If you're seeing this message, it means we're having trouble loading external resources on our website.
If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
To log in and use all the features of Khan Academy, please enable JavaScript in your browser.

High school biology
Course: high school biology > unit 9.
- Biogeochemical cycles overview
- The water cycle
The carbon cycle
- The nitrogen cycle
- Biogeochemical cycles review
- Biogeochemical cycles
Want to join the conversation?
- Upvote Button navigates to signup page
- Downvote Button navigates to signup page
- Flag Button navigates to signup page

Video transcript

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

17.2A: Carbon Cycle
- Last updated
- Save as PDF
- Page ID 5820

- John W. Kimball
- Tufts University & Harvard
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
The concentration of carbon in living matter (18%) is almost 100 times greater than its concentration in the earth (0.19%). So living things extract carbon from their nonliving environment. For life to continue, this carbon must be recycled. Carbon exists in the nonliving environment as:
- carbon dioxide (CO 2 ) in the atmosphere and dissolved in water (forming HCO 3 − )
- carbonate rocks (limestone and coral = CaCO 3 )
- deposits of coal, petroleum, and natural gas derived from once-living things
- dead organic matter, e.g., humus in the soil
Carbon enters the biotic world through the action of primarily photoautotrophs , like plants and algae, that use the energy of light to convert carbon dioxide to organic matter and to a small extent, chemoautotrophs - bacteria and archaea that do the same but use the energy derived from an oxidation of molecules in their substrate. Carbon returns to the atmosphere and water by respiration (as CO 2 ), burning, and decay (producing CO 2 if oxygen is present, methane (CH 4 ) if it is not.
The uptake and return of CO 2 are not in balance
The carbon dioxide content of the atmosphere is gradually and steadily increasing. The graph shows the CO 2 concentration at the summit of Mauna Loa in Hawaii from 1958 through 1999. The values are in parts per million (ppm). The seasonal fluctuation is caused by the increased uptake of CO 2 by plants in the summer. (In March 2015, its average worldwide concentration reached 400 ppm.)
The increase in CO 2 probably began with the start of the industrial revolution. Samples of air trapped over the centuries in the glacial ice of Greenland show no change in CO 2 content until 300 years ago. Since measurements of atmospheric CO 2 began late in the nineteenth century, its concentration has risen over 20%. This increase is surely "anthropogenic"; that is, caused by human activities:
- burning fossil fuels (coal, oil, natural gas) which returns to the atmosphere carbon that has been locked within the earth for millions of years.
- clearing and burning of forests, especially in the tropics. In recent decades, large areas of the Amazon rain forest have been cleared for agriculture and cattle grazing.
Where is the missing carbon?
Curiously, the increase in atmospheric CO 2 is only about one-half of what would have been expected from the amount of fossil fuel consumption and forest burning. Where has the rest gone? Research has shown that increased CO 2 levels lead to increased net production by photoautotrophs. There is evidence that at least some of the missing CO 2 has been incorporated by: (1) increased growth of forests, especially in North America, (2) increased amounts of photoautotrophic plankton in the oceans, and (3) uptake by desert soils (mechanism as yet unknown)
The Greenhouse Effect and Global Warming
Despite these "sinks" for our greatly-increased CO 2 production, the concentration of atmospheric CO 2 continues to rise? Should we be worried? Carbon dioxide is transparent to light but rather opaque to heat rays. Therefore, CO 2 in the atmosphere retards the radiation of heat from the earth back into space - the "greenhouse effect". Has the increase in carbon dioxide led to global warming?
Some evidence:
- Careful monitoring shows that the global air temperature in 2014 was 0.57°C higher than the average from 1961–1990, and that 14 of the 15 warmest years since records began being kept late in the 19th Century have occurred in this century (including 2005 and 2010 as well as 2014).
- Many glaciers and ice sheets are receding.
- Woody shrubs are now growing in areas of northern Alaska that 50 years ago were barren tundra.
- Many angiosperms in temperate climates are flowering earlier in the spring than they used to.
- Many species of birds and butterflies are moving north and breeding earlier in the spring.
Will continued increase in carbon dioxide lead to more global warming and, if so, how much? At this point, the answer depends on what assumptions you plug into your computer models. But as the different models have been improved, they seem to be converging on a consensus: a doubling of the CO 2 concentration (expected by the end of this century) will cause the earth to warm somewhere in the range of 1.1–6.4°C.
Other Greenhouse Gases
Although their levels in the atmosphere are much lower than that of CO 2 ,
- methane (CH 4 )
- nitrous oxide (N 2 O)
- hydrofluorocarbons (HFCs)
are also potent greenhouse gases.
Although methane ("marsh gas") is released by natural processes (e.g. from decay occurring in swamps), human activities now account for some 60% of the total.
- mining, processing, and use of coal, oil, and natural gas
- release from landfills
- growing rice in paddies
- burning forests
- raising cattle (fermentation in their rumens produces methane that is expelled from their GI tract)
So burning of the tropical rain forest adds to the atmospheric methane budget in two ways:
- incomplete combustion during burning
- release from the GI tract of the cattle that are later placed on the cleared land.
The methane concentration in Arctic air is presently some 1.9 parts per million, the highest level seen such measurements began. Although this concentration is far less than that of CO 2 , methane is 28 times as potent a greenhouse gas. The marked warming of the earth that occurred at the end of the Paleocene epoch is thought to have been caused by the release of large amounts of methane from the sea floor.
Geosciences Department Penn State
- ORIENTATION
- INSTRUCTOR INFORMATION
- EARTH SCIENCES PROGRAM
- LIBRARY RESOURCES
- GETTING HELP
Carbon Cycle and Atmospheric CO2
The Global Carbon Cycle
The biogeochemical cycle in which carbon is exchanged between Earth’s terrestrial biosphere , hydrosphere , geosphere , and atmosphere is called the carbon cycle . The global carbon budget is the balance of the fluxes of carbon between these four reservoirs . The terms source or sink define whether the net carbon flux is out of or into the reservoir, respectively.
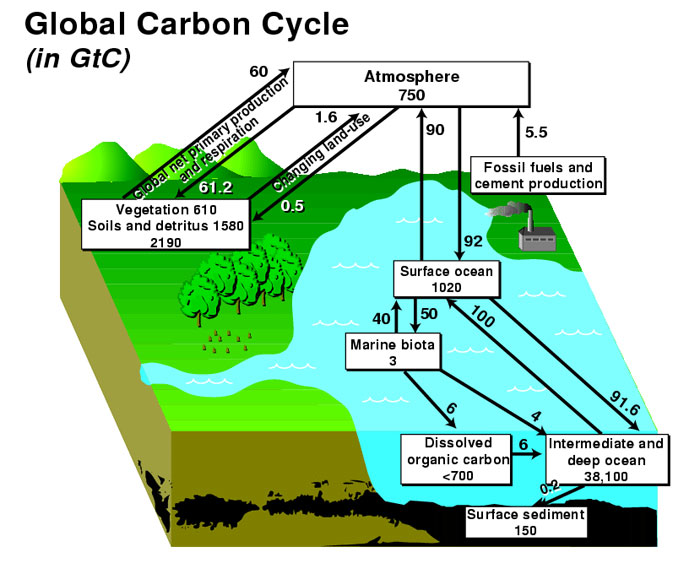
Atmospheric carbon dioxide
The carbon cycle is of interest to understanding climate because it includes two of the most important greenhouse gases: carbon dioxide (CO 2 ) and methane (CH 4 ). Most atmospheric carbon is in the form of CO 2 , while CH 4 is present only in trace concentrations. Because CO 2 is chemically inert, it is relatively well mixed within the atmosphere away from forest canopies, whereas CH 4 is chemically active and is removed quickly from the atmosphere through oxidation to CO 2 and water. The overall atmospheric concentration of these greenhouse gases has increased during the past century and contributed to global warming.
Carbon is removed from the atmosphere through:
- the photosynthetic conversion of carbon dioxide into carbohydrates by plants, releasing oxygen in the process;
- the formation of carbonic acid as circulating ocean surface waters cool near the poles, thereby absorbing more atmospheric CO 2 ;
- the conversion of reduced carbon to organic tissues or carbonates to hard body parts, such as shells, by marine biota in ocean surface waters; and
- the reaction between carbonic acid and silicate rock that leads to the production of bicarbonate ions, which are carried to the ocean and eventually deposited in marine carbonate sediments.
Carbon can be input to the atmosphere by a variety of processes, including:
- plant and animal respiration, an exothermic reaction that involves the breakdown of organic molecules into CO 2 and water;
- decay by fungi and bacteria that break down carbon compounds in dead animals and plants and convert the carbon to CO 2 if oxygen is present, or methane if not;
- organic matter combustion (including deforestation and burning fossil fuels), which oxidizes carbon-producing CO 2 ;
- cement production, when limestone (calcium carbonate) is heated to produce lime (calcium oxide), a component of cement, and CO 2 is released;
- release of dissolved CO 2 back to the atmosphere through warming of surface ocean water; and
- release of water vapor, carbon dioxide, and sulfur dioxide from volcanic eruptions and metamorphism.
Oceanic Carbon
Inorganic carbon is readily exchanged between the atmosphere and ocean, exerting an important control on the pH of ocean water. Carbon is released to the atmosphere at oceanic upwelling sites, whereas regions of downwelling transfer carbon from the atmosphere to the ocean. When carbon (CO 2 ) enters the ocean, carbonic acid is formed by the reaction: CO 2 + H 2 O = H 2 CO 3. Carbonic acid dissociates to form bicarbonate ions (HCO 3 - ), the form in which most of the carbon in the oceans exists; lesser amounts of carbon exist as carbonic acid (H 2 CO 3 or dissolved CO 2 ), and carbonate ions (CO 3 2- ) paired with calcium and magnesium and other cations. Marine organisms build their skeletons and shells out of the minerals calcite and aragonite (CaCO 3 ) through the incorporation of bicarbonate ions. These minerals dissolve after the death of the organism, but some of the material settles to the sea floor where it can be buried and stored in the form of limestone.
Carbon in the Biosphere
Carbon is an essential part of life on Earth. It plays an important role in the structure, biochemistry, and nutrition of all living cells. Autotrophs are organisms that produce their own organic compounds using carbon dioxide from the air or water they live in. This lifestyle requires an external source of energy, for example, the absorption of solar radiation in the process called photosynthesis, or the exploitation of chemical energy sources in a process called chemosynthesis. The most important autotrophs for the carbon cycle are trees in forests and phytoplankton in the ocean.
Large quantities of carbon pass between the atmosphere and biosphere on short time-scales: the removal of atmospheric carbon occurs during photosynthesis, following the reaction CO 2 + H 2 O = CH 2 O + O 2 , while most carbon leaves the biosphere through respiration, a reversal of the previous reaction in which an amount of energy equivalent to that absorbed during photosynthesis is released as heat. When oxygen is present, aerobic respiration occurs, which releases carbon dioxide into the surrounding air or water. Otherwise, anaerobic respiration occurs and releases methane into the surrounding environment, which eventually makes its way into the atmosphere or hydrosphere.
The biosphere is capable of storing ~10% of atmospheric carbon at any given time. However, carbon storage in the biosphere is influenced by a number of processes on different time-scales: while net primary productivity follows seasonal and annual cycles, carbon can be stored up to several hundreds of years in trees and up to thousands of years in soils. Changes in those long-term carbon pools may thus affect global climate change (view this example of changes to soil) .
Carbon in the Geosphere
Organic and inorganic carbon reservoirs in Earth's crust are large with long residence times. Carbon enters the geosphere through the biosphere when dead organic matter (such as peat or marine algae) becomes incorporated into fossil fuels like coal and organic-matter-rich oil and gas source rocks, and when shells of calcium carbonate become limestone through the process of sedimentation briefly described above. These carbon reservoirs can remain intact, that is, the carbon can remain stored within them, for many millions of years. Eventually, most rocks are uplifted and subjected to exposure to the atmosphere where they are weathered and eroded, or they are subducted, metamorphosed, and erupted through volcanoes, returning the stored carbon back into the atmosphere, ocean, and biosphere. Our society's dependence on fossil fuels bypasses this natural process by moving as much carbon from the geosphere to the atmosphere in a single year as what might otherwise require hundreds of thousands or millions of years. In Pennsylvania, when we strip mine and burn coal we are in effect releasing the atmospheric carbon dioxide and stored energy of the sun that has been buried for over 300,000,000 years!
Watch this!
On a trip to Bear Meadows, near the University Park campus of Penn State, I describe atmospheric carbon and organic carbon storage in the Critical Zone.
Video: A Trip to Bear Meadows (5:40)
TIM WHITE: I've traveled to Bear Meadows, a national natural landmark, about eight miles southeast of Penn State's main campus in central Pennsylvania. I've brought you here today to describe the relationship between atmospheric carbon dioxide content and organic carbon storage at the surface of the Earth within the critical zone. Let's go have a look at the bog.
Bear Meadows is a peat bog that began forming nearly 10,000 years ago, shortly after northwestern and northeastern Pennsylvania were covered by vast ice sheets that extended far to the north into the Hudson Bay region and Canada. The closest those ice sheets reached to State College and the Penn State campus here was northeast of Williamsport, nearly 30 miles away. One of the tasks I'd like to accomplish while we're here is to probe the peat in the bog and have a look at what it's made of, and so to do that I'm going to walk along the edge of the bog-- in the background you can see where I'm going to go-- and I'm going to get my soil auger so I have a way to probe and sample the peat.
Recall that plants use water, atmospheric carbon dioxide, and energy from the sun to build their organic framework through photosynthesis. Therefore, plants draw down atmospheric carbon dioxide as part of their life habit. When plants die, the organic matter is oxidized and carbon dioxide is returned to the atmosphere.
This wet, boggy setting is perfect for the preservation of organic matter and organic carbon. If you look beneath the water's surface, you can clearly see various organic constituents related to the plants that live around in the bog. I can see maple leaves and abundant roots associated with these grasses. If plants die and their various organic constituents are preserved, as is the case here in the bog, the original carbon in the atmospheric carbon dioxide that was photosynthesized by the living plant is also stored or preserved, rather than being returned to the atmosphere through decay. We call the site of such processes a carbon sink.
I'm probing the peat with an auger to see how deep I can get to the base of the bog to see if I can get a peat sample from the base of the bog here. You can see that I've penetrated over a meter of the peat and I've obtained a sample from the base of the bog. And you can see that the peat down there is made up of a fine organic muck, along with a number of roots and some grass leaves, similar to what you'd find right here at the surface.
Further out in the bog, there are places where 15 feet of peat have accumulated over the last 8,000 or so years. That means that 8,000 years ago, carbon was removed from the ancient atmosphere, stored here in the peat, and remains today. One last thought before we leave and that relates to the time scale of organic carbon storage in the critical zone. I've told you that organic matter has been accumulating here over the last 8,000 years, but this setting exists within the Appalachian Mountains and the fate of mountains ultimately is to be eroded, which is happening to these mountains today. So while this organic carbon remains stored here now, over long geologic time scales-- hundreds of thousands to millions of years-- this peat will be oxidized and that ancient organic carbon would be returned to our modern atmosphere.
Next, visit a Pennsylvania coal seam and nearby electric generating station to learn about the relationship between Bear Meadows and the burning of coal.
Video: Visit to a Pennsylvania Coal Seam (02:52)
Coal Seams and Electricity Generation near Shawville, PA
[Tim is standing next to a coal seam.]
The geologic, economic, and cultural history of Pennsylvania is steeped in this state's naturally occurring abundance of coal. To give students an opportunity to view coal in its natural setting, I like to come here, Curwensville Dam, about 45 miles northwest of State College.
[Here you can see two coal seams, one directly in front of me and one a little bit further up on the slope. Coal is peat that's been deeply buried in the earth and subjected to high pressures and temperatures. Remember at Bear Meadows, we looked at peat and we were able to see the various plant constituents from which it's made.
We also discussed that those plant constituents at the bottom of the bog were 8,000 years old and contained 8,000-year-old atmospheric carbon dioxide. These coal seams are over 300 million years old. They formed from vast peat swamps that existed along the margins of a large interior seaway that flooded much of North America at the time. So when we dig up and mine these coals, and use them to fire our electric generating stations and the computers you're using today to view this video, we're releasing 300 million-year-old carbon dioxide into our present-day atmosphere.
[looking out a car window at the Shawville Generating Station]
We're approaching the Shawville Generating Station. At this power plant, which is just about 15 miles from the Curwensville coal outcrop we just visited, coal is burned to produce electricity for local and regional consumption.
[Dr. White standing in front of the generating station]
At numerous coal-fired electric generating stations around the state, coal is burned to produce electricity. And it's through this process that Pennsylvania produces 1% of total global human carbon dioxide emissions in any given year.
In subsequent lessons, we will examine and discuss the effects of anthropogenic carbon dioxide emissions and climate change on critical zone processes.

New to InTeGrate?
- Unit 2: The Carbon Cycle
Callan Bentley (Northern Virginia Community College)
This activity was selected for the On the Cutting Edge Reviewed Teaching Collection
This activity has received positive reviews in a peer review process involving five review categories. The five categories included in the process are
For more information about the peer review process itself, please see https://serc.carleton.edu/teachearth/activity_review.html .
- Reviewed: January 20, 2015 -- Reviewed by the InTeGrate Materials Review Process
- First Publication: July 15, 2016
- Reviewed: May 10, 2019 -- Reviewed by the On the Cutting Edge Activity Review Process
Students will explore the different aspects of the carbon cycle on Earth. This includes the original source of all the carbon on our planet, the near ubiquity of carbon, the six principle reservoirs of carbon in the Earth system, and the movement (flux) of carbon between reservoirs. Students will approach the chemical history of carbon by personifying the "journey" of specific carbon atoms throughout geologic time.
The unit emphasizes the grand challenges of energy resources and climate change by grounding these issues in a solid understanding of carbon from a systems thinking perspective. The point here is for students to gain a more robust appreciation for the movement of carbon between atmosphere and geosphere, between hydrosphere and biosphere. The unit provides dynamic understanding of how perturbations to one sphere or changes in the amount of carbon in a given reservoir can have implications throughout the Earth system.

Expand for more detail and links to related resources
Activity Classification and Connections to Related Resources Collapse
Grade level.
View Standards Details »


Science and Engineering Practices
Using Mathematics and Computational Thinking: Apply mathematical concepts and/or processes (e.g., ratio, rate, percent, basic operations, simple algebra) to scientific and engineering questions and problems. MS-P5.4:
Constructing Explanations and Designing Solutions: Construct an explanation using models or representations. MS-P6.2:
Cross Cutting Concepts
Scale, Proportion and Quantity: Proportional relationships (e.g., speed as the ratio of distance traveled to time taken) among different types of quantities provide information about the magnitude of properties and processes. MS-C3.3:
Energy and Matter: Changes of energy and matter in a system can be described in terms of energy and matter flows into, out of, and within that system. HS-C5.2:
Energy and Matter: Energy drives the cycling of matter within and between systems. HS-C5.4:
Disciplinary Core Ideas
Earth’s Materials and Systems: All Earth processes are the result of energy flowing and matter cycling within and among the planet’s systems. This energy is derived from the sun and Earth’s hot interior. The energy that flows and matter that cycles produce chemical and physical changes in Earth’s materials and living organisms. MS-ESS2.A1:
The Universe and Its Stars: Other than the hydrogen and helium formed at the time of the Big Bang, nuclear fusion within stars produces all atomic nuclei lighter than and including iron, and the process releases electromagnetic energy. Heavier elements are produced when certain massive stars achieve a supernova stage and explode. HS-ESS1.A4:
Structure and Properties of Matter: The structure and interactions of matter at the bulk scale are determined by electrical forces within and between atoms. HS-PS1.A3:

Learning Goals
- Students will be able to describe the ultimate source of all carbon in the universe.
- Students will be able to quantify movements (fluxes) associated with carbon reservoirs on Earth.
- Students will be able to create an illustration of Earth's carbon cycle that clearly shows and distinguishes reservoirs and movements (fluxes).
Context for Use
This unit is designed to be applicable in an introductory level college Earth science course or environmental science course. This unit consists of classroom activities, discussions, and an out-of-class project. It would be appropriate for classes ranging in size from 5–50. In larger introductory courses, students could play in teams, or the activities could be done piecemeal during lab sections.
It requires dice (1 die per student or team), scissors, and string. It should take 50 minutes (not including the out-of-class assessment project). The activities are designed to be flexible and adaptable for use in other circumstances.
Description and Teaching Materials
The overall flow of this unit is as follows:
Pre-class preparation Students watch a series of videos about the carbon cycle. (20 min, before the day of the lesson) Optional: There is an activity to accompany the video which instructors may assign. (an additional 25 min before the day of the lesson)
In-class lesson components:
- Engagement activity : Students brainstorm where carbon is found on Earth. (5 min)
- The origin of carbon: Instructor presents information on nucleosynthesis (fusion) in stars. (10 min)
- Carbon cycle game: Students explore the concepts of reservoirs and fluxes with a game of chance. (15 min)
- Sizing up the reservoirs: Students quantify the size of principle carbon reservoirs in the Earth system by drawing circles. (20 min) During class, this comprehensive PowerPoint can help guide instructors through the sequence of activities: Comprehensive Unit 2 PowerPoint (PowerPoint 2007 (.pptx) 8.7MB Aug11 16)
Post-class assessment
- Homework: Students complete draw/create a graphical representation of the carbon cycle as a homework assignment. (20–60 min, after class )
- Summative assessment: Quiz: can be administered at the start of the next class, or as part of a larger whole-module assessment. (10 min)
All of the following activities can take place during class time, with the exception of the pre-class preparation (NPR "Climate Connections" videos) and the post-class assessment (carbon cycle diagram/Prezi).
Pre-class preparation: Before the day of the lesson, students will be introduced to the subject via a series of short videos. In the context of climate change, the videos examine why carbon is such an important element, and who it bonds with and why. To do this, students will be assigned to watch some or all of the five videos in the "Climate Connections" series by National Geographic and National Public Radio. There is helpful information in the instructor's guide to the activity that assists instructors in deciding which of the videos to assign (link below). If instructors wish, they can use the worksheet to assign students the activity. The students should use the series' cartoon character carbon, hydrogen, and oxygen atoms to show bonding arrangements, and then to tell small stories about how bonds form or break (photosynthesis, respiration, geological carbon sequestration, and fossil fuel burning). Instructors could offer bonus points for added details to make the stories dramatic and memorable. As written, the students turn in the worksheet as a homework assignment to be collected by the teacher, but it could also be assigned to be performed in class — if there were enough time for the performance. (Time estimate: 20 min before class; 45 min total with activity option) × div[id^='image-'] {position:static}div[id^='image-'] div.hover{position:static} Odd Todd's "carbon" cartoon character. Reproduced with permission of the artist. Provenance: Todd Rosenberg, "Odd Todd" http://www.oddtoddstudios.com/ Reuse: If you wish to use this item outside this site in ways that exceed fair use (see http://fairuse.stanford.edu/) you must seek permission from its creator. Link to videos on YouTube: Episode 1 of 5 — Introduction Episode 2 of 5 — Making Carbon Bonds Episode 3 of 5 — Breaking Carbon Bonds Episode 4 of 5 — How Carbon Makes Us Rich Episode 5 of 5 — How Carbon Makes Us Warm
Student worksheet (simplified version):
Word format: "Climate Connections" video worksheet (Microsoft Word 233kB Aug11 16) PDF format: "Climate Connections" video worksheet (Acrobat (PDF) 257kB Aug11 16)
Student worksheet (more advanced version):
Word format: Climate Connections Chemistry Worksheet (Microsoft Word 2007 (.docx) 235kB Aug11 16) PDF format: Climate Connections Chemistry Worksheet (Acrobat (PDF) 277kB Aug11 16)
Instructor's guide to activity:
Word format: Instructor’s Guide to Climate Connections Chemistry Worksheet (Microsoft Word 2007 (.docx) 67kB Aug11 16) PDF format: Instructor’s Guide to Climate Connections Chemistry Worksheet (Acrobat (PDF) 136kB Aug11 16)
2. Engagement activity:
Students identify the many substances in the Earth system and in everyday life that contain appreciable amounts of carbon. Discussion occurs in the "think/pair/share" format , or simply "pair/share" for the sake of a more expedited learning activity. As instructors facilitate the final large-group discussion, they can encourage students to compare and contrast some of the listed components [for instance, soda pop vs. seltzer water: only the soda contains sugar (a carbon compound), but both are carbonated with dissolved CO 2 ]. The results of this activity can serve as a formative assessment for the instructor. (Time estimate: 5 min)
Engagement activity handout: Word format: Engage: Where's the carbon? (Microsoft Word 2007 (.docx) 27kB Jun3 14) PDF format: Engage: Where's the carbon? (Acrobat (PDF) 68kB Jun3 14)
Instructor's key:
Word format: Engage: Where's the carbon? (Instructor's copy with answers) -- private instructor-only file Hide Engage: Where's the carbon? (Instructor's copy with answers) This file is only accessible to verified educators. If you are a teacher or faculty member and would like access to this file please enter your email address to be verified as belonging to an educator. Email Adress Submit PDF format: Engage: Where's the carbon? (Instructor's copy with answers) -- private instructor-only file Hide Engage: Where's the carbon? (Instructor's copy with answers) This file is only accessible to verified educators. If you are a teacher or faculty member and would like access to this file please enter your email address to be verified as belonging to an educator. Email Adress Submit
3. The origin of carbon:
Where does carbon come from? A brief instructional presentation on stellar nucleosynthesis — how carbon forms from the thermonuclear fusion of smaller atoms. Guiding questions address issues such as how carbon got from the middle of a star to Earth: i.e., the nebular theory of solar system formation. The presentation is available as a PowerPoint slideshow, or as a video (Quicktime or YouTube formats available). (Time estimate: 10 min)
PowerPoint presentation (Note that these slides are already included in the "Comprehensive Unit 2" PowerPoint, if you downloaded that above): Steller nucleosynthesis of carbon by thermonuclear fusion (PowerPoint 8.1MB Aug11 16)
PDF version of slideshow:
Steller nucleosynthesis of carbon by thermonuclear fusion (Acrobat (PDF) 15.1MB Aug11 16)
presenter notes (PDF):
Presenter notes: Steller nucleosynthesis of carbon by thermonuclear fusion (Acrobat (PDF) 2.2MB Aug1 14)
Video of presentation:
Windows Media Player version to download and play on PCs: Video of Stellar C nucleosynthesis presentation ( 7.9MB Jun2 16) YouTube video link: https://youtu.be/vY_wQz55YSA
The instructor may wish to invoke a short discussion at this point to emphasize that chemists and biologists (and often geologists) tend to think of atoms as eternal unchanging entities, but in reality, atoms are constructed from smaller building blocks. While thermonuclear fission is generally well appreciated as being the cause of radioactivity, thermonuclear fusion is less intuitive, given how different the interior of a star is from our everyday experience. Atoms can be created, and destroyed, and we need to allow our minds the latitude to appreciate that if we are to understand carbon.
Next up is an exploration of the major reservoirs and fluxes of carbon in the Earth system. This will be accomplished with two activities.
4. Carbon cycle game: The first activity is a game wherein students play carbon atoms that move through the Earth system between reservoirs via various fluxes . The game builds small student-generated dramas of carbon's epic journey. In the game, students roll dice, and depending on which number comes up, they follow a path from one reservoir to another through geologic time. The six reservoirs modeled are: mantle, crust, ocean, atmosphere, vegetation & soils, and fossil fuels. Instructors introduce the game with a definition of "reservoir" and "flux." Students can play (journey between reservoirs via fluxes) as long as the instructor sees fit. The random influence of the dice will generate countless possible pathways through the carbon cycle. Students can "report out" at the conclusion of the game to tell the most interesting "twists" in their carbon atom's personal voyage through the carbon cycle. Instructors should debrief the activity by emphasizing again the idea of carbon as a system, with places of residence (reservoirs) and processes of transfer (fluxes). An optional extension would be to explore which of these fluxes occur rapidly, and which are more likely to occur at slow rates. (Time estimate: 15 min) Pre-game terminology definitions by instructor: very brief lecture notes to define "reservoir" and "flux" prior to the start of the game. (Time estimate: 4 min) Instruction before the Carbon Cycle game (Microsoft Word 440kB Aug11 16) Instruction before the Carbon Cycle game (Acrobat (PDF) 459kB Aug11 16)
"Game rolls" handout
Carbon cycle dice game (Microsoft Word 201kB Jun12 17) Carbon cycle dice game (Acrobat (PDF) 314kB Aug6 18)
5. Sizing up the reservoirs and calculating the magnitude of fluxes:
The second activity is to quantify the size of the six principle carbon reservoirs. Here, students scale a series of circles to various sizes commensurate with estimates of the size of the carbon load of that reservoir. Depending on the math abilities of the students, the instructors can opt to provide the radii of the circles to be created, or ask the students to calculate the radii from the size of the reservoir. A follow-up activity quantifies the size of the fluxes between reservoirs, and students will be able to rank the amount of change in the carbon in each reservoir as a percentage of total. This information can be used to predict the growth of the carbon proportion of the atmospheric reservoir at several hypothetical points in the future — an introduction to the crudest form of climate modeling. (Time estimate: 20 min) "Reservoirs and fluxes" activity handout Reservoirs and fluxes activity (Microsoft Word 149kB Aug11 16) Reservoirs and fluxes activity (Acrobat (PDF) 206kB Aug11 16)
Instructor's copy (with answers)
Reservoirs and fluxes activity - Instructor's copy -- private instructor-only file Hide Reservoirs and fluxes activity - Instructor's copy This file is only accessible to verified educators. If you are a teacher or faculty member and would like access to this file please enter your email address to be verified as belonging to an educator. Email Adress Submit Instructor's copy of the 'reservoirs and fluxes' activity -- private instructor-only file Hide Instructor's copy of the 'reservoirs and fluxes' activity This file is only accessible to verified educators. If you are a teacher or faculty member and would like access to this file please enter your email address to be verified as belonging to an educator. Email Adress Submit
If instructors want to make students do their own calculations, they can modify this Excel spreadsheet for distribution:
Area gigatonne calculation (Excel 2007 (.xlsx) 11kB Jun3 14)
If instructors want to provide the reservoir circles pre-drawn, here is a PDF showing their relative size:
Sizing Up Reservoir Graphic (Acrobat (PDF) 322kB Mar14 16) (This is a PDF with the reservoirs drawn to scale. Be forewarned: some are tiny! To read the lower right corner more easily, remember that you can select your level of zoom on a PDF.)
The unit concludes with two assessments — see the Assessment section below.
Teaching Notes and Tips
Emphasis should be put on the temporary residence of a single given carbon atom in any reservoir, and the more or less eternal nature of the carbon atom ( 14 C's radioactive decay aside). The same carbon that powers our bodies today was part of minerals and ancient life in the past. It has been part of the air, and the crust, and dissolved in the ocean. Ultimately, it, like everything but hydrogen, was forged in incredibly powerful reactions in the heart of long-dead stars.
The video version of the "carbon nucleosynthesis" presentation could be used as an optional pre-class assignment if instructors want more flexibility during class.
If the classroom lacks actual dice for the carbon cycle game, there is a free dice-rolling app that could substitute for students with iPads or iPhones.
For the reservoirs & fluxes activity, you will need a wide open space and a big piece of paper â€" the biggest circle is 2.6 meters in diameter! If the instructor is short on time or materials, the instructor could prepare the circles before class. Successful deployments of this activity used four sheets of butcher paper taped together (prepared in advance), unfolded cardboard shipping boxes, or the back side of old topographic maps. Alternatively, a parking lot and chalk could work, or partial circles drawn on a chalkboard or whiteboard.
Three possible extensions include:
Elemental abundance activity:
(optional) Expanding on the relationship between carbon in the universe and carbon in our bodies, students will engage in an elemental abundance comparison. In this activity, they will use an Excel spreadsheet of elemental abundances in the Milky Way galaxy and compare them with the elements that make up people, coming to the conclusion (through a discussion facilitated by the instructor) that people (and life in general) are anomalously enriched in carbon relative to galactic background levels. It is suggested that students work on the initial calculations in pairs or small groups (determined by number of computers available). (Time estimate: 10 min)
Elemental abundance worksheet: Elemental abundance comparison activity (Microsoft Word 2007 (.docx) 29kB Aug11 16) Elemental abundance comparison activity (Acrobat (PDF) 61kB Aug11 16)
Elemental abundance spreadsheet:
Elemental abundance comparison spreadsheet (Excel 2007 (.xlsx) 22kB Jun3 14) (student copy for distribution) Elemental abundance comparison spreadsheet: Instructor's copy -- private instructor-only file Hide Elemental abundance comparison spreadsheet: Instructor's copy This file is only accessible to verified educators. If you are a teacher or faculty member and would like access to this file please enter your email address to be verified as belonging to an educator. Email Adress Submit (instructor's copy with answers)
The Suess Effect
A discussion of the Suess effect (isotopically light carbon as a signature of fossil fuel sources). The atmosphere has become progressively more enriched in 14 C- and 13 C-depleted CO 2 since the Industrial Revolution. Corals have incorporated this light carbon into their skeletal material in a measurable way. If the population of students in the class is sufficiently advanced as to read primary literature, discussing The 13C Suess effect in scleractinian corals mirror changes in the anthropogenic CO2 inventory of the surface oceans (Swart, et al., 2010) would be a good place to start.
Read and discuss CO2 Rising by Tyler Volk
A reading of (portions of) Tyler Volk's book CO 2 Rising , and ensuing discussion. This book provides a terrific, accessible approach to the carbon cycle by chronicling the journeys of "Dave," a single carbon atom. Over time, Dave is incorporated into a limestone, the ocean, the atmosphere (and passes through the measurement apparatus at Mauna Loa), a wheat plant, a beer, and the author, Tyler Volk, who exhales Dave back into the atmosphere again. The book is recommended as background "perspective" reading for anyone contemplating teaching this unit, or any curriculum about the carbon cycle. I think students would find it engaging and useful for visualizing the complexity of the carbon cycle in the Earth system.
There are several ungraded (formative) and two graded (summative) means of assessment for this unit. (Ungraded assessments include keeping track of correct and insightful observations and statements during discussions of unit material and activities, as well as incorrect or "off-base" comments. In particular, the initial "engagement" activity should provide baseline formative assessment for the instructor.) If time and student achievement level permits, the optional graded homework assignment (#6, below) could be assigned as a summative assessment. It could also work as an in-class group activity, if time permits.
6. Post-class homework summative assignment (optional):
Students will provide their instructors a formative assessment as they create a graphical representation of the carbon cycle, as a paper poster, a PowerPoint presentation, or the dynamic, zoomable medium called a Prezi. Synthesizing material from each of the previous activities into something visual that can be understood "at a glance" will provide instructors with a "read" on whether students are assimilating unit lessons. Based on the accuracy and quality of these graphic representations (scored with a rubric provided with the activity), instructors can address any misconceptions before moving on. (Time estimate for this optional assessment: 30 min; to be assigned as out-of-class work) Assignment: Unit 2 assessment: graphic representation of the carbon cycle (Microsoft Word 2007 (.docx) 19kB Aug6 18) Unit 2 assessment: graphic representation of the carbon cycle (Acrobat (PDF) 74kB Jun3 14)
Scoring rubric:
Unit 2 assessment grading rubric (Microsoft Word 34kB Aug11 16) Unit 2 assessment grading rubric (Acrobat (PDF) 80kB Aug18 16)
7. Summative assessment (in-class quiz):
The summative assessment for this unit, which can also function as a "pre-test" or a part of a larger, whole-module exam, is a quiz covering key aspects of the carbon cycle. Some of these questions are basic recall, while others ask students to apply their understanding. (Time estimate: 10 min) Quiz: Unit 2 quiz -- private instructor-only file Hide Unit 2 quiz This file is only accessible to verified educators. If you are a teacher or faculty member and would like access to this file please enter your email address to be verified as belonging to an educator. Email Adress Submit Unit 2 quiz -- private instructor-only file Hide Unit 2 quiz This file is only accessible to verified educators. If you are a teacher or faculty member and would like access to this file please enter your email address to be verified as belonging to an educator. Email Adress Submit
Instructor's key for scoring the quiz:
Unit 2 quiz instructor's key -- private instructor-only file Hide Unit 2 quiz instructor's key This file is only accessible to verified educators. If you are a teacher or faculty member and would like access to this file please enter your email address to be verified as belonging to an educator. Email Adress Submit Unit 2 quiz instructor's key -- private instructor-only file Hide Unit 2 quiz instructor's key This file is only accessible to verified educators. If you are a teacher or faculty member and would like access to this file please enter your email address to be verified as belonging to an educator. Email Adress Submit
References and Resources
"Climate Connections" video series by National Geographic and National Public Radio, featuring Robert Krulwich with cartoons by Odd Todd.
CO 2 Rising by Tyler Volk. The MIT Press (September 24, 2010) ISBN-13: 978-0262515214. An excellent overview of the carbon cycle, with an emphasis on the transformations experienced by an individual carbon atom ("Dave") that is at various points part of the oceans, a limestone, the air, a wheat plant, a beer, and the author's body, then exhaled back into the air, and so on. It also does a great job incrementally building up an understanding of pre-historic atmospheric carbon variations with a series of additive graphs. It could make an excellent book for students to read and discuss. At the very least, it is a good conceptual/philosophical background for instructors who see climate change as a big issue, but want a better grounding on the basics of carbon reservoirs and fluxes. See a more detailed review of it.
« Previous Page Next Page »
- Instructor Materials: Overview of the Carbon, Climate and Energy Resources Module
- Unit 1: Identifying Misconceptions & Logical Fallacies
- Unit 3: Geologic Record of Past Climate
- Unit 4: Fossil Fuel Formation
- Unit 5: Modern CO 2 Accumulation
- Unit 6: Moving Forward: Evaluating Impacts of Modern-day Proposals Affecting the Carbon-cycle and Climate
- Student Materials
Teaching Themes
Already used some of these materials in a course? Let us know and join the discussion »
Considering using these materials with your students? Get advice for using GETSI modules in your courses » Get pointers and learn about how it's working for your peers in their classrooms »

- About this Site
- Accessibility
Reuse of InTeGrate Materials
We encourage the reuse and dissemination of the material on this site for noncommercial purposes as long as attribution to the original material on the InTeGrate site is retained.
Material on this page is offered under a Creative Commons license unless otherwise noted below.
Show terms of use for text on this page »
Show terms of use for media on this page »

Learn more about Citing, Reusing and Adapting InTeGrate materials for your classroom
- Short URL: https://serc.carleton.edu/152854 What's This?
Disclaimer: Any opinions, findings, conclusions or recommendations expressed in this website are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Course title
Pre-requisite, course description.
Biology 1-2
Project: Modeling the Carbon Cycle: Students develop a scientific model on a poster that illustrates the role of photosynthesis and cellular respiration in the carbon cycle. The model should also show how carbon cycles the Earth?s four spheres. Once completed; students write a multi-paragraph paper describing the content of the model in more detail.
Lab: Interdependence of Organisms : Students complete a virtual lab; accompanied with a lab report; where they explore the interdependence of organisms by performing an experiment with lima bean plants and earthworms. They write a lab report on their experiment.
Project: Analyzing Factors That Affect Carrying Capacity and Biodiversity Students use simulations to analyze how climate; resources; and habitat size affect carrying capacity. Then they look at another simulation to analyze the effects of certain factors that affect biodiversity and populations. Finally; students will write and revise a conclusion on how human activity affects biodiversity
Project: Evaluating Human Impact on the Environment: Students use; evaluate; and improve a simulation to highlight human impact on coral reef ecosystems. Then they propose and evaluate real-world solutions to reduce the negative effects of human activity on this ecosystem and its biodiversity.
Lab: Diffusion Across a Semi-permeable Membrane: Students complete a virtual lab; accompanied with a lab report; where they explore how materials move across a semipermeable membrane.
Project: Analyzing Cellular Respiration: Students gather evidence to construct an explanation of how energy and matter move through the environment under aerobic and anaerobic conditions. Then they conduct additional guided research to revise their explanations based on new information discovered.
Lab: Building Proteins from RNA : Students complete a virtual lab; accompanied with a lab report; where they explore the molecular process of building proteins from the information carried by RNA using a laboratory procedure.
Project: Analyzing Genetic Variation: Students analyze claims about the causes of inherited genetic variation. You will then make your own claim based on prior knowledge. Next; they defend the claim by conducting research to gather information that supports it. Finally; students present claim and defense in a type-written paper
Lab: Mouse Genetics (One Trait ): Students complete a virtual lab; accompanied with a lab report; where they explore the relationship between genotype and phenotype.
Lab: Mouse Genetics (two Traits): Students complete a virtual lab; accompanied with a lab report; where they explore the law of independent assortment by examining a dihybrid cross in mice.
Lab: Natural Selection: Students complete a virtual lab; accompanied with a lab report; where they explore natural selection using a laboratory simulation
School country
School state, school city, school / district address, school zip code, requested competency code, date submitted, approved competency code, approved date, deferred reason.
Per ABOR Requirements: "A Laboratory Science course is defined as a course in w hich at least 1 class period each week is devoted to providing an opportunity for students to manipulate equipment; materials; or specimens; to develop skills in observation and analysis; and to discover; demonstrate; illustrate; or test scientific principles or concepts; such as chemistry; physics; earth sciences and biology." Please advise how manipulation of equipment; materials or specimens was performed once a week in this course.
Online / Virtual
- EO Explorer

- Global Maps
The Slow Carbon Cycle
Through a series of chemical reactions and tectonic activity, carbon takes between 100-200 million years to move between rocks, soil, ocean, and atmosphere in the slow carbon cycle. On average, 10 13 to 10 14 grams (10–100 million metric tons) of carbon move through the slow carbon cycle every year. In comparison, human emissions of carbon to the atmosphere are on the order of 10 15 grams, whereas the fast carbon cycle moves 10 16 to 10 17 grams of carbon per year.
The movement of carbon from the atmosphere to the lithosphere (rocks) begins with rain. Atmospheric carbon combines with water to form a weak acid—carbonic acid—that falls to the surface in rain. The acid dissolves rocks—a process called chemical weathering—and releases calcium, magnesium, potassium, or sodium ions. Rivers carry the ions to the ocean.

Rivers carry calcium ions—the result of chemical weathering of rocks—into the ocean, where they react with carbonate dissolved in the water. The product of that reaction, calcium carbonate, is then deposited onto the ocean floor, where it becomes limestone. ( Photograph ©2009 Greg Carley. )
In the ocean, the calcium ions combine with bicarbonate ions to form calcium carbonate, the active ingredient in antacids and the chalky white substance that dries on your faucet if you live in an area with hard water. In the modern ocean, most of the calcium carbonate is made by shell-building (calcifying) organisms (such as corals) and plankton (like coccolithophores and foraminifera). After the organisms die, they sink to the seafloor. Over time, layers of shells and sediment are cemented together and turn to rock, storing the carbon in stone—limestone and its derivatives.

Limestone, or its metamorphic cousin, marble, is rock made primarily of calcium carbonate. These rock types are often formed from the bodies of marine plants and animals, and their shells and skeletons can be preserved as fossils. Carbon locked up in limestone can be stored for millions—or even hundreds of millions—of years. ( Photograph ©2008 Rookuzz (Hmm). )
Only 80 percent of carbon-containing rock is currently made this way. The remaining 20 percent contain carbon from living things (organic carbon) that have been embedded in layers of mud. Heat and pressure compress the mud and carbon over millions of years, forming sedimentary rock such as shale. In special cases, when dead plant matter builds up faster than it can decay, layers of organic carbon become oil, coal, or natural gas instead of sedimentary rock like shale.

This coal seam in Scotland was originally a layer of sediment, rich in organic carbon. The sedimentary layer was eventually buried deep underground, and the heat and pressure transformed it into coal. Coal and other fossil fuels are a convenient source of energy, but when they are burned, the stored carbon is released into the atmosphere. This alters the balance of the carbon cycle, and is changing Earth’s climate. ( Photograph ©2010 Sandchem. )
The slow cycle returns carbon to the atmosphere through volcanoes. Earth’s land and ocean surfaces sit on several moving crustal plates. When the plates collide, one sinks beneath the other, and the rock it carries melts under the extreme heat and pressure. The heated rock recombines into silicate minerals, releasing carbon dioxide.
When volcanoes erupt, they vent the gas to the atmosphere and cover the land with fresh silicate rock to begin the cycle again. At present, volcanoes emit between 130 and 380 million metric tons of carbon dioxide per year. For comparison, humans emit about 30 billion tons of carbon dioxide per year—100–300 times more than volcanoes—by burning fossil fuels.
Chemistry regulates this dance between ocean, land, and atmosphere. If carbon dioxide rises in the atmosphere because of an increase in volcanic activity, for example, temperatures rise, leading to more rain, which dissolves more rock, creating more ions that will eventually deposit more carbon on the ocean floor. It takes a few hundred thousand years to rebalance the slow carbon cycle through chemical weathering.

Carbon stored in rocks is naturally returned to the atmosphere by volcanoes. In this photograph, Russia’s Kizimen Volcano vents ash and volcanic gases in January 2011. Kizimen is located on the Kamchatka Peninsula, where the Pacific Plate is subducting beneath Asia. (Photograph ©2011 Artyom Bezotechestvo/ Photo Kamchatka. )
However, the slow carbon cycle also contains a slightly faster component: the ocean. At the surface, where air meets water, carbon dioxide gas dissolves in and ventilates out of the ocean in a steady exchange with the atmosphere. Once in the ocean, carbon dioxide gas reacts with water molecules to release hydrogen, making the ocean more acidic. The hydrogen reacts with carbonate from rock weathering to produce bicarbonate ions.
Before the industrial age, the ocean vented carbon dioxide to the atmosphere in balance with the carbon the ocean received during rock weathering. However, since carbon concentrations in the atmosphere have increased, the ocean now takes more carbon from the atmosphere than it releases. Over millennia, the ocean will absorb up to 85 percent of the extra carbon people have put into the atmosphere by burning fossil fuels, but the process is slow because it is tied to the movement of water from the ocean’s surface to its depths.
In the meantime, winds, currents, and temperature control the rate at which the ocean takes carbon dioxide from the atmosphere. (See The Ocean’s Carbon Balance on the Earth Observatory.) It is likely that changes in ocean temperatures and currents helped remove carbon from and then restore carbon to the atmosphere over the few thousand years in which the ice ages began and ended.
Atmosphere Land

IMAGES
VIDEO
COMMENTS
OCR Gateway A Biology (New Specification - First exams in 2018) revision summary on B4.1.7 - The Carbon Cycle for the higher and foundation tier.
The following diagram shows the carbon cycle. Which arrow in the diagram represents cellular respiration? Learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more. Khan Academy is a nonprofit with the mission of providing a free, world-class education for anyone, anywhere.
The carbon cycle. Carbon is crucial for life, forming molecules like glucose, ATP, amino acids, and DNA. The carbon cycle involves plants taking in carbon dioxide, fixing carbon, and creating organic molecules. Animals eat plants, metabolize the molecules, and release carbon dioxide. This cycle keeps carbon circulating in the biosphere.
The uptake and return of CO 2 are not in balance. Figure 17.2.1.2 Carbon dioxide concentration. The carbon dioxide content of the atmosphere is gradually and steadily increasing. The graph shows the CO 2 concentration at the summit of Mauna Loa in Hawaii from 1958 through 1999. The values are in parts per million (ppm).
Term. Definition. carbon cycle. biogeochemical cycle through which carbon is recycled through the atmosphere, biosphere, and geosphere. cellular respiration. a biochemical process in which cells break down glucose and oxygen to make carbon dioxide, water, and ATP for energy. fossil fuel.
O2-CO2 cycle separate from other events of carbon cycle (e.g., plant and animal growth, decay, food chains). Macroscopic events (e.g., growth, breathing) are associated with specific organs (e.g., stomach, lungs) rather than cellular processes. Energy as cause to make things happen and energy can be used up to make things happen.
Lab 5: Carbon Cycle Modeling (Introduction) The goals of this lab are to: Evaluate how permafrost melting amplifies warming under the different emission scenarios. Please make sure that you read the Introduction to the lab. Skipping it will result in a lot of confusion and a lower score on the assignment.
The Carbon Cycle. Carbon is one of the most common elements found in living organisms. Chains of carbon molecules form the backbones of many organic molecules, such as carbohydrates, proteins, and lipids. Carbon is constantly cycling between living organisms and the atmosphere ( Figure below ). The cycling of carbon occurs through the carbon cycle.
The amount of inorganic C far exceeds the amount of organic C on Earth (Table 7.1; Figure 7.1), and participates in a number of chemical transformations.The major molecules in the inorganic part of the carbon cycle are: CO 2, a gas that readily dissolves in water; HCO 3 − (bicarbonate), which exists only as a dissolved form; and CO 3 = (carbonate), which can be dissolved or solid and forms ...
When carbon dioxide dissolves in water (H 2 O), it forms an acid called carbonic acid (H 2 CO 3 ). The reaction is given by the equation: CO 2 + H 2 O ↔ H 2 CO 3. The double-headed arrow indicates that the reaction can occur in either direction, depending on the conditions and the amount of carbon dioxide present.
Atmospheric carbon dioxide. The carbon cycle is of interest to understanding climate because it includes two of the most important greenhouse gases: carbon dioxide (CO 2) and methane (CH 4).Most atmospheric carbon is in the form of CO 2, while CH 4 is present only in trace concentrations. Because CO 2 is chemically inert, it is relatively well mixed within the atmosphere away from forest ...
Next up is an exploration of the major reservoirs and fluxes of carbon in the Earth system. This will be accomplished with two activities. 4. Carbon cycle game: The first activity is a game wherein students play carbon atoms that move through the Earth system between reservoirs via various fluxes.
Lesson Plan contains background, and game activity "Carbon Process". Has a real world connection Click Create Assignment to assign this modality to your LMS.
Photosynthesis is an example of a biological process that occurs on a much shorter timescale. Important processes in the carbon cycle include photosynthesis (autotrophs taking in CO2 gas and converting the carbon into sugars), respiration (aerobic or anaerobic, converting sugars back into CO2), combustion (chemical reaction turning pure carbon ...
Course description. Biology 1-2. Sem 1: Project: Modeling the Carbon Cycle: Students develop a scientific model on a poster that illustrates the role of photosynthesis and cellular respiration in the carbon cycle. The model should also show how carbon cycles the Earth?s four spheres. Once completed; students write a multi-paragraph paper describing the content of the model in more detail.
The Slow Carbon Cycle. Through a series of chemical reactions and tectonic activity, carbon takes between 100-200 million years to move between rocks, soil, ocean, and atmosphere in the slow carbon cycle. On average, 10 13 to 10 14 grams (10-100 million metric tons) of carbon move through the slow carbon cycle every year.
FlexBook Platform®, FlexBook®, FlexLet® and FlexCard™ are registered trademarks of CK-12 Foundation.
17.1.2 What is the carbon cycle. Skip to main content. General Biology Start typing, then use the up and down arrows to select an option from the list.? Get exam ready ... What is bio magnification? Siddhi Makhija. 846. views. 01:50. Biological Magnification. Patrick Haney. 538. views. 04:09. Bioaccumulation & Biomagnificaiton ...
Modeling the Carbon Cycle Assignment Summary: For this assignment, you will develop a scientific model (digitally or by hand) that illustrates the role of photosynthesis and cellular respiration in the carbon cycle. Your model should also show how carbon cycles the Earth's four spheres. Once you have completed your model, you will write an explanation with a few paragraphs describing the ...
APES: 1.4-1.7 Biogeochemical cycles. How does the burning of fossil fuels contribute to the net increase in atmospheric carbon? Click the card to flip 👆. carbon that has been sequestered underground is added to the carbon cycling between the atmosphere and the biosphere. Click the card to flip 👆.
2.1.7 modeling the carbon cycle 1,Compare the amount of carbon released into the atmosphere by the various human activities how much carbon is released into the atmosphere each year by all human activities in the simulation combined 2, what are the effects of increasing atmospheric carbon on natural ecosystems? 3, nearly half of earths land, consist of Articulture ecosystems of this ...
Carbon Cycle Lesson Plan 2. Community Contributed. Students will experience how carbon is found in fossil fuels and how it travels in many forms. This lesson includes a background, introduction, procedures for the activity with extensions. Download.
1.4-1.7 Reading Assignment. Energy flows directionally through ecosystems, entering as sunlight and leaving as heat during the transfers between trophic levels. Rather than flowing through an ecosystem, the matter that makes up living organisms is conserved and recycled. The six most common elements associated with organic molecules—carbon ...