Masks Strongly Recommended but Not Required in Maryland, Starting Immediately
Due to the downward trend in respiratory viruses in Maryland, masking is no longer required but remains strongly recommended in Johns Hopkins Medicine clinical locations in Maryland. Read more .
- Vaccines
- Masking Guidelines
- Visitor Guidelines

Understanding Clinical Trials
Clinical research: what is it.

Your doctor may have said that you are eligible for a clinical trial, or you may have seen an ad for a clinical research study. What is clinical research, and is it right for you?
Clinical research is the comprehensive study of the safety and effectiveness of the most promising advances in patient care. Clinical research is different than laboratory research. It involves people who volunteer to help us better understand medicine and health. Lab research generally does not involve people — although it helps us learn which new ideas may help people.
Every drug, device, tool, diagnostic test, technique and technology used in medicine today was once tested in volunteers who took part in clinical research studies.
At Johns Hopkins Medicine, we believe that clinical research is key to improve care for people in our community and around the world. Once you understand more about clinical research, you may appreciate why it’s important to participate — for yourself and the community.
What Are the Types of Clinical Research?
There are two main kinds of clinical research:
Observational Studies
Observational studies are studies that aim to identify and analyze patterns in medical data or in biological samples, such as tissue or blood provided by study participants.
Clinical Trials
Clinical trials, which are also called interventional studies, test the safety and effectiveness of medical interventions — such as medications, procedures and tools — in living people.
Clinical research studies need people of every age, health status, race, gender, ethnicity and cultural background to participate. This will increase the chances that scientists and clinicians will develop treatments and procedures that are likely to be safe and work well in all people. Potential volunteers are carefully screened to ensure that they meet all of the requirements for any study before they begin. Most of the reasons people are not included in studies is because of concerns about safety.
Both healthy people and those with diagnosed medical conditions can take part in clinical research. Participation is always completely voluntary, and participants can leave a study at any time for any reason.
“The only way medical advancements can be made is if people volunteer to participate in clinical research. The research participant is just as necessary as the researcher in this partnership to advance health care.” Liz Martinez, Johns Hopkins Medicine Research Participant Advocate
Types of Research Studies
Within the two main kinds of clinical research, there are many types of studies. They vary based on the study goals, participants and other factors.
Biospecimen studies
Healthy volunteer studies.
Clinical trials study the safety and effectiveness of interventions and procedures on people’s health. Interventions may include medications, radiation, foods or behaviors, such as exercise. Usually, the treatments in clinical trials are studied in a laboratory and sometimes in animals before they are studied in humans. The goal of clinical trials is to find new and better ways of preventing, diagnosing and treating disease. They are used to test:
Drugs or medicines

New types of surgery

Medical devices

New ways of using current treatments

New ways of changing health behaviors

New ways to improve quality of life for sick patients

Goals of Clinical Trials
Because every clinical trial is designed to answer one or more medical questions, different trials have different goals. Those goals include:
Treatment trials
Prevention trials, screening trials, phases of a clinical trial.
In general, a new drug needs to go through a series of four types of clinical trials. This helps researchers show that the medication is safe and effective. As a study moves through each phase, researchers learn more about a medication, including its risks and benefits.
Is the medication safe and what is the right dose? Phase one trials involve small numbers of participants, often normal volunteers.
Does the new medication work and what are the side effects? Phase two trials test the treatment or procedure on a larger number of participants. These participants usually have the condition or disease that the treatment is intended to remedy.
Is the new medication more effective than existing treatments? Phase three trials have even more people enrolled. Some may get a placebo (a substance that has no medical effect) or an already approved treatment, so that the new medication can be compared to that treatment.
Is the new medication effective and safe over the long term? Phase four happens after the treatment or procedure has been approved. Information about patients who are receiving the treatment is gathered and studied to see if any new information is seen when given to a large number of patients.
“Johns Hopkins has a comprehensive system overseeing research that is audited by the FDA and the Association for Accreditation of Human Research Protection Programs to make certain all research participants voluntarily agreed to join a study and their safety was maximized.” Gail Daumit, M.D., M.H.S., Vice Dean for Clinical Investigation, Johns Hopkins University School of Medicine
Is It Safe to Participate in Clinical Research?
There are several steps in place to protect volunteers who take part in clinical research studies. Clinical Research is regulated by the federal government. In addition, the institutional review board (IRB) and Human Subjects Research Protection Program at each study location have many safeguards built in to each study to protect the safety and privacy of participants.
Clinical researchers are required by law to follow the safety rules outlined by each study's protocol. A protocol is a detailed plan of what researchers will do in during the study.
In the U.S., every study site's IRB — which is made up of both medical experts and members of the general public — must approve all clinical research. IRB members also review plans for all clinical studies. And, they make sure that research participants are protected from as much risk as possible.
Earning Your Trust
This was not always the case. Many people of color are wary of joining clinical research because of previous poor treatment of underrepresented minorities throughout the U.S. This includes medical research performed on enslaved people without their consent, or not giving treatment to Black men who participated in the Tuskegee Study of Untreated Syphilis in the Negro Male. Since the 1970s, numerous regulations have been in place to protect the rights of study participants.
Many clinical research studies are also supervised by a data and safety monitoring committee. This is a group made up of experts in the area being studied. These biomedical professionals regularly monitor clinical studies as they progress. If they discover or suspect any problems with a study, they immediately stop the trial. In addition, Johns Hopkins Medicine’s Research Participant Advocacy Group focuses on improving the experience of people who participate in clinical research.
Clinical research participants with concerns about anything related to the study they are taking part in should contact Johns Hopkins Medicine’s IRB or our Research Participant Advocacy Group .
Learn More About Clinical Research at Johns Hopkins Medicine
For information about clinical trial opportunities at Johns Hopkins Medicine, visit our trials site.
Video Clinical Research for a Healthier Tomorrow: A Family Shares Their Story
Clinical Research for a Healthier Tomorrow: A Family Shares Their Story


Top 17 Clinical Research Organizations (CRO) in 2023
In clinical research and treatment development, clinical research organizations (CROs) are frequently a sponsor’s most important partner and ally.
Depending on the nature of the clinical trial, and your existing capabilities as a sponsor to run the trial, the CRO company of your choice will typically be responsible for facilitating most of the micro and macro processes that go into designing and running a successful clinical trial.
When contracting a CRO to help you with your trial, you are transferring over a large portion of responsibility into the hands of your clinical research partner. The CRO of your choice will have the responsibility to control a variety of factors and processes of a clinical trial, and depending on their expertise, team structures, service offerings, internal resources and many other capabilities.
Your ability to find and contract a top CRO company that is the right fit for your unique trial will be a determinant of whether or not you will be able to operate a high-quality clinical trial that meets your expected timelines, budget and delivers a top-notch patient experience.
At ClaraHealth (a patient-centric recruitment acceleration platform) , we have put together an extensive list of the top CRO companies in the US and around the world.
This is not a cro rankings list, but rather a compiled list of some of the top clinical research organizations around the world. We have highlighted their strengths and core service offerings to make it easier for you to find the right fit clinical research partner.
In addition, we’ve put together a list of 9 fundamental questions to ask the prospective clinical research organization , which will help you to save time and ensure a right fit in picking the CRO.
Formerly known as Quintiles and IMS Health, IQVIA is one of the largest CROs in the world, with a large range of service offerings to help advance clinical research.
The company was founded in North Carolina in 1982, and has since grown to over 88,000 employees in more than 100 countries.
Some clinical trial solutions offered by IQVIA include:
- Assistance with protocol design
- Design of phase 1 clinical trials
- Assessment and improvement of phase 2 and 3 clinical trials
- Site identification & selection
- Patient recruitment
- Access to global laboratories via their wholly owned subsidiary Q2 Solutions
Parexel is a global clinical research organization that was founded in 1982, and specializes in conducting clinical studies on behalf of its pharmaceutical partners in order to accelerate and ensure the drug approval process of up-and-coming potential treatments. It currently operates in more than 50 countries, and is run by more than 18,000 employees around the world.
The company has a wide range of service offerings, covering nearly every type of clinical trial service to assist sponsors in running successful clinical studies.
Some clinical trial solutions offered by Parexel include:
- Clinical trial design and development for early phase, phase 2 & 3, and late phase clinical trials
- Clinical data management
- Decentralized clinical trials
- Clinical supply chain management
- Medical writing
- Regulatory affairs consulting
- Pharmacovigilance
3. PRA Health Sciences
PRA Health Sciences is one of the largest contract research organizations in the world. Founded in 1976 under the name “Anti-Inflammatory Drug Study Group”, the company was renamed to PRA in 1982. PRA Health Sciences employees more than 17,000 people, and provides coverage to more than 90 countries.
In 2021, PRA Health Sciences was acquired by the Ireland-headquartered global CRO leader ICON, which is also reviewed in this list.
Some clinical trial solutions offered by PRA Health Sciences include:
- Decentralized Clinical Trials Platform
- Protocol Consultation & Study Design
- Onsite Support services
- Customized Solutions for Biotech (such as asset valuation, regulatory strategy, engagement and support, drug development strategy and funding solutions)
- Clinical Diagnostics
- Site Commercial Solutions
- PRA’s Laboratories for Drug Development
Headquartered in Ireland, ICON was founded in 1990 in Dublin by co-founders John Climax and Ronan Lambre. The company has since grown to be one of the largest CROs in the world. As of September 2020, the company employs more than 15,000 people in 94 locations and across 40 countries.
ICON offers clinical research services which include consulting, clinical development and commercialization across a wide range of therapeutic areas.
In 2021, ICON acquired PRA Health Sciences, which is another CRO and global leader in clinical research services.
Some clinical trial solutions offered by ICON:
- Commercial Positioning
- Early Phase
- Functional Services Provision
- Laboratories
- Language Services
- Medical Imaging
- Real World Intelligence
- Site & Patient Solutions
- COVID-19 Clinical Operations
5. Syneos Health
Formerly known as InVentiv Health Incorporated and INC Research, Syneos Health is a publicly listed and global contract research organization. The company is based in Morrisville, North Carolina, and specializes in assisting companies with late-stage clinical trials. Syneos Health currently employs more than 25,000 people, and has offices across 91 locations.
In early 2018, INC Research was acquired inVentiv Health, and the merged company was named Syneos Health.
Some clinical trial solutions offered by Syneos Health include:
- Decentralized Clinical Trials Solutions
- Bioanalytical Solutions
- Phase II-III/Phase IIIb-IIIV
- Medical Device Diagnostics
- Clinical Data Management
- Clinical Project Management
- Clinical Monitoring
- Drug Safety & Pharmacovigilance
- Site and Patient Access
6. Labcorp Drug Development (Formerly Covance)
Formerly known as Covance and renamed to Labcorp Drug Development in early 2021, this CRO is one of the largest contract research organizations in the world. The company claims to provide the world’s largest central laboratory network, and has been rated as one of the best places to work for LGBTQ+ equality by the Human Rights Campaign organization in 2018 to 2021. Currently, Labcorp employs over 70,000 people and is able to support clinical research efforts in almost 100 countries around the world.
Some clinical trial solutions offered by Labcorp Drug Development include:
- Preclinical Services
- Clinical Trials
- Clinical Trial Laboratory Services
- Post-Marketing Solutions
- Medical Devices
- Data & Technology
Also known as Pharmaceutical Product Development, PPD is a large global contract research organization headquartered in Wilmington, North Carolina. Started as a one-person consulting firm in 1985, PPD has grown to over 27,000 employees worldwide, and provides a wide range of clinical research services to pharmaceutical and biotech companies.
Some clinical trial solutions offered by PPD include:
- Clinical Development
- Early Development
- Peri- and Post-Approval
- PPD Biotech
- PPD Laboratories
- Product Development and Consulting
- Site and Patient Centric Solutions
8. Fisher Clinical Services
Part of Thermo Fisher Scientific, Fisher Clinical Services is a global clinical research organization with headquarters in Center Valley, Philadelphia.
The company has been in the business of clinical supply chain management for over 20 years, and is focused exclusively on working with the packaging and distribution requirements of clinical trials across the globe.
Some clinical trial solutions offered by Fisher Clinical Services include:
- Biologistics Management
- Cell & Gene Therapy
- Clinical Ancillary Management
- Clinical Label Services
- Clinical Trial Packaging & Storage
- Clinical Supply Optimization Services
- Cold Chain Management & Expertise
- Direct-to-Patient
- Distribution & Logistics
- Strategic Comparator Sourcing
- Public Health Research
Established in 1997 under the name Kiecana Clinical Research, KCR is a full-service contract research organization that provides a variety of services for clinical monitoring, safety & pharmacovigilance, clinical project management, quality assurance and regulatory affairs.
KCR operates globally, and has offices in North America, Western Europe, Central Europe and Eartern Europe. The company currently employs more than 700 staff.
Some clinical trial solutions offered by KCR include:
- Trial Execution
10. Medpace
Founded in 1992 and based in Cincinnati, Ohio, Medpace is a midsize clinical contract research organization. The company has operations in over 45 countries, and employs over 2,800 people. Medpace provides support services for Phase I-IV clinical trials for pharmaceutical and biotechnology companies, which include central laboratory services and regulatory services.
Some clinical trial solutions offered by Medpace include:
- Biostatistics and Data Sciences
- Clinical Trial Management
- Drug Safety and Pharmacovigilance
- Medical Writing
- Quality Assurance
- Regulatory Affairs
- Risk-Based Monitoring
- Medpace Laboratories
11. Clintec
Now in business for over 22 years, Clintec is a medium-sized global contract research organization for pharmaceutical, biotech and medical device industries, with large expertise in oncology and rare diseases.
The company provides the flexibility and agility of a smaller-sized CRO, while also having a wide global coverage that large CRO companies are known for. Clintec is based in more than 50 countries, and was acquired by the leading global CRO IQVIA in late 2018.
Some clinical trial solutions offered by Clintec include:
- Project Management
- Data Management
- Biostatistics
- Global Feasibilities
- Patient Recruitment & Retention
12. Worldwide Clinical Trials
Bringing over 30 years of experience to the clinical research market, Worldwide Clinical Trials is a leading medium-sized global contract research organization. Founded by physicians with a dedication and commitment to advancing medical research, Worldwide Clinical Trials was the first customer-centric CRO.
Currently the company has coverage in more than 60 countries, and has extensive experience in a wide range of therapeutic areas, including central nervous system, metabolic, cardiovascular, oncology, rare diseases and general medicine.
Some clinical trial solutions offered by Worldwide Clinical Trials include:
- Bioanalytical Lab
- Early Phase Development
- Clinical Phase IIB-II Clinical Trials
- Phase IIIB-IV Clinical Trials
- Trial Management Technologies
Named #1 CRO in the world for operational excellence at the 2021 CRO Leadership Awards, CTI Clinical Trial And Consulting Services is a medium-sized global contract research organization that has been serving pharmaceutical companies since 1999.
Based in Covington, Kentucky, CTI has offices around the world in more than 60 countries, with coverage in North America, Europe, Latin America, Middle-East, Africa, and Asia-Pacific regions.
Some clinical trial solutions offered by CTI include:
- Feasibility
- Regulatory Affairs Study Start-Up
- Medical Monitoring
- Safety & Pharmacovigilance
- Clinical Services
14. Wuxi AppTec
Founded in 2000 as WuXi PharmaTech in the city of Wuxi, China, Wuxi AppTec has grown from a single laboratory into a leading global contract research organization with more than 28,000 employees, including 23,000 scientists and more than 30 research & development and manufacturing sites around the world.
With offices in Asia, U.S, Europe and the Middle East, the company is able to provide coverage to more than 30 countries around the world.
Some clinical trial solutions offered by Wuxi AppTec include:
- Small Molecule Drug R&D and Manufacturing
- Cell Therapy and Gene Therapy
- Drug R&D and Medical Device Testing
- Clinical Services (Phase I-IV)
15. Advanced Clinical
Founded in 1994 and based out of Deerfield, Illinois, Advanced Clinical is a midsize and full-service CRO that helps sponsors with running clinical trials. The company employs more than 700 staff, and offers a wide variety of services across many therapeutic areas. Advanced Clinical has global representation in over 50 countries around the world.
Some clinical trial solutions offered by Advanced Clinical include:
- eTMF & Document Management
- Global Medical Services
- Quality & Validation
16. Pharm-Olam
Pharm-Olam is a leading midsize CRO with global headquarters located in Houston, Texas and its European headquarters in Bracknell, United Kingdom. The company employs more than 800 staff, and has 25 offices around the world, with a global coverage in more than 60 countries.
The company has therapeutic expertise in 5 areas, including Rare & Orphan Disease, Infectious Disease & Vaccine, Oncology-Hematology, Allergy and Autoimmune.
Some clinical trial solutions offered by Pharm-Olam include:
- Study Feasibility
- Site Activation
- Patient Recruitment
- Medical Affairs
- Compliance & Training
- Clinical Monitoring & Operations
17. Clinipace
Founded in 2003 and based out of Morrisville, North Carolina, Clinipace is a global midsize full-service CRO with a focus on solution customization for clinical trials. The company has a large global coverage in more than 50 countries, and has offices in North America, South America, Europe and Asia-Pacific regions.
Clinipace’s therapeutic focus areas include Oncology, Nephrology and Urology, Rare Disease, Gastroenterology and Women’s Health. The company also has complete therapeutic expertise in Infectious Disease & Vaccines, Cardiology, CNS, Immunology, and Respiratory.
Some clinical trial solutions offered by Clinipace include:
- Clinical Analytics
- Clinical Technology and Ecosystem
- Functional Service Partnership (FSP)
- Regulatory & Strategic Product Development
9 Fundamental Questions To Ask A Top CRO Company Before Signing The Contract
1. which services does the cro provide.
CROs offload a lot of operational tasks from trial sponsors, which can touch any component of clinical trial operations. From formulating an overall study strategy and implementing technologies to support the operational processes of the trial, to picking and identifying sites, and supporting patients during the trial, the range of clinical services offered by a CRO tends to be vast and inclusive of all the typical services and support you will require for running a successful clinical trial.
However, not all CROs are the same in their service offerings, or are able to offer the same depth of capability within a seemingly same clinical trial support process. For this reason it is important to understand exactly which kind of clinical services and support you are looking to receive from the prospective CRO when running your clinical trial.
While services such as clinical monitoring and clinical trial management are offered by the majority of CROs, the specific needs of each trial are unique, and for this reason it is important to first identify what will be the unique services your trial requires. Completing this internal analysis first will help you to understand the extent to which a potential CRO partner will be able to provide all of these services.
Some CROs specialize in specific clinical trial functions which the company may label as a “core services”, in which case this is a sign the company will have more expertise, experience, and will be set up in a way to maximize their capabilities in providing support for these services compared to other services that the CRO offers.
For example, a CRO may include patient recruitment as part of its “core services”, which implies that they are highly skilled in and have the necessary infrastructure to design and implement a high-quality patient recruitment strategy.
Clara Health CRO Support Services: At Clara Health our specialty services include technology-augmented digital and patient advocacy recruitment, as well as patient support via our signature patient recruitment platform, which we use to upgrade clinical trials and deliver results sponsors look for in their recruitment and retention campaigns.
At Clara, we work alongside CROs to supplement and support clinical trials with modern and personalized capabilities that CROs do not typically have the bandwidth, corporate structure or infrastructure to support.
If you would like to learn more about exactly how our platform can upgrade your unique trial, feel free to book a Free 30 Minute Consultation Session Here with one of our in-house experts.
2. What Related Experience Does The CRO Have?
It is helpful to ask the prospective CRO company if they have any relevant experience in running clinical trials that would be an asset in designing and running your study. Previous experience in a related therapeutic area or in running a trial with a similar design allows CROs to have a deeper understanding into potential opportunities and challenges, increasing the likelihood of your clinical study being successful.
For example, if a sponsor is planning to run a trial in oncology, for the purpose of site identification and selection it would be valuable to partner with a CRO vendor that has expertise in this area, as they likely already have a good understanding of which sites will lead to optimal results.
However, it is also important to consider all factors when selecting a CRO vendor and not to rely on therapeutic experience as the sole qualifier for whether or not a potential CRO is a fit for your trial. While previous experience is beneficial, some sponsors close themselves off from working with vendors that have not worked in their therapeutic area, which significantly limits options when choosing a CRO partner that is truly a good fit for their clinical study.
This can impact the end result of your clinical study, as sponsors that are not successful in choosing a CRO vendor that is the right overall fit may face difficulties if the needs of their clinical study aren’t being properly met.
Clara Health: We have worked to provide support for clinical trials across a wide range of therapeutic areas and trial designs. Our specialty is filling in the gaps that CROs traditionally did not have to think about, which include digital patient recruitment, patient advocacy recruitment, and technology-augmented patient support.
Additionally, we are constantly building our proprietary data and running tests in a variety of therapeutic areas. These research efforts allow us to have a detailed understanding of the expected level of difficulty when recruiting particular patient populations, as well as allow us to predict with accuracy which segments of the targeted population will be likely to qualify in a particular study.
3. What Are The Communication Workflows & Expectations For Performing And Delivering Contracted Services?
It is important that you clarify what the expectations for communication will be between your prospective CRO vendor and your internal teams, as you will most likely be working with the CRO of your choice for the entire duration of your clinical trial.
There are a vast variety of factors and success determinants for a clinical trial, which are continuously undergoing change as the study unfolds. For this reason, it is recommended that you work with a CRO that is proactive in their communication, so that you are kept up to date with information about important changes as your clinical trial progresses.
A vendor that is proactive rather than reactive in their communication and approach to dealing with arising issues is one of the most important qualities in CRO. Challenging situations will naturally arise, and the promptness with which they are taken care of will significantly impact your clinical trial’s degree of success. Therefore, seeking a vendor that is able to match the standard of communication that you as a sponsor would like to experience throughout the duration of your partnership is one of the most critical steps in determining which CRO is the right fit for your clinical trial.
We’ve included a few additional questions pertaining to the communication structure and reporting expectations that you can ask a prospective CRO vendor to determine the degree of fit in this particular category:
Communication Expectations:
- If we were to move forward with you, which of your team members will be our main point of contact?
- How available will you be outside of the scheduled meetings to address any of our concerns or additional requests?
- What will be the frequency at which update meetings will be conducted, and who will be present at those meetings?
- Which clinical study processes will be reported on, and what will be the workflow for how we will receive this information?
- What will be the cadence at which we will receive progress reports?
- Would we be able to access metrics electronically via an interactive dashboard, or will you send us formal reports?
Clara Health: At Clara Health, we directly interact and actively work with several key stakeholders involved in running a clinical trial, which includes sponsors, CROs, sites, and patients. This unique position allows us to have a centralized perspective which helps us to see all the moving parts of a clinical trial at the same time, which helps to identify issues and relay this vital information and insight back to the sponsor (or other appropriate stakeholders) in the shortest time possible.
The ability to access this perspective allows us to gather the most accurate, complete, and up-to-date information about how the clinical trial is unfolding, and quickly becomes very valuable to sponsors for their clinical trial.
As an example, we may receive feedback from patients about having an unsatisfactory experience with a particular study site. We are able to aggregate and analyze this information, and relay our findings back to the sponsor and the study site to improve the experience for other patients.
4. What Is The CRO’s Client Satisfaction Record?
It is a good practice to request information or metrics from the prospective CRO vendor that can point to the degree of satisfaction of their past clients. Prior to signing the contract, vendors will naturally do their best to uplift their image and future value to you during their sales conversations with you and your team. It can be tricky to get an objective understanding of what the partnership experience will actually entail, especially when there are multiple vendors fighting for your commitment.
We recommend that you ask the prospective vendor to provide success metrics regarding areas of clinical trial operations that are going to be important for your trial.
For example, you may be interested in learning about the vendor’s relationship to finances, in which case it will be useful to ask them about situations in which they went over the planned budget, and investigate into the reasons behind that. Alternatively you may be concerned about potential delays in timelines, in which case it would be helpful to learn about metrics regarding the CRO’s ability to meet timeline expectations.
You may also request to talk to the prospective CRO’s past clients, which will help you to gain insight into what the relationship was like and give you the opportunity to examine if the way in which the particular CRO manages its relationships and performs its services meets the expectations that you would have for your potential relationship and for your clinical trial.
Clara Health: At Clara Health, our relationships with our partners and with our patients are most important to us. In the unique position where we fit in the clinical trial process, we have the opportunity to directly co-create the clinical trial patient experience with a variety of stakeholders, including sponsors, sites, CROs, and patients.
Our company’s values and culture have been directed and developed to be such that the client and patient experience is at the top of priority for all of our internal teams, and we work to provide the best quality of care to all stakeholders.
We have many testimonials from every type of partner we’ve worked with which we can happily share with you.
5. How Do You Adapt When Encountering Challenges With Running A Clinical Trial?
It is inevitable that challenges and unforeseen changes will arise throughout the operational clinical trial process, and for this reason it is important to work with a CRO vendor that can provide you with evidence of their flexibility and ability to adapt to sudden changes.
The ideal CRO partner is one that is highly consultative throughout the entire process, and has an ability and the initiative to deal with challenges at their seed stage, prior to them turning into major obstacles for the success of your trial.
CROs naturally have a large reach, and there are a lot of different clinical trial mechanisms and processes that are under their control. They are able to monitor and respond to what is going on in every key link in the chain of the clinical trial operation.
It is reasonable to expect this level of oversight from a CRO, and additional questions that can help you gain insight into this include:
- What are some examples where the CRO was effective at monitoring the health of clinical trials they’ve helped operate in the past?
- How quickly does the CRO respond to challenges or opportunities for improving the clinical trial experience?
- How well does the CRO gather & process information from study sites, study teams, patients & the sponsor, and what are their typical data analysis workflows?
It is also recommended to speak to the prospective CROs past clients to help you gain insight into how well they respond and adapt to the naturally arising challenges in clinical trials.
Clara Health: While CROs do have a large reach within the clinical trial, no CRO has complete visibility into every clinical process. They are not typically set up to support full visibility, which can manifest as a potential threat to your clinical trial as it unfolds. This is especially true for parts of the clinical trial processes that CROs naturally do not specialize and often subcontract, such as clinical trial recruitment.
At Clara, we are in a unique position in relation to other key partners involved in operating the clinical trial. We are in direct and frequent contact with patients, CROs, study sites, study teams, and the sponsor, and have a very deep understanding of the patient pipeline. This allows us the unique ability to go very deep into specific parts of the recruitment chain and investigate what is working and what is not working.
In addition, Clara functions as a resource for all partners in the clinical trial. For example, we work directly with site teams to ensure that they have access to a 3rd party that they can relay their needs to and receive fast support in case there is anything they require that can improve the patient recruitment process.
6. Which Parts Of Operating The Clinical Trial Will You Be Outsourcing?
Since there are so many processes and mechanisms that go into operating a clinical trial, CROs will always outsource some parts of running and managing the study. While you can expect that the prospective CRO will subcontract some of the work, it is important to find out which exact parts the clinical study will be outsourced.
There are certain basic and key clinical processes (such as site selection) that CROs almost always help with, and if you find that these parts of your trial are going to be subcontracted to another company, it is recommended to find out why the CROs operations are set up this way and how this would impact the service you will receive.
Ultimately what matters to you as a partner and client is that the quality of service and care that you will receive will be up to standard, and meet what was promised and what you are expecting. While this trust is important after you have signed the contract, it is recommended that prior to entering into such a significant commitment that you have evidence and the conviction that the CRO of your choice is truly the right fit and will deliver the quality of service that was being discussed.
Since it is impossible to predict exactly what the quality of this relationship and services performed will actually be like in practice, it is recommended that you understand the details of what will be done for your trial and how. Investigating how the CRO outsources and subcontracts services for a clinical trial will help you to gain necessary insight that you would need to make the correct vendor selection decision.
Clara Health: At Clara, we maximize the effectiveness of the digital component across the entire digital & recruitment spectrum, which is added on top of the existing capabilities of the CROs and other vendors involved in operating your clinical trial. In addition, we offer services that augment the CROs efforts, which has the potential to significantly improve the patient experience, operations flows, recruitment and retention performance, which is so important in ensuring the success of a clinical trial.
For example, if a CRO wants to have a great site relationship, we are able to come in as a third party on behalf of the sponsor and CRO and act as a resource and additional support for sites.
In another example, If a sponsor wants to have great relationships with the patient community, Clara is able to come in on behalf of the sponsor and develop these relationships while being perceived more neutrally by the patient community.
7. Do You Have Experience Running International Trials?
If you are planning on operating an international clinical trial, it is recommended to work with a CRO that has extensive experience in this area. While many CROs will offer near-global coverage, the level of experience with specific geographic locations can significantly vary from one vendor to another.
It is important to work with a CRO that has experience running clinical trials in the specific countries and regions you are planning to conduct your research in. Being compliant with the local rules and regulations for clinical testing is a very complex process that requires existing understanding and familiarity in order to ensure logistical smoothness and to mitigate legal risks. In operating a clinical trial, there are a multitude of clinical services and processes, which can greatly vary across the many regions in which you can conduct clinical testing.
A CRO that is lacking experience in operating international trials or operating in particular regions where you plan on conducting research may not be able to meet your desired quality and agility expectations, and therefore may not be the right fit for your international clinical trial.
Clara Health: In the past, we have provided international patient recruitment and digitally-augmented trial support services for clinical trials in the EU, Canada, UK, Australia and South America.
Clara Health is fully compliant to operate international studies everywhere in the world, with the exception of Russia and China.
8. What Is Your Relationship With Patients?
Patient-centric approach to designing and operating a clinical trial is becoming more and more crucial in the clinical research space. The ability of a sponsor and their CRO partner to understand the needs and characteristics of their target patient community is a significant determinant of whether or not the study will be a success.
A sponsor that has close and authentic relationships with the patient community tends to have a deeper understanding of how to create the best clinical trial experience that will attract patients and keep their interest throughout the clinical trial.
In addition, strong relationships with patients allow sponsors and CROs to forecast recruitment and patient retention pipeline with much higher accuracy. This ability is critical for ensuring the success of the trial and mitigating the risk of low enrollment. After an understanding of the patient population is acquired, sponsors gain the necessary insight to design a clinical trial that is not only favorable to their research results, but is also practical and will result in the enrollment numbers they are looking for.
While many CROs have already recognized the importance of patient-centricity and evolved the ways in which they design and operate clinical trials, other CROs have not yet made such a pivot in their values. It is important to understand the degree of importance the prospective CRO places on creating a favorable patient experience, and what kind of infrastructure the company has to support it.
At Clara, we recommend choosing a CRO partner that is adapting to the patient-centric model which is becoming more and more important for running a successful clinical trial.
Clara Health: Since early stages of our development, we’ve had a dedicated patient advocacy team that has been integral in shaping our company’s vision and operations. We have built our entire platform and recruitment infrastructure around creating the best experience for patients. Our teams, corporate values, service offerings and company infrastructure all work in the service of the patient.
In addition, over the many years of being in business we have heavily invested in building authentic patient community relationships that span across a variety of therapeutic areas. This has given us a unique ability to receive feedback directly from patients that is genuine and authentic around marketing materials, strategy for patient recruitment, and other services that we build for specific trials.
This ability to build partnerships with the patient community in an authentic way gives us a very unique ability to engage with the patient community on behalf of a pharmaceutical company, allowing our sponsor & CRO partners the opportunity to start conversations with patients through our in-house patient advocacy team.
If you would like to learn how Clara can help you to build a strong & authentic relationship with your target patient community, get in touch with us and we’d be happy to share our capabilities and previous results with you as they relate to your current or upcoming clinical trial.
9. How Is The CRO Going To Utilize Patient Input For Developing The Trial?
In the initial stages of clinical trial design, sponsors often determine the ideal patient profiles that would help them to drive the most favorable research outcomes for their study. While it is important for the success of your trial to determine who your ideal patients are, very often these projections do not match up with what is viable in practice.
At Clara, we often encounter study protocols that are not set up realistically for successful recruitment to be possible.
Common mistakes that are made when determining trial eligibility criteria and trial design include:
- Overestimating the interest in the clinical trial from the target patient population
- A lack of patient focus in the trial design
- A lack of convenience for patients in their participation
- Complicated and/or inefficient study experience flows
- Crafting the eligibility criteria around the patient population that is most likely to lead to favorable study outcomes, without conducting sufficient research to more accurately estimate the recruitment and retention difficulty of the group for a particular study
It is natural for there to be a “push & pull” between the research ideal and the real world practicality. It is important to determine the correct balance between these two sides for your trial, as going too far in either direction will decrease the chance of your clinical study’s success.
The nature of the industry as it is right now is such that there is excess research idealization and not enough emphasis on patient centricity. This distorted orientation has resulted in many clinical trials being unsuccessful, negatively impacting sponsors, patients and the entire clinical trials industry.
The ideal CRO partner should help you make sure that your protocol design sets your study up for success. The CRO should be able to help you determine the proper balance between the research ideal and the real world practicality, and back up their findings with sufficient research and patient data that can project your trial being a success.
Clara Health: When formulating a recruitment and retention plan for our clients, we begin with conducting thorough research into the target trial patient population. This allows us to get a clear understanding of which recruitment channels will yield the best results and what kind of marketing materials will resonate with the prospective study participants.
To ensure accuracy and real-world applicability of our research, we consult and collaborate with our internal patient advocacy and patient support teams, as well as with our clients and patients representing the target trial patient profiles. We then tie our findings back with any existing proprietary data that we have in connection with the therapeutic area or the prospective target patient group.
Our unique position within the clinical recruitment chain gives us the presence and deep-rooted access needed to effectively tap into any of the three patient traffic sources: digital recruitment, offline recruitment, or patient advocacy recruitment.
Once a recruitment campaign has gone live, we constantly monitor, analyze and optimize our performance to make sure that the processes we have in place are as efficient as possible and drive the greatest results. In addition, we have the capability to layer in any traditional advertising (such as billboard ads) if requested by the study sponsor.

2024: An Endeavor into the Enigma - Uncovering the Substance of CROs
Reflecting on the complexities inherent in the acronyms CRO (Clinical Research Organization) and CRA (Clinical Research Associate) indeed reveals a multifaceted landscape within the realm of clinical research. For those deeply entrenched in the nuances of clinical inquiry, understanding the operational mechanisms of CROs emerges as a pivotal journey delving into the uncharted territories of healthcare advancement. Whether venturing into the realms of clinical research for the first time or seeking a renewed perspective, delving into the semantic intricacies of CROs and unlocking their transformative potential in healthcare becomes an essential exploration, propelling organizations towards unparalleled developmental heights.
The Essence of a CRO:
Clinical Research Organizations, abbreviated as CROs, epitomize entities entrusted with the management of clinical trials on behalf of pharmaceutical and biotechnological giants. Their mandates encompass the selection of patients, procurement of informed consent, aggregation of data, and the persistent oversight of safety protocols. CROs are broadly categorized into two overarching classifications: full-service CROs, offering a spectrum of services essential for clinical trials, and specialized CROs, focusing their expertise on specific areas such as patient enrollment or data management.
The Era of CRO Dominance:
A seismic shift characterizes the ascendancy of CROs in recent chronicles. The tally of clinical trials orchestrated by CROs surged from 9,000 in 2006 to a staggering 26,000 in 2016. This remarkable ascent fundamentally attributes itself to the accessible expertise and efficiency that CROs demonstrate, all at a cost-effective rate compared to traditional avenues of research. Moreover, the integration of CROs into the operational framework enables pharmaceutical and biotechnological entities to sharpen their core competencies while concurrently mitigating the looming specter of clinical setbacks.
Navigating the Intersection of Costs and Benefits:
However, amidst the commendable merits inherent in CRO engagement, a caveat looms ominously. Skepticism burgeons regarding the equivalence of expertise, with concerns revolving around the potential discrepancy in data quality vis-à-vis conventional research entities. A lingering unease surfaces, questioning the parity of commitment to patient safety between CROs and their traditional counterparts. Navigating through the intricate labyrinth of CRO utilization necessitates a judicious assessment, a meticulous weighing of the benefits against the potential pitfalls in the steadfast pursuit of advancing the uncharted territories of healthcare improvement.
Clinical investigate organizations, recognized as contract inquire about substances, expand outsourced administrations to the pharmaceutical and biotechnology divisions. These organizations organize clinical trials for inventive pharmaceuticals and restorative approaches. The engagement of CROs can be started either by the pharmaceutical companies themselves or by the scholastic teach initiating the trials. The CRO industry has been encountering exponential development in later a long time. In 2024, the worldwide CRO administrations showcase gathered an evaluated $25.1 billion in 2024, with projections showing a compound yearly development rate of 7.5% over the resulting five a long time. Clinical inquire about administrations constituted the most considerable share of this advertise, measuring to $19.8 billion in 2024. Multiple variables contribute to the thriving of the CRO industry. At first, there has been a essential surge in the around the world conduct of clinical trials. Hence, a move from minute, casual trials to sweeping, formal trials overseen by commercial CROs has been watched. Thirdly, there is a recognizable uptick in the outsourcing slant among pharmaceutical and biotechnology enterprises. The essential on-screen characters in the CRO division include major multinational substances such as QuintilesIMS, Covance, and Parexel. At the same time, an developing unexpected of littler CROs specializes in specific restorative spaces or particular trial categories, extending the industry's scope and diversity. So, what absolutely constitutes the modus operandi of a CRO in 2024? In pith, a CRO outfits a comprehensive cluster of administrations including consider conceptualization, ponder execution, watchfulness, information administration, and expository interests. Furthermore, they habitually apportion administrative backing administrations like creating entries and conducting administrative audits. The vital advantage of locks in a CRO dwells in its capacity to speed up the medicate advancement direction. By subcontracting select or whole clinical trial endeavors, pharmaceutical enterprises in 2024 stand to abridge consumptions and rescue time. CROs, blessed with capability in executing clinical trials, adeptly encourage effective and compelling trial executions, subsequently progressing the field of pharmaceutical investigate.
What are the benefits of Clinical Research Organizations?
There are many benefits to working with a Clinical Research Organization (CRO). Perhaps the most important benefit is that CROs can help you to move your clinical research program forward faster and more efficiently. They have the experience and expertise to help you manage all aspects of your study, from start to finish. This can save you a lot of time and money, as CROs know how to navigate the complex world of clinical research.
Another major benefit of using a CRO is that they can help you to reduce risk. By using their experience and knowledge, CROs can help you to select the right study participants, design the study correctly, and implement best practices throughout the study. This can help to minimize any potential risks involved in conducting your study.
Finally, CROs can also help sponsors save money. They have established relationships with vendors and suppliers, so they can often get better deals on study materials and services than you would be able to negotiate on your own. This can result in significant cost savings for your clinical research program.
What are the challenges of Clinical Research Organizations?
The burgeoning domain of Clinical Investigate Organizations (CROs) experiences a heap of challenges in the year 2024. The quick development of this industry applies colossal weight on CROs to speed up and streamline considers, frequently coming about in hurried and substandard work. Concurrently, the basic to reduce costs holds on, coupled with the heightening complexities of administrative orders. Besides, hooking with furious competition from financially beneficial countries includes an extra layer of complexity, given their capacity to give cost-effective services.
These multifaceted challenges have accelerated various misgivings with respect to the quality and security guidelines inside the CRO space. A chronicled occasion in 2013 seen the FDA issuing a cautionary note to a CRO locked in in sedate company considers. The reprobation highlighted different deficiencies in the CRO's endeavors, including insufficient consider plan, subpar information administration, and a shortage of satisfactory observing protocols.
In a more later occurrence, circa 2013, another FDA caution resulted, provoked by a quiet casualty amid a clinical trial conducted by a CRO. The administrative body perceived insufficiencies in the CRO's oversight, showing in an insulant checked ponder and the disappointment to report grave antagonistic events.
These outlines only scratch the surface of the horde security worries that have tormented the CRO scene all through the a long time. The need of exacting quality control measures and inadequately observation can possibly result in extreme wounds or indeed fatalities for people taking an interest in clinical trials. Tending to these security concerns orders a concerted exertion. The FDA has spread direction archives and administrative goals pointed at improving the caliber of CRO endeavors. At the same time, pharmaceutical substances must work out increased acumen in selecting capable CROs whereas giving persevering oversight. Planned patients are empowered to attempt exhaustive investigate some time recently enlisting in clinical trials and ought to posture related inquiries with respect to the trial's procedural conduct.
Innovative Approaches to Better Clinical Research Organizations in the Year 2024
In the scene of clinical investigate organizations (CROs) as of 2024, there is a essential require for upgrade to guarantee the viability of clinical trials. The necessarily part of CROs in the arranging, execution, and documentation of clinical inquire about thinks about requires a sharp center on advancement strategies.
To move the advancement of CROs in 2024, one vital road includes the outsourcing of clinical inquire about preparing. By entrusting this pivotal viewpoint to outside substances, CROs can ensure comprehensive preparing in best hones, cultivating the conveyance of trials of the most elevated quality. This approach too serves to keep CROs side by side of cutting-edge advances and winning patterns inside the field, contributing to their ceaseless refinement.
Another essential road for the upgrade of CROs lies in the enlargement of straightforwardness. This means the basic of making operational subtle elements of CROs freely open. Such straightforwardness envelops illustrating the strategies utilized by CROs, uncovering the results of their considers, and uncovering the charge structures they execute. By grasping straightforwardness, the moral operation of CROs is shielded, guaranteeing impartial treatment of patients.
In the broader setting, the change of CROs in 2024 is a multifaceted endeavor. Outsourcing clinical inquire about preparing and cultivating straightforwardness rise as linchpin procedures to accomplish this overarching objective.
While CROs irrefutably constitute a significant aspect of the clinical inquire about prepare, their adequacy pivots on tending to characteristic challenges. Moved forward communication and straightforwardness with supports, destinations, and patients are basic for CRO viability. Emphasizing quality over amount is vital to maintain a sterling notoriety inside the industry. If diving more profound into the complexities of clinical investigate organizations interests you, consider selecting in our clinical inquire about certification course for an smart investigation of their working.
A Comprehensive Guide to PMP Certification
The meaning of triage: a guide for the clinical research professional.
Clinical Research Organization (CRO): How they work?
- Author Company: Clariwell Global Services
- Author Name: Zain Malik
- Author Email: [email protected]
- Author Telephone: +917709551477
- Author Website: https://www.clariwell.in/best-clinical-research-courses-in-pune-with-100-percent-job-guarantee
A clinical research organization (CRO) is often called a contract research organization (CRO). CRO is a service organization that provides support to pharmaceutical and biotechnology industries in the form of outsourced clinical research courses and services for both medical devices and drugs.
The main functions required to conduct clinical researches, which are usually departments of the clinical research organization, are:
- The function of medical operations: People working in this area are given clinical research training from various clinical research institutes. This sector includes medically qualified people who are capable of constructing and designing clinical studies, clinical trials, and their protocols to provide medical-related input throughout the study. This includes roles like medical monitor, clinical research physician, advisors, etc.
- Clinical operations: This is the most organized and the largest team in any contract research organization. It consists of medical research associates, clinical trial assistants, managers, etc. This is the team of the CRO, which selects the clinical trial sites and locations, assists the studies, monitors the studies, etc.
- Data Management: This team helps in managing and designing various tools and databases. This is done in order to collect data from the trial. They help in ensuring that the data collected from the trials is accurate and is suitable for analysis. To keep track and organized, this team uses sophisticated software and modern technologies.
- Biostatistics: This team plays a major role in the outcome of the trial. They help in analyzing the study data and figuring out whether the study has yielded positive results or negative results while adhering strictly to all the protocols. They also generate statistical tables, figures, and graphs with their interpretations which are all then passed down to the medical writers to compile into reports.
- Writing team: these writers compile all the data into medical reports that can easily be understood by the public as well. They also manage the study protocols, study analysis reports, promotional material, etc.
- Quality assurance: The audits to ensure that the guidelines, standard operations, and regulation procedures are followed throughout are assessed by these trained professionals. This department is entirely dedicated to ensuring the quality of the product.
- Human Resources: Every clinical research organization has a dedicated human resource organization that is responsible for hiring trained professionals for various job roles and positions within the organization. They have to maintain a certain level of talent in the talent pool available.
- IT team: This is generally considered to be the support staff of the Clinical research organizations. They take care of all the IT-related needs like maintenance and purchases of laptops, desktops, telephones, software, etc.
- The finance team: All the monetary controls and the finance part, including the administration of the whole CRO, is managed by this department of the CRO.
- Training and Development: This is a dedicated department to training and developing in the CRO. It focuses on the professional development of all its employees and conducts routine training to make sure that their staffs remain up to date with all the developments in technology. It acts as a clinical research institute that provides the best clinical research courses and clinical research training .
You are leaving PharmiWeb.com

Disclaimer: You are now leaving PharmiWeb.com website and are going to a website that is not operated by us. We are not responsible for the content or availability of linked sites.
ABOUT THIRD PARTY LINKS ON OUR SITE
PharmiWeb.com offers links to other third party websites that may be of interest to our website visitors. The links provided in our website are provided solely for your convenience and may assist you in locating other useful information on the Internet. When you click on these links you will leave the PharmiWeb.com website and will be redirected to another site. These sites are not under the control of PharmiWeb.com.
PharmiWeb.com is not responsible for the content of linked third party websites. We are not an agent for these third parties nor do we endorse or guarantee their products. We make no representation or warranty regarding the accuracy of the information contained in the linked sites. We suggest that you always verify the information obtained from linked websites before acting upon this information.
Also, please be aware that the security and privacy policies on these sites may be different than PharmiWeb.com policies, so please read third party privacy and security policies closely.
If you have any questions or concerns about the products and services offered on linked third party websites, please contact the third party directly.
- Clinical Trials
About Clinical Studies
Research: it's all about patients.
Mayo's mission is about the patient, the patient comes first. So the mission and research here, is to advance how we can best help the patient, how to make sure the patient comes first in care. So in many ways, it's a cycle. It can start with as simple as an idea, worked on in a laboratory, brought to the patient bedside, and if everything goes right, and let's say it's helpful or beneficial, then brought on as a standard approach. And I think that is one of the unique characteristics of Mayo's approach to research, that patient-centeredness. That really helps to put it in its own spotlight.
At Mayo Clinic, the needs of the patient come first. Part of this commitment involves conducting medical research with the goal of helping patients live longer, healthier lives.
Through clinical studies, which involve people who volunteer to participate in them, researchers can better understand how to diagnose, treat and prevent diseases or conditions.
Types of clinical studies
- Observational study. A type of study in which people are observed or certain outcomes are measured. No attempt is made by the researcher to affect the outcome — for example, no treatment is given by the researcher.
- Clinical trial (interventional study). During clinical trials, researchers learn if a new test or treatment works and is safe. Treatments studied in clinical trials might be new drugs or new combinations of drugs, new surgical procedures or devices, or new ways to use existing treatments. Find out more about the five phases of non-cancer clinical trials on ClinicalTrials.gov or the National Cancer Institute phases of cancer trials .
- Medical records research. Medical records research involves the use of information collected from medical records. By studying the medical records of large groups of people over long periods of time, researchers can see how diseases progress and which treatments and surgeries work best. Find out more about Minnesota research authorization .
Clinical studies may differ from standard medical care
A health care provider diagnoses and treats existing illnesses or conditions based on current clinical practice guidelines and available, approved treatments.
But researchers are constantly looking for new and better ways to prevent and treat disease. In their laboratories, they explore ideas and test hypotheses through discovery science. Some of these ideas move into formal clinical trials.
During clinical studies, researchers formally and scientifically gather new knowledge and possibly translate these findings into improved patient care.
Before clinical trials begin
This video demonstrates how discovery science works, what happens in the research lab before clinical studies begin, and how a discovery is transformed into a potential therapy ready to be tested in trials with human participants:
How clinical trials work
Trace the clinical trial journey from a discovery research idea to a viable translatable treatment for patients:
See a glossary of terms related to clinical studies, clinical trials and medical research on ClinicalTrials.gov.
Watch a video about clinical studies to help you prepare to participate.
Let's Talk About Clinical Research
Narrator: This presentation is a brief introduction to the terms, purposes, benefits and risks of clinical research.
If you have questions about the content of this program, talk with your health care provider.
What is clinical research?
Clinical research is a process to find new and better ways to understand, detect, control and treat health conditions. The scientific method is used to find answers to difficult health-related questions.
Ways to participate
There are many ways to participate in clinical research at Mayo Clinic. Three common ways are by volunteering to be in a study, by giving permission to have your medical record reviewed for research purposes, and by allowing your blood or tissue samples to be studied.
Types of clinical research
There are many types of clinical research:
- Prevention studies look at ways to stop diseases from occurring or from recurring after successful treatment.
- Screening studies compare detection methods for common conditions.
- Diagnostic studies test methods for early identification of disease in those with symptoms.
- Treatment studies test new combinations of drugs and new approaches to surgery, radiation therapy and complementary medicine.
- The role of inheritance or genetic studies may be independent or part of other research.
- Quality of life studies explore ways to manage symptoms of chronic illness or side effects of treatment.
- Medical records studies review information from large groups of people.
Clinical research volunteers
Participants in clinical research volunteer to take part. Participants may be healthy, at high risk for developing a disease, or already diagnosed with a disease or illness. When a study is offered, individuals may choose whether or not to participate. If they choose to participate, they may leave the study at any time.
Research terms
You will hear many terms describing clinical research. These include research study, experiment, medical research and clinical trial.
Clinical trial
A clinical trial is research to answer specific questions about new therapies or new ways of using known treatments. Clinical trials take place in phases. For a treatment to become standard, it usually goes through two or three clinical trial phases. The early phases look at treatment safety. Later phases continue to look at safety and also determine the effectiveness of the treatment.
Phase I clinical trial
A small number of people participate in a phase I clinical trial. The goals are to determine safe dosages and methods of treatment delivery. This may be the first time the drug or intervention is used with people.
Phase II clinical trial
Phase II clinical trials have more participants. The goals are to evaluate the effectiveness of the treatment and to monitor side effects. Side effects are monitored in all the phases, but this is a special focus of phase II.
Phase III clinical trial
Phase III clinical trials have the largest number of participants and may take place in multiple health care centers. The goal of a phase III clinical trial is to compare the new treatment to the standard treatment. Sometimes the standard treatment is no treatment.
Phase IV clinical trial
A phase IV clinical trial may be conducted after U.S. Food and Drug Administration approval. The goal is to further assess the long-term safety and effectiveness of a therapy. Smaller numbers of participants may be enrolled if the disease is rare. Larger numbers will be enrolled for common diseases, such as diabetes or heart disease.
Clinical research sponsors
Mayo Clinic funds clinical research at facilities in Rochester, Minnesota; Jacksonville, Florida; and Arizona, and in the Mayo Clinic Health System. Clinical research is conducted in partnership with other medical centers throughout the world. Other sponsors of research at Mayo Clinic include the National Institutes of Health, device or pharmaceutical companies, foundations and organizations.
Clinical research at Mayo Clinic
Dr. Hugh Smith, former chair of Mayo Clinic Board of Governors, stated, "Our commitment to research is based on our knowledge that medicine must be constantly moving forward, that we need to continue our efforts to better understand disease and bring the latest medical knowledge to our practice and to our patients."
This fits with the term "translational research," meaning what is learned in the laboratory goes quickly to the patient's bedside and what is learned at the bedside is taken back to the laboratory.
Ethics and safety of clinical research
All clinical research conducted at Mayo Clinic is reviewed and approved by Mayo's Institutional Review Board. Multiple specialized committees and colleagues may also provide review of the research. Federal rules help ensure that clinical research is conducted in a safe and ethical manner.
Institutional review board
An institutional review board (IRB) reviews all clinical research proposals. The goal is to protect the welfare and safety of human subjects. The IRB continues its review as research is conducted.
Consent process
Participants sign a consent form to ensure that they understand key facts about a study. Such facts include that participation is voluntary and they may withdraw at any time. The consent form is an informational document, not a contract.
Study activities
Staff from the study team describe the research activities during the consent process. The research may include X-rays, blood tests, counseling or medications.
Study design
During the consent process, you may hear different phrases related to study design. Randomized means you will be assigned to a group by chance, much like a flip of a coin. In a single-blinded study, participants do not know which treatment they are receiving. In a double-blinded study, neither the participant nor the research team knows which treatment is being administered.
Some studies use an inactive substance called a placebo.
Multisite studies allow individuals from many different locations or health care centers to participate.
Remuneration
If the consent form states remuneration is provided, you will be paid for your time and participation in the study.
Some studies may involve additional cost. To address costs in a study, carefully review the consent form and discuss questions with the research team and your insurance company. Medicare may cover routine care costs that are part of clinical trials. Medicaid programs in some states may also provide routine care cost coverage, as well.
When considering participation in a research study, carefully look at the benefits and risks. Benefits may include earlier access to new clinical approaches and regular attention from a research team. Research participation often helps others in the future.
Risks/inconveniences
Risks may include side effects. The research treatment may be no better than the standard treatment. More visits, if required in the study, may be inconvenient.
Weigh your risks and benefits
Consider your situation as you weigh the risks and benefits of participation prior to enrolling and during the study. You may stop participation in the study at any time.
Ask questions
Stay informed while participating in research:
- Write down questions you want answered.
- If you do not understand, say so.
- If you have concerns, speak up.
Website resources are available. The first website lists clinical research at Mayo Clinic. The second website, provided by the National Institutes of Health, lists studies occurring in the United States and throughout the world.
Additional information about clinical research may be found at the Mayo Clinic Barbara Woodward Lips Patient Education Center and the Stephen and Barbara Slaggie Family Cancer Education Center.
Clinical studies questions
- Phone: 800-664-4542 (toll-free)
- Contact form
Cancer-related clinical studies questions
- Phone: 855-776-0015 (toll-free)
International patient clinical studies questions
- Phone: 507-284-8884
- Email: [email protected]
Clinical Studies in Depth
Learning all you can about clinical studies helps you prepare to participate.
- Institutional Review Board
The Institutional Review Board protects the rights, privacy, and welfare of participants in research programs conducted by Mayo Clinic and its associated faculty, professional staff, and students.
More about research at Mayo Clinic
- Research Faculty
- Laboratories
- Core Facilities
- Centers & Programs
- Departments & Divisions
- Postdoctoral Fellowships
- Training Grant Programs
- Publications
Mayo Clinic Footer
- Request Appointment
- About Mayo Clinic
- About This Site
Legal Conditions and Terms
- Terms and Conditions
- Privacy Policy
- Notice of Privacy Practices
- Notice of Nondiscrimination
- Manage Cookies
Advertising
Mayo Clinic is a nonprofit organization and proceeds from Web advertising help support our mission. Mayo Clinic does not endorse any of the third party products and services advertised.
- Advertising and sponsorship policy
- Advertising and sponsorship opportunities
Reprint Permissions
A single copy of these materials may be reprinted for noncommercial personal use only. "Mayo," "Mayo Clinic," "MayoClinic.org," "Mayo Clinic Healthy Living," and the triple-shield Mayo Clinic logo are trademarks of Mayo Foundation for Medical Education and Research.
- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español
You are here
Nih clinical research trials and you, what is clinical research.
Clinical research occurs in many formats and can involve anyone. Learn how you can participate and contribute to medical advances.
This page last reviewed on September 29, 2016
Connect with Us
- More Social Media from NIH
Therapeutic Expertise
Participate

Explore end-to-end solutions throughout development — from portfolio optimization and regulatory strategy, to Phase I-IV clinical trials, market access planning, and more.
- Portfolio management and asset valuation
- Early development and innovation
- Integrated clinical development
- Approval and access
- Value substantiation lifecycle management
HOW WE DO IT
- Delivery models
- Operational excellence
- Building patient insights into assets, profile and claims
- Portfolio optimization
- Asset valuation and indication prioritization
- Early evidence review
- Model-based drug development
- Integrated development strategy and planning
- Phase I Clinical Trials
- Proof of Concept Studies: Phase IB-IIA
- Patient Engagement Strategy and Enrollment Solutions
- Patient Inclusion
- Site Alliance Network and KOL Engagement
- Protocol Optimization
- Regulatory Strategy
- Market Access Strategy and Delivery
- Biomarker and Genomic Medicine Strategy
- Clinical Trial Supply & Logistics
- Medical Communications
- Phase IIB-IV Clinical Trials
- Real World Evidence
- Protocol-Driven, Customized Site Solution Strategy
- Regulatory Strategy, Submissions, Compliance, and Outsourcing
- Clinical Development Technology Optimization
- Global Regulatory Submissions and Outsourcing
- Compliance and Risk Management
- Real-World Evidence, Market Access Strategy and Planning
- Regulatory Compliance, Drug Safety and Pharmacovigilance
- Lifecycle Optimization
- Gain an advantage through FSP
- Leveraging AI and digital in clinical development

Parexel Biotech provides the end-to-end capabilities you’ll need to succeed. We're fast, flexible, and dedicated to impacting patient lives.

Utilize our expertise across therapeutic areas, combining innovative trial designs, leading clinical and regulatory expertise, global reach, and a passion for changing patient lives.
- Neuroscience
- General medicine
- Infectious disease & vaccines
- Inflammation & immunology
Cross-Therapeutic Expertise
- Cell & gene therapies
- Rare diseases

Our experts help you stay at the forefront of the industry - and ahead of change.
New Medicines, Novel Insights
- Advancing precision oncology
- Advancing rare disease drug development
- Accelerating development of cell and gene therapies
- Achieving patient-guided drug development
Discussions on Diversity
- Chapter 1 Bridging the Gap
- Chapter 2 Beyond the Binary
The Regulatory Navigator
- BIOSECURE Act: Implications for US-based drug developers
- EU-CTR transitions: To meet the deadline, act now
- Potency assurance for CGT products: FDA's new draft guidance
Latest Report

New Medicines, Novel Insights: Advancing precision oncology

Thinking about joining a clinical trial? Learn the drug development process, what it’s like to participate, how to find a trial, and answers to frequently asked questions.
INTERESTED IN PARTICIPATING?
- Healthy Volunteers
- Patient Volunteers
- Patient Advocacy Groups
HEAR FROM REAL PATIENTS
- Patient Stories
TRIAL SITES

Want to collaborate with us to offer clinical trials at your site? We would welcome the opportunity to discuss.
We are one of the largest CROs in the world, speeding life-changing medicine to market by engaging patients With Heart ™. Learn about who we are, what we do, and what we believe.
- Leadership team
- Global reach
Our DE&I strategy
- Compliance, tax & privacy
- Meet us at an event
- Board of Directors
- Our ESG strategy
- Compliance & Ethics
- Tax Strategy
- Privacy Policy
- Supplier Data Privacy Requirements
- Terms of Use
- French Gender Pay Data
- UK Gender Pay Data
- UK Modern Slavery Act Statement
- EU-U.S. Data Privacy Framework (EU-U.S. DPF)
- Supplier Diversity Policy
What can we help you find today?
At Parexel, we speed your life-changing medicines to patients
Parexel is among the world’s largest clinical research organizations (CROs), providing the full range of Phase I to IV clinical development services to help lifesaving treatments reach patients faster. Leveraging the breadth of our clinical, regulatory, and therapeutic expertise, our team of more than 21,000 global professionals works in partnership with biopharmaceutical leaders, emerging innovators, and sites to design and deliver clinical trials with patients in mind, increasing access and participation to make clinical research a care option for anyone, anywhere.
Our depth of industry knowledge and strong track record gained over the past 40 years is moving the industry forward and advancing clinical research in healthcare’s most complex areas, while our innovation ecosystem offers quality solutions to make every phase of the clinical trial process more efficient. Our top-notch people, insight, and focus on operational excellence allow us to work every day to treat patients with dignity and continuously learn from their experiences, so every trial makes a difference.
This approach continues to earn us recognition industrywide, with Parexel being named “Best Contract Research Organization” in November 2023 by an independent panel for Citeline, “Top CRO to Work With” by investigative sites worldwide in the 2023 WCG CenterWatch Global Site Relationship Benchmark Survey and recipient of the 2023 Society for Clinical Research Sites (SCRS) Eagle Award for advancing the clinical research profession through strong site partnerships.
Read the transcript
It starts with people. They may come from anywhere. But they’re driven by a common goal: Their search for a brighter future.
They inspire us to learn from their lives across language and race, ability, ethnicity, and community.
So we design clinical trials that give them a voice, honor their sacrifice, and treat them as equals.
We are more than 21,000 professionals working with passion and perseverance to open doors. To lead change. And find new ways to work together.
We make participation easier and partnerships more productive to get to results faster and treatments sooner.
So every patient’s step forward brings them one step closer to a cure, to care, to hope.
Delivered with heart.

Leading the industry in trial inclusivity and accessibility
Within clinical research, patient diversity is critical. Inclusive studies move us closer to healthcare equity and more accurately reflect real-world populations — resulting in a far greater understanding of how the treatment will affect the people who need it. Our approach to designing more diverse, accessible trials is leading the industry forward.
Our family of brands
Parexel Academy
Your trusted partner in learning. Parexel Academy is committed to building and enhancing capabilities within the clinical research workforce so that, together, we make a positive impact on the lives of patients around the world.

Health Advances
As a trusted strategic advisor to healthcare and life science executives, Health Advances catalyzes your success with insight, innovation, and integrity.
The Medical Affairs Company
The industry’s leading provider of comprehensive outsourced medical affairs solutions.
Patients First
Empowerment and accountability.
Each of us, no matter what we do at Parexel, contributes to the development of a therapy that ultimately will benefit a patient. We take our work personally, we do it with empathy, and we're committed to making a difference.
From the smallest detail to the largest, we take quality seriously. We focus on the details while never losing sight of the big picture to drive the best possible outcome.
In our quest for innovation, we recognize and uphold the importance of all people, from our employees to our clients and the patients we all serve.
We follow our hearts, we do the right thing, and we have the courage to own the outcome.
Awards & Recognition
2024 ViE Best Contract Research Organization
Parexel was selected by a distinguished industry advisory board for its range of services in niche and core therapeutic areas, methods of performance improvement, attention to and quality of relationships with clients, reaching milestones and final outcomes, and building and maintaining existing and long-term partnerships. The annual ViE Awards, organized by Terrapin, celebrate the industry’s most outstanding achievements and showcase excellence in the global vaccine industry.
2023 Scrip Award
Parexel was named “Best Contract Research Organization” in the Full-Service Provider category at the 19th Annual Scrip Awards. The annual Scrip Awards, organized by Citeline, are designed to celebrate and recognize the very best innovations and achievements in global biopharma.
2023 HBA ACE Award
Parexel was recognized with the 2023 Healthcare Businesswomen’s Association’s (HBA) “Advancement. Commitment. Engagement (ACE) Award,” which recognizes companies for their creation and implementation of initiatives that deliver impactful outcomes designed to close the gender gap in the healthcare ecosystem. Parexel was one of two companies chosen as a result of the strong outcomes from its business initiative “Priority: Advancing Women in Leadership.”
2023 Eagle Award by Society of Clinical Research Sites
The SCRS Eagle Award recognizes the sponsor and CRO committed to outstanding leadership, professionalism, integrity, passion and dedication to advancing the clinical research profession through strong site partnerships. Recipients of the Eagle Award are selected based on votes cast from the global site community.
Ecovadis 2023
For the second year in a row, Parexel has received a “silver” rating in the 2023 EcoVadis Sustainability Rating Program. Ecovadis is the world’s largest and most trusted provider of business sustainability ratings. A silver rating is awarded to organizations with a structured and proactive sustainability approach with strong reporting on KPIs and actions.
2023 CRO Leadership Awards
Parexel has been recognized with CRO Leadership Awards for the 12 th consecutive year across all five categories – Capabilities, Compatibility, Expertise, Quality, and Reliability – for exceeding customer expectations in different customer segments. Winning CROs are chosen based on feedback from sponsor companies that they have worked on an outsourced project within the previous 18 months.
2023 WCG CenterWatch Global Site Relationship Benchmark Survey
Parexel was ranked as the “Top CRO to Work With” by investigative sites worldwide in the 2023 WCG CenterWatch Global Site Relationship Benchmark Survey for the second straight time. Among 34 CROs, Parexel received the highest average rating across all 26 performance attributes evaluated in the survey. In addition to receiving the highest average rating across all attributes, Parexel ranked highest on four out of the five attributes considered the most important to investigative sites and was selected as the CRO that investigative sites were most willing to recommend to a colleague.
2022 Catalyst Awards
Parexel was named a 2022 Catalyst Award winner by the global nonprofit organization Catalyst. Parexel was recognized for its Leveraging Gender Partnership to Advance Women in Leadership initiative that has evolved the company culture to one where women have the right resources and training to succeed.
FlexJobs’ Top 100 Companies to Watch for Remote Jobs in 2022
Parexel was recognized as a company to watch on FlexJobs' 10th annual list of the Top 100 Companies to Watch for Remote Jobs in 2023. Parexel is one of only five companies to have made the FlexJobs' list each year since its inception in 2014.
Human Rights Campaign Corporate Equality Index 2022
Parexel is proud to be featured on the Human Rights Campaign’s 2022 Corporate Equality Index, the premier survey benchmarking tool on how corporations across the US and beyond are adopting equitable workplace policies, practices and benefits for LGBTQ+ employees
Management Team
Meet our management team
Our ESG Strategy
Explore our environmental, social, and governance (ESG) report
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.15(2); 2023 Feb
- PMC10023071

Clinical Trials and Clinical Research: A Comprehensive Review
Venkataramana kandi.
1 Clinical Microbiology, Prathima Institute of Medical Sciences, Karimnagar, IND
Sabitha Vadakedath
2 Biochemistry, Prathima Institute of Medical Sciences, Karimnagar, IND
Clinical research is an alternative terminology used to describe medical research. Clinical research involves people, and it is generally carried out to evaluate the efficacy of a therapeutic drug, a medical/surgical procedure, or a device as a part of treatment and patient management. Moreover, any research that evaluates the aspects of a disease like the symptoms, risk factors, and pathophysiology, among others may be termed clinical research. However, clinical trials are those studies that assess the potential of a therapeutic drug/device in the management, control, and prevention of disease. In view of the increasing incidences of both communicable and non-communicable diseases, and especially after the effects that Coronavirus Disease-19 (COVID-19) had on public health worldwide, the emphasis on clinical research assumes extremely essential. The knowledge of clinical research will facilitate the discovery of drugs, devices, and vaccines, thereby improving preparedness during public health emergencies. Therefore, in this review, we comprehensively describe the critical elements of clinical research that include clinical trial phases, types, and designs of clinical trials, operations of trial, audit, and management, and ethical concerns.
Introduction and background
A clinical trial is a systematic process that is intended to find out the safety and efficacy of a drug/device in treating/preventing/diagnosing a disease or a medical condition [ 1 , 2 ]. Clinical trial includes various phases that include phase 0 (micro-dosing studies), phase 1, phase 2, phase 3, and phase 4 [ 3 ]. Phase 0 and phase 2 are called exploratory trial phases, phase 1 is termed the non-therapeutic phase, phase 3 is known as the therapeutic confirmatory phase, and phase 4 is called the post-approval or the post-marketing surveillance phase. Phase 0, also called the micro-dosing phase, was previously done in animals but now it is carried out in human volunteers to understand the dose tolerability (pharmacokinetics) before being administered as a part of the phase 1 trial among healthy individuals. The details of the clinical trial phases are shown in Table Table1 1 .
This table has been created by the authors.
MTD: maximum tolerated dose; SAD: single ascending dose; MAD: multiple ascending doses; NDA: new drug application; FDA: food and drug administration
Clinical research design has two major types that include non-interventional/observational and interventional/experimental studies. The non-interventional studies may have a comparator group (analytical studies like case-control and cohort studies), or without it (descriptive study). The experimental studies may be either randomized or non-randomized. Clinical trial designs are of several types that include parallel design, crossover design, factorial design, randomized withdrawal approach, adaptive design, superiority design, and non-inferiority design. The advantages and disadvantages of clinical trial designs are depicted in Table Table2 2 .
There are different types of clinical trials that include those which are conducted for treatment, prevention, early detection/screening, and diagnosis. These studies address the activities of an investigational drug on a disease and its outcomes [ 4 ]. They assess whether the drug is able to prevent the disease/condition, the ability of a device to detect/screen the disease, and the efficacy of a medical test to diagnose the disease/condition. The pictorial representation of a disease diagnosis, treatment, and prevention is depicted in Figure Figure1 1 .

This figure has been created by the authors.
The clinical trial designs could be improvised to make sure that the study's validity is maintained/retained. The adaptive designs facilitate researchers to improvise during the clinical trial without interfering with the integrity and validity of the results. Moreover, it allows flexibility during the conduction of trials and the collection of data. Despite these advantages, adaptive designs have not been universally accepted among clinical researchers. This could be attributed to the low familiarity of such designs in the research community. The adaptive designs have been applied during various phases of clinical trials and for different clinical conditions [ 5 , 6 ]. The adaptive designs applied during different phases are depicted in Figure Figure2 2 .

The Bayesian adaptive trial design has gained popularity, especially during the Coronavirus Disease-19 (COVID-19) pandemic. Such designs could operate under a single master protocol. It operates as a platform trial wherein multiple treatments can be tested on different patient groups suffering from disease [ 7 ].
In this review, we comprehensively discuss the essential elements of clinical research that include the principles of clinical research, planning clinical trials, practical aspects of clinical trial operations, essentials of clinical trial applications, monitoring, and audit, clinical trial data analysis, regulatory audits, and project management, clinical trial operations at the investigation site, the essentials of clinical trial experiments involving epidemiological, and genetic studies, and ethical considerations in clinical research/trials.
A clinical trial involves the study of the effect of an investigational drug/any other intervention in a defined population/participant. The clinical research includes a treatment group and a placebo wherein each group is evaluated for the efficacy of the intervention (improved/not improved) [ 8 ].
Clinical trials are broadly classified into controlled and uncontrolled trials. The uncontrolled trials are potentially biased, and the results of such research are not considered as equally as the controlled studies. Randomized controlled trials (RCTs) are considered the most effective clinical trials wherein the bias is minimized, and the results are considered reliable. There are different types of randomizations and each one has clearly defined functions as elaborated in Table Table3 3 .
Principles of clinical trial/research
Clinical trials or clinical research are conducted to improve the understanding of the unknown, test a hypothesis, and perform public health-related research [ 2 , 3 ]. This is majorly carried out by collecting the data and analyzing it to derive conclusions. There are various types of clinical trials that are majorly grouped as analytical, observational, and experimental research. Clinical research can also be classified into non-directed data capture, directed data capture, and drug trials. Clinical research could be prospective or retrospective. It may also be a case-control study or a cohort study. Clinical trials may be initiated to find treatment, prevent, observe, and diagnose a disease or a medical condition.
Among the various types of clinical research, observational research using a cross-sectional study design is the most frequently performed clinical research. This type of research is undertaken to analyze the presence or absence of a disease/condition, potential risk factors, and prevalence and incidence rates in a defined population. Clinical trials may be therapeutic or non-therapeutic type depending on the type of intervention. The therapeutic type of clinical trial uses a drug that may be beneficial to the patient. Whereas in a non-therapeutic clinical trial, the participant does not benefit from the drug. The non-therapeutic trials provide additional knowledge of the drug for future improvements. Different terminologies of clinical trials are delineated in Table Table4 4 .
In view of the increased cost of the drug discovery process, developing, and low-income countries depend on the production of generic drugs. The generic drugs are similar in composition to the patented/branded drug. Once the patent period is expired generic drugs can be manufactured which have a similar quality, strength, and safety as the patented drug [ 9 ]. The regulatory requirements and the drug production process are almost the same for the branded and the generic drug according to the Food and Drug Administration (FDA), United States of America (USA).
The bioequivalence (BE) studies review the absorption, distribution, metabolism, and excretion (ADME) of the generic drug. These studies compare the concentration of the drug at the desired location in the human body, called the peak concentration of the drug (Cmax). The extent of absorption of the drug is measured using the area under the receiver operating characteristic curve (AUC), wherein the generic drug is supposed to demonstrate similar ADME activities as the branded drug. The BE studies may be undertaken in vitro (fasting, non-fasting, sprinkled fasting) or in vivo studies (clinical, bioanalytical, and statistical) [ 9 ].
Planning clinical trial/research
The clinical trial process involves protocol development, designing a case record/report form (CRF), and functioning of institutional review boards (IRBs). It also includes data management and the monitoring of clinical trial site activities. The CRF is the most significant document in a clinical study. It contains the information collected by the investigator about each subject participating in a clinical study/trial. According to the International Council for Harmonisation (ICH), the CRF can be printed, optical, or an electronic document that is used to record the safety and efficacy of the pharmaceutical drug/product in the test subjects. This information is intended for the sponsor who initiates the clinical study [ 10 ].
The CRF is designed as per the protocol and later it is thoroughly reviewed for its correctness (appropriate and structured questions) and finalized. The CRF then proceeds toward the print taking the language of the participating subjects into consideration. Once the CRF is printed, it is distributed to the investigation sites where it is filled with the details of the participating subjects by the investigator/nurse/subject/guardian of the subject/technician/consultant/monitors/pharmacist/pharmacokinetics/contract house staff. The filled CRFs are checked for their completeness and transported to the sponsor [ 11 ].
Effective planning and implementation of a clinical study/trial will influence its success. The clinical study majorly includes the collection and distribution of the trial data, which is done by the clinical data management section. The project manager is crucial to effectively plan, organize, and use the best processes to control and monitor the clinical study [ 10 , 11 ].
The clinical study is conducted by a sponsor or a clinical research organization (CRO). A perfect protocol, time limits, and regulatory requirements assume significance while planning a clinical trial. What, when, how, and who are clearly planned before the initiation of a study trial. Regular review of the project using the bar and Gantt charts, and maintaining the timelines assume increased significance for success with the product (study report, statistical report, database) [ 10 , 11 ].
The steps critical to planning a clinical trial include the idea, review of the available literature, identifying a problem, formulating the hypothesis, writing a synopsis, identifying the investigators, writing a protocol, finding a source of funding, designing a patient consent form, forming ethics boards, identifying an organization, preparing manuals for procedures, quality assurance, investigator training and initiation of the trial by recruiting the participants [ 10 ].
The two most important points to consider before the initiation of the clinical trial include whether there is a need for a clinical trial, if there is a need, then one must make sure that the study design and methodology are strong for the results to be reliable to the people [ 11 ].
For clinical research to envisage high-quality results, the study design, implementation of the study, quality assurance in data collection, and alleviation of bias and confounding factors must be robust [ 12 ]. Another important aspect of conducting a clinical trial is improved management of various elements of clinical research that include human and financial resources. The role of a trial manager to make a successful clinical trial was previously reported. The trial manager could play a key role in planning, coordinating, and successfully executing the trial. Some qualities of a trial manager include better communication and motivation, leadership, and strategic, tactical, and operational skills [ 13 ].
Practical aspects of a clinical trial operations
There are different types of clinical research. Research in the development of a novel drug could be initiated by nationally funded research, industry-sponsored research, and clinical research initiated by individuals/investigators. According to the documents 21 code of federal regulations (CFR) 312.3 and ICH E-6 Good Clinical Practice (GCP) 1.54, an investigator is an individual who initiates and conducts clinical research [ 14 ]. The investigator plan, design, conduct, monitor, manage data, compile reports, and supervise research-related regulatory and ethical issues. To manage a successful clinical trial project, it is essential for an investigator to give the letter of intent, write a proposal, set a timeline, develop a protocol and related documents like the case record forms, define the budget, and identify the funding sources.
Other major steps of clinical research include the approval of IRBs, conduction and supervision of the research, data review, and analysis. Successful clinical research includes various essential elements like a letter of intent which is the evidence that supports the interest of the researcher to conduct drug research, timeline, funding source, supplier, and participant characters.
Quality assurance, according to the ICH and GCP guidelines, is necessary to be implemented during clinical research to generate quality and accurate data. Each element of the clinical research must have been carried out according to the standard operating procedure (SOP), which is written/determined before the initiation of the study and during the preparation of the protocol [ 15 ].
The audit team (quality assurance group) is instrumental in determining the authenticity of the clinical research. The audit, according to the ICH and GCP, is an independent and external team that examines the process (recording the CRF, analysis of data, and interpretation of data) of clinical research. The quality assurance personnel are adequately trained, become trainers if needed, should be good communicators, and must handle any kind of situation. The audits can be at the investigator sites evaluating the CRF data, the protocol, and the personnel involved in clinical research (source data verification, monitors) [ 16 ].
Clinical trial operations are governed by legal and regulatory requirements, based on GCPs, and the application of science, technology, and interpersonal skills [ 17 ]. Clinical trial operations are complex, time and resource-specific that requires extensive planning and coordination, especially for the research which is conducted at multiple trial centers [ 18 ].
Recruiting the clinical trial participants/subjects is the most significant aspect of clinical trial operations. Previous research had noted that most clinical trials do not meet the participant numbers as decided in the protocol. Therefore, it is important to identify the potential barriers to patient recruitment [ 19 ].
Most clinical trials demand huge costs, increased timelines, and resources. Randomized clinical trial studies from Switzerland were analyzed for their costs which revealed approximately 72000 USD for a clinical trial to be completed. This study emphasized the need for increased transparency with respect to the costs associated with the clinical trial and improved collaboration between collaborators and stakeholders [ 20 ].
Clinical trial applications, monitoring, and audit
Among the most significant aspects of a clinical trial is the audit. An audit is a systematic process of evaluating the clinical trial operations at the site. The audit ensures that the clinical trial process is conducted according to the protocol, and predefined quality system procedures, following GCP guidelines, and according to the requirements of regulatory authorities [ 21 ].
The auditors are supposed to be independent and work without the involvement of the sponsors, CROs, or personnel at the trial site. The auditors ensure that the trial is conducted by designated professionally qualified, adequately trained personnel, with predefined responsibilities. The auditors also ensure the validity of the investigational drug, and the composition, and functioning of institutional review/ethics committees. The availability and correctness of the documents like the investigational broacher, informed consent forms, CRFs, approval letters of the regulatory authorities, and accreditation of the trial labs/sites [ 21 ].
The data management systems, the data collection software, data backup, recovery, and contingency plans, alternative data recording methods, security of the data, personnel training in data entry, and the statistical methods used to analyze the results of the trial are other important responsibilities of the auditor [ 21 , 22 ].
According to the ICH-GCP Sec 1.29 guidelines the inspection may be described as an act by the regulatory authorities to conduct an official review of the clinical trial-related documents, personnel (sponsor, investigator), and the trial site [ 21 , 22 ]. The summary report of the observations of the inspectors is performed using various forms as listed in Table Table5 5 .
FDA: Food and Drug Administration; IND: investigational new drug; NDA: new drug application; IRB: institutional review board; CFR: code of federal regulations
Because protecting data integrity, the rights, safety, and well-being of the study participants are more significant while conducting a clinical trial, regular monitoring and audit of the process appear crucial. Also, the quality of the clinical trial greatly depends on the approach of the trial personnel which includes the sponsors and investigators [ 21 ].
The responsibility of monitoring lies in different hands, and it depends on the clinical trial site. When the trial is initiated by a pharmaceutical industry, the responsibility of trial monitoring depends on the company or the sponsor, and when the trial is conducted by an academic organization, the responsibility lies with the principal investigator [ 21 ].
An audit is a process conducted by an independent body to ensure the quality of the study. Basically, an audit is a quality assurance process that determines if a study is carried out by following the SPOs, in compliance with the GCPs recommended by regulatory bodies like the ICH, FDA, and other local bodies [ 21 ].
An audit is performed to review all the available documents related to the IRB approval, investigational drug, and the documents related to the patient care/case record forms. Other documents that are audited include the protocol (date, sign, treatment, compliance), informed consent form, treatment response/outcome, toxic response/adverse event recording, and the accuracy of data entry [ 22 ].
Clinical trial data analysis, regulatory audits, and project management
The essential elements of clinical trial management systems (CDMS) include the management of the study, the site, staff, subject, contracts, data, and document management, patient diary integration, medical coding, monitoring, adverse event reporting, supplier management, lab data, external interfaces, and randomization. The CDMS involves setting a defined start and finishing time, defining study objectives, setting enrolment and termination criteria, commenting, and managing the study design [ 23 ].
Among the various key application areas of clinical trial systems, the data analysis assumes increased significance. The clinical trial data collected at the site in the form of case record form is stored in the CDMS ensuring the errors with respect to the double data entry are minimized.
Clinical trial data management uses medical coding, which uses terminologies with respect to the medications and adverse events/serious adverse events that need to be entered into the CDMS. The project undertaken to conduct the clinical trial must be predetermined with timelines and milestones. Timelines are usually set for the preparation of protocol, designing the CRF, planning the project, identifying the first subject, and timelines for recording the patient’s data for the first visit.
The timelines also are set for the last subject to be recruited in the study, the CRF of the last subject, and the locked period after the last subject entry. The planning of the project also includes the modes of collection of the data, the methods of the transport of the CRFs, patient diaries, and records of severe adverse events, to the central data management sites (fax, scan, courier, etc.) [ 24 ].
The preparation of SOPs and the type and timing of the quality control (QC) procedures are also included in the project planning before the start of a clinical study. Review (budget, resources, quality of process, assessment), measure (turnaround times, training issues), and control (CRF collection and delivery, incentives, revising the process) are the three important aspects of the implementation of a clinical research project.
In view of the increasing complexity related to the conduct of clinical trials, it is important to perform a clinical quality assurance (CQA) audit. The CQA audit process consists of a detailed plan for conducting audits, points of improvement, generating meaningful audit results, verifying SOP, and regulatory compliance, and promoting improvement in clinical trial research [ 25 ]. All the components of a CQA audit are delineated in Table Table6 6 .
CRF: case report form; CSR: clinical study report; IC: informed consent; PV: pharmacovigilance; SAE: serious adverse event
Clinical trial operations at the investigator's site
The selection of an investigation site is important before starting a clinical trial. It is essential that the individuals recruited for the study meet the inclusion criteria of the trial, and the investigator's and patient's willingness to accept the protocol design and the timelines set by the regulatory authorities including the IRBs.
Before conducting clinical research, it is important for an investigator to agree to the terms and conditions of the agreement and maintain the confidentiality of the protocol. Evaluation of the protocol for the feasibility of its practices with respect to the resources, infrastructure, qualified and trained personnel available, availability of the study subjects, and benefit to the institution and the investigator is done by the sponsor during the site selection visit.
The standards of a clinical research trial are ensured by the Council for International Organizations of Medical Sciences (CIOMS), National Bioethics Advisory Commission (NBAC), United Nations Programme on Human Immunodeficiency Virus/Acquired Immunodeficiency Syndrome (HIV/AIDS) (UNAIDS), and World Medical Association (WMA) [ 26 ].
Recommendations for conducting clinical research based on the WMA support the slogan that says, “The health of my patient will be my first consideration.” According to the International Code of Medical Ethics (ICME), no human should be physically or mentally harmed during the clinical trial, and the study should be conducted in the best interest of the person [ 26 ].
Basic principles recommended by the Helsinki declaration include the conduction of clinical research only after the prior proof of the safety of the drug in animal and lab experiments. The clinical trials must be performed by scientifically, and medically qualified and well-trained personnel. Also, it is important to analyze the benefit of research over harm to the participants before initiating the drug trials.
The doctors may prescribe a drug to alleviate the suffering of the patient, save the patient from death, and gain additional knowledge of the drug only after obtaining informed consent. Under the equipoise principle, the investigators must be able to justify the treatment provided as a part of the clinical trial, wherein the patient in the placebo arm may be harmed due to the unavailability of the therapeutic/trial drug.
Clinical trial operations greatly depend on the environmental conditions and geographical attributes of the trial site. It may influence the costs and targets defined by the project before the initiation. It was noted that one-fourth of the clinical trial project proposals/applications submit critical data on the investigational drug from outside the country. Also, it was noted that almost 35% of delays in clinical trials owing to patient recruitment with one-third of studies enrolling only 5% of the participants [ 27 ].
It was suggested that clinical trial feasibility assessment in a defined geographical region may be undertaken for improved chances of success. Points to be considered under the feasibility assessment program include if the disease under the study is related to the population of the geographical region, appropriateness of the study design, patient, and comparator group, visit intervals, potential regulatory and ethical challenges, and commitments of the study partners, CROs in respective countries (multi-centric studies) [ 27 ].
Feasibility assessments may be undertaken at the program level (ethics, regulatory, and medical preparedness), study level (clinical, regulatory, technical, and operational aspects), and at the investigation site (investigational drug, competency of personnel, participant recruitment, and retention, quality systems, and infrastructural aspects) [ 27 ].
Clinical trials: true experiments
In accordance with the revised schedule "Y" of the Drugs and Cosmetics Act (DCA) (2005), a drug trial may be defined as a systematic study of a novel drug component. The clinical trials aim to evaluate the pharmacodynamic, and pharmacokinetic properties including ADME, efficacy, and safety of new drugs.
According to the drug and cosmetic rules (DCR), 1945, a new chemical entity (NCE) may be defined as a novel drug approved for a disease/condition, in a specified route, and at a particular dosage. It also may be a new drug combination, of previously approved drugs.
A clinical trial may be performed in three types; one that is done to find the efficacy of an NCE, a comparison study of two drugs against a medical condition, and the clinical research of approved drugs on a disease/condition. Also, studies of the bioavailability and BE studies of the generic drugs, and the drugs already approved in other countries are done to establish the efficacy of new drugs [ 28 ].
Apart from the discovery of a novel drug, clinical trials are also conducted to approve novel medical devices for public use. A medical device is defined as any instrument, apparatus, appliance, software, and any other material used for diagnostic/therapeutic purposes. The medical devices may be divided into three classes wherein class I uses general controls; class II uses general and special controls, and class III uses general, special controls, and premarket approvals [ 28 ].
The premarket approval applications ensure the safety and effectiveness, and confirmation of the activities from bench to animal to human clinical studies. The FDA approval for investigational device exemption (IDE) for a device not approved for a new indication/disease/condition. There are two types of IDE studies that include the feasibility study (basic safety and potential effectiveness) and the pivotal study (trial endpoints, randomization, monitoring, and statistical analysis plan) [ 28 ].
As evidenced by the available literature, there are two types of research that include observational and experimental research. Experimental research is alternatively known as the true type of research wherein the research is conducted by the intervention of a new drug/device/method (educational research). Most true experiments use randomized control trials that remove bias and neutralize the confounding variables that may interfere with the results of research [ 28 ].
The variables that may interfere with the study results are independent variables also called prediction variables (the intervention), dependent variables (the outcome), and extraneous variables (other confounding factors that could influence the outside). True experiments have three basic elements that include manipulation (that influence independent variables), control (over extraneous influencers), and randomization (unbiased grouping) [ 29 ].
Experiments can also be grouped as true, quasi-experimental, and non-experimental studies depending on the presence of specific characteristic features. True experiments have all three elements of study design (manipulation, control, randomization), and prospective, and have great scientific validity. Quasi-experiments generally have two elements of design (manipulation and control), are prospective, and have moderate scientific validity. The non-experimental studies lack manipulation, control, and randomization, are generally retrospective, and have low scientific validity [ 29 ].
Clinical trials: epidemiological and human genetics study
Epidemiological studies are intended to control health issues by understanding the distribution, determinants, incidence, prevalence, and impact on health among a defined population. Such studies are attempted to perceive the status of infectious diseases as well as non-communicable diseases [ 30 ].
Experimental studies are of two types that include observational (cross-sectional studies (surveys), case-control studies, and cohort studies) and experimental studies (randomized control studies) [ 3 , 31 ]. Such research may pose challenges related to ethics in relation to the social and cultural milieu.
Biomedical research related to human genetics and transplantation research poses an increased threat to ethical concerns, especially after the success of the human genome project (HGP) in the year 2000. The benefits of human genetic studies are innumerable that include the identification of genetic diseases, in vitro fertilization, and regeneration therapy. Research related to human genetics poses ethical, legal, and social issues (ELSI) that need to be appropriately addressed. Most importantly, these genetic research studies use advanced technologies which should be equally available to both economically well-placed and financially deprived people [ 32 ].
Gene therapy and genetic manipulations may potentially precipitate conflict of interest among the family members. The research on genetics may be of various types that include pedigree studies (identifying abnormal gene carriers), genetic screening (for diseases that may be heritable by the children), gene therapeutics (gene replacement therapy, gene construct administration), HGP (sequencing the whole human genome/deoxyribonucleic acid (DNA) fingerprinting), and DNA, cell-line banking/repository [ 33 ]. The biobanks are established to collect and store human tissue samples like umbilical tissue, cord blood, and others [ 34 ].
Epidemiological studies on genetics are attempts to understand the prevalence of diseases that may be transmitted among families. The classical epidemiological studies may include single case observations (one individual), case series (< 10 individuals), ecological studies (population/large group of people), cross-sectional studies (defined number of individuals), case-control studies (defined number of individuals), cohort (defined number of individuals), and interventional studies (defined number of individuals) [ 35 ].
Genetic studies are of different types that include familial aggregation (case-parent, case-parent-grandparent), heritability (study of twins), segregation (pedigree study), linkage study (case-control), association, linkage, disequilibrium, cohort case-only studies (related case-control, unrelated case-control, exposure, non-exposure group, case group), cross-sectional studies, association cohort (related case-control, familial cohort), and experimental retrospective cohort (clinical trial, exposure, and non-exposure group) [ 35 ].
Ethics and concerns in clinical trial/research
Because clinical research involves animals and human participants, adhering to ethics and ethical practices assumes increased significance [ 36 ]. In view of the unethical research conducted on war soldiers after the Second World War, the Nuremberg code was introduced in 1947, which promulgated rules for permissible medical experiments on humans. The Nuremberg code suggests that informed consent is mandatory for all the participants in a clinical trial, and the study subjects must be made aware of the nature, duration, and purpose of the study, and potential health hazards (foreseen and unforeseen). The study subjects should have the liberty to withdraw at any time during the trial and to choose a physician upon medical emergency. The other essential principles of clinical research involving human subjects as suggested by the Nuremberg code included benefit to the society, justification of study as noted by the results of the drug experiments on animals, avoiding even minimal suffering to the study participants, and making sure that the participants don’t have life risk, humanity first, improved medical facilities for participants, and suitably qualified investigators [ 37 ].
During the 18th world medical assembly meeting in the year 1964, in Helsinki, Finland, ethical principles for doctors practicing research were proposed. Declaration of Helsinki, as it is known made sure that the interests and concerns of the human participants will always prevail over the interests of the society. Later in 1974, the National Research Act was proposed which made sure that the research proposals are thoroughly screened by the Institutional ethics/Review Board. In 1979, the April 18th Belmont report was proposed by the national commission for the protection of human rights during biomedical and behavioral research. The Belmont report proposed three core principles during research involving human participants that include respect for persons, beneficence, and justice. The ICH laid down GCP guidelines [ 38 ]. These guidelines are universally followed throughout the world during the conduction of clinical research involving human participants.
ICH was first founded in 1991, in Brussels, under the umbrella of the USA, Japan, and European countries. The ICH conference is conducted once every two years with the participation from the member countries, observers from the regulatory agencies, like the World Health Organization (WHO), European Free Trade Association (EFTA), and the Canadian Health Protection Branch, and other interested stakeholders from the academia and the industry. The expert working groups of the ICH ensure the quality, efficacy, and safety of the medicinal product (drug/device). Despite the availability of the Nuremberg code, the Belmont Report, and the ICH-GCP guidelines, in the year 1982, International Ethical Guidelines for Biomedical Research Involving Human Subjects was proposed by the CIOMS in association with WHO [ 39 ]. The CIOMS protects the rights of the vulnerable population, and ensures ethical practices during clinical research, especially in underdeveloped countries [ 40 ]. In India, the ethical principles for biomedical research involving human subjects were introduced by the Indian Council of Medical Research (ICMR) in the year 2000 and were later amended in the year 2006 [ 41 ]. Clinical trial approvals can only be done by the IRB approved by the Drug Controller General of India (DGCI) as proposed in the year 2013 [ 42 ].
Current perspectives and future implications
A recent study attempted to evaluate the efficacy of adaptive clinical trials in predicting the success of a clinical trial drug that entered phase 3 and minimizing the time and cost of drug development. This study highlighted the drawbacks of such clinical trial designs that include the possibility of type 1 (false positive) and type 2 (false negative) errors [ 43 ].
The usefulness of animal studies during the preclinical phases of a clinical trial was evaluated in a previous study which concluded that animal studies may not completely guarantee the safety of the investigational drug. This is noted by the fact that many drugs which passed toxicity tests in animals produced adverse reactions in humans [ 44 ].
The significance of BE studies to compare branded and generic drugs was reported previously. The pharmacokinetic BE studies of Amoxycillin comparing branded and generic drugs were carried out among a group of healthy participants. The study results have demonstrated that the generic drug had lower Cmax as compared to the branded drug [ 45 ].
To establish the BE of the generic drugs, randomized crossover trials are carried out to assess the Cmax and the AUC. The ratio of each pharmacokinetic characteristic must match the ratio of AUC and/or Cmax, 1:1=1 for a generic drug to be considered as a bioequivalent to a branded drug [ 46 ].
Although the generic drug development is comparatively more beneficial than the branded drugs, synthesis of extended-release formulations of the generic drug appears to be complex. Since the extended-release formulations remain for longer periods in the stomach, they may be influenced by gastric acidity and interact with the food. A recent study suggested the use of bio-relevant dissolution tests to increase the successful production of generic extended-release drug formulations [ 47 ].
Although RCTs are considered the best designs, which rule out bias and the data/results obtained from such clinical research are the most reliable, RCTs may be plagued by miscalculation of the treatment outcomes/bias, problems of cointerventions, and contaminations [ 48 ].
The perception of healthcare providers regarding branded drugs and their view about the generic equivalents was recently analyzed and reported. It was noted that such a perception may be attributed to the flexible regulatory requirements for the approval of a generic drug as compared to a branded drug. Also, could be because a switch from a branded drug to a generic drug in patients may precipitate adverse events as evidenced by previous reports [ 49 ].
Because the vulnerable population like drug/alcohol addicts, mentally challenged people, children, geriatric age people, military persons, ethnic minorities, people suffering from incurable diseases, students, employees, and pregnant women cannot make decisions with respect to participating in a clinical trial, ethical concerns, and legal issues may prop up, that may be appropriately addressed before drug trials which include such groups [ 50 ].
Conclusions
Clinical research and clinical trials are important from the public health perspective. Clinical research facilitates scientists, public health administrations, and people to increase their understanding and improve preparedness with reference to the diseases prevalent in different geographical regions of the world. Moreover, clinical research helps in mitigating health-related problems as evidenced by the current Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) pandemic and other emerging and re-emerging microbial infections. Clinical trials are crucial to the development of drugs, devices, and vaccines. Therefore, scientists are required to be up to date with the process and procedures of clinical research and trials as discussed comprehensively in this review.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
- Investigators
- Clinical Data Management
- Medical Device CRO
Selecting a CRO
WHAT IS A CRO?
- Request for Proposal

What is a Contract Research Organization?
A Contract Research Organization (CRO), sometimes known as a Clinical Research Organization, is an organization contracted by another company to take the lead in managing that company’s trials and complex medical testing responsibilities. Contract Research Organizations reduce the cost of research and development to help businesses and institutions meet the needs of the evolving medical device and pharma industry.
Topics covered below:
- What is Clinical Outsourcing?
- What Does a Contract Research Organization Do?
Benefits of Outsourcing to a CRO
- Working With a CRO
- Contract Research and Manufacturing Services (CRAMS)
What Is Clinical Outsourcing?
Clinical outsourcing occurs when a company hires another company made up of researchers and experts to conduct comprehensive and complex medical research so that the hiring company does not have to staff experts in-house, or maintain the required infrastructure and office space. This enables the hiring company to receive expert medical testing while also saving both time and money.
To reduce the cost of research and development, drug companies are increasingly outsourcing their medical testing responsibilities to alleviate the growing and ongoing cost of maintaining medical facilities and full-time staff year-round.
What Does A Contract Research Organization Do?
Contract Research Organizations (CROs) conduct clinical trials and research support services for biotechnology, medical device, and pharmaceutical industries, as well as universities, government organizations, and foundations.
CROs are able to provide a wide range of clinical research services to medical sponsors, including, but not limited to:
- Procedures and Logistics
- Resource Allocation Management
- Database Design
- Data Entry and Validation
- Database Maintenance and Archival
- Security Procedures
- Site Recruitment
- Patient Recruitment
- Study Monitoring
- Site Management
- Audits/FDA inspection support
- Clinical SOP Development
- GCP Seminars
- Medicine and Disease Coding
- Validation Programming
- Market Assessments
- Market Research
- Strategic Planning Consultation
- Medical Product Registration
- Product Launch
- Product Marketing
- Product Sales
- Safety and Efficacy Summaries
- Quality Reporting
- Statistical Methodology Consultation
- Statistical Analysis Plan Development
- Statistical Analysis Reports
- Final Study Reports
- Clinical Study Protocol Documents
- “Instructions for Use”
- Manuscripts
- PowerPoint Presentations
- White Papers
Outsourcing to a Contract Research Organization can bring multiple benefits to clinical professionals and institutions.
When choosing to outsource clinical work, consider:
Time Savings
Cost Savings
Advanced Technological Needs
Evolving and Complex Regulatory Requirements
Outsourcing to a CRO saves critical time in the trial and development phase. Working with a CRO to conduct a trial often significantly reduces the time it takes compared to completing the trial in-house. CROs are already set up with all of the necessary tools and resources needed as well as a team of in-house experts who are experienced in all areas of clinical testing, development, and compliance.
There are significant cost savings in hiring a CRO. A faster trial process alone offers medical institutions a reduction in costs. There are also financial savings in each long-term purchase an entity would incur in order to run sufficient research trials. Full-time staff and medical facilities are costly year-round, especially when they are not needed during all parts of the year.
Working with a CRO gives hiring companies access to the most advanced technology and systems for data management, product development, research analysis, and other clinical research services. Clinical research is a rapidly changing industry. It is essential that software and hardware IT capabilities, as well as Internet-based applications, are the best in the industry to facilitate the acceleration of clinical trials while maintaining comprehensive quality control.
The FDA and other relevant regulatory authorities require intricate and accurate data for approvals. CROs work within clinical compliance on a daily basis, which gives them intricate knowledge of regulatory requirements and audits such as Good Clinical Practice (GCP) audits or Good Laboratory Practice (GLP) audits. CROs work with hiring companies to optimize audit results through careful review of any previous issues, close inspection of infrastructure, and adherence to current protocols.
Working with a CRO
There is a wide variety in CROs. Some are large, publicly owned companies with global coverage and a range of comprehensive services, while other CROs are small, privately owned companies that specialize in a specific niche area. Comparing one CRO to another can be very difficult, as CRO budgets and services often vary significantly from one company to the next. When selecting a CRO, take into account the company’s previous experience, including the types of projects completed, the clients they have worked with, any niche services they provide, as well as their overall track record in the industry.
Before you decide, read our guide on how to efficiently and effectively evaluate Clinical Research Companies and download our CRO Evaluation Checklist .
Sponsor’s Responsibility
A business, organization, or institution may transfer any or all of their clinical trials and research responsibilities over to a Contract Research Organization, but the responsibility remains with the original company hiring the CRO. The quality and integrity of the clinical research data continue to reside with the entity sponsoring the work. CROs should be backed by a spotless track record of quality assurance and quality control.
Get It In Writing
When hiring a CRO, always ensure each delegated task is outlined in writing and signed by both parties. All agreements should be thoroughly documented by all of the involved parties to avoid any costly misunderstandings or complications. It should be very clear which organization is responsible for each aspect of the medical research, development, or other clinical services. Any services or components that are not specified in the agreement will remain the responsibility of the hiring entity.
What Is Contract Research And Manufacturing Services?
Contract Research and Manufacturing Services (CRAMS) is a broader clinical outsourcing term that includes CROs as well as CMOs, Contract Manufacturing Organizations.
Contract Manufacturing Organizations (CMOs) are similar to CROs, in that they are also hired by another pharmaceutical company on a contract basis. The purview of a CMO is in the development of a drug through to its manufacturing.
They all make up a rapidly expanding segment of the biotechnological and pharmaceutical industry. Outsourcing specialized clinical research and manufacturing work continues to develop as organizations aim to meet the needs of an evolving industry.
Promedica Intl. (PMI) delivers the quality and consistent results of a large Clinical Research Organization with the efficiencies and responsiveness of a small CRO. PMI can support your needs with a range of specialized services, including Project Management, Product Development Planning, Clinical Study Management, Product Commercialization, Medical Writing, Biostatistics, Data Management, and Research Compliance & Education.
Learn more about our wide range of clinical services , contact our team , or submit a Request for Proposal .
PMI’s quality control procedures for statistical output involve a combination of independent programming and internal review to verify accuracy and completeness of tables, figures, and listings.
CORPORATE INFORMATION
News & Events
Audits/FDA Inspections Data Management GCP Seminars
Clinical Information Technology
Employment Opportunities Contract Consulting
Request for Proposal Selecting a CRO Visiting PMI Study Opportunities Links & Resources

Site Map | Privacy Policy | Disclaimer
© 2019-2024 Promedica International, a California Corporation
Asia & Oceania
Middle east & africa.
- United States
- Asia Pacific
- Australia & NZ
- Southeast Asia
- Czech Republic
- Deutschland
- España
- Switzerland
- United Kingdom

EMEA Thought Leadership
Developing IQVIA’s positions on key trends in the pharma and life sciences industries, with a focus on EMEA.
- Middle East and Africa
- Research & Development
- Real World Evidence
- Commercialization
- Safety & Regulatory Compliance
- Technologies
LIFE SCIENCE SEGMENTS
- Pharmaceutical Manufacturers
- Emerging Biopharma
- Consumer Health
HEALTHCARE SEGMENTS
- Information Partner Services
- Financial Institutions
- Public Health and Government
- Patient Associations
THERAPEUTIC AREAS
- Cardiovascular
- Cell and Gene Therapy
- Central Nervous System
- GI & Hepatology
- Infectious Diseases and Vaccines
- Rare Diseases

Impacting People's Lives
"We strive to help improve outcomes and create a healthier, more sustainable world for people everywhere.

Harness the power to transform clinical development
Reimagine clinical development by intelligently connecting data, technology, and analytics to optimize your trials. The result? Faster decision making and reduced risk so you can deliver life-changing therapies faster.
Research & Development Quick Links
- Clinical Trials
- Functional Services
- Decentralized Trials
- Therapeutic Expertise
- Site and Investigators

Real World Evidence. Real Confidence. Real Results.
Generate and disseminate evidence that answers crucial clinical, regulatory and commercial questions, enabling you to drive smarter decisions and meet your stakeholder needs with confidence.
Real World Evidence Quick Links
- Real World & Health Data Sets
- Medical Affairs
- Health Data Apps & AI
- Health Data Transformation
- Study Design
- Evidence Networks
- Health Economics & Value
- Regulatory and Safety
- Natural Language Processing
- Real World Evidence Library

See markets more clearly. Opportunities more often.
Elevate commercial models with precision and speed using AI-driven analytics and technology that illuminate hidden insights in data.
Commercialization Quick Links
- COVID-19 & Commercialization
- Launch Strategy & Management
- Brand Strategy & Management
- Pricing & Market Access
- Healthcare Professional Engagement
- Patient Engagement and Support
- Promotional Strategy

Service driven. Tech-enabled. Integrated compliance.
Orchestrate your success across the complete compliance lifecycle with best-in-class services and solutions for safety, regulatory, quality and medical information.
Safety & Regulatory Compliance Quick Links
- Safety Pharmacovigilance
- Regulatory Compliance
- Quality Compliance
- Medical Information
- Commercial Compliance

Intelligence that transforms life sciences end-to-end.
When your destination is a healthier world, making intelligent connections between data, technology, and services is your roadmap.
Technology Quick Links
- Orchestrated Clinical Trials
- Enterprise Information Management
- Performance Management & Insights
- Provider Reference Data Network
- Customer Engagement
- Safety, Regulatory, Quality Compliance
- Partner Programs
- Technology Insights
CLINICAL PRODUCTS
- Planning Suite
- Clinical Trial Payments
- Investigator Site Portal
- One Home for Sites
- Patient Engagement Suite
- Electronic Clinical Outcome Assessment (eCOA)
- Interactive Response Technology (IRT)
- Clinical Data Analytics Solutions

COMMERCIAL PRODUCTS
- Information Management
- Promotional Engagement
- Orchestrated Analytics
- Next Best Action
- Customer Engagement (OCE)
COMPLIANCE, SAFETY, REG PRODUCTS
- IQVIA Vigilance Platform
- SmartSolve eQMS
- Safety & Pharmacovigilance
REAL WORLD PRODUCTS
- Data Profiling
- Patient Profiling
- HCP Profiling
- Market Profiling

"Data in a Day
BLOGS, WHITE PAPERS & CASE STUDIES
Explore our library of insights, thought leadership, and the latest topics & trends in healthcare.
THE IQVIA INSTITUTE
An in-depth exploration of the global healthcare ecosystem with timely research, insightful analysis, and scientific expertise.

INSTITUTE REPORT
"Global Trends in R&D 2024

EMEA Insights
"IQVIA thought leadership with a focus on EMEA.

WHITE PAPER
"DCTs Deliver Big ROI

"Capturing value at scale: The $4 billion RWE imperative
FEATURED INNOVATIONS
- IQVIA Connected Intelligence™
- IQVIA Healthcare-grade AI™
- Human Data Science Cloud
- IQVIA Innovation Hub
- Patient Experience powered by Apple
Commitment to Public Health
- Code of Conduct
- Environmental Social Governance
- Executive Team
NEWS & RESOURCES
- Events & Webinars
- Industry Analyst Reports
- COVID-19 Resources
- Our Locations

INVESTOR RELATIONS
"Visit our investor relations site for more information.

Unlock your potential to drive healthcare forward
By making intelligent connections between your needs, our capabilities, and the healthcare ecosystem, we can help you be more agile, accelerate results, and improve patient outcomes.

IQVIA AI is Healthcare-grade AI
Building on a rich history of developing AI for healthcare, IQVIA AI connects the right data, technology, and expertise to address the unique needs of healthcare. It's what we call Healthcare-grade AI.

Your healthcare data deserves more than just a cloud.
The IQVIA Human Data Science Cloud is our unique capability designed to enable healthcare-grade analytics, tools, and data management solutions to deliver fit-for-purpose global data at scale.

Innovations make an impact when bold ideas meet powerful partnerships
The IQVIA Innovation Hub connects start-ups with the extensive IQVIA network of assets, resources, clients, and partners. Together, we can help lead the future of healthcare with the extensive IQVIA network of assets, resources, clients, and partners.

Proven, faster DCT solutions
IQVIA Decentralized Trials deliver purpose-built clinical services and technologies that engage the right patients wherever they are. Our hybrid and fully virtual solutions have been used more than any others.

IQVIA Patient Experience Solutions powered by Apple
Empowering patients to personalize their healthcare and connecting them to caregivers has the potential to change the care delivery paradigm. IQVIA and Apple are collaborating to bring this exciting future of personalized care directly to devices patients already have and use.
WORKING AT IQVIA
Our mission is to accelerate innovation for a healthier world. Together, we can solve customer challenges and improve patient lives.
LIFE AT IQVIA
Careers, culture and everything in between. Find out what’s going on right here, right now.

WE’RE HIRING
"Improving human health requires brave thinkers who are willing to explore new ideas and build on successes. Unleash your potential with us.
OUR MISSION
Accelerating innovation for a healthier world, powering smarter healthcare for everyone, everywhere.
IQVIA Connected Intelligence
Environmental, Social and Governance
Innovation Hub
Third Party Access Program
Creating a healthier world
We want to help make extraordinary things possible for patients. We are inspired by their stories, and it is our mission to accelerate innovations that enable better outcomes. Every day, we work alongside customers and partners to unleash potential and help improve health around the world.

In pursuit of the extraordinary
We are focused on driving healthcare forward, supported by four key principles.
We strive to make a positive impact in everything we do.
We approach every problem with curiosity and an open-mind to better support our customers’ innovations.
Collaboration
We aim to bring out the best in each other and our diverse expertise. We maximize our positive impact by working together.
We are always learning and seeking opportunities for personal, professional, and organizational growth.
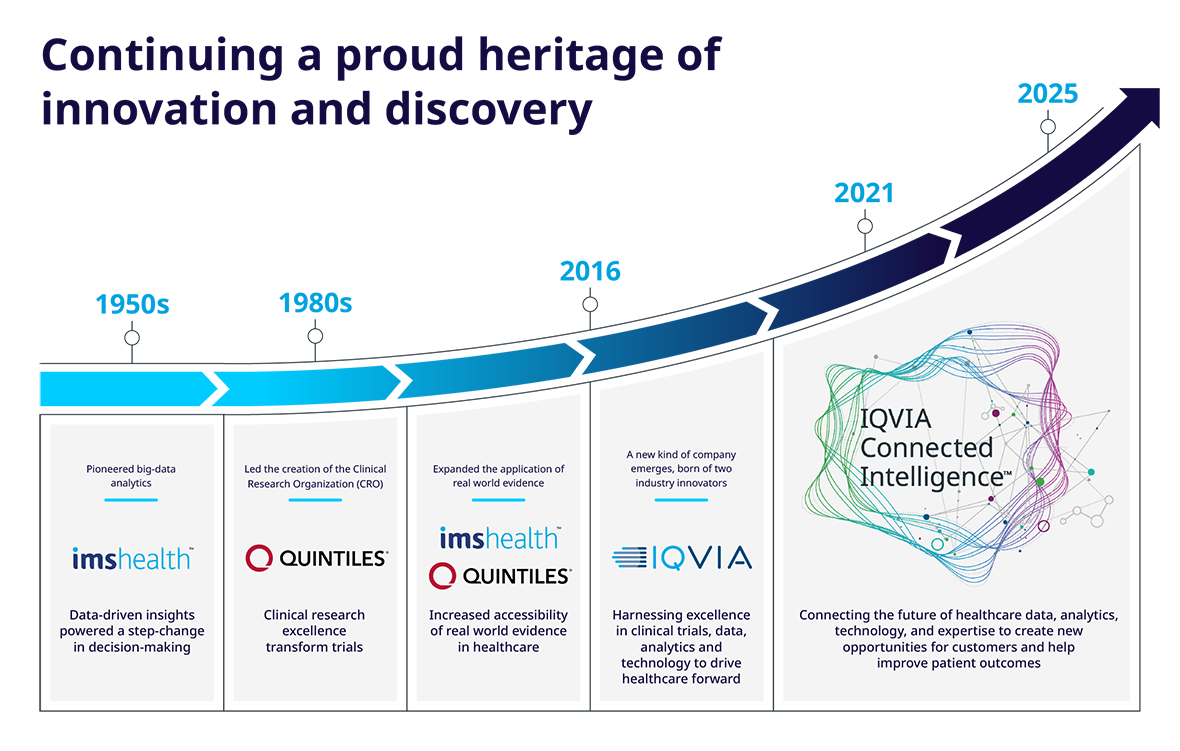
Intelligent connections to accelerate results
In today’s healthcare environment, it’s not only about how much data, information, and technology you have at your fingertips – it’s what you do with it.
IQVIA is focused on making intelligent connections for customers across the entire healthcare ecosystem to help you drive healthcare forward.
Whether that means partnering with novel technology companies to boost patient engagement, leveraging AI & machine learning to accelerate results, or using decentralized trials to reach the right patients wherever they are – we are always looking for smarter ways to move you forward.
IQVIA is a leading global provider of advanced analytics, technology solutions, and clinical research services to the life sciences industry. IQVIA creates intelligent connections across all aspects of healthcare through its analytics, transformative technology, big data resources and extensive domain expertise. IQVIA Connected Intelligence™ delivers powerful insights with speed and agility — enabling customers to accelerate the clinical development and commercialization of innovative medical treatments that improve healthcare outcomes for patients. With approximately 86,000 employees, IQVIA conducts operations in more than 100 countries.
Explore More
Code of Conduct
Featured Innovations
The IQVIA Institute
Insights Library
For this browsing session please remember my choice and don't ask again.

What Is a CRO?
Pharmaceutical, biotechnology and medical device companies initiate, manage and fund clinical trials and are ultimately responsible for the clinical trial process. They are referred to as sponsors. Clinical trial sponsors also include government agencies, such as the National Institutes of Health (NIH) in the United States, and non-profit organizations.
The research and development process for a single drug can require the participation of thousands of people. It may take as long as 15 years to bring a new drug to market and the average cost can exceed $1 billion. It is a long, complex and expensive process, and many sponsors are not equipped, resourced or prepared to perform all trial-related duties and functions themselves.
For this reason, sponsors may choose to outsource all or part of the trial-related tasks to a company called a clinical research organization or contract research organization (CRO) to manage the clinical trial on their behalf, without sponsors having to maintain a staff for these services. CROs have the capacity to conduct the day-to-day research activities that are either not possible or too expensive for a sponsor to achieve in-house. CROs are experts in the space and active partners in clinical research.
- A CRO's Role in Pharmaceutical Development
- Functions of a CRO
- Clinical Research and Trial Management
- Global and Specialized CRO Services
“ It may take as long as 15 years to bring a new drug to market and the average cost can exceed $1 billion . “
CROs in the United States
the size that the global CRO market size is expected to reach, in dollars, in 2023
What Is a CRO’s role in pharmaceutical development?
CROs play a major role in ensuring safe, ethical clinical trials that are essential to developing new, life-changing drugs and medical devices that benefit millions of patients worldwide .
The services that a CRO may offer can cover the full timeline of the study:
- The development and revision of protocols for trials
- The adaptation of the necessary documentation to the applicable rules
- Obtaining the necessary approvals from clinical research ethics committees and regulatory authorities
- The design and preparation of case report forms
- The determination of the sample
- The selection of the best researchers and research centers
- The final negotiation of the contracts among all parties (the CRO, vendors and sites).
- Once regulatory approval is obtained and the trial begins with site selection and patient recruitment, the CRO can provide monitoring , which consists of controlling compliance with the protocol and the procedures established for the development of the study. Likewise, pharmacovigilance services include detection and action in case of any adverse event (AE).
- The last steps to ensure the success of any clinical study are the statistical analysis and management of the trial data , the generation of reports (including the clinical study report for submission to regulatory authorities for drug approval) and the control and storage of the documentation.
CRO structure
A Contract Research Organization (CRO) acts as a bridge between the sponsor, the one who contracts the services, and the rest of the actors involved in the clinical trial.
(Leon Research, https://leonresearch.com/what-is-a-cro-and-how-can-it-help-you-in-your-clinical-trial/ )
Functions of a Clinical Research Organization
Typically, a CRO provides scientific, clinical and business continuity for clinical trial sponsors. The CRO can be involved in a range of services from pre-clinical research (which takes place before human trials) to post-marketing surveillance (which takes place after a product has been approved for use).
Other administrative activities may include strategic consulting and protocol development, laboratory and analytical services, project management, trial logistics, medicine and disease coding, statistical analysis and reporting, validation programming, safety and efficacy summaries and the final study report.
Clinical research and trial management
A sponsor hires a CRO to plan, coordinate, execute and manage the life cycle of the clinical trial safely and efficiently. Serving as the main contact between the sponsor and other stakeholders throughout the trial, the CRO communicates with ethics and compliance committees, regulatory personnel, vendors, physicians and research coordinators.
Clinical trials conducted by CROs are completed on average 30 days faster than those conducted by sponsor companies. 1
Every day counts for both patients (who may be anxiously waiting on therapies that could improve or even save their lives) and sponsors (because time spent in the development process counts against the patent protection period after the drug goes to market, affecting sales revenue).
With more than 35 years of experience, the PPD clinical research business of Thermo Fisher Scientific offers an established drug development platform, patient enrollment expertise and robust laboratory services . Our broad range of clinical development, analytical and patient and advisory services – including full service to functional service provider services , digital and decentralized trials, and laboratories – enables customers to drive innovation and increase drug development productivity. We are recognized for accelerating promising medicines from early development through regulatory approval and market access. Our flexible, custom-tailored solutions serve pharmaceutical, biotech, medical device, academic, government and public health organizations in clinical research and development .
Global and specialized CRO services
Most large CROs are full-service, providing complete clinical trial management support, but there are some companies specializing in a specific type of study (e.g., research with medical devices or observational studies) and also CROs specializing in a specific therapeutic area, such as oncology or ophthalmology.
Global CROs, large companies with operations all over the world, provide greater, integrated geographical coverage, with more service offerings and capabilities, while local companies may sometimes have more in-depth knowledge of a particular country’s nuances and demographics as they relate to clinical development.
The global network consisting of clinical sites within the PPD clinical research business of Thermo Fisher Scientific and non-owned partner sites offers access to a diverse pool of healthy volunteers and specialty populations around the world. Our experienced staff provides external oversight of a trial, working directly with the institutions where clinical studies are conducted – hospitals and clinics, called investigational sites. We provide training to the principal investigator and the site staff, and ensure that the safety, rights and well-being of patients are protected; that the conduct of the trial is in compliance with regulatory requirements; and that reported trial data are accurate and complete.
What is the difference between a CRO and a sponsor?
Although the sponsoring company may transfer the trial functions to a CRO, the sponsor owns the investigational product and needs to ensure results are factual and scientifically backed.
According to the definition of the International Council on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) , a CRO is: “A person or an organization (commercial, academic or other) contracted by the sponsor to perform one or more of a sponsor’s trial-related duties and functions.”
The sponsor maintains a central role throughout the clinical trial process. Although regulatory tasks differ globally, they include initiating the trial process by submitting an application and ensuring approval is obtained (directly or indirectly).
The ICH Guideline for GCP specifies the responsibilities a CRO can take over from a sponsor :
- 5.2.1: A sponsor may transfer any or all of the sponsor’s trial-related duties and functions to a CRO, but the ultimate responsibility for the quality and integrity of the trial data always resides with the sponsor. The CRO should implement quality assurance and quality control.
- 5.2.2: Any trial-related duty and function that is transferred to and assumed by a CRO should be specified in writing. The sponsor should ensure oversight of any trial-related duties and functions carried out on its behalf, including trial-related duties and functions that are subcontracted to another party by the sponsor’s contracted CRO(s).
- 5.2.3: Any trial-related duties and functions not specifically transferred to and assumed by a CRO are retained by the sponsor.
- 5.2.4: All references to a sponsor in this guideline also apply to a CRO to the extent that a CRO has assumed the trial-related duties and functions of a sponsor.
What are the advantages of outsourcing to a CRO?
A CRO can offer substantial cost savings for a sponsor by reducing the time it takes to conduct a trial compared to a sponsor doing so in-house. This also eliminates the need for staff, infrastructure and lab and office space for the sponsor to run the trials themselves. Finally, a full-service CRO can provide access to a global network of clinical research sites, meaning it can quickly identify suitable sites for clinical trials in multiple countries. Geographical reach or therapeutic focus can be important in patient recruitment, especially in specific populations, such as underserved and diverse , or for rare diseases that affect a small subset of people.
Working with a CRO gives sponsors access to the most advanced technology and systems for data management, product development, research analysis and other clinical research services. CROs are quick to adopt the latest technologies, enabling them to provide strategic insights and advice, and to offer novel and distinctive ways to use advanced tools and adapt them to each sponsor’s needs to ensure optimal clinical trial performance. Given that CROs work with many different sponsors, they often have a breadth of experience beyond even a single large pharmaceutical company.
CROs adhere to clinical compliance on a daily basis, which requires intricate in-house knowledge of regulatory requirements and audits such as GCP (Good Clinical Practice) or GLP (Good Laboratory Practice) audits.
Sponsors conducting clinical research in a niche area such as rare disease may choose to work with a specialty CRO or a global CRO with offering such services.
Global Network
The PPD clinical research business of Thermo Fisher Scientific has an extensive global network of investigators, clinicians and partners with a wide range of products, technologies and services that can be leveraged for clinical trial conduct. Our goal is to deliver considerable time and cost savings in drug development, critical to accelerating the delivery of life-changing therapies. Our experts consider the end before the beginning by envisioning and planning for commercialization and market acceptance well before the first patient is enrolled in a clinical trial. And before trials are underway, we design for post-approval, real-world patient observation that provides support for payer authorizations and may lead to new ideas and products .
How does a sponsor select a CRO?
When selecting a CRO, here are some questions to consider:
- Can the CRO offer the services that the sponsor needs?
- Does the CRO have related experience and a good track record (i.e., similar projects and references or testimonials from satisfied clients)?
- Is the CRO financially stable?
- Does the CRO have a good system for employee training and a low staff turnover rate?
- Does the CRO have the key staff members required and can it set up a team to deliver qualified results?
- Does the CRO have the required infrastructure?
- Does the CRO have a robust quality assurance system?
- Is the CRO’s team responsive and willing to work with you and communicate throughout the project?
- Does the CRO have specialty services, laboratories, digital and decentralized solutions, a clinical supplies network and other capabilities important to your drug development program?
- Will the CRO help you recruit and manage safety boards or committees?
- Will the CRO conduct audits to help you prepare to pass FDA inspections?
- Can the CRO provide general site support and project management help in addition to clinical monitoring?
- Does the CRO function as a vendor or a true partner ?
Drive your drug development program forward
Partner with a leading, global CRO that leverages a proven track record and deep expertise to keep the patient at the forefront of your trial.
The PPD clinical research business of Thermo Fisher Scientific accelerates your success with a dynamic, forward-thinking approach, a comprehensive suite of capabilities and true partnership with our customers.
- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
U.S. Food and Drug Administration
- Search
- Menu
- For Patients
- Learn About Drug and Device Approvals
- The Drug Development Process
Step 3: Clinical Research
While preclinical research answers basic questions about a drug’s safety, it is not a substitute for studies of ways the drug will interact with the human body. “Clinical research” refers to studies, or trials, that are done in people. As the developers design the clinical study, they will consider what they want to accomplish for each of the different Clinical Research Phases and begin the Investigational New Drug Process (IND), a process they must go through before clinical research begins.
On this page you will find information on:
Designing Clinical Trials
Clinical Research Phase Studies
The Investigational New Drug Process
Asking for FDA Assistance
FDA IND Review Team
Researchers design clinical trials to answer specific research questions related to a medical product. These trials follow a specific study plan, called a protocol , that is developed by the researcher or manufacturer. Before a clinical trial begins, researchers review prior information about the drug to develop research questions and objectives. Then, they decide:
Who qualifies to participate (selection criteria)
How many people will be part of the study
How long the study will last
Whether there will be a control group and other ways to limit research bias
How the drug will be given to patients and at what dosage
What assessments will be conducted, when, and what data will be collected
How the data will be reviewed and analyzed
Clinical trials follow a typical series from early, small-scale, Phase 1 studies to late-stage, large scale, Phase 3 studies.
What are the Clinical Trial Phases?
Watch this video to learn about the three phases of clinical trials.
Study Participants: 20 to 100 healthy volunteers or people with the disease/condition.
Length of Study: Several months
Purpose: Safety and dosage
During Phase 1 studies, researchers test a new drug in normal volunteers (healthy people). In most cases, 20 to 80 healthy volunteers or people with the disease/condition participate in Phase 1. However, if a new drug is intended for use in cancer patients, researchers conduct Phase 1 studies in patients with that type of cancer.
Phase 1 studies are closely monitored and gather information about how a drug interacts with the human body. Researchers adjust dosing schemes based on animal data to find out how much of a drug the body can tolerate and what its acute side effects are.
As a Phase 1 trial continues, researchers answer research questions related to how it works in the body, the side effects associated with increased dosage, and early information about how effective it is to determine how best to administer the drug to limit risks and maximize possible benefits. This is important to the design of Phase 2 studies.
Approximately 70% of drugs move to the next phase
Study Participants: Up to several hundred people with the disease/condition.
Length of Study: Several months to 2 years
Purpose: Efficacy and side effects
In Phase 2 studies, researchers administer the drug to a group of patients with the disease or condition for which the drug is being developed. Typically involving a few hundred patients, these studies aren't large enough to show whether the drug will be beneficial.
Instead, Phase 2 studies provide researchers with additional safety data. Researchers use these data to refine research questions, develop research methods, and design new Phase 3 research protocols.
Approximately 33% of drugs move to the next phase
Study Participants: 300 to 3,000 volunteers who have the disease or condition
Length of Study: 1 to 4 years
Purpose: Efficacy and monitoring of adverse reactions
Researchers design Phase 3 studies to demonstrate whether or not a product offers a treatment benefit to a specific population. Sometimes known as pivotal studies, these studies involve 300 to 3,000 participants.
Phase 3 studies provide most of the safety data. In previous studies, it is possible that less common side effects might have gone undetected. Because these studies are larger and longer in duration, the results are more likely to show long-term or rare side effects
Approximately 25-30% of drugs move to the next phase
Study Participants: Several thousand volunteers who have the disease/condition
Purpose: Safety and efficacy
Phase 4 trials are carried out once the drug or device has been approved by FDA during the Post-Market Safety Monitoring
Learn more about Clinical Trials .
Drug developers, or sponsors , must submit an Investigational New Drug (IND) application to FDA before beginning clinical research.
In the IND application, developers must include:
Animal study data and toxicity (side effects that cause great harm) data
Manufacturing information
Clinical protocols (study plans) for studies to be conducted
Data from any prior human research
Information about the investigator
Drug developers are free to ask for help from FDA at any point in the drug development process, including:
Pre-IND application, to review FDA guidance documents and get answers to questions that may help enhance their research
After Phase 2, to obtain guidance on the design of large Phase 3 studies
Any time during the process, to obtain an assessment of the IND application
Even though FDA offers extensive technical assistance, drug developers are not required to take FDA’s suggestions. As long as clinical trials are thoughtfully designed, reflect what developers know about a product, safeguard participants, and otherwise meet Federal standards, FDA allows wide latitude in clinical trial design.
The review team consists of a group of specialists in different scientific fields. Each member has different responsibilities.
Project Manager: Coordinates the team’s activities throughout the review process, and is the primary contact for the sponsor.
Medical Officer: Reviews all clinical study information and data before, during, and after the trial is complete.
Statistician: Interprets clinical trial designs and data, and works closely with the medical officer to evaluate protocols and safety and efficacy data.
Pharmacologist: Reviews preclinical studies.
Pharmakineticist: Focuses on the drug’s absorption, distribution, metabolism, and excretion processes.Interprets blood-level data at different time intervals from clinical trials, as a way to assess drug dosages and administration schedules.
Chemist: Evaluates a drug’s chemical compounds. Analyzes how a drug was made and its stability, quality control, continuity, the presence of impurities, etc.
Microbiologist: Reviews the data submitted, if the product is an antimicrobial product, to assess response across different classes of microbes.
The FDA review team has 30 days to review the original IND submission. The process protects volunteers who participate in clinical trials from unreasonable and significant risk in clinical trials. FDA responds to IND applications in one of two ways:
Approval to begin clinical trials.
Clinical hold to delay or stop the investigation. FDA can place a clinical hold for specific reasons, including:
Participants are exposed to unreasonable or significant risk.
Investigators are not qualified.
Materials for the volunteer participants are misleading.
The IND application does not include enough information about the trial’s risks.
A clinical hold is rare; instead, FDA often provides comments intended to improve the quality of a clinical trial. In most cases, if FDA is satisfied that the trial meets Federal standards, the applicant is allowed to proceed with the proposed study.
The developer is responsible for informing the review team about new protocols, as well as serious side effects seen during the trial. This information ensures that the team can monitor the trials carefully for signs of any problems. After the trial ends, researchers must submit study reports.
This process continues until the developer decides to end clinical trials or files a marketing application. Before filing a marketing application, a developer must have adequate data from two large, controlled clinical trials.
- Program Overview
The SOCRA Certified Clinical Research Professional (CCRP) program is your gateway to excellence in clinical research. Elevate your career with our internationally recognized certification, tailored for professionals dedicated to upholding the highest standards in the field. Join a community committed to ethical practices, continuous learning, and advancing global health.
The Society of Clinical Research Associates (SOCRA) established the Certification Program for Clinical Research Professionals in order to create an internationally accepted standard of knowledge, education, and experience by which clinical research professionals will be recognized by the clinical research community. Those individuals so recognized may use the "Certified Clinical Research Professional" or "CCRP ® " designation.
Path to Certification
CCRP certification is awarded upon meeting two criteria: a successful written application and a passing CCRP examination score. The benefits of obtaining certification are numerous. It not only validates knowledge, skills, and abilities but also enhances credibility and peer recognition. Career advancement and increased earning potential become tangible outcomes, reflecting a commitment to standards, compliance, and integrity.
Scope and Standards of Practice
The standards upon which this certification program is based have been set forth by SOCRA to promote recognition and continuing excellence in the ethical conduct of clinical trials. It is the goal of SOCRA to encourage members, and assure the competency of certified members, in their knowledge, understanding, and application of the conduct of clinical investigations involving humans in accordance with the ICH Guidelines, the U.S. Code of Federal Regulations, and the ethical principles that guide clinical research. Members are expected to adhere to national, state, local and provincial regulations and to international guidelines published by the International Conference for Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) and all applicable federal, state and local laws and policies.
Standards of Practice include an understanding of and application of basic concepts of Good Clinical (Research) Practice, including:
- The Nuremberg Code
- The Belmont Report
- The Declaration of Helsinki
- 21 U.S. Code of Federal Regulations – Parts 11, 50, 56, 312, 812
- 45 U.S. Code of Federal Regulations - Part 46
- ICH Harmonised Guideline for Good Clinical Practice E6(R2), and
- ICH Clinical Safety Data Management: Definitions and Standards for Expedited Reporting (E2A)
- 42 CFR Part 11 (ClinicalTrials.gov)
Certification Exam
The SOCRA Certification Examination is offered in two formats: paper and pencil (at SOCRA sponsored sites), and computer based (at Prometric testing centers or through Home Proctoring).
SOCRA Sponsored Sites: Paper and Pencil
- Hosted exams offered in various location throughout the US and Canada.
- Visit the paper and pencil exam schedule for dates and locations.
- A complete application must be received by the deadline date as stated on the examination schedule.
- Score reports mailed to you in 4-6 weeks after exam.
Computer Based Testing: Testing Centers and Remote Proctoring
- Offered at Prometric testing centers throughout the world or through Home Proctoring
- Click here for a list of test centers.
- Allow 2-4 weeks for application processing.
- Once application is approved, schedule exam at a testing center. Exam sessions are available at least 6 weeks in advance.
- Score reports received immediately upon completion of exam.
Candidate Handbook
For more information, please view the Candidate Handbook.
Certification
- CCRP Certification Quick Facts
- Definition of a Clinical Research Professional
- Certification Program Policies
- Removal of CCRP® Credential
- Verify Certification
- Exam Overview
- Candidate Eligibility
- Application and Fee
- Computer Based Testing Exams
- Paper and Pencil Exams
- Refunds, Rescheduling and Retesting
- SOCRA Sponsored Exam Schedule
- Preparing for the Exam
- Preparation Resources
- Examination Results
- Host an Exam at Your Site
- Apply Online
- Exam Schedule SOCRA Sponsored Sites
- Requirements for Maintaining Certification
- Continuing Education Requirements
- Descriptions of Acceptable CE
- CE Recordkeeping Requirements
- Request for SOCRA CE for Courses / Workshops
- Installment Plan Payment
- Renewal of Certification
- Recertification Audit
- Recertification Learning Module
- Accreditation
Summary of Certification Activities
11,145 CCRPs (as of 12/31/2022)
- 1,391 candidates took CCRP exam
- 73% passed CCRP exam
- 2,649 CCRPs recertified
- 946 candidates took CCRP exam
- 65% passed CCRP exam
- 2,783 CCRPs recertified
- 2,060 candidates took CCRP exam
- 70% passed CCRP exam
- 3,801 CCRPs recertified
- 1,980 candidates took CCRP exam
- 71% passed CCRP exam
- 3,188 CCRPs recertified
- 104 exam sites hosted
- 2,175 candidates took CCRP exam
- 2,491 CCRPs recertified
- 91 exam sites hosted
- 2,141 candidates took CCRP exam
- 2,421CCRPs recertified
Translating intent to impact.
The triad of trust: Navigating real-world healthcare data integration
Ensuring the validity of clinical outcomes assessment data.
The value of rater training
Advancements in Artificial Intelligence for site selection
Using human-enabled AI to enhance decision-making and minimise risk
A multifaceted risk factor: Addressing obesity's impact across the disease spectrum
Considerations for clinical trial design focused on obesity treatments.
Optimising biotech funding whitepaper series
Navigating biotech's challenges and embracing a promising tomorrow.
2023 biotech sector survey
EMA guideline on computerised systems and electronic data in clinical trials
Key considerations on the impact of the new framework of globally applicable standards.
Featured Solutions
Blended solutions
Bespoke, seamless solutions to meet unique sponsor challenges.
Digital Health Technologies
ICON acquires HumanFirst, a cloud-based technology company for life sciences supporting precision measurement in patient centred clinical research.
ICON provides full service outsourcing and flexible support for biotech specific needs such as due diligence and asset valuation.
Cardiac Safety Solutions
End-to-end cardiac safety solutions, including ECG, event monitoring, BPM, long-term Holter monitoring, ECHO and MUGA studies.
Early Clinical and Bioanalytical Solutions
Innovative early clinical solutions that will advance your drug development strategy.
Site & Patient Solutions
Transforming recruitment through patient-centric trials and real-world, real-time data.
Market Access
Expertise in mission-critical pricing, market access, and reimbursement.
Strategic approaches for first-in-human (FIH) studies and early clinical development
29 May 2024. Register today.
Unlocking precision medicine: An overview of Illumina’s TSO500
17 June 2024. Register today.
The future of pharmacovigilance: Exploring automation and AI in literature surveillance
Watch the webinar.
ICON Insights
- 10 May 2024
Integrating Performance Studies of In-Vitro Diagnostics into Clinical Trials: A Complex Challenge
SDoH data analysis for proactive outcome improvement: A multilayered approach
- 09 May 2024
Navigating the complexities of healthcare data types
- 01 May 2024
Cross-border initiative to empower rare disease researchers
- 26 Apr 2024
How AI could transform literature surveillance for pharmacovigilance
- 25 Apr 2024
Complexities of running clinical trials in retinal disorders and ways to overcome them
- 01 Apr 2024
SDoH data for efficient, patient-centred clinical trials
Insights from PHUSE US Connect 2024
- 27 Mar 2024
Diabesity: Overlapping pathophysiology informs multi-indication treatment
What’s happening in icon, how can we help.
- Cell and Gene Therapies
- Early Clinical
- Medical Device
- Rare & Orphan Diseases
- Real World Evidence
- Site & Patient Recruitment
- Strategic Solutions

- Log in to ECRI account Client Login
- How We Help Independent Device Evaluation Patient Safety Advisory Services Supply Chain Intelligence ISMP Medication Safety
- Who We Serve Acute Care Ambulatory Care Senior Care Insurers Payers Manufacturers Government
- How We Help Newsletters Education Consulting Memberships
- Who We Serve Acute Care Ambulatory Care Senior Care Insurers Manufacturers Government
- Resources Alerts & Articles Guidance & Tools Events
- Company About Us Careers Awards News Contact Us
- Report an error Report an Error
- Resources Blog Events ECRI Now Podcast On-Demand Webinars Thought Leadership
- Company About Us Careers News Request A Speaker Contact Us
Your cart is empty

Impactful improvements. Safer, equitable healthcare for all.
Despite our best efforts, challenges in safe care persist. Today, one in every four patients will suffer an adverse event during care, a quarter of which are avoidable. We can and must do better. Together, focusing on impactful improvements, we can build a safer, equitable healthcare system for all.
Our Mission and Vision
We're dedicated to advancing evidence-based healthcare globally for the benefit of patients everywhere. We see a world where safe, high-quality healthcare is accessible to everyone.
As one of the foremost independent healthcare patient safety experts, we leverage expertise, spend and clinical data to lead the global effort to dramatically reduce preventable harm, make healthcare more transparent and accessible.

how it all began
A Passion For Preventing Harm
In 1963, tragedy struck during an ER internship when a defibrillator malfunctioned, costing the life of a four-year-old boy despite Dr. Joel Nobel's pleas for repairs. This spurred Dr. Nobel to create the MAX cart - an efficient emergency response system that has since saved countless lives - and led to the founding of ECRI and its first-of-its-kind independent device testing lab grounded in human factors engineering principles. At ECRI, this spirit of passionate ingenuity fuels our unwavering commitment to our mission, resonating through every aspect of our work.

ECRI Founded
Founded as Emergency Care Research Institute (ECRI), focusing on research in emergency medicine, resuscitation, and related biomedical engineering studies.

Collaboration with World Health Organization (WHO)
Designated a World Health Organization (WHO) Collaborating Center in recognition of the ECRI’s achievements in advancing the safety and cost-effectiveness of patient care.

Evidence-Based Practice Center (EPC) Designation
Designated as one of nine Evidence-based Practice Centers by the U.S. Agency for Healthcare Research and Quality (AHRQ).

Patient Safety Organization (PSO) Designation
Designated a Patient Safety Organization (PSO) by the U.S. Department of Health and Human Services under the Patient Safety and Quality Improvement Act of 2005.

ISMP joins ECRI Family
The Institute for Safe Medication Practices (ISMP) joins forces with ECRI creating one of the largest healthcare quality and safety entities in the world.

Safeguarding the Future: Shaping a Post-Pandemic World
ECRI's ground-breaking COVID-19 free resource center provided life-saving information to healthcare professionals across the globe and has helped shape the way we approach our work in safety across the continuum of care.
A Message From Our CEO

Get ECRI's CEO, Marcus Schabacker's perspective on the key challenges and emerging concerns facing healthcare.
Our leadership.

Marcus Schabacker, MD, PhD
President and chief executive officer.

Peter Catalano, MBA, CPA
Chief financial officer.

Darryl Goss
Chief technical officer.

Dheerendra Kommala, MD
Chief medical officer.

Stuart Morris-Hipkins, MSc, ACMA, CGMA
Chief solutions officer.

Bevin O’Neil
Chief strategy officer.

Lea Rubini, MSOD, SHRM-SCP
Chief human resources officer.

Randy White
Chief legal officer.
Dr. Marcus Schabacker became president and chief executive officer of ECRI in January 2018. Dr. Schabacker is a board-certified anesthesiologist and intensive care specialist with more than 35 years of healthcare experience in complex global environments, and more than 20 years of senior leadership responsibilities serving the medical device and pharmaceutical industries across the healthcare value chain.
After his medical and academic training at the Medical University of Lubeck, Germany, Dr. Schabacker served as senior medical officer and head of the intensive care and anesthesia department at the Mafikeng General Hospital, North-West Province, South Africa. His work there was part of a humanitarian aid program to support the African National Congress government under Nelson Mandela in the restructuring and buildup of a rural healthcare system in post-apartheid South Africa.
Upon his return from Africa, Dr. Schabacker joined the medical device industry and held roles of increasing responsibility in medical affairs, preclinical and clinical development, regulatory affairs, quality, research and development, and patient safety. His experience includes designing, transforming, and leading organizations of up to 4,000 employees across five continents to provide safe and effective products to patients and healthcare providers worldwide.
In his last corporate role prior to joining ECRI, Dr. Schabacker served as corporate vice president and chief scientific officer at Baxter. During his clinical years, and his time as an industry thought leader, Dr. Schabacker was focused on patient safety and enhancing patient care.
Dr. Schabacker is a national and international thought leader on patient safety, medical technology, and health equity issues. A sought-after independent expert by health and business reporters, he is often quoted in major news outlets, including USA Today, NY Times, Wall Street Journal, and Modern Healthcare. His broadcast experience includes live and recorded interviews on CNN, CNN International, and CBS-Chicago, plus network-affiliated radio shows. He has presented at national and international conferences.
Dr. Schabacker achieved his board certification in anesthesia and intensive care, as well as a doctorate in medicine from the Medical University, Lubeck, Germany. He also received certifications in emergency medicine and disaster medicine. He is an affiliate assistant professor at The Stritch School of Medicine at Loyola University Chicago. Dr. Schabacker serves as a member of the Life Sciences Advisory Board of Philadelphia. In 2019, Dr. Schabacker was elected into the Fellowship of The College of Physicians of Philadelphia. He has been recognized by the Philadelphia Business Journal as a Healthcare Leader in 2021.
Peter Catalano joined ECRI in 2016 as chief financial officer. He is responsible for financial reporting and controls, development and implementation of sound financial plans and policies, financial analysis, cash management, and the conduct of relationships with lending institutions, creditors, and the financial community.
Catalano began his career at Coopers & Lybrand in Philadelphia. Prior to joining ECRI, Catalano was CFO of asset management within SunGard, and before that he was vice president of global finance position at Ellucian, SunGard's higher education division. Catalano brings extensive experience in service-oriented companies, and he has held high level finance roles in both venture-backed and multi-billion dollar companies. In addition to his finance responsibilities, his achievements include many operational initiatives such as system implementations, customer relationship management (CRM) optimization, and commission streamlining.
Catalano is a graduate of Villanova University and received his MBA from Lehigh University. He is a licensed CPA in the state of Pennsylvania, and is a member of both AICPA and PICPA.
Dheerendra Kommala, MD, is Chief Medical Officer at ECRI, responsible for ECRI’s Medical Office. He joined ECRI in 2019 as Chief Strategy Officer responsible for setting the organization’s strategic direction.
Dr. Kommala brings more than 20 years of experience as an academic clinician, researcher, and chief medical officer. He successfully introduced new products and services to markets throughout the world by working collaboratively with major health systems, industry leaders, clinicians, and patients. Throughout his career, he has been a vocal advocate for patient safety and a visionary leader managing large teams.
Prior to joining ECRI, Dr. Kommala was global vice president of medical affairs for Baxter Healthcare. Previous experience included working as chief medical officer/global vice president of medical affairs for ConvaTec, and as associate medical director of Global Pharmaceutical Research for Renal Care, Abbott Laboratories.
Dr. Kommala received his initial medical training in India, and completed a fellowship in nephrology at the University of Missouri, Columbia School of Medicine.
Stuart Morris-Hipkins joined ECRI in 2021 as Chief Solutions Officer. He serves as a member of ECRI’s Executive Committee and is responsible for sales, marketing, membership services, and the safety and technology business lines.
Morris-Hipkins brings over 20 years of global leadership experience from a variety of different healthcare sectors and industries.
Morris-Hipkins has led multibillion businesses with full leadership responsibility as CEO/President, as well as startups in more than 120 countries. Aside from leadership roles, he has led strategy development, business transformation, mergers and acquisitions (M&A), and revenue growth projects by partnering with healthcare customers globally.
Prior to joining ECRI, Morris-Hipkins founded AFTRR Business Link, Inc., and assisted organizations with strategy, growth, and due diligence projects. Previous experience includes working as president of global solutions at Owens & Minor, chairman at Fusion5 and executive and commercial leadership roles at Smith & Nephew, PLC, and Smiths Group PLC.
Morris-Hipkins holds a Bachelor of Science degree in Mathematics and Chemistry with Honors from Oxford Brookes University, UK, and a Master of Science degree in Atmospheric Sciences from the University of East Anglia, UK. He also holds the professional qualification of Chartered Global Management Accountant (CGMA, ACMA).
Stuart is a citizen of the United Kingdom and the United States.
Bevin O'Neil is the chief strategy officer at ECRI, a nonprofit organization focused on advancing effective, evidenced-based healthcare globally. In her role, O’Neil is responsible for developing and executing its global strategy. O’Neil has over two decades of private equity, fundraising and operating experience, and has sat on several boards.
Prior to ECRI, O’Neil was the founder and managing partner of Incline GEP, which focused on supporting entrepreneurs with emerging consumer brands. From 2011 to 2013, O’Neil served on the drug access team of Clinton Health Access Initiative (CHAI), responsible for improving sustainable access to pediatric HIV drugs and diagnostics for the developing world utilizing a market-based approach.
Earlier in her career, O’Neil was a Principal at Avista Capital Partners, founded by former DLJ Merchant Banking partners. At Avista, O’Neil launched the consumer silo and executed five private equity investments and related tack-on acquisitions in healthcare and consumer, four of which were corporate carve-outs requiring intense operational and infrastructure building. Prior to Avista, O'Neil was a senior manager in business development at Tumi, an Oaktree Capital Management portfolio company. In addition, O'Neil served various roles in the private equity groups of Guggenheim Partners, Oaktree Capital Management, and DLJ Merchant Banking.
O’Neil received a BBA from the University of Michigan.
Lea Rubini joined ECRI in January 2022 as Chief Human Resources Officer. She is a dynamic and trusted leader with over 20 years of global experience across numerous industries. She is known for her inclusive style, business acumen, and ability to quickly move between strategies and tactics.
Throughout her career, Rubini has applied her expertise in driving employee engagement, creating inclusive environments, and affecting change to seamlessly drive business results, transform operating models, and evolve culture. Rubini has proven experience leading global teams in human resources, change management, process improvement, and talent management.
Prior to joining ECRI, Rubini held leadership positions of increasing responsibility in the healthcare, pharmaceutical and consumer goods industries, including at Penn Medicine, Johnson & Johnson, Bristol Myers Squibb, and Campbell Soup Company, to list a few.
Rubini holds a Master’s degree in organizational dynamics from the University of Pennsylvania and a Bachelor's degree in labor and industrial relations from Penn State University. She is certified to facilitate the Hogan Leadership Assessment, Talent X7 Leadership Assessment, and LIFO Behavioral Styles Assessment. She holds coaching and organizational change certificates from the University of Pennsylvania and is a SHRM Senior Certified Professional (SHRM-SCP).
Darryl Goss joined ECRI in February 2024 as Chief Technology Officer. Goss has nearly 30 years of experience in senior leadership positions at public and private equity firms enhancing technological capabilities in the healthcare, pharmaceutical, and chemical industries.
Goss leads the technological transformation and delivery of ECRI’s externally facing products and services. He is driven to provide a high-quality member experience and help healthcare decision-makers leverage data to improve the safety and efficiency of patient care.
For ECRI’s information technology programming, Goss directs internally facing business operations for the organization’s global and mostly remote workforce. Before being named CTO, Goss served as a member of ECRI’s external IT Tech Advisory Council, which was established to offer expert advice on technologies, data strategy and tech roadmaps.
Prior to joining ECRI, Goss served as Chief Executive Officer of Inform Diagnostics, a provider of anatomic pathology laboratory services; President of Sigma-Aldrich Fine Chemicals (SAFC) Hitech, a manufacturing division for pharmaceutical and tech organizations; and Vice President and General Manager of Europe and Asia Pacific for SAFC's life science services. As a healthcare consultant, he was engaged by private equity firms to evaluate and improve company operations, lead strategic planning, and initiate technology investments.
Randy White joined ECRI in 2019 as chief legal officer. He serves as a member of ECRI’s Executive Committee and is also ECRI’s chief privacy officer. Overseeing ECRI’s legal department and outside counsel, Randy is responsible for all of ECRI’s legal affairs in the United States, Europe, and Asia Pacific.
Prior to his current role, Randy spent twenty years as a partner at Fox Rothschild LLP, a nationwide Am Law 100 firm. A partner in Fox’s litigation and labor and employment departments, Randy advised and litigated on behalf of executives, directors, and publicly and privately held companies across the country. At Fox, Randy also served as outside general counsel for nonprofits, start-ups, and middle market companies, overseeing transaction and litigation teams.
Randy holds a juris doctor with honors from the Dickinson School of Law and a bachelor’s degree in government from St. Lawrence University. He is a member of the bars of the Commonwealths of Massachusetts and Pennsylvania, and has been admitted to practice before numerous federal courts.
How We Work
A team of 500 strong, united by shared values and the passion to accomplish better. OneECRI embodies our commitment to harness the full potential of our collective expertise and resources for the benefit of every client and the communities they serve. Across c-suite and frontline leaders alike, we collaborate with you to turn intentions into action and develop the capabilities you need to sustain the impactful changes we've achieved together.
Passionate about making healthcare better? So are we.
Join us on our journey to make safe, cost-effective, exceptional care for all a reality.

living our values
At ECRI, our values aren't just words—they're actions that touch lives every day. Through ECRI Cares, our volunteer program, our team comes together to make a real difference. From ensuring safe housing to supporting vulnerable mothers and babies, we live by our motto: Serving Together. Making a Difference. And through our work with Floating Doctors, we extend this impact globally, using our expertise to create lasting change in communities around the world.
Our Board of Trustees
Richard M. Arons, SCD
David L. Mayer, PHD
Robert M. Crane
Daniel Farber Huang
Karen Fisher, JD
Cary Greene, MBA
Cristina Jimenez
Michael Prokopsis, MBA
Shikha Anand, MD, MPH
Marcus Schabacker, MD, PHD
OUR PRESENCE
TRUSTED ACROSS THE GLOBE
5200 Butler Pike Plymouth Meeting, PA 19462 610.825.6000 [email protected]
ECRI2 Falcon GateShire, Park Welwyn Garden, CityAL7 1TW, United Kingdom +44 (0) 1707 831001 [email protected] www.ecri.org.uk
West Wing, Level 12, Wisma Consplant 2 No. 7, Jalan SS16/1, 47500 Subang Jaya, Selangor, MALAYSIA +60-3-5651 5500 [email protected]
P.O. Box 25886, Abu Dhabi United Arab Emirates +971 54 3237509 [email protected]
Get in Touch
Learn more about our impactful work can benefit you and your organization.
Career Conversations with Merck Take Deep Dives into Research Profession Roles
Blog May 23, 2024

Julia Buoscio, Associate Clinical Research Associate, Merck
In anticipation of the May 29 “ Merck Journeys: Career Conversations within Clinical Research ” virtual career event being held by Merck in partnership with ACRP, we asked Julia Buoscio, an Associate Clinical Research Associate based in the New York City area with the company, to share some details on her early-career progression since completing an internship with Merck and transitioning to a full-time position.
Buoscio will be joined by Merck representatives in Clinical Data Management, Clinical Science and Study Management, and Clinical Research Associate roles as they describe career options and opportunities at the company and industrywide employment trends in drug research and development during the event, which is free for ACRP members and nonmembers.
ACRP: Please tell us a little about what drew you into science in the first place, how you found your internship with Merck, what you thought of this introduction to clinical research, and your career growth into your current role since then.
Buoscio: I have always had an interest in science and medicine. While completing my undergraduate degree at Bucknell University, I was on the pre-med track, as I felt medical school would align most with my passions. After a few shadowing experiences and externship opportunities, I reconsidered whether this path was for me. I knew I had a passion for healthcare, but I was unsure what other career paths would satisfy that.
After doing extensive research online and searching for internship opportunities, I came across the Clinical Science & Study Management (CSSM) Internship position at Merck. I was unfamiliar with the field of clinical research prior to this but was beyond excited to discover a field that highlighted my passion for advancing medicine further to improve patient care. I applied and was accepted to this internship in the summer of 2021, and my internship experience reinforced my passion for the field. I applied for a full-time position as a Clinical Trial Coordinator and joined Merck in the summer of 2022. A year later, I began my current position as an Associate Clinical Research Associate (ACRA).
ACRP: What kinds of transferable skills have allowed you to grow and transition as new career opportunities have presented themselves?
Buoscio: I believe some important skills that can be applied to a variety of roles include project management, organization, and proactivity. However, I think the most valuable skills (especially for those early in their career) are the ability to recognize what you don’t know and to seek out that information by asking questions.
When I first started the ACRA role, I had a lot to learn. I had to learn all of Merck’s standard operating procedures and site processes, the complexity of each protocol I was working on, the various systems we were using (electronic data capture, electronic trial master file, vendor systems, etc.), and more. There was a point where I was hesitant to ask questions because I didn’t want to seem unprepared or that I was lacking information. However, I have learned that getting comfortable asking questions is a vital skill to recognize and develop in your career. I am grateful to be surrounded by such amazing colleagues who have been so open to hopping on a quick call with me or guiding me in the right direction of a resource when I didn’t know how to navigate a situation. The skill of “asking questions” has proven itself to be very valuable to me and has enabled me to grow and develop through my career.
ACRP: Have there been elements of the Merck culture, career development tools, or side opportunities within the company that have helped support your growth and development there?
Buoscio: My favorite part about Merck is that the company offers “Gigs,” which are opportunities that employees apply to and complete at the same time as their regular role. Gigs provide development experiences for employees to strengthen and grow skills or gain exposure to a new function/division/region that’s in alignment with their developmental goals or career aspirations. During my first year at Merck, I recognized certain skills outside the scope of my day-to-day job that I wanted to further develop, such as project management and leadership. I searched for Gigs on our “Opportunity Marketplace” and came across an event planning position through one of our Employee Business Resource Groups called “Next Gen Network” (NGN). I applied and interviewed for the position and was thrilled to be accepted.
In this Gig position, I managed and coordinated all event planning activities for a United States Regional Leadership Summit for about 50 NGN chapter leads across the U.S. and Canada. I consulted with the U.S. Leadership Team to determine objectives and requirements for the event; I managed the budget and booked all the venues, hotels, and activities for the group; I attended the meeting and facilitated workshops; and I conducted an after-action review post-summit and presented on lessons learned from the summit.
My Gig helped me develop transferable skills, enhance productivity with a high workload, become more efficient at multitasking, and even improve my confidence in my ability to learn new things. I am so grateful that Merck offers Gigs and provides employees the opportunity to enhance skills and capabilities through meaningful work beyond the scope of one’s typical role.
ACRP: Please share the top few pieces of advice you have for someone who is interested in exploring a clinical career at Merck.
Buoscio: My main piece of advice is to recognize the value of networking and learn to excel at it. I think the best way to learn more about clinical research and opportunities within the field is by talking to individuals already embedded in it—learning about their career path, hearing about their current role, and asking for any guidance they have to offer.
Before I joined the company, I connected with individuals on LinkedIn and asked if they were open to chatting briefly on a 1-on-1 Zoom/Teams call. Everyone offered their own unique perspective which allowed me to learn so much from these conversations. The people at Merck are truly what make the company so special, and I have found that everyone at the company has been very open to connecting to offer advice and guidance. I also think it’s beneficial to take advantage of resources offered online—ACRP offers some great courses that provide a thorough overview of clinical research and can prepare you for a variety of positions within the field.
Edited by Gary Cramer
Sorry, we couldn't find any jobs that match your criteria.

Data Professional Reflects on Her ‘Journey Back into Clinical Research’ During AAPI Heritage Month

Health Equity Expert Values AAPI Heritage Month as Chance to Advocate for Diversity in Clinical Trials
Jobs in the acrp career center.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 13 May 2024
Long-term weight loss effects of semaglutide in obesity without diabetes in the SELECT trial
- Donna H. Ryan 1 ,
- Ildiko Lingvay ORCID: orcid.org/0000-0001-7006-7401 2 ,
- John Deanfield 3 ,
- Steven E. Kahn 4 ,
- Eric Barros ORCID: orcid.org/0000-0001-6613-4181 5 ,
- Bartolome Burguera 6 ,
- Helen M. Colhoun ORCID: orcid.org/0000-0002-8345-3288 7 ,
- Cintia Cercato ORCID: orcid.org/0000-0002-6181-4951 8 ,
- Dror Dicker 9 ,
- Deborah B. Horn 10 ,
- G. Kees Hovingh 5 ,
- Ole Kleist Jeppesen 5 ,
- Alexander Kokkinos 11 ,
- A. Michael Lincoff ORCID: orcid.org/0000-0001-8175-2121 12 ,
- Sebastian M. Meyhöfer 13 ,
- Tugce Kalayci Oral 5 ,
- Jorge Plutzky ORCID: orcid.org/0000-0002-7194-9876 14 ,
- André P. van Beek ORCID: orcid.org/0000-0002-0335-8177 15 ,
- John P. H. Wilding ORCID: orcid.org/0000-0003-2839-8404 16 &
- Robert F. Kushner 17
Nature Medicine ( 2024 ) Cite this article
44k Accesses
3 Citations
2423 Altmetric
Metrics details
- Health care
- Medical research
In the SELECT cardiovascular outcomes trial, semaglutide showed a 20% reduction in major adverse cardiovascular events in 17,604 adults with preexisting cardiovascular disease, overweight or obesity, without diabetes. Here in this prespecified analysis, we examined effects of semaglutide on weight and anthropometric outcomes, safety and tolerability by baseline body mass index (BMI). In patients treated with semaglutide, weight loss continued over 65 weeks and was sustained for up to 4 years. At 208 weeks, semaglutide was associated with mean reduction in weight (−10.2%), waist circumference (−7.7 cm) and waist-to-height ratio (−6.9%) versus placebo (−1.5%, −1.3 cm and −1.0%, respectively; P < 0.0001 for all comparisons versus placebo). Clinically meaningful weight loss occurred in both sexes and all races, body sizes and regions. Semaglutide was associated with fewer serious adverse events. For each BMI category (<30, 30 to <35, 35 to <40 and ≥40 kg m − 2 ) there were lower rates (events per 100 years of observation) of serious adverse events with semaglutide (43.23, 43.54, 51.07 and 47.06 for semaglutide and 50.48, 49.66, 52.73 and 60.85 for placebo). Semaglutide was associated with increased rates of trial product discontinuation. Discontinuations increased as BMI class decreased. In SELECT, at 208 weeks, semaglutide produced clinically significant weight loss and improvements in anthropometric measurements versus placebo. Weight loss was sustained over 4 years. ClinicalTrials.gov identifier: NCT03574597 .
Similar content being viewed by others

Effects of a personalized nutrition program on cardiometabolic health: a randomized controlled trial

Two-year effects of semaglutide in adults with overweight or obesity: the STEP 5 trial

What is the pipeline for future medications for obesity?
The worldwide obesity prevalence, defined by body mass index (BMI) ≥30 kg m − 2 , has nearly tripled since 1975 (ref. 1 ). BMI is a good surveillance measure for population changes over time, given its strong correlation with body fat amount on a population level, but it may not accurately indicate the amount or location of body fat at the individual level 2 . In fact, the World Health Organization defines clinical obesity as ‘abnormal or excessive fat accumulation that may impair health’ 1 . Excess abnormal body fat, especially visceral adiposity and ectopic fat, is a driver of cardiovascular (CV) disease (CVD) 3 , 4 , 5 , and contributes to the global chronic disease burden of diabetes, chronic kidney disease, cancer and other chronic conditions 6 , 7 .
Remediating the adverse health effects of excess abnormal body fat through weight loss is a priority in addressing the global chronic disease burden. Improvements in CV risk factors, glycemia and quality-of-life measures including personal well-being and physical functioning generally begin with modest weight loss of 5%, whereas greater weight loss is associated with more improvement in these measures 8 , 9 , 10 . Producing and sustaining durable and clinically significant weight loss with lifestyle intervention alone has been challenging 11 . However, weight-management medications that modify appetite can make attaining and sustaining clinically meaningful weight loss of ≥10% more likely 12 . Recently, weight-management medications, particularly those comprising glucagon-like peptide-1 receptor agonists, that help people achieve greater and more sustainable weight loss have been developed 13 . Once-weekly subcutaneous semaglutide 2.4 mg, a glucagon-like peptide-1 receptor agonist, is approved for chronic weight management 14 , 15 , 16 and at doses of up to 2.0 mg is approved for type 2 diabetes treatment 17 , 18 , 19 . In patients with type 2 diabetes and high CV risk, semaglutide at doses of 0.5 mg and 1.0 mg has been shown to significantly lower the risk of CV events 20 . The SELECT trial (Semaglutide Effects on Heart Disease and Stroke in Patients with Overweight or Obesity) studied patients with established CVD and overweight or obesity but without diabetes. In SELECT, semaglutide was associated with a 20% reduction in major adverse CV events (hazard ratio 0.80, 95% confidence interval (CI) 0.72 to 0.90; P < 0.001) 21 . Data derived from the SELECT trial offer the opportunity to evaluate the weight loss efficacy, in a geographically and racially diverse population, of semaglutide compared with placebo over 208 weeks when both are given in addition to standard-of-care recommendations for secondary CVD prevention (but without a focus on targeting weight loss). Furthermore, the data allow examination of changes in anthropometric measures such as BMI, waist circumference (WC) and waist-to-height ratio (WHtR) as surrogates for body fat amount and location 22 , 23 . The diverse population can also be evaluated for changes in sex- and race-specific ‘cutoff points’ for BMI and WC, which have been identified as anthropometric measures that predict cardiometabolic risk 8 , 22 , 23 .
This prespecified analysis of the SELECT trial investigated weight loss and changes in anthropometric indices in patients with established CVD and overweight or obesity without diabetes, who met inclusion and exclusion criteria, within a range of baseline categories for glycemia, renal function and body anthropometric measures.
Study population
The SELECT study enrolled 17,604 patients (72.3% male) from 41 countries between October 2018 and March 2021, with a mean (s.d.) age of 61.6 (8.9) years and BMI of 33.3 (5.0) kg m − 2 (ref. 21 ). The baseline characteristics of the population have been reported 24 . Supplementary Table 1 outlines SELECT patients according to baseline BMI categories. Of note, in the lower BMI categories (<30 kg m − 2 (overweight) and 30 to <35 kg m − 2 (class I obesity)), the proportion of Asian individuals was higher (14.5% and 7.4%, respectively) compared with the proportion of Asian individuals in the higher BMI categories (BMI 35 to <40 kg m − 2 (class II obesity; 3.8%) and ≥40 kg m − 2 (class III obesity; 2.2%), respectively). As the BMI categories increased, the proportion of women was higher: in the class III BMI category, 45.5% were female, compared with 20.8%, 25.7% and 33.0% in the overweight, class I and class II categories, respectively. Lower BMI categories were associated with a higher proportion of patients with normoglycemia and glycated hemoglobin <5.7%. Although the proportions of patients with high cholesterol and history of smoking were similar across BMI categories, the proportion of patients with high-sensitivity C-reactive protein ≥2.0 mg dl −1 increased as the BMI category increased. A high-sensitivity C-reactive protein >2.0 mg dl −1 was present in 36.4% of patients in the overweight BMI category, with a progressive increase to 43.3%, 57.3% and 72.0% for patients in the class I, II and III obesity categories, respectively.
Weight and anthropometric outcomes
Percentage weight loss.
The average percentage weight-loss trajectories with semaglutide and placebo over 4 years of observation are shown in Fig. 1a (ref. 21 ). For those in the semaglutide group, the weight-loss trajectory continued to week 65 and then was sustained for the study period through week 208 (−10.2% for the semaglutide group, −1.5% for the placebo group; treatment difference −8.7%; 95% CI −9.42 to −7.88; P < 0.0001). To estimate the treatment effect while on medication, we performed a first on-treatment analysis (observation period until the first time being off treatment for >35 days). At week 208, mean weight loss in the semaglutide group analyzed as first on-treatment was −11.7% compared with −1.5% for the placebo group (Fig. 1b ; treatment difference −10.2%; 95% CI −11.0 to −9.42; P < 0.0001).
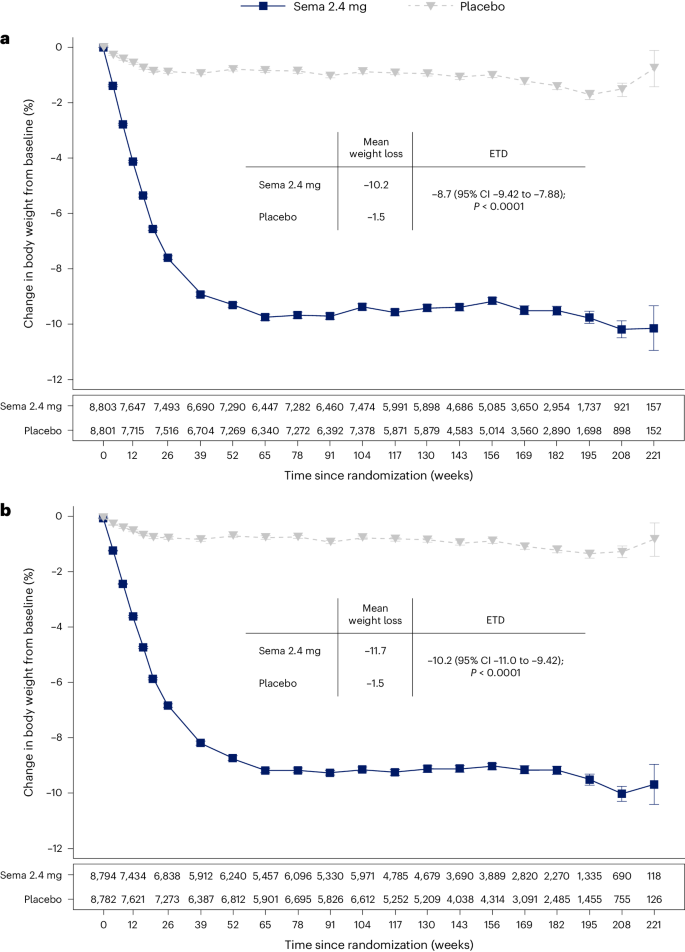
a , b , Observed data from the in-trial period ( a ) and first on-treatment ( b ). The symbols are the observed means, and error bars are ±s.e.m. Numbers shown below each panel represent the number of patients contributing to the means. Analysis of covariance with treatment and baseline values was used to estimate the treatment difference. Exact P values are 1.323762 × 10 −94 and 9.80035 × 10 −100 for a and b , respectively. P values are two-sided and are not adjusted for multiplicity. ETD, estimated treatment difference; sema, semaglutide.
Categorical weight loss and individual body weight change
Among in-trial (intention-to-treat principle) patients at week 104, weight loss of ≥5%, ≥10%, ≥15%, ≥20% and ≥25% was achieved by 67.8%, 44.2%, 22.9%, 11.0% and 4.9%, respectively, of those treated with semaglutide compared with 21.3%, 6.9%, 1.7%, 0.6% and 0.1% of those receiving placebo (Fig. 2a ). Individual weight changes at 104 weeks for the in-trial populations for semaglutide and placebo are depicted in Fig. 2b and Fig. 2c , respectively. These waterfall plots show the variation in weight-loss response that occurs with semaglutide and placebo and show that weight loss is more prominent with semaglutide than placebo.
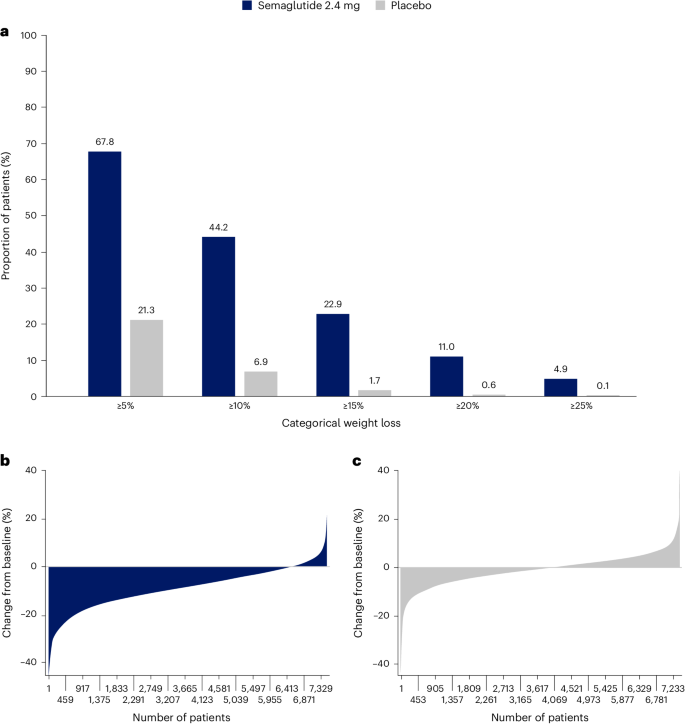
a , Categorical weight loss from baseline at week 104 for semaglutide and placebo. Data from the in-trial period. Bars depict the proportion (%) of patients receiving semaglutide or placebo who achieved ≥5%, ≥10%, ≥15%, ≥20% and ≥25% weight loss. b , c , Percentage change in body weight for individual patients from baseline to week 104 for semaglutide ( b ) and placebo ( c ). Each patient’s percentage change in body weight is plotted as a single bar.
Change in WC
WC change from baseline to 104 weeks has been reported previously in the primary outcome paper 21 . The trajectory of WC change mirrored that of the change in body weight. At week 208, average reduction in WC was −7.7 cm with semaglutide versus −1.3 cm with placebo, with a treatment difference of −6.4 cm (95% CI −7.18 to −5.61; P < 0.0001) 21 .
WC cutoff points
We analyzed achievement of sex- and race-specific cutoff points for WC by BMI <35 kg m − 2 or ≥35 kg m − 2 , because for BMI >35 kg m − 2 , WC is more difficult technically and, thus, less accurate as a risk predictor 4 , 25 , 26 . Within the SELECT population with BMI <35 kg m − 2 at baseline, 15.0% and 14.3% of the semaglutide and placebo groups, respectively, were below the sex- and race-specific WC cutoff points. At week 104, 41.2% fell below the sex- and race-specific cutoff points for the semaglutide group, compared with only 18.0% for the placebo group (Fig. 3 ).
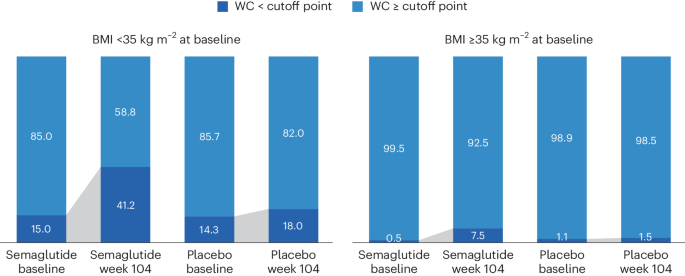
WC cutoff points; Asian women <80 cm, non-Asian women <88 cm, Asian men <88 cm, non-Asian men <102 cm.
Waist-to-height ratio
At baseline, mean WHtR was 0.66 for the study population. The lowest tertile of the SELECT population at baseline had a mean WHtR <0.62, which is higher than the cutoff point of 0.5 used to indicate increased cardiometabolic risk 27 , suggesting that the trial population had high WCs. At week 208, in the group randomized to semaglutide, there was a relative reduction of 6.9% in WHtR compared with 1.0% in placebo (treatment difference −5.87% points; 95% CI −6.56 to −5.17; P < 0.0001).
BMI category change
At week 104, 52.4% of patients treated with semaglutide achieved improvement in BMI category compared with 15.7% of those receiving placebo. Proportions of patients in the BMI categories at baseline and week 104 are shown in Fig. 4 , which depicts in-trial patients receiving semaglutide and placebo. The BMI category change reflects the superior weight loss with semaglutide, which resulted in fewer patients being in the higher BMI categories after 104 weeks. In the semaglutide group, 12.0% of patients achieved a BMI <25 kg m − 2 , which is considered the healthy BMI category, compared with 1.2% for placebo; per study inclusion criteria, no patients were in this category at baseline. The proportion of patients with obesity (BMI ≥30 kg m − 2 ) fell from 71.0% to 43.3% in the semaglutide group versus 71.9% to 67.9% in the placebo group.
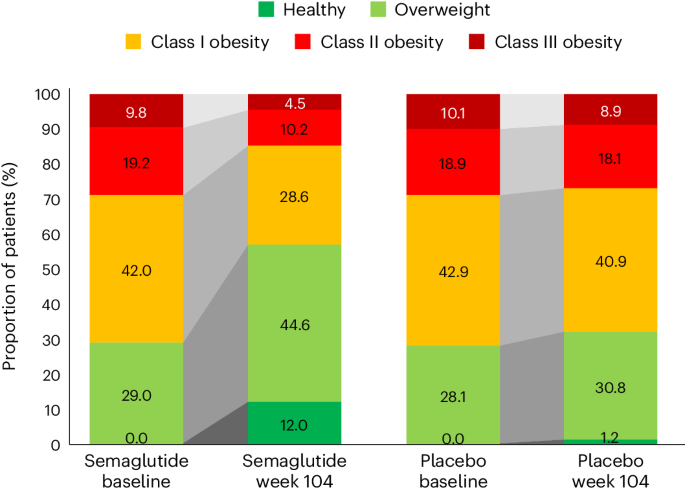
In the semaglutide group, 12.0% of patients achieved normal weight status at week 104 (from 0% at baseline), compared with 1.2% (from 0% at baseline) for placebo. BMI classes: healthy (BMI <25 kg m − 2 ), overweight (25 to <30 kg m − 2 ), class I obesity (30 to <35 kg m − 2 ), class II obesity (35 to <40 kg m − 2 ) and class III obesity (BMI ≥40 kg m − 2 ).
Weight and anthropometric outcomes by subgroups
The forest plot illustrated in Fig. 5 displays mean body weight percentage change from baseline to week 104 for semaglutide relative to placebo in prespecified subgroups. Similar relationships are depicted for WC changes in prespecified subgroups shown in Extended Data Fig. 1 . The effect of semaglutide (versus placebo) on mean percentage body weight loss as well as reduction in WC was found to be heterogeneous across several population subgroups. Women had a greater difference in mean weight loss with semaglutide versus placebo (−11.1% (95% CI −11.56 to −10.66) versus −7.5% in men (95% CI −7.78 to −7.23); P < 0.0001). There was a linear relationship between age category and degree of mean weight loss, with younger age being associated with progressively greater mean weight loss, but the actual mean difference by age group is small. Similarly, BMI category had small, although statistically significant, associations. Those with WHtR less than the median experienced slightly lower mean body weight change than those above the median, with estimated treatment differences −8.04% (95% CI −8.37 to −7.70) and −8.99% (95% CI −9.33 to −8.65), respectively ( P < 0.0001). Patients from Asia and of Asian race experienced slightly lower mean weight loss (estimated treatment difference with semaglutide for Asian race −7.27% (95% CI −8.09 to −6.46; P = 0.0147) and for Asia −7.30 (95% CI −7.97 to −6.62; P = 0.0016)). There was no difference in weight loss with semaglutide associated with ethnicity (estimated treatment difference for Hispanic −8.53% (95% CI −9.28 to −7.76) or non-Hispanic −8.52% (95% CI −8.77 to 8.26); P = 0.9769), glycemic status (estimated treatment difference for prediabetes −8.53% (95% CI −8.83 to −8.24) or normoglycemia −8.48% (95% CI −8.88 to −8.07; P = 0.8188) or renal function (estimated treatment difference for estimated glomerular filtration rate (eGFR) <60 or ≥60 ml min −1 1.73 m − 2 being −8.50% (95% CI −9.23 to −7.76) and −8.52% (95% CI −8.77 to −8.26), respectively ( P = 0.9519)).
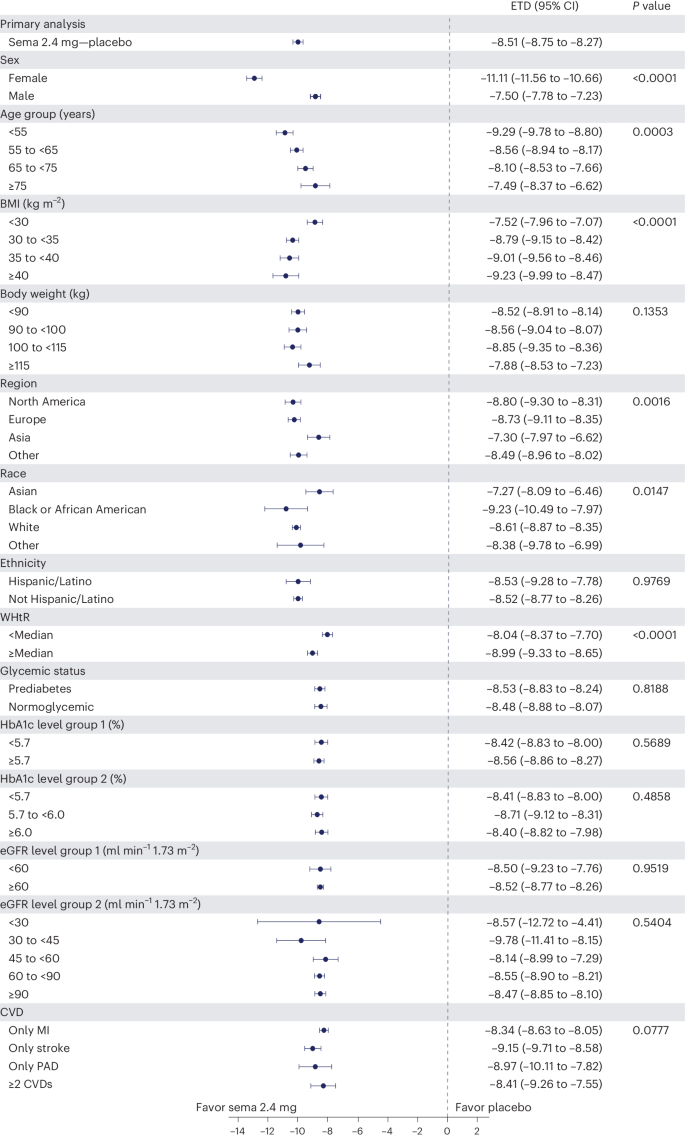
Data from the in-trial period. N = 17,604. P values represent test of no interaction effect. P values are two-sided and are not adjusted for multiplicity. The dots show estimated treatment differences, and the error bars show 95% CIs. Details of the statistical models are available in Methods . ETD, estimated treatment difference; HbA1c, glycated hemoglobin; MI, myocardial infarction; PAD, peripheral artery disease; sema, semaglutide.
Safety and tolerability according to baseline BMI category
We reported in the primary outcome of the SELECT trial that adverse events (AEs) leading to permanent discontinuation of the trial product occurred in 1,461 patients (16.6%) in the semaglutide group and 718 patients (8.2%) in the placebo group ( P < 0.001) 21 . For this analysis, we evaluated the cumulative incidence of AEs leading to trial product discontinuation by treatment assignment and by BMI category (Fig. 6 ). For this analysis, with death modeled as a competing risk, we tracked the proportion of in-trial patients for whom drug was withdrawn or interrupted for the first time (Fig. 6 , left) or cumulative discontinuations (Fig. 6 , right). Both panels of Fig. 6 depict a graded increase in the proportion discontinuing semaglutide, but not placebo. For lower BMI classes, discontinuation rates are higher in the semaglutide group but not the placebo group.
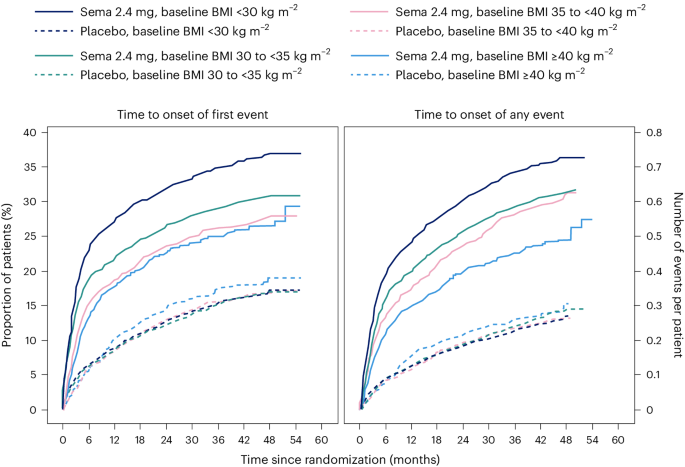
Data are in-trial from the full analysis set. sema, semaglutide.
We reported in the primary SELECT analysis that serious adverse events (SAEs) were reported by 2,941 patients (33.4%) in the semaglutide arm and by 3,204 patients (36.4%) in the placebo arm ( P < 0.001) 21 . For this study, we analyzed SAE rates by person-years of treatment exposure for BMI classes (<30 kg m − 2 , 30 to <35 kg m − 2 , 35 to <40 kg m − 2 , and ≥40 kg m − 2 ) and provide these data in Supplementary Table 2 . We also provide an analysis of the most common categories of SAEs. Semaglutide was associated with lower SAEs, primarily driven by CV event and infections. Within each obesity class (<30 kg m − 2 , 30 to <35 kg m − 2 , 35 to <40 kg m − 2 , and ≥40 kg m − 2 ), there were fewer SAEs in the group receiving semaglutide compared with placebo. Rates (events per 100 years of observation) of SAEs were 43.23, 43.54, 51.07 and 47.06 for semaglutide and 50.48, 49.66, 52.73 and 60.85 for placebo, with no evidence of heterogeneity. There was no detectable difference in hepatobiliary or gastrointestinal SAEs comparing semaglutide with placebo in any of the four BMI classes we evaluated.
The analyses of weight effects of the SELECT study presented here reveal that patients assigned to once-weekly subcutaneous semaglutide 2.4 mg lost significantly more weight than those receiving placebo. The weight-loss trajectory with semaglutide occurred over 65 weeks and was sustained up to 4 years. Likewise, there were similar improvements in the semaglutide group for anthropometrics (WC and WHtR). The weight loss was associated with a greater proportion of patients receiving semaglutide achieving improvement in BMI category, healthy BMI (<25 kg m − 2 ) and falling below the WC cutoff point above which increased cardiometabolic risk for the sex and race is greater 22 , 23 . Furthermore, both sexes, all races, all body sizes and those from all geographic regions were able to achieve clinically meaningful weight loss. There was no evidence of increased SAEs based on BMI categories, although lower BMI category was associated with increased rates of trial product discontinuation, probably reflecting exposure to a higher level of drug in lower BMI categories. These data, representing the longest clinical trial of the effects of semaglutide versus placebo on weight, establish the safety and durability of semaglutide effects on weight loss and maintenance in a geographically and racially diverse population of adult men and women with overweight and obesity but not diabetes. The implications of weight loss of this degree in such a diverse population suggests that it may be possible to impact the public health burden of the multiple morbidities associated with obesity. Although our trial focused on CV events, many chronic diseases would benefit from effective weight management 28 .
There were variations in the weight-loss response. Individual changes in body weight with semaglutide and placebo were striking; still, 67.8% achieved 5% or more weight loss and 44.2% achieved 10% weight loss with semaglutide at 2 years, compared with 21.3% and 6.9%, respectively, for those receiving placebo. Our first on-treatment analysis demonstrated that those on-drug lost more weight than those in-trial, confirming the effect of drug exposure. With semaglutide, lower BMI was associated with less percentage weight loss, and women lost more weight on average than men (−11.1% versus −7.5% treatment difference from placebo); however, in all cases, clinically meaningful mean weight loss was achieved. Although Asian patients lost less weight on average than patients of other races (−7.3% more than placebo), Asian patients were more likely to be in the lowest BMI category (<30 kg m − 2 ), which is known to be associated with less weight loss, as discussed below. Clinically meaningful weight loss was evident in the semaglutide group within a broad range of baseline categories for glycemia and body anthropometrics. Interestingly, at 2 years, a significant proportion of the semaglutide-treated group fell below the sex- and race-specific WC cutoff points, especially in those with BMI <35 kg m − 2 , and a notable proportion (12.0%) fell below the BMI cutoff point of 25 kg m − 2 , which is deemed a healthy BMI in those without unintentional weight loss. As more robust weight loss is possible with newer medications, achieving and maintaining these cutoff point targets may become important benchmarks for tracking responses.
The overall safety profile did not reveal any new signals from prior studies, and there were no BMI category-related associations with AE reporting. The analysis did reveal that tolerability may differ among specific BMI classes, since more discontinuations occurred with semaglutide among lower BMI classes. Potential contributors may include a possibility of higher drug exposure in lower BMI classes, although other explanations, including differences in motivation and cultural mores regarding body size, cannot be excluded.
Is the weight loss in SELECT less than expected based on prior studies with the drug? In STEP 1, a large phase 3 study of once-weekly subcutaneous semaglutide 2.4 mg in individuals without diabetes but with BMI >30 kg m − 2 or 27 kg m − 2 with at least one obesity-related comorbidity, the mean weight loss was −14.9% at week 68, compared with −2.4% with placebo 14 . Several reasons may explain the observation that the mean treatment difference was −12.5% in STEP 1 and −8.7% in SELECT. First, SELECT was designed as a CV outcomes trial and not a weight-loss trial, and weight loss was only a supportive secondary endpoint in the trial design. Patients in STEP 1 were desirous of weight loss as a reason for study participation and received structured lifestyle intervention (which included a −500 kcal per day diet with 150 min per week of physical activity). In the SELECT trial, patients did not enroll for the specific purpose of weight loss and received standard of care covering management of CV risk factors, including medical treatment and healthy lifestyle counseling, but without a specific focus on weight loss. Second, the respective study populations were quite different, with STEP 1 including a younger, healthier population with more women (73.1% of the semaglutide arm in STEP 1 versus 27.7% in SELECT) and higher mean BMI (37.8 kg m − 2 versus 33.3 kg m − 2 , respectively) 14 , 21 . Third, major differences existed between the respective trial protocols. Patients in the semaglutide treatment arm of STEP 1 were more likely to be exposed to the medication at the full dose of 2.4 mg than those in SELECT. In SELECT, investigators were allowed to slow, decrease or pause treatment. By 104 weeks, approximately 77% of SELECT patients on dose were receiving the target semaglutide 2.4 mg weekly dose, which is lower than the corresponding proportion of patients in STEP 1 (89.6% were receiving the target dose at week 68) 14 , 21 . Indeed, in our first on-treatment analysis at week 208, weight loss was greater (−11.7% for semaglutide) compared with the in-trial analysis (−10.2% for semaglutide). Taken together, all these issues make less weight loss an expected finding in SELECT, compared with STEP 1.
The SELECT study has some limitations. First, SELECT was not a primary prevention trial, and the data should not be extrapolated to all individuals with overweight and obesity to prevent major adverse CV events. Although the data set is rich in numbers and diversity, it does not have the numbers of individuals in racial subgroups that may have revealed potential differential effects. SELECT also did not include individuals who have excess abnormal body fat but a BMI <27 kg m − 2 . Not all individuals with increased CV risk have BMI ≥27 kg m − 2 . Thus, the study did not include Asian patients who qualify for treatment with obesity medications at lower BMI and WC cutoff points according to guidelines in their countries 29 . We observed that Asian patients were less likely to be in the higher BMI categories of SELECT and that the population of those with BMI <30 kg m − 2 had a higher percentage of Asian race. Asian individuals would probably benefit from weight loss and medication approaches undertaken at lower BMI levels in the secondary prevention of CVD. Future studies should evaluate CV risk reduction in Asian individuals with high CV risk and BMI <27 kg m − 2 . Another limitation is the lack of information on body composition, beyond the anthropometric measures we used. It would be meaningful to have quantitation of fat mass, lean mass and muscle mass, especially given the wide range of body size in the SELECT population.
An interesting observation from this SELECT weight loss data is that when BMI is ≤30 kg m − 2 , weight loss on a percentage basis is less than that observed across higher classes of BMI severity. Furthermore, as BMI exceeds 30 kg m − 2 , weight loss amounts are more similar for class I, II and III obesity. This was also observed in Look AHEAD, a lifestyle intervention study for weight loss 30 . The proportion (percentage) of weight loss seems to be less, on average, in the BMI <30 kg m − 2 category relative to higher BMI categories, despite their receiving of the same treatment and even potentially higher exposure to the drug for weight loss 30 . Weight loss cannot continue indefinitely. There is a plateau of weight that occurs after weight loss with all treatments for weight management. This plateau has been termed the ‘set point’ or ‘settling point’, a body weight that is in harmony with the genetic and environmental determinants of body weight and adiposity 31 . Perhaps persons with BMI <30 kg m − 2 are closer to their settling point and have less weight to lose to reach it. Furthermore, the cardiometabolic benefits of weight loss are driven by reduction in the abnormal ectopic and visceral depots of fat, not by reduction of subcutaneous fat stores in the hips and thighs. The phenotype of cardiometabolic disease but lower BMI (<30 kg m − 2 ) may be one where reduction of excess abnormal and dysfunctional body fat does not require as much body mass reduction to achieve health improvement. We suspect this may be the case and suggest further studies to explore this aspect of weight-loss physiology.
In conclusion, this analysis of the SELECT study supports the broad use of once-weekly subcutaneous semaglutide 2.4 mg as an aid to CV event reduction in individuals with overweight or obesity without diabetes but with preexisting CVD. Semaglutide 2.4 mg safely and effectively produced clinically significant weight loss in all subgroups based on age, sex, race, glycemia, renal function and anthropometric categories. Furthermore, the weight loss was sustained over 4 years during the trial.
Trial design and participants
The current work complies with all relevant ethical regulations and reports a prespecified analysis of the randomized, double-blind, placebo-controlled SELECT trial ( NCT03574597 ), details of which have been reported in papers describing study design and rationale 32 , baseline characteristics 24 and the primary outcome 21 . SELECT evaluated once-weekly subcutaneous semaglutide 2.4 mg versus placebo to reduce the risk of major adverse cardiac events (a composite endpoint comprising CV death, nonfatal myocardial infarction or nonfatal stroke) in individuals with established CVD and overweight or obesity, without diabetes. The protocol for SELECT was approved by national and institutional regulatory and ethical authorities in each participating country. All patients provided written informed consent before beginning any trial-specific activity. Eligible patients were aged ≥45 years, with a BMI of ≥27 kg m − 2 and established CVD defined as at least one of the following: prior myocardial infarction, prior ischemic or hemorrhagic stroke, or symptomatic peripheral artery disease. Additional inclusion and exclusion criteria can be found elsewhere 32 .
Human participants research
The trial protocol was designed by the trial sponsor, Novo Nordisk, and the academic Steering Committee. A global expert panel of physician leaders in participating countries advised on regional operational issues. National and institutional regulatory and ethical authorities approved the protocol, and all patients provided written informed consent.
Study intervention and patient management
Patients were randomly assigned in a double-blind manner and 1:1 ratio to receive once-weekly subcutaneous semaglutide 2.4 mg or placebo. The starting dose was 0.24 mg once weekly, with dose increases every 4 weeks (to doses of 0.5, 1.0, 1.7 and 2.4 mg per week) until the target dose of 2.4 mg was reached after 16 weeks. Patients who were unable to tolerate dose escalation due to AEs could be managed by extension of dose-escalation intervals, treatment pauses or maintenance at doses below the 2.4 mg per week target dose. Investigators were allowed to reduce the dose of study product if tolerability issues arose. Investigators were provided with guidelines for, and encouraged to follow, evidence-based recommendations for medical treatment and lifestyle counseling to optimize management of underlying CVD as part of the standard of care. The lifestyle counseling was not targeted at weight loss. Additional intervention descriptions are available 32 .
Sex, race, body weight, height and WC measurements
Sex and race were self-reported. Body weight was measured without shoes and only wearing light clothing; it was measured on a digital scale and recorded in kilograms or pounds (one decimal with a precision of 0.1 kg or lb), with preference for using the same scale throughout the trial. The scale was calibrated yearly as a minimum unless the manufacturer certified that calibration of the weight scales was valid for the lifetime of the scale. Height was measured without shoes in centimeters or inches (one decimal with a precision of 0.1 cm or inches). At screening, BMI was calculated by the electronic case report form. WC was defined as the abdominal circumference located midway between the lower rib margin and the iliac crest. Measures were obtained in a standing position with a nonstretchable measuring tape and to the nearest centimeter or inch. The patient was asked to breathe normally. The tape touched the skin but did not compress soft tissue, and twists in the tape were avoided.
The following endpoints relevant to this paper were assessed at randomization (week 0) to years 2, 3 and 4: change in body weight (%); proportion achieving weight loss ≥5%, ≥10%, ≥15% and ≥20%; change in WC (cm); and percentage change in WHtR (cm cm −1 ). Improvement in BMI category (defined as being in a lower BMI class) was assessed at week 104 compared with baseline according to BMI classes: healthy (BMI <25 kg m − 2 ), overweight (25 to <30 kg m − 2 ), class I obesity (30 to <35 kg m − 2 ), class II obesity (35 to <40 kg m − 2 ) and class III obesity (≥40 kg m − 2 ). The proportions of individuals with BMI <35 or ≥35 kg m − 2 who achieved sex- and race-specific cutoff points for WC (indicating increased metabolic risk) were evaluated at week 104. The WC cutoff points were as follows: Asian women <80 cm, non-Asian women <88 cm, Asian men <88 cm and non-Asian men <102 cm.
Overall, 97.1% of the semaglutide group and 96.8% of the placebo group completed the trial. During the study, 30.6% of those assigned to semaglutide did not complete drug treatment, compared with 27.0% for placebo.
Statistical analysis
The statistical analyses for the in-trial period were based on the intention-to-treat principle and included all randomized patients irrespective of adherence to semaglutide or placebo or changes to background medications. Continuous endpoints were analyzed using an analysis of covariance model with treatment as a fixed factor and baseline value of the endpoint as a covariate. Missing data at the landmark visit, for example, week 104, were imputed using a multiple imputation model and done separately for each treatment arm and included baseline value as a covariate and fit to patients having an observed data point (irrespective of adherence to randomized treatment) at week 104. The fit model is used to impute values for all patients with missing data at week 104 to create 500 complete data sets. Rubin’s rules were used to combine the results. Estimated means are provided with s.e.m., and estimated treatment differences are provided with 95% CI. Binary endpoints were analyzed using logistic regression with treatment and baseline value as a covariate, where missing data were imputed by first using multiple imputation as described above and then categorizing the imputed data according to the endpoint, for example, body weight percentage change at week 104 of <0%. Subgroup analyses for continuous and binary endpoints also included the subgroup and interaction between treatment and subgroup as fixed factors. Because some patients in both arms continued to be followed but were off treatment, we also analyzed weight loss by first on-treatment group (observation period until first time being off treatment for >35 days) to assess a more realistic picture of weight loss in those adhering to treatment. CIs were not adjusted for multiplicity and should therefore not be used to infer definitive treatment effects. All statistical analyses were performed with SAS software, version 9.4 TS1M5 (SAS Institute).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Data will be shared with bona fide researchers who submit a research proposal approved by the independent review board. Individual patient data will be shared in data sets in a deidentified and anonymized format. Information about data access request proposals can be found at https://www.novonordisk-trials.com/ .
Obesity and overweight. World Health Organization https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (2021).
Cornier, M. A. et al. Assessing adiposity: a scientific statement from the American Heart Association. Circulation 124 , 1996–2019 (2011).
Article PubMed Google Scholar
Afshin, A. et al. Health effects of overweight and obesity in 195 countries over 25 years. N. Engl. J. Med. 377 , 13–27 (2017).
Jensen, M. D. et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J. Am. Coll. Cardiol. 63 , 2985–3023 (2014).
Poirier, P. et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 113 , 898–918 (2006).
Dai, H. et al. The global burden of disease attributable to high body mass index in 195 countries and territories, 1990–2017: an analysis of the Global Burden of Disease Study. PLoS Med. 17 , e1003198 (2020).
Article PubMed PubMed Central Google Scholar
Ndumele, C. E. et al. Cardiovascular–kidney–metabolic health: a presidential advisory from the American Heart Association. Circulation 148 , 1606–1635 (2023).
Garvey, W. T. et al. American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr. Pr. 22 , 1–203 (2016).
Article Google Scholar
Ryan, D. H. & Yockey, S. R. Weight loss and improvement in comorbidity: differences at 5%, 10%, 15%, and over. Curr. Obes. Rep. 6 , 187–194 (2017).
Wing, R. R. et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care 34 , 1481–1486 (2011).
Article CAS PubMed PubMed Central Google Scholar
Wadden, T. A., Tronieri, J. S. & Butryn, M. L. Lifestyle modification approaches for the treatment of obesity in adults. Am. Psychol. 75 , 235–251 (2020).
Tchang, B. G. et al. Pharmacologic treatment of overweight and obesity in adults. in (eds. Feingold, K. R. et al.) Endotext https://www.ncbi.nlm.nih.gov/books/NBK279038/ (MDText.com, 2000).
Müller, T. D., Blüher, M., Tschöp, M. H. & DiMarchi, R. D. Anti-obesity drug discovery: advances and challenges. Nat. Rev. Drug Discov. 21 , 201–223 (2022).
Wilding, J. P. H. et al. Once-weekly semaglutide in adults with overweight or obesity. N. Engl. J. Med. 384 , 989–1002 (2021).
Article CAS PubMed Google Scholar
Wegovy (semaglutide) summary of product characteristics. European Medicines Agency https://www.ema.europa.eu/en/documents/product-information/wegovy-epar-product-information_en.pdf (2023).
WEGOVY (semaglutide) prescribing information. Food and Drug Administration https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/215256s007lbl.pdf (2023).
Sorli, C. et al. Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double-blind, randomised, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol. 5 , 251–260 (2017).
Ozempic (semaglutide) summary of product characteristics. European Medicines Agency https://www.ema.europa.eu/en/documents/product-information/ozempic-epar-product-information_en.pdf (2023).
OZEMPIC (semaglutide) prescribing information. Food and Drug Administration https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209637lbl.pdf (2017).
Marso, S. P. et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 375 , 1834–1844 (2016).
Lincoff, A. M. et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. N. Engl. J. Med. 389 , 2221–2232 (2023).
Ross, R. et al. Waist circumference as a vital sign in clinical practice: a consensus statement from the IAS and ICCR Working Group on Visceral Obesity. Nat. Rev. Endocrinol. 16 , 177–189 (2020).
Snijder, M. B., van Dam, R. M., Visser, M. & Seidell, J. C. What aspects of body fat are particularly hazardous and how do we measure them? Int. J. Epidemiol. 35 , 83–92 (2006).
Lingvay, I. et al. Semaglutide for cardiovascular event reduction in people with overweight or obesity: SELECT study baseline characteristics. Obesity 31 , 111–122 (2023).
Basset, J. The Asia-Pacific perspective: redefining obesity and its treatment. International Diabetes Institute, World Health Organization Regional Office for the Western Pacific, International Association for the Study of Obesity & International Obesity Task Force https://www.vepachedu.org/TSJ/BMI-Guidelines.pdf (2000).
Hu, F. in Obesity Epidemiology (ed. Hu, F.) 53–83 (Oxford University Press, 2008).
Browning, L. M., Hsieh, S. D. & Ashwell, M. A systematic review of waist-to-height ratio as a screening tool for the prediction of cardiovascular disease and diabetes: 0·5 could be a suitable global boundary value. Nutr. Res. Rev. 23 , 247–269 (2010).
Sattar, N. et al. Treating chronic diseases without tackling excess adiposity promotes multimorbidity. Lancet Diabetes Endocrinol. 11 , 58–62 (2023).
Obesity classification. World Obesity https://www.worldobesity.org/about/about-obesity/obesity-classification (2022).
Unick, J. L. et al. Effectiveness of lifestyle interventions for individuals with severe obesity and type 2 diabetes: results from the Look AHEAD trial. Diabetes Care 34 , 2152–2157 (2011).
Speakman, J. R. et al. Set points, settling points and some alternative models: theoretical options to understand how genes and environments combine to regulate body adiposity. Dis. Model. Mech. 4 , 733–745 (2011).
Ryan, D. H. et al. Semaglutide effects on cardiovascular outcomes in people with overweight or obesity (SELECT) rationale and design. Am. Heart J. 229 , 61–69 (2020).
Download references
Acknowledgements
Editorial support was provided by Richard Ogilvy-Stewart of Apollo, OPEN Health Communications, and funded by Novo Nordisk A/S, in accordance with Good Publication Practice guidelines ( www.ismpp.org/gpp-2022 ).
Author information
Authors and affiliations.
Pennington Biomedical Research Center, Baton Rouge, LA, USA
Donna H. Ryan
Department of Internal Medicine/Endocrinology and Peter O’ Donnell Jr. School of Public Health, University of Texas Southwestern Medical Center, Dallas, TX, USA
Ildiko Lingvay
Institute of Cardiovascular Science, University College London, London, UK
John Deanfield
VA Puget Sound Health Care System and University of Washington, Seattle, WA, USA
Steven E. Kahn
Novo Nordisk A/S, Søborg, Denmark
Eric Barros, G. Kees Hovingh, Ole Kleist Jeppesen & Tugce Kalayci Oral
Endocrinology and Metabolism Institute, Cleveland Clinic, Cleveland, OH, USA
Bartolome Burguera
Institute of Genetics and Cancer, University of Edinburgh, Edinburgh, UK
Helen M. Colhoun
Obesity Unit, Department of Endocrinology, Hospital das Clínicas, University of São Paulo, São Paulo, Brazil
Cintia Cercato
Internal Medicine Department D, Hasharon Hospital-Rabin Medical Center, Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel
Dror Dicker
Center for Obesity Medicine and Metabolic Performance, Department of Surgery, University of Texas McGovern Medical School, Houston, TX, USA
Deborah B. Horn
First Department of Propaedeutic Internal Medicine, School of Medicine, National and Kapodistrian University of Athens, Athens, Greece
Alexander Kokkinos
Department of Cardiovascular Medicine, Cleveland Clinic, and Cleveland Clinic Lerner College of Medicine of Case Western Reserve University, Cleveland, OH, USA
A. Michael Lincoff
Institute of Endocrinology & Diabetes, University of Lübeck, Lübeck, Germany
Sebastian M. Meyhöfer
Cardiovascular Division, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA
Jorge Plutzky
University of Groningen, University Medical Center Groningen, Department of Endocrinology, Groningen, the Netherlands
André P. van Beek
Department of Cardiovascular and Metabolic Medicine, University of Liverpool, Liverpool, UK
John P. H. Wilding
Northwestern University Feinberg School of Medicine, Chicago, IL, USA
Robert F. Kushner
You can also search for this author in PubMed Google Scholar
Contributions
D.H.R., I.L. and S.E.K. contributed to the study design. D.B.H., I.L., D.D., A.K., S.M.M., A.P.v.B., C.C. and J.P.H.W. were study investigators. D.B.H., I.L., D.D., A.K., S.M.M., A.P.v.B., C.C. and J.P.H.W. enrolled patients. D.H.R. was responsible for data analysis and manuscript preparation. All authors contributed to data interpretation, review, revisions and final approval of the manuscript.
Corresponding author
Correspondence to Donna H. Ryan .
Ethics declarations
Competing interests.
D.H.R. declares having received consulting honoraria from Altimmune, Amgen, Biohaven, Boehringer Ingelheim, Calibrate, Carmot Therapeutics, CinRx, Eli Lilly, Epitomee, Gila Therapeutics, IFA Celtics, Novo Nordisk, Pfizer, Rhythm, Scientific Intake, Wondr Health and Zealand Pharma; she declares she received stock options from Calibrate, Epitomee, Scientific Intake and Xeno Bioscience. I.L. declares having received research funding (paid to institution) from Novo Nordisk, Sanofi, Mylan and Boehringer Ingelheim. I.L. received advisory/consulting fees and/or other support from Altimmune, AstraZeneca, Bayer, Biomea, Boehringer Ingelheim, Carmot Therapeutics, Cytoki Pharma, Eli Lilly, Intercept, Janssen/Johnson & Johnson, Mannkind, Mediflix, Merck, Metsera, Novo Nordisk, Pharmaventures, Pfizer, Regeneron, Sanofi, Shionogi, Structure Therapeutics, Target RWE, Terns Pharmaceuticals, The Comm Group, Valeritas, WebMD and Zealand Pharma. J.D. declares having received consulting honoraria from Amgen, Boehringer Ingelheim, Merck, Pfizer, Aegerion, Novartis, Sanofi, Takeda, Novo Nordisk and Bayer, and research grants from British Heart Foundation, MRC (UK), NIHR, PHE, MSD, Pfizer, Aegerion, Colgate and Roche. S.E.K. declares having received consulting honoraria from ANI Pharmaceuticals, Boehringer Ingelheim, Eli Lilly, Merck, Novo Nordisk and Oramed, and stock options from AltPep. B.B. declares having received honoraria related to participation on this trial and has no financial conflicts related to this publication. H.M.C. declares being a stockholder and serving on an advisory panel for Bayer; receiving research grants from Chief Scientist Office, Diabetes UK, European Commission, IQVIA, Juvenile Diabetes Research Foundation and Medical Research Council; serving on an advisory board and speaker’s bureau for Novo Nordisk; and holding stock in Roche Pharmaceuticals. C.C. declares having received consulting honoraria from Novo Nordisk, Eli Lilly, Merck, Brace Pharma and Eurofarma. D.D. declares having received consulting honoraria from Novo Nordisk, Eli Lilly, Boehringer Ingelheim and AstraZeneca, and received research grants through his affiliation from Novo Nordisk, Eli Lilly, Boehringer Ingelheim and Rhythm. D.B.H. declares having received research grants through her academic affiliation from Novo Nordisk and Eli Lilly, and advisory/consulting honoraria from Novo Nordisk, Eli Lilly and Gelesis. A.K. declares having received research grants through his affiliation from Novo Nordisk and Pharmaserve Lilly, and consulting honoraria from Pharmaserve Lilly, Sanofi-Aventis, Novo Nordisk, MSD, AstraZeneca, ELPEN Pharma, Boehringer Ingelheim, Galenica Pharma, Epsilon Health and WinMedica. A.M.L. declares having received honoraria from Novo Nordisk, Eli Lilly, Akebia Therapeutics, Ardelyx, Becton Dickinson, Endologix, FibroGen, GSK, Medtronic, Neovasc, Provention Bio, ReCor, BrainStorm Cell Therapeutics, Alnylam and Intarcia for consulting activities, and research funding to his institution from AbbVie, Esperion, AstraZeneca, CSL Behring, Novartis and Eli Lilly. S.M.M. declares having received consulting honoraria from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Daichii-Sankyo, esanum, Gilead, Ipsen, Eli Lilly, Novartis, Novo Nordisk, Sandoz and Sanofi; he declares he received research grants from AstraZeneca, Eli Lilly and Novo Nordisk. J.P. declares having received consulting honoraria from Altimmune, Amgen, Esperion, Merck, MJH Life Sciences, Novartis and Novo Nordisk; he has received a grant, paid to his institution, from Boehringer Ingelheim and holds the position of Director, Preventive Cardiology, at Brigham and Women’s Hospital. A.P.v.B. is contracted via the University of Groningen (no personal payment) to undertake consultancy for Novo Nordisk, Eli Lilly and Boehringer Ingelheim. J.P.H.W. is contracted via the University of Liverpool (no personal payment) to undertake consultancy for Altimmune, AstraZeneca, Boehringer Ingelheim, Cytoki, Eli Lilly, Napp, Novo Nordisk, Menarini, Pfizer, Rhythm Pharmaceuticals, Sanofi, Saniona, Tern Pharmaceuticals, Shionogi and Ysopia. J.P.H.W. also declares personal honoraria/lecture fees from AstraZeneca, Boehringer Ingelheim, Medscape, Napp, Menarini, Novo Nordisk and Rhythm. R.F.K. declares having received consulting honoraria from Novo Nordisk, Weight Watchers, Eli Lilly, Boehringer Ingelheim, Pfizer, Structure and Altimmune. E.B., G.K.H., O.K.J. and T.K.O. are employees of Novo Nordisk A/S.
Peer review
Peer review information.
Nature Medicine thanks Christiana Kartsonaki, Peter Rossing, Naveed Sattar and Vikas Sridhar for their contribution to the peer review of this work. Primary Handling Editor: Sonia Muliyil, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended data fig. 1 effect of semaglutide treatment or placebo on waist circumference from baseline to week 104 by subgroups..
Data from the in-trial period. N = 17,604. P values represent test of no interaction effect. P values are two-sided and not adjusted for multiplicity. The dots show estimated treatment differences and the error bars show 95% confidence intervals. Details of the statistical models are available in Methods . BMI, body mass index; CI, confidence interval; CV, cardiovascular; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; ETD, estimated treatment difference; HbA1c, glycated hemoglobin; MI, myocardial infarction; PAD, peripheral artery disease; sema, semaglutide.
Supplementary information
Reporting summary, supplementary tables 1 and 2.
Supplementary Table 1. Baseline characteristics by BMI class. Data are represented as number and percentage of patients. Renal function categories were based on the eGFR as per Chronic Kidney Disease Epidemiology Collaboration. Albuminuria categories were based on UACR. Smoking was defined as smoking at least one cigarette or equivalent daily. The category ‘Other’ for CV inclusion criteria includes patients where it is unknown if the patient fulfilled only one or several criteria and patients who were randomized in error and did not fulfill any criteria. Supplementary Table 2. SAEs according to baseline BMI category. P value: two-sided P value from Fisher’s exact test for test of no difference.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Ryan, D.H., Lingvay, I., Deanfield, J. et al. Long-term weight loss effects of semaglutide in obesity without diabetes in the SELECT trial. Nat Med (2024). https://doi.org/10.1038/s41591-024-02996-7
Download citation
Received : 01 March 2024
Accepted : 12 April 2024
Published : 13 May 2024
DOI : https://doi.org/10.1038/s41591-024-02996-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
To revisit this article, visit My Profile, then View saved stories .
- Backchannel
- Newsletters
- WIRED Insider
- WIRED Consulting
Emily Mullin
Neuralink’s First User Is ‘Constantly Multitasking’ With His Brain Implant

In 2016, Noland Arbaugh suffered a spinal cord injury while swimming in a lake. The details are fuzzy, but what he remembers is rushing toward the water with his friends, diving in, and hitting his head on something—or someone. He floated to the surface, unable to move.
Doctors later confirmed that he was paralyzed from the neck down. Arbaugh went from being a self-sufficient college student to moving back in with his parents and relying on them for his daily needs. He learned how to get around in a wheelchair and use a mouth-held stick to operate an iPad, but the hardest adjustment was feeling like he was a burden on his family.
The year 2016 was also when Elon Musk cofounded the brain implant startup Neuralink . This January, Arbaugh became the first person to receive the company’s investigational device , dubbed Telepathy, as part of a clinical trial. Known as a brain-computer interface , or BCI, it decodes intended movement signals in the brain and translates them into computer commands. Arbaugh just has to think about moving a cursor on his laptop screen and it moves.
The experimental device has given Arbaugh, now 30, a sense of independence. Before, using a mouth-stick required someone to position him upright. If he dropped his mouth-stick, it needed to be picked up for him. And he couldn’t use it for long or he’d develop sores. With the Neuralink device, he has nearly full control of a computer. He can browse the web and play computer games whenever he wants, and Neuralink says he has set the human record for cursor control with a BCI .

Arbaugh isn’t the first person to get a BCI; one recipient, Nathan Copeland, has had one for nine years . Beyond Neuralink, several other companies are working to commercialize BCIs to help people with paralysis, mental health disorders, and even blindness. Arbaugh recently spoke with WIRED via Zoom to talk about his experience in the Neuralink study. This interview has been edited for length and clarity.

By Justin Pot

By Nena Farrell
By Charlie Wood

By Angela Watercutter
Emily Mullin: Before you got the implant, what was your day-to-day life like?
Noland Arbaugh: I was lying in bed most days, all day. I didn’t get up a whole lot unless I had something to do. I got up to take showers every other day. I got up when people would come to my house to see me, but outside of that I was just in bed.
Right before my “initiation” into Neuralink, I was trying to learn a lot more. I was trying to get my life back on track, because I had kind of done nothing for five years after my accident. So I started learning languages and stuff. The two years before the Neuralink trial I was actively trying to better myself.
You’ve said you found out about the Neuralink trial from a friend. Had you heard of brain-computer interfaces before then?
Musk is a very showy person, and Neuralink has livestreamed some flashy updates over the years. What was your initial impression of the company?
I was blown away at what they were trying to do. You know, Elon Musk, he has had such an impact on the world, whether people think that’s good or bad. It was really cool knowing that he was a part of something like this. I really feel like we’re kindred spirits with our mentality of wanting to better humanity.
It was very cool to see the richest man in the world, possibly one of the most powerful men in the world, taking an interest—you just don’t see this kind of funding go into things for handicapped people.
You had to go through a pretty extensive screening process to find out if you were eligible for the Neuralink trial. What was that like?
It took about a month. I applied and within a day I’d gotten an email back that said I’d been selected and they wanted me to go through the first interview. I did a bunch of Zoom interviews. I had to do a lot of medical screenings. They asked about my medical history and family history, and I had to do a psych screening.
At the very end, about a month in, I went to the hospital they had selected. I did a full day of screening, which was eight hours of tests, including brain scans, different head scans, blood tests, and urine tests. I did another psychoanalysis and then memory tests to see if I was all there cognitively and also just to get a baseline, so if anything changes they would be able to know where I was when I began. That was a long day.
After that, it was just a waiting game.
When they told you that you had been selected for the trial and were going to get the Neuralink implant, how did you feel?
The whole time I tried to keep my expectations really level. They told me throughout this whole process that at any point, if I don’t meet one of their criteria, they’ll move on in a different direction. I tried to push down any expectation I had just because I didn’t want to get my hopes up and be let down. It was hard to not get excited. But I think I needed that to keep me grounded through the whole process.
Did you have concerns or fears at any point about getting brain surgery?
There were a couple of things that gave me pause, but I wouldn’t call them concerns. It was more that I needed to think through it and sort through my feelings and emotions and see if I was really ready to undertake what was coming my way.
The first is that I’m a quadriplegic, and all I really have is my brain. So letting someone go in there and mess around, it’s a big commitment. If something goes wrong, that’s kind of it for me. But I knew I wanted to help out, and I didn’t want to let my fears get in the way of that.
The second thing that gave me pause was that I didn’t know if I wanted to be the first one to get this in my brain if anything would go wrong with the implant. What if it breaks or stops working and I only have it for a day, a week? I thought maybe someone else should get it first, and I’ll get the better version of it.

Did Neuralink prepare you for the possibility that the implant might not work?
I knew there were a lot of risks going in, and I knew it might not work. I didn’t anticipate any of that though. I had complete faith in Neuralink.
The day after your surgery, Musk posted on X that the device was showing neuron spike detection. Was it really that fast?
I was lying in my hospital bed right after surgery, and they came in and woke up the implant for the first time. They showed me a screen with different channels on it, and they said they were real-time signals that the Neuralink was picking up in my brain. So I knew it was working.
My first instinct was to just start playing around, moving my fingers, to see if I could notice any big spikes. Every time I moved my index finger, there was a big yellow spike, and I did it three or four times. I was just lying there thinking, “That’s so cool.” I moved my finger and it jumped, and everyone in the room was just geeking out.
Once they started putting me in the app and letting me do things like calibration and body mapping and I got cursor control for the first time, it was very intuitive. It wasn’t hard at all, and I think it’s only going to improve from here.
By body mapping, you mean that you would think about moving your hand or your finger in a certain way and Neuralink would correlate that with a certain neural signal?
Yeah, so in the body mapping, there were visualizations of a hand moving on a screen. There were different actions that they had me perform, like push your hand forward, pull your hand back, and so I did that for a while. We would do the action during body mapping, and they said that same action will be how you control the cursor. We did finger presses, like pushing down with each of my fingers 10 times. Then they would say, “OK, this finger got the best signal, and so that's the one we're going to use for the click.” So every time I went to click, I used that finger. It was very intuitive.
You’re not actually moving your finger then, just thinking about it?
Exactly. Even though I can’t move it, I can still try to move it, and it feels like it should be moving. The signal is still happening in my brain.
What does it feel like to be using the device? Do you have to concentrate really hard?
No, it’s very easy. I’m constantly multitasking when I’m in sessions or when I’m playing around. I’ll throw on an audiobook or throw something on my TV and then play a game at the same time. It takes very little brain power. What I’m thinking the whole time is just where I want the cursor to go.
What devices are you able to use the Neuralink app on?
It’s just on a Macbook right now, but they’re planning on moving it onto other devices. It will move to a phone pretty soon, and we’ll continue going from there.
Neuralink put out a blog post recently about your first 100 days with the device, and it mentioned that some of the implant’s threads, which are dotted with electrodes that read your neural activity, pulled out of your brain. Did you notice a difference in functionality when that happened?
I could tell right away that something was wrong. I just started losing control of the cursor. That was about three weeks in, I would say. I thought it was something on their end, like they had changed something in the software that made it perform worse.
Were you aware that it was possible that the threads could come out ?
I didn’t have any knowledge that it was possible. I don’t think they saw it in any of the animal trials. I had heard that it had maybe happened in one of the monkeys but that it was much different. It was never anticipated that it would happen in me.
But there were a lot of things that they didn’t expect with the human brain, like just how much it moves. It threw off a lot of their calculations for how things should be going.

How long did it take to recalibrate and get back to the cursor speed you were at before?
It took maybe two weeks. I remember the day that it happened. I was playing on it, and things just got better. It was just one little tweak that they had made on the software side, and from that point on things kept getting better and better.
Are you worried at all that more threads could pull out and the implant could stop working altogether?
Yeah, I’ve had fears about that. I’ve mentioned it to them. They have been very upfront with me and said that they don’t see any evidence of that. It seems like the threads have stabilized, and even some that were pulled out of my brain had found their way back in. I’m not worried about it now.
How has your life changed since getting the implant?
It’s just made me more independent, and that helps not only me but everyone around me. It makes me feel less helpless and like less of a burden. I love the fact that the people around me don’t have to wait on me so much. Outside of being completely healed, I believe what most quadriplegics want is independence.
What do you wish you could do with your implant that you can’t do right now? What does Telepathy 2.0 look like?
I mentioned this in [Neuralink’s] all-hands meeting, and I think it would be so freakin’ cool if I had a [Tesla] Optimus robot that I could control with it that would do basically everything for me and be a caretaker. It would eliminate probably 90 percent of the things that I need other people for. On top of that, it could connect to other things. I could connect to a car—a Tesla would be pretty cool, because they’re already self-driving. I would just need to find some way to get into it and set an address. Right now it’s a mission to get me anywhere. There are so many people, so many moving parts that are involved. If I could do all that on my own, man, it would change everything.

There are now a few dozen people around the world who have gotten BCIs. Have you met any of them?
No, I haven’t. I would like to. Maybe I need to take that first step and reach out. Maybe they’re all mad because I’ve been breaking world records .
You’ll have the implant for at least a year as part of the trial. Is there a scenario where you’d want to have it taken out?
My thinking through this whole process has been, it would benefit Neuralink if I left it in as long as possible, because I’ll have the longest case study of anyone. I would like to do that if it benefits them. That being said, if after a year I or Neuralink feels as if they’ve gotten what they can from me, and I’ve given what I can, then we’ll see. It also depends on how functional it is. I don’t expect it to lose any more function, but I never know what the future holds.
What has Neuralink told you about the possibility of getting an improved model?
I would love one, but they haven’t promised me anything. They’re not allowed to because it’s seen as an incentive. Since it’s a voluntary study, I’m not allowed to be incentivized at all in any way.
I hope that being the first short-lists me in some way, but if this is the extent of my participation, then that would be enough for me.
Neuralink is looking for a second trial participant . What would you say to that person?
I’m excited to have a buddy in this, someone to compare notes with. It will be nice to get a different perspective. I only have a few months on the next participant, but I want to help out in any way I can and be available for any questions they have. I guess my role in all this is sort of like a big brother.
What do you think is the next frontier for BCIs?
Being able to translate language in real time, I think that’s doable.
I know that BCIs don’t write into the brain yet, they just read. There’s no way to insert knowledge in there. But I think if we’re already at the reading step, then maybe writing comes next. That is a little bit of a scarier notion and something that I feel like a lot of people might not be too happy with. Maybe that’s something that needs to be thought out a bit more and taken a bit more carefully. But I think it’s a real possibility, and it’s a real bright future.
You Might Also Like …
In your inbox: Will Knight's Fast Forward explores advances in AI
Indian voters are being bombarded with millions of deepfakes
They bought tablets in prison —and found a broken promise
The one thing that’s holding back the heat pump
It's always sunny: Here are the best sunglasses for every adventure

Geraldine Castro

Max G. Levy

Jonathan O'Callaghan

Andrea J. Arratibel
Dogs play a key role in veterinary college’s brain cancer trial
- Marjorielee Christianson
21 May 2024
- Share on Facebook
- Share on Twitter
- Copy address link to clipboard

Lucy, with her boundless puppy-like energy even at 12 years old, is more than just a pet to Susan Ketcham. She's now part of a research project that could transform the way we treat brain cancer – in both dogs and humans.
This study at Virginia Tech's Virginia-Maryland College of Veterinary Medicine explores an innovative therapy called histotripsy. It's a leap forward from traditional cancer treatments, harnessing the power of focused ultrasound to break down tumors with precision.
When Lucy began experiencing seizures last July, Ketcham, a clinical nurse specialist, knew something more serious was the cause. The diagnosis of a brain tumor was devastating, but Ketcham was determined to explore all treatment options available. She discovered the histotripsy trial during her search and quickly reached out.
"Being in human medicine myself," said Ketcham. "I work in operating rooms and am very familiar with focused ultrasound, so I was eager to learn more."
Collaborative mission, translational impact
The trial is led by John Rossmeisl , the Dr. and Mrs. Dorsey Taylor Mahin Professor of Neurology and Neurosurgery, and Rell Parker , an iTHRIV scholar and assistant professor of neurology and neurosurgery.
Also on the team is Lauren Ruger , a postdoctoral associate in Eli Vlaisavljevich’s lab in the Department of Biomedical Engineering and Mechanics where histotripsy is extensively researched. She's adapting the equipment used in the study to make it safe and effective for their canine patients.
“I had wanted to be a veterinarian when I was younger before deciding to become an engineer,” said Ruger. “So I love having the opportunity to use my skills as an engineer to influence animal health.”
This trial offers an essential stepping stone in developing less invasive treatment options for brain cancer and is supported by the Focused Ultrasound Foundation and the Canine Health Foundation, highlighting the widespread commitment to results across species.

Hope for histotripsy
“Histotripsy uses acoustic energy, or sound waves, to modify tissue,” said Rossmeisl. “The intent is to cause a mechanical disruption of the tissue – killing cancer cells."
The technology was developed by researchers at the University of Michigan in the early 2000s.
The advantage is precision. Unlike traditional surgery, histotripsy can focus its impact on the tumor itself. "We could potentially treat these hard-to-reach brain tumors we normally can’t access with traditional surgery,” said Parker.
"We really don't have great ways to treat brain cancers in patients,” said Rossmeisl. “Even when you do surgery, radiation, or chemotherapy alone or in combination, usually, you're not creating a cure."
However, there is hope that histotripsy could be used to activate the body’s immune response and have it attack cancer cells, called the abscopal effect. Clinicians also see fewer side effects compared to other traditional treatment options.
About the procedure
The study currently still involves surgery to access brain tumors, which is the gold standard of care for this type of diagnosis. This allows for direct targeting of the tumor with the histotripsy transducer, delivering focused sound waves for precise treatment.
“When we do the surgery, we can see the tumor via ultrasound,” Parker said. “We can see that we're treating the appropriate cells, and then we also do an MRI to ensure that we've targeted the right area.”
After the histotripsy treatment, surgeons carefully remove the treated tumor. This tissue provides crucial insights into the technique's effect on cancer cells, helping researchers refine the technology for future applications.
“It gives us the advantage of being able to look at the tissue that's been broken down to ensure that we're getting the desired effect from the histotripsy therapy,” said Ruger.
While the science is complex, the stories of patients like Lucy are reminders of why this work matters. "The recovery was quick, the incision was small," Ketcham said. "She's back to her playful self, and knowing she's helped advance science and technology is amazing."
Parker added: "We’re happy to say that the procedure has been safe for our patients, and we've been able to treat them appropriately."
Looking toward the future of treatment
A long-term goal of this study is to develop a completely non-invasive treatment that would eliminate the need for surgery. The team is in the early stages of exploring this possibility, citing several challenges to realizing a solution that could be widely available.
“Transmitting ultrasound through the bones of the skull is very difficult,” said Ruger. “And then accurately focusing it in only the areas you want to treat with histotripsy adds another layer of complexity.”
However, the technique can be successfully applied through the skin, explained Rossmeisl. "That would be a paradigm changer. We would make surgically treating tumors a lot more widely available.”
“Even though I love neurosurgery,” he added. “Anytime I can do something that doesn't require putting the patient through a complex and invasive procedure and get them home quicker, that's always a good thing.”
To learn more about eligibility criteria or enroll your dog in the trial, please contact John Rossmeisl at [email protected] or 540-231-4621 or Mindy Quigley, clinical trials coordinator at [email protected] or 540-231-1363.

Andrew Mann
540-231-9005
- Biomedical Engineering and Mechanics
- Blacksburg, Va.
- Brain Cancer
- Cancer Research
- College of Engineering
- Faculty Excellence
- Good Health and Well-Being
- Industry, Innovation, and Infrastructure
- Office of Postdoctoral Affairs
- Small Animal Clinical Sciences
- Success Story
- Top News - Virginia-Maryland College of Veterinary Medicine
- University Distinguished Professor
- Veterinary Clinical Research Office
- Veterinary Teaching Hospital
- Virginia-Maryland College of Veterinary Medicine
Related Content
Study record managers: refer to the Data Element Definitions if submitting registration or results information.
Search for terms

- Advanced Search
- See Studies by Topic
- See Studies on Map
- How to Search
- How to Use Search Results
- How to Find Results of Studies
- How to Read a Study Record

- Learn About Studies
- Other Sites About Studies
- Glossary of Common Site Terms

- Submit Studies to ClinicalTrials.gov PRS
- Why Should I Register and Submit Results?
- FDAAA 801 and the Final Rule
- How to Apply for a PRS Account
- How to Register Your Study
- How to Edit Your Study Record
- How to Submit Your Results
- Frequently Asked Questions
- Support Materials
- Training Materials

- Selected Publications
- Clinical Alerts and Advisories
- Trends, Charts, and Maps
- Downloading Content for Analysis

- ClinicalTrials.gov Background
- About the Results Database
- History, Policies, and Laws
- ClinicalTrials.gov Modernization
- Media/Press Resources
- Linking to This Site
- Terms and Conditions
- Search Results
- Study Record Detail

An Open Comparative Study of the Effectiveness and Incomparable Study of the Immunogenicity and Safety of the Vaccine (CoviVac) for Adults Aged 60 Years and Older
- Study Details
- Tabular View
- No Results Posted

Inclusion Criteria:
Volunteers must meet the following inclusion criteria:
Type of participants
• Healthy volunteers or volunteers with a history of stable diseases that do not meet any of the criteria for non-inclusion in the study.
Other inclusion criteria
- Written informed consent of volunteers to participate in a clinical trial
- Volunteers who are able to fulfill the Protocol requirements (i.e., fill out a self-observation Diary, come to control visits).
Exclusion Criteria:
SARS-CoV-2 infection • A case of established COVID-19 disease confirmed by PCR and/or ELISA in the last 6 months.
Diseases or medical conditions
- Serious post-vaccination reaction (temperature above 40 C, hyperemia or edema more than 8 cm in diameter) or complication (collapse or shock-like condition that developed within 48 hours after vaccination; convulsions, accompanied or not accompanied by a feverish state) to any previous vaccination.
- Burdened allergic history (anaphylactic shock, Quincke's edema, polymorphic exudative eczema, serum sickness in the anamnesis, hypersensitivity or allergic reactions to the introduction of any vaccines in the anamnesis, known allergic reactions to vaccine components, etc.).
- Guillain-Barre syndrome (acute polyradiculitis) in the anamnesis.
- The axillary temperature at the time of vaccination is more than 37.0 ° C.
- Acute infectious diseases (recovery earlier than 4 weeks before vaccination) according to anamnesis.
- Donation of blood or plasma (in the amount of 450 ml or more) less than 2 months before inclusion in the study.
- Severe and/or uncontrolled diseases of the cardiovascular, bronchopulmonary, neuroendocrine systems, gastrointestinal tract, liver, kidneys, hematopoietic, immune systems.
- Is registered at the dispensary for tuberculosis, leukemia, oncological diseases, autoimmune diseases.
- Any confirmed or suspected immunosuppressive or immunodeficiency condition in the anamnesis.
- Splenectomy in the anamnesis.
- Neutropenia (decrease in the absolute number of neutrophils less than 1000/mm3), agranulocytosis, significant blood loss, severe anemia (hemoglobin less than 80 g/l) according to anamnesis.
- Anorexia according to anamnesis.
Prior or concomitant therapy
- Vaccination with any vaccine carried out within 30 days before vaccination / the first dose of the studied vaccine or planned administration within 30 days after vaccination / the last dose of the studied vaccine.
- Prior vaccination with an experimental or registered vaccine that may affect the interpretation of the study data (any coronavirus or SARS vaccines).
- Long-term use (more than 14 days) of immunosuppressants or other immunomodulatory drugs (immunoregulatory peptides, cytokines, interferons, immune system effector proteins (immunoglobulins), interferon inducers (cycloferon) during the six months preceding the study, according to anamnesis.
- Treatment with systemic glucocorticosteroids (≥ 20 mg of prednisone, or an analog, for more than 15 days during the last month).
- Volunteers who received immunoglobulin preparations or blood transfusion during the last 3 months prior to the start of the study according to anamnesis.
Other non-inclusion criteria
• Participation in any other clinical trial within the last 3 months.
Exclusion criteria:
- Withdrawal of Informed consent by a volunteer;
- The volunteer was included in violation of the inclusion/non-inclusion criteria of the Protocol;
- Any condition of a volunteer that requires, in the reasoned opinion of a medical researcher, the withdrawal of a volunteer from the study;
- Taking unauthorized medications (see section 6.2);
- The volunteer refuses to cooperate or is undisciplined (for example, failure to attend a scheduled visit without warning the researcher and/or loss of communication with the volunteer), or dropped out of observation;
- For administrative reasons (termination of the study by the Sponsor or regulatory authorities), as well as in case of gross violations of the Protocol that may affect the results of the study.
- For Patients and Families
- For Researchers
- For Study Record Managers
- Customer Support
- Accessibility
- Viewers and Players
- Freedom of Information Act
- HHS Vulnerability Disclosure
- U.S. National Library of Medicine
- U.S. National Institutes of Health
- U.S. Department of Health and Human Services

COMMENTS
Clinical research is a branch of medical research that involves people and aims to determine the effectiveness ( efficacy) and safety of medications, devices, diagnostic products, and treatment regimens intended for improving human health. [1] [2] These research procedures are designed for the prevention, treatment, diagnosis or understanding ...
Clinical research is the comprehensive study of the safety and effectiveness of the most promising advances in patient care. Clinical research is different than laboratory research. It involves people who volunteer to help us better understand medicine and health.
1. IQVIA. Formerly known as Quintiles and IMS Health, IQVIA is one of the largest CROs in the world, with a large range of service offerings to help advance clinical research. The company was founded in North Carolina in 1982, and has since grown to over 88,000 employees in more than 100 countries.
The Essence of a CRO: Clinical Research Organizations, abbreviated as CROs, epitomize entities entrusted with the management of clinical trials on behalf of pharmaceutical and biotechnological giants. Their mandates encompass the selection of patients, procurement of informed consent, aggregation of data, and the persistent oversight of safety ...
A clinical research organization (CRO) is often called a contract research organization (CRO). CRO is a service organization that provides support to pharmaceutical and biotechnology industries in the form of outsourced clinical research courses and services for both medical devices and drugs. The main functions required to conduct clinical ...
Clinical trials are conducted for many reasons: to determine whether a new drug or device is safe and effective for people to use. to study different ways to use standard treatments or current ...
Clinical trials are part of clinical research and at the heart of all medical advances. Clinical trials look at new ways to prevent, detect, or treat disease. Clinical trials can study: New drugs or new combinations of drugs; New ways of doing surgery; New medical devices; New ways to use existing treatments; New ways to change behaviors to ...
Observational study. A type of study in which people are observed or certain outcomes are measured. No attempt is made by the researcher to affect the outcome — for example, no treatment is given by the researcher. Clinical trial (interventional study). During clinical trials, researchers learn if a new test or treatment works and is safe.
What is Clinical Research? Clinical research occurs in many formats and can involve anyone. Learn how you can participate and contribute to medical advances. This page last reviewed on September 29, 2016.
Who we are. Parexel is among the world's largest clinical research organizations (CROs), providing the full range of Phase I to IV clinical development services to help lifesaving treatments reach patients faster. Leveraging the breadth of our clinical, regulatory, and therapeutic expertise, our team of more than 21,000 global professionals ...
Clinical research is an alternative terminology used to describe medical research. Clinical research involves people, and it is generally carried out to evaluate the efficacy of a therapeutic drug, a medical/surgical procedure, or a device as a part of treatment and patient management. ... Type of clinical trial: Definition: Randomized trial ...
Clinical research involves studying health and illness in people through observational studies or clinical trials. Participating in a trial or study has many potential benefits and also some possible risks. Learn about the benefits and risks of participating in clinical research and how your safety is protected.
A Contract Research Organization (CRO), sometimes known as a Clinical Research Organization, is an organization contracted by another company to take the lead in managing that company's trials and complex medical testing responsibilities. Contract Research Organizations reduce the cost of research and development to help businesses and ...
IQVIA is a leading global provider of advanced analytics, technology solutions, and clinical research services to the life sciences industry. IQVIA creates intelligent connections across all aspects of healthcare through its analytics, transformative technology, big data resources and extensive domain expertise.
Definition, regulatory aspects. The International Council on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, a 2015 Swiss NGO of pharmaceutical companies and others, defined a contract research organization (CRO), specifically pertaining to clinical trials services as:: 10 "A person or an organization (commercial, academic, or other) contracted by the ...
With more than 35 years of experience, the PPD clinical research business of Thermo Fisher Scientific offers an established drug development platform, patient enrollment expertise and robust laboratory services.Our broad range of clinical development, analytical and patient and advisory services - including full service to functional service provider services, digital and decentralized ...
Step 3: Clinical Research. While preclinical research answers basic questions about a drug's safety, it is not a substitute for studies of ways the drug will interact with the human body ...
The SOCRA Certified Clinical Research Professional (CCRP) program is your gateway to excellence in clinical research. Elevate your career with our internationally recognized certification, tailored for professionals dedicated to upholding the highest standards in the field. Join a community committed to ethical practices, continuous learning ...
ICON is the world's leading clinical research organisation, providing outsourced clincal development and commercialisation services to the pharmaceutical, ... ICON acquires HumanFirst, a cloud-based technology company for life sciences supporting precision measurement in patient centred clinical research.
Dr. Marcus Schabacker became president and chief executive officer of ECRI in January 2018. Dr. Schabacker is a board-certified anesthesiologist and intensive care specialist with more than 35 years of healthcare experience in complex global environments, and more than 20 years of senior leadership responsibilities serving the medical device and pharmaceutical industries across the healthcare ...
Angela Kaindl, Professor of Pediatrics at Charité Berlin, whose research focus is on neuropediatrics, introduced me to the highly complex and interesting research topic of epilepsy. As it is often the case, the highly specialized clinical studies cannot be transferred one-to-one to application in everyday clinical practice, so further ...
In anticipation of the May 29 "Merck Journeys: Career Conversations within Clinical Research" virtual career event being held by Merck in partnership with ACRP, we asked Julia Buoscio, an Associate Clinical Research Associate based in the New York City area with the company, to share some details on her early-career progression since completing an internship with Merck and transitioning to ...
The POA includes 91 cancer centers, academic institutions, research consortia and healthcare systems, including 43 NCI-designated cancer centers, collaborating to advance precision oncology and ...
A prespecified analysis of the SELECT trial revealed that patients assigned to once-weekly subcutaneous semaglutide 2.4 mg lost significantly more weight than those receiving placebo and showed ...
Noland Arbaugh is the first to get Elon Musk's brain device. The 30-year-old speaks to WIRED about what it's like to use a computer with his mind—and gain a new sense of independence.
She's now part of a research project that could transform the way we treat brain cancer - in both dogs and humans. ... Ketcham, a clinical nurse specialist, knew something more serious was the cause. The diagnosis of a brain tumor was devastating, but Ketcham was determined to explore all treatment options available. She discovered the ...
P earls, it is said, represent purity. They may soon stand for something else: business dynamism. In Greenville, South Carolina, two locals have created earrings that look like jewels, but contain ...
Major companies include: Elektrostal metallurgical factory; Elektrostal chemical-mechanical factory; Elektrostal Heavy Engineering Works, JSC is a designer and manufacturer of equipment for producing seamless hot-rolled, cold-rolled and welded steel materials and metallurgical equipment.
Choosing to participate in a study is an important personal decision. Talk with your doctor and family members or friends about deciding to join a study. To learn more about this study, you or your doctor may contact the study research staff using the contacts provided below. For general information, Learn About Clinical Studies.
Natural language processing (NLP) is a form of artificial intelligence ( AI) that allows computers to understand human language, whether it be written, spoken, or even scribbled. As AI-powered devices and services become increasingly more intertwined with our daily lives and world, so too does the impact that NLP has on ensuring a seamless ...