Yale University
- Academic programs
- Health & medicine
- Working at Yale
- Yale & New Haven
- Yale & the World
- Giving to Yale
- Prospective students
- Foundations & corporations


CIRA eBulletin
- Core Structure
- Affiliating with CIRA
- Community Advisory Board
- CIRA Highlights
- Directions/Parking
- AIDS Research Centers
- Affiliated Projects
- International Research
- HIV/AIDS in humanitarian crises
- AIDS Science Day 2023
- Criminalization of HIV Exposure Work Group
- Research Snapshot Series
- Qualitative Research Discussion Group (QRDG)
- COVID-19/HIV Resources
- Mpox Resources
- Video Archive
- eBulletin Archive
- 2024 Pilot Projects in HIV Research
- International Visiting Fellow Program
- HIV Health Equity & Social Justice Fellowship (HESJF)
- Peer Review
- Job Listings
- Yale AIDS Prevention Training Program (Y-APT)
- Research Education Institute for Diverse Scholars (REIDS)
- Training Mentors
- R3EDI: Rigorous, Rapid and Relevant Evidence aDaptationand Implementation to Ending the HIV Epidemic (EHE)
- New England HIV Implementation Science Network
- IS Resources
- HIV Implementation Science to Optimize Research Impact (HISTORI)
- News & Media
NIH Strategic Plan for HIV and HIV-Related Research FY 2021-2025

The NIH Office of AIDS Research (OAR) presents the NIH Strategic Plan for HIV and HIV-Related Research (the Plan) for fiscal years (FY) 2021–2025. The Plan serves as the guiding framework for OAR to allocate funds that advance the NIH-wide HIV research agenda and ensure investment of resources in the highest priority areas of scientific opportunity.
- More information
- Strategic Plan
Published: Monday, January 27, 2020
Recent Publications by CIRA Affiliates
Social support as a mediator between mental health and stigma among newly HIV-positive men who have sex with men Yafang Zhao, Kaveh Khoshnood , Yu Sheng International Journal of STD and AIDS . 2024. Feb 5:9564624241227653. doi: 10.1177/09564624241227653. Online ahead of print.
Feasibility of a Mobile Health Intervention for Providing a Continuum of HIV Services for MSM: Pilot Study of the WeTest Program in 3 Cities in China Zhihui Zhu, Xiaoyan Lu, Pan Gao, Xiaodong Wang, Xuejiao Hu, Nianhua Xie, Cong Liu, Yue Zhao, Yanqiu Zhao, Zhen Dai, Hongbo Zhang, Jun Wang, Yehuan Sun, Tao Liu, Shufang Sun , Cui Yang, Nickolas Zaller, Zhihua Zhang, Don Operario Current HIV Research . 2024. Jan 19. doi: 10.2174/011570162X280190240105063449. Online ahead of print.
Time to blood pressure control and predictors among patients receiving integrated treatment for hypertension and HIV based on an adapted WHO HEARTS implementation strategy at a large urban HIV clinic in Uganda Willington Amutuhaire, Fred Collins Semitala, Isaac Derick Kimera, Christabellah Namugenyi, Frank Mulindwa, Rebecca Ssenyonjo, Rodgers Katwesigye, Frank Mugabe, Gerald Mutungi, Isaac Ssinabulya, Jeremy I Schwartz , Anne R Katahoire, Lewis S Musoke, George A Yendewa, Chris T Longenecker, Martin Muddu Journal of Human Hypertension . 2024. Feb 1. doi: 10.1038/s41371-024-00897-3. Online ahead of print.
The Potential Role of Undetectable = Untransmittable (U = U) in Reducing HIV Stigma among Sexual Minority Men in the US Sarah K Calabrese , David A Kalwicz, Myra A Zaheer, John F Dovidio , Alex Garner, Maria Cecilia Zea, Carla Treloar, Martin Holt, Anthony K J Smith, James MacGibbon, Djordje X Modrakovic, Sharanya Rao, Lisa A Eaton AIDS and Behavior . 2024. Feb;28(2):741-757. doi: 10.1007/s10461-023-04263-1. Epub 2024 Jan 29.
Black Adolescent Females' Perceptions of PrEP for HIV Risk Reduction Mariana Budge, Ijeoma Opara , Veronica U Weser, Brandon E Sands, Kimberly D Hieftje Journal of the International Association of Providers of AIDS Care . 2023. Jan-Dec:22:23259582231206934. doi: 10.1177/23259582231206934.
Copyright © 2024 Yale University. All rights reserved. Privacy policy.
Center for Interdisciplinary Research on AIDS at Yale University 135 College Street, Suite 200, New Haven, CT 06510-2483 Ph: 203-764-4333
CIRA is supported by National Institute of Mental Health Grant No. P30MH062294. Trace Kershaw, Ph.D., Principal Investigator
- What Are HIV and AIDS?
- How Is HIV Transmitted?
- Who Is at Risk for HIV?
- Symptoms of HIV
- U.S. Statistics
- Impact on Racial and Ethnic Minorities
- Global Statistics
- HIV and AIDS Timeline
- In Memoriam
- Supporting Someone Living with HIV
- Standing Up to Stigma
- Getting Involved
- HIV Treatment as Prevention
- Pre-exposure Prophylaxis (PrEP)
- Post-exposure Prophylaxis (PEP)
- Preventing Sexual Transmission of HIV
- Alcohol and HIV Risk
- Substance Use and HIV Risk
- Preventing Perinatal Transmission of HIV
- HIV Vaccines
- Long-acting HIV Prevention Tools
- Microbicides
- Who Should Get Tested?
- HIV Testing Locations
- HIV Testing Overview
- Understanding Your HIV Test Results
- Living with HIV
- Talking About Your HIV Status
- Locate an HIV Care Provider
- Types of Providers
- Take Charge of Your Care
- What to Expect at Your First HIV Care Visit
- Making Care Work for You
- Seeing Your Health Care Provider
- HIV Lab Tests and Results
- Returning to Care
- HIV Treatment Overview
- Viral Suppression and Undetectable Viral Load
- Taking Your HIV Medicine as Prescribed
- Tips on Taking Your HIV Medication Every Day
- Paying for HIV Care and Treatment
- Other Health Issues of Special Concern for People Living with HIV
- Alcohol and Drug Use
- Coronavirus (COVID-19) and People with HIV
- Hepatitis B & C
- Vaccines and People with HIV
- Flu and People with HIV
- Mental Health
- Mpox and People with HIV
- Opportunistic Infections
- Sexually Transmitted Infections
- Syphilis and People with HIV
- HIV and Women's Health Issues
- Aging with HIV
- Emergencies and Disasters and HIV
- Employment and Health
- Exercise and Physical Activity
- Food Safety and Nutrition
- Housing and Health
- Traveling Outside the U.S.
- Civil Rights
- Workplace Rights
- Limits on Confidentiality
- National HIV/AIDS Strategy (2022-2025)
- Implementing the National HIV/AIDS Strategy
- Prior National HIV/AIDS Strategies (2010-2021)
- Key Strategies
- Priority Jurisdictions
- HHS Agencies Involved
- Learn More About EHE
- Ready, Set, PrEP
- Ready, Set, PrEP Pharmacies
- Ready, Set, PrEP Resources
- AHEAD: America’s HIV Epidemic Analysis Dashboard
- HIV Prevention Activities
- HIV Testing Activities
- HIV Care and Treatment Activities
- HIV Research Activities
- Activities Combating HIV Stigma and Discrimination
- The Affordable Care Act and HIV/AIDS
- HIV Care Continuum
- Syringe Services Programs
- Finding Federal Funding for HIV Programs
- Fund Activities
- The Fund in Action
- About PACHA
- Members & Staff
- Subcommittees
- Prior PACHA Meetings and Recommendations
- I Am a Work of Art Campaign
- Awareness Campaigns
- Global HIV/AIDS Overview
- U.S. Government Global HIV/AIDS Activities
- U.S. Government Global-Domestic Bidirectional HIV Work
- Global HIV/AIDS Organizations
- National Black HIV/AIDS Awareness Day February 7
- HIV Is Not A Crime Awareness Day February 28
- National Women and Girls HIV/AIDS Awareness Day March 10
- National Native HIV/AIDS Awareness Day March 20
- National Youth HIV & AIDS Awareness Day April 10
- HIV Vaccine Awareness Day May 18
- National Asian & Pacific Islander HIV/AIDS Awareness Day May 19
- HIV Long-Term Survivors Awareness Day June 5
- National HIV Testing Day June 27
- Zero HIV Stigma July 21
- Southern HIV/AIDS Awareness Day August 20
- National Faith HIV/AIDS Awareness Day August 27
- National African Immigrants and Refugee HIV/AIDS and Hepatitis Awareness Day September 9
- National HIV/AIDS and Aging Awareness Day September 18
- National Gay Men's HIV/AIDS Awareness Day September 27
- National Latinx AIDS Awareness Day October 15
- World AIDS Day December 1
- Event Planning Guide
- U.S. Conference on HIV/AIDS (USCHA)
- National Ryan White Conference on HIV Care & Treatment
- AIDS 2020 (23rd International AIDS Conference Virtual)
Want to stay abreast of changes in prevention, care, treatment or research or other public health arenas that affect our collective response to the HIV epidemic? Or are you new to this field?
HIV.gov curates learning opportunities for you, and the people you serve and collaborate with.
Stay up to date with the webinars, Twitter chats, conferences and more in this section.
Share Your Input on the Next NIH Strategic Plan for HIV Research by March 28
- Share on Facebook
- Share on Twitter
- Share on LinkedIn
- Share on Email

The NIH Office of AIDS Research (OAR) is seeking input to inform the development of the next multi-year NIH Strategic Plan for HIV and HIV-Related Research , which will span 2026-2030. OAR leads the effort across NIH Institutes, Centers, and Offices to establish HIV research priorities and develop the Strategic Plan. As part of that process, OAR has issued a Request for Information to gather ideas and recommendations from interested constituents.
“The NIH Strategic Plan for HIV and HIV-Related Research guides the world’s largest public investment in HIV research, building on scientific progress and opportunities for advancing research to end the HIV pandemic,” observed Diana Finzi, Ph.D., Acting Director, NIH Office of AIDS Research. “Partner and public input helps OAR ensure that the next Strategic Plan is informed by broad and deep expertise across the HIV research community. We invite all interested parties to respond to the open Request for Information until March 28, 2024.”
OAR plans to adopt a new framework for the next plan that consists of four strategic goals:
- Enhance discovery and advance HIV science through fundamental research.
- Advance the development and assessment of novel interventions for HIV prevention, treatment, and cure.
- Optimize public health impact of HIV discoveries through translation, dissemination, and implementation of research findings.
- Build research workforce and infrastructure capacity to enhance sustainability of HIV scientific discovery.
OAR invites researchers, health care professionals, advocates and health advocacy organizations, scientific or professional organizations, public health officials, government agencies, and community members to provide input through the online process. Respondents are invited to propose research priorities within each goal and provide additional feedback on the new framework.
View additional details and submit your feedback online until March 28, 2024.
Related HIV.gov Blogs
- NIH National Institutes of Health
- OAR NIH Office of AIDS Research
- Research Agenda
- Español (Spanish)
Webpage (HTML) Topic Funding Opportunities Tools and Information Services Research Research Organizations Prevention Research Treatment Research Vaccine Research Resource Link https://www.oar.nih.gov/hiv-policy-and-research/strategic-plan Source National Institutes of Health
The Federal Register
The daily journal of the united states government, request access.
Due to aggressive automated scraping of FederalRegister.gov and eCFR.gov, programmatic access to these sites is limited to access to our extensive developer APIs.
If you are human user receiving this message, we can add your IP address to a set of IPs that can access FederalRegister.gov & eCFR.gov; complete the CAPTCHA (bot test) below and click "Request Access". This process will be necessary for each IP address you wish to access the site from, requests are valid for approximately one quarter (three months) after which the process may need to be repeated.
An official website of the United States government.
If you want to request a wider IP range, first request access for your current IP, and then use the "Site Feedback" button found in the lower left-hand side to make the request.
April 21, 2023
Related Announcements
- October 9, 2020 - Administrative Supplements to Existing NIH Grants and Cooperative Agreements (Parent Admin Supp Clinical Trial Optional). See NOFO PA-20-272 .
National Institute on Alcohol Abuse and Alcoholism ( NIAAA )
National Institute of Allergy and Infectious Diseases ( NIAID )
National Institute of Arthritis and Musculoskeletal and Skin Diseases ( NIAMS )
National Institute of Diabetes and Digestive and Kidney Diseases ( NIDDK )
National Institute on Drug Abuse ( NIDA )
National Institute of Mental Health ( NIMH )
National Institute of Neurological Disorders and Stroke ( NINDS )
National Institute on Minority Health and Health Disparities ( NIMHD )
National Center for Complementary and Integrative Health ( NCCIH )
National Cancer Institute ( NCI )
All applications to this funding opportunity announcement should fall within the mission of the Institutes/Centers. The following NIH Offices may co-fund applications assigned to those Institutes/Centers.
Office of Research on Women's Health ( ORWH )
The National Institute on Aging (NIA) and NIH Office of AIDS Research (OAR) with participating NIH Institutes and Centers announce the availability of administrative supplements to support research on HIV/AIDS and aging. Eligible parent awards include existing projects funded by the participating Institutes and Centers listed above with or without an existing focus on HIV/AIDS as long as other eligibility criteria are met and are within scope of the original project. Supplemental projects may involve a variety of scientific approaches and methods provided that they are focused on HIV/AIDS, can be conducted within one year of the supplement award, address previously unforeseen research opportunities, and fit within the scope of the parent grant. Within scope means that the proposed aims do not add to or expand the existing study in a manner that would be more appropriate for a competing peer-reviewed mechanism and/or revision application. Examples of potential supplemental studies could include:
- Addition of participants with HIV to an ongoing study where such participants were either not included originally or not enrolled in sufficient numbers to make meaningful comparisons between groups
- Addition of older adult participants to an ongoing study involving subjects with HIV where such participants were either not included originally or not enrolled in sufficient numbers to make meaningful comparisons between groups
- Exploration of measures relevant to HIV and aging
- Development of enhanced approaches to improve the recruitment and retention of aging individuals with HIV
Depending on the parent project, not all of the above approaches would be considered within scope, and other approaches may also be possible. Prospective applicants are strongly encouraged to discuss their proposed aims with their program officer to verify eligibility criteria and fit within the scope of their existing award.
Grantees whose awarding IC is not listed above are encouraged to reach out to their program officer.
Scientific topics of interest include, but are not limited to:
- Understanding the impact of HIV infection and pathogenesis in the context of aging-related genetic, molecular, and cellular changes and physiological outcomes.
- Understanding molecular mechanisms that may be common to multiple comorbid conditions related to aging with HIV.
- Understanding the contributions of individual, interpersonal, social, structural, institutional, and healthcare system factors to the physical, psychological, and economic well-being of persons aging with HIV and to social inequalities and health disparities.
- Understanding relationships between HIV infection and cognitive decline, especially in Alzheimer’s disease (AD) and Alzheimer’s disease-related dementias (ADRD) and other neurological impairments in people with HIV and advancing age.
- Enhancing assessment and treatment of older individuals with HIV and comorbidities, polypharmacy, frailty, disability, or disparities in health outcomes.
Research Objectives
The specific research objective of this Notice of Special Interest (NOSI) is to accelerate new knowledge related to the science of HIV and aging, and to expand the pool of researchers conducting studies at the intersection of HIV and aging. Applications should focus on one or more Key HIV Research Areas as outlined in the FY2021-2025 NIH Strategic Plan for HIV and HIV-Related Research . Applications must be focused on HIV-relevant research for consideration through this NOSI. Applicants may consider leveraging existing HIV cohorts and resources, such as NA-ACCORD , MACS/WIHS Combined Cohort Study , Veteran Aging Cohort Study , and the National NeuroAIDS Tissue Consortium . Specific interests of participating ICs are described below:
National Institute on Aging (NIA)
NIA welcomes research supplements that fall within NIA’s mission to support genetic, biological, clinical, behavioral, social, and economic research on aging. NIA encourages applicants to address priorities outlined in the NIA Health Disparities Research Framework . Investigators interested in analyses of existing datasets may consider using NIA-supported observational and interventional studies such as those listed in the Aging Research Biobank, NIA’s Research Resources page , and those listed in NOT-AG-21-020 . NIA-supported Research Centers may be particularly useful for accessing recruitment resources, analytic capabilities, specimens, specialized expertise, and other aging-related research resources. More information about each of these Centers programs can be found at their respective links: Alzheimer's Disease Research Centers , Artificial Intelligence and Technology Collaboratories , Centers on the Demography and Economics of Aging , Claude D. Pepper Older Americans Independence Centers , Nathan Shock Centers of Excellence in the Basic Biology of Aging , Edward R. Roybal Centers for Translation Research in the Behavioral and Social Sciences of Aging , and Resource Centers for Minority Aging Research . Coordination among all of NIA's Centers programs is facilitated through the NIA Research Centers Collaborative Network .
National Institute of Alcohol Abuse and Alcoholism (NIAAA)
NIAAA seeks supplements to address alcohol use among aging individuals with varying patterns of alcohol use. Levels of alcohol use that may be harmful could be substantially lower in people with HIV (PWH), and clinical studies focused on understanding how alcohol exacerbates geriatric syndromes like falls, delirium, frailty, and complications from polypharmacy are of particular interest.
While alcohol use contributes to overall frailty, it can also exacerbate HIV pathogenesis in multiple organ systems. Improvement in health outcomes of PWH who drink may be complicated by multiple comorbidities associated with continued heavy alcohol use and a range of alcohol use disorders (AUD). These comorbidities include diabetes, cardiovascular disease (CVD), cancers, and other skeletal and metabolic injury. Supplements to advance cross-cutting basic research on these complications are also of interest.
National Institute of Allergy and Infectious Diseases (NIAID)
NIAID welcomes research topics aimed at elucidating the interactions of aging with HIV-related coinfections and the effects of aging on achieving durable viral suppression. Research may include such topics as viral hepatitis, tuberculosis, other coinfections with significant impact on the health of people living with HIV (PLWH), HIV reservoirs, and sustaining virologic response.
National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS)
NIAMS seeks research supplements focusing on aging and HIV-associated comorbidities within the mission areas of systemic rheumatic, musculoskeletal, and skin diseases. NIAMS supports studies on how HIV infection or HIV treatment impact the earlier onset or greater risk of diseases and conditions that are associated with aging and relevant to the NIAMS mission. These include, but are not limited to, osteoporosis, osteopenia, bone fracture, musculoskeletal frailty and pain, rheumatic and dermatological manifestations. We also support studies focused on the elucidation of pathophysiology and management strategies of these comorbidities among older people living with HIV/AIDS. NIAMS will not support clinical trial applications in response to this NOSI.
National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
NIDDK invites research topics aimed at elucidating effects of aging on HIV-related comorbidities and coinfections within its mission. These include loss of gastrointestinal immune and microbial homeostasis, enteropathy, noncommunicable liver and biliary diseases, viral hepatitis, nutritional inadequacies, obesity, metabolic/endocrine dysfunction and perturbations, kidney disease, benign genitourinary diseases and disorders, and hematologic sequelae. NIDDK also encourages studies that address how these comorbidities and coinfections in older people impact HIV reservoirs in anatomical sites relevant to its mission. These sites include the gastrointestinal mucosa, the liver, adipose tissue, the kidney, and the male genital tract.
National Institute on Drug Abuse (NIDA)
NIDA is interested in research to explore the impact of substance use and substance use disorders (SUD) in aging people with HIV. Addictive substances of interest include: cannabinoids, nicotine, cocaine, stimulants, opioids, prescription drugs, or combinations of these drugs.
The research areas of interest include but are not limited to:
- Identifying mechanisms underlying vulnerability or resilience to HIV-related neuropathogenesis or neurocognitive impairment as a function of SUD history in aging people with HIV.
- Leveraging existing epidemiological data (i.e., MWCCS: MACS/WIHS Combined Cohort Study) to elucidate effects of short- and long-term cannabis use in older adult populations with HIV.
- Generating multi-omic (genomic, epigenomic, proteomic, metabolomic, microbiome) data as a resource for studying HIV and SUD in aging people living with HIV.
- Examining long-term HIV infection and substance use outcomes, as well as medical consequences, among aging persons living with HIV in order to enhance healthcare access and utilization.
- Research to identify biomarkers for and mechanisms that contribute to vulnerability to HIV exposure, aggravate HIV pathologies, regulate HIV persistence, or increase the risk for comorbidities including SUD and other poor health outcomes in aging people with HIV.
National Institute of Mental Health (NIMH)
NIMH will support research supplements focused on the Division of AIDS Research’s priorities in people aging with HIV. NIMH will support studies that focus on HIV/AIDS-related differences in behavioral risk, cognitive and social functioning, or mental health issues in the context of aging populations with HIV. For instance, studies that examine how social and contextual characteristics guide the development of effective multi-level HIV/AIDS social and behavioral interventions in at-risk and infected populations of older adults throughout the lifespan are of interest to NIMH. NIMH is also interested in epidemiological, behavioral, biological, and treatment studies of age-associated central nervous system (CNS) complications in people with HIV. Further, NIMH is interested in basic and clinical studies in aging populations with HIV related to CNS viral dynamics (reservoir), immune dysfunction/inflammation, and alterations in neuronal circuitry. It is strongly recommended that applicants consult with the Program Officer listed on their grant before submitting the research supplement.
National Institute of Neurological Disorders and Stroke (NINDS)
NINDS supports basic, translational, and clinical research on the brain and nervous system and uses that knowledge to reduce the burden of neurological disease. In the context of HIV disease, NINDS is particularly interested in the neurological complications of HIV infection that affect the brain, spinal cord, and peripheral nervous system. For the purposes of this notice, specific topics of interest might include (but would not be limited to): the long-term consequences of latent HIV in the CNS as it pertains to the modulation of chronic neuroinflammation and cognitive impairment in aging; the mechanisms by which chronic HIV exacerbates long-term blood-brain barrier damage and cerebrovascular dysfunction; studies of the mechanisms by which chronic HIV primes the CNS for neurodegeneration, including in the context of AD/ADRD; potential interactions between chronic HIV infection and other neurological disorders within the mission of NINDS; the long-term effect of chronic antiretroviral therapy (ART) exposure throughout the lifespan on the central and peripheral nervous systems; mechanisms of HIV-associated peripheral neuropathy and chronic pain; and studies that address the question of whether chronic HIV infection and exposure to ART cause accelerated aging of the CNS. It is strongly encouraged that potential NINDS applicants contact the program officer listed at the bottom of this Notice to discuss proposals prior to submission.
NINDS urges investigators to follow the NIH guidance for rigor and transparency in grant applications ( https://grants.nih.gov/policy/reproducibility/guidance.htm ) and additionally recommends the research practices described at https://www.ninds.nih.gov/Funding/grant_policy to ensure that robust experiments are designed, potential experimenter biases are minimized, results and analyses are transparently reported, and results are interpreted carefully. These recommended research practices include, where applicable: rationale for the chosen model(s) and primary/secondary endpoints, clear descriptions of tools and parameters, blinding, randomization, ensuring adequate sample size, pre-specified inclusion/exclusion criteria, handling of missing data and outliers, appropriate controls, preplanned analyses, appropriate quantitative techniques, clear indication of exploratory vs. confirmatory components of the study, consideration of limitations, and plans for transparent reporting of all methods, analyses, and results so that other investigators can evaluate the quality of the work and potentially perform replications.
National Institute on Minority Health and Health Disparities (NIMHD)
The mission of the NIMHD is to lead scientific research to improve minority health and reduce health disparities. Studies must include a focus on one or more of the following NIH-designated populations that experience health disparities in the United States: African Americans, Latinos/Hispanics, American Indians and Alaska Natives, Asian Americans, Native Hawaiians and other Pacific Islanders, less privileged socioeconomic groups, underserved rural populations, and sexual and gender minorities. Comparison groups/populations may also be included as appropriate for the research questions posed. Proposed studies should utilize multi-level approaches as identified in the NIMHD Research Framework and address the relevant social determinants of health using measures available in the PhenX Toolkit , as appropriate. In the context of this NOSI, interdisciplinary approaches are encouraged to elucidate the biological, behavioral, clinical, and social mechanisms of aging in populations that experience health disparities, and to address the growing health concerns and improve health outcomes in PWH. Studies that address care coordination challenges among PWH with multiple comorbidities to optimize care delivery in diverse care settings, especially rural settings, are also encouraged. Finally, studies that are focused on HIV and women from racial and ethnic minority populations, including but not limited to addressing access to care, stigma, discrimination, and intimate partner violence, especially in cis and transgender women, are encouraged. Please note that NIMHD will not support animal studies.
National Center for Complementary and Integrative Health (NCCIH)
NCCIH is interested in supporting research on complementary and integrative approaches to improve resilience, restore health, manage HIV-related comorbidities, and reduce health disparities for PWH as they age. In the context of this NOSI, NCCIH is particularly interested in encouraging applications that propose expanding existing clinical trials to include greater numbers of older PWH, performing secondary analyses of datasets that include older PWH, and conducting basic and mechanistic studies to explain age-related health outcomes in PWH. Proposals should support a whole person health framework and address priorities outlined in the NCCIH Strategic Plan FY 2021–?2025 .
National Cancer Institute (NCI)
NCI welcomes research supplements that help to understand how aging in the presence of chronic HIV infection affects the risk, spectrum, and biology of cancer (AIDS-defining and non-AIDS-defining cancers). Supplements that are focused on the interplay between host factors and immune perturbations in older individuals living with HIV who develop cancer is also encouraged.
The Office of Research on Women's Health (ORWH)
ORWH is part of the Office of the Director, NIH, and works with the 27 NIH Institutes and Centers to advance rigorous research of relevance to the health of women. ORWH does not award grants but co-funds women’s health-related applications and research projects that have received an award from one of the participating NIH Institutes and Centers listed in the announcement. Applications seeking ORWH co-funding, in response to this Notice, should ensure that the proposed work is aligned with at least one goal and objective outlined in the Trans-NIH Strategic Plan for Women’s Health Research .
ORWH supports research projects that address topics of relevance to aging, HIV, and women across the lifespan – including cisgender, transgender, and gender diverse women – and individuals assigned female at birth. Intersectional approaches to women, aging, and HIV are encouraged. Areas of interest include:
- Understanding the influence of sex on HIV infection, pathogenesis and treatment
- The influence of comorbidity among older populations of women with HIV
- Impact of HIV on menopause
- Understanding the role of HIV in violence, trauma, and mental health in aging women with HIV
- Addition of participants to existing studies to enable meaningful sex and/or gender comparisons
Applications will be evaluated according to the following criteria:
- Does the administrative supplement fit within the aims of the parent grant?
- Is the administrative supplement focused on Key NIH HIV Research Areas ? Does it have an HIV/AIDS research component?
- Will the administrative supplement result in advancing knowledge and/or collaborations leading to additional research activity at the intersection of HIV and aging?
Application and Submission Information
Applications for this initiative must be submitted using the following opportunity or its subsequent reissued equivalent.
- PA-20-272 - Administrative Supplements to Existing NIH Grants and Cooperative Agreements (Parent Admin Supp Clinical Trial Optional)
Administrative supplements may only be used to meet increased costs that are within the scope of the approved award, but were unforeseen when the new or renewal application or grant progress report for non-competing continuation support was submitted.
All instructions in the SF424 (R&R) Application Guide and PA-20-272 must be followed, with the following additions:
- Application Due Date(s) – May 22, 2023, by 5:00 PM local time of applicant organization.
- For funding consideration, applicants must include “NOT-AG-23-008” (without quotation marks) in the Agency Routing Identifier field (box 4B) of the SF424 R&R form. Applications without this information in box 4B will not be considered for this initiative.
- Administrative supplement requests may be for one year of support only.
- The proposed budget period must be within the project period of the parent award. Active awards with project end dates in FY 2024 or later are eligible. The award may not be in a terminal no-cost extension or going into a no-cost extension in FY 2023.
- Individual requests can be no more than $250,000 in direct costs exclusive of Facilities and Administrative costs on sub-contracts, cannot exceed the direct costs of the parent award, and must reflect the actual needs of the proposed project.
- Requests must also adhere to the funding caps associated with the respective parent NOFO (e.g., salary caps on K’s, SBIR/STTR limits, etc.).
- Applicants may apply for more than one supplement to a given parent grant/award, provided they are scientifically distinct. However, though supplement requests are not limited to one per award, we will consider substantial additional funding to an award as beyond the scope of the funded award.
- The Research Strategy section of the application is limited to 6 pages.
Funding decisions of applications submitted in response to this Notice are subject to program priorities and availability of funds.
Please direct all inquiries to the following Scientific/Research Contacts:
Stacy Carrington-Lawrence, Ph.D. National Institute on Aging (NIA) Division of Aging Biology (DAB) Telephone: 301-496-6402 Email: [email protected]
Melissa Gerald, Ph.D. National Institute on Aging (NIA) Division of Behavioral and Social Research (DBSR) Telephone: 301-496-3136 Email: [email protected]
Basil Eldadah, M.D., Ph.D. National Institute on Aging (NIA) Division of Geriatrics and Clinical Gerontology (DGCG) Telephone: 301-496-6761 Email: [email protected]
Maja Maric, Ph.D. National Institute on Aging (NIA) Division of Neuroscience (DN) Telephone: 301-496-9350 Email: [email protected]
Geetanjali Bansal, Ph.D. Office of AIDS Research (OAR) Telephone: 240-669-5073 Email: [email protected]
Kendall J. Bryant, Ph.D. Coordinator, HIV/AIDS and Alcohol Research National Institute on Alcohol Abuse and Alcoholism (NIAAA) Email: [email protected]
Robert C. Palmer, MS, MSc National Institute of Allergy and Infectious Diseases (NIAID) Telephone: 240-292-4842 Email: [email protected]
Heiyoung Park, Ph.D. National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) Telephone: 301-594-5032 E-mail: [email protected]
Peter Perrin, Ph.D. National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Telephone: 301-451-3759 Email: [email protected]
Vasundhara Varthakavi, DVM, Ph.D. National Institute on Drug Abuse (NIDA) Telephone: 240-669-5020 E-mail: [email protected]
Vasudev R Rao, MBBS, MS National Institute of Mental Health (NIMH) Telephone: 301-825-3259 Email: [email protected]
William P. Daley, Ph.D. National Institutes of Neurological Disorders and Stroke (NINDS) Telephone: 301-496-1431 Email: [email protected]
Seema N. Desai, Ph.D. National Institute on Minority Health and Health Disparities (NIMHD) Telephone: 301-827-6698 Email: [email protected]
Sekai Chideya-Chihota, Ph.D. National Center for Complementary and Integrative Health (NCCIH) Telephone: 240-552-2994 Email: [email protected]
Geraldina Dominguez, Ph.D. National Cancer Institute (NCI) Telephone: 301-920-6044 Email: [email protected]
Chyren Hunter, Ph.D. Office of Research on Women's Health (ORWH) Telephone: 301-402-4158 E-mail: [email protected]

Note: For help accessing PDF, RTF, MS Word, Excel, PowerPoint, Audio or Video files, see Help Downloading Files .

An official website of the United States government
Here's how you know
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
Office of AIDS Research
Nih hiv research funding opportunity: advancing hiv service delivery through pharmacies and pharmacists.

Two newly published research funding opportunities from NIH are now available to support the advancement of HIV service delivery through pharmacies and pharmacists.
This notice of funding opportunity (NOFO) solicits research designed to capacitate, transform, and scale the delivery of HIV testing, prevention, and care services through pharmacists and pharmacies in US and/or global settings. This includes the opportunity to advance training curricula that enables pharmacy students, pharmacists, pharmacies, and pharmacy systems to deliver the spectrum of needed HIV services with ease, equity, and effectiveness.
The NIH institutes and offices that are co-sponsoring the two requests for applications (RFAs) under the NOFO have collectively set aside funds in FY25 to support relevant, high priority research. These RFAs reflect themes identified in the June 2023 NIH-sponsored meeting, “ Pharmacy-Centered Research: Current Landscape and Future Frontiers .”
Please find further information about the NOFO below.
Links to NOFO: Advancing HIV service delivery through pharmacies and pharmacists (R01 Clinical Trial Optional) RFA-MH-25-185 Advancing HIV service delivery through pharmacies and pharmacists (R21 Clinical Trial Optional) RFA-MH-25-186
Mechanisms offered: R01 and R21
Due Date: Applications are due by August 13, 2024, 5:00 p.m . local time of the applicant organization.
Webinar: NIH will hold a pre-application informational webinar on the RFAs. Additional details about the webinar will be published in the NIH Guide and posted on this webpage when available.
Participating Institutes, Centers, and Offices (ICOs): NIH Office of AIDS Research ( OAR ) National Institute of Mental Health ( NIMH ) National Institute of Allergy and Infectious Diseases ( NIAID ) National Institute on Drug Abuse ( NIDA ) National Institute on Alcohol Abuse and Alcoholism ( NIAAA ) National Heart, Lung, and Blood Institute ( NHLBI ) National Institute of Diabetes and Digestive and Kidney Diseases ( NIDDK ) Eunice Kennedy Shriver National Institute of Child Health and Human Development ( NICHD ) National Institute on Minority Health and Health Disparities ( NIMHD)
This page last reviewed on May 8, 2024

An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.

Strategic Plan: Fiscal Years 2024-2028
National Institute on Alcohol Abuse and Alcoholism (NIAAA)
Cross-cutting research programs.
Cross-cutting research programs are NIAAA-managed research portfolios that include topics spanning multiple research goals outlined in this strategic plan. These programs complement the Cross-Cutting Research Themes and Supporting the Mission sections of this strategic plan.
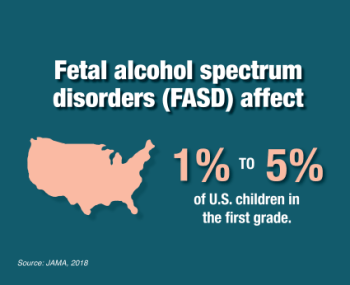
Fetal Alcohol Spectrum Disorders
Fetal alcohol spectrum disorders (FASD) represent the broad range of neurodevelopmental impairments and other physical effects that result from prenatal exposure to alcohol. NIAAA supports a robust FASD research program to advance study of the causes, mechanisms, diagnosis, prevention, and treatment of FASD.
Research to reduce stigma around prenatal alcohol exposure, to increase education and awareness of FASD, and to develop culturally appropriate prevention strategies are important components of FASD prevention. Basic research on the biological mechanisms that underlie prenatal alcohol exposure and contribute to FASD can lead to the identification of diagnostic biomarkers as well as potential treatment targets, paving the way for earlier diagnosis and treatment. NIAAA is also facilitating efforts to reach consensus on a single research classification system for FASD to harmonize research efforts across the globe and accelerate scientific progress
Objective 1: Advance the Prevention of Prenatal Alcohol Exposure
Preventing and reducing alcohol use during pregnancy is essential to preventing prenatal alcohol exposure and related consequences, including FASD. Studies have shown that alcohol screening, brief intervention, and referral to treatment (SBIRT) approaches are an effective tool for addressing alcohol and other substance use in primary and prenatal care settings.
NIAAA encourages research and other activities to prevent and reduce prenatal alcohol exposure and the prevalence of FASD—for example:
- Designing and evaluating cost-effective models to help women at high risk of having a child with FASD (i.e., who misuse alcohol, have alcohol use disorder (AUD) , or already have another child with FASD) to reduce or abstain from drinking during pregnancy and, consequently, reduce the incidence of FASD
- Developing culturally informed interventions to prevent alcohol-exposed pregnancies
- Improving implementation and enhancing scalability of effective preventive interventions (e.g., alcohol SBIRT) for alcohol misuse and its negative consequences in women
- Identifying the causes of FASD-related stigma and exploring strategies to reduce it in order to improve prevention efforts
- Promoting collaboration with federal and nonfederal organizations to raise public awareness about FASD and advance implementation of prevention interventions in various clinical and nonclinical settings
Objective 2: Improve Early Identification and Treatment Interventions for Fetal Alcohol Spectrum Disorders
Improved diagnostic techniques for FASD could enable more accurate diagnosis, expand access to assessment for FASD, allow for earlier intervention, and likely reduce costs and overall burden of FASD across the life span. Early identification holds promise for better outcomes, but limited availability of FASD diagnostic services remains a barrier to care.
NIAAA encourages research to enhance FASD screening, diagnosis, and treatment—for example:
- Improving implementation of existing evidence-based FASD diagnostic approaches across the life span as well as early intervention and care for individuals with FASD across a range of populations and settings, such as for children and parents in the child welfare system and individuals in the juvenile justice system
- Developing novel technologies and methods to facilitate earlier identification of children affected by prenatal alcohol exposure, as early as the fetal and newborn periods
- Assessing combinations of evidence-based therapeutic approaches for individuals with FASD
- Understanding and addressing health disparities concerning prenatal alcohol exposure in different populations
- Developing and evaluating novel prenatal and postnatal therapeutic approaches, medications, and dietary supplements to combat prenatal alcohol exposure and FASD
- Pursuing various interventions to mitigate the neurocognitive and behavioral deficits associated with FASD across the life span
Objective 3: Identify Mechanisms, Diagnostic Biomarkers, and Potential Treatment Targets for Fetal Alcohol Spectrum Disorders
Alcohol can disrupt prenatal development through a variety of mechanisms. Understanding the biological effects of prenatal alcohol exposure that emerge across the life span, from prenatal development to adulthood, can inform preventive and therapeutic strategies to mitigate the consequences of prenatal alcohol exposure.
NIAAA encourages research to advance foundational knowledge of the mechanisms mediating FASD—for example:
- Identifying and characterizing the biological mechanisms underlying the harmful effects of prenatal alcohol exposure across the life span, including health consequences that persist or emerge in adults with FASD
- Further defining periods of susceptibility to alcohol exposure (e.g., periconception, specific months or trimester, entire pregnancy, or time points during postnatal development)
- Elucidating the potential paternal contribution to the etiology of FASD
- Refining and advancing biomarkers of prenatal alcohol exposure
- Defining the long-term health impacts of prenatal alcohol exposure in later adulthood
Alcohol and HIV
Alcohol is an important contributor to the HIV pandemic. In the United States, many people with HIV engage in alcohol misuse or have AUD. Alcohol can affect behaviors that increase the likelihood of acquiring or transmitting HIV to others. Alcohol may also speed HIV progression in people living with the disease, influence their engagement and retention in HIV treatment, and increase their susceptibility to organ damage and coinfections. These effects may shift across the life span, particularly as people living with HIV enter midlife and older adulthood.
In coordination with the NIH Office of AIDS Research and the NIH Strategic Plan for HIV and HIV-Related Research , NIAAA continues to advance basic, translational, and clinical research to improve health outcomes among people living with HIV, including women and underserved populations who experience health disparities. NIAAA will enhance collaboration with partners within and outside NIH to translate and disseminate research findings to maximize the public health impact of NIAAA-supported HIV research, and to strengthen the research and workforce capacity to sustain alcohol-HIV research.
Objective 1: Understand the Biological and Behavioral Mechanisms of Alcohol Misuse and HIV
The mechanisms through which alcohol misuse interacts with HIV to increase morbidity and mortality are not well understood. While HIV can be treated effectively with antiretroviral medications, alcohol misuse contributes to or exacerbates adverse medication interactions, chronic inflammation, liver and other organ injury, cardiovascular and neurological problems, and other pathologies.
Several biological or behavioral phenotypes of HIV and alcohol have been identified that may have markedly different disease courses, biological underpinnings, and treatment responses. Collaborative, multidisciplinary research will be key to better understanding the interactions between alcohol misuse and HIV.
NIAAA encourages research to better understand the relationship between alcohol misuse and HIV to inform the development of effective interventions—for example:
- Determining how alcohol misuse confers a biological risk for HIV infection
- Determining how current and past alcohol misuse in the context of HIV infection and the use of antiretroviral medications affect HIV disease progression and the development of organ and tissue injury (e.g., gut, liver, lung, and brain)
- Improving the understanding of the biological, clinical, and socio-behavioral aspects of aging through the lens of HIV infection and alcohol misuse
Objective 2: Prevent Alcohol Misuse and Treat Alcohol Use Disorder Among People Living With HIV or at High Risk of Acquiring HIV
As noted above, alcohol misuse contributes to poor health outcomes among people living with HIV and reduces the effectiveness of strategies designed to prevent new HIV infections. For example, the use of pre-exposure prophylaxis (PrEP) is highly effective in preventing HIV among those at high risk. However, alcohol misuse may reduce a person’s likelihood of following PrEP medication regimens.
Alcohol misuse may also affect an individual’s adherence to antiretroviral therapy and contribute to HIV transmission. Evidence-based approaches, such as brief interventions, contingency management, risk and harm reduction interventions, and stepped care approaches, have been demonstrated to prevent and reduce alcohol misuse among people living with HIV. Stepped care approaches that integrate treatment for alcohol misuse and HIV have also been shown to improve HIV-related outcomes. Strategies to identify and address alcohol misuse among people with HIV or at high risk for HIV can contribute to improved health and a reduced incidence of HIV.
NIAAA encourages alcohol-related research to prevent and reduce HIV infection, progression, and transmission—for example:
- Enhancing routine alcohol SBIRT to reduce HIV transmission and disease progression
- Developing strategies that address alcohol-related barriers to effective use of PrEP, especially among women and other populations with historically low usage rates
- Developing novel interventions and enhancing implementation of existing evidence-based prevention and treatment interventions that address both alcohol misuse and HIV
- Expanding access to and options for AUD treatment for people living with HIV
Objective 3: Address Alcohol-Related Comorbidities Among People Living With HIV
Many comorbidities, coinfections, and medical complications are associated with chronic alcohol misuse and HIV disease progression. These factors combine with the natural processes of aging to increase frailty and present challenges for medical management. Addressing comorbid medical complications is critical for improving quality of life and longevity.
NIAAA encourages research to address alcohol-related comorbidity among individuals with HIV—for example:
- Promoting the integration of care for HIV, alcohol misuse or AUD, and co-occurring health conditions and medical complications in different settings
- Developing a comprehensive model for alcohol misuse or AUD and disease outcomes among older adults with HIV, including expanded biomarker assays
- Evaluating the efficacy and effectiveness of interventions for reducing alcohol misuse in improving co-occurring pain, stress, and other mental health concerns among people living with HIV
niaaa.nih.gov
An official website of the National Institutes of Health and the National Institute on Alcohol Abuse and Alcoholism
Putting science to work for the health of women
Advancing Women’s Health Research: From Policy to Action
Advancing women’s health research: from policy to action , by dr. janine a. clayton.

White House Actions to Advance Women’s Health Research
On March 18, 2024, President Joe Biden signed a new Executive Order (EO) that builds upon the White House Initiative on Women’s Health Research . The EO outlined strategies to improve women’s health research by integrating women’s health across the federal research portfolio, prioritizing funding, galvanizing new research on women’s midlife health, and assessing areas needing further support. ORWH Deputy Director Vivian Ota Wang, Ph.D., and I were honored to attend the historic signing, alongside NIH Director Monica Bertagnolli, M.D., and Deputy Director for Program Coordination, Planning, and Strategic Initiatives Tara A. Schwetz, Ph.D., and other NIH leaders. A key aspect of this initiative was the announcement of a new NIH-wide effort to invest $200 million in Fiscal Year 2025 specifically for interdisciplinary women’s health research. This substantial investment is aligned with the administration’s commitment to advancing our understanding of women’s health issues and developing targeted interventions and treatments.
As part of the White House Initiative on Women’s Health Research, First Lady Dr. Jill Biden and I visited Research Triangle Park, home to North Carolina State University, Duke University, and the University of North Carolina at Chapel Hill, to promote the EO, specifically focusing on challenges women face in midlife like menopause and heart disease. Our collaborative efforts with renowned institutions and health care professionals emphasized the need to address disparities in health care, advocating for innovative research and health care solutions that prioritize women’s health.
Moreover, in alignment with the White House Initiative on Women’s Health Research, ORWH issued a NIH-wide Notice of Special Interest to highlight interest in receiving research applications focused on diseases and health conditions that predominantly affect women (e.g., autoimmune diseases, depressive disorders, Alzheimer’s disease and Alzheimer’s disease-related dementias, gender-based violence), present and progress differently in women (e.g., cardiovascular disease, HIV, reproductive aging and its implications), or are female specific (e.g., uterine fibroids, endometriosis, menopause). I strongly encourage applications incorporate an intersectional and/or multidimensional approach to gender-related social and structural variables, including race, ethnicity, socioeconomic status, and state and federal policies.
Dr. Elizabeth Barr Named Associate Director for Interdisciplinary Research
Congratulations to Elizabeth Barr, Ph.D. on being named Associate Director for Interdisciplinary Research at ORWH. Dr. Barr joined ORWH in 2019, where she has coordinated efforts to advance intersectional health research on gender as a social and structural variable, managed the ORWH interprofessional education program, and led efforts to advance HIV research for women. Dr. Barr’s background is in gender and women’s studies, community-led HIV research, and reproductive justice. Dr. Barr completed her Ph.D. in communications, with training in science and technology studies, at the University of Wisconsin–Madison and her M.S. in women’s and gender studies at Towson University. Prior to joining ORWH, Dr. Barr led interdisciplinary, cross-sector projects to increase women’s engagement in clinical research and served on the faculties of Towson University and the University of Maryland, Baltimore County. Dr. Barr is widely respected in the field of interdisciplinary research as demonstrated by her accomplishments above, and we look forward to her continued contributions to ORWH!
Advisory Committee on Research on Women’s Health
The 60th Meeting of the NIH Advisory Committee on Research on Women's Health (ACRWH), held on April 12, 2024, provided a forum for ACRWH members to give guidance and make recommendations on priority issues affecting women's health and sex differences research. Carolyn Mazure, Ph.D., White House Initiative on Women’s Health Research chair, provided an insightful update on the White House Women’s Health Research Initiative. Dawn Corbett, M.P.H., NIH inclusion policy officer, Office of Extramural Research, presented a comprehensive inclusion update on NIH clinical research. The event hosted a robust panel titled Middle-Life Health of Women and Menopause, of expert researchers that discussed how menopause can affect the health of women. The Office of Disease Prevention will host a Pathways to Prevention (P2P) workshop in 2025 to identify research gaps in the menopausal tradition and to promote well-being through midlife and beyond. Visit the ACRWH event page for the videocast recording.
HIV & Women Scientific Workshop
In March 2024, the NIH Office of AIDS Research (OAR) and ORWH hosted a two-day virtual workshop to review the state of the science on HIV and women and inform the future research agenda. The workshop, which is part of the OAR-ORWH joint HIV and Women Signature Program , featured a diverse array of HIV-related topics of relevance to women, girls, and gender-diverse people, including prevention, treatment, cure, and social and structural determinants of health. Aligned with the signature program’s focus on intersectional, data-driven, and equity-informed approaches to HIV and women, the workshop included community members on the organizing committee and community speakers on every panel. We were also happy to feature work from established and emerging investigators, highlighting opportunities for ongoing collaborations and connections. Over 700 people watched the workshop live, and it is archived via videocast for viewing on-demand.
National Minority Health Month
April marks National Minority Health Month (NMHM), a time to raise awareness about the importance of improving the health of racial and ethnic minority communities and reducing health disparities. The National Institute on Minority Health and Health Disparities celebrated this month with a series of events, including a Minority Health Walk, Run, Roll 5K and a Fireside Chat featuring the Honorable Louis W. Sullivan, M.D. Additionally, the Health and Human Services Office of Minority Health (OMH) has emphasized this year’s theme, “Be the Source for Better Health: Improving Health Outcomes Through Our Cultures, Communities, and Connections,” focusing on understanding how the unique environments, cultures, histories, and circumstances (known as social determinants of health) of racial and ethnic minority and American Indian and Alaska Native populations impact their overall health. I encourage you to learn more about health equity by using the resources made available online by OMH.
As we commemorate NMHM, I look forward to the upcoming release of the Health of Women of U3 Populations Data Book. This resource holds immense promise in offering valuable insights into the complex interplay of cultural, racial, socioeconomic, and geographical factors that can influence the health status of women. By highlighting the challenges these communities face, this data book emphasizes the urgent need for equitable health care practices to promote health equity for all.
Black Maternal Health Week
This year, we observed Black Maternal Health Week from April 11 to 17. This week serves as a crucial platform to spotlight and address the disparities in health outcomes experienced by Black mothers. Research and data have consistently shown stark racial and ethnic disparities in maternal mortality rates, with Non-Hispanic Native Hawaiian and Pacific Islander, Black or African American, American Indian, and Alaska Native women facing mortality rates two to four times higher than Non-Hispanic White women in pregnancy-related causes. Recognizing the urgent need for action, the Health Resources and Services Administration Maternal and Child Health Bureau has spearheaded a comprehensive campaign during Black Maternal Health Week aimed at raising awareness, fostering meaningful action, and advocating for positive change in Black maternal health. In response to the disparate maternal mortality rates, the IMPROVE initiative was developed to support research on how to reduce preventable maternal mortality, decrease severe maternal morbidity, and promote health equity. Central to this effort is a toolkit featuring a range of resources strategically designed to empower individuals and organizations, providing them with the tools necessary to amplify their initiatives, forge collaborations, and celebrate achievements in the ongoing quest for equitable maternal health care for Black mothers. It is imperative these efforts continue beyond this designated week, fostering sustained awareness, advocacy, and policy changes to ensure lasting improvements in Black maternal health outcomes.
Looking Forward
In line with ORWH’s mission to advance women in science careers, we launched a prize competition aimed at enhancing gender diversity among faculty members in higher education. This initiative underscores our commitment to breaking down barriers and fostering transformative change. The resulting NIH Prize for Enhancing Faculty Gender Diversity in Biomedical and Behavioral Science has not only encouraged innovative strategies but has also led to the development of a toolkit. This toolkit highlights best practices and links them with evidence of their impact, fostering a culture of accountability and innovation. For more information on how the toolkit was developed, click here .
Looking ahead, ORWH is excited to celebrate National Women’s Health Week by hosting the 8th Annual Vivian W. Pinn Symposium and the first ever NIH Women’s Health Roundtable event. The Vivian W. Pinn Symposium honors the first full-time director of the office, Vivian W. Pinn, M.D., and serves as a critical forum for experts across sectors to communicate and collaborate for the advancement of women’s health. The roundtable “ Future Directions in Menopause Research: Optimizing Midlife Health of Women ,” is scheduled for May 16, 2024, from 11 a.m. to 1 p.m. EDT. This event serves as the launch for the NIH Women’s Health Roundtable Series as part of the White House Women’s Health Research Initiative. The series will engage the extramural research community to bring greater awareness to female-specific health conditions, as well as diseases and conditions that present differently in women. I encourage all researchers, health care professionals, policymakers, and advocates to join us at this impactful roundtable as we collaborate to foster transformative discoveries and interventions in women's health. Your participation and insights are invaluable in driving positive change and advancing our collective mission to shape the future of women’s health research and improve outcomes for women.
The recent initiatives and collaborative efforts serve as a catalyst for continuous dialogue, innovation, and enhancing research. As we navigate the intricate landscape of health care disparities and societal complexities, ORWH’s commitment remains unwavering—to expand and accelerate women’s health research initiatives that generate new data and discoveries which will promote health equity. Together, we strive to create a future where health outcomes are improved for all populations of women, paving the way for more equitable, inclusive health care for everyone.
- Previous Message
- Back to Top
Director’s Messages
April 30, 2024
March 27, 2024
February 27, 2024
January 25, 2024

- U.S. Department of Health & Human Services HHS
- National Institutes of Health NIH
- Division of Program Coordination, Planning, and Strategic Initiatives DPCPSI

Dietary Supplements for Immune Function and Infectious Diseases
This is a general overview. For more in-depth information, see our health professional fact sheet .
How does your immune system work?
Your immune system is made up of cells , tissues , and organs that help fight viruses , bacteria , and other germs that cause infections and other diseases. For example, your skin helps prevent germs from getting inside your body. Cells that line your digestive tract also help protect against harmful germs that cause diseases. White blood cells try to destroy substances they recognize as foreign to your body. Some white blood cells also recognize germs they have been exposed to before and develop antibodies to defend against them in the future.
What do we know about specific dietary supplement ingredients and immune function?
Your immune system needs certain vitamins and minerals to work properly. These include vitamin C , vitamin D , and zinc . Herbal supplements , probiotics, and other dietary supplement ingredients might also affect your immune system.
Eating a variety of nutritious foods can give you enough vitamins, minerals, and other nutrients for a healthy immune system. However, you might wonder whether taking certain dietary supplements can improve your body’s immune system and its ability to fight infections.
This fact sheet describes what we know about the effectiveness and safety of common vitamins, minerals, and other dietary supplement ingredients that might affect immune function .
Dietary supplement ingredients are presented in each section in alphabetical order.
The health professional version of this fact sheet includes more details and references to the scientific literature .
Vitamins and Minerals
Getting enough vitamins and minerals through the foods and beverages you consume is important for a healthy immune system. It’s especially important to get enough of vitamins A, B6, B12, C, D, E, and K as well as folate , copper , iodine , iron , magnesium , selenium , and zinc.
If your diet doesn’t include adequate amounts of certain vitamins and minerals, your immune system will not be able to function as well as it could, you might be more likely to get infections, and you might not recover as well. If your health care provider determines that you are not getting enough of a specific nutrient, vitamin and mineral supplements can help increase intakes to recommended amounts. In most cases, however, if you don’t have a deficiency , increasing your intake of vitamins and minerals through dietary supplements doesn’t help prevent infections or help you recover from them any faster.
Vitamin A is an essential nutrient found in many foods. It exists in two different forms:
- Preformed vitamin A is found in fish, organ meats (such as liver ), dairy products, and eggs.
- Provitamin A carotenoids are turned into vitamin A by your body. They are found in fruits, vegetables, and other plant-based products. The most common provitamin A carotenoid in foods and dietary supplements is beta-carotene .
Vitamin A is important for healthy immune function as well as vision, reproduction, growth, and development.
Vitamin A deficiency is rare in the United States, but it is common in many low- and middle-income countries.
The recommended daily amount (known as Recommended Dietary Allowance or RDA) ranges from 300 to 1,200 microgram (mcg) retinol activity equivalents (RAE) for infants , children, and teens, depending on age, and from 700 to 1,300 mcg RAE for adults.
Does it work?
Diarrhea in children.
Children with a vitamin A deficiency are more likely to get diarrhea caused by germs. These children also have a higher chance of dying of diarrhea, especially in sub-Saharan Africa and south Asia.
Research suggests that vitamin A supplements lower the risk and severity of diarrhea in children in low- and middle-income countries. However, vitamin A supplementation might not help very young infants in these countries.

HIV infection
HIV infection can decrease your appetite and weaken your body’s ability to use nutrients from food. HIV can also increase the risk of related health problems, such as diarrhea and respiratory diseases.
It’s not clear if vitamin A supplements lower the risk of spreading HIV or keep the disease from getting worse. Some studies in young children with HIV have found that vitamin A supplements help lower the risk of death. However, it’s not clear whether vitamin A supplements affect the risk of diarrhea or respiratory infections in young children with HIV. Other studies in adults with HIV have found that vitamin A supplements do not improve immune function.
Research in pregnant people with HIV has found that vitamin A supplements do not help reduce the chance of passing HIV from mother to infant. However, one study found that pregnant people with HIV who took vitamin A were more likely to carry their babies to full-term.
Measles in children
In low- and middle-income countries where vitamin A deficiency is common, children with measles are more likely to have severe symptoms and may die from the disease. In these children, vitamin A supplements might help prevent measles, but it’s unclear whether they lower the risk of dying from measles.
Pneumonia and other respiratory infections in children
Is it safe.
Preformed vitamin A is safe at daily intakes up to 600 to 2,800 mcg for infants, children, and teens, depending on age, and up to 3,000 mcg for adults. There are no upper limits for beta-carotene and other forms of provitamin A.
Getting too much preformed vitamin A can cause severe headache, blurred vision, nausea , dizziness, muscle aches, and problems with coordination. In severe cases, getting too much preformed vitamin A can even lead to coma and death.
If you are pregnant, taking too much preformed vitamin A can cause birth defects, including abnormal eyes, skull, lungs , and heart. If you are or might be pregnant or breastfeeding, you should not take high-dose supplements of preformed vitamin A.
High intakes of beta-carotene (provitamin A) do not cause the same problems as preformed vitamin A. Consuming high amounts of beta-carotene can turn the skin yellow-orange, but this condition is harmless and goes away when you eat less of it. However, several studies have shown that smokers, former smokers, and people exposed to asbestos who take high-dose beta-carotene supplements have a higher risk of lung cancer and death.
Vitamin A supplements might interact with some medications such as orlistat (used for weight loss), acitretin (used to treat psoriasis ), and bexarotene (used to treat the skin effects of T-cell lymphoma ).
More information about vitamin A is available in the ODS consumer fact sheet on vitamin A .
Vitamin C is an essential nutrient found in citrus fruits and many other fruits and vegetables. Vitamin C is an antioxidant and is important for healthy immune function. The body also needs vitamin C to make collagen .
The RDA ranges from 15 to 115 milligrams (mg) for infants, children, and teens, depending on age, and from 75 to 120 mg for nonsmoking adults. People who smoke need 35 mg more than the RDA per day.
Common cold
Taking vitamin C regularly might help decrease cold symptoms and reduce the number of days a cold lasts. It might also help reduce the risk of getting a cold in people who undergo extreme physical stress, such as marathon runners and soldiers stationed in very cold locations. However, taking vitamin C after coming down with a cold may not be helpful.
Research suggests that vitamin C supplements might be more effective in people who do not get enough vitamin C from foods and beverages.
Sepsis (using intravenous vitamin C, not vitamin C supplements)
Sepsis is a life-threatening complication of an infection that can damage the body’s organs and tissues. It’s not clear whether high-dose intravenous (IV) vitamin C helps treat sepsis, and in some cases it might be harmful. In some studies, IV vitamin C reduced the risk of death, but in other studies it did not affect the risk of death or the amount of organ damage. Other research suggests that IV vitamin C might increase the risk of death or organ damage.
Vitamin C is safe at daily intakes up to 400 to 1,800 mg for children and teens, depending on age, and up to 2,000 mg for adults. Taking higher amounts of vitamin C can cause diarrhea, nausea, and stomach cramps, and it might also cause false readings on blood sugar monitors, which are used by people with diabetes . In people with hemochromatosis (an iron overload disorder ), high amounts of vitamin C might cause iron build-up in the body, which can damage body tissues.
Vitamin C supplements might decrease the effectiveness of radiation therapy and chemotherapy .
More information about vitamin C is available in the ODS consumer fact sheet on vitamin C .
For information about vitamin C and COVID-19, see the ODS consumer fact sheet, Dietary Supplements in the Time of COVID-19 .
Vitamin D is an essential nutrient that is naturally present in fatty fish and fish liver oils and in small amounts in beef liver, egg yolks, and cheese. It’s also added to some foods, such as fortified milk. Your body can also make vitamin D when your skin is exposed to the sun. Vitamin D is important for healthy bones and immune function.
The RDA ranges from 10 to 15 mcg (400 International Units [ IU ] to 600 IU) for infants, children, and teens, depending on age, and from 15 to 20 mcg (600 to 800 IU) for adults.
Flu, pneumonia, and other respiratory infections
People with low vitamin D levels might be more likely to get respiratory infections and might have a higher chance of dying from these infections. Some studies suggest that taking vitamin D supplements regularly might slightly reduce the risk of getting a respiratory infection, especially in people with low vitamin D levels. However, other studies have not found that taking vitamin D supplements reduces the risk of respiratory infections. In addition, vitamin D supplements do not appear to help treat respiratory infections.
People with HIV have a higher risk of vitamin D deficiency partly because many HIV medications cause the body to break down vitamin D faster than normal. Having a vitamin D deficiency might also worsen HIV infection. However, studies haven’t shown that vitamin D supplements improve the health of people with HIV.
Vitamin D is safe at daily intakes up to 25 to 100 mcg (1,000 to 4,000 IU) for infants, children, and teens, depending on age, and up to 100 mcg (4,000 IU) for adults. Taking higher amounts can cause nausea, vomiting, muscle weakness, confusion, pain, loss of appetite, dehydration, excessive urination and thirst, and kidney stones . Extremely high doses can cause kidney failure , damaged blood vessels and heart valves, heart rhythm problems, and death.
Vitamin D supplements might interact with some medications such as orlistat (used for weight loss), statins (used to lower cholesterol levels), thiazide diuretics (used for high blood pressure ), and steroids.
More information about vitamin D is available in the ODS consumer fact sheet on vitamin D .
For information about vitamin D and COVID-19, see the ODS consumer fact sheet, Dietary Supplements in the Time of COVID-19 .
Vitamin E (also called alpha-tocopherol ) is an essential nutrient found in nuts, seeds, vegetable oils, and green leafy vegetables. It acts as an antioxidant and helps your immune system function properly. Vitamin E deficiency is rare.
The RDA is 4 to 15 mg for infants, children, and teens, depending on age, and 15 to 19 mg for adults.
Pneumonia and other respiratory infections
It’s not clear whether vitamin E supplements reduce the risk or severity of respiratory infections. Some studies have found that vitamin E supplements might help but others have not, and the effects might depend on whether someone has low vitamin E levels. One study in people who had normal vitamin E levels found that those who took high-dose vitamin E supplements had worse respiratory symptoms and were sick longer.
Vitamin E from food is safe at any level. In supplements, vitamin E is safe at daily intakes up to 200 to 800 mg for children and teens, depending on age, and up to 1,000 mg for adults. Taking higher amounts can increase the risk of bleeding and stroke .
Vitamin E supplements might interact with blood thinners and might reduce the effectiveness of radiation therapy and chemotherapy.
More information about vitamin E is available in the ODS consumer fact sheet on vitamin E .
For information about vitamin E and COVID-19, see the ODS consumer fact sheet, Dietary Supplements in the Time of COVID-19 .
Selenium is an essential mineral found in many foods, including Brazil nuts, seafood, meat, poultry , eggs, dairy products, bread, cereals, and other grain products. It acts as an antioxidant and is important for reproduction, thyroid gland function, and DNA production.
The RDA ranges from 15 to 70 micrograms (mcg) for infants, children, and teens, depending on age, and from 55 to 70 mcg for adults.
People with HIV have higher risk of selenium deficiency than other people, and this might worsen their infection and increase the risk of death. However, it’s not clear whether taking selenium supplements improves the health of people with HIV. Some studies have found that selenium supplements might improve immune function slightly in people with HIV, but other studies have not.
Selenium is safe at daily intakes up to 45 to 400 mcg for infants, children, and teens, depending on age, and up to 400 mcg for adults. Taking higher amounts can cause a garlic odor in the breath, a metallic taste in the mouth, hair and nail loss or brittleness, skin rash, nausea, diarrhea, fatigue , irritability, and nervous system problems.
Selenium might interact with cisplatin (a drug used in chemotherapy).
More information about selenium is available in the ODS consumer fact sheet on selenium .
For information about selenium and COVID-19, see the ODS consumer fact sheet, Dietary Supplements in the Time of COVID-19 .
Zinc is an essential nutrient found in seafood, meat, beans, nuts, whole grains , and dairy products. It’s important for a healthy immune system, making proteins and DNA, healing wounds, and for proper sense of taste.
The RDA ranges from 2 to 13 mg for infants, children, and teens, depending on age, and from 8 to 12 mg for adults.
Some studies suggest that zinc lozenges and zinc syrup speed recovery from the common cold if you start taking them at the start of a cold. However, these products don’t seem to affect the severity of cold symptoms. More research is needed to determine the best dose and form of zinc for the common cold as well as how often and how long it should be taken.
Pneumonia in children
Some studies in lower income countries show that zinc supplements lower the risk of pneumonia in young children. However, zinc doesn’t seem to speed recovery or reduce the number of deaths from pneumonia.
Studies show that zinc supplements help shorten the duration of diarrhea in children in low-income countries, where zinc deficiency is common. The World Health Organization and UNICEF recommend that children with diarrhea take zinc for 10 to 14 days (20 mg/day, or 10 mg/day for infants under 6 months). However, it’s not clear if zinc supplements help children with diarrhea who already get enough zinc, such as most children in the United States.
Many people with HIV have low zinc levels. This occurs because they have trouble absorbing zinc from food and they often have diarrhea, which increases zinc loss. Some studies have found that supplemental zinc decreases diarrhea and complications of HIV, but other studies have not. Zinc supplements do not appear to reduce the risk of death in people with HIV.
Zinc is safe at daily intakes up to 4 to 34 mg for infants, children, and teens, depending on age, and up to 40 mg for adults. Taking higher amounts can cause nausea, vomiting, loss of appetite, stomach cramps, diarrhea, and headaches. High intakes of zinc over a long time can cause low blood levels of copper and impair immune function.
Zinc supplements might interact with antibiotics , penicillamine (used to treat rheumatoid arthritis ), and thiazide diuretics (used to treat high blood pressure).
More information about zinc is available in the ODS consumer fact sheet on zinc .
For information about zinc and COVID-19, see the ODS consumer fact sheet, Dietary Supplements in the Time of COVID-19 .
Andrographis
Andrographis is an herb native to Southeast Asia. It might help your body fight viruses, reduce inflammation , and strengthen your immune system.
Common cold and other respiratory infections
Some studies have found that taking andrographis after getting a cold or other respiratory infection might lessen the severity of symptoms and shorten the length of time symptoms last. However, additional studies are needed to confirm these findings.
No safety concerns have been reported when andrographis is used as directed. Side effects of andrographis can include nausea, vomiting, dizziness, skin rashes, diarrhea, and fatigue.
Andrographis might decrease blood pressure and thin the blood, so it could interact with blood pressure and blood thinning medications.
Andrographis might also decrease the effectiveness of medications that suppress the immune system. Andrographis might affect fertility, so some scientists recommend avoiding it if you are pregnant or planning to have a baby.
For information about andrographis and COVID-19, see the ODS consumer fact sheet, Dietary Supplements in the Time of COVID-19 .
Echinacea is an herb that grows in North America and Europe. It might help stop the growth or spread of some types of viruses and other germs. It might also help strengthen your immune system and reduce inflammation.
Common cold and flu
Studies have found that echinacea might slightly reduce the risk of catching a cold, but it doesn’t reduce the severity of symptoms or shorten the length of time symptoms last.
It’s unclear whether echinacea is helpful for the flu.
Echinacea appears to be safe. Side effects can include stomach upset, diarrhea, trouble sleeping, and skin rashes. In rare cases, echinacea might cause allergic reactions.
Echinacea might reduce the effectiveness of some medications, including medications that suppress the immune system. Scientists don’t know if echinacea is safe to take during pregnancy.
For information about echinacea and COVID-19, see the ODS consumer fact sheet, Dietary Supplements in the Time of COVID-19 .
Elderberry (European Elder)
Elderberry (or elder berry) is the fruit of a tree that grows in North America, Europe, and parts of Africa and Asia. Elderberry might help your body fight viruses and other germs, reduce inflammation, and strengthen your immune system.
Elderberry doesn’t appear to reduce the risk of coming down with the common cold. However, some studies have found that elderberry might help relieve symptoms of colds and flu and help people recover quicker.
Elderberry flowers and ripe fruit appear to be safe to eat. However, the bark, leaves, seeds, and raw or unripe elderberry fruit can be poisonous and can cause nausea, vomiting, diarrhea, and dehydration. Cooked elderberry fruit and properly manufactured supplements do not have this safety concern.
Elderberry might affect insulin and blood sugar levels. It might also reduce the effectiveness of medications that suppress the immune system. Scientists don’t know if elderberry is safe to take during pregnancy.
For information about elderberry and COVID-19, see the ODS consumer fact sheet, Dietary Supplements in the Time of COVID-19 .
Garlic is a vegetable that has been used in cooking throughout history . It is also available as a dietary supplement.
Garlic might help your body fight viruses and other germs.
Only a few studies have looked at whether garlic supplements help prevent the common cold or flu, and it’s not clear if garlic is helpful.
Garlic is considered safe. Side effects can include bad breath, body odor, and skin rash.
Garlic might interact with blood thinners and blood pressure medications.
Ginseng ( Panax ginseng or Panax quinquefolius ) is a plant used in traditional Chinese medicine. It might help your body fight viruses, reduce inflammation, and strengthen your immune system.
Another botanical , eleuthero ( Eleutherococus senticosus ), has sometimes been called Siberian ginseng, but it is not related to true ginseng.
Common cold, flu, and other respiratory infections
Ginseng might reduce the risk of coming down with the common cold, flu, or other respiratory infections. However, it’s unclear whether ginseng helps relieve symptoms or affects the length of time symptoms last.
Ginseng appears to be safe. Side effects can include headache, trouble sleeping, and digestive upset. However, high doses (more than 2.5 grams [g]/day) of ginseng might cause insomnia , rapid heartbeat, high blood pressure, and nervousness.
Ginseng might interact with diabetes medications, stimulants , and medications that suppress the immune system.
For information about ginseng and COVID-19, see the ODS consumer fact sheet, Dietary Supplements in the Time of COVID-19 .
Tea and tea catechins
Tea ( Camellia sinensis ) is a popular beverage that may have health benefits. Tea extracts are also available as dietary supplements.
Green, black, and oolong tea leaves are processed in different ways. Green tea is made from dried and steamed tea leaves, and black and oolong teas are made from fermented tea leaves.
Tea, especially green tea, has high amounts of substances called catechins. Catechins might help fight viruses and other germs.
Flu and other respiratory infections
Based on only a few studies, it’s unclear whether tea or tea catechins are helpful for the flu or other respiratory infections. Some studies have found that tea and tea catechins might reduce the risk of coming down with upper respiratory infections. They might also reduce the length and severity of some symptoms but not other symptoms.
Tea is safe to drink. Side effects of green tea extract can include nausea, constipation , stomach discomfort, and increased blood pressure. Some green tea extracts might damage your liver, especially if you take them on an empty stomach.
Tea also contains caffeine, which can disturb your sleep and cause nervousness, jitteriness, and shakiness. Safe doses of caffeine for healthy adults are up to 400 to 500 mg/day and up to 200 mg/day for people who are pregnant.
Tea might interact with atorvastatin (a cholesterol-lowering drug) and stimulants, such as bitter orange or ephedrine.
Other Ingredients
Glutamine is an amino acid found in many foods including beef, fish, poultry, dried beans, eggs, rice, grains, and dairy products. Your body makes enough glutamine to meet your needs, except under rare conditions (for example, if you are critically ill in an intensive care unit [ICU] or have had major surgery).
Glutamine helps your immune system work properly.
Critical illness (giving glutamine as an IV or tube feeding)
It’s unclear whether glutamine helps people who are critically ill. Some studies in hospitalized patients who were critically ill or had undergone major surgery found that glutamine given as an IV or tube feeding reduced the risk of getting an infection, but it did not reduce the risk of death.
Glutamine is considered safe. Side effects can include nausea, bloating, burping, pain, gas, and vomiting. These side effects are more likely to occur with higher doses of glutamine.
No interactions between glutamine and medications have been reported.
N-acetylcysteine and glutathione
N-acetylcysteine (NAC) is similar to cysteine, an amino acid. It acts as an antioxidant and helps reduce mucus in the respiratory tract .
NAC raises levels in your body of a substance called glutathione, which also acts as an antioxidant. NAC and glutathione might also help your body fight viruses and other germs, reduce inflammation, and strengthen your immune system.
People with HIV may have low levels of glutathione, which might increase the risk of certain diseases including tuberculosis . However, there is very little research on NAC supplements in people with HIV. Therefore, scientists don’t know whether it’s helpful.
NAC appears to be safe. Side effects can include nausea, vomiting, stomach pain, diarrhea, indigestion, and heartburn.
NAC might interact with blood thinners and blood pressure medications. Taking NAC with nitroglycerine (used to treat chest pain) might cause low blood pressure and severe headaches.
For information about NAC and COVID-19, see the ODS consumer fact sheet, Dietary Supplements in the Time of COVID-19 .
Omega-3 fatty acids
Omega-3s are types of fats, including alpha linolenic acid (ALA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA). ALA is found mainly in plant oils, such as flaxseed, soybean , and canola oils. EPA and DHA are found mainly in fatty fish and fish oils.
Omega-3s are important for healthy cell membranes and proper function of the heart, lungs, brain, immune system, and endocrine system .
The recommended amount of omega-3s for infants is 0.5 g per day, and 0.7 to 1.6 g per day of ALA for children, teens, and adults, depending on age. EPA and DHA do not have individual recommendations.
Omega-3s might help your body fight viruses and other germs, reduce inflammation, and strengthen your immune system.
Acute respiratory distress syndrome (giving omega-3s as an IV or tube feeding)
Acute respiratory distress syndrome (ARDS) is a serious lung condition that can lead to death. In people who do recover, ARDS often causes long-term physical and mental health problems.
Researchers have studied whether giving omega-3s as an IV or tube feeding is helpful for people with ARDS, but results from these studies are not clear. Some studies have found that omega-3s given in this manner might help the lungs work better, but they don’t appear to lower the risk of dying from ARDS. In addition, it’s not clear whether omega-3s given in this manner affect the length of time people are hospitalized with ARDS and need a ventilator to help them breathe.
Respiratory infections in infants and young children
The immune system continues to develop in babies after birth, and their immune cells contain the omega-3s EPA and DHA. However, it’s not clear whether adding omega-3s to infant formula improves immune function or reduces the risk of getting respiratory infections.
A study in school-age children found that children who consumed milk with added EPA and DHA had fewer upper respiratory infections than those who did not consume omega-3s. In another study, however, using an infant formula containing DHA and another fatty acid had no effect on the risk of respiratory infections in infants.
Omega-3s are considered safe. Side effects can include a bad taste in the mouth, bad breath, heartburn, nausea, digestive discomfort, diarrhea, headache, and smelly sweat. Omega-3s might interact with blood thinners, blood pressure medications, and medications that suppress the immune system.
More information about omega-3s is available in the ODS consumer fact sheet on omega-3 fatty acids .
For information about omega-3s and COVID-19, see the ODS consumer fact sheet, Dietary Supplements in the Time of COVID-19 .
Probiotics are live microorganisms (bacteria and yeasts) that provide health benefits. They are naturally present in certain fermented foods, added to some food products, and available as dietary supplements. Probiotics act mostly in the stomach and intestines . They might improve immune function and help fight viruses.
Acute diarrhea in infants and children
Acute infectious diarrhea in infants and children causes loose or liquid stools and three or more bowel movements within 24 hours. This condition is often caused by a viral infection and can last for up to a week. Some infants and children also develop fever and vomiting. Some studies have shown that probiotics shorten acute diarrhea by about 1 day, but other studies do not.
Some studies have reported that two strains of probiotics— Lactobacillus rhamnosus GG (LGG) and Saccharomyces boulardii —were most likely to benefit children with acute infectious diarrhea, but other studies have not.
Probiotics might reduce the risk of some respiratory infections and shorten the length of illness. Some studies in infants, children, and adults have found that probiotics reduce the risk of getting a cold and help relieve some symptoms, such as fever and cough. Other studies in children reported fewer sick days from school and quicker recovery. However, formulations of probiotics vary, and the effects of one product may not be the same as another.
Ventilator-associated pneumonia
It’s not clear whether probiotics help people who are critically ill. Some studies have found that probiotics lower the risk of developing pneumonia in people who are critically ill and need a ventilator to help them breathe, but other studies have not.
Probiotics are considered safe for most people. Side effects can include gas and other digestive symptoms. In people who are very ill or have immune system problems, probiotics might cause severe illness. Probiotics might also cause infections or even life-threatening illness in preterm infants. Although probiotics don’t appear to interact with medications, taking antibiotics or antifungal medications might decrease the effectiveness of some probiotics.
More information about probiotics is available in the ODS consumer fact sheet on probiotics .
For information about probiotics and COVID-19, see the ODS consumer fact sheet, Dietary Supplements in the Time of COVID-19 .
Do dietary supplements interact with medications or other supplements?
Yes, some supplements can interact or interfere with medicines you take.
Tell your doctor, pharmacist , and other health care providers about any dietary supplements and prescription or over-the-counter medicines you take. They can tell you if the dietary supplements might interact with your medicines or if the medicines might interfere with how your body absorbs , uses, or breaks down nutrients.
Where can I find out more about dietary supplements and immune function?
- Office of Dietary Supplements (ODS) Health Professional Fact Sheet on Dietary Supplements for Immune Function and Infectious Diseases
- Herbs at a Glance , National Center for Complementary and Integrative Health
- ODS Frequently Asked Questions: Which brand(s) of dietary supplements should I purchase?
This fact sheet by the National Institutes of Health (NIH) Office of Dietary Supplements (ODS) provides information that should not take the place of medical advice. We encourage you to talk to your health care providers (doctor, registered dietitian, pharmacist, etc.) about your interest in, questions about, or use of dietary supplements and what may be best for your overall health. Any mention in this publication of a specific brand name is not an endorsement of the product.
Updated: November 14, 2023

Transforming the understanding and treatment of mental illnesses.
Información en español
Celebrating 75 Years! Learn More >>
- Science News
- Meetings and Events
- Social Media
- Press Resources
- Email Updates
- Innovation Speaker Series
Mental Health Matters Podcast: Pathways to Recovery: Psychosis and Schizophrenia
May 9, 2024 • 75th Anniversary
Subscribe to the Mental Health Matters Podcast
Episode summary.
Psychosis, a condition marked by a loss of touch with reality, is distressing for those who experience it and their loved ones. If left untreated, psychosis can have serious impacts on people's lives. But the good news is there's hope. In this episode, we talk with Dr. Robert Heinssen, a leader in the development and adoption of coordinated specialty care for treating psychosis. We learn about the signs and symptoms of psychosis, discuss coordinated specialty care, and explore how NIMH research in psychosis and schizophrenia fundamentally changed the health care landscape.
Dr. Heinssen : People may start not so much by hearing voices, but they might hear sounds or their name being called, and they can look around and check and see, "Well, I don't know where that came from." They may become a little bit anxious and attentive of what's going on in their environment, and maybe they start being a little afraid and suspicious that people around them might be observing them or monitoring them in some way. So changes in all of those areas would be some of the early signs that something is amiss.
Dr. Gordon : Psychosis, a condition marked by a loss of touch with reality, is distressing for both those who experience it and their loved ones. If left untreated, psychosis can have serious impacts on people's lives. But the good news is there's hope. Hello, and welcome to Mental Health Matters, a National Institute of Mental Health podcast. I'm Dr. Joshua Gordon, Director of NIMH, and today we'll be talking with Dr. Robert Heinssen, a leader in the development and adoption of coordinated specialty care for treating psychosis. In this episode, we'll learn about the signs and symptoms of psychosis, talk about coordinated specialty care, and discuss how NIMH research in psychosis and schizophrenia fundamentally changed the healthcare landscape. Welcome to Dr. Robert Heinssen of the National Institute of Mental Health. Bob, it's a pleasure to have you here today.
Dr. Heinssen : Dr. Gordon, thank you for this opportunity, and I am equally pleased to be here with you.
Dr. Gordon : Today we're going to talk about psychosis, which is a serious mental condition, of course. Can you talk about what that is exactly?
Dr. Heinssen : Sure. So, psychosis is a condition that affects an individual's perception of reality, their thinking, and their functioning. So to unpack that a little bit, people who are experiencing psychosis may see or hear things that aren't apparent to other individuals. They may have difficulties in their thinking in terms of memory or concentration. Those difficulties may impede their ability to converse with somebody in a fluent way, which has some impact on the person's social relationships, interpersonal relationships. And that can get to be a problem in situations like school and work.
Dr. Gordon : It sounds like it's a real challenge and a burden for those who have it. What causes psychosis?
Dr. Heinssen : Well, there are a variety of pathways to psychosis. You could have a medical condition or an acute infection, a fever that would, in some cases, cause some of these symptoms. Sometimes abuse of substances or alcohol can cause psychotic symptoms. And there are some conditions that are independent of those causes that are mental disorders that start usually in late adolescence and, if untreated, can progress into the person's young adult and adult life.
Dr. Gordon : One of the mental illnesses we often think of as associated with psychosis is schizophrenia. Can you talk about the relationship between psychosis in general and schizophrenia specifically?
Dr. Heinssen : Sure. So, schizophrenia is a very disabling condition, but it includes among the symptoms of that condition, psychosis is a central feature. To make a diagnosis of schizophrenia, professionals require a period of time where psychotic symptoms are present before they'll make the determination that it's clearly schizophrenia. So, for a young person, some of those cognitive problems and perceptual problems might become a barrier to them functioning effectively in a school situation. The anxiety and distress that they feel may cause them to withdraw from other people around them, their friends, other students, even their family members. And that would create the social difficulty and isolation that is often seen later in schizophrenia. And again, without intervention, this can spiral into a set of symptoms and then functional problems that really impede the person's ability to achieve expected developmental milestones of education, relationships, work, and so forth.
Dr. Gordon : So psychosis, and in particular the psychosis that accompanies schizophrenia can be devastating. It can really prevent people from being able to lead normal lives.
Dr. Heinssen : I think devastating is the right word. It's devastating for the individual who is experiencing these symptoms. It's devastating to family members who observe changes and often are unsure of what is driving those changes.
Dr. Gordon : What's one thing you'd want people to understand about psychosis?
Dr. Heinssen : One thing I'd want people to understand is that behind the symptoms are human beings that have hopes and dreams and aspirations just like the rest of us. The symptoms sometimes create a barrier between the person with psychosis and others. If you look behind those symptoms, you see the promise of a human being, of an individual who wants the same things that you want out of life and has the same aspirations, the same type of goals, and the same type of prospects. So if we look beyond the symptoms to the human being, we'll perhaps have more compassion to offer this kind of assistance that help people get to the kind of futures that we all want.
Dr. Gordon : Can people recover from a psychotic episode? Can people get better?
Dr. Heinssen : So, Josh, a little bit of history here. If you asked me that question in 1990, I, like most mental health professionals, would say, "Well, people will get better with treatment. Their chances for full recovery are probably slight." And that was the message that healthcare professionals delivered in the late 1980s, early 1990s. But through research that has identified a critical period for intervention in the early stages of psychosis, we now have a very different story, a much more optimistic story that early intervention can really help people gain control of these symptoms, to get back on track with things like school and relationships and work. And with enduring care, over time, they can expect to lead productive, fulfilling, and meaningful life. So yes. Short answer, yes, recovery is possible.
Dr. Gordon : Tell me about ways that we might be able to help people recognize that something is wrong.
Dr. Heinssen : It's not always clear that something that it's a start of an illness or a disorder, it could just be people might say the child is going through a phase or having a rough spot, but educating the individuals who are experiencing the condition, the parents or caregivers who were surrounding them, the health care providers that would be the first professionals to come in contact and then mobilizing our healthcare system so that there are open doors when individuals present for a consultation and potential care that they have rapid access to those services, that the services can rapidly perform an evaluation and then, when indicated, can rapidly start the treatment. All of those things reduce the interval between the onset of symptoms and the initiation of treatment. And we know from NIMH-supported research that intervals, often called the duration of untreated psychosis, is an important factor in determining how well people will respond to treatment and to what extent they will recover in the long term.
Dr. Gordon : So it's important to detect risk for psychosis early because getting people into treatment early means that they can have better outcomes.
Dr. Heinssen : Yes, time is our enemy. The faster we can identify and intervene, the much more potent are our existing interventions.
Dr. Gordon : Let's say someone does have psychosis. They have schizophrenia, so not necessarily the first episode. What's the role of medication and other forms of treatment for individuals with psychosis?
Dr. Heinssen : We do have individual treatments that do help with components of the experience of psychosis. So antipsychotic medications are an effective strategy for dealing with some of the perceptual problems, the hallucinations that people might be seeing or hearing things that others don't see. They are all also affected with some of the distortions and thinking, some of these ideas that others are monitoring them, there are suspiciousness or worries in that regard. Antipsychotic medications can help with that component of psychosis. Psychotherapies, particularly cognitive and behavioral psychotherapies, are a very effective way of dealing with the distress that people feel and for managing the disruption that has occurred in their lives.
The cognitive and behavioral therapies can help the person, one, come to terms with the experience that they're having. It can reinstill an optimism that recovery is possible and it can help them map out a treatment plan that will set out a roadmap and goals for resuming their normal activities. In addition to that, we know that family education and support is very crucial in helping the family be a support to the individual and help them as they negotiate or navigate their recovery from these episodes.
Dr. Gordon : An important effort in understanding how best to care for these young people who are experiencing their first episode of psychosis was funded by NIMH. The recovery after an initial schizophrenia episode or RAISE Study. What were the main findings? What was involved in getting that off the ground?
Dr. Heinssen : So, a little historical context. By the late 1990s, researchers throughout the world were actually recognizing this idea that early intervention in this critical window could make a real difference. It could help people recover from their initial episode of psychosis and could put them onto a path of more normal functioning. So there were a number of research studies that were exploring how this could be done, and in those, they were testing various models that had these multiple components to address the various symptoms of psychosis. So a number of us at NIMH were looking at these results and thinking, if this could work in the United States, this really could be a very big advance for young people with psychosis and their families.
So three research aims, feasibility, effectiveness, and scalability, were at the heart of the recovery after an initial schizophrenia episode initiative. And NIMH launched that in 2008 and we ended up funding two studies. One of them was a comparative effectiveness study. This took place in 34 community clinics throughout the United States. And 17 of these clinic programs were assigned to that type of coordinated treatment. And then the remaining 17 clinics provided treatment as usual. That study addressed the questions of feasibility and effectiveness. And then a second study, an implementation study, explored what would be potential barriers to implementing such a coordinated approach in public health settings across the U.S.
And that study identified barriers, but more importantly, they developed approaches to be able to surmount those barriers and make it possible that this intervention would be able to be delivered with fidelity and to be done on a broad scale. We had stunning success in both studies. Coordinated treatment was more effective than treatment as usual in terms of improving quality of life and reducing distressing symptoms, and helping individuals return to school and work. These programs led to the adoption of coordinated specialty care by the states of New York and Maryland immediately after the research was concluded. So those two studies set a foundation for a broad implementation of this new approach across the United States.
Dr. Gordon : Can you talk about what effect the RAISE Study has had on mental health care, in particular for health care for first-episode psychosis in the U.S.?
Dr. Heinssen : Sure. I'll start this by saying that NIMH science has been a very critical element in this success story. But it's one element and one thing that I learned is as terrific as our science is, if we don't have partnerships with key stakeholders, it's not a given that science will actually make its way into the healthcare system. So in this case, we did this study in the context of growing awareness among federal partners, among advocacy groups, among private foundations, that early intervention was really a new concept that should be exploited.
And we worked very hard with all of those stakeholders to be able to translate the science into new clinical practice. And hats off to our partners at the Substance Abuse and Mental Health Services Administration who embraced this new scientific approach and partnered with us in disseminating the new approaches, in training people in being able to implement them, and then providing the resources or channeling the resources that Congress provided through their community mental health block grant to fund these new programs, these coordinated specialty care programs via a set aside to their block grant program.
So all of this came together, the science, the partnerships, and the coordinated effort to move the science into practice. And today, it's a much different world. In 2008, we know that there were only two states that had committed to early intervention as a state policy in mental health care. And we estimate that there were only about a dozen high-quality specialty care programs in the United States at that time. A dozen years later, there are over 350 of these programs in all 50 states, and they today serve tens of thousands of young people each year.
Dr. Gordon : Can you compare what treatment and prognosis is like for individuals experiencing their first episode of psychosis before and after RAISE?
Dr. Heinssen : So if we look back in the United States in the late 1990s and early 2000s, in academic circles, there was recognition that early intervention was the way to go, and people were studying ways to do that, but that message had not gone to the broader community. So if you were somebody experienced a first episode psychosis, in all likelihood, your symptoms would really not be recognized until some sort of crisis occurred that required either an emergency department visit or an unplanned inpatient treatment, or in some cases, contact with the police that might result in arrest and involuntary commitment to one of these treatment facilities.
Once you got into treatment, the chances were that you were going to be evaluated and treated by somebody who didn't have a wealth of experience in early psychosis. So your care would likely be fragmented. It may not have been up to the guidelines that existed for medication treatment at that point, and it would not have been continuous. You would have been discharged from an inpatient facility and left on your own, perhaps with the help of your family, to try to navigate outpatient services. Now, today, the best-case scenario is very different. In these programs that have established referral networks in the community, very often, a person can be referred to a first-episode psychosis program before an initial hospitalization. So the differences are really kind of night and day, and the outcomes are also night and day.
Dr. Gordon : Early intervention is key. Before RAISE, before these clinics, did people have to wait a long time for care in order to get it?
Dr. Heinssen : Yeah. In the United States, believe it or not, a person could be psychotic for anywhere between one and three years.
Dr. Gordon : Years?
Dr. Heinssen : Years.
Dr. Gordon : Wow.
Dr. Heinssen : In the RAISE program, the average amount of time a person waited was 18 months. And think about that. Psychotic symptoms, they're very dramatic, and they're very disabling, and they are associated with a lot of distress and pain. And for people experiencing those symptoms for that period of time, it's just astounding that was the state of care.
Dr. Gordon : So RAISE showed us that we can reduce the time to treat psychosis, and we can get people better and keep them better if we get them into a coordinated specialty care treatment and ensure continuity of care. What has NIMH been up to try to solidify that success of RAISE, to make sure that we're doing the best we can to treat individuals in their first episode of psychosis?
Dr. Heinssen : So the results of the RAISE study, the principal results started to become available around 2014, 2015. I think at that point there were maybe somewhere around 50 or 60 of these programs nationwide. So we started thinking there's 50 or 60 of these programs, and it would be great if we had 50 excellent programs in the United States. But imagine what would happen if you linked those programs together so that they could talk to one another, they could share data, they could share learning. So that became the beginning of an idea that we imagined, an Early Psychosis Intervention Network, or EPINET. And the idea started in 2015. And then we took some lessons learned from the RAISE program in developing that idea. We started funding regional networks in 2019.
So this would be a network of like-minded programs that offered services within a defined area. And then we have linked those regional networks through a national data coordinating center. And so together, this enterprise embraces 8 regional networks, 101 community clinics throughout the United States, well, in 17 states. And we're anticipating that somewhere between 3,000 and 4,000 young people with first-episode psychosis will be enrolled in these programs.
Dr. Gordon : Wow. So 100 clinics, thousands of individuals, all participating in an effort to really understand how best to take care of people with first-episode psychosis, what are they learning?
Dr. Heinssen : I like to think of this. If you're a person with cancer and you go to a cancer clinic that's affiliated with a research program, you go in there and you know that information about your care is going to be utilized by that clinic to help them improve the quality of the care that they offer and then also to offer you opportunities to participate in research that may benefit others by generating increased knowledge about cancer and its treatment. We're building that same kind of culture within the EPINET clinics that people come in there and they're first struck with this idea that this is a different experience. The person who's entering this program will know that I'm in a program that looks at its procedures continuously with an eye towards improvement. That's the kind of system that a person will be entering in.
Dr. Gordon : So these learning health care systems, these clinics, they're not just providing care, they're asking the questions that will help them provide better care in the future. Bob, what's it like for a patient who's in one of these first episode psychosis clinics, from their point of view, what are they getting?
Dr. Heinssen : So they're getting access to a treatment team. So it all starts with conversations about what's happened and what's been disrupted for you. What would you like to return to or what would you like to get out of treatment? And then here are some of the tools that we have that we can make available to you. And then that conversation with the treatment team leads to an individualized treatment plan that usually is a combination of the medication, the psychotherapy, the family intervention, and these rehabilitation or supported employment and education activities. Then the interesting thing, what I hear from people who run these programs, young people are interested in getting back on track with their lives. And they embrace this as their job. Their job is to get better and get back on track.
Dr. Gordon : So how long will a person stay in one of these first-episode psychosis clinics? How long will they be in a program like that?
Dr. Heinssen : I would say between one and three years is probably typical. Most programs organize themselves around two years. Important to note that the treatment plans are individualized. So this is not like you're going through a program and that you have the same program over the course of a year or two years. You have an individualized treatment plan, and that plan adjusts continuously based on your recovery, your emerging needs, and so forth.
Dr. Gordon : What inspires you as a scientist?
Dr. Heinssen : So before I came to NIMH, I worked in a psychiatric hospital that was the center of a community treatment program for serious mental illness, for schizophrenia. And I ran a treatment program for adults with serious mental illness, several hundred adults. And these programs, I thought they were quite good in embracing a lot of the science-based treatments that were available at that time. I was very proud of the fact that we were able to move people out of hospital settings into the community and to help them lead independent lives in the community. One day, I was giving a talk about this treatment program and what was available to people with serious mental illness, and I gave the talk. I was very proud to talk about our programs, about how they were science-based, the outcomes we had achieved. And I was feeling very good about this meeting. There was a long line of people who wanted to speak with me afterwards.
The last person was somebody who looked very familiar to me. The woman introduced herself and she said, " Dr. Heinssen , you might remember me. My son so-and-so was in your program." I immediately remembered who she was talking about, and I was thinking, "Boy, this is going to be great. He did so well." He was a young man who had had schizophrenia for several years by the time he came into our program. But we had helped him to achieve his goal of returning to college. He was going to community college. He was taking a course. He was living in an assisted living facility, but he was living in the community. And he was also working part-time in a grocery store. And I thought this was a great...this is a great outcome. And I was waiting for this feedback, boy, what a great outcome. And the woman started crying and she said, "I know I should be grateful, he's doing so much better than he was but we had hoped for so much more."
And that really arrested me thinking that on the one hand, we did the best that we could do given the current state of knowledge, but hubris was not called for in this situation. Humility was called for. I had the opportunity to come to NIMH very shortly after that, and when I had the opportunity to jump on to this early intervention research, that mother's story was in the back of my mind. Her voice was ringing in my ears that we had hoped for so much more. And I thought, "There's so much more that we need to do." That was the initial impetus and that's been the thing that has kept me in the race for as long as I have been, that it is only through research and then through the hard work of implementing the research that we can hope for better outcomes. That really has been the motivation for me over these 22 years. So to that woman, if you hear this podcast, thank you very much. You changed the whole trajectory of my career.
Dr. Gordon : This concludes this episode of Mental Health Matters. I'd like to thank our guest, Dr. Robert Heinssen, for joining us today. And I'd like to thank you for listening. If you enjoyed this podcast, please subscribe and tell a friend to tune in. If you'd like to know more about psychosis or coordinated specialty care, please visit nimh.gov. We hope you'll join us for the next podcast.
Faster approach for starting extended-release naltrexone to treat opioid use disorder shown effective
NIH-supported clinical trial addresses important barrier to opioid use disorder treatment

Starting people with opioid use disorder on extended-release, injectable naltrexone (XR-naltrexone) within five to seven days of seeking treatment is more effective than the standard treatment method of starting within 10-15 days, but requires closer medical supervision, according to results from a clinical trial supported by the National Institutes of Health’s (NIH) National Institute on Drug Abuse (NIDA). Published in JAMA Network Open , the findings suggest that this rapid treatment protocol could make XR-naltrexone more viable as a treatment option for opioid use disorder, which continues to take lives at an alarming rate.
“When someone is ready to seek treatment for opioid use disorder, it is crucial that they receive it as quickly as possible,” said Nora Volkow, M.D., NIDA director. “This study paves the way for more timely care with one of the three medications for opioid use disorder we have available, better supporting people in their ability to choose the treatment option that will work best for them.”
XR-naltrexone is one of three Food and Drug Administration-approved medications for the treatment of opioid use disorder . It works by binding to and blocking opioid receptors in the brain, which reduces opioid cravings and prevents the euphoric and sedative effects of opioids. However, starting treatment with XR-naltrexone has traditionally required patients to go through a seven to 10-day opioid-free period, to avoid experiencing painful withdrawal symptoms caused when naltrexone abruptly stops the effects of opioids in the brain. During this waiting period, patients are at high risk of returning to opioid use or discontinuing treatment. This has been a significant barrier to implementation of XR-naltrexone.
To address this challenge, researchers tested the effectiveness of a more rapid procedure to start people with opioid use disorder on XR-naltrexone. Between March 2021 and September 2022, the study enrolled and followed 415 patients with opioid use disorder who were admitted at six community-based inpatient addiction facilities across the U.S. and who chose treatment with XR-naltrexone. Every 14 weeks, the sites were randomized to either provide the standard XR-naltrexone procedure, or the more rapid procedure.
In the study, standard XR-naltrexone prescribing included a three- to five-day treatment period with buprenorphine to ease withdrawal symptoms, followed by a seven- to 10-day opioid-free period. The rapid procedure consisted of one day of buprenorphine (up to 10 mg), a 24-hour opioid-free period, and a gradual increase in low-dose oral naltrexone for three to four days prior to getting an injection of XR-naltrexone. Doctors also used medications such as clonidine and clonazepam throughout the process to manage withdrawal symptoms.
The study found that patients on the rapid five to seven-day treatment procedure were significantly more likely to receive a first injection of XR-naltrexone compared to those on the standard seven to 15-day treatment procedure (62.7% vs. 35.8%). Withdrawal severity was generally low and comparable across the two groups. Targeted safety events and serious adverse events (such as a fall or overdose) were infrequent overall but occurred more on rapid procedure (5.3% and 6.7%) than on standard procedure (2.1% and 1.6%), and the rapid procedure required more staff attention. This indicates that closer monitoring and greater clinical expertise may be needed if patients start treatment with the rapid procedure.
Though the shorter wait-time improved the proportion of people who started on XR-naltrexone overall, these findings underscore that challenges remain in starting patients on XR-naltrexone and also keeping them in treatment long term. Across both the standard and rapid procedures, the most commonly reported reason that participants did not receive a first dose of XR-naltrexone was that they chose to leave the treatment unit early. The authors also note that only about 10% of all patients entering treatment chose XR-naltrexone. These findings reaffirm that a small but sizable proportion of people with opioid use disorder do opt for treatment with XR-naltrexone when presented with all three medication choices, and that it is important to support research into making this evidence-based treatment option more viable for those who choose it.
“Time has been an important barrier that we’ve seen hinder the use of extended-release naltrexone for opioid use disorder in the past, both among individuals and treatment providers,” said Matisyahu Shulman, M.D., a clinician researcher at New York State Psychiatric Institute and Columbia University Irving Medical Center, New York City, and lead author on the study. “We hope that these findings can help encourage more treatment settings to offer extended-release naltrexone as a safe and effective option for patients, to help prevent overdose and support recovery.”
The authors note that future studies should explore sustainability, feasibility, and health economic aspects of this more rapid treatment protocol for XR-naltrexone. Despite cost savings from fewer days on the rapid procedure, the resources needed for intensive monitoring should also be considered.
In 2022, over 107,000 people died of a drug overdose, with 75% of those deaths involving an opioid. The overall rise in overdose deaths is largely attributable to the proliferation in the drug supply of illicit fentanyl, a highly potent synthetic opioid. Decades of research have shown the overwhelming benefit of three existing medications for opioid use disorder: methadone, buprenorphine, and XR-naltrexone.
The study, known as the Surmounting Withdrawal to Initiate Fast Treatment with Naltrexone (SWIFT) study , was conducted at six sites within the NIDA Clinical Trials Network and funded through NIH’s Helping to End Addiction Long-Term Initiative (or NIH HEAL initiative) . The study was led by researchers at New York State Psychiatric Institute and Columbia University Irving Medical Center.
If you or someone you know is struggling or in crisis, help is available. Call or text 988 or chat at 988lifeline.org . To learn how to get support for mental health, drug or alcohol issues, visit FindSupport.gov . If you are ready to locate a treatment facility or provider, you can go directly to FindTreatment.gov or call 800-662-HELP (4357) .
- M Shulman, et al. Rapid Initiation of Injection Naltrexone for Opioid Use Disorder: A Stepped-wedge, Cluster-Randomized Clinical Trial . JAMA Network Open . DOI: 10.1001/jamanetworkopen.2024.9744 (2024)..
About the National Institute on Drug Abuse (NIDA): NIDA is a component of the National Institutes of Health, U.S. Department of Health and Human Services. NIDA supports most of the world’s research on the health aspects of drug use and addiction. The Institute carries out a large variety of programs to inform policy, improve practice, and advance addiction science. For more information about NIDA and its programs, visit www.nida.nih.gov .
About the National Institutes of Health (NIH): NIH, the nation’s medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit www.nih.gov .
About substance use disorders: Substance use disorders are chronic, treatable conditions from which people can recover. In 2022, nearly 49 million people in the United States had at least one substance use disorder. Substance use disorders are defined in part by continued use of substances despite negative consequences. They are also relapsing conditions, in which periods of abstinence (not using substances) can be followed by a return to use. Stigma can make individuals with substance use disorders less likely to seek treatment. Using preferred language can help accurately report on substance use and addiction. View NIDA’s online guide .
NIH…Turning Discovery Into Health®
Related Articles

Analysis of social media language using AI models predicts depression severity for white Americans, but not Black Americans

Law enforcement seizures of psilocybin mushrooms rose dramatically between 2017-2022

Reduced drug use is a meaningful treatment outcome for people with stimulant use disorders

IMAGES
VIDEO
COMMENTS
The current plan, FY 2021-2025: NIH Strategic Plan for HIV and HIV-Related Research, focuses on four strategic goals: Advance rigorous and innovative research to end the HIV pandemic and improve the health of people with, at risk for, or affected by HIV across the lifespan. Ensure that the NIH HIV research portfolio remains flexible and ...
The Plan informs the scientific community, the public, Congress, people with HIV, and organizations working in HIV about the NIH HIV/AIDS research agenda. Per the NIH Revitalization Act of 1993, the Plan is reviewed annually and revised as appropriate. FY 2019−2020. (PDF, 2.1MB) FY 2021−2025. (PDF, 4.8MB)
The FY 2021-2025 Plan serves as the guiding framework for OAR to allocate funds to the NIH Institutes, Centers, and Offices that advance the NIH-wide HIV research agenda and ensure investment of resources in the highest priority areas. For example, one emergent activity utilizing the Plan is NIH-wide support for the Ending the HIV Epidemic: A ...
The NIH Office of AIDS Research (OAR) leads the effort across NIH to establish HIV research priorities and develop the NIH Strategic Plan for HIV and HIV-Related Research This plan guides the largest public investment in HIV research, building on scientific progress and opportunities for advancing research to end to the HIV pandemic.. As part of the process, OAR seeks input from all interested ...
The NIH Office of AIDS Research (OAR) presents the NIH Strategic Plan for HIV and HIV-Related Research (the Plan) for fiscal years (FY) 2021-2025. The Plan serves as the guiding framework for OAR to allocate funds that advance the NIH-wide HIV research agenda and ensure investment of resources in the highest priority areas of scientific ...
The NIH Office of AIDS Research (OAR) is seeking input to inform the development of the next multi-year NIH Strategic Plan for HIV and HIV-Related Research, which will span 2026-2030. OAR leads the effort across NIH Institutes, Centers, and Offices to establish HIV research priorities and develop the Strategic Plan.
NIH Strategic Plan for HIV and HIV-Related Research NIH Strategic Plan for HIV and HIV-Related Research. Webpage (HTML) Topic. Funding Opportunities. Tools and Information Services. Research. Research Organizations. Prevention Research. Treatment Research. Vaccine Research. Resource Link.
Request for Information (RFI) To Inform Development of the FY 2026-2030 NIH Strategic Plan for HIV and HIV-Related Research. A Notice by the National Institutes of Health on 02/15/2024. ... and other interested constituents on the development of the fiscal year (FY) 2026-2030 NIH Strategic Plan for HIV and HIV-Related Research (the Plan).
Snapshot of NIH HIV-Related Stigma Research. Stigma is a key research area within the NIH HIV Research Program as stated in the FY 2021 - 2025 NIH Strategic Plan for HIV and HIV-Related Research [] and as reflected in the increase of NIH-funded projects in this area of science in recent fiscal years (FYs).The NIH OAR, which oversees, coordinates, and manages the NIH HIV Research Program ...
Substance use plays a significant role in HIV transmission and health outcomes for people living with HIV. The opioid crisis has been associated with marked increases in the number of people who inject drugs in the United States. This has resulted in several localized HIV transmission hot spots, driven by risky injection practices, poor support for syringe exchange programs, and limited access ...
The update of the NIH HIV research priorities was informed by stakeholder input via a 2019 request for information NOT-OD-19-078, Listening Sessions hosted by OAR at sites in different locations, the OAR Advisory Council and the NIH AIDS Executive Committee, the NIH Plan for HIV and HIV-Related Research, and input from NIH leadership ...
Applications should focus on one or more Key HIV Research Areas as outlined in the FY2021-2025 NIH Strategic Plan for HIV and HIV-Related Research. Applications must be focused on HIV-relevant research for consideration through this NOSI. ... should ensure that the proposed work is aligned with at least one goal and objective outlined in the ...
OAR proposes a new framework based on the HIV research-to-practice continuum for priority setting. A New Framework for NIH HIV Research OAR is adopting a new framework for the next Strategic Plan (FY 2026‒2030) that consists of four strategic goals: Goal 1. Enhance discovery and advance HIV science through fundamental research.
Two newly published research funding opportunities from NIH are now available to support the advancement of HIV service delivery through pharmacies and pharmacists. This notice of funding opportunity (NOFO) solicits research designed to capacitate, transform, and scale the delivery of HIV testing, prevention, and care services through ...
We strongly encourage potential applicants to arrange a prior consultation with Dr. Betty Poon, our scientific/research contact for this initiative, at [email protected] or 240-669-5024. In the prior consultation, we can advise whether your proposed program meets the goals of this NOFO and discuss matters related to responsiveness.
Evaluating the efficacy and effectiveness of interventions for reducing alcohol misuse in improving co-occurring pain, stress, and other mental health concerns among people living with HIV. Cross-cutting research programs are NIAAA-managed research portfolios that include topics spanning multiple research goals outlined in this strategic plan.
The roundtable " Future Directions in Menopause Research: Optimizing Midlife Health of Women ," is scheduled for May 16, 2024, from 11 a.m. to 1 p.m. EDT. This event serves as the launch for the NIH Women's Health Roundtable Series as part of the White House Women's Health Research Initiative. The series will engage the extramural ...
Vitamin C is an antioxidant and is important for healthy immune function. The body also needs vitamin C to make collagen. The RDA ranges from 15 to 115 milligrams (mg) for infants, children, and teens, depending on age, and from 75 to 120 mg for nonsmoking adults. People who smoke need 35 mg more than the RDA per day.
In this episode, we talk with Dr. Robert Heinssen, a leader in the development and adoption of coordinated specialty care for treating psychosis. We learn about the signs and symptoms of psychosis, discuss coordinated specialty care, and explore how NIMH research in psychosis and schizophrenia fundamentally changed the health care landscape.
Starting people with opioid use disorder on extended-release, injectable naltrexone (XR-naltrexone) within five to seven days of seeking treatment is more effective than the standard treatment method of starting within 10-15 days, but requires closer medical supervision, according to results from a clinical trial supported by the National Institutes of Health's (NIH) National Institute on ...