and opportunities for Nuclear Theory. Most importantly, it is our hope that this collection will confer a big picture, including references to basic material, that will be valuable for young researches who intend to enter this exciting discipline.
The richness of applications in modern ab initio nuclear theory can be appreciated in Hergert ’s contribution that provides us with a general overview of the most successful microscopic many-body approaches currently in use. Traditionally, the refinement and sophistication of these computational tools has given fundamental support to advance the theories of nuclear forces. Quantum Monte Carlo (QMC) techniques allow to solve the many-body Schrödinger equation with high accuracy for light nuclei up to masses A ∼ 16–40. Gandolfi et al. discuss the use of QMC methods (namely, Variational, Green’s Function, and Auxiliary Diffusion Monte Carlo methods) in combination with local chiral interactions in coordinate space. QMC methods are used in lattice effective field theory, where the EFT Lagrangian is implemented in momentum space with nucleons and pions placed on a lattice. Lee discusses the basic features of this approach and its high potential for understanding clustering phenomena.
For heavier isotopes, ab initio theories can be pushed to masses A ∼ 140 provided that one retains only the relevant nuclear excitations, as it is done through all-orders resummations. Among these methods, the self-consistent Green’s function (SCGF) theory gives direct access to the spectral information probed by a wide range of experiments as reviewed in detail by Somà ’s contributions. Once in the region of the nuclear chart that corresponds to medium masses, open shell isotopes become the next challenge to be addressed by the theory. In fact, resolving the degeneracy in uncorrelated systems requires large scale configuration mixing. Coraggio and Itaco demonstrate how this can be handled by projecting the correlated many-body states into a shell model Hamiltonian, using the so-called “Q-box” formalism. A similar strategy is shared by other computational frameworks, such as coupled cluster and in-medium SRG, that are touched upon in the contribution by Hergert . A less conventional approach to open shells is to break SU(1) symmetry (in short, allowing for breaking particle number conservation). This is discussed by Somà within SCGFs and by Tichai et al. in the framework of many-body perturbation theory.
The remainder of this topical review focuses on selected open challenges in Nuclear Theory that require an ab initio approach. Two contributions show different aspect of studying infinite nucleon systems and the implications for astrophysical scenarios. Tews covers QMC calculations of the equation of state (EoS) of dense matter in neutron stars. With the recent observation of star mergers and the birth of multi-messenger astronomy, it has become of prime importance to understand the finite temperature properties of the EoS. Rios discusses this topic and how the structure of neutron matter depends on temperature, using SCGF theory.
In the quest for physics beyond the Standard Model, Nuclear Theory, and in particular accurate calculations of neutrino-nucleus interactions at all energy scaler, plays a crucial role. This is carefully analyzed by Rocco ’s contribution that address this challenge with emphasis on impacts to neutrino oscillations experimental programs. The last contribution of this Topical Review addresses one of the hardest open challenges in the interpretation of experimental data: the lack of a truly first-principles theory that can describe consistently both structure and reaction processes. Rotureau highlights recent steps in deriving an ab inito optical potential using the coupled cluster method (that, together with SCGF, is one of the two possible approaches to this problem).
We are really grateful to all the scientists participating in this project and hope that the reader will enjoy this Topical Review.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor declared a past co-authorship with one of the authors SP.
Keywords: Ab initio (calculations), nuclear theory, nuclear reactions, effective field theories, many-body physics, nuclear structure
Citation: Coraggio L, Pastore S and Barbieri C (2021) Editorial: The Future of Nuclear Structure: Challenges and Opportunities in the Microscopic Description of Nuclei. Front. Phys. 8 :626976. doi: 10.3389/fphy.2020.626976
Received: 07 November 2020; Accepted: 20 November 2020; Published: 05 February 2021.
Edited and Reviewed by:
Copyright © 2021 Coraggio, Pastore and Barbieri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luigi Coraggio, [email protected] ; Saori Pastore, [email protected] ; Carlo Barbieri, [email protected]
This article is part of the Research Topic
The Future of Nuclear Structure: Challenges and Opportunities in the Microscopic Description of Nuclei

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

2.2: Atomic Structure
- Last updated
- Save as PDF
- Page ID 221331
Learning Objectives
- State the modern atomic theory.
- Learn how atoms are constructed.
The smallest piece of an element that maintains the identity of that element is called an atom . Individual atoms are extremely small. It would take about fifty million atoms in a row to make a line that is 1 cm long. The period at the end of a printed sentence has several million atoms in it. Atoms are so small that it is difficult to believe that all matter is made from atoms—but it is.
The concept that atoms play a fundamental role in chemistry is formalized by the modern atomic theory , first stated by John Dalton, an English scientist, in 1808. It consists of three parts:
- All matter is composed of atoms.
- Atoms of the same element are the same; atoms of different elements are different.
- Atoms combine in whole-number ratios to form compounds.
These concepts form the basis of chemistry. Although the word atom comes from a Greek word that means "indivisible," we understand now that atoms themselves are composed of smaller parts called subatomic particles . The first part to be discovered was the electron , a tiny subatomic particle with a negative charge. It is often represented as e − , with the right superscript showing the negative charge. Later, two larger particles were discovered. The proton is a more massive (but still tiny) subatomic particle with a positive charge, represented as p + . The neutron is a subatomic particle with about the same mass as a proton, but no charge. It is represented as either n or n 0 . We now know that all atoms of all elements are composed of electrons, protons, and (with one exception) neutrons. Table \(\PageIndex{1}\) summarizes the properties of these three subatomic particles.
How are these particles arranged in atoms? They are not arranged at random. Experiments by Ernest Rutherford in England in the 1910s pointed to a nuclear model with at oms that has the protons and neutrons in a central nucleus with the electrons in orbit about the nucleus . The relatively massive protons and neutrons are collected in the center of an atom, in a region called the nucleus of the atom (plural nuclei ). The electrons are outside the nucleus and spend their time orbiting in space about the nucleus. (Figure \(\PageIndex{1}\)).
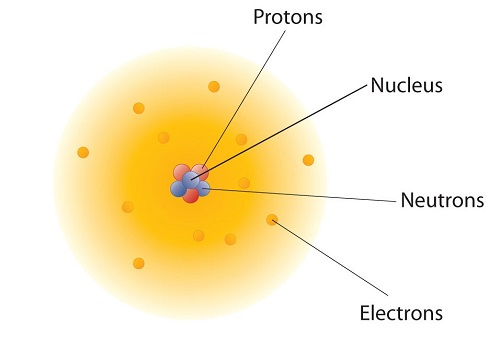
The modern atomic theory states that atoms of one element are the same, while atoms of different elements are different. What makes atoms of different elements different? The fundamental characteristic that all atoms of the same element share is the number of protons . All atoms of hydrogen have one and only one proton in the nucleus; all atoms of iron have 26 protons in the nucleus. This number of protons is so important to the identity of an atom that it is called the atomic number. The number of protons in an atom is the atomic number of the element. Thus, hydrogen has an atomic number of 1, while iron has an atomic number of 26. Each element has its own characteristic atomic number.
Atoms of the same element can have different numbers of neutrons, however. Atoms of the same element (i.e., atoms with the same number of protons) with different numbers of neutrons are called isotopes . Most naturally occurring elements exist as isotopes. For example, most hydrogen atoms have a single proton in their nucleus. However, a small number (about one in a million) of hydrogen atoms have a proton and a neutron in their nuclei. This particular isotope of hydrogen is called deuterium. A very rare form of hydrogen has one proton and two neutrons in the nucleus; this isotope of hydrogen is called tritium. The sum of the number of protons and neutrons in the nucleus is called the mass number of the isotope.
Neutral atoms have the same number of electrons as they have protons, so their overall charge is zero. However, as we shall see later, this will not always be the case.
Example \(\PageIndex{1}\):
- The most common carbon atoms have six protons and six neutrons in their nuclei. What are the atomic number and the mass number of these carbon atoms?
- An isotope of uranium has an atomic number of 92 and a mass number of 235. What are the number of protons and neutrons in the nucleus of this atom?
- If a carbon atom has six protons in its nucleus, its atomic number is 6. If it also has six neutrons in the nucleus, then the mass number is 6 + 6, or 12.
- If the atomic number of uranium is 92, then that is the number of protons in the nucleus. Because the mass number is 235, then the number of neutrons in the nucleus is 235 − 92, or 143.
Exercise \(\PageIndex{1}\)
The number of protons in the nucleus of a tin atom is 50, while the number of neutrons in the nucleus is 68. What are the atomic number and the mass number of this isotope?
Atomic number = 50, mass number = 118
When referring to an atom, we simply use the element's name: the term sodium refers to the element as well as an atom of sodium. But it can be unwieldy to use the name of elements all the time. Instead, chemistry defines a symbol for each element. The atomic symbol is a one- or two-letter representation of the name of an element. By convention, the first letter of an element's symbol is always capitalized, while the second letter (if present) is lowercase. Thus, the symbol for hydrogen is H, the symbol for sodium is Na, and the symbol for nickel is Ni. Most symbols come from the English name of the element, although some symbols come from an element's Latin name. (The symbol for sodium, Na, comes from its Latin name, natrium .) Table \(\PageIndex{2}\) lists some common elements and their symbols. You should memorize the symbols in Table \(\PageIndex{2}\), as this is how we will be representing elements throughout chemistry.
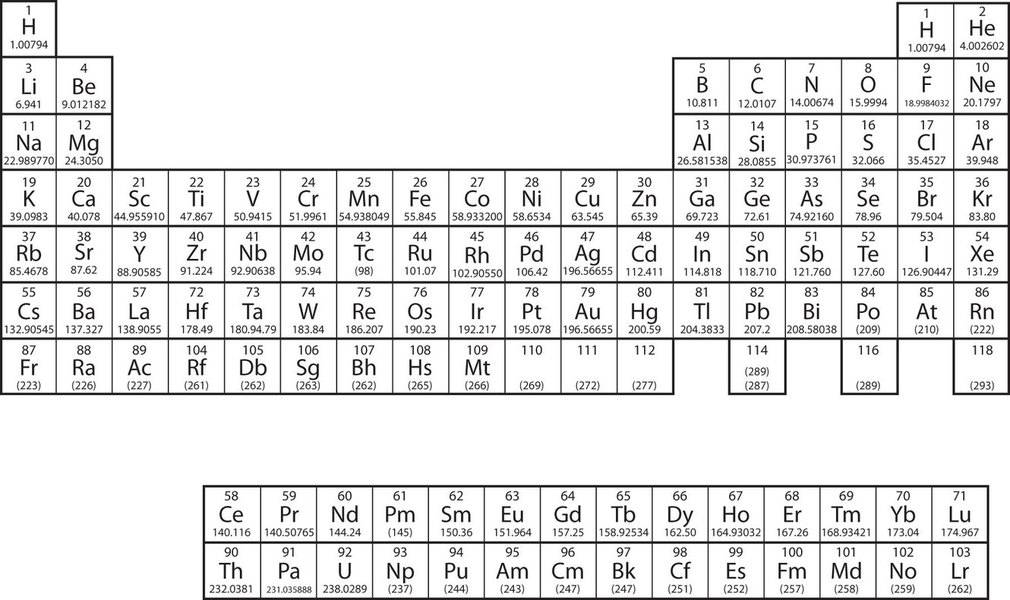
The elements are grouped together in a special chart called the periodic table of all the elements . A simple periodic table is shown in Figure \(\PageIndex{2}\), while one may view a more extensive periodic table from another source. The elements on the periodic table are listed in order of ascending atomic number. The periodic table has a special shape that will become important to us when we consider the organization of electrons in atoms (Chapter 8). One immediate use of the periodic table helps us identify metals and nonmetals. Nonmetals are in the upper right-hand corner of the periodic table, on one side of the heavy line splitting the right-side part of the chart. All other elements are metals.
There is an easy way to represent isotopes using the atomic symbols. We use the construction:
\[\ce{_{Z}^{A}X}\nonumber \]
where \(X\) is the symbol of the element, \(A\) is the mass number, and \(Z\) is the atomic number. Thus, for the isotope of carbon that has 6 protons and 6 neutrons, the symbol is:
\[\ce{_{6}^{12}C}\nonumber \]
where \(C\) is the symbol for the element, 6 represents the atomic number, and 12 represents the mass number.
Example \(\PageIndex{2}\):
- What is the symbol for an isotope of uranium that has an atomic number of 92 and a mass number of 235?
- How many protons and neutrons are in \(\ce{_{26}^{56}Fe}\)
- The symbol for this isotope is \(\ce{_{92}^{235}U}\)
- This iron atom has 26 protons and 56 − 26 = 30 neutrons.
Exercise \(\PageIndex{2}\)
How many protons are in \(\ce{_{11}^{23} Na}\)
It is also common to state the mass number after the name of an element to indicate a particular isotope. Carbon-12 represents an isotope of carbon with 6 protons and 6 neutrons, while uranium-238 is an isotope of uranium that has 146 neutrons.

A Short History of the Atomic Structure
The basic idea that matter is made up of tiny indivisible particles is very old, appearing in many ancient cultures such as Greece and India. The word atom is derived from the ancient Greek word atomos , which means "indivisible". This ancient idea was based on philosophical reasoning rather than scientific reasoning, and modern atomic theory was developed throughout a few centuries of research and experimentation.
The first atomic theory based on experimentation was stated by John Dalton, an English scientist, in 1808. By studying the chemical composition of different oxides, Dalton noticed that elements combined in very specific patterns given by small whole numbers. This observation prompted him to support that all matter is made of atoms, although Dalton had no idea about the actual structure of the atoms.
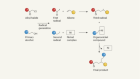
In 1897, J. J. Thomson discovered that the existence of the first subatomic particle. He renamed these new negatively charged particles as electrons . To explain the overall neutral charge of the atom, Thomson concluded that these electrons must be embedded in an uniform sea of positive charge. In this "plum pudding atomic model", the electrons were seen as embedded in the positive charge like raisins in a plum pudding.
Between 1908 and 1913, Ernest Rutherford and his colleagues Hans Geiger and Ernest Marsden came to have doubts about the Thomson model. Rutheford performed a series of experiments in which they bombarded thin foils of metal with positively charged alpha particles. They spotted alpha particles being deflected by angles greater than 90°. To explain this, Rutherford proposed that the positive charge of the atom is not distributed throughout the atom's volume as Thomson believed, but is concentrated in a tiny nucleus at the center. Rutherford's atomic model introduced the existence of the positively charged nucleus surrounded by the negatively charged electrons.
In 1913, after studying atomic spectra produced by elements. the physicist Niels Bohr proposed a model in which the electrons of an atom were assumed to orbit the nucleus but could only do so in a finite set of orbits, and could jump between these orbits only in discrete changes of energy corresponding to absorption or radiation of a photon. This quantized atomic model, also known as the planetary model of the atom, was used to explain why the electrons' orbits are stable and why elements absorb and emit electromagnetic radiation in discrete lines. The Bohr model of the atom was the first complete physical model of the atom. It described the overall structure of the atom and how atoms bond to each other. Bohr's planetary atomi model was not perfect and was soon superseded by the more accurate Schrödinger model, but it is sufficient to understand most chemical and physical properties discussed in this course.
In 1924, Louis de Broglie had proposed that all particles behave like waves to some extent, including the electron. In 1926 Erwin Schrödinger used this idea to develop the Schrödinger model of the atom. In this model, electrons are considered electromagnetic waves mechanics rather than particles. According to this model, it is mathematically impossible to obtain precise values for both the position and the energy (momentum) of an electron at a given point in time. This idea became the uncertainty principle, formulated by Werner Heisenberg in 1927. Because it is impossible to determine the position and the energy of an electron in an atom, Schrödinger's atomic model is based on the "probability" of finding an electron in a certain region around the nucleus. Thus, the orbits in the planetary model of the atom were discarded in favor of atomic orbitals, which are zones around the nucleus where a given electron is most likely to be observed.

Figure \(\PageIndex{2}\):A summary of atomic models. Image by Compound Interest (2016) https://www.compoundchem.com/2016/10/13/atomicmodels/. Shared under CC BY-NC-ND 4.0 license
Key Takeaways
- Chemistry is based on the modern atomic theory, which states that all matter is composed of atoms.
- Atoms themselves are composed of protons, neutrons, and electrons.
- Each element has its own atomic number, which is equal to the number of protons in its nucleus.
- Isotopes of an element contain different numbers of neutrons.
- Elements are represented by an atomic symbol.
- The periodic table is a chart that organizes all the elements.
Citations and attributions
" Atomic Theory" by LibreTexts is licensed under CC BY-NC-SA .
Wikipedia contributors. (2021, May 6). Atom. In Wikipedia, The Free Encyclopedia . Retrieved 15:20, May 18, 2021, from https://en.wikipedia.org/w/index.php?title=Atom&oldid=1021695004
Wikipedia contributors. (2021, May 8). J. J. Thomson. In Wikipedia, The Free Encyclopedia . Retrieved 15:21, May 18, 2021, from https://en.wikipedia.org/w/index.php?title=J._J._Thomson&oldid=1022092604
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
- We're Hiring!
- Help Center
Atomic Structure
- Most Cited Papers
- Most Downloaded Papers
- Newest Papers
- Save to Library
- Last »
- X-ray production Follow Following
- Nucleic Acid Follow Following
- Theoretical and Space Physics Follow Following
- Space Physics Follow Following
- Organic Compound Follow Following
- Hyperfine Structure Follow Following
- Electromagnetic Field Follow Following
- Theoretical Physics Follow Following
- Amino Acid Profile Follow Following
- Mathematical Sciences Follow Following
Enter the email address you signed up with and we'll email you a reset link.
- Academia.edu Publishing
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024
Historical Teaching of Atomic and Molecular Structure
- First Online: 30 December 2013
Cite this chapter

- José Antonio Chamizo 2 &
- Andoni Garritz 2
6296 Accesses
1 Citations
1 Altmetric
Besides the presentation and conclusions, the chapter is divided into two equally important sections. The first one describes the modern development of atomic and molecular structure, emphasising some of the philosophical problems that have been taken, and those that have to be faced in its understanding. The second discusses the alternative conceptions and difficulties of students of different educational levels and also the different approaches to its historical or philosophical teaching. Finally, we recognise the necessity for science teachers to assume a specific historical-philosophical position.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
- Durable hardcover edition
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
See also Moreno-Ramírez et al. ( 2010 ).
In German he says ‘Die Energie eines Resonators ändert sich durch Absorption und Emision sprungweise, und zwar ein ganzzahliges Vielfache von (R/N)βν’ (Einstein 1906 , p. 202).
See, for example, Lee et al. ( 1993 ), Novick and Nussbaum ( 1978 , 1981 ), Nussbaum ( 1985 ), Valanides ( 2000 ), and Wightman et al. ( 1987 ).
As can be seen in Birk and Kurtz ( 1999 ), Boo ( 1998 ), Furió and Calatayud ( 1996 ), Griffiths and Preston ( 1992 ), Hund ( 1977 ), Kutzelnigg ( 1984 ), Magnasco ( 2004 ), Özmen ( 2004 ), and Sutcliffe ( 1996 ).
For example, Coll and Treagust ( 2002 ), Niaz ( 2001 ), and Peterson et al. ( 1989 ).
Such as in Coll and Treagust ( 2003a ) and De Posada ( 1997 , 1999 ).
See, for example, Butts and Smith ( 1987 ), Coll and Treagust ( 2003b ), and Taber ( 1994 , 1997 ).
Such as Dobson et al. ( 2000 ), Petri and Niedderer ( 1998 ), Shiland ( 1995 , 1997 ), and Tsaparlis and Papaphotis ( 2002 , 2009 ).
For example, Hadzidaki et al. ( 2000 ), Johnston et al. ( 1998 ), Kalkanis et al. ( 2003 ), Michelini et al. ( 2000 ), Paoloni ( 1982 ), and Wittmann et al. ( 2002 ).
As can be seen in Ardac ( 2002 ), Melrose and Scerri ( 1996 ), Niaz and Fernández ( 2008 ), and Scerri ( 1991 ).
For example, Cervellati and Perugini ( 1981 ), Conceicao and Koscinski ( 2003 ), Ogilvie ( 1994 ), Scerri ( 2000a ), Taber ( 2002a , b ; 2005 ), and Tsaparlis ( 1997a ).
For example, Buchwald and Warwick ( 2001 ), Giunta ( 2010 ), Marinacci ( 1995 ), Nye ( 1993 ), Snow ( 1981 ), and Toulmin and Goodfield ( 1962 ).
Achinstein, P. (2001). Who really discovered the electron? In Buchwald J.Z. & Warwick A. (eds.) Histories of the Electron. The Birth of Microphysics , (Chapter 13 pp. 403–424), Cambridge, Massachusetts: The MIT Press.
Google Scholar
Adúriz-Bravo A. (2012) A ‘Semantic’ View of Scientific Models for Science Education, Science & Education , Online First, 17 January.
Anderson, P. W. (1972). More Is Different, Science , 177(4047), 393–396. Aug. 4.
Arabatzis, T. (2001). The Zeeman Effect and the Discovery of the Electron? In Buchwald J.Z. & Warwick A. (eds.) Histories of the Electron. The Birth of Microphysics , (Chapter 5 pp. 171–193), Cambridge, Massachusetts: The MIT Press.
Ardac, D. (2002). Solving quantum number problems: An examination of novice performance in terms of conceptual based requirements, Journal of Chemical Education , 79(4), 510–3.
Atkins, P., de Paula, J., & Friedman, R. (2008). Quanta, Matter and Change: A Molecular Approach to Physical Chemistry , Oxford: Oxford University Press.
Ayar, M., & Yalvac, B. (2010). A sociological standpoint to authentic scientific practices and its role in school science teaching, Ahi Evran Uni. Kirsehir Journal of Education (KEFAD) 11, 113–127.
Baggott, J. (2011). The Quantum Story. A History in 40 Moments . Oxford: Oxford University Press.
Bensaude-Vincent, B. (1999). Atomism and Positivism: A legend about French Chemistry, Annals of Science , 56, 81–94.
Bent, H. A. (1984). Should orbitals be X-rated in beginning chemistry courses? Journal of Chemical Education , 61(5), 421–423.
Birk, J., & Kurtz, M. (1999). Effect of experience on retention and elimination of misconceptions about molecular structure and bonding, Journal of Chemical Education , 76(1), 124–128.
Bishop, D. M. (1973). Group theory and chemistry , Oxford, UK: Clarendon Press.
Boo, H. K. (1998). Students’ Understandings of Chemical Bonds and the Energetics of Chemical Reactions, Journal of Research in Science Teaching , 35(5), 569–581.
Branch, G.E.K. (1984). Gilbert Newton Lewis, 1875–1946, Journal of Chemical Education , 61(1), 18–21.
Bucat, R., & Mocerino, M. (2009). Learning at the Sub-micro Level: Structural Representations, in Gilbert, J. K. & Treagust, D. (Eds.) Multiple Representations in Chemical Education , (Chapter 1, pp. 11–29), Secaucus, NJ, USA: Springer.
Buchwald, J. Z. & Warwick, A. (ed) (2001). Histories of the electron. The Birth of microphysics , Cambridge Massachusetts: The MIT Press.
Butts, B., & Smith, R. (1987). HSC chemistry students’ understanding of the structure and properties of molecular and ionic compounds, Research in Science Education , 17, 192–201.
Campbell, J. A. (1962). Chemical Education Material Study . Berkeley, CA, USA: Lawrence Hall of Science.
Cervellati, R. & Perugini, D. (1981). The understanding of the atomic orbital concept by Italian high school students, Journal of Chemical Education , 58(7), 568–9.
Chalmers, A. (1998). Retracing the Ancient Steps to atomic theory, Science & Education , 7(1), 69–84.
Chamizo, J.A. (1992). El maestro de lo infinitamente pequeño . John Dalton [The master of the infinitely small. John Dalton], México: Conaculta-Pangea.
Chamizo, J.A. (2001) El curriculum oculto en la enseñanza de la química, Educación Química , 12(4), 194–198.
Chamizo, J. A. (2007). Teaching modern chemistry through ‘historical recurrent teaching models’, Science & Education , 16(2), 197–216.
Chamizo, J.A. (2011). A new definition of Models and Modelling for chemistry Teaching, Science & Education OnLine First 01 November, special issue on [Philosophical Considerations in Teaching of Chemistry] edited by Sibel Erduran.
Chamizo, J.A. (2012). Heuristic Diagrams as a Tool to teach History of Science, Science & Education , 21(5), 745–762. OnLine First 23th August, 2011.
Christie, M. & Christie, J. R. (2000). ‘Laws’ and ‘Theories’ in Chemistry Do not Obey The rules in Bhushan N. & Rosenfeld S. (ed) Of Minds and Molecules. New Philosophical Perspectives on Chemistry , New York: Oxford University Press.
Coll, R. K., & Treagust, D. F. (2002). Exploring tertiary students’ understanding of covalent bonding, Research in Science and Technological Education , 20, 241–267.
Coll, R. K., & Treagust, D. F. (2003a). Learners’ mental models of metallic bonding: A cross-age study, Science Education , 87(5), 685–707.
Coll, R. K., & Treagust, D. F. (2003b). Investigation of secondary school, undergraduate, and graduate learners’ mental models of ionic bonding, Journal of Research in Science Teaching , 40(5), 464–486.
Conceicao, J., & Koscinski, J. T. (2003). Exploring Atomic and Molecular Orbital in Freshman Chemistry using Computational Chemistry, The Chemical Educator , 8, 378–382.
Cotton, F. A. (1963). Chemical Applications of Group Theory , New York: John Wiley & Sons.
Cruz, D., Chamizo, J. A. y Garritz, A. (1986). Estructura atómica. Un enfoque químico [Atomic structure. A chemical approach], Wilmington, DE, USA: Addison Wesley Iberoamericana.
De Posada, J. M. (1997). Conceptions of high school students concerning the internal structure of metals and their electric conduction: structure and evolution, Science Education , 81(4), 445–467.
De Posada, J. M. (1999). The presentation of metallic bonding in high school science textbooks during three decades: science educational reforms and substantive changes of tendencies, Science Education , 83, 423–447.
Develaki, M. (2007). ‘The Model-Based view of Scientific Theories and the structuring of school science, Science & Education , 16(7–8), 725–749.
Didis, N. & SakirErkoc, S. (2009). ‘History of Science for Science Courses: “Spin” Example from Physics, Latin American Journal of Physics Education , 3, 9–12.
Dirac, P.A.M. (1929). Quantum Mechanics of Many-Electron Systems, Proceedings of the Royal Society (London) A123, 714–733.
Dobson, K., Lawrence, I., & Britton, P. (2000). The A to B of quantum physics, Physics Education , 35, 400–5.
Doyle M. (ed) (1993). Historical Science Experiments on File, Facts on File , New York.
Duschl, R. A. (1994). Research on the History and Philosophy of Science, in Gabel D. (Ed.) Handbook of Research on Science Teaching and Learning , (pp. 443–465) New York: MacMillan.
Early, J. E. (2004). Would Introductory Chemistry Courses work better with a new Philosophical basis? Foundations of Chemistry , 6, 137–160.
Echeverria, J. Introducción a la Metodología de la Ciencia , [Introduction to Science’s Methodology] Madrid: Cátedra, 2003.
Eggen, P.O., Kvittingen, L., Lykknes, A., & Wittje, R. (2012). Reconstructing Iconic Experiments on Electrochemistry: Experiences from a History of Science Course. Science & Education , 21, 179–189.
Einstein, A. (1906). Zur Theorie der Lichterzeugung und Lichtabsorption, Annals of Physics , 325, 199–206.
Einstein, A. (1909). Zum gegenwärtigen Stand des Strahlungsproblems, Phys. Zeitschr. 10, 185–193.
Einstein, A. (1926; 1944; 1948). Letters to Max Born; The Born-Einstein Letters, translated by Irene Born , New York: Walker and Company, 1971. Taken from the URL http://www.spaceandmotion.com/quantum-theory-albert-einstein-quotes.htm
Erduran, S., & Scerri, E. (2002). ‘The nature of chemical knowledge and chemical education’, in Gilbert J.K. et al. (eds.) Chemical Education: Towards Research-based Practice , Kluwer, Dordrecht.
Erduran, S. (2005). Applying the Philosophical Concept of Reduction to the Chemistry of Water: Implications for Chemical Education, Science & Education , 14: 161–171.
Feldman, B. (2001). The Nobel Prize: A History of Genius, Controversy, and Prestige , New York, USA: Arcade Publishing, Reed Business Information, Inc.
Feynman, R. (1985). The Strange Theory of Light and Matter . London: Penguin.
Furió, C. & Calatayud, M. L. (1996). Difficulties with the Geometry and Polarity of Molecules. Beyond Misconceptions, Journal of Chemical Education , 73(1), 36–41.
Gagliardi, R. (1988) Cómo utilizar la historia de las ciencias en la enseñanza de las ciencias, [How to use history of sciences in the teaching of sciences], Enseñanza de las Ciencias , 6, 291–296.
Garritz, A. (2013). Teaching the Philosophical Interpretations of Quantum Mechanics and Quantum Chemistry through Controversies. Accepted for publication in the special issue on [Philosophical Considerations in Teaching of Chemistry] edited by Sibel Erduran, Science & Education , 22(7), 1787–1808.
Gault, C. (1991) History of science, individual development and science teaching, Research in Science Education , 21, 133–140.
Gell-Mann, M. (1994). The Quark and the Jaguar: adventures in the simple and the complex , New York, USA: Freeman.
Giere, R. N. (1999). Science without laws , Chicago, USA: University of Chicago Press.
Gilbert, J. K. (2006). On the Nature of “Context” in Chemical Education, International Journal of Science Education , 28(9), 957–976.
Gillespie, R. J. (1991). What is wrong with the general chemistry course? Journal of Chemical Education , 68(3), 192–4.
Giunta, C. (2010). Atoms in Chemistry: From Dalton’s predecessors to Complex Atoms and Beyond , American Chemical Society-Oxford University Press, Washington.
Griffiths, A. K., & Preston, K. R. (1992). Grade-12 students’ misconceptions relating to fundamental characteristics of atoms and molecules, Journal of Research in Science Teaching , 29, 611–628.
Grosslight, L., Unger, C., Jay, E., & Smith, C. (1991). Understanding models and their use in scienceconceptions of middle and high school students and experts. Journal of Research in Science Teaching , 28, 799–822.
Hacking, I. (1983). Representing and Intervening , Cambridge, UK: Cambridge University Press.
Hadzidaki, P., Kalkanis, G. & Stavrou, D. (2000). Quantum mechanics: A systemic component of the modern physics paradigm, Physics Education , 35, 386–392.
Hargittai, M. & Hargittai, I. (2009). Group Symmetry through the Eyes of a Chemist , 3rd edition, Dordrecht, The Netherlands: Springer.
Harré, R. (2004). Modelling: Gateway to the Unknown , Amsterdam: Elsevier.
Harris, D. C. & Bertolucci, M. D. (1978). Symmetry and spectroscopy. An introduction to vibrational and electronic spectroscopy , New York: Dover.
Harrison, A. G., & Treagust, D. F. (1996). Secondary students’ mental models of atoms and molecules: Implications for teaching science, Science Education , 80, 509–534.
Hawkes, S. J. (1992). Why should they know that? Journal of Chemical Education , 69(3), 178–181.
Heilbron, J. L. & Kuhn, T. S. (1969). The Genesis of the Bohr Atom, Historical Studies in the Physical Sciences . 1(3–4), 211–290.
Herrestein-Smith, B. (1981). Narrative Versions, Narrative Theories. In W. Mitchel (Ed.), On Narrative , (pp 209–232) Chicago: University of Chicago Press.
Hoffmann, R. (1998) Qualitative thinking in the age of modern computational chemistry-or what Liones Salem knows, Journal of Molecular Structure , 424: 1–6
Hohenberg, P. & Kohn, W. (1964). Inhomogeneous electron gas, Physical Review , 136, B864–71.
Holbrow, C. H., Amato, J. C., Galvez, E. J. & Lloyd, J. N. (1995). Modernizing Introductory Physics, American Journal of Physics , 63, 1078–1090.
Hund, F. (1977). Early History of the Quantum Mechanical Treatment of the Chemical Bond, Angewandte Chemie , International Edition in English, 16, 87–91.
Husbands, C. (2003). What is history teaching? Language, ideas and meaning in learning about the past. Buckingham: Open University Press.
Izquierdo, M. & Adúriz, A. (2009). Physical construction of the chemical atom: Is it Convenient to go All the Way Back? Science & Education , 18(3–4), 443–455.
Izquierdo, M. (2010). La transformación del átomo químico en una partícula física ¿se puede realizar el proceso inverso? In Chamizo J.A. (ed) Historia y Filosofía de la Química [History and philosophy of chemistry], (pp 195–209) México: Siglo XXI-UNAM.
Jensen, W. B. (1980). The Lewis acid–base concepts , New York, Wiley.
Jensen, W. B. (1998). Logic, History, and the Chemistry Textbook. I. Does Chemistry Have a Logical Structure? Journal of Chemical Education , 75(6), 679–687; II. Can We Unmuddle the Chemistry Textbook? 75(7), 817–828; III. One Chemical Revolution or Three? 75(8), 961–969.
Jensen, W.B (ed) (2002). Mendeleev on the Periodic Law. Selected Writings, 1869–1905 , New York, Dover.
Jensen, W.B. (2010). Four Centuries of Atomic Theory in Giunta C. (ed) Atoms in Chemistry: From Dalton’s predecessors to Complex Atoms and Beyond , American Chemical Society-Oxford University Press, Washington.
Jensen, W. P., Palenik, G. J., & Suh, I. (2003). The History of Molecular Structure Determination Viewed through the Nobel Prizes, Journal of Chemical Education , 80(7), 753–761.
Johnston, I. D., Crawford, K., & Fletcher, P. R. (1998). Student difficulties in learning quantum mechanics, International Journal of Science Education , 20(5), 427–446.
Justi, R., & Gilbert, J. (2000). History and philosophy of science through models: some challenges in the case of ‘the atom, International Journal of Science Education , 22(9), 993–1009.
Kalkanis, G., Hadzidaki, P., & Stavrou, D. (2003). An instructional model for a radical conceptual change towards quantum mechanics concepts, Science Education , 87, 257–280.
Karakostas, V. & Hadzidaki, P. (2005). Realism vs. Constructivism in Contemporary Physics: The Impact of the Debate on the Understanding of Quantum Theory and its Instructional Process, Science & Education , 14(7–8), 607–629.
Kauffman, G. B. & Kauffman, L. M. (1996). An Interview with Linus Pauling, Journal of Chemical Education , 73(1), 29–32.
Kauffman, G. B. (1999). From Triads to Catalysis: Johann Wolfgang Döbereiner (1780–1849) on the 150th Anniversary of His Death, The Chemical Educator , 4, 186–197.
Kauffman, G. B. (2004). Sir William Ramsay: Noble Gas Pioneer. On the 100th Anniversary of His Nobel Prize, The Chemical Educator , 9, 378–383.
Kauffman, G. B. (2006). Radioactivity and Isotopes: A Retrospective View of Frederick Soddy (1877.1956) on the 50th Anniversary of His Death, The Chemical Educator , 11, 289–297.
Kauffman, G. B. (2010). The 150th Anniversary of the First International Congress of Chemists, Karlsruhe, Germany, September 3–5, 1860, The Chemical Educator , 15, 309–320.
Klassen, S. (2007). The Construction and Analysis of a Science Story: A Proposed Methodology, Proccedings of the International History and Philosophy of Science Teaching Group Conference , Calgary, Canada.
Klassen, S. (2008). The Photoelectric Effect: Rehabilitating the Story for the Physics Classroom’ Proceedings of the Second International Conference on Story in Science Teaching , Munich, Germany.
Kleppner, D., & Jackiw, R. (2000). One Hundred Years of Quantum Physics, Science , 289(5481), 893–898.
Kohn, W., & Sham, L. J. (1965). Self-consistent equations including exchange and correlation effects, Physical Review ,140, A1133–8.
Kuhn, T. S. (1969). The structure of scientific revolutions , Chicago: University of Chicago Press.
Kuhn, T. S. (1978). Black-Body Theory and the Quantum Discontinuity 1894–1912 , Oxford, UK: Oxford University Press.
Kutzelnigg, W. (1984). Chemical Bonding in Higher Main Group Elements, Angew. Chem. Int. Ed. Engl. 23, 272–295.
Langmuir, I. (1919). The Arrangement of Electrons in Atoms and Molecules, J.Am. Chem.Soc , 41, 868–934
Laloë, F. (2001). Do we really understand quantum mechanics? Strange correlations, paradoxes, and theorems, American Journal of Physics , 69, 655–701.
Laudan, L. (1997). Progress and its Problems: Toward a theory of scientific growth , Berkeley: University of California Press.
Lee, O., Eichinger, D. C., Anderson, C. W., Berkheimer, G. D., & Blakeslee, T.D. (1993). Changing Middle School Student’s Conception of Matter and Molecules, Journal of Research in Science Teaching , 30(3), 249–270.
Lewis, G. N. (1923). Valence and the Structure of Atoms and Molecules , New York: Dover.
Lombardi, O. & Labarca, M. (2005). The Ontological Autonomy of The Chemical World, Foundations of CHemistry , 7, 125–148.
Martin B. & Richards E. (1995). Scientific knowledge, controversy, and public decision-making, in Published in Jasanoff, S., Markle, G.E., Petersen, J.C. & Pinch T. (eds.), Handbook of Science and Technology Studies (Newbury Park, CA: Sage.
Matthews, M. R. (1994/2014). Science teaching: The role of history and philosophy of science. London: Routledge.
Matthews, M.R. (1992). History, Philosophy and Science Teaching: The Present Rapprochement, Science & Education 1(1), 11–48.
Magnasco, V. (2004). A Model for the Chemical Bond, Journal of Chemical Education , 81(3), 427–435.
Marinacci, B. (1995) (Ed) Linus Pauling in his own words , Simon&Schuster, New York
Melrose, M. P., & Scerri, E. R. (1996). Why the 4s Orbital Is Occupied before the 3d, Journal of Chemical Education , 73(6), 498–503.
Metz, D., Klassen, S., Mcmillan, B., Clough, M., & Olson, J. (2007). Building a Foundation for the Use of Historical Narratives, Science & Education , 16(3–5), 313–334.
Morrison M. (2001). History and Metaphysics: On the Reality of Spin, In Buchwald J.Z. & Warwick A. (eds.) Histories of the Electron. The Birth of Microphysics , (Chapter 14 pp. 425–450), Cambridge, Massachusetts: The MIT Press.
Michelini, M., Ragazzon, R., Santi, L., & Stefanel, A. (2000). Proposal for quantum physics in secondary school, Physics Education , 35(6), 406–410.
Moreno-Ramírez, J. E., Gallego-Badillo, R. and Pérez-Miranda, R. (2010). El modelo semicuántico de Bohr en los libros de texto [The semi-quantum Bohr’s model in textbooks], Ciência & Educação , 16(3), 611–629.
Nachtrieb N.H. (1975) Interview with Robert S. Mulliken, Journal of Chemical Education , 52(9), 560–563.
Nash, L. K. (1957). “The Atomic-Molecular Theory.” In James Bryant Conant (Ed.) Harvard Case Histories in Experimental Science , Vol. 1. Cambridge, MA, USA: Harvard University Press.
Niaz, M. (2000). The oil drop experiment: a rational reconstruction of the Millikan-Ehrenhaft controversy and its implications for chemistry textbooks, Journal of Research in Science Teaching , 37(5), 480–508.
Niaz, M. (2001). A rational reconstruction of the origin of the covalent bond and its implications for general chemistry textbooks, International Journal of Science Education , 23, 623–641.
Niaz, M. (2009). Critical Appraisal of Physical Science as a Human Enterprise. Dynamics of Scientific Progress . Dordrecht, The Netherlands: Springer Academic Publishers.
Niaz, M. (2010). Science curriculum and teacher education: The role of presuppositions, contradictions, controversies and speculations vs. Kuhn’s normal science, Teaching and Teacher Education , 26, 891–899.
Niaz, M., & Fernández, R. (2008). Understanding quantum numbers in general chemistry textbooks, International Journal of Science Education , 30(7), 869–901.
Norris, S., Guilbert, M., Smith, M., Shaharam, H., & Phillips, L. (2005). A theoretical Framework for Narrative Explanation in Science, Science Education , 89(4) 535–554.
Novick, S., & Nussbaum, J. (1978). Junior High School Pupils’ Understanding of the Particulate Nature of Matter: An Interview Study, Science Education , 62[3], 273–281.
Novick, S., & Nussbaum, J. (1981). Pupils’ Understanding of the Particulate Nature of Matter: A Cross-Age Study, Science Education , 65[2], 187–196.
Nuffield Foundation (1967). Chemistry. Handbook for teachers , London: Longmans/Penguin Books.
Nussbaum, J. (1985). The Particulate Nature of Matter in the Gaseous Phase. In R. Driver, E. Guesne y A. Tiberghien (Eds.), Children's Ideas in Science , (pp. 125–144) Philadelphia: Open University Press.
Nye, M. J. (1993). From Chemical Philosophy to Theoretical Chemistry , University of California Press, Berkeley
Ogilvie, J. F. (1994). The Nature of the Chemical Bond 1993. There are No Such Things as Orbitals!, in E. S. Kryachko and J. L. Calais (eds.), Conceptual Trends in Quantum Chemistry , (pp. 171–198), Dordrecht, The Netherlands: Kluwer.
Özmen, H. (2004). Some Student Misconceptions in Chemistry: A Literature Review of Chemical Bonding, Journal of Science Education and Technology , 13(2), 147–159.
Pagliaro, M. (2010). On shapes, molecules and models: An insight into chemical methodology, European Journal of Chemistry , 1, 276–281.
Panusch, M., Singh, R., & Heering, P. (2008). How Robert A. Millikan got the Physics Nobel Prize’. Proceedings of the Second International Conference on Story in Science Teaching , Munich, Germany.
Paoloni, L. (1982). Classical mechanics and quantum mechanics: an elementary approach to the comparison of two viewpoints, European Journal of Science Education , 4, 241–251.
Paraskevopoulou, E. and Koliopoulos, D. (2011). Teaching the Nature of Science Through the Millikan-Ehrenhaft Dispute, Science & Education , 20(10), 943–960. Published online 26 September 2010.
Park, E: J. & Light, G. (2009). Identifying Atomic Structure as a Threshold Concept: Student mental models and troublesomeness, International Journal of Science Education , 31(2), 895–930.
Peterson, R. F., Treagust, D. F., & Garnett, P. (1989). Development and application of a diagnostic instrument to evaluate grade 11 and 12 students’ concepts of covalent bonding and structure following a course of instruction, Journal of Research in Science Teaching , 26(4), 301–314.
Petri, J., & Niedderer, H. (1998). A learning pathway in high-school level quantum atomic physics, International Journal of Science Education , 20(9), 1075–1088.
Piaget, J. & Garcia, R. (1983). Psychogenesis and the history of science . New York, Columbia University Press.
Pospiech, G. (2000). Uncertainty and complementarity: The heart of quantum physics, Physics Education , 35(6), 393–399.
Popper, K. (1969). Conjectures and Refutations , London: Routledge and Kegan Paul.
Primas, H. (1983) Chemistry, Quantum Mechanics and Reductionism: Perspectives in theoretical chemistry , Berlin, Springer.
Purser, G. H. (2001). Lewis structure in General Chemistry: Agreement between electron density calculations and Lewis structures, Journal of Chemical Education , 78(7), 981–983.
Reichenbach, H. (1938). Experience and prediction: an analysis of the foundations and the structure of knowledge . Chicago: University of Chicago Press.
Reichenbach, H. (1978, [1929]) The aims and methods of physical knowledge pp 81–125 in Hans Reichenbach: Selected writings 1909–1953 (M. Reichenbach and R.S. Cohen, Eds; principal translations by E.H. Schneewind), volumen II, Dordrecht: Reidel.
Reish, G. A. (2005). How the Cold War transformed Philosophy of Science. To the Icy Slopes of Logic , New York, Cambridge University Press (Versión en español Cómo la Guerra fria transformó la filosofía de la ciencia. Hacia las heladas laderas de la lógica , Buenos Aires, Universidad de Quilmes Editorial, 2009).
Rodríguez, M., & Niaz, M. (2004). A Reconstruction of Structure of the Atom and Its Implications for General Physics Textbooks: A History and Philosophy of Science Perspective, Journal of Science Education and Technology , Vol. 13, No. 3.
Scerri, E. R. (1991). Electronic Configurations, Quantum Mechanics and Reduction, British Journal for the Philosophy of Science , 42(3), 309–25.
Scerri, E. R. (2000a). Have Orbitals Really Been Observed? Journal of Chemical Education , 77(11), 1492–4.
Scerri, E. R. (2000b). The failure of Reduction and How to Resist Disunity of the Sciences in the Context of Chemical Education, Science & Education , 9, 405–425.
Scerri, E. R. (2001). The Recently Claimed Observation of Atomic Orbitals and Some Related Philosophical Issues, Philosophy of Science, 68 (Proceedings) S76-S88, N. Koertge, ed. Philosophy of Science Association, East Lansing, MI
Scerri, E. R. (2007). The Periodic Table: Its Story and Its Significance , Oxford University Press, New York.
Scheffel, L., Brockmeier, W., & Parchmann, L. (2009). Historical material in macro-micro thinking: Conceptual change in chemistry education and the history of chemistry. In Gilbert, J. K. & Treagust, D. F. (Eds.). (2009). Multiple representations in chemical education (pp. 215–250). Springer.
Schummer, J. (1998). The Chemical Core of Chemistry I: A Conceptual Approach, HYLE-International Journal for Philosophy of Chemistry , 4, 129–162.
Schummer, J. (1999). Coping with the Growth of Chemical Knowledge: Challenges for Chemistry Documentation, Education, and Working Chemists, Educación Química , 10(2), 92–101.
Schummer, J. (2008). The philosophy of chemistry in Fritz Allhoff (Ed.), Philosophies of the Sciences , (pp. 163–183), Albany, NY, USA: Blackwell-Wiley.
Schwab, J. J. (1962). The teaching of science as enquiry. In J. J. Schwab & P. F. Brandwein (Eds.), The teaching of science. Cambridge: Harvard University Press.
Seok P. & Jin S. (2011) What Teachers of Science Need to Know about Models: An overview, International Journal of Science Education , 33(8), 1109–1130.
Segrè, E. (2007). From X-rays to Quarks: Modern Physicists and Their Discoveries , New York, USA: Dover Publications.
Shahbazian, S. & Zahedi, M. (2006). The Role of Observables and Non-Observables in Chemistry: A Critique of Chemical Language, Foundations of Chemistry , 8, 37–52.
Shiland, T. W. (1995). What’s the use of all this theory? The role of quantum mechanics in high school chemistry textbooks, Journal of Chemical Education , 72(3), 215–219.
Shiland, T. W. (1997). Quantum mechanics and conceptual change in high school chemistry textbooks, Journal of Research in Science Teaching , 34(5), 535–545.
Shrigley, R.L. & Koballa, T. R. (1989). Anecdotes: What Research Suggests about Their Use in the Science Classroom, School Science and Mathematics , 89, 293–298.
Silberstein, M. (2002). Reduction, Emergence and explanation, en Machamer P., and Silberstein, M., Philosophy of Science , Oxford: Blackwell.
Slater, J. C. (1951). A Simplification of the Hartree-Fock Method, Physical Review , 81, 385–390.
Snooks, R. J. (2006). Another Scientific Practice separating chemistry from Physics: Thought Experiments, Foundations of Chemistry , 8, 255–270.
Snow, C.P. (1981). The Physicists. A generation that changed the world , Macmillan, London
Spence, J. C. H., O’Keeffe, M. and Zuo, J. M. (2001). Have orbitals really been observed? Letter in Journal of Chemical Education , 78(7), 877.
Stewart, I. (2007). Why Beauty is Truth. The history of symmetry . Basic Books.
Stinner, A. (2008). Teaching Modern Physics using Selected Nobel Lectures APS Physics Forum on Education, fall.
Stinner, A. & Williams, H. (1998). History and Philosophy of Science in the Science Curriculum, a chapter in The International Handbook of Science Education . Dordrecht: Kluwer Academic Publishers.
Strong, I. E. (1962). Chemical Systems. Chemical Bond Approach Project , New York, USA: Chemical Education Publishing Company.
Styer, D. F. (2000). The Strange World of Quantum Mechanics . Cambridge: Cambridge University Press.
Sutcliffe, B. T. (1996). The Development of the Idea of a Chemical Bond, International Journal of Quantum Chemistry , 58, 645–55.
Taber, K. S. (1994). Misunderstanding the ionic bond, Education in Chemistry , 31(4), 100–103.
Taber, K. S. (1997). Student understanding of ionic bonding: molecular versus electrostatic framework? School Science Review , 78(285), 85–95.
Taber, K. S. (2002a). Conceptualizing Quanta: Illuminating the Ground State of Student Understanding of Atomic Orbitals, Chemistry Education: Research and Practice , 3(2), 145–158.
Taber, K. S. (2002b). Compounding Quanta: Probing the Frontiers of Student Understanding Of Molecular Orbitals, Chemistry Education: Research and Practice , 3(2), 159–173.
Taber, K.S. (2003). The Atom in the Chemistry Curriculum: Fundamental Concept, Teaching Model or Epistemological Obstacle, Foundations of Chemistry , 5, 43–84.
Taber, K. S. (2005). Learning Quanta: Barriers to Stimulating Transitions in Student Understanding of Orbital Ideas, Science Education , 89, 94–116.
Talanquer, V. (2011, online), School Chemistry: The Need for Transgression, Science & Education , published online 17th September.
Tapio, I. (2007). Models and Modelling in Physics Education: A critical Re-analysis of Philosophical Underpinnings and Suggestions for Revisions, Science & Education , 16, 751–773.
Teichmann, J. (2008). Anecdotes Can Tell Stories—How? And What is Good and What is Bad about Such Stories? Proceedings of the Second International Conference on Story in Science Teaching , Munich, Germany.
Thomson, J. J. (1904). Electricity and matter , Westminster, UK: Archibald Constable & Co. Ltd.
Toulmin, S. (1961). Foresight and Understanding: An Enquiry Into the Aims of Science , Bloomington: Indiana University Press.
Toulmin, S. (1972). Human Understanding , Princeton: Princeton University Press.
Tsaparlis, G., & Papaphotis, G. (2002). Quantum-Chemical Concepts: Are They Suitable for Secondary Students? Chemistry Education: Research and Practice , 3(2), 129–144.
Tsaparlis, G. (1997a). Atomic orbitals, molecular orbitals and related concepts: Conceptual difficulties among chemistry students. Research in Science Education , 27, 271–287.
Tsaparlis, G. (1997b). Atomic and Molecular Structure in Chemical Education, Journal of Chemical Education , 74(8), 922–5.
Tsaparlis, G. (2001). Towards a meaningful introduction to the Schrödinger equation through historical and heuristic approaches, Chemistry Education: Research and Practice in Europe , 2, 203–213.
Tsaparlis, G., & Papaphotis, G. (2009). High-school Students’ Conceptual Difficulties and Attempts at Conceptual Change: The case of basic quantum chemical concepts, International Journal of Science Education , 31(7), 895–930.
Toulmin S. & Goodfield J. (1962). The Architecture of Matter , The University of Chicago Press, Chicago
Valanides, N. (2000). Primary student teachers’ understanding of the particulate nature of matter and its transformations during dissolving, Chemistry Education: Research and Practice in Europe , 1, 249–262.
Van Aalsvoort, J. (2004) ‘Logical positivism as a tool to analyse the problem of chemistry’s lack of relevance in secondary school chemical education’, International Journal of Science Education , 26, 1151–1168.
Van Brakel, J. (2000). Philosophy of Chemistry , Leuven University Press, Louvain.
Van Berkel, B. (2005). The Structure of Current School Chemistry. A Quest for Conditions for Escape , Centrum voor Didactiek van Wiskunde en Natuurwetenschappen, University of Utrech CD-β Press, Utrech.
van Berkel, B., de Vos, W., Veronk, A. H., & Pilot, A. (2000). Normal science education and its dangers: The case of school chemistry. Science & Education , 9, 123–159.
Velmulapalli, G. K. & Byerly H. (1999) Remnants of Reductionism, Foundations of Chemistry 1, 17–41.
Viana, H. E. B. & Porto, P. A. (2010). The development of Dalton’s Atomic Theory as a Case Study in the History of Science: Reflections for Educators in Chemistry, Science & Education , 19(1), 75–90.
Wandersee, J. H., & Griffard, P. B. (2002). The history of chemistry: Potential and actual contributions to chemical education in Gilbert J. et al. (eds), Chemical Education: Towards Research-based Practice , (Chapter 2, pp. 29–46), Dordrecht, The Netherlands: Kluwer.
Weyer, J. (1974) Chemiegeschichtsschreibung von Wiegleb (1790) bis Partington (1970); Gerstenberg: Hildescheim
Wightman, T., Johnston, K., & Scott, P. (1987). Children’s learning in science project in the classroom. Approaches to teaching the particulate theory of matter , Centre for Studies in Science and Mathematics Education: University of Leeds.
Wisniak, J. (2013). Gustav Charles Bonaventure Chancel, Educación Química , 24(1), 23–30.
Wittmann, M. C., Steinberg, R. N., & Redish, E. F. (2002). Investigating student understanding of quantum physics: Spontaneous models of conductivity, American Journal of Physics , 70, 218–226.
Woolley, R.G. (1978). Must a molecule have a shape? Journal of the American Chemical Society , 100, 1073–1078.
Yager, R. E. (2004). Science is Not Written, But It Can Be Written About, in W. Saul (Ed.), Crossing Borders in Literacy and Science Instruction , (pp. 95–107) Washington: NSTA.
Zuo, J.; Kim, M.; O’Keeffe, M.; Spence, J. (1999). Direct observation of d holes and Cu-Cu bonding in. Cu 2 O, Nature , 401, 49–56.
Download references
Author information
Authors and affiliations.
Seminario de Investigación Educativa en Química, Facultad de Química, Universidad Nacional Autónoma de México, Avenida Universidad 3000, Delegación Coyoacán, México, DF, 04510, México
José Antonio Chamizo & Andoni Garritz
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to José Antonio Chamizo .
Editor information
Editors and affiliations.
School of Education, University of New South Wales, Sydney, New South Wales, Australia
Michael R. Matthews
Rights and permissions
Reprints and permissions
Copyright information
© 2014 Springer Science+Business Media Dordrecht
About this chapter
Chamizo, J.A., Garritz, A. (2014). Historical Teaching of Atomic and Molecular Structure. In: Matthews, M. (eds) International Handbook of Research in History, Philosophy and Science Teaching. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-7654-8_12
Download citation
DOI : https://doi.org/10.1007/978-94-007-7654-8_12
Published : 30 December 2013
Publisher Name : Springer, Dordrecht
Print ISBN : 978-94-007-7653-1
Online ISBN : 978-94-007-7654-8
eBook Packages : Humanities, Social Sciences and Law Education (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research

- Next Article
Niels Bohr’s First 1913 Paper: Still Relevant, Still Exciting, Still Puzzling
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Reprints and Permissions
- Cite Icon Cite
- Search Site
Kenneth W. Ford; Niels Bohr’s First 1913 Paper: Still Relevant, Still Exciting, Still Puzzling. Phys. Teach. 1 November 2018; 56 (8): 500–502. https://doi.org/10.1119/1.5064553
Download citation file:
- Ris (Zotero)
- Reference Manager
Many teachers like to introduce the Bohr atom toward the end of an introductory physics course. This is an excellent idea, given the historic importance of Bohr’s 1913 work, which provided the bridge from Planck’s quantized interaction of matter and radiation (1900) to the full theory of quantum mechanics (1925-28). Unfortunately, the version of the Bohr atom that appears in many textbooks and is no doubt often presented to students is more wrong than right and may leave both teachers and students wondering why, more than a hundred years later, it is still being taught. This “pedagogic” version postulates that an electron in a stationary state moves in a circular orbit with an angular momentum that is an integral multiple of h /2π ( L = nh /2π = nħ )— ħ for the lowest-energy state, 2 ħ for the next state, and so on. This picture of the hydrogen atom is wrong in two senses. First it doesn’t conform to our present understanding of the hydrogen atom. This, in itself, is not a reason to scrap it, for the historical development of quantum physics is certainly of interest. But second, it doesn’t conform to the essence of what Bohr actually did. That is a reason not to teach about circular orbits and L = nħ .
Sign in via your Institution
Citing articles via.
- Online ISSN 1943-4928
- Print ISSN 0031-921X
- For Researchers
- For Librarians
- For Advertisers
- Our Publishing Partners
- Physics Today
- Conference Proceedings
- Special Topics
pubs.aip.org
- Privacy Policy
- Terms of Use
Connect with AIP Publishing
This feature is available to subscribers only.
Sign In or Create an Account
- Diversity & Inclusion
- Community Values
- Visiting MIT Physics
- People Directory
- Faculty Awards
- History of MIT Physics
- Policies and Procedures
- Departmental Committees
- Academic Programs Team
- Finance Team
- Meet the Academic Programs Team
- Prospective Students
- Requirements
- Employment Opportunities
- Research Opportunities
- Graduate Admissions
- Doctoral Guidelines
- Financial Support
- Graduate Student Resources
- PhD in Physics, Statistics, and Data Science
- MIT LEAPS Program
- for Undergraduate Students
- for Graduate Students
- Mentoring Programs Info for Faculty
- Non-degree Programs
- Student Awards & Honors
- Astrophysics Observation, Instrumentation, and Experiment
- Astrophysics Theory
Atomic Physics
- Condensed Matter Experiment
- Condensed Matter Theory
- High Energy and Particle Theory
- Nuclear Physics Experiment
- Particle Physics Experiment
- Quantum Gravity and Field Theory
- Quantum Information Science
- Strong Interactions and Nuclear Theory
- Center for Theoretical Physics
- Affiliated Labs & Centers
- Program Founder
- Competition
- Donor Profiles
- Patrons of Physics Fellows Society
- Giving Opportunties
- physics@mit Journal: Fall 2023 Edition
- Events Calendar
- Physics Colloquia
- Search for: Search
Atomic physics seeks to understand and control the quantum behavior of matter at the smallest scale. What are the fundamental properties of single atoms, ions, molecules, and photons? Once we combine them together, what are the laws governing emergent many-body phenomena such as superfluidity and magnetism? Can we create and study new states of matter that have never existed in nature before? And how can we exert control over quantum properties to engineer useful systems such as precise clocks, new sensors, fundamentally improved measurements, and new paradigms of quantum computation?
At MIT, researchers trap and manipulate individual ions, photons, spins, and clouds of atoms and molecules. They cool gases down to temperatures a million times colder than deep space, control and image matter at the level of single atoms, hunt for dark matter, tame single photons of light, and control atom-light interactions to generate entanglement and correlations among quantum particles. This research is made possible by an ever-expanding atomic physics toolbox, including ultra-stable lasers, atom and ion traps, nanofabrication of photonic structures, high-resolution microscopes, and optical tweezers.
The group is anchored by the NSF Center for Ultracold Atoms (CUA), a joint effort between atomic physicists at MIT and Harvard, and is also vitally involved in educational activities.
Current Faculty

Emeritus Faculty

Affiliated Labs & Centers
- MIT–Harvard Center for Ultracold Atoms
Related News
MIT physicists capture the first sounds of heat “sloshing” in a superfluid
Coldest: how a letter to Einstein and advances in laser-cooling technology led physicists to new quantum states of matter

Richard Fletcher named a 2023 Packard Fellow

IMAGES
VIDEO
COMMENTS
The structure of the atom. Nobel Lecture, December 11, 1922. Ladies and Gentlemen. Today, as a consequence of the great honour the Swedish Academy of Sciences has done me in awarding me this year's No-bel Prize for Physics for my work on the structure of the atom, it is my duty to give an account of the results of this work and I think that I ...
PDF | On Apr 18, 2018, Ramesh Duraisamy published Atomic Structure & Basic Concepts of Chemistry | Find, read and cite all the research you need on ResearchGate
The research described in this paper is an investigation into the conceptions held about atomic orbitals, hybridization and related concepts by prospective chemistry teachers.
Atomic and molecular physics articles from across Nature Portfolio. Atom; RSS Feed; Definition. Atomic and molecular physics it the study of the properties, dynamics and interactions of the basic ...
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications. ... Accurate atomic structure and ...
Atoms. Atoms is an international, peer-reviewed, open access journal on all aspects of the atom published monthly online by MDPI. Open Access — free for readers, with article processing charges (APC) paid by authors or their institutions. High Visibility: indexed within Scopus, ESCI (Web of Science), Astrophysics Data System, Inspec, CAPlus ...
Figure 1 | 3D atomic positions and local chemical order of medium- and high-entropy alloy nanoparticles. a-d, Experimental atomic models of two MEAs (a, b) and two HEAs (c, d) obtained using ...
The distribution of representations of the atomic structure in tasks 1, 2a and 2b is presented in Table 4. When students represented atomic structure independent of any context (task 1), the nuclear and Bohr's models (categories C and D) were the most frequent, whereas the quantum mechanical model (category E) appeared only in 1.9% of the ...
Progress in the macroscopic (or, ab initio) theory has been quite notable and it has been supported by two major pillars: First, thanks to the advent of Effective Field Theories (EFTs), we can now systematically develop nuclear Hamiltonians that are rooted in the fundamental properties and symmetries of the underlying theory of QCD. Second, advances in computational resources and novel ...
Atomic radius is an important periodic descriptor used in understanding a variety of physico-chemical and bio-chemical processes. Numerous scales are suggested to define atomic radii. The aim of the current study is to find out the most reliable and universal scale among different (experimental and theoretical) scales of radii. For this, we have used different types of radii to compute some ...
Systematic Procedure for Drawing Lewis Structures Based on Electron Pairing Priority and the Explicit Use of Donor Bonds: An Alternative to the Normal Procedure Which Can Be Pen and Paper Based or Automated on a PC in User Interactive 3D. Journal of Chemical Education 2019, 96 (7) , 1412-1417.
Figure 2.2.1 2.2. 1: The Structure of the Atom. Atoms have protons and neutrons in the center, making the nucleus, while the electrons orbit the nucleus. The modern atomic theory states that atoms of one element are the same, while atoms of different elements are different.
Atomic-scale observation of the grain-boundary structure of Yb-doped and heat-treated silicon nitride ceramics. The effect of secondary sintering additives and/or a post-sintering heat treatment on the semicrystalline atomic structure of the intergranular phase in silicon nitride ceramics is investigated.
The purpose of this paper is to argue that history and philosophy of chemistry and physics are central strategies in the teaching of atomic and molecular structure, from the Dalton model (for an earlier approach see Chalmers 1998) to modern quantum mechanics and quantum chemistry.Therefore, in addition to the presentation and conclusions, the chapter is divided into two equally important sections.
Many teachers like to introduce the Bohr atom toward the end of an introductory physics course. This is an excellent idea, given the historic importance of Bohr's 1913 work, which provided the bridge from Planck's quantized interaction of matter and radiation (1900) to the full theory of quantum mechanics (1925-28).
Atomic models, J.J. Thomson's "plum pudding" model. In 1897, Joseph John Thomson (1856-1940) announced the discovery of a corpuscle. Others. soon called it →electron, despite Thomson's ...
Research paper. Atomic fine-structure calculations performed with a finite-nuclear-mass approach and with all-electron explicitly correlated Gaussian functions. Author links open overlay panel Andrzej Kędziorski a, Monika Stanke a, Ludwik Adamowicz b c.
Pyrophyllite is extensively used in the high-pressure synthesis industry as a pressure-transmitting medium because of its outstanding pressure transmission, machinability, and insulation. Therefore, the atomic structure, electronic, and mechanical behavior of pyrophyllite [Al4Si8O20(OH)4] under high pressure should be discussed deeply and systematically. In the present paper, the lattice ...
Coronaviruses have caused several epidemics and a global pandemic since the early 2000s (1-3), necessitating fundamental studies of the structure and function of the proteins of these viruses.The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus encodes 29 proteins, 4 of which are structural: the spike protein (S), the nucleocapsid protein (N), the matrix protein (M), and ...
This research is made possible by an ever-expanding atomic physics toolbox, including ultra-stable lasers, atom and ion traps, nanofabrication of photonic structures, high-resolution microscopes, and optical tweezers. The group is anchored by the NSF Center for Ultracold Atoms (CUA), a joint effort between atomic physicists at MIT and Harvard ...
atom, the basic building block of all matter and chemistry. Atoms can combine with other atoms to form molecules but cannot be divided into smaller parts by ordinary chemical processes. Most of the atom is empty space. The rest consists of three basic types of subatomic particles: protons, neutrons, and electrons.
The emergence of atomic-level structure in language models shows a high-resolution picture of protein structure encoded by evolution into protein sequences that can be captured with unsupervised learning. Our current models are very far from the limit of scale in parameters, sequence data, and computing power that can in principle be applied.
This paper presents a study of soils structure and composition using up to date technique, such as scanning. electronic microscopy, atomic force microscopy, X-ray diffraction, X-ray uorescence, as ...