Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 13 June 2017

Sol-gel Autocombustion Synthesis of Nanocrystalline High-entropy Alloys
- Fan Zhang 1 ,
- Hang Ping 1 ,
- Jieyang Zhou 1 ,
- Liwen Lei 1 ,
- Jingjing Xie 1 ,
- Jinyong Zhang 1 ,
- Weimin Wang 1 &
- Zhengyi Fu 1
Scientific Reports volume 7 , Article number: 3421 ( 2017 ) Cite this article
12k Accesses
92 Citations
Metrics details
- Nanoparticle synthesis
- Synthesis and processing
A reduction in the particle size is expected to improve the properties and increase the application potential of high-entropy alloys. Therefore, in this study, a novel sol–gel autocombustion technique was first used to synthesize high-entropy alloys. The average grain size of the prepared nanocrystalline CoCrCuNiAl high-entropy alloys showed was 14 nm with an excellent and uniform dispersion, exhibiting a distinct magnetic behavior similar to the superparamagnetic behavior. We show that the metal nitrates first form (Co,Cu,Mg,Ni,Zn)O high-entropy oxides, and then in situ reduce to CoCrCuNiAl high-entropy alloys by the reducing gases, and the chelation between citric acid and the metal ions and the in situ chemical reactions are the dominant reaction mechanisms. We demonstrate that the sol–gel autocombustion process is an efficient way to synthesize solid solution alloys eluding the restriction of a high mixing entropy.
Similar content being viewed by others
Partial liquid metal dealloying to synthesize nickel-containing porous and composite ferrous and high-entropy alloys
Retrosynthetic design of core–shell nanoparticles for thermal conversion to monodisperse high-entropy alloy nanoparticles
Dual heterogeneous structures lead to ultrahigh strength and uniform ductility in a Co-Cr-Ni medium-entropy alloy
Introduction.
Over the past years, high-entropy alloys (HEAs) have received more and more attention owing to their unique compositions, microstructures and adjustable properties 1 . In contrast to traditional alloys based on only one or two principal elements, HEAs were originally defined as multicomponent alloys composed of five or more principal elements in an equal or near-equal ratio 2 , 3 . The resulting high mixing entropy was demonstrated to facilitate the formation of simple solid solutions rather than complex phases 4 . HEAs typically exhibit excellent physicochemical properties such as special electrical and magnetic properties 5 , 6 , a high strength as well as promising resistances to wear, oxidation and corrosion 7 , 8 , 9 , making them promising candidates for future engineering applications.
A variety of synthesis techniques have aided the development of HEAs such as arc melting 10 , laser cladding 11 and thin-film sputtering 12 . However, these fabrication routes are sometimes unsuitable for industrial manufacturing, because they might prove uneconomic or impose restrictions on the shape (bulk or film) or the size of the final product. Metallic nanoparticles have attracted significant attention from both the academia and the industry because of their unique physicochemical properties (e.g., their magnetic, catalytic and biomedical properties) arising from their much smaller size compared to their bulk counterparts 13 , 14 . To date, HEA nanoparticles are generally synthesized via mechanical alloying (MA). However, when using this method, the grains are usually larger than 30 nm and the nanograins tend to agglomerate and form particles larger than 3 μm 15 , 16 . A size reduction of the particles is expected to improve the properties and increase the application potential of HEAs. This, however, requires the development of a new synthesis method to prepare nanocrystalline HEAs.
Sol–gel autocombustion is a rapid and economical synthesis technique for the fabrication of particulate products and has been widely used for the synthesis of a variety of metal and alloy nanoparticles, forming nano-sized, homogeneous, and highly reactive powders through mixing different elements at the atomic level 17 . For instance, Yang et al . successfully synthesized pure Co, Ni, Cu, Ag, and Bi metals with a grain size of several nanometers via sol–gel autocombustion 18 . Pure metal atoms can be used as building blocks in the construction of different types of alloys. In previous studies, CoNi, NiFe, and even immiscible NiAg nanoalloys have been successfully prepared by the sol–gel autocombustion method 19 , 20 , 21 , indicating a great potential for the synthesis of nanocrystalline HEAs.
In this study, the feasibility of this approach was demonstrated using the CoCrCuNiAl HEA as an example. As one of the first identified HEAs 22 , CoCrCuNiAl contains five metals with different crystal structures (Cu, Ni and Al crystallize in the face-centered cubic (FCC) structure, Cr in the body-centered cubic (BCC), and Co in the hexagonal close-packed (HCP) structure) and consists of simple solid solutions of the FCC and BCC structure. In this study, CoCrCuNiAl HEA was synthesized via sol-gel autocombustion, and the effect of the fuel-oxidant ratio on the combustion process and the final products was investigated. In addition, the microstructure and the magnetic properties of the prepared CoCrCuNiAl HEA were studied.
Results and Discussion
The sol–gel autocombustion technique is primarily used to synthesize nanosized oxides via an exothermic reaction between oxidants (typically metal nitrates) and fuel (such as organic amines, urea and acids) 23 . In this study, a nitrate–citric acid system was used, with the nitrate ions acting as the oxidants and the citric acid as the fuel. To further understand the reaction process, a thermogravimetric–differential scanning calorimetry–mass spectrometry (TG–DSC–MS) analysis was performed to analyze the combustion reaction, and the gas composition of the dried gel with a fuel-oxidant ratio of 1:1. As shown in Fig. 1 , the endothermic peaks appeared at ∼ 70 and 130 °C. They correspond to the evaporation of the free and the bound water in the dried gels, respectively. Another endothermic peak was observed at a temperature of 205 °C and may be ascribed to the decomposition of NH 4 NO 3 formed during the adjustment of the pH value. A strong exothermal peak associated with a sharp decrease in the weight was observed at 231 °C, related to the combustion reaction. No additional peaks but only a small reduction in weight were observed after the combustion process, indicating the formation of a stable phase. As shown in Fig. 1b and c , during the combustion process, gases such as CO 2 , NO 2 , and NO are released, and the metal oxides will be formed simultaneously through the decomposition of the nitrates. Meanwhile, reduction gases such as H 2 , CH 4 , and NH 3 are released as well and play an important role in the reduction of the metal oxides according to a previous study 24 . The combustion reaction can be simply described as follows (where M denotes the metal atom/ions and n is the metal valence):
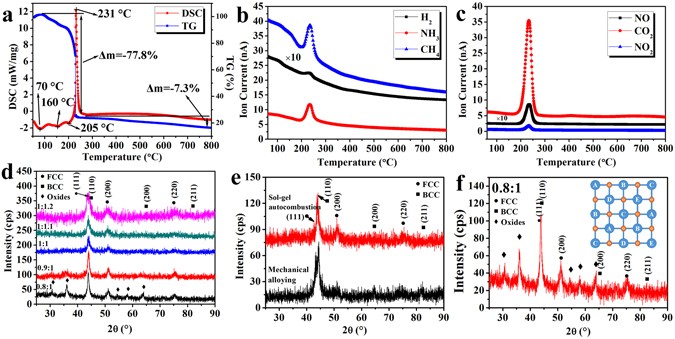
( a – c ) TG–DSC–MA results obtained for the dried gel prepared from the sol with a fuel–oxidant ratio of 1:1; ( d ) XRD patterns revealing the crystal structures of the combustion products of the dried gels for different fuel-oxidant ratios; ( e ) comparison of XRD patterns of CoCrCuNiAl HEAs synthesized by sol–gel autocombustion and MA; ( f ) XRD patterns of the combustion product of the gels with a fuel–oxidant ratio of 0.8:1 and the schematic illustration of HEOs.
The TG–DSC–MS results show that the gas phases play an important role in the mechanism behind the sol–gel autocombustion process. Different fuel-oxidant ratios will promote the formation of different gas components, which will significantly affect the composition and structure of the final product. To optimize the fuel–oxidant ratio, reagents with different fuel-oxidant ratios (0.8:1, 0.9:1, 1:1, 1.1:1, and 1.2:1) were dissolved in deionized water to obtain the sols. The dried gels were then activated by combustion at a temperature of 300 °C based on the TG–DSC–MS results.
The XRD patterns obtained for the synthesized CoCrCuNiAl HEAs powders are shown in Fig. 1d . Oxide phase was detected in the combustion product of the gels with a fuel–oxidant ratio of 0.8:1, so the volume of reducing gases created by the fuel was not enough to completely reduce the metal oxides to pure alloys. When the fuel–oxidant ratio was increased to 0.9:1, only weak oxide peaks were detected, and when the fuel-oxidant ratio was increased to 1:1, only peaks corresponding to an FCC structure ((111), (200), and (220)) and a BCC structure ((110), (200) and (211)) could be identified. Further increasing the fuel–oxidant ratio to 1.1:1 and 1.2:1 did not result in an obvious change in the XRD patterns. Combustion product of the gel with fuel-oxidant ratio of 1:1 was annealed at 1,000 °C in a flowing argon atmosphere with a soaking time of 1 h and cooled naturally to the room temperature. A Comparison of the XRD results before and after annealing is shown in Supplementary Figure S1 . No oxide peak is detected after annealing, indicating no amorphous oxide in the sol–gel combustion product. In consequence, simple solid solutions rather than complex phases were formed, indicating successful synthesis of HEAs via the sol–gel autocombustion method, and the lowest fuel-oxidant ratio to synthesize pure CoCrCuNiAl HEAs was determined to be a 1:1 ratio. For comparison, a CoCrCuNiAl HEA was also prepared by conventional mechanical alloying (MA). More detailed information on the prepared CoCrCuNiAl HEA via MA is provided in Supplementary Figure S2 . As shown in Fig. 1e , both of the XRD patterns of the CoCrCuNiAl HEAs synthesized by MA and sol–gel autocombustion show the same phases, further confirming the successful synthesis of HEAs by the sol–gel autocombustion.
There are two possible routes of the synthesis of CoCrCuNiAl HEAs in the autocombustion process. (i) different metal oxides are reduced to metals by the reducing gases and then form HEAs as the building blocks. (ii) different metal oxides form complex multicomponent oxides, followed by in situ reduction to HEAs by the reducing gases. According to thermodynamics, it is impossible to obtain metallic aluminum and chromium in the autocombustion process because Al 2 O 3 and Cr 2 O 3 cannot be reduced to metals by any type of the gases formed in the combustion. Therefore, the synthesis of CoCrCuNiAl HEAs is through the second route. Recently, (Co,Cu,Mg,Ni,Zn)O high-entropy oxides (HEOs) were synthesized by pyrolyzing nitrates of the individual metals according to the report of Sarkar et al . 25 . Similar with HEAs, HEOs containing five or more metals in equiatomic amounts will be formed because of the high mixing entropy. Hence, the oxide phase in the XRD patterns of the combustion product with fuel–oxidant ratios less than 1:1 is likely to be (Co,Cr,Cu,Ni,Al)O HEO. In the HEOs, the metallic elements are bonded together and ordered in thermodynamics to form the structure of A–B–C–D–E–O (where A, B, C, D, and E denote the metallic elements and O represents oxygen), as shown in Fig. 1f . There is always an intermediate anion separating neighboring cation lattice sites, and no single component metal oxide phases exist in the (Co,Cr,Cu,Ni,Al)O HEOs, so that the reduction of Al 3+ and Cr 3+ can be realized. The dried gels with fuel–oxidant ratios of 0.8:1 and 0.9:1 were also activated through combustion in a tube furnace under a flowing hydrogen atmosphere. The XRD patterns of the combustion products are shown in Supplementary Figure S3 . No oxide phase was detected in the combustion product, so the external H 2 can further reduce the oxide phases, validating the above hypothesis.
Transmission electron microscopy (TEM) was used to study the microstructure of the prepared CoCrCuNiAl HEAs. The TEM micrograph revealing the detailed microstructure of the CoCrCuNiAl HEA prepared from a sol with a fuel–oxidant ratio of 1:1 is shown in Fig. 2a , and the TEM images of the combustion products obtained from sols with fuel–oxidant ratios of 0.8:1, 0.9:1, 1.1:1, and 1.2:1 are shown in Supplementary Figure S4 , indicating unifromly dispersed HEA nanoparticles. The average grain size of the nanoparticles was calculated to be 14 nm based on a statistical analysis of the size of the particles shown in Fig. 2b . It is worth noting that the grain size of the CoCrCuNiAl HEA synthesized in this study via the sol–gel autocombustion is smaller than the sizes of all HEA nanoparticles so far reported in literature. Furthermore, the nanoparticles show an excellent uniform dispersion. The rings in the SAED pattern reveal that the nanocrystalline CoCrCuNiAl HEA powder prepared via the sol–gel autocombustion consists of a BCC phase and a FCC phase and is in agreement with the XRD results.
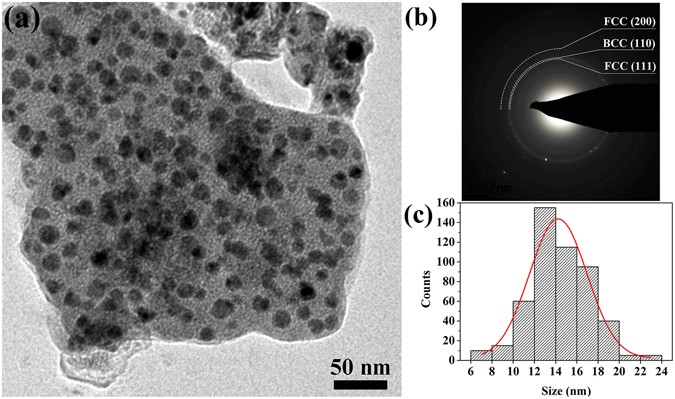
( a ) TEM image of the prepared CoCrCuNiAl HEA; ( b ) corresponding SAED pattern; ( c ) histogram plot of the particle size distribution.
Based on the above results, it can be claimed that nanocrystalline CoCrCuNiAl HEA with an average grain size of 14 nm was synthesized via the sol–gel autocombustion. According to the knowledge about HEAs, the different metals should homogeneously share a common lattice to form simple solid solutions. Herein, we suggest that the formation of nanocrystalline HEAs is attibuted to the chelation between citric acid and metal ions. Citric acid has three carboxyl (-COOH) groups and one hydroxyl group (-OH), providing four lone pair electrons to form a tetradentate ligand. However, one metal ion cannot be chelated with more than two of the carboxyl groups, and another carboxyl group can only chelate with other metal ions 25 . The dried gels with a fuel–oxidant ratio of 1:1 were studied by Fourier Transform infrared spectroscopy (FTIR) in the wavelength range from 440 to 4000 cm −1 , and the resulting spectra are shown in Fig. 3 . The band at 1380 cm −1 is commonly assigned to the NO − ions, whereas the bands at 1400 and 1631 cm −1 are because of the symmetric vibrations, ν s (COO − ), and antisymmetric stretching vibrations, ν as (COO − ), of the citrate molecules, respectively. The band at 3175 cm −1 is linked to the hydroxyl group (-OH), and the broad bands ∼ 3500 cm −1 can be assigned to water molecules. The distance between the antisymmetric and symmetric stretching vibration peaks, Δ( ν as (COO − )- ν s (COO − )), was larger than 200 cm −1 , indicating that some carboxylate groups in the citrate molecules are coordinated to metal ions in a monodentate configuration 26 . Therefore, in the sols, Co 2+ , Cu 2+ , and Ni 2+ will form tetradentate complexes with an α-hydroxyl group, an α-carboxyl group, and a β-carboxyl group of the citric acid, as well as one of the β-carboxyl groups of another citric acid molecule; Cr 3+ and Al 3+ will form hexadentate complexes with an α-hydroxyl group, an α-carboxyl group, and a β-carboxyl group of the citric acid, as well as three β-carboxyl groups of three other citric acid molecules. Because of the chelation of the citric acid and the metal ions, a 3D network will form in the sols, which remains intact during the transformation of the sols into gels, as indicated by the spectra shown in Fig. 3 . As a result, the Co 2+ , Cr 3+ , Cu 2+ , Ni 2+ , and Al 3+ ions will be homogeneously distributed in the dried gels, and no interface will exist between the metal ions. Compared to traditional HEAs synthesis techniques requiring long periods of time for the diffusion of the atoms to form solid solutions, the in situ chemical reactions described in Eq. ( 1 ) groups (4) only require a short-range diffusion of the atoms to form solid solutions.
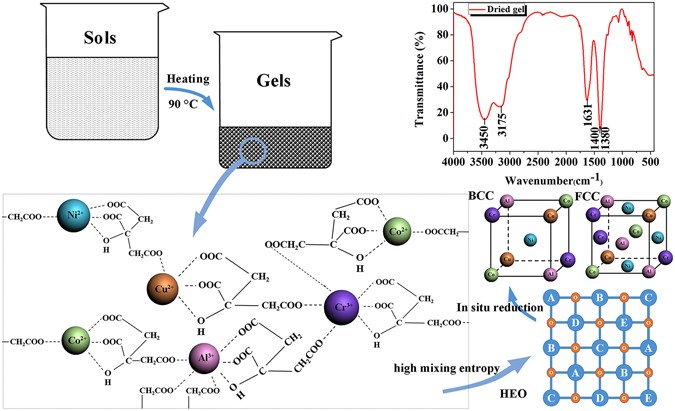
Schematic illustration of the sol–gel combustion process and the FTIR pattern for the dried gel.
To better understand the results, an equiatomic CoCuNi alloy and an equiatomic CoCrCuNi alloy were also synthesized via the sol–gel autocombustion. As shown in Fig. 4 , both of these alloys feature an FCC structure. More information on the CoCuNi and CoCrCuNi alloys can be found in Supplementary Figure S5 . A comparison of the XRD patterns of equiatomic CoCuNi alloy and equiatomic CoCrCuNi alloy synthesized by the sol–gel autocombustion and MA are shown in Fig. 4 . Some additional peaks still can be detected after mechanical alloying for 60 h; however, there are no additional peaks, and the background is low and flat, and peak widths are narrow in 2θ space in the XRD patterns of equiatomic CoCuNi alloy and CoCrCuNi alloy synthesized by the sol–gel autocombustion, indicating the successful synthesis of ternary and quaternary equiatomic solid solution alloys. According to Boltzmann’s hypothesis regarding the relationship between the entropy of a system and the system complexity, for a random solid solution, the configurational entropy of mixing is represented by the following Eq.
where R is the gas constant (8.314 J/K mol) and \({c}_{i}\) is the molar content of the i th component 27 . Thus, for an equiatomic alloy with n components, the configurational entropy of mixing is:
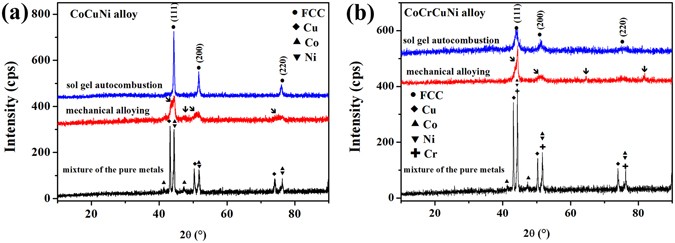
XRD patterns obtained for the equiatomic CoCuNi and CoCrCuNi alloys prepared via sol-gel autocombustion and mechanical alloying, respectively.
For an equiatomic alloy with 3, 4, and 5 principal components, Δ S mix is 1.10 R , 1.39 R , and 1.61 R , respectively. In fact, complex phases or intermetallics easily form in alloy systems with multiple principal components according to traditional metallurgical knowledge 28 , and simple solid solutions will be formed when the alloys contain more than five principal elements because of the high mixing entropy (>1.61 R ) according to the knowledge about HEAs. However, CoCuNi (ternary, \({\rm{\Delta }}{S}_{mix}=1.10R\) ) and CoCrCuNi (quaternary, \({\rm{\Delta }}{S}_{mix}=1.39R\) ) equiatomic solid solution alloys without a high mixing entropy were synthesized in this study. Therefore, according to the above discussion, some equiatomic solid solution alloys can be synthesized eluding the restriction of a high mixing entropy, which further indicates the superiority of the sol–gel autocombustion technique for the preparation of solid solution HEAs.
The magnetic properties of the CoCrCuNiAl HEAs synthesized via the sol–gel autocombustion and mechanical alloying were measured at room temperature. The obtained magnetic hysteresis loops are shown in Fig. 5 . The HEA powder prepared via MA showed a ferromagnetic behavior, and the saturated magnetizations ( M S ), remanence ratio ( M R / M S ) and coercivity force ( H C ) were measured to be 2.521 emu/g, 17.3%, and 238.867 Oe, respectively. However, compared to the magnetic properties of the CoCrCuNiAl HEA synthesized via MA, the corresponding HEA synthesized via the sol–gel autocombustion showed a distinctive soft magnetic behavior and higher saturated magnetizations (13.372 emu/g), a lower remanence ratio (1.93%), and a lower coercivity force (10.528 Oe). Furthermore, the HEA powder synthesized via the sol–gel autocombustion showed a behavior similar to a superparamagnetic behavior because of the low remanence ratio. In general, magnetic nanoparticles with diameters <10 nm exhibited a superparamagnetic behavior 29 . The CoCrCuNiAl HEA synthesized via the sol–gel autocombustion had an average grain size of 14 nm, and can easily lead to the observed magnetic behavior similar to a superparamagnetic behavior. This makes HEAs prepared via the sol–gel autocombustion promising candidates for future applications in some special conditions.
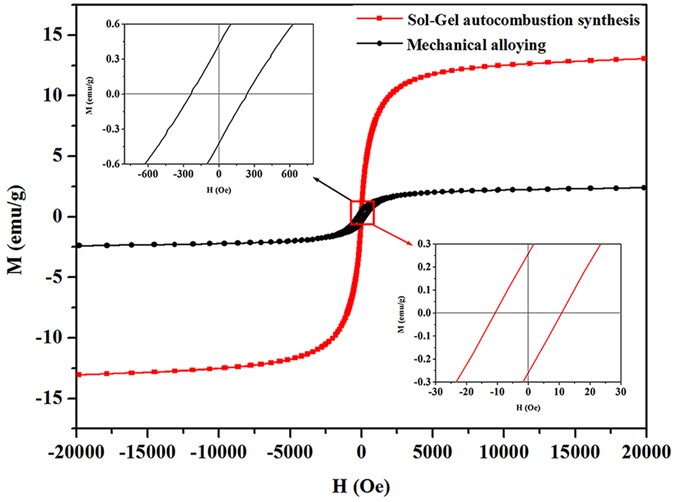
Comparison of the magnetic hysteresis curves obtained for the CoCrCuNiAl HEAs prepared via the sol–gel autocombustion and mechanical alloying, respectively.
Conclusions
In summary, CoCrCuNiAl HEAs were successfully synthesized via the sol–gel autocombustion. The optimal fuel–oxidant ratio for the synthesis of CoCrCuNiAl HEAs using nitrate ions as the oxidants and citric acid as the fuel was determined to be 1:1. Solid solutions with an FCC and BCC structure and an average grain size of 14 nm were obtained after the combustion process, and the HEA nanoparticles showed an excellent uniform dispersion. The synthesis of the nanocrystalline HEAs was associated with the chelation between citric acid and metal ions and the in situ chemical reactions. The nanopowders exhibited a distinctive magnetic behavior similar to a superparamagnetic behavior, demonstrating the great application potential of HEAs synthesized via the sol–gel autocombustion.
Preparation of the HEAs by sol–gel autocombustion
Analytical grade Co(NO 3 ) 2 ·6H 2 O, Cr(NO 3 ) 3 ·9H 2 O, Cu(NO 3 ) 2 ·3H 2 O, Ni(NO 3 ) 2 ·6H 2 O, Al(NO 3 ) 3 ·9H 2 O, citric acid, ethanol, and ammonia (all procured from Sinopharm, China) were used as the starting materials in this study. The metal nitrates were first dissolved in distilled water at an equiatomic ratio and stirred thoroughly at room temperature for 1 h. Then, citric acid was added to the solution as coordinating agent with the molar ratio of citric acid to the total amount of metal ions selected to 0.8:1, 0.9:1, 1:1, 1.1:1, or 1.2:1. After homogenizing the solution through magnetic stirring, the pH value was adjusted to 7 by adding ammonia, and finally the sols were annealed at 90 °C for 48 h to form the dried gels. The dried gels were activated by combustion at 300 °C in a tube furnace under a protective argon atmosphere or under a flowing hydrogen atmosphere.
Preparation of the HEAs by mechanical alloying
For comparison, CoCuNi, CoCrCuNi, and CoCrCuNiAl HEAs were also prepared via mechanical alloying (MA). Co, Cr, Cu, Ni, and Al metal powders were used as the starting materials in this case. The powders were milled in a high-energy planetary ball mill (QM-BP, Nanjing Nan-Da Instrument Plant) at 250 rev/min with a ball-to-powder weight ratio of 15:1 for 60 h, and n-heptane was used as the process-controlling agent.
Characterization of alloys
X-ray diffraction (XRD, Rigaku Ultima III) measurements were performed using Cu Kα radiation in the 2θ range from 10 to 90° at a scanning rate of 4°/min to analyze the crystal structure of the obtained HEAs powders. A thermogravimetric analysis combined with differential scanning calorimetry and dynamic mass spectrometry (TG–DSC–MS, Netzsch STA449F3, Germany) was used to perform an in situ thermogravimetric and gas-phase analysis of the reactive gels under a high-purity argon atmosphere using a heating rate of 10 K/min. Transmission electron microscopy (TEM, Philips M12) was used in conjunction with selected area electron diffraction (SAED) to analyze the microstructure of the powders. The gels were evaluated by Fourier Transform infrared spectroscopy (FTIR, Thermo Scientific Nicolet 6700) from 4000 to 400 cm −1 , at a resolution of 4 cm −1 with 32 scans. The magnetic properties of the HEAs at room temperature were measured using a Physical Property Measurement System (PPMS, Quantum Design PPMS-9T), and the magnetic field was varied from −20000 to 20000 Oe.
Zhang, Y. et al . Microstructures and properties of high-entropy alloys. Prog. Mater Sci. 61 , 1–93 (2014).
Article Google Scholar
Cantor, B., Chang, I. T. H., Knight, P. & Vincent, A. J. B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 375–377 , 213–218 (2004).
Yeh, J. W. et al . Nanostructured high-entropy alloys with multiple principal elements: novel alloy design concepts and outcomes. Adv. Eng. Mater. 6 , 299–303 (2004).
Article CAS Google Scholar
Zhang, Y., Zhou, Y. J., Lin, J. P., Chen, G. L. & Liaw, P. K. Solid-solution phase formation rules for multi-component alloys. Adv. Eng. Mater. 10 , 534–538 (2008).
Koželj, P. et al . Discovery of a Superconducting High-Entropy Alloy. Phys. Rev. Lett. 113 , 107001 (2014).
Article ADS PubMed Google Scholar
Zhang, Y., Zuo, T. T., Cheng, Y. Q. & Liaw, P. K. High-entropy Alloys with High Saturation Magnetization, Electrical Resistivity, and Malleability. Sci. Rep. 3 , 1455 (2013).
Article ADS PubMed PubMed Central Google Scholar
Gludovatz, B. et al . Fracture-resistant high-entropy alloy for cryogenic applications. Science 345 , 1153–1158 (2014).
Article ADS CAS PubMed Google Scholar
Lim, X. Z. Mixed-up metals make for stronger, tougher, stretchier alloys. Nature 533 , 306–307 (2016).
Li, Z. M., Pradeep, K. G., Deng, Y., Raabe, D. & Tasan, C. C. Metastable high-entropy dual-phase alloys overcome the strength-ductility trade-off. Nature 534 , 227–230 (2016).
Santodonato, L. J. et al . Deviation from high-entropy configurations in the atomic distributions of a multi-principal-element alloy. Nat. Commun 6 , 5964 (2015).
Article PubMed Google Scholar
Qiu, X. W. & Liu, C. G. Microstructure and properties of Al 2 CrFeCoCuTiNi x high-entropy alloys prepared by laser cladding. J. Alloys Compd. 553 , 216–220 (2013).
Zou, Y., Ma, H. & Spolenak, R. Ultrastrong ductile and stable high-entropy alloys at small scales. Nat. Commun. 6 , 7748 (2015).
Alivisatos, A. P. Semiconductor Clusters, Nanocrystals, and Quantum Dots. Science 271 , 933–937 (1996).
Article ADS CAS Google Scholar
Guo, T. & Tan, Y. W. Formation of one-dimensional Ag-Au solid solution colloids with Au nanorods as seeds, their alloying mechanisms, and surface plasmon resonances. Nanoscale 5 , 561–569 (2013).
Zhang, K. B. et al . Nanocrystalline CoCrFeNiCuAl high-entropy solid solution synthesized by mechanical alloying. J. Alloys Compd 485 , 31–34 (2009).
Zhang, K. B. et al . Characterization of nanocrystalline CoCrFeNiTiAl high-entropy solid solution processed by mechanical alloying. J. Alloys Compd. 495 , 33–38 (2010).
Kessler, V. G., Seisenbaeva, G. A., Unell, M. & Hakansson, S. Chemically Triggered Biodelivery Using Metal-Organic Sol-Gel Synthesis. Angew. Chem. Int. Ed. 47 , 8506–8509 (2008).
Jiang, Y. W., Yang, S. G., Hua, Z. H. & Huang, H. B. Sol-Gel Autocombustion Synthesis of Metals and Metal Alloys. Angew. Chem. Int. Ed. 48 , 8529–8531 (2009).
Hua, Z. H., Cao, Z. W., Deng, Y., Jiang, Y. W. & Yang, S. G. Sol-gel autocombustion synthesis of Co-Ni alloy powder. Mater. Chem. Phys. 126 , 542–545 (2011).
Li, P. Y., Syed, J. A. & Meng, X. K. Sol-gel preparation and characterization of NiCo and Ni 3 Fe nanoalloys. J. Alloys Compd. 512 , 47–51 (2012).
Jiang, Y. W., Yang, S. G., Hua, Z. H., Gong, J. F. & Zhao, X. N. Sol-gel auto-combustion synthesis of totally immiscible NiAg alloy. Mater. Res. Bull. 46 , 2531–2536 (2011).
Yeh, J. W., Chang, S. Y., Hong, Y. D., Chen, S. K. & Lin, S. J. Anomalous decrease in X-ray diffraction intensities of Cu-Ni-Al-Co-Cr-Fe-Si alloy systems with multi-principal elements. Mater. Chem. Phys. 103 , 41–46 (2007).
Yue, Z., Zhou, J., Li, L., Zhang, H. & Gui, Z. Synthesis of nanocrystalline NiCuZn ferrite powders by sol-gel combustion method. J. Magn. Magn. Mater. 208 , 55–60 (2000).
Manukyan, K. V. et al . Solution Combustion Synthesis of Nano-Crystalline Metallic Materials: Mechanistic Studies. J. Phys. Chem. C 117 , 24417–24427 (2013).
Sarkar, A. et al . Nanocrystalline multicomponent entropy stabilised transition metaloxides. J. Eur. Ceram. Soc 37 , 747–754 (2017).
Francis, A. J., Dodge, C. J. & Gillow, J. B. Biodegradation of metal citrate complexes and implications for toxic-metal mobility. Nature 356 , 140–142 (1992).
Kaliva, M. et al . A Unique Dinuclear Mixed V(V) Oxo-peroxo Complex in the Structural Speciation of the Ternary V(V)-Peroxo-citrate System. Potential Mechanistic and Structural Insight into the Aqueous Synthetic Chemistry of Dinuclear V(V)-Citrate Species with H 2 O 2 . Inorg. Chem. 50 , 11423–11436 (2011).
Article CAS PubMed Google Scholar
Greer, A. L. Confusion by design. Nature 366 , 303–304 (1993).
Article ADS Google Scholar
Shinde, S. R. et al . Co-occurrence of superparamagnetism and anomalous Hall effect in highly reduced cobalt-doped rutile TiO 2−δ films. Phys. Rev. Lett. 92 , 166601 (2004).
Download references
Acknowledgements
This work was financially supported by the Ministry of Science and Technology of the People’s Republic of China (2015DFR50650), the National Natural Science Foundation of China (51502220, 51521001, 51672197), and the Self-determined and Innovative Research Funds of WUT(2017II17XZ), and the Open Project Program of Key Laboratory of Inorganic Functional Materials and Devices, Chinese Academy of Sciences (Grant No.: KLIFMD201606).
Author information
Authors and affiliations.
State Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology, Wuhan, 430070, China
Bo Niu, Fan Zhang, Hang Ping, Na Li, Jieyang Zhou, Liwen Lei, Jingjing Xie, Jinyong Zhang, Weimin Wang & Zhengyi Fu
You can also search for this author in PubMed Google Scholar
Contributions
B. Niu and F. Zhang designed the research, carried out most experiments, analyzed data and wrote the manuscript. H. Ping, N. Li, and J.Y. Zhou performed the characterization. L.W. Lie, J.J. Xie and J.Y. Zhang supervised the research and analyzed the data. W.M.W. and Z.Y. Fu developed the theoretical model. F. Zhang and Z.Y. Fu proposed the idea and initiated the project. All authors discussed the results and revised the manuscript.
Corresponding authors
Correspondence to Fan Zhang or Zhengyi Fu .
Ethics declarations
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supporting information, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Niu, B., Zhang, F., Ping, H. et al. Sol-gel Autocombustion Synthesis of Nanocrystalline High-entropy Alloys. Sci Rep 7 , 3421 (2017). https://doi.org/10.1038/s41598-017-03644-6
Download citation
Received : 10 March 2017
Accepted : 02 May 2017
Published : 13 June 2017
DOI : https://doi.org/10.1038/s41598-017-03644-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Preparation of high-entropy alloy bifunctional catalysts with rare earth ce coordination and efficient water splitting research.
- Zhang Wenyu
Ionics (2024)
Functional two-dimensional high-entropy materials
- Srinivasa Kartik Nemani
- Mohammad Torkamanzadeh
- Babak Anasori
Communications Materials (2023)
High entropy materials based electrocatalysts for water splitting: Synthesis strategies, catalytic mechanisms, and prospects
- Guoqing Guan
Nano Research (2023)
High-entropy catalysts for electrochemical water-electrolysis of hydrogen evolution and oxygen evolution reactions
Frontiers in Energy (2023)
Review of electrodeposition methods for the preparation of high-entropy alloys
- Zahra Shojaei
- Gholam Reza Khayati
- Esmaeel Darezereshki
International Journal of Minerals, Metallurgy and Materials (2022)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

11.6: Combustion Reactions
- Last updated
- Save as PDF
- Page ID 53784
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)

How do you cook the perfect marshmallow?
Roasting marshmallows over an open fire is a favorite past-time for campers, outdoor cook-outs, and just gathering around a fire in the back yard. The trick is to get the marshmallow a nice golden brown without catching it on fire. Too often we are not successful and we see the marshmallow burning on the stick – a combustion reaction taking place right in front of us.
Combustion Reactions
A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Combustion reactions must involve \(\ce{O_2}\) as one reactant. The combustion of hydrogen gas produces water vapor:
\[2 \ce{H_2} \left( g \right) + \ce{O_2} \left( g \right) \rightarrow 2 \ce{H_2O} \left( g \right)\nonumber \]
Notice that this reaction also qualifies as a combination reaction.
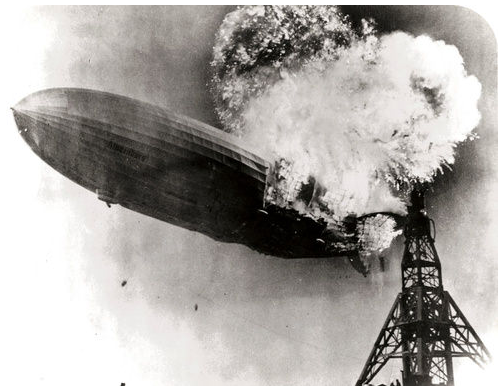
The Hindenberg was a hydrogen-filled airship that suffered an accident upon its attempted landing in New Jersey in 1937. The hydrogen immediately combusted in a huge fireball, destroying the airship and killing 36 people. The chemical reaction was a simple one: hydrogen combining with oxygen to produce water.
Many combustion reactions occur with a hydrocarbon, a compound made up solely of carbon and hydrogen. The products of the combustion of hydrocarbons are carbon dioxide and water. Many hydrocarbons are used as fuel because their combustion releases very large amounts of heat energy. Propane \(\left( \ce{C_3H_8} \right)\) is a gaseous hydrocarbon that is commonly used as the fuel source in gas grills.
\[\ce{C_3H_8} \left( g \right) + 5 \ce{O_2} \left( g \right) \rightarrow 3 \ce{CO_2} \left( g \right) + 4 \ce{H_2O} \left( g \right)\nonumber \]
Example \(\PageIndex{1}\): Combustion Reactions
Ethanol can be used as a fuel source in an alcohol lamp. The formula for ethanol is \(\ce{C_2H_5OH}\). Write the balanced equation for the combustion of ethanol.
Step 1: Plan the problem .
Ethanol and oxygen are the reactants. As with a hydrocarbon, the products of the combustion of an alcohol are carbon dioxide and water.
Step 2: Solve .
Write the skeleton equation:
\[\ce{C_2H_5OH} \left( l \right) + \ce{O_2} \left( g \right) \rightarrow \ce{CO_2} \left( g \right) + \ce{H_2O} \left( g \right)\nonumber \]
Balance the equation.
\[\ce{C_2H_5OH} \left( l \right) + 3 \ce{O_2} \left( g \right) \rightarrow 2 \ce{CO_2} \left( g \right) + 3 \ce{H_2O} \left( g \right)\nonumber \]
Step 3: Think about your result.
Combustion reactions must have oxygen as a reactant. Note that the water produced is in the gas state, rather than the liquid state, because of the high temperatures that accompany a combustion reaction.
- Combustion reaction is defined and examples are given.
- What is needed for a combustion reaction to take place?
- What is formed in any combustion reaction?
- Mercury reacts with oxygen to form mercuric oxide. Is this a combustion reaction?
- What are the products of any combustion reaction involving a hydrocarbon?
Open Access is an initiative that aims to make scientific research freely available to all. To date our community has made over 100 million downloads. It’s based on principles of collaboration, unobstructed discovery, and, most importantly, scientific progression. As PhD students, we found it difficult to access the research we needed, so we decided to create a new Open Access publisher that levels the playing field for scientists across the world. How? By making research easy to access, and puts the academic needs of the researchers before the business interests of publishers.
We are a community of more than 103,000 authors and editors from 3,291 institutions spanning 160 countries, including Nobel Prize winners and some of the world’s most-cited researchers. Publishing on IntechOpen allows authors to earn citations and find new collaborators, meaning more people see your work not only from your own field of study, but from other related fields too.
Brief introduction to this section that descibes Open Access especially from an IntechOpen perspective
Want to get in touch? Contact our London head office or media team here
Our team is growing all the time, so we’re always on the lookout for smart people who want to help us reshape the world of scientific publishing.
Home > Books > Developments in Combustion Technology
Solution Combustion Synthesis: Applications in Oxide Electronics
Reviewed: 30 June 2016 Published: 05 October 2016
DOI: 10.5772/64761
Cite this chapter
There are two ways to cite this chapter:
From the Edited Volume
Developments in Combustion Technology
Edited by Konstantinos G. Kyprianidis and Jan Skvaril
To purchase hard copies of this book, please contact the representative in India: CBS Publishers & Distributors Pvt. Ltd. www.cbspd.com | [email protected]
Chapter metrics overview
2,757 Chapter Downloads
Impact of this chapter
Total Chapter Downloads on intechopen.com
Total Chapter Views on intechopen.com
Oxide-based electronics have been well established as an alternative to silicon technology; however, typical processing requires complex, high-vacuum equipment, which is a major drawback, particularly when targeting low-cost applications. The possibility to deposit the materials by low-cost techniques such as inkjet printing has drawn tremendous interest in solution processible materials for electronic applications; however, high processing temperatures still required. To overcome this issue, solution combustion synthesis has been recently pursued. Taking advantage of the exothermic nature of the reaction as a source of energy for localized heating, the precursor solutions can be converted into oxides at lower process temperatures. Theoretically, this can be applied to any metal ions to produce the desired oxide, opening unlimited possibilities to materials’ composition and combinations. Solution combustion synthesis has been applied for the production of semiconductor thin films based on ZnO, In2O3, SnO2 and combinations of these oxides, and also for high κ dielectrics (Al2O3). All of which are required for numerous electronic devices and applications such as fully oxide-based thin-film transistors (TFTs). The properties of produced thin films are highly dependent on the precursor solution characteristics; hence, the influence of several processing parameters; organic fuel, solvent and annealing temperature was studied. Although precursor solution degradation/oxide formation mechanisms are not yet fully understood, particularly for thin films, we demonstrate that high-performance devices are obtained with combustion solution-based metal oxide thin films. The results clearly show that solution combustion synthesis is becoming one of the most promising methods for low-temperature flexible electronics.
- solution processing
- combustion synthesis
- environmentally friendly
- metal oxide materials
- thin-film transistors
- semiconductor oxides
- dielectric oxides
Author Information
Rita branquinho *.
- i3N/CENIMAT, Department of Materials Science, Faculty of Science and Technology, NOVA University of Lisbon and CEMOP/UNINOVA, Caparica, Portugal

Emanuel Carlos
Daniela salgueiro, pedro barquinha, rodrigo martins, elvira fortunato.
*Address all correspondence to: [email protected]
1. Introduction
1.1. solution combustion synthesis.
Solution combustion synthesis (SCS) is a popular method for the preparation of a wide variety of materials due to its simplicity, broad range of applicability and the possibility of easily obtaining products in the desired composition. This method has been widely used for the development of oxide powder materials; including perovskites, spinels, ferrites; for diverse applications, such as catalysis and solid oxide fuel cells [ 1 – 3 ] and is becoming one of the most convenient methods for the preparation of simple and multicomponent oxides for electronic applications [ 4 ].
SCS is based on a redox system that requires a solution that upon heating to moderate temperatures leads to the development of a strongly exothermic redox reaction, which generally provides the energy for the metal oxide formation. The ignition temperature of the exothermic reaction is significantly lower than the final combustion temperature which results in the material formation; thus allowing the conversion of solution precursors into oxides at lower process temperatures [ 1 – 3 ].
The precursor solution for combustion synthesis is typically constituted by metal nitrates, which are employed simultaneously as metal source and oxidizing agents, and an organic fuel that acts as a reducing agent. However when using metal chlorides, as a metal ion source, a combustion aid is required to provide the oxidizing nitrate ions; in this case, ammonium nitrate is typically used. The most frequently applied fuels are urea, acetylacetone and glycine, amongst others, since these can form stable complexes with metal ions to increase solubility and prevent selective precipitation of the metal ions in solution. This process produces oxide materials of good compositional homogeneity, which is especially important in the synthesis of multicomponent oxides. Historically, water is the most widely used solvent for the combustion synthesis of powder materials [ 1 – 3 ]. Recently, the pursuit of environmental friendly solvents for electronic applications, such as ethanol and water, is growing [ 5 – 7 ]; however, organic solvents, including 2-methoxyethanol and acetonitrile, are currently the most widely used for the production of oxide thin films for electronic applications purposes [ 5 ].
The resulting solution is then heated to evaporate the solvent and when ignition temperature is reached the exothermic reaction takes place. During the combustion reaction, the fuel is oxidized by the nitrate ions. The precursor materials are converted into the metal oxide, and gaseous H 2 O, CO 2 and N 2 are the remaining products formed in the combustion reaction. This process can in theory be applied to any desired metal ion.
The solution combustion synthesis of a metal oxide can be represented by the combination of metal nitrate decomposition reaction and fuel oxidation reaction. As an example, respective equations for the combustion synthesis of Al 2 O 3 from aluminium nitrate and using urea as fuel are given as follows [Eq. ( 1 ) and ( 2 )] [ 6 ].
The overall combustion reaction can thus be written as Eq. ( 3 )
Note that these are theoretical reaction equations that do not consider possible secondary reactions such as nitrates decomposition, urea hydrolysis, thermal decomposition and also fuel-oxidizer adducts; however, the overall reaction allows the calculation of a stoichiometric condition that can be used as a reference [ 3 , 6 ].
There are many essential variables in solution combustion synthesis, including metal ion, metal precursor type and concentration, content of organic fuel, combustion aid and oxidizer/fuel proportion; which is determinant for the thermodynamics of the oxide formation [ 2 ].
A concept for determining the stoichiometric proportion of oxidizer and fuel for SCS was adopted from the Jain method [ 8 ], which is based on propellant chemistry and allows the calculation of the reducing/oxidizing valences (OV/RV) of a redox mixture. In this method, oxygen and nitrogen are considered oxidizers with the respective valence of −2 and 0. On the other hand, carbon, hydrogen and metal ions are considered as reducing elements with their final valences of +4 and +1 and the corresponding metal valence, respectively. Consequently, urea has a reducing valence of +6 (RV = 4 + 4 × 1 + 2 × 0 − 2) whilst aluminium nitrate has an oxidizing valence of −15 (OV = 3 + 2 × 0 − 3 × 6), resulting in a 15/6 (or 5/2) reducing/oxidizing valences for an optimal stoichiometric redox mixture of urea-aluminium nitrate.
The relation between the redox stoichiometry and the molar ratio of reagents can be determined by the reducing/oxidizing valences (RV/OV) of the reagents and is given by Eq. ( 4 )
The oxygen consumption or production is controlled by the fuel/oxidizer ratio ( Ф ) which also depends on n; the number of moles of fuel per mole of oxidizer [ 1 – 3 ]. The optimal stoichiometry composition of the redox mixture is obtained for Ф = 1 that corresponds to a condition in which the reaction does not require any molecular oxygen to occur. For the given example of urea-aluminium nitrate redox mixture, 5 moles of urea are required per 2 moles of aluminium nitrate to assure redox stoichiometry of the aluminium oxide formation reaction, as depicted in Eq. ( 3 ) [ 6 ].
A fuel-lean condition is obtained when Ф < 1 and upon reaction molecular oxygen is produced; whereas when Ф > 1 molecular oxygen is required to fully convert the fuel and the redox mixture is in a fuel-rich regime [ 3 ].
1.2. Solution-based oxide electronics
The evolution from rigid silicon-based electronics to flexible electronics requires the use of new materials with novel functionalities that allow non-conventional, low-cost and environmental friendly processing technologies [ 9 , 10 ]. Metal oxide-based electronics have been well established as an alternative to silicon technology, demonstrating exceptional electronic performance as active semiconductor components, which can be tuned for the applications, where high transparency/electrical conductivity is demanded [ 11 , 12 ]. The major investment of several high-profile companies: SHARP, SAMSUNG, LG, BOE has led to the commercialization of oxide-based display backplanes in a very short period of time, and the global market is expected to increase [ 9 , 10 ]. The current typical processing techniques require complex high-vacuum equipment which is a major drawback, particularly when targeting low-cost applications.
Recently, the demands for low-cost flexible electronics has led to a remarkable development of solution-based production methods and solution-processed inorganic metal oxide semiconductor materials for high-performance thin-film transistors (TFTs), and such devices have demonstrated competitive results when compared with materials obtained by physical techniques [ 4 , 13 ].
Several solution-based chemical synthetic routes have been exploited for the preparation of these oxides because of their simplicity, versatility, and scale-up capability; however, the application of solution combustion synthesis to the production of thin films for TFT applications was first reported in 2011 [ 4 ]. Since then, significant research efforts have been put on the development of semiconductor materials such as indium oxide and indium, zinc tin and gallium-based multicomponent oxides [ 13 – 16 ] and more recently of dielectric materials [ 5 , 6 , 17 ]. However, most of the research as focussed on the use of toxic solvents (2-methoxyethanol) and scarce materials (indium), which can be a major drawback in the upscaling of this technology. Consequently, the challenge remains to unveil the combination of solution combustion synthesis processing parameters that allow an environmentally friendly production of high-quality insulator and semiconductor thin films at low temperature and their combination in fully combustion solution-processed TFTs.
In this work, we focus on the environmentally friendly solution combustion synthesis of oxide-based materials for electronic applications, including insulator and semiconductor thin films by studying the influence of synthetic and processing parameters, such as fuel type; solvent and annealing temperature, on their electrical properties. Fully combustion solution-processed indium-free AlO x /ZTO TFTs using ethanol as solvent were successfully produced demonstrating extremely promising performance for electronic applications.
2. Experimental details
2.1. precursor solution preparation and characterization.
The preparation of all precursor solutions was performed in a similar manner. Typically, the metal salts were dissolved in 25 mL of solvent, either 2-methoxyethanol (2-ME, C 3 H 8 O 2 , Fluka, 99%), ethanol (C 2 H 6 O, Merck, 99.5%) or deionized water, to yield solutions with the desired metal ion concentration. Then, the fuel (urea; CO(NH 2 ) 2 , Sigma, 98%) and ,if required, the combustion aid (ammonium nitrate; NH 4 NO 3 , Roth, 98%) were added to the prepared solutions which were maintained under constant stirring until complete dissolution. The urea to metal nitrate molar proportion was determined by the Jain method, as described in Section 1.1, to guarantee the redox stoichiometry of the reaction for each material; namely 1.6:1 for Zn 2+ metal ions and 2.5:1 for In 3+ and Al 3+ metal ions. In the case of metal chloride precursors, a combustion aid is required and the molar proportion of metal ion:NH 4 NO 3 :urea used was 1:1:1 for tin chloride solutions.
Semiconductor ZTO precursor solutions were prepared by mixing zinc oxide and tin oxide precursor solutions in a 2:1 proportion. Zinc oxide precursor solution was prepared as described earlier in 2-methoxyethanol or ethanol to yield solutions with 0.05 M concentration. Tin oxide precursor solutions were prepared by mixing tin chloride (SnCl 2 ⋅2H 2 O, Sigma, 98%), urea and ammonium nitrate to yield solutions with 0.05 M concentration. Individual solutions were magnetically stirred for 12 h before ZTO solution preparation.
Dielectric aluminium oxide precursor solutions were prepared by dissolving aluminium nitrate nonahydrate (Al(NO 3 ) 3 ⋅9H 2 O, Fluka, 98%) and urea in 2-methoxyethanol, ethanol or water, to yield solutions with 0.1 M concentration. Prior to their use, all solutions where magnetically stirred for at least 15 min and filtrated through a 0.45 µm hydrophilic filter. All reagents were used without further purification.
Thermal characterization of precursor solutions was performed by thermogravimetry and differential scanning calorimetry (TG-DSC). TG-DSC analyses were performed on precursors dried for 12 h at 80°C under air atmosphere up to 550°C with a 10°C/min heating rate in an aluminium crucible using a Simultaneous Thermal Analyzer, Netzsch (TGA-DSC—STA 449 F3 Jupiter).
2.2. Thin-film deposition and characterization
Prior to deposition all substrates (silicon wafer and soda-lime glass) were cleaned in an ultrasonic bath at 60°C in acetone for 15 min, then in 2-isopropanol for 15 min. and dried under N 2 ; followed by a 30 min. UV/Ozone surface activation step using a PSD-UV Novascan system. Thin films were deposited by spin coating the precursor solutions at 2000 rpm for 35 s (Laurell Technologies) followed by an immediate hotplate annealing in ambient conditions; this procedure was repeated to obtain the desired thickness.
The films’ structure was assessed by glancing angle X-ray diffraction (GAXRD) performed by an X’Pert PRO PANalytical powder diffractometer using with Cu Kα line radiation (λ = 1.540598 Å) with angle of incidence of the X-ray beam fixed at 0.9°. The surface morphology was investigated by atomic force microscopy (AFM, Asylum MFP3D) and scanning electron microscopy (SEM-FIB, Zeiss Auriga Crossbeam microscope). Cross section of produced films and devices was performed by focussed ion beam (FIB). In FIB milling experiments, Ga + ions were accelerated to 30 kV at 5 pA and the etching depth was around 250 nm. Spectroscopic ellipsometry measurements of thin films deposited on silicon substrates were made over an energy range of 1.5–6.0 eV with an incident angle of 70° using a Jobin Yvon Uvisel system to determine film thickness.
2.3. Electronic device fabrication and characterization
Metal-insulator-semiconductor (MIS) capacitors were produced by AlO x thin-film deposition onto p-type silicon substrates (1–10 Ω cm) as described earlier (see section 2.2). Aluminium gate electrodes (100 nm thick) with an area of 8.7 × 10 −3 cm 2 were deposited by thermal evaporation via shadow mask. A 100-nm-thick aluminium film was also deposited on the back of the silicon wafer to improve electrical contact. Electrical characterization was performed measuring both the capacitance-voltage and capacitance-frequency characteristics in the range off 10 kHz to 1 MHz of the devices using a semiconductor characterization system (Keithley 4200SCS).
Thin-film transistors (TFTs) were produced in a staggered bottom-gate, top-contact structure by depositing AlO x thin films onto p-type silicon substrates (1–10 Ω cm) as described earlier. The semiconductor layer was deposited by sequentially spin coating four layers of semiconductor precursor solution 0.05 M onto the AlO x thin films and annealed in air at 350°C for 30 min after each layer deposition. For comparison, AlO x /IGZO TFTs were also produced with sputtered IGZO thin films. IGZO thin films were fabricated in a AJA ATC-1300F sputtering system using a IGZO target with 2:1:1 composition (in In 2 O 3 :Ga 2 O 3 :ZnO molar ratio). Deposition was carried out in Ar+O 2 atmosphere at a pressure of 2.3 mTorr and r.f. power of 100 W, to obtain a 30 nm thick film. Post-processing annealing was performed in air on a hotplate at 180°C for 1 h. Finally, source and drain aluminium electrodes (100 nm thick) were deposited by e-beam evaporation via shadow mask onto annealed films, defining a channel width ( W ) and length ( L ) of 1400 µm and 10 µm, respectively ( W / L = 14). Electrical characterization was performed by the measurement of current-voltage characteristics of the devices using a semiconductor parameter analyser (Agilent 4155C) attached to a microprobe station (Cascade M150) inside a dark box, at room temperature. The saturation mobility (µ SAT ) was determined from the following equation: [ 18 ]
where C i is the gate dielectric capacitance per unit area, W and L are the channel width and length, V G is the gate voltage and V T is the threshold voltage, which was determined in the saturation region by linear fitting of a I D 1/2 vs. V G plot.
The core of oxide-based electronics for displays applications are thin-film transistors (TFTs). Figure 1 shows a schematic representation of a TFT structure and respective transfer plot. TFTs are three terminal field-effect devices, whose working principle relies on the modulation of the current flowing in a semiconductor placed between two electrodes (source and drain). A dielectric layer is inserted between the semiconductor and a transversal electrode (gate), being the current modulation achieved by the capacitive injection of carriers close to the dielectric/semiconductor interface, known as field effect [ 12 ].

Schematic representation of a bottom-gate TFT structure and typical field effect electrical characteristics plot.
The semiconductor and gate insulator are equally important material components in field-effect transistors; consequently, the development of both materials by solution techniques is essential [ 6 ].
3.1. Amorphous semiconductor oxides
Amorphous oxide semiconductors (AOSs) have drawn significant attention in the field of flat panel displays during the last decade, due to their high carrier mobility when compared to amorphous silicon (a-Si). Indium-gallium-zinc oxide (IGZO) is the most explored semiconductor due to its superior field effect mobility and enhanced electrical performance [ 11 , 12 ]. However, alternative semiconductor materials that rely on abundant and non-toxic elements are required due to environmental demands. Zinc-tin-oxide (ZTO) is a promising indium and gallium-free alternative and impressive results have already been obtained in TFTs applications [ 19 , 20 ].
Several solution-processed ZTO-based TFTs have been reported; however, processing temperature above 400°C and a toxic organic solvent, such as acetonitrile, 2-ethoxyethanol or 2-methoxyethanol are usually required [ 5 ]. The use of non-toxic solvents has been pursued and ethanol [ 5 ] and water [ 7 ] based ZTO TFTs have already been reported.
The decomposition of urea [Eq. ( 2 )] and zinc nitrate [Eq. ( 6 )] is represented as follows.
The overall ZnO formation reaction can thus be written as follows Eq. ( 7 ).
Using the Jain method, the reducing valence of urea is +6 and the oxidizing valence of zinc nitrate is –10. In order to assure the redox stoichiometry 5/3 (or 1.6), moles of urea are required per mole of zinc nitrate.
The tin oxide formation can be represented by the following equation:
In the combustion synthesis of tin oxide, a combustion aid, ammonium nitrate, was added to the precursor solution as commonly performed for chlorine-based metal salts. According to Jain’s method, the oxidizer valence of ammonium nitrate (NH 4 NO 3 ) is −2 and to achieve Ф = 1 0.3 mol of urea per mole of ammonium nitrate are required. In this case, a fuel-rich condition, Ф > 1, was used to ensure the full oxidation of the metal precursor.
ZTO precursor solution was prepared after mixing individual of zinc and tin oxide precursor solutions of 0.05 M concentration in a 2:1 proportion, respectively. Thermal analysis of ZTO precursor solutions was performed to investigate the influence of solvent on their decomposition behaviour. TG-DSC measurements were performed on precursors dried for 12 h at 80°C. Figure 2 shows the DSC results for ZTO precursors up to 350°C as above this temperature no further events were observed.
The combustion reaction of the organic fuel with the metal nitrates typically leads to an intense exothermic peak with corresponding abrupt mass loss. For ZTO precursors, these were observed at 125°C and 108°C for 2-ME and ethanol-based solutions, respectively. A smaller peak is observed at 275°C which can be related to secondary reactions [ 3 , 4 ] that can occur during thermal decomposition of the reagents.

TG-DSC analysis of ZTO-based precursor solutions using 2-methoxyethanol (2-ME) and ethanol as solvent. Adapted from [ 5 ].
The precursor solution degradation mechanism is expected to be different in bulk and thin-film form which explains the need for higher thin-film processing temperature than the one determined by thermal analysis. Devices annealed at temperatures below 350°C did not show effective gate modulation as such, the processing temperature was fixed at this temperature although the minimum temperature required for full degradation of the precursors is 275°C.
Structural characterization of the films ( Figure 3a ) indicates that amorphous films are obtained up to 350°C for both precursor solution solvents, as no diffraction peaks were observed. Morphological surface analysis show that smooth and uniform films are obtained regardless of solvent; however, 2-ME-based ZTO films demonstrate a lower surface roughness, 0.2 nm, when compared to ethanol-based films; 5 nm.

Structural and morphological analysis of 2-methoxyethanol (2-ME) and ethanol-based ZTO thin films produced at 350°C: a) XRD, b) AFM, c) SEM.
Combustion solution-based ZTO TFTs were produced by spin-coating precursor solutions onto commercial Si/SiO 2 substrates. Electrical characterization of TFTs was performed by measuring the transfer and output characteristics of the devices in ambient conditions in the dark. Figure 4 shows a cross section SEM image of ZTO TFTs produced on Si/SiO 2 , with Al source-drain electrodes, obtained after FIB milling and electrical characterization of 2-ME and ethanol-based ZTO TFTs.

Electrical and morphological characterization of ZTO/SiO 2 TFTs produced at 350°C: transfer and output characteristics of a) 2-methoxyethanol (2-ME) and b) ethanol-based ZTO/SiO 2 TFTs. c) SEM-FIB cross section of a ZTO/SiO 2 TFT.
The analysis of the electrical characteristics reveals that ZTO TFTs show an I on / I off above 10 6 , saturation mobility of 2 cm 2 /V⋅s, low subthreshold swing and low hysteresis. Output characteristics exhibit saturation suggesting a low background carrier concentration and anticipate the absence of transconductance degradation as V GS increases, revealing good dielectric/semiconductor interface properties and no significant carrier injection problems. These devices exhibit high operational stability under positive gate bias stress [ 21 ]. Combustion synthesis-based ZTO thin films thus demonstrate high potential for alternative and environmental friendly solution-based electronic applications, allowing their use as reliable switching elements in active matrices.
3.2. Dielectric oxides
Insulator materials suitable for TFT gate dielectric applications must fulfil some criteria, namely i) high bang gap, with favourable conduction band offset to avoid high gate leakage; ii) high dielectric constant (high-κ) the added capacitance can compensate interface traps, thus improving the transistor performance and allow low operating voltage; iii) good interface properties, which can be achieved using amorphous dielectrics [ 11 , 22 , 23 ].
Solution-based high-κ dielectrics, such as Al 2 O 3 , HfO 2 , ZrO 2 , have demonstrated high performance and suitability for the application in metal oxide semiconductor-based TFTs [ 5 , 6 , 17 , 24 – 28 ]. Amongst these, aluminium oxide is one of the most developed materials from solution synthesis since several aluminium precursors salts are readily availability at low cost. The influence of processing parameters on the solution combustion synthesis of aluminium oxide was studied.
The decomposition reactions for aluminium, urea and the overall aluminium oxide formation reaction are already represented by Eq. ( 1 ), ( 2 ) and ( 3 ), respectively. According to Jain’s method and to ensure redox stoichiometry, 2.5 mol of urea per mole of aluminium nitrate were used to prepare aluminium oxide precursor solutions with 0.1 M concentration. The influence of several processing parameters such as fuel, solvent and annealing temperature on the properties of dielectric AlO x thin films was studied.
Thermal analysis of AlO x precursor solutions was performed to investigate the influence of solvent on their decomposition behaviour. Figure 5 shows the DSC results for AlO x precursors prepared with 2-methoxethanol, ethanol and water.

TG-DSC analysis of AlO x -based precursor solutions: a) non-combustion AlO x precursor using 2-methoxyethanol (2-ME) and b) combustion AlO x precursor using 2-ME, ethanol and water as solvent.
Thermal behaviour of AlO x precursor solutions is strongly affected by the presence of urea, which acts as fuel; when no fuel is added only endothermic peaks corresponding to solvent evaporation (water at 95°C and 2-methoxyethanol at 125°C) which is the main component of the solution with a corresponding 60% mass loss and subsequent organics decomposition (235°C) are observed, meaning that additional energy must be supplied to promote oxide formation. On the other hand, when urea is added in a stoichiometric proportion an intense exothermic peak with corresponding abrupt mass loss at about 180°C is observed. This exothermic event is attributed to the combustion reaction of the organic fuel with the metal nitrates; a smaller peak at 250°C is also observed and can be related to the degradation of residual organics. Thermal behaviour of combustion AlO x precursor solutions does not shown significant variations when using different solvents. The influence of solvent in the oxide formation reaction is apparently more significant for multicomponent oxides, such as ZTO ( Figure 2 ).
Thin films of AlO x were produced by spin-coating precursor solution onto a substrate (either soda-lime glass or Si) and annealing on a hotplate for 30 min. The influence of several processing parameters on the structural and morphological properties of dielectric AlO x thin films obtained from 0.1 M concentration solutions was studied. XRD, SEM and AFM characterization of the AlO x thin films was performed and Figure 6 shows typical data obtained for AlO x thin films produced at 350°C from 2-methoxyethanol, ethanol and water-based precursor solutions.

Structural and morphological analysis of combustion solution AlO x thin films produced at 350°C: a) XRD, b) SEM and c) AFM of water-based films. Inset show gate current density.
Generally, structural and morphological properties of AlO x thin films did not vary significantly regardless of processing parameters within the studied range. The variation of precursor solution’s solvent does not influence the structural properties of the films, which is in agreement with that observed for DSC measurements. Morphological properties are slightly influenced by the solvent used with rms film roughness increasing from 0.3 nm when using 2ME or ethanol to 0.9 nm for water-based films. Fuel content and annealing temperature also do not affect structural and morphological properties as amorphous, highly uniform and smooth AlO x thin films with rms roughness of below 1 nm were consistently obtained at 350°C. The properties demonstrated by these aluminium oxide thin films produced from solution synthesis are highly desirable for electronic applications as low surface roughness leads to enhanced interface properties.
The effect of processing parameters on the electrical properties of these films was studied by assessing the electrical performance of capacitors and thin-film transistors comprising the solution-based AlO x films.
Solution-based AlO x capacitors were produced by spin-coating AlO x precursor solutions onto commercial Si substrates followed by aluminium gate contact deposition by thermal evaporation. The effect of processing parameters on the electrical performance of these devices was studied and Figure 7 shows capacitance-voltage measurements for different fuel, using either urea, citric acid or no fuel; and annealing temperature, 350, 250 and 200°C. The effect of precursor solution solvent variation has already been reported elsewhere [ 6 ].

Electrical characterization of AlO x capacitors produced with different parameters: a) fuel variation in 2-ME; b) temperature variation for 2-methoxyethanol-based films. Inset show gate current density.
The electrical characteristics of AlO x capacitors produced with no fuel show a non-uniform capacitance variation at −3 to −2 V indicating the presence of organic residues within the film such as nitrate groups; these ions favour water adsorption leading to higher capacitance due to the high dielectric constant of water, as previously observed [ 6 , 29 ]. To improve film properties, when a non-combustion synthesis route is applied, either higher temperature or UV-assisted annealing is currently being used [ 26 , 30 ]. When citric acid is added as fuel low capacitance and high current density is observed, this is consistent with more porous AlO x films and in fact their application as gate dielectric in TFTs was not successful as only short-circuited devices were obtained. Urea leads to consistent one domain capacitance characteristics and low current density within TFT operating voltage range as required for gate dielectric applications. The film properties are highly affected by processing temperature due to inefficient organics removal; at 250°C a two domain system is obtained, similar to that observed for non-combustion synthesis route, and at 200°C large hysteresis indicates an increase in charge defects concentration consistent with organic impurities within the film. Although from DSC analysis, the ignition temperature of AlO x combustion reaction was determined to be 180°C, still higher temperature is required to obtain thin films with desirable dielectric properties. This inconsistency between thermal analysis and electrical properties obtained at low temperature is attributed to the difference between bulk and film decomposition processes.
IGZO TFTs comprising combustion solution-based AlO x gate dielectric were produced by sputtering IGZO semiconductor layer onto annealed AlO x thin films and finally evaporating aluminium source/drain contacts. The use of a standard sputtered semiconductor allowed the assessment of the TFTs performance variation solely due to the dielectric layer’s processing parameters influence. Electrical characterization of TFTs was performed by measuring the electrical characteristics of the devices in ambient conditions in the dark ( Figure 8 ).

Electrical characterization of IGZO/AlO x TFTs produced at different temperatures using a) 2-methoxyethanol and b) water as solvent.
TFT performance is assessed trough the analysis of the turn-on voltage ( V ON ), the threshold voltage ( V T ), drain current on-off ratio ( I ON /I OFF ), subthreshold slope ( S ) and saturation mobility ( µ SAT ), which was calculated using AlO x capacitance at the 10 kHz in order to minimize overestimation. Electrical characteristics of these devices are presented in Table 1 .
Electrical characteristics of solution-based IGZO/AlO x TFTs.
Despite the non-ideal capacitance-voltage characteristics obtained for low-temperature AlO x thin films, IGZO/AlO x TFTs were successfully produced. Similarly to what is obtained for capacitors the TFTs performance is highly dependent on annealing temperature. For both solvents used, 2-methoxyethanol and water, enhanced properties are obtained for 350°C and similar trend with temperature variation is observed which is in agreement with DSC-TG analysis. For lower temperatures, a hysteresis increase and on/off current ratio decrease is observed, being more significant for 200°C, due to oxide charge defects within the dielectric as a consequence of incomplete organic residues removal. Nevertheless, successful gate modulation at low operation voltage, due to the higher capacitance achieved with very thin AlO x films, was obtained for all devices which demonstrated close to zero V ON , high I ON , high saturation mobility and low subthreshold swing as required for electronic applications.
3.3. Fully solution-based oxide TFTs
The realization of printed electronics requires all solution-based devices; consequently, semiconductor and dielectric materials must be combined in TFTs. Fully combustion-solution-based TFTs comprising the developed dielectric and semiconductor materials have been successfully fabricated. Figure 9 shows transfer characteristics of these devices [ 5 , 6 ].

Transfer characteristics of fully combustion solution-based bottom gate TFTs produced at 350°C on highly doped p-Si (gate) with a) 2ME-based ZTO and AlO x , b) ethanol-based ZTO and AlO x , and c) 2ME-based GZTO and water-based AlO x . Adapted from [ 5 , 6 ].
The combination of solution-processed dielectric and semiconductors yielded TFTs with a good overall performance, demonstrating very low clockwise hysteresis, close to zero V ON and I ON /I OFF above 10 4 . Generally, 2ME-based devices show better performance when compared to more environmental friendly ethanol; saturation mobility increases from 0.8 cm 2 /V⋅s to 2.3 cm 2 /V⋅s for ZTO/AlO x TFTs, also 2ME GZTO shows µ SAT of 1.3 cm 2 /V⋅s when combined with water-based AlO x . These differences in device performance are attributed to trapped charges at the semiconductor-insulator interface as consequence of the semiconductor films’ composition and rougher morphology of ethanol-based films [ 5 , 6 ]. The high I OFF observed mainly arises from the use of non-patterned semiconductor layers which can be improved by implementing patterning techniques. The combination of AlO x dielectrics and ZTO-based semiconductors yielded very promising results for application in fully solution-processed electronics; however, the processing temperature required for good TFT performance was 350°C which is still too high for flexible substrates. Nevertheless, these results are within the state-of-the-art for solution-processed ZTO TFTs using different solvents ( Table 2 ).
Selected processing details for reported solution-based ZTO TFTs.

Transfer characteristics of fully combustion-solution-based InO x /AlO x TFTs produced from 2ME precursors using a maximum processing temperature of 200°C.
The use of indium-free semiconductor materials, such as ZTO and GZTO, although environmentally relevant somehow lead to a compromise of solution-processed TFTs performance both in saturation mobility and processing temperature.
Fully solution-based TFTs were obtained at maximum processing temperature of 200°C when combining the developed 2-methoxyethanol-based AlO x dielectric with solution-based indium oxide semiconductor produced using UV-assisted low-temperature annealing as reported in [ 36 ]. Transfer characteristics of low-temperature InO x /AlO x TFTs are depicted in Figure 10 .
In O x /AlO x TFTs showed higher saturation mobility (µ SAT = 5.6 cm 2 V −1 s −1 ) when compared to indium-free devices processed at higher temperatures and otherwise similar electrical performance whilst at the same time being compatible with polymeric substrates for flexible electronics applications.
4. Conclusions
The application of solution combustion synthesis to prepare oxide materials for electronic devices was successfully achieved for dielectric and semiconductor thin films using varied processing parameters. We have clearly demonstrated that dielectric AlO x thin films can be obtained using green solvents such as ethanol and water and successfully combined with ethanol-based ZTO semiconductor layers to yield fully combustion solution-based TFTs. Although electrical properties of these solution-processed TFTs are still far for what is required in AMTFT displays applications, successful preparation and combination of dielectric and semiconductor oxides films by a relatively low-temperature solution process represents a significant achievement and an advancement towards environmentally friendly production process. The facile applicability of SCS to other metal precursors opens numerous possibilities for low temperature synthesis of oxides materials and thin films required for the development of all solution-based devices. The mechanisms behind precursor degradation/oxide formation process are not yet fully understood so the challenge remains to unravel the ideal combination of solvent, oxidizer and organic fuel to obtain high-quality materials and properties. Nevertheless, this versatile synthesis method combined with innovative low-temperature annealing processes will continue to play a major role in the future development flexible electronics.
Acknowledgments
This work was partly funded by FEDER funds through the COMPETE 2020 Programme and National Funds through FCT—Portuguese Foundation for Science and Technology under the projects POCI-01-0145-FEDER-007688, Reference UID/CTM/50025 and EXCL/CTM-NAN/0201/2012; European Community FP7 2007-2013 project i-FLEXIS Grant Agreement n. 611070. D. Salgueiro acknowledges FCT-MEC for doctoral grant SFRH/BD/110427/2015. Authors would like to acknowledge J. V. Pinto for XRD, D. Nunes for SEM-FIB and T. Sequeira for AFM measurements.
- 1. M. Epifani, E. Melissano, G. Pace, and M. Schioppa, “Precursors for the combustion synthesis of metal oxides from the sol-gel processing of metal complexes,” J. Eur. Ceram. Soc., vol. 27, no. 1, pp. 115–123, 2007.
- 2. Z. Shao, W. Zhou, and Z. Zhu, “Advanced synthesis of materials for intermediate-temperature solid oxide fuel cells,” Prog. Mater. Sci., vol. 57, no. 4, pp. 804–874, 2012.
- 3. S. L. Gonzalez-Cortes and F. E. Imbert, “Fundamentals, properties and applications of solid catalysts prepared by solution combustion synthesis (SCS),” Appl. Catal. A Gen., vol. 452, pp. 117–131, 2013.
- 4. M.-G. Kim, M. G. Kanatzidis, A. Facchetti, and T. J. Marks, “Low-temperature fabrication of high-performance metal oxide thin-film electronics via combustion processing,” Nat. Mater., vol. 10, no. 5, pp. 382–388, 2011.
- 5. R. Branquinho, D. Salgueiro, A. Santa, and A. Kiazadeh, “Towards environmental friendly solution-based ZTO/AlO x TFTs,” Semicond. Sci. Technol., vol. 30, no. 2, p. 24007, 2015.
- 6. R. Branquinho, D. Salgueiro, L. Santos, P. Barquinha, L. Pereira, R. F. D. P. Martins, and E. Fortunato, “Aqueous combustion synthesis of aluminium oxide thin films and application as gate dielectric in GZTO solution-based TFTs,” ACS Appl. Mater. Interfaces, vol. 6, pp. 195592–195599, 2014.
- 7. J. H. Park, W. J. Choi, J. Y. Oh, S. S. Chae, W. S. Jang, S. J. Lee, K. M. Song, and H. K. Baik, “Low-temperature, aqueous-solution-processed zinc tin oxide thin film transistor,” Jpn. J. Appl. Phys., vol. 50, no. 7, p. 070201, 2011.
- 8. S. R. Jain, K. C. Adiga, and V. R. Pai Verneker, “A new approach to thermochemical calculations of condensed fuel-oxidizer mixtures,” Combust. Flame, vol. 40, pp. 71–79, 1981.
- 9. E. Fortunato, P. Barquinha, R. Branquinho, D. Salgueiro, E. Carlos, A. Liu, F. K. Shan, and R. Martins, “Is the new oxide electronics (r)evolution solution based?,” J. Phys. D Appl. Phys., 2016, in press.
- 10. J. F. Wager, “Oxide TFTs: A Progress Report,” Inf. Disp. (1975), vol. 32, no. 1, pp. 16–21, 2016.
- 11. P. Barquinha, L. Pereira, R. Martins, and E. Fortunato, Transparent Electronics: From Materials to Devices. John Wiley & Sons, Inc., Hoboken, New Jersey, 2012.
- 12. E. Fortunato, P. Barquinha, and R. Martins, “Oxide semiconductor thin-film transistors: a review of recent advances,” Adv. Mater., vol. 24, no. 22, pp. 2945–2986, 2012.
- 13. B. Du Ahn, H.-J. Jeon, J. Sheng, J. Park, and J.-S. Park, “A review on the recent developments of solution processes for oxide thin film transistors,” Semicond. Sci. Technol., vol. 30, no. 6, p. 064001, 2015.
- 14. Y. H. Kang, S. Jeong, J. M. Ko, J.-Y. Lee, Y. Choi, C. Lee, and S. Y. Cho, “Two-component solution processing of oxide semiconductors for thin-film transistors via self-combustion reaction,” J. Mater. Chem. C, vol. 2, no. 21, p. 4247, 2014.
- 15. J. W. Hennek, M.-G. Kim, M. G. Kanatzidis, A. Facchetti, and T. J. Marks, “Exploratory combustion synthesis: amorphous indium yttrium oxide for thin-film transistors,” J. Am. Chem. Soc., vol. 134, no. 23, pp. 9593–9596, 2012.
- 16. J. W. Hennek, J. Smith, A. Yan, M.-G. Kim, W. Zhao, V. P. Dravid, A. Facchetti, and T. J. Marks, “Oxygen getter” effects on microstructure and carrier transport in low temperature combustion-processed a-InXZnO (X = Ga, Sc, Y, La) transistors,” J. Am. Chem. Soc., vol. 135, no. 29, pp. 10729–10741, 2013.
- 17. E. J. Bae, Y. H. Kang, M. Han, C. Lee, and S. Y. Cho, “Soluble oxide gate dielectrics prepared using the self-combustion reaction for high-performance thin-film transistors,” J. Mater. Chem. C, vol. 2, no. 28, pp. 5695–5703, 2014.
- 18. D. K. Schroder, Semiconductor Material and Device Characterization, 3rd ed. John Wiley & Sons, Inc., Hoboken, New Jersey, 2006.
- 19. J. S. Lee, Y.-J. Kwack, W.-S. Choi, and C. Woon-Seop, “Low-temperature solution-processed zinc-tin-oxide thin-film transistor and its stability,” J. Korean Phys. Soc., vol. 59, no. 5, p. 3055, 2011.
- 20. R. D. Chandra, M. Rao, K. Zhang, R. R. Prabhakar, C. Shi, J. Zhang, S. G. Mhaisalkar, and N. Mathews, “Tuning electrical properties in amorphous zinc tin oxide thin films for solution processed electronics.,” ACS Appl. Mater. Interfaces, vol. 6, no. 2, pp. 773–7, 2014.
- 21. A. Kiazadeh, D. Salgueiro, R. Branquinho, J. Pinto, H. L. Gomes, P. Barquinha, R. Martins, and E. Fortunato, “Operational stability of solution based zinc tin oxide/SiO2 thin film transistors under gate bias stress,” APL Mater., vol. 3, no. 6, p. 062804, 2015.
- 22. L. Pereira, P. Barquinha, E. Fortunato, R. Martins, D. Kang, C. J. Kim, H. Lim, I. Song, and Y. Park, “High k dielectrics for low temperature electronics,” Thin Solid Films, vol. 516, no. 7, pp. 1544–1548, 2008.
- 23. P. Barquinha, L. Pereira, G. Gonçalves, R. Martins, E. Fortunato, D. Kuscer, M. Kosec, A. Vilà, A. Olziersky, and J. R. Morante, “Low-temperature sputtered mixtures of high κ and high bandgap dielectrics for GIZO TFTs,” J. Soc. Inf. Disp., vol. 18, no. 10, pp. 762–772, 2010.
- 24. Y. B. Yoo, J. H. Park, K. H. Lee, H. W. Lee, K. M. Song, S. J. Lee, and H. K. Baik, “Solution-processed high-k HfO2 gate dielectric processed under softening temperature of polymer substrates,” J. Mater. Chem. C, vol. 1, no. 8, p. 1651, 2013.
- 25. J. Ko, J. Kim, S. Y. Park, E. Lee, K. Kim, K.-H. Lim, and Y. S. Kim, “Solution-processed amorphous hafnium-lanthanum oxide gate insulator for oxide thin-film transistors,” J. Mater. Chem. C, vol. 2, no. 6, pp. 1050–1056, 2014.
- 26. S. Park, K.-H. Kim, J.-W. Jo, S. Sung, K.-T. Kim, W.-J. Lee, J. Kim, H. J. Kim, G.-R. Yi, Y.-H. Kim, M.-H. Yoon, and S. K. Park, “In-depth studies on rapid photochemical activation of various sol-gel metal oxide films for flexible transparent electronics,” Adv. Funct. Mater., vol. 25, pp. 2807–2815, 2015.
- 27. C. Avis and J. Jang, “High-performance solution processed oxide TFT with aluminum oxide gate dielectric fabricated by a sol-gel method,” J. Mater. Chem., vol. 21, no. 29, pp. 10649–10652, 2011.
- 28. C. Avis, Y. G. Kim, and J. Jang, “Solution processed hafnium oxide as a gate insulator for low-voltage oxide thin-film transistors,” J. Mater. Chem., vol. 22, no. 34, p. 17415, 2012.
- 29. J. H. Park, K. Kim, Y. B. Yoo, S. Y. Park, K.-H. Lim, K. H. Lee, H. K. Baik, and Y. S. Kim, “Water adsorption effects of nitrate ion coordinated Al2O3 dielectric for high performance metal-oxide thin-film transistor,” J. Mater. Chem. C, vol. 1, no. 43, pp. 7166–7174, 2013.
- 30. Y.-H. Kim, J.-S. Heo, T.-H. Kim, S. K. Park, M.-H. Yoon, J. Kim, M. S. Oh, G.-R. Yi, Y.-Y. Noh, and S. K. Park, “Flexible metal-oxide devices made by room-temperature photochemical activation of sol-gel films,” Nature, vol. 489, no. 7414, pp. 128–132, 2012.
- 31. T.-J. Ha and A. Dodabalapur, “Photo stability of solution-processed low-voltage high mobility zinc-tin-oxide/ZrO2 thin-film transistors for transparent display applications,” Appl. Phys. Lett., vol. 102, no. 12, p. 123506, 2013.
- 32. Y. G. Kim, C. Avis, and J. Jang, “Low voltage driven, stable solution-processed zinc-tin-oxide TFT with HfOy and AlOx stack gate dielectric,” ECS Solid State Lett., vol. 1, no. 2, pp. Q23–Q25, 2012.
- 33. J.-S. Lee, S.-M. Song, D.-W. Kang, Y.-H. Kim, J.-Y. Kwon, and M.-K. Han, “Effects of ultra-violet treatment on electrical characteristics of solution-processed oxide thin-film transistors,” ECS Trans., vol. 50, no. 8, pp. 121–127, 2013.
- 34. W. Hu and R. L. Peterson, “Charge transport in solution-processed zinc tin oxide thin film transistors,” J. Mater. Res., vol. 27, no. 17, pp. 2286–2292, 2012.
- 35. C. Kim, N.-H. Lee, Y.-K. Kwon, and B. Kang, “Effects of film thickness and Sn concentration on electrical properties of solution-processed zinc tin oxide thin film transistors,” Thin Solid Films, vol. 544, pp. 129–133, 2013.
- 36. J. Leppäniemi, H. Majumdar, K. Ojanperä, T. Kololuoma, J. Dahl, M. Tuominen, P. Laukkanen, and A. Alastalo, “Rapid low-temperature processing of metal-oxide thin film transistors with combined far ultraviolet and thermal annealing,” Appl. Phys. Lett., vol. 113514, pp. 1–10, 2014.
© 2016 The Author(s). Licensee IntechOpen. This chapter is distributed under the terms of the Creative Commons Attribution 3.0 License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Continue reading from the same book
Edited by Konstantinos Kyprianidis
Published: 05 October 2016
By Emília Hroncová, Juraj Ladomerský, Ján Valíček and...
2720 downloads
By Paweł Madejski, Tomasz Janda, Norbert Modliński an...
2863 downloads
By Guan-Fu Pan, Hong-De Xia and Zhen-Yu Tian
1650 downloads

Cool green-emissive Y 2 Si 2 O 7 :Tb 3+ nanophosphor: auto-combustion synthesis and structural and photoluminescence characteristics with good thermal stability for lighting applications †

First published on 22nd May 2024
1 Introduction
Few studies have been conducted on yttrium orthosilicates (YSO) and for a long time, the focus of research on the luminous characteristics of RE-activated yttrium silicates was only on yttrium pyrosilicates (YPS). The magnetic and electrical characteristics of binary disilicates, particularly those of rare earth disilicates, have been thoroughly studied. 20 Specifically, on activation with RE elements, these materials show optimal performance for luminous applications such as plasma displays, laser materials, and high energy nanophosphors. 21 These phosphors provide very efficient luminescence when excited by UV and cathode rays. Because of their chemical stability, yttrium disilicates, also known as yttrium pyrosilicates (Y 2 Si 2 O 7 ), are among the most suitable silicates. 22 The photoluminescence of Ce 3+ and Eu 3+ was particularly explored with respect to the luminescence characteristics of rare earth-doped Y 2 Si 2 O 7 . Nowadays, the green phosphor is quite popular in the lighting industry. As a result, interest in an effective new green phosphor has grown. The green color-emitter trivalent terbium (Tb 3+ ) is one of the rare earth ions that are most frequently employed as an active center. Its many emission peaks encompass a broad wavelength range from blue to orange light. The ground state spectrum characteristics of the Tb 3+ ion are 7 F j ( j = 0–6), while its outermost electron arrangement is 4f 8 . The 5 D 4 → 7 F j ( j = 0–6) transitions are often the source of the distinctive green emission of Tb 3+ ions, while the concurrently existing 5 D 3 → 7 F j transitions that produce blue or ultraviolet light are also conceivable. 23,24 Moreover, the luminescence of 4f-electrons is mainly a characteristic inherent to the RE ions and remains unaffected by the crystal field surrounding the luminescent center. This is because the 4f-electrons within the inner layer of Tb 3+ are protected by the outer electrons of 5s 2 5p 6 . Consequently, it is believed that Tb 3+ -activated phosphors would show outstanding stability for the particular emission. This work presents a thorough exploration of the structural and optical features of Tb 3+ -doped Y 2 Si 2 O 7 (YPS) phosphor that is produced using the gel-combustion process. X-ray diffraction, transmission electron microscopy, energy dispersive X-ray spectroscopy, diffuse reflectance spectroscopy and photoluminescence spectroscopy were employed to comprehensively examine the structural, morphological, and spectroscopic aspects of the prepared series of Y 2− x Si 2 O 7 : x Tb 3+ ( x = 0.01–0.06 mole) samples to ascertain the suitability of the prepared phosphors in pc-WLEDs.
2 Experimental
2.1 synthesis and characterization, 3 results and discussion, 3.1 xrd evaluation, 3.2 morphological analysis, 3.3 edx investigation, 3.4 photoluminescence study.
In above equation, x represents the doping concentration of Tb 3+ ions, I signifies the PL intensity of samples, and M denotes the electric multipole index. The values of M such as M = 6, 8 and 10 correspond to dipole–dipole, dipole–quadrupole and quadrupole–quadrupole interactions, respectively. 43,44 In Fig. 11 , the graph between log( I / x ) and log( x ) is depicted, and the slope of the fitted line is determined to be −2.28. Consequently, the calculated value of M is 6.48, agreeing closely with the value of 6. Hence, the dipole–dipole interaction is identified as the primary reason responsible for the CQ effect in the Y 2 Si 2 O 7 :Tb 3+ phosphors.
3.5 Luminescence lifetime
Table 4 contains the calculated values of lifetime, quantum efficiency and non-radiative transitions for all the synthesized YPS: x Tb 3+ ( x = 0.01–0.06 mole) nanophosphors.
3.6 Optical band gap analysis
The band gap ( E g ) of the sample is indicated by extrapolating the resulting curve to the hν axis ( x -axis), where [ F ( R ) hν ] 2 = 0. This is achieved through a plot of [ F ( R ) hν ] 2 versus hν , as illustrated in Fig. 15 . The band gaps for the host YPS and YPS:0.04Tb 3+ phosphor are 5.61 eV and 5.79 eV, respectively. Burstein–Moss (B–M) theory results in a continual rise of the optical energy gap from the host to the doped sample.
3.7 Temperature-dependent luminescence
3.8 photometric investigation, 4 conclusions, data availability, author contributions, conflicts of interest, acknowledgements.
- R. Naik, S. C. Prashantha, H. Nagabhushana, S. C. Sharma, B. M. Nagabhushana, H. P. Nagaswarupa and H. B. Premkumar, Low temperature synthesis and photoluminescence properties of red emitting Mg 2 SiO 4 :Eu 3+ nanophosphor for near UV-light emitting diodes, Sens. Actuators, B , 2014, 195 , 140–149, DOI: 10.1016/j.snb.2014.01.018 .
- D. Singh, V. Tanwar, S. Bhagwan and I. Singh, Recent Advancements in Luminescent Materials and Their Potential Applications, Adv. Magn. Opt. Mater. , 2016, 317–352, DOI: 10.1002/9781119241966.ch10 .
- V. Singh, S. Kaur and M. Jayasimhadri, Luminescence properties of orange emitting CaAl 4 O 7 :Sm 3+ phosphor for solid state lighting applications, Solid State Sci. , 2020, 101 , 106049, DOI: 10.1016/j.solidstatesciences.2019.106049 .
- I. Gupta, P. Kumar, S. Singh, S. Bhagwan, S. K. Chhikara and D. Singh, Crystal configuration, spectroscopic and optical characteristics of Er 3+ doped YAlO 3 perovskites for advanced photonic appliances, Inorg. Chim. Acta , 2022, 543 , 121183, DOI: 10.1016/j.ica.2022.121183 .
- J. Chen, H. Xiang, J. Wang, R. Wang, Y. Li, Q. Shan and H. Zeng, Perovskite white light emitting diodes: progress, challenges, and opportunities, ACS Nano , 2021, 15 , 17150–17174, DOI: 10.1021/acsnano.1c06849 .
- D. Singh and S. Sheoran, Synthesis and luminescent characteristics of M 3 Y 2 Si 3 O 12 :Eu 3+ (M = Ca, Mg, Sr and Ba) nanomaterials for display applications, J. Mater. Sci.: Mater. Electron. , 2017, 27 , 12707–12718, DOI: 10.1007/s10854-016-5405-5 .
- I. Gupta, S. Singh, S. Bhagwan and D. Singh, Rare earth (RE) doped phosphors and their emerging applications: a review, Ceram. Int. , 2021, 47 , 19282–19303, DOI: 10.1016/j.ceramint.2021.03.308 .
- D. Singh, V. Tanwar, A. P. Simantilleke, S. Bhagwan, B. Mari, P. S. Kadyan and I. Singh, Synthesis and enhanced luminescent characterization of SrAl 4 O 7 :Eu 2+ , RE 3+ (RE = Nd, Dy) nanophosphors for light emitting applications, J. Mater. Sci.: Mater. Electron. , 2016, 27 , 5303–5308, DOI: 10.1007/s10854-016-4428-2 .
- R. Fouad and M. Saif, Synthesis, spectroscopic and photoluminescence studies of novel Eu 3+ nanophosphor complex as fluorescent sensor for highly sensitive detection of latent fingerprints and anti-counterfeiting, J. Mol. Struct. , 2020, 1217 , 128472, DOI: 10.1016/j.molstruc.2020.128472 .
- P. Kumar, S. Singh, I. Gupta, K. Nehra, V. Kumar and D. Singh, Structural refinement and optical characteristics of single-phase Gd 3 Al 5 O 12 :Er 3+ nanophosphors for luminescent applications, J. Lumin. , 2022, 252 , 119338, DOI: 10.1016/j.jlumin.2022.119338 .
- G. L. Bhagyalekshmi and D. N. Rajendran, Luminescence dynamics of Eu 3+ activated and co-activated defect spinel zinc titanate nanophosphor for applications in WLEDs, J. Alloys Compd. , 2021, 850 , 156660, DOI: 10.1016/j.jallcom.2020.156660 .
- P. Kumar, D. Singh, I. Gupta, S. Singh, S. Nehra and R. Kumar, A study of phase evolution, crystallographic and down-conversion luminescent behaviour of monoclinic Y 4 Al 2 O 9 :Dy 3+ nanophosphors for white light applications, Opt. Mater. , 2023, 138 , 113677, DOI: 10.1016/j.optmat.2023.113677 .
- P. Kumar, S. Singh, I. Gupta, V. Kumar and D. Singh, Er 3+ -activated LaAlO 3 perovskite phosphor: crystal structure and down conversion photoluminescent behaviour for optoelectronic devices, Inorg. Chem. Commun. , 2022, 1265 , 109578, DOI: 10.1016/j.inoche.2022.109578 .
- V. Tanwar, S. Singh, I. Gupta, P. Kumar, H. Kumar, B. Mari and D. Singh, Preparation and luminescence characterization of Eu (III)-activated Forsterite for optoelectronic applications., J. Mol. Struct. , 2022, 1250 , 131802, DOI: 10.1016/j.molstruc.2021.131802 .
- P. Kumar, D. Singh, I. Gupta and H. Kumar, Influence of Dy 3+ ion concentration on structural, photoluminescence and energy transfer mechanism of promising GdSr 2 AlO 5 nanophosphors for white light applications, Ceram. Int. , 2023, 49 , 29010–29024, DOI: 10.1016/j.ceramint.2023.06.173 .
- X. Yang, Y. Zhang, X. Zhang, J. Chen, H. Huang, D. Wang and B. Lei, Facile synthesis of the desired red phosphor Li 2 Ca 2 Mg 2 Si 2 N 6 : Eu 2+ for high CRI white LEDs and plant growth LED device, J. Am. Ceram. Soc. , 2020, 103 , 1773–1781, DOI: 10.1111/jace.16858 .
- P. Kumar, S. Singh, I. Gupta, V. Kumar and D. Singh, Luminous LaAlO 3 :Dy 3+ perovskite nanomaterials: synthesis, structural, and luminescence characteristics for white light-emitting diodes, Luminescence , 2022, 37 , 1932–1941, DOI: 10.1002/bio.4377 .
- Y. Bai, Z. Jia, J. Gao, L. Wu, Y. Kong, Y. Zhang and J. Xu, A novel red-emitting phosphor K 2 MgGeO 4 : Eu 3+ for WLEDs: zero-thermal quenching induced by heterovalent substitution, J. Mater. , 2022, 10 , 15957–15966, 10.1039/D2TC03500F .
- T. S. Dhapodkar, A. R. Kadam, N. Brahme and S. J. Dhoble, Efficient white light-emitting Mg 21 Ca 4 Na 4 (PO 4 ) 18 :Dy 3+ , Tb 3+ , Eu 3+ triple-doped glasses: a multipurpose glasses for WLEDs, solar cell efficiency enhancement, and smart windows applications, Mater. Today Chem. , 2022, 24 , 100938, DOI: 10.1016/j.mtchem.2022.100938 .
- P. Kantuptim, H. Fukushima, H. Kimura, D. Nakauchi, T. Kato, M. Koshimizu and T. Yanagida, VUV-and X-ray-induced properties of Lu 2 Si 2 O 7 , Y 2 Si 2 O 7 , and Gd 2 Si 2 O 7 single crystals, Sens. Mater. , 2021, 33 , 2195, DOI: 10.18494/SAM.2021.3316 .
- X. Xu, Z. Xiao, Y. Wang, Y. Yan, J. Shen, Y. Nie and F. Lai, Structure and upconversion luminescence properties of Pr 3+ -doped Y 2 Si 2 O 7 phosphor prepared by different methods, Opt. Mater. , 2022, 134 , 113191, DOI: 10.1016/j.optmat.2022.113191 .
- S. Liu, H. Cui, M. Liu, J. Chen, M. Wen, S. Li and X. Sun, Spherical red-emitting X1-Y 2 SiO 5 :Eu and α-Y 2 Si 2 O 7 :Eu phosphors with high color purity: the evolution of morphology, phase and photoluminescence upon annealing, Ceram. Int. , 2022, 48 , 8641–8652, DOI: 10.1016/j.ceramint.2021.12.075 .
- P. Kumar, S. Singh, I. Gupta, A. Dalal, V. Kumar and D. Singh, Preparation, structural and photometric properties of single-phased Gd 3 Al 5 O 12 :Tb 3+ green-emitting phosphors for solid state lighting purpose, J. Mater. Sci. Eng. B , 2023, 288 , 116189, DOI: 10.1016/j.mseb.2022.116189 .
- H. Tang, D. Yang, H. Li, M. Li and J. Zhu, A narrow-band RbBaBP 2 O 8 : Tb 3+ green phosphor with high thermal stability for backlighting display application, J. Lumin. , 2023, 257 , 119733, DOI: 10.1016/j.jlumin.2023.119733 .
- I. Gupta, S. Singh, P. Kumar, S. Bhagwan, V. Kumar and D. Singh, Structural, morphological and optoelectronic aspects of YAlO 3 :Dy 3+ doped nanocrystalline materials for NUV energized WLEDs, Curr. Appl. Phys. , 2022, 43 , 78–89, DOI: 10.1016/j.cap.2022.08.011 .
- P. Kumar, D. Singh and H. Kumar, Red-emitting Eu 3+ activated LaSr 2 AlO 5 phosphor for wLEDs: crystal structure, photoluminescence, thermal stability, Judd-Ofelt calculation and band-gap analyses, Mater. Res. Bull. , 2024, 173 , 112683, DOI: 10.1016/j.materresbull.2024.112683 .
- V. Kahlenberg, W. Wertl, D. M. Többens, R. Kaindl, P. Schuster and H. Schottenberger, Rietveld analysis and Raman spectroscopic investigations on α-Y 2 Si 2 O 7 , Z. Anorg. Allg. Chem. , 2008, 634 , 1166–1172, DOI: 10.1002/zaac.200700548 .
- J. Sokolnicki, Upconversion luminescence from Er 3+ in nanocrystalline Y 2 Si 2 O 7 :Er 3+ and Y 2 Si 2 O 7 : Yb 3+ , Er 3+ phosphors, Mater. Chem. Phys. , 2011, 131 , 306–312, DOI: 10.1016/j.matchemphys.2011.09.046 .
- P. Kumar, S. Singh, I. Gupta, A. Hooda, V. Kumar and D. Singh, Reddish-orange color tunable Sm 3+ activated Gd 3 Al 5 O 12 phosphors: crystallographic and photophysical investigation for lighting applications, J. Mol. Struct. , 2023, 1271 , 134074, DOI: 10.1016/j.molstruc.2022.134074 .
- N. Huang, G. Lu, B. Bai, H. Zhao, W. Yao, C. Cao and A. Xie, Preparation, crystal structure, and photoluminescence properties of Tb 3+ activated Lu 2 (MoO 4 ) 3 green-emitting phosphors, J. Lumin. , 2024, 269 , 120475, DOI: 10.1016/j.jlumin.2024.120475 .
- F. T. L. Muniz, M. R. Miranda, C. Morilla dos Santos and J. M. Sasaki, The Scherrer equation and the dynamical theory of X-ray diffraction, Acta Crystallogr., Sect. A: Found. Adv. , 2016, 72 , 385–390, DOI: 10.1107/S205327331600365X .
- P. Kumar, S. Singh, I. Gupta, V. Kumar and D. Singh, Structural and optical characterization of trivalent samarium-activated LaAlO 3 nanocrystalline materials for solid-state lighting, J. Mol. Struct. , 2022, 1265 , 133362, DOI: 10.1016/j.molstruc.2022.133362 .
- C. Zuo, S. Yang, Z. Cao, H. Yu and X. Wei, Excellent energy storage and hardness performance of Sr 0.7 Bi 0.2 TiO 3 ceramics fabricated by solution combustion-synthesized nanopowders, Chem. Eng. J. , 2022, 442 , 136330, DOI: 10.1016/j.cej.2022.136330 .
- I. Gupta, D. Singh, P. Kumar, S. Singh, S. Bhagwan and V. Kumar, Crystallographic and luminescence studies of Gd 2 Si 2 O 7 :Er 3+ nanomaterials for NUV energized lighting applications, J. Mol. Struct. , 2023, 1287 , 135595, DOI: 10.1016/j.molstruc.2023.135595 .
- D. Navami, G. P. Darshan, D. R. Lavanya, H. B. Premkumar, S. C. Sharma, H. Adarsha and H. Nagabhushana, Design of green emitting CaZrO 3 :Tb 3+ nanophosphor: luminescence based platform for real-time ultrasensitive detection of latent fingerprints and anti-counterfeiting applications, Opt. Mater. , 2021, 122 , 111474, DOI: 10.1016/j.optmat.2021.111474 .
- I. Gupta, D. Singh, S. Singh, P. Kumar, S. Bhagwan and V. Kumar, Phase recognition and spectroscopic characteristics of single-phase Tb 3+ doped Gd 4 Al 2 O 9 nanophosphors for NUV energized advanced photonic appliances, J. Lumin. , 2022, 252 , 119327, DOI: 10.1016/j.jlumin.2022.119327 .
- P. Kumar, D. Singh, I. Gupta, S. Singh, S. Nehra and R. Kumar, Combustion derived single phase Y 4 Al 2 O 9 :Tb 3+ nanophosphor: crystal chemistry and optical analysis for solid state lighting applications, RSC Adv. , 2023, 13 , 7752–7765, 10.1039/D3RA00735A .
- I. Gupta, D. Singh, S. Singh, P. Kumar, S. Bhagwan, V. Kumar and S. K. Chhikara, Crystallographic, morphological and photoluminescent investigations of Tb 3+ -doped YAlO 3 perovskites for lighting applications, Luminescence , 2023, 38 , 585–599, DOI: 10.1002/bio.4486 .
- P. Kumar, D. Singh and I. Gupta, Physical insights into crystal structure and optical response of green light emitting Tb 3+ activated GdSr 2 AlO 5 nanophosphors for optical displays, Mater. Res. Bull. , 2023, 167 , 112413, DOI: 10.1016/j.materresbull.2023.112413 .
- U. Berwal, V. Singh and R. Sharma, Structural and optical studies on Dy 3+ doped Gd 2 Ti 2 O 7 pyrochlore as white light emission, Ceram. Int. , 2023, 49 , 8897–8906, DOI: 10.1016/j.ceramint.2022.11.045 .
- N. Hussain, S. Rubab and V. Kumar, Spectroscopic characterizations and investigation of Judd-Ofelt intensity parameters of Dy 3+ doped Ba 2 La 8 (SiO 4 ) 6 O 2 near white light emitting phosphor, Ceram. Int. , 2023, 49 , 15341–15348, DOI: 10.1016/j.ceramint.2023.01.118 .
- P. Kumar, D. Singh, I. Gupta, S. Singh, S. Nehra and R. Kumar, Realization of warm reddish-orange light emitter single phase Y 4 Al 2 O 9 :Sm 3+ nanophosphors for indoor lighting applications, J. Lumin. , 2023, 257 , 119703, DOI: 10.1016/j.jlumin.2023.119703 .
- Y. Tian, B. Chen, B. Tian, R. Hua, J. Sun, L. Cheng, H. Zhong, X. Li, J. Zhang, Y. Zheng, T. Yu, L. Huang and Q. Meng, Concentration-dependent luminescence and energy transfer of flower-like Y 2 (MoO 4 ) 3 :Dy 3+ phosphor, J. Alloys Compd. , 2011, 509 , 6096–6101, DOI: 10.1016/j.jallcom.2011.03.034 .
- I. Gupta, S. Singh, P. Kumar, S. Bhagwan, V. Tanwar, S. Nehra and D. Singh, Synthetic, structural and optical characteristic of novel color tunable reddish-orange Gd 4 Al 2 O 9 : Sm 3+ nanocrystalline materials for solid-state photonic appliances, Inorg. Chem. Commun. , 2023, 148 , 110332, DOI: 10.1016/j.inoche.2022.110332 .
- I. Gupta, D. Singh, S. Singh, P. Kumar, S. Bhagwan and V. Kumar, Structural, optical and Judd-Ofelt analyses of Gd 2-x Eu x Si 2 O 7 nanocrystals for lighting applications, Chem. Phys. Lett. , 2023, 826 , 140670, DOI: 10.1016/j.cplett.2023.140670 .
- Y. Tian, B. Chen, R. Hua, J. Sun, L. Cheng, H. Zhong, X. Li, J. Zhang, Y. Zheng, T. Yu, L. Huang and H. Yu, Optical transition, electron-phonon coupling and fluorescent quenching of La 2 (MoO 4 ) 3 :Eu 3+ phosphor, J. Appl. Phys. , 2011, 109 , 053511, DOI: 10.1063/1.3551584 .
- J. L. Cheng, H. Tang, X. Yu, Z. Wang, X. Mi and X. Zhang, Synthesis and photoluminescence properties of NUV-excited NaBi(MoO 4 ) 2 : Sm 3+ phosphors for white light emitting diodes, Opt. Laser Technol. , 2022, 147 , 107659, DOI: 10.1016/j.optlastec.2021.107659 .
- P. Kumar, D. Singh and I. Gupta, UV excitable GdSr 2 AlO 5 :Eu 3+ red emitting nanophosphors: structure refinement, photoluminescence, Judd-Ofelt analysis and thermal stability for w-LEDs, J. Alloys Compd. , 2023, 966 , 171410, DOI: 10.1016/j.jallcom.2023.171410 .
- M. Luo, X. Sha, B. Chen, X. Zhang, H. Yu, X. Li, J. Zhang, S. Xu, Y. Cao, Y. Wang, X. Wang, Y. Zhang, D. Gao and L. Wang, Optical transition properties, internal quantum efficiencies, and temperature sensing of Er 3+ doped BaGd 2 O 4 phosphor with low maximum phonon energy, J. Am. Ceram. Soc. , 2022, 105 , 3353–3363, DOI: 10.1111/jace.18299 .
- P. Kumar, D. Singh, I. Gupta and H. Kumar, Synthesis, crystallographic structure, down shifting luminescence of Er(III) activated GdSr 2 AlO 5 nanophosphors: an efficient green emitter for solid state lighting, Mater. Sci. Semicond. Process. , 2023, 167 , 107765, DOI: 10.1016/j.mssp.2023.107765 .
- P. Kumar, D. Singh and I. Gupta, Gadolinium-based Sm 3+ activated GdSr 2 AlO 5 nanophosphor: synthesis, crystallographic and opto-electronic analysis for warm wLEDs, RSC Adv. , 2023, 13 , 7703–7718, 10.1039/D3RA00636K .
- N. Ma, W. Li, B. Devakumar, Z. Zhang and X. Huang, Utilizing energy transfer strategy to produce efficient green luminescence in Ca 2 LuHf 2 Al 3 O 12 :Ce 3+ , Tb 3+ garnet phosphors for high-quality near-UV-pumped warm-white LEDs, J. Colloid Interface Sci. , 2021, 601 , 365–377, DOI: 10.1016/j.jcis.2021.05.108 .
- S. Wang, B. Devakumar, Q. Sun, J. Liang, L. Sun and X. Huang, Highly efficient near- UV-excitable Ca 2 YHf 2 Al 3 O 12 :Ce 3+ ,Tb 3+ green-emitting garnet phosphors with potential application in high color rendering warm-white LEDs, J. Mater. Chem. C , 2020, 8 , 4408–4420, 10.1039/D0TC00130A .
- F. Liao, Y. Zhang and J. Hu, Enhancement of green emission from Ca 14 Al 10 Zn 6 O 35 :Tb 3+ phosphors via cross-relaxation energy transfer by Li + ions, J. Lumin. , 2021, 231 , 117791, DOI: 10.1016/j.jlumin.2020.117791 .
- W. W. Wu, Y. P. Zhang and J. X. Hu, Ba 3 YB 3 O 9 based phosphor ceramic plates with excellent thermal stability for wLED applications, Dalton Trans. , 2021, 50 , 5287–5300, 10.1039/D1DT00194A .
- H. Tang, D. Yang, H. Li, M. Li and J. Zhu, A narrow-band RbBaBP 2 O 8 :Tb 3+ green phosphor with high thermal stability for backlighting display application, J. Lumin. , 2023, 257 , 119733, DOI: 10.1016/j.jlumin.2023.119733 .
- K. Rawat, A. K. Vishwakarma and K. Jha, Thermally stable Ca 2 Ga 2 SiO 7 :Tb 3+ green emitting phosphor for tricolor w-LEDs application, Mater. Res. Bull. , 2020, 124 , 110750, DOI: 10.1016/j.materresbull.2019.110750 .
- F. Chi, L. Pan, B. Jiang, Z. Ji, J. Cheng, B. Wang and S. Liu, Luminescence properties of multicolor emitting La 4 GeO 8 :Tb 3+ , Eu 3+ phosphors, Ceram. Int. , 2023, 49 , 2522–2530, DOI: 10.1016/j.ceramint.2022.09.230 .
- Y. K. Zheng, T. S. Yang, Y. F. Xiang, K. Xiong, D. Yang, Z. Y. Fang, S. Q. Yang and J. Zhu, Ba 3 (ZnB 5 O 10 )PO 4 :Tb 3+ green phosphor: microwave-assisted sintering synthesis and thermally stable photoluminescence, J. Alloys Compd. , 2022, 911 , 165087, DOI: 10.1016/j.jallcom.2022.165087 .
- J. L. Song, H. Li, Y. F. Xiang and J. Zhu, Content and temperature quenching of Tb 3+ - activated Bi 3 TeBO 9 green phosphor excited by NUV/VIS light, J. Solid State Chem. , 2022, 313 , 123317, DOI: 10.1016/j.jssc.2022.123317 .
- J. F. Yang, H. Y. Wu, L. N. Song, X. X. Wang, J. C. Dong, S. C. Gan and L. C. Zou, A novel synthesis route to monodisperse Na 5 Lu 9 F 32 :Tb 3+ phosphors with superior thermal stability, J. Lumin. , 2018, 204 , 533–538, DOI: 10.1016/j.jlumin.2018.08.061 .
- S. Limbu, L. R. Singh and G. S. Okram, The effect of lithium on structural and luminescence performance of tunable light-emitting nanophosphors for white LEDs, RSC Adv. , 2020, 10 , 35619–35635, 10.1039/D0RA05433J .
- P. Kumar, D. Singh and H. Kumar, Gel-combustion synthesis, structural and optoelectronic analyses of Tb 3+ activated LaSr 2 AlO 5 nanophosphors: a green emitter for displays, Mater. Sci. Semicond. Process. , 2024, 174 , 108162, DOI: 10.1016/j.mssp.2024.108162 .
- I. Gupta, D. Singh, S. Singh, P. Kumar, S. Bhagwan and V. Kumar, Structural and photophysical measurements of Er 3+ doped Gd 4 Al 2 O 9 nanophosphors for NUV excitable solid-state lighting applications, Chem. Phys. Lett. , 2023, 814 , 140350, DOI: 10.1016/j.cplett.2023.140350 .
- P. Kumar, S. Singh, I. Gupta, K. Nehra, V. Kumar and D. Singh, Structural and luminescent behaviour of Dy(III) activated Gd 3 Al 5 O 12 nanophosphors for white-LEDs applications, Mater. Chem. Phys. , 2023, 295 , 127035, DOI: 10.1016/j.matchemphys.2022.127035 .
- B. Deng, Y. Yang, W. Chen, X. Xie, R. Yang, C. Li and R. Yu, Synthesis and characterization of thermostable Dy 3+- doped La 5 NbMo 2 O 16 yellow-emitting phosphors for w-LEDs, J. Mater. Sci.: Mater. Electron. , 2022, 33 , 23042–23053, DOI: 10.1007/s10854-022-09071-2 .
Modeling of Volumetric Synthesis of Composite with Regard the Subsequent Structurization
- Published: 27 May 2024
Cite this article

- N. V. Bukrina 1 &
- A. G. Knyazeva 1
Explore all metrics
The paper proposes a two-dimensional thermokinetic model of the composite synthesis process in the mode of dynamic thermal explosion taking into account the structurization of the synthesis product. Structurization in the model is understood as a transition from amorphous to crystalline state. The model takes into account the heating of the reactor walls by thermal radiation from the device, the temperature of which can vary at different rates. Chemical reactions are described by a summary scheme that corresponds to the synthesis of Ni 3 Al composite. The kinetic law takes into account a possible strong inhibition of the rate of the reaction with the accumulation of the synthesis product, which is typical for reactions controlled by diffusion at the particle level. The effective thermophysical properties of the components of mixture and reaction products in the reactor depend on the properties of the constituents of the initial mixture and the fraction of reaction product. The properties of the latter also depend on the degree of structurization. The structurization process is described by a special additional parameter whose evolution is modeled by a reversible reaction. It is assumed that the structurization process starts from the moment of product appearance and continues during the cooling of the system. It is shown that synthesis results in the formation of a composite containing the initial components and the reaction product with the matrix partly in the amorphous state and partly in the structured state. The dynamics of synthesis and structurization are influenced by heating conditions and reactor size.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others
Numerical Study of the Effect of the Type of Refractory Particles on Product Formation under Conditions of Bulk Synthesis

Influence of Inert Particles on the Physical Regularities of Bulk Synthesis of Composite
Numerical Study of the Two-Stage Process of Synthesis of a Composite Controlled by a Moving Source under Conditions of Conjugate Heat Exchange
S. Rogachev and A. S. Mukasyan, Combustion for Material Synthesis, CRC Press, Boca Raton (2014).
Book Google Scholar
A. A. Shavnev, M. L. Vaganova, O. Y. Sorokin, et al ., Phys. Mesomech., 23 , No. 1, 78–85 (2020); DOI https://doi.org/10.24411/1683-805X-2020-11007 .
Article Google Scholar
M. S. Antipov, P. M. Bazhin, A. S. Konstantinov, et al ., Phys. Mesomech., 26 , No. 6, 691–700 (2023); https://doi.org/10.1134/S1029959923060085 .
Yu. A. Shishkina, G. A. Baglyuk, A. A. Mamonova, and I. B. Tikhonova, Powder Metall. Met. Ceram., 52 , 154–160, (2013); https://doi.org/10.1007/s11106-013-9508-8 .
V. Filimonov, Al. Sitnikov, V. Yakovlev, et al ., Appl. Mech. Mater., 621 , 71–76 (2014); https://doi.org/10.4028/www.scientific.net/AMM.621.71 .
Q. Zhang, Sh. Fu, D. Wan, et al., J. Eur. Ceram. Soc . , 42 , No. 9, 3780–3786 (2022); https://doi.org/10.1016/j.jeurceramsoc.2022.03.019 .
E. R. Saifullin and A. G. Knyazeva, Comput. Contin. Mech., 15 , No. 1, 5–18; https://doi.org/10.7242/1999-6691/2022.15.1.1 .
S. I. Khudyayev and O. V. Ushakovskiy, Math. Models Comput. Simul., 14 , No. 7, 53–73 (2002).
Google Scholar
N. A. Belyaeva, Math. Models Comput. Simul., 18 , No. 6, 3–14 (2006).
A. M. Stolin, S. I. Khudyayev , Proc. USSR Acad. Sci., 260 , No. 5, 1180–1184 (1981).
V. E. Ovcharenko, E. N. Boyangin, A. Pshenichnikov, and T. A. Krilova, Mater. Sci. Forum, 906 , 95–100 (2017); DOI: https://doi.org/10.4028/www.scientific.net/MSF.906.95 .
N. V. Bukrina and A. G Knyazeva, High Temp. Mater. Process., 24 , No. 1, 65–79 (2020); DOI: https://doi.org/10.1615/HighTempMatProc.2020033859 .
A. P. Aldushin, T. M. Martem’yanova, A. G. Merzhanov, et al ., Combust. Explos. Shock Waves, 8 , 159–167 (1972); https://doi.org/10.1007/BF00740444 .
S. Grigor’ev and E. Z. Meilikhov, Physical Quantities . A Handbook [in Russian], Energoatomizdat, Moscow (1991).
A. Bakinovskii, A. G. Knyazeva, M. G. Krinitcyn, et al . Int. J. Self-Propag. High-Temp. Synth., 28 , No. 4, 245–255 (2019); DOI: https://doi.org/10.3103/S1061386219040034 .
Zh. Li, X. Cai, X. Ren, et al ., Combust. Sci. Technol. , 192 , No. 3, 486–492 (2020); DOI: https://doi.org/10.1080/00102202.2019.1576652 .
L. Thiers, A. S. Mukasyan, and A. Varma, Comb. Flame, 131 , Nos. 1–2, 198–209 (2002); https://doi.org/10.1016/S0010-2180(02)00402-9 .
V. E. Zarko and A. G. Knyazeva, Comb. Flame, 221 , 453–461 (2020); https://doi.org/10.1016/j.combustflame.2020.08.022 .
Download references
Author information
Authors and affiliations.
Institute of Strength Physics and Materials Science of the Siberian Branch of the Russian Academy of Sciences, Tomsk, Russia
N. V. Bukrina & A. G. Knyazeva
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to N. V. Bukrina .
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Bukrina, N.V., Knyazeva, A.G. Modeling of Volumetric Synthesis of Composite with Regard the Subsequent Structurization. Russ Phys J (2024). https://doi.org/10.1007/s11182-024-03171-8
Download citation
Received : 04 February 2024
Published : 27 May 2024
DOI : https://doi.org/10.1007/s11182-024-03171-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- high-temperature synthesis
- thermal explosion
- structurization
- mathematical modeling
- Find a journal
- Publish with us
- Track your research
Accessibility Links
- Skip to content
- Skip to search IOPscience
- Skip to Journals list
- Accessibility help
- Accessibility Help
Click here to close this panel.

As a society-owned publisher with a legacy of serving scientific communities, we are committed to offering a home to all scientifically valid and rigorously reviewed research. In doing so, we aim to accelerate the dissemination of scientific knowledge and the advancement of scholarly communications to benefit all.
Physica Scripta supports this mission and actively demonstrates our core values of inclusive publishing and trusted science . To find out more about these values and how they can help you publish your next paper with us, visit our journal scope .
Purpose-led Publishing is a coalition of three not-for-profit publishers in the field of physical sciences: AIP Publishing, the American Physical Society and IOP Publishing.
Together, as publishers that will always put purpose above profit, we have defined a set of industry standards that underpin high-quality, ethical scholarly communications.
We are proudly declaring that science is our only shareholder.
Laser-based synthesis of silver nanoparticles embedded in cellulose paper for catalytic reduction of azo dyes and SERS sensing of pesticides
Ammara Malik 1 , Shafqat Hussain 2 , Ameenah N. AlAhmadi 3 , Abdel-Haleem Abdel-Aty 4 , Zia Ur Rehman 5 and Hamza Qayyum 6
What is an Accepted Manuscript?
Article metrics
Permissions.
Get permission to re-use this article
Share this article
Author e-mails.
Author affiliations
1 Department of Physics, COMSATS University Islamabad, Dept. of Physics, COMSATS University Islamabad, Park Rd, Islamabad, Islamabad, 45550, PAKISTAN
2 Physics, PINSTECH, Nilore, Islamabad, Islamabad, Islamabad, 45650, PAKISTAN
3 Physics Department, Umm Al-Qura University, AlAwli - Makkah, Makkah, Makkah, Makkah, 24230, SAUDI ARABIA
4 University of Bisha, Department of Physics, College of Sciences, University of Bisha, Bisha 61922, Saudi Arabia, Bisha, 61922, SAUDI ARABIA
5 Pakistan Institute of Nuclear Science and Technology, Physics Division, Pakistan Institute of Nuclear Science and Technology, Nilore, Islamabad 45650, Pakistan, Islamabad, 45650, PAKISTAN
6 Department of Physics, COMSATS University Islamabad, Park Rd, Islamabad, Islamabad Capital Territory 45550, Islamabad, Islamabad, 45550, PAKISTAN
Shafqat Hussain https://orcid.org/0000-0003-3415-5847
Ameenah N. AlAhmadi https://orcid.org/0000-0001-6775-3785
Abdel-Haleem Abdel-Aty https://orcid.org/0000-0002-6763-2569
Hamza Qayyum https://orcid.org/0000-0001-5830-7265
- Received 10 March 2024
- Revised 13 May 2024
- Accepted 30 May 2024
- Accepted Manuscript online 30 May 2024
In this study, highly reactive bare silver nanoparticles (Ag NPs) are synthesized using the Pulsed Laser Ablation in Liquid (PLAL) technique. Ag NPs are then coated on the filter paper using the dip coating method. This process converts filter paper into a versatile substrate for catalysis and surface-enhanced Raman Spectroscopy (SERS) based sensing. The successful synthesis of spherical Ag NPs and their effective embedding into the filter paper was confirmed using UV-Visible absorption spectroscopy, scanning electron microscopy (SEM), and Energy Dispersive X-ray analysis (EDX). SEM images revealed that the Ag NPs were embedded in the filter paper and attached to the cellulose fibers. The use of Ag NPs embedded filter paper as a catalyst substrate for the reduction of both cationic and anionic dyes demonstrated that higher concentrations of Ag NPs on the filter paper resulted in a faster reduction. In particular, filter paper impregnated with 52 µg of Ag NPs demonstrated a complete reduction of methylene blue and methyl orange in less than a minute and 4 minutes, respectively. To demonstrate the practical sensing capability of the Ag NPs embedded filter paper, it was utilized as a SERS substrate. This enabled the detection of trace levels of Rhodamine 6G (R6G) and the pesticide molecule chlorpyrifos, demonstrating its potential real-world applications.
Export citation and abstract BibTeX RIS

During the embargo period (the 12 month period from the publication of the Version of Record of this article), the Accepted Manuscript is fully protected by copyright and cannot be reused or reposted elsewhere.
As the Version of Record of this article is going to be / has been published on a subscription basis, this Accepted Manuscript will be available for reuse under a CC BY-NC-ND 3.0 licence after the 12 month embargo period.
After the embargo period, everyone is permitted to use copy and redistribute this article for non-commercial purposes only, provided that they adhere to all the terms of the licence https://creativecommons.org/licences/by-nc-nd/3.0
Although reasonable endeavours have been taken to obtain all necessary permissions from third parties to include their copyrighted content within this article, their full citation and copyright line may not be present in this Accepted Manuscript version. Before using any content from this article, please refer to the Version of Record on IOPscience once published for full citation and copyright details, as permissions may be required. All third party content is fully copyright protected, unless specifically stated otherwise in the figure caption in the Version of Record.

IMAGES
VIDEO
COMMENTS
Combustion synthesis (also known as self-propagating high-temperature synthesis or SHS) is a simple and cost-effective method for producing a wide range of sophisticated materials, including ceramics and composites [25,26]. In conventional processing, the chemical reaction is done by long-term heating, whereas in combustion, it is done by the ...
SCS: Solution combustion synthesis (SCS) is a low cost, simple, fast and energy efficient method to produce oxides with desired morphology. The final properties of the oxide materials can be fine-tuned by controlling crucial parameters (fuel type, metal precursors, stoichiometry, pH). The growing application of printed metal oxide thin films at ...
Solution combustion is an exciting phenomenon, which involves propagation of self-sustained exothermic reactions along an aqueous or sol-gel media. This process allows for the synthesis of a variety of nanoscale materials, including oxides, metals, alloys, and sulfides. This Review focuses on the analysis of new approaches and results in the field of solution combustion synthesis (SCS ...
Combustion synthesis is an attractive and popular synthesis method for the preparation of rare-earth-activated phosphors. Advanced materials such as powders, ceramics, intermetallics, composites, and functional materials can be manufactured by this method [30].This method has gained more popularity and attention in the research world due to its advanced properties such as easy synthesis method ...
Emulsion combustion synthesis: This method developed by Takatori et al. utilizes formation of emulsion and then combustion is carried out by introducing it into the flame. After combustion, the resulting powder is collected. The emulsion combustion method is a highly scalable production method for synthesis of nanopowders with special powder ...
Solution combustion synthesis is a technique in which the main solid phase products form during exothermic redox reactions between components of a precursor, metal nitrates (oxidizer), and organic ...
Combustion synthesis is the technology that synthesizes compound materials through self-sustained combustion after ignition by exothermic chemical reactions between dissimilar substances in the powder and powdered compact. It is also known as self-propagating combustion high temperature synthesis and self-propagating high temperature synthesis.
Combustion synthesis is used to synthesize useful materials via combustion reactions. Compared to many other material synthesis approaches, combustion synthesis is furnace-free and more efficient, with negligible energy consumption, short processing times, large productivities, and low costs of production. The unique conditions of combustion ...
The combustion synthesis process is subdivided into two distinct modes of ignition. The first is termed thermal explosion, in which the whole volume of the compact is heated to the ignition ...
Combustion product of the gel with fuel-oxidant ratio of 1:1 was annealed at 1,000 °C in a flowing argon atmosphere with a soaking time of 1 h and cooled naturally to the room temperature.
The solution combustion synthesis technique is a versatile method for the production of powders used in a variety of applications. It has been used to produce hundreds of compounds, thus demonstrating its versatility, especially for the preparation of oxides, and is now a workhorse technique in materials science.
Combustion Synthesis is a technology that ca n be used to produce specia lity materials with unique properties, particularly metals a nd ceramics. These two materi al classes can be
This brief review discusses principles and some latest developments in electrically activated combustion synthesis. Processes discussed include field-activated combustion synthesis (FACS), field-activated pressure-assisted synthesis (FAPAS), reactive spark plasma sintering (R-SPS), electrothermal explosion (ETE), and electrostatic-field-activated combustion synthesis (EFACS). These processes ...
Solution Combustion Synthesis. Singanahally T. Aruna, in Concise Encyclopedia of Self-Propagating High-Temperature Synthesis, 2017 Abstract. Solution combustion synthesis (SCS) has become one of the most widely used methods for the preparation of a plethora of oxide materials. Advantages of the SCS process are that it is fast, uses relatively simple equipment, permits control over many aspects ...
A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Combustion reactions must involve O2 O 2 as one reactant. The combustion of hydrogen gas produces water vapor: 2H2(g) +O2(g) → 2H2O(g) 2 H 2 ( g) + O 2 ( g) → 2 H 2 O ( g) Notice that this reaction also qualifies ...
The solution combustion synthesis is a process intensification (PI) approach to synthesize metal oxides. 46,48-51 SCS-synthesized metal oxides often show more advanced performance than their calcined counterparts. 52 In general, the SCS of metal oxides is a possible combination of solid- and gas-state reactions between the precursor powders ...
Solution combustion synthesis. Solution combustion synthesis (SCS) is a popular method for the preparation of a wide variety of materials due to its simplicity, broad range of applicability and the possibility of easily obtaining products in the desired composition. This method has been widely used for the development of oxide powder materials ...
combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the form of flame.The rate or speed at which the reactants combine is high, in part because of the nature of the chemical reaction itself and in part because more energy is generated than can escape into the surrounding medium, with the result that the ...
Nanosized β-SiC powder was prepared by combustion synthesis on a large scale, which was carried out in a N2 atmosphere with silicon powder and carbon black as the reactants. Mechanical activation played an important role in enhancing the reactivity of the reactants, and a higher ball/charge weight ratio and a prolonged activation time were favorable for complete reaction.
2.1 Synthesis and characterization Employing ecologically-sound gel-combustion synthesis, triclinic symmetry type Y 2− x Si 2 O 7: x Tb 3+ phosphors with variable dopant ion content as x = 0.01-0.06 mole were developed, yielding a product that exhibits excellent crystallinity and homogeneity .
Volume combustion synthesis. Volume combustion synthesis (VCS) is method of chemical synthesis in which the reactants are heated uniformly in a controlled manner until a reaction ignites throughout the volume of the reaction chamber. [1] The VCS mode is typically used for weakly exothermic reactions that require preheating prior to ignition.
Understanding the catalytic mechanism of support effects is crucial for tunable catalysis. Here, Zhang et al. propose a redox-based kinetics strategy by decoupling the reduction step and oxidation step to highlight the important roles of low-valence Cu species and oxygen vacancies in Cu catalysts for catalytic hydrogen combustion.
The combustion of fossil fuels has led to an unprecedentedly high concentration of carbon dioxide in the atmosphere, triggering issues such as global warming, ocean acidification, and glacier melting. ... Synthesis gas prodn. through the catalytic reverse water-gas shift (RWGS) reaction is an attractive option for the conversion of CO2 to fuels ...
It is shown that synthesis results in the formation of a composite containing the initial components and the reaction product with the matrix partly in the amorphous state and partly in the structured state. The dynamics of synthesis and structurization are influenced by heating conditions and reactor size. ... Combustion for Material Synthesis ...
The successful synthesis of spherical Ag NPs and their effective embedding into the filter paper was confirmed using UV-Visible absorption spectroscopy, scanning electron microscopy (SEM), and Energy Dispersive X-ray analysis (EDX). SEM images revealed that the Ag NPs were embedded in the filter paper and attached to the cellulose fibers.