
Chemistry Steps

General Chemistry
- Chemical Equilibrium
This equilibrium practice problem set includes questions on writing the equilibrium constant of given chemical reactions, determining the value of the equilibrium constant based on the concentrations and partial pressures of gases , deriving a new expression for an equilibrium constant from separate reactions , converting between K c and K p , c alculating the quotient and determining the course of the reaction, c alculations of concentrations based on the quotient and equilibrium constant, as well as working on equilibrium reactions based on the Le Châtelier’s principle .
The links to the corresponding articles are provided herein:
- Equilibrium Constant
- K p and Partial Pressure
- K p and K c Relationship
- K Changes with Chemical Equation
- Equilibrium Constant K from Two Reactions
- Reaction Quotient – Q
- ICE Table – Calculating Equilibrium Concentrations
- ICE Table Practice Problems
- Le Châtelier’s principle
- Le Châtelier’s principle Practice Problems
- Chemical Equilibrium Practice Problems
Write the equilibrium expression ( K ) for each of the following reactions.
A) N 2 ( g ) + O 2 ( g ) ⇆ 2NO( g )
B) 2NOCl( g ) ⇆ 2NO( g ) + Cl 2 ( g )
C) CH 3 COOH( aq ) ⇆ H + ( aq ) + CH 3 COO 2 – ( aq )
D) 2CuS( s ) + 3O 2 ( g ) ⇆ 2CuO( s ) + 2SO 2 ( g )
E) CO 2 ( g ) + 3H 2 ( g ) ⇆ CH 3 OH( g ) + H 2 O( g )
F) 2SO 3 ( g ) ⇆ 2SO 2 ( g ) + O 2 ( g )
G) CaCO 3 ( s ) ⇆ CaO( s ) + CO 2 ( g )
H) Fe 2 O 3 ( s ) + 3CO( g ) ⇆ 2Fe( s ) + 3CO 2 ( g )
I) 4HCl( g ) + O 2 ( g ) ⇆ 2Cl 2 ( g ) + 2H 2 O( g )
J) CO 3 2- ( aq ) + H 2 O( l ) ⇆ HCO 3 – ( aq ) + OH – ( aq )
K) P 4 (s) + 5O 2 (g) ⇆ P 4 O 10 (s)
l) 2H + ( aq ) + Zn(s) ⇆ H 2 ( g ) + Zn 2+ ( aq )
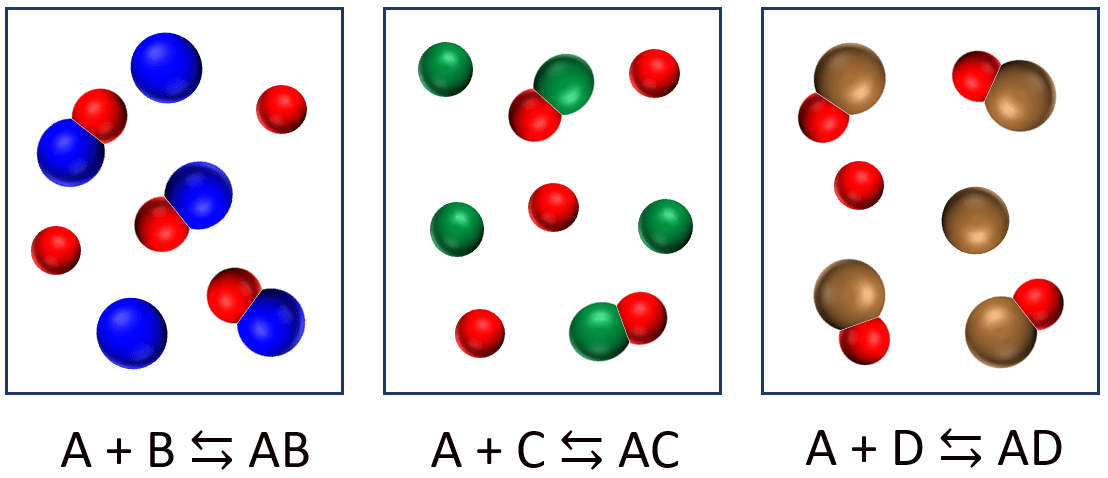
Tree different reactions are represented in the diagram below. The reaction equations can be written as A + X ⇆ AX (X = B, C, or D).
(a) Which reaction has the largest equilibrium constant? (b) Which reaction has the smallest equilibrium constant?
The following graph represents an initial mixture of N 2 and H 2 at high temperature and pressure:
The gases react to form ammonia gas (NH 3 ) as represented by the following concentration profile:
a) Label each plot on the graph as H 2 , N 2 , or NH 3 , and explain your answers.
b) What information do the relative shapes of the plots tell us?
c) At what time is equilibrium reached?
At a particular temperature, it is determined for the reaction
2NO( g ) + 2H 2 ( g ) ⇆ N 2 ( g ) + 2H 2 O( g )
that at equilibrium, the concentrations are as follows: [NO( g )] = 3.2 x 10 -3 M , [H 2 ( g )] = 6.7 x 10 -6 M, [N 2 ( g )] = 4.8 x 10 -2 M, and [H 2 O( g )] = 2.4 x 10 -2 M. What is the value of the equilibrium constant K for the reaction at this temperature?
Given the equilibrium concentrations, calculate the value of the equilibrium constant K for the reaction between CO 2 and H 2 that produces methanol and water at high temperature.
CO 2 ( g ) + 3H 2 ( g ) ⇆ CH 3 OH( g ) + H 2 O( g )
[CO 2 ] = 0.061 M , [H 2 ] = 0.079 M , [CH 3 OH] = 4.7 x 10 2 M , and [H 2 O] = 5.7 x 10 4 M Calculate the value of K for the reaction.
The reaction for converting methane to acetylene has an equilibrium constant of K = 0.154 at 2000 K.
2CH 4 (g) ⇆ C 2 H 2 (g) + 3H 2 (g)
Calculate the equilibrium constant for this process if the reaction is represented as follows:
a) CH 4 (g) ⇆ 1/2C 2 H 2 (g) + 3/2H 2 (g)
b) 4CH 4 (g) ⇆ 2C 2 H 2 (g) + 6H 2 (g)
c) 6CH 4 (g) ⇆ 3C 2 H 2 (g) + 9H 2 (g)
The following reaction has an equilibrium constant of K p = 4.42 x 10 -5 at 298 K:
CH 3 OH( g ) ⇆ CO ( g ) + 2H 2 ( g )
a) CO( g ) + 2H 2 ( g ) ⇆ CH 3 OH( g )
b) 2CH 3 OH( g ) ⇆ 2CO( g ) + 4H 2 ( g )
c) 1/2CH 3 OH( g ) ⇆ 1/2CO( g ) + H 2 ( g )
The equilibrium constant values for the reactions below were determined at a certain temperature:
S( s ) + O 2 ( g ) ⇆ SO 2 ( g ) K a = 3.2 x 10 45
2S( s ) + 3O 2 ( g ) ⇆ 2SO 3 ( g ) K b = 3.2 x 10 124
Using these data, determine the equilibrium constant K c for the following reaction:
2SO 2 ( g ) + O 2 ( g ) ⇆ 2SO 3 ( g ) K c = ?
2NO(g) ⇆ N 2 (g) + O 2 (g) K a = 3.8 x 10 32
NO( g ) + 1/2Cl 2 ( g ) ⇆ NOCl( g ) K b = 6.4
1/2N 2 (g) + 1/2O 2 (g) + 1/2Cl 2 ( g ) ⇆ NOCl( g ) Kc = ?
The following equilibrium pressures were observed at a certain temperature for the Haber process
3H 2 ( g ) + N 2 ( g ) ⇆ 2NH 3 ( g )
P( NH 3 ) = 5.2 x 10 9 atm
P( N 2 ) = 6.1 x 10 2 atm
P( H 2 ) = 4.7 x 10 3 atm
Calculate the value for the equilibrium constant K p at this temperature.
Consider the following reactions:
1) H 2 ( g ) + I 2 ( g ) ⇆ 2HI( g )
2) H 2 ( g ) + I 2 ( s ) ⇆ 2HI( g )
In which reaction are the K and K p equal?
At 300 K, the equilibrium concentrations for the following reaction are [CH 3 OH] = 0.240 M , [CO] = 0.350 M , and [H 2 ] = 1.65 M for the reaction
CH 3 OH( g ) ⇆ CO( g ) + 2H 2 ( g )
Calculate K p at this temperature.
Given the equilibrium constant, calculate K p for each of the following reactions at 298 K.
a) N 2 O 4 ( g ) ⇆ 2NO 2 ( g ) K c = 4.6 x 10 -4
b) 3H 2 ( g ) + N 2 ( g ) ⇆ 2NH 3 ( g ) K c = 6.7 x 10 9
c) H 2 ( g ) + B 2 ( g ) ⇆ 2HBr( g ) K c = 5.20 x 10 18
Calculate the K c for each reaction at 298 K.
a) CO( g ) + Cl 2 ( g ) ⇆ COCl 2 ( g ) K p = 5.3 x 10 6
b) CH 4 ( g ) + H 2 O( g ) ⇆ CO( g ) + 3H 2 ( g ) K p = 7.7 x 10 8
c) 2SO 2 ( g ) + O 2 ( g ) ⇆ 2SO 3 ( g ) K p = 6.26 x 10 5
Consider the reaction between nitrogen and hydrogen gases:
Using the data given in the table, complete the missing numbers assuming that all concentrations are measured at equilibrium.
Consider the following reaction at 298 K:
2NO( g ) + Cl 2 ( g ) ⇆ 2NOCl( g ) K p = 31.6
In a reaction mixture at equilibrium, the partial pressure of NO is 128 torr and that of Cl 2 is 176 torr. Calculate the partial pressure of NOCl at equilibrium.
The equilibrium constant for the following reaction at 600 o C is determined to be K c = 0.495:
H 2 O( g ) + CO( g ) ⇆ H 2 ( g ) + CO 2 ( g )
Calculate the number of H 2 moles that are present at equilibrium if a mixture of 0.400 mole CO and 0.500 mole H 2 O is heated to 600°C in a 10.0-L container.
The equilibrium constant K c for the following reaction at 800°C is 3.74 x 10 5
H 2 ( g ) + I 2 ( g ) ⇆ 2HI( g )
If 6.25 moles of HI were initially added to a 15.0-L empty vessel, what would the concentrations of H 2 , I 2 , and HI be at equilibrium.
The equilibrium constant for the following reaction at 700 K is K p = 6.7 x 10 -3
CO( g ) + 2H 2 ( g ) ⇆ CH 3 OH( g )
A reaction mixture contains 0.248 atm of H 2 , 0.085 atm of CO, and 0.598 atm of CH 3 OH. Is the reaction mixture at equilibrium? If not, in what direction will the reaction proceed?
For the reaction shown below, K c = 0.654 at 600 K.
N 2 O 4 ( g ) ⇆ 2NO 2 ( g )
If initially, 0.0600 M of N 2 O 4 are present in the reaction vessel, what are the equilibrium concentrations of the gases at 600 K?
For the reaction shown below, K c = 255 at 800 K.
PCl 3 ( g ) + Cl 2 ( g ) ⇆ PCl 5 ( g )
If a reaction mixture initially contains 0.3500 M PCl 3 and 0.375 M Cl 2 at 800 K, what are the equilibrium concentrations of all the species in the mixture?
Consider the following reaction characterized with K p = 2.34 x 10 -4 at 250 K:
I 2 ( g ) + Cl 2 ( g ) ⇆ 2ICl( g )
A reaction mixture initially contains I 2 with partial pressure of 655 torr and Cl 2 with partial pressure of 864 torr at 250 K. Calculate the equilibrium partial pressure of ICl.
Consider the following reversible reaction:
POCl 3 (g) ⇌ POCl(g) + Cl 2 (g) K c = 0.650
The following initial amounts of reactants and products were mixed: [POCl 3 ] = 0.650 M, [POCl] = 0.450 M, and [Cl 2 ] = 0.250 M.
Calculate the reaction quotient, Q c, and determine the equilibrium concentration of POCl?
Consider the following equilibrium:
Initially, there are 0.15 moles of NO and 0.25 moles of H 2 , in a 10.0-L container. If there are 0.056 moles of NO at equilibrium, how many moles of N 2 are present at equilibrium?
2NOCl( g ) ⇆ 2NO( g ) + Cl 2 ( g )
2.00 mole of pure NOCl and 1.65 mole of pure Cl 2 are placed in a 1.00-L container. Calculate the equilibrium concentration of NO( g ) considering that with K = 2.4 x 10 –6 .
Consider the following equilibrium process
2SO 3 ( g ) ⇆ 2SO 2 ( g ) + O 2 ( g ) Δ H ° = 2198 kJ/mol
How will the concentrations of SO 2 , O 2 , and SO 3 be affected in each scenario?
(a) the temperature is increased
(b) the pressure is decreased by increasing the volume of the container
(c) the concentration of O 2 is increased
(d) a is added catalyst
(e) an inert gas is added at constant volume
Suppose you need to increase the amount of C 3 H 6 Cl 2 produced in the following exothermic reaction:
C 3 H 6 ( g ) + Cl 2 ( g ) ⇆ C 3 H 6 Cl 2 ( g )
Which of the following strategies will work once the reaction mixture reaches equilibrium?
a) decreasing the reaction volume
b) removing C 3 H 6 Cl 2 from the reaction mixture as it forms
c) adding a catalyst
d) adding Cl 2
e) increasing the temperature
Predict the shift in the equilibrium position that will occur for each of the following reactions at equilibrium when the volume of the reaction container is increased.
1) PCl 3 ( g ) + Cl 2 ( g ) ⇆ PCl 5 ( g )
2) 2NBr 3 ( g ) ⇆ N 2 ( g ) + 3Br 2 ( g )
3) CO( g ) + Cl 2 ( g ) ⇆ COCl 2 ( g )
4) H 2 ( g ) + B 2 ( g ) ⇆ 2HBr( g )
5) MgCO 3 ( s ) ⇆ MgO( s ) + CO 2 ( g )
Consider the following equilibrium process for the commercial production of hydrogen:
CO( g ) + H 2 O( g ) ⇆ CO 2 ( g ) + H 2 ( g ) Δ H ° = +42 kJ/mol
Predict the direction of the shift in equilibrium when
(a) the temperature is raised.
(b) more CO gas is added to the reaction mixture.
(c) some CO 2 is removed from the mixture.
(d) the pressure on the gases is increased by changing the volume of the container.
(e) a catalyst is added to the reaction mixture
Consider this reaction at equilibrium:
N 2 + O 2 ( g ) ⇆ 2NO( g )
Predict whether the reaction will shift left, shift right, or remain unchanged after each disturbance.
a) NO is added to the reaction mixture.
b) N 2 is added to the reaction mixture.
c) NO is removed from the reaction mixture.
2 thoughts on “Chemical Equilibrium Practice Problems”
it is very helpful
Glad to hear that. Thanks.
Leave a Comment Cancel reply
Notify me of followup comments via e-mail. You can also subscribe without commenting.
Log In | Join AACT | Renew Membership
Save Your Favorite AACT Resources! ×
Log in or join now to start building your personalized "My Favorites" page. Easily save all the resources you love by logging in and clicking on the star icon next to any resource title.
- AACT member benefits »
- Forgot User Name or Password?
Equilibrium Unit Plan Mark as Favorite (21 Favorites)
LESSON PLAN in Le Châtelier's Principle , Establishing Equilibrium , Equilibrium Constants , Reaction Quotient , Unit Plans . Last updated December 22, 2020.
The AACT high school classroom resource library and multimedia collection has everything you need to put together a unit plan for your classroom: lessons, activities, labs, projects, videos, simulations, and animations. We constructed a unit plan using AACT resources that is designed to teach equilibrium to your students.
Grade Level
High School
By the end of this unit, students should be able to
- Recognize when equilibrium is reached.
- Recognize that at equilibrium the rate of the forward and reverse reactions are equal.
- Recognize that the concentration of products and reactants remain constant at equilibrium.
- Understand that equilibrium can be approached from many starting points and both directions.
- Understand the concept of dynamic equilibrium.
- Understand the meaning of K, and what its value signifies.
- Know how to use Q to see if reaction conditions have reached equilibrium.
- Compare K to Q in order to predict which direction a system must proceed to reach equilibrium.
- Perform calculations involving K and manipulations of K.
- Use a graph to recognize the establishment of chemical equilibrium.
- Understand how equilibrium shifts.
- Predict what will happen if a system at equilibrium is disturbed.
- Recognize that a change in concentration does not change K but a change in temperature does.
- Qualitatively assess how a stress on a system changes Q.
- Qualitatively compare Q vs K to determine how a system will respond to a stress.
- When appropriate, use Le Chatelier’s principle to explain how a system will respond to a stress.
- Understand that a chemical change is taking place.
- Recognize that an indicator causes the color change.
- Realize that an acid base reaction is taking place.
- Recognize the limiting and excess reactants during the reaction.
- Determine how changing pressure can affect the equilibrium shift of a chemical reaction.
- Analyze how changing temperature can affect the equilibrium shift of a chemical reaction.
- Interpret how changing concentration can affect the equilibrium shift of a chemical reaction.
- Explain the molecular changes that occur when an equilibrium system is disturbed
- Relate the symbolic representation to the particle representation for changes in an equilibrium system.
Chemistry Topics
This unit supports students’ understanding of
- Chemical Equilibrium
- Establishing Equilibrium
- Reversible Reactions
- Equilibrium Constant (K)
- Reaction Quotient (Q)
- Le Chatelier’s Principle
- Acid/base neutralization reaction
- Limiting Reactant
- Reaction Rates
- Temperature
- Concentration
Teacher Preparation : See individual resources.
Lesson : 8-12 class periods, depending on class level.
- Refer to the materials list given with each individual activity.
- Refer to the safety instructions given with each individual activity.
Teacher Notes
- Some of the resources in this unit plan were written for AP Chemistry. However, most can easily be adapted for an on-level or honors class.
- The activities shown below are listed in the order that they should be completed.
- The teacher notes, student handouts, and additional materials can be accessed on the page for each individual activity.
- Please note that most of these resources are AACT member benefits.
Classroom Resources
- Start the unit with an introduction by having your students collect data that models chemical equilibrium with the Equilibrium Introduction classroom resource. By the end of this activity, students should be able to recognize when equilibrium is reached and that at equilibrium the rate of the forward and reverse reactions are equal. They will also understand that equilibrium can be approached from many starting points and from both directions. Additionally, they will learn that the concentration of products and reactants remain constant at equilibrium. This lesson is based on the article Equilibrium: A Teaching/Learning Activity by Audrey H. Wilson from the Journal of Chemical Education , Vol. 75, No. 9, September 1998.
- Then use the lesson plan, Discovering Equilibrium , to allow your students to manipulate sets of given conditions to discover what equilibrium is, and how equilibrium is established from different starting conditions. By the end of this lesson, students will be able to understand the concept of dynamic equilibrium, the meaning of K, compare K to Q in order to predict which direction a system must proceed to reach equilibrium, perform calculations involving K, and use a graph to recognize the establishment of chemical equilibrium. Your AP Chemistry students can refer back to this activity as the foundation framework for the rest of Essential Knowledge 6.A, 6.B.1 and 6.B.2.
- Another hands-on activity to help students visualize equilibrium is the Dynamic Equilibrium Simulation . In this activity, students explore equilibrium using paper clips to mimic a chemical reaction. By the end of this activity, students will be able to better understand what it means for a system to reach equilibrium. This lesson includes alignment with the AP Chemistry Big Ideas.
- Help your students to further visualize equilibrium with our Equilibrium Animation which shows the dissociation of water and its interaction with a piece of chalk (calcium carbonate) at the particle level.
- Use our Le Châtelier’s Principle demonstration to introduce this topic and allow your students to witness how different stresses cause the equilibrium of a system to shift. This will allow students to better understand how equilibrium shifts and to recognize that a change in concentration does not change K but a change in temperature does. It will also help them understand how to use Q to see if reaction conditions have reached equilibrium. This lesson includes alignment with the AP Chemistry Big Ideas.
- A green alternative to this demonstration would be to use the lab, A Greener Le Châtelier's Principle to explore the concept using non-toxic materials, while still visualizing the equilibrium shifts through color changes. Traditional equilibrium experiments and Le Châtelier’s Principle are observed using chemicals, such as cobalt (IV) chloride and iron (III) thiocyanate, which undergo color changes as the equilibrium position shifts. While these reactions are very effective, they utilize reagents that are toxic.
- The AACT simulation, Predicting Shifts in Equilibrium: Q vs K allows students to take a 15 question quiz to help them reinforce their understanding of shifts in equilibrium through the comparison of Q and K. Each quiz question has two parts. The first part requires the student to calculate the value of the reaction quotient, Q. The second portion of the question requires students to compare the value of Q to the equilibrium constant, K, and to predict which way the reaction will shift to reach equilibrium. The simulation includes five different reactions which each have three scenarios: Q > K, Q = K, and Q < K.
- Part two of the lesson plan uses the demonstration, Milk of Magnesia Magic to connect the concept of equilibrium to indicators, acid/base chemistry, limiting reactants, and reaction rates. In this demonstration, students observe a color change in a milk of magnesia solution as vinegar is added. By the end of this demonstration, students should be able to understand that a chemical change is taking place, recognize that an indicator causes a color change, realize that an acid base reaction occurs, identify the limiting and excess reactants during the reaction, and apply Le Châtelier’s principle to explain the color change. This lesson includes alignment with the AP Chemistry Big Ideas.
- Help your students can gain a better understanding of equilibrium and how a reaction progresses over time with the activity, Equilibrium Particulate View . They will learn how to relate the equilibrium constant to the amount of products and reactants present at equilibrium. Then use the activity, Le Châtelier's Principle Particulate View to provide students with more practice predicting how a stress to a reaction system will shift the equilibrium.
- Use the Le Châtelier’s Soda lab next to allow your students to observe how the equilibrium of a chemical reaction is affected when a change in pressure, temperature, and concentration is applied to the system. By the end of this lab, students should be able to determine how changing pressure, temperature, or concentration can affect the equilibrium shift of a chemical reaction. This lesson includes alignment with the AP Chemistry Big Ideas and NGSS performance expectations.
- Students often form misconceptions about equilibrium and what happens at the particle level during the process. Use the activity, Equilibriumin a Beaker to help them model equilibrium reactions using plastic chips to represent atoms. The goal of the lesson is to connect the symbolic model of an equilibrium reaction to its particle model. Read more about this activity in an article from the November 2018 issue of Chemistry Solutions . This lesson includes alignment with the AP Chemistry Big Ideas and NGSS performance expectations.
- Students understand the connections between the equilibrium constant (K) and the reaction quotient (Q) as well as how they determine the favorability of a reaction with the lesson plan, Making Connections in Kinetics, Equilibrium, and Thermochemistry . Additionally students will be able to determine if a reaction is kinetically favored or thermodynamically favored. This lesson includes alignment with the AP Chemistry Big Ideas.
- In the Kinetics and Equilibrium lab , students investigate the reaction of the hydrogen sulfite ion (HSO 3 - ) and the iodate ion (IO 3 - ) to determine the effect that changing concentration and temperature has on the reaction rate. This lesson includes alignment with the AP Chemistry Big Ideas and NGSS performance expectations.
Find what you need to study
7.1 Introduction to Equilibrium
6 min read • january 21, 2023
Dylan Black
Attend a live cram event
Review all units live with expert teachers & students
Reversible Reactions
Welcome to the introduction to equilibrium ! Equilibrium is an incredibly important topic both for the AP exam and for chemistry in general. Equilibrium takes what you know about reactions from unit 4 and unit 5 and expands it into the realm of reversible reactions . That is reactions that can go both forward (reactants turning into products) and backward (products turning into reactants) at the same time. This unit will focus on describing how reactions can do this and to what extent they do.
Image From GIPHY
Many observable processes in nature are reversible, such as:
The evaporation and condensation of water; H₂O(l) ⇌ H₂O(g)
The dissolution and precipitation of a salt; NH₄Cl(s) ⇌ NH₄⁺(aq) + Cl⁻(aq)
Acid-base reactions; H₂CO₃ + HCO₃⁻ ⇌ H₂O + CO₂
Redox reactions; Zn + Cu²⁺ ⇌ Zn²⁺ + Cu
All of these reactions can go back and forth, forming both products and reactants.
Arrows in Chemistry
The concept of reversible reactions introduces a new arrow that you can use: the double arrow (⇌). This symbol is used in a chemical equation of a reversible reaction and also indicates a system in equilibrium , which we will discuss soon.
When you are completing the free-response questions on the AP Chemistry exam, make sure you are using the right arrow:
The single arrow shows a reaction proceeding in one direction (→)
The double arrow shows a reversible reaction at equilibrium (⇌)
What Is Equilibrium?
Let’s begin by asking the question, “what even is equilibrium ?” In most textbooks, equilibrium is defined as the point in a reaction where the rate of the forward reaction is equal to the rate of the reverse reaction . Let’s break this down a bit.
Suppose we have a reaction in which A, a reactant, is turned into B, a product. As concentrations of A decrease, the rate of reaction A → B will decrease. However, there will be a secondary reaction occurring simultaneously, that being the reaction B → A as concentrations of B build up. In many instances, this second reaction is incredibly slow and does not happen much. However, in many other instances, this second reaction is actually the driving reaction. We’ll talk a bit more about how to measure how “far forward” a reaction goes by using equilibrium constants .
Going back to arrows, we can represent the two reactions by using a double arrow on the reaction A → B to describe that this reaction is reversible : A ⇌ B. This notation tells us that this reaction will settle at an equilibrium . Now that we’ve covered that, we’re ready to dive more into how the rates of these two reactions relate.
Representing Equilibrium Graphically
As we mentioned before, equilibrium is the point at which the forward reaction and reverse reactions continue at the same rate. At this point, the production of products equals the production of reactants and so concentrations of the two remain the same. The below graphic shows us what happens at equilibrium :
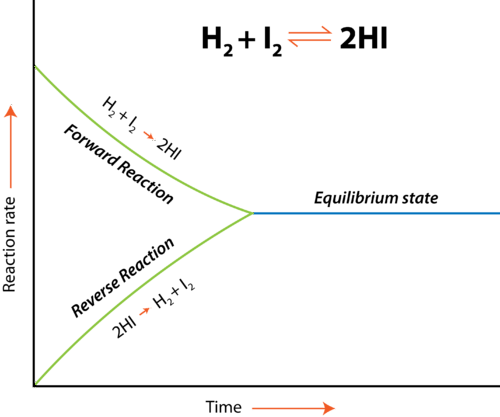
Image From LibreTexts
In this example, we have the equilibrium reaction H₂+I₂ ⇌ 2HI. We have two reactions each occurring at different rates, one being the forward reaction and the other being the reverse reaction . However, note the point at which the two become the same. That area is called the equilibrium state . At this point, the rate at which products are created equals the rate at which reactants are created and therefore the concentrations present in your reaction are equal. It’s important to note that equilibrium doesn’t mean the concentrations themselves are the same, but rather just the rates of each reaction.
A Closed System
Equilibrium can only occur in a closed system , which is a system that does not exchange matter or energy with its surroundings. This is because in a closed system , the concentrations of reactants and products are fixed and cannot change. The only way for the concentrations to change is through the forward and reverse reactions. As the forward and reverse reactions proceed, the concentrations of the reactants and products change until they reach a state where the rate of the forward reaction is equal to the rate of the reverse reaction .
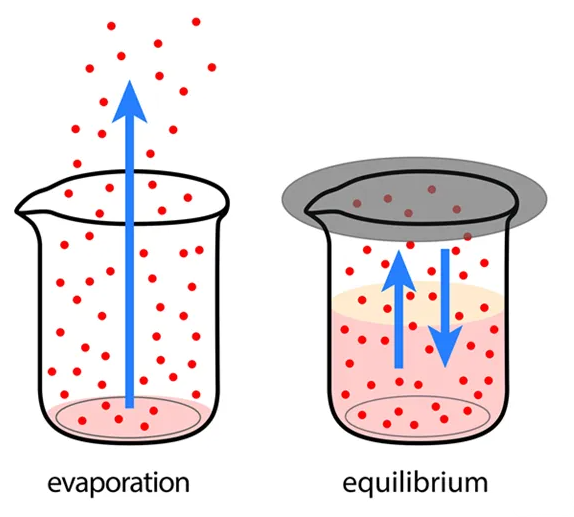
Image Courtesy of Shalom Education
In an open system , matter and/or energy can be exchanged with the surroundings, which can affect the concentrations of the reactants and products, making it impossible for the system to reach equilibrium .
Measuring Equilibrium: Kc
Now that we understand what equilibrium means in terms of reaction rate, let’s discuss how we actually measure how “far forward” a reaction goes. When doing calculations with equilibrium , we use the equilibrium constant , K for a reaction. K describes the ratio between the rate of the forward reaction and the rate of the reverse reaction , as well as the ratio between the equilibrium concentrations of products and reactants. If we have the reversible reaction A ⇌ B, we can describe how far forward this reaction goes before equilibrium by writing out the rate laws for the forward and reverse reactions:
A → B ⇒ R = k₁[A]
B → A ⇒ R = k₂[B]
At equilibrium , k₁[A] = k₂[B]. We can describe the ratio k₁/k₂ as a representation of how far forward the product-creating reaction goes vs. how far forward the reactant-creating reaction goes. We can also represent this value as [B]/[A] where [B] and [A] are the concentrations of B and A at equilibrium . This value is known as Kc or sometimes simply K . The formula for K is as follows:
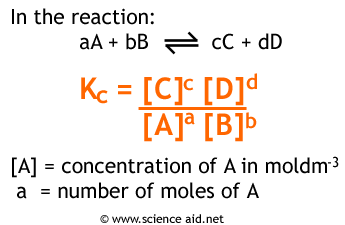
Image From ScienceAid
K is a unitless value that changes based on temperature (since k1 and k2 change based on temperature ). However, the initial reactant and product concentrations do NOT change K. No matter where you start a reaction (assuming constant temperature), you will find that K remains constant. This will also be true for KP which we’ll discuss next.
Measuring Equilibrium: Kp
There is another way of measuring equilibrium , specifically when discussing gasses. Like we use concentrations, we can also use partial pressures to describe how many products were formed and how many reactants remain at equilibrium . This value is known as Kp . Kp is the same calculation as Kc just with partial pressures instead of concentrations.
In this study guide, we're focusing on what Kc and Kp conceptually represent, but later in this unit , we'll be doing some calculations with them! For now, make sure you’re comfortable understanding what they mean in a non-mathematical way as well like we’ve described here.
There is also a relationship between Kp and Kc that is described by the formula Kp= Kc(RT)^(Δn) where Δn denotes the difference in stoichiometric coefficients of the products and the stoichiometric coefficients of the reactants. This won’t be too common on the exam (in fact it probably won’t show up), but it’s nice to know!
Common Misconception: Equilibrium Stopped Reaction
Let’s close this guide out with a common misconception that students have about equilibrium that hinders their understanding of equilibrium as a balancing of rates. Many students believe that at equilibrium the reaction stops completely. This could not be further from the truth! In fact, reversible reactions never “end”. Equilibrium is a dynamic process in which reactants are still turned into products and vice versa, not the point at which a reaction stops. What this means is that the reactants and products are constantly interconverting. The system is constantly active, but there is no observable change because concentrations remain constant.
Key Terms to Review ( 12 )
Closed System
Double Arrow
Dynamic Process
Equilibrium
Equilibrium Constant (K)
Equilibrium State
Forward Reaction
Open System
Partial Pressures
Reverse Reaction

Stay Connected
© 2024 Fiveable Inc. All rights reserved.
AP® and SAT® are trademarks registered by the College Board, which is not affiliated with, and does not endorse this website.

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

V: Chemical Equilibrium
- Last updated
- Save as PDF
- Page ID 43440
- 5.1: The Nature of Chemical Equilibrium At equilibrium, the forward and reverse reactions of a system proceed at equal rates. Chemical equilibrium is a dynamic process consisting of forward and reverse reactions that proceed at equal rates. At equilibrium, the composition of the system no longer changes with time. The composition of an equilibrium mixture is independent of the direction from which equilibrium is approached.
- 5.2: The Empirical Law of Mass Action The law of mass action describes a system at equilibrium in terms of the concentrations of the products and the reactants. For a system involving one or more gases, either the molar concentrations of the gases or their partial pressures can be used. The equilibrium constant can be defined in terms of forward and reverse rate constants: \(K=k_f/k_r\). The equilibrium constant expression (law of mass action): \[K=\dfrac{[C]^c[D]^d}{[A]^a[B]^b} \]
- 5.3: Thermodynamic Description of the Equilibrium State In this unit we introduce a new thermodynamic function, the free energy, which turns out to be the single most useful criterion for predicting the direction of a chemical reaction and the composition of the system at equilibrium. As we will explain near the bottom of this page, the term "free energy", although still widely used, is rather misleading, so we will often refer to it as "Gibbs energy."
- 5.4: The Law of Mass Action for Related and Simultaneous Equilibria Chemists frequently need to know the equilibrium constant for a reaction that has not been previously studied. In such cases, the desired reaction can often be written as the sum of other reactions for which the equilibrium constants are known. The equilibrium constant for the unknown reaction can then be calculated from the tabulated values for the other reactions.
- 5.5: Gas-Phase and Heterogeneous Reaction Equilibria An equilibrated system that contains products and reactants in a single phase is a homogeneous equilibrium; a system whose reactants, products, or both are in more than one phase is a heterogeneous equilibrium.
- 5.6: The Direction of Change in Chemical Reactions: Empirical Description Systems at equilibrium can be disturbed by changes to temperature, concentration, and, in some cases, volume and pressure; volume and pressure changes will disturb equilibrium if the number of moles of gas is different on the reactant and product sides of the reaction. The system's response to these disturbances is described by Le Châtelier's principle: The system will respond in a way that counteracts the disturbance. Not all changes to the system result in a disturbance of the equilibrium.
- 5.7: The Direction of Change in Chemical Reactions - Thermodynamic Explanation A negative value for ΔG indicates a spontaneous process; a positive ΔG indicates a nonspontaneous process; and a ΔG of zero indicates that the system is at equilibrium. A number of approaches to the computation of free energy changes are possible.
- 5.8: Distribution between Immiscible Phases - Extraction and Separation Processes A partitioning of a compound exist between a mixture of two immiscible phases at equilibrium, which is a measure of the difference in solubility of the compound in these two phases. If one of the solvents is a gas and the other a liquid, the "gas/liquid partition coefficient" is the same as the dimensionless form of the Henry's law constant. A solute can partition when one or both solvents is a solid (e.g., solid solution).
- 5.E: Chemical Equilibrium (Exercises) These are homework exercises to accompany the Textmap created for "Principles of Modern Chemistry" by Oxtoby et al.
chemistry equilibrium
All Formats
Resource types, all resource types.
- Rating Count
- Price (Ascending)
- Price (Descending)
- Most Recent
Chemistry equilibrium

Equilibrium and Solutions Chemistry Homework Page Unit Bundle


AP Chemistry Power Point and Guided Notes: Chemical Equilibrium

CHEMICAL EQUILIBRIUM ~ Digital Resource for Google Slides ~ Chemistry

- Google Apps™
- Internet Activities

AP Chemistry LeChatelier's Principle ( Equilibrium ) Lab

AP Chemistry Equilibrium FULL UNIT Plan All types (General, Acid Base, Ksp)

NSW HSC Chemistry Module 5: Equilibrium and Acid Reactions
AP Chemistry Unit 7: Equilibrium Learning Guide and Review

- Google Drive™ folder

NGSS Regents Chemistry - Unit 8: Kinetics & Equilibrium (Complete Unit)

Chemical Equilibrium and Equilibrium Constants (10-LESSON CHEMISTRY BUNDLE)

Acid-Base Equilibria and Buffer Solutions (9-LESSON CHEMISTRY BUNDLE)

Le Chatelier's Principle and Factors Affecting Equilibrium ( CHEMISTRY BUNDLE)

AP Chemistry Equilibrium (Complete Chapter) Bundle

AP Chemistry Power Point: Chemical Equilibrium

AP Chemistry Acid Base Equilibrium Unit BUNDLE (PowerPoint, Guided Notes, Test)!

Solubility Equilibria and Precipitation Reactions (7-LESSON CHEMISTRY BUNDLE)

AP Chemistry Game Bundle -- Stoichiometry, Equilibrium , Periodic Trends, More!

AP Chemistry Equilibrium Homework Handout with ANSWER KEY
AP Chemistry Unit 7: Equilibrium PowerPoint and Guided Notes

AP Chemistry Unit 7 Test- Equilibrium

- Word Document File

318PG SHORT ANSWER Grade 12 Chemistry ALL UNITS Organic Bonding Equilibria Redox

AP Chemistry Equilibrium Escape Room Breakout Box

AP Chemistry Equilibrium Unit BUNDLE (PowerPoint, Guided Notes, Unit Test)!

Notes & Practice #2: Acid-Base Equilibria (Unit 8-Advanced Chemistry ).

AP Chemistry Equilibrium Worksheets (full answer keys)

- We're hiring
- Help & FAQ
- Privacy policy
- Student privacy
- Terms of service
- Tell us what you think
- Study Guides
- Homework Questions
04+-+Chemical+Equilibrium+and+Le+Chatelier+Copper+-+F23Pre-Lab (1)

IMAGES
VIDEO
COMMENTS
Equilibria and Equilibrium Constants (Worksheet) is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. This discussion worksheet addresses equilibria and equilibrium constants. A basic introduction to equilibrium constants should be given before hand including: the law of mass action, reaction ….
K = [NO2]2 [N2O4] b. K = [NO2] [N2O4]1 2. Although the equilibrium constant expressions have a 2:1 ratio of concentration for the products to the concentration of the reactants for the same species involved to get the K value for a. We would need to square it to get the K value for b. 3. K ′ = [NH3] [N2]1 2[H2]3 2.
1. Set up "RICE" TABLE—write a balanced reaction, place initial concentrations into the table, determine the change in initial concentrations in terms of x, calculate the equilibrium concentration expressions (0.25 M - x). 2. Set up the equilibrium expression and set it equal to its value, if given.
2. A mixture of 9.22 moles of A, 10.11 moles of B, and 27.83 moles of C is placed in a one-liter container at a certain temperature. The reaction is allowed to reach equilibrium. At equilibrium the number of moles of B is 18.32. Calculate the equilibrium constant for the reaction: A (g) + 2 B (g) 3 C (g. Ans: 0.832.
14.1: The Concept of Equilibrium. At equilibrium, the forward and reverse reactions of a system proceed at equal rates. Chemical equilibrium is a dynamic process consisting of forward and reverse reactions that proceed at equal rates. At equilibrium, the composition of the system no longer changes with time.
For the equilibrium: 2 NO (g) N2 (g) + 02 (g) at 300 K, the equilibrium constant, Kc, is O. 185. If 1.45 moles each of N2 (g) and 02 (g) are introduced in a container that has a volume of 6.00 liters and allowed to reach equilibrium at 300 K, what are the concentrations of N2 (g 02(g),and NO (g) at equilibrium? Setup: 00 Ans: 0.112M 1021- 0.112 M
The equilibrium constant for the following reaction at 600 o C is determined to be K c = 0.495: H 2 O ( g) + CO ( g) ⇆ H 2 ( g) + CO 2 ( g) Calculate the number of H 2 moles that are present at equilibrium if a mixture of 0.400 mole CO and 0.500 mole H 2 O is heated to 600°C in a 10.0-L container. answer.
This introduction to chemical equilibrium has students look at the rate of change by changing the temperature, concentration, and activation energy. ... or homework packet handout for students. My Kinetics and Equilibrium Unit covers these lessons:Kinetics; Reaction Rate Factors, Catalysts, and Activation EnergyEnergy in Chemical ...
The AACT high school classroom resource library and multimedia collection has everything you need to put together a unit plan for your classroom: lessons, activities, labs, projects, videos, simulations, and animations. We constructed a unit plan using AACT resources that is designed to teach equilibrium to your students.
Welcome to the introduction to. equilibrium. ! Equilibrium. is an incredibly important topic both for the AP exam and for chemistry in general. Equilibrium. takes what you know about reactions from unit 4 and unit 5 and expands it into the realm of. reversible reactions. That is reactions that can go both forward (reactants turning into ...
For the reaction: 2NO + Cl2 ↔ 2NOCl, the pressures at equilibrium are 1.2 atm, 0.30 atm, and 0.05 atm respectively. Calculate Kp at 100K. The equilibrium constant for the reaction CO + H2O(l) ↔ CO2 + H2 is K=4.0 at a given T. At equilibrium, the mixture contains 0.80mol CO, 0.35mol water and 2.2mol CO2.
This is an 8-page guided notes packet used to explain some of the fundamental concepts used as a basis of equilibrium. It covers various topics including: the molecular implications, equilibrium constant, equilibrium pressures, LeChatlier's Principle, using the ICE method of solving equilibrium problems, and much more. Fully editable!
Consider the reaction of chloromethane with OH- in aque- ous solution: CH Cl1aq2 + OH-1aq2 ∆kf CH OH1aq2 + Cl-1aq2 At 25 °C, the rate constant for the forward reaction is 6 * 10-6 M-1 s-1, and the equilibrium constant Kc is 1 * 1016. Calculate the rate constant for the reverse reac- tion at 25 °C. 238.
At 550 K, a sample with a NO2 concentration of 0.250 M decomposes at the rate of 1.17 M/min. (a) Write the rate law (rate expression). (b) Use the concentration and rate given above to find k, with the correct units. 4. The reaction shown below is first order in both reactants: ICl (g) + 1⁄2 H2 (g) 1⁄2 I2 (g) +HCl (g) (a) Write the rate law ...
Introduction to Equilibrium. Sometimes, when a chemical reaction takes place, it proceeds for a period of time and then seems to stop before all the reactants are consumed. But the reaction does not actually stop. Instead, the reaction reaches a point of chemical equilibrium in which the reverse reaction is converting products into reactants as ...
Chemical equilibrium is a dynamic process consisting of forward and reverse reactions that proceed at equal rates. At equilibrium, the composition of the system no longer changes with time. The composition of an equilibrium mixture is independent of the direction from which equilibrium is approached. 5.2: The Empirical Law of Mass Action
Kp (do not think that Kp and Kc is the same) what is the Equilibrium Constant Kp formula? Kp = Kc (RT)^ (change in n) R= 0.0821. (change in n)= the sum of gaseous product in the equation minus the sum of coefficients of the gaseous reactants. what direction will the reaction go if Kc is very large Kc<100?
All of my packets are aligned with (but not limited by) the NGSS and NYS Regents Physical Setting/ Chemistry Curriculum. This file contains the Student, Key, and Teacher versions of my Note Packet and the Student and Key versions of my Practice Packet for the unit on Kinetics & Equilibrium, all in Adobe format.The Key for the Note Packet also contains links to my vodcasts for every lesson ...
View Packet 13 - Chemical Equilibrium Calculations.pdf from CHEM 1032 at Temple University. CHEM 1032, General Chemistry Recitation Packets Series, 2020 Packet 13: Chemical Equilibrium ... homework. Auditoría de estados financieros al 31 de diciembre 2020 No de cuenta Cuenta. Technological University of North Guanajuato. MATEMATICA 2002.
4 Types of Chemical Reactions Notes Synthesis- two or more elements or compounds combine to form one compound. Decomposition-a single compound decomposes into two or more elements or smaller compounds. Single Replacement- a metal will replace a less active metal in an ionic compound OR a nonmetal will replace a less active nonmetal. Double Replacement- the metals in ionic compounds switch places.
View Packet 11 - Chemical Equilibrium-1.pdf from CHEM 1032 at Temple University. CHEM 1032, General Chemistry Recitation Packets Series, 2020 Packet 11: Chemical Equilibrium Part 1: Prior knowledge ... homework. Lab Report 6 worksheets 312 F21.pdf. Solutions Available. Texas A&M University. GENE 312. Aspirin lab report 5 (1).pdf.
Chemistry 104 Discussion Activity and Lab Preparation Packet Chemical Equilibrium Ion Packet 5. Considering again the product favored equilibrium system AB draw how the forward and reverse an? reaction rates change as the reaction proceeds to equilibrium for the following situations: a. The equilibrium is product-favored. Initially only A is ...
Chemical Equilibrium and Le Châtelier's Principle Initial Conditions: MX 2 ∙ 4 H 2 O dissolved in water. Equilibrium: [M (H 2 O) 4 ] 2+ (aq) + 4 Cl - (aq) ⇌ [MCl 4 ] 2- (aq) + 6 H 2 O (l) 1. Experimental Step: Solid MX 2 ∙ 4 H 2 O is added to water to create a salt solution. Observation: The light blue MX 2 ∙ 4 H 2 O crystals dissolved ...