- Preferences


INTRODUCTION TO CLINICAL RESEARCH Lecture 1: Who, What, Why and Where of Clinical Research - PowerPoint PPT Presentation

INTRODUCTION TO CLINICAL RESEARCH Lecture 1: Who, What, Why and Where of Clinical Research
Introduction to clinical research lecture 1: who, what, why and where of clinical research richard j. barohn, m.d. professor and chairman department of neurology – powerpoint ppt presentation.
- Richard J. Barohn, M.D.
- Professor and Chairman
- Department of Neurology
- Director, General Clinical Research Center
- University of Kansas Medical School
- August 24, 2006
- Types of clinical research
- Retrospective looking back at data previously collected
- - Case Reports
- - Case Series
- Prospective make plan for future data collection
- - Observational
- - Interventional
- - Device/technique
- Can involve direct participant contact
- - Measure a clinical end-point
- Obtain tissue samples from participant to study in the laboratory
- Can be data-mining of clinical databases
- - Never see participant
- - Example Medicare database
- Survival (mortality)
- Physiologic parameter
- Example quantitative strength, breathing (vital capacity)
- Clinical scales
- Example Mini Mental Status
- Blood or other body tissue measurement
- Example - Glucose Level
- - Hormone Level
- Open-label Trial
- Everyone receives research intervention
- Controlled Trial
- Some receive research intervention, some do not
- Control group - Ones that do not receive research intervention
- Control group can be getting another intervention
- Comparing new drug to older one
- Or control group can get placebo (inactive intervention)
- Randomized Trials
- Participants randomly allocated to active intervention or control group
- Blinded usually double blind
- Doctor and participant do not know which group subject is in
- Gold Standard Randomized Double-Blinded Controlled Trial
- Most proceeded by beneficial results in laboratory models
- All multicenter with other ALS centers
- COX-2 inhibitor (glutamate inhibitor) (MDA)
- Celebrex trial just completed no benefit
- ? improves strength in athletes (NIH)
- Minocycline oral (cell death inhibitor) (NIH)
- Ceftriaxone intravenous (cell death inhibitor) (NIH)
- CoQ10 (antioxidant) (NIH)
- Arimoclomol (increases heat shock protein) (Biotech investigator initiated)
- Celebrex/creatine vs mino/creatine (ALSA)
- Pre-Clinical Animal and In Vitro Lab Studies
- Phase I Safety (normals disease)
- Phase II Preliminary Efficacy Data with
- additional safety
- usually lt 100 patients
- Phase III Pivotal efficacy trial
- Large, often multicenter
- Phase IV - Post-marketing
- In Academic Health Centers
- Faculty MD, DO, PhD are Principal Investigators and Co-Investigators in Schools of Medicine, Nursing, Allied Health
- Trainees (students, residents, fellows) work under Faculty
- Outside of Academic Health Centers
- Private practice health care providers
- Pharmaceutical companies, example Quintiles
- Biomedical device companies
- Investigator
- Clinical Research Coordinators
- (Example nurse, respiratory therapist, RD)
- Clinical Research Evaluator
- Research Assistant
- Biostatistician
- Data Manager
- Research Pharmacy
- If laboratory based
- Lab research personnel (students, post doctorate, technicians)
- Federal Grants
- Example National Institutes of Health
- FDA-Orphan Drug Grant Program
- Foundations
- Example Muscular Dystrophy Association
- Internal-funding at medical center
- Example Research Institute of KUMC
- Private Donations
- Non-Funded Research
- Local Institutional Support
- KUMC Research Institute
- Disease-Related Foundations
- Standard Research Grants
- Career Development Grants
- NIH (NIH.gov)
- Apply to NIH for
- K23 Clinical Research
- K08 Lab/Translational
- R03 Pilot Program
- R21 Developmental/Exploratory
- Institutional Awarded Training Grants
- T32 Training
- K12 Career Development
- VA Career Development Awards
- Merit Grants
- GOAL RO-1 THE BIG ONE
- Food Drug Administration (FDA)
- PI applies to get IND for drug trial
- Local Human Subjects Committee (Institutional Review Board)
- Local HIPPA Compliance Program
- General Clinical Research Center
- Research Subject Advocate
- Safety Monitors
- Data Safety Monitoring Boards
- Local KUMC DSMB
- National NIH DSMB
- Research Institute Clinical Trials Office
- Serve as centralized sponsor contact point for KUMC
- Prepares consent with PI input
- Prepares and negotiates trial budgets
- Submits protocol/consent to HSC if there is a sponsor
- Contact Laurie Kemble at 588-1242 or http//www2.kumc.edu/researchinstitute/
- Human Subjects Committee
- KUMCs Institutional Review Board
- Committed to ethical, legal and safe conduct in all research involving human participants
- Meets 2nd and 4th Tuesdays
- Submissions by noon Friday, 7 business days prior to meeting
- Submission of materials
- Contact Karen Blackwell at 588-0942 or http//www.kumc.edu/hipaa/
- Focus on steering mechanism not the
- drive train or brakes, as they are
- not usually accessible to mentors.
- The mentor should
- Be interested
- Have experience
- Be available
- Limit number of mentees
- Commit substantial time
- Be vigilant but not intrusive
- In academic health centers
- Departmental clinical space
- Designated research space
- Example GCRC
- Hoglund Brian Imaging Center
- Veterans Administration Medical Centers
- Hospitals not affiliated with AHC
- NIH in Bethesda intramural
- Private health care provider offices
- Industry offices
- Advance science/improve knowledge
- Obtain access to a potential Rx at the earliest possible time
- Current Rx not working
- It helps maintain hope - better than not doing anything
- Desire to meet and speak with researchers
- To help others
- Reimbursement to subject/patient
- Painful unpleasant procedures
- Fear of going off current medication
- New treatment may not be better
- Time commitment/travel
- Side effects
- Fear of the unknown
- Fear of being assigned to placebo
- They accept the disease is their fate
- Efforts futile
- Privacy concerns publication, etc.
- Resentment of medical personnel
- Distrust of science community
- Dont want to be a guinea pig
- Dont agree with focus of research
- Long wait to get research results
- Be considered a partner in the research
- Be treated with respect / taken seriously, kept informed and up-to-date
- Do not want to be talked down to
- Full disclosure of side effects
- Option to stop involvement in study
- Support from research team - availability
- After study, want to know results and if they were on drug or placebo
- Realistic idea when results will be available
- No cost to participant
- Confidentiality
- Enough time and comfortable area
- To ask questions
- In which to do the study
- A NIH-supported multidisciplinary research unit which facilitates investigator-initiated clinical studies and trials conducted by full-time faculty of the AHC.
- Provides clinical research infrastructure to investigators who receive funding from federal agencies, private foundations, other peer-reviewed sources.
- Also, can include investigator initiated unfunded pilot studies and industry sponsored studies.
- This is at no cost to the PIs for Investigator-Initiated Trials
- Provide clinical investigators from the SOM, SON, and SOAH with a modern, state-of-the-art facility in which clinical research could be conducted
- Enhance multidisciplinary research across departments and the three schools
- Enable and train junior faculty and trainees to become more involved in clinical research
- Apply for federal funding to support the GCRC
- Outpatient Unit
- Inpatient Unit
- Scatter Beds
- Metabolic Kitchen
- Informatics Core
- VOTING MEMBERS
- Matthew Mayo, PhD
- Jared Grantham, MD
- Marge Bott, RN, PhD
- Richard McCallum, MD
- Stephen Williamson, MD
- Richard Dubinsky, MD
- Barbara Lukert, MD
- Kevin Latinis, MD, PhD
- Debra Sullivan, PhD, RD, LD
- Patrick Moriarty, MD
- Jo Ann Harris, MD
- John Ferraro, PhD
- Andrea Charbonneau, MD
- Kathryn Ellerbeck, MD
- Ossama Tawfik, PhD, MD
- Barbara Quaney, PT, PhD
- Kathleen Gustafson, PhD
- NON-VOTING EX OFFICIO MEMBERS
- Barbara Atkinson, MD Executive Dean SOM
- Richard J. Barohn, MD Program Director
- Curt Hagedorn, MD Associate Director
- Jeff Burns, MD Assistant Director
- Patricia Kluding, PT, PhD Assistant Director
- Paul Terranova, PhD
- Susan Schmitz, BA, CCRC Administrative Director
- Judy Otey, RN, BSN Nurse Manager
- Ed Ellerbeck, MD, MPH
- Jon Jackson
- Jo Denton, MSN
- Laurie Kemble, BS, CRT
- For GAC approved studies
- Space to see patients
- Biostat support study design
- Data management
- Nurse support
- Administrative support
- Specimen collection/storage
- Common equipment
- No cost for above services to PIs on investigator initiated studies
- Industry sponsored with charges for space/resources
- Space/resources for industry studies
- Permanent space for research coordinators
- Overnight stay on GCRC unit
- GCRC Planning Committee formed 2002
- Construction/Remodeling began June 1, 2004
- Completed October 2004
- 6, 000 square feet in Delp
- Announce GAC will accept research applications from investigators, September 2004
- Began doing studies on GCRC January 2005
- Approval of 53 on-going studies by GAC as of June 2006
- GCRC NIH grant submitted June 1, 2006
- 2 million (direct costs) per year for 3 years
- Contact Judy Otey, RN, BSN
- GCRC Administrative Director
- 913-588-0984 alt. 2460
- jotey_at_kumc.edu
- Nicole Ladesich, BS
- GCRC Senior Coordinator
- 913-588-0976 alt. 2294
- nladesich_at_kumc.edu
- Visit Website http//gcrc.kumc.edu
- Recruiting and retaining researchers
- Explosion in clinical demands, reduced financial margins ? limit time, diluted value
- Regulatory burden
- Fragmented training
- Complexity of training
- No real HOME for clinical researchers
- Limitations / barriers due to NIH funding mechanisms, review, program structures
- Clinical Research covers all studies of diseases and trials of
- treatments in human subjects
- Translational Research describes the steps between a
- fundamental discovery and its application in clinical
- medicine. For example
- Testing a new anti-cancer drug in humans for the first time
- Identifying best practices in the diagnosis, prevention, or management of a disease and enhancing their adoption by the community
- Purpose Forge a transformative and integrative academic home for clinical and translational science
- The home must be a Center, Department, or Institute.
- Encompass all components of clinical research (education, career development, clinical research infrastructure)
- Promote multidisciplinary research teams
- Create an incubator for innovative research tools
- Catalyze the application of new knowledge to clinical practice
- Degree granting capabilities in Clinical Research
- Masters and/or PhD
- No current GCRC funding
- Current K12 applicability?
- Small pool of clinical mentors, current clinical R01s
- Limited culture for CR
- GCRC infrastructure
- Institutional support
- Research Institute
- SOM / SON / SOAH
- Bioinformatics Center (Mayo)
- K-BRIN (Hunt)
- KU Lawrence (Georg and others)
- Intro to CR Course
- K30 (MSCR program)
- KU Lawrence
- Life Span Institute
- Drug Development
- Introduction to CR Course
- No Clinical T32
- Letter of Intent February 27, 2006
- Grant Application March 27, 2006
- Planning grant 150,000 for 1 year
- Full CTSA up to 6 million with pediatrics
- 4 million without pediatrics
- Existing K30, T32, and GCRC are in addition
- 5 year award RFA offered annually
- Goal 60 CTSAs to be awarded by 2012
- Will replace all GCRCs and NCRR / Roadmap K12s
- Planning Steering Committee
- Barbara Atkinson, MD
- Richard J. Barohn, MD
- Governance Planning Sub-Committee
- Grant Writing Planning Sub-Committee
- Lauren Aaronson, PhD, RN
- Education Planning Committee
- Chair Ed Ellerbeck, MD, MPH
- Clinical Research Resources Planning Committee
- Regulatory Planning Committee
- Jim Voogt, PhD
- John Finley, JD, MPH
- Novel Methods Translational Technologies Planning Committee
- Curt Hagedorn, MD
- Health Disparities Research Planning Committee
- Patricia Thomas, MD
- Kirby Randolph, PhD
- Community Participant Planning Committee
- Joshua Freeman, MD
PowerShow.com is a leading presentation sharing website. It has millions of presentations already uploaded and available with 1,000s more being uploaded by its users every day. Whatever your area of interest, here you’ll be able to find and view presentations you’ll love and possibly download. And, best of all, it is completely free and easy to use.
You might even have a presentation you’d like to share with others. If so, just upload it to PowerShow.com. We’ll convert it to an HTML5 slideshow that includes all the media types you’ve already added: audio, video, music, pictures, animations and transition effects. Then you can share it with your target audience as well as PowerShow.com’s millions of monthly visitors. And, again, it’s all free.
About the Developers
PowerShow.com is brought to you by CrystalGraphics , the award-winning developer and market-leading publisher of rich-media enhancement products for presentations. Our product offerings include millions of PowerPoint templates, diagrams, animated 3D characters and more.
- All Resource
PPT Templates
Single slides.
- Pitch Deck 209 templates
- Animation 326 templates
- Vertical Report 316 templates
- Business 803 templates
- Finance 56 templates
- Construction 45 templates
- IT/Commerce 171 templates
- Medical 64 templates
- Education 45 templates
- Lifestyle 394 templates
- Pitch Decks 138 templates
- Business 541 templates
- Finance 20 templates
- Construction 75 templates
- IT/Commerce 73 templates
- Medical 27 templates
- Lifestyle 578 templates
- Pitch Decks 140 templates
- Business 469 templates
- Finance 19 templates
- Construction 64 templates
- IT/Commerce 72 templates
- Medical 29 templates
- Education 39 templates
- Lifestyle 490 templates
- Cover 266 templates
- Agenda 97 templates
- Overview 216 templates
- CEO 28 templates
- Our Team 142 templates
- Organization 48 templates
- History 38 templates
- Vision, Mission 109 templates
- Problem, Solution 193 templates
- Opportunity 154 templates
- Business Model 158 templates
- Product, Services 299 templates
- Technology 65 templates
- Market 155 templates
- Prices 56 templates
- Customers 55 templates
- Competitor 113 templates
- Business Process 151 templates
- Analysis 222 templates
- Strategy 120 templates
- Marketing, Sales 61 templates
- Profit, Loss 69 templates
- Financials 247 templates
- Timeline 122 templates
- Proposal 40 templates
- Contact Us 272 templates
- Break Slides 16 templates
- List 361 templates
- Process 351 templates
- Cycle 177 templates
- Hierarchy 98 templates
- Relationship 152 templates
- Matrix 86 templates
- Pyramid 67 templates
- Tables 145 templates
- Map 96 templates
- Puzzles 163 templates
- Graph 217 templates
- Infographics 436 templates
- SWOT 111 templates
- Icon 418 templates
- Theme Slides 138 templates
- Mockup 42 templates
- Column 315 templates
- Line 199 templates
- Pie 139 templates
- Bar 179 templates
- Area 130 templates
- X Y,Scatter 16 templates
- Stock 59 templates
- Surface 3 templates
- Doughnut 256 templates
- Bubble 65 templates
- Radar 83 templates
- Free PPT Templates 2,101 templates
- Free Keynote 2,017 templates
- Free Google Slides 2,098 templates
- Free Theme Slides 35 templates
- Free Diagram 126 templates
- Free Chart 49 templates
- New Updates
Result for ' clinical research '
217 Templates are available.
- Sort by Accuracy
- Sort by Newest

Medical research PowerPoint Presentations Samples
Built-in custom color palette Data charts (editable via Excel) 100% vector (fully editable maps, infographic, icons) Free images and artwork Smart and innovative presentation slides Modern layouts based on master slides

Scientific research Professional PPT
Easy customization 100% fully editable PowerPoint slides Fully editable content (graphics and text) via PowerPoint - No Photoshop needed! Vector icons 100% editable Free images and artwork Professional business presentation

Market research PPT Templates Design
Possible to change shape and color properties Easy to customize without graphic design skills Professional and unique slides Created with high quality slides Creatively crafted slides

Fashion research Book Layout Design powerpoint presentation download
Highly editable presentation template. Replaceable the image into placeholder Professional and unique slides Creatively crafted slides Easy color change

Medical Scientific research Annual Report presentation slide design
Easy to edit and customize Shapes and text are 100% editable Professionally designed Premade color variation Modern layouts based on master slides

Scientific research slides presentation
Scalable vectorial PowerPoint shapes and PowerPoint icons Shapes and text are 100% editable Possible to change shape and color properties Easy to customize without graphic design skills High quality, editable pre-designed slides

Genetic research - Free Design Template
Free images and artwork Landscape orientation style Clean, modern, and creative slides Latest Templates support version

Google Slides Templates Free Download - Genetic research
Modern and clean design Professional business presentation All images included Easy to change colors

research into drugs and vaccines to combat COVID-19 PowerPoint Slide
covid19, virus, research, vaccines, drug, technology, technology trends, retention technology, how to use, production process

Market research Single Slide
Market, Market Size, Market Trend, Market Needs, Market distribution, Market Research, Market Insight

Market research Slide
Modern and clean design Fully editable content (graphics and text) via PowerPoint - No Photoshop needed! 16:9 aspect ratio Best investors pitch deck Ready to use presentation slides on data analytics

Market research Presentation Slide
Quick and easy to customize Compatible with all major Microsoft PowerPoint versions, Keynote and Google Slides Best investors pitch deck Suitable for creative projects Ready to use presentation slides on data analytics

Market research PowerPoint Design
Drag & drop image placeholders Completely editable presentation template Professional and unique slides Creatively crafted slides Ready to use presentation slides on data analytics

World Market research Page Template
Quick and easy to customize Premium & modern multipurpose For professionals and educators Professionally designed infographic templates Changable into PDF, JPG, and PNG formats

Food Market research Single Page
Easy to edit and customize 100% fully editable PowerPoint slides Professional and unique slides Professionally designed infographic templates Readily available in both 4:3 and 16:9 aspect ratio

Market research Chart Simple Slide
All elements are editable Drag & drop image placeholders 100% fully editable PowerPoint slides Modern business plan Changable into PDF, JPG, and PNG formats

Market research Template
Quick and easy to customize Built-in custom color palette Possible to change shape and color properties Beautiful presentation decks and templates Professionally designed infographic templates

Market research Single Deck
Market Insight, Market Forecasts, Market Needs, Market Expansion, Market Size

clinical Trial Roadmap
Easy to edit and customize Quick and easy to customize Dark & light backgrounds

Business research Topics - Free Professional PowerPoint Templates
Quick and easy to customize Fully editable content (graphics and text) via PowerPoint - No Photoshop needed! Presentation photos are included; Landscape orientation style Changable into PDF, JPG, and PNG formats
1 / 11 page
Free Slides
Slide Members
All Rights Reserved 2024 © Copyright Slide Members
Information
- Privacy Policy
- Terms & Conditions
Recent Slides
- 26+ Latest weekly update Powerpoint Templates & Google slides
- 19+ Recently Powerpoint Templates & Google slides Update
- 9+ New Powerpoint Templates & Google Slides Update
- Log In Username Enter your ACP Online username. Password Enter the password that accompanies your username. Remember me Forget your username or password ?
- Privacy Policy
- Career Connection
- Member Forums
© Copyright 2024 American College of Physicians, Inc. All Rights Reserved. 190 North Independence Mall West, Philadelphia, PA 19106-1572 800-ACP-1915 (800-227-1915) or 215-351-2600
If you are unable to login, please try clearing your cookies . We apologize for the inconvenience.
Preparing the Research Presentation
If you have never presented a paper at a scientific meeting, you should read this article. Even if you have presented before, it is likely that this article contains information that will improve your presentation. This article contains a set of practical, proven steps that will guide your preparation of the presentation. Our assumptions are that you will schedule appropriate planning and preparation time, are interested in doing the best job possible, and know that a quality presentation is a combination of good research and communication skills. This and subsequent articles will focus on planning, preparation, creating visual aids (slides), and presentation skills for a scientific presentation. The intent of this series of articles is to help you make a favorable impression at the scientific meeting and reap the rewards, personal and professional, of a job well done.
To begin with, you need to create an outline of the topics you might present at the meeting. Your outline should follow the IMRAC format (introduction, methods, results, and conclusion). This format is chosen because your audience understands it and expects it. If you have already prepared a paper for publication, it can be a rich source of content for the topic outline.
To get you started, we have prepared a generic outline to serve as an example. We recognize that a generic outline does not necessarily adapt to all research designs, but we ask you to think, "How can I adapt this to my situation?" To help you visualize the content you might include in the outline, two types of examples have been included, one that describes a cross-sectional study using a survey methodology (example A), and a second using a combination of a case-control and cohort designs (example B).
Use the Preparing the Research Presentation Checklist to assist you in preparing the topic outline.

Introduction to Clinical Research Design
Mar 30, 2019
730 likes | 898 Views
Introduction to Clinical Research Design. Lee E. Morrow, MD, MS Assistant Professor of Medicine Creighton University. Descriptive Describe incidence of outcomes over time Case Reports Case Series Registries Cross Sections. Analytic Analyze associations between predictors and outcomes

Share Presentation
- intervention studies
- time period
- ms assistant professor
- absent select controls population

Presentation Transcript
Introduction to Clinical Research Design Lee E. Morrow, MD, MS Assistant Professor of Medicine Creighton University
Descriptive Describe incidence of outcomes over time Case Reports Case Series Registries Cross Sections Analytic Analyze associations between predictors and outcomes Observational Cohort Studies Case-Control Studies Experimental Clinical Trials Clinical Research Designs
Descriptive Studies • Often a first step in research • Doesn’t always have a specific hypothesis to be tested • Causality usually cannot be determined • Examples: • Case Reports/Series • Registries • Cross Sectional Studies
Case Reports/Series • Definition: A single/series of patients with or without a disease or exposure of interest for whom data are collected in any fashion • Sources: Clinics, hospitals, disease registries • Limitations: Not randomly selected, bias due to selection factors inherent in the source, not representative of the population from which they are selected
Case Reports/Series • Benefits: Easy to do, useful for exploring relationships and/or generating hypotheses • Key Point: Associations seen in case series are highly likely to be biased and frequently do NOT hold up in more rigorous studies
Cross-Sectional Studies • Definition: A study based on a sample selected at one point or period in time Risk Factor Present Risk Factor Absent Population
Cross-Sectional Studies • Definition: A study based on a sample selected at one point or period in time Risk Factor Present Risk Factor Absent Sample Population
Cross-Sectional Studies • Definition: A study based on a sample selected at one point or period in time Disease No Disease Risk Factor Present Disease No Disease Risk Factor Absent Sample Population
Cross-Sectional Studies • If looking at a specified moment in time: point prevalence • If looking at a specified moment in time plus all new cases during the specified time period: period prevalence • If looking only at new cases during the specified time period: incidence
Cross-Sectional Studies Which cases are included in 7/1/02 point prevalence? Which cases are included in 7/1/02-6/30/03 period prevalence? Which cases are included in incidence? 1 2 3 4 5 6 7 8 9 7/1/02 6/30/03
Cross-Sectional Studies 7/1/02 point prevalence cases: 1, 2, 8 7/1/02-6/30/03 period prevalence cases: 1, 2, 3, 4, 6, 8, 9 incidence cases: 3, 4, 6, 9 1 2 3 4 5 6 7 8 9 7/1/02 6/30/03
Cross-Sectional Studies Assuming N=100, calculate the 7/1/02 point prevalence. Calculate the 7/1/02-6/30/03 period prevalence. Calculate the incidence rate. 1 2 3 4 5 6 7 8 9 7/1/02 6/30/03
Cross-Sectional Studies 7/1/02 point prevalence: 3% 7/1/02-6/30/03 period prevalence: 7% incidence rate: 4% 1 2 3 4 5 6 7 8 9 7/1/02 6/30/03
Cross-Sectional Studies • Limitations: • Exposure and outcome are assessed at the same time by the investigator (no temporality) • Sample selection is not based on exposure or outcome • Prevalence estimate is affected by duration of disease: disease with longer duration is more likely to be detected • Must consider “at risk” population only
Cross-Sectional Studies • Benefits • Easy • Cheap • Gives a “snap-shot” of exposure and outcome • Good for hypothesis generation
Analytic Studies • Involve a specific hypothesis that can be tested using a statistical model • Involve assessing exposures as a predictor of outcomes • Examples: • Observational: Cohort Studies, Case-Control Studies • Experimental: Clinical Trials
Cohort Studies • Involve following a group (cohort) of subjects over time • Usually analytic but may be descriptive • Was a treatment specifically initiated for evaluation? • No: Simple Cohort Study • Yes: Clinical Trial • Randomized • Non-Randomized
Cohort Studies • Prospective Cohort Studies • Investigator defines sample and predictor variables before any outcomes have occurred • Retrospective Cohort Studies • Investigator defines sample and collects information about predictor variables after the outcomes have occurred
Prospective Cohort Studies Is a given Risk Factor associated with a given Disease? The Present Risk Factor Present Risk Factor Absent Population
Prospective Cohort Studies Is a given Risk Factor associated with a given Disease? The Present Risk Factor Present Risk Factor Absent Sample Population
Prospective Cohort Studies Is a given Risk Factor associated with a given Disease? The Present The Future Risk Factor Present Disease No Disease Disease No Disease Risk Factor Absent Sample Population
Retrospective Cohort Studies Is a given Risk Factor associated with a given Disease? The Present Disease No Disease Disease No Disease
Retrospective Cohort Studies Is a given Risk Factor associated with a given Disease? The Past The Present Risk Factor Present Disease No Disease Disease No Disease Risk Factor Absent Sample Population
Cohort Studies in General • Strengths • Powerful strategy for directly measuring the incidence of a disease • Can examine multiple outcomes and multiple exposures • Easier to establish temporal relationship: improves inference for causality
Cohort Studies in General • Weaknesses • Attrition of the sample • Level of exposure may change over time • Inability to identify presence of confounders and effect modifiers • Susceptible to follow-up bias: there may be a difference in the exposure-disease relationship for those who follow-up and those who do not • Cost and feasibility vs. representativeness: general population sample vs. restricted cohort sample
Prospective Cohort Studies • Strengths • Allows opportunity for complete and accurate measurement of risk factors • Uniquely valuable for studying the antecedents of fatal diseases • End-point unknown: can take a long time for sufficient number of cases to develop • Observer bias • Weaknesses • Expensive and inefficient for rare diseases • Observer bias
Retrospective Cohort Studies • Strengths • Much less costly and time consuming • Observer bias • Weaknesses • Less control over the nature and quality of predictor variable data collected • Incomplete data sets • Observer bias, recall bias
Risk Ratios in Cohort Studies • The Risk Ratio (RR) is the ratio of the incidence of disease in exposed persons to the incidence of disease in non-exposed persons Cumulative Incidence in Exposed RR = Cumulative Incidence in Non-Exposed
Risk Ratios in Cohort Studies • RR calculation requires incidence data • Used in cohort and intervention studies • Not used in Case-Control Diseased - + a b + a/(a+b) RR = Exposed c/(c+d) c - d
Risk Ratios in Cohort Studies • Is a measure of the strength of association between exposure and outcome: does not imply causality…
Case-Control Studies • Compares people with disease (cases) to people without disease (controls) with respect to history of exposure • If exposure is different between cases and controls, an association exists between exposure and disease • Cases must represent the population of all cases while controls must represent the population of all non-diseased
Case-Control Studies The Present Population with Disease Population without Disease
Case-Control Studies The Present Population with Disease Risk Factor Present Risk Factor Absent Population without Disease
Case-Control Studies The Present Select Cases Population with Disease Risk Factor Present Risk Factor Absent Select Controls Population without Disease
Case-Control Studies The Present The Past D+/RF+ D+/RF- Population with Disease Risk Factor Present Risk Factor Absent Population without Disease D-/RF+ D-/RF-
Case-Control Studies • Strengths • Shorter study period is possible • Rare diseases are more easily studied • Less expensive • Multiple risk factors may be studied • Particularly useful for studying new diseases about which little is known
Case-Control Studies • Weaknesses • Choice of appropriate controls is usually very difficult (selection bias) • Cases and controls do not usually come from the same population (selection bias) • May be difficult to assess whether exposure preceded disease (recall bias) • Incidence rates cannot be calculated directly
Odds Ratios in Case-Control Studies • The Odds Ratio (OR) provides an estimate of the Risk Ratio (RR) for Case-Control studies • OR is a good estimate of the RR if the disease is “rare” (incidence <10% per year in the population) • Is a measure of the strength of association between exposure and outcome: does not imply causality…
Nested Case-Control Studies • Select disease cases from within a cohort study • Controls are selected from non-diseased cases within the same cohort, within the same time period as the cases develop • If controls are randomly selected from within the cohort (i.e.: includes diseased subjects in the case group and the control group) it is a Case-Cohort Study
A Few Words About Controls • The most difficult aspect of Case-Control Studies is selecting appropriate controls • Matching is often used to eliminate the effect of potential confounders • Technically speaking, matching reduces the variance of the OR! • Matching is difficult to do correctly and may paradoxically worsen analysis problems if done incorrectly • Impossible to match for unknown confounders
Clinical Trials • Definition: A clinical trial is a scientific experiment involving human subjects which is designed to evaluate the effects of intervention(s) against a particular disease in order to elucidate the most appropriate care for future subjects
Clinical Trials • Controlled* or Uncontrolled • Is there a concurrent comparison group? • Randomized* or Nonrandomized • Are subjects randomly allocated to the control and experimental groups? • Parallel Group or Crossover • Parallel group implies each subject receives only one of the interventions • Crossover implies each subject receives successively each of the interventions *Hence the terminology RCT
Clinical Trials: Randomization • Participants are randomly assigned to “Exposure” or “No Exposure” • Randomization refers to assigning subject to an intervention arm without regard for baseline characteristics • Goal of randomization is to equalize all other exposures that may confound or bias the association between Treatment and Outcome
Clinical Trials: Blinding • Single Blinding: examiners do not know treatment assignment • Double Blinding: examiners and subjects do not know treatment assignment • Triple Blinding: examiners, subjects, and statisticians do not know treatment assignments • Blinding is not always possible…
Clinical Trials • Advantages • Minimizes confounding and bias through randomization • Allows clear assessment of temporal association • Permits a test of causality between exposure and disease
Clinical Trials • Disadvantages • Ethical considerations of treatment or with-holding treatment • Harms (drug side effects, emotional distress) may outweigh benefits • Expensive and time-consuming • Loss to follow up • Non-adherence to group assignment • Possible early termination • Cannot always randomize an exposure
Quasi-Experimentation • This is essentially a clinical trial without randomization • Not possible to randomize: patients being enrolled in a rare disease trial at a site which does not have access to a given intervention • Not ethical to randomize: patients with cancer who have already failed the chemo in one arm of a trial cannot ethically be randomized to that arm • Uses statistical deductive processes to rule out threats to plausibility • Causal inference is less strong
Factorial Designs Intervention - X a b Y • This is essentially an attempt to evaluate multiple interventions concurrently • Given costs and inconvenience of recruiting, this is particularly appealing • Not a valid model if interaction, adds complexity, potential for polypharmacy, reviewer skepticism Intervention c - d CellIntervention a X + Y b Y + Placebo c X + Placebo d Placebo
Example 1: Design Type? • Investigators obtained lists of RNs age 25-42 in the 11 most populous U.S. states • They mailed baseline questionnaires about diet and other risk factors • Follow-up questionnaires were sent every 2 years for 20 years assessing additional risk factors and the development of disease outcomes
Example 1: Nurses’ Health Study • Prospective Cohort Study • Assembled a cohort • Assessed baseline risk factors • In the future assessed disease outcomes • Repeated Cross Sectional Study • Described changes over time in characteristics of the same study population
- More by User

Introduction to Research Design
Introduction to Research Design. Threats to Internal Validity One Group Pretest-Posttest Design. O X O. Campbell & Stanley “pre-experimental.” Wuensch “experimental.” OK design if can achieve “experimental isolation,” as in the chemistry lab.
230 views • 10 slides

Introduction to Research Design. External Validity Simple Research Designs Extraneous Variable Control. External Validity. The extent to which the results of the study can generalize beyond the specifics of the experimental situation. Would you get the same results if you
439 views • 19 slides

Introduction to the Clinical Research Centers and Clinical Research Unit
Introduction to the Clinical Research Centers and Clinical Research Unit . Agenda. Overview Administrative Issues Role of the Research Subject Advocate Nursing Policies and Procedures Bionutrition Specimen Processing Gateway Services Statistics, Information Technologies
662 views • 41 slides

Introduction to Research Design and Exploratory Research
Introduction to Research Design and Exploratory Research. Jeremy Kees, Ph.D. Formulate Problem. Stages in the Research Process. Determine Research Design. Design Data Collection Method and Forms. Design Sample and Collect Data. Analyze and Interpret the Data.
1.27k views • 30 slides

Introduction to Research Design. Threats to Internal Validity Two or More Groups Social Threats. Selection. Comparison groups are selected, or subjects selected into group, such that the groups differ on the criterion variable prior to administration of the treatment .
547 views • 21 slides

Introduction to Clinical Research Methodology
Introduction to Clinical Research Methodology. Introduction Overview of the Scientific Method Criteria Supporting the Causal Nature of an Association Outline of Available Research Designs. From The Book of Daniel, Chapter One.
1.58k views • 30 slides

Introduction to Qualitative Research Design
Introduction to Qualitative Research Design. By: S. Babar Ali. QUALITATIVE RESEARCH: GROWING IN POPULARITY IN HEALTH AND MEDICINE .
3.44k views • 37 slides

Introduction to Clinical Research and Research Questions
Introduction to Clinical Research and Research Questions . Thomas B. Newman, MD,MPH Professor of Epidemiology & Biostatistics and Pediatrics, UCSF Epi 150.03, August 2, 2009. Outline. Anatomy and Physiology of Research Research questions Examples. Anatomy of research: What it’s made of.
960 views • 46 slides

Introduction to Research Ethics: Research vs. Clinical Therapy
Introduction to Research Ethics: Research vs. Clinical Therapy. 4 October 2012 Joal Hill, JD, MPH, PhD. Objectives. Define research Distinguish research from “experimental” (clinical innovation) Distinguish research from practice Who should review/approve? And why?. Research (legal).
482 views • 28 slides

Introduction to Design of Genomic Clinical Trials
Introduction to Design of Genomic Clinical Trials. Richard Simon, D.Sc. Chief, Biometric Research Branch National Cancer Institute http://brb.nci.nih.gov. Biometric Research Branch Website brb.nci.nih.gov. Powerpoint presentations Reprints & Technical Reports BRB-ArrayTools software
436 views • 36 slides

Introduction to Clinical Research and Research Questions. Thomas B. Newman, MD,MPH Professor of Epidemiology & Biostatistics and Pediatrics, UCSF Epi 150.03, August 1, 2011. Outline. Anatomy and Physiology of Research Research questions Examples. Anatomy of research: What it’s made of.
666 views • 47 slides

INTRODUCTION TO RESEARCH DESIGN
INTRODUCTION TO RESEARCH DESIGN. Jonas Pontusson 04.11.2011. what is this about?. • common challenges that we all face in developing and executing a research project: • research questions • theory building (note: focus on causal arguments)
779 views • 53 slides

Introduction to Clinical Research
Introduction to Clinical Research. Clinical Research Practice 1. This Course Will Introduce You To:. The basics of clinical research, types of clinical trials and why clinical research is necessary.
6.35k views • 56 slides

Introduction to Clinical Research and Research Questions. Thomas B. Newman, MD,MPH Professor of Epidemiology & Biostatistics and Pediatrics, UCSF Epi 150.03, August 1, 2012. Outline. Anatomy and Physiology of Research Research questions Examples: jaundice in newborns.
834 views • 55 slides

Introduction to Research Design. Module 6 Darcy Freedman, MPH, PhD June 18, 2014. Assumptions in Scientific Research. Nature is orderly and regular To some extent, events are consistent and predictable Events or conditions have one or more causes that can be discovered
1.53k views • 52 slides

Introduction to the Clinical Research Centers and Clinical Research Unit. Agenda. Overview Administrative Issues Role of the Research Subject Advocate Nursing Policies and Procedures Bionutrition Specimen Processing Gateway Services Statistics, Information Technologies
540 views • 41 slides

INTRODUCTION TO CLINICAL RESEARCH Scientific Concepts for Clinical Research
INTRODUCTION TO CLINICAL RESEARCH Scientific Concepts for Clinical Research Karen Bandeen-Roche, Ph.D. July 12, 2010. Acknowledgements. Scott Zeger Marie Diener-West ICTR Leadership / Team. Section 1: The Science of Clinical Investigation.
659 views • 30 slides

Clinical research is a basic term which is given to all the researches which are carried out in humans and also enables the doctors to find some better ways that determines the safety and effectiveness of medications as well as devices for their use. Visit - www.dysmech.com/skilling-entrepreneurship/clinical-research
90 views • 0 slides

An Introduction to Clinical Trials: Design Issues
An Introduction to Clinical Trials: Design Issues. Edgar R Miller III PhD, MD Welch Center for Prevention, Epidemiology and Clinical Research Johns Hopkins University School of Medicine and Bloomberg School of Public Health. Type of Studies. Non-experimental (Observational) Case report
1.46k views • 139 slides

Introduction to Research Design. What Is Research Design?. The structure of research. Elements of a Design. Observations or measures Treatments or programs Groups Assignment to group Time. Observations or Measures.
183 views • 17 slides

Clinical Research Courses Introduces to Adaptive Design Clinical Trials
Adaptive clinical trials have been used exhaustively in medical development. Presently, it is being taken to drug development.. Adaptive design helps to minimize the number of patients as well as the number of trials overall. Furthermore, it can be used to get informative trial results. . Finally, there may be an increase in acceptability due to stakeholders due to the flexibility of adaptive design. Clinical research courses would tell one more about adaptive clinical trials.
97 views • 9 slides

- Customer Favourites
Clinical Research
Powerpoint Templates
Icon Bundle
Kpi Dashboard
Professional
Business Plans
Swot Analysis
Gantt Chart
Business Proposal
Marketing Plan
Project Management
Business Case
Business Model
Cyber Security
Business PPT
Digital Marketing
Digital Transformation
Human Resources
Product Management
Artificial Intelligence
Company Profile
Acknowledgement PPT
PPT Presentation
Reports Brochures
One Page Pitch
Interview PPT
All Categories

- You're currently reading page 1

Stages // require(['jquery'], function ($) { $(document).ready(function () { //removes paginator if items are less than selected items per page var paginator = $("#limiter :selected").text(); var itemsPerPage = parseInt(paginator); var itemsCount = $(".products.list.items.product-items.sli_container").children().length; if (itemsCount ? ’Stages’ here means the number of divisions or graphic elements in the slide. For example, if you want a 4 piece puzzle slide, you can search for the word ‘puzzles’ and then select 4 ‘Stages’ here. We have categorized all our content according to the number of ‘Stages’ to make it easier for you to refine the results.
Category // require(['jquery'], function ($) { $(document).ready(function () { //removes paginator if items are less than selected items per page var paginator = $("#limiter :selected").text(); var itemsperpage = parseint(paginator); var itemscount = $(".products.list.items.product-items.sli_container").children().length; if (itemscount.
- Anatomy (11)
- Business Plan Word (1)
- Business Plans (1)
- Business Slides (925)
- Circular (27)
- Cluster (4)
Got any suggestions?
We want to hear from you! Send us a message and help improve Slidesgo
Top searches
Trending searches

11 templates

66 templates

teacher appreciation

9 templates

memorial day
12 templates

pediatrician
27 templates
Clinical Trial Infographics
Free google slides theme, powerpoint template, and canva presentation template.
These Clinical Trial Infographics are simply great for medical purposes: talking about treatments, steps, and diseases is simple if you use these timelines, arrows, bars and circle charts, banners and text blocks.
Features of these infographics
- 100% editable and easy to modify
- 29 different infographics to boost your presentations
- Include icons and Flaticon’s extension for further customization
- Designed to be used in Google Slides, Canva, and Microsoft PowerPoint and Keynote
- 16:9 widescreen format suitable for all types of screens
- Include information about how to edit and customize your infographics
How can I use the infographics?
Am I free to use the templates?
How to attribute the infographics?
Attribution required If you are a free user, you must attribute Slidesgo by keeping the slide where the credits appear. How to attribute?
Related posts on our blog.

How to Add, Duplicate, Move, Delete or Hide Slides in Google Slides

How to Change Layouts in PowerPoint

How to Change the Slide Size in Google Slides
Related presentations.

Premium template
Unlock this template and gain unlimited access


IMAGES
VIDEO
COMMENTS
Clinical Research Presentation. Sep 16, 2009 • Download as PPT, PDF •. 49 likes • 28,400 views. AI-enhanced description. D. deepikashankar. Clinical research involves conducting research studies in human volunteers to answer health questions and find new treatments. It typically involves several phases from preclinical testing in animals ...
Introduction to clinical research. Clinical research involves systematic studies in human subjects to improve quality of life. Clinical trials are a form of clinical research that experimentally tests medications, devices, or biologics to evaluate safety and effectiveness. There are different phases of clinical trials, from small early phase ...
Introduction Clinical research: Is the scientific study that involve people. • Individual volunteer to participate in carefully conducted studies which ultimately uncover improved methods and knowledge on screening, diagnosis, treatment and prevention of diseases. INTRODUCTION 4. 5.
July 2013 JHU Intro to Clinical Research 31 Main Points Once Again A clinical investigation is a search for truth - how a treatment affects population, not only your sample. ... Bandeen-Roche_Scientific Concepts for Clinical Research_Lecture 1 2013.ppt [Compatibility Mode]
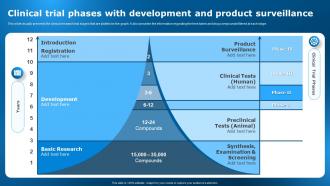
Typically, trials progress through distinct phases: Phase 1: Small-scale studies assess safety and dosage in healthy volunteers. Phase 2: Larger groups with the target condition receive the intervention, evaluating its efficacy and safety. Phase 3: Even larger trials confirm effectiveness and compare the intervention to existing treatments or ...
Presentation Transcript. This Course Will Introduce You To: • The basics of clinical research, types of clinical trials and why clinical research is necessary. • Good Clinical Practice and Good Laboratory Practice that guide the conduct of clinical research. • The importance of protecting participants and the informed consent procedures.
therapies for treating these diseases. Clinical trials represent an essential component of evidence based medical research. Clinical trials are research studies involving people (healthy volunteers or patients) that test the safety and efficacy of a new treatment. A 'treatment' in this context could mean: A medicine.
2.Possible side effects of experimental vaccines could include fever, chills, rash, aches and pains, nausea, headache, dizziness, and fatigue. Injections can cause pain, soreness, redness, and swelling on the part of the body where the vaccine shot is given. Clinical Research Activity. With your group:
Justify the number of subjects, sample size. Have a statistical plan. Measurable results rather than plausible reasoning are required to support conclusions. Retrospective. Participants are selected on the basis of presence or absence. of an event/condition of interest. Subjects can be identified from hospital records or other data sources.
INTRODUCTION TO CLINICAL RESEARCH Lecture 1: Who, What, Why and Where of Clinical Research Richard J. Barohn, M.D. Professor and Chairman Department of Neurology - A free PowerPoint PPT presentation (displayed as an HTML5 slide show) on PowerShow.com - id: 3bcf47-NDFmM
Content of this Powerpoint Presentation. Slide 1: This slide introduces Clinical Research Trial Stages. State your company name and begin. Slide 2: This slide shows the various steps involved in the clinical trial process. Slide 3: This slide indicates the key steps involved in the clinical drug investigation process.
Human Immunology Research Office • To request assistance: • Contact office to set up meeting to discuss project, assistance requested, etc • Complete research outline (template will be supplied by the office) • Contact information: Location: E1540 BST Phone: 412-624-6611 Email: [email protected].
clinical research PPT Templates Download over 6,300+ complete free templates in high resolution. Ready-Made Slide Variety of templates for each industries. ... Medical research PowerPoint Presentations Samples. Built-in custom color palette Data charts (editable via Excel) 100% vector (fully editable maps, infographic, icons)
To begin with, you need to create an outline of the topics you might present at the meeting. Your outline should follow the IMRAC format (introduction, methods, results, and conclusion). This format is chosen because your audience understands it and expects it. If you have already prepared a paper for publication, it can be a rich source of ...
Our presentation template for MS PowerPoint and Google Slides is the perfect pick to present information in a well-structured fashion. Usage This 100% editable deck is ideal for medical researchers and will help them illustrate the types, objectives, benefits, and widespread community of clinical research.
PowerPoint presentations can and should be used by all members of the clinical development team including the principal investigator, site staff, and central laboratory. ... Template 12: Drug Discovery And Clinical Research PPT Slide . This PowerPoint presentation template can help you provide an in-depth clinical development business strategy ...
Clinical and pharma icons. To make your presentation slides more appealing, this template has medical icons on it so you can use them as visual aids during the explanation of each clinical trial phase. Get your presentation custom designed by us, starting at just $10 per slide. STEP 1. UPLOAD PRESENTATION.
Mar 30, 2019. 730 likes | 898 Views. Introduction to Clinical Research Design. Lee E. Morrow, MD, MS Assistant Professor of Medicine Creighton University. Descriptive Describe incidence of outcomes over time Case Reports Case Series Registries Cross Sections. Analytic Analyze associations between predictors and outcomes. Download Presentation.
Clinical Trials - An Introduction. Oct 10, 2011 •. 449 likes • 196,945 views. Dr Purnendu Sekhar Das. This presentation will give an overview of the Clinical Research/Drug Development and licensing (US FDA) process. Please feel free to provide feedback. Read more. Health & Medicine Business. 1 of 51.
74 Best Clinical Research-Themed Templates. CrystalGraphics creates templates designed to make even average presentations look incredible. Below you'll see thumbnail sized previews of the title slides of a few of our 74 best clinical research templates for PowerPoint and Google Slides. The text you'll see in in those slides is just example ...
Download the "Endometrial Cancer Detection Breakthrough" presentation for PowerPoint or Google Slides. Treating diseases involves a lot of prior research and clinical trials. But whenever there's a new discovery, a revolutionary finding that opens the door to new treatments, vaccines or ways to prevent illnesses, it's great news. Should ...
Free Google Slides theme, PowerPoint template, and Canva presentation template. Detail the latest research in the field of medicine on these slides and let the community know! Going back to basics, this template is formal and simple. There're mostly rectangular shapes and enough room for your own content.
Diabetes clinical research ppt powerpoint presentation model layout. Slide 1 of 2. Researcher icon for performing clinical trail. Slide 1 of 2. Half yearly clinical research roadmap for human medicine. Slide 1 of 7. Drug progress model template powerpoint templates microsoft. Slide 1 of 5. Research icon science experiment test tubes.
Clinical Trial Infographics. Free Google Slides theme, PowerPoint template, and Canva presentation template. These Clinical Trial Infographics are simply great for medical purposes: talking about treatments, steps, and diseases is simple if you use these timelines, arrows, bars and circle charts, banners and text blocks.