Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 14 October 2016

Genetic drift, selection and the evolution of the mutation rate
- Michael Lynch 1 ,
- Matthew S. Ackerman 1 ,
- Jean-Francois Gout 1 ,
- Hongan Long 1 ,
- Way Sung 1 ,
- W. Kelley Thomas 2 &
- Patricia L. Foster 1
Nature Reviews Genetics volume 17 , pages 704–714 ( 2016 ) Cite this article
34k Accesses
476 Citations
99 Altmetric
Metrics details
- Evolutionary genetics
- Experimental evolution
- Genetic variation
- Genome evolution
- Mutagenesis
- Next-generation sequencing
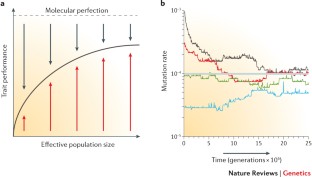
As one of the few cellular traits that can be quantified across the tree of life, DNA-replication fidelity provides an excellent platform for understanding fundamental evolutionary processes. Furthermore, because mutation is the ultimate source of all genetic variation, clarifying why mutation rates vary is crucial for understanding all areas of biology. A potentially revealing hypothesis for mutation-rate evolution is that natural selection primarily operates to improve replication fidelity, with the ultimate limits to what can be achieved set by the power of random genetic drift. This drift-barrier hypothesis is consistent with comparative measures of mutation rates, provides a simple explanation for the existence of error-prone polymerases and yields a formal counter-argument to the view that selection fine-tunes gene-specific mutation rates.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
176,64 € per year
only 14,72 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
A mutation rate model at the basepair resolution identifies the mutagenic effect of polymerase III transcription

Molecular and evolutionary processes generating variation in gene expression
The rate and molecular spectrum of mutation are selectively maintained in yeast
Rosenberg, S. M. Evolving responsively: adaptive mutation. Nat. Rev. Genet. 2 , 504–515 (2001).
CAS PubMed Google Scholar
Galhardo, R. S., Hastings, P. J. & Rosenberg, S. M. Mutation as a stress response and the regulation of evolvability. Crit. Rev. Biochem. Mol. Biol. 42 , 399–435 (2007).
CAS PubMed PubMed Central Google Scholar
Martincorena, I., Seshasayee, A. S. & Luscombe, N. M. Evidence of non-random mutation rates suggests an evolutionary risk management strategy. Nature 485 , 95–98 (2012).
Paul, S., Million-Weaver, S., Chattopadhyay, S., Sokurenko, E. & Merrikh, H. Accelerated gene evolution through replication-transcription conflicts. Nature 495 , 512–515 (2013).
Ram, Y. & Hadany, L. Stress-induced mutagenesis and complex adaptation. Proc. Biol. Sci. 281 , 20141025 (2014).
PubMed PubMed Central Google Scholar
Lynch, M. The cellular, developmental, and population-genetic determinants of mutation-rate evolution. Genetics 180 , 933–943 (2008). This paper provides an overview of population-genetic theory for the selective disadvantage of a mutator allele associated with the indirect effects of linked deleterious mutations under arbitrary degrees of recombination, and also for the direct effects of somatic mutation. This paper also considers the expected frequencies of mutator alleles under mutation–selection balance.
Lynch, M. The lower bound to the evolution of mutation rates. Genome Biol. Evol. 3 , 1107–1118 (2011). This article develops the theory associated with the drift-barrier hypothesis for the lower bound to mutation-rate evolution, as well as an overview of empirical observations on the error rates associated with various DNA polymerases.
MacLean, R. C., Torres-Barceló, C. & Moxon, R. Evaluating evolutionary models of stress-induced mutagenesis in bacteria. Nat. Rev. Genet. 14 , 221–227 (2013). This study provides an overview of evolutionary theory in the context of stress-induced mutagenesis and presents supportive data for the idea that the error-prone nature of polymerases associated with such activities have arrived at such a condition by genetic drift.
Kimura, M. On the evolutionary adjustment of spontaneous mutation rates. Genet. Res. 9 , 23–34 (1967). This is a classical paper in which the selective disadvantage of mutator alleles associated with linked mutation load was first considered.
Google Scholar
Kondrashov, A. S. Modifiers of mutation-selection balance: general approach and the evolution of mutation rates. Genet. Res. 66 , 53–70 (1995).
Dawson, K. J. The dynamics of infinitesimally rare alleles, applied to the evolution of mutation rates and the expression of deleterious mutations. Theor. Pop. Biol. 55 , 1–22 (1999).
CAS Google Scholar
Lynch, M. et al. Spontaneous deleterious mutation. Evolution 53 , 645–663 (1999).
PubMed Google Scholar
Baer, C. F., Miyamoto, M. M. & Denver, D. R. Mutation rate variation in multicellular eukaryotes: causes and consequences. Nat. Rev. Genet. 8 , 619–631 (2007).
Eyre-Walker, A. & Keightley, P. D. The distribution of fitness effects of new mutations. Nat. Rev. Genet. 8 , 610–618 (2007). This article provides a broad overview of methods for the estimation of the distribution of fitness effects of de novo mutations, and the implications derived from population-genetic data.
Hall, D. W., Fox, S., Kuzdzal-Fick, J. J., Strassmann, J. E. & Queller, D. C. The rate and effects of spontaneous mutation on fitness traits in the social amoeba, Dictyostelium discoideum . G3 (Bethesda) 8 , 1115–1127 (2013).
Lynch, M. Evolutionary layering and the limits to cellular perfection. Proc. Natl Acad. Sci. USA 109 , 18851–18856 (2012). This paper demonstrates that when selection operates on the overall perfection of a process involving multiple levels, the alternative components are free to drift so long as the level of refinement of the entire system remains at the drift barrier; this degree of interaction can lead to an evolutionary situation in which complex systems are ultimately no more efficient than simpler systems, but maintain an illusion of adaptive robustness.
Drake, J. W., Charlesworth, B., Charlesworth, D. & Crow, J. F. Rates of spontaneous mutation. Genetics 148 , 1667–1686 (1998). This article provides an early comprehensive overview of the rate and fitness effects of mutations in diverse organisms.
Sniegowski, P. D., Gerrish, P. J., Johnson, T. & Shaver, A. The evolution of mutation rates: separating causes from consequences. Bioessays 22 , 1057–1066 (2000).
André, J. B. & Godelle, B. The evolution of mutation rate in finite asexual populations. Genetics 172 , 611–626 (2006).
Bessman, M. J., Muzyczka, N., Goodman, M. F. & Schnaar, R. L. Studies on the biochemical basis of spontaneous mutation. II. The incorporation of base and its analogue into DNA by wild type, mutator and antimutator DNA polymerases. J. Mol. Biol. 88 , 409–421 (1974).
Loh, E., J. Choe, J. & Loeb, L. A. Highly tolerated amino acid substitutions increase the fidelity of Escherichia coli DNA polymerase I. J. Biol. Chem. 282 , 12201–12209 (2007). This paper is gives an empirical demonstration of the relative ease of obtaining antimutator alleles by mutations in a DNA polymerase.
Tian, W., Hwang, Y. T. & Hwang, C. B. The enhanced DNA replication fidelity of a mutant herpes simplex virus type 1 DNA polymerase is mediated by an improved nucleotide selectivity and reduced mismatch extension ability. J. Virol. 82 , 8937–8941 (2008).
Loh, E., Salk, J. J. & Loeb, L. A. Optimization of DNA polymerase mutation rates during bacterial evolution. Proc. Natl Acad. Sci. USA 107 , 1154–1159 (2010).
Lynch, M. & Marinov, G. F. The bioenergetic cost of a gene. Proc. Natl Acad. Sci. USA 112 , 15690–15695 (2015).
Casjens, S. The diverse and dynamic structure of bacterial genomes. Annu. Rev. Genet. 32 , 339–377 (1998).
Cox, R. A. Quantitative relationships for specific growth rates and macromolecular compositions of Mycobacterium tuberculosis, Streptomyces coelicolor A3(2) and Escherichia coli B/r: an integrative theoretical approach. Microbiology 150 , 1413–1426 (2004).
Mira, A., Ochman, H. & Moran, N. A. Deletional bias and the evolution of bacterial genomes. Trends Genet. 17 , 589–596 (2001).
Vieira-Silva, S., Touchon, M. & Rocha, E. P. No evidence for elemental-based streamlining of prokaryotic genomes. Trends Ecol. Evol. 25 , 319–320 (2010).
Lynch, M. Evolution of the mutation rate. Trends Genet. 26 , 345–352 (2010).
Drake, J. W. A constant rate of spontaneous mutation in DNA-based microbes. Proc. Natl Acad. Sci. USA 88 , 7160–7164 (1991). A classical paper that first suggested that there is an inverse relationship between the mutation rate per site, u , and the number of nucleotides per genome in microbes, leading to a constant expected total number of mutations per genome.
Rosche, W. A. & Foster, P. L. Determining mutation rates in bacterial populations. Methods 20 , 4–17 (2000). This article provides a broad overview of methods for estimating microbial mutation rates using reporter constructs.
Nachman, M. W. & Crowell, S. L. Estimate of the mutation rate per nucleotide in humans. Genetics 156 , 297–304 (2000). This study presents a first attempt to estimate the human mutation rate from the level of molecular divergence between orthologous human and chimpanzee sequences.
Kibota, T. & Lynch, M. Estimate of the genomic mutation rate deleterious to overall fitness in Escherichia coli . Nature 381 , 694–696 (1996).
Campbell, C. D. et al. Estimating the human mutation rate using autozygosity in a founder population. Nat. Genet. 44 , 1277–1281 (2012).
Kong, A. et al. Rate of de novo mutations and the importance of father's age to disease risk. Nature 488 , 471–475 (2012). This paper describes one of the first attempts to estimate the human mutation rate by comparing the genomic sequences of parents and offspring.
Venn, O. et al. Strong male bias drives germline mutation in chimpanzees. Science 344 , 1272–1275 (2014).
Keightley, P. D., Ness, R. W., Halligan, D. L. & Haddrill, P. R. Estimation of the spontaneous mutation rate per nucleotide site in a Drosophila melanogaster full-sib family. Genetics 196 , 313–320 (2014).
Keightley, P. D. et al. Estimation of the spontaneous mutation rate in Heliconius melpomene. Mol. Biol. Evol. 32 , 239–243 (2015). This study uses population-genetic data to arrive at the conclusion that the average newborn human acquires about two new mutations.
Sung, W., Ackerman, M. S., Miller, S. F., Doak, T. G. & Lynch, M. The drift-barrier hypothesis and mutation-rate evolution. Proc. Natl Acad. Sci. USA 109 , 18488–18492 (2012).
Lynch, M. The Origins of Genome Architecture (Sinauer Assoc., 2007).
Kimura, M. The Neutral Theory of Molecular Evolution (Cambridge Univ. Press, 1983).
Massey, S. E. The proteomic constraint and its role in molecular evolution. Mol. Biol. Evol. 25 , 2557–2565 (2008).
Siepel, A. et al. Evolutionarily conserved elements in vertebrate, insect, worm, and yeast genomes. Genome Res. 15 , 1034–1050 (2005).
Halligan, D. L. & Keightley, P. D. Ubiquitous selective constraints in the Drosophila genome revealed by a genome-wide interspecies comparison. Genome Res. 16 , 875–884 (2006).
Keightley, P. D. Rates and fitness consequences of new mutations in humans. Genetics 190 , 295–304 (2012).
Lindblad-Toh, K. et al. A high-resolution map of human evolutionary constraint using 29 mammals. Nature 478 , 476–482 (2011).
Rands, C. M., Meader, S., Ponting, C. P. & Lunter, G. 8. 2% of the human genome is constrained: variation in rates of turnover across functional element classes in the human lineage. PLoS Genet. 10 , e1004525 (2014).
Radman, M., Taddei, F. & Matic, I. Evolution-driving genes. Res. Microbiol. 151 , 91–95 (2000).
Tenaillon, O., Taddei, F., Radman, M. & Matic, I. Second-order selection in bacterial evolution: selection acting on mutation and recombination rates in the course of adaptation. Res. Microbiol. 152 , 11–16 (2001).
Earl, D. J. & Deem, M. W. Evolvability is a selectable trait. Proc. Natl Acad. Sci. USA 101 , 11531–11536 (2004).
Foster, P. L. Stress-induced mutagenesis in bacteria. Crit. Rev. Biochem. Mol. Biol. 42 , 373–397 (2007).
Gerrish, P. J., Colato, A., Perelson, A. S. & Sniegowski, P. D. Complete genetic linkage can subvert natural selection. Proc. Natl Acad. Sci. USA 104 , 6266–6271 (2007).
Gerrish, P. J., Colato, A. & Sniegowski, P. D. Genomic mutation rates that neutralize adaptive evolution and natural selection. J. R. Soc. Interface 10 , 20130329 (2013).
Kondrashov, A. S. Direct estimates of human per nucleotide mutation rates at 20 loci causing Mendelian diseases. Hum. Mutat. 21 , 12–27 (2003).
Lynch, M. Rate, molecular spectrum, and consequences of spontaneous mutations in man. Proc. Natl Acad. Sci. USA 107 , 961–968 (2009).
Lynch, M. The origins of eukaryotic gene structure. Mol. Biol. Evol. 23 , 450–468 (2006).
Sung, W. et al. Evolution of the insertion-deletion mutation rate across the tree of life. G3 (Bethesda) 6 , 2583–2591 (2016).
Lynch, M. et al. Genome-wide linkage-disequilibrium profiles from single individuals. Genetics 198 , 269–281 (2014).
Leigh, E. G. Jr Natural selection and mutability. Amer. Nat. 104 , 301–305 (1970).
Orr, H. A. The rate of adaptation in asexuals. Genetics 155 , 961–968 (2000).
Johnson, T. & Barton, N. H. The effect of deleterious alleles on adaptation in asexual populations. Genetics 162 , 395–411 (2002).
Sturtevant, A. H. Essays on evolution. I. On the effects of selection on mutation rate. Quart. Rev. Biol. 12 , 464–476 (1937).
Johnson, T. Beneficial mutations, hitchhiking and the evolution of mutation rates in sexual populations. Genetics 151 , 1621–1631 (1999).
Lee, H., Popodi, E., Tang, H. & Foster, P. L. Rate and molecular spectrum of spontaneous mutations in the bacterium Escherichia coli as determined by whole-genome sequencing. Proc. Natl Acad. Sci. USA 109 , E2774–E2783 (2012).
Lujan, S. A. et al. Heterogeneous polymerase fidelity and mismatch repair bias genome variation and composition. Genome Res. 24 , 1751–1764 (2014).
Sung, W. et al. Asymmetric context-dependent mutation patterns revealed through mutation accumulation experiments. Mol. Biol. Evol. 32 , 1672–1683 (2015). This study uses MA-WGS data from several bacterial species to demonstrate the strong dependency of site-specific mutation rates on the identity of neighbouring nucleotides.
Chen, X., Yang, J. R. & Zhang, J. Nascent RNA folding mitigates transcription-associated mutagenesis. Genome Res. 26 , 50–59 (2016).
Kashi, Y. & King, D. G. Simple sequence repeats as advantageous mutators in evolution. Trends Genet. 22 , 253–259 (2006).
Moxon, R., Bayliss, C. & Hood, D. Bacterial contingency loci: the role of simple sequence DNA repeats in bacterial adaptation. Annu. Rev. Genet. 40 , 307–333 (2006). This paper presents an overview of the evidence suggesting that some loci may have special sequence features, potentially maintained by selection, that enhance mutagenicity.
Zhou, K., Aertsen, A. & Michiels, C. W. The role of variable DNA tandem repeats in bacterial adaptation. FEMS Microbiol. Rev. 38 , 119–141 (2014).
Haerty, W. & Golding, G. B. Genome-wide evidence for selection acting on single amino acid repeats. Genome Res. 20 , 755–760 (2010).
Scala, C. et al. Amino acid repeats cause extraordinary coding sequence variation in the social amoeba Dictyostelium discoideum . PLoS ONE 7 , e46150 (2012).
Lin, C. H., Lian, C. Y., Hsiung, C. A. & Chen, F. C. Changes in transcriptional orientation are associated with increases in evolutionary rates of enterobacterial genes. BMC Bioinformatics 12 (Suppl. 9), 19 (2011).
Foster, P. L. et al. On the mutational topology of the bacterial genome. G3 (Bethesda) 3 , 399–407 (2013). This article demonstrates large-scale spatial variation in the mutation rate over the E. coli genome.
Long, H. et al. Mutation rate, spectrum, topology, and context-dependency in the DNA mismatch repair deficient Pseudomonas fluorescens ATCC948. Genome Biol. Evol. 7 , 262–271 (2015).
Lang, G. I. & Murray, A. W. Mutation rates across budding yeast chromosome VI are correlated with replication timing. Genome Biol. Evol. 3 , 799–811 (2011).
Stamatoyannopoulos, J. A. et al. Human mutation rate associated with DNA replication timing. Nat. Genet. 41 , 393–395 (2009).
Chen, X. et al. Nucleosomes suppress spontaneous mutations base-specifically in eukaryotes. Science 335 , 1235–1238 (2012).
Ganesan, A., Spivak, G. & Hanawalt, P. C. Transcription-coupled DNA repair in prokaryotes. Prog. Mol. Biol. Transl. Sci. 110 , 25–40 (2012). This paper presents a broad overview of the mechanism of TCR in bacteria.
Jinks-Robertson, S. & Bhagwat, A. S. Transcription-associated mutagenesis. Annu. Rev. Genet. 48 , 341–359 (2014). This article discusses an overview of observations on the association between transcription and mutagenesis.
Park, C., Qian, W. & Zhang, J. Genomic evidence for elevated mutation rates in highly expressed genes. EMBO Rep. 13 , 1123–1129 (2012). This study provides evidence that highly expressed genes have elevated mutation rates.
Chen, X. & Zhang, J. Yeast mutation accumulation experiment supports elevated mutation rates at highly transcribed sites. Proc. Natl Acad. Sci. USA 111 , E4062 (2014).
Green, P. et al. Transcription-associated mutational asymmetry in mammalian evolution. Nat. Genet. 33 , 514–517 (2003).
Polak, P. & Arndt, P. F. Transcription induces strand-specific mutations at the 5′ end of human genes. Genome Res. 18 , 1216–1223 (2008).
Haines, N. M., Kim, Y. I., Smith, A. J. & Savery, N. J. Stalled transcription complexes promote DNA repair at a distance. Proc. Natl Acad. Sci. USA 111 , 4037–4042 (2014).
Eyre-Walker, A. & Bulmer, M. Synonymous substitution rates in enterobacteria. Genetics 140 , 1407–1412 (1995).
Chen, X. & Zhang, J. No gene-specific optimization of mutation rate in Escherichia coli . Mol. Biol. Evol. 30 , 1559–1562 (2013).
Merrikh, H., Zhang, Y., Grossman, A. D. & Wang, J. D. Replication-transcription conflicts in bacteria. Nat. Rev. Microbiol. 10 , 449–458 (2012).
Helmrich, A., Ballarino, M., Nudler, E. & Tora, L. Transcription-replication encounters, consequences and genomic instability. Nat. Struct. Mol. Biol. 20 , 412–418 (2013).
Fijalkowska, I. J., Jonczyk, P., Tkaczyk, M. M., Bialoskorska, M. & Schaaper, R. M. Unequal fidelity of leading strand and lagging strand DNA replication on the Escherichia coli chromosome. Proc. Natl Acad. Sci. USA 95 , 10020–10025 (1998).
Wang, J. D., Berkmen, M. B. & Grossman, A. D. Genome-wide coorientation of replication and transcription reduces adverse effects on replication in Bacillus subtilis . Proc. Natl Acad. Sci. USA 104 , 5608–5613 (2007).
Srivatsan, A., Tehranchi, A., MacAlpine, D. M. & Wang, J. D. Co-orientation of replication and transcription preserves genome integrity. PLoS Genet. 6 , e1000810 (2010).
Rocha, E. P. The replication-related organization of bacterial genomes. Microbiology 150 , 1609–1627 (2004).
Rocha, E. P. Is there a role for replication fork asymmetry in the distribution of genes in bacterial genomes? Trends Microbiol. 10 , 393–395 (2002).
Drummond, D. A., Bloom, J. D., Adami, C., Wilke, C. O. & Arnold, F. H. Why highly expressed proteins evolve slowly. Proc. Natl Acad. Sci. USA 102 , 14338–14343 (2005). This paper provides evidence that highly expressed genes experience a higher level of purifying selection against mutations that induce problems in translation and folding.
Gout, J. F., Kahn, D., Duret, L. & Paramecium Post-Genomics Consortium. The relationship among gene expression, the evolution of gene dosage, and the rate of protein evolution. PLoS Genet. 6 , e1000944 (2010).
Park, C., Chen, X., Yang, J. R. & Zhang, J. Differential requirements for mRNA folding partially explain why highly expressed proteins evolve slowly. Proc. Natl Acad. Sci. USA 110 , E678–E686 (2013).
Chen, X. & Zhang, J. Why are genes encoded on the lagging strand of the bacterial genome? Genome Biol. Evol. 5 , 2436–2439 (2013).
Szczepanik, D. et al. Evolution rates of genes on leading and lagging DNA strands. J. Mol. Evol. 52 , 426–433 (2001).
McDonald, M. J., Hsieh, Y. Y., Yu, Y. H., Chang, S. L. & Leu, J. Y. The evolution of low mutation rates in experimental mutator populations of Saccharomyces cerevisiae . Curr. Biol. 22 , 1235–1240 (2012).
Turrientes, M. C. et al. Normal mutation rate variants arise in a mutator (mut S) Escherichia coli population. PLoS ONE 8 , e72963 (2013).
Wielgoss, S. et al. Mutation rate dynamics in a bacterial population reflect tension between adaptation and genetic load. Proc. Natl Acad. Sci. USA 110 , 222–227 (2013).
Williams, L. N., Herr, A. J. & Preston, B. D. Emergence of DNA polymerase antimutators that escape error-induced extinction in yeast. Genetics 193 , 751–770 (2013).
Lynch, M. Mutation and human exceptionalism: our future genetic load. Genetics 202 , 869–875 (2016).
Behjati, S. et al. Genome sequencing of normal cells reveals developmental lineages and mutational processes. Nature 513 , 422–425 (2014).
Denamur, E. & Matic, I. Evolution of mutation rates in bacteria. Mol. Microbiol. 60 , 820–827 (2006).
Desai, M. M. & Fisher, D. S. Beneficial mutation selection balance and the effect of linkage on positive selection. Genetics 176 , 1759–1798 (2007). This article develops a general theory for considering the roles that beneficial mutations have in driving mutation-rate evolution.
Raynes, Y. & Sniegowski, P. D. Experimental evolution and the dynamics of genomic mutation rate modifiers. Heredity 113 , 375–380 (2014).
Giraud, A. et al. Costs and benefits of high mutation rates: adaptive evolution of bacteria in the mouse gut. Science 291 , 2606–2608 (2001). This study considers the interplay between the short-term advantages and long-term disadvantages of mutator alleles.
Oliver, A., Baquero, F. & Blázquez, J. The mismatch repair system ( mutS, mutL and uvrD genes) in Pseudomonas aeruginosa: molecular characterization of naturally occurring mutants. Mol. Microbiol. 43 , 1641–1650 (2002).
Pal, C., Maciá, M. D., Oliver, A., Schachar, I. & Buckling, A. Coevolution with viruses drives the evolution of bacterial mutation rates. Nature 450 , 1079–1081 (2007).
Harris, K. Evidence for recent, population-specific evolution of the human mutation rate. Proc. Natl Acad. Sci. USA 112 , 3439–3444 (2015).
Download references
Acknowledgements
Support was provided by the US Army Research Office Multidisciplinary University Research Initiative (MURI) awards W911NF-09-1-0444 to M.L., P.L.F., H. Tang and S. Finkel, and W911NF-14-1-0411 to M.L., P.L.F., A. Drummond, J. Lennon and J. McKinlay; and the US National Institutes of Health Research Project grant R01-GM036827 to M.L. and W.K.T. We thank R. Ness for providing information, and A. Kondrashov and two anonymous reviewers for their comments.
Author information
Authors and affiliations.
Department of Biology, Indiana University, Bloomington, 47401, Indiana, USA
Michael Lynch, Matthew S. Ackerman, Jean-Francois Gout, Hongan Long, Way Sung & Patricia L. Foster
Department of Molecular, Cellular, and Biomedical Sciences, University of New Hampshire, Durham, 03824, New Hampshire, USA
W. Kelley Thomas
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Michael Lynch .
Ethics declarations
Competing interests.
The authors declare no competing financial interests.
Supplementary information
Supplementary information s1 (table).
Summary of mutation-rate estimates, and data sources. (XLSX 43 kb)
PowerPoint slides
Powerpoint slide for fig. 1, powerpoint slide for fig. 2, powerpoint slide for fig. 3, powerpoint slide for fig. 4, powerpoint slide for fig. 5.
A mutation having detrimental effects on the fitness of an organism.
The idea that the ability of natural selection to refine a phenotype is ultimately limited by the noise created by random genetic drift, which itself is a consequence of finite population size and the stochastic effects of linked mutations.
( N e ). A measure of the size of a population from the standpoint of the reliability of allele-frequency transmission across generations; generally, one to several orders of magnitude below the actual population size, owing to variation in family size, a wide range of other demographic features and the hitch-hiking effects of linked mutations.
The process by which a genetic variant at an initially polymorphic site increases in frequency until it attains a frequency of 1.0 in the population.
Brothers and sisters sharing the same mother and father.
An alteration of the nucleotide sequence at one chromosomal location resulting from the acquisition of information from a homologous sequence elsewhere in the genome during genetic recombination; such events are not always accompanied by chromosomal crossing over.
A strand of nascent DNA that is synthesized in the opposite direction of the progressive opening of the DNA on a parental chromosome, resulting in discontinuous replication fragments that must be stitched together.
A strand of nascent DNA that is synthesized in one continuous flow in the same direction as the progression of the opening of the DNA on a parental chromosome.
An equilibrium allele frequency that results from the opposing pressures of natural selection and mutation, one tending to remove variation and the other creating it.
Genomic sites within protein-coding regions at which nucleotide substitutions have no effect on the encoded amino acid, owing to the redundancy of the genetic code.
DNA-level changes arising within the somatic cells of multicellular organisms, and therefore not transmissible across generations but having direct effects on fitness.
Genetic variation among individuals within a population.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Lynch, M., Ackerman, M., Gout, JF. et al. Genetic drift, selection and the evolution of the mutation rate. Nat Rev Genet 17 , 704–714 (2016). https://doi.org/10.1038/nrg.2016.104
Download citation
Published : 14 October 2016
Issue Date : November 2016
DOI : https://doi.org/10.1038/nrg.2016.104
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Spontaneous mutations and mutational responses to penicillin treatment in the bacterial pathogen streptococcus pneumoniae d39.
- Wanyue Jiang
- Tongtong Lin
- Hongan Long
Marine Life Science & Technology (2024)
Deciphering climate resilience in Indian cattle breeds by selection signature analyses
- Sonali Sonejita Nayak
- Manjit Panigrahi
- Triveni Dutt
Tropical Animal Health and Production (2024)
Effects of urban-induced mutations on ecology, evolution and health
- Marc T. J. Johnson
- Irtaqa Arif
- Kristin M. Winchell
Nature Ecology & Evolution (2024)
Cisplatin exposure alters tRNA-derived small RNAs but does not affect epimutations in C. elegans
- Manon Fallet
- Rachel Wilson
- Peter Sarkies
BMC Biology (2023)
Genome structure-based Juglandaceae phylogenies contradict alignment-based phylogenies and substitution rates vary with DNA repair genes
- Ya-Mei Ding
- Xiao-Xu Pang
- Wei-Ning Bai
Nature Communications (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Learning mitigates genetic drift
Affiliations.
- 1 Faculty of Science, Research Centre for Toxic Compounds in the Environment, Masaryk University, Kamenice 5, Building A29, 62500, Brno, Czech Republic. [email protected].
- 2 Department of Experimental Biology, Faculty of Science, Masaryk University, Kamenice 5, 62500, Brno, Czech Republic. [email protected].
- 3 Faculty of Science, Institute of Cell Biology, University of Bern, Baltzerstrasse 4, 3012, Bern, Switzerland. [email protected].
- 4 Faculty of Science, Research Centre for Toxic Compounds in the Environment, Masaryk University, Kamenice 5, Building A29, 62500, Brno, Czech Republic.
- 5 Department of Mathematics, Faculty of Science, Centre for Mathematical Biology, University of South Bohemia, Branišovská 1760, 37005, České Budějovice, Czech Republic.
- 6 Department of Ecology, Biology Centre, Institute of Entomology, The Czech Academy of Sciences, Branišovská 31, 37005, České Budějovice, Czech Republic.
- PMID: 36437294
- PMCID: PMC9701794
- DOI: 10.1038/s41598-022-24748-8
Genetic drift is a basic evolutionary principle describing random changes in allelic frequencies, with far-reaching consequences in various topics ranging from species conservation efforts to speciation. The conventional approach assumes that genetic drift has the same effect on all populations undergoing the same changes in size, regardless of different non-reproductive behaviors and history of the populations. However, here we reason that processes leading to a systematic increase of individuals` chances of survival, such as learning or immunological memory, can mitigate loss of genetic diversity caused by genetic drift even if the overall mortality rate in the population does not change. We further test this notion in an agent-based model with overlapping generations, monitoring allele numbers in a population of prey, either able or not able to learn from successfully escaping predators' attacks. Importantly, both these populations start with the same effective size and have the same and constant overall mortality rates. Our results demonstrate that even under these conditions, learning can mitigate loss of genetic diversity caused by drift, by creating a pool of harder-to-die individuals that protect alleles they carry from extinction. Furthermore, this effect holds regardless if the population is haploid or diploid or whether it reproduces sexually or asexually. These findings may be of importance not only for basic evolutionary theory but also for other fields using the concept of genetic drift.
© 2022. The Author(s).
PubMed Disclaimer
Conflict of interest statement
The authors declare no competing interests.
Intensity of predation modulates interaction…
Intensity of predation modulates interaction of learning and genetic drift. Temporal dynamics of…
Effectiveness of learning dictates its…
Effectiveness of learning dictates its effect on genetic drift. Temporal dynamics of the…
Immediate learning affects drift in…
Immediate learning affects drift in a distinct way from stepwise learning. Temporal dynamics…
Restriction of maximal possible age…
Restriction of maximal possible age makes effects of learning smaller but still noticeable.…
Learning often prolongs generation time.…
Learning often prolongs generation time. The heatmaps show the mean difference in generation…
Effect of learning on genetic…
Effect of learning on genetic drift in a diploid sexually reproducing population. Temporal…
Similar articles
- Diploidy and the selective advantage for sexual reproduction in unicellular organisms. Kleiman M, Tannenbaum E. Kleiman M, et al. Theory Biosci. 2009 Nov;128(4):249-85. doi: 10.1007/s12064-009-0077-9. Epub 2009 Nov 10. Theory Biosci. 2009. PMID: 19902285
- Diffusion approximations for one-locus multi-allele kin selection, mutation and random drift in group-structured populations: a unifying approach to selection models in population genetics. Lessard S. Lessard S. J Math Biol. 2009 Nov;59(5):659-96. doi: 10.1007/s00285-008-0248-1. Epub 2009 Jan 21. J Math Biol. 2009. PMID: 19156416
- Effects of genetic drift on variance components under a general model of epistasis. Barton NH, Turelli M. Barton NH, et al. Evolution. 2004 Oct;58(10):2111-32. doi: 10.1111/j.0014-3820.2004.tb01591.x. Evolution. 2004. PMID: 15562679
- Foundational errors in the Neutral and Nearly-Neutral theories of evolution in relation to the Synthetic Theory: is a new evolutionary paradigm necessary? Valenzuela CY. Valenzuela CY. Biol Res. 2013;46(2):101-19. doi: 10.4067/S0716-97602013000200001. Biol Res. 2013. PMID: 23959008 Review.
- The genetics of evolutionary radiations. Naciri Y, Linder HP. Naciri Y, et al. Biol Rev Camb Philos Soc. 2020 Aug;95(4):1055-1072. doi: 10.1111/brv.12598. Epub 2020 Mar 31. Biol Rev Camb Philos Soc. 2020. PMID: 32233014 Review.
- IL-10 (-1082 G/A) polymorphism in Bataknese with schizophrenia. Mardhiyah SA, Effendy E, Nasution NM. Mardhiyah SA, et al. J Taibah Univ Med Sci. 2023 Sep 11;19(1):64-69. doi: 10.1016/j.jtumed.2023.08.011. eCollection 2024 Feb. J Taibah Univ Med Sci. 2023. PMID: 37868103 Free PMC article.
- Lequime S, Fontaine A, Gouilh MA, Moltini-Conclois I, Lambrechts L. Genetic drift, purifying selection and vector genotype shape dengue virus intra-host genetic diversity in mosquitoes. PLOS Genet. 2016;12:e1006111. doi: 10.1371/journal.pgen.1006111. - DOI - PMC - PubMed
- Lynch M, et al. Genetic drift, selection and the evolution of the mutation rate. Nat. Rev. Genet. 2016;17:704–714. doi: 10.1038/nrg.2016.104. - DOI - PubMed
- Wang J, Santiago E, Caballero A. Prediction and estimation of effective population size. Heredity. 2016;117:193–206. doi: 10.1038/hdy.2016.43. - DOI - PMC - PubMed
- Wright S. Inbreeding and homozygosis. Proc. Natl. Acad. Sci. U. S. A. 1933;19:411–420. doi: 10.1073/pnas.19.4.411. - DOI - PMC - PubMed
- Wright S. Evolution in mendelian populations. Genetics. 1931;16:97–159. doi: 10.1093/genetics/16.2.97. - DOI - PMC - PubMed
Publication types
- Search in MeSH
Grants and funding
- CZ.02.1.01/0.0/0.0/15_003/0000469/Ministerstvo Školství, Mládeže a Tělovýchovy
- 857560/Horizon 2020 Framework Programme
LinkOut - more resources
Full text sources.
- Bern Open Repository and Information System
- Europe PubMed Central
- Nature Publishing Group
- PubMed Central

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Loading metrics
Open Access
Peer-reviewed
Research Article
Genetic drift and selection in many-allele range expansions
Roles Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliation School of Engineering and Applied Sciences, Harvard University, Cambridge, Massachusetts, United States of America
Roles Conceptualization, Formal analysis, Methodology, Software, Supervision, Validation, Visualization, Writing – review & editing
Current address: Department of Physics and Astronomy, University of Tennessee, Knoxville, Tennessee, United States of America
Affiliation Department of Physics and Astronomy, University of Pennsylvania, Philadelphia, Pennsylvania, United States of America
Roles Conceptualization, Formal analysis, Investigation, Methodology, Software, Supervision, Validation, Visualization, Writing – review & editing
Affiliations Living Systems Institute, University of Exeter, Exeter, United Kingdom, Physics and Astronomy, College of Engineering, Mathematics and Physical Sciences, University of Exeter, Exeter, United Kingdom, Department of Physics, Harvard University, Cambridge, Massachusetts, United States of America
Roles Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing
Affiliations FAS Center for Systems Biology, Harvard University, Cambridge, Massachusetts, United States of America, Department of Molecular and Cellular Biology, Harvard University, Cambridge, Massachusetts, United States of America
Roles Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing
Affiliations School of Engineering and Applied Sciences, Harvard University, Cambridge, Massachusetts, United States of America, Department of Physics, Harvard University, Cambridge, Massachusetts, United States of America, FAS Center for Systems Biology, Harvard University, Cambridge, Massachusetts, United States of America, Department of Molecular and Cellular Biology, Harvard University, Cambridge, Massachusetts, United States of America
- Bryan T. Weinstein,
- Maxim O. Lavrentovich,
- Wolfram Möbius,
- Andrew W. Murray,
- David R. Nelson

- Published: December 1, 2017
- https://doi.org/10.1371/journal.pcbi.1005866
- See the preprint
- Reader Comments
We experimentally and numerically investigate the evolutionary dynamics of four competing strains of E. coli with differing expansion velocities in radially expanding colonies. We compare experimental measurements of the average fraction, correlation functions between strains, and the relative rates of genetic domain wall annihilations and coalescences to simulations modeling the population as a one-dimensional ring of annihilating and coalescing random walkers with deterministic biases due to selection. The simulations reveal that the evolutionary dynamics can be collapsed onto master curves governed by three essential parameters: (1) an expansion length beyond which selection dominates over genetic drift; (2) a characteristic angular correlation describing the size of genetic domains; and (3) a dimensionless constant quantifying the interplay between a colony’s curvature at the frontier and its selection length scale. We measure these parameters with a new technique that precisely measures small selective differences between spatially competing strains and show that our simulations accurately predict the dynamics without additional fitting. Our results suggest that the random walk model can act as a useful predictive tool for describing the evolutionary dynamics of range expansions composed of an arbitrary number of genotypes with different fitnesses.
Author summary
Population expansions occur naturally during the spread of invasive species and have played a role in our evolutionary history when humans migrated out of Africa. We use a colony of non-motile bacteria expanding into unoccupied, nutrient-rich territory on an agar plate as a model system to explore how an expanding population’s spatial structure impacts its evolutionary dynamics. Spatial structure is present in expanding microbial colonies because daughter cells migrate only a small distance away from their mothers each generation. Generally, the constituents of expansions occurring in nature and in the lab have different genetic compositions (genotypes, or alleles if a single gene differs), each instilling different fitnesses, which compete to proliferate at the frontier. Here, we show that a random-walk model can accurately predict the dynamics of four expanding strains of E. coli with different fitnesses; each strain represents a competing allele. Our results can be extended to describe any number of competing genotypes with different fitnesses in a naturally occurring expansion as long as the underlying motility of the organisms does not cause our model to break down. Our model can also be used to precisely measure small selective differences between spatially competing genotypes in controlled laboratory settings.
Citation: Weinstein BT, Lavrentovich MO, Möbius W, Murray AW, Nelson DR (2017) Genetic drift and selection in many-allele range expansions. PLoS Comput Biol 13(12): e1005866. https://doi.org/10.1371/journal.pcbi.1005866
Editor: Jeff Gore, MIT, UNITED STATES
Received: June 8, 2017; Accepted: November 1, 2017; Published: December 1, 2017
Copyright: © 2017 Weinstein et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All of the experimental data used to create the figures and tables in this paper and its supplemental materials can be publicly accessed in a Dryad repository at the following DOI: 10.5061/dryad.n9r96 .
Funding: Research by BTW is supported by the Department of Energy Office of Science Graduate Fellowship Program (DOE SCGF), made possible in part by the American Recovery and Reinvestment Act of 2009, administered by ORISE-ORAU under contract no. DE-AC05-06OR23100, by the US Department of Energy (DOE) under Grant No. DE-FG02-87ER40328, as well as Harvard University’s Institute for Applied Computational Science (IACS) Student Fellowship. BTW, AWM, and DRN benefitted from support from the Human Frontiers Science Program Grant RGP0041/2014 and from the National Science Foundation, through grants DMR1608501 and via the Harvard Materials Science and Engineering Center, through grant DMR1435999. MOL acknowledges support from NSF grant DMR-1262047, the UPenn MRSEC under Award No. NSF-DMR-1120901, the US Department of Energy, Office of Basic Energy Sciences, Division of Materials Sciences and Engineering under Grant No. DE-FG02-05ER46199, and from the Simons Foundation for the collaboration "Cracking the Glass Problem’’ (Grant No. 454945). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Introduction
A competition between stochastic and deterministic effects underlies evolution. In a well-mixed system such as a shaken culture of the yeast microorganism Saccharomyces cerevisiae , stochastic competition between individuals, mutations, and selection dictate the dynamics of the population [ 1 ]. In spatially structured environments, active or passive dispersal of individuals also plays an important role. The local “well-mixed” dynamics must be coupled to the motion of individuals, leading to strikingly different evolutionary dynamics, even in the absence of selection [ 2 – 7 ].
A model laboratory system that can be used to explore the coupling between local “well-mixed” effects and spatial deterministic and stochastic dynamics is a microbial range expansion [ 8 ], in which a population expands into an unoccupied region of a hard agar Petri dish. Non-motile microbes expand outwards from their initial position due to a combination of growth coupled with random pushing by neighboring cells and leave behind a record of their genetic competition as they cannot move and cease reproducing once the population becomes too dense [ 8 ]. A frozen genetic pattern of four competing strains of E. coli marked by different fluorescent colors can be seen in Fig 1 . Spatial structure is present in the frozen genetic patterns because the microbes at the expanding frontier produce daughter cells of the same color that migrate only a small fraction of the front circumference within a generation. Hallatschek et al. [ 8 ] identified the key role of genetic drift in producing these sectored patterns; the small population size at the front of an expanding population [ 9 , 10 ] enhances number fluctuations (i.e. genetic drift), eventually leading to the local fixation of one strain past a critical expansion radius R 0 . The decrease in genetic diversity as the small number of individuals at the frontier expands is referred to as the “Founder effect” [ 11 ].
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pcbi.1005866.g001
Outside of the laboratory, range expansions occur naturally during the spread of invasive species such as the bank vole in Ireland [ 12 ] or the cane toad in Australia [ 13 ], and played a role in the evolutionary history of humans when migrating out of Africa [ 14 ]. In these natural expansions, populations may have many competing genotypes, or alleles, each instilling a different fitness. Even if a population is originally clonal, mutations may create new alleles that compete with one another to proliferate, a phenomenon known as clonal interference [ 15 ].
An allele’s fitness is often determined by its corresponding expansion velocity. Faster expanding individuals will colonize more territory and will block slower strains from expanding, resulting in the increased abundance of ‘faster’ alleles at the frontier [ 13 , 16 , 17 ]. If the curvature of a microbial colony can be neglected and its front is sufficiently smooth, it has been shown both theoretically and experimentally that the domain wall of a faster expanding strain will displace a slower expanding strain at a constant rate per length expanded after an initial transient, resulting in a characteristic triangular shape [ 17 ] as shown on the right side of Fig 1 . If the curvature of the expansion is not negligible, the sector boundaries will trace logarithmic spirals [ 17 ].
Even in the most simple scenario when de-novo mutations and mutualistic or antagonistic interactions are ignored, the dynamics of many competing alleles with varying fitnesses at the front of a range expansion have neither been quantified theoretically nor explored in laboratory experiments. Prior laboratory experiments focused on the dynamics of a single sector of a more fit strain (representing a competing alelle) of yeast sweeping through a less fit strain [ 17 ] in regimes where stochastic wandering of genetic boundaries was not expected to be important. Recent experimental work studied how fast a single more fit strain swept through a less fit strain in a range expansion and compared the dynamics to the same strains in a well mixed test tube [ 9 ].
In this paper, we experimentally and numerically investigate the dynamics of four competing strains (alleles) of E. coli with varying selective advantages initially distributed randomly at the front of a radial range expansion. The eCFP (blue) and eYFP-labeled (yellow) strains expanded the fastest, followed by the non-fluorescent (black) strain, and finally the mCherry-labeled (red) strain. The differences in expansion speeds are reflected in Fig 1 as follows: the yellow/blue bulges at the front of the expansion are larger than the black bulges which are larger than the red bulges. The significant random undulations at the frontier, however, significantly mask the selection-induced bulges.
Experimental results
We begin by reporting our measurements of the average fraction of each strain, the two-point correlation functions between strains, and the relative rates of annihilations and coalescences as a function of length expanded for our four competing strains of E. coli . As discussed in the Materials and Methods, we found that our eCFP and eYFP strains had the fastest expansion velocities followed by the black strain and finally the mCherry strain (see Table 1 ). We expected that our experimental measurements would reflect this hierarchy of speeds; faster expanding strains should have a larger fitness than slower expanding ones. To illustrate the presence of selection, we used neutral theory (discussed in detail in S1 Appendix ) as a null expectation; selection caused deviations from the neutral predictions. To calibrate neutral theory to our experiments we fit R 0 and D w , two model parameters illustrated in Fig 1 , following the procedures discussed in the Materials and Methods. The fit values of R 0 and D w can be seen in Table 2 . In later sections, we show how to predict the average fraction, two-point correlation functions, and relative rates of annihilation and coalescences using our random-walk model and simulation.
https://doi.org/10.1371/journal.pcbi.1005866.t001
https://doi.org/10.1371/journal.pcbi.1005866.t002
Average fractions.
We measured the average fraction versus radial length expanded in two separate sets of experiments where we inoculated different fractions of our eYFP, eCFP, and mCherry strains. In one experiment, we inoculated the eYFP, eCFP, and mCherry strains with equal initial fractions of 33% while in the other we inoculated 80% of the mCherry strain and 10% each of the eCFP and eYFP strains. We conducted 20 replicates in each case and calculated the average fraction of each strain using our image analysis package. Fig 2 displays the trajectories of the 20 expansions and the mean trajectory (the average fraction) as ternary composition diagrams for both sets of initial conditions [ 37 ].
The red dot indicates the composition at the radius R 0 = 3.50 mm where distinct domain walls form and the blue dot indicates the composition at the end of the experiment. The red dots are dispersed about the initial inoculated fractions due to the stochastic dynamics at the early stages of the range expansions when R < R 0 . The highly stochastic trajectories illustrate the importance of genetic drift at the frontier in the E. coli range expansions. The smaller ternary diagrams display the average fraction over all expansions vs. length expanded for each set of experiments. For both initial conditions, we see a small systematic drift away from the mCherry vertex indicating that the mCherry strain has a lower fitness, in agreement with the independent radial expansion velocities of each strain (see Table 1 ). Note that two replicates on the right resulted in the complete extinction of eCFP due to strong spatial diffusion, indicated by the trajectories pinned on the absorbing line connecting the eYFP and mCherry vertices.
https://doi.org/10.1371/journal.pcbi.1005866.g002
In both sets of experiments, we observed a systematic drift away from the mCherry vertex as a function of radius as illustrated by the mean trajectories shown as insets. We witnessed two cases where the 10% initial inoculant of the eCFP strain became extinct, represented by the pinning of trajectories to the absorbing boundary connecting the eYFP and mCherry vertex, a consequence of the strong genetic drift at the frontiers of our E. coli range expansions. These measurements indicate that the mCherry strain was less fit than the eCFP and eYFP strains, consistent with the order of the radial expansion velocities.
Two-point correlation functions.
We measured the correlation functions between each pair of strains in three sets of experiments where we inoculated equal well-mixed fractions of the eCFP, eYFP, and black strains, then eCFP, eYFP, and mCherry, and then finally all four strains. We conducted 20 replicates of each experiment, measured all two-point correlation functions at the final radius of R = 10 mm corresponding to a length expanded of L = R − R 0 = 6.5 mm, and averaged the results. In Fig 3 , we plotted the neutral correlation function prediction and compared it to the experimentally measured correlation functions.
The shaded regions in these plots indicate standard errors of the mean. Using the measured diffusion coefficient D w and initial radius where domain walls form R 0 (see Table 2 ), we also plot the theoretical neutral two-point correlation functions (black dashed line; see eq. (S1.3)). The colors of each plotted correlation function were chosen to correspond to their composite strain colors; for example, two-point correlation correlation functions associated with mCherry were red or were blended with red. The subscripts correspond to the color of each strain: C = eCFP, Y = eYFP, R = mCherry, and B = Black. As judged by the magnitude of the deviation from neutral predictions, the black strain has a small selective disadvantage relative to eCFP and eYFP and the mCherry strain has an even greater disadvantage, in agreement with the independent radial expansion velocities of each strain (see Table 1 ).
https://doi.org/10.1371/journal.pcbi.1005866.g003
The two-point correlation functions in the experiment between eCFP, eYFP, and the black strains (first column of Fig 3 ) are consistent with the order of radial expansion velocities (see Table 1 ). The correlation between the eCFP and eYFP strains plateaued at a higher value than the neutral prediction while the correlation between eCFP and black plateaued at a lower value, indicating that the eCFP and eYFP strains were more fit. The self-correlation for the black strain, F BB , also plateaued at a value below eCFP, eYFP, and the neutral prediction, further indicating that it had a smaller fitness. The self-correlation data was more noisy than the correlation between strains, however; we consistently found that correlations between strains were better at detecting fitness differences than self-correlations.
In contrast, combining eCFP, eYFP, and mCherry in one set of experiments and all four strains in another revealed that mCherry had a larger fitness defect. Correlation functions including mCherry always plateaued at a significantly smaller value than correlation functions excluding it. Furthermore, off-diagonal (bottom-row of Fig 3 ) correlation functions involving the mCherry strain had a smaller slope at zero angular separation, indicating that less mCherry domain walls were present and that the mCherry strain was less fit than the others. The two-point correlation functions were thus consistent with the black strain having a small selective disadvantage relative to eCFP and eYFP and the mCherry strain having a larger disadvantage relative to all others.
Annihilation asymmetry.
To gain insight into the behavior of Δ P , for the case of q neutral colors in equal proportions, we have lim q →∞ Δ P ( q ) = −1 (only coalescences), Δ P ( q = 3) = 0 (equal numbers of annihilations and coalescences), and Δ P ( q = 2) = 1 (only annihilations). The quantity Δ P thus provides a simple way to characterize the annihilation/coalescence difference in a single curve that varies smoothly between −1 and 1 as 2 ≤ q < ∞. In S1 Appendix we develop and discuss the case when strains are inoculated in non-equal proportions (see supplementary equations (S1.8)–(S1.10)); in that scenario, it is useful to define a “fractional q ” by inverting eq (3) to read q = (3 + Δ P )/(1 + Δ P ) (i.e. a fractional q can be evaluated for a given Δ P ).
To experimentally quantify the annihilation asymmetry, we examined the average cumulative difference in annihilations and coalescences vs. the average cumulative number of domain wall collisions as colonies expanded; Δ P is given by the slope of this quantity and can be seen in Fig 4 (see Supplementary S1 Fig for a display of cumulative count vs. length expanded). Regardless of which strains were inoculated and their selective differences, our results were consistent with the neutral theory prediction in eq (3) for q = 2, q = 3, and q = 4 as judged by the overlap of the black dashed line with the shaded standard error of the mean in each case. Δ P appeared to be constant as a function of length. We also tested an initial condition where we inoculated strains in unequal proportions: we inoculated 10% of eCFP and eYFP and 80% of mCherry. This experiment again matched the neutral prediction of Δ P ≈ 0.51 (and correspondingly q ≈ 2.33) within error. Evidentally, as discussed in more detail below, certain observables like the average fraction and two-point correlation functions show stronger signatures of selection than others like the annihilation asymmetry.
The slope of this plot gives the annihilation asymmetry Δ P . The shaded regions represent the standard error of the mean between many experiments. We use the notation C = eCFP, Y = eYFP, B = black, and R = mCherry. Despite the presence of selection, Δ P was consistent with the standard neutral theory prediction of eq (3) for q = 2, q = 3, and q = 4 (equal initial fractions of q strains), as judged by the overlap of the black dashed lines with the shaded areas in every case. We also explored an initial condition where we inoculated unequal fractions of three strains; we inoculated 10% of both eCFP and eYFP and 80% of mCherry. Our experiments agreed with the prediction of Δ P ≈ 0.51, or an effective q ≈ 2.33, from the neutral theory developed in supplementary equations (S1.8)–(S1.10).
https://doi.org/10.1371/journal.pcbi.1005866.g004
Simulation results
Key parameters.
If κ ≳ 1, inflation does not appreciably slow selective sweeps as L I approaches the linear selection length scale L s . In contrast, if κ ≪ 1, the inflationary selection length scale L I will be many times larger than the linear selection length scale L s , indicating that selection will be weak compared to inflation and diffusion (but will ultimately dominate at very large lengths expanded). The three black points correspond to measurements of the κ ij that govern the dynamics of our competing strains; N stands for the two selectively neutral strains (eCFP and eYFP), B for black, and R for mCherry (red). See the Predicting experimental results with simulation section for more details.
https://doi.org/10.1371/journal.pcbi.1005866.g005
Collapsing the evolutionary dynamics with the key parameters.
https://doi.org/10.1371/journal.pcbi.1005866.g006
We now consider the collapsed curves F ( L / L s , κ ) and Δ P ( L / L s , κ ) as a function of the parameter κ as seen in Fig 6 . κ had a pronounced effect on both quantities. For κ ≳ 5 the dynamics of F and Δ P approached the dynamics of a linear expansion at all L / L s , illustrated by the bright pink line on the left and the bright pink dots on the right of Fig 6 ; the more fit strain swept so quickly through the less fit strain that the colony’s radial expansion could be ignored. As κ decreased, the less fit strain was squeezed out more slowly due to the inflation of the frontier, resulting in slower transitions from q = 3 to q = 2 colors and consequently slower transitions from Δ P = 0 to Δ P = 1. For κ ≪ 1, Δ P barely shifted from 0 over the course of the simulation. Interestingly, Δ P peaked at a finite L / L s for small κ ; it is not clear what causes this effect, but it may be related to the transition from linear to inflation-dominated dynamics as L increases.
Predicting experimental results with simulations
A major goal of this paper is to test if the annihilating and coalescing random-walk model can predict the experimental evolutionary dynamics of our four competing strains (alleles) with different fitnesses (radial expansion velocities). To the best of our knowledge, analytical results for the random-walk model are unavailable (as discussed in S1 Appendix ); we consequently used our simulations to predict the dynamics. In this section we quantify the three key parameter combinations for our experimental expansions and then use them to predict the evolutionary dynamics of all four of our competing E. coli strains in an independent experiment.
https://doi.org/10.1371/journal.pcbi.1005866.g007
To determine the best-fitting value of L s , we calculated the sum of the squared displacements weighted by the inverse of the experimental standard error squared between experiment and simulation. The best-fitting L s was determined by finding the value which minimized the weighted sum of squares. To estimate the error in our fit, we assigned each potential value of L s a probability proportional to the inverse of the weighted sum of squares, normalized the probability distribution, and set the error in our fit of L s to the confidence intervals of the probability distribution.
“CI” stands for confidence interval.
https://doi.org/10.1371/journal.pcbi.1005866.t003
To test that the resulting L s and κ could accurately predict the experimental dynamics at all L and not just the L where the correlation functions were fit, we plotted the experimental average fraction and correlation functions (solid lines, Fig 8 ) as we varied L and compared their values to those predicted by simulation (dashed lines, Fig 8 ). Fig 8 uses the same set of experimental data as that from Fig 7 . The simulation using the fit parameters always closely tracked the experimental values at all L , suggesting that our fitting technique was robust and could be used to describe the dynamics of our strains.
The shaded region is the standard error of the mean. The simulated dynamics closely match the experimental dynamics, suggesting that our fitting technique to extract L s is robust and can be used to describe the dynamics of our strains at all L .
https://doi.org/10.1371/journal.pcbi.1005866.g008
No additional fitting parameters were used. The shaded region is the standard error of the mean. The simulated dynamics closely matched the experimental dynamics except at small lengths expanded ( L ≲ 3 mm) where the black strain introduced significant image analysis artifacts (see Supplementary S5 Fig ).
https://doi.org/10.1371/journal.pcbi.1005866.g009
The quantitative agreement between our model and our experiments suggests that the one-dimensional annihilating-coalescing random walk model can indeed be used to predict the dynamics of many competing strains with different fitnesses in a range expansion.
https://doi.org/10.1371/journal.pcbi.1005866.t004
Materials and methods
We used four E. coli strains (labelled BW001, BW002, BW003, and BW012) with a DH5 α background and plasmids whose sequences coded for spectrally distinguishable fluorescent proteins. The unique colors were obtained by using the plasmid vector pTrc99a [ 39 ] and the open reading frame for the respective fluorescent proteins. Strains BW001, BW002, and BW003 expressed eCFP (cyan/blue), Venus YFP (yellow), and mCherry (red) respectively, and were identical to the E. coli strains eWM282, eWM284, and eWM40 used in Ref. [ 40 ]. Note that these three strains were isogenic and differed only by the open reading frames corresponding to their respective fluorescent proteins. The final strain, BW012, was a mutated descendant of strain BW002 (yellow) that fluoresced at a decreased intensity, appearing black, while retaining its ampicillin resistance from the pTrc99a vector. Throughout this work, no additional mutations were introduced or observed. We therefore consider that these four strains correspond to four different alleles. Throughout the paper, we refer to the strains as eCFP, eYFP, mCherry, and black.
Experimental setup
To prepare saturated cultures, strains were inoculated in 10mL of 2xYT media and were shaken for approximately 16 hours at 37°C. After vortexing each saturated culture and obtaining their concentration via optical density (OD-600) measurements, appropriate volumes (e.g., 1:1:1 mixtures of three strains) were added to an Eppendorf tube with a final volume of 1mL. The Eppendorf tube was then vortexed to uniformly mix the strains. A volume of 2 μ L was taken from the vortexed tube and placed on center of a 100 mm diameter Petri dish containing 35 mL of lysogeny broth (LB), ampicillin at a concentration of 100 μ g/mL, and 1.25% w/v bacto-agar. The carrier fluid in the resulting circular drop evaporated within 2-3 minutes, depositing a circular “homeland” of well-mixed bacteria onto the plate.
After inoculation, plates were stored for 8 days upside down (to avoid condensation) in a Rubbermaid 7J77 box at 37°C with a beaker filled with water; the water acted as a humidifier and prevented the plates from drying out. The plates were occasionally removed from the box and imaged (at roughly 24 hour intervals) using the brightfield channel to determine the radius of the colony as a function of time. On the eighth day, the plates were imaged in both fluorescent and brightfield channels. The number of replicate plates used are stated next to the respective experimental results. If we noticed that a mutation had occurred during an expansion (mutations usually presented themselves as unexpected large bulges at the front of a colony or as distortions in fluorescent intensity), we discounted the colony.
Image acquisition and analysis
We imaged our range expansions with a Zeiss SteREO Lumar.V12 stereoscope in four channels: eCFP, eYFP, mCherry (fluorescent channels), and brightfield. In order to analyze a colony with a maximum radius of approximately 10 mm using a single image, we stitched four images together with an overlap of 20% using AxioVision 4.8.2, the software accompanying the microscope. We blended the overlapping areas of the images to lessen the impact of background inhomogeneities. An example of a stitched image can be seen on the left side of Fig 10 . Stitching introduced small artifacts such as vertical lines near the center of our expansions; we verified that these did not affect our results.
Images were acquired for four overlapping quadrants and stitched together to obtain a single image with a large field of view. Overlapping regions were blended to minimize inhomogeneities. To obtain the binary masks, pixels with fluorescence above background noise were marked as “on.” A visual comparison of the raw data and the masks confirm that our binary masks accurately reflect the location and shape of individual sectors.
https://doi.org/10.1371/journal.pcbi.1005866.g010
To extract the local fraction of each strain per pixel, we first created binary masks for each fluorescence channel indicating if the corresponding E. coli strain was present. We utilized the “Enhance Local Contrast” (CLAHE) algorithm [ 41 ] in Fiji [ 42 ], an open-source image analysis platform, to help correct for inhomogeneities in background illumination. After applying the CLAHE algorithm, a combination of automatic thresholding and manual tracing yielded a binary mask of each channel, an example of which is shown in Fig 10 ; the image on the left is an overlay of an experimental range expansion’s fluorescent channels and the image on the right is the overlay of the corresponding binary masks. A small amount of manual tracing was required near the edges of our colonies because our fluorescent lamp provided uneven illumination; resulting dark regions could barely be identified above background noise. As we mainly used manual tracing near the edge of the colonies where the monoclonal sectors were well defined, we found that our procedure was very reproducible. To alleviate this problem, future work could utilize brighter strains or a more advanced imaging setup.
We mapped the binary images to the local fraction of each E. coli strain in the following way: if N binary masks (corresponding to N colors) were “on” at a pixel, the local fraction of their corresponding channels was assigned to be 1/ N . Although this assignment produces inaccuracies (i.e., if one strain occupied 90% of a pixel and the other occupied 10%, our algorithm would register both as 50%), domain boundaries were the only areas besides the homeland and the early stages of the range expansions where multiple strains were colocalized. The black strain was defined to be present at pixels reached by the range expansion in which no other strains were present. Although this definition introduced errors at radii close to the homeland with significant color overlap, the error became negligible at large radii as quantified in Supplementary S5 Fig . Once we determined the fraction of each strain at each pixel, we were able to extract quantities such as the total fraction of each strain in the colony and spatial correlations between strains at a given expansion radius.
The mask in Fig 10 highlights that sector boundaries can be used to determine local strain abundance. Although it is possible to extract the position of every domain wall from each strains’ local fraction, it is challenging to actually track a single wall due to collisions between walls. To address this problem, we created a binary mask of the edges in our images and labelled the edges of each domain. Annihilations and coalescences were counted manually within Fiji [ 42 ]; automated measures were not accurate enough.
It is worth pointing out that in this paper, we ignore the three-dimensional structure of our colonies and describe them by our two-dimensional images taken with the stereoscope. We justify this approximation because the initial diameter of our colonies is at least a factor of 10 larger than their height (less than 1 mm as judged by a ruler), so they are effectively two-dimensional, and because the strain composition of our colonies does not vary with height inside the colony. We confirmed that strain composition does not vary with height by using a confocal microscope to probe the internal structure and also by taking a pipette tip, scratching it through a sector, growing the cells touched by the tip in overnight culture, and verifying that plated single colonies from the culture were the same color as the sector.
Measuring radial expansion velocities u i
We used the average expansion velocity of each strain for radii R > R 0 as a proxy for selective advantage, similar to previous work [ 17 , 35 ]. In three independent sets of experiments using different batches of agar plates (the main source of variability in our experiments), we measured the diameter of 12 expansions of each strain approximately every 24 hours following the protocol for range expansions with two or more strains. To account for biological variance, sets of four of the 12 colonies were created from independent single colonies; no statistical difference was seen between biological replicates. The diameters were determined by manually fitting a circle to a brightfield image of the expansion three times and averaging the measured diameters. Fig 11 shows the average radius increasing with time for each strain from one of our experiments. In every experiment, the eCFP and eYFP strains had the fastest expansion velocities (the respective datapoints overlap in Fig 11 ), followed by the black strain, and then finally the mCherry strain. The expansion velocity slowly decreased as a function of time; we attribute this to nutrient depletion in the plates.
The error bars (comparable to symbol size at early times) are the standard errors of the mean calculated from 12 replicate expansions for each strain. The eYFP and eCFP strains had the fastest expansion velocities (data points overlap in the plot) followed by black and then mCherry. R 0 is the radius at which expansions with competing strains typically demix into one color locally; R 0 is approximately 1.75 times the initial inoculant radius of 2 mm (see Fig 1 ).
https://doi.org/10.1371/journal.pcbi.1005866.g011
The radial expansion velocity of each strain was obtained by using linear regression to fit the radius versus time for radii greater than R 0 . We calculated the average radial expansion velocity between the three sets of plates and reported its error as the standard error of the mean; see Table 1 . Additionally, we quantified the dimensionless selective advantage of each strain relative to the slowest growing mCherry strain following [ 17 ] via s iR = u i / u R − 1 where the R indicates the mCherry strain (red) in each experiment. The selective advantages were consistent, within error, when we calculated the velocities u i and u R over different time intervals. We averaged s iR across our three experiments and reported its error as the standard error of the mean as seen in Table 1 .
The eCFP and eYFP strains had an average selective advantage of 9%, similar to the experiments of Weber et al. [ 35 ] which found, despite the fact that they used different E. coli strains and plasmids, that the expression of mCherry decreased the expansion velocity of their strains by approximately 15% in certain “fast growth” environmental conditions. Our black strain had an approximately 6% enhancement over the mCherry strain. Differences in radial expansion velocities of this magnitude have been used to study yeast S. cerevisiae and E. coli range expansions in the past [ 9 , 17 ]. To investigate the source of this fitness defect, we took the plasmids from our original strains, inserted them into a different set of clonal DH5 α cells, and inoculated the new eCFP, eYFP, and mCherry strains in equal proportions in a range expansion. We saw that the average mCherry fraction decreased by 10% at a radius expanded of R = 10 mm, matching the results of Fig 2 , suggesting that the presence of the plasmids was responsible for the fitness defect.
Comparing well-mixed fitness to fitness from expansion velocities

Measuring the local fixation radius R 0
When calibrating our model to experiment, the precise value of R 0 did not matter as long as each strain’s local fraction could be accurately measured at that radius. Therefore, to maximize the length over which we could quantify range expansion growth, we defined the local fixation radius R 0 as the minimum radius where our image analysis package became accurate. For R < R 0 , our package predicted equal fractions of each strain due to the overlap of each channel in the homeland (see Fig 10 ). Therefore, to determine R 0 , we inoculated radial expansions with three strains in unequal proportions; we used 10% of two strains and 80% of another. The minimum radius where the fractions agreed with their inoculated values was R 0 = 3.50 ± 0.05 mm as seen in Supplementary S6 Fig . We found that this value of R 0 worked for all colonies.
Measuring the domain wall diffusion coefficient D w
We fit H ( ϕ , L ) to our experimentally measured heterozygosity of two neutral strains (eCFP and eYFP) on three independent sets of agar plates each with 14 range expansions. We averaged the heterozygosity at each L as can be seen in Fig 12 (error bars were omitted for readability; the same figure with error bars can be found as Supplementary S7 Fig ). As we had previously measured R 0 = 3.50 ± 0.05 mm, and H 0 = 1/2 for two neutral strains inoculated at equal fractions, D w is the single free parameter in eq (11) . We consequently fit D w at each L with non-linear least-squares, averaged the D w from the three independent experiments, and found D w = 0.100 ± 0.005 mm; the reported error is the standard error of the mean between the experiments. The value of the diffusion constant is on the same order of magnitude as that from previous work [ 18 ].
The dashed lines are the theoretical fits of the heterozygosity with a constant D w = 0.100 ± 0.005 mm. The theoretical curves track our experimental data, suggesting that a diffusive approximation to domain boundary motion is justified.
https://doi.org/10.1371/journal.pcbi.1005866.g012
Fig 12 shows the Voter model’s fit (dashed lines) together with the experimental heterozygosity (solid lines) for one set of plates using our values of D w and R 0 . The fit closely matches the experimental heterozygosity suggesting that a diffusive description of E. coli domain motion is justified. We use this value of D w for all strains. In principle, D w may depend on ij , the particular domain wall type. However, we checked that the measured value of D w did not vary for our all ij (all strain) combinations by examining the variance in domain wall position versus length expanded; the variances agreed within error and were thus consistent with a constant D w . The two-point correlation functions in the main-text were well fit by a constant D w as well. Unlike the Voter model and our simulations, the experimental heterozygosity at zero separation H ( L , ϕ = 0) fails to vanish due to overlap between strains at domain boundaries; this effect is less pronounced at large radii because the effective angular width of boundaries decreased. The discrepancy between the theoretical and experimental heterozygosity is larger at small lengths expanded because the overlap between strains is larger; our image analysis is consequently less accurate.
Measuring the domain wall velocities
https://doi.org/10.1371/journal.pcbi.1005866.g013
Simulation methods
Lattice simulations of range expansions, especially radial ones, can suffer from artifacts arising from the preferred directions of the lattice. It is possible to use an amorphous Bennett model lattice [ 44 ] to mitigate some of these effects [ 32 ]. Instead, we developed a simple off-lattice method that treats the domain walls as annihilating and coalescing random walkers moving along the edge of an inflating ring. The basic idea of the simulation is illustrated in Fig 14 . We incorporate both the random, diffusive motion of the domain walls as well as deterministic movement due to selection. The radial expansion procedure is most easily understood by first considering a linear range expansion simulation for which the simulation steps are as follows:
- Create a line of N 0 microbes of width a at the linear frontier. Assign each microbe one of the q potential alleles.
- Identify genetic domain walls by locating neighbors with different alleles; assign type ij to each wall where i and j are the strains to the left and right respectively. Assign a relative “growth rate” r ij to each wall characterizing the bias in the probability that strain i divides into new territory before strain j . Two such domain walls are shown in a radial expansion in Fig 14 .
- (b) If the hopping domain wall collides with another wall, react the walls instantaneously with an appropriate annihilation or coalescence depending on whether the leftmost and rightmost strains are the same or different respectively.
- Increment the elapsed time by Δ t = 1/ N generations, where N is the number of domain walls at the beginning of the jump, and increment the length expanded by the colony by Δ L = a Δ t = a / N , where a (the cell width) is the distance that the colony expands per generation. Note that this length increment Δ L could also be some set by a different length scale d , i.e. Δ L = d / N (in our experiments, colonies typically expand further than a cell width during each generation due to growth behind the front). This does not change our analysis and we choose d = a for simplicity.
- Repeat steps 3 and 4 until no domain walls remain or until the simulation has run for the desired number of generations.
The initial population is a circle of cells of radius R 0 = N 0 a /2 π , where N 0 is the initial number of cells and a is a cell width. During each time step (generation), the expansion advances a distance a ; the radius consequently grows according to R ( t ) = R 0 + at where t is the time in generations. The dashed circle shows the population after one generation time. Each domain wall position is tracked on the inflating ring (solid lines). At each time step, domain walls (two shown) hop to the left or right with probability P l and P r , respectively, with an angular jump length δϕ ≡ a / R ( t ), and the position is updated (dashed lines). After each domain wall movement, the time in generations is incremented by 1/ N where N is the number of domain walls present. For a linear simulation, the radius is simply not inflated in time, i.e. R ( t ) = R 0 .
https://doi.org/10.1371/journal.pcbi.1005866.g014
In contrast to algorithms that follow the position and state of every organism at the front of a colony, our algorithm only tracks the positions of domain walls and is consequently much faster per generation as the sectors coarsen, allowing for simulations of larger colonies. Fig 15 displays a radial and linear simulation with three neutral colors and a fourth red color with a selective disadvantage comparable to our experiments. We check that our simulation correctly reproduces the behavior of a single more fit domain wall sweeping through a less fit strain as we vary simulation parameters in Supplementary S8 Fig . Our implementation of this algorithm and examples of how to use it are available on GitHub [ 34 ].
https://doi.org/10.1371/journal.pcbi.1005866.g015
Supporting information
S1 appendix. supplemental theory..
https://doi.org/10.1371/journal.pcbi.1005866.s001
S2 Appendix. Quantifying the discrepancy between radial expansion velocity and wall velocity.
https://doi.org/10.1371/journal.pcbi.1005866.s002
S1 Fig. Average cumulative annihilations and coalescences for two, three, and four strains.
All strains were inoculated in equal fractions except for the experiment with 10% of eCFP, 10% of eYFP and 80% of mCherry. The annihilation and coalescence rates (the slope of the respective curves) decrease as radius increases as there are less domain walls due to previous collisions and also because inflation decreases the probability of two walls colliding per length expanded. As the number of colors increases, coalescences occur more often than annihilations.
https://doi.org/10.1371/journal.pcbi.1005866.s003
S2 Fig. Collapse of F ij .
https://doi.org/10.1371/journal.pcbi.1005866.s004
S3 Fig. Collapsed average fraction and annihilation asymmetry on a linear scale.
Identical to Fig 6 except the y -axis of F ( L / L s , κ ) is placed on a linear scale, which may be useful for comparison with experiments.
https://doi.org/10.1371/journal.pcbi.1005866.s005
S4 Fig. Collapse of average fraction and annihilation asymmetry.
https://doi.org/10.1371/journal.pcbi.1005866.s006
S5 Fig. Image processing artifacts introduced by using a non-fluorescent (i.e. black) strain.
To estimate the image analysis artifacts introduced by using a non-fluorescent, black strain we performed an experiment with three fluorescent strains (eCFP, eYFP, and mCherry in equal initial proportions) and analyzed the data twice: once where we included all three fluorescent channels and once where we excluded the eCFP channel and treated it as if it were a black strain. We compared the black-substituted average fractions F i (the dashed lines) to the real fractions as a function of radius (the solid lines). At a small radius relative to R 0 = 3.5 mm, the error from introducing a black strain was large; this is likely because we defined black as the absence of any other channels and channels typically had large overlaps close to the homeland. At large radius, the error from introducing a black strain was negligible.
https://doi.org/10.1371/journal.pcbi.1005866.s007
S6 Fig. Determining R 0 .
To fit the radius R 0 where our image analysis package became accurate, we inoculated 80% of mCherry, 10% of eCFP, and 10% of eYFP in 10 range expansions and tabulated the average fraction of each strain. The inoculated fractions are illustrated by dashed lines. As seen in the plot, at a radius of approximately R 0 = 3.50 ± 0.05 mm the measured average fractions were closest to the inoculated fractions. Our image analysis package inaccurately predicted fractions in the homeland because of significant overlap between the strains.
https://doi.org/10.1371/journal.pcbi.1005866.s008
S7 Fig. Error bars when fitting D w .
The same as the right side of Fig 12 except with error bars; the shaded areas are the standard error of the mean.
https://doi.org/10.1371/journal.pcbi.1005866.s009
S8 Fig. Confirming simulation accuracy.
https://doi.org/10.1371/journal.pcbi.1005866.s010
Acknowledgments
BTW would like to thank Matti Gralka, Paula Villa Martin, Miguel A Muñoz, Severine Atis, Markus F. Weber, Kirill Korolev, and Steven Weinstein for helpful discussion and advice.
- View Article
- PubMed/NCBI
- Google Scholar
- 11. Hartl DL, Clark AG. Principles of Population Genetics. 4th ed. Sunderland, MA: Sinauer Associates, Inc.; 2007. Available from: http://www.sinauer.com/principles-of-population-genetics.html .
- 21. Krapivsky PL, Redner S, Ben-Naim E. A Kinetic View of Statistical Physics. Cambridge: Cambridge University Press; 2010. Available from: http://ebooks.cambridge.org/ref/id/CBO9780511780516 .
- 34. Weinstein B. Range Expansions on GitHub; 2016. Available from: https://github.com/Range-Expansions .
- 37. Harper M. Python-ternary: A python library for ternary plots; 2011. Available from: https://github.com/marcharper/python-ternary .
- 41. Zuiderveld K. Contrast limited adaptive histogram equalization. Graphics Gems IV. 1994; p. 474–485. https://doi.org/10.1016/B978-0-12-336156-1.50061-6
- Introduction
- Conclusions
- Article Information
A, Odds ratios for AF in UK Biobank cohort are stratified by PRS quintile and carrier status of rare predicted loss-of-function (pLOF) variants in AF-associated genes. The group with a low PRS (0%-20%) and no pLOF variant is considered as the reference, with the base line representing the reference odds ratio of 1.0. B, Absolute risk of AF in shown by age, based on PRS quintile and carrier-status of rare pLOF variants in AF-associated genes. Date of inclusion was used as the index date.
Absolute 10-year risk of AF by sex. The top 2 subpanels show the 10-year risk of AF for individuals with and without a predicted loss-of-function (pLOF) variant in AF-associated genes. The bottom 4 subpanels show the 10-year risk of AF in individuals according to 2 common risk factors: obesity and hypertension. The individual tiles denote absolute risk of AF within 10 years. Q indicates quintile.
Estimates from cause-specific Cox regressions according to predisposition for AF. First-incident AF, cardiomyopathy, heart failure, and all-cause mortality were considered competing events. The figure shows results from the full cohort and subgroup analyses on individuals diagnosed with AF, or cardiomyopathy or heart failure during follow-up.
eTable 1. Odds ratio for AF according to PRS and pLOF variants
eTable 2. Odds ratio for AF according to PRS and pLOF variants (unrelated individuals)
eTable 3. Variant carriers in study cohort for incident AF, HF and cardiomyopathy
eTable 4. Hazard ratios ratio for incident AF according to genetic risk and clinical risk factors
eTable 5. Cumulative incidence of AF by age 80
eTable 6. Cumulative incidence of AF by age 70
eTable 7. Cumulative incidence of AF by age 60
eTable 8. Cumulative incidence of AF by age 80 (unrelated individuals)
eTable 9. Cumulative incidence of AF by age 80 (excluding TTN pLOF variants)
eFigure 1. Flowchart of study design
eFigure 2. Manhattan plot of gene-based test for rare pLOF variants
eFigure 3. Quantile-Quantile plot of gene-based test for rare pLOF variants
eFigure 4. Cardiac expression of AF associated genes
eFigure 5. Tissue-specific RNA expression of C10orf71
eFigure 6. Results from gene-based association test with AF in independent replication cohort
eFigure 7. Ten-year risk of AF (unrelated individuals)
eFigure 8. Ten-year risk of AF (excluding TTN pLOF variants)
eFigure 9. Forest plot of hazard ratios for AF, cardiomyopathy, and HF (unrelaed individuals)
eFigure 10. Forest plot of hazard ratios for AF, cardiomyopathy, and HF (30-day grace period)
eReferences
eTable 10. Gene-based burden test (pLOF variants)
eTable 11. Variants in gene masks (pLOF variants)
eTable 12. Gene-based burden test (missense variants, additive model)
eTable 13. Gene-based burden test (missense variants, ACATO model)
eTable 14. LOVO analysis (pLOF variants)
eTable 15. LOVO analysis (missense variants)
eTable 16. Gene-based burden test, excluding cardiomyoopathy (pLOF variants)
eTable 17. Gene-based burden test, excluding cardiomyopathy (missense variants)
Geisinger MyCode Community Health Initiative and Regeneron Genetics Center (RGC) Research Team members
Data sharing statement
See More About
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Others Also Liked
- Download PDF
- X Facebook More LinkedIn
Vad OB , Monfort LM , Paludan-Müller C, et al. Rare and Common Genetic Variation Underlying Atrial Fibrillation Risk. JAMA Cardiol. Published online June 26, 2024. doi:10.1001/jamacardio.2024.1528
Manage citations:
© 2024
- Permissions
Rare and Common Genetic Variation Underlying Atrial Fibrillation Risk
- 1 Department of Cardiology, The Heart Centre, Copenhagen University Hospital – Rigshospitalet, Copenhagen, Denmark
- 2 Department of Biomedical Sciences, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark
- 3 Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Denmark
- 4 Regeneron Genetics Center, Tarrytown, New York
Question What is the combined contribution of rare and common genetic variation to atrial fibrillation (AF) risk?
Findings In this genetic association study, rare genetic variants, predicted to cause loss of function, in 6 genes were associated with AF. Together, these rare variants and a polygenic risk score for AF were associated with a considerable risk of incident atrial fibrillation; rare variants were also associated with heart failure and cardiomyopathy, and a higher risk of cardiomyopathy following AF diagnosis.
Meaning The findings suggest that assessing both rare and common genetic variation may aid in atrial fibrillation prevention and risk stratification.
Importance Atrial fibrillation (AF) has a substantial genetic component. The importance of polygenic risk is well established, while the contribution of rare variants to disease risk warrants characterization in large cohorts.
Objective To identify rare predicted loss-of-function (pLOF) variants associated with AF and elucidate their role in risk of AF, cardiomyopathy (CM), and heart failure (HF) in combination with a polygenic risk score (PRS).
Design, Setting, and Participants This was a genetic association and nested case-control study. The impact of rare pLOF variants was evaluated on the risk of incident AF. HF and CM were assessed in cause-specific Cox regressions. End of follow-up was July 1, 2022. Data were analyzed from January to October 2023. The UK Biobank enrolled 502 480 individuals aged 40 to 69 years at inclusion in the United Kingdom between March 13, 2006, and October 1, 2010. UK residents of European ancestry were included. Individuals with prior diagnosis of AF were excluded from analyses of incident AF.
Exposures Rare pLOF variants and an AF PRS.
Main Outcomes and Measures Risk of AF and incident HF or CM prior to and subsequent to AF diagnosis.
Results A total of 403 990 individuals (218 489 [54.1%] female) with a median (IQR) age of 58 (51-63) years were included; 24 447 were diagnosed with incident AF over a median (IQR) follow-up period of 13.3 (12.4-14.0) years. Rare pLOF variants in 6 genes ( TTN , RPL3L , PKP2 , CTNNA3 , KDM5B , and C10orf71 ) were associated with AF. Of these, TTN , RPL3L , PKP2 , CTNNA3 , and KDM5B replicated in an external cohort. Combined with high PRS, rare pLOF variants conferred an odds ratio of 7.08 (95% CI, 6.03-8.28) for AF. Carriers with high PRS also had a substantial 10-year risk of AF (16% in female individuals and 24% in male individuals older than 60 years). Rare pLOF variants were associated with increased risk of CM both prior to AF (hazard ratio [HR], 3.13; 95% CI, 2.24-4.36) and subsequent to AF (HR, 2.98; 95% CI, 1.89-4.69).
Conclusions and Relevance Rare and common genetic variation were associated with an increased risk of AF. The findings provide insights into the genetic underpinnings of AF and may aid in future genetic risk stratification.
Atrial fibrillation (AF) is the most common cardiac arrhythmia, and it is associated with an increased risk of stroke, heart failure (HF), and premature death. 1 While large genome-wide association studies (GWASs) have uncovered parts of the complex genetic component of AF and identified associations with primarily common genetic variants, 2 , 3 it is not always possible to pinpoint a specific causal gene based on the associated locus identified in GWASs.
On the other hand, rare coding variants are in general considered to have large effect sizes on disease risk and prognosis, which might be clinically relevant for the carrier. Several recent studies have suggested genetic testing in some subpopulations of patients with AF (eg, those with early-onset AF). 4 , 5 Genetic studies on familial AF have identified associations with several genes, including the ion-channel gene KCNQ1 6 and the sarcomere gene MYL4 . 7 However, it is difficult to assess the impact of these variants on AF in the general population, and only a few of these findings have been replicated in large-scale, population-based cohort studies.
By examining whole-exome sequencing data on more than 400 000 individuals, more than 30 000 of whom were diagnosed with AF, we aimed to identify novel gene associations driven by rare variation. Moreover, we aimed to elucidate how such variants influenced risk of incident AF and progression to more severe cardiac disease in combination with polygenic risk. A deeper understanding of genetic causes of AF in the general population may elucidate novel targets for therapeutics and explore the potential of future genetic risk stratification.
The study followed the Strengthening the Reporting of Observational Studies in Epidemiology ( STROBE ) reporting guideline. The study was conducted in the UK Biobank. All participants gave informed written consent. The UK Biobank received ethical approval from North West Multi-Centre Research Ethics Committee. The UK Biobank is a large, population-based cohort study including genetic and clinical information on almost 500 000 individuals representing the general population. The biobank and details on whole-exome sequencing, quality control, and variant annotation have previously been described in detail. 8 Further filtering, quality control, and phenotype definitions are described in the eAppendix in Supplement 1 . A flowchart of participant selection for the study cohort and subsequent analyses is shown in eFigure 1 in Supplement 1 .
Rare coding variants were collapsed into gene-based burden masks. Only variants with minor allele frequencies less than 1% were included in the analyses. Gene masks with a cumulative allele count less than 10 were excluded. In our primary analysis, we focused on rare predicted loss-of-function (pLOF) variants across 17 979 genes. Secondary gene-based tests for association with rare missense variants have been described in the eAppendix in Supplement 1 . The burden tests were conducted using the genome-wide regression tests in regenie, 9 adjusting for age at inclusion, sex, and 10 first principal components. We applied Firth logistic regression when the standard logistic regression P was less than .01. We considered associations statistically significant at the P < 2.77 × 10 −6 (corresponding to correction for testing in 18 000 genes). Sensitivity analyses (eAppendix in Supplement 1 ) included a leave-one-variant-out approach and subgroup analyses without individuals diagnosed with cardiomyopathies at inclusion or during follow-up. We replicated significant associations in an external dataset of 17 910 individuals with AF and 149 348 control individuals (eAppendix in Supplement 1 ).
For genes in which pLOF variants were significantly associated with AF, we also evaluated the protein and RNA expression. The methodology behind this analysis has been described in detail in the eAppendix in Supplement 1 .
The titin protein is expressed in several different isoforms, all encoded by the TTN gene. 10 As a secondary analysis, analyses were conducted, for gene-sets including only the predominant cardiac isoforms of titin (NB2 and NB2A) and constitutively expressed exons (percentage spliced in more than 90% [ TTN -PSI90]) respectively.
To estimate the impact of common genetic variation, we obtained polygenic risk score (PRS) weights from a previously published and validated AF PRS. 11 The PRS weights were calculated using the PRS–continuous shrinkage (CS)-auto method, and were based on summary statistics from the Atrial Fibrillation Consortium study, 12 which did not overlap with the UK Biobank. We then calculated PRSs for each individual in the UK Biobank cohort using PLINK 13 based on the number of risk alleles and the posterior effect size of the variants. The PRS was normalized by scaling to a mean of 0, with an SD equal to 1. Odds ratios (ORs) for AF were calculated per 1-SD increase in PRS using Firth logistic regression models (the logistf packing in R version 1.25.0 [R Foundation]), adjusted for sex, age at inclusion, and principal components 1 to 10. Area under curve (AUC) was estimated using a receiver operator characteristic (ROC) curve.
The PRS was assessed separately in carriers and noncarriers of rare pLOF variants in genes associated with AF (defined as individuals with a pLOF variant in TTN , RPL3L , PKP2 , CTNNA3 , or KDM5B ). For TTN , we only included those with variants in constitutively spliced-in cardiac exons ( TTN -PSI90). To assess polygenic risk and rare variants in aggregate, the cohort was stratified into groups based on PRS quintile and carrier status for subsequent analyses of OR for AF and absolute risk of AF. To avoid results being driven by relatedness, we conducted a sensitivity analysis on a subset of unrelated individuals (>third degree), described in the eAppendix in Supplement 1 .
To assess hazard ratios (HRs) and absolute risk of AF we designed a nested case-control study, with inclusion date as index date and excluding individuals with AF, HF, or cardiomyopathy diagnosed prior to inclusion. The cohort was stratified into 10 groups based on PRS and carrier status of pLOF variants, as described above. HRs were calculated using a Cox regression model and adjusted for sex, age at inclusion, and clinical risk factors at baseline (obesity, hypertension, ischemic heart disease [IHD], and HF). Individuals were followed up with until the date of AF diagnosis and censored at death or end of follow-up (July 1, 2022), whichever came first. Obesity was defined as body mass index (BMI) of 30 or greater (calculated as weight in kilograms divided by height in meters squared) at inclusion. Other phenotype definitions are described in the eAppendix in Supplement 1 .
Crude cumulative incidences were estimated using the Aalen-Johansen estimator (prodlim package in R version 2019.11.13), accounting for all-cause mortality as a competing risk. Models were constructed as time-to-event analyses, with date of inclusion as index date and age in years as the time scale. We then further stratified by age groups (above or below 60 years at inclusion) and estimated 10-year risk of AF, using time since inclusion as the time scale. To assess the interplay between polygenic risk and clinical risk factors, another model was constructed by stratifying the cohort based on these age groups and 2 common and modifiable risk factors for AF: obesity and hypertension. 14 In sensitivity analyses, we examined a subset of unrelated individuals and investigated models without TTN variants among the pLOF variants.
Using cause-specific Cox regressions, we evaluated how genetic predisposition to AF influenced the risk of HF and cardiomyopathy. Genetic predisposition was represented by all pLOF variants in the 5 genes described above; all pLOF variants, excluding those in TTN ; and the PRS for AF (per SD increase). The methodology behind these analyses is described in detail in the eAppendix in Supplement 1 .
We conducted gene-based association tests across the exome of 403 990 individuals. Baseline characteristics of the cohort are summarized in Table 1 . The median (IQR) age at inclusion was 58 (51-63) years, and 218 489 participants (54.1%) were female. A total of 6677 individuals had an AF diagnosis at inclusion in the biobank, and 24 447 individuals were diagnosed with AF by the end of follow-up. The 31 124 individuals with a diagnosis of AF were defined as cases in the gene-based burden tests, while the remaining 372 871 individuals were considered controls.
We identified significant associations between AF and pLOF variants in the genes PKP2 (OR, 2.12; 95% CI, 1.60-2.82; P = 2.21 × 10 −07 ), CTNNA3 (OR, 2.79; 95% CI, 1.88-4.14; P = 3.74 × 10 −07 ), C10orf71 (OR, 2.33; 95% CI, 1.69-3.39; P = 7.83 × 10 −07 ), and KDM5B (OR, 2.70; 95% CI, 1.80-4.06; P = 1.76 × 10 −06 ) and replicated the previously reported association between AF and pLOF variants in the genes TTN (OR, 1.77; 95% CI, 1.60-1.95; P = 3.38 × 10 −30 ) 15 and RPL3L (OR, 1.56; 95% CI, 1.38-1.77; P = 1.69 × 10 −12 ). 16 A Manhattan plot and quantile-quantile plot of associations are provided in eFigures 2 and 3 in Supplement 1 . Significant genetic associations are summarized in Table 2 .
Full results from the gene-based burden test of pLOF variants have been summarized in eTable 10 in Supplement 2 . Protein and RNA expression analyses revealed that the associated genes were predominantly expressed in cardiomyocytes, except KDM5B , which was expressed across all cell types (eAppendix and eFigure 4 in Supplement 1 ). As the C10orf71 gene had not previously been associated with cardiovascular disease, we examined tissue-specific expression of the gene and found that it was almost exclusively expressed in muscle tissue (eFigure 5 in Supplement 1 ). In an external replication cohort of 17 910 individuals with AF and 149 348 control individuals, we found replicated associations for TTN , PKP2 , CTNNA3, RPL3L , and KDM5B (eAppendix and eFigure 6 in Supplement 1 ). The variants contributing to each gene mask are summarized in eTable 11 in Supplement 2 .
Secondary analyses on pLOF variants in TTN cardiac isoforms (N2BA and N2B) and constitutively expressed cardiac exons found an even greater OR for AF (OR, 2.17; 95% CI, 1.93-2.43; P = 2.07 × 10 −40 and OR, 3.85; 95% CI, 3.25-4.55; P = 1.09 × 10 −55 ). Secondary gene-based tests for rare missense variants replicated one previously known association with the gene UBE4B (OR, 1.22; 95% CI, 1.12-1.31; P = 5.90 × 10 −7 ) (eTables 11 and 12 in Supplement 2 ). Sensitivity analyses did not substantially alter the results, except for pLOF variants in RPL3L and missense variants in UBE4B (eAppendix and eTables 4-7 in Supplement 1 ). We note that for RPL3L the association was predominantly driven by a pLOF variant, while for UBE4B it was driven by a missense variant.
We evaluated polygenic risk using an externally derived PRS. A 1-SD increase in this PRS was associated with an OR of 1.53 (95% CI, 1.51-1.55; P < .001) for AF. The addition of the PRS yielded an AUC of 0.76 (95% CI, 0.75-0.76) compared to an AUC of 0.74 (95% CI, 0.74-0.74) in a reference model adjusted for sex, age at inclusion, and principal components 1 to 10.
A 1-SD increase in PRS was associated with elevated OR estimates for AF in carriers of rare pLOF variants (OR, 1.69; 95% CI, 1.54-1.85; P < .001) compared with noncarriers (OR, 1.53; 95% CI, 1.51-1.55; P < .001). When assessing PRSs and rare pLOF variants in aggregate, we observed a dose-response–like increase in OR from the group with lowest genetic risk to the group with the highest risk. The group with a PRS in the top quintile and a rare pLOF variant had a markedly increased OR for AF of 7.08 (95% CI, 6.03-8.28) compared with noncarriers with low PRSs. Results have been illustrated in Figure 1 A and summarized in eTable 1 in Supplement 1 . Similar estimates were observed in a subset of unrelated individuals (eTable 2 in Supplement 1 ).
After excluding individuals with prevalent AF, HF, or cardiomyopathy at baseline, 5032 individuals (1.27%) were carriers of a rare pLOF variant (eTable 3 in Supplement 1 ). During a median (IQR) follow-up period of 13.3 (12.4-14.0) years, 24 061 individuals were diagnosed with incident AF, while 23 907 died before AF diagnosis or end of follow-up. The group with both a PRS in the top quintile and a rare pLOF variant had an HR of 4.78 for incident AF (95% CI, 4.06-5.63; P < .001) compared with noncarriers with low PRSs. Estimates for all model covariates are provided in eTable 4 in Supplement 1 .
This trend was also observed for an absolute risk of AF, where pLOF variant carriers with a high PRS had a cumulative AF incidence of 28.55% (95% CI, 24.5-33.2) by age 80 years ( Figure 1 B). Comparatively, noncarriers with middle (40%-60%) and low (0%-20%) PRSs, had absolute risks of 12.1% (95% CI, 11.7-12.5%) and 8.1% (95% CI, 7.8-8.4%), respectively (eTables 5-7 in Supplement 1 ). Sensitivity analyses in unrelated individuals and models excluding pLOF variants in TTN did not substantially alter the results (eTables 8 and 9 in Supplement 1 ).
Concordant results were observed in analyses on 10-year absolute risk of AF ( Figure 2 ). Individuals with a high PRS for AF consistently had a higher 10-year risk of AF across age groups and sex. The risk of AF was more pronounced in individuals with BMI of 30 or greater and hypertension. The highest risk was found in individuals older than 60 years at inclusion with high PRSs who also carried a rare pLOF variant (15% and 25% in female individuals and male individuals, respectively). Sensitivity analyses in a subset of unrelated individuals (>third degree) and models not considering TTN pLOF variants did not substantially alter these estimates (eFigures 7 and 8 in Supplement 1 ).
When regarding AF, cardiomyopathy, and HF as competing events, rare pLOF variants in AF-associated genes conferred an increased HR for incident AF (HR, 1.85; 95% CI, 1.69-2.02; P < .001), incident cardiomyopathy (HR, 3.13; 95% CI, 2.24-4.36; P < .001), and incident HF (HR, 1.51; 95% CI, 1.26-1.82; P < .001). When not considering TTN variants, the effect estimates for rare pLOF variants were attenuated for AF (HR, 1.60, 95% CI, 1.44-1.78; P < .001) and no longer statistically significant for cardiomyopathy (HR, 1.39; 95% CI, 0.80-2.40; P = .24) and HF (HR, 1.01; 95% CI, 0.79-1.29, P = .95). The AF PRS was associated with AF (HR per SD, 1.45; 95% CI, 1.43-1.47; P < .001) but not with cardiomyopathy (HR per SD, 0.99; 95% CI, 0.92-1.06; P = .73) or HF (HR per SD, 1.02; 95% CI, 0.99-1.05; P = .12). Sensitivity analyses in a subset of unrelated individuals showed similar results (eFigure 9 in Supplement 1 ).
We identified 21 154 individuals diagnosed with AF after inclusion into UK Biobank, and no prior diagnosis of HF or cardiomyopathy. Regarding cardiomyopathy and HF as competing events, we observed an increased HR for cardiomyopathy in carriers of rare pLOF variants in AF-associated genes (HR, 2.98; 95% CI, 1.89-4.69; P < .001). We did not observe significant effects of pLOF variants when excluding TTN variants (HR, 1.01; 95% CI, 0.42-2.45; P = .98) or for the AF PRS (HR per SD, 1.06; 95% CI, 0.94-1.19; P = .34). We examined the risk of incident AF in another subgroup of 7625 individuals with incident cardiomyopathy or HF during follow-up, without a prior diagnosis of AF. Here, we found no significant associations between pLOF variants or AF PRS and incident AF, although we noted increased estimates in carriers of pLOF variants in AF-associated genes excluding TTN (HR, 1.73; 95% CI, 1.03-2.89; P = .04). Sensitivity analyses in unrelated individuals and the application of a 30-day grace period did not substantially alter the results (eFigure 10 in Supplement 1 ). All estimates are shown in Figure 3 .
In this genetic association study, we examined large-scale, whole-exome sequencing data on more than 400 000 individuals in the UK Biobank, including approximately 31 000 individuals with AF. Whole-exome sequencing of large population-based cohorts offers several advantages compared to former genetic approaches. The method enables identification of rare coding variants with large effect sizes that are often not picked up with GWAS genotyping. Using a gene-based burden test, we identified several genes in which rare pLOF variants conveyed a considerably increased OR for AF. We demonstrated that polygenetic risk of AF and rare pLOF variants were associated with an increased absolute risk of incident AF and investigated the combined effect of an AF PRS and clinical risk factors (obesity and hypertension). Individuals with a pLOF variant had a substantial absolute risk of AF comparable to those with both BMI of 30 or greater and hypertension. Consequently, our results indicate a benefit in including both common and rare genetic variation in AF risk stratification.
Our study identified several novel associations between pLOF variants and with AF in key genes in ventricular cardiomyopathies, as well as genes not previously linked with heart disease. Among these were novel associations with the genes CTNNA3 and KDM5B . The CTNNA3 gene encodes the cytoskeletal protein catenin α3, which interacts with cardiomyocyte desmosomes and plays a role in cell adhesion. 17 Variants in CTNNA3 have been putatively associated with arrhythmogenic right ventricle cardiomyopathy 18 and rare variants in CTNNA3 have also been associated with familial AF. 19 The KDM5B gene has not previously been associated with arrhythmias. However, the gene encodes lysine-specific demethylase 5B, a protein involved in histone methylation. This protein is thought to play a role in cardiac fibrosis, 20 a common substrate in reentry arrhythmia mechanisms. We also replicated an association with pLOF variants in the RPL3L gene, which is involved in ribosomal function and muscle growth. 21 Sensitivity analyses revealed that the association was primarily driven by a splice-donor variant, which has previously been reported in an AF GWAS. 16
Results also showed an association with the C10orf71 gene that did not replicate in the external dataset. While a locus near the C10orf71 gene was recently associated with AF, 22 knowledge of the C10orf71 gene and the functions of the encoded protein cardiac-enriched FHL2-interacting protein (CEFIP) is relatively sparse. One study 23 has suggested that the protein may locate to sarcomere Z-discs and contribute to the regulation of cardiomyocyte hypertrophy, which is consistent with proteomics and single-cell sequencing data showing expression in both atria, with a predominant expression in cardiomyocytes. As the genetic association did not externally replicate, its potential role in AF remains uncertain until validated in other studies.
TTN is a well-established dilated cardiomyopathy gene, 24 and its association with AF has been hypothesized to be partly driven by an atrial cardiomyopathy. 15 Interestingly, we also identified an AF association with pLOF variants in the PKP2 gene, which plays a major role in arrhythmogenic right ventricle cardiomyopathy. 25 Common variants in a locus near PKP2 have previously been associated with AF in GWASs, and our study corroborates these findings with evidence of a role of pLOF variants in PKP2 in AF.
Given the discovered associations with several cardiomyopathy genes and previous reports of increased mortality in rare variant carriers, 5 we examined whether carriers of rare pLOF variants in AF-associated genes were at a greater risk of cardiomyopathy or HF. AF and HF often coexist, and their temporal relationship can be complex and challenging to disentangle. 26 In an effort to elucidate the role of genetic variation in this context, we conducted cause-specific Cox regressions with AF, HF, and cardiomyopathy as competing events. We found that both rare pLOF variants and the AF PRS associated with incident AF prior to a potential diagnosis of HF or cardiomyopathy. Genetic predisposition for AF was also associated with incident cardiomyopathy or HF prior to AF, where we noted high HRs for cardiomyopathy in pLOF variant carriers. Moreover, rare pLOF variants were associated with an increased risk of cardiomyopathy in individuals with AF but not vice versa. These findings indicate that for some variant carriers, AF may be the first disease manifestation preceding more severe cardiac disease.
While the prevalence of rare pLOF variants is relatively low in the general population, their contribution to disease risk and their associations with more severe cardiac disease may justify genetic testing in specific patient groups. 27 Previous research has shown a higher proportion of rare variants in younger patients with AF 4 and found an increased mortality in variant carriers. 5 Based in part on these findings, recent guidelines on AF from the American College of Cardiology and American Heart Association 28 suggest that genetic testing or surveillance for cardiomyopathy may be reasonable in patients with AF with onset before 45 years of age. Our findings align with these recommendations and may contribute to a better understanding of specific genes in which rare pLoF variants convey a large risk of AF. Although population-wide genetic screening for rare variants is unlikely in the near future, integrating genetic perspectives may aid in AF risk stratification as sequencing becomes cheaper and more readily available. Future studies focused on identifying patient or population groups that may benefit from genetic testing are warranted.
The reported results should be interpreted with respect to the study limitations. First, in order to avoid bias from population stratification, we only included individuals of European ancestry and our results may therefore not be generalizable to other populations. Second, correcting for thousands of independent tests led us to apply a strict significance threshold, which may have limited our power to detect potential genes associated with AF. Third, the gene-based association tests and analyses on absolute risk of AF were observational findings. Hence, they may be biased by residual confounding and confounding by indication and cannot be assumed to represent causal relationships. Although 5 of the 6 associated genes were replicated in an external cohort, they should also be validated in functional studies and in other cohorts and population groups to better understand the pathophysiological mechanisms leading to arrhythmia. Additionally, it should be noted that the study design of the UK Biobank may also introduce healthy volunteer bias.
In summary, our study identified novel genetic associations with AF, including several hallmark genes of major cardiomyopathy subtypes. The genes identified were involved in diverse cellular processes, including sarcomere and desmosome structure, while the associations with genes involved in ribosomal function, histone methylation and ubiquitination hint at novel arrhythmia mechanisms. We showed an interplay between rare and common genetic variation and demonstrated a substantial absolute risk of AF in individuals with a high PRS carrying a rare pLOF variant. These findings may contribute to possible future genetic risk stratification and improved clinical practice.
Accepted for Publication: April 16, 2024.
Published Online: June 26, 2024. doi:10.1001/jamacardio.2024.1528
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2024 Vad OB et al. JAMA Cardiology .
Corresponding Author: Morten S. Olesen, MSc, PhD, Department of Biomedical Sciences, Faculty of Health and Medical Sciences, University of Copenhagen, Blegdamsvej 3B, 2200 Copenhagen N, Denmark ( [email protected] ).
Author Contributions: Dr Olesen had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design : Vad, Paludan-Müller, Svendsen, Olesen.
Acquisition, analysis, or interpretation of data : Vad, Monfort, Paludan-Müller, Kahnert, Diederichsen, Andreasen, Lotta, Nielsen, Lundby, Svendsen.
Drafting of the manuscript : Vad, Paludan-Müller, Andreasen, Nielsen, Lundby, Olesen.
Critical review of the manuscript for important intellectual content : Monfort, Kahnert, Diederichsen, Lotta, Lundby, Svendsen.
Statistical analysis : Vad, Monfort, Paludan-Müller, Kahnert, Nielsen.
Obtained funding : Lotta, Lundby, Svendsen.
Administrative, technical, or material support : Diederichsen, Lotta, Nielsen, Lundby.
Supervision : Diederichsen, Andreasen, Lotta, Lundby, Svendsen, Olesen.
Conflict of Interest Disclosures: Dr Vad reported grants from Research Foundation at Rigshospitalet, the Department of Clinical Medicine, University of Copenhagen, and Skibsreder Per Henriksen, R, og hustrus fond during the conduct of the study. Dr Diederichsen reported personal fees from Bristol Myers Squibb, Pfizer, Bayer, Cortrium, and Acesion Pharma and grants from Abbott and Boston Scientific outside the submitted work. Dr Lotta reported other from Regeneron Pharmaceuticals (employment, stocks, and stock options) during the conduct of the study and outside the submitted work. Dr Nielsen reported other from Regeneron Pharmaceuticals (employment, stocks, and stock options) during the conduct of the study and outside the submitted work. Dr Lundby reported grants from Novo Nordisk during the conduct of the study. Dr Svendsen reported personal fees from Medtronic (speaker fee and member of advisory board) and Vital Beats (member of advisory board) outside the submitted work. No other disclosures were reported.
Funding/Support: This work was supported by the Novo Nordisk Foundation (to Dr Olesen; NNF17OC0031204), the John and Birthe Meyer Foundation (Drs Svendsen and Olesen), the Research Foundation at Rigshospitalet (Drs Vad and Paludan-Müller), the Department of Clinical Medicine at the University of Copenhagen (Dr Vad), the Villadsen Family Foundation (Dr Olesen), the Arvid Nilsson Foundation (Dr Svendsen), and Skibsreder Per Henriksens R., og hustrus fond (Drs Vad and Svendsen).
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Group Information: The members of the Geisinger MyCode Community Health Initiative and the Regeneron Genetics Center (RGC) Research Team appear in Supplement 3 .
Data Sharing Statement: See Supplement 4 .
Additional Contributions: We would like to thank the participants and investigators of the UK Biobank. Our study incorporated publicly available data based on the Atrial Fibrillation Consortium study, and data from the Human Protein Atlas and Genotype-Tissue Expression project, and we would like to acknowledge the investigators and participants of these studies.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- CBE Life Sci Educ
- v.11(3); Fall 2012
Biology Undergraduates’ Misconceptions about Genetic Drift
T. m. andrews.
*Department of Ecology, Montana State University, Bozeman, MT 59717
R. M. Price
§ Interdisciplinary Arts and Sciences, University of Washington, Bothell, WA 98011
‖ BEACON Center for the Study of Evolution in Action, Michigan State University, East Lansing, MI 48824
T. L. McElhinny
¶ Department of Geological Science, Michigan State University, East Lansing, MI 48824
A. Thanukos
# University of California Museum of Paleontology, Berkeley, CA 94720
K. E. Perez
@ Department of Biology, University of Wisconsin, La Crosse, La Crosse, WI 54601
C. F. Herreid
**Department of Biological Sciences, State University of New York at Buffalo, Buffalo, NY 14260
D. R. Terry
‡‡ Division of Education, Alfred University, Alfred, NY 14802
P. P. Lemons
§§ Department of Plant Biology, University of Georgia, Athens, GA 30602
Associated Data
This study explores biology undergraduates’ misconceptions about genetic drift. We use qualitative and quantitative methods to describe students’ definitions, identify common misconceptions, and examine differences before and after instruction on genetic drift. We identify and describe five overarching categories that include 16 distinct misconceptions about genetic drift. The accuracy of students’ conceptions ranges considerably, from responses indicating only superficial, if any, knowledge of any aspect of evolution to responses indicating knowledge of genetic drift but confusion about the nuances of genetic drift. After instruction, a significantly greater number of responses indicate some knowledge of genetic drift ( p = 0.005), but 74.6% of responses still contain at least one misconception. We conclude by presenting a framework that organizes how students’ conceptions of genetic drift change with instruction. We also articulate three hypotheses regarding undergraduates’ conceptions of evolution in general and genetic drift in particular. We propose that: 1) students begin with undeveloped conceptions of evolution that do not recognize different mechanisms of change; 2) students develop more complex, but still inaccurate, conceptual frameworks that reflect experience with vocabulary but still lack deep understanding; and 3) some new misconceptions about genetic drift emerge as students comprehend more about evolution.
INTRODUCTION
Biology educators have articulated the importance of teaching undergraduates the mechanisms of evolution. In a national survey, more than 300 college biology faculty agreed on the importance of evolution instruction in introductory biology sequences, including instruction on evolutionary mechanisms and phylogenetics ( Gregory et al. , 2011 ). In fact, evolution was the most agreed upon topic, with 89% of faculty agreeing it was an essential topic for biology students to learn. Similarly, in the report BIO2010, the Committee on Undergraduate Biology Education noted that students should understand that “all living things have evolved from a common ancestor through processes that include natural selection and genetic drift acting on heritable genetic variation” ( National Research Council, 2003 ). Evolution is a core concept in biology, and it is also one of the most challenging concepts for students to learn.
Most of the research on students’ conceptual difficulties with evolution has focused on natural selection, but understanding random evolutionary processes is also particularly challenging ( Garvin-Doxas and Klymkowsky, 2008 ; Klymkowsky and Garvin-Doxas, 2008 ; Mead and Scott, 2010 ). In the development of the Biology Concept Inventory, “deep-seated, and often unaddressed, misconceptions about random processes” emerged as factors contributing to student difficulties in learning evolutionary and molecular biology ( Garvin-Doxas and Klymkowsky, 2008 ; Klymkowsky and Garvin-Doxas, 2008 ). This is not surprising, because probability and randomness perplex students of all ages (e.g., Fischbein and Schnarch, 1997 ; Taleb, 2005 ; Lecoutre et al. , 2006 ). Students are challenged by both the terminology associated with random evolutionary processes ( Mead and Scott, 2010 ) and the conceptual complexities of these processes ( Garvin-Doxas and Klymkowsky, 2008 ; Klymkowsky and Garvin-Doxas, 2008 ). Indeed, the most tenacious misconception in biology may be the idea that all processes serve a purpose ( Gregory, 2009 ; Kelemen and Rosset, 2009 ; Mead and Scott, 2010 ). This idea is so deep-seated that students fail to even consider random processes as responsible for biological patterns ( Garvin-Doxas and Klymkowsky, 2008 ). The fact that random processes confound students is particularly worrisome, because random processes occur at every level of the biological world, from gene expression ( Cai et al. , 2006 ) to clade diversification and extinction ( Raup et al. , 1973 ).
Despite these obstacles, understanding random processes such as genetic drift is essential for a deep understanding of the theory of evolution. In contrast to natural selection, genetic drift is nonselective and therefore results in nonadaptive changes in populations ( Beatty, 1992 ). Genetic drift occurs in any finite population and therefore occurs in every population all the time ( Futuyma, 2005 ; Barton et al. , 2007 ). Of particular concern to conservation biologists, drift may overwhelm selection in small populations ( Frankham et al. , 2002 ). Drift reduces the amount of genetic variation within populations and tends to increase genetic variation among populations ( Frankham et al. , 2002 ; Futuyma, 2005 ; Barton et al. , 2007 ). Genetic drift is also the theoretical framework for neutral evolution ( Barton et al. , 2007 ; Masel, 2012 ). Thus, biology undergraduates should be able to explain the random process of genetic drift and predict how drift impacts populations ( Masel, 2012 ).
The teaching and learning of genetic drift has been largely overlooked in biology education research. A search in ERIC (performed April 23, 2012) for the term “genetic drift” in the text of any article written over the past 45 yr produced only 13 papers. Most of these papers described methods for teaching genetic drift, but did not report data on how effective those methods are at changing students’ conceptions of drift (e.g., Maret and Rissing, 1998 ; Staub, 2002 ; Young and Young, 2003 ). In contrast, numerous studies have focused on undergraduates’ scientifically inaccurate conceptions about natural selection (e.g., Bishop and Anderson, 1990 ; Settlage, 1994 ; Jensen and Finley, 1996 ; Anderson et al. , 2002 ; Abraham et al. , 2009 ; Gregory, 2009 ; Kalinowski et al. , 2010 ; Andrews et al. , 2011 ). An ERIC search for the term “natural selection” produced 317 papers.
In the present study, we used a mixed-methods approach to: 1) describe undergraduates’ definitions of genetic drift, 2) identify the most common misconceptions in those definitions, 3) examine differences in students’ definitions before and after receiving instruction on genetic drift, and 4) propose a framework for future research that interprets students’ misconceptions and illustrates how undergraduates’ understanding of genetic drift progresses.
Our methodology was mixed. Our qualitative analytical methods aligned with grounded theory and were supplemented with statistical analysis to compare student responses before and after instruction.
In grounded theory, the central question is: “What theory emerges from systematic comparative analysis and is grounded in fieldwork so as to explain what has been and is observed?” ( Patton, 2002 , p. 133). In practice, grounded theory aims to derive descriptions from the data, as opposed to approaching the data with preliminary explanations. Those data are read and re-read, and from these readings investigators establish categories that explain the data. Once categories are established, investigators review data again and assign units of data, such as quotes from student responses, to categories. Thus, categories serve to organize detailed descriptions of the data. In this way, grounded theory is analogous to inductive science, in which careful and repeated observations enable descriptions. Additionally, grounded theory, like inductive science, may produce hypotheses that can be tested with additional research (i.e., deductive science). Grounded theory was developed by sociologists and is traditionally used to analyze interview data with the goal of developing theories about human actions, interactions, and social processes ( Creswell, 2007 ). In our study, we analyze qualitative data from written responses, focusing on participants’ conceptions, rather than the broader context in which the participants are acting.
For this study, we synthesized data collected during two distinct research projects ( Table 1 ). Authors from a National Evolutionary Synthesis Center (NESCent) working group (T.M.A., R.M.P., L.S.M., T.L.M., A.T., and K.E.P.) collected data in preparation for the development of a concept inventory on genetic drift. Authors affiliated with the National Center for Case Study Teaching in Science (C.F.H, D.R.T., and P.P.L.) collected data during a study of the effectiveness of a series of case studies, including one on genetic drift and other evolutionary mechanisms. Combining data sets allowed us to analyze misconceptions about genetic drift from a broad range of students and to capitalize on the different strengths of each project ( Table 1 ). The case study data set allowed us to test for differences between responses collected before and after instruction, whereas the concept inventory data set provided information about misconceptions that occur after more than one exposure to genetic drift instruction, since many of the participants were biology majors enrolled in courses for which introductory biology was a prerequisite. We describe methods used to collect both data sets in the Supplemental Material.
The data in this study came from two distinct collaborations
| Project | Level of courses | Data | Sample size |
|---|---|---|---|
| Concept inventory | Upper-division biology courses | Interview and written surveys about genetic drift | 37 |
| Case study | Introductory biology for majors and nonmajors | Open-ended question before and after instruction about natural selection and genetic drift | 319 |
Qualitative Analysis
Our analysis focused on the misconceptions in student responses. Because our community of researchers lacks a consensus on how to characterize knowledge that conflicts with expert ideas, we need to define how we used the term misconception in this study (see Gilbert and Watts, 1983 ; Tanner and Allen, 2005 ). We defined a misconception as a scientifically inaccurate idea about a scientific concept. These inaccuracies may occur before and after instruction. We did not distinguish between ideas generated during data collection and deeply held ideas. We considered the term misconception to be equivalent to the term alternative conception , and to be a particular kind of preconception or naïve conception ( Gilbert and Watts, 1983 ).
In the literature on natural selection misconceptions, the term misconception is often defined more narrowly than we have defined it in this study. For example, natural selection misconceptions have been referred to as “deeply rooted” and as “intuitive interpretations of the world” ( Cunningham and Wescott, 2009 ; Gregory, 2009 ). However, natural selection misconceptions have been explored in depth, leading to more precise definitions of natural selection misconceptions. In contrast, few, if any, studies have focused on students’ conceptions of genetic drift. We have used a broad definition of misconceptions that encompasses all students’ inaccurate ideas, because considerably more research will be necessary to identify which inaccurate ideas are intuitive, common across diverse populations, and deeply held.
Rigor in qualitative research has been defined as the “attempt to make data and explanatory schemes as public and replicable as possible” (Norman Denzin, as quoted in Anfara et al. , 2002 , p. 7). Therefore, two authors followed this systematic approach:
- We (T.M.A. and P.P.L.) independently identified student misconceptions about genetic drift in the concept inventory data set and case study data set, respectively. Thereafter, we combined data sets and completed all analyses in the same place and time, which allowed us to immediately deliberate on any ambiguities.
- We agreed on an initial list of misconceptions about genetic drift. To create this initial list, we analyzed a subsample of student responses from the combined data set to establish the characteristics of misconceptions.
- We each coded ∼40 responses, identifying the misconceptions in each response to establish that we could reliably classify misconceptions. We discussed any discrepancies until we reached consensus. At this preliminary stage, we identified three general types of student responses: responses that did not address genetic drift, despite explicit instructions to do so; responses containing misconceptions about genetic drift; and responses indicating at least some knowledge of genetic drift. These general types were not mutually exclusive.
- Using the initial list of misconceptions produced in step 3, we began coding the full data set. Any idea we could not classify after discussion was coded as undetermined. Coding the full data set was necessarily iterative. Throughout this process, new misconceptions emerged, our descriptions of existing misconceptions were refined and sometimes subdivided, and the data were recoded accordingly. In all cases, new misconceptions were closely related to misconceptions from our initial list, so it was only necessary to reanalyze responses previously coded as containing a misconception closely related to the newly emerged misconception and responses previously coded as undetermined.
- After all responses had been analyzed and coded at least once, we re-read all of the responses containing the same misconception, and discussed at length the characteristics delineating each misconception.
- Toward the end of our analysis, we tested our list of misconceptions to ensure it was exhaustive. We drew a new, random sample of 30 responses from the case study project data set, including questionnaires completed before and after instruction from all six sections, and coded this sample. We found no misconceptions we could not classify with our final coding system. We therefore concluded our list of misconceptions included all but the rarest genetic drift misconceptions held by participating students.
As we analyzed the data, we looked for overarching categories that would enable us to build a framework for future research on students’ conceptions about genetic drift. This is the end product of a grounded theory study ( Creswell, 2007 ; Glaser and Strauss, 2010 ). We designed the framework to facilitate the interpretation of undergraduates’ misconceptions about genetic drift and to hypothesize how undergraduates’ understanding of drift may progress. To build the framework, three investigators (T.M.A., R.M.P., and P.P.L.) iteratively grouped the full set of misconceptions and named the resulting clusters. We worked to propose a final framework that was derived from the data, not from explanations about student conceptions that we held prior to data analysis. This process continued until all three investigators agreed that the framework was true to the data and suggested testable hypotheses about genetic drift.
Statistical Analyses
We used 319 responses collected as part of the case study project to examine differences in students’ conceptions about genetic drift before and after instruction. As described in the Supplemental Material, we used a systematic sampling design to select student responses. We sampled different students’ responses before and after instruction, even though this precluded using matched pairs. This approach, which allowed us to include more students, ensured a breadth of responses, even though it limited statistical power by not controlling for individual variation among students. All statistical analyses were conducted in R, an open-source statistical analysis program (R Project for Statistical Computing, 2011).
To assess the hypothesis that instruction improved students’ understanding of genetic drift, we tested three predictions generated by this hypothesis. We predicted that before and after instruction the following would be different:
- Number of students who did not address drift
- Number of responses that indicated some knowledge of the definition of genetic drift
- Number of responses containing at least one misconception
We tested these predictions with Fisher's exact tests ( Ramsey and Schafer, 2002 ); for predictions 2 and 3, we excluded responses that did not address drift. The Fisher's exact test is more precise than a chi-squared test when some cells in a contingency table have small sample sizes ( Ramsey and Schafer, 2002 ). A small p value resulting from this test indicates that the counts of responses in the two categories are not independent. In other words, a small p value resulting from the tests described above would suggest that instruction influenced students’ responses.
Finally, we used descriptive statistics to examine differences between the frequency of misconceptions before and after instruction in introductory biology courses and among upper-division students. We did not pursue additional statistical analysis for individual misconceptions or categories of misconceptions, as there were small sample sizes for some misconceptions and a lack of independence among groups resulting from responses containing more than one misconception.
Out of 356 student responses analyzed, few defined or attempted to apply the concept of genetic drift without using misconceptions. Even though questions from both data sets specifically asked students to define genetic drift, 31.5% ( n = 112) of responses failed to address drift at all ( Table 2 ). Among responses that addressed drift ( n = 244), only 11.5% ( n = 28) indicated some knowledge of the definition of genetic drift. Overall, 83.2% ( n = 203) of the responses that addressed drift contained at least one misconception ( Table 2 ). Some responses ( n = 25) hinted at knowledge of genetic drift (e.g., included the term random or chance ), but were too vague to be fully evaluated. Note that, because some responses indicated knowledge of genetic drift but also contained misconceptions, the percentages provided here sum to greater than 100%.
Frequency of different types of responses observed in full data set ( n = 356), in only those responses that addressed drift ( n = 244), and before ( n = 85) and after ( n = 122) introductory instruction
| Responses that… | % Full data set | % Addressed drift | % Before instruction | % After instruction | Value |
|---|---|---|---|---|---|
| did not address drift | 31.5 | NA | 46.5 | 23.8 | < 0.0001 |
| contained at least one misconception | 57.0 | 83.2 | 99.0 | 74.6 | < 0.0001 |
| hinted at knowledge of genetic drift, but were too vague to evaluate | 7.0 | 10.2 | 1.0 | 17.0 | NA |
| indicated some knowledge of genetic drift | 7.9 | 11.5 | 1.0 | 11.0 | 0.005 |
a p Values indicate significance of Fisher's exact tests comparing counts of responses before and after instruction.
b Values in a column may sum to greater than 100%, because a response could indicate knowledge of drift and contain a misconception.
c The first cell in this column is calculated from all responses collected before instruction ( n = 159). The rest of the cells in this column are calculated from the responses that addressed drift ( n = 85).
d The first cell in this column is calculated from all responses collected before instruction ( n = 160). The rest of the cells in this column are calculated from the responses that addressed drift ( n = 122).
Categories of Student Misconceptions Regarding Genetic Drift
In responses that addressed drift ( n = 244), we identified five overarching categories of misconceptions: Novice Genetics, Novice Evolution, Associating Genetic Drift with Other Evolutionary Mechanisms, Associating Genetic Drift with Population Boundaries, and Developing Genetic Drift Comprehension. These overarching categories are further divided into 16 distinct misconceptions that we describe below and summarize in Table 3 . We also describe the frequency of each misconception ( Table 3 ). We further divide the frequency of each misconception into those collected before and after introductory genetic drift instruction (case study data set) and those collected from students enrolled in upper-division biology courses (concept inventory data set) ( Table 3 ).
Categories of misconceptions, student quotes, and the frequency with which students employed these misconceptions a
| Misconceptions | Student quotes | % of Total ( = 244) | % Before instruction ( = 85) | % After instruction ( = 122) | % Upper division ( = 37) |
|---|---|---|---|---|---|
| 12.7 | 22.4 | 9.0 | 5.4 | ||
| shared traits or genes. | “Genetic drift [is] when it's the same species but different characteristics.” | 7.4 | 14.1 | 4.9 | 0.0 |
| “Genetic drift because both species [have] distinctive commonalities.” | |||||
| gradual genetic change in a population. | “Genetic drift is where the amount of present alleles change[s] gradually over time.” | 4.1 | 5.9 | 3.3 | 5.4 |
| “Genetic drift is a change in genes over time.” | |||||
| when genes or traits are passed from one individual to another. | “Genetic drift is the passing down of traits while natural selection does not have anything to do with genetics.” | 1.2 | 2.4 | 0.8 | 0.0 |
| 20.9 | 31.8 | 14.7 | 13.5 | ||
| … | |||||
| acclimation to the environment that may result from a need to survive. | “It was probably genetic drift. As the butterflies adapted to their new habitat they had to physically change in order for survival.” | 15.6 | 25.9 | 11.5 | 2.7 |
| “The evolution of the two butterflies is genetic drift because they developed to their surroundings.” | |||||
| change resulting from mating between individuals from different species. | “[Genetic drift occurred when] certain butterflies with each gene and characteristics came together in a certain spot and they mated forming new types of butterflies.” | 4.5 | 5.9 | 1.6 | 10.8 |
| when natural selection cannot or is not occurring. | “[Genetic drift is] the genetic changes that occur when a population is not under selection.” | 0.8 | 0.0 | 1.6 | 0.0 |
| 18.8 | 13.0 | 13.1 | 48.6 | ||
| random mutation. | “[Genetic drift occurs when] due to random mutations, genetic structure can change over time.” | 7.4 | 4.7 | 5.7 | 18.9 |
| “The definition of genetic drift is random chance mutation.” | |||||
| gene flow. | “The movement of genes from one population of a species to another or from one locality to another.” | 5.7 | 7.1 | 4.1 | 8.1 |
| “Genetic drift is a chance occurrence that brings genes into a population.” | |||||
| natural selection. | “Genetic drift occurs to eliminate the less adaptable trait that is not well suitable to the environment.” | 4.5 | 1.2 | 2.5 | 13.5 |
| any change in allele frequencies. | “[Genetic drift is] the process of changing allele frequencies within a population.” | 1.2 | 0.0 | 0.8 | 8.1 |
| 32.8 | 33.0 | 36.1 | 21.6 | ||
| migration with or without acclimation to the environment. | “Genetic drift is when the population moves to a location more suitable to its characteristics.” | 14.8 | 16.5 | 15.6 | 8.1 |
| “[Genetic drift occurred] as certain ancestral butterflies moved to different areas, they changed to better suit their new environment.” | |||||
| the separation of populations with or without acclimation to the environment. | “[Genetic drift occurs due to] isolation of a population or species by whatever means.” | 10.2 | 9.4 | 10.7 | 10.8 |
| “Genetic drift occurs when a sect of a species is separated from the other and changes to adapt to their new environment.” | |||||
| speciation. | “I believe [it was genetic drift] because I believe at one point both species were one, then separated.” | 7.8 | 7.1 | 9.8 | 2.7 |
| “It was genetic drift because some genes changed to create this new species.” | |||||
| 8.6 | 0.0 | 12.3 | 18.9 | ||
| a change in genes caused by an isolated event, often a catastrophe. | “Genetic drift involves a natural disaster that dramatically changes the genes in that area.” | 4.5 | 0.0 | 8.2 | 2.7 |
| limited to small populations. | “Genetic drift is genetics in a smaller populations.” | 2.5 | 0.0 | 3.3 | 8.1 |
| when an allele is fixed in a population. | “This is when alleles from one population either die out or become the only allele present. It occurs because of random processes. The alleles just happen to die out or become the most prevalent because of chance.” | 1.6 | 0.0 | 0.8 | 8.1 |
a Frequencies are based on the subset of responses that addressed drift ( n = 244), not the total number of responses ( n = 356).
b Responses from the case study project.
c Responses from the concept inventory project.
Our detailed description of the misconceptions begins with the most novice overarching categories (Novice Genetics and Novice Evolution) and concludes with the most advanced category (Developing Genetic Drift Comprehension). The two categories presented in the middle (Associating Genetic Drift with Other Evolutionary Mechanisms, Associating Genetic Drift with Population Boundaries) do not represent a progression; rather, some responses in each category range from novice to developing comprehension. Within the overarching categories of misconceptions, we have listed misconceptions in decreasing order from highest to lowest percentage of responses that addressed drift ( Table 3 ). It is important to recognize that although some misconceptions we describe indicated more advanced knowledge than others, responses in the most advanced category still differ in key ways from an expert's conception of genetic drift.
We use quotes from students to illustrate the misconceptions encompassed by each overarching category. In the interest of brevity, we include the most salient sections of a response, rather than complete responses. In some cases, we may have used additional information included in a response to analyze a student's conceptions in order to classify his or her misconceptions. We have lightly edited some quotes for clarity, but have left grammatical and syntax errors when they do not hinder the interpretation of a quote.
Category 1: Novice Genetics.
Although a number of definitions for genetic drift exist ( Masel, 2012 ), biologists generally define genetic drift as a change in the allele frequencies within a population resulting from random sampling error from generation to generation ( Futuyma, 2005 ; Barton et al. , 2007 ). Some responses in our sample recognized genetic drift was associated with genetics, but did not recognize it as an evolutionary mechanism. These definitions of genetic drift tended to be vague and brief, indicating only superficial knowledge of genetics.
The most common misconception in Novice Genetics was the idea that genetic drift is, or results in, shared traits or shared genes. In some cases, responses stated or implied that genetic drift causes some differences among individuals, but natural selection causes many differences among individuals:
“Genetic drift is more likely [than natural selection] because they share many of the same habitats and seem to be similar.”
“Genetic drift equals family members…I would have to assume these two butterflies are similar in DNA because of similar shape and habits but not full related because of color and preferred areas to be like meadows and forests.”
Some responses in Novice Genetics vaguely described genetic drift as gradual genetic change in a population without describing a mechanism of change:
“Genetic drift = gradual change in genes.”
“[This is genetic drift because] their similar characteristics indicate that over time the genetics of the species slowly changed.”
A few responses in Novice Genetics defined genetic drift as occurring when genes or traits are passed from one individual to another. Responses were not always specific about the units between which traits or genes were passed. Some described genes passing from parent to offspring through reproduction, but others described the transmission of traits between individuals:
“Genetic drift is when certain desirable characteristics that may occur through mutation are passed on to offspring.”
“Genetic drift is the flow of genes from one individual to another.”
Category 2: Novice Evolution.
Responses in the Novice Evolution category defined genetic drift as an evolutionary mechanism but conflated the definition of genetic drift with novice conceptions of evolution. The answers indicated little or no knowledge of random occurrences. The most common misconception in Novice Evolution has also been identified and described in studies of students’ misconceptions regarding natural selection (e.g., Bishop and Anderson, 1990 ; Nehm and Reilly, 2007 ). These responses defined genetic drift as the process, or result, of the environment causing change over time, attributing this change to “adaptation,” by which they seemed to mean acclimation to environmental characteristics. Some responses containing this misconception explicitly stated that change resulted from a need to survive:
“Genetic drift is the most reasonable answer because the sun brings out brightness like the bright butterfly and the shade is dark like the darker butterfly.”
“Genetic drift is genes change over time to fit world changes.”
“Genetic drift [occurred] because the butterflies[’] color changed depending on where they spent the most time.”
“Genetic drift is when a species changes due to a specific need to survive or thrive.”
Another misconception in Novice Evolution defined genetic drift as an evolutionary mechanism in which change results from mating between individuals from different species:
“The butterflies were the same color and liked the same environments but began breeding with butterflies of different kinds, possibly because of food scarcity or wind currents.”
“Genetic drift is change due to breeding.”
Lastly, a few responses in Novice Evolution contained the misconception that genetic drift is a mechanism of evolutionary change that occurs when natural selection cannot or is not occurring. The descriptions in these responses were so superficial that despite the use of key terms like natural selection , the responses failed to indicate any understanding of evolutionary processes. This misconception was not common, but was very clearly articulated in two responses collected from students in different courses in response to different questions:
“[Genetic drift is] the genetic changes that occur when a population is not under selection.”
Category 3: Associating Genetic Drift with Other Evolutionary Mechanisms.
Biologists recognize natural and sexual selection, mutation, gene flow, and genetic drift as distinct evolutionary mechanisms. Responses in Associating Genetic Drift with Other Evolutionary Mechanisms confused genetic drift with other evolutionary mechanisms or with evolution in general. The definitions in these responses indicated developing comprehension of evolution, but did not indicate knowledge of genetic drift.
The most common misconception in Associating Genetic Drift with Other Evolutionary Mechanisms defined genetic drift as random mutation. About half of these responses explained that genetic drift results from mutations, while the other half defined genetic drift as the process of mutation or the accumulation of mutations over time. In some cases, students specified a precise mechanism of mutation:
“Genetic drift = change in a population due to mutation.”
“Genetic drift is the drifting of genes during mutations. A base pair is usually cutoff, that alters the gene sequence leading to changed genes.”
Another misconception in this category defined genetic drift as gene flow. Specifically, these responses described genetic drift as the process of alleles entering or leaving populations or as the process of alleles from different populations “mixing.” Some responses described the movement of genes, rather than the movement of alleles. Notably, Nehm and Reilly (2007) identified this misconception in undergraduates’ responses to an open-response item designed to measure knowledge of natural selection:
“Genetic drift involves the movement of alleles out of populations/gene pools to new environments.”
“Gene exchange between different populations of animals. Results in an increase or decrease of a specific type of gene.”
The third misconception in Associating Genetic Drift with Other Evolutionary Mechanisms defined genetic drift as natural selection. In some cases, these definitions of natural selection were nuanced and accurate; in other cases, responses were less detailed, but implied or described an interaction between traits and the environment resulting in differential reproductive success, survival, or fitness. One response defined genetic drift as sexual selection:
“Genetic drift occurs because survival of the fittest so if some alleles that are passed down to offspring provide a benefit, those alleles are more likely to get passed on to their offspring.”
“Genetic drift is the gradual change in the frequency of specific alleles in a population to be more or less common [and]…occurs when there is a change in the environment that makes specific traits more or less favorable for fitness.”
Finally, a few responses in this category defined genetic drift as any change in allele frequencies:
“Genetic drift is when there is a change in the allele frequency of a population.” “Drift is the alteration of genes by anything, including chance.”
Category 4: Associating Genetic Drift with Boundaries between Populations.
Biologists recognize the founder effect to be one scenario in which genetic drift can occur. Essentially, when a small random sample of individuals from a larger population become the founders of a new population, they are likely to carry only a fraction of the genetic variation of the original population ( Futuyma, 2005 ). Additionally, founding populations are often small and are therefore likely to be further impacted by genetic drift for many generations following the founding event. Moreover, genetic drift and natural selection can lead to reproductive isolation in a peripheral population, such as a founding population. This process is called peripatric speciation ( Futuyma, 2005 ). No responses in Associating genetic drift with boundaries between populations came close to indicating knowledge of the nuanced concepts just described. However, these responses defined genetic drift as movement, separation, and/or speciation, which hinted at knowledge of, or at least exposure to, founder effect as an example of genetic drift.
The most common misconception in Associating Genetic Drift with Population Boundaries defined genetic drift as migration, by which responses typically seemed to mean emigration. In the interest of preserving student ideas, we have also used the term migration to describe emigration or immigration. In some cases, responses described migration followed by adaptation to the environment. The descriptions of adaptation in these responses were similar to those in Novice Evolution in that they described adaptation as acclimation to environmental characteristics, but these responses were distinct in that they also discussed the movement of individuals. The units discussed in these responses included individuals, species, and populations. Some responses discussed individuals or groups moving to locations better suited to their traits.
The terms migration and gene flow are often used interchangeably by experts, who recognize that migration is an evolutionary process only when it leads to a change in allele frequencies. There was no indication that students understood this subtlety. The responses in this category differed from those that defined genetic drift as gene flow, because they did not mention the movement of alleles or genes:
“Genetic drift is when a certain species migrates to another location.”
“Genetic drift would be where members of a population with different traits move to an environment that fits those traits.”
“Genetic drift would take place if the butterflies would have migrated to another climate and adapted to their surroundings by the means of migrations.”
A similar misconception defined genetic drift as the separation or isolation of populations. In some cases, responses discussed separation followed by adaptation, by which they seemed to mean acclimation, to a new environment:
“Genetic drift generally happens when part of a species population is separated and become[s] distinguished and change[s].”
“Genetic drift is when members of the same species get separated by environmental forces and over time develop differently.”
The third misconception in this category defined genetic drift as speciation. While it is possible for genetic drift to contribute to speciation, these responses did not provide an explanation for how speciation would occur. About half of these responses defined genetic drift as speciation following the separation of populations:
“These species of butterflies were once the same then slowly over time began shifting into one species that prefer sunny meadows and another that prefers dense woodlands.”
“Genetic drift occurs when an offshoot of a population starts to develop traits that separate it from the original population, usually by a chance act.”
“Genetic drift happens when two species become isolated from each other or no longer reproduce, creating a cross breeds.”
Category 5: Developing Genetic Drift Comprehension.
Biologists recognize many nuances of the process of genetic drift. For example, genetic drift can result from random sampling of gametes during sexual reproduction, as well as random sampling of individuals, and their gametes, resulting from a population bottleneck ( Futuyma, 2005 ). Experts recognize drift occurs in all finite populations, but is likely to have a more pronounced impact given a small effective population size ( Barton et al. , 2007 ). Experts also know genetic drift can , but does not always, lead to the fixation of alleles, and that genetic drift tends to decrease genetic variation within a population and increase variation among populations ( Frankham et al. , 2002 ).
Responses in Developing Genetic Drift Comprehension indicated some knowledge of genetic drift. However, the definitions in this category placed inaccurate limitations on the circumstances under which genetic drift can occur.
The most common misconception in Developing Genetic Drift Comprehension defined genetic drift as, or as resulting from, an isolated event, often a catastrophe. These responses did not recognize genetic drift as a process occurring each generation:
“Genetic drift is where there is some event that decreases the variation in a population.”
Another misconception in Developing Genetic Drift Comprehension limited genetic drift to small populations:
“Genetic drift is a change in allele frequency due to a random genetic occurrence in a small population.”
The least common response in Developing Genetic Drift Comprehension described genetic drift as allele fixation, rather than describing fixation as a potential result of genetic drift:
“[Genetic drift is] when an allele gets fixed on a population.”
“[Genetic drift is] allele fixation due to limited gene pool.”
“[Genetic drift is when] a random event knocks out one genotype.”
Vague Responses That Hinted at Knowledge of Genetic Drift
Responses that hinted at knowledge of genetic drift used terms such as random or chance but otherwise did not indicate knowledge of genetic drift. In some cases, the term random or chance was embedded in misconceptions, but in most cases these responses were simply too vague to evaluate:
“Genetic drift is all about chances to the outcome of the offspring.”
Responses Indicating Some Knowledge of Genetic Drift
Responses indicating some knowledge of drift ranged considerably in quality. Some responses provided precise and nuanced definitions of genetic drift, others gave brief but accurate descriptions of drift, and some responses included misconceptions.
The following quote was one of the most articulate responses in our sample. In particular, the subtle and precise language differentiates this response from responses containing misconceptions. Though the response discusses an event or catastrophe leading to genetic drift—like responses in Developing Genetic Drift Comprehension—the use of the introductory clause “for instance” suggests that the student recognizes this is one example of drift, rather than the only circumstance under which drift takes place:
“Genetic drift is evolution that occurs purely by chance. For instance, an F 1 generation could have 10 red flowers, 10 pink flowers, and 10 white flowers. If all the white flowers are accidentally killed or something happens, their genes will not be passed on to future generations.”
In contrast, the next quote demonstrates how a response can indicate some knowledge of genetic drift and contain a misconception. The first sentence of the response confuses genetic drift with selection, while the second sentence indicates knowledge of genetic drift:
“[Genetic drift occurs when] through sexual or natural selection, certain alleles are favored. Additionally, it may just so happen that an allele becomes more or less prevalent though it neither helps nor harms individuals within a population.”
Results of Statistical Analyses
We used statistical analyses to address three predictions about student learning. We tested these predictions using data from the case study project ( n = 319), because this project collected data before and after introductory-level genetic drift instruction. We predicted that 1) the number of students who did not address drift, 2) the number of responses that indicated some knowledge of the definition of genetic drift, and 3) the number of responses containing at least one misconception would all be different before and after instruction.
All three of the predictions about student learning were supported by our data ( Table 2 ). In all cases, students exhibited more knowledge of genetic drift after instruction. The number of responses that did not address drift was significantly different before and after instruction (Fisher's test, p < 0.0001; Table 2 ), suggesting that students in these courses did not address drift before instruction because they had little or no knowledge of the concept. To test our second and third predictions, we examined only the responses from the case study data set in which students addressed drift ( n = 207). The number of responses indicating some knowledge of genetic drift was different before and after instruction ( p = 0.005; Table 2 ). Additionally, the number of responses containing at least one misconception was different before and after instruction ( p < 0.0001; Table 2 ).
When we examined the frequency of student responses containing each of the 16 distinct misconceptions at different stages of instruction, we noticed that while some misconceptions were less common among students who had received genetic drift instruction, other misconceptions were more common following instruction ( Table 3 ). Specifically, the misconceptions in Novice Genetics and Novice Evolution were less common after introductory instruction and among upper-division students, whereas misconceptions in Developing Genetic Drift Comprehension were absent before instruction, but increasingly common with more instruction ( Table 3 ). The frequency of misconceptions in Associating Genetic Drift with Other Evolutionary Mechanisms and Associating Genetic Drift with Population Boundaries remained about the same before and after introductory instruction, but among upper-division students these two categories diverged ( Table 3 ). Misconceptions in Associating Genetic Drift with Other Evolutionary Mechanisms were substantially more common among upper-division students than among introductory students, whereas misconceptions in Associating Genetic Drift with Population Boundaries were less common among upper-division students than among introductory students ( Table 3 ).
Our observations represent the first effort, to our knowledge, to describe students’ conceptions of genetic drift and how those conceptions change over time. Among students who addressed genetic drift in their responses, nearly all (99%) undergraduates in introductory biology courses had misconceptions about genetic drift before instruction, and almost 75% retained misconceptions after explicit genetic drift instruction ( Table 2 ). Furthermore, undergraduates who had completed introductory biology and were enrolled in upper-division biology courses for biology majors still had serious misconceptions about genetic drift ( Table 3 ).
To facilitate future research on student conceptions of genetic drift, we propose a framework to interpret students’ conceptions about genetic drift and to describe how those conceptions change as students learn. This framework suggests three hypotheses regarding undergraduates’ conceptions of genetic drift. The rest of this paper presents the framework and hypotheses, followed by implications for instruction and future research.
Our framework includes the five broad categories of misconceptions identified during our qualitative analysis. The arrows between categories of misconceptions in our framework represent ways in which students’ conceptions may be changing as they learn ( Figure 1 ). At one end of the framework are two categories of misconceptions most common among students before genetic drift instruction (Novice Genetics and Novice Evolution). Responses including misconceptions in these categories indicated no knowledge of genetic drift and only superficial—if any—knowledge of evolution. In the middle of the framework are two categories of misconceptions (Associating Genetic Drift with Population Boundaries and Associating Genetic Drift with Other Evolutionary Mechanisms) that were more common in students’ responses after some genetic drift instruction. These responses tended to use appropriate terminology about evolution, but did so in a way that revealed misconceptions and was often imprecise and disorganized. At the other end of the framework is the category of misconceptions indicating some knowledge of genetic drift, but also some confusion (Developing Genetic Drift Comprehension). Misconceptions in this category were most common among upper-division students who presumably had the most exposure to genetic drift.

This framework hypothesizes how students’ conceptions of genetic drift change over time. Each circle represents an overarching category of misconceptions. Arrows represent the ways in which students’ conceptions may be changing as they learn. (I) Students enter introductory biology with undeveloped conceptions of evolution that do not distinguish among mechanisms of evolutionary change. (II) Students’ conceptual frameworks of evolution grow more complex, but are still highly inaccurate. (III) Students reject some misconceptions but form new ones regarding inaccurate constraints on when drift occurs.
We did not include a stage representing Expertise in Genetic Drift in our framework, because we derived our framework solely from our data. The standard for expertise would be for students to comprehend genetic drift without misconceptions and to correctly apply their comprehension to novel problems dealing with drift. Students in our data set did not demonstrate this level of expertise. For example, we asked the participants in the concept inventory study to explain experimental results using their knowledge of genetic drift and none were able to do so.
On the basis of framework, we propose three hypotheses regarding undergraduates’ conceptions of genetic drift. First, we hypothesize that most students enter introductory biology courses with an undeveloped conception of evolution that does not distinguish among mechanisms of evolutionary change ( Figure 1 , I). Common misconceptions documented in studies of students’ conceptions of natural selection were actually common misconceptions about genetic drift as well ( Bishop and Anderson, 1990 ; Nehm and Reilly, 2007 ; Gregory, 2009 ). For example, students defined genetic drift as acclimation to the environment. The fact that these common misconceptions are associated with drift, as well as natural selection, suggests they are actually misconceptions about evolution in general. It appears that students who know nothing about genetic drift are using the context of the question or cues in class to associate genetic drift with evolution. They are then defining genetic drift as they would define evolution or natural selection, perhaps because they think all evolution is natural selection ( Jakobi, 2010 ). If misconceptions in Novice Evolution do in fact become less common after instruction, as our data suggest ( Table 3 ), that would support the hypothesis that students begin with a simplistic conception of evolution that grows more complex as they learn.
Second, we hypothesize that students’ conceptual frameworks of evolution grow more complex as they learn, but the added complexity is not necessarily more accurate than their previous, less complex, conceptual frameworks, nor is it expertly organized ( Figure 1 , II). Students seem to be gaining knowledge of biology vocabulary and concepts, but still lack deep understanding of concepts and scientifically accurate connections among concepts. Their definitions of genetic drift mix misconceptions, imprecise terminology, and irrelevant information with some accurate information. Responses containing misconceptions in the two categories at the center of our framework illustrate this confusion ( Figure 1 ).
Student conceptions probably do not skip from the novice to the developing comprehension end of our framework, but instead must move through the muddled intermediate stage ( Figure 1 ). The challenge for us as instructors is to move students through this stage effectively and efficiently, especially in introductory courses. An exciting area of future research will be to test the efficacy of teaching modules geared to addressing this issue.
Third, we hypothesize that genetic drift instruction leads to the rejection of some misconceptions and the formation of new ones (e.g., Yip, 1998 ). We observed that after instruction, fewer students had misconceptions in Novice Genetics and Novice Evolution, but more students had misconceptions in Developing Genetic Drift Comprehension ( Figure 1 , III). Among upper-division students, 48.6% had misconceptions in Associating Genetic Drift with Other Evolutionary Mechanisms. This is a substantially larger percentage than we observed among introductory students before (13.0%) or after (13.1%) instruction, suggesting additional genetic drift instruction revealed or generated misconceptions in this category. This result is simultaneously encouraging and discouraging. It is encouraging, because it indicates students’ ideas are changing. But it is discouraging in that most students still had misconceptions as upper-division biology undergraduates. It remains to be seen, through additional research, what conditions contribute to the development of Expertise in Genetic Drift (i.e., understanding and application without misconceptions).
Implications for Instruction
Our observations suggest that genetic drift is a challenging topic for students to learn. We have not found any exercises to teach genetic drift that have been assessed for impact on student learning, but a number of scholars have proposed ideas for teaching genetic drift and improving an instructor's degree of comfort with the concept (e.g., Staub, 2002 ; Young and Young, 2003 ; Masel, 2011 [includes a description of the classic experiment by Peter Buri], 2012).
Though it remains unclear what strategies might effectively facilitate student learning of genetic drift, our observations indicate one potential problem to avoid. Instruction that provides limited examples of genetic drift in action may inadvertently teach students that drift occurs only in such cases. For example, if instruction focuses on the founder effect, students may extrapolate that genetic drift only occurs when individuals move from one location to another or when a subset of a population is isolated from the larger population. Alternatively, students may assume genetic drift only occurs in small populations when scenarios used in class focus exclusively on drift within small populations.
Implications for Future Research
Evidence is accumulating that the student misconception that need is a rationale for change is common across biology concepts. Though biological explanations including the term “need” are not necessarily illegitimate ( Zohar and Ginossar, 1998 ), teleological reasoning commonly results in misconceptions. The most common misconception we observed among students was defining genetic drift as acclimation to the environment and, in many cases, describing acclimation as resulting from a need to survive. The most common misconception about natural selection is also the idea that individuals or populations change because they need to ( Gregory, 2009 ). This misconception extends beyond conceptions of evolution as well. When asked to explain pictures of biological phenomena, such as a plant growing toward the sun or a group of birds flying in a V formation, the most common idea provided by elementary and secondary school students was that organisms changed because they needed to ( Southerland et al. , 2001 ). Adults who had taken multiple college-level science courses also commonly explained natural phenomena as existing to fulfill a need ( Kelemen and Rosset, 2009 ). If this single (albeit tenacious) misconception is affecting students’ ability to learn concepts throughout biology, instruction specifically designed to help students think critically about this sort of reasoning could have an impressive impact on student learning. Future research can explicitly focus on determining the pervasiveness of the idea that need is a rationale for change in biological systems and on effective strategies for changing this misconception to a scientifically accurate explanation.
Future research is also necessary to fill out and refine our framework of how students learn genetic drift. Interviews will be valuable to gain deeper insight about student conceptions and how they change with instruction. A broader student population would also be valuable. For example, studying a larger sample of advanced undergraduates will be necessary to understand how student conceptions of genetic drift progress, including how instruction reveals or creates new misconceptions. Furthermore, different questions are likely to elucidate additional misconceptions ( Nehm and Ha, 2011 ).
Finally, future research can document how experts define genetic drift, as well as outlining the key concepts and skills needed to demonstrate expertise in genetic drift. Genetic drift is fundamental to evolution, yet often overlooked. For example, Teaching about Evolution and the Nature of Science ( National Academy of Sciences Working Group on Teaching Evolution, 1998 ) outlines the major themes in evolution (Ch. 2) but never mentions genetic drift. In the more recent Vision and Change ( American Association for the Advancement of Science, 2011 ), evolution is included in the list of core concepts that all undergraduates should understand but genetic drift is hardly mentioned. To correct this oversight, genetic drift experts and biology education experts need to collaborate to describe what a student who has a complete understanding of genetic drift should be able to do with that knowledge. It would also be useful for experts to think about the necessary scaffolds for learning genetic drift, as well as the recommended timing of scaffolding, for example, in high school biology, undergraduate introductory biology, and undergraduate advanced biology. We have begun to address this aim by uncovering misconceptions about genetic drift among biology undergraduates, and future research on student conceptions of drift has the potential to be just as fruitful.
Supplementary Material
Acknowledgments.
This material is based on work supported by the National Science Foundation (NSF) under grant number DUE-0920264 awarded to the National Center for Case Study Teaching in Science. Additional support was provided by the National Evolutionary Synthesis Center (NSF grant number EF-0905606). Any opinions, findings, conclusions, or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the NSF. We thank the students who participated in this study and their instructors. We also thank Erin L. Dolan, Steven T. Kalinowski, our reviewers, and the other members of the EvoCI Toolkit Working Group. Susan C. Alberts wrote the original draft of the butterfly question used as part of the case study project. This is a publication of both the University of Georgia Science Education Research Group and the EvoCI Toolkit Working Group at the NESCent.
† National Evolutionary Synthesis Center Working Group member.
‡ Present address: Division of Biological Sciences, University of Georgia, Athens, GA 30602.
†† Affiliate of the National Center for Case Study Teaching in Science.
- Abraham JK, Meir E, Perry J, Herron JC, Maruca S, Stal D. Addressing undergraduate student misconceptions about natural selection with an interactive simulated laboratory. Evol Educ Outreach. 2009; 2 :393–404. [ Google Scholar ]
- American Association for the Advancement of Science. Vision and Change in Undergraduate Biology Education: A Call to Action. Washington, DC: American Association for the Advancement of Science; 2011. [ Google Scholar ]
- Anderson DL, Fisher KM, Norman GJ. Development and evaluation of the conceptual inventory of natural selection. J Res Sci Teach. 2002; 39 :952–978. [ Google Scholar ]
- Andrews TM, Kalinowski ST, Leonard MJ. “Are humans evolving?” A classroom discussion to change students’ misconceptions about natural selection. Evol Educ Outreach. 2011; 4 :456–466. [ Google Scholar ]
- Anfara VA, Jr., Brown KM, Mangione TL. Qualitative analysis on stage: making the research process more public. Educ Res. 2002; 31 :28–38. [ Google Scholar ]
- Barton NH, Briggs DEG, Eisen JA, Goldstein DB, Patel NH. Evolution. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2007. [ Google Scholar ]
- Beatty J. Random drift. In: Keller EF, Lloyd EA, editors. Keywords in Evolutionary Biology. Cambridge, MA: Harvard University Press; 1992. [ Google Scholar ]
- Bishop B, Anderson C. Student conceptions of natural selection and its role in evolution. J Res Sci Teach. 1990; 27 :415–427. [ Google Scholar ]
- Cai L, Friedman N, Xie XS. Stochastic protein expression in individual cells at the single molecule level. Nature. 2006; 440 :358–362. [ PubMed ] [ Google Scholar ]
- Creswell JW. Qualitative Inquiry and Research Design: Choosing Among Five Approaches. 2nd ed. Thousand Oaks, CA: Sage; 2007. [ Google Scholar ]
- Cunningham DL, Wescott DJ. Still more “fancy” than “fact” in students’ conceptions of evolution. Evol Educ Outreach. 2009; 2 :505–517. [ Google Scholar ]
- Fischbein E, Schnarch D. The evolution with age of probabilistic, intuitively based misconceptions. J Res Math Educ. 1997; 28 :96. [ Google Scholar ]
- Frankham R, Ballou JD, Briscoe DA. Introduction to Conservation Genetics. Cambridge, UK: Cambridge University Press; 2002. [ Google Scholar ]
- Futuyma DJ. Evolution. Sunderland, MA: Sinauer Associates; 2005. [ Google Scholar ]
- Garvin-Doxas K, Klymkowsky M. Understanding randomness and its impact on student learning: lessons learned from building the Biology Concept Inventory (BCI) CBE Life Sci Educ. 2008; 7 :227–233. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Gilbert JK, Watts DM. Concepts, misconceptions and alternative conceptions: changing perspectives in science education. Stud Sci Educ. 1983; 10 :61–98. [ Google Scholar ]
- Glaser BG, Strauss AL. The Discovery of Grounded Theory: Strategies for Qualitative Research. New Brunswick, NJ: Aldine Transaction; 2010. [ Google Scholar ]
- Gregory E, Ellis JP, Orenstein AN. A proposal for a common minimal topic set in introductory biology courses for majors. Am Biol Teach. 2011; 73 :16–21. [ Google Scholar ]
- Gregory TR. Understanding natural selection: essential concepts and common misconceptions. Evol Educ Outreach. 2009; 2 :156–175. [ Google Scholar ]
- Jakobi SR. “Little monkey on the grass…” How people for and against evolution fail to understand the theory of evolution. Evol Educ Outreach. 2010; 3 :416–419. [ Google Scholar ]
- Jensen MS, Finley FN. Changes in students’ understanding of evolution resulting from different curricular and instructional strategies. J Res Sci Teach. 1996; 33 :879–900. [ Google Scholar ]
- Kalinowski ST, Leonard MJ, Andrews TM. Nothing in evolution makes sense except in the light of DNA. CBE Life Sci Educ. 2010; 9 :87–97. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kelemen D, Rosset E. The human function compunction: teleological explanation in adults. Cognition. 2009; 111 :138–143. [ PubMed ] [ Google Scholar ]
- Klymkowsky M, Garvin-Doxas K. Recognizing student misconceptions through Ed's Tools and the Biology Concept Inventory. PLoS Biol. 2008; 6 :14–17. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lecoutre M-P, Rovira K, Lecoutre B, Poitevineau J. People's intuition about randomness and probability: an empirical study. Stat Educ Res J. 2006; 5 :20–35. [ Google Scholar ]
- Maret TJ, Rissing SW. Exploring genetic drift and natural selection through a simulation activity. Am Biol Teach. 1998; 60 :681–683. [ Google Scholar ]
- Masel J. Genetic drift. Current Biol. 2011; 21 :R837–R838. [ PubMed ] [ Google Scholar ]
- Masel J. Rethinking Hardy-Weinberg and genetic drift in undergraduate biology. BioEssays. 2012 doi: 10.1002/bies.201100178. [ PubMed ] [ Google Scholar ]
- Mead LS, Scott EC. Problem concepts in evolution part II: cause and chance. Evol Educ Outreach. 2010; 3 :261–264. [ Google Scholar ]
- National Academy of Sciences Working Group on Teaching Evolution. Teaching about Evolution and the Nature of Science. Washington, DC: National Academies Press; 1998. [ Google Scholar ]
- National Research Council. BIO2010: Transforming Undergraduate Education for Future Research Biologists. Washington, DC: National Academies Press; 2003. [ PubMed ] [ Google Scholar ]
- Nehm RH, Ha M. Item features effects in evolution assessment. J Res Sci Teach. 2011; 48 :237–256. [ Google Scholar ]
- Nehm RH, Reilly L. Biology majors’ knowledge and misconceptions of natural selection. BioScience. 2007; 57 :263–272. [ Google Scholar ]
- Patton M. Qualitative Research and Evaluation Methods. 3rd ed. Newbury Park, CA: Sage; 2002. [ Google Scholar ]
- Ramsey F, Schafer D. The Statistical Sleuth: A Course in Methods of Data Analysis. 2nd ed. Pacific Grove, CA: Duxbury; 2002. [ Google Scholar ]
- Raup DM, Gould SJ, Schopf TJM, Simberloff DS. Stochastic models of phylogeny and the evolution of diversity. J Geol. 1973; 81 :525–542. [ Google Scholar ]
- R Project for Statistical Computing (2011). The R Project Home Page. www.r-project.org (accessed 9 December 2011)
- Settlage J. Conceptions of natural selection—a snapshot of the sense-making process. J Res Sci Teach. 1994; 31 :449–457. [ Google Scholar ]
- Southerland SA, Abrams E, Cummins CL, Anzelmo J. Understanding explanations of biological phenomena: conceptual frameworks or p-prims. Sci Educ. 2001; 85 :328–348. [ Google Scholar ]
- Staub NL. Teaching evolutionary mechanisms: genetic drift and M&M's ® BioScience. 2002; 52 :373–377. [ Google Scholar ]
- Taleb NN. Fooled by Randomness: The Hidden Role of Chance in Life and in the Markets. New York: Random House; 2005. [ Google Scholar ]
- Tanner K, Allen D. Approaches to biology teaching and learning: understanding wrong answers—teaching toward conceptual change. Cell Biol Educ. 2005; 4 :112–117. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Yip D. Identification of misconceptions in novice biology teachers and remedial strategies for improving biology learning. Int J Sci Educ. 1998; 4 :461–477. [ Google Scholar ]
- Young HJ, Young TP. A hands-on exercise to demonstrate evolution by natural selection and genetic drift. Am Biol Teach. 2003; 65 :444–448. [ Google Scholar ]
- Zohar A, Ginossar S. Lifting the taboo regarding teleology and anthropomorphism in biology education—heretical suggestions. Sci Educ. 1998; 82 :679–697. [ Google Scholar ]
- DOI: 10.1631/fitee.2300170
- Corpus ID: 271012884
PEGA: probabilistic environmental gradient-driven genetic algorithm considering epigenetic traits to balance global and local optimizations
- Zhiyu Duan , Shunkun Yang , +1 author Minghao Yang
- Published in Frontiers Inf. Technol… 1 June 2024
- Computer Science, Engineering, Biology
- Frontiers Inf. Technol. Electron. Eng.
20 References
Dynamic hybrid mechanism-based differential evolution algorithm and its application, mutation bias reflects natural selection in arabidopsis thaliana, inverse design of soft materials via a deep learning–based evolutionary strategy, an improved gray wolf optimization algorithm to solve engineering problems, a biological perspective on evolutionary computation, a review on genetic algorithm: past, present, and future, ant lion optimizer: a comprehensive survey of its variants and applications, evolutionary algorithms and their applications to engineering problems, a genetic programming hyper-heuristic approach for the multi-skill resource constrained project scheduling problem, a binary social spider algorithm for continuous optimization task, related papers.
Showing 1 through 3 of 0 Related Papers

IMAGES
VIDEO
COMMENTS
Volume 2. Charles J. Goodnight, Gil Rosenthal, in Encyclopedia of Animal Behavior (Second Edition), 2019 Phase 1: The Phase of Genetic Drift. During this first phase, evolution in small populations is dominated by genetic drift (Fig. 2(a)).Genetic drift is a function of population size: in very small populations, even selected alleles tend to behave as if they are neutral.
Genetic drift is a basic evolutionary principle describing random changes in allelic frequencies, with far-reaching consequences in various topics ranging from species conservation efforts to ...
Genetic drift can introduce alleles that are slightly deleterious to populations either when drift is strong or when there are many mutations whose effect are small (Schultz and Lynch 1997; Whitlock 2003). Since genetic drift should be relatively less important for the largest population, our results suggest that the number of these mutations ...
The theory outlined in Box 1 also predicts detectable levels (certainly two- to threefold) of variation in the mutation rate among lineages experiencing identical levels of selection and random ...
These experts did not necessarily teach or research the topic of genetic drift. We used a Welch's t test to compare the mean scores between experts and undergraduates. This analysis excludes one statement (19 in Supplemental Material, Genetic Drift Inventory 1.0) because we included the incorrect stem for one statement in the version of the ...
Abstract. Changes in pathogen genetic variation within hosts alter the severity and spread of infectious diseases, with important implications for clinical disease and public health. Genetic drift may play a strong role in shaping pathogen variation, but analyses of drift in pathogens have oversimplified pathogen population dynamics, either by ...
There is a long-standing debate about the role of genetic drift versus selection in evolutionary change (1-7).While this debate has sometimes been contentious, the answers to these questions are quantitative, describing the relative contributions of basic evolutionary forces to allele frequency change and how this differs across species and different functional categories.
This increased amount of genetic variation is due to the introduction of slightly deleterious alleles by genetic drift and this process is more efficient when migration load is higher. The incorporation of genetic drift also selects for fitness sets that exhibit allele-frequency equilibria with larger domains of attraction: they are "more stable."
Introduction. New disease mutations arise in heterozygotes and either drift to higher frequencies or are rapidly purged from the population, depending on the strength of selection and the demographic history of the population [1-6].Elucidating the relative contributions of mutation, natural selection and genetic drift will help to understand why disease alleles persist in humans.
Genetic drift is a basic evolutionary principle describing random changes in allelic frequencies, with far-reaching consequences in various topics ranging from species conservation efforts to speciation. ... 4 Faculty of Science, Research Centre for Toxic Compounds in the Environment, Masaryk University, Kamenice 5, Building A29, 62500, Brno ...
The allele 120I (P = 1 × 10 −10 ) had stable frequency (23%) over time. Genetic drift is a non-directional change in allele frequency that occurs by chance between generations by decreasing or ...
Hallatschek et al. [ 8] identified the key role of genetic drift in producing these sectored patterns; the small population size at the front of an expanding population [ 9, 10] enhances number fluctuations (i.e. genetic drift), eventually leading to the local fixation of one strain past a critical expansion radius R0.
Discover the world's research. 25+ million members; 160+ million publication pages; 2.3+ billion citations; ... increases and the stochastic effects of genetic drift (black arrows) decrease. The
Highlights. Less than 1.6% of cell lines in pharmacogenomic studies have genetic identity issues. A median of 4.5%-6.1% of the total genome is drifted between homonymous cell lines. Genetic drift is present within each dataset between different data types. Small association between total genetic drift and discordances in drug response.
Genetic drift, like any other evolutionary force, can only operate as an evolutionary force when there is genetic variability. Genetic drift causes its most dramatic and rapid changes in small populations. The chapter consider some examples of founder and bottleneck effects. Disassortative mating can strongly interact with drift-induced linkage ...
Abstract. The dynamics of extinction and (re)colonization in habitat patches are characterizing features of dynamic metapopulations, causing them to evolve differently than large, stable populations. The propagule model, which assumes genetic bottlenecks during colonization, posits that newly founded subpopulations have low genetic diversity ...
If populations have diversified through random evolutionary processes such as genetic drift, evolutionary theory predicts a proportional relationship between within-group and between-group phenotypic variation ( 14 - 16 ); a nonproportional relationship indicates the action of nonrandom processes such as differential selection.
The paucity of genetic diversity found in generation F 58 suggested that relatively few genetic lineages may have survived into the later stages of CCII. To understand the composition of the genomes of individual progeny, we conducted a genotyping by sequencing (GBS) experiment on CCII parents and 878 total progeny sampled across generations F ...
Introduction. When colonizing new areas, species may be subjected to novel selection pressures and develop new adaptations that differentiate them from their ancestors (Gillespie & Roderick, 2002; Kawecki, 2008; Barker et al., 2009).The genetic composition of a colonizing population may also differ from that of the ancestral population simply due to genetic drift, if colonization occurs ...
Genetic drift is a basic evolutionary principle describing random changes in allelic frequencies, with far-reaching consequences in various topics ranging from species conservation efforts to speciation. ... This work was financially supported from Operational Programme Research, Development and Education—Project CETOCOEN PLUS (No. CZ.02.1.01 ...
Genomic research has evolved from seeking to understand the fundamentals of the human genetic code to examining the ways in which this code varies among people, and then applying this knowledge to ...
Semantic Scholar extracted view of "Genetic drift" by J. Masel. ... Search 219,620,744 papers from all fields of science. Search. Sign In Create Free Account. DOI: 10.1016/j.cub.2011.08.007; Corpus ID: 17619958; Genetic drift ... AI-powered research tool for scientific literature, based at the Allen Institute for AI. Learn More.
Therefore, genetic duplication is predicted to proceed in a nearly neutral manner based on mutation pressure and genetic drift. In addition, "concerted evolution" in minisatellites used as markers for hyper-polymorphisms, and in other sequences such as rRNA genes can be explained well by Ohno's theory (Hillis et al. 1991 ; Jeffreys et al ...
Several recent studies have suggested genetic testing in some subpopulations of patients with AF (eg, those with early-onset AF). 4,5 Genetic studies on familial AF have identified associations with several genes, including the ion-channel gene KCNQ1 6 and the sarcomere gene MYL4. 7 However, it is difficult to assess the impact of these ...
This framework suggests three hypotheses regarding undergraduates' conceptions of genetic drift. The rest of this paper presents the framework and hypotheses, followed by implications for instruction and future research. ... and future research on student conceptions of drift has the potential to be just as fruitful. Supplementary Material.
This Perspective highlights where major differences still exist, and where the field of evolutionary computation could attempt to approach features from biological evolution more closely, namely neutrality and random drift, complex genotype-to-phenotype mappings with rich environmental interactions and major organizational transitions.