Skip to Content
- Conquer Cancer
- ASCO Journals
- f Cancer.net on Facebook
- t Cancer.net on Twitter
- q Cancer.net on YouTube
- g Cancer.net on Google
- Types of Cancer
- Navigating Cancer Care
- Coping With Cancer
- Understanding the Publication and Format of Cancer Research Studies
- Understanding Cancer Research Study Design and How to Evaluate Results
- How Are Cancer Drugs Discovered and Developed?
- Drug Approval and Labeling in the United States
- Evaluating Cancer Information on the Internet
- How Patient Advocates Help Cancer Research: An Expert Q&A
- Journals and Magazines
- Clinical Trials
- ASCO Annual Meetings
- Health Disparities and Cancer
- For Patient Advocates
- Public Policy Advocacy
- Cancer Awareness Dates
- Survivorship
Research studies answer key questions about how cancer works in the body. They also show what tests and treatments may work best. To help improve cancer care, scientists share the results of their studies with other scientists and doctors. The main way they do so is by publishing them in medical journals.
Scientists may publish their own cancer research, which can be done in a laboratory or with volunteers in a clinic. Studies that involve people are known as clinical trials . Or they may write a review article. A review article looks at all of the published research on a certain topic.
Most cancer research studies are written for scientists and medical doctors. But people with cancer may read them to learn about their disease and treatment options. Research studies use scientific terms that some people may not know. Talk with your health care team if you have questions about research you find.

How is cancer research published?
Different medical journals often focus on different topics, such as clinical cancer research. These journals present new scientific findings and the research methods used.
Most journals publish in print and online. This includes the American Society of Clinical Oncology journals . Journals usually publish on a specific schedule, such as weekly, biweekly, monthly, or quarterly.
For articles published in scientific journals, the phrase “peer reviewed" means that the article has undergone a process in which qualified experts have reviewed it and provided feedback to the authors to improve the scientific quality and integrity of the article. The reviewers were not part of the study. These experts decide whether the research data and results are reliable. Learn more about the importance of peer review in research quality .
How is a cancer research study formatted?
Most cancer research studies include background information, the researcher's methods, results, and the meaning of the findings. Studies published in many journals present this data in a certain format known as Introduction, Methods, Results, and Discussion (IMRAD).
The IMRAD format allows other scientists to do similar studies to see if there is the same result, a scientific principle called replication. The International Committee of Medical Journal Editors supports IMRAD. But some journals may use other names for the format's sections, which are described below.
Introduction. This section explains why a study was done. It also states the research question. For example, "Does this treatment help people with stage IV colon cancer live longer?"
Methods. This is where researchers describe how they answered the research question. To do this, they explain the study's design. This may include how, how much, and how often people in the study received treatment. The researchers also state what result they were measuring. For instance, this may be how long the participants lived without the cancer progressing ("progression-free survival") or if the tumors shrank. They also show how they studied the data.
Results. This section shares the main study findings. Tables and graphics may show the data in different ways. The results section also gives general information about study volunteers, such as the age range and sex. It explains why the volunteers were chosen and the type and stage of cancer they have.
Discussion. This section is also known as the conclusion. It describes what the results mean in relation to the study's purpose. It also looks at the importance of the results and how they may affect cancer research and care. For instance, the results may confirm or challenge earlier research.
What is a cancer research study abstract?
An abstract is a summary that is at the beginning of published cancer research studies. It shares the study's main data. This allows readers to quickly learn about the most important parts of the research. Researchers often share their abstracts at scientific meetings, sometimes even before they have been published in a journal.
How can I find cancer research studies?
There are many ways to find cancer research studies. One way is to visit a journal's website. Then you can use either the search function or the online archive to find a study. An archive stores older studies.
You can also use large, online databases that provide study abstracts. One popular database cancer researchers and doctors use is PubMed . PubMed is a service of the U.S. National Library of Medicine. Another online database you can use to search for studies in Google Scholar . These databases include millions of citations from a wide range of medical journals. A citation is a reference to a source that provides information. This includes the study title, author names, and journal title.
PubMed and Google Scholar can be hard to use because they include so many studies. You can make it easier by searching for a certain cancer topic. If you cannot find studies on that topic, try including more medical terms in your search. For example, try "renal cell carcinoma" instead of "kidney cancer." You can also include the word "review" along with the type of cancer to find review articles. Be as specific as you can about the topic you are interested in.
Abstracts can often be read online for free. However, sometimes you may not be able to read the full study if you do not subscribe to the journal that published it. Sometimes there may be a way to pay a one-time fee to read a study. For printed copies of medical journals, visit a local library or university.
Related Resources
Understanding Cancer Research Study Designs and How to Evaluate Results What to Know When Searching for Cancer Information Online: An Expert Perspective Evaluating Cancer Information on the Internet
Major Milestones Against Cancer
Research and Advocacy
More in this section.
Timely. Trusted. Compassionate.
Comprehensive information for people with cancer, families, and caregivers, from the American Society of Clinical Oncology (ASCO), the voice of the world's oncology professionals.
Find a Cancer Doctor
2022 Abstracts and Posters
2022 cri meeting abstracts.
First Place
Successful Methods of Addressing Clinical Research Staff Turnover [ Abstract ] [ Poster ] N. Nahmias, J. Sanchez, A. Olier-Pino, A. Allred, K. Aviles, L. Corrales Sylvester Comprehensive Cancer Center, University of Miami Health System
Second Place
Starting Off on the Right Foot: Elevating the Voice of Community Stakeholders During the IIT Development Process [ Abstract ] E. Monari, S. Szurek, A. Ivey, T. George, A. Anderson, E. Shenkman, C. Evans, A. Lawson-Ross University of Florida Health Cancer Center
Third Place
Clinical Research Coordinator Workload Estimation and Tracking [ Abstract ] M. Repede, D. Beighley, K. Putz, A. Fritsche, G. Nowakowski Mayo Clinic Cancer Center
Abstracts are organized by category and completion status, then in alphabetical order by cancer center.
Categories:
Clinical trial operations.
1. Clinical Trial Office Response to COVID-19 at an Academic Comprehensive Cancer Center [ Abstract ] E. Bentlyewski, F. Brogan, R. Shelton, J. Jurcic, A. Lassman Herbert Irving Comprehensive Cancer Center, Columbia University Irving Medical Center
Finance/CCSG/PRMS
19. Creation of a Consort Diagram to Visualize Participant Enrollment and Allocation at the Memorial Sloan Kettering Data and Safety Monitoring Committee [ Abstract ] [ Poster ] C. Kolenut, K. Napolitano, X. Lekperic, S. Hanley, K.Tan, E. O’Reilly, S. Slovin Memorial Sloan Kettering Cancer Center 20. Research Portfolio Management: The Protocol Performance Monitoring Dashboard [ Abstract ] [ Poster ] J. Migliacci, X. Lekperic, B. Seko, K. Kaufman, K. Napolitano, S. Hanley, A. Rodavitch Memorial Sloan Kettering Cancer Center 21. PRMC Member Workload Survey After Charter Alignment With NCI Requirements [ Abstract ] [ Poster ] B. Hughes, C. Allen, T. Herzog, C. Vollmer, M. Marcum, N. Kurtzweil University of Cincinnati Cancer Center 22. Impact of the SRMC Zero Tolerance Policy on DSG Trial Portfolios [ Abstract ] [ Poster ] J. Walsh, T. Guinn, Jr., T. George, A. Anderson, A. Ivey University of Florida Health Cancer Center 23. * Taking a Closer Look: Standardizing Disease Focus Groups to Strengthen Trial Portfolios [ Abstract ] [ Poster ] L. Neal Hollings Cancer Center, Medical University of South Carolina 24. Automating and Streamlining the 2-Stage Scientific Review Process [ Abstract ] [ Poster ] T. Baxter, J. Welter, M. Voss, M. Golafshar, T. DeWees, J. Clikeman, A. Fritsche, J. Summer Bolster, A. Dispenzieri Mayo Clinic Cancer Center 25. Clinical Research: Following the Money Phase III [ Abstract ] [ Poster ] R. Geary, P. Eggleton, M. Kovak, M. Birrer, A. Smith, Z. Feng, N. Pruss UAMS Winthrop P. Rockefeller Cancer Institute Information Technology Research Systems, University of Arkansas for Medical Sciences 26. Monitoring Study Enrollment Demographics: PRMS-COE Collaboration at University of Colorado Cancer Center (UCCC) [ Abstract ] [ Poster ] D. McCollister, D. Pacheco, A. Henningham, E. Borrayo, C. Cost University of Colorado Cancer Center Back to top
Investigator-Initiated Trials
27. * Development of a Multisite Investigator-Initiated Trial Coordinating Center at Cedars-Sinai Cancer [ Abstract ] [ Poster ] E. Hautamaki, D. Ngo, A. Tan, P. Chang Cedars-Sinai Cancer 28. Development of a Workload Assessment Tool for Investigator-Initiated Trial Protocol Development Based on the Ontario Protocol Assessment Level Scale [ Abstract ] [ Poster ] E. Hautamaki Cedars-Sinai Cancer 29. Building IND Infrastructure to Ensure Compliance and Enable Growth [ Abstract ] [ Poster ] J. Morrison, N. Babadi, E. Crecelius, S. Scott, R. Johnson, S. Boyle, M. Retter, A. Camp, L. Kiefer, C. Lee UNC Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill 30. Development of an Investigator-Initiated Trial Intake Process at Cedars-Sinai Cancer [ Abstract ] [ Poster ] E. Hautamaki, P. Chang, D. Ngo, A. Tan Cedars-Sinai Cancer 31. Streamlining Data Collection: Implementation of an EDC FHIR Lab Interface [ Abstract ] [ Poster ] E. Crecelius, M. O’Dwyer, L. Logan, S. Balu, J. Frank, R. Johnson, R. Church, C. Lee, J. Morrison UNC Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill 32. Starting Off on the Right Foot: Elevating the Voice of Community Stakeholders During the IIT Development Process [ Abstract ] [ Poster ] E. Monari, S. Szurek, A. Ivey, T. George, A. Anderson, E. Shenkman, C. Evans, A. Lawson-Ross University of Florida Health Cancer Center 33. UF’s IIT Think Tank Experiment [ Abstract ] [ Poster ] E. Monari, A. Ivey, T. George, A. Anderson University of Florida Health Cancer Center Back to top
Quality Assurance, Remote Monitoring, and Auditing
34. Proactive Quality Assurance Through Dual Review of Eligibility and Consent [ Abstract ] [ Poster ] K. Thorne Huntsman Cancer Institute, University of Utah 35. Transforming Risk Management: Technological Evolution of MSK’s Clinical Research Quality Assurance Program [ Abstract ] [ Poster ] A. Granobles, M. Satter, S. Puleio, F. Puma, N. Brosnan, K. Yataghene Memorial Sloan Kettering Cancer Center 36. * Virtual Monitoring and Auditing Digitization in Decentralized Clinical Trials: Source Document Verification, System Scheduling, and Real Time Protocol Performance Feedback [ Abstract ] [ Poster ] M. Buckley, J. Lengfellner, M. Latif, K. Yataghene, C. Houston, S. Terzulli, N. Cimaglia, P. Sabbatini Memorial Sloan Kettering Cancer Center 37. Automating Data Safety Monitoring Committee (DSMC) Progress Reports [ Abstract ] [ Poster ] T. McSpadden, S. Grolnic University of Colorado Cancer Center 38. Introducing a Quality Management System Into the Mayo Clinic Cancer Center Clinical Research Office [ Abstract ] [ Poster ] K. Alexander, K. Croghan, A. Fritsche, J. Summer Bolster, J. Welter Mayo Clinic Cancer Center 39. Preparing and Sharing Subject Cases for Remote NCTN Audit [ Abstract ] [ Poster ] K. Rygalski, M. Russell, D. Kitterman University of Illinois Cancer Center 40. Standardized Quality Metrics in Cancer Clinical Trials: A Qualitative Study [ Abstract ] H.A. Forbes McClellan, A. Anglemyer, E. Davis, A. Dumont, K. Shaddox, R. Simons, J. Stern Vanderbilt-Ingram Cancer Center Back to top
41. Digitalizing and Automating Clinical Research Protocol Regulatory Binders for Greater Efficiencies [ Abstract ] M. Buckley, R. Lehrman, J. Lengfellner, M. Latif, K. Yataghene, C. Houston, S. Terzulli, P. Sabbatini Memorial Sloan Kettering Cancer Center 42. * Delegation of Authority – A Simplified Process [ Abstract ] [ Poster ] B. Scanlan, A. Holley, M. Kovak, B. Lehman, P. Newman, R. Perry, D. Wade, M. Birrer UAMS Winthrop P. Rockefeller Cancer Institute 43. Optimization of a Regulatory eBinder Platform [ Abstract ] S. Rebar, K. Lopez, D. Cervantes Fred Hutchinson Cancer Research Center 44. Simplifying and Improving Training and Delegation Documentation [ Abstract ] [ Poster ] R. Kingsford, L. Hayes, L. Lujan Huntsman Cancer Institute, University of Utah 45. Supporting Virtual Clinical Trials: How the Generation of DOAs in PIMS has Enabled Clinical Trial Compliance in a Remote World [ Abstract ] P. Lim Memorial Sloan Kettering Cancer Center Back to top
Training and Career Development
46. Investing in Investigator Training: Developing Tools to Close the Gap [ Abstract ] [ Poster ] L. Valanejad Kiefer, N. H. Babadi, M. Robinson, A. Camp, C. Lee, J. K. Morrison UNC Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill 47. The Effectiveness of an Innovative Competency-Based Education and Training Program on Decreasing Audit Findings [ Abstract ] [ Poster ] E. Dawkins, S. Cole, N. Nahimas, P. Seo, and J. Brown Sylvester Comprehensive Cancer Center, University of Miami Health System 48. Comprehensive Application of Supplemental Phantom Educational Resources (CASPER): a Friendly Phantom Patient to Guide the Way for New Study Coordinators [ Abstract ] [ Poster ] E. Cunningham, L. Dunham, B. Olsen Barbara Ann Karmanos Cancer Institute, Wayne State University 49. Implementation of Small Group Trainings to Expedite Initial Onboarding for Clinical Research Staff and Increase Connection Between New Employee s [ Abstract ] [ Poster ] D. Kreitner, M. Wanchoo, D. Castro, C. Burgin OHSU Knight Cancer Institute 50. * Staffing Pipeline Creation: Clinical Research Internship for Undergraduate BIPOC Students [ Abstract ] [ Poster ] T. Cummings, A. Walens, A. Leak-Bryant, V. Carlisle, M. Haines, C. Lee UNC Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill 51. Using Surveys to Evaluate Staff Onboarding Experiences: Pandemic to Present [ Abstract ] [ Poster ] C.L. Allen, P. Rose, M. Marcum, N. Kurtzweil University of Cincinnati Cancer Center 52. Piloting a New Investigator E-Learning Onboarding Program [ Abstract ] [ Poster ] J. Thomas, M. Murphy, T. George, A. Anderson, E. Monari, A. Ivey University of Florida Health Cancer Center Back to top
Trial Recruitment & Community Outreach and Engagement
Back to top
Trial Start-up and Activation
62. Collaboration to Develop Recommendations to Improve Trial Activation Timelines [ Abstract ] T. Werner, T. Lin, C. Houston, D. Otap, M. Nashawati, L. Ashmore, E. Buell, A. Zafirovski, K. Much Huntsman Cancer Institute, University of Utah; The University of Kansas Cancer Center; Memorial Sloan Kettering Cancer Center; Mays Cancer Center at UT Health San Antonio MD Anderson; Robert H. Lurie Comprehensive Cancer Center of Northwestern University; AbbVie; Genentech; Janssen Oncology; Merck 63. * Practical Benefits of Defining and Implementing Structured Intake and New Study Assignment in a Centralized Start-up Model [ Abstract ] [ Poster ] A. McCauley, M. Winkler, M. Poduri, M. Hibbert Fred Hutchinson Cancer Research Center 64. Four Years and Beyond: Progress With the Committee on Radiation [ Abstract ] [ Poster ] A. Andreatta, C. Ryan, S. Hanley, A. Rodavitch, P. Zanzonico, L. Dauer, M. Williamson Memorial Sloan Kettering Cancer Center 65. It’s About Time: A Simplified Approach to NCI Trial Activation [ Abstract ] [ Poster ] J. Balletti, L. Gaffney, M. Warren, S. Hanley, E. Valentino, A. Rodavitch, J. Migliacci Memorial Sloan Kettering Cancer Center 66. Strategies to Expedite Activation of Expanded Access Protocols at Memorial Sloan Kettering Cancer Center [ Abstract ] [ Poster ] X. Lekperic, E. Valentino, S. Hanley, A. Rodavitch Memorial Sloan Kettering Cancer Center 67. Enhancing 1 st Stage Protocol Review – A Quantitative Approach [ Abstract ] [ Poster ] L. Wall, A. Spratt, R. Szmulewitz The University of Chicago Medicine Comprehensive Cancer Center 68. Evaluation of a Prioritization Matrix for Electronic Order Build in an Investigational Drug Service [ Abstract ] A. Smith, K. Bottenberg, J. Rudolph, K. Redic University of Michigan Rogel Cancer Center 69. Clinical Trial Research Group (CTRG) Guidelines for Trial Portfolio Management [ Abstract ] [ Poster ] J. Moehle, L. Lujan, S. Sharry, N. Agarwal, H. Colman, D. Gaffney, T. Werner Huntsman Cancer Institute, University of Utah 70. Technology and Centralization in Early Study Start-up Activities [ Abstract ] [ Poster ] E. Lebleu, L. Lujan, J. Moehle, T. Werner Huntsman Cancer Institute, University of Utah 71. Enhancing Transparency and Interoperability: Developing an Enterprise-Level Portal to Streamline Trial Activation Processes [ Abstract ] P. Arlen, M. Santiago, K. Williams, L. Thyssen, G. Degennaro, A. Ward, N. Reyes, C. Valdivia Sylvester Comprehensive Cancer Center, University of Miami Health System
72. Improving Trial Activation Timelines: A Comprehensive Process Improvement Project [ Abstract ] [ Poster ] P. Arlen, L. Thyssen, K. Williams Sylvester Comprehensive Cancer Center, University of Miami Health System 73. Value Stream Mapping: Maximizing Value, Minimizing Waste, and Improving Flow Across the Clinical Trial Activation Process [ Abstract ] P. Arlen, L. Thyssen, K. Williams Sylvester Comprehensive Cancer Center, University of Miami Health System 74. Implementation of a Feasibility Committee – University of Cincinnati Cancer Center (UCCC) Study Operations & Administrative Review (SOAR) [ Abstract ] [ Poster ] A. Kastl, M. Marcum University of Cincinnati Cancer Center Back to top
*Honorable Mention
Vitamin D regulates microbiome-dependent cancer immunity
Affiliations.
- 1 Immunobiology Laboratory, The Francis Crick Institute, London NW1 1AT, UK.
- 2 Cancer Immunosurveillance Group, Cancer Research UK Manchester Institute, The University of Manchester, Manchester M20 4BX, UK.
- 3 Inflammatory Cell Dynamics Section, Laboratory of Integrative Cancer Immunology (LICI), Center for Cancer Research (CCR), National Cancer Institute (NCI), Bethesda, MD 20892, USA.
- 4 Department of Immunology and Inflammation, Imperial College London, London SW7 2AZ, UK.
- 5 Bioinformatics and Biostatistics STP, The Francis Crick Institute, London NW1 1AT, UK.
- 6 MRC Toxicology Unit, University of Cambridge, Cambridge CB2 1QR, UK.
- 7 Basic Science Program, Frederick National Laboratory for Cancer Research, Frederick, MD 21701, USA.
- 8 Microbiome and Genetics Core, LICI, CCR, NCI, Bethesda, MD 20892, USA.
- 9 National Center of Excellence for Molecular Prediction of Inflammatory Bowel Disease, PREDICT, Faculty of Medicine, Aalborg University, Department of Gastroenterology and Hepatology, Aalborg University Hospital, A DK-2450 Copenhagen, Denmark.
- 10 Metabolomics STP, The Francis Crick Institute, London NW1 1AT, UK.
- 11 Cancer Inflammation and Immunity Group, Cancer Research UK Manchester Institute, The University of Manchester, Manchester M20 4BX, UK.
- 12 Tumor Immunogenomics and Immunosurveillance (TIGI) Lab, UCL Cancer Institute, London WC1E 6DD, UK.
- 13 AhRimmunity Laboratory, The Francis Crick Institute, London NW1 1AT, UK.
- 14 Experimental Histopathology, The Francis Crick Institute, London NW1 1AT, UK.
- 15 Department of Pathobiology and Population Sciences, The Royal Veterinary College, North Mimms, Hatfield, Hertfordshire AL9 7TA, UK.
- 16 Genetic Mechanisms of Disease Laboratory, The Francis Crick Institute, London NW1 1AT, UK.
- 17 Institute of Liver and Digestive Health, Division of Medicine, Royal Free Hospital, University College London, London NW3 2QG, UK.
- PMID: 38662827
- DOI: 10.1126/science.adh7954
A role for vitamin D in immune modulation and in cancer has been suggested. In this work, we report that mice with increased availability of vitamin D display greater immune-dependent resistance to transplantable cancers and augmented responses to checkpoint blockade immunotherapies. Similarly, in humans, vitamin D-induced genes correlate with improved responses to immune checkpoint inhibitor treatment as well as with immunity to cancer and increased overall survival. In mice, resistance is attributable to the activity of vitamin D on intestinal epithelial cells, which alters microbiome composition in favor of Bacteroides fragilis , which positively regulates cancer immunity. Our findings indicate a previously unappreciated connection between vitamin D, microbial commensal communities, and immune responses to cancer. Collectively, they highlight vitamin D levels as a potential determinant of cancer immunity and immunotherapy success.
Publication types
- Research Support, N.I.H., Extramural
- Research Support, Non-U.S. Gov't
- Bacteroides fragilis*
- Gastrointestinal Microbiome* / drug effects
- Immune Checkpoint Inhibitors / pharmacology
- Immune Checkpoint Inhibitors / therapeutic use
- Immunotherapy
- Intestinal Mucosa / immunology
- Intestinal Mucosa / metabolism
- Intestinal Mucosa / microbiology
- Mice, Inbred C57BL
- Neoplasms / immunology
- Neoplasms / microbiology
- Vitamin D* / pharmacology
- Immune Checkpoint Inhibitors

Journal of Cancer Research and Clinical Oncology
- The official journal of the German Cancer Society (Deutsche Krebsgesellschaft)
- Fully open access (OA) as of January 1, 2024
- Alwin Krämer
Latest articles
Dynamic change in the peritoneal cancer index based on ct after chemotherapy in the overall survival prediction of gastric cancer patients with peritoneal metastasis.
- Yi-Yuan Wei
- Jie-Yuan Cai

Regulation of VEGF-A expression and VEGF-A-targeted therapy in malignant tumors
Two-headed UNetEfficientNets for parallel execution of segmentation and classification of brain tumors: incorporating postprocessing techniques with connected component labelling
- Hari Mohan Rai
- Serhii Dashkevych
Systematic assessment of the influence of quality of studies on mistletoe in cancer care on the results of a meta-analysis on overall survival
- Jorina Hofinger
- Lukas Kaesmann
- Jutta Huebner

Abrogating PDK4 activates autophagy-dependent ferroptosis in breast cancer via ASK1/JNK pathway
- Wenbiao Shi
- Linjun Yang
Journal updates
Journal of cancer research and clinical oncology is now fully open access.
We are excited to announce that the Journal of Cancer Research and Clinical Oncology has now become a fully open-access (OA) journal as of January 2024. This means that we will only be publishing articles as Open Access meaning content will be freely available to readers worldwide, enabling the widest possible dissemination and reuse.
Journal information
- Biological Abstracts
- CAB Abstracts
- Chemical Abstracts Service (CAS)
- Current Contents/Life Sciences
- Google Scholar
- INIS Atomindex
- Japanese Science and Technology Agency (JST)
- OCLC WorldCat Discovery Service
- Pathway Studio
- Science Citation Index Expanded (SCIE)
- Semantic Scholar
- TD Net Discovery Service
- UGC-CARE List (India)
Rights and permissions
Springer policies
© Springer-Verlag GmbH Germany, part of Springer Nature
- Find a journal
- Publish with us
- Track your research
- Introduction
- Conclusions
- Article Information
The figure shows the percentage of women who answered that having the other risk factor put a woman at greater risk compared with dense breasts. Family history is defined as having a mother or sister who has or had breast cancer. Other race included women identifying as mixed race or another race or ethnicity. Data were missing for the following categories: being overweight or obese, 23; having 1 or more drinks of alcohol per day, 23; first-degree family history of breast cancer, 15; never having children, 27; having a breast biopsy, 32; and race and ethnicity, 1.
eAppendix 1. Breast Cancer Risk Factors
eAppendix 2. Survey Instrument Content
eAppendix 3. Interview Guide
Data Sharing Statement
See More About
Sign up for emails based on your interests, select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Get the latest research based on your areas of interest.
Others also liked.
- Download PDF
- X Facebook More LinkedIn
Beidler LB , Kressin NR , Wormwood JB , Battaglia TA , Slanetz PJ , Gunn CM. Perceptions of Breast Cancer Risks Among Women Receiving Mammograph Screening. JAMA Netw Open. 2023;6(1):e2252209. doi:10.1001/jamanetworkopen.2022.52209
Manage citations:
© 2024
- Permissions
Perceptions of Breast Cancer Risks Among Women Receiving Mammograph Screening
- 1 The Dartmouth Institute for Health Policy and Clinical Practice, Geisel School of Medicine, Dartmouth College, Hanover, New Hampshire
- 2 Section of General Internal Medicine, Boston University Chobanian and Avedesian School of Medicine, Boston, Massachusetts
- 3 Department of Psychology, University of New Hampshire, Durham
- 4 Department of Radiology, Boston University Chobanian and Avedisian School of Medicine, Boston, Massachusetts
- 5 Dartmouth Cancer Center, The Dartmouth Institute for Health Policy and Clinical Practice, Geisel School of Medicine, Dartmouth College, Hanover, New Hampshire
Question How do women perceive the breast cancer risk associated with breast density, and how do they plan to mitigate their risk?
Findings In this qualitative study of women aged 40 to 76 years, family history was perceived as the greatest risk factor for breast cancer. In interviews, few women perceived breast density as a risk factor, and one-third thought that they could not take any actions to reduce their breast cancer risk.
Meaning Despite laws that require women to be notified about breast density, women did not describe a strong understanding of the risk associated with breast density relative to other breast cancer risk factors.
Importance Breast density is an independent risk factor for breast cancer. Despite the proliferation of mandated written notifications about breast density following mammography, there is little understanding of how women perceive the relative breast cancer risk associated with breast density.
Objective To assess women’s perception of breast density compared with other breast cancer risks and explore their understanding of risk reduction.
Design, Setting, and Participants This mixed-methods qualitative study used telephone surveys and semistructured interviews to investigate perceptions about breast cancer risk among a nationally representative, population-based sample of women. Eligible study participants were aged 40 to 76 years, reported having recently undergone mammography, had no history of prior breast cancer, and had heard of breast density. Survey participants who had been informed of their personal breast density were invited for a qualitative interview. Survey administration spanned July 1, 2019, to April 30, 2020, with 2306 women completing the survey. Qualitative interviews were conducted from February 1 to May 30, 2020.
Main Outcomes and Measures Respondents compared the breast cancer risk associated with breast density with 5 other risk factors. Participants qualitatively described what they thought contributed to breast cancer risk and ways to reduce risk.
Results Of the 2306 women who completed the survey, 1858 (166 [9%] Asian, 503 [27%] Black, 268 [14%] Hispanic, 792 [43%] White, and 128 [7%] other race or ethnicity; 358 [19%] aged 40-49 years, 906 [49%] aged 50-64 years, and 594 [32%] aged ≥65 years) completed the revised risk perception questions and were included in the analysis. Half of respondents thought breast density to be a greater risk than not having children (957 [52%]), having more than 1 alcoholic drink per day (975 [53%]), or having a prior breast biopsy (867 [48%]). Most respondents felt breast density was a lesser risk than having a first-degree relative with breast cancer (1706 [93%]) or being overweight or obese (1188 [65%]). Of the 61 women who were interviewed, 6 (10%) described breast density as contributing to breast cancer risk, and 43 (70%) emphasized family history as a breast cancer risk factor. Of the interviewed women, 17 (28%) stated they did not know whether it was possible to reduce their breast cancer risk.
Conclusions and Relevance In this qualitative study of women of breast cancer screening age, family history was perceived as the primary breast cancer risk factor. Most interviewees did not identify breast density as a risk factor and did not feel confident about actions to mitigate breast cancer risk. Comprehensive education about breast cancer risks and prevention strategies is needed.
Dense breasts, in which breasts are composed of more glandular tissue relative to fatty tissue, is an independent, nonmodifiable risk factor for breast cancer and can mask cancer on mammograms. 1 Dense breast tissue is present in 40% to 50% of women undergoing screening mammography 2 and is associated with a 1.2 to 4.0 times higher risk of breast cancer (depending on degree of density) compared with a 2.0 times higher risk associated with a first-degree family history of breast cancer. 3 - 6 Other known risk factors include obesity, alcohol consumption, parity, and having a prior breast biopsy (eAppendix 1 in Supplement 1 ). 3 , 7 , 8 Although how much each risk factor or combination of factors affects overall breast cancer risk has not been completely characterized, 7 knowledge about personal risk is necessary to promote engagement in prevention, particularly for modifiable contributors, such as alcohol consumption and obesity.
Aiming to increase awareness and empower women to make informed choices about supplemental screening, laws enacted across 38 states mandate that women receive written notification about their personal breast density and its potential health implications. 9 Although laws vary among states, 9 they share an underlying goal of informing women about their personal breast cancer risk to promote informed decision-making about breast cancer screening and early detection.
Prior studies 10 - 17 have evaluated the association of breast density notification laws with women’s awareness of their individual breast density, masking bias, and the risks associated with breast density. Qualitative studies have found that few women are aware of the legislation around breast density notification, 15 that some women find breast density notifications to be confusing, 17 and that, although most women understand that breast density could mask cancer on a mammogram, few know that breast density is an independent breast cancer risk. 13 Cross-sectional surveys have found variation in women’s knowledge about breast density as a risk factor 10 - 12 , 14 , 16 ; variation in knowledge across racial and ethnic groups, income, and educational levels 11 , 14 ; that most women were aware of masking bias 11 , 14 , 16 ; and that women in states that mandated breast density notification were more likely to report having dense breasts. 14
Although the current literature explores women’s knowledge about breast density, a systematic review 18 noted that little is known about whether women understand the risk associated with breast density compared with other risk factors or their approaches to mitigating risk. We used a national survey and qualitative interviews to examine how women perceive breast density’s cancer risk relative to other breast cancer risk factors and their understanding of actions they could take to reduce breast cancer risk.
This mixed-methods qualitative study included survey data from a national, random-digit-dialing telephone survey coupled with semistructured interviews with a subset of survey respondents. Survey questions examined women’s perception of breast density in relation to other known breast cancer risks; interviews explored women’s understanding of breast cancer risk factors and actions to mitigate risk. This mixed-methods approach allowed us to examine the scope of awareness and understanding. On the basis of prior literature demonstrating differences in perceptions by sociodemographic characteristics, 11 , 14 we examined whether risk perceptions varied by self-reported race and ethnicity and by literacy level (high literacy [HL] vs low literacy [LL]). This study was reviewed by the Boston University Medical Campus Institutional Review Board, which determined that the study met federal exemption criteria and provided a waiver of documentation of informed consent. Approval was for the qualitative interviews (survey work was conducted by an external survey firm) and at the time of transcription. All interview data was deidentified. The study followed the Standards for Reporting Qualitative Research ( SRQR ) for reporting qualitative data. 19
The sampling frame consisted of 2306 participants who completed a national, random-digit-dialing survey of the effect of states’ breast density notification laws on knowledge about breast cancer risks associated with breast density. Eligible participants were aged 40 to 76 years, reported having undergone mammography in the prior 2 years, had no history of breast cancer, and had heard of breast density. Within the population-based sampling, efforts were made to ensure a sufficient sample of women from diverse racial and ethnic backgrounds, from states with and without breast density notification laws, and with lower literacy levels, as detailed in prior publications. 20 , 21 Participants were asked in the survey to self-identify their race or ethnicity. We collected race and ethnicity data to allow for oversampling across some groups to ensure that we could conduct analyses that compared findings across groups.
After completing the survey, women who reported knowing their breast density were invited to participate in a qualitative interview. Those who responded affirmatively were called to schedule an interview. We purposively sampled equal numbers of women who identified as Black, Hispanic, White, or other race or ethnicity as well as those with HL vs LL. In the survey, participants were asked to self-identify their race from a list that included Asian, Black or African American, Native American, Pacific Islander, White, mixed race, or some other race. For these analyses, anyone who responded that they were Native American, Pacific Islander, mixed race, or some other race were classified as other race. For the qualitative interviews, we included respondents who were Asian in the other race category.
Breast density awareness and breast cancer risk questions were adapted from measures used in prior surveys, 10 , 11 , 22 with modified measures tested by patient advisory group members. Advisory group members also reviewed the interview guide. The survey firm, SSRS, conducted all surveys using a standardized interview approach (eAppendix 2 in Supplement 1 ). The cooperation rate for the overall survey was 85%. 20 Survey administration spanned July 1, 2019, to April 30, 2020, and took approximately 10 minutes. Qualitative interviews were conducted from February 1 to May 30, 2020, and lasted 30 to 45 minutes. Qualitative interviews followed a flexible, semistructured interview guide (eAppendix 3 in Supplement 1 ) and were audiorecorded and transcribed. All data were collected via telephone by trained interviewers.
This mixed-methods qualitative study focused on women’s perceptions of breast cancer risks, examining how women rate certain risks relative to the risk of breast density. Women were asked to compare the risk of breast density with 5 other breast cancer risk factors (having a first-degree relative with breast cancer, being overweight or obese, having more than 1 alcoholic drink per day, never having children, or having a prior breast biopsy). A review of data from the first 448 survey participants revealed that wording of the risk perceptions questions was confusing. We revised the questions and excluded those participants from analyses due to identified measurement error and incompatibility of responses with subsequent risk questions. For each risk factor, participants were asked the question, “Which do you think puts someone at greater risk for developing breast cancer? Having dense breasts or…” Risk factors were elicited in a random order to minimize ordering bias.
We characterized the proportion of women who said having dense breasts puts someone at a greater risk for developing breast cancer vs the alternative risk factor or “don’t know”; participants with missing responses were excluded from analyses (<1%). Bivariate χ 2 analyses assessed whether the proportion of women who said having dense breasts puts someone at greater risk for developing breast cancer was associated with participants’ race and ethnicity (coded as Asian, Black, Hispanic, White, and other category not listed) or literacy level (HL or LL). Low literacy was defined as either having less than a high school education or reporting sometimes, often, or always needing assistance to complete medical forms using the validated Single Item Literacy Screener. 23 We used SPSS statistics software, version 26 (IBM Inc). 24 Statistical significance was defined at α = .05. We followed the American Association for Public Opinion Research ( AAPOR ) reporting guidelines for survey data. 25
Women were asked in an open-ended fashion what they thought contributed to breast cancer risk and how they could reduce their breast cancer risk. To organize and support analyses, we developed an analytic memo that described all observed themes. 26 We used a matrix coding approach to guide development of themes and justify inclusion or exclusion of interviewees within themes. 27 This approach includes arranging data within a table where individual participants represent rows and themes represent columns. We analyzed whether themes varied across literacy levels or across racial and ethnic groups. Qualitative analyses were overseen by a doctoral-level health services researcher (C.M.G.) with expertise in qualitative methods. Two masters-level trained research coordinators and 1 doctoral student participated in data collection and analysis, including co-coding and consensus determination meetings.
Of the 2306 women who responded to the survey, 1858 (166 [9%] Asian, 503 [27%] Black, 268 [14%] Hispanic, 792 [43%] White, and 128 [7%] other race; 358 [19%] aged 40-49 years, 906 [49%] aged 50-64 years, and 594 [32%] aged ≥65 years) completed the revised risk perception questions and were included in the analysis ( Table 1 ). In comparing risk factors with the risk associated with breast density, 1706 women (93%) viewed family history of breast cancer as the greater risk, and 1188 (65%) felt that being overweight or obese was a greater risk than breast density. Half of respondents thought that breast density was a greater risk than not having children (957 [52%]), having more than 1 alcoholic drink per day (975 [53%]), or having a prior breast biopsy (867 [48%]) ( Figure ). A higher proportion of women with LL compared with women with HL rated breast density as a higher risk than family history (13% vs 7%; χ 2 1 = 12.99, P < .001), alcohol consumption (60% vs 53%; χ 2 1 = 5.41, P = .02), and never having children (60% vs 51%; χ 2 1 = 7.39, P = .007). A higher proportion of Black women (290 [58%]) and Hispanic women (153 [58%]) rated dense breast as a higher risk than alcohol consumption compared with women of other races (χ 2 4 13.63, P = .009). A total of 289 Black (58%) and 153 Hispanic (58%) women also rated dense breasts as a higher risk than nulliparity than women who identified as Asian (74 [45%]), White (377 [48%]), and other race (64 [52%]) (χ 2 4 = 17.48, P = .002).
Among 61 women interviewed, few women perceived breast density as contributing to their risk of developing breast cancer. Most women correctly noted that breast density could make mammograms harder to read: “It’s difficult to detect subsequent lumps or potential problem areas because of the dense breast tissue.” (Black woman, HL, respondent 7). When asked about their personal risk factors for breast cancer, few women noted that breast density could be a risk factor. One woman described her concern by saying, “Maybe 10% more worried than I was before because of the dense tissue issues. Just a slight uptick, but it’s not overwhelming” (Hispanic woman, HL, respondent 17).
Women most frequently and confidently emphasized family history of cancer or genetic factors as contributing to their own breast cancer risk ( Table 2 ), and many viewed this as conferring very high levels of risk. One woman estimated her own risk as “probably 50/50 at this point since my mother had breast cancer” (Black woman, HL, respondent 5). Concurrently, women who had no known family history seemed to minimize the possibility of developing cancer: “I’m not worried about it because it does not run in my family. So I don’t have to worry about dodging that bullet” (Hispanic woman, LL, respondent 23).
Table 2 displays risk factors cited by women, ordered by the prevalence of the theme across participants. Reported risk factors included diet, lifestyle, smoking and environmental exposures, breast density, obesity, alcohol consumption, and reproductive history. Unlike family history, most women did not voice confidence in their understanding of other risk factors. Instead, they spoke about a series of behaviors and exposures that they perceived as related to their health overall: “We blame smoking for everything. So I’m sure smoking’s on there” (Black woman, HL, respondent 5). Few women stated that they had no knowledge of what breast cancer risk factors were: “I have no idea. All the stuff that’s been here on the news. This chemical, that chemical...” (Black woman, HL, respondent 8). We did not observe differences in understanding or perception of personal breast cancer risk by health literacy level or by racial or ethnic group.
When asked about actions that could reduce their breast cancer risk, many women described detection methods, such as breast self-examinations and mammograms, as prevention strategies. Among women who discussed mammograms or breast self-examinations, a small subset noted that screening methods would not prevent cancer but were useful for potentially detecting breast cancer earlier: “Well, if I go for my annual mammogram and do self-breast examination, I will catch whatever’s growing in my breast will be nipped in the bud...It will be taken care of before it gets out of control” (White woman, LL, respondent 54).
Women’s descriptions of risk mitigation focused on mammography, with descriptions conflating early detection and prevention. Other ideas for reducing personal breast cancer risk included improving diet, maintaining a healthy weight, quitting smoking, avoiding secondhand smoke, limiting alcohol, and exercising ( Table 3 ). Many women suggested behaviors that they thought could improve their overall health but expressed less certainty about the direct effect on their breast cancer risk: “I try to eat a healthier lifestyle, more in the vegetable fields, less in any kind of…dairy or red meat portions. I do exercise more, but I did that for my general health, not for breast cancer” (Hispanic woman, HL, respondent 17).
Many women (17 [28%]) stated that they were not sure if it was possible to reduce their breast cancer risk or that they did not know what actions they could take to reduce their risk: “Do people even know how to prevent breast cancer? I couldn’t even say” (woman of other race, HL, respondent 30). Neither health literacy level nor race or ethnicity appeared to differentiate how women perceived actions that they could take to reduce their breast cancer risk.
This mixed-methods qualitative study demonstrated that women perceived family history as the strongest risk factor for breast cancer, with mixed perceptions about other lifestyle or clinical risk factors in relation to breast cancer risk. Among interview respondents who knew their breast density, few women noted breast density as a breast cancer risk factor. Few women understood options for mitigating their personal breast cancer risk.
Despite breast density being associated with a 1.2 to 4 times higher risk of breast cancer, 1 , 5 , 6 few women perceived breast density to be a strong personal risk factor. This finding is not surprising because prior studies 11 , 14 have shown variable rates of women indicating that breast density contributed to breast cancer risk (23%-66%). Qualitative studies 13 , 17 , 28 , 29 of women receiving breast density notifications found that women did not fully understand the clinical term breast density . It is possible that notification language stressing the normality of dense breast tissue in the population confers a sense of reassurance that may contribute to the downplaying of breast density as a risk factor. 13 , 29
In both interviews and surveys, women perceived family history as highly deterministic of future breast cancer. Women without a family history believed they were safe or had limited risk based on this factor alone. Other studies 30 , 31 have similarly found that women with family histories of breast cancer perceived their personal risk of cancer to be higher than the estimated risk associated with their family history. The emphasis on family history may be in part a result of clinical elicitation of family and genetic risk factors, including the increased emphasis on genetic testing for BRCA1/2 genes, both clinically and in popular media. 32 , 33 A 2021 systematic review 34 found that in primary care, family history is often the only risk factor elicited to counsel patients on breast cancer risk. Thus, frequent health messaging around family history and breast cancer risk may play a role in how this sample of women perceived their own breast cancer risk. Interviewed women displayed little confidence in their ability to modify their cancer risk, suggesting a need for more comprehensive education about which risk factors are amenable to intervention.
Few women identified ways in which they could reduce their breast cancer risk. When mentioned, these actions included participating in regular screening, diet and exercise, and avoiding tobacco ( Table 3 ). Many women suggested that breast self-examinations were important to maintaining their breast health, but these examinations are no longer recommended because of a lack of evidence of benefit. 35 (p179) 36 Women also suggested actions that they thought were generally healthy lifestyle changes, but they were not confident these actions would alter their breast cancer risk. Women may benefit from general guidance and information about cancer prevention strategies, such as tools that can help patients understand overall cancer risk and prevention options. 37 Clinical treatments, such as chemoprevention agents, are available to reduce breast cancer risk for women at elevated risk (>1.7% 5-year risk as determined by a validated risk model) 38 , 39 but were not mentioned by any interviewees. This finding is not unexpected because chemoprevention is significantly underused by the eligible population, 40 - 42 despite being recommended for women at elevated risk. 43
This study has some limitations. Despite efforts to include a racially and ethnically diverse sample on the telephone survey panel, nonresponse bias could have influenced findings. The survey did not ask about women’s perception of the absolute risk associated with each risk factor, limiting our ability to draw conclusions about the accuracy of women’s risk perceptions. Interviewees reported being informed of their personal breast density, but we were unable to verify the nature or timing of this notification. We defined low literacy using a single-item literacy scale combined with educational level, which is an imprecise way to measure literacy, limiting our ability to draw conclusions about the direct effect of literacy on risk perception.
Our study, coupled with prior research, 12 , 14 , 18 , 20 suggests that understanding of breast density’s contribution to breast cancer risk remains underappreciated by many women. Most notifications encourage women to speak with their physicians, yet prior studies 15 , 44 - 47 found that many clinicians do not feel comfortable counseling on the implications of breast density and cancer risk. Efforts to communicate breast density in part are intended to align with evidence suggesting that breast cancer screening services should be tailored to personal risk to maximize the benefits and avoid undue harms, 48 - 50 rendering conversations about risk imperative. Women with dense breasts, and thus some elevated risk, are ideal candidates for risk assessment. Tools that incorporate breast density in risk measures, such as one from the Breast Cancer Surveillance Consortium, 51 , 52 can inform future screening behaviors, including the opportunity for supplemental screening. Supplemental screening not only can lead to increased rates of cancer detection but also may result in more false-positive results and recall appointments. 53 - 55 Because supplemental screening is not recommended for women at average risk, 55 clinicians should use risk assessment to guide discussions with patients about tradeoffs associated with supplemental screening.
Despite available guidance on breast cancer risk assessment to inform screening decisions, 56 , 57 such assessments are underused in primary care. 58 - 60 Reported barriers include inadequate time, lack of integrated tools, and uncertainty in interpreting results for decision-making. 58 A review 61 of interventions involving the use of risk assessment tools in primary care concluded that more comprehensive interventions that combined risk assessment with decision support were more likely to have an effect on behavior. In some cases, it may be beneficial to develop partnerships between primary care and radiology to help counsel women on appropriate supplemental screening and/or preventive measures. 62 In summary, future laws or regulations involving breast density notifications should ensure that communications promote a more comprehensive understanding of breast cancer risk to inform choices about screening and prevention.
Accepted for Publication: December 2, 2022.
Published: January 23, 2023. doi:10.1001/jamanetworkopen.2022.52209
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2023 Beidler LB et al. JAMA Network Open .
Corresponding Author: Christine M. Gunn, PhD, Dartmouth Cancer Center, The Dartmouth Institute for Health Policy and Clinical Practice, Geisel School of Medicine, Dartmouth College, 1 Medical Center Dr, Williamson Translational Research Bldg, Level 5, Lebanon, NH 03765 ( [email protected] ).
Author Contributions: Dr Wormwood had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Kressin, Battaglia, Gunn.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Beidler, Gunn.
Critical revision of the manuscript for important intellectual content: Kressin, Wormwood, Battaglia, Slanetz, Gunn.
Statistical analysis: Wormwood.
Obtained funding: Kressin.
Supervision: Slanetz, Gunn.
Conflict of Interest Disclosures: Dr Battaglia reported receiving grants from Boston Medical Center during the conduct of the study. Dr Slanetz reported receiving royalties from Wolters-Kluwer outside the submitted work and serving as subspecialty chair of the American College of Radiology Appropriateness Criteria Breast Imaging Panels. Dr Gunn reported receiving grants from the American Cancer Society during the conduct of the study and receiving grants from the National Cancer Institute and consultation fees from Gilead Sciences outside the submitted work.
Funding/Support: This study was supported by grant RSG-133017-CPHPS from the American Cancer Society (principal investigator, Dr Kressin). Dr Gunn was funded in part by the National Cancer Institute (K07CA221899; principal investigator, Dr Gunn).
Role of the Funder/Sponsor: The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
Disclaimer: The views expressed here do not necessarily reflect the views of the American Cancer Society.
Data Sharing Statement: See Supplement 2 .
Additional Contributions: Ariel Maschke, MA, Magdalena Pankowska, MPH, and Cristina Araujo Brinkerhoff, MA (Section of General Internal Medicine, Boston University Chobanian and Avedisian School of Medicine) contributed to qualitative data collection activities. They were all employed by Boston Medical Center at the time of their involvement with the project, and their role on the project was associated with their positions; they were not compensated for their work.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
Abstracting a Cancer Case
A tumor abstract summarizes the important information about a patient’s reportable tumor. Cancer Registrars must understand the contents of a medical record to be able to extrapolate required data items for the cancer abstract. The Abstracting a Cancer Case module discusses information contained in patient medical records and how a cancer registrar can utilize the medical record to recount the patient’s cancer journey in the tumor abstract. Thorough documentation of the cancer patient’s diagnosis, extent of disease and treatment supports case consolidation, quality review, and cancer research and clinical studies.
In this module you will learn to
- Identify the guidelines for abstracting a reportable cancer diagnosis.
- Describe the types of information commonly contained in most medical records.
- Identify medical procedures used assist in the diagnosis and staging of certain cancers.
- Identify pathologic examinations that contain cancer information of a tumor abstract.
Updated : December 28, 2023
ORIGINAL RESEARCH article
Relationships between body image, dyadic coping and post-traumatic growth in breast cancer patients: a cross-sectional study.

- 1 Affiliated Hospital of Jiangnan University, Wuxi, Jiangsu Province, China
- 2 Shanghai Medical College, Fudan University, Shanghai, Shanghai Municipality, China
The final, formatted version of the article will be published soon.
Select one of your emails
You have multiple emails registered with Frontiers:
Notify me on publication
Please enter your email address:
If you already have an account, please login
You don't have a Frontiers account ? You can register here
Background: The diagnosis and treatment of cancer triggers not only a negative psychological response for the patient, but also a positive psychological outcome. Positive dyadic coping, as a form of coping for mental health outcomes, can maintain or reestablish internal stability between the patient and his or her spouse, resulting in positive physical and psychological changes. However, there is a paucity of research on body image, dyadic coping, and post-traumatic growth in breast cancer patients. The purpose of this study was to explore the relationship and pathways between body image, dyadic coping, and post-traumatic growth (PTG) in breast cancer patients. Methods: A cross-sectional study was conducted from November 2022 to November 2023 at a tertiary care hospital in Wuxi, Jiangsu, China. This study was conducted among 154 breast cancer patients treated at the Affiliated Hospital of Jiangnan University, all of whom completed demographic and clinical information questionnaires, Body image self-rating questionnaire for breast cancer (BISQ-BC), Dyadic Coping Inventory (DCI) and Post Traumatic Growth Inventory (PTGI). A Pearson correlation analysis was used to explore the relationship between body image, dyadic coping, and post-traumatic growth. Structural equation modeling was used to analyze the path relationships among the three and to explore the mediating role of dyadic coping. Results: Body image were negatively correlated with post-traumatic growth (r = -0.462, p < 0.01); Body image was negatively correlated with dyadic coping (r = -0.308, p < 0.01); And dyadic coping was positively associated with post-traumatic growth (r = 0.464, p < 0.01); The structural equation modeling results supported the mediation model with the following model fit indices, chi-square to degrees of freedom ratio (χ2/df=2.05), goodness of fit index (GFI=0.93), comparative fit index (CFI=0.99), canonical fit index (NFI=0.93), incremental fit index (IFI=0.99), non-canonical fit index (TLI=0.99) and the root mean square of the difference in approximation error (RMSEA=0.03). Body image and dyadic coping directly affected post-traumatic growth (β= -0.33, p<0.05; β= 0.43, p<0.05); And body image indirectly influenced post-traumatic growth through dyadic coping (β=-0.17, p<0.05). Conclusion: Interconnections between body image, dyadic coping, and post-traumatic growth in breast cancer patients.
Keywords: Cross-sectional study, breast cancer, Nursing, body image, Dyadic coping, posttraumatic growth
Received: 10 Jan 2024; Accepted: 29 Apr 2024.
Copyright: © 2024 Wang, Wang, Tong, Zhuang, Xu, Wu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Yibo Wu, Affiliated Hospital of Jiangnan University, Wuxi, Jiangsu Province, China Ling Chen, Affiliated Hospital of Jiangnan University, Wuxi, Jiangsu Province, China
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
- Open access
- Published: 19 April 2024

Central venous access device terminologies, complications, and reason for removal in oncology: a scoping review
- Kerrie Curtis 1 , 2 , 3 ,
- Karla Gough 1 , 4 , 5 ,
- Meinir Krishnasamy 1 , 4 , 5 , 6 ,
- Elena Tarasenko 3 ,
- Geoff Hill 7 &
- Samantha Keogh 8
BMC Cancer volume 24 , Article number: 498 ( 2024 ) Cite this article
311 Accesses
1 Altmetric
Metrics details
Lack of agreed terminology and definitions in healthcare compromises communication, patient safety, optimal management of adverse events, and research progress. The purpose of this scoping review was to understand the terminologies used to describe central venous access devices (CVADs), associated complications and reasons for premature removal in people undergoing cancer treatment. It also sought to identify the definitional sources for complications and premature removal reasons. The objective was to map language and descriptions used and to explore opportunities for standardisation.
A systematic search of MedLine, PubMed, Cochrane, CINAHL Complete and Embase databases was performed. Eligibility criteria included, but were not limited to, adult patients with cancer, and studies published between 2017 and 2022. Articles were screened and data extracted in Covidence. Data charting included study characteristics and detailed information on CVADs including terminologies and definitional sources for complications and premature removal reasons. Descriptive statistics, tables and bar graphs were used to summarise charted data.
From a total of 2363 potentially eligible studies, 292 were included in the review. Most were observational studies ( n = 174/60%). A total of 213 unique descriptors were used to refer to CVADs, with all reasons for premature CVAD removal defined in 84 (44%) of the 193 studies only, and complications defined in 56 (57%) of the 292 studies. Where available, definitions were author-derived and/or from national resources and/or other published studies.
Substantial variation in CVAD terminology and a lack of standard definitions for associated complications and premature removal reasons was identified. This scoping review demonstrates the need to standardise CVAD nomenclature to enhance communication between healthcare professionals as patients undergoing cancer treatment transition between acute and long-term care, to enhance patient safety and rigor of research protocols, and improve the capacity for data sharing.
Peer Review reports
Central venous access devices (CVADs) are critical for effective and efficient management of patients with malignancies because they facilitate urgent, acute or prolonged access to the bloodstream for the administration of prescribed and supportive therapies and repeated blood sampling [ 1 ]. However, they also present considerable risk of complications and many are removed prematurely before the end of prescribed therapy. Premature removal rates of up to 50% are reported in this patient cohort [ 1 , 2 , 3 ]. Complications can be related to the coagulopathic and inflammatory processes of the disease process [ 4 ], adverse effects of prescribed therapies including prolonged and profound immunosuppression [ 3 ], and adverse effects of supportive therapies such as blood products [ 1 ]. CVAD complications and premature removal may lead to delays in treatment, reduced treatment efficacy and subsequent survival due to interruptions in schedules [ 5 ], and increased morbidity from CVAD complications (e.g., infection, mortality and healthcare expenditure) [ 1 ].
Lack of standardised nomenclature in healthcare has been shown to negatively impact patient safety, patient experience and health system efficiency [ 6 ]. The lack of a common language impairs communication and interoperability between individuals and organisations [ 6 ]. The potential for complex systems such as electronic health records (EHR) to accurately capture clinical management of patients’ care and health outcomes [ 7 ] and to inform and support research is reliant on agreed nomenclature. This enables data sharing, robust data analysis, and meets the requirements of a learning health system [ 8 ]. An example of a common global language used in healthcare is the systematised nomenclature of medicine clinical terms (SNOMED CT). SNOMED CT is a comprehensive and precise medical terminology system that is coded and linked, facilitating homogenous data entry, encoding of existing data, mapping of free text, analysis of clinical data, and interoperability between systems and organisations [ 9 ].
To date, there is no consensus on CVAD terminology and no standardised definitions for CVAD associated complications and reasons for premature removal. This is imperative to advance the quality and safety of clinical assessment and management, and to drive robust, impactful research for patients undergoing cancer treatment. A scoping review fits well with reviews that map and synthesise available evidence about a given topic and identify gaps and similarities in the published literature [ 10 ]. The aim of this review was to understand the terminologies used to describe CVADs, associated complications and reasons for premature removal in people undergoing cancer treatment. It also sought to identify the definitional sources for complications and premature removal reasons. The objective was to map language and descriptions used and to explore opportunities for standardisation.
An a priori protocol for this scoping review aligning with the five stages of Arksey and O’Malley’s scoping review framework, including identification of the research question and relevant studies, selection of studies, documentation of the data, and collating and summarising the results, was developed. Reporting was guided by the PRISMA Extension for Scoping Reviews, PRISMA-ScR [ 11 ].
Eligibility criteria
Adult patients with cancer over the age of 18 years and with any type of CVAD in situ, for example short-term centrally inserted central catheters (CICCs), or longer term CVADs, for example peripherally inserted central catheters (PICCs) or totally implantable venous access devices (TIVADs) were eligible for inclusion. In keeping with the broad aims of a scoping review, study designs included experimental, quasi-experimental, observational, systematic reviews, meta-analyses, quality improvement and surveys. Studies were limited to English and publications after the 2016 edition of the Infusion Therapy Standards of Practice [ 12 ].
Information sources
The search was executed in the MedLine, PubMed, Cochrane, CINAHL Complete and Embase databases for a comprehensive approach to the topic.
Population, concept, and context
The search strategy was developed in collaboration with a medical librarian to address the question: how are reasons for premature removal and CVAD-related complications defined in the published literature? A second question was established in response to the diversity of CVAD terminologies noted during development of the search strategy: what CVAD terminology is evident in the published literature? The broader approach of a scoping review aligns with a less restrictive search strategy based on the population, concept and context (PCC) format compared to the precise research questions, and inclusion and exclusion criteria required for a systematic review [ 13 ]. The population for this review was broad, including all patients with haematological and solid tumours as this cohort requires insertion of a CVAD for the administration of prescribed therapies for treatment of their disease.
The concept in this scoping review included the various CVAD-related complications and reasons for premature removal. This was not restricted to the more commonly reported issues of infection and thrombosis and included subject headings and key terms for clinically relevant problems such as occlusion, catheter migration, skin impairment, CVAD damage or rupture, and accidental dislodgement. Categorical descriptors (e.g., equipment failure, device removal, accidental injuries, and death) were also included.
The context was patients with any type of CVAD in situ as the different CVAD types serve different functions according to the goals of treatment, and type and length of prescribed therapies. CVADs included CICCs, PICCs, tunnelled cuffed-centrally inserted central catheters, totally implantable venous access ports, and apheresis and haemodialysis catheters. Subject headings (e.g., central venous catheters or catheterization, central venous), descriptors (e.g., cuff, tunnelled, implanted), trade names commonly used in the literature (e.g., Hickman™ or Infusaport™) were included.
The search was established for the MEDLINE database (Table 1 ), then adapted for PUBMed – National Institutes of Health (NIH), EMBASE, CINAHL and the Cochrane Library.
Subject headings and key words were combined using Boolean operators AND/OR. The search limiters applied were publication dates before 2017, non-English language, and studies in animals (including mice, mouse, rat(s), porcine, pig(s), sheep, murine, canine or rabbit) or in vitro. Excluded study designs were qualitative studies, study protocols and study reports with limited information including conference abstracts, letters to the editor, educational, posters and case studies.
Selection of sources of evidence
The search was executed in May 2022. Studies were collated and screened for duplicates in EndNote X9 by one reviewer (KC). Eligible studies were imported into Covidence, a web-based platform that streamlines the process of systematic and other literature reviews [ 14 ], during which a further 125 duplicate records were excluded (total of 5230 duplicate studies). Paired independent review of 100% of studies at title and abstract was undertaken (KC, ET), as well as at full text level (KC, ET), reasons for exclusions were noted, and the eligible studies moved forward for data extraction.
Data charting process
Data were extracted in Covidence using an a priori template established for this review by one author (KC). Data included key study (i.e., year, title, authors, country where the study took place, study design, aims and objectives, and participant details including number and diagnoses) and device (i.e., CVAD terminologies and abbreviations, terminologies used to describe CVAD complications and definitional sources, and terminologies used to describe CVAD removal reasons and definitional sources) details. Form fields were primarily free text to accurately capture the nuances in terminologies and definitional sources for premature removals and complications.
The data charting process was undertaken independently by two authors for 20% of the studies (KC, ET). Any conflicts were discussed and resolved between the two reviewers. Level of agreement was high so individual data extraction was completed for the remainder of the studies (KC).
Synthesis of results
Study data were stratified according to whether only one or multiple reasons for premature removal, or only one or multiple complications were reported. Data from studies reporting complications that did not indicate whether the complication resulted in premature removal were reported separately.
Definitional sources for complications and removal reasons were categorised as follows: national resources or guidelines (e.g., Centers for Disease Control and Prevention-National Healthcare Safety Network (CDC-NHSN), Infectious Diseases Society of America (IDSA) guidelines), other published studies, author-derived, or a combination of the first three categories. Descriptive statistics, primarily counts and percentages, tables and bar graphs were used to summarise charted data.
The search identified 31,877 records. After removing duplicates ( n = 5230) and irrelevant studies ( n = 24,390) in Endnote X9, 2363 study titles and abstracts, and then 341 full texts were screened for eligibility in Covidence. A total of 292 eligible studies were identified (Fig. 1 ).
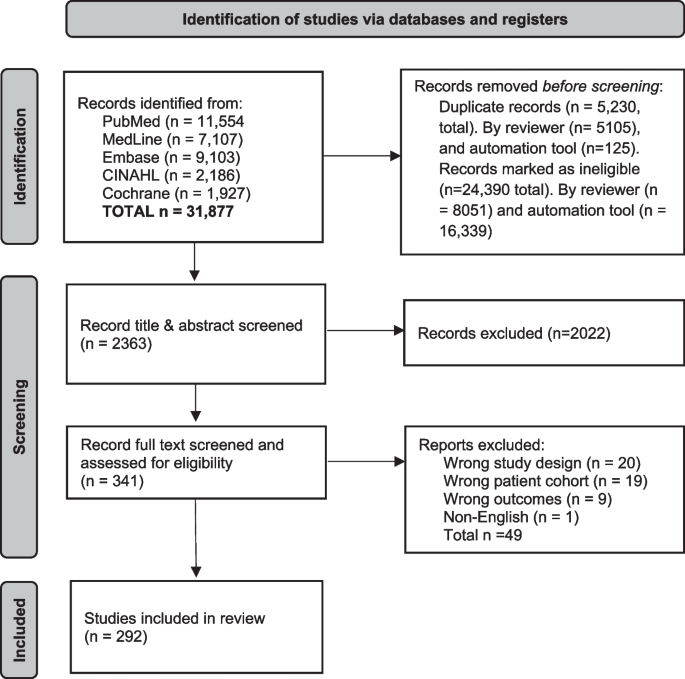
PRISMA flow diagram
Central venous access device nomenclature, and taxonomy of complications and reasons for premature removal in patients with cancer: a scoping review.
Characteristics of sources of evidence
Characteristics of the included studies are detailed in Supplement Information, Additional files 3 due to the volume of studies summarised. Of the 292 studies in this review, 193 (66%) reported on premature removal related to complications ( [ 2 , 3 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 , 144 , 145 , 146 , 147 , 148 , 149 , 150 , 151 , 152 , 153 , 154 , 155 , 156 , 157 , 158 , 159 , 160 , 161 , 162 , 163 , 164 , 165 , 166 , 167 , 168 , 169 , 170 , 171 , 172 , 173 , 174 , 175 , 176 , 177 , 178 , 179 , 180 , 181 , 182 , 183 , 184 , 185 , 186 , 187 , 188 , 189 , 190 , 191 , 192 , 193 , 194 , 195 , 196 , 197 , 198 , 199 , 200 , 201 , 202 , 203 , 204 , 205 ]. The remainder ( n = 99/34%) reported on complications only [ 206 , 207 , 208 , 209 , 210 , 211 , 212 , 213 , 214 , 215 , 216 , 217 , 218 , 219 , 220 , 221 , 222 , 223 , 224 , 225 , 226 , 227 , 228 , 229 , 230 , 231 , 232 , 233 , 234 , 235 , 236 , 237 , 238 , 239 , 240 , 241 , 242 , 243 , 244 , 245 , 246 , 247 , 248 , 249 , 250 , 251 , 252 , 253 , 254 , 255 , 256 , 257 , 258 , 259 , 260 , 261 , 262 , 263 , 264 , 265 , 266 , 267 , 268 , 269 , 270 , 271 , 272 , 273 , 274 , 275 , 276 , 277 , 278 , 279 , 280 , 281 , 282 , 283 , 284 , 285 , 286 , 287 , 288 , 289 , 290 , 291 , 292 , 293 , 294 , 295 , 296 , 297 , 298 , 299 , 300 , 301 , 302 , 303 , 304 ] Characteristics are summarised using counts and percentages.
Samples included patients with solid tumours only ( n = 93), haematological malignancies and solid tumours ( n = 92), and haematological malignancies only ( n = 56). The remainder were described as cancer patients ( n = 51). Studies were conducted in China, ( n = 61), the United States of America (USA) ( n = 41), Italy ( n = 25), Japan and Korea (both n = 15), and Australia, Germany and Turkey (all n = 13). Twelve were multinational. According to the Joanna Briggs Institute’s levels of evidence [ 13 ], most studies were level 4 observational, descriptive studies ( n = 174). The remainder were level 3 observational, analytical designs ( n = 61), level 2 quasi-experimental designs ( n = 31), level 1 experimental designs ( n = 24) and level 5 expert opinion, bench research ( n = 2).
CVAD terminologies
A total of 213 unique descriptors were extracted from the included studies: 14 unique terms for CVADs, 104 for totally implantable venous access ports, 25 for peripherally inserted central catheters, 41 for tunnelled cuffed centrally inserted central catheters, 27 for centrally inserted central catheters, and two for femorally inserted central catheters. This did not include spelling variations, hyphenation, or use of capitals, or the use of multiple different terms for the device in the same study. The greatest variation was related to the descriptive nature of the names. For example, for totally implantable venous access ports the descriptors included combinations of totally or fully, subcutaneously or tunnelled, implanted or implantable; chest, arm, subclavian, internal jugular, brachial, groin or centrally inserted; devices, catheters, ports or systems; central venous, vascular or venous access; single or dual chamber; chemotherapy or infusion; traditional or power-injectable; PICC, peripherally inserted or peripheral central ports; variations on port, portacath, portacath and the various trade names.
Premature CVAD removal related to complications
Of the 193 studies that reported on premature removals, 128 (66%) identified multiple types of complications including catheter occlusion, malposition, dislodgement, fracture, local bleeding, infection, or skin necrosis. The remainder ( n = 65, 34%) identified one complication only, most commonly infection ( n = 18) or thrombosis ( n = 14).
In studies reporting on multiple reasons for premature removal, definitional sources were not provided in 45 (35%) studies, for one reason only in 37 (29%) studies, and for all reasons in 46 (36%) studies. In studies that reported one premature removal reason only, the definition was provided in 47 (72%) studies, and not provided in 18 (28%) studies. The definitional sources in these studies included local national resources or guidelines in 21 (45%) studies, author-derived definitions in 19 (40%), definitions from other published studies in six (13%) and a combination of these sources in one (2%) study. The definitional sources in studies with multiple reasons for removal included a combination of national guidelines or resources, definitions from other published studies or author-derived definitions (Fig. 2 ).
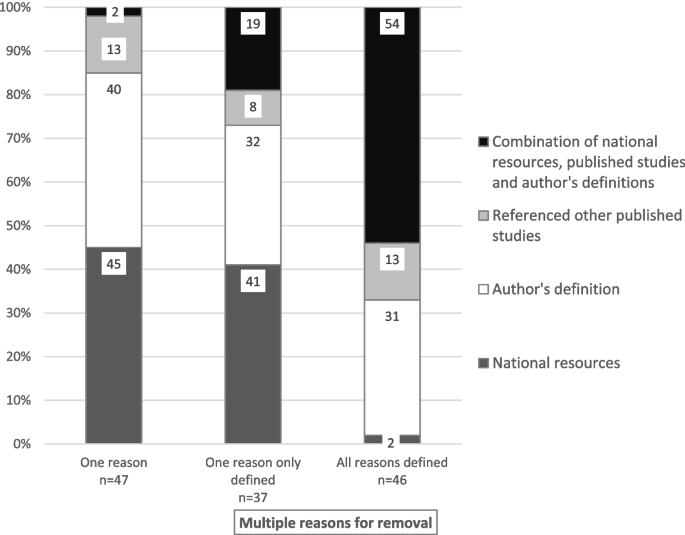
Definitional sources for premature CVA removal where provided
CVAD complications
Of the 99 studies that reported CVAD-related complications, 49 (49%) reported one complication and 50 (51%) reported on multiple complications. Complication definitions were provided in 36 (73%) studies reporting one complication, and no definitions provided in 13 (27%). For studies that reported on multiple complications, all complications were defined in 20 (40%) studies, only one and not all complications in 14 (28%) studies, and no complication definitions were provided in 16 (32%) studies.
Definitional sources in studies that reported one type of complication were from national resources or guidelines in 16 (44%) studies (e.g., CDC-NHSN or IDSA), author-derived in 14 (39%), and from other published studies in six (17%) studies (Fig. 3 ). Comparatively, of the studies that reported on multiple complications, fewer referenced national resources ( n = 2, 10%); more were author-derived ( n = 10, 50%) or used a combination of sources ( n = 8, 40%) when all complications were defined. Definitional sources were from national resources in three [ 21 ] studies, author-derived in eight (57%) studies, other published studies in one (7%) and a combination of sources in two (14%) studies that defined only one of the multiple complications.
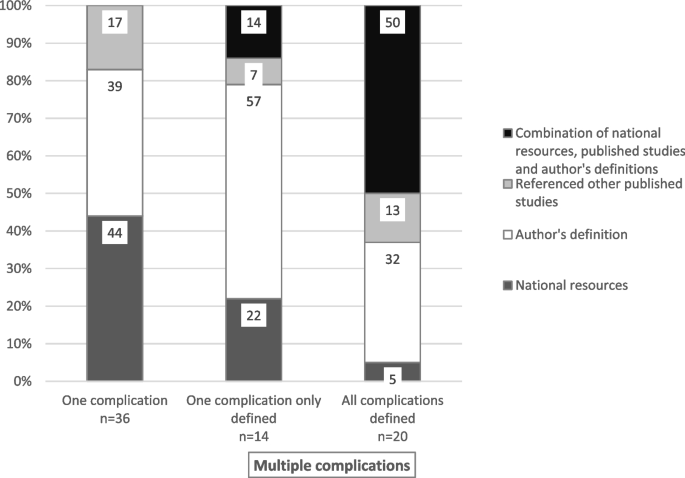
Definitional sources for CVAD complications where provided
This review identified considerable variation in CVAD terminology related to reason for removal and the actual device itself. This included over 200 unique names for the different types of CVADs, with the greatest variation evident for totally implantable venous access devices or ports with over 100 unique names. In addition to inconsistency with definitions and device terminology between studies, inconsistencies were also observed within the same study, underscoring the complexity and confusion in this clinical issue.
Terminologies were used interchangeably such as central venous catheters (CVC) and central venous access devices . CVC was also used to describe the multi-lumen catheter most commonly used in critical care units. Despite the term central venous catheter being used more frequently as the term to describe all types of devices, it does not accurately describe or reflect the wide variety of implanted, cuffed or tunnelled catheters and devices, or contemporary innovations in insertion techniques; for example, tunnelling PICCs. The term central venous access device is more inclusive, intuitive, and reflective of the diversity in contemporary clinical practice [ 305 ].
Similar findings have previously been reported in other research. In a Delphi consensus study about a minimum dataset for vascular access, no standardised CVADs terms were identified [ 306 ]. The authors advocated for development of a vascular access minimum dataset to overcome lack of clarity in the literature that hampers robust data collection, analysis and interoperability within and across countries, ultimately adversely affecting patient outcomes [ 6 , 306 ]. In response to their findings, Schults et al. (2020) subsequently developed a common set of descriptors (nomenclature) for commonly used vascular access devices [ 306 ]. However, these descriptors did not include CVADs commonly used in cancer care (e.g., tunnelled cuffed centrally inserted central catheters, apheresis catheters), and contemporary insertion techniques (e.g. tunnelled peripherally inserted central catheters). A more comprehensive set of descriptors need to be developed to represent CVADs used in cancer care.
Considerable variation in CVAD nomenclature evident in this review is problematic. A lack of standardised nomenclature impairs communication and interoperability between healthcare professionals and organisations locally and globally, and fractures data sharing, linkage, analysis and the evidence base from clinical practice [ 6 , 306 ]. The World Health Organization states that standardised nomenclature is essential for recording and surveillance of all types of medical devices including CVADs [ 307 ], and in the systematic review of 20 papers by Gildow and Lazar (2022), standardised nomenclature was shown to be associated with reduced clinical errors and patient injury, improved communication and opportunity for standardisation of clinical care [ 308 ].
Most studies reported multiple reasons for premature device removal as opposed to a single reason for removal. Research investigating multiple reasons for removal reflects the increasing complexity of care and treatment for people with cancer, the majority of whom require CVAD support. The multiplicity of treatment and supporting therapies that commonly characterise care for a person with cancer, compounded by patient, clinician, therapy, and workplace related factors, come together to compound risk of premature CVAD removal. The interplay between one or more of these factors increases the risk of premature removal increasing morbidity and mortality, and cost of care [ 4 , 309 , 310 , 311 ].
The only consistently defined premature removal reason was infection. Nearly all studies cited national sources for catheter-related blood stream infection (CRBSI) or the surveillance definition for central line-associated blood stream infection (CLABSI), with the majority citing CDC [ 312 ] or IDSA [ 313 ] from the USA. There was no consistency in definitions for any other reason for premature removal. This is an important finding with overt implications for quality and safety of care. Heterogeneity of terminology and definitions impair standardised clinical management by causing confusion and permitting an inconsistent approach for the different members of the healthcare team and clinical specialties, and consequently negatively impacts quality and safety of patients [ 314 ]. Standardised nomenclature, clinical procedures and standardisation of care have been shown to reduce errors and patient injury by improving communication and dissemination of evidence to inform clinical practices [ 308 ].
The infinite potential for utilising routinely collected patient management data and outcomes captured in EHR systems for clinical research into improving patient care and outcomes [ 315 ] cannot be realised when such variation exists. Consistency in EHR data is key to the efficient and effective collation and linkage of data required for the development of a reliable big data set [ 308 , 315 ]. Clinical data, expertise and knowledge integrated with current evidence are the cornerstones of a learning health system which aims to provide informed, safer, higher quality clinical care [ 8 ]. Also, consistent data and definitions are required for meta-analyses in quantitative research [ 218 ].
Standardised nomenclature in healthcare is complex requiring a multifaceted response. Strategies require collaboration, consensus, communication, and implementation by multidisciplinary professionals including clinicians, health economists, and health service researchers, strategists, and implementation science professionals. This includes commitment by journals, national peak bodies and associations to use the standardised nomenclature as consistency at a system level is required to provide the guidance for the end users. Furthermore, regular review of nomenclature is required so it accurately reflects contemporary evidence in the literature, clinical practice, emerging technology and products.
As EHRs become increasingly prevalent across health services, they offer opportunity for standardisation of clinical nomenclature. For example, different standardised global clinical languages such as SNOMED CT or International Classification of Diseases 10 th Revision are translatable and already have equivalent codes for use in EHRs. Leveraging the opportunity of EHRs will require close collaboration between EHR development teams and all end users of the EHR systems.
Limitations
There are a number of limitations of this scoping review. Limiting the patient cohort to patients with cancer may restrict the applicability to other patient cohorts. However, this was considered to have minimal impact as CVADs are used across multiple patient cohorts. The date range was five years after the 2016 edition of the Infusion Therapy Standards of Practice [ 12 ], so all descriptors and definitions may not be captured; however, it reflects contemporary practice, policy and research. The volume of studies did not allow for analysis beyond the absolute numbers of the different types of CVADs and categories of resources for definitions of CVAD complications and reasons for removal. Establishing consistent definitions for each type of premature removal or complication was not possible. The exclusion of non-English studies is important to acknowledge as a limitation when considering the results and findings of this review.
Conclusions
Standardised CVAD nomenclature and definitions for premature CVAD removal and complications do not exist. This impacts effective and accurate communication and has been shown to hamper safe, effective cancer care. It also prevents interoperability between individuals and organisations globally to inform research to reduce the incidence and impact of CVAD complications and premature removal on cancer and patients’ experience of care, health outcomes and health system costs. Collaboration, consensus, and standardisation is required to deliver quality CVAD care.
Availability of data and materials
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Zakhour R, Chaftari AM, Raad II. Catheter-related infections in patients with haematological malignancies: novel preventive and therapeutic strategies. Lancet Infect Dis. 2016;16(11):e241–50.
Article PubMed Google Scholar
Moss JG, Wu O, Bodenham AR, Agarwal R, Menne TF, Jones BL, et al. Central venous access devices for the delivery of systemic anticancer therapy (CAVA): a randomised controlled trial. Lancet. 2021;398(10298):403–15.
Mariggiò E, Iori AP, Micozzi A, Chistolini A, Latagliata R, Berneschi P, et al. Peripherally inserted central catheters in allogeneic hematopoietic stem cell transplant recipients. Support Care Cancer. 2020;28(9):4193–9.
Levi M, Sivapalaratnam S. An overview of thrombotic complications of old and new anticancer drugs. Thromb Res. 2020;191 Suppl 1:S17–21.
van Waart H, Stuiver MM, van Harten WH, Geleijn E, Kieffer JM, Buffart LM, et al. Effect of low-intensity physical activity and moderate- to high-intensity physical exercise during adjuvant chemotherapy on physical fitness, fatigue, and chemotherapy completion rates: results of the PACES randomized clinical trial. J Clin Oncol. 2015;33(17):1918–27.
World Health Organisation. Health products policy and standards. Nomenclature of medical devices. 2021. Retrieved from: https://www.who.int/teams/health-product-policy-and-standards/assistive-and-medical-technology/medical-devices/nomenclature . Accessed 15 Dec 2022.
Cornet R, de Keizer N. Forty years of SNOMED: a literature review. BMC Med Inf Decis Mak. 2008;8 Suppl 1(Suppl 1):S2.
Article Google Scholar
Agency for Healthcare Research and Quality. About learning health systems Rockville, MD. 2019. Available from: https://www.ahrq.gov/learning-health-systems/about.html .
Gaudet-Blavignac C, Foufi V, Bjelogrlic M, Lovis C. Use of the systematized nomenclature of medicine clinical terms (SNOMED CT) for processing free text in health care: systematic scoping review. J Med Internet Res. 2021;23(1):e24594.
Article PubMed PubMed Central Google Scholar
Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32.
Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, et al. PRISMA Extension for Scoping Reviews (PRISMAScR): Checklist and Explanation. Ann Intern Med. 2018;169:467–473.
Gorski L, Hadaway L, Hagle ME, McGoldrick M, Orr M, Doellman D. Infusion therapy: standards of practice. J Infus Nurs. 2016;39(1S):S1–159.
The Joanna Briggs Institute. Joanna Briggs Institute Reviewers’ Manual: 2015 edition / supplement2015. Available from: https://reben.com.br/revista/wp-content/uploads/2020/10/Scoping.pdf .
Covidence. Veritas health innovation. 2023. Available from: https://www.covidence.org .
Google Scholar
Aghamohammadi D, Fakhari S, Ataei Y, Bilehjani E, Jafari M. Totally implantable venous access port infection in northwest of Iran. Crescent J Med Biol Sci. 2017;4(3):126–30.
Anbar R, Avci D, Cetinkaya A. Port catheter complications and thrombosis issues: assessment of 114 patients with port catheter implantation by single surgeon. Biomedical Res Therapy. 2017;4(12):1898–910.
Aribas BK, Tiken R, Aribas O, Uylar T, Akdulum I, Turker I, et al. Factors on patency periods of subcutaneous central venous Port: long-term results of 1,408 patients. Iran J Radiol. 2017;14(2):1.
Bai X, Gu X, Cheng L, Yuan Q, Jing J, Jin Y, et al. Clinical diagnosis and treatment of peripherally inserted central catheter related upper extremity deep venous thrombosis. Biomedical Res (India). 2017;28(22):9707–11.
CAS Google Scholar
Busch JD, Vens M, Herrmann J, Adam G, Ittrich H. Material failure of silicone catheter lines: a retrospective review of partial and complete ruptures in 553 patients. AJR Am J Roentgenol. 2017;208(2):464–9.
Chaftari P, Chaftari AM, Adachi J, Hachem R, Raad S, Natividad E, et al. Improvement in the diagnosis of catheter-related bloodstream infections in a tertiary cancer center. Am J Infect Control. 2017;45(3):e34–39.
Chan RJ, Northfield S, Larsen E, Mihala G, Ullman A, Hancock P, et al. Central venous access device securement and dressing effectiveness for peripherally inserted central catheters in adult acute hospital patients (CASCADE): a pilot randomised controlled trial. Trials. 2017;18(1):458.
Chang DH, Mammadov K, Hickethier T, Borggrefe J, Hellmich M, Maintz D, et al. Fibrin sheaths in central venous port catheters: treatment with low-dose, single injection of urokinase on an outpatient basis. Ther Clin Risk Manag. 2017;13:111–5.
Article CAS PubMed PubMed Central Google Scholar
Chen MH, Hwang WL, Chang KH, Chiang LCJ, Teng CLJ. Application of peripherally inserted central catheter in acute myeloid leukaemia patients undergoing induction chemotherapy. Eur J Cancer Care. 2017;26(6):e12627.
Cornillon J, Martignoles JA, Tavernier-Tardy E, Gire M, Martinez P, Tranchan C, et al. Prospective evaluation of systematic use of peripherally inserted central catheters (PICC lines) for the home care after allogeneic hematopoietic stem cells transplantation. Support Care Cancer. 2017;25(9):2843–7.
Article CAS PubMed Google Scholar
Diaz JA, Rai SN, Wu X, Chao JH, Dias AL, Kloecker GH. Phase II trial on extending the maintenance flushing interval of Implanted ports. J Oncol Pract. 2017;13(1):e22–28.
Fang S, Jiang Y, Yang J, Song L, Liu Y. Comparison of three types of central venous catheters in patients with malignant tumor receiving chemotherapy. Patient Prefer Adherence. 2017;11:1197–204.
Grau D, Clarivet B, Lotthe A, Bommart S, Parer S. Complications with peripherally inserted central catheters (PICCs) used in hospitalized patients and outpatients: a prospective cohort study. Antimicrob Resist Infect Control. 2017;6:18.
Hashimoto Y, Fukuta T, Maruyama J, Omura H, Tanaka T. Experience of peripherally inserted central venous catheter in patients with hematologic diseases. Intern Med (Tokyo Japan). 2017;56(4):389–93.
Hyo-Cheol K, Saebeom H, Hoyong J. Malfunction of totally implantable central venous ports. Iran J Radiol. 2017;14(1):1.
Kakkos A, Bresson L, Hudry D, Cousin S, Lervat C, Bogart E, et al. Complication-related removal of totally implantable venous access port systems: does the interval between placement and first use and the neutropenia-inducing potential of chemotherapy regimens influence their incidence? A four-year prospective study of 4045 patients. Eur J Surg Oncol. 2017;43(4):689–95.
Kang J, Chen W, Sun W, Ge R, Li H, Ma E, et al. Peripherally inserted central catheter-related complications in cancer patients: a prospective study of over 50,000 catheter days. J Vasc Access. 2017;18(2):153–7.
Kang JR, Long LH, Yan SW, Wei WW, Jun HZ, Chen W. Peripherally inserted central catheter-related vein thrombosis in patients with lung cancer. Clin Appl Thromb Hemost. 2017;23(2):181–6.
Kao PF, Weng JH, Tyan YS, Yang SF, Tsao TC. The incidence of totally implantable venous access devices insertion and the associated abnormalities in patients with cancer revealed in (18)F-FDG PET-CT imaging. Acad Radiol. 2017;24(12):1588–95.
Liscynesky C, Johnston J, Haydocy KE, Stevenson KB. Prospective evaluation of peripherally inserted central catheter complications in both inpatient and outpatient settings. Am J Infect Control. 2017;45(9):1046–9.
Lo Priore E, Fliedner M, Heverhagen JT, Novak U, Marschall J. The role of a surveillance programme for intro-ducing peripherally inserted central catheters: a 2-year observational study in an academic hospital. Swiss Med Wkly. 2017;147:w14441.
PubMed Google Scholar
Longo R, Llorens M, Goetz C, Platini C, Eid N, Sellies J, et al. Taurolidine/citrate lock therapy for primary prevention of catheter-related infections in cancer patients: results of a prospective, randomized, phase IV trial (ATAPAC). Oncology. 2017;93(2):99–105.
Luong NV, Kroll MH, Vu K. Recurrence of venous thromboembolism among adults acute leukemia patients treated at the University of Texas MD Anderson cancer center: incidence and risk factors. Thromb Res. 2017;156:14–9.
Nakamura T, Sato T, Watanabe M, Sasaki J, Asari Y, Torii S. Complications after implantation of subcutaneous central venous ports (PowerPort). Annals Med Surg. 2017;17:1.
Paquet F, Boucher LM, Valenti D, Lindsay R. Impact of arm selection on the incidence of PICC complications: results of a randomized controlled trial. J. 2017;18(5):408–14.
Rickard CM, Marsh NM, Webster J, Gavin NC, Chan RJ, McCarthy AL, et al. Peripherally InSerted CEntral catheter dressing and securement in patients with cancer: the PISCES trial. Protocol for a 2 × 2 factorial, superiority randomised controlled trial. BMJ Open. 2017;7(6):e015291.
Seo TS, Song MG, Kim JS, Choi CW, Seo JH, Oh SC, et al. Long-term clinical outcomes of the single-incision technique for implantation of implantable venous access ports via the axillary vein. J Vasc Access. 2017;18(4):345–51.
Solinas G, Platini F, Trivellato M, Rigo C, Alabiso O, Galetto AS. Port in oncology practice: 3-monthly locking with normal saline for catheter maintenance, a preliminary report. J Vasc Access. 2017;18(4):325–7.
Tabatabaie O, Kasumova GG, Eskander MF, Critchlow JF, Tawa NE, Tseng JF. Totally implantable venous access devices: a review of complications and management strategies. Am J Clin Oncol. 2017;40(1):94–105.
Verboom MC, Ouwerkerk J, Gelderblom H, Steeghs N, Kerst JM, Lutjeboer J, et al. Central venous access related adverse events after trabectedin infusions in soft tissue sarcoma patients// experience and management in a nationwide multi-center study. Clin Sarcoma Res. 2017;7(1):2.
Wang YC, Lin PL, Chou WH, Lin CP, Huang CH. Long-term outcomes of totally implantable venous access devices. Support Care Cancer. 2017;25(7):2049–54.
Webster J, Larsen E, Marsh N, Choudhury A, Harris P, Rickard CM. Chlorhexidine gluconate or polyhexamethylene biguanide disc dressing to reduce the incidence of central-line-associated bloodstream infection: a feasibility randomized controlled trial (the CLABSI trial). J Hosp Infect. 2017;Date of Publication: January 29.
Xie J, Xu L, Xu X, Huang Y. Complications of peripherally inserted central catheters in advanced cancer patients undergoing combined radiotherapy and chemotherapy. J Clin Nurs. 2017;26(23–24):4726–33.
Zerla PA, Canelli A, Cerne L, Caravella G, Gilardini A, De Luca G, et al. Evaluating safety, efficacy, and cost-effectiveness of PICC securement by subcutaneously anchored stabilization device. J Vasc Access. 2017;18(3):238–42.
Zhou H, Yang B, Wang C, Qin Y. Analysis and clinical significance of venography findings in complications associated with peripherally inserted central catheters. Biomedical Res (India). 2017;28(15):6619–25.
Alfonso Alvarez-Rodriguez J, Garcia-Suarez M, Fernandez-Garcia D, Mendez-Martinez C, Gomez-Salgado J. Analysis of peripheral central venous access ports at the forearm: an observational study. Eur J Cancer Care. 2018;27(6):e12929.
Alkindi SY, Chai-Adisaksopha C, Cheah M, Linkins L-A. Management of cancer-associated upper extremity deep vein thrombosis with and without venous catheters at a tertiary care center. Thromb Res. 2018;166:92–5.
Bouzidi H, Emirian A, Marty A, Chachaty E, Laplanche A, Gachot B, et al. Differential time to positivity of central and peripheral blood cultures is inaccurate for the diagnosis of Staphylococcus aureus long-term catheter-related sepsis. J Hosp Infect. 2018;99(2):192–9.
Burbridge B, Plewes C, Stoneham G, Szkup P, Otani R, Babyn P, et al. Randomized clinical trial evaluating complications and complication-related removal of arm-situated power-injectable and non-power-injectable totally implanted venous access devices among cancer patients. J Vasc Interv Radiol. 2018;29(5):648–656.e3.
Chaftari AM, Hachem R, Raad S, Jiang Y, Natividad E, Chaftari P, et al. Unnecessary removal of central venous catheters in Cancer patients with bloodstream infections. Infect Control Hosp Epidemiol. 2018;39(2):222–5.
Chopra V, Kaatz S, Grant P, Swaminathan L, Boldenow T, Conlon A, et al. Risk of venous thromboembolism following peripherally inserted central catheter exchange: an analysis of 23,000 hospitalized patients. Am J Med. 2018;131(6):651–60.
Davies GA, Lazo-Langner A, Gandara E, Rodger M, Tagalakis V, Louzada M, et al. A prospective study of Rivaroxaban for central venous catheter associated upper extremity deep vein thrombosis in cancer patients (catheter 2). Thromb Res. 2018;162:88–92.
Decousus H, Bourmaud A, Fournel P, Bertoletti L, Labruyère C, Presles E, et al. Cancer-associated thrombosis in patients with implanted ports: a prospective multicenter French cohort study (ONCOCIP). Blood. 2018;132(7):707–16.
El-Balat A, Schmeil I, Karn T, Holtrich U, Mavrova-Risteska L, Rody A, et al. Catheter-related complications of subcutaneous implantable venous access devices in breast cancer patients. Vivo. 2018;32(5):1275–81.
Htun KT, Ma MJY, Lee AYY. Incidence and outcomes of catheter related thrombosis (CRT) in patients with acute leukemia using a platelet-adjusted low molecular weight heparin regimen. J Thromb Thrombolysis. 2018;46(3):386–92.
Imaoka Y, Kuranishi F, Ogawa Y. Usefulness of totally implantable central venous access devices in elderly patients: a retrospective study. Ann Nutr Metab. 2018;72(2):112–6.
Kato Y, Hagihara M, Kurumiya A, Takahashi T, Sakata M, Shibata Y, et al. Impact of mucosal barrier injury laboratory-confirmed bloodstream infection (MBI-LCBI) on central line-associated bloodstream infections (CLABSIs) in department of hematology at single university hospital in Japan. J Infect Chemotherapy: Official J Japan Soc Chemother. 2018;24(1):31–5.
Kiesow L, Mahnken AH, Keulers AR. Port Implantation in patients with severe thrombocytopenia is safe with interventional radiology. Cardiovasc Interv Radiol. 2018;41(1):80–6.
Lee YM, Lee MS, Park KH, Moon C, Kim YJ, Lee HJ. Clinical impact of delayed catheter removal for patients with central-venous-catheter-related Gram-negative bacteraemia. J Hosp Infect. 2018;99(1):106–13.
Liu K, Zhou Y, Xie W, Chen X, Wang H, Gu Z, et al. Handgrip exercise reduces peripherally-inserted central catheter-related venous thrombosis in patients with solid cancers: a randomized controlled trial. Int J Nurs Stud. 2018;86:99–106.
Madabhavi I, Patel A, Anand A, Kataria P, Kadakol N, Sarkar M. Use of tunneled-cuffed central catheters in patients with cancer: a single-center experience. JAVA - J Association Vascular Access. 2018;23(1):23–9.
Madabhavi I, Patel A, Anand A, Sarkar M, Kataria P, Kadakol N. A study of the use of peripherally inserted central catheters in cancer patients: a single-center experience. J Vascular Nursing: Official Publication Soc Peripheral Vascular Nurs. 2018;36(3):149–56.
Nucci M, Braga PR, Nouer SA, Anaissie E. Time of catheter removal in candidemia and mortality. Brazilian J Infect Diseases. 2018;22(6):455–61.
Ohtake S, Nakagawa M, Uchino Y, Miura K, Iriyama N, Hatta Y, et al. 1% chlorhexidine-alcohol for preventing central venous catheter-related infection during intensive chemotherapy for patients with haematologic malignancies. J Infect Chemother. 2018;24(7):544–8.
Raad S, Chaftari AM, Hachem RY, Shah P, Natividad E, Cleeland CS, et al. Removal and insertion of central venous catheters in cancer patients is associated with high symptom burden. Expert Rev Med Dev. 2018;15(8):591–6.
Article CAS Google Scholar
Samuelson C, Kaur H, Kritsotakis EI, Goode SD, Nield A, Partridge D. A daily topical decontamination regimen reduces catheter-related bloodstream infections in haematology patients. J Infect. 2018;76(2):132–9.
Tippit D, Ananthula A, Siegel E, Ochoa D, Hill E, Merrill A, et al. Upper-extremity deep vein thrombosis in patients with breast cancer with chest versus arm central venous port catheters. Breast Cancer. 2018;12:1178223418771909.
PubMed PubMed Central Google Scholar
Vermeulin T, Lucas M, Marini H, Di Fiore F, Loeb A, Lottin M, et al. Totally implanted venous access-associated adverse events in oncology: results from a prospective 1-year surveillance programme. Bull Cancer. 2018;105(11):1003–11.
Voog E, Bourgeois H, Domont J, Denis F, Emmanuel E, Dupuis O, et al. Totally implantable venous access ports: a prospective long-term study of early and late complications in adult patients with cancer. Support Care Cancer. 2018;26(1):81–9.
Yang S-S, Ahn MS. A comparison between upper arm and chest for optimal site of totally implanted venous access ports in patients with female breast cancer. Ann Vasc Surg. 2018;50:128–34.
Yanik F, Karamustafaoglu YA, Karatas A, Yoruk Y. Experience in totally implantable venous port catheter: analysis of 3,000 patients in 12 years. Turk gogus kalp damar cerrahisi dergisi. 2018;26(3):422–8.
Zhang S, Kobayashi K, Faridnia M, Skummer P, Zhang D, Karmel MI. Clinical predictors of port infections in adult patients with hematologic malignancies. J Vascular Interventional Radiology: JVIR. 2018;29(8):1148–55.
Ahmad A, Hjerming M, Kjeldsen L, Bjerrum OW, Moser C, Classen V, et al. Hydrochloric acid prolongs the lifetime of central venous catheters in haematologic patients with bacteraemia. Dan Med J. 2019;66(5):A5544.
Ammar G, Almashaikh E, Ibdah A, Shajrawi W, Awawdeh S, Al Mousa A, et al. Impact of early dressing removal on tunneled central venous catheters: a piloting study. Asian Pac J Cancer Prev. 2019;20(9):2693–7.
Campagna S, Berchialla P, Gonella S, Dimonte V, Mussa B, Morano G, et al. Can peripherally inserted central catheters be safely placed in patients with Cancer receiving chemotherapy? A retrospective study of almost 400,000 catheter-days. Oncologist. 2019;24(9):e953–959.
Campagna S, Gonella S, Berchialla P, Rigo C, Morano G, Zerla PA, et al. A retrospective study of the safety of over 100,000 peripherally-inserted central catheters days for parenteral supportive treatments. Res Nurs Health. 2019;42(3):198–204.
Chou PL, Fu JY, Cheng CH, Chu Y, Wu CF, Ko PJ, et al. Current port maintenance strategies are insufficient: view based on actual presentations of implanted ports. Med (Baltim). 2019;98(44):e17757.
da Costa ACC, Ribeiro JM, Vasques CI, De Luca Canto G, Porporatti AL, Dos Reis PED. Interventions to obstructive long-term central venous catheter in cancer patients: a meta-analysis. Supportive care cancer: Official J Multinational Association Supportive Care Cancer. 2019;27(2):407–21.
Fares J, Khalil M, Chaftari AM, Hachem R, Jiang Y, Kantarjian HM, et al. Impact of catheter management on clinical outcome in adult cancer patients with Gram-negative bacteremia. Open Forum Infect Dis. 2019;6(10):ofz357.
Gray KL, Benson HL, Pearce CL, Steidley IG, Bachman AM, Adamski J. Implementation and 2-year outcomes of the first FDA-approved implantable apheresis vascular access device. Transfusion. 2019;59(11):3461–7.
Harrold K, Martin A, Bhuva N. A prospective audit evaluating use of urokinase in oncology patients with occluded central venous access devices. Br J Nurs. 2019;28(19):S30–36.
Hong S, Seo TS, Song MG, Seol HY, Suh SI, Ryoo IS. Clinical outcomes of totally implantable venous access port placement via the axillary vein in patients with head and neck malignancy. J Vasc Access. 2019;20(2):134–9.
Kim IJ, Shim DJ, Byeon JH, Lee JH, Kim ET, Lee HJ, et al. Impact of subcutaneous tunnels on peripherally inserted catheter placement: a multicenter retrospective study. Eur Radiol. 2019;29(5):2716–23.
Li G, Zhang Y, Ma H, Zheng J. Arm port vs chest port: a systematic review and meta-analysis. Cancer Manage Res. 2019;11:6099–112.
Nezami N, Groenwald M, Silin D, Latich I, Xing M, Kokabi N. Risk factors of infection and role of antibiotic prophylaxis in totally implantable venous access port placement: propensity score matching. Cardiovasc Interv Radiol. 2019;42(9):1302–10.
Picardi M, Della Pepa R, Cerchione C, Pugliese N, Mortaruolo C, Trastulli F, et al. A frontline approach with peripherally inserted versus centrally inserted central venous catheters for remission induction chemotherapy phase of Acute myeloid leukemia: a randomized comparison. Clin Lymphoma Myeloma Leuk. 2019;19(4):e184–194.
Ruiz-Giardin JM, Ochoa Chamorro I, Velazquez Rios L, Jaqueti Aroca J, Garcia Arata MI, SanMartin Lopez JV, et al. Blood stream infections associated with central and peripheral venous catheters. BMC Infect Dis. 2019;19(1):841.
Seckold T, Walker S, Dwyer T, Signal T. Peripherally inserted central catheter postinsertion complications: a retrospective study. J Association Vascular Access. 2019;24(1):Oct–20.
Suleman A, Jarvis V, Hadziomerovic A, Carrier M, McDiarmid S. Implanted vascular access device related deep vein thrombosis in oncology patients: a prospective cohort study. Thromb Res. 2019;177:117–21.
Balsorano P, Romagnoli S, Pinelli F, Virgili G, Villa G, De Gaudio AR, et al. Peripherally inserted central catheter-related thrombosis rate in modern vascular access era-when insertion technique matters: a systematic review and meta-analysis. J Vasc Access. 2020;21(1):45–54.
Bertoglio S, Cafiero F, Meszaros P, Varaldo E, Blondeaux E, Molinelli C, et al. PICC-PORT totally implantable vascular access device in breast cancer patients undergoing chemotherapy. J Vasc Access. 2020;21(4):460–6.
Calò F, Retamar P, Martínez Pérez-Crespo PM, Lanz-García J, Sousa A, Goikoetxea J, et al. Catheter-related bloodstream infections: predictive factors for Gram-negative bacteria aetiology and 30 day mortality in a multicentre prospective cohort. J Antimicrob Chemother. 2020;75(10):3056–61.
Carvalho Castanho LE, Nogueira dos Santos B, Salles Margatho A, Merizio Martins Braga FT, Diniz PE, de Oliveira MC, et al. Chlorhexidine gel dressing in hematopoietic stem cell transplantation. Acta Paulista de Enfermagem. 2020;33(3):1.
Chen Y, Chen H, Yang J, Jin W, Fu D, Liu M, et al. Patterns and risk factors of peripherally inserted central venous catheter-related symptomatic thrombosis events in patients with malignant tumors receiving chemotherapy. J Vascular Surg Venous Lymphatic Disorders. 2020;8(6):919–29.
Choksi A, Finnegan K, Etezadi V. Does systemic antibiotic prophylaxis prior to the placement of totally implantable venous access devices reduce early infection? A retrospective study of 1,485 cases at a large academic institution. Am J Infect Control. 2020;48(1):95–9.
Clatot F, Fontanilles M, Lefebvre L, Lequesne J, Veyret C, Alexandru C, et al. Randomized phase II trial evaluating the safety of peripherally inserted central catheters vs implanted port catheters during adjuvant chemotherapy in early breast cancer patients. Ann Oncol. 2019;30:v739.
Dai C, Li J, Li QM, Guo X, Fan YY, Qin HY. Effect of tunneled and nontunneled peripherally inserted central catheter placement: a randomized controlled trial. J Vasc Access. 2020;21(4):511–519.
de Silveira CP, Braga RC, Galvao FTMM, dos Reis CM, Ferreira PED, Clark EB. AM. Dressings for the central venous catheter to prevent infection in patients undergoing hematopoietic stem cell transplantation: a systematic review and meta-analysis. Support Care in Cancer. 2020;28(2):425–438.
de la Cruz-Hernández I, Cornejo-Juárez P, Tellez-Miranda O, Barrera-Pérez L, Sandoval-Hernández S, Vilar-Compte D, et al. Microbiology and prevalence of E2SKAPE-resistant strains in catheter-related bloodstream infections in patients with cancer. Am J Infect Control. 2020;48(1):40–5.
de Mooij CEM, van der Velden WJFM, van Groningen LFJ, Blijlevens NMA, Verweij PE, Meijer C, et al. Surveillance of catheter-related bloodstream infections in haemato-oncology patients: comparison of two definitions. J Hosp Infect. 2020;105(4):686–90.
Gudiol C, Arnan M, Aguilar-Guisado M, Royo-Cebrecos C, Sanchez-Orteg I, Montero I, et al. A randomized, double-blind, placebo-controlled trial (TAURCAT study) of citrate lock solution for prevention of endoluminal central venous catheter infection in neutropenic hematological patients. Antimicrob Agents Chemother. 2020;64(2):10.
Haggstrom L, Parmar G, Brungs D. Central venous catheter thrombosis in cancer: a multi-centre retrospective study investigating risk factors and contemporary trends in management. Clin Med Insights Oncol. 2020;14:1.17955E + 15.
Heidenreich D, Hansen E, Kreil S, Nolte F, Jawhar M, Hecht de Gutierrez A, et al. Influence of the insertion site on central venous catheter-related complications in patients undergoing allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transpl. 2020;26(6):1189–94.
Ince ME, Ozkan G, Ors N, Yildirim AK, Doganci S. Complications and pitfalls of central venous port catheters: experience with 782 patients with cancer. Ir J Med Sci. 2020;189(4):1371–7.
Inoue S, Yoshida T, Nishino T, Goto M, Nishioka K, Fujimoto K, et al. Safe central venous catheters for esophageal cancer treatment. J Med Investig. 2020;67(34):298–303.
Jiang M, Cui XW, Li CL, Pan CQ, Dietrich CF. Risk of venous thromboembolism associated with totally implantable venous access ports in cancer patients: a systematic review and meta-analysis. J Thromb Haemost. 2020;18(9):2253–2273.
Kao CY, Cheng YC, Chen CCC, Chai JW, Fu CH, Chen JL, et al. Outcome analysis in 270 radiologically guided implantations of totally implantable venous access ports via basilic vein. J Chin Med Association. 2020;83(3):295–301.
Kikuchi M, Sato T, Okada S, Abe N, Sato A, Suzuki Y. Maintenance antisepsis in reducing the rate of late-onset central venous catheter-related bloodstream infection: a comparison of 0.05% and 1% chlorhexidine. J Infect Chemotherapy: Official J Japan Soc Chemother. 2020;26(2):188–93.
Lingegowda D, Gehani A, Sen S, Mukhopadhyay S, Ghosh P. Centrally inserted tunnelled peripherally inserted central catheter: off-label use for venous access in oncology patients. J Vasc Access. 2020;21(5):773–7.
Low XZ, Tay KH, Leong S, Lo RHG, Zhuang KD, Chua JME, et al. Repurposing the power injectable peripherally inserted central catheter as a tunnelled, non-cuffed, centrally inserted central venous catheter in oncological patients for short- to mid-term vascular access: a pilot study. J Vasc Access. 2021;(3):457–461.
Lv L, Xu J, Bai C, Gong J, Ma W, Sun X. Cluster nursing in the prevention of PICC-related venous thrombosis and its influence on tumor patients’ coagulation functions. Int J Clin Exp Med. 2020;13(12):10005–11.
Malek AE, Raad II. Preventing catheter-related infections in cancer patients: a review of current strategies. Expert Rev Anti Infect Ther. 2020;18(6):531–8.
McParlan D, Edgar L, Gault M, Gillespie S, Menelly R, Reid M. Intravascular catheter migration: a cross-sectional and health-economic comparison of adhesive and subcutaneous engineered stabilisation devices for intravascular device securement. J Vasc Access. 2020;21(1):33–8.
Mielke D, Wittig A, Teichgraber U. Peripherally inserted central venous catheter (PICC) in outpatient and inpatient oncological treatment. Support care cancer. 2020;28(10):4753–60.
Mollee P, Abro E, Van Kuilenburg R, Joubert W, Okano S, Looke D, et al. Catheter-associated bloodstream infections in adults with cancer: a prospective randomized controlled trial. J Hosp Infect. 2020;106(2):335–42.
Park S, Moon S, Pai H, Kim B. Appropriate duration of peripherally inserted central catheter maintenance to prevent central line-associated bloodstream infection. PLoS One. 2020;15(6):e0234966.
Pu YL, Li ZS, Zhi XX, Shi YA, Meng AF, Cheng F, et al. Complications and costs of peripherally inserted central venous catheters compared with Implantable port catheters for cancer patients: a meta-analysis. Cancer Nurs. 2020;43(6):455–67.
Santacatalina-Roig E, Espinar-de Las Heras E, Ballesteros-Lizondo JM, Ibanez-Puchades I, Pescador-Marco JL. Peripherally inserted central catheter in haematopoietic stem cell transplantation. Infusion of haematopoietic cells and complications. Enfirm Clin (Engl Ed). 2020;30(5):295–301.
Tan L, Sun Y, Zhu L, Lei X, Liang D, Rao N, et al. Risk factors of catheter-related thrombosis in early-stage breast cancer patients: a single-center retrospective study. Cancer Manage Res. 2019;11:8379–89.
Tang T, Li H, Wang J, Li C, Geng C. The causes and managements of catheter misplacement in implantable vascular access devices: a retrospective analysis of 8534 patients in a single center. Int J Clin Exp Med. 2019;12(9):11864–8.
Taxbro K, Hammarskjöld F, Thelin B, Lewin F, Hagman H, Hanberger H, et al. Clinical impact of peripherally inserted central catheters vs implanted port catheters in patients with cancer: an open-label, randomised, two-centre trial. Br J Anaesth. 2019;122(6):734–41.
Ullman AJ, Mihala G, O’Leary K, Marsh N, Woods C, Bugden S, et al. Skin complications associated with vascular access devices: a secondary analysis of 13 studies involving 10,859 devices. Int J Nurs Stud. 2019;91:Jun–13.
Velioglu Y, Yuksel A, Sinmaz E. Complications and management strategies of totally implantable venous access port insertion through percutaneous subclavian vein. Turk Gogus kalp damar cerrahisi dergisi. 2019;27(4):499–507.
Xiong ZY, Luo Z, Chen HY. Prevalence of idle peripherally inserted central catheters in adult patients: a multicenter cross-sectional study. J Vasc Access. 2019;20(6):677–82.
Yildiz A, Albayrak M, Sahin O, Pala C, Ozturk HBA, Gunes G, et al. Incidence and risk factors of port related infections in patients with hematological malignancy. Int J Clin Exp Med. 2019;12(1):989–96.
Zabicki B, Limphaibool N, Veilemand Holstad MJ, Perkowska K. Central venous access ports in the interventional radiology suite - one-centre experience. Pol J Radiol. 2019;84:e328–334.
Zanwar S, Gokarn A, Devadas SK, Punatar S, Khurana S, Bonda A, et al. Antibiotic lock therapy for salvage of tunneled central venous catheters with catheter colonization and catheter-related bloodstream infection. Transpl Infect Disease. 2019;21(1):e13017.
Simonetti G, Sommariva A, Lusignani M, Anghileri E, Ricci CB, Eoli M, et al. Prospective observational study on the complications and tolerability of a peripherally inserted central catheter (PICC) in neuro-oncological patients. Supportive care cancer: Official J Multinational Association Supportive Care Cancer. 2020;28(6):2789–95.
Skummer P, Kobayashi K, DeRaddo JS, Blackburn T, Schoeneck M, Patel J, et al. Risk factors for early port infections in adult oncologic patients. J Vascular Interventional Radiology: JVIR. 2020;31(9):1427–36.
Slaughter E, Keogh SJ, Kynoch K, Brodribb M. Evaluating the impact of central venous catheter materials and design on thrombosis: a systematic review and meta-analysis. Worldviews evidence-based Nurs. 2020;17(5):376–84.
Song X, Lu H, Chen F, Bao Z, Li S, Li S, et al. A longitudinal observational retrospective study on risk factors and predictive model of PICC associated thrombosis in cancer patients. Sci Rep. 2020;10(1):10090.
Tang L, Kim CY, Martin JG, Pabon-Ramos WM, Sag AA, Suhocki PV, et al. Length of stay predicts risk of early infection for hospitalized patients undergoing central venous port placement. J Vascular Interventional Radiology: JVIR. 2020;31(3):454–61.
Tsuruta S, Goto Y, Miyake H, Nagai H, Yoshioka Y, Yuasa N, et al. Late complications associated with totally implantable venous access port implantation via the internal jugular vein. Supportive care cancer: Official J Multinational Association Supportive Care Cancer. 2020;28(6):2761–8.
Wang GD, Wang HZ, Shen YF, Dong J, Wang XP, Wang XZ, et al. The influence of venous characteristics on peripherally inserted central catheter-related symptomatic venous thrombosis in cancer patients. Cancer Manage Res. 2020;12:11909–20.
Yin L, Li J. Central venous catheter insertion in colorectal cancer patients, PICC or PC? Cancer Manage Res. 2020;12:5813–8.
Yin YX, Gao W, Li XY, Lu W, Deng QH, Zhao CY, et al. Randomized multicenter study on long-term complications of peripherally inserted central catheters positioned by electrocardiographic technique. Phlebology. 2020;35(8):614–622.
Akhtar N, Lee L. Utilization and complications of central venous access devices in oncology patients. Curr Oncol (Toronto Ont). 2021;28(1):367–77.
Annetta MG, Ostroff M, Marche B, Emoli A, Musarò A, Celentano D, et al. Chest-to-arm tunneling: a novel technique for medium/long term venous access devices. J Vasc Access. 2023;24(1):92–8.
Clari M, Spoto M, Franceschi G, Acuto M, Tonella S, Caristia S, et al. Short versus long timing of flushing of totally implantable venous access devices when not used routinely: a systematic review and Meta-analysis. Cancer Nurs. 2021;44(3):205–213.
Corti F, Brambilla M, Manglaviti S, Di Vico L, Pisanu MN, Facchinetti C, et al. Comparison of outcomes of central venous catheters in patients with solid and hematologic neoplasms: an Italian real-world analysis. Tumori. 2021;107(1):17–25.
Cruz-Aguilar R, Carney J, Mondaini V, Vehreschild M, Griskaitis M, Salmanton-García J, et al. A quality improvement study on the reduction of central venous catheter-associated bloodstream infections by use of self-disinfecting venous access caps (STERILE). Am J Infect Control. 2021;49(5):586–592.
D’Souza PC, Kumar S, Kakaria A, Al-Sukaiti R, Al-Baimani K, Hamid RS, et al. Complications and management of totally implantable central venous access ports in cancer patients at a University Hospital in Oman. Sultan Qaboos Univ Med J. 2021;21(1):e103–109.
Egnatios D, Gloria C. Implanted port patency: comparing heparin and normal saline. Clin J Oncol Nurs. 2021;25(2):169–73.
Kara H, Arikan AE, Dulgeroglu O, Uras C, Icten GE, Tutar B, et al. Detachment and embolization of totally implantable central venous access devices: diagnosis and management. Acta Chir Belg. 2022;122(4):240–247.
Lee YM, Ryu BH, Hong SI, Cho OH, Hong KW, Bae IG, et al. Clinical impact of early reinsertion of a central venous catheter after catheter removal in patients with catheter-related bloodstream infections. Infect Control Hosp Epidemiol. 2021;42(2):162–8.
Lichtenstein T, Rau K, Hokamp NG, Maintz D, Mammadov K, Do TD, et al. Long-term follow-up and clinical relevance of incidental findings of fibrin sheath and thrombosis on computed tomography scans of cancer patients with port catheters. Ther Clin Risk Manag. 2021;17:111–8.
Michell H, Nezami N, Morris C, Hong K. Dual-chambered venous access port as alternative access for extracorporeal apheresis therapy. J Vasc Access. 2021;22(2):173–7.
Sachs OA, Chugh P, He K, Moseley JM, Oneal PB, Whang E, Kristo G. Survival and Complications After Placement of Central Venous Access Ports for Palliative Chemotherapy: A Single-Institution Retrospective Analysis. The American Journal of Hospice and Palliative Care. 2022;39(1):34–38.
Oh SB, Park K, Kim JJ, Oh SY, Jung KS, Park BS, et al. Safety and feasibility of 3-month interval access and flushing for maintenance of totally implantable central venous port system in colorectal cancer patients after completion of curative intended treatments. Med (Baltim). 2021;100(2):e24156.
Park EJ, Park K, Kim JJ, Oh SB, Jung KS, Oh SY, et al. Safety, efficacy, and patient satisfaction with initial peripherally inserted central catheters compared with usual intravenous access in terminally Ill cancer patients: a randomized phase II study. Cancer Res Treat. 2021;53(3):881–888.
Piredda A, Radice D, Zencovich C, Cerri M, Aventino L, Naccarato F, et al. Safe use of peripherally inserted Central catheters for chemotherapy of solid malignancies in adult patients: a 1-year monocentric, prospectively-assessed, unselected cohort of 482 patients. J Vasc Access. 2020;1:E12973.
Rixecker T, Lesan V, Ahlgrimm M, Thurner L, Bewarder M, Murawski N, et al. Insertion site of central venous catheter correlates with catheter-related infectious events in patients undergoing intensive chemotherapy. Bone Marrow Transplant. 2021;56(1):195–201.
Shibata J, Hiramatsu K, Shibata Y, Aoba T, Fujii M, Arimoto A, et al. Impact of chest subcutaneous fat on the occurrence of central venous port-related infectious complications in cancer patients. Support Care Cancer. 2021;29(9):5291–5398.
Tumay LV, Guner OS. Availability of totally implantable venous access devices in cancer patients is high in the long term: a seven-year follow-up study. Support Care Cancer. 2021;29(7):3531–3538.
Xiao MF, Xiao CQ, Li J, Dai C, Fan YY, Cao HJ, et al. Subcutaneous tunneling technique to improve outcomes for patients undergoing chemotherapy with peripherally inserted central catheters: a randomized controlled trial. J Int Med Res. 2021;49(4):3.00061E + 15.
Yan W, Zhang C, Luo C, Li Z. Management of outpatient with totally implantable venous access ports during the COVID-19 epidemic. Medicine. 2021;100(7):e24720.
Sze Yong T, Vijayanathan AA, Chung E, Ng WL, Yaakup NA, Sulaiman N. Comparing catheter related bloodstream infection rate between cuffed tunnelled and non-cuffed tunnelled peripherally inserted central catheter. J Vasc Access. 2022;23(2):225–231.
Gür Ö, DonbaloĞLu MO, GÜRkan S. Comparison of clinical follow-up and complications according to cancer types in patients with permanent port catheter insertion due to Malignancy. Duzce Med J. 2018;20(3):59–62.
Ban T, Fujiwara SI, Murahashi R, Nakajima H, Ikeda T, Matsuoka S, et al. Risk factors for complications associated with peripherally inserted central catheters during induction chemotherapy for acute myeloid leukemia. Intern Med. 2022;61(7):989–95.
Baumann Kreuziger L, Gaddh M, Onadeko O, George G, Wang TF, Oo TH, et al. Treatment of catheter-related thrombosis in patients with hematologic malignancies: a venous thromboEmbolism Network U.S. retrospective cohort study. Thromb Res. 2021;202:155–61.
Bertoglio S, Annetta MG, Brescia F, Emoli A, Fabiani F, Fino M, et al. A multicenter retrospective study on 4480 implanted PICC-ports: a GAVeCeLT project. J Vasc Access. 2022;24(5):1114–1120.
Böll B, Schalk E, Buchheidt D, Hasenkamp J, Kiehl M, Kiderlen TR, et al. Central venous catheter-related infections in hematology and oncology: 2020 updated guidelines on diagnosis, management, and prevention by the infectious diseases working party (AGIHO) of the German society of hematology and medical oncology (DGHO). Ann Hematol. 2021;100(1):239–59.
Brescia F, Pittiruti M, Roveredo L, Zanier C, Morabito A, Santarossa E, et al. Subcutaneously anchored securement for peripherally inserted central catheters: Immediate, early, and late complications. J Vasc Access. 2023;24(1):82–6.
Caris MG, de Jonge NA, Punt HJ, Salet DM, de Jong VMT, Lissenberg-Witte BI, et al. Indwelling time of peripherally inserted central catheters and incidence of bloodstream infections in haematology patients: a cohort study. Antimicrob Resist Infect Control. 2022;11(1):37.
Chen K, Beeraka NM, Gu Y, Li J, Sinelnikov M, Han N, et al. Totally implantable venous access port systems: implant depth-based complications in breast cancer therapy - a comparative study. Curr Pharm Des. 2021;27(46):4671–6.
Chen P, Zhu B, Wan G, Qin L. The incidence of asymptomatic thrombosis related to peripherally inserted central catheter in adults: a systematic review and meta-analysis people’s. Nurs Open. 2021;8(5):2249–61.
Cotogni P, Mussa B, Degiorgis C, De Francesco A, Pittiruti M. Comparative complication rates of 854 central venous access devices for home parenteral nutrition in cancer patients: a prospective study of over 169,000 catheter-days. JPEN J Parenter Enter Nutr. 2021;45(4):768–76.
El Boghdadly Z, Zhao Q, Koutou J, Lustberg ME, Ludwig M, Liscynesky C, et al. Evaluation of central line salvage for mucosal barrier injury laboratory-confirmed bloodstream infection (MBI-LCBI) management practices in patients with hematologic malignancies. Leuk Lymphoma. 2022;63(6):1455–63.
González S, Jiménez P, Saavedra P, Macías D, Loza A, León C, et al. Five-year outcome of peripherally inserted central catheters in adults: a separated infectious and thrombotic complications analysis. Infect Control Hosp Epidemiol. 2021;42(7):833–41.
Guan X, Yan H, Zhang J, Li Y, Zhou Y. Risk factors of infection of totally implantable venous access port: a retrospective study. J Vasc Access. 2023;24(6):1340–1348.
Hashimoto Y, Hosoda R, Omura H, Tanaka T. Catheter-related bloodstream infection associated with multiple insertions of the peripherally inserted central catheter in patients with hematological disorders. Sci Rep. 2021;11(1):12209.
Heidenreich D, Hansen E, Kreil S, Nolte F, Jawhar M, Hecht A, et al. The insertion site is the main risk factor for central venous catheter-related complications in patients with hematologic malignancies. Am J Hematol. 2022;97(3):303–10.
Huang C, Wu Z, Huang W, Zhang X, Lin X, Luo J, et al. Identifying the impact of the zone insertion method(TM) (ZIM(TM)): a randomized controlled trial. J Vasc Access. 2021;24(4):1–10. https://doi.org/10.1177/11297298211052528 .
Johns J, Wahlrab L, Elefritz JL. Acutely ill hematology/oncology patients with central-line associated bloodstream infections and the impact of timing of catheter removal on outcomes. Am J Infect Control. 2022;50(7):749–54.
Kim TH, Choi YW, Ahn MS, Choi YS, Lee HW, Jeong SH, et al. Early removal of central venous catheter may not impact the in-hospital mortality in patients with acute leukemia. Ann Hematol. 2021;100(11):2825–30.
Kinoshita M, Takao S, Hiraoka J, Takechi K, Akagawa Y, Osaki K, et al. Risk factors for unsuccessful removal of central venous access ports implanted in the forearm of adult oncologic patients. Jpn J Radiol. 2022;40(4):412–8.
Krümpelmann U, Boseila A, Löhnert M, Kaup O, Clarenbach JJ, Görner M. An analysis of totally implantable central venous port system infections in an urban tertiary referral center. J Chemother. 2021;33(4):228–37.
Kumwenda MJ, Dougherty L, Jackson A, Hill S. Prospective audit to study urokinaSe use to restore Patency in occluded centRal venous caTheters in haematology and oncology patients (PASSPORT 2). J Vasc Access. 2021;22(4):568–74.
Liu B, Wu Z, Lin C, Li L, Kuang X. Applicability of TIVAP versus PICC in non-hematological malignancies patients: a meta-analysis and systematic review. PLoS One. 2021;16(8):e0255473.
Martinez J, Capela R. Infusion pump flow rates in central venous catheters: thrombus reflux and aspiration Clot. Onconews. 2021;(42):16–20.
McKeown C, Ricciuti A, Agha M, Raptis A, Hou JZ, Farah R, et al. A prospective study of the use of central venous catheters in patients newly diagnosed with acute myeloid leukemia treated with induction chemotherapy. Support Care Cancer. 2022;30(2):1673–9.
Mittal GS, Sundriyal D, Naik NB, Sehrawat A. Totally implantable venous access device (Chemoport) in Oncology: study of 168 polyurethane chemoport catheter system. South Asian J Cancer. 2021;10(4):261–4.
Moralar DG, Turkmen UA, Bilen A, Turkmen S, Feyizi H, Altan HA. Our central venous port catheter system practice - a retrospective study. J Pak Med Assoc. 2021;71(5):1442–5.
Pike S, Tan K, Burbridge B. Complications associated with totally implanted venous access devices in the arm versus the chest: a short-term retrospective study. Can Assoc Radiol J. 2022;73(3):581–8.
Pinelli F, Balsorano P, Mura B, Pittiruti M. Reconsidering the GAVeCeLT Consensus on catheter-related thrombosis, 13 years later. J Vasc Access. 2021;22(4):501–8.
Pinelli F, Pittiruti M, Van Boxtel T, Barone G, Biffi R, Capozzoli G, et al. GAVeCeLT-WoCoVA Consensus on subcutaneously anchored securement devices for the securement of venous catheters: current evidence and recommendations for future research. J Vasc Access. 2021;22(5):716–25.
Platanaki C, Zareifopoulos N, Lagadinou M, Tsiotsios K, Velissaris D. Correlation of positive blood cultures with peripherally inserted central catheter line infection in oncology patients. Cureus. 2021;13(1):e12858.
Ploton G, Brebion N, Guyomarch B, Pistorius MA, Connault J, Hersant J, et al. Predictive factors of venous recanalization in upper-extremity vein thrombosis. PLoS One. 2021;16(5):e0251269.
Ranch-Lundin M, Schedin A, Björkhem-Bergman L. Equal effect of Vancomycin lock with or without heparin in treatment of central venous catheter related blood stream infections - an observational study in palliative home care. Infect Dis (Lond). 2021;53(9):719–23.
Rejane Rabelo-Silva E, Lourenço SA, Maestri RN, Candido da Luz C, Carlos Pupin V, Bauer Cechinel R, et al. Patterns, appropriateness and outcomes of peripherally inserted central catheter use in Brazil: a multicentre study of 12 725 catheters. BMJ Qual Saf. 2022;31(9):652–61.
Rockholt MM, Thorarinsdottir HR, Lazarevic V, Rundgren M, Kander T. Central venous catheter-related complications in hematologic patients: an observational study. Acta Anaesthesiol Scand. 2022;66(4):473–82.
Sacks OA, Chugh P, He K, Moseley JM, Oneal PB, Whang E, et al. Survival and complications after placement of central venous access ports for palliative chemotherapy: a single-institution retrospective analysis. Am J Hosp Palliat Care. 2022;39(1):34–8.
Sapkota S, Sannur R, Naik R. Analysis of peripherally inserted central catheter line in cancer patients: a single-center experience. South Asian J Cancer. 2020;9(4):253–6.
Shih YH, Teng CJ, Chen TC, Chang KH, Chen MH. Dual-lumen power injectable peripherally inserted central catheters in allogeneic hematopoietic stem cell transplantation: a prospective observational study. J Clin Nurs. 2022;31(11–12):1654–61.
Snarski E, Stringer J, Mikulska M, Gil L, Tridello G, Bosman P, et al. Risk of infectious complications in adult patients after allogeneic hematopoietic stem cell transplantation depending on the site of central venous catheter insertion-multicenter prospective observational study, from the IDWP EBMT and Nurses Group of EBMT. Bone Marrow Transpl. 2021;56(12):2929–33.
Ullman AJ, Paterson RS, Schults JA, Kleidon TM, August D, O’Malley M, et al. Do antimicrobial and antithrombogenic peripherally inserted central catheter (PICC) materials prevent catheter complications? An analysis of 42,562 hospitalized medical patients. Infect Control Hosp Epidemiol. 2022;43(4):427–34.
Wan R, Gu L, Yin B, Cai S, Zhou R, Yang W. A six-year study of complications related to peripherally inserted central catheters: a multi-center retrospective cohort study in China. Perfusion. 2023;38(4):689–97.
Winkler MA, Spencer TR, Siddiqi N, Wallace JE, Gallien JZ, Elbalasi H, et al. Clinical experience with a chlorhexidine-coated PICC: a prospective, multicenter, observational study. J Vasc Access. 2021;25(1):225–231.
Yang WJ, Song MG, Seo TS, Park SJ. Effectiveness of mechanical recanalization for intraluminal occlusion of totally implantable venous access ports. J Vasc Access. 2023;24(3):430–5.
Yun WS, Yang SS. Comparison of peripherally inserted central catheters and totally implanted venous access devices as chemotherapy delivery routes in oncology patients: a retrospective cohort study. Sci Prog. 2021;104(2):368504211011871.
Zhang Y, Zhao R, Jiang N, Shi Y, Wang Q, Sheng Y. A retrospective observational study on maintenance and complications of totally implantable venous access ports in 563 patients: prolonged versus short flushing intervals. Int J Nurs Sci. 2021;8(3):252–6.
Broadhurst D, Moureau N, Ullman AJ. Management of central venous Access device-Associated skin impairment: an evidence-based Algorithm. J Wound Ostomy Cont Nurs. 2017;44(3):211–20.
Busch JD, Herrmann J, Adam G, Ittrich H, Vens M, Mahler C. Complication rates observed in silicone and polyurethane catheters of totally implanted central venous Access devices implanted in the upper arm. J Vasc Interv Radiol. 2017;28(8):1177–83.
Chong HY, Lai NM, Apisarnthanarak A, Chaiyakunapruk N. Comparative efficacy of antimicrobial central venous catheters in reducing catheter-related bloodstream infections in adults: abridged cochrane systematic review and network meta-analysis. Clin Infect Diseases: Official Publication Infect Dis Soc Am. 2017;64(suppl2):S131–40.
Jones D, Wismayer K, Bozas G, Palmer J, Elliott M, Maraveyas A. The risk of venous thromboembolism associated with peripherally inserted central catheters in ambulant cancer patients. Thromb J. 2017;15:25.
Kramer RD, Mann J, Rogers MAM, Saint S, Chopra V, Conte M. Are antimicrobial peripherally inserted central catheters associated with reduction in central line-associated bloodstream infection? A systematic review and meta-analysis. Am J Infect Control. 2017;45(2):108–14.
Lin WY, Lin CP, Hsu CH, Lee YH, Lin YT, Hsu MC, et al. Right or left? Side selection for a totally implantable vascular access device: a randomised observational study. Br J Cancer. 2017;117(7):932–7.
Madabhavi I, Patel A, Anand A, Panchal H, Parikh S, Sarkar M. A study of Use of PORT catheter in patients with cancer: a single-center experience. Clin Med Insights: Oncol. 2017;11:1179554917691031.
McDiarmid S, Scrivens N, Carrier M, Sabri E, Toye B, Huebsch L, et al. Outcomes in a nurse-led peripherally inserted central catheter program: a retrospective cohort study. CMAJ Open. 2017;5(3):E535–539.
Milani A, Mazzocco K, Gandini S, Pravettoni G, Libutti L, Zencovich C, et al. Incidence and determinants of port occlusions in cancer outpatients. Cancer Nurs. 2017;40(2):102–7.
Patel PA, Boehm S, Zhou Y, Zhu C, Peterson KE, Grayes A, et al. Prospective observational study on central line-associated bloodstream infections and central venous catheter occlusions using a negative displacement connector with an alcohol disinfecting cap. Am J Infect Control. 2017;45(2):115–20.
Suleman A, McDiarmid S. A retrospective analysis of catheter-related upper extremity deep vein thrombosis in peripherally inserted catheters with and without a dermatotomy. JAVA - J Association Vascular Access. 2017;22(4):178–81.
Tabatabaie O, Kasumova GG, Kent TS, Eskander MF, Fadayomi AB, Ng SC, et al. Upper extremity deep venous thrombosis after port insertion: what are the risk factors? Surgery. 2017;162(2):437–44.
Takashima M, Ray-Barruel G, Ullman A, Keogh S, Rickard CM. Randomized controlled trials in central vascular access devices: a scoping review. PLoS One. 2017;12(3):e0174164.
Voor in ’t holt AF, Helder OK, Vos MC, Schafthuizen L, Sülz S, van den Hoogen A, et al. Antiseptic barrier cap effective in reducing central line-associated bloodstream infections: a systematic review and meta-analysis. Int J Nurs Stud. 2017;69:34–40.
Wang XJ. Preventive effect of dexamethasone solution pre-treated catheter on PICC-induced phlebitis. Biomedical Res (India). 2017;28(12):5310–4.
Yu L, Zhang R, Li J, Yan X, Jin K, Li W, et al. Incidence and risk factors for peripherally inserted central catheter-related vein thrombosis in lung cancer patients. Int J Clin Exp Med. 2017;10(8):12440–6.
Zhang M, Kang L, Li Q. A comparative study on the use of different connectors in tube sealing in elderly tumor patients with PICC. Int J Clin Exp Med. 2017;10(6):9488–94.
Brito ARO, Nishinari K, Saad PF, Saad KR, Pereira MAT, Emidio SCD, et al. Comparison between saline solution containing heparin versus saline solution in the lock of totally implantable catheters. Ann Vasc Surg. 2018;47:85–9.
Hallam C, Jackson T, Rajgopal A, Russell B. Establishing catheter-related bloodstream infection surveillance to drive improvement. J Infect Prev. 2018;19(4):160–6.
Huang V. Effect of a patency bundle on central venous catheter complications among hospitalized adult patients: a best practice implementation project. JBI Database Syst Reviews Implement Rep. 2018;16(2):565–86.
Huihan Z, Yu H, Qin W, Yanping Y. Medical adhesive–related skin Injury Prevalence at the peripherally inserted central catheter insertion site: a cross-sectional, multiple-center study. J Wound Ostomy Cont Nurs. 2018;45(1):22–5.
Iftikhar R, Chaudhry QUN, Satti TM, Mahmood SK, Satti HS, Ghafoor T, et al. Noble Metal Coated Central venous catheters are not Superior to uncoated catheters in preventing infectious and non-infectious complications in immunocompromised patients. J Ayub Med Coll Abbottabad: JAMC. 2018;30(Suppl 1):S647–651.
Kim JH, Hong YS, Kim SY, Kim K-P, Choi KE, Kim TW, et al. Increased incidence of chemoport-related thrombosis in patients with colorectal cancer receiving bevacizumab: a single-institutional experience. Chin J Cancer Res. 2018;30(4):460–7.
Koo CM, Vissapragada R, Sharp R, Nguyen P, Ung T, Solanki C, et al. ABO blood group related venous thrombosis risk in patients with peripherally inserted central catheters. Br J Radiol. 2018;91(1082):20170560.
Lam PW, Volling C, Chan T, Wiggers JB, Castellani L, Wright J, et al. Impact of defaulting to single-lumen peripherally inserted central catheters on patient outcomes: an interrupted time series study. Clin Infect Dis. 2018;67(6):954–7.
Lopez-Briz E, Ruiz Garcia V, Cabello JB, Bort-Marti S, Carbonell Sanchis R, Burls A. Heparin versus 0.9% sodium chloride locking for prevention of occlusion in central venous catheters in adults. Cochrane Database Syst Reviews. 2018;2018(7):CD008462.
Lv Y, Hou Y, Yu L, Xu D, Song J, Shang H, et al. Risk associated with central catheters for malignant tumor patients: a systematic review and meta-analysis. Oncotarget. 2018;9(15):12376–88.
Mansour A, Khozouz O, Saadeh SS, Abunasser M, Abdel-Razeq N, Taqash A. Clinical course and complications of catheter and non-catheter-related upper extremity deep vein thrombosis in patients with cancer. Clin Appl Thromb Hemost. 2018;24(8):1234–40.
McDonald MK, Culos KA, Gatwood KS, Prow C, Chen H, Savani BN, et al. Defining incidence and risk factors for catheter-associated bloodstream infections in an outpatient adult hematopoietic cell transplantation program. Biology of blood and marrow transplantation: journal of the American Society for Blood and marrow transplantation. 2018;24(10):2081–7.
Rasero L, Golin L, Ditta S, Di Massimo DS, Dal Molin A, Piemonte G. Effects of prolonged flushing interval in totally implantable venous access devices (TIVADs). Br J Nurs. 2018;27(8):S4–10.
Spires SS, Rebeiro PF, Miller M, Koss K, Wright PW, Talbot TR. Medically attended catheter complications are common in patients with outpatient central venous catheters. Infect Control Hosp Epidemiol. 2018;39(4):439–44.
Wu S, Li W, Zhang Q, Li S, Wang L. Comparison of complications between peripheral arm ports and central chest ports: a meta-analysis. J Adv Nurs. 2018;74(11):2484–96.
Yu XY, Xu JL, Li D, Jiang ZF. Late complications of totally implantable venous access ports in patients with cancer: risk factors and related nursing strategies. Medicine (Baltimore). 2018;97(38):e12427.
Agrawal SK, Gautam H, Choudhary AH, Das BK, Kumar L, Kapil A. Central line-associated bloodstream infections in cancer patients: an experience from a tertiary care cancer centre. Ind J Med Microbiol. 2019;37(3):376–80.
Bademler S, Ucuncu M, Yildirim I, Karanlik H. Risk factors for complications in cancer patients with totally implantable access ports: a retrospective study and review of the literature. J Int Med Res. 2019;47(2):702–9.
Dang FP, Li HJ, Tian JH. Comparative efficacy of 13 antimicrobial dressings and different securement devices in reducing catheter-related bloodstream infections: a bayesian network meta-analysis. Medicine (Baltimore). 2019;98(14):e14940.
Dinçer M, Kocakuşak A, Hut A, Gür Ü, Çıtlak G, Akıncı M. Comparison of two different central venous Access device insertion techniques: no evil in details. Med Bull Haseki / Haseki Tip Bulteni. 2019;57(1):Sep–14.
Eldeeb H, Al-Asadi O, Almusarhed M. Predictive risk factors of venous thromboembolism (VTE) associated with peripherally inserted central catheters (PICC) in ambulant solid cancer patients: retrospective single centre cohort study. Thromb J. 2019;17(1):191.
Fornaro C, Piubeni M, Tovazzi V, Cosentini D, Gelmi M, Rota G, et al. Eight-week interval in flushing and locking port-a-cath in cancer patients: a single-institution experience and systematic review. Eur J Cancer Care (Engl). 2019;28(2):e12978.
Fu X, Lu P, Wang C, Ye G. Analysis of the risk factors of peripherally inserted central catheter-associated venous thrombosis after chemotherapy in patients with lung cancer. Int J Clin Exp Med. 2019;12(5):5852–9.
Hill S, Hamblett I, Brady S, Vasileukaya S, Zuzuarregui I, Martin F. Central venous access device-related sheaths: a predictor of infective and thrombotic incidence? Br J Nurs. 2019;28(19):S10–18.
Isom C, Bream P, Gallagher K, Walia S, Ahmed R, Kauffmann R. Placement of subcutaneous central venous ports in breast Cancer patients: does side matter? J Surg Res. 2019;244:296–301.
Kagan E, Salgado CD, Banks AL, Marculescu CE, Cantey JR. Peripherally inserted central catheter-associated bloodstream infection: risk factors and the role of antibiotic-impregnated catheters for prevention. Am J Infect Control. 2019;47(2):191–5.
Lee IJ, Kim HB, Choi YJ, Lee JH, Kim ET, Shim DJ, et al. Prevalence and predictors of peripherally inserted central catheter-associated bloodstream infections in adults: a multicenter cohort study. PLoS One. 2019;14(3):e0213555.
Liu S, Cong L, Xiao Z, Song Y, Lou T, Ma Y, et al. Risk factors associated with peripherally inserted central catheter-related venous thrombosis in hospitalized patients of advanced age. J Int Med Res. 2019;48(1):300060518820744.
Liu Z, Chen J, Zan L, Ding S, Yi H, Yan C. Exploring the risk factors of thrombosis and bloodstream infections in peripherally inserted central catheter (PICC) patients. J Biomaterials Tissue Eng. 2019;9(7):929–34.
Okazaki M, Oyama K, Kinoshita J, Miyashita T, Tajima H, Takamura H, et al. Incidence of and risk factors for totally implantable vascular access device complications in patients with gastric cancer: a retrospective analysis. Mol Clin Oncol. 2019;11(4):343–8.
Russo R, Oliveira MS, Shikanai-Yasuda MA, Mendes ET, Levin AS, Costa SF, et al. Bloodstream infection in hematopoietic stem cell transplantation outpatients: risk factors for hospitalization and death. Rev Inst Med Trop Sao Paulo. 2019;61:e3.
Sengul T, Ocakci AF, Guven B, Kaya N. Connectors as a risk factor for blood-associated infections (3-way stopcock and needleless connector): a randomized-experimental study. Am J Infect Control. 2020;48(3):275–280.
Skelton Iv WP, Franke AJ, Welniak S, Bosse RC, Ayoub F, Murphy M, et al. Investigation of complications following port insertion in a cancer patient population: a retrospective analysis. Clin Med Insights: Oncol. 2019;13:N.PAG–N.PAG.
Suttle RD, Buffington HM, Madden WT, Dawson MA. Central line care: empowering patients to prevent infection and Injury Via EPIC2. Clin J Oncol Nurs. 2019;23(1):E10–16.
Ziegler M, Landsburg D, Kucharczuk C, Gorman T, Bink K, Stadtmauer EA, et al. Fluoroquinolone prophylaxis is highly effective for the prevention of central line-associated bloodstream infections in autologous stem cell transplant patients. Biol Blood Marrow Transplant. 2019;25(5):1004–10.
Bessis S, Cassir N, Meddeb L, Remacle AB, Soussan J, Vidal V, et al. Early mortality attributable to PICC-lines in 4 public hospitals of Marseille from 2010 to 2016 (revised V3). Medicine (Baltimore). 2020;99(1):e18494.
Beypinar I, Demir H, Uysal M, Araz M, Beypinar D. The comparison of central venous port catheters in gastrointestinal cancer treatment. J Oncological Sci. 2020;6(1):10–4.
Huang W, Xu J. The role of sterile chitosan-based dressing in reducing complications related to a peripherally inserted central catheter in patients with hematological tumors. Annals Palliat Med. 2020;9(4):2037–44.
Jiang M, Li CL, Pan CQ, Yu L. The risk of bloodstream infection associated with totally implantable venous access ports in cancer patient: a systematic review and meta-analysis. Support Care Cancer. 2020;28(1):361–72.
Karapanou A, Sampanis MA, Vieru A-M, Daikos GL, Samarkos M, Pantazatou A, et al. Failure of central venous catheter insertion and care bundles in a high central line-associated bloodstream infection rate, high bed occupancy hospital. Am J Infect Control. 2020;48(7):770–6.
Kitamura H, Kimura S, Kubota Y, Komukai S, Yoshida H, Kaneko Y, et al. Venue of catheter insertion does not significantly impact the event of central line-associated bloodstream infection in patients with haematological diseases. Infect Prev Pract. 2020;2(2):100050.
Kukla ME, Childs CA, Puig-Asensio M, Marra AR, Perencevich EN, Schweizer ML. Effectiveness of chlorhexidine dressings to prevent catheter-related bloodstream infections. Does one size fit all? A systematic literature review and meta-analysis. Infect Control Hosp Epidemiol. 2020;41(12):1388–1395.
Lin Y, Zeng Z, Zheng J, Lin R, Liu S, Gao X. The Caprini thrombosis risk model predicts the risk of PICC-related upper extremity venous thrombosis in cancer patients. J Vasc Surg Venous Lymphat Disord. 2021;9(5):1151–1158.
Liu Y, Li LL, Xu L, Feng DD, Cao Y, Mao XY, et al. Comparison between arm port and chest port for optimal vascular access port in patients with breast cancer: a systematic review and meta-analysis. Biomed Res Int. 2020;2020:9082924.
Qi F, Cheng H, Yuan X, Zhang L. Comparison of PICC and TIVAP in chemotherapy for patients with thyroid cancer. Oncol Lett. 2020;20(2):1657–62.
Rowe MS, Arnold K, Spencer TR. Catheter securement impact on PICC-related CLABSI: a university hospital perspective. Am J Infect Control. 2020;48(12):1497–500.
Scrivens N, Sabri E, McDiarmid S, Bredeson C. Comparison of complication rates and incidences associated with different peripherally inserted central catheters (PICC) in patients with hematological malignancies: a retrospective cohort study. Leuk Lymphoma. 2020;61(1):156–164.
Song Y, Liu S, Lou T, Ma Y, Wang N, Yong Q, et al. Risk factors associated with peripherally inserted central catheter-related venous thrombosis in hospitalized patients of advanced age. The Journal of international medical research. 2020;48(1):3.00061E + 14.
Wang G, Li Y, Wu C, Guo L, Hao L, Liao H, et al. The clinical features and related factors of PICC-related upper extremity asymptomatic venous thrombosis in cancer patients: a prospective study. Medicine. 2020;99(12):e19409.
Webber JR. Sticking it to them–tissue adhesive reduces PICC Migration and microbial contamination. Vascular Access. 2020;14(1):13–7.
Ying S, Liping Z, Yanhong D, Zhulin G, Liang G. Impact of arm choice for peripherally inserted central catheter (PICC) insertion on patients: a cross-sectional study. Contemp Nurse. 2020;56(1):80–9.
Chen XS, Wu XH, Chen LC, Zhang TT, Liu GL. Heparin versus 0.9% saline solution to maintain patency of totally implanted venous access ports in cancer patients: a systematic review and meta-analysis. Int J Nurs Pract. 2021;27:e12913.
Furuhashi S, Morita Y, Ida S, Muraki R, Kitajima R, Suzuki K, et al. Risk factors for totally implantable central venous access port-related infection in patients with malignancy. Anticancer Res. 2021;41(3):1547–53.
Kleidon TM, Horowitz J, Ratz D, Chopra V, Rickard CM, Ullman AJ, et al. Peripherally inserted central catheter thrombosis after Placement via Electrocardiography vs traditional methods. Am J Med. 2021;134(2):e79–88.
Rickard CM, Flynn J, Larsen E, Mihala G, Playford EG, Shaw J, et al. Needleless connector decontamination for prevention of central venous access device infection: a pilot randomized controlled trial. Am J Infect Control. 2021;49(2):269–73.
Schears GJ, Ferko N, Syed I, Arpino JM, Alsbrooks K. Peripherally inserted central catheters inserted with current best practices have low deep vein thrombosis and central line-associated bloodstream infection risk compared with centrally inserted central catheters: a contemporary meta-analysis. J Vasc Access. 2021;22(1):Sep–25.
Sharp R, Carr P, Childs J, Scullion A, Young M, Flynn T, et al. Catheter to vein ratio and risk of peripherally inserted central catheter (PICC)-associated thrombosis according to diagnostic group: a retrospective cohort study. BMJ Open. 2021;11(7):e045895.
Silva SRD, Reichembach MT, Pontes L, Souza G, Kusma S. Heparin solution in the prevention of occlusions in Hickman® catheters a randomized clinical trial. Rev Latinoam Enferm. 2021;29:e3385.
Tian L, Yin X, Zhu Y, Zhang X, Zhang C. Analysis of factors causing skin damage in the application of peripherally inserted central catheter in cancer patients. J Oncol. 2021;2021:6628473.
Wang G, Wang H, Shen Y, Dong J, Wang X, Wang X, et al. Association between ABO blood group and venous thrombosis related to the peripherally inserted central catheters in cancer patients. J Vasc Access. 2021;22(4):590–596.
Wu X, Zhang T, Chen L, Chen X. Prolonging the flush-lock interval of totally implantable venous access ports in patients with cancer: a systematic review and meta-analysis. J Vasc Access. 2021;22(5):814–821.
Xiong ZY, Zhou HM, Li SY. Prolonged flushing and locking interval for totally implantable vascular access device: a systematic review and meta-analysis. J Vasc Access. 2021;22(6):969–978.
Zhang Y, Chen J, Zhao R, Zhang S. Blood sampling from peripherally inserted central catheter is effective and safe for patients with head and neck cancers. J Vasc Access. 2021;22(3):424–431.
Zhong J, Wang B, Huang Q. Study on treating tumor patients with a peripherally inserted central catheter. Int J Clin Exp Med. 2021;14(1):683–9.
Zhao Y, Bian L, Yang J. Intervention efficacy of MARSI nursing management on skin injury at peripherally inserted central catheter insertion site on oncological patients. Int Wound J. 2022;19(8):2055–61.
Belloni S, Caruso R, Cattani D, Mandelli G, Donizetti D, Mazzoleni B, et al. Occurrence rate and risk factors for long-term central line-associated bloodstream infections in patients with cancer: a systematic review. Worldviews Evid Based Nurs. 2022;19(2):100–11.
Capozzi VA, Monfardini L, Sozzi G, Armano G, Butera D, Scarpelli E, et al. Peripherally inserted central venous catheters (PICC) versus totally implantable venous access device (PORT) for chemotherapy administration: a meta-analysis on gynecological cancer patients. Acta Biomed. 2021;92(5):e2021257.
CAS PubMed PubMed Central Google Scholar
Chopra V, O’Malley M, Horowitz J, Zhang Q, McLaughlin E, Saint S, et al. Improving peripherally inserted central catheter appropriateness and reducing device-related complications: a quasiexperimental study in 52 Michigan hospitals. BMJ Qual Saf. 2022;31(1):23–30.
Feng Y, Zheng R, Fu Y, Xiang Q, Yue Z, Li J, et al. Assessing the thrombosis risk of peripherally inserted central catheters in cancer patients using Caprini risk assessment model: a prospective cohort study. Support Care Cancer. 2021;29(9):5047–55.
Gilardi E, Piano A, Chellini P, Fiori B, Dolcetti L, Pittiruti M, et al. Reduction of bacterial colonization at the exit site of peripherally inserted central catheters: a comparison between chlorhexidine-releasing sponge dressings and cyano-acrylate. J Vasc Access. 2021;22(4):597–601.
He E, Ye K, Zheng H. Clinical effect and safety of venous access ports and peripherally inserted central catheters in patients receiving tumor chemotherapy: a systematic review and meta-analysis. Ann Palliat Med. 2021;10(8):9105–13.
Hoppe A, Rupa-Matysek J, Małecki B, Dytfeld D, Hoppe K, Gil L. Risk factors for catheter-related thrombosis in multiple myeloma patients undergoing autologous stem cell transplantation. Med (Kaunas). 2021;57(10):1020.
Jabaley T, Xiong N, Conley S, Mazeika T, Johnson D, Biggins BA, et al. Transitioning from heparin to saline locks for central venous access devices in oncology: an evidence-based practice approach. Can Oncol Nurs J. 2022;32(2):286–93.
Jones M, Okano S, Looke D, Kennedy G, Pavilion G, Clouston J, et al. Catheter-associated bloodstream infection in patients with cancer: comparison of left- and right-sided insertions. J Hosp Infect. 2021;118:70–6.
Liu GD, Ma WJ, Liu HX, Tang L, Tan YH. Risk factors associated with catheter-related venous thrombosis: a meta-analysis. Public Health. 2022;205:45–54.
Liu X, Tao S, Ji H, Chen S, Gu Y, Jin X. Risk factors for peripherally inserted central catheter (PICC)-associated infections in patients receiving chemotherapy and the preventive effect of a self-efficacy intervention program: a randomized controlled trial. Ann Palliat Med. 2021;10(9):9398–405.
Peng SY, Wei T, Li XY, Yuan Z, Lin Q. A model to assess the risk of peripherally inserted central venous catheter-related thrombosis in patients with breast cancer: a retrospective cohort study. Support Care Cancer. 2022;30(2):1127–37.
Pénichoux J, Rio J, Kammoun L, Vermeulin T, Pepin LF, Camus V, et al. Retrospective analysis of the safety of peripherally inserted catheters versus implanted port catheters during first-line treatment for patients with diffuse large B-cell lymphoma. Eur J Haematol. 2022;109(1):41–9.
Perek S, Khatib A, Izhaki N, Khalaila AS, Brenner B, Horowitz NA. A prediction model for central venous catheter-related thrombosis in patients with newly-diagnosed acute myeloid leukemia: a derivation cohort analysis. Eur J Intern Med. 2022;101:68–75.
Simonetti G, Bersani A, Tramacere I, Lusignani M, Gaviani P, Silvani A. The role of body mass index in the development of thromboembolic events among cancer patients with PICCs: a systematic review. J Vasc Nurs. 2022;40(1):11–6.
Trezza C, Califano C, Iovino V, D’Ambrosio C, Grimaldi G, Pittiruti M. Incidence of fibroblastic sleeve and of catheter-related venous thrombosis in peripherally inserted central catheters: a prospective study on oncological and hematological patients. J Vasc Access. 2021;22(3):444–9.
Zhao H, He Y, Huang H, Ling Y, Zhou X, Wei Q, et al. Prevalence of medical adhesive-related skin injury at peripherally inserted central catheter insertion site in oncology patients. J Vasc Access. 2018;19(1):23–7.
Curtis K, Gavin N, Fuller F. Cancer Nurses Society of Australia, vascular access devices: evidence-based clinical practice guidelines, 2021. In: Cancer Nurses Society of Australia, editor. https://www.cnsa.org.au/practiceresources/vascular-access-guidelines2021 .
Schults J, Kleidon T, Chopra V, Cooke M, Paterson R, Ullman AJ, et al. International recommendations for a vascular access minimum dataset: a Delphi consensus-building study. BMJ Qual Saf. 2021;30(9);722–730.
World Health Organization. Health products policy and standards. Nomenclature of medical devices 2021. Available from: https://www.who.int/teams/health-product-policy-and-standards/assistive-and-medical-technology/medical-devices/nomenclature .
Gildow C, Lazar B. The implementation of standard terminology in electronic health record systems and the effect on patient outcomes: a systematic literature review. Int J Acad Health Med Res. 2022;6(7):132–8.
Gouin-Thibault I, Achkar A, Samama MM. The thrombophilic state in cancer patients. Acta Haematol. 2001;106(1–2):33–42.
Jiang D, Lee AI. Thrombotic risk from chemotherapy and other cancer therapies. In: Soff GA, editor. Thrombosis and hemostasis in cancer. Cancer treatment and research. Springer Nature; Switzerland AG. 2019.
Levi M. Clinical characteristics of disseminated intravascular coagulation in patients with solid and hematological cancers. Thromb Res. 2018;164 Suppl 1:S77–81.
Centers for Disease Control and Prevention. National Healthcare Safety Network (NHSN) patient safety component manual. 2020. Retrieved from https://www.cdc.gov/nhsn/pdfs/pscmanual/pcsmanual_current . Accessed 15 Dec 2022.
Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O’Grady NP, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the infectious diseases society of America. Clin Infect Dis. 2009;49(1):1–45.
Rowley S, Clare S. Standardizing the critical clinical competency of aseptic, sterile, and clean techniques with a single international standard: Aseptic non Touch technique (ANTT®). JAVA. 2019;24(4):12–7.
Roos LL, Wall-Wieler E, Burchill C, Hamm NC, Hamad AF, Lix LM. Record linkage and big data—enhancing information and improving design. J Clin Epidemiol. 2022;150:18–24.
Download references
Acknowledgements
No funding was received for the development or publication of the scoping review.
Author information
Authors and affiliations.
Department of Nursing, University of Melbourne, Melbourne, Australia
Kerrie Curtis, Karla Gough & Meinir Krishnasamy
Peter MacCallum Cancer Centre, Melbourne, Australia
Kerrie Curtis
Austin Health, Melbourne, Australia
Kerrie Curtis & Elena Tarasenko
Department of Health Services Research, Peter MacCallum Cancer Centre, Melbourne, Australia
Karla Gough & Meinir Krishnasamy
Sir Peter MacCallum Department of Oncology, University of Melbourne, Melbourne, Australia
Victorian Comprehensive Cancer Centre Alliance, Melbourne, Australia
Meinir Krishnasamy
Royal Melbourne Hospital, Melbourne, Australia
Centre for Healthcare Transformation, Queensland University of Technology, Brisbane, Australia
Samantha Keogh
You can also search for this author in PubMed Google Scholar
Contributions
KC, MK, SK and KG conceived the scoping review and participated in its design. KC and GH developed and conducted the literature search strategy. KC and ET conducted the data screening and extraction. KC and KG carried out the data analyses. KC drafted the manuscript and MK, SK, and KG revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Kerrie Curtis .
Ethics declarations
Ethics approve and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1..
Scoping review protocol.
Additional file 2.
Search strategy.
Additional file 3.
Included studies.
Additional file 4.
Summary of CVAD terminology.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Curtis, K., Gough, K., Krishnasamy, M. et al. Central venous access device terminologies, complications, and reason for removal in oncology: a scoping review. BMC Cancer 24 , 498 (2024). https://doi.org/10.1186/s12885-024-12099-8
Download citation
Received : 27 September 2023
Accepted : 08 March 2024
Published : 19 April 2024
DOI : https://doi.org/10.1186/s12885-024-12099-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Central venous catheters
- Catheters indwelling
- Central venous access device
- Device removal
- Complication
- Premature removal
- Terminology
ISSN: 1471-2407
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 21 August 1943
“Abstracts from Cancer Research”
Nature volume 152 , page 212 ( 1943 ) Cite this article
5812 Accesses
Metrics details
THE problems of the nature and possible treatment of malignant disease are studied in many laboratories and results are published in different journals. It is difficult for any worker to keep in touch with all the published work. Excellent summaries of papers were published for many years in the Cancer Review , the publication of which was unfortunately suspended about ten years ago. Since then abstracts have been available in the American Journal of Cancer , which has now been replaced by Cancer Research . The “Abstracts from Cancer Research” are published separately and the first volume covers the year 1941. The summaries are grouped under the main headings of experimental research (including carcinogenic compounds, hormones, viruses, genetics, physical factors, radiation, chemosurgery, biochemistry and nutrition-chemotherapy, immunology, leukæmia, transplantation and tissue culture), comparative oncology, clinical and pathological reports, statistics and cancer control and public health. The production seems admirable and should be of great value to cancer research workers. This new journal is published in America by the International Cancer Research Foundation, hut Prof. E. L. Keimaway and Dr. W. E. Gye arrange for the abstracting of articles appearing in publications in the British Commonwealth.
Article PDF
Rights and permissions.
Reprints and permissions
About this article
Cite this article.
“Abstracts from Cancer Research”. Nature 152 , 212 (1943). https://doi.org/10.1038/152212c0
Download citation
Issue Date : 21 August 1943
DOI : https://doi.org/10.1038/152212c0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Select "Patients / Caregivers / Public" or "Researchers / Professionals" to filter your results. To further refine your search, toggle appropriate sections on or off.
American Association for Cancer Research (AACR)
Research-driven advances in liquid biopsy are beginning to show how these less invasive tests can be used for early cancer detection, treatment selection, and patient monitoring.
Support Lifesaving Cancer Research. Donate Now.
Cancer is not a single disease, but rather a collection of diseases all characterized by the uncontrolled proliferation of cells.

Take our Cancer Prevention Quiz to test your knowledge and learn more about cancer risk factors and risk reduction.

More than 299,000 men in the U.S. are expected to be diagnosed with prostate cancer this year. Read about prevention, screening, and treatment options for this type of cancer.

Isabella Snow Fraser was diagnosed with a rare type of cancer when she was just six years old. Cancer immunotherapy helped Bella to reclaim her childhood.
The report highlights the AACR’s impact on the cancer community during the past year while advancing our mission to prevent and cure all cancers.

Whether honoring a special person or a special day, a donation to the American Association for Cancer Research has a lasting impact.

The official news website of the AACR Annual Meeting 2024. Stay up to date on the latest developments from the most important cancer meeting in the world.
The AACR Cancer Progress Report 2023 provides a comprehensive overview of the latest research-driven advances against the collection of devastating diseases called cancer.
The AACR and its more than 58,000 members worldwide are advancing a scientifically bold agenda against the collection of diseases we call cancer.
A new wave of research-driven discoveries and technological innovations are delivering – and will propel additional – transformative advances to save more lives from cancer..
By the Numbers
percent decrease of the overall age-adjusted cancer death rate in the U.S. from 1991 to 2020
therapeutics were approved for new or expanded uses by the FDA from Aug. 1, 2022, to July 31, 2023
million cancer survivors in the U.S. are living with, through, and beyond their disease thanks to research
cancer diagnoses in the United States are associated with preventable risk factors
Your donation to the American Association for Cancer Research helps our more than 58,000 members worldwide drive progress against cancer.

- The Progression of Cancer
- AACR Project GENIE®: Powering Precision Medicine
- Patient Advocacy

IMAGES
COMMENTS
Cancer Research publishes original studies, reviews, and opinion pieces on cancer biology and drug response. Browse the current issue or search by keywords, abstracts, or authors.
Publication of Abstracts in Cancer Research. All presented abstracts were published in a two-part online-only supplement to the AACR journal Cancer Research. Regular abstracts were published on April 4, 2023, and clinical trial and late-breaking abstracts were published on April 14, 2023.
Find out how to submit and present your abstract at the AACR Annual Meeting 2024 in San Diego, California. Learn about the deadlines, formats, publication opportunities and guidelines for regular, late-breaking and clinical trial abstracts.
the abstracts presented at the AACR Annual Meeting 2021 have been published in an online-only Proceedings supplement to the July 1 issue of the AACR journal Cancer Research. View the Supplement 615 Chestnut St., 17th Floor
A total of 496 participants were assigned to receive pembrolizumab and 498 to receive placebo. As of September 15, 2023, the median follow-up was 57.2 months.
The adherence to the American Association for the Study of Liver Diseases 2018 guidelines in the management of hepatocellular carcinoma and its impact on survival. Manne, Ashish; Mulekar, Madhuri; Escobar, Daisy; More. Journal of Cancer Research and Therapeutics. 19 (5):1103-1108, Jul-Sep 2023. Abstract.
What is a cancer research study abstract? An abstract is a summary that is at the beginning of published cancer research studies. It shares the study's main data. This allows readers to quickly learn about the most important parts of the research. Researchers often share their abstracts at scientific meetings, sometimes even before they have ...
According to the International Agency for Research on Cancer (IARC), in 2020 there were approximately 19.3 million new cases of cancer, and 10 million deaths by this disease, 6 while 23.8 million cases and 13.0 million deaths are projected to occur by 2030. 73 In this regard, it is clear the increasing role that environmental factors ...
In recent years, precision oncology has made important strides in advancing treatment for patients with cancer, as described in several reviews 1,2,3,4,5,6.Much of the focus in the field has been ...
2022 CRI Meeting Abstracts. 2022 Abstracts and Posters. First Place. Successful Methods of Addressing Clinical Research Staff Turnover [ Abstract] [ Poster] N. Nahmias, J. Sanchez, A. Olier-Pino, A. Allred, K. Aviles, L. Corrales. Sylvester Comprehensive Cancer Center, University of Miami Health System.
2 Cancer Immunosurveillance Group, Cancer Research UK Manchester Institute, The University of Manchester, Manchester M20 4BX, UK. 3 Inflammatory Cell Dynamics Section, ... Abstract A role for vitamin D in immune modulation and in cancer has been suggested. In this work, we report that mice with increased availability of vitamin D display ...
The Journal of Cancer Research and Clinical Oncology publishes content within the fields of experimental and clinical oncology. Topics covered include, but are not limited to: carcinogenesis, molecular biology, developments in tumor therapy, general and laboratory diagnoses, diagnostic and experimental pathology, oncologic surgery, and epidemiology.
American Association for Cancer Research. From molecules to medicine, from proteomics to prevention, the journals of the AACR cover the full spectrum of cancer science and medicine. To submit your manuscript, please select the journal that aligns with your research and follow the instructions to access the correct submission site.
Esophageal cancer imaging - reporting and data system (ECI-RADS) and post-therapy ECI-RADS (pECI-RADS): Comprehensive synoptic reporting formats for esophageal cancer imaging: A narrative review. Chakrabarty, Nivedita; Mahajan, Abhishek. Cancer Research, Statistics, and Treatment. 5 (3):562-568, Jul-Sep 2022. Abstract.
Colorectal cancer (CRC) is the third most common cancer and the second leading cause of cancer death worldwide with more than 1.9 million incident cases and nearly 1 million deaths in 2020 (1, 2).There is convincing evidence that individuals with overweight or obesity [body mass index (BMI) ≥ 25 kg/m 2] have a higher risk of CRC ().Waist or hip circumferences (WC or HC) and waist-to-hip ...
AACR Annual Meeting 2022. April 8 - 13, 2022 Ernest N. Morial Convention Center New Orleans, Louisiana. Home > Cancer Researchers / Other Health Care Professionals > Meetings > Meetings and Workshops Calendar > AACR Annual Meeting 2022 > Abstracts.
Dense breasts, in which breasts are composed of more glandular tissue relative to fatty tissue, is an independent, nonmodifiable risk factor for breast cancer and can mask cancer on mammograms. 1 Dense breast tissue is present in 40% to 50% of women undergoing screening mammography 2 and is associated with a 1.2 to 4.0 times higher risk of ...
Abstracting a Cancer Case. A tumor abstract summarizes the important information about a patient's reportable tumor. Cancer Registrars must understand the contents of a medical record to be able to extrapolate required data items for the cancer abstract. ... and cancer research and clinical studies. In this module you will learn to. Identify ...
Abstract: Now a day' s cancer is the most prevalent life threatening disease which is sp reading because of the. lifestyl e we are living. ... cancer research remains important scientific ...
However, there is a paucity of research on body image, dyadic coping, and post-traumatic growth in breast cancer patients. The purpose of this study was to explore the relationship and pathways between body image, dyadic coping, and post-traumatic growth (PTG) in breast cancer patients.
Individuals interested in submitting an abstract for presentation during the conference must do so through the AACR online abstract submission service below. Abstract submissions will only be accepted electronically; paper abstracts will not be accepted. The abstract submission deadline is Monday, July 1, 2024, 1 p.m. ET. ABSTRACT CATEGORIES NOTIFICATION OF ABSTRACT STATUS All individuals ...
Lack of agreed terminology and definitions in healthcare compromises communication, patient safety, optimal management of adverse events, and research progress. The purpose of this scoping review was to understand the terminologies used to describe central venous access devices (CVADs), associated complications and reasons for premature removal in people undergoing cancer treatment.
The AACR Annual Meeting is the focal point of the cancer research community, where scientists, clinicians, other health care professionals, survivors, patients, and advocates gather to share the latest advances in cancer science and medicine. From population science and prevention; to cancer biology, translational, and clinical studies; to ...
About the Journal. Clinical Cancer Research publishes articles that focus on innovative clinical and translational research bridging the laboratory and the clinic. Topics include targeted therapies; mechanisms of drug sensitivity and resistance; pharmacogenetics and pharmacogenomics; personalized medicine; immunotherapy; gene therapy ...
The "Abstracts from Cancer Research" are published separately and the first volume covers the year 1941. The summaries are grouped under the main headings of experimental research (including ...
Abstracts. Abstracts presented at the AACR Virtual Annual Meetings have been published in an online-only supplement to the AACR journal Cancer Research. Clinical trial and late-breaking abstract deadline: January 30, 2020. Final deadline for clinical trial placeholder abstracts: February 20, 2020.
Your donation to the American Association for Cancer Research helps our more than 58,000 members worldwide drive progress against cancer. The AACR is the first and largest cancer research organization. Our mission is to prevent and cure cancer through research, education, communication, collaboration, funding, and advocacy. Our 58,000 members ...