Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 06 August 2021

Advancement in long-distance bird migration through individual plasticity in departure
- Jesse R. Conklin ORCID: orcid.org/0000-0002-1414-0587 1 ,
- Simeon Lisovski ORCID: orcid.org/0000-0002-6399-0035 2 &
- Phil F. Battley ORCID: orcid.org/0000-0002-8590-8098 3
Nature Communications volume 12 , Article number: 4780 ( 2021 ) Cite this article
8796 Accesses
25 Citations
44 Altmetric
Metrics details
- Animal migration
- Evolutionary ecology
Globally, bird migration is occurring earlier in the year, consistent with climate-related changes in breeding resources. Although often attributed to phenotypic plasticity, there is no clear demonstration of long-term population advancement in avian migration through individual plasticity. Using direct observations of bar-tailed godwits ( Limosa lapponica ) departing New Zealand on a 16,000-km journey to Alaska, we show that migration advanced by six days during 2008–2020, and that within-individual advancement was sufficient to explain this population-level change. However, in individuals tracked for the entire migration (50 total tracks of 36 individuals), earlier departure did not lead to earlier arrival or breeding in Alaska, due to prolonged stopovers in Asia. Moreover, changes in breeding-site phenology varied across Alaska, but were not reflected in within-population differences in advancement of migratory departure. We demonstrate that plastic responses can drive population-level changes in timing of long-distance migration, but also that behavioral and environmental constraints en route may yet limit adaptive responses to global change.
Similar content being viewed by others

Carryover effects of long-distance avian migration are weaker than effects of breeding environment in a partially migratory bird
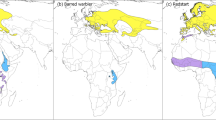
Limited flexibility in departure timing of migratory passerines at the East-Mediterranean flyway
Individuals departing non-breeding areas early achieve earlier breeding and higher breeding success
Introduction.
The current pace and scale of environmental change tests the capacity of organisms to adapt. For example, climate change is altering the timing of annual cycles in animals and plants worldwide 1 , 2 . In general, the timing of bird migration and breeding are advancing 3 , 4 , as expected with earlier availability of seasonal resources for reproduction 5 . However, weak or inadequate phenological responses are commonly observed 6 , as are general declines in migratory populations 7 , 8 , implying limitations to the capacity for adaptation. For long-distance migrants, this is complicated by changes that may vary geographically in degree or direction 9 , or include interactions with non-climate-related effects such as direct habitat alteration by humans 10 . Currently, the specific mechanisms driving adaptive population changes are poorly understood.
Observed population-level changes in avian migration phenology are generally attributed to phenotypic plasticity 11 , but the additional or interacting role of evolutionary responses are rarely tested and difficult to exclude 12 , 13 . Individuals are often relatively consistent in migration timing 14 , but both observational and experimental studies show that individual timing is flexible and responsive to environmental conditions, body state, and social context 15 , 16 , 17 . Observed magnitudes of plasticity may be sufficient to account for documented population-level phenology changes 18 , particularly in long-lived species that may use lifelong experience to adjust annual routines 19 , 20 . However, data to directly address this question are scarce, due to the difficulty of simultaneously collecting long-term population-wide information and repeated individual data for any stage of migration. One such study found that arrival at breeding grounds did not change within adult individuals over time, but that population advancement occurred through the recruitment of young individuals with increasingly early migration timing 21 . To our knowledge, there has been no demonstration of long-term, directional population change in migration timing resulting from individual plasticity.
The annual migration of bar-tailed godwits ( Limosa lapponica baueri ) includes three flights of 6000–12,000 km each, including two of the longest non-stop flights recorded in birds 22 , 23 . Across the Alaska breeding range (Fig. 1 ), there is a latitudinal cline in which northern-breeding godwits are smaller and migrate later than southern breeders 24 , 25 ; the timing differences reflect the increasingly later snowmelt, and thus the availability of tundra breeding sites, at higher latitudes. At non-breeding sites in New Zealand, annually consistent individual differences in departure on the 10,000-km non-stop flight to the Yellow Sea region of Asia are maintained across a one-month period 26 , 27 , and are generally retained throughout the northward migration 24 , 27 . In the non-breeding season, bar-tailed godwits present a unique opportunity to directly observe both population-level and individual behavior, due to their relatively large size, high site fidelity and longevity, gregarious use of open habitats, and conspicuous migration departure during daylight hours 15 .
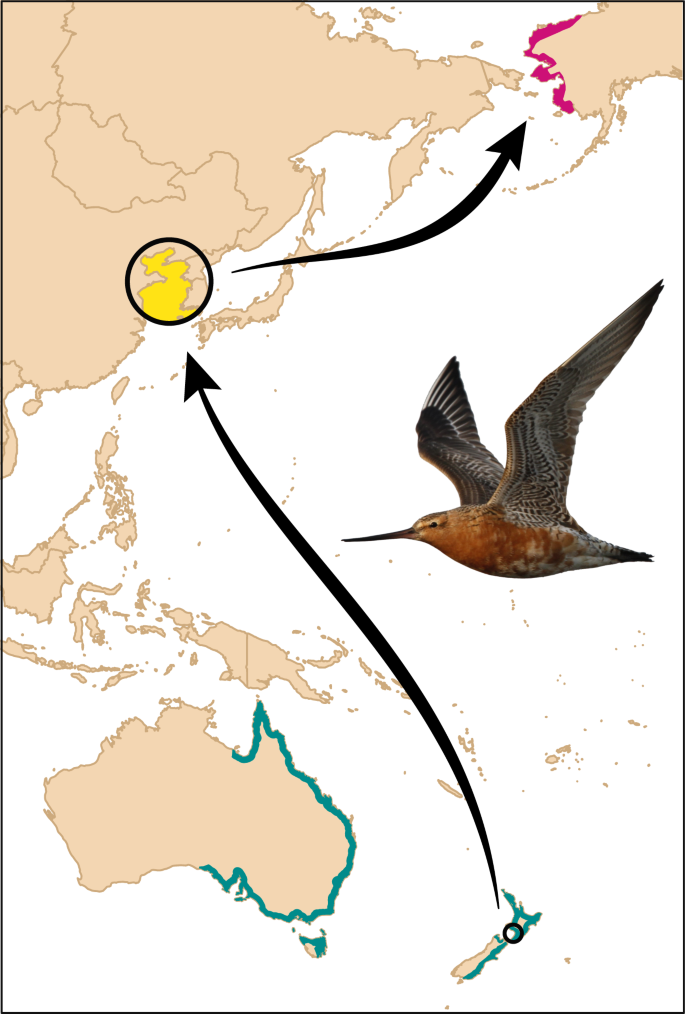
Field site in New Zealand indicated by a circle. Map made with Natural Earth. Photo: P. Battley.
Here, we use 13 years of directly observed migratory departures by bar-tailed godwits from a small, intensively-monitored non-breeding site in New Zealand (Fig. 1 ) to: (1) describe the magnitude of population-level change in the initiation of migration, and (2) assess the relative contributions of individual plasticity and between-individual differences to the population trend. By tracking a subset of individuals with light-level geolocators for the complete northward migration, we quantify the extent to which between-year changes in departure persist to later stages, including the timing of arrival and breeding in Alaska. To evaluate expected responses, we describe phenology changes in the species’ Alaska breeding range during the same period, using remotely sensed environmental data. We demonstrate that directional, population-level change in timing of bird migration can occur predominantly through within-individual changes: across 13 years, bar-tailed godwits advanced their departure from New Zealand by nearly 0.5 days per year, and within-individual advancement alone was sufficient to explain the trend.
During 2008–2020, we documented all migratory departures from the Manawatu River estuary in New Zealand, which included 10–14 flocks containing 128–251 total godwits per year. Over this period, mean northward departure advanced at –0.484 d/yr ± SE 0.035 ( R 2 = 0.073, F 1,2421 = 192.7, p < 0.0001), from ca. 21 to 15 March (Fig. 2a ).
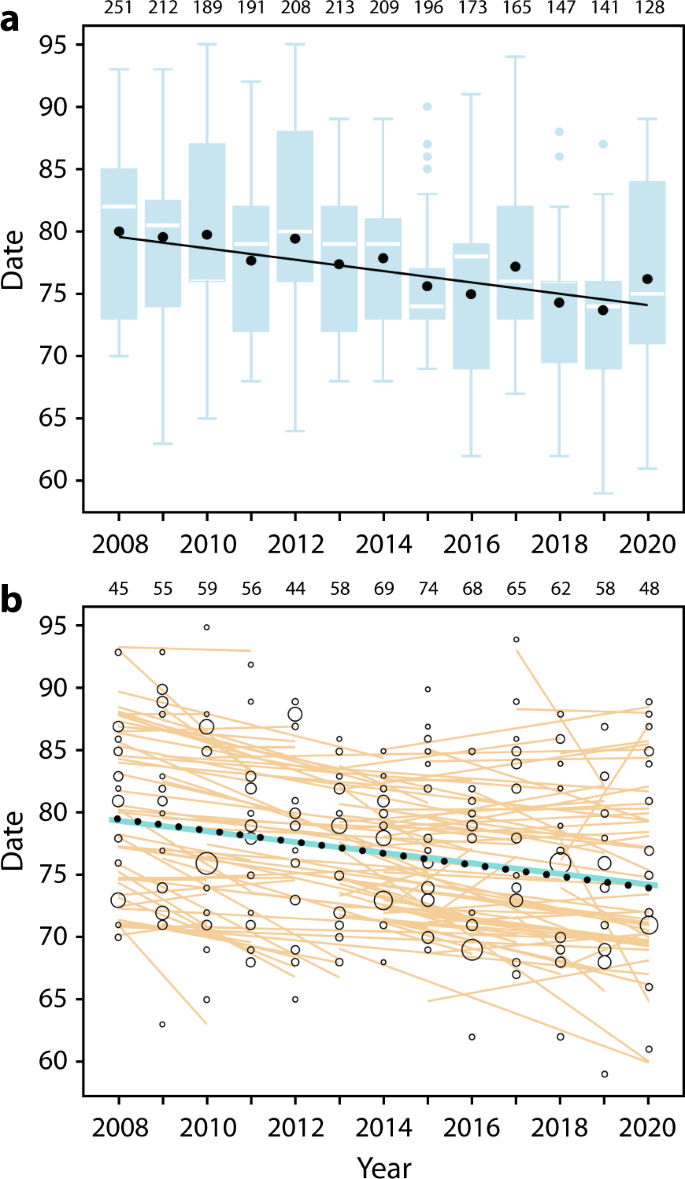
a Population-level advancement in departure. In blue: distribution of departures ( n = 128–251 godwits per year, total = 2,423 departures; sample sizes above panel); boxplots indicate 25–75th percentile range with median departure date (white lines); whiskers extend to 1.5× interquartile range; outliers (dots) beyond this. In black: mean departure dates (filled circles) plus linear slope (solid line). Date: 1 = 1 January. b Within-individual advancement of departure date ( n = 124 marked individuals observed in 3–13 years each; sample sizes above panel). Open circles = all departures; size scaled to the proportion of total migrating individuals that year (0.006–0.422 per day). Orange lines indicate within-individual slopes. Overall advancement was shown by the solid blue line (within-individual slope) and black dotted line (between-individual slope); see Supplementary Table 1a .
Based on individually-identifiable godwits in these flocks (124 marked individuals observed in 3–13 years each), plastic responses were sufficient to explain the population-level advancement in departure: the overall within-individual trend (−0.428 d/yr ± SE 0.100) was not distinguishable from the between-individual trend (−0.463 d/yr ± SE 0.082, p = 0.78; Fig. 2b and Supplementary Table 1a ). Within-individual trends ranged from −6.5 to +5.5 d/yr, and were advancing in 69% of individuals (86 of 124; Supplementary Fig. 1 ). Despite the overall advancement, consistent between-individual differences were retained (individual repeatability of departure during 2008–2020: r = 0.782 ± SE 0.021, F = 23.0, d.f. = 123, p < 0.0001).
Population-level advancement could potentially occur through a changing composition of the study population; for example, through lower survival or lower recruitment of later-migrating (i.e., smaller, northern-breeding) birds across the study period. However, we found no evidence of a changing proportion of northern- and southern-breeders across annual samples: mean body size was constant over time (Supplementary Fig. 2 ) and the likelihood of returning the next year was unrelated to departure date (generalized linear model: slope = −0.0005, Z = −0.28, d.f. = 746, p = 0.78).
During the same period (2008–2020), advancement in the timing of snowmelt (date when 33% snow-free) within the entire Alaska breeding range (−0.651 d/yr ± SE 0.001; Supplementary Table 2 ) was similar in magnitude to the advancement in godwit departure from New Zealand, although characterized by large between-year variation. However, changes varied regionally: snowmelt advanced at −1.926 d/yr ± SE 0.003 in southern (<64°N) Alaska, compared to −0.235 d/yr ± SE 0.002 in northern (>64°N) Alaska (Fig. 3c and Supplementary Table 2 ). Advancement of spring green-up (date of greatest increase in Normalized Difference Vegetation Index, NDVI) was also much greater in southern (−2.296 d/yr ± SE 0.031) than in northern Alaska (−0.467 d/yr ± SE 0.014; Fig. 3d and Supplementary Table 2 ).

a Breeding latitude of 36 geolocator-tracked individuals (orange dots; lines indicate individuals tracked in multiple years) in four years of tracking (2008, 2009, 2013, 2014; n = 12–13 per year), and assigned to Alaska region (North >64°N, n = 16; South <64°N, n = 20). The known breeding range is shown in orange. Map made with Natural Earth. b Within- and between - individual advancement of directly observed departure date from New Zealand during 2008–2020 for individuals with known breeding locations in northern (upper; n = 15 individuals observed in 3–13 years each) and southern Alaska (lower; n = 19). Date: 1 = 1 January. Figure details as described in Fig. 2b . The within-individual slope is indicated in the panel (see Supplementary Table 1b ). c Timing of snowmelt (date when 33% snow-free) in the Alaska breeding range north (upper) and south (lower) of 64°N. d Start of spring green - up (date of greatest increase in Normalized Difference Vegetation Index, NDVI) in the Alaska breeding range north (upper) and south (lower) of 64°N. For c and d , black lines indicate median date across region, and shaded areas indicate 95% quantile range. Dotted lines indicate the 13-year trend (2008–2020; slope indicated in the panel), and orange lines indicate the trend during the period of geolocator-tracking (2008–2014).
To determine whether advancement in departure from New Zealand differed by breeding destination, we reduced the data set of directly observed departures to 34 individuals with known breeding locations, determined from geolocator-tracking in four years of the study (2008, 2009, 2013, 2014; Fig. 3a and Supplementary Fig. 3 ). For this sample, within-individual advancement of departure from New Zealand during 2008–2020 was similar for godwits that bred in northern Alaska (−0.500 d/yr ± SE 0.206, n = 15) and southern Alaska (−0.522 d/yr ± SE 0.209, n = 19; Fig. 3b and Supplementary Table 1b ).
Geolocator-tracking (50 complete northward migration tracks of 36 individuals) also showed that the advancement in departure from New Zealand did not lead to earlier arrival in Alaska (Fig. 4 and Supplementary Table 3 ). During 2008–2014, despite advancement of the first flight from New Zealand to the Yellow Sea (departure: −0.845 d/yr ± SE 0.85; arrival: −0.885 d/yr ± SE 0.267; both p ≤ 0.005), the subsequent flight to Alaska did not significantly advance (departure: −0.333 d/yr ± SE 0.257; arrival: −0.205 d/yr ± SE 0.233; both p ≥ 0.20). This difference is explained by the increasing duration of the stopover in the Yellow Sea (+0.693 d/yr ± SE 0.302, p = 0.026). The date that godwits started incubating their first clutch ( n = 41 nests of 29 individuals) did not change across 2008–2014 (+0.066 d/yr ± SE 0.402, p = 0.87). Differences in regional trends in the timing of snowmelt and NDVI in Alaska during the shorter period of geolocator-tracking (2008–2014) were similar to the longer-term (2008–2020) trends (Fig. 3c, d and Supplementary Table 2 ).
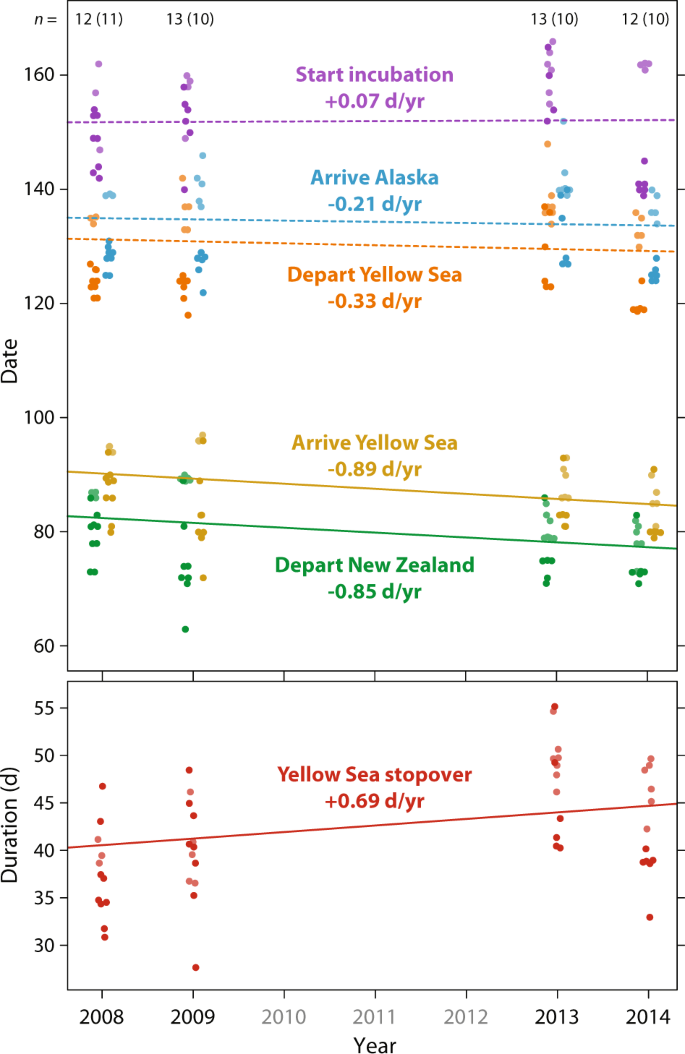
Points for overlapping stages and individuals are offset for clarity. Within each stage (distinguished by color), dark and pale dots indicate individuals breeding in southern (<64°N) and northern (>64°N) Alaska, respectively (Fig. 2a ). Lines indicate significant (solid) and non-significant (dashed) slopes during 2008–2014, derived from linear mixed models (see Supplementary Table 3 ). Numbers across the top of the plot indicate the number of tracked birds per year (smaller sample of incubating birds in parenthesis). Date: 1 = January.
Our findings contrast with the expectation that inter-generational or evolutionary processes are required to explain observed phenological changes in bird migration 28 , 29 . In our analysis of migratory departure from New Zealand, we aimed to explicitly separate phenotypic flexibility (i.e., reversible plastic changes within a post-development adult 30 ) from between-individual shifts in the population over time 31 , which may occur through developmental plasticity or micro-evolution through selection on different phenotypes. The statistically indistinguishable within- and between-individual slopes (Fig. 2b and Supplementary Table 1 ) indicate that no selection or generation shift is required to explain the advancement in migration timing of bar-tailed godwits.
It is commonly claimed that weak or insufficient responses by migratory birds to advancing phenologies at breeding grounds (in terms of timing of arrival or breeding) may reflect an inherent lack of flexibility in the initiation of migration 32 , 33 . This claim is particularly invoked for longer-distance migrants, which may face greater temporal or physiological constraints and stronger canalization of migratory behavior 11 . The magnitude of the advance in the departure of godwits from New Zealand is comparable to or greater than recent population-level changes observed at the passage or terminal sites of both short- and long-distance migrants (advances of typically 0.1–0.5 d/yr 34 , 35 , 36 ). Therefore, our results do not support limited plasticity in the initiation of migration as an explanation for insufficient population responses to advancing phenology of breeding resources. This may be an unexpected finding in an extreme long-distance migrant that shows unusually high individual repeatability in migration timing 14 , 26 , 27 , but demonstrates that repeatability does not strictly imply consistency 27 , and that neither precludes potential flexibility in new circumstances.
Although developmental or evolutionary changes are unnecessary to explain our results, we cannot exclude that some also occurred during our study. A longer time series is required to specifically address generational change in this species, as our study period did not involve a complete turnover of individuals. On average, godwits in this population will migrate to Alaska nine times (adult annual survival ca. 88% 37 ), and some individuals surpass 25 years of age. In our study, most individuals (93%) were of unknown age, having been first marked as adults (≥3 years old), and 19 individuals contributed 10–13 years of data. Therefore, we do not know whether the degree of plasticity we observed would persist and be sufficient to address multi-decadal change in this population 4 , or would potentially reach a functional limit in a longer-term study 38 .
Advancement of migration timing is generally assumed to reflect a response to global climate change, particularly at breeding grounds, and it is possible that godwits are departing New Zealand earlier in an attempt to track advancing conditions in Alaska. Indeed, snowmelt across their breeding range as a whole advanced at a similar rate (−0.65 d/yr) as the departure from New Zealand (−0.48 d/yr). However, earlier departure by godwits did not lead to earlier arrival in Alaska (Fig. 4 ), and the more rapid advancement of breeding conditions in southern Alaska was not reflected in a relatively greater response by southern-breeding godwits (Fig. 3b ). This suggests that the advancement in migration timing was not simply, and perhaps not at all, a response to conditions in Alaska.
After departing New Zealand, migrating godwits stage on the shores of the Yellow Sea in East Asia, a region threatened by habitat loss and decreased food supplies. Intertidal mudflats in the Yellow Sea, used by this and many other migratory shorebird populations on the East Asian-Australasian Flyway, have been significantly lost and degraded in recent decades 39 , coincident with general population declines 40 and decreasing survival, including in bar-tailed godwits 37 , 41 . At the most important northward stopover site for Alaska-breeding godwits, the Yalu Jiang National Nature Reserve in northeast China, benthic prey and godwit intake rates plummeted during 2011–2013 and prey levels have not recovered subsequently 42 . In our geolocator-tracked godwits, earlier departure from New Zealand in 2013–2014 versus 2008–2009 was completely countered by a prolonged stopover in Asia. This raises two alternative, non-mutually-exclusive explanations for the observed change in New Zealand.
The first is that advancing departure from New Zealand is a response to advancing breeding phenology in Alaska, but that deteriorating conditions in Asia prevented a similar advancement of the second flight to Alaska. Lower habitat quality at staging sites could decrease fueling rates 43 , 44 and thus increase the required length of stay in Asia. In this scenario, the consistent timing of arrival in Alaska is maladaptive, resulting from reversible-state effects 45 of disruptive conditions along the migratory route. A second alternative is that advancing departure from New Zealand is actually a response to conditions in Asia rather than Alaska, as a mechanism to allow more time to refuel in Asia while maintaining a consistent arrival date in Alaska. In this scenario, the increased length of stay in Asia is the adaptive response, successfully preventing later arrival in Alaska.
With current data, we cannot exclude either of these scenarios, nor can we rule out an additional role of changing conditions in New Zealand. However, we find this last possibility unlikely to drive a directional trend in timing, because previous work has shown departure from New Zealand to be generally insensitive to seasonal carry-over effects 46 , and days with advantageous wind conditions for migration are quite common 15 . Currently, we have no evidence that the length of stay in New Zealand, which is approximately six months, has changed over our study period. Further, any resource-based hypothesis for advancing departure would need to reconcile earlier fueling for a 10,000-km non-stop flight with both a decreasing study population (Fig. 2a ) and declining benthic prey resources at the study site (PFB & JRC unpubl. data).
If individual godwits are advancing their migration timing in New Zealand based on changing conditions in Asia or Alaska, it implies the existence of feedback mechanisms that allow modification of circannual cycles according to past events or conditions (i.e., learning). For godwits, there are no conceivable within-year cues on the non-breeding grounds in New Zealand for stopover or breeding conditions in the northern hemisphere (ca. 10,000 and 16,000 km along the migration route, respectively). However, as the average godwit will experience numerous migrations, adults have ample opportunity to use previously experienced conditions at staging and breeding sites to inform migratory decisions 19 , 47 , enabling intermediate-term (i.e., year-to-year) plastic responses. Because godwits migrate in flocks and are highly social in the non-breeding season, individuals may be additionally influenced by the behavior and experiences of others 20 , 48 . Also, young individuals departing New Zealand on their first northward migration typically join flocks of experienced adults; this may represent a mechanism for incremental, inter-generational change, without the need for replacement through natural selection 20 . Such flexible modifications to endogenous annual rhythms may be common in long-lived species, but less important for short-lived species experiencing only 1–3 migrations. Precisely what mechanisms allow long-distance migrants to track long-term environmental changes, which are often more stochastic than incremental (e.g., Fig. 3c, d ), remain poorly understood 49 .
Currently, we lack data to address whether the relatively constant timing of Alaska arrival has affected fitness through decreased reproductive success. However, we can derive some insights from geolocator-derived nest incubation behavior (Fig. 4 ). After arrival in southwest Alaska, bar-tailed godwits spend up to two weeks refueling at coastal sites before moving to tundra breeding sites 22 , 24 , and this period represents an opportunity to gain information about local environmental conditions and potentially compensate for sub-optimal arrival timing. According to geolocator data, in 2008, 2009, and 2013, northern-breeding godwits started incubation ca. 18 days (annual means 16.5–20.5 days) after arrival in Alaska, compared to 23 days (annual means 20.3–27.5 days) for southern-breeding birds. In these years, the timing of snowmelt in both regions was close to the 13-year trend (Fig. 3c ). However, in 2014, snowmelt was unusually late in northern Alaska, and unusually early in the south (Fig. 3c ). As expected, northern breeders delayed the start of incubation (mean 24.0 days after arrival), while southern breeders started incubation much sooner after arrival (mean 15.7 days). This implies flexibility in the system to respond to local phenology after arrival in Alaska when necessary, but a longer time series, including a greater range of environmental fluctuations, is required to understand the limits of this flexibility.
The surprisingly large reaction norm in New Zealand departure may imply that other stages of the migration would be similarly plastic, given the right environmental conditions. However, it is also conceivable, particularly in trans-hemispheric migrations in which distant locations are linked by one or few flights 23 , that different stages of the migration have been shaped by very different regimes of selection, and thus respond differently to change 50 . Such modularity is demonstrated by different endocrine and time-keeping mechanisms among annual-cycle stages such as molt, migratory fueling, and breeding 51 , 52 , and in the common observation that temporal variation decreases with increasing proximity to breeding areas 53 , 54 . It is also clear that en route conditions can de-couple timing of departure and arrival, such that variation observed at the migratory destination may not reflect the degree of potential flexibility in earlier stages 55 , 56 , as we found in this study.
The expectation that long-distance migrants may be limited in their capacity to respond to change stems, in part, from the understanding that migration timing is set by endogenous programs that are entrained by daylength 32 , 57 , in birds without relevant within-season cues about destination conditions 49 . While there are clearly fundamental endogenous components to migration, there is increasing evidence for substantial capacity for change within certain timing windows that are presumably set by heritable processes and photoperiod 58 . In New Zealand, there is latitudinal variation in the timing of godwit migration, with birds in the southern part of the non-breeding range (with longer photoperiods) departing significantly earlier than those further north 59 . Across all studied sites, larger (southern-breeding) birds migrated earlier on average than smaller (northern-breeding) birds, implying both external (photoperiodic) control of the population migration timing, and internal (i.e., endogenous program) control of individual schedules. The current study indicates that, overlaid on this framework of individual- and population-specific timing, adult godwits can advance their migration timing, presumably in response to past conditions throughout the annual cycle.
In summary, we have demonstrated population-level advancement in the initiation of avian migration that is explicable by within-individual changes in post-development adults. This shows that, at decadal scales and for long-lived species, evolutionary or other inter-generational mechanisms are not necessarily required for population adaptation to phenological changes in annual conditions and resources. However, a lack of earlier arrival at breeding grounds suggests that flexibility at departure can still be disrupted by conditions or control mechanisms that vary across stages of migration 9 , 51 , 55 . Although most studies of phenological change in migratory birds are focused on adaptation to climate effects on the breeding grounds, our results additionally highlight the potential for phenological responses to human-induced changes en route, which may be largely independent of climate effects 60 . It is not clear that the observed degree of phenotypic flexibility, or subsequent evolution of phenological traits 61 , will be sufficient to track current and future environmental change 6 , 62 , 63 . However, it may offer some optimism regarding timely adaptation by migratory and other apparently time-constrained species, provided the critical habitats and resources they require are preserved 56 .
Study site and population
The Manawatu River estuary (40.47°S, 175.22°E; Fig. 1 ) is a small (ca. 1 × 2 km) intertidal mudflat area on the west coast of the North Island, New Zealand. Since 2006, we have captured bar-tailed godwits by cannon-net or mist-net, and marked individuals with a numbered metal band, plus either a unique combination of the white flag and four colorbands, or an engraved white flag with a field-readable three-digit alphabetical code. During 2008–2020, 35% (range: 23–48% per year) of adult (i.e., migratory) individuals were marked in the estuary’s highly site-faithful population of 170–280 godwits.
At capture, godwits were aged (≥/<3 years) by plumage or state of primary feather molt; because godwits ≥3 years cannot be precisely aged, the great majority of birds in our studies are of unknown age. Due to sexual dimorphism (females > males), most (89%) individuals were sexed by bill length (length of exposed culmen: >99 mm = female; <88 mm = male), but intermediate birds (88–99 mm) cannot be sexed by this measure 25 ; however, conspicuous dimorphism in plumage before departure from New Zealand allowed unambiguous sexing of the remaining individuals. In this population, size and migration timing in both sexes vary along a latitudinal cline in the Alaska breeding range (60–71°N; higher latitude = smaller and later migration) 24 , 25 .
Directly observed migratory departures
The local godwit population is highly approachable and can usually be observed entirely from one of several vantage points along the perimeter of the mudflat. Each year, a single observer (occasionally two) monitored the flock daily from ca. 1 March until all migratory individuals had departed (29 Mar–5 Apr). During surveys, the observer watched and listened for migratory behavior, which included distinct vocalizations and low circling flights expressing intent to depart. Flocks typically engaged in conspicuous calling, preening, bathing, and short exploratory flights for 0.5–4 h before actual departure 15 ; during this time, the observer recorded all marked individuals involved, using a 20–60× spotting scope and digital camera with a 400 mm lens. All flying flocks were watched and/or photographed until they resettled or disappeared from sight. After each departure, the observer quickly surveyed the estuary to account for all remaining marked godwits. In addition, we conducted daily high-tide surveys to confirm the size and composition of the remaining flock; the average daily resighting probability of marked godwits was 88% (range 51–100% per individual) before departure.
We recorded departures of 44–81 marked individuals per year (174 total individuals). In 86% of these departures, the bird was directly observed preparing and/or departing with an observed flock, and therefore exact time and flock size were known. The remaining individuals were assigned a departure date based on the last day they were recorded at the estuary. For 10% of cases, this coincided with an observed departing flock of partially or completely unknown individual composition; therefore, we considered a time and flock size to be known. For the remaining 4% of cases, the individual’s disappearance coincided with a decrease in local flock size unexplained by observed departures; we considered these departures unobserved, and calculated flock size based on successive high-tide counts. 98% of observed departures occurred during 13:00–21:00, and unobserved departures likely occurred at night or early morning. By including unobserved departures, annual totals represent a virtually complete accounting of flocks and individuals migrating from the site.
We are confident that observed movements out of the estuary represented a migratory departure from New Zealand. Site fidelity of marked godwits is extremely high for the entire non-breeding season (September–March), and nonmigratory movements in and out of the estuary were rare. Departures were easily distinguished from local movements by both altitude and direction; departing flocks always flew NW/NNW and slowly ascended toward the ocean before disappearing from view. Furthermore, for all departures captured by both direct observation and geolocator tracking ( n = 51; see below), conductivity (wetness) data recorded by geolocators confirmed a ca. 7-day dry period (indicating a non-stop flight to Asia) starting on the same day (0–2.5 h difference) as the observed departure 64 .
All statistics were calculated in the software R 3.5.1 65 . Using departures of all godwits ( n = 128–251 total individuals in 12–17 flocks on 10–14 days per year), we analyzed phenology change across years using a linear model in the R package lme4 v.1.1.21 66 .
Including only individuals observed departing New Zealand in ≥3 years ( n = 124 individuals; 44–74 per year), we distinguished the contributions of within- and between-individual variation using within-subject centering 31 in a linear mixed model in lme4. To explore within-population variation in these effects, we performed the same analysis separately for the two regions in the Alaska breeding range (North, South; Fig. 3 ), including 34 individuals with known breeding locations from geolocator tracking (see below).
To quantify the proportion of individuals (≥3 observations) that showed an advancement in their departure timing, we obtained individual posterior distributions via direct simulations of 1000 values from the joint posterior distribution of ordinary linear model parameters using the function sim from the R package arm v.1.11-2 67 . The 2.5% and 97.5% quantiles of the simulated values across individuals were used to define the range of slopes. The proportion of negative slopes within-individual simulations was used to estimate the percentage of individuals showing advanced departure dates.
We calculated repeatability (intra-class correlation coefficient, r ± SE) 68 of departure date from New Zealand for 124 individuals observed 3–13 years each.
Geolocator tracking and analysis
In 2008–2009 and 2013–2014, a subset of individuals was tracked for the full migration to Alaska using light-level geolocators. The units (1.0–1.5 g) were mounted to a colorband on the tibia; attachment plus all individual markings represented 1–2% of lean body mass. We deployed geolocators in March 2008 ( n = 17 MK14, British Antarctic Survey, UK), October 2008 ( n = 19 MK14), February 2013 ( n = 15 MK4093, Biotrack, UK, and n = 24 Intigeo-C65K, Migrate Technology, UK), and November 2013 ( n = 22 Intigeo-C65K) and recaptured birds during the following non-breeding season(s) to retrieve the units. This resulted in the following number of complete northward tracks (including breeding location; see below): 12 in 2008, 13 in 2009, 13 in 2013, and 12 in 2014; 25 individuals were tracked in 1 year, 11 in 2 years, and 1 in 3 years.
Godwits depart New Zealand near the austral autumnal equinox, when light-level geolocation is least effective for describing movements, particularly for flights in a north-south direction 69 . However, geolocators also recorded conductivity (i.e., contact with salt water); wet-dry transitions can thus identify extended periods of flight between intertidal habitats with high precision 64 . From conductivity data, we derived four parameters for each northward track: dates of departure from New Zealand (NZdep), arrival and departure from the Yellow Sea region of Asia (YSarr, YSdep), and first arrival in Alaska (AKarr). Most godwits spend up to two weeks in coastal SW Alaska before moving to breeding sites 22 , 24 , but some individuals show no discernible shift in light or conductivity data during this period. Therefore, we did not analyze arrival at the ultimate breeding site. We derived a fifth timing parameter, duration of the stopover in the Yellow Sea (YSdur), as the difference (in days) between YSarr and YSdep.
Because the geolocators were leg-mounted, light-level data in Alaska also indicated periods of nest incubation, when the unit was shaded by the sitting bird 24 , 27 . During the breeding season, geolocators registered nights as regular, clearly demarcated periods of darkness 0–4.5 h in length; these did not appear at all if birds were north of 64°N. Days appeared as continuous light, irregularly broken by a brief (<1 h) shading events, most likely corresponding to behaviors such as wading or sitting. Within 6–25 days of apparent arrival on breeding grounds, most godwits (41 of 50 total tracks) displayed a conspicuous pattern of incubation, in which semi-regular shading events of 4–13 h were overlaid on the day/night pattern for periods up to 25 d. Bar-tailed godwits incubate tundra nests bi-parentally, and so both sexes demonstrate this pattern. We considered the first day of this period to be the start of incubation (IncSt), and analyzed this timing parameter along with the five migration parameters (above).
For godwits tracked in 2008–2009 using MK14 geolocators, breeding locations are published previously 24 (Supplementary Fig. 3a ). We used BASTrak 70 software (British Antarctic Survey, UK) to produce twice-daily location estimates, and calculated mean latitude and longitude (plus 95% range of estimates) during stationary periods in the breeding season, after removing light-dark transitions clearly affected by behavioral shading events such as incubation. Based on periods when the bird’s true location was known (at the deployment site in New Zealand, and in some cases additionally in Asia), we used sun angles of 3.0–3.6° below the horizon to represent twilight for calibration of light-level data for location estimates. For three individuals that traveled north of the Arctic Circle, narrow-range light sensitivity of MK14 geolocators precluded location estimation in 24-h daylight; however, longitude estimates immediately before and after the breeding season were east of 160°W, strongly indicating breeding destinations on the central North Slope of Alaska. Therefore, we assumed a breeding latitude of 70.2°N (the mid-point of the known breeding range in this region; see Supplementary Fig. 3a ) for these individuals. For individuals tracked in multiple years ( n = 6), the location derived from the year with the best available data (i.e., longer uninterrupted stationary breeding period) is shown in Supplementary Fig. 3a .
For godwits tracked in 2013–2014 using Intigeo-C65K geolocators, which recorded full-range light levels, we estimated breeding locations using the R package PolarGeolocation v.0.1.0 71 . To calibrate the maximum light curve and the error distribution of light recordings from the maximum light curve, we used the longest possible period for each bird, i.e., all days when the bird was at the Manawatu River estuary (range 38–161 days; mean = 127 days). Next, we selected a time period during the breeding season that included a clear pattern of breeding (incubation inferred from shading patterns); although including incubation increases uncertainty in the breeding-site estimate, we chose such periods to ensure that we did not include periods spent away from the breeding site in the location estimation. The duration of the period used to estimate breeding locations was 12–48 days (mean = 28 days). PolarGeolocation estimates a gridded likelihood surface from which we can derive confidence levels of our best location estimate; these confidence intervals depend on the extent of the grid. To make the confidence estimates comparable across individuals, we ran a first estimate using a grid with 50 km resolution and a radius of 1500 km around the approximate center of the breeding range (162°W, 62°N). Next, we re-centered the mask around the best estimate and re-ran the simulation using the same radius of 1500 km, and the same likelihood contour level of 0.05 to describe the confidence intervals for all birds (Supplementary Fig. 3b ). For individuals tracked in multiple years ( n = 4), the location derived from the first year of tracking is shown in Supplementary Fig. 3b .
To estimate breeding sites from four Biotrack MK4093 geolocators in 2013, we performed the simple threshold approach. First, we defined sunrise and sunset times using a light intensity threshold of 2 arbitrary units, and used the periods the individual bird was at the deployment site in New Zealand for calibration and calculation of a reference zenith angle 69 that was then used to estimate locations via the thresholdPath function in the R Package SGAT v.0.1.3 72 . All loggers showed dark periods during the breeding season (i.e., remained south of the Arctic Circle), allowing estimation of locations throughout the year. We extracted the locations for the period including incubation and calculated the median location and the 95% credibility interval.
Bar-tailed godwits breeding in northern and southern Alaska differ in size and migration timing 24 , 25 , and potentially experience very different temporal trends in conditions during the breeding season. Therefore, tracked individuals were grouped into two regional groups, comprising those breeding >64°N (North) or <64°N (South). Individuals tracked in multiple years and/or with different geolocator types allow assessment of repeatability of breeding location estimates; however, we note that the uncertainty measures we show in Supplementary Fig. 3 for the three estimation methods are not strictly comparable. For six individuals with breeding locations derived in both 2008 and 2009 from MK14 geolocators, location estimates differed by 24–124 km, respectively. For four individuals tracked with Intigeo units in both 2013 and 2014, location estimates differed by 50–190 km, respectively. For one individual tracked with both MK14 and Intigeo loggers, the best estimates for 2008 and 2014 differed by approximately 78 km. These differences fall within the expected error of geolocation, and in no case affected an individual’s assignment to the northern or southern groups. The final set of tracked godwits with known breeding latitude included 36 individuals (North = 16, South = 20; Fig. 2a and Supplementary Fig. 3 ).
To describe the rate of change across 2008–2014 in six migration and breeding phenology parameters (NZdep, YSarr, YSdep, AKarr, IncSt, and YSdur; see above), we used linear mixed models including individual as a random effect and breeding region (North or South) as a fixed factor (Fig. 4 , Supplementary Table 3 ). To account for unequal samples of northern and southern breeders across years, we initially included the interaction of year*breeding region in the models; this interaction factor was non-significant for all phenology parameters ( p = 0.08–0.85), and so we excluded it from the final models.
Environmental data and analysis
In Arctic-breeding shorebirds, the timing of breeding closely follows the retreat of snow-cover from tundra nest sites in the spring 73 . We compared two indices of breeding phenology (timing of snowmelt and spring green-up), summarized separately for the two regions (North, South) of the Alaska breeding range of bar-tailed godwits. We did this for two temporal periods: one encompassing the entire study period (2008–2020), and a shorter-term related directly to the period in which we tracked individuals with geolocators (2008–2014).
Remotely sensed IMS Daily Northern Hemisphere Snow and Ice Analysis data for the period 2008–2020 on a scale of 4 × 4 km were downloaded 74 . Next, grid cells falling in the subspecies’ breeding range were extracted. Then, data from cells that did not include measures of sea ice were modeled using a maximum likelihood fit ( mle2 function from R package bbmle v.1.0.23.1) of the asymmetric Gaussian model function 75 with a binomial error distribution. The model fit is a compromise between the first snow-free day and the day when the pixel remained snow-free in late spring and summer (for more details and comparison between different snow-melt indices see Supplementary Methods ). Using these year-specific fits, the date of snowmelt was then determined as the date on which the fitted curve predicted 1/3 of the cell area to be snow-free.
Normalized Difference Vegetation Index (NDVI)
From the NOAA STAR Vegetation Health Product data set, weekly noise-removed NDVI data on a scale of 4 × 4 km grid cells were downloaded for the period 2008–2020 76 . Next, cells located in the subspecies’ breeding range were selected for further analysis. To identify the annual day when the vegetation starts to grow (green-up day), a penalized cubic smoothing spline was fitted to the yearly NDVI values, allowing daily interpolation and the calculation of when the curve exceeds 15% of the greatest amplitude during spring 77 . To ensure a robust fit of the smoothing spline and to remove potential artifacts of single pixels, the NDVI values of the surrounding pixels within a radius of 15 km were taken into account. However, the distance to the focal cell was used to weigh the importance of the model fit (with a Gaussian decline over distance with a standard deviation of 4 km). Because the presence of snow and ice can significantly affect NDVI values, the model was used to interpolate daily NDVI values only for days that were snow-free in at least one of the pixels within the 15 km radius. Next, the day of the maximum NDVI was established, and the day when the increasing NDVI curve (start to maximum) exceeded 15% of the amplitude was extracted as the NDVI start date. More detailed information and a comparison between the estimated NDVI start date and the higher spatial resolution MODIS derived green-up day (also based on a 15% threshold) are in Supplementary Methods .
Snowmelt and NDVI start were highly correlated across the breeding range ( t 160221 = 664.95, p < 0.001, R 2 = 0.856) as well as in the northern ( t 120852 = 494.19, p < 0.001, R 2 = 0.817) and southern ( t 39367 = 285.65, p < 0.001, R 2 = 0.821) regions separately.
Ethics statement
Fieldwork was conducted with Massey University Animal Ethics Committee approval (#07/163, 12/90, and 16/117) and appropriate New Zealand Department of Conservation permits (Banding permit 2007/39, 35503-FAU, 38111-FAU).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The behavioral data generated in this study are available on Zenodo ( https://doi.org/10.5281/zenodo.5016733 ). The environmental data used in this study are available from NSIDC ( https://doi.org/10.7265/N52R3PMC ) and NOAA ( ftp://ftp.star.nesdis.noaa.gov/pub/corp/scsb/wguo/ ).
Code availability
The code for environmental analyses performed in this study is available on Zenodo ( https://doi.org/10.5281/zenodo.5025715 ).
Parmesan, C. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 37 , 637–669 (2006).
Article Google Scholar
Thackeray, S. J. et al. Phenological sensitivity to climate across taxa and trophic levels. Nature 535 , 241–245 (2016).
Article ADS CAS PubMed Google Scholar
Gordo, O. Why are bird migration dates shifting? A review of weather and climate effects on avian migratory phenology. Clim. Res. 35 , 37–58 (2007).
Charmantier, A. et al. Adaptive phenotypic plasticity in response to climate change in a wild bird population. Science 320 , 800–803 (2008).
Carey, C. The impacts of climate change on the annual cycles of birds. Philos. Trans. R. Soc. B Biol. Sci. 364 , 3321–3330 (2009).
Radchuk, V. et al. Adaptive responses of animals to climate change are most likely insufficient. Nat. Commun. 10 , 1–14 (2019).
Article CAS Google Scholar
Both, C. et al. Avian population consequences of climate change are most severe for long-distance migrants in seasonal habitats. Proc. R. Soc. B Biol. Sci. 277 , 1259–1266 (2010).
Saino, N. et al. Climate warming, ecological mismatch at arrival and population decline in migratory birds. Proc. R. Soc. B Biol. Sci. 278 , 835–842 (2011).
Ahola, M. et al. Variation in climate warming along the migration route uncouples arrival and breeding dates. Glob. Chang. Biol. 10 , 1610–1617 (2004).
Article ADS Google Scholar
Mantyka-Pringle, C. S., Martin, T. G. & Rhodes, J. R. Interactions between climate and habitat loss effects on biodiversity: a systematic review and meta-analysis. Glob. Chang. Biol. 18 , 1239–1252 (2012).
Knudsen, E. et al. Challenging claims in the study of migratory birds and climate change. Biol. Rev. 86 , 928–946 (2011).
Article PubMed Google Scholar
Charmantier, A. & Gienapp, P. Climate change and timing of avian breeding and migration: evolutionary versus plastic changes. Evol. Appl. 7 , 15–28 (2014).
Gienapp, P., Leimu, R. & Merilä, J. Responses to climate change in avian migration time–microevolution versus phenotypic plasticity. Clim. Res. 35 , 25–35 (2007).
Both, C., Bijlsma, R. G. & Ouwehand, J. Repeatability in spring arrival dates in pied flycatchers varies among years and sexes. Ardea 104 , 3–21 (2016).
Conklin, J. R. & Battley, P. F. Impacts of wind on individual migration schedules of New Zealand bar-tailed godwits. Behav. Ecol. 22 , 854–861 (2011).
Studds, C. E. & Marra, P. P. Rainfall-induced changes in food availability modify the spring departure programme of a migratory bird. Proc. R. Soc. B Biol. Sci. 278 , 3437–3443 (2011).
Cooper, N. W., Sherry, T. W., Marra, P. P. & Inouye, B. D. Experimental reduction of winter food decreases body condition and delays migration in a long-distance migratory bird. Ecology 96 , 1933–1942 (2015).
Fraser, K. C., Shave, A., de Greef, E., Siegrist, J. & Garroway, C. J. Individual variability in migration timing can explain long-term, population-level advances in a songbird. Front. Ecol. Evol. 7 , 324 (2019).
Sergio, F. et al. Individual improvements and selective mortality shape lifelong migratory performance. Nature 515 , 410–413 (2014).
Mueller, T., O’Hara, R. B., Converse, S. J., Urbanek, R. P. & Fagan, W. F. Social learning of migratory performance. Science 341 , 999–1003 (2013).
Gill, J. A. et al. Why is timing of bird migration advancing when individuals are not? Proc. R. Soc. B Biol. Sci. 281 , 20132161 (2014).
Battley, P. F. et al. Contrasting extreme long-distance migration patterns in bar-tailed godwits Limosa lapponica . J. Avian Biol. 43 , 21–32 (2012).
Conklin, J. R., Senner, N. R., Battley, P. F. & Piersma, T. Extreme migration and the individual quality spectrum. J. Avian Biol. 48 , 19–36 (2017).
Conklin, J. R., Battley, P. F., Potter, M. A. & Fox, J. W. Breeding latitude drives individual schedules in a trans-hemispheric migrant bird. Nat. Commun. 1 , 67 (2010).
Article ADS PubMed CAS Google Scholar
Conklin, J. R., Battley, P. F., Potter, M. A. & Ruthrauff, D. R. Geographic variation in morphology of Alaska-breeding bar-tailed godwits ( Limosa lapponica ) is not maintained on their nonbreeding grounds in New Zealand. Auk 128 , 363–373 (2011).
Battley, P. F. Consistent annual schedules in a migratory shorebird. Biol. Lett. 2 , 517–520 (2006).
Article PubMed PubMed Central Google Scholar
Conklin, J. R., Battley, P. F. & Potter, M. A. Absolute consistency: Individual versus population variation in annual-cycle schedules of a long-distance migrant bird. PLoS ONE 8 , e54535 (2013).
Article ADS CAS PubMed PubMed Central Google Scholar
Van Buskirk, J., Mulvihill, R. S. & Leberman, R. C. Phenotypic plasticity alone cannot explain climate-induced change in avian migration timing. Ecol. Evol. 2 , 2430–2437 (2012).
Gill, J. A., Alves, J. A. & Gunnarsson, T. G. Mechanisms driving phenological and range change in migratory species. Philos. Trans. R. Soc. B Biol. Sci. 374 , 20180047 (2019).
Piersma, T. & Drent, J. Phenotypic flexibility and the evolution of organismal design. Trends Ecol. Evol. 18 , 228–233 (2003).
van de Pol, M. & Wright, J. A simple method for distinguishing within- versus between-subject effects using mixed models. Anim. Behav. 77 , 753–758 (2009).
Gwinner, E. Circadian and circannual programmes in avian migration. J. Exp. Biol. 199 , 39–48 (1996).
Article CAS PubMed Google Scholar
Schmaljohann, H. & Both, C. The limits of modifying migration speed to adjust to climate change. Nat. Clim. Chang. 7 , 573–576 (2017).
Jonzén, N. et al. Rapid advance of spring arrival dates in long-distance migratory birds. Science 312 , 1959–1961 (2006).
Rubolini, D., Møller, A., Rainio, K. & Lehikoinen, E. Intraspecific consistency and geographic variability in temporal trends of spring migration phenology among European bird species. Clim. Res. 35 , 135–146 (2007).
Mills, A. M. Changes in the timing of spring and autumn migration in North American migrant passerines during a period of global warming. Ibis 147 , 259–269 (2005).
Conklin, J. R. et al. Declining adult survival of New Zealand Bar-tailed Godwits during 2005–2012 despite apparent population stability. Emu 116 , 147–157 (2016).
DeWitt, T. J., Sih, A. & Wilson, D. S. Cost and limits of phenotypic plasticity. Trends Ecol. Evol. 13 , 77–81 (1998).
Murray, N. J., Clemens, R. S., Phinn, S. R., Possingham, H. P. & Fuller, R. A. Tracking the rapid loss of tidal wetlands in the Yellow Sea. Front. Ecol. Environ. 12 , 267–272 (2014).
Studds, C. E. et al. Rapid population decline in migratory shorebirds relying on Yellow Sea tidal mudflats as stopover sites. Nat. Commun. 8 , 14895 (2017).
Piersma, T. et al. Simultaneous declines in summer survival of three shorebird species signals a flyway at risk. J. Appl. Ecol. 53 , 479–490 (2016).
Zhang, S.-D. et al. Persistent use of a shorebird staging site in the Yellow Sea despite severe declines in food resources implies a lack of alternatives. Bird. Conserv. Int. 28 , 534–548 (2018).
Weber, T. P., Ens, B. J. & Houston, A. I. Optimal avian migration: a dynamic model of fuel stores and site use. Evol. Ecol. 12 , 377–401 (1998).
Newton, I. Can conditions experienced during migration limit the population levels of birds? J. Ornithol. 147 , 146–166 (2006).
Senner, N. R., Conklin, J. R. & Piersma, T. An ontogenetic perspective on individual differences. Proc. R. Soc. B Biol. Sci. 282 , 20151050 (2015).
Conklin, J. R. & Battley, P. F. Carry-over effects and compensation: late arrival on non-breeding grounds affects wing moult but not plumage or schedules of departing bar-tailed godwits Limosa lapponica baueri . J. Avian Biol. 43 , 252–263 (2012).
Teitelbaum, C. S. et al. Experience drives innovation of new migration patterns of whooping cranes in response to global change. Nat. Commun. 7 , 12793 (2016).
Kendal, R. L., Coolen, I., van Bergen, Y. & Laland, K. N. Trade-offs in the adaptive use of social and asocial learning. Adv. Study Behav. 35 , 333–379 (2005).
Winkler, D. W. et al. Cues, strategies, and outcomes: How migrating vertebrates track environmental change. Mov. Ecol. 2 , 10 (2014).
Schmaljohann, H., Lisovski, S. & Bairlein, F. Flexible reaction norms to environmental variables along the migration route and the significance of stopover duration for total speed of migration in a songbird migrant. Front. Zool. 14 , 1–16 (2017).
Wingfield, J. C. Flexibility in annual cycles of birds: implications for endocrine control mechanisms. J. Ornithol. 146 , 291–304 (2005).
Karagicheva, J. et al. Seasonal time keeping in a long-distance migrating shorebird. J. Biol. Rhythms 31 , 509–521 (2016).
Farmer, A. H. & Wiens, J. A. Models and reality: time-energy trade-offs in Pectoral Sandpiper ( Calidris melanotos ) migration. Ecology 80 , 2566–2580 (1999).
Google Scholar
Battley, P. F. et al. Do body condition and plumage during fuelling predict northwards departure dates of Great Knots Calidris tenuirostris from north-west Australia? Ibis 146 , 46–60 (2004).
Both, C. Flexibility of timing of avian migration to climate change masked by environmental constraints en route. Curr. Biol. 20 , 243–248 (2010).
Rakhimberdiev, E. et al. Fuelling conditions at staging sites can mitigate Arctic warming effects in a migratory bird. Nat. Commun. 9 , 4263 (2018).
Article ADS PubMed PubMed Central CAS Google Scholar
Coppack, T. & Pulido, F. Photoperiodic response and the adaptability of avian life cycles to environmental change. Adv. Ecol. Res. 35 , 131–150 (2004).
Åkesson, S. & Helm, B. Endogenous programs and flexibility in bird migration. Front. Ecol. Evol. 8 , 1–20 (2020).
Battley, P. F. et al. Interacting roles of breeding geography and early-life settlement in godwit migration timing. Front. Ecol. Evol. 8 , 1–14 (2020).
Merilä, J. & Hendry, A. P. Climate change, adaptation, and phenotypic plasticity: the problem and the evidence. Evol. Appl. 7 , 1–14 (2014).
Badyaev, A. V. Stress-induced variation in evolution: from behavioural plasticity to genetic assimilation. Proc. R. Soc. B Biol. Sci. 272 , 877–886 (2005).
Vedder, O., Bouwhuis, S. & Sheldon, B. C. Quantitative assessment of the importance of phenotypic plasticity in adaptation to climate change in wild bird populations. PLoS Biol. 11 , e1001605 (2013).
Article CAS PubMed PubMed Central Google Scholar
Phillimore, A. B., Leech, D. I., Pearce-Higgins, J. W. & Hadfield, J. D. Passerines may be sufficiently plastic to track temperature-mediated shifts in optimum lay date. Glob. Chang. Biol. 22 , 3259–3272 (2016).
Article ADS PubMed Google Scholar
Battley, P. F. & Conklin, J. R. Geolocator wetness data accurately detect periods of migratory flight in two species of shorebird. Wader Study 124 , 112–119 (2017).
R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2018).
Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67 , 1–48 (2015).
Gelman, A. & Hill, J. Data Analysis Using Regression and Hierarchical/Multilevel Models (Cambridge, 2007).
Lessells, C. M. & Boag, P. T. Unrepeatable repeatabilities: a common mistake. Auk 104 , 116–121 (2007).
Lisovski, S. et al. Geolocation by light: accuracy and precision affected by environmental factors. Methods Ecol. Evol. 3 , 603–612 (2012).
Fox, J. Geolocator Manual, Version 8 (British Antarctic Survey, 2010).
Lisovski, S. Light-level geolocation in polar regions with 24-hour daylight. Wader Study 125 , 129–134 (2018).
Wotherspoon, S. J., Sumner, M. D. & Lisovski, S. Package SGAT: Solar/Satellite Geolocation for Animal Tracking . GitHub Repository. http://github.com/swotherspoon/ (2013).
Smith, P. A., Gilchrist, H. G., Forbes, M. R., Martin, J.-L. & Allard, K. Inter-annual variation in the breeding chronology of arctic shorebirds: effects of weather, snow melt and predators. J. Avian Biol. 41 , 292–304 (2010).
NSIDC. IMS Daily Northern Hemisphere Snow and Ice Analysis at 1 km, 4 km, and 24 km Resolutions, Version 1 . https://doi.org/10.7265/N52R3PMC (2019).
Jönsson, P. & Eklundh, L. Seasonality extraction by function fitting to time-series of satellite sensor data. IEEE Trans. Geosci. Remote Sens. 40 , 1824–1832 (2002).
NOAA. NOAA Center for Satellite Applications and Research . ftp://ftp.star.nesdis.noaa.gov/pub/corp/scsb/wguo/ (2018).
Bolton, D. K. et al. Continental-scale land surface phenology from harmonized Landsat 8 and Sentinel-2 imagery. Remote Sens. Environ. 240 , 111685 (2020).
Download references
Acknowledgements
We thank colleagues for helpful discussions and comments on previous drafts: Christiaan Both, Bart Kempenaers, Jelle Loonstra, Theunis Piersma, Eldar Rakhimberdiev, Joost Tinbergen, Mo Verhoeven, and especially Yvonne Verkuil. We thank David Melville, Adrian Riegen, Rob Schuckard, and many volunteers for trapping and banding assistance, and Iris Bontekoe for fieldwork in 2018. The collection of long-term departure data was supported by Chris & Neville Hopkins, David & Lucile Packard Foundation, Dobberke Foundation for Comparative Psychology, Manawatu Estuary Trust, Marsden Fund (Royal Society of New Zealand), Massey University Doctoral Scholarship, New Zealand Department of Conservation, Ornithological Society of New Zealand, Pacific Shorebird Migration Project, Pūkorokoro Miranda Naturalist’s Trust, and Royal Netherlands Academy of Arts & Sciences (KNAW). We acknowledge support by the Open Access Publication Funds of Alfred-Wegener-Institut Helmholtz-Zentrum für Polar- und Meeresforschung and of Massey University.
Author information
Authors and affiliations.
Conservation Ecology Group, Groningen Institute for Evolutionary Life Sciences (GELIFES), University of Groningen, Groningen, The Netherlands
Jesse R. Conklin
Alfred Wegener Institute, Helmholtz Centre for Polar and Marine Research, Telegrafenberg, Potsdam, Germany
Simeon Lisovski
Wildlife and Ecology Group, School of Agriculture and Environment, Massey University, Palmerston North, New Zealand
Phil F. Battley
You can also search for this author in PubMed Google Scholar
Contributions
J.R.C. and P.F.B. conceived the study. J.R.C. collected and analyzed the observational data. J.R.C. and P.F.B. deployed and retrieved geolocators. All authors analyzed geolocator data. S.L. analyzed the environmental data. J.R.C. wrote the manuscript with contributions from S.L. and P.F.B. All authors reviewed and edited the manuscript.

Corresponding authors
Correspondence to Jesse R. Conklin , Simeon Lisovski or Phil F. Battley .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Peer review information Nature Communications thanks Richard Fuller, Aldina Franco, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, peer review file, reporting summary, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Conklin, J.R., Lisovski, S. & Battley, P.F. Advancement in long-distance bird migration through individual plasticity in departure. Nat Commun 12 , 4780 (2021). https://doi.org/10.1038/s41467-021-25022-7
Download citation
Received : 23 December 2020
Accepted : 08 July 2021
Published : 06 August 2021
DOI : https://doi.org/10.1038/s41467-021-25022-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Impacts on avian migratory patterns due to climate change and hormonal disruption: a review.
- Sachin Kumar
- Tejdeep Kaur Kler
- Tanvi Sahni
Mitigation and Adaptation Strategies for Global Change (2024)
Improving Primary Healthcare Education through lessons from a flock of birds
- Ioanna Tsiligianni
- Antonios Christodoulakis
- Siân Williams
npj Primary Care Respiratory Medicine (2023)
A partial migrant relies upon a range-wide cue set but uses population-specific weighting for migratory timing
- Paweł Brzęk
- Jesko Partecke
Movement Ecology (2021)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
Mapping Global Diversity Patterns for Migratory Birds
* E-mail: [email protected]
Affiliations Department of Zoology, University of Cambridge, Cambridge, United Kingdom, Centre d’Ecologie Fonctionnelle et Evolutive, CNRS-CEFE UMR5175, Montpellier, France
Affiliation Department of Zoology, University of Cambridge, Cambridge, United Kingdom
Affiliation BirdLife International, Wellbrook Court, Cambridge, United Kingdom
Affiliation Centre d’Ecologie Fonctionnelle et Evolutive, CNRS-CEFE UMR5175, Montpellier, France
- Marius Somveille,
- Andrea Manica,
- Stuart H. M. Butchart,
- Ana S. L. Rodrigues

- Published: August 7, 2013
- https://doi.org/10.1371/journal.pone.0070907
- Reader Comments
Nearly one in five bird species has separate breeding and overwintering distributions, and the regular migrations of these species cause a substantial seasonal redistribution of avian diversity across the world. However, despite its ecological importance, bird migration has been largely ignored in studies of global avian biodiversity, with few studies having addressed it from a macroecological perspective. Here, we analyse a dataset on the global distribution of the world’s birds in order to examine global spatial patterns in the diversity of migratory species, including: the seasonal variation in overall species diversity due to migration; the contribution of migratory birds to local bird diversity; and the distribution of narrow-range and threatened migratory birds. Our analyses reveal a striking asymmetry between the Northern and Southern hemispheres, evident in all of the patterns investigated. The highest migratory bird diversity was found in the Northern Hemisphere, with high inter-continental turnover in species composition between breeding and non-breeding seasons, and extensive regions (at high latitudes) where migratory birds constitute the majority of the local avifauna. Threatened migratory birds are concentrated mainly in Central and Southern Asia, whereas narrow-range migratory species are mainly found in Central America, the Himalayas and Patagonia. Overall, global patterns in the diversity of migratory birds indicate that bird migration is mainly a Northern Hemisphere phenomenon. The asymmetry between the Northern and Southern hemispheres could not have easily been predicted from the combined results of regional scale studies, highlighting the importance of a global perspective.
Citation: Somveille M, Manica A, Butchart SHM, Rodrigues ASL (2013) Mapping Global Diversity Patterns for Migratory Birds. PLoS ONE 8(8): e70907. https://doi.org/10.1371/journal.pone.0070907
Editor: Tapio Mappes, University of Jyväskylä, Finland
Received: March 1, 2013; Accepted: June 24, 2013; Published: August 7, 2013
Copyright: © 2013 Somveille et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Funding: The authors have no support or funding to report.
Competing interests: The authors have declared that no competing interests exist.
Introduction
Bird migration is a phenomenon that has long fascinated scientists and other observers. An estimated 1,855 bird species (19% of extant species) are migratory, making regular cyclical movements beyond their breeding distribution, with predictable timing and destinations [1] . Much attention has been devoted to bird migration, as exemplified by the nearly 2800 references cited in a recent book on the subject [2] , and the 4539 articles in the Web of Science under the topic “bird migration”. This extensive literature has concentrated on aspects such as the behavioural adaptations of migration (e.g. [3] ), the evolution of migration (e.g. [4] ) and the conservation status of migratory species (e.g. [1] ). However, very few studies have investigated bird migration using a macroecological approach.
Macroecology, the study of broad-scale spatial patterns in biodiversity [5] , has developed considerably in recent years, and as one of the better-studied taxonomic groups, birds have had a key role in this development. From continental to global scales, bird data have been used to investigate, for example: drivers of species richness patterns [6] , [7] ; the global distributions of range sizes [8] and body sizes [9] , the spatial turnover in species [10] , and species extinction risk [11] ; and the congruence between richness and endemism [12] , [13] . However, despite this extensive body of work, most macroecological studies considered bird species only in their breeding distributions. Very few have addressed one of the most striking features of avian biogeography: the fact that, for a substantial proportion of the species, distributions vary seasonally, and accordingly so do macroecological patterns. The few previous studies that have analysed bird migration from a macroecological perspective (see Appendix S1 for a review), focus mainly on Europe and North America, and often analyse just a subset of the local bird community (e.g. only breeding species; Table S1 in Appendix S1 ). None are global in scale, and only two span the equator. Nonetheless, these studies reveal some macroecological patterns, in particular an increase in the absolute numbers and in the proportion of migratory species with latitude and increasing climatic seasonality (e.g. [14] – [19] ).
We investigated whether these regional patterns can generalise to the global scale by mapping global patterns in the diversity of migratory birds. We used a newly released dataset of digital distribution maps for the world’s birds [20] , in which breeding and non-breeding ranges are mapped separately. We mapped global spatial patterns in the seasonal variation in species richness due to migration, the diversity of migratory species, the contribution of migratory birds to local bird diversity, the distribution of narrow-range migratory birds, and of threatened migratory birds. The last two have never (to our knowledge) been investigated, and the first three had only been previously assessed through taxonomically and/or regionally restricted studies. Information on the global distribution of these features of avian communities could shed light on how avian communities are structured, and in particular how they adjust to seasonal environments in high latitudes, with many species vacating during the winter and visiting to exploit the food supply during the summer.
Materials and Methods
Spatial data.
Data on the distribution of bird species were derived from BirdLife International and NatureServe (2011) [20] , a global dataset compiled as part of the International Union for Nature Conservation (IUCN) Red List assessments for birds [21] . This dataset comprised Geographic Information System (GIS) shapefiles of the distributions of all 9783 extant bird species whose distributions are known. These distribution polygons represent moderately coarse generalizations of species’ distributions derived from the locations of known records, with interpolation by experts (see [22] for further details). Polygons were coded according to species’ presence (1– extant; 2– probably extant; 3– possibly extant; 4– possibly extinct; 5– extinct), origin (1– native; 2– reintroduced; 3– introduced; 4– vagrant; 5– origin uncertain) and seasonality (1– resident; 2– breeding season; 3– non-breeding season; 4– passage; 5– seasonal occurrence uncertain). In this analysis, only polygons coded as presence 1 or 2, origin 1 or 2, and seasonality 1, 2 or 3 were included.
For the purpose of the present study, migratory species were defined as those mapped with at least one polygon coded as breeding or non-breeding (seasonality 2 or 3; some such species also had polygons coded as seasonality 1 for resident populations). Hence, this analysis focuses on species whose annual movements result in predictable, large-scale, changes in bird diversity. It does not cover other forms of migration, such as partial migration (in which only a proportion of a population migrates while the rest remain as residents; [1] , [23] , fine-scale altitudinal migration (not captured in the maps analysed), differential migration (where migrant individuals comprise just one age-class or sex; [24] , and nomadic or irruptive species (in which the movements are not predictable seasonally or geographically; [1] , [2] . This study concentrated on geographical patterns over land and therefore marine species were excluded from the analysis (but the terrestrial part of coastal species’ distributions was included).
BirdLife International had also coded, in a separate database, the migratory status of each species. As a cross-validation analysis, we checked whether species classified in this database as “full migrants” (those for which a substantial proportion of the global or a regional population makes regular cyclical movements beyond the breeding distribution, with predictable timing and destinations) matched those that we coded as migratory based on the distribution maps. We resolved any discrepancies (either by improving the maps, or by amending the classification in the database). Our analyses were based on the corrected map dataset (which was incorporated into BirdLife International and NatureServe, 2012; available upon request from the website in reference [25] ).
To investigate the temporal variation between seasons in species distribution, while being globally consistent, we focussed on occupancy in two months, January and July, approximating to the middle of the summer season in the Southern and Northern Hemispheres, respectively. For each species, we assigned occupancy during the two focal months to each component of the distribution ( Figure 1 ). For this purpose, we considered all species whose entire distribution falls within latitudes superior to 30° N as breeding in July and non-breeding in January, and vice-versa for species at latitudes superior to 30° S. For the remaining species, we obtained information on their breeding season from the literature (in particular from the Handbook of the Birds of the World collection; [26] ). The few species for which this classification was not applicable were treated for analytical purposes as if they were resident (52 species, mainly tropical). This includes species for which the description of migratory behaviour in the literature was too vague, too conflicting or based on too few observations, as well as species that follow a more complex migration pattern (i.e. partial migrants, differential migrants, nomadic and irruptive species). The non-permanent distribution of each migratory species was defined as the area where the species only occurs seasonally ( Figure 1E ).
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
This figure is illustrating: (A) the complete distribution (in brown); (B) subdivision into breeding (red), resident (green) and non-breeding (blue) components; (C) July distribution (including breeding and resident components); (D) January distribution (including non-breeding and resident components); (E) non-permanent distribution (breeding and non-breeding components). Photo by JJ Harrison/Wikimedia Commons.
https://doi.org/10.1371/journal.pone.0070907.g001
Coastline boundaries were obtained from VMAP, a vector-based collection of GIS data collated by the United States National Geospatial-Intelligence Agency ( https://www1.nga.mil/ ). Antarctica was excluded because no land species occur in this part of the world. The spatial units employed in these analyses were equal-area, equal-shape hexagons, obtained from a geodesic discrete global grid system, defined on an icosahedron and projected to the sphere using the inverse Icosahedral Snyder Equal Area (ISEA) Projection [27] . Hexagons with an area of ∼23,322 km 2 (corresponding to an ISEA Aperture 3, resolution 7 hexagon grid), were used in all analyses, for a total of 7604 land hexagons.
We produced eight maps to analyse global diversity patterns in birds:
- A) Total species richness: mapped as the number of all bird species (migratory and non-migratory) whose distributions intersect with each hexagon. This map does not correspond to the real species richness at any particular time, because migratory species are double-counted in their breeding and non-breeding distributions, but represents the variation in the total species pool across regions.
- B) Species richness in January: mapped as the number of bird species (migratory and non-migratory) whose January distributions intersect with each hexagon.
- C) Species richness in July: mapped as the number of bird species (migratory and non-migratory) whose July distributions intersect with each hexagon.
- D) Difference in species richness: mapped as the number of bird species in July (map B) minus the number of bird species in January (map C) in each hexagon. This map represents the change in species richness between the two opposite seasons due to migration.
- E) Total number of non-permanent species (referred hereinafter, for simplicity, as the total number of migratory species): mapped as the number of migratory species whose non-permanent distributions (see Figure 1E ) intersect with each hexagon. This represents the spatial variation in the number of species that are only present in each site during part of the year.
- F) Fraction of non-permanent species (referred hereinafter as the fraction of migratory species): mapped as the number of migratory species whose non-permanent distributions overlap each hexagon (map E) divided by the total number of species (map A). This represents the fraction of the total species pool that is only present during part of the year.
- G) Richness in threatened migratory species: mapped as the number of migratory bird species that are threatened (that is, classified as Vulnerable, Endangered or Critically Endangered according to the IUCN Red List; [21] ), per hexagon. As in Map A, species are counted in both their breeding and non-breeding distributions.
- H) Richness in narrow-range migratory species. Narrow-range migratory species were defined as those in the lower quartile of migratory species in terms of range size (e.g., [28] , and were identified separately for each season (July and January). Separate maps of richness in narrow-range migratory species were then obtained for January and for July, and combined into a final map representing the maximum richness in narrow-range species across seasons.
The global distribution of bird species richness (including both migratory and non-migratory species), is dominated by a concentration of species richness in the tropical regions, especially the tropical Andes in South America, the Eastern Arc mountains of East Africa, and the Himalayan slopes in Asia ( Figure 2A ). The variation in richness between January ( Figure 2B ) and July ( Figure 2C ) is most prominent in the Northern Hemisphere (North America and Eurasia), where species richness is substantially higher in July, but overall this variation is obscured by the magnitude of the latitudinal differences. The seasonal differences become much clearer when mapped directly ( Figure 3A ). The northern part of the Northern Hemisphere is richer in bird species in July than in January, but in the southern part of the Northern Hemisphere, the reverse is true. A transition zone where the difference in species richness between seasons is small or null (mapped as a pale line in Figure 3A ) is found around latitudes 30° to 40° N, crossing North America, the Mediterranean region, Central Asia, the Himalayas and Southern China. In contrast, there is no corresponding transition zone in the Southern Hemisphere. Instead, south of the Northern Hemisphere transition zone, richness is nearly always higher in January, with large seasonal differences in diversity in Central America, South Asia and South-east Asia compared with much smaller seasonal differences in the Sahara, the Arabian Peninsula, South America and Oceania.
https://doi.org/10.1371/journal.pone.0070907.g002
(A) Difference in local species richness between July and January, with positive values (in red) indicating areas that are richer in July, and negative values (in blue) indicating areas that are richer in January; (B) richness in migratory species (i.e., non permanent-species, that are only present seasonally in each area); and (C) the proportion of migratory (non-permanent) species.
https://doi.org/10.1371/journal.pone.0070907.g003
Richness in migratory species is considerably higher in the Northern Hemisphere ( Figure 3B ), with consistently high numbers outside arid regions (Sahara, Arabian Peninsula, Tibetan Plateau) and at high latitudes (Greenland, Northern Russia, Northern Canada). In contrast, richness in migratory species is generally low throughout the Southern Hemisphere. The proportion of migratory species shows a clear latitudinal gradient, increasing towards higher latitudes ( Figure 3C ). There is, however, a strong asymmetry between the two hemispheres, with higher values in the Northern Hemisphere.
The strong asymmetry between hemispheres is most striking when values in the difference in species richness, in the absolute number of migratory species, and in the proportion of migratory species are plotted against latitude ( Figure 4 ). These results are consistent across major migratory flyways (see Appendix S2 ). The difference in local species richness between January and July is rather uniform (slightly negative) at southern latitudes, whereas at northern latitudes it switches between strongly negative values (<100 species, around latitude 20°N) and strongly positive values (>150 species, around 55°N), with a transition zone around 35°N ( Figure 4A ). Richness in migratory species is uniformly lower (<100 species) at southern latitudes, whereas in the Northern Hemisphere it reaches up to 200 species between 20 and 55°N, decreasing sharply towards zero at 80°N. Finally, the proportion of migratory species is generally low south of the equator (generally <25%, with a slight increasing trend towards the south), whereas in northern latitudes there is a constant and marked increase from the equator northwards, reaching 100% around 80°N.
(A) Difference in local species richness between July and January; (B) richness in migratory (non-permanent) species; and (C) proportion of migratory (non-permanent) species.
https://doi.org/10.1371/journal.pone.0070907.g004
Among the species we defined as migratory, 132 are globally threatened ( Figure 5A ). They are mostly concentrated along two parallel bands in Asia, a northern one through Kazakhstan, Mongolia and southern Russia, and a southern band extending from Pakistan, along the Himalayan slopes into southern China and southern Japan, with a smaller concentration in Eastern Africa. Overall, 48% of all threatened migratory species are found in Eurasia, 14% in North America, 12% in South America, 17% in Africa, and 20% in Australasia (the total is larger than 100% because of shared species). Three in every four threatened migratory species also have a narrow range during at least part of the year, particularly those in the Americas and in Australia (see Table S2 in File S2 ).
(A) Richness in threatened migratory species; (B) richness in narrow-range migratory species.
https://doi.org/10.1371/journal.pone.0070907.g005
The majority (76%) of species classified as narrow-range migratory species was qualified as such in both seasons. The criteria of “narrow” was relative to all migratory bird species, rather than to all birds; in absolute terms, narrow-range migratory species (maximum range size 3.3 million km 2 in July and 3 million km 2 in January) have on average substantially wider distributions than narrow-range birds (across all birds “restricted-range species” are defined as those having a breeding distribution size less than 50,000 km 2 [29] . Richness in narrow-range migratory species is highest in Central America, the Himalayas, and also in Patagonia, and to a lesser extent in South-East Asia and North-East Asia ( Figure 5B ). In Central America, Patagonia and South-East Asia, these species are found mainly in January, while in the Himalayas and North-East Asia they occur mainly in July (see Figure S3 in File S1 ).
The results obtained in this study should be interpreted within the context of the limitations in the available data. Species were mapped as polygons that are coarse generalizations of their distribution, and may include relatively extensive areas from which the species is absent, potentially overestimating the species’ true area of occupancy [30] . For some species, finer scale distribution data are available (e.g., [31] ), but the data we used represent the best available dataset for all species globally compiled in a consistent manner, thus minimising the degree to which variations in data quality affect the spatial patterns obtained. Even so, distributions maps for species in Europe and North America are likely to be more accurate than those in other regions. However, such limitations are not expected to significantly affect the global patterns obtained, given the coarse spatial resolution of the analyses (cell area c. 23,322 km 2 ; [32] . Although lower data quality may result in an underestimation of the total number of birds in the tropics and in the Southern Hemisphere, it would be necessary for hundreds of migratory species to be unmapped for the observed asymmetrical patterns to be offset. This is highly unlikely, as birds are the best known class of organisms (with only 69 species, <1%, classified as Data Deficient, compared with 15% for mammals; [21] ). Indeed, lower data quality is likely to minimise rather than exaggerate the observed asymmetry between the northern and southern hemispheres, as coarse distribution maps may overestimate local species richness.
Classification of migratory species was based on the mapped polygons, distinguishing breeding, non-breeding, and resident areas, but this is an oversimplification of the diversity in bird migratory behaviour [33] , excluding partial, differential and nomadic or irruptive migrants. Nonetheless, we probably included nearly all species whose annual movements affect the large-scale spatial patterns of diversity of migratory species investigated here. By treating as resident 52 species with complex migratory behaviour or for which there was uncertainty about their distributions (see methods) our estimates of numbers and proportions of migratory species are conservative.
Our results confirm those of Newton and Dale (1997; [34] ) in that the difference in species richness between summer and winter is positive in the south and negative in the north of the Western Palearctic region, with a transition around 35°N. Although Newton and Dale only examined species that breed in the Western Palearctic, such species correspond to the vast majority of all migrants in the region (given that the Western Palearctic is the northernmost part of the African-Eurasian flyway, and few species occur as non-breeders in the region while breeding elsewhere). We show that this pattern generalises to the entire Palearctic and Nearctic regions as well, but that there is no corresponding pattern in the Southern Hemisphere ( Figure 4A ). Moreover, when juxtaposing the spatial pattern in the difference ( Figure 3A ) with the total number of migratory species ( Figure 3B ), it becomes clear that the transition area around 35°N (with similar numbers of migratory species in July and January) is an area rich in migratory species, corresponding therefore a region of high seasonal species turnover (in contrast, in areas such as the Sahara, Greenland and central Australia, the small differences between July and January are a consequence of the overall low richness in migratory species). This reveals that the strong migration dynamics in the Northern Hemisphere is dominated by intra-continental (rather than inter-continental) movements, with most species migrating between lower and higher latitudes within the hemisphere. In contrast, the slightly negative values observed in the Southern Hemisphere are mainly due to the relatively few long-distance migrants that cross the equator for the non-breeding season, creating a uniform low enrichment of birds in January. Our results confirm that, globally, bird migration is mainly a Northern Hemisphere phenomenon.
We found a hump-shaped relationship between the absolute number of migratory species and latitude in the Northern Hemisphere, with an increase between the equator and 50°N, followed by a decrease towards the North Pole ( Figure 4B ). This pattern is very different from that for the distribution of total avian species richness ( Figure 2A ): bird species in general are concentrated in the tropics, whereas migratory species are concentrated in the northern temperate latitudes. Somewhat unexpectedly, it also differs from the results in previous studies ( [18] , [35] ) which reported a general increase in the absolute number of migratory species with increasing latitude on North America and Europe. The discrepancy may be explained by the fact that these previous studies only looked at the breeding distributions of migratory birds. Indeed, many of the migratory species in the Northern Hemisphere winter in the southern part of this hemisphere, and so high overall numbers of migratory species are also found at relatively low latitudes.
Our study confirms previous results that the proportion of migratory species increases with latitude ( [14] , [15] ,b; Figure 3C ), and we show that this trend generalises to the global scale. Bird communities at higher latitudes are therefore more influenced by migratory species than those at lower latitudes. However, although this applies to both hemispheres, there is a strong asymmetry in the magnitude of the effect: the local percentage of migratory species reaches a maximum of 60% in the Southern Hemisphere whereas in the Northern Hemisphere percentages are often above this value. This asymmetry is not simply explained by an absence of land at high latitudes in the Southern Hemisphere, as it is found at equivalent latitudes (e.g., about 60% of species at 40°N are migratory, but just 20% at 40°S; Figure 4C ). Above 50°N, most communities are composed primarily by migratory species (>50%), which therefore have a major influence on community dynamics (e.g. intra-specific competition [36] ) and ecosystem function (e.g., long-distance dispersal of seeds [37] and of pathogens [38] ).
Global distribution patterns of threatened and narrow-range migratory species are very different from those for all migratory bird species. The latter are concentrated in the tropical Andes, Atlantic Forests of Brazil, the eastern Himalayas, eastern Madagascar, and the archipelagos of South-East Asia [39] (BirdLife International, 2008) whereas threatened migratory species are concentrated mostly in Asia and to a lesser extent in eastern Africa ( Figure 5A ). The two latitudinal bands of high diversity of threatened migratory species in Asia are mainly caused by the same species being counted in both their breeding and non-breeding distributions. The local richness in threatened migratory species is much higher in the Eastern Hemisphere than in the Western Hemisphere ( Figure 5A ), even though the total numbers of threatened migratory species by continent do not reflect this. This is explained by threatened migratory species in the Americas having generally smaller distributions (with lower degree of overlap and so lower richness per hexagon) than in the Eastern Hemisphere.
The majority of threatened migratory species are waterbirds, and these are primarily threatened by widespread degradation and destruction of wetlands [1] . In particular, industrialisation around the Yellow Sea, driven by rapid growth in the population and economy of China and South Korea, affects species on their breeding grounds (e.g., Chinese Egret), non-breeding grounds (e.g., Hooded Crane) or during their migration along the East Asian-Australasian Flyway (e.g., Great Knot, Far Eastern Curlew and Spoon-billed Sandpiper). Furthermore, the intensification of agriculture, combined with the degradation of rivers by pollution and transport in southern Asia, affects many species on their wintering grounds (e.g. Manchurian Reed Warbler, Wood Snipe, Asian Finfoot and Indian Skimmer). The region around the Black and Caspian Seas is another area with many threats to migratory species, such as the intensification of agriculture in Eastern Europe or hunting in Middle East and South-West Asia (e.g. Houbara Bustard; [40] ).
When defined across all bird species, narrow-range species are concentrated in the tropics (e.g., northern Andes, Eastern Arc of Africa, and archipelagos of South-East Asia; [13] , [29] ). In contrast, narrow-range migratory species are mostly absent from those areas and are concentrated in Central America, the Himalayas and Patagonia ( Figure 5B ). In the Americas, the concentration of migratory species in Patagonia and Central America in January may reflect the more restricted areas of land in these regions, concentrating migrants from the central/northern parts of South America and from North America, respectively. In Asia, the concentration of migrants in the Himalayas in July may reflect the availability of seasonally rich habitats at different altitudes within a narrow latitudinal band, with a large region to the south in South Asia to support migrants in the non-breeding season.
As in previous studies ( [13] for birds, [41] for mammals, and [42] for multiple taxa), we found little congruence between areas with high richness, endemism and threat for migratory bird species. The Himalayan region, however, stands out as an exception, suggesting that it is of particular importance for the conservation of migratory birds at the global scale. Other regions highlighted by at least two of these aspects of diversity include south-east Russia, Japan, southern Myanmar, Central America, eastern China, the Rift Valley, north-west India, and Mongolia. Many of these also fall within existing regions of global conservation priority [43] . However, whereas the majority of the latter occur in tropical regions, regions of importance for migratory birds are mainly found in temperate regions in the Northern Hemisphere.
Overall, we found a striking asymmetry between the Northern and Southern Hemispheres in all the patterns investigated ( Figures 3 , 4 and 5 ). This was not readily apparent from the combined results of previous regional-scale studies, highlighting the importance of a global perspective for understanding biodiversity patterns. There are a number of (non-mutually exclusive) hypotheses that might explain this asymmetry. First, the evolution of migration from the tropics towards northern areas (as proposed by the “southern-home” theory; [4] might have been favoured by the greater extent of continental mass in the Northern Hemisphere. The shape of the continental mass, with the Southern Hemisphere being dominated by the V-shaped South America and Africa, and isolated islands in Oceania, might have further enhanced this effect. Second, climate seasonality is more extreme in the Northern than in the Southern Hemisphere due to the relative amount of land versus ocean (given the buffering effect of the ocean on climate), making it more challenging for species to remain all year round. Third, differences in climatic history might have provided different opportunities for the evolution of migration in the two hemispheres. Indeed, the Northern Hemisphere has been characterised by much greater long-term climatic variability than the Southern Hemisphere (e.g. more extensive recent glaciations; [44] . Finally, present and past habitat geography could have favoured a more pronounced evolution of migration in the northern hemisphere, for example if important migration routes have evolved between tropical forests and the large temperate forests once occurring in the Northern Hemisphere [45] . Future studies explicitly testing these hypotheses, through their predictions on the spatial patterns of migratory bird diversity, will shed light on the origin and evolution of bird migration.
Supporting Information
Appendix s1. literature review. synthesis of the published literature on bird migration from a macroecological perspective. information is synthesized in a table (table s1)..
https://doi.org/10.1371/journal.pone.0070907.s001
Appendix S2. Analysis across flyways. Migratory species diversity as a function of latitude across the three major global migratory flyways. The major global flyways are described in Figure S1. Figure S2 shows the diversity in migratory species plotted against latitude and across flyways.
https://doi.org/10.1371/journal.pone.0070907.s002
File S1. Figure S3, Global patterns of species diversity for narrow-range migratory species. (A) Richness in narrow-range migratory species in July. (B) Richness in narrow-range migratory species in January.
https://doi.org/10.1371/journal.pone.0070907.s003
File S2. Table S2, Summary of geographical location, distribution and major threats for threatened migratory species.
https://doi.org/10.1371/journal.pone.0070907.s004
Author Contributions
Conceived and designed the experiments: AM AR MS. Analyzed the data: MS. Wrote the paper: MS SB AR AM. Co-coordinated the data collection: SB. Verified and corrected the data for migratory species: MS.
- View Article
- Google Scholar
- 2. Newton I (2008) The Migration Ecology of Birds. 1st ed. London, UK: Academic Press.
- 5. Gaston KJ, Blackburn TM (2000) Pattern and Process in Macroecology. Oxford, UK: Blackwell Scientific.
- 20. Birdlife International, NatureServe (2011) Bird species distribution maps of the world. BirdLife International, Cambridge, United Kingdom and NatureServe, Arlington, United States.
- 21. IUCN (2012) The IUCN Red List of Threatened Species. Version 2012.1. IUCN. Available: http://www.iucnredlist.org . Accessed 2013 Jul 4.
- 25. Birdlife International, NatureServe (2012) Bird species distribution maps of the world. BirdLife International, Cambridge, United Kingdom and NatureServe, Arlington, United States. Available: http://www.birdlife.org/datazone/info/spcdownload . Accessed 2013 Jul 4.
- 26. Del Hoyo J, Elliott A, Sargatal J (1992–2011) The Handbook of the Birds of the World. Barcelona: Lynx Edicions.
- 29. Strattersfield AJ, Crosby MJ, Long AJ, Wege DC (1998) Endemic Bird Areas of the World. Priorities for biodiversity conservation. BirdLife Conservation Series 7. Cambridge, UK: BirdLife International.
- 31. Sauer JR, Hines JE, Fallon JE, Pardieck KL, Ziolkowski Jr DJ, et al.. (2011) The North American Breeding Bird Survey, Results and Analysis 1966–2010. Version 12.07.2011 USGS Patuxent Wildlife Research Center, Laurel, MD.
- 39. BirdLife International (2000) Threatened Birds of the World. Lynx Edicions and BirdLife International, Barcelona and Cambridge.

IMAGES
VIDEO
COMMENTS
In the following sections, we focus on five key research areas in bird migration research — navigation, social influences, individual development, costs and the environment and conservation.
Using a dataset on the geographical distributions of the world's birds, I mapped global patterns of migratory bird diversity, revealing strong spatial features.
Here, we compile a global dataset on the annual cycle of migratory birds obtained by tracking 1531 individuals and 177 populations from 186 species, and investigate how body mass, a key...
These results highlight the strong flexibility of the global bird migration system and offer a baseline in the context of on-going anthropogenic climate change.
We found that 35% of the overall 343 important sites that strongly connect the migration network across the annual cycle of global migratory birds are uncovered by protected areas. This leads to nearly 87% of 1,862 migratory bird species being at risk.
We demonstrate that directional, population-level change in timing of bird migration can occur predominantly through within-individual changes: across 13 years, bar-tailed godwits advanced...
Here, we used over 390 million community-contributed bird observations to derive order-specific, spatially explicit estimates of annual migration networks for 26 bird orders across the world.
Given the strong continental migratory connectivity of Grasshopper Sparrows and the partial migration behavior of Eastern Meadowlarks, our research highlights the need for regional management strategies that consider the full annual cycle of these two grassland-dependent bird species.
We used data on the distribution of the world's bird species to derive global empirical patterns of: the number of breeding migrants, the number of non-breeding migrants and the difference in seasonal diversity.
Here, we analyse a dataset on the global distribution of the world’s birds in order to examine global spatial patterns in the diversity of migratory species, including: the seasonal variation in overall species diversity due to migration; the contribution of migratory birds to local bird diversity; and the distribution of narrow-range and ...