

Write an Error-free Research Protocol As Recommended by WHO: 21 Elements You Shouldn’t Miss!
Principal Investigator: Did you draft the research protocol?
Student: Not yet. I have too many questions about it. Why is it important to write a research protocol? Is it similar to research proposal? What should I include in it? How should I structure it? Is there a specific format?
Researchers at an early stage fall short in understanding the purpose and importance of some supplementary documents, let alone how to write them. Let’s better your understanding of writing an acceptance-worthy research protocol.
Table of Contents
What Is Research Protocol?
The research protocol is a document that describes the background, rationale, objective(s), design, methodology, statistical considerations and organization of a clinical trial. It is a document that outlines the clinical research study plan. Furthermore, the research protocol should be designed to provide a satisfactory answer to the research question. The protocol in effect is the cookbook for conducting your study
Why Is Research Protocol Important?
In clinical research, the research protocol is of paramount importance. It forms the basis of a clinical investigation. It ensures the safety of the clinical trial subjects and integrity of the data collected. Serving as a binding document, the research protocol states what you are—and you are not—allowed to study as part of the trial. Furthermore, it is also considered to be the most important document in your application with your Institution’s Review Board (IRB).
It is written with the contributions and inputs from a medical expert, a statistician, pharmacokinetics expert, the clinical research coordinator, and the project manager to ensure all aspects of the study are covered in the final document.
Is Research Protocol Same As Research Proposal?
Often misinterpreted, research protocol is not similar to research proposal. Here are some significant points of difference between a research protocol and a research proposal:
What Are the Elements/Sections of a Research Protocol?
According to Good Clinical Practice guidelines laid by WHO, a research protocol should include the following:

1. General Information
- Protocol title, protocol identifying number (if any), and date.
- Name and address of the funder.
- Name(s) and contact details of the investigator(s) responsible for conducting the research, the research site(s).
- Responsibilities of each investigator.
- Name(s) and address(es) of the clinical laboratory(ies), other medical and/or technical department(s) and/or institutions involved in the research.
2. Rationale & Background Information
- The rationale and background information provides specific reasons for conducting the research in light of pertinent knowledge about the research topic.
- It is a statement that includes the problem that is the basis of the project, the cause of the research problem, and its possible solutions.
- It should be supported with a brief description of the most relevant literatures published on the research topic.
3. Study Objectives
- The study objectives mentioned in the research proposal states what the investigators hope to accomplish. The research is planned based on this section.
- The research proposal objectives should be simple, clear, specific, and stated prior to conducting the research.
- It could be divided into primary and secondary objectives based on their relativity to the research problem and its solution.
4. Study Design
- The study design justifies the scientific integrity and credibility of the research study.
- The study design should include information on the type of study, the research population or the sampling frame, participation criteria (inclusion, exclusion, and withdrawal), and the expected duration of the study.
5. Methodology
- The methodology section is the most critical section of the research protocol.
- It should include detailed information on the interventions to be made, procedures to be used, measurements to be taken, observations to be made, laboratory investigations to be done, etc.
- The methodology should be standardized and clearly defined if multiple sites are engaged in a specified protocol.
6. Safety Considerations
- The safety of participants is a top-tier priority while conducting clinical research .
- Safety aspects of the research should be scrutinized and provided in the research protocol.
7. Follow-up
- The research protocol clearly indicate of what follow up will be provided to the participating subjects.
- It must also include the duration of the follow-up.
8. Data Management and Statistical Analysis
- The research protocol should include information on how the data will be managed, including data handling and coding for computer analysis, monitoring and verification.
- It should clearly outline the statistical methods proposed to be used for the analysis of data.
- For qualitative approaches, specify in detail how the data will be analysed.
9. Quality Assurance
- The research protocol should clearly describe the quality control and quality assurance system.
- These include GCP, follow up by clinical monitors, DSMB, data management, etc.
10. Expected Outcomes of the Study
- This section indicates how the study will contribute to the advancement of current knowledge, how the results will be utilized beyond publications.
- It must mention how the study will affect health care, health systems, or health policies.
11. Dissemination of Results and Publication Policy
- The research protocol should specify not only how the results will be disseminated in the scientific media, but also to the community and/or the participants, the policy makers, etc.
- The publication policy should be clearly discussed as to who will be mentioned as contributors, who will be acknowledged, etc.
12. Duration of the Project
- The protocol should clearly mention the time likely to be taken for completion of each phase of the project.
- Furthermore a detailed timeline for each activity to be undertaken should also be provided.
13. Anticipated Problems
- The investigators may face some difficulties while conducting the clinical research. This section must include all anticipated problems in successfully completing their projects.
- Furthermore, it should also provide possible solutions to deal with these difficulties.
14. Project Management
- This section includes detailed specifications of the role and responsibility of each investigator of the team.
- Everyone involved in the research project must be mentioned here along with the specific duties they have performed in completing the research.
- The research protocol should also describe the ethical considerations relating to the study.
- It should not only be limited to providing ethics approval, but also the issues that are likely to raise ethical concerns.
- Additionally, the ethics section must also describe how the investigator(s) plan to obtain informed consent from the research participants.
- This section should include a detailed commodity-wise and service-wise breakdown of the requested funds.
- It should also include justification of utilization of each listed item.
17. Supplementary Support for the Project
- This section should include information about the received funding and other anticipated funding for the specific project.
18. Collaboration With Other Researchers or Institutions
- Every researcher or institute that has been a part of the research project must be mentioned in detail in this section of the research protocol.
19. Curriculum Vitae of All Investigators
- The CVs of the principal investigator along with all the co-investigators should be attached with the research protocol.
- Ideally, each CV should be limited to one page only, unless a full-length CV is requested.
20. Other Research Activities of Investigators
- A list of all current research projects being conducted by all investigators must be listed here.
21. References
- All relevant references should be mentioned and cited accurately in this section to avoid plagiarism.
How Do You Write a Research Protocol? (Research Protocol Example)
Main Investigator
Number of Involved Centers (for multi-centric studies)
Indicate the reference center
Title of the Study
Protocol ID (acronym)
Keywords (up to 7 specific keywords)
Study Design
Mono-centric/multi-centric
Perspective/retrospective
Controlled/uncontrolled
Open-label/single-blinded or double-blinded
Randomized/non-randomized
n parallel branches/n overlapped branches
Experimental/observational
Endpoints (main primary and secondary endpoints to be listed)
Expected Results
Analyzed Criteria
Main variables/endpoints of the primary analysis
Main variables/endpoints of the secondary analysis
Safety variables
Health Economy (if applicable)
Visits and Examinations
Therapeutic plan and goals
Visits/controls schedule (also with graphics)
Comparison to treatment products (if applicable)
Dose and dosage for the study duration (if applicable)
Formulation and power of the studied drugs (if applicable)
Method of administration of the studied drugs (if applicable)
Informed Consent
Study Population
Short description of the main inclusion, exclusion, and withdrawal criteria
Sample Size
Estimated Duration of the Study
Safety Advisory
Classification Needed
Requested Funds
Additional Features (based on study objectives)
Click Here to Download the Research Protocol Example/Template
Be prepared to conduct your clinical research by writing a detailed research protocol. It is as easy as mentioned in this article. Follow the aforementioned path and write an impactful research protocol. All the best!
Clear as template! Please, I need your help to shape me an authentic PROTOCOL RESEARCH on this theme: Using the competency-based approach to foster EFL post beginner learners’ writing ability: the case of Benin context. I’m about to start studies for a master degree. Please help! Thanks for your collaboration. God bless.
Rate this article Cancel Reply
Your email address will not be published.

Enago Academy's Most Popular Articles
![research study protocol definition What is Academic Integrity and How to Uphold it [FREE CHECKLIST]](https://www.enago.com/academy/wp-content/uploads/2024/05/FeatureImages-59-210x136.png)
Ensuring Academic Integrity and Transparency in Academic Research: A comprehensive checklist for researchers
Academic integrity is the foundation upon which the credibility and value of scientific findings are…

- Publishing Research
- Reporting Research
How to Optimize Your Research Process: A step-by-step guide
For researchers across disciplines, the path to uncovering novel findings and insights is often filled…

- Industry News
- Trending Now
Breaking Barriers: Sony and Nature unveil “Women in Technology Award”
Sony Group Corporation and the prestigious scientific journal Nature have collaborated to launch the inaugural…

Achieving Research Excellence: Checklist for good research practices
Academia is built on the foundation of trustworthy and high-quality research, supported by the pillars…

- Promoting Research
Plain Language Summary — Communicating your research to bridge the academic-lay gap
Science can be complex, but does that mean it should not be accessible to the…
Choosing the Right Analytical Approach: Thematic analysis vs. content analysis for…
Research Recommendations – Guiding policy-makers for evidence-based decision making
Demystifying the Role of Confounding Variables in Research

Sign-up to read more
Subscribe for free to get unrestricted access to all our resources on research writing and academic publishing including:
- 2000+ blog articles
- 50+ Webinars
- 10+ Expert podcasts
- 50+ Infographics
- 10+ Checklists
- Research Guides
We hate spam too. We promise to protect your privacy and never spam you.
I am looking for Editing/ Proofreading services for my manuscript Tentative date of next journal submission:

As a researcher, what do you consider most when choosing an image manipulation detector?
- Search Menu
Sign in through your institution
- Browse content in Arts and Humanities
- Browse content in Archaeology
- Anglo-Saxon and Medieval Archaeology
- Archaeological Methodology and Techniques
- Archaeology by Region
- Archaeology of Religion
- Archaeology of Trade and Exchange
- Biblical Archaeology
- Contemporary and Public Archaeology
- Environmental Archaeology
- Historical Archaeology
- History and Theory of Archaeology
- Industrial Archaeology
- Landscape Archaeology
- Mortuary Archaeology
- Prehistoric Archaeology
- Underwater Archaeology
- Urban Archaeology
- Zooarchaeology
- Browse content in Architecture
- Architectural Structure and Design
- History of Architecture
- Residential and Domestic Buildings
- Theory of Architecture
- Browse content in Art
- Art Subjects and Themes
- History of Art
- Industrial and Commercial Art
- Theory of Art
- Biographical Studies
- Byzantine Studies
- Browse content in Classical Studies
- Classical Literature
- Classical Reception
- Classical History
- Classical Philosophy
- Classical Mythology
- Classical Art and Architecture
- Classical Oratory and Rhetoric
- Greek and Roman Papyrology
- Greek and Roman Archaeology
- Greek and Roman Epigraphy
- Greek and Roman Law
- Late Antiquity
- Religion in the Ancient World
- Digital Humanities
- Browse content in History
- Colonialism and Imperialism
- Diplomatic History
- Environmental History
- Genealogy, Heraldry, Names, and Honours
- Genocide and Ethnic Cleansing
- Historical Geography
- History by Period
- History of Emotions
- History of Agriculture
- History of Education
- History of Gender and Sexuality
- Industrial History
- Intellectual History
- International History
- Labour History
- Legal and Constitutional History
- Local and Family History
- Maritime History
- Military History
- National Liberation and Post-Colonialism
- Oral History
- Political History
- Public History
- Regional and National History
- Revolutions and Rebellions
- Slavery and Abolition of Slavery
- Social and Cultural History
- Theory, Methods, and Historiography
- Urban History
- World History
- Browse content in Language Teaching and Learning
- Language Learning (Specific Skills)
- Language Teaching Theory and Methods
- Browse content in Linguistics
- Applied Linguistics
- Cognitive Linguistics
- Computational Linguistics
- Forensic Linguistics
- Grammar, Syntax and Morphology
- Historical and Diachronic Linguistics
- History of English
- Language Evolution
- Language Reference
- Language Variation
- Language Families
- Language Acquisition
- Lexicography
- Linguistic Anthropology
- Linguistic Theories
- Linguistic Typology
- Phonetics and Phonology
- Psycholinguistics
- Sociolinguistics
- Translation and Interpretation
- Writing Systems
- Browse content in Literature
- Bibliography
- Children's Literature Studies
- Literary Studies (Romanticism)
- Literary Studies (American)
- Literary Studies (Modernism)
- Literary Studies (Asian)
- Literary Studies (European)
- Literary Studies (Eco-criticism)
- Literary Studies - World
- Literary Studies (1500 to 1800)
- Literary Studies (19th Century)
- Literary Studies (20th Century onwards)
- Literary Studies (African American Literature)
- Literary Studies (British and Irish)
- Literary Studies (Early and Medieval)
- Literary Studies (Fiction, Novelists, and Prose Writers)
- Literary Studies (Gender Studies)
- Literary Studies (Graphic Novels)
- Literary Studies (History of the Book)
- Literary Studies (Plays and Playwrights)
- Literary Studies (Poetry and Poets)
- Literary Studies (Postcolonial Literature)
- Literary Studies (Queer Studies)
- Literary Studies (Science Fiction)
- Literary Studies (Travel Literature)
- Literary Studies (War Literature)
- Literary Studies (Women's Writing)
- Literary Theory and Cultural Studies
- Mythology and Folklore
- Shakespeare Studies and Criticism
- Browse content in Media Studies
- Browse content in Music
- Applied Music
- Dance and Music
- Ethics in Music
- Ethnomusicology
- Gender and Sexuality in Music
- Medicine and Music
- Music Cultures
- Music and Media
- Music and Culture
- Music and Religion
- Music Education and Pedagogy
- Music Theory and Analysis
- Musical Scores, Lyrics, and Libretti
- Musical Structures, Styles, and Techniques
- Musicology and Music History
- Performance Practice and Studies
- Race and Ethnicity in Music
- Sound Studies
- Browse content in Performing Arts
- Browse content in Philosophy
- Aesthetics and Philosophy of Art
- Epistemology
- Feminist Philosophy
- History of Western Philosophy
- Metaphysics
- Moral Philosophy
- Non-Western Philosophy
- Philosophy of Language
- Philosophy of Mind
- Philosophy of Perception
- Philosophy of Action
- Philosophy of Law
- Philosophy of Religion
- Philosophy of Science
- Philosophy of Mathematics and Logic
- Practical Ethics
- Social and Political Philosophy
- Browse content in Religion
- Biblical Studies
- Christianity
- East Asian Religions
- History of Religion
- Judaism and Jewish Studies
- Qumran Studies
- Religion and Education
- Religion and Health
- Religion and Politics
- Religion and Science
- Religion and Law
- Religion and Art, Literature, and Music
- Religious Studies
- Browse content in Society and Culture
- Cookery, Food, and Drink
- Cultural Studies
- Customs and Traditions
- Ethical Issues and Debates
- Hobbies, Games, Arts and Crafts
- Natural world, Country Life, and Pets
- Popular Beliefs and Controversial Knowledge
- Sports and Outdoor Recreation
- Technology and Society
- Travel and Holiday
- Visual Culture
- Browse content in Law
- Arbitration
- Browse content in Company and Commercial Law
- Commercial Law
- Company Law
- Browse content in Comparative Law
- Systems of Law
- Competition Law
- Browse content in Constitutional and Administrative Law
- Government Powers
- Judicial Review
- Local Government Law
- Military and Defence Law
- Parliamentary and Legislative Practice
- Construction Law
- Contract Law
- Browse content in Criminal Law
- Criminal Procedure
- Criminal Evidence Law
- Sentencing and Punishment
- Employment and Labour Law
- Environment and Energy Law
- Browse content in Financial Law
- Banking Law
- Insolvency Law
- History of Law
- Human Rights and Immigration
- Intellectual Property Law
- Browse content in International Law
- Private International Law and Conflict of Laws
- Public International Law
- IT and Communications Law
- Jurisprudence and Philosophy of Law
- Law and Society
- Law and Politics
- Browse content in Legal System and Practice
- Courts and Procedure
- Legal Skills and Practice
- Primary Sources of Law
- Regulation of Legal Profession
- Medical and Healthcare Law
- Browse content in Policing
- Criminal Investigation and Detection
- Police and Security Services
- Police Procedure and Law
- Police Regional Planning
- Browse content in Property Law
- Personal Property Law
- Study and Revision
- Terrorism and National Security Law
- Browse content in Trusts Law
- Wills and Probate or Succession
- Browse content in Medicine and Health
- Browse content in Allied Health Professions
- Arts Therapies
- Clinical Science
- Dietetics and Nutrition
- Occupational Therapy
- Operating Department Practice
- Physiotherapy
- Radiography
- Speech and Language Therapy
- Browse content in Anaesthetics
- General Anaesthesia
- Neuroanaesthesia
- Clinical Neuroscience
- Browse content in Clinical Medicine
- Acute Medicine
- Cardiovascular Medicine
- Clinical Genetics
- Clinical Pharmacology and Therapeutics
- Dermatology
- Endocrinology and Diabetes
- Gastroenterology
- Genito-urinary Medicine
- Geriatric Medicine
- Infectious Diseases
- Medical Toxicology
- Medical Oncology
- Pain Medicine
- Palliative Medicine
- Rehabilitation Medicine
- Respiratory Medicine and Pulmonology
- Rheumatology
- Sleep Medicine
- Sports and Exercise Medicine
- Community Medical Services
- Critical Care
- Emergency Medicine
- Forensic Medicine
- Haematology
- History of Medicine
- Browse content in Medical Skills
- Clinical Skills
- Communication Skills
- Nursing Skills
- Surgical Skills
- Medical Ethics
- Browse content in Medical Dentistry
- Oral and Maxillofacial Surgery
- Paediatric Dentistry
- Restorative Dentistry and Orthodontics
- Surgical Dentistry
- Medical Statistics and Methodology
- Browse content in Neurology
- Clinical Neurophysiology
- Neuropathology
- Nursing Studies
- Browse content in Obstetrics and Gynaecology
- Gynaecology
- Occupational Medicine
- Ophthalmology
- Otolaryngology (ENT)
- Browse content in Paediatrics
- Neonatology
- Browse content in Pathology
- Chemical Pathology
- Clinical Cytogenetics and Molecular Genetics
- Histopathology
- Medical Microbiology and Virology
- Patient Education and Information
- Browse content in Pharmacology
- Psychopharmacology
- Browse content in Popular Health
- Caring for Others
- Complementary and Alternative Medicine
- Self-help and Personal Development
- Browse content in Preclinical Medicine
- Cell Biology
- Molecular Biology and Genetics
- Reproduction, Growth and Development
- Primary Care
- Professional Development in Medicine
- Browse content in Psychiatry
- Addiction Medicine
- Child and Adolescent Psychiatry
- Forensic Psychiatry
- Learning Disabilities
- Old Age Psychiatry
- Psychotherapy
- Browse content in Public Health and Epidemiology
- Epidemiology
- Public Health
- Browse content in Radiology
- Clinical Radiology
- Interventional Radiology
- Nuclear Medicine
- Radiation Oncology
- Reproductive Medicine
- Browse content in Surgery
- Cardiothoracic Surgery
- Gastro-intestinal and Colorectal Surgery
- General Surgery
- Neurosurgery
- Paediatric Surgery
- Peri-operative Care
- Plastic and Reconstructive Surgery
- Surgical Oncology
- Transplant Surgery
- Trauma and Orthopaedic Surgery
- Vascular Surgery
- Browse content in Science and Mathematics
- Browse content in Biological Sciences
- Aquatic Biology
- Biochemistry
- Bioinformatics and Computational Biology
- Developmental Biology
- Ecology and Conservation
- Evolutionary Biology
- Genetics and Genomics
- Microbiology
- Molecular and Cell Biology
- Natural History
- Plant Sciences and Forestry
- Research Methods in Life Sciences
- Structural Biology
- Systems Biology
- Zoology and Animal Sciences
- Browse content in Chemistry
- Analytical Chemistry
- Computational Chemistry
- Crystallography
- Environmental Chemistry
- Industrial Chemistry
- Inorganic Chemistry
- Materials Chemistry
- Medicinal Chemistry
- Mineralogy and Gems
- Organic Chemistry
- Physical Chemistry
- Polymer Chemistry
- Study and Communication Skills in Chemistry
- Theoretical Chemistry
- Browse content in Computer Science
- Artificial Intelligence
- Computer Architecture and Logic Design
- Game Studies
- Human-Computer Interaction
- Mathematical Theory of Computation
- Programming Languages
- Software Engineering
- Systems Analysis and Design
- Virtual Reality
- Browse content in Computing
- Business Applications
- Computer Games
- Computer Security
- Computer Networking and Communications
- Digital Lifestyle
- Graphical and Digital Media Applications
- Operating Systems
- Browse content in Earth Sciences and Geography
- Atmospheric Sciences
- Environmental Geography
- Geology and the Lithosphere
- Maps and Map-making
- Meteorology and Climatology
- Oceanography and Hydrology
- Palaeontology
- Physical Geography and Topography
- Regional Geography
- Soil Science
- Urban Geography
- Browse content in Engineering and Technology
- Agriculture and Farming
- Biological Engineering
- Civil Engineering, Surveying, and Building
- Electronics and Communications Engineering
- Energy Technology
- Engineering (General)
- Environmental Science, Engineering, and Technology
- History of Engineering and Technology
- Mechanical Engineering and Materials
- Technology of Industrial Chemistry
- Transport Technology and Trades
- Browse content in Environmental Science
- Applied Ecology (Environmental Science)
- Conservation of the Environment (Environmental Science)
- Environmental Sustainability
- Environmentalist Thought and Ideology (Environmental Science)
- Management of Land and Natural Resources (Environmental Science)
- Natural Disasters (Environmental Science)
- Nuclear Issues (Environmental Science)
- Pollution and Threats to the Environment (Environmental Science)
- Social Impact of Environmental Issues (Environmental Science)
- History of Science and Technology
- Browse content in Materials Science
- Ceramics and Glasses
- Composite Materials
- Metals, Alloying, and Corrosion
- Nanotechnology
- Browse content in Mathematics
- Applied Mathematics
- Biomathematics and Statistics
- History of Mathematics
- Mathematical Education
- Mathematical Finance
- Mathematical Analysis
- Numerical and Computational Mathematics
- Probability and Statistics
- Pure Mathematics
- Browse content in Neuroscience
- Cognition and Behavioural Neuroscience
- Development of the Nervous System
- Disorders of the Nervous System
- History of Neuroscience
- Invertebrate Neurobiology
- Molecular and Cellular Systems
- Neuroendocrinology and Autonomic Nervous System
- Neuroscientific Techniques
- Sensory and Motor Systems
- Browse content in Physics
- Astronomy and Astrophysics
- Atomic, Molecular, and Optical Physics
- Biological and Medical Physics
- Classical Mechanics
- Computational Physics
- Condensed Matter Physics
- Electromagnetism, Optics, and Acoustics
- History of Physics
- Mathematical and Statistical Physics
- Measurement Science
- Nuclear Physics
- Particles and Fields
- Plasma Physics
- Quantum Physics
- Relativity and Gravitation
- Semiconductor and Mesoscopic Physics
- Browse content in Psychology
- Affective Sciences
- Clinical Psychology
- Cognitive Psychology
- Cognitive Neuroscience
- Criminal and Forensic Psychology
- Developmental Psychology
- Educational Psychology
- Evolutionary Psychology
- Health Psychology
- History and Systems in Psychology
- Music Psychology
- Neuropsychology
- Organizational Psychology
- Psychological Assessment and Testing
- Psychology of Human-Technology Interaction
- Psychology Professional Development and Training
- Research Methods in Psychology
- Social Psychology
- Browse content in Social Sciences
- Browse content in Anthropology
- Anthropology of Religion
- Human Evolution
- Medical Anthropology
- Physical Anthropology
- Regional Anthropology
- Social and Cultural Anthropology
- Theory and Practice of Anthropology
- Browse content in Business and Management
- Business Ethics
- Business History
- Business Strategy
- Business and Technology
- Business and Government
- Business and the Environment
- Comparative Management
- Corporate Governance
- Corporate Social Responsibility
- Entrepreneurship
- Health Management
- Human Resource Management
- Industrial and Employment Relations
- Industry Studies
- Information and Communication Technologies
- International Business
- Knowledge Management
- Management and Management Techniques
- Operations Management
- Organizational Theory and Behaviour
- Pensions and Pension Management
- Public and Nonprofit Management
- Strategic Management
- Supply Chain Management
- Browse content in Criminology and Criminal Justice
- Criminal Justice
- Criminology
- Forms of Crime
- International and Comparative Criminology
- Youth Violence and Juvenile Justice
- Development Studies
- Browse content in Economics
- Agricultural, Environmental, and Natural Resource Economics
- Asian Economics
- Behavioural Finance
- Behavioural Economics and Neuroeconomics
- Econometrics and Mathematical Economics
- Economic History
- Economic Methodology
- Economic Systems
- Economic Development and Growth
- Financial Markets
- Financial Institutions and Services
- General Economics and Teaching
- Health, Education, and Welfare
- History of Economic Thought
- International Economics
- Labour and Demographic Economics
- Law and Economics
- Macroeconomics and Monetary Economics
- Microeconomics
- Public Economics
- Urban, Rural, and Regional Economics
- Welfare Economics
- Browse content in Education
- Adult Education and Continuous Learning
- Care and Counselling of Students
- Early Childhood and Elementary Education
- Educational Equipment and Technology
- Educational Strategies and Policy
- Higher and Further Education
- Organization and Management of Education
- Philosophy and Theory of Education
- Schools Studies
- Secondary Education
- Teaching of a Specific Subject
- Teaching of Specific Groups and Special Educational Needs
- Teaching Skills and Techniques
- Browse content in Environment
- Applied Ecology (Social Science)
- Climate Change
- Conservation of the Environment (Social Science)
- Environmentalist Thought and Ideology (Social Science)
- Natural Disasters (Environment)
- Social Impact of Environmental Issues (Social Science)
- Browse content in Human Geography
- Cultural Geography
- Economic Geography
- Political Geography
- Browse content in Interdisciplinary Studies
- Communication Studies
- Museums, Libraries, and Information Sciences
- Browse content in Politics
- African Politics
- Asian Politics
- Chinese Politics
- Comparative Politics
- Conflict Politics
- Elections and Electoral Studies
- Environmental Politics
- European Union
- Foreign Policy
- Gender and Politics
- Human Rights and Politics
- Indian Politics
- International Relations
- International Organization (Politics)
- International Political Economy
- Irish Politics
- Latin American Politics
- Middle Eastern Politics
- Political Behaviour
- Political Economy
- Political Institutions
- Political Theory
- Political Methodology
- Political Communication
- Political Philosophy
- Political Sociology
- Politics and Law
- Politics of Development
- Public Policy
- Public Administration
- Quantitative Political Methodology
- Regional Political Studies
- Russian Politics
- Security Studies
- State and Local Government
- UK Politics
- US Politics
- Browse content in Regional and Area Studies
- African Studies
- Asian Studies
- East Asian Studies
- Japanese Studies
- Latin American Studies
- Middle Eastern Studies
- Native American Studies
- Scottish Studies
- Browse content in Research and Information
- Research Methods
- Browse content in Social Work
- Addictions and Substance Misuse
- Adoption and Fostering
- Care of the Elderly
- Child and Adolescent Social Work
- Couple and Family Social Work
- Direct Practice and Clinical Social Work
- Emergency Services
- Human Behaviour and the Social Environment
- International and Global Issues in Social Work
- Mental and Behavioural Health
- Social Justice and Human Rights
- Social Policy and Advocacy
- Social Work and Crime and Justice
- Social Work Macro Practice
- Social Work Practice Settings
- Social Work Research and Evidence-based Practice
- Welfare and Benefit Systems
- Browse content in Sociology
- Childhood Studies
- Community Development
- Comparative and Historical Sociology
- Economic Sociology
- Gender and Sexuality
- Gerontology and Ageing
- Health, Illness, and Medicine
- Marriage and the Family
- Migration Studies
- Occupations, Professions, and Work
- Organizations
- Population and Demography
- Race and Ethnicity
- Social Theory
- Social Movements and Social Change
- Social Research and Statistics
- Social Stratification, Inequality, and Mobility
- Sociology of Religion
- Sociology of Education
- Sport and Leisure
- Urban and Rural Studies
- Browse content in Warfare and Defence
- Defence Strategy, Planning, and Research
- Land Forces and Warfare
- Military Administration
- Military Life and Institutions
- Naval Forces and Warfare
- Other Warfare and Defence Issues
- Peace Studies and Conflict Resolution
- Weapons and Equipment

- < Previous chapter
- Next chapter >
15 Clinical trial protocols: study protocol
- Published: February 2016
- Cite Icon Cite
- Permissions Icon Permissions
This chapter defines protocols and the requirements to be considered when writing a protocol, The protocol is one of the most important documents and must be clear, concise and well written. The chapter describes the procedures before embarking on writing a protocol, who should be involved and describes all the main elements to be included. The ICH E6 guidelines give a complete list of the content of a protocol so this chapter centres on the main decisions/considerations. The main considerations are outlined in a skeleton protocol which includes for example objectives and end points, subject population, study design, study methodology, assessments and timings and statistical considerations. Once these main decisions have been made the first full draft protocol can be written by elaborating every detail and adding the administrative sections as set out in the company/institution SOPs and the ICH GCP guidelines. The process of protocol approval is described along with the requirements for protocol amendments. The additional requirements to be considered in writing protocols for healthcare studies is set out especially the ethical requirements that have to be addressed.
Signed in as
Institutional accounts.
- GoogleCrawler [DO NOT DELETE]
- Google Scholar Indexing
Personal account
- Sign in with email/username & password
- Get email alerts
- Save searches
- Purchase content
- Activate your purchase/trial code
- Add your ORCID iD
Institutional access
Sign in with a library card.
- Sign in with username/password
- Recommend to your librarian
- Institutional account management
- Get help with access
Access to content on Oxford Academic is often provided through institutional subscriptions and purchases. If you are a member of an institution with an active account, you may be able to access content in one of the following ways:
IP based access
Typically, access is provided across an institutional network to a range of IP addresses. This authentication occurs automatically, and it is not possible to sign out of an IP authenticated account.
Choose this option to get remote access when outside your institution. Shibboleth/Open Athens technology is used to provide single sign-on between your institution’s website and Oxford Academic.
- Click Sign in through your institution.
- Select your institution from the list provided, which will take you to your institution's website to sign in.
- When on the institution site, please use the credentials provided by your institution. Do not use an Oxford Academic personal account.
- Following successful sign in, you will be returned to Oxford Academic.
If your institution is not listed or you cannot sign in to your institution’s website, please contact your librarian or administrator.
Enter your library card number to sign in. If you cannot sign in, please contact your librarian.
Society Members
Society member access to a journal is achieved in one of the following ways:
Sign in through society site
Many societies offer single sign-on between the society website and Oxford Academic. If you see ‘Sign in through society site’ in the sign in pane within a journal:
- Click Sign in through society site.
- When on the society site, please use the credentials provided by that society. Do not use an Oxford Academic personal account.
If you do not have a society account or have forgotten your username or password, please contact your society.
Sign in using a personal account
Some societies use Oxford Academic personal accounts to provide access to their members. See below.
A personal account can be used to get email alerts, save searches, purchase content, and activate subscriptions.
Some societies use Oxford Academic personal accounts to provide access to their members.
Viewing your signed in accounts
Click the account icon in the top right to:
- View your signed in personal account and access account management features.
- View the institutional accounts that are providing access.
Signed in but can't access content
Oxford Academic is home to a wide variety of products. The institutional subscription may not cover the content that you are trying to access. If you believe you should have access to that content, please contact your librarian.
For librarians and administrators, your personal account also provides access to institutional account management. Here you will find options to view and activate subscriptions, manage institutional settings and access options, access usage statistics, and more.
Our books are available by subscription or purchase to libraries and institutions.
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Rights and permissions
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Clin Diagn Res
- v.10(11); 2016 Nov
Protocol Writing in Clinical Research
Azzam al-jundi.
1 Professor, Department of Orthodontics, King Saud bin Abdul Aziz University for Health Sciences, College of Dentistry, Riyadh, Kingdom of Saudi Arabia.
Salah Sakka
2 Associate Professor, Department of Oral Surgery, Al Farabi Dental College, Riyadh, Kingdom of Saudi Arabia.
Writing a research proposal is probably one of the most challenging and difficult task as research is a new area for the majority of postgraduates and new researchers. The purpose of this article is to summarize the most important steps and necessary guidelines for producing a standard research protocol. Academic and administrative success of any project is usually determined by acquiring a grant for the related field of research. Hence, the quality of a protocol is primarily required to achieve success in this scientific competition.
What is A Protocol?
Clinical research is conducted according to a plan (a protocol) or an action plan. The protocol demonstrates the guidelines for conducting the trial. It illustrates what will be made in the study by explaining each essential part of it and how it is carried out. It also describes the eligibility of the participants, the length of the study, the medications and the related tests.
A protocol is directed by a chief researcher. The health of the participants’ will be regularly checked by members of the research team to ultimately ensure the study’s safety and effectiveness.
Purpose of a Research Proposal
[ Table/Fig-1 ] outlines the key aims of the protocol.
[Table/Fig-1]:
Aims of the protocol.
Benefits of the Proposal to a Researcher
[ Table/Fig-2 ] outlines the key benefits of the protocol.
[Table/Fig-2]:
Benefits of the protocol.
The key points of the proposal should include justification for the need of the project and a detailed plan for the investigation [ 1 , 2 ]:
What is the question? (Hypothesis) What is it to be investigated?
- Why is the study important? (Significance)
- Where and when it will take place?
- What is the methodology? (Procedures and methods to be used).
- How are you going to do it? (Research design)
- Proposed time table and budget.
- Resources required (technical, scientific, and financial).
Drafting the protocol correctly will increase the likelihood that the conclusions drawn from the research are scientifically sound. Recommendations and suggestions should be sought from colleagues and experts so that researchers can develop their plans. However, once the study is launched, the protocol should not be altered during the progression of the study or trials. If the changes during progress of study are minor, then that part of the study should be excluded from the analysis. Unless unexpected complications occur during the conduct of the trial, it is advisable to reconsider and rewrite the protocol where the whole process is started again provided that the original research topic is still considered to be relevant. If complications are anticipated, it is suitable to run a pilot study, to check the feasibility of the study and find answers to the potential areas of the trial.
What is A Protocol Review?
Clinical trials must be approved and monitored by an Institutional Review Board that ensures that the risks are negligible and are worth any potential benefits. It is an independent committee that consists of physicians, dentists, statisticians, and members of the community.
The committee ensures that clinical trials are ethical and that the rights of all participants are protected. The board must initially approve and periodically review the research.
Components of a Research Protocol: The topics that should be covered in a protocol are shown in [ Table/Fig-3 ] [ 3 , 4 ].
[Table/Fig-3]:
The components of the protocol.
Writing The Protocol
Protocol writing allows the researcher to review and critically evaluate the published literature on the interested topic, plan and review the project steps and serves as a guide throughout the investigation. The proposal is an inevitable document that enables the researcher to monitor the progress of the project [ 5 ].
1) Title of the Study: Title of proposal should be accurate, short, concise, and identify [ 2 , 6 ].
What is the study about, Who are the targets, Where is the setting of the study and When it is launched, if applicable-
It should make the main objective clear, convey the main purpose of the research and mention the target population. Carry maximum information about the topic in a few words; it is a good practice to keep the title to within 12-15 words. It should convey the idea about the area of research and what methods are going to be used in a compact, relevant, accurate, attractive, easy to understand, and informative way.
2) Administrative Details: The following administrative details and a protocol content summary should follow the title page:
- Contents page list of relevant sections and sub-sections with corresponding page number.
- Signature page is signed by senior members of the research team and dated to confirm that the version concerned has been approved by them.
- Contact details for the research team members listing postal, e-mail addresses and telephone numbers.
3) Project Summary: The summary should be distinctive, concise and should sum up all the essentials of the protocol.
4) Introduction (Background): The background to the project should be concise and refer to the subject straight forwardly. In writing the review, attention should be drawn to the positives, negatives and limitations of the studies quoted [ 7 – 9 ]. Introduction is concluded by explaining how the present study will benefit the community. The literature review should logically lead to the statement of the aims of the proposed project and end with the aims and objectives of the study. The review should include the most recent publications in the field and the topic of the research is selected only after completing the literature review and finding some gaps in it.
Introduction should briefly answer the importance of the topic, the gaps/lacunae in the literature, the purpose of the study and benefits for the society, from the study.
The research question should be described precisely and concisely. It is going to be the basis of designing the project. The definition of the problem should be clear so that a reader can straight forwardly recognize the real meaning of it.
5) Study Objectives (Aims): The aims should be explicitly stated. These should be confined to the intention of the project and they should arise from the literature review. State the goal you need to achieve.
The study aims or objectives emerge from the study questions/ hypothesis. They are answers to what are the possible responses to the research question or hypothesis under analysis and measure. Aims should be logical and coherent, feasible, concise, realistic, considering local conditions, phrased to clearly meet the purpose of the study and related to what the specific research is intended to accomplish. For example, to evaluate knowledge level regarding dental caries in primary school children in KSA (this is not detailed). The following should be added: Causes, treatment, preventive measures, etc.
The objectives should be (SMART objective): Specific, Measurable, Achievable, Relevant and Time based [ 10 ].
Specific Aims: Details of each objective that will finally lead to the achievement of the goal should be stated. Specific aims one by one should be listed concisely. It is good practice not to include too many aims in the study (2-5 best); too many objectives often lead to inaccurate and poorly defined results. Furthermore, aims should be achievable, realistic and specific with no general and ambiguous statements. They should be stated in action verbs that illustrate their purpose: i.e., “to determine, to compare, to verify, to calculate, to reduce, to describe, etc.”
Secondary Objectives (Optional): These are referred to as ancillary and minor objectives that could be studied during the course of the study.
The formulation of objectives helps to focus the study and to avoid the collection of any unnecessary data and hence organize the study in clear and distinct stages.
Hypothesis: It is a statement based on sound scientific theory that recognizes the predicted correlation between two or additional assessable variables [ 11 ]. It is always developed in response to the purpose statement or to answer the research questions posed. Furthermore, hypothesis transforms research questions into a format amendable to testing or into a statement that predicts an expected outcome.
Types of hypothesis statements:
- Null hypothesis: A null hypothesis is a statement that there is no actual relationship between variables (H0 or HN). It may be read as there is no difference between the groups to be compared and no relationship between the exposure and outcome under investigation. H0 states the contradictory of what the researchers expect. The final conclusion of the investigators will either keep a null hypothesis or reject it in support of an alternative hypothesis. It does not essentially mean that H0 is accurate when not rejecting it as there might not be an adequate proof against it.
- Alternative hypothesis: An alternative hypothesis is a statement that suggests a potential outcome that the researcher may expect (H1 or HA). This hypothesis is derived from previous studies where an evident difference between the groups to be compared is present. It is recognized only when a null hypothesis is rejected. Practically, hypotheses are stated in the null form, because they have their inferential statistics. Such hypotheses of no difference will be challenged by researchers and the result of the statistical testing gives the probability that the hypothesis of no difference is true or false [ 12 ].
Aims should be logically linked and arranged according to the tested hypothesis statement.
- Research question: Is there a difference in fluoride release between the Compomer and Glass- ionomer cement?
- Null Hypothesis: There is no difference in fluoride release between the Compomer and Glass- ionomer cement.
- Alternate Hypothesis: There is a difference in fluoride release between the Compomer and Glass-ionomer cement.
The statement of the problem should provide a summary of exactly what the project is trying to achieve.
- What exactly do you want to study?
- Why is it worth studying?
- Does the proposed study have theoretical and/or practical significance?
- Does it contribute to a new understanding of a phenomenon? (i.e., Does it address new or little known material or does it treat familiar material in a new way or does it challenge an existing understanding or extend existing knowledge?)
The justification of the research should be a convincing statement for the need to do it:
- How does the research relate to the priorities of the region and the country?
- What knowledge and information will be obtained?
- What is the ultimate purpose that the knowledge obtained from the study will serve?
- How will the results be disseminated?
- How will the results be used, and who will be the beneficiaries?
6) Methods and Materials: It should describe in detail the ‘Where’, ‘Who’, ‘How’ the research will be conducted. It explains the study design and procedures and techniques used to achieve the proposed objectives. It defines the variables and demonstrates in detail how the variables will be measured. It details the proposed methodology for data gathering and processing.
Methodology composes an important part of the protocol. It assures that the hypothesis will be confirmed or rejected. It also refers to a thorough strategy to attain the objectives [ 13 ].
The methods and materials are divided into various subheadings:
a) Study design (cross-sectional, case-control, intervention study, RCT, etc.): Proper explanation should be given as to why a particular design was chosen (on the basis of proposed objectives and availability of resources).
A study design is in fact the researcher’s general plan to acquire the answer (s) to the hypothesis being tested. Here, strategies will be applied to develop balanced, correct, objective and meaningful information [ 14 ]. It explains the methods that will be used to collect and analyze data. Proper selection of the study design is important to attain reliable and valid scientific results.
Ethics, logistic concerns, economic features and scientific thorough-ness will determine the design of the study. Here, a chief concern is given to the legality of the results including potential bias mystifying issues.
Randomized controlled clinical trial is the best to document a causal relationship between an exposure and its outcome [ Table/Fig-4 ].
[Table/Fig-4]:
Suitable research design depends on the purpose of the study.
b) Study population (Study subjects): Where are you going to do the research and who is the study population (why doing research in this place and why selecting this population?).
It describes in detail about the study subjects, all aspects of the selection procedure and sample size calculation. Proper definition of eligibility, inclusion, exclusion and discontinuation criteria of the study subjects should be stated. Allocation of subjects to study arms should be explained and described in details bearing in mind the concealment and randomization process [ 15 , 16 ].
c) Sample size: Sample size calculation is recommended for economical and ethical reasons [ 16 – 18 ]. The calculation of the sample size must be explained including the power of the sample. The sampling technique should be mentioned, e.g., randomization that will be used in order to obtain a representative sample for your target population. Each step involved in the recruitment of the study subjects should be described according to the selection criteria (inclusion and exclusion criteria).
“Informed consent” should be mentioned (Permission granted in full knowledge of the possible consequences).
d) Proposed intervention: Full description of proposed intervention should be given. Here, all the activities and actions should be recorded and thoroughly explained in their order of occurrence.
- When using drugs, both scientific and brand name should be mentioned followed by the name of the manufacturing company, city, and country. Drug route, dosage, frequency of administration, and total duration of treatment with the drug should be mentioned.
- When using apparatus its name should be given followed by the name of the manufacturer, city and country.
Involved personnel should precisely define:
- Who will be responsible for the interventions?
- What activities each personnel will perform and with what frequency and intensity?
e) Data collection methods, instruments used:
Data collection tools are:
- Retrospective data (medical records) [ 19 ]
- Questionnaires [ 20 ]
- Interviews (Structured, Semi-Structured)
- Laboratory test (literature or personal knowledge should be referenced, if established test, or description should be provided in details, if not established)
- Clinical examinations
- Description of instruments, tools used for data collection, as well as the methods used to test the validity and reliability of the instrument should be provided [ 21 ].
7) Data Management and Analysis Plan: This section should be written following statistical advice from a statistician. The analysis plan and which statistical tests will be used to check the significance to the research question/hypothesis with appropriate references should be described. Names of variables that will be used in the analyses and the name of statistical analysis that will be performed to assess the outcome should be listed [ 22 – 25 ].
If computer programs are to be applied, it is important to mention the software used and its version.
8) Project Management: Work plan-A work plan is an outline of activities of all the phases of the research to be carried out according to an anticipated time schedule.
Proper time table for accomplishing each major step of the study should be defined. Assigning time frame to each step in the trial will be helpful in organizing the structure of the research trial. The personnel (investigators, assistants, laboratory technicians etc.) involved in the study or data collection should be properly trained.
9) Strengths and Limitations: It is important to mention the strengths or limitations of the study, i.e., what study can achieve or cannot achieve is important, so as to prevent wasteful allocation of resources.
10) Ethical Considerations (Issues for Ethical Review and Approvals): It should indicate whether the procedures to be followed are in accord with the Declaration of Helsinki. In any case, study should not start unless approval from ethics committee is received [ 26 ].
The following points should be explained:
- The benefits and risks for the subjects involved. The physical, social and psychological implications of the research.
- Details of the information to be given to the study patients including alternative treatments/approaches.
- Information should be provided on the free informed consent of the participants. Information form should contain: Justification for research, outline of study, risks, confidentiality, and voluntary participation should be told patients about the freedom to withdraw from the study whenever they wish to. Confidentiality indicates how the personal information obtained from the patient will be kept secret (Data safety).
11) Operational Planning and Budgeting (Budget Summary): Outline the budget requirement showing head wise expenditure for the study-manpower, transportation, instruments, laboratory tests, and cost of the drug. Budget estimate is to be attached in the annexure. All costs including personnel, consumables, equipment, supplies, communication, and funds for patients and data processing are all included in the budget. Each item should be justified.
12) Reference System: Referencing is the regular method of recognizing information taken from other researchers’ work. A proper citation will enable the readers to follow-up any reference of interest. Plagiarism refers to claiming and acquiring someone else’s ideas, an action that is considered a criminal action.
Failure to reference an idea that you have found in your research, or to acknowledge the work of other team members in a team assignment falls under the category of plagiarism. Therefore referencing is an extremely important aspect of the research protocol. The two most commonly used citation systems in clinical writing are the Vancouver system and the Harvard system [ 27 , 28 ]. The choice of referencing system is dependent upon the funding organizations where the research protocol is being submitted. These frequently identify their preferred system of referencing and this should be strictly adhered to. The most common style used in the dental literature is Vancouver style.
13) Annexure:
The following annexes are to be attached at the end of the protocol:
- Informed consent form.
- Letters from ethics committees.
- Study questionnaire (copies of any questionnaires or draft questionnaires).
- Case Record Forms (CRFs).
- Budget details.
- Curriculum Vitae (CV) of the chief investigator and co-investigator and their role in the study. It will ensure that the role of each investigator is well defined.
How to Judge A Good Protocol?
The protocol should adequately answer the research question. The research design must be sound enough to yield the expected knowledge. It should provide enough detail (methodology) that can allow another investigator to do the study and arrive at comparable conclusions. Here, the proposed number of participants is reasonably justified and the scientific design is adequately described.
Common Mistakes (Common Pitfalls to Avoid)
Incorporating insufficient elements regarding proposed studies and inadequate explanation for the implication of the problem must be shunned as well as suggesting far more work than can be practically done during the study period. Furthermore, underpowered sample size should be justified, invalid or unreliable instrumentation should be tested and improper statistics should be adequately analyzed.
The most difficult stage of conducting a research project is the preparation of a protocol that results in a short yet comprehensive document that clearly summarizes the project. Such proposal is considered successful when it is clear, free of typographical errors, accurate and easy to read.
It is important to understand the steps in developing a research protocol in order to perform an appropriate study and obtain reliable results. Extra time spent to write a good protocol will save failures at a later stage besides helping analysis. If the protocol is poorly prepared and not adhered to, it is unlikely that the project will yield the information that you hope for and in all probability the chances of selling your idea to the reviewers of a granting agency would be less.
Financial or Other Competing Interests
- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español
You are here
Nih clinical research trials and you.
The NIH Clinical Trials and You website is a resource for people who want to learn more about clinical trials. By expanding the below questions, you can read answers to common questions about taking part in a clinical trial.
What are clinical trials and why do people participate?
Clinical research is medical research that involves people like you. When you volunteer to take part in clinical research, you help doctors and researchers learn more about disease and improve health care for people in the future. Clinical research includes all research that involves people. Types of clinical research include:

- Epidemiology, which improves the understanding of a disease by studying patterns, causes, and effects of health and disease in specific groups.
- Behavioral, which improves the understanding of human behavior and how it relates to health and disease.
- Health services, which looks at how people access health care providers and health care services, how much care costs, and what happens to patients as a result of this care.
- Clinical trials, which evaluate the effects of an intervention on health outcomes.
What are clinical trials and why would I want to take part?
Clinical trials are part of clinical research and at the heart of all medical advances. Clinical trials look at new ways to prevent, detect, or treat disease. Clinical trials can study:
- New drugs or new combinations of drugs
- New ways of doing surgery
- New medical devices
- New ways to use existing treatments
- New ways to change behaviors to improve health
- New ways to improve the quality of life for people with acute or chronic illnesses.
The goal of clinical trials is to determine if these treatment, prevention, and behavior approaches are safe and effective. People take part in clinical trials for many reasons. Healthy volunteers say they take part to help others and to contribute to moving science forward. People with an illness or disease also take part to help others, but also to possibly receive the newest treatment and to have added (or extra) care and attention from the clinical trial staff. Clinical trials offer hope for many people and a chance to help researchers find better treatments for others in the future

Why is diversity and inclusion important in clinical trials?
People may experience the same disease differently. It’s essential that clinical trials include people with a variety of lived experiences and living conditions, as well as characteristics like race and ethnicity, age, sex, and sexual orientation, so that all communities benefit from scientific advances.
See Diversity & Inclusion in Clinical Trials for more information.
How does the research process work?
The idea for a clinical trial often starts in the lab. After researchers test new treatments or procedures in the lab and in animals, the most promising treatments are moved into clinical trials. As new treatments move through a series of steps called phases, more information is gained about the treatment, its risks, and its effectiveness.
What are clinical trial protocols?
Clinical trials follow a plan known as a protocol. The protocol is carefully designed to balance the potential benefits and risks to participants, and answer specific research questions. A protocol describes the following:
- The goal of the study
- Who is eligible to take part in the trial
- Protections against risks to participants
- Details about tests, procedures, and treatments
- How long the trial is expected to last
- What information will be gathered
A clinical trial is led by a principal investigator (PI). Members of the research team regularly monitor the participants’ health to determine the study’s safety and effectiveness.
What is an Institutional Review Board?
Most, but not all, clinical trials in the United States are approved and monitored by an Institutional Review Board (IRB) to ensure that the risks are reduced and are outweighed by potential benefits. IRBs are committees that are responsible for reviewing research in order to protect the rights and safety of people who take part in research, both before the research starts and as it proceeds. You should ask the sponsor or research coordinator whether the research you are thinking about joining was reviewed by an IRB.
What is a clinical trial sponsor?
Clinical trial sponsors may be people, institutions, companies, government agencies, or other organizations that are responsible for initiating, managing or financing the clinical trial, but do not conduct the research.
What is informed consent?
Informed consent is the process of providing you with key information about a research study before you decide whether to accept the offer to take part. The process of informed consent continues throughout the study. To help you decide whether to take part, members of the research team explain the details of the study. If you do not understand English, a translator or interpreter may be provided. The research team provides an informed consent document that includes details about the study, such as its purpose, how long it’s expected to last, tests or procedures that will be done as part of the research, and who to contact for further information. The informed consent document also explains risks and potential benefits. You can then decide whether to sign the document. Taking part in a clinical trial is voluntary and you can leave the study at any time.
What are the types of clinical trials?
There are different types of clinical trials.
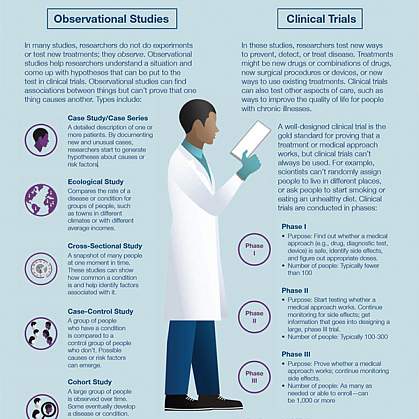
- Prevention trials look for better ways to prevent a disease in people who have never had the disease or to prevent the disease from returning. Approaches may include medicines, vaccines, or lifestyle changes.
- Screening trials test new ways for detecting diseases or health conditions.
- Diagnostic trials study or compare tests or procedures for diagnosing a particular disease or condition.
- Treatment trials test new treatments, new combinations of drugs, or new approaches to surgery or radiation therapy.
- Behavioral trials evaluate or compare ways to promote behavioral changes designed to improve health.
- Quality of life trials (or supportive care trials) explore and measure ways to improve the comfort and quality of life of people with conditions or illnesses.
What are the phases of clinical trials?
Clinical trials are conducted in a series of steps called “phases.” Each phase has a different purpose and helps researchers answer different questions.
- Phase I trials : Researchers test a drug or treatment in a small group of people (20–80) for the first time. The purpose is to study the drug or treatment to learn about safety and identify side effects.
- Phase II trials : The new drug or treatment is given to a larger group of people (100–300) to determine its effectiveness and to further study its safety.
- Phase III trials : The new drug or treatment is given to large groups of people (1,000–3,000) to confirm its effectiveness, monitor side effects, compare it with standard or similar treatments, and collect information that will allow the new drug or treatment to be used safely.
- Phase IV trials : After a drug is approved by the FDA and made available to the public, researchers track its safety in the general population, seeking more information about a drug or treatment’s benefits, and optimal use.
What do the terms placebo, randomization, and blinded mean in clinical trials?
In clinical trials that compare a new product or therapy with another that already exists, researchers try to determine if the new one is as good, or better than, the existing one. In some studies, you may be assigned to receive a placebo (an inactive product that resembles the test product, but without its treatment value).
Comparing a new product with a placebo can be the fastest and most reliable way to show the new product’s effectiveness. However, placebos are not used if you would be put at risk — particularly in the study of treatments for serious illnesses — by not having effective therapy. You will be told if placebos are used in the study before entering a trial.
Randomization is the process by which treatments are assigned to participants by chance rather than by choice. This is done to avoid any bias in assigning volunteers to get one treatment or another. The effects of each treatment are compared at specific points during a trial. If one treatment is found superior, the trial is stopped so that the most volunteers receive the more beneficial treatment. This video helps explain randomization for all clinical trials .
" Blinded " (or " masked ") studies are designed to prevent members of the research team and study participants from influencing the results. Blinding allows the collection of scientifically accurate data. In single-blind (" single-masked ") studies, you are not told what is being given, but the research team knows. In a double-blind study, neither you nor the research team are told what you are given; only the pharmacist knows. Members of the research team are not told which participants are receiving which treatment, in order to reduce bias. If medically necessary, however, it is always possible to find out which treatment you are receiving.
Who takes part in clinical trials?
Many different types of people take part in clinical trials. Some are healthy, while others may have illnesses. Research procedures with healthy volunteers are designed to develop new knowledge, not to provide direct benefit to those taking part. Healthy volunteers have always played an important role in research.
Healthy volunteers are needed for several reasons. When developing a new technique, such as a blood test or imaging device, healthy volunteers help define the limits of "normal." These volunteers are the baseline against which patient groups are compared and are often matched to patients on factors such as age, gender, or family relationship. They receive the same tests, procedures, or drugs the patient group receives. Researchers learn about the disease process by comparing the patient group to the healthy volunteers.
Factors like how much of your time is needed, discomfort you may feel, or risk involved depends on the trial. While some require minimal amounts of time and effort, other studies may require a major commitment of your time and effort, and may involve some discomfort. The research procedure(s) may also carry some risk. The informed consent process for healthy volunteers includes a detailed discussion of the study's procedures and tests and their risks.
A patient volunteer has a known health problem and takes part in research to better understand, diagnose, or treat that disease or condition. Research with a patient volunteer helps develop new knowledge. Depending on the stage of knowledge about the disease or condition, these procedures may or may not benefit the study participants.
Patients may volunteer for studies similar to those in which healthy volunteers take part. These studies involve drugs, devices, or treatments designed to prevent,or treat disease. Although these studies may provide direct benefit to patient volunteers, the main aim is to prove, by scientific means, the effects and limitations of the experimental treatment. Therefore, some patient groups may serve as a baseline for comparison by not taking the test drug, or by receiving test doses of the drug large enough only to show that it is present, but not at a level that can treat the condition.
Researchers follow clinical trials guidelines when deciding who can participate, in a study. These guidelines are called Inclusion/Exclusion Criteria . Factors that allow you to take part in a clinical trial are called "inclusion criteria." Those that exclude or prevent participation are "exclusion criteria." These criteria are based on factors such as age, gender, the type and stage of a disease, treatment history, and other medical conditions. Before joining a clinical trial, you must provide information that allows the research team to determine whether or not you can take part in the study safely. Some research studies seek participants with illnesses or conditions to be studied in the clinical trial, while others need healthy volunteers. Inclusion and exclusion criteria are not used to reject people personally. Instead, the criteria are used to identify appropriate participants and keep them safe, and to help ensure that researchers can find new information they need.
What do I need to know if I am thinking about taking part in a clinical trial?

Risks and potential benefits
Clinical trials may involve risk, as can routine medical care and the activities of daily living. When weighing the risks of research, you can think about these important factors:
- The possible harms that could result from taking part in the study
- The level of harm
- The chance of any harm occurring
Most clinical trials pose the risk of minor discomfort, which lasts only a short time. However, some study participants experience complications that require medical attention. In rare cases, participants have been seriously injured or have died of complications resulting from their participation in trials of experimental treatments. The specific risks associated with a research protocol are described in detail in the informed consent document, which participants are asked to consider and sign before participating in research. Also, a member of the research team will explain the study and answer any questions about the study. Before deciding to participate, carefully consider risks and possible benefits.
Potential benefits
Well-designed and well-executed clinical trials provide the best approach for you to:
- Help others by contributing to knowledge about new treatments or procedures.
- Gain access to new research treatments before they are widely available.
- Receive regular and careful medical attention from a research team that includes doctors and other health professionals.
Risks to taking part in clinical trials include the following:
- There may be unpleasant, serious, or even life-threatening effects of experimental treatment.
- The study may require more time and attention than standard treatment would, including visits to the study site, more blood tests, more procedures, hospital stays, or complex dosage schedules.
What questions should I ask if offered a clinical trial?
If you are thinking about taking part in a clinical trial, you should feel free to ask any questions or bring up any issues concerning the trial at any time. The following suggestions may give you some ideas as you think about your own questions.
- What is the purpose of the study?
- Why do researchers think the approach may be effective?
- Who will fund the study?
- Who has reviewed and approved the study?
- How are study results and safety of participants being monitored?
- How long will the study last?
- What will my responsibilities be if I take part?
- Who will tell me about the results of the study and how will I be informed?
Risks and possible benefits
- What are my possible short-term benefits?
- What are my possible long-term benefits?
- What are my short-term risks, and side effects?
- What are my long-term risks?
- What other options are available?
- How do the risks and possible benefits of this trial compare with those options?
Participation and care
- What kinds of therapies, procedures and/or tests will I have during the trial?
- Will they hurt, and if so, for how long?
- How do the tests in the study compare with those I would have outside of the trial?
- Will I be able to take my regular medications while taking part in the clinical trial?
- Where will I have my medical care?
- Who will be in charge of my care?
Personal issues
- How could being in this study affect my daily life?
- Can I talk to other people in the study?
Cost issues
- Will I have to pay for any part of the trial such as tests or the study drug?
- If so, what will the charges likely be?
- What is my health insurance likely to cover?
- Who can help answer any questions from my insurance company or health plan?
- Will there be any travel or child care costs that I need to consider while I am in the trial?
Tips for asking your doctor about trials
- Consider taking a family member or friend along for support and for help in asking questions or recording answers.
- Plan what to ask — but don't hesitate to ask any new questions.
- Write down questions in advance to remember them all.
- Write down the answers so that they’re available when needed.
- Ask about bringing a tape recorder to make a taped record of what's said (even if you write down answers).
This information courtesy of Cancer.gov.
How is my safety protected?

Ethical guidelines
The goal of clinical research is to develop knowledge that improves human health or increases understanding of human biology. People who take part in clinical research make it possible for this to occur. The path to finding out if a new drug is safe or effective is to test it on patients in clinical trials. The purpose of ethical guidelines is both to protect patients and healthy volunteers, and to preserve the integrity of the science.
Informed consent
Informed consent is the process of learning the key facts about a clinical trial before deciding whether to participate. The process of providing information to participants continues throughout the study. To help you decide whether to take part, members of the research team explain the study. The research team provides an informed consent document, which includes such details about the study as its purpose, duration, required procedures, and who to contact for various purposes. The informed consent document also explains risks and potential benefits.
If you decide to enroll in the trial, you will need to sign the informed consent document. You are free to withdraw from the study at any time.
Most, but not all, clinical trials in the United States are approved and monitored by an Institutional Review Board (IRB) to ensure that the risks are minimal when compared with potential benefits. An IRB is an independent committee that consists of physicians, statisticians, and members of the community who ensure that clinical trials are ethical and that the rights of participants are protected. You should ask the sponsor or research coordinator whether the research you are considering participating in was reviewed by an IRB.
Further reading
For more information about research protections, see:
- Office of Human Research Protection
- Children's Assent to Clinical Trial Participation
For more information on participants’ privacy and confidentiality, see:
- HIPAA Privacy Rule
- The Food and Drug Administration, FDA’s Drug Review Process: Ensuring Drugs Are Safe and Effective
For more information about research protections, see: About Research Participation
What happens after a clinical trial is completed?
After a clinical trial is completed, the researchers carefully examine information collected during the study before making decisions about the meaning of the findings and about the need for further testing. After a phase I or II trial, the researchers decide whether to move on to the next phase or to stop testing the treatment or procedure because it was unsafe or not effective. When a phase III trial is completed, the researchers examine the information and decide whether the results have medical importance.
Results from clinical trials are often published in peer-reviewed scientific journals. Peer review is a process by which experts review the report before it is published to ensure that the analysis and conclusions are sound. If the results are particularly important, they may be featured in the news, and discussed at scientific meetings and by patient advocacy groups before or after they are published in a scientific journal. Once a new approach has been proven safe and effective in a clinical trial, it may become a new standard of medical practice.
Ask the research team members if the study results have been or will be published. Published study results are also available by searching for the study's official name or Protocol ID number in the National Library of Medicine's PubMed® database .
How does clinical research make a difference to me and my family?

Only through clinical research can we gain insights and answers about the safety and effectiveness of treatments and procedures. Groundbreaking scientific advances in the present and the past were possible only because of participation of volunteers, both healthy and those with an illness, in clinical research. Clinical research requires complex and rigorous testing in collaboration with communities that are affected by the disease. As research opens new doors to finding ways to diagnose, prevent, treat, or cure disease and disability, clinical trial participation is essential to help us find the answers.
This page last reviewed on October 3, 2022
Connect with Us
- More Social Media from NIH
This site uses session cookies and persistent cookies to improve the content and structure of the site.
By clicking “ Accept All Cookies ”, you agree to the storing of cookies on this device to enhance site navigation and content, analyse site usage, and assist in our marketing efforts.
By clicking ' See cookie policy ' you can review and change your cookie preferences and enable the ones you agree to.
By dismissing this banner , you are rejecting all cookies and therefore we will not store any cookies on this device.
The research protocol is an essential part of a research project.
It is a full description of the research study and will act as a ‘manual’ for members of the research team to ensure everyone adheres to the methods outlined. As the study gets underway, it can then be used to monitor the study’s progress and evaluate its outcomes.
No two research protocols will be the same, but there are common elements and items that need to be addressed. Use the templates below to see examples of the kinds of headings your protocol needs to contain.
The protocol should describe as much detail about the research project as possible, to enable the review bodies to fully understand your study. It should be kept up to date as the research evolves, and include a version number and approval history.
Publishing your protocol
In the interests of transparency we would encourage you to make your protocol publicly available. This will:
- create an early scientific record of your methodology;
- help with review and publication of your study results (some journals require protocol publication); and
- potentially reduce duplication of research effort.
Many publishers now offer a protocol publication service.
Protocol templates
To help you we have provided templates that can support you with developing your research protocol. You may of course use your own template but we recommend that you check it against our examples to ensure that they capture the relevant elements.
Related links
- Outcome measures
- Costing templates
- Qualitative protocol guidance and template: consultation
- Privacy notice
- Terms & conditions
- Accessibility statement
- Feedback or concerns

- Planning a Research Project
- Getting Started
- Study Designs
- Data Management
- Budgets & Funding This link opens in a new window
- Writing Proposals This link opens in a new window
- Ethics Approval

Clinical Resource Guides
Databases and Apps A-Z
Education and Training
Equipment Booking
Keeping up to date
Research Repository
Research Toolkit
What does the library have?
What the library can do for you
- Systematic Review Guide
- Quality Improvement Projects
- Research and Grant Proposals
- Literature Searching Guide
- Grey Literature Guide
- Data Collection & Storage
- Statistics for Research
- Writing, Referencing & Publishing
- Communicating Research Findings
- Research Promotion & Impact
- Templates & examples
- Registration & publication
- Monash Health requirements
What is a study protocol?
A protocol is a detailed plan for a research study, outlining the specifics of how the research will be conducted. It is an essential document that helps to ensure research is conducted in a safe and ethical manner, and that the integrity of the research is preserved throughout the life of the study.
Common elements of a protocol
Melbourne Children's Trials Centre. (2017). Developing, amending and complying with research protocols. https://www.mcri.edu.au/images/research/training-resources/research-process-resources/guideline_developing_research_protocols.pdf
See the Data Management page in this guide for information on creating a data management plan (DMP), plus online tools and templates.
Literature review
Protocols draw on relevant literature at multiple points, e.g. in background information, when providing a rationale for the study, and when discussing known risks and benefits. If you haven't yet completed a literature search, now is the time to do one!
The Library offers training and guidance on conducting literature reviews, including a webinar on Advanced Literature Searching and our Literature Searching Guide .
Library research support
The Library offers various forms of research support depending on the purpose of your research. Our research support includes:
- Research consultations
- Literature search service
- Literature review report service
- Feedback on your literature search strategy
- Co-authorship for systematic reviews and other evidence synthesis projects
Complete this online form to request research support and learn more about each service offered.
Request research support
For an overview of Library research support, click the link below.
Library Research Support Services
Additional resources
- Protocol writing in clinical research (2016) by A. Al-Jundi & S. Sakka
- Write an error-free research protocol as recommended by WHO: 21 elements you shouldn't miss! (2022) by U. Bhosale
- How to Write a Research Protocol: Tips and Tricks (2018) by M. Cameli
- Guidance: Developing a protocol for a clinical research project (n.d.) by the Clinical Research Development Office, RCH
Protocol templates
The Murdoch Children's Research Institute (MCRI) provides annotated protocol templates for the following study designs:
- Clinical trial - drug/device intervention
- Clinical trial - intervention is not drug/device
- Observational studies
- Clinical audits / Quality assurance
To access the templates, click the blue button below and scroll down to the section on Clinical Research Development Office (CRDO) resources.
MCRI protocol templates
Qualitative studies
The NHS Health Research Authority offers a protocol template for qualitative research at the bottom of the page linked below.
NHS Qualitative protocol template
Example protocols
- RCT - A clinical investigation evaluating efficacy of a full-thickness placental allograft (Revita) in lumbar microdiscectomy outcomes
- Observational study - Prospective observational long-term safety registry of Multiple Sclerosis patients who have participated in cladribine clinical trials (PREMIERE)
- Systematic review and meta-analysis - Protocol for a systematic review and meta-analysis on the associations between shift work and sickness absence
Tip: The results of your literature search may include published protocols relevant to your research question.
Journal requirements
Journals that publish study protocols provide guidelines for the content required in protocol manuscripts. It can be helpful to review these guidelines even if you do not intend to publish your protocol in a peer-reviewed journal.
Search for the manuscript guidelines for your preferred journal(s) or review the examples below.
- BMC Nursing
Registering or publishing your protocol
Researchers are encouraged to submit their protocol for publication in a scholarly journal, and/or register their protocol in a relevant database. Many funding agencies also grant recipients to register or publish their study protocol.
Where to register your protocol
Your study design generally determines where you register your protocol. See the table below for options on where to register your protocol.
Where to publish your protocol
There are two main options:
- E.g. Journal of Clinical Epidemiology , Journal of Allied Health , Journal of Clinical Nursing
- E.g. BMC Trials , JMIR Research Protocols , Contemporary Clinical Trials
Be aware of predatory publishers - Check our our Writing, Referencing & Publishing Guide or our Predatory Publishing A-Z for more information.

Relevant Prompt documents include:
- Publication and dissemination of research findings
- Intellectual Property
- Research Ethics and Governance – Protocol and Investigational Brochure Content, Design, Amendments and Compliance
- Privacy and Confidentiality in Research
- Research Ethics and Governance – Authorship for Research
Visit the Forms Library from Research Support Services for links and more information.
Research - Forms Library
Protocol amendments
Once your protocol is finalised and ethics approval has been granted, you must notify the Monash Health Research Support Services team regarding protocol amendments .
Monash Health acknowledges the Traditional Custodians of the land, the Wurundjeri and Boonwurrung peoples, and we pay our respects to them, their culture and their Elders past, present and future.
We are committed to creating a safe and welcoming environment that embraces all backgrounds, cultures, sexualities, genders and abilities.
- << Previous: Study Designs
- Next: Data Management >>
- Last Updated: May 15, 2024 10:55 AM
- URL: https://monashhealth.libguides.com/planning_research
The Research Protocol
- First Online: 17 December 2015
Cite this chapter

- Gilberto Lopes M.D., M.B.A. 2 ,
- Gustavo Werutsky 3 &
- Patricia Moretto M.D., M.Sc. 4
825 Accesses
2 Altmetric
The research protocol is defined as the most important document in clinical research which helps the researchers and the scientists to understand the necessity of the study and the way of execution and completion.
The protocol outlines the rationale for the study, its objective, the methodology used, and how the data will be managed and analyzed. It highlights how ethical issues have been considered, and where appropriate, how gender and minority issues are being addressed.
An additional step, after writing the protocol, particularly in large studies with teams of investigators, is to develop what may be called the operations manual for the study.
The full research protocol development takes usually 4 months, but is variable depending on the complexity of the research.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Available as EPUB and PDF
- Read on any device
- Instant download
- Own it forever
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Conselho Nacional de Saúde (Brasil). Resolução n o 466, de 12 de dezembro de 2012. Brasília, 2012 [citado 2014 Mar 11]. http://www.conselho.saude.gov.br/web_comissoes/conep/index.html . Assessed November 2014.
IARC cancer statistics. http://globocan.iarc.fr/ . Assessed November 2014. OR http://www.iarc.fr/en/media-centre/pr/2014/pdfs/pr224_E.pdf . Assessed Nov 2014.
WHO. Cancer fact sheet. Geneva: WHO; 2014. Accessed Nov 2014.
Google Scholar
IAEA. A silent crisis: cancer treatment in developing countries. Vienna: Division of Public Information, IAEA; 2003.
Emanuel EJ, Wendler D, Killen J, Grady C. What makes clinical research in developing countries ethical? The benchmarks of ethical research. J Infect Dis. 2004;189(5):930–7. Epub 2004 Feb 17.
Download references
Author information
Authors and affiliations.
Oncoclinicas do Brasil, Rua Maranhão 569/4, São Paulo, SP, 01240-001, Brazil
Gilberto Lopes M.D., M.B.A.
Latin American Cooperative Oncology Group, 6681, 99A/806 Ipiranga Avenue, Porto Alegre, RS, 90619-900, Brazil
Gustavo Werutsky
Oncology, Caxias do Sul University Foundation, Caxias do Sul General Hospital, Rua Prof. Antônio Vignoli, 255 - Bairro Petrópolis, Caxias do Sul, RS, 95070-561, Brazil
Patricia Moretto M.D., M.Sc.
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Gilberto Lopes M.D., M.B.A. .
Editor information
Editors and affiliations.
South African Medical Research Council, Francie van Zyl, Parrow, Cape Town, South Africa
Daniela Cristina Stefan
Barriers to Research in Developing Countries and Possible Steps to Overcome Them
The rise in the number of cancer cases, 14 million new cancer cases globally every year, with the majority of cases occurring in developing countries [ 1 ], has a great impact in those countries and must be addressed. Additionally, treatment options are both limited and expensive, and trial participation is an opportunity for better cancer care.
There are four key components to cancer control and, therefore, areas in need of research: prevention, early detection, diagnosis and treatment, and palliation, as stated by WHO [ 2 ]. In each of these areas, developing countries face major challenges, not only in dealing with the ongoing problems, but also with research opportunities.
The International Agency for Research on Cancer (IARC) uses the term “developing country” for less-developed regions such as: all regions of Africa, Asia (excluding Japan), Latin America and the Caribbean, Melanesia, Micronesia, and Polynesia [ 1 ].
In order to overcome barriers to doing research, and so that the results will have a chance to impact on local problems, some points have to be taken into consideration:
Choose a Relevant Topic to Your Community
Make sure that the many pressing problems in developing countries are being addressed, so local patients are able to see direct benefits. The research question has to be relevant for the population where it is tested. This is an ethics question, as well as a question that will improve accrual.
There is a broad consensus that efforts and scarce resources in developing countries should focus first and foremost on prevention and awareness rising, and secondly on early detection, treatment, and research. However, this should not prevent the progress and improvement of research capabilities in these countries.
Will the Protocol Be Feasible?
If the issue being looked at is technology, it should be noted that most health equipment used in developing nations is manufactured in the first world. Sometimes the equipment does not work on arrival, with lack of parts or trained technicians to fix it or do maintenance. Technical, social, cultural, and economic factors must be considered when looking at new technologies for these countries.
Researchers should also remember that in some countries there is limited access to radiotherapy, so research questions involving concurrent or sequential treatments may not be the best research option.
According to the International Atomic Energy Agency (IAEA) [ 3 ], although the developing world constitutes 85 % of the world’s population, it only has 2200 radiation therapy machines compared with the 4500 in the developed world. Those developing countries that have access to radiotherapy face considerable financial investment constrains and for up to 5 years are required to provide the necessary training, equipment set-up and maintenance, protocols, and quality control. Therefore, even if budget is not a concern for a specific study, creating a Radiotherapy Service for research purposes in a developing country would take a lot of planning, resources, and time.
In order to do the scans as planned in a protocol, radiologists with RECIST knowledge and time to do proper comparative reports are also needed and are not always available. Even with a central review, the delay in getting the images as planned could result in wrong clinical decisions and protocol violations.
The same applies to health workers, where it is sometimes necessary to train locals and adapt to the capabilities of the country. Obviously, if feasible, the improvement in capabilities would be welcome, as in addition to the research they could offer new treatments to patients and open the way for further research.
Funding and Economic Incentives
The challenges are many and substantial such as insufficient political priority and funding among donor agencies and governments of developing countries that have many competing priorities.
However, the local investigator must be familiarized with potential local and international sources: universities, government incentives, international for-profit initiatives, and creative not-for-profit partnerships.
Involvement in research with pharmaceutical companies, which can be lucrative to the institutions where it is done, is an obvious way to start, but usually some capabilities must already be in place to attract this kind of research. A pressing challenge is getting industry partners to invest in the clinical development of drugs that offer limited commercial opportunities. Despite the fact that the cost to approve drugs is lower in developing countries, it is still high and is a problem that universities and public institutions cannot overcome alone.
Intellectual Property
Partnership with industry may be necessary to develop early research in universities, and intellectual property licensing should be viewed as a requirement to attract industry partners. However, researchers must pay attention to patent systems before concluding agreements with pharmaceutical companies (who want a patent system that will protect their investments for as long as possible) and be careful, clearly stating on contracts who has ownership of the data collected, of publications or abstracts arising from study results, and of information obtained based on new data analyses.
Encouragement and Structure
In some developing countries, in addition to other research barriers, such as lack of diagnostic, treatment, and monitoring capacity, there is lack of academic staff (oncology and hematology expertise, specialized cancer nurses, pharmacists), qualified monitors, lack of encouragement to conduct and publish research, as well as lack of a research publishing infrastructure. Training personnel abroad, in other research centers, or in-house training before starting a project may be needed.
Planning and Accrual
Researchers may have problems with proper calculation for accrual, as most cancer registries in low- and middle-income countries have shortcomings and screening programs are largely absent. This can lead to erroneous assumptions regarding the relative frequencies of the varying levels of different cancers based on global trends, which can be misleading. These issues are major challenges for the future, but setting up an institutional cancer registry could be a good start in research planning.
In order to properly choose the study population, the researcher should take into consideration the contrasts in patient’s disease profiles: most cancer patients in developing countries have advanced or incurable cancers at the time their initial presentation, a different profile from that seen in developed countries. The prevalence and incidence can be also markedly different in comparison to developed countries for each disease site.
Developed countries often have relatively high rates of lung, colorectal, breast, and prostate cancer because of the earlier onset of the tobacco epidemic, the earlier exposure to occupational carcinogens, and the Western diet and lifestyle. In contrast, up to a fourth of cancers in developing countries are associated with chronic infections. Liver cancer is often causally associated with infection by the hepatitis B virus (HBV), cervical cancer is associated with infection by certain types of human papillomavirus (HPV), and stomach cancer is associated with Helicobacter pylori infection.
Weak referral systems can also make the accrual slow. This should be anticipated and should encourage creative solutions to strengthen it by the researchers. In some countries there are few cancer services. This can be helpful in the sense that it aggregates most of the cases in a few centers. However, if the health system is a mix of public and private, this dilutes the number of cases, increasing the numbers of institutions necessary for the accrual, and increasing the budget.
Issues During the Trial and for Follow-Up
There is sometimes a lack of awareness of the signs and symptoms of cancer and side effects from treatments, a lack of money to travel to a hospital, which could result in sub-notification of important events or delays in the notification and treatment of important side effects.
As health systems in developing countries are not set up for chronic disease management, chronic diseases such as cancer are often lost to follow-up. Therefore, organizing proper follow-up for patients enrolled in the trials could be a problem.
Need for Palliative Care Research
In some developing countries, there is lack of trained personnel to administer palliative care or pain killers such as morphine. This is particularly startling given that approximately 70 % of the patients seen at these hospitals are at such an advanced stage of cancer upon arrival that they are beyond cure and palliative care and pain management is the only benefit they can still receive. Studies in the palliative setting, which should be relatively easy to accrue, could have a major impact in the quality of life of patients and in the training of local health workers.
Ethics Barriers
The ethics principles in clinical research were established in order to avoid previous abuses/misconducts, such as the Tuskegee Study of Untreated Syphilis (TSUS).
Based on the influential codes of ethics and regulations that guide ethical clinical research (Nuremberg Code Footnote 1 ; Declaration of Helsinki 1 , Belmont Report 1 , CIOMS 1 , U.S. Common Rule 1 ), seven main principles have been described as guiding the conduct of ethical research 1 : social and clinical value; scientific validity; fair subject selection; favorable risk-benefit ratio; independent review; informed consent; and respect for potential and enrolled subjects.
The trial should follow the ethics rules from the country where it was originally written and in the other countries where the research will be developed. Some areas of ethics controversy in developing countries are: the standard of care to be used (as the local standard of care could mean no treatment or less than the “worldwide best”) and the quality of informed consent (due to lack of knowledge, language/cultural barriers, high workload, and time constraints). The discussion about valid science, social benefits, and favorable risk:benefit ratio to justify less than the worldwide best care has to take place early on in the protocol design to avoid an unethical and/or unfeasible trial. Efforts should be taken to improve the quality of informed consent, such as researchers training and observance to the GCP.
Not all the population in developing countries is vulnerable, but a great deal of them is at increased risk of exploitation due to poverty, limited healthcare services, illiteracy, cultural and linguistic differences, and limited understanding of the nature of scientific research. To make things worse, regulatory infrastructures, which could minimize this risk, may lack effectiveness or be nonexistent. As suggested by Emanuael et al. [ 4 ], “collaborative partnerships between researchers and sponsors in developed countries and researchers, policy makers, and communities in developing countries help to minimize the possibility of exploitation by ensuring that a developing country determines for itself whether the research is acceptable and responsive to the community’s health problems.”
Lastly, the many differences between developed and developing countries, in addition to the various sociocultural groups existing in a country, must be taken into consideration during the research protocol conception and execution. Specifically, issues to keep private information confidential, to find the person responsible for giving consent, and how best to explain the clinical trial purpose, benefits, risks, and involved procedures could arise due to local peculiarities.
Rights and permissions
Reprints and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Lopes, G., Werutsky, G., Moretto, P. (2016). The Research Protocol. In: Stefan, D. (eds) Cancer Research and Clinical Trials in Developing Countries. Springer, Cham. https://doi.org/10.1007/978-3-319-18443-2_5
Download citation
DOI : https://doi.org/10.1007/978-3-319-18443-2_5
Published : 17 December 2015
Publisher Name : Springer, Cham
Print ISBN : 978-3-319-18442-5
Online ISBN : 978-3-319-18443-2
eBook Packages : Medicine Medicine (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
What is a clinical trial protocol?
Affiliation.
- 1 Department of Medicine and Public Health, Section of Psychiatry and Clinical Psychology, University of Verona, Italy. [email protected]
- PMID: 20815294
Trial protocols are documents that describe the objectives, design, methodology, statistical considerations and aspects related to the organization of clinical trials. Trial protocols provide the background and rationale for conducting a study, highlighting specific research questions that are addressed, and taking into consideration ethical issues. Trial protocols must meet a standard that adheres to the principles of Good Clinical Practice, and are used to obtain ethics approval by local Ethics Committees or Institutional Review Boards.
- Clinical Protocols*
- Clinical Trials as Topic / standards*

Clinical study protocol
A clinical study or research protocol refers to the document that describes what the study will do, how it will be done, and why it is being done. It explains how many people will be in the study, who is eligible to take part in it, what study therapy or other interventions will be given, what tests will be done and how often, and what information will be collected. A protocol guides the study and associated data collection and analysis in a productive and standardized manner and is carefully designed to safeguard the participants’ health and answer specific research questions.
Sourced From NCImetathesaurus U.S. Food and Drug Administration (FDA) Patient-Focused Drug Development Glossary Learn More NIH Clinical Research Trials and You The Basics: What are clinical trial protocols? UCSF Clinical Research Resource HUB: Clinical trial protocol development

- Policy & Compliance
- Clinical Trials
NIH's Definition of a Clinical Trial
This page provides information, tools, and resources about the definition of a clinical trial. Correctly identifying whether a study is considered by NIH to be a clinical trial is crucial to how you will:
- Select the right NIH funding opportunity for your research study
- Write the research strategy and human subjects sections of your grant application and contract proposal
- Comply with appropriate policies and regulations, including registration and reporting in ClinicalTrials.gov
Note: Misclassified clinical trial applications may be withdrawn.
In 2016, NIH launched a multi-faceted effort to enhance its stewardship over clinical trials. The goal of this effort is to encourage advances in the design, conduct, and oversight of clinical trials while elevating the entire biomedical research enterprise to a new level of transparency and accountability. The NIH definition of a clinical trial was revised in 2014 in anticipation of these stewardship reforms to ensure a clear and responsive definition of a clinical trial. Learn more about why NIH has made changes to improve clinical trial stewardship.
NIH Definition of a Clinical Trial
A research study in which one or more human subjects are prospectively assigned prospectively assigned The term "prospectively assigned" refers to a pre-defined process (e.g., randomization) specified in an approved protocol that stipulates the assignment of research subjects (individually or in clusters) to one or more arms (e.g., intervention, placebo, or other control) of a clinical trial. to one or more interventions interventions An "intervention" is defined as a manipulation of the subject or subject’s environment for the purpose of modifying one or more health-related biomedical or behavioral processes and/or endpoints. Examples include: drugs/small molecules/compounds; biologics; devices; procedures (e.g., surgical techniques); delivery systems (e.g., telemedicine, face-to-face interviews); strategies to change health-related behavior (e.g., diet, cognitive therapy, exercise, development of new habits); treatment strategies; prevention strategies; and, diagnostic strategies. (which may include placebo or other control) to evaluate the effects of those interventions on health-related biomedical or behavioral outcomes. health-related biomedical or behavioral outcomes. A "health-related biomedical or behavioral outcome" is defined as the pre-specified goal(s) or condition(s) that reflect the effect of one or more interventions on human subjects’ biomedical or behavioral status or quality of life. Examples include: positive or negative changes to physiological or biological parameters (e.g., improvement of lung capacity, gene expression); positive or negative changes to psychological or neurodevelopmental parameters (e.g., mood management intervention for smokers; reading comprehension and /or information retention); positive or negative changes to disease processes; positive or negative changes to health-related behaviors; and, positive or negative changes to quality of life.

DECISION TOOL
Your human subjects study may meet the NIH definition of a clinical trial.
FIND OUT HERE
Use the following four questions to determine the difference between a clinical study and a clinical trial:
- Does the study involve human participants?
- Are the participants prospectively assigned to an intervention?
- Is the study designed to evaluate the effect of the intervention on the participants?
- Is the effect being evaluated a health-related biomedical or behavioral outcome?
Note that if the answers to the 4 questions are yes, your study meets the NIH definition of a clinical trial, even if…
- You are studying healthy participants
- Your study does not have a comparison group (e.g., placebo or control)
- Your study is only designed to assess the pharmacokinetics, safety, and/or maximum tolerated dose of an investigational drug
- Your study is utilizing a behavioral intervention
- Only one aim or sub-aim of your study meets the clinical trial definition
Studies intended solely to refine measures are not considered clinical trials. Studies that involve secondary research with biological specimens or health information are not clinical trials.
Resources to Clarify the Definition
Case studies.
These simplified case studies illustrate the differences between clinical trials and clinical studies.
These FAQs further clarify the application of the clinical trial definition.
Decision Tree
Print this decision tree for an easy reference for the four questions that identify a clinical trial.
Related Guide Notice
NOT-OD-15-015 Notice of Revised NIH Definition of “Clinical Trial”
This page last updated on: August 8, 2017
- Bookmark & Share
- E-mail Updates
- Help Downloading Files
- Privacy Notice
- Accessibility
- National Institutes of Health (NIH), 9000 Rockville Pike, Bethesda, Maryland 20892
- NIH... Turning Discovery Into Health
A .gov website belongs to an official government organization in the United States.
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Infection Prevention and Long-term Care Facility Residents
- Nursing Home Infection Preventionist Training
- PPE in Nursing Homes
- Respiratory Virus Toolkit
- Healthcare-Associated Infections (HAIs)
- The resources on this page can keep you and your loved ones safe.
Long-term care facilities provide many services, both medical and personal care, to people who are unable to live without help.
If you live in a nursing home, assisted living facility or other long-term care facility, you have a higher risk of getting an infection. There are steps you can take to reduce your risk:
- Tell your healthcare provider if you think you have an infection or if your infection is getting worse.
- Take antibiotics exactly as prescribed and tell your healthcare provider if you have any side effects, such as diarrhea.
- Keep your hands clean. Remind staff and visitors to keep their hands clean.
- Get vaccinated against flu and other infections to avoid complications.
Resources for staying safe in long-term care facilities
About C. diff
About Carbapenem-resistant Enterobacterales
Catheter-associated Urinary Tract Infection Basics
Methicillin-resistant Staphylococcus aureus (MRSA) Basics

Healthy Habits: Antibiotic Do's and Don'ts
About Sepsis

About Norovirus
Resources for family members and caregivers
- Medicare resources for long-term care residents and caregivers.
- Find a nursing home in your area .
This website provides resources for patients, families and caregivers on the prevention of infections in nursing homes and assisted living facilities.
For Everyone
Health care providers.

IMAGES
VIDEO
COMMENTS
Open in a separate window. First section: Description of the core center, contacts of the investigator/s, quantification of the involved centers. A research protocol must start from the definition of the coordinator of the whole study: all the details of the main investigator must be reported in the first paragraph.
A study protocol is an important document that specifies the research plan for a clinical study. It should be written in detail and researchers should aim to publish their study protocols as it is encouraged by many funders. The spirit 2013 statement provides a useful checklist on what should be included in a research protocol . In this paper ...
The research protocol is a document that describes the background, rationale, objective (s), design, methodology, statistical considerations and organization of a clinical trial. It is a document that outlines the clinical research study plan. Furthermore, the research protocol should be designed to provide a satisfactory answer to the research ...
Those using the protocol include all study site staff (investigators, study site coordinators, research nurses, pharmacists and dispensing staff, laboratory staff, and staff from other departments if any special procedures are required, e.g. radiographs, endoscopies, etc.) as well as study monitors, data managers, statisticians, auditors ...
Writing the research protocol. 5.1 Introduction. After proper and complete planning of the study, the plan should be written down. The protocol is the detailed plan of the study. Every research study should have a protocol, and the protocol should be written. The written protocol: •.
In terms of the "research pipeline", the detailed protocol writing usually follows the definition of the overall research question and must be informed by a detailed literature review to ensure all research procedures and interventions are up to date and the study methodology is relevant to answer the research question.
The study protocol serves as a comprehensive guide and also represents the main document for external evaluation of the study (e.g., ethical committee, grant authorities). However, the purpose of the study protocol is to give a concise description of the study idea, plan, and further analysis. The writing style should be brief and concise.
Definition. The study protocol is "the most important document in clinical trials, since it ensures the quality and integrity of the clinical investigation in terms of its planning, execution, conduct, and the analysis of the data" (Chow & Chang, 2007 ). The study protocol is a comprehensive plan of action that contains information ...
A research protocol is the road map you will follow in writing a grant proposal and carrying out your research. This chapter provides a long list of elements that may be included, such as study design, safety considerations, quality assurance, and ethical outcomes. Also included in the chapter are sections on what makes a good research protocol ...
The study protocol is an integral part of your multicentre collaborative project. This is a document that defines and justifies the research question; outlines the aims, objectives and outcome ...
09/21/2022. Every clinical investigation begins with the development of a clinical protocol. The protocol is a document that describes how a clinical trial will be conducted (the objective (s), design, methodology, statistical considerations and organization of a clinical trial,) and ensures the safety of the trial subjects and integrity of the ...
Abstract. A research protocol is best viewed as a key to open the gates between the researcher and his/her research objectives. Each gate is defended by a gatekeeper whose role is to protect the resources and principles of a domain: the ethics committee protects participants and the underlying tenets of good practice, the postgraduate office protects institutional academic standards, the ...
Protocol writing allows the researcher to review and critically evaluate the published literature on the interested topic, plan and review the project steps and serves as a guide throughout the investigation. The proposal is an inevitable document that enables the researcher to monitor the progress of the project [ 5 ].
Clinical trials follow a plan known as a protocol. The protocol is carefully designed to balance the potential benefits and risks to participants, and answer specific research questions. A protocol describes the following: The goal of the study; Who is eligible to take part in the trial; Protections against risks to participants
A research protocol is a physical record that details a research study. This document helps researchers conduct their studies as smoothly as possible without interruptions or impediments. A well-written research protocol addresses the research question and describes specific elements of the study, such as its methodology, design and objectives.
The research protocol is an essential part of a research project. It is a full description of the research study and will act as a 'manual' for members of the research team to ensure everyone adheres to the methods outlined. As the study gets underway, it can then be used to monitor the study's progress and evaluate its outcomes.
A protocol is a detailed plan for a research study, outlining the specifics of how the research will be conducted. It is an essential document that helps to ensure research is conducted in a safe and ethical manner, and that the integrity of the research is preserved throughout the life of the study. A protocol -- or Project Description -- is a ...
The research protocol is defined as the most important document in clinical research which helps the researchers and the scientists to understand the necessity of the study and the way of execution and completion. The protocol outlines the rationale for the study, its objective, the methodology used, and how the data will be managed and analyzed.
The protocol must explicitly address the issues likely to be. raised by these gatekeepers, demonstrating evidence of a clear understanding of the issues involved and tha t all components. of the ...
Clinical Trials as Topic / standards*. Humans. Trial protocols are documents that describe the objectives, design, methodology, statistical considerations and aspects related to the organization of clinical trials. Trial protocols provide the background and rationale for conducting a study, highlighting specific research questions that are ...
Clinical study protocol. A clinical study or research protocol refers to the document that describes what the study will do, how it will be done, and why it is being done. It explains how many people will be in the study, who is eligible to take part in it, what study therapy or other interventions will be given, what tests will be done and how ...
Protocol (science) In natural and social science research, a protocol is most commonly a predefined procedural method in the design and implementation of an experiment. Protocols are written whenever it is desirable to standardize a laboratory method to ensure successful replication of results by others in the same laboratory or by other ...
NIH Definition of a Clinical Trial. A research study in which one or more human subjects are prospectively assigned prospectively assigned The term "prospectively assigned" refers to a pre-defined process (e.g., randomization) specified in an approved protocol that stipulates the assignment of research subjects (individually or in clusters) to one or more arms (e.g., intervention, placebo, or ...
There are steps you can take to reduce your risk: Tell your healthcare provider if you think you have an infection or if your infection is getting worse. Take antibiotics exactly as prescribed and tell your healthcare provider if you have any side effects, such as diarrhea. Keep your hands clean. Remind staff and visitors to keep their hands clean.