

More teens than ever are overdosing. Psychologists are leading new approaches to combat youth substance misuse
“Just Say No” didn’t work , but experts are employing new holistic programs to help steer kids away—or at least keep them from dying—from illicit substances.
Vol. 55 No. 2 Print version: page 48
- Substance Use, Abuse, and Addiction

For years, students in middle and high schools across the country were urged to “just say no” to drugs and alcohol. But it’s no secret that the Drug Abuse Resistance Education (D.A.R.E.) program, which was typically delivered by police officers who urged total abstinence, didn’t work. A meta-analysis found the program largely ineffective and one study even showed that kids who completed D.A.R.E. were more likely than their peers to take drugs ( Ennett, S. T., et al., American Journal of Public Health , Vol. 84, No. 9, 1994 ; Rosenbaum, D. P., & Hanson, G. S., Journal of Research in Crime and Delinquency , Vol. 35, No. 4, 1998 ).
“We know that the ‘Just Say No’ campaign doesn’t work. It’s based in pure risks, and that doesn’t resonate with teens,” said developmental psychologist Bonnie Halpern-Felsher, PhD, a professor of pediatrics and founder and executive director of several substance use prevention and intervention curriculums at Stanford University. “There are real and perceived benefits to using drugs, as well as risks, such as coping with stress or liking the ‘high.’ If we only talk about the negatives, we lose our credibility.”
Partially because of the lessons learned from D.A.R.E., many communities are taking a different approach to addressing youth substance use. They’re also responding to very real changes in the drug landscape. Aside from vaping, adolescent use of illicit substances has dropped substantially over the past few decades, but more teens are overdosing than ever—largely because of contamination of the drug supply with fentanyl, as well as the availability of stronger substances ( Most reported substance use among adolescents held steady in 2022, National Institute on Drug Abuse ).
“The goal is to impress upon youth that far and away the healthiest choice is not to put these substances in your body, while at the same time acknowledging that some kids are still going to try them,” said Aaron Weiner, PhD, ABPP, a licensed clinical psychologist based in Lake Forest, Illinois, and immediate past-president of APA’s Division 50 (Society of Addiction Psychology). “If that’s the case, we want to help them avoid the worst consequences.”
While that approach, which incorporates principles of harm reduction, is not universally accepted, evidence is growing for its ability to protect youth from accidental overdoses and other consequences of substance use, including addiction, justice involvement, and problems at school. Psychologists have been a key part of the effort to create, test, and administer developmentally appropriate, evidence-based programs that approach prevention in a holistic, nonstigmatizing way.
“Drugs cannot be this taboo thing that young people can’t ask about anymore,” said Nina Christie, PhD, a postdoctoral research fellow in the Center on Alcohol, Substance Use, and Addictions at the University of New Mexico. “That’s just a recipe for young people dying, and we can’t continue to allow that.”
Changes in drug use
In 2022, about 1 in 3 high school seniors, 1 in 5 sophomores, and 1 in 10 eighth graders reported using an illicit substance in the past year, according to the National Institute on Drug Abuse’s (NIDA) annual survey ( Monitoring the Future: National Survey Results on Drug Use, 1975–2022: Secondary School Students , NIDA, 2023 [PDF, 7.78MB] ). Those numbers were down significantly from prepandemic levels and essentially at their lowest point in decades.
Substance use during adolescence is particularly dangerous because psychoactive substances, including nicotine, cannabis, and alcohol, can interfere with healthy brain development ( Winters, K. C., & Arria, A., Prevention Research , Vol. 18, No. 2, 2011 ). Young people who use substances early and frequently also face a higher risk of developing a substance use disorder in adulthood ( McCabe, S. E., et al., JAMA Network Open , Vol. 5, No. 4, 2022 ). Kids who avoid regular substance use are more likely to succeed in school and to avoid problems with the juvenile justice system ( Public policy statement on prevention, American Society of Addiction Medicine, 2023 ).
“The longer we can get kids to go without using substances regularly, the better their chances of having an optimal life trajectory,” Weiner said.
The drugs young people are using—and the way they’re using them—have also changed, and psychologists say this needs to inform educational efforts around substance use. Alcohol and cocaine are less popular than they were in the 1990s; use of cannabis and hallucinogens, which are now more salient and easier to obtain, were higher than ever among young adults in 2021 ( Marijuana and hallucinogen use among young adults reached all-time high in 2021, NIDA ).
“Gen Z is drinking less alcohol than previous generations, but they seem to be increasingly interested in psychedelics and cannabis,” Christie said. “Those substances have kind of replaced alcohol as the cool thing to be doing.”
Young people are also seeing and sharing content about substance use on social media, with a rise in posts and influencers promoting vaping on TikTok and other platforms ( Vassey, J., et al., Nicotine & Tobacco Research , 2023 ). Research suggests that adolescents and young adults who see tobacco or nicotine content on social media are more likely to later start using it ( Donaldson, S. I., et al., JAMA Pediatrics , Vol. 176, No. 9, 2022 ).
A more holistic view
Concern for youth well-being is what drove the well-intentioned, but ultimately ineffective, “mad rush for abstinence,” as Robert Schwebel, PhD, calls it. Though that approach has been unsuccessful in many settings, a large number of communities still employ it, said Schwebel, a clinical psychologist who created the Seven Challenges Program for treating substance use in youth.
But increasingly, those working to prevent and treat youth substance use are taking a different approach—one that aligns with principles Schwebel helped popularize through Seven Challenges.
A key tenet of modern prevention and treatment programs is empowering youth to make their own decisions around substance use in a developmentally appropriate way. Adolescents are exploring their identities (including how they personally relate to drugs), learning how to weigh the consequences of their actions, and preparing for adulthood, which involves making choices about their future. The Seven Challenges Program, for example, uses supportive journaling exercises, combined with counseling, to help young people practice informed decision-making around substance use with those processes in mind.
“You can insist until you’re blue in the face, but that’s not going to make people abstinent. They ultimately have to make their own decisions,” Schwebel said.
Today’s prevention efforts also tend to be more holistic than their predecessors, accounting for the ways drug use relates to other addictive behaviors, such as gaming and gambling, or risky choices, such as fighting, drag racing, and having unprotected sex. Risk factors for substance use—which include trauma, adverse childhood experiences, parental history of substance misuse, and personality factors such as impulsivity and sensation seeking—overlap with many of those behaviors, so it often makes sense to address them collectively.
[ Related: Psychologists are innovating to tackle substance use ]
“We’ve become more sophisticated in understanding the biopsychosocial determinants of alcohol and drug use and moving beyond this idea that it’s a disease and the only solution is medication,” said James Murphy, PhD, a professor of psychology at the University of Memphis who studies addictive behaviors and how to intervene.
Modern prevention programs also acknowledge that young people use substances to serve a purpose—typically either social or emotional in nature—and if adults expect them not to use, they should help teens learn to fulfill those needs in a different way, Weiner said.
“Youth are generally using substances to gain friends, avoid losing them, or to cope with emotional problems that they’re having,” he said. “Effective prevention efforts need to offer healthy alternatives for achieving those goals.”
Just say “know”
At times, the tenets of harm reduction and substance use prevention seem inherently misaligned. Harm reduction, born out of a response to the AIDS crisis, prioritizes bodily autonomy and meeting people where they are without judgment. For some harm reductionists, actively encouraging teens against using drugs could violate the principle of respecting autonomy, Weiner said.
On the other hand, traditional prevention advocates may feel that teaching adolescents how to use fentanyl test strips or encouraging them not to use drugs alone undermines the idea that they can choose not to use substances. But Weiner says both approaches can be part of the solution.
“It doesn’t have to be either prevention or harm reduction, and we lose really important tools when we say it has to be one or the other,” he said.
In adults, harm reduction approaches save lives, prevent disease transmission, and help people connect with substance use treatment ( Harm Reduction, NIDA, 2022 ). Early evidence shows similar interventions can help adolescents improve their knowledge and decision-making around drug use ( Fischer, N. R., Substance Abuse Treatment, Prevention, and Policy , Vol. 17, 2022 ). Teens are enthusiastic about these programs, which experts often call “Just Say Know” to contrast them with the traditional “Just Say No” approach. In one pilot study, 94% of students said a “Just Say Know” program provided helpful information and 92% said it might influence their approach to substance use ( Meredith, L. R., et al., The American Journal of Drug and Alcohol Abuse , Vol. 47, No. 1, 2021 ).
“Obviously, it’s the healthiest thing if we remove substance use from kids’ lives while their brains are developing. At the same time, my preference is that we do something that will have a positive impact on these kids’ health and behaviors,” said Nora Charles, PhD, an associate professor and head of the Youth Substance Use and Risky Behavior Lab at the University of Southern Mississippi. “If the way to do that is to encourage more sensible and careful engagement with illicit substances, that is still better than not addressing the problem.”
One thing not to do is to overly normalize drug use or to imply that it is widespread, Weiner said. Data show that it’s not accurate to say that most teens have used drugs in the past year or that drugs are “just a part of high school life.” In fact, students tend to overestimate how many of their peers use substances ( Dumas, T. M., et al., Addictive Behaviors , Vol. 90, 2019 ; Helms, S. W., et al., Developmental Psychology , Vol. 50, No. 12, 2014 ).
A way to incorporate both harm reduction and traditional prevention is to customize solutions to the needs of various communities. For example, in 2022, five Alabama high school students overdosed on a substance laced with fentanyl, suggesting that harm reduction strategies could save lives in that community. Other schools with less reported substance use might benefit more from a primary prevention-style program.
At Stanford, Halpern-Felsher’s Research and Education to Empower Adolescents and Young Adults to Choose Health (REACH) Lab has developed a series of free, evidence-based programs through community-based participatory research that can help populations with different needs. The REACH Lab offers activity-based prevention, intervention, and cessation programs for elementary, middle, and high school students, including curricula on alcohol, vaping, cannabis, fentanyl, and other drugs ( Current Problems in Pediatric and Adolescent Health Care , Vol. 52, No. 6, 2022 ). They’re also working on custom curricula for high-risk groups, including sexual and gender minorities.
The REACH Lab programs, including the comprehensive Safety First curriculum , incorporate honest discussion about the risks and benefits of using substances. For example: Drugs are one way to cope with stress, but exercise, sleep, and eating well can also help. Because many young people care about the environment, one lesson explores how cannabis and tobacco production causes environmental harm.
The programs also dispel myths about how many adolescents are using substances and help them practice skills, such as how to decline an offer to use drugs in a way that resonates with them. They learn about the developing brain in a positive way—whereas teens were long told they can’t make good decisions, Safety First empowers them to choose to protect their brains and bodies by making healthy choices across the board.
“Teens can make good decisions,” Halpern-Felsher said. “The equation is just different because they care more about certain things—peers, relationships—compared to adults.”
Motivating young people
Because substance use and mental health are so intertwined, some programs can do prevention successfully with very little drug-focused content. In one of the PreVenture Program’s workshops for teens, only half a page in a 35-page workbook explicitly mentions substances.
“That’s what’s fascinating about the evidence base for PreVenture,” said clinical psychologist Patricia Conrod, PhD, a professor of psychiatry at the University of Montreal who developed the program. “You can have quite a dramatic effect on young people’s substance use without even talking about it.”
PreVenture offers a series of 90-minute workshops that apply cognitive behavioral insights upstream (addressing the root causes of a potential issue rather than waiting for symptoms to emerge) to help young people explore their personality traits and develop healthy coping strategies to achieve their long-term goals.
Adolescents high in impulsivity, hopelessness, thrill-seeking, or anxiety sensitivity face higher risks of mental health difficulties and substance use, so the personalized material helps them practice healthy coping based on their personality type. For example, the PreVenture workshop that targets anxiety sensitivity helps young people learn to challenge cognitive distortions that can cause stress, then ties that skill back to their own goals.
The intervention can be customized to the needs of a given community (in one trial, drag racing outstripped substance use as the most problematic thrill-seeking behavior). In several randomized controlled trials of PreVenture, adolescents who completed the program started using substances later than peers who did not receive the intervention and faced fewer alcohol-related harms ( Newton, N. C., et al., JAMA Network Open , Vol. 5, No. 11, 2022 ). The program has also been shown to reduce the likelihood that adolescents will experiment with illicit substances, which relates to the current overdose crisis in North America, Conrod said ( Archives of General Psychiatry , Vol. 67, No. 1, 2010 ).
“People shouldn’t shy away from a targeted approach like this,” Conrod said. “Young people report that having the words and skills to manage their traits is actually helpful, and the research shows that at behavioral level, it really does protect them.”
As young people leave secondary school and enter college or adult life, about 30% will binge drink, 8% will engage in heavy alcohol use, and 20% will use illicit drugs ( Alcohol and Young Adults Ages 18 to 24, National Institute on Alcohol Abuse and Alcoholism, 2023 ; SAMHSA announces national survey on drug use and health (NSDUH) results detailing mental illness and substance use levels in 2021 ). But young people are very unlikely to seek help, even if those activities cause them distress, Murphy said. For that reason, brief interventions that leverage motivational interviewing and can be delivered in a school, work, or medical setting can make a big difference.
In an intervention Murphy and his colleagues are testing, young adults complete a questionnaire about how often they drink or use drugs, how much money they spend on substances, and negative things that have happened as a result of those choices (getting into an argument or having a hangover, for example).
In an hour-long counseling session, they then have a nonjudgmental conversation about their substance use, where the counselor gently amplifies any statements the young person makes about negative outcomes or a desire to change their behavior. Participants also see charts that quantify how much money and time they spend on substances, including recovering from being intoxicated, and how that stacks up against other things they value, such as exercise, family time, and hobbies.
“For many young people, when they look at what they allocate to drinking and drug use, relative to these other things that they view as much more important, it’s often very motivating,” Murphy said.
A meta-analysis of brief alcohol interventions shows that they can reduce the average amount participants drink for at least 6 months ( Mun, E.Y., et al., Prevention Science , Vol. 24, No. 8, 2023 ). Even a small reduction in alcohol use can be life-altering, Murphy said. The fourth or fifth drink on a night out, for example, could be the one that leads to negative consequences—so reducing intake to just three drinks may make a big difference for young people.
Conrod and her colleagues have also adapted the PreVenture Program for university students; they are currently testing its efficacy in a randomized trial across multiple institutions.
Christie is also focused on the young adult population. As a policy intern with Students for Sensible Drug Policy, she created a handbook of evidence-based policies that college campuses can use to reduce harm among students but still remain compliant with federal law. For example, the Drug Free Schools and Communities Act mandates that higher education institutions formally state that illegal drug use is not allowed on campus but does not bar universities from taking an educational or harm reduction-based approach if students violate that policy.
“One low-hanging fruit is for universities to implement a Good Samaritan policy, where students can call for help during a medical emergency and won’t get in trouble, even if illegal substance use is underway,” she said.
Ultimately, taking a step back to keep the larger goals in focus—as well as staying dedicated to prevention and intervention approaches backed by science—is what will help keep young people healthy and safe, Weiner said.
“What everyone can agree on is that we want kids to have the best life they can,” he said. “If we can start there, what tools do we have available to help?”
Further reading
Public Policy Statement on Prevention American Society of Addiction Medicine, 2023
Listen to young people: How to implement harm reduction in the collegiate setting Christie, N. C., 2023
Brief alcohol interventions for young adults: Strengthening effects and disentangling mechanisms to build personalized interventions for widespread uptake Special issue of Psychology of Addictive Behaviors , 2022
Addressing adolescent substance use with a public health prevention framework: The case for harm reduction Winer, J. M., et al., Annals of Medicine , 2022
A breath of knowledge: Overview of current adolescent e-cigarette prevention and cessation programs Liu, J., et al., Current Addiction Reports , 2020
Recommended Reading
Six things psychologists are talking about.
The APA Monitor on Psychology ® sister e-newsletter offers fresh articles on psychology trends, new research, and more.
Welcome! Thank you for subscribing.
Speaking of Psychology
Subscribe to APA’s audio podcast series highlighting some of the most important and relevant psychological research being conducted today.
Subscribe to Speaking of Psychology and download via:

Contact APA
You may also like.

Essay on Effect of Drugs on Youth
Students are often asked to write an essay on Effect of Drugs on Youth in their schools and colleges. And if you’re also looking for the same, we have created 100-word, 250-word, and 500-word essays on the topic.
Let’s take a look…
100 Words Essay on Effect of Drugs on Youth
Introduction.
Drugs have a significant influence on youth, often leading to harmful consequences. Young people are more susceptible to addiction due to their developing brains.
Physical Impact
Drugs can severely damage the health of young people. They can lead to heart diseases, lung problems, and other serious illnesses.
Mental Impact
Drugs can also cause mental health issues like depression, anxiety, and even psychosis. They can affect memory and learning abilities, hindering academic performance.
Social Consequences
Drug use can lead to strained relationships, isolation, and legal problems. It can also lead to risky behaviors, affecting the future of the youth.
250 Words Essay on Effect of Drugs on Youth
The allure and consequences of drug use.
The youth, often considered the backbone of society, are increasingly falling prey to the menace of drug abuse. The reasons behind this are manifold – peer pressure, curiosity, stress, and the desire for an ‘escape’ from reality. These substances, while offering temporary relief, have devastating long-term effects.
Physical and Psychological Impact
Drugs interfere with the normal functioning of the brain, leading to physical and psychological dependencies. Prolonged usage can cause severe health issues like liver damage, cardiovascular diseases, and even brain damage. Psychologically, drugs can lead to anxiety, depression, and suicidal tendencies.
Impact on Education and Career
Drug abuse also affects academic performance and career prospects. Concentration levels drop, grades plummet, and the ability to perform even simple tasks diminishes. This leads to a vicious cycle of poor performance and increased drug use.
The social implications are equally alarming. Drug abuse can lead to isolation, as relationships with family and friends deteriorate. It can also lead to criminal activities, as individuals resort to unlawful means to fund their addiction.
Conclusion: The Need for Intervention
The effects of drug abuse on youth are far-reaching and destructive. It is crucial to create awareness about the dangers of drug use, promote healthy coping mechanisms, and provide support for those struggling with addiction. This is not just an individual fight, but a societal one that requires collective action and commitment.
500 Words Essay on Effect of Drugs on Youth
Physical health consequences.
Youth is a critical period for physical development. Drug abuse can significantly hinder this process, leading to severe health problems. Drugs like alcohol, marijuana, and opioids can cause long-lasting damage to the brain, liver, heart, and other organs. The damage can be immediate, like alcohol poisoning, or long-term, such as liver cirrhosis or heart disease.
Mental Health Implications
The effects of drugs on youth extend beyond the physical to the psychological realm. Drugs can alter brain chemistry, leading to mental health disorders such as depression, anxiety, and psychosis. Moreover, the dependence on drugs can exacerbate feelings of isolation, loneliness, and low self-esteem, creating a vicious cycle of substance use and emotional pain.
Academic Performance and Future Prospects
Social relationships and crime.
Drug use can strain relationships with family and friends, leading to social isolation. Additionally, the illicit nature of drug use can expose youth to criminal activities and legal problems. The association between drug use and crime is well-documented, with young drug users more likely to engage in criminal behavior, further limiting their opportunities.
Prevention and Intervention
Given the profound impact of drugs on youth, prevention and intervention strategies are crucial. Schools, families, and communities need to work together to educate young people about the dangers of drug use. Early intervention programs can help identify at-risk youth, providing them with the necessary support to overcome potential drug problems.
If you’re looking for more, here are essays on other interesting topics:
Apart from these, you can look at all the essays by clicking here .
Leave a Reply Cancel reply
Save my name, email, and website in this browser for the next time I comment.
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Risk and protective factors of drug abuse among adolescents: a systematic review
Azmawati mohammed nawi, rozmi ismail, fauziah ibrahim, mohd rohaizat hassan, mohd rizal abdul manaf, norhayati ibrahim, nurul shafini shafurdin.
- Author information
- Article notes
- Copyright and License information
Corresponding author.
Received 2021 Jun 10; Accepted 2021 Sep 22; Collection date 2021.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Drug abuse is detrimental, and excessive drug usage is a worldwide problem. Drug usage typically begins during adolescence. Factors for drug abuse include a variety of protective and risk factors. Hence, this systematic review aimed to determine the risk and protective factors of drug abuse among adolescents worldwide.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) was adopted for the review which utilized three main journal databases, namely PubMed, EBSCOhost, and Web of Science. Tobacco addiction and alcohol abuse were excluded in this review. Retrieved citations were screened, and the data were extracted based on strict inclusion and exclusion criteria. Inclusion criteria include the article being full text, published from the year 2016 until 2020 and provided via open access resource or subscribed to by the institution. Quality assessment was done using Mixed Methods Appraisal Tools (MMAT) version 2018 to assess the methodological quality of the included studies. Given the heterogeneity of the included studies, a descriptive synthesis of the included studies was undertaken.
Out of 425 articles identified, 22 quantitative articles and one qualitative article were included in the final review. Both the risk and protective factors obtained were categorized into three main domains: individual, family, and community factors. The individual risk factors identified were traits of high impulsivity; rebelliousness; emotional regulation impairment, low religious, pain catastrophic, homework completeness, total screen time and alexithymia; the experience of maltreatment or a negative upbringing; having psychiatric disorders such as conduct problems and major depressive disorder; previous e-cigarette exposure; behavioral addiction; low-perceived risk; high-perceived drug accessibility; and high-attitude to use synthetic drugs. The familial risk factors were prenatal maternal smoking; poor maternal psychological control; low parental education; negligence; poor supervision; uncontrolled pocket money; and the presence of substance-using family members. One community risk factor reported was having peers who abuse drugs. The protective factors determined were individual traits of optimism; a high level of mindfulness; having social phobia; having strong beliefs against substance abuse; the desire to maintain one’s health; high paternal awareness of drug abuse; school connectedness; structured activity and having strong religious beliefs.
The outcomes of this review suggest a complex interaction between a multitude of factors influencing adolescent drug abuse. Therefore, successful adolescent drug abuse prevention programs will require extensive work at all levels of domains.
Keywords: Risk factor; Protective factor; Drug abuse, substance, adolescent
Introduction
Drug abuse is a global problem; 5.6% of the global population aged 15–64 years used drugs at least once during 2016 [ 1 ]. The usage of drugs among younger people has been shown to be higher than that among older people for most drugs. Drug abuse is also on the rise in many ASEAN (Association of Southeast Asian Nations) countries, especially among young males between 15 and 30 years of age. The increased burden due to drug abuse among adolescents and young adults was shown by the Global Burden of Disease (GBD) study in 2013 [ 2 ]. About 14% of the total health burden in young men is caused by alcohol and drug abuse. Younger people are also more likely to die from substance use disorders [ 3 ], and cannabis is the drug of choice among such users [ 4 ].
Adolescents are the group of people most prone to addiction [ 5 ]. The critical age of initiation of drug use begins during the adolescent period, and the maximum usage of drugs occurs among young people aged 18–25 years old [ 1 ]. During this period, adolescents have a strong inclination toward experimentation, curiosity, susceptibility to peer pressure, rebellion against authority, and poor self-worth, which makes such individuals vulnerable to drug abuse [ 2 ]. During adolescence, the basic development process generally involves changing relations between the individual and the multiple levels of the context within which the young person is accustomed. Variation in the substance and timing of these relations promotes diversity in adolescence and represents sources of risk or protective factors across this life period [ 6 ]. All these factors are crucial to helping young people develop their full potential and attain the best health in the transition to adulthood. Abusing drugs impairs the successful transition to adulthood by impairing the development of critical thinking and the learning of crucial cognitive skills [ 7 ]. Adolescents who abuse drugs are also reported to have higher rates of physical and mental illness and reduced overall health and well-being [ 8 ].
The absence of protective factors and the presence of risk factors predispose adolescents to drug abuse. Some of the risk factors are the presence of early mental and behavioral health problems, peer pressure, poorly equipped schools, poverty, poor parental supervision and relationships, a poor family structure, a lack of opportunities, isolation, gender, and accessibility to drugs [ 9 ]. The protective factors include high self-esteem, religiosity, grit, peer factors, self-control, parental monitoring, academic competence, anti-drug use policies, and strong neighborhood attachment [ 10 – 15 ].
The majority of previous systematic reviews done worldwide on drug usage focused on the mental, psychological, or social consequences of substance abuse [ 16 – 18 ], while some focused only on risk and protective factors for the non-medical use of prescription drugs among youths [ 19 ]. A few studies focused only on the risk factors of single drug usage among adolescents [ 20 ]. Therefore, the development of the current systematic review is based on the main research question: What is the current risk and protective factors among adolescent on the involvement with drug abuse? To the best of our knowledge, there is limited evidence from systematic reviews that explores the risk and protective factors among the adolescent population involved in drug abuse. Especially among developing countries, such as those in South East Asia, such research on the risk and protective factors for drug abuse is scarce. Furthermore, this review will shed light on the recent trends of risk and protective factors and provide insight into the main focus factors for prevention and control activities program. Additionally, this review will provide information on how these risk and protective factors change throughout various developmental stages. Therefore, the objective of this systematic review was to determine the risk and protective factors of drug abuse among adolescents worldwide. This paper thus fills in the gaps of previous studies and adds to the existing body of knowledge. In addition, this review may benefit certain parties in developing countries like Malaysia, where the national response to drugs is developing in terms of harm reduction, prison sentences, drug treatments, law enforcement responses, and civil society participation.
This systematic review was conducted using three databases, PubMed, EBSCOhost, and Web of Science, considering the easy access and wide coverage of reliable journals, focusing on the risk and protective factors of drug abuse among adolescents from 2016 until December 2020. The search was limited to the last 5 years to focus only on the most recent findings related to risk and protective factors. The search strategy employed was performed in accordance with the Preferred Reporting Items for a Systematic Review and Meta-analysis (PRISMA) checklist.
A preliminary search was conducted to identify appropriate keywords and determine whether this review was feasible. Subsequently, the related keywords were searched using online thesauruses, online dictionaries, and online encyclopedias. These keywords were verified and validated by an academic professor at the National University of Malaysia. The keywords used as shown in Table 1 .
The search strings
| Database Search string | |
|---|---|
| PubMed | adolescent OR teenager OR teen OR youth OR school-going children OR youngster OR pediatric* AND abuse OR addiction OR dependence OR habituation OR overdose OR misuse OR overuse OR use AND drug OR narcotic OR opioid OR psychoactive substance OR amphetamine OR cannabis OR ecstasy OR heroin OR cocaine OR hallucinogen* OR depressant OR stimulant OR marijuana OR illicit drug OR tranquilizers OR sedatives OR LSD OR Fentanyl OR illegal drug OR street drug OR club drug OR recreational drug OR substances AND risk factor OR protective factor OR predictive factor OR determinant OR cause |
| EBSCOhost | TX (“adolescent” OR “teenager” OR “teen’ OR youth” OR “school-going children” OR “youngster” OR pediatric) AND TX (“abuse” OR “addiction” OR “dependence” OR “habituation” OR “overdose” OR “misuse” OR “overuse” OR “use”) AND TX (“drug” OR “narcotic” OR “opioid” OR “psychoactive substance” OR “amphetamine” OR “cannabis” OR “ecstasy” OR “heroin” OR “cocaine” OR “hallucinogens” OR “depressant” OR “stimulant” OR “marijuana” OR “illicit drug” OR “tranquilizers” OR “sedatives” OR “LSD” OR “Fentanyl” OR “illegal drug” OR “street drug” OR “recreational drug” OR “substances”) AND TX (“risk factor” OR “protective factor” OR “predictive factor” OR “determinant” OR “cause”) |
| WoS | TS = (((“adolescent” OR “teenager” OR “teen’ OR youth” OR “school-going children” OR “youngster” OR pediatric*) AND (“abuse” OR “ad-diction” OR “dependence” OR “habituation” OR “overdose” OR “misuse” OR “overuse” OR “use*”) AND (“drug” OR “narcotic” OR “opioid” OR “psychoactive substance” OR “amphetamine” OR “cannabis” OR “ecstasy” OR “heroin” OR “cocaine” OR “hallucinogens” OR “depressant” OR “stimulant” OR “marijuana” OR “illicit drug” OR “tranquilizers” OR “sedatives” OR “LSD” OR “Fentanyl” OR “illegal drug” OR “street drug” OR “recreational drug” OR “sub-stances”) AND (“risk factor” OR “protective factor” OR “predictive factor” OR “determinant” OR “cause”) |
Selection criteria
The systematic review process for searching the articles was carried out via the steps shown in Fig. 1 . Firstly, screening was done to remove duplicate articles from the selected search engines. A total of 240 articles were removed in this stage. Titles and abstracts were screened based on the relevancy of the titles to the inclusion and exclusion criteria and the objectives. The inclusion criteria were full text original articles, open access articles or articles subscribed to by the institution, observation and intervention study design and English language articles. The exclusion criteria in this search were (a) case study articles, (b) systematic and narrative review paper articles, (c) non-adolescent-based analyses, (d) non-English articles, and (e) articles focusing on smoking (nicotine) and alcohol-related issues only. A total of 130 articles were excluded after title and abstract screening, leaving 55 articles to be assessed for eligibility. The full text of each article was obtained, and each full article was checked thoroughly to determine if it would fulfil the inclusion criteria and objectives of this study. Each of the authors compared their list of potentially relevant articles and discussed their selections until a final agreement was obtained. A total of 22 articles were accepted to be included in this review. Most of the excluded articles were excluded because the population was not of the target age range—i.e., featuring subjects with an age > 18 years, a cohort born in 1965–1975, or undergraduate college students; the subject matter was not related to the study objective—i.e., assessing the effects on premature mortality, violent behavior, psychiatric illness, individual traits, and personality; type of article such as narrative review and neuropsychiatry review; and because of our inability to obtain the full article—e.g., forthcoming work in 2021. One qualitative article was added to explain the domain related to risk and the protective factors among the adolescents.
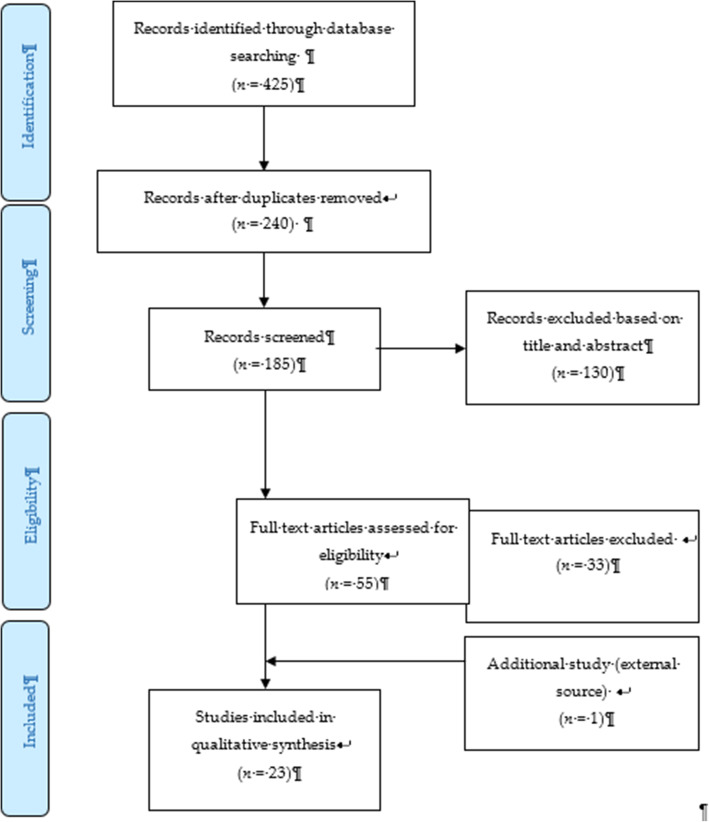
PRISMA flow diagram showing the selection of studies on risk and protective factors for drug abuse among adolescents.2.2. Operational Definition
Drug-related substances in this context refer to narcotics, opioids, psychoactive substances, amphetamines, cannabis, ecstasy, heroin, cocaine, hallucinogens, depressants, and stimulants. Drugs of abuse can be either off-label drugs or drugs that are medically prescribed. The two most commonly abused substances not included in this review are nicotine (tobacco) and alcohol. Accordingly, e-cigarettes and nicotine vape were also not included. Further, “adolescence” in this study refers to members of the population aged between 10 to 18 years [ 21 ].
Data extraction tool
All researchers independently extracted information for each article into an Excel spreadsheet. The data were then customized based on their (a) number; (b) year; (c) author and country; (d) titles; (e) study design; (f) type of substance abuse; (g) results—risks and protective factors; and (h) conclusions. A second reviewer crossed-checked the articles assigned to them and provided comments in the table.
Quality assessment tool
By using the Mixed Method Assessment Tool (MMAT version 2018), all articles were critically appraised for their quality by two independent reviewers. This tool has been shown to be useful in systematic reviews encompassing different study designs [ 22 ]. Articles were only selected if both reviewers agreed upon the articles’ quality. Any disagreement between the assigned reviewers was managed by employing a third independent reviewer. All included studies received a rating of “yes” for the questions in the respective domains of the MMAT checklists. Therefore, none of the articles were removed from this review due to poor quality. The Cohen’s kappa (agreement) between the two reviewers was 0.77, indicating moderate agreement [ 23 ].
The initial search found 425 studies for review, but after removing duplicates and applying the criteria listed above, we narrowed the pool to 22 articles, all of which are quantitative in their study design. The studies include three prospective cohort studies [ 24 – 26 ], one community trial [ 27 ], one case-control study [ 28 ], and nine cross-sectional studies [ 29 – 45 ]. After careful discussion, all reviewer panels agreed to add one qualitative study [ 46 ] to help provide reasoning for the quantitative results. The selected qualitative paper was chosen because it discussed almost all domains on the risk and protective factors found in this review.
A summary of all 23 articles is listed in Table 2 . A majority of the studies (13 articles) were from the United States of America (USA) [ 25 – 27 , 29 – 31 , 34 , 36 – 45 ], three studies were from the Asia region [ 32 , 33 , 38 ], four studies were from Europe [ 24 , 28 , 40 , 44 ], and one study was from Latin America [ 35 ], Africa [ 43 ] and Mediterranean [ 45 ]. The number of sample participants varied widely between the studies, ranging from 70 samples (minimum) to 700,178 samples (maximum), while the qualitative paper utilized a total of 100 interviewees. There were a wide range of drugs assessed in the quantitative articles, with marijuana being mentioned in 11 studies, cannabis in five studies, and opioid (six studies). There was also large heterogeneity in terms of the study design, type of drug abused, measurements of outcomes, and analysis techniques used. Therefore, the data were presented descriptively.
Study characteristic and main findings
| No | Year | Authors/ Country | Study objectives | Study design | Types of substance abuse | Result / findings Risk factors /Protective factors | Conclusion |
|---|---|---|---|---|---|---|---|
| 1 | 2020 | Dash et al. (USA) | To capture a time-sensitive report of the intersection of prescription opioid receipt and contextual risks for opioid misuse related to pain experience, mental health symptoms, and substance use at the adolescent and parental levels. | Cross-sectional | Opiod | Risk Factors 1) Pain catastrophe 2) Mother history of chronic pain (parents reported keeping opioids at home) and parent anxiety | Opioids at home as a risk factors for adolescent misuse |
| 2 | 2020 | Osborne et al. (USA) | To examine peer influence and parental guidance, in addition to peer and parental sources of alcohol, on patterns of prescription opioid use | Cross-sectional | Opiod | Risk factors 1) Close friend who used other substances 2) Alcoholic parents Protective Factors 1) Increased number of close friends | Increased number of close friends was a protective factor against prescription opioid |
| 3 | 2020 | Zuckermann et al.(Canada) | To investigate demographic and behavioral risk factors for non-medical use of prescription opioids. | Cross-sectional study | Opiod: oxycodone, fentanyl, other prescription pain relievers | Risk factors 1) lack of homework completion Protective Factors 1) School connectedness | School connectedness may lower the risk of non-medical use of prescription opioids, indicating that a school-based focus is justified. |
| 4 | 2020 | Spillane et al. (USA) | To examines the role of perceived availability and engagement in structured and unstructured activities on adolescent alcohol and marijuana use controlling for substance availability | Cross sectional | Marijuana | Risk Factors 1) Availability of unstructured activities | Perceived availability of and engagement in unstructured activities may present a risk, while perceived availability of and engagement in structured activities may serve as a protective factor for youth substance use |
| 5 | 2020 | Afifi et al.(Beirut) | To explore the association between bullying victimization and substance use in adolescents with low and high levels of religiosity. | Cross-sectional | Substance use | Risk Factors 1) Lower religiosity levels who had been bullied | Religiosity may be a potential moderator of the association between being bullied and substance use |
| 6 | 2019 | Marin S et al. (Iran) | To examine the relationship between optimistic explanatory style and cigarette smoking, hookah smoking, and illicit drug use among high school students in Sonqor county, Iran | Cross-sectional | Opium Cannabis Ecstasy Methamphetamine | Protective Factors 1) Optimism trait of an individual measured using Children Attributional Style Questionnaire (CASQ). 2) Higher scores of optimism protected students from using illicit drugs (Model 3: OR = 0.90, 95% CI: 0.85–0.95, < 0.001). 3) Negative-stability and negative-globality domains of optimism were significantly higher among advanced-stage smokers and illicit drug users. | Optimism was found to be a protective factor against substance abuse. |
| 7 | 2019 | Schleimer et al. (Latin America: Chile, Uruguay, and Argentina) | 1) To estimate associations between perceived availability and perceived risk of marijuana use and past-month marijuana use 2) To describe how these associations changed over time | Cross-sectional | Marijuana | Risk Factors 1) No/ Low perceived risk increase the odds of past-month marijuana use by 8.22 times compared to those who perceived moderate/great risk. 2) High perceived availability of drug: consistently associated with higher odds of past-month marijuana use. Protective Factors 1) Moderate/ High perceived risk of substance use. 2) Low perceived availability | Perceived risk and availability of marijuana are significant risk factors for adolescent marijuana use in the Southern Cone. |
| 8 | 2019 | Guttmannova et al. (USA) | To examine a set of marijuana-specific risk factors from multiple domains of development for marijuana use over the course of adolescence | Community Randomized-Controlled Trial | Marijuana | Risk Factors 1) Perception of lax community enforcement of marijuana laws regarding adolescent use 2) Low perception of harm 3) Rebelliousness traits 4) Parents with low education | A greater frequency of marijuana use was predicted among the identified risk factors. |
| 9 | 2019 | Doggett et al. (Canada) | To examine the association between various types of screen time sedentary behavior (STSBs) and cannabis use | Cross-sectional | Cannabis | Risk Factors 1) Total screen time sedentary behavior (internet use, messaging, playing video games, watching TV | STSB is a risk factor for the tendency for individuals to use substances as a coping mechanism. |
| 10 | 2017 | Wilson et al. (USA) | To examine associations among levels of trait mindfulness and opioid use behaviors. | Cross sectional | Opioid | - Study using a convenience sample of 112 youth (ages 14–24) was recruited during an episode of inpatient detoxification and residential treatment for opioid use disorders. - Youth had difficulties in emotion regulation (m = 104.2; SD = 2.41) and low mindfulness (m = 19.1; SD = 0.59). Risk Factors 1) Difficulty in regulating emotions Protective Factors 1) High level of mindfulness | Majority of youth presenting with opioid use disorders have impairments in emotion regulation and deficits in trait mindfulness. |
| 11 | 2017 | Li et al. (Macau) | To identify culturally relevant predictors of synthetic drug use among adolescents in Macao. | Cross sectional | Ketamine Ecstasy/MDMA Methamphetamine Tranquilizers Hybrid synthetic drugs | - The rates of synthetic use among male adolescents were higher than those among female adolescents for lifetime use (1.79% vs. 1.04%), past-year use (1.29% vs. 0.70%), and past-month use (1.03% vs. 0.44%). - Synthetic drug use was the most prevalent among fifth and sixth graders at the elementary school level. Risk Factors 1) Peer usage 2) Recreational use of time 3) Attitudes towards synthetic drugs 4) Availability of synthetic drugs | The investigated risk factors contribute to adolescent drug abuse. |
| 12 | 2017 | Luk et al. (USA) | To examine both direct and indirect effects of multiple parenting dimensions on substance use behaviors across Asian-Pacific Islander (API) and European American youth. | Prospective Cohort | Marijuana | - Mother’s knowledge predicted fewer externalizing problems in Grade 8, which in turn predicted fewer substance use problems in Grades 9 and 12. - Father’s warmth predicted better academic achievement in Grade 8, which in turn predicted fewer substance use problems in Grades 9 and 12, as well as alcohol and marijuana dependence in Grade 12. Risk Factors 1) Mother’s psychological control Protective Factors 1) Father’s knowledge | Promoting father’s knowledge of adolescents’ whereabouts can reduce substance use risks among both European and API Americans. |
| 13 | 2017 | De Pedro et al. (USA) | This study aims to fill this gap in the literature and inform programs aimed at reducing substance use among LGB youth | Cross-sectional | Marijuana, inhalants, prescription pain medication, and other illegal drugs | Protective Factors 1) school connectedness and school adult support | The results indicate a need for substance use prevention programs that integrate school connectedness and adult support in school |
| 14 | 2017 | Dorard et al. (France) | To investigate alexithymia in young outpatient cannabis misusers to determine whether the levels of alexithymia and the state and traits of anxiety and depression predict cannabis misuse by adolescents | Case control | Cannabis | - Study done on 120 young patients with cannabis dependence or abuse (DSM-IV-TR criteria evaluated with the MINI) and seeking treatment in an addiction unit + another 110 healthy control subjects. - Used self-reports for measuring alexithymia (TAS-20;BVAQ-B), depression (BDI-13), and states and traits of anxiety (STAI). - 35.3% of cannabis users were alexithymia Risk Factors 1) Difficulty in identifying feelings Protective Factors 1) Difficulty in describing feelings | Lower rate of alexithymics than in previous reports among substance abusers but higher than those reported in the control |
| 15 | 2017 | Kobulsky (USA) | To examine the relations between child physical and sexual abuse and early substance use among youths investigated by child protective services | Cohort | Marijuana Inhalants Hard drugs NMPD | - Significant indirect effects of physical abuse severity on early substance use were found through externalizing behavior problems in girls, with a significantly stronger relation found only between externalizing problems and early substance use in girls. Risk Factors 1) Girls: Physical abuse severity, externalizing problems | Significant gender differences in the effect of early substance from physical abuse. |
| 16 | 2017 | Chuang et al. (USA) | To examine the potential relationship between two self-reported risk factors (impulsivity and the presence of one or more behavioral addictions) and tobacco, alcohol, and marijuana use—or susceptibility to use these drugs in the future among nonusers—in an adolescent population | Cross-sectional | Marijuana | - Adolescents who had either impulsivity alone or at least two behavioral addictions alone were more likely to have used tobacco, alcohol, or marijuana compared to individuals who had neither risk factor (OR = 2.50–4.13), and- Individuals who endorsed both impulsivity and three or more behavioral addictions were the most likely to have used these drugs (OR = 9.40–10.13) Risk Factors 1) High impulsivity combined with more than 3 behavioral addictions. | High impulsivity was related to behavioral addictions in adolescents, and a combination of these two factors increased risk for drug use |
| 17 | 2016 | Khoddam, et al. (USA) | To study whether the relationship of conduct problems and several internalizing disorders with future substance use is redundant, incremental, or interactive in adolescents. | Cross-sectional | Marijuana | Risk Factors 1) Conduct Problems (CPs) 2) Major depressive disorder Protective Factors 1) Social phobia | CPs are a risk factor for substance use, as well as the nuanced interplay of internalizing-externalizing problems in the developmental psychopathology of adolescent drug use vulnerability. |
| 18 | 2016 | Gabrielli et al. (USA) | To identify the relations between maltreatment and SU behavior in a population known for a significant risk of SU behaviour—youth in foster care. | Cross-sectional | Alcohol Marijuana Cocaine Stimulants LSD Tranquilizers Opiates PCP Sniffed gases/fumes Prescribed drugs | - 31% of participants reported past-year substance abuse. - Age of substance abuse onset was 11.08 years (Sd = 2.21 years) - Structural model with maltreatment predicting substance abuse severity demonstrated strong model fit with a significant path between maltreatment and substance abuse. Risk Factors 1) Maltreatment during stay in foster care. | Findings revealed a robust relationship between maltreatment, indicated by the severity and chronicity of experiences across types of maltreatment and substance use behavior severity. |
| 19 | 2016 | Traube et al. (USA) | 1) To untangle two aspects of time in the growth process of polysubstance use: age or development and the length of time in the Child Welfare System (CWS). 2) To determine residential status as either a risk or protective factor | Cross-sectional | Alcohol Marijuana | - Analysis using longitudinal data from the National Survey of Child and Adolescent Well-Being ( = 1178). - Time- invariant characteristics of ethnicity and gender were not related to polysubstance use. - Increased proportions of the sample reporting the use of alcohol and marijuana (from 16 to 26% and from 9 to 18%, respectively). Risk Factors 1) Duration of stay in Child Welfare System (CWS) | Findings indicated that children who enter child welfare when they are older than age 15 are at increased risk of substance use, although those who enter the CWS at a young age may be at greater risk over time. |
| 20 | 2016 | Cecil et al. (UK) | 1) To determine DNAm patterns at birth that are associated with adolescent substance use? 2) To identify DNAm markers that are associated with genetic and environmental influences | Cohort | Cannabis | - The sample comprised 244 youth (51% female) from the Avon Longitudinal Study of Parents and Children (ALSPAC). - At birth, epigenetic variation across a tightly interconnected genetic network ( = 65 loci; qo0.05) was associated with greater levels of substance use during adolescence, as well as an earlier age of onset among users. - Several of the identified loci were associated with known methylation quantitative trait loci. - Collectively, these 65 loci were also found to partially mediate the effect of prenatal maternal tobacco smoking on adolescent substance use. Risk Factors 1) Prenatal tobacco smoking | Tobacco exposure during pregnancy may increase the risk of future substance use. |
| 21 | 2016 | Ogunsola et al. (Nigeria) | To compare the prevalence of substance use among in-school adolescents in urban and rural areas of Osun State, Nigeria, and identified risk and protective factors. | Cross-sectional | Substances use | Risk Factors 1) Private school attendance 2) having friends who use substances 3) mother having had tertiary education Protective Factors 1) Parental disapproval of substance use | The risk and protective factors for adolescent substance use somewhat differ for rural and urban areas |
| 22 | 2015 | Miech et al. (USA) | To determine whether e-cigarette use is part of a pattern towards extensive substance use. | Cross-sectional | Marijuana Prescription drugs | - The distribution of e-cigarette use is consistent with the distribution of most other substances. - Youth who use e-cigarettes are, on average, highly likely to use other substances, as well. Risk Factors 1) E-cigarette smokers | Exposure to e-cigarettes within the past 30-days, increases the prevalence of marijuana use and prescription drug use among adolescents. |
| 23 | 2018 | El Kazdouh et al. (Morocco) | To explore and understand factors that protect or influence substance use in adolescents. | Focus Group Discussion (FGD) analysis via Thematic Analysis | Any illicit drug | Risk Factors 1) Perceived benefits of drug abuse 2) Perceived availability of drugs (cheaper price) 3) Lack of parental supervision 4) Peer pressure from those who do drugs Protective Factors 1) Strong belief in maintaining good health 2) Good family support in giving advice 3) Strong religious beliefs | There are many interplay factors that contribute to the risk of developing drug abuse problems and protecting adolescents from drug abuse. Key prevention activities need to be targeted at each level to ensure healthy behaviors among adolescents. |
After thorough discussion and evaluation, all the findings (both risk and protective factors) from the review were categorized into three main domains: individual factors, family factors, and community factors. The conceptual framework is summarized in Fig. 2 .
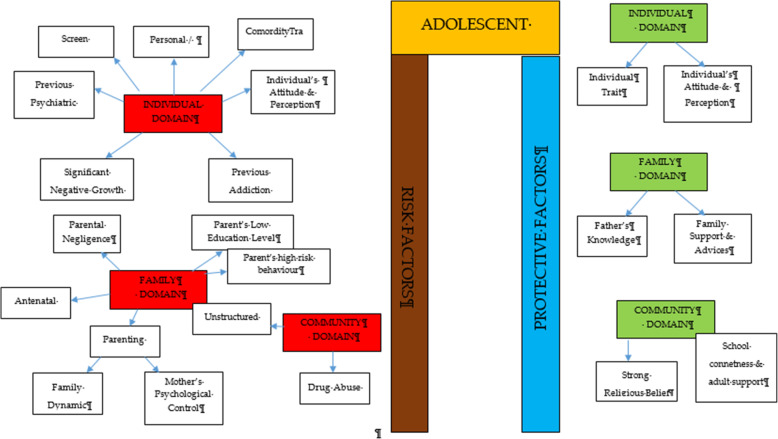
Conceptual framework of risk and protective factors related to adolescent drug abuse
DOMAIN: individual factor
Risk factors.
Almost all the articles highlighted significant findings of individual risk factors for adolescent drug abuse. Therefore, our findings for this domain were further broken down into five more sub-domains consisting of personal/individual traits, significant negative growth exposure, personal psychiatric diagnosis, previous substance history, comorbidity and an individual’s attitude and perception.
Personal/individual traits
Chuang et al. [ 29 ] found that adolescents with high impulsivity traits had a significant positive association with drug addiction. This study also showed that the impulsivity trait alone was an independent risk factor that increased the odds between two to four times for using any drug compared to the non-impulsive group. Another longitudinal study by Guttmannova et al. showed that rebellious traits are positively associated with marijuana drug abuse [ 27 ]. The authors argued that measures of rebelliousness are a good proxy for a youth’s propensity to engage in risky behavior. Nevertheless, Wilson et al. [ 37 ], in a study involving 112 youths undergoing detoxification treatment for opioid abuse, found that a majority of the affected respondents had difficulty in regulating their emotions. The authors found that those with emotional regulation impairment traits became opioid dependent at an earlier age. Apart from that, a case-control study among outpatient youths found that adolescents involved in cannabis abuse had significant alexithymia traits compared to the control population [ 28 ]. Those adolescents scored high in the dimension of Difficulty in Identifying Emotion (DIF), which is one of the key definitions of diagnosing alexithymia. Overall, the adjusted Odds Ratio for DIF in cannabis abuse was 1.11 (95% CI, 1.03–1.20).
Significant negative growth exposure
A history of maltreatment in the past was also shown to have a positive association with adolescent drug abuse. A study found that a history of physical abuse in the past is associated with adolescent drug abuse through a Path Analysis, despite evidence being limited to the female gender [ 25 ]. However, evidence from another study focusing at foster care concluded that any type of maltreatment might result in a prevalence as high as 85.7% for the lifetime use of cannabis and as high as 31.7% for the prevalence of cannabis use within the last 3-months [ 30 ]. The study also found significant latent variables that accounted for drug abuse outcomes, which were chronic physical maltreatment (factor loading of 0.858) and chronic psychological maltreatment (factor loading of 0.825), with an r 2 of 73.6 and 68.1%, respectively. Another study shed light on those living in child welfare service (CWS) [ 35 ]. It was observed through longitudinal measurements that proportions of marijuana usage increased from 9 to 18% after 36 months in CWS. Hence, there is evidence of the possibility of a negative upbringing at such shelters.
Personal psychiatric diagnosis
The robust studies conducted in the USA have deduced that adolescents diagnosed with a conduct problem (CP) have a positive association with marijuana abuse (OR = 1.75 [1.56, 1.96], p < 0.0001). Furthermore, those with a diagnosis of Major Depressive Disorder (MDD) showed a significant positive association with marijuana abuse.
Previous substance and addiction history
Another study found that exposure to e-cigarettes within the past 30 days is related to an increase in the prevalence of marijuana use and prescription drug use by at least four times in the 8th and 10th grades and by at least three times in the 12th grade [ 34 ]. An association between other behavioral addictions and the development of drug abuse was also studied [ 29 ]. Using a 12-item index to assess potential addictive behaviors [ 39 ], significant associations between drug abuse and the groups with two behavioral addictions (OR = 3.19, 95% CI 1.25,9.77) and three behavioral addictions (OR = 3.46, 95% CI 1.25,9.58) were reported.
Comorbidity
The paper by Dash et al. (2020) highlight adolescent with a disease who needs routine medical pain treatment have higher risk of opioid misuse [ 38 ]. The adolescents who have disorder symptoms may have a risk for opioid misuse despite for the pain intensity.
Individual’s attitudes and perceptions
In a study conducted in three Latin America countries (Argentina, Chile, and Uruguay), it was shown that adolescents with low or no perceived risk of taking marijuana had a higher risk of abuse (OR = 8.22 times, 95% CI 7.56, 10.30) [ 35 ]. This finding is in line with another study that investigated 2002 adolescents and concluded that perceiving the drug as harmless was an independent risk factor that could prospectively predict future marijuana abuse [ 27 ]. Moreover, some youth interviewed perceived that they gained benefits from substance use [ 38 ]. The focus group discussion summarized that the youth felt positive personal motivation and could escape from a negative state by taking drugs. Apart from that, adolescents who had high-perceived availability of drugs in their neighborhoods were more likely to increase their usage of marijuana over time (OR = 11.00, 95% CI 9.11, 13.27) [ 35 ]. A cheap price of the substance and the availability of drug dealers around schools were factors for youth accessibility [ 38 ]. Perceived drug accessibility has also been linked with the authorities’ enforcement programs. The youth perception of a lax community enforcement of laws regarding drug use at all-time points predicted an increase in marijuana use in the subsequent assessment period [ 27 ]. Besides perception, a study examining the attitudes towards synthetic drugs based on 8076 probabilistic samples of Macau students found that the odds of the lifetime use of marijuana was almost three times higher among those with a strong attitude towards the use of synthetic drugs [ 32 ]. In addition, total screen time among the adolescent increase the likelihood of frequent cannabis use. Those who reported daily cannabis use have a mean of 12.56 h of total screen time, compared to a mean of 6.93 h among those who reported no cannabis use. Adolescent with more time on internet use, messaging, playing video games and watching TV/movies were significantly associated with more frequent cannabis use [ 44 ].
Protective factors
Individual traits.
Some individual traits have been determined to protect adolescents from developing drug abuse habits. A study by Marin et al. found that youth with an optimistic trait were less likely to become drug dependent [ 33 ]. In this study involving 1104 Iranian students, it was concluded that a higher optimism score (measured using the Children Attributional Style Questionnaire, CASQ) was a protective factor against illicit drug use (OR = 0.90, 95% CI: 0.85–0.95). Another study found that high levels of mindfulness, measured using the 25-item Child Acceptance and Mindfulness Measure, CAMM, lead to a slower progression toward injectable drug abuse among youth with opioid addiction (1.67 years, p = .041) [ 37 ]. In addition, the social phobia trait was found to have a negative association with marijuana use (OR = 0.87, 95% CI 0.77–0.97), as suggested [ 31 ].
According to El Kazdouh et al., individuals with a strong belief against substance use and those with a strong desire to maintain their health were more likely to be protected from involvement in drug abuse [ 46 ].
DOMAIN: family factors
The biological factors underlying drug abuse in adolescents have been reported in several studies. Epigenetic studies are considered important, as they can provide a good outline of the potential pre-natal factors that can be targeted at an earlier stage. Expecting mothers who smoke tobacco and alcohol have an indirect link with adolescent substance abuse in later life [ 24 , 39 ]. Moreover, the dynamic relationship between parents and their children may have some profound effects on the child’s growth. Luk et al. examined the mediator effects between parenting style and substance abuse and found the maternal psychological control dimension to be a significant variable [ 26 ]. The mother’s psychological control was two times higher in influencing her children to be involved in substance abuse compared to the other dimension. Conversely, an indirect risk factor towards youth drug abuse was elaborated in a study in which low parental educational level predicted a greater risk of future drug abuse by reducing the youth’s perception of harm [ 27 , 43 ]. Negligence from a parental perspective could also contribute to this problem. According to El Kazdouh et al. [ 46 ], a lack of parental supervision, uncontrolled pocket money spending among children, and the presence of substance-using family members were the most common negligence factors.
While the maternal factors above were shown to be risk factors, the opposite effect was seen when the paternal figure equipped himself with sufficient knowledge. A study found that fathers with good information and awareness were more likely to protect their adolescent children from drug abuse [ 26 ]. El Kazdouh et al. noted that support and advice could be some of the protective factors in this area [ 46 ].
DOMAIN: community factors
Risk factor.
A study in 2017 showed a positive association between adolescent drug abuse and peers who abuse drugs [ 32 , 39 ]. It was estimated that the odds of becoming a lifetime marijuana user was significantly increased by a factor of 2.5 ( p < 0.001) among peer groups who were taking synthetic drugs. This factor served as peer pressure for youth, who subconsciously had desire to be like the others [ 38 ]. The impact of availability and engagement in structured and unstructured activities also play a role in marijuana use. The findings from Spillane (2000) found that the availability of unstructured activities was associated with increased likelihood of marijuana use [ 42 ].
Protective factor
Strong religious beliefs integrated into society serve as a crucial protective factor that can prevent adolescents from engaging in drug abuse [ 38 , 45 ]. In addition, the school connectedness and adult support also play a major contribution in the drug use [ 40 ].
The goal of this review was to identify and classify the risks and protective factors that lead adolescents to drug abuse across the three important domains of the individual, family, and community. No findings conflicted with each other, as each of them had their own arguments and justifications. The findings from our review showed that individual factors were the most commonly highlighted. These factors include individual traits, significant negative growth exposure, personal psychiatric diagnosis, previous substance and addiction history, and an individual’s attitude and perception as risk factors.
Within the individual factor domain, nine articles were found to contribute to the subdomain of personal/ individual traits [ 27 – 29 , 37 – 40 , 43 , 44 ]. Despite the heterogeneity of the study designs and the substances under investigation, all of the papers found statistically significant results for the possible risk factors of adolescent drug abuse. The traits of high impulsivity, rebelliousness, difficulty in regulating emotions, and alexithymia can be considered negative characteristic traits. These adolescents suffer from the inability to self-regulate their emotions, so they tend to externalize their behaviors as a way to avoid or suppress the negative feelings that they are experiencing [ 41 , 47 , 48 ]. On the other hand, engaging in such behaviors could plausibly provide a greater sense of positive emotions and make them feel good [ 49 ]. Apart from that, evidence from a neurophysiological point of view also suggests that the compulsive drive toward drug use is complemented by deficits in impulse control and decision making (impulsive trait) [ 50 ]. A person’s ability in self-control will seriously impaired with continuous drug use and will lead to the hallmark of addiction [ 51 ].
On the other hand, there are articles that reported some individual traits to be protective for adolescents from engaging in drug abuse. Youth with the optimistic trait, a high level of mindfulness, and social phobia were less likely to become drug dependent [ 31 , 33 , 37 ]. All of these articles used different psychometric instruments to classify each individual trait and were mutually exclusive. Therefore, each trait measured the chance of engaging in drug abuse on its own and did not reflect the chance at the end of the spectrum. These findings show that individual traits can be either protective or risk factors for the drugs used among adolescents. Therefore, any adolescent with negative personality traits should be monitored closely by providing health education, motivation, counselling, and emotional support since it can be concluded that negative personality traits are correlated with high risk behaviours such as drug abuse [ 52 ].
Our study also found that a history of maltreatment has a positive association with adolescent drug abuse. Those adolescents with episodes of maltreatment were considered to have negative growth exposure, as their childhoods were negatively affected by traumatic events. Some significant associations were found between maltreatment and adolescent drug abuse, although the former factor was limited to the female gender [ 25 , 30 , 36 ]. One possible reason for the contrasting results between genders is the different sample populations, which only covered child welfare centers [ 36 ] and foster care [ 30 ]. Regardless of the place, maltreatment can happen anywhere depending on the presence of the perpetrators. To date, evidence that concretely links maltreatment and substance abuse remains limited. However, a plausible explanation for this link could be the indirect effects of posttraumatic stress (i.e., a history of maltreatment) leading to substance use [ 53 , 54 ]. These findings highlight the importance of continuous monitoring and follow-ups with adolescents who have a history of maltreatment and who have ever attended a welfare center.
Addiction sometimes leads to another addiction, as described by the findings of several studies [ 29 , 34 ]. An initial study focused on the effects of e-cigarettes in the development of other substance abuse disorders, particularly those related to marijuana, alcohol, and commonly prescribed medications [ 34 ]. The authors found that the use of e-cigarettes can lead to more severe substance addiction [ 55 ], possibly through normalization of the behavior. On the other hand, Chuang et al.’s extensive study in 2017 analyzed the combined effects of either multiple addictions alone or a combination of multiple addictions together with the impulsivity trait [ 29 ]. The outcomes reported were intriguing and provide the opportunity for targeted intervention. The synergistic effects of impulsiveness and three other substance addictions (marijuana, tobacco, and alcohol) substantially increased the likelihood for drug abuse from 3.46 (95%CI 1.25, 9.58) to 10.13 (95% CI 3.95, 25.95). Therefore, proper rehabilitation is an important strategy to ensure that one addiction will not lead to another addiction.
The likelihood for drug abuse increases as the population perceives little or no harmful risks associated with the drugs. On the opposite side of the coin, a greater perceived risk remains a protective factor for marijuana abuse [ 56 ]. However, another study noted that a stronger determinant for adolescent drug abuse was the perceived availability of the drug [ 35 , 57 ]. Looking at the bigger picture, both perceptions corroborate each other and may inform drug use. Another study, on the other hand, reported that there was a decreasing trend of perceived drug risk in conjunction with the increasing usage of drugs [ 58 ]. As more people do drugs, youth may inevitably perceive those drugs as an acceptable norm without any harmful consequences [ 59 ].
In addition, the total spent for screen time also contribute to drug abuse among adolescent [ 43 ]. This scenario has been proven by many researchers on the effect of screen time on the mental health [ 60 ] that leads to the substance use among the adolescent due to the ubiquity of pro-substance use content on the internet. Adolescent with comorbidity who needs medical pain management by opioids also tend to misuse in future. A qualitative exploration on the perspectives among general practitioners concerning the risk of opioid misuse in people with pain, showed pain management by opioids is a default treatment and misuse is not a main problem for the them [ 61 ]. A careful decision on the use of opioids as a pain management should be consider among the adolescents and their understanding is needed.
Within the family factor domain, family structures were found to have both positive and negative associations with drug abuse among adolescents. As described in one study, paternal knowledge was consistently found to be a protective factor against substance abuse [ 26 ]. With sufficient knowledge, the father can serve as the guardian of his family to monitor and protect his children from negative influences [ 62 ]. The work by Luk et al. also reported a positive association of maternal psychological association towards drug abuse (IRR 2.41, p < 0.05) [ 26 ]. The authors also observed the same effect of paternal psychological control, although it was statistically insignificant. This construct relates to parenting style, and the authors argued that parenting style might have a profound effect on the outcomes under study. While an earlier literature review [ 63 ] also reported such a relationship, a recent study showed a lesser impact [ 64 ] with regards to neglectful parenting styles leading to poorer substance abuse outcomes. Nevertheless, it was highlighted in another study that the adolescents’ perception of a neglectful parenting style increased their odds (OR 2.14, p = 0.012) of developing alcohol abuse, not the parenting style itself [ 65 ]. Altogether, families play vital roles in adolescents’ risk for engaging in substance abuse [ 66 ]. Therefore, any intervention to impede the initiation of substance use or curb existing substance use among adolescents needs to include parents—especially improving parent–child communication and ensuring that parents monitor their children’s activities.
Finally, the community also contributes to drug abuse among adolescents. As shown by Li et al. [ 32 ] and El Kazdouh et al. [ 46 ], peers exert a certain influence on other teenagers by making them subconsciously want to fit into the group. Peer selection and peer socialization processes might explain why peer pressure serves as a risk factor for drug-abuse among adolescents [ 67 ]. Another study reported that strong religious beliefs integrated into society play a crucial role in preventing adolescents from engaging in drug abuse [ 46 ]. Most religions devalue any actions that can cause harmful health effects, such as substance abuse [ 68 ]. Hence, spiritual beliefs may help protect adolescents. This theme has been well established in many studies [ 60 , 69 – 72 ] and, therefore, could be implemented by religious societies as part of interventions to curb the issue of adolescent drug abuse. The connection with school and structured activity did reduce the risk as a study in USA found exposure to media anti-drug messages had an indirect negative effect on substances abuse through school-related activity and social activity [ 73 ]. The school activity should highlight on the importance of developmental perspective when designing and offering school-based prevention programs [75].
Limitations
We adopted a review approach that synthesized existing evidence on the risk and protective factors of adolescents engaging in drug abuse. Although this systematic review builds on the conclusion of a rigorous review of studies in different settings, there are some potential limitations to this work. We may have missed some other important factors, as we only included English articles, and article extraction was only done from the three search engines mentioned. Nonetheless, this review focused on worldwide drug abuse studies, rather than the broader context of substance abuse including alcohol and cigarettes, thereby making this paper more focused.
Conclusions
This review has addressed some recent knowledge related to the individual, familial, and community risk and preventive factors for adolescent drug use. We suggest that more attention should be given to individual factors since most findings were discussed in relation to such factors. With the increasing trend of drug abuse, it will be critical to focus research specifically on this area. Localized studies, especially those related to demographic factors, may be more effective in generating results that are specific to particular areas and thus may be more useful in generating and assessing local control and prevention efforts. Interventions using different theory-based psychotherapies and a recognition of the unique developmental milestones specific to adolescents are among examples that can be used. Relevant holistic approaches should be strengthened not only by relevant government agencies but also by the private sector and non-governmental organizations by promoting protective factors while reducing risk factors in programs involving adolescents from primary school up to adulthood to prevent and control drug abuse. Finally, legal legislation and enforcement against drug abuse should be engaged with regularly as part of our commitment to combat this public health burden.
Acknowledgements
The authors acknowledge The Ministry of Higher Education Malaysia and The Universiti Kebangsaan Malaysia, (UKM) for funding this study under the Long-Term Research Grant Scheme-(LGRS/1/2019/UKM-UKM/2/1). We also thank the team for their commitment and tireless efforts in ensuring that manuscript was well executed.
Authors’ contributions
Manuscript concept, and drafting AMN and RI; model development, FI, NI and NA.; Editing manuscript MRH, MRAN, NSS,; Critical revision of manuscript for important intellectual content, all authors. The authors read and approved the final manuscript.
Financial support for this study was obtained from the Ministry of Higher Education, Malaysia through the Long-Term Research Grant Scheme-(LGRS/1/2019/UKM-UKM/2/1). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data availability and materials
All data generated or analysed during this study are included in this published article.

Declarations
Ethics approval and consent to participate.
This study was approved by the Ethics Committee of the Secretariat of Research Ethics, Universiti Kebangsaan Malaysia, Faculty of Medicine, Cheras, Kuala Lumpur (Reference no. UKMPPI/111/8/JEP-2020.174(2). Dated 27 Mac 2020.
Consent for publication
Not applicable.
Competing interests
The authors AMN, RI, FI, MRM, MRAM, NA, NI NSS declare that they have no conflict of interest relevant to this work.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
- 1. Nation, U . World Drug Report 2018 (United Nations publication, Sales No. E.18X.XI.9. United Nation publication) 2018. [ Google Scholar ]
- 2. Degenhardt L, Stockings E, Patton G, Hall WD, Lynskey M. The increasing global health priority of substance use in young people. Lancet Psychiatry. 2016;3(3):251–264. doi: 10.1016/S2215-0366(15)00508-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 3. Ritchie H, Roser M. Drug Use - Our World in Data: Global Change Data Lab; 2019. https://ourworldindata.org/drug-use [10 June 2020]
- 4. Holm S, Sandberg S, Kolind T, Hesse M. The importance of cannabis culture in young adult cannabis use. J Subst Abus. 2014;19(3):251–256. [ Google Scholar ]
- 5. Luikinga SJ, Kim JH, Perry CJ. Developmental perspectives on methamphetamine abuse: exploring adolescent vulnerabilities on brain and behavior. Progress Neuro Psychopharmacol Biol Psychiatry. 2018;87(Pt A):78–84. doi: 10.1016/j.pnpbp.2017.11.010. [ DOI ] [ PubMed ] [ Google Scholar ]
- 6. Ismail R, Ghazalli MN, Ibrahim N. Not all developmental assets can predict negative mental health outcomes of disadvantaged youth: a case of suburban Kuala Lumpur. Mediterr J Soc Sci. 2015;6(1):452–459. doi: 10.5901/mjss.2015.v6n5s1p452. [ DOI ] [ Google Scholar ]
- 7. Crews F, He J, Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacol Biochem Behav. 2007;86(2):189–199. doi: 10.1016/j.pbb.2006.12.001. [ DOI ] [ PubMed ] [ Google Scholar ]
- 8. Schulte MT, Hser YI. Substance use and associated health conditions throughout the lifespan. Public Health Rev. 2013;35(2). 10.1007/bf03391702 Technosdar Ltd. [ DOI ] [ PMC free article ] [ PubMed ]
- 9. Somani S, Meghani S. Substance Abuse among Youth: A Harsh Reality. 2016. [ Google Scholar ]
- 10. Drabble L, Trocki KF, Klinger JL. Religiosity as a protective factor for hazardous drinking and drug use among sexual minority and heterosexual women: findings from the National Alcohol Survey. Drug Alcohol Depend. 2016;161:127–134. doi: 10.1016/j.drugalcdep.2016.01.022. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 11. Goliath V, Pretorius B. Peer risk and protective factors in adolescence: Implications for drug use prevention. Soc Work. 2016;52(1):113–129. doi: 10.15270/52-1-482. [ DOI ] [ Google Scholar ]
- 12. Guerrero LR, Dudovitz R, Chung PJ, Dosanjh KK, Wong MD. Grit: a potential protective factor against substance use and other risk behaviors among Latino adolescents. Acad Pediatr. 2016;16(3):275–281. doi: 10.1016/j.acap.2015.12.016. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 13. National Institutes on Drug Abuse. What are risk factors and protective factors? National Institute on Drug Abuse (NIDA); 2003. Retrieved from https://www.drugabuse.gov/publications/preventing-drug-use-among-children-adolescents/chapter-1-risk-factors-protective-factors/what-are-risk-factors
- 14. Nguyen NN, Newhill CE. The role of religiosity as a protective factor against marijuana use among African American, White, Asian, and Hispanic adolescents. J Subst Abus. 2016;21(5):547–552. doi: 10.3109/14659891.2015.1093558. [ DOI ] [ Google Scholar ]
- 15. Schinke S, Schwinn T, Hopkins J, Wahlstrom L. Drug abuse risk and protective factors among Hispanic adolescents. Prev Med Rep. 2016;3:185–188. doi: 10.1016/j.pmedr.2016.01.012. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 16. Macleod J, Oakes R, Copello A, Crome PI, Egger PM, Hickman M, Oppenkowski T, et al. Psychological and social sequelae of cannabis and other illicit drug use by young people: a systematic review of longitudinal, general population studies. Lancet. 2004;363(9421):1579–1588. doi: 10.1016/S0140-6736(04)16200-4. [ DOI ] [ PubMed ] [ Google Scholar ]
- 17. Moore TH, Zammit S, Lingford-Hughes A, Barnes TR, Jones PB, Burke M, Lewis G. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370(9584):319–328. doi: 10.1016/S0140-6736(07)61162-3. [ DOI ] [ PubMed ] [ Google Scholar ]
- 18. Semple DM, McIntosh AM, Lawrie SM. Cannabis as a risk factor for psychosis: systematic review. J Psychopharmacol. 2005;19(2):187–194. doi: 10.1177/0269881105049040. [ DOI ] [ PubMed ] [ Google Scholar ]
- 19. Nargiso JE, Ballard EL, Skeer MR. A systematic review of risk and protective factors associated with nonmedical use of prescription drugs among youth in the united states: A social ecological perspective. J Stud Alcohol Drugs. 2015;76(1):5–20. doi: 10.15288/jsad.2015.76.5. [ DOI ] [ PubMed ] [ Google Scholar ]
- 20. Guxensa M, Nebot M, Ariza C, Ochoa D. Factors associated with the onset of cannabis use: a systematic review of cohort studies. Gac Sanit. 2007;21(3):252–260. doi: 10.1157/13106811. [ DOI ] [ PubMed ] [ Google Scholar ]
- 21. Susan MS, Peter SA, Dakshitha W, George CP. The age of adolescence. Lancet Child Adolesc Health. 2018;2(Issue 3):223–228. doi: 10.1016/S2352-4642(18)30022-1. [ DOI ] [ PubMed ] [ Google Scholar ]
- 22. Hong QN, Fàbregues S, Bartlett G, Boardman F, Cargo M, Dagenais P, Gagnon MP, Griffiths F, Nicolau B, O’Cathain A, Rousseau MC, Vedel I, Pluye P. The mixed methods appraisal tool (MMAT) version 2018 for information professionals and researchers. Educ Inf. 2018;34(4):285–291. doi: 10.3233/EFI-180221. [ DOI ] [ Google Scholar ]
- 23. McHugh ML. Interrater reliability: The kappa statistic. Biochem Med. 2012;22(3):276–282. doi: 10.11613/bm.2012.031. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 24. Cecil CAM, Walton E, Smith RG, Viding E, McCrory EJ, Relton CL, Suderman M, Pingault JB, McArdle W, Gaunt TR, Mill J, Barker ED. DNA methylation and substance-use risk: a prospective, genome-wide study spanning gestation to adolescence. Transl Psychiatry. 2016;6(12):e976. doi: 10.1038/tp.2016.247. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 25. Kobulsky JM. Gender differences in pathways from physical and sexual abuse to early substance use. Child Youth Serv Rev. 2017;83:25–32. doi: 10.1016/j.childyouth.2017.10.027. [ DOI ] [ Google Scholar ]
- 26. Luk JW, King KM, McCarty CA, McCauley E, Stoep A. Prospective effects of parenting on substance use and problems across Asian/Pacific islander and European American youth: Tests of moderated mediation. J Stud Alcohol Drugs. 2017;78(4):521–530. doi: 10.15288/jsad.2017.78.521. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 27. Guttmannova K, Skinner ML, Oesterle S, White HR, Catalano RF, Hawkins JD. The interplay between marijuana-specific risk factors and marijuana use over the course of adolescence. Prev Sci. 2019;20(2):235–245. doi: 10.1007/s11121-018-0882-9. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 28. Dorard G, Bungener C, Phan O, Edel Y, Corcos M, Berthoz S. Is alexithymia related to cannabis use disorder? Results from a case-control study in outpatient adolescent cannabis abusers. J Psychosom Res. 2017;95:74–80. doi: 10.1016/j.jpsychores.2017.02.012. [ DOI ] [ PubMed ] [ Google Scholar ]
- 29. Chuang CWI, Sussman S, Stone MD, Pang RD, Chou CP, Leventhal AM, Kirkpatrick MG. Impulsivity and history of behavioral addictions are associated with drug use in adolescents. Addict Behav. 2017;74:41–47. doi: 10.1016/j.addbeh.2017.05.021. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 30. Gabrielli J, Jackson Y, Brown S. Associations between maltreatment history and severity of substance use behavior in youth in Foster Care. Child Maltreat. 2016;21(4):298–307. doi: 10.1177/1077559516669443. [ DOI ] [ PubMed ] [ Google Scholar ]
- 31. Khoddam R, Jackson NJ, Leventhal AM. Internalizing symptoms and conduct problems: redundant, incremental, or interactive risk factors for adolescent substance use during the first year of high school? Drug Alcohol Depend. 2016;169:48–55. doi: 10.1016/j.drugalcdep.2016.10.007. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 32. Li SD, Zhang X, Tang W, Xia Y. Predictors and implications of synthetic drug use among adolescents in the gambling Capital of China. SAGE Open. 2017;7(4):215824401773303. doi: 10.1177/2158244017733031. [ DOI ] [ Google Scholar ]
- 33. Marin S, Heshmatian E, Nadrian H, Fakhari A, Mohammadpoorasl A. Associations between optimism, tobacco smoking and substance abuse among Iranian high school students. Health Promot Perspect. 2019;9(4):279–284. doi: 10.15171/hpp.2019.38. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 34. Miech RA, O’Malley PM, Johnston LD, Patrick ME. E-cigarettes and the drug use patterns of adolescents. Nicotine Tob Res. 2015;18(5):654–659. doi: 10.1093/ntr/ntv217. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 35. Schleimer JP, Rivera-Aguirre AE, Castillo-Carniglia A, Laqueur HS, Rudolph KE, Suárez H, Ramírez J, Cadenas N, Somoza M, Brasesco MV, Martins SS, Cerdá M. Investigating how perceived risk and availability of marijuana relate to marijuana use among adolescents in Argentina, Chile, and Uruguay over time. Drug Alcohol Depend. 2019;201:115–126. doi: 10.1016/j.drugalcdep.2019.03.029. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 36. Traube DE, Yarnell LM, Schrager SM. Differences in polysubstance use among youth in the child welfare system: toward a better understanding of the highest-risk teens. Child Abuse Negl. 2016;52:146–157. doi: 10.1016/j.chiabu.2015.11.020. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 37. Wilson JD, Vo H, Matson P, Adger H, Barnett G, Fishman M. Trait mindfulness and progression to injection use in youth with opioid addiction. Subst Use Misuse. 2017;52(11):1486–1493. doi: 10.1080/10826084.2017.1289225. [ DOI ] [ PubMed ] [ Google Scholar ]
- 38. Dash GF, Feldstein Ewing SW, Murphy C, Hudson KA, Wilson AC. Contextual risk among adolescents receiving opioid prescriptions for acute pain in pediatric ambulatory care settings. Addict Behav. 2020;104:106314. doi: 10.1016/j.addbeh.2020.106314. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 39. Osborne V, Serdarevic M, Striley CW, Nixon SJ, Winterstein AG, Cottler LB. Age of first use of prescription opioids and prescription opioid non-medical use among older adolescents. Substance Use Misuse. 2020;55(14):2420–2427. doi: 10.1080/10826084.2020.1823420. [ DOI ] [ PubMed ] [ Google Scholar ]
- 40. Zuckermann AME, Qian W, Battista K, Jiang Y, de Groh M, Leatherdale ST. Factors influencing the non-medical use of prescription opioids among youth: results from the COMPASS study. J Subst Abus. 2020;25(5):507–514. doi: 10.1080/14659891.2020.1736669. [ DOI ] [ Google Scholar ]
- 41. De Pedro KT, Esqueda MC, Gilreath TD. School protective factors and substance use among lesbian, gay, and bisexual adolescents in California public schools. LGBT Health. 2017;4(3):210–216. doi: 10.1089/lgbt.2016.0132. [ DOI ] [ PubMed ] [ Google Scholar ]
- 42. Spillane NS, Schick MR, Kirk-Provencher KT, Hill DC, Wyatt J, Jackson KM. Structured and unstructured activities and alcohol and marijuana use in middle school: the role of availability and engagement. Substance Use Misuse. 2020;55(11):1765–1773. doi: 10.1080/10826084.2020.1762652. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 43. Ogunsola OO, Fatusi AO. Risk and protective factors for adolescent substance use: a comparative study of secondary school students in rural and urban areas of Osun state, Nigeria. Int J Adolesc Med Health. 2016;29(3). 10.1515/ijamh-2015-0096. [ DOI ] [ PubMed ]
- 44. Doggett A, Qian W, Godin K, De Groh M, Leatherdale ST. Examining the association between exposure to various screen time sedentary behaviours and cannabis use among youth in the COMPASS study. SSM Population Health. 2019;9:100487. doi: 10.1016/j.ssmph.2019.100487. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 45. Afifi RA, El Asmar K, Bteddini D, Assi M, Yassin N, Bitar S, Ghandour L. Bullying victimization and use of substances in high school: does religiosity moderate the association? J Relig Health. 2020;59(1):334–350. doi: 10.1007/s10943-019-00789-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 46. El Kazdouh H, El-Ammari A, Bouftini S, El Fakir S, El Achhab Y. Adolescents, parents and teachers’ perceptions of risk and protective factors of substance use in Moroccan adolescents: a qualitative study. Substance Abuse Treat Prevent Policy. 2018;13(1):–31. 10.1186/s13011-018-0169-y. [ DOI ] [ PMC free article ] [ PubMed ]
- 47. Sussman S, Lisha N, Griffiths M. Prevalence of the addictions: a problem of the majority or the minority? Eval Health Prof. 2011;34(1):3–56. doi: 10.1177/0163278710380124. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 48. Aldao A, Nolen-Hoeksema S, Schweizer S. Emotion-regulation strategies across psychopathology: a meta-analytic review. Clin Psychol Rev. 2010;30(2):217–237. doi: 10.1016/j.cpr.2009.11.004. [ DOI ] [ PubMed ] [ Google Scholar ]
- 49. Ricketts T, Macaskill A. Gambling as emotion management: developing a grounded theory of problem gambling. Addict Res Theory. 2003;11(6):383–400. doi: 10.1080/1606635031000062074. [ DOI ] [ Google Scholar ]
- 50. Williams AD, Grisham JR. Impulsivity, emotion regulation, and mindful attentional focus in compulsive buying. Cogn Ther Res. 2012;36(5):451–457. doi: 10.1007/s10608-011-9384-9. [ DOI ] [ Google Scholar ]
- 51. National Institutes on Drug Abuse . Drugs, brains, and behavior the science of addiction national institute on drug abuse (nida) 2014. [ Google Scholar ]
- 52. Hokm Abadi ME, Bakhti M, Nazemi M, Sedighi S, Mirzadeh Toroghi E. The relationship between personality traits and drug type among substance abuse. J Res Health. 2018;8(6):531–540. doi: 10.29252/jrh.8.6.531. [ DOI ] [ Google Scholar ]
- 53. Longman-Mills S, Haye W, Hamilton H, Brands B, Wright MGM, Cumsille F, Mann R, Khenti A. Psychological maltreatment and its relationship with substance abuse among university students in Kingston, Jamaica. Florianopolis: Texto Contexto Enferm; 2015. pp. 63–68. [ Google Scholar ]
- 54. Rosenkranz SE, Muller RT, Henderson JL. The role of complex PTSD in mediating childhood maltreatment and substance abuse severity among youth seeking substance abuse treatment. Psychol Trauma Theory Res Pract Policy. 2014;6(1):25–33. doi: 10.1037/a0031920. [ DOI ] [ Google Scholar ]
- 55. Krishnan-Sarin S, Morean M, Kong G, et al. E-Cigarettes and “dripping” among high-school youth. Pediatrics. 2017;139(3). 10.1542/peds.2016-3224. [ DOI ] [ PMC free article ] [ PubMed ]
- 56. Adinoff B. Neurobiologic processes in drug reward and addiction. Harvard review of psychiatry. NIH Public Access. 2004;12(6):305–320. doi: 10.1080/10673220490910844. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 57. Kandel D, Kandel E. The gateway hypothesis of substance abuse: developmental, biological and societal perspectives. Acta Paediatrica. 2014;104(2):130–7. [ DOI ] [ PubMed ]
- 58. Dempsey RC, McAlaney J, Helmer SM, Pischke CR, Akvardar Y, Bewick BM, et al. Normative perceptions of Cannabis use among European University students: associations of perceived peer use and peer attitudes with personal use and attitudes. J Stud Alcohol Drugs. 2016;77(5):740–748. doi: 10.15288/jsad.2016.77.740. [ DOI ] [ PubMed ] [ Google Scholar ]
- 59. Cioffredi L, Kamon J, Turner W. Effects of depression, anxiety and screen use on adolescent substance use. Prevent Med Rep. 2021;22:101362. doi: 10.1016/j.pmedr.2021.101362. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 60. Luckett T, NewtonJohn T, Phillips J, et al. Risk of opioid misuse in people with cancer and pain and related clinical considerations:a qualitative study of the perspectives of Australian general practitioners. BMJ Open. 2020;10(2):e034363. doi: 10.1136/bmjopen-2019-034363. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 61. Lipari RN. Trends in Adolescent Substance Use and Perception of Risk from Substance Use. The CBHSQ Report. Substance Abuse Mental Health Serv Admin. 2013; Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/27656743 . [ PubMed ]
- 62. Muchiri BW, dos Santos MML. Family management risk and protective factors for adolescent substance use in South Africa. Substance Abuse. 2018;13(1):24. doi: 10.1186/s13011-018-0163-4. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 63. Becoña E, Martínez Ú, Calafat A, Juan M, Fernández-Hermida JR, Secades-Villa R. Parental styles and drug use: a review. In: Drugs: Education, Prevention and Policy: Taylor & Francis; 2012. 10.3109/09687637.2011.631060.
- 64. Berge J, Sundel K, Ojehagen A, Hakansson A. Role of parenting styles in adolescent substance use: results from a Swedish longitudinal cohort study. BMJ Open. 2016;6(1):e008979. doi: 10.1136/bmjopen-2015-008979. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 65. Opara I, Lardier DT, Reid RJ, Garcia-Reid P. “It all starts with the parents”: a qualitative study on protective factors for drug-use prevention among black and Hispanic girls. Affilia J Women Soc Work. 2019;34(2):199–218. doi: 10.1177/0886109918822543. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 66. Martínez-Loredo V, Fernández-Artamendi S, Weidberg S, Pericot I, López-Núñez C, Fernández-Hermida J, Secades R. Parenting styles and alcohol use among adolescents: a longitudinal study. Eur J Invest Health Psychol Educ. 2016;6(1):27–36. doi: 10.1989/ejihpe.v6i1.146. [ DOI ] [ Google Scholar ]
- 67. Baharudin MN, Mohamad M, Karim F. Drug-abuse inmates maqasid shariah quality of lifw: a conceotual paper. Hum Soc Sci Rev. 2020;8(3):1285–1294. doi: 10.18510/hssr.2020.83131. [ DOI ] [ Google Scholar ]
- 68. Henneberger AK, Mushonga DR, Preston AM. Peer influence and adolescent substance use: a systematic review of dynamic social network research. Adolesc Res Rev. 2020;6(1):57–73. doi: 10.1007/s40894-019-00130-0. [ DOI ] [ Google Scholar ]
- 69. Gomes FC, de Andrade AG, Izbicki R, Almeida AM, de Oliveira LG. Religion as a protective factor against drug use among Brazilian university students: a national survey. Rev Bras Psiquiatr. 2013;35(1):29–37. doi: 10.1016/j.rbp.2012.05.010. [ DOI ] [ PubMed ] [ Google Scholar ]
- 70. Kulis S, Hodge DR, Ayers SL, Brown EF, Marsiglia FF. Spirituality and religion: intertwined protective factors for substance use among urban American Indian youth. Am J Drug Alcohol Abuse. 2012;38(5):444–449. doi: 10.3109/00952990.2012.670338. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 71. Miller L, Davies M, Greenwald S. Religiosity and substance use and abuse among adolescents in the national comorbidity survey. J Am Acad Child Adolesc Psychiatry. 2000;39(9):1190–1197. doi: 10.1097/00004583-200009000-00020. [ DOI ] [ PubMed ] [ Google Scholar ]
- 72. Moon SS, Rao U. Social activity, school-related activity, and anti-substance use media messages on adolescent tobacco and alcohol use. J Hum Behav Soc Environ. 2011;21(5):475–489. doi: 10.1080/10911359.2011.566456. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 73. Simone A. Onrust, Roy Otten, Jeroen Lammers, Filip smit, school-based programmes to reduce and prevent substance use in different age groups: what works for whom? Systematic review and meta-regression analysis. Clin Psychol Rev. 2016;44:45–59. doi: 10.1016/j.cpr.2015.11.002. [ DOI ] [ PubMed ] [ Google Scholar ]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
- View on publisher site
- PDF (1.3 MB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Substance Abuse in Teenagers Essay
Introduction, substance abuse issue, causes of substance abuse.
I picked this topic because teenage drug addiction has prevailed in society, making it a growing problem. Teenagers frequently experiment with various activities and substances that often result in abuse and addiction. Brain development in adolescents is more vulnerable to drug deficits, which concerns society. Substance abuse causes injury, sexually transmitted diseases, teenage pregnancies, poor mental health, and suicide. The essay presents the issues and the causes of substance abuse among adolescents.
The prevalence of drug use is higher in boys than in girls. For example, a survey conducted by Molinaro et al. (2011) presents cannabis as five times more prevalent than other drugs. Figure 1 below shows the rate of use of cannabis by male adolescents.
A comparison of the two figures shows more males using cannabis. The prevalence of cannabis in 2009 in females is 24.7, while in males, it is 33.8, which affirms that males are more subject to drug addiction than females (Molinaro et al., 2011). The results show men being the bigger addicts to heroin, cocaine, stimulants, and hallucinogens.
Drug consumption also exhibits a dynamic evolution over time influenced by cultural, political, and economic factors like changing laws and market price volatility (Molinaro et al., 2011). Despite significant legislative and social communication efforts in the field, the prevalence of drug use remained relatively unchanged for the commonly used drugs like cocaine and cannabis, with a decrease in heroin overbalanced by a significant increase in hallucinogen and stimulant use (Molinaro et al., 2011). Between 2005 and 2008, the trend for cannabis use and availability decreased while its price increased, whereas the prevalence of cocaine and stimulant use increased significantly (Molinaro et al., 2011). In spite of the various social communication and legislative initiatives to prevent substance misuse, the situation has not improved considerably.
There are various reasons why teenagers and young adults become involved with drugs. Regrettably, the root of substance abuse often goes deeper than experimentation. The availability of illegal drugs for adolescents predicts increased substance use as an adult. According to a survey conducted between 1999 and 2009, cannabis has been the most widely available illicit substance, with cocaine’s availability rising since 2006, where one out of every five students reported easy access to the drug (Molinaro et al., 2011). Substance availability influences the use of substances by adolescents as they can obtain them easily. Cannabis is a consistently available illicit drug that adolescents abuse.
An increase in the prices of illicit substances affects the rates of abuse. The cheaper the illegal drugs are, the easier it is for adolescents to access them. An example is from the survey conducted by Molinaro et al. (2011) on cannabis.
Cannabis use and availability decreased in 2006, and its price increased, although cocaine and stimulant use prevalence increased significantly from 2005 to 2006, and their price decreased (Molinaro et al., 2011). The prices set for drugs influence their abuse. The higher the set costs, the less their abuse and inversely.
Drug usage is common among high school students, with cannabis being the most common and heroin being the least. Girls are less susceptible to illicit substance use than boys. The root of substance abuse often goes deeper than experimentation. Substance availability influences the use of substances by adolescents as they can obtain them easily. In spite of the various social communication and legislative initiatives to prevent substance misuse, the situation has not improved considerably.
Molinaro, S., Siciliano, V., Curzio, O., Denoth, F., Salvadori, S., & Mariani, F. (2011). Illegal substance use among Italian high school students: Trends over 11 years (1999–2009) . PloS one , 6 (6), e20482. Web.
- Drug Addiction: Overview of the Main Principles and Recovery Plan
- Tyler Skaggs’s Death Reminding About Opioid Crisis
- Cannabis Dependence and Psychiatric Disorders: Outline
- Heroin, Its History, Production, and Effects
- Experimentation on Animals
- How Opioid Addiction Affects the United States
- Why Marilyn Monroe Was Addicted to Substance Abuse
- Substance Use Prevention Among Youth
- The Role of Mitochondria in Cocaine Addiction
- Drug Laws Influnce on Different Population Groups
- Chicago (A-D)
- Chicago (N-B)
IvyPanda. (2023, January 5). Substance Abuse in Teenagers. https://ivypanda.com/essays/substance-abuse-in-teenagers/
"Substance Abuse in Teenagers." IvyPanda , 5 Jan. 2023, ivypanda.com/essays/substance-abuse-in-teenagers/.
IvyPanda . (2023) 'Substance Abuse in Teenagers'. 5 January.
IvyPanda . 2023. "Substance Abuse in Teenagers." January 5, 2023. https://ivypanda.com/essays/substance-abuse-in-teenagers/.
1. IvyPanda . "Substance Abuse in Teenagers." January 5, 2023. https://ivypanda.com/essays/substance-abuse-in-teenagers/.
Bibliography
IvyPanda . "Substance Abuse in Teenagers." January 5, 2023. https://ivypanda.com/essays/substance-abuse-in-teenagers/.
- To find inspiration for your paper and overcome writer’s block
- As a source of information (ensure proper referencing)
- As a template for you assignment
IvyPanda uses cookies and similar technologies to enhance your experience, enabling functionalities such as:
- Basic site functions
- Ensuring secure, safe transactions
- Secure account login
- Remembering account, browser, and regional preferences
- Remembering privacy and security settings
- Analyzing site traffic and usage
- Personalized search, content, and recommendations
- Displaying relevant, targeted ads on and off IvyPanda
Please refer to IvyPanda's Cookies Policy and Privacy Policy for detailed information.
Certain technologies we use are essential for critical functions such as security and site integrity, account authentication, security and privacy preferences, internal site usage and maintenance data, and ensuring the site operates correctly for browsing and transactions.
Cookies and similar technologies are used to enhance your experience by:
- Remembering general and regional preferences
- Personalizing content, search, recommendations, and offers
Some functions, such as personalized recommendations, account preferences, or localization, may not work correctly without these technologies. For more details, please refer to IvyPanda's Cookies Policy .
To enable personalized advertising (such as interest-based ads), we may share your data with our marketing and advertising partners using cookies and other technologies. These partners may have their own information collected about you. Turning off the personalized advertising setting won't stop you from seeing IvyPanda ads, but it may make the ads you see less relevant or more repetitive.
Personalized advertising may be considered a "sale" or "sharing" of the information under California and other state privacy laws, and you may have the right to opt out. Turning off personalized advertising allows you to exercise your right to opt out. Learn more in IvyPanda's Cookies Policy and Privacy Policy .
- Type 2 Diabetes
- Heart Disease
- Digestive Health
- Multiple Sclerosis
- Diet & Nutrition
- Health Insurance
- Public Health
- Patient Rights
- Caregivers & Loved Ones
- End of Life Concerns
- Health News
- Thyroid Test Analyzer
- Doctor Discussion Guides
- Hemoglobin A1c Test Analyzer
- Lipid Test Analyzer
- Complete Blood Count (CBC) Analyzer
- What to Buy
- Editorial Process
- Meet Our Medical Expert Board
Teenage Drug Addiction: An Overview
- Substance Use Statistics
- Why Teens Use Drugs
- Drug Effects
- Specific Health Risks
- Symptoms and Warning Signs
- Four Stages of Addiction
Many teens experiment with substances but don’t continue to use them. For some adolescents, however, trying a substance like alcohol, marijuana, or illicit drugs leads to regular use. Once withdrawal and cravings set in, a teen dealing with addiction and dependence may not be able to stop using a substance, even if they want to.
Caregivers can prevent teen drug abuse by knowing the signs and talking to their children about the consequences of using substances. This article reviews statistics, risk factors, health effects, signs, and treatment for teenage drug addiction .
Sturti / Getty Images
Teenage Substance Use Statistics
Public health experts track the rates of substance use in people of all ages. One group that they pay particular attention to is teens.
Basic Statistics
Here are some of the key statistics from the Monitoring the Future survey, which has been tracking youth substance use in the United States for over 40 years.
In 2023, here’s how many teens in the U.S. reported any illicit drug use in the last year:
- Eighth graders: 10.9%
- 10th graders: 19.2%
- 12th graders: 31.2%
In addition:
- By the time they reach 12th grade, 21.3% of teens have tried an illicit drug at least once.
- From 2016 to 2020, drug use among eighth graders increased by 61%.
- In a year, around 4,477 15-to-24-year-olds die of illicit drug overdoses (about 11.2% of all overdose deaths are in this age group).
Substances Used
Here is how many teens reported using a specific substance in the last year:
- Eighth graders: 15.1%
- 10th graders: 30.6%
- 12th graders: 45.7%
- Eighth graders: 8.3%
- 10th graders: 17.8%
- 12th graders: 29%
- Any illicit drugs:
- 10th graders: 19.8%
- 12th graders: 31.2 %
- Cigarettes:
- Eighth graders: 5.8%
- 10th graders: 9.4%
- 12 t thgraders: 15%
- Vaping nicotine (e-cigarettes):
- Eighth graders: 11.4%
- 10th graders: 17.6%
- 12th graders: 23.2%
Prescription Medications
Alcohol is the most commonly abused substance among teens, but rates of nicotine and prescription medication abuse are increasing. Examples of prescription drugs teens may misuse include stimulants like Adderall and benzodiazepines like Xanax .
What Causes Teens to Use Drugs?
The reasons why any person uses drugs are complex, and the same is true for teens. Wanting to fit in with peers, feeling overwhelmed by their changing brains and bodies, and pressure to perform in school or sports are just a few reasons why teens may start experimenting with drugs. Teens may not seek drugs out but are instead introduced to substances by someone they know, such as a friend, teammate, or even a family member.
In addition, teens often don’t know or understand the dangers of substance abuse. They may see occasional use as being safe and don’t believe they could become addicted to drugs or face consequences. They may also assume that they can stop using if they want to.
Other risk factors for drug use in teens include:
- Family history of substance use
- Academic pressure
- Adverse childhood events ( ACES )
- Lack of supervision
- Mental health disorders
- Peer pressure
- Desire to escape (e.g., external situation like home life or internal situation like complex feelings)
- Social acceptance (e.g., fitting in with peers)
- Low self-esteem
- Increased access to substances
- Transitional periods (e.g., starting puberty or attending a new school)
While drug use can lead to mental health disorders, sometimes it’s the other way around. Teens may use substances to self-medicate or numb emotional pain.
What Are the Effects of Using Drugs During Adolescence?
The body sends out a “feel good” chemical called dopamine when using a substance. This response tells the brain that it is worth using the substance again to get that feeling. As a result, a person starts having cravings for the substance. Addiction happens when cravings don’t stop, withdrawal occurs without the substance, and use continues even when there are negative consequences. Since the physical and mental urge to use is so strong, it becomes very hard to stop using a substance.
Teenagers who misuse substances can experience drug dependence ( substance use disorder ). Developmentally, adolescents are at the highest risk for drug dependence and severe addiction.
Effects on Brain Development and Growth
The human brain continues to develop until about the age of 25. Using substances during adolescence can change brain structure and negatively affect brain functions like learning, processing emotions, and decision-making. It can also lead to the following:
- More risky behaviors : Substance abuse makes teens more likely to engage in risky behaviors like unprotected sex (or "condomless sex") or dangerous driving.
- Higher risk for adult health problems : Teenagers who abuse substances have a higher risk of heart disease, high blood pressure, and sleep disorders.
- Mental health disorders : It is common for teens with substance abuse disorders to have mental health conditions (and vice versa).
- Impaired academic performance : Substance use affects a teen’s concentration and memory, which may negatively affect their schoolwork.
Substance Misuse and Mental Health
A study showed that 60% of teens in a community-based substance use treatment program were also diagnosed with a mental health disorder.
What Are the Health Risks of Drug Abuse?
Drug and alcohol use can lead to substance use disorder as well as the specific health risks of the substance being abused.
Alcohol use can lead to an increased risk of:
- Liver disease, cirrhosis, and cancer
- Heart disease and stroke
- Depression
- Lack of focus
- Alcohol poisoning
- Increased risky behavior
Alcohol Statistics
In the United States, 29.5 million people ages 12 and older have an alcohol use disorder.
Marijuana can impair concentration, worsen mental health, interfere with prescription medications, lead to risky sexual behaviors, or contribute to dangerous driving. Smoking marijuana can also negatively affect lung health.
Marijuana is often thought of as not being "as bad" as other drugs and, in some cases, even good for you. However, marijuana can be harmful to teens because their brains are still developing. Marijuana use in teens is linked to difficulty with problem-solving, memory and learning issues, impaired coordination, and problems with maintaining attention.
Vaping and Edible Marijuana Use Is on the Rise
Recent data shows a shift from teens smoking marijuana to using vaping devices and edibles instead.
Opioids include legal prescription medications such as hydrocodone, oxycontin, and fentanyl, as well as illegal drugs such as heroin. These drugs carry a high risk of overdose and death. The annual rate of opioid overdose deaths for those aged 15 to 24 years is 12.6 per 100,000 people.
Over-the-Counter and Prescription Medications
Over-the-counter (OTC) and prescription medications can be misused more easily than others because they’re often easy for teens to obtain. Diet pills, caffeine pills, and cold and flu products with dextromethorphan are just a few examples of OTC substances teens may use. They may also have access to family member’s prescriptions for drugs like opiate painkillers and stimulants or get them from friends who do.
There are serious health risks to misusing OTC cold and cough products, including increased blood pressure, loss of consciousness, and overdose. There can also be legal issues if a teen is using someone else’s prescriptions.
Tobacco can lead to multiple chronic illnesses, including:
- Lung disease
- Heart disease
- Vision loss
- Decreased fertility
E-Cigarettes (Vaping)
Vaping is attractive to teens because e-cigarettes are often flavored like fruit, candy, or mint. These products may contain nicotine or other synthetic substances that damage the brain and lungs. The teenage brain is vulnerable to the harmful effects of nicotine, including anxiety and addiction.
E-cigarettes come in a variety of shapes and sizes and might be disguised as everyday items, such as:
- USB Flash Drives
- Hoodie (sweatshirt) strings
- Smartwatches
- Toys (e.g., fidget spinners)
- Phone cases
Cocaine carries a risk of overdose and withdrawal. It causes decreased impulse control and poor decision-making. Withdrawal symptoms from cocaine include restlessness, paranoia, and irritability. Snorting cocaine can cause nosebleeds and a loss of smell. Using cocaine can lead to heart attacks, lung problems, strokes, seizures, and coma.
Cocaine Can Be Fatal With First Use
There have been reports of people dying the first time they use cocaine, often from sudden cardiac arrest, respiratory arrest, or seizures.
Ecstasy (MDMA)
Ecstasy is a stimulant that causes an increased heart rate, blurred vision, and nausea. It can also lead to brain swelling, seizures, and organ damage.
Ecstasy is also known as:
Inhalants are fumes from gases, glue, aerosols, or solvents that can damage the brain, heart, lungs, kidneys, and liver. Using inhalants even once can lead to overdose, suffocation, seizures, and death.
Methamphetamine
Methamphetamine (crystal meth) is a highly addictive stimulant that has multiple health consequences, including:
- Severe weight loss
- Lack of sleep
- Dental problems
- Change in brain structure
- Paranoia and hallucinations
Disease Transmission Risk
Injecting drugs with shared needles increases the risk of contracting HIV, hepatitis B, and hepatitis C.
What Are the Signs a Teen Is Using Drugs?
Being on the lookout for drug paraphernalia and signs and symptoms of drug abuse can help adults recognize at-risk teens.
Behavioral warning signs of drug use in teens include:
- Personality changes
- Irritability
- Difficulty sleeping
- Inappropriate or odd behavior (e.g., laughing randomly)
- Loss of interest in hobbies or extracurricular activities
- Avoiding eye contact
- Acting secretive or like they’re hiding something
- Staying out late
- Social withdrawal (e.g., from family, friends)
- Poor academic performance
- Hanging out with new friends or no longer hanging out with their usual friend group
- Poor hygiene
- Skipping school
- Isolation (e.g., staying in their room, refusing family meals)
Not All Warning Signs Indicate Drug Use
These warning signs do not necessarily mean a teen is using drugs. Other health problems like allergies, sinus infections, hormone imbalances, or mental disorders can also cause these symptoms in teens.
Physical signs of drug use in teens may include:
- Persistent cough
- Dilated pupils
- Increased or decreased energy
- Sleeping all the time or not at all
- Mood swings
- Memory problems
- Talking very fast or slowly
- Runny nose or nosebleeds
- Increased/decreased appetite
- Weight loss
- Smells like smoke or alcohol (e.g., on clothes, skin, or breath)
Other than behavior and physical signs in a teen, you should also be aware of objects that can be used to do drugs. Examples of drug paraphernalia include:
- Mirrors with white powder
- Razorblades
- Rolled dollar bills
- Crack pipes and spoons
- Needles and syringes
- Rolling paper
Substance Abuse Screening
The American Academy of Pediatrics (AAP) recommends that teens be screened at each annual medical exam appointment with questionnaires that ask them about substance use and their knowledge of the risks.
What Are the Four Stages of Drug Addiction?
You should also be aware of the four stages of addiction. The earlier teen drug use is recognized, the sooner they can get help.
- Experimentation: A teen tries one or more substances. Some teens will only try a substance once. Others will continue to experiment and increase their use.
- Regular or “social” use: A teen begins to use one or more substances regularly. At this stage, they may limit their use to just when they’re with friends or only in situations where they feel it’s needed—e.g., before a test.
- Risky use: A teen continues to use a substance that they have regularly been using, even if it’s caused problems for them at school, at home, and in their relationships. They crave the substance, both physically and mentally. At this stage, the substance has become central to a teen’s life, and they’ll take risks to get and use it.
- Dependence and Addiction: A teen is addicted to a substance, and most of their time and energy is devoted to getting and using it. At this stage, they would need intervention and treatment to quit, as they may not be able to stop on their own, even if they wanted to.
How Can Parents Prevent Teenage Drug Use?
While they may not express it, teens do value bonds with the adults in their lives. Nurturing that connection with them includes being involved in their lives and having open, honest communication.
How to Talk to Your Teen About Drug Use
Open communication starts by showing an interest in and talking to your teen about everything. This dialogue builds trust and respect, making it easier for you to talk about difficult topics.
Giving teens your undivided attention, without distractions, helps them feel special and heard. This quality time could be during chores, dinner, walks, car rides, or a fun family game night.
Here are some general tips to keep in mind when you’re talking about drugs with your teen:
- Stay curious and show interest.
- Ask open-ended questions.
- Actively listen.
- Don’t interrupt.
- Give compliments.
- Stay up late to talk.
- Chat over their favorite food.
If you’re trying to start a conversation with your teen because you think they may be using drugs, their response to being confronted will determine how you’ll need to approach the conversation.
If your teen admits to using drugs, stay calm. Be supportive and willing to listen. Find out as much as you can about their drug use—what substances they’re using, how often they’re using them, and how they’re getting them. Be clear that the risks of drugs are serious and that drug use will not be tolerated. At the same time, make sure that you reassure your teen that you love them and that you want to help.
If your teen denies using drugs and you think they are lying , communicate the negative consequences of drug and alcohol use. Be clear that you want them to be safe and that experimenting with substances is dangerous—even if it’s just one time. If you are not able to keep the line of communication open with your teen, talk to their healthcare provider. They can help connect you to resources and support you in taking more decisive action, like drug testing.
Other Strategies
Talking to your teen openly and often is key, but there are also other steps you can take:
- Model responsible behavior for them.
- Stay involved with their activities but let them express their boundaries.
- Meet their friends and their parents.
- Teach them how to make good decisions when under pressure.
Protect Teens From Prescription Medications
Prescription drugs are generally safe when they're taken as prescribed. However, any time a person takes medication for reasons other than what they were prescribed for, it is considered medication abuse. Strategies to protect teens from prescription medication misuse include:
- Storing prescription medications in a safe place
- Locking up controlled substances
- Getting rid of old medications
Safe Medication Disposal
Do not dispose of medications by flushing them down the toilet or pouring them down the sink. Medications can be crushed and mixed into the trash (to keep them away from children and pets) or returned to your local pharmacy or community drug take-back program.
Drug Addiction Treatment for Teens
Even if the adults in their lives try to prevent it, some teens will develop substance use disorders. Support for teens with drug addiction includes treating withdrawal or underlying mental health conditions, and addressing emotional needs, usually with a qualified mental health professional such as a psychiatrist or psychologist.
Treatment for teens experiencing substance use disorder includes a combination of the following:
- Outpatient clinics
- 12-step programs
- Inpatient mental health or substance use units
- Medications
- Therapy (individual, group, or family)
Substance Use Helpline
If your teen is struggling with substance use or addiction, contact the Substance Abuse and Mental Health Services Administration (SAMHSA) National Helpline at 1-800-662-4357 for information on support and treatment facilities in your area.
If you are having suicidal thoughts, dial 988 to contact the 988 Suicide & Crisis Lifeline and connect with a trained counselor. If you or a loved one are in immediate danger, call 911 .
Talk to your teen’s healthcare provider about what treatment would be best for them. Here are a few topics to discuss:
- Underlying health problems
- Benefits of treatment
- Credentials of team members
- Side effects
- Family involvement
- Schoolwork during treatment
- Length of treatment
- Follow-up care
Experimenting with drugs or alcohol is tempting for teenagers because they may not know or understand the dangers of using substances—even just once. Academic pressure, low self-esteem, and peer pressure are just a few factors that increase their risk of substance use.
Caregivers need to have an open line of communication with their teens and teach them about the risks of using drugs. It’s also important to know the signs of drug use and intervene early to help teens who are at risk for or have already developed substance use disorders.
While drug use may increase the risk of mental health disorders, it’s also important to note that these disorders can lead to substance abuse to self-medicate or numb the emotional pain. If you suspect that a teenager is experiencing either, consult a pediatrician or mental health professional as soon as possible.
Frequently Asked Questions
Depending on the substance and severity, a tube may be placed through the nose to suction drugs from the stomach. Activated charcoal is given through the tube to bind with the drug to release it from the body, decreasing the amount released into the bloodstream. If an antidote (reversal agent) such as Narcan is available for that substance, it may be given.
National surveys from the National Institute on Drug Abuse show adolescent drug use rates have remained steady. However, the survey’s detected a shift in the types of drugs used by teens. Alcohol is still the most often abused substance, but the rates are decreasing. Instead, nicotine use and misuse of prescription medications are on the rise.
University of Michigan. Teen drug use remains below pre-pandemic levels .
National Center for Drug Abuse Statistics. Drug use among youth: facts & statistics .
Monitoring the Future. National Survey Results on Drug Use, 1975-2023: Secondary School Students .
NCDAS. Drug use among youth: facts & statistics .
Monitoring the Future. Alcohol: Trends in last 12 months prevalence of use in 8 th , 10 th , and 12 th grade .
Monitoring the Future. Marijuana: Trends in last 12 months prevalence of use in 8 th , 10 th , and 12 th grade .
Monitoring the Future. Any illicit drug: Trends in last 12 months prevalence of use in 8 th , 10 th , and 12 th grade .
Monitoring the Future. Cigarettes: Trends in last 12 months prevalence of use in 8 th , 10 th , and 12 th grade .
Monitoring the Future. Vape nicotine (e-cigarettes): Trends in last 12 months prevalence of use in 8 th , 10 th , and 12 th grade .
DEA. Prescription for disaster: How teens abuse medicines .
National Institute of Health: National Institute on Drug Abuse, Advancing Addiction Science. NIH-funded study finds overall rate of drug use among 10-14 year-olds remained stable during the 2020 COVID-19 pandemic .
Scholastic and the National Institute of Drug Abuse (NIDA). How nicotine affects the teen brain .
Steinfeld M, Torregrossa MM. Consequences of adolescent drug use . Translational Psychiatry . 2023;13(1). doi:10.1038/s41398-023-02590-4
University of Rochester Medical Center. Understanding the teen brain .
National Institute of Health: National Institute on Drug Abuse, Advancing Addiction Science. Common comorbidities with substance use disorders research report: part 1: the connection between substance use disorders and mental illness .
National Institute on Alcohol Abuse and Alcoholism. Alcohol use in the United States .
NIH. Alcohol use disorder (AUD) in the United States: Age groups and demographic characteristics .
American Lung Association. Marijuana and lung health .
Centers for Disease Control and Prevention. Cannabis and teens .
Sharma P, Mathews DB, Nguyen QA, Rossmann GL, A Patten C, Hammond CJ. Old dog, new tricks: A review of identifying and addressing youth cannabis vaping in the pediatric clinical setting . Clin Med Insights Pediatr . 2023;17:11795565231162297. Published 2023 Mar 25. doi:10.1177/11795565231162297
NCDAS. Drug overdose death rates .
NIDA. Over-the-counter medicines .
Centers for Disease Control and Prevention. Health effects of cigarette smoking .
Center for Disease Control and Prevention. E-cigarette use among youth .
NYC Health. Cocaine .
Nemours Teens Health. What Is MDMA (ecstasy)?
Medline Plus. Inhalants .
National Institute of Health: National Institute on Drug Abuse, Advancing Addiction Science. Methamphetamine drug facts .
CDC. Injection drug use .
Levy S, Williams JF, Ryan S, et al. Substance Use Screening, Brief Intervention, and Referral to Treatment . Pediatrics . 2016;138(1). doi:10.1542/peds.2016-1211
AAP. Bright Futures Toolkit: Links to Commonly Used Screening Instruments and Tools .
Orlando Recovery Center. The four stages of addiction – what are they?
Casa Palmera. The four stages of drug addiction .
Partnership to End Addiction. Connecting with your teenager to prevent drug use .
SAMHSA. Talking with teens about alcohol and other drugs .
American Academy of Child & Adolescent Psychiatry (AACAP). Substance abuse treatment for children and adolescents: questions to ask .
National Council Against Prescription Drug Abuse (NCAPDA). Drug overdose response: know the signs .
American Academy of Child & Adolescent Psychiatry (AACAP). Teens: alcohol and other drugs .
Substance Abuse and Mental Health Services Administration (SAMHSA). Tips for teens: cocaine .
By Brandi Jones, MSN-ED RN-BC Jones is a registered nurse and freelance health writer with more than two decades of healthcare experience.
- Skip to main content
- Skip to secondary menu
- Skip to primary sidebar
- Skip to footer
A Plus Topper
Improve your Grades
Essay on Drug Addiction | Drug Addiction Essay for Students and Children in English
February 12, 2024 by Veerendra
Essay on Drug Addiction: Addiction refers to the harmful need to consume substances that have damaging consequences on the user. Addiction affects not just the body but also on the person’s mental health and soundness of mind. Addiction is one of the most severe health problems faced around the world and is termed as a chronic disease. A widespread disorder ranges from drugs, alcohol addiction to gambling, and even phone addiction.
You can read more Essay Writing about articles, events, people, sports, technology many more.
One of the most unfortunate yet common addictions that affect millions today is drug addiction. Also referred to as substance – use disorder, it is the addiction to substances that harm neurological functioning and a person’s behavior. The essay provides relevant information on this topic.
Long and Short Essay on Drug Addiction in English for Students and Kids
There are two essays listed below. The long essay consists of 500 words and a short essay of 200 words.
Long Essay on Drug Addiction in English 500 words
Drug addiction, also known as substance–use disorder, refers to the dangerous and excessive intake of legal and illegal drugs. This leads to many behavioral changes in the person as well as affects brain functions. Drug addiction includes abusing alcohol, cocaine, heroin, opioid, painkillers, and nicotine, among others. Drugs like these help the person feel good about themselves and induce ‘dopamine’ or the happiness hormone. As they continue to use the drug, the brain starts to increase dopamine levels, and the person demands more.
Drug addiction has severe consequences. Some of the signs include anxiety, paranoia, increased heart rate, and red eyes. They are intoxicated and unable to display proper coordination and have difficulty in remembering things. A person who is addicted cannot resist using them and unable to function correctly without ingesting them. It causes damage to the brain, their personal and professional relationships. It affects mental cognition; they are unable to make proper decisions, cannot retain information, and make poor judgments. They tend to engage in reckless activities such as stealing or driving under the influence. They also make sure that there is a constant supply and are willing to pay a lot of money even if they are unable to afford it and tend to have erratic sleep patterns.
Drug addiction also causes a person to isolate themselves and have either intense or no food cravings. They stop taking care of their hygiene. Drug addiction affects a person’s speech and experience hallucinations. They are unable to converse and communicate properly; they speak fast and are hyperactive. Those addicted have extreme mood swings. They can go from feeling happy to feeling sad quickly and are incredibly secretive. They begin to lose interest in activities they once loved. Substance abusers also undergo withdrawal symptoms. Withdrawal symptoms refer to the symptoms that occur when they stop taking the drug. Some withdrawal symptoms include nausea, fatigue, and tremors. They stop and starting using again, an endless cycle that could be life-threatening. Drug addiction can be fatal if not treated timely. It can cause brain damage and seizures as well as overdose, heart diseases, respiratory problems, damage to the liver and kidneys, vomiting, lung diseases, and much more.
Though chronic, treatment is available for drug addiction. Many techniques are used, such as behavioral counseling, medication to treat the addiction, and providing treatment not just for substance abuse but also for many factors that accompany addiction such as stress, anxiety, and depression. Many devices have developed to overcome addiction. There are rehabilitation centers to help people. After treatment, there are numerous follow-ups to ensure that the cycle does not come back. The most important is having family and friends to support the effect. It will help them build confidence and come over their addiction.
The United Nations celebrates International Day against Drug Abuse and Illicit Trafficking on the 26th of June. Drug addiction impacts millions and needs to be treated carefully to prevent further harm to the individual and letting them live a better life.
Short Essay on Drug Addiction in English 250 words
Drug addiction refers to taking substances that are harmful to our bodies. They cause changes to a person’s behavior as well. Many people take these drugs to feel happier and better about themselves. These dangerous substances make the brain produce a chemical that makes us happy, called dopamine. Producing large amounts of these causes the person to take the drug consistently.
Some of the drugs include alcohol, nicotine, and other unhealthy substances. Taking these substances can lead to many symptoms. These include unable to think correctly, cannot remember things, and unable to speak clearly. They steal and keep secrets from their close ones. Those addicted cannot sleep; they become happy and sad quickly. They stop doing the activities that they liked doing. They are not aware of their surroundings. Taking these dangerous substances can cause many health problems such as vomiting, unable to breathe, brain, and lung damage. It also affects their family, friends, and work.
Drug addiction is life-threatening. However, people with this addiction can be treated and helped with therapy, counseling, and taking medicines along with rehab centers. They do follow-ups to ensure that they never retake these drugs. They must have their family and friends to support them as they recover.
10 lines About Drug Addiction Essay in English
- Drug addiction refers to taking harmful substances that affect a person’s brain functions and behavior. It involves taking legal and illegal drugs, and the person is unable to stop using them. It is also referred to as substance- use disorders
- Harmful drugs include alcohol, cocaine, heroin, opioids, painkillers, nicotine, etc.
- The harmful drugs cause an excessive release of dopamine or the happy hormone, which causes the person to take more.
- Drug addiction can affect mental cognition, including decision making, judgments, and memory. It also causes speech problems.
- It can cause anxiety paranoia and increased blood pressure. They have erratic sleep patterns and isolate themselves. It causes problems in their personal and professional relationships.
- Those addicted become moody, hyperactive, and hallucinate. They also engage in reckless activities.
- They experience withdrawal symptoms when they try to stop using substances. These include nausea, fatigue, and tremors.
- It can have many effects on the body, such as brain damage, seizures, liver and kidney damage, respiratory and lung issues.
- Treatment is available. It includes behavioral therapy, medication, rehabilitation, as well as a follow-up to prevent relapse.
- The United Nations celebrates International Day against Drug Abuse and Illicit Trafficking on the 26th of June.
Frequently Asked Questions on Drug Addiction Essay
Question 1. What is drug addiction?
Answer: Drug addiction, also known as substance – use disorder, refers to the dangerous and excessive intake of legal and illegal drugs. This leads to many behavioral changes in the person as well as affects brain functions.
Question 2. Why does drug addiction occur?
Answer: People become addicted to these drugs because they want to feel happier. The drugs cause a chemical called dopamine, which induces happiness to be released. The brain starts to increase dopamine levels, and thus the person becomes addicted to the drug to match the increasing levels.
Question 3. What is the difference between dependence and addiction?
Answer: Dependence and addiction vary. While dependence is an intense craving for the drug by the body, addiction also refers to the changes in behavior and bodily functions due to repeated use of the drug, which has severe consequences.
Question 4. Can we treat drug addiction?
Answer: Yes, drug addiction can be treated. The various treatment methods are behavioral counseling, medication, and treatment of anxiety and depression. There are rehabilitation centers available. This is followed by a check-up to prevent relapse.
- Picture Dictionary
- English Speech
- English Slogans
- English Letter Writing
- English Essay Writing
- English Textbook Answers
- Types of Certificates
- ICSE Solutions
- Selina ICSE Solutions
- ML Aggarwal Solutions
- HSSLive Plus One
- HSSLive Plus Two
- Kerala SSLC
- Distance Education

IMAGES
VIDEO
COMMENTS
Drug addiction among youth is a complex issue that requires collective effort and understanding. By addressing the root causes and implementing effective prevention strategies, we can hope to curb this growing problem.
The global landscape of drug abuse and addiction is a complex issue that has significant implications on the youth. The impact of drugs on youth is far-reaching, affecting not just their physical health, but also their mental well-being, academic performance, and future prospects.
Aside from vaping, adolescent use of illicit substances has dropped substantially over the past few decades, but more teens are overdosing than ever—largely because of contamination of the drug supply with fentanyl, as well as the availability of stronger substances (Most reported substance use among adolescents held steady in 2022, National ...
The effects of drug abuse on youth are far-reaching and destructive. It is crucial to create awareness about the dangers of drug use, promote healthy coping mechanisms, and provide support for those struggling with addiction.
Adolescents are significant long-term contributors to the crisis due to their susceptibilities to drug abuse and impressionable age. This review examines the particular vulnerabilities of the adolescent brain to drug abuse and the risk and protective factors thereof, especially in light of the Rat Park studies.
The outcomes of this review suggest a complex interaction between a multitude of factors influencing adolescent drug abuse. Therefore, successful adolescent drug abuse prevention programs will require extensive work at all levels of domains. Keywords: Risk factor, Protective factor, Drug abuse, substance, adolescent.
More good news is that drug use and addiction are preventable. Teachers, parents, and health care providers have crucial roles in educating young people and preventing drug use and...
Substance abuse causes injury, sexually transmitted diseases, teenage pregnancies, poor mental health, and suicide. The essay presents the issues and the causes of substance abuse among adolescents. Get a custom essay on Substance Abuse in Teenagers. 189 writers online. Learn More.
Many teens have a problem with substance abuse, such as alcohol, marijuana, and prescription drugs. Learn the warning signs and what puts teens at risk.
One of the most unfortunate yet common addictions that affect millions today is drug addiction. Also referred to as substance – use disorder, it is the addiction to substances that harm neurological functioning and a person’s behavior. The essay provides relevant information on this topic.