Human Genetic Engineering: Key Principles and Issues Essay
Introduction.
Improving the quality and duration of human life are the key priorities of the world’s developed economies and countries. For more effective prevention, diagnosis, and treatment of socially significant diseases, along with the rehabilitation of patients, technological breakthroughs in the field of biomedicine are necessary. They are primarily associated with the creation of fundamentally new drugs, products for cell and gene therapy, and tools for precise molecular diagnostics. Human genetic engineering is one such method, formulating a significant breakthrough in the field of medicine. However, it is a controversial aspect in terms of ethical issues, as genetic changes can lead to unforeseen consequences. At the same time, it makes it possible to cure complex diseases or correct some problems for a person.
Genetic engineering is a recent breakthrough in humanity in the field of medicine, formulating one of the most complex processes. Genetic engineering technologies include the construction of functionally active genetic structures, their introduction into the human body, and integration into the genome (Wheale & Schomber, 2019). It allows one to develop new, in some cases, unique genetic, biochemical, and physiological properties. The creation of new biopharmaceuticals and cell cultures producing biologically active molecules in the future will provide the medical market with affordable, innovative drugs and diagnostic tools. However, there is a possibility that genetic engineering procedures will have a significant price, and only some people will be able to afford such treatment.
Point effects are required for the effective treatment of many diseases, primarily of an immune nature, sometimes at the level of individual cells. The creation of target-oriented drugs, including conjugated and DNA vaccines, will increase the effectiveness of treating oncological, rheumatic, and infectious diseases, as well as disorders of the nervous system (Wheale & Schomber, 2019). The first direction in the development of the trend is associated with the use of recombinant DNA to obtain biological products with desired therapeutic properties and high rates of bioavailability and specificity of action (Wheale & Schomber, 2019). As a result, new drugs will appear that are effective in diseases caused by immune system disorders. The creation of diagnostic biosensors formulates another direction for therapeutic cellular products, and specific molecular fragments obtained based on genetic engineering technologies. These solutions could increase the diagnostic value of portable tests being brought to the medical device market.
Implementing new genes into a microorganism, plant, animal, or human body opens new possibilities to gain new body characteristics. These treats have never been enjoyed by the object before and could promote better living or treatment. One can reorganize these genotypes by transforming DNA, a molecule that is responsible for transfer, custody, and pass from one breed to another descent, and execution of the genetic program for the evolving and performing of living entities (Wheale & Schomber, 2019). Moreover, transformations occur in ribonucleic acid, one of the key molecules in all living entities’ cells.
The key aspects of standard genetics were founded in the middle of the 19th century due to the tests of the Czech-Austrian scientist and biologist Gregor Mendel (Wheale & Schomber, 2019). The foundations of transferring of ancestral features from parental entities to their scions, outlined by him based on the experiments on plants in 1865, unfortunately, were not significantly popular among the cotemporaries (Wheale & Schomber, 2019). After several decades, the followers of this trend returned to focus on the aspect of genetic engineering, and the issue began to be studied more carefully. Therefore, nowadays, genetic engineering is applied in many areas, and on its basis, an independent area of healthcare area has been formed, which is one of the contemporary parts of biotechnology.
The medicines that are currently under clinical experiments are remedies that potentially can cure cardiovascular disease, arthrosis, AIDS, and oncology. Several hundred companies engaged in genetic design are promoting the manufacturing of medicines and diagnostics. Nowadays, human insulin obtained by means of retransmitted DNA is actively utilized by many healthcare providers. Human insulin-cloned genes were implemented into a bacterial cell (Wheale & Schomber, 2019). Since 1982, various companies in developed countries have been producing genetically designed insulin (Wheale & Schomber, 2019). In addition, many new diagnostic drugs have already been implemented into healthcare practice.
Despite the apparent positive effects of these discoveries and the possibility of improving the level of a cure for diseases and the quality of life of people, genetic engineering has other aspects. Speaking from an ethical point of view, it can have negative consequences as genetic changes can be used for devastating effects. Thus, organizations have introduced various restrictions and moratoriums on experiments, introducing more humane principles. In addition, genetic engineering brings humanity closer to the possibility of cloning, which opens up many options. For example, one could grow a clone for later organ transplantation or use it as a donor. However, whether such a procedure is ethically acceptable remains an open question as opinions differ.
There are many options for the development of events in the field of genetic engineering, and not all of them have been studied. Therefore, they must be consistently fixed and regulated, as bad scenarios of the development of events cause most fears. As a rule, it all starts with helping people and inventing new drugs, and then a person may come to desire to change his child’s hair or eyes or to create an army of universal soldiers who are not afraid of pain and do not know fear. Modern society is so heterogeneous culturally and economically that any methods that can significantly change the genome can create conditions for class and species stratification (Wheale & Schomber, 2019). For example, representatives of the rich world will be able to significantly prolong their lives and not be afraid of any diseases, unlike less wealthy people, and this is a serious ground for conflicts and clashes.
However, many incurable diseases occur due to pathological changes in the cell genome. Traditional drugs are not effective enough in their treatment due to low specificity and, in some cases, significant toxic effects on the body. Moreover, they do not act on the very cause of such diseases, namely somatic mutations of the genome. It is expected that one will be able to target gene expression, interrupting the sequence of pathological changes in the cell (Wheale & Schomber, 2019). In addition, the person will be able to control the critical mechanisms of development, and treatment of oncological diseases will become possible with the help of technologies for the therapeutic use of RNA interference.
To conclude, human genetic engineering is one of the major medical breakthroughs, giving many opportunities for healing and improving life. Genetic engineering is a new direction in the field of molecular biology, which has become widespread in many areas of medicine and biology relatively recently. However, despite the positive effects, it has some controversial ethical aspects. Genetic engineering provides limitless opportunities for humans, including creating their own fearless army, which can be used for crimes. In addition, errors during surgery at the gene level can lead to irreversible negative consequences.
Wheale, P., & Schomberg, R. (2019). The social management of genetic engineering . Routledge.
- Chicago (A-D)
- Chicago (N-B)
IvyPanda. (2024, February 11). Human Genetic Engineering: Key Principles and Issues. https://ivypanda.com/essays/human-genetic-engineering-key-principles-and-issues/
"Human Genetic Engineering: Key Principles and Issues." IvyPanda , 11 Feb. 2024, ivypanda.com/essays/human-genetic-engineering-key-principles-and-issues/.
IvyPanda . (2024) 'Human Genetic Engineering: Key Principles and Issues'. 11 February.
IvyPanda . 2024. "Human Genetic Engineering: Key Principles and Issues." February 11, 2024. https://ivypanda.com/essays/human-genetic-engineering-key-principles-and-issues/.
1. IvyPanda . "Human Genetic Engineering: Key Principles and Issues." February 11, 2024. https://ivypanda.com/essays/human-genetic-engineering-key-principles-and-issues/.
Bibliography
IvyPanda . "Human Genetic Engineering: Key Principles and Issues." February 11, 2024. https://ivypanda.com/essays/human-genetic-engineering-key-principles-and-issues/.
- Discussion: Human Genome Sequencing
- Human Genome Project vs. Human Proteome Project
- The Human Genome Project and Its Revolutionary Insight into the Genetic Blue Print of the Human Body
- Human Genome Sequencing and Experiments
- Genome: Bioethics and Genetic Engineering
- Molecular Components of the DNA Molecule
- Footprints of Non-Sentient Design Inside the Human Genome
- Molecular Genetics: Gene Sequence Homology
- Molecular Genetics and Biological Inheritance
- Sequencing Bacterial Genome
- Natural Selection: The Roles of History, Chance, and Natural Selection
- Meg Tirrell's "Unlocking My Genome: Was It Worth It?"
- Ethical Issues with Fetal Anomalies
- Preimplantation Genetic Diagnosis and Manipulation
- Hutchinson-Gilford Progeria Syndrome

Want to create or adapt books like this? Learn more about how Pressbooks supports open publishing practices.

18 Human Genetic Engineering
Melissa Nolan
18.1 Introduction
- Gene – units of heredity transferred from a parent to offspring, contained in DNA
- Gene editing – deliberate manipulation of the genetic material to achieve desired results
- Human Genetic Engineering (HGE) – gene editing applied to human cells
- CRISPR/Cas9 – gene editing technology derived from bacterial immune system
- Germline gene editing – a process which alters the genome of an embryo, so that the entire organism has altered genes and can pass those genes down to offspring
- Somatic gene editing – a process which alters the genome of just a few cells in an organism, so that the altered genes are not passed down to offspring
- Recombinant DNA – genetic material from multiple sources
- Congenital disease – a disease present from birth, typically caused by some genetic factor
- Eugenics – the study of how to arrange reproduction within a human population to increase the occurrence of heritable characteristics regarded as desirable
- Technological determinism – technology determines the development of its social structure and cultural values or regulations
- Cultural determinism – the culture we are raised presents certain issues which necessitate the development of a specific technology
- Legacy thinking – using thinking strategies and actions which are outdated and no longer serve the purpose they once did
- Antibodies – proteins produced by an organism as a result of their immune response
Learning Objectives
By the end of this chapter, students should be able to:
- Understand the basic principles of gene editing and their applications.
- Understand the history of gene editing
- Identify potential ethical concerns regarding human genetic engineering
Those beautiful blue eyes you inherited from your mother are actually a result of a complex science known as Genetics. The scientific field of genetics studies genes in our DNA. Genes are units of heredity transferred from a parent to offspring and determine some characteristic of offspring. Your genes are responsible for coding all of your traits- including hair color, eye color, and so on. In recent years, scientists began exploring the concept of gene editing , which is the deliberate manipulation of genetic material to achieve desired results. Gene editing can potentially alter any given trait in an organism- from height to hair texture to susceptibility for certain diseases.
Gene editing applied to humans is referred to as Human Genetic Engineering , or HGE. There is extensive debate in and out of the scientific community regarding the ethics of HGE. Much of this debate stems from how this technology will affect society, and vice versa. Individuals may harbor concerns about the rise of “designer babies” or scientists “playing God” by determining the traits of an individual. On the contrary, HGE presents potential cures to diseases caused by genetic mutations. Human Genetic Engineering (HGE) is a novel technology which presents various ethical concerns and potential consequences. HGE should be approached cautiously and with extensive governmental regulation given it’s history, it’s current state, and the potential it has to change the world in the future.
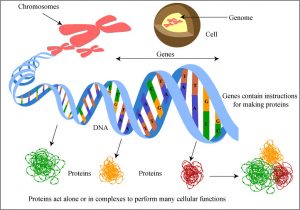
“ Genetic Encoding of Proteins ” by MIT OpenCourseWare is licensed under CC BY-NC-SA 2.0
18.2 What is HGE
Key Takeaway
HGE encompasses a variety of methods which all work to produce a deliberate change in the human genome. The most common and prevalent way to edit the human genome is via CRISPR/Cas9 . CRISPR stands for clustered regularly interspaced short palindromic repeats, and Cas9 is a protein that functions as scissors to cut DNA/genes. The CRISPR/Cas9 system originally developed as a part of a bacteria’s immune system, which can recognize repeats in DNA of invading viruses, then cut them out. Since then, scientists have harnessed the CRISPR/Cas9 system to cut DNA sequences of their choice and then insert new DNA sequences in their place.
The CRISPR/Cas9 system allows for designer genomes, and rapid engineering of any cell’s programming. With the use of CRISPR/Cas9, scientists can cut out certain traits from an individual’s cells and insert new traits into those same cells.
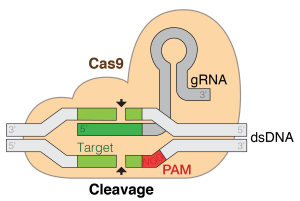
“ CRISPR Cas9 System ” by Marius Walter is licensed under CC-BY-SA-4.0
18.3 History of Gene Editing
Gene therapy concepts were initially introduced in the 1960s, utilizing outdated methods, such as recombinant DNA technology and viral vectors, to edit microorganisms’ genomes. Recombinant DNA consists of genetic material from multiple sources. The first experiments involved transferring a genome from one bacteria to another via a viral vector. Soon after was the first successful transformation of human cells with foreign DNA. The success of the experiment prompted public concern over the ethics of gene therapy, and led to political regulation. In the gene therapy report of the President’s Commission in the United States, germline genome editing was deemed problematic over somatic genome editing . Also, non-medical genome editing was deemed problematic over medical genome editing. Germline genome editing occurs when scientists alter the genome of an embryo, so that the entire organism has altered genes and the traits can be passed to offspring. Somatic genome editing involves editing only a few cells in the entire organism so that traits can not be passed down to offspring. In response to the report, the rDNA Advisory Committee of the National Institutes of Health was formed and proposed the first guidelines for the gene therapy clinical trials. This is an example of technological determinism , in which technology determines the development of its social structure and cultural values or regulations.
In the past few decades, gene editing has advanced exponentially, introducing state-of-the-art technologies such as the CRISPR/Cas9 system, which was developed to induce gene modifications at very specific target sites. Thus, gene editing became a major focus for medical research (Tamura, 2020). Gene editing has led to the potential for development of treatment strategies for a variety of diseases and cancers. So far, somatic genome editing has shown promise in treating leukemia, melanoma, and a variety of other diseases. In this way, HGE may be demonstrative of cultural determinism , in which the culture we are raised presents certain issues which necessitate the development of a specific technology.
“ DNA CRISPR Scissors ” by Max Pixel is licensed under CC0 1.0
18.4. Impact of HGE on society
Somatic genome editing in HGE via the CRISPR/Cas9 system has proven to be effective at editing specific genome sites. Since 2015, genome editing technologies have been used in over 30 human clinical trials and have shown positive patient outcomes. The treatment of disease may be a positive benefit of HGE, but there are also various potential risks. Various forms of deliberative democracies formed in recent years to address scientific and ethical concerns in HGE. Deliberative democracies affirm the need to justify technological decisions made by citizens and their representatives with experts in the field via deliberation. Overall, the consensus remains that the pros and cons of HGE are not equivalent enough to justify widespread use of the technology.
18.4.1 Pros of HGE
Current human clinical trials show successful transformation of human immune cells to HIV-resistant cells. This implies that HGE may be the cure for HIV(Hu, 2019). Other successful somatic genome editing trials treated myeloma, leukemia, sickle cell disease, various forms of epithelial cancers, and hemophilia. Thus, gene editing has provided novel treatment options for congenital diseases and cancers (Tamaura, 2020). Congenital diseases are those present from birth, and typically have a genetic cause. For these reasons, scientific summits concluded HGE is ethical for research regarding somatic genome editing in congenital diseases and cancers.
18.4.2 Cons of HGE
There are many safety concerns regarding CRISPR applications, mainly in germline genome editing. As a result of technological determinism, a leading group of CRISPR/Cas9 scientists and ethicists met for the international Summit on Human Gene Editing. The summit determined that heritable genome research trials may be permitted only following extensive research on risks and benefits of HGE. However, the summit concluded that federal funding cannot be used to support research involving human embryos with germline editing techniques. These decisions were made to avoid potential risks such as the following.
The major concerns regarding germline genome editing in HGE include: serious injury or disability, a blurry line between therapeutic applications of HGE and medical applications, misapplications, potential for eugenics ( the study of how to arrange reproduction within a human population to increase the occurrence of heritable characteristics regarded as desirable), and inequitable access to the technology.
18.5. Future Outlook
The future of HGE is uncertain and requires immense forethought. The American Society of Human Genetics workgroup developed a position statement on human germline engineering. The statement argues that it is inappropriate to perform germline gene editing that culminates in human pregnancy; and that in vitro(outside of an organism) germline editing should be permitted with appropriate oversight. It also states future clinical human germline editing requires ethical justification, compelling medical rationale, and evidence that supports its clinical usage. Many of these decisions were made based on the potential concerts over the future possibilities of the technology.
At the societal level, there may be concerns related to eugenics, social justice, and accessibility to technology. Eugenics could potentially reinforce prejudice and enforce exclusivity in certain physical traits. Traits can be preselected for, thus labeling some as ‘good’ and others as ‘unfavorable’. This may perpetuate existing racist ideals, for example.
Moreover, germline genome editing may also increase the amount of inequality in a society. Human germline editing is likely to be very expensive and access may be limited to certain geographic regions, health systems, or socioeconomic statuses. Even if human genetic engineering is only used for medical purposes, genetic disease could become an artifact of class, location, or ethnic group. Therefore, preclinical trials are necessary to establish validity, safety, and efficacy before any wide scale studies are initiated.
Others argue that HGE may lessen genetic diversity in a human population, creating a biological monoculture that could lead to disease susceptibility and eventual extinction. Analyses have predicted that there will be negligible effect on diversity and will more likely ensure the health and longevity of humans (Russel, 2010). Legacy thinking may be responsible for the hesitations towards continuing forward with HGE, as there are also many potential pros for genetic engineering. Legacy thinking is using outdated thinking strategies and actions which may not be useful anymore.
In an alternative modernity, we can imagine HGE as an end-all for most congenital diseases and cancers. Moreover, it may be used in germline gene editing to prevent certain birth defects or heritable diseases. So, although HGE has a variety of potential risk factors, there is also great promise for novel medical therapies in the coming decades. The continued use of this technology should be approached cautiously and with extensive governmental regulation, allowing for research regarding its medical applications only.
In 2016, germline gene editing was proven feasible and effective in chickens by leading researchers in genetic engineering, Dimitrov and colleagues. In this study, scientists used CRISPR/Cas9 to target the gene for an antibody / immunoglobulin commonly produced in chickens. Antibodies are proteins produced in immune response. In the resulting population, the chickens grew normally and healthily with modified antibodies which conferred drug resistance. This study was the first to prove that germline editing is both feasible and effective.
Chapter Summary
HGE is a rapidly expanding field of research which presents novel possibilities for the coming decades. HGE utilizes CRISPR/Cas9 gene editing tools to cut out specific genes and replace them with a newly designed gene. As important as this technology is, it is also important to recognize how new it is. Gene therapy research began in the 1960’s, with somatic cell editing only commencing in the past two decades. This has presented many advantages for the potential treatment of congenital diseases, but also presents various risks. Those risks stem from germline gene editing and include eugenics and inequitable access to the technology creating large socio economic divides. In the future, more regulation should be placed on the advancement of HGE research before larger-scale studies take place.
Review Questions
1. What is the primary technology proposed for use in HGE?
A. Recombinant DNA technology
B. CRISPR/Cas9
C. Bacterial Transformation
D. Immunoglobulin
2. When was gene therapy concepts first introduced?
3. What is a major ethical concern regarding HGE addressed in this chapter?
A. Potential for ageism
B. Gene editing is only 50% effective
C. HGE can only be used in Caucasians
D. Potential for eugenics
Food for Thought
- Would you personally employ germline genome editing for your own children? In what scenarios? Why or why not?
- Should technologies such as this be federally regulated or should scientific freedom preside? Justify your reasoning.
Baltimore, D. et. al.(2015). A prudent path forward for genomic engineering and germline gene modification. Science. https://doi.org/10.1126/science.aab1028
Brokowski, C., & Adli, M. (2019). CRISPR Ethics: Moral Considerations for Applications of a Powerful Tool. Journal of Molecular Biology. https://doi.org/10.1016/j.jmb.2018.05.044
Cong, L., Ran, F., & Zhang, F. (2013). Multiplex Genome Engineering Using CRISPR/Cas9 Systems. Science. https://doi.org/10.1126/science.1231143
Dimitrov, L., et. al. (2016). Germline Gene Editing in Chickens by Efficient CRISPR-Mediated Homologous Recombination in Primordial Germ Cells. Plos One. https://doi.org/10.1371/journal.pone.0154303
Hu, C. (2019). Safety of Transplantation of CRISPR CCR5 Modified CD34+ Cells in HIV-Infected Subjects with Hematological Malignancies. U.S National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT03164135
Ormond, K., et. al.(2017). Human Germline Genome Editing. AJHG. https://doi.org/10.1016/j.ajhg.2017.06.012
Russell P.(2010) The Evolutionary Biological Implications of Human Genetic Engineering, The Journal of Medicine and Philosophy: A Forum for Bioethics and Philosophy of Medicine. https://doi.org/10.1093/jmp/jhq004
Tamura, R., & Toda, M. (2020). Historic Overview of Genetic Engineering Technologies for Human Gene Therapy. Neurologia medico-chirurgica. https://doi.org/10.2176/nmc.ra.2020-0049
Thomas, C. (2020). CRISPR-Edited Allogeneic Anti-CD19 CAR-T Cell Therapy for Relapsed/Refractory B Cell Non-Hodgkin Lymphoma. ClinicalTrials. https://clinicaltrials.gov/show/NCT04637763
Units of heredity transferred from a parent to offspring, contained in DNA.
Deliberate manipulation of the genetic material to achieve desired results.
Gene editing applied to human cells.
Gene editing technology derived from bacterial immune system.
genetic material from multiple sources
A process which alters the genome of an embryo, so that the entire organism has altered genes and can pass those genes down to offspring.
A process which alters the genome of just a few cells in an organism, so that the altered genes are not passed down to offspring.
The idea that a society's technology determines the development of its social and cultural values.
The culture we are raised presents certain issues which necessitate the development of a specific technology.
A disease present from birth, typically caused by some genetic factor.
The study of how to arrange reproduction within a human population to increase the occurrence of heritable characteristics regarded as desirable.
Using thinking strategies and actions which are outdated and no longer serve the purpose they once did.
Proteins produced by an organism as a result of their immune response,
Technology: Where it Started and Where it’s Going Copyright © by Melissa Nolan is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License , except where otherwise noted.
Share This Book

Arguing For and Against Genetic Engineering
Harvard philosopher Michael Sandel recently spoke at Stanford on the subject of his new book, The Case against Perfection: Ethics in the Age of Genetic Engineering. He focused on the “ethical problems of using biomedical technologies to determine and choose from the genetic material of human embryos,” an issue that has inspired much debate.
Having followed Sandel’s writings on genetic enhancement for several years, I think that this issue deserves special thought. For many years, the specter of human genetic engineering has haunted conservatives and liberals alike. Generally, their main criticisms run thus:
First, genetic engineering limits children’s autonomy to shape their own destinies. Writer Dinesh D’Souza articulates this position in a 2001 National Review Online article: “If parents are able to remake a child’s genetic makeup, they are in a sense writing the genetic instructions that shape his entire life. If my parents give me blue eyes instead of brown eyes, if they make me tall instead of medium height, if they choose a passive over an aggressive personality, their choices will have a direct, lifelong effect on me.” In other words, genetic enhancement is immoral because it artificially molds people’s lives, often pointing their destinies in directions that they themselves would not freely choose. Therefore, it represents a fundamental violation of their rights as human beings.
Second, some fear that genetic engineering will lead to eugenics. In a 2006 column, writer Charles Colson laments: “British medical researchers recently announced plans to use cutting-edge science to eliminate a condition my family is familiar with: autism. Actually, they are not ‘curing’ autism or even making life better for autistic people. Their plan is to eliminate autism by eliminating autistic people. There is no in utero test for autism as there is for Down syndrome…[Prenatal] testing, combined with abortion-on-demand, has made people with Down syndrome an endangered population…This utilitarian view of life inevitably leads us exactly where the Nazis were creating a master race. Can’t we see it?” The logic behind this argument is that human genetic enhancement perpetuates discrimination against the disabled and the “genetically unfit,” and that this sort of discrimination is similar to the sort that inspired the eugenics of the Third Reich.
A third argument is that genetic engineering will lead to vast social inequalities. This idea is expressed in the 1997 cult film Gattaca, which portrays a society where the rich enjoy genetic enhancements—perfect eyesight, improved height, higher intelligence—that the poor cannot afford. Therefore, the main character Vincent, a man from a poor background who aspires to be an astronaut, finds it difficult to achieve his goal because he is short-sighted and has a “weak heart.” This discrepancy is exacerbated by the fact that his brother, who is genetically-engineered, enjoys perfect health and is better able to achieve his dreams. To many, Gattaca is a dystopia where vast gaps between the haves and have-nots will become intolerable, due to the existence of not just material, but also genetic inequalities.
The critics are right that a world with genetic engineering will contain inequalities. On the other hand, it is arguable that a world without genetic engineering, like this one, is even more unequal. In Gattaca, a genetically “fit” majority of people can aspire to be astronauts, but an unfortunate “unfit” minority cannot. In the real world, the situation is the other way round: the majority of people don’t have the genes to become astronauts, and only a small minority with perfect eyesight and perfect physical fitness—the Neil Armstrong types—would qualify.
The only difference is that in the real world, we try to be polite about the unpleasant realities of life by insisting that the Average Joe has, at least theoretically, a Rocky-esque chance of becoming an astronaut. In that sense, our covert discrimination is much more polite than the overt discrimination of the Gattaca variety. But it seems that our world, where genetic privilege exists naturally among a tiny minority, could conceivably be less equal (and less socially mobile) than a world with genetic engineering, where genetic enhancements would be potentially available to the majority of people, giving them a chance to create better futures for themselves. Supporters of human genetic engineering thus ask the fair question: Are natural genetic inequalities, doled out randomly and sometimes unfairly by nature, more just than engineered ones, which might be earned through good old fashioned American values like hard work, determination, and effort?
“But,” the critics ask, “wouldn’t genetic engineering lead us to eugenics?” The pro-genetic engineering crowd thinks not. They suggest that genetic engineering, if done on a purely decentralized basis by free individuals and couples, will not involve any form of coercion. Unlike the Nazi eugenics program of the 1930s, which involved the forced, widespread killing of “unfit” peoples and disabled babies, the de facto effect of genetic engineering is to cure disabilities, not kill the disabled. This is a key moral difference. As pointed out by biologist Robert Sinsheimer, genetic engineering would “permit in principle the conversion of all the ‘unfit’ to the highest genetic level.” Too often, women choose to abort babies because pre-natal testing shows that they have Down syndrome or some other ailment. If anything, genetic engineering should be welcomed by pro-life groups because by converting otherwise-disabled babies into normal, healthy ones, it would reduce the number of abortions.
In addition, the world of Gattaca, for all its faults, features a world that, far from being defined along Hitler-esque racial lines, has in fact transcended racism. Being blond-haired and blue-eyed loses its racially elitist undertones because such traits are easily available on the genetic supermarket. Hair color, skin color, and eye color become a subjective matter of choice, no more significant than the color of one’s clothes. If anything, genetic engineering will probably encourage, not discourage, racial harmony and diversity.
It is true that genetic engineering may limit children’s autonomy to shape their own destinies. But it is equally true that all people’s destinies are already limited by their natural genetic makeup, a makeup that they are born with and cannot change. A short person, for example, would be unlikely to join the basketball team because his height makes it difficult for him to compete with his tall peers. An ugly person would be unable to achieve her dream of becoming a famous actress because the lead roles are reserved for the beautiful. A myopic kid who wears glasses will find it difficult to become a pilot. A student with an IQ of 75 will be unlikely to get into Harvard however hard he tries. In some way or another, our destinies are limited by the genes we are born with.
In this sense, it is arguable that genetic engineering might help to level the playing field. Genetic engineering could give people greater innate capacity to fulfill their dreams and pursue their own happiness. Rather than allow peoples’ choices to be limited by their genetic makeup, why not give each person the capability of becoming whatever he or she wants to, and let his or her eventual success be determined by effort, willpower, and perseverance? America has long represented the idea that people can shape their own destinies. To paraphrase Dr. King, why not have a society where people are judged not by the genes they inherit, but by the content of their character?
Looking at both sides, the genetic engineering controversy does raise questions that should be answered, not shouted down. Like all major scientific advances, it probably has some negative effects, and steps must be taken to ameliorate these outcomes. For example, measures should also be taken to ensure that genetic engineering’s benefits are, at least to some extent, available to the poor. As ethicists Maxwell Mehlman and Jeffrey Botkin suggest in their book Access to the Genome: The Challenge to Equality, the rich could be taxed on genetic enhancements, and the revenue from these taxes could be used to help pay for the genetic enhancement of the poor. To some extent, this will help to ameliorate the unequal effects of genetic engineering, allowing its benefits to be more equitably distributed. In addition, caution must be taken in other areas, such as ensuring that the sanctity of human life is respected at all times. In this respect, pro-life groups like Focus on the Family can take a leading role in ensuring that scientific advances do not come at the expense of moral ethics.
At the same time, we should not allow our fear of change to prevent our society from exploring this promising new field of science, one that promises so many medical and social benefits. A strategy that defines itself against the core idea of scientific progress cannot succeed. Instead of attempting to bury our heads in the sand, we should seek to harness genetic engineering for its positive benefits, even as we take careful steps to ameliorate its potential downsides.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
Genetic engineering articles from across Nature Portfolio
Genetic engineering is the act of modifying the genetic makeup of an organism. Modifications can be generated by methods such as gene targeting, nuclear transplantation, transfection of synthetic chromosomes or viral insertion. Selective breeding is not considered a form of genetic engineering.
Latest Research and Reviews

Joint genotypic and phenotypic outcome modeling improves base editing variant effect quantification
BEAN is a Bayesian approach for analyzing base editing screens with improved effect size quantification and variant classification. Applied to low-density lipoprotein (LDL)-associated common variants and saturation base editing of LDLR , BEAN identifies new LDL uptake genes and offers insights into variant structure–pathogenicity mechanisms.
- Jayoung Ryu
- Luca Pinello
Genome engineering with Cas9 and AAV repair templates generates frequent concatemeric insertions of viral vectors
AAV vectors form difficult-to-detect concatemers at Cas9 target sites.
- Fabian P. Suchy
- Daiki Karigane
- Hiromitsu Nakauchi
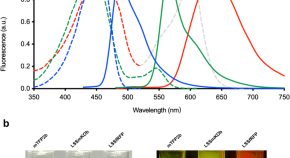
UFObow: A single-wavelength excitable Brainbow for simultaneous multicolor ex-vivo and in-vivo imaging of mammalian cells
UFObow is a single-wavelength excitable Brainbow technique, incorporating three newly developed blue-excitable fluorescent proteins. This method facilitates mapping of immune cells’ spatial distribution at a single-cell resolution.
- Fangfang Yang
Gene targeting in adult organs using in vivo cleavable donor plasmids for CRISPR-Cas9 and CRISPR-Cas12a
- Riki Ishibashi
- Ritsuko Maki
- Fumiko Toyoshima
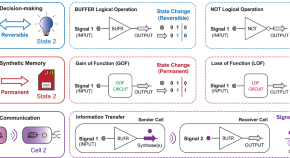
Engineering intelligent chassis cells via recombinase-based MEMORY circuits
The unification of decision-making, communication, and memory would enable the programming of intelligent biotic systems. Here, the authors achieve this goal by engineering E. coli chassis cells with an array of inducible recombinases that mediate diverse genetic programs.
- Brian D. Huang
- Corey J. Wilson
Edible mycelium bioengineered for enhanced nutritional value and sensory appeal using a modular synthetic biology toolkit
Fungi have the potential to produce sustainable foods for a growing population, but current products are based on a small number of strains with inherent limitations. Here, the authors develop genetic tools for an edible fungus and engineer its nutritional value and sensory appeal for alternative meat applications.
- Vayu Maini Rekdal
- Casper R. B. van der Luijt
- Jay D. Keasling
News and Comment
Quest: my postdoc home.
For a postdoctoral fellowship, it’s advisable to be selective about lab choice and to be clear about expectations.
- Vivien Marx
Exploitation of the microbiome for crop breeding
Rhizosphere microbiomes are shaped by both the environment and the host. A recent study of the maize microbiome reveals how plants recruit a specific microbiome to alleviate abiotic stress, and provides clues for precision microbiome engineering in agriculture.
- Jiayong Shen
- Mingxing Wang
Affinity maturation of CRISPR-engineered B cell receptors in vivo
CRISPR–Cas12a was used to directly replace mouse antibody variable chain genes with human versions in primary B cells. The edited cells underwent affinity maturation in vivo, improving the potency of HIV-1 and SARS-CoV-2 neutralizing antibodies without loss of bioavailability. Affinity maturation of edited cells also enables new vaccine models and adaptive B cell therapies.
A leap for directed evolution
- Rita Strack
Plasma membrane damage causes cellular senescence
Suda and colleagues explore the enduring consequences of plasma membrane injury in budding yeast and mammalian cells. Their findings highlight that membrane damage induces irreversible cell-cycle arrest and premature cellular senescence, whereas upregulation of plasma membrane repair suppresses them.
- Stine Lauritzen Sønder
- Jesper Nylandsted
Packaging and delivery of genome-editing tools
A study in Nature Biotechnology reports a platform that combines lentivirus capabilities with antibody recognition for targeted cell delivery and genome editing.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
January 16, 2019
Human Gene Editing: Great Power, Great Responsibility
Modifying the human germline has profound implications and must be approached with extraordinary care
By E. Paul Zehr
This article was published in Scientific American’s former blog network and reflects the views of the author, not necessarily those of Scientific American
We are at the point where our technology will soon surpass our humanity. It used to be that what we had in our jeans was just what we had in our genes. But we no longer are reliant on choosing our parents wisely. It was always going to happen. The new gene editing techniques were always going to be used to alter the genome in non-medically indicated cases. But it wasn’t anticipated we’d so soon have nontherapeutic application in human embryos.
On November 28, 2018, He Jiankui, from the Southern University of Science and Technology in Guangdong China, revealed that he had performed ex vivo gene editing on two human embryos. This was presented at the International Summit on Human Genome Editing in Hong Kong. It was not a therapeutic, medically indicated procedure, but, regardless, it was unethical and illegal in most countries.
As an actual practicing scientist and as a human, I strongly advocate for advancement of science and leveraging our advances to enhance our species. Despite that, and somewhat ironically, when I began writing my most recent book, Chasing Captain America: How Advances in Science, Engineering, and Biotechnology Will Produce a Superhuman —a book explicitly focused on examining the science of altering human biology—I was skeptical about enhancing humanity. I challenged my perspective while writing and came to think we have an obligation to modify human form and function so we have the best chance to flourish on Earth and in space. Given the recently revealed experiments in which human embryos underwent nontherapeutic gene edits and were brought to term, we need to consider deeply the implications of this and ensure that what we do and how we proceed are grounded in ethical principles agreed upon by all of us.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing . By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
The idea of genetic engineering contained in gene editing is really no different in outcome than the pioneering work of Gregor Mendel in the mid-19th century and his detailed experiments with plants, particularly beans and peas. Mendel’s detailed observations of more than 10,000 plants taken over just about 10 years were published in 1866 and revealed the targeted changes in a living organism that could be obtained by breeding for desired characteristics.
Instead of producing desired characteristics, most of the biomedical work on gene therapy in our modern age focuses on therapeutic, medically indicated applications in inherited diseases and cancers. Many of these medical conditions arise because of dysfunctions in cellular metabolism, growth and viability. Of course, it is probably natural that along with the therapeutic application, there’s been interest in applications not aimed at “curing” disease but rather altering human performance in the otherwise “healthy.”
Gene editing techniques generally involve proteins that cut DNA, such as those employed in CRISPR-Cas9, transcription activator-like effector nucleases (TALENs) and zinc-finger nucleases. The most commonly used Cas enzyme, Cas9, comes from Streptococcus pyogenes —the one that gives you strep throat and was proven viable in mouse and human cells in 2013. The basic process is that the CRISPR molecule is programmed to search for a specific nucleotide sequence among the 3 billion in the human genome. Once the correct sequence is identified, CRISPR unwinds the coils of DNA coils and “snips” the sequence out of the strand. DNA strands are then repaired in the case of a gene deletion, or, for an insertion, a new sequence can be included to alter the genome.
Performed in an embryonic germ line cell, an egg or a sperm cell, gene “edits” will be part of the genetic code that goes to the next generation. But there can be errors—in other words, editing more than intended—with targeting associated with the guide RNA used to target the deletions. It is the presence of these “off-target repeats” that indicates extreme caution and a need for better regulation before techniques like CRISPR can have safe clinical application.
As such, we as scientists and society must also balance the potential good associated with new techniques and the prospect of doing something just because we could. Gene editing places great power over altering the fundamental principles of biology, and our whole society needs to part of the discussion on what is okay to do and what is not. And we need to move quickly but not in a hurry.
It’s critical to think about the path ahead—which one to take and to where—before we arrive. Scientists and engineers at work right now are working to enable the realization of our common futures. But guiding the implementation of that future is the right and responsibility of us all and cannot be entrusted exclusively to those at work in the field and laboratories, nor to those who attempt to regulate their work, our lawmakers and bureaucrats.
The future we invent can be bright—but there are strings attached. The most important string is that we need input from as many sectors in our society as possible. The decisions that are made will literally affect the future of our species and cannot be made in isolation from our society as a whole.
Science works as a machine of chance effects with experimental outcomes; tested against a backdrop of random occurrences and biological evolution is the emergence of chance survival characteristics expanding over millions of years. There is a pace and timing to adaptations. Yet, any modifying of the human germ line—editing sperm or egg cells—has direct implications for the next generation and must be done carefully in light of regulations specifically addressing this kind of experimentation. In many countries there is a de facto moratorium on human germ line and embryo editing because such work is illegal. It is also completely unethical, not least of all because of lack of consent.
Eike-Henner Kluge from the University of Victoria has written that “germ line alteration would be performed without the consent of those who are most affected: namely, future generations.” And C.S. Lewis, when he wasn’t enthralling us with the Chronicles of Narnia, wrote in 1965’s The Abolition of Man that if a society gains power to make descendants “what it pleases, all men who live after it are patients of that power … the rule of a few hundreds of men over billions upon billions of men.”
All of us citizens, scientists, engineers and future users of human enhancement methodologies must proceed with conviction but also caution, with purpose but also extreme care. It’s critical to appreciate the implications of the power of science as articulated by Richard Dawkins that “science is the most powerful way to do whatever it is you want to do. If you want to do good, it’s the most powerful way of doing good. If you want to do evil, it’s the most powerful way to do evil.” Never before have we—or any other species on this planet—had such influence and so much power over the fundamental nature of our own biology.
The nontherapeutic use of gene editing on human embryos was and remains unethical and illegal on every level. Yet, now we need to leverage attention on gene editing and human enhancement into a real conversation about the future our species. As the late Stan Lee wrote back in 1962 in Amazing Fantasy , the first comic book featuring Spider-Man, “with great power there must also come—great responsibility!”
Both must be exercised judiciously here and now in real life.
Home — Essay Samples — Science — Technology & Engineering — Genetic Engineering
Essays on Genetic Engineering
What makes a good genetic engineering essay topic.
When it comes to writing a captivating genetic engineering essay, the topic you choose is paramount. It not only grabs the reader's attention but also allows for effective exploration of the subject matter. So, how can you brainstorm and select a standout essay topic? Here are some recommendations:
- Brainstorm: Kickstart your ideas by brainstorming topics related to genetic engineering. Consider the latest advancements, ethical concerns, controversial issues, or potential future applications. Jot down any ideas that come to mind.
- Research: Once you have a list of potential topics, conduct thorough research to gather relevant information and understand different perspectives. This will help you evaluate the feasibility and depth of each topic.
- Consider Interest: Choose a topic that genuinely piques your interest. Writing about something you are passionate about will make the entire process more enjoyable and motivate you to delve deeper into the subject matter.
- Relevance: Ensure that the chosen topic is relevant to genetic engineering. It should align with the scope of the subject and allow you to explore various aspects related to it.
- Uniqueness: Strive for a unique and imaginative topic that stands out from the ordinary. Steer clear of generic subjects and instead focus on specific areas or emerging trends within genetic engineering.
- Controversy: Controversial topics often generate more interest and discussion. Consider exploring ethical dilemmas, potential risks, or societal impacts of genetic engineering to add a thought-provoking element to your essay.
- Depth and Scope: Assess the depth and scope of each topic. Make sure it provides enough material for a comprehensive essay without being too broad or too narrow.
- Audience Appeal: Keep your target audience in mind. Choose a topic that would captivate readers, whether they are experts in the field or individuals with limited knowledge about genetic engineering.
- Originality: Strive for originality in your topic selection. Look for unique angles, lesser-known areas, or innovative applications of genetic engineering that can make your essay stand out.
- Personal Connection: If possible, choose a topic that connects with your personal experiences or future aspirations. This will enhance your engagement and make your essay more meaningful.
Igniting Thought: The Finest Genetic Engineering Essay Topics
Below are some of the most captivating genetic engineering essay topics to consider:
- Genetic Engineering and the Future of Human Evolution
- The Ethical Dilemmas of Designer Babies
- Genetic Engineering in Agriculture: Balancing Benefits and Concerns
- CRISPR-Cas9: Unleashing Revolutionary Potential in Genetic Engineering
- The Potential of Genetic Engineering in Cancer Treatment
- Genetic Engineering's Role in Creating Sustainable Food Sources
- Genetic Engineering and Animal Welfare: Navigating Ethical Considerations
- Genetic Engineering and its Impact on Biodiversity
- The Social and Economic Implications of Genetic Engineering
- Genetic Engineering's Influence on Human Longevity
- Enhancing Athletic Performance: The Power of Genetic Engineering
- Genetic Engineering Techniques for Disease Prevention and Treatment
- Genetic Engineering's Role in Environmental Conservation
- Genetic Engineering and the Preservation of Endangered Species
- The Psychological and Societal Effects of Genetic Engineering
- The Pros and Cons of Genetic Engineering for Non-Medical Purposes
- Exploring the Potential Risks and Benefits of Genetic Engineering in Space Exploration
- Genetic Engineering and the Creation of Biofuels
- The Morality of Genetic Engineering: Insights from Religious and Philosophical Perspectives
- Genetic Engineering's Role in Combating Climate Change
Thought-Provoking Genetic Engineering Essay Questions
Consider these stimulating questions for your genetic engineering essay:
- How does genetic engineering impact the concept of natural selection?
- What are the potential consequences of genetic engineering on human genetic diversity?
- Is it ethically justifiable to use genetic engineering for cosmetic purposes?
- How does genetic engineering contribute to the development of personalized medicine?
- What are the social implications of genetically modifying animals for human consumption?
- How does the use of genetic engineering in agriculture affect food security?
- Should genetic engineering be used to resurrect extinct species?
- What are the potential risks and benefits of genetically modifying viruses for medical purposes?
- How does genetic engineering influence the balance between individual rights and societal well-being?
- Can genetic engineering be the solution to eradicating genetic diseases?
Provocative Genetic Engineering Essay Prompts
Here are some imaginative and engaging prompts for your genetic engineering essay:
- Imagine a world where genetic engineering has eliminated all hereditary diseases. Discuss the potential benefits and drawbacks of such a scenario.
- You have been granted the ability to genetically engineer one aspect of yourself. What would you choose and why?
- Write a fictional story set in a future where genetic engineering is widespread and explore the consequences it has on society.
- Reflect on the ethical considerations of genetically modifying animals for entertainment purposes, such as creating glow-in-the-dark pets.
- Create a persuasive argument for or against the use of genetic engineering in enhancing human intelligence.
Answering Your Genetic Engineering Essay Queries
Q: Can I write about the history of genetic engineering?
A: Absolutely! Exploring the historical context of genetic engineering can provide valuable insights and set the foundation for your essay.
Q: How can I make my genetic engineering essay engaging for readers with limited scientific knowledge?
A: Simplify complex concepts and terminologies, provide relevant examples, and use relatable analogies to help readers grasp the information more easily.
Q: Can I express my personal opinion in a genetic engineering essay?
A: Yes, expressing your personal opinion is encouraged as long as you support it with logical reasoning and evidence from reputable sources.
Q: Are there any potential risks associated with genetic engineering that I should discuss in my essay?
A: Yes, incorporating a discussion on the potential risks and ethical concerns surrounding genetic engineering is essential to provide a balanced perspective.
Q: Can I include interviews or case studies in my genetic engineering essay?
A: Absolutely! Interviews or case studies can add depth and real-life examples to support your arguments and make your essay more compelling.
Remember, when writing your genetic engineering essay, let your creativity shine through while maintaining a formal and engaging tone.
The Ethics of Genetic Engineering in Human Enhancement
Exploring the pros and cons of genetic engineering, made-to-order essay as fast as you need it.
Each essay is customized to cater to your unique preferences
+ experts online
Pros and Cons of Genetic Engineering: The Need for Proper Regulation
Genetic engineering, ethical issues of genetic engineering, the dangers of genetic engineering to humanity, let us write you an essay from scratch.
- 450+ experts on 30 subjects ready to help
- Custom essay delivered in as few as 3 hours
The Use and Ethics of Genetic Engineering
The potential and consequences of genetic engineering, the issue of the use of genetic modification of humans, reasons why genetic engineering should be banned, get a personalized essay in under 3 hours.
Expert-written essays crafted with your exact needs in mind
Genetic Engineering: an Overview of The Dna/rna and The Crispr/cas9 Technology
Review of human germline engineering, positional cloning of genetic disorders, engineering american society: the lesson of eugenics, bioethical issues related to genetic engineering, cloning and ethical controversies related to it, genetic editing as a possibility of same-sex parents to have children, adhering to natural processes retains the integrity of a natural human race , genetically modified organisms: soybeans, gene silencing to produce milk with reduced blg proteins, the role of crispr-cas9 gene drive in mosquitoes, the life of gregor mendel and his contributions to science, eugenics, its history and modern development, morphological operation hsv color space tree detetction, cytogenetics: analysis of comparative genomic hybridization and its implications, genetically engineered eucalyptus tree and crispr, review of the process of dna extraction, review of the features of the process of cloning, heterologous gene expression as an approach for fungal secondary metabolite discovery, review of the genetic algorithm searches.
Genetic engineering (also called genetic modification) is a process that uses laboratory-based technologies to alter the DNA makeup of an organism.
Genetic engineering as the direct manipulation of DNA by humans outside breeding and mutations has only existed since the 1970s. In 1972, Paul Berg created the first recombinant DNA molecules by combining DNA from the monkey virus SV40 with that of the lambda virus. The first field trials of genetically engineered plants occurred in France and the US in 1986, tobacco plants were engineered to be resistant to herbicides.
It is a set of technologies used to change the genetic makeup of cells, including the transfer of genes within and across species boundaries to produce improved or novel organisms. New DNA is obtained by either isolating and copying the genetic material of interest using recombinant DNA methods or by artificially synthesising the DNA. Used in research and industry, genetic engineering has been applied to the production of cancer therapies, brewing yeasts, genetically modified plants and livestock, and more.
Relevant topics
- Engineering
- Space Exploration
- Natural Selection
- Charles Darwin
- Mathematics in Everyday Life
- Time Travel
- Stephen Hawking
By clicking “Check Writers’ Offers”, you agree to our terms of service and privacy policy . We’ll occasionally send you promo and account related email
No need to pay just yet!
We use cookies to personalyze your web-site experience. By continuing we’ll assume you board with our cookie policy .
- Instructions Followed To The Letter
- Deadlines Met At Every Stage
- Unique And Plagiarism Free
Genetic Engineering Essay Guide With 70 Hot Topics
Genetic engineering has been a subject of heated debate. You will find many essays on genetic engineering, asking you to debate for or against, discuss its ethical implications, or emerging congenital disease.
With all these at hand, you may be tempted to opt-out immediately. However, this top-notch guide seeks to make genetics essay writing as fun and as straightforward as possible. Ride along to see the magic!

What Is An Essay on Genetic Engineering?
Now, genetic engineering in itself is the use of biotechnology to manipulate an organism’s genes directly. Therefore, essays on genetics will require students to explore the set of technologies used to change cells’ genetic makeup. These include the transfer of genes within and across species boundaries to produce novel or improved organisms.
We have various areas of genetic engineering, such as:
- Human genetic engineering definition: Deals with genetic engineering techniques applied to humans
- Genetic engineering in plants: Concentrates on genetically modified plant species
Genetic engineering is mostly applied in medicine and thus its technicality. I know this is a field that most students approach with reverence and uttermost humility. Nonetheless, it doesn’t have to be that way. The next few lines might change your opinion on genetic engineering forever!
Why is genetic engineering necessary?
Importance of Genetic Engineering
It is essential in the following ways:
- Ensures that seed companies can protect modified seed varieties as intellectual property.
- Leads to production o organisms with better traits
- Helps maintain the ecosystem
You can see why this field is unavoidable regardless of the negative talk behind it.
Genetically Engineering Plants and Animals – Essay Sample
Young in practice, a little over forty years old, genetic engineering has provided the scientific community with an abundance of knowledge once thought absurd. Genetic engineering means deliberately changing the genome of an organism to acquire some desired traits during its cultivation. On the whole, genetic engineering has a multitude of advantages and disadvantages when it comes to using it on animals and plants; the most prominent advantages include disease resistance, increased crop yields, and a decrease in need for pesticides and antibiotics, whereas disadvantages include the potential for emergence of stronger pathogens, as well as various unexpected consequences. This current paper discusses the pros and cons of using genetic engineering on plants, animals, and provides a synthesis, arguing that, despite its disadvantages, it still serves as a pivotal advantage not only within the scientific community, but also society.
The Advantages of Using Genetic Engineering
The impact of genetic engineering on society can be seen at various aspects, affecting various aspects of social and physical organic life, especially in terms of human beings. The practice consists of the specific selection and removal of genes from organic organisms and inserting them into another. The practice, though still young in practice and not yet deemed completely socially acceptable, makes the possibility of curing diseases once thought incurable a reality, thereby inherently improving the life of both humans and non-human animals. It has many positive effects on society, an example being in Uganda bananas, a main source of caloric intake, are susceptible to the emergence of new diseases that affects their production because of the disease’s potency. Ugandan scientists have successfully used a genetic modification, inserting a pepper gene into bananas, which prevents the fruit from getting the disease (Bohanec, 2015). Furthermore, through genetic engineering, tissue, skin cells, and other forms of organic matter can be grown and used in replacing damaged, worn, or malfunctioning organs and tissues thereby prolonging human life and benefiting their quality of life. The practice helps better advance both the scientific and medical field, both of which are essential in discovering how to better life on Earth.
Genetic engineering, as previously mentioned, can be used to grow and replace damaged tissue or organs, aiding in the betterment and prolonging of human life; it can cure diseases once though incurable, an example being AIDS and cancer. Millions of people around the world suffer from AIDS and cancer, both posing a severe risk to the overall health of the person. More than 900,000 lives were taken by AIDS in 2017 (UNAIDS, 2018). Similarly, over 600,000 were taken by cancer in the following year (NIH, 2018). Genetic engineering makes the possibility of eradicating these diseases a reality. In theory, genetic engineering can help those who suffer from these diseases live longer, healthier, fuller lives by eradicating the disease in its entirety. Though it would not be an easy feat, nor a cheap one, it could still help further advance and better human life and prolong the human life span. People would no longer live in fear of dying from these prolific diseases. Furthermore, genetic engineering, despite the naysayers and opposers of the practice, is another step in organic evolution. From plants to animals, the practice has the chance to achieve strides within scientific history that can greatly benefit the planet in its entirety. From eliminating hunger, to eradicating once prolific diseases, genetic engineering can provide a better, longer, and higher quality of life and tackle bounds once thought impossible the scientific community.
Genetically engineered plants and animals may provide a wide array of benefits that might be pivotal for humanity in the modern world. These benefits include the possibility of developing such plant cultivars that would be resistant to a wide variety of pathogens and diseases caused by microorganisms such as viruses (Ginn, Alexander, Edelstein, Abedi, & Wixon, 2013). If such plant cultivars are created, it might become unnecessary to use chemicals in order to battle these plant diseases. This is clearly a major benefit, since it means better preserving the natural environment and avoiding the use of chemicals that may contaminate soils and waters, as well as kill wildlife.
The Disadvantages of Using Genetic Engineering
The use of genetic engineering to alter plants and animals used in agriculture and husbandry may also have a variety of adverse consequences. For instance, it should be noted that high rates of resistance to disease might have a serious flip side. More specifically, the pathogenic microorganisms (such as bacteria and viruses) can usually mutate quickly in order to adapt to the new conditions. This means that if new cultivars or breeds of plants or animals with high resistance to diseases are created, the pathogens may adapt to these changes in their “hosts” and turn stronger, thus becoming capable of infecting the new cultivars or breeds (Ayres, n.d.). This might again necessitate the use of chemicals or antibiotics; only now stronger drugs or pesticides would be needed. In addition, the old cultivars or breeds may also become infected by the new microorganism strains, and these strains will probably cause more severe diseases in the “original” plants and animals and will be more difficult to cure or prevent.
Another negative possibility is accidentally creating some invasive species that may harm the local ecosystems. For instance, if new plants are made in such a manner that the local species of animals cannot eat them, and then humans lose control over their growth, the new plants may pose a danger to the original plants growing in the given ecosystem, therefore disrupting the ecosystem. For example, in 1984 a patch of seaweed labelled as Caulerpa taxifolia was bred with another robust strain of seaweed identified by scientists as Caulerpa taxifolia (Vahl) C. Agandh . The initial objective was to breed an aquarium plant, however, after a sample escaped in 1984 into the Mediterranean Sea, being found off the coast of both the United States and Australia in 2000, it was found that the strain’s taste was subpar to marine wild life. It was eventually poisoned by the California state government to avoid further damage to marine life and the marine ecosystem and was consequently outlawed by hundreds of countries. The World Conservation Union named it one of the 100 World’s Worst Invasive Alien Species, despite it being manmade (Cellania, 2008).
Finally, there is always the risk of “going too far” when practicing genetic engineering (Bruce & Bruce, 2013). Indeed, it should be noted that the humanity has used various methods of cultivation for millennia in order to breed for specific traits. For example, in 1956, Warwick Kerr, a Brazilian geneticist, imported an aggressive breed of African honeybee to breed with a European species to aid in the decreasing bee population epidemic. Provoked by even the smallest of instigation, after over 26 swarms of the aggressive bee escaped from the apiary in Sao Paulo, they wreaked havoc in North and South America, found in the United States in the early 90s. Nevertheless, genetic engineering is a fast and radical method to change organisms, and very little, if any, data is available to predict the potential adverse impacts of its utilization. It may be difficult to tell when (if at any point) one must stop the process of genetic engineering to avoid unexpected adverse influences of its utilization.
Genetic engineering, despite its disadvantages, can help progress humanity in ways that once seemed impossible. With the environmental and physical epidemics surrounding the planet, the practice can serve as a benefit to resolving the hunger crisis, the preservation of endangered plant and animal species, bringing certain species back from extinction, and so much more. It should be stressed that the utilization of biotechnology and genetic engineering may bring a wide array of significant benefits, which may be of great use to the humanity nowadays. The creation of breeds and cultivars which are immune to disease, resistant to harsh environmental conditions, are cheap to grow, and provide better nutritional value for people might be extremely helpful in reducing the amount of chemicals, pesticides, and antibiotics needed to grow these animals or plants, and, consequently, to help preserve the environment. However, it should also be remembered that genetic engineering might have a wide array of adverse impacts, such as the emergence of new, stronger pathogens, the creation of invasive species, and a multitude of negative consequences that no one knew to expect.
Genetic Engineering Essay Structure
A top-rated genetic engineering essay comes in the manner outlined below:
- Genetic engineering essay introduction: Provide context for your paper by giving a well-researched background on the subject of discussion. Include the thesis statement which will provide the direction of your writing.
- Body: Discuss the main points in detail with relevant examples and evidence from authentic and reliable sources. You can use diagrams or illustrations to support your argument if need be.
- Genetic engineering conclusion: Finalize your paper with a summative statement and a restatement of the thesis statement while showing the genetic engineering process’s implication. Does it add any value to society?
Armed with this great treasure of knowledge, you are good to begin writing your paper. However, we have quality genetic engineering essay topics from expert writers to start you off:
Interesting Genetic Engineering Persuasive Essay Topics
- How human curiosity has led to new advancements and technologies in genetics
- History of genetically modified food
- Discuss the process of genetic engineering in crops
- Evaluate the acceptance of genetically modified crops worldwide
- Analyze the leading countries implementing genetic engineering
- Does genetic engineering produce a desired characteristic?
- What are the legal implications of genetic engineering
- The role of scientists in making the world a better place
- Why coronavirus is a game-changer in the field of genetic engineering
- The effectiveness of genetic engineering as a course in college
Great Topics on the Disadvantages of Genetic Engineering in Humans
- Why changing the sequence of nucleotides of the DNA affects human code structure
- Impact of genetic engineering human lifespans
- Genetic engineering and population control
- Ethical questions to consider in human genetic engineering
- Unintended side effects on humans
- Increasing the risk of allergies
- The foundation of new weapon technologies
- Disadvantages of trait selection before birth
- The greater risk of stillbirth
- Why ladies are at risk with genetic engineering
Why is Genetic Engineering Good Essay Topics
- Genetic engineering and disease prevention
- The creation of a healthy and better society
- Production of drought-resistant crops
- Crop pollen spreads further than expected
- Survival of human species
- Birth of healthy children with desirable traits
- Solving food insecurity problems globally
- Elimination of fertility issues for couples
- Medical advancements as a result of genetic engineering
- Reducing the prevalence of schizophrenia and depression
Good Genetic Engineering Topics
- The development of genetic engineering in the modern world
- Application of ethics in genetic engineering
- Societal class versus genetic engineering
- Impact of genetic engineering on natural selection and adaptation
- Detection of toxins from GMO foods
- Social effects of genetic engineering
- Why people are becoming increasingly resistant to antibiotics
- How gene editing affects the human germline
- Medical treatment opportunities in genetic engineering
- The relationship between molecular cloning and genetic engineering
Impressive Genetic Engineering Research Paper Topics
- Impact of genetic engineering on food supply
- The taste of GMO food versus ordinary food
- GMOs and their need for environmental resources
- Why genetic engineering may face out the use of pesticides
- Reduced cost of living and longer shelf life.
- Growth rates of plants and animals
- Application of genetic engineering on soil bacteria
- New allergens in the food supply
- Production of new toxins
- Enhancement of the environment for toxic fungi
Latest Genetic Engineering Ideas
- The discovery of vaccines through genetic engineering
- Biological warfare on the rise
- Change in herbicide use patterns
- Mutation effects in plants and animals
- Impact of gene therapies
- Does genetic engineering always lead to the desired phenotype?
- Genetic engineering in mass insulin production
- Role of genetic engineering in human growth hormones
- Treating infertility
- Development of monoclonal antibodies
Pro and Cons of Genetic Engineering in Humans Topic Ideas
- Possibility of increased economic inequality
- Increased human suffering
- The emergence of large-scale eugenic programmes
- Rise of totalitarian control over human lives
- The concentration of toxic metals in genetic engineering
- Creation of animal models of human diseases
- Using somatic gene therapy on Parkinson’s disease
- Production of allergens in the food supply
- Redesigning the world through genetic engineering
- Bioterrorism: A study of the issue of emerging infectious diseases
I believe that by now explain genetic engineering in a sentence and write an essay on it effortlessly. If this still seems complicated for you, we have professional essay writers at your disposal.
You are sure of trustful, custom essay writing help at the convenience of your bedroom study table. It is cheap and high-quality.
Place your order online today!

Leave a Reply Cancel reply
Save my name, email, and website in this browser for the next time I comment.
Post Comment
Get it done risk-free
With top experts across the board, 10 days to request free revisions, and a 60-day money-back guarantee, sleep tight while we handle your.
- Search Menu
- Special Issues
- Author Guidelines
- Submission Site
- Open Access
- About Journal of Law and the Biosciences
- About the Duke University School of Law
- About the Harvard Law School
- About Stanford Law School
- Editorial Board
- Advertising and Corporate Services
- Journals Career Network
- Self-Archiving Policy
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
I. i. introduction, ii. the germline gene editing science race, iii. iii. legal approaches to human germline editing, iv. iv. from prohibition to regulation of hgge, v. v. the blurring boundary between treatment and enhancement, vi. vi. individual and collective dimensions of human rights law, vii. vii. from human rights to self-regulation by the scientific community, viii. viii. conclusion.
- < Previous
Rewriting the human genome, rewriting human rights law? Human rights, human dignity, and human germline modification in the CRISPR era
Britta van Beers is professor of Biolaw and Bioethics at VU University Amsterdam, Faculty of Law, Department of Legal Theory. Britta van Beers teaches and writes about the legal–philosophical aspects of the governance of biomedical technologies. She is particularly intrigued by the legal and philosophical questions raised by assisted reproductive technologies, such as wrongful birth and wrongful life claims, selective reproduction, and reproductive tourism. In more recent work, she has focused on issues related to personalized medicine and human gene editing. She has actively contributed to public debates on new technologies by writing essays and op-eds, and speaking as an expert for the Dutch Senate and House of Representatives. Her publications include the volumes Personalised Medicine, Individual Choice and the Common Good (co-edited with Sigrid Sterckx and Donna Dickenson, Cambridge University Press 2018), Symbolic Legislation and Developments in Biolaw (co-edited with Bart van Klink and Lonneke Poort, Springer 2016), and Humanity across International Law and Biolaw (co-edited with Wouter Werner and Luigi Corrias, Cambridge University Press 2014).
- Article contents
- Figures & tables
- Supplementary Data
Britta C van Beers, Rewriting the human genome, rewriting human rights law? Human rights, human dignity, and human germline modification in the CRISPR era, Journal of Law and the Biosciences , Volume 7, Issue 1, January-June 2020, lsaa006, https://doi.org/10.1093/jlb/lsaa006
- Permissions Icon Permissions
In most legal orders, human germline modification is either prohibited or severely restricted. A recurring thought in these legal frameworks is that heritable genome editing would result in practices that are at odds with principles of human rights, such as dignity, justice, and equality. However, now that CRISPR is bringing heritable genome editing within human reach, the question has risen as to whether these human rights bans still make sense. The call is growing louder to lift the ban on heritable genome editing for therapeutic purposes as soon as the technology is safe for introduction in the clinic. This article critically examines these recent proposals from a human rights perspective. First, it examines the question as to how realistic the proposed distinction between the therapeutic and the nontherapeutic uses of human germline modification is in the CRISPR era. Second, it argues that these proposals rely on a one-dimensional understanding of the meaning of human rights for this issue. Finally, it suggests that this one-dimensional understanding paves the way for a regime of self-regulation by the scientific community that leaves little room for public debate on the question as to whether or how human germline modification fits in the long-term aspirations of society.
In November 2018, biophysicist, He Jiankui, announced on YouTube 1 that two genetically modified babies, ‘beautiful little Chinese girls named Lulu and Nana’, had come ‘crying into the world as healthy as any other babies’. 2 The Chinese scientist had used the genetic cut-copy-paste technology CRISPR-Cas9 to modify the DNA of human embryos and then implanted these for a pregnancy. With his ‘genetic surgery’, as he calls it himself, He had targeted a gene called CCR5 in an effort to create babies who are resistant to infection from HIV. In total, he involved eight couples in this project, of whom the male partner is HIV-positive. Two days after his YouTube announcement, while speaking during the International Summit on Human Genome Editing in Hong Kong, He informed a stunned audience that a third genetically modified baby was on its way. 3
The news on the Chinese ‘CRISPR babies’ sent a shockwave throughout the world. Members of the global scientific community responded with a mixture of indignation and horror upon learning how He had defied scientific conventions, ignored basic rules for research on human subjects, and violated multiple norms of medical practice. The technology that He used is still in a very experimental stage, and much work needs to be done to make it safe and effective. Indeed, his data suggest that the procedure was only partially successful, if at all, resulting in a mosaic of altered and unaltered cells for both embryos 4 and off-target genetic changes. 5 Moreover, the on-target modifications lead to a novel genetic variation of CCR5 that is similar but not identical to the known mutation of CCR5 that confers natural HIV resistance. 6 As such, He and his team created genetic ‘changes that had never been seen in humans before’. 7 Yet He chose to go ahead, thereby exposing the health of the twins to huge risks. As Jennifer Doudna, one of the inventors of CRISPR-Cas9, summarizes the scientific upheaval: ‘He’s fateful decision to ignore the basic medical mantra of “do no harm” and risk the unintended consequences will likely be remembered as one of the most shocking misapplications of any scientific tool in our history.’ 8
Nevertheless, it is clear that the birth of a genetically modified baby ‘was something everyone in the burgeoning, multibillion-dollar field of genome editing knew would come one day.’ 9 In contrast, for the public at large, the news served as a wake-up call on the possibilities of human germline gene editing (HGGE) and its potentially far-reaching implications for the future of human reproduction. If He’s claims are true, then it can be said that he has single-handedly brought humankind a significant step closer to taking genetic fate into its own hands. Admittedly, reproductive technologies such as noninvasive prenatal testing (NIPT) and preimplantation genetic diagnosis (PGD) have made it technologically possible to genetically select a certain type of child for quite some time. Yet in those cases of selective reproduction, the child’s entire genetic profile is still the outcome of a biological recombination of parental genes. CRISPR opens up the possibility of genetically modifying one’s offspring. That means that it becomes possible to override the outcomes of the genetic lottery.
How should legal orders respond to the birth of the ‘CRISPR babies’? Interestingly, both national and international legal systems have long anticipated the arrival of this groundbreaking technology. Already since the late 1990s, when the first legal frameworks for the regulation of biomedical developments came into existence, the use of HGGE technologies for reproductive purposes has been prohibited in many national and international jurisdictions. A recurring thought in these legal frameworks is that genetically modifying offspring results or may result in practices that are at odds with human rights and their underlying principles, such as dignity, justice, and equality. However, now that HGGE has come within human reach, a worldwide debate has erupted about the question as to whether these human rights bans still make sense in the CRISPR era. As I will discuss hereafter, the first cracks in existing human rights legal frameworks are starting to appear. Moreover, in scientific, political, and academic circles, the call is growing louder to move from prohibition to regulation of HGGE. According to these recent proposals, the ban on heritable genome editing can be lifted for therapeutic purposes as soon as the technology is safe for introduction in the clinic.
In this article, I examine the shift that is currently taking place in the discussion on human rights and HGGE. I argue that many of the calls to lift or reconsider existing bans and restrictions on HGGE are rooted in a novel, but impoverished understanding of the meaning of human rights for this issue. To substantiate that claim, I compare the understanding of human rights which underlies these recent proposals with the human rights approaches to HGGE as contained in existing legal frameworks in this field, such as UNESCO’s Universal Declaration on the Human Genome and Human Rights (1997) and the Council of Europe’s Convention of Human Rights and Biomedicine (1997).
I start with an overview of the technological (Section II) and legal developments (Section III) that preceded the birth of the first CRISPR babies. This is followed by an examination of proposals to allow HGGE for therapeutic purposes once the technology is safe for clinical application (Section IV). In the remainder of the article, I offer a critical analysis of these proposals on three levels. First, I examine the question as to how realistic the proposed distinction between the therapeutic and the nontherapeutic uses of human germline editing is in the CRISPR era (Section V). Second, I argue that these proposals rely on a one-dimensional understanding of the meaning of human rights for this issue (Section VI). Finally, I suggest that this one-dimensional understanding paves the way for a regime of self-regulation by the scientific community that leaves little room for public debate on the question as to whether or how HGGE fits in the long-term aspirations of society (Section VII).
For a few years now, 10 scientists worldwide are using CRISPR-Cas9 to modify the genetic code of organisms. As CRISPR offers the possibility of cut, copy, and paste with the letters C, G, A, and T in which DNA is encoded, this process is also known as gene editing. This revolutionary technology is relatively cheap and easy to use. Even amateurs have discovered CRISPR. Do-It-Yourself CRISPR kits are available online, offering so-called biohackers the opportunity to experiment with DNA in their home labs and garages, including their own DNA. 11 Members of the biohacking movement have embraced CRISPR as a means to bring an end to the biotech sector’s monopoly on genetic engineering and thereby ‘democratize’ the life sciences. Yet, also for the biotech sector, the possibilities of CRISPR are myriad. The commercial stakes are high in the pursuit of CRISPR applications, as is strikingly illustrated by the CRISPR ‘patent wars’ 12 that are currently taking place.
Correspondingly, CRISPR is already having a real impact on the world of plants and animals. Various types of apes, dogs, birds, insects, and fish have been ‘welcomed to the CRISPR zoo’. 13 Wild plans are made, ranging from bringing back extinct animals such as the mammoth to producing ‘micropigs’ that grow to only around 15 kilograms, as a new type of pet. Indeed, as a 2016 article in the journal Nature on the subject concludes, ‘the CRISPR zoo is expanding fast and the question now is how to navigate the way forward’. 14
In the light of the birth of the genetically modified babies in China, these last words have acquired a new urgency. If, at some point in the future, the technology is deemed safe for application on human life, would there be convincing reasons against welcoming the human species to the CRISPR zoo too? If there are not, how can legal orders guide this process of human ‘self-domestication’ 15 into the right direction, ensuring a sustainable, responsible, and equitable usage of this technology? Will existing legal bans on genetic modification have to make place for regulatory frameworks in that process? If so, will the formulation of a set of ‘rules for the human zoo’ be necessary in order to actively confront the challenges of human genetic modification, as German philosopher Peter Sloterdijk argued two decades ago in his provocative essay of the same name? 16
In this discussion, two possible applications of CRISPR on humans should be distinguished. CRISPR can be used for the purpose of ‘somatic gene editing’. This type of genetic intervention affects the genes in the targeted cells of existing patients. As such, somatic modifications are not inherited by future generations. This article focuses on a different, more radical form of genetic modification: the use of CRISPR to alter the DNA of human embryos or gametes. This type of intervention is commonly known as human germline gene editing because it involves the genetic modification of germ cells. Unlike somatic gene editing, HGGE affects all body cells of the future individuals in question, from their brains and organs to their vessels and skin. Moreover, because the changes will equally come to expression in their gametes, the genetic modifications are also inherited by their offspring and their offspring’s offspring. In that sense, rewriting the human germline also means ‘rewriting the gene pool of future generations’. 17
Despite legal bans on germline editing, breakthroughs in this field have followed one after the other in recent years as part of what appears to be an international science race. That race took off in April 2015, when Chinese stem cell researcher Junjiu Huang and his team published an article in which they described their attempts to genetically modify human embryos. Although the researchers had used nonviable embryos for their study (thereby excluding the possibility of initiating a pregnancy), 18 and although they had failed to repair the targeted genetic deficiency, their CRISPR experiment created a huge upheaval: they had broken the taboo on using CRISPR to genetically modify human life.
Exactly a year later, in April 2016, a genetically modified Jordanian baby was born in Mexico. The boy’s birth did not become known until September 2016, when John Zhang, the New York-based Chinese–American fertility doctor who was responsible for the genetic modification, came forward with the news. 19 Zhang had not used CRISPR for the modification, but a procedure called ‘human nuclear genome transfer’ (HNGT) also known as ‘mitochondrial replacement therapy’. 20
First, Zhang and his team transplanted the nucleus of the intending mother’s egg cell into an enucleated egg cell donated by a third party. They then fertilized the resulting, composite egg cell with the intending father’s sperm. This particular HNGT technique is known as ‘maternal spindle transfer’. Because the gametes of three parties are brought together during HNGT, popular media referred to the event as the birth of the first ‘three parent baby’.
The parents had contacted Zhang because the mother is carrier of a mitochondrial disorder: Leigh Syndrome. By replacing her dysfunctional mitochondrial DNA with the egg donor’s healthy mitochondrial DNA, Zhang aimed to prevent the transmission of the mitochondrial disorder to the boy. How successful the intervention was, especially given possibly adverse long-term effects on the boy’s health, 21 remains to be seen.
It should be noted at this point that only 15–20 per cent of all mitochondrial diseases are caused by mutations in mitochondrial DNA. For the remaining 80–85 per cent, nuclear genome transfer is useless. 22 However, HNGT can also be used for other purposes. Indeed, a few months after the birth of the Jordanian boy, in January 2017, another ‘three parent baby’ was born, a Ukrainian girl, as part of a fertility treatment. This time, a different HNGT technique was used, pronuclear transfer, which involves transferring the nuclear material of the intending mother’s fertilized egg into a fertilized enucleated donor egg. The Ukrainian fertility clinic in question had used HNGT because the 34-year-old intending mother had been suffering from ‘unexplained infertility’. 23 In a similar vein, the aforementioned John Zhang was making plans to offer HNGT on a commercial basis for the rejuvenation of egg cells through his start-up ‘Darwin Life’, 24 until the US Food and Drug Administration sent him a warning. 25
HNGT has a much smaller genetic impact than CRISPR germline editing. It only affects mitochondrial DNA, which constitutes less than 1 per cent of a person’s total DNA, thereby leaving the nuclear DNA unaffected. Even so, there are convincing reasons to regard HNGT as a form of human germline genetic modification. 26 First, it is hard to deny that the procedure affects the genetic composition of germline cells. Second, if daughters are born, as was the case in Ukraine, they will pass on these genetic changes to their offspring (inheritance is generally 27 through the maternal line).
In the summer of 2017, the first successful CRISPR modification of embryonic nuclear DNA took place. The Kazakh–American biologist Shoukhrat Mitalipov and his American–Chinese–South-Korean team managed to repair a genetic mutation that is linked to a serious heart disease. 28 The resulting ‘CRISPR embryos’ were, however, not implanted for a pregnancy. With the birth of the Chinese ‘CRISPR babies’ in November 2018, this last step now also seems to have been taken.
Although He’s actions were widely condemned, the science race still appears to be in full swing. In June 2019, the Russian molecular biologist Denis Rebrikov announced his intentions to genetically modify human embryos for reproductive purposes before the end of the year, targeting the same gene as He did. 29 In addition, he unfolded plans to use HGGE to prevent the transmission of deafness. 30 Rebrikov is already conducting experiments on human egg cells to be able to achieve this goal. 31 His long-term plans include using HGGE to target genes related to dwarfism and blindness. 32
From a legal perspective, the widespread condemnation of He’s efforts to create genetically modified babies is quite understandable. ‘Globally’, as Françoise Baylis writes, ‘the political consensus on heritable human genome editing—such as it is—inclines toward an outright ban, and if not a ban, at least a moratorium.’ 33 Interestingly, the oft-heard expression that the law inevitably lags behind technological developments proves false in the case of HGGE. Most existing legal bans and restrictions have been effective for quite a while. Indeed, from the very first debates on the regulation of biomedical developments, the possibility of genetically designing children played a vital role within the public imagination. 34 Moreover, in that context, human rights and human dignity are often invoked as main frame of reference. However, even if the first bans on HGGE were established already in the late 1990s and are typically rooted in human rights discourse, these legal frameworks are currently under pressure. The first cracks are starting to appear ever since CRISPR and HNGT put human germline genetic modification back on the legal–political agenda. In this section, I first explore national legal frameworks in this field (Section III.A). I then examine the international legal landscape (Section III.B). Finally, I address several pressing questions with regard to these legal frameworks (Section III.C).
III.A. III.A. National legal approaches to human germline modification
Most countries with legal frameworks for the regulation of biomedical developments either ban or severely restrict HGGE technologies. 35 Admittedly, the scope, means, and nature of these national bans and restrictions vary greatly. 36 At one end of the spectrum, there are countries where human germline modifications are categorically prohibited and accompanied by criminal sanctions, such as many European countries, Australia, Canada, and Brazil. 37 At the other end of the spectrum, there are countries which allow, for example, HGGE for research purposes. Yet, also these more permissive national orders, of which China, the USA, and the UK are the most prominent, have laws and regulations that impose strict limits to the use of this technology. A brief look at the regulatory situations in the latter three countries can make that clear.
As mentioned earlier, the first attempt at human germline modification was made in China, and the first genetically modified babies were born in China. Hence, one would expect the Chinese rules on germline editing to be lax. However, China equally bans genetically modifying offspring. A Chinese ministerial guideline provides that ‘gene manipulation on human gametes, zygotes and embryos for the purpose of reproduction is banned.’ 38 The National Health and Family Planning Commission is responsible for the enforcement of this rule. Accordingly, Chinese officials have denounced He Jiankui’s actions as ‘extremely abominable in nature’ and in violation of Chinese laws and science ethics. 39 For a long time, it was unclear how He Jiankui would be punished. The existing rules do not mention any penalties for violating the aforementioned ban. Nevertheless, in December 2019, He Jiankui was sentenced to 3 years in prison. Moreover, in response to the scandal, Chinese authorities have proposed to tighten the rules and introduce penalties. 40
Also in the USA, the most prolific country with regard to basic genome editing research, 41 several legal limits to HGGE are in place. These limits are part of what has been called ‘a complex regulatory and statutory web concerned with human embryo research in general and human germline modification in particular.’ 42 Although HGGE is not formally prohibited, currently several mechanisms, taken together, practically impede the clinical introduction of this technology. First, the National Institutes of Health, which is responsible for research funding in the USA, has stated that it ‘will not fund any use of gene-editing technologies in human embryos’. 43 Second, the US Food and Drug Administration, which has the authority to regulate products and drugs involving gene editing, including human gene editing, has so far stood in the way of using HGGE for reproductive purposes and is also not likely to change its policy in the near future. Since December 2015, US Congress has regularly added an amendment to the FDA’s funding bill, a so-called ‘bill rider’, making it impossible for the FDA to consider any application which involves ‘research in which a human embryo is intentionally created or modified to include a heritable genetic modification’. 44 Without the FDA’s approval, implantation of a genetically modified human embryo is illegal in the USA. However, genetically modifying human embryos for research purposes are permitted, even though such experiments remain ineligible for public funding. 45
Finally, the UK legal situation is worth mentioning in this context. The UK can be said to be at the forefront of germline editing because of its status as first country in the world to explicitly permit HNGT. In 2015, after many years of political debate, the UK Parliament gave green light to the clinical use of HNGT, 46 resulting in the ‘Mitochondrial Donation Regulations 2015’. 47 The UK’s decision to legalize HNGT for the purpose of preventing mitochondrial disorders was much discussed worldwide. As legal scholar Samvel Varvaštian explains the controversy: ‘the UK has not only become the first state to explicitly allow mitochondrial donation, but the first to openly challenge the fragile global policy with regard to germline gene modification.’ 48
Nevertheless, HGGE for reproductive purposes, in general, remains prohibited in the UK. According to the ‘Human Fertilisation and Embryology Act 1990’ (HFE Act), all uses of gametes and embryos outside the body are prohibited unless carried out on the basis of a license issued by the Human Fertilisation and Embryology Authority. Some activities cannot be licensed according to the HFE Act and are therefore absolutely prohibited. One of these activities is placing embryos or gametes other than ‘permitted embryos or gametes’ in a woman. According to Section 3ZA(4b) of the HFE Act, an embryo can only qualify as a ‘permitted embryo’ if ‘no nuclear or mitochondrial DNA of any cell of the embryo has been altered’. However, in 2008, an opening was created for HNGT when, during the 2008 revision of the Act, Section 3ZA(5) was added, which stipulates the following:
Regulations may provide that an egg can be a permitted egg, or an embryo can be a permitted embryo, even though the egg or embryo has had applied to it in prescribed circumstances a prescribed process designed to prevent the transmission of serious mitochondrial disease.
This is exactly what happened in 2015: Parliament voted in favor of regulations that make an exception to the general ban on germline editing to allow HNGT to prevent passing on serious mitochondrial diseases. 49 Nevertheless, also under this regulation, the use of HNGT for fertility treatment remains off-limits.
In sum, despite the wide variety of regulatory frameworks, there is broad consensus that HGGE is a technology with potentially far reaching consequences and that bans and restrictions are in order. Moreover, in most legal orders, the ban on reproductive HGGE appears to be firmly established: an extensive legislative procedure will be needed to lift it. The latter is also the case for the UK. The possibility that was built into the HFE Act in 2008 to amend the definition of ‘permitted embryo’ was, as explained, a constrained one. If the UK government ever wanted to lift the ban on editing the nuclear DNA of human embryos, it would have to go through the much more drastic process of changing its primary legislation.
However, this is not the case for all countries, as the legal situations in China and the USA indicate. In these countries, the existing legal frameworks can be adapted much more easily. As the Chinese prohibition is contained in a ministerial guideline, it can presumably be amended without having to pass through a legislative, parliamentary procedure. Moreover, as the UK Nuffield Council on Bioethics writes, ‘encouraged by international competition and given its Confucian traditions, China appears to be a candidate to lift the ban on intergenerational genome editing if sufficient evidence was adduced to support a move into clinical use.’ 50
As to the US restrictions on HGGE, these may be changed without any rigorous legislative revisions, for example, if the NIH changes its funding policy or if the FDA would be able to consider an application for human germline editing. Indeed, in June 2019, several Democratic lawmakers proposed to eliminate the bill rider which currently stands in the way of the FDA to consider trials for HGGE. 51
If the bans in these countries were lifted, and the Rubicon thus be crossed, it is likely that other countries will follow their example in today’s competitive ‘knowledge economy’. Moreover, it can be expected that medical tourism will bolster this legal ‘domino effect’, thereby creating the risk of a race to the bottom. This makes the question as to the international legal norms in this field all the more urgent.
III.B. III.B. International legal approaches to human germline modification
Characteristic for existing international human rights frameworks on biomedical technologies is the thought that with the application of germline genetic modification, a fundamental line would be crossed for humankind from which there is no turning back. 52 A striking illustration is the legal approach chosen by the Council of Europe, whose European Convention on Human Rights is effective in 47 states. Already in 1982, the Parliamentary Assembly of the Council of Europe considered in its ‘Recommendation on genetic engineering’ that ‘the rights to life and to human dignity protected by Articles 2 and 3 of the European Convention on Human Rights imply the right to inherit a genetic pattern which has not been artificially changed’. 53 These words are an early expression of the idea that human rights are of special importance within the regulation of human genetic technologies and that restrictions to the use of genetic technologies may be in order to ensure the protection of the fundamental values and rights protected by human rights discourse. 54 However, the Recommendation left the question unanswered as to what the exact scope of the proposed restrictions to germline interventions should be. 55 Instead, it recommended that the Committee of Ministers performs this task at a later stage by drawing up a European agreement on the topic, based on the rights and principles that are contained in the European Convention on Human Rights. 56
In 1997, this European agreement would take on the form of the ‘Convention for the Protection of Human Rights and Dignity of the Human Being with regard to the Application of Biology and Medicine’. As this treaty was opened for signature in Oviedo, Spain, it is more commonly known as the ‘Oviedo Convention’. The Oviedo Convention is the first international legally binding instrument in the field of biomedical law. According to Article 13 of this convention, ‘an intervention seeking to modify the human genome may only be undertaken for preventive, diagnostic or therapeutic purposes and only if its aim is not to introduce any modification in the genome of any descendants.’ The emphasized words imply a categorical ban on using HGGE for reproductive purposes.
According to the Oviedo Convention’s Explanatory Report, the prohibition contained in Article 13 goes back to the thought that misuse of HGGE ‘may endanger not only the individual but also the species itself.’ This sentence is a direct reference to the following, central phrase within the convention’s preamble: ‘convinced of the need to respect the human being both as an individual and as a member of the human species and recognising the importance of ensuring the dignity of the human being’. In the light of this reference, it appears that human dignity is one of the prohibition’s underlying principles, even if it is not mentioned explicitly by the Explanatory Report at this point. Indeed, the concept of human dignity is generally interpreted as a principle which protects the interests of both the individual and humanity, as is discussed more elaborately in Section VI. This raises the question as to how human dignity would be at stake in the context of the ban on HGGE. An answer is provided by the subsequent phrase in the Explanatory Report: ‘the ultimate fear is of intentional modification of the human genome so as to produce individuals or entire groups endowed with particular characteristics and required qualities’. 57
More recently, the ban on reproductive HGGE has been reaffirmed by both the Council of Europe’s Parliamentary Assembly 58 and Committee on Bioethics. 59 At this point, it should be noted, however, that the Oviedo Convention has actually entered into force in only 29 of the 47 Council of Europe’s member states. The states that are missing in this list have diverging reasons for not signing or ratifying the convention. Germany, for example, abstained from signing because the treaty was deemed too permissive. 60 The UK, on the contrary, did not sign because the convention was considered too restrictive. 61 Another interesting case is Ukraine, where the first baby after pronuclear transfer was born, as described above. This country signed the Oviedo Convention but failed to ratify it.
Nevertheless, member states that have not signed or ratified the Oviedo Convention are still faced with a ban on HGGE, albeit in a different form, if they are also European Union member states. 62 For over two decades, the EU has regarded germline gene modification as conflicting with fundamental values of the European legal order. ‘There is’, in the words of the preamble of the ‘Biotech Directive’ (1998), ‘a consensus within the Community that interventions in the human germ line and the cloning of human beings offend against “ordre public” and morality’. 63 Correspondingly, Article 6 of the Biotech Directive excludes from patentability ‘processes for modifying the germ line genetic identity of human beings’ and ‘processes for cloning human beings’. Also, within this context, human dignity played an important role in the legislative process. Recital 16 of the Biotech Directive emphasizes that ‘patent law must be applied so as to respect the fundamental principles safeguarding the dignity and integrity of the person’. The Court of Justice has taken this to mean that ‘the European Union legislature intended to exclude any possibility of patentability where respect for human dignity could thereby be affected’. 64
Within the EU ‘Charter of Fundamental Rights’ (2000), the interconnections between human rights, human dignity, and biomedical developments in EU law are further elaborated. The Charter’s first chapter, entitled Human dignity , includes a ‘prohibition of eugenic practices, in particular those aiming at the selection of persons’ [Article 3(2) sub b]. Of more direct importance to human germline editing are the EU rules on clinical trials that were introduced in 2001. According to both the ‘Clinical Trials Directive’ 65 and its successor, the ‘Clinical Trials Regulation’, 66 ‘no gene therapy clinical trials may be carried out which result in modifications to the subject’s germ line genetic identity.’ 67 As a clinical trial is indispensable for the safe introduction of HGGE in the clinic, it could be said that the reproductive use of germline modification technologies is effectively blocked by this legal provision. Even so, there is discussion on the question as to whether the EU rules on clinical trials formally apply to trials involving HGGE, as those will be discussed in the next subsection.
Also outside Europe international human rights norms have been established for HGGE, with UNESCO’s ‘Universal Declaration on the Human Genome and Human Rights’ (1997) as main example. The declaration is not a legally binding instrument. Like the Oviedo Convention, it expresses the thought that germline editing touches upon the collective interests of humanity, albeit in a slightly different way. As Article 1 states, ‘The human genome underlies the fundamental unity of all members of the human family, as well as the recognition of their inherent dignity and diversity. In a symbolic sense, it is the heritage of humanity’. What these words mean for HGGE is suggested by Article 24, in which the International Bioethics Committee (IBC) is assigned the task to disseminate the principles set out in the declaration. According to this provision, the task also entails giving ‘advice concerning the follow-up of this Declaration, in particular regarding the identification of practices that could be contrary to human dignity, such as germ-line intervention.’
If there were any remaining doubts on UNESCO’s position on HGGE, these are taken away in a recent report. In this report from 2015, the IBC offers reflection on the relation between human rights and the human genome in the light of recent technological developments, including not only the rise of personalized medicine, NIPT, and direct-to-consumer genetic testing but also human germline editing. As to the latter, the IBC expresses its support for the Oviedo Convention’s ban. Building on Article 1 of the Declaration and its notion of the human genome as the heritage of humanity, the IBC states that making any genetic changes which are passed on to descendants should be prohibited. 68 According to the committee, ‘the alternative would be to jeopardize the inherent and therefore equal dignity of all human beings and renew eugenics, disguised as the fulfillment of the wish for a better, improved life’. 69
III.C. III.C. Persisting ambiguities and current discussions
Although various formulations are used within the international legal frameworks in this field, it is clear that human dignity emerges as a core idea. According to UNESCO, the Council of Europe, and the EU, this legal principle is compromised as soon as eugenic practices emerge, human reproduction degenerates into the production of humans, or children are reduced to objects of design.
Yet, many questions remain. UNESCO’s IBC, for example, is ambiguous about its exact position on HGGE. As discussed, the IBC supports the Council of Europe’s ban on the use of this technology for reproductive purposes. However, in its report’s final recommendations, the IBC appears to take a slightly different position than the Council of Europe by advocating a differentiated approach to human cloning 70 and HGGE. In the IBC’s words:
The IBC reaffirms the necessity for a ban on human cloning for reproductive purposes and recommends a moratorium on genome editing of the human germline. There is no medical or ethical argument to support the former. As to the latter, the concerns about the safety of the procedure and its ethical implications are so far prevailing’ (emphasis added). 71
Unfortunately, what the exact implications of the IBC’s distinction between ‘ban’ and ‘moratorium’ are in this context and what its words ‘so far’ allude to remains unexplained. Does this mean that the IBC could change its position depending on the circumstances and that it does not endorse a categorical ban? If so, then these recommendations are at odds with the IBC’s firmly expressed concerns about HGGE, dignity and justice, as expressed earlier in the report.
Furthermore, discussion is possible on the exact scope of the various existing prohibitions, also in EU law. For example, what is exactly meant by the term ‘eugenic practices’ to which the EU Charter of Fundamental Rights refers? According to the explanatory memorandum, ‘the reference to eugenic practices, in particular those aiming at the selection of persons, is related to possible situations in which selection programs are organized and implemented, involving campaigns for sterilization, forced pregnancy, and compulsory ethnic marriage among others’. 72 This list seems to suggest that cases in which selective reproduction is the outcome of individual, voluntary decision-making, in other words, instances of ‘liberal eugenics’, do not qualify as ‘eugenic practices’ under this provision. At the same time, the words ‘among others’ indicate that this list of possible eugenic situations is not exhaustive.
More specific questions have risen about both the EU Biotech Directive’s and the Clinical Trials Regulation’s references to ‘the germ line genetic identity’. When can it be said that a germline intervention affects the future person’s germline genetic identity? Is this the case for all types of germline interventions, or only more radical ones? A possible line of reasoning is that HNGT falls into a category of germline interventions that do not affect the future person’s genetic identity, and therefore remains outside the scope of both provisions. However, as a number of ethicists point out, this line of reasoning has serious weaknesses. 73 In their words, ‘modification of the mitochondrial DNA is not substantively different from modification of the nuclear DNA in terms of its effects on the identity of the future person’. 74
Nevertheless, both UK and Dutch legislature, for example, have come to a different conclusion. In the UK and The Netherlands, modifications of nuclear embryonic DNA are banned, whereas modifications of nuclear mitochondrial DNA are not. It seems that the lawmakers of both EU states (at the time, the UK still was an EU member state) have interpreted the EU legislation’s references to ‘genetic identity’ to mean that only modifications of nuclear embryonic DNA are targeted by the current bans, thus leaving the possibility open for member states to permit modifications of mitochondrial DNA. 75 Even so, despite the absence of a national legal ban, The Netherlands has, as of yet, neither explicitly nor actively endorsed this technology. This is due to other legal provisions that impede the use of HNGT. 76 In the UK, on the contrary, clinical trials were given the green light in December 2016 by the HFEA, which was hailed by the journal Nature as a ‘historic decision’. 77
However, media reports from 2019 suggest that the UK was probably not even the first EU member state to permit clinical trials in this field. 78 Equally in 2016, Greek health authorities gave permission for clinical trials involving HNGT. This information was not shared with the general public until a Spanish–Greek team of fertility doctors announced in April 2019 that a genetically modified baby had been born in Greece after an IVF procedure with HNGT. This boy is not only the ‘first three parent baby born in clinical trial to treat infertility’, as media headlined, 79 but also the first ‘three parent baby’ born in the EU. The purpose of the nuclear genome transfer had not been to prevent passing on mitochondrial disorders, which is the sole purpose for which HNGT can be used in the UK. Instead, like the Ukrainian case, the fertility doctors sought to increase the chances of a successful fertility treatment. The team that initiated the procedure, the Spanish company Embryotools, had decided to move the trials to Greece because of legal hurdles in Spain. 80 The Greek National Authority of Assisted Reproduction formally approved these clinical trials at the end of 2016, 81 apparently assuming that EU clinical trials legislation permits this kind of germline intervention.
In addition to these discussions about the meaning of ‘genetic identity’, there is disagreement about the meaning of the terms ‘clinical trials’ and ‘subject’ as used in the EU Clinical Trials Directive 82 and Regulation. 83 For example, in its 2018 report on HGGE, the British Nuffield Council of Ethics raises the question as to whether HGGE clinical trials would fall within the EU law’s definition of a clinical trial, given the centrality of the notion ‘subject’ in that definition. 84 The authors of the report have doubts because the germline intervention is performed on embryos and not fully grown individuals. 85 Against this view, the argument can be made that the subject in question is not so much the embryo but rather the future person that will be born after the genetic intervention.
During the parliamentary debates on HNGT, the UK Department of Health used a different line of reasoning to arrive at the same conclusion. It argued that trials for HNGT are actually, not clinical trials in the sense of the EU Clinical Trials Regulation and that, therefore, the ban does not apply to this situation. In essence, the Department argued that when Article 90 of that Regulation posits that ‘no gene therapy clinical trials may be carried out which result in modifications to the subject’s germ line genetic identity’, the Regulation does not mean to imply anything for trials in the field of germline interventions. For this position, the Department offers two reasons.
First, this regulation is formally known as the ‘Regulation on clinical trials on medicinal products for human use’ (emphasis added). According to the Department, no medicinal products are involved with HNGT. Ergo , the Regulation does not apply. Second, the Health Department points out that Article 2(1) defines clinical trials as having ‘the objective of ascertaining the safety and/or efficacy of […] medicinal products’. The Department argues that the licenses which the HFEA issues to clinics to perform HNGT cannot qualify as ‘clinical trials’ because they ‘will not be licensed with the objective of ascertaining the safety and/or efficacy” of the treatment. The primary objective will be to prevent the transmission of serious mitochondrial disease’.
Nonetheless, the licenses that the HFEA has issued in the meantime are widely perceived as official permissions to perform clinical trials in this field. 86 Furthermore, the Health Department’s interpretation is questionable because the broad wording of Article 90 suggests ‘that it was intended to prohibit all gene therapy clinical trials involving germline editing, irrespective of whether they relate to medicinal products.’ 87 Finally, the Department’s position that no medicinal products are involved in case of HNGT can be challenged in the light of ongoing discussions about the meaning of the term ‘medicinal products’. 88 Varvaštian observes in this context ‘that the compliance of the Mitochondrial Donation Regulations 2015 with the EU legislation on clinical trials could indeed be questioned by the European Court of Justice, should a case be brought before it.’ 89
Such a court decision could have a huge impact. Either the UK and Greece rely on a wrong interpretation of Article 90 and would have to stop their clinical trials (that is, as long as the UK is still a member state), or the UK and Greece are right, in which case the ruling would not only affect the governance of HNGT, but, possibly, of HGGE at large. After all, many of the arguments made to allow HNGT trials under the Clinical Trials Regulation, equally remove clinical trials for HGGE from the scope of the clinical trials directive.
A final important question that needs to be addressed is what the existing prohibitive or restrictive legal approaches to germline modification imply for research in this field. For example, although EU law prohibits clinical trials in which the subject’s germ line genetic identity is affected, the question remains unanswered as to what the rules are for preclinical or basic research in this field. According to the European Group on Ethics in Science and New Technologies (EGE), which is the European Commission’s advisory body on ethical matters, not only the clinical application of this technology would be ground for serious concerns, but also the research activities in this field, ‘given the profound potential consequences of this research for humanity’. 90 However, the EGE fails to indicate how these concerns should be translated in either law or policy and what this means for the various stages of research.
The Oviedo Convention is less ambiguous in that regard. This treaty contains, what could be called, a de facto ban on research in this field. Article 18(2) of the Oviedo Convention prohibits the creation of embryos for research purposes. Nonetheless, research aimed at developing HGGE requires one-cell stage embryos, which can only be obtained by creating embryos for research purposes. 91 Embryos that are left over from an IVF treatment are not suitable for this purpose. This means that this kind of research is practically impossible in those countries that have ratified the Oviedo Convention.
In several countries that have not signed the Oviedo Convention, such as Germany and Canada, a more explicit, overall prohibitive approach can be found. 92 In these countries, editing the human germline involves crossing a red line, regardless of whether the intervention serves research or reproductive purposes. Isasi, Kleiderman, and Knoppers suggest that this type of prohibitive approach, with ‘upstream’ limitations on research activities in this field, is ultimately based on ‘a critical attitude toward science because of fears of commodification of potential life’, 93 be it unborn life (ban on creating research embryos) or the life of the person-to-be (ban on germline editing). However, one can also imagine a different line of reasoning: research in this field is prohibited because it can be regarded as lacking purpose as long as the reproductive use of HGGE is categorically prohibited in these countries.
As discussed in the previous section, both national and international legal orders have been well prepared for the rise of HGGE technologies. In this case, it can be said that law outpaced technology, instead of, as is often claimed, the other way around. At the same time, it is clear that the first cracks are becoming visible within the existing legal frameworks in this field. A clear sign is that two EU member states, the UK and Greece, are currently at the global forefront of HNGT, even though the European legal order is among the strictest when it comes to the governance of genetic modification technologies.
For now, the challenges to existing human rights frameworks mostly take on the form of legal-technical disputes on the exact scope and meaning of terms such as ‘germline genetic identity’ and ‘clinical trials’ or the distinction between bans and moratoria. Yet, it is quite evident that behind this façade of legal technicalities a substantial, normative shift is taking place. Indeed, where most legal discussions have so far focused on the terms used within existing laws, discussions elsewhere focus on the question as to whether it is time to revise these laws altogether.
Ironically, just when technological developments in this field have finally caught up with existing legal frameworks and legal bans and restrictions could thus start to play the role as originally intended by the legislatures, these frameworks are being called into question by various parties. A straightforward explanation for this situation is offered by legal scholar Henry Greely: at the time, when the bans and restrictions on this technology were called into existence, many people were still in favor because ‘it wasn’t hard to renounce something that you could not do.’ 94
Whatever the reason is, fact is that the call for a revision of existing laws on HGGE is growing louder, especially among scientific and medical-professional bodies, academies, and societies. 95 Examples are manifestos and open letters from groups of biomedical scientists, 96 statements from medical-professional organizations, 97 advisory reports from various national science academies, 98 concluding statements from international summits organized by science academies, 99 and recommendations from health and ethics councils. 100
What these proposals have in common is that they ‘do not favor an international treaty that would ban all clinical uses of germline editing’, 101 such as the Oviedo Convention’s ban. Such a prohibitive approach would be ‘too rigid’. 102 Instead, they advocate a ‘responsible,’ 103 ‘prudent path forward’. 104 This entails defining a ‘translational pathway to germline editing’, 105 along with developing a ‘pathway to effective governance’ 106 of this technology. According to this line of thought, bans on HGGE for reproductive purposes will be needed for the time being, for example through a self-imposed temporary ban (‘moratorium’) 107 from the science community. However, the idea is that this ban can be lifted as soon as clinical requirements are met. From that moment on, a governance model for HGGE will replace the existing bans on this technology. In essence, the proposals for regulation instead of prohibition rest on three tiers.
First, basic and preclinical research in this area should be facilitated in order to make a safe introduction of this technology in the clinic possible. On a practical level, this also means that bans on the creation of embryos for research purposes, such as, for example, contained in Article 18 of the Oviedo Convention, have to be lifted. Second, as soon as HGGE is found safe enough for introduction in the clinic, these proposals recommend lifting the ban on this technology. From then on, HGGE should be made available for strictly therapeutic purposes, that is, to eliminate serious genetic diseases and conditions. Reproductive use of HGGE for nonmedical reasons, such as improving intelligence or appearance, must remain prohibited, at least initially. 108 Third, the proposals underline the importance of a public debate on the issue. That public debate should focus on the conditions under which reproductive HGGE should be allowed.
How should these proposals for a regulatory pathway to reproductive HGGE be viewed from a human rights perspective? Evidently, they conflict with the existing bans on the clinical use of this technology, as contained in the Oviedo Convention and as suggested by UNESCO’s IBC. Nevertheless, both the Council of Europe’s Committee on Bioethics 109 and UNESCO’s IBC 110 have stressed the need for an ongoing public debate about the human rights questions raised by these technological developments. Indeed, Article 28 111 of the Oviedo Convention explicitly recognizes the general need for public debate on such questions. Moreover, it is widely recognized that even fundamental rights and their underlying principles are open to dynamic or evolutive interpretation. 112 Therefore, notwithstanding the current human rights bans on genetically modifying offspring, the need for a debate on the meaning of human rights for this technology has never been more urgent.
By means of this article, I hope to contribute to the debate. Hereafter, I formulate three concerns about the recent proposals for a regulatory pathway to HGGE, using human rights discourse as my main frame of reference. First, I argue that this model is based on a distinction between healing and enhancing offspring that may be useful and realistic enough for the regulation of PGD but is much more problematic in the case of human germline editing (Section V). Second, although these proposals also refer to human rights, they rely on an impoverished understanding of what human rights and human dignity mean in the context of biolaw (Section VI). Third and finally, I discuss how this impoverished understanding of human rights sets the stage for a type of deliberation on HGGE in which the voice of the scientific community dominates at the cost of more public perspectives (Section VII).
Have existing bans on HGGE become ‘outdated’ 113 in the light of recent technological developments? Should we ‘update’ these legal frameworks ‘to recognize, permit, and regulate new techniques to allow safe HGGE for therapeutic and preventive aims’? 114 At first sight, these thoughts, which can be recognized in publications from, for example, the US National Academies of Sciences, Engineering and Medicine (NASEM), the UK Nuffield Council on Bioethics, the Hinxton Group, the European Society of Human Reproduction and Embryology (ESHRE), the European Society of Human Genetics (ESHG), and several prominent scholars, 115 seem convincing. After all, how can one oppose the prevention of grave diseases?
Moreover, under most of these proposals, the use of HGGE with the purpose of enhancing offspring remains off-limits. From a human rights perspective, the distinction between healing and enhancing is a highly relevant one. For example, while Article 13 of the Oviedo Convention categorically bans heritable genome editing, it also states that human genetic modifications in general can only be undertaken for preventive, diagnostic, or therapeutic purposes. Similarly, Article 14 prohibits ‘the use of techniques of medically assisted procreation […] for the purpose of choosing a future child’s sex, except where serious hereditary sex-related disease is to be avoided.’
Furthermore, it can be argued that a regulatory model will be more realistic and effective than a prohibitive model. Alta Charo, for example, writes that ‘calling for a moratorium may feel satisfying, [but] it does little to stop rogue actors, nor does it help scientists who […] wish to pursue the technology cautiously and responsibly.’ According to this pragmatic line of reasoning, a comprehensive regulatory approach ‘will do more to control and guide this technology than a moratorium or formal ban.’ 116 In a similar vein, several authors fear that a prohibitive approach would fuel CRISPR tourism. 117
Finally, the fear for a slippery slope toward eugenics seems unfounded if a sound regulatory framework is established with strict and transparent limits to the use of HGGE. Indeed, the proposed regulatory frameworks show strong resemblance to the regulatory schemes that have been developed in many countries to regulate PGD. Hence, some refer to this governance model for HGGE as the ‘PGD model’. 118 Genetically selecting embryos for reproductive purposes through PGD are, typically, only permitted according to medically determined requirements, such as the gravity of the genetic condition for which PGD is used and whether it is untreatable. 119 These legal limits of PGD are usually established through a regulatory mix of national laws and medical-professional guidelines and appear to work quite effectively and convincingly in these countries.
However, in order to make the PGD model truly effective and feasible in case of HGGE, a clear demarcation of the terms ‘serious disease or condition’ is necessary. 120 If this turns out to be practically impossible, then those who, like Lanphier et al. , ‘oppose germline modification on the grounds that permitting even unambiguously therapeutic interventions could start us down a path toward nontherapeutic genetic enhancement’ 121 have a valid point. 122
Upon closer inspection, there are several substantial differences between PGD and human germline editing that suggest that it is problematic to apply the PGD model without reservations to germline editing. First, the risk of a slippery slope is much greater in case of HGGE. Contrary to PGD, HGGE breaks with the principle of reproduction through genetic recombination. Consequently, the number of possible choices increases exponentially. For example, as discussed, the Chinese genetically modified twins have a novel variation of the CCR5 gene that has not been seen in humans before. Moreover, in theory, it is possible to introduce not only nonparental DNA but also even nonhuman DNA. The Nuffield Council offers an interesting list of several possibilities opened up by CRISPR, including creating ‘supersenses or superabilities’ and ‘tolerance for adverse environmental conditions (such as those that might be envisaged as a result of climate change or in space flight)’. As the Nuffield Council explains, ‘what opens up these possibilities is a change in perspective from one focused on the achievement of a limited purpose—one that may have animated the initial research and innovation—to a vision animated by the exploitation of a technology to secure the maximum value from its use.’ 123 Accordingly, it can be said that the eugenic potential of HGGE exceeds that of PGD multiple times. 124
Second, compared with PGD, it will be even more difficult to define what ‘therapeutic’ means in case of HGGE. The Chinese CRISPR babies offer a striking illustration of that difficulty. Did He Jiankui’s intervention serve a medical purpose? The biophysicist sought to make the babies resistant to infection from HIV. In other words, he was not trying to cure them from a disease. Instead, he exposed these babies, with whom in principle nothing was wrong, to serious health risks. At the same time, the intervention could perhaps be labeled as medical, because its main purpose was the prevention of a disease: AIDS. 125
If the possible side effects of the genetic intervention are also taken into account, the discussion becomes even more complex. According to neurobiologists, it is quite likely that He’s intervention also affected various brain functions. More specifically, the inhibition of that particular gene has been linked to greater recovery of neurological impairments and improvement of cognitive functions. 126 It is, therefore, possible that He’s genetic modifications also resulted in human enhancement, more specifically, cognitive enhancement. 127 Such ‘incidental enhancements’ complicate line drawing substantially. 128
Third, in many cases of HGGE, it will be hard, if not impossible, to decide whether the interference yields an overall positive impact on the future child’s health, with positive effects outweighing the negative ones. Again, He’s ‘genetic surgery’ offers the perfect example of the complexities. Even if his genetic modification turns out to be successful, with off-target effects kept to a minimum, there still is a reason to doubt whether it has truly benefitted the twins. According to geneticists, the intended genetic mutation may provide resistance against HIV infection, but it also increases the vulnerability to infection from the West Nile virus and influenza. 129 As such, the Chinese case offers a glimpse of the complex trade-offs and vexing dilemmas with which the reproductive use of CRISPR would confront parents-to-be. 130
In short, HGGE is likely to result in a further blurring of the medical boundary between healing and enhancing. Moreover, it can be argued, as the Nuffield Council does, that ‘we have to take care when applying categories such as “therapy” and “enhancement” […] to the anticipation of people who do not yet (and may never) exist.’ 131
What does all of this imply for the governance of HGGE? For the Nuffield Council, the conclusion is that the medical boundary is too problematic to be able to function as a red line for human germline modification. 132 The advisory body proposes a new legal-ethical standard: the genetic intervention should serve the welfare of the future child. As the Nuffield Council explains, this means that ‘there is no a priori reason that preferences beyond the avoidance of disease should not also be consistent with the welfare of the future person.’ 133
It has to be said that the Nuffield Council’s proposal for governing HGGE is more realistic than the PGD model that is recommended by most scientific and professional bodies and organizations. However, the UK proposal is also much more radical: the medical boundary (which only allows interventions of a therapeutic or preventive nature) is abandoned, with the result that human enhancement also becomes one of the possibilities. 134 This is also problematic from a human rights perspective. As UNESCO’s IBC writes:
The goal of enhancing individuals and the human species by engineering the genes related to some characteristics and traits […] impinges upon the principle of respect for human dignity in several ways. It weakens the idea that the differences among human beings, regardless of the measure of their endowment, are exactly what the recognition of their equality presupposes and therefore protects. It introduces the risk of new forms of discrimination and stigmatization for those who cannot afford such enhancement or simply do not want to resort to it. 135
Evidently, another conclusion is also possible. If it is practically impossible to draw a sharp line between therapy and enhancement in this context, is the promise of a strictly regulated and limited use of HGGE not fundamentally illusory? Moreover, if HGGE remains prohibited, the interests of prospective parents with serious genetic diseases in their families can continue to be taken seriously. In almost all cases, PGD already offers these couples the possibility of reproducing without passing on their genetic disorders, with the added benefit that such an arrangement is considerably less risky and radical in nature.
A second set of questions elicited by the recent proposals to revise current laws on human germline editing relates to their understanding of the concept of human rights and human dignity. While this understanding tacitly informs many of these proposals, it is explicitly addressed by legal scholar Robin Alta Charo, who is a member of the organizing committee of the Hong Kong International Summit on Human Genome Editing and co-author of the 2017 NASEM report. In an essay she wrote on the occasion of a symposium the seventieth anniversary of the Universal Declaration on Human Rights, Alta Charo argues ‘that the current human rights law on germline editing misunderstands both the mechanisms of genetics and the moral basis for human rights, suggesting a more nuanced approach as we move forward and keep pace with new gene-editing technologies.’ 136 In other words, Alta Charo is open about the fact that her interpretation breaks with existing human rights approaches to this issue. Interestingly, as will be discussed hereafter, her exploration of the moral basis of human rights even leads her to suggest that, ultimately, the concept of humanity may not be necessary for the proper functioning of human rights discourse at all.
It seems Alta Charo is not alone in her line of reasoning. In a similar vein, the recent calls for a regulatory pathway to HGGE do not or hardly make mention of humankind, human dignity, or the human species. To come to a better understanding of the normative shift that is thus taking place in this context, I first describe the vision of human rights and human dignity as implied by the existing human rights law on germline editing. I then juxtapose it with the vision on human rights as unfolded in the recent proposals to replace the bans on genetically modifying offspring with regulatory schemes.
Within the Oviedo Convention and the Universal Declaration on the Human Genome and Human Rights, fundamental interests of both an individual and a collective nature are protected. According to its Explanatory Report, the Oviedo Convention aims to address concerns about biomedical developments at three levels: the level of the individual; the level of society; and the level of the human species. 137 Similarly, UNESCO’s IBC states that the rise of HGGE necessitates reflection about the possible consequences of this technology ‘on human rights and freedoms as well as on the future of humanity itself.’ 138 Moreover, the IBC stresses that ‘ethics is not simply a matter of individual morality but it involves society as a whole’ and ‘must therefore also pursue the common good’. 139 In that context, the committee warns for ‘a radical conception of autonomy, according to which any medical progress should be at the disposal of patients, who are turned into consumers (clients).’ 140
Accordingly, human dignity is explained within this approach as a legal principle that not only affords protection to individual rights and freedoms (referred to within scholarly literature as ‘the individual dimension of human dignity’ or ‘dignity as empowerment’) but also to the collective interests of humanity upon which human rights are built (‘collective dimension of human dignity’ or ‘dignity as constraint’). 141 Concerns about both dimensions of human dignity can be detected in the Council of Europe’s and UNESCO’s approach to human germline editing.
As to dignity’s individual dimension, both human rights bodies, evidently, subscribe to the necessity of clinical safety. They also underline that new genetic technologies ‘are likely to offer unprecedented tools against diseases.’ 142 However, they are concerned that HGGE may give rise to new forms of discrimination and may impact negatively on the self-perception and sense of freedom of the resulting individuals. UNESCO’s IBC, for example, expresses the fear that social-economic inequalities will become engrained on a genetic level 143 and expresses concern about ‘the significant effects on the life of individuals who could be considered designed on demand by someone else without their consent’. 144
As to dignity’s collective dimension, these human rights bodies stress that, while genome editing in general is ‘one of the most promising undertakings of science for the sake of all humankind’, 145 reproductive HGGE may lead to a renewal of eugenics. 146 More specifically, as discussed above, international bans on this technology aim to prevent practices in which individuals or entire groups are produced with particular characteristics and required qualities. 147 The underlying logic appears to be that human dignity is affected by HGGE to the extent that this technology opens up the possibility of one person designing the other.
Within this approach, the collective and individual dimensions of human dignity are regarded as inextricably and fundamentally connected, even though it is clear that certain tensions may arise between both sides. The underlying thought which unites both dimensions is that fundamental freedoms cannot be exercised to the detriment of the collective foundations of these freedoms, which is the humanity of humankind. The IBC expresses this fundamental thought in the context of germline editing as follows: ‘the human genome [is] one of the premises of freedom itself and not simply […] raw material to manipulate at leisure.’ 148 The other way around, collective dignity should always serve the long-term goal of protecting individual dignity, 149 just as human dignity as empowerment and human dignity as constraint both serve to protect the individual. Without this caveat, there is a danger that individual freedoms are sacrificed to preserve ‘the dignity of the whole’. 150 Accordingly, Article 2 of the Oviedo Convention states that ‘the interests and welfare of the human being shall prevail over the sole interest of society or science.’
In line with the emphasis on both individual and collective interests, a recurring thought within this approach is that ‘the human genome, metaphorically speaking, belongs to all of us’, 151 and that, ‘in a symbolic sense, it is the heritage of humanity’ (Article 1 Universal Declaration on the Human Genome and Human Rights). As these technologies touch upon the future of human reproduction, they merit a broad international and societal debate. In other words, from this perspective, ‘no one has the moral warrant to go it alone’ when it comes to HGGE. 152
In contrast, many who advocate lifting the ban on HGGE neglect or even negate the collective dimensions of human rights. More specifically, whereas the Oviedo Convention addresses concerns at the level of the individual, of society, and of the human species, the recent proposals rely exclusively on the first two. In this vein, the Nuffield Council’s main line of reasoning with regard to HGGE rests on two principles: the principle of the welfare of the future person and the principle of social justice and solidarity. Accordingly, it recommends lifting the ban on HGGE, first, for purposes that are in line with the future child’s welfare (i.e. level of the individual) 153 and, second, in circumstances in which it cannot be reasonably expected to produce or exacerbate social inequalities (i.e. level of society). 154 As to concerns at the level of the human species, the Nuffield Council actively dismisses human dignity as a guiding principle for the governance of HGGE, 155 because of reasons that will be discussed below.
A similar line of reasoning can be detected in the NASEM report on human gene editing. The authors of this report describe their own normative framework as follows:
The committee focused on principles that are aimed at protecting and promoting the health and well-being of individuals; approaching novel technologies with careful attention to constantly evolving information; respecting individual rights; guarding against unwanted societal effects; and equitably distributing information, burdens, and benefits. 156
No mention is made of the various collective interests of humankind. Moreover, where the report does mention human dignity, it brings only dignity’s individual dimension to the fore, as in the following sentence: ‘The principle of respect for persons requires recognition of the personal dignity of all individuals, acknowledgment of the centrality of personal choice, and respect for individual decisions’ (emphasis added). 157
In brief, in both the NASEM and Nuffield Council report, the collective dimensions of human rights discourse and human dignity are pushed aside. What remains are considerations related to safety risks, individual rights, reproductive autonomy, and social justice. How can this normative shift be explained? The Nuffield Council invokes the well-known line of thinking that human dignity is a vague or even useless concept 158 and that it does not have added value to the functioning of human rights: ‘Whereas human dignity has been advanced by some as the basis of human rights, the coherent functioning of human rights discourse does not depend on accepting this claim’. 159 Moreover, even if it can be agreed that human dignity is the basis of human rights, then it would still not be at stake with HGGE. This is because ‘entitlement to human rights does not depend on the possession of a “human genome”’. 160 To suggest otherwise, would be to engage in ‘genomic essentialism’, the authors of the report argue. 161 Other authors, 162 including Alta Charo, 163 argue along similar lines.
I agree that such essentialism would indeed be problematic: the human species has evolved over time and it would be reductionist to understand both dignity and humanity in genetic terms. However, that is not what the existing human rights law in this field claims to protect. Indeed, Article 3 of the Universal Declaration on the Human Genome and Human states that ‘the human genome, which by its nature evolves, is subject to mutations.’ Accordingly, as discussed in Section III.B, the ban on HGGE as contained in human rights law is not so much about the preservation of the human genome, but about the fear that HGGE would give rise to new forms of discrimination and eugenics. 164
Furthermore, questions can be asked about the NASEM’s and Nuffield Council’s own understanding of humanity and dignity. As already discussed, in these reports, human dignity is equated with the protection of individual freedoms, thereby ignoring the collective dimension of that principle. This does impoverish not only human rights discourse but also the public debate on this issue, as will be discussed in the next section.
Moreover, these proposals appear to pave the way for a more transhumanist or posthumanist approach. As already discussed, the Nuffield Council is not willing to hold on to the medical boundary between healing and enhancing. Additionally, when discussing transhumanism in its report, the UK ethics body suggests that if human germline editing were to result in ‘the self-overcoming of the human species’, 165 this would not necessarily be problematic. Indeed, it may be warranted because of the huge impact that humankind is having on the environment in an age that is already being referred to as the ‘Anthropocene’. According to the Nuffield Council, if certain parts of the world became inhabitable because of air pollution or climate change, ‘genome editing could offer a remedy to this predicament by allowing the introduction of characteristics that will fit future generations better for the conditions in which they may be required to live.’ 166 Even though the authors of the report admit that ‘such a project would be reckless at present’, they argue that the precautionary principle ‘would at least seem to mandate further research and development of genome editing technologies as a way of hedging against future threats.’ 167 In other words, based on this remarkable view of the role that the precautionary principle would have to play in the Anthropocene, the authors argue that a good response to the negative impact that humans are having on the natural world would be to also subject ‘human nature’ to human intervention. This is in sharp contrast with views held by, for example, the German Ethics Council. According to the Council, given the fact that the current geological epoch is already referred to as the Anthropocene, and given the manner in which the human genome is strongly linked to individual and collective self-images of humankind, more extensive reflection processes are needed to be able to bear responsibility for the heavy decisions to come. 168
In a similar vein, Peter Mills, assistant director of the Nuffield Council, openly questions the humanist foundations of human rights discourse altogether. He argues that:
whatever the ground of ‘human’ rights is, it should be seen as a threshold rather than a property exclusive to natural kind or class. It follows, therefore, that such rights as are currently enjoyed by humans should equally extend to a non-human, a posthuman or even an artificial intelligence, who is capable of being welcomed into our moral community. 169
Alta Charo agrees with Mills on this. In the closing words of her essay on germline engineering and human rights, she raises the possibility of a human rights discourse that dispenses with the notion of the human altogether:
Understanding that Homo sapiens is a species with blurry boundaries, and that we carry within us genetic traits that trace back to Neanderthals and even far more primitive life forms, should make us question whether germline editing in any way undermines the basis for according human rights, and indeed, whether being human is essential to human rights at all. 170
Evidently, the idea of a human rights approach without a notion of the human is a radical departure from the humanist foundations of human rights discourse. Moreover, Alta Charo’s, Mills’, and the Nuffield Council’s line of argumentation raises the question as to whether their understanding of human rights and human dignity does not give rise to a self-destructive dynamic within human rights discourse: what, initially, appears as merely a reinterpretation of humanist notions such as human rights and freedoms gives rise, in the long run, to the possibility of an abandonment of humanism altogether or even a self-overcoming of the human species. 171
The neglect of the more communal or collective dimensions of human rights, as they come to expression in principles such as human dignity or the view of the human genome as heritage of humankind, also has repercussions for the terms in which the public deliberation about this issue is framed. Instead of asking the question which kind of future the human community wants to build for itself, the issue is narrowed to questions of safety risk, reproductive rights of prospective parents, and health interests of future children. Evidently, the health and rights of those directly involved in HGGE are of great concern and need to be taken very seriously. However, more is at stake. Human rights law in this field does aim to protect not only the rights of prospective parents and their future offspring but also the rights and interests of future generations. It does aim to protect not only our health but also our humanity. In other words, ‘human genome editing raises questions that cannot be dealt with only in terms of medical ethics principles relating to safety, informed consent and individual reproductive rights.’ 172
Moreover, because this technology may determine the future of human reproduction, it is a collective responsibility of society to take ‘stock of alternative imaginable futures’ and to decide ‘which ones are worth pursuing and which ones should be regulated, or even prevented.’ 173 As such, HGGE is one of the most striking examples of the more general need to ‘reclaim biotechnology for the common good.’ 174 Accordingly, the future of HGGE deserves a broad, democratic debate in which society’s long-term aspirations and visions of the good life can be explored and imagined. 175
However, so far, a certain perspective dominates. Characteristic for the recent proposals to move from prohibiting to regulating HGGE is the central position afforded to biomedical professionals and scientific bodies in that process. Not only is self-regulation by the biomedical community propagated in the form of ‘the Asilomar 176 model’, 177 but also the voice of biomedical researchers and professionals becomes decisive within regulation as soon as it is based primarily on medical standards such as clinical safety and prevention of serious diseases. 178 Indeed, the position taken by certain scientific bodies has gradually become more outspoken over the years. Even the birth of the genetically modified twins in China has not been able to break this trend thus far. Especially, striking in that regard is the changing view on the need and purpose of broad societal discussion and consensus. The evolving position of the national science academies of the USA, China, and the UK can serve as an example.
In December 2015, the US National Academy of Sciences, the US National Academy of Medicine, the Chinese Academy of Sciences, and the UK Royal Society together hosted the first International Summit on Human Gene Editing in Washington, DC. During this 3-day event, experts from around the world came together to discuss the scientific, ethical, and legal questions raised by human gene-editing. The event was concluded with a so-called summit statement, which included recommendations with regard to HGGE. According to the organizing committee:
it would be irresponsible to proceed with any clinical use of germline editing unless and until (i) the relevant safety and efficacy issues have been resolved, based on appropriate understanding and balancing of risks, potential benefits, and alternatives, and (ii) there is broad societal consensus about the appropriateness of the proposed application. 179
Moreover, the committee stressed the need for an international forum to discuss these matters and called upon national academies of China, the UK, and the USA to take the lead in this. Accordingly, in the following years, national science bodies from the USA and UK issued reports on the subject.
First, the NASEM published a report on gene editing in February 2017. The report indicates a subtle, yet important shift. Whereas the 2015 Summit statement endorses a ‘not allowed, unless’ approach to human germline editing, the NASEM seem to switch to ‘allowed, if’, as becomes clear in the following excerpt from the report’s public summary:
Given both the technical and societal concerns, the committee concludes there is a need for caution in any move toward germline editing, but that caution does not mean prohibition. It recommends that germline editing research trials might be permitted, but only after much more research to meet appropriate risk/benefit standards for authorizing clinical trials. Even then, germline editing should only be permitted for compelling reasons and under strict oversight. 180
Even if the NASEM equally underlines the importance of input by the public, a partially new stance now seems to be taken compared with the 2015 Summit statement, which narrows the scope of the propagated public debate considerably. The tacit normative shift in the NASEM approach to human germline editing is aptly described in a 2017 report on human germline editing from the German Ethics Council:
It is clear that the US-American academies are no longer focusing on a partially fundamental, partially risk-related strong rejection of germline therapy by genome editing but on a fundamental permission guided by individual formal and material criteria. […] Apparently, speculations now concentrate less on whether but rather only on when the first genetically modified [babies] by genome editing will be born. 181
In July 2018, the UK Nuffield Council on Bioethics issued its report on HGGE, in which, as discussed in the previous sections, the Nuffield Council takes an even more permissive approach than the NASEM by also leaving the door open to HGGE for enhancement purposes.
In November 2018, the Second International Summit on Human Gene Editing took place in Hong Kong, only days, as mentioned, after He’s shocking announcement. A day before the 3-day summit’s official kick-off, the organizing committee issued a statement in response to the news on the Chinese CRISPR babies. 182 The statement, in which little of the general indignation about He’s actions can be recognized, was criticized by many as rather ‘bland’. 183 A stronger condemnation followed, however, in the committee’s concluding statement. Even so, it is evident from the words used in that concluding statement that the trend toward a more permissive approach has not been reversed by the news from China. On the contrary, unlike its predecessor, this summit’s organizing committee proposes that a ‘translational pathway’ be developed toward the clinical use of this technology.
The organizing committee concludes that the scientific understanding and technical requirements for clinical practice remain too uncertain and the risks too great to permit clinical trials of germline editing at this time. Progress over the last three years and the discussions at the current summit, however, suggest that it is time to define a rigorous, responsible translational pathway toward such trials. A translational pathway to germline editing will require adhering to widely accepted standards for clinical research, including criteria articulated in genome editing guidance documents published in the last three years [reference at this point to aforementioned NASEM and Nuffield Council reports]. Such a pathway will require establishing standards for preclinical evidence and accuracy of gene modification, assessment of competency for practitioners of clinical trials, enforceable standards of professional behavior, and strong partnerships with patients and patient advocacy groups. 184
The first summit’s requirement of ‘broad societal consensus’ is barely mentioned anymore beyond underlining the need for ‘strong partnerships with patients and patient advocacy groups’. Therefore, as Greely writes, the second summit’s concluding statement can be read as saying: ‘There are a lot of technical things scientists need to figure out before this can be done. The public should have a chance to comment, but they will not make the decisions. We will.’ 185
In brief, although the need for public debate and democratic deliberation on the matter is formally recognized, the common tenor within the scientific community is that the main question to be answered is not whether HGGE should be pursued, but how and under which circumstances. 186 Moreover, the general thought seems to be that the answer to the ‘how question’ can also largely be provided by the scientific community itself, for example, through the erection of self-regulating oversight bodies and the development of protocols.
Although scientists will need to be involved in the decision-making on this issue, primarily to provide inside information on the latest technological developments and on possible health risks, the dominance of the voice of scientists in current debates on heritable genome editing is a reason for concern. For Petra De Sutter, a Belgian professor of gynecology and Rapporteur for the Council of Europe’s Parliamentary Assembly, the risk of conflicts of interest is the main problem:
There is a natural tendency for scientists to want to be the pioneers of genetic technology developments, to endeavour to publish papers thereon and to reap economic benefits from their research (for example by participating in technological companies). This raises the question of possible conflicts of interest. In my opinion, science provides knowledge, but it should not be left to scientists alone to decide on research policies (for example on where to set the limits of such research) and how the research is used. 187
Others argue that proposals for self-regulation rely on an outdated understanding of scientific practice according to which the development of new technologies takes place in a political and legal vacuum. 188 The danger is that reckless scientists such as He Jiankui will interpret such an approach to science as an encouragement to further push the boundaries of what is legally and ethically accepted. Commercially, they have a reason to persist in that thought. He, for example, raised around 40 million dollars from investors for his biotech start-ups.
Furthermore, the birth of the genetically modified twins does not give reason for much optimism on the capacity of the scientific community to prevent human germline editing from spiraling out of control. In the wake of the scandal, several doubts have been expressed. For example, why did the various scientists, whom He had consulted for his CRISPR experiment, remain silent about the dangerous path that he had embarked upon? 189 Moreover, can it be said that ‘the implicit endorsement of reproductive gene editing in [the NASEM and Nuffield] reports facilitated the work of rogue actors such as He, providing ethical cover for his work?’ 190 Indeed, He has defended his actions by claiming that they are in line with the guidelines from the NASEM report on gene editing. 191 Perhaps, this can explain in part why He chose to defy the existing Chinese guidelines and regulations. 192 The Chinese biophysicist argues that he genetically modified the twins only to prevent a serious disease in the absence of reasonable alternatives, exactly as prescribed by the NASEM. Although He’s rather loose interpretation of these guidelines can be regarded as mere rationalization, his claim is not entirely without ground. Responding to the birth of the genetically modified twins, Victor Dzau, president of the US National Academy of Medicine, admitted that the existing guidelines are not clear enough and too open for interpretation. 193 As such, the event did serve as a ‘wake-up call from Hong Kong’ for the NASEM. 194
All of this suggests that attempts at self-regulation from the biomedical community have so far not only failed to prevent He’s reckless experiment with the twins but also even helped to create ‘an increasingly permissive climate among elite scientists that may well have emboldened He’ 195 in the first place. At worst, this scientific elitism may take on the shape of outright contempt for the idea of legal rules and democratic deliberation itself. A striking example is the approach taken by the Russian scientist Denis Rebrikov, who, as discussed, plans to follow in He’s footsteps to create genetically modified babies. In an interview with SCIENCE, he offers the following views on the regulation of scientific progress and human enhancement:
‘We cannot stop progress with words on paper. So even if we say, let us not do the nuclear physics, because it can make a bomb, a lot of scientists will still do this. We cannot stop it. A lot of groups will try to do experiments with embryos to transfer to women, and maybe it will not be in my group, but we will see in the next years that they will have some results, and they will publish it. That’s maybe the problem for humans on the planet, that we cannot stop the progress. […] [Human enhancement] will be the next step. But in 20 to 30 years. Now, I’m opposed to it. In 2040, I’ll support it. I’m not against the idea itself. And these people who are opposed want to have all these things in their children but only by “divine providence,” not by science. They are liars or stupid.’ 196
Should individuals have the possibility to change the genetic constitution of their descendants? Since the rise of CRISPR, this question is no longer theoretical. Traditionally, national and international legal orders have answered this question with a clear and adamant ‘no’. Most of the existing bans and restrictions on this technology go back to the human rights frameworks that were adopted in the late 1990s to regulate biomedical developments. A recurring idea in these frameworks is that reproductive HGGE is at odds with the human rights principles of freedom, equality, and dignity, as this technology would make it possible to produce individuals to fit certain requirements. In other words, it could open the door for unprecedented forms and practices of eugenics.
However, now that CRISPR has taken the biotechnology world by storm, these provisions are under increasing pressure. Even the uproar created by He Jiankui’s attempts at genetically modifying offspring has not been able to break this trend. Especially, among scientific and medical-professional bodies, academies, and societies, the view is gaining ground that the existing bans should be lifted and that reproductive gene editing should be allowed for therapeutic purposes as soon as the technology is safe for clinical application.
How should these proposals be viewed from a human rights perspective? Interestingly, in most of the reports, articles, and manifestos that advocate a regulatory pathway approach to HGGE, human rights are equally invoked. The Nuffield Council’s report, for example, explicitly refers to human rights discourse as its prime ethical framework, 197 while the NASEM report states that its overarching framework is ‘embedded within the larger context of international conventions and norms for protection of human rights’. 198 In other words, these proposals rely on human rights to justify why the existing human rights ban on reproductive gene editing should be lifted. This suggests that conflicting views on the meaning of human rights and human dignity are at the heart of current legal-ethical debates on HGGE. As such, HGGE indeed touches on the normative foundations of human rights law.
It is widely recognized that the meaning of human rights and their underlying principles may evolve over time. In this vein, the European Court of Human Rights has characterized the European Convention on Human Rights as a ‘living instrument, which must be interpreted in the light of present day conditions’, even when it comes to core human rights such as the prohibition on torture and inhuman or degrading treatments or punishments. 199 The same line of thinking necessarily applies to the principles underlying these rights, such as human dignity.
Similarly, the meaning of human rights and human dignity for the issue of human germline editing is not set in stone and may evolve over time. This is also recognized by bioethics committees of both the Council of Europe and UNESCO, when they emphasize the vital importance of a public debate on this matter. In this article, I have aimed to contribute to that debate by addressing three human rights concerns about the recent proposals for a regulatory pathway approach to reproductive gene editing.
First, I have discussed the distinction that many of these proposals make between human germline editing for therapeutic and nontherapeutic purposes. According to this line of thought, using genetic modification for enhancement purposes would conflict with human rights law, whereas using this technology to prevent serious diseases and conditions would not. Although the medical boundary between healing and enhancing can indeed be recognized in the human rights frameworks for the regulation of biomedical technologies, I have argued that the medical boundary will be much harder to maintain in case of HGGE than PGD. Hence, once the ban on HGGE is lifted, it will be hard to prevent the practice from gradually sliding down toward more eugenic applications.
Second, I have focused on the view and concept of human rights that underlie the recent proposals to go from prohibition to regulation of heritable gene editing. Some authors have argued that editing the human germline is tantamount to editing human nature and that this would therefore disrupt the foundation of human rights. 200 This paper goes back to a different thought, namely, that proposals to lift the ban on germline editing are mostly rooted in a one-dimensional reading of human rights that radically parts with existing human rights discourse on biomedical technologies. Existing human rights approaches, as laid down in international law documents from the Council of Europe and UNESCO, address concerns at the level of the individual, society, and humanity. The recent proposals, however, only take into account the individual and societal dimensions of human rights discourse. References to humanity, humankind, human dignity, or the idea of the human genome as heritage of humanity are conspicuously absent. If human dignity is mentioned, it is reduced to a principle that only protects individual freedoms and rights. In other words, the collective dimension of human dignity is ignored. For a proper debate on the meaning of human rights for heritable genome editing, both sides need to be taken into account. A careful balance needs to be found between, on the one hand, the health and rights of prospective parents and their future offspring and, on the other hand, the long-term interests of society, future generations, and humankind.
Third, I have argued that this dismissal of the collective dimensions of human rights discourse also has repercussions for the public deliberation on this matter. Once the issue is framed as one concerning exclusively the health and rights of those directly involved, vital questions are lost from view. Questions such as: what kind of lives do we wish for future generations? And: how can we protect our humanity in an increasingly technological and data-obsessed society? Moreover, the way in which the issue is reframed, with a strong focus on medical standards such as safety and the medical boundary between healing and enhancing, bolsters the tendency to leave the discussion and governance to the scientific community itself.
From this perspective, democratic interests appear to be at stake as well. In a way, CRISPR is turning democracy upside-down. In a ‘CRISPR democracy’, 201 citizens take the place of scientists when they, as biohackers, start tinkering with DNA, under the guise of ‘democratizing’ the life sciences. The other way around, scientists take the place of citizens when their voice becomes decisive in the governance of highly controversial technologies that may have a lasting impact on no less than the future of humankind.
The He Lab, About Lulu and Nana: Twin Girls Born Healthy After Gene Surgery As Single-Cell Embryos , www.youtube.com/watch?v=th0vnOmFltc (accessed July 19, 2019).
Antonio Regalado, Chinese Scientists Are Creating CRISPR Babies , MIT Technology Review, Nov. 25, 2018.
Charlotte Jee, A Second CRISPR Pregnancy Is Already Under Way, Claims Chinese Scientist , MIT Technology Review, Nov. 28, 2018.
Gina Kolata & Pam Belluck, Why Are Scientists So Upset About the First Crispr Babies? , New York Times, Dec. 5, 2018; Kiran Musunuru, We need to know what happened to CRISPR twins Lulu and Nana , MIT Technology Review, Dec. 3, 2019.
Henry Greely, CRISPR’d babies: human germlinegenome editing in the ‘He Jiankui affair’ , 6 J. L. & Biosci. 111, 116–117 (2019); Antonio Regalado, China’s CRISPR babies: Read exclusive excerpts from the unseen original research , MIT Technology Review, Dec. 3, 2019.
Greely, CRISPR’d babies, supra note 5, at 117.
Jennifer Doudna, He Jiankui , Time Magazine, Apr. 29, 2019.
Sharon Begley & Andrew Joseph, The CRISPR shocker: How Genome-Editing Scientist He Jiankui Rose From Obscurity to Stun the World, STAT, Dec. 18, 2018.
In 2012, CRISPR-Cas9 was mentioned for the first time in scientific literature (Martin Jinek et al., A Program-mable Dual-RNA-Guided DNA Endonuclease in Adaptive Bacterial Immunity , 337 Science 816 (2012)).
Alex Pearlman, Biohackers Are Using CRISPR On Their DNA and We Cannot Stop It , New Scientist, Nov. 15, 2017.
For a brief overview of these patent wars, see Ana Nordberg et al., Cutting Edges and Weaving Threads in the Gene Editing (Я)evolution: Reconciling Scientific Progress With Legal, Ethical, and Social Concerns , 5 J. L. & Biosci. 35, 68–71 (2018).
Sara Reardon, ‘Welcome to the CRISPR Zoo’, 531 Nature 160 (2016).
Id . at 163.
Peter Sloterdijk, Regeln für den Menschenpark. Ein Antwortschreiben zu Heideggers Brief über den Humanismus (1999). For an English translation, see Peter Sloterdijk, Rules for the Human Zoo: A Response to the Letter on Humanism , 27 Environ. Plann. D 12 (2009).
David Cyranoski, The CRISPR-Baby Scandal: What’s Next for Human Gene-Editing , 566 Nature 440 (2019).
David Cyranoski & Sara Reardon, Chinese Scientists Genetically Modify Human Embryos , 250 Nature 593 (2015).
Jessica Hamzelou, World’s First Baby Born With New ‘3 Parent’ Technique , New Scientist , Sept. 27, 2016.
I use the term ‘human nuclear genome transfer’ instead of ‘mitochondrial replacement therapy’ for reasons explained by Françoise Baylis, Human Nuclear Genome Transfer (So-Called Mitochondrial Replacement): Clearing the Underbrush , 31 Bioethics 7 (2017).
See Sara Reardon, Genetic Details of Controversial ‘Three-Parent Baby’ Revealed , 544 Nature 17 (2017); Steve Connor, When Replacement Becomes Reversion , 35 Nat. Biotechnol . 1012 (2017).
Françoise Baylis & Alana Cattapan, Personalised Medicine and the Politics of Human Nuclear Genome Transfer , in Personalised Medicine, Individual Choice and the Common Good 26 (Britta van Beers, Sigrid Sterckx & Donna Dickenson eds., 2018).
Susan Scutti, Controversial IVF Technique Produces a Baby Girl; And for Some, That’s a Problem , CNN, Jan. 18, 2018, https://edition.cnn.com/2017/01/18/health/ivf-three-parent-baby-girl-ukraine-bn/index.html (accessed July 19, 2019).
Emily Mullin, The Fertility Doctor Trying to Commercialize Three-Parent Babies , MIT Technology Review, June 13, 2017.
The FDA sent the following letter to Zhang: https://www.fda.gov/media/106739/download?source=govdelivery&utm_medium=email&utm_source=govdelivery (accessed July 19, 2019).
Many scientists also regard nuclear genome transfer as germline genetic modification (e.g. Guido de Wert et al., Responsible Innovation in Human Germline Gene Editing: Background Document to the Recommendations of the ESHG and ESHRE , 26 Eur. J. Hum. Genet. 550 (2018)). However, the UK Government maintained that while MRTs ‘do result in germ-line modification, the techniques [do not] constitute genetic modification’ (see Rosamund Scott & Stephen Wilkinson, Germline Genetic Modification and Identity: the Mitochondrial and Nuclear Genomes , 37 OJLS 886, 887 (2017)).
Although the rule is that mitochondrial disorders are generally inherited through the maternal line, occasionally, these disorders may be transmitted by the father as well (see: Thomas McWilliams & Anu Suomalainen, Mitochondrial DNA Can Be Inherited from Fathers, Not Just Mothers , 565 Nature 296 (2019)).
Heidi Ledford, CRISPR Fixes Disease Gene in Viable Human Embryos , 548 Nature 13 (2017).
David Cyranoski, Russian Biologist Plans More CRISPR-Edited Babies , 570 Nature 145 (2019).
Michael Le Page, Five Couples Lined Up for CRISPR Babies to Avoid Deafness , Scientist, July 4, 2019.
David Cyranoski, Russian Scientist Edits Human Eggs in Effort to Alter Deafness Gene , 574 Nature 465 (2019).
Jon Cohen, Russian Geneticist Answers Challenges to His Plan to Make Gene-Edited Babies , Science, June 13, 2019.
Françoise Baylis, Human Genome Editing: Our Future Belongs to All of Us , 35 Issues Sci. Technol. 42, 42 (2019).
Rinie van Est et al., Rules for the digital human park: Two paradigmatic cases of breeding and taming human beings—Human germline editing and persuasive technology 15 (2017).
Rosario Isasi, Erika Kleiderman & Bartha Knoppers, Editing Policy to Fit the Genome? , 351 Science 337 (2016); Motoko Araki & Tetsuya Ishii, International Regulatory Landscape and Integration of Corrective Genome Editing Into In Vitro Fertilization , 12 Reprod. Biol. Endocrinol. 108 (2014).
As Isasi, Kleiderman and Knoppers characterize the legal diversity: ‘Internationally, policies extend across a continuum that distinguishes between degrees of permissiveness, that is, between legally binding legislation and regulatory and/or professional guidance or research versus clinical applications’ (Isasi, Kleiderman & Knoppers, supra note 35, at 337).
Artt. 3.7 & 3.9 of the 2003 ‘Technical Norms of Human Assisted Reproductive Technologies’ (see Nuffield Council on Bioethics, Genome Editing and Human Reproduction: Social and Ethical Issues 111 (2018); Di Zhang, & Reidar K. Lie, Ethical Issues in Human Germline Gene Editing: A Perspective from China , 36(1–4) Monash Bioethics Review, 23 (2018).
Research Activities of Persons Halted Over Gene-Edited Babies Incident , XinhuaNet, Nov. 29, 2018, http://www.xinhuanet.com/english/2018-11/29/c_137640174.htm (accessed July 19, 2019).
Ian Sample, ‘Chinese scientist who edited babies’ genes jailed for three years’, Guardian, Dec. 31, 2019, https://www.theguardian.com/world/2019/dec/30/gene-editing-chinese-scientist-he-jiankui-jailed-three-years (accessed Mar. 24, 2020); David Cyranoski, ‘China to Tighten Rules on Gene Editing in Humans’, Nature, Mar. 6, 2019, https://www.nature.com/articles/d41586-019-00773-y (accessed July 19, 2019).
Nuffield Council, supra note 38, at 109.
I. Glenn Cohen & Eli Adashi, The FDA Is Prohibited From Going Germline , 353 Science 545 (2016).
See statement by NIH Director Francis Collins, Statement on NIH Funding of Research Using Gene-editing Technologies in Human Embryos , Apr. 28, 2015, https://www.nih.gov/about-nih/who-we-are/nih-director/statements/statement-nih-funding-research-using-gene-editing-technologies-human-embryos (accessed July 19, 2019).
Greely, CRISPR’d babies, supra note 5, at 128–129. For a critical discussion of this legal situation, see Glenn Cohen & Adashi, supra note 42. Also see Nuffield Council, supra note 38, at 109–110.
Glenn Cohen & Adashi, supra note 42.
Rowena Mason & Hannah Devlin, MPs Vote in Favour of ‘Three-Person Embryo’ Law , Guardian, Feb. 3, 2015.
The Human Fertilisation and Embryology (Mitochondrial Donation) Regulations 2015 (S.I 2015 No. 572).
Samvel Varvaštian , UK’s Legalisation of Mitochondrial Donation in IVF Treatment: A Challenge to the International Community or a Promotion of Life-saving Medical Innovation to Be Followed by Others? , 22 Eur. J. Health L. 405, 424 (2015).
Nuffield Council, supra note 38, at 112.
Lev Facher, Why Democrats Reopened the Debate About Germline Gene Editing , STAT, June 18, 2019, https://www.statnews.com/2019/06/18/democrats-reopened-debate-about-germline-editing/ (accessed July 1, 2019).
Roberto Andorno & Alicia Ely Yamin, The Right to Design Babies? Human Rights and Bioethics , Open Global Rights, Jan. 8, 2019, https://www.openglobalrights.org/the-right-to-design-babies-human-rights-and-bioethics/ (accessed July 19, 2019).
Parliamentary Assembly of the Council of Europe, Recommendation on Genetic Engineering , Recommendation 934 (1982), sub 4a.
Roberto Andorno, Biomedicine and International Human Rights Law: In Search of a Global Consensus , 80 Bulletin of the World Health Organization 959 (2002).
According to Scott & Wilkinson, supra note 26, this Recommendation leaves the door open for forms of germline editing that aims to prevent diseases, because of par. 4c: ‘the explicit recognition of this right must not impede development of the therapeutic applications of genetic engineering (gene therapy), which holds great promise for the treatment and eradication of certain diseases which are genetically transmitted’.
Parliamentary Assembly of the Council of Europe, Recommendation on Genetic Engineering , supra note 53, sub 4a & 7a.
Explanatory report to the Convention on Human Rights and Biomedicine, European Treaty Series, nr. 164, sub 89, https://rm.coe.int/16800ccde5 (accessed Aug. 1, 2019).
Parliamentary Assembly of the Council of Europe, The Use of New Genetic Technologies in Human Beings , Recommendation 2115 (2017), sub 3.
Committee on Bioethics (DH-BIO), Statement on Genome Editing Technologies , Dec. 2, 2015, https://rm.coe.int/168049034a (accessed Aug. 1, 2019).
Roberto Andorno, The Oviedo Convention: A European Legal Framework at the Intersection of Human Rights and Health Law , 2 J. Int. Biotech. L . 134 (2005).
28 out of 47 Council of Europe member states are EU member states.
Directive 98/44/EC of the European Parliament and of the Council on the legal protection of biotechnological inventions, C/2016/6997, OJ C 411, preamble sub 40.
Case C-34/10, Oliver Brüstle v. Greenpeace e. V , EU:C:2011:669, sub 34.
Directive 2001/20/EC of the European Parliament and of the Council of Apr. 4, 2001 on the approximation of the laws, regulations, and administrative provisions of the member states relating to the implementation of good clinical practice in the conduct of clinical trials on medicinal products for human use, OJ L 121, 1.5.2001, at 34 (hereafter: Clinical Trials Directive).
Regulation (EU) No 536/2014 of the European Parliament and of the Council of Apr. 16, 2014 on clinical trials on medical products for human use, and repealing Directive 2001/20/EC (hereafter: Clinical Trials Regulation).
Previously art. 9 par. 6 Clinical Trials Directive; replaced by art. 90 Clinical Trials Regulation.
International Bioethics Committee, Report of the IBC on Updating Its Reflection on the Human Genome and Human Rights , UNESCO, SHS/YES/IBC-22/15/2 REV.2 (Paris, October 2, 2015), sub 107.
Id. sub 107.
A prohibition on reproductive cloning can be found in the Additional Protocol to the Oviedo Convention on the Prohibition of Cloning Human Beings.
Id. sub 111.
Explanations Relating to the Charter of Fundamental Rights , 2007/C 303/02.
E.g. Annelien Bredenoord et al., Ethics of Modifying the Mitochondrial Genome , 37 J. Med. Ethics 97 (2011); Scott & Wilkinson, supra note 26.
Bredenoord et al., supra note 73, at 97.
For a further analysis of how this disctinction between nuclear and mitochondrial DNA has affected the UK’s regulation of HNGT, see Scott & Wilkinson, supra note 26, especially at 897–898. For the Dutch legislature’s position, see the travaux préparatoires of the Dutch Embryo Act (Parliamentary document Kamerstukken II 2000/01, 27 423, nr. 5, p. 99–100).
According to the Dutch minister of Health, this has to do with the Dutch ban on creating embryos for research purposes. This ban has made it impossible for Dutch scientists to do research in this field ensuring that the technology is safe for introduction in the clinic (see Parliamentary document Kamerstukken II 2016/17, 29 323, nr. 105, p. 12–13). For a further analysis of the Dutch policy in this field, see Britta van Beers, Charlotte de Kluiver & Rick Maas, The Regulation of Human Germline Genome Modification In the Netherlands , in: Human Germline Modification and the Right to Science. A Comparative Study of National Laws and Policies 309–344 (Andrea Boggio, Cesare Romano, & Jessica Almqvist (eds.), 2020).
Ewen Callaway, Historic Decision Allows UK Researchers to Trial ‘Three Person’ Babies , Nature (2016), https://www.nature.com/news/historic-decision-allows-uk-researchers-to-trial-three-person-babies-1.21182 (accessed Aug. 1, 2019).
Emily Mullin, Pregnancy Reported in the First Known Trial of ‘Three-Person IVF’ for Infertility , STAT, Jan. 24, 2019.
Helen Thomson, ‘First 3-parent baby born in clinical trial to treat infertility’, New Scientist, Apr. 11, 2019, https://www-newscientist-com.vu-nl.idm.oclc.org/article/2199441-first-3-parent-baby-born-in-clinical-trial-to-treat-infertility/ (accessed July 19, 2019).
Embryotools Achieves the World’s First Pregnancy With a New Nuclear Transfer Technique for Treating Infertility , Jan. 17, 2019, http://www.pcb.ub.edu/portal/en/noticies/-/noticia/no_embryotools-aconsegueix-el-primer-embaras-del-mon-amb-una-nova-tecnica-de-transferencia-nuclear-per-tractar-la-infertilitat (accessed July 19, 2019).
Mullin, Pregnancy Reported in the First Known Trial , supra note 78.
For full reference, supra note 65.
For full reference , supra note 66.
See Art. 2(2) Clinical Trials Regulation; and Art. 2(a) Clinical Trials Directive.
Nuffield Council, supra note 38, at 122–123.
See for example the title of the article in Nature in which the news on the licences is shared (Callaway, supra note 77).
Rumiana Yotova, The Regulation of Genome Editing and Human Reproduction Under International Law, EU Law and Comparative Law (background report for the Nuffield Council on Bioethics) (2017).
Varvaštian, supra note 48, at 421–422 (note 92).
Varvaštian, supra note 48, at 99.
European Group on Ethics in Science and New Technologies, Statement on Gene Editing , https://ec.europa.eu/research/ege/pdf/gene_editing_ege_statement.pdf (accessed July 19, 2019).
De Wert et al, supra note 26, at 456.
Isasi, Kleiderman & Knoppers, supra note 35, at 337.
Quoted in Antonio Regalado , Engineering the Perfect Baby , MIT Technology Review, Mar. 5, 2015.
For an overview of the ethics statements on HGGE, see Carolyn Brokowski, Do CRISPR Germline Ethics Statements Cut It? , 1 CRISPR Journal 115 (2018); and Nuffield Council, supra note 38, at 129–132;
E.g. David Baltimore et al., A Prudent Path Forward for Genomic Engineering and Germline Gene Modification , 348 Science 36 (2015); Hinxton Group Statement, Statement on genome editing technologies and human germline genetic modification , 2015, http://www.hinxtongroup.org/Hinxton2015_Statement.pdf (accessed July 19, 2019); George Daley, Robin Lovell-Badge, Julie Steffann, After the Storm: A Responsible Path for Genome Editing , 380 New Eng. J. Med. 897 (2019); Eric Lander et al., Adopt a Moratorium on Heritable Genome Editing , 567 Nature 165 (2019).
De Wert et al., supra note 26.
E.g. in the US: National Academies of Sciences, Engingeering and Medicine, Human Genome Editing: Science, Ethics, and Governance (2017); in Germany: Leopoldina, ACATECH, & UNION, The Opportunities and Limits of Genome Editing (2015); and in the Netherlands: KNAW, Genome Editing. Position Paper of the Royal Netherlands Academy of Arts and Sciences (2016).
E.g. Organizing Committee for the International Summit on Gene Editing, On Human Gene Editing: International Summit Statement , Washington Dec. 1–3, 2015, http://www8.nationalacademies.org/onpinews/newsitem.aspx?RecordID=12032015a (accessed July 19, 2019); Federation of European Academies of Medicine, The Application of Genome Editing in Humans , October 2017, https://www.feam.eu/the-application-of-genome-editing-in-humans/ (accessed July 19, 2019); Organizing Committee for the International Summit on Gene Editing, On Human Genome Editing II. Statement by the Organizing Committee of the Second International Summit on Human Genome Editing , Hong Kong Nov, 29, 2018, http://www8.nationalacademies.org/onpinews/newsitem.aspx?RecordID=11282018b (accessed July 19, 2019).
E.g. Nuffield Council, supra note 38; Netherlands Commission on Genetic Modification (COGEM) & Health Council of the Netherlands (Gezondheidsraad), Editing Human DNA: Moral and Social Implications of Germline Genetic Modification (2017).
Lander et al., supra note 96, at 168.
See the subtitle of Daley, Lovell-Badge, Steffann, supra note 96: ‘A Responsible Path for Genome Editing’.
See the title of Baltimore et al., supra note 96: ‘A Prudent Path Forward for Genomic Engineering and Germline Gene Modification’.
See statement from Organizing Committee for the International Summit on Gene Editing, supra note 99.
Robin Alta Charo, Rogues and Regulation of Germline Editing , 380 New Eng. J. of Med. 977 (2019).
There is disagreement among those who propagate a pathway approach as to the need of a moratorium (for more details on this discussion, see Eli Adashi & I. Glenn Cohen, Heritable Genome Editing: Is a Moratorium Needed?, 322 JAMA 104 (2019)).
The UK Nuffield Council on Bioethics takes a different stance and rejects the medical boundary between healing and enhancing as a useful red line. This will be discussed in further detail in Section V.
Committee on Bioethics, supra note 59.
International Bioethics Committee, supra note 68, sub 117 & 118.
‘Parties to this Convention shall see to it that the fundamental questions raised by the developments of biology and medicine are the subject of appropriate public discussion in the light, in particular, of relevant medical, social, economic, ethical and legal implications, and that their possible application is made the subject of appropriate consultation.’
See, e.g., ECtHR, Apr. 25, 1978, Tyrer v. UK , application no. 5856/72.
Peter Sykora & Arthur Caplan, The Council of Europe Should Not Reaffirm the Ban on Germline Genome Editing in Humans , 18 EMBO Reports 1871 (2017).
E.g. Isasi, Kleiderman & Knoppers, supra note 35; Alta Charo, Rogues , supra note 93; Sykora & Caplan, supra note 99.
Alta Charo, Rogues , supra note 106, at 976.
E.g. Eli Adashi & I. Glenn Cohen, ‘Germline Editing: Could Ban Encourage Medical Tourism?’, 569 Nature 40 (2019). Elsewhere, I critically discuss the pragmatic line of reasoning with regard to reproductive tourism, see Britta van Beers, Is Europe ‘Giving in to Baby Markets’? Reproductive Tourism in Europe and the Gradual Erosion of Existing Legal Limits to Reproductive Markets , 23 Med. L. Rev. 103 (2015).
Isasi, Kleiderman & Knoppers, supra note 35, at 339.
For an analysis of and possible human rights approach to this issue, see Erika Kleiderman, Vardit Ravitsky & Bartha Knoppers, The ‘Serious’ Factor in Germline Modification , 45 J Med Ethics 508 (2019).
Edward Lanphier et al., Do not Edit the Human Germline , 519 Nature 411 (2015).
Likewise, the European Group on Ethics writes in its 2016 Statement on Gene Editing that ‘the blurring of the lines between clinical applications in pursuit of therapeutic or enhancement goals […], must be considered’ (see European Group on Ethics, supra note 90).
Nuffield Council, supra note 38, at 47.
However, according to the Nuffield Council, concerns about function creep and slippery slopes can be countered through reliable regulation (see Nuffield Council, supra note 38, at 53–55).
For a more elaborate discussion of the difficulty to define ‘therapeutic’ in the context of HGGE, see Eric T. Juengst, Crowdsourcing the Moral Limits of Human Gene Editing? , 47(3) Hastings Cent. Rep. 15, 21 (2017).
Mary Joy et al., CCR5 Is a Therapeutic Target for Recovery after Stroke and Traumatic Brain Injury , 176 Cell 1143 (2019).
Antonio Regalado, ‘China’s CRISPR twins might have had their brains inadvertently enhanced’, MIT Technology Review, Feb. 21, 2019.
For this expression, and for an elaborate discussion of this issue, see Eric T. Juengst et al., Is Enhancement the Price of Prevention in Human Gene Editing? , 1 CRISPR Journal 351, 353 (2018).
Kolata & Belluck, supra note 4.
Given these complexities, future parents may come to rely on algorithms for reproductive decisionmaking, not only in the case of ‘easy PGD’ (a combination of in vitro gametogenesis, PGD and genome sequencing, see Henry Greely, The End of Sex and the Future of Human Reproduction (2016)), as Sonia Suter has set out in a recent article ( The Tyranny of Choice , 5 J. Law Biosci . 262 (2018) but also in the context of heritable germline editing.
Nuffield Council, supra note 38, at 71.
Id. at 71 and 91–92.
For a comparison between the NASEM report and the Nuffield Council Report, see Eli Adashi & I. Glenn Cohen, The Ethics of Heritable Genome Editing: New Considerations in a Controversial Area , 320 JAMA 2531 (2018).
International Bioethics Committee, supra note 68, sub 111.
Robin Alta Charo, Germline Engineering and Human Rights , 112 AJIL Unbound, 344 (2018).
Explanatory Report Oviedo Convention, supra note 57, sub 14.
International Bioethics Committee, supra note 68, sub 34.
Id. sub 30.
Id. sub 28.
E.g. Deryck Beyleveld & Roger Brownsword, Human Dignity in Bioethics and Biolaw (2002); Roberto Andorno, Human Dignity and Human Rights as a Common Ground for a Global Bioethics, 34 J. Med. Philos. 223 (2009); Micha Werner, ‘Individual and collective dignity’, in: The Cambridge Handbook of Human Dignity: Interdisciplinary Perspectives 343 (Marcus Duewell, Jens Braarvig, Roger Brownsword & Dietmar Mieth eds., 2014).
International Bioethics Committee, supra note 68, sub 128.
Id . sub 108.
Id . sub 105.
Id . sub 103.
Id . sub 107.
Explanatory Report Oviedo Convention, supra note 57, sub 89.
Andorno, Human Dignity , supra note 141, at 233; German Ethics Council, Intervening in the Human Germline: Executive Summary & Recommendations sub 55 (2019).
Andorno, Human Dignity , supra note 141, at 233; Teresa Iglesias, The Dignity of the Individual: Issues of Bioethics and Law 3 (2001).
Baylis, Human Genome Editing, supra note 33, at 44.
Katie Hasson & Marcy Darnovsky, Gene-Edited Babies: No One Has the Moral Warrant to Go It Alone , Guardian, Nov. 27, 2018. Also see International Bioethics Committee, supra note 68, sub 116.
Nuffield Council, supra note 38, at 75.
Nuffield Council, supra note 38, at 87.
Especially see box 3.4 in the report, that is entirely devoted to this argument (Nuffield Council, supra note 38, at 93–94).
National Academies, supra note 98, at 32.
At this point the Nuffield Council refers to Ruth Macklin, Human Dignity Is a Useless Concept , 327 British Med J 1419 (2003); and Mirko Bagaric & James Allan, The Vacuous Concept of Dignity , 5 J. Human Rights 257 (2006).
‘Whereas human dignity has been advanced by some as the basis of human rights, the coherent functioning of human rights discourse does not depend on accepting this claim’ (Nuffield Council, supra note 38, at 94).
Nuffield Council, supra note 38, at 96.
E.g. Iñigo de Miguel Beriain, Human Dignity and Gene Editing, EMBO Reports, e46789, (2018); Peter Mills, Genome Editing, Human Rights and the ‘Posthuman’ , 3 October 2017, http://nuffieldbioethics.org/blog/genome-editing-human-rights-posthuman (accessed July 19, 2019).
Alta Charo, Germline Engineering , supra note 136, at 348–349.
Françoise Baylis & Lisa Ikemoto, The Council of Europe and the Prohibition on Human Germline Genome Editing , 18 EMBO Reports 2084 (2017).
Nuffield Council, supra note 38, at 91.
Id. at 90–91.
German Ethics Council, supra note 149, at 4.
Mills, supra note 162.
Alta Charo, Germline Engineering , supra note 136, at 349.
This is also one of the central theses of Harari’s bestseller Homo Deus : ‘The rise of humanism also contains the seeds of its downfall. While the attempt to upgrade into gods takes humanism to its logical conclusion, it simultaneously exposes humanism’s inherent flaws’ (Yuval Harari, Homo Deus: A Brief History of Tomorrow 65 (2016)).
Van Est et al., supra note 34, at 15.
Sheila Jasanoff, J. Benjamin Hurlbut & Krishanu Saha , CRISPR Democracy: Gene Editing and the Need for Inclusive Deliberation , 32 Issues Sci. Technol. 25 (2015).
See Donna Dickenson, Me Medicine vs. We Medicine: Reclaiming Biotechnology for the Common Good (2013); and Britta van Beers, Sigrid Sterckx and Donna Dickenson (eds.), Personalised Medicine, Individual Choice and the Common Good (2018).
Elsewhere, I offer further reflection on the need for public imagination in the debate on human germline genetic modification (see Britta van Beers, Imagining Future People in Biomedical Law: From Technological Utopias to Legal Dystopias Within the Regulation of Human Genetic Modification Technologies , in: Risk and the Regulation of Uncertainty in International Law 117 (Monika Ambrus, Rosemary Rayfuse & Wouter Werner eds. 2017).
Jasanoff, Hurlbut & Saha, supra note 173.
See, for example, Baltimore et al., supra note 96.
On Human Gene Editing: International Summit Statement , http://www8.nationalacademies.org/onpinews/newsitem.aspx?RecordID=12032015a (accessed Aug. 1, 2019).
See Report Highlights , p. 3, available at: https://www.nap.edu/resource/24623/Human-Genome-Editing-highlights.pdf (accessed Aug. 1, 2019). Also see National Academies, supra note 98, at 134–135 and 189–190.
Deutscher Ethikrat, Germline Intervention in the Human Embryo: German Ethics Council Calls for Global Political Debate and International Regulation 3 (2017).
Statement from the Organizing Committee on Reported Human Embryo Genome Editing , http://www8.nationalacademies.org/onpinews/newsitem.aspx?RecordID=11262018 (accessed Aug. 1, 2019).
E.g. Ed Yong, The CRISPR Baby Scandal Gets Worse by the Day , The Atlantic, Dec. 3, 2018, https://www.theatlantic.com/science/archive/2018/12/15-worrying-things-about-crispr-babies-scandal/577234/ (accessed July 19, 2019).
Statement by the Organizing Committee of the Second International Summit on Human Genome Editing , http://www8.nationalacademies.org/onpinews/newsitem.aspx?RecordID=11282018b (accessed Aug. 1, 2019).
Henry Greely, How Should Science Respond to CRISPR’d Babies? , 35 Issues Sci. Technol. 32, 36 (2019).
J. Benjamin Hurlbut, Human Genome Editing: Ask Whether, Not How , 565 Nature 135 (2019); Donna Dickenson & Marcy Darnovsky, Did a Permissive Scientific Culture Encourage the ‘CRISPR Babies’ Experiment? , 37 Nat. Biotechnol . 355 (2019).
Petra De Sutter, The Use of New Genetic Technologies in Human Beings (Explanatory Memorandum to Recommendation 2115 of the Parliamentary Assembly of the Council of Europe) , doc. 14328, May 24, 2017, sub 32.
Natalie Kofler, Why Were Scientists Silent Over Gene-Edited Babies? , 566 Nature 427 (2019).
Hasson & Darnovsky, supra note 152.
Antonio Regalado, Rogue Chinese CRISPR Scientist Cited US Report As His Green Light , MIT Technology Review, Nov. 27, 2018.
Another important factor is the failure to implement and enforce the Chinese regulations, see Zhang & Lie, supra note 38; and Erika Kleiderman & Ubaka Ogbogu, Realigning Gene Editing with Clinical Research Ethics: What the “CRISPR Twins” Debacle Means for Chinese and International Research Ethics Governance , 26(4) Accountability in Research 257.
Sharon Begley, After ‘CRISPR babies’, International Medical Leaders Aim to Tighten Genome Editing Guidelines , STAT, Jan. 24, 2019.
Victor Dzau, Marcia McNutt & Chunli Bai, Wake-Up Call From Hong Kong , 362 Science 1215 (2018).
Dickenson & Darnovsky, supra note 186, at 356.
Cohen, supra note 32.
Nuffield Council, supra note 38, at 59.
National Academies, supra note 98, at 29.
ECtHR, Apr. 25, 1978, Tyrer v. UK , application no. 5856/72.
E.g. Francis Fukuyama, Our Posthuman Future (2002); George Annas, Lori Andrews & Rosario Isasi, Protecting the Endangered Human: Toward an International Treaty Prohibiting Cloning and Inheritable Alterations , 28 Am. J.L. & Med. 151 (2002).
See title of Jasanoff, Hurlbut & Saha, supra note 173.
Author notes
Email alerts, citing articles via.
- Recommend to your Library
Affiliations

- Online ISSN 2053-9711
- Copyright © 2024 Oxford University Press and Harvard, Duke and Stanford Law Schools
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Eur J Hum Genet
- v.26(1); 2018 Jan
One small edit for humans, one giant edit for humankind? Points and questions to consider for a responsible way forward for gene editing in humans
Heidi c. howard.
1 Centre for Research Ethics and Bioethics, Uppsala University, Uppsala, Sweden
Carla G. van El
2 Department of Clinical Genetics, Section Community Genetics and EMGO Institute for Health and Care Research, VU University Medical Center, Amsterdam, The Netherlands
Francesca Forzano
3 Department of Clinical Genetics, Great Ormond Street Hospital, London, UK
Dragica Radojkovic
4 Laboratory for Molecular Genetics, Institute of Molecular Genetics and Genetic Engineering, University of Belgrade, Belgrade, Serbia
Emmanuelle Rial-Sebbag
5 UMR 1027, Inserm, Faculté de médecine Université Toulouse 3, Paul Sabatier, Toulouse France
Guido de Wert
7 Department of Health, Ethics and Society, Research Schools CAPHRI and GROW, Maastricht University, Maastricht, The Netherlands
Pascal Borry
6 Centre for Biomedical Ethics and Law, Department of Public Health and Primary Care, Leuven Institute for Genomics and Society, KU Leuven, Kapucijnenvoer 35 Box 7001, 3000 Leuven, Belgium
Martina C. Cornel
Gene editing, which allows for specific location(s) in the genome to be targeted and altered by deleting, adding or substituting nucleotides, is currently the subject of important academic and policy discussions. With the advent of efficient tools, such as CRISPR-Cas9, the plausibility of using gene editing safely in humans for either somatic or germ line gene editing is being considered seriously. Beyond safety issues, somatic gene editing in humans does raise ethical, legal and social issues (ELSI), however, it is suggested to be less challenging to existing ethical and legal frameworks; indeed somatic gene editing is already applied in (pre-) clinical trials. In contrast, the notion of altering the germ line or embryo such that alterations could be heritable in humans raises a large number of ELSI; it is currently debated whether it should even be allowed in the context of basic research. Even greater ELSI debates address the potential use of germ line or embryo gene editing for clinical purposes, which, at the moment is not being conducted and is prohibited in several jurisdictions. In the context of these ongoing debates surrounding gene editing, we present herein guidance to further discussion and investigation by highlighting three crucial areas that merit the most attention, time and resources at this stage in the responsible development and use of gene editing technologies: (1) conducting careful scientific research and disseminating results to build a solid evidence base; (2) conducting ethical, legal and social issues research; and (3) conducting meaningful stakeholder engagement, education and dialogue.
Introduction
Gene editing, which allows for specific location(s) in the genome to be targeted and changed by deleting, adding or substituting nucleotides, is currently the subject of much academic, industry and policy discussions. While not new per se, gene editing has become a particularly salient topic primarily due to a relatively novel tool called CRISPR-Cas9. This specific tool distinguishes itself from its counterparts, (e.g., zinc-finger nucleases and TAL effector nucleases (TALENs)) due to a mixture of increased efficiency (number of sites altered), specificity (at the exact location targeted), ease of use and accessibility for researchers (e.g., commercially available kits), as well as a relatively affordable price [ 1 ]. These attributes make CRISPR-Cas9 an extremely useful and powerful tool that can (and has) been used in research in order to alter the genes in cells from a large range of different organisms, including plants, non-human animals and microorganisms, as well as in human cells [ 2 ]. Ultimately, CRISPR-Cas9 is becoming increasingly available to a larger number of scientists, who have used it, or intend to use it for a myriad of reasons in many different research domains. When such powerful and potentially disruptive technologies or tools (begin to) show a tendency to become widely used, it is common for debate and discussion to erupt. Germane to this debate is the fact that with the advent of CRISPR-Cas9 and other similar tools (e.g., CRISPR Cpf1), the possibility of using the technique of gene editing in a potentially safe and effective manner in humans—whether for somatic or germ line/heritable 1 gene editing—has become feasible in the near to medium future.
With some clinical trials underway, somatic genetic editing for therapeutic purposes is certainly much closer to being offered in the clinic. For example, several clinical trials on HIV are ongoing [ 3 , 4 ]; in 2015 an infant with leukaemia was treated with modified immunes cells (using TALENs) from a healthy donor [ 5 ]. Moreover, in the autumn of 2016, a Chinese group became 'the first to inject a person with cells that contain genes edited using the CRISPR-Cas9 technique' within the context of a clinical trial for aggressive lung cancer [ 6 ]. With such tools, gene editing is being touted as a feasible approach to treat or even cure certain single-gene diseases such as beta-thalassaemia and sickle-cell disease through somatic gene editing [ 3 ].
Beyond somatic cell gene editing, there is also discussion that through the manipulation of germ line cells or embryos, gene editing could be used to trans-generationally 'correct' or avoid single-gene disorders entirely. Notably, (ethical) concerns about heritable gene editing in humans were heightened when in April 2015, a group at Sun Yat-sen University in Guangzhou, China, led by Dr. Junjiu Huang reported they had successfully used gene editing in human embryos [ 7 ]. They used CRISPR-Cas9 to modify the beta-globin gene in non-viable (triplonuclear) spare embryos from in vitro fertility treatments. The authors concluded that while the experiments were successful overall, it is difficult to predict all the intended and unintended outcomes of gene editing in embryos (e.g., mosaicism, off-target events) and that 'clinical applications of the CRISPR-Cas9 system may be premature at this stage' [ 7 ]. Partly in anticipation/response to these experiments and to the increasing use of CRISPR-Cas9 in many different areas, a number of articles were published [ 2 , 8 – 14 ] and meetings were organized [ 9 , 10 , 15 – 17 ] in order to further discuss the scientific, ethical, legal, policy and social issues of gene editing, particularly regarding heritable human gene editing and the responsible way forward.
Internationally, some first position papers on human gene editing were published in 2015 and 2016. Interestingly, these different recommendations and statements do not entirely concur with one another. The United Nations Educational, Scientific and Cultural Organisation (UNESCO) called for a temporary ban on any use of germ line gene editing [ 18 ]. The Society for Developmental Biology 'supports a voluntary moratorium by members of the scientific community on all manipulation of pre- implantation human embryos by genome editing ' [ 19 ]. The Washington Summit (2015) organizers (National Academy of Sciences, the U.S. National Academy of Medicine, the Chinese Academy of Sciences and the U.K.’s Royal Society) recommended against any use of it in the clinic at present [ 17 ] and specified that with increasing scientific knowledge and advances, this stance 'should be revisited on regular basis' [ 17 ]. Indeed, this was done, to some extent, in a follow-up report by the US National Academy of Sciences and National Academy of Medicine, in which the tone of the recommendations appear much more open towards allowing germ line modifications in the clinic [ 20 , 21 ]. Meanwhile, the 'Hinxton group' also stated that gene editing 'is not sufficiently developed to consider human genome editing for clinical reproductive purposes at this time' [ 22 ] and they proposed a set of general recommendations to move the science of gene editing ahead in an established and accepted regulatory framework. Despite these differences, at least two arguments are consistent throughout these guidance documents: (1) the recognition of the need for further research regarding the risks and benefits; and (2) the recognition of the need for on-going discussion and/or education involving a wide range of stakeholders (including lay publics) regarding the potential clinical use and ethical and societal issues and impacts of heritable gene editing. It should be noted, however, that in the 2017 National Academies of Science, and of Medicine Report, the role of public engagement (PE) and dialogue was presented within the context of having to discuss the use of gene editing for enhancement vs. therapy (rather than somatic vs. heritable gene editing, which was the case in the 2015 summit report) [ 20 , 21 ].
Although many stakeholders, including scientists, clinicians and patients are enthusiastic about the present and potential future applications of these more efficient tools in both the research and clinical contexts, there are also important concerns about moving forward with gene editing technologies for clinical use in humans, and to some extent, for use in the laboratory as well. As we have learned from other ethically sensitive areas in the field of genetics and genomics, such as newborn screening, reproductive genetics or return of results, normative positions held by different stakeholders may be dissimilar and even completely incompatible. This might be influenced by various factors, such as commercial pressure, a technological imperative, ideological or political views, or personal values. Furthermore, it is clear that associated values often differ between different stakeholder groups, different cultures and countries (e.g., where some may be more/less liberal), making widespread or global agreement on such criteria very difficult, if not impossible to reach [ 23 , 24 ].
From this perspective, it was important to study the opportunities and challenges created by the use of gene editing (with CRISPR-Cas9 and other similar tools) within the Public and Professional Policy Committee (PPPC) 2 of the European Society of Human Genetics (ESHG; https://www.eshg.org/pppc.0.html ). Our committee advances that ESHG members and related stakeholders should be aware of, and if possible, take part in the current debates surrounding gene editing. Although not all genetics researchers will necessarily use gene editing in their research, and while gene editing as a potential treatment strategy, may appear, initially, somewhat separate from the diagnostics-focused present day Genetics Clinic, we believe that these stakeholders have an important role to play in the discussions around the development of these tools. For one, their expertise in the science of genetics and in dealing with patients with genetic diseases makes them a rare set of stakeholders who are particularly well placed to not only understand the molecular aspects and critically assess the scientific discourse, but also understand current clinic/hospital/health system resources, as well as human/patient needs. Furthermore, in more practical terms, one could consider that clinical genetics laboratories could be involved in the genome sequencing needed to verify for off-target events in somatic gene editing; and that clinical geneticists and/or genetic counsellors could be involved in some way in the offer of such treatment, especially in any counselling related to the genetic condition for which treatment is sought.
The PPPC is an interdisciplinary group of clinicians and researchers with backgrounds in different fields of expertise including Genetics, Health Law, Bioethics, Philosophy, Sociology, Health Policy, Psychology, as well as Health Economics. As a first step, a sub-committee was assigned the task to specifically study the subject of gene editing (including attending international meetings on the subject) and report back to the remaining members. Subsequently, all PPPC members contributed to a collective discussion during the January 2016 PPPC meeting in Zaandam, The Netherlands (15–16 January 2016). At this meeting, a decision was reached to develop an article outlining the main areas that need to be addressed in order to proceed responsibly with human gene editing, including a review of the critical issues for a multidisciplinary audience and the formulation of crucial questions that require answers as we move forward. A first draft of the article was developed by the sub-committee. This draft was further discussed during the 2016 ESHG annual meeting in Barcelona (21–24 May 2016). A second draft was developed and sent out for comments by all PPPC members and a final draft of the article was concluded based on these comments. Although the work herein acts as guidance for further discussion, reflection and research, the ESHG will be publishing separate recommendations on germ line gene editing (accepted during the 2017 annual meeting in Copenhagen, Denmark).
In the context of the ongoing discussion and debate surrounding gene editing, we present herein three crucial areas that merit the most attention at this stage in the responsible development and use of these gene editing technologies, particularly for uses that directly or indirectly affect humans:
- Conducting careful scientific research to build an evidence base.
- Conducting ethical, legal and social issues (ELSI) research.
- Conducting meaningful stakeholder engagement, education, and dialogue (SEED).
Although the main focus of this discussion article is on the use of gene editing in humans (or in human cells) in research and in the clinic for both somatic and heritable gene editing, we also briefly mention the use of gene editing in non-humans as this will also affect humans indirectly.
Conduct ongoing responsible scientific research to build a solid evidence base
The benefits, as well as risks and negative impacts encountered when conducting gene editing in any research context should be adequately monitored and information about these should be made readily available. Particular attention should be paid to the dissemination of the information by reporting and/or publishing both the 'successful' and 'unsuccessful' experiments including the benefits and risks involved in experiments using gene editing in both human and non-human cells and organisms (Table 1 ).
Example of questions that should be addressed regarding building a scientific evidence base for gene editing
An evidence base regarding actual (and potential) health risks and benefits relevant to the use of gene editing in the human context still needs to be built. Therefore, a discussion needs to be held regarding what type of monitoring, reporting and potential proactive search for any physically based risks and benefits should be conducted by researchers using gene editing. Hereby, various questions emerge: are the current expectations and practices of sharing the results of academic and commercial research adequate for the current and future field of gene editing? Should there be a specific system established for the (systematic) monitoring of some types of basic and (pre-) clinical research? If so, which stakeholders/agencies should or could be responsible for this? How could or should an informative long-term medical surveillance of human patients be organized? Following treatment, would patients be obliged to commit to lifelong follow-up? And, if relevant, how could long-term consequences be monitored for future generations? For example, if heritable gene editing was allowed, from logistical and ELSI perspectives, there would be many challenges in attempting to ensure that the initial patients (in whom gene editing was conducted), as well as their offspring would report for some form of follow-up medical check-ups to assess the full impact of gene editing on future generations while still respecting these individuals’ autonomy.
Although the availability of results and potential monitoring are especially important in a biomedical context for all experiments and assays conducted in human cells, and especially in any ex vivo or in vivo trials with humans, relevant and useful information (to the human context and/or affecting humans) can also be gleaned from the results of experiments with non-human animals and even plants. Furthermore, as clearly explained by Caplan et al. [ 2 ], gene editing in insects, plants and non-human animals are currently taking place and may have very concrete and important impacts on human health long before any gene editing experiments are used in any regular way in the health-care setting. As such, while keeping a focus on human use, there should also be monitoring of the results in non-human and non-model organism experiments and potential applications [ 2 ]. Effects might include change of the ecosystem, of microbial environment, (including the microbiome, of parasites and zoonosis, which can involve new combinations with some disappearing, and/or new unexpected ones appearing), change to vegetation, which has a reflection on our vegetal food and on animals’ food and natural niche [ 25 ]. All this will have an impact on the environment, and consequently on organisms (including humans) who are exposed to this altered environment, hence the monitoring of risks and benefits is very important. Especially with gene editing of organisms for human consumption (in essence, genetically modified organisms), it will be important to note that the absence of obvious harms does not mean that there are no harms. Proper studies must be conducted and information regarding these should be made readily available.
Ongoing reflection, research and dialogue on the ELSI of gene editing as it pertains to humans
Research on the ELSI and impacts of human gene editing should be conducted in tandem with the basic scientific research, as well as with any implementations of gene editing in the clinic. Appropriate resources and priority should be granted to support and promote ELSI research; it should be performed unabated, in a meaningful way and by individuals from a diverse range of disciplines (Table 2 ).
Example of questions needed to be addressed for the ethical, legal, and social issues research (ELSI) of gene editing
Ongoing research, reflection and dialogue should address all ELSI 3 salient to gene editing. With respect to gene editing in humans, both somatic and germ line/heritable embryonic gene editing contexts should be addressed. As stated above, we should also study the ELSI of gene editing in non-human and non experimental/model organisms, including issues surrounding the potential (legal and logistical related to implementation) confusions surrounding the use of the terms genetically modified organisms vs. the term gene-edited organisms.
Somatic gene editing
Although somatic gene editing is not free from ethical, legal and social implications—it is, in many respects, similar to more traditional 'gene therapy' approaches in humans—it has been suggested that in many cases, the use of somatic gene editing does not challenge existing ethical, legal and social frameworks as much as heritable gene editing. However, as with any new experimental therapeutic, the unknowns still outweigh what is known and issues of risk assessment and safety, risk/benefit calculation, patient monitoring (potentially for long periods), reimbursement, equity in access to new therapies and the potential for the unjustified draining of resources from more pressing (albeit less novel) therapies, particular protection for vulnerable populations (e.g., fetuses, children (lacking competencies)), and informed consent remain important to study further [ 26 ].
Furthermore, as with any new (disruptive) technology or application, there often remains a gap to be filled between the setting of abstract principles or guidelines and how to apply these in practice. Indeed, important questions and uncertainties surrounding somatic gene editing both in research and in the clinic remain, including, but not limited to: do the established (national and international) legal and regulatory frameworks (e.g., Regulation (EC) no. 1394/2007 on advanced therapy medicinal products) need further shaping/revisions to appropriately address somatic gene editing (including not just issues with the products per se but also for issues related to potential health tourism)? And if so, how would this best be accomplished? Do present clinical trial principles and protocols suffice? How exactly will trials in somatic gene editing be conducted and evaluated? Do we need particular protection or status for patients in such trials? What procedures will be instilled for patients receiving such treatments (e.g., consent, genetic counselling, follow-up monitoring)? Furthermore, to what extent will commercial companies be able to, or be allowed to offer, potentially upon consumer request, treatments based on techniques where so much uncertainty regarding harms remains? Importantly, which health-care professionals will be involved in the provision of somatic gene therapy and the care of patients who undergo such treatments? Who will decide on roles and responsibilities in this novel context? And, based on what criteria will the eligible diseases/populations to be treated be chosen? Indeed, these questions can also all be applied to the context of heritable gene editing, which is discussed below.
Germ line/heritable gene editing
With respect to germ line or heritable gene editing in humans, the ELSI are more challenging than for somatic gene editing, yet they are not all new per se either. Some of these previously discussed concerns include, but are not limited to: issues addressing sanctity of human life, and respect for human dignity, the moral status of the human embryo, individual autonomy, respect and protection for vulnerable persons, respect for cultural and biological diversity and pluralism, disability rights, protection of future generations, equitable access to new technologies and health care, the potential reduction of human genetic variation, stakeholder roles and responsibilities in decision making, as well as how to conduct 'globally responsible' science [ 16 , 2 , 11 , 18 ]. Discussions and debates over some of these topics have been held numerous times in the last three decades, especially within the context of in vitro fertilization, transgenic animals, cloning, pre-implantation genetic diagnosis (PGD), research with stem cells and induced pluripotent stem cells, as well as related to the large scope of discussion around 'enhancement' [ 13 ]. Although it is important to identify and reflect on more general ELSI linked with heritable gene editing and these different contexts, it is also vital to reflect on the ELSI that may be (more) specific to this novel approach. For example, would the fact that for the first time a human (scientist or clinician) would be directly editing the nuclear DNA of another human in a heritable way cause some form of segregation of types of humans? Creators and the created? [ 27 ] Clearly, we need time for additional reflection and discussion on such topics. Distinguishing the ELSI between different yet related contexts will allow for a deeper understanding of the issues and the rationale behind their (un)acceptability by different stakeholders.
A major contextual difference in the current discussions regarding germ line/heritable gene editing is that we have never been so close to having the technology to perform it in humans in a potentially safe and effective manner. Hence, as we move closer to this technical possibility and as we work out the scientific issues of efficiency and safety, the discussions orient themselves increasingly towards the ELSI regarding whether or not we want to even use heritable gene editing in a laboratory or clinical setting, and if so, how we want it to be used, by whom and based on which criteria? This includes, but is not limited to the following questions: should gene editing of human germ line cells, gametes and embryos be allowed in basic research—for the further understanding of human biology (e.g., human development) and without the intention of being used for creating modified human life? Some jurisdictions, such as the UK, have already answered this question, and are allowing this technique in the research setting in human cells in vitro (they will not be placed in a human body, the research will only involve studying the human embryos outside of the body) whereby researchers need to apply for permission to conduct such research. Some believe that allowing this will inevitably lead to the technology being used in the clinic (the so-called 'slippery slope' argument). This, then, brings us to the question at the centre of the debate: should gene editing of germ line cells, gametes or embryos or any other cell that results in a heritable alteration be allowed in humans in a clinical setting? Germane to this issue is another vital question: what, if any, principles or reasoning would justify the use of hereditary gene editing in humans in a clinical context given the current ban on such techniques in many jurisdictions? The new EU clinical trial Regulation (536/2014 Art 90 al.2.) does not allow germ line modification in humans. Should there be leeway for reconsidering this ban in the future in view of the possible benefits of therapeutic germ line gene editing? Should we first understand the risks and benefits of somatic gene editing before even seriously considering heritable gene editing? If we consider that it could be used in some situations, should we only consider using germ line gene editing in the clinic if there are absolutely no other alternatives? Should already established and potentially safer 4 reproductive alternatives, like PGD, be the approaches of choice before even considering germ line gene editing? If we do entertain its use, what, if any criteria, will be safe enough according to different stakeholders (scientists, ethicists, clinicians, policy makers, patients, general public) for it to be legitimate to consider using gene editing for reproductive use? Who will set this safety threshold and based on what risk/benefit calculations? Furthermore, if ever allowed, should heritable human gene editing be permitted only for specific medical purposes with a particular high chance of developing a disease (e.g., only when parents have a-near-100% risk of having a child affected with a serious disorder), and if so, would it matter if the risk is not 100%, but (much) lower? In addition, how can we, or should we define/demarcate medical reasons from enhancement? And, as was posed above for the use in somatic cells, for what medical conditions will gene editing be considered appropriate for use? What will the criteria be and who will decide?
Taking a step back and looking at the issues from a more general perspective, such ELSI research and reflection will need to address, among others, questions that fall under the following themes:
- the balance of risks and benefits for individual patients and also for the larger community and ecosystem as a whole;
- the ethical, governance and legislative frameworks;
- the motivations and interests 'pushing' gene editing to be used;
- the roles and responsibilities of different stakeholders in ensuring the ethically acceptable use of gene editing, including making sure that every stakeholder voice is heard;
- the commercial presence, influence, and impact on (the use of) gene editing;
- the rationale behind the allocation of resources for health care and research and if and which kind of shift might be expected with the new technologies on the rise.
Additional overarching issues relating to ELSI include the need to take a historical perspective and consider previous attempts to deal with genetic technologies and what or how we can learn from these; the need to consider how group actors could or should accept a shared global responsibility when it comes to the governance of gene editing; the potential eugenic tendencies related to new technologies used to eliminate disease phenotypes; the responsibility of current society for future generations; the way different stakeholders may perceive and desire to eliminate (genetic) risk and/or uncertainty by using new technologies such as gene editing; and the potential role(s) different stakeholders, including 'experts', may inadvertently play in propagating a false sense of control over human health.
Although the human context is where much of the attention currently resides, and is indeed, the focus of this article, as mentioned above, we also stress that many concerns and ELSI also stem from the use of gene editing in non-human organisms (plants, insects and microorganisms), the study of which, could inform the human context. More importantly, given that the use of gene editing in these organisms is currently taking place in laboratories and, if released, some of these gene-edited organisms could have a large impact on the environment and society [ 2 ], the ELSI of gene editing in non-human organisms should also be seriously addressed. In this respect, the current debates over definitions and whether plants and non-human animals in which gene editing is performed are considered (legally) genetically modified organisms (GMOs) are particularly important to consider; indeed, this legal stance may be a misleading way to describe the scientific differences in practice. Moreover, the manipulation of definitions may also be used to circumvent the negative press and opinions surrounding GMOs in Europe. Last, but not least, the use of gene editing for the creation of biologic weapons is a possibility that must be discussed and adequately managed [ 2 ].
In order to ensure that the appropriate ELSI research is conducted to answer these myriad questions, ELSI researchers must ensure adequate understanding of scientific facts and possibilities of gene editing, ensure appropriate use of robust methods [ 29 ] to answer specific ELSI questions, as well as learn from previous research on related themes such as (traditional) gene therapy, reproductive technologies, and GMOs. Furthermore, funding will have to be prioritized for ELSI research. National and European funding agencies should ensure that ELSI funding is given in certain proportion to how much gene editing research is being conducted in the laboratory and (pre) clinical domain. In practice, this will mean ensuring that there are adequate review panels for stand-alone ELSI grants, which do not usually fall within any one traditional academic field (e.g., philosophy, law or social sciences). The requirement of including ELSI work packages within science grants may also be useful if such work packages are conducted by ELSI experts (and this is verified by the funding agencies), that they are given enough budget to conduct research and not only offer services, and that the ELSI work package is not co-opted by the science agenda. Spending money on ELSI research has already allowed for the information to be used in more applied ways. Among others, ELSI research has contributed to helping individual researchers understand what kind of research they are (not) allowed to do in certain countries or regions; helped to design appropriate consent forms for research and clinic; and has helped inform policy decisions.
As ELSI are identified, studied and discussed, it will be of utmost importance to communicate these with as many publics as relevant and possible in a clear and comprehensive way so that the largest number of different stakeholders can understand and engage in a discussion about these issues. With respect to engaging non-academic and non-expert audiences in meaningful dialogue, the challenges are greater. Yet, as this is a vital element of conducting science and preparing clinical applications in a responsible manner and stretches beyond the academic focus of ELSI we propose to distinguish a third domain dedicated to such stakeholder engagement, education and dialogue (SEED) described below.
Stakeholder engagement, education and dialogue (SEED)
To deliver socially responsible research (and health care), an ongoing robust and meaningful multidisciplinary dialogue among a diverse group of stakeholders, including lay publics, should be initiated and maintained to discuss scientific and ethically relevant issues related to gene editing. Publics must not only be asked to engage in the discussion, but they should also be given proper information and education regarding the known facts, as well as the uncertainties regarding the use of gene editing in research and in the clinic. In this way, the two focal areas described above will feed into these SEED goals. Stakeholders should also be given the tools to be able to reflect on the ethically relevant issues in order to help informed decision making. Appropriate resources and prioritization should be granted to support and promote SEED (Table 3 ).
Examples of questions to be answered regarding stakeholder, engagement, education and dialogue (SEED) for gene editing
As mentioned in the introduction, the statements addressing gene editing published by different groups and organizations have highlighted the need for an ongoing discussion about human gene editing among all stakeholders, including experts, and the general public(s) [ 8 , 9 , 17 ], In calling for an 'ongoing international forum to discuss the potential clinical uses of gene editing', the organizing committee of the International Summit on Human Gene Editing stated that
'The forum should be inclusive among nations and engage a wide range of perspectives and expertise – including from biomedical scientists, social scientists, ethicists, health care providers, patients and their families, people with disabilities, policymakers, regulators, research funders, faith leaders, public interest advocates, industry representatives, and members of the general public' [ 17 ].
Hence, this implies that not only should different expertise be represented in this ongoing discussion, but lay publics should also be included. For this to be a meaningful and impactful endeavour, all stakeholders involved should be appropriately informed and educated about the basic science and possibilities of gene editing. Academic/professional silos, differences in language, definitions, approaches and general lack of experience with multi- and inter-disciplinary work are all barriers to involving different expert stakeholders in meaningful exchange and dialogue. Some first constructive steps have included the posting online of meeting and conference presentations on gene editing (e.g., the 3 days of the Washington Summit ( http://www.nationalacademies.org/gene-editing/Gene-Edit-Summit/index.htm .), Eurordis webinars and meetings aimed at informing patients, http://www.eurordis.org/tv ). Beyond this, one important barrier to having a truly meaningful and inclusive multidisciplinary discussion about new technologies is the (potential) lack of knowledge and/or understanding of different publics [ 30 ]. Indeed, it is not reasonable for experts to expect that all concerned stakeholders are properly informed about the science and/or the social and ethical issues, which are important requisites for having meaningful and productive conversations about responsible gene editing. Furthermore, a pitfall we must avoid is using PE with the aim of persuading or gaining acceptance of technologies instead of 'true participation' [ 31 ] and as a means to allow for supporting informed opinions.
Another critical issue is the role and influence of different stakeholders, including the media, in educating and informing the public. What are the roles and responsibilities of different stakeholders in setting up and maintaining responsible engagement and dialogue? What will, and what should be the role of scientists in popular media communications and other SEED activities? Where will the funding for these activities come from? Financial and temporal resources will have to be reserved for such SEED regarding gene editing. Resources will also be needed to conduct further research on the best way to engage different publics and to study whether engagement strategies are successful.
Moreover, before engaging different publics and asking for their feedback, whichever stakeholders take on this task must seriously reflect on the precise reasons for which lay publics are being engaged. What is the goal? And, what method of engagement will best meet these goals? There is also a need for honest evaluation of engagement efforts to report on their impacts and outcomes. Indeed, the purposes of PE in science can vary widely, including, among others, informing, consulting and/or collaborating; [ 32 ] clearly each of these implies different levels of participation by publics, and by extension, different levels of influence on a topic. Importantly, there are a long list of questions that also need to be answered for PE (Table 3 ), including but not limited to how different voices will be weighed and if or how they will be used in any policy or decision making.
The value of PE in the form of public dialogue in a democratic society, (and we would specify its contribution to responsible science) is very well summarized by Mohr and Raman (2012) in a perspective piece on the UK Stem Cell Dialogue: [ 31 ]
'The value of public dialogue in a democratic society is twofold. From a normative perspective, the process of PE is in itself a good thing in that the public should be consulted on decisions in which they have a stake. From a substantive standpoint, PE generates manifold perspectives, visions, and values that are relevant to the science and technologies in question, and could potentially lead to more socially robust outcomes (which may differ from the outcomes envisaged by sponsors or scientists)' [ 31 ].
Particularly for the purposes of gene editing, we consider SEED a way to try to ensure that decisions on a subject that is filled with uncertainties, and could have important implications for society for generations to come, is not left in the hands of a few. We want to underline the need for: lay publics to be informed to support transparency; lay publics to be educated to support autonomy and informed opinion/decision making; different voices and concerns to be heard and considered through ongoing dialogue to help ensure that no one stakeholder group pursue their interests unchecked. Although it is beyond the scope of this article to go into any detail, it is important to take the time to learn from past and ongoing engagement efforts in science in general [ 32 ], as well as in biomedicine, including areas like stem cell research [ 31 ] and genetics [ 30 , 33 ]. For example, we can learn about: how PE can generate value and impact for a society, as well as how to conceive of and evaluate a PE programme [ 32 ]; the nuances around 'representative samples' and if they really are representative [ 31 ]; how letting citizens be the 'architects' rather than just participants of engagement (activities) could help to ward against the generation of 'predetermined outcomes' [ 31 ]; the utility of deliberative PE to 'offer useful information to policy makers [ 30 ]. Given all the different reasons for PE, and given the higher standards expected for PE in recent years [ 34 ] it is to be expected that each PE activity will have to be adjusted for the specific context. There are, also, useful tools for PE from a European funded project called 'PE2020, Public Engagement Innovations for Horizon 2020' [ 35 ], which has as an aim to 'to identify, analyse and refine innovative public engagement (PE) tools and instruments for dynamic governance in the field of Science in Society (SiS)' [ 35 ].
As already mentioned above for ELSI research, funding agencies will have to prioritize resources for these SEED activities, and the strategies we outlined for ELSI, could also apply for SEED.
In the midst of a plethora of debate over gene editing, different stakeholder views, preferences, agendas and messages, it is crucial to focus our limited resources, including human resources, time and finances on the most important areas that will enable and support the responsible use of gene editing. We have identified the following three areas that merit an equitable distribution of attention and resources in the immediate and medium-term future:
- Conducting ELSI research.
Indeed, one way to ensure that each of these three important areas receive adequate financial support to conduct the necessary work would be for international and national funding agencies to announce specific funding calls on gene editing. They could also encourage or require that scientific projects focused on gene editing include ELSI and SEED along with the scientific work packages. Furthermore, understandably, priorities need to be made with respect to resource allocation in the biomedical sciences, especially in such uncertain financial contexts, however, as expressed at the World Science Forum in Budapest in November 2011, we must ward against scarce funding being funnelled to single disciplines since it is common knowledge that much of the most valuable work is now multidisciplinary [ 36 ]. Moreover, at such a time funding entities must not 'expel' the social sciences 'from the temple' but rather, the hard sciences should 'invite them in to help public engagement' [ 36 ].
Acknowledgements
We thank all members of the Public and Professional Policy Committee of the ESHG for their valuable feedback and generosity in discussions. Members of PPPC in 2015–2017 were Caroline Benjamin, Pascal Borry, Angus Clarke, Martina Cornel, Carla van El, Florence Fellmann, Francesca Forzano, Heidi Carmen Howard, Hulya Kayserili, Bela Melegh, Alvaro Mendes, Markus Perola, Dragica Radijkovic, Maria Soller, Emmanuelle Rial-Sebbag and Guido de Wert. We also thank the anonymous reviewers for their constructive comments, which have helped to improve the article. Part of this work has been supported by the Swedish Foundation for Humanities and Social Science under grant M13-0260:1, and the CHIP ME COST Action IS1303.
Compliance with ethical standards
Conflict of interest.
The authors declare that they have no competing interests.
1 In this category, we include the editing of germ line cells, or embryonic cells, or even somatic cells that are edited and promoted to then become germ line cells in such a way that the alterations would be heritable.
2 This group studies salient ethical, legal, social, policy and economic aspects relating to genetics and genomics.
3 Herein, the terms 'ethical', 'legal' and 'social' are used in a broad sense, where, for example, issues such as economic evaluations, public health prioritization and other related areas would also be included. Indeed the first goal of 'SEED' (see below) is also, to some extent, part of ELSI research, however, given the paucity of meaningful PE in the past, combined with strong consensus regarding the current need and importance of such activities, we have chosen to highlight it separately. We also wish to stress the difference between academic ELSI research and the work of ethics review committees. Although both deal with ethical and legal issues, the former has as a main goal to advance research and does not act as a policing body, nor does it have an agenda per se. Furthemore, ELSI research does not only identify issues to be addressed but also works with scientists and policy makers to address the issues responsibly.
4 It is important to note that despite attempts at addressing these issues, even for technologies such as PGD [ 28 ].
Genetic Engineering: A Serious Threat to Human Society
Scientists have been trying to create synthetic life, life created in lab, for many years. The first breakthrough in this process happened about thirty years ago when genetic engineers began to genetically modify organisms (Savulescu). These engineers physically move genes across species in order to improve an organism or to cause an organism to function differently. Even though this process sounds as if it happens only in fantasy games, genetically modified organisms are common. For example, genetically modified crops are used every day in the world’s food supply and genetically modified bacteria have been used in medicine, chemical manufacturing, and bio warfare (Pickrell). Slowly, genetic engineering has become a powerful tool in many different fields. Recently, genetic engineering’s potential power increased when Craig Venter, a famous geneticist and entrepreneurs, recreated a living organism out of synthetic chemicals. His success proved to genetic engineers that functioning genomes can be made purely of synthetic chemicals. This power would allow genetic engineers to build new artificial genomes instead of having to modify naturally existing genomes. Genetic engineers now have the chance to broaden their fields’ applications. However, genetic engineering is unpredictable and dangerous, and broadening the application of genetic engineering only furthers the risks. Genetically engineered organisms pose lethal and economic risks to human society.
The availability of genomic information and genetic engineering technology creates a lethal threat to humanity because terrorists can use both the information and technology to recreate deadly pathogens, such as the poliovirus. The naturally occurring poliovirus killed and paralyzed millions of people for many years. In 1988, a worldwide vaccination campaign against the virus nearly exterminated it from the environment, and this solved the poliovirus epidemic. However, in 2002, well intentioned scientists decided to recreate the poliovirus for research means. Using the genomic sequence of the poliovirus found on a public database and commercially available machines, these scientists synthesized fragments of viral genomes into a functional poliovirus (Avise 7). These scientists proved that deadly pathogens can be recreated from genetic engineering techniques. Also, the information and technology used in genetic engineering is readily available and relativity cheap (Kuzma and Tanji 3). Mixing the power to recreate a deadly pathogen with the public availability of genetic engineering information and technology creates a lethal risk to humanity when terrorist exist in society. Terrorist could use genetic engineering to reinstate the poliovirus into the environment, and the virus would kill and paralyze more people. Luckily, these scientists were filled with good intent; however, there is nothing to prevent terrorists from harming innocent lives. Recreating deadly pathogens makes genetic engineering dangerous enough; however, genetic engineers also have the potential to improve the effectiveness of deadly pathogens, such as Y. pestis.
Genetic engineers can make deadly pathogens, such as Y. pestis, resistant to modern antibiotics, and these pathogens could kill innocent people if used as a weapon. Y. pestis, also known as the black plague, wreaked havoc on humanity during the Middle Ages by killing millions of people. In response to a Y. pestis threat during the 20th century, scientists developed an effective vaccine for the pathogen. However, genetic engineers at Biopreparat, a Russian biological warfare agency, engineered a new Y. pestis strain with genetic resistance to modern antibiotics and natural human immunity (Avise 6). The genetically engineered Y. pestis was more deadly and effective than the natural Y. pestis that killed millions of people during the Middle Ages. Biopreparat’s research proved that deadly pathogens can be genetically engineered into superior forms that are resistant to modern medicine. If this strain of Y. pestis was released, a black plague would devastate current human society. Militaries could use the same genetic engineering techniques that Biopreparat used to create deadly biological weapons. With this ability to make deadly pathogens resistant to modern medicine, genetically engineered organisms become lethal weapons that cannot be stopped. Other than lethal weapons, genetically engineered organisms can produce lethal chemical compounds when they are used as a manufacturing tool in the chemical industry.
Showa Denko’s genetically modified bacteria produced a lethal L-tryptophan amino acid that killed and disabled people who took the company’s food supplements. In 1989, an epidemic of eosinophilia myalgia syndrome, a syndrome that is characterized by a high eosinophil count and severe muscle pain, struck the United States (Genetic Engineering: Too Good to Go Wrong 9). This epidemic killed a hundred people and physically disabled ten thousand patients, some of which were paralyzed. Doctors eventually discovered that L-tryptophan, an amino acid used as a food supplement, was causing the epidemic. In 1990, the Journal of the American Medical Association reported that only people who took the L-tryptophan supplement made by Showa Denko, a Japanese biotech company, came down with EMS. Showa Denko’s genetically engineered organisms produced corrupted forms of L-tryptophan that were dangerous to human health (Smith 4).
Many chemical companies want to use genetically engineered organisms to produce chemicals because it is cheaper than normal manufacturing methods. If chemical companies begin to rely on genetically engineered organisms to produce food and medical chemicals, the public could be at risk for another dangerous outbreak of lethal chemicals. Using genetically engineered organisms to cutting down manufacturing costs seems as if it will help the economy; however, genetically engineered organisms, specifically anti-material organisms, can hurt economies more than help them.
Genetic engineers possess the ability to create anti-material organisms that can degrade infrastructure and man-made materials, and malicious people can use these organisms to tear down society’s infrastructures and economies. In nature, there are many organisms with the ability to degrade infrastructure and man-made materials. These microbes cost governments and industries millions of dollars in biodeterioration and biodegradation damages. For instance, bacteria are the leading cause of road and runway deterioration. In Houston, Texas, microbes have been known to degrade the concrete in the city’s sewage systems, and the city has spent millions of dollars trying to contain the problem. High-tech companies, such as airlines and fuel companies, constantly have their facilities and machinery being degraded away by anti-material organisms. These natural organisms cause enough damage to infrastructure, and fixing the damage is expensive and time consuming (Sunshine Project 2). Similarly to the artificially made poliovirus, genetic engineers have the potential to recreate or improve these naturally occurring anti-material organisms. In theory, malicious people could unleash genetically engineered anti-material organisms on infrastructures worldwide, and this would create an expensive cleanup project for governments and companies. With these expensive damages, genetically engineered organisms can destroy economies. The same economic and environmental dangers of anti-material organisms can also be seen in genetically modified crops.
Genetically modified crops will negatively impact the economy and environment because engineered genetic resistance is ineffective at stopping natural parasites in the long term. Farmers use genetically modified crops because these crops contain a genetic resistance to parasites, such as insect pests and microbes. In evolution, two organisms that are in a parasitic relationship evolve in a balance with each other. When genetically modified plants are placed into a natural environment, parasites will evolve in a direction that allows them to bypass the genetic resistance engineered into the crops. Since the majority of crop parasites go through successive generations at a fast pace, these parasites will quickly evolve into a population that can surpass the genetic resistance. This evolutionary process makes the benefits of genetically modified crops short lived. Farmers, who pay more for genetically modified seed than natural seed, then have to pay for harmful and expensive pesticides to protect their crops. In the end, farmers will lose money due to the increased costs of buying genetically modified crops and dangerous pesticides. Also, dangerous chemicals, such as DDT, will be reintroduced into the environment (Avise 73). The ineffectiveness of genetically modified crops creates an economic and environmental risk to human society in the long run since farmers will be losing more money and introducing dangerous chemicals into the environment.
Genetically engineered organisms pose an enormous risk to human society on a lethal and economic front. Natural lethal pathogens, such as the poliovirus and Y. pestis, can be recreated or improved, and malicious people could use these genetically engineered pathogens to kill millions of people. Chemicals manufactured by genetically modified bacteria have proven to be harmful to human health, which was the case during the EMS epidemic in the United States. On an economic front, genetically engineered organisms increase costs instead of minimizing them, and they harm the environment. Anti-material organisms can be created to deteriorate infrastructures, and this would cost governments and industries millions of dollars in repair costs. Also, genetically modified crops in the long term will cost farmers more money than they save because the advantages of the genetically modified crops will be nullified by evolving parasites. Genetically engineered organisms have a huge potential to harm society. However, researching new methods and applications of genetic engineering will not stop because scientists believe in the vast opportunities of the field. In order to keep human society safe, scientists must exhaust all options before turning to the power of genetic engineering. It is an unwise idea to rely on genetic engineering since it is unpredictable and imprecise form of engineering.
Bibliography
Avise, John C. The Hope, Hype, & Reality of Genetic Engineering . Oxford: Oxford UP, 2004. Web. 14 Nov. 2010.
Benner, Steven. "Q&A: Life, Synthetic Biology and Risk." BMC Biology 8 (2010): 77. Biomed Central . 2010. Web. 13 Oct. 2010. <http://www.biomedcentral.com/1741-7007/8/77>.
“Debate: Artificial life.” http://debatepedia.idebate.org/en/index.php/Debate:_Artificial_life. N.p., 2010. Web. 20 Sept. 2010. <http://debatepedia.idebate.org/en/index.php/Debate:_Artificial_life>.
"Genetic Engineering: Too Good to Go Wrong?" Green Peace . 2000. Web. 14 Nov. 2010. <http://archive.greenpeace.org/comms/97/geneng/getoogoo.html#3gen>.
Hurlbert, R. E. "Biological Weapons; Malignant Biology." Washington State University. 2000. Web. 13 Oct. 2010.
Institute for Responsible Technology. "State of the Science on the Health Risks of GM Foods." Responsible Technology . 2007. Web. 13 Oct. 2010. <http://www.saynotogmos.org/paper.pdf>.
Kuzma, Jennifer, and Todd Tanji. "Unpackaging Synthetic Biology." Regulation & Governance 4.1 (2010): 92-112. Wiley Online Library. 2010. Web. 13 Oct. 2010. <http://onlinelibrary.wiley.com/doi/10.1111/j.1748-5991.2010.01071.x/full>.
Pickrell, John. “Introduction: GM Organisms.” New Scientist . N.p., 2010. Web. 20 Sept. 2010. <http://www.newscientist.com/article/dn9921-instant-expert-gm-organisms.html>.
Savulescu, Julian. “A matter of synthetic life and death: Venter’s artificial organism invention is fraught with peril.” New York Daily News . N.p., 2010. Web. 20 Sept. 2010. <http://www.nydailynews.com/>.
Smith, Jeffrey M. "Scrambling and Gambling with the Genome." Say No To GMOs! Aug. 2005. Web. 13 Oct. 2010. <http://www.saynotogmos.org>.
Sunshine Project. "Non-Lethal Weapons Research in the US." Sunshine Project . Mar. 2002. Web. 13 Oct. 2010. <http://www.sunshine-project.org/publications/bk/bk9en.html>.
Union of Concerned Scientists. "Risks of Genetic Engineering." Union of Concerned Scientists . 2010. Web. 14 Nov. 2010. <http://www.ucsusa.org/food_and_agriculture/science_and_impacts/impacts_genetic_engineering/risks_of_genetic_engineeringeering.html#New_Allergens_in_the_Food_Supply>.
Articles copyright © 2024 the original authors. No part of the contents of this Web journal may be reproduced or transmitted in any form without permission from the author or the Academic Writing Program of the University of Maryland. The views expressed in these essays do not represent the views of the Academic Writing Program or the University of Maryland.
- Study Guides
- Homework Questions
The Ethics of Genetic Engineering

IMAGES
VIDEO
COMMENTS
Genetic engineering is a recent breakthrough in humanity in the field of medicine, formulating one of the most complex processes. Genetic engineering technologies include the construction of functionally active genetic structures, their introduction into the human body, and integration into the genome (Wheale & Schomber, 2019).
Introduction. Gene therapy is a therapeutic strategy using genetic engineering techniques to treat various diseases. 1, 2) In the early 1960s, gene therapy first progressed with the development of recombinant DNA (rDNA) technology, 1) and was further developed using various genetic engineering tools, such as viral vectors. 3 - 5) More than ...
Human Genetic Engineering (HGE) - gene editing applied to human cells. CRISPR/Cas9 - gene editing technology derived from bacterial immune system. Germline gene editing - a process which alters the genome of an embryo, so that the entire organism has altered genes and can pass those genes down to offspring.
Genetic engineering opens new possibilities for biomedical enhancement requiring ethical, societal and practical considerations to evaluate its implications for human biology, human evolution and our natural environment. In this Commentary, we consider human enhancement, and in particular, we explore genetic enhancement in an evolutionary context.
The logic behind this argument is that human genetic enhancement perpetuates discrimination against the disabled and the "genetically unfit," and that this sort of discrimination is similar to the sort that inspired the eugenics of the Third Reich. A third argument is that genetic engineering will lead to vast social inequalities.
Conclusion. Genetic engineering is a powerful tool with diverse applications and immense potential to improve human health, agriculture, industry, and the environment. It has revolutionized medicine by enabling the development of therapies for genetic disorders and the production of pharmaceuticals.
Genes influence health and disease, as well as human traits and behavior. Researchers are just beginning to use genetic technology to unravel the genomic contributions to these different ...
3 A gene drive is a genetic engineering technology—adding, deleting, disrupting, or modifying genes—to rapidly spread a particular genetic trait to an entire offspring population.
genetic engineering, the artificial manipulation, modification, and recombination of DNA or other nucleic acid molecules in order to modify an organism or population of organisms. The term genetic engineering is generally used to refer to methods of recombinant DNA technology, which emerged from basic research in microbial genetics.
Genetic engineering is the use of molecular biology technology to modify DNA sequence(s) in genomes, using a variety of approaches. For example, homologous recombination can be used to target specific sequences in mouse embryonic stem (ES) cell genomes or other cultured cells, but it is cumbersome, poorly efficient, and relies on drug positive/negative selection in cell culture for success.
Genetic engineering is the act of modifying the genetic makeup of an organism. ... CRISPR-Cas12a was used to directly replace mouse antibody variable chain genes with human versions in primary B ...
Gene editing places great power over altering the fundamental principles of biology, and our whole society needs to part of the discussion on what is okay to do and what is not. And we need to ...
Genetic Engineering. 2 pages / 835 words. Genetic engineering, also known as genetic modification, is the direct manipulation of DNA to alter an organism's characteristics (phenotype) in a particular way. It is a set of technologies used to change the genetic makeup of cells to produce improved or novel organisms.
Interesting Genetic Engineering Persuasive Essay Topics. How human curiosity has led to new advancements and technologies in genetics. History of genetically modified food. Discuss the process of genetic engineering in crops. Evaluate the acceptance of genetically modified crops worldwide. Analyze the leading countries implementing genetic ...
Artificial manipulation of genes is a relatively new science, and a number of watershed moments have provided the foundation for the current state of genetic engineering. Researchers first discovered that nonspecific alterations to Drosophila DNA could be introduced using radiation 1 and chemicals 2 in 1927 and 1947, respectively.
Human genetic engineering simply eliminates the "undesirable" traits and encourages specific "desirable" traits. With the endless possibilities of choosing what to eliminate, inevitably the "desirable" traits are picked and chosen on whim decisions such as blonde hair, blue eyes, a slender figure, and tall height (Act For Libraries).
Essay Writing Service. The opponents of human genetic engineering often refer to a number of dangers involved in artificially manipulating genetic codes which result in such medical issues as Down's syndrome, Hemophilia, and even anxiety and stress.
Already in 1982, the Parliamentary Assembly of the Council of Europe considered in its 'Recommendation on genetic engineering' that 'the rights to life and to human dignity protected by Articles 2 and 3 of the European Convention on Human Rights imply the right to inherit a genetic pattern which has not been artificially changed'. 53 ...
Human Genetic Engineering Essay. Genetic engineering is a practice commonly used in food to produce yields of superior size or quality. Recently this technology has been tested on humans. The human race will now be able to improve upon itself and their offspring. With this technology disease can be disposed of and normal people can become ...
Internationally, some first position papers on human gene editing were published in 2015 and 2016. Interestingly, these different recommendations and statements do not entirely concur with one another. The United Nations Educational, Scientific and Cultural Organisation (UNESCO) called for a temporary ban on any use of germ line gene editing .
Genetically engineered organisms pose lethal and economic risks to human society. The availability of genomic information and genetic engineering technology creates a lethal threat to humanity because terrorists can use both the information and technology to recreate deadly pathogens, such as the poliovirus. The naturally occurring poliovirus ...
Really the most useful application of human genetic engineering is preventing hereditary diseases, disabilities and defects/doodlers. Examples include: Down syndrome, Diabetes, color blindness and even allergies. Stopping these diseases/doodlers before the baby Is even born can help prevent a lot of Issues from happening In the child's future ...
The Ethics of Genetic Engineering Introduction: Advances in genetic engineering technologies, such as CRISPR-Cas9, have opened new possibilities for manipulating the genetic makeup of living organisms, including humans. While genetic engineering holds promise for addressing medical conditions, enhancing agricultural productivity, and promoting environmental sustainability, it also raises ...