

The Ultimate Guide to Qualitative Research - Part 1: The Basics

- Introduction and overview
- What is qualitative research?
- What is qualitative data?
- Examples of qualitative data
- Qualitative vs. quantitative research
- Mixed methods
- Qualitative research preparation
- Theoretical perspective
- Theoretical framework
- Literature reviews
Research question
- Conceptual framework
- Conceptual vs. theoretical framework
Data collection
- Qualitative research methods
- Focus groups
- Observational research
What is a case study?
Applications for case study research, what is a good case study, process of case study design, benefits and limitations of case studies.
- Ethnographical research
- Ethical considerations
- Confidentiality and privacy
- Power dynamics
- Reflexivity
Case studies
Case studies are essential to qualitative research , offering a lens through which researchers can investigate complex phenomena within their real-life contexts. This chapter explores the concept, purpose, applications, examples, and types of case studies and provides guidance on how to conduct case study research effectively.

Whereas quantitative methods look at phenomena at scale, case study research looks at a concept or phenomenon in considerable detail. While analyzing a single case can help understand one perspective regarding the object of research inquiry, analyzing multiple cases can help obtain a more holistic sense of the topic or issue. Let's provide a basic definition of a case study, then explore its characteristics and role in the qualitative research process.
Definition of a case study
A case study in qualitative research is a strategy of inquiry that involves an in-depth investigation of a phenomenon within its real-world context. It provides researchers with the opportunity to acquire an in-depth understanding of intricate details that might not be as apparent or accessible through other methods of research. The specific case or cases being studied can be a single person, group, or organization – demarcating what constitutes a relevant case worth studying depends on the researcher and their research question .
Among qualitative research methods , a case study relies on multiple sources of evidence, such as documents, artifacts, interviews , or observations , to present a complete and nuanced understanding of the phenomenon under investigation. The objective is to illuminate the readers' understanding of the phenomenon beyond its abstract statistical or theoretical explanations.
Characteristics of case studies
Case studies typically possess a number of distinct characteristics that set them apart from other research methods. These characteristics include a focus on holistic description and explanation, flexibility in the design and data collection methods, reliance on multiple sources of evidence, and emphasis on the context in which the phenomenon occurs.
Furthermore, case studies can often involve a longitudinal examination of the case, meaning they study the case over a period of time. These characteristics allow case studies to yield comprehensive, in-depth, and richly contextualized insights about the phenomenon of interest.
The role of case studies in research
Case studies hold a unique position in the broader landscape of research methods aimed at theory development. They are instrumental when the primary research interest is to gain an intensive, detailed understanding of a phenomenon in its real-life context.
In addition, case studies can serve different purposes within research - they can be used for exploratory, descriptive, or explanatory purposes, depending on the research question and objectives. This flexibility and depth make case studies a valuable tool in the toolkit of qualitative researchers.
Remember, a well-conducted case study can offer a rich, insightful contribution to both academic and practical knowledge through theory development or theory verification, thus enhancing our understanding of complex phenomena in their real-world contexts.
What is the purpose of a case study?
Case study research aims for a more comprehensive understanding of phenomena, requiring various research methods to gather information for qualitative analysis . Ultimately, a case study can allow the researcher to gain insight into a particular object of inquiry and develop a theoretical framework relevant to the research inquiry.
Why use case studies in qualitative research?
Using case studies as a research strategy depends mainly on the nature of the research question and the researcher's access to the data.
Conducting case study research provides a level of detail and contextual richness that other research methods might not offer. They are beneficial when there's a need to understand complex social phenomena within their natural contexts.
The explanatory, exploratory, and descriptive roles of case studies
Case studies can take on various roles depending on the research objectives. They can be exploratory when the research aims to discover new phenomena or define new research questions; they are descriptive when the objective is to depict a phenomenon within its context in a detailed manner; and they can be explanatory if the goal is to understand specific relationships within the studied context. Thus, the versatility of case studies allows researchers to approach their topic from different angles, offering multiple ways to uncover and interpret the data .
The impact of case studies on knowledge development
Case studies play a significant role in knowledge development across various disciplines. Analysis of cases provides an avenue for researchers to explore phenomena within their context based on the collected data.
This can result in the production of rich, practical insights that can be instrumental in both theory-building and practice. Case studies allow researchers to delve into the intricacies and complexities of real-life situations, uncovering insights that might otherwise remain hidden.
Types of case studies
In qualitative research , a case study is not a one-size-fits-all approach. Depending on the nature of the research question and the specific objectives of the study, researchers might choose to use different types of case studies. These types differ in their focus, methodology, and the level of detail they provide about the phenomenon under investigation.
Understanding these types is crucial for selecting the most appropriate approach for your research project and effectively achieving your research goals. Let's briefly look at the main types of case studies.
Exploratory case studies
Exploratory case studies are typically conducted to develop a theory or framework around an understudied phenomenon. They can also serve as a precursor to a larger-scale research project. Exploratory case studies are useful when a researcher wants to identify the key issues or questions which can spur more extensive study or be used to develop propositions for further research. These case studies are characterized by flexibility, allowing researchers to explore various aspects of a phenomenon as they emerge, which can also form the foundation for subsequent studies.
Descriptive case studies
Descriptive case studies aim to provide a complete and accurate representation of a phenomenon or event within its context. These case studies are often based on an established theoretical framework, which guides how data is collected and analyzed. The researcher is concerned with describing the phenomenon in detail, as it occurs naturally, without trying to influence or manipulate it.
Explanatory case studies
Explanatory case studies are focused on explanation - they seek to clarify how or why certain phenomena occur. Often used in complex, real-life situations, they can be particularly valuable in clarifying causal relationships among concepts and understanding the interplay between different factors within a specific context.

Intrinsic, instrumental, and collective case studies
These three categories of case studies focus on the nature and purpose of the study. An intrinsic case study is conducted when a researcher has an inherent interest in the case itself. Instrumental case studies are employed when the case is used to provide insight into a particular issue or phenomenon. A collective case study, on the other hand, involves studying multiple cases simultaneously to investigate some general phenomena.
Each type of case study serves a different purpose and has its own strengths and challenges. The selection of the type should be guided by the research question and objectives, as well as the context and constraints of the research.
The flexibility, depth, and contextual richness offered by case studies make this approach an excellent research method for various fields of study. They enable researchers to investigate real-world phenomena within their specific contexts, capturing nuances that other research methods might miss. Across numerous fields, case studies provide valuable insights into complex issues.
Critical information systems research
Case studies provide a detailed understanding of the role and impact of information systems in different contexts. They offer a platform to explore how information systems are designed, implemented, and used and how they interact with various social, economic, and political factors. Case studies in this field often focus on examining the intricate relationship between technology, organizational processes, and user behavior, helping to uncover insights that can inform better system design and implementation.
Health research
Health research is another field where case studies are highly valuable. They offer a way to explore patient experiences, healthcare delivery processes, and the impact of various interventions in a real-world context.

Case studies can provide a deep understanding of a patient's journey, giving insights into the intricacies of disease progression, treatment effects, and the psychosocial aspects of health and illness.
Asthma research studies
Specifically within medical research, studies on asthma often employ case studies to explore the individual and environmental factors that influence asthma development, management, and outcomes. A case study can provide rich, detailed data about individual patients' experiences, from the triggers and symptoms they experience to the effectiveness of various management strategies. This can be crucial for developing patient-centered asthma care approaches.
Other fields
Apart from the fields mentioned, case studies are also extensively used in business and management research, education research, and political sciences, among many others. They provide an opportunity to delve into the intricacies of real-world situations, allowing for a comprehensive understanding of various phenomena.
Case studies, with their depth and contextual focus, offer unique insights across these varied fields. They allow researchers to illuminate the complexities of real-life situations, contributing to both theory and practice.
Whatever field you're in, ATLAS.ti puts your data to work for you
Download a free trial of ATLAS.ti to turn your data into insights.
Understanding the key elements of case study design is crucial for conducting rigorous and impactful case study research. A well-structured design guides the researcher through the process, ensuring that the study is methodologically sound and its findings are reliable and valid. The main elements of case study design include the research question , propositions, units of analysis, and the logic linking the data to the propositions.
The research question is the foundation of any research study. A good research question guides the direction of the study and informs the selection of the case, the methods of collecting data, and the analysis techniques. A well-formulated research question in case study research is typically clear, focused, and complex enough to merit further detailed examination of the relevant case(s).
Propositions
Propositions, though not necessary in every case study, provide a direction by stating what we might expect to find in the data collected. They guide how data is collected and analyzed by helping researchers focus on specific aspects of the case. They are particularly important in explanatory case studies, which seek to understand the relationships among concepts within the studied phenomenon.
Units of analysis
The unit of analysis refers to the case, or the main entity or entities that are being analyzed in the study. In case study research, the unit of analysis can be an individual, a group, an organization, a decision, an event, or even a time period. It's crucial to clearly define the unit of analysis, as it shapes the qualitative data analysis process by allowing the researcher to analyze a particular case and synthesize analysis across multiple case studies to draw conclusions.
Argumentation
This refers to the inferential model that allows researchers to draw conclusions from the data. The researcher needs to ensure that there is a clear link between the data, the propositions (if any), and the conclusions drawn. This argumentation is what enables the researcher to make valid and credible inferences about the phenomenon under study.
Understanding and carefully considering these elements in the design phase of a case study can significantly enhance the quality of the research. It can help ensure that the study is methodologically sound and its findings contribute meaningful insights about the case.
Ready to jumpstart your research with ATLAS.ti?
Conceptualize your research project with our intuitive data analysis interface. Download a free trial today.
Conducting a case study involves several steps, from defining the research question and selecting the case to collecting and analyzing data . This section outlines these key stages, providing a practical guide on how to conduct case study research.
Defining the research question
The first step in case study research is defining a clear, focused research question. This question should guide the entire research process, from case selection to analysis. It's crucial to ensure that the research question is suitable for a case study approach. Typically, such questions are exploratory or descriptive in nature and focus on understanding a phenomenon within its real-life context.
Selecting and defining the case
The selection of the case should be based on the research question and the objectives of the study. It involves choosing a unique example or a set of examples that provide rich, in-depth data about the phenomenon under investigation. After selecting the case, it's crucial to define it clearly, setting the boundaries of the case, including the time period and the specific context.
Previous research can help guide the case study design. When considering a case study, an example of a case could be taken from previous case study research and used to define cases in a new research inquiry. Considering recently published examples can help understand how to select and define cases effectively.
Developing a detailed case study protocol
A case study protocol outlines the procedures and general rules to be followed during the case study. This includes the data collection methods to be used, the sources of data, and the procedures for analysis. Having a detailed case study protocol ensures consistency and reliability in the study.
The protocol should also consider how to work with the people involved in the research context to grant the research team access to collecting data. As mentioned in previous sections of this guide, establishing rapport is an essential component of qualitative research as it shapes the overall potential for collecting and analyzing data.
Collecting data
Gathering data in case study research often involves multiple sources of evidence, including documents, archival records, interviews, observations, and physical artifacts. This allows for a comprehensive understanding of the case. The process for gathering data should be systematic and carefully documented to ensure the reliability and validity of the study.
Analyzing and interpreting data
The next step is analyzing the data. This involves organizing the data , categorizing it into themes or patterns , and interpreting these patterns to answer the research question. The analysis might also involve comparing the findings with prior research or theoretical propositions.
Writing the case study report
The final step is writing the case study report . This should provide a detailed description of the case, the data, the analysis process, and the findings. The report should be clear, organized, and carefully written to ensure that the reader can understand the case and the conclusions drawn from it.
Each of these steps is crucial in ensuring that the case study research is rigorous, reliable, and provides valuable insights about the case.
The type, depth, and quality of data in your study can significantly influence the validity and utility of the study. In case study research, data is usually collected from multiple sources to provide a comprehensive and nuanced understanding of the case. This section will outline the various methods of collecting data used in case study research and discuss considerations for ensuring the quality of the data.
Interviews are a common method of gathering data in case study research. They can provide rich, in-depth data about the perspectives, experiences, and interpretations of the individuals involved in the case. Interviews can be structured , semi-structured , or unstructured , depending on the research question and the degree of flexibility needed.
Observations
Observations involve the researcher observing the case in its natural setting, providing first-hand information about the case and its context. Observations can provide data that might not be revealed in interviews or documents, such as non-verbal cues or contextual information.
Documents and artifacts
Documents and archival records provide a valuable source of data in case study research. They can include reports, letters, memos, meeting minutes, email correspondence, and various public and private documents related to the case.

These records can provide historical context, corroborate evidence from other sources, and offer insights into the case that might not be apparent from interviews or observations.
Physical artifacts refer to any physical evidence related to the case, such as tools, products, or physical environments. These artifacts can provide tangible insights into the case, complementing the data gathered from other sources.
Ensuring the quality of data collection
Determining the quality of data in case study research requires careful planning and execution. It's crucial to ensure that the data is reliable, accurate, and relevant to the research question. This involves selecting appropriate methods of collecting data, properly training interviewers or observers, and systematically recording and storing the data. It also includes considering ethical issues related to collecting and handling data, such as obtaining informed consent and ensuring the privacy and confidentiality of the participants.
Data analysis
Analyzing case study research involves making sense of the rich, detailed data to answer the research question. This process can be challenging due to the volume and complexity of case study data. However, a systematic and rigorous approach to analysis can ensure that the findings are credible and meaningful. This section outlines the main steps and considerations in analyzing data in case study research.
Organizing the data
The first step in the analysis is organizing the data. This involves sorting the data into manageable sections, often according to the data source or the theme. This step can also involve transcribing interviews, digitizing physical artifacts, or organizing observational data.
Categorizing and coding the data
Once the data is organized, the next step is to categorize or code the data. This involves identifying common themes, patterns, or concepts in the data and assigning codes to relevant data segments. Coding can be done manually or with the help of software tools, and in either case, qualitative analysis software can greatly facilitate the entire coding process. Coding helps to reduce the data to a set of themes or categories that can be more easily analyzed.
Identifying patterns and themes
After coding the data, the researcher looks for patterns or themes in the coded data. This involves comparing and contrasting the codes and looking for relationships or patterns among them. The identified patterns and themes should help answer the research question.
Interpreting the data
Once patterns and themes have been identified, the next step is to interpret these findings. This involves explaining what the patterns or themes mean in the context of the research question and the case. This interpretation should be grounded in the data, but it can also involve drawing on theoretical concepts or prior research.
Verification of the data
The last step in the analysis is verification. This involves checking the accuracy and consistency of the analysis process and confirming that the findings are supported by the data. This can involve re-checking the original data, checking the consistency of codes, or seeking feedback from research participants or peers.
Like any research method , case study research has its strengths and limitations. Researchers must be aware of these, as they can influence the design, conduct, and interpretation of the study.
Understanding the strengths and limitations of case study research can also guide researchers in deciding whether this approach is suitable for their research question . This section outlines some of the key strengths and limitations of case study research.
Benefits include the following:
- Rich, detailed data: One of the main strengths of case study research is that it can generate rich, detailed data about the case. This can provide a deep understanding of the case and its context, which can be valuable in exploring complex phenomena.
- Flexibility: Case study research is flexible in terms of design , data collection , and analysis . A sufficient degree of flexibility allows the researcher to adapt the study according to the case and the emerging findings.
- Real-world context: Case study research involves studying the case in its real-world context, which can provide valuable insights into the interplay between the case and its context.
- Multiple sources of evidence: Case study research often involves collecting data from multiple sources , which can enhance the robustness and validity of the findings.
On the other hand, researchers should consider the following limitations:
- Generalizability: A common criticism of case study research is that its findings might not be generalizable to other cases due to the specificity and uniqueness of each case.
- Time and resource intensive: Case study research can be time and resource intensive due to the depth of the investigation and the amount of collected data.
- Complexity of analysis: The rich, detailed data generated in case study research can make analyzing the data challenging.
- Subjectivity: Given the nature of case study research, there may be a higher degree of subjectivity in interpreting the data , so researchers need to reflect on this and transparently convey to audiences how the research was conducted.
Being aware of these strengths and limitations can help researchers design and conduct case study research effectively and interpret and report the findings appropriately.

Ready to analyze your data with ATLAS.ti?
See how our intuitive software can draw key insights from your data with a free trial today.

Site Search
- How to Search
- Advisory Group
- Editorial Board
- OEC Fellows
- History and Funding
- Using OEC Materials
- Collections
- Research Ethics Resources
- Ethics Projects
- Communities of Practice
- Get Involved
- Submit Content
- Open Access Membership
- Become a Partner
Using Case Studies in Teaching Research Ethics
An essay exploring how to effectively use case studies to teach research ethics.
It is widely believed that discussing case studies is the most effective method of teaching the responsible conduct of research (Kovac 1996; Macrina and Munro 1995), probably because discussing case studies is an effective way to get students involved in the issues. (I use the word “student” to cover all those who study, including faculty members and other professionals.)
Case studies are stories, Some of the many forms case studies can take are described in the Appendix. and narrative – the telling of stories – is a fundamental human tool for organizing, understanding, and explaining experience. Alasdair MacIntyre offers an amusing example of how one might make sense of a nonsensical event by embedding it into a story.
I am standing waiting for a bus and the young man standing next to me suddenly says, ‘The name of the common wild duck is Histrionicus histrionicus histrionicus.’ There is no problem as to the meaning of the sentence he uttered; the problem is, how to answer the question, what was he doing in uttering it? Suppose he just uttered such sentences at random intervals; this would be one possible form of madness. We would render his act of utterance intelligible if one of the following turned out to be true: He has mistaken me for someone who yesterday had approached him in the library and asked: ‘Do you by any chance know the Latin name of the common wild duck?’ Or he has just come from a session with his psychotherapist who has urged him to break down his shyness by talking to strangers. ‘But what shall I say?’ ‘Oh, anything at all.’ Or he is a Soviet spy waiting at a prearranged rendez-vous and uttering the ill-chosen code sentence which will identify him to his contact. In each case the act of utterance becomes intelligible by finding its place in a narrative. [MacIntyre 1981:195-196, italics in original] The young man is mistaken, by the way. Ducks belong to the family Anatidae, not Histrionicus.
Just as unintelligible actions invite us to put them into a story, stories invite us to interpret them. Stories imply causality, intention, and meaning; in the forms of parables, fables, and allegories, stories are favored vehicles for moral and religious instruction worldwide.
An in-depth discussion of a case is the closest approximation to actually confronting an ethical problem that can easily be set up in a classroom. Experience is the best teacher, but we can’t predict whether or when our students will face an actual ethical conflict in research, and we would not want to wish such an experience on them. Although a good case discussion is not the same as dealing with a real ethical problem, it can be an approximation of such an experience, just as watching a film about the decline and death of an aged friend can be a highly affecting approximation of the actual experience. Watching the film The Dresser can bring a person to real tears; discussing a case can bring a student to genuine ethical development.
The value of case study discussion can be illustrated with an anecdote. In the first year of the Teaching Research Ethics Workshop, we might have spent a bit too much time talking about using case studies and how to lead case study discussions. By Wednesday (the workshop began on a Sunday that year), one participant complained, saying something like, “Aren’t you going to talk about anything but cases? I’ve used them and students get bored with them.”
We spent less time on case studies thereafter, but I mention the incident because of an evaluation we did in the third year of the workshop. We hired an external evaluator to talk to past workshop participants about its impact on them. I asked our evaluator to talk to several specific participants, including the one who had complained about case studies. To my complete surprise, the report showed that this participant “identified mastery of the case study approach as having had the greatest direct impact” on his teaching. The other past participants interviewed made similar comments.
Like all teaching techniques, case study discussion can be done well or poorly, and I hope to provide some guidance to help you avoid the worst pitfalls. I will assume that you already know how to lead a discussion and limit my comments to considerations pertaining directly to using case studies in research ethics. My comments are rooted in what has worked for me with the assumption that most of it will work for you, too – but probably not all of it. Teaching is an art, and success depends a great deal on the skills and personality of the teacher.
Much of what follows might sound dogmatic, but that should be taken as a stylistic quirk. I could add all the hedges and exceptions of which I can think, but that would only muddy things. Use your own judgment and take the advice for what it’s worth. Also note that this is general advice; some cases are designed to be used in a particular way (see Bebeau et al. 1995).
Preparing to lead a case study discussion is much the same as preparing to teach anything – figure out what you want to accomplish, how much time you can spend on it, and the like.
In the classroom, start by laying out ground rules. In many settings this step does not have to be overt – if it is a group you have been meeting with already, and you have established a tone of respect and openness, there’s no need to go over this again. If the group has not established this kind of rapport, then it is important to make it clear that everyone’s opinion will be heard – and challenged – respectfully.
You might also want to offer your students some strategies and tactics before plunging into the discussion.
Strategies cover the broad direction for the discussion. For example, you can tell your students that you want them to:
- Decide which of two positions to defend – “Should Peterson copy the notes? Why or why not?”
- Solve a problem – “What should Peterson do?”
- Take a role – “What would you do if you were Peterson?” • Think about how the problem could have been avoided – “What went wrong here?”
Clearly these are not mutually exclusive, and there are probably other strategies you could use.
It is often also helpful to suggest some tactics . Sometimes students see a case study (or ethics) as an inchoate mass – or as too well integrated to analyze. It can be useful to give them some specific things to dig out of the case.
For example, in Moral Reasoning in Scientific Research: Cases for Teaching and Assessment , which I developed along with Mickey Bebeau, Karen Muskavitch, and several other colleagues (Bebeau et al. 1995), we suggest that students try to identify (a) the ethical issues and points of conflict, (b) the interested parties, (c) the likely consequences of the proposed course of action, and (d) the moral obligations of the protagonist.
Lucinda Peach (included in Penslar 1995) offers a different approach, suggesting the value of paying attention to six factors: facts; interpretations of the facts; consequences; obligations; rights; and virtues (or character). I have found it particularly helpful to point out the distinction between the facts presented in the case and the interpretations of facts that are sometimes made unconsciously.
When the time comes to start the actual discussion, I always distribute a copy of the case study to all students, and I often also display it using an overhead projector. If a case is at all complex or subtle, or has more than one or two characters, it is very difficult to take part in the discussion without having the case on hand for reference.
I usually ask one or more students to volunteer to read the case aloud . If there are several characters in the case, I often take the part of narrator and ask students to read the parts of the characters. Reading the case aloud ensures that everyone finishes at the same time; asking students to take part gets their voices heard early.
Then I give students a chance to ask any questions of factual clarification they might have. The answers might already be in the case, but they aren’t always. I don’t always answer all of these questions at this point, saying instead, “Let’s make sure we get to that when we discuss the case.” For example, if a student were to ask: “What kind of student is Peterson? Is she any good?” I would want to wait until the discussion period, when I would respond by asking, “What difference does it make?” (Not to imply that it doesn’t make a difference, but to see why the students think it does.)
I often then give students a few minutes to write some thoughts – perhaps to answer the strategic question, or identify the tactical elements I had already outlined. I usually don’t collect the papers; I do collect the papers when I use Moral Reasoning in Scientific Research; it’s part of the method outlined in the booklet. the object here is to give students a chance to collect their thoughts and make a commitment, however tentative, to a few of them. Ideas that remain only half-formed in the mind often fly away when the discussion begins, but the written ideas are there for the students’ reference.
If the group isn’t too large, I find it very useful to go around the room and ask every student to make one short response to the case. When the strategy is to defend a position, I first ask them each to answer the first question – “Should Peterson copy the notes?” – yes or no. I tally their answers on the board. Then I go around again and ask each student to offer one reason for their answer. (If the responses are unbalanced – say 10 yes and 2 no – I give the students who said “no” the chance to state their case first.) In larger groups, I get a random sample of responses.
Then I plunge into the discussion, trying to be as quiet as I can and to get the students to talk as much as possible. My part is to keep things orderly, to clarify points in the case (including relevant rules and regulations), and to gently direct the discussion toward profitable paths. I usually write main points on the board.
Finally, the case should be brought to some kind of closure . Sometimes this means describing what I take to be the areas of agreement and disagreement and the relative weight of each (“Almost everyone agrees on X, but we’re still pretty divided on Y”). Sometimes it even includes a pronouncement: “It would be wrong for Peterson to copy the notes.” But I would generally qualify the pronouncement by describing some of Peterson’s other options.
Case study discussion can work even if you use it only once, but the more often a group discusses cases, the better. Using case studies is not the only technique for teaching responsible science, but it is, I think, one of the best.
Works Cited
Barnbaum, Deborah R., and Michael Byron. 2001. Research Ethics: Text and Readings . Upper Saddle River, NJ: Prentice-Hall.
Bebeau, Muriel J., et al. 1995. Moral Reasoning in Scientific Research : Cases for Teaching and Assessment. Bloomington, IN.: Poynter Center. http://poynter.indiana.edu/mr-main.shtml
Elliott, Deni, and Judy E. Stern, eds. 1997. Research Ethics: A Reader . Hanover, CT: University Press of New England.
Harris, Charles E. Jr., Michael S. Pritchard, and Michael J. Rabins. 1995. Engineering Ethics: Concepts and Cases . Belmont: Wadsworth Publishing Company.
King, Nancy M. P., Gail E. Henderson, and Jane Stein, eds. 1999. Beyond Regulations: Ethics in Human Subjects Research . Chapel Hill: The University of North Carolina Press.
Kovac, Jeffrey. 1996. “Scientific ethics in chemical education.” Journal of Chemical Education 73(10): 926-928.
MacIntyre, Alasdair. 1981. After Virtue: A Study in Moral Theory . Notre Dame: University of Notre Dame Press.
Macrina, Francis L. 2000. Scientific Integrity: An Introductory Text with Cases . 2nd ed. Washington, DC: ASM Press.
Macrina, Francis L. and Cindy L. Munro. 1995. “The case-study approach to teaching scientific integrity in nursing and the biomedical sciences.” Journal of Professional Nursing 11(1): 40- 44.
Orlans, F. Barbara, et al. 1998. The Human Use of Animals: Case Studies in Ethical Choice . New York: Oxford University Press. Penslar, Robin Levin. 1995. Research Ethics: Cases and Materials. Bloomington: Indiana University Press.
Seebauer, Edmund G., and Robert L. Barry. 2001. Fundamentals of Ethics for Scientists and Engineers . Oxford: Oxford University Press.
Schrag, Brian, ed. 1997-2002. Research Ethics: Cases and Commentaries . Six volumes. Bloomington, IN: Association for Practical and Professional Ethics.
Appendix: Types of case studies
I don’t know of any thorough typology of case studies, but it is clear that case studies take many forms. Here are some of the forms that I have come across. The list is not intended to be exhaustive, and the descriptive names are my own – they should not be construed as definitive or in common use.
Illustrative cases are perhaps the most common form. They are included in textbooks written specifically for instruction in the responsible conduct of research and are generally found at the end of each chapter to illustrate the chapter’s major points. For examples, see Barnbaum and Byron 2001; Elliott and Stern 1997; Harris, Pritchard, and Rabins 1995; Macrina 2000; and Seebauer and Barry 2001.
Historical case studies start with a particular controversy, event, or series of related events. Good examples can be found in The Human Use of Animals (Orlans et al. 1998). The first case, “Baboon-human liver transplants: The Pittsburgh case,” describes an operation performed in 1992 at the University of Pittsburgh to replace a dying man’s defective liver with a healthy liver from a baboon. The case itself is presented in two pages, followed by about a page of historical context. The bulk of the chapter, about eight pages, consists of commentary on the ethical issues raised by the case. (See also King et.al 1999.) Historical cases are good because they are real, not made up, and students cannot dismiss them by saying, “That would never happen.” On the other hand, though, some students will view historical cases as settled and over with; the very fact that they have been written up can seem to imply that the issues raised have all be solved.
Historical synopses are shorter, often focusing on a well-known event. Fundamentals of Ethics for Scientists and Engineers (Seebauer and Barry 2001), for example, includes sixteen “real-life cases,” generally one or two pages long with a few questions for discussion. The first three cases are titled “Destruction of the Spaceship Challenger,” “Toxic Waste at Love Canal,” and “Dow Corning Corp. and Breast Implants.”
Journalistic case studies are historical case studies written by journalists for mass consumption. A recent example, “The Stuttering Doctor’s ‘Monster Study’,” can be found in the New York Times Magazine (Reynolds 2003). It is the story of Wendell Johnson’s research in the late 1930’s that involved inducing stuttering in orphans. Journalistic accounts generally are written in a more literary, less academic style – they are often more passionate and viscerally engaging than case studies prepared by philosophers and ethicists.
Cases with commentary present the case study first and then follow it with one or more commentaries. The six-volume series Research Ethics: Cases and Commentaries (Schrag 1996- 2002) presents a short case (about two-four pages) followed by a commentary by the case’s author and a second commentary by another expert. (See also King et.al 1999.)
Dramatic cases are formatted like a script, which allows the characters’ voices to carry most of the story. I find them very good for conveying subtleties.
Trigger tapes are short videos intended to trigger discussion. Among the best available are the five videos in the series “Integrity in Scientific Research” (see http://www.aaas.org/spp/video/ ).
Finally, a series of casuistic cases presents several very short, related cases, each one in some way a variation or elaboration of one or more of the previous cases in the series. The first one or two cases are generally straightforward, presenting, for example, a clear-cut case of cheating and a clear-cut case of acceptable sharing of information. Later cases are less straightforward, pushing the boundaries that make the earlier cases clear-cut. Excellent examples can be found in Penslar 1995 (see, e.g., Chapters 5 and 6). This book also includes examples of many of the other kinds of case studies described here.
Portions of this paper are adapted from a presentation at the Planning Workshop for a Guide for Teaching Responsible Science, sponsored by the National Academy of Sciences, the National Science Foundation, and the National Institutes of Health, February 1997.
Copyright 2003, 2007, Kenneth D. Pimple, Ph.D. All rights reserved.
Permission is hereby granted to reproduce and distribute copies of this work for nonprofit educational purposes, provided that copies are distributed at or below cost, and that the author, source, and copyright notice are included on each copy. This permission is in addition to rights of reproduction granted under Sections 107, 108, and other provisions of the U.S. Copyright Act.
Also available at the TeachRCR.us site.
Related Resources
Submit Content to the OEC Donate

This material is based upon work supported by the National Science Foundation under Award No. 2055332. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
McCombs School of Business
- Español ( Spanish )
Videos Concepts Unwrapped View All 36 short illustrated videos explain behavioral ethics concepts and basic ethics principles. Concepts Unwrapped: Sports Edition View All 10 short videos introduce athletes to behavioral ethics concepts. Ethics Defined (Glossary) View All 58 animated videos - 1 to 2 minutes each - define key ethics terms and concepts. Ethics in Focus View All One-of-a-kind videos highlight the ethical aspects of current and historical subjects. Giving Voice To Values View All Eight short videos present the 7 principles of values-driven leadership from Gentile's Giving Voice to Values. In It To Win View All A documentary and six short videos reveal the behavioral ethics biases in super-lobbyist Jack Abramoff's story. Scandals Illustrated View All 30 videos - one minute each - introduce newsworthy scandals with ethical insights and case studies. Video Series
Curated Resources UT Star Icon
Professional Ethics
Professionals work in a wide variety of settings and across many different industries including business, science, medicine, education, art, and public service.
Many professions have Codes of Conduct that specify ethical behavior and expectations particular to that industry. In addition, professionals must make ethical judgments in their area of specialty that fall outside their specific Code of Conduct.
The resources in this section offer insights that apply to a wide range of professionals as they seek to develop standards of ethical decision-making and behavior in their careers. Often, professionals need to apply moral reasoning to their interactions with co-workers, clients, and the general public to solve problems that arise in their work. Professionals also need to be on lookout for social and organizational pressures and situational factors that could cause them to err, unknowingly, in their ethical judgments and actions.
No profession is free from ethical dilemmas. All professionals will face ethical issues regardless of their career trajectory or the role they play within an organization. While Codes of Conduct are essential, and a good starting point for ethical conduct, they are no substitute for a well-rounded education in behavioral and applied ethics.
Start Here: Videos

Role Morality
Role morality is the tendency we have to use different moral standards for the different roles we play in society.

Bounded Ethicality
Bounded ethicality explains how social pressures and psychological processes cause us to behave in ways that are inconsistent with our own values.

Being Your Best Self, Part 4: Moral Action
Moral action means transforming the intent to do the right thing into reality. This involves moral ownership, moral efficacy, and moral courage.
Start Here: Cases

Freedom vs. Duty in Clinical Social Work
What should social workers do when their personal values come in conflict with the clients they are meant to serve?

High Stakes Testing
In the wake of the No Child Left Behind Act, parents, teachers, and school administrators take different positions on how to assess student achievement.
Healthcare Obligations: Personal vs. Institutional
A medical doctor must make a difficult decision when informing patients of the effectiveness of flu shots while upholding institutional recommendations.
Teaching Notes
Begin by viewing the “Start Here” videos. They introduce key topics that commonly emerge in our careers, such as making ethical decisions based on the role we’re playing at work. The four-part video, Being Your Best Self , describes the four components of ethical decision-making and action. To help strengthen ethical decision-making skills, watch the behavioral ethics videos in the “Additional Videos” section to learn about the psychological biases that can often lead to making poor choices.
Read through these videos’ teaching notes for details and related ethics concepts. Watch the “Related Videos” and/or read the related Case Study. The video’s “Additional Resources” offer further reading and a bibliography.
To use these resources in the classroom, show a video in class, assign a video to watch outside of class, or embed a video in an online learning module such as Canvas. Then, prompt conversation in class to encourage peer-to-peer learning. Ask students to answer the video’s “Discussion Questions,” and to reflect on the ideas and issues raised by the students in the video. How do their experiences align? How do they differ? The videos also make good writing prompts. Ask students to watch a video and apply the ethics concept to course content.
The case studies offer examples of professionals facing tough ethical decisions or ethically questionable situations in their careers in teaching, science, politics, and social services. Cases are an effective way to introduce ethics topics, and for people to learn how to spot ethical issues.
Select a case study from the Cases Series or find one in the “Additional Cases” section that resonates with your industry or profession. Then, reason through the ethical dimensions presented, and sketch the ethical decision-making process outlined by the case. Challenge yourself (and/or your team at work) to develop strategies to avoid these ethical pitfalls. Watch the case study’s “Related Videos” and “Related Terms” for further understanding.
To use the case studies in the classroom, ask students to read a video’s “Case Study” and answer the case study “Discussion Questions.” Then, follow the strategy outlined in the previous paragraph, challenging students to develop strategies to avoid the ethical pitfalls presented in the case.
Ethics Unwrapped blogs are also useful prompts to engage colleagues or students in discussions about ethics. Learning about ethics in the context of real-world (often current) events can enliven conversation and make ethics relevant and concrete. Share a blog in a meeting or class or post one to the company intranet or the class’s online learning module. To spur discussion, try to identify the ethical issues at hand and to name the ethics concepts related to the blog (or current event in the news). Dig more deeply into the topic using the Additional Resources listed at the end of the blog post.
Remember to review video, case study, and blogs’ relevant glossary terms. In this way, you will become familiar with all the ethics concepts contained in these material. Share this vocabulary with your colleagues or students, and use it to expand and enrich ethics and leadership conversations. To dive deeper in the glossary, watch “Related” glossary videos.
Many of the concepts covered in Ethics Unwrapped operate in tandem with each other. As you watch more videos, you will become more fluent in ethics and see the interrelatedness of ethics concepts more readily. You also will be able to spot ethical issues more easily – at least, that is the hope! It will also be easier to express your ideas and thoughts about what is and isn’t ethical and why. Hopefully, you will also come to realize the interconnectedness of ethics and leadership, and the essential role ethics plays in developing solid leadership skills that can advance your professional career.
Additional Videos
- Self-serving Bias
- Moral Equilibrium
- Conflict of Interest
- In It To Win: The Jack Abramoff Story
- In It To Win: Jack & Framing
- In It To Win: Jack & Rationalizations
- In It To Win: Jack & Self-Serving Bias
- In It To Win: Jack & Role Morality
- In It To Win: Jack & Moral Equilibrium
- Intro to GVV
- GVV Pillar 1: Values
- GVV Pillar 2: Choice
- GVV Pillar 3: Normalization
- GVV Pillar 4: Purpose
- GVV Pillar 5: Self-Knowledge & Alignment
- GVV Pillar 6: Voice
- GVV Pillar 7: Reasons & Rationalizations
- Obedience to Authority
- Loss Aversion
- Intro to Behavioral Ethics
- Moral Muteness
- Moral Myopia
- Being Your Best Self, Part 1: Moral Awareness
- Being Your Best Self, Part 2: Moral Decision Making
- Being Your Best Self, Part 3: Moral Intent
- Legal Rights & Ethical Responsibilities
Additional Cases
Liberal arts & fine arts.
- A Million Little Pieces
- Approaching the Presidency: Roosevelt & Taft
- Pardoning Nixon
Science & Engineering
- Retracting Research: The Case of Chandok v. Klessig
- Arctic Offshore Drilling
Social Science
- The CIA Leak
- Edward Snowden: Traitor or Hero?
- The Costco Model
- The Collapse of Barings Bank
- Teaching Blackface: A Lesson on Stereotypes
- Cyber Harassment
- Cheating: Atlanta’s School Scandal
Communication & Journalism
- Dr. V’s Magical Putter
- Limbaugh on Drug Addiction
- Reporting on Robin Williams
- Covering Yourself? Journalists and the Bowl Championship
- Sports Blogs: The Wild West of Sports Journalism?
- Cheney v. U.S. District Court
- Negotiating Bankruptcy
- Patient Autonomy & Informed Consent
- Prenatal Diagnosis & Parental Choice
Public Policy & Administration
- Gaming the System: The VA Scandal
- Krogh & the Watergate Scandal
Stay Informed
Support our work.
- Privacy Policy

Home » Ethical Considerations – Types, Examples and Writing Guide
Ethical Considerations – Types, Examples and Writing Guide
Table of Contents

Ethical Considerations
Ethical considerations in research refer to the principles and guidelines that researchers must follow to ensure that their studies are conducted in an ethical and responsible manner. These considerations are designed to protect the rights, safety, and well-being of research participants, as well as the integrity and credibility of the research itself
Some of the key ethical considerations in research include:
- Informed consent: Researchers must obtain informed consent from study participants, which means they must inform participants about the study’s purpose, procedures, risks, benefits, and their right to withdraw at any time.
- Privacy and confidentiality : Researchers must ensure that participants’ privacy and confidentiality are protected. This means that personal information should be kept confidential and not shared without the participant’s consent.
- Harm reduction : Researchers must ensure that the study does not harm the participants physically or psychologically. They must take steps to minimize the risks associated with the study.
- Fairness and equity : Researchers must ensure that the study does not discriminate against any particular group or individual. They should treat all participants equally and fairly.
- Use of deception: Researchers must use deception only if it is necessary to achieve the study’s objectives. They must inform participants of the deception as soon as possible.
- Use of vulnerable populations : Researchers must be especially cautious when working with vulnerable populations, such as children, pregnant women, prisoners, and individuals with cognitive or intellectual disabilities.
- Conflict of interest : Researchers must disclose any potential conflicts of interest that may affect the study’s integrity. This includes financial or personal relationships that could influence the study’s results.
- Data manipulation: Researchers must not manipulate data to support a particular hypothesis or agenda. They should report the results of the study objectively, even if the findings are not consistent with their expectations.
- Intellectual property: Researchers must respect intellectual property rights and give credit to previous studies and research.
- Cultural sensitivity : Researchers must be sensitive to the cultural norms and beliefs of the participants. They should avoid imposing their values and beliefs on the participants and should be respectful of their cultural practices.
Types of Ethical Considerations
Types of Ethical Considerations are as follows:
Research Ethics:
This includes ethical principles and guidelines that govern research involving human or animal subjects, ensuring that the research is conducted in an ethical and responsible manner.
Business Ethics :
This refers to ethical principles and standards that guide business practices and decision-making, such as transparency, honesty, fairness, and social responsibility.
Medical Ethics :
This refers to ethical principles and standards that govern the practice of medicine, including the duty to protect patient autonomy, informed consent, confidentiality, and non-maleficence.
Environmental Ethics :
This involves ethical principles and values that guide our interactions with the natural world, including the obligation to protect the environment, minimize harm, and promote sustainability.
Legal Ethics
This involves ethical principles and standards that guide the conduct of legal professionals, including issues such as confidentiality, conflicts of interest, and professional competence.
Social Ethics
This involves ethical principles and values that guide our interactions with other individuals and society as a whole, including issues such as justice, fairness, and human rights.
Information Ethics
This involves ethical principles and values that govern the use and dissemination of information, including issues such as privacy, accuracy, and intellectual property.
Cultural Ethics
This involves ethical principles and values that govern the relationship between different cultures and communities, including issues such as respect for diversity, cultural sensitivity, and inclusivity.
Technological Ethics
This refers to ethical principles and guidelines that govern the development, use, and impact of technology, including issues such as privacy, security, and social responsibility.
Journalism Ethics
This involves ethical principles and standards that guide the practice of journalism, including issues such as accuracy, fairness, and the public interest.
Educational Ethics
This refers to ethical principles and standards that guide the practice of education, including issues such as academic integrity, fairness, and respect for diversity.
Political Ethics
This involves ethical principles and values that guide political decision-making and behavior, including issues such as accountability, transparency, and the protection of civil liberties.
Professional Ethics
This refers to ethical principles and standards that guide the conduct of professionals in various fields, including issues such as honesty, integrity, and competence.
Personal Ethics
This involves ethical principles and values that guide individual behavior and decision-making, including issues such as personal responsibility, honesty, and respect for others.
Global Ethics
This involves ethical principles and values that guide our interactions with other nations and the global community, including issues such as human rights, environmental protection, and social justice.
Applications of Ethical Considerations
Ethical considerations are important in many areas of society, including medicine, business, law, and technology. Here are some specific applications of ethical considerations:
- Medical research : Ethical considerations are crucial in medical research, particularly when human subjects are involved. Researchers must ensure that their studies are conducted in a way that does not harm participants and that participants give informed consent before participating.
- Business practices: Ethical considerations are also important in business, where companies must make decisions that are socially responsible and avoid activities that are harmful to society. For example, companies must ensure that their products are safe for consumers and that they do not engage in exploitative labor practices.
- Environmental protection: Ethical considerations play a crucial role in environmental protection, as companies and governments must weigh the benefits of economic development against the potential harm to the environment. Decisions about land use, resource allocation, and pollution must be made in an ethical manner that takes into account the long-term consequences for the planet and future generations.
- Technology development : As technology continues to advance rapidly, ethical considerations become increasingly important in areas such as artificial intelligence, robotics, and genetic engineering. Developers must ensure that their creations do not harm humans or the environment and that they are developed in a way that is fair and equitable.
- Legal system : The legal system relies on ethical considerations to ensure that justice is served and that individuals are treated fairly. Lawyers and judges must abide by ethical standards to maintain the integrity of the legal system and to protect the rights of all individuals involved.
Examples of Ethical Considerations
Here are a few examples of ethical considerations in different contexts:
- In healthcare : A doctor must ensure that they provide the best possible care to their patients and avoid causing them harm. They must respect the autonomy of their patients, and obtain informed consent before administering any treatment or procedure. They must also ensure that they maintain patient confidentiality and avoid any conflicts of interest.
- In the workplace: An employer must ensure that they treat their employees fairly and with respect, provide them with a safe working environment, and pay them a fair wage. They must also avoid any discrimination based on race, gender, religion, or any other characteristic protected by law.
- In the media : Journalists must ensure that they report the news accurately and without bias. They must respect the privacy of individuals and avoid causing harm or distress. They must also be transparent about their sources and avoid any conflicts of interest.
- In research: Researchers must ensure that they conduct their studies ethically and with integrity. They must obtain informed consent from participants, protect their privacy, and avoid any harm or discomfort. They must also ensure that their findings are reported accurately and without bias.
- In personal relationships : People must ensure that they treat others with respect and kindness, and avoid causing harm or distress. They must respect the autonomy of others and avoid any actions that would be considered unethical, such as lying or cheating. They must also respect the confidentiality of others and maintain their privacy.
How to Write Ethical Considerations
When writing about research involving human subjects or animals, it is essential to include ethical considerations to ensure that the study is conducted in a manner that is morally responsible and in accordance with professional standards. Here are some steps to help you write ethical considerations:
- Describe the ethical principles: Start by explaining the ethical principles that will guide the research. These could include principles such as respect for persons, beneficence, and justice.
- Discuss informed consent : Informed consent is a critical ethical consideration when conducting research. Explain how you will obtain informed consent from participants, including how you will explain the purpose of the study, potential risks and benefits, and how you will protect their privacy.
- Address confidentiality : Describe how you will protect the confidentiality of the participants’ personal information and data, including any measures you will take to ensure that the data is kept secure and confidential.
- Consider potential risks and benefits : Describe any potential risks or harms to participants that could result from the study and how you will minimize those risks. Also, discuss the potential benefits of the study, both to the participants and to society.
- Discuss the use of animals : If the research involves the use of animals, address the ethical considerations related to animal welfare. Explain how you will minimize any potential harm to the animals and ensure that they are treated ethically.
- Mention the ethical approval : Finally, it’s essential to acknowledge that the research has received ethical approval from the relevant institutional review board or ethics committee. State the name of the committee, the date of approval, and any specific conditions or requirements that were imposed.
When to Write Ethical Considerations
Ethical considerations should be written whenever research involves human subjects or has the potential to impact human beings, animals, or the environment in some way. Ethical considerations are also important when research involves sensitive topics, such as mental health, sexuality, or religion.
In general, ethical considerations should be an integral part of any research project, regardless of the field or subject matter. This means that they should be considered at every stage of the research process, from the initial planning and design phase to data collection, analysis, and dissemination.
Ethical considerations should also be written in accordance with the guidelines and standards set by the relevant regulatory bodies and professional associations. These guidelines may vary depending on the discipline, so it is important to be familiar with the specific requirements of your field.
Purpose of Ethical Considerations
Ethical considerations are an essential aspect of many areas of life, including business, healthcare, research, and social interactions. The primary purposes of ethical considerations are:
- Protection of human rights: Ethical considerations help ensure that people’s rights are respected and protected. This includes respecting their autonomy, ensuring their privacy is respected, and ensuring that they are not subjected to harm or exploitation.
- Promoting fairness and justice: Ethical considerations help ensure that people are treated fairly and justly, without discrimination or bias. This includes ensuring that everyone has equal access to resources and opportunities, and that decisions are made based on merit rather than personal biases or prejudices.
- Promoting honesty and transparency : Ethical considerations help ensure that people are truthful and transparent in their actions and decisions. This includes being open and honest about conflicts of interest, disclosing potential risks, and communicating clearly with others.
- Maintaining public trust: Ethical considerations help maintain public trust in institutions and individuals. This is important for building and maintaining relationships with customers, patients, colleagues, and other stakeholders.
- Ensuring responsible conduct: Ethical considerations help ensure that people act responsibly and are accountable for their actions. This includes adhering to professional standards and codes of conduct, following laws and regulations, and avoiding behaviors that could harm others or damage the environment.

Advantages of Ethical Considerations
Here are some of the advantages of ethical considerations:
- Builds Trust : When individuals or organizations follow ethical considerations, it creates a sense of trust among stakeholders, including customers, clients, and employees. This trust can lead to stronger relationships and long-term loyalty.
- Reputation and Brand Image : Ethical considerations are often linked to a company’s brand image and reputation. By following ethical practices, a company can establish a positive image and reputation that can enhance its brand value.
- Avoids Legal Issues: Ethical considerations can help individuals and organizations avoid legal issues and penalties. By adhering to ethical principles, companies can reduce the risk of facing lawsuits, regulatory investigations, and fines.
- Increases Employee Retention and Motivation: Employees tend to be more satisfied and motivated when they work for an organization that values ethics. Companies that prioritize ethical considerations tend to have higher employee retention rates, leading to lower recruitment costs.
- Enhances Decision-making: Ethical considerations help individuals and organizations make better decisions. By considering the ethical implications of their actions, decision-makers can evaluate the potential consequences and choose the best course of action.
- Positive Impact on Society: Ethical considerations have a positive impact on society as a whole. By following ethical practices, companies can contribute to social and environmental causes, leading to a more sustainable and equitable society.
About the author
Muhammad Hassan
Researcher, Academic Writer, Web developer
You may also like

Critical Analysis – Types, Examples and Writing...

Research Methods – Types, Examples and Guide

Delimitations in Research – Types, Examples and...

Chapter Summary & Overview – Writing Guide...

Thesis Format – Templates and Samples

Implications in Research – Types, Examples and...
Ethical Considerations In Psychology Research
Saul Mcleod, PhD
Editor-in-Chief for Simply Psychology
BSc (Hons) Psychology, MRes, PhD, University of Manchester
Saul Mcleod, PhD., is a qualified psychology teacher with over 18 years of experience in further and higher education. He has been published in peer-reviewed journals, including the Journal of Clinical Psychology.
Learn about our Editorial Process
Olivia Guy-Evans, MSc
Associate Editor for Simply Psychology
BSc (Hons) Psychology, MSc Psychology of Education
Olivia Guy-Evans is a writer and associate editor for Simply Psychology. She has previously worked in healthcare and educational sectors.
On This Page:
Ethics refers to the correct rules of conduct necessary when carrying out research. We have a moral responsibility to protect research participants from harm.
However important the issue under investigation, psychologists must remember that they have a duty to respect the rights and dignity of research participants. This means that they must abide by certain moral principles and rules of conduct.
What are Ethical Guidelines?
In Britain, ethical guidelines for research are published by the British Psychological Society, and in America, by the American Psychological Association. The purpose of these codes of conduct is to protect research participants, the reputation of psychology, and psychologists themselves.
Moral issues rarely yield a simple, unambiguous, right or wrong answer. It is, therefore, often a matter of judgment whether the research is justified or not.
For example, it might be that a study causes psychological or physical discomfort to participants; maybe they suffer pain or perhaps even come to serious harm.
On the other hand, the investigation could lead to discoveries that benefit the participants themselves or even have the potential to increase the sum of human happiness.
Rosenthal and Rosnow (1984) also discuss the potential costs of failing to carry out certain research. Who is to weigh up these costs and benefits? Who is to judge whether the ends justify the means?
Finally, if you are ever in doubt as to whether research is ethical or not, it is worthwhile remembering that if there is a conflict of interest between the participants and the researcher, it is the interests of the subjects that should take priority.
Studies must now undergo an extensive review by an institutional review board (US) or ethics committee (UK) before they are implemented. All UK research requires ethical approval by one or more of the following:
- Department Ethics Committee (DEC) : for most routine research.
- Institutional Ethics Committee (IEC) : for non-routine research.
- External Ethics Committee (EEC) : for research that s externally regulated (e.g., NHS research).
Committees review proposals to assess if the potential benefits of the research are justifiable in light of the possible risk of physical or psychological harm.
These committees may request researchers make changes to the study’s design or procedure or, in extreme cases, deny approval of the study altogether.
The British Psychological Society (BPS) and American Psychological Association (APA) have issued a code of ethics in psychology that provides guidelines for conducting research. Some of the more important ethical issues are as follows:
Informed Consent
Before the study begins, the researcher must outline to the participants what the research is about and then ask for their consent (i.e., permission) to participate.
An adult (18 years +) capable of being permitted to participate in a study can provide consent. Parents/legal guardians of minors can also provide consent to allow their children to participate in a study.
Whenever possible, investigators should obtain the consent of participants. In practice, this means it is not sufficient to get potential participants to say “Yes.”
They also need to know what it is that they agree to. In other words, the psychologist should, so far as is practicable, explain what is involved in advance and obtain the informed consent of participants.
Informed consent must be informed, voluntary, and rational. Participants must be given relevant details to make an informed decision, including the purpose, procedures, risks, and benefits. Consent must be given voluntarily without undue coercion. And participants must have the capacity to rationally weigh the decision.
Components of informed consent include clearly explaining the risks and expected benefits, addressing potential therapeutic misconceptions about experimental treatments, allowing participants to ask questions, and describing methods to minimize risks like emotional distress.
Investigators should tailor the consent language and process appropriately for the study population. Obtaining meaningful informed consent is an ethical imperative for human subjects research.
The voluntary nature of participation should not be compromised through coercion or undue influence. Inducements should be fair and not excessive/inappropriate.
However, it is not always possible to gain informed consent. Where the researcher can’t ask the actual participants, a similar group of people can be asked how they would feel about participating.
If they think it would be OK, then it can be assumed that the real participants will also find it acceptable. This is known as presumptive consent.
However, a problem with this method is that there might be a mismatch between how people think they would feel/behave and how they actually feel and behave during a study.
In order for consent to be ‘informed,’ consent forms may need to be accompanied by an information sheet for participants’ setting out information about the proposed study (in lay terms), along with details about the investigators and how they can be contacted.
Special considerations exist when obtaining consent from vulnerable populations with decisional impairments, such as psychiatric patients, intellectually disabled persons, and children/adolescents. Capacity can vary widely so should be assessed individually, but interventions to improve comprehension may help. Legally authorized representatives usually must provide consent for children.
Participants must be given information relating to the following:
- A statement that participation is voluntary and that refusal to participate will not result in any consequences or any loss of benefits that the person is otherwise entitled to receive.
- Purpose of the research.
- All foreseeable risks and discomforts to the participant (if there are any). These include not only physical injury but also possible psychological.
- Procedures involved in the research.
- Benefits of the research to society and possibly to the individual human subject.
- Length of time the subject is expected to participate.
- Person to contact for answers to questions or in the event of injury or emergency.
- Subjects” right to confidentiality and the right to withdraw from the study at any time without any consequences.
Debriefing after a study involves informing participants about the purpose, providing an opportunity to ask questions, and addressing any harm from participation. Debriefing serves an educational function and allows researchers to correct misconceptions. It is an ethical imperative.
After the research is over, the participant should be able to discuss the procedure and the findings with the psychologist. They must be given a general idea of what the researcher was investigating and why, and their part in the research should be explained.
Participants must be told if they have been deceived and given reasons why. They must be asked if they have any questions, which should be answered honestly and as fully as possible.
Debriefing should occur as soon as possible and be as full as possible; experimenters should take reasonable steps to ensure that participants understand debriefing.
“The purpose of debriefing is to remove any misconceptions and anxieties that the participants have about the research and to leave them with a sense of dignity, knowledge, and a perception of time not wasted” (Harris, 1998).
The debriefing aims to provide information and help the participant leave the experimental situation in a similar frame of mind as when he/she entered it (Aronson, 1988).
Exceptions may exist if debriefing seriously compromises study validity or causes harm itself, like negative emotions in children. Consultation with an institutional review board guides exceptions.
Debriefing indicates investigators’ commitment to participant welfare. Harms may not be raised in the debriefing itself, so responsibility continues after data collection. Following up demonstrates respect and protects persons in human subjects research.
Protection of Participants
Researchers must ensure that those participating in research will not be caused distress. They must be protected from physical and mental harm. This means you must not embarrass, frighten, offend or harm participants.
Normally, the risk of harm must be no greater than in ordinary life, i.e., participants should not be exposed to risks greater than or additional to those encountered in their normal lifestyles.
The researcher must also ensure that if vulnerable groups are to be used (elderly, disabled, children, etc.), they must receive special care. For example, if studying children, ensure their participation is brief as they get tired easily and have a limited attention span.
Researchers are not always accurately able to predict the risks of taking part in a study, and in some cases, a therapeutic debriefing may be necessary if participants have become disturbed during the research (as happened to some participants in Zimbardo’s prisoners/guards study ).
Deception research involves purposely misleading participants or withholding information that could influence their participation decision. This method is controversial because it limits informed consent and autonomy, but can provide otherwise unobtainable valuable knowledge.
Types of deception include (i) deliberate misleading, e.g. using confederates, staged manipulations in field settings, deceptive instructions; (ii) deception by omission, e.g., failure to disclose full information about the study, or creating ambiguity.
The researcher should avoid deceiving participants about the nature of the research unless there is no alternative – and even then, this would need to be judged acceptable by an independent expert. However, some types of research cannot be carried out without at least some element of deception.
For example, in Milgram’s study of obedience , the participants thought they were giving electric shocks to a learner when they answered a question wrongly. In reality, no shocks were given, and the learners were confederates of Milgram.
This is sometimes necessary to avoid demand characteristics (i.e., the clues in an experiment that lead participants to think they know what the researcher is looking for).
Another common example is when a stooge or confederate of the experimenter is used (this was the case in both the experiments carried out by Asch ).
According to ethics codes, deception must have strong scientific justification, and non-deceptive alternatives should not be feasible. Deception that causes significant harm is prohibited. Investigators should carefully weigh whether deception is necessary and ethical for their research.
However, participants must be deceived as little as possible, and any deception must not cause distress. Researchers can determine whether participants are likely distressed when deception is disclosed by consulting culturally relevant groups.
Participants should immediately be informed of the deception without compromising the study’s integrity. Reactions to learning of deception can range from understanding to anger. Debriefing should explain the scientific rationale and social benefits to minimize negative reactions.
If the participant is likely to object or be distressed once they discover the true nature of the research at debriefing, then the study is unacceptable.
If you have gained participants’ informed consent by deception, then they will have agreed to take part without actually knowing what they were consenting to. The true nature of the research should be revealed at the earliest possible opportunity or at least during debriefing.
Some researchers argue that deception can never be justified and object to this practice as it (i) violates an individual’s right to choose to participate; (ii) is a questionable basis on which to build a discipline; and (iii) leads to distrust of psychology in the community.
Confidentiality
Protecting participant confidentiality is an ethical imperative that demonstrates respect, ensures honest participation, and prevents harms like embarrassment or legal issues. Methods like data encryption, coding systems, and secure storage should match the research methodology.
Participants and the data gained from them must be kept anonymous unless they give their full consent. No names must be used in a lab report .
Researchers must clearly describe to participants the limits of confidentiality and methods to protect privacy. With internet research, threats exist like third-party data access; security measures like encryption should be explained. For non-internet research, other protections should be noted too, like coding systems and restricted data access.
High-profile data breaches have eroded public trust. Methods that minimize identifiable information can further guard confidentiality. For example, researchers can consider whether birthdates are necessary or just ages.
Generally, reducing personal details collected and limiting accessibility safeguards participants. Following strong confidentiality protections demonstrates respect for persons in human subjects research.
What do we do if we discover something that should be disclosed (e.g., a criminal act)? Researchers have no legal obligation to disclose criminal acts and must determine the most important consideration: their duty to the participant vs. their duty to the wider community.
Ultimately, decisions to disclose information must be set in the context of the research aims.
Withdrawal from an Investigation
Participants should be able to leave a study anytime if they feel uncomfortable. They should also be allowed to withdraw their data. They should be told at the start of the study that they have the right to withdraw.
They should not have pressure placed upon them to continue if they do not want to (a guideline flouted in Milgram’s research).
Participants may feel they shouldn’t withdraw as this may ‘spoil’ the study. Many participants are paid or receive course credits; they may worry they won’t get this if they withdraw.
Even at the end of the study, the participant has a final opportunity to withdraw the data they have provided for the research.
Ethical Issues in Psychology & Socially Sensitive Research
There has been an assumption over the years by many psychologists that provided they follow the BPS or APA guidelines when using human participants and that all leave in a similar state of mind to how they turned up, not having been deceived or humiliated, given a debrief, and not having had their confidentiality breached, that there are no ethical concerns with their research.
But consider the following examples:
a) Caughy et al. 1994 found that middle-class children in daycare at an early age generally score less on cognitive tests than children from similar families reared in the home.
Assuming all guidelines were followed, neither the parents nor the children participating would have been unduly affected by this research. Nobody would have been deceived, consent would have been obtained, and no harm would have been caused.
However, consider the wider implications of this study when the results are published, particularly for parents of middle-class infants who are considering placing their young children in daycare or those who recently have!
b) IQ tests administered to black Americans show that they typically score 15 points below the average white score.
When black Americans are given these tests, they presumably complete them willingly and are not harmed as individuals. However, when published, findings of this sort seek to reinforce racial stereotypes and are used to discriminate against the black population in the job market, etc.
Sieber & Stanley (1988) (the main names for Socially Sensitive Research (SSR) outline 4 groups that may be affected by psychological research: It is the first group of people that we are most concerned with!
- Members of the social group being studied, such as racial or ethnic group. For example, early research on IQ was used to discriminate against US Blacks.
- Friends and relatives of those participating in the study, particularly in case studies, where individuals may become famous or infamous. Cases that spring to mind would include Genie’s mother.
- The research team. There are examples of researchers being intimidated because of the line of research they are in.
- The institution in which the research is conducted.
salso suggest there are 4 main ethical concerns when conducting SSR:
- The research question or hypothesis.
- The treatment of individual participants.
- The institutional context.
- How the findings of the research are interpreted and applied.
Ethical Guidelines For Carrying Out SSR
Sieber and Stanley suggest the following ethical guidelines for carrying out SSR. There is some overlap between these and research on human participants in general.
Privacy : This refers to people rather than data. Asking people questions of a personal nature (e.g., about sexuality) could offend.
Confidentiality: This refers to data. Information (e.g., about H.I.V. status) leaked to others may affect the participant’s life.
Sound & valid methodology : This is even more vital when the research topic is socially sensitive. Academics can detect flaws in methods, but the lay public and the media often don’t.
When research findings are publicized, people are likely to consider them fact, and policies may be based on them. Examples are Bowlby’s maternal deprivation studies and intelligence testing.
Deception : Causing the wider public to believe something, which isn’t true by the findings, you report (e.g., that parents are responsible for how their children turn out).
Informed consent : Participants should be made aware of how participating in the research may affect them.
Justice & equitable treatment : Examples of unjust treatment are (i) publicizing an idea, which creates a prejudice against a group, & (ii) withholding a treatment, which you believe is beneficial, from some participants so that you can use them as controls.
Scientific freedom : Science should not be censored, but there should be some monitoring of sensitive research. The researcher should weigh their responsibilities against their rights to do the research.
Ownership of data : When research findings could be used to make social policies, which affect people’s lives, should they be publicly accessible? Sometimes, a party commissions research with their interests in mind (e.g., an industry, an advertising agency, a political party, or the military).
Some people argue that scientists should be compelled to disclose their results so that other scientists can re-analyze them. If this had happened in Burt’s day, there might not have been such widespread belief in the genetic transmission of intelligence. George Miller (Miller’s Magic 7) famously argued that we should give psychology away.
The values of social scientists : Psychologists can be divided into two main groups: those who advocate a humanistic approach (individuals are important and worthy of study, quality of life is important, intuition is useful) and those advocating a scientific approach (rigorous methodology, objective data).
The researcher’s values may conflict with those of the participant/institution. For example, if someone with a scientific approach was evaluating a counseling technique based on a humanistic approach, they would judge it on criteria that those giving & receiving the therapy may not consider important.
Cost/benefit analysis : It is unethical if the costs outweigh the potential/actual benefits. However, it isn’t easy to assess costs & benefits accurately & the participants themselves rarely benefit from research.
Sieber & Stanley advise that researchers should not avoid researching socially sensitive issues. Scientists have a responsibility to society to find useful knowledge.
- They need to take more care over consent, debriefing, etc. when the issue is sensitive.
- They should be aware of how their findings may be interpreted & used by others.
- They should make explicit the assumptions underlying their research so that the public can consider whether they agree with these.
- They should make the limitations of their research explicit (e.g., ‘the study was only carried out on white middle-class American male students,’ ‘the study is based on questionnaire data, which may be inaccurate,’ etc.
- They should be careful how they communicate with the media and policymakers.
- They should be aware of the balance between their obligations to participants and those to society (e.g. if the participant tells them something which they feel they should tell the police/social services).
- They should be aware of their own values and biases and those of the participants.
Arguments for SSR
- Psychologists have devised methods to resolve the issues raised.
- SSR is the most scrutinized research in psychology. Ethical committees reject more SSR than any other form of research.
- By gaining a better understanding of issues such as gender, race, and sexuality, we are able to gain greater acceptance and reduce prejudice.
- SSR has been of benefit to society, for example, EWT. This has made us aware that EWT can be flawed and should not be used without corroboration. It has also made us aware that the EWT of children is every bit as reliable as that of adults.
- Most research is still on white middle-class Americans (about 90% of research is quoted in texts!). SSR is helping to redress the balance and make us more aware of other cultures and outlooks.
Arguments against SSR
- Flawed research has been used to dictate social policy and put certain groups at a disadvantage.
- Research has been used to discriminate against groups in society, such as the sterilization of people in the USA between 1910 and 1920 because they were of low intelligence, criminal, or suffered from psychological illness.
- The guidelines used by psychologists to control SSR lack power and, as a result, are unable to prevent indefensible research from being carried out.
American Psychological Association. (2002). American Psychological Association ethical principles of psychologists and code of conduct. www.apa.org/ethics/code2002.html
Baumrind, D. (1964). Some thoughts on ethics of research: After reading Milgram’s” Behavioral study of obedience.”. American Psychologist , 19 (6), 421.
Caughy, M. O. B., DiPietro, J. A., & Strobino, D. M. (1994). Day‐care participation as a protective factor in the cognitive development of low‐income children. Child development , 65 (2), 457-471.
Harris, B. (1988). Key words: A history of debriefing in social psychology. In J. Morawski (Ed.), The rise of experimentation in American psychology (pp. 188-212). New York: Oxford University Press.
Rosenthal, R., & Rosnow, R. L. (1984). Applying Hamlet’s question to the ethical conduct of research: A conceptual addendum. American Psychologist, 39(5) , 561.
Sieber, J. E., & Stanley, B. (1988). Ethical and professional dimensions of socially sensitive research. American psychologist , 43 (1), 49.
The British Psychological Society. (2010). Code of Human Research Ethics. www.bps.org.uk/sites/default/files/documents/code_of_human_research_ethics.pdf
Further Information
- MIT Psychology Ethics Lecture Slides
BPS Documents
- Code of Ethics and Conduct (2018)
- Good Practice Guidelines for the Conduct of Psychological Research within the NHS
- Guidelines for Psychologists Working with Animals
- Guidelines for ethical practice in psychological research online
APA Documents
APA Ethical Principles of Psychologists and Code of Conduct
Related Articles
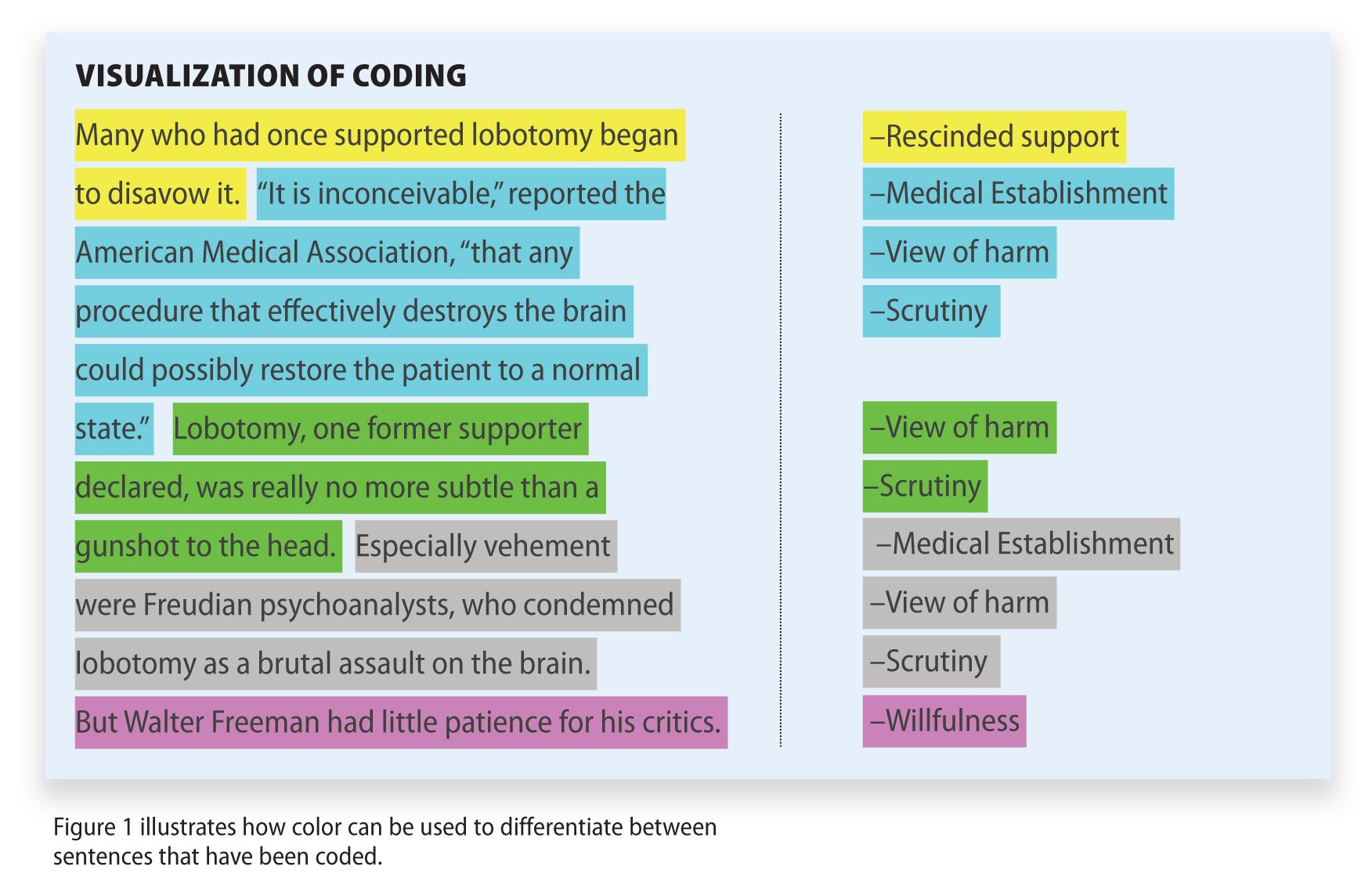
Research Methodology
Qualitative Data Coding

What Is a Focus Group?
Cross-Cultural Research Methodology In Psychology

What Is Internal Validity In Research?

Research Methodology , Statistics
What Is Face Validity In Research? Importance & How To Measure

Criterion Validity: Definition & Examples
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Med Princ Pract
- v.30(1); 2021 Feb

Principles of Clinical Ethics and Their Application to Practice
An overview of ethics and clinical ethics is presented in this review. The 4 main ethical principles, that is beneficence, nonmaleficence, autonomy, and justice, are defined and explained. Informed consent, truth-telling, and confidentiality spring from the principle of autonomy, and each of them is discussed. In patient care situations, not infrequently, there are conflicts between ethical principles (especially between beneficence and autonomy). A four-pronged systematic approach to ethical problem-solving and several illustrative cases of conflicts are presented. Comments following the cases highlight the ethical principles involved and clarify the resolution of these conflicts. A model for patient care, with caring as its central element, that integrates ethical aspects (intertwined with professionalism) with clinical and technical expertise desired of a physician is illustrated.
Highlights of the Study
- Main principles of ethics, that is beneficence, nonmaleficence, autonomy, and justice, are discussed.
- Autonomy is the basis for informed consent, truth-telling, and confidentiality.
- A model to resolve conflicts when ethical principles collide is presented.
- Cases that highlight ethical issues and their resolution are presented.
- A patient care model that integrates ethics, professionalism, and cognitive and technical expertise is shown.
Introduction
A defining responsibility of a practicing physician is to make decisions on patient care in different settings. These decisions involve more than selecting the appropriate treatment or intervention.
Ethics is an inherent and inseparable part of clinical medicine [ 1 ] as the physician has an ethical obligation (i) to benefit the patient, (ii) to avoid or minimize harm, and to (iii) respect the values and preferences of the patient. Are physicians equipped to fulfill this ethical obligation and can their ethical skills be improved? A goal-oriented educational program [ 2 ] (Table (Table1) 1 ) has been shown to improve learner awareness, attitudes, knowledge, moral reasoning, and confidence [ 3 , 4 ].
Goals of ethics education
Ethics, Morality, and Professional Standards
Ethics is a broad term that covers the study of the nature of morals and the specific moral choices to be made. Normative ethics attempts to answer the question, “Which general moral norms for the guidance and evaluation of conduct should we accept, and why?” [ 5 ]. Some moral norms for right conduct are common to human kind as they transcend cultures, regions, religions, and other group identities and constitute common morality (e.g., not to kill, or harm, or cause suffering to others, not to steal, not to punish the innocent, to be truthful, to obey the law, to nurture the young and dependent, to help the suffering, and rescue those in danger). Particular morality refers to norms that bind groups because of their culture, religion, profession and include responsibilities, ideals, professional standards, and so on. A pertinent example of particular morality is the physician's “accepted role” to provide competent and trustworthy service to their patients. To reduce the vagueness of “accepted role,” physician organizations (local, state, and national) have codified their standards. However, complying with these standards, it should be understood, may not always fulfill the moral norms as the codes have “often appeared to protect the profession's interests more than to offer a broad and impartial moral viewpoint or to address issues of importance to patients and society” [ 6 ].
Bioethics and Clinical (Medical) Ethics
A number of deplorable abuses of human subjects in research, medical interventions without informed consent, experimentation in concentration camps in World War II, along with salutary advances in medicine and medical technology and societal changes, led to the rapid evolution of bioethics from one concerned about professional conduct and codes to its present status with an extensive scope that includes research ethics, public health ethics, organizational ethics, and clinical ethics.
Hereafter, the abbreviated term, ethics, will be used as I discuss the principles of clinical ethics and their application to clinical practice.
The Fundamental Principles of Ethics
Beneficence, nonmaleficence, autonomy, and justice constitute the 4 principles of ethics. The first 2 can be traced back to the time of Hippocrates “to help and do no harm,” while the latter 2 evolved later. Thus, in Percival's book on ethics in early 1800s, the importance of keeping the patient's best interest as a goal is stressed, while autonomy and justice were not discussed. However, with the passage of time, both autonomy and justice gained acceptance as important principles of ethics. In modern times, Beauchamp and Childress' book on Principles of Biomedical Ethics is a classic for its exposition of these 4 principles [ 5 ] and their application, while also discussing alternative approaches.
Beneficence
The principle of beneficence is the obligation of physician to act for the benefit of the patient and supports a number of moral rules to protect and defend the right of others, prevent harm, remove conditions that will cause harm, help persons with disabilities, and rescue persons in danger. It is worth emphasizing that, in distinction to nonmaleficence, the language here is one of positive requirements. The principle calls for not just avoiding harm, but also to benefit patients and to promote their welfare. While physicians' beneficence conforms to moral rules, and is altruistic, it is also true that in many instances it can be considered a payback for the debt to society for education (often subsidized by governments), ranks and privileges, and to the patients themselves (learning and research).
Nonmaleficence
Nonmaleficence is the obligation of a physician not to harm the patient. This simply stated principle supports several moral rules − do not kill, do not cause pain or suffering, do not incapacitate, do not cause offense, and do not deprive others of the goods of life. The practical application of nonmaleficence is for the physician to weigh the benefits against burdens of all interventions and treatments, to eschew those that are inappropriately burdensome, and to choose the best course of action for the patient. This is particularly important and pertinent in difficult end-of-life care decisions on withholding and withdrawing life-sustaining treatment, medically administered nutrition and hydration, and in pain and other symptom control. A physician's obligation and intention to relieve the suffering (e.g., refractory pain or dyspnea) of a patient by the use of appropriate drugs including opioids override the foreseen but unintended harmful effects or outcome (doctrine of double effect) [ 7 , 8 ].
The philosophical underpinning for autonomy, as interpreted by philosophers Immanuel Kant (1724–1804) and John Stuart Mill (1806–1873), and accepted as an ethical principle, is that all persons have intrinsic and unconditional worth, and therefore, should have the power to make rational decisions and moral choices, and each should be allowed to exercise his or her capacity for self-determination [ 9 ]. This ethical principle was affirmed in a court decision by Justice Cardozo in 1914 with the epigrammatic dictum, “Every human being of adult years and sound mind has a right to determine what shall be done with his own body” [ 10 ].
Autonomy, as is true for all 4 principles, needs to be weighed against competing moral principles, and in some instances may be overridden; an obvious example would be if the autonomous action of a patient causes harm to another person(s). The principle of autonomy does not extend to persons who lack the capacity (competence) to act autonomously; examples include infants and children and incompetence due to developmental, mental or physical disorder. Health-care institutions and state governments in the US have policies and procedures to assess incompetence. However, a rigid distinction between incapacity to make health-care decisions (assessed by health professionals) and incompetence (determined by court of law) is not of practical use, as a clinician's determination of a patient's lack of decision-making capacity based on physical or mental disorder has the same practical consequences as a legal determination of incompetence [ 11 ].
Detractors of the principle of autonomy question the focus on the individual and propose a broader concept of relational autonomy (shaped by social relationships and complex determinants such as gender, ethnicity and culture) [ 12 ]. Even in an advanced western country such as United States, the culture being inhomogeneous, some minority populations hold views different from that of the majority white population in need for full disclosure, and in decisions about life support (preferring a family-centered approach) [ 13 ].
Resistance to the principle of patient autonomy and its derivatives (informed consent, truth-telling) in non-western cultures is not unexpected. In countries with ancient civilizations, rooted beliefs and traditions, the practice of paternalism ( this term will be used in this article, as it is well-entrenched in ethics literature, although parentalism is the proper term ) by physicians emanates mostly from beneficence. However, culture (a composite of the customary beliefs, social forms, and material traits of a racial, religious or social group) is not static and autonomous, and changes with other trends over passing years. It is presumptuous to assume that the patterns and roles in physician-patient relationships that have been in place for a half a century and more still hold true. Therefore, a critical examination of paternalistic medical practice is needed for reasons that include technological and economic progress, improved educational and socioeconomic status of the populace, globalization, and societal movement towards emphasis on the patient as an individual, than as a member of a group. This needed examination can be accomplished by research that includes well-structured surveys on demographics, patient preferences on informed consent, truth-telling, and role in decision-making.
Respecting the principle of autonomy obliges the physician to disclose medical information and treatment options that are necessary for the patient to exercise self-determination and supports informed consent, truth-telling, and confidentiality.
Informed Consent
The requirements of an informed consent for a medical or surgical procedure, or for research, are that the patient or subject (i) must be competent to understand and decide, (ii) receives a full disclosure, (iii) comprehends the disclosure, (iv) acts voluntarily, and (v) consents to the proposed action.
The universal applicability of these requirements, rooted and developed in western culture, has met with some resistance and a suggestion to craft a set of requirements that accommodate the cultural mores of other countries [ 14 ]. In response and in vigorous defense of the 5 requirements of informed consent, Angell wrote, “There must be a core of human rights that we would wish to see honored universally, despite variations in their superficial aspects …The forces of local custom or local law cannot justify abuses of certain fundamental rights, and the right of self-determination on which the doctrine of informed consent is based, is one of them” [ 15 ].
As competence is the first of the requirements for informed consent, one should know how to detect incompetence. Standards (used singly or in combination) that are generally accepted for determining incompetence are based on the patient's inability to state a preference or choice, inability to understand one's situation and its consequences, and inability to reason through a consequential life decision [ 16 ].
In a previously autonomous, but presently incompetent patient, his/her previously expressed preferences (i.e., prior autonomous judgments) are to be respected [ 17 ]. Incompetent (non-autonomous) patients and previously competent (autonomous), but presently incompetent patients would need a surrogate decision-maker. In a non-autonomous patient, the surrogate can use either a substituted judgment standard (i.e., what the patient would wish in this circumstance and not what the surrogate would wish), or a best interests standard (i.e., what would bring the highest net benefit to the patient by weighing risks and benefits). Snyder and Sulmasy [ 18 ], in their thoughtful article, provide a practical and useful option when the surrogate is uncertain of the patient's preference(s), or when patient's preferences have not kept abreast of scientific advances. They suggest the surrogate use “substituted interests,” that is, the patient's authentic values and interests, to base the decision.
Truth-Telling
Truth-telling is a vital component in a physician-patient relationship; without this component, the physician loses the trust of the patient. An autonomous patient has not only the right to know (disclosure) of his/her diagnosis and prognosis, but also has the option to forgo this disclosure. However, the physician must know which of these 2 options the patient prefers.
In the United States, full disclosure to the patient, however grave the disease is, is the norm now, but was not so in the past. Significant resistance to full disclosure was highly prevalent in the US, but a marked shift has occurred in physicians' attitudes on this. In 1961, 88% of physicians surveyed indicated their preference to avoid disclosing a diagnosis [ 19 ]; in 1979, however, 98% of surveyed physicians favored it [ 20 ]. This marked shift is attributable to many factors that include − with no order of importance implied − educational and socioeconomic progress, increased accountability to society, and awareness of previous clinical and research transgressions by the profession.
Importantly, surveys in the US show that patients with cancer and other diseases wish to have been fully informed of their diagnoses and prognoses. Providing full information, with tact and sensitivity, to patients who want to know should be the standard. The sad consequences of not telling the truth regarding a cancer include depriving the patient of an opportunity for completion of important life-tasks: giving advice to, and taking leave of loved ones, putting financial affairs in order, including division of assets, reconciling with estranged family members and friends, attaining spiritual order by reflection, prayer, rituals, and religious sacraments [ 21 , 22 ].
In contrast to the US, full disclosure to the patient is highly variable in other countries [ 23 ]. A continuing pattern in non-western societies is for the physician to disclose the information to the family and not to the patient. The likely reasons for resistance of physicians to convey bad news are concern that it may cause anxiety and loss of hope, some uncertainty on the outcome, or belief that the patient would not be able to understand the information or may not want to know. However, this does not have to be a binary choice, as careful understanding of the principle of autonomy reveals that autonomous choice is a right of a patient, and the patient, in exercising this right, may authorize a family member or members to make decisions for him/her.
Confidentiality
Physicians are obligated not to disclose confidential information given by a patient to another party without the patient's authorization. An obvious exception (with implied patient authorization) is the sharing necessary of medical information for the care of the patient from the primary physician to consultants and other health-care teams. In the present-day modern hospitals with multiple points of tests and consultants, and the use of electronic medical records, there has been an erosion of confidentiality. However, individual physicians must exercise discipline in not discussing patient specifics with their family members or in social gatherings [ 24 ] and social media. There are some noteworthy exceptions to patient confidentiality. These include, among others, legally required reporting of gunshot wounds and sexually transmitted diseases and exceptional situations that may cause major harm to another (e.g., epidemics of infectious diseases, partner notification in HIV disease, relative notification of certain genetic risks, etc.).
Justice is generally interpreted as fair, equitable, and appropriate treatment of persons. Of the several categories of justice, the one that is most pertinent to clinical ethics is distributive justice . Distributive justice refers to the fair, equitable, and appropriate distribution of health-care resources determined by justified norms that structure the terms of social cooperation [ 25 ]. How can this be accomplished? There are different valid principles of distributive justice. These are distribution to each person (i) an equal share, (ii) according to need, (iii) according to effort, (iv) according to contribution, (v) according to merit, and (vi) according to free-market exchanges. Each principle is not exclusive, and can be, and are often combined in application. It is easy to see the difficulty in choosing, balancing, and refining these principles to form a coherent and workable solution to distribute medical resources.
Although this weighty health-care policy discussion exceeds the scope of this review, a few examples on issues of distributive justice encountered in hospital and office practice need to be mentioned. These include allotment of scarce resources (equipment, tests, medications, organ transplants), care of uninsured patients, and allotment of time for outpatient visits (equal time for every patient? based on need or complexity? based on social and or economic status?). Difficult as it may be, and despite the many constraining forces, physicians must accept the requirement of fairness contained in this principle [ 26 ]. Fairness to the patient assumes a role of primary importance when there are conflicts of interests. A flagrant example of violation of this principle would be when a particular option of treatment is chosen over others, or an expensive drug is chosen over an equally effective but less expensive one because it benefits the physician, financially, or otherwise.
Conflicts between Principles
Each one of the 4 principles of ethics is to be taken as a prima facie obligation that must be fulfilled, unless it conflicts, in a specific instance, with another principle. When faced with such a conflict, the physician has to determine the actual obligation to the patient by examining the respective weights of the competing prima facie obligations based on both content and context. Consider an example of a conflict that has an easy resolution: a patient in shock treated with urgent fluid-resuscitation and the placement of an indwelling intravenous catheter caused pain and swelling. Here the principle of beneficence overrides that of nonmaleficence. Many of the conflicts that physicians face, however, are much more complex and difficult. Consider a competent patient's refusal of a potentially life-saving intervention (e.g., instituting mechanical ventilation) or request for a potentially life-ending action (e.g., withdrawing mechanical ventilation). Nowhere in the arena of ethical decision-making is conflict as pronounced as when the principles of beneficence and autonomy collide.
Beneficence has enjoyed a historical role in the traditional practice of medicine. However, giving it primacy over patient autonomy is paternalism that makes a physician-patient relationship analogous to that of a father/mother to a child. A father/mother may refuse a child's wishes, may influence a child by a variety of ways − nondisclosure, manipulation, deception, coercion etc., consistent with his/her thinking of what is best for the child. Paternalism can be further divided into soft and hard .
In soft paternalism, the physician acts on grounds of beneficence (and, at times, nonmaleficence) when the patient is nonautonomous or substantially nonautonomous (e.g., cognitive dysfunction due to severe illness, depression, or drug addiction) [ 27 ]. Soft paternalism is complicated because of the difficulty in determining whether the patient was nonautonomous at the time of decision-making but is ethically defensible as long as the action is in concordance with what the physician believes to be the patient's values. Hard paternalism is action by a physician, intended to benefit a patient, but contrary to the voluntary decision of an autonomous patient who is fully informed and competent, and is ethically indefensible.
On the other end of the scale of hard paternalism is consumerism, a rare and extreme form of patient autonomy, that holds the view that the physician's role is limited to providing all the medical information and the available choices for interventions and treatments while the fully informed patient selects from the available choices. In this model, the physician's role is constrained, and does not permit the full use of his/her knowledge and skills to benefit the patient, and is tantamount to a form of patient abandonment and therefore is ethically indefensible.
Faced with the contrasting paradigms of beneficence and respect for autonomy and the need to reconcile these to find a common ground, Pellegrino and Thomasma [ 28 ] argue that beneficence can be inclusive of patient autonomy as “the best interests of the patients are intimately linked with their preferences” from which “are derived our primary duties to them.”
One of the basic and not infrequent reasons for disagreement between physician and patient on treatment issues is their divergent views on goals of treatment. As goals change in the course of disease (e.g., a chronic neurologic condition worsens to the point of needing ventilator support, or a cancer that has become refractory to treatment), it is imperative that the physician communicates with the patient in clear and straightforward language, without the use of medical jargon, and with the aim of defining the goal(s) of treatment under the changed circumstance. In doing so, the physician should be cognizant of patient factors that compromise decisional capacity, such as anxiety, fear, pain, lack of trust, and different beliefs and values that impair effective communication [ 29 ].
The foregoing theoretical discussion on principles of ethics has practical application in clinical practice in all settings. In the resource book for clinicians, Jonsen et al. [ 30 ] have elucidated a logical and well accepted model (Table (Table2), 2 ), along the lines of the systematic format that practicing physicians have been taught and have practiced for a long time (Chief Complaint, History of Present Illness, Past History, pertinent Family and Social History, Review of Systems, Physical Examination and Laboratory and Imaging studies). This practical approach to problem-solving in ethics involves:
- Clinical assessment (identifying medical problems, treatment options, goals of care)
- Patient (finding and clarifying patient preferences on treatment options and goals of care)
- Quality of life (QOL) (effects of medical problems, interventions and treatments on patient's QOL with awareness of individual biases on what constitutes an acceptable QOL)
- Context (many factors that include family, cultural, spiritual, religious, economic and legal).
Application of principles of ethics in patient care
Using this model, the physician can identify the principles that are in conflict, ascertain by weighing and balancing what should prevail, and when in doubt, turn to ethics literature and expert opinion.
Illustrative Cases
There is a wide gamut of clinical patient encounters with ethical issues, and some, especially those involving end-of-life care decisions, are complex. A few cases (Case 1 is modified from resource book [ 30 ]) are presented below as they highlight the importance of understanding and weighing the ethical principles involved to arrive at an ethically right solution. Case 6 was added during the revision phase of this article as it coincided with the outbreak of Coronavirus Infectious Disease-2019 (COVID-19) that became a pandemic rendering a discussion of its ethical challenges necessary and important.
A 20-year old college student living in the college hostel is brought by a friend to the Emergency Department (ED) because of unrelenting headache and fever. He appeared drowsy but was responsive and had fever (40°C), and neck rigidity on examination. Lumbar puncture was done, and spinal fluid appeared cloudy and showed increased white cells; Gram stain showed Gram-positive diplococci. Based on the diagnosis of bacterial meningitis, appropriate antibiotics were begun, and hospitalization was instituted. Although initial consent for diagnosis was implicit, and consent for lumbar puncture was explicit, at this point, the patient refuses treatment without giving any reason, and insists to return to his hostel. Even after explanation by the physician as to the seriousness of his diagnosis, and the absolute need for prompt treatment (i.e., danger to life without treatment), the patient is adamant in his refusal.
Comment . Because of this refusal, the medical indications and patient preferences (see Table Table2) 2 ) are at odds. Is it ethically right to treat against his will a patient who is making a choice that has dire consequences (disability, death) who gives no reason for this decision, and in whom a clear determination of mental incapacity cannot be made (although altered mental status may be presumed)? Here the principle of beneficence and principle of autonomy are in conflict. The weighing of factors: (1) patient may not be making a reasoned decision in his best interest because of temporary mental incapacity; and (2) the severity of life-threatening illness and the urgency to treat to save his life supports the decision in favor of beneficence (i.e., to treat).
A 56-year old male lawyer and current cigarette smoker with a pack-a-day habit for more than 30 years, is found to have a solitary right upper lobe pulmonary mass 5 cm in size on a chest radiograph done as part of an insurance application. The mass has no calcification, and there are no other pulmonary abnormalities. He has no symptoms, and his examination is normal. Tuberculosis skin test is negative, and he has no history of travel to an endemic area of fungal infection. As lung cancer is the most probable and significant diagnosis to consider, and early surgical resection provides the best prospects for cure, the physician, in consultation with the thoracic surgeon, recommends bronchoscopic biopsy and subsequent resection. The patient understands the treatment plan, and the significance of not delaying the treatment. However, he refuses, and states that he does not think he has cancer; and is fearful that the surgery would kill him. Even after further explanations on the low mortality of surgery and the importance of removing the mass before it spreads, he continues to refuse treatment.
Comment . Even though the physician's prescribed treatment, that is, removal of the mass that is probably cancer, affords the best chance of cure, and delay in its removal increases its chance of metastases and reaching an incurable stage − the choice by this well informed and mentally competent patient should be respected. Here, autonomy prevails over beneficence. The physician, however, may not abandon the patient and is obligated to offer continued outpatient visits with advice against making decision based on fear, examinations, periodic tests, and encouragement to seek a second opinion.
A 71-year-old man with very severe chronic obstructive pulmonary disease (COPD) is admitted to the intensive care unit (ICU) with pneumonia, sepsis, and respiratory failure. He is intubated and mechanically ventilated. For the past 2 years, he has been on continuous oxygen treatment and was short of breath on minimal exertion. In the past 1 year, he had 2 admissions to the ICU; on both occasions he required intubation and mechanical ventilation. Presently, even with multiple antibiotics, intravenous fluid hydration, and vasopressors, his systolic blood pressure remains below 60 mm Hg, and with high flow oxygen supplementation, his oxygen saturation stays below 80%; his arterial blood pH is 7.0. His liver enzymes are elevated. He is anuric, and over next 8 h his creatinine has risen to 5 mg/dL and continues to rise. He has drifted into a comatose state. The intensivist suggests discontinuation of vasopressors and mechanical ventilation as their continued use is futile. The patient has no advance care directives or a designated health-care proxy.
Comment . The term “futility” is open to different definitions [ 31 ] and is often controversial, and therefore, some experts suggest the alternate term, “clinically non-beneficial interventions” [ 32 ]. However, in this case the term futility is appropriate to indicate that there is evidence of physiological futility (multisystem organ failure in the setting of preexisting end stage COPD, and medical interventions would not reverse the decline). It is appropriate then to discuss the patient's condition with his family with the goal of discontinuing life-sustaining interventions. These discussions should be done with sensitivity, compassion and empathy. Palliative care should be provided to alleviate his symptoms and to support the family until his death and beyond in their bereavement.
A 67-year old widow, an immigrant from southern India, is living with her son and his family in Wisconsin, USA. She was experiencing nausea, lack of appetite and weight loss for a few months. During the past week, she also had dark yellow urine, and yellow coloration of her skin. She has basic knowledge of English. She was brought to a multi-specialty teaching hospital by her son, who informed the doctor that his mother has “jaundice,” and instructed that, if any serious life-threatening disease was found, not to inform her. He asked that all information should come to him, and if there is any cancer not to treat it, since she is older and frail. Investigations in the hospital reveals that she has pancreatic cancer, and chemotherapy, while not likely to cure, would prolong her life.
Comment . In some ancient cultures, authority is given to members of the family (especially senior men) to make decisions that involve other members on marriage, job, and health care. The woman in this case is a dependent of her son, and given this cultural perspective, the son can rightfully claim to have the authority to make health-care decisions for her. Thus, the physician is faced with multiple tasks that may not be consonant. To respect cultural values [ 33 ], to directly learn the patient's preferences, to comply with the American norm of full disclosure to the patient, and to refuse the son's demands.
The principle of autonomy provides the patient the option to delegate decision-making authority to another person. Therefore, the appropriate course would be to take the tactful approach of directly informing the patient (with a translator if needed), that the diagnosed disease would require decisions for appropriate treatment. The physician should ascertain whether she would prefer to make these decisions herself, or whether she would prefer all information to be given to her son, and all decisions to be made by him.
A 45-year-old woman had laparotomy and cholecystectomy for abdominal pain and multiple gall stones. Three weeks after discharge from the hospital, she returned with fever, abdominal pain, and tenderness. She was given antibiotics, and as her fever continued, laparotomy and exploration were undertaken; a sponge left behind during the recent cholecystectomy was found. It was removed, the area cleansed, and incision closed. Antibiotics were continued, and she recovered without further incident and was discharged. Should the surgeon inform the patient of his error?
Comment . Truth-telling, a part of patient autonomy is very much applicable in this situation and disclosure to patient is required [ 34 , 35 , 36 ]. The mistake caused harm to the patient (morbidity and readmission, and a second surgery and monetary loss). Although the end result remedied the harm, the surgeon is obligated to inform the patient of the error and its consequences and offer an apology. Such errors are always reported to the Operating Room Committees and Surgical Quality Improvement Committees of US Hospitals. Hospital-based risk reduction mechanisms (e.g., Risk Management Department) present in most US hospitals would investigate the incident and come up with specific recommendations to mitigate the error and eliminate them in the future. Many institutions usually make financial settlements to obviate liability litigation (fees and hospital charges waived, and/or monetary compensation made to the patient). Elsewhere, if such mechanisms do not exist, it should be reported to the hospital. Acknowledgment from the hospital, apologies from the institution and compensation for the patient are called for. Whether in US or elsewhere, a malpractice suit is very possible in this situation, but a climate of honesty substantially reduces the threat of legal claims as most patients trust their physicians and are not vindictive.
The following scenario is at a city hospital during the peak of the COVID-19 pandemic: A 74-year-old woman, residing in an assisted living facility, is brought to the ED with shortness of breath and malaise. Over the past 4 days she had been experiencing dry cough, lack of appetite, and tiredness; 2 days earlier, she stopped eating and started having a low-grade fever. A test for COVID-19 undertaken by the assisted living facility was returned positive on the morning of the ED visit.
She, a retired nurse, is a widow; both of her grown children live out-of-state. She has had hypertension for many years, controlled with daily medications. Following 2 strokes, she was moved to an assisted living facility 3 years ago. She recovered most of her functions after the strokes and required help only for bathing and dressing. She is able to answer questions appropriately but haltingly, because of respiratory distress. She has tachypnea (34/min), tachycardia (120/min), temperature of 101°F, BP 100/60 and 90% O 2 saturation (on supplemental O 2 of 4 L/min). She has dry mouth and tongue and rhonchi on lung auscultation. Her respiratory rate is increasing on observation and she is visibly tiring.
Another patient is now brought in by ambulance; this is a 22-year-old man living in an apartment and has had symptoms of “flu” for a week. Because of the pandemic, he was observing the recommended self-distancing, and had no known exposure to coronavirus. He used saline gargles, acetaminophen, and cough syrup to alleviate his sore throat, cough, and fever. In the past 2 days, his symptoms worsened, and he drove himself to a virus testing station and got tested for COVID-19; he was told that he would be notified of the results. He returned to his apartment and after a sleepless night with fever, sweats, and persistent cough, he woke up and felt drained of all strength. The test result confirmed COVID-19. He then called for an ambulance.
He has been previously healthy. He is a non-smoker and uses alcohol rarely. He is a second-year medical student. He is single, and his parents and sibling live hundreds of miles away.
On examination, he has marked tachypnea (>40/min), shallow breathing, heart rate of 128/min, temperature of 103°F and O 2 saturation of 88 on pulse oximetry. He appears drowsy and is slow to respond to questions. He is propped up to a sitting position as it is uncomfortable for him to be supine. Accessory muscles of neck and intercostals are contracting with each breath, and on auscultation, he has basilar crackles and scattered rhonchi. His O 2 saturation drops to 85 and he is in respiratory distress despite nebulized bronchodilator treatment.
Both of these patients are in respiratory failure, clinically and confirmed by arterial blood gases, and are in urgent need of intubation and mechanical ventilation. However, only one ventilator is available; who gets it?
Comment . The decision to allocate a scarce and potentially life-saving equipment (ventilator) is very difficult as it directly addresses the question “Who shall live when not everyone can live? [ 5 ]. This decision cannot be emotion-driven or arbitrary; nor should it be based on a person's wealth or social standing. Priorities need to be established ethically and must be applied consistently in the same institution and ideally throughout the state and the country. The general social norm to treat all equally or to treat on a first come, first saved basis is not the appropriate choice here. There is a consensus among clinical ethics scholars, that in this situation, maximizing benefits is the dominant value in making a decision [ 37 ]. Maximizing benefits can be viewed in 2 different ways; in lives saved or in life-years saved; they differ in that the first is non-utilitarian while the second is utilitarian. A subordinate consideration is giving priority to patients who have a better chance of survival and a reasonable life expectancy. The other 2 considerations are promoting and rewarding instrumental value (benefit to others) and the acuity of illness. Health-care workers (physicians, nurses, therapists etc.) and research participants have instrumental value as their work benefits others; among them those actively contributing are of more value than those who have made their contributions. The need to prioritize the sickest and the youngest is also a recognized value when these are aligned with the dominant value of maximizing benefits. In the context of COVID-19 pandemic, Emanuel et al. [ 37 ] weighed and analyzed these values and offered some recommendations. Some ethics scholars opine that in times of a pandemic, the burden of making a decision as to who gets a ventilator and who does not (often a life or death choice) should not be on the front-line physicians, as it may cause a severe and life-long emotional toll on them [ 35 , 36 ]. The toll can be severe for nurses and other front-line health-care providers as well. As a safeguard, they propose that the decision should rest on a select committee that excludes doctors, nurses and others who are caring for the patient(s) under consideration [ 38 ].
Both patients described in the case summaries have comparable acuity of illness and both are in need of mechanical ventilator support. However, in the dominant value of maximizing benefits the two patients differ; in terms of life-years saved, the second patient (22-year-old man) is ahead as his life expectancy is longer. Additionally, he is more likely than the older woman, to survive mechanical ventilation, infection, and possible complications. Another supporting factor in favor of the second patient is his potential instrumental value (benefit to others) as a future physician.
Unlike the other illustrative cases, the scenario of these 2 cases, does not lend itself to a peaceful and fully satisfactory resolution. The fairness of allocating a scarce and potentially life-saving resource based on maximizing benefits and preference to instrumental value (benefit to others) is open to question. The American College of Physicians has stated that allocation decisions during resource scarcity should be made “based on patient need, prognosis (determined by objective scientific measure and informed clinical judgment) and effectiveness (i.e., likelihood that the therapy will help the patient to recover), … to maximize the number of patients who will recover” [ 39 ].
This review has covered basics of ethics founded on morality and ethical principles with illustrative examples. In the following segment, professionalism is defined, its alignment with ethics depicted, and virtues desired of a physician (inclusive term for medical doctor regardless of type of practice) are elucidated. It concludes with my vision of an integrated model for patient care.
The core of professionalism is a therapeutic relationship built on competent and compassionate care by a physician that meets the expectation and benefits a patient. In this relationship, which is rooted in the ethical principles of beneficence and nonmaleficence, the physician fulfills the elements shown in Table Table3. 3 . Professionalism “demands placing the interest of patients above those of the physician, setting and maintaining standards of competence and integrity, and providing expert advice to society on matters of health” [ 26 , 40 ].
Physicians obligations
Drawing on several decades of experience in teaching and mentoring, I envisage physicians with qualities of both “heart” and “head.” Ethical and humanistic values shape the former, while knowledge (e.g., by study, research, practice) and technical skills (e.g., medical and surgical procedures) form the latter. Figure Figure1 1 is a representation of this model. Morality that forms the base of the model and ethical principles that rest on it were previously explained. Virtues are linked, some more tightly than others, to the principles of ethics. Compassion, a prelude to caring, presupposes sympathy, is expressed in beneficence. Discernment is especially valuable in decision-making when principles of ethics collide. Trustworthiness leads to trust, and is a needed virtue when patients, at their most vulnerable time, place themselves in the hands of physicians. Integrity involves the coherent integration of emotions, knowledge and aspirations while maintaining moral values. Physicians need both professional integrity and personal integrity, as the former may not cover all scenarios (e.g., prescribing ineffective drugs or expensive drugs when effective inexpensive drugs are available, performing invasive treatments or experimental research modalities without fully informed consent, any situation where personal monetary gain is placed over patient's welfare). Conscientiousness is required to determine what is right by critical reflection on good versus bad, better versus good, logical versus emotional, and right versus wrong.

Integrated model of patient care.
In my conceptualized model of patient care (Fig. (Fig.1), 1 ), medical knowledge, skills to apply that knowledge, technical skills, practice-based learning, and communication skills are partnered with ethical principles and professional virtues. The virtues of compassion, discernment, trustworthiness, integrity, and conscientiousness are the necessary building blocks for the virtue of caring. Caring is the defining virtue for all health-care professions. In all interactions with patients, besides the technical expertise of a physician, the human element of caring (one human to another) is needed. In different situations, caring can be expressed verbally and non-verbally (e.g., the manner of communication with both physician and patient closely seated, and with unhurried, softly spoken words); a gentle touch especially when conveying “bad news”; a firmer touch or grip to convey reassurance to a patient facing a difficult treatment choice; to hold the hand of a patient dying alone). Thus, “caring” is in the center of the depicted integrated model, and as Peabody succinctly expressed it nearly a hundred years ago, “The secret of the care of the patient is caring for the patient” [ 41 ].
Conflict of Interest Statement
The author declares that he has no conflicts of interest.
- Bipolar Disorder
- Therapy Center
- When To See a Therapist
- Types of Therapy
- Best Online Therapy
- Best Couples Therapy
- Best Family Therapy
- Managing Stress
- Sleep and Dreaming
- Understanding Emotions
- Self-Improvement
- Healthy Relationships
- Student Resources
- Personality Types
- Guided Meditations
- Verywell Mind Insights
- 2024 Verywell Mind 25
- Mental Health in the Classroom
- Editorial Process
- Meet Our Review Board
- Crisis Support
What Is a Case Study?
Weighing the pros and cons of this method of research
Kendra Cherry, MS, is a psychosocial rehabilitation specialist, psychology educator, and author of the "Everything Psychology Book."
:max_bytes(150000):strip_icc():format(webp)/IMG_9791-89504ab694d54b66bbd72cb84ffb860e.jpg)
Cara Lustik is a fact-checker and copywriter.
:max_bytes(150000):strip_icc():format(webp)/Cara-Lustik-1000-77abe13cf6c14a34a58c2a0ffb7297da.jpg)
Verywell / Colleen Tighe
- Pros and Cons
What Types of Case Studies Are Out There?
Where do you find data for a case study, how do i write a psychology case study.
A case study is an in-depth study of one person, group, or event. In a case study, nearly every aspect of the subject's life and history is analyzed to seek patterns and causes of behavior. Case studies can be used in many different fields, including psychology, medicine, education, anthropology, political science, and social work.
The point of a case study is to learn as much as possible about an individual or group so that the information can be generalized to many others. Unfortunately, case studies tend to be highly subjective, and it is sometimes difficult to generalize results to a larger population.
While case studies focus on a single individual or group, they follow a format similar to other types of psychology writing. If you are writing a case study, we got you—here are some rules of APA format to reference.
At a Glance
A case study, or an in-depth study of a person, group, or event, can be a useful research tool when used wisely. In many cases, case studies are best used in situations where it would be difficult or impossible for you to conduct an experiment. They are helpful for looking at unique situations and allow researchers to gather a lot of˜ information about a specific individual or group of people. However, it's important to be cautious of any bias we draw from them as they are highly subjective.
What Are the Benefits and Limitations of Case Studies?
A case study can have its strengths and weaknesses. Researchers must consider these pros and cons before deciding if this type of study is appropriate for their needs.
One of the greatest advantages of a case study is that it allows researchers to investigate things that are often difficult or impossible to replicate in a lab. Some other benefits of a case study:
- Allows researchers to capture information on the 'how,' 'what,' and 'why,' of something that's implemented
- Gives researchers the chance to collect information on why one strategy might be chosen over another
- Permits researchers to develop hypotheses that can be explored in experimental research
On the other hand, a case study can have some drawbacks:
- It cannot necessarily be generalized to the larger population
- Cannot demonstrate cause and effect
- It may not be scientifically rigorous
- It can lead to bias
Researchers may choose to perform a case study if they want to explore a unique or recently discovered phenomenon. Through their insights, researchers develop additional ideas and study questions that might be explored in future studies.
It's important to remember that the insights from case studies cannot be used to determine cause-and-effect relationships between variables. However, case studies may be used to develop hypotheses that can then be addressed in experimental research.
Case Study Examples
There have been a number of notable case studies in the history of psychology. Much of Freud's work and theories were developed through individual case studies. Some great examples of case studies in psychology include:
- Anna O : Anna O. was a pseudonym of a woman named Bertha Pappenheim, a patient of a physician named Josef Breuer. While she was never a patient of Freud's, Freud and Breuer discussed her case extensively. The woman was experiencing symptoms of a condition that was then known as hysteria and found that talking about her problems helped relieve her symptoms. Her case played an important part in the development of talk therapy as an approach to mental health treatment.
- Phineas Gage : Phineas Gage was a railroad employee who experienced a terrible accident in which an explosion sent a metal rod through his skull, damaging important portions of his brain. Gage recovered from his accident but was left with serious changes in both personality and behavior.
- Genie : Genie was a young girl subjected to horrific abuse and isolation. The case study of Genie allowed researchers to study whether language learning was possible, even after missing critical periods for language development. Her case also served as an example of how scientific research may interfere with treatment and lead to further abuse of vulnerable individuals.
Such cases demonstrate how case research can be used to study things that researchers could not replicate in experimental settings. In Genie's case, her horrific abuse denied her the opportunity to learn a language at critical points in her development.
This is clearly not something researchers could ethically replicate, but conducting a case study on Genie allowed researchers to study phenomena that are otherwise impossible to reproduce.
There are a few different types of case studies that psychologists and other researchers might use:
- Collective case studies : These involve studying a group of individuals. Researchers might study a group of people in a certain setting or look at an entire community. For example, psychologists might explore how access to resources in a community has affected the collective mental well-being of those who live there.
- Descriptive case studies : These involve starting with a descriptive theory. The subjects are then observed, and the information gathered is compared to the pre-existing theory.
- Explanatory case studies : These are often used to do causal investigations. In other words, researchers are interested in looking at factors that may have caused certain things to occur.
- Exploratory case studies : These are sometimes used as a prelude to further, more in-depth research. This allows researchers to gather more information before developing their research questions and hypotheses .
- Instrumental case studies : These occur when the individual or group allows researchers to understand more than what is initially obvious to observers.
- Intrinsic case studies : This type of case study is when the researcher has a personal interest in the case. Jean Piaget's observations of his own children are good examples of how an intrinsic case study can contribute to the development of a psychological theory.
The three main case study types often used are intrinsic, instrumental, and collective. Intrinsic case studies are useful for learning about unique cases. Instrumental case studies help look at an individual to learn more about a broader issue. A collective case study can be useful for looking at several cases simultaneously.
The type of case study that psychology researchers use depends on the unique characteristics of the situation and the case itself.
There are a number of different sources and methods that researchers can use to gather information about an individual or group. Six major sources that have been identified by researchers are:
- Archival records : Census records, survey records, and name lists are examples of archival records.
- Direct observation : This strategy involves observing the subject, often in a natural setting . While an individual observer is sometimes used, it is more common to utilize a group of observers.
- Documents : Letters, newspaper articles, administrative records, etc., are the types of documents often used as sources.
- Interviews : Interviews are one of the most important methods for gathering information in case studies. An interview can involve structured survey questions or more open-ended questions.
- Participant observation : When the researcher serves as a participant in events and observes the actions and outcomes, it is called participant observation.
- Physical artifacts : Tools, objects, instruments, and other artifacts are often observed during a direct observation of the subject.
If you have been directed to write a case study for a psychology course, be sure to check with your instructor for any specific guidelines you need to follow. If you are writing your case study for a professional publication, check with the publisher for their specific guidelines for submitting a case study.
Here is a general outline of what should be included in a case study.
Section 1: A Case History
This section will have the following structure and content:
Background information : The first section of your paper will present your client's background. Include factors such as age, gender, work, health status, family mental health history, family and social relationships, drug and alcohol history, life difficulties, goals, and coping skills and weaknesses.
Description of the presenting problem : In the next section of your case study, you will describe the problem or symptoms that the client presented with.
Describe any physical, emotional, or sensory symptoms reported by the client. Thoughts, feelings, and perceptions related to the symptoms should also be noted. Any screening or diagnostic assessments that are used should also be described in detail and all scores reported.
Your diagnosis : Provide your diagnosis and give the appropriate Diagnostic and Statistical Manual code. Explain how you reached your diagnosis, how the client's symptoms fit the diagnostic criteria for the disorder(s), or any possible difficulties in reaching a diagnosis.
Section 2: Treatment Plan
This portion of the paper will address the chosen treatment for the condition. This might also include the theoretical basis for the chosen treatment or any other evidence that might exist to support why this approach was chosen.
- Cognitive behavioral approach : Explain how a cognitive behavioral therapist would approach treatment. Offer background information on cognitive behavioral therapy and describe the treatment sessions, client response, and outcome of this type of treatment. Make note of any difficulties or successes encountered by your client during treatment.
- Humanistic approach : Describe a humanistic approach that could be used to treat your client, such as client-centered therapy . Provide information on the type of treatment you chose, the client's reaction to the treatment, and the end result of this approach. Explain why the treatment was successful or unsuccessful.
- Psychoanalytic approach : Describe how a psychoanalytic therapist would view the client's problem. Provide some background on the psychoanalytic approach and cite relevant references. Explain how psychoanalytic therapy would be used to treat the client, how the client would respond to therapy, and the effectiveness of this treatment approach.
- Pharmacological approach : If treatment primarily involves the use of medications, explain which medications were used and why. Provide background on the effectiveness of these medications and how monotherapy may compare with an approach that combines medications with therapy or other treatments.
This section of a case study should also include information about the treatment goals, process, and outcomes.
When you are writing a case study, you should also include a section where you discuss the case study itself, including the strengths and limitiations of the study. You should note how the findings of your case study might support previous research.
In your discussion section, you should also describe some of the implications of your case study. What ideas or findings might require further exploration? How might researchers go about exploring some of these questions in additional studies?
Need More Tips?
Here are a few additional pointers to keep in mind when formatting your case study:
- Never refer to the subject of your case study as "the client." Instead, use their name or a pseudonym.
- Read examples of case studies to gain an idea about the style and format.
- Remember to use APA format when citing references .
Crowe S, Cresswell K, Robertson A, Huby G, Avery A, Sheikh A. The case study approach . BMC Med Res Methodol . 2011;11:100.
Crowe S, Cresswell K, Robertson A, Huby G, Avery A, Sheikh A. The case study approach . BMC Med Res Methodol . 2011 Jun 27;11:100. doi:10.1186/1471-2288-11-100
Gagnon, Yves-Chantal. The Case Study as Research Method: A Practical Handbook . Canada, Chicago Review Press Incorporated DBA Independent Pub Group, 2010.
Yin, Robert K. Case Study Research and Applications: Design and Methods . United States, SAGE Publications, 2017.
By Kendra Cherry, MSEd Kendra Cherry, MS, is a psychosocial rehabilitation specialist, psychology educator, and author of the "Everything Psychology Book."
- IMD Directory
- Industry Insights
- Workforce Development
- Business 4.0
- Industrial Fabrication & Automation
- Manufacturing Showcase
- Safety & Maintenance
- Case Studies
- IMD Industry Insights Podcast
- Industry Updates
- Product News
- Talking Shop
- White Papers
- IMD Monthly Issues
- IMD Quarterly Issues
- Surplus Buying and Selling
- IMD Auctions
- Total Industrial Plant Solutions
- Login or Register
- Advertise Now
- eNewsletter Subscription

Exploring the three pillars of modern manufacturing
Plasma metal cutting: what fabricators need to know, u.s. manufacturing surge: growth strategies amid workforce challenges, an uncommon perspective on industrial design-build, empowering american manufacturing, unlocking customer experience: the critical role of your supply, gearing up for industry 5.0 with new welding solutions, design advantages for profitable and sustainable cooling lubricant ultra-fine filtration, what is ethical manufacturing and why is it so important.
Businesses have a big impact on their community, whether that involves how they care for the environment or how they contribute to the lives of their staff. This is particularly true for manufacturers. Their presence is vital in supporting communities and providing essential products for consumers and other businesses, but their environmental impact can be large. The challenge for manufacturers is how to achieve an ethical status while improving their business processes. From eliminating waste to developing staff, ethical manufacturing has many forms. Here, we explore the topic of ethical manufacturing, understanding what it means, why it’s important, and how a business can achieve a positive impact in the world.
What Does Ethical Manufacturing Mean?

An ethical manufacturer has oversight and cares about each section of their business and their own supply chain, prioritising the well-being of both customers and staff, as well as the environment in which they work, shop, and source materials.
Ethical businesses want to operate in the best interest of workers. The health and happiness of staff become priorities, going beyond the standard legal requirements. This means that safety is not sacrificed, and workers are treated fairly. In turn, this can benefit a business through a boost in productivity and staff retention.
Ethical production may include material and energy use. Is renewable energy used as part of the manufacturing process? Are materials recycled or are products designed to minimize the amount of waste produced in manufacturing?
This is key for many manufacturing businesses that have waste management issues, such as fashion and clothing manufacturers. An estimated 92 million tons of textile waste is created annually from the fashion industry. Ethical clothing manufacturers must ensure that their processes decrease or limit their waste through the design of their garments or by reusing materials. This process can be extended and translated across other manufacturing industries.
Why Is Ethical Manufacturing Important for Businesses?
Ethical production is important for staff, customers, and environmental well-being. But it can also have significant benefits for businesses. By utilizing sustainable processes and materials, manufacturers are ensuring that their future is secure. For example, some toilet paper companies will plant trees to replace the ones used for their production. This action is circular, as it means that their impact on the environment is limited and that they have future materials to continue their manufacturing.
Also, ethical businesses that provide a positive work environment are likely to improve productivity. Ethical businesses can continue the development of staff and their skills, improving their work. Oxford University found that happy workers are 13 percent more productive than those who are not. Training staff could also help retention, as 93 percent of employees said they would stay at a company longer if it invested in their career. Ethical manufacturing businesses do not just have to rely on material sustainability – the ethical impact can extend to their workers as well.
Sustainability and ethical manufacturing are also key to attracting customers. 92 per cent of millennials said they were more likely to purchase from an ethical company, displaying a need to know that their corporate mission is genuine and from a well-founded place. From a business perspective, ethical manufacturing has clear financial benefits. Aligning your production, staff development, and customer outreach within a framework of ethical manufacturing can lead to improved quality, more productivity, and increased revenue.
Becoming an Ethical Business

This is achieved at many layers within a business. Sustainably sourced materials may include recycled goods or items sourced from another local manufacturer. This reduces waste and limits transport needs, which can have a negative environmental impact.
In the workplace, training, development, and a prioritization of safety can help staff feel happier. Investment in staff is key to creating a positive working culture. This can be achieved through understanding the ambitions of workers and finding ways to help encourage their development through educational courses. Using ethical workwear suppliers to encourage an inclusive work culture will also contribute to your standing. Suitable workwear clothing can also improve safety during the manufacturing process. Combined, training and positive culture can help to boost productivity and your status as an ethical business.
Finally, you should display your status as an ethical manufacturer proudly. Remind customers that your business is acting with the best intentions and processes. By doing this, your customers will use and promote your brand so that your investment in the environment and community provides positive returns.
https://www.fashionrevolution.org/waste-is-it-really-in-fashion/ https://learning.linkedin.com/resources/workplace-learning-report-2018 https://www.entrepreneur.com/article/341699
RELATED ARTICLES MORE FROM AUTHOR
Siemens controls help tire cord manufacturer improve performance of wire draw system, those who adapt the fastest win, recent articles, rego-fix powers ed carpenter racing with precision toolholding, combilift launches national forklift safety campaign: “lift your standards by lowering..., toyota material handling adds to its north american headquarters with nearly..., pps cf: the newest factor 4 material for industrial 3d printing, heule features innovative back spot facing tools in imts booth #431364, ipg introduces ima brand e-co flex sealmatic fully automatic random wat..., research finds 90% of manufacturers are utilizing ai but many still..., mazak celebrates 50 years of kentucky manufacturing excellence, global manufacturing industry recovery in 2025 to follow sluggish 2024, walter unveils the xtra∙tec® xt m5250 helical milling cutter with bc....
- About Industrial Machinery Digest
- Privacy Policy
- Subscribe Now – Print
- Subscribe Now – Digital
- Open access
- Published: 31 May 2024
Public involvement and engagement in scientific research and higher education: the only way is ethics?
- Claire Nollett 1 ,
- Matthias Eberl 2 , 3 ,
- Jim Fitzgibbon 4 ,
- Natalie Joseph-Williams 5 , 6 &
- Sarah Hatch 7
Research Involvement and Engagement volume 10 , Article number: 50 ( 2024 ) Cite this article
111 Accesses
26 Altmetric
Metrics details
Involving and engaging the public in scientific research and higher education is slowly becoming the norm for academic institutions in the United Kingdom and elsewhere. Driven by a wide range of stakeholders including regulators, funders, research policymakers and charities public involvement and public engagement are increasingly seen as essential in delivering open and transparent activity that is relevant and positively impacts on our society. It is obvious that any activities involving and engaging members of the public should be conducted safely and ethically. However, it is not clear whether conducting activities ethically means they require ethical approval from a research ethics committee.
Although there is some guidance available from government organisations (e.g. the UK Health Research Authority) to suggest ethical approval is not required for such activities, requests from funders and publishers to have ethical approval in place is commonplace in the authors’ experience. We explore this using case studies from our own institution.
We conclude that any public-facing activity with the purpose to systemically investigate knowledge, attitudes and experiences of members of the public as research and as human participants requires prior approval from an ethics committee. In contrast, engaging and involving members of the public and drawing on lived experience to inform aspects of research and teaching does not. However, lack of clarity around this distinction often results in the academic community seeking ethical approval ‘just in case’, leading to wasted time and resources and erecting unnecessary barriers for public involvement and public engagement. Instead, ethical issues and risks should be appropriately considered and mitigated by the relevant staff within their professional roles, be it academic or a professional service. Often this can involve following published guidelines and conducting an activity risk assessment, or similar. Moving forward, it is critical that academic funders and publishers acknowledge the distinction and agree on an accepted approach to avoid further exacerbating the problem.
Plain English summary
Involving and engaging members of the public is recognised best practice in university research and teaching. Involvement and engagement activities (for instance, working with the public to design a research study) continue to increase in priority and are an important part of an academic’s role. However, there is often confusion amongst researchers and educators around whether involving the public in these activities requires prior ethical approval, similar to what would be the case when inviting members of the public to participate in a clinical research study, or to donate samples such as blood for experiments. As an example, sometimes researchers are asked for ethical approval by scientific journals when trying to publish the findings from their public involvement and engagement work, when in fact this is not needed. The ongoing uncertainty about the difference between actual research on one hand and public involvement and engagement on the other hand wastes precious time and resources, and is a barrier for scientists to working with the public. We have developed guidance for academic staff on when ethical approval is and is not required, using examples from our own experience. We wrote this article to bring awareness to this problem; share our views with the wider academic community; encourage discussion around the problem and possible solutions; and ultimately contribute to educating on when research ethics approval is needed, and when not.
Peer Review reports
Public involvement (PI) is ‘important, expected and possible in all types of health and social care research’ [ 1 ]. It is now commonly embedded and reported in health research papers in the UK, with approximately half mentioning public involvement activities [ 2 ]. Public engagement (PE) is also encouraged and recognised by funders and other stakeholders across the higher education sector to raise awareness, increase trust and transparency, share knowledge, foster learning and deliver positive impact to society [ 3 ].
In 2019, the UK Standards Partnership published the UK Standards for Public Involvement ‘to help researchers and organisations improve the quality and consistency of public involvement in health and care research’ [ 4 ], and a large knowledge base is developing around how to do public involvement well. However, PI is not without its challenges, as identified both in the literature e.g [ 5 ]. and through our own experience as academic researchers, professional services staff and members of several national public involvement committees. Key issues include how to efficiently pay and reimburse public contributors within organisations, how to effectively evaluate the impact, and how to provide inclusive opportunities and reach under-served groups to increase the diversity of those involved [ 6 ].
The Research Excellence Framework (REF) 2029, the UK’s national assessment of the quality of research produced by its higher education institutions held every 6–7 years, will see a 25% weighting of returns with respect to the social, economic and political influence of the research conducted. The 2029 round will in fact be the first REF assessment where impact will be measured as “ Engagement and Impact” (our emphasis), alongside an accompanying statement to evidence engagement and impact activity beyond case studies [ 7 ]. As with PI, researchers face challenges in delivering PE including achieving the inclusion of under-served communities [ 8 ] and how to evaluate impact [ 3 ].
With individual researchers and their host institutions increasingly embracing PI and PE as part of their research and scholarship activities, there is one issue that we have found particularly contentious with researchers, employers, funders and publishers across both involvement and engagement and that is the focus of this commentary: the role of ethical approval in PI and PE activity.
Public involvement, sometimes referred to as Patient & Public Involvement (PPI) in health and social care research, is defined as ‘research being carried out ‘with’ or ‘by’ members of the public, rather than ‘to’, ‘about’ or ‘for’ them’ [ 9 ]. PE, adopting the UK’s National Coordinating Centre for Public Engagement’s definition, is a ‘myriad of ways in which the activity and benefits of higher education and research can be shared with the public’ [ 10 ]. PE is by definition a two-way process, involving interaction and listening, with the goal of generating mutual benefit. Both PI and PE are distinct from human participation in research whereby a member of the public agrees via informed consent to be a participant in research, e.g. receiving a study intervention, donating samples or sharing lived experiences. Whilst health and social care research involving human participants requires approval from a research ethics committee (REC), PI and PE activities typically do not.
In the UK, ethical approval is granted by a REC under the auspices of the National Health Service (NHS) for research on patients or healthcare professionals, or a local review committee or panel for research that does not include NHS patients. In academic research, this would usually be a university or school REC (referred to here as an Institutional Review Board, IRB). Other countries may use different approaches but the general need for RECs to approve research with human participants is ubiquitous. With regard to public involvement, the UK Health Research Authority (HRA) that is responsible for all NHS RECs explicitly states that ‘You do not need to submit an application to a Research Ethics Committee in order to involve the public in the planning or the design stage of research, even if the people involved are patients’ [ 11 ]. This advice would also apply to university ethics committees. However, despite this clear distinction, we have encountered and become aware of situations in which investigators were asked to acquire ethical approval for activities with the public – including PI, PE and impact activities. This highlights a potential misunderstanding of the nature of PI and PE, and their role alongside research. Whilst either activity can raise ethical considerations for the individuals involved, the requests to acquire research ethics approval for PI and PE need to be challenged within the academic community to increase awareness, understanding of and best practice around these activities. Seeking unnecessary approval adds a heavy additional burden on researchers which effectively acts as a barrier to carrying out PI and PE; can significantly delay timely activities; and uses valuable resources.
We propose that the requests to gain ethical approval for PI and PE activities stem largely from three main issues.
Firstly, ‘grey’ areas, such as a blurring of the boundary between qualitative research and PI and PE activities, including confusion amongst the research community over the differences between research involvement, engagement and participation.
Secondly, a perception amongst the research community that it is best to seek ethical approval ‘just in case’ or to ‘be on the safe side’, e.g. if asked by journal editors when trying to publish, rather than complete appropriate risk assessments to address any ethical considerations when carrying out PI and PE.
And finally, lack of knowledge of an alternative recognised process on how to evidence that PI and PE activities with the public have been conducted in an ethical manner, if not approved by an NHS REC or local IRB.
Despite guidance indicating other ways to address ethical concerns in PI and PE [ 12 , 13 , 14 , 15 , 16 , 17 , 18 ], researchers, funders and publishers appear to be turning increasingly to university IRBs as the (perceived) ultimate arbiters of deciding ethical issues related to PI and PE activities. We see the need to highlight this as a growing problem and suggest ways the issues above can be overcome. We will firstly explore in more detail the distinction between qualitative research and PI and PE activities before outlining examples from our own experience around the three issues identified, and then proceeding to make recommendations for moving forwards.
Public involvement and engagement vs. qualitative research
Distinguishing between whether activities with members of the public constitute PI and PE or qualitative research (and therefore require ethical approval) is a particularly ‘grey’ area [ 19 ]. This is especially true when consulting with a number of people at one time in what is usually referred to as a ‘focus group’. Going forward, it may be helpful to distinguish between ‘focus groups’, which are used for research, and ‘discussion groups’ used for PI and PE [ 20 ].
Several authors and organisations have described the difference between the two activities and developed useful side-by-side comparisons [ 19 , 20 ]. In focus groups which are part of research, people attending are research participants who receive a standard Participant Information Sheet and provide informed consent. Their input will usually be recorded via an audio device, transcribed verbatim, treated as ‘data’, and systematically be analysed to answer a research question. For this, ethical approval is usually required. On the other hand, the contributions of people attending PI discussion groups will be recorded only as key points (e.g. a list of key themes emerging or key priorities discussed by the group in relation to a specific topic) to help shape and guide the research itself, such as agreeing which research outcome measures to use, helping to shape the intervention or the development of data collection materials like participant information sheets or interview guides. PI discussion groups do not require ethical approval but should be conducted in an ethical manner. Those involved should still be provided with information about the activity up front to ensure they are clear what their involvement will entail, and they may be asked to provide agreement or consent, but not in the formally documented way required for research. This is discussed in more detail in the recommendations.
Another grey area concerns whether direct quotes gathered from people in a discussion group can be used in a publication. Whilst ethical approval is not required for this, we do advise gaining documented agreement if you wish to do this, e.g. an email from the group member agreeing to quotes being used in a publication to illustrate the key points identified (not as data). In some cases, researchers will need to combine PI activities with a qualitative research approach and there may be confusion regarding which activities require approval. For example, an investigator may wish to interview new mothers as research participants to get their views on motherhood (research participation). This would require ethical approval. But prior to interviews, they may want to involve a separate group of new mothers in a discussion to help shape the topic guide for the interviews (PI). This would not need ethical approval [ 21 ].
The extent of the problem - examples from our own experience
Through requesting examples from colleagues on their experiences, we uncovered many different situations within our own institution highlighting a difference of opinion on whether research ethics should be sought for PI and PE activity. We here outline three examples, giving the background to the project, the activity undertaken and the issues encountered.
Writing a training program with charity service users and staff – request from charity and publication to seek ethical approval from the university IRB for the project .
This project involved service users and charity staff in writing a mental health training curriculum for staff to identify depression in service users. Staff and service user input was sought through online meetings and email feedback. The attendees gave their opinions (based on their lived experience) on what should be included in the curriculum, and the key points were summarised to inform curriculum development. The information they gave was not treated as data to answer a research question and was not systematically analysed using qualitative methods. In this respect, HRA state that ’if you are collecting opinions rather than study data, your activity is likely an involvement activity’ [ 22 ].
Regardless of the above considerations, the project lead was asked by third sector organisations to seek university IRB approval, to ensure the service users would be treated in an ethical manner. An academic colleague agreed this was a good idea ‘just in case’ it was questioned by others, in particular by a journal editor when seeking to publish (which indeed it was). However, we view this as unnecessary given the activity was not classed as research and therefore not in the remit of the IRB. The IRB provided written agreement that ethical review was not required for this project and the project team agreed a standard engagement risk assessment would consider and address any ethical issues.
Co-producing an educational online resource for school children – request from publication to seek ethical approval for the project .
This co-production project working with researchers, a PI and PE professional, school teachers and web designers aimed to develop an educational online resource for school age children and their teachers. This interdisciplinary team of experts were involved in four online workshops to support the delivery and development of a website that would support teachers and enhance learning. All individuals involved fully signed up to the coproduction focus of the project and provided verbal agreement to take part in the workshops and off-line discussions. However, when trying to publish the co-production process, the journal editor stressed that according to journal policy ‘research involving human subjects, human material or human data must have been approved by an appropriate ethics committee’.
The authors explained that the project did not involve human subjects, human material or human data (as it was not research) and therefore in their opinion did not require ethical approval. The journal editor disagreed, arguing that the project was a research study that collected and analysed data, and that the teachers and web designers involved in this project were human participants of the study and data had been generated of their opinions. The editor recommended seeking either retrospective ethical approval or else removing all human data. The team saw no alternative but to withdraw their original manuscript and submit the work elsewhere.
Co-production project involving people from minority ethnic backgrounds in discussion about inclusive health research – project investigators not comfortable including quotes from public contributors due to lack of informed consent.
This project involving researchers, an artist, charity project workers serving the most ethnically diverse ward in Wales and local residents aimed to answer the question: ‘How can people from minority ethnic backgrounds influence health research in terms of both what and how this research is done?’ Eight co-production workshops drawing on the participatory democracy approach were held and delivered a set of recommendations for the health research community. In advance of these workshops, a university IRB Chair helped to clarify that ethical approval was not needed.
When publishing this work, researchers did not include quotes obtained from the workshops as informed consent had not been sought (as it was not research) [ 23 ]. On reflection, the authors would like to have gained agreement for the residents’ quotes to be used, in the absence of the requirement for documented informed consent.
Identified exceptions
Whilst PI and PE activities do not generally require ethical approval, there are at least two example scenarios where approval is required. Firstly, for example, when systematically comparing two methods of involvement and/or engagement to understand which is better i.e. answering a research question about PI/PE to produce generalisable or transferable findings. Secondly, when public members come into direct contact with study participants or their data e.g. if assisting with conducting research interviews or analysing the transcripts. In this situation, ethical approval is required because human participants are involved in the research.
Recommendations for moving forwards
We encourage the research community, including researchers, publishers, reviewers, funders and ethics committees to better appreciate the difference between PI and PE and research involving human participants; to recognise that all involved stakeholders operate within professional boundaries; and to work together to agree an alternative accepted approach when the PI and PE activity raises ethical considerations (e.g. when working with vulnerable groups or publishing of public contributor quotes). The responsibility of determining whether research ethic approval is required falls on the individuals/team planning the activity. We understand that it is tempting to seek research ethical approval for PI and PE activity ‘just in case’ or ‘to be on the safe side’, but we do believe this is detrimental for several reasons including:
Sustains the confusion between qualitative research and PI and PE activity, and the different purposes of each.
Wastes valuable researcher and committee time and resources.
Undermines the importance of the research ethics approval process.
Delays PI and PE activities in the research process, potentially leading to missing out on the benefits of earlier involvement.
Undermines coproduction principles such as equality and shared responsibility between researchers and members of the public. The process of acquiring ethical approval itself asserts a hierarchy whereby a researcher is identified as Chief/Principal investigator, and other members of the team are listed below an identified leader.
Acts as an additional barrier and disincentive to researchers carrying out PI and PE activity.
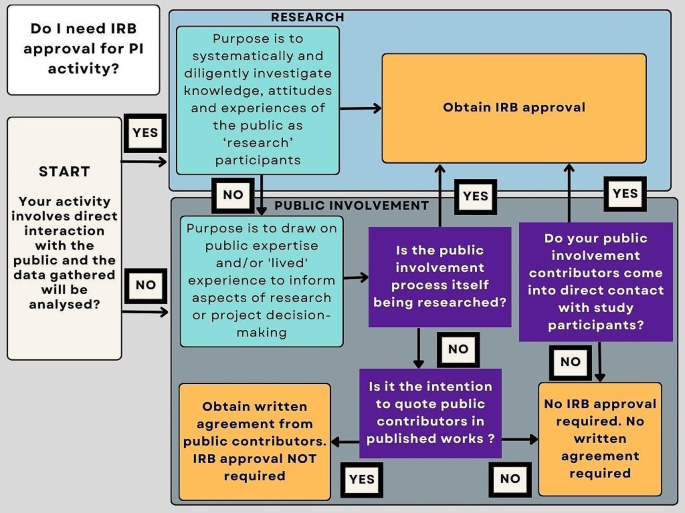
Simple flow diagram to support researchers to decide on the need for research ethical approval via an IRB
There is a need to address this growing problem, via education and generating solutions acceptable to the community as a whole, providing confidence in decisions made and assurances that the health and safety and any risks associated with the proposed PI and PE activity have been carefully considered and approved. Here we present key recommendations for those conducting public involvement and engagement activities based on our internal guidance (Appendix 1) for alternative courses of action moving forwards when faced with these challenges.
Purpose - Consider the purpose of the activity. Is it to answer a scientific or clinical question (research) or help shape, guide or disseminate the research (PI/PE)? If you are unsure if your project is research, you can consult the UK Health Research Authority’s ‘Is my study research’ decision tool. Following response to three questions, (1. Are the participants in your study randomised to different groups? 2. Does your study protocol demand changing treatment/care/services from accepted standards for any of the patients/service users involved? 3. Is your study designed to produce generalisable or transferable findings? ) The tool confirms if your study would be considered as research. This result can be downloaded and further advice can be sought [ 24 ]. The HRA table ‘Defining Research’ can also help provide clarification [ 25 ].
Internally, a simple flow diagram (Fig. 1 ) has been created to support researchers in making a decision on the need for research ethics approval when carrying out public involvement activity.
Risk assessment – To ensure PI and PE activities are conducted in a safe and ethical manner, particularly when engaging and/or involving ‘vulnerable’ groups, refer to published guidance on conducting ethical PI&E [ 12 , 13 , 14 , 15 , 16 ], consider completing a specifically designed PI and PE risk assessment (See Appendix 2 for an example) or using the PIRIT tool [ 26 ]to assess your planned activities and undertake adequate training (See Appendix 2 for an example). Use the same considerations as you might for research or teaching e.g. what to do if an individual becomes upset in a discussion group, how to support them, where to refer them. Also consider safety, protection of anonymity and confidentiality of personal data. Use the UK Standards on Public Involvement [ 4 ] to guide your thinking around accessibility and inclusivity when completing the assessment. If possible, involve a public contributor and have this signed off by a senior academic/responsible member of staff in your organisation.
Adequate information and agreement to take part – Ensure that public members being invited to take part in PI and PE activity agree for you to use their anonymous quotes in any output. But understand that standard Participant Information Sheets and Informed Consent Forms are not required as formal consent is not required.
Language – To avoid confusion for reviewers and publishers, think carefully about the language you use to describe your PI and PE activities. For example, use the term ‘discussion group’ rather than ‘focus group’; refer to members of the public as ‘attendees’ not ‘participants’ and input as ‘contributions’ rather than ‘data’; and ‘summarising key points or themes’ as opposed to ‘thematic analysis’ when describing your activities (if that is indeed what you are doing).
Written confirmation – Some institutions have established infrastructure to support researchers through a self-assessment process for governance and ethics, providing a confirmatory statement as to whether ethical approval is required if challenged by funders and publishers [ 27 ]. However, not all institutions have this facility and until this area of contention is resolved, some individuals may wish to seek written confirmation from their local IRB. In our experience, a letter confirming approval is not required is acceptable by journal editors. Liaise with your local IRB to determine if this is within their remit.
Training – The development and inclusion of training for researchers and support staff is required on when to seek ethical approval and how to effectively manage ethical, risks, and health and safety aspects of PI and PE in a considered, widely accepted and non-burdensome way.
Conclusions
Our experience suggests that ambiguity remains in the academic community about whether ethical approval is needed for PI and PE activities. We believe this stems from (1) the grey area between qualitative research and PI and PE activities; (2) seeking approval ‘just in case’ they are requested by funders, publishers or authorities (based on previous experience) (3) funders, publishers and authorities not being clear in the distinction and equally asking for approval ‘just in case’ and (4) a lack of an alternative recognised way to evidence that ethical issues have been considered and mitigated against. We have used real world examples to demonstrate the issues encountered in a single institution and make several recommendations aimed at researchers for addressing this area of contention going forward. We appreciate that our views may be framed by our experience of conducting PI&E in a healthcare context and in the UK, and the experiences of researchers in other disciplines and countries may vary significantly.
We hope this commentary triggers debate in the community to highlight, educate and clarify the position surrounding research ethics and PI and PE activity amongst researchers, funders and journal editors. Our experience shows that this issue is effectively acting as a barrier to researchers conducting PI and PE activity and publishing PI and PE learning. An alternative recognised process needs to be established by the community to resolve this growing detrimental development.
Data availability
Not applicable.
Abbreviations
Health Research Authority
Institutional review board
- Public engagement
- Public involvement
Research Ethics Committee
Research Excellence Framework
Health Research Authority. Putting people first - embedding public involvement in health and social care research 2023 [ https://www.hra.nhs.uk/planning-and-improving-research/best-practice/public-involvement/putting-people-first-embedding-public-involvement-health-and-social-care-research/
Lang I, King A, Jenkins G, Boddy K, Khan Z, Liabo K. How common is patient and public involvement (PPI)? Cross-sectional analysis of frequency of PPI reporting in health research papers and associations with methods, funding sources and other factors. BMJ Open. 2022;12:e063356.
Article PubMed PubMed Central Google Scholar
Eberl M, Joseph-Williams N, Nollett C, Fitzgibbon J, Hatch S. Overcoming the disconnect between scientific research and the public. Immunol Cell Biol. 2023;101(7):590.
Article PubMed Google Scholar
UK Public Involvement Standards Development Partnership. UK Standards for Public Involvement 2019 [ https://drive.google.com/file/d/1U-IJNJCfFepaAOruEhzz1TdLvAcHTt2Q/view
Staniszewska S, Denegri S, Matthews R, Minogue V. Reviewing progress in public involvement in NIHR research: developing and implementing a new vision for the future. BMJ Open. 2018;8(7):e017124.
Hatch S, Fitzgibbon J, Tonks AJ, Forty L. Diversity in patient and public involvement in healthcare research and education—realising the potential. Health Expect. 2023;27(1):e13896.
Research Excellence Framework. Research Excellence Framework 2029 2024 [ https://www.ref.ac.uk/
Nguyen Thanh H, Cheah P, Chambers M. Identifying ‘hard-to-reach’ groups and strategies to engage them in biomedical research: perspectives from engagement practitioners in Southeast Asia. Wellcome Open Res. 2019;4(102).
National Institute for Health and Care Research. Briefing notes for researchers-public involvement in NHS, health and social care research. 2021.
National Co-ordinating Centre for Public Engagement. Introducing Public Engagament 2024 [ https://www.publicengagement.ac.uk/introducing-public-engagement
Health Research Authority. What do I need to do? 2020 [ https://www.hra.nhs.uk/planning-and-improving-research/best-practice/public-involvement/what-do-i-need-do/
Pandya-Wood R, Barron D, Elliott J. A framework for public involvement at the design stage of NHS health and social care research: time to develop ethically conscious standards. Res Involv Engagem. 2017;3(3).
Canadian Institutes of Health Research. Ethics Guidance for Developing Partnerships with Patients and Researchers 2020 [ https://cihr-irsc.gc.ca/e/51910.html#2
Hersh D, Israel M. C. S. The ethics of patient and public involvement across the research process: towards partnership with people with aphasia. Aphaisology. 2021.
Abma T, Groot B, Widdershoven B. The Ethics of Public and Service User Involvement in Health Research: the need for participatory reflection on everyday ethical issues. Am J Bioeth. 2019;19:23–5.
Groot B, Abma T. Ethics framework for citizen science and public and patient participation in research. BMC Med Ethics. 2022;23.
National Institute for Health and Care Research. Ethical dimensions of community engagement and involvement in global health research 2021 [ https://www.nihr.ac.uk/documents/ethical-dimensions-of-community-engagement-and-involvement-in-global-health-research/28258#references
Martineau JT, Minyaoui A, Boivin A. Partnering with patients in healthcare research: a scoping review of ethical issues, challenges, and recommendations for practice. BMC Med Ethics. 2020;21.
Hanley B, Staley K, Stewart D, Barber R. Qualitative research and patient and public involvement in health and social care research: What are the key differences? 2019 [ https://www.learningforinvolvement.org.uk/content/resource/qualitative-research-and-patient-and-public-involvement-in-health-and-social-care-research-what-are-the-key-differences/
Doria N, Condran B, Curtis Maillet LB, Dowling D, Levy L. A. Sharpening the focus: differentiating between focus groups for patient engagement vs. Qualitative Res Res Involv Engagem. 2018;4.
Morgan H, Thomson G, Crossland N, Dykes F, Hoddinott P, ‘BIBS’ study team. Combining PPI with qualitative research to engage ‘harder-to-reach’ populations: service user groups as co-applicants on a platform study for a trial. Res Involv Engagem. 2016;24(7).
Health Research Authority. Accessing study support and advice services 2024 [ https://www.hra.nhs.uk/planning-and-improving-research/research-planning/access-study-support-advice-services/
Bridges S, Lamont-Robinson C, Herbert A, Din M, Smith C, Ahmed N et al. Talking trials: an arts-based exploration of attitudes to clinical trials amongst minority ethnic members of the South Riverside Community of Cardiff Health expectations. 2023(26):3.
Health Research Authority. Is my study research? 2024 [ https://www.hra-decisiontools.org.uk/research/
Health Research Authority. Defining Research Table 2022 [ https://www.hra-decisiontools.org.uk/research/docs/DefiningResearchTable_Oct2022.pdf
Marie Curie Research Centre. Public Involvement in Research Impact Toolkit (PIRIT) [ https://www.cardiff.ac.uk/marie-curie-research-centre/patient-and-public-involvement/public-involvement-in-research-impact-toolkit-pirit
University of Surrey. Ethics Guide 2022 [ https://issuu.com/universityofsurrey/docs/surrey_ethics_cop_v20_single?fr=sNDM5NzUwNzU0OTI
Download references
Acknowledgements
We are grateful to our colleagues Martina Svobodoba, Sarah Bridges and Dr Vicky Shepherd for providing useful insights and resources from their experiences and to Dr Emma Yhnell for her helpful review and comment on the first draft.
Author information
Authors and affiliations.
Centre for Trials Research, Cardiff University, 7th Floor, Neuadd Meirionnydd, Heath Park Campus, Cardiff, UK
Claire Nollett
Division of Infection and Immunity, School of Medicine, Cardiff University, Cardiff, UK
Matthias Eberl
Systems Immunity Research Institute, Cardiff University, Cardiff, UK
School of Medicine, Lead Public Contributor, Cardiff University, Cardiff, UK
Jim Fitzgibbon
Division of Population Medicine, School of Medicine, Cardiff University, Cardiff, UK
Natalie Joseph-Williams
Health and Care Research Wales Evidence Centre, Cardiff, UK
Public Involvement and Engagement Team, School of Medicine, Cardiff University, Cardiff, UK
Sarah Hatch
You can also search for this author in PubMed Google Scholar
Contributions
CN and SH drafted the first version; CN, SH, ME and NJW added case studies; ME, NJW and JF contributed to revised versions and all authors read and approved the final manuscript.
Corresponding authors
Correspondence to Claire Nollett or Sarah Hatch .
Ethics declarations
Ethics approval and consent to participate.
Not applicable – no participants involved.
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Authors information
CN is Academic Lead for Public Involvement and Engagement in the Centre for Trials Research, Cardiff University. SH is the Public Involvement and Engagement Manager for the School of Medicine, Cardiff University, alongside researchers ME and NJW who are the Joint Academic Leads for Public Involvement and Engagement in the School of Medicine, Cardiff University. ME is also the Engagement Lead for the Systems Immunity Research Institute at Cardiff University and the Engagement Secretary for the British Society for Immunology. JF was the Lead Public Contributor in the School of Medicine at the time of writing.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Supplementary material 2, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Nollett, C., Eberl, M., Fitzgibbon, J. et al. Public involvement and engagement in scientific research and higher education: the only way is ethics?. Res Involv Engagem 10 , 50 (2024). https://doi.org/10.1186/s40900-024-00587-x
Download citation
Received : 15 February 2024
Accepted : 24 May 2024
Published : 31 May 2024
DOI : https://doi.org/10.1186/s40900-024-00587-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Research ethics committee
- Ethical approval
Research Involvement and Engagement
ISSN: 2056-7529
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
By 'ethical evidence' we mean results and findings that have been generated by research and other activities during which the standards of research ethics and integrity have been upheld ... Ethics case studies allow such reflection to facilitate the development of ethical decision-making skills. This volume has major interests in ethics and ...
Case studies play a significant role in knowledge development across various disciplines. Analysis of cases provides an avenue for researchers to explore phenomena within their context based on the collected data. Analysis of qualitative data from case study research can contribute to knowledge development.
Case Studies. More than 70 cases pair ethics concepts with real world situations. From journalism, performing arts, and scientific research to sports, law, and business, these case studies explore current and historic ethical dilemmas, their motivating biases, and their consequences. Each case includes discussion questions, related videos, and ...
Ethical considerations in research are a set of principles that guide your research designs and practices. Scientists and researchers must always adhere to a certain code of conduct when collecting data from people. The goals of human research often include understanding real-life phenomena, studying effective treatments, investigating ...
The term ethics may refer to the philosophical study of the concepts of moral right and wrong and moral good and bad, to any philosophical theory of what is morally right and wrong or morally good and bad, and to any system or code of moral rules, principles, or values. The last may be associated with particular religions, cultures, professions, or virtually any other group that is at least ...
An essay exploring how to effectively use case studies to teach research ethics. It is widely believed that discussing case studies is the most effective method of teaching the responsible conduct of research (Kovac 1996; Macrina and Munro 1995), probably because discussing case studies is an effective way to get students involved in the issues.
Revised on November 20, 2023. A case study is a detailed study of a specific subject, such as a person, group, place, event, organization, or phenomenon. Case studies are commonly used in social, educational, clinical, and business research. A case study research design usually involves qualitative methods, but quantitative methods are ...
Challenge yourself (and/or your team at work) to develop strategies to avoid these ethical pitfalls. Watch the case study's "Related Videos" and "Related Terms" for further understanding. To use the case studies in the classroom, ask students to read a video's "Case Study" and answer the case study "Discussion Questions."
Secondly, ethics refers to the study and development of one's ethical standards. As mentioned above, feelings, laws, and social norms can deviate from what is ethical. So it is necessary to constantly examine one's standards to ensure that they are reasonable and well-founded. Ethics also means, then, the continuous effort of studying our own ...
Ethical Considerations. Ethical considerations in research refer to the principles and guidelines that researchers must follow to ensure that their studies are conducted in an ethical and responsible manner. These considerations are designed to protect the rights, safety, and well-being of research participants, as well as the integrity and credibility of the research itself
casuistry, in ethics, a case-based method of reasoning. It is particularly employed in field-specific branches of professional ethics such as business ethics and bioethics. Casuistry typically uses general principles in reasoning analogically from clear-cut cases, called paradigms, to vexing cases. Similar cases are treated similarly.
The research team. There are examples of researchers being intimidated because of the line of research they are in. The institution in which the research is conducted. salso suggest there are 4 main ethical concerns when conducting SSR: The research question or hypothesis. The treatment of individual participants.
Ethics is a broad term that covers the study of the nature of morals and the specific moral choices to be made. ... Case 6 was added during the revision phase of this article as it coincided with the outbreak of Coronavirus Infectious Disease-2019 (COVID-19) that became a pandemic rendering a discussion of its ethical challenges necessary and ...
Justice means giving each person what he or she deserves or, in more traditional terms, giving each person his or her due. Justice and fairness are closely related terms that are often today used interchangeably. There have, however, also been more distinct understandings of the two terms.
The Markkula Center for Applied Ethics brings these traditions of ethical thinking to bear on real world problems. It has programs focusing on ethics in health care, business, technology, journalism, government, leadership, and other areas. As part of its Social Sector Ethics program, the Center has launched Standards for Excellence®, an ...
A case study is an in-depth study of one person, group, or event. In a case study, nearly every aspect of the subject's life and history is analyzed to seek patterns and causes of behavior. Case studies can be used in many different fields, including psychology, medicine, education, anthropology, political science, and social work.
The issue of ethics in a case study research is also gaining currency of late, and now constitutes an important component of the training of case study researchers. Yin (2014, p. 95) says that ethics in case study can be buttressed in the field by conducting the study with extra care and sensitivity towards the participants by adopting the ...
At its simplest, ethics is a system of moral principles. They affect how people make decisions and lead their lives. Ethics is concerned with what is good for individuals and society and is also ...
Ethics are at the heart of professionalism. To create cultures of transparency and trust, practitioners should demonstrate strong standards of integrity when advising business leaders. This factsheet explores what ethical practice means and why it matters in an organisational context. It outlines the trade-offs involved in upholding ethical ...
Case Study 1. Fred and Tamara, a married couple in their 30's, are applying for a business loan to help them realize their long-held dream of owning and operating their own restaurant. Fred is a highly promising graduate of a prestigious culinary school, and Tamara is an accomplished accountant.
Ethical manufacturing is a holistic approach to the manufacturing process that focuses on good health for all involved. This means that a product's design, creation, and use maintain sustainable standards and that the item and the process of making these has a positive impact on communities. An ethical manufacturer has oversight and cares ...
However, there is often confusion amongst researchers and educators around whether involving the public in these activities requires prior ethical approval, similar to what would be the case when inviting members of the public to participate in a clinical research study, or to donate samples such as blood for experiments.
Question: Describe the ethical issues involved in the situation discussed in the case study.Describe the steps Dr. Jamison should take with regard to her discovery of potential ethical concerns.Provide recommendations for the group's advertising needs that would meet ethical standards.